User login
Which Revascularization Strategy for Multivessel Coronary Disease?
Study Overview
Objective. To compare percutaneous coronary intervention (PCI) using second-generation drug-eluting stents (everolimus-eluting stents) with coronary artery bypass grafting (CABG) among patients with multivessel coronary disease.
Design. Observational registry study with propensity-score matching.
Setting and participants. The study relies on patients identified from the Cardiac Surgery Reporting System (CSRS) and Percutaneous Coronary Intervention Reporting System (PCIRS) registries of the New York State Department of Health. These 2 registries were linked to the New York State Vital Statistics Death registry and to the Statewide Planning and Research Cooperative System registry (SPARCS) to obtain further information like dates of admission, surgery, discharge, and death. Subjects were eligible for inclusion if they had multivessel disease (defined as severe stenosis [≥ 70%] in at least 2 diseased major epicardial coronary arteries) and if they had undergone either PCI with implantation of an everolimus-eluting stent or CABG. Subjects were excluded if they had revascularization within 1 year before index procedure; previous cardiac surgery; severe left main coronary artery disease (degree of stenosis ≥ 50%); PCI with a stent other than an everolimus-eluting stent; myocardial infarction without 24 hours before the index procedure; and unstable hemodynamics or cardiogenic shock.
Main outcome measures. The primary outcome of the study was all-cause mortality. Various secondary outcomes included rates of myocardial infarction, stroke, and repeat vascularization.
Main results. Among 116,915 patients assessed for eligibility, 82,096 were excluded. Among 34,819 who met inclusion criteria, 18,446 were included in the propensity score–matched analysis. With a 1:1 matching algorithm, 9223 were in the PCI with everolimus-eluting stent group and 9223 were in the CABG group. Short-term outcomes (in hospital or ≤ 30 days after the index procedure) favored PCI with everolimus-eluting stents over CABG, with a significantly lower risk of death (0.6% vs. 1.1%; hazard ratio [HR], 0.49; 95% confidence interval [CI], 0.35 to 0.69; P < 0.002) as well as stroke (0.2% vs 1.2%; HR, 0.18; 95% CI, 0.11 to 0.29; P < 0.001). The 2 groups had similar rates of myocardial infarction in the short-term (0.5% and 0.4%; HR, 1.37; 95% CI, 0.89 to 2.12; P = 0.16). After a mean follow-up of 2.9 years, there was a similar annual death rate between groups: 3.1% for PCI and 2.9% for CABG (HR, 1.04; 95% CI, 0.93 to 1.17; P = 0.50). PCI with everolimus-eluting stents was associated with a higher risk of a first myocardial infarction than was CABG (1.9% vs 1.1% per year; HR, 1.51; 95% CI, 1.29 to 1.77; P < 0.001). PCI with everolimus-eluting stents was associated with a lower risk of a first stroke than CABG (0.7% vs. 1.0% per year; HR, 0.62; 95% CI, 0.50 to 0.76; P < 0.001). Finally, PCI with everolimus-eluting stents was associated with a higher risk of a first repeat-revascularization procedure than CABG (7.2% vs. 3.1% per year; HR, 2.35; 95% CI, 2.14 to 2.58; P < 0.001).
Conclusion. In the setting of newer stent technology with second-generation everolimus-eluting stents, the risk of death associated with PCI was similar to that associated with CABG for multivessel coronary artery disease. In the long-term, PCI was associated with a higher risk of myocardial infarction and repeat revascularization, whereas CABG was associated with an increased risk of stroke. In the short-term, PCI had lower risks of both death and stroke.
Commentary
Coronary artery disease is a major public health problem. For patients for whom revascularization is deemed to be appropriate, a choice must be made between PCI and CABG. In previous studies that compared PCI and CABG, CABG was shown to have less need for repeat revascularizations as well as mortality benefits [1–3]. However, these prior studies compared CABG with older generations of stents. In the past decade, stent technologies have improved, as the bare-metal stent era gave way to the first generation of of drug-eluting stents (with sirolimus or paclitaxel), to be followed by second-generation drug-eluting stents (with everolimus or zotarolimus) [4].
In this article, Bangalore and colleagues addressed the issue of whether the use of second-generation drug-eluting stents close the outcome gap that favors CABG over PCI in patients with multivessel coronary artery disease. In patients who were considered to have had complete revascularization performed during PCI (ie, revascularization of all major vessels with clinically significant stenosis), they noted mitigation of the outcome differences between the PCI group and the CABG group. They conclude that the decision-making process by patients and their providers regarding revascularization be placed in the context of individual values and preferences.
One major limitation is that the study is an observational study from registry data. Despite the use of sophisticated statistical techniques including propensity score matching to adjust for confounders that are implicit in any nonrandomized comparison of treatment strategies, observational studies suffer from the definitely proof of causality. These limitations are especially important when the two groups being compared have modest differences in outcome.
Applications for Clinical Practice
This observational study, together with a recent randomized clinical trial in which CABG was compared with PCI with the use of everolimus-eluting stents from the BEST trial [5], provided new insights of the 2 revascularization strategies. Clinicians should engage and empower patients with a shared decision-making approach. The early hazard of CABG in stroke and death may be unacceptable to some patients, whereas others might want to avoid the later hazards of PCI in repeat procedure or having a myocardial infarction. Until a definitive study is available, patients should be informed of the best current knowledge of the pros and cons of the two revascularization strategies.
—Ka Ming Gordon Ngai, MD, MPH
1. Farooq V, van Klaveren D, Steyerberg EW, et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet 2013;381: 639–50.
2. Hannan EL, Racz MJ, Arani DT, et al. A comparison of short- and long-term outcomes for balloon angioplasty and coronary stent placement. J Am Coll Cardiol 2000;36:395–403.
3. Hannan EL, Racz MJ, Walford G, et al. Long-term outcomes of coronary-artery bypass grafting versus stent implantation. N Engl J Med 2005;352: 2174–83.
4. Harrington RA. Selecting revascularization strategies in patients with coronary disease. N Engl J Med 2015;372: 1261–3.
5. Park SJ, Ahn JM, Kim YH, et al. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med 2015;372:1204–12.
Study Overview
Objective. To compare percutaneous coronary intervention (PCI) using second-generation drug-eluting stents (everolimus-eluting stents) with coronary artery bypass grafting (CABG) among patients with multivessel coronary disease.
