User login
Sports Purpura From Floorball, Indoor Climbing, and Archery
To the Editor:
Sports purpura can be broken down into different types including traumatic purpura,1 exercise-induced cutaneous vasculitis,2 occurrence of coincidental systemic purpura,3 and other conditions.4-6 Traumatic purpura results from brutal contact with an opponent, the court, the equipment, or the ball. Three cases of sports purpura related to equipment and balls are reported.
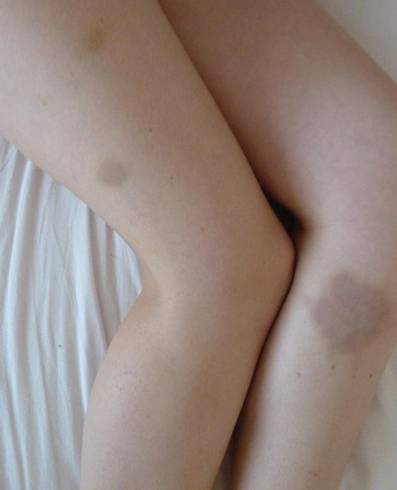
An otherwise healthy 27-year-old woman presented with multiple ecchymotic round patches on her legs. The largest patch was 70 mm and displayed a heterogeneous Swiss cheese–like pattern with discrete whiter round areas within the patch (Figure 1). She reported that she played as a defender in a second division floorball team weekly, acknowledging frequent body contacts and being hit on the legs with the sticks and balls. Purpura was diagnosed due to hits from the floorball.
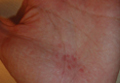
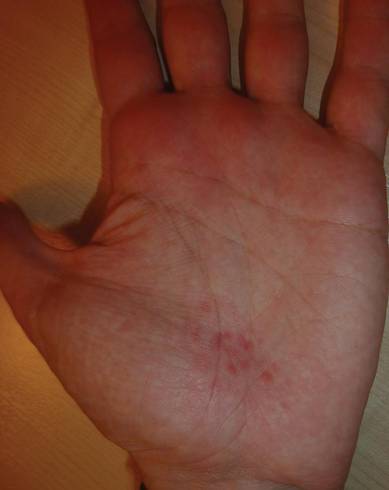
A 32-year-old healthy man presented with purpuric petechiae of the left palm after indoor climbing. He had been regularly climbing indoors for 3 years and denied a history of similar eruptions. The lesions were painless, noninfiltrated, and did not disappear after pressure (Figure 2). Lesions presumably were due to repeated friction on the climbing hold. Petechiae took a transiently golden hue before resolving within a week.
| 
|
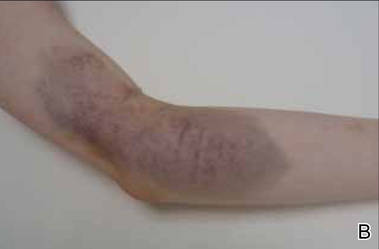
A 26-year-old right-handed woman injured the left forearm while practicing target archery. She was not wearing an arm guard at the time of the injury. Once released, the bowstring scraped the volar aspect of the forearm, causing a painful warm ecchymotic and swollen plaque. She denied neurologic or vascular symptoms. The hematoma rapidly evolved from red to blue (Figure 3) and spontaneously resolved within weeks.
|
|
Purpura related to the high-velocity impact of sport balls has been previously reported with ping-pong,7 paintball,8,9 racquetball, squash,10 and baseball. Floorball, one of the most popular team sports in Finland, is played indoors and resembles ice hockey. The players use graphite compound sticks and a light hollow plastic ball. Except for the goalkeeper, players do not wear specific protective gear. Accidental body contact, including a direct hit from the floorball stick or ball, are frequent.11 The ball weighs 23 g, measures 72 mm in diameter, and has 26 holes that are 11 mm in diameter. The fastest shot was recorded at 127 miles per hour.12 The cutaneous imprint from the ball impact on bare skin, as shown with patient 1, initially is annular,8-10 but the bruise later takes an unusual design due to the peculiar shape of the ball. This complication is no stranger to floorball players but has been rarely reported. The diagnosis is easy, the condition is benign and asymptomatic, and it resolves when the season is over; therefore, players commonly will not seek medical attention. Of note, lower limb injuries, including joint sprains, muscle strains, and soft-tissue contusions, are frequent in female athletes.11 Additional causes of purpura include collision with another player or with boards and stick hits.
Palmar petechiae from indoor climbing is similar to black palm from weight lifting.13 Although the typical black discoloration is absent, the mechanisms of friction and brutal trauma, clinical presentation, and evolution are similar.
Lastly, archery-induced hematomas are caused by the absence of an arm guard, which protects the wrist and forearm when the string snaps back.14 This complication is not often reported but is known by archers. Because archers usually wear protective gear, these injuries are expected to occur in novices or when safety measures are not respected.
1. Aguayo-Leiva I, Vano-Galvan S, Arrazola JM. A purpuric rash. Aust Fam Physician. 2009;38:889-890.
2. Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423-427.
3. Leonard JC, Rieger M. Idiopathic thrombocytopenic purpura presenting in a high school football player: a case report. J Athl Train. 1998;33:269-270.
4. Nordlind K, Bondesson L, Johansson SG, et al. Purpura provoked by cold exposure in a skier. Dermatologica. 1983;167:101-103.
5. Latenser BA, Hempstead RW. Exercise-associated solar purpura in an atypical location. Cutis. 1985;35:365-366.
6. Allan SJ, Humphreys F, Buxton PK. Annular purpura and step aerobics. Clin Exp Dermatol. 1994;19:418.
7. Scott MJ Jr, Scott MJ 3rd. Ping pong patches. Cutis. 1989;43:363-364.
8. Aboutalebi S, Stetson CL. Paintball purpura. J Am Acad Dermatol. 2005;53:901-902.
9. Levsky ME, Crowe M. What is your diagnosis? paintball purpura. Cutis. 2005;75:148, 157-158.
10. Barazi H, Adams BB. Sports purpura. Int J Dermatol. 2006;45:1443.
11. Pasanen K, Parkkari J, Kannus P, et al. Injury risk in female floorball: a prospective one-season follow-up [published online ahead of print May 9, 2007]. Scand J Med Sci Sports. 2008;18:49-54.
12. New world record. Floorball Central Web site. http://www.floorballcentral.com/2010/11/new-world -record.html. Published November 5, 2010. Accessed April 8, 2015.
13. Izumi AK. Letter: pigmented palmar petechiae (black palm). Arch Dermatol. 1974;109:261.
14. Rayan GM. Archery-related injuries of the hand, forearm, and elbow. South Med J. 1992;85:961-964.
To the Editor:
Sports purpura can be broken down into different types including traumatic purpura,1 exercise-induced cutaneous vasculitis,2 occurrence of coincidental systemic purpura,3 and other conditions.4-6 Traumatic purpura results from brutal contact with an opponent, the court, the equipment, or the ball. Three cases of sports purpura related to equipment and balls are reported.
An otherwise healthy 27-year-old woman presented with multiple ecchymotic round patches on her legs. The largest patch was 70 mm and displayed a heterogeneous Swiss cheese–like pattern with discrete whiter round areas within the patch (Figure 1). She reported that she played as a defender in a second division floorball team weekly, acknowledging frequent body contacts and being hit on the legs with the sticks and balls. Purpura was diagnosed due to hits from the floorball.
A 32-year-old healthy man presented with purpuric petechiae of the left palm after indoor climbing. He had been regularly climbing indoors for 3 years and denied a history of similar eruptions. The lesions were painless, noninfiltrated, and did not disappear after pressure (Figure 2). Lesions presumably were due to repeated friction on the climbing hold. Petechiae took a transiently golden hue before resolving within a week.

| 
|
A 26-year-old right-handed woman injured the left forearm while practicing target archery. She was not wearing an arm guard at the time of the injury. Once released, the bowstring scraped the volar aspect of the forearm, causing a painful warm ecchymotic and swollen plaque. She denied neurologic or vascular symptoms. The hematoma rapidly evolved from red to blue (Figure 3) and spontaneously resolved within weeks.
|
|
Purpura related to the high-velocity impact of sport balls has been previously reported with ping-pong,7 paintball,8,9 racquetball, squash,10 and baseball. Floorball, one of the most popular team sports in Finland, is played indoors and resembles ice hockey. The players use graphite compound sticks and a light hollow plastic ball. Except for the goalkeeper, players do not wear specific protective gear. Accidental body contact, including a direct hit from the floorball stick or ball, are frequent.11 The ball weighs 23 g, measures 72 mm in diameter, and has 26 holes that are 11 mm in diameter. The fastest shot was recorded at 127 miles per hour.12 The cutaneous imprint from the ball impact on bare skin, as shown with patient 1, initially is annular,8-10 but the bruise later takes an unusual design due to the peculiar shape of the ball. This complication is no stranger to floorball players but has been rarely reported. The diagnosis is easy, the condition is benign and asymptomatic, and it resolves when the season is over; therefore, players commonly will not seek medical attention. Of note, lower limb injuries, including joint sprains, muscle strains, and soft-tissue contusions, are frequent in female athletes.11 Additional causes of purpura include collision with another player or with boards and stick hits.
Palmar petechiae from indoor climbing is similar to black palm from weight lifting.13 Although the typical black discoloration is absent, the mechanisms of friction and brutal trauma, clinical presentation, and evolution are similar.
Lastly, archery-induced hematomas are caused by the absence of an arm guard, which protects the wrist and forearm when the string snaps back.14 This complication is not often reported but is known by archers. Because archers usually wear protective gear, these injuries are expected to occur in novices or when safety measures are not respected.
To the Editor:
Sports purpura can be broken down into different types including traumatic purpura,1 exercise-induced cutaneous vasculitis,2 occurrence of coincidental systemic purpura,3 and other conditions.4-6 Traumatic purpura results from brutal contact with an opponent, the court, the equipment, or the ball. Three cases of sports purpura related to equipment and balls are reported.
An otherwise healthy 27-year-old woman presented with multiple ecchymotic round patches on her legs. The largest patch was 70 mm and displayed a heterogeneous Swiss cheese–like pattern with discrete whiter round areas within the patch (Figure 1). She reported that she played as a defender in a second division floorball team weekly, acknowledging frequent body contacts and being hit on the legs with the sticks and balls. Purpura was diagnosed due to hits from the floorball.
A 32-year-old healthy man presented with purpuric petechiae of the left palm after indoor climbing. He had been regularly climbing indoors for 3 years and denied a history of similar eruptions. The lesions were painless, noninfiltrated, and did not disappear after pressure (Figure 2). Lesions presumably were due to repeated friction on the climbing hold. Petechiae took a transiently golden hue before resolving within a week.

| 
|
A 26-year-old right-handed woman injured the left forearm while practicing target archery. She was not wearing an arm guard at the time of the injury. Once released, the bowstring scraped the volar aspect of the forearm, causing a painful warm ecchymotic and swollen plaque. She denied neurologic or vascular symptoms. The hematoma rapidly evolved from red to blue (Figure 3) and spontaneously resolved within weeks.
|
|
Purpura related to the high-velocity impact of sport balls has been previously reported with ping-pong,7 paintball,8,9 racquetball, squash,10 and baseball. Floorball, one of the most popular team sports in Finland, is played indoors and resembles ice hockey. The players use graphite compound sticks and a light hollow plastic ball. Except for the goalkeeper, players do not wear specific protective gear. Accidental body contact, including a direct hit from the floorball stick or ball, are frequent.11 The ball weighs 23 g, measures 72 mm in diameter, and has 26 holes that are 11 mm in diameter. The fastest shot was recorded at 127 miles per hour.12 The cutaneous imprint from the ball impact on bare skin, as shown with patient 1, initially is annular,8-10 but the bruise later takes an unusual design due to the peculiar shape of the ball. This complication is no stranger to floorball players but has been rarely reported. The diagnosis is easy, the condition is benign and asymptomatic, and it resolves when the season is over; therefore, players commonly will not seek medical attention. Of note, lower limb injuries, including joint sprains, muscle strains, and soft-tissue contusions, are frequent in female athletes.11 Additional causes of purpura include collision with another player or with boards and stick hits.
Palmar petechiae from indoor climbing is similar to black palm from weight lifting.13 Although the typical black discoloration is absent, the mechanisms of friction and brutal trauma, clinical presentation, and evolution are similar.
Lastly, archery-induced hematomas are caused by the absence of an arm guard, which protects the wrist and forearm when the string snaps back.14 This complication is not often reported but is known by archers. Because archers usually wear protective gear, these injuries are expected to occur in novices or when safety measures are not respected.
1. Aguayo-Leiva I, Vano-Galvan S, Arrazola JM. A purpuric rash. Aust Fam Physician. 2009;38:889-890.
2. Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423-427.
3. Leonard JC, Rieger M. Idiopathic thrombocytopenic purpura presenting in a high school football player: a case report. J Athl Train. 1998;33:269-270.
4. Nordlind K, Bondesson L, Johansson SG, et al. Purpura provoked by cold exposure in a skier. Dermatologica. 1983;167:101-103.
5. Latenser BA, Hempstead RW. Exercise-associated solar purpura in an atypical location. Cutis. 1985;35:365-366.
6. Allan SJ, Humphreys F, Buxton PK. Annular purpura and step aerobics. Clin Exp Dermatol. 1994;19:418.
7. Scott MJ Jr, Scott MJ 3rd. Ping pong patches. Cutis. 1989;43:363-364.
8. Aboutalebi S, Stetson CL. Paintball purpura. J Am Acad Dermatol. 2005;53:901-902.
9. Levsky ME, Crowe M. What is your diagnosis? paintball purpura. Cutis. 2005;75:148, 157-158.
10. Barazi H, Adams BB. Sports purpura. Int J Dermatol. 2006;45:1443.
11. Pasanen K, Parkkari J, Kannus P, et al. Injury risk in female floorball: a prospective one-season follow-up [published online ahead of print May 9, 2007]. Scand J Med Sci Sports. 2008;18:49-54.
12. New world record. Floorball Central Web site. http://www.floorballcentral.com/2010/11/new-world -record.html. Published November 5, 2010. Accessed April 8, 2015.
13. Izumi AK. Letter: pigmented palmar petechiae (black palm). Arch Dermatol. 1974;109:261.
14. Rayan GM. Archery-related injuries of the hand, forearm, and elbow. South Med J. 1992;85:961-964.
1. Aguayo-Leiva I, Vano-Galvan S, Arrazola JM. A purpuric rash. Aust Fam Physician. 2009;38:889-890.
2. Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423-427.
3. Leonard JC, Rieger M. Idiopathic thrombocytopenic purpura presenting in a high school football player: a case report. J Athl Train. 1998;33:269-270.
4. Nordlind K, Bondesson L, Johansson SG, et al. Purpura provoked by cold exposure in a skier. Dermatologica. 1983;167:101-103.
5. Latenser BA, Hempstead RW. Exercise-associated solar purpura in an atypical location. Cutis. 1985;35:365-366.
6. Allan SJ, Humphreys F, Buxton PK. Annular purpura and step aerobics. Clin Exp Dermatol. 1994;19:418.
7. Scott MJ Jr, Scott MJ 3rd. Ping pong patches. Cutis. 1989;43:363-364.
8. Aboutalebi S, Stetson CL. Paintball purpura. J Am Acad Dermatol. 2005;53:901-902.
9. Levsky ME, Crowe M. What is your diagnosis? paintball purpura. Cutis. 2005;75:148, 157-158.
10. Barazi H, Adams BB. Sports purpura. Int J Dermatol. 2006;45:1443.
11. Pasanen K, Parkkari J, Kannus P, et al. Injury risk in female floorball: a prospective one-season follow-up [published online ahead of print May 9, 2007]. Scand J Med Sci Sports. 2008;18:49-54.
12. New world record. Floorball Central Web site. http://www.floorballcentral.com/2010/11/new-world -record.html. Published November 5, 2010. Accessed April 8, 2015.
13. Izumi AK. Letter: pigmented palmar petechiae (black palm). Arch Dermatol. 1974;109:261.
14. Rayan GM. Archery-related injuries of the hand, forearm, and elbow. South Med J. 1992;85:961-964.
NASPAG: Parity, postpartum status predict adolescent LARC use
ORLANDO – The decision to use long-acting reversible contraception appears largely reactionary among adolescent girls, as the only factors significantly associated with the decision in a recent cross-sectional study were increased parity and postpartum status.
The findings could help with future efforts to identify and remove barriers to long-acting reversible contraceptive (LARC) use among adolescents, according to Dr. Lisa Moon, a third-year resident at the University of Oklahoma, Oklahoma City.
Of 209 adolescents included in the study, 66 used oral contraceptive (OC) pills, and 143 used LARC methods. Levonorgestrel intrauterine devices were used most often (77 subjects), followed by etonogestrel implants (61 subjects). Five of the adolescents used a copper intrauterine device (IUD), Dr. Moon reported at the annual meeting of the North American Society for Pediatric and Adolescent Gynecology.
A breakdown of the findings by age showed that with the exception of those aged 15 years, LARC use increased with increasing age; 1 subject was aged 14 years, and she used OCs; 5 were aged 15 years, and all used a LARC; 15 were aged 16 years, and 9 (60%) used a LARC; 44 were aged 17 years, and 28 (64%) used a LARC; 62 were aged 18 years and 44 (71%) used a LARC; and 82 were aged 19 years, and 57 (70%) used a LARC.
Multivariate analysis showed that having previously given birth and postpartum status were significant predictors of LARC vs. OC use (odds ratios, 3.5 and 3.9, respectively). Age, race, marital status, and documented citizenship were not associated with choice of contraception.
The vast majority of adolescent pregnancies – about 82% – are unplanned, and 50% of teens with unplanned pregnancies report having used some form of contraception at the time of pregnancy. LARC methods have the potential to improve teen pregnancy rates because non-LARC methods have been reported to have a more than 20-fold greater risk of failure; that risk was almost doubled in adolescents, but while 8.5% of U.S. women use such methods, 4.5% of those aged 15-19 years do so, Dr. Moon said (N. Engl. J. Med. 2012;366:1998-2007).
Lack of familiarity with LARCs, misperceptions, cost, lack of access, health care provider concerns, and confidentiality concerns are possible barriers to increased LARC use, she noted.
Confusion about recommendations for LARC use also may play a role, she said, noting that as recently as 2004, a World Health Organization report stated that “While there are no restrictions based on age or parity for IUDs, many adolescents still will not qualify as candidates, because of the risk of exposure to STIs [sexually transmitted infections]. Ideal candidates for IUDs are in long-term mutually monogamous relationships, are parous, and do not have unexplained vaginal bleeding,”
That view has changed. In 2012, both the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists released recommendations promoting LARC use among adolescents, and a 2013 WHO report stated that “LARC methods are appropriate for most women, including adolescent and nulliparous women.”
The ACOG recommendation specifically notes that LARC methods should be first line for all women (Obstet. Gynecol. 2012;120:983-8).
“It takes a little bit of time for that information to percolate out to our community clinics, which is where we get a little bit behind sometimes in our recommendations,” Dr. Moon noted.
That is concerning, given that a 2010 survey of physicians showed that 30.7% agreed that IUDs were appropriate for teenagers, 49.6% said they would offer an IUD to an unmarried teenager with one child, and 19% said they would offer an IUD to a nulliparous unmarried teenager (Contraception 2010;81:112-6).
“There’s kind of this disconnect between what we know is effective and reliable for preventing pregnancy in our teen population, and what we recommend to them,” Dr. Moon said.
The findings have prompted a deeper look into barriers to adolescent LARC use in Oklahoma, which ranks 48th in the nation for teen pregnancy rates among 15- to 17-year olds (22.8 births per 1,000 vs. 14.1 nationally), 50th for unplanned pregnancies among 18- and 19-year olds (83.1 per 1,000 vs. 51.4 nationally), and 49th overall (MMWR 2013;62:249-55).
“What’s most staggering to me is that 20% of those are to teens who are already parents, which highlights this unmet need that we have in our state,” she said.
The current findings demonstrate that parity and postpartum status predict LARC choice, but they don’t explain why that is, Dr. Moon said.
To characterize barriers to LARC use, as well as biases on the part of both patients and physicians, researchers are currently meeting with focus groups of primary care practitioners to identify provider biases, and focus groups of adolescent are planned, she said.
“Our hope is that with education and identifying some of those barriers, we can catch these people – before they get pregnant – and get them the contraception that they need,” she said.
Dr. Moon reported having no relevant financial disclosures.
ORLANDO – The decision to use long-acting reversible contraception appears largely reactionary among adolescent girls, as the only factors significantly associated with the decision in a recent cross-sectional study were increased parity and postpartum status.
The findings could help with future efforts to identify and remove barriers to long-acting reversible contraceptive (LARC) use among adolescents, according to Dr. Lisa Moon, a third-year resident at the University of Oklahoma, Oklahoma City.
Of 209 adolescents included in the study, 66 used oral contraceptive (OC) pills, and 143 used LARC methods. Levonorgestrel intrauterine devices were used most often (77 subjects), followed by etonogestrel implants (61 subjects). Five of the adolescents used a copper intrauterine device (IUD), Dr. Moon reported at the annual meeting of the North American Society for Pediatric and Adolescent Gynecology.
A breakdown of the findings by age showed that with the exception of those aged 15 years, LARC use increased with increasing age; 1 subject was aged 14 years, and she used OCs; 5 were aged 15 years, and all used a LARC; 15 were aged 16 years, and 9 (60%) used a LARC; 44 were aged 17 years, and 28 (64%) used a LARC; 62 were aged 18 years and 44 (71%) used a LARC; and 82 were aged 19 years, and 57 (70%) used a LARC.
Multivariate analysis showed that having previously given birth and postpartum status were significant predictors of LARC vs. OC use (odds ratios, 3.5 and 3.9, respectively). Age, race, marital status, and documented citizenship were not associated with choice of contraception.
The vast majority of adolescent pregnancies – about 82% – are unplanned, and 50% of teens with unplanned pregnancies report having used some form of contraception at the time of pregnancy. LARC methods have the potential to improve teen pregnancy rates because non-LARC methods have been reported to have a more than 20-fold greater risk of failure; that risk was almost doubled in adolescents, but while 8.5% of U.S. women use such methods, 4.5% of those aged 15-19 years do so, Dr. Moon said (N. Engl. J. Med. 2012;366:1998-2007).
