User login
Survey Shows Five-Year Decrease in Employee Benefits, Paid Time Off for Hospitalists
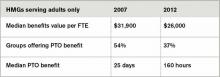
For the first time in several years, SHM included questions about employee benefits and paid time off in its 2012 State of Hospital Medicine survey. The median value of benefits per physician FTE reported by HM groups serving adults only was $26,000, according to the 2012 survey. But what a surprise it was when survey respondents in 2007 reported median benefits of $31,900.
I admit to being flummoxed by the decrease. The definition of “benefits” was identical in both surveys. The only difference is that in 2007, SHM collected actual benefit cost for each individual on the individual questionnaire; in 2012, we asked for the average benefits per FTE for the group. One possible explanation is that some respondents simply guessed about the average, because they didn’t have to report data for individual doctors. Of course, it’s also possible that groups are requiring physicians to pay a higher proportion of insurance premiums or are reducing retirement plan contributions due to the weak economy. But in the work I do with hospitalist groups around the country, I rarely see benefit costs below about $35,000.
Source: 2012 State of Hospital Medicine report
Another interesting finding from the 2012 survey is that 37% of adult medicine groups reported offering paid time off (PTO), down from 54% in 2007. Even among groups using a seven-on/seven-off schedule, the PTO rate was only 44%. Does this represent a survey design or respondent input error, differences in respondent populations, or an actual shift in the prevalence of PTO benefits? I suspect it’s the latter, because the median amount of PTO time awarded has also declined. In 2007, adult HMGs reported a median of 25 PTO days annually. In 2012, the median for those groups offering PTO was 160 hours of PTO, which represents somewhere around 13 to 20 days, depending on shift length.
Why might PTO benefits be declining? I suppose it could be belt-tightening associated with the poor economy. But I think many HM groups simply have found PTO benefits difficult to administer and fraught with unintended consequences. Many groups are so thinly staffed that for someone to take a PTO day, someone else must work extra to cover. Then, when the covering doctor takes PTO, the first doctor must work extra—effectively offsetting the value of PTO. And if a hospitalist takes PTO and also works extra shifts in the same pay period, do these two offset each other? Or does the doctor get paid for both the PTO days and the extra shift days?
For clinicians such as hospitalists, whose work is defined in highly variable, shift-based schedules that include a lot of night and weekend work, it becomes very difficult to determine which of the days not worked were PTO days versus just days the doctor wasn’t scheduled.
Personally, I don’t think it makes much sense for most hospitalists to have PTO. Don’t get me wrong—I think hospitalists should be paid well and have generous amounts of time off in exchange for long, challenging workdays and a disproportionate amount of night and weekend work. But arbitrarily assigning some of the days not worked as PTO while others are just unscheduled days seems unnecessarily complex.
Time will tell if the specialty as a whole agrees with me or not.
Leslie Flores is a principal in Nelson Flores Hospital Medicine Consultants and a member of SHM’s Practice Analysis Committee.
For the first time in several years, SHM included questions about employee benefits and paid time off in its 2012 State of Hospital Medicine survey. The median value of benefits per physician FTE reported by HM groups serving adults only was $26,000, according to the 2012 survey. But what a surprise it was when survey respondents in 2007 reported median benefits of $31,900.
I admit to being flummoxed by the decrease. The definition of “benefits” was identical in both surveys. The only difference is that in 2007, SHM collected actual benefit cost for each individual on the individual questionnaire; in 2012, we asked for the average benefits per FTE for the group. One possible explanation is that some respondents simply guessed about the average, because they didn’t have to report data for individual doctors. Of course, it’s also possible that groups are requiring physicians to pay a higher proportion of insurance premiums or are reducing retirement plan contributions due to the weak economy. But in the work I do with hospitalist groups around the country, I rarely see benefit costs below about $35,000.
Source: 2012 State of Hospital Medicine report
Another interesting finding from the 2012 survey is that 37% of adult medicine groups reported offering paid time off (PTO), down from 54% in 2007. Even among groups using a seven-on/seven-off schedule, the PTO rate was only 44%. Does this represent a survey design or respondent input error, differences in respondent populations, or an actual shift in the prevalence of PTO benefits? I suspect it’s the latter, because the median amount of PTO time awarded has also declined. In 2007, adult HMGs reported a median of 25 PTO days annually. In 2012, the median for those groups offering PTO was 160 hours of PTO, which represents somewhere around 13 to 20 days, depending on shift length.
Why might PTO benefits be declining? I suppose it could be belt-tightening associated with the poor economy. But I think many HM groups simply have found PTO benefits difficult to administer and fraught with unintended consequences. Many groups are so thinly staffed that for someone to take a PTO day, someone else must work extra to cover. Then, when the covering doctor takes PTO, the first doctor must work extra—effectively offsetting the value of PTO. And if a hospitalist takes PTO and also works extra shifts in the same pay period, do these two offset each other? Or does the doctor get paid for both the PTO days and the extra shift days?
For clinicians such as hospitalists, whose work is defined in highly variable, shift-based schedules that include a lot of night and weekend work, it becomes very difficult to determine which of the days not worked were PTO days versus just days the doctor wasn’t scheduled.
Personally, I don’t think it makes much sense for most hospitalists to have PTO. Don’t get me wrong—I think hospitalists should be paid well and have generous amounts of time off in exchange for long, challenging workdays and a disproportionate amount of night and weekend work. But arbitrarily assigning some of the days not worked as PTO while others are just unscheduled days seems unnecessarily complex.
Time will tell if the specialty as a whole agrees with me or not.
Leslie Flores is a principal in Nelson Flores Hospital Medicine Consultants and a member of SHM’s Practice Analysis Committee.
For the first time in several years, SHM included questions about employee benefits and paid time off in its 2012 State of Hospital Medicine survey. The median value of benefits per physician FTE reported by HM groups serving adults only was $26,000, according to the 2012 survey. But what a surprise it was when survey respondents in 2007 reported median benefits of $31,900.
I admit to being flummoxed by the decrease. The definition of “benefits” was identical in both surveys. The only difference is that in 2007, SHM collected actual benefit cost for each individual on the individual questionnaire; in 2012, we asked for the average benefits per FTE for the group. One possible explanation is that some respondents simply guessed about the average, because they didn’t have to report data for individual doctors. Of course, it’s also possible that groups are requiring physicians to pay a higher proportion of insurance premiums or are reducing retirement plan contributions due to the weak economy. But in the work I do with hospitalist groups around the country, I rarely see benefit costs below about $35,000.
Source: 2012 State of Hospital Medicine report
Another interesting finding from the 2012 survey is that 37% of adult medicine groups reported offering paid time off (PTO), down from 54% in 2007. Even among groups using a seven-on/seven-off schedule, the PTO rate was only 44%. Does this represent a survey design or respondent input error, differences in respondent populations, or an actual shift in the prevalence of PTO benefits? I suspect it’s the latter, because the median amount of PTO time awarded has also declined. In 2007, adult HMGs reported a median of 25 PTO days annually. In 2012, the median for those groups offering PTO was 160 hours of PTO, which represents somewhere around 13 to 20 days, depending on shift length.
Why might PTO benefits be declining? I suppose it could be belt-tightening associated with the poor economy. But I think many HM groups simply have found PTO benefits difficult to administer and fraught with unintended consequences. Many groups are so thinly staffed that for someone to take a PTO day, someone else must work extra to cover. Then, when the covering doctor takes PTO, the first doctor must work extra—effectively offsetting the value of PTO. And if a hospitalist takes PTO and also works extra shifts in the same pay period, do these two offset each other? Or does the doctor get paid for both the PTO days and the extra shift days?
For clinicians such as hospitalists, whose work is defined in highly variable, shift-based schedules that include a lot of night and weekend work, it becomes very difficult to determine which of the days not worked were PTO days versus just days the doctor wasn’t scheduled.
Personally, I don’t think it makes much sense for most hospitalists to have PTO. Don’t get me wrong—I think hospitalists should be paid well and have generous amounts of time off in exchange for long, challenging workdays and a disproportionate amount of night and weekend work. But arbitrarily assigning some of the days not worked as PTO while others are just unscheduled days seems unnecessarily complex.
Time will tell if the specialty as a whole agrees with me or not.
Leslie Flores is a principal in Nelson Flores Hospital Medicine Consultants and a member of SHM’s Practice Analysis Committee.
Hospital Medicine Advocates Aid in Securing $10 Million for National Quality Forum
Hospitalists on the Hill
WHEN: May 16, 2013
WHERE: Washington, D.C.
HOW: Register today at www.hospitalmedicine2013.org/onthehill Space is limited.