Design. Observational registry study with propensity-score matching.
Setting and participants. The study relies on patients identified from the Cardiac Surgery Reporting System (CSRS) and Percutaneous Coronary Intervention Reporting System (PCIRS) registries of the New York State Department of Health. These 2 registries were linked to the New York State Vital Statistics Death registry and to the Statewide Planning and Research Cooperative System registry (SPARCS) to obtain further information like dates of admission, surgery, discharge, and death. Subjects were eligible for inclusion if they had multivessel disease (defined as severe stenosis [≥ 70%] in at least 2 diseased major epicardial coronary arteries) and if they had undergone either PCI with implantation of an everolimus-eluting stent or CABG. Subjects were excluded if they had revascularization within 1 year before index procedure; previous cardiac surgery; severe left main coronary artery disease (degree of stenosis ≥ 50%); PCI with a stent other than an everolimus-eluting stent; myocardial infarction without 24 hours before the index procedure; and unstable hemodynamics or cardiogenic shock.
Main outcome measures. The primary outcome of the study was all-cause mortality. Various secondary outcomes included rates of myocardial infarction, stroke, and repeat vascularization.
Main results. Among 116,915 patients assessed for eligibility, 82,096 were excluded. Among 34,819 who met inclusion criteria, 18,446 were included in the propensity score–matched analysis. With a 1:1 matching algorithm, 9223 were in the PCI with everolimus-eluting stent group and 9223 were in the CABG group. Short-term outcomes (in hospital or ≤ 30 days after the index procedure) favored PCI with everolimus-eluting stents over CABG, with a significantly lower risk of death (0.6% vs. 1.1%; hazard ratio [HR], 0.49; 95% confidence interval [CI], 0.35 to 0.69; P < 0.002) as well as stroke (0.2% vs 1.2%; HR, 0.18; 95% CI, 0.11 to 0.29; P < 0.001). The 2 groups had similar rates of myocardial infarction in the short-term (0.5% and 0.4%; HR, 1.37; 95% CI, 0.89 to 2.12; P = 0.16). After a mean follow-up of 2.9 years, there was a similar annual death rate between groups: 3.1% for PCI and 2.9% for CABG (HR, 1.04; 95% CI, 0.93 to 1.17; P = 0.50). PCI with everolimus-eluting stents was associated with a higher risk of a first myocardial infarction than was CABG (1.9% vs 1.1% per year; HR, 1.51; 95% CI, 1.29 to 1.77; P < 0.001). PCI with everolimus-eluting stents was associated with a lower risk of a first stroke than CABG (0.7% vs. 1.0% per year; HR, 0.62; 95% CI, 0.50 to 0.76; P < 0.001). Finally, PCI with everolimus-eluting stents was associated with a higher risk of a first repeat-revascularization procedure than CABG (7.2% vs. 3.1% per year; HR, 2.35; 95% CI, 2.14 to 2.58; P < 0.001).
Conclusion. In the setting of newer stent technology with second-generation everolimus-eluting stents, the risk of death associated with PCI was similar to that associated with CABG for multivessel coronary artery disease. In the long-term, PCI was associated with a higher risk of myocardial infarction and repeat revascularization, whereas CABG was associated with an increased risk of stroke. In the short-term, PCI had lower risks of both death and stroke.
Commentary
Coronary artery disease is a major public health problem. For patients for whom revascularization is deemed to be appropriate, a choice must be made between PCI and CABG. In previous studies that compared PCI and CABG, CABG was shown to have less need for repeat revascularizations as well as mortality benefits [1–3]. However, these prior studies compared CABG with older generations of stents. In the past decade, stent technologies have improved, as the bare-metal stent era gave way to the first generation of of drug-eluting stents (with sirolimus or paclitaxel), to be followed by second-generation drug-eluting stents (with everolimus or zotarolimus) [4].
In this article, Bangalore and colleagues addressed the issue of whether the use of second-generation drug-eluting stents close the outcome gap that favors CABG over PCI in patients with multivessel coronary artery disease. In patients who were considered to have had complete revascularization performed during PCI (ie, revascularization of all major vessels with clinically significant stenosis), they noted mitigation of the outcome differences between the PCI group and the CABG group. They conclude that the decision-making process by patients and their providers regarding revascularization be placed in the context of individual values and preferences.
One major limitation is that the study is an observational study from registry data. Despite the use of sophisticated statistical techniques including propensity score matching to adjust for confounders that are implicit in any nonrandomized comparison of treatment strategies, observational studies suffer from the definitely proof of causality. These limitations are especially important when the two groups being compared have modest differences in outcome.
Applications for Clinical Practice
This observational study, together with a recent randomized clinical trial in which CABG was compared with PCI with the use of everolimus-eluting stents from the BEST trial [5], provided new insights of the 2 revascularization strategies. Clinicians should engage and empower patients with a shared decision-making approach. The early hazard of CABG in stroke and death may be unacceptable to some patients, whereas others might want to avoid the later hazards of PCI in repeat procedure or having a myocardial infarction. Until a definitive study is available, patients should be informed of the best current knowledge of the pros and cons of the two revascularization strategies.
—Ka Ming Gordon Ngai, MD, MPH
Study Overview
Objective. To compare percutaneous coronary intervention (PCI) using second-generation drug-eluting stents (everolimus-eluting stents) with coronary artery bypass grafting (CABG) among patients with multivessel coronary disease.
Design. Observational registry study with propensity-score matching.
Setting and participants. The study relies on patients identified from the Cardiac Surgery Reporting System (CSRS) and Percutaneous Coronary Intervention Reporting System (PCIRS) registries of the New York State Department of Health. These 2 registries were linked to the New York State Vital Statistics Death registry and to the Statewide Planning and Research Cooperative System registry (SPARCS) to obtain further information like dates of admission, surgery, discharge, and death. Subjects were eligible for inclusion if they had multivessel disease (defined as severe stenosis [≥ 70%] in at least 2 diseased major epicardial coronary arteries) and if they had undergone either PCI with implantation of an everolimus-eluting stent or CABG. Subjects were excluded if they had revascularization within 1 year before index procedure; previous cardiac surgery; severe left main coronary artery disease (degree of stenosis ≥ 50%); PCI with a stent other than an everolimus-eluting stent; myocardial infarction without 24 hours before the index procedure; and unstable hemodynamics or cardiogenic shock.