Lack of familiarity with LARCs, misperceptions, cost, lack of access, health care provider concerns, and confidentiality concerns are possible barriers to increased LARC use, she noted.
Confusion about recommendations for LARC use also may play a role, she said, noting that as recently as 2004, a World Health Organization report stated that “While there are no restrictions based on age or parity for IUDs, many adolescents still will not qualify as candidates, because of the risk of exposure to STIs [sexually transmitted infections]. Ideal candidates for IUDs are in long-term mutually monogamous relationships, are parous, and do not have unexplained vaginal bleeding,”
That view has changed. In 2012, both the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists released recommendations promoting LARC use among adolescents, and a 2013 WHO report stated that “LARC methods are appropriate for most women, including adolescent and nulliparous women.”
The ACOG recommendation specifically notes that LARC methods should be first line for all women (Obstet. Gynecol. 2012;120:983-8).
“It takes a little bit of time for that information to percolate out to our community clinics, which is where we get a little bit behind sometimes in our recommendations,” Dr. Moon noted.
That is concerning, given that a 2010 survey of physicians showed that 30.7% agreed that IUDs were appropriate for teenagers, 49.6% said they would offer an IUD to an unmarried teenager with one child, and 19% said they would offer an IUD to a nulliparous unmarried teenager (Contraception 2010;81:112-6).
“There’s kind of this disconnect between what we know is effective and reliable for preventing pregnancy in our teen population, and what we recommend to them,” Dr. Moon said.
The findings have prompted a deeper look into barriers to adolescent LARC use in Oklahoma, which ranks 48th in the nation for teen pregnancy rates among 15- to 17-year olds (22.8 births per 1,000 vs. 14.1 nationally), 50th for unplanned pregnancies among 18- and 19-year olds (83.1 per 1,000 vs. 51.4 nationally), and 49th overall (MMWR 2013;62:249-55).
“What’s most staggering to me is that 20% of those are to teens who are already parents, which highlights this unmet need that we have in our state,” she said.
The current findings demonstrate that parity and postpartum status predict LARC choice, but they don’t explain why that is, Dr. Moon said.
To characterize barriers to LARC use, as well as biases on the part of both patients and physicians, researchers are currently meeting with focus groups of primary care practitioners to identify provider biases, and focus groups of adolescent are planned, she said.
“Our hope is that with education and identifying some of those barriers, we can catch these people – before they get pregnant – and get them the contraception that they need,” she said.
Dr. Moon reported having no relevant financial disclosures.
ORLANDO – The decision to use long-acting reversible contraception appears largely reactionary among adolescent girls, as the only factors significantly associated with the decision in a recent cross-sectional study were increased parity and postpartum status.
The findings could help with future efforts to identify and remove barriers to long-acting reversible contraceptive (LARC) use among adolescents, according to Dr. Lisa Moon, a third-year resident at the University of Oklahoma, Oklahoma City.
Of 209 adolescents included in the study, 66 used oral contraceptive (OC) pills, and 143 used LARC methods. Levonorgestrel intrauterine devices were used most often (77 subjects), followed by etonogestrel implants (61 subjects). Five of the adolescents used a copper intrauterine device (IUD), Dr. Moon reported at the annual meeting of the North American Society for Pediatric and Adolescent Gynecology.
A breakdown of the findings by age showed that with the exception of those aged 15 years, LARC use increased with increasing age; 1 subject was aged 14 years, and she used OCs; 5 were aged 15 years, and all used a LARC; 15 were aged 16 years, and 9 (60%) used a LARC; 44 were aged 17 years, and 28 (64%) used a LARC; 62 were aged 18 years and 44 (71%) used a LARC; and 82 were aged 19 years, and 57 (70%) used a LARC.
Multivariate analysis showed that having previously given birth and postpartum status were significant predictors of LARC vs. OC use (odds ratios, 3.5 and 3.9, respectively). Age, race, marital status, and documented citizenship were not associated with choice of contraception.
The vast majority of adolescent pregnancies – about 82% – are unplanned, and 50% of teens with unplanned pregnancies report having used some form of contraception at the time of pregnancy. LARC methods have the potential to improve teen pregnancy rates because non-LARC methods have been reported to have a more than 20-fold greater risk of failure; that risk was almost doubled in adolescents, but while 8.5% of U.S. women use such methods, 4.5% of those aged 15-19 years do so, Dr. Moon said (N. Engl. J. Med. 2012;366:1998-2007).
Lack of familiarity with LARCs, misperceptions, cost, lack of access, health care provider concerns, and confidentiality concerns are possible barriers to increased LARC use, she noted.
Confusion about recommendations for LARC use also may play a role, she said, noting that as recently as 2004, a World Health Organization report stated that “While there are no restrictions based on age or parity for IUDs, many adolescents still will not qualify as candidates, because of the risk of exposure to STIs [sexually transmitted infections]. Ideal candidates for IUDs are in long-term mutually monogamous relationships, are parous, and do not have unexplained vaginal bleeding,”
That view has changed. In 2012, both the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists released recommendations promoting LARC use among adolescents, and a 2013 WHO report stated that “LARC methods are appropriate for most women, including adolescent and nulliparous women.”
The ACOG recommendation specifically notes that LARC methods should be first line for all women (Obstet. Gynecol. 2012;120:983-8).
“It takes a little bit of time for that information to percolate out to our community clinics, which is where we get a little bit behind sometimes in our recommendations,” Dr. Moon noted.
That is concerning, given that a 2010 survey of physicians showed that 30.7% agreed that IUDs were appropriate for teenagers, 49.6% said they would offer an IUD to an unmarried teenager with one child, and 19% said they would offer an IUD to a nulliparous unmarried teenager (Contraception 2010;81:112-6).
“There’s kind of this disconnect between what we know is effective and reliable for preventing pregnancy in our teen population, and what we recommend to them,” Dr. Moon said.
The findings have prompted a deeper look into barriers to adolescent LARC use in Oklahoma, which ranks 48th in the nation for teen pregnancy rates among 15- to 17-year olds (22.8 births per 1,000 vs. 14.1 nationally), 50th for unplanned pregnancies among 18- and 19-year olds (83.1 per 1,000 vs. 51.4 nationally), and 49th overall (MMWR 2013;62:249-55).
“What’s most staggering to me is that 20% of those are to teens who are already parents, which highlights this unmet need that we have in our state,” she said.
The current findings demonstrate that parity and postpartum status predict LARC choice, but they don’t explain why that is, Dr. Moon said.
To characterize barriers to LARC use, as well as biases on the part of both patients and physicians, researchers are currently meeting with focus groups of primary care practitioners to identify provider biases, and focus groups of adolescent are planned, she said.
“Our hope is that with education and identifying some of those barriers, we can catch these people – before they get pregnant – and get them the contraception that they need,” she said.
Dr. Moon reported having no relevant financial disclosures.
AT THE NASPAG ANNUAL MEETING
Key clinical point: LARC methods are underutilized in adolescents.
Major finding: Significant predictors of LARC vs. OC use were previous childbirth and postpartum status (odds ratios, 3.5 and 3.9, respectively).
Data source: A cross-sectional study of 209 adolescents.
Disclosures: Dr. Moon reported having no relevant financial disclosures.
ACOG, SMFM, and others address safety concerns in labor and delivery
At least half of all cases of maternal morbidity and mortality could be prevented, or so studies suggest.1,2
The main stumbling block?
Faulty communication.
That’s the word from the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, the American College of Nurse-Midwives, and the Association of Women’s Health, Obstetric and Neonatal Nurses.3
In a joint “blueprint” to transform communication and enhance the safety culture in intrapartum care, these organizations, led by Audrey Lyndon, PhD, RN, FAAN, from the University of California, San Francisco, School of Nursing, describe the extent of the problem, steps that various team members can take to improve safety, notable success stories, and communication strategies.3 In this article, the joint blueprint is summarized, with a focus on steps obstetricians can take to improve the intrapartum safety culture.
Scope of the problem
A study of more than 3,282 physicians, midwives, and registered nurses produced a troubling statistic: More than 90% of respondents said that they had “witnessed shortcuts, missing competencies, disrespect, or performance problems” during the preceding year of practice.4 Few of these clinicians reported that they had discussed their concerns with the parties involved.
A second study of 1,932 clinicians found that 34% of physicians, 40% of midwives, and 56% of registered nurses had witnessed patients being put at risk within the preceding 2 years by other team members’ inattentiveness or lack of responsiveness.5
These findings suggest that health care providers often witness weak links in intrapartum safety but do not always address or report them. Among the reasons team members may be hesitant to speak up when they perceive a potential problem:
- feelings of resignation or inability to change the situation
- fear of retribution or ridicule
- fear of interpersonal or intrateam conflict.
Although Lyndon and colleagues acknowledge that it is impossible to eliminate adverse outcomes entirely or completely eradicate human error, they argue that significant improvements can be made by adopting a number of manageable strategies.
Recommended strategies
Lyndon and colleagues describe some of the challenges of effective communication in a health care setting:
Lyndon and colleagues go on to mention a number of strategies to improve communication, boost safety, and reduce medical errors.
1. Remember that the patient is part of the team
The patient and her family play a key role in identifying the potential for harm during labor and delivery, Lyndon and colleagues assert. They should be considered members of the intrapartum team, care should be patient-focused, and any communications from the patient should not only be heard but fully considered. In fact, explicit elicitation of her experience and concerns is recommended.
2. Consider that you might be part of the problem
It is human nature to attribute a communication problem to the other people involved, rather than take responsibility for it oneself. One potential solution to this mindset is team training, where all members are encouraged to communicate clearly and listen attentively. Organizations that have been successful at improving their culture of safety have implemented such training, as well as the use of checklists, training in fetal heart-rate monitoring, formation of a patient safety committee, external review of safety practices, and designation of a key clinician to lead the safety program and oversee team training.
3. Structure handoffs
The team should standardize handoffs so that they occur smoothly and all channels of communication remain open and clear.
“Having structured formats for debriefing and handoffs are steps in the right direction, but solving the problem of communication breakdowns is more complicated than standardizing the flow and format of information transfer,” Lyndon and colleagues assert. “Indeed, solving communication breakdowns is a matter of individual, group, organizational, and professional responsibility for creating and sustaining an environment of mutual respect, curiosity, and accountability for behavior and performance.”3
4. Learn to communicate responsibly
“Differences of opinion about clinical assessments, goals of care, and the pathway to optimal outcomes are bound to occur with some regularity in the dynamic environment of labor and delivery,” note Lyndon and colleagues. “Every person has the responsibility to contribute to improving how we relate to and communicate with each other. Collectively, we must create environments in which every team member (woman, family member, physician, midwife, nurse, unit clerk, patient care assistant, or scrub tech) is comfortable expressing and discussing concerns about safety or performance, is encouraged to do so, and has the support of the team to articulate the rationale for and urgency of the concern without fear of put-downs, retribution, or receiving poor-quality care.”3
5. Be persistent and proactive
When team members have differing expectations and communication styles, useful approaches include structured communication tools such as situation, background, assessment, recommendation (SBAR); structured handoffs; board rounds; huddles; attentive listening; and explicit elicitation of the patient’s concerns and desires.3
If someone fails to pay attention to a concern you raise, be persistent about restating that concern until you elicit a response.
If someone exhibits disruptive behavior, point to or establish a code of conduct that clearly describes professional behavior.
If there is a difference of opinion on patient management, such as fetal monitoring and interpretation, conduct regular case reviews and standardize a plan for notification of complications.
6. If you’re a team leader, set clear goals
Then ask team members what will be needed to achieve the outcomes desired.
“Team leaders need to develop outstanding skills for listening and eliciting feedback and cross-monitoring (being aware of each other’s actions and performance) from other team members,” note Lyndon and colleagues.
7. Increase public awareness of safety concepts
When these concepts and best practices are made known to the public, women and families become “empowered” to speak up when they have concerns about care.
And when they do speak up, it pays to listen.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Geller SE, Rosenberg D, Cox SM, et al. The continuum of maternal morbidity and mortality: factors associated with severity. Am J Obstet Gynecol. 2004;191(3):939–944.
2. Mitchell C, Lawton E, Morton C, McCain C, Holtby S, Main E. California Pregnancy-Associated Mortality Review: mixed methods approach for improved case identification, cause of death analyses and translation of findings. Matern Child Health J. 2014;18(3):518–526.
3. Lyndon A, Johnson MC, Bingham D, et al. Transforming communication and safety culture in intrapartum care: a multi-organization blueprint. Obstet Gynecol. 2015;125(5):1049–1055.
4. Maxfield DG, Lyndon A, Kennedy HP, O’Keeffe DF, Ziatnik MG. Confronting safety gaps across labor and delivery teams. Am J Obstet Gynecol. 2013;209(5):402–408.e3.
5. Lyndon A, Zlatnik MG, Maxfield DG, Lewis A, McMillan C, Kennedy HP. Contributions of clinical disconnections and unresolved conflict to failures in intrapartum safety. J Obstet Gynecol Neonatal Nurs. 2014;43(1):2–12.
At least half of all cases of maternal morbidity and mortality could be prevented, or so studies suggest.1,2
The main stumbling block?
Faulty communication.
That’s the word from the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, the American College of Nurse-Midwives, and the Association of Women’s Health, Obstetric and Neonatal Nurses.3
In a joint “blueprint” to transform communication and enhance the safety culture in intrapartum care, these organizations, led by Audrey Lyndon, PhD, RN, FAAN, from the University of California, San Francisco, School of Nursing, describe the extent of the problem, steps that various team members can take to improve safety, notable success stories, and communication strategies.3 In this article, the joint blueprint is summarized, with a focus on steps obstetricians can take to improve the intrapartum safety culture.
Scope of the problem
A study of more than 3,282 physicians, midwives, and registered nurses produced a troubling statistic: More than 90% of respondents said that they had “witnessed shortcuts, missing competencies, disrespect, or performance problems” during the preceding year of practice.4 Few of these clinicians reported that they had discussed their concerns with the parties involved.
A second study of 1,932 clinicians found that 34% of physicians, 40% of midwives, and 56% of registered nurses had witnessed patients being put at risk within the preceding 2 years by other team members’ inattentiveness or lack of responsiveness.5
These findings suggest that health care providers often witness weak links in intrapartum safety but do not always address or report them. Among the reasons team members may be hesitant to speak up when they perceive a potential problem:
- feelings of resignation or inability to change the situation
- fear of retribution or ridicule
- fear of interpersonal or intrateam conflict.
Although Lyndon and colleagues acknowledge that it is impossible to eliminate adverse outcomes entirely or completely eradicate human error, they argue that significant improvements can be made by adopting a number of manageable strategies.
Recommended strategies
Lyndon and colleagues describe some of the challenges of effective communication in a health care setting:
Lyndon and colleagues go on to mention a number of strategies to improve communication, boost safety, and reduce medical errors.
1. Remember that the patient is part of the team
The patient and her family play a key role in identifying the potential for harm during labor and delivery, Lyndon and colleagues assert. They should be considered members of the intrapartum team, care should be patient-focused, and any communications from the patient should not only be heard but fully considered. In fact, explicit elicitation of her experience and concerns is recommended.
2. Consider that you might be part of the problem
It is human nature to attribute a communication problem to the other people involved, rather than take responsibility for it oneself. One potential solution to this mindset is team training, where all members are encouraged to communicate clearly and listen attentively. Organizations that have been successful at improving their culture of safety have implemented such training, as well as the use of checklists, training in fetal heart-rate monitoring, formation of a patient safety committee, external review of safety practices, and designation of a key clinician to lead the safety program and oversee team training.
3. Structure handoffs
The team should standardize handoffs so that they occur smoothly and all channels of communication remain open and clear.
“Having structured formats for debriefing and handoffs are steps in the right direction, but solving the problem of communication breakdowns is more complicated than standardizing the flow and format of information transfer,” Lyndon and colleagues assert. “Indeed, solving communication breakdowns is a matter of individual, group, organizational, and professional responsibility for creating and sustaining an environment of mutual respect, curiosity, and accountability for behavior and performance.”3
4. Learn to communicate responsibly
“Differences of opinion about clinical assessments, goals of care, and the pathway to optimal outcomes are bound to occur with some regularity in the dynamic environment of labor and delivery,” note Lyndon and colleagues. “Every person has the responsibility to contribute to improving how we relate to and communicate with each other. Collectively, we must create environments in which every team member (woman, family member, physician, midwife, nurse, unit clerk, patient care assistant, or scrub tech) is comfortable expressing and discussing concerns about safety or performance, is encouraged to do so, and has the support of the team to articulate the rationale for and urgency of the concern without fear of put-downs, retribution, or receiving poor-quality care.”3
5. Be persistent and proactive
When team members have differing expectations and communication styles, useful approaches include structured communication tools such as situation, background, assessment, recommendation (SBAR); structured handoffs; board rounds; huddles; attentive listening; and explicit elicitation of the patient’s concerns and desires.3
If someone fails to pay attention to a concern you raise, be persistent about restating that concern until you elicit a response.
If someone exhibits disruptive behavior, point to or establish a code of conduct that clearly describes professional behavior.
If there is a difference of opinion on patient management, such as fetal monitoring and interpretation, conduct regular case reviews and standardize a plan for notification of complications.
6. If you’re a team leader, set clear goals
Then ask team members what will be needed to achieve the outcomes desired.
“Team leaders need to develop outstanding skills for listening and eliciting feedback and cross-monitoring (being aware of each other’s actions and performance) from other team members,” note Lyndon and colleagues.
7. Increase public awareness of safety concepts
When these concepts and best practices are made known to the public, women and families become “empowered” to speak up when they have concerns about care.
And when they do speak up, it pays to listen.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
At least half of all cases of maternal morbidity and mortality could be prevented, or so studies suggest.1,2
The main stumbling block?
Faulty communication.
That’s the word from the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, the American College of Nurse-Midwives, and the Association of Women’s Health, Obstetric and Neonatal Nurses.3
In a joint “blueprint” to transform communication and enhance the safety culture in intrapartum care, these organizations, led by Audrey Lyndon, PhD, RN, FAAN, from the University of California, San Francisco, School of Nursing, describe the extent of the problem, steps that various team members can take to improve safety, notable success stories, and communication strategies.3 In this article, the joint blueprint is summarized, with a focus on steps obstetricians can take to improve the intrapartum safety culture.
Scope of the problem
A study of more than 3,282 physicians, midwives, and registered nurses produced a troubling statistic: More than 90% of respondents said that they had “witnessed shortcuts, missing competencies, disrespect, or performance problems” during the preceding year of practice.4 Few of these clinicians reported that they had discussed their concerns with the parties involved.
A second study of 1,932 clinicians found that 34% of physicians, 40% of midwives, and 56% of registered nurses had witnessed patients being put at risk within the preceding 2 years by other team members’ inattentiveness or lack of responsiveness.5
These findings suggest that health care providers often witness weak links in intrapartum safety but do not always address or report them. Among the reasons team members may be hesitant to speak up when they perceive a potential problem:
- feelings of resignation or inability to change the situation
- fear of retribution or ridicule
- fear of interpersonal or intrateam conflict.
Although Lyndon and colleagues acknowledge that it is impossible to eliminate adverse outcomes entirely or completely eradicate human error, they argue that significant improvements can be made by adopting a number of manageable strategies.
Recommended strategies
Lyndon and colleagues describe some of the challenges of effective communication in a health care setting:
Lyndon and colleagues go on to mention a number of strategies to improve communication, boost safety, and reduce medical errors.
1. Remember that the patient is part of the team
The patient and her family play a key role in identifying the potential for harm during labor and delivery, Lyndon and colleagues assert. They should be considered members of the intrapartum team, care should be patient-focused, and any communications from the patient should not only be heard but fully considered. In fact, explicit elicitation of her experience and concerns is recommended.
2. Consider that you might be part of the problem
It is human nature to attribute a communication problem to the other people involved, rather than take responsibility for it oneself. One potential solution to this mindset is team training, where all members are encouraged to communicate clearly and listen attentively. Organizations that have been successful at improving their culture of safety have implemented such training, as well as the use of checklists, training in fetal heart-rate monitoring, formation of a patient safety committee, external review of safety practices, and designation of a key clinician to lead the safety program and oversee team training.
3. Structure handoffs
The team should standardize handoffs so that they occur smoothly and all channels of communication remain open and clear.
“Having structured formats for debriefing and handoffs are steps in the right direction, but solving the problem of communication breakdowns is more complicated than standardizing the flow and format of information transfer,” Lyndon and colleagues assert. “Indeed, solving communication breakdowns is a matter of individual, group, organizational, and professional responsibility for creating and sustaining an environment of mutual respect, curiosity, and accountability for behavior and performance.”3
4. Learn to communicate responsibly
“Differences of opinion about clinical assessments, goals of care, and the pathway to optimal outcomes are bound to occur with some regularity in the dynamic environment of labor and delivery,” note Lyndon and colleagues. “Every person has the responsibility to contribute to improving how we relate to and communicate with each other. Collectively, we must create environments in which every team member (woman, family member, physician, midwife, nurse, unit clerk, patient care assistant, or scrub tech) is comfortable expressing and discussing concerns about safety or performance, is encouraged to do so, and has the support of the team to articulate the rationale for and urgency of the concern without fear of put-downs, retribution, or receiving poor-quality care.”3
5. Be persistent and proactive
When team members have differing expectations and communication styles, useful approaches include structured communication tools such as situation, background, assessment, recommendation (SBAR); structured handoffs; board rounds; huddles; attentive listening; and explicit elicitation of the patient’s concerns and desires.3
If someone fails to pay attention to a concern you raise, be persistent about restating that concern until you elicit a response.
If someone exhibits disruptive behavior, point to or establish a code of conduct that clearly describes professional behavior.
If there is a difference of opinion on patient management, such as fetal monitoring and interpretation, conduct regular case reviews and standardize a plan for notification of complications.
6. If you’re a team leader, set clear goals
Then ask team members what will be needed to achieve the outcomes desired.
“Team leaders need to develop outstanding skills for listening and eliciting feedback and cross-monitoring (being aware of each other’s actions and performance) from other team members,” note Lyndon and colleagues.
7. Increase public awareness of safety concepts
When these concepts and best practices are made known to the public, women and families become “empowered” to speak up when they have concerns about care.
And when they do speak up, it pays to listen.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
1. Geller SE, Rosenberg D, Cox SM, et al. The continuum of maternal morbidity and mortality: factors associated with severity. Am J Obstet Gynecol. 2004;191(3):939–944.
