User login
Should we still use electrocardiography to diagnose pericardial disease?
Yes. Acute pericarditis has a unique clinical presentation, physical findings, and electrocardiographic (ECG) changes. ECG is always ordered to look for ischemic changes in patients with chest pain. Acute pericarditis develops in stages, which makes it easy to differentiate from early repolarization and, more significantly, myocardial infarction. The ECG changes, along with the clinical presentation and physical findings, can make the diagnosis of pericarditis.
In atypical and complicated cases, advanced imaging studies (ie, echocardiography and cardiac magnetic resonance imaging) have been used to confirm the diagnosis and to follow the course of the disease. However, ECG remains a useful, cost-effective test.
PERICARDIAL DISEASE IS DIVERSE
The pericardium is a thin layer that covers the heart and separates it from other structures in the mediastinum.
Pericardial syndromes include acute, recurrent, constrictive, and effusive-constrictive pericarditis, as well as pericardial effusion with or without tamponade. Causes include viral or bacterial infection, postpericardiotomy syndrome (Dressler syndrome), postmyocardial infarction, primary and metastatic tumors, trauma, uremia, radiation, and autoimmune disease, but pericardial syndromes can also be idiopathic.1
Acute pericarditis is the most common pericardial syndrome and occurs in all age groups. Once diagnosed, it can easily be treated with antiinflammatory drugs. However, recurrent pericarditis, reported in 30% of patients experiencing a first attack of pericarditis, can be difficult to manage, can have a significant impact on the patient’s health, and can be life-threatening.2
CHANGES OF ACUTE PERICARDITIS DEVELOP IN STAGES
Pericarditis can be diagnosed on the basis of ECG changes, clinical signs and symptoms, and laboratory and imaging findings.3 ECG criteria of acute pericarditis have been published.4,5
The characteristic chest pain in acute pericarditis is usually sudden in onset and sharp and occurs over the anterior chest wall. The pain is exacerbated by inspiration and decreases when the patient sits up and leans forward.4
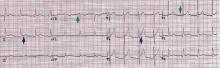
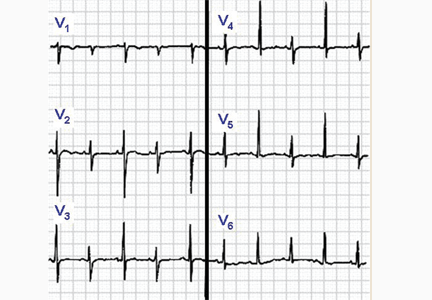
ECG classically shows a widespread saddle-shaped (upward concave) ST-segment elevation in the precordial and limb leads, reflecting subepicardial inflammation. PR-segment depression (with PR-segment elevation in lead aVR) can accompany or precede the ST changes and is known as the “discordant ST-PR segment sign” (Figures 1 and 2). These changes are seen in 60% of patients.
The ECG changes develop in stages, making them easy to differentiate from early repolarization and, more significantly, from myocardial infarction. Four stages are apparent1,4,6–9:
- Stage I occurs in a few hours to days, with diffuse, up-sloping ST-segment elevation and upright T waves, the result of an alteration in ventricular repolarization caused by pericardial inflammation. Because of alteration in repolarization of the atrium secondary to inflammation, the PR segment is elevated in aVR and depressed in the rest of the limb and chest leads.
- Stage II—the ST and PR segments normalize.
- Stage III—widespread T-wave inversion.
- Stage IV—normalization of the T waves.
There is no pathologic Q-wave formation or loss of R-wave progression in acute pericarditis.
The ECG changes of pericarditis vary widely from one patient to another, depending on the extent and severity of pericardial inflammation and the timing of the patient’s presentation. Changes vary in duration. In some cases, ST elevation returns to baseline within a few days without T-wave inversions; in other cases, T-wave inversions can persist for weeks to months. Sometimes the abnormalities resolve by the time symptoms develop.
ASSOCIATED CONDITIONS
Myocardial involvement
In acute myocarditis, findings on ECG can be normal unless the pericardium is involved. Changes that can be seen in myocarditis and that indicate a deeper involvement of inflammation include ST-segment abnormalities, arrhythmias (eg, premature ventricular or atrial contractions), pathologic Q waves, intraventricular conduction delay, and right or left bundle branch block.1,10–12
Elevated troponin and new focal or global left ventricular dysfunction on cardiac imaging indicates myocarditis, especially in a patient with a normal coronary angiogram.10–13
Pericardial effusion: Tachycardia and low QRS voltage
Pericardial effusion is often a complication of pericarditis, but it can also develop from other conditions, such as myxedema, uremia, malignancy, connective tissue disease, aortic dissection, and postpericardiotomy syndrome, and it can also be iatrogenic.
The most common ECG sign of pericardial effusion is tachycardia and low voltage of the QRS complexes. Low voltage is defined as a total amplitude of the QRS complexes in each of the six limb leads less than or equal to 5 mm, and less than or equal to 10 mm in V1 through V6. However, low voltage is not always present in the chest leads.
Mechanisms proposed to explain low QRS voltage associated with pericardial effusion include internal short-circuiting of the electrical currents by accumulated fluids within the pericardial sac, greater distance of the heart from body surface electrodes, reduced cardiac size caused by effusion, and change in the generation and propagation of electrical current in the myocardium.14,15
Cardiac tamponade: Tachycardia, electrical alternans, low QRS voltage
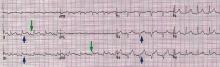
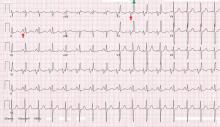
Sinus tachycardia and electrical alternans are specific but not sensitive signs of pericardial tamponade (Figure 3).16,17 Electrical alternans is characterized by beat-to-beat alterations in the axis of QRS complexes in the limb and precordial leads as a result of the mechanical swinging of the heart in a large pericardial effusion.17 There is evidence to suggest that low QRS voltage is more the result of the tamponade than the effusion.18
Treating tamponade with pericardiocentesis, surgical creation of a fistula (“window”) between the pericardial space and the pleural cavity, or anti-inflammatory drugs can resolve low QRS voltage within 1 week.
DIFFERENTIAL DIAGNOSIS OF ACUTE PERICARDITIS
Acute myocardial infarction
ECG changes in acute pericarditis differ from those in acute myocardial infarction in many ways.
ST-segment elevation in pericarditis rarely exceeds 5 mm, in contrast to acute myocardial infarction, in which ST elevation at the J point has to be more than 2 mm and in two anatomically contiguous leads.19
In pericarditis, the changes occur more slowly and in stages, reflecting the evolving inflammation of different areas of the pericardium.
The ST segment is elevated diffusely in the precordial and limb leads in pericarditis, indicating involvement of more than one coronary vascular territory, differentiating it from characteristic regional changes in myocardial infarction.19,20
If concomitant atrial injury is present with acute pericarditis, then PR elevation in aVR with PR depression in other leads may be seen.
Finally, pathologic Q waves or high-grade heart block reflects acute myocardial infarction.
Early repolarization: Elevation of the J point
Early repolarization is sometimes seen in healthy young people, especially in black men.
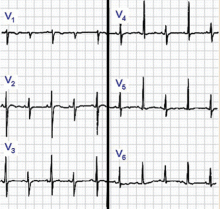
Early repolarization is characterized by elevation of the J point (ie, the junction between the end of the QRS complex and the beginning of the ST segment). Elevation of the J point causes elevation of the ST segment in the mid to lateral precordial leads (V3–V6) with an up-right T wave.21
Acute pericarditis tends to cause ST-segment elevation in both the limb and precordial leads, whereas ST elevation in early repolarization mainly involves the lateral chest leads.
The PR segment is more prominent in acute pericarditis, especially in lead aVR.
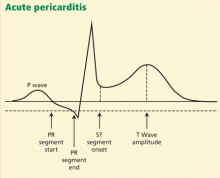
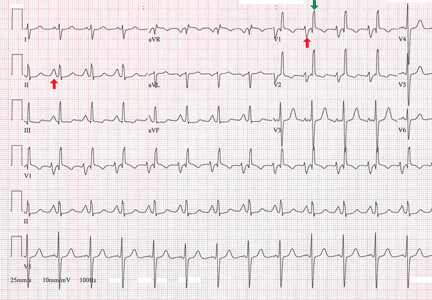
Another finding that strongly favors acute pericarditis is the ratio of the height of the ST-segment junction to the height of the apex of the T wave of more than 0.25 in leads I, V4, V5, and V6 (Figure 4).5,8,22
- Imazio M, Trinchero R. Triage and management of acute pericarditis. Int J Cardiol 2007; 118:286–294.
- Little WC, Freeman GL. Pericardial disease. Circulation 2006; 113:1622–1632.
- Imazio M, Spodick DH, Brucato A, Trinchero R, Markel G, Adler Y. Diagnostic issues in the clinical management of pericarditis. Int J Clin Pract 2010; 64:1384–1392.
- Spodick DH. Acute pericarditis: current concepts and practice. JAMA 2003; 289:1150–1153.
- Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet 2004; 363:717–727.
- Shabetai R. Acute pericarditis. Cardiol Clin 1990; 8:639–644.
- Baljepally R, Spodick DH. PR-segment deviation as the initial electrocardiographic response in acute pericarditis. Am J Cardiol 1998; 81:1505–1506.
- Spodick DH. Diagnostic electrocardiographic sequences in acute pericarditis. Significance of PR segment and PR vector changes. Circulation 1973; 48:575–580.
- Spodick D, editor. The Pericardium: A Comprehensive Textbook. New York, NY: Marcel Dekker; 1997:46–64.
- Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation 1997; 95:163–168.
- Sarda L, Colin P, Boccara F, et al. Myocarditis in patients with clinical presentation of myocardial infarction and normal coronary angiograms. J Am Coll Cardiol 2001; 37:786–792.
- Spodick DH. Arrhythmias during acute pericarditis. A prospective study of 100 consecutive cases. JAMA 1976; 235:39–41.
- Imazio M, Trinchero R. Myopericarditis: etiology, management, and prognosis. Int J Cardiol 2008; 127:17–26.
- Toney JC, Kolmen SN. Cardiac tamponade: fluid and pressure effects on electrocardiographic changes. Proc Soc Exp Biol Med 1966; 121:642–648.
- Karatay CM, Fruehan CT, Lighty GW, Spear RM, Smulyan H. Acute pericardial distension in pigs: effect of fluid conductance on body surface electrocardiogram QRS size. Cardiovasc Res 1993; 27:1033–1038.
- Spodick DH. Acute cardiac tamponade. Pathologic physiology, diagnosis and management. Prog Cardiovasc Dis 1967; 10:64–96.
- Eisenberg MJ, de Romeral LM, Heidenreich PA, Schiller NB, Evans GT. The diagnosis of pericardial effusion and cardiac tamponade by 12-lead ECG. A technology assessment. Chest 1996; 110:318–324.
- Bruch C, Schmermund A, Dagres N, et al. Changes in QRS voltage in cardiac tamponade and pericardial effusion: reversibility after pericardiocentesis and after anti-inflammatory drug treatment. J Am Coll Cardiol 2001; 38:219–226.
- Wang K, Asinger RW, Marriott HJ. ST-segment elevation in conditions other than acute myocardial infarction. N Engl J Med 2003; 349:2128–2135.
- Brady WJ, Perron A, Ullman E. Errors in emergency physician interpretation of ST-segment elevation in emergency department chest pain patients. Acad Emerg Med 2000; 7:1256–1260.
- Kambara H, Phillips J. Long-term evaluation of early repolarization syndrome (normal variant RS-T segment elevation). Am J Cardiol 1976; 38:157–166.
- Ginzton LE, Laks MM. The differential diagnosis of acute pericarditis from the normal variant: new electrocardiographic criteria. Circulation 1982; 65:1004–1009.
Yes. Acute pericarditis has a unique clinical presentation, physical findings, and electrocardiographic (ECG) changes. ECG is always ordered to look for ischemic changes in patients with chest pain. Acute pericarditis develops in stages, which makes it easy to differentiate from early repolarization and, more significantly, myocardial infarction. The ECG changes, along with the clinical presentation and physical findings, can make the diagnosis of pericarditis.
In atypical and complicated cases, advanced imaging studies (ie, echocardiography and cardiac magnetic resonance imaging) have been used to confirm the diagnosis and to follow the course of the disease. However, ECG remains a useful, cost-effective test.
PERICARDIAL DISEASE IS DIVERSE
The pericardium is a thin layer that covers the heart and separates it from other structures in the mediastinum.
Pericardial syndromes include acute, recurrent, constrictive, and effusive-constrictive pericarditis, as well as pericardial effusion with or without tamponade. Causes include viral or bacterial infection, postpericardiotomy syndrome (Dressler syndrome), postmyocardial infarction, primary and metastatic tumors, trauma, uremia, radiation, and autoimmune disease, but pericardial syndromes can also be idiopathic.1
Acute pericarditis is the most common pericardial syndrome and occurs in all age groups. Once diagnosed, it can easily be treated with antiinflammatory drugs. However, recurrent pericarditis, reported in 30% of patients experiencing a first attack of pericarditis, can be difficult to manage, can have a significant impact on the patient’s health, and can be life-threatening.2
CHANGES OF ACUTE PERICARDITIS DEVELOP IN STAGES
Pericarditis can be diagnosed on the basis of ECG changes, clinical signs and symptoms, and laboratory and imaging findings.3 ECG criteria of acute pericarditis have been published.4,5
The characteristic chest pain in acute pericarditis is usually sudden in onset and sharp and occurs over the anterior chest wall. The pain is exacerbated by inspiration and decreases when the patient sits up and leans forward.4
ECG classically shows a widespread saddle-shaped (upward concave) ST-segment elevation in the precordial and limb leads, reflecting subepicardial inflammation. PR-segment depression (with PR-segment elevation in lead aVR) can accompany or precede the ST changes and is known as the “discordant ST-PR segment sign” (Figures 1 and 2). These changes are seen in 60% of patients.
The ECG changes develop in stages, making them easy to differentiate from early repolarization and, more significantly, from myocardial infarction. Four stages are apparent1,4,6–9:
- Stage I occurs in a few hours to days, with diffuse, up-sloping ST-segment elevation and upright T waves, the result of an alteration in ventricular repolarization caused by pericardial inflammation. Because of alteration in repolarization of the atrium secondary to inflammation, the PR segment is elevated in aVR and depressed in the rest of the limb and chest leads.
- Stage II—the ST and PR segments normalize.
- Stage III—widespread T-wave inversion.
- Stage IV—normalization of the T waves.
There is no pathologic Q-wave formation or loss of R-wave progression in acute pericarditis.
The ECG changes of pericarditis vary widely from one patient to another, depending on the extent and severity of pericardial inflammation and the timing of the patient’s presentation. Changes vary in duration. In some cases, ST elevation returns to baseline within a few days without T-wave inversions; in other cases, T-wave inversions can persist for weeks to months. Sometimes the abnormalities resolve by the time symptoms develop.
ASSOCIATED CONDITIONS
Myocardial involvement
In acute myocarditis, findings on ECG can be normal unless the pericardium is involved. Changes that can be seen in myocarditis and that indicate a deeper involvement of inflammation include ST-segment abnormalities, arrhythmias (eg, premature ventricular or atrial contractions), pathologic Q waves, intraventricular conduction delay, and right or left bundle branch block.1,10–12
Elevated troponin and new focal or global left ventricular dysfunction on cardiac imaging indicates myocarditis, especially in a patient with a normal coronary angiogram.10–13
Pericardial effusion: Tachycardia and low QRS voltage
Pericardial effusion is often a complication of pericarditis, but it can also develop from other conditions, such as myxedema, uremia, malignancy, connective tissue disease, aortic dissection, and postpericardiotomy syndrome, and it can also be iatrogenic.
The most common ECG sign of pericardial effusion is tachycardia and low voltage of the QRS complexes. Low voltage is defined as a total amplitude of the QRS complexes in each of the six limb leads less than or equal to 5 mm, and less than or equal to 10 mm in V1 through V6. However, low voltage is not always present in the chest leads.
Mechanisms proposed to explain low QRS voltage associated with pericardial effusion include internal short-circuiting of the electrical currents by accumulated fluids within the pericardial sac, greater distance of the heart from body surface electrodes, reduced cardiac size caused by effusion, and change in the generation and propagation of electrical current in the myocardium.14,15
Cardiac tamponade: Tachycardia, electrical alternans, low QRS voltage
Sinus tachycardia and electrical alternans are specific but not sensitive signs of pericardial tamponade (Figure 3).16,17 Electrical alternans is characterized by beat-to-beat alterations in the axis of QRS complexes in the limb and precordial leads as a result of the mechanical swinging of the heart in a large pericardial effusion.17 There is evidence to suggest that low QRS voltage is more the result of the tamponade than the effusion.18
Treating tamponade with pericardiocentesis, surgical creation of a fistula (“window”) between the pericardial space and the pleural cavity, or anti-inflammatory drugs can resolve low QRS voltage within 1 week.
DIFFERENTIAL DIAGNOSIS OF ACUTE PERICARDITIS
Acute myocardial infarction
ECG changes in acute pericarditis differ from those in acute myocardial infarction in many ways.
ST-segment elevation in pericarditis rarely exceeds 5 mm, in contrast to acute myocardial infarction, in which ST elevation at the J point has to be more than 2 mm and in two anatomically contiguous leads.19
In pericarditis, the changes occur more slowly and in stages, reflecting the evolving inflammation of different areas of the pericardium.
The ST segment is elevated diffusely in the precordial and limb leads in pericarditis, indicating involvement of more than one coronary vascular territory, differentiating it from characteristic regional changes in myocardial infarction.19,20
If concomitant atrial injury is present with acute pericarditis, then PR elevation in aVR with PR depression in other leads may be seen.
Finally, pathologic Q waves or high-grade heart block reflects acute myocardial infarction.
Early repolarization: Elevation of the J point
Early repolarization is sometimes seen in healthy young people, especially in black men.
Early repolarization is characterized by elevation of the J point (ie, the junction between the end of the QRS complex and the beginning of the ST segment). Elevation of the J point causes elevation of the ST segment in the mid to lateral precordial leads (V3–V6) with an up-right T wave.21
Acute pericarditis tends to cause ST-segment elevation in both the limb and precordial leads, whereas ST elevation in early repolarization mainly involves the lateral chest leads.
The PR segment is more prominent in acute pericarditis, especially in lead aVR.
Another finding that strongly favors acute pericarditis is the ratio of the height of the ST-segment junction to the height of the apex of the T wave of more than 0.25 in leads I, V4, V5, and V6 (Figure 4).5,8,22
Yes. Acute pericarditis has a unique clinical presentation, physical findings, and electrocardiographic (ECG) changes. ECG is always ordered to look for ischemic changes in patients with chest pain. Acute pericarditis develops in stages, which makes it easy to differentiate from early repolarization and, more significantly, myocardial infarction. The ECG changes, along with the clinical presentation and physical findings, can make the diagnosis of pericarditis.
In atypical and complicated cases, advanced imaging studies (ie, echocardiography and cardiac magnetic resonance imaging) have been used to confirm the diagnosis and to follow the course of the disease. However, ECG remains a useful, cost-effective test.
PERICARDIAL DISEASE IS DIVERSE
The pericardium is a thin layer that covers the heart and separates it from other structures in the mediastinum.
Pericardial syndromes include acute, recurrent, constrictive, and effusive-constrictive pericarditis, as well as pericardial effusion with or without tamponade. Causes include viral or bacterial infection, postpericardiotomy syndrome (Dressler syndrome), postmyocardial infarction, primary and metastatic tumors, trauma, uremia, radiation, and autoimmune disease, but pericardial syndromes can also be idiopathic.1
Acute pericarditis is the most common pericardial syndrome and occurs in all age groups. Once diagnosed, it can easily be treated with antiinflammatory drugs. However, recurrent pericarditis, reported in 30% of patients experiencing a first attack of pericarditis, can be difficult to manage, can have a significant impact on the patient’s health, and can be life-threatening.2
CHANGES OF ACUTE PERICARDITIS DEVELOP IN STAGES
Pericarditis can be diagnosed on the basis of ECG changes, clinical signs and symptoms, and laboratory and imaging findings.3 ECG criteria of acute pericarditis have been published.4,5
The characteristic chest pain in acute pericarditis is usually sudden in onset and sharp and occurs over the anterior chest wall. The pain is exacerbated by inspiration and decreases when the patient sits up and leans forward.4
ECG classically shows a widespread saddle-shaped (upward concave) ST-segment elevation in the precordial and limb leads, reflecting subepicardial inflammation. PR-segment depression (with PR-segment elevation in lead aVR) can accompany or precede the ST changes and is known as the “discordant ST-PR segment sign” (Figures 1 and 2). These changes are seen in 60% of patients.
The ECG changes develop in stages, making them easy to differentiate from early repolarization and, more significantly, from myocardial infarction. Four stages are apparent1,4,6–9:
- Stage I occurs in a few hours to days, with diffuse, up-sloping ST-segment elevation and upright T waves, the result of an alteration in ventricular repolarization caused by pericardial inflammation. Because of alteration in repolarization of the atrium secondary to inflammation, the PR segment is elevated in aVR and depressed in the rest of the limb and chest leads.
- Stage II—the ST and PR segments normalize.
- Stage III—widespread T-wave inversion.
- Stage IV—normalization of the T waves.
There is no pathologic Q-wave formation or loss of R-wave progression in acute pericarditis.
The ECG changes of pericarditis vary widely from one patient to another, depending on the extent and severity of pericardial inflammation and the timing of the patient’s presentation. Changes vary in duration. In some cases, ST elevation returns to baseline within a few days without T-wave inversions; in other cases, T-wave inversions can persist for weeks to months. Sometimes the abnormalities resolve by the time symptoms develop.
ASSOCIATED CONDITIONS
Myocardial involvement
In acute myocarditis, findings on ECG can be normal unless the pericardium is involved. Changes that can be seen in myocarditis and that indicate a deeper involvement of inflammation include ST-segment abnormalities, arrhythmias (eg, premature ventricular or atrial contractions), pathologic Q waves, intraventricular conduction delay, and right or left bundle branch block.1,10–12
Elevated troponin and new focal or global left ventricular dysfunction on cardiac imaging indicates myocarditis, especially in a patient with a normal coronary angiogram.10–13
Pericardial effusion: Tachycardia and low QRS voltage
Pericardial effusion is often a complication of pericarditis, but it can also develop from other conditions, such as myxedema, uremia, malignancy, connective tissue disease, aortic dissection, and postpericardiotomy syndrome, and it can also be iatrogenic.
The most common ECG sign of pericardial effusion is tachycardia and low voltage of the QRS complexes. Low voltage is defined as a total amplitude of the QRS complexes in each of the six limb leads less than or equal to 5 mm, and less than or equal to 10 mm in V1 through V6. However, low voltage is not always present in the chest leads.
Mechanisms proposed to explain low QRS voltage associated with pericardial effusion include internal short-circuiting of the electrical currents by accumulated fluids within the pericardial sac, greater distance of the heart from body surface electrodes, reduced cardiac size caused by effusion, and change in the generation and propagation of electrical current in the myocardium.14,15
Cardiac tamponade: Tachycardia, electrical alternans, low QRS voltage
Sinus tachycardia and electrical alternans are specific but not sensitive signs of pericardial tamponade (Figure 3).16,17 Electrical alternans is characterized by beat-to-beat alterations in the axis of QRS complexes in the limb and precordial leads as a result of the mechanical swinging of the heart in a large pericardial effusion.17 There is evidence to suggest that low QRS voltage is more the result of the tamponade than the effusion.18
Treating tamponade with pericardiocentesis, surgical creation of a fistula (“window”) between the pericardial space and the pleural cavity, or anti-inflammatory drugs can resolve low QRS voltage within 1 week.
DIFFERENTIAL DIAGNOSIS OF ACUTE PERICARDITIS
Acute myocardial infarction
ECG changes in acute pericarditis differ from those in acute myocardial infarction in many ways.
ST-segment elevation in pericarditis rarely exceeds 5 mm, in contrast to acute myocardial infarction, in which ST elevation at the J point has to be more than 2 mm and in two anatomically contiguous leads.19
In pericarditis, the changes occur more slowly and in stages, reflecting the evolving inflammation of different areas of the pericardium.
The ST segment is elevated diffusely in the precordial and limb leads in pericarditis, indicating involvement of more than one coronary vascular territory, differentiating it from characteristic regional changes in myocardial infarction.19,20
If concomitant atrial injury is present with acute pericarditis, then PR elevation in aVR with PR depression in other leads may be seen.
Finally, pathologic Q waves or high-grade heart block reflects acute myocardial infarction.
Early repolarization: Elevation of the J point
Early repolarization is sometimes seen in healthy young people, especially in black men.
Early repolarization is characterized by elevation of the J point (ie, the junction between the end of the QRS complex and the beginning of the ST segment). Elevation of the J point causes elevation of the ST segment in the mid to lateral precordial leads (V3–V6) with an up-right T wave.21
Acute pericarditis tends to cause ST-segment elevation in both the limb and precordial leads, whereas ST elevation in early repolarization mainly involves the lateral chest leads.
The PR segment is more prominent in acute pericarditis, especially in lead aVR.
Another finding that strongly favors acute pericarditis is the ratio of the height of the ST-segment junction to the height of the apex of the T wave of more than 0.25 in leads I, V4, V5, and V6 (Figure 4).5,8,22
- Imazio M, Trinchero R. Triage and management of acute pericarditis. Int J Cardiol 2007; 118:286–294.
- Little WC, Freeman GL. Pericardial disease. Circulation 2006; 113:1622–1632.
- Imazio M, Spodick DH, Brucato A, Trinchero R, Markel G, Adler Y. Diagnostic issues in the clinical management of pericarditis. Int J Clin Pract 2010; 64:1384–1392.
- Spodick DH. Acute pericarditis: current concepts and practice. JAMA 2003; 289:1150–1153.
- Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet 2004; 363:717–727.
- Shabetai R. Acute pericarditis. Cardiol Clin 1990; 8:639–644.
- Baljepally R, Spodick DH. PR-segment deviation as the initial electrocardiographic response in acute pericarditis. Am J Cardiol 1998; 81:1505–1506.
- Spodick DH. Diagnostic electrocardiographic sequences in acute pericarditis. Significance of PR segment and PR vector changes. Circulation 1973; 48:575–580.
- Spodick D, editor. The Pericardium: A Comprehensive Textbook. New York, NY: Marcel Dekker; 1997:46–64.
- Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation 1997; 95:163–168.
- Sarda L, Colin P, Boccara F, et al. Myocarditis in patients with clinical presentation of myocardial infarction and normal coronary angiograms. J Am Coll Cardiol 2001; 37:786–792.
- Spodick DH. Arrhythmias during acute pericarditis. A prospective study of 100 consecutive cases. JAMA 1976; 235:39–41.
- Imazio M, Trinchero R. Myopericarditis: etiology, management, and prognosis. Int J Cardiol 2008; 127:17–26.
- Toney JC, Kolmen SN. Cardiac tamponade: fluid and pressure effects on electrocardiographic changes. Proc Soc Exp Biol Med 1966; 121:642–648.
- Karatay CM, Fruehan CT, Lighty GW, Spear RM, Smulyan H. Acute pericardial distension in pigs: effect of fluid conductance on body surface electrocardiogram QRS size. Cardiovasc Res 1993; 27:1033–1038.
- Spodick DH. Acute cardiac tamponade. Pathologic physiology, diagnosis and management. Prog Cardiovasc Dis 1967; 10:64–96.
- Eisenberg MJ, de Romeral LM, Heidenreich PA, Schiller NB, Evans GT. The diagnosis of pericardial effusion and cardiac tamponade by 12-lead ECG. A technology assessment. Chest 1996; 110:318–324.
- Bruch C, Schmermund A, Dagres N, et al. Changes in QRS voltage in cardiac tamponade and pericardial effusion: reversibility after pericardiocentesis and after anti-inflammatory drug treatment. J Am Coll Cardiol 2001; 38:219–226.
- Wang K, Asinger RW, Marriott HJ. ST-segment elevation in conditions other than acute myocardial infarction. N Engl J Med 2003; 349:2128–2135.
- Brady WJ, Perron A, Ullman E. Errors in emergency physician interpretation of ST-segment elevation in emergency department chest pain patients. Acad Emerg Med 2000; 7:1256–1260.
- Kambara H, Phillips J. Long-term evaluation of early repolarization syndrome (normal variant RS-T segment elevation). Am J Cardiol 1976; 38:157–166.
- Ginzton LE, Laks MM. The differential diagnosis of acute pericarditis from the normal variant: new electrocardiographic criteria. Circulation 1982; 65:1004–1009.
- Imazio M, Trinchero R. Triage and management of acute pericarditis. Int J Cardiol 2007; 118:286–294.
- Little WC, Freeman GL. Pericardial disease. Circulation 2006; 113:1622–1632.
- Imazio M, Spodick DH, Brucato A, Trinchero R, Markel G, Adler Y. Diagnostic issues in the clinical management of pericarditis. Int J Clin Pract 2010; 64:1384–1392.
- Spodick DH. Acute pericarditis: current concepts and practice. JAMA 2003; 289:1150–1153.
- Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet 2004; 363:717–727.
- Shabetai R. Acute pericarditis. Cardiol Clin 1990; 8:639–644.
- Baljepally R, Spodick DH. PR-segment deviation as the initial electrocardiographic response in acute pericarditis. Am J Cardiol 1998; 81:1505–1506.
- Spodick DH. Diagnostic electrocardiographic sequences in acute pericarditis. Significance of PR segment and PR vector changes. Circulation 1973; 48:575–580.
- Spodick D, editor. The Pericardium: A Comprehensive Textbook. New York, NY: Marcel Dekker; 1997:46–64.
- Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation 1997; 95:163–168.
- Sarda L, Colin P, Boccara F, et al. Myocarditis in patients with clinical presentation of myocardial infarction and normal coronary angiograms. J Am Coll Cardiol 2001; 37:786–792.
- Spodick DH. Arrhythmias during acute pericarditis. A prospective study of 100 consecutive cases. JAMA 1976; 235:39–41.
- Imazio M, Trinchero R. Myopericarditis: etiology, management, and prognosis. Int J Cardiol 2008; 127:17–26.
- Toney JC, Kolmen SN. Cardiac tamponade: fluid and pressure effects on electrocardiographic changes. Proc Soc Exp Biol Med 1966; 121:642–648.
- Karatay CM, Fruehan CT, Lighty GW, Spear RM, Smulyan H. Acute pericardial distension in pigs: effect of fluid conductance on body surface electrocardiogram QRS size. Cardiovasc Res 1993; 27:1033–1038.
- Spodick DH. Acute cardiac tamponade. Pathologic physiology, diagnosis and management. Prog Cardiovasc Dis 1967; 10:64–96.
- Eisenberg MJ, de Romeral LM, Heidenreich PA, Schiller NB, Evans GT. The diagnosis of pericardial effusion and cardiac tamponade by 12-lead ECG. A technology assessment. Chest 1996; 110:318–324.
- Bruch C, Schmermund A, Dagres N, et al. Changes in QRS voltage in cardiac tamponade and pericardial effusion: reversibility after pericardiocentesis and after anti-inflammatory drug treatment. J Am Coll Cardiol 2001; 38:219–226.
- Wang K, Asinger RW, Marriott HJ. ST-segment elevation in conditions other than acute myocardial infarction. N Engl J Med 2003; 349:2128–2135.
- Brady WJ, Perron A, Ullman E. Errors in emergency physician interpretation of ST-segment elevation in emergency department chest pain patients. Acad Emerg Med 2000; 7:1256–1260.
- Kambara H, Phillips J. Long-term evaluation of early repolarization syndrome (normal variant RS-T segment elevation). Am J Cardiol 1976; 38:157–166.
- Ginzton LE, Laks MM. The differential diagnosis of acute pericarditis from the normal variant: new electrocardiographic criteria. Circulation 1982; 65:1004–1009.
Resistant hypertension: Diagnostic strategies and management
Poor control of blood pressure is one of the most common risk factors for death worldwide, responsible for 62% of cases of cerebral vascular disease and 49% of cases of ischemic heart disease as well as 7.1 million deaths annually. As our population ages and the prevalence of obesity, diabetes, and chronic kidney disease increases, resistant hypertension will be seen more often in general practice.
Using a case study, this article will provide a strategy for diagnosing and treating resistant hypertension.
CASE: A WOMAN WITH LONG-STANDING HIGH BLOOD PRESSURE
A 37-year-old woman was referred for help with managing difficult-to-control hypertension. She had been diagnosed with hypertension at age 32, and it was well controlled until about 2 years ago. Various combinations of antihypertensive drugs had been tried, and a search for a cause of secondary hypertension revealed no clues.
On examination, her blood pressure averaged 212/124 mm Hg, and her heart rate was 109 beats per minute. Her medications were:
- Amlodipine (Norvasc), a calcium channel blocker, 10 mg once daily
- Valsartan (Diovan), an angiotensin II receptor antagonist, 160 mg once daily
- Carvedilol (Coreg), a beta-blocker, 25 mg twice daily
- Labetalol (Normodyne), a beta-blocker, 400 mg three times daily
- Clonidine (Catapres), a sympatholytic agent, 0.05 mg three times daily
- Doxazosin (Cardura), a peripheral alpha-blocker, 16 mg once daily
- Xylometazoline (Xylomet), an alpha agonist nasal spray for nasal congestion.
She had previously been taking spironolactone (Aldactone), hydralazine (Apresoline), and hydrochlorothiazide, but they were discontinued because of adverse effects.
Does this patient have resistant hypertension? How should her condition be managed?
RESISTANT HYPERTENSION DEFINED
The seventh Joint National Committee and the American Heart Association define resistant hypertension as an office blood pressure above the appropriate goal of therapy (< 140/90 mm Hg for most patients, and < 130/80 mm Hg for those with ischemic heart disease, diabetes, or renal insufficiency) despite the use of three or more antihypertensive drugs from different classes at full dosages, one of which is a diuretic.1,2
In this definition, the number of antihypertensive drugs required is arbitrary. More importantly, the concept of resistant hypertension is focused on identifying patients who may have a reversible cause of hypertension, as well as those who could benefit from special diagnostic or therapeutic intervention because of persistently high blood pressure.
This definition does not apply to patients who have recently been diagnosed with hypertension.
Resistant hypertension is not synonymous with uncontrolled hypertension, which includes all cases of hypertension that is not optimally controlled despite treatment, including apparent resistance (ie, pseudoresistance) and true resistance (defined below).
COMMON, BUT ITS PREVALENCE IS HARD TO PINPOINT
The prevalence of resistant hypertension is unknown because of inadequate sample sizes in published studies. However, it is common and is likely to become more common with the aging of the population and with the increasing prevalence of obesity, diabetes mellitus, and chronic kidney disease.