Main outcome measures. The primary outcome of the study was all-cause mortality. Various secondary outcomes included rates of myocardial infarction, stroke, and repeat vascularization.
Main results. Among 116,915 patients assessed for eligibility, 82,096 were excluded. Among 34,819 who met inclusion criteria, 18,446 were included in the propensity score–matched analysis. With a 1:1 matching algorithm, 9223 were in the PCI with everolimus-eluting stent group and 9223 were in the CABG group. Short-term outcomes (in hospital or ≤ 30 days after the index procedure) favored PCI with everolimus-eluting stents over CABG, with a significantly lower risk of death (0.6% vs. 1.1%; hazard ratio [HR], 0.49; 95% confidence interval [CI], 0.35 to 0.69; P < 0.002) as well as stroke (0.2% vs 1.2%; HR, 0.18; 95% CI, 0.11 to 0.29; P < 0.001). The 2 groups had similar rates of myocardial infarction in the short-term (0.5% and 0.4%; HR, 1.37; 95% CI, 0.89 to 2.12; P = 0.16). After a mean follow-up of 2.9 years, there was a similar annual death rate between groups: 3.1% for PCI and 2.9% for CABG (HR, 1.04; 95% CI, 0.93 to 1.17; P = 0.50). PCI with everolimus-eluting stents was associated with a higher risk of a first myocardial infarction than was CABG (1.9% vs 1.1% per year; HR, 1.51; 95% CI, 1.29 to 1.77; P < 0.001). PCI with everolimus-eluting stents was associated with a lower risk of a first stroke than CABG (0.7% vs. 1.0% per year; HR, 0.62; 95% CI, 0.50 to 0.76; P < 0.001). Finally, PCI with everolimus-eluting stents was associated with a higher risk of a first repeat-revascularization procedure than CABG (7.2% vs. 3.1% per year; HR, 2.35; 95% CI, 2.14 to 2.58; P < 0.001).
Conclusion. In the setting of newer stent technology with second-generation everolimus-eluting stents, the risk of death associated with PCI was similar to that associated with CABG for multivessel coronary artery disease. In the long-term, PCI was associated with a higher risk of myocardial infarction and repeat revascularization, whereas CABG was associated with an increased risk of stroke. In the short-term, PCI had lower risks of both death and stroke.
Commentary
Coronary artery disease is a major public health problem. For patients for whom revascularization is deemed to be appropriate, a choice must be made between PCI and CABG. In previous studies that compared PCI and CABG, CABG was shown to have less need for repeat revascularizations as well as mortality benefits [1–3]. However, these prior studies compared CABG with older generations of stents. In the past decade, stent technologies have improved, as the bare-metal stent era gave way to the first generation of of drug-eluting stents (with sirolimus or paclitaxel), to be followed by second-generation drug-eluting stents (with everolimus or zotarolimus) [4].
In this article, Bangalore and colleagues addressed the issue of whether the use of second-generation drug-eluting stents close the outcome gap that favors CABG over PCI in patients with multivessel coronary artery disease. In patients who were considered to have had complete revascularization performed during PCI (ie, revascularization of all major vessels with clinically significant stenosis), they noted mitigation of the outcome differences between the PCI group and the CABG group. They conclude that the decision-making process by patients and their providers regarding revascularization be placed in the context of individual values and preferences.
One major limitation is that the study is an observational study from registry data. Despite the use of sophisticated statistical techniques including propensity score matching to adjust for confounders that are implicit in any nonrandomized comparison of treatment strategies, observational studies suffer from the definitely proof of causality. These limitations are especially important when the two groups being compared have modest differences in outcome.
Applications for Clinical Practice
This observational study, together with a recent randomized clinical trial in which CABG was compared with PCI with the use of everolimus-eluting stents from the BEST trial [5], provided new insights of the 2 revascularization strategies. Clinicians should engage and empower patients with a shared decision-making approach. The early hazard of CABG in stroke and death may be unacceptable to some patients, whereas others might want to avoid the later hazards of PCI in repeat procedure or having a myocardial infarction. Until a definitive study is available, patients should be informed of the best current knowledge of the pros and cons of the two revascularization strategies.
—Ka Ming Gordon Ngai, MD, MPH
1. Farooq V, van Klaveren D, Steyerberg EW, et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet 2013;381: 639–50.
2. Hannan EL, Racz MJ, Arani DT, et al. A comparison of short- and long-term outcomes for balloon angioplasty and coronary stent placement. J Am Coll Cardiol 2000;36:395–403.
3. Hannan EL, Racz MJ, Walford G, et al. Long-term outcomes of coronary-artery bypass grafting versus stent implantation. N Engl J Med 2005;352: 2174–83.
4. Harrington RA. Selecting revascularization strategies in patients with coronary disease. N Engl J Med 2015;372: 1261–3.
5. Park SJ, Ahn JM, Kim YH, et al. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med 2015;372:1204–12.
1. Farooq V, van Klaveren D, Steyerberg EW, et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet 2013;381: 639–50.
2. Hannan EL, Racz MJ, Arani DT, et al. A comparison of short- and long-term outcomes for balloon angioplasty and coronary stent placement. J Am Coll Cardiol 2000;36:395–403.
3. Hannan EL, Racz MJ, Walford G, et al. Long-term outcomes of coronary-artery bypass grafting versus stent implantation. N Engl J Med 2005;352: 2174–83.
4. Harrington RA. Selecting revascularization strategies in patients with coronary disease. N Engl J Med 2015;372: 1261–3.
5. Park SJ, Ahn JM, Kim YH, et al. Trial of everolimus-eluting stents or bypass surgery for coronary disease. N Engl J Med 2015;372:1204–12.
Decision Making in Venous Thromboembolism
From the Brigham and Women’s Hospital and Dana-Farber Cancer Institute, Boston, MA.
Abstract
- Objective: To review the diagnosis and management of venous thromboembolism (VTE).
- Methods: Review of the literature.
- Results: VTE and its associated complications account for significant morbidity and mortality. Various imaging modalities can be employed to support a diagnosis of a VTE and are used based on clinical suspicion arising from the presence of signs and symptoms. Clinical decision rules have been developed that can help determine which patients warrant further testing. Anticoagulation, the mainstay of VTE treatment, increases bleeding risk, necessitating tailored treatment strategies that must incorporate etiology, risk, benefit, cost, and patient preference.