The American Taxpayer Relief Act, which was signed into law Jan. 3 to stave off the fiscal cliff, contained a little-mentioned provision that reauthorized $10 million in Medicare funding for the National Quality Forum (NQF). Hospitalists, taking on the role of policy advocates, helped secure this reauthorization and score a victory for national quality-improvement (QI) efforts.
The NQF is a nonprofit organization established to create consensus around national priorities and measures for performing reporting and improvement in healthcare. It receives funding through a variety of sources ranging from public funds, private organizations and membership dues. Since 2009, it has received $10 million annually from a U.S. Department of Health and Human Services contract. That contract, a significant portion of NQF funding, was set to end in 2012.
The Stand for Quality coalition, composed of healthcare organizations supportive of the NQF, helps ensure that Congress sustains funding and support of performance measurement reporting and QI. SHM historically has been a strong proponent of the NQF, pitching in at the start of Stand for Quality four years ago this month. As it became clear that the federal contract for the NQF was not likely to be renewed by Congress for the 2013 calendar year, the member organizations of Stand for Quality were asked to step up to the plate in any way they could.
Jumping at the opportunity to assist, SHM linked hospitalists to their members of Congress in support of the NQF. Through members of SHM's Public Policy and Performance Measurement and Reporting committees, SHM connected with congressional offices and urged them to join a sign-on letter from the office of U.S. Sen. Mark Begich (D-Alaska) office in support of continued NQF funding. Letters between members of Congress are a way that legislators internally lobby on behalf of a particular issue. Hearing from their constituents can help persuade a legislator to act.
Sharing professional expertise also can inform a legislator's decisions. SHM members have a wealth of specialized knowledge and experiences that greatly benefit health policy discussions. In December 2012, SHM was asked to join NQF staffers and several other organizations on Capitol Hill for visits with select members of Congress, including several physician-legislators. These meetings were designed to raise the profile of the important work NQF is doing for QI and to make sure that NQF funding was not forgotten in any debt deal.
Mangla Gulati, MD, FACP, FHM, a hospitalist and SHM member, participated in these visits on behalf of hospital medicine and provided much-needed physician input on the importance of NQF's work.
The benefits of in-person visits are mutual: Members of Congress learn more about the real-life effects of a particular policy and, according to Dr. Gulati, "the visits to the Hill were a very valuable experience and shed a whole new light on the challenges we face in medicine."
Taken together, these advocacy tactics—coalition-building, contacting congressional offices, and in-person visits in Washington—were crucial to securing NQF's funding reauthorization and are critical tools for the advocacy work at SHM. The most concrete and impactful advocacy includes positioning hospitalists on the front line and sharing their perspectives and experiences with policymakers and their staffs.
We hope you'll heed this call and join SHM in Washington, D.C., May 16 for Hospitalists on the Hill, part of SHM's annual meeting. For more information and to register, go to www.hospitalmedicine2013.org/onthehill.
Joshua Lapps is SHM's government relations specialist.
Hospitalists on the Hill
WHEN: May 16, 2013
WHERE: Washington, D.C.
HOW: Register today at www.hospitalmedicine2013.org/onthehill Space is limited.
The American Taxpayer Relief Act, which was signed into law Jan. 3 to stave off the fiscal cliff, contained a little-mentioned provision that reauthorized $10 million in Medicare funding for the National Quality Forum (NQF). Hospitalists, taking on the role of policy advocates, helped secure this reauthorization and score a victory for national quality-improvement (QI) efforts.
The NQF is a nonprofit organization established to create consensus around national priorities and measures for performing reporting and improvement in healthcare. It receives funding through a variety of sources ranging from public funds, private organizations and membership dues. Since 2009, it has received $10 million annually from a U.S. Department of Health and Human Services contract. That contract, a significant portion of NQF funding, was set to end in 2012.
The Stand for Quality coalition, composed of healthcare organizations supportive of the NQF, helps ensure that Congress sustains funding and support of performance measurement reporting and QI. SHM historically has been a strong proponent of the NQF, pitching in at the start of Stand for Quality four years ago this month. As it became clear that the federal contract for the NQF was not likely to be renewed by Congress for the 2013 calendar year, the member organizations of Stand for Quality were asked to step up to the plate in any way they could.
Jumping at the opportunity to assist, SHM linked hospitalists to their members of Congress in support of the NQF. Through members of SHM's Public Policy and Performance Measurement and Reporting committees, SHM connected with congressional offices and urged them to join a sign-on letter from the office of U.S. Sen. Mark Begich (D-Alaska) office in support of continued NQF funding. Letters between members of Congress are a way that legislators internally lobby on behalf of a particular issue. Hearing from their constituents can help persuade a legislator to act.
Sharing professional expertise also can inform a legislator's decisions. SHM members have a wealth of specialized knowledge and experiences that greatly benefit health policy discussions. In December 2012, SHM was asked to join NQF staffers and several other organizations on Capitol Hill for visits with select members of Congress, including several physician-legislators. These meetings were designed to raise the profile of the important work NQF is doing for QI and to make sure that NQF funding was not forgotten in any debt deal.
Mangla Gulati, MD, FACP, FHM, a hospitalist and SHM member, participated in these visits on behalf of hospital medicine and provided much-needed physician input on the importance of NQF's work.
The benefits of in-person visits are mutual: Members of Congress learn more about the real-life effects of a particular policy and, according to Dr. Gulati, "the visits to the Hill were a very valuable experience and shed a whole new light on the challenges we face in medicine."
Taken together, these advocacy tactics—coalition-building, contacting congressional offices, and in-person visits in Washington—were crucial to securing NQF's funding reauthorization and are critical tools for the advocacy work at SHM. The most concrete and impactful advocacy includes positioning hospitalists on the front line and sharing their perspectives and experiences with policymakers and their staffs.
We hope you'll heed this call and join SHM in Washington, D.C., May 16 for Hospitalists on the Hill, part of SHM's annual meeting. For more information and to register, go to www.hospitalmedicine2013.org/onthehill.
Joshua Lapps is SHM's government relations specialist.
Hospitalists on the Hill
WHEN: May 16, 2013
WHERE: Washington, D.C.
HOW: Register today at www.hospitalmedicine2013.org/onthehill Space is limited.
The American Taxpayer Relief Act, which was signed into law Jan. 3 to stave off the fiscal cliff, contained a little-mentioned provision that reauthorized $10 million in Medicare funding for the National Quality Forum (NQF). Hospitalists, taking on the role of policy advocates, helped secure this reauthorization and score a victory for national quality-improvement (QI) efforts.
The NQF is a nonprofit organization established to create consensus around national priorities and measures for performing reporting and improvement in healthcare. It receives funding through a variety of sources ranging from public funds, private organizations and membership dues. Since 2009, it has received $10 million annually from a U.S. Department of Health and Human Services contract. That contract, a significant portion of NQF funding, was set to end in 2012.
The Stand for Quality coalition, composed of healthcare organizations supportive of the NQF, helps ensure that Congress sustains funding and support of performance measurement reporting and QI. SHM historically has been a strong proponent of the NQF, pitching in at the start of Stand for Quality four years ago this month. As it became clear that the federal contract for the NQF was not likely to be renewed by Congress for the 2013 calendar year, the member organizations of Stand for Quality were asked to step up to the plate in any way they could.
Jumping at the opportunity to assist, SHM linked hospitalists to their members of Congress in support of the NQF. Through members of SHM's Public Policy and Performance Measurement and Reporting committees, SHM connected with congressional offices and urged them to join a sign-on letter from the office of U.S. Sen. Mark Begich (D-Alaska) office in support of continued NQF funding. Letters between members of Congress are a way that legislators internally lobby on behalf of a particular issue. Hearing from their constituents can help persuade a legislator to act.
Sharing professional expertise also can inform a legislator's decisions. SHM members have a wealth of specialized knowledge and experiences that greatly benefit health policy discussions. In December 2012, SHM was asked to join NQF staffers and several other organizations on Capitol Hill for visits with select members of Congress, including several physician-legislators. These meetings were designed to raise the profile of the important work NQF is doing for QI and to make sure that NQF funding was not forgotten in any debt deal.
Mangla Gulati, MD, FACP, FHM, a hospitalist and SHM member, participated in these visits on behalf of hospital medicine and provided much-needed physician input on the importance of NQF's work.
The benefits of in-person visits are mutual: Members of Congress learn more about the real-life effects of a particular policy and, according to Dr. Gulati, "the visits to the Hill were a very valuable experience and shed a whole new light on the challenges we face in medicine."
Taken together, these advocacy tactics—coalition-building, contacting congressional offices, and in-person visits in Washington—were crucial to securing NQF's funding reauthorization and are critical tools for the advocacy work at SHM. The most concrete and impactful advocacy includes positioning hospitalists on the front line and sharing their perspectives and experiences with policymakers and their staffs.