In small studies, the prevalence of resistance in hypertensive patients ranged from 5% in general medical practice to more than 50% in nephrology clinics. In the National Health and Nutrition Examination Survey in 2003 to 2004, only 58% of people being treated for hypertension had achieved blood pressure levels lower than 140/90 mm Hg,3 and the control rate in those with diabetes mellitus or chronic kidney disease was less than 40%.4
Isolated systolic hypertension—elevated systolic pressure with normal diastolic pressure—increases in prevalence with age in those with treated, uncontrolled hypertension. It accounted for 29.1% of cases of treated, uncontrolled hypertension in patients ages 25 to 44, 66.1% of cases in patients ages 45 to 64, and 87.6% of cases in patients age 65 and older.5
Even in clinical trials, in which one would expect excellent control of hypertension, rates of control ranged from 45% to 82%.6–10
APPARENT RESISTANCE VS TRUE RESISTANCE
Resistant hypertension can be divided arbitrarily into two broad categories: apparent resistance and true resistance, with the prevalence of apparent resistance being considerably higher. Each broad category has a long list of possible causes; most are readily identifiable in the course of a thorough history and physical examination and routine laboratory testing. If resistance to therapy persists, referral to a hypertension specialist is a logical next step.
Detecting pseudoresistance
Causes of apparent resistance include improper technique in measuring blood pressure, such as not having the patient rest before measurement, allowing the patient to have coffee or to smoke just before measurement, or not positioning the patient’s arm at the level of the heart during measurement.
Many elderly patients have calcified arteries that are hard to compress, leading to erroneously high systolic blood pressure measurements, a situation called pseudohypertension and a cause of pseudoresistance. The only way to measure blood pressure accurately in such cases is intra-arterially. These patients often do not have target-organ disease, which would be expected with high systolic pressure.
The white-coat phenomenon is another common cause of apparent resistance. It is defined as persistently elevated clinic or office blood pressure (> 140/90 mm Hg), together with normal daytime ambulatory blood pressure (the “white-coat effect” is the difference between those blood pressures).
Finally, poor patient adherence to treatment is estimated to account for 40% of cases of resistant hypertension.4,5,11 Poor adherence is difficult to prove because patients often claim they are compliant, but certain clues are indicative. For example, patients taking a diuretic should have increased uric acid levels, so normal uric acid levels in a patient on a diuretic could be a clue that he or she is not taking the medication. If poor adherence is suspected, patients should be admitted to the hospital to take the medications under close observation.
Many factors can contribute to true resistance
Many cases of resistant hypertension are drug-induced, particularly in patients taking a nonsteroidal anti-inflammatory drug or a cyclooxygenase II inhibitor. Use of ginseng, ma huang, and bitter lemon should also be suspected. Drugs or herbal preparations contributing to high blood pressure should be discontinued or minimized.
Alcohol intake in excess of two drinks (1 oz of alcohol) per day for men and half that amount for women can also contribute to hypertension.
Volume overload is common and has many causes, including a compensatory response to vasodilators, excessive salt intake, or an undetected reduction in the glomerular filtration rate causing retention of salt and water.
Drug considerations
A common cause of apparent resistant hypertension is physicians not following blood pressure treatment guidelines by not increasing the dosage when needed or by prescribing inappropriate drug combinations.
We commonly see furosemide (Lasix) being misused, ie, being prescribed once daily for hypertension. (It has a shorter duration of action than thiazide diuretics, the usual class of diuretics used for hypertension.)
For a patient who is already on many medications but whose hypertension is not responding, the first step should be to give a diuretic of an appropriate class in an appropriate dosage.
Diuretics are often inappropriately stopped if a patient develops hypokalemia. Potassium supplementation should always be an adjunct to diuretic therapy. Potassium itself is a potent vasodilator and, given as a supplement, has been shown to reduce stroke risk in rats.
The combination of an angiotensin receptor blocker and an angiotensin-converting enzyme inhibitor should not be used for patients with true resistant hypertension. The direct renin inhibitor aliskiren (Tekturna) should not be used in combination with these drugs, and the combination of aliskiren and valsartan (Valturna) has now been taken off the market.
Spironolactone (Aldactone) is sometimes used for resistant hypertension in the belief that in some cases primary aldosteronism is the underlying cause. A study in 1,400 participants confirms that it lowers blood pressure,9 but the reason is unclear: the blood pressure response was unrelated to levels of renin, angiotensin, or the plasma aldosterone-to-renin ratio.
Identify secondary causes of hypertension
Patients should be evaluated for kidney disease, which is the most common secondary medical reason for resistant hypertension. For patients with poor renal function (estimated glomerular filtration rate < 50 mL/minute), hydrochlorothiazide is not effective against hypertension, but chlorthalidone is. In addition, patients with poor renal function should be given loop diuretics such as furosemide two or three times daily, or the long-acting drug torsemide (Demadex) should be used instead.
Genetic variation can cause different rates of metabolism of drugs, contributing to resistant hypertension. Certain people metabolize hydralazine very fast, making it less effective. The same is true for some beta-blockers.
Obesity and diabetes can also contribute to resistant hypertension.
Ancillary neurohumoral studies are occasionally indicated to rule out identifiable causes of secondary hypertension that may be correctable. There are many identifiable causes of hypertension, but detailing each is beyond the scope of this article.
Patients should be tested for thyroid disease. Hypothyroidism can cause high blood pressure, although usually diastolic rather than systolic hypertension. Hyperthyroidism can cause marked systolic hypertension.
Table 1 provides a step-by-step guide for evaluating and managing patients with resistant hypertension.
EXPERIMENTAL DRUG THERAPY
Endothelin receptor antagonists are currently under investigation for the treatment of resistant hypertension. The protein endothelin-1 (ET-1) is a potent vasoconstrictor (30–50 times more potent than angiotensin II and norepinephrine) and has a long duration of action. ET-1 binds to two receptors with opposing effects: ET-A promotes vasoconstriction, and ET-B promotes vasodilation and clears ET-1.
Darusentan, a selective blocker of ET-A, was tested in the phase III DORADO trial, which was discontinued because the initial results did not meet primary outcome measures. Initial findings had indicated that it might not be as useful as hoped. Side effects included headache, flushing, and edema.
EXPERIMENTAL NONPHARMACOLOGIC THERAPIES
Electrical stimulation of carotid sinus baroreceptors is being tried under the assumption that a high sympathoexcitatory state contributes to resistant hypertension. Devices are placed around the carotid artery bifurcation, and stimulation is believed to increase the depressor influences that modulate blood pressure. Large-scale trials are under way, but it is too early to tell if the approach will be useful. Patients complain of neck pain from the device.
Renal denervation is another experimental approach.12 The kidney has a central role in blood pressure regulation: efferent nerves regulate renal vascular resistance, renal blood flow, and renin release from the juxtaglomerular apparatus; afferent nerves modulate sympathetic output from the central nervous system. The results of the Renal Denervation in Patients With Uncontrolled Hypertension (Symplicity HTN) trials 1 and 2 have been encouraging. The Symplicity HTN-3 trial will begin soon in the United States.
OUR PATIENT UNDERGOES ADDITIONAL STUDIES
To rule out the white-coat effect in our patient, we measured her blood pressure with an automated device that takes several readings without the clinician in the room. (This topic has been reviewed by Vidt et al in this journal13). The average of the automated readings was 183/113 mm Hg, and her average pulse was 109 beats per minute, arguing against a white-coat effect.
Her blood pressure was also markedly elevated (average 198/129) during 24-hour ambulatory blood pressure monitoring.
Findings on physical examination were unremarkable except for grade III hypertensive retinopathy. She had no carotid or abdominal bruits. Her peripheral pulses were strong and synchronous bilaterally.
Laboratory testing found the patient had normal serum electrolyte levels and good renal function but relatively low urinary sodium, 90 mmol/day (normal 40–220), and very low renin activity, 0.7 μg/L/h (normal up-right 0.8–5.8 μg/L/h, supine 0.5–1.8 μg/L/h), calling into question the wisdom of treatment with an angiotensin receptor blocker.
Hemodynamic studies were performed using impedance cardiography and found very high systemic vascular resistance with normal cardiac output, indicating that the patient had a high preload, which could be from hypervolemia or intense venous constriction. It is especially interesting that her vascular resistance was high despite her treatment regimen that included an angiotensin receptor blocker and a vasodilator, perhaps an indication of nonadherence with her medications.
Diuresis reduces her blood pressure
The patient was admitted to the hospital, and because her laboratory results indicated that plasma renin activity was suppressed, the angiotensin receptor blocker valsartan was discontinued.
On day 1, her weight was 162 lb and average blood pressure was 194/128 mm Hg. After 4 days of diuresis with escalating doses of furosemide, her weight was 153 lb and blood pressures ranged from 140 to 158 over 82 to 98 mm Hg. Her heart rate was 90 beats per minute. The hospital stay showed that volume overload was one of the factors maintaining her hypertension. She was discharged on metoprolol succinate (Toprol-XL) 100 mg twice daily and furosemide 80 mg twice daily.
Her blood pressure fluctuates widely after discharge
Over the next 5 days after discharge, the patient’s blood pressure rose steadily to 180/122 mm Hg, her heart rate was in excess of 100 beats per minute, and her weight increased to 158 lb. Blood screening found that the level of metoprolol was undetectable, and a diuretic screen showed no furosemide in the urine. Both the patient and her husband were adamant that she was taking her medications.
Hydrochlorothiazide 25 mg daily was added, and nadolol (Corgard) 80 mg once daily was started in place of metoprolol. On a return visit, her blood pressure and heart rate were finally good at 138/86 mm Hg and 60 beats per minute (sitting) and 134/92 and 63 (standing).
On 24-hour monitoring, some fluctuations of elevated blood pressure were still evident, with an average of 142/91 mm Hg, so nifedipine (Procardia) 60 mg daily was added.
Her final list of medications is hydrochlorothiazide 25 mg, nadolol 80 mg, and nifedipine XL 60 mg, all taken once daily.
Volume overload complicated by nonadherence
In summary, the main pathogenetic mechanism that sustained this patient’s hypertension was volume overload. Her urinary sodium level indicated that she was not taking excessive amounts of sodium. The volume overload may have been a compensatory response to the concomitant use of peripheral vasodilators plus sympatholytic agents.
In addition, she was not adherent to her antihypertensive regimen. The fact that her heart rate was 109 beats per minute despite having a drug regimen that included five sympathetic blocking agents was a strong clue. She eventually admitted that she did not like taking diuretics because they made her skin wrinkle.
In general, in a case like this, I try to minimize the number of drugs and give a diuretic as well as different classes of appropriate drugs.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2007; 51:1403–1419.
- Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment and control of hypertension among United States adults 1999–2004. Hypertension 2007; 49:69–75.
- Sarafidis PA, Li S, Chen SC, et al. Hypertension awareness, treatment, and control in chronic kidney disease. Am J Med 2008; 121:332–340.
- Sarafidis PA, Bakris GL. State of hypertension management in the United States: confluence of risk factors and the prevalence of resistant hypertension. J Clin Hypertens (Greenwich) 2008; 10:130–139.
- Jamerson K, Bakris GL, Dahlöf B, et al; for the ACCOMPLISH Investigators. Exceptional early blood pressure control rates: the ACCOMPLISH trial. Blood Pressure 2007; 16:80–86.
- Dahlöf B, Devereux RB, Kjeldsen S, et al; for the LIFE study group. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–003.
- Cushman WC, Ford CE, Cutler JA, et al; for the ALLHAT Collaborative Research Group. Success and predictors of blood pressure control in diverse North American settings: the Antihypertensive and Lipid-Lowering and Treatment to Prevent Heart Attack Trial (ALLHAT). J Clin Hypertens (Greenwich) 2002; 4:393–404.
- Chapman N, Dobson J, Wilson S, et al; on behalf of the Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 2007; 49:839–845.
- Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al; INVEST Investigators. A calcium antagonist vs a noncalcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003; 290:2805–2816.
- Calhoun DA, Jones D, Textor S, et al. AHA Scientific Statement. Resistant hypertension: diagnosis, evaluation, and treatment. Circulation 2008; 17:e510–e526.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension: guarded optimism. Cleve Clin J Med 2012; 79:501–510.
- Vidt DG, Lang RS, Seballos RJ, Misra-Hebert A, Campbell J, Bena JF. Taking blood pressure: too important to trust to humans? Cleve Clin J Med 2010; 77:683–688.
Poor control of blood pressure is one of the most common risk factors for death worldwide, responsible for 62% of cases of cerebral vascular disease and 49% of cases of ischemic heart disease as well as 7.1 million deaths annually. As our population ages and the prevalence of obesity, diabetes, and chronic kidney disease increases, resistant hypertension will be seen more often in general practice.
Using a case study, this article will provide a strategy for diagnosing and treating resistant hypertension.
CASE: A WOMAN WITH LONG-STANDING HIGH BLOOD PRESSURE
A 37-year-old woman was referred for help with managing difficult-to-control hypertension. She had been diagnosed with hypertension at age 32, and it was well controlled until about 2 years ago. Various combinations of antihypertensive drugs had been tried, and a search for a cause of secondary hypertension revealed no clues.
On examination, her blood pressure averaged 212/124 mm Hg, and her heart rate was 109 beats per minute. Her medications were:
- Amlodipine (Norvasc), a calcium channel blocker, 10 mg once daily
- Valsartan (Diovan), an angiotensin II receptor antagonist, 160 mg once daily
- Carvedilol (Coreg), a beta-blocker, 25 mg twice daily
- Labetalol (Normodyne), a beta-blocker, 400 mg three times daily
- Clonidine (Catapres), a sympatholytic agent, 0.05 mg three times daily
- Doxazosin (Cardura), a peripheral alpha-blocker, 16 mg once daily
- Xylometazoline (Xylomet), an alpha agonist nasal spray for nasal congestion.
She had previously been taking spironolactone (Aldactone), hydralazine (Apresoline), and hydrochlorothiazide, but they were discontinued because of adverse effects.
Does this patient have resistant hypertension? How should her condition be managed?
RESISTANT HYPERTENSION DEFINED
The seventh Joint National Committee and the American Heart Association define resistant hypertension as an office blood pressure above the appropriate goal of therapy (< 140/90 mm Hg for most patients, and < 130/80 mm Hg for those with ischemic heart disease, diabetes, or renal insufficiency) despite the use of three or more antihypertensive drugs from different classes at full dosages, one of which is a diuretic.1,2
In this definition, the number of antihypertensive drugs required is arbitrary. More importantly, the concept of resistant hypertension is focused on identifying patients who may have a reversible cause of hypertension, as well as those who could benefit from special diagnostic or therapeutic intervention because of persistently high blood pressure.
This definition does not apply to patients who have recently been diagnosed with hypertension.
Resistant hypertension is not synonymous with uncontrolled hypertension, which includes all cases of hypertension that is not optimally controlled despite treatment, including apparent resistance (ie, pseudoresistance) and true resistance (defined below).
COMMON, BUT ITS PREVALENCE IS HARD TO PINPOINT
The prevalence of resistant hypertension is unknown because of inadequate sample sizes in published studies. However, it is common and is likely to become more common with the aging of the population and with the increasing prevalence of obesity, diabetes mellitus, and chronic kidney disease.
In small studies, the prevalence of resistance in hypertensive patients ranged from 5% in general medical practice to more than 50% in nephrology clinics. In the National Health and Nutrition Examination Survey in 2003 to 2004, only 58% of people being treated for hypertension had achieved blood pressure levels lower than 140/90 mm Hg,3 and the control rate in those with diabetes mellitus or chronic kidney disease was less than 40%.4
Isolated systolic hypertension—elevated systolic pressure with normal diastolic pressure—increases in prevalence with age in those with treated, uncontrolled hypertension. It accounted for 29.1% of cases of treated, uncontrolled hypertension in patients ages 25 to 44, 66.1% of cases in patients ages 45 to 64, and 87.6% of cases in patients age 65 and older.5
Even in clinical trials, in which one would expect excellent control of hypertension, rates of control ranged from 45% to 82%.6–10
APPARENT RESISTANCE VS TRUE RESISTANCE
Resistant hypertension can be divided arbitrarily into two broad categories: apparent resistance and true resistance, with the prevalence of apparent resistance being considerably higher. Each broad category has a long list of possible causes; most are readily identifiable in the course of a thorough history and physical examination and routine laboratory testing. If resistance to therapy persists, referral to a hypertension specialist is a logical next step.
Detecting pseudoresistance
Causes of apparent resistance include improper technique in measuring blood pressure, such as not having the patient rest before measurement, allowing the patient to have coffee or to smoke just before measurement, or not positioning the patient’s arm at the level of the heart during measurement.
Many elderly patients have calcified arteries that are hard to compress, leading to erroneously high systolic blood pressure measurements, a situation called pseudohypertension and a cause of pseudoresistance. The only way to measure blood pressure accurately in such cases is intra-arterially. These patients often do not have target-organ disease, which would be expected with high systolic pressure.
The white-coat phenomenon is another common cause of apparent resistance. It is defined as persistently elevated clinic or office blood pressure (> 140/90 mm Hg), together with normal daytime ambulatory blood pressure (the “white-coat effect” is the difference between those blood pressures).
Finally, poor patient adherence to treatment is estimated to account for 40% of cases of resistant hypertension.4,5,11 Poor adherence is difficult to prove because patients often claim they are compliant, but certain clues are indicative. For example, patients taking a diuretic should have increased uric acid levels, so normal uric acid levels in a patient on a diuretic could be a clue that he or she is not taking the medication. If poor adherence is suspected, patients should be admitted to the hospital to take the medications under close observation.
Many factors can contribute to true resistance
Many cases of resistant hypertension are drug-induced, particularly in patients taking a nonsteroidal anti-inflammatory drug or a cyclooxygenase II inhibitor. Use of ginseng, ma huang, and bitter lemon should also be suspected. Drugs or herbal preparations contributing to high blood pressure should be discontinued or minimized.
Alcohol intake in excess of two drinks (1 oz of alcohol) per day for men and half that amount for women can also contribute to hypertension.
Volume overload is common and has many causes, including a compensatory response to vasodilators, excessive salt intake, or an undetected reduction in the glomerular filtration rate causing retention of salt and water.
Drug considerations
A common cause of apparent resistant hypertension is physicians not following blood pressure treatment guidelines by not increasing the dosage when needed or by prescribing inappropriate drug combinations.
We commonly see furosemide (Lasix) being misused, ie, being prescribed once daily for hypertension. (It has a shorter duration of action than thiazide diuretics, the usual class of diuretics used for hypertension.)
For a patient who is already on many medications but whose hypertension is not responding, the first step should be to give a diuretic of an appropriate class in an appropriate dosage.
Diuretics are often inappropriately stopped if a patient develops hypokalemia. Potassium supplementation should always be an adjunct to diuretic therapy. Potassium itself is a potent vasodilator and, given as a supplement, has been shown to reduce stroke risk in rats.
The combination of an angiotensin receptor blocker and an angiotensin-converting enzyme inhibitor should not be used for patients with true resistant hypertension. The direct renin inhibitor aliskiren (Tekturna) should not be used in combination with these drugs, and the combination of aliskiren and valsartan (Valturna) has now been taken off the market.
Spironolactone (Aldactone) is sometimes used for resistant hypertension in the belief that in some cases primary aldosteronism is the underlying cause. A study in 1,400 participants confirms that it lowers blood pressure,9 but the reason is unclear: the blood pressure response was unrelated to levels of renin, angiotensin, or the plasma aldosterone-to-renin ratio.
Identify secondary causes of hypertension
Patients should be evaluated for kidney disease, which is the most common secondary medical reason for resistant hypertension. For patients with poor renal function (estimated glomerular filtration rate < 50 mL/minute), hydrochlorothiazide is not effective against hypertension, but chlorthalidone is. In addition, patients with poor renal function should be given loop diuretics such as furosemide two or three times daily, or the long-acting drug torsemide (Demadex) should be used instead.
Genetic variation can cause different rates of metabolism of drugs, contributing to resistant hypertension. Certain people metabolize hydralazine very fast, making it less effective. The same is true for some beta-blockers.
Obesity and diabetes can also contribute to resistant hypertension.
Ancillary neurohumoral studies are occasionally indicated to rule out identifiable causes of secondary hypertension that may be correctable. There are many identifiable causes of hypertension, but detailing each is beyond the scope of this article.
Patients should be tested for thyroid disease. Hypothyroidism can cause high blood pressure, although usually diastolic rather than systolic hypertension. Hyperthyroidism can cause marked systolic hypertension.
Table 1 provides a step-by-step guide for evaluating and managing patients with resistant hypertension.
EXPERIMENTAL DRUG THERAPY
Endothelin receptor antagonists are currently under investigation for the treatment of resistant hypertension. The protein endothelin-1 (ET-1) is a potent vasoconstrictor (30–50 times more potent than angiotensin II and norepinephrine) and has a long duration of action. ET-1 binds to two receptors with opposing effects: ET-A promotes vasoconstriction, and ET-B promotes vasodilation and clears ET-1.
Darusentan, a selective blocker of ET-A, was tested in the phase III DORADO trial, which was discontinued because the initial results did not meet primary outcome measures. Initial findings had indicated that it might not be as useful as hoped. Side effects included headache, flushing, and edema.
EXPERIMENTAL NONPHARMACOLOGIC THERAPIES
Electrical stimulation of carotid sinus baroreceptors is being tried under the assumption that a high sympathoexcitatory state contributes to resistant hypertension. Devices are placed around the carotid artery bifurcation, and stimulation is believed to increase the depressor influences that modulate blood pressure. Large-scale trials are under way, but it is too early to tell if the approach will be useful. Patients complain of neck pain from the device.
Renal denervation is another experimental approach.12 The kidney has a central role in blood pressure regulation: efferent nerves regulate renal vascular resistance, renal blood flow, and renin release from the juxtaglomerular apparatus; afferent nerves modulate sympathetic output from the central nervous system. The results of the Renal Denervation in Patients With Uncontrolled Hypertension (Symplicity HTN) trials 1 and 2 have been encouraging. The Symplicity HTN-3 trial will begin soon in the United States.
OUR PATIENT UNDERGOES ADDITIONAL STUDIES
To rule out the white-coat effect in our patient, we measured her blood pressure with an automated device that takes several readings without the clinician in the room. (This topic has been reviewed by Vidt et al in this journal13). The average of the automated readings was 183/113 mm Hg, and her average pulse was 109 beats per minute, arguing against a white-coat effect.
Her blood pressure was also markedly elevated (average 198/129) during 24-hour ambulatory blood pressure monitoring.
Findings on physical examination were unremarkable except for grade III hypertensive retinopathy. She had no carotid or abdominal bruits. Her peripheral pulses were strong and synchronous bilaterally.
Laboratory testing found the patient had normal serum electrolyte levels and good renal function but relatively low urinary sodium, 90 mmol/day (normal 40–220), and very low renin activity, 0.7 μg/L/h (normal up-right 0.8–5.8 μg/L/h, supine 0.5–1.8 μg/L/h), calling into question the wisdom of treatment with an angiotensin receptor blocker.
Hemodynamic studies were performed using impedance cardiography and found very high systemic vascular resistance with normal cardiac output, indicating that the patient had a high preload, which could be from hypervolemia or intense venous constriction. It is especially interesting that her vascular resistance was high despite her treatment regimen that included an angiotensin receptor blocker and a vasodilator, perhaps an indication of nonadherence with her medications.
Diuresis reduces her blood pressure
The patient was admitted to the hospital, and because her laboratory results indicated that plasma renin activity was suppressed, the angiotensin receptor blocker valsartan was discontinued.
On day 1, her weight was 162 lb and average blood pressure was 194/128 mm Hg. After 4 days of diuresis with escalating doses of furosemide, her weight was 153 lb and blood pressures ranged from 140 to 158 over 82 to 98 mm Hg. Her heart rate was 90 beats per minute. The hospital stay showed that volume overload was one of the factors maintaining her hypertension. She was discharged on metoprolol succinate (Toprol-XL) 100 mg twice daily and furosemide 80 mg twice daily.
Her blood pressure fluctuates widely after discharge
Over the next 5 days after discharge, the patient’s blood pressure rose steadily to 180/122 mm Hg, her heart rate was in excess of 100 beats per minute, and her weight increased to 158 lb. Blood screening found that the level of metoprolol was undetectable, and a diuretic screen showed no furosemide in the urine. Both the patient and her husband were adamant that she was taking her medications.
Hydrochlorothiazide 25 mg daily was added, and nadolol (Corgard) 80 mg once daily was started in place of metoprolol. On a return visit, her blood pressure and heart rate were finally good at 138/86 mm Hg and 60 beats per minute (sitting) and 134/92 and 63 (standing).
On 24-hour monitoring, some fluctuations of elevated blood pressure were still evident, with an average of 142/91 mm Hg, so nifedipine (Procardia) 60 mg daily was added.
Her final list of medications is hydrochlorothiazide 25 mg, nadolol 80 mg, and nifedipine XL 60 mg, all taken once daily.
Volume overload complicated by nonadherence
In summary, the main pathogenetic mechanism that sustained this patient’s hypertension was volume overload. Her urinary sodium level indicated that she was not taking excessive amounts of sodium. The volume overload may have been a compensatory response to the concomitant use of peripheral vasodilators plus sympatholytic agents.
In addition, she was not adherent to her antihypertensive regimen. The fact that her heart rate was 109 beats per minute despite having a drug regimen that included five sympathetic blocking agents was a strong clue. She eventually admitted that she did not like taking diuretics because they made her skin wrinkle.
In general, in a case like this, I try to minimize the number of drugs and give a diuretic as well as different classes of appropriate drugs.
Poor control of blood pressure is one of the most common risk factors for death worldwide, responsible for 62% of cases of cerebral vascular disease and 49% of cases of ischemic heart disease as well as 7.1 million deaths annually. As our population ages and the prevalence of obesity, diabetes, and chronic kidney disease increases, resistant hypertension will be seen more often in general practice.
Using a case study, this article will provide a strategy for diagnosing and treating resistant hypertension.
CASE: A WOMAN WITH LONG-STANDING HIGH BLOOD PRESSURE
A 37-year-old woman was referred for help with managing difficult-to-control hypertension. She had been diagnosed with hypertension at age 32, and it was well controlled until about 2 years ago. Various combinations of antihypertensive drugs had been tried, and a search for a cause of secondary hypertension revealed no clues.
On examination, her blood pressure averaged 212/124 mm Hg, and her heart rate was 109 beats per minute. Her medications were:
- Amlodipine (Norvasc), a calcium channel blocker, 10 mg once daily
- Valsartan (Diovan), an angiotensin II receptor antagonist, 160 mg once daily
- Carvedilol (Coreg), a beta-blocker, 25 mg twice daily
- Labetalol (Normodyne), a beta-blocker, 400 mg three times daily
- Clonidine (Catapres), a sympatholytic agent, 0.05 mg three times daily
- Doxazosin (Cardura), a peripheral alpha-blocker, 16 mg once daily
- Xylometazoline (Xylomet), an alpha agonist nasal spray for nasal congestion.
She had previously been taking spironolactone (Aldactone), hydralazine (Apresoline), and hydrochlorothiazide, but they were discontinued because of adverse effects.
Does this patient have resistant hypertension? How should her condition be managed?
RESISTANT HYPERTENSION DEFINED
The seventh Joint National Committee and the American Heart Association define resistant hypertension as an office blood pressure above the appropriate goal of therapy (< 140/90 mm Hg for most patients, and < 130/80 mm Hg for those with ischemic heart disease, diabetes, or renal insufficiency) despite the use of three or more antihypertensive drugs from different classes at full dosages, one of which is a diuretic.1,2
In this definition, the number of antihypertensive drugs required is arbitrary. More importantly, the concept of resistant hypertension is focused on identifying patients who may have a reversible cause of hypertension, as well as those who could benefit from special diagnostic or therapeutic intervention because of persistently high blood pressure.
This definition does not apply to patients who have recently been diagnosed with hypertension.
Resistant hypertension is not synonymous with uncontrolled hypertension, which includes all cases of hypertension that is not optimally controlled despite treatment, including apparent resistance (ie, pseudoresistance) and true resistance (defined below).
COMMON, BUT ITS PREVALENCE IS HARD TO PINPOINT
The prevalence of resistant hypertension is unknown because of inadequate sample sizes in published studies. However, it is common and is likely to become more common with the aging of the population and with the increasing prevalence of obesity, diabetes mellitus, and chronic kidney disease.
In small studies, the prevalence of resistance in hypertensive patients ranged from 5% in general medical practice to more than 50% in nephrology clinics. In the National Health and Nutrition Examination Survey in 2003 to 2004, only 58% of people being treated for hypertension had achieved blood pressure levels lower than 140/90 mm Hg,3 and the control rate in those with diabetes mellitus or chronic kidney disease was less than 40%.4
Isolated systolic hypertension—elevated systolic pressure with normal diastolic pressure—increases in prevalence with age in those with treated, uncontrolled hypertension. It accounted for 29.1% of cases of treated, uncontrolled hypertension in patients ages 25 to 44, 66.1% of cases in patients ages 45 to 64, and 87.6% of cases in patients age 65 and older.5
Even in clinical trials, in which one would expect excellent control of hypertension, rates of control ranged from 45% to 82%.6–10
APPARENT RESISTANCE VS TRUE RESISTANCE
Resistant hypertension can be divided arbitrarily into two broad categories: apparent resistance and true resistance, with the prevalence of apparent resistance being considerably higher. Each broad category has a long list of possible causes; most are readily identifiable in the course of a thorough history and physical examination and routine laboratory testing. If resistance to therapy persists, referral to a hypertension specialist is a logical next step.
Detecting pseudoresistance
Causes of apparent resistance include improper technique in measuring blood pressure, such as not having the patient rest before measurement, allowing the patient to have coffee or to smoke just before measurement, or not positioning the patient’s arm at the level of the heart during measurement.
Many elderly patients have calcified arteries that are hard to compress, leading to erroneously high systolic blood pressure measurements, a situation called pseudohypertension and a cause of pseudoresistance. The only way to measure blood pressure accurately in such cases is intra-arterially. These patients often do not have target-organ disease, which would be expected with high systolic pressure.
The white-coat phenomenon is another common cause of apparent resistance. It is defined as persistently elevated clinic or office blood pressure (> 140/90 mm Hg), together with normal daytime ambulatory blood pressure (the “white-coat effect” is the difference between those blood pressures).
Finally, poor patient adherence to treatment is estimated to account for 40% of cases of resistant hypertension.4,5,11 Poor adherence is difficult to prove because patients often claim they are compliant, but certain clues are indicative. For example, patients taking a diuretic should have increased uric acid levels, so normal uric acid levels in a patient on a diuretic could be a clue that he or she is not taking the medication. If poor adherence is suspected, patients should be admitted to the hospital to take the medications under close observation.
Many factors can contribute to true resistance
Many cases of resistant hypertension are drug-induced, particularly in patients taking a nonsteroidal anti-inflammatory drug or a cyclooxygenase II inhibitor. Use of ginseng, ma huang, and bitter lemon should also be suspected. Drugs or herbal preparations contributing to high blood pressure should be discontinued or minimized.
Alcohol intake in excess of two drinks (1 oz of alcohol) per day for men and half that amount for women can also contribute to hypertension.
Volume overload is common and has many causes, including a compensatory response to vasodilators, excessive salt intake, or an undetected reduction in the glomerular filtration rate causing retention of salt and water.
Drug considerations
A common cause of apparent resistant hypertension is physicians not following blood pressure treatment guidelines by not increasing the dosage when needed or by prescribing inappropriate drug combinations.
We commonly see furosemide (Lasix) being misused, ie, being prescribed once daily for hypertension. (It has a shorter duration of action than thiazide diuretics, the usual class of diuretics used for hypertension.)
For a patient who is already on many medications but whose hypertension is not responding, the first step should be to give a diuretic of an appropriate class in an appropriate dosage.
Diuretics are often inappropriately stopped if a patient develops hypokalemia. Potassium supplementation should always be an adjunct to diuretic therapy. Potassium itself is a potent vasodilator and, given as a supplement, has been shown to reduce stroke risk in rats.
The combination of an angiotensin receptor blocker and an angiotensin-converting enzyme inhibitor should not be used for patients with true resistant hypertension. The direct renin inhibitor aliskiren (Tekturna) should not be used in combination with these drugs, and the combination of aliskiren and valsartan (Valturna) has now been taken off the market.
Spironolactone (Aldactone) is sometimes used for resistant hypertension in the belief that in some cases primary aldosteronism is the underlying cause. A study in 1,400 participants confirms that it lowers blood pressure,9 but the reason is unclear: the blood pressure response was unrelated to levels of renin, angiotensin, or the plasma aldosterone-to-renin ratio.
Identify secondary causes of hypertension
Patients should be evaluated for kidney disease, which is the most common secondary medical reason for resistant hypertension. For patients with poor renal function (estimated glomerular filtration rate < 50 mL/minute), hydrochlorothiazide is not effective against hypertension, but chlorthalidone is. In addition, patients with poor renal function should be given loop diuretics such as furosemide two or three times daily, or the long-acting drug torsemide (Demadex) should be used instead.
Genetic variation can cause different rates of metabolism of drugs, contributing to resistant hypertension. Certain people metabolize hydralazine very fast, making it less effective. The same is true for some beta-blockers.
Obesity and diabetes can also contribute to resistant hypertension.
Ancillary neurohumoral studies are occasionally indicated to rule out identifiable causes of secondary hypertension that may be correctable. There are many identifiable causes of hypertension, but detailing each is beyond the scope of this article.
Patients should be tested for thyroid disease. Hypothyroidism can cause high blood pressure, although usually diastolic rather than systolic hypertension. Hyperthyroidism can cause marked systolic hypertension.
Table 1 provides a step-by-step guide for evaluating and managing patients with resistant hypertension.
EXPERIMENTAL DRUG THERAPY
Endothelin receptor antagonists are currently under investigation for the treatment of resistant hypertension. The protein endothelin-1 (ET-1) is a potent vasoconstrictor (30–50 times more potent than angiotensin II and norepinephrine) and has a long duration of action. ET-1 binds to two receptors with opposing effects: ET-A promotes vasoconstriction, and ET-B promotes vasodilation and clears ET-1.
Darusentan, a selective blocker of ET-A, was tested in the phase III DORADO trial, which was discontinued because the initial results did not meet primary outcome measures. Initial findings had indicated that it might not be as useful as hoped. Side effects included headache, flushing, and edema.
EXPERIMENTAL NONPHARMACOLOGIC THERAPIES
Electrical stimulation of carotid sinus baroreceptors is being tried under the assumption that a high sympathoexcitatory state contributes to resistant hypertension. Devices are placed around the carotid artery bifurcation, and stimulation is believed to increase the depressor influences that modulate blood pressure. Large-scale trials are under way, but it is too early to tell if the approach will be useful. Patients complain of neck pain from the device.
Renal denervation is another experimental approach.12 The kidney has a central role in blood pressure regulation: efferent nerves regulate renal vascular resistance, renal blood flow, and renin release from the juxtaglomerular apparatus; afferent nerves modulate sympathetic output from the central nervous system. The results of the Renal Denervation in Patients With Uncontrolled Hypertension (Symplicity HTN) trials 1 and 2 have been encouraging. The Symplicity HTN-3 trial will begin soon in the United States.
OUR PATIENT UNDERGOES ADDITIONAL STUDIES
To rule out the white-coat effect in our patient, we measured her blood pressure with an automated device that takes several readings without the clinician in the room. (This topic has been reviewed by Vidt et al in this journal13). The average of the automated readings was 183/113 mm Hg, and her average pulse was 109 beats per minute, arguing against a white-coat effect.
Her blood pressure was also markedly elevated (average 198/129) during 24-hour ambulatory blood pressure monitoring.
Findings on physical examination were unremarkable except for grade III hypertensive retinopathy. She had no carotid or abdominal bruits. Her peripheral pulses were strong and synchronous bilaterally.
Laboratory testing found the patient had normal serum electrolyte levels and good renal function but relatively low urinary sodium, 90 mmol/day (normal 40–220), and very low renin activity, 0.7 μg/L/h (normal up-right 0.8–5.8 μg/L/h, supine 0.5–1.8 μg/L/h), calling into question the wisdom of treatment with an angiotensin receptor blocker.
Hemodynamic studies were performed using impedance cardiography and found very high systemic vascular resistance with normal cardiac output, indicating that the patient had a high preload, which could be from hypervolemia or intense venous constriction. It is especially interesting that her vascular resistance was high despite her treatment regimen that included an angiotensin receptor blocker and a vasodilator, perhaps an indication of nonadherence with her medications.
Diuresis reduces her blood pressure
The patient was admitted to the hospital, and because her laboratory results indicated that plasma renin activity was suppressed, the angiotensin receptor blocker valsartan was discontinued.
On day 1, her weight was 162 lb and average blood pressure was 194/128 mm Hg. After 4 days of diuresis with escalating doses of furosemide, her weight was 153 lb and blood pressures ranged from 140 to 158 over 82 to 98 mm Hg. Her heart rate was 90 beats per minute. The hospital stay showed that volume overload was one of the factors maintaining her hypertension. She was discharged on metoprolol succinate (Toprol-XL) 100 mg twice daily and furosemide 80 mg twice daily.
Her blood pressure fluctuates widely after discharge
Over the next 5 days after discharge, the patient’s blood pressure rose steadily to 180/122 mm Hg, her heart rate was in excess of 100 beats per minute, and her weight increased to 158 lb. Blood screening found that the level of metoprolol was undetectable, and a diuretic screen showed no furosemide in the urine. Both the patient and her husband were adamant that she was taking her medications.
Hydrochlorothiazide 25 mg daily was added, and nadolol (Corgard) 80 mg once daily was started in place of metoprolol. On a return visit, her blood pressure and heart rate were finally good at 138/86 mm Hg and 60 beats per minute (sitting) and 134/92 and 63 (standing).
On 24-hour monitoring, some fluctuations of elevated blood pressure were still evident, with an average of 142/91 mm Hg, so nifedipine (Procardia) 60 mg daily was added.
Her final list of medications is hydrochlorothiazide 25 mg, nadolol 80 mg, and nifedipine XL 60 mg, all taken once daily.
Volume overload complicated by nonadherence
In summary, the main pathogenetic mechanism that sustained this patient’s hypertension was volume overload. Her urinary sodium level indicated that she was not taking excessive amounts of sodium. The volume overload may have been a compensatory response to the concomitant use of peripheral vasodilators plus sympatholytic agents.
In addition, she was not adherent to her antihypertensive regimen. The fact that her heart rate was 109 beats per minute despite having a drug regimen that included five sympathetic blocking agents was a strong clue. She eventually admitted that she did not like taking diuretics because they made her skin wrinkle.
In general, in a case like this, I try to minimize the number of drugs and give a diuretic as well as different classes of appropriate drugs.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2007; 51:1403–1419.
- Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment and control of hypertension among United States adults 1999–2004. Hypertension 2007; 49:69–75.
- Sarafidis PA, Li S, Chen SC, et al. Hypertension awareness, treatment, and control in chronic kidney disease. Am J Med 2008; 121:332–340.
- Sarafidis PA, Bakris GL. State of hypertension management in the United States: confluence of risk factors and the prevalence of resistant hypertension. J Clin Hypertens (Greenwich) 2008; 10:130–139.
- Jamerson K, Bakris GL, Dahlöf B, et al; for the ACCOMPLISH Investigators. Exceptional early blood pressure control rates: the ACCOMPLISH trial. Blood Pressure 2007; 16:80–86.
- Dahlöf B, Devereux RB, Kjeldsen S, et al; for the LIFE study group. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–003.
- Cushman WC, Ford CE, Cutler JA, et al; for the ALLHAT Collaborative Research Group. Success and predictors of blood pressure control in diverse North American settings: the Antihypertensive and Lipid-Lowering and Treatment to Prevent Heart Attack Trial (ALLHAT). J Clin Hypertens (Greenwich) 2002; 4:393–404.
- Chapman N, Dobson J, Wilson S, et al; on behalf of the Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 2007; 49:839–845.
- Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al; INVEST Investigators. A calcium antagonist vs a noncalcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003; 290:2805–2816.
- Calhoun DA, Jones D, Textor S, et al. AHA Scientific Statement. Resistant hypertension: diagnosis, evaluation, and treatment. Circulation 2008; 17:e510–e526.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension: guarded optimism. Cleve Clin J Med 2012; 79:501–510.
- Vidt DG, Lang RS, Seballos RJ, Misra-Hebert A, Campbell J, Bena JF. Taking blood pressure: too important to trust to humans? Cleve Clin J Med 2010; 77:683–688.
- Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2007; 51:1403–1419.
- Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment and control of hypertension among United States adults 1999–2004. Hypertension 2007; 49:69–75.
- Sarafidis PA, Li S, Chen SC, et al. Hypertension awareness, treatment, and control in chronic kidney disease. Am J Med 2008; 121:332–340.
- Sarafidis PA, Bakris GL. State of hypertension management in the United States: confluence of risk factors and the prevalence of resistant hypertension. J Clin Hypertens (Greenwich) 2008; 10:130–139.
- Jamerson K, Bakris GL, Dahlöf B, et al; for the ACCOMPLISH Investigators. Exceptional early blood pressure control rates: the ACCOMPLISH trial. Blood Pressure 2007; 16:80–86.
- Dahlöf B, Devereux RB, Kjeldsen S, et al; for the LIFE study group. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–003.
- Cushman WC, Ford CE, Cutler JA, et al; for the ALLHAT Collaborative Research Group. Success and predictors of blood pressure control in diverse North American settings: the Antihypertensive and Lipid-Lowering and Treatment to Prevent Heart Attack Trial (ALLHAT). J Clin Hypertens (Greenwich) 2002; 4:393–404.
- Chapman N, Dobson J, Wilson S, et al; on behalf of the Anglo-Scandinavian Cardiac Outcomes Trial Investigators. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension 2007; 49:839–845.
- Pepine CJ, Handberg EM, Cooper-DeHoff RM, et al; INVEST Investigators. A calcium antagonist vs a noncalcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA 2003; 290:2805–2816.
- Calhoun DA, Jones D, Textor S, et al. AHA Scientific Statement. Resistant hypertension: diagnosis, evaluation, and treatment. Circulation 2008; 17:e510–e526.
- Thomas G, Shishehbor MH, Bravo EL, Nally JV. Renal denervation to treat resistant hypertension: guarded optimism. Cleve Clin J Med 2012; 79:501–510.
- Vidt DG, Lang RS, Seballos RJ, Misra-Hebert A, Campbell J, Bena JF. Taking blood pressure: too important to trust to humans? Cleve Clin J Med 2010; 77:683–688.
KEY POINTS
- Resistant hypertension is arbitrarily divided into two categories: apparent resistance (pseudoresistant hypertension) and true resistance. Apparent resistance is much more common.
- Common causes of true resistant hypertension are volume overload, excessive alcohol use, some drugs (eg, nonsteroidal anti-inflammatory drugs), and some over-the-counter supplements.
- Volume overload commonly results from excess sodium intake, kidney disease, or a counterregulatory response to arterial vasodilation.
- To address volume overload, an appropriate diuretic at an adequate dosage is a cornerstone of therapy, along with potassium supplementation.
- Hospitalization may be needed to monitor drug intake if poor compliance is suspected.
Dermatitis in an intestinal transplant candidate
A 36-year-old woman on total parenteral nutrition because of short-bowel syndrome presented with a 2-week history of skin lesions on the face, arms, and legs, but no fever. Examination revealed prominent vesicular lesions on the left arm (Figure 1), face, palms, and soles. Cultures of biopsy specimens were negative for viral, bacterial, and fungal organisms.
Q: Which is the most likely diagnosis?
- Herpes simplex infection
- Varicella zoster infection
- Coxsackievirus infection
- Micronutrient deficiency
- Pemphigus vulgaris
A: Micronutrient deficiency is most likely the cause of her lesions—specifically, severe zinc deficiency, as she was found to have a serum zinc concentration of 12 μg/dL (reference range 55–150). Biopsy specimens showed characteristic intraepidermal blistering with necrosis and minimal inflammation. Serum levels of other micronutrients (iron, copper, selenium) were normal.
Her total parenteral nutrition regimen contained no zinc. Zinc supplementation was started, and a few days later the lesions began to resolve.
Herpes viral infections can cause similar blistering lesions, but this diagnosis was unlikely given the negative viral culture and direct fluorescence antibody test. Coxsackievirus infection is most often seen in children and typically causes fever and mouth sores, which this patient did not have. Lesions of pemphigus vulgaris typically exhibit the Nikolsky sign, ie, they are flaccid, they rupture easily, and the surrounding superficial skin separates from the deeper layers with rubbing or minor trauma. Our patient’s blisters were tense, with a negative Nikolsky sign, and skin biopsy was not consistent with pemphigus vulgaris.
Dermatitis can result from zinc deficiency, which can occur in conditions that cause severe malnutrition due to malabsorption or reduced dietary intake—eg, inflammatory bowel disease, anorexia nervosa, chronic alcoholism, and cystic fibrosis. The lesions can be complicated by secondary bacterial infection, which can cause significant morbidity. Zinc deficiency can also suppress cell-mediated and humoral immunity.
Zinc deficiency can be diagnosed on the basis of clinical findings, skin biopsy, and serum zinc levels. Other micronutrient deficiencies can coexist and should be ruled out. Perioral and acral skin lesions are typically more prominent. Zinc supplementation usually produces rapid resolution of the lesions.
Our patient’s presentation highlights the importance of monitoring micronutrient levels, including zinc, in patients on long-term total parenteral nutrition. Nutritional deficiencies should be considered as a possible cause of dermatitis in such patients.
- Gehrig KA, Dinulos JG. Acrodermatitis due to nutritional deficiency. Curr Opin Pediatr 2010; 22:107–112.
A 36-year-old woman on total parenteral nutrition because of short-bowel syndrome presented with a 2-week history of skin lesions on the face, arms, and legs, but no fever. Examination revealed prominent vesicular lesions on the left arm (Figure 1), face, palms, and soles. Cultures of biopsy specimens were negative for viral, bacterial, and fungal organisms.
Q: Which is the most likely diagnosis?
- Herpes simplex infection
- Varicella zoster infection
- Coxsackievirus infection
- Micronutrient deficiency
- Pemphigus vulgaris
A: Micronutrient deficiency is most likely the cause of her lesions—specifically, severe zinc deficiency, as she was found to have a serum zinc concentration of 12 μg/dL (reference range 55–150). Biopsy specimens showed characteristic intraepidermal blistering with necrosis and minimal inflammation. Serum levels of other micronutrients (iron, copper, selenium) were normal.
Her total parenteral nutrition regimen contained no zinc. Zinc supplementation was started, and a few days later the lesions began to resolve.
Herpes viral infections can cause similar blistering lesions, but this diagnosis was unlikely given the negative viral culture and direct fluorescence antibody test. Coxsackievirus infection is most often seen in children and typically causes fever and mouth sores, which this patient did not have. Lesions of pemphigus vulgaris typically exhibit the Nikolsky sign, ie, they are flaccid, they rupture easily, and the surrounding superficial skin separates from the deeper layers with rubbing or minor trauma. Our patient’s blisters were tense, with a negative Nikolsky sign, and skin biopsy was not consistent with pemphigus vulgaris.
Dermatitis can result from zinc deficiency, which can occur in conditions that cause severe malnutrition due to malabsorption or reduced dietary intake—eg, inflammatory bowel disease, anorexia nervosa, chronic alcoholism, and cystic fibrosis. The lesions can be complicated by secondary bacterial infection, which can cause significant morbidity. Zinc deficiency can also suppress cell-mediated and humoral immunity.
Zinc deficiency can be diagnosed on the basis of clinical findings, skin biopsy, and serum zinc levels. Other micronutrient deficiencies can coexist and should be ruled out. Perioral and acral skin lesions are typically more prominent. Zinc supplementation usually produces rapid resolution of the lesions.
Our patient’s presentation highlights the importance of monitoring micronutrient levels, including zinc, in patients on long-term total parenteral nutrition. Nutritional deficiencies should be considered as a possible cause of dermatitis in such patients.
A 36-year-old woman on total parenteral nutrition because of short-bowel syndrome presented with a 2-week history of skin lesions on the face, arms, and legs, but no fever. Examination revealed prominent vesicular lesions on the left arm (Figure 1), face, palms, and soles. Cultures of biopsy specimens were negative for viral, bacterial, and fungal organisms.
Q: Which is the most likely diagnosis?
- Herpes simplex infection
- Varicella zoster infection
- Coxsackievirus infection
- Micronutrient deficiency
- Pemphigus vulgaris
A: Micronutrient deficiency is most likely the cause of her lesions—specifically, severe zinc deficiency, as she was found to have a serum zinc concentration of 12 μg/dL (reference range 55–150). Biopsy specimens showed characteristic intraepidermal blistering with necrosis and minimal inflammation. Serum levels of other micronutrients (iron, copper, selenium) were normal.
Her total parenteral nutrition regimen contained no zinc. Zinc supplementation was started, and a few days later the lesions began to resolve.
Herpes viral infections can cause similar blistering lesions, but this diagnosis was unlikely given the negative viral culture and direct fluorescence antibody test. Coxsackievirus infection is most often seen in children and typically causes fever and mouth sores, which this patient did not have. Lesions of pemphigus vulgaris typically exhibit the Nikolsky sign, ie, they are flaccid, they rupture easily, and the surrounding superficial skin separates from the deeper layers with rubbing or minor trauma. Our patient’s blisters were tense, with a negative Nikolsky sign, and skin biopsy was not consistent with pemphigus vulgaris.
Dermatitis can result from zinc deficiency, which can occur in conditions that cause severe malnutrition due to malabsorption or reduced dietary intake—eg, inflammatory bowel disease, anorexia nervosa, chronic alcoholism, and cystic fibrosis. The lesions can be complicated by secondary bacterial infection, which can cause significant morbidity. Zinc deficiency can also suppress cell-mediated and humoral immunity.
Zinc deficiency can be diagnosed on the basis of clinical findings, skin biopsy, and serum zinc levels. Other micronutrient deficiencies can coexist and should be ruled out. Perioral and acral skin lesions are typically more prominent. Zinc supplementation usually produces rapid resolution of the lesions.
Our patient’s presentation highlights the importance of monitoring micronutrient levels, including zinc, in patients on long-term total parenteral nutrition. Nutritional deficiencies should be considered as a possible cause of dermatitis in such patients.
- Gehrig KA, Dinulos JG. Acrodermatitis due to nutritional deficiency. Curr Opin Pediatr 2010; 22:107–112.
- Gehrig KA, Dinulos JG. Acrodermatitis due to nutritional deficiency. Curr Opin Pediatr 2010; 22:107–112.
It’s all in the P wave
A 49-year-old man with rheumatic mitral valve stenosis, which had been diagnosed 3 years previously, presented to the outpatient department with worsening exertional dyspnea, fatigue, and cough.
At rest, he appeared comfortable; his pulse rate was 94 bpm and his blood pressure was 117/82 mm Hg. Cardiac auscultation revealed a loud first heart sound, a mid-diastolic murmur with presystolic accentuation at the cardiac apex, and a pansystolic murmur at the left lower sternal border that increased in intensity with inspiration. A prominent left parasternal heave was present.
His 12-lead electrocardiogram is shown in Figure 1.
Transthoracic echocardiography confirmed severe mitral stenosis with an estimated mitral valve area of 0.7 cm2 without significant mitral regurgitation. In addition, right ventricular dilatation with moderately severe systolic dysfunction and 4+ (severe) tricuspid regurgitation were present. On the basis of the peak tricuspid regurgitant velocity, the right ventricular systolic pressure was calculated to be 80 mm Hg, consistent with severe pulmonary hypertension. The left ventricular end-diastolic volume was reduced and the ejection fraction was normal.
On right heart catheterization, the pulmonary artery pressure was 92/51 mm Hg.
Q: Electrocardiographic findings that support a diagnosis of pulmonary hypertension include which of the following?
- QRS complex axis of +110°
- R/S (QRS complex) ratio greater than 1 in lead V1
- Sum of the amplitudes of the R wave in lead V1 and the S wave in lead V6 greater than 1.0 mV
- All of the above
A: The correct answer is all of the above. Regardless of the cause, patients with long-standing pulmonary hypertension possess varying degrees of right ventricular hypertrophy that may be accompanied by right ventricular enlargement and systolic dysfunction. A QRS complex axis of 110° or more, an R/S (QRS complex) ratio greater than 1 in lead V1, and the sum of the amplitudes of the R wave in lead V1 and the S wave in lead V6 greater than 1.0 mV all support right ventricular hypertrophy.1
As noted in this electrocardiogram, T-wave inversion in leads V1 and V2 supports a right ventricular repolarization abnormality secondary to the hypertrophy.2
Q: Important electrocardiographic findings in this patient that support secondary pulmonary hypertension due to mitral stenosis include which of the following?
- Tall peaked P waves in lead II of at least 0.25 mV and positive P waves in V1 greater than 0.15 mV
- Prolonged P waves of at least 120 ms in lead II and terminal negative P waves in V1 greater than 40 ms
- Right ventricular hypertrophy
- All of the above
A: The correct answer is prolonged P waves of at least 120 ms in lead II and terminal negative P waves in V1 greater than 40 ms.
Abnormal surface electrocardiographic findings reflecting atrial enlargement or slowed atrial conduction are difficult to differentiate and are best characterized as “atrial abnormalities.” On surface electrocardiography, an atrial abnormality is represented by a P wave morphology that is best studied in leads II and V1. In lead II, a tall peaked P wave of at least 0.25 mV supports right atrial abnormality, and a prolonged P wave (≥ 120 ms) supports left atrial abnormality. In lead V1, right atrial abnormality is suggested by a positive P wave in V1 greater than 0.15 mV, and a terminally negative P wave greater than 40 ms in duration and greater than 0.1 mV deep supports left atrial abnormality.3
It is well recognized that the pathophysiology of pulmonary hypertension involves both the right ventricle and the right atrium.4,5 Therefore, irrespective of the cause of pulmonary hypertension, electrocardiography may additionally reveal right atrial abnormality.6
When the findings suggest pulmonary hypertension (ie, right ventricular hypertrophy with or without right atrial abnormality), it is also important to evaluate for concurrent left atrial abnormality. If present, concomitant left atrial abnormality is a valuable, more specific clue that may help characterize secondary pulmonary hypertension from left-sided heart disease, as illustrated in this example with long-standing severe mitral stenosis.2
- Hancock EW, Deal BJ, Mirvis DM, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009; 53:992–1002.
- Goldberger AL. Atrial and ventricular enlargement. In: Clinical Electrocardiography: A Simplified Approach. 7th ed. Philadelphia, PA: Mosby Elsevier; 2006:59–71.
- Bayés-de-Luna A, Goldwasser D, Fiol M, Bayés-Genis A. Surface electrocardiography. In: Hurst’s The Heart. 13th ed. New York, NY: McGraw-Hill Medical; 2011.
- Cioffi G, de Simone G, Mureddu G, Tarantini L, Stefenelli C. Right atrial size and function in patients with pulmonary hypertension associated with disorders of respiratory system or hypoxemia. Eur J Echocardiogr 2007; 8:322–331.
- Raymond RJ, Hinderliter AL, Willis PW, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol 2002; 39:1214–1219.
- Al-Naamani K, Hijal T, Nguyen V, Andrew S, Nguyen T, Huynh T. Predictive values of the electrocardiogram in diagnosing pulmonary hypertension. Int J Cardiol 2008; 127:214–218.
A 49-year-old man with rheumatic mitral valve stenosis, which had been diagnosed 3 years previously, presented to the outpatient department with worsening exertional dyspnea, fatigue, and cough.
At rest, he appeared comfortable; his pulse rate was 94 bpm and his blood pressure was 117/82 mm Hg. Cardiac auscultation revealed a loud first heart sound, a mid-diastolic murmur with presystolic accentuation at the cardiac apex, and a pansystolic murmur at the left lower sternal border that increased in intensity with inspiration. A prominent left parasternal heave was present.
His 12-lead electrocardiogram is shown in Figure 1.
Transthoracic echocardiography confirmed severe mitral stenosis with an estimated mitral valve area of 0.7 cm2 without significant mitral regurgitation. In addition, right ventricular dilatation with moderately severe systolic dysfunction and 4+ (severe) tricuspid regurgitation were present. On the basis of the peak tricuspid regurgitant velocity, the right ventricular systolic pressure was calculated to be 80 mm Hg, consistent with severe pulmonary hypertension. The left ventricular end-diastolic volume was reduced and the ejection fraction was normal.
On right heart catheterization, the pulmonary artery pressure was 92/51 mm Hg.
Q: Electrocardiographic findings that support a diagnosis of pulmonary hypertension include which of the following?
- QRS complex axis of +110°
- R/S (QRS complex) ratio greater than 1 in lead V1
- Sum of the amplitudes of the R wave in lead V1 and the S wave in lead V6 greater than 1.0 mV
- All of the above
A: The correct answer is all of the above. Regardless of the cause, patients with long-standing pulmonary hypertension possess varying degrees of right ventricular hypertrophy that may be accompanied by right ventricular enlargement and systolic dysfunction. A QRS complex axis of 110° or more, an R/S (QRS complex) ratio greater than 1 in lead V1, and the sum of the amplitudes of the R wave in lead V1 and the S wave in lead V6 greater than 1.0 mV all support right ventricular hypertrophy.1
As noted in this electrocardiogram, T-wave inversion in leads V1 and V2 supports a right ventricular repolarization abnormality secondary to the hypertrophy.2
Q: Important electrocardiographic findings in this patient that support secondary pulmonary hypertension due to mitral stenosis include which of the following?
- Tall peaked P waves in lead II of at least 0.25 mV and positive P waves in V1 greater than 0.15 mV
- Prolonged P waves of at least 120 ms in lead II and terminal negative P waves in V1 greater than 40 ms
- Right ventricular hypertrophy
- All of the above
A: The correct answer is prolonged P waves of at least 120 ms in lead II and terminal negative P waves in V1 greater than 40 ms.
Abnormal surface electrocardiographic findings reflecting atrial enlargement or slowed atrial conduction are difficult to differentiate and are best characterized as “atrial abnormalities.” On surface electrocardiography, an atrial abnormality is represented by a P wave morphology that is best studied in leads II and V1. In lead II, a tall peaked P wave of at least 0.25 mV supports right atrial abnormality, and a prolonged P wave (≥ 120 ms) supports left atrial abnormality. In lead V1, right atrial abnormality is suggested by a positive P wave in V1 greater than 0.15 mV, and a terminally negative P wave greater than 40 ms in duration and greater than 0.1 mV deep supports left atrial abnormality.3
It is well recognized that the pathophysiology of pulmonary hypertension involves both the right ventricle and the right atrium.4,5 Therefore, irrespective of the cause of pulmonary hypertension, electrocardiography may additionally reveal right atrial abnormality.6
When the findings suggest pulmonary hypertension (ie, right ventricular hypertrophy with or without right atrial abnormality), it is also important to evaluate for concurrent left atrial abnormality. If present, concomitant left atrial abnormality is a valuable, more specific clue that may help characterize secondary pulmonary hypertension from left-sided heart disease, as illustrated in this example with long-standing severe mitral stenosis.2
A 49-year-old man with rheumatic mitral valve stenosis, which had been diagnosed 3 years previously, presented to the outpatient department with worsening exertional dyspnea, fatigue, and cough.
At rest, he appeared comfortable; his pulse rate was 94 bpm and his blood pressure was 117/82 mm Hg. Cardiac auscultation revealed a loud first heart sound, a mid-diastolic murmur with presystolic accentuation at the cardiac apex, and a pansystolic murmur at the left lower sternal border that increased in intensity with inspiration. A prominent left parasternal heave was present.
His 12-lead electrocardiogram is shown in Figure 1.
Transthoracic echocardiography confirmed severe mitral stenosis with an estimated mitral valve area of 0.7 cm2 without significant mitral regurgitation. In addition, right ventricular dilatation with moderately severe systolic dysfunction and 4+ (severe) tricuspid regurgitation were present. On the basis of the peak tricuspid regurgitant velocity, the right ventricular systolic pressure was calculated to be 80 mm Hg, consistent with severe pulmonary hypertension. The left ventricular end-diastolic volume was reduced and the ejection fraction was normal.
On right heart catheterization, the pulmonary artery pressure was 92/51 mm Hg.
Q: Electrocardiographic findings that support a diagnosis of pulmonary hypertension include which of the following?
- QRS complex axis of +110°
- R/S (QRS complex) ratio greater than 1 in lead V1
- Sum of the amplitudes of the R wave in lead V1 and the S wave in lead V6 greater than 1.0 mV
- All of the above
A: The correct answer is all of the above. Regardless of the cause, patients with long-standing pulmonary hypertension possess varying degrees of right ventricular hypertrophy that may be accompanied by right ventricular enlargement and systolic dysfunction. A QRS complex axis of 110° or more, an R/S (QRS complex) ratio greater than 1 in lead V1, and the sum of the amplitudes of the R wave in lead V1 and the S wave in lead V6 greater than 1.0 mV all support right ventricular hypertrophy.1
As noted in this electrocardiogram, T-wave inversion in leads V1 and V2 supports a right ventricular repolarization abnormality secondary to the hypertrophy.2
Q: Important electrocardiographic findings in this patient that support secondary pulmonary hypertension due to mitral stenosis include which of the following?
- Tall peaked P waves in lead II of at least 0.25 mV and positive P waves in V1 greater than 0.15 mV
- Prolonged P waves of at least 120 ms in lead II and terminal negative P waves in V1 greater than 40 ms
- Right ventricular hypertrophy
- All of the above
A: The correct answer is prolonged P waves of at least 120 ms in lead II and terminal negative P waves in V1 greater than 40 ms.
Abnormal surface electrocardiographic findings reflecting atrial enlargement or slowed atrial conduction are difficult to differentiate and are best characterized as “atrial abnormalities.” On surface electrocardiography, an atrial abnormality is represented by a P wave morphology that is best studied in leads II and V1. In lead II, a tall peaked P wave of at least 0.25 mV supports right atrial abnormality, and a prolonged P wave (≥ 120 ms) supports left atrial abnormality. In lead V1, right atrial abnormality is suggested by a positive P wave in V1 greater than 0.15 mV, and a terminally negative P wave greater than 40 ms in duration and greater than 0.1 mV deep supports left atrial abnormality.3
It is well recognized that the pathophysiology of pulmonary hypertension involves both the right ventricle and the right atrium.4,5 Therefore, irrespective of the cause of pulmonary hypertension, electrocardiography may additionally reveal right atrial abnormality.6
When the findings suggest pulmonary hypertension (ie, right ventricular hypertrophy with or without right atrial abnormality), it is also important to evaluate for concurrent left atrial abnormality. If present, concomitant left atrial abnormality is a valuable, more specific clue that may help characterize secondary pulmonary hypertension from left-sided heart disease, as illustrated in this example with long-standing severe mitral stenosis.2
- Hancock EW, Deal BJ, Mirvis DM, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009; 53:992–1002.
- Goldberger AL. Atrial and ventricular enlargement. In: Clinical Electrocardiography: A Simplified Approach. 7th ed. Philadelphia, PA: Mosby Elsevier; 2006:59–71.
- Bayés-de-Luna A, Goldwasser D, Fiol M, Bayés-Genis A. Surface electrocardiography. In: Hurst’s The Heart. 13th ed. New York, NY: McGraw-Hill Medical; 2011.
- Cioffi G, de Simone G, Mureddu G, Tarantini L, Stefenelli C. Right atrial size and function in patients with pulmonary hypertension associated with disorders of respiratory system or hypoxemia. Eur J Echocardiogr 2007; 8:322–331.
- Raymond RJ, Hinderliter AL, Willis PW, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol 2002; 39:1214–1219.
- Al-Naamani K, Hijal T, Nguyen V, Andrew S, Nguyen T, Huynh T. Predictive values of the electrocardiogram in diagnosing pulmonary hypertension. Int J Cardiol 2008; 127:214–218.
- Hancock EW, Deal BJ, Mirvis DM, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009; 53:992–1002.
- Goldberger AL. Atrial and ventricular enlargement. In: Clinical Electrocardiography: A Simplified Approach. 7th ed. Philadelphia, PA: Mosby Elsevier; 2006:59–71.
- Bayés-de-Luna A, Goldwasser D, Fiol M, Bayés-Genis A. Surface electrocardiography. In: Hurst’s The Heart. 13th ed. New York, NY: McGraw-Hill Medical; 2011.
- Cioffi G, de Simone G, Mureddu G, Tarantini L, Stefenelli C. Right atrial size and function in patients with pulmonary hypertension associated with disorders of respiratory system or hypoxemia. Eur J Echocardiogr 2007; 8:322–331.
- Raymond RJ, Hinderliter AL, Willis PW, et al. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol 2002; 39:1214–1219.
- Al-Naamani K, Hijal T, Nguyen V, Andrew S, Nguyen T, Huynh T. Predictive values of the electrocardiogram in diagnosing pulmonary hypertension. Int J Cardiol 2008; 127:214–218.
Odynophagia, peripheral facial nerve paralysis, mucocutaneous lesions
A 54-year-old woman presented with a 7-day history of odynophagia, pharyngeal swelling, and painful skin lesions on her left ear. She had been on antiretroviral therapy for human immunodeficiency virus infection but had not been fully compliant with the treatment.
On examination, she had painful erythematous vesicles and pustules on the left auricle and in the external auditory canal (Figure 1), as well as small vesicles and circumscribed erosions on the left anterior twothirds of her tongue (Figure 2) and left palate. Facial sensory function was normal; however, she had lagophthalmos, a flattened nasolabial fold, ptosis of the oral commissure, and a loss of the forehead wrinkles on the left side of her face—all signs of peripheral facial nerve paralysis.
Q: Which is the most likely diagnosis?
- Ramsay Hunt syndrome
- Herpes simplex
- Contact dermatitis
- Malignant external otitis
- Erysipelas
A: This patient had Ramsay Hunt syndrome, also known as herpes zoster oticus. It is a rare complication of herpes zoster in which the reactivation of latent varicella-zoster virus infection in the geniculate ganglion causes the triad of ipsilateral facial paralysis, ear pain, and vesicles in the auditory canal and auricle. Taste perception, hearing (eg, tinnitus, hyperacusis), and lacrimation can be affected.1
Ramsay Hunt syndrome is generally considered a polycranial neuropathy of cranial nerves VII (facial) and VIII (acoustic). In some cases other cranial neuropathies may be present and may involve cranial nerves V (trigeminal), IX (glossopharyngeal), and X (vagus). Vestibular disturbances such as vertigo are also often reported. It is more severe in patients with immune deficiency. Because the classic symptoms are not always present at the onset, the syndrome can be misdiagnosed.
DIAGNOSIS
Once the vesicular rash caused by herpes zoster has appeared, the diagnosis is usually readily apparent. The other main disease to consider in the differential diagnosis is herpes simplex. Herpes zoster infection is characterized by a painful sensory prodrome, dermatomal distribution, and lack of a history of a similar rash. However, if the patient has had a similar vesicular rash in the same location, then recurrent zosteriform herpes simplex should be considered. A noninfectious cause to consider is contact dermatitis. However, contact dermatitis usually produces intense itch rather than pain.
If the clinical presentation is uncertain, then viral culture, direct immunofluorescence testing, and a polymerase chain reaction assay is indicated to confirm the diagnosis. Polymerase chain reaction testing is the most sensitive test.3
TREATMENT
The rapid start of antiviral therapy is particularly critical in immunocompromised patients,4 even if the vesicles have been present for 72 hours. Immunocompromised patients with Ramsay Hunt syndrome and other forms of complicated herpes zoster infection should be hospitalized for intravenous acyclovir therapy.
Corticosteroids and oral acyclovir (10 mg/kg three times daily for 7 days) are commonly used in Ramsay Hunt syndrome. In a recent review,5 combination therapy with a corticosteroid and intravenous acyclovir did not show a benefit over corticosteroids alone in promoting resolution of facial neuropathy after 6 months.5 However, randomized clinical trials are needed to evaluate both therapies.
Although antiviral therapy reduces pain associated with acute neuritis, pain syndromes associated with herpes zoster can still be severe. Nonsteroidal antiinflammatory drugs and acetaminophen are useful for mild pain, either alone or in combination with a weak opioid analgesic (eg, tramadol, codeine). For moderate to severe pain that disturbs sleep, a stronger opioid analgesic (eg, oxycodone, morphine) may be necessary.6
Vestibular suppressants may be helpful if vestibular symptoms are severe. Temporary relief of otalgia may be achieved by applying a local anesthetic to the trigger point, if in the external auditory canal. Carbamazepine may be helpful, especially in cases of idiopathic geniculate neuralgia.7
OTHER CONSIDERATIONS
Once drug therapy is started, the patient should be seen at 2 weeks, 6 weeks, and 3 months to monitor the evolution of nerve paralysis.8
- Mishell JH, Applebaum EL. Ramsay-Hunt syndrome in a patient with HIV infection. Otolaryngol Head Neck Surg 1990; 102:177–179.
- Adour KK. Otological complications of herpes zoster. Ann Neurol 1994; 35(suppl):S62–S64.
- Stránská R, Schuurman R, de Vos M, van Loon AM. Routine use of a highly automated and internally controlled real-time PCR assay for the diagnosis of herpes simplex and varicella-zoster virus infections. J Clin Virol 2004; 30:39–44.
- Miller GG, Dummer JS. Herpes simplex and varicella zoster viruses: forgotten but not gone. Am J Transplant 2007; 7:741–747.
- Uscategui T, Dorée C, Chamberlain IJ, Burton MJ. Antiviral therapy for Ramsay Hunt syndrome (herpes zoster oticus with facial palsy) in adults. Cochrane Database Syst Rev 2008;(4):CD006851.
- Dworkin RH, Barbano RL, Tyring SK, et al. A randomized, placebo-controlled trial of oxycodone and of gabapentin for acute pain in herpes zoster. Pain 2009; 142:209–217.
- Edelsberg JS, Lord C, Oster G. Systematic review and meta-analysis of efficacy, safety, and tolerability data from randomized controlled trials of drugs used to treat postherpetic neuralgia. Ann Pharmacother 2011; 45:1483–1490.
- Ryu EW, Lee HY, Lee SY, Park MS, Yeo SG. Clinical manifestations and prognosis of patients with Ramsay Hunt syndrome. Am J Otolaryngol 2012; 33:313–318.
A 54-year-old woman presented with a 7-day history of odynophagia, pharyngeal swelling, and painful skin lesions on her left ear. She had been on antiretroviral therapy for human immunodeficiency virus infection but had not been fully compliant with the treatment.
On examination, she had painful erythematous vesicles and pustules on the left auricle and in the external auditory canal (Figure 1), as well as small vesicles and circumscribed erosions on the left anterior twothirds of her tongue (Figure 2) and left palate. Facial sensory function was normal; however, she had lagophthalmos, a flattened nasolabial fold, ptosis of the oral commissure, and a loss of the forehead wrinkles on the left side of her face—all signs of peripheral facial nerve paralysis.
Q: Which is the most likely diagnosis?
- Ramsay Hunt syndrome
- Herpes simplex
- Contact dermatitis
- Malignant external otitis
- Erysipelas
A: This patient had Ramsay Hunt syndrome, also known as herpes zoster oticus. It is a rare complication of herpes zoster in which the reactivation of latent varicella-zoster virus infection in the geniculate ganglion causes the triad of ipsilateral facial paralysis, ear pain, and vesicles in the auditory canal and auricle. Taste perception, hearing (eg, tinnitus, hyperacusis), and lacrimation can be affected.1
Ramsay Hunt syndrome is generally considered a polycranial neuropathy of cranial nerves VII (facial) and VIII (acoustic). In some cases other cranial neuropathies may be present and may involve cranial nerves V (trigeminal), IX (glossopharyngeal), and X (vagus). Vestibular disturbances such as vertigo are also often reported. It is more severe in patients with immune deficiency. Because the classic symptoms are not always present at the onset, the syndrome can be misdiagnosed.
DIAGNOSIS
Once the vesicular rash caused by herpes zoster has appeared, the diagnosis is usually readily apparent. The other main disease to consider in the differential diagnosis is herpes simplex. Herpes zoster infection is characterized by a painful sensory prodrome, dermatomal distribution, and lack of a history of a similar rash. However, if the patient has had a similar vesicular rash in the same location, then recurrent zosteriform herpes simplex should be considered. A noninfectious cause to consider is contact dermatitis. However, contact dermatitis usually produces intense itch rather than pain.
If the clinical presentation is uncertain, then viral culture, direct immunofluorescence testing, and a polymerase chain reaction assay is indicated to confirm the diagnosis. Polymerase chain reaction testing is the most sensitive test.3
TREATMENT
The rapid start of antiviral therapy is particularly critical in immunocompromised patients,4 even if the vesicles have been present for 72 hours. Immunocompromised patients with Ramsay Hunt syndrome and other forms of complicated herpes zoster infection should be hospitalized for intravenous acyclovir therapy.
Corticosteroids and oral acyclovir (10 mg/kg three times daily for 7 days) are commonly used in Ramsay Hunt syndrome. In a recent review,5 combination therapy with a corticosteroid and intravenous acyclovir did not show a benefit over corticosteroids alone in promoting resolution of facial neuropathy after 6 months.5 However, randomized clinical trials are needed to evaluate both therapies.
Although antiviral therapy reduces pain associated with acute neuritis, pain syndromes associated with herpes zoster can still be severe. Nonsteroidal antiinflammatory drugs and acetaminophen are useful for mild pain, either alone or in combination with a weak opioid analgesic (eg, tramadol, codeine). For moderate to severe pain that disturbs sleep, a stronger opioid analgesic (eg, oxycodone, morphine) may be necessary.6
Vestibular suppressants may be helpful if vestibular symptoms are severe. Temporary relief of otalgia may be achieved by applying a local anesthetic to the trigger point, if in the external auditory canal. Carbamazepine may be helpful, especially in cases of idiopathic geniculate neuralgia.7
OTHER CONSIDERATIONS
Once drug therapy is started, the patient should be seen at 2 weeks, 6 weeks, and 3 months to monitor the evolution of nerve paralysis.8
A 54-year-old woman presented with a 7-day history of odynophagia, pharyngeal swelling, and painful skin lesions on her left ear. She had been on antiretroviral therapy for human immunodeficiency virus infection but had not been fully compliant with the treatment.
On examination, she had painful erythematous vesicles and pustules on the left auricle and in the external auditory canal (Figure 1), as well as small vesicles and circumscribed erosions on the left anterior twothirds of her tongue (Figure 2) and left palate. Facial sensory function was normal; however, she had lagophthalmos, a flattened nasolabial fold, ptosis of the oral commissure, and a loss of the forehead wrinkles on the left side of her face—all signs of peripheral facial nerve paralysis.
Q: Which is the most likely diagnosis?
- Ramsay Hunt syndrome
- Herpes simplex
- Contact dermatitis
- Malignant external otitis
- Erysipelas
A: This patient had Ramsay Hunt syndrome, also known as herpes zoster oticus. It is a rare complication of herpes zoster in which the reactivation of latent varicella-zoster virus infection in the geniculate ganglion causes the triad of ipsilateral facial paralysis, ear pain, and vesicles in the auditory canal and auricle. Taste perception, hearing (eg, tinnitus, hyperacusis), and lacrimation can be affected.1
Ramsay Hunt syndrome is generally considered a polycranial neuropathy of cranial nerves VII (facial) and VIII (acoustic). In some cases other cranial neuropathies may be present and may involve cranial nerves V (trigeminal), IX (glossopharyngeal), and X (vagus). Vestibular disturbances such as vertigo are also often reported. It is more severe in patients with immune deficiency. Because the classic symptoms are not always present at the onset, the syndrome can be misdiagnosed.
DIAGNOSIS
Once the vesicular rash caused by herpes zoster has appeared, the diagnosis is usually readily apparent. The other main disease to consider in the differential diagnosis is herpes simplex. Herpes zoster infection is characterized by a painful sensory prodrome, dermatomal distribution, and lack of a history of a similar rash. However, if the patient has had a similar vesicular rash in the same location, then recurrent zosteriform herpes simplex should be considered. A noninfectious cause to consider is contact dermatitis. However, contact dermatitis usually produces intense itch rather than pain.
If the clinical presentation is uncertain, then viral culture, direct immunofluorescence testing, and a polymerase chain reaction assay is indicated to confirm the diagnosis. Polymerase chain reaction testing is the most sensitive test.3
TREATMENT
The rapid start of antiviral therapy is particularly critical in immunocompromised patients,4 even if the vesicles have been present for 72 hours. Immunocompromised patients with Ramsay Hunt syndrome and other forms of complicated herpes zoster infection should be hospitalized for intravenous acyclovir therapy.
Corticosteroids and oral acyclovir (10 mg/kg three times daily for 7 days) are commonly used in Ramsay Hunt syndrome. In a recent review,5 combination therapy with a corticosteroid and intravenous acyclovir did not show a benefit over corticosteroids alone in promoting resolution of facial neuropathy after 6 months.5 However, randomized clinical trials are needed to evaluate both therapies.
Although antiviral therapy reduces pain associated with acute neuritis, pain syndromes associated with herpes zoster can still be severe. Nonsteroidal antiinflammatory drugs and acetaminophen are useful for mild pain, either alone or in combination with a weak opioid analgesic (eg, tramadol, codeine). For moderate to severe pain that disturbs sleep, a stronger opioid analgesic (eg, oxycodone, morphine) may be necessary.6
Vestibular suppressants may be helpful if vestibular symptoms are severe. Temporary relief of otalgia may be achieved by applying a local anesthetic to the trigger point, if in the external auditory canal. Carbamazepine may be helpful, especially in cases of idiopathic geniculate neuralgia.7
OTHER CONSIDERATIONS
Once drug therapy is started, the patient should be seen at 2 weeks, 6 weeks, and 3 months to monitor the evolution of nerve paralysis.8
- Mishell JH, Applebaum EL. Ramsay-Hunt syndrome in a patient with HIV infection. Otolaryngol Head Neck Surg 1990; 102:177–179.
- Adour KK. Otological complications of herpes zoster. Ann Neurol 1994; 35(suppl):S62–S64.
- Stránská R, Schuurman R, de Vos M, van Loon AM. Routine use of a highly automated and internally controlled real-time PCR assay for the diagnosis of herpes simplex and varicella-zoster virus infections. J Clin Virol 2004; 30:39–44.
- Miller GG, Dummer JS. Herpes simplex and varicella zoster viruses: forgotten but not gone. Am J Transplant 2007; 7:741–747.
- Uscategui T, Dorée C, Chamberlain IJ, Burton MJ. Antiviral therapy for Ramsay Hunt syndrome (herpes zoster oticus with facial palsy) in adults. Cochrane Database Syst Rev 2008;(4):CD006851.
- Dworkin RH, Barbano RL, Tyring SK, et al. A randomized, placebo-controlled trial of oxycodone and of gabapentin for acute pain in herpes zoster. Pain 2009; 142:209–217.
- Edelsberg JS, Lord C, Oster G. Systematic review and meta-analysis of efficacy, safety, and tolerability data from randomized controlled trials of drugs used to treat postherpetic neuralgia. Ann Pharmacother 2011; 45:1483–1490.
- Ryu EW, Lee HY, Lee SY, Park MS, Yeo SG. Clinical manifestations and prognosis of patients with Ramsay Hunt syndrome. Am J Otolaryngol 2012; 33:313–318.
- Mishell JH, Applebaum EL. Ramsay-Hunt syndrome in a patient with HIV infection. Otolaryngol Head Neck Surg 1990; 102:177–179.
- Adour KK. Otological complications of herpes zoster. Ann Neurol 1994; 35(suppl):S62–S64.
- Stránská R, Schuurman R, de Vos M, van Loon AM. Routine use of a highly automated and internally controlled real-time PCR assay for the diagnosis of herpes simplex and varicella-zoster virus infections. J Clin Virol 2004; 30:39–44.
- Miller GG, Dummer JS. Herpes simplex and varicella zoster viruses: forgotten but not gone. Am J Transplant 2007; 7:741–747.
- Uscategui T, Dorée C, Chamberlain IJ, Burton MJ. Antiviral therapy for Ramsay Hunt syndrome (herpes zoster oticus with facial palsy) in adults. Cochrane Database Syst Rev 2008;(4):CD006851.
- Dworkin RH, Barbano RL, Tyring SK, et al. A randomized, placebo-controlled trial of oxycodone and of gabapentin for acute pain in herpes zoster. Pain 2009; 142:209–217.
- Edelsberg JS, Lord C, Oster G. Systematic review and meta-analysis of efficacy, safety, and tolerability data from randomized controlled trials of drugs used to treat postherpetic neuralgia. Ann Pharmacother 2011; 45:1483–1490.
- Ryu EW, Lee HY, Lee SY, Park MS, Yeo SG. Clinical manifestations and prognosis of patients with Ramsay Hunt syndrome. Am J Otolaryngol 2012; 33:313–318.
Functional anatomy of the facial nerve revealed by Ramsay Hunt syndrome
Varicella-zoster virus (VZV) is a highly neurotropic and ubiquitous alpha-herpesvirus. Primary infection causes varicella (chickenpox), after which the virus becomes latent in ganglionic neurons along the entire neuraxis. Reactivation decades later usually results in zoster (shingles), pain with a dermatomal distribution, and rash. Unlike herpes simplex virus type 1 (HSV-1), which becomes latent exclusively in cranial nerve ganglia and reactivates to produce recurrent vesicular lesions around the mouth, and unlike HSV type 2, which becomes latent exclusively in sacral ganglia and reactivates to produce genital herpes, VZV may reactivate from any ganglia to cause zoster anywhere on the body.
Reactivation of VZV from the geniculate (facial nerve) ganglion leads to the Ramsay Hunt syndrome, ie, facial paralysis accompanied by a rash around the ear (zoster oticus). The syndrome is the second most common cause of atraumatic facial paralysis after Bell palsy (idiopathic facial paralysis). Importantly, virus reactivation from the geniculate ganglion may also be accompanied by zoster rash on the hard palate or on the anterior two-thirds of the tongue (Figure 1).1 A rash in any of these three skin or mucosal sites in a patient with facial paralysis indicates geniculate ganglionitis. To his credit, Dr. J. Ramsay Hunt recognized that although there is no somatic sensory facial branch to the oropharynx or tongue, virus can still spread from a seventh cranial nerve element to the pharynx or, via special sensory fibers, to the tongue, thus providing an anatomic explanation for zoster rash in patients with facial paralysis (geniculate zoster) not only around the ear, but also on the hard palate or on the anterior two-thirds of the tongue.2
In geniculate ganglionitis, a rash is usually seen in one but not all three of these skin and mucosal sites. Yet in this issue of the Cleveland Clinic Journal of Medicine, Grillo et al3 describe a patient with facial palsy and rash in all three sites. This remarkable finding underscores the importance of distinguishing Ramsay Hunt syndrome from Bell palsy by checking for rash on the ear, tongue, and hard palate in any patient with acute unilateral peripheral facial weakness. Ramsay Hunt syndrome results from active VZV replication in the geniculate ganglion and requires treatment with antiviral drugs, whereas Bell palsy is usually treated with steroids. Steroid treatment of Ramsay Hunt syndrome misdiagnosed as Bell palsy can potentiate the viral infection. This may partially explain why the outcome of facial paralysis in Ramsay Hunt syndrome is not as good as in idiopathic Bell palsy, in which more than 70% of patients recover full facial function.
Although only cranial nerve VII (facial) was involved in their patient, Grillo et al correctly noted the frequent involvement of other cranial nerves in Ramsay Hunt syndrome. For example, dizziness, vertigo, or hearing loss indicative of involvement of cranial nerve VIII (acoustic) is most likely due to the close proximity of the geniculate ganglion and facial nerve to the vestibulocochlear nerve in the bony facial canal. Patients with this syndrome may also develop dysarthria or dysphagia indicative of lower cranial nerve involvement, reflecting the shared derivation of the facial, glossopharyngeal, and vagus nerves from the same branchial arch. Magnetic resonance imaging, not usually performed in patients with Ramsay Hunt syndrome, may show enhancement in the geniculate ganglion as well as in the intracanalicular and tympanic segments of the facial nerve during its course through the facial canal.
The report by Grillo et al comes at an auspicious time, 100 years after an enlightening series of papers by Dr. Hunt from 1907 to 1915 in which he described herpetic inflammation of the geniculate ganglion,4 the sensory system of the facial nerve,5 and ultimately the syndrome that bears his name.2,6 Dr. Hunt received his doctorate from the University of Pennsylvania in 1893 and later became instructor at Cornell University School of Medicine. In 1924, he became full professor at Columbia University School of Medicine. A clinician of Olympian stature, he is also credited with describing two additional syndromes (clinical features produced by carotid artery occlusion and dyssynergia cerebellaris progressiva), although the best known is zoster oticus with peripheral facial palsy.
Importantly, some patients develop peripheral facial paralysis without any rash but with a fourfold rise in antibody to VZV or in association with the presence of VZV DNA in auricular skin, blood mononuclear cells, middle ear fluid, or saliva, indicating that a proportion of patients with Bell palsy have “Ramsay Hunt syndrome zoster sine herpete” or, more accurately, “geniculate zoster sine herpete.” Treatment of such patients with acyclovir-prednisone within 7 days of onset has been shown to improve the outcome of facial palsy.
Because it is now clear that geniculate ganglionitis may present with facial palsy and zoster rash in any or all of three sites, it may be time to call peripheral facial paralysis associated with zoster rash on the ear, tongue, or palate exactly what it is: geniculate zoster. After all, zoster rash on the face is called trigeminal zoster, and zoster rash on the chest is called thoracic zoster. Most important, however, is the recognition that facial paralysis in association with rash on the ear, tongue, or hard palate reflects geniculate zoster and requires immediate antiviral treatment.
- Sweeney CJ, Gilden DH. Ramsay Hunt syndrome. J Neurol Neurosurg Psychiatry 2001; 71:149–154.
- Hunt JR. The symptom-complex of the acute posterior poliomyelitis of the geniculate, auditory, glossopharyngeal and pneumogastric ganglia. Arch Intern Med 1910; 5:631–675.
- Grillo E, Miguel-Morrondo A, Vano-Galvan S, Jaen P. A 54-year-old woman with odynophagia, peripheral facial nerve paralysis and mucocutaneous lesions. Cleve Clin J Med 2013; 80:76–77.
- Hunt JR. On herpetic inflammations of the geniculate ganglion: a new syndrome and its complications. J Nerv Ment Dis 1907; 34:73–96.
- Hunt JR. The sensory system of the facial nerve and its symptomatology. J Nerv Ment Dis 1909; 36:321–350.
- Hunt JR. The sensory field of the facial nerve: a further contribution to the symptomatology of the geniculate ganglion. Brain 1915; 38:418–446.
Varicella-zoster virus (VZV) is a highly neurotropic and ubiquitous alpha-herpesvirus. Primary infection causes varicella (chickenpox), after which the virus becomes latent in ganglionic neurons along the entire neuraxis. Reactivation decades later usually results in zoster (shingles), pain with a dermatomal distribution, and rash. Unlike herpes simplex virus type 1 (HSV-1), which becomes latent exclusively in cranial nerve ganglia and reactivates to produce recurrent vesicular lesions around the mouth, and unlike HSV type 2, which becomes latent exclusively in sacral ganglia and reactivates to produce genital herpes, VZV may reactivate from any ganglia to cause zoster anywhere on the body.
Reactivation of VZV from the geniculate (facial nerve) ganglion leads to the Ramsay Hunt syndrome, ie, facial paralysis accompanied by a rash around the ear (zoster oticus). The syndrome is the second most common cause of atraumatic facial paralysis after Bell palsy (idiopathic facial paralysis). Importantly, virus reactivation from the geniculate ganglion may also be accompanied by zoster rash on the hard palate or on the anterior two-thirds of the tongue (Figure 1).1 A rash in any of these three skin or mucosal sites in a patient with facial paralysis indicates geniculate ganglionitis. To his credit, Dr. J. Ramsay Hunt recognized that although there is no somatic sensory facial branch to the oropharynx or tongue, virus can still spread from a seventh cranial nerve element to the pharynx or, via special sensory fibers, to the tongue, thus providing an anatomic explanation for zoster rash in patients with facial paralysis (geniculate zoster) not only around the ear, but also on the hard palate or on the anterior two-thirds of the tongue.2

In geniculate ganglionitis, a rash is usually seen in one but not all three of these skin and mucosal sites. Yet in this issue of the Cleveland Clinic Journal of Medicine, Grillo et al3 describe a patient with facial palsy and rash in all three sites. This remarkable finding underscores the importance of distinguishing Ramsay Hunt syndrome from Bell palsy by checking for rash on the ear, tongue, and hard palate in any patient with acute unilateral peripheral facial weakness. Ramsay Hunt syndrome results from active VZV replication in the geniculate ganglion and requires treatment with antiviral drugs, whereas Bell palsy is usually treated with steroids. Steroid treatment of Ramsay Hunt syndrome misdiagnosed as Bell palsy can potentiate the viral infection. This may partially explain why the outcome of facial paralysis in Ramsay Hunt syndrome is not as good as in idiopathic Bell palsy, in which more than 70% of patients recover full facial function.
Although only cranial nerve VII (facial) was involved in their patient, Grillo et al correctly noted the frequent involvement of other cranial nerves in Ramsay Hunt syndrome. For example, dizziness, vertigo, or hearing loss indicative of involvement of cranial nerve VIII (acoustic) is most likely due to the close proximity of the geniculate ganglion and facial nerve to the vestibulocochlear nerve in the bony facial canal. Patients with this syndrome may also develop dysarthria or dysphagia indicative of lower cranial nerve involvement, reflecting the shared derivation of the facial, glossopharyngeal, and vagus nerves from the same branchial arch. Magnetic resonance imaging, not usually performed in patients with Ramsay Hunt syndrome, may show enhancement in the geniculate ganglion as well as in the intracanalicular and tympanic segments of the facial nerve during its course through the facial canal.
The report by Grillo et al comes at an auspicious time, 100 years after an enlightening series of papers by Dr. Hunt from 1907 to 1915 in which he described herpetic inflammation of the geniculate ganglion,4 the sensory system of the facial nerve,5 and ultimately the syndrome that bears his name.2,6 Dr. Hunt received his doctorate from the University of Pennsylvania in 1893 and later became instructor at Cornell University School of Medicine. In 1924, he became full professor at Columbia University School of Medicine. A clinician of Olympian stature, he is also credited with describing two additional syndromes (clinical features produced by carotid artery occlusion and dyssynergia cerebellaris progressiva), although the best known is zoster oticus with peripheral facial palsy.
Importantly, some patients develop peripheral facial paralysis without any rash but with a fourfold rise in antibody to VZV or in association with the presence of VZV DNA in auricular skin, blood mononuclear cells, middle ear fluid, or saliva, indicating that a proportion of patients with Bell palsy have “Ramsay Hunt syndrome zoster sine herpete” or, more accurately, “geniculate zoster sine herpete.” Treatment of such patients with acyclovir-prednisone within 7 days of onset has been shown to improve the outcome of facial palsy.
Because it is now clear that geniculate ganglionitis may present with facial palsy and zoster rash in any or all of three sites, it may be time to call peripheral facial paralysis associated with zoster rash on the ear, tongue, or palate exactly what it is: geniculate zoster. After all, zoster rash on the face is called trigeminal zoster, and zoster rash on the chest is called thoracic zoster. Most important, however, is the recognition that facial paralysis in association with rash on the ear, tongue, or hard palate reflects geniculate zoster and requires immediate antiviral treatment.
Varicella-zoster virus (VZV) is a highly neurotropic and ubiquitous alpha-herpesvirus. Primary infection causes varicella (chickenpox), after which the virus becomes latent in ganglionic neurons along the entire neuraxis. Reactivation decades later usually results in zoster (shingles), pain with a dermatomal distribution, and rash. Unlike herpes simplex virus type 1 (HSV-1), which becomes latent exclusively in cranial nerve ganglia and reactivates to produce recurrent vesicular lesions around the mouth, and unlike HSV type 2, which becomes latent exclusively in sacral ganglia and reactivates to produce genital herpes, VZV may reactivate from any ganglia to cause zoster anywhere on the body.
Reactivation of VZV from the geniculate (facial nerve) ganglion leads to the Ramsay Hunt syndrome, ie, facial paralysis accompanied by a rash around the ear (zoster oticus). The syndrome is the second most common cause of atraumatic facial paralysis after Bell palsy (idiopathic facial paralysis). Importantly, virus reactivation from the geniculate ganglion may also be accompanied by zoster rash on the hard palate or on the anterior two-thirds of the tongue (Figure 1).1 A rash in any of these three skin or mucosal sites in a patient with facial paralysis indicates geniculate ganglionitis. To his credit, Dr. J. Ramsay Hunt recognized that although there is no somatic sensory facial branch to the oropharynx or tongue, virus can still spread from a seventh cranial nerve element to the pharynx or, via special sensory fibers, to the tongue, thus providing an anatomic explanation for zoster rash in patients with facial paralysis (geniculate zoster) not only around the ear, but also on the hard palate or on the anterior two-thirds of the tongue.2

In geniculate ganglionitis, a rash is usually seen in one but not all three of these skin and mucosal sites. Yet in this issue of the Cleveland Clinic Journal of Medicine, Grillo et al3 describe a patient with facial palsy and rash in all three sites. This remarkable finding underscores the importance of distinguishing Ramsay Hunt syndrome from Bell palsy by checking for rash on the ear, tongue, and hard palate in any patient with acute unilateral peripheral facial weakness. Ramsay Hunt syndrome results from active VZV replication in the geniculate ganglion and requires treatment with antiviral drugs, whereas Bell palsy is usually treated with steroids. Steroid treatment of Ramsay Hunt syndrome misdiagnosed as Bell palsy can potentiate the viral infection. This may partially explain why the outcome of facial paralysis in Ramsay Hunt syndrome is not as good as in idiopathic Bell palsy, in which more than 70% of patients recover full facial function.
Although only cranial nerve VII (facial) was involved in their patient, Grillo et al correctly noted the frequent involvement of other cranial nerves in Ramsay Hunt syndrome. For example, dizziness, vertigo, or hearing loss indicative of involvement of cranial nerve VIII (acoustic) is most likely due to the close proximity of the geniculate ganglion and facial nerve to the vestibulocochlear nerve in the bony facial canal. Patients with this syndrome may also develop dysarthria or dysphagia indicative of lower cranial nerve involvement, reflecting the shared derivation of the facial, glossopharyngeal, and vagus nerves from the same branchial arch. Magnetic resonance imaging, not usually performed in patients with Ramsay Hunt syndrome, may show enhancement in the geniculate ganglion as well as in the intracanalicular and tympanic segments of the facial nerve during its course through the facial canal.
The report by Grillo et al comes at an auspicious time, 100 years after an enlightening series of papers by Dr. Hunt from 1907 to 1915 in which he described herpetic inflammation of the geniculate ganglion,4 the sensory system of the facial nerve,5 and ultimately the syndrome that bears his name.2,6 Dr. Hunt received his doctorate from the University of Pennsylvania in 1893 and later became instructor at Cornell University School of Medicine. In 1924, he became full professor at Columbia University School of Medicine. A clinician of Olympian stature, he is also credited with describing two additional syndromes (clinical features produced by carotid artery occlusion and dyssynergia cerebellaris progressiva), although the best known is zoster oticus with peripheral facial palsy.
Importantly, some patients develop peripheral facial paralysis without any rash but with a fourfold rise in antibody to VZV or in association with the presence of VZV DNA in auricular skin, blood mononuclear cells, middle ear fluid, or saliva, indicating that a proportion of patients with Bell palsy have “Ramsay Hunt syndrome zoster sine herpete” or, more accurately, “geniculate zoster sine herpete.” Treatment of such patients with acyclovir-prednisone within 7 days of onset has been shown to improve the outcome of facial palsy.
Because it is now clear that geniculate ganglionitis may present with facial palsy and zoster rash in any or all of three sites, it may be time to call peripheral facial paralysis associated with zoster rash on the ear, tongue, or palate exactly what it is: geniculate zoster. After all, zoster rash on the face is called trigeminal zoster, and zoster rash on the chest is called thoracic zoster. Most important, however, is the recognition that facial paralysis in association with rash on the ear, tongue, or hard palate reflects geniculate zoster and requires immediate antiviral treatment.
- Sweeney CJ, Gilden DH. Ramsay Hunt syndrome. J Neurol Neurosurg Psychiatry 2001; 71:149–154.
- Hunt JR. The symptom-complex of the acute posterior poliomyelitis of the geniculate, auditory, glossopharyngeal and pneumogastric ganglia. Arch Intern Med 1910; 5:631–675.
- Grillo E, Miguel-Morrondo A, Vano-Galvan S, Jaen P. A 54-year-old woman with odynophagia, peripheral facial nerve paralysis and mucocutaneous lesions. Cleve Clin J Med 2013; 80:76–77.
- Hunt JR. On herpetic inflammations of the geniculate ganglion: a new syndrome and its complications. J Nerv Ment Dis 1907; 34:73–96.
- Hunt JR. The sensory system of the facial nerve and its symptomatology. J Nerv Ment Dis 1909; 36:321–350.
- Hunt JR. The sensory field of the facial nerve: a further contribution to the symptomatology of the geniculate ganglion. Brain 1915; 38:418–446.
- Sweeney CJ, Gilden DH. Ramsay Hunt syndrome. J Neurol Neurosurg Psychiatry 2001; 71:149–154.
- Hunt JR. The symptom-complex of the acute posterior poliomyelitis of the geniculate, auditory, glossopharyngeal and pneumogastric ganglia. Arch Intern Med 1910; 5:631–675.
- Grillo E, Miguel-Morrondo A, Vano-Galvan S, Jaen P. A 54-year-old woman with odynophagia, peripheral facial nerve paralysis and mucocutaneous lesions. Cleve Clin J Med 2013; 80:76–77.
- Hunt JR. On herpetic inflammations of the geniculate ganglion: a new syndrome and its complications. J Nerv Ment Dis 1907; 34:73–96.
- Hunt JR. The sensory system of the facial nerve and its symptomatology. J Nerv Ment Dis 1909; 36:321–350.
- Hunt JR. The sensory field of the facial nerve: a further contribution to the symptomatology of the geniculate ganglion. Brain 1915; 38:418–446.
A weirder than weird story, and yet…
At first, the idea of collecting feces, putting it in a blender, and then transferring it into the gastrointestinal (GI) tract of another person might seem to be the creation of a third-grade boy writing a composition on the grossest thing he could think of. And yet, as Agito et al describe in this issue, this very procedure may hold promise for some patients suffering from recurrent and recalcitrant Clostridium difficile infection—and may help open the book on a new area of clinical biology.
The complete story on the biology of primary and recurrent C difficile infection has yet to be fully elaborated. For most patients, the plotline involves an alteration of their resident bacteria by an antibiotic that permits the overgrowth of C difficile, including spore-forming strains that can generate a significant amount of toxin. If the depletion of competitive intestinal bacteria allows for unfettered growth of this toxic bacterium, then it is predictable that replenishing the intestinal microbiome will permit balanced bacterial growth and control of C difficile multiplication.
But the C difficile story is only part of a biologic anthology that is still being written. The microbial biome accounts for probably 90% of the DNA that each of us carries. This microbial DNA, although diverse since it represents nuclear material from many species of bacteria, is not distributed randomly among individuals. There are at least several enterotypes (patterns of gut bacterial ecosystems) that can be identified by molecular techniques. The GI microbiome patterns of couples and household contacts are more similar than would be expected by chance alone, and patterns are seemingly influenced by dietary intake (carnivores differ from vegans) and perhaps by the host’s unique immune responsiveness. Our intestinal microbiome may exert a greater influence on our overall health than we previously thought.
The gut microbiome not only participates in digestion of what we eat and synthesizes some necessary nutritional factors, it also generates small molecules capable of regulating aspects of our systemic immune response. Altering the microbiome, by fecal transplantation or other means, may well contribute to the development or suppression of inflammatory disorders as diverse as spondylitis, atherosclerosis, immune thrombocytopenia, and allergies.
Soon, peptic ulcer disease may not be the only condition treated by therapies directed at bacteria within our GI tract. This is an evolving story that may seem weird but is worth following.
At first, the idea of collecting feces, putting it in a blender, and then transferring it into the gastrointestinal (GI) tract of another person might seem to be the creation of a third-grade boy writing a composition on the grossest thing he could think of. And yet, as Agito et al describe in this issue, this very procedure may hold promise for some patients suffering from recurrent and recalcitrant Clostridium difficile infection—and may help open the book on a new area of clinical biology.
The complete story on the biology of primary and recurrent C difficile infection has yet to be fully elaborated. For most patients, the plotline involves an alteration of their resident bacteria by an antibiotic that permits the overgrowth of C difficile, including spore-forming strains that can generate a significant amount of toxin. If the depletion of competitive intestinal bacteria allows for unfettered growth of this toxic bacterium, then it is predictable that replenishing the intestinal microbiome will permit balanced bacterial growth and control of C difficile multiplication.
But the C difficile story is only part of a biologic anthology that is still being written. The microbial biome accounts for probably 90% of the DNA that each of us carries. This microbial DNA, although diverse since it represents nuclear material from many species of bacteria, is not distributed randomly among individuals. There are at least several enterotypes (patterns of gut bacterial ecosystems) that can be identified by molecular techniques. The GI microbiome patterns of couples and household contacts are more similar than would be expected by chance alone, and patterns are seemingly influenced by dietary intake (carnivores differ from vegans) and perhaps by the host’s unique immune responsiveness. Our intestinal microbiome may exert a greater influence on our overall health than we previously thought.
The gut microbiome not only participates in digestion of what we eat and synthesizes some necessary nutritional factors, it also generates small molecules capable of regulating aspects of our systemic immune response. Altering the microbiome, by fecal transplantation or other means, may well contribute to the development or suppression of inflammatory disorders as diverse as spondylitis, atherosclerosis, immune thrombocytopenia, and allergies.
Soon, peptic ulcer disease may not be the only condition treated by therapies directed at bacteria within our GI tract. This is an evolving story that may seem weird but is worth following.
At first, the idea of collecting feces, putting it in a blender, and then transferring it into the gastrointestinal (GI) tract of another person might seem to be the creation of a third-grade boy writing a composition on the grossest thing he could think of. And yet, as Agito et al describe in this issue, this very procedure may hold promise for some patients suffering from recurrent and recalcitrant Clostridium difficile infection—and may help open the book on a new area of clinical biology.
The complete story on the biology of primary and recurrent C difficile infection has yet to be fully elaborated. For most patients, the plotline involves an alteration of their resident bacteria by an antibiotic that permits the overgrowth of C difficile, including spore-forming strains that can generate a significant amount of toxin. If the depletion of competitive intestinal bacteria allows for unfettered growth of this toxic bacterium, then it is predictable that replenishing the intestinal microbiome will permit balanced bacterial growth and control of C difficile multiplication.
But the C difficile story is only part of a biologic anthology that is still being written. The microbial biome accounts for probably 90% of the DNA that each of us carries. This microbial DNA, although diverse since it represents nuclear material from many species of bacteria, is not distributed randomly among individuals. There are at least several enterotypes (patterns of gut bacterial ecosystems) that can be identified by molecular techniques. The GI microbiome patterns of couples and household contacts are more similar than would be expected by chance alone, and patterns are seemingly influenced by dietary intake (carnivores differ from vegans) and perhaps by the host’s unique immune responsiveness. Our intestinal microbiome may exert a greater influence on our overall health than we previously thought.
The gut microbiome not only participates in digestion of what we eat and synthesizes some necessary nutritional factors, it also generates small molecules capable of regulating aspects of our systemic immune response. Altering the microbiome, by fecal transplantation or other means, may well contribute to the development or suppression of inflammatory disorders as diverse as spondylitis, atherosclerosis, immune thrombocytopenia, and allergies.
Soon, peptic ulcer disease may not be the only condition treated by therapies directed at bacteria within our GI tract. This is an evolving story that may seem weird but is worth following.
Fecal microbiota transplantation for recurrent C difficile infection: Ready for prime time?
If you had a serious disease, would you agree to an alternative treatment that was cheap, safe, and effective—but seemed disgusting? Would you recommend it to patients?
Such a disease is recurrent Clostridium difficile infection, and such a treatment is fecal microbiota transplantation—instillation of blenderized feces from a healthy donor (ideally, the patient’s spouse or “significant other”) into the patient’s colon to restore a healthy population of bacteria.1,2 The rationale behind this procedure is simple: antibiotics and other factors disrupt the normal balance of the colonic flora, allowing C difficile to proliferate, but the imbalance can be corrected by reintroducing the normal flora.1
In this article, we will review how recurrent C difficile infection occurs and the importance of the gut microbiota in resisting colonization with this pathogen. We will also describe the protocol used for fecal microbiota transplantation.
C DIFFICILE INFECTION OFTEN RECURS
C difficile is the most common cause of hospital-acquired diarrhea and an important cause of morbidity and death in hospitalized patients.3,4 The cost of this infection is estimated to be more than $1.1 billion per year and its incidence is rising, partly because of the emergence of more-virulent strains that make treatment of recurrent infection more difficult.5,6
C difficile infection is characterized by diarrhea associated with findings suggestive of pseudomembranous colitis or, in fulminant cases, ileus or megacolon.7 Recurrent C difficile infection is defined as the return of symptoms within 8 weeks after successful treatment.7
C difficile produces two types of toxins. Toxin A is an enterotoxin, causing increased intestinal permeability and fluid secretion, while toxin B is a cytotoxin, causing intense colonic inflammation. People who have a poor host immune response to these toxins tend to develop more diarrhea and colonic inflammation.8
A more virulent strain of C difficile has emerged. Known as BI/NAP1/027, this strain is resistant to quinolones, and it also produces a binary toxin that has a partial gene deletion that allows for increased production of toxins A and B in vitro.9,10 More cases of severe and recurrent C difficile infection have been associated with the increasing number of people infected with this hypervirulent strain.9,10
C difficile infection recurs in about 20% to 30% of cases after antibiotic treatment for it, usually within 30 days, and the risk of a subsequent episode doubles after two or more occurrences.10,11 Metronidazole (Flagyl) and vancomycin are the primary treatments; alternative treatments include fidaxomicin (Dificid), 10 rifaximin (Xifaxan),12 nitazoxanide,13 and tolevamer (a novel polymer that binds C difficile toxins).14
Table 1 summarizes the treatment regimen for C difficile infection in adults, based on clinical practice guidelines from the US Centers for Disease Control and Prevention (CDC).7
THE NORMAL GUT MICROBIOTA KEEPS PATHOGENS OUT
Immediately after birth, the sterile human gut becomes colonized by a diverse community of microorganisms.15 This gut microbiota performs various functions, such as synthesizing vitamin K and vitamin B complex, helping digest food, maintaining the mucosal integrity of the gut, and priming the mucosal immune response to maintain homeostasis of commensal microbiota.16
However, the most important role of the gut microbiota is “colonization resistance” or preventing exogenous or potentially pathogenic organisms from establishing a colony within the gut.17 It involves competition for nutrients and occupation of binding sites on the gut epithelium by indigenous flora.16 Other factors such as the mucosal barrier, salivation, swallowing, gastric acidity, desquamation of mucosal membrane cells, intestinal motility, and secretion of antibodies also play major roles in colonization resistance.17
ANTIBIOTICS DISRUPT THE GUT FLORA
Physical or chemical injuries (the latter by antimicrobial or antineoplastic agents, eg) may disrupt the gut microbiota. In this situation, opportunistic pathogens such as C difficile colonize the gut mucosa, stimulate an immune reaction, and release toxins that cause diarrhea and inflammation.18C difficile will try to compete for nutrients and adhesion sites until it dominates the intestinal tract.
When C difficile spores are ingested, they replicate in the gut and eventually release toxins. Antibiotic therapy may eliminate C difficile bacteria but not the spores; hence, C difficile infection can recur after the antibiotic is discontinued unless the indigenous bacteria can restrain C difficile from spreading.19
HOW DOES FECAL MICROBIOTA TRANSPLANTATION WORK?
Fecal microbiota transplantation involves instilling processed stool that contains essential intestinal bacteria (eg, Bacteroides species) from a healthy screened donor into the diseased gastrointestinal tract of a suitable recipient (Figure 1).1
The aim of this procedure is to reestablish the normal composition of the gut flora, restore balance in metabolism, and stimulate both the acquired and the humoral immune responses in the intestinal mucosa after disruption of the normal flora.20–23 One study showed that patients who have recurrent C difficile infections have fewer protective microorganisms (ie, Firmicutes and Bacteriodetes) in their gut, but after fecal microbiota transplantation their microbiota was found to be similar to that of the donor, and their symptoms promptly resolved.18
STUDIES UP TO NOW
The principle of transplanting donor stool to treat various gastrointestinal diseases has been practiced in veterinary medicine for decades in a process known as transfaunation.24 Fecal microbiota transplantation was first performed in humans in the late 1950s in patients with fulminant pseudomembranous colitis that did not respond to standard antibiotic therapy for C difficile infection.25 Since then, a number of case reports and case series have described instillation of donor stool via nasogastric tube,26 via colonoscope,27–31 and via enema.32 Regardless of the protocols used, disease resolution has been shown in 92% of cases and few adverse effects have been reported, even though transmission of infectious pathogens is theoretically possible.33
A recent multicenter long-term follow-up study34 showed that diarrhea resolved within 90 days after fecal microbiota transplantation in 70 (91%) of 77 patients, while resolution of C difficile infection after a further course of antibiotics with or without repeating fecal microbiota transplantation was seen in 76 (98%) of 77 patients.34 Some patients were reported to have improvement of preexisting allergies, and a few patients developed peripheral neuropathy and autoimmune diseases such as Sjögren syndrome, idiopathic thrombocytopenic purpura, and rheumatoid arthritis.33
As the important role of the gut microbiota in resisting colonization by C difficile is becoming more recognized, scientists are beginning to understand and explore the additional potential benefits of fecal microbiota transplantation on other microbiotarelated dysfunctions.2 The Human Microbiome Project is focusing on characterizing and understanding the role of the microbial components of the human genetic and metabolic landscape in relation to human health and disease.35 Earlier observational studies showed fecal microbiota transplantation to be beneficial in inflammatory bowel disease, 36,37 irritable bowel syndrome,38,39 multiple sclerosis,40 rheumatologic40 and autoimmune diseases,41 and metabolic syndrome,42 likely owing to the role of the microbiota in immunity and energy metabolism. Although these reports may provide insight into the unexplored possibilities of fecal microbiota transplantation, further clinical investigations with randomized controlled trials are still necessary.
THE CURRENT PROTOCOL FOR FECAL MICROBIOTA TRANSPLANTATION
As yet, there is no standardized protocol for fecal microbiota transplantation, since no completed randomized trial supporting its efficacy and safety has been published. However, a group of experts in infectious disease and gastroenterology have published a formal standard practice guideline,19 as summarized below.
Primary indications for fecal microbiota transplantation
- Recurrent C difficile infection—at least three episodes of mild to moderate C difficile infection and failure of a 6- to 8-week taper with vancomycin with or without an alternative antibiotic such as rifaximin or nitazoxanide, or at least two episodes of severe C difficile infection resulting in hospitalization and associated with significant morbidity
- Mild to moderate C difficile infection not responding to standard therapy for at least 1 week
- Severe or fulminant C difficile colitis that has not responded to standard therapy after 48 hours.
Who is a likely donor?
The gut microbiota is continuously replenished with bacteria from the environment in which we live, and we constantly acquire organisms from people who live in that same environment. Hence, the preferred donor is someone who has intimate physical contact with the recipient.33,43,44 The preferred stool donor (in order of preference) is a spouse or significant partner, a family household member, or any other healthy donor.26,36
Who should not be a donor?
It is the responsibility of the physician performing the fecal microbiota transplantation to make sure that the possibility of transmitting disease to the recipient is minimized. Extensive history-taking and physical examination must never be omitted, since not all diseases or conditions can be detected by laboratory screening alone, especially if testing was done during the early stage or window period of a given disease.19 Nevertheless, the donor’s blood and stool should be screened for transmissible diseases such as human immunodeficiency virus (HIV), hepatitis, syphilis, enteric bacteria, parasites, and C difficile.
The recipient has the option to be tested for transmissible diseases such as HIV and hepatitis in order to avoid future questions about transmission after fecal microbiota transplantation. A positive screening test must always be verified with confirmatory testing.19
Table 2 summarizes the exclusion criteria and screening tests performed for donors according to the practice guidelines for fecal microbiota transplantation formulated by Bakken et al.19
Preprocedure instructions and stool preparation
The physician should orient both the donor and recipient regarding “do’s and don’ts” before fecal microbiota transplantation. Table 3 summarizes the preprocedure instructions and steps for stool preparation.
Route of administration
The route of administration may vary depending on the clinical situation. Upper-gastrointestinal administration is performed via nasogastric or nasojejunal tube or gastroscopy. Lower-gastrointestinal administration is performed via colonoscopy (the route of choice) or retention enema.
The upper-gastrointestinal route (nasogastric tube, jejunal catheter, or gastroscope). The nasogastric or nasojejunal tube or gastroscope is inserted into the upper-gastrointestinal tract, and positioning is confirmed by radiography. From 25 to 50 mL of stool suspension is drawn up in a syringe and instilled into the tubing followed by flushing with 25 mL of normal saline.26 Immediately after instillation, the tube is removed and the patient is allowed to go home and continue with his or her usual diet.
This approach is easier to perform, costs less, and poses lower risk of intestinal perforation than the colonoscopic approach. Disadvantages include the possibility that stool suspension may not reach distal areas of the colon, especially in patients with ileus and small-bowel obstruction. There is also a higher risk of bacterial overgrowth in elderly patients who have lower gastric acid levels.33
The lower-gastrointestinal route (colonoscopy, retention enema). Colonoscopy is currently considered the first-line approach for fecal microbiota transplantation.45 After giving informed consent, the patient undergoes standard colonoscopy under sedation. An initial colonoscopic examination is performed, and biopsy specimans are obtained if necessary. Approximately 20 mL of stool suspension is drawn up in a syringe and injected via the biopsy channel of the colonoscope every 5 to 10 cm as the scope is withdrawn, for a total volume of 250 to 500 mL.19,27 The patient should be advised to refrain from defecating for 30 to 45 minutes after fecal microbiota transplantation.46
This approach allows direct visualization of the entire colon, allowing instillation of stool suspension in certain areas where C difficile may predominate or hide (eg, in diverticuli).27,47 One disadvantage to this route of administration is the risk of colon perforation, especially if the patient has toxic colitis.
Instillation via retention enema may be done at home with a standard enema kit.32 Disadvantages include the need for multiple instillations over 3 to 5 days,36 back-leakage of stool suspension causing discomfort to patients, and stool suspension reaching only to the splenic flexure.48
MEASUREMENT OF OUTCOME
Fecal microbiota transplantation is considered successful if symptoms resolve and there is no relapse within 8 weeks. Testing for C difficile in asymptomatic patients is not recommended since patients can be colonized with C difficile without necessarily developing disease.19 There is currently no consensus on treatment recommendations for patients who do not respond to fecal microbiota transplantation, although some reports showed resolution of diarrhea after a repeat 2-week standard course of oral vancomycin26 or repeated instillation of feces collected from new donors.49
IS IT READY FOR PRIME TIME?
Fecal microbiota transplantation has been used primarily as an alternative treatment for recurrent C difficile infection, although other indications for its use are currently being identified and studied. This procedure is now being done in several specialized centers in the United States and abroad, and although the protocol may vary by institution, the clinical outcomes have been consistently promising.
The Fecal Therapy to Eliminate Associated Long-standing Diarrhea (FECAL) trial, currently underway, is the first randomized trial to assess the efficacy of fecal microbiota transplantation for treatment of recurrent C difficile infection.50 Clinical trials such as this one should satisfy our doubts about the efficacy of fecal microbiota transplantation and hopefully pave the way for its application in the near future.
An increasing number of patients are learning to overcome the “yuck factor” associated with fecal microbiota transplantation once they understand its safety and benefits.51 Moreover, the Human Microbiome Project is attempting to identify specific organisms in stool that may specifically treat C difficile infection, hence eliminating the need for whole-stool transplantation in the near future. Although fecal microbiota transplantation is still in its infancy, its low cost, safety, and effectiveness in treating recurrent C difficile infection will likely lead to the procedure becoming widely adopted in mainstream clinical practice.
Editor’s note: On January 16, 2013, after this article was completed, a randomized controlled trial of fecal microbiota transplantation was published in the New England Journal of Medicine. That trial, “Duodenal infusion of donor feces for recurrent Clostridium difficile,” found: “The infusion of donor feces was significantly more effective for the treatment of recurrent C difficile infection than the use of vancomycin.” The study is available online at http://www.nejm.org/doi/full/10.1056/NEJMoa1205037 (subscription required).
- Brandt L, Reddy S. Fecal microbiota transplantation for recurrent Clostridium difficile infection. J Clin Gastroenterol 2011; 45(suppl):S159–S167.
- Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol 2011; 9:88–96.
- Lipp MJ, Nero DC, Callahan MA. The impact of hospital-acquired Clostridium difficile. J Gastroenterol Hepatol 2012; 27:1733–1737.
- Kyne L, Sougioultzis S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol 2002; 23:653–659.
- Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis 2002; 34:346–353.
- Gorbach SL. Antibiotics and Clostridium difficile. N Engl J Med 1999; 341:1690–1691.
- Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–455.
- Beales IL. Intravenous immunoglobulin for recurrent Clostridium difficile diarrhoea. Gut 2002; 51:456.
- O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology 2009; 136:1913–1924.
- Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–431.
- Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med 2008; 359:1932–1940.
- Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis 2007; 44:846–848.
- Musher DM, Logan N, Hamill RJ, et al Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis 2006; 43:421–427.
- Louie TJ, Peppe J, Watt CK, et al. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile-associated diarrhea. Clin Infect Dis 2006; 43:411–420.
- Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher JH. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol 2011; 9:27–38.
- Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol 1996; 4:430–435.
- Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother 1994; 38:409–414.
- Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol 2010; 44:354–360.
- Bakken JS, Borody T, Brandt LJ, et al; Fecal Microbiota Transplantation Workgroup. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011; 9:1044–1049.
- Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–307.
- McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994; 271:1913–1918.
- Neish AS, Gewirtz AT, Rao AS, et al. Non-pathogenic bacteria may block epithelial responses: Attenuation of IKB ubiquitination as a novel, physiologic mode of antiinflammation. Gastroenterology 2000; 118:A3754.
- Helwig U, Rizzello F, Cifone G, et al. Elevated IL-10 levels in pouch-tissue after probiotic therapy. Immunol Lett. 1999; 69:159.
- Rager KD, George LW, House JK, DePeters EJ. Evaluation of rumen transfaunation after surgical correction of left-sided displacement of the abomasum in cows. J Am Vet Med Assoc 2004; 225:915–920.
- Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958; 44:854–859.
- Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis 2003; 36:580–585.
- Yoon SS, Brandt LJ. Treatment of refractory/recurrent C. difficile-associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol 2010; 44:562–566.
- Mattila E, Uusitalo-Seppälä R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology 2012; 142:490–496.
- Garborg K, Waagsbø B, Stallemo A, Matre J, Sundøy A. Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea. Scand J Infect Dis 2010; 42:857–861.
- Mellow MH, Kanatzar A. Colonoscopic fecal bacteriotherapy in the treatment of recurrent Clostridium difficile infection–results and follow-up. J Okla State Med Assoc 2011; 104:89–91.
- Rohlke F, Surawicz CM, Stollman N. Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol 2010; 44:567–570.
- Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol 2010; 8:471–473.
- Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 2011; 53:994–1002.
- Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol 2012; 107:1079–1087.
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 2007; 449:804–810.
- Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol 2003; 37:42–47.
- Borody TJ, Torres M, Campbell J, et al. Reversal of inflammatory bowel disease (IBD) with recurrent fecal microbiota transplants (FMT). Am J Gastroenterol 2011; 106:S352.
- Andrews P, Borody TJ, Shortis NP, Thompson S. Bacteriotherapy for chronic constipation—long term follow-up. (abstract). Gastroenterology 1995; 108:A563.
- Borody TJ. Bacteriotherapy for chronic fatigue syndrome: a long-term follow up study. Presented at the 1995 Chronic Fatigue Syndrome National Consensus Conference.
- Borody TJ, Leis S, Campbell J, et al. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS) (abstract). Am J Gastroenterol 2011; 106:S352.
- Borody TJ, Campbell J, Torres M, et al. Reversal of idiopathic thrombocytopenic purpura (ITP) with fecal microbiota transplantation (FMT) (abstract). Am J Gastroenterol 2011; 106:S352.
- Vrieze AF, Holleman MJ, Serlie MT, Ackermans GM, Dallinga-Thie GM, Groen AK. Metabolic effects of transplanting gut microbiota from lean donors to subjects with metabolic syndrome (abstract). Diabetologia 2010; 53:S44.
- Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe 2009; 15:285–289.
- Bjørneklett A. [To repair an ecosystem] (In Norwegian). Tidsskr Nor Laegeforen 1998; 118:1026.
- Brandt LJ, Borody TJ, Campbell J. Endoscopic fecal microbiota transplantation: “first-line” treatment for severe Clostridium difficile infection? J Clin Gastroenterol 2011; 45:655–657.
- Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol 2012; 46:145–149.
- Thanjan AJ, Southern W, Anand N, et al. Is Clostridium difficile infection (CDI) more difficult to eradicate in patients with diverticulosis? (abstract) Am J Gastroenterol 2008; 103:S195.
- Persky SE, Brandt LJ. Treatment of recurrent Clostridium difficile-associated diarrhea by administration of donated stool directly through a colonoscope. Am J Gastroenterol 2000; 95:3283–3285.
- Nieuwdorp M, van Nood E, Speelman P, et al. [Treatment of recurrent Clostridium difficile-associated diarrhoea with a suspension of donor faeces] (In Dutch). Ned Tijdschr Geneeskd 2008; 152:1927–1932.
- van Nood E, Speelman P, Kuijper EJ, Keller JJ. Struggling with recurrent Clostridium difficile infections: is donor faeces the solution? Euro Surveill 2009; 14. doi:pii:19316.
- Kahn SA, Gorawara-Bhat R, Rubin DT. Fecal bacteriotherapy for ulcerative colitis: patients are ready, are we? Inflamm Bowel Dis 2012; 18:676–684.
If you had a serious disease, would you agree to an alternative treatment that was cheap, safe, and effective—but seemed disgusting? Would you recommend it to patients?
Such a disease is recurrent Clostridium difficile infection, and such a treatment is fecal microbiota transplantation—instillation of blenderized feces from a healthy donor (ideally, the patient’s spouse or “significant other”) into the patient’s colon to restore a healthy population of bacteria.1,2 The rationale behind this procedure is simple: antibiotics and other factors disrupt the normal balance of the colonic flora, allowing C difficile to proliferate, but the imbalance can be corrected by reintroducing the normal flora.1
In this article, we will review how recurrent C difficile infection occurs and the importance of the gut microbiota in resisting colonization with this pathogen. We will also describe the protocol used for fecal microbiota transplantation.
C DIFFICILE INFECTION OFTEN RECURS
C difficile is the most common cause of hospital-acquired diarrhea and an important cause of morbidity and death in hospitalized patients.3,4 The cost of this infection is estimated to be more than $1.1 billion per year and its incidence is rising, partly because of the emergence of more-virulent strains that make treatment of recurrent infection more difficult.5,6
C difficile infection is characterized by diarrhea associated with findings suggestive of pseudomembranous colitis or, in fulminant cases, ileus or megacolon.7 Recurrent C difficile infection is defined as the return of symptoms within 8 weeks after successful treatment.7
C difficile produces two types of toxins. Toxin A is an enterotoxin, causing increased intestinal permeability and fluid secretion, while toxin B is a cytotoxin, causing intense colonic inflammation. People who have a poor host immune response to these toxins tend to develop more diarrhea and colonic inflammation.8
A more virulent strain of C difficile has emerged. Known as BI/NAP1/027, this strain is resistant to quinolones, and it also produces a binary toxin that has a partial gene deletion that allows for increased production of toxins A and B in vitro.9,10 More cases of severe and recurrent C difficile infection have been associated with the increasing number of people infected with this hypervirulent strain.9,10
C difficile infection recurs in about 20% to 30% of cases after antibiotic treatment for it, usually within 30 days, and the risk of a subsequent episode doubles after two or more occurrences.10,11 Metronidazole (Flagyl) and vancomycin are the primary treatments; alternative treatments include fidaxomicin (Dificid), 10 rifaximin (Xifaxan),12 nitazoxanide,13 and tolevamer (a novel polymer that binds C difficile toxins).14
Table 1 summarizes the treatment regimen for C difficile infection in adults, based on clinical practice guidelines from the US Centers for Disease Control and Prevention (CDC).7
THE NORMAL GUT MICROBIOTA KEEPS PATHOGENS OUT
Immediately after birth, the sterile human gut becomes colonized by a diverse community of microorganisms.15 This gut microbiota performs various functions, such as synthesizing vitamin K and vitamin B complex, helping digest food, maintaining the mucosal integrity of the gut, and priming the mucosal immune response to maintain homeostasis of commensal microbiota.16
However, the most important role of the gut microbiota is “colonization resistance” or preventing exogenous or potentially pathogenic organisms from establishing a colony within the gut.17 It involves competition for nutrients and occupation of binding sites on the gut epithelium by indigenous flora.16 Other factors such as the mucosal barrier, salivation, swallowing, gastric acidity, desquamation of mucosal membrane cells, intestinal motility, and secretion of antibodies also play major roles in colonization resistance.17
ANTIBIOTICS DISRUPT THE GUT FLORA
Physical or chemical injuries (the latter by antimicrobial or antineoplastic agents, eg) may disrupt the gut microbiota. In this situation, opportunistic pathogens such as C difficile colonize the gut mucosa, stimulate an immune reaction, and release toxins that cause diarrhea and inflammation.18C difficile will try to compete for nutrients and adhesion sites until it dominates the intestinal tract.
When C difficile spores are ingested, they replicate in the gut and eventually release toxins. Antibiotic therapy may eliminate C difficile bacteria but not the spores; hence, C difficile infection can recur after the antibiotic is discontinued unless the indigenous bacteria can restrain C difficile from spreading.19
HOW DOES FECAL MICROBIOTA TRANSPLANTATION WORK?
Fecal microbiota transplantation involves instilling processed stool that contains essential intestinal bacteria (eg, Bacteroides species) from a healthy screened donor into the diseased gastrointestinal tract of a suitable recipient (Figure 1).1
The aim of this procedure is to reestablish the normal composition of the gut flora, restore balance in metabolism, and stimulate both the acquired and the humoral immune responses in the intestinal mucosa after disruption of the normal flora.20–23 One study showed that patients who have recurrent C difficile infections have fewer protective microorganisms (ie, Firmicutes and Bacteriodetes) in their gut, but after fecal microbiota transplantation their microbiota was found to be similar to that of the donor, and their symptoms promptly resolved.18
STUDIES UP TO NOW
The principle of transplanting donor stool to treat various gastrointestinal diseases has been practiced in veterinary medicine for decades in a process known as transfaunation.24 Fecal microbiota transplantation was first performed in humans in the late 1950s in patients with fulminant pseudomembranous colitis that did not respond to standard antibiotic therapy for C difficile infection.25 Since then, a number of case reports and case series have described instillation of donor stool via nasogastric tube,26 via colonoscope,27–31 and via enema.32 Regardless of the protocols used, disease resolution has been shown in 92% of cases and few adverse effects have been reported, even though transmission of infectious pathogens is theoretically possible.33
A recent multicenter long-term follow-up study34 showed that diarrhea resolved within 90 days after fecal microbiota transplantation in 70 (91%) of 77 patients, while resolution of C difficile infection after a further course of antibiotics with or without repeating fecal microbiota transplantation was seen in 76 (98%) of 77 patients.34 Some patients were reported to have improvement of preexisting allergies, and a few patients developed peripheral neuropathy and autoimmune diseases such as Sjögren syndrome, idiopathic thrombocytopenic purpura, and rheumatoid arthritis.33
As the important role of the gut microbiota in resisting colonization by C difficile is becoming more recognized, scientists are beginning to understand and explore the additional potential benefits of fecal microbiota transplantation on other microbiotarelated dysfunctions.2 The Human Microbiome Project is focusing on characterizing and understanding the role of the microbial components of the human genetic and metabolic landscape in relation to human health and disease.35 Earlier observational studies showed fecal microbiota transplantation to be beneficial in inflammatory bowel disease, 36,37 irritable bowel syndrome,38,39 multiple sclerosis,40 rheumatologic40 and autoimmune diseases,41 and metabolic syndrome,42 likely owing to the role of the microbiota in immunity and energy metabolism. Although these reports may provide insight into the unexplored possibilities of fecal microbiota transplantation, further clinical investigations with randomized controlled trials are still necessary.
THE CURRENT PROTOCOL FOR FECAL MICROBIOTA TRANSPLANTATION
As yet, there is no standardized protocol for fecal microbiota transplantation, since no completed randomized trial supporting its efficacy and safety has been published. However, a group of experts in infectious disease and gastroenterology have published a formal standard practice guideline,19 as summarized below.
Primary indications for fecal microbiota transplantation
- Recurrent C difficile infection—at least three episodes of mild to moderate C difficile infection and failure of a 6- to 8-week taper with vancomycin with or without an alternative antibiotic such as rifaximin or nitazoxanide, or at least two episodes of severe C difficile infection resulting in hospitalization and associated with significant morbidity
- Mild to moderate C difficile infection not responding to standard therapy for at least 1 week
- Severe or fulminant C difficile colitis that has not responded to standard therapy after 48 hours.
Who is a likely donor?
The gut microbiota is continuously replenished with bacteria from the environment in which we live, and we constantly acquire organisms from people who live in that same environment. Hence, the preferred donor is someone who has intimate physical contact with the recipient.33,43,44 The preferred stool donor (in order of preference) is a spouse or significant partner, a family household member, or any other healthy donor.26,36
Who should not be a donor?
It is the responsibility of the physician performing the fecal microbiota transplantation to make sure that the possibility of transmitting disease to the recipient is minimized. Extensive history-taking and physical examination must never be omitted, since not all diseases or conditions can be detected by laboratory screening alone, especially if testing was done during the early stage or window period of a given disease.19 Nevertheless, the donor’s blood and stool should be screened for transmissible diseases such as human immunodeficiency virus (HIV), hepatitis, syphilis, enteric bacteria, parasites, and C difficile.
The recipient has the option to be tested for transmissible diseases such as HIV and hepatitis in order to avoid future questions about transmission after fecal microbiota transplantation. A positive screening test must always be verified with confirmatory testing.19
Table 2 summarizes the exclusion criteria and screening tests performed for donors according to the practice guidelines for fecal microbiota transplantation formulated by Bakken et al.19
Preprocedure instructions and stool preparation
The physician should orient both the donor and recipient regarding “do’s and don’ts” before fecal microbiota transplantation. Table 3 summarizes the preprocedure instructions and steps for stool preparation.
Route of administration
The route of administration may vary depending on the clinical situation. Upper-gastrointestinal administration is performed via nasogastric or nasojejunal tube or gastroscopy. Lower-gastrointestinal administration is performed via colonoscopy (the route of choice) or retention enema.
The upper-gastrointestinal route (nasogastric tube, jejunal catheter, or gastroscope). The nasogastric or nasojejunal tube or gastroscope is inserted into the upper-gastrointestinal tract, and positioning is confirmed by radiography. From 25 to 50 mL of stool suspension is drawn up in a syringe and instilled into the tubing followed by flushing with 25 mL of normal saline.26 Immediately after instillation, the tube is removed and the patient is allowed to go home and continue with his or her usual diet.
This approach is easier to perform, costs less, and poses lower risk of intestinal perforation than the colonoscopic approach. Disadvantages include the possibility that stool suspension may not reach distal areas of the colon, especially in patients with ileus and small-bowel obstruction. There is also a higher risk of bacterial overgrowth in elderly patients who have lower gastric acid levels.33
The lower-gastrointestinal route (colonoscopy, retention enema). Colonoscopy is currently considered the first-line approach for fecal microbiota transplantation.45 After giving informed consent, the patient undergoes standard colonoscopy under sedation. An initial colonoscopic examination is performed, and biopsy specimans are obtained if necessary. Approximately 20 mL of stool suspension is drawn up in a syringe and injected via the biopsy channel of the colonoscope every 5 to 10 cm as the scope is withdrawn, for a total volume of 250 to 500 mL.19,27 The patient should be advised to refrain from defecating for 30 to 45 minutes after fecal microbiota transplantation.46
This approach allows direct visualization of the entire colon, allowing instillation of stool suspension in certain areas where C difficile may predominate or hide (eg, in diverticuli).27,47 One disadvantage to this route of administration is the risk of colon perforation, especially if the patient has toxic colitis.
Instillation via retention enema may be done at home with a standard enema kit.32 Disadvantages include the need for multiple instillations over 3 to 5 days,36 back-leakage of stool suspension causing discomfort to patients, and stool suspension reaching only to the splenic flexure.48
MEASUREMENT OF OUTCOME
Fecal microbiota transplantation is considered successful if symptoms resolve and there is no relapse within 8 weeks. Testing for C difficile in asymptomatic patients is not recommended since patients can be colonized with C difficile without necessarily developing disease.19 There is currently no consensus on treatment recommendations for patients who do not respond to fecal microbiota transplantation, although some reports showed resolution of diarrhea after a repeat 2-week standard course of oral vancomycin26 or repeated instillation of feces collected from new donors.49
IS IT READY FOR PRIME TIME?
Fecal microbiota transplantation has been used primarily as an alternative treatment for recurrent C difficile infection, although other indications for its use are currently being identified and studied. This procedure is now being done in several specialized centers in the United States and abroad, and although the protocol may vary by institution, the clinical outcomes have been consistently promising.
The Fecal Therapy to Eliminate Associated Long-standing Diarrhea (FECAL) trial, currently underway, is the first randomized trial to assess the efficacy of fecal microbiota transplantation for treatment of recurrent C difficile infection.50 Clinical trials such as this one should satisfy our doubts about the efficacy of fecal microbiota transplantation and hopefully pave the way for its application in the near future.
An increasing number of patients are learning to overcome the “yuck factor” associated with fecal microbiota transplantation once they understand its safety and benefits.51 Moreover, the Human Microbiome Project is attempting to identify specific organisms in stool that may specifically treat C difficile infection, hence eliminating the need for whole-stool transplantation in the near future. Although fecal microbiota transplantation is still in its infancy, its low cost, safety, and effectiveness in treating recurrent C difficile infection will likely lead to the procedure becoming widely adopted in mainstream clinical practice.
Editor’s note: On January 16, 2013, after this article was completed, a randomized controlled trial of fecal microbiota transplantation was published in the New England Journal of Medicine. That trial, “Duodenal infusion of donor feces for recurrent Clostridium difficile,” found: “The infusion of donor feces was significantly more effective for the treatment of recurrent C difficile infection than the use of vancomycin.” The study is available online at http://www.nejm.org/doi/full/10.1056/NEJMoa1205037 (subscription required).
If you had a serious disease, would you agree to an alternative treatment that was cheap, safe, and effective—but seemed disgusting? Would you recommend it to patients?
Such a disease is recurrent Clostridium difficile infection, and such a treatment is fecal microbiota transplantation—instillation of blenderized feces from a healthy donor (ideally, the patient’s spouse or “significant other”) into the patient’s colon to restore a healthy population of bacteria.1,2 The rationale behind this procedure is simple: antibiotics and other factors disrupt the normal balance of the colonic flora, allowing C difficile to proliferate, but the imbalance can be corrected by reintroducing the normal flora.1
In this article, we will review how recurrent C difficile infection occurs and the importance of the gut microbiota in resisting colonization with this pathogen. We will also describe the protocol used for fecal microbiota transplantation.
C DIFFICILE INFECTION OFTEN RECURS
C difficile is the most common cause of hospital-acquired diarrhea and an important cause of morbidity and death in hospitalized patients.3,4 The cost of this infection is estimated to be more than $1.1 billion per year and its incidence is rising, partly because of the emergence of more-virulent strains that make treatment of recurrent infection more difficult.5,6
C difficile infection is characterized by diarrhea associated with findings suggestive of pseudomembranous colitis or, in fulminant cases, ileus or megacolon.7 Recurrent C difficile infection is defined as the return of symptoms within 8 weeks after successful treatment.7
C difficile produces two types of toxins. Toxin A is an enterotoxin, causing increased intestinal permeability and fluid secretion, while toxin B is a cytotoxin, causing intense colonic inflammation. People who have a poor host immune response to these toxins tend to develop more diarrhea and colonic inflammation.8
A more virulent strain of C difficile has emerged. Known as BI/NAP1/027, this strain is resistant to quinolones, and it also produces a binary toxin that has a partial gene deletion that allows for increased production of toxins A and B in vitro.9,10 More cases of severe and recurrent C difficile infection have been associated with the increasing number of people infected with this hypervirulent strain.9,10
C difficile infection recurs in about 20% to 30% of cases after antibiotic treatment for it, usually within 30 days, and the risk of a subsequent episode doubles after two or more occurrences.10,11 Metronidazole (Flagyl) and vancomycin are the primary treatments; alternative treatments include fidaxomicin (Dificid), 10 rifaximin (Xifaxan),12 nitazoxanide,13 and tolevamer (a novel polymer that binds C difficile toxins).14
Table 1 summarizes the treatment regimen for C difficile infection in adults, based on clinical practice guidelines from the US Centers for Disease Control and Prevention (CDC).7
THE NORMAL GUT MICROBIOTA KEEPS PATHOGENS OUT
Immediately after birth, the sterile human gut becomes colonized by a diverse community of microorganisms.15 This gut microbiota performs various functions, such as synthesizing vitamin K and vitamin B complex, helping digest food, maintaining the mucosal integrity of the gut, and priming the mucosal immune response to maintain homeostasis of commensal microbiota.16
However, the most important role of the gut microbiota is “colonization resistance” or preventing exogenous or potentially pathogenic organisms from establishing a colony within the gut.17 It involves competition for nutrients and occupation of binding sites on the gut epithelium by indigenous flora.16 Other factors such as the mucosal barrier, salivation, swallowing, gastric acidity, desquamation of mucosal membrane cells, intestinal motility, and secretion of antibodies also play major roles in colonization resistance.17
ANTIBIOTICS DISRUPT THE GUT FLORA
Physical or chemical injuries (the latter by antimicrobial or antineoplastic agents, eg) may disrupt the gut microbiota. In this situation, opportunistic pathogens such as C difficile colonize the gut mucosa, stimulate an immune reaction, and release toxins that cause diarrhea and inflammation.18C difficile will try to compete for nutrients and adhesion sites until it dominates the intestinal tract.
When C difficile spores are ingested, they replicate in the gut and eventually release toxins. Antibiotic therapy may eliminate C difficile bacteria but not the spores; hence, C difficile infection can recur after the antibiotic is discontinued unless the indigenous bacteria can restrain C difficile from spreading.19
HOW DOES FECAL MICROBIOTA TRANSPLANTATION WORK?
Fecal microbiota transplantation involves instilling processed stool that contains essential intestinal bacteria (eg, Bacteroides species) from a healthy screened donor into the diseased gastrointestinal tract of a suitable recipient (Figure 1).1
The aim of this procedure is to reestablish the normal composition of the gut flora, restore balance in metabolism, and stimulate both the acquired and the humoral immune responses in the intestinal mucosa after disruption of the normal flora.20–23 One study showed that patients who have recurrent C difficile infections have fewer protective microorganisms (ie, Firmicutes and Bacteriodetes) in their gut, but after fecal microbiota transplantation their microbiota was found to be similar to that of the donor, and their symptoms promptly resolved.18
STUDIES UP TO NOW
The principle of transplanting donor stool to treat various gastrointestinal diseases has been practiced in veterinary medicine for decades in a process known as transfaunation.24 Fecal microbiota transplantation was first performed in humans in the late 1950s in patients with fulminant pseudomembranous colitis that did not respond to standard antibiotic therapy for C difficile infection.25 Since then, a number of case reports and case series have described instillation of donor stool via nasogastric tube,26 via colonoscope,27–31 and via enema.32 Regardless of the protocols used, disease resolution has been shown in 92% of cases and few adverse effects have been reported, even though transmission of infectious pathogens is theoretically possible.33
A recent multicenter long-term follow-up study34 showed that diarrhea resolved within 90 days after fecal microbiota transplantation in 70 (91%) of 77 patients, while resolution of C difficile infection after a further course of antibiotics with or without repeating fecal microbiota transplantation was seen in 76 (98%) of 77 patients.34 Some patients were reported to have improvement of preexisting allergies, and a few patients developed peripheral neuropathy and autoimmune diseases such as Sjögren syndrome, idiopathic thrombocytopenic purpura, and rheumatoid arthritis.33
As the important role of the gut microbiota in resisting colonization by C difficile is becoming more recognized, scientists are beginning to understand and explore the additional potential benefits of fecal microbiota transplantation on other microbiotarelated dysfunctions.2 The Human Microbiome Project is focusing on characterizing and understanding the role of the microbial components of the human genetic and metabolic landscape in relation to human health and disease.35 Earlier observational studies showed fecal microbiota transplantation to be beneficial in inflammatory bowel disease, 36,37 irritable bowel syndrome,38,39 multiple sclerosis,40 rheumatologic40 and autoimmune diseases,41 and metabolic syndrome,42 likely owing to the role of the microbiota in immunity and energy metabolism. Although these reports may provide insight into the unexplored possibilities of fecal microbiota transplantation, further clinical investigations with randomized controlled trials are still necessary.
THE CURRENT PROTOCOL FOR FECAL MICROBIOTA TRANSPLANTATION
As yet, there is no standardized protocol for fecal microbiota transplantation, since no completed randomized trial supporting its efficacy and safety has been published. However, a group of experts in infectious disease and gastroenterology have published a formal standard practice guideline,19 as summarized below.
Primary indications for fecal microbiota transplantation
- Recurrent C difficile infection—at least three episodes of mild to moderate C difficile infection and failure of a 6- to 8-week taper with vancomycin with or without an alternative antibiotic such as rifaximin or nitazoxanide, or at least two episodes of severe C difficile infection resulting in hospitalization and associated with significant morbidity
- Mild to moderate C difficile infection not responding to standard therapy for at least 1 week
- Severe or fulminant C difficile colitis that has not responded to standard therapy after 48 hours.
Who is a likely donor?
The gut microbiota is continuously replenished with bacteria from the environment in which we live, and we constantly acquire organisms from people who live in that same environment. Hence, the preferred donor is someone who has intimate physical contact with the recipient.33,43,44 The preferred stool donor (in order of preference) is a spouse or significant partner, a family household member, or any other healthy donor.26,36
Who should not be a donor?
It is the responsibility of the physician performing the fecal microbiota transplantation to make sure that the possibility of transmitting disease to the recipient is minimized. Extensive history-taking and physical examination must never be omitted, since not all diseases or conditions can be detected by laboratory screening alone, especially if testing was done during the early stage or window period of a given disease.19 Nevertheless, the donor’s blood and stool should be screened for transmissible diseases such as human immunodeficiency virus (HIV), hepatitis, syphilis, enteric bacteria, parasites, and C difficile.
The recipient has the option to be tested for transmissible diseases such as HIV and hepatitis in order to avoid future questions about transmission after fecal microbiota transplantation. A positive screening test must always be verified with confirmatory testing.19
Table 2 summarizes the exclusion criteria and screening tests performed for donors according to the practice guidelines for fecal microbiota transplantation formulated by Bakken et al.19
Preprocedure instructions and stool preparation
The physician should orient both the donor and recipient regarding “do’s and don’ts” before fecal microbiota transplantation. Table 3 summarizes the preprocedure instructions and steps for stool preparation.
Route of administration
The route of administration may vary depending on the clinical situation. Upper-gastrointestinal administration is performed via nasogastric or nasojejunal tube or gastroscopy. Lower-gastrointestinal administration is performed via colonoscopy (the route of choice) or retention enema.
The upper-gastrointestinal route (nasogastric tube, jejunal catheter, or gastroscope). The nasogastric or nasojejunal tube or gastroscope is inserted into the upper-gastrointestinal tract, and positioning is confirmed by radiography. From 25 to 50 mL of stool suspension is drawn up in a syringe and instilled into the tubing followed by flushing with 25 mL of normal saline.26 Immediately after instillation, the tube is removed and the patient is allowed to go home and continue with his or her usual diet.
This approach is easier to perform, costs less, and poses lower risk of intestinal perforation than the colonoscopic approach. Disadvantages include the possibility that stool suspension may not reach distal areas of the colon, especially in patients with ileus and small-bowel obstruction. There is also a higher risk of bacterial overgrowth in elderly patients who have lower gastric acid levels.33
The lower-gastrointestinal route (colonoscopy, retention enema). Colonoscopy is currently considered the first-line approach for fecal microbiota transplantation.45 After giving informed consent, the patient undergoes standard colonoscopy under sedation. An initial colonoscopic examination is performed, and biopsy specimans are obtained if necessary. Approximately 20 mL of stool suspension is drawn up in a syringe and injected via the biopsy channel of the colonoscope every 5 to 10 cm as the scope is withdrawn, for a total volume of 250 to 500 mL.19,27 The patient should be advised to refrain from defecating for 30 to 45 minutes after fecal microbiota transplantation.46
This approach allows direct visualization of the entire colon, allowing instillation of stool suspension in certain areas where C difficile may predominate or hide (eg, in diverticuli).27,47 One disadvantage to this route of administration is the risk of colon perforation, especially if the patient has toxic colitis.
Instillation via retention enema may be done at home with a standard enema kit.32 Disadvantages include the need for multiple instillations over 3 to 5 days,36 back-leakage of stool suspension causing discomfort to patients, and stool suspension reaching only to the splenic flexure.48
MEASUREMENT OF OUTCOME
Fecal microbiota transplantation is considered successful if symptoms resolve and there is no relapse within 8 weeks. Testing for C difficile in asymptomatic patients is not recommended since patients can be colonized with C difficile without necessarily developing disease.19 There is currently no consensus on treatment recommendations for patients who do not respond to fecal microbiota transplantation, although some reports showed resolution of diarrhea after a repeat 2-week standard course of oral vancomycin26 or repeated instillation of feces collected from new donors.49
IS IT READY FOR PRIME TIME?
Fecal microbiota transplantation has been used primarily as an alternative treatment for recurrent C difficile infection, although other indications for its use are currently being identified and studied. This procedure is now being done in several specialized centers in the United States and abroad, and although the protocol may vary by institution, the clinical outcomes have been consistently promising.
The Fecal Therapy to Eliminate Associated Long-standing Diarrhea (FECAL) trial, currently underway, is the first randomized trial to assess the efficacy of fecal microbiota transplantation for treatment of recurrent C difficile infection.50 Clinical trials such as this one should satisfy our doubts about the efficacy of fecal microbiota transplantation and hopefully pave the way for its application in the near future.
An increasing number of patients are learning to overcome the “yuck factor” associated with fecal microbiota transplantation once they understand its safety and benefits.51 Moreover, the Human Microbiome Project is attempting to identify specific organisms in stool that may specifically treat C difficile infection, hence eliminating the need for whole-stool transplantation in the near future. Although fecal microbiota transplantation is still in its infancy, its low cost, safety, and effectiveness in treating recurrent C difficile infection will likely lead to the procedure becoming widely adopted in mainstream clinical practice.
Editor’s note: On January 16, 2013, after this article was completed, a randomized controlled trial of fecal microbiota transplantation was published in the New England Journal of Medicine. That trial, “Duodenal infusion of donor feces for recurrent Clostridium difficile,” found: “The infusion of donor feces was significantly more effective for the treatment of recurrent C difficile infection than the use of vancomycin.” The study is available online at http://www.nejm.org/doi/full/10.1056/NEJMoa1205037 (subscription required).
- Brandt L, Reddy S. Fecal microbiota transplantation for recurrent Clostridium difficile infection. J Clin Gastroenterol 2011; 45(suppl):S159–S167.
- Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol 2011; 9:88–96.
- Lipp MJ, Nero DC, Callahan MA. The impact of hospital-acquired Clostridium difficile. J Gastroenterol Hepatol 2012; 27:1733–1737.
- Kyne L, Sougioultzis S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol 2002; 23:653–659.
- Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis 2002; 34:346–353.
- Gorbach SL. Antibiotics and Clostridium difficile. N Engl J Med 1999; 341:1690–1691.
- Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–455.
- Beales IL. Intravenous immunoglobulin for recurrent Clostridium difficile diarrhoea. Gut 2002; 51:456.
- O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology 2009; 136:1913–1924.
- Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–431.
- Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med 2008; 359:1932–1940.
- Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis 2007; 44:846–848.
- Musher DM, Logan N, Hamill RJ, et al Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis 2006; 43:421–427.
- Louie TJ, Peppe J, Watt CK, et al. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile-associated diarrhea. Clin Infect Dis 2006; 43:411–420.
- Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher JH. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol 2011; 9:27–38.
- Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol 1996; 4:430–435.
- Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother 1994; 38:409–414.
- Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol 2010; 44:354–360.
- Bakken JS, Borody T, Brandt LJ, et al; Fecal Microbiota Transplantation Workgroup. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011; 9:1044–1049.
- Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–307.
- McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994; 271:1913–1918.
- Neish AS, Gewirtz AT, Rao AS, et al. Non-pathogenic bacteria may block epithelial responses: Attenuation of IKB ubiquitination as a novel, physiologic mode of antiinflammation. Gastroenterology 2000; 118:A3754.
- Helwig U, Rizzello F, Cifone G, et al. Elevated IL-10 levels in pouch-tissue after probiotic therapy. Immunol Lett. 1999; 69:159.
- Rager KD, George LW, House JK, DePeters EJ. Evaluation of rumen transfaunation after surgical correction of left-sided displacement of the abomasum in cows. J Am Vet Med Assoc 2004; 225:915–920.
- Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958; 44:854–859.
- Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis 2003; 36:580–585.
- Yoon SS, Brandt LJ. Treatment of refractory/recurrent C. difficile-associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol 2010; 44:562–566.
- Mattila E, Uusitalo-Seppälä R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology 2012; 142:490–496.
- Garborg K, Waagsbø B, Stallemo A, Matre J, Sundøy A. Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea. Scand J Infect Dis 2010; 42:857–861.
- Mellow MH, Kanatzar A. Colonoscopic fecal bacteriotherapy in the treatment of recurrent Clostridium difficile infection–results and follow-up. J Okla State Med Assoc 2011; 104:89–91.
- Rohlke F, Surawicz CM, Stollman N. Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol 2010; 44:567–570.
- Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol 2010; 8:471–473.
- Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 2011; 53:994–1002.
- Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol 2012; 107:1079–1087.
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 2007; 449:804–810.
- Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol 2003; 37:42–47.
- Borody TJ, Torres M, Campbell J, et al. Reversal of inflammatory bowel disease (IBD) with recurrent fecal microbiota transplants (FMT). Am J Gastroenterol 2011; 106:S352.
- Andrews P, Borody TJ, Shortis NP, Thompson S. Bacteriotherapy for chronic constipation—long term follow-up. (abstract). Gastroenterology 1995; 108:A563.
- Borody TJ. Bacteriotherapy for chronic fatigue syndrome: a long-term follow up study. Presented at the 1995 Chronic Fatigue Syndrome National Consensus Conference.
- Borody TJ, Leis S, Campbell J, et al. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS) (abstract). Am J Gastroenterol 2011; 106:S352.
- Borody TJ, Campbell J, Torres M, et al. Reversal of idiopathic thrombocytopenic purpura (ITP) with fecal microbiota transplantation (FMT) (abstract). Am J Gastroenterol 2011; 106:S352.
- Vrieze AF, Holleman MJ, Serlie MT, Ackermans GM, Dallinga-Thie GM, Groen AK. Metabolic effects of transplanting gut microbiota from lean donors to subjects with metabolic syndrome (abstract). Diabetologia 2010; 53:S44.
- Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe 2009; 15:285–289.
- Bjørneklett A. [To repair an ecosystem] (In Norwegian). Tidsskr Nor Laegeforen 1998; 118:1026.
- Brandt LJ, Borody TJ, Campbell J. Endoscopic fecal microbiota transplantation: “first-line” treatment for severe Clostridium difficile infection? J Clin Gastroenterol 2011; 45:655–657.
- Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol 2012; 46:145–149.
- Thanjan AJ, Southern W, Anand N, et al. Is Clostridium difficile infection (CDI) more difficult to eradicate in patients with diverticulosis? (abstract) Am J Gastroenterol 2008; 103:S195.
- Persky SE, Brandt LJ. Treatment of recurrent Clostridium difficile-associated diarrhea by administration of donated stool directly through a colonoscope. Am J Gastroenterol 2000; 95:3283–3285.
- Nieuwdorp M, van Nood E, Speelman P, et al. [Treatment of recurrent Clostridium difficile-associated diarrhoea with a suspension of donor faeces] (In Dutch). Ned Tijdschr Geneeskd 2008; 152:1927–1932.
- van Nood E, Speelman P, Kuijper EJ, Keller JJ. Struggling with recurrent Clostridium difficile infections: is donor faeces the solution? Euro Surveill 2009; 14. doi:pii:19316.
- Kahn SA, Gorawara-Bhat R, Rubin DT. Fecal bacteriotherapy for ulcerative colitis: patients are ready, are we? Inflamm Bowel Dis 2012; 18:676–684.
- Brandt L, Reddy S. Fecal microbiota transplantation for recurrent Clostridium difficile infection. J Clin Gastroenterol 2011; 45(suppl):S159–S167.
- Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol 2011; 9:88–96.
- Lipp MJ, Nero DC, Callahan MA. The impact of hospital-acquired Clostridium difficile. J Gastroenterol Hepatol 2012; 27:1733–1737.
- Kyne L, Sougioultzis S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol 2002; 23:653–659.
- Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis 2002; 34:346–353.
- Gorbach SL. Antibiotics and Clostridium difficile. N Engl J Med 1999; 341:1690–1691.
- Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–455.
- Beales IL. Intravenous immunoglobulin for recurrent Clostridium difficile diarrhoea. Gut 2002; 51:456.
- O’Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology 2009; 136:1913–1924.
- Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364:422–431.
- Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med 2008; 359:1932–1940.
- Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis 2007; 44:846–848.
- Musher DM, Logan N, Hamill RJ, et al Nitazoxanide for the treatment of Clostridium difficile colitis. Clin Infect Dis 2006; 43:421–427.
- Louie TJ, Peppe J, Watt CK, et al. Tolevamer, a novel nonantibiotic polymer, compared with vancomycin in the treatment of mild to moderately severe Clostridium difficile-associated diarrhea. Clin Infect Dis 2006; 43:411–420.
- Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher JH. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol 2011; 9:27–38.
- Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol 1996; 4:430–435.
- Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother 1994; 38:409–414.
- Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol 2010; 44:354–360.
- Bakken JS, Borody T, Brandt LJ, et al; Fecal Microbiota Transplantation Workgroup. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011; 9:1044–1049.
- Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–307.
- McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994; 271:1913–1918.
- Neish AS, Gewirtz AT, Rao AS, et al. Non-pathogenic bacteria may block epithelial responses: Attenuation of IKB ubiquitination as a novel, physiologic mode of antiinflammation. Gastroenterology 2000; 118:A3754.
- Helwig U, Rizzello F, Cifone G, et al. Elevated IL-10 levels in pouch-tissue after probiotic therapy. Immunol Lett. 1999; 69:159.
- Rager KD, George LW, House JK, DePeters EJ. Evaluation of rumen transfaunation after surgical correction of left-sided displacement of the abomasum in cows. J Am Vet Med Assoc 2004; 225:915–920.
- Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958; 44:854–859.
- Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis 2003; 36:580–585.
- Yoon SS, Brandt LJ. Treatment of refractory/recurrent C. difficile-associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol 2010; 44:562–566.
- Mattila E, Uusitalo-Seppälä R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology 2012; 142:490–496.
- Garborg K, Waagsbø B, Stallemo A, Matre J, Sundøy A. Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea. Scand J Infect Dis 2010; 42:857–861.
- Mellow MH, Kanatzar A. Colonoscopic fecal bacteriotherapy in the treatment of recurrent Clostridium difficile infection–results and follow-up. J Okla State Med Assoc 2011; 104:89–91.
- Rohlke F, Surawicz CM, Stollman N. Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol 2010; 44:567–570.
- Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol 2010; 8:471–473.
- Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 2011; 53:994–1002.
- Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol 2012; 107:1079–1087.
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature 2007; 449:804–810.
- Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol 2003; 37:42–47.
- Borody TJ, Torres M, Campbell J, et al. Reversal of inflammatory bowel disease (IBD) with recurrent fecal microbiota transplants (FMT). Am J Gastroenterol 2011; 106:S352.
- Andrews P, Borody TJ, Shortis NP, Thompson S. Bacteriotherapy for chronic constipation—long term follow-up. (abstract). Gastroenterology 1995; 108:A563.
- Borody TJ. Bacteriotherapy for chronic fatigue syndrome: a long-term follow up study. Presented at the 1995 Chronic Fatigue Syndrome National Consensus Conference.
- Borody TJ, Leis S, Campbell J, et al. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS) (abstract). Am J Gastroenterol 2011; 106:S352.
- Borody TJ, Campbell J, Torres M, et al. Reversal of idiopathic thrombocytopenic purpura (ITP) with fecal microbiota transplantation (FMT) (abstract). Am J Gastroenterol 2011; 106:S352.
- Vrieze AF, Holleman MJ, Serlie MT, Ackermans GM, Dallinga-Thie GM, Groen AK. Metabolic effects of transplanting gut microbiota from lean donors to subjects with metabolic syndrome (abstract). Diabetologia 2010; 53:S44.
- Bakken JS. Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe 2009; 15:285–289.
- Bjørneklett A. [To repair an ecosystem] (In Norwegian). Tidsskr Nor Laegeforen 1998; 118:1026.
- Brandt LJ, Borody TJ, Campbell J. Endoscopic fecal microbiota transplantation: “first-line” treatment for severe Clostridium difficile infection? J Clin Gastroenterol 2011; 45:655–657.
- Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol 2012; 46:145–149.
- Thanjan AJ, Southern W, Anand N, et al. Is Clostridium difficile infection (CDI) more difficult to eradicate in patients with diverticulosis? (abstract) Am J Gastroenterol 2008; 103:S195.
- Persky SE, Brandt LJ. Treatment of recurrent Clostridium difficile-associated diarrhea by administration of donated stool directly through a colonoscope. Am J Gastroenterol 2000; 95:3283–3285.
- Nieuwdorp M, van Nood E, Speelman P, et al. [Treatment of recurrent Clostridium difficile-associated diarrhoea with a suspension of donor faeces] (In Dutch). Ned Tijdschr Geneeskd 2008; 152:1927–1932.
- van Nood E, Speelman P, Kuijper EJ, Keller JJ. Struggling with recurrent Clostridium difficile infections: is donor faeces the solution? Euro Surveill 2009; 14. doi:pii:19316.
- Kahn SA, Gorawara-Bhat R, Rubin DT. Fecal bacteriotherapy for ulcerative colitis: patients are ready, are we? Inflamm Bowel Dis 2012; 18:676–684.
KEY POINTS
- Fecal microbiota transplantation involves instilling gut microbiota from a healthy donor into the diseased gut of a patient who has recurrent or recalcitrant episodes of diarrhea despite antibiotic treatment for C difficile infection. The instillation can be done via nasogastric tube, endoscope, or enema.
- Donor screening is necessary to prevent transmission of communicable diseases to the recipient.
- Recently published studies indicate that this procedure is effective for treating recurrent C difficile infection. Randomized clinical trials to assess its efficacy and safety are underway.
- The field of microbiota therapy is rapidly progressing. More physicians are learning to embrace the concept of fecal microbiota transplantation, and patients are beginning to overcome the “yuck factor” and accept its benefits.
New and Noteworthy Information—February
Patients with multiple sclerosis (MS) disease activity have a higher rate of thinning of the ganglion cell/inner plexiform (GCIP) layer of the eye, researchers reported in the January 1 Neurology. Annual rates of GCIP thinning may be highest among patients with new gadolinium-enhancing lesions, new T2 lesions, and disease duration of less than five years. The investigators performed spectral-domain optical coherence tomography scans every six months on 164 patients with MS and 59 healthy controls. The mean follow-up time was 21.1 months. Annual GCIP thinning occurred 42% faster in patients with relapses, 54% faster in patients with new gadolinium-enhanced lesions, and 36% faster in patients with new T2 lesions.
Vaccination with a monovalent AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine does not appear to be associated with an increased risk of epileptic seizures, according to research published in the December 28, 2012, BMJ. Researchers studied 373,398 people with and without epilepsy who had received the vaccine. The primary end point was admission to a hospital or outpatient hospital care with epileptic seizures. The investigators found no increased risk of seizures in patients with epilepsy and a nonsignificantly decreased risk of seizures in persons without epilepsy during the initial seven-day risk period. During the subsequent 23-day risk period, people without epilepsy had a nonsignificantly increased risk of seizures, but patients with epilepsy had no increase in risk of seizures.
Variations in some genes associated with risk for psychiatric disorders may be observed as differences in brain structure in neonates, according to a study published in the January 2 online Cerebral Cortex. Investigators performed automated region-of-interest volumetry and tensor-based morphometry on 272 newborns who had had high-resolution MRI scans. The group found that estrogen receptor alpha (rs9340799) predicted intracranial volume. Polymorphisms in estrogen receptor alpha (rs9340799), as well as in disrupted-in-schizophrenia 1 (DISC1, rs821616), catechol-O-methyltransferase (COMT), neuregulin 1, apolipoprotein E, and brain-derived neurotrophic factor, were significantly associated with local variation in gray matter volume. “The results highlight the importance of prenatal brain development in mediating psychiatric risk,” noted the authors.
Four months after mild traumatic brain injury (TBI), white matter abnormalities may persist in children, even if cognitive symptoms have resolved, according to research published in the December 12, 2012, Journal of Neuroscience. The magnitude and duration of these abnormalities also appear to be greater in children with mild TBI than in adults with mild TBI. Researchers performed fractional anisotropy, axial diffusivity, and radial diffusivity on 15 children with semiacute mild TBI and 15 matched controls. Post-TBI cognitive dysfunction was observed in the domains of attention and processing speed. Increased anisotropy identified patients with pediatric mild TBI with 90% accuracy but was not associated with neuropsychologic deficits. Anisotropic diffusion may provide an objective biomarker of pediatric mild TBI.
The FDA has approved Eliquis (apixaban) for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. In a phase III clinical trial, Eliquis, an oral anticoagulant, reduced the risk of stroke or systemic embolism by 21%, compared with warfarin. The drug primarily reduced the risk of hemorrhagic stroke and ischemic stroke that converted to hemorrhagic stroke, and it also decreased the risks of major bleeding and all-cause mortality, compared with warfarin. Eliquis inhibits Factor Xa, a blood-clotting protein, thus decreasing thrombin generation and blood clots. The recommended dose is 5 mg twice daily. For patients age 80 or older and those who weigh 60 kg or less, the recommended dose is 2.5 mg twice daily. Eliquis is manufactured by Bristol-Myers Squibb (New York City) and comarketed with Pfizer (New York City).
Intermittent fasting, together with a ketogenic diet, may reduce seizures in children with epilepsy to a greater extent than the ketogenic diet alone, investigators reported in the November 30, 2012, online Epilepsy Research. The researchers placed six children with an incomplete response to a ketogenic diet on an intermittent fasting regimen. The children, ages 2 to 7, fasted on alternate days. Four children had transient improvement in seizure control, but they also had hunger-related adverse reactions. Three patients adhered to the combined intermittent fasting and ketogenic diet regimen for two months. The ketogenic diet and intermittent fasting may not share the same anticonvulsant mechanisms, noted the authors.
The available evidence does not support the use of cannabis extract to treat multiple sclerosis (MS), according to a review published in the December 2012 Drug and Therapeutics Bulletin. Researchers concluded that the trial data for nabiximols, a mouth spray for patients with MS containing dronabinol and cannabidiol, were limited. In the trials, which were the basis for the drug’s approval, symptoms decreased in a slightly higher number of patients taking nabiximols, compared with patients taking placebo. The drug was used for relatively short periods (ie, six weeks to four months) in many of these studies, however, and no study compared nabiximols with another active ingredient. One properly designed trial with a sufficient number of patients showed no difference in symptom relief between participants who took nabiximols and those who did not.
Baseline depression was associated with mild cognitive impairment (MCI) and dementia in individuals 65 or older, researchers reported in the December 31, 2012, Archives of Neurology. Depression may coincide with cognitive impairment, but may not precede it, the study authors noted. The investigators studied 2,160 community-dwelling Medicare recipients in New York City. The team defined depression as a score of 4 or more on the Center for Epidemiological Studies Depression scale. MCI, dementia, and progression from MCI to dementia were the study’s main outcome measures. Baseline depression was associated with an increased risk of incident dementia, but not with incident MCI. Participants with MCI and comorbid depression at baseline had a higher risk of progression to dementia, but not Alzheimer’s disease.
Consumption of fructose resulted in a smaller increase in systemic glucose, insulin, and glucagon-like polypeptide 1 levels than consumption of glucose, according to research published in the January 2 JAMA. Glucose ingestion was associated with a significantly greater reduction in hypothalamic cerebral blood flow than fructose ingestion. Researchers performed MRIs of 20 healthy adults at baseline and after ingestion of a glucose or fructose drink. The blinded study had a random-order crossover design. Compared with baseline, glucose ingestion increased functional connectivity between the hypothalamus and the thalamus and striatum. Fructose increased connectivity between the hypothalamus and thalamus, but not the striatum. Fructose reduced regional cerebral blood flow in the thalamus, hippocampus, posterior cingulate cortex, fusiform, and visual cortex.
Research published in the January 7 online Epilepsia provides evidence for a shared genetic susceptibility to epilespsy and migraine with aura. Compared with migraine without aura, the prevalence of migraine with aura was significantly increased among patients with epilepsy who have two or more first-degree relatives with epilepsy. Investigators studied the prevalence of a history of migraine in 730 participants in the Epilepsy Phenome/Genome Project. Eligible participants were 12 or older, had nonacquired focal epilepsy or generalized epilepsy, and had one or more relative epilepsy of unknown cause. The researchers collected information on migraine with and without aura using an instrument validated for individuals 12 and older. The team also interviewed participants about the history of seizure disorders in nonenrolled family members.
Higher exposure to benomyl is associated with an increased risk for Parkinson’s disease, according to an epidemiologic study published in the December 24, 2012, online Proceedings of the National Academy of Sciences. In primary mesencephalic neurons, benomyl exposure inhibits aldehyde dehydrogenase (ALDH) and alters dopamine homeostasis. Investigators tested the effects of benomyl in cell cultures and confirmed that the chemical damaged or destroyed dopaminergic neurons. The researchers also found that benomyl caused the loss of dopaminergic neurons in zebrafish. The ALDH model for Parkinson’s disease etiology may help explain the selective vulnerability of dopaminergic neurons and describe the mechanism through which environmental toxicants contribute to Parkinson’s disease pathogenesis, the authors theorized.
Patients with a history of traumatic brain injury (TBI) and loss of consciousness may have an increased risk for future TBI and loss of consciousness, according to a study published in the November 21, 2012, online Journal of Neurology, Neurosurgery, and Psychiatry. Researchers are conducting an ongoing study of 4,225 nondemented adults age 65 and older. Participants are seen every two years, and 14% have reported a lifetime history of TBI and loss of consciousness. Individuals reporting a first injury before age 25 had an adjusted hazard ratio of 2.54 for TBI and loss of consciousness, compared with a hazard ratio of 3.79 for adults with first injury after age 55.
—Erik Greb
Patients with multiple sclerosis (MS) disease activity have a higher rate of thinning of the ganglion cell/inner plexiform (GCIP) layer of the eye, researchers reported in the January 1 Neurology. Annual rates of GCIP thinning may be highest among patients with new gadolinium-enhancing lesions, new T2 lesions, and disease duration of less than five years. The investigators performed spectral-domain optical coherence tomography scans every six months on 164 patients with MS and 59 healthy controls. The mean follow-up time was 21.1 months. Annual GCIP thinning occurred 42% faster in patients with relapses, 54% faster in patients with new gadolinium-enhanced lesions, and 36% faster in patients with new T2 lesions.
Vaccination with a monovalent AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine does not appear to be associated with an increased risk of epileptic seizures, according to research published in the December 28, 2012, BMJ. Researchers studied 373,398 people with and without epilepsy who had received the vaccine. The primary end point was admission to a hospital or outpatient hospital care with epileptic seizures. The investigators found no increased risk of seizures in patients with epilepsy and a nonsignificantly decreased risk of seizures in persons without epilepsy during the initial seven-day risk period. During the subsequent 23-day risk period, people without epilepsy had a nonsignificantly increased risk of seizures, but patients with epilepsy had no increase in risk of seizures.
Variations in some genes associated with risk for psychiatric disorders may be observed as differences in brain structure in neonates, according to a study published in the January 2 online Cerebral Cortex. Investigators performed automated region-of-interest volumetry and tensor-based morphometry on 272 newborns who had had high-resolution MRI scans. The group found that estrogen receptor alpha (rs9340799) predicted intracranial volume. Polymorphisms in estrogen receptor alpha (rs9340799), as well as in disrupted-in-schizophrenia 1 (DISC1, rs821616), catechol-O-methyltransferase (COMT), neuregulin 1, apolipoprotein E, and brain-derived neurotrophic factor, were significantly associated with local variation in gray matter volume. “The results highlight the importance of prenatal brain development in mediating psychiatric risk,” noted the authors.
Four months after mild traumatic brain injury (TBI), white matter abnormalities may persist in children, even if cognitive symptoms have resolved, according to research published in the December 12, 2012, Journal of Neuroscience. The magnitude and duration of these abnormalities also appear to be greater in children with mild TBI than in adults with mild TBI. Researchers performed fractional anisotropy, axial diffusivity, and radial diffusivity on 15 children with semiacute mild TBI and 15 matched controls. Post-TBI cognitive dysfunction was observed in the domains of attention and processing speed. Increased anisotropy identified patients with pediatric mild TBI with 90% accuracy but was not associated with neuropsychologic deficits. Anisotropic diffusion may provide an objective biomarker of pediatric mild TBI.
The FDA has approved Eliquis (apixaban) for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. In a phase III clinical trial, Eliquis, an oral anticoagulant, reduced the risk of stroke or systemic embolism by 21%, compared with warfarin. The drug primarily reduced the risk of hemorrhagic stroke and ischemic stroke that converted to hemorrhagic stroke, and it also decreased the risks of major bleeding and all-cause mortality, compared with warfarin. Eliquis inhibits Factor Xa, a blood-clotting protein, thus decreasing thrombin generation and blood clots. The recommended dose is 5 mg twice daily. For patients age 80 or older and those who weigh 60 kg or less, the recommended dose is 2.5 mg twice daily. Eliquis is manufactured by Bristol-Myers Squibb (New York City) and comarketed with Pfizer (New York City).
Intermittent fasting, together with a ketogenic diet, may reduce seizures in children with epilepsy to a greater extent than the ketogenic diet alone, investigators reported in the November 30, 2012, online Epilepsy Research. The researchers placed six children with an incomplete response to a ketogenic diet on an intermittent fasting regimen. The children, ages 2 to 7, fasted on alternate days. Four children had transient improvement in seizure control, but they also had hunger-related adverse reactions. Three patients adhered to the combined intermittent fasting and ketogenic diet regimen for two months. The ketogenic diet and intermittent fasting may not share the same anticonvulsant mechanisms, noted the authors.
The available evidence does not support the use of cannabis extract to treat multiple sclerosis (MS), according to a review published in the December 2012 Drug and Therapeutics Bulletin. Researchers concluded that the trial data for nabiximols, a mouth spray for patients with MS containing dronabinol and cannabidiol, were limited. In the trials, which were the basis for the drug’s approval, symptoms decreased in a slightly higher number of patients taking nabiximols, compared with patients taking placebo. The drug was used for relatively short periods (ie, six weeks to four months) in many of these studies, however, and no study compared nabiximols with another active ingredient. One properly designed trial with a sufficient number of patients showed no difference in symptom relief between participants who took nabiximols and those who did not.
Baseline depression was associated with mild cognitive impairment (MCI) and dementia in individuals 65 or older, researchers reported in the December 31, 2012, Archives of Neurology. Depression may coincide with cognitive impairment, but may not precede it, the study authors noted. The investigators studied 2,160 community-dwelling Medicare recipients in New York City. The team defined depression as a score of 4 or more on the Center for Epidemiological Studies Depression scale. MCI, dementia, and progression from MCI to dementia were the study’s main outcome measures. Baseline depression was associated with an increased risk of incident dementia, but not with incident MCI. Participants with MCI and comorbid depression at baseline had a higher risk of progression to dementia, but not Alzheimer’s disease.
Consumption of fructose resulted in a smaller increase in systemic glucose, insulin, and glucagon-like polypeptide 1 levels than consumption of glucose, according to research published in the January 2 JAMA. Glucose ingestion was associated with a significantly greater reduction in hypothalamic cerebral blood flow than fructose ingestion. Researchers performed MRIs of 20 healthy adults at baseline and after ingestion of a glucose or fructose drink. The blinded study had a random-order crossover design. Compared with baseline, glucose ingestion increased functional connectivity between the hypothalamus and the thalamus and striatum. Fructose increased connectivity between the hypothalamus and thalamus, but not the striatum. Fructose reduced regional cerebral blood flow in the thalamus, hippocampus, posterior cingulate cortex, fusiform, and visual cortex.
Research published in the January 7 online Epilepsia provides evidence for a shared genetic susceptibility to epilespsy and migraine with aura. Compared with migraine without aura, the prevalence of migraine with aura was significantly increased among patients with epilepsy who have two or more first-degree relatives with epilepsy. Investigators studied the prevalence of a history of migraine in 730 participants in the Epilepsy Phenome/Genome Project. Eligible participants were 12 or older, had nonacquired focal epilepsy or generalized epilepsy, and had one or more relative epilepsy of unknown cause. The researchers collected information on migraine with and without aura using an instrument validated for individuals 12 and older. The team also interviewed participants about the history of seizure disorders in nonenrolled family members.
Higher exposure to benomyl is associated with an increased risk for Parkinson’s disease, according to an epidemiologic study published in the December 24, 2012, online Proceedings of the National Academy of Sciences. In primary mesencephalic neurons, benomyl exposure inhibits aldehyde dehydrogenase (ALDH) and alters dopamine homeostasis. Investigators tested the effects of benomyl in cell cultures and confirmed that the chemical damaged or destroyed dopaminergic neurons. The researchers also found that benomyl caused the loss of dopaminergic neurons in zebrafish. The ALDH model for Parkinson’s disease etiology may help explain the selective vulnerability of dopaminergic neurons and describe the mechanism through which environmental toxicants contribute to Parkinson’s disease pathogenesis, the authors theorized.
Patients with a history of traumatic brain injury (TBI) and loss of consciousness may have an increased risk for future TBI and loss of consciousness, according to a study published in the November 21, 2012, online Journal of Neurology, Neurosurgery, and Psychiatry. Researchers are conducting an ongoing study of 4,225 nondemented adults age 65 and older. Participants are seen every two years, and 14% have reported a lifetime history of TBI and loss of consciousness. Individuals reporting a first injury before age 25 had an adjusted hazard ratio of 2.54 for TBI and loss of consciousness, compared with a hazard ratio of 3.79 for adults with first injury after age 55.
—Erik Greb
Patients with multiple sclerosis (MS) disease activity have a higher rate of thinning of the ganglion cell/inner plexiform (GCIP) layer of the eye, researchers reported in the January 1 Neurology. Annual rates of GCIP thinning may be highest among patients with new gadolinium-enhancing lesions, new T2 lesions, and disease duration of less than five years. The investigators performed spectral-domain optical coherence tomography scans every six months on 164 patients with MS and 59 healthy controls. The mean follow-up time was 21.1 months. Annual GCIP thinning occurred 42% faster in patients with relapses, 54% faster in patients with new gadolinium-enhanced lesions, and 36% faster in patients with new T2 lesions.
Vaccination with a monovalent AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine does not appear to be associated with an increased risk of epileptic seizures, according to research published in the December 28, 2012, BMJ. Researchers studied 373,398 people with and without epilepsy who had received the vaccine. The primary end point was admission to a hospital or outpatient hospital care with epileptic seizures. The investigators found no increased risk of seizures in patients with epilepsy and a nonsignificantly decreased risk of seizures in persons without epilepsy during the initial seven-day risk period. During the subsequent 23-day risk period, people without epilepsy had a nonsignificantly increased risk of seizures, but patients with epilepsy had no increase in risk of seizures.
Variations in some genes associated with risk for psychiatric disorders may be observed as differences in brain structure in neonates, according to a study published in the January 2 online Cerebral Cortex. Investigators performed automated region-of-interest volumetry and tensor-based morphometry on 272 newborns who had had high-resolution MRI scans. The group found that estrogen receptor alpha (rs9340799) predicted intracranial volume. Polymorphisms in estrogen receptor alpha (rs9340799), as well as in disrupted-in-schizophrenia 1 (DISC1, rs821616), catechol-O-methyltransferase (COMT), neuregulin 1, apolipoprotein E, and brain-derived neurotrophic factor, were significantly associated with local variation in gray matter volume. “The results highlight the importance of prenatal brain development in mediating psychiatric risk,” noted the authors.
Four months after mild traumatic brain injury (TBI), white matter abnormalities may persist in children, even if cognitive symptoms have resolved, according to research published in the December 12, 2012, Journal of Neuroscience. The magnitude and duration of these abnormalities also appear to be greater in children with mild TBI than in adults with mild TBI. Researchers performed fractional anisotropy, axial diffusivity, and radial diffusivity on 15 children with semiacute mild TBI and 15 matched controls. Post-TBI cognitive dysfunction was observed in the domains of attention and processing speed. Increased anisotropy identified patients with pediatric mild TBI with 90% accuracy but was not associated with neuropsychologic deficits. Anisotropic diffusion may provide an objective biomarker of pediatric mild TBI.
The FDA has approved Eliquis (apixaban) for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. In a phase III clinical trial, Eliquis, an oral anticoagulant, reduced the risk of stroke or systemic embolism by 21%, compared with warfarin. The drug primarily reduced the risk of hemorrhagic stroke and ischemic stroke that converted to hemorrhagic stroke, and it also decreased the risks of major bleeding and all-cause mortality, compared with warfarin. Eliquis inhibits Factor Xa, a blood-clotting protein, thus decreasing thrombin generation and blood clots. The recommended dose is 5 mg twice daily. For patients age 80 or older and those who weigh 60 kg or less, the recommended dose is 2.5 mg twice daily. Eliquis is manufactured by Bristol-Myers Squibb (New York City) and comarketed with Pfizer (New York City).
Intermittent fasting, together with a ketogenic diet, may reduce seizures in children with epilepsy to a greater extent than the ketogenic diet alone, investigators reported in the November 30, 2012, online Epilepsy Research. The researchers placed six children with an incomplete response to a ketogenic diet on an intermittent fasting regimen. The children, ages 2 to 7, fasted on alternate days. Four children had transient improvement in seizure control, but they also had hunger-related adverse reactions. Three patients adhered to the combined intermittent fasting and ketogenic diet regimen for two months. The ketogenic diet and intermittent fasting may not share the same anticonvulsant mechanisms, noted the authors.
The available evidence does not support the use of cannabis extract to treat multiple sclerosis (MS), according to a review published in the December 2012 Drug and Therapeutics Bulletin. Researchers concluded that the trial data for nabiximols, a mouth spray for patients with MS containing dronabinol and cannabidiol, were limited. In the trials, which were the basis for the drug’s approval, symptoms decreased in a slightly higher number of patients taking nabiximols, compared with patients taking placebo. The drug was used for relatively short periods (ie, six weeks to four months) in many of these studies, however, and no study compared nabiximols with another active ingredient. One properly designed trial with a sufficient number of patients showed no difference in symptom relief between participants who took nabiximols and those who did not.
Baseline depression was associated with mild cognitive impairment (MCI) and dementia in individuals 65 or older, researchers reported in the December 31, 2012, Archives of Neurology. Depression may coincide with cognitive impairment, but may not precede it, the study authors noted. The investigators studied 2,160 community-dwelling Medicare recipients in New York City. The team defined depression as a score of 4 or more on the Center for Epidemiological Studies Depression scale. MCI, dementia, and progression from MCI to dementia were the study’s main outcome measures. Baseline depression was associated with an increased risk of incident dementia, but not with incident MCI. Participants with MCI and comorbid depression at baseline had a higher risk of progression to dementia, but not Alzheimer’s disease.
Consumption of fructose resulted in a smaller increase in systemic glucose, insulin, and glucagon-like polypeptide 1 levels than consumption of glucose, according to research published in the January 2 JAMA. Glucose ingestion was associated with a significantly greater reduction in hypothalamic cerebral blood flow than fructose ingestion. Researchers performed MRIs of 20 healthy adults at baseline and after ingestion of a glucose or fructose drink. The blinded study had a random-order crossover design. Compared with baseline, glucose ingestion increased functional connectivity between the hypothalamus and the thalamus and striatum. Fructose increased connectivity between the hypothalamus and thalamus, but not the striatum. Fructose reduced regional cerebral blood flow in the thalamus, hippocampus, posterior cingulate cortex, fusiform, and visual cortex.
Research published in the January 7 online Epilepsia provides evidence for a shared genetic susceptibility to epilespsy and migraine with aura. Compared with migraine without aura, the prevalence of migraine with aura was significantly increased among patients with epilepsy who have two or more first-degree relatives with epilepsy. Investigators studied the prevalence of a history of migraine in 730 participants in the Epilepsy Phenome/Genome Project. Eligible participants were 12 or older, had nonacquired focal epilepsy or generalized epilepsy, and had one or more relative epilepsy of unknown cause. The researchers collected information on migraine with and without aura using an instrument validated for individuals 12 and older. The team also interviewed participants about the history of seizure disorders in nonenrolled family members.
Higher exposure to benomyl is associated with an increased risk for Parkinson’s disease, according to an epidemiologic study published in the December 24, 2012, online Proceedings of the National Academy of Sciences. In primary mesencephalic neurons, benomyl exposure inhibits aldehyde dehydrogenase (ALDH) and alters dopamine homeostasis. Investigators tested the effects of benomyl in cell cultures and confirmed that the chemical damaged or destroyed dopaminergic neurons. The researchers also found that benomyl caused the loss of dopaminergic neurons in zebrafish. The ALDH model for Parkinson’s disease etiology may help explain the selective vulnerability of dopaminergic neurons and describe the mechanism through which environmental toxicants contribute to Parkinson’s disease pathogenesis, the authors theorized.
Patients with a history of traumatic brain injury (TBI) and loss of consciousness may have an increased risk for future TBI and loss of consciousness, according to a study published in the November 21, 2012, online Journal of Neurology, Neurosurgery, and Psychiatry. Researchers are conducting an ongoing study of 4,225 nondemented adults age 65 and older. Participants are seen every two years, and 14% have reported a lifetime history of TBI and loss of consciousness. Individuals reporting a first injury before age 25 had an adjusted hazard ratio of 2.54 for TBI and loss of consciousness, compared with a hazard ratio of 3.79 for adults with first injury after age 55.
—Erik Greb
Pregnant Woman, 39, With Hypertension and New-Onset Proteinuria
A 39-year-old black woman, gravida 1, para 0, with an intrauterine pregnancy of 34 weeks and three days (according to last menstrual period and nine-week ultrasound) presented to her Ob-Gyn office for a routine prenatal visit. She was found to have an elevated blood pressure with new onset of 2+ proteinuria. The patient was sent to the labor and delivery unit at the adjoining hospital for serial blood pressure readings, laboratory work, and fetal monitoring.
The patient’s previous medical history was limited to sinusitis. She was taking no prescription medications, and her only listed allergy was to pineapple. Initial lab studies revealed elevations in liver enzymes, lactate dehydrogenase (LDH), uric acid, and serum creatinine, as well as thrombocytopenia (see Table 11-5). She also had a critically low blood glucose level, which conflicted with a normal follow-up reading.
At this point, the patient was thought to have HELLP syndrome6 (ie, hemolysis, elevated liver enzymes, low platelet count), or possibly acute fatty liver of pregnancy (AFLP).2,4,7-11 Additional labs were drawn immediately to confirm or rule out AFLP. These included repeat serum glucose (following a second reading with normal results), a serum ammonia level, prothrombin time (PT), and partial thromboplastin time (PTT). The most reliable values to distinguish AFLP from HELLP are profound hypoglycemia (found in 94% of women with AFLP12) and an elevated serum ammonia level.4
Given the serious nature of either diagnosis, immediate delivery of the infant was deemed necessary. Because the patient’s cervix was not found favorable for induction, she underwent low-transverse cesarean delivery without complications. She was noted to have essentially normal anatomy with the exception of a small subserosal fibroid posteriorly. Meconium-stained amniotic fluid was present. A male infant was delivered, weighing 5 lb with 1-minute and 5-minute Apgar scores of 8 and 9, respectively.
Postoperatively, the patient remained in the recovery area, where she received intensive monitoring. She experienced fluctuations in blood glucose, ranging from 33 to 144 mg/dL; she was started on 5% dextrose in lactated Ringer’s solution and treated with IV dextrose 50 g. While the patient was in surgical recovery, results from the second set of labs, drawn before surgery, were returned; findings included an elevated ammonia level and an abnormal coagulation panel, including PT of 25.3 sec, PTT of 48.4 sec, and a fibrinogen level of 116 mg/dL, confirming the suspected diagnosis of AFLP.
Magnesium sulfate, which had been started immediately postop, was discontinued on confirmation of the diagnosis of AFLP. The patient was initially somnolent as a result of general anesthesia but gradually returned to a fully normal sensorium by early morning on postop day 1. Postoperatively, the patient’s hemoglobin was found to be low (8.6 g/dL; reference range, 13.5 to 18.5 g/dL), so she was transfused with two units of packed red blood cells (PRBCs) and given fresh frozen plasma (FFP) to correct this coagulopathy. The patient’s platelets were also low at 82,000/mm3 (reference range, 140,000 to 340,000/mm3).
On postop day 1, the patient’s serum creatinine rose to 4.2 mg/dL and her total bilirubin increased to 14.4 mg/dL (reference ranges, 0.6 to 1.2 mg/dL and < 1.0 mg/dL, respectively). Given the multiple systems affected by AFLP and the need for intensive supportive care, the patient was transferred to the ICU.
On her arrival at the ICU, the patient’s vital signs were initially stable, and she was alert and oriented. However, within the next few hours, she became hypotensive and encephalopathic. She required aggressive fluid resuscitation and multiple transfusions of PRBCs and FFP due to persistent anemia and coagulopathy. Her vital signs were stabilized, but she continued to need blood transfusions.
Postop day 2, the patient became less responsive and was soon unable to follow commands or speak clearly. Her breathing remained stable with just 3 L of oxygen by nasal cannula, but in order to prevent aspiration and in consideration of a postoperative ileus, it was necessary to place a nasogastric tube with low intermittent suction. This produced a bloody return, but no intervention other than close monitoring and transfusion was performed at that time.
Abdominal ultrasound showed ascites and mild left-sided hydronephrosis with no gallstones. The common bile duct measured 3 mm in diameter.
Although liver biopsy is considered the gold standard for a confirmed diagnosis of AFLP,13,14 this procedure was contraindicated by the patient’s coagulopathy. Concern was also expressed by one consultant that the patient might have thrombotic thrombocytopenic purpura (TTP) in addition to AFLP. TTP can manifest with similar findings, such as anemia, thrombocytopenia, neurologic symptoms, and renal abnormalities, but usually fever is involved, and the patient was afebrile. A catheter was placed for hemodialysis and therapeutic plasma exchange (TPE). Given that TTP-associated mortality is significantly decreased by use of TPE,15 this intervention was deemed prudent. The patient underwent TPE on three consecutive days, postop days 2 through 4.
The patient’s mental status began to improve, and by postop day 6, she was able to follow commands and engage in brief conversations. By postop day 9, she had returned almost completely to her baseline mental status.
The patient’s liver function test results and total bilirubin, ammonia, and creatinine levels all improved over the first few postoperative days but began to rise again by day 6. In response to worsening renal and hepatic functioning, the decision was made on postop day 9 to transfer the patient to a hospital with liver transplantation capabilities, should this procedure become necessary.
Discussion
AFLP is a rare condition specific to pregnancy, affecting 1/7,000 to 1/20,000 pregnancies. Due to the low incidence of this disease, randomized controlled trials to study it are not possible. Instead, clinicians must learn either from individual case studies or from retrospective syntheses of cases reported over time.1,2,7 Fortunately, the wealth of information gleaned over the past 30 years has significantly reduced AFLP-associated maternal and fetal mortality and morbidity rates. In the 1980s, maternal and fetal mortality rates as high as 85% were reported.3 Worldwide, maternal mortality associated with AFLP has decreased significantly to 7% to 18%, whereas the fetal mortality rate has fallen to between 9% and 23%.1,16,17
Common trends among women who have developed AFLP include nulliparity, multiparity, and advanced maternal age. One retrospective study of 57 women who had developed AFLP revealed that 35 cases (61%) involved first-time pregnancies. It also showed that 10 (18%) of the women had twins, and 14 (25%) were older than 35.2 In another study of 35 cases of AFLP, 40% of the women were nulliparous, and 11.4% were multiparous, including one triplet gestation.12 In a third, smaller study, 80% of women affected by AFLP were multiparous.10 Currently, there is no known evidence linking any maternal behavior to development of AFLP.
Presentation
Women who present with AFLP often experience vague, nonspecific symptoms, leading to misdiagnosis or delayed diagnosis. Objective measurements, including physical exam findings, laboratory studies, and other diagnostic tests, will help with a diagnosis. The most frequent initial symptoms are nausea and vomiting (in 70% of patients) and abdominal pain (50% to 80%), epigastric or right upper-quadrant.3 Other common symptoms include fatigue, malaise, anorexia, weight gain, polyuria, and polydipsia.2,3,9,18,19
Because the presenting symptoms in AFLP can be vague, clinicians should complete a thorough physical exam to differentiate accurately among conditions associated with pregnancy. Physical signs present in women with AFLP can include jaundice, ascites, edema, confusion, abdominal tenderness, and fever. More severe cases can present with multisystem involvement, including acute renal failure, gastrointestinal bleeding, pancreatitis, coagulopathy, and hepatic encephalopathy.3,4,9,18
Diagnostic Tests
Relevant laboratory tests include a complete blood count (CBC), liver studies, chemistry, coagulation studies, and urinalysis (see Table 1). Viral causes should be ruled out by way of a hepatitis panel.3 In AFLP, the CBC may show elevated white blood cells, decreased hemoglobin and hematocrit, and decreased platelets. Liver studies show elevated hepatic aminotransferase, bilirubin, LDH, and ammonia levels. Chemistry results show elevated blood urea nitrogen and creatinine, and decreased blood glucose. Coagulation factors are affected, and prolonged PTT, decreased fibrinogen, and proteinurea may also be found.9
Though invasive and not often necessary4,13 (and not possible for the case patient), the definitive diagnostic test for AFLP is liver biopsy.13,14 Biopsy reveals a microvesicular fatty infiltration of the hepatocytes as minute fat droplets surrounding a centrally located nucleus. These fatty infiltrates stain with oil red O, specific for fat. Inflammation is present in 50% of cases. There may also be a picture similar to cholestasis with bile thrombi or deposits within the hepatocytes.20
Due to the risk for hemorrhage and the critical status of women with AFLP, biopsy is often not possible. Ultrasonography may show increased echogenicity; CT may show decreased or diffuse attenuation in the liver. These imaging studies, though possibly helpful in severe cases, often yield false-negative results.3,20
In the absence of another explanation for the patient’s symptoms, the Swansea criteria are used for diagnosis of AFLP.1 Six or more of the following criteria must be present to confirm this diagnosis: vomiting, abdominal pain, polydipsia or polyuria, encephalopathy, leukocytosis, elevated bilirubin, elevated liver enzymes, elevated ammonia, hypoglycemia, renal dysfunction, coagulopathy, elevated uric acid, ascites on ultrasound, and microvesicular steatosis on liver biopsy.1,2,5
Pathophysiology
Normal functions of the liver include metabolism, protein synthesis, and manufacturing of blood coagulation proteins. These functions are disturbed in the presence of AFLP. Thus, women with this disease experience signs and symptoms related directly to the dysfunction of these processes.20-22
Disturbances in the hepatocytes due to excess fatty acids impair the liver’s ability to convert unconjugated bilirubin into conjugated bilirubin, causing plasma levels of unconjugated bilirubin to rise. This increase in bilirubin explains the jaundiced appearance of women with AFLP. AFLP is often thought to occur in conjunction with preeclampsia in many, but not all, patients. Thrombocytopenia in these patients is felt to be secondary to peripheral vascular consumption. Conjugated bilirubin levels may also be increased due to decreased flow of conjugated bilirubin into the common bile duct.21
Another liver function that is disrupted is that of glycogen storage and conversion to glucose, and the liver’s ability to convert nutrients into glycogen is also impaired. Decreased storage of glycogen, along with the liver’s inability to break down previously stored glycogen, causes a decrease in serum glucose levels. Women with AFLP often require treatment with IV dextrose in response to marked hypoglycemia.16,21,23
The liver dysfunction associated with AFLP reduces adequate production of clotting factors and coagulation proteins. Thrombocytopenia, elevated clotting times, and bleeding are all problems seen in AFLP. Mild to moderate elevations in serum aminotransferases and elevated LDH also occur in patients with AFLP.23,24
Genetic Factor
There is little known about the etiology of AFLP, although recent data point to a genetic component that was found in as many as 62% of mothers in one study and in 25% of infants in another study.20-22 Fatty acid oxidation (FAO) is one of the processes of hepatic mitochondria, a process that relies on several enzymes. When FAO is interrupted, fatty acids are deposited in the liver cells, as seen in histologic studies of AFLP.25,26 The common thread in women with this disease is a mutation in one of the enzymes needed for FAO. This enzyme is the long-chain 3-hydroxyacyl-CoA dehydrogenase. Deficiencies in this enzyme are common in mothers with AFLP and their infants.3,16,20,23,27
Differential Diagnosis
Several complications of pregnancy that involve the liver may, on presentation, mimic AFLP.16,20,23,24,28 The most common are hyperemesis gravidarum and intrahepatic cholestasis of pregnancy23 (see Table 216,20,23,24,28); others are preeclampsia/eclampsia and HELLP syndrome. It is important to distinguish between the signs and symptoms associated with each of these disorders in order to provide the most effective treatment. Hepatitis serologies are important in the differential diagnosis when liver enzyme levels are exceptionally high.4,16,22,28
Treatment
The most effective treatment for AFLP is delivery of the infant; often, this alone causes the signs and symptoms of AFLP to resolve.8,21,27,29 In two of three cases in a small study by Aso et al,8 early delivery of the fetus led to complete resolution of symptoms and return to normal liver function. One of these patients was sent home four days after delivery; the other, 14 days later. Other patients may require more invasive treatment and support.8
Management in the ICU is often required to provide appropriate supportive care to the mother after delivery. Acute respiratory distress syndrome, pancreatitis, hemorrhage, encephalopathy, renal failure, and continual liver failure are among the severe complications associated with AFLP.4,8,10 Many women require intubation, dialysis, fluid resuscitation, blood product transfusion, and vasopressor therapy.3,8,11 Prophylactic antibiotics, IV steroids, and glucose may all be required in the supportive care and recovery of a mother with AFLP.3,8,11
TPE has also been useful in instances of severe complications.1,3,6 In one retrospective study, Martin et al1 recommended administration of TPE in patients with AFLP under the following circumstances:
(1) Deteriorating central nervous system abnormalities, such as sensorium changes or coma;
(2) Persistent coagulopathy requiring continued and aggressive blood product support with plasma, red cells, and/or cryoprecipitate;
(3) Advanced renal dysfunction that compromised fluid management;
(4) Progressive cardiopulmonary compromise; and/or
(5) Ongoing fluid management concerns, including significant ascites, edema, anuria/oliguria, and/or fluid overload.1
In rare cases, liver transplantation is needed in patients with AFLP. Westbrook et al18 reviewed 54 cases of liver disease in pregnancy in one UK hospital between 1997 and 2008. Of these, six patients with encephalopathy or elevated lactate were listed for liver transplant, including just one with a diagnosis of AFLP. This woman never actually underwent transplant but recovered in response to medical management alone.18 According to data reported in June 2011 by the Organ Procurement and Transplantation Network,30 liver transplantation was needed in only three US patients with AFLP between 2000 and 2011. Further retrospective studies on outcomes from transplant versus medical management should be considered to guide future decision making involving this invasive therapy.
The Case Patient
This 39-year-old patient presented during a routine prenatal visit with proteinuria and hypertension, possibly indicative of preeclampsia. Because of the serious nature of this potential diagnosis in pregnancy, she was admitted for monitoring and further testing. Although the diagnosis of AFLP was not confirmed until later, the patient’s preliminary lab studies showed elevated liver enzymes and low platelet counts, signifying the need for prompt intervention and delivery of the infant. At this point, the patient met criteria for HELLP syndrome, but AFLP was suspected after the initial finding of profound hypoglycemia led to further testing.
As an older mother experiencing pregnancy for the first time, this patient fit the profile for AFLP. She initially responded well after delivery of her infant but continued to experience complications. On the days that the patient was treated with TPE, her total bilirubin and liver enzymes were at their lowest. Perhaps this treatment should be considered in more cases of AFLP.
The patient was transferred to a hospital with liver transplantation capabilities, but she ultimately recovered without undergoing transplant.
Conclusion
For the primary obstetric care provider, being aware of the possible complications associated with pregnancy is important. Though uncommon, AFLP is a serious complication that should be ruled out in women who present with vague symptoms such as nausea, vomiting, and abdominal pain in the third trimester of pregnancy. The reduction in AFLP-associated morbidity and mortality during the past 20 years is a direct result of increased early recognition and therapeutic delivery.
Referral to a maternal fetal medicine specialist, gastroenterologist, hematologist, and/or nephrologist may be necessary and appropriate in the management of a woman with AFLP. Further study is indicated for use of TPE in more severe cases of AFLP, particularly in women affected by persistent thrombocytopenia and anemia.
The author would like to thank C. Leanne Browning, MD, obstetrics/gynecology, for her invaluable guidance and advice on this project.
References
1. Martin JN Jr, Briery CM, Rose CH, et al. Postpartum plasma exchange as adjunctive therapy for severe acute fatty liver of pregnancy. J Clin Apher. 2009;23(4):138-143.
2. Knight M, Nelson-Piercy C, Kurinczuk JJ; UK Obstetric Surveillance System. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57(7):951-956.
3. Barsoom MJ, Tierney BJ. Acute fatty liver of pregnancy (2011). http://emedicine.medscape.com/article/1562425-overview. Accessed January 21, 2013.
4. Ko HH, Yoshida E. Acute fatty liver of pregnancy. Can J Gastroenterol. 2006;20(1):25-30.
5. Rathi U, Bapat M, Rathi P, Abraham P. Effect of liver disease on maternal and fetal outcome: a prospective study. Indian J Gastroenterol. 2007;26(2):59-63.
6. Myers L. Postpartum plasma exchange in a woman with suspected thrombotic thrombocytopenic purpura (TTP) vs hemolysis, elevated liver enzymes, and low platelet syndrome (HELLP): a case study. Nephrol Nurs J. 2010;37(4):399-402.
7. Vigil-de Gracia P. Acute fatty liver and HELLP syndrome: two distinct pregnancy disorders. Int J Gynaecol Obstet. 2001;73(3):215-220.
8. Aso K, Hojo S, Yumoto Y, et al. Three cases of acute fatty liver of pregnancy: postpartum clinical course depends on interval between onset of symptoms and termination of pregnancy. J Matern Fetal Neonatal Med. 2010;23(9):1047-1049.
9. Wei Q, Zhang L, Liu X. Clinical diagnosis and treatment of acute fatty liver of pregnancy: a literature review and 11 new cases. J Obstet Gynaecol Res. 2010;36(4):751-756.
10. Barber MA, Eguiluz I, Martin A, et al. Acute fatty liver of pregnancy: analysis of five consecutive cases from a tertiary centre. J Obstet Gynaecol. 2010;30(3):241-243.
11. Ajayi AO, Alao MO. Case report: acute fatty liver of pregnancy in a 30-year-old Nigerian primigravida. Niger J Clin Pract. 2008;11(4):389-391.
12. Vigíl-de Gracia P, Montufar-Rueda C. Acute fatty liver of pregnancy: diagnosis, treatment, and outcome based on 35 consecutive cases. J Matern Fetal Neonatal Med. 2011;24(9):1143-1146.
13. Dey M, Reema K. Acute fatty liver of pregnancy. N Am J Med Sci. 2012;4(11):611-612.
14. Castro MA, Goodwin TM, Shaw KJ, et al. Disseminated intravascular coagulation and antithrombin III depression in acute fatty liver of pregnancy. Am J Obstet Gynecol. 1996;174(1 pt 1):211-216.
15. Altuntas F, Aydogdu I, Kabukcu S, et al. Therapeutic plasma exchange for the treatment of thrombotic thrombocytopenic purpura: a retrospective multicenter study. Transfus Apher Sci. 2007;36(1):57-67.
16. Hay JE. Liver disease in pregnancy. Hepatology. 2008;47(3):1067-1076.
17. Wand S, Waeschle RM, Von Ahsen N, et al. Acute fatty liver failure due to acute fatty liver of pregnancy. Minerva Anesthesiol. 2012;78(4):503-506.
18. Westbrook RH, Yeoman AD, Joshi D, et al. Outcomes of severe pregnancy-related liver disease: refining the role of transplantation. Am J Transplant. 2010;10(11):2520-2526.
19. Fesenmeier MF, Coppage KH, Lambers DS, et al. Acute fatty liver of pregnancy in 3 tertiary care centers. Am J Obstet Gynecol. 2005;192(5):1416-1419.
20. Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol. 2011;35(3):182-193.
21. Huether SE. Alterations of digestive function. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1452-1515.
22. Huether SE. Structure and function of the digestive system. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1420-1451.
23. Schutt VA, Minuk GY. Liver diseases unique to pregnancy. Best Pract Res Clin Gastroenterol. 2007;21(5):771-792.
24. Pan C, Perumalswami PV. Pregnancy-related liver diseases. Clin Liver Dis. 2011;15(1):199-208.
25. Ibdah JA. Acute fatty liver of pregnancy: an update on pathogenesis and clinical implications. World J Gastroenterol. 2006;12(46):7397-7404.
26. Browning MF, Levy HL, Wilkins-Haug LE, et al. Fetal fatty acid oxidation defects and maternal liver disease in pregnancy. Obstet Gynecol. 2006;107(1):115-120.
27. Dekker RR, Schutte JM, Stekelenburg J, et al. Maternal mortality and severe maternal morbidity from acute fatty liver of pregnancy in the Netherlands. Eur J Obstet Gynecol Reprod Biol. 2011;157(1):27-31.
28. Lee NM, Brady CW. Liver disease in pregnancy. World J Gastroenterol. 2009;15(8):897-906.
29. Vora KS, Shah VR, Parikh GP. Acute fatty liver of pregnancy: a case report of an uncommon disease. Indian J Crit Care Med. 2009;13(1):34-36.
30. Organ Procurement and Transplantation Network, Scientific Registry of Transplant Recipients. OPTN/SRTR 2011 Annual Data Report: Liver. http://srtr.transplant.hrsa.gov/annual_reports/2011/pdf/03_%20liver_12.pdf. Accessed January 18, 2013.
A 39-year-old black woman, gravida 1, para 0, with an intrauterine pregnancy of 34 weeks and three days (according to last menstrual period and nine-week ultrasound) presented to her Ob-Gyn office for a routine prenatal visit. She was found to have an elevated blood pressure with new onset of 2+ proteinuria. The patient was sent to the labor and delivery unit at the adjoining hospital for serial blood pressure readings, laboratory work, and fetal monitoring.
The patient’s previous medical history was limited to sinusitis. She was taking no prescription medications, and her only listed allergy was to pineapple. Initial lab studies revealed elevations in liver enzymes, lactate dehydrogenase (LDH), uric acid, and serum creatinine, as well as thrombocytopenia (see Table 11-5). She also had a critically low blood glucose level, which conflicted with a normal follow-up reading.
At this point, the patient was thought to have HELLP syndrome6 (ie, hemolysis, elevated liver enzymes, low platelet count), or possibly acute fatty liver of pregnancy (AFLP).2,4,7-11 Additional labs were drawn immediately to confirm or rule out AFLP. These included repeat serum glucose (following a second reading with normal results), a serum ammonia level, prothrombin time (PT), and partial thromboplastin time (PTT). The most reliable values to distinguish AFLP from HELLP are profound hypoglycemia (found in 94% of women with AFLP12) and an elevated serum ammonia level.4
Given the serious nature of either diagnosis, immediate delivery of the infant was deemed necessary. Because the patient’s cervix was not found favorable for induction, she underwent low-transverse cesarean delivery without complications. She was noted to have essentially normal anatomy with the exception of a small subserosal fibroid posteriorly. Meconium-stained amniotic fluid was present. A male infant was delivered, weighing 5 lb with 1-minute and 5-minute Apgar scores of 8 and 9, respectively.
Postoperatively, the patient remained in the recovery area, where she received intensive monitoring. She experienced fluctuations in blood glucose, ranging from 33 to 144 mg/dL; she was started on 5% dextrose in lactated Ringer’s solution and treated with IV dextrose 50 g. While the patient was in surgical recovery, results from the second set of labs, drawn before surgery, were returned; findings included an elevated ammonia level and an abnormal coagulation panel, including PT of 25.3 sec, PTT of 48.4 sec, and a fibrinogen level of 116 mg/dL, confirming the suspected diagnosis of AFLP.
Magnesium sulfate, which had been started immediately postop, was discontinued on confirmation of the diagnosis of AFLP. The patient was initially somnolent as a result of general anesthesia but gradually returned to a fully normal sensorium by early morning on postop day 1. Postoperatively, the patient’s hemoglobin was found to be low (8.6 g/dL; reference range, 13.5 to 18.5 g/dL), so she was transfused with two units of packed red blood cells (PRBCs) and given fresh frozen plasma (FFP) to correct this coagulopathy. The patient’s platelets were also low at 82,000/mm3 (reference range, 140,000 to 340,000/mm3).
On postop day 1, the patient’s serum creatinine rose to 4.2 mg/dL and her total bilirubin increased to 14.4 mg/dL (reference ranges, 0.6 to 1.2 mg/dL and < 1.0 mg/dL, respectively). Given the multiple systems affected by AFLP and the need for intensive supportive care, the patient was transferred to the ICU.
On her arrival at the ICU, the patient’s vital signs were initially stable, and she was alert and oriented. However, within the next few hours, she became hypotensive and encephalopathic. She required aggressive fluid resuscitation and multiple transfusions of PRBCs and FFP due to persistent anemia and coagulopathy. Her vital signs were stabilized, but she continued to need blood transfusions.
Postop day 2, the patient became less responsive and was soon unable to follow commands or speak clearly. Her breathing remained stable with just 3 L of oxygen by nasal cannula, but in order to prevent aspiration and in consideration of a postoperative ileus, it was necessary to place a nasogastric tube with low intermittent suction. This produced a bloody return, but no intervention other than close monitoring and transfusion was performed at that time.
Abdominal ultrasound showed ascites and mild left-sided hydronephrosis with no gallstones. The common bile duct measured 3 mm in diameter.
Although liver biopsy is considered the gold standard for a confirmed diagnosis of AFLP,13,14 this procedure was contraindicated by the patient’s coagulopathy. Concern was also expressed by one consultant that the patient might have thrombotic thrombocytopenic purpura (TTP) in addition to AFLP. TTP can manifest with similar findings, such as anemia, thrombocytopenia, neurologic symptoms, and renal abnormalities, but usually fever is involved, and the patient was afebrile. A catheter was placed for hemodialysis and therapeutic plasma exchange (TPE). Given that TTP-associated mortality is significantly decreased by use of TPE,15 this intervention was deemed prudent. The patient underwent TPE on three consecutive days, postop days 2 through 4.
The patient’s mental status began to improve, and by postop day 6, she was able to follow commands and engage in brief conversations. By postop day 9, she had returned almost completely to her baseline mental status.
The patient’s liver function test results and total bilirubin, ammonia, and creatinine levels all improved over the first few postoperative days but began to rise again by day 6. In response to worsening renal and hepatic functioning, the decision was made on postop day 9 to transfer the patient to a hospital with liver transplantation capabilities, should this procedure become necessary.
Discussion
AFLP is a rare condition specific to pregnancy, affecting 1/7,000 to 1/20,000 pregnancies. Due to the low incidence of this disease, randomized controlled trials to study it are not possible. Instead, clinicians must learn either from individual case studies or from retrospective syntheses of cases reported over time.1,2,7 Fortunately, the wealth of information gleaned over the past 30 years has significantly reduced AFLP-associated maternal and fetal mortality and morbidity rates. In the 1980s, maternal and fetal mortality rates as high as 85% were reported.3 Worldwide, maternal mortality associated with AFLP has decreased significantly to 7% to 18%, whereas the fetal mortality rate has fallen to between 9% and 23%.1,16,17
Common trends among women who have developed AFLP include nulliparity, multiparity, and advanced maternal age. One retrospective study of 57 women who had developed AFLP revealed that 35 cases (61%) involved first-time pregnancies. It also showed that 10 (18%) of the women had twins, and 14 (25%) were older than 35.2 In another study of 35 cases of AFLP, 40% of the women were nulliparous, and 11.4% were multiparous, including one triplet gestation.12 In a third, smaller study, 80% of women affected by AFLP were multiparous.10 Currently, there is no known evidence linking any maternal behavior to development of AFLP.
Presentation
Women who present with AFLP often experience vague, nonspecific symptoms, leading to misdiagnosis or delayed diagnosis. Objective measurements, including physical exam findings, laboratory studies, and other diagnostic tests, will help with a diagnosis. The most frequent initial symptoms are nausea and vomiting (in 70% of patients) and abdominal pain (50% to 80%), epigastric or right upper-quadrant.3 Other common symptoms include fatigue, malaise, anorexia, weight gain, polyuria, and polydipsia.2,3,9,18,19
Because the presenting symptoms in AFLP can be vague, clinicians should complete a thorough physical exam to differentiate accurately among conditions associated with pregnancy. Physical signs present in women with AFLP can include jaundice, ascites, edema, confusion, abdominal tenderness, and fever. More severe cases can present with multisystem involvement, including acute renal failure, gastrointestinal bleeding, pancreatitis, coagulopathy, and hepatic encephalopathy.3,4,9,18
Diagnostic Tests
Relevant laboratory tests include a complete blood count (CBC), liver studies, chemistry, coagulation studies, and urinalysis (see Table 1). Viral causes should be ruled out by way of a hepatitis panel.3 In AFLP, the CBC may show elevated white blood cells, decreased hemoglobin and hematocrit, and decreased platelets. Liver studies show elevated hepatic aminotransferase, bilirubin, LDH, and ammonia levels. Chemistry results show elevated blood urea nitrogen and creatinine, and decreased blood glucose. Coagulation factors are affected, and prolonged PTT, decreased fibrinogen, and proteinurea may also be found.9
Though invasive and not often necessary4,13 (and not possible for the case patient), the definitive diagnostic test for AFLP is liver biopsy.13,14 Biopsy reveals a microvesicular fatty infiltration of the hepatocytes as minute fat droplets surrounding a centrally located nucleus. These fatty infiltrates stain with oil red O, specific for fat. Inflammation is present in 50% of cases. There may also be a picture similar to cholestasis with bile thrombi or deposits within the hepatocytes.20
Due to the risk for hemorrhage and the critical status of women with AFLP, biopsy is often not possible. Ultrasonography may show increased echogenicity; CT may show decreased or diffuse attenuation in the liver. These imaging studies, though possibly helpful in severe cases, often yield false-negative results.3,20
In the absence of another explanation for the patient’s symptoms, the Swansea criteria are used for diagnosis of AFLP.1 Six or more of the following criteria must be present to confirm this diagnosis: vomiting, abdominal pain, polydipsia or polyuria, encephalopathy, leukocytosis, elevated bilirubin, elevated liver enzymes, elevated ammonia, hypoglycemia, renal dysfunction, coagulopathy, elevated uric acid, ascites on ultrasound, and microvesicular steatosis on liver biopsy.1,2,5
Pathophysiology
Normal functions of the liver include metabolism, protein synthesis, and manufacturing of blood coagulation proteins. These functions are disturbed in the presence of AFLP. Thus, women with this disease experience signs and symptoms related directly to the dysfunction of these processes.20-22
Disturbances in the hepatocytes due to excess fatty acids impair the liver’s ability to convert unconjugated bilirubin into conjugated bilirubin, causing plasma levels of unconjugated bilirubin to rise. This increase in bilirubin explains the jaundiced appearance of women with AFLP. AFLP is often thought to occur in conjunction with preeclampsia in many, but not all, patients. Thrombocytopenia in these patients is felt to be secondary to peripheral vascular consumption. Conjugated bilirubin levels may also be increased due to decreased flow of conjugated bilirubin into the common bile duct.21
Another liver function that is disrupted is that of glycogen storage and conversion to glucose, and the liver’s ability to convert nutrients into glycogen is also impaired. Decreased storage of glycogen, along with the liver’s inability to break down previously stored glycogen, causes a decrease in serum glucose levels. Women with AFLP often require treatment with IV dextrose in response to marked hypoglycemia.16,21,23
The liver dysfunction associated with AFLP reduces adequate production of clotting factors and coagulation proteins. Thrombocytopenia, elevated clotting times, and bleeding are all problems seen in AFLP. Mild to moderate elevations in serum aminotransferases and elevated LDH also occur in patients with AFLP.23,24
Genetic Factor
There is little known about the etiology of AFLP, although recent data point to a genetic component that was found in as many as 62% of mothers in one study and in 25% of infants in another study.20-22 Fatty acid oxidation (FAO) is one of the processes of hepatic mitochondria, a process that relies on several enzymes. When FAO is interrupted, fatty acids are deposited in the liver cells, as seen in histologic studies of AFLP.25,26 The common thread in women with this disease is a mutation in one of the enzymes needed for FAO. This enzyme is the long-chain 3-hydroxyacyl-CoA dehydrogenase. Deficiencies in this enzyme are common in mothers with AFLP and their infants.3,16,20,23,27
Differential Diagnosis
Several complications of pregnancy that involve the liver may, on presentation, mimic AFLP.16,20,23,24,28 The most common are hyperemesis gravidarum and intrahepatic cholestasis of pregnancy23 (see Table 216,20,23,24,28); others are preeclampsia/eclampsia and HELLP syndrome. It is important to distinguish between the signs and symptoms associated with each of these disorders in order to provide the most effective treatment. Hepatitis serologies are important in the differential diagnosis when liver enzyme levels are exceptionally high.4,16,22,28
Treatment
The most effective treatment for AFLP is delivery of the infant; often, this alone causes the signs and symptoms of AFLP to resolve.8,21,27,29 In two of three cases in a small study by Aso et al,8 early delivery of the fetus led to complete resolution of symptoms and return to normal liver function. One of these patients was sent home four days after delivery; the other, 14 days later. Other patients may require more invasive treatment and support.8
Management in the ICU is often required to provide appropriate supportive care to the mother after delivery. Acute respiratory distress syndrome, pancreatitis, hemorrhage, encephalopathy, renal failure, and continual liver failure are among the severe complications associated with AFLP.4,8,10 Many women require intubation, dialysis, fluid resuscitation, blood product transfusion, and vasopressor therapy.3,8,11 Prophylactic antibiotics, IV steroids, and glucose may all be required in the supportive care and recovery of a mother with AFLP.3,8,11
TPE has also been useful in instances of severe complications.1,3,6 In one retrospective study, Martin et al1 recommended administration of TPE in patients with AFLP under the following circumstances:
(1) Deteriorating central nervous system abnormalities, such as sensorium changes or coma;
(2) Persistent coagulopathy requiring continued and aggressive blood product support with plasma, red cells, and/or cryoprecipitate;
(3) Advanced renal dysfunction that compromised fluid management;
(4) Progressive cardiopulmonary compromise; and/or
(5) Ongoing fluid management concerns, including significant ascites, edema, anuria/oliguria, and/or fluid overload.1
In rare cases, liver transplantation is needed in patients with AFLP. Westbrook et al18 reviewed 54 cases of liver disease in pregnancy in one UK hospital between 1997 and 2008. Of these, six patients with encephalopathy or elevated lactate were listed for liver transplant, including just one with a diagnosis of AFLP. This woman never actually underwent transplant but recovered in response to medical management alone.18 According to data reported in June 2011 by the Organ Procurement and Transplantation Network,30 liver transplantation was needed in only three US patients with AFLP between 2000 and 2011. Further retrospective studies on outcomes from transplant versus medical management should be considered to guide future decision making involving this invasive therapy.
The Case Patient
This 39-year-old patient presented during a routine prenatal visit with proteinuria and hypertension, possibly indicative of preeclampsia. Because of the serious nature of this potential diagnosis in pregnancy, she was admitted for monitoring and further testing. Although the diagnosis of AFLP was not confirmed until later, the patient’s preliminary lab studies showed elevated liver enzymes and low platelet counts, signifying the need for prompt intervention and delivery of the infant. At this point, the patient met criteria for HELLP syndrome, but AFLP was suspected after the initial finding of profound hypoglycemia led to further testing.
As an older mother experiencing pregnancy for the first time, this patient fit the profile for AFLP. She initially responded well after delivery of her infant but continued to experience complications. On the days that the patient was treated with TPE, her total bilirubin and liver enzymes were at their lowest. Perhaps this treatment should be considered in more cases of AFLP.
The patient was transferred to a hospital with liver transplantation capabilities, but she ultimately recovered without undergoing transplant.
Conclusion
For the primary obstetric care provider, being aware of the possible complications associated with pregnancy is important. Though uncommon, AFLP is a serious complication that should be ruled out in women who present with vague symptoms such as nausea, vomiting, and abdominal pain in the third trimester of pregnancy. The reduction in AFLP-associated morbidity and mortality during the past 20 years is a direct result of increased early recognition and therapeutic delivery.
Referral to a maternal fetal medicine specialist, gastroenterologist, hematologist, and/or nephrologist may be necessary and appropriate in the management of a woman with AFLP. Further study is indicated for use of TPE in more severe cases of AFLP, particularly in women affected by persistent thrombocytopenia and anemia.
The author would like to thank C. Leanne Browning, MD, obstetrics/gynecology, for her invaluable guidance and advice on this project.
References
1. Martin JN Jr, Briery CM, Rose CH, et al. Postpartum plasma exchange as adjunctive therapy for severe acute fatty liver of pregnancy. J Clin Apher. 2009;23(4):138-143.
2. Knight M, Nelson-Piercy C, Kurinczuk JJ; UK Obstetric Surveillance System. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57(7):951-956.
3. Barsoom MJ, Tierney BJ. Acute fatty liver of pregnancy (2011). http://emedicine.medscape.com/article/1562425-overview. Accessed January 21, 2013.
4. Ko HH, Yoshida E. Acute fatty liver of pregnancy. Can J Gastroenterol. 2006;20(1):25-30.
5. Rathi U, Bapat M, Rathi P, Abraham P. Effect of liver disease on maternal and fetal outcome: a prospective study. Indian J Gastroenterol. 2007;26(2):59-63.
6. Myers L. Postpartum plasma exchange in a woman with suspected thrombotic thrombocytopenic purpura (TTP) vs hemolysis, elevated liver enzymes, and low platelet syndrome (HELLP): a case study. Nephrol Nurs J. 2010;37(4):399-402.
7. Vigil-de Gracia P. Acute fatty liver and HELLP syndrome: two distinct pregnancy disorders. Int J Gynaecol Obstet. 2001;73(3):215-220.
8. Aso K, Hojo S, Yumoto Y, et al. Three cases of acute fatty liver of pregnancy: postpartum clinical course depends on interval between onset of symptoms and termination of pregnancy. J Matern Fetal Neonatal Med. 2010;23(9):1047-1049.
9. Wei Q, Zhang L, Liu X. Clinical diagnosis and treatment of acute fatty liver of pregnancy: a literature review and 11 new cases. J Obstet Gynaecol Res. 2010;36(4):751-756.
10. Barber MA, Eguiluz I, Martin A, et al. Acute fatty liver of pregnancy: analysis of five consecutive cases from a tertiary centre. J Obstet Gynaecol. 2010;30(3):241-243.
11. Ajayi AO, Alao MO. Case report: acute fatty liver of pregnancy in a 30-year-old Nigerian primigravida. Niger J Clin Pract. 2008;11(4):389-391.
12. Vigíl-de Gracia P, Montufar-Rueda C. Acute fatty liver of pregnancy: diagnosis, treatment, and outcome based on 35 consecutive cases. J Matern Fetal Neonatal Med. 2011;24(9):1143-1146.
13. Dey M, Reema K. Acute fatty liver of pregnancy. N Am J Med Sci. 2012;4(11):611-612.
14. Castro MA, Goodwin TM, Shaw KJ, et al. Disseminated intravascular coagulation and antithrombin III depression in acute fatty liver of pregnancy. Am J Obstet Gynecol. 1996;174(1 pt 1):211-216.
15. Altuntas F, Aydogdu I, Kabukcu S, et al. Therapeutic plasma exchange for the treatment of thrombotic thrombocytopenic purpura: a retrospective multicenter study. Transfus Apher Sci. 2007;36(1):57-67.
16. Hay JE. Liver disease in pregnancy. Hepatology. 2008;47(3):1067-1076.
17. Wand S, Waeschle RM, Von Ahsen N, et al. Acute fatty liver failure due to acute fatty liver of pregnancy. Minerva Anesthesiol. 2012;78(4):503-506.
18. Westbrook RH, Yeoman AD, Joshi D, et al. Outcomes of severe pregnancy-related liver disease: refining the role of transplantation. Am J Transplant. 2010;10(11):2520-2526.
19. Fesenmeier MF, Coppage KH, Lambers DS, et al. Acute fatty liver of pregnancy in 3 tertiary care centers. Am J Obstet Gynecol. 2005;192(5):1416-1419.
20. Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol. 2011;35(3):182-193.
21. Huether SE. Alterations of digestive function. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1452-1515.
22. Huether SE. Structure and function of the digestive system. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1420-1451.
23. Schutt VA, Minuk GY. Liver diseases unique to pregnancy. Best Pract Res Clin Gastroenterol. 2007;21(5):771-792.
24. Pan C, Perumalswami PV. Pregnancy-related liver diseases. Clin Liver Dis. 2011;15(1):199-208.
25. Ibdah JA. Acute fatty liver of pregnancy: an update on pathogenesis and clinical implications. World J Gastroenterol. 2006;12(46):7397-7404.
26. Browning MF, Levy HL, Wilkins-Haug LE, et al. Fetal fatty acid oxidation defects and maternal liver disease in pregnancy. Obstet Gynecol. 2006;107(1):115-120.
27. Dekker RR, Schutte JM, Stekelenburg J, et al. Maternal mortality and severe maternal morbidity from acute fatty liver of pregnancy in the Netherlands. Eur J Obstet Gynecol Reprod Biol. 2011;157(1):27-31.
28. Lee NM, Brady CW. Liver disease in pregnancy. World J Gastroenterol. 2009;15(8):897-906.
29. Vora KS, Shah VR, Parikh GP. Acute fatty liver of pregnancy: a case report of an uncommon disease. Indian J Crit Care Med. 2009;13(1):34-36.
30. Organ Procurement and Transplantation Network, Scientific Registry of Transplant Recipients. OPTN/SRTR 2011 Annual Data Report: Liver. http://srtr.transplant.hrsa.gov/annual_reports/2011/pdf/03_%20liver_12.pdf. Accessed January 18, 2013.
A 39-year-old black woman, gravida 1, para 0, with an intrauterine pregnancy of 34 weeks and three days (according to last menstrual period and nine-week ultrasound) presented to her Ob-Gyn office for a routine prenatal visit. She was found to have an elevated blood pressure with new onset of 2+ proteinuria. The patient was sent to the labor and delivery unit at the adjoining hospital for serial blood pressure readings, laboratory work, and fetal monitoring.
The patient’s previous medical history was limited to sinusitis. She was taking no prescription medications, and her only listed allergy was to pineapple. Initial lab studies revealed elevations in liver enzymes, lactate dehydrogenase (LDH), uric acid, and serum creatinine, as well as thrombocytopenia (see Table 11-5). She also had a critically low blood glucose level, which conflicted with a normal follow-up reading.
At this point, the patient was thought to have HELLP syndrome6 (ie, hemolysis, elevated liver enzymes, low platelet count), or possibly acute fatty liver of pregnancy (AFLP).2,4,7-11 Additional labs were drawn immediately to confirm or rule out AFLP. These included repeat serum glucose (following a second reading with normal results), a serum ammonia level, prothrombin time (PT), and partial thromboplastin time (PTT). The most reliable values to distinguish AFLP from HELLP are profound hypoglycemia (found in 94% of women with AFLP12) and an elevated serum ammonia level.4
Given the serious nature of either diagnosis, immediate delivery of the infant was deemed necessary. Because the patient’s cervix was not found favorable for induction, she underwent low-transverse cesarean delivery without complications. She was noted to have essentially normal anatomy with the exception of a small subserosal fibroid posteriorly. Meconium-stained amniotic fluid was present. A male infant was delivered, weighing 5 lb with 1-minute and 5-minute Apgar scores of 8 and 9, respectively.
Postoperatively, the patient remained in the recovery area, where she received intensive monitoring. She experienced fluctuations in blood glucose, ranging from 33 to 144 mg/dL; she was started on 5% dextrose in lactated Ringer’s solution and treated with IV dextrose 50 g. While the patient was in surgical recovery, results from the second set of labs, drawn before surgery, were returned; findings included an elevated ammonia level and an abnormal coagulation panel, including PT of 25.3 sec, PTT of 48.4 sec, and a fibrinogen level of 116 mg/dL, confirming the suspected diagnosis of AFLP.
Magnesium sulfate, which had been started immediately postop, was discontinued on confirmation of the diagnosis of AFLP. The patient was initially somnolent as a result of general anesthesia but gradually returned to a fully normal sensorium by early morning on postop day 1. Postoperatively, the patient’s hemoglobin was found to be low (8.6 g/dL; reference range, 13.5 to 18.5 g/dL), so she was transfused with two units of packed red blood cells (PRBCs) and given fresh frozen plasma (FFP) to correct this coagulopathy. The patient’s platelets were also low at 82,000/mm3 (reference range, 140,000 to 340,000/mm3).
On postop day 1, the patient’s serum creatinine rose to 4.2 mg/dL and her total bilirubin increased to 14.4 mg/dL (reference ranges, 0.6 to 1.2 mg/dL and < 1.0 mg/dL, respectively). Given the multiple systems affected by AFLP and the need for intensive supportive care, the patient was transferred to the ICU.
On her arrival at the ICU, the patient’s vital signs were initially stable, and she was alert and oriented. However, within the next few hours, she became hypotensive and encephalopathic. She required aggressive fluid resuscitation and multiple transfusions of PRBCs and FFP due to persistent anemia and coagulopathy. Her vital signs were stabilized, but she continued to need blood transfusions.
Postop day 2, the patient became less responsive and was soon unable to follow commands or speak clearly. Her breathing remained stable with just 3 L of oxygen by nasal cannula, but in order to prevent aspiration and in consideration of a postoperative ileus, it was necessary to place a nasogastric tube with low intermittent suction. This produced a bloody return, but no intervention other than close monitoring and transfusion was performed at that time.
Abdominal ultrasound showed ascites and mild left-sided hydronephrosis with no gallstones. The common bile duct measured 3 mm in diameter.
Although liver biopsy is considered the gold standard for a confirmed diagnosis of AFLP,13,14 this procedure was contraindicated by the patient’s coagulopathy. Concern was also expressed by one consultant that the patient might have thrombotic thrombocytopenic purpura (TTP) in addition to AFLP. TTP can manifest with similar findings, such as anemia, thrombocytopenia, neurologic symptoms, and renal abnormalities, but usually fever is involved, and the patient was afebrile. A catheter was placed for hemodialysis and therapeutic plasma exchange (TPE). Given that TTP-associated mortality is significantly decreased by use of TPE,15 this intervention was deemed prudent. The patient underwent TPE on three consecutive days, postop days 2 through 4.
The patient’s mental status began to improve, and by postop day 6, she was able to follow commands and engage in brief conversations. By postop day 9, she had returned almost completely to her baseline mental status.
The patient’s liver function test results and total bilirubin, ammonia, and creatinine levels all improved over the first few postoperative days but began to rise again by day 6. In response to worsening renal and hepatic functioning, the decision was made on postop day 9 to transfer the patient to a hospital with liver transplantation capabilities, should this procedure become necessary.
Discussion
AFLP is a rare condition specific to pregnancy, affecting 1/7,000 to 1/20,000 pregnancies. Due to the low incidence of this disease, randomized controlled trials to study it are not possible. Instead, clinicians must learn either from individual case studies or from retrospective syntheses of cases reported over time.1,2,7 Fortunately, the wealth of information gleaned over the past 30 years has significantly reduced AFLP-associated maternal and fetal mortality and morbidity rates. In the 1980s, maternal and fetal mortality rates as high as 85% were reported.3 Worldwide, maternal mortality associated with AFLP has decreased significantly to 7% to 18%, whereas the fetal mortality rate has fallen to between 9% and 23%.1,16,17
Common trends among women who have developed AFLP include nulliparity, multiparity, and advanced maternal age. One retrospective study of 57 women who had developed AFLP revealed that 35 cases (61%) involved first-time pregnancies. It also showed that 10 (18%) of the women had twins, and 14 (25%) were older than 35.2 In another study of 35 cases of AFLP, 40% of the women were nulliparous, and 11.4% were multiparous, including one triplet gestation.12 In a third, smaller study, 80% of women affected by AFLP were multiparous.10 Currently, there is no known evidence linking any maternal behavior to development of AFLP.
Presentation
Women who present with AFLP often experience vague, nonspecific symptoms, leading to misdiagnosis or delayed diagnosis. Objective measurements, including physical exam findings, laboratory studies, and other diagnostic tests, will help with a diagnosis. The most frequent initial symptoms are nausea and vomiting (in 70% of patients) and abdominal pain (50% to 80%), epigastric or right upper-quadrant.3 Other common symptoms include fatigue, malaise, anorexia, weight gain, polyuria, and polydipsia.2,3,9,18,19
Because the presenting symptoms in AFLP can be vague, clinicians should complete a thorough physical exam to differentiate accurately among conditions associated with pregnancy. Physical signs present in women with AFLP can include jaundice, ascites, edema, confusion, abdominal tenderness, and fever. More severe cases can present with multisystem involvement, including acute renal failure, gastrointestinal bleeding, pancreatitis, coagulopathy, and hepatic encephalopathy.3,4,9,18
Diagnostic Tests
Relevant laboratory tests include a complete blood count (CBC), liver studies, chemistry, coagulation studies, and urinalysis (see Table 1). Viral causes should be ruled out by way of a hepatitis panel.3 In AFLP, the CBC may show elevated white blood cells, decreased hemoglobin and hematocrit, and decreased platelets. Liver studies show elevated hepatic aminotransferase, bilirubin, LDH, and ammonia levels. Chemistry results show elevated blood urea nitrogen and creatinine, and decreased blood glucose. Coagulation factors are affected, and prolonged PTT, decreased fibrinogen, and proteinurea may also be found.9
Though invasive and not often necessary4,13 (and not possible for the case patient), the definitive diagnostic test for AFLP is liver biopsy.13,14 Biopsy reveals a microvesicular fatty infiltration of the hepatocytes as minute fat droplets surrounding a centrally located nucleus. These fatty infiltrates stain with oil red O, specific for fat. Inflammation is present in 50% of cases. There may also be a picture similar to cholestasis with bile thrombi or deposits within the hepatocytes.20
Due to the risk for hemorrhage and the critical status of women with AFLP, biopsy is often not possible. Ultrasonography may show increased echogenicity; CT may show decreased or diffuse attenuation in the liver. These imaging studies, though possibly helpful in severe cases, often yield false-negative results.3,20
In the absence of another explanation for the patient’s symptoms, the Swansea criteria are used for diagnosis of AFLP.1 Six or more of the following criteria must be present to confirm this diagnosis: vomiting, abdominal pain, polydipsia or polyuria, encephalopathy, leukocytosis, elevated bilirubin, elevated liver enzymes, elevated ammonia, hypoglycemia, renal dysfunction, coagulopathy, elevated uric acid, ascites on ultrasound, and microvesicular steatosis on liver biopsy.1,2,5
Pathophysiology
Normal functions of the liver include metabolism, protein synthesis, and manufacturing of blood coagulation proteins. These functions are disturbed in the presence of AFLP. Thus, women with this disease experience signs and symptoms related directly to the dysfunction of these processes.20-22
Disturbances in the hepatocytes due to excess fatty acids impair the liver’s ability to convert unconjugated bilirubin into conjugated bilirubin, causing plasma levels of unconjugated bilirubin to rise. This increase in bilirubin explains the jaundiced appearance of women with AFLP. AFLP is often thought to occur in conjunction with preeclampsia in many, but not all, patients. Thrombocytopenia in these patients is felt to be secondary to peripheral vascular consumption. Conjugated bilirubin levels may also be increased due to decreased flow of conjugated bilirubin into the common bile duct.21
Another liver function that is disrupted is that of glycogen storage and conversion to glucose, and the liver’s ability to convert nutrients into glycogen is also impaired. Decreased storage of glycogen, along with the liver’s inability to break down previously stored glycogen, causes a decrease in serum glucose levels. Women with AFLP often require treatment with IV dextrose in response to marked hypoglycemia.16,21,23
The liver dysfunction associated with AFLP reduces adequate production of clotting factors and coagulation proteins. Thrombocytopenia, elevated clotting times, and bleeding are all problems seen in AFLP. Mild to moderate elevations in serum aminotransferases and elevated LDH also occur in patients with AFLP.23,24
Genetic Factor
There is little known about the etiology of AFLP, although recent data point to a genetic component that was found in as many as 62% of mothers in one study and in 25% of infants in another study.20-22 Fatty acid oxidation (FAO) is one of the processes of hepatic mitochondria, a process that relies on several enzymes. When FAO is interrupted, fatty acids are deposited in the liver cells, as seen in histologic studies of AFLP.25,26 The common thread in women with this disease is a mutation in one of the enzymes needed for FAO. This enzyme is the long-chain 3-hydroxyacyl-CoA dehydrogenase. Deficiencies in this enzyme are common in mothers with AFLP and their infants.3,16,20,23,27
Differential Diagnosis
Several complications of pregnancy that involve the liver may, on presentation, mimic AFLP.16,20,23,24,28 The most common are hyperemesis gravidarum and intrahepatic cholestasis of pregnancy23 (see Table 216,20,23,24,28); others are preeclampsia/eclampsia and HELLP syndrome. It is important to distinguish between the signs and symptoms associated with each of these disorders in order to provide the most effective treatment. Hepatitis serologies are important in the differential diagnosis when liver enzyme levels are exceptionally high.4,16,22,28
Treatment
The most effective treatment for AFLP is delivery of the infant; often, this alone causes the signs and symptoms of AFLP to resolve.8,21,27,29 In two of three cases in a small study by Aso et al,8 early delivery of the fetus led to complete resolution of symptoms and return to normal liver function. One of these patients was sent home four days after delivery; the other, 14 days later. Other patients may require more invasive treatment and support.8
Management in the ICU is often required to provide appropriate supportive care to the mother after delivery. Acute respiratory distress syndrome, pancreatitis, hemorrhage, encephalopathy, renal failure, and continual liver failure are among the severe complications associated with AFLP.4,8,10 Many women require intubation, dialysis, fluid resuscitation, blood product transfusion, and vasopressor therapy.3,8,11 Prophylactic antibiotics, IV steroids, and glucose may all be required in the supportive care and recovery of a mother with AFLP.3,8,11
TPE has also been useful in instances of severe complications.1,3,6 In one retrospective study, Martin et al1 recommended administration of TPE in patients with AFLP under the following circumstances:
(1) Deteriorating central nervous system abnormalities, such as sensorium changes or coma;
(2) Persistent coagulopathy requiring continued and aggressive blood product support with plasma, red cells, and/or cryoprecipitate;
(3) Advanced renal dysfunction that compromised fluid management;
(4) Progressive cardiopulmonary compromise; and/or
(5) Ongoing fluid management concerns, including significant ascites, edema, anuria/oliguria, and/or fluid overload.1
In rare cases, liver transplantation is needed in patients with AFLP. Westbrook et al18 reviewed 54 cases of liver disease in pregnancy in one UK hospital between 1997 and 2008. Of these, six patients with encephalopathy or elevated lactate were listed for liver transplant, including just one with a diagnosis of AFLP. This woman never actually underwent transplant but recovered in response to medical management alone.18 According to data reported in June 2011 by the Organ Procurement and Transplantation Network,30 liver transplantation was needed in only three US patients with AFLP between 2000 and 2011. Further retrospective studies on outcomes from transplant versus medical management should be considered to guide future decision making involving this invasive therapy.
The Case Patient
This 39-year-old patient presented during a routine prenatal visit with proteinuria and hypertension, possibly indicative of preeclampsia. Because of the serious nature of this potential diagnosis in pregnancy, she was admitted for monitoring and further testing. Although the diagnosis of AFLP was not confirmed until later, the patient’s preliminary lab studies showed elevated liver enzymes and low platelet counts, signifying the need for prompt intervention and delivery of the infant. At this point, the patient met criteria for HELLP syndrome, but AFLP was suspected after the initial finding of profound hypoglycemia led to further testing.
As an older mother experiencing pregnancy for the first time, this patient fit the profile for AFLP. She initially responded well after delivery of her infant but continued to experience complications. On the days that the patient was treated with TPE, her total bilirubin and liver enzymes were at their lowest. Perhaps this treatment should be considered in more cases of AFLP.
The patient was transferred to a hospital with liver transplantation capabilities, but she ultimately recovered without undergoing transplant.
Conclusion
For the primary obstetric care provider, being aware of the possible complications associated with pregnancy is important. Though uncommon, AFLP is a serious complication that should be ruled out in women who present with vague symptoms such as nausea, vomiting, and abdominal pain in the third trimester of pregnancy. The reduction in AFLP-associated morbidity and mortality during the past 20 years is a direct result of increased early recognition and therapeutic delivery.
Referral to a maternal fetal medicine specialist, gastroenterologist, hematologist, and/or nephrologist may be necessary and appropriate in the management of a woman with AFLP. Further study is indicated for use of TPE in more severe cases of AFLP, particularly in women affected by persistent thrombocytopenia and anemia.
The author would like to thank C. Leanne Browning, MD, obstetrics/gynecology, for her invaluable guidance and advice on this project.
References
1. Martin JN Jr, Briery CM, Rose CH, et al. Postpartum plasma exchange as adjunctive therapy for severe acute fatty liver of pregnancy. J Clin Apher. 2009;23(4):138-143.
2. Knight M, Nelson-Piercy C, Kurinczuk JJ; UK Obstetric Surveillance System. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57(7):951-956.
3. Barsoom MJ, Tierney BJ. Acute fatty liver of pregnancy (2011). http://emedicine.medscape.com/article/1562425-overview. Accessed January 21, 2013.
4. Ko HH, Yoshida E. Acute fatty liver of pregnancy. Can J Gastroenterol. 2006;20(1):25-30.
5. Rathi U, Bapat M, Rathi P, Abraham P. Effect of liver disease on maternal and fetal outcome: a prospective study. Indian J Gastroenterol. 2007;26(2):59-63.
6. Myers L. Postpartum plasma exchange in a woman with suspected thrombotic thrombocytopenic purpura (TTP) vs hemolysis, elevated liver enzymes, and low platelet syndrome (HELLP): a case study. Nephrol Nurs J. 2010;37(4):399-402.
7. Vigil-de Gracia P. Acute fatty liver and HELLP syndrome: two distinct pregnancy disorders. Int J Gynaecol Obstet. 2001;73(3):215-220.
8. Aso K, Hojo S, Yumoto Y, et al. Three cases of acute fatty liver of pregnancy: postpartum clinical course depends on interval between onset of symptoms and termination of pregnancy. J Matern Fetal Neonatal Med. 2010;23(9):1047-1049.
9. Wei Q, Zhang L, Liu X. Clinical diagnosis and treatment of acute fatty liver of pregnancy: a literature review and 11 new cases. J Obstet Gynaecol Res. 2010;36(4):751-756.
10. Barber MA, Eguiluz I, Martin A, et al. Acute fatty liver of pregnancy: analysis of five consecutive cases from a tertiary centre. J Obstet Gynaecol. 2010;30(3):241-243.
11. Ajayi AO, Alao MO. Case report: acute fatty liver of pregnancy in a 30-year-old Nigerian primigravida. Niger J Clin Pract. 2008;11(4):389-391.
12. Vigíl-de Gracia P, Montufar-Rueda C. Acute fatty liver of pregnancy: diagnosis, treatment, and outcome based on 35 consecutive cases. J Matern Fetal Neonatal Med. 2011;24(9):1143-1146.
13. Dey M, Reema K. Acute fatty liver of pregnancy. N Am J Med Sci. 2012;4(11):611-612.
14. Castro MA, Goodwin TM, Shaw KJ, et al. Disseminated intravascular coagulation and antithrombin III depression in acute fatty liver of pregnancy. Am J Obstet Gynecol. 1996;174(1 pt 1):211-216.
15. Altuntas F, Aydogdu I, Kabukcu S, et al. Therapeutic plasma exchange for the treatment of thrombotic thrombocytopenic purpura: a retrospective multicenter study. Transfus Apher Sci. 2007;36(1):57-67.
16. Hay JE. Liver disease in pregnancy. Hepatology. 2008;47(3):1067-1076.
17. Wand S, Waeschle RM, Von Ahsen N, et al. Acute fatty liver failure due to acute fatty liver of pregnancy. Minerva Anesthesiol. 2012;78(4):503-506.
18. Westbrook RH, Yeoman AD, Joshi D, et al. Outcomes of severe pregnancy-related liver disease: refining the role of transplantation. Am J Transplant. 2010;10(11):2520-2526.
19. Fesenmeier MF, Coppage KH, Lambers DS, et al. Acute fatty liver of pregnancy in 3 tertiary care centers. Am J Obstet Gynecol. 2005;192(5):1416-1419.
20. Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol. 2011;35(3):182-193.
21. Huether SE. Alterations of digestive function. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1452-1515.
22. Huether SE. Structure and function of the digestive system. In: McCance KL, Huether SE, eds. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. St. Louis, MO: Mosby; 2009:1420-1451.
23. Schutt VA, Minuk GY. Liver diseases unique to pregnancy. Best Pract Res Clin Gastroenterol. 2007;21(5):771-792.
24. Pan C, Perumalswami PV. Pregnancy-related liver diseases. Clin Liver Dis. 2011;15(1):199-208.
25. Ibdah JA. Acute fatty liver of pregnancy: an update on pathogenesis and clinical implications. World J Gastroenterol. 2006;12(46):7397-7404.
26. Browning MF, Levy HL, Wilkins-Haug LE, et al. Fetal fatty acid oxidation defects and maternal liver disease in pregnancy. Obstet Gynecol. 2006;107(1):115-120.
27. Dekker RR, Schutte JM, Stekelenburg J, et al. Maternal mortality and severe maternal morbidity from acute fatty liver of pregnancy in the Netherlands. Eur J Obstet Gynecol Reprod Biol. 2011;157(1):27-31.
28. Lee NM, Brady CW. Liver disease in pregnancy. World J Gastroenterol. 2009;15(8):897-906.
29. Vora KS, Shah VR, Parikh GP. Acute fatty liver of pregnancy: a case report of an uncommon disease. Indian J Crit Care Med. 2009;13(1):34-36.
30. Organ Procurement and Transplantation Network, Scientific Registry of Transplant Recipients. OPTN/SRTR 2011 Annual Data Report: Liver. http://srtr.transplant.hrsa.gov/annual_reports/2011/pdf/03_%20liver_12.pdf. Accessed January 18, 2013.