2. Mitchell C, Lawton E, Morton C, McCain C, Holtby S, Main E. California Pregnancy-Associated Mortality Review: mixed methods approach for improved case identification, cause of death analyses and translation of findings. Matern Child Health J. 2014;18(3):518–526.
3. Lyndon A, Johnson MC, Bingham D, et al. Transforming communication and safety culture in intrapartum care: a multi-organization blueprint. Obstet Gynecol. 2015;125(5):1049–1055.
4. Maxfield DG, Lyndon A, Kennedy HP, O’Keeffe DF, Ziatnik MG. Confronting safety gaps across labor and delivery teams. Am J Obstet Gynecol. 2013;209(5):402–408.e3.
5. Lyndon A, Zlatnik MG, Maxfield DG, Lewis A, McMillan C, Kennedy HP. Contributions of clinical disconnections and unresolved conflict to failures in intrapartum safety. J Obstet Gynecol Neonatal Nurs. 2014;43(1):2–12.
1. Geller SE, Rosenberg D, Cox SM, et al. The continuum of maternal morbidity and mortality: factors associated with severity. Am J Obstet Gynecol. 2004;191(3):939–944.
2. Mitchell C, Lawton E, Morton C, McCain C, Holtby S, Main E. California Pregnancy-Associated Mortality Review: mixed methods approach for improved case identification, cause of death analyses and translation of findings. Matern Child Health J. 2014;18(3):518–526.
3. Lyndon A, Johnson MC, Bingham D, et al. Transforming communication and safety culture in intrapartum care: a multi-organization blueprint. Obstet Gynecol. 2015;125(5):1049–1055.
4. Maxfield DG, Lyndon A, Kennedy HP, O’Keeffe DF, Ziatnik MG. Confronting safety gaps across labor and delivery teams. Am J Obstet Gynecol. 2013;209(5):402–408.e3.
5. Lyndon A, Zlatnik MG, Maxfield DG, Lewis A, McMillan C, Kennedy HP. Contributions of clinical disconnections and unresolved conflict to failures in intrapartum safety. J Obstet Gynecol Neonatal Nurs. 2014;43(1):2–12.
Clinicians are adept at estimating uterine size prior to benign hysterectomy
In a poster presented at the 2015 ACOG Annual Clinical Meeting in San Francisco, Neal Marc Lonky, MD, and colleagues from the Southern California Permanente Group assessed the clinical acumen of physicians in estimating uterine size prior to elective hysterectomy for benign indications. They found that the correlation between estimates and actual uterine weight was 0.79 (P<.001), with a very low conversion rate for the surgery.1
Lonky and colleagues collected preoperative uterine estimates and actual specimen weights prospectively for 1,079 cases of benign hysterectomy. The surgeries were performed by 186 primary surgeons and assistant surgeons at 10 Kaiser Permanente Southern California medical centers. Surgeons based the route of hysterectomy on estimates of uterine size, which were calculated using bimanual examination, ultrasonography, or both. Linear regression was used to measure and compare the relationship between estimated uterine size and the pelvic specimen weight.
Uterine size estimates ranged from 4 cm to 40 cm, and specimen weights ranged from 2 g to 4,607 g. The mean (SD) estimate of uterine size was 11.7 (4.43) cm, and the mean actual specimen weight was 334.6 (401.42) g.
The mean age of women in the sample was 47.2 (8.35) years. Overall, 379 women (35.1%) were Hispanic, 325 (30.1%) were non-Hispanic white, 281 (26.0%) were non-Hispanic black, and 81 (7.5%) were Asian/Pacific Islander. The mean body mass index (BMI) was 30.0 (6.37) kg/m2, with a range of 16.8 to 67.9 kg/m2.
“This is real world research,” said Dr. Lonky. “It’s called comparative effectiveness research. Basically, all patients who are undergoing the procedure are entered in the registry, and the clinical acumen of the physician—either using or not using ultrasound—is assessed.”
“We looked at whether or not we had a bias toward one patient age group, race/ethnicity, BMI, or estimated uterine size. But there were no clusters, so this was truly a random distribution,” said Dr. Lonky.
“These findings may be population-specific to my group of doctors,” he added. “They should be replicated in other settings. It may be that residents are not going to be as linear.”
Reference
- Lonky NM, Chiu V, Mohan Y. Clinical utility of the estimation of uterine size in planning hysterectomy approach. Obstet Gynecol. 2015;125(5 suppl):19S.
In a poster presented at the 2015 ACOG Annual Clinical Meeting in San Francisco, Neal Marc Lonky, MD, and colleagues from the Southern California Permanente Group assessed the clinical acumen of physicians in estimating uterine size prior to elective hysterectomy for benign indications. They found that the correlation between estimates and actual uterine weight was 0.79 (P<.001), with a very low conversion rate for the surgery.1
Lonky and colleagues collected preoperative uterine estimates and actual specimen weights prospectively for 1,079 cases of benign hysterectomy. The surgeries were performed by 186 primary surgeons and assistant surgeons at 10 Kaiser Permanente Southern California medical centers. Surgeons based the route of hysterectomy on estimates of uterine size, which were calculated using bimanual examination, ultrasonography, or both. Linear regression was used to measure and compare the relationship between estimated uterine size and the pelvic specimen weight.
Uterine size estimates ranged from 4 cm to 40 cm, and specimen weights ranged from 2 g to 4,607 g. The mean (SD) estimate of uterine size was 11.7 (4.43) cm, and the mean actual specimen weight was 334.6 (401.42) g.
The mean age of women in the sample was 47.2 (8.35) years. Overall, 379 women (35.1%) were Hispanic, 325 (30.1%) were non-Hispanic white, 281 (26.0%) were non-Hispanic black, and 81 (7.5%) were Asian/Pacific Islander. The mean body mass index (BMI) was 30.0 (6.37) kg/m2, with a range of 16.8 to 67.9 kg/m2.
“This is real world research,” said Dr. Lonky. “It’s called comparative effectiveness research. Basically, all patients who are undergoing the procedure are entered in the registry, and the clinical acumen of the physician—either using or not using ultrasound—is assessed.”
“We looked at whether or not we had a bias toward one patient age group, race/ethnicity, BMI, or estimated uterine size. But there were no clusters, so this was truly a random distribution,” said Dr. Lonky.
“These findings may be population-specific to my group of doctors,” he added. “They should be replicated in other settings. It may be that residents are not going to be as linear.”
In a poster presented at the 2015 ACOG Annual Clinical Meeting in San Francisco, Neal Marc Lonky, MD, and colleagues from the Southern California Permanente Group assessed the clinical acumen of physicians in estimating uterine size prior to elective hysterectomy for benign indications. They found that the correlation between estimates and actual uterine weight was 0.79 (P<.001), with a very low conversion rate for the surgery.1
Lonky and colleagues collected preoperative uterine estimates and actual specimen weights prospectively for 1,079 cases of benign hysterectomy. The surgeries were performed by 186 primary surgeons and assistant surgeons at 10 Kaiser Permanente Southern California medical centers. Surgeons based the route of hysterectomy on estimates of uterine size, which were calculated using bimanual examination, ultrasonography, or both. Linear regression was used to measure and compare the relationship between estimated uterine size and the pelvic specimen weight.
Uterine size estimates ranged from 4 cm to 40 cm, and specimen weights ranged from 2 g to 4,607 g. The mean (SD) estimate of uterine size was 11.7 (4.43) cm, and the mean actual specimen weight was 334.6 (401.42) g.
The mean age of women in the sample was 47.2 (8.35) years. Overall, 379 women (35.1%) were Hispanic, 325 (30.1%) were non-Hispanic white, 281 (26.0%) were non-Hispanic black, and 81 (7.5%) were Asian/Pacific Islander. The mean body mass index (BMI) was 30.0 (6.37) kg/m2, with a range of 16.8 to 67.9 kg/m2.
“This is real world research,” said Dr. Lonky. “It’s called comparative effectiveness research. Basically, all patients who are undergoing the procedure are entered in the registry, and the clinical acumen of the physician—either using or not using ultrasound—is assessed.”
“We looked at whether or not we had a bias toward one patient age group, race/ethnicity, BMI, or estimated uterine size. But there were no clusters, so this was truly a random distribution,” said Dr. Lonky.
“These findings may be population-specific to my group of doctors,” he added. “They should be replicated in other settings. It may be that residents are not going to be as linear.”
Reference
- Lonky NM, Chiu V, Mohan Y. Clinical utility of the estimation of uterine size in planning hysterectomy approach. Obstet Gynecol. 2015;125(5 suppl):19S.
Reference
- Lonky NM, Chiu V, Mohan Y. Clinical utility of the estimation of uterine size in planning hysterectomy approach. Obstet Gynecol. 2015;125(5 suppl):19S.
Hospital Medicine 2015 Photo Gallery - Day One
Photographs from the first day of Hospital Medicine 2015, which took place March 29-April 1 at the Gaylord National Hotel and Conference Center in National Harbor, Md.
Photos by Manuel Noguera
[gallery ids="9553,9555,9556,9557,9558,9559,9560,9561,9562,9563,9564,9565,9566,9567,9568,9569,9570,9571,9572,9573,9574,9575,9576,9577,9578,9579,9580,9581,9582,9583,9584"]
Photographs from the first day of Hospital Medicine 2015, which took place March 29-April 1 at the Gaylord National Hotel and Conference Center in National Harbor, Md.
Photos by Manuel Noguera
[gallery ids="9553,9555,9556,9557,9558,9559,9560,9561,9562,9563,9564,9565,9566,9567,9568,9569,9570,9571,9572,9573,9574,9575,9576,9577,9578,9579,9580,9581,9582,9583,9584"]
Photographs from the first day of Hospital Medicine 2015, which took place March 29-April 1 at the Gaylord National Hotel and Conference Center in National Harbor, Md.
Photos by Manuel Noguera
[gallery ids="9553,9555,9556,9557,9558,9559,9560,9561,9562,9563,9564,9565,9566,9567,9568,9569,9570,9571,9572,9573,9574,9575,9576,9577,9578,9579,9580,9581,9582,9583,9584"]
Is the use of a containment bag at minimally invasive hysterectomy or myomectomy effective at reducing tissue spillage?
Tissue extraction during laparoscopic or robot-assisted laparoscopic gynecologic surgery raises safety concerns for dissemination of tissue during the open, or uncontained, electromechanical morcellation process. Researchers from Brigham & Women’s Hospital in Boston, Massachusetts, investigated whether contained tissue extraction using power morcellators entirely within a bag is safe and practical for preventing tissue spillage. Goggins and colleagues presented their findings in a poster at the 2015 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in San Francisco, California.
A total of 76 women at 4 institutions underwent laparoscopic or robotic multiport surgery (42 hysterectomy; 34 myomectomy). The average (SD) age and body mass index of the women were 43.16 (8.53) years and 26.47 kg/m2 (5.93), respectively. After surgical dissection, each specimen was placed into a containment bag that also included blue dye. The bag was insufflated intracorporeally and electromechanical morcellation and extraction of tissue were performed. The bag was evaluated visually for dye leakage or tears before and after the procedure.
Results
In one case, there was a tear in the bag before morcellation; no bag tears occurred during the morcellation process. Spillage of dye or tissue was noted in 7 cases, although containment bags were intact in each instance. One patient experienced intraoperative blood loss (3600 mL), and that procedure was converted to open radical hysterectomy. The most common pathologic finding was benign leiomyoma.
Conclusion
Goggins and colleagues concluded, “Contained tissue extraction using electromechanical morcellation and intracorporeally insufflated bags may provide a safe alternative to uncontained morcellation by decreasing the spread of tissue in the peritoneal cavity while allowing for the traditional benefits of laparoscopy.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Goggins ER, Greenberg JA, Cohen SL, Morris SN, Brown DN, Einarsson JI. Efficacy of contained tissue extraction for minimizing tissue dissemination during laparoscopic hysterectomy and myomectomy. Obstet Gynecol. 2015;125(5)(suppl):29S.
Tissue extraction during laparoscopic or robot-assisted laparoscopic gynecologic surgery raises safety concerns for dissemination of tissue during the open, or uncontained, electromechanical morcellation process. Researchers from Brigham & Women’s Hospital in Boston, Massachusetts, investigated whether contained tissue extraction using power morcellators entirely within a bag is safe and practical for preventing tissue spillage. Goggins and colleagues presented their findings in a poster at the 2015 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in San Francisco, California.
A total of 76 women at 4 institutions underwent laparoscopic or robotic multiport surgery (42 hysterectomy; 34 myomectomy). The average (SD) age and body mass index of the women were 43.16 (8.53) years and 26.47 kg/m2 (5.93), respectively. After surgical dissection, each specimen was placed into a containment bag that also included blue dye. The bag was insufflated intracorporeally and electromechanical morcellation and extraction of tissue were performed. The bag was evaluated visually for dye leakage or tears before and after the procedure.
Results
In one case, there was a tear in the bag before morcellation; no bag tears occurred during the morcellation process. Spillage of dye or tissue was noted in 7 cases, although containment bags were intact in each instance. One patient experienced intraoperative blood loss (3600 mL), and that procedure was converted to open radical hysterectomy. The most common pathologic finding was benign leiomyoma.
Conclusion
Goggins and colleagues concluded, “Contained tissue extraction using electromechanical morcellation and intracorporeally insufflated bags may provide a safe alternative to uncontained morcellation by decreasing the spread of tissue in the peritoneal cavity while allowing for the traditional benefits of laparoscopy.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Tissue extraction during laparoscopic or robot-assisted laparoscopic gynecologic surgery raises safety concerns for dissemination of tissue during the open, or uncontained, electromechanical morcellation process. Researchers from Brigham & Women’s Hospital in Boston, Massachusetts, investigated whether contained tissue extraction using power morcellators entirely within a bag is safe and practical for preventing tissue spillage. Goggins and colleagues presented their findings in a poster at the 2015 Annual Clinical Meeting of the American College of Obstetricians and Gynecologists in San Francisco, California.
A total of 76 women at 4 institutions underwent laparoscopic or robotic multiport surgery (42 hysterectomy; 34 myomectomy). The average (SD) age and body mass index of the women were 43.16 (8.53) years and 26.47 kg/m2 (5.93), respectively. After surgical dissection, each specimen was placed into a containment bag that also included blue dye. The bag was insufflated intracorporeally and electromechanical morcellation and extraction of tissue were performed. The bag was evaluated visually for dye leakage or tears before and after the procedure.
Results
In one case, there was a tear in the bag before morcellation; no bag tears occurred during the morcellation process. Spillage of dye or tissue was noted in 7 cases, although containment bags were intact in each instance. One patient experienced intraoperative blood loss (3600 mL), and that procedure was converted to open radical hysterectomy. The most common pathologic finding was benign leiomyoma.
Conclusion
Goggins and colleagues concluded, “Contained tissue extraction using electromechanical morcellation and intracorporeally insufflated bags may provide a safe alternative to uncontained morcellation by decreasing the spread of tissue in the peritoneal cavity while allowing for the traditional benefits of laparoscopy.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Goggins ER, Greenberg JA, Cohen SL, Morris SN, Brown DN, Einarsson JI. Efficacy of contained tissue extraction for minimizing tissue dissemination during laparoscopic hysterectomy and myomectomy. Obstet Gynecol. 2015;125(5)(suppl):29S.
Reference
- Goggins ER, Greenberg JA, Cohen SL, Morris SN, Brown DN, Einarsson JI. Efficacy of contained tissue extraction for minimizing tissue dissemination during laparoscopic hysterectomy and myomectomy. Obstet Gynecol. 2015;125(5)(suppl):29S.
Patients may need extended VTE prophylaxis, doc says

Photo by Andre E.X. Brown
SEATTLE—Patients who undergo surgery for lung cancer may have a higher risk of developing venous thromboembolism (VTE) than we thought, according to a
new study.
About 12% of the patients studied developed deep vein thrombosis (DVT), pulmonary embolism (PE), or both, although they had received VTE prophylaxis until hospital discharge.
Only about 21% of these patients showed symptoms of VTE, and the clots conferred a higher risk of mortality at 30 days.
“This study shows that a significant proportion of lung cancer surgery patients are at risk of VTE and indicates a need for future research into minimizing the occurrence of DVT and PE,” said investigator Yaron Shargall, MD, of McMaster University in Hamilton, Ontario, Canada.
“It is possible that extended use of blood thinners beyond hospital discharge may reduce the number of patients who experience these life-threatening events and may help to reduce the rates of death after lung surgery.”
Dr Shargall presented this viewpoint at the 95th Annual Meeting of the American Association for Thoracic Surgery.
For their study, he and his colleagues evaluated 157 patients who underwent thoracic surgery for primary lung cancer (89.9%) or metastatic cancer (6.3%).
All patients received unfractionated heparin or low-molecular-weight heparin and graduated compression stockings as VTE prophylaxis from the time of surgery until leaving the hospital.
Two weeks later, these patients were evaluated for signs and symptoms of VTE. The investigators evaluated clinical outcomes at 30 ± 5 days post-operatively using CT pulmonary angiography and bilateral Doppler venous ultrasonography.
Patients who had developed symptoms suggestive of VTE within the 30 days after surgery underwent urgent CT-PE examination and had a repeat scan 30 days after surgery if the first scan was negative. Patients with VTE were monitored and treated.
In all, there were 19 VTEs, a 12.1% incidence rate. These included 14 PEs (8.9%), 3 DVTs (1.9%), and 1 combined PE/DVT. One patient developed a massive left atrial thrombus originating from a surgical stump and died.
For all 157 patients, the 30-day mortality rate was 0.64%. For those with VTE, it was 5.2%.
“This demonstrates the clinical importance and relative fatality of VTE following lung cancer surgery,” Dr Shargall said.
All of the patients who were diagnosed with a VTE had undergone anatomic resections (lobectomy or segmentectomy), and most had primary lung cancer. The clots tended to form on the same side as the lung surgery. The majority of patients developed lung clots without forming DVTs beforehand.
The investigators examined factors that might distinguish patients who developed VTEs from those who did not and could not find differences in patient age, lung function, hospital length of stay, comorbidities, lung cancer stage, smoking status, or Caprini Score.
Among patients diagnosed with a VTE, only 4 (21.1%) showed symptoms. All the events were diagnosed after the patient left the hospital and only because these patients were screened for VTEs as part of the study. ![]()

Photo by Andre E.X. Brown
SEATTLE—Patients who undergo surgery for lung cancer may have a higher risk of developing venous thromboembolism (VTE) than we thought, according to a
new study.
About 12% of the patients studied developed deep vein thrombosis (DVT), pulmonary embolism (PE), or both, although they had received VTE prophylaxis until hospital discharge.
Only about 21% of these patients showed symptoms of VTE, and the clots conferred a higher risk of mortality at 30 days.
“This study shows that a significant proportion of lung cancer surgery patients are at risk of VTE and indicates a need for future research into minimizing the occurrence of DVT and PE,” said investigator Yaron Shargall, MD, of McMaster University in Hamilton, Ontario, Canada.
“It is possible that extended use of blood thinners beyond hospital discharge may reduce the number of patients who experience these life-threatening events and may help to reduce the rates of death after lung surgery.”
Dr Shargall presented this viewpoint at the 95th Annual Meeting of the American Association for Thoracic Surgery.
For their study, he and his colleagues evaluated 157 patients who underwent thoracic surgery for primary lung cancer (89.9%) or metastatic cancer (6.3%).
All patients received unfractionated heparin or low-molecular-weight heparin and graduated compression stockings as VTE prophylaxis from the time of surgery until leaving the hospital.
Two weeks later, these patients were evaluated for signs and symptoms of VTE. The investigators evaluated clinical outcomes at 30 ± 5 days post-operatively using CT pulmonary angiography and bilateral Doppler venous ultrasonography.
Patients who had developed symptoms suggestive of VTE within the 30 days after surgery underwent urgent CT-PE examination and had a repeat scan 30 days after surgery if the first scan was negative. Patients with VTE were monitored and treated.
In all, there were 19 VTEs, a 12.1% incidence rate. These included 14 PEs (8.9%), 3 DVTs (1.9%), and 1 combined PE/DVT. One patient developed a massive left atrial thrombus originating from a surgical stump and died.
For all 157 patients, the 30-day mortality rate was 0.64%. For those with VTE, it was 5.2%.
“This demonstrates the clinical importance and relative fatality of VTE following lung cancer surgery,” Dr Shargall said.
All of the patients who were diagnosed with a VTE had undergone anatomic resections (lobectomy or segmentectomy), and most had primary lung cancer. The clots tended to form on the same side as the lung surgery. The majority of patients developed lung clots without forming DVTs beforehand.
The investigators examined factors that might distinguish patients who developed VTEs from those who did not and could not find differences in patient age, lung function, hospital length of stay, comorbidities, lung cancer stage, smoking status, or Caprini Score.
Among patients diagnosed with a VTE, only 4 (21.1%) showed symptoms. All the events were diagnosed after the patient left the hospital and only because these patients were screened for VTEs as part of the study. ![]()

Photo by Andre E.X. Brown
SEATTLE—Patients who undergo surgery for lung cancer may have a higher risk of developing venous thromboembolism (VTE) than we thought, according to a
new study.
About 12% of the patients studied developed deep vein thrombosis (DVT), pulmonary embolism (PE), or both, although they had received VTE prophylaxis until hospital discharge.
Only about 21% of these patients showed symptoms of VTE, and the clots conferred a higher risk of mortality at 30 days.
“This study shows that a significant proportion of lung cancer surgery patients are at risk of VTE and indicates a need for future research into minimizing the occurrence of DVT and PE,” said investigator Yaron Shargall, MD, of McMaster University in Hamilton, Ontario, Canada.
“It is possible that extended use of blood thinners beyond hospital discharge may reduce the number of patients who experience these life-threatening events and may help to reduce the rates of death after lung surgery.”
Dr Shargall presented this viewpoint at the 95th Annual Meeting of the American Association for Thoracic Surgery.
For their study, he and his colleagues evaluated 157 patients who underwent thoracic surgery for primary lung cancer (89.9%) or metastatic cancer (6.3%).
All patients received unfractionated heparin or low-molecular-weight heparin and graduated compression stockings as VTE prophylaxis from the time of surgery until leaving the hospital.
Two weeks later, these patients were evaluated for signs and symptoms of VTE. The investigators evaluated clinical outcomes at 30 ± 5 days post-operatively using CT pulmonary angiography and bilateral Doppler venous ultrasonography.
Patients who had developed symptoms suggestive of VTE within the 30 days after surgery underwent urgent CT-PE examination and had a repeat scan 30 days after surgery if the first scan was negative. Patients with VTE were monitored and treated.
In all, there were 19 VTEs, a 12.1% incidence rate. These included 14 PEs (8.9%), 3 DVTs (1.9%), and 1 combined PE/DVT. One patient developed a massive left atrial thrombus originating from a surgical stump and died.
For all 157 patients, the 30-day mortality rate was 0.64%. For those with VTE, it was 5.2%.
“This demonstrates the clinical importance and relative fatality of VTE following lung cancer surgery,” Dr Shargall said.
All of the patients who were diagnosed with a VTE had undergone anatomic resections (lobectomy or segmentectomy), and most had primary lung cancer. The clots tended to form on the same side as the lung surgery. The majority of patients developed lung clots without forming DVTs beforehand.
The investigators examined factors that might distinguish patients who developed VTEs from those who did not and could not find differences in patient age, lung function, hospital length of stay, comorbidities, lung cancer stage, smoking status, or Caprini Score.
Among patients diagnosed with a VTE, only 4 (21.1%) showed symptoms. All the events were diagnosed after the patient left the hospital and only because these patients were screened for VTEs as part of the study. ![]()
Simpler, more cost-effective way to grow stem cells

Photo courtesy of University
of Texas at El Paso
Researchers say they have developed a protocol to prepare human induced pluripotent stem (hiPS) cells using chemically fixed feeder cells.
This method saves time and money by avoiding the need for colony formation of live feeder cells, which is required by current conventional methods.
The new protocol challenges the theory that live feeder cells are required to provide nutrients to growing stem cells.
“We’ve proved an important phenomenon,” said Binata Joddar, PhD, of the University of Texas at El Paso. “And it suggests that these feeder cells, which are difficult to grow, may not be important at all for stem cell growth.”
Dr Joddar and her colleagues described the phenomenon in Journal of Materials Chemistry B.
Using 2.5% glutaraldehyde (GA) or formaldehyde (FA) for 10 minutes, the researchers prepared a niche matrix from autologus human dermal fibroblast (HDF) feeder cells.
They then introduced hiPS cells to the niche matrix, which adhered to and were maintained as colonies on the fixed feeder cells.
The colony doubling times of the cells grown this way were similar to those of hiPS cells grown on mitomycin-C-treated HDF or SNL feeders. (SNL cells are derived from mouse fibroblast STO cells transformed with a neomycin resistance gene.)
But the colony doubling time for the hiPS cells was shorter with the fixed feeder than for cells cultured on laminin-5.
The researchers also discovered that the average number of colonies per passage was signficiantly higher for hiPS cells cultured on fixed feeder cells compared to those cultured without feeders.
They noted hiPS cells cultured on gelatin did not grow beyond the first passage.
The team concluded that the two types of chemically fixed HDF feeder cells (HDF-glutaraldehyde and HDF-formaldehyde) can be used as substitutes for mitomycin-C-treated HDF feeders to culture hiPS cells.
This new method would not extend the doubling time, would save preparation time, and would avoid labor-intensive protocols to prepare.
In addition, after chemical fixation, the feeder cells are non-viable and cannot release active growth factors or chemokines into the cell culture. Therefore, fixed feeder cells can be refrigerated for long-term storage prior to use.
“Because feeder cells don’t need to stay alive in the process, we can store them at room temperature and spend less time cultivating them,” Dr Joddar said.
“This makes me think that we [could] use a nanomanufacturing approach to grow stem cells. We could mimic feeder cells’ nanotopology with 3-D printing techniques and skip using feeder cells altogether in the future.” ![]()

Photo courtesy of University
of Texas at El Paso
Researchers say they have developed a protocol to prepare human induced pluripotent stem (hiPS) cells using chemically fixed feeder cells.
This method saves time and money by avoiding the need for colony formation of live feeder cells, which is required by current conventional methods.
The new protocol challenges the theory that live feeder cells are required to provide nutrients to growing stem cells.
“We’ve proved an important phenomenon,” said Binata Joddar, PhD, of the University of Texas at El Paso. “And it suggests that these feeder cells, which are difficult to grow, may not be important at all for stem cell growth.”
Dr Joddar and her colleagues described the phenomenon in Journal of Materials Chemistry B.
Using 2.5% glutaraldehyde (GA) or formaldehyde (FA) for 10 minutes, the researchers prepared a niche matrix from autologus human dermal fibroblast (HDF) feeder cells.
They then introduced hiPS cells to the niche matrix, which adhered to and were maintained as colonies on the fixed feeder cells.
The colony doubling times of the cells grown this way were similar to those of hiPS cells grown on mitomycin-C-treated HDF or SNL feeders. (SNL cells are derived from mouse fibroblast STO cells transformed with a neomycin resistance gene.)
But the colony doubling time for the hiPS cells was shorter with the fixed feeder than for cells cultured on laminin-5.
The researchers also discovered that the average number of colonies per passage was signficiantly higher for hiPS cells cultured on fixed feeder cells compared to those cultured without feeders.
They noted hiPS cells cultured on gelatin did not grow beyond the first passage.
The team concluded that the two types of chemically fixed HDF feeder cells (HDF-glutaraldehyde and HDF-formaldehyde) can be used as substitutes for mitomycin-C-treated HDF feeders to culture hiPS cells.
This new method would not extend the doubling time, would save preparation time, and would avoid labor-intensive protocols to prepare.
In addition, after chemical fixation, the feeder cells are non-viable and cannot release active growth factors or chemokines into the cell culture. Therefore, fixed feeder cells can be refrigerated for long-term storage prior to use.
“Because feeder cells don’t need to stay alive in the process, we can store them at room temperature and spend less time cultivating them,” Dr Joddar said.
“This makes me think that we [could] use a nanomanufacturing approach to grow stem cells. We could mimic feeder cells’ nanotopology with 3-D printing techniques and skip using feeder cells altogether in the future.” ![]()

Photo courtesy of University
of Texas at El Paso
Researchers say they have developed a protocol to prepare human induced pluripotent stem (hiPS) cells using chemically fixed feeder cells.
This method saves time and money by avoiding the need for colony formation of live feeder cells, which is required by current conventional methods.
The new protocol challenges the theory that live feeder cells are required to provide nutrients to growing stem cells.
“We’ve proved an important phenomenon,” said Binata Joddar, PhD, of the University of Texas at El Paso. “And it suggests that these feeder cells, which are difficult to grow, may not be important at all for stem cell growth.”
Dr Joddar and her colleagues described the phenomenon in Journal of Materials Chemistry B.
Using 2.5% glutaraldehyde (GA) or formaldehyde (FA) for 10 minutes, the researchers prepared a niche matrix from autologus human dermal fibroblast (HDF) feeder cells.
They then introduced hiPS cells to the niche matrix, which adhered to and were maintained as colonies on the fixed feeder cells.
The colony doubling times of the cells grown this way were similar to those of hiPS cells grown on mitomycin-C-treated HDF or SNL feeders. (SNL cells are derived from mouse fibroblast STO cells transformed with a neomycin resistance gene.)
But the colony doubling time for the hiPS cells was shorter with the fixed feeder than for cells cultured on laminin-5.
The researchers also discovered that the average number of colonies per passage was signficiantly higher for hiPS cells cultured on fixed feeder cells compared to those cultured without feeders.
They noted hiPS cells cultured on gelatin did not grow beyond the first passage.
The team concluded that the two types of chemically fixed HDF feeder cells (HDF-glutaraldehyde and HDF-formaldehyde) can be used as substitutes for mitomycin-C-treated HDF feeders to culture hiPS cells.
This new method would not extend the doubling time, would save preparation time, and would avoid labor-intensive protocols to prepare.
In addition, after chemical fixation, the feeder cells are non-viable and cannot release active growth factors or chemokines into the cell culture. Therefore, fixed feeder cells can be refrigerated for long-term storage prior to use.
“Because feeder cells don’t need to stay alive in the process, we can store them at room temperature and spend less time cultivating them,” Dr Joddar said.
“This makes me think that we [could] use a nanomanufacturing approach to grow stem cells. We could mimic feeder cells’ nanotopology with 3-D printing techniques and skip using feeder cells altogether in the future.” ![]()
Patient Satisfaction Variance Prediction
The Affordable Care Act of 2010 mandates that government payments to hospitals and physicians must depend, in part, on metrics that assess the quality and efficiency of healthcare being provided to encourage value‐based healthcare.[1] Value in healthcare is defined by the delivery of high‐quality care at low cost.[2, 3] To this end, Hospital Value‐Based Purchasing (HVBP) and Physician Value‐Based Payment Modifier programs have been developed by the Centers for Medicare & Medicaid Services (CMS). HVBP is currently being phased in and affects CMS payments for fiscal year (FY) 2013 for over 3000 hospitals across the United States to incentivize healthcare delivery value. The final phase of implementation will be in FY 2017 and will then affect 2% of all CMS hospital reimbursement. HVBP is based on objective measures of hospital performance as well as a subjective measure of performance captured under the Patient Experience of Care domain. This subjective measure will remain at 30% of the aggregate score until FY 2016, when it will then be 25% the aggregate score moving forward.[4] The program rewards hospitals for both overall achievement and improvement in any domain, so that hospitals have multiple ways to receive financial incentives for providing quality care.[5] Even still, there appears to be a nonrandom pattern of patient satisfaction scores across the country with less favorable scores clustering in densely populated areas.[6]
Value‐Based Purchasing and other incentive‐based programs have been criticized for increasing disparities in healthcare by penalizing larger hospitals (including academic medical centers, safety‐net hospitals, and others that disproportionately serve lower socioeconomic communities) and favoring physician‐based specialty hospitals.[7, 8, 9] Therefore, hospitals that serve indigent and elderly populations may be at a disadvantage.[9, 10] HVBP portends significant economic consequences for the majority of hospitals that rely heavily on Medicare and Medicaid reimbursement, as most hospitals have large revenues but low profit margins.[11] Higher HVBP scores are associated with for profit status, smaller size, and location in certain areas of the United States.[12] Jha et al.[6] described Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) scores regional geographic variability, but concluded that poor satisfaction was due to poor quality.
The Patient Experience of Care domain quantifies patient satisfaction using the validated HCAHPS survey, which is provided to a random sample of patients continuously throughout the year at 48 hours to 6 weeks after discharge. It is a publically available standardized survey instrument used to measure patients perspectives on hospital care. It assesses the following 8 dimensions: nurse communication, doctor communication, hospital staff responsiveness, pain management, medicine communication, discharge information, hospital cleanliness and quietness, and overall hospital rating, of which the last 2 dimensions each have 2 measures (cleanliness and quietness) and (rating 9 or 10 and definitely recommend) to give a total of 10 distinct measures.
The United States is a complex network of urban, suburban, and rural demographic areas. Hospitals exist within a unique contextual and compositional meshwork that determines its caseload. The top population density decile of the United States lives within 37 counties, whereas half of the most populous parts of the United States occupy a total of 250 counties out of a total of 3143 counties in the United States. If the 10 measures of patient satisfaction (HCAHPS) scores were abstracted from hospitals and viewed according to county‐level population density (separated into deciles across the United States), a trend would be apparent (Figure 1). Greater population density is associated with lower patient satisfaction in 9 of 10 categories. On the state level, composite scores of overall patient satisfaction (amount of positive scores) of hospitals show a 12% variability and a significant correlation with population density (r=0.479; Figure 2). The lowest overall satisfaction scores are obtained from hospitals located in the population‐dense regions of Washington, DC, New York State, California, Maryland, and New Jersey (ie, 63%65%), and the best scores are from Louisiana, South Dakota, Iowa, Maine, and Vermont (ie, 74%75%). The average patient satisfaction score is 71%2.9%. Lower patient satisfaction scores appear to cluster in population‐dense areas and may be associated with greater heterogeneous patient demographics and economic variability in addition to population density.
These observations are surprising considering that CMS already adjusts HCAHPS scores based on patient‐mix coefficients and mode of collection.[13, 14, 15, 16, 17, 18] Adjustments are updated multiple times per year and account for survey collection either by telephone, email, or paper survey, because the populations that select survey forms will differ. Previous studies have shown that demographic features influence the patient evaluation process. For example, younger and more educated patients were found to provide less positive evaluations of healthcare.[19]
This study examined whether patients perceptions of healthcare (pattern of patient satisfaction) as quantified under the patient experience domain of HVBP were affected and predicted by population density and other demographic factors that are outside the control of individual hospitals. In addition, hospital‐level data (eg, number of hospital beds) and county‐level data such as race, age, gender, overall population, income, time spent commuting to work, primary language, and place of birth were analyzed for correlation with patient satisfaction scores. Our study demonstrates that demographic and hospital‐level data can predict patient satisfaction scores and suggests that CMS may need to modify its adjustment formulas to eliminate bias in HVBP‐based reimbursement.
METHODS
Data Collection
Publically available data were obtained from Hospital Compare,[20] American Hospital Directory,[21] and the US Census Bureau[22] websites. Twenty relevant US Census data categories were selected by their relevance for this study out of the 50 publically reported US Census categories, and included the following: county population, county population density, percent of population change over 1 year, poverty level (percent), income level per capita, median household income, average household size, travel time to work, percentage of high school or college graduates, non‐English primary language spoken at home, percentage of residents born outside of the United States, population percent in same residence for over 1 year, gender, race (white alone, white alone (not Hispanic or Latino), black or African American alone), population over 65 years old, and population under 18 years old.
HCAHPS Development
The HCAHPS survey is 32 questions in length, comprised of 10 evaluative dimensions. All short‐term, acute care, nonspecialty hospitals are invited to participate in the HCAHPS survey.
Data Analysis
Statistical analyses used the Statistical Package for Social Sciences version 16.0 for Windows (SPSS Inc., Chicago, IL). Data were checked for statistical assumptions, including normality, linearity of relationships, and full range of scores. Categories in both the Hospital Compare (HCAHPS) and US Census datasets were analyzed to assess their distribution curves. The category of population densities (per county) was converted to a logarithmic scale to account for a skewed distribution and long tail in the area of low population density. Data were subsequently merged into an Excel (Microsoft, Redmond, WA) spreadsheet using the VLookup function such that relevant 2010 census county data were added to each hospital's Hospital Compare data. Linear regression modeling was performed. Bivariate analysis was conducted (ENTER method) to determine the significant US Census data predictors for each of the 10 Hospital Compare dimensions including the composite overall satisfaction score. Significant predictors were then analyzed in a multivariate model (BACKWORDS method) for each Hospital Compare dimension and the composite average positive score. Models were assessed by determinates of correlation (adjusted R2) to assess for goodness of fit. Statistically significant predictor variables for overall patient satisfaction scores were then ranked according to their partial regression coefficients (standardized ).
A patient satisfaction predictive model was sought based upon significant predictors of aggregate percent positive HCAHPS scores. Various predictor combinations were formed based on their partial coefficients (ie, standardized coefficients); combinations were assessed based on their R2 values and assessed for colinearity. Combinations of partial coefficients included the 2, 4, and 8 most predictive variables as well the 2 most positive and negative predictors. They were then incorporated into a multivariate analysis model (FORWARD method) and assessed based on their adjusted R2 values. A 4‐variable combination (the 2 most predictive positive partial coefficients plus the 2 most predictive negative partial coefficients) was selected as a predictive model, and a formula predictive of the composite overall satisfaction score was generated. This formula (predicted patient satisfaction formula [PPSF]) predicts hospital patient satisfaction HCAHPS scores based on the 4 predictive variables for particular county and hospital characteristics.
| B | SE | t | P | ||
|---|---|---|---|---|---|
| |||||
| Educational attainmentbachelor's degree | 0.157 | 0.018 | 0.27 | 8.612 | <0.001 |
| White alone percent 2012 | 0.09 | 0.012 | 0.235 | 7.587 | <0.001 |
| Resident population percent under 18 years | 0.404 | 0.0444 | 0.209 | 9.085 | <0.001 |
| Black or African American alone percent 2012 | 0.083 | 0.014 | 0.191 | 5.936 | <0.001 |
| Median household income 20072011 | 0.00003 | 0.00 | 0.062 | 2.027 | 0.043 |
| Population density (log) 2010 | 0.277 | 0.083 | 0.087 | 3.3333 | 0.001 |
| Average travel time to work | 0.107 | 0.024 | 0.088 | 4.366 | <0.001 |
| Educational attainmenthigh school | 0.082 | 0.026 | 0.088 | 3.147 | 0.002 |
| Average household size | 2.58 | 0.727 | 0.107 | 3.55 | <0.001 |
| Total females percent 2012 | 0.423 | 0.067 | 0.107 | 6.296 | <0.001 |
| Percent nonEnglish speaking at home 20072011 | 0.052 | 0.018 | 0.14 | 2.929 | 0.003 |
| No. of hospital beds | 0.006 | 0.00 | 0.213 | 12.901 | <0.001 |
| Adjusted R2 | 0.222 | ||||
The PPSF was then modified by weighting with the partial coefficient () to remove the bias in patient satisfaction generated by demographic and structural factors over which individual hospitals have limited or no control. This formula generated a Weighted Individual (hospital) Predicted Patient Satisfaction Score (WIPPSS). Application of this formula narrowed the predicted distribution of patient satisfaction for all hospitals across the country.
To create an adjusted score with direct relevance to the reported patient satisfaction scores, the reported scores were multiplied by an adjustment factor that defines the difference between individual hospital‐weighted scores and the national mean HCAHPS score across the United States. This formula, the Weighted Individual (hospital) Patient Satisfaction Adjustment Score (WIPSAS), represents a patient satisfaction score adjusted for demographic and structural factors that can be utilized for interhospital comparisons across all areas of the country.
where PSrep=patient satisfaction reported score, PSUSA=mean reported score for United States (71.84), and WIPPSSX=WIPPSS for individual hospital.
Application of Data Analysis
PPSF, WIPPSS, and WIPSAS were calculated for all HCAHPS‐participating hospitals and compared with averaged raw HCAHPS scores across the United States. WIPSAS and raw scores were specifically analyzed for New York State to demonstrate exactly how adjustments would change state‐level rankings.
RESULTS
Complete HCAHPS scores were obtained from 3907 hospitals out of a total 4621 hospitals listed by the Hospital Compare website (85%). The majority of hospitals (2884) collected over 300 surveys, fewer hospitals (696) collected 100 to 299 surveys, and fewer still (333) collected <100 surveys. In total, results were available from at least 934,800 individual surveys, by the most conservative estimate. Missing HCAHPS hospital data averaged 13.4 (standard deviation [SD] 12.2) hospitals per state. County‐level data were obtained from all 3144 county or county equivalents across the United States (100%). Multivariate regression modeling across all HCAHPS dimensions found that between 10 and 16 of the 20 predictors (US Census categories) were statistically significant and predictive of individual HCAHPS dimension scores and the aggregate percent positive score as demonstrated in Table 2. For example, county percentage of bachelors degrees positively predicts for positive doctor communication scores, and hospital beds negatively predicts for quiet dimension. The strongest positive and negative predictive variables by model regression coefficients for each HCAHPS dimension are also listed in Table 2.
| Average Positive Scores | Nurse Communication | Doctor Communication | Help | Pain | Explain Meds | Clean | Quiet | Discharge Explain | Recommend 9/10 | Definitely Recommend | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||
| Educationalbachelor's | 0.27 | 0.19 | 0.45 | 0.10 | 0.10 | 0.05 | 0.08 | 0.33 | 0.15 | 0.27 | 0.416 |
| Hospital beds | 0.21 | 0.16 | 0.19 | 0.26 | 0.16 | 0.17 | 0.27 | 0.26 | 0.06 | 0.11 | |
| Population density 2010 | 0.09 | 0.07 | 0.28 | 0.20 | 0.08 | 0.23 | 0.14 | 0.19 | 0.22 | 0.07 | * |
| White alone percent | 0.24 | 0.25 | 0.09 | 0.16 | 0.23 | 0.07 | 0.16 | 0.17 | 0.31 | 0.317 | |
| Total females percent | 0.11 | 0.05 | 0.06 | 0.07 | 0.06 | 0.03 | 0.05 | 0.09 | 0.12 | 0.09 | |
| African American alone | 0.19 | 0.19 | 0.09 | 0.23 | 0.09 | 0.07 | 0.34 | * | 0.09 | 0.084 | |
| Average travel time to work | 0.09 | 0.10 | * | 0.09 | 0.06 | 0.04 | 0.08 | * | 0.12 | 0.17 | 0.16 |
| Foreign‐born percent | * | 0.16 | 0.14 | 0.06 | 0.12 | 0.08 | 0.06 | 0.13 | 0.18 | * | * |
| Average household size | 0.11 | 0.05 | 0.15 | 0.07 | * | 0.07 | * | 0.01 | * | 0.07 | 0.076 |
| NonEnglish speaking | 0.14 | 0.12 | 0.50 | 0.07 | * | * | * | * | * | 0.34 | 0.28 |
| Educationhigh school | 0.09 | 0.09 | 0.40 | * | 0.27 | 0.06 | 0.08 | * | |||
| Household income | 0.06 | * | 0.35 | 0.08 | * | * | 0.16 | 0.41 | 0.265 | ||
| Population 65 years and over | * | 0.14 | 0.14 | 0.12 | * | 0.11 | 0.15 | * | 0.10 | ||
| White, not Hispanic/Latino | * | * | 0.20 | * | * | * | 0.09 | 0.13 | 0.09 | 0.22 | 0.25 |
| Population under 18 | 0.21 | 0.15 | 0.08 | 0.11 | 0.20 | ||||||
| Population (county) | * | 0.06 | 0.08 | * | 0.03 | 0.05 | * | * | 0.06 | * | * |
| All ages in poverty | 0.24 | 0.10 | 0.22 | 0.08 | * | 0.281 | |||||
| 1 year at same residence | * | 0.13 | 0.12 | 0.11 | 0.10 | * | 0.04 | * | * | ||
| Per capita income | * | 0.07 | * | * | * | * | * | 0.09 | * | ||
| Population percent change | * | * | * | * | * | * | 0.05 | * | * | ||
| Adjusted R2 | 0.22 | 0.25 | 0.30 | 0.30 | 0.12 | 0.17 | 0.23 | 0.30 | 0.19 | 0.14 | 0.15 |
Table 1 highlights multivariate regression modeling of the composite average positive score, which produced an adjusted R2 of 0.222 (P<0.001). All variables were significant and predicted change of the composite HCAHPS except for place of birthforeign born (not listed in the table). Table 1 ranks variables from most positive to most negative predictors.
Other HCAHPS domains demonstrated statistically significant models (P<0.001) and are listed by their coefficients of determination (ie, adjusted R2) (Table 2). The best‐fit dimensions were help (adjusted R2=0.304), quiet (adjusted R2=0.299), doctor communication (adjusted R2=0.298), nurse communication (adjusted R2=0.245), and clean (adjusted R2=0.232). Models that were not as strongly predictive as the composite score included pain (adjusted R2=0.124), overall 9/10 (adjusted R2=0.136), definitely recommend (adjusted R2=0.150), and explained meds (adjusted R2=0.169).
A predictive formula for average positive scores was created by determination of the most predictive partial coefficients and the best‐fit model. Bachelor's degree and white only were the 2 greatest positive predictors, and number of hospital beds and nonEnglish speaking were the 2 greatest negative predictors. The PPSF (predictive formula) was chosen out of various combinations of predictors (Table 1), because its coefficient of determination (adjusted R2=0.155) was closest to the overall model's coefficient of determination (adjusted R2=0.222) without demonstrating colinearity. Possible predictive formulas were based on the predictors standardized and included the following combinations: the 2 greatest overall predictors (adjusted R2=0.051), the 2 greatest negative and positive predictors (adjusted R2=0.098), the 4 greatest overall predictors (adjusted R2=0.117), and the 8 greatest overall predictors (adjusted R2=0.201), which suffered from colinearity (household size plus nonEnglish speaking [Pearson=0.624] and under 18 years old [Pearson=0.708]). None of the correlated independent variables (eg, poverty and median income) were placed in the final model.
The mean WIPSAS scores closely corresponded with the national average of HCAHPS scores (71.6 vs 71.84) but compressed scores into a narrower distribution (SD 5.52 vs 5.92). The greatest positive and negative changes were by 8.51% and 2.25%, respectively. Essentially, a smaller number of hospitals in demographically challenged areas were more significantly impacted by the WIPSAS adjustment than the larger number of hospitals in demographically favorable areas. Large hospitals in demographically diverse counties saw the greatest positive change (e.g., Texas, California, and New York), whereas smaller hospitals in demographically nondiverse areas saw comparatively smaller decrements in the overall WIPSAS scores. The WIPSAS had the most beneficial effect on urban and rural safety‐net hospitals that serve diverse populations including many academic medical centers. This is illustrated by the reranking of the top 10 and bottom 10 hospitals in New York State by the WIPSAS (Table 3). For example, 3 academic medical centers in New York State, Montefiore Medical Center, New York Presbyterian Hospital, and Mount Sinai Hospital, were moved from the 46th, 43rd, and 42nd (out of 167 hospitals) respectively into the top 10 in patient satisfaction utilizing the WIPSAS methodology. Reported patient satisfaction scores, PPSF, WIPPSS, and WIPSAS scores for each hospital in the United States are available online (see Supporting Table S1 in the online version of this article).
| Ten Highest Ranked New York State Hospitals by HCAHPS | Ten Highest Ranked New York State Hospitals After WIPSAS |
|---|---|
| |
| 1. River Hospital, Inc. | 1. River Hospital, Inc. |
| 2. Westfield Memorial Hospital, Inc. | 2. Westfield Memorial Hospital, Inc. |
| 3. Clifton Fine Hospital | 3. Clifton Fine Hospital |
| 4. Hospital For Special Surgery | 4. Hospital For Special Surgery |
| 5. Delaware Valley Hospital, Inc. | 5. New YorkPresbyterian Hospital |
| 6. Putnam Hospital Center | 6. Delaware Valley Hospital, Inc. |
| 7. Margaretville Memorial Hospital | 7. Montefiore Medical Center |
| 8. Community Memorial Hospital, Inc. | 8. St. Francis Hospital, Roslyn |
| 9. Lewis County General Hospital | 9. Putnam Hospital Center |
| 10. St. Francis Hospital, Roslyn | 10. Mount Sinai Hospital |
DISCUSSION
The HVBP program is an incentive program that is meant to enhance the quality of care. This study illustrates healthcare inequalities in patient satisfaction that are not accounted for by the current CMS adjustments, and shows that education, ethnicity, primary language, and number of hospital beds are predictive of how patients evaluate their care via patient satisfaction scores. Hospitals that treat a disproportionate percentage of nonEnglish speaking, nonwhite, noneducated patients in large facilities are not meeting patient satisfaction standards. This inequity is not ameliorated by the adjustments currently performed by CMS, and has financial consequences for those hospitals that are not meeting national standards in patient satisfaction. These hospitals, which often include academic medical centers in urban areas, may therefore be penalized under the existing HVBP reimbursement models.
Using only 4 demographic and hospital‐specific predictors (ie, hospital beds, percent nonEnglish speaking, percent bachelors degrees, percent white), it is possible to utilize a simple formula to predict patient satisfaction with a significant degree of correlation to the reported scores available through Hospital Compare.
Our initial hypothesis that population density predicted lower patient satisfaction scores was confirmed, but these aforementioned demographic and hospital‐based factors were stronger independent predictors of HCAHPS scores. The WIPSAS is a representation of patient satisfaction and quality‐of‐care delivery across the country that accounts for nonrandom variation in patient satisfaction scores.
For hospitals in New York State, WIPSAS resulted in the placement of 3 urban‐based academic medical centers in the top 10 in patient satisfaction, when previously, based on the raw scores, their rankings were between 42nd and 46th statewide. Prior studies have suggested that large, urban, teaching, and not‐for‐profit hospitals were disadvantaged based on their hospital characteristics and patient features.[10, 11, 12] Under the current CMS reimbursement methodologies, these institutions are more likely to receive financial penalties.[8] The WIPSAS is a simple method to assess hospitals performance in the area of patient satisfaction that accounts for the demographic and hospital‐based factors (eg, number of beds) of the hospital. Its incorporation into CMS reimbursement calculations, or incorporation of a similar adjustment formula, should be strongly considered to account for predictive factors in patient satisfaction that could be addressed to enhance their scores.
Limitations for this study are the approximation of county‐level data for actual individual hospital demographic information and the exclusion of specialty hospitals, such as cancer centers and children's hospitals, in HCAHPS surveys. Repeated multivariate analyses at different time points would also serve to identify how CMS‐specific adjustments are recalibrated over time. Although we have primarily reported on the composite percent positive score as a surrogate for all HCAHPS dimensions, an individual adjustment formula could be generated for each dimension of the patient experience of care domain.
Although patient satisfaction is a component of how quality should be measured, further emphasis needs to be placed on nonrandom patient satisfaction variance so that HVBP can serve as an incentivizing program for at‐risk hospitals. Regional variation in scoring is not altogether accounted for by the current CMS adjustment system. Because patient satisfaction scores are now directly linked to reimbursement, further evaluation is needed to enhance patient satisfaction scoring paradigms to account for demographic and hospital‐specific factors.
Disclosure
Nothing to report.
- , , . Will choice‐based reform work for Medicare? Evidence from the Federal Employees Health Benefits Program. Health Serv Res. 2006;41:1741–1761.
- H.R. 3590. Patient Protection and Affordable Care Act 2010 (2010).
- . The quality of care. How can it be assessed? JAMA. 1988;260(12):1743–1748.
- Lake Superior Quality Innovation Network. FY 2017 Value‐Based Purchasing domain weighting. Available at: http://www.stratishealth.org/documents/VBP‐FY2017.pdf. Accessed March 13, 2015.
- Hospital Value‐Based Purchasing Program. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/Hospital‐Value‐Based‐Purchasing. Accessed December 1st, 2013.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- , . Providers must lead the way in making value the overarching goal Harvard Bus Rev. October 2013:3–19.
- , , . The effect of financial incentives on hospitals that serve poor patients. Ann Intern Med. 2010;153(5):299–306.
- , . Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342–343.
- . Will value‐based purchasing increase disparities in care? N Engl J Med. 2013;369(26):2472–2474.
- , , . Hospital conversions, margins, and the provision of uncompensated care. Health Aff (Millwood). 2000;19(6):187–194.
- , , , , , . Association between value‐based purchasing score and hospital characteristics. BMC Health Serv Res. 2012;12:464.
- , , , et al. Effects of survey mode, patient mix, and nonresponse on CAHPS hospital survey scores. Health Serv Res. 2009;44(2 pt 1):501–518.
- , , , , . Patient satisfaction measurement strategies: a comparison of phone and mail methods. Jt Comm J Qual Improv. 2001;27(7):349–361.
- , , . Comparing telephone and mail responses to the CAHPS survey instrument. Consumer Assessment of Health Plans Study. Med Care. 1999;37(3 suppl):MS41–MS49.
- , , , , , . Evaluating patients' experiences with individual physicians: a randomized trial of mail, internet, and interactive voice response telephone administration of surveys. Med Care. 2006;44(2):167–174.
- , , , , . Case‐mix adjustment of the CAHPS Hospital Survey. Health Serv Res. 2005;40(6 pt 2):2162–2181.
- Mode and patient‐mix adjustments of CAHPS hospital survey (HCAHPS). Available at: http://www.hcahpsonline.org/modeadjustment.aspx. Accessed December 1, 2013.
- , , , , , . Adjusting performance measures to ensure equitable plan comparisons. Health Care Financ Rev. 2001;22(3):109–126.
- Official Hospital Compare Data. Displaying datasets in Patient Survey Results category. Available at: https://data.medicare.gov/data/hospital‐compare/Patient%20Survey%20Results. Accessed December 1, 2013.
- Hospital statistics by state. American Hospital Directory, Inc. website. Available at: http://www.ahd.com/state_statistics.html. Accessed December 1, 2013.
- U.S. Census Download Center. Available at: http://factfinder.census.gov/faces/nav/jsf/pages/download_center.xhtml. Accessed December 1, 2013.
The Affordable Care Act of 2010 mandates that government payments to hospitals and physicians must depend, in part, on metrics that assess the quality and efficiency of healthcare being provided to encourage value‐based healthcare.[1] Value in healthcare is defined by the delivery of high‐quality care at low cost.[2, 3] To this end, Hospital Value‐Based Purchasing (HVBP) and Physician Value‐Based Payment Modifier programs have been developed by the Centers for Medicare & Medicaid Services (CMS). HVBP is currently being phased in and affects CMS payments for fiscal year (FY) 2013 for over 3000 hospitals across the United States to incentivize healthcare delivery value. The final phase of implementation will be in FY 2017 and will then affect 2% of all CMS hospital reimbursement. HVBP is based on objective measures of hospital performance as well as a subjective measure of performance captured under the Patient Experience of Care domain. This subjective measure will remain at 30% of the aggregate score until FY 2016, when it will then be 25% the aggregate score moving forward.[4] The program rewards hospitals for both overall achievement and improvement in any domain, so that hospitals have multiple ways to receive financial incentives for providing quality care.[5] Even still, there appears to be a nonrandom pattern of patient satisfaction scores across the country with less favorable scores clustering in densely populated areas.[6]
Value‐Based Purchasing and other incentive‐based programs have been criticized for increasing disparities in healthcare by penalizing larger hospitals (including academic medical centers, safety‐net hospitals, and others that disproportionately serve lower socioeconomic communities) and favoring physician‐based specialty hospitals.[7, 8, 9] Therefore, hospitals that serve indigent and elderly populations may be at a disadvantage.[9, 10] HVBP portends significant economic consequences for the majority of hospitals that rely heavily on Medicare and Medicaid reimbursement, as most hospitals have large revenues but low profit margins.[11] Higher HVBP scores are associated with for profit status, smaller size, and location in certain areas of the United States.[12] Jha et al.[6] described Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) scores regional geographic variability, but concluded that poor satisfaction was due to poor quality.
The Patient Experience of Care domain quantifies patient satisfaction using the validated HCAHPS survey, which is provided to a random sample of patients continuously throughout the year at 48 hours to 6 weeks after discharge. It is a publically available standardized survey instrument used to measure patients perspectives on hospital care. It assesses the following 8 dimensions: nurse communication, doctor communication, hospital staff responsiveness, pain management, medicine communication, discharge information, hospital cleanliness and quietness, and overall hospital rating, of which the last 2 dimensions each have 2 measures (cleanliness and quietness) and (rating 9 or 10 and definitely recommend) to give a total of 10 distinct measures.
The United States is a complex network of urban, suburban, and rural demographic areas. Hospitals exist within a unique contextual and compositional meshwork that determines its caseload. The top population density decile of the United States lives within 37 counties, whereas half of the most populous parts of the United States occupy a total of 250 counties out of a total of 3143 counties in the United States. If the 10 measures of patient satisfaction (HCAHPS) scores were abstracted from hospitals and viewed according to county‐level population density (separated into deciles across the United States), a trend would be apparent (Figure 1). Greater population density is associated with lower patient satisfaction in 9 of 10 categories. On the state level, composite scores of overall patient satisfaction (amount of positive scores) of hospitals show a 12% variability and a significant correlation with population density (r=0.479; Figure 2). The lowest overall satisfaction scores are obtained from hospitals located in the population‐dense regions of Washington, DC, New York State, California, Maryland, and New Jersey (ie, 63%65%), and the best scores are from Louisiana, South Dakota, Iowa, Maine, and Vermont (ie, 74%75%). The average patient satisfaction score is 71%2.9%. Lower patient satisfaction scores appear to cluster in population‐dense areas and may be associated with greater heterogeneous patient demographics and economic variability in addition to population density.


These observations are surprising considering that CMS already adjusts HCAHPS scores based on patient‐mix coefficients and mode of collection.[13, 14, 15, 16, 17, 18] Adjustments are updated multiple times per year and account for survey collection either by telephone, email, or paper survey, because the populations that select survey forms will differ. Previous studies have shown that demographic features influence the patient evaluation process. For example, younger and more educated patients were found to provide less positive evaluations of healthcare.[19]
This study examined whether patients perceptions of healthcare (pattern of patient satisfaction) as quantified under the patient experience domain of HVBP were affected and predicted by population density and other demographic factors that are outside the control of individual hospitals. In addition, hospital‐level data (eg, number of hospital beds) and county‐level data such as race, age, gender, overall population, income, time spent commuting to work, primary language, and place of birth were analyzed for correlation with patient satisfaction scores. Our study demonstrates that demographic and hospital‐level data can predict patient satisfaction scores and suggests that CMS may need to modify its adjustment formulas to eliminate bias in HVBP‐based reimbursement.
METHODS
Data Collection
Publically available data were obtained from Hospital Compare,[20] American Hospital Directory,[21] and the US Census Bureau[22] websites. Twenty relevant US Census data categories were selected by their relevance for this study out of the 50 publically reported US Census categories, and included the following: county population, county population density, percent of population change over 1 year, poverty level (percent), income level per capita, median household income, average household size, travel time to work, percentage of high school or college graduates, non‐English primary language spoken at home, percentage of residents born outside of the United States, population percent in same residence for over 1 year, gender, race (white alone, white alone (not Hispanic or Latino), black or African American alone), population over 65 years old, and population under 18 years old.
HCAHPS Development
The HCAHPS survey is 32 questions in length, comprised of 10 evaluative dimensions. All short‐term, acute care, nonspecialty hospitals are invited to participate in the HCAHPS survey.
Data Analysis
Statistical analyses used the Statistical Package for Social Sciences version 16.0 for Windows (SPSS Inc., Chicago, IL). Data were checked for statistical assumptions, including normality, linearity of relationships, and full range of scores. Categories in both the Hospital Compare (HCAHPS) and US Census datasets were analyzed to assess their distribution curves. The category of population densities (per county) was converted to a logarithmic scale to account for a skewed distribution and long tail in the area of low population density. Data were subsequently merged into an Excel (Microsoft, Redmond, WA) spreadsheet using the VLookup function such that relevant 2010 census county data were added to each hospital's Hospital Compare data. Linear regression modeling was performed. Bivariate analysis was conducted (ENTER method) to determine the significant US Census data predictors for each of the 10 Hospital Compare dimensions including the composite overall satisfaction score. Significant predictors were then analyzed in a multivariate model (BACKWORDS method) for each Hospital Compare dimension and the composite average positive score. Models were assessed by determinates of correlation (adjusted R2) to assess for goodness of fit. Statistically significant predictor variables for overall patient satisfaction scores were then ranked according to their partial regression coefficients (standardized ).
A patient satisfaction predictive model was sought based upon significant predictors of aggregate percent positive HCAHPS scores. Various predictor combinations were formed based on their partial coefficients (ie, standardized coefficients); combinations were assessed based on their R2 values and assessed for colinearity. Combinations of partial coefficients included the 2, 4, and 8 most predictive variables as well the 2 most positive and negative predictors. They were then incorporated into a multivariate analysis model (FORWARD method) and assessed based on their adjusted R2 values. A 4‐variable combination (the 2 most predictive positive partial coefficients plus the 2 most predictive negative partial coefficients) was selected as a predictive model, and a formula predictive of the composite overall satisfaction score was generated. This formula (predicted patient satisfaction formula [PPSF]) predicts hospital patient satisfaction HCAHPS scores based on the 4 predictive variables for particular county and hospital characteristics.
| B | SE | t | P | ||
|---|---|---|---|---|---|
| |||||
| Educational attainmentbachelor's degree | 0.157 | 0.018 | 0.27 | 8.612 | <0.001 |
| White alone percent 2012 | 0.09 | 0.012 | 0.235 | 7.587 | <0.001 |
| Resident population percent under 18 years | 0.404 | 0.0444 | 0.209 | 9.085 | <0.001 |
| Black or African American alone percent 2012 | 0.083 | 0.014 | 0.191 | 5.936 | <0.001 |
| Median household income 20072011 | 0.00003 | 0.00 | 0.062 | 2.027 | 0.043 |
| Population density (log) 2010 | 0.277 | 0.083 | 0.087 | 3.3333 | 0.001 |
| Average travel time to work | 0.107 | 0.024 | 0.088 | 4.366 | <0.001 |
| Educational attainmenthigh school | 0.082 | 0.026 | 0.088 | 3.147 | 0.002 |
| Average household size | 2.58 | 0.727 | 0.107 | 3.55 | <0.001 |
| Total females percent 2012 | 0.423 | 0.067 | 0.107 | 6.296 | <0.001 |
| Percent nonEnglish speaking at home 20072011 | 0.052 | 0.018 | 0.14 | 2.929 | 0.003 |
| No. of hospital beds | 0.006 | 0.00 | 0.213 | 12.901 | <0.001 |
| Adjusted R2 | 0.222 | ||||
The PPSF was then modified by weighting with the partial coefficient () to remove the bias in patient satisfaction generated by demographic and structural factors over which individual hospitals have limited or no control. This formula generated a Weighted Individual (hospital) Predicted Patient Satisfaction Score (WIPPSS). Application of this formula narrowed the predicted distribution of patient satisfaction for all hospitals across the country.
To create an adjusted score with direct relevance to the reported patient satisfaction scores, the reported scores were multiplied by an adjustment factor that defines the difference between individual hospital‐weighted scores and the national mean HCAHPS score across the United States. This formula, the Weighted Individual (hospital) Patient Satisfaction Adjustment Score (WIPSAS), represents a patient satisfaction score adjusted for demographic and structural factors that can be utilized for interhospital comparisons across all areas of the country.
where PSrep=patient satisfaction reported score, PSUSA=mean reported score for United States (71.84), and WIPPSSX=WIPPSS for individual hospital.
Application of Data Analysis
PPSF, WIPPSS, and WIPSAS were calculated for all HCAHPS‐participating hospitals and compared with averaged raw HCAHPS scores across the United States. WIPSAS and raw scores were specifically analyzed for New York State to demonstrate exactly how adjustments would change state‐level rankings.
RESULTS
Complete HCAHPS scores were obtained from 3907 hospitals out of a total 4621 hospitals listed by the Hospital Compare website (85%). The majority of hospitals (2884) collected over 300 surveys, fewer hospitals (696) collected 100 to 299 surveys, and fewer still (333) collected <100 surveys. In total, results were available from at least 934,800 individual surveys, by the most conservative estimate. Missing HCAHPS hospital data averaged 13.4 (standard deviation [SD] 12.2) hospitals per state. County‐level data were obtained from all 3144 county or county equivalents across the United States (100%). Multivariate regression modeling across all HCAHPS dimensions found that between 10 and 16 of the 20 predictors (US Census categories) were statistically significant and predictive of individual HCAHPS dimension scores and the aggregate percent positive score as demonstrated in Table 2. For example, county percentage of bachelors degrees positively predicts for positive doctor communication scores, and hospital beds negatively predicts for quiet dimension. The strongest positive and negative predictive variables by model regression coefficients for each HCAHPS dimension are also listed in Table 2.
| Average Positive Scores | Nurse Communication | Doctor Communication | Help | Pain | Explain Meds | Clean | Quiet | Discharge Explain | Recommend 9/10 | Definitely Recommend | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||
| Educationalbachelor's | 0.27 | 0.19 | 0.45 | 0.10 | 0.10 | 0.05 | 0.08 | 0.33 | 0.15 | 0.27 | 0.416 |
| Hospital beds | 0.21 | 0.16 | 0.19 | 0.26 | 0.16 | 0.17 | 0.27 | 0.26 | 0.06 | 0.11 | |
| Population density 2010 | 0.09 | 0.07 | 0.28 | 0.20 | 0.08 | 0.23 | 0.14 | 0.19 | 0.22 | 0.07 | * |
| White alone percent | 0.24 | 0.25 | 0.09 | 0.16 | 0.23 | 0.07 | 0.16 | 0.17 | 0.31 | 0.317 | |
| Total females percent | 0.11 | 0.05 | 0.06 | 0.07 | 0.06 | 0.03 | 0.05 | 0.09 | 0.12 | 0.09 | |
| African American alone | 0.19 | 0.19 | 0.09 | 0.23 | 0.09 | 0.07 | 0.34 | * | 0.09 | 0.084 | |
| Average travel time to work | 0.09 | 0.10 | * | 0.09 | 0.06 | 0.04 | 0.08 | * | 0.12 | 0.17 | 0.16 |
| Foreign‐born percent | * | 0.16 | 0.14 | 0.06 | 0.12 | 0.08 | 0.06 | 0.13 | 0.18 | * | * |
| Average household size | 0.11 | 0.05 | 0.15 | 0.07 | * | 0.07 | * | 0.01 | * | 0.07 | 0.076 |
| NonEnglish speaking | 0.14 | 0.12 | 0.50 | 0.07 | * | * | * | * | * | 0.34 | 0.28 |
| Educationhigh school | 0.09 | 0.09 | 0.40 | * | 0.27 | 0.06 | 0.08 | * | |||
| Household income | 0.06 | * | 0.35 | 0.08 | * | * | 0.16 | 0.41 | 0.265 | ||
| Population 65 years and over | * | 0.14 | 0.14 | 0.12 | * | 0.11 | 0.15 | * | 0.10 | ||
| White, not Hispanic/Latino | * | * | 0.20 | * | * | * | 0.09 | 0.13 | 0.09 | 0.22 | 0.25 |
| Population under 18 | 0.21 | 0.15 | 0.08 | 0.11 | 0.20 | ||||||
| Population (county) | * | 0.06 | 0.08 | * | 0.03 | 0.05 | * | * | 0.06 | * | * |
| All ages in poverty | 0.24 | 0.10 | 0.22 | 0.08 | * | 0.281 | |||||
| 1 year at same residence | * | 0.13 | 0.12 | 0.11 | 0.10 | * | 0.04 | * | * | ||
| Per capita income | * | 0.07 | * | * | * | * | * | 0.09 | * | ||
| Population percent change | * | * | * | * | * | * | 0.05 | * | * | ||
| Adjusted R2 | 0.22 | 0.25 | 0.30 | 0.30 | 0.12 | 0.17 | 0.23 | 0.30 | 0.19 | 0.14 | 0.15 |
Table 1 highlights multivariate regression modeling of the composite average positive score, which produced an adjusted R2 of 0.222 (P<0.001). All variables were significant and predicted change of the composite HCAHPS except for place of birthforeign born (not listed in the table). Table 1 ranks variables from most positive to most negative predictors.
Other HCAHPS domains demonstrated statistically significant models (P<0.001) and are listed by their coefficients of determination (ie, adjusted R2) (Table 2). The best‐fit dimensions were help (adjusted R2=0.304), quiet (adjusted R2=0.299), doctor communication (adjusted R2=0.298), nurse communication (adjusted R2=0.245), and clean (adjusted R2=0.232). Models that were not as strongly predictive as the composite score included pain (adjusted R2=0.124), overall 9/10 (adjusted R2=0.136), definitely recommend (adjusted R2=0.150), and explained meds (adjusted R2=0.169).
A predictive formula for average positive scores was created by determination of the most predictive partial coefficients and the best‐fit model. Bachelor's degree and white only were the 2 greatest positive predictors, and number of hospital beds and nonEnglish speaking were the 2 greatest negative predictors. The PPSF (predictive formula) was chosen out of various combinations of predictors (Table 1), because its coefficient of determination (adjusted R2=0.155) was closest to the overall model's coefficient of determination (adjusted R2=0.222) without demonstrating colinearity. Possible predictive formulas were based on the predictors standardized and included the following combinations: the 2 greatest overall predictors (adjusted R2=0.051), the 2 greatest negative and positive predictors (adjusted R2=0.098), the 4 greatest overall predictors (adjusted R2=0.117), and the 8 greatest overall predictors (adjusted R2=0.201), which suffered from colinearity (household size plus nonEnglish speaking [Pearson=0.624] and under 18 years old [Pearson=0.708]). None of the correlated independent variables (eg, poverty and median income) were placed in the final model.
The mean WIPSAS scores closely corresponded with the national average of HCAHPS scores (71.6 vs 71.84) but compressed scores into a narrower distribution (SD 5.52 vs 5.92). The greatest positive and negative changes were by 8.51% and 2.25%, respectively. Essentially, a smaller number of hospitals in demographically challenged areas were more significantly impacted by the WIPSAS adjustment than the larger number of hospitals in demographically favorable areas. Large hospitals in demographically diverse counties saw the greatest positive change (e.g., Texas, California, and New York), whereas smaller hospitals in demographically nondiverse areas saw comparatively smaller decrements in the overall WIPSAS scores. The WIPSAS had the most beneficial effect on urban and rural safety‐net hospitals that serve diverse populations including many academic medical centers. This is illustrated by the reranking of the top 10 and bottom 10 hospitals in New York State by the WIPSAS (Table 3). For example, 3 academic medical centers in New York State, Montefiore Medical Center, New York Presbyterian Hospital, and Mount Sinai Hospital, were moved from the 46th, 43rd, and 42nd (out of 167 hospitals) respectively into the top 10 in patient satisfaction utilizing the WIPSAS methodology. Reported patient satisfaction scores, PPSF, WIPPSS, and WIPSAS scores for each hospital in the United States are available online (see Supporting Table S1 in the online version of this article).
| Ten Highest Ranked New York State Hospitals by HCAHPS | Ten Highest Ranked New York State Hospitals After WIPSAS |
|---|---|
| |
| 1. River Hospital, Inc. | 1. River Hospital, Inc. |
| 2. Westfield Memorial Hospital, Inc. | 2. Westfield Memorial Hospital, Inc. |
| 3. Clifton Fine Hospital | 3. Clifton Fine Hospital |
| 4. Hospital For Special Surgery | 4. Hospital For Special Surgery |
| 5. Delaware Valley Hospital, Inc. | 5. New YorkPresbyterian Hospital |
| 6. Putnam Hospital Center | 6. Delaware Valley Hospital, Inc. |
| 7. Margaretville Memorial Hospital | 7. Montefiore Medical Center |
| 8. Community Memorial Hospital, Inc. | 8. St. Francis Hospital, Roslyn |
| 9. Lewis County General Hospital | 9. Putnam Hospital Center |
| 10. St. Francis Hospital, Roslyn | 10. Mount Sinai Hospital |
DISCUSSION
The HVBP program is an incentive program that is meant to enhance the quality of care. This study illustrates healthcare inequalities in patient satisfaction that are not accounted for by the current CMS adjustments, and shows that education, ethnicity, primary language, and number of hospital beds are predictive of how patients evaluate their care via patient satisfaction scores. Hospitals that treat a disproportionate percentage of nonEnglish speaking, nonwhite, noneducated patients in large facilities are not meeting patient satisfaction standards. This inequity is not ameliorated by the adjustments currently performed by CMS, and has financial consequences for those hospitals that are not meeting national standards in patient satisfaction. These hospitals, which often include academic medical centers in urban areas, may therefore be penalized under the existing HVBP reimbursement models.
Using only 4 demographic and hospital‐specific predictors (ie, hospital beds, percent nonEnglish speaking, percent bachelors degrees, percent white), it is possible to utilize a simple formula to predict patient satisfaction with a significant degree of correlation to the reported scores available through Hospital Compare.
Our initial hypothesis that population density predicted lower patient satisfaction scores was confirmed, but these aforementioned demographic and hospital‐based factors were stronger independent predictors of HCAHPS scores. The WIPSAS is a representation of patient satisfaction and quality‐of‐care delivery across the country that accounts for nonrandom variation in patient satisfaction scores.
For hospitals in New York State, WIPSAS resulted in the placement of 3 urban‐based academic medical centers in the top 10 in patient satisfaction, when previously, based on the raw scores, their rankings were between 42nd and 46th statewide. Prior studies have suggested that large, urban, teaching, and not‐for‐profit hospitals were disadvantaged based on their hospital characteristics and patient features.[10, 11, 12] Under the current CMS reimbursement methodologies, these institutions are more likely to receive financial penalties.[8] The WIPSAS is a simple method to assess hospitals performance in the area of patient satisfaction that accounts for the demographic and hospital‐based factors (eg, number of beds) of the hospital. Its incorporation into CMS reimbursement calculations, or incorporation of a similar adjustment formula, should be strongly considered to account for predictive factors in patient satisfaction that could be addressed to enhance their scores.
Limitations for this study are the approximation of county‐level data for actual individual hospital demographic information and the exclusion of specialty hospitals, such as cancer centers and children's hospitals, in HCAHPS surveys. Repeated multivariate analyses at different time points would also serve to identify how CMS‐specific adjustments are recalibrated over time. Although we have primarily reported on the composite percent positive score as a surrogate for all HCAHPS dimensions, an individual adjustment formula could be generated for each dimension of the patient experience of care domain.
Although patient satisfaction is a component of how quality should be measured, further emphasis needs to be placed on nonrandom patient satisfaction variance so that HVBP can serve as an incentivizing program for at‐risk hospitals. Regional variation in scoring is not altogether accounted for by the current CMS adjustment system. Because patient satisfaction scores are now directly linked to reimbursement, further evaluation is needed to enhance patient satisfaction scoring paradigms to account for demographic and hospital‐specific factors.
Disclosure
Nothing to report.
The Affordable Care Act of 2010 mandates that government payments to hospitals and physicians must depend, in part, on metrics that assess the quality and efficiency of healthcare being provided to encourage value‐based healthcare.[1] Value in healthcare is defined by the delivery of high‐quality care at low cost.[2, 3] To this end, Hospital Value‐Based Purchasing (HVBP) and Physician Value‐Based Payment Modifier programs have been developed by the Centers for Medicare & Medicaid Services (CMS). HVBP is currently being phased in and affects CMS payments for fiscal year (FY) 2013 for over 3000 hospitals across the United States to incentivize healthcare delivery value. The final phase of implementation will be in FY 2017 and will then affect 2% of all CMS hospital reimbursement. HVBP is based on objective measures of hospital performance as well as a subjective measure of performance captured under the Patient Experience of Care domain. This subjective measure will remain at 30% of the aggregate score until FY 2016, when it will then be 25% the aggregate score moving forward.[4] The program rewards hospitals for both overall achievement and improvement in any domain, so that hospitals have multiple ways to receive financial incentives for providing quality care.[5] Even still, there appears to be a nonrandom pattern of patient satisfaction scores across the country with less favorable scores clustering in densely populated areas.[6]
Value‐Based Purchasing and other incentive‐based programs have been criticized for increasing disparities in healthcare by penalizing larger hospitals (including academic medical centers, safety‐net hospitals, and others that disproportionately serve lower socioeconomic communities) and favoring physician‐based specialty hospitals.[7, 8, 9] Therefore, hospitals that serve indigent and elderly populations may be at a disadvantage.[9, 10] HVBP portends significant economic consequences for the majority of hospitals that rely heavily on Medicare and Medicaid reimbursement, as most hospitals have large revenues but low profit margins.[11] Higher HVBP scores are associated with for profit status, smaller size, and location in certain areas of the United States.[12] Jha et al.[6] described Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) scores regional geographic variability, but concluded that poor satisfaction was due to poor quality.
The Patient Experience of Care domain quantifies patient satisfaction using the validated HCAHPS survey, which is provided to a random sample of patients continuously throughout the year at 48 hours to 6 weeks after discharge. It is a publically available standardized survey instrument used to measure patients perspectives on hospital care. It assesses the following 8 dimensions: nurse communication, doctor communication, hospital staff responsiveness, pain management, medicine communication, discharge information, hospital cleanliness and quietness, and overall hospital rating, of which the last 2 dimensions each have 2 measures (cleanliness and quietness) and (rating 9 or 10 and definitely recommend) to give a total of 10 distinct measures.
The United States is a complex network of urban, suburban, and rural demographic areas. Hospitals exist within a unique contextual and compositional meshwork that determines its caseload. The top population density decile of the United States lives within 37 counties, whereas half of the most populous parts of the United States occupy a total of 250 counties out of a total of 3143 counties in the United States. If the 10 measures of patient satisfaction (HCAHPS) scores were abstracted from hospitals and viewed according to county‐level population density (separated into deciles across the United States), a trend would be apparent (Figure 1). Greater population density is associated with lower patient satisfaction in 9 of 10 categories. On the state level, composite scores of overall patient satisfaction (amount of positive scores) of hospitals show a 12% variability and a significant correlation with population density (r=0.479; Figure 2). The lowest overall satisfaction scores are obtained from hospitals located in the population‐dense regions of Washington, DC, New York State, California, Maryland, and New Jersey (ie, 63%65%), and the best scores are from Louisiana, South Dakota, Iowa, Maine, and Vermont (ie, 74%75%). The average patient satisfaction score is 71%2.9%. Lower patient satisfaction scores appear to cluster in population‐dense areas and may be associated with greater heterogeneous patient demographics and economic variability in addition to population density.


These observations are surprising considering that CMS already adjusts HCAHPS scores based on patient‐mix coefficients and mode of collection.[13, 14, 15, 16, 17, 18] Adjustments are updated multiple times per year and account for survey collection either by telephone, email, or paper survey, because the populations that select survey forms will differ. Previous studies have shown that demographic features influence the patient evaluation process. For example, younger and more educated patients were found to provide less positive evaluations of healthcare.[19]
This study examined whether patients perceptions of healthcare (pattern of patient satisfaction) as quantified under the patient experience domain of HVBP were affected and predicted by population density and other demographic factors that are outside the control of individual hospitals. In addition, hospital‐level data (eg, number of hospital beds) and county‐level data such as race, age, gender, overall population, income, time spent commuting to work, primary language, and place of birth were analyzed for correlation with patient satisfaction scores. Our study demonstrates that demographic and hospital‐level data can predict patient satisfaction scores and suggests that CMS may need to modify its adjustment formulas to eliminate bias in HVBP‐based reimbursement.
METHODS
Data Collection
Publically available data were obtained from Hospital Compare,[20] American Hospital Directory,[21] and the US Census Bureau[22] websites. Twenty relevant US Census data categories were selected by their relevance for this study out of the 50 publically reported US Census categories, and included the following: county population, county population density, percent of population change over 1 year, poverty level (percent), income level per capita, median household income, average household size, travel time to work, percentage of high school or college graduates, non‐English primary language spoken at home, percentage of residents born outside of the United States, population percent in same residence for over 1 year, gender, race (white alone, white alone (not Hispanic or Latino), black or African American alone), population over 65 years old, and population under 18 years old.
HCAHPS Development
The HCAHPS survey is 32 questions in length, comprised of 10 evaluative dimensions. All short‐term, acute care, nonspecialty hospitals are invited to participate in the HCAHPS survey.
Data Analysis
Statistical analyses used the Statistical Package for Social Sciences version 16.0 for Windows (SPSS Inc., Chicago, IL). Data were checked for statistical assumptions, including normality, linearity of relationships, and full range of scores. Categories in both the Hospital Compare (HCAHPS) and US Census datasets were analyzed to assess their distribution curves. The category of population densities (per county) was converted to a logarithmic scale to account for a skewed distribution and long tail in the area of low population density. Data were subsequently merged into an Excel (Microsoft, Redmond, WA) spreadsheet using the VLookup function such that relevant 2010 census county data were added to each hospital's Hospital Compare data. Linear regression modeling was performed. Bivariate analysis was conducted (ENTER method) to determine the significant US Census data predictors for each of the 10 Hospital Compare dimensions including the composite overall satisfaction score. Significant predictors were then analyzed in a multivariate model (BACKWORDS method) for each Hospital Compare dimension and the composite average positive score. Models were assessed by determinates of correlation (adjusted R2) to assess for goodness of fit. Statistically significant predictor variables for overall patient satisfaction scores were then ranked according to their partial regression coefficients (standardized ).
A patient satisfaction predictive model was sought based upon significant predictors of aggregate percent positive HCAHPS scores. Various predictor combinations were formed based on their partial coefficients (ie, standardized coefficients); combinations were assessed based on their R2 values and assessed for colinearity. Combinations of partial coefficients included the 2, 4, and 8 most predictive variables as well the 2 most positive and negative predictors. They were then incorporated into a multivariate analysis model (FORWARD method) and assessed based on their adjusted R2 values. A 4‐variable combination (the 2 most predictive positive partial coefficients plus the 2 most predictive negative partial coefficients) was selected as a predictive model, and a formula predictive of the composite overall satisfaction score was generated. This formula (predicted patient satisfaction formula [PPSF]) predicts hospital patient satisfaction HCAHPS scores based on the 4 predictive variables for particular county and hospital characteristics.
| B | SE | t | P | ||
|---|---|---|---|---|---|
| |||||
| Educational attainmentbachelor's degree | 0.157 | 0.018 | 0.27 | 8.612 | <0.001 |
| White alone percent 2012 | 0.09 | 0.012 | 0.235 | 7.587 | <0.001 |
| Resident population percent under 18 years | 0.404 | 0.0444 | 0.209 | 9.085 | <0.001 |
| Black or African American alone percent 2012 | 0.083 | 0.014 | 0.191 | 5.936 | <0.001 |
| Median household income 20072011 | 0.00003 | 0.00 | 0.062 | 2.027 | 0.043 |
| Population density (log) 2010 | 0.277 | 0.083 | 0.087 | 3.3333 | 0.001 |
| Average travel time to work | 0.107 | 0.024 | 0.088 | 4.366 | <0.001 |
| Educational attainmenthigh school | 0.082 | 0.026 | 0.088 | 3.147 | 0.002 |
| Average household size | 2.58 | 0.727 | 0.107 | 3.55 | <0.001 |
| Total females percent 2012 | 0.423 | 0.067 | 0.107 | 6.296 | <0.001 |
| Percent nonEnglish speaking at home 20072011 | 0.052 | 0.018 | 0.14 | 2.929 | 0.003 |
| No. of hospital beds | 0.006 | 0.00 | 0.213 | 12.901 | <0.001 |
| Adjusted R2 | 0.222 | ||||
The PPSF was then modified by weighting with the partial coefficient () to remove the bias in patient satisfaction generated by demographic and structural factors over which individual hospitals have limited or no control. This formula generated a Weighted Individual (hospital) Predicted Patient Satisfaction Score (WIPPSS). Application of this formula narrowed the predicted distribution of patient satisfaction for all hospitals across the country.
To create an adjusted score with direct relevance to the reported patient satisfaction scores, the reported scores were multiplied by an adjustment factor that defines the difference between individual hospital‐weighted scores and the national mean HCAHPS score across the United States. This formula, the Weighted Individual (hospital) Patient Satisfaction Adjustment Score (WIPSAS), represents a patient satisfaction score adjusted for demographic and structural factors that can be utilized for interhospital comparisons across all areas of the country.
where PSrep=patient satisfaction reported score, PSUSA=mean reported score for United States (71.84), and WIPPSSX=WIPPSS for individual hospital.
Application of Data Analysis
PPSF, WIPPSS, and WIPSAS were calculated for all HCAHPS‐participating hospitals and compared with averaged raw HCAHPS scores across the United States. WIPSAS and raw scores were specifically analyzed for New York State to demonstrate exactly how adjustments would change state‐level rankings.
RESULTS
Complete HCAHPS scores were obtained from 3907 hospitals out of a total 4621 hospitals listed by the Hospital Compare website (85%). The majority of hospitals (2884) collected over 300 surveys, fewer hospitals (696) collected 100 to 299 surveys, and fewer still (333) collected <100 surveys. In total, results were available from at least 934,800 individual surveys, by the most conservative estimate. Missing HCAHPS hospital data averaged 13.4 (standard deviation [SD] 12.2) hospitals per state. County‐level data were obtained from all 3144 county or county equivalents across the United States (100%). Multivariate regression modeling across all HCAHPS dimensions found that between 10 and 16 of the 20 predictors (US Census categories) were statistically significant and predictive of individual HCAHPS dimension scores and the aggregate percent positive score as demonstrated in Table 2. For example, county percentage of bachelors degrees positively predicts for positive doctor communication scores, and hospital beds negatively predicts for quiet dimension. The strongest positive and negative predictive variables by model regression coefficients for each HCAHPS dimension are also listed in Table 2.
| Average Positive Scores | Nurse Communication | Doctor Communication | Help | Pain | Explain Meds | Clean | Quiet | Discharge Explain | Recommend 9/10 | Definitely Recommend | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||
| Educationalbachelor's | 0.27 | 0.19 | 0.45 | 0.10 | 0.10 | 0.05 | 0.08 | 0.33 | 0.15 | 0.27 | 0.416 |
| Hospital beds | 0.21 | 0.16 | 0.19 | 0.26 | 0.16 | 0.17 | 0.27 | 0.26 | 0.06 | 0.11 | |
| Population density 2010 | 0.09 | 0.07 | 0.28 | 0.20 | 0.08 | 0.23 | 0.14 | 0.19 | 0.22 | 0.07 | * |
| White alone percent | 0.24 | 0.25 | 0.09 | 0.16 | 0.23 | 0.07 | 0.16 | 0.17 | 0.31 | 0.317 | |
| Total females percent | 0.11 | 0.05 | 0.06 | 0.07 | 0.06 | 0.03 | 0.05 | 0.09 | 0.12 | 0.09 | |
| African American alone | 0.19 | 0.19 | 0.09 | 0.23 | 0.09 | 0.07 | 0.34 | * | 0.09 | 0.084 | |
| Average travel time to work | 0.09 | 0.10 | * | 0.09 | 0.06 | 0.04 | 0.08 | * | 0.12 | 0.17 | 0.16 |
| Foreign‐born percent | * | 0.16 | 0.14 | 0.06 | 0.12 | 0.08 | 0.06 | 0.13 | 0.18 | * | * |
| Average household size | 0.11 | 0.05 | 0.15 | 0.07 | * | 0.07 | * | 0.01 | * | 0.07 | 0.076 |
| NonEnglish speaking | 0.14 | 0.12 | 0.50 | 0.07 | * | * | * | * | * | 0.34 | 0.28 |
| Educationhigh school | 0.09 | 0.09 | 0.40 | * | 0.27 | 0.06 | 0.08 | * | |||
| Household income | 0.06 | * | 0.35 | 0.08 | * | * | 0.16 | 0.41 | 0.265 | ||
| Population 65 years and over | * | 0.14 | 0.14 | 0.12 | * | 0.11 | 0.15 | * | 0.10 | ||
| White, not Hispanic/Latino | * | * | 0.20 | * | * | * | 0.09 | 0.13 | 0.09 | 0.22 | 0.25 |
| Population under 18 | 0.21 | 0.15 | 0.08 | 0.11 | 0.20 | ||||||
| Population (county) | * | 0.06 | 0.08 | * | 0.03 | 0.05 | * | * | 0.06 | * | * |
| All ages in poverty | 0.24 | 0.10 | 0.22 | 0.08 | * | 0.281 | |||||
| 1 year at same residence | * | 0.13 | 0.12 | 0.11 | 0.10 | * | 0.04 | * | * | ||
| Per capita income | * | 0.07 | * | * | * | * | * | 0.09 | * | ||
| Population percent change | * | * | * | * | * | * | 0.05 | * | * | ||
| Adjusted R2 | 0.22 | 0.25 | 0.30 | 0.30 | 0.12 | 0.17 | 0.23 | 0.30 | 0.19 | 0.14 | 0.15 |
Table 1 highlights multivariate regression modeling of the composite average positive score, which produced an adjusted R2 of 0.222 (P<0.001). All variables were significant and predicted change of the composite HCAHPS except for place of birthforeign born (not listed in the table). Table 1 ranks variables from most positive to most negative predictors.
Other HCAHPS domains demonstrated statistically significant models (P<0.001) and are listed by their coefficients of determination (ie, adjusted R2) (Table 2). The best‐fit dimensions were help (adjusted R2=0.304), quiet (adjusted R2=0.299), doctor communication (adjusted R2=0.298), nurse communication (adjusted R2=0.245), and clean (adjusted R2=0.232). Models that were not as strongly predictive as the composite score included pain (adjusted R2=0.124), overall 9/10 (adjusted R2=0.136), definitely recommend (adjusted R2=0.150), and explained meds (adjusted R2=0.169).
A predictive formula for average positive scores was created by determination of the most predictive partial coefficients and the best‐fit model. Bachelor's degree and white only were the 2 greatest positive predictors, and number of hospital beds and nonEnglish speaking were the 2 greatest negative predictors. The PPSF (predictive formula) was chosen out of various combinations of predictors (Table 1), because its coefficient of determination (adjusted R2=0.155) was closest to the overall model's coefficient of determination (adjusted R2=0.222) without demonstrating colinearity. Possible predictive formulas were based on the predictors standardized and included the following combinations: the 2 greatest overall predictors (adjusted R2=0.051), the 2 greatest negative and positive predictors (adjusted R2=0.098), the 4 greatest overall predictors (adjusted R2=0.117), and the 8 greatest overall predictors (adjusted R2=0.201), which suffered from colinearity (household size plus nonEnglish speaking [Pearson=0.624] and under 18 years old [Pearson=0.708]). None of the correlated independent variables (eg, poverty and median income) were placed in the final model.
The mean WIPSAS scores closely corresponded with the national average of HCAHPS scores (71.6 vs 71.84) but compressed scores into a narrower distribution (SD 5.52 vs 5.92). The greatest positive and negative changes were by 8.51% and 2.25%, respectively. Essentially, a smaller number of hospitals in demographically challenged areas were more significantly impacted by the WIPSAS adjustment than the larger number of hospitals in demographically favorable areas. Large hospitals in demographically diverse counties saw the greatest positive change (e.g., Texas, California, and New York), whereas smaller hospitals in demographically nondiverse areas saw comparatively smaller decrements in the overall WIPSAS scores. The WIPSAS had the most beneficial effect on urban and rural safety‐net hospitals that serve diverse populations including many academic medical centers. This is illustrated by the reranking of the top 10 and bottom 10 hospitals in New York State by the WIPSAS (Table 3). For example, 3 academic medical centers in New York State, Montefiore Medical Center, New York Presbyterian Hospital, and Mount Sinai Hospital, were moved from the 46th, 43rd, and 42nd (out of 167 hospitals) respectively into the top 10 in patient satisfaction utilizing the WIPSAS methodology. Reported patient satisfaction scores, PPSF, WIPPSS, and WIPSAS scores for each hospital in the United States are available online (see Supporting Table S1 in the online version of this article).
| Ten Highest Ranked New York State Hospitals by HCAHPS | Ten Highest Ranked New York State Hospitals After WIPSAS |
|---|---|
| |
| 1. River Hospital, Inc. | 1. River Hospital, Inc. |
| 2. Westfield Memorial Hospital, Inc. | 2. Westfield Memorial Hospital, Inc. |
| 3. Clifton Fine Hospital | 3. Clifton Fine Hospital |
| 4. Hospital For Special Surgery | 4. Hospital For Special Surgery |
| 5. Delaware Valley Hospital, Inc. | 5. New YorkPresbyterian Hospital |
| 6. Putnam Hospital Center | 6. Delaware Valley Hospital, Inc. |
| 7. Margaretville Memorial Hospital | 7. Montefiore Medical Center |
| 8. Community Memorial Hospital, Inc. | 8. St. Francis Hospital, Roslyn |
| 9. Lewis County General Hospital | 9. Putnam Hospital Center |
| 10. St. Francis Hospital, Roslyn | 10. Mount Sinai Hospital |
DISCUSSION
The HVBP program is an incentive program that is meant to enhance the quality of care. This study illustrates healthcare inequalities in patient satisfaction that are not accounted for by the current CMS adjustments, and shows that education, ethnicity, primary language, and number of hospital beds are predictive of how patients evaluate their care via patient satisfaction scores. Hospitals that treat a disproportionate percentage of nonEnglish speaking, nonwhite, noneducated patients in large facilities are not meeting patient satisfaction standards. This inequity is not ameliorated by the adjustments currently performed by CMS, and has financial consequences for those hospitals that are not meeting national standards in patient satisfaction. These hospitals, which often include academic medical centers in urban areas, may therefore be penalized under the existing HVBP reimbursement models.
Using only 4 demographic and hospital‐specific predictors (ie, hospital beds, percent nonEnglish speaking, percent bachelors degrees, percent white), it is possible to utilize a simple formula to predict patient satisfaction with a significant degree of correlation to the reported scores available through Hospital Compare.
Our initial hypothesis that population density predicted lower patient satisfaction scores was confirmed, but these aforementioned demographic and hospital‐based factors were stronger independent predictors of HCAHPS scores. The WIPSAS is a representation of patient satisfaction and quality‐of‐care delivery across the country that accounts for nonrandom variation in patient satisfaction scores.
For hospitals in New York State, WIPSAS resulted in the placement of 3 urban‐based academic medical centers in the top 10 in patient satisfaction, when previously, based on the raw scores, their rankings were between 42nd and 46th statewide. Prior studies have suggested that large, urban, teaching, and not‐for‐profit hospitals were disadvantaged based on their hospital characteristics and patient features.[10, 11, 12] Under the current CMS reimbursement methodologies, these institutions are more likely to receive financial penalties.[8] The WIPSAS is a simple method to assess hospitals performance in the area of patient satisfaction that accounts for the demographic and hospital‐based factors (eg, number of beds) of the hospital. Its incorporation into CMS reimbursement calculations, or incorporation of a similar adjustment formula, should be strongly considered to account for predictive factors in patient satisfaction that could be addressed to enhance their scores.
Limitations for this study are the approximation of county‐level data for actual individual hospital demographic information and the exclusion of specialty hospitals, such as cancer centers and children's hospitals, in HCAHPS surveys. Repeated multivariate analyses at different time points would also serve to identify how CMS‐specific adjustments are recalibrated over time. Although we have primarily reported on the composite percent positive score as a surrogate for all HCAHPS dimensions, an individual adjustment formula could be generated for each dimension of the patient experience of care domain.
Although patient satisfaction is a component of how quality should be measured, further emphasis needs to be placed on nonrandom patient satisfaction variance so that HVBP can serve as an incentivizing program for at‐risk hospitals. Regional variation in scoring is not altogether accounted for by the current CMS adjustment system. Because patient satisfaction scores are now directly linked to reimbursement, further evaluation is needed to enhance patient satisfaction scoring paradigms to account for demographic and hospital‐specific factors.
Disclosure
Nothing to report.
- , , . Will choice‐based reform work for Medicare? Evidence from the Federal Employees Health Benefits Program. Health Serv Res. 2006;41:1741–1761.
- H.R. 3590. Patient Protection and Affordable Care Act 2010 (2010).
- . The quality of care. How can it be assessed? JAMA. 1988;260(12):1743–1748.
- Lake Superior Quality Innovation Network. FY 2017 Value‐Based Purchasing domain weighting. Available at: http://www.stratishealth.org/documents/VBP‐FY2017.pdf. Accessed March 13, 2015.
- Hospital Value‐Based Purchasing Program. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/Hospital‐Value‐Based‐Purchasing. Accessed December 1st, 2013.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- , . Providers must lead the way in making value the overarching goal Harvard Bus Rev. October 2013:3–19.
- , , . The effect of financial incentives on hospitals that serve poor patients. Ann Intern Med. 2010;153(5):299–306.
- , . Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342–343.
- . Will value‐based purchasing increase disparities in care? N Engl J Med. 2013;369(26):2472–2474.
- , , . Hospital conversions, margins, and the provision of uncompensated care. Health Aff (Millwood). 2000;19(6):187–194.
- , , , , , . Association between value‐based purchasing score and hospital characteristics. BMC Health Serv Res. 2012;12:464.
- , , , et al. Effects of survey mode, patient mix, and nonresponse on CAHPS hospital survey scores. Health Serv Res. 2009;44(2 pt 1):501–518.
- , , , , . Patient satisfaction measurement strategies: a comparison of phone and mail methods. Jt Comm J Qual Improv. 2001;27(7):349–361.
- , , . Comparing telephone and mail responses to the CAHPS survey instrument. Consumer Assessment of Health Plans Study. Med Care. 1999;37(3 suppl):MS41–MS49.
- , , , , , . Evaluating patients' experiences with individual physicians: a randomized trial of mail, internet, and interactive voice response telephone administration of surveys. Med Care. 2006;44(2):167–174.
- , , , , . Case‐mix adjustment of the CAHPS Hospital Survey. Health Serv Res. 2005;40(6 pt 2):2162–2181.
- Mode and patient‐mix adjustments of CAHPS hospital survey (HCAHPS). Available at: http://www.hcahpsonline.org/modeadjustment.aspx. Accessed December 1, 2013.
- , , , , , . Adjusting performance measures to ensure equitable plan comparisons. Health Care Financ Rev. 2001;22(3):109–126.
- Official Hospital Compare Data. Displaying datasets in Patient Survey Results category. Available at: https://data.medicare.gov/data/hospital‐compare/Patient%20Survey%20Results. Accessed December 1, 2013.
- Hospital statistics by state. American Hospital Directory, Inc. website. Available at: http://www.ahd.com/state_statistics.html. Accessed December 1, 2013.
- U.S. Census Download Center. Available at: http://factfinder.census.gov/faces/nav/jsf/pages/download_center.xhtml. Accessed December 1, 2013.
- , , . Will choice‐based reform work for Medicare? Evidence from the Federal Employees Health Benefits Program. Health Serv Res. 2006;41:1741–1761.
- H.R. 3590. Patient Protection and Affordable Care Act 2010 (2010).
- . The quality of care. How can it be assessed? JAMA. 1988;260(12):1743–1748.
- Lake Superior Quality Innovation Network. FY 2017 Value‐Based Purchasing domain weighting. Available at: http://www.stratishealth.org/documents/VBP‐FY2017.pdf. Accessed March 13, 2015.
- Hospital Value‐Based Purchasing Program. Available at: http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instruments/Hospital‐Value‐Based‐Purchasing. Accessed December 1st, 2013.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- , . Providers must lead the way in making value the overarching goal Harvard Bus Rev. October 2013:3–19.
- , , . The effect of financial incentives on hospitals that serve poor patients. Ann Intern Med. 2010;153(5):299–306.
- , . Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342–343.
- . Will value‐based purchasing increase disparities in care? N Engl J Med. 2013;369(26):2472–2474.
- , , . Hospital conversions, margins, and the provision of uncompensated care. Health Aff (Millwood). 2000;19(6):187–194.
- , , , , , . Association between value‐based purchasing score and hospital characteristics. BMC Health Serv Res. 2012;12:464.
- , , , et al. Effects of survey mode, patient mix, and nonresponse on CAHPS hospital survey scores. Health Serv Res. 2009;44(2 pt 1):501–518.
- , , , , . Patient satisfaction measurement strategies: a comparison of phone and mail methods. Jt Comm J Qual Improv. 2001;27(7):349–361.
- , , . Comparing telephone and mail responses to the CAHPS survey instrument. Consumer Assessment of Health Plans Study. Med Care. 1999;37(3 suppl):MS41–MS49.
- , , , , , . Evaluating patients' experiences with individual physicians: a randomized trial of mail, internet, and interactive voice response telephone administration of surveys. Med Care. 2006;44(2):167–174.
- , , , , . Case‐mix adjustment of the CAHPS Hospital Survey. Health Serv Res. 2005;40(6 pt 2):2162–2181.
- Mode and patient‐mix adjustments of CAHPS hospital survey (HCAHPS). Available at: http://www.hcahpsonline.org/modeadjustment.aspx. Accessed December 1, 2013.
- , , , , , . Adjusting performance measures to ensure equitable plan comparisons. Health Care Financ Rev. 2001;22(3):109–126.
- Official Hospital Compare Data. Displaying datasets in Patient Survey Results category. Available at: https://data.medicare.gov/data/hospital‐compare/Patient%20Survey%20Results. Accessed December 1, 2013.
- Hospital statistics by state. American Hospital Directory, Inc. website. Available at: http://www.ahd.com/state_statistics.html. Accessed December 1, 2013.
- U.S. Census Download Center. Available at: http://factfinder.census.gov/faces/nav/jsf/pages/download_center.xhtml. Accessed December 1, 2013.
© 2015 Society of Hospital Medicine
Nurse Case Management Fails to Yield Improvements in Blood Pressure and Glycemic Control
Study Overview
Objective. To determine the effectiveness of a nurse-led, telephone-delivered behavioral intervention for diabetes (DM) and hypertension (HTN) versus an attention control within primary care community practices.
Study design. A 9-site, 2-arm randomized controlled trial.
Setting and participants. Study participants were recruited from 9 community practices within the Duke Primary Care Research Consortium. The practices were chosen because they traditionally operate outside of the academic context. Subjects were required to have both type 2 DM and HTN, as indicated by their medications and confirmed by administrative data as well as patient self-reporting. Participants had to have been seen at participating practices for at least 1 year and have poorly controlled DM (indicated by most recent A1c ≥ 7.5%), but they were not required to have poorly controlled HTN. Exclusion criteria included fewer than 1 primary care clinic visit during the previous year, serious comorbid illness, type 1 diabetes, inability to receive a telephone intervention in English, residence in a nursing home, and participation in another hypertension or diabetes study [1]. Participants were randomly assigned using a computer-generated randomization sequence [1] to either the intervention or control groups at a 1:1 ratio, stratified by clinic and baseline blood pressure (BP) control.
Intervention. A single nurse with extensive experience in case management delivered both the behavioral intervention and attention control by telephone. In both arms, calls were conducted once every 2 months over a 24-month period.
The calls in the intervention arm consisted of tailored behavior-modifying techniques according to patient barriers. This content was divided into a series of modules relevant to behaviors associated with improving control of BP or blood sugar, including physical activity, weight reduction, sodium intake, smoking cessation, medication adherence, and others. These modules were scheduled according to patient needs (based on certain parameters such as high body mass index or use of insulin) and preferences [1].
The calls in the attention control were not tailored but rather consisted of didactic health-related information unrelated to HTN or DM (eg, flu shots, skin cancer prevention). This content was also highly scripted and designed to limit the potential for interaction between the nurse and patient.
Main outcome measures. A1c and systolic blood pressure (SBP) were primary outcomes. Key secondary outcomes were diastolic blood pressure (DBP), overall BP control, weight, physical activity, self-efficacy, and medication adherence. Study staff obtained measurements at baseline and 6, 12, and 24 months [1].
Results. The researchers assessed 2601 patients for eligibility and excluded 2224. Most patients were excluded for not meeting inclusion criteria (n = 1156), in particular because of improved HbA1c control (n = 983), and 1064 declined to participate. They randomized 377 patients—193 to the intervention arm and 184 to the attention control arm. Participants had an average age of 58.7, 49.1% had an education level of high school or less, 50.1% were non-white, and 54.9% were unemployed/retired. Patient characteristics in the intervention and control arms were similar at baseline. Seventy-eight percent of patients completed the 12-month follow-up and 70% (263) reached the 24-month endpoint. Patients in the intervention arm completed 78% of scheduled calls while patients in the control group completed 81%.
After adjusting for stratification variables, the estimated mean A1c and SBP were similar between arms at 24 months (intervention 0.1% higher than control, 95% CI −0.3 % to 0.5 %, P = 0.50 for A1c; intervention 0.9 mm Hg lower than control, 95% CI −5.4 to 3.5, P = 0.69 for SBP). There were also no significant differences between arms in mean A1c or SBP at 6 or 12 months. However, A1c levels did improve within each arm at the end of the study, with the intervention group improving by approx-imately 0.5% and the control group improving by approximately 0.6%. In terms of secondary outcomes, there were no significant differences between arms in DBP, weight, physical activity, or BP control rates throughout the 2-year study period.
Conclusion. Overall, the intervention and control groups did not differ significantly in terms of A1c, SBP, or any of the secondary outcomes at any point during the 2-year study.
Commentary
The prevalence of type 2 diabetes and its comorbidities (such as hypertension and obesity) have increased due to a variety of factors including an aging population and an increasingly sedentary lifestyle. Several nurse management programs for DM and HTN have been shown to be efficacious in reducing blood sugar levels [2–4] and promoting BP control [5,6]. However, these interventions were conducted in tightly controlled academic settings, and it is unclear how well these programs may translate into community settings. The aim of this study was to test the effectiveness of a nurse-led behavioral telephone intervention for the comanagement of DM and HTN within non–academically affiliated community practices. Results indicated no significant differences between the intervention and control groups for A1c levels or SBP at any point during the 2-year study, but A1c levels did improve for both arms.
Despite this being a negative study, it is a unique and important contribution to the literature. It is the only trial as of yet that has tested the effectiveness of a nurse management intervention targeting both DM and HTN in a real-world, community setting. This novel approach is supported by data that suggests BP control is actually more cost-effective than intensive glycemic control in treating patients with type 2 diabetes [7]. There were several strengths to the study design, including the use of intention-to-treat analysis, stratified randomization, a diverse patient population, and blinding of the study staff who took BP and A1c measurements. Furthermore, a single nurse conducted all telephone calls, ensuring that differences in counseling skill levels would not affect the results of the study. The few weaknesses of the study included the fact that the nurse who delivered the intervention (as well as the patients) could not be blinded to treatment allocation, and the income of study participants was not reported.
The reasons for the negative outcomes of this study are unclear. The authors claim that similar interventions within academic settings have been shown to be effective and speculate that time and financial pressures of community practices may be reasons that the intervention was not successful. However, the “successful” interventions that they cite were quite different from and more intensive than this intervention. For instance, many of these studies used at least 1 call per month [3,4,8], and one even conducted several calls each week [3]. Furthermore, a DM study conducted by Blackberry et al in a community setting with less than 1 call per month (8 calls over 18 months) similarly failed to produce significant results [9], and therefore more frequent calls may be necessary in DM and HTN interventions. In a systematic review, Eakin et al demonstrated that 12 or more calls in a 6- to 12-month period were associated with better outcomes in physical activity and diet interventions [10], and this may also translate to closely related DM and HTN interventions.
In addition to the infrequent calls, this intervention also lacked communication and integration with patients’ primary care teams. Several studies have demonstrated that integration with primary care teams can improve outcomes in DM and HTN interventions [11,12], and nearly all of the successful studies cited by the authors also included at least some form of communication with patients’ primary care providers (PCPs) [2–4,5,8]. In many of these studies the nurse also had prescribing rights to alter medications [2,3,5]. The nurse in this study met monthly with an expert team of clinicians to discuss patient issues but did not communicate directly with any of the patients’ PCPs [1]. The authors acknowledge that this lack of integration may have contributed to their negative results and point to the fact that it is harder to integrate interventions within community practices that often lack internal integration. However, Walsh, Harris, and Roberts demonstrated that integration between primary and secondary care teams was both feasible and effective for a diabetes initiative within community practices [13].
An additional important feature not present in this intervention was self-monitoring of BP levels. Home self-monitoring of BP has been demonstrated to significantly improve BP levels [14], and 2 of the successful studies in academic settings cited by the authors also included a BP self-monitoring component [5,6]. In one of these studies [6], Bosworth et al conducted a 2 × 2 randomized trial to improve HTN control in which the arms consisted of (a) usual care, (b) bimonthly nurse administered telephone intervention only (this arm was highly similar to the intervention arm in this study), (c) BP monitoring 3 times a week only, and (d) a combination of the telephone intervention with the BP monitoring. Interestingly, the only arm that was successful relative to usual care was the combination of the telephone intervention and BP self-monitoring; the arm consisting only of bi-monthly telephone calls (very similar to this intervention) failed despite the study taking place in an academic setting (it was also less effective than BP monitoring only). Thus, the addition of self-monitoring to a nurse case management telephone intervention can achieve better results.
Applications for Clinical Practice
A telephone-based intervention delivered by a trained nurse for co-management of DM and HTN was not more effective than an attention control delivered by the same nurse in a community setting. This may have been due to several factors, including low intensity marked by less than 1 call per month, a lack of integration with other members of the primary care team, and lack of a BP self-monitoring component. Future studies are needed to determine the optimal type and duration of nurse case management interventions targeting glucose and BP control for diabetic patients in community settings.
—Sandeep Sikerwar, BA, and Melanie Jay, MD, MS
1. Crowley MJ, Bosworth HB, Coffman CJ, et al. Tailored Case Management for Diabetes and Hypertension (TEACH-DM) in a community population: study design and baseline sample characteristics. Contemp Clin Trials 2013;36:298–306.
2. Aubert RE, Herman WH, Waters J, et al. Nurse case management to improve glycemic control in diabetic patients in a health maintenance organization. A randomized, controlled trial. Ann Intern Med 1998;129:605–12.
3. Thompson DM, Kozak SE, Sheps S. Insulin adjustment by a diabetes nurse educator improves glucose control in insulin-requiring diabetic patients: a randomized trial. CMAJ 1999;161:959–62.
4. Weinberger M, Kirkman MS, Samsa GP, et al. A nurse-coordinated intervention for primary care patients with non-insulin-dependent diabetes mellitus: impact on glycemic control and health-related quality of life. J Gen Intern Med 1995;10:59–66.
5. Bosworth HB, Powers BJ, Olsen MK, et al. Home blood pressure management and improved blood pressure control: results from a randomized controlled trial. Arch Intern Med 2011;171:1173–80.
6. Bosworth HB, Olsen MK, Grubber JM, et al. Two self-management interventions to improve hypertension control: a randomized trial. Ann Intern Med 2009;151:687–95.
7. CDC Diabetes Cost-effectiveness Group. Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction for type 2 diabetes. JAMA 2002;287:2542–51.
8. Mons U, Raum E, Krämer HU, et al. Effectiveness of a supportive telephone counseling intervention in type 2 diabetes patients: randomized controlled study. PLoS One 2013;8:e77954.
9. Blackberry ID, Furler JS, Best JD, et al. Effectiveness of general practice based, practice nurse led telephone coaching on glycaemic control of type 2 diabetes: the Patient Engagement and Coaching for Health (PEACH) pragmatic cluster randomised controlled trial. BMJ 2013;347:f5272.
10. Eakin EG, Lawler SP, Vandelanotte C, Owen N. Telephone interventions for physical activity and dietary behavior change: a systematic review. Am J Prev Med 2007;32:419–34.
11. Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA 2006;296:427–40.
12. Katon WJ, Lin EHB, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611–20.
13. Walsh JL, Harris BHL, Roberts AW. Evaluation of a community diabetes initiative: Integrating diabetes care. Prim Care Diabetes 2014 Dec 11.
14. Halme L, Vesalainen R, Kaaja M, Kantola I. Self-monitoring of blood pressure promotes achievement of blood pressure target in primary health care. Am J Hypertens 2005;18:1415–20.
Study Overview
Objective. To determine the effectiveness of a nurse-led, telephone-delivered behavioral intervention for diabetes (DM) and hypertension (HTN) versus an attention control within primary care community practices.
Study design. A 9-site, 2-arm randomized controlled trial.
Setting and participants. Study participants were recruited from 9 community practices within the Duke Primary Care Research Consortium. The practices were chosen because they traditionally operate outside of the academic context. Subjects were required to have both type 2 DM and HTN, as indicated by their medications and confirmed by administrative data as well as patient self-reporting. Participants had to have been seen at participating practices for at least 1 year and have poorly controlled DM (indicated by most recent A1c ≥ 7.5%), but they were not required to have poorly controlled HTN. Exclusion criteria included fewer than 1 primary care clinic visit during the previous year, serious comorbid illness, type 1 diabetes, inability to receive a telephone intervention in English, residence in a nursing home, and participation in another hypertension or diabetes study [1]. Participants were randomly assigned using a computer-generated randomization sequence [1] to either the intervention or control groups at a 1:1 ratio, stratified by clinic and baseline blood pressure (BP) control.
Intervention. A single nurse with extensive experience in case management delivered both the behavioral intervention and attention control by telephone. In both arms, calls were conducted once every 2 months over a 24-month period.
The calls in the intervention arm consisted of tailored behavior-modifying techniques according to patient barriers. This content was divided into a series of modules relevant to behaviors associated with improving control of BP or blood sugar, including physical activity, weight reduction, sodium intake, smoking cessation, medication adherence, and others. These modules were scheduled according to patient needs (based on certain parameters such as high body mass index or use of insulin) and preferences [1].
The calls in the attention control were not tailored but rather consisted of didactic health-related information unrelated to HTN or DM (eg, flu shots, skin cancer prevention). This content was also highly scripted and designed to limit the potential for interaction between the nurse and patient.
Main outcome measures. A1c and systolic blood pressure (SBP) were primary outcomes. Key secondary outcomes were diastolic blood pressure (DBP), overall BP control, weight, physical activity, self-efficacy, and medication adherence. Study staff obtained measurements at baseline and 6, 12, and 24 months [1].
Results. The researchers assessed 2601 patients for eligibility and excluded 2224. Most patients were excluded for not meeting inclusion criteria (n = 1156), in particular because of improved HbA1c control (n = 983), and 1064 declined to participate. They randomized 377 patients—193 to the intervention arm and 184 to the attention control arm. Participants had an average age of 58.7, 49.1% had an education level of high school or less, 50.1% were non-white, and 54.9% were unemployed/retired. Patient characteristics in the intervention and control arms were similar at baseline. Seventy-eight percent of patients completed the 12-month follow-up and 70% (263) reached the 24-month endpoint. Patients in the intervention arm completed 78% of scheduled calls while patients in the control group completed 81%.
After adjusting for stratification variables, the estimated mean A1c and SBP were similar between arms at 24 months (intervention 0.1% higher than control, 95% CI −0.3 % to 0.5 %, P = 0.50 for A1c; intervention 0.9 mm Hg lower than control, 95% CI −5.4 to 3.5, P = 0.69 for SBP). There were also no significant differences between arms in mean A1c or SBP at 6 or 12 months. However, A1c levels did improve within each arm at the end of the study, with the intervention group improving by approx-imately 0.5% and the control group improving by approximately 0.6%. In terms of secondary outcomes, there were no significant differences between arms in DBP, weight, physical activity, or BP control rates throughout the 2-year study period.
Conclusion. Overall, the intervention and control groups did not differ significantly in terms of A1c, SBP, or any of the secondary outcomes at any point during the 2-year study.
Commentary
The prevalence of type 2 diabetes and its comorbidities (such as hypertension and obesity) have increased due to a variety of factors including an aging population and an increasingly sedentary lifestyle. Several nurse management programs for DM and HTN have been shown to be efficacious in reducing blood sugar levels [2–4] and promoting BP control [5,6]. However, these interventions were conducted in tightly controlled academic settings, and it is unclear how well these programs may translate into community settings. The aim of this study was to test the effectiveness of a nurse-led behavioral telephone intervention for the comanagement of DM and HTN within non–academically affiliated community practices. Results indicated no significant differences between the intervention and control groups for A1c levels or SBP at any point during the 2-year study, but A1c levels did improve for both arms.
Despite this being a negative study, it is a unique and important contribution to the literature. It is the only trial as of yet that has tested the effectiveness of a nurse management intervention targeting both DM and HTN in a real-world, community setting. This novel approach is supported by data that suggests BP control is actually more cost-effective than intensive glycemic control in treating patients with type 2 diabetes [7]. There were several strengths to the study design, including the use of intention-to-treat analysis, stratified randomization, a diverse patient population, and blinding of the study staff who took BP and A1c measurements. Furthermore, a single nurse conducted all telephone calls, ensuring that differences in counseling skill levels would not affect the results of the study. The few weaknesses of the study included the fact that the nurse who delivered the intervention (as well as the patients) could not be blinded to treatment allocation, and the income of study participants was not reported.
The reasons for the negative outcomes of this study are unclear. The authors claim that similar interventions within academic settings have been shown to be effective and speculate that time and financial pressures of community practices may be reasons that the intervention was not successful. However, the “successful” interventions that they cite were quite different from and more intensive than this intervention. For instance, many of these studies used at least 1 call per month [3,4,8], and one even conducted several calls each week [3]. Furthermore, a DM study conducted by Blackberry et al in a community setting with less than 1 call per month (8 calls over 18 months) similarly failed to produce significant results [9], and therefore more frequent calls may be necessary in DM and HTN interventions. In a systematic review, Eakin et al demonstrated that 12 or more calls in a 6- to 12-month period were associated with better outcomes in physical activity and diet interventions [10], and this may also translate to closely related DM and HTN interventions.
In addition to the infrequent calls, this intervention also lacked communication and integration with patients’ primary care teams. Several studies have demonstrated that integration with primary care teams can improve outcomes in DM and HTN interventions [11,12], and nearly all of the successful studies cited by the authors also included at least some form of communication with patients’ primary care providers (PCPs) [2–4,5,8]. In many of these studies the nurse also had prescribing rights to alter medications [2,3,5]. The nurse in this study met monthly with an expert team of clinicians to discuss patient issues but did not communicate directly with any of the patients’ PCPs [1]. The authors acknowledge that this lack of integration may have contributed to their negative results and point to the fact that it is harder to integrate interventions within community practices that often lack internal integration. However, Walsh, Harris, and Roberts demonstrated that integration between primary and secondary care teams was both feasible and effective for a diabetes initiative within community practices [13].
An additional important feature not present in this intervention was self-monitoring of BP levels. Home self-monitoring of BP has been demonstrated to significantly improve BP levels [14], and 2 of the successful studies in academic settings cited by the authors also included a BP self-monitoring component [5,6]. In one of these studies [6], Bosworth et al conducted a 2 × 2 randomized trial to improve HTN control in which the arms consisted of (a) usual care, (b) bimonthly nurse administered telephone intervention only (this arm was highly similar to the intervention arm in this study), (c) BP monitoring 3 times a week only, and (d) a combination of the telephone intervention with the BP monitoring. Interestingly, the only arm that was successful relative to usual care was the combination of the telephone intervention and BP self-monitoring; the arm consisting only of bi-monthly telephone calls (very similar to this intervention) failed despite the study taking place in an academic setting (it was also less effective than BP monitoring only). Thus, the addition of self-monitoring to a nurse case management telephone intervention can achieve better results.
Applications for Clinical Practice
A telephone-based intervention delivered by a trained nurse for co-management of DM and HTN was not more effective than an attention control delivered by the same nurse in a community setting. This may have been due to several factors, including low intensity marked by less than 1 call per month, a lack of integration with other members of the primary care team, and lack of a BP self-monitoring component. Future studies are needed to determine the optimal type and duration of nurse case management interventions targeting glucose and BP control for diabetic patients in community settings.
—Sandeep Sikerwar, BA, and Melanie Jay, MD, MS
Study Overview
Objective. To determine the effectiveness of a nurse-led, telephone-delivered behavioral intervention for diabetes (DM) and hypertension (HTN) versus an attention control within primary care community practices.
Study design. A 9-site, 2-arm randomized controlled trial.
Setting and participants. Study participants were recruited from 9 community practices within the Duke Primary Care Research Consortium. The practices were chosen because they traditionally operate outside of the academic context. Subjects were required to have both type 2 DM and HTN, as indicated by their medications and confirmed by administrative data as well as patient self-reporting. Participants had to have been seen at participating practices for at least 1 year and have poorly controlled DM (indicated by most recent A1c ≥ 7.5%), but they were not required to have poorly controlled HTN. Exclusion criteria included fewer than 1 primary care clinic visit during the previous year, serious comorbid illness, type 1 diabetes, inability to receive a telephone intervention in English, residence in a nursing home, and participation in another hypertension or diabetes study [1]. Participants were randomly assigned using a computer-generated randomization sequence [1] to either the intervention or control groups at a 1:1 ratio, stratified by clinic and baseline blood pressure (BP) control.
Intervention. A single nurse with extensive experience in case management delivered both the behavioral intervention and attention control by telephone. In both arms, calls were conducted once every 2 months over a 24-month period.
The calls in the intervention arm consisted of tailored behavior-modifying techniques according to patient barriers. This content was divided into a series of modules relevant to behaviors associated with improving control of BP or blood sugar, including physical activity, weight reduction, sodium intake, smoking cessation, medication adherence, and others. These modules were scheduled according to patient needs (based on certain parameters such as high body mass index or use of insulin) and preferences [1].
The calls in the attention control were not tailored but rather consisted of didactic health-related information unrelated to HTN or DM (eg, flu shots, skin cancer prevention). This content was also highly scripted and designed to limit the potential for interaction between the nurse and patient.
Main outcome measures. A1c and systolic blood pressure (SBP) were primary outcomes. Key secondary outcomes were diastolic blood pressure (DBP), overall BP control, weight, physical activity, self-efficacy, and medication adherence. Study staff obtained measurements at baseline and 6, 12, and 24 months [1].
Results. The researchers assessed 2601 patients for eligibility and excluded 2224. Most patients were excluded for not meeting inclusion criteria (n = 1156), in particular because of improved HbA1c control (n = 983), and 1064 declined to participate. They randomized 377 patients—193 to the intervention arm and 184 to the attention control arm. Participants had an average age of 58.7, 49.1% had an education level of high school or less, 50.1% were non-white, and 54.9% were unemployed/retired. Patient characteristics in the intervention and control arms were similar at baseline. Seventy-eight percent of patients completed the 12-month follow-up and 70% (263) reached the 24-month endpoint. Patients in the intervention arm completed 78% of scheduled calls while patients in the control group completed 81%.
After adjusting for stratification variables, the estimated mean A1c and SBP were similar between arms at 24 months (intervention 0.1% higher than control, 95% CI −0.3 % to 0.5 %, P = 0.50 for A1c; intervention 0.9 mm Hg lower than control, 95% CI −5.4 to 3.5, P = 0.69 for SBP). There were also no significant differences between arms in mean A1c or SBP at 6 or 12 months. However, A1c levels did improve within each arm at the end of the study, with the intervention group improving by approx-imately 0.5% and the control group improving by approximately 0.6%. In terms of secondary outcomes, there were no significant differences between arms in DBP, weight, physical activity, or BP control rates throughout the 2-year study period.
Conclusion. Overall, the intervention and control groups did not differ significantly in terms of A1c, SBP, or any of the secondary outcomes at any point during the 2-year study.
Commentary
The prevalence of type 2 diabetes and its comorbidities (such as hypertension and obesity) have increased due to a variety of factors including an aging population and an increasingly sedentary lifestyle. Several nurse management programs for DM and HTN have been shown to be efficacious in reducing blood sugar levels [2–4] and promoting BP control [5,6]. However, these interventions were conducted in tightly controlled academic settings, and it is unclear how well these programs may translate into community settings. The aim of this study was to test the effectiveness of a nurse-led behavioral telephone intervention for the comanagement of DM and HTN within non–academically affiliated community practices. Results indicated no significant differences between the intervention and control groups for A1c levels or SBP at any point during the 2-year study, but A1c levels did improve for both arms.
Despite this being a negative study, it is a unique and important contribution to the literature. It is the only trial as of yet that has tested the effectiveness of a nurse management intervention targeting both DM and HTN in a real-world, community setting. This novel approach is supported by data that suggests BP control is actually more cost-effective than intensive glycemic control in treating patients with type 2 diabetes [7]. There were several strengths to the study design, including the use of intention-to-treat analysis, stratified randomization, a diverse patient population, and blinding of the study staff who took BP and A1c measurements. Furthermore, a single nurse conducted all telephone calls, ensuring that differences in counseling skill levels would not affect the results of the study. The few weaknesses of the study included the fact that the nurse who delivered the intervention (as well as the patients) could not be blinded to treatment allocation, and the income of study participants was not reported.
The reasons for the negative outcomes of this study are unclear. The authors claim that similar interventions within academic settings have been shown to be effective and speculate that time and financial pressures of community practices may be reasons that the intervention was not successful. However, the “successful” interventions that they cite were quite different from and more intensive than this intervention. For instance, many of these studies used at least 1 call per month [3,4,8], and one even conducted several calls each week [3]. Furthermore, a DM study conducted by Blackberry et al in a community setting with less than 1 call per month (8 calls over 18 months) similarly failed to produce significant results [9], and therefore more frequent calls may be necessary in DM and HTN interventions. In a systematic review, Eakin et al demonstrated that 12 or more calls in a 6- to 12-month period were associated with better outcomes in physical activity and diet interventions [10], and this may also translate to closely related DM and HTN interventions.
In addition to the infrequent calls, this intervention also lacked communication and integration with patients’ primary care teams. Several studies have demonstrated that integration with primary care teams can improve outcomes in DM and HTN interventions [11,12], and nearly all of the successful studies cited by the authors also included at least some form of communication with patients’ primary care providers (PCPs) [2–4,5,8]. In many of these studies the nurse also had prescribing rights to alter medications [2,3,5]. The nurse in this study met monthly with an expert team of clinicians to discuss patient issues but did not communicate directly with any of the patients’ PCPs [1]. The authors acknowledge that this lack of integration may have contributed to their negative results and point to the fact that it is harder to integrate interventions within community practices that often lack internal integration. However, Walsh, Harris, and Roberts demonstrated that integration between primary and secondary care teams was both feasible and effective for a diabetes initiative within community practices [13].
An additional important feature not present in this intervention was self-monitoring of BP levels. Home self-monitoring of BP has been demonstrated to significantly improve BP levels [14], and 2 of the successful studies in academic settings cited by the authors also included a BP self-monitoring component [5,6]. In one of these studies [6], Bosworth et al conducted a 2 × 2 randomized trial to improve HTN control in which the arms consisted of (a) usual care, (b) bimonthly nurse administered telephone intervention only (this arm was highly similar to the intervention arm in this study), (c) BP monitoring 3 times a week only, and (d) a combination of the telephone intervention with the BP monitoring. Interestingly, the only arm that was successful relative to usual care was the combination of the telephone intervention and BP self-monitoring; the arm consisting only of bi-monthly telephone calls (very similar to this intervention) failed despite the study taking place in an academic setting (it was also less effective than BP monitoring only). Thus, the addition of self-monitoring to a nurse case management telephone intervention can achieve better results.
Applications for Clinical Practice
A telephone-based intervention delivered by a trained nurse for co-management of DM and HTN was not more effective than an attention control delivered by the same nurse in a community setting. This may have been due to several factors, including low intensity marked by less than 1 call per month, a lack of integration with other members of the primary care team, and lack of a BP self-monitoring component. Future studies are needed to determine the optimal type and duration of nurse case management interventions targeting glucose and BP control for diabetic patients in community settings.
—Sandeep Sikerwar, BA, and Melanie Jay, MD, MS
1. Crowley MJ, Bosworth HB, Coffman CJ, et al. Tailored Case Management for Diabetes and Hypertension (TEACH-DM) in a community population: study design and baseline sample characteristics. Contemp Clin Trials 2013;36:298–306.
2. Aubert RE, Herman WH, Waters J, et al. Nurse case management to improve glycemic control in diabetic patients in a health maintenance organization. A randomized, controlled trial. Ann Intern Med 1998;129:605–12.
3. Thompson DM, Kozak SE, Sheps S. Insulin adjustment by a diabetes nurse educator improves glucose control in insulin-requiring diabetic patients: a randomized trial. CMAJ 1999;161:959–62.
4. Weinberger M, Kirkman MS, Samsa GP, et al. A nurse-coordinated intervention for primary care patients with non-insulin-dependent diabetes mellitus: impact on glycemic control and health-related quality of life. J Gen Intern Med 1995;10:59–66.
5. Bosworth HB, Powers BJ, Olsen MK, et al. Home blood pressure management and improved blood pressure control: results from a randomized controlled trial. Arch Intern Med 2011;171:1173–80.
6. Bosworth HB, Olsen MK, Grubber JM, et al. Two self-management interventions to improve hypertension control: a randomized trial. Ann Intern Med 2009;151:687–95.
7. CDC Diabetes Cost-effectiveness Group. Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction for type 2 diabetes. JAMA 2002;287:2542–51.
8. Mons U, Raum E, Krämer HU, et al. Effectiveness of a supportive telephone counseling intervention in type 2 diabetes patients: randomized controlled study. PLoS One 2013;8:e77954.
9. Blackberry ID, Furler JS, Best JD, et al. Effectiveness of general practice based, practice nurse led telephone coaching on glycaemic control of type 2 diabetes: the Patient Engagement and Coaching for Health (PEACH) pragmatic cluster randomised controlled trial. BMJ 2013;347:f5272.
10. Eakin EG, Lawler SP, Vandelanotte C, Owen N. Telephone interventions for physical activity and dietary behavior change: a systematic review. Am J Prev Med 2007;32:419–34.
11. Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA 2006;296:427–40.
12. Katon WJ, Lin EHB, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611–20.
13. Walsh JL, Harris BHL, Roberts AW. Evaluation of a community diabetes initiative: Integrating diabetes care. Prim Care Diabetes 2014 Dec 11.
14. Halme L, Vesalainen R, Kaaja M, Kantola I. Self-monitoring of blood pressure promotes achievement of blood pressure target in primary health care. Am J Hypertens 2005;18:1415–20.
1. Crowley MJ, Bosworth HB, Coffman CJ, et al. Tailored Case Management for Diabetes and Hypertension (TEACH-DM) in a community population: study design and baseline sample characteristics. Contemp Clin Trials 2013;36:298–306.
2. Aubert RE, Herman WH, Waters J, et al. Nurse case management to improve glycemic control in diabetic patients in a health maintenance organization. A randomized, controlled trial. Ann Intern Med 1998;129:605–12.
3. Thompson DM, Kozak SE, Sheps S. Insulin adjustment by a diabetes nurse educator improves glucose control in insulin-requiring diabetic patients: a randomized trial. CMAJ 1999;161:959–62.
4. Weinberger M, Kirkman MS, Samsa GP, et al. A nurse-coordinated intervention for primary care patients with non-insulin-dependent diabetes mellitus: impact on glycemic control and health-related quality of life. J Gen Intern Med 1995;10:59–66.
5. Bosworth HB, Powers BJ, Olsen MK, et al. Home blood pressure management and improved blood pressure control: results from a randomized controlled trial. Arch Intern Med 2011;171:1173–80.
6. Bosworth HB, Olsen MK, Grubber JM, et al. Two self-management interventions to improve hypertension control: a randomized trial. Ann Intern Med 2009;151:687–95.
7. CDC Diabetes Cost-effectiveness Group. Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction for type 2 diabetes. JAMA 2002;287:2542–51.
8. Mons U, Raum E, Krämer HU, et al. Effectiveness of a supportive telephone counseling intervention in type 2 diabetes patients: randomized controlled study. PLoS One 2013;8:e77954.
9. Blackberry ID, Furler JS, Best JD, et al. Effectiveness of general practice based, practice nurse led telephone coaching on glycaemic control of type 2 diabetes: the Patient Engagement and Coaching for Health (PEACH) pragmatic cluster randomised controlled trial. BMJ 2013;347:f5272.
10. Eakin EG, Lawler SP, Vandelanotte C, Owen N. Telephone interventions for physical activity and dietary behavior change: a systematic review. Am J Prev Med 2007;32:419–34.
11. Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA 2006;296:427–40.
12. Katon WJ, Lin EHB, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611–20.
13. Walsh JL, Harris BHL, Roberts AW. Evaluation of a community diabetes initiative: Integrating diabetes care. Prim Care Diabetes 2014 Dec 11.
14. Halme L, Vesalainen R, Kaaja M, Kantola I. Self-monitoring of blood pressure promotes achievement of blood pressure target in primary health care. Am J Hypertens 2005;18:1415–20.