We hope you'll heed this call and join SHM in Washington, D.C., May 16 for Hospitalists on the Hill, part of SHM's annual meeting. For more information and to register, go to www.hospitalmedicine2013.org/onthehill.
Joshua Lapps is SHM's government relations specialist.
We Welcome the Newest SHM Members
- D. Davis, MD, Alabama
- V. Palabindala, Alabama
- V. Do, Arizona
- G. Khera, MD, Arizona
- A. Afrashteh, MD, California
- P. Alegarbes, California
- J. Close, California
- B. Davis, DO , California
- J. Eng, MD, California
- C. Liao, MD, California
- A. Manoharan, MBBS, California
- K. Martinez, California
- K. Mothkuri, MD, California
- M. Ochner, MD, MPH, California
- T. Ososkova, MD, California
- H. Selke, MD, California
- M. Sethi, MD, California
- S. Sonti, MD, California
- C. Tsay, California
- D. Virnich, MD, MBA, California
- A. Montoya, FNP, Colorado
- J. Nickelsen, MD, Colorado
- V. Kota, MD, Connecticut
- S. Kim, MD, Delaware
- N. Serafimova, MD, Delaware
- S. Brulte, MD, Florida
- A. Camacho, AN P, Florida
- E. Carter, MD, Florida
- C. Cesa, MD, Florida
- K. Eaton, PA-C, Florida
- N. Harris, MD, Florida
- T. Jones, MD, Florida
- A. Karmand, MD, Florida
- A. Laila, MD, Florida
- M. Lane, MD, Florida
- L. Leisch, MD, Florida
- V. Ngo, MD, Florida
- H. Patel, DO , Florida
- M. Pop, MD, Florida
- A. Rahman, MD, Florida
- J. Whynot, MD, Florida
- P. Amene, MBBS, Georgia
- A. Bawa, MD, Georgia
- J. Dee, Georgia
- C. Henritz, DO , Georgia
- Y. Imran, MD, Georgia
- J. Mikell, MD, Georgia
- D. Nagarajan, MD, Georgia
- L. Porter, MD, Georgia
- K. Thykeson, MD, Idaho
- C. Beveridge, Illinois
- R. Helfrich, MD, Illinois
- R. Kellum, MD, Illinois
- T. Mahmood, MD, Illinois
- D. Patel, MD, Illinois
- M. Regala, MD, Illinois
- H. Sandhu, MD, Illinois
- U. Tekin, MD, Illinois
- D. Azad, MD, FACP, MPH, Indiana
- J. Light, MD, Indiana
- P. Marpu, MD, Indiana
- N. Paul, ACNP, Indiana
- C. Bowers, MD, Kansas
- L. Fanucchi, MD, MPH, Kentucky
- S. Kad, MD, FACP, MPH, MS, USAR , Kentucky
- D. Davis, MD, Alabama
- V. Palabindala, Alabama
- V. Do, Arizona
- G. Khera, MD, Arizona
- A. Afrashteh, MD, California
- P. Alegarbes, California
- J. Close, California
- B. Davis, DO , California
- J. Eng, MD, California
- C. Liao, MD, California
- A. Manoharan, MBBS, California
- K. Martinez, California
- K. Mothkuri, MD, California
- M. Ochner, MD, MPH, California
- T. Ososkova, MD, California
- H. Selke, MD, California
- M. Sethi, MD, California
- S. Sonti, MD, California
- C. Tsay, California
- D. Virnich, MD, MBA, California
- A. Montoya, FNP, Colorado
- J. Nickelsen, MD, Colorado
- V. Kota, MD, Connecticut
- S. Kim, MD, Delaware
- N. Serafimova, MD, Delaware
- S. Brulte, MD, Florida
- A. Camacho, AN P, Florida
- E. Carter, MD, Florida
- C. Cesa, MD, Florida
- K. Eaton, PA-C, Florida
- N. Harris, MD, Florida
- T. Jones, MD, Florida
- A. Karmand, MD, Florida
- A. Laila, MD, Florida
- M. Lane, MD, Florida
- L. Leisch, MD, Florida
- V. Ngo, MD, Florida
- H. Patel, DO , Florida
- M. Pop, MD, Florida
- A. Rahman, MD, Florida
- J. Whynot, MD, Florida
- P. Amene, MBBS, Georgia
- A. Bawa, MD, Georgia
- J. Dee, Georgia
- C. Henritz, DO , Georgia
- Y. Imran, MD, Georgia
- J. Mikell, MD, Georgia
- D. Nagarajan, MD, Georgia
- L. Porter, MD, Georgia
- K. Thykeson, MD, Idaho
- C. Beveridge, Illinois
- R. Helfrich, MD, Illinois
- R. Kellum, MD, Illinois
- T. Mahmood, MD, Illinois
- D. Patel, MD, Illinois
- M. Regala, MD, Illinois
- H. Sandhu, MD, Illinois
- U. Tekin, MD, Illinois
- D. Azad, MD, FACP, MPH, Indiana
- J. Light, MD, Indiana
- P. Marpu, MD, Indiana
- N. Paul, ACNP, Indiana
- C. Bowers, MD, Kansas
- L. Fanucchi, MD, MPH, Kentucky
- S. Kad, MD, FACP, MPH, MS, USAR , Kentucky
- D. Davis, MD, Alabama
- V. Palabindala, Alabama
- V. Do, Arizona
- G. Khera, MD, Arizona
- A. Afrashteh, MD, California
- P. Alegarbes, California
- J. Close, California
- B. Davis, DO , California
- J. Eng, MD, California
- C. Liao, MD, California
- A. Manoharan, MBBS, California
- K. Martinez, California
- K. Mothkuri, MD, California
- M. Ochner, MD, MPH, California
- T. Ososkova, MD, California
- H. Selke, MD, California
- M. Sethi, MD, California
- S. Sonti, MD, California
- C. Tsay, California
- D. Virnich, MD, MBA, California
- A. Montoya, FNP, Colorado
- J. Nickelsen, MD, Colorado
- V. Kota, MD, Connecticut
- S. Kim, MD, Delaware
- N. Serafimova, MD, Delaware
- S. Brulte, MD, Florida
- A. Camacho, AN P, Florida
- E. Carter, MD, Florida
- C. Cesa, MD, Florida
- K. Eaton, PA-C, Florida
- N. Harris, MD, Florida
- T. Jones, MD, Florida
- A. Karmand, MD, Florida
- A. Laila, MD, Florida
- M. Lane, MD, Florida
- L. Leisch, MD, Florida
- V. Ngo, MD, Florida
- H. Patel, DO , Florida
- M. Pop, MD, Florida
- A. Rahman, MD, Florida
- J. Whynot, MD, Florida
- P. Amene, MBBS, Georgia
- A. Bawa, MD, Georgia
- J. Dee, Georgia
- C. Henritz, DO , Georgia
- Y. Imran, MD, Georgia
- J. Mikell, MD, Georgia
- D. Nagarajan, MD, Georgia
- L. Porter, MD, Georgia
- K. Thykeson, MD, Idaho
- C. Beveridge, Illinois
- R. Helfrich, MD, Illinois
- R. Kellum, MD, Illinois
- T. Mahmood, MD, Illinois
- D. Patel, MD, Illinois
- M. Regala, MD, Illinois
- H. Sandhu, MD, Illinois
- U. Tekin, MD, Illinois
- D. Azad, MD, FACP, MPH, Indiana
- J. Light, MD, Indiana
- P. Marpu, MD, Indiana
- N. Paul, ACNP, Indiana
- C. Bowers, MD, Kansas
- L. Fanucchi, MD, MPH, Kentucky
- S. Kad, MD, FACP, MPH, MS, USAR , Kentucky
HMX Term of the Month: Achievement Points
Awarded to a hospital by comparing an individual hospital’s performance measure rates during a certain period with all hospitals’ rates during the baseline period.
Awarded to a hospital by comparing an individual hospital’s performance measure rates during a certain period with all hospitals’ rates during the baseline period.
Awarded to a hospital by comparing an individual hospital’s performance measure rates during a certain period with all hospitals’ rates during the baseline period.
Fellow in Hospital Medicine Spotlight: Katherine Hochman, MD, MBA, FHM
Dr. Hochman is assistant chief of medicine service and director of the hospitalist program at New York University Medical Center (NYUMC) in New York City. She is a clinical assistant professor at New York University School of Medicine. She earned her Fellow in Hospital Medicine designation in 2008.
Undergraduate education: University of Pennsylvania, Philadelphia.