- Conclusion: Further study is needed to understand individual patient risks and to identify treatments that will lead to improved patient outcomes.
Venous thromboembolism (VTE) and its associated complications account for significant morbidity and mortality. Each year between 100 and 180 persons per 100,000 in Western countries develop VTE. The majority of VTEs are classified as either pulmonary embolism (PE), which accounts for one third of the events, or deep vein thrombosis (DVT), which is responsible for the remaining two thirds. Between 20% and 30% of those patients diagnosed with thrombotic events will die within the first month after diagnosis [1].PE is a common consequence of DVT; 40% of patients who are diagnosed with DVT will be subsequently found to have PE upon further imaging. This high rate of association is also seen in those who present with PE, 70% of whom will also be found to have concomitant DVT [2,3].
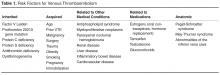
Anatomic risk factors include Paget-Schroetter syndrome (compression of upper extremity veins due to abnormalities at the thoracic outlet), May-Thurner syndrome (significant compression of the left common iliac vein by the right common iliac artery), and abnormalities of the inferior vena cava [14–16].Medications that are associated with increased risk of VTE include but are not limited to estrogen (both in oral contraceptives as well as hormone replacement therapy) [17,18],the selective estrogen receptor modulator tamoxifen [19],testosterone [20],and glucocorticoids [21].It is important to note that many patients with VTE have more than one acquired risk factor for thrombosis [22],and also that acquired risk factors are more likely to lead to VTE in the setting of underlying inherited thrombophilic conditions [23].
Pathogenesis
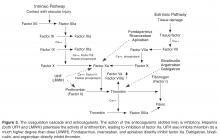
Abnormalities in both coagulation factors and the vascular bed are at the core of the pathogenesis of VTE. The multifaceted etiology of thrombosis was first described in 1856 by Virchow, who defined a triad of defects in the vessel wall, platelets, and coagulation proteins [24].Usually the vessel wall is lined with endothelial cells that provide a nonthrombotic surface and limit platelet aggregation through release of prostacyclins and nitric oxide. When the endothelial lining is compromised, the homeostatic surveillance system is disturbed and platelet activation and the coagulation system are initiated. Tissue factor exposure in the damaged area of the vessel leads to activation of the coagulation cascade. Collagen that is present in the area of the wound is also exposed and can activate platelets, which provide the phospholipid surface upon which the coagulation cascade occurs. Platelets initially tether to the exposed collagen through binding of glycoprotein Ib-V-IX in association with von Willebrand factor [25].The thrombus is initiated as more platelets are recruited to exposed collagen of the injured endothelium through aggregation in response to the binding of GPIIIb/IIa with fibrinogen. This process is self-perpetuating as these activated platelets release additional proteins such as adenosine diphosphate (ADP), serotonin, and thromboxane A2, all of which fuel the recruitment and activation of additional platelets [26].
Diagnosis
The key to decreasing the morbidity and mortality associated with VTE is timely diagnosis and early initiation of therapy. Various imaging modalities can be employed to support a diagnosis of a VTE and are used based on clinical suspicion arising from the presence of signs and symptoms. DVT is usually associated with pain in calf or thigh, unilateral swelling, tenderness, and redness. PE can present as chest pain, shortness of breath, syncope, hemoptysis, and/or cardiac palpitations.
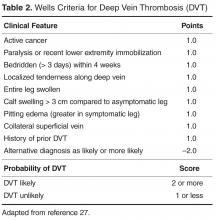
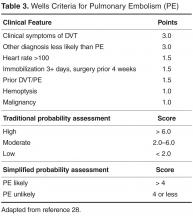
Decision Rules
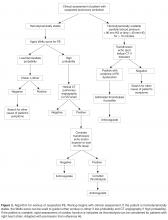
Clinical decision rules based on signs, symptoms, and risk factors have been developed to estimate the pretest probability of PE or DVT and to help determine which patients warrant further testing. These clinical decision rules include the Wells criteria (separate rules for DVT and PE) [27,28],as well as the Geneva score [29],which is focused on identifying patients with a likelihood of having a PE. In general, these clinical rules are applied at presentation to predict the risk of VTE, and patients who score high are evaluated by imaging modalities, while those with lower scores should be considered for further stratification based on D-dimer testing. The goal of clinical assessment and use of a decision rule is to identify patients at low risk of VTE to reduce the number of imaging studies performed. Most of the decision rules focus on the use of noninvasive evaluations that are easily implemented, including clinical history and presentation, abnormalities in oxygen saturation, chest radiography findings, and electro-cardiography.
D-Dimer Testing
D-dimer testing is at the core of all predictive models for VTE. D-dimer is a fibrin degradation product that is detectable in the blood during active fibrinolysis and occurs after clot formation. The concentration of D-dimer increases in patients with active clot. D-dimer testing is usually performed as a quantitative ELISA or automated turbidometric assay and is highly sensitive (> 95%) in excluding a diagnosis of VTE if results are in the normal range [30].The presence of a normal D-dimer and a low probability based on clinical assessment criteria can be integrated to determine which patients have a low (generally < 99%) likelihood of having VTE [31].It should be noted that other factors can lead to an increased D-dimer, including malignancy, trauma, critical illness, disseminated intravascular coagulation, pregnancy, infection, and postoperative status, which can produce false-positive results and cloud the utility of the test in excluding those at low risk of VTE from undergoing imaging [32–34].Additionally, D-dimer values naturally increase with age and recent work has shown utility of an age-adjusted D-dimer threshold, though this method is not yet widespread in clinical practice [35,36].
Imaging
After application of a clinical prediction rule, the mainstay of diagnosis of VTE is imaging. For DVT the use of ultrasonography is considered the gold standard, with both high sensitivity (89–100%) and specificity (86–100%), especially when the DVT is located proximally [37–39].We generally recommend compression ultrasound starting with the proximal veins but expanding to include the whole leg if the proximal studies are negative [40–42].Other diagnostic options include computed tomography (CT) venography, which is not first line as it is highly invasive and exposes the patient to iodine-based contrast dyes, and magnetic resonance venography (MRV), which offers superb visualization for diagnosis of pelvic vein thrombosis but is limited because of availability and cost issues.
Helical CT pulmonary angiography (CTPA) is the diagnostic test of choice in PE, with high sensitivity (96%) and specificity (95%), and has replaced conventional ventilation perfusion (VQ) scanning or other methods such as magnetic resonance pulmonary angiography in most settings [43,44].CTPA should be avoided in patients who have severe chronic kidney disease or a contrast allergy, and is often avoided in patients who are pregnant due to potential risk of radiation exposure, and in such situations VQ scanning may be employed.
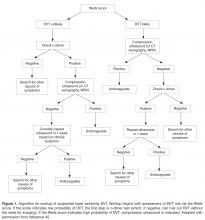
Algorithmic Approach to Workup
Of note, there are multiple clinical situations in which the application of a clinical prediction rule followed by D-dimer testing and/or imaging cannot be “standardized” with such algorithms. These include situations where D-dimer may be falsely positive (as above), situations in which alternative imaging strategies should be used to avoid contrast exposure in workup of PE (as above), and workup of suspected upper extremity DVT. Upper extremity ultrasound comprises about 10% of all DVT and frequently occurs in the setting of risk factors such as central venous catheters or pacemakers; specific upper-extremity risk-assessment rules have been developed [47,48].
Acute Treatment Options
The first step in treatment is identification of patients who are at high risk of
In standard cases of DVT and PE without hemodynamic compromise, the current standard of care is to initiate parenteral anticoagulation. The immediate goal of therapy is to treat rapidly with anticoagulants to prevent the thrombus from propagating further and to prevent DVT from embolization to the lungs or other vascular beds. The initial treatment of VTE has been extensively discussed and guidelines have been established with recommendations for initiation of anticoagulation; the American College of Chest Physicians (ACCP) released the 9th edition of their guidelines in 2012 based on consensus agreements derived from primary data [51].
Heparin-based drugs are the mainstay of initial treatment. These drugs act by potentiating antithrombin and therefore inactivating thrombin and other coagulation factors such as Xa. Unfractionated heparin (UFH) can be administered as an initial bolus followed by a continuous infusion with dosing being based on weight and titrated to activated partial thromboplastin time (aPTT) or the anti-factor Xa level. Alternatively, patients may be treated with a low molecular weight heparin (LMWH) administered subcutaneously in fixed weight-adjusted doses, which obviates the need for monitoring in most cases [52].LMWHs work in a similar manner to UFH but have more anti-Xa activity in comparison to anti-thrombin activity. LMWH appears to be more effective than UFH for initial treatment of VTE and has been associated with lower risk of major hemorrhage [53].The options for treatment of VTE have expanded in recent years with the approval of fondparinux, a pentasaccharide specifically targeted to inhibit factor Xa. Fondaparinux has been shown to have similar efficacy to LMWH in patients with DVT [54],and while it has not been evaluated directly against LMWH for initial treatment of PE it has been shown to be at least as effective and safe as UFH [55].
Both LMWH and fondaparinux are cleared renally and therefore have increased bleeding risk in patients with renal impairment. In patients with creatinine clearance of less than 30 mL/min, dose reduction or lengthening of dosing interval may be appropriate. Anti-factor Xa activity can be used as a functional assay to monitor and titrate the level of anticoagulation in patients treated with UFH, LMWH, and fondaparinux. Monitoring is useful in the setting of impaired renal function (as above) in addition to extremes of body weight and pregnancy. When used for monitoring of UFH, the anti-factor Xa activity can be measured at any time during administration with a therapeutic goal range of 0.3–0.7 international units (IU)/mL. When used for LMWH, a “peak” anti-factor Xa should be measured approximately 4 hours after dosing, with therapeutic goals depending on preparation and schedule of treatment but generally between 0.6 to 1.0 IU/mL for twice daily and around 1.0 -2.0 IU/mL for once-daily [56].For patients on dialysis, we generally use intravenous UFH for acute treatment of VTE, though recent work has shown that enoxaparin (doses of 0.4 to 1 mg/kg/day) was as safe as UFH with respect to bleeding and was associated with shorter hospital length of stay [57].For long-term treatment of VTE, warfarin is generally preferred based on clinical experience with this agent, though small studies have suggested that parenteral agents may be useful alternatives to warfarin [58].
In many patients who are clinically stable without significant medical comorbidities, outpatient administration of these medications without hospitalization is considered safe. Patients with DVT are often safe to manage as outpatients unless significant clot burden is present and thrombolysis is being considered. For PE, the pulmonary embolism severity index (PESI) and simplified index (sPESI) may be useful to risk-stratify patients and identify those at low risk of complications who may be suitable for outpatient treatment [59,60].Studies have shown that hemodynamically stable patients who did not require supplemental oxygenation or have contraindications to LMWH therapy were safely managed as outpatients with low risk of recurrent VTE and bleeding [61,62].One exception may be patients with intermediate risk PE, who are hemodynamically stable but have evidence of right ventricular dysfunction and may be better served by an initial in-hospital observation period, especially if thrombolysis is being considered.
Most patients who present with VTE are transitioned to warfarin for long-term therapy. Warfarin can be started on the same day as parenteral anticoagulation. Both drugs are overlapped for at least 5 days, with a target INR of 2.0–3.0. Patients may achieve the target INR level quickly because factor VII has a short half-life and the level drops quickly; however, the overlap of 5 days is essential even when the INR is in the target range because a full anticoagulant affect is not achieved until prothrombin levels decline, and this is a slow process due to the long half-life of prothrombin. Warfarin also causes rapid decrease in levels of natural anticoagulants such as protein C and protein S, which further exacerbates the net hypercoagulable state in the short-term. Warfarin without a bridging parenteral agent carries a risk of warfarin-induced skin necrosis [63]and is not effective as an initial anticoagulant treatment in acute VTE as there is a relatively high risk of symptomatic clot extension or recurrent VTE compared to warfarin with use of a bridging agent [64].In specific cases such as cancer-associated VTE (see discussion below), LMWH is preferred to warfarin for long-term active therapy.
Long-Term Active Therapy After Acute Treatment
Duration of Anticoagulation
Recommended duration of anticoagulation depends on a myriad of factors including severity of VTE, risk of recurrence, bleeding risk, and lifestyle modification issues, as well as on the safety and availability of alternative therapies such as low-intensity warfarin, aspirin, or the new oral anticoagulants. The decision tree for length of treatment starts with whether the VTE was a provoked or a spontaneous event. Provoked events occur when the event is associated with an identifiable risk factor, such as immobilization from prolonged medical illness or surgical intervention, pregnancy or oral contraceptive use, and prolonged air travel.
Consensus guidelines suggest that 3 months of anti-coagulation are generally sufficient treatment for a provoked VTE [51,65,66]. Data from multiple studies and a meta-analysis suggests that less than 3 months of anticoagulation (4 to 6 weeks in most trials) is associated with an approximately 1.5-fold higher risk of recurrent VTE than 3 months [67,68].However, data from this meta-analysis also suggests that anticoagulation for longer than 3 months (6 to 12 months in most trials) is not associated with higher rates of recurrent VTE. We generally anticoagulate for 3 months in patients with provoked VTE.
Determining the duration of anticoagulation is more complex in patients with idiopathic/unprovoked VTE. Kearon and colleagues found that in patients with first idiopathic VTE, patients who were anticoagulated for 24 months versus 3 months had lower risk of recurrent VTE (1.3% per patient-year with 24 months versus 27.4% per patient-year with 3 months) [69].Similar studies and meta-analyses have demonstrated decreased recurrence rates in patients anticoagulated for a prolonged period of time. However, one study of prolonged anticoagulation revealed that at 3 years there was no difference in recurrence rate in patients with PE who were anticoagulated for 6 months versus 1 year [70].The likelihood of recurrent DVT in patients with first episode of idiopathic proximal DVT treated with either 3 months or 12 months of warfarin was similar after treatment was discontinued [71].Prolonged periods of anticoagulation do not directly influence risk of recurrence but instead may only delay occurrence of a second event [72].For that reason, the decision is essentially whether to anticoagulate for 3 months or to continue therapy indefinitely [73]. Current guidelines recommend continuing anticoagulation for 3 months in those at high risk of bleeding, and continuing for an extended duration in those at low or moderate bleeding risk [51]. Patients' values and perferences should be entertained and decisions made on a patient-by-patient basis.
For patients at high risk of recurrent VTE, we generally recommend indefinite anticoagulation unless the patient has a significantly elevated bleeding risk or strongly prefers to discontinue anticoagulation and compliance concerns are evident. High-risk patients are those who have suffered from multiple episodes of recurrent VTE, those who have clotted while being anticoagulated, and those with acquired risk factors, such as antiphospholipid antibodies and malignancy. Other high-risk groups are those with high-risk thrombophilias such as deficiency of protein S, protein C, or antithrombin, homozygous factor V Leiden or prothrombin gene mutations, and compound heterozygous factor V Leiden/prothrombin gene mutation in the setting of an unprovoked event. Further discussion of models for risk assessment of recurrence is provided below.
Assessment of Bleeding Risk
The bleeding risk associated with the use of anticoagulation must be weighed against the risk of clotting events when determining duration of anticoagulation, especially in those patients for whom indefinite anticoagulation is a consideration. Risk of bleeding while on anticoagulation is approximately 1–3% per 100 patient-years [74],but concomitant medical conditions such as renal failure, diabetes-related cerebrovascular disease, malignancy, advanced age, and use of antiplatelet agents all increase the risk of bleeding. Bleeding risk is highest when patients first initiate anticoagulation and is approximately 10 times the risk in the first month of therapy than after the first year of therapy [75].
Risk assessment models such as the RIETE score may be helpful when indefinite anticoagulation is a possibility [76].The RIETE score encompasses 6 risk factors (age > 75 years, recent bleeding, cancer, creatinine level > 1.2 mg/dL, anemia, or PE at baseline) to categorize patients into low risk (0 points, 0.3% risk of bleeding), intermediate risk (1–4 points, 2.6% risk of bleeding) and high risk (> 4 points, 6.2% risk of bleeding) within 3 months of anticoagulant therapy. The ACCP has developed a more extensive list of 17 potential risk factors for bleeding to categorize patients into low risk (no risk factors, 0.8%/year risk of bleeding), intermediate risk (1 risk factor, 1.6%/year risk of bleeding) and high risk (2 or more risk factors, >6.5%/year risk of bleeding) categories [77].The RIETE score is simpler to use but was not developed for assessing risk of bleeding during indefinite therapy, while the ACCP risk categorization predicts a yearly risk and is therefore applicable for long-term risk assessment but is more cumbersome to use. In practice, we generally use a clinical gestalt of a patient’s clinical risk factors (particularly age, renal or hepatic dysfunction, and frequent falls) to assess if they may be at high risk of bleeding and if the risk of indefinite anticoagulation may thus outweigh the potential benefit.
We also note that several scoring systems (HAS-BLED, HEMORR2HAGES, and ATRIA scores) have been developed to predict those at high risk of bleeding on anticoagulation for atrial fibrillation [78–80].These scores generally include similar clinical risk factors to those in the RIETE and ACCP scoring systems. Several studies have compared the HAS-BLED, HEMORR2HAGES, and ATRIA scores and a systematic review and meta-analysis concluded that the HAS-BLED score is recommended, due to increased sensitivity and ease of application [81].However, as these scores have not been validated for anticoagulation in the setting of VTE, we do not use them in this capacity.
Risk Stratification for Recurrent VTE
When predicting risk of recurrent VTE, clinical risk factors including obesity, male gender, and underlying thrombophilia (including the “high risk” inherited thrombophilias identified above) must taken into consideration. Location of the thrombus must also be considered; it has also been demonstrated that patients with DVT involving the iliofemoral veins are at higher risk of recurrence than those without iliac involvement [82].Other factors that may be useful in risk stratification include D-dimer level and ultrasound to search for residual venous thrombosis.
D-dimer Levels
D-dimer levels are one of the more promising methods for assessing the risk of recurrent VTE after cessation of anticoagulation, especially in the case of idiopathic VTE where indefinite anticoagulation should be considered but may pose either risk of bleeding or significant inconvenience to patients. A normal D-dimer measured 1 month after cessation of anticoagulation offers a high negative predictive value for risk of recurrence [83].A number of studies have demonstrated that patients with elevated D-dimer 1 month after anticoagulation cessation are at increased risk for a recurrent event [84–86].Two predictive models that have been developed incorporate D-dimer testing into decision making [87,88].The DASH predictive model relies on the D-dimer result in addition to age, male sex, and use of hormone therapy as a method of risk stratification for recurrent VTE in patients with a first unprovoked event. Using this scoring system, patients with a score of 0 or 1 had a recurrence rate of 3.1%, those with a score of 2 a recurrence rate of 6.4%, and those with a score of 3 or greater a recurrence rate of 12.3%. The authors postulate that by using this assessment scheme they can avoid lifelong anticoagulation in 51% of patients. The Vienna prediction model uses male sex, location of VTE (proximal DVT and PE are at higher risk), and D-dimer level to predict risk of recurrent VTE. This model has recently been updated to include a “dynamic” component to predict risk of recurrence of VTE from multiple random time points [89].
Overall, D-dimer may be useful for risk stratification. We often employ the method of stopping anticoagulation in patients with unprovoked VTE after 3 months (if the patient has no identifiable clinical risk factors that place them at high risk of recurrence) and testing D-dimer 1 month after cessation of anticoagulation. An elevated D-dimer is a solid reason to restart anticoagulation (potentially on an indefinite basis), while a negative D-dimer provides support for withholding further anticoagulation in the absence of other significant risk factors for recurrence. However, lack of agreement regarding assay cut-points as well as multiple reasons other than VTE for D-dimer elevation may limit widespread use of this method. We generally use a cutpoint of 250 ug/L as “negative,” though at least one study showed that cut-points of 250 ug/L versus 500 ug/L did not change the utility of this method [90].In our practice, risk prediction models are most useful to provide patients with additional information and a visual presentation to support our recommendation. This is particularly true of the Vienna prediction rule, which is available in a printable nomogram which can be distributed to patients and completed together during the clinic visit.
Imaging Analysis
Imaging analysis may also assist with risk stratification. Clinical assessment modules have been developed that incorporate repeat imaging studies for assessment of recannulization of affected veins. In patients with residual vein thrombosis (RVT) at the time anticoagulation was stopped, the hazard ratio for recurrence was 2.4 compared to those without RVT [91].There are a number of ways RVT could impact recurrence, including inpaired venous flow leading to stasis and activation of the coagulation cascade. Subsequent studies used serial ultrasound to determine when to stop anticoagulation. In one study, patients were anticoagulated for 3 months and for those that had RVT, anticoagulation was continued for up to 9 months for provoked and 21 months for unprovoked VTE. In comparison to fixed dosing of 6 months of anti-coagulation, those who had their length of anticoagulation tailored to ultrasonography findings had a lower rate of recurrent VTE [92].Limitations to using RVT in clinical decision-making include lack of a standard definition of RVT and variability in both timing of ultrasound (operator variability) and interpretation of results [93].
Other Options
Another option in patients who are being considered for indefinite anticoagulation is to decrease the intensity of anticoagulation. Since this would theoretically lower the risk of bleeding, the perceived benefit of long-term, low-intensity anticoagulation would be reduction in both bleeding and clotting risk. The PREVENT trial randomized patients who had received full-dose anticoagulation for a median of 6.5 months to either low-intensity warfarin (INR goal of 1.5-2.0 instead of 2.0-3.0) or placebo. In the anticoagulation group, there was a 64% risk reduction in recurrent VTE (hazard ratio 0.36, 95% CI 0.19 to 0.67) but an increased risk of bleeding (hazard ratio 1.92, 95% CI 1.26 to 2.93) [94].The ELATE study randomized patients with unprovoked VTE who had completed 3 or more months of full-intensity warfarin therapy (target INR 2.0–3.0) to continue therapy with either low-intensity warfarin (target INR 1.5–2.0) or full-intensity warfarin (target INR 2.0-3.0). Compared to the low-intensity group, the conventional-intensity group had lower rates of recurrent VTE and no increased rates of major bleeding [95].This study, however, has been criticized because of its overall low bleeding rate in both treatment groups.
Aspirin is an option in patients in whom long-term anticoagulation is untenable. The ASPIRE trial demonstrated that in patients with unprovoked VTE who had completed a course of initial anticoagulation, aspirin 100 mg daily reduced the risk of major vascular events compared to placebo with no increase in bleeding [96].However, aspirin was not associated with a significant reduction in risk of VTE alone (only the composite vascular event endpoint). The WARFASA trial, however, demonstrated that aspirin 100 mg daily was associated with a significant reduction in recurrent VTE compared to placebo after 6 to 18 months of anticoagulation without an increase in major bleeding [97].The absolute risk of recurrence was 11% in the placebo group and 5.9% in the aspirin group. More recently, the INSPIRE collaboration analyzed data from both trials and found that aspirin after initial anticoagulation reduced the risk of recurrent VTE by approximately 42% with a low rate of major bleeding [98].The absolute risk reduction was even larger in men and older patients. For this reason, we recommend aspirin to those patients in whom indefinite anticoagulation may be warranted from the standpoint of reducing risk of recurrent VTE but in whom the risk of bleeding precludes its use.
Hypercoagulable States In Specific Populations
Inherited Thrombophilias
Patients with a hereditary thrombophilia are at increased risk for incident VTE [99].These inherited mutations result in either a loss of normal anticoagulant function or gain of a prothrombotic state. Hereditary disorders associated with VTE include deficiency of antithrombin, protein C, or protein S, or the presence of factor V Leiden and/or prothrombin G20210A mutations. Although deficiency of protein C, protein S, or antithrombin is uncommon and affects only 0.5% of the population, these states have been associated with a 10-fold increased risk of thrombosis in comparison to the general population. Factor V Leiden and prothrombin gene mutation are less likely to be associated with incident thrombosis (2 to 5-fold increased risk of VTE) and are more prevalent in the Caucasian population [100].Though these hereditary thrombophilias increase risk of VTE, prophylactic anti-coagulation prior to a first VTE is not generally indicated.
Data regarding the impact of the inherited thrombophilias on risk of recurrent VTE is less well defined. While some data suggest that inherited thrombophilias are associated with increased risk of recurrent VTE, the degree of impact may be clinically modest especially in those with heterozygous factor V Leiden or prothrombin gene mutations [101].Ideally, a clinical trial would be designed to assess whether hereditary thrombophilia testing is beneficial for patients with VTE in decision-making regarding length of anticoagulation, type of anticoagulation, and risk of recurrence. If a patient with a low-risk inherited thrombophilia has a DVT in the setting of an additional provoking risk factor (surgery, pregnancy, etc), a 3-month course of anticoagulation followed by D-dimer assessment as above is reasonable. If a patient with an inherited thrombophilia experiences an idiopathic VTE, or if a patient with a “high-risk” thrombophilia as described above experiences any type of VTE, we generally recommend indefinite anticoagulation in the absence of high bleeding risk, though again this is a very patient-dependent choice.
Acquired Thrombophilias
Antiphospholipid Syndrome
Antibodies directed against proteins that bind phospho-lipids are associated with an acquired hypercoagulable state. The autoantibodies are categorized as antiphospho-lipid antibodies (APLAs), which include anticardiolipin antibodies (IgG and IgM), beta-2 glycoprotein 1 antibodies (anti-B2 GP), and lupus anticoagulant. These antibodies can form autonomously, as seen in primary disorders, or in association with autoimmune disease as a secondary disorder.
Criteria have been developed to distinguish antiphospholipid-associated clotting disorders from other forms of thrombophilia. The updated Sapporo criteria depend on both laboratory and clinical diagnostic criteria [102].The laboratory diagnosis of APLAs requires the presence of lupus anticoagulants, anticardiolipin antibodies, or anti-B2 GP on at least 2 assays at least 12 weeks apart with elevation above the 99th percentile of the testing laboratory’s normal distribution [103].Testing for lupus anticoagulant is based on 3 stages, the first of which is inhibition of phospholipid-dependent coagulation tests with prolonged clotting time (eg, aPTT or dilute Russell’s viper venom time). The diagnosis is confirmed by a secondary test in which excess hexagonal phase phospholipids are added to incubate with the patient’s plasma to absorb the APLA [104].The presence of anticardiolipin antibodies and anti beta-2 GP antibodies is determined using ELISA based immunoassays. Unlike most other thrombophilias, antiphospholipid syndrome is associated with both arterial and venous thromboembolic events and may be an indication for lifelong anticoagulation after a first thrombotic event. We generally recommend indefinite anticoagulation in the absence of significant bleeding risk.
Cancer-Associated Hypercoagulable State
Patients with cancer have a propensity for thromboembolic events. The underlying mechanisms responsible for cancer-associated clotting events are multifactorial and an area of intense research. Tumor cells can initiate activation of the clotting cascade through release of tissue factor and other pro-coagulant molecules [105].Type and stage of cancer impact risk of VTE, and the tumor itself can compress vasculature leading to venous stasis. Furthermore, chemotherapy, hormone therapy, antiangiogenic drugs, erythropoietin agents, and indwelling central venous catheters all are associated with increased risk of thrombotic events. Approximately 25% of all cancer patients will experience a thrombotic event during the course of their disease [106]. In fact, the presence of a spontaneous clot may be a harbinger of underlying malignancy [107].Approximately 10% of patients who present with an idiopathic VTE are diagnosed with cancer in the next 1 to 2 years.
The utility of extensive cancer screening in patients with spontaneous clotting events is often debated. The small studies that have addressed cancer associated clots have not demonstrated any mortality benefit with extensive screening. A prospective cohort study addressed the utility of limited versus extensive screening [108].In this study, all patients underwent a series of basic screening tests such as history taking, physical examination, chest radiograph, and basic laboratory parameters. Approximately half of the patients underwent additional testing (CT of chest and abdomen and mammography for women). Screening did not result in increased survival or fewer cancer-related deaths. 3.5 % of patients in the extensive screening group were diagnosed with malignancy in comparison to 2.4% in the limited screening group. During follow-up, cancer was diagnosed in 3.7% and 5.0% in the extensive and limited screening groups, respectively. The authors concluded that the low yield of extensive screening and lack of survival benefit did not warrant routinely ordering cancer screening tests above and beyond age-appropriate screening in patients with idiopathic VTE. However, it is known that identification of occult malignancy at an earlier stage of disease is beneficial, and cancer diagnosed within one year of an episode of VTE is generally more advanced and associated with a poorer prognosis [109].It is our practice to take a through history from patients with unprovoked clots particularly focusing on symptoms suggestive of an underlying cancer. We recommend that patients be up to date with all age-appropriate cancer screening.
Heparin-based products (rather than warfarin) are recommended for long-term treatment of cancer-associated DVT. Several trials, most prominently the CLOT trial, have demonstrated that LMWH is associated with reduced risk of recurrent VTE compared with warfarin in cancer patients [110].Fondaparinux may be a reasonable alternative if a patient is unable to tolerate a LMWH. In terms of treatment duration, patients with cancer-associated VTE should be anticoagulated indefinitely as long as they continue to have evidence of active malignancy and/or remain on antineoplastic treatment [111].
Heparin has potential anticancer effects beyond its anticoagulation properties. It is believed that heparin use in patients with cancer can influence cancer progression by acting as an antimetastatic agent. The molecular mechanisms underlying this significant observation are not completely understood, although the first documented benefit of these drugs dates back to the 1970s [112].Overall, LMWH have been associated with improved overall survival in cancer patients and this effect appears to be distinct from its ability to prevent life-threatening VTE episodes [113].
Estrogen-Related Thromboembolic Disease
Pregnancy is a well-established acquired hypercoagulable state, and thromboembolic disease accounts for significant morbidity and mortality in pregnancy and the postpartum period. Approximately 1 in 1000 women will suffer from a thrombotic event during pregnancy or shortly after delivery [8]. The etiology of the tendency to clot during pregnancy is multifactorial and mainly reflects venous stasis due to vasculature compression by the uterus, changes in coagulation factors as the pregnancy progresses, and endothelial damage during delivery, especially Cesarean section. Both factor VIII and von Willebrand factor levels increase, especially in the final months of pregnancy. Simultaneously, levels of the natural anticoagulant protein S diminish, leading to an acquired resistance to activated protein C which results in increased thrombin generation and therefore a hypercoagulable state [114].The risk of thrombosis in pregnancy is clearly heightened in women with inherited thrombophilias, especially in the postpartum period [115].
Similarly to pregnancy, hormone-based contraceptive agents and estrogen replacement therapies are also associated with increased thrombotic risk. Over the years, drug manufacturers have tried to mitigate the clotting risk associated with these drugs by reducing the amount of estrogen and altering the type of progesterone used, yet a risk still remains, resulting in a VTE incidence 2 to 7 times higher in this population [116].The risk is highest in the first 4 months of use and is unaffected by duration of use; risk extends for 3 months after cessation of estