User login
Survey of Academic PHM Programs in the US
Pediatric hospital medicine (PHM) is a relatively new field that has been growing rapidly over the past 20 years.[1] The field has been increasingly recognized for its contributions to high‐quality patient care, patient safety, systems improvement, medical education, and research.[2, 3, 4, 5, 6, 7, 8, 9] However, there appears to be significant variation among programs, even in basic factors such as how clinical effort is defined, the extent of in‐house coverage provided, and the scope of clinical services provided, and there exists a paucity of data describing these variations.[8]
Most previously published work did not specifically focus on academic programs,[2, 3, 8, 9] and specifically targeted hospital leadership,[2] practicing hospitalists,[3] residents,[7] and pediatric residency or clerkship directors,[4, 7] rather than hospitalist directors.[9] Furthermore, previous work focused on specific aspects of PHM programs such as education,[4, 7] value,[2] work environment,[9] and clinical practice,[3] rather than a more comprehensive approach.
We conducted a survey of academic PHM programs to learn about the current state and variation among programs across multiple domains (organizational, administrative, and financial). We speculated that:
- Many institutions currently lacking an academic PHM program were planning on starting a program in the next 3 years.
- Variability exists in hospitalist workload among programs.
- In programs providing clinical coverage at more than 1 site, variability exists in the relationship between the main site and satellite site(s) in terms of decision making, scheduling, and reporting of performance.
METHODS
Sample
We used the online American Medical Association Fellowship and Residency Electronic Interactive Database (FREIDA) to identify all 198 accredited pediatric residency training programs in the United States. A total of 246 hospitals were affiliated with these programs, and all of these were targeted for the survey. In addition, academic PHM program leaders were targeted directly with email invitations through the American Academy of Pediatrics (AAP) Section on Hospital Medicine LISTSERV.
Survey Instrument
A 49‐question online survey on the administrative, organizational, and financial aspects of academic PHM programs was developed with the input of academic PHM hospital leaders from Cincinnati Children's Hospital Medical Center and St. Louis Children's Hospital. First, the survey questions were developed de novo by the researchers. Then, multiple hospitalist leaders from each institution took the survey and gave feedback on content and structure. Using this feedback, changes were made and then tested by the leaders taking the new version of the survey. This process was repeated for 3 cycles until consensus was reached by the researchers on the final version of the survey. The survey contained questions that asked if the program provided coverage at a single site or at multiple sites and utilized a combination of open‐ended and fixed‐choice questions. For some questions, more than 1 answer was permitted. For the purposes of this survey, we utilized the following definitions adapted from the Society of Hospital Medicine. A hospitalist was defined as a physician who specializes in the practice of hospital medicine.[10] An academic PHM program was defined as any hospitalist practice associated with a pediatric residency program.[11] A nocturnist was defined as a hospitalist who predominantly works a schedule providing night coverage.[12]
Survey Administration
SurveyMonkey, an online survey software, was used to administer the survey. In June 2011, letters were mailed to all 246 hospitals affiliated with an accredited pediatric residency program as described above. These were addressed to either the hospital medicine director (if identified using the institutions Web site) or pediatric residency director. The letter asked the recipient to either participate in the survey or forward the survey to the physician best able to answer the survey. The letters included a description of the study and a link to the online survey. Of note, there was no follow‐up on this process. We also distributed the direct link to the survey and a copy of the letter utilizing the AAP Section on Hospital Medicine LISTSERV. Two reminders were sent through the LISTSERV in the month after the initial request. All respondents were informed that they would receive the deidentified raw data as an incentive to participate in the survey. Respondents were defined as those answering the first question, Does your program have an academic hospitalist program?
Statistical Analysis
Completed survey responses were extracted to Microsoft Excel (Microsoft Corp., Redmond, WA) for data analysis. Basic statistics were utilized to determine response rates for each question. Data were stratified for program type (single site or at multiple sites). For some questions, data were further stratified for the main site of multiple‐site programs for comparison to single‐site programs. In a few instances, more than 1 physician from a particular program responded to the survey. For these, the most appropriate respondent (PHM director, residency director, senior hospitalist) was identified utilizing the programs' publicly available Web site; only that physician's answers were used in the analysis.
Human Subjects Protection
This study was determined to be exempt from review by the Cincinnati Children's Hospital Medical Center and Washington University in St. Louis institutional review boards. All potential responders received written information about the survey. Survey design allowed for anonymous responses with voluntary documentation of program name and responders' contact information. The willingness to respond was qualified as implied consent. Data were deidentified prior to analysis and prior to sharing with the survey participants.
RESULTS
Response Rates
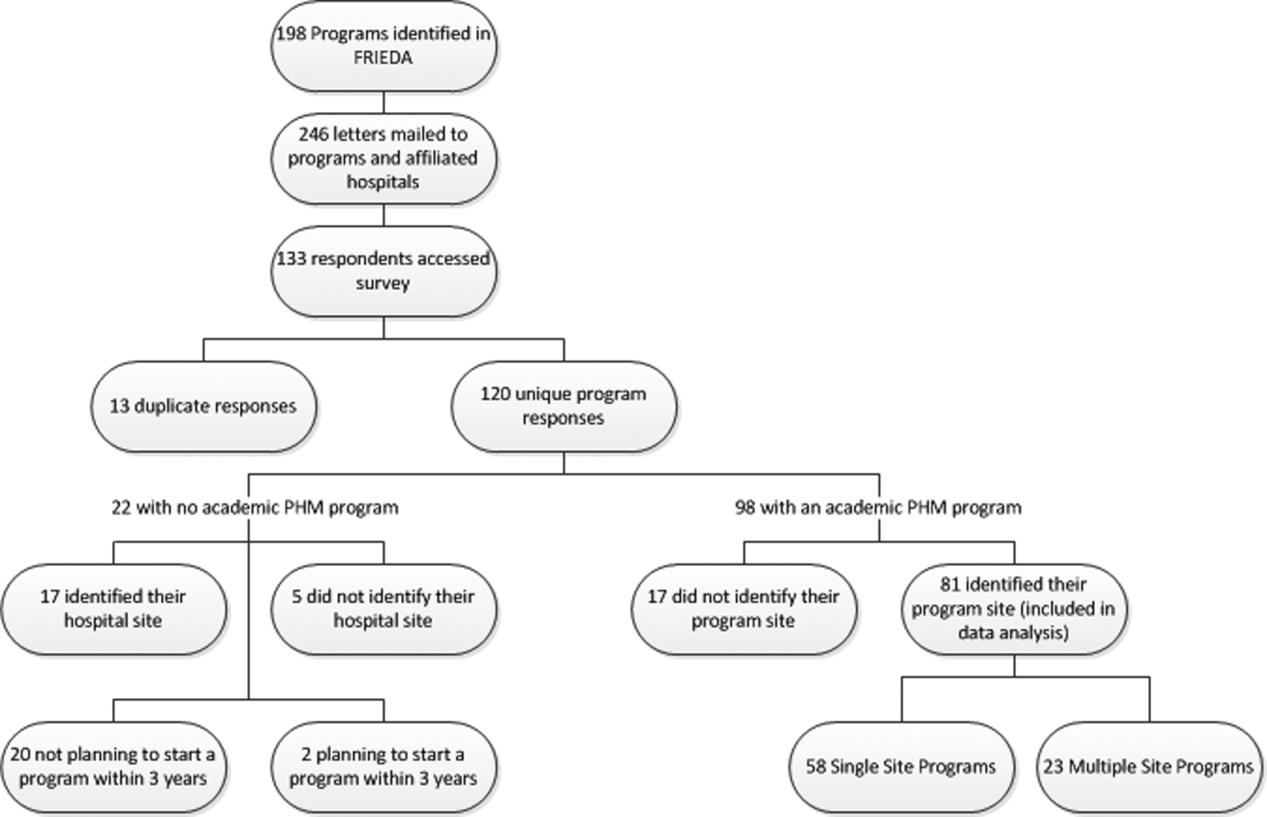
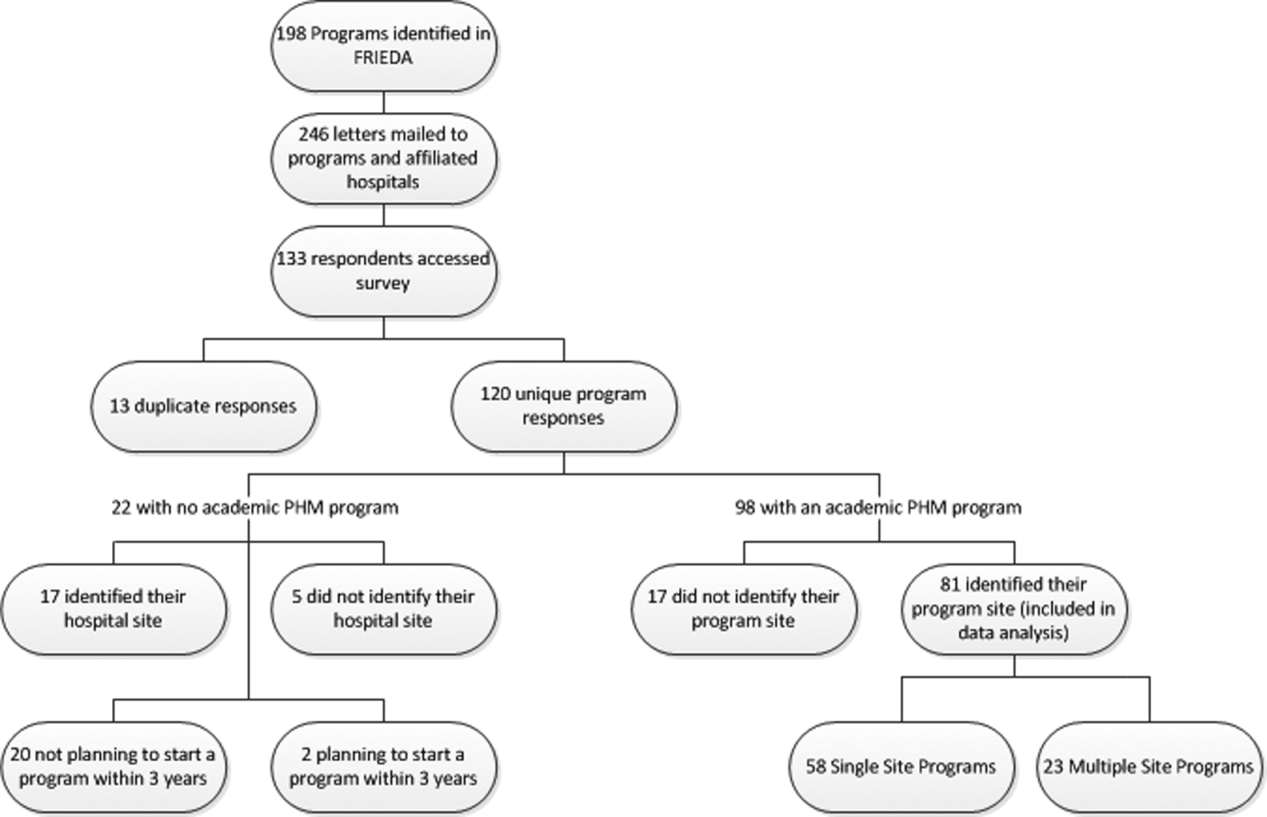
A total of 133 responses were received. Duplicate responses from the same program (13/133) were eliminated from the analysis. This yielded an overall response rate of 48.8% (120/246). A total of 81.7% (98/120) of institutions reported having an academic PHM program. Of the 18.3% (22/120) of institutions reporting not having a program, 9.1% (2/22) reported planning on starting a program in the next 3 years. Of the 98 respondents with an academic PHM program, 17 answered only the first survey question, Does your program have an academic hospitalist program? The remaining 81 completed surveys were left for further analysis. All of these respondents identified their program, and therefore we are certain that there were no duplicate responses in the analytic dataset. Of these, 23 (28%) indicated that their programs provided clinical care at multiple sites, and 58 (72%) indicated that their program provided care at a single site (Figure 1).
Administrative
Respondents reported wide variation for the definition of a 1.0 full‐time employee (FTE) hospitalist in their group. This included the units used (hours/year, weeks/year, shifts/year) as well as actual physician workload (Table 1). Weeks/year was the most common unit utilized by programs to define workload (66% of single‐site programs, 48% of multiple‐site programs), followed by hours/year (19%, 22%) and shifts/year (14%, 22%). The mean and median workload per FTE is represented (Table 1). The large ranges and the standard deviations from the mean indicate variability in workload per FTE (Table 1).
| Single‐Site Program | Multiple‐Site Programs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Programs | Mean | Median | SD | Range | % Programs | Mean | Median | SD | Range | |
| ||||||||||
| Weeks on service | 66 | 27.14 | 26 | 8.1 | 1246 | 48 | 27.2 | 24 | 9.6 | 1736 |
| Hours/year | 19 | 1886.25 | 1880 | 231.2 | 16002300 | 22 | 1767.33 | 1738 | 109.0 | 16641944 |
| Shifts/year* | 14 | 183 | 191 | 52.2 | 182240 | 22 | 191 | 184 | 38.3 | 155214 |
Scheduled in‐house hospitalist coverage also varied. Daytime coverage was defined as until 3 to 5 pm, evening coverage was defined a until 10 pm to midnight, and 24‐hour coverage was defined a 24/7. Programs reported plans to increase in‐house coverage with the implementation of the 2011 Accreditation Council for Graduate Medical Education (ACGME) resident work hours restrictions.[13] Among single‐site programs, there was a planned 50% increase in day/evening coverage (14% to 21%), with a planned decrease in day‐only coverage, and no change in 24/7 coverage (Table 2). Among the main sites of multiple‐site programs, there was a planned 50% increase in 24/7 in‐house coverage (35% to 52%), with a planned decrease in day‐only coverage, and no change in day/evening coverage (Table 3). Among the satellite sites of multiple‐site programs, there was a planned 9% increase in 24/7 coverage (41% to 50%), with a planned decrease in day‐only coverage, and no change in day/evening coverage (Table 2). Most programs reported that all hospitalists share night coverage (87% single site, 89% multiple sites) (Table 2). Multiple‐site programs were more likely than single‐site programs to use nocturnists, moonlighters, and incentives for those providing evening or night coverage (Table 2).
| Single Site (n=58) | Main Site of Multiple‐Site Programs (n=23) | |||
|---|---|---|---|---|
| Proportion | Response Rate | Proportion | Response Rate | |
| ||||
| Organizational | ||||
| Night shifts | .79 (46/58) | .83 (19/23) | ||
| All share nights | .87 (40/46) | .89 (17/19) | ||
| Nocturnists | .09 (4/46) | .26 (5/19) | ||
| Moonlighters | .04 (2/46) | .12 (2/19) | ||
| Night shift incentives | .74 (43/58) | .78 (18/23) | ||
| Financial | .12 (5/43) | .28 (5/18) | ||
| Time | .12 (5/43) | .22 (4/18) | ||
| No incentives | .79 (34/43) | .61 (11/18) | ||
| In‐house hospitalist coverage pre July 2011a | 1.0 (58/58) | 1.0 (23/23) | ||
| 24/7 | .29 (17/58) | .35 (8/23) | ||
| Day and evening | .14 (8/58) | .17 (4/23) | ||
| Day only | .57 (33/58) | .48 (11/23) | ||
| In‐house hospitalist coverage post July 2011a | 1.0 (58/58) | 1.0 (23/23) | ||
| 24/7 | .29 (17/58) | .52 (12/23) | ||
| Day and evening | .21 (12/58) | .17 (4/23) | ||
| Day only | .50 (29/58) | .30 (7/23) | ||
| Administrative | ||||
| Own division | .32 (18/57) | .98 (57/58) | .74 (17/23) | 1.0 (23/23) |
| Part of another division | .68 (39/57) | .26 (6/23) | ||
| Financial | ||||
| Revenues>expenses | .26 (14/53) | .91 (53/58) | .04 (1/23) | .04 (19/23) |
| Incentives supplement base salary | .45 (25/55) | .95 (55/58) | .48 (10/21) | .91 (21/23) |
| Metrics used to determine incentivesb | .47 (27/58) | .52 (12/23) | ||
| RVUs/MD | .85 (23/27) | .83 (10/12) | ||
| Costs/discharge | .19 (5/27) | .08 (1/12) | ||
| Financial reportingb | .81 (47/58) | .04 (19/23) | ||
| Charges | .64 (30/47) | .68 (13/19) | ||
| Collections | .66 (31/47) | .68 (13/19) | ||
| RVUs | .77 (36/47) | .47 (9/19) | ||
| Main Site (n=23) | Satellite Sites (n=51) | |||
|---|---|---|---|---|
| Proportion | Response Rate | Proportion | Response Rate | |
| In‐house hospitalist coverage pre July 2011 | 1.0 (23/23) | .80 (41/51) | ||
| 24/7 | .35 (8/23) | .41 (17/41) | ||
| Day and evening | .17 (4/23) | .10 (4/41) | ||
| Day only | .48 (11/23) | .49 (20/41) | ||
| In‐house hospitalist coverage post July 2011 | 1.0 (23/23) | |||
| 24/7 | .52 (12/23) | .50 (19/38) | .75 (38/51) | |
| Day and evening | .17 (4/23) | .11 (4/38) | ||
| Day only | .30 (7/23) | .39 (15/38) | ||
| Night shift coverage | .83 (19/23) | .78 (18/23) | ||
| All share nights | .89 (17/19) | .94 (17/18) | ||
| Nocturnists | .26 (5/19) | .22 (4/18) | ||
| Moonlighters | .12 (2/19) | .17 (3/18) | ||
The vast majority of multiple‐site programs reported that their different clinical sites are considered parts of a single hospitalist program (96%), and that there is a designated medical director for each site (83%). However, only 70% of multiple‐site programs report that decisions concerning physician coverage are made as a group, and only 65% report that scheduling is done centrally. In addition, there is variability in how quality, safety, and patient satisfaction is reported (group vs site). The majority of programs report sharing revenues and expenses among the sites (Table 4).
| Proportion | Response Rate | |
|---|---|---|
| Sites regularly collaborate on: | 1.0 (23/23) | |
| Quality improvement projects | .74 (17/23) | |
| Safety initiatives | .74 (17/23) | |
| Research | .48 (11/23) | |
| Have a designated hospitalist medical director for each site | .83 (19/23) | 1.0 (23/23) |
| Different sites considered parts of a single hospitalist program | .96 (22/23) | 1.0 (23/23) |
| Make decisions on program/coverage/hour changes as a group | .70 (16/23) | 1.0 (23/23) |
| Scheduling done centrally | .65 (15/23) | 1.0 (23/23) |
| Report or track the following as individual sites: | ||
| Quality measures | .43 (9/21) | .91 (21/23) |
| Safety measures | .48 (10/21) | .91 (21/23) |
| Patient satisfaction | .50 (10/20) | .87 (20/23) |
| Report or track the following as a group: | ||
| Quality measures | .33 (7/21) | .91 (21/23) |
| Safety measures | .33 (7/21) | .91 (21/23) |
| Patient satisfaction | .30 (6/20) | .87 (20/23) |
| Report or track the following as both individual sites and as a group: | ||
| Quality measures | .24 (5/21) | .91 (21/23) |
| Safety measures | .19 (4/21) | .91 (21/23) |
| Patient satisfaction | .25 (4/20) | .87 (20/23) |
| Sites share revenues and expenses | .67 (14/21) | .91 (21/23) |
Organizational
Of the single‐site programs that answered the question Is your hospital medicine program considered its own division or a section within another division? 32% reported that their programs were considered its own division, and 68% reported that they were a part of another division, predominately (62%) general pediatrics, but also a few (6% combined) within emergency medicine, critical care, physical medicine and rehabilitation, and infectious diseases. Of the multiple‐site programs, a majority of 74% programs were their own division, and 26% were part of another division (Table 2). Respondents reported that their satellite sites included pediatric units in small community hospitals, small pediatric hospitals, large nonpediatric hospitals with pediatric units, rehabilitation facilities, and Shriner orthopedic hospitals.
Financial
Of the single‐site programs that answered the question Do patient revenues produced by your hospitalist group cover all expenses? only 26% reported that revenues exceeded expenses. Of the multiple‐site programs responding to this question, only 4% reported that the main site of their programs had revenues greater than expenses (Table 2). Programs used a combination of metrics to report revenue, and relative value unit (RVU)/medical doctor (MD) is the most commonly used metric to determine incentive pay (Table 2).
DISCUSSION
Our study demonstrates that academic PHM programs are common, which is consistent with previous data.[4, 7, 9, 14] The data support our belief that more institutions are planning on starting PHM programs. However, there exist much variability in a variety of program factors.[2, 3, 8, 9, 14] The fact that up to 35% of categorical pediatric residents are considering a career as a hospitalist further highlights the need for better data on PHM programs.[7]
We demonstrated that variability existed in hospitalist workload at academic PHM programs. We found considerable variation in the workload per hospitalist (large ranges and standard deviations), as well as variability in how an FTE is defined (hours/year, weeks/year, shifts/year) (Table 1). In addition, survey respondents might have interpreted certain questions differently, and this might have led to increased variability in the data. For example, the question concerning the definition of an FTE was worded as A clinical FTE is defined as. Some of the reported variation in workload might be partially explained by hospitalists having additional nonclinical responsibilities within hospital medicine or another field, including protected time for quality improvement, medical education, research, or administrative activities. Furthermore, some hospitalists might have clinical responsibilities outside of hospital medicine. Given that most PHM programs lack a formal internal definition of what it means to be a hospitalist,[7] it is not surprising to find such variation between programs. The variability in the extent of in‐house coverage provided by academic PHM programs, as well as institutional plans for increased coverage with the 2011 residency work‐hours restrictions is also described, and is consistent with other recently published data.[14] This is likely to continue, as 70% of academic PHM programs reported an anticipated increase in coverage in the near future,[14] suggesting that academic hospitalists are being used to help fill gaps in coverage left by changes in resident staffing.
Our data describe the percentage of academic programs that have a distinct division of hospital medicine. The fact that multisite programs were more likely to report being a distinct division might reflect the increased complexities of providing care at more than 1 site, requiring a greater infrastructure. This might be important in institutional planning as well as academic and financial expectations of academic pediatric hospitalists.
We also demonstrated that programs with multiple sites differ as far as the degree of integration of the various sites, with variation reported in decision making, scheduling, and how quality, safety, and patient satisfaction are reported (Table 4). Whether or not increased integration between the various clinical sites of a multiple‐site program is associated with better performance and/or physician satisfaction are questions that need to be answered. However, academic PHM directors would likely agree that there are great challenges inherent in managing these programs. These challenges include professional integration (do hospitalists based at satellite sites feel that they are academically supported?), clinical work/expectations (fewer resources and fewer learners at satellite sites likely affects workload), and administrative issues (physician scheduling likely becomes more complex as the number of sites increases). As programs continue to grow and provide clinical services in multiple geographic sites, it will become more important to understand how the different sites are coordinated to identify and develop best practices.
Older studies have described that the majority of PHM programs (70%78%) reported that professional revenues do not cover expenses, unfortunately these results were not stratified for program type (academic vs community).[2, 9]
Our study describes that few academic PHM programs (26% of single site, 4% of multiple‐site programs) report revenues (defined in our survey as only the collections from professional billing) in excess of expenses. This is consistent with prior studies that have included both academic and community PHM programs.[2] Therefore, it appears to be common for PHM programs to require institutional funding to cover all program expenses, as collections from professional billing are not generally adequate for this purpose. We believe that this is a critical point for both hospitalists and administrators to understand. However, it is equally important that they be transparent about the importance and value of the nonrevenue‐generating work performed by PHM programs. It has been reported that the vast majority of pediatric hospitalists are highly involved in education, quality improvement work, practice guideline development, and other work that is vitally important to institutions.[3] Furthermore, although one might expect PHM leaders to believe that their programs add value beyond the professional revenue collected,[9] even hospital leadership has been reported to perceive that PHM programs add value in several ways, including increased patient satisfaction (94%), increased referring MD satisfaction (90%), decreased length of stay (81%), and decreased costs (62%).[2] Pediatric residency and clerkship directors report that pediatric hospitalists are more accessible than other faculty (84% vs 64%) and are associated with an increase in the practice of evidence‐based medicine (76% vs 61%).[4] Therefore, there is strong evidence supporting that pediatric hospitalist programs provide important value that is not evident on a balance sheet.
In addition, our data also indicate that programs currently use a variety of metrics in combination to report productivity, and there is no accepted gold standard for measuring the performance of a hospitalist or hospitalist program (Table 2). Given that hospitalists generally cannot control how many patients they see, and given the fact that hospitalists are strongly perceived to provide value to their institutions beyond generating clinical revenue, metrics such as RVUs and charges likely do not accurately represent actual productivity.[2] Furthermore, it is likely that the metrics currently used underestimate actual productivity as they are not designed to take into account confounding factors that might affect hospitalist productivity. For example, consider an academic hospitalist who has clinical responsibilities divided between direct patient care and supervisory patient care (such as a team with some combination of residents, medical students, and physician extenders). When providing direct patient care, the hospitalist is likely responsible or all of the tasks usually performed by residents, including writing all patient notes and prescriptions, all communication with families, nurses, specialists, and primary care providers; and discharge planning. Conversely, when providing supervisory care, it is likely that the tasks are divided among the team members, and the hospitalist has the additional responsibility for providing teaching. However, the hospitalist might be responsible for more complex and acute patients. These factors are not adequately measured by RVUs or professional billing. Furthermore, these metrics do not capture the differences in providing in‐house daytime versus evening/night coverage, and do not measure the work performed while being on call when outside of the hospital. It is important for PHM programs and leaders to develop a better representation of the value provided by hospitalists, and for institutional leaders to understand this value, because previous work has suggested that the majority of hospital leaders do not plan to phase out the subsidy of hospitalists over time, as they do not anticipate the program(s) will be able to covercosts.[2] Given the realities of decreasing reimbursement and healthcare reform, it is unlikely to become more common for PHM programs to generate enough professional revenue to cover expenses.
The main strength of this descriptive study is the comprehensive nature of the survey, including many previously unreported data. In addition, the data are consistent with previously published work, which validates the quality of the data.
This study has several limitations including a low response rate and the exclusion of some hospitals or programs because they provided insufficient data for analysis. However, a post hoc analysis demonstrated that the majority of the institutions reporting that they did not have an academic PHM program (18/22), and those that were excluded due to insufficient data (12/17) were either smaller residency programs (<60 residents) or hospitals that were not the main site of a residency program. Therefore, our data likely are a good representation of academic PHM programs at larger academic institutions. Another potential weakness is that, although PHM program directors and pediatric residency directors were targeted, the respondent might not have been the person with the best knowledge of the program, which could have produced inaccurate data, particularly in terms of finances. However, the general consistency of our findings with previous work, particularly the high percentage of institutions with academic PHM programs,[4, 7, 9, 14] the low percentage of programs with revenues greater than expenses,[2, 9] and the trend toward increased in‐house coverage associated with the 2011 ACGME work‐hour restrictions,[14] supports the validity of our other results. In addition, survey respondents might have interpreted certain questions differently, specifically the questions concerning the definition of an FTE, and this might have led to increased variability in the data.
CONCLUSIONS
Academic PHM programs exist in the vast majority of academic centers, and more institutions are planning on starting programs in the next few years. There appears to be variability in a number of program factors, including hospitalist workload, in‐house coverage, and whether the program is a separate division or a section within another academic division. Many programs are currently providing care at more than 1 site. Programs uncommonly reported that their revenues exceeded their expenses. These data are the most comprehensive data existing for academic PHM programs.
Acknowledgment
Disclosure: Nothing to report.
- . Pediatric hospital medicine: historical perspectives, inspired future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):107–112.
- , , . Assessing the value of pediatric hospitalist programs: the perspective of hospital leaders. Acad Pediatr. 2009;9(3):192–196.
- , . Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179–186.
- , , . Hospitalists' involvement in pediatrics training: perspectives from pediatric residency program and clerkship directors. Acad Med. 2009;84(11):1617–1621.
- , . Research in pediatric hospital medicine: how research will impact clinical care. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):127–130.
- , . Pediatric hospitalists in medical education: current roles and future directions. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):120–126.
- , , , , . Pediatric hospitalists' influences on education and career plans. J Hosp Med. 2012;7(4):282–286.
- , . Pediatric hospitalist systems versus traditional models of care: effect on quality and cost outcomes. J Hosp Med. 2012;7(4):350–357.
- , , , . Characteristics of the pediatric hospitalist workforce: its roles and work environment. Pediatrics. 2007;120(33):33–39.
- Society of Hospital Medicine. Definition of a hospitalist and hospital medicine. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Hospitalist_Definition7(4):299–303.
Pediatric hospital medicine (PHM) is a relatively new field that has been growing rapidly over the past 20 years.[1] The field has been increasingly recognized for its contributions to high‐quality patient care, patient safety, systems improvement, medical education, and research.[2, 3, 4, 5, 6, 7, 8, 9] However, there appears to be significant variation among programs, even in basic factors such as how clinical effort is defined, the extent of in‐house coverage provided, and the scope of clinical services provided, and there exists a paucity of data describing these variations.[8]
Most previously published work did not specifically focus on academic programs,[2, 3, 8, 9] and specifically targeted hospital leadership,[2] practicing hospitalists,[3] residents,[7] and pediatric residency or clerkship directors,[4, 7] rather than hospitalist directors.[9] Furthermore, previous work focused on specific aspects of PHM programs such as education,[4, 7] value,[2] work environment,[9] and clinical practice,[3] rather than a more comprehensive approach.
We conducted a survey of academic PHM programs to learn about the current state and variation among programs across multiple domains (organizational, administrative, and financial). We speculated that:
- Many institutions currently lacking an academic PHM program were planning on starting a program in the next 3 years.
- Variability exists in hospitalist workload among programs.
- In programs providing clinical coverage at more than 1 site, variability exists in the relationship between the main site and satellite site(s) in terms of decision making, scheduling, and reporting of performance.
METHODS
Sample
We used the online American Medical Association Fellowship and Residency Electronic Interactive Database (FREIDA) to identify all 198 accredited pediatric residency training programs in the United States. A total of 246 hospitals were affiliated with these programs, and all of these were targeted for the survey. In addition, academic PHM program leaders were targeted directly with email invitations through the American Academy of Pediatrics (AAP) Section on Hospital Medicine LISTSERV.
Survey Instrument
A 49‐question online survey on the administrative, organizational, and financial aspects of academic PHM programs was developed with the input of academic PHM hospital leaders from Cincinnati Children's Hospital Medical Center and St. Louis Children's Hospital. First, the survey questions were developed de novo by the researchers. Then, multiple hospitalist leaders from each institution took the survey and gave feedback on content and structure. Using this feedback, changes were made and then tested by the leaders taking the new version of the survey. This process was repeated for 3 cycles until consensus was reached by the researchers on the final version of the survey. The survey contained questions that asked if the program provided coverage at a single site or at multiple sites and utilized a combination of open‐ended and fixed‐choice questions. For some questions, more than 1 answer was permitted. For the purposes of this survey, we utilized the following definitions adapted from the Society of Hospital Medicine. A hospitalist was defined as a physician who specializes in the practice of hospital medicine.[10] An academic PHM program was defined as any hospitalist practice associated with a pediatric residency program.[11] A nocturnist was defined as a hospitalist who predominantly works a schedule providing night coverage.[12]
Survey Administration
SurveyMonkey, an online survey software, was used to administer the survey. In June 2011, letters were mailed to all 246 hospitals affiliated with an accredited pediatric residency program as described above. These were addressed to either the hospital medicine director (if identified using the institutions Web site) or pediatric residency director. The letter asked the recipient to either participate in the survey or forward the survey to the physician best able to answer the survey. The letters included a description of the study and a link to the online survey. Of note, there was no follow‐up on this process. We also distributed the direct link to the survey and a copy of the letter utilizing the AAP Section on Hospital Medicine LISTSERV. Two reminders were sent through the LISTSERV in the month after the initial request. All respondents were informed that they would receive the deidentified raw data as an incentive to participate in the survey. Respondents were defined as those answering the first question, Does your program have an academic hospitalist program?
Statistical Analysis
Completed survey responses were extracted to Microsoft Excel (Microsoft Corp., Redmond, WA) for data analysis. Basic statistics were utilized to determine response rates for each question. Data were stratified for program type (single site or at multiple sites). For some questions, data were further stratified for the main site of multiple‐site programs for comparison to single‐site programs. In a few instances, more than 1 physician from a particular program responded to the survey. For these, the most appropriate respondent (PHM director, residency director, senior hospitalist) was identified utilizing the programs' publicly available Web site; only that physician's answers were used in the analysis.
Human Subjects Protection
This study was determined to be exempt from review by the Cincinnati Children's Hospital Medical Center and Washington University in St. Louis institutional review boards. All potential responders received written information about the survey. Survey design allowed for anonymous responses with voluntary documentation of program name and responders' contact information. The willingness to respond was qualified as implied consent. Data were deidentified prior to analysis and prior to sharing with the survey participants.
RESULTS
Response Rates
A total of 133 responses were received. Duplicate responses from the same program (13/133) were eliminated from the analysis. This yielded an overall response rate of 48.8% (120/246). A total of 81.7% (98/120) of institutions reported having an academic PHM program. Of the 18.3% (22/120) of institutions reporting not having a program, 9.1% (2/22) reported planning on starting a program in the next 3 years. Of the 98 respondents with an academic PHM program, 17 answered only the first survey question, Does your program have an academic hospitalist program? The remaining 81 completed surveys were left for further analysis. All of these respondents identified their program, and therefore we are certain that there were no duplicate responses in the analytic dataset. Of these, 23 (28%) indicated that their programs provided clinical care at multiple sites, and 58 (72%) indicated that their program provided care at a single site (Figure 1).

Administrative
Respondents reported wide variation for the definition of a 1.0 full‐time employee (FTE) hospitalist in their group. This included the units used (hours/year, weeks/year, shifts/year) as well as actual physician workload (Table 1). Weeks/year was the most common unit utilized by programs to define workload (66% of single‐site programs, 48% of multiple‐site programs), followed by hours/year (19%, 22%) and shifts/year (14%, 22%). The mean and median workload per FTE is represented (Table 1). The large ranges and the standard deviations from the mean indicate variability in workload per FTE (Table 1).
| Single‐Site Program | Multiple‐Site Programs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Programs | Mean | Median | SD | Range | % Programs | Mean | Median | SD | Range | |
| ||||||||||
| Weeks on service | 66 | 27.14 | 26 | 8.1 | 1246 | 48 | 27.2 | 24 | 9.6 | 1736 |
| Hours/year | 19 | 1886.25 | 1880 | 231.2 | 16002300 | 22 | 1767.33 | 1738 | 109.0 | 16641944 |
| Shifts/year* | 14 | 183 | 191 | 52.2 | 182240 | 22 | 191 | 184 | 38.3 | 155214 |
Scheduled in‐house hospitalist coverage also varied. Daytime coverage was defined as until 3 to 5 pm, evening coverage was defined a until 10 pm to midnight, and 24‐hour coverage was defined a 24/7. Programs reported plans to increase in‐house coverage with the implementation of the 2011 Accreditation Council for Graduate Medical Education (ACGME) resident work hours restrictions.[13] Among single‐site programs, there was a planned 50% increase in day/evening coverage (14% to 21%), with a planned decrease in day‐only coverage, and no change in 24/7 coverage (Table 2). Among the main sites of multiple‐site programs, there was a planned 50% increase in 24/7 in‐house coverage (35% to 52%), with a planned decrease in day‐only coverage, and no change in day/evening coverage (Table 3). Among the satellite sites of multiple‐site programs, there was a planned 9% increase in 24/7 coverage (41% to 50%), with a planned decrease in day‐only coverage, and no change in day/evening coverage (Table 2). Most programs reported that all hospitalists share night coverage (87% single site, 89% multiple sites) (Table 2). Multiple‐site programs were more likely than single‐site programs to use nocturnists, moonlighters, and incentives for those providing evening or night coverage (Table 2).
| Single Site (n=58) | Main Site of Multiple‐Site Programs (n=23) | |||
|---|---|---|---|---|
| Proportion | Response Rate | Proportion | Response Rate | |
| ||||
| Organizational | ||||
| Night shifts | .79 (46/58) | .83 (19/23) | ||
| All share nights | .87 (40/46) | .89 (17/19) | ||
| Nocturnists | .09 (4/46) | .26 (5/19) | ||
| Moonlighters | .04 (2/46) | .12 (2/19) | ||
| Night shift incentives | .74 (43/58) | .78 (18/23) | ||
| Financial | .12 (5/43) | .28 (5/18) | ||
| Time | .12 (5/43) | .22 (4/18) | ||
| No incentives | .79 (34/43) | .61 (11/18) | ||
| In‐house hospitalist coverage pre July 2011a | 1.0 (58/58) | 1.0 (23/23) | ||
| 24/7 | .29 (17/58) | .35 (8/23) | ||
| Day and evening | .14 (8/58) | .17 (4/23) | ||
| Day only | .57 (33/58) | .48 (11/23) | ||
| In‐house hospitalist coverage post July 2011a | 1.0 (58/58) | 1.0 (23/23) | ||
| 24/7 | .29 (17/58) | .52 (12/23) | ||
| Day and evening | .21 (12/58) | .17 (4/23) | ||
| Day only | .50 (29/58) | .30 (7/23) | ||
| Administrative | ||||
| Own division | .32 (18/57) | .98 (57/58) | .74 (17/23) | 1.0 (23/23) |
| Part of another division | .68 (39/57) | .26 (6/23) | ||
| Financial | ||||
| Revenues>expenses | .26 (14/53) | .91 (53/58) | .04 (1/23) | .04 (19/23) |
| Incentives supplement base salary | .45 (25/55) | .95 (55/58) | .48 (10/21) | .91 (21/23) |
| Metrics used to determine incentivesb | .47 (27/58) | .52 (12/23) | ||
| RVUs/MD | .85 (23/27) | .83 (10/12) | ||
| Costs/discharge | .19 (5/27) | .08 (1/12) | ||
| Financial reportingb | .81 (47/58) | .04 (19/23) | ||
| Charges | .64 (30/47) | .68 (13/19) | ||
| Collections | .66 (31/47) | .68 (13/19) | ||
| RVUs | .77 (36/47) | .47 (9/19) | ||
| Main Site (n=23) | Satellite Sites (n=51) | |||
|---|---|---|---|---|
| Proportion | Response Rate | Proportion | Response Rate | |
| In‐house hospitalist coverage pre July 2011 | 1.0 (23/23) | .80 (41/51) | ||
| 24/7 | .35 (8/23) | .41 (17/41) | ||
| Day and evening | .17 (4/23) | .10 (4/41) | ||
| Day only | .48 (11/23) | .49 (20/41) | ||
| In‐house hospitalist coverage post July 2011 | 1.0 (23/23) | |||
| 24/7 | .52 (12/23) | .50 (19/38) | .75 (38/51) | |
| Day and evening | .17 (4/23) | .11 (4/38) | ||
| Day only | .30 (7/23) | .39 (15/38) | ||
| Night shift coverage | .83 (19/23) | .78 (18/23) | ||
| All share nights | .89 (17/19) | .94 (17/18) | ||
| Nocturnists | .26 (5/19) | .22 (4/18) | ||
| Moonlighters | .12 (2/19) | .17 (3/18) | ||
The vast majority of multiple‐site programs reported that their different clinical sites are considered parts of a single hospitalist program (96%), and that there is a designated medical director for each site (83%). However, only 70% of multiple‐site programs report that decisions concerning physician coverage are made as a group, and only 65% report that scheduling is done centrally. In addition, there is variability in how quality, safety, and patient satisfaction is reported (group vs site). The majority of programs report sharing revenues and expenses among the sites (Table 4).
| Proportion | Response Rate | |
|---|---|---|
| Sites regularly collaborate on: | 1.0 (23/23) | |
| Quality improvement projects | .74 (17/23) | |
| Safety initiatives | .74 (17/23) | |
| Research | .48 (11/23) | |
| Have a designated hospitalist medical director for each site | .83 (19/23) | 1.0 (23/23) |
| Different sites considered parts of a single hospitalist program | .96 (22/23) | 1.0 (23/23) |
| Make decisions on program/coverage/hour changes as a group | .70 (16/23) | 1.0 (23/23) |
| Scheduling done centrally | .65 (15/23) | 1.0 (23/23) |
| Report or track the following as individual sites: | ||
| Quality measures | .43 (9/21) | .91 (21/23) |
| Safety measures | .48 (10/21) | .91 (21/23) |
| Patient satisfaction | .50 (10/20) | .87 (20/23) |
| Report or track the following as a group: | ||
| Quality measures | .33 (7/21) | .91 (21/23) |
| Safety measures | .33 (7/21) | .91 (21/23) |
| Patient satisfaction | .30 (6/20) | .87 (20/23) |
| Report or track the following as both individual sites and as a group: | ||
| Quality measures | .24 (5/21) | .91 (21/23) |
| Safety measures | .19 (4/21) | .91 (21/23) |
| Patient satisfaction | .25 (4/20) | .87 (20/23) |
| Sites share revenues and expenses | .67 (14/21) | .91 (21/23) |
Organizational
Of the single‐site programs that answered the question Is your hospital medicine program considered its own division or a section within another division? 32% reported that their programs were considered its own division, and 68% reported that they were a part of another division, predominately (62%) general pediatrics, but also a few (6% combined) within emergency medicine, critical care, physical medicine and rehabilitation, and infectious diseases. Of the multiple‐site programs, a majority of 74% programs were their own division, and 26% were part of another division (Table 2). Respondents reported that their satellite sites included pediatric units in small community hospitals, small pediatric hospitals, large nonpediatric hospitals with pediatric units, rehabilitation facilities, and Shriner orthopedic hospitals.
Financial
Of the single‐site programs that answered the question Do patient revenues produced by your hospitalist group cover all expenses? only 26% reported that revenues exceeded expenses. Of the multiple‐site programs responding to this question, only 4% reported that the main site of their programs had revenues greater than expenses (Table 2). Programs used a combination of metrics to report revenue, and relative value unit (RVU)/medical doctor (MD) is the most commonly used metric to determine incentive pay (Table 2).
DISCUSSION
Our study demonstrates that academic PHM programs are common, which is consistent with previous data.[4, 7, 9, 14] The data support our belief that more institutions are planning on starting PHM programs. However, there exist much variability in a variety of program factors.[2, 3, 8, 9, 14] The fact that up to 35% of categorical pediatric residents are considering a career as a hospitalist further highlights the need for better data on PHM programs.[7]
We demonstrated that variability existed in hospitalist workload at academic PHM programs. We found considerable variation in the workload per hospitalist (large ranges and standard deviations), as well as variability in how an FTE is defined (hours/year, weeks/year, shifts/year) (Table 1). In addition, survey respondents might have interpreted certain questions differently, and this might have led to increased variability in the data. For example, the question concerning the definition of an FTE was worded as A clinical FTE is defined as. Some of the reported variation in workload might be partially explained by hospitalists having additional nonclinical responsibilities within hospital medicine or another field, including protected time for quality improvement, medical education, research, or administrative activities. Furthermore, some hospitalists might have clinical responsibilities outside of hospital medicine. Given that most PHM programs lack a formal internal definition of what it means to be a hospitalist,[7] it is not surprising to find such variation between programs. The variability in the extent of in‐house coverage provided by academic PHM programs, as well as institutional plans for increased coverage with the 2011 residency work‐hours restrictions is also described, and is consistent with other recently published data.[14] This is likely to continue, as 70% of academic PHM programs reported an anticipated increase in coverage in the near future,[14] suggesting that academic hospitalists are being used to help fill gaps in coverage left by changes in resident staffing.
Our data describe the percentage of academic programs that have a distinct division of hospital medicine. The fact that multisite programs were more likely to report being a distinct division might reflect the increased complexities of providing care at more than 1 site, requiring a greater infrastructure. This might be important in institutional planning as well as academic and financial expectations of academic pediatric hospitalists.
We also demonstrated that programs with multiple sites differ as far as the degree of integration of the various sites, with variation reported in decision making, scheduling, and how quality, safety, and patient satisfaction are reported (Table 4). Whether or not increased integration between the various clinical sites of a multiple‐site program is associated with better performance and/or physician satisfaction are questions that need to be answered. However, academic PHM directors would likely agree that there are great challenges inherent in managing these programs. These challenges include professional integration (do hospitalists based at satellite sites feel that they are academically supported?), clinical work/expectations (fewer resources and fewer learners at satellite sites likely affects workload), and administrative issues (physician scheduling likely becomes more complex as the number of sites increases). As programs continue to grow and provide clinical services in multiple geographic sites, it will become more important to understand how the different sites are coordinated to identify and develop best practices.
Older studies have described that the majority of PHM programs (70%78%) reported that professional revenues do not cover expenses, unfortunately these results were not stratified for program type (academic vs community).[2, 9]
Our study describes that few academic PHM programs (26% of single site, 4% of multiple‐site programs) report revenues (defined in our survey as only the collections from professional billing) in excess of expenses. This is consistent with prior studies that have included both academic and community PHM programs.[2] Therefore, it appears to be common for PHM programs to require institutional funding to cover all program expenses, as collections from professional billing are not generally adequate for this purpose. We believe that this is a critical point for both hospitalists and administrators to understand. However, it is equally important that they be transparent about the importance and value of the nonrevenue‐generating work performed by PHM programs. It has been reported that the vast majority of pediatric hospitalists are highly involved in education, quality improvement work, practice guideline development, and other work that is vitally important to institutions.[3] Furthermore, although one might expect PHM leaders to believe that their programs add value beyond the professional revenue collected,[9] even hospital leadership has been reported to perceive that PHM programs add value in several ways, including increased patient satisfaction (94%), increased referring MD satisfaction (90%), decreased length of stay (81%), and decreased costs (62%).[2] Pediatric residency and clerkship directors report that pediatric hospitalists are more accessible than other faculty (84% vs 64%) and are associated with an increase in the practice of evidence‐based medicine (76% vs 61%).[4] Therefore, there is strong evidence supporting that pediatric hospitalist programs provide important value that is not evident on a balance sheet.
In addition, our data also indicate that programs currently use a variety of metrics in combination to report productivity, and there is no accepted gold standard for measuring the performance of a hospitalist or hospitalist program (Table 2). Given that hospitalists generally cannot control how many patients they see, and given the fact that hospitalists are strongly perceived to provide value to their institutions beyond generating clinical revenue, metrics such as RVUs and charges likely do not accurately represent actual productivity.[2] Furthermore, it is likely that the metrics currently used underestimate actual productivity as they are not designed to take into account confounding factors that might affect hospitalist productivity. For example, consider an academic hospitalist who has clinical responsibilities divided between direct patient care and supervisory patient care (such as a team with some combination of residents, medical students, and physician extenders). When providing direct patient care, the hospitalist is likely responsible or all of the tasks usually performed by residents, including writing all patient notes and prescriptions, all communication with families, nurses, specialists, and primary care providers; and discharge planning. Conversely, when providing supervisory care, it is likely that the tasks are divided among the team members, and the hospitalist has the additional responsibility for providing teaching. However, the hospitalist might be responsible for more complex and acute patients. These factors are not adequately measured by RVUs or professional billing. Furthermore, these metrics do not capture the differences in providing in‐house daytime versus evening/night coverage, and do not measure the work performed while being on call when outside of the hospital. It is important for PHM programs and leaders to develop a better representation of the value provided by hospitalists, and for institutional leaders to understand this value, because previous work has suggested that the majority of hospital leaders do not plan to phase out the subsidy of hospitalists over time, as they do not anticipate the program(s) will be able to covercosts.[2] Given the realities of decreasing reimbursement and healthcare reform, it is unlikely to become more common for PHM programs to generate enough professional revenue to cover expenses.
The main strength of this descriptive study is the comprehensive nature of the survey, including many previously unreported data. In addition, the data are consistent with previously published work, which validates the quality of the data.
This study has several limitations including a low response rate and the exclusion of some hospitals or programs because they provided insufficient data for analysis. However, a post hoc analysis demonstrated that the majority of the institutions reporting that they did not have an academic PHM program (18/22), and those that were excluded due to insufficient data (12/17) were either smaller residency programs (<60 residents) or hospitals that were not the main site of a residency program. Therefore, our data likely are a good representation of academic PHM programs at larger academic institutions. Another potential weakness is that, although PHM program directors and pediatric residency directors were targeted, the respondent might not have been the person with the best knowledge of the program, which could have produced inaccurate data, particularly in terms of finances. However, the general consistency of our findings with previous work, particularly the high percentage of institutions with academic PHM programs,[4, 7, 9, 14] the low percentage of programs with revenues greater than expenses,[2, 9] and the trend toward increased in‐house coverage associated with the 2011 ACGME work‐hour restrictions,[14] supports the validity of our other results. In addition, survey respondents might have interpreted certain questions differently, specifically the questions concerning the definition of an FTE, and this might have led to increased variability in the data.
CONCLUSIONS
Academic PHM programs exist in the vast majority of academic centers, and more institutions are planning on starting programs in the next few years. There appears to be variability in a number of program factors, including hospitalist workload, in‐house coverage, and whether the program is a separate division or a section within another academic division. Many programs are currently providing care at more than 1 site. Programs uncommonly reported that their revenues exceeded their expenses. These data are the most comprehensive data existing for academic PHM programs.
Acknowledgment
Disclosure: Nothing to report.
Pediatric hospital medicine (PHM) is a relatively new field that has been growing rapidly over the past 20 years.[1] The field has been increasingly recognized for its contributions to high‐quality patient care, patient safety, systems improvement, medical education, and research.[2, 3, 4, 5, 6, 7, 8, 9] However, there appears to be significant variation among programs, even in basic factors such as how clinical effort is defined, the extent of in‐house coverage provided, and the scope of clinical services provided, and there exists a paucity of data describing these variations.[8]
Most previously published work did not specifically focus on academic programs,[2, 3, 8, 9] and specifically targeted hospital leadership,[2] practicing hospitalists,[3] residents,[7] and pediatric residency or clerkship directors,[4, 7] rather than hospitalist directors.[9] Furthermore, previous work focused on specific aspects of PHM programs such as education,[4, 7] value,[2] work environment,[9] and clinical practice,[3] rather than a more comprehensive approach.
We conducted a survey of academic PHM programs to learn about the current state and variation among programs across multiple domains (organizational, administrative, and financial). We speculated that:
- Many institutions currently lacking an academic PHM program were planning on starting a program in the next 3 years.
- Variability exists in hospitalist workload among programs.
- In programs providing clinical coverage at more than 1 site, variability exists in the relationship between the main site and satellite site(s) in terms of decision making, scheduling, and reporting of performance.
METHODS
Sample
We used the online American Medical Association Fellowship and Residency Electronic Interactive Database (FREIDA) to identify all 198 accredited pediatric residency training programs in the United States. A total of 246 hospitals were affiliated with these programs, and all of these were targeted for the survey. In addition, academic PHM program leaders were targeted directly with email invitations through the American Academy of Pediatrics (AAP) Section on Hospital Medicine LISTSERV.
Survey Instrument
A 49‐question online survey on the administrative, organizational, and financial aspects of academic PHM programs was developed with the input of academic PHM hospital leaders from Cincinnati Children's Hospital Medical Center and St. Louis Children's Hospital. First, the survey questions were developed de novo by the researchers. Then, multiple hospitalist leaders from each institution took the survey and gave feedback on content and structure. Using this feedback, changes were made and then tested by the leaders taking the new version of the survey. This process was repeated for 3 cycles until consensus was reached by the researchers on the final version of the survey. The survey contained questions that asked if the program provided coverage at a single site or at multiple sites and utilized a combination of open‐ended and fixed‐choice questions. For some questions, more than 1 answer was permitted. For the purposes of this survey, we utilized the following definitions adapted from the Society of Hospital Medicine. A hospitalist was defined as a physician who specializes in the practice of hospital medicine.[10] An academic PHM program was defined as any hospitalist practice associated with a pediatric residency program.[11] A nocturnist was defined as a hospitalist who predominantly works a schedule providing night coverage.[12]
Survey Administration
SurveyMonkey, an online survey software, was used to administer the survey. In June 2011, letters were mailed to all 246 hospitals affiliated with an accredited pediatric residency program as described above. These were addressed to either the hospital medicine director (if identified using the institutions Web site) or pediatric residency director. The letter asked the recipient to either participate in the survey or forward the survey to the physician best able to answer the survey. The letters included a description of the study and a link to the online survey. Of note, there was no follow‐up on this process. We also distributed the direct link to the survey and a copy of the letter utilizing the AAP Section on Hospital Medicine LISTSERV. Two reminders were sent through the LISTSERV in the month after the initial request. All respondents were informed that they would receive the deidentified raw data as an incentive to participate in the survey. Respondents were defined as those answering the first question, Does your program have an academic hospitalist program?
Statistical Analysis
Completed survey responses were extracted to Microsoft Excel (Microsoft Corp., Redmond, WA) for data analysis. Basic statistics were utilized to determine response rates for each question. Data were stratified for program type (single site or at multiple sites). For some questions, data were further stratified for the main site of multiple‐site programs for comparison to single‐site programs. In a few instances, more than 1 physician from a particular program responded to the survey. For these, the most appropriate respondent (PHM director, residency director, senior hospitalist) was identified utilizing the programs' publicly available Web site; only that physician's answers were used in the analysis.
Human Subjects Protection
This study was determined to be exempt from review by the Cincinnati Children's Hospital Medical Center and Washington University in St. Louis institutional review boards. All potential responders received written information about the survey. Survey design allowed for anonymous responses with voluntary documentation of program name and responders' contact information. The willingness to respond was qualified as implied consent. Data were deidentified prior to analysis and prior to sharing with the survey participants.
RESULTS
Response Rates
A total of 133 responses were received. Duplicate responses from the same program (13/133) were eliminated from the analysis. This yielded an overall response rate of 48.8% (120/246). A total of 81.7% (98/120) of institutions reported having an academic PHM program. Of the 18.3% (22/120) of institutions reporting not having a program, 9.1% (2/22) reported planning on starting a program in the next 3 years. Of the 98 respondents with an academic PHM program, 17 answered only the first survey question, Does your program have an academic hospitalist program? The remaining 81 completed surveys were left for further analysis. All of these respondents identified their program, and therefore we are certain that there were no duplicate responses in the analytic dataset. Of these, 23 (28%) indicated that their programs provided clinical care at multiple sites, and 58 (72%) indicated that their program provided care at a single site (Figure 1).

Administrative
Respondents reported wide variation for the definition of a 1.0 full‐time employee (FTE) hospitalist in their group. This included the units used (hours/year, weeks/year, shifts/year) as well as actual physician workload (Table 1). Weeks/year was the most common unit utilized by programs to define workload (66% of single‐site programs, 48% of multiple‐site programs), followed by hours/year (19%, 22%) and shifts/year (14%, 22%). The mean and median workload per FTE is represented (Table 1). The large ranges and the standard deviations from the mean indicate variability in workload per FTE (Table 1).
| Single‐Site Program | Multiple‐Site Programs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Programs | Mean | Median | SD | Range | % Programs | Mean | Median | SD | Range | |
| ||||||||||
| Weeks on service | 66 | 27.14 | 26 | 8.1 | 1246 | 48 | 27.2 | 24 | 9.6 | 1736 |
| Hours/year | 19 | 1886.25 | 1880 | 231.2 | 16002300 | 22 | 1767.33 | 1738 | 109.0 | 16641944 |
| Shifts/year* | 14 | 183 | 191 | 52.2 | 182240 | 22 | 191 | 184 | 38.3 | 155214 |
Scheduled in‐house hospitalist coverage also varied. Daytime coverage was defined as until 3 to 5 pm, evening coverage was defined a until 10 pm to midnight, and 24‐hour coverage was defined a 24/7. Programs reported plans to increase in‐house coverage with the implementation of the 2011 Accreditation Council for Graduate Medical Education (ACGME) resident work hours restrictions.[13] Among single‐site programs, there was a planned 50% increase in day/evening coverage (14% to 21%), with a planned decrease in day‐only coverage, and no change in 24/7 coverage (Table 2). Among the main sites of multiple‐site programs, there was a planned 50% increase in 24/7 in‐house coverage (35% to 52%), with a planned decrease in day‐only coverage, and no change in day/evening coverage (Table 3). Among the satellite sites of multiple‐site programs, there was a planned 9% increase in 24/7 coverage (41% to 50%), with a planned decrease in day‐only coverage, and no change in day/evening coverage (Table 2). Most programs reported that all hospitalists share night coverage (87% single site, 89% multiple sites) (Table 2). Multiple‐site programs were more likely than single‐site programs to use nocturnists, moonlighters, and incentives for those providing evening or night coverage (Table 2).
| Single Site (n=58) | Main Site of Multiple‐Site Programs (n=23) | |||
|---|---|---|---|---|
| Proportion | Response Rate | Proportion | Response Rate | |
| ||||
| Organizational | ||||
| Night shifts | .79 (46/58) | .83 (19/23) | ||
| All share nights | .87 (40/46) | .89 (17/19) | ||
| Nocturnists | .09 (4/46) | .26 (5/19) | ||
| Moonlighters | .04 (2/46) | .12 (2/19) | ||
| Night shift incentives | .74 (43/58) | .78 (18/23) | ||
| Financial | .12 (5/43) | .28 (5/18) | ||
| Time | .12 (5/43) | .22 (4/18) | ||
| No incentives | .79 (34/43) | .61 (11/18) | ||
| In‐house hospitalist coverage pre July 2011a | 1.0 (58/58) | 1.0 (23/23) | ||
| 24/7 | .29 (17/58) | .35 (8/23) | ||
| Day and evening | .14 (8/58) | .17 (4/23) | ||
| Day only | .57 (33/58) | .48 (11/23) | ||
| In‐house hospitalist coverage post July 2011a | 1.0 (58/58) | 1.0 (23/23) | ||
| 24/7 | .29 (17/58) | .52 (12/23) | ||
| Day and evening | .21 (12/58) | .17 (4/23) | ||
| Day only | .50 (29/58) | .30 (7/23) | ||
| Administrative | ||||
| Own division | .32 (18/57) | .98 (57/58) | .74 (17/23) | 1.0 (23/23) |
| Part of another division | .68 (39/57) | .26 (6/23) | ||
| Financial | ||||
| Revenues>expenses | .26 (14/53) | .91 (53/58) | .04 (1/23) | .04 (19/23) |
| Incentives supplement base salary | .45 (25/55) | .95 (55/58) | .48 (10/21) | .91 (21/23) |
| Metrics used to determine incentivesb | .47 (27/58) | .52 (12/23) | ||
| RVUs/MD | .85 (23/27) | .83 (10/12) | ||
| Costs/discharge | .19 (5/27) | .08 (1/12) | ||
| Financial reportingb | .81 (47/58) | .04 (19/23) | ||
| Charges | .64 (30/47) | .68 (13/19) | ||
| Collections | .66 (31/47) | .68 (13/19) | ||
| RVUs | .77 (36/47) | .47 (9/19) | ||
| Main Site (n=23) | Satellite Sites (n=51) | |||
|---|---|---|---|---|
| Proportion | Response Rate | Proportion | Response Rate | |
| In‐house hospitalist coverage pre July 2011 | 1.0 (23/23) | .80 (41/51) | ||
| 24/7 | .35 (8/23) | .41 (17/41) | ||
| Day and evening | .17 (4/23) | .10 (4/41) | ||
| Day only | .48 (11/23) | .49 (20/41) | ||
| In‐house hospitalist coverage post July 2011 | 1.0 (23/23) | |||
| 24/7 | .52 (12/23) | .50 (19/38) | .75 (38/51) | |
| Day and evening | .17 (4/23) | .11 (4/38) | ||
| Day only | .30 (7/23) | .39 (15/38) | ||
| Night shift coverage | .83 (19/23) | .78 (18/23) | ||
| All share nights | .89 (17/19) | .94 (17/18) | ||
| Nocturnists | .26 (5/19) | .22 (4/18) | ||
| Moonlighters | .12 (2/19) | .17 (3/18) | ||
The vast majority of multiple‐site programs reported that their different clinical sites are considered parts of a single hospitalist program (96%), and that there is a designated medical director for each site (83%). However, only 70% of multiple‐site programs report that decisions concerning physician coverage are made as a group, and only 65% report that scheduling is done centrally. In addition, there is variability in how quality, safety, and patient satisfaction is reported (group vs site). The majority of programs report sharing revenues and expenses among the sites (Table 4).
| Proportion | Response Rate | |
|---|---|---|
| Sites regularly collaborate on: | 1.0 (23/23) | |
| Quality improvement projects | .74 (17/23) | |
| Safety initiatives | .74 (17/23) | |
| Research | .48 (11/23) | |
| Have a designated hospitalist medical director for each site | .83 (19/23) | 1.0 (23/23) |
| Different sites considered parts of a single hospitalist program | .96 (22/23) | 1.0 (23/23) |
| Make decisions on program/coverage/hour changes as a group | .70 (16/23) | 1.0 (23/23) |
| Scheduling done centrally | .65 (15/23) | 1.0 (23/23) |
| Report or track the following as individual sites: | ||
| Quality measures | .43 (9/21) | .91 (21/23) |
| Safety measures | .48 (10/21) | .91 (21/23) |
| Patient satisfaction | .50 (10/20) | .87 (20/23) |
| Report or track the following as a group: | ||
| Quality measures | .33 (7/21) | .91 (21/23) |
| Safety measures | .33 (7/21) | .91 (21/23) |
| Patient satisfaction | .30 (6/20) | .87 (20/23) |
| Report or track the following as both individual sites and as a group: | ||
| Quality measures | .24 (5/21) | .91 (21/23) |
| Safety measures | .19 (4/21) | .91 (21/23) |
| Patient satisfaction | .25 (4/20) | .87 (20/23) |
| Sites share revenues and expenses | .67 (14/21) | .91 (21/23) |
Organizational
Of the single‐site programs that answered the question Is your hospital medicine program considered its own division or a section within another division? 32% reported that their programs were considered its own division, and 68% reported that they were a part of another division, predominately (62%) general pediatrics, but also a few (6% combined) within emergency medicine, critical care, physical medicine and rehabilitation, and infectious diseases. Of the multiple‐site programs, a majority of 74% programs were their own division, and 26% were part of another division (Table 2). Respondents reported that their satellite sites included pediatric units in small community hospitals, small pediatric hospitals, large nonpediatric hospitals with pediatric units, rehabilitation facilities, and Shriner orthopedic hospitals.
Financial
Of the single‐site programs that answered the question Do patient revenues produced by your hospitalist group cover all expenses? only 26% reported that revenues exceeded expenses. Of the multiple‐site programs responding to this question, only 4% reported that the main site of their programs had revenues greater than expenses (Table 2). Programs used a combination of metrics to report revenue, and relative value unit (RVU)/medical doctor (MD) is the most commonly used metric to determine incentive pay (Table 2).
DISCUSSION
Our study demonstrates that academic PHM programs are common, which is consistent with previous data.[4, 7, 9, 14] The data support our belief that more institutions are planning on starting PHM programs. However, there exist much variability in a variety of program factors.[2, 3, 8, 9, 14] The fact that up to 35% of categorical pediatric residents are considering a career as a hospitalist further highlights the need for better data on PHM programs.[7]
We demonstrated that variability existed in hospitalist workload at academic PHM programs. We found considerable variation in the workload per hospitalist (large ranges and standard deviations), as well as variability in how an FTE is defined (hours/year, weeks/year, shifts/year) (Table 1). In addition, survey respondents might have interpreted certain questions differently, and this might have led to increased variability in the data. For example, the question concerning the definition of an FTE was worded as A clinical FTE is defined as. Some of the reported variation in workload might be partially explained by hospitalists having additional nonclinical responsibilities within hospital medicine or another field, including protected time for quality improvement, medical education, research, or administrative activities. Furthermore, some hospitalists might have clinical responsibilities outside of hospital medicine. Given that most PHM programs lack a formal internal definition of what it means to be a hospitalist,[7] it is not surprising to find such variation between programs. The variability in the extent of in‐house coverage provided by academic PHM programs, as well as institutional plans for increased coverage with the 2011 residency work‐hours restrictions is also described, and is consistent with other recently published data.[14] This is likely to continue, as 70% of academic PHM programs reported an anticipated increase in coverage in the near future,[14] suggesting that academic hospitalists are being used to help fill gaps in coverage left by changes in resident staffing.
Our data describe the percentage of academic programs that have a distinct division of hospital medicine. The fact that multisite programs were more likely to report being a distinct division might reflect the increased complexities of providing care at more than 1 site, requiring a greater infrastructure. This might be important in institutional planning as well as academic and financial expectations of academic pediatric hospitalists.
We also demonstrated that programs with multiple sites differ as far as the degree of integration of the various sites, with variation reported in decision making, scheduling, and how quality, safety, and patient satisfaction are reported (Table 4). Whether or not increased integration between the various clinical sites of a multiple‐site program is associated with better performance and/or physician satisfaction are questions that need to be answered. However, academic PHM directors would likely agree that there are great challenges inherent in managing these programs. These challenges include professional integration (do hospitalists based at satellite sites feel that they are academically supported?), clinical work/expectations (fewer resources and fewer learners at satellite sites likely affects workload), and administrative issues (physician scheduling likely becomes more complex as the number of sites increases). As programs continue to grow and provide clinical services in multiple geographic sites, it will become more important to understand how the different sites are coordinated to identify and develop best practices.
Older studies have described that the majority of PHM programs (70%78%) reported that professional revenues do not cover expenses, unfortunately these results were not stratified for program type (academic vs community).[2, 9]
Our study describes that few academic PHM programs (26% of single site, 4% of multiple‐site programs) report revenues (defined in our survey as only the collections from professional billing) in excess of expenses. This is consistent with prior studies that have included both academic and community PHM programs.[2] Therefore, it appears to be common for PHM programs to require institutional funding to cover all program expenses, as collections from professional billing are not generally adequate for this purpose. We believe that this is a critical point for both hospitalists and administrators to understand. However, it is equally important that they be transparent about the importance and value of the nonrevenue‐generating work performed by PHM programs. It has been reported that the vast majority of pediatric hospitalists are highly involved in education, quality improvement work, practice guideline development, and other work that is vitally important to institutions.[3] Furthermore, although one might expect PHM leaders to believe that their programs add value beyond the professional revenue collected,[9] even hospital leadership has been reported to perceive that PHM programs add value in several ways, including increased patient satisfaction (94%), increased referring MD satisfaction (90%), decreased length of stay (81%), and decreased costs (62%).[2] Pediatric residency and clerkship directors report that pediatric hospitalists are more accessible than other faculty (84% vs 64%) and are associated with an increase in the practice of evidence‐based medicine (76% vs 61%).[4] Therefore, there is strong evidence supporting that pediatric hospitalist programs provide important value that is not evident on a balance sheet.
In addition, our data also indicate that programs currently use a variety of metrics in combination to report productivity, and there is no accepted gold standard for measuring the performance of a hospitalist or hospitalist program (Table 2). Given that hospitalists generally cannot control how many patients they see, and given the fact that hospitalists are strongly perceived to provide value to their institutions beyond generating clinical revenue, metrics such as RVUs and charges likely do not accurately represent actual productivity.[2] Furthermore, it is likely that the metrics currently used underestimate actual productivity as they are not designed to take into account confounding factors that might affect hospitalist productivity. For example, consider an academic hospitalist who has clinical responsibilities divided between direct patient care and supervisory patient care (such as a team with some combination of residents, medical students, and physician extenders). When providing direct patient care, the hospitalist is likely responsible or all of the tasks usually performed by residents, including writing all patient notes and prescriptions, all communication with families, nurses, specialists, and primary care providers; and discharge planning. Conversely, when providing supervisory care, it is likely that the tasks are divided among the team members, and the hospitalist has the additional responsibility for providing teaching. However, the hospitalist might be responsible for more complex and acute patients. These factors are not adequately measured by RVUs or professional billing. Furthermore, these metrics do not capture the differences in providing in‐house daytime versus evening/night coverage, and do not measure the work performed while being on call when outside of the hospital. It is important for PHM programs and leaders to develop a better representation of the value provided by hospitalists, and for institutional leaders to understand this value, because previous work has suggested that the majority of hospital leaders do not plan to phase out the subsidy of hospitalists over time, as they do not anticipate the program(s) will be able to covercosts.[2] Given the realities of decreasing reimbursement and healthcare reform, it is unlikely to become more common for PHM programs to generate enough professional revenue to cover expenses.
The main strength of this descriptive study is the comprehensive nature of the survey, including many previously unreported data. In addition, the data are consistent with previously published work, which validates the quality of the data.
This study has several limitations including a low response rate and the exclusion of some hospitals or programs because they provided insufficient data for analysis. However, a post hoc analysis demonstrated that the majority of the institutions reporting that they did not have an academic PHM program (18/22), and those that were excluded due to insufficient data (12/17) were either smaller residency programs (<60 residents) or hospitals that were not the main site of a residency program. Therefore, our data likely are a good representation of academic PHM programs at larger academic institutions. Another potential weakness is that, although PHM program directors and pediatric residency directors were targeted, the respondent might not have been the person with the best knowledge of the program, which could have produced inaccurate data, particularly in terms of finances. However, the general consistency of our findings with previous work, particularly the high percentage of institutions with academic PHM programs,[4, 7, 9, 14] the low percentage of programs with revenues greater than expenses,[2, 9] and the trend toward increased in‐house coverage associated with the 2011 ACGME work‐hour restrictions,[14] supports the validity of our other results. In addition, survey respondents might have interpreted certain questions differently, specifically the questions concerning the definition of an FTE, and this might have led to increased variability in the data.
CONCLUSIONS
Academic PHM programs exist in the vast majority of academic centers, and more institutions are planning on starting programs in the next few years. There appears to be variability in a number of program factors, including hospitalist workload, in‐house coverage, and whether the program is a separate division or a section within another academic division. Many programs are currently providing care at more than 1 site. Programs uncommonly reported that their revenues exceeded their expenses. These data are the most comprehensive data existing for academic PHM programs.
Acknowledgment
Disclosure: Nothing to report.
- . Pediatric hospital medicine: historical perspectives, inspired future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):107–112.
- , , . Assessing the value of pediatric hospitalist programs: the perspective of hospital leaders. Acad Pediatr. 2009;9(3):192–196.
- , . Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179–186.
- , , . Hospitalists' involvement in pediatrics training: perspectives from pediatric residency program and clerkship directors. Acad Med. 2009;84(11):1617–1621.
- , . Research in pediatric hospital medicine: how research will impact clinical care. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):127–130.
- , . Pediatric hospitalists in medical education: current roles and future directions. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):120–126.
- , , , , . Pediatric hospitalists' influences on education and career plans. J Hosp Med. 2012;7(4):282–286.
- , . Pediatric hospitalist systems versus traditional models of care: effect on quality and cost outcomes. J Hosp Med. 2012;7(4):350–357.
- , , , . Characteristics of the pediatric hospitalist workforce: its roles and work environment. Pediatrics. 2007;120(33):33–39.
- Society of Hospital Medicine. Definition of a hospitalist and hospital medicine. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Hospitalist_Definition7(4):299–303.
- . Pediatric hospital medicine: historical perspectives, inspired future. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):107–112.
- , , . Assessing the value of pediatric hospitalist programs: the perspective of hospital leaders. Acad Pediatr. 2009;9(3):192–196.
- , . Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179–186.
- , , . Hospitalists' involvement in pediatrics training: perspectives from pediatric residency program and clerkship directors. Acad Med. 2009;84(11):1617–1621.
- , . Research in pediatric hospital medicine: how research will impact clinical care. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):127–130.
- , . Pediatric hospitalists in medical education: current roles and future directions. Curr Probl Pediatr Adolesc Health Care. 2012;42(5):120–126.
- , , , , . Pediatric hospitalists' influences on education and career plans. J Hosp Med. 2012;7(4):282–286.
- , . Pediatric hospitalist systems versus traditional models of care: effect on quality and cost outcomes. J Hosp Med. 2012;7(4):350–357.
- , , , . Characteristics of the pediatric hospitalist workforce: its roles and work environment. Pediatrics. 2007;120(33):33–39.
- Society of Hospital Medicine. Definition of a hospitalist and hospital medicine. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Hospitalist_Definition7(4):299–303.
Copyright © 2013 Society of Hospital Medicine
Preventing the intergenerational transmission of trauma
Intergenerational trauma often proves to be a prevailing feature of family systems.
The trauma of the Nazi concentration camps, for example, can be re-experienced in the lives of the children of camp survivors. Even the grandchildren of Holocaust survivors have been found to suffer from the effects of trauma. These effects manifest through characteristics such as increased suspiciousness of others, anger, and irritability in these individuals compared with controls (J. Relig. Health 2011;50:321-9).
Such intergenerational trauma has been found among urban American Indian and Alaska Native populations who have been involved in culturally specific sobriety maintenance programs (Am. Indian Alsk. Native Ment. Health Res. 2011;18:17-40). Likewise, a body of research supports the notion that untreated intergenerational trauma tied to generations of slavery in the United States continues to negatively affect many in the black community.
Other kinds of trauma can be passed down through the generations, as well. Take the trauma of a combat soldier; victim of or prisoner of war; survivor of a mass shooting or of child abuse; witness of genocide; or survivor of colonial suppression, slavery, or political totalitarianism. People who have experienced these traumas can pass down the consequences to subsequent generations.
We know that people who suffer trauma firsthand often develop posttraumatic stress disorder symptoms (PTSD) symptoms such as fearfulness, nightmares, flashbacks, sorrow, and difficulty with emotional closeness. However, it also is clear that compared with controls, the children of veterans with PTSD have shown an inability to experience appropriate emotional responses to situations and difficulty in solving problems effectively both within and outside the family unit (Aust. N.Z. J. Psychiatry 2001;35:345-51).
The trauma of childhood abuse also is transmitted down through the influences of the other members of the family, especially their children.
Another group known to suffer from the effects of intergenerational trauma is the children of alcoholics. This is a group that has demonstrated an increased need to care for others and keep secrets. They might use lying as a normal coping style and sometimes experience difficulty being children. Such behaviors are understood as a direct consequence of the experience of the family dysfunction. The question about trauma is: How do the symptoms of PTSD get "passed down" through the next generations, when the younger family members were not exposed to any trauma?
Various mechanisms have been considered, with individual psychological mechanisms and family dynamics being the most commonly cited mechanisms. Other factors have been suggested, such as the role of cultural and societal factors in the perpetuation of symptoms. Children and young adults might develop retaliatory fantasies "to right the wrongs done to their families." These types of beliefs and fantasies fuel many sectarian struggles around the world.
Individual psychological mechanisms commonly considered to be important are projection and identification. The parent with PTSD projects unwanted aspects of himself onto the child, who takes up the projection and identifies with it; this is called projective identification. Fear of the cold or the dark in the father then becomes the child’s fear instead. Children who are closest to the traumatized parent will be most affected.
Other postulated mechanisms focus on affect regulation. Parents who have difficulty with emotional regulation will have difficulty bonding appropriately with their child. On the other hand, emotional numbing might be present, which interferes with the development of a strong bond between parent and child.
One study of male Vietnam veterans found that "emotional numbing" and the quality of their relationship with their children remained significant even after investigators controlled for numerous factors, including the fathers’ family-of-origin stressors, combat exposure, depression, and substance abuse (J. Trauma Stress 2002;15:351-7). In other words, the children then suffer from secondary trauma.
Trauma-affected families also might have difficulty setting appropriate boundaries between parent and child so that the child becomes the caregiver of sorts and protector of the parent. The fears of the parent can become the fears of the child. It might be confusing for the child when a parent says: "Shh! Did you hear that noise," implying that "they" will get us, without really specifying the who and why, thus depriving the child of a rational explanation of his or her own experiences.
However, sometimes, trauma is not transmitted intergenerationally, a series of meta-analyses shows (Attach. Hum. Dev. 2008;10:105-21). Instead, these families are able to develop resilience and adapt well in the face of adversity – and achieve posttraumatic growth. How do we help the families with trauma become these resilient families?
Here is a list of nine points that can help guide the family psychiatrist:
1. The ability to regulate emotions, especially negative affect, is key to maintaining an understandable emotional climate for others in the family. Frequent unexplained emotional outbursts are difficult for other family members to understand. For children, it is especially important for them to understand that any emotional dysregulation is not caused by their behavior but by the parent’s experience of prior trauma.
2. The family should have an understanding of the meaning and cause of the traumatic events.
The traumatic events must be symbolized in a way that allows conversation and discussion about the past. The mention of wartime trauma can be phrased in a way that allows for the experience of pain, and then recovery, with hope and resilience as the message. A narrative story is important, with a good ending that the parent has survived, has overcome difficulties, and is here in the present with the child.
3. The parent must have "worked through" the trauma to the extent that he can internally symbolize his experiences enough to be able to talk about them and relay them to his
offspring in a coherent narrative with a positive message.
4. Open communication about the trauma prevents any unsymbolized, unspoken aspects of the trauma from being driven into unconsciousness, where they become dark fearful secrets that haunt the imagination and awaken the children, even as adults, at night.
5. Being able to access public accounts of the traumatic events is helpful to widen the family’s understanding of how others are affected, thus reducing the fearfulness of being alone with the trauma. Families should be encouraged to access these sources in order to understand the global aspects of trauma and the associated suffering and recovery.
6. For many families, having suffered trauma means that they must always be prepared for disaster. This, too, can be framed in a positive way, more like the scout motto of "be prepared," rather than the fearful posture of the survivalist.
7. A family fleeing from trauma might experience displacement through immigration and have no sense of home. This can be modulated by reestablishing and developing a new sense of community, and developing strong social and family rootedness. Sometimes, this involves a religious or spiritual group affiliation.
8. Family members who have suffered trauma often can identify skills that helped them survive. Hope, education, community, art – these values can be transmitted as the positive legacy of trauma. Helping families identify with positive resilient features of surviving trauma does not mean forgetting about the trauma but identifying the aspects that help the family go forward, enabling them to develop a narrative that allows recovery and growth.
9. If the child or other family members develop ongoing secondary PTSD or have enduring feelings of survivor guilt, persecution, and so on that are not resolved by family intervention, individual assessment might be needed.
In conclusion, despite the many illuminating case reports and anecdotes about the intergenerational transmission of trauma (for example, see J. Marital Fam. Ther. 2004;30:45-59), the message to families must be resilience focused. The question for these families becomes: "What did you do to manage the trauma and survive?"
Using a narrative framework, we can help these families identify the factors that can contribute to resilience, and build a future for the family that does not transmit traumatic symptoms but rather transmits the ability to move forward, despite traumatic symptoms.
E-mail Dr. Heru at [email protected].
Intergenerational trauma often proves to be a prevailing feature of family systems.
The trauma of the Nazi concentration camps, for example, can be re-experienced in the lives of the children of camp survivors. Even the grandchildren of Holocaust survivors have been found to suffer from the effects of trauma. These effects manifest through characteristics such as increased suspiciousness of others, anger, and irritability in these individuals compared with controls (J. Relig. Health 2011;50:321-9).
Such intergenerational trauma has been found among urban American Indian and Alaska Native populations who have been involved in culturally specific sobriety maintenance programs (Am. Indian Alsk. Native Ment. Health Res. 2011;18:17-40). Likewise, a body of research supports the notion that untreated intergenerational trauma tied to generations of slavery in the United States continues to negatively affect many in the black community.
Other kinds of trauma can be passed down through the generations, as well. Take the trauma of a combat soldier; victim of or prisoner of war; survivor of a mass shooting or of child abuse; witness of genocide; or survivor of colonial suppression, slavery, or political totalitarianism. People who have experienced these traumas can pass down the consequences to subsequent generations.
We know that people who suffer trauma firsthand often develop posttraumatic stress disorder symptoms (PTSD) symptoms such as fearfulness, nightmares, flashbacks, sorrow, and difficulty with emotional closeness. However, it also is clear that compared with controls, the children of veterans with PTSD have shown an inability to experience appropriate emotional responses to situations and difficulty in solving problems effectively both within and outside the family unit (Aust. N.Z. J. Psychiatry 2001;35:345-51).
The trauma of childhood abuse also is transmitted down through the influences of the other members of the family, especially their children.
Another group known to suffer from the effects of intergenerational trauma is the children of alcoholics. This is a group that has demonstrated an increased need to care for others and keep secrets. They might use lying as a normal coping style and sometimes experience difficulty being children. Such behaviors are understood as a direct consequence of the experience of the family dysfunction. The question about trauma is: How do the symptoms of PTSD get "passed down" through the next generations, when the younger family members were not exposed to any trauma?
Various mechanisms have been considered, with individual psychological mechanisms and family dynamics being the most commonly cited mechanisms. Other factors have been suggested, such as the role of cultural and societal factors in the perpetuation of symptoms. Children and young adults might develop retaliatory fantasies "to right the wrongs done to their families." These types of beliefs and fantasies fuel many sectarian struggles around the world.
Individual psychological mechanisms commonly considered to be important are projection and identification. The parent with PTSD projects unwanted aspects of himself onto the child, who takes up the projection and identifies with it; this is called projective identification. Fear of the cold or the dark in the father then becomes the child’s fear instead. Children who are closest to the traumatized parent will be most affected.
Other postulated mechanisms focus on affect regulation. Parents who have difficulty with emotional regulation will have difficulty bonding appropriately with their child. On the other hand, emotional numbing might be present, which interferes with the development of a strong bond between parent and child.
One study of male Vietnam veterans found that "emotional numbing" and the quality of their relationship with their children remained significant even after investigators controlled for numerous factors, including the fathers’ family-of-origin stressors, combat exposure, depression, and substance abuse (J. Trauma Stress 2002;15:351-7). In other words, the children then suffer from secondary trauma.
Trauma-affected families also might have difficulty setting appropriate boundaries between parent and child so that the child becomes the caregiver of sorts and protector of the parent. The fears of the parent can become the fears of the child. It might be confusing for the child when a parent says: "Shh! Did you hear that noise," implying that "they" will get us, without really specifying the who and why, thus depriving the child of a rational explanation of his or her own experiences.
However, sometimes, trauma is not transmitted intergenerationally, a series of meta-analyses shows (Attach. Hum. Dev. 2008;10:105-21). Instead, these families are able to develop resilience and adapt well in the face of adversity – and achieve posttraumatic growth. How do we help the families with trauma become these resilient families?
Here is a list of nine points that can help guide the family psychiatrist:
1. The ability to regulate emotions, especially negative affect, is key to maintaining an understandable emotional climate for others in the family. Frequent unexplained emotional outbursts are difficult for other family members to understand. For children, it is especially important for them to understand that any emotional dysregulation is not caused by their behavior but by the parent’s experience of prior trauma.
2. The family should have an understanding of the meaning and cause of the traumatic events.
The traumatic events must be symbolized in a way that allows conversation and discussion about the past. The mention of wartime trauma can be phrased in a way that allows for the experience of pain, and then recovery, with hope and resilience as the message. A narrative story is important, with a good ending that the parent has survived, has overcome difficulties, and is here in the present with the child.
3. The parent must have "worked through" the trauma to the extent that he can internally symbolize his experiences enough to be able to talk about them and relay them to his
offspring in a coherent narrative with a positive message.
4. Open communication about the trauma prevents any unsymbolized, unspoken aspects of the trauma from being driven into unconsciousness, where they become dark fearful secrets that haunt the imagination and awaken the children, even as adults, at night.
5. Being able to access public accounts of the traumatic events is helpful to widen the family’s understanding of how others are affected, thus reducing the fearfulness of being alone with the trauma. Families should be encouraged to access these sources in order to understand the global aspects of trauma and the associated suffering and recovery.
6. For many families, having suffered trauma means that they must always be prepared for disaster. This, too, can be framed in a positive way, more like the scout motto of "be prepared," rather than the fearful posture of the survivalist.
7. A family fleeing from trauma might experience displacement through immigration and have no sense of home. This can be modulated by reestablishing and developing a new sense of community, and developing strong social and family rootedness. Sometimes, this involves a religious or spiritual group affiliation.
8. Family members who have suffered trauma often can identify skills that helped them survive. Hope, education, community, art – these values can be transmitted as the positive legacy of trauma. Helping families identify with positive resilient features of surviving trauma does not mean forgetting about the trauma but identifying the aspects that help the family go forward, enabling them to develop a narrative that allows recovery and growth.
9. If the child or other family members develop ongoing secondary PTSD or have enduring feelings of survivor guilt, persecution, and so on that are not resolved by family intervention, individual assessment might be needed.
In conclusion, despite the many illuminating case reports and anecdotes about the intergenerational transmission of trauma (for example, see J. Marital Fam. Ther. 2004;30:45-59), the message to families must be resilience focused. The question for these families becomes: "What did you do to manage the trauma and survive?"
Using a narrative framework, we can help these families identify the factors that can contribute to resilience, and build a future for the family that does not transmit traumatic symptoms but rather transmits the ability to move forward, despite traumatic symptoms.
E-mail Dr. Heru at [email protected].
Intergenerational trauma often proves to be a prevailing feature of family systems.
The trauma of the Nazi concentration camps, for example, can be re-experienced in the lives of the children of camp survivors. Even the grandchildren of Holocaust survivors have been found to suffer from the effects of trauma. These effects manifest through characteristics such as increased suspiciousness of others, anger, and irritability in these individuals compared with controls (J. Relig. Health 2011;50:321-9).
Such intergenerational trauma has been found among urban American Indian and Alaska Native populations who have been involved in culturally specific sobriety maintenance programs (Am. Indian Alsk. Native Ment. Health Res. 2011;18:17-40). Likewise, a body of research supports the notion that untreated intergenerational trauma tied to generations of slavery in the United States continues to negatively affect many in the black community.
Other kinds of trauma can be passed down through the generations, as well. Take the trauma of a combat soldier; victim of or prisoner of war; survivor of a mass shooting or of child abuse; witness of genocide; or survivor of colonial suppression, slavery, or political totalitarianism. People who have experienced these traumas can pass down the consequences to subsequent generations.
We know that people who suffer trauma firsthand often develop posttraumatic stress disorder symptoms (PTSD) symptoms such as fearfulness, nightmares, flashbacks, sorrow, and difficulty with emotional closeness. However, it also is clear that compared with controls, the children of veterans with PTSD have shown an inability to experience appropriate emotional responses to situations and difficulty in solving problems effectively both within and outside the family unit (Aust. N.Z. J. Psychiatry 2001;35:345-51).
The trauma of childhood abuse also is transmitted down through the influences of the other members of the family, especially their children.
Another group known to suffer from the effects of intergenerational trauma is the children of alcoholics. This is a group that has demonstrated an increased need to care for others and keep secrets. They might use lying as a normal coping style and sometimes experience difficulty being children. Such behaviors are understood as a direct consequence of the experience of the family dysfunction. The question about trauma is: How do the symptoms of PTSD get "passed down" through the next generations, when the younger family members were not exposed to any trauma?
Various mechanisms have been considered, with individual psychological mechanisms and family dynamics being the most commonly cited mechanisms. Other factors have been suggested, such as the role of cultural and societal factors in the perpetuation of symptoms. Children and young adults might develop retaliatory fantasies "to right the wrongs done to their families." These types of beliefs and fantasies fuel many sectarian struggles around the world.
Individual psychological mechanisms commonly considered to be important are projection and identification. The parent with PTSD projects unwanted aspects of himself onto the child, who takes up the projection and identifies with it; this is called projective identification. Fear of the cold or the dark in the father then becomes the child’s fear instead. Children who are closest to the traumatized parent will be most affected.
Other postulated mechanisms focus on affect regulation. Parents who have difficulty with emotional regulation will have difficulty bonding appropriately with their child. On the other hand, emotional numbing might be present, which interferes with the development of a strong bond between parent and child.
One study of male Vietnam veterans found that "emotional numbing" and the quality of their relationship with their children remained significant even after investigators controlled for numerous factors, including the fathers’ family-of-origin stressors, combat exposure, depression, and substance abuse (J. Trauma Stress 2002;15:351-7). In other words, the children then suffer from secondary trauma.
Trauma-affected families also might have difficulty setting appropriate boundaries between parent and child so that the child becomes the caregiver of sorts and protector of the parent. The fears of the parent can become the fears of the child. It might be confusing for the child when a parent says: "Shh! Did you hear that noise," implying that "they" will get us, without really specifying the who and why, thus depriving the child of a rational explanation of his or her own experiences.
However, sometimes, trauma is not transmitted intergenerationally, a series of meta-analyses shows (Attach. Hum. Dev. 2008;10:105-21). Instead, these families are able to develop resilience and adapt well in the face of adversity – and achieve posttraumatic growth. How do we help the families with trauma become these resilient families?
Here is a list of nine points that can help guide the family psychiatrist:
1. The ability to regulate emotions, especially negative affect, is key to maintaining an understandable emotional climate for others in the family. Frequent unexplained emotional outbursts are difficult for other family members to understand. For children, it is especially important for them to understand that any emotional dysregulation is not caused by their behavior but by the parent’s experience of prior trauma.
2. The family should have an understanding of the meaning and cause of the traumatic events.
The traumatic events must be symbolized in a way that allows conversation and discussion about the past. The mention of wartime trauma can be phrased in a way that allows for the experience of pain, and then recovery, with hope and resilience as the message. A narrative story is important, with a good ending that the parent has survived, has overcome difficulties, and is here in the present with the child.
3. The parent must have "worked through" the trauma to the extent that he can internally symbolize his experiences enough to be able to talk about them and relay them to his
offspring in a coherent narrative with a positive message.
4. Open communication about the trauma prevents any unsymbolized, unspoken aspects of the trauma from being driven into unconsciousness, where they become dark fearful secrets that haunt the imagination and awaken the children, even as adults, at night.
5. Being able to access public accounts of the traumatic events is helpful to widen the family’s understanding of how others are affected, thus reducing the fearfulness of being alone with the trauma. Families should be encouraged to access these sources in order to understand the global aspects of trauma and the associated suffering and recovery.
6. For many families, having suffered trauma means that they must always be prepared for disaster. This, too, can be framed in a positive way, more like the scout motto of "be prepared," rather than the fearful posture of the survivalist.
7. A family fleeing from trauma might experience displacement through immigration and have no sense of home. This can be modulated by reestablishing and developing a new sense of community, and developing strong social and family rootedness. Sometimes, this involves a religious or spiritual group affiliation.
8. Family members who have suffered trauma often can identify skills that helped them survive. Hope, education, community, art – these values can be transmitted as the positive legacy of trauma. Helping families identify with positive resilient features of surviving trauma does not mean forgetting about the trauma but identifying the aspects that help the family go forward, enabling them to develop a narrative that allows recovery and growth.
9. If the child or other family members develop ongoing secondary PTSD or have enduring feelings of survivor guilt, persecution, and so on that are not resolved by family intervention, individual assessment might be needed.
In conclusion, despite the many illuminating case reports and anecdotes about the intergenerational transmission of trauma (for example, see J. Marital Fam. Ther. 2004;30:45-59), the message to families must be resilience focused. The question for these families becomes: "What did you do to manage the trauma and survive?"
Using a narrative framework, we can help these families identify the factors that can contribute to resilience, and build a future for the family that does not transmit traumatic symptoms but rather transmits the ability to move forward, despite traumatic symptoms.
E-mail Dr. Heru at [email protected].
How Should Physicians Assess and Manage Pressure Ulcers in the Hospitalized Patient?
The Case
An 85-year-old woman with stroke, functional quadriplegia, and diabetes mellitus presents with altered mental status. She is febrile (38.5°C) with leukocytosis (14,400 cells/mm3) and has a 5 cm x 4 cm x 2 cm Stage III malodorous sacral ulcer without surrounding erythema, tunneling, or pain. The ulcer base is partially covered by green slough. How should this pressure ulcer be evaluated and treated?
Overview
Pressure ulcers in vulnerable populations, such as the elderly and those with limited mobility, are exceedingly common. In the acute-care setting, the incidence of pressure ulcers ranges from 0.4% to 38%, with 2.5 million cases treated annually at an estimated cost of $11 billion per year.1,2 Moreover, as of Oct. 1, 2008, the Centers for Medicare & Medicaid Services (CMS) guideline states that hospitals will no longer receive additional payment when a hospitalized patient develops Stage III or IV pressure ulcers that are not present on admission.
A pressure ulcer is a localized injury to skin and underlying soft tissue over a bony prominence due to sustained external pressure.3 Prolonged pressure on these weight-bearing areas leads to reduced blood flow, ischemia, cell death, and necrosis of local tissues.4 Risk factors for developing pressure ulcers include increased external pressure, shear, friction, moisture, poor perfusion, immobility, incontinence, malnutrition, and impaired mental status.4 Inadequately treated pressure ulcers can lead to pain, tunneling, fistula formation, disfigurement, infection, prolonged hospitalization, lower quality of life, and increased mortality.4
Because of the significant morbidities and high costs associated with the care of pressure ulcers in acute care, hospitalists must be familiar with the assessment and treatment of pressure ulcers in vulnerable patients.
Review of the Data
The management of pressure ulcers in the hospitalized patient starts with a comprehensive assessment of the patient’s medical comorbidities, risk factors, and wound-staging. Considerations must be given to differentiate an infected pressure ulcer from a noninfected ulcer. These evaluations then guide the appropriate treatments of pressure ulcers, including the prevention of progression or formation of new ulcers, debridement, application of wound dressing, and antibiotic use.
Assessing pressure ulcer stage. The National Pressure Ulcer Advisory Panel (NPUAP) Classification System is the most commonly used staging tool. It describes four stages of pressure ulcers (see Table 1).3 A Stage 1 pressure ulcer is characterized by intact skin with nonblanchable erythema and may be discolored, painful, soft, firm, and warmer or cooler compared to adjacent area. A Stage II pressure ulcer presents with partial thickness skin loss with a shallow red-pink wound bed without slough, or as an intact or ruptured serum-filled blister. Stage II pressure ulcers do not include skin tears, tape burns, macerations, or excoriations. A Stage III pressure ulcer has full thickness skin loss with or without visible subcutaneous fat. Bone, tendon, or muscle are not exposed or directly palpable. Slough may be present but it does not obscure the depth of ulcer. Deep ulcers can develop in anatomical regions with high adiposity, such as the pelvic girdle. A Stage IV pressure ulcer has full thickness tissue loss with exposed and palpable bone, tendon, or muscle. Slough, eschar, undermining, and tunneling may be present. The depth of a Stage IV ulcer varies depending on anatomical location and adiposity. Stage IV ulcers also create a nidus for osteomyelitis.
NPUAP describes two additional categories of pressure ulcers: unstageable and deep tissue injury.3 An unstageable ulcer has full thickness skin or tissue loss of unknown depth because the wound base is completely obscured by slough or eschar. The ulcer can only be accurately categorized as Stage III or IV after sufficient slough or eschar is removed to identify wound depth. Lastly, suspected deep tissue injury describes a localized area of discolored intact skin (purple or maroon) or blood-filled blister due to damage of underlying tissue from pressure or shear.
Diagnosing infected pressure ulcers. Pressure ulcer infection delays wound healing and increases risks for sepsis, cellulitis, osteomyelitis, and death.5,6 Clinical evidence of soft tissue involvement, such as erythema, warmth, tenderness, foul odor, or purulent discharge, and systemic inflammatory response (fever, tachycardia, or leukocytosis) are suggestive of a wound infection.3,5 However, these clinical signs may be absent and thus make the distinction between chronic wound and infected pressure ulcer difficult.7 Delayed healing with friable granulation tissue and increased pain in a treated wound may be the only signs of a pressure ulcer infection.3,5,7
Routine laboratory tests (i.e. white blood cell count, C-reactive protein, and erythrocyte sedimentation rate) are neither sensitive nor specific in diagnosing wound infection. Moreover, because pressure ulcers are typically colonized with ≥105 organisms/mL of normal skin flora and bacteria from adjacent gastrointestinal or urogenital environments, swab cultures identify colonizing organisms and are not recommended as a diagnostic test for pressure ulcer microbiologic evaluation.5,6 If microbiological data are needed to guide antibiotic use, cultures of blood, bone, or deep tissue biopsied from a surgically debrided wound should be used.5 Importantly, a higher index of suspicion should be maintained for infection of Stage III or IV pressure ulcers because they are more commonly infected than Stage I or II ulcers.3
Prevention. The prevention of wound progression is essential in treating acute, chronic, or infected pressure ulcers. Although management guidelines are limited by few high-quality, randomized controlled trials, NPUAP recommends a number of prevention strategies targeting risk factors that contribute to pressure ulcer development.2,3,8
For all bed-bound and chair-bound persons with impaired ability to self-reposition, risk assessment for pressure ulcer should be done on admission and repeated every 24 hours. The presence of such risk factors as immobility, shear, friction, moisture, incontinence, and malnutrition should be used to guide preventive treatments. Pressure relief on an ulcer can be achieved by repositioning the immobile patient at one- to two-hour intervals. Pressure-redistributing support surfaces (static, overlays, or dynamic) reduce tissue pressure and decrease overall incidence of pressure ulcers. Due to a lack of relative efficacy data, the selection of a support surface should be determined by the patient’s individual needs in order to reduce pressure and shear.3 For instance, dynamic support is an appropriate surface for an immobile patient with multiple or nonhealing ulcers. Shearing force and friction can be reduced by limiting head-of-bed elevation to <30° and using such transfer aids as bed linens while repositioning patients. The use of pillows, foam wedges, or other devices should be used to eliminate direct contact of bony prominences or reduce pressure on heels.8
Skin care should be optimized to limit excessive dryness or moisture. This includes using moisturizers for dry skin, particularly for the sacrum, and implementing bowel and bladder programs and absorbent underpads in patients with bowel or bladder incontinence.2 Given that patients with pressure ulcers are in a catabolic state, those who are nutritionally compromised may benefit from nutritional supplementation.3 Lastly, appropriate use of local and systemic pain regimen for painful pressure ulcers can improve patient cooperation in repositioning, dressing change, and quality of life.
Debridement. Wound debridement removes necrotic tissue often present in infected or chronic pressure ulcers, reduces risk for further infection, and promotes granulation tissue formation and wound healing. Debridement, however, is not indicated for ulcers of an ischemic limb or dry eschar of the heel, due to propensity for complications.3,4 The five common debridement methods are sharp, mechanical, autolytic, enzymatic, and biosurgical. The debridement method of choice is determined by clinician preference and availability.4
Sharp debridement results in rapid removal of large amounts of nonviable necrotic tissues and eschar using sharp instruments and, therefore, is indicated if wound infection or sepsis is present. Mechanical debridement by wet-to-dry dressing or whirlpool nonselectively removes granulation tissue and, thus, should be used cautiously. Autolytic debridement uses occlusive dressings (i.e. hydrocolloid or hydrogel) to maintain a moist wound environment in order to optimize the body’s inherent ability to selectively self-digest necrotic tissues. Enzymatic debridement with concentrated topical proteolytic enzymes (i.e. collagenase) digests necrotic tissues and achieves faster debridement than autolysis while being less invasive than surgical intervention. Biosurgery utilizes maggots (i.e. Lucilia sericata) that produce enzymes to effectively debride necrotic tissues.
Wound care and dressing. Pressure ulcers should be cleansed with each dressing change using such physiologic solutions as normal saline. Cleansing with antimicrobial solutions for ulcers with large necrotic debris or infection needs to be thoughtfully administered due to the potential impairment on wound healing.4 Wound dressing should maintain a moist wound environment to allow epithelialization and limit excessive exudates in order to prevent maceration. Although there are many categories of moisture retentive dressings, their comparative effectiveness remain unclear.4 Table 2 summarizes characteristics of common wound dressings and their applications.
Antibiotic use. Topical antibiotics are appropriate for Stage III or IV ulcers with signs of local infection, including periwound erythema and friable granulation tissue.4 The Agency for Health Care Policy and Research recommends a two-week trial of a topical antibiotic, such as silver sulfadiazine, for pressure ulcers that fail to heal after two to four weeks of optimal care.6 Systemic antibiotics should be used for patients who demonstrate evidence of systemic infection including sepsis, osteomyelitis, or cellulitis with associated fever and leukocytosis. The choice of systemic antibiotics should be based on cultures from blood, bone, or deep tissue biopsied from a surgically debrided wound.4,6
Back to the Case
The patient was hospitalized for altered mental status. She was at high risk for the progression of her sacral ulcer and the development of new pressure ulcers due to immobility, incontinence, malnutrition, and impaired mental status. The sacral wound was a chronic, Stage III pressure ulcer without evidence of local tissue infection. However, the presence of leukocytosis and fever were suggestive of an underlying infection. Her urine analysis was consistent with a urinary tract infection.
Trimethoprim/sulfamethoxazole was administered with subsequent resolution of leukocytosis, fever, and delirium. The sacral ulcer was cleansed with normal saline and covered with hydrocolloid dressing every 72 hours in order to maintain a moist wound environment and facilitate autolysis. Preventive interventions guided by her risk factors for pressure ulcer were implemented. Interventions included:
- Daily skin and wound assessment;
- Pressure relief with repositioning every two hours;
- Use of a dynamic support surface;
- Head-of-bed elevation of no more than <30° to reduce shear and friction;
- Use of transfer aids;
- Use of devices to eliminate direct contact of bony prominences;
- Optimizing skin care with moisturizers for dry skin and frequent changing of absorbent under pads for incontinence; and
- Consulting nutrition service to optimize nutritional intake.
Bottom Line
Assessments of pressure ulcer stage, wound infection, and risk factors guide targeted therapeutic interventions that facilitate wound healing and prevent new pressure ulcer formation.
Dr. Prager is a fellow in the Brookdale Department of Geriatrics and Palliative Medicine at Mount Sinai School of Medicine in New York City. Dr. Ko is a hospitalist and an assistant professor in the Brookdale Department of Geriatrics and Palliative Medicine at Mount Sinai.
References
- Pressure ulcers in America: prevalence, incidence, and implications for the future. An executive summary of the National Pressure Ulcer Advisory Panel monograph. Adv Skin Wound Care. 2001;14(4):208-215.
- Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA. 2006;296(8):974-984.
- European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel. Treatment of Pressure Ulcers: Quick Reference Guide. Washington, D.C.: National Pressure Ulcer Advisory Panel; 2009.
- Bates-Jensen BM. Chapter 58. Pressure Ulcers. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York: McGraw-Hill; 2009.
- Livesley NJ, Chow AW. Infected pressure ulcers in elderly individuals. Clin Infect Dis. 2002;35(11):1390-1396.
- Agency for Health Care Policy and Research (AHCPR). Treatment of Pressure Ulcers. Clinical Practice Guideline Number 15. U.S. Department of Health and Human Services. 1994.
- Reddy M, Gill SS, Wu W, Kalkar SR, Rochon PA. Does this patient have an infection of a chronic wound? JAMA. 2012;307(6):605-611.
- National Pressure Ulcer Advisory Panel. Pressure Ulcer Prevention Points. National Pressure Ulcer Advisory Panel website. Available at: http://www.npuap.org/resources/educational-and-clinical-resources/pressure-ulcer-prevention-points/. Accessed Aug. 1, 2012.
- Reuben DB, Herr KA, Pacala JT, et al. Skin Ulcers. In: Geriatrics At Your Fingertips. 12th ed. New York: The American Geriatrics Society; 2010.
The Case
An 85-year-old woman with stroke, functional quadriplegia, and diabetes mellitus presents with altered mental status. She is febrile (38.5°C) with leukocytosis (14,400 cells/mm3) and has a 5 cm x 4 cm x 2 cm Stage III malodorous sacral ulcer without surrounding erythema, tunneling, or pain. The ulcer base is partially covered by green slough. How should this pressure ulcer be evaluated and treated?
Overview
Pressure ulcers in vulnerable populations, such as the elderly and those with limited mobility, are exceedingly common. In the acute-care setting, the incidence of pressure ulcers ranges from 0.4% to 38%, with 2.5 million cases treated annually at an estimated cost of $11 billion per year.1,2 Moreover, as of Oct. 1, 2008, the Centers for Medicare & Medicaid Services (CMS) guideline states that hospitals will no longer receive additional payment when a hospitalized patient develops Stage III or IV pressure ulcers that are not present on admission.
A pressure ulcer is a localized injury to skin and underlying soft tissue over a bony prominence due to sustained external pressure.3 Prolonged pressure on these weight-bearing areas leads to reduced blood flow, ischemia, cell death, and necrosis of local tissues.4 Risk factors for developing pressure ulcers include increased external pressure, shear, friction, moisture, poor perfusion, immobility, incontinence, malnutrition, and impaired mental status.4 Inadequately treated pressure ulcers can lead to pain, tunneling, fistula formation, disfigurement, infection, prolonged hospitalization, lower quality of life, and increased mortality.4
Because of the significant morbidities and high costs associated with the care of pressure ulcers in acute care, hospitalists must be familiar with the assessment and treatment of pressure ulcers in vulnerable patients.
Review of the Data
The management of pressure ulcers in the hospitalized patient starts with a comprehensive assessment of the patient’s medical comorbidities, risk factors, and wound-staging. Considerations must be given to differentiate an infected pressure ulcer from a noninfected ulcer. These evaluations then guide the appropriate treatments of pressure ulcers, including the prevention of progression or formation of new ulcers, debridement, application of wound dressing, and antibiotic use.
Assessing pressure ulcer stage. The National Pressure Ulcer Advisory Panel (NPUAP) Classification System is the most commonly used staging tool. It describes four stages of pressure ulcers (see Table 1).3 A Stage 1 pressure ulcer is characterized by intact skin with nonblanchable erythema and may be discolored, painful, soft, firm, and warmer or cooler compared to adjacent area. A Stage II pressure ulcer presents with partial thickness skin loss with a shallow red-pink wound bed without slough, or as an intact or ruptured serum-filled blister. Stage II pressure ulcers do not include skin tears, tape burns, macerations, or excoriations. A Stage III pressure ulcer has full thickness skin loss with or without visible subcutaneous fat. Bone, tendon, or muscle are not exposed or directly palpable. Slough may be present but it does not obscure the depth of ulcer. Deep ulcers can develop in anatomical regions with high adiposity, such as the pelvic girdle. A Stage IV pressure ulcer has full thickness tissue loss with exposed and palpable bone, tendon, or muscle. Slough, eschar, undermining, and tunneling may be present. The depth of a Stage IV ulcer varies depending on anatomical location and adiposity. Stage IV ulcers also create a nidus for osteomyelitis.
NPUAP describes two additional categories of pressure ulcers: unstageable and deep tissue injury.3 An unstageable ulcer has full thickness skin or tissue loss of unknown depth because the wound base is completely obscured by slough or eschar. The ulcer can only be accurately categorized as Stage III or IV after sufficient slough or eschar is removed to identify wound depth. Lastly, suspected deep tissue injury describes a localized area of discolored intact skin (purple or maroon) or blood-filled blister due to damage of underlying tissue from pressure or shear.
Diagnosing infected pressure ulcers. Pressure ulcer infection delays wound healing and increases risks for sepsis, cellulitis, osteomyelitis, and death.5,6 Clinical evidence of soft tissue involvement, such as erythema, warmth, tenderness, foul odor, or purulent discharge, and systemic inflammatory response (fever, tachycardia, or leukocytosis) are suggestive of a wound infection.3,5 However, these clinical signs may be absent and thus make the distinction between chronic wound and infected pressure ulcer difficult.7 Delayed healing with friable granulation tissue and increased pain in a treated wound may be the only signs of a pressure ulcer infection.3,5,7
Routine laboratory tests (i.e. white blood cell count, C-reactive protein, and erythrocyte sedimentation rate) are neither sensitive nor specific in diagnosing wound infection. Moreover, because pressure ulcers are typically colonized with ≥105 organisms/mL of normal skin flora and bacteria from adjacent gastrointestinal or urogenital environments, swab cultures identify colonizing organisms and are not recommended as a diagnostic test for pressure ulcer microbiologic evaluation.5,6 If microbiological data are needed to guide antibiotic use, cultures of blood, bone, or deep tissue biopsied from a surgically debrided wound should be used.5 Importantly, a higher index of suspicion should be maintained for infection of Stage III or IV pressure ulcers because they are more commonly infected than Stage I or II ulcers.3
Prevention. The prevention of wound progression is essential in treating acute, chronic, or infected pressure ulcers. Although management guidelines are limited by few high-quality, randomized controlled trials, NPUAP recommends a number of prevention strategies targeting risk factors that contribute to pressure ulcer development.2,3,8
For all bed-bound and chair-bound persons with impaired ability to self-reposition, risk assessment for pressure ulcer should be done on admission and repeated every 24 hours. The presence of such risk factors as immobility, shear, friction, moisture, incontinence, and malnutrition should be used to guide preventive treatments. Pressure relief on an ulcer can be achieved by repositioning the immobile patient at one- to two-hour intervals. Pressure-redistributing support surfaces (static, overlays, or dynamic) reduce tissue pressure and decrease overall incidence of pressure ulcers. Due to a lack of relative efficacy data, the selection of a support surface should be determined by the patient’s individual needs in order to reduce pressure and shear.3 For instance, dynamic support is an appropriate surface for an immobile patient with multiple or nonhealing ulcers. Shearing force and friction can be reduced by limiting head-of-bed elevation to <30° and using such transfer aids as bed linens while repositioning patients. The use of pillows, foam wedges, or other devices should be used to eliminate direct contact of bony prominences or reduce pressure on heels.8
Skin care should be optimized to limit excessive dryness or moisture. This includes using moisturizers for dry skin, particularly for the sacrum, and implementing bowel and bladder programs and absorbent underpads in patients with bowel or bladder incontinence.2 Given that patients with pressure ulcers are in a catabolic state, those who are nutritionally compromised may benefit from nutritional supplementation.3 Lastly, appropriate use of local and systemic pain regimen for painful pressure ulcers can improve patient cooperation in repositioning, dressing change, and quality of life.
Debridement. Wound debridement removes necrotic tissue often present in infected or chronic pressure ulcers, reduces risk for further infection, and promotes granulation tissue formation and wound healing. Debridement, however, is not indicated for ulcers of an ischemic limb or dry eschar of the heel, due to propensity for complications.3,4 The five common debridement methods are sharp, mechanical, autolytic, enzymatic, and biosurgical. The debridement method of choice is determined by clinician preference and availability.4
Sharp debridement results in rapid removal of large amounts of nonviable necrotic tissues and eschar using sharp instruments and, therefore, is indicated if wound infection or sepsis is present. Mechanical debridement by wet-to-dry dressing or whirlpool nonselectively removes granulation tissue and, thus, should be used cautiously. Autolytic debridement uses occlusive dressings (i.e. hydrocolloid or hydrogel) to maintain a moist wound environment in order to optimize the body’s inherent ability to selectively self-digest necrotic tissues. Enzymatic debridement with concentrated topical proteolytic enzymes (i.e. collagenase) digests necrotic tissues and achieves faster debridement than autolysis while being less invasive than surgical intervention. Biosurgery utilizes maggots (i.e. Lucilia sericata) that produce enzymes to effectively debride necrotic tissues.
Wound care and dressing. Pressure ulcers should be cleansed with each dressing change using such physiologic solutions as normal saline. Cleansing with antimicrobial solutions for ulcers with large necrotic debris or infection needs to be thoughtfully administered due to the potential impairment on wound healing.4 Wound dressing should maintain a moist wound environment to allow epithelialization and limit excessive exudates in order to prevent maceration. Although there are many categories of moisture retentive dressings, their comparative effectiveness remain unclear.4 Table 2 summarizes characteristics of common wound dressings and their applications.
Antibiotic use. Topical antibiotics are appropriate for Stage III or IV ulcers with signs of local infection, including periwound erythema and friable granulation tissue.4 The Agency for Health Care Policy and Research recommends a two-week trial of a topical antibiotic, such as silver sulfadiazine, for pressure ulcers that fail to heal after two to four weeks of optimal care.6 Systemic antibiotics should be used for patients who demonstrate evidence of systemic infection including sepsis, osteomyelitis, or cellulitis with associated fever and leukocytosis. The choice of systemic antibiotics should be based on cultures from blood, bone, or deep tissue biopsied from a surgically debrided wound.4,6
Back to the Case
The patient was hospitalized for altered mental status. She was at high risk for the progression of her sacral ulcer and the development of new pressure ulcers due to immobility, incontinence, malnutrition, and impaired mental status. The sacral wound was a chronic, Stage III pressure ulcer without evidence of local tissue infection. However, the presence of leukocytosis and fever were suggestive of an underlying infection. Her urine analysis was consistent with a urinary tract infection.
Trimethoprim/sulfamethoxazole was administered with subsequent resolution of leukocytosis, fever, and delirium. The sacral ulcer was cleansed with normal saline and covered with hydrocolloid dressing every 72 hours in order to maintain a moist wound environment and facilitate autolysis. Preventive interventions guided by her risk factors for pressure ulcer were implemented. Interventions included:
- Daily skin and wound assessment;
- Pressure relief with repositioning every two hours;
- Use of a dynamic support surface;
- Head-of-bed elevation of no more than <30° to reduce shear and friction;
- Use of transfer aids;
- Use of devices to eliminate direct contact of bony prominences;
- Optimizing skin care with moisturizers for dry skin and frequent changing of absorbent under pads for incontinence; and
- Consulting nutrition service to optimize nutritional intake.
Bottom Line
Assessments of pressure ulcer stage, wound infection, and risk factors guide targeted therapeutic interventions that facilitate wound healing and prevent new pressure ulcer formation.
Dr. Prager is a fellow in the Brookdale Department of Geriatrics and Palliative Medicine at Mount Sinai School of Medicine in New York City. Dr. Ko is a hospitalist and an assistant professor in the Brookdale Department of Geriatrics and Palliative Medicine at Mount Sinai.
References
- Pressure ulcers in America: prevalence, incidence, and implications for the future. An executive summary of the National Pressure Ulcer Advisory Panel monograph. Adv Skin Wound Care. 2001;14(4):208-215.
- Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA. 2006;296(8):974-984.
- European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel. Treatment of Pressure Ulcers: Quick Reference Guide. Washington, D.C.: National Pressure Ulcer Advisory Panel; 2009.
- Bates-Jensen BM. Chapter 58. Pressure Ulcers. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York: McGraw-Hill; 2009.
- Livesley NJ, Chow AW. Infected pressure ulcers in elderly individuals. Clin Infect Dis. 2002;35(11):1390-1396.
- Agency for Health Care Policy and Research (AHCPR). Treatment of Pressure Ulcers. Clinical Practice Guideline Number 15. U.S. Department of Health and Human Services. 1994.
- Reddy M, Gill SS, Wu W, Kalkar SR, Rochon PA. Does this patient have an infection of a chronic wound? JAMA. 2012;307(6):605-611.
- National Pressure Ulcer Advisory Panel. Pressure Ulcer Prevention Points. National Pressure Ulcer Advisory Panel website. Available at: http://www.npuap.org/resources/educational-and-clinical-resources/pressure-ulcer-prevention-points/. Accessed Aug. 1, 2012.
- Reuben DB, Herr KA, Pacala JT, et al. Skin Ulcers. In: Geriatrics At Your Fingertips. 12th ed. New York: The American Geriatrics Society; 2010.
The Case
An 85-year-old woman with stroke, functional quadriplegia, and diabetes mellitus presents with altered mental status. She is febrile (38.5°C) with leukocytosis (14,400 cells/mm3) and has a 5 cm x 4 cm x 2 cm Stage III malodorous sacral ulcer without surrounding erythema, tunneling, or pain. The ulcer base is partially covered by green slough. How should this pressure ulcer be evaluated and treated?
Overview
Pressure ulcers in vulnerable populations, such as the elderly and those with limited mobility, are exceedingly common. In the acute-care setting, the incidence of pressure ulcers ranges from 0.4% to 38%, with 2.5 million cases treated annually at an estimated cost of $11 billion per year.1,2 Moreover, as of Oct. 1, 2008, the Centers for Medicare & Medicaid Services (CMS) guideline states that hospitals will no longer receive additional payment when a hospitalized patient develops Stage III or IV pressure ulcers that are not present on admission.
A pressure ulcer is a localized injury to skin and underlying soft tissue over a bony prominence due to sustained external pressure.3 Prolonged pressure on these weight-bearing areas leads to reduced blood flow, ischemia, cell death, and necrosis of local tissues.4 Risk factors for developing pressure ulcers include increased external pressure, shear, friction, moisture, poor perfusion, immobility, incontinence, malnutrition, and impaired mental status.4 Inadequately treated pressure ulcers can lead to pain, tunneling, fistula formation, disfigurement, infection, prolonged hospitalization, lower quality of life, and increased mortality.4
Because of the significant morbidities and high costs associated with the care of pressure ulcers in acute care, hospitalists must be familiar with the assessment and treatment of pressure ulcers in vulnerable patients.
Review of the Data
The management of pressure ulcers in the hospitalized patient starts with a comprehensive assessment of the patient’s medical comorbidities, risk factors, and wound-staging. Considerations must be given to differentiate an infected pressure ulcer from a noninfected ulcer. These evaluations then guide the appropriate treatments of pressure ulcers, including the prevention of progression or formation of new ulcers, debridement, application of wound dressing, and antibiotic use.
Assessing pressure ulcer stage. The National Pressure Ulcer Advisory Panel (NPUAP) Classification System is the most commonly used staging tool. It describes four stages of pressure ulcers (see Table 1).3 A Stage 1 pressure ulcer is characterized by intact skin with nonblanchable erythema and may be discolored, painful, soft, firm, and warmer or cooler compared to adjacent area. A Stage II pressure ulcer presents with partial thickness skin loss with a shallow red-pink wound bed without slough, or as an intact or ruptured serum-filled blister. Stage II pressure ulcers do not include skin tears, tape burns, macerations, or excoriations. A Stage III pressure ulcer has full thickness skin loss with or without visible subcutaneous fat. Bone, tendon, or muscle are not exposed or directly palpable. Slough may be present but it does not obscure the depth of ulcer. Deep ulcers can develop in anatomical regions with high adiposity, such as the pelvic girdle. A Stage IV pressure ulcer has full thickness tissue loss with exposed and palpable bone, tendon, or muscle. Slough, eschar, undermining, and tunneling may be present. The depth of a Stage IV ulcer varies depending on anatomical location and adiposity. Stage IV ulcers also create a nidus for osteomyelitis.
NPUAP describes two additional categories of pressure ulcers: unstageable and deep tissue injury.3 An unstageable ulcer has full thickness skin or tissue loss of unknown depth because the wound base is completely obscured by slough or eschar. The ulcer can only be accurately categorized as Stage III or IV after sufficient slough or eschar is removed to identify wound depth. Lastly, suspected deep tissue injury describes a localized area of discolored intact skin (purple or maroon) or blood-filled blister due to damage of underlying tissue from pressure or shear.
Diagnosing infected pressure ulcers. Pressure ulcer infection delays wound healing and increases risks for sepsis, cellulitis, osteomyelitis, and death.5,6 Clinical evidence of soft tissue involvement, such as erythema, warmth, tenderness, foul odor, or purulent discharge, and systemic inflammatory response (fever, tachycardia, or leukocytosis) are suggestive of a wound infection.3,5 However, these clinical signs may be absent and thus make the distinction between chronic wound and infected pressure ulcer difficult.7 Delayed healing with friable granulation tissue and increased pain in a treated wound may be the only signs of a pressure ulcer infection.3,5,7
Routine laboratory tests (i.e. white blood cell count, C-reactive protein, and erythrocyte sedimentation rate) are neither sensitive nor specific in diagnosing wound infection. Moreover, because pressure ulcers are typically colonized with ≥105 organisms/mL of normal skin flora and bacteria from adjacent gastrointestinal or urogenital environments, swab cultures identify colonizing organisms and are not recommended as a diagnostic test for pressure ulcer microbiologic evaluation.5,6 If microbiological data are needed to guide antibiotic use, cultures of blood, bone, or deep tissue biopsied from a surgically debrided wound should be used.5 Importantly, a higher index of suspicion should be maintained for infection of Stage III or IV pressure ulcers because they are more commonly infected than Stage I or II ulcers.3
Prevention. The prevention of wound progression is essential in treating acute, chronic, or infected pressure ulcers. Although management guidelines are limited by few high-quality, randomized controlled trials, NPUAP recommends a number of prevention strategies targeting risk factors that contribute to pressure ulcer development.2,3,8
For all bed-bound and chair-bound persons with impaired ability to self-reposition, risk assessment for pressure ulcer should be done on admission and repeated every 24 hours. The presence of such risk factors as immobility, shear, friction, moisture, incontinence, and malnutrition should be used to guide preventive treatments. Pressure relief on an ulcer can be achieved by repositioning the immobile patient at one- to two-hour intervals. Pressure-redistributing support surfaces (static, overlays, or dynamic) reduce tissue pressure and decrease overall incidence of pressure ulcers. Due to a lack of relative efficacy data, the selection of a support surface should be determined by the patient’s individual needs in order to reduce pressure and shear.3 For instance, dynamic support is an appropriate surface for an immobile patient with multiple or nonhealing ulcers. Shearing force and friction can be reduced by limiting head-of-bed elevation to <30° and using such transfer aids as bed linens while repositioning patients. The use of pillows, foam wedges, or other devices should be used to eliminate direct contact of bony prominences or reduce pressure on heels.8
Skin care should be optimized to limit excessive dryness or moisture. This includes using moisturizers for dry skin, particularly for the sacrum, and implementing bowel and bladder programs and absorbent underpads in patients with bowel or bladder incontinence.2 Given that patients with pressure ulcers are in a catabolic state, those who are nutritionally compromised may benefit from nutritional supplementation.3 Lastly, appropriate use of local and systemic pain regimen for painful pressure ulcers can improve patient cooperation in repositioning, dressing change, and quality of life.
Debridement. Wound debridement removes necrotic tissue often present in infected or chronic pressure ulcers, reduces risk for further infection, and promotes granulation tissue formation and wound healing. Debridement, however, is not indicated for ulcers of an ischemic limb or dry eschar of the heel, due to propensity for complications.3,4 The five common debridement methods are sharp, mechanical, autolytic, enzymatic, and biosurgical. The debridement method of choice is determined by clinician preference and availability.4
Sharp debridement results in rapid removal of large amounts of nonviable necrotic tissues and eschar using sharp instruments and, therefore, is indicated if wound infection or sepsis is present. Mechanical debridement by wet-to-dry dressing or whirlpool nonselectively removes granulation tissue and, thus, should be used cautiously. Autolytic debridement uses occlusive dressings (i.e. hydrocolloid or hydrogel) to maintain a moist wound environment in order to optimize the body’s inherent ability to selectively self-digest necrotic tissues. Enzymatic debridement with concentrated topical proteolytic enzymes (i.e. collagenase) digests necrotic tissues and achieves faster debridement than autolysis while being less invasive than surgical intervention. Biosurgery utilizes maggots (i.e. Lucilia sericata) that produce enzymes to effectively debride necrotic tissues.
Wound care and dressing. Pressure ulcers should be cleansed with each dressing change using such physiologic solutions as normal saline. Cleansing with antimicrobial solutions for ulcers with large necrotic debris or infection needs to be thoughtfully administered due to the potential impairment on wound healing.4 Wound dressing should maintain a moist wound environment to allow epithelialization and limit excessive exudates in order to prevent maceration. Although there are many categories of moisture retentive dressings, their comparative effectiveness remain unclear.4 Table 2 summarizes characteristics of common wound dressings and their applications.
Antibiotic use. Topical antibiotics are appropriate for Stage III or IV ulcers with signs of local infection, including periwound erythema and friable granulation tissue.4 The Agency for Health Care Policy and Research recommends a two-week trial of a topical antibiotic, such as silver sulfadiazine, for pressure ulcers that fail to heal after two to four weeks of optimal care.6 Systemic antibiotics should be used for patients who demonstrate evidence of systemic infection including sepsis, osteomyelitis, or cellulitis with associated fever and leukocytosis. The choice of systemic antibiotics should be based on cultures from blood, bone, or deep tissue biopsied from a surgically debrided wound.4,6
Back to the Case
The patient was hospitalized for altered mental status. She was at high risk for the progression of her sacral ulcer and the development of new pressure ulcers due to immobility, incontinence, malnutrition, and impaired mental status. The sacral wound was a chronic, Stage III pressure ulcer without evidence of local tissue infection. However, the presence of leukocytosis and fever were suggestive of an underlying infection. Her urine analysis was consistent with a urinary tract infection.
Trimethoprim/sulfamethoxazole was administered with subsequent resolution of leukocytosis, fever, and delirium. The sacral ulcer was cleansed with normal saline and covered with hydrocolloid dressing every 72 hours in order to maintain a moist wound environment and facilitate autolysis. Preventive interventions guided by her risk factors for pressure ulcer were implemented. Interventions included:
- Daily skin and wound assessment;
- Pressure relief with repositioning every two hours;
- Use of a dynamic support surface;
- Head-of-bed elevation of no more than <30° to reduce shear and friction;
- Use of transfer aids;
- Use of devices to eliminate direct contact of bony prominences;
- Optimizing skin care with moisturizers for dry skin and frequent changing of absorbent under pads for incontinence; and
- Consulting nutrition service to optimize nutritional intake.
Bottom Line
Assessments of pressure ulcer stage, wound infection, and risk factors guide targeted therapeutic interventions that facilitate wound healing and prevent new pressure ulcer formation.
Dr. Prager is a fellow in the Brookdale Department of Geriatrics and Palliative Medicine at Mount Sinai School of Medicine in New York City. Dr. Ko is a hospitalist and an assistant professor in the Brookdale Department of Geriatrics and Palliative Medicine at Mount Sinai.
References
- Pressure ulcers in America: prevalence, incidence, and implications for the future. An executive summary of the National Pressure Ulcer Advisory Panel monograph. Adv Skin Wound Care. 2001;14(4):208-215.
- Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. JAMA. 2006;296(8):974-984.
- European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel. Treatment of Pressure Ulcers: Quick Reference Guide. Washington, D.C.: National Pressure Ulcer Advisory Panel; 2009.
- Bates-Jensen BM. Chapter 58. Pressure Ulcers. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York: McGraw-Hill; 2009.
- Livesley NJ, Chow AW. Infected pressure ulcers in elderly individuals. Clin Infect Dis. 2002;35(11):1390-1396.
- Agency for Health Care Policy and Research (AHCPR). Treatment of Pressure Ulcers. Clinical Practice Guideline Number 15. U.S. Department of Health and Human Services. 1994.
- Reddy M, Gill SS, Wu W, Kalkar SR, Rochon PA. Does this patient have an infection of a chronic wound? JAMA. 2012;307(6):605-611.
- National Pressure Ulcer Advisory Panel. Pressure Ulcer Prevention Points. National Pressure Ulcer Advisory Panel website. Available at: http://www.npuap.org/resources/educational-and-clinical-resources/pressure-ulcer-prevention-points/. Accessed Aug. 1, 2012.
- Reuben DB, Herr KA, Pacala JT, et al. Skin Ulcers. In: Geriatrics At Your Fingertips. 12th ed. New York: The American Geriatrics Society; 2010.
The disability application process
Being rheumatologists, we all probably get letters on fancy lawyer letterheads asking for our office notes for a patient who is applying for disability. Then we receive more requests asking the physician to please describe in detail how much or how little work the patient can be expected to perform. Not infrequently, I even receive requests for disability assessments for patients I’ve never heard of, only to find out that I am seeing them as new patients in the coming days.
So I was excited to learn that subspecialty grand rounds was going to be given by a disability lawyer. He did a good job of explaining the law and breaking down the process.
Disability, as defined by the law, is "the inability to engage in any substantial gainful activity by reason of any medically determinable physical or mental impairment which ... has lasted or can be expected to last for a continuous period of not less than 12 months."
The process of getting disability is lengthy. According to the Social Security Administration, for the past 3 years, roughly 35% of first-time applications were granted. Of applicants who were denied, only half submitted a request for reconsideration, and of those, only 12%-14% were granted disability status.
After the agency denies an applicant twice, the applicant’s next recourse is to request a hearing. At this point an administrative law judge reviews the information, hears the case, and makes a decision. Data from the past 3 years reveals that about 60% succeed at this stage. If denied, the applicant can appeal this decision, and it goes to the same judge.
If, at the end of all that, the patient still does not get disability, he can either elevate the appeal to a federal judge or file a new application altogether.
Documentation has to be submitted to the effect that the patient cannot perform his or her past work. Then the question is whether, given his age, education, and work experience, he is able to perform other kinds of work.
The gold standard for evidence of a patient’s ability to work is a physician’s description of the patient’s functional limits. This is where those onerous forms come in. In the pesky and interminable "residual functional capacity" questionnaires, we are asked to estimate how much or how little standing/sitting/lifting the patient can do in a given amount of time.
But while the disability lawyer clarified the process of getting disability, he nonetheless interpreted the process through his own perspective. I felt that the following points he made oversimplified the important questions that physicians must answer:
• Disability is really difficult to obtain. Therefore, people who are willing to put up with the process must really be in bad shape.
This is a bogus bifurcation, a fallacy of apriorism. People will do unpleasant things, like wait a very long time to get disability, if they perceive it as the better of two unpleasant options.
• He asks his audience, "Would you hire them?"
I would counter that there may be other issues that would lead an employer to not hire someone, and the law specifically states that disability status is denied or granted "regardless of ... whether or not he would be hired if he applied for work."
• It is not easy, if you have chronic pain, to lift a 10-pound weight frequently throughout the day, which is how the Department of Labor defines "light work."
But the same paragraph that defines light work as the frequent lifting of objects weighing up to 10 pounds also says, "Even though the weight lifted may be very little, a job is in this category when it requires a good deal of walking or standing, or when it involves sitting most of the time with some pushing and pulling of arm or leg controls."
I think these sorts of points illustrate a line of reasoning that does not serve patients well when they seek legal advice on applying for disability. They paint a picture that gives doctors reasons to be skeptical of the process (especially when the doctor has not even met the patient yet) and may encourage patients to seek disability when they still have the ability "to engage in any substantial gainful activity."
Dr. Chan practices rheumatology in Pawtucket, R.I. E-mail her at [email protected]
Being rheumatologists, we all probably get letters on fancy lawyer letterheads asking for our office notes for a patient who is applying for disability. Then we receive more requests asking the physician to please describe in detail how much or how little work the patient can be expected to perform. Not infrequently, I even receive requests for disability assessments for patients I’ve never heard of, only to find out that I am seeing them as new patients in the coming days.
So I was excited to learn that subspecialty grand rounds was going to be given by a disability lawyer. He did a good job of explaining the law and breaking down the process.
Disability, as defined by the law, is "the inability to engage in any substantial gainful activity by reason of any medically determinable physical or mental impairment which ... has lasted or can be expected to last for a continuous period of not less than 12 months."
The process of getting disability is lengthy. According to the Social Security Administration, for the past 3 years, roughly 35% of first-time applications were granted. Of applicants who were denied, only half submitted a request for reconsideration, and of those, only 12%-14% were granted disability status.
After the agency denies an applicant twice, the applicant’s next recourse is to request a hearing. At this point an administrative law judge reviews the information, hears the case, and makes a decision. Data from the past 3 years reveals that about 60% succeed at this stage. If denied, the applicant can appeal this decision, and it goes to the same judge.
If, at the end of all that, the patient still does not get disability, he can either elevate the appeal to a federal judge or file a new application altogether.
Documentation has to be submitted to the effect that the patient cannot perform his or her past work. Then the question is whether, given his age, education, and work experience, he is able to perform other kinds of work.
The gold standard for evidence of a patient’s ability to work is a physician’s description of the patient’s functional limits. This is where those onerous forms come in. In the pesky and interminable "residual functional capacity" questionnaires, we are asked to estimate how much or how little standing/sitting/lifting the patient can do in a given amount of time.
But while the disability lawyer clarified the process of getting disability, he nonetheless interpreted the process through his own perspective. I felt that the following points he made oversimplified the important questions that physicians must answer:
• Disability is really difficult to obtain. Therefore, people who are willing to put up with the process must really be in bad shape.
This is a bogus bifurcation, a fallacy of apriorism. People will do unpleasant things, like wait a very long time to get disability, if they perceive it as the better of two unpleasant options.
• He asks his audience, "Would you hire them?"
I would counter that there may be other issues that would lead an employer to not hire someone, and the law specifically states that disability status is denied or granted "regardless of ... whether or not he would be hired if he applied for work."
• It is not easy, if you have chronic pain, to lift a 10-pound weight frequently throughout the day, which is how the Department of Labor defines "light work."
But the same paragraph that defines light work as the frequent lifting of objects weighing up to 10 pounds also says, "Even though the weight lifted may be very little, a job is in this category when it requires a good deal of walking or standing, or when it involves sitting most of the time with some pushing and pulling of arm or leg controls."
I think these sorts of points illustrate a line of reasoning that does not serve patients well when they seek legal advice on applying for disability. They paint a picture that gives doctors reasons to be skeptical of the process (especially when the doctor has not even met the patient yet) and may encourage patients to seek disability when they still have the ability "to engage in any substantial gainful activity."
Dr. Chan practices rheumatology in Pawtucket, R.I. E-mail her at [email protected]
Being rheumatologists, we all probably get letters on fancy lawyer letterheads asking for our office notes for a patient who is applying for disability. Then we receive more requests asking the physician to please describe in detail how much or how little work the patient can be expected to perform. Not infrequently, I even receive requests for disability assessments for patients I’ve never heard of, only to find out that I am seeing them as new patients in the coming days.
So I was excited to learn that subspecialty grand rounds was going to be given by a disability lawyer. He did a good job of explaining the law and breaking down the process.
Disability, as defined by the law, is "the inability to engage in any substantial gainful activity by reason of any medically determinable physical or mental impairment which ... has lasted or can be expected to last for a continuous period of not less than 12 months."
The process of getting disability is lengthy. According to the Social Security Administration, for the past 3 years, roughly 35% of first-time applications were granted. Of applicants who were denied, only half submitted a request for reconsideration, and of those, only 12%-14% were granted disability status.
After the agency denies an applicant twice, the applicant’s next recourse is to request a hearing. At this point an administrative law judge reviews the information, hears the case, and makes a decision. Data from the past 3 years reveals that about 60% succeed at this stage. If denied, the applicant can appeal this decision, and it goes to the same judge.
If, at the end of all that, the patient still does not get disability, he can either elevate the appeal to a federal judge or file a new application altogether.
Documentation has to be submitted to the effect that the patient cannot perform his or her past work. Then the question is whether, given his age, education, and work experience, he is able to perform other kinds of work.
The gold standard for evidence of a patient’s ability to work is a physician’s description of the patient’s functional limits. This is where those onerous forms come in. In the pesky and interminable "residual functional capacity" questionnaires, we are asked to estimate how much or how little standing/sitting/lifting the patient can do in a given amount of time.
But while the disability lawyer clarified the process of getting disability, he nonetheless interpreted the process through his own perspective. I felt that the following points he made oversimplified the important questions that physicians must answer:
• Disability is really difficult to obtain. Therefore, people who are willing to put up with the process must really be in bad shape.
This is a bogus bifurcation, a fallacy of apriorism. People will do unpleasant things, like wait a very long time to get disability, if they perceive it as the better of two unpleasant options.
• He asks his audience, "Would you hire them?"
I would counter that there may be other issues that would lead an employer to not hire someone, and the law specifically states that disability status is denied or granted "regardless of ... whether or not he would be hired if he applied for work."
• It is not easy, if you have chronic pain, to lift a 10-pound weight frequently throughout the day, which is how the Department of Labor defines "light work."
But the same paragraph that defines light work as the frequent lifting of objects weighing up to 10 pounds also says, "Even though the weight lifted may be very little, a job is in this category when it requires a good deal of walking or standing, or when it involves sitting most of the time with some pushing and pulling of arm or leg controls."
I think these sorts of points illustrate a line of reasoning that does not serve patients well when they seek legal advice on applying for disability. They paint a picture that gives doctors reasons to be skeptical of the process (especially when the doctor has not even met the patient yet) and may encourage patients to seek disability when they still have the ability "to engage in any substantial gainful activity."
Dr. Chan practices rheumatology in Pawtucket, R.I. E-mail her at [email protected]
Low Concordance for Site of Death
At the turn of the 20th century, most deaths in the United States occurred at home. By the 1960s, over 70% of deaths occurred in an institutional setting, reflecting an evolution of medical technology.[1, 2, 3] With the birth of the hospice movement in the 1970s, dying patients had the opportunity to have both death at home and aggressive symptom control at the end of life. Although there has been a slow decline in the proportion of deaths that occur in the hospital over the past 2 decades,[3] the overwhelming majority of persons state that they would prefer to die at home. However, recent findings suggest that most people will die in an institutional setting.[3, 4, 5, 6]
Although good data exist describing population preferences for location of death, and we know, based on death records, where deaths occur in the United States, there are few studies that examine concordance between preferred and actual site of death at the individual patient level. Furthermore, although factors have been identified that predict death at home, factors predicting concordance between preferred and actual site of death are not well described.[3, 6, 7, 8, 9, 10, 11, 12, 13]
Regardless of where death ultimately occurs, most adults will experience multiple hospitalizations within the last years of their life. Understanding the preferences and subsequent experiences of this population is of particular relevance to hospitalist physicians who are in a unique position to elicit goals from seriously ill patients and help match patient preferences with their medical care. In this observational study, we sought to determine preferences for site of death in a cohort of adult patients admitted to the hospital for medical illness, and then follow those patients to determine where death occurred for those who died. We also sought to explore factors that may predict concordance between preferred and actual site of death. We hypothesized that ethnic diversity and lower socioeconomic status would be associated with a lower likelihood of concordance between preferred and actual site of death. We also hypothesized that advanced care planning would be associated with a higher likelihood of concordance. The Colorado Multi‐Institutional Review Board approved this study.
METHODS
Participants were recruited from 3 hospitals affiliated with the University of Colorado School of Medicine Internal Medicine Residency program, including the Denver Veterans' Administration Center (DVAMC), Denver Health Medical Center (DHMC), and University of Colorado Hospital (UCH). The DVAMC is a large urban Veterans Administration hospital, serving veterans from the Denver metro area, and is a tertiary referral center for veterans in rural Colorado, Wyoming, and parts of Montana. DHMC, the safety‐net hospital for the Denver area, serves over 25% of the residents in the city and county of Denver, including such special populations as the indigent, chronically mentally ill, and persons with polysubstance dependence. UCH had 350 licensed beds at the time of our study and serves as the Rocky Mountain region's only academic tertiary, specialty care, and referral center. At the time of this study, there was limited inpatient palliative care services at the DVAMC and UH, and no palliative care services at DHMC. Participants were screened on the first day following admission to the adult general medical service. Participants were recruited on 96 postadmission days between February 2004 and June 2006. Recruitment days varied from Monday through Friday, to include admissions from the weekend and throughout the year to reduce potential bias due to seasonal trends of diseases such as influenza. Patients were excluded if they died or were discharged within the first 24 hours of admission, were pregnant, jailed, or unable to give informed consent. All other patients were approached and invited to participate in a brief survey.
After informed consent was obtained, participants completed a bedside interview that included self‐identified ethnicity and the Berkman‐Syme Social Network Index,[14] a brief questionnaire quantifying social support from spouse or domestic partner, family, friends, and other religious or secular organizations. Baseline socioeconomic measures (eg, income, employment, home ownership, car ownership) and questions related to the last days of life were also included. Participants were asked the following question, If you were very sick, with an illness that could not be cured, and in bed most of the time, where would you spend the last days of your life if you could chose?
For each participant, we performed a detailed chart review to determine demographic data, presence of advance directives, and CARING criteria (Cancer, Admissions 2, Residence in a nursing home, Intensive care unit admit with multiorgan failure, 2 Noncancer hospice Guidelines), a set of prognostic criteria identifying patients at an index hospitalization who have a high burden of illness and are at risk for death in the following year.[15] We then followed patients for 5 years. If participants died within the follow‐up period, we collected the date and location of death using medical records, death certificates, or in a few cases when official death records were unavailable, direct contact with the family. Participants were considered alive if they had a clinic visit or MD/RN phone contact within 3 months prior to the final collection point date.
Analysis
SAS 9.1 (SAS Institute Inc., Cary, NC) was used for all analyses. Simple frequencies and means statistics were used to determine rates of descriptive characteristics of the sample as well as rates of the measured outcomes, preferred place to spend last days of life, and actual site of death. Agreement or concordance between preferred and actual site of death was calculated. For the purposes of the analysis, we assumed all persons who stated they had no preference died in a place concordant with their wishes. To calculate agreement by preferred and actual site, participants who expressed a preference and died (n=111) in hospital, nursing home, home, or hospice setting were included in the analysis, and participants (n=4) who died in an unknown or other locations were excluded (eg, motel room).
Logistic Regression Modeling
2 tests were performed for all categorical variables to determine a significant association with outcome variables. Preferred place of death and concordance between preferred and actual site of death were modeled using predictive variables selected if univariable association demonstrated a P0.25. This standard cutoff was selected to broadly identify candidate variables for logistic regression modeling.[16] A stepwise algorithm was used to select significant predictors that would remain in the model.
In lieu of fitting a multinomial logit model for preferred site of death of home vs hospital vs nursing home or hospice facility as preferred site of death, 3 logit models (although only 2 may be sufficient to estimate the underlying multinomial logit model[17]) were considered with outcome categories: home vs nursing home or hospice facility, and hospital vs nursing home or hospice facility and home vs hospital.
For the logistic regression modeling of concordance, we included the full sample of patients who died during the follow‐up period (n=123). We classified participants as dying in a place concordant with their wishes or not concordant.
RESULTS
Study Population
Subjects were recruited on 96 post‐admission days totaling 842 admissions. Three hundred thirty‐one patients (39%) were ineligible for study participation (n=175 discharged within 24 hours, n=76 unable to consent, n=78 ineligible for other reasons [eg, prisoner, pregnant, under 18 years old], n=2 died within 24 hours of admission). Only 53 of the remaining 511 (10%) patients refused; 458 patients (90%) gave informed consent to participate. Characteristics of the study population are depicted in Table 1. There were very few missing cases (<3%), that is persons without a recent clinic follow‐up date, contact, or a confirmed date of death. These persons were considered alive. Overall, the sample population was ethnically diverse, slightly older than middle age, mostly male (due to the inclusion of the Veterans Administration hospital), and of low socioeconomic status.
| Mean age (SD), y | 57.9 (14.8) |
|---|---|
| |
| Mean time to death (SD), d | 339.5 (348.4) |
| Ethnicity | |
| African American | 19% (88) |
| Caucasian | 52% (239) |
| Latino | 22% (102) |
| Other | 6% (29) |
| Spanish language only | 6% (27) |
| Female gender | 35% (159) |
| Admitted to DVAMC | 41% (188) |
| Admitted to DHMC | 38% (174) |
| Admitted to UCH | 21% (96) |
| CARING criteria | |
| Cancer diagnosis | 11% (51) |
| Admitted to hospital 2 times in the past year for chronic illness | 40% (181) |
| Resident in a nursing home | 2% (9) |
| Noncancer hospice guidelines (meeting 2) | 13% (59) |
| Income <$30,000/year | 84% (377) |
| No greater than high school education | 55% (248) |
| Home owner | 26% (120) |
| Rents home | 39% (177) |
| Unstable living situationa | 34% (156) |
| Low social supportb | 36% (165) |
| Uninsured | 18% (81) |
| Regular primary care provider | 73% (330) |
Preferred Site of Death
When asked where they preferred to spend the last days of their life, 75% of patients (n=343) stated they would like to be at home. In the hospital was the preferred location for 10% of patients, whereas 6% stated a nursing home and 4% a hospice inpatient facility. Two percent stated they had no preference, and 3% refused to answer (Figure 1)
We found that in the univariable analysis the following factors were associated with preference for site of death at a significance level of P<0.25: unstable housing, hospital setting, income level, ethnicity, CARING criteria, presence of an advance directive, education level, married, primary care provider, and presence of public insurance. Results of the logit models (home vs nursing home or hospice facility, and hospital vs nursing home or hospice facility and home vs hospital) are presented in Table 2.
| Adjusted Odds Ratio (95% Confidence Interval) | |||
|---|---|---|---|
| Home vs Nursing Home/Hospice Facility | Hospital vs Nursing Home/Hospice Facility | Home vs Hospital | |
| |||
| Low income | 2.71 (1.305.67) | 3.05 (1.019.24) | 0.99 (0.422.37) |
| Married | 2.44 (1.145.21) | 2.40 (0.876.62) | 0.82 (0.421.57) |
| CARING criteria | 0.58 (0.301.14) | 0.44 (0.181.09) | 0.89 (0.471.66) |
Patients with income <$30,000/year were more likely to prefer home (or hospital) over a nursing home or hospice facility. Being married was predictive of preferring home over nursing home or hospice facility. Patients meeting 1 of the CARING criteria trended toward being less likely (P=0.11 for home and P=0.08 for hospital) to prefer home (or hospital) vs nursing home or hospice facility. However, there were no significant predictors for a preference for home or hospital when directly comparing the 2 locations, as expected from observing similar effects of variables in the other 2 logit models.
Actual Site of Death
One hundred twenty‐three patients died during the follow‐up period (26% of the total sample). Of those who died, the mean age was 64 years (standard deviation 13), 82% had annual incomes <$30,000, 73% were men, and 77% met at least 1 of the CARING criteria suggesting advanced medical illness. The distribution of ethnicities of the deceased subsample was similar to that of the overall cohort. Complete death records were obtained for 121 patients. Only 31% (n=38) died at home, whereas 35% (n=42) died in a hospital, 20% (n=24) died in a nursing home, and 12% (n=14) died in an inpatient hospice facility (Figure 1).
In univariable analysis, there were no associations at a 25% significance level between actual site of death and ethnicity, gender, age, severity of illness, high vs low social support, high or low socioeconomic status, stable vs unstable housing, or presence of a completed advance directive in the medical record.
Concordance Between Preferred and Actual Site of Death
Overall, 37% of the patients died where they stated they would prefer to die, including the 2 with no preference. Concordance rates for each site of death are presented in Table 3. We examined sociodemographic variables, disease severity, advance‐care planning, primary care provider, health insurance, and hospital site to look for associations with concordance. We found that female gender was positively associated with concordance (odds ratio [OR], 3.30; 95% confidence interval [CI], 1.25‐8.72). CARING criteria (P=0.06) and Latino ethnicity (vs all other ethnicity categories, P=0.12) also showed trends for association. Restricting to those who preferred home, the associations became stronger (OR, 4.62; 95% CI, 1.44‐14.79 for female; OR, 7.72; 95% CI, 1.67‐35.71 for CARING criteria), and the trend for the negative association between Latino ethnicity and concordance remained (P=0.12). Results of the model are shown in Table 4.
| Actual Site of Death, n (Row %) | Row Total, % Out of 111 | ||||
|---|---|---|---|---|---|
| Hospital | Nursing Home | Home | Hospice Facility | ||
| Preferred hospital | 5 (42%) | 3 (25%) | 2 (17%) | 2 (17%) | 12 (11%) |
| Preferred nursing home | 1 (13%) | 5 (63%) | 2 (25%) | 0 | 8 (7%) |
| Preferred home | 30 (34%) | 15 (17%) | 31 (35%) | 12 (14%) | 88 (79%) |
| Preferred hospice facility | 3 (100%) | 0 | 0 | 0 | 3 (3%) |
| Adjusted Odds Ratio (95% Confidence Interval) | |||
|---|---|---|---|
| All | Home (Using Same Variables) | Home (Using Only Significant Variables) | |
| |||
| Female gender | 3.30 (1.258.72) | 4.62 (1.4414.79) | 3.57 (1.2410.34) |
| CARING criteria | 3.09 (0.979.81) | 7.72 (1.6735.71) | 5.93 (1.4124.91) |
| Latino vs African American/Caucasian/other | 0.43 (0.151.24) | 0.35 (0.091.30) | |
DISCUSSION
We found, similarly to previous reports in the literature, the majority of patients preferred to die at home. We did not find a significant difference in preferences or location of death by ethnicity or illness severity. Lower‐income patients and married patients were more likely to prefer to be at home over a nursing home or a hospice facility in the last days of life. We found that the minority of patients died at their stated preferred site of death, and female gender was the only predictive variable we found to distinguish those patients who died in a place concordant with their wishes compared to those who did not.
In the literature, previous studies have reported concordance rates between preferred and actual site of death that range from 30% to 90%.[12, 13, 18, 19, 20, 21, 22, 23, 24] We found a concordance rate at the lowest end of this spectrum. In trying to understand our findings and place them in context, it is helpful to examine other studies. Many of these studies focused solely on cancer patients.[13, 18, 19, 20, 21, 22, 23] Cancer follows a more predictable trajectory of decline compared to other common life‐threatening illnesses, such as cardiac disease, emphysema, or liver failure, that often involve periods of acute deteriorations and plateaus throughout illness progression. The more predictable trajectory may explain the overall higher concordance rates found in the studies involving cancer patients.
The majority of studies in the literature examining concordance between preferred and actual site of death recruited the study sample from home health or home palliative care programs that were providing support and care to participants.[10, 12, 13, 18, 22, 25, 26, 27] The high concordance rates reported may be the result of the patients in the sample receiving services at home aimed at eliciting preferences and providing support at home. Our observational study is unique in that we elicited patient preferences from a diverse group of hospitalized adults. Patients had a broad range of medical illness and were at various stages in their disease trajectory. This allowed our findings to be more generalizable, a major strength of our study.
The only variable associated with concordance that we identified to predict concordance between preferred and actual site of death was female gender. Women have been shown to be more active in medical decision making, which may explain our findings.[28] Female gender and illness severity (as measured by the CARING criteria) were found to be associated with concordance when the preference is for death at home. For persons with more advanced medical illness, they may have had more opportunity to consider their preferences and talk about these preferences. It is even possible that our interview prompted some participants to have discussions with their families or providers.
Variables with high face validity, such as high social support, higher education, and completing an advance directive, did not demonstrate any effect on the outcome of concordance. Other studies have shown that low functional status, Caucasian ethnicity, home care, higher education, and social support have been associated with a greater likelihood for a home death.[3, 6, 9] However, although studies specifically examining concordance between preferred and actual site of death have looked at predictors for home death, we were unable to find predictors for concordance across all preferences in the literature. We can conclude from our findings that the factors that influence concordance of preferences for site of death are extremely complex and difficult to capture and measure. This is extremely unsatisfying in the face of the low concordance rate of 30% we identified.
Latino ethnicity showed a trend toward having a negative association with concordance between preferred and actual site of death. This trend persisted whether it was concordance overall or for concordance with those who preferred a death at home. In the literature, Latinos have been found to be less likely to complete advance directives, use hospice services at the end of life, and are more likely to experience a hospital death.[29, 30, 31, 32, 33] As care at the end of life continues to improve, careful attention should be paid to ensure that these kinds of gaps do not widen any further.
We interviewed patients at an index hospitalization. Patients had an acute medical illness or an exacerbation of a chronic medical illness and required at least 24 hours of hospitalization to be eligible for inclusion. Our bedside interview made use of an opportune time to question patients, a time when it may have been easier for patients to visualize severe illness at the end of life, rather than asking this question during a time of wellness. Although participants overwhelmingly stated they preferred to be at home, for those who died, decisions were made in their care that did not allow for this preference.
Our follow‐up after the initial bedside interview only included death records of where and when participants died. We do not have the details and narrative of the conversations that may have taken place that led to the care decisions that determined participants' actual place of death. We do not know if preferences were elicited or discussed, and care decisions then negotiated, to best meet the goals and preferences expressed at that time. We also do not know if the conversations did not occur and the default of medical intervention and cure‐focused care dictated how participants spent the last days of their life. There is evidence that when conversations about goals and preferences do occur, concordance between preferences and care received are high.[12, 21]
We were unable to identify any predictors beyond gender in this cohort of adults hospitalized with a broad spectrum of severe medical illness to predict concordance with stated preferences and actual site of death. We can conclude then, based on our findings and supported by the literature, that the default trends toward institutional end‐of‐life experiences. To shift to a more patient‐centered approach, away from the default, healthcare providers need to embrace a palliative approach and incorporate preferences and goals into the discussions about next steps of care to facilitate the peaceful death that the majority of patients imagine for themselves. Hospitalist physicians have a unique opportunity at an index hospitalization to start the conversation about preferences for care including where patients would want to spend the last days of their life.
Our study does have some limitations. We elicited preferences at a single point in time, at an index hospitalization. It is possible that participants' preferences changed over the course of their illness. However, in Agar et al.'s study of longitudinal patient preferences for site of death and place of care, most preferences remained stable over time.[18] We also did not have data that included palliative care involvement, homecare or hospice utilization, or cause of death. All of these variables may be important predictors of concordance. Issues of symptom management and lack of caregiver may also dictate place of death, even when goals and care are aligned. We do not have data to address these components of end‐of‐life decision making.
CONCLUSION
Patients continue to express a preference for death at home. However, the majority of patients experienced an institutional death. Furthermore, few participants achieved concordance with where they preferred to die and where they actually died. Female gender was the sole factor associated with concordance between preferred and actual site of death. Incorporating a palliative approach that elicits goals and helps match goals to care, may offer the best opportunity to help people die where they chose.
Disclosures: This research was supported by the Brookdale National Fellowship Award and the NIA/Beeson grant 5K23AG028957. All authors have seen and agree with the contents of the article. This submission was not under review by any other publication. The authors have no financial interest or other potential conflicts of interest.
- Field MJ, Cassel CK, eds. Committee on Care at the End of Life. Approaching Death: Improving Care at the End of Life. Washington DC: National Academy Press; 1997.
- , . Demography and epidemiology of dying in the U.S. with emphasis on deaths of older persons. Hosp J. 1998;13:49–60.
- , , , . Factors associated with site of death: a national study of where people die. Med Care. 2003;41:323–335.
- , , , , , . Terminal cancer care and patient's preferences for place of death: a prospective study. BMJ. 1990;301:415–417.
- , , , et al. Influence of patient preferences and local health system characteristics on the place of death. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Risks and Outcomes of Treatment. J Am Geriatr Soc. 1998;46:1242–1250.
- , , , , , . Where people die: a multilevel approach to understanding influences on site of death in America. Med Care Res Rev. 2007;64:351–378.
- , , , , , . Dying at home or in an institution using death certificates to explore the factors associated with place of death. Health Policy. 2006;78:319–329.
- , . How do cancer patients who die at home differ from those who die elsewhere? Palliat Med. 1998;12:279–286.
- , . Factors influencing death at home in terminally ill patients with cancer: systematic review [published correction appears in BMJ. 2006;332:1012]. BMJ 2006;332:515–521.
- , , , , , . Predictive factors for home deaths among cancer patients in Swedish palliative home care. Support Care Cancer. 2003;11:560–567.
- , . Systemic adenosine infusions alleviated neuropathic pain. Pain. 2001;94:121–122.
- , , . Prevalence, effectiveness, and predictors of planning their place of death among older persons followed in community‐based long term care. J Am Geriatr Soc. 2000;48:943–948.
- , , , , . Preferences for place of care and place of death among informal caregivers of the terminally ill. Palliat Med. 2005;19:492–499.
- , . Social networks, host resistance, and mortality: a nine‐year follow‐up study of Alameda County residents. Am J Epidemiol. 1979;109:186–204.
- , , , , , . A practical tool to identify patients who may benefit from a palliative approach: the CARING criteria. J Pain Symptom Manage. 2006;31:285–292.
- , . Applied Logistic Regression. 2nd ed. New York, NY: Wiley‐Interscience; 2000.
- , . Calculation of polychotmous logistic regression parameters using individualized regressions. Biometrika. 1984;71:11–18.
- , , , , , . Preference for place of care and place of death in palliative care: are these different questions? Palliat Med. 2008;22(7):787–795.
- , , . Place of death: preferences among cancer patients and their carers. Soc Sci Med. 2004;58:2431–2444.
- , . Determinants of congruence between the preferred and actual place of death for terminally ill cancer patients. J Palliat Care. 2003;19:230–237.
- , , . Factors associated with location of death (home or hospital) of patients referred to a palliative care team. CMAJ. 1995;152:361–367.
- , , , et al. Proxy perspectives regarding end‐of‐life care for persons with cancer. Cancer. 2008;112:1854–1861.
- , , , , , . Actual and preferred place of death of cancer patients. Results from the Italian survey of the dying of cancer (ISDOC). J Epidemiol Community Health. 2006;60:412–416.
- , , , . Family reports of barriers to optimal care of the dying. Nurs Res. 2000;49:310–317.
- , , . Place of death: preferences among cancer patients and their carers. Soc Sci Med. 2004;58(12):2431–2444.
- , . Where do elderly patients prefer to die? Place of death and patient characteristics of 100 elderly patients under the care of a home health care team. J Am Geriatr Soc. 1983;31:457–461.
- , , , , , . Predictors of home death in palliative care cancer patients. J Palliat Care. 2000;16:23–28.
- , . Patient preferences for medical decision making: who really wants to participate? Med Care. 2000;38:335–341.
- , , , et al. Racial and ethnic differences in advance care planning among patients with cancer: impact of terminal illness acknowledgment, religiousness, and treatment preferences. J Clin Oncol. 2008;26:4131–4137.
- , , , . Influence of ethnicity on advance directives and end‐of‐life decisions. JAMA. 1997;277:298–299.
- , , , . Differences in end‐of‐life decision making among black and white ambulatory cancer patients. J Gen Intern Med. 1996;11:651–656.
- , , . Hospice usage by minorities in the last year of life: results from the National Mortality Feedback Survey. J Am Geriatr Soc. 2003;51:970–978.
- , , , , , . Place of death: correlations with quality of life of patients with cancer and predictors of bereaved caregivers' mental health. J Clin Oncol. 2010;28:4457–4464.
At the turn of the 20th century, most deaths in the United States occurred at home. By the 1960s, over 70% of deaths occurred in an institutional setting, reflecting an evolution of medical technology.[1, 2, 3] With the birth of the hospice movement in the 1970s, dying patients had the opportunity to have both death at home and aggressive symptom control at the end of life. Although there has been a slow decline in the proportion of deaths that occur in the hospital over the past 2 decades,[3] the overwhelming majority of persons state that they would prefer to die at home. However, recent findings suggest that most people will die in an institutional setting.[3, 4, 5, 6]
Although good data exist describing population preferences for location of death, and we know, based on death records, where deaths occur in the United States, there are few studies that examine concordance between preferred and actual site of death at the individual patient level. Furthermore, although factors have been identified that predict death at home, factors predicting concordance between preferred and actual site of death are not well described.[3, 6, 7, 8, 9, 10, 11, 12, 13]
Regardless of where death ultimately occurs, most adults will experience multiple hospitalizations within the last years of their life. Understanding the preferences and subsequent experiences of this population is of particular relevance to hospitalist physicians who are in a unique position to elicit goals from seriously ill patients and help match patient preferences with their medical care. In this observational study, we sought to determine preferences for site of death in a cohort of adult patients admitted to the hospital for medical illness, and then follow those patients to determine where death occurred for those who died. We also sought to explore factors that may predict concordance between preferred and actual site of death. We hypothesized that ethnic diversity and lower socioeconomic status would be associated with a lower likelihood of concordance between preferred and actual site of death. We also hypothesized that advanced care planning would be associated with a higher likelihood of concordance. The Colorado Multi‐Institutional Review Board approved this study.
METHODS
Participants were recruited from 3 hospitals affiliated with the University of Colorado School of Medicine Internal Medicine Residency program, including the Denver Veterans' Administration Center (DVAMC), Denver Health Medical Center (DHMC), and University of Colorado Hospital (UCH). The DVAMC is a large urban Veterans Administration hospital, serving veterans from the Denver metro area, and is a tertiary referral center for veterans in rural Colorado, Wyoming, and parts of Montana. DHMC, the safety‐net hospital for the Denver area, serves over 25% of the residents in the city and county of Denver, including such special populations as the indigent, chronically mentally ill, and persons with polysubstance dependence. UCH had 350 licensed beds at the time of our study and serves as the Rocky Mountain region's only academic tertiary, specialty care, and referral center. At the time of this study, there was limited inpatient palliative care services at the DVAMC and UH, and no palliative care services at DHMC. Participants were screened on the first day following admission to the adult general medical service. Participants were recruited on 96 postadmission days between February 2004 and June 2006. Recruitment days varied from Monday through Friday, to include admissions from the weekend and throughout the year to reduce potential bias due to seasonal trends of diseases such as influenza. Patients were excluded if they died or were discharged within the first 24 hours of admission, were pregnant, jailed, or unable to give informed consent. All other patients were approached and invited to participate in a brief survey.
After informed consent was obtained, participants completed a bedside interview that included self‐identified ethnicity and the Berkman‐Syme Social Network Index,[14] a brief questionnaire quantifying social support from spouse or domestic partner, family, friends, and other religious or secular organizations. Baseline socioeconomic measures (eg, income, employment, home ownership, car ownership) and questions related to the last days of life were also included. Participants were asked the following question, If you were very sick, with an illness that could not be cured, and in bed most of the time, where would you spend the last days of your life if you could chose?
For each participant, we performed a detailed chart review to determine demographic data, presence of advance directives, and CARING criteria (Cancer, Admissions 2, Residence in a nursing home, Intensive care unit admit with multiorgan failure, 2 Noncancer hospice Guidelines), a set of prognostic criteria identifying patients at an index hospitalization who have a high burden of illness and are at risk for death in the following year.[15] We then followed patients for 5 years. If participants died within the follow‐up period, we collected the date and location of death using medical records, death certificates, or in a few cases when official death records were unavailable, direct contact with the family. Participants were considered alive if they had a clinic visit or MD/RN phone contact within 3 months prior to the final collection point date.
Analysis
SAS 9.1 (SAS Institute Inc., Cary, NC) was used for all analyses. Simple frequencies and means statistics were used to determine rates of descriptive characteristics of the sample as well as rates of the measured outcomes, preferred place to spend last days of life, and actual site of death. Agreement or concordance between preferred and actual site of death was calculated. For the purposes of the analysis, we assumed all persons who stated they had no preference died in a place concordant with their wishes. To calculate agreement by preferred and actual site, participants who expressed a preference and died (n=111) in hospital, nursing home, home, or hospice setting were included in the analysis, and participants (n=4) who died in an unknown or other locations were excluded (eg, motel room).
Logistic Regression Modeling
2 tests were performed for all categorical variables to determine a significant association with outcome variables. Preferred place of death and concordance between preferred and actual site of death were modeled using predictive variables selected if univariable association demonstrated a P0.25. This standard cutoff was selected to broadly identify candidate variables for logistic regression modeling.[16] A stepwise algorithm was used to select significant predictors that would remain in the model.
In lieu of fitting a multinomial logit model for preferred site of death of home vs hospital vs nursing home or hospice facility as preferred site of death, 3 logit models (although only 2 may be sufficient to estimate the underlying multinomial logit model[17]) were considered with outcome categories: home vs nursing home or hospice facility, and hospital vs nursing home or hospice facility and home vs hospital.
For the logistic regression modeling of concordance, we included the full sample of patients who died during the follow‐up period (n=123). We classified participants as dying in a place concordant with their wishes or not concordant.
RESULTS
Study Population
Subjects were recruited on 96 post‐admission days totaling 842 admissions. Three hundred thirty‐one patients (39%) were ineligible for study participation (n=175 discharged within 24 hours, n=76 unable to consent, n=78 ineligible for other reasons [eg, prisoner, pregnant, under 18 years old], n=2 died within 24 hours of admission). Only 53 of the remaining 511 (10%) patients refused; 458 patients (90%) gave informed consent to participate. Characteristics of the study population are depicted in Table 1. There were very few missing cases (<3%), that is persons without a recent clinic follow‐up date, contact, or a confirmed date of death. These persons were considered alive. Overall, the sample population was ethnically diverse, slightly older than middle age, mostly male (due to the inclusion of the Veterans Administration hospital), and of low socioeconomic status.
| Mean age (SD), y | 57.9 (14.8) |
|---|---|
| |
| Mean time to death (SD), d | 339.5 (348.4) |
| Ethnicity | |
| African American | 19% (88) |
| Caucasian | 52% (239) |
| Latino | 22% (102) |
| Other | 6% (29) |
| Spanish language only | 6% (27) |
| Female gender | 35% (159) |
| Admitted to DVAMC | 41% (188) |
| Admitted to DHMC | 38% (174) |
| Admitted to UCH | 21% (96) |
| CARING criteria | |
| Cancer diagnosis | 11% (51) |
| Admitted to hospital 2 times in the past year for chronic illness | 40% (181) |
| Resident in a nursing home | 2% (9) |
| Noncancer hospice guidelines (meeting 2) | 13% (59) |
| Income <$30,000/year | 84% (377) |
| No greater than high school education | 55% (248) |
| Home owner | 26% (120) |
| Rents home | 39% (177) |
| Unstable living situationa | 34% (156) |
| Low social supportb | 36% (165) |
| Uninsured | 18% (81) |
| Regular primary care provider | 73% (330) |
Preferred Site of Death
When asked where they preferred to spend the last days of their life, 75% of patients (n=343) stated they would like to be at home. In the hospital was the preferred location for 10% of patients, whereas 6% stated a nursing home and 4% a hospice inpatient facility. Two percent stated they had no preference, and 3% refused to answer (Figure 1)

We found that in the univariable analysis the following factors were associated with preference for site of death at a significance level of P<0.25: unstable housing, hospital setting, income level, ethnicity, CARING criteria, presence of an advance directive, education level, married, primary care provider, and presence of public insurance. Results of the logit models (home vs nursing home or hospice facility, and hospital vs nursing home or hospice facility and home vs hospital) are presented in Table 2.
| Adjusted Odds Ratio (95% Confidence Interval) | |||
|---|---|---|---|
| Home vs Nursing Home/Hospice Facility | Hospital vs Nursing Home/Hospice Facility | Home vs Hospital | |
| |||
| Low income | 2.71 (1.305.67) | 3.05 (1.019.24) | 0.99 (0.422.37) |
| Married | 2.44 (1.145.21) | 2.40 (0.876.62) | 0.82 (0.421.57) |
| CARING criteria | 0.58 (0.301.14) | 0.44 (0.181.09) | 0.89 (0.471.66) |
Patients with income <$30,000/year were more likely to prefer home (or hospital) over a nursing home or hospice facility. Being married was predictive of preferring home over nursing home or hospice facility. Patients meeting 1 of the CARING criteria trended toward being less likely (P=0.11 for home and P=0.08 for hospital) to prefer home (or hospital) vs nursing home or hospice facility. However, there were no significant predictors for a preference for home or hospital when directly comparing the 2 locations, as expected from observing similar effects of variables in the other 2 logit models.
Actual Site of Death
One hundred twenty‐three patients died during the follow‐up period (26% of the total sample). Of those who died, the mean age was 64 years (standard deviation 13), 82% had annual incomes <$30,000, 73% were men, and 77% met at least 1 of the CARING criteria suggesting advanced medical illness. The distribution of ethnicities of the deceased subsample was similar to that of the overall cohort. Complete death records were obtained for 121 patients. Only 31% (n=38) died at home, whereas 35% (n=42) died in a hospital, 20% (n=24) died in a nursing home, and 12% (n=14) died in an inpatient hospice facility (Figure 1).
In univariable analysis, there were no associations at a 25% significance level between actual site of death and ethnicity, gender, age, severity of illness, high vs low social support, high or low socioeconomic status, stable vs unstable housing, or presence of a completed advance directive in the medical record.
Concordance Between Preferred and Actual Site of Death
Overall, 37% of the patients died where they stated they would prefer to die, including the 2 with no preference. Concordance rates for each site of death are presented in Table 3. We examined sociodemographic variables, disease severity, advance‐care planning, primary care provider, health insurance, and hospital site to look for associations with concordance. We found that female gender was positively associated with concordance (odds ratio [OR], 3.30; 95% confidence interval [CI], 1.25‐8.72). CARING criteria (P=0.06) and Latino ethnicity (vs all other ethnicity categories, P=0.12) also showed trends for association. Restricting to those who preferred home, the associations became stronger (OR, 4.62; 95% CI, 1.44‐14.79 for female; OR, 7.72; 95% CI, 1.67‐35.71 for CARING criteria), and the trend for the negative association between Latino ethnicity and concordance remained (P=0.12). Results of the model are shown in Table 4.
| Actual Site of Death, n (Row %) | Row Total, % Out of 111 | ||||
|---|---|---|---|---|---|
| Hospital | Nursing Home | Home | Hospice Facility | ||
| Preferred hospital | 5 (42%) | 3 (25%) | 2 (17%) | 2 (17%) | 12 (11%) |
| Preferred nursing home | 1 (13%) | 5 (63%) | 2 (25%) | 0 | 8 (7%) |
| Preferred home | 30 (34%) | 15 (17%) | 31 (35%) | 12 (14%) | 88 (79%) |
| Preferred hospice facility | 3 (100%) | 0 | 0 | 0 | 3 (3%) |
| Adjusted Odds Ratio (95% Confidence Interval) | |||
|---|---|---|---|
| All | Home (Using Same Variables) | Home (Using Only Significant Variables) | |
| |||
| Female gender | 3.30 (1.258.72) | 4.62 (1.4414.79) | 3.57 (1.2410.34) |
| CARING criteria | 3.09 (0.979.81) | 7.72 (1.6735.71) | 5.93 (1.4124.91) |
| Latino vs African American/Caucasian/other | 0.43 (0.151.24) | 0.35 (0.091.30) | |
DISCUSSION
We found, similarly to previous reports in the literature, the majority of patients preferred to die at home. We did not find a significant difference in preferences or location of death by ethnicity or illness severity. Lower‐income patients and married patients were more likely to prefer to be at home over a nursing home or a hospice facility in the last days of life. We found that the minority of patients died at their stated preferred site of death, and female gender was the only predictive variable we found to distinguish those patients who died in a place concordant with their wishes compared to those who did not.
In the literature, previous studies have reported concordance rates between preferred and actual site of death that range from 30% to 90%.[12, 13, 18, 19, 20, 21, 22, 23, 24] We found a concordance rate at the lowest end of this spectrum. In trying to understand our findings and place them in context, it is helpful to examine other studies. Many of these studies focused solely on cancer patients.[13, 18, 19, 20, 21, 22, 23] Cancer follows a more predictable trajectory of decline compared to other common life‐threatening illnesses, such as cardiac disease, emphysema, or liver failure, that often involve periods of acute deteriorations and plateaus throughout illness progression. The more predictable trajectory may explain the overall higher concordance rates found in the studies involving cancer patients.
The majority of studies in the literature examining concordance between preferred and actual site of death recruited the study sample from home health or home palliative care programs that were providing support and care to participants.[10, 12, 13, 18, 22, 25, 26, 27] The high concordance rates reported may be the result of the patients in the sample receiving services at home aimed at eliciting preferences and providing support at home. Our observational study is unique in that we elicited patient preferences from a diverse group of hospitalized adults. Patients had a broad range of medical illness and were at various stages in their disease trajectory. This allowed our findings to be more generalizable, a major strength of our study.
The only variable associated with concordance that we identified to predict concordance between preferred and actual site of death was female gender. Women have been shown to be more active in medical decision making, which may explain our findings.[28] Female gender and illness severity (as measured by the CARING criteria) were found to be associated with concordance when the preference is for death at home. For persons with more advanced medical illness, they may have had more opportunity to consider their preferences and talk about these preferences. It is even possible that our interview prompted some participants to have discussions with their families or providers.
Variables with high face validity, such as high social support, higher education, and completing an advance directive, did not demonstrate any effect on the outcome of concordance. Other studies have shown that low functional status, Caucasian ethnicity, home care, higher education, and social support have been associated with a greater likelihood for a home death.[3, 6, 9] However, although studies specifically examining concordance between preferred and actual site of death have looked at predictors for home death, we were unable to find predictors for concordance across all preferences in the literature. We can conclude from our findings that the factors that influence concordance of preferences for site of death are extremely complex and difficult to capture and measure. This is extremely unsatisfying in the face of the low concordance rate of 30% we identified.
Latino ethnicity showed a trend toward having a negative association with concordance between preferred and actual site of death. This trend persisted whether it was concordance overall or for concordance with those who preferred a death at home. In the literature, Latinos have been found to be less likely to complete advance directives, use hospice services at the end of life, and are more likely to experience a hospital death.[29, 30, 31, 32, 33] As care at the end of life continues to improve, careful attention should be paid to ensure that these kinds of gaps do not widen any further.
We interviewed patients at an index hospitalization. Patients had an acute medical illness or an exacerbation of a chronic medical illness and required at least 24 hours of hospitalization to be eligible for inclusion. Our bedside interview made use of an opportune time to question patients, a time when it may have been easier for patients to visualize severe illness at the end of life, rather than asking this question during a time of wellness. Although participants overwhelmingly stated they preferred to be at home, for those who died, decisions were made in their care that did not allow for this preference.
Our follow‐up after the initial bedside interview only included death records of where and when participants died. We do not have the details and narrative of the conversations that may have taken place that led to the care decisions that determined participants' actual place of death. We do not know if preferences were elicited or discussed, and care decisions then negotiated, to best meet the goals and preferences expressed at that time. We also do not know if the conversations did not occur and the default of medical intervention and cure‐focused care dictated how participants spent the last days of their life. There is evidence that when conversations about goals and preferences do occur, concordance between preferences and care received are high.[12, 21]
We were unable to identify any predictors beyond gender in this cohort of adults hospitalized with a broad spectrum of severe medical illness to predict concordance with stated preferences and actual site of death. We can conclude then, based on our findings and supported by the literature, that the default trends toward institutional end‐of‐life experiences. To shift to a more patient‐centered approach, away from the default, healthcare providers need to embrace a palliative approach and incorporate preferences and goals into the discussions about next steps of care to facilitate the peaceful death that the majority of patients imagine for themselves. Hospitalist physicians have a unique opportunity at an index hospitalization to start the conversation about preferences for care including where patients would want to spend the last days of their life.
Our study does have some limitations. We elicited preferences at a single point in time, at an index hospitalization. It is possible that participants' preferences changed over the course of their illness. However, in Agar et al.'s study of longitudinal patient preferences for site of death and place of care, most preferences remained stable over time.[18] We also did not have data that included palliative care involvement, homecare or hospice utilization, or cause of death. All of these variables may be important predictors of concordance. Issues of symptom management and lack of caregiver may also dictate place of death, even when goals and care are aligned. We do not have data to address these components of end‐of‐life decision making.
CONCLUSION
Patients continue to express a preference for death at home. However, the majority of patients experienced an institutional death. Furthermore, few participants achieved concordance with where they preferred to die and where they actually died. Female gender was the sole factor associated with concordance between preferred and actual site of death. Incorporating a palliative approach that elicits goals and helps match goals to care, may offer the best opportunity to help people die where they chose.
Disclosures: This research was supported by the Brookdale National Fellowship Award and the NIA/Beeson grant 5K23AG028957. All authors have seen and agree with the contents of the article. This submission was not under review by any other publication. The authors have no financial interest or other potential conflicts of interest.
At the turn of the 20th century, most deaths in the United States occurred at home. By the 1960s, over 70% of deaths occurred in an institutional setting, reflecting an evolution of medical technology.[1, 2, 3] With the birth of the hospice movement in the 1970s, dying patients had the opportunity to have both death at home and aggressive symptom control at the end of life. Although there has been a slow decline in the proportion of deaths that occur in the hospital over the past 2 decades,[3] the overwhelming majority of persons state that they would prefer to die at home. However, recent findings suggest that most people will die in an institutional setting.[3, 4, 5, 6]
Although good data exist describing population preferences for location of death, and we know, based on death records, where deaths occur in the United States, there are few studies that examine concordance between preferred and actual site of death at the individual patient level. Furthermore, although factors have been identified that predict death at home, factors predicting concordance between preferred and actual site of death are not well described.[3, 6, 7, 8, 9, 10, 11, 12, 13]
Regardless of where death ultimately occurs, most adults will experience multiple hospitalizations within the last years of their life. Understanding the preferences and subsequent experiences of this population is of particular relevance to hospitalist physicians who are in a unique position to elicit goals from seriously ill patients and help match patient preferences with their medical care. In this observational study, we sought to determine preferences for site of death in a cohort of adult patients admitted to the hospital for medical illness, and then follow those patients to determine where death occurred for those who died. We also sought to explore factors that may predict concordance between preferred and actual site of death. We hypothesized that ethnic diversity and lower socioeconomic status would be associated with a lower likelihood of concordance between preferred and actual site of death. We also hypothesized that advanced care planning would be associated with a higher likelihood of concordance. The Colorado Multi‐Institutional Review Board approved this study.
METHODS
Participants were recruited from 3 hospitals affiliated with the University of Colorado School of Medicine Internal Medicine Residency program, including the Denver Veterans' Administration Center (DVAMC), Denver Health Medical Center (DHMC), and University of Colorado Hospital (UCH). The DVAMC is a large urban Veterans Administration hospital, serving veterans from the Denver metro area, and is a tertiary referral center for veterans in rural Colorado, Wyoming, and parts of Montana. DHMC, the safety‐net hospital for the Denver area, serves over 25% of the residents in the city and county of Denver, including such special populations as the indigent, chronically mentally ill, and persons with polysubstance dependence. UCH had 350 licensed beds at the time of our study and serves as the Rocky Mountain region's only academic tertiary, specialty care, and referral center. At the time of this study, there was limited inpatient palliative care services at the DVAMC and UH, and no palliative care services at DHMC. Participants were screened on the first day following admission to the adult general medical service. Participants were recruited on 96 postadmission days between February 2004 and June 2006. Recruitment days varied from Monday through Friday, to include admissions from the weekend and throughout the year to reduce potential bias due to seasonal trends of diseases such as influenza. Patients were excluded if they died or were discharged within the first 24 hours of admission, were pregnant, jailed, or unable to give informed consent. All other patients were approached and invited to participate in a brief survey.
After informed consent was obtained, participants completed a bedside interview that included self‐identified ethnicity and the Berkman‐Syme Social Network Index,[14] a brief questionnaire quantifying social support from spouse or domestic partner, family, friends, and other religious or secular organizations. Baseline socioeconomic measures (eg, income, employment, home ownership, car ownership) and questions related to the last days of life were also included. Participants were asked the following question, If you were very sick, with an illness that could not be cured, and in bed most of the time, where would you spend the last days of your life if you could chose?
For each participant, we performed a detailed chart review to determine demographic data, presence of advance directives, and CARING criteria (Cancer, Admissions 2, Residence in a nursing home, Intensive care unit admit with multiorgan failure, 2 Noncancer hospice Guidelines), a set of prognostic criteria identifying patients at an index hospitalization who have a high burden of illness and are at risk for death in the following year.[15] We then followed patients for 5 years. If participants died within the follow‐up period, we collected the date and location of death using medical records, death certificates, or in a few cases when official death records were unavailable, direct contact with the family. Participants were considered alive if they had a clinic visit or MD/RN phone contact within 3 months prior to the final collection point date.
Analysis
SAS 9.1 (SAS Institute Inc., Cary, NC) was used for all analyses. Simple frequencies and means statistics were used to determine rates of descriptive characteristics of the sample as well as rates of the measured outcomes, preferred place to spend last days of life, and actual site of death. Agreement or concordance between preferred and actual site of death was calculated. For the purposes of the analysis, we assumed all persons who stated they had no preference died in a place concordant with their wishes. To calculate agreement by preferred and actual site, participants who expressed a preference and died (n=111) in hospital, nursing home, home, or hospice setting were included in the analysis, and participants (n=4) who died in an unknown or other locations were excluded (eg, motel room).
Logistic Regression Modeling
2 tests were performed for all categorical variables to determine a significant association with outcome variables. Preferred place of death and concordance between preferred and actual site of death were modeled using predictive variables selected if univariable association demonstrated a P0.25. This standard cutoff was selected to broadly identify candidate variables for logistic regression modeling.[16] A stepwise algorithm was used to select significant predictors that would remain in the model.
In lieu of fitting a multinomial logit model for preferred site of death of home vs hospital vs nursing home or hospice facility as preferred site of death, 3 logit models (although only 2 may be sufficient to estimate the underlying multinomial logit model[17]) were considered with outcome categories: home vs nursing home or hospice facility, and hospital vs nursing home or hospice facility and home vs hospital.
For the logistic regression modeling of concordance, we included the full sample of patients who died during the follow‐up period (n=123). We classified participants as dying in a place concordant with their wishes or not concordant.
RESULTS
Study Population
Subjects were recruited on 96 post‐admission days totaling 842 admissions. Three hundred thirty‐one patients (39%) were ineligible for study participation (n=175 discharged within 24 hours, n=76 unable to consent, n=78 ineligible for other reasons [eg, prisoner, pregnant, under 18 years old], n=2 died within 24 hours of admission). Only 53 of the remaining 511 (10%) patients refused; 458 patients (90%) gave informed consent to participate. Characteristics of the study population are depicted in Table 1. There were very few missing cases (<3%), that is persons without a recent clinic follow‐up date, contact, or a confirmed date of death. These persons were considered alive. Overall, the sample population was ethnically diverse, slightly older than middle age, mostly male (due to the inclusion of the Veterans Administration hospital), and of low socioeconomic status.
| Mean age (SD), y | 57.9 (14.8) |
|---|---|
| |
| Mean time to death (SD), d | 339.5 (348.4) |
| Ethnicity | |
| African American | 19% (88) |
| Caucasian | 52% (239) |
| Latino | 22% (102) |
| Other | 6% (29) |
| Spanish language only | 6% (27) |
| Female gender | 35% (159) |
| Admitted to DVAMC | 41% (188) |
| Admitted to DHMC | 38% (174) |
| Admitted to UCH | 21% (96) |
| CARING criteria | |
| Cancer diagnosis | 11% (51) |
| Admitted to hospital 2 times in the past year for chronic illness | 40% (181) |
| Resident in a nursing home | 2% (9) |
| Noncancer hospice guidelines (meeting 2) | 13% (59) |
| Income <$30,000/year | 84% (377) |
| No greater than high school education | 55% (248) |
| Home owner | 26% (120) |
| Rents home | 39% (177) |
| Unstable living situationa | 34% (156) |
| Low social supportb | 36% (165) |
| Uninsured | 18% (81) |
| Regular primary care provider | 73% (330) |
Preferred Site of Death
When asked where they preferred to spend the last days of their life, 75% of patients (n=343) stated they would like to be at home. In the hospital was the preferred location for 10% of patients, whereas 6% stated a nursing home and 4% a hospice inpatient facility. Two percent stated they had no preference, and 3% refused to answer (Figure 1)

We found that in the univariable analysis the following factors were associated with preference for site of death at a significance level of P<0.25: unstable housing, hospital setting, income level, ethnicity, CARING criteria, presence of an advance directive, education level, married, primary care provider, and presence of public insurance. Results of the logit models (home vs nursing home or hospice facility, and hospital vs nursing home or hospice facility and home vs hospital) are presented in Table 2.
| Adjusted Odds Ratio (95% Confidence Interval) | |||
|---|---|---|---|
| Home vs Nursing Home/Hospice Facility | Hospital vs Nursing Home/Hospice Facility | Home vs Hospital | |
| |||
| Low income | 2.71 (1.305.67) | 3.05 (1.019.24) | 0.99 (0.422.37) |
| Married | 2.44 (1.145.21) | 2.40 (0.876.62) | 0.82 (0.421.57) |
| CARING criteria | 0.58 (0.301.14) | 0.44 (0.181.09) | 0.89 (0.471.66) |
Patients with income <$30,000/year were more likely to prefer home (or hospital) over a nursing home or hospice facility. Being married was predictive of preferring home over nursing home or hospice facility. Patients meeting 1 of the CARING criteria trended toward being less likely (P=0.11 for home and P=0.08 for hospital) to prefer home (or hospital) vs nursing home or hospice facility. However, there were no significant predictors for a preference for home or hospital when directly comparing the 2 locations, as expected from observing similar effects of variables in the other 2 logit models.
Actual Site of Death
One hundred twenty‐three patients died during the follow‐up period (26% of the total sample). Of those who died, the mean age was 64 years (standard deviation 13), 82% had annual incomes <$30,000, 73% were men, and 77% met at least 1 of the CARING criteria suggesting advanced medical illness. The distribution of ethnicities of the deceased subsample was similar to that of the overall cohort. Complete death records were obtained for 121 patients. Only 31% (n=38) died at home, whereas 35% (n=42) died in a hospital, 20% (n=24) died in a nursing home, and 12% (n=14) died in an inpatient hospice facility (Figure 1).
In univariable analysis, there were no associations at a 25% significance level between actual site of death and ethnicity, gender, age, severity of illness, high vs low social support, high or low socioeconomic status, stable vs unstable housing, or presence of a completed advance directive in the medical record.
Concordance Between Preferred and Actual Site of Death
Overall, 37% of the patients died where they stated they would prefer to die, including the 2 with no preference. Concordance rates for each site of death are presented in Table 3. We examined sociodemographic variables, disease severity, advance‐care planning, primary care provider, health insurance, and hospital site to look for associations with concordance. We found that female gender was positively associated with concordance (odds ratio [OR], 3.30; 95% confidence interval [CI], 1.25‐8.72). CARING criteria (P=0.06) and Latino ethnicity (vs all other ethnicity categories, P=0.12) also showed trends for association. Restricting to those who preferred home, the associations became stronger (OR, 4.62; 95% CI, 1.44‐14.79 for female; OR, 7.72; 95% CI, 1.67‐35.71 for CARING criteria), and the trend for the negative association between Latino ethnicity and concordance remained (P=0.12). Results of the model are shown in Table 4.
| Actual Site of Death, n (Row %) | Row Total, % Out of 111 | ||||
|---|---|---|---|---|---|
| Hospital | Nursing Home | Home | Hospice Facility | ||
| Preferred hospital | 5 (42%) | 3 (25%) | 2 (17%) | 2 (17%) | 12 (11%) |
| Preferred nursing home | 1 (13%) | 5 (63%) | 2 (25%) | 0 | 8 (7%) |
| Preferred home | 30 (34%) | 15 (17%) | 31 (35%) | 12 (14%) | 88 (79%) |
| Preferred hospice facility | 3 (100%) | 0 | 0 | 0 | 3 (3%) |
| Adjusted Odds Ratio (95% Confidence Interval) | |||
|---|---|---|---|
| All | Home (Using Same Variables) | Home (Using Only Significant Variables) | |
| |||
| Female gender | 3.30 (1.258.72) | 4.62 (1.4414.79) | 3.57 (1.2410.34) |
| CARING criteria | 3.09 (0.979.81) | 7.72 (1.6735.71) | 5.93 (1.4124.91) |
| Latino vs African American/Caucasian/other | 0.43 (0.151.24) | 0.35 (0.091.30) | |
DISCUSSION
We found, similarly to previous reports in the literature, the majority of patients preferred to die at home. We did not find a significant difference in preferences or location of death by ethnicity or illness severity. Lower‐income patients and married patients were more likely to prefer to be at home over a nursing home or a hospice facility in the last days of life. We found that the minority of patients died at their stated preferred site of death, and female gender was the only predictive variable we found to distinguish those patients who died in a place concordant with their wishes compared to those who did not.
In the literature, previous studies have reported concordance rates between preferred and actual site of death that range from 30% to 90%.[12, 13, 18, 19, 20, 21, 22, 23, 24] We found a concordance rate at the lowest end of this spectrum. In trying to understand our findings and place them in context, it is helpful to examine other studies. Many of these studies focused solely on cancer patients.[13, 18, 19, 20, 21, 22, 23] Cancer follows a more predictable trajectory of decline compared to other common life‐threatening illnesses, such as cardiac disease, emphysema, or liver failure, that often involve periods of acute deteriorations and plateaus throughout illness progression. The more predictable trajectory may explain the overall higher concordance rates found in the studies involving cancer patients.
The majority of studies in the literature examining concordance between preferred and actual site of death recruited the study sample from home health or home palliative care programs that were providing support and care to participants.[10, 12, 13, 18, 22, 25, 26, 27] The high concordance rates reported may be the result of the patients in the sample receiving services at home aimed at eliciting preferences and providing support at home. Our observational study is unique in that we elicited patient preferences from a diverse group of hospitalized adults. Patients had a broad range of medical illness and were at various stages in their disease trajectory. This allowed our findings to be more generalizable, a major strength of our study.
The only variable associated with concordance that we identified to predict concordance between preferred and actual site of death was female gender. Women have been shown to be more active in medical decision making, which may explain our findings.[28] Female gender and illness severity (as measured by the CARING criteria) were found to be associated with concordance when the preference is for death at home. For persons with more advanced medical illness, they may have had more opportunity to consider their preferences and talk about these preferences. It is even possible that our interview prompted some participants to have discussions with their families or providers.
Variables with high face validity, such as high social support, higher education, and completing an advance directive, did not demonstrate any effect on the outcome of concordance. Other studies have shown that low functional status, Caucasian ethnicity, home care, higher education, and social support have been associated with a greater likelihood for a home death.[3, 6, 9] However, although studies specifically examining concordance between preferred and actual site of death have looked at predictors for home death, we were unable to find predictors for concordance across all preferences in the literature. We can conclude from our findings that the factors that influence concordance of preferences for site of death are extremely complex and difficult to capture and measure. This is extremely unsatisfying in the face of the low concordance rate of 30% we identified.
Latino ethnicity showed a trend toward having a negative association with concordance between preferred and actual site of death. This trend persisted whether it was concordance overall or for concordance with those who preferred a death at home. In the literature, Latinos have been found to be less likely to complete advance directives, use hospice services at the end of life, and are more likely to experience a hospital death.[29, 30, 31, 32, 33] As care at the end of life continues to improve, careful attention should be paid to ensure that these kinds of gaps do not widen any further.
We interviewed patients at an index hospitalization. Patients had an acute medical illness or an exacerbation of a chronic medical illness and required at least 24 hours of hospitalization to be eligible for inclusion. Our bedside interview made use of an opportune time to question patients, a time when it may have been easier for patients to visualize severe illness at the end of life, rather than asking this question during a time of wellness. Although participants overwhelmingly stated they preferred to be at home, for those who died, decisions were made in their care that did not allow for this preference.
Our follow‐up after the initial bedside interview only included death records of where and when participants died. We do not have the details and narrative of the conversations that may have taken place that led to the care decisions that determined participants' actual place of death. We do not know if preferences were elicited or discussed, and care decisions then negotiated, to best meet the goals and preferences expressed at that time. We also do not know if the conversations did not occur and the default of medical intervention and cure‐focused care dictated how participants spent the last days of their life. There is evidence that when conversations about goals and preferences do occur, concordance between preferences and care received are high.[12, 21]
We were unable to identify any predictors beyond gender in this cohort of adults hospitalized with a broad spectrum of severe medical illness to predict concordance with stated preferences and actual site of death. We can conclude then, based on our findings and supported by the literature, that the default trends toward institutional end‐of‐life experiences. To shift to a more patient‐centered approach, away from the default, healthcare providers need to embrace a palliative approach and incorporate preferences and goals into the discussions about next steps of care to facilitate the peaceful death that the majority of patients imagine for themselves. Hospitalist physicians have a unique opportunity at an index hospitalization to start the conversation about preferences for care including where patients would want to spend the last days of their life.
Our study does have some limitations. We elicited preferences at a single point in time, at an index hospitalization. It is possible that participants' preferences changed over the course of their illness. However, in Agar et al.'s study of longitudinal patient preferences for site of death and place of care, most preferences remained stable over time.[18] We also did not have data that included palliative care involvement, homecare or hospice utilization, or cause of death. All of these variables may be important predictors of concordance. Issues of symptom management and lack of caregiver may also dictate place of death, even when goals and care are aligned. We do not have data to address these components of end‐of‐life decision making.
CONCLUSION
Patients continue to express a preference for death at home. However, the majority of patients experienced an institutional death. Furthermore, few participants achieved concordance with where they preferred to die and where they actually died. Female gender was the sole factor associated with concordance between preferred and actual site of death. Incorporating a palliative approach that elicits goals and helps match goals to care, may offer the best opportunity to help people die where they chose.
Disclosures: This research was supported by the Brookdale National Fellowship Award and the NIA/Beeson grant 5K23AG028957. All authors have seen and agree with the contents of the article. This submission was not under review by any other publication. The authors have no financial interest or other potential conflicts of interest.
- Field MJ, Cassel CK, eds. Committee on Care at the End of Life. Approaching Death: Improving Care at the End of Life. Washington DC: National Academy Press; 1997.
- , . Demography and epidemiology of dying in the U.S. with emphasis on deaths of older persons. Hosp J. 1998;13:49–60.
- , , , . Factors associated with site of death: a national study of where people die. Med Care. 2003;41:323–335.
- , , , , , . Terminal cancer care and patient's preferences for place of death: a prospective study. BMJ. 1990;301:415–417.
- , , , et al. Influence of patient preferences and local health system characteristics on the place of death. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Risks and Outcomes of Treatment. J Am Geriatr Soc. 1998;46:1242–1250.
- , , , , , . Where people die: a multilevel approach to understanding influences on site of death in America. Med Care Res Rev. 2007;64:351–378.
- , , , , , . Dying at home or in an institution using death certificates to explore the factors associated with place of death. Health Policy. 2006;78:319–329.
- , . How do cancer patients who die at home differ from those who die elsewhere? Palliat Med. 1998;12:279–286.
- , . Factors influencing death at home in terminally ill patients with cancer: systematic review [published correction appears in BMJ. 2006;332:1012]. BMJ 2006;332:515–521.
- , , , , , . Predictive factors for home deaths among cancer patients in Swedish palliative home care. Support Care Cancer. 2003;11:560–567.
- , . Systemic adenosine infusions alleviated neuropathic pain. Pain. 2001;94:121–122.
- , , . Prevalence, effectiveness, and predictors of planning their place of death among older persons followed in community‐based long term care. J Am Geriatr Soc. 2000;48:943–948.
- , , , , . Preferences for place of care and place of death among informal caregivers of the terminally ill. Palliat Med. 2005;19:492–499.
- , . Social networks, host resistance, and mortality: a nine‐year follow‐up study of Alameda County residents. Am J Epidemiol. 1979;109:186–204.
- , , , , , . A practical tool to identify patients who may benefit from a palliative approach: the CARING criteria. J Pain Symptom Manage. 2006;31:285–292.
- , . Applied Logistic Regression. 2nd ed. New York, NY: Wiley‐Interscience; 2000.
- , . Calculation of polychotmous logistic regression parameters using individualized regressions. Biometrika. 1984;71:11–18.
- , , , , , . Preference for place of care and place of death in palliative care: are these different questions? Palliat Med. 2008;22(7):787–795.
- , , . Place of death: preferences among cancer patients and their carers. Soc Sci Med. 2004;58:2431–2444.
- , . Determinants of congruence between the preferred and actual place of death for terminally ill cancer patients. J Palliat Care. 2003;19:230–237.
- , , . Factors associated with location of death (home or hospital) of patients referred to a palliative care team. CMAJ. 1995;152:361–367.
- , , , et al. Proxy perspectives regarding end‐of‐life care for persons with cancer. Cancer. 2008;112:1854–1861.
- , , , , , . Actual and preferred place of death of cancer patients. Results from the Italian survey of the dying of cancer (ISDOC). J Epidemiol Community Health. 2006;60:412–416.
- , , , . Family reports of barriers to optimal care of the dying. Nurs Res. 2000;49:310–317.
- , , . Place of death: preferences among cancer patients and their carers. Soc Sci Med. 2004;58(12):2431–2444.
- , . Where do elderly patients prefer to die? Place of death and patient characteristics of 100 elderly patients under the care of a home health care team. J Am Geriatr Soc. 1983;31:457–461.
- , , , , , . Predictors of home death in palliative care cancer patients. J Palliat Care. 2000;16:23–28.
- , . Patient preferences for medical decision making: who really wants to participate? Med Care. 2000;38:335–341.
- , , , et al. Racial and ethnic differences in advance care planning among patients with cancer: impact of terminal illness acknowledgment, religiousness, and treatment preferences. J Clin Oncol. 2008;26:4131–4137.
- , , , . Influence of ethnicity on advance directives and end‐of‐life decisions. JAMA. 1997;277:298–299.
- , , , . Differences in end‐of‐life decision making among black and white ambulatory cancer patients. J Gen Intern Med. 1996;11:651–656.
- , , . Hospice usage by minorities in the last year of life: results from the National Mortality Feedback Survey. J Am Geriatr Soc. 2003;51:970–978.
- , , , , , . Place of death: correlations with quality of life of patients with cancer and predictors of bereaved caregivers' mental health. J Clin Oncol. 2010;28:4457–4464.
- Field MJ, Cassel CK, eds. Committee on Care at the End of Life. Approaching Death: Improving Care at the End of Life. Washington DC: National Academy Press; 1997.
- , . Demography and epidemiology of dying in the U.S. with emphasis on deaths of older persons. Hosp J. 1998;13:49–60.
- , , , . Factors associated with site of death: a national study of where people die. Med Care. 2003;41:323–335.
- , , , , , . Terminal cancer care and patient's preferences for place of death: a prospective study. BMJ. 1990;301:415–417.
- , , , et al. Influence of patient preferences and local health system characteristics on the place of death. SUPPORT Investigators. Study to Understand Prognoses and Preferences for Risks and Outcomes of Treatment. J Am Geriatr Soc. 1998;46:1242–1250.
- , , , , , . Where people die: a multilevel approach to understanding influences on site of death in America. Med Care Res Rev. 2007;64:351–378.
- , , , , , . Dying at home or in an institution using death certificates to explore the factors associated with place of death. Health Policy. 2006;78:319–329.
- , . How do cancer patients who die at home differ from those who die elsewhere? Palliat Med. 1998;12:279–286.
- , . Factors influencing death at home in terminally ill patients with cancer: systematic review [published correction appears in BMJ. 2006;332:1012]. BMJ 2006;332:515–521.
- , , , , , . Predictive factors for home deaths among cancer patients in Swedish palliative home care. Support Care Cancer. 2003;11:560–567.
- , . Systemic adenosine infusions alleviated neuropathic pain. Pain. 2001;94:121–122.
- , , . Prevalence, effectiveness, and predictors of planning their place of death among older persons followed in community‐based long term care. J Am Geriatr Soc. 2000;48:943–948.
- , , , , . Preferences for place of care and place of death among informal caregivers of the terminally ill. Palliat Med. 2005;19:492–499.
- , . Social networks, host resistance, and mortality: a nine‐year follow‐up study of Alameda County residents. Am J Epidemiol. 1979;109:186–204.
- , , , , , . A practical tool to identify patients who may benefit from a palliative approach: the CARING criteria. J Pain Symptom Manage. 2006;31:285–292.
- , . Applied Logistic Regression. 2nd ed. New York, NY: Wiley‐Interscience; 2000.
- , . Calculation of polychotmous logistic regression parameters using individualized regressions. Biometrika. 1984;71:11–18.
- , , , , , . Preference for place of care and place of death in palliative care: are these different questions? Palliat Med. 2008;22(7):787–795.
- , , . Place of death: preferences among cancer patients and their carers. Soc Sci Med. 2004;58:2431–2444.
- , . Determinants of congruence between the preferred and actual place of death for terminally ill cancer patients. J Palliat Care. 2003;19:230–237.
- , , . Factors associated with location of death (home or hospital) of patients referred to a palliative care team. CMAJ. 1995;152:361–367.
- , , , et al. Proxy perspectives regarding end‐of‐life care for persons with cancer. Cancer. 2008;112:1854–1861.
- , , , , , . Actual and preferred place of death of cancer patients. Results from the Italian survey of the dying of cancer (ISDOC). J Epidemiol Community Health. 2006;60:412–416.
- , , , . Family reports of barriers to optimal care of the dying. Nurs Res. 2000;49:310–317.
- , , . Place of death: preferences among cancer patients and their carers. Soc Sci Med. 2004;58(12):2431–2444.
- , . Where do elderly patients prefer to die? Place of death and patient characteristics of 100 elderly patients under the care of a home health care team. J Am Geriatr Soc. 1983;31:457–461.
- , , , , , . Predictors of home death in palliative care cancer patients. J Palliat Care. 2000;16:23–28.
- , . Patient preferences for medical decision making: who really wants to participate? Med Care. 2000;38:335–341.
- , , , et al. Racial and ethnic differences in advance care planning among patients with cancer: impact of terminal illness acknowledgment, religiousness, and treatment preferences. J Clin Oncol. 2008;26:4131–4137.
- , , , . Influence of ethnicity on advance directives and end‐of‐life decisions. JAMA. 1997;277:298–299.
- , , , . Differences in end‐of‐life decision making among black and white ambulatory cancer patients. J Gen Intern Med. 1996;11:651–656.
- , , . Hospice usage by minorities in the last year of life: results from the National Mortality Feedback Survey. J Am Geriatr Soc. 2003;51:970–978.
- , , , , , . Place of death: correlations with quality of life of patients with cancer and predictors of bereaved caregivers' mental health. J Clin Oncol. 2010;28:4457–4464.
Copyright © 2013 Society of Hospital Medicine
Deterioration Alerts on Medical Wards
Timely interventions are essential in the management of complex medical conditions such as new‐onset sepsis in order to prevent rapid progression to severe sepsis and septic shock.[1, 2, 3, 4, 5] Similarly, rapid identification and appropriate treatment of other medical and surgical conditions have been associated with improved outcomes.[6, 7, 8] We previously developed a real‐time, computerized prediction tool (PT) using recursive partitioning regression tree analysis for the identification of impending sepsis for use on general hospital wards.[9] We also showed that implementation of a real‐time computerized sepsis alert on hospital wards based on the PT resulted in increased use of early interventions, including antibiotic escalation, intravenous fluids, oxygen therapy, and diagnostics in patients identified as at risk.[10]
The first goal of this study was to develop an updated PT for use on hospital wards that could be used to predict subsequent global clinical deterioration and the need for a higher level of care. The second goal was to determine whether simply providing a real‐time alert to nursing staff based on the updated PT resulted in any demonstrable changes in patient outcomes.
METHODS
Study Location
The study was conducted at Barnes‐Jewish Hospital, a 1250‐bed academic medical center in St. Louis, Missouri. Eight adult medicine wards were assessed from July 2007 through December 2011. The medicine wards are closed areas with patient care delivered by dedicated house staff physicians under the supervision of a board‐certified attending physician. The study was approved by the Washington University School of Medicine Human Studies Committee.
Study Period
The period from July 2007 through January 2010 was used to train and retrospectively test the prediction model. The period from January 2011 through December 2011 was used to prospectively validate the model during a randomized trial using alerts generated from the prediction model.
Patients
Electronically captured clinical data were housed in a centralized clinical data repository. This repository cataloged 28,927 hospital visits from 19,116 distinct patients between July 2007 and January 2010. It contained a rich set of demographic and medical data for each of the visits, such as patient age, manually collected vital‐sign data, pharmacy data, laboratory data, and intensive care unit (ICU) transfer. This study served as a proof of concept for our vision of using machine learning to identify at‐risk patients and ultimately to perform real‐time event detection and interventions.
Algorithm Overview
Details regarding the predictive model development have been previously described.[11] To predict ICU transfer for patients housed on general medical wards, we used logistic regression, employing a novel framework to analyze the data stream from each patient, assigning scores to reflect the probability of ICU transfer to each patient.
Before building the model, several preprocessing steps were applied to eliminate outliers and find an appropriate representation of patients' states. For each of 36 input variables we specified acceptable ranges based on the domain knowledge of the medical experts on our team. For any value that was outside of the medically conceivable range, we replaced it by the mean value for that patient, if available. Values for every continuous parameter were scaled so that all measurements lay in the interval [0, 1] and were normalized by the minimum and maximum of the parameter. To capture the temporal effects in our data, we retained a sliding window of all the collected data points within the last 24 hours. We then subdivided these data into a series of 6 sequential buckets of 4 hours each.
To capture variations within a bucket, we computed 3 values for each feature in the bucket: the minimum, maximum, and mean data points. Each of the resulting 3n values was input to the logistic regression equation as separate variables. To deal with missing data points within the buckets, we used the patients' most recent reading from any time earlier in the hospital stay, if available. If no prior values existed, we used mean values calculated over the entire historical dataset. Bucket 6 max/min/mean represents the most recent 4‐hour window from the preceding 24‐hour time period for the maximum, minimum, and mean values, respectively. By itself, logistic regression does not operate on time‐series data. That is, each variable input to the logistic equation corresponds to exactly 1 data point (eg, a blood‐pressure variable would consist of a single blood‐pressure reading). In a clinical application, however, it is important to capture unusual changes in vital‐sign data over time. Such changes may precede clinical deterioration by hours, providing a chance to intervene if detected early enough. In addition, not all readings in time‐series data should be treated equally; the value of some kinds of data may change depending on their age. For example, a patient's condition may be better reflected by a blood‐oxygenation reading collected 1 hour ago than a reading collected 12 hours ago. This is the rationale for our use of a sliding window of all collected data points within the last 24 hours performed in a real‐time basis.
The algorithm was first implemented in MATLAB (Natick, MA). For the purposes of training, we used a single 24‐hour window of data from each patient. For patients admitted to ICU, this window was 26 hours to 2 hours prior to ICU admission; for all other patients, this window consisted of the first 24 hours of their hospital stay. The dataset's 36 input variables were divided into buckets and min/mean/max features wherever applicable, resulting in 398 variables. The first half of the dataset was used to train the model. We then used the second half of the dataset as the validation dataset. We generated a predicted outcome for each case in the validation data, using the model parameter coefficients derived from the training data. We also employed bootstrap aggregation to improve classification accuracy and to address overfitting. We then applied various threshold cut‐points to convert these predictions into binary values and compared the results against the ICU transfer outcome. A threshold of 0.9760 for specificity was chosen to achieve a sensitivity of approximately 40%. These operating characteristics were chosen in turn to generate a manageable number of alerts per hospital nursing unit per day (estimated at 12 per nursing unit per day). At this cut‐point the C‐statistic was 0.8834, with an overall accuracy of 0.9292.
In order to train the logistic model, we used a single 24‐hour window of data for each patient. However, in a system that predicts patients' outcomes in real time, scores are recomputed each time new data are entered into the database. Hence, patients have a series of scores over the length of their hospital stay, and an alert is triggered when any one of these scores is above the chosen threshold.
Once the model was developed, we implemented it in an internally developed, Java‐based clinical decision support rules engine, which identified when new data relevant to the model were available in a real‐time central data repository. The rules engine queried the data repository to acquire all data needed to evaluate the model. The score was calculated with each relevant new data point, and an alert was generated when the score exceeded the cut‐point threshold. We then prospectively validated these alerts on patients on 8 general medical wards at Barnes Jewish Hospital. Details regarding the architecture of our clinical decision support system have been previously published.[12] The sensitivity and positive predictive values for ICU transfer for these alerts were tracked during an intervention trial that ran from January 24, 2011, through December 31, 2011. Four general medical wards were randomized to the intervention group and 4 wards were randomized to the control group. The 8 general medical wards were ordered according to their alert rates based upon the historical data from July 2007 through January 2010, creating 4 pairs of wards in ascending order of alert rate. Within each of the 4 pairs, 1 member of the pair was randomized to the intervention group and the other to the control group using a random number generator.
Real‐time automated alerts generated 24 hours per day, 7 days per week from the predictive algorithm were sent to the charge‐nurse pager on the intervention units. Alerts were also generated and stored in the database on the control units, but these alerts were not sent to the charge nurse on those units. The alerts were sent to the charge nurses on the respective wards, as these individuals were thought to be in the best position to perform the initial assessment of the alerted patients, especially during evening hours when physician staffing was reduced. The charge nurses assessed the intervention‐group patients and were instructed to contact the responsible physician (hospitalist or internal medicine house officer) to inform them of the alert, or to call the rapid response team (RRT) if the patient's condition already appeared to be significantly deteriorating.
Descriptive statistics for algorithm sensitivity and positive predictive value and for patient outcomes were performed. Associations between alerts and the primary outcome, ICU transfer, were determined, as well as the impact of alerts in the intervention group compared with the control group, using [2] tests. The same analyses were performed for patient death. Differences in length of stay (LOS) were assessed using the Wilcoxon rank sum test.
RESULTS
Predictive Model
The variables with the greatest coefficients contributing to the PT model included respiratory rate, oxygen saturation, shock index, systolic blood pressure, anticoagulation use, heart rate, and diastolic blood pressure. A complete list of variables is provided in the Appendix (see Supporting Information in the online version of this article). All but 1 are routinely collected vital‐sign measures, and all but 1 occur in the 4‐hour period immediately prior to the alert (bucket 6).
Prospective Trial
Patient characteristics are presented in Table 1. Patients were well matched for race, sex, age, and underlying diagnoses. All alerts reported to the charge nurses were to be associated with a call from the charge nurse to the responsible physician caring for the alerted patient. The mean number of alerts per alerted patient was 1.8 (standard deviation=1.7). Patients meeting the alert threshold were at nearly 5.3‐fold greater risk of ICU transfer (95% confidence interval [CI]: 4.6‐6.0) than those not satisfying the alert threshold (358 of 2353 [15.2%; 95% CI: 13.8%‐16.7%] vs 512 of 17678 [2.9%; 95% CI: 2.7%‐3.2%], respectively; P<0.0001). Patients with alerts were at 8.9‐fold greater risk of death (95% CI: 7.4‐10.7) than those without alerts (244 of 2353 [10.4%; 95% CI: 9.2%‐11.7%] vs 206 of 17678 [1.2%; 95% CI: 1.0%‐1.3%], respectively; P<0.0001). Operating characteristics of the PT from the prospective trial are shown in Table 2. Alerts occurred a median of 25.5 hours prior to ICU transfer (interquartile range, 7.00‐81.75) and 8 hours prior to death (interquartile range, 4.09‐15.66).
| Study Group | ||||||
|---|---|---|---|---|---|---|
| Control (N=10,120) | Intervention (N=9911) | |||||
| ||||||
| Race | N | % | N | % | ||
| White | 5,062 | 50 | 4,934 | 50 | ||
| Black | 4,864 | 48 | 4,790 | 48 | ||
| Other | 194 | 2 | 187 | 2 | ||
| Sex | ||||||
| F | 5,355 | 53 | 5,308 | 54 | ||
| M | 4,765 | 47 | 4,603 | 46 | ||
| Age at discharge, median (IQR), y | 57 (4469) | 57 (4470) | ||||
| Top 10 ICD‐9 descriptions and counts, n (%) | ||||||
| 1 | Diseases of the digestive system | 1,774 (17.5) | Diseases of the digestive system | 1,664 (16.7) | ||
| 2 | Diseases of the circulatory system | 1,252 (12.4) | Diseases of the circulatory system | 1,253 (12.6) | ||
| 3 | Diseases of the respiratory system | 1,236 (12.2) | Diseases of the respiratory system | 1,210 (12.2) | ||
| 4 | Injury and poisoning | 864 (8.5) | Injury and poisoning | 849 (8.6) | ||
| 5 | Endocrine, nutritional, and metabolic diseases, and immunity disorders | 797 (7.9) | Diseases of the genitourinary system | 795 (8.0) | ||
| 6 | Diseases of the genitourinary system | 762 (7.5) | Endocrine, nutritional, and metabolic diseases, and immunity disorders | 780 (7.9) | ||
| 7 | Infectious and parasitic diseases | 555 (5.5) | Infectious and parasitic diseases | 549 (5.5) | ||
| 8 | Neoplasms | 547 (5.4) | Neoplasms | 465 (4.7) | ||
| 9 | Diseases of the blood and blood‐forming organs | 426 (4.2) | Diseases of the blood and blood‐forming organs | 429 (4.3) | ||
| 10 | Symptoms, signs, and ill‐defined conditions and factors influencing health status | 410 (4.1) | Diseases of the musculoskeletal system and connective tissue | 399 (4.0) | ||
| Sensitivity, % | Specificity, % | PPV, % | NPV, % | Positive Likelihood Ratio | Negative Likelihood Ratio | |||
|---|---|---|---|---|---|---|---|---|
| ||||||||
| ICU Transfer | Yes (N=870) | No (N=19,161) | ||||||
| Alert | 358 | 1,995 | 41.1 (95% CI: 37.944.5) | 89.6 (95% CI: 89.290.0) | 15.2 (95% CI: 13.816.7) | 97.1 (95% CI: 96.897.3) | 3.95 (95% CI: 3.614.30) | 0.66 (95% CI: 0.620.70) |
| No Alert | 512 | 17,166 | ||||||
| Death | Yes (N=450) | No (N=19,581) | ||||||
| Alert | 244 | 2109 | 54.2 (95% CI: 49.658.8) | 89.2 (95% CI: 88.889.7) | 10.4 (95% CI: 9.211.7) | 98.8 (95% CI: 98.799.0) | 5.03 (95% CI: 4.585.53) | 0.51 (95% CI: 0.460.57) |
| No Alert | 206 | 17,472 | ||||||
Among patients identified by the PT, there were no differences in the proportion of patients who were transferred to the ICU or who died in the intervention group as compared with the control group (Table 3). In addition, although there was no difference in LOS in the intervention group compared with the control group, identification by the PT was associated with a significantly longer median LOS (7.01 days vs 2.94 days, P<0.001). The largest numbers of patients who were transferred to the ICU or died did so in the first hospital day, and 60% of patients who were transferred to the ICU did so in the first 4 days, whereas deaths were more evenly distributed across the hospital stay.
| Outcomes by Alert Statusa | ||||||||
|---|---|---|---|---|---|---|---|---|
| Alert Study Group | No‐Alert Study Group | |||||||
| Intervention, N=1194 | Control, N=1159 | Intervention, N=8717 | Control, N=8961 | |||||
| N | % | N | % | N | % | N | % | |
| ||||||||
| ICU Transfer | ||||||||
| Yes | 192 | 16 | 166 | 14 | 252 | 3 | 260 | 3 |
| No | 1002 | 84 | 993 | 86 | 8465 | 97 | 8701 | 97 |
| Death | ||||||||
| Yes | 127 | 11 | 117 | 10 | 96 | 1 | 110 | 1 |
| No | 1067 | 89 | 1042 | 90 | 8621 | 99 | 8851 | 99 |
| LOS from admit to discharge, median (IQR), da | 7.07 (3.9912.15) | 6.92 (3.8212.67) | 2.97 (1.775.33) | 2.91 (1.745.19) | ||||
DISCUSSION
We have demonstrated that a relatively simple hospital‐specific method for generating a PT derived from routine laboratory and hemodynamic values is capable of predicting clinical deterioration and the need for ICU transfer, as well as hospital mortality, in non‐ICU patients admitted to general hospital wards. We also found that the PT identified a sicker patient population as manifest by longer hospital LOS. The methods used in generating this real‐time PT are relatively simple and easily executed with the use of an electronic medical record (EMR) system. However, our data also showed that simply providing an alert to nursing units based on the PT did not result in any demonstrable improvement in patient outcomes. Moreover, our PT and intervention in their current form have substantial limitations, including low sensitivity and positive predictive value, high possibility of alert fatigue, and no clear clinical impact. These limitations suggest that this approach has limited applicability in its current form.
Unplanned ICU transfers occurring as early as within 8 hours of hospitalization are relatively common and associated with increased mortality.[13] Bapoje et al evaluated a total of 152 patients over 1 year who had unplanned ICU transfers.[14] The most common reason was worsening of the problem for which the patient was admitted (48%). Other investigators have also attempted to identify predictors for clinical deterioration resulting in unplanned ICU transfer that could be employed in a PT or early warning system (EWS). Keller et al evaluated 50 consecutive general medical patients with unplanned ICU transfers between 2003 and 2004.[15] Using a case‐control methodology, these investigators found shock index values>0.85 to be the best predictor for subsequent unplanned ICU transfer (P<0.02; odds ratio: 3.0).
Organizations such as the Institute for Healthcare Improvement have called for the development and implementation of EWSs in order to direct the activities of RRTs and improve outcomes.[16] Escobar et al carried out a retrospective case‐control study using as the unit of analysis 12‐hour patient shifts on hospital wards.[17] Using logistic regression and split validation, they developed a PT for ICU transfer from clinical variables available in their EMR. The EMR derived PT had a C‐statistic of 0.845 in the derivation dataset and 0.775 in the validation dataset, concluding that EMR‐based detection of impending deterioration outside the ICU is feasible in integrated healthcare delivery systems.
We found that simply providing an alert to nursing units did not result in any demonstrable improvements in the outcomes of high‐risk patients identified by our PT. This may have been due to simply relying on the alerted nursing staff to make phone calls to physicians and not linking a specific and effective patient‐directed intervention to the PT. Other investigators have similarly observed that the use of an EWS or PT may not result in outcome improvements.[18] Gao et al performed an analysis of 31 studies describing hospital track and trigger EWSs.[19] They found little evidence of reliability, validity, and utility of these systems. Peebles et al showed that even when high‐risk non‐ICU patients are identified, delays in providing appropriate therapies occur, which may explain the lack of efficacy of EWSs and RRTs.[20] These observations suggest that there is currently a paucity of validated interventions available to improve outcome in deteriorating patients, despite our ability to identify patients who are at risk for such deterioration.
As a result of mandates from quality‐improvement organizations, most US hospitals currently employ RRTs for emergent mobilization of resources when a clinically deteriorating patient is identified on a hospital ward.[21] However, as noted above, there is limited evidence to suggest that RRTs contribute to improved patient outcomes.[22, 23, 24, 25, 26, 27] The potential importance of this is reflected in a recent report suggesting that 2900 US hospitals now have rapid‐response systems in place without clear demonstration of their overall efficacy.[28] Linking rapid‐response interventions with a validated real‐time alert may represent a way of improving the effectiveness of such interventions.[29, 30, 31, 32, 33, 34] Our data showed that hospital LOS was statistically longer among alerted patients compared with nonalerted patients. This supports the conclusion that the alerts helped identify a sicker group of patients, but the nursing alerts did not appear to change outcomes. This finding also seems to refute the hypothesis that simply linking an intervention to a PT will improve outcomes, albeit the intervention we employed may not have been robust enough to influence patient outcomes.
The development of accurate real‐time EWSs holds the potential to identify patients at risk for clinical deterioration at an earlier point in time when rescue interventions can be implemented in a potentially more effective manner in both adults and children.[35] Unfortunately, the ideal intervention to be applied in this situation is unknown. Our experience suggests that successful interventions will require a more integrated approach than simply providing an alert with general management principles. As a result of our experience, we are undertaking a randomized clinical trial in 2013 to determine whether linking a patient‐specific intervention to a PT will result in improved outcomes. The intervention we will be testing is to have the RRT immediately notified about alerted patients so as to formally evaluate them and to determine the need for therapeutic interventions, and to administer such interventions as needed and/or transfer the alerted patients to a higher level of care as deemed necessary. Additionally, we are updating our PT with more temporal data to determine if this will improve its accuracy. One of these updates will include linking the PT to wirelessly obtained continuous oximetry and heart‐rate data, using minimally intrusive sensors, to establish a 2‐tiered EWS.[11]
Our study has several important limitations. First, the PT was developed using local data, and thus the results may not be applicable to other settings. However, our model shares many of the characteristics identified in other clinical‐deterioration PTs.[15, 17] Second, the positive prediction value of 15.2% for ICU transfer may not be clinically useful due to the large number of false‐positive results. Moreover, the large number of false positives could result in alert fatigue, causing alerts to be ignored. Third, although the charge nurses were supposed to call the responsible physicians for the alerted patients, we did not determine whether all these calls occurred or whether they resulted in any meaningful changes in monitoring or patient treatment. This is important because lack of an effective intervention or treatment would make the intervention group much more like our control group. Future studies are needed to assess the impact of an integrated intervention (eg, notification of experienced RRT members with adequate resource access) to determine if patient outcomes can be impacted by the use of an EWS. Finally, we did not compare the performance of our PT to other models such as the modified early warning score (MEWS).
An additional limitation to consider is that our PT offered no new information to the nurse manager, or the PT did not change the opinions of the charge nurses. This is supported by a recent study of 63 serious adverse outcomes in a Belgian teaching hospital where death was the final outcome.[36] Survey results revealed that nurses were often unaware that their patients were deteriorating before the crisis. Nurses also reported threshold levels for concern for abnormal vital signs that suggested they would call for assistance relatively late in clinical crises. The limited ability of nursing staff to identify deteriorating patients is also supported by a recent simulation study demonstrating that nurses did identify that patients were deteriorating, but as each patient deteriorated staff performance declined, with a reduction in all observational records and actions.[37]
In summary, we have demonstrated that a relatively simple hospital‐specific PT could accurately identify patients on general medicine wards who subsequently developed clinical deterioration and the need for ICU transfer, as well as hospital mortality. However, no improvements in patient outcomes were found from reporting this information to nursing wards on a real‐time basis. The low positive predictive value of the alerts, local development of the EWS, and absence of improved outcomes substantially limits the broader application of this system in its current form. Continued efforts are needed to identify and implement systems that will not only accurately identify high‐risk patients on general wards but also intervene to improve their outcomes.
Acknowledgments
Disclosures: This study was funded in part by the Barnes‐Jewish Hospital Foundation and by Grant No. UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
- , , , et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299:2294–2303.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
- , , , et al. Before‐after study of a standardized hospital order set for the management of septic shock. Crit Care Med. 2007;34:2707–2713.
- , , . Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327.
- , , , et al. Septic shock: an analysis of outcomes for patients with onset on hospital wards versus intensive care units. Crit Care Med. 1998;26:1020–1024.
- , , , , . Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity. J Gen Intern Med. 2003;18:77–83.
- , , , , , . Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med. 2011;155:226–233.
- , , , . Comprehensive stroke centers overcome the weekend versus weekday gap in stroke treatment and mortality. Stroke. 2011;42:2403–2409.
- , , , , , . Hospital‐wide impact of a standardized order set for the management of bacteremic severe sepsis. Crit Care Med. 2009;37:819–824.
- , , , et al. Implementation of a real‐time computerized sepsis alert in non–intensive care unit patients. Crit Care Med. 2011;39:469–473.
- , , , et al. Toward a two‐tier clinical warning system for hospitalized patients. AMIA Annu Symp Proc. 2011;2011:511–519.
- , , , , , . Migrating toward a next‐generation clinical decision support application: the BJC HealthCare experience. AMIA Annu Symp Proc. 2007;344–348.
- , , , . Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med. 2011;7:224–230.
- , , , . Unplanned transfers to a medical intensive care unit: causes and relationship to preventable errors in care. J Hosp Med. 2011;6:68–72.
- , , , , , . Unplanned transfers to the intensive care unit: the role of the shock index. J Hosp Med. 2010;5:460–465.
- Institute for Healthcare Improvement. Early warning systems: the next level of rapid response. Available at: http://www.ihi.org/IHI/Programs/AudioAndWebPrograms/ExpeditionEarlyWarningSystemsTheNextLevelofRapidResponse.htmplayerwmp. Accessed April 6, 2011.
- , , , , , . Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7:388–395.
- , , . Early warning systems: the next level of rapid response. Nursing. 2012;42:38–44.
- , , , et al. Systematic review and evaluation of physiological track and trigger warning systems for identifying at‐risk patients on the ward. Intensive Care Med. 2007;33:667–679.
- , , , . Timing and teamwork—an observational pilot study of patients referred to a Rapid Response Team with the aim of identifying factors amenable to re‐design of a Rapid Response System. Resuscitation. 2012;83:782–787.
- , , , . Rapid response: a quality improvement conundrum. J Hosp Med. 2009;4:255–257.
- , , , et al. Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30:1398–1404.
- . Out of our reach? Assessing the impact of introducing critical care outreach service. Anaesthesiology. 2003;58:882–885.
- , , . Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ. 2003;327:1014–1016.
- , , . Reducing mortality and avoiding preventable ICU utilization: analysis of a successful rapid response program using APR DRGs [published online ahead of print March 10, 2010]. J Healthc Qual. doi: 10.1111/j.1945‐1474.2010.00084.x.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised control trial. Lancet. 2005;365:2091–2097.
- , , , , , . The impact of the introduction of critical care outreach services in England: a multicentre interrupted time‐series analysis. Crit Care. 2007;11:R113.
- . Rapid response systems now established at 2,900 hospitals. Hospitalist News. March 2010;3:1.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170:18–26.
- , , , et al. Outreach and early warning systems (EWS) for the prevention of intensive care admission and death of critically ill adult patients on general hospital wards. Cochrane Database Syst Rev. 2007;3:CD005529.
- , , . Rapid‐response teams. N Engl J Med. 2011;365:139–146.
- , , . Early warning systems. Hosp Chron. 2012;7(suppl 1):37–43.
- , , , et al. Grand challenges in clinical decision support. J Biomed Inform. 2008;41(2):387–392.
- , , , et al. Utility of commonly captured data from an EHR to identify hospitalized patients at risk for clinical deterioration. AMIA Annu Symp Proc. 2007;404–408.
- , , , , , . Sensitivity of the pediatric early warning score to identify patient deterioration. Pediatrics. 2010;125(4)e763–e769.
- , , , . In‐hospital mortality after serious adverse events on medical and surgical nursing units: a mixed methods study [published online ahead of print July 24, 2012]. J Clin Nurs. doi: 10.1111/j.1365‐2702.2012.04154.x.
- , , , et al. Managing deteriorating patients: registered nurses' performance in a simulated setting. Open Nurs J. 2011;5:120–126.
Timely interventions are essential in the management of complex medical conditions such as new‐onset sepsis in order to prevent rapid progression to severe sepsis and septic shock.[1, 2, 3, 4, 5] Similarly, rapid identification and appropriate treatment of other medical and surgical conditions have been associated with improved outcomes.[6, 7, 8] We previously developed a real‐time, computerized prediction tool (PT) using recursive partitioning regression tree analysis for the identification of impending sepsis for use on general hospital wards.[9] We also showed that implementation of a real‐time computerized sepsis alert on hospital wards based on the PT resulted in increased use of early interventions, including antibiotic escalation, intravenous fluids, oxygen therapy, and diagnostics in patients identified as at risk.[10]
The first goal of this study was to develop an updated PT for use on hospital wards that could be used to predict subsequent global clinical deterioration and the need for a higher level of care. The second goal was to determine whether simply providing a real‐time alert to nursing staff based on the updated PT resulted in any demonstrable changes in patient outcomes.
METHODS
Study Location
The study was conducted at Barnes‐Jewish Hospital, a 1250‐bed academic medical center in St. Louis, Missouri. Eight adult medicine wards were assessed from July 2007 through December 2011. The medicine wards are closed areas with patient care delivered by dedicated house staff physicians under the supervision of a board‐certified attending physician. The study was approved by the Washington University School of Medicine Human Studies Committee.
Study Period
The period from July 2007 through January 2010 was used to train and retrospectively test the prediction model. The period from January 2011 through December 2011 was used to prospectively validate the model during a randomized trial using alerts generated from the prediction model.
Patients
Electronically captured clinical data were housed in a centralized clinical data repository. This repository cataloged 28,927 hospital visits from 19,116 distinct patients between July 2007 and January 2010. It contained a rich set of demographic and medical data for each of the visits, such as patient age, manually collected vital‐sign data, pharmacy data, laboratory data, and intensive care unit (ICU) transfer. This study served as a proof of concept for our vision of using machine learning to identify at‐risk patients and ultimately to perform real‐time event detection and interventions.
Algorithm Overview
Details regarding the predictive model development have been previously described.[11] To predict ICU transfer for patients housed on general medical wards, we used logistic regression, employing a novel framework to analyze the data stream from each patient, assigning scores to reflect the probability of ICU transfer to each patient.
Before building the model, several preprocessing steps were applied to eliminate outliers and find an appropriate representation of patients' states. For each of 36 input variables we specified acceptable ranges based on the domain knowledge of the medical experts on our team. For any value that was outside of the medically conceivable range, we replaced it by the mean value for that patient, if available. Values for every continuous parameter were scaled so that all measurements lay in the interval [0, 1] and were normalized by the minimum and maximum of the parameter. To capture the temporal effects in our data, we retained a sliding window of all the collected data points within the last 24 hours. We then subdivided these data into a series of 6 sequential buckets of 4 hours each.
To capture variations within a bucket, we computed 3 values for each feature in the bucket: the minimum, maximum, and mean data points. Each of the resulting 3n values was input to the logistic regression equation as separate variables. To deal with missing data points within the buckets, we used the patients' most recent reading from any time earlier in the hospital stay, if available. If no prior values existed, we used mean values calculated over the entire historical dataset. Bucket 6 max/min/mean represents the most recent 4‐hour window from the preceding 24‐hour time period for the maximum, minimum, and mean values, respectively. By itself, logistic regression does not operate on time‐series data. That is, each variable input to the logistic equation corresponds to exactly 1 data point (eg, a blood‐pressure variable would consist of a single blood‐pressure reading). In a clinical application, however, it is important to capture unusual changes in vital‐sign data over time. Such changes may precede clinical deterioration by hours, providing a chance to intervene if detected early enough. In addition, not all readings in time‐series data should be treated equally; the value of some kinds of data may change depending on their age. For example, a patient's condition may be better reflected by a blood‐oxygenation reading collected 1 hour ago than a reading collected 12 hours ago. This is the rationale for our use of a sliding window of all collected data points within the last 24 hours performed in a real‐time basis.
The algorithm was first implemented in MATLAB (Natick, MA). For the purposes of training, we used a single 24‐hour window of data from each patient. For patients admitted to ICU, this window was 26 hours to 2 hours prior to ICU admission; for all other patients, this window consisted of the first 24 hours of their hospital stay. The dataset's 36 input variables were divided into buckets and min/mean/max features wherever applicable, resulting in 398 variables. The first half of the dataset was used to train the model. We then used the second half of the dataset as the validation dataset. We generated a predicted outcome for each case in the validation data, using the model parameter coefficients derived from the training data. We also employed bootstrap aggregation to improve classification accuracy and to address overfitting. We then applied various threshold cut‐points to convert these predictions into binary values and compared the results against the ICU transfer outcome. A threshold of 0.9760 for specificity was chosen to achieve a sensitivity of approximately 40%. These operating characteristics were chosen in turn to generate a manageable number of alerts per hospital nursing unit per day (estimated at 12 per nursing unit per day). At this cut‐point the C‐statistic was 0.8834, with an overall accuracy of 0.9292.
In order to train the logistic model, we used a single 24‐hour window of data for each patient. However, in a system that predicts patients' outcomes in real time, scores are recomputed each time new data are entered into the database. Hence, patients have a series of scores over the length of their hospital stay, and an alert is triggered when any one of these scores is above the chosen threshold.
Once the model was developed, we implemented it in an internally developed, Java‐based clinical decision support rules engine, which identified when new data relevant to the model were available in a real‐time central data repository. The rules engine queried the data repository to acquire all data needed to evaluate the model. The score was calculated with each relevant new data point, and an alert was generated when the score exceeded the cut‐point threshold. We then prospectively validated these alerts on patients on 8 general medical wards at Barnes Jewish Hospital. Details regarding the architecture of our clinical decision support system have been previously published.[12] The sensitivity and positive predictive values for ICU transfer for these alerts were tracked during an intervention trial that ran from January 24, 2011, through December 31, 2011. Four general medical wards were randomized to the intervention group and 4 wards were randomized to the control group. The 8 general medical wards were ordered according to their alert rates based upon the historical data from July 2007 through January 2010, creating 4 pairs of wards in ascending order of alert rate. Within each of the 4 pairs, 1 member of the pair was randomized to the intervention group and the other to the control group using a random number generator.
Real‐time automated alerts generated 24 hours per day, 7 days per week from the predictive algorithm were sent to the charge‐nurse pager on the intervention units. Alerts were also generated and stored in the database on the control units, but these alerts were not sent to the charge nurse on those units. The alerts were sent to the charge nurses on the respective wards, as these individuals were thought to be in the best position to perform the initial assessment of the alerted patients, especially during evening hours when physician staffing was reduced. The charge nurses assessed the intervention‐group patients and were instructed to contact the responsible physician (hospitalist or internal medicine house officer) to inform them of the alert, or to call the rapid response team (RRT) if the patient's condition already appeared to be significantly deteriorating.
Descriptive statistics for algorithm sensitivity and positive predictive value and for patient outcomes were performed. Associations between alerts and the primary outcome, ICU transfer, were determined, as well as the impact of alerts in the intervention group compared with the control group, using [2] tests. The same analyses were performed for patient death. Differences in length of stay (LOS) were assessed using the Wilcoxon rank sum test.
RESULTS
Predictive Model
The variables with the greatest coefficients contributing to the PT model included respiratory rate, oxygen saturation, shock index, systolic blood pressure, anticoagulation use, heart rate, and diastolic blood pressure. A complete list of variables is provided in the Appendix (see Supporting Information in the online version of this article). All but 1 are routinely collected vital‐sign measures, and all but 1 occur in the 4‐hour period immediately prior to the alert (bucket 6).
Prospective Trial
Patient characteristics are presented in Table 1. Patients were well matched for race, sex, age, and underlying diagnoses. All alerts reported to the charge nurses were to be associated with a call from the charge nurse to the responsible physician caring for the alerted patient. The mean number of alerts per alerted patient was 1.8 (standard deviation=1.7). Patients meeting the alert threshold were at nearly 5.3‐fold greater risk of ICU transfer (95% confidence interval [CI]: 4.6‐6.0) than those not satisfying the alert threshold (358 of 2353 [15.2%; 95% CI: 13.8%‐16.7%] vs 512 of 17678 [2.9%; 95% CI: 2.7%‐3.2%], respectively; P<0.0001). Patients with alerts were at 8.9‐fold greater risk of death (95% CI: 7.4‐10.7) than those without alerts (244 of 2353 [10.4%; 95% CI: 9.2%‐11.7%] vs 206 of 17678 [1.2%; 95% CI: 1.0%‐1.3%], respectively; P<0.0001). Operating characteristics of the PT from the prospective trial are shown in Table 2. Alerts occurred a median of 25.5 hours prior to ICU transfer (interquartile range, 7.00‐81.75) and 8 hours prior to death (interquartile range, 4.09‐15.66).
| Study Group | ||||||
|---|---|---|---|---|---|---|
| Control (N=10,120) | Intervention (N=9911) | |||||
| ||||||
| Race | N | % | N | % | ||
| White | 5,062 | 50 | 4,934 | 50 | ||
| Black | 4,864 | 48 | 4,790 | 48 | ||
| Other | 194 | 2 | 187 | 2 | ||
| Sex | ||||||
| F | 5,355 | 53 | 5,308 | 54 | ||
| M | 4,765 | 47 | 4,603 | 46 | ||
| Age at discharge, median (IQR), y | 57 (4469) | 57 (4470) | ||||
| Top 10 ICD‐9 descriptions and counts, n (%) | ||||||
| 1 | Diseases of the digestive system | 1,774 (17.5) | Diseases of the digestive system | 1,664 (16.7) | ||
| 2 | Diseases of the circulatory system | 1,252 (12.4) | Diseases of the circulatory system | 1,253 (12.6) | ||
| 3 | Diseases of the respiratory system | 1,236 (12.2) | Diseases of the respiratory system | 1,210 (12.2) | ||
| 4 | Injury and poisoning | 864 (8.5) | Injury and poisoning | 849 (8.6) | ||
| 5 | Endocrine, nutritional, and metabolic diseases, and immunity disorders | 797 (7.9) | Diseases of the genitourinary system | 795 (8.0) | ||
| 6 | Diseases of the genitourinary system | 762 (7.5) | Endocrine, nutritional, and metabolic diseases, and immunity disorders | 780 (7.9) | ||
| 7 | Infectious and parasitic diseases | 555 (5.5) | Infectious and parasitic diseases | 549 (5.5) | ||
| 8 | Neoplasms | 547 (5.4) | Neoplasms | 465 (4.7) | ||
| 9 | Diseases of the blood and blood‐forming organs | 426 (4.2) | Diseases of the blood and blood‐forming organs | 429 (4.3) | ||
| 10 | Symptoms, signs, and ill‐defined conditions and factors influencing health status | 410 (4.1) | Diseases of the musculoskeletal system and connective tissue | 399 (4.0) | ||
| Sensitivity, % | Specificity, % | PPV, % | NPV, % | Positive Likelihood Ratio | Negative Likelihood Ratio | |||
|---|---|---|---|---|---|---|---|---|
| ||||||||
| ICU Transfer | Yes (N=870) | No (N=19,161) | ||||||
| Alert | 358 | 1,995 | 41.1 (95% CI: 37.944.5) | 89.6 (95% CI: 89.290.0) | 15.2 (95% CI: 13.816.7) | 97.1 (95% CI: 96.897.3) | 3.95 (95% CI: 3.614.30) | 0.66 (95% CI: 0.620.70) |
| No Alert | 512 | 17,166 | ||||||
| Death | Yes (N=450) | No (N=19,581) | ||||||
| Alert | 244 | 2109 | 54.2 (95% CI: 49.658.8) | 89.2 (95% CI: 88.889.7) | 10.4 (95% CI: 9.211.7) | 98.8 (95% CI: 98.799.0) | 5.03 (95% CI: 4.585.53) | 0.51 (95% CI: 0.460.57) |
| No Alert | 206 | 17,472 | ||||||
Among patients identified by the PT, there were no differences in the proportion of patients who were transferred to the ICU or who died in the intervention group as compared with the control group (Table 3). In addition, although there was no difference in LOS in the intervention group compared with the control group, identification by the PT was associated with a significantly longer median LOS (7.01 days vs 2.94 days, P<0.001). The largest numbers of patients who were transferred to the ICU or died did so in the first hospital day, and 60% of patients who were transferred to the ICU did so in the first 4 days, whereas deaths were more evenly distributed across the hospital stay.
| Outcomes by Alert Statusa | ||||||||
|---|---|---|---|---|---|---|---|---|
| Alert Study Group | No‐Alert Study Group | |||||||
| Intervention, N=1194 | Control, N=1159 | Intervention, N=8717 | Control, N=8961 | |||||
| N | % | N | % | N | % | N | % | |
| ||||||||
| ICU Transfer | ||||||||
| Yes | 192 | 16 | 166 | 14 | 252 | 3 | 260 | 3 |
| No | 1002 | 84 | 993 | 86 | 8465 | 97 | 8701 | 97 |
| Death | ||||||||
| Yes | 127 | 11 | 117 | 10 | 96 | 1 | 110 | 1 |
| No | 1067 | 89 | 1042 | 90 | 8621 | 99 | 8851 | 99 |
| LOS from admit to discharge, median (IQR), da | 7.07 (3.9912.15) | 6.92 (3.8212.67) | 2.97 (1.775.33) | 2.91 (1.745.19) | ||||
DISCUSSION
We have demonstrated that a relatively simple hospital‐specific method for generating a PT derived from routine laboratory and hemodynamic values is capable of predicting clinical deterioration and the need for ICU transfer, as well as hospital mortality, in non‐ICU patients admitted to general hospital wards. We also found that the PT identified a sicker patient population as manifest by longer hospital LOS. The methods used in generating this real‐time PT are relatively simple and easily executed with the use of an electronic medical record (EMR) system. However, our data also showed that simply providing an alert to nursing units based on the PT did not result in any demonstrable improvement in patient outcomes. Moreover, our PT and intervention in their current form have substantial limitations, including low sensitivity and positive predictive value, high possibility of alert fatigue, and no clear clinical impact. These limitations suggest that this approach has limited applicability in its current form.
Unplanned ICU transfers occurring as early as within 8 hours of hospitalization are relatively common and associated with increased mortality.[13] Bapoje et al evaluated a total of 152 patients over 1 year who had unplanned ICU transfers.[14] The most common reason was worsening of the problem for which the patient was admitted (48%). Other investigators have also attempted to identify predictors for clinical deterioration resulting in unplanned ICU transfer that could be employed in a PT or early warning system (EWS). Keller et al evaluated 50 consecutive general medical patients with unplanned ICU transfers between 2003 and 2004.[15] Using a case‐control methodology, these investigators found shock index values>0.85 to be the best predictor for subsequent unplanned ICU transfer (P<0.02; odds ratio: 3.0).
Organizations such as the Institute for Healthcare Improvement have called for the development and implementation of EWSs in order to direct the activities of RRTs and improve outcomes.[16] Escobar et al carried out a retrospective case‐control study using as the unit of analysis 12‐hour patient shifts on hospital wards.[17] Using logistic regression and split validation, they developed a PT for ICU transfer from clinical variables available in their EMR. The EMR derived PT had a C‐statistic of 0.845 in the derivation dataset and 0.775 in the validation dataset, concluding that EMR‐based detection of impending deterioration outside the ICU is feasible in integrated healthcare delivery systems.
We found that simply providing an alert to nursing units did not result in any demonstrable improvements in the outcomes of high‐risk patients identified by our PT. This may have been due to simply relying on the alerted nursing staff to make phone calls to physicians and not linking a specific and effective patient‐directed intervention to the PT. Other investigators have similarly observed that the use of an EWS or PT may not result in outcome improvements.[18] Gao et al performed an analysis of 31 studies describing hospital track and trigger EWSs.[19] They found little evidence of reliability, validity, and utility of these systems. Peebles et al showed that even when high‐risk non‐ICU patients are identified, delays in providing appropriate therapies occur, which may explain the lack of efficacy of EWSs and RRTs.[20] These observations suggest that there is currently a paucity of validated interventions available to improve outcome in deteriorating patients, despite our ability to identify patients who are at risk for such deterioration.
As a result of mandates from quality‐improvement organizations, most US hospitals currently employ RRTs for emergent mobilization of resources when a clinically deteriorating patient is identified on a hospital ward.[21] However, as noted above, there is limited evidence to suggest that RRTs contribute to improved patient outcomes.[22, 23, 24, 25, 26, 27] The potential importance of this is reflected in a recent report suggesting that 2900 US hospitals now have rapid‐response systems in place without clear demonstration of their overall efficacy.[28] Linking rapid‐response interventions with a validated real‐time alert may represent a way of improving the effectiveness of such interventions.[29, 30, 31, 32, 33, 34] Our data showed that hospital LOS was statistically longer among alerted patients compared with nonalerted patients. This supports the conclusion that the alerts helped identify a sicker group of patients, but the nursing alerts did not appear to change outcomes. This finding also seems to refute the hypothesis that simply linking an intervention to a PT will improve outcomes, albeit the intervention we employed may not have been robust enough to influence patient outcomes.
The development of accurate real‐time EWSs holds the potential to identify patients at risk for clinical deterioration at an earlier point in time when rescue interventions can be implemented in a potentially more effective manner in both adults and children.[35] Unfortunately, the ideal intervention to be applied in this situation is unknown. Our experience suggests that successful interventions will require a more integrated approach than simply providing an alert with general management principles. As a result of our experience, we are undertaking a randomized clinical trial in 2013 to determine whether linking a patient‐specific intervention to a PT will result in improved outcomes. The intervention we will be testing is to have the RRT immediately notified about alerted patients so as to formally evaluate them and to determine the need for therapeutic interventions, and to administer such interventions as needed and/or transfer the alerted patients to a higher level of care as deemed necessary. Additionally, we are updating our PT with more temporal data to determine if this will improve its accuracy. One of these updates will include linking the PT to wirelessly obtained continuous oximetry and heart‐rate data, using minimally intrusive sensors, to establish a 2‐tiered EWS.[11]
Our study has several important limitations. First, the PT was developed using local data, and thus the results may not be applicable to other settings. However, our model shares many of the characteristics identified in other clinical‐deterioration PTs.[15, 17] Second, the positive prediction value of 15.2% for ICU transfer may not be clinically useful due to the large number of false‐positive results. Moreover, the large number of false positives could result in alert fatigue, causing alerts to be ignored. Third, although the charge nurses were supposed to call the responsible physicians for the alerted patients, we did not determine whether all these calls occurred or whether they resulted in any meaningful changes in monitoring or patient treatment. This is important because lack of an effective intervention or treatment would make the intervention group much more like our control group. Future studies are needed to assess the impact of an integrated intervention (eg, notification of experienced RRT members with adequate resource access) to determine if patient outcomes can be impacted by the use of an EWS. Finally, we did not compare the performance of our PT to other models such as the modified early warning score (MEWS).
An additional limitation to consider is that our PT offered no new information to the nurse manager, or the PT did not change the opinions of the charge nurses. This is supported by a recent study of 63 serious adverse outcomes in a Belgian teaching hospital where death was the final outcome.[36] Survey results revealed that nurses were often unaware that their patients were deteriorating before the crisis. Nurses also reported threshold levels for concern for abnormal vital signs that suggested they would call for assistance relatively late in clinical crises. The limited ability of nursing staff to identify deteriorating patients is also supported by a recent simulation study demonstrating that nurses did identify that patients were deteriorating, but as each patient deteriorated staff performance declined, with a reduction in all observational records and actions.[37]
In summary, we have demonstrated that a relatively simple hospital‐specific PT could accurately identify patients on general medicine wards who subsequently developed clinical deterioration and the need for ICU transfer, as well as hospital mortality. However, no improvements in patient outcomes were found from reporting this information to nursing wards on a real‐time basis. The low positive predictive value of the alerts, local development of the EWS, and absence of improved outcomes substantially limits the broader application of this system in its current form. Continued efforts are needed to identify and implement systems that will not only accurately identify high‐risk patients on general wards but also intervene to improve their outcomes.
Acknowledgments
Disclosures: This study was funded in part by the Barnes‐Jewish Hospital Foundation and by Grant No. UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Timely interventions are essential in the management of complex medical conditions such as new‐onset sepsis in order to prevent rapid progression to severe sepsis and septic shock.[1, 2, 3, 4, 5] Similarly, rapid identification and appropriate treatment of other medical and surgical conditions have been associated with improved outcomes.[6, 7, 8] We previously developed a real‐time, computerized prediction tool (PT) using recursive partitioning regression tree analysis for the identification of impending sepsis for use on general hospital wards.[9] We also showed that implementation of a real‐time computerized sepsis alert on hospital wards based on the PT resulted in increased use of early interventions, including antibiotic escalation, intravenous fluids, oxygen therapy, and diagnostics in patients identified as at risk.[10]
The first goal of this study was to develop an updated PT for use on hospital wards that could be used to predict subsequent global clinical deterioration and the need for a higher level of care. The second goal was to determine whether simply providing a real‐time alert to nursing staff based on the updated PT resulted in any demonstrable changes in patient outcomes.
METHODS
Study Location
The study was conducted at Barnes‐Jewish Hospital, a 1250‐bed academic medical center in St. Louis, Missouri. Eight adult medicine wards were assessed from July 2007 through December 2011. The medicine wards are closed areas with patient care delivered by dedicated house staff physicians under the supervision of a board‐certified attending physician. The study was approved by the Washington University School of Medicine Human Studies Committee.
Study Period
The period from July 2007 through January 2010 was used to train and retrospectively test the prediction model. The period from January 2011 through December 2011 was used to prospectively validate the model during a randomized trial using alerts generated from the prediction model.
Patients
Electronically captured clinical data were housed in a centralized clinical data repository. This repository cataloged 28,927 hospital visits from 19,116 distinct patients between July 2007 and January 2010. It contained a rich set of demographic and medical data for each of the visits, such as patient age, manually collected vital‐sign data, pharmacy data, laboratory data, and intensive care unit (ICU) transfer. This study served as a proof of concept for our vision of using machine learning to identify at‐risk patients and ultimately to perform real‐time event detection and interventions.
Algorithm Overview
Details regarding the predictive model development have been previously described.[11] To predict ICU transfer for patients housed on general medical wards, we used logistic regression, employing a novel framework to analyze the data stream from each patient, assigning scores to reflect the probability of ICU transfer to each patient.
Before building the model, several preprocessing steps were applied to eliminate outliers and find an appropriate representation of patients' states. For each of 36 input variables we specified acceptable ranges based on the domain knowledge of the medical experts on our team. For any value that was outside of the medically conceivable range, we replaced it by the mean value for that patient, if available. Values for every continuous parameter were scaled so that all measurements lay in the interval [0, 1] and were normalized by the minimum and maximum of the parameter. To capture the temporal effects in our data, we retained a sliding window of all the collected data points within the last 24 hours. We then subdivided these data into a series of 6 sequential buckets of 4 hours each.
To capture variations within a bucket, we computed 3 values for each feature in the bucket: the minimum, maximum, and mean data points. Each of the resulting 3n values was input to the logistic regression equation as separate variables. To deal with missing data points within the buckets, we used the patients' most recent reading from any time earlier in the hospital stay, if available. If no prior values existed, we used mean values calculated over the entire historical dataset. Bucket 6 max/min/mean represents the most recent 4‐hour window from the preceding 24‐hour time period for the maximum, minimum, and mean values, respectively. By itself, logistic regression does not operate on time‐series data. That is, each variable input to the logistic equation corresponds to exactly 1 data point (eg, a blood‐pressure variable would consist of a single blood‐pressure reading). In a clinical application, however, it is important to capture unusual changes in vital‐sign data over time. Such changes may precede clinical deterioration by hours, providing a chance to intervene if detected early enough. In addition, not all readings in time‐series data should be treated equally; the value of some kinds of data may change depending on their age. For example, a patient's condition may be better reflected by a blood‐oxygenation reading collected 1 hour ago than a reading collected 12 hours ago. This is the rationale for our use of a sliding window of all collected data points within the last 24 hours performed in a real‐time basis.
The algorithm was first implemented in MATLAB (Natick, MA). For the purposes of training, we used a single 24‐hour window of data from each patient. For patients admitted to ICU, this window was 26 hours to 2 hours prior to ICU admission; for all other patients, this window consisted of the first 24 hours of their hospital stay. The dataset's 36 input variables were divided into buckets and min/mean/max features wherever applicable, resulting in 398 variables. The first half of the dataset was used to train the model. We then used the second half of the dataset as the validation dataset. We generated a predicted outcome for each case in the validation data, using the model parameter coefficients derived from the training data. We also employed bootstrap aggregation to improve classification accuracy and to address overfitting. We then applied various threshold cut‐points to convert these predictions into binary values and compared the results against the ICU transfer outcome. A threshold of 0.9760 for specificity was chosen to achieve a sensitivity of approximately 40%. These operating characteristics were chosen in turn to generate a manageable number of alerts per hospital nursing unit per day (estimated at 12 per nursing unit per day). At this cut‐point the C‐statistic was 0.8834, with an overall accuracy of 0.9292.
In order to train the logistic model, we used a single 24‐hour window of data for each patient. However, in a system that predicts patients' outcomes in real time, scores are recomputed each time new data are entered into the database. Hence, patients have a series of scores over the length of their hospital stay, and an alert is triggered when any one of these scores is above the chosen threshold.
Once the model was developed, we implemented it in an internally developed, Java‐based clinical decision support rules engine, which identified when new data relevant to the model were available in a real‐time central data repository. The rules engine queried the data repository to acquire all data needed to evaluate the model. The score was calculated with each relevant new data point, and an alert was generated when the score exceeded the cut‐point threshold. We then prospectively validated these alerts on patients on 8 general medical wards at Barnes Jewish Hospital. Details regarding the architecture of our clinical decision support system have been previously published.[12] The sensitivity and positive predictive values for ICU transfer for these alerts were tracked during an intervention trial that ran from January 24, 2011, through December 31, 2011. Four general medical wards were randomized to the intervention group and 4 wards were randomized to the control group. The 8 general medical wards were ordered according to their alert rates based upon the historical data from July 2007 through January 2010, creating 4 pairs of wards in ascending order of alert rate. Within each of the 4 pairs, 1 member of the pair was randomized to the intervention group and the other to the control group using a random number generator.
Real‐time automated alerts generated 24 hours per day, 7 days per week from the predictive algorithm were sent to the charge‐nurse pager on the intervention units. Alerts were also generated and stored in the database on the control units, but these alerts were not sent to the charge nurse on those units. The alerts were sent to the charge nurses on the respective wards, as these individuals were thought to be in the best position to perform the initial assessment of the alerted patients, especially during evening hours when physician staffing was reduced. The charge nurses assessed the intervention‐group patients and were instructed to contact the responsible physician (hospitalist or internal medicine house officer) to inform them of the alert, or to call the rapid response team (RRT) if the patient's condition already appeared to be significantly deteriorating.
Descriptive statistics for algorithm sensitivity and positive predictive value and for patient outcomes were performed. Associations between alerts and the primary outcome, ICU transfer, were determined, as well as the impact of alerts in the intervention group compared with the control group, using [2] tests. The same analyses were performed for patient death. Differences in length of stay (LOS) were assessed using the Wilcoxon rank sum test.
RESULTS
Predictive Model
The variables with the greatest coefficients contributing to the PT model included respiratory rate, oxygen saturation, shock index, systolic blood pressure, anticoagulation use, heart rate, and diastolic blood pressure. A complete list of variables is provided in the Appendix (see Supporting Information in the online version of this article). All but 1 are routinely collected vital‐sign measures, and all but 1 occur in the 4‐hour period immediately prior to the alert (bucket 6).
Prospective Trial
Patient characteristics are presented in Table 1. Patients were well matched for race, sex, age, and underlying diagnoses. All alerts reported to the charge nurses were to be associated with a call from the charge nurse to the responsible physician caring for the alerted patient. The mean number of alerts per alerted patient was 1.8 (standard deviation=1.7). Patients meeting the alert threshold were at nearly 5.3‐fold greater risk of ICU transfer (95% confidence interval [CI]: 4.6‐6.0) than those not satisfying the alert threshold (358 of 2353 [15.2%; 95% CI: 13.8%‐16.7%] vs 512 of 17678 [2.9%; 95% CI: 2.7%‐3.2%], respectively; P<0.0001). Patients with alerts were at 8.9‐fold greater risk of death (95% CI: 7.4‐10.7) than those without alerts (244 of 2353 [10.4%; 95% CI: 9.2%‐11.7%] vs 206 of 17678 [1.2%; 95% CI: 1.0%‐1.3%], respectively; P<0.0001). Operating characteristics of the PT from the prospective trial are shown in Table 2. Alerts occurred a median of 25.5 hours prior to ICU transfer (interquartile range, 7.00‐81.75) and 8 hours prior to death (interquartile range, 4.09‐15.66).
| Study Group | ||||||
|---|---|---|---|---|---|---|
| Control (N=10,120) | Intervention (N=9911) | |||||
| ||||||
| Race | N | % | N | % | ||
| White | 5,062 | 50 | 4,934 | 50 | ||
| Black | 4,864 | 48 | 4,790 | 48 | ||
| Other | 194 | 2 | 187 | 2 | ||
| Sex | ||||||
| F | 5,355 | 53 | 5,308 | 54 | ||
| M | 4,765 | 47 | 4,603 | 46 | ||
| Age at discharge, median (IQR), y | 57 (4469) | 57 (4470) | ||||
| Top 10 ICD‐9 descriptions and counts, n (%) | ||||||
| 1 | Diseases of the digestive system | 1,774 (17.5) | Diseases of the digestive system | 1,664 (16.7) | ||
| 2 | Diseases of the circulatory system | 1,252 (12.4) | Diseases of the circulatory system | 1,253 (12.6) | ||
| 3 | Diseases of the respiratory system | 1,236 (12.2) | Diseases of the respiratory system | 1,210 (12.2) | ||
| 4 | Injury and poisoning | 864 (8.5) | Injury and poisoning | 849 (8.6) | ||
| 5 | Endocrine, nutritional, and metabolic diseases, and immunity disorders | 797 (7.9) | Diseases of the genitourinary system | 795 (8.0) | ||
| 6 | Diseases of the genitourinary system | 762 (7.5) | Endocrine, nutritional, and metabolic diseases, and immunity disorders | 780 (7.9) | ||
| 7 | Infectious and parasitic diseases | 555 (5.5) | Infectious and parasitic diseases | 549 (5.5) | ||
| 8 | Neoplasms | 547 (5.4) | Neoplasms | 465 (4.7) | ||
| 9 | Diseases of the blood and blood‐forming organs | 426 (4.2) | Diseases of the blood and blood‐forming organs | 429 (4.3) | ||
| 10 | Symptoms, signs, and ill‐defined conditions and factors influencing health status | 410 (4.1) | Diseases of the musculoskeletal system and connective tissue | 399 (4.0) | ||
| Sensitivity, % | Specificity, % | PPV, % | NPV, % | Positive Likelihood Ratio | Negative Likelihood Ratio | |||
|---|---|---|---|---|---|---|---|---|
| ||||||||
| ICU Transfer | Yes (N=870) | No (N=19,161) | ||||||
| Alert | 358 | 1,995 | 41.1 (95% CI: 37.944.5) | 89.6 (95% CI: 89.290.0) | 15.2 (95% CI: 13.816.7) | 97.1 (95% CI: 96.897.3) | 3.95 (95% CI: 3.614.30) | 0.66 (95% CI: 0.620.70) |
| No Alert | 512 | 17,166 | ||||||
| Death | Yes (N=450) | No (N=19,581) | ||||||
| Alert | 244 | 2109 | 54.2 (95% CI: 49.658.8) | 89.2 (95% CI: 88.889.7) | 10.4 (95% CI: 9.211.7) | 98.8 (95% CI: 98.799.0) | 5.03 (95% CI: 4.585.53) | 0.51 (95% CI: 0.460.57) |
| No Alert | 206 | 17,472 | ||||||
Among patients identified by the PT, there were no differences in the proportion of patients who were transferred to the ICU or who died in the intervention group as compared with the control group (Table 3). In addition, although there was no difference in LOS in the intervention group compared with the control group, identification by the PT was associated with a significantly longer median LOS (7.01 days vs 2.94 days, P<0.001). The largest numbers of patients who were transferred to the ICU or died did so in the first hospital day, and 60% of patients who were transferred to the ICU did so in the first 4 days, whereas deaths were more evenly distributed across the hospital stay.
| Outcomes by Alert Statusa | ||||||||
|---|---|---|---|---|---|---|---|---|
| Alert Study Group | No‐Alert Study Group | |||||||
| Intervention, N=1194 | Control, N=1159 | Intervention, N=8717 | Control, N=8961 | |||||
| N | % | N | % | N | % | N | % | |
| ||||||||
| ICU Transfer | ||||||||
| Yes | 192 | 16 | 166 | 14 | 252 | 3 | 260 | 3 |
| No | 1002 | 84 | 993 | 86 | 8465 | 97 | 8701 | 97 |
| Death | ||||||||
| Yes | 127 | 11 | 117 | 10 | 96 | 1 | 110 | 1 |
| No | 1067 | 89 | 1042 | 90 | 8621 | 99 | 8851 | 99 |
| LOS from admit to discharge, median (IQR), da | 7.07 (3.9912.15) | 6.92 (3.8212.67) | 2.97 (1.775.33) | 2.91 (1.745.19) | ||||
DISCUSSION
We have demonstrated that a relatively simple hospital‐specific method for generating a PT derived from routine laboratory and hemodynamic values is capable of predicting clinical deterioration and the need for ICU transfer, as well as hospital mortality, in non‐ICU patients admitted to general hospital wards. We also found that the PT identified a sicker patient population as manifest by longer hospital LOS. The methods used in generating this real‐time PT are relatively simple and easily executed with the use of an electronic medical record (EMR) system. However, our data also showed that simply providing an alert to nursing units based on the PT did not result in any demonstrable improvement in patient outcomes. Moreover, our PT and intervention in their current form have substantial limitations, including low sensitivity and positive predictive value, high possibility of alert fatigue, and no clear clinical impact. These limitations suggest that this approach has limited applicability in its current form.
Unplanned ICU transfers occurring as early as within 8 hours of hospitalization are relatively common and associated with increased mortality.[13] Bapoje et al evaluated a total of 152 patients over 1 year who had unplanned ICU transfers.[14] The most common reason was worsening of the problem for which the patient was admitted (48%). Other investigators have also attempted to identify predictors for clinical deterioration resulting in unplanned ICU transfer that could be employed in a PT or early warning system (EWS). Keller et al evaluated 50 consecutive general medical patients with unplanned ICU transfers between 2003 and 2004.[15] Using a case‐control methodology, these investigators found shock index values>0.85 to be the best predictor for subsequent unplanned ICU transfer (P<0.02; odds ratio: 3.0).
Organizations such as the Institute for Healthcare Improvement have called for the development and implementation of EWSs in order to direct the activities of RRTs and improve outcomes.[16] Escobar et al carried out a retrospective case‐control study using as the unit of analysis 12‐hour patient shifts on hospital wards.[17] Using logistic regression and split validation, they developed a PT for ICU transfer from clinical variables available in their EMR. The EMR derived PT had a C‐statistic of 0.845 in the derivation dataset and 0.775 in the validation dataset, concluding that EMR‐based detection of impending deterioration outside the ICU is feasible in integrated healthcare delivery systems.
We found that simply providing an alert to nursing units did not result in any demonstrable improvements in the outcomes of high‐risk patients identified by our PT. This may have been due to simply relying on the alerted nursing staff to make phone calls to physicians and not linking a specific and effective patient‐directed intervention to the PT. Other investigators have similarly observed that the use of an EWS or PT may not result in outcome improvements.[18] Gao et al performed an analysis of 31 studies describing hospital track and trigger EWSs.[19] They found little evidence of reliability, validity, and utility of these systems. Peebles et al showed that even when high‐risk non‐ICU patients are identified, delays in providing appropriate therapies occur, which may explain the lack of efficacy of EWSs and RRTs.[20] These observations suggest that there is currently a paucity of validated interventions available to improve outcome in deteriorating patients, despite our ability to identify patients who are at risk for such deterioration.
As a result of mandates from quality‐improvement organizations, most US hospitals currently employ RRTs for emergent mobilization of resources when a clinically deteriorating patient is identified on a hospital ward.[21] However, as noted above, there is limited evidence to suggest that RRTs contribute to improved patient outcomes.[22, 23, 24, 25, 26, 27] The potential importance of this is reflected in a recent report suggesting that 2900 US hospitals now have rapid‐response systems in place without clear demonstration of their overall efficacy.[28] Linking rapid‐response interventions with a validated real‐time alert may represent a way of improving the effectiveness of such interventions.[29, 30, 31, 32, 33, 34] Our data showed that hospital LOS was statistically longer among alerted patients compared with nonalerted patients. This supports the conclusion that the alerts helped identify a sicker group of patients, but the nursing alerts did not appear to change outcomes. This finding also seems to refute the hypothesis that simply linking an intervention to a PT will improve outcomes, albeit the intervention we employed may not have been robust enough to influence patient outcomes.
The development of accurate real‐time EWSs holds the potential to identify patients at risk for clinical deterioration at an earlier point in time when rescue interventions can be implemented in a potentially more effective manner in both adults and children.[35] Unfortunately, the ideal intervention to be applied in this situation is unknown. Our experience suggests that successful interventions will require a more integrated approach than simply providing an alert with general management principles. As a result of our experience, we are undertaking a randomized clinical trial in 2013 to determine whether linking a patient‐specific intervention to a PT will result in improved outcomes. The intervention we will be testing is to have the RRT immediately notified about alerted patients so as to formally evaluate them and to determine the need for therapeutic interventions, and to administer such interventions as needed and/or transfer the alerted patients to a higher level of care as deemed necessary. Additionally, we are updating our PT with more temporal data to determine if this will improve its accuracy. One of these updates will include linking the PT to wirelessly obtained continuous oximetry and heart‐rate data, using minimally intrusive sensors, to establish a 2‐tiered EWS.[11]
Our study has several important limitations. First, the PT was developed using local data, and thus the results may not be applicable to other settings. However, our model shares many of the characteristics identified in other clinical‐deterioration PTs.[15, 17] Second, the positive prediction value of 15.2% for ICU transfer may not be clinically useful due to the large number of false‐positive results. Moreover, the large number of false positives could result in alert fatigue, causing alerts to be ignored. Third, although the charge nurses were supposed to call the responsible physicians for the alerted patients, we did not determine whether all these calls occurred or whether they resulted in any meaningful changes in monitoring or patient treatment. This is important because lack of an effective intervention or treatment would make the intervention group much more like our control group. Future studies are needed to assess the impact of an integrated intervention (eg, notification of experienced RRT members with adequate resource access) to determine if patient outcomes can be impacted by the use of an EWS. Finally, we did not compare the performance of our PT to other models such as the modified early warning score (MEWS).
An additional limitation to consider is that our PT offered no new information to the nurse manager, or the PT did not change the opinions of the charge nurses. This is supported by a recent study of 63 serious adverse outcomes in a Belgian teaching hospital where death was the final outcome.[36] Survey results revealed that nurses were often unaware that their patients were deteriorating before the crisis. Nurses also reported threshold levels for concern for abnormal vital signs that suggested they would call for assistance relatively late in clinical crises. The limited ability of nursing staff to identify deteriorating patients is also supported by a recent simulation study demonstrating that nurses did identify that patients were deteriorating, but as each patient deteriorated staff performance declined, with a reduction in all observational records and actions.[37]
In summary, we have demonstrated that a relatively simple hospital‐specific PT could accurately identify patients on general medicine wards who subsequently developed clinical deterioration and the need for ICU transfer, as well as hospital mortality. However, no improvements in patient outcomes were found from reporting this information to nursing wards on a real‐time basis. The low positive predictive value of the alerts, local development of the EWS, and absence of improved outcomes substantially limits the broader application of this system in its current form. Continued efforts are needed to identify and implement systems that will not only accurately identify high‐risk patients on general wards but also intervene to improve their outcomes.
Acknowledgments
Disclosures: This study was funded in part by the Barnes‐Jewish Hospital Foundation and by Grant No. UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
- , , , et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299:2294–2303.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
- , , , et al. Before‐after study of a standardized hospital order set for the management of septic shock. Crit Care Med. 2007;34:2707–2713.
- , , . Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327.
- , , , et al. Septic shock: an analysis of outcomes for patients with onset on hospital wards versus intensive care units. Crit Care Med. 1998;26:1020–1024.
- , , , , . Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity. J Gen Intern Med. 2003;18:77–83.
- , , , , , . Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med. 2011;155:226–233.
- , , , . Comprehensive stroke centers overcome the weekend versus weekday gap in stroke treatment and mortality. Stroke. 2011;42:2403–2409.
- , , , , , . Hospital‐wide impact of a standardized order set for the management of bacteremic severe sepsis. Crit Care Med. 2009;37:819–824.
- , , , et al. Implementation of a real‐time computerized sepsis alert in non–intensive care unit patients. Crit Care Med. 2011;39:469–473.
- , , , et al. Toward a two‐tier clinical warning system for hospitalized patients. AMIA Annu Symp Proc. 2011;2011:511–519.
- , , , , , . Migrating toward a next‐generation clinical decision support application: the BJC HealthCare experience. AMIA Annu Symp Proc. 2007;344–348.
- , , , . Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med. 2011;7:224–230.
- , , , . Unplanned transfers to a medical intensive care unit: causes and relationship to preventable errors in care. J Hosp Med. 2011;6:68–72.
- , , , , , . Unplanned transfers to the intensive care unit: the role of the shock index. J Hosp Med. 2010;5:460–465.
- Institute for Healthcare Improvement. Early warning systems: the next level of rapid response. Available at: http://www.ihi.org/IHI/Programs/AudioAndWebPrograms/ExpeditionEarlyWarningSystemsTheNextLevelofRapidResponse.htmplayerwmp. Accessed April 6, 2011.
- , , , , , . Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7:388–395.
- , , . Early warning systems: the next level of rapid response. Nursing. 2012;42:38–44.
- , , , et al. Systematic review and evaluation of physiological track and trigger warning systems for identifying at‐risk patients on the ward. Intensive Care Med. 2007;33:667–679.
- , , , . Timing and teamwork—an observational pilot study of patients referred to a Rapid Response Team with the aim of identifying factors amenable to re‐design of a Rapid Response System. Resuscitation. 2012;83:782–787.
- , , , . Rapid response: a quality improvement conundrum. J Hosp Med. 2009;4:255–257.
- , , , et al. Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30:1398–1404.
- . Out of our reach? Assessing the impact of introducing critical care outreach service. Anaesthesiology. 2003;58:882–885.
- , , . Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ. 2003;327:1014–1016.
- , , . Reducing mortality and avoiding preventable ICU utilization: analysis of a successful rapid response program using APR DRGs [published online ahead of print March 10, 2010]. J Healthc Qual. doi: 10.1111/j.1945‐1474.2010.00084.x.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised control trial. Lancet. 2005;365:2091–2097.
- , , , , , . The impact of the introduction of critical care outreach services in England: a multicentre interrupted time‐series analysis. Crit Care. 2007;11:R113.
- . Rapid response systems now established at 2,900 hospitals. Hospitalist News. March 2010;3:1.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170:18–26.
- , , , et al. Outreach and early warning systems (EWS) for the prevention of intensive care admission and death of critically ill adult patients on general hospital wards. Cochrane Database Syst Rev. 2007;3:CD005529.
- , , . Rapid‐response teams. N Engl J Med. 2011;365:139–146.
- , , . Early warning systems. Hosp Chron. 2012;7(suppl 1):37–43.
- , , , et al. Grand challenges in clinical decision support. J Biomed Inform. 2008;41(2):387–392.
- , , , et al. Utility of commonly captured data from an EHR to identify hospitalized patients at risk for clinical deterioration. AMIA Annu Symp Proc. 2007;404–408.
- , , , , , . Sensitivity of the pediatric early warning score to identify patient deterioration. Pediatrics. 2010;125(4)e763–e769.
- , , , . In‐hospital mortality after serious adverse events on medical and surgical nursing units: a mixed methods study [published online ahead of print July 24, 2012]. J Clin Nurs. doi: 10.1111/j.1365‐2702.2012.04154.x.
- , , , et al. Managing deteriorating patients: registered nurses' performance in a simulated setting. Open Nurs J. 2011;5:120–126.
- , , , et al. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299:2294–2303.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
- , , , et al. Before‐after study of a standardized hospital order set for the management of septic shock. Crit Care Med. 2007;34:2707–2713.
- , , . Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327.
- , , , et al. Septic shock: an analysis of outcomes for patients with onset on hospital wards versus intensive care units. Crit Care Med. 1998;26:1020–1024.
- , , , , . Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity. J Gen Intern Med. 2003;18:77–83.
- , , , , , . Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med. 2011;155:226–233.
- , , , . Comprehensive stroke centers overcome the weekend versus weekday gap in stroke treatment and mortality. Stroke. 2011;42:2403–2409.
- , , , , , . Hospital‐wide impact of a standardized order set for the management of bacteremic severe sepsis. Crit Care Med. 2009;37:819–824.
- , , , et al. Implementation of a real‐time computerized sepsis alert in non–intensive care unit patients. Crit Care Med. 2011;39:469–473.
- , , , et al. Toward a two‐tier clinical warning system for hospitalized patients. AMIA Annu Symp Proc. 2011;2011:511–519.
- , , , , , . Migrating toward a next‐generation clinical decision support application: the BJC HealthCare experience. AMIA Annu Symp Proc. 2007;344–348.
- , , , . Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med. 2011;7:224–230.
- , , , . Unplanned transfers to a medical intensive care unit: causes and relationship to preventable errors in care. J Hosp Med. 2011;6:68–72.
- , , , , , . Unplanned transfers to the intensive care unit: the role of the shock index. J Hosp Med. 2010;5:460–465.
- Institute for Healthcare Improvement. Early warning systems: the next level of rapid response. Available at: http://www.ihi.org/IHI/Programs/AudioAndWebPrograms/ExpeditionEarlyWarningSystemsTheNextLevelofRapidResponse.htmplayerwmp. Accessed April 6, 2011.
- , , , , , . Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7:388–395.
- , , . Early warning systems: the next level of rapid response. Nursing. 2012;42:38–44.
- , , , et al. Systematic review and evaluation of physiological track and trigger warning systems for identifying at‐risk patients on the ward. Intensive Care Med. 2007;33:667–679.
- , , , . Timing and teamwork—an observational pilot study of patients referred to a Rapid Response Team with the aim of identifying factors amenable to re‐design of a Rapid Response System. Resuscitation. 2012;83:782–787.
- , , , . Rapid response: a quality improvement conundrum. J Hosp Med. 2009;4:255–257.
- , , , et al. Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004;30:1398–1404.
- . Out of our reach? Assessing the impact of introducing critical care outreach service. Anaesthesiology. 2003;58:882–885.
- , , . Effect of the critical care outreach team on patient survival to discharge from hospital and readmission to critical care: non‐randomised population based study. BMJ. 2003;327:1014–1016.
- , , . Reducing mortality and avoiding preventable ICU utilization: analysis of a successful rapid response program using APR DRGs [published online ahead of print March 10, 2010]. J Healthc Qual. doi: 10.1111/j.1945‐1474.2010.00084.x.
- , , , et al. Introduction of the medical emergency team (MET) system: a cluster‐randomised control trial. Lancet. 2005;365:2091–2097.
- , , , , , . The impact of the introduction of critical care outreach services in England: a multicentre interrupted time‐series analysis. Crit Care. 2007;11:R113.
- . Rapid response systems now established at 2,900 hospitals. Hospitalist News. March 2010;3:1.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170:18–26.
- , , , et al. Outreach and early warning systems (EWS) for the prevention of intensive care admission and death of critically ill adult patients on general hospital wards. Cochrane Database Syst Rev. 2007;3:CD005529.
- , , . Rapid‐response teams. N Engl J Med. 2011;365:139–146.
- , , . Early warning systems. Hosp Chron. 2012;7(suppl 1):37–43.
- , , , et al. Grand challenges in clinical decision support. J Biomed Inform. 2008;41(2):387–392.
- , , , et al. Utility of commonly captured data from an EHR to identify hospitalized patients at risk for clinical deterioration. AMIA Annu Symp Proc. 2007;404–408.
- , , , , , . Sensitivity of the pediatric early warning score to identify patient deterioration. Pediatrics. 2010;125(4)e763–e769.
- , , , . In‐hospital mortality after serious adverse events on medical and surgical nursing units: a mixed methods study [published online ahead of print July 24, 2012]. J Clin Nurs. doi: 10.1111/j.1365‐2702.2012.04154.x.
- , , , et al. Managing deteriorating patients: registered nurses' performance in a simulated setting. Open Nurs J. 2011;5:120–126.
Copyright © 2013 Society of Hospital Medicine
Subspecialties Working Together in Multidisciplinary Cosmetic Centers
Dr. Feldman discusses a multidisciplinary approach to cosmetic medicine and reviews the findings from his survey of physicians from different specialties. For more information, read Dr. Feldman's article in the July 2012 issue, "Academic Physicians' Attitudes Toward Implementation of Multidisciplinary Cosmetic Centers and the Challenges of Subspecialties Working Together."
Dr. Feldman discusses a multidisciplinary approach to cosmetic medicine and reviews the findings from his survey of physicians from different specialties. For more information, read Dr. Feldman's article in the July 2012 issue, "Academic Physicians' Attitudes Toward Implementation of Multidisciplinary Cosmetic Centers and the Challenges of Subspecialties Working Together."
Dr. Feldman discusses a multidisciplinary approach to cosmetic medicine and reviews the findings from his survey of physicians from different specialties. For more information, read Dr. Feldman's article in the July 2012 issue, "Academic Physicians' Attitudes Toward Implementation of Multidisciplinary Cosmetic Centers and the Challenges of Subspecialties Working Together."
'I never know when to call palliative care'
This morbidity and mortality conference was like any other: First, we reviewed the case of a patient with a complication from anticoagulation and then another with an anastomotic leak. Finally, we discussed an elderly patient who had a major emergent procedure to treat complications from an underlying life-limiting condition, only to die in hospital weeks later after developing insurmountable medical, surgical, and infectious complications.
This elderly patient was a man in his mid-70s recently diagnosed with recurrent melanoma. He came to our emergency department with peritonitis and hypotension. His wife of 52 years sat beside him. She was tearful and afraid. "We want everything done," she said. Eight weeks ago, he was working full time and playing golf. But, 6 weeks ago he became confused. A CT scan revealed brain metastasis. He spent 5 of the last 6 weeks in the ICU and in a step-down unit, or a nursing home after a series of complications from his brain biopsy. Now he was back in the hospital with a bowel perforation.
Before our surgical team even saw him, he was told he needed surgery, or he would die. We discussed the surgical risks including the likelihood of a protracted ICU stay, and the high risk he would never go home. Still, he and his wife were unprepared for death and so we went to the operating room.
The following weeks were fraught with complications. His symptoms – including delirium, tumor headaches, and pain – were all difficult to manage on his cocktail of steroids, opiates, and antipsychotics. Occasionally, he would mumble something about dying but we couldn’t determine if he was lucid. His symptoms and "talk about death" were distressing for his family. All in all, our team spent almost an hour each day answering their questions and tending to their anxiety and suffering. It took a high emotional toll on our entire team, as we each worried to ourselves that we were doing more harm than good.
One organ system failed after the other. And finally, after two operations, 10 different consultants, and 3 weeks in the hospital, we stopped talking about organ systems and told the family that this man was dying. That day we consulted palliative care to help us with "goals of care." The next day, he became oliguric, and we shifted our focus to comfort. He died within hours surrounded by his loving extended family.
When we discussed this case at M&M, there were no objections to the decision to operate or how we managed his laundry list of complications. His death was deemed "nonpreventable." But, at then end of the discussion, a colleague asked, with some exasperation, "I never know when to call palliative care. How do you decide when the patient is dying?"
In retrospect, it is clear that this patient was dying when we met him in the emergency department. He was malnourished and disabled from his cancer and treatment. His bowel perforation was caused by the steroids prescribed to treat his underlying terminal disease. The best outcome we could hope for was a good quality of life in his last days, a peaceful and dignified death, and an uncomplicated bereavement for his survivors. Our emergency, life-saving surgery was, in fact, palliative. Death was near, but we just didn’t want him to die this way.
According to the American College of Surgeons code of professional conduct, surgeons play a pivotal role in facilitating the transition from curative to palliative treatment for the patients and the entire health care team. Furthermore, "effective palliation obligates sensitive discussion with patients and their families." These conversations can be particularly onerous for surgeons because we take on tremendous sense of personal responsibility for postoperative outcomes. Once we commit to operating on a patient, their death, especially if it follows complications, can be equated with personal defeat. We may benefit from consulting specialists who can help us set the stage, and smooth the transition for our patients and their families. Surgeons may also personally benefit from the support of other providers to help us cope with these emotionally difficult cases.
Palliative care is a multidisciplinary model of care to address the physical, intellectual, emotional, social, and spiritual needs of patients and families facing serious illness. The goal of palliative care is to support the best possible quality of life for patients at all stages of serious illness, through providing aggressive symptom management, psychosocial and spiritual care, and grief and bereavement counseling before and after death. Palliative care seeks to be life affirming and is based on the understanding of death as a normal life process. It can and should be delivered along with life-prolonging treatment.
In this case, palliative care should have been offered in the emergency department as soon as this patient was admitted to our service. The patient, and his family, would have benefited from a team of physicians, nurses, pharmacists, social workers, and chaplains with the time and expertise to manage distressing symptoms from his cancer, and attend to the grief and suffering that characterized his final weeks. Earlier palliative care may have also steered us away from the slog of high-burden treatments that ultimately offered him little benefit. For the surgeons, palliative care would have provided additional resources to take the best possible care of our patient who, whether or not he made it home, was near the end of his life from an advanced illness.
Dr. Zara Cooper is an ACS Fellow, and assistant professor of surgery, Harvard Medical School, and department of surgery, division of trauma, burns and critical care at Brigham and Women’s Hospital, Boston. Dr. Cooper has no disclosures relevant to this editorial.
This morbidity and mortality conference was like any other: First, we reviewed the case of a patient with a complication from anticoagulation and then another with an anastomotic leak. Finally, we discussed an elderly patient who had a major emergent procedure to treat complications from an underlying life-limiting condition, only to die in hospital weeks later after developing insurmountable medical, surgical, and infectious complications.
This elderly patient was a man in his mid-70s recently diagnosed with recurrent melanoma. He came to our emergency department with peritonitis and hypotension. His wife of 52 years sat beside him. She was tearful and afraid. "We want everything done," she said. Eight weeks ago, he was working full time and playing golf. But, 6 weeks ago he became confused. A CT scan revealed brain metastasis. He spent 5 of the last 6 weeks in the ICU and in a step-down unit, or a nursing home after a series of complications from his brain biopsy. Now he was back in the hospital with a bowel perforation.
Before our surgical team even saw him, he was told he needed surgery, or he would die. We discussed the surgical risks including the likelihood of a protracted ICU stay, and the high risk he would never go home. Still, he and his wife were unprepared for death and so we went to the operating room.
The following weeks were fraught with complications. His symptoms – including delirium, tumor headaches, and pain – were all difficult to manage on his cocktail of steroids, opiates, and antipsychotics. Occasionally, he would mumble something about dying but we couldn’t determine if he was lucid. His symptoms and "talk about death" were distressing for his family. All in all, our team spent almost an hour each day answering their questions and tending to their anxiety and suffering. It took a high emotional toll on our entire team, as we each worried to ourselves that we were doing more harm than good.
One organ system failed after the other. And finally, after two operations, 10 different consultants, and 3 weeks in the hospital, we stopped talking about organ systems and told the family that this man was dying. That day we consulted palliative care to help us with "goals of care." The next day, he became oliguric, and we shifted our focus to comfort. He died within hours surrounded by his loving extended family.
When we discussed this case at M&M, there were no objections to the decision to operate or how we managed his laundry list of complications. His death was deemed "nonpreventable." But, at then end of the discussion, a colleague asked, with some exasperation, "I never know when to call palliative care. How do you decide when the patient is dying?"
In retrospect, it is clear that this patient was dying when we met him in the emergency department. He was malnourished and disabled from his cancer and treatment. His bowel perforation was caused by the steroids prescribed to treat his underlying terminal disease. The best outcome we could hope for was a good quality of life in his last days, a peaceful and dignified death, and an uncomplicated bereavement for his survivors. Our emergency, life-saving surgery was, in fact, palliative. Death was near, but we just didn’t want him to die this way.
According to the American College of Surgeons code of professional conduct, surgeons play a pivotal role in facilitating the transition from curative to palliative treatment for the patients and the entire health care team. Furthermore, "effective palliation obligates sensitive discussion with patients and their families." These conversations can be particularly onerous for surgeons because we take on tremendous sense of personal responsibility for postoperative outcomes. Once we commit to operating on a patient, their death, especially if it follows complications, can be equated with personal defeat. We may benefit from consulting specialists who can help us set the stage, and smooth the transition for our patients and their families. Surgeons may also personally benefit from the support of other providers to help us cope with these emotionally difficult cases.
Palliative care is a multidisciplinary model of care to address the physical, intellectual, emotional, social, and spiritual needs of patients and families facing serious illness. The goal of palliative care is to support the best possible quality of life for patients at all stages of serious illness, through providing aggressive symptom management, psychosocial and spiritual care, and grief and bereavement counseling before and after death. Palliative care seeks to be life affirming and is based on the understanding of death as a normal life process. It can and should be delivered along with life-prolonging treatment.
In this case, palliative care should have been offered in the emergency department as soon as this patient was admitted to our service. The patient, and his family, would have benefited from a team of physicians, nurses, pharmacists, social workers, and chaplains with the time and expertise to manage distressing symptoms from his cancer, and attend to the grief and suffering that characterized his final weeks. Earlier palliative care may have also steered us away from the slog of high-burden treatments that ultimately offered him little benefit. For the surgeons, palliative care would have provided additional resources to take the best possible care of our patient who, whether or not he made it home, was near the end of his life from an advanced illness.
Dr. Zara Cooper is an ACS Fellow, and assistant professor of surgery, Harvard Medical School, and department of surgery, division of trauma, burns and critical care at Brigham and Women’s Hospital, Boston. Dr. Cooper has no disclosures relevant to this editorial.
This morbidity and mortality conference was like any other: First, we reviewed the case of a patient with a complication from anticoagulation and then another with an anastomotic leak. Finally, we discussed an elderly patient who had a major emergent procedure to treat complications from an underlying life-limiting condition, only to die in hospital weeks later after developing insurmountable medical, surgical, and infectious complications.
This elderly patient was a man in his mid-70s recently diagnosed with recurrent melanoma. He came to our emergency department with peritonitis and hypotension. His wife of 52 years sat beside him. She was tearful and afraid. "We want everything done," she said. Eight weeks ago, he was working full time and playing golf. But, 6 weeks ago he became confused. A CT scan revealed brain metastasis. He spent 5 of the last 6 weeks in the ICU and in a step-down unit, or a nursing home after a series of complications from his brain biopsy. Now he was back in the hospital with a bowel perforation.
Before our surgical team even saw him, he was told he needed surgery, or he would die. We discussed the surgical risks including the likelihood of a protracted ICU stay, and the high risk he would never go home. Still, he and his wife were unprepared for death and so we went to the operating room.
The following weeks were fraught with complications. His symptoms – including delirium, tumor headaches, and pain – were all difficult to manage on his cocktail of steroids, opiates, and antipsychotics. Occasionally, he would mumble something about dying but we couldn’t determine if he was lucid. His symptoms and "talk about death" were distressing for his family. All in all, our team spent almost an hour each day answering their questions and tending to their anxiety and suffering. It took a high emotional toll on our entire team, as we each worried to ourselves that we were doing more harm than good.
One organ system failed after the other. And finally, after two operations, 10 different consultants, and 3 weeks in the hospital, we stopped talking about organ systems and told the family that this man was dying. That day we consulted palliative care to help us with "goals of care." The next day, he became oliguric, and we shifted our focus to comfort. He died within hours surrounded by his loving extended family.
When we discussed this case at M&M, there were no objections to the decision to operate or how we managed his laundry list of complications. His death was deemed "nonpreventable." But, at then end of the discussion, a colleague asked, with some exasperation, "I never know when to call palliative care. How do you decide when the patient is dying?"
In retrospect, it is clear that this patient was dying when we met him in the emergency department. He was malnourished and disabled from his cancer and treatment. His bowel perforation was caused by the steroids prescribed to treat his underlying terminal disease. The best outcome we could hope for was a good quality of life in his last days, a peaceful and dignified death, and an uncomplicated bereavement for his survivors. Our emergency, life-saving surgery was, in fact, palliative. Death was near, but we just didn’t want him to die this way.
According to the American College of Surgeons code of professional conduct, surgeons play a pivotal role in facilitating the transition from curative to palliative treatment for the patients and the entire health care team. Furthermore, "effective palliation obligates sensitive discussion with patients and their families." These conversations can be particularly onerous for surgeons because we take on tremendous sense of personal responsibility for postoperative outcomes. Once we commit to operating on a patient, their death, especially if it follows complications, can be equated with personal defeat. We may benefit from consulting specialists who can help us set the stage, and smooth the transition for our patients and their families. Surgeons may also personally benefit from the support of other providers to help us cope with these emotionally difficult cases.
Palliative care is a multidisciplinary model of care to address the physical, intellectual, emotional, social, and spiritual needs of patients and families facing serious illness. The goal of palliative care is to support the best possible quality of life for patients at all stages of serious illness, through providing aggressive symptom management, psychosocial and spiritual care, and grief and bereavement counseling before and after death. Palliative care seeks to be life affirming and is based on the understanding of death as a normal life process. It can and should be delivered along with life-prolonging treatment.
In this case, palliative care should have been offered in the emergency department as soon as this patient was admitted to our service. The patient, and his family, would have benefited from a team of physicians, nurses, pharmacists, social workers, and chaplains with the time and expertise to manage distressing symptoms from his cancer, and attend to the grief and suffering that characterized his final weeks. Earlier palliative care may have also steered us away from the slog of high-burden treatments that ultimately offered him little benefit. For the surgeons, palliative care would have provided additional resources to take the best possible care of our patient who, whether or not he made it home, was near the end of his life from an advanced illness.
Dr. Zara Cooper is an ACS Fellow, and assistant professor of surgery, Harvard Medical School, and department of surgery, division of trauma, burns and critical care at Brigham and Women’s Hospital, Boston. Dr. Cooper has no disclosures relevant to this editorial.
Dabigatran noninferior to warfarin for preventing recurrent VTE
Credit: Andre E.X. Brown
New research suggests dabigatran is noninferior to warfarin as extended prophylaxis for recurrent venous thromboembolism (VTE), and warfarin presents a significantly higher risk of bleeding.
These results are from the RE-MEDY study, which compared the 2 drugs as long-term prophylaxis in patients who had received at least 3 months of VTE treatment.
The data appear in an NEJM article alongside results of the RE-SONATE study, which compared dabigatran and placebo in a similar patient population.
Both of these randomized, double-blind studies were sponsored by the makers of dabigatran, Boehringer Ingelheim.
In the RE-MEDY trial, 2856 patients were randomized in a 1:1 ratio to receive dabigatran or warfarin for up to 36 months. Patients either received active dabigatran at 150 mg twice daily and a warfarin-like placebo or active warfarin and a dabigatran-like placebo. The warfarin dose was adjusted to maintain an INR of 2.0 to 3.0.
In the RE-SONATE trial, 1343 patients were randomized to receive treatment for 6 months. They were assigned in a 1:1 ratio to receive dabigatran at 150 mg twice daily or a matching placebo.
Extended follow-up to evaluate the long-term risk of VTE recurrence took place 12 months after the completion of study treatment.
In RE-MEDY, recurrent VTE occurred in 1.8% of patients in the dabigatran arm and 1.3% of patients in the warfarin arm (P=0.01 for noninferiority).
In RE-SONATE, recurrent VTE occurred in 0.4% of patients in the dabigatran arm and 5.6% of patients in the placebo arm (P<0.001 for superiority).
The rate of clinically relevant or major bleeding was lower with dabigatran than with warfarin—at 5.6% and 10.2%, respectively (P<0.001).
But the rate of clinically relevant or major bleeding was higher with dabigatran than with placebo, at 5.3% and 1.8%, respectively (P=0.001).
“[These results] suggest dabigatran is a good option to prevent deep vein thrombosis and pulmonary embolism from happening again after an initial event,” said lead study author Sam Schulman, MD, PhD, of McMaster University in Hamilton, Ontario, Canada.
“They reinforce the efficacy and favorable safety profile of dabigatran seen in the RE-COVER trials, where dabigatran showed similar efficacy and a significant reduction in clinically relevant bleeding versus warfarin in the treatment of acute venous thromboembolism.” ![]()

Credit: Andre E.X. Brown
New research suggests dabigatran is noninferior to warfarin as extended prophylaxis for recurrent venous thromboembolism (VTE), and warfarin presents a significantly higher risk of bleeding.
These results are from the RE-MEDY study, which compared the 2 drugs as long-term prophylaxis in patients who had received at least 3 months of VTE treatment.
The data appear in an NEJM article alongside results of the RE-SONATE study, which compared dabigatran and placebo in a similar patient population.
Both of these randomized, double-blind studies were sponsored by the makers of dabigatran, Boehringer Ingelheim.
In the RE-MEDY trial, 2856 patients were randomized in a 1:1 ratio to receive dabigatran or warfarin for up to 36 months. Patients either received active dabigatran at 150 mg twice daily and a warfarin-like placebo or active warfarin and a dabigatran-like placebo. The warfarin dose was adjusted to maintain an INR of 2.0 to 3.0.
In the RE-SONATE trial, 1343 patients were randomized to receive treatment for 6 months. They were assigned in a 1:1 ratio to receive dabigatran at 150 mg twice daily or a matching placebo.
Extended follow-up to evaluate the long-term risk of VTE recurrence took place 12 months after the completion of study treatment.
In RE-MEDY, recurrent VTE occurred in 1.8% of patients in the dabigatran arm and 1.3% of patients in the warfarin arm (P=0.01 for noninferiority).
In RE-SONATE, recurrent VTE occurred in 0.4% of patients in the dabigatran arm and 5.6% of patients in the placebo arm (P<0.001 for superiority).
The rate of clinically relevant or major bleeding was lower with dabigatran than with warfarin—at 5.6% and 10.2%, respectively (P<0.001).
But the rate of clinically relevant or major bleeding was higher with dabigatran than with placebo, at 5.3% and 1.8%, respectively (P=0.001).
“[These results] suggest dabigatran is a good option to prevent deep vein thrombosis and pulmonary embolism from happening again after an initial event,” said lead study author Sam Schulman, MD, PhD, of McMaster University in Hamilton, Ontario, Canada.
“They reinforce the efficacy and favorable safety profile of dabigatran seen in the RE-COVER trials, where dabigatran showed similar efficacy and a significant reduction in clinically relevant bleeding versus warfarin in the treatment of acute venous thromboembolism.” ![]()

Credit: Andre E.X. Brown
New research suggests dabigatran is noninferior to warfarin as extended prophylaxis for recurrent venous thromboembolism (VTE), and warfarin presents a significantly higher risk of bleeding.
These results are from the RE-MEDY study, which compared the 2 drugs as long-term prophylaxis in patients who had received at least 3 months of VTE treatment.
The data appear in an NEJM article alongside results of the RE-SONATE study, which compared dabigatran and placebo in a similar patient population.
Both of these randomized, double-blind studies were sponsored by the makers of dabigatran, Boehringer Ingelheim.
In the RE-MEDY trial, 2856 patients were randomized in a 1:1 ratio to receive dabigatran or warfarin for up to 36 months. Patients either received active dabigatran at 150 mg twice daily and a warfarin-like placebo or active warfarin and a dabigatran-like placebo. The warfarin dose was adjusted to maintain an INR of 2.0 to 3.0.
In the RE-SONATE trial, 1343 patients were randomized to receive treatment for 6 months. They were assigned in a 1:1 ratio to receive dabigatran at 150 mg twice daily or a matching placebo.
Extended follow-up to evaluate the long-term risk of VTE recurrence took place 12 months after the completion of study treatment.
In RE-MEDY, recurrent VTE occurred in 1.8% of patients in the dabigatran arm and 1.3% of patients in the warfarin arm (P=0.01 for noninferiority).
In RE-SONATE, recurrent VTE occurred in 0.4% of patients in the dabigatran arm and 5.6% of patients in the placebo arm (P<0.001 for superiority).
The rate of clinically relevant or major bleeding was lower with dabigatran than with warfarin—at 5.6% and 10.2%, respectively (P<0.001).
But the rate of clinically relevant or major bleeding was higher with dabigatran than with placebo, at 5.3% and 1.8%, respectively (P=0.001).
“[These results] suggest dabigatran is a good option to prevent deep vein thrombosis and pulmonary embolism from happening again after an initial event,” said lead study author Sam Schulman, MD, PhD, of McMaster University in Hamilton, Ontario, Canada.
“They reinforce the efficacy and favorable safety profile of dabigatran seen in the RE-COVER trials, where dabigatran showed similar efficacy and a significant reduction in clinically relevant bleeding versus warfarin in the treatment of acute venous thromboembolism.” ![]()
The Society of Hospital Medicine’s "Choosing Wisely" Recommendations for Hospitalists
SHM has joined the American Board of Internal Medicine (ABIM) Foundation’s Choosing Wisely campaign, a multiyear effort to spark national dialogue about waste in the healthcare system and the kinds of common treatments that doctors and patients should think twice about before deciding to pursue. Ad hoc subcommittees of SHM’s Hospital Quality and Patient Safety Committee created lists of five adult and five pediatric treatments that hospitalists and their patients should question (see below). Those lists were shared alongside 15 other medical specialty societies at a Feb. 21 news conference in Washington, D.C.
Adult Hospitalist "Avoid List"
1. Do not place, or leave in place, urinary catheters for incontinence or convenience or monitoring of output for non-critically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days for urologic procedures; use weights instead to monitor diuresis).
2. Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for GI complications.
3. Avoid transfusions of red blood cells for arbitrary hemoglobin or hematocrit thresholds and in the absence of symptoms or active coronary disease, heart failure or stroke.
4. Do not order continuous telemetry monitoring outside of the ICU without using a protocol that governs continuation.
5. Do not perform repetitive CBC and chemistry testing in the face of clinical and lab stability.
Pediatric HospitalIST "Avoid List"
1. Don’t order chest radiographs in children with uncomplicated asthma or bronchiolitis.
2. Don’t routinely use bronchodilators in children with bronchiolitis.
3. Don’t use systemic corticosteroids in children under 2 years of age with an uncomplicated lower respiratory tract infection.
4. Don’t treat gastroesophageal reflux in infants routinely with acid suppression therapy.
5. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen.
For complete recommendations and references, visit SHM's website.
SHM has joined the American Board of Internal Medicine (ABIM) Foundation’s Choosing Wisely campaign, a multiyear effort to spark national dialogue about waste in the healthcare system and the kinds of common treatments that doctors and patients should think twice about before deciding to pursue. Ad hoc subcommittees of SHM’s Hospital Quality and Patient Safety Committee created lists of five adult and five pediatric treatments that hospitalists and their patients should question (see below). Those lists were shared alongside 15 other medical specialty societies at a Feb. 21 news conference in Washington, D.C.
Adult Hospitalist "Avoid List"
1. Do not place, or leave in place, urinary catheters for incontinence or convenience or monitoring of output for non-critically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days for urologic procedures; use weights instead to monitor diuresis).
2. Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for GI complications.
3. Avoid transfusions of red blood cells for arbitrary hemoglobin or hematocrit thresholds and in the absence of symptoms or active coronary disease, heart failure or stroke.
4. Do not order continuous telemetry monitoring outside of the ICU without using a protocol that governs continuation.
5. Do not perform repetitive CBC and chemistry testing in the face of clinical and lab stability.
Pediatric HospitalIST "Avoid List"
1. Don’t order chest radiographs in children with uncomplicated asthma or bronchiolitis.
2. Don’t routinely use bronchodilators in children with bronchiolitis.
3. Don’t use systemic corticosteroids in children under 2 years of age with an uncomplicated lower respiratory tract infection.
4. Don’t treat gastroesophageal reflux in infants routinely with acid suppression therapy.
5. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen.
For complete recommendations and references, visit SHM's website.
SHM has joined the American Board of Internal Medicine (ABIM) Foundation’s Choosing Wisely campaign, a multiyear effort to spark national dialogue about waste in the healthcare system and the kinds of common treatments that doctors and patients should think twice about before deciding to pursue. Ad hoc subcommittees of SHM’s Hospital Quality and Patient Safety Committee created lists of five adult and five pediatric treatments that hospitalists and their patients should question (see below). Those lists were shared alongside 15 other medical specialty societies at a Feb. 21 news conference in Washington, D.C.
Adult Hospitalist "Avoid List"
1. Do not place, or leave in place, urinary catheters for incontinence or convenience or monitoring of output for non-critically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days for urologic procedures; use weights instead to monitor diuresis).
2. Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for GI complications.
3. Avoid transfusions of red blood cells for arbitrary hemoglobin or hematocrit thresholds and in the absence of symptoms or active coronary disease, heart failure or stroke.
4. Do not order continuous telemetry monitoring outside of the ICU without using a protocol that governs continuation.
5. Do not perform repetitive CBC and chemistry testing in the face of clinical and lab stability.
Pediatric HospitalIST "Avoid List"
1. Don’t order chest radiographs in children with uncomplicated asthma or bronchiolitis.
2. Don’t routinely use bronchodilators in children with bronchiolitis.
3. Don’t use systemic corticosteroids in children under 2 years of age with an uncomplicated lower respiratory tract infection.
4. Don’t treat gastroesophageal reflux in infants routinely with acid suppression therapy.
5. Don’t use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen.
For complete recommendations and references, visit SHM's website.