Medical school: University of Miami Miller School of Medicine.
Notable: In 2004, Dr. Hochman was the first and only hospitalist at NYUMC. Today, there are 23.5 hospitalists in the program, thanks to her work in founding the NYU Hospitalist Group. Although she and her team struggled with recruiting hospitalists to work nights and weekends, her directorship of the NYU Hospitalist Scholars program, which combines clinical work and research mentorship, has helped the group attract physicians for those shifts.
As director, she and other hospitalists have created the NYC Hospitalist Directors’ Consortium, which meets regularly through SHM. Dr. Hochman has mentored and passed on her hospitalist passion to dozens of graduate students, residents, and post-doctoral fellows. As a result of her mentorship and dedication to education, she was awarded the 2003 Firm Chief Award for Outstanding Medical Student Teaching and the 2005 NYU Teacher of the Year Award.
FYI: A mother of three, Dr. Hochman still finds time to follow her passion for museum visits. Her favorite haunt is the Museum of Modern Art. She has even staged innovative team-building events using themed museum tours. She also coaches an indoor soccer club.
Quotable: "The SHM fellowship is an important distinction for me. It shows a continued commitment to the field of hospital medicine."
Dr. Hochman is assistant chief of medicine service and director of the hospitalist program at New York University Medical Center (NYUMC) in New York City. She is a clinical assistant professor at New York University School of Medicine. She earned her Fellow in Hospital Medicine designation in 2008.
Undergraduate education: University of Pennsylvania, Philadelphia.
Medical school: University of Miami Miller School of Medicine.
Notable: In 2004, Dr. Hochman was the first and only hospitalist at NYUMC. Today, there are 23.5 hospitalists in the program, thanks to her work in founding the NYU Hospitalist Group. Although she and her team struggled with recruiting hospitalists to work nights and weekends, her directorship of the NYU Hospitalist Scholars program, which combines clinical work and research mentorship, has helped the group attract physicians for those shifts.
As director, she and other hospitalists have created the NYC Hospitalist Directors’ Consortium, which meets regularly through SHM. Dr. Hochman has mentored and passed on her hospitalist passion to dozens of graduate students, residents, and post-doctoral fellows. As a result of her mentorship and dedication to education, she was awarded the 2003 Firm Chief Award for Outstanding Medical Student Teaching and the 2005 NYU Teacher of the Year Award.
FYI: A mother of three, Dr. Hochman still finds time to follow her passion for museum visits. Her favorite haunt is the Museum of Modern Art. She has even staged innovative team-building events using themed museum tours. She also coaches an indoor soccer club.
Quotable: "The SHM fellowship is an important distinction for me. It shows a continued commitment to the field of hospital medicine."
Dr. Hochman is assistant chief of medicine service and director of the hospitalist program at New York University Medical Center (NYUMC) in New York City. She is a clinical assistant professor at New York University School of Medicine. She earned her Fellow in Hospital Medicine designation in 2008.
Undergraduate education: University of Pennsylvania, Philadelphia.
Medical school: University of Miami Miller School of Medicine.
Notable: In 2004, Dr. Hochman was the first and only hospitalist at NYUMC. Today, there are 23.5 hospitalists in the program, thanks to her work in founding the NYU Hospitalist Group. Although she and her team struggled with recruiting hospitalists to work nights and weekends, her directorship of the NYU Hospitalist Scholars program, which combines clinical work and research mentorship, has helped the group attract physicians for those shifts.
As director, she and other hospitalists have created the NYC Hospitalist Directors’ Consortium, which meets regularly through SHM. Dr. Hochman has mentored and passed on her hospitalist passion to dozens of graduate students, residents, and post-doctoral fellows. As a result of her mentorship and dedication to education, she was awarded the 2003 Firm Chief Award for Outstanding Medical Student Teaching and the 2005 NYU Teacher of the Year Award.
FYI: A mother of three, Dr. Hochman still finds time to follow her passion for museum visits. Her favorite haunt is the Museum of Modern Art. She has even staged innovative team-building events using themed museum tours. She also coaches an indoor soccer club.
Quotable: "The SHM fellowship is an important distinction for me. It shows a continued commitment to the field of hospital medicine."
Is Your Hospital Medicine Group a Good Candidate for Project BOOST?
Does your team have:
- Eagerness to improve their discharge processes and reduce unnecessary readmissions and avoidable adverse events in the post-discharge period?
- A multidisciplinary team in place capable of working collaboratively to redesign existing care processes?
- A dedicated leader to manage the process of tailoring the BOOST intervention to your site’s needs and implementing BOOST?
- Support of at least one executive sponsor who can meet with the team monthly?
- Access to data support personnel needed to collect baseline and post-implementation data?
Does your team have:
- Eagerness to improve their discharge processes and reduce unnecessary readmissions and avoidable adverse events in the post-discharge period?
- A multidisciplinary team in place capable of working collaboratively to redesign existing care processes?
- A dedicated leader to manage the process of tailoring the BOOST intervention to your site’s needs and implementing BOOST?
- Support of at least one executive sponsor who can meet with the team monthly?
- Access to data support personnel needed to collect baseline and post-implementation data?
Does your team have:
- Eagerness to improve their discharge processes and reduce unnecessary readmissions and avoidable adverse events in the post-discharge period?
- A multidisciplinary team in place capable of working collaboratively to redesign existing care processes?
- A dedicated leader to manage the process of tailoring the BOOST intervention to your site’s needs and implementing BOOST?
- Support of at least one executive sponsor who can meet with the team monthly?
- Access to data support personnel needed to collect baseline and post-implementation data?
Soaring Medicare Costs for Unplanned Hospitalizations Underscore Need to Reduce Readmissions
- According to research published in the New England Journal of Medicine, about 1 in 5 hospitalized Medicare beneficiaries were readmitted within 30 days after discharge. Unplanned rehospitalizations cost Medicare $17.4 billion in 2004.
- The Project BOOST toolkit has been downloaded more than 4,000 times.
- Project BOOST has been implemented at more than 150 sites nationwide.
- Early data from six sites that have implemented Project BOOST reveal a reduction in 30-day readmission rates to 11.2% from 14.2%, as well as a 21% reduction in 30-day, all-cause readmission rates.
Source: www.hospitalmedicine.org
- According to research published in the New England Journal of Medicine, about 1 in 5 hospitalized Medicare beneficiaries were readmitted within 30 days after discharge. Unplanned rehospitalizations cost Medicare $17.4 billion in 2004.
- The Project BOOST toolkit has been downloaded more than 4,000 times.
- Project BOOST has been implemented at more than 150 sites nationwide.
- Early data from six sites that have implemented Project BOOST reveal a reduction in 30-day readmission rates to 11.2% from 14.2%, as well as a 21% reduction in 30-day, all-cause readmission rates.
Source: www.hospitalmedicine.org
- According to research published in the New England Journal of Medicine, about 1 in 5 hospitalized Medicare beneficiaries were readmitted within 30 days after discharge. Unplanned rehospitalizations cost Medicare $17.4 billion in 2004.
- The Project BOOST toolkit has been downloaded more than 4,000 times.
- Project BOOST has been implemented at more than 150 sites nationwide.
- Early data from six sites that have implemented Project BOOST reveal a reduction in 30-day readmission rates to 11.2% from 14.2%, as well as a 21% reduction in 30-day, all-cause readmission rates.
Source: www.hospitalmedicine.org
Hospitalists Urged to Help Reduce 30-Day Readmission Rate
For hospitals across the country, 2013 is the year to address readmissions and find practical solutions. In January, the Journal of the American Medical Association dedicated
an entire issue to the vexing problem of hospital readmissions. In his audio summary of the issue, JAMA editor Howard Bauchner, MD, notes that it “came together organically,” based on increased submissions and attention to 30-day readmissions.
Among nearly a dozen articles focused on readmissions, discharge, and transitions of care, Project BOOST principal investigator Mark V. Williams, MD, FACP, MHM, makes the case for a community-based approach in an editorial titled “A Requirement to Reduce Readmissions: Take Care of the Patient, Not Just the Disease.” In the piece, he advocates for “broad patient-centered approaches that engage all members of a care team, especially front-line clinicians and use proven quality-improvement [QI] methods.” He goes on to link the concepts to the principles taught by Project BOOST.
After all, readmissions are expensive, and not just for hospitals, which is why private insurers and the Centers for Medicare & Medicaid Services (CMS) are investing resources to improve discharge processes, reduce readmissions, and reduce costs.
Many adverse events that happen after discharge are predictable using assessment tools and methods in the Project BOOST program, Dr. Williams says. Hospitalists can—and should, according to many—improve the system to protect patients.
And while systemwide change doesn’t happen overnight, it does have to start somewhere, as leaders at the 150-plus Project BOOST sites nationwide can attest Now is the time to begin planning to join the Project BOOST 2013 cohort. Applications will be accepted through this summer; training will begin in the fall. But participation is limited, and successful applicants often need time to prepare their applications, which must include letters of support from a site executive and the development of a multidisciplinary team. For more information, visit www.hospitalmedicine.org/boost.
Brendon Shank is SHM’s associate vice president of communications.
For hospitals across the country, 2013 is the year to address readmissions and find practical solutions. In January, the Journal of the American Medical Association dedicated
an entire issue to the vexing problem of hospital readmissions. In his audio summary of the issue, JAMA editor Howard Bauchner, MD, notes that it “came together organically,” based on increased submissions and attention to 30-day readmissions.
Among nearly a dozen articles focused on readmissions, discharge, and transitions of care, Project BOOST principal investigator Mark V. Williams, MD, FACP, MHM, makes the case for a community-based approach in an editorial titled “A Requirement to Reduce Readmissions: Take Care of the Patient, Not Just the Disease.” In the piece, he advocates for “broad patient-centered approaches that engage all members of a care team, especially front-line clinicians and use proven quality-improvement [QI] methods.” He goes on to link the concepts to the principles taught by Project BOOST.
After all, readmissions are expensive, and not just for hospitals, which is why private insurers and the Centers for Medicare & Medicaid Services (CMS) are investing resources to improve discharge processes, reduce readmissions, and reduce costs.
Many adverse events that happen after discharge are predictable using assessment tools and methods in the Project BOOST program, Dr. Williams says. Hospitalists can—and should, according to many—improve the system to protect patients.
And while systemwide change doesn’t happen overnight, it does have to start somewhere, as leaders at the 150-plus Project BOOST sites nationwide can attest Now is the time to begin planning to join the Project BOOST 2013 cohort. Applications will be accepted through this summer; training will begin in the fall. But participation is limited, and successful applicants often need time to prepare their applications, which must include letters of support from a site executive and the development of a multidisciplinary team. For more information, visit www.hospitalmedicine.org/boost.
Brendon Shank is SHM’s associate vice president of communications.
For hospitals across the country, 2013 is the year to address readmissions and find practical solutions. In January, the Journal of the American Medical Association dedicated
an entire issue to the vexing problem of hospital readmissions. In his audio summary of the issue, JAMA editor Howard Bauchner, MD, notes that it “came together organically,” based on increased submissions and attention to 30-day readmissions.
Among nearly a dozen articles focused on readmissions, discharge, and transitions of care, Project BOOST principal investigator Mark V. Williams, MD, FACP, MHM, makes the case for a community-based approach in an editorial titled “A Requirement to Reduce Readmissions: Take Care of the Patient, Not Just the Disease.” In the piece, he advocates for “broad patient-centered approaches that engage all members of a care team, especially front-line clinicians and use proven quality-improvement [QI] methods.” He goes on to link the concepts to the principles taught by Project BOOST.
After all, readmissions are expensive, and not just for hospitals, which is why private insurers and the Centers for Medicare & Medicaid Services (CMS) are investing resources to improve discharge processes, reduce readmissions, and reduce costs.
Many adverse events that happen after discharge are predictable using assessment tools and methods in the Project BOOST program, Dr. Williams says. Hospitalists can—and should, according to many—improve the system to protect patients.
And while systemwide change doesn’t happen overnight, it does have to start somewhere, as leaders at the 150-plus Project BOOST sites nationwide can attest Now is the time to begin planning to join the Project BOOST 2013 cohort. Applications will be accepted through this summer; training will begin in the fall. But participation is limited, and successful applicants often need time to prepare their applications, which must include letters of support from a site executive and the development of a multidisciplinary team. For more information, visit www.hospitalmedicine.org/boost.
Brendon Shank is SHM’s associate vice president of communications.
Shaun Frost: Why Hospital Patients' Expectations Should Dictate Their Care
It is difficult to disagree that patients and their families deserve to be satisfied with the care they receive, and furthermore that a satisfying care experience is the foundation upon which the ability to heal is based. Mention the subject of patient satisfaction, however, to care providers, and prepare for many to respond negatively. For most, this frustration likely stems from the challenges associated with satisfaction measurement, and the application of this measurement to provider performance reporting and reimbursement. Perhaps by focusing so intently on quantifying how happy patients are with their care, we have distracted ourselves from the real goal of creating patient experiences that enable optimal healing.
In some respects, healthcare’s preoccupation with satisfaction measurements seems analogous to administering a final examination before teaching the course material. If so, at this juncture, it would be prudent to back up and examine the curriculum required to master the subject matter necessary to perform well on the test. To this end, it is necessary to:
Identify the contributors to a satisfying patient experience, and Focus specifically on understanding patient expectations.
Expectation Examination
An essential reading on the subject of patient experience and satisfaction is a July/August 2005 article in The Hospitalist titled “Patient Satisfaction: the Hospitalist’s Role,” in which Patrick J. Torcson, MD, MMM, FACP, SFHM, introduces “The First Law of Service.”1 According to this law, satisfaction can be mathematically defined as equal to patients’ perceptions of the care they received minus their expectations for that care (satisfaction=perception–expectation). Accordingly, if perception meets or exceeds expectation, an associated degree of satisfaction will be generated.
Both perceptions and expectations can be affected to create satisfaction. Remodeling a hospital lobby is an example of an effort to primarily influence patient perception. When considering efforts to influence patient expectations, it is useful to think of universal versus individual patient requirements, needs, desires, values, and goals. Examples of universal patient expectations (meaning expectations held by all or a majority of patients) would include receiving warm meals at scheduled times, having call lights answered in a timely manner, understanding the side effects of medications, and receiving instructions at the time of discharge. Examples of individual patient expectations (meaning expectations that are personally held by individual patients because of reasons unique to individual circumstances not common to everyone) would be need for low-cost medications due to economic hardship, prioritization of functional improvement versus pain elimination, and tolerance of treatment-related side effects.
It might be fair to say that in its pursuit to create satisfying patient experiences, our healthcare system has focused more on influencing perception and universal patient expectations than it has on addressing unique, personally held patient interests. In the future, we should attend more diligently to individual expectations. By doing so, patients will be better engaged, providers will be better informed, and satisfaction will follow.
Shared Decision-Making
You wouldn’t think of retaining a real estate agent to assist you in purchasing a home without informing that person about your personal needs. In order to satisfy you, the agent must understand what you expect in regard to such issues as price, square footage, yard size, community amenities, school district, proximity to work, etc. Just as your needs in shopping for a home can only be met by considering your personal expectations, your patient’s needs can only be met by understanding their individual healthcare requirements.
Unfortunately, understanding an individual patient’s expectations about their healthcare is more challenging than outlining a list of requirements for their ideal home. Although the reasons for this are multiple (see “Barriers to Understanding Patient Expectations,” left), the solution in large part rests in the application of shared decision-making (SDM).
SDM is defined as a collaborative communication process between provider and patient intended to help the patient decide among multiple acceptable healthcare choices in accordance with their preferences and values. SDM has been demonstrated to positively impact patient satisfaction, as well as care quality, resource utilization, and healthcare costs. A cornerstone feature of SDM is the use of decision aids to assist patients in identifying their personal healthcare expectations while simultaneously educating them about how those expectations apply to care plan options. Decision aids also benefit care providers by creating a standardized framework by which to solicit patients’ input regarding their preferences. When navigated appropriately, SDM balances the clinician’s expertise and knowledge with the patient’s goals and values.
Recent investigations into the application of SDM to HM practice have touted its effectiveness (e.g. when applied to low-risk chest pain evaluations) and questioned the creation of unintended negative consequences (e.g. on hospital resource consumption and affordability).2,3 Despite limited data in the HM setting, several literature reviews examining the effectiveness of SDM across various care sites consistently linked it to greater patient satisfaction.4
It is important to realize that policymakers are lauding the promise of SDM and incorporating its use into rules, regulations, and funding opportunities. For example, the Centers for Medicare & Medicaid Services (CMS) requires SDM to participate in its accountable-care organization (ACO) programs, and several states recently have enacted legislation to promote SDM. Expect thus to experience future pressure to apply SDM in your hospitalist practice. Organizations dedicated to the advancement of SDM include the Society for Participatory Medicine, the Informed Medical Decisions Foundation, the Society for Medical Decision Making, and the Mayo Clinic. More information and resources are available on their websites.
Conclusions
Satisfaction surveys are tools for measuring the quality of patient care experiences. Although satisfaction surveying is an inexact science, and the application of survey results to performance evaluation is challenging, we must remember that the goal is to optimize patient experience. Necessary in the creation of a satisfying patient experience is a robust understanding of patient expectations. SDM is a promising communication strategy that can help both providers and patients better identify the personally held values and goals that determine patient care expectations.
Dr. Frost is president of SHM.
References
- Torcson, P. Patient satisfaction: the hospitalist’s role. The Hospitalist website. Available at: http://www.the-hospitalist.org/details/article/256805/Patient_Satisfaction_the_Hospitalists_Role.html. Accessed Jan. 30, 2013.
- Hess E, et al. The chest pain choice decision aid. A randomized trial. Circ Cardiovasc Qual Outcomes. 2012;5:251-259.
- Tak, HJ, Meltzer, D. Effect of patient preference in medical decision-making on inpatient care [abstract]. J Hosp Med. 2012;7(Suppl 2):91.
- Hostetter, M, Klein S. Helping patients make better treatment choices with decision aids. The Commonwealth Fund website. Available at: http://www.commonwealthfund.org/Newsletters/Quality-Matters/2012/October-November/In-Focus.aspx. Accessed Jan. 30, 2013.
It is difficult to disagree that patients and their families deserve to be satisfied with the care they receive, and furthermore that a satisfying care experience is the foundation upon which the ability to heal is based. Mention the subject of patient satisfaction, however, to care providers, and prepare for many to respond negatively. For most, this frustration likely stems from the challenges associated with satisfaction measurement, and the application of this measurement to provider performance reporting and reimbursement. Perhaps by focusing so intently on quantifying how happy patients are with their care, we have distracted ourselves from the real goal of creating patient experiences that enable optimal healing.
In some respects, healthcare’s preoccupation with satisfaction measurements seems analogous to administering a final examination before teaching the course material. If so, at this juncture, it would be prudent to back up and examine the curriculum required to master the subject matter necessary to perform well on the test. To this end, it is necessary to:
Identify the contributors to a satisfying patient experience, and Focus specifically on understanding patient expectations.
Expectation Examination
An essential reading on the subject of patient experience and satisfaction is a July/August 2005 article in The Hospitalist titled “Patient Satisfaction: the Hospitalist’s Role,” in which Patrick J. Torcson, MD, MMM, FACP, SFHM, introduces “The First Law of Service.”1 According to this law, satisfaction can be mathematically defined as equal to patients’ perceptions of the care they received minus their expectations for that care (satisfaction=perception–expectation). Accordingly, if perception meets or exceeds expectation, an associated degree of satisfaction will be generated.
Both perceptions and expectations can be affected to create satisfaction. Remodeling a hospital lobby is an example of an effort to primarily influence patient perception. When considering efforts to influence patient expectations, it is useful to think of universal versus individual patient requirements, needs, desires, values, and goals. Examples of universal patient expectations (meaning expectations held by all or a majority of patients) would include receiving warm meals at scheduled times, having call lights answered in a timely manner, understanding the side effects of medications, and receiving instructions at the time of discharge. Examples of individual patient expectations (meaning expectations that are personally held by individual patients because of reasons unique to individual circumstances not common to everyone) would be need for low-cost medications due to economic hardship, prioritization of functional improvement versus pain elimination, and tolerance of treatment-related side effects.
It might be fair to say that in its pursuit to create satisfying patient experiences, our healthcare system has focused more on influencing perception and universal patient expectations than it has on addressing unique, personally held patient interests. In the future, we should attend more diligently to individual expectations. By doing so, patients will be better engaged, providers will be better informed, and satisfaction will follow.
Shared Decision-Making
You wouldn’t think of retaining a real estate agent to assist you in purchasing a home without informing that person about your personal needs. In order to satisfy you, the agent must understand what you expect in regard to such issues as price, square footage, yard size, community amenities, school district, proximity to work, etc. Just as your needs in shopping for a home can only be met by considering your personal expectations, your patient’s needs can only be met by understanding their individual healthcare requirements.
Unfortunately, understanding an individual patient’s expectations about their healthcare is more challenging than outlining a list of requirements for their ideal home. Although the reasons for this are multiple (see “Barriers to Understanding Patient Expectations,” left), the solution in large part rests in the application of shared decision-making (SDM).
SDM is defined as a collaborative communication process between provider and patient intended to help the patient decide among multiple acceptable healthcare choices in accordance with their preferences and values. SDM has been demonstrated to positively impact patient satisfaction, as well as care quality, resource utilization, and healthcare costs. A cornerstone feature of SDM is the use of decision aids to assist patients in identifying their personal healthcare expectations while simultaneously educating them about how those expectations apply to care plan options. Decision aids also benefit care providers by creating a standardized framework by which to solicit patients’ input regarding their preferences. When navigated appropriately, SDM balances the clinician’s expertise and knowledge with the patient’s goals and values.
Recent investigations into the application of SDM to HM practice have touted its effectiveness (e.g. when applied to low-risk chest pain evaluations) and questioned the creation of unintended negative consequences (e.g. on hospital resource consumption and affordability).2,3 Despite limited data in the HM setting, several literature reviews examining the effectiveness of SDM across various care sites consistently linked it to greater patient satisfaction.4
It is important to realize that policymakers are lauding the promise of SDM and incorporating its use into rules, regulations, and funding opportunities. For example, the Centers for Medicare & Medicaid Services (CMS) requires SDM to participate in its accountable-care organization (ACO) programs, and several states recently have enacted legislation to promote SDM. Expect thus to experience future pressure to apply SDM in your hospitalist practice. Organizations dedicated to the advancement of SDM include the Society for Participatory Medicine, the Informed Medical Decisions Foundation, the Society for Medical Decision Making, and the Mayo Clinic. More information and resources are available on their websites.
Conclusions
Satisfaction surveys are tools for measuring the quality of patient care experiences. Although satisfaction surveying is an inexact science, and the application of survey results to performance evaluation is challenging, we must remember that the goal is to optimize patient experience. Necessary in the creation of a satisfying patient experience is a robust understanding of patient expectations. SDM is a promising communication strategy that can help both providers and patients better identify the personally held values and goals that determine patient care expectations.
Dr. Frost is president of SHM.
References
- Torcson, P. Patient satisfaction: the hospitalist’s role. The Hospitalist website. Available at: http://www.the-hospitalist.org/details/article/256805/Patient_Satisfaction_the_Hospitalists_Role.html. Accessed Jan. 30, 2013.
- Hess E, et al. The chest pain choice decision aid. A randomized trial. Circ Cardiovasc Qual Outcomes. 2012;5:251-259.
- Tak, HJ, Meltzer, D. Effect of patient preference in medical decision-making on inpatient care [abstract]. J Hosp Med. 2012;7(Suppl 2):91.
- Hostetter, M, Klein S. Helping patients make better treatment choices with decision aids. The Commonwealth Fund website. Available at: http://www.commonwealthfund.org/Newsletters/Quality-Matters/2012/October-November/In-Focus.aspx. Accessed Jan. 30, 2013.
It is difficult to disagree that patients and their families deserve to be satisfied with the care they receive, and furthermore that a satisfying care experience is the foundation upon which the ability to heal is based. Mention the subject of patient satisfaction, however, to care providers, and prepare for many to respond negatively. For most, this frustration likely stems from the challenges associated with satisfaction measurement, and the application of this measurement to provider performance reporting and reimbursement. Perhaps by focusing so intently on quantifying how happy patients are with their care, we have distracted ourselves from the real goal of creating patient experiences that enable optimal healing.
In some respects, healthcare’s preoccupation with satisfaction measurements seems analogous to administering a final examination before teaching the course material. If so, at this juncture, it would be prudent to back up and examine the curriculum required to master the subject matter necessary to perform well on the test. To this end, it is necessary to:
Identify the contributors to a satisfying patient experience, and Focus specifically on understanding patient expectations.
Expectation Examination
An essential reading on the subject of patient experience and satisfaction is a July/August 2005 article in The Hospitalist titled “Patient Satisfaction: the Hospitalist’s Role,” in which Patrick J. Torcson, MD, MMM, FACP, SFHM, introduces “The First Law of Service.”1 According to this law, satisfaction can be mathematically defined as equal to patients’ perceptions of the care they received minus their expectations for that care (satisfaction=perception–expectation). Accordingly, if perception meets or exceeds expectation, an associated degree of satisfaction will be generated.
Both perceptions and expectations can be affected to create satisfaction. Remodeling a hospital lobby is an example of an effort to primarily influence patient perception. When considering efforts to influence patient expectations, it is useful to think of universal versus individual patient requirements, needs, desires, values, and goals. Examples of universal patient expectations (meaning expectations held by all or a majority of patients) would include receiving warm meals at scheduled times, having call lights answered in a timely manner, understanding the side effects of medications, and receiving instructions at the time of discharge. Examples of individual patient expectations (meaning expectations that are personally held by individual patients because of reasons unique to individual circumstances not common to everyone) would be need for low-cost medications due to economic hardship, prioritization of functional improvement versus pain elimination, and tolerance of treatment-related side effects.
It might be fair to say that in its pursuit to create satisfying patient experiences, our healthcare system has focused more on influencing perception and universal patient expectations than it has on addressing unique, personally held patient interests. In the future, we should attend more diligently to individual expectations. By doing so, patients will be better engaged, providers will be better informed, and satisfaction will follow.
Shared Decision-Making
You wouldn’t think of retaining a real estate agent to assist you in purchasing a home without informing that person about your personal needs. In order to satisfy you, the agent must understand what you expect in regard to such issues as price, square footage, yard size, community amenities, school district, proximity to work, etc. Just as your needs in shopping for a home can only be met by considering your personal expectations, your patient’s needs can only be met by understanding their individual healthcare requirements.
Unfortunately, understanding an individual patient’s expectations about their healthcare is more challenging than outlining a list of requirements for their ideal home. Although the reasons for this are multiple (see “Barriers to Understanding Patient Expectations,” left), the solution in large part rests in the application of shared decision-making (SDM).
SDM is defined as a collaborative communication process between provider and patient intended to help the patient decide among multiple acceptable healthcare choices in accordance with their preferences and values. SDM has been demonstrated to positively impact patient satisfaction, as well as care quality, resource utilization, and healthcare costs. A cornerstone feature of SDM is the use of decision aids to assist patients in identifying their personal healthcare expectations while simultaneously educating them about how those expectations apply to care plan options. Decision aids also benefit care providers by creating a standardized framework by which to solicit patients’ input regarding their preferences. When navigated appropriately, SDM balances the clinician’s expertise and knowledge with the patient’s goals and values.
Recent investigations into the application of SDM to HM practice have touted its effectiveness (e.g. when applied to low-risk chest pain evaluations) and questioned the creation of unintended negative consequences (e.g. on hospital resource consumption and affordability).2,3 Despite limited data in the HM setting, several literature reviews examining the effectiveness of SDM across various care sites consistently linked it to greater patient satisfaction.4
It is important to realize that policymakers are lauding the promise of SDM and incorporating its use into rules, regulations, and funding opportunities. For example, the Centers for Medicare & Medicaid Services (CMS) requires SDM to participate in its accountable-care organization (ACO) programs, and several states recently have enacted legislation to promote SDM. Expect thus to experience future pressure to apply SDM in your hospitalist practice. Organizations dedicated to the advancement of SDM include the Society for Participatory Medicine, the Informed Medical Decisions Foundation, the Society for Medical Decision Making, and the Mayo Clinic. More information and resources are available on their websites.
Conclusions
Satisfaction surveys are tools for measuring the quality of patient care experiences. Although satisfaction surveying is an inexact science, and the application of survey results to performance evaluation is challenging, we must remember that the goal is to optimize patient experience. Necessary in the creation of a satisfying patient experience is a robust understanding of patient expectations. SDM is a promising communication strategy that can help both providers and patients better identify the personally held values and goals that determine patient care expectations.
Dr. Frost is president of SHM.
References
- Torcson, P. Patient satisfaction: the hospitalist’s role. The Hospitalist website. Available at: http://www.the-hospitalist.org/details/article/256805/Patient_Satisfaction_the_Hospitalists_Role.html. Accessed Jan. 30, 2013.
- Hess E, et al. The chest pain choice decision aid. A randomized trial. Circ Cardiovasc Qual Outcomes. 2012;5:251-259.
- Tak, HJ, Meltzer, D. Effect of patient preference in medical decision-making on inpatient care [abstract]. J Hosp Med. 2012;7(Suppl 2):91.
- Hostetter, M, Klein S. Helping patients make better treatment choices with decision aids. The Commonwealth Fund website. Available at: http://www.commonwealthfund.org/Newsletters/Quality-Matters/2012/October-November/In-Focus.aspx. Accessed Jan. 30, 2013.
Win Whitcomb: Hospital Value-Based Purchasing Program Adds Measure in Efficiency Domain
HVBP’s First Efficiency Measure
Move over, cost, LOS—make room for ‘Medicare spending per beneficiary’
The unwritten rule in hospitalist circles is that lower cost and length of stay (LOS) mean higher efficiency, with hospitalists (me included) often pointing to one or both of these as a yardstick of performance in the efficiency domain. But if we lower hospital length of stay and costs while shifting costs to post-hospital care, have we solved anything?
This very question was raised by Kuo and Goodwin’s observational study that revealed that decreased hospital costs and LOS were offset by higher utilization and costs after discharge under hospitalist care.1
Efficiency As a Domain of Quality
Using the Institute of Medicine’s (IOM) six domains of quality as a framework (see Table 1, right), the Centers for Medicare & Medicaid Services’ (CMS) Hospital Value-Based Purchasing (HVBP) program seeks to encourage enhanced quality in all of the IOM domains. For HVBP 2015, we see the addition of the first measure in the domain of efficiency.
You may be saying to yourself, “It’s only 2013. Why should I worry about HVBP 2015?” Here’s why: The measurement periods for HVBP 2015 are May to December 2011 (baseline) and May to December 2013 (performance). Hospitals can succeed under HVBP if they demonstrate improvement from baseline or attainment, which indicates maintenance of a high level of performance despite no substantial improvement from baseline. Medicare spending per beneficiary (MSPB) will make up 20% of the 2015 HVBP incentive pool for hospitals.
Medicare Spending Per Beneficiary Instead of Costs or LOS
Why did CMS choose to use MSPB as a measure and not simply hospital costs or length of stay? Measuring efficiency of hospital care has proven to be a sticky wicket. If one simply measures and rewards decreased hospital costs and/or length of stay (which you might legitimately argue is exactly what the current DRG system does by paying a case rate to the hospital), one runs the risk of shifting costs to settings beyond the four walls of the hospital, or even fueling higher rates of hospital readmissions. Also, physician costs and other costs (therapy, home care) are not accounted for; they are accounted for under MSPB (described below). Finally, hospital costs and LOS vary substantially across regions and by severity of illness of the patients being analyzed. This makes it difficult to compare, for example, LOS in California versus Massachusetts.
The MSPB is designed to be a comprehensive and equitable metric:
- It seeks to eliminate cost-shifting among settings by widening the time period from three days prior to 30 days post-inpatient-care episode;
- It looks at the full cost of care by including expenses from both Medicare Part A (hospital) and Part B (doctors, PT, OT, home health, others);
- It incorporates risk adjustment by taking into account differences in patient health status; and
- It seeks to level the playing field by using a price standardization methodology that factors in geographic payment differences in wages, practice costs, and payments for indirect medical education and disproportionate share hospitals (those that treat large numbers of the indigent population).
Driving High Performance in Medicare Spending Per Beneficiary
Hospitalists straddle the Part A and Part B elements of Medicare; they have one foot in the hospital and one foot in the physician practice world. They should be able to improve their hospitals’ performance under the MSPB yardstick. Since the performance measurement period starts in May, now is the time to sharpen your focus on MSPB.
Here are the top priorities for MSPB that I recommend for hospitalists:
Reduction of marginally beneficial resource utilization. This is a process of analyzing resource (e.g. pharmacy, radiology, laboratory, blood products) utilization for the purpose of minimizing costly practices that do not benefit the patient. This is an essential practice of a high-functioning hospitalist program. Through its participation in the Choosing Wisely campaign (see “Stop! Think Twice Before You Order,” p. 46), SHM has helped hospitalists have conversations about these practices with patients.
Hospital throughput. Work on “front end” throughput with the ED by having a process in place to quickly evaluate and facilitate potential admissions. Work with case management to assure timely (and early in the day) discharges.
Safe-discharge processes. We reviewed key elements of a safe discharge last month and provided a link to SHM’s Project BOOST (www.hospitalmedicine.org/boost). From the MSPB perspective: A safe discharge minimizes exorbitant spending after discharge.
Documentation integrity. Because MSPB is risk-adjusted, the more the record reflects patient severity of illness, the better your hospital will perform, all else being equal. Work collaboratively with your documentation integrity professionals!
Much of the success of the HM specialty has been built on the tenet that the hospitalist model delivers efficient inpatient care. In the coming years, the specialty’s contribution will increasingly be gauged by the MSPB measure.
Reference
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is a co-founder and past president of SHM. Email him at [email protected].
HVBP’s First Efficiency Measure
Move over, cost, LOS—make room for ‘Medicare spending per beneficiary’
The unwritten rule in hospitalist circles is that lower cost and length of stay (LOS) mean higher efficiency, with hospitalists (me included) often pointing to one or both of these as a yardstick of performance in the efficiency domain. But if we lower hospital length of stay and costs while shifting costs to post-hospital care, have we solved anything?
This very question was raised by Kuo and Goodwin’s observational study that revealed that decreased hospital costs and LOS were offset by higher utilization and costs after discharge under hospitalist care.1
Efficiency As a Domain of Quality
Using the Institute of Medicine’s (IOM) six domains of quality as a framework (see Table 1, right), the Centers for Medicare & Medicaid Services’ (CMS) Hospital Value-Based Purchasing (HVBP) program seeks to encourage enhanced quality in all of the IOM domains. For HVBP 2015, we see the addition of the first measure in the domain of efficiency.
You may be saying to yourself, “It’s only 2013. Why should I worry about HVBP 2015?” Here’s why: The measurement periods for HVBP 2015 are May to December 2011 (baseline) and May to December 2013 (performance). Hospitals can succeed under HVBP if they demonstrate improvement from baseline or attainment, which indicates maintenance of a high level of performance despite no substantial improvement from baseline. Medicare spending per beneficiary (MSPB) will make up 20% of the 2015 HVBP incentive pool for hospitals.
Medicare Spending Per Beneficiary Instead of Costs or LOS
Why did CMS choose to use MSPB as a measure and not simply hospital costs or length of stay? Measuring efficiency of hospital care has proven to be a sticky wicket. If one simply measures and rewards decreased hospital costs and/or length of stay (which you might legitimately argue is exactly what the current DRG system does by paying a case rate to the hospital), one runs the risk of shifting costs to settings beyond the four walls of the hospital, or even fueling higher rates of hospital readmissions. Also, physician costs and other costs (therapy, home care) are not accounted for; they are accounted for under MSPB (described below). Finally, hospital costs and LOS vary substantially across regions and by severity of illness of the patients being analyzed. This makes it difficult to compare, for example, LOS in California versus Massachusetts.
The MSPB is designed to be a comprehensive and equitable metric:
- It seeks to eliminate cost-shifting among settings by widening the time period from three days prior to 30 days post-inpatient-care episode;
- It looks at the full cost of care by including expenses from both Medicare Part A (hospital) and Part B (doctors, PT, OT, home health, others);
- It incorporates risk adjustment by taking into account differences in patient health status; and
- It seeks to level the playing field by using a price standardization methodology that factors in geographic payment differences in wages, practice costs, and payments for indirect medical education and disproportionate share hospitals (those that treat large numbers of the indigent population).
Driving High Performance in Medicare Spending Per Beneficiary
Hospitalists straddle the Part A and Part B elements of Medicare; they have one foot in the hospital and one foot in the physician practice world. They should be able to improve their hospitals’ performance under the MSPB yardstick. Since the performance measurement period starts in May, now is the time to sharpen your focus on MSPB.
Here are the top priorities for MSPB that I recommend for hospitalists:
Reduction of marginally beneficial resource utilization. This is a process of analyzing resource (e.g. pharmacy, radiology, laboratory, blood products) utilization for the purpose of minimizing costly practices that do not benefit the patient. This is an essential practice of a high-functioning hospitalist program. Through its participation in the Choosing Wisely campaign (see “Stop! Think Twice Before You Order,” p. 46), SHM has helped hospitalists have conversations about these practices with patients.
Hospital throughput. Work on “front end” throughput with the ED by having a process in place to quickly evaluate and facilitate potential admissions. Work with case management to assure timely (and early in the day) discharges.
Safe-discharge processes. We reviewed key elements of a safe discharge last month and provided a link to SHM’s Project BOOST (www.hospitalmedicine.org/boost). From the MSPB perspective: A safe discharge minimizes exorbitant spending after discharge.
Documentation integrity. Because MSPB is risk-adjusted, the more the record reflects patient severity of illness, the better your hospital will perform, all else being equal. Work collaboratively with your documentation integrity professionals!
Much of the success of the HM specialty has been built on the tenet that the hospitalist model delivers efficient inpatient care. In the coming years, the specialty’s contribution will increasingly be gauged by the MSPB measure.
Reference
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is a co-founder and past president of SHM. Email him at [email protected].
HVBP’s First Efficiency Measure
Move over, cost, LOS—make room for ‘Medicare spending per beneficiary’
The unwritten rule in hospitalist circles is that lower cost and length of stay (LOS) mean higher efficiency, with hospitalists (me included) often pointing to one or both of these as a yardstick of performance in the efficiency domain. But if we lower hospital length of stay and costs while shifting costs to post-hospital care, have we solved anything?
This very question was raised by Kuo and Goodwin’s observational study that revealed that decreased hospital costs and LOS were offset by higher utilization and costs after discharge under hospitalist care.1
Efficiency As a Domain of Quality
Using the Institute of Medicine’s (IOM) six domains of quality as a framework (see Table 1, right), the Centers for Medicare & Medicaid Services’ (CMS) Hospital Value-Based Purchasing (HVBP) program seeks to encourage enhanced quality in all of the IOM domains. For HVBP 2015, we see the addition of the first measure in the domain of efficiency.
You may be saying to yourself, “It’s only 2013. Why should I worry about HVBP 2015?” Here’s why: The measurement periods for HVBP 2015 are May to December 2011 (baseline) and May to December 2013 (performance). Hospitals can succeed under HVBP if they demonstrate improvement from baseline or attainment, which indicates maintenance of a high level of performance despite no substantial improvement from baseline. Medicare spending per beneficiary (MSPB) will make up 20% of the 2015 HVBP incentive pool for hospitals.
Medicare Spending Per Beneficiary Instead of Costs or LOS
Why did CMS choose to use MSPB as a measure and not simply hospital costs or length of stay? Measuring efficiency of hospital care has proven to be a sticky wicket. If one simply measures and rewards decreased hospital costs and/or length of stay (which you might legitimately argue is exactly what the current DRG system does by paying a case rate to the hospital), one runs the risk of shifting costs to settings beyond the four walls of the hospital, or even fueling higher rates of hospital readmissions. Also, physician costs and other costs (therapy, home care) are not accounted for; they are accounted for under MSPB (described below). Finally, hospital costs and LOS vary substantially across regions and by severity of illness of the patients being analyzed. This makes it difficult to compare, for example, LOS in California versus Massachusetts.
The MSPB is designed to be a comprehensive and equitable metric:
- It seeks to eliminate cost-shifting among settings by widening the time period from three days prior to 30 days post-inpatient-care episode;
- It looks at the full cost of care by including expenses from both Medicare Part A (hospital) and Part B (doctors, PT, OT, home health, others);
- It incorporates risk adjustment by taking into account differences in patient health status; and
- It seeks to level the playing field by using a price standardization methodology that factors in geographic payment differences in wages, practice costs, and payments for indirect medical education and disproportionate share hospitals (those that treat large numbers of the indigent population).
Driving High Performance in Medicare Spending Per Beneficiary
Hospitalists straddle the Part A and Part B elements of Medicare; they have one foot in the hospital and one foot in the physician practice world. They should be able to improve their hospitals’ performance under the MSPB yardstick. Since the performance measurement period starts in May, now is the time to sharpen your focus on MSPB.
Here are the top priorities for MSPB that I recommend for hospitalists:
Reduction of marginally beneficial resource utilization. This is a process of analyzing resource (e.g. pharmacy, radiology, laboratory, blood products) utilization for the purpose of minimizing costly practices that do not benefit the patient. This is an essential practice of a high-functioning hospitalist program. Through its participation in the Choosing Wisely campaign (see “Stop! Think Twice Before You Order,” p. 46), SHM has helped hospitalists have conversations about these practices with patients.
Hospital throughput. Work on “front end” throughput with the ED by having a process in place to quickly evaluate and facilitate potential admissions. Work with case management to assure timely (and early in the day) discharges.
Safe-discharge processes. We reviewed key elements of a safe discharge last month and provided a link to SHM’s Project BOOST (www.hospitalmedicine.org/boost). From the MSPB perspective: A safe discharge minimizes exorbitant spending after discharge.
Documentation integrity. Because MSPB is risk-adjusted, the more the record reflects patient severity of illness, the better your hospital will perform, all else being equal. Work collaboratively with your documentation integrity professionals!
Much of the success of the HM specialty has been built on the tenet that the hospitalist model delivers efficient inpatient care. In the coming years, the specialty’s contribution will increasingly be gauged by the MSPB measure.
Reference
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is a co-founder and past president of SHM. Email him at [email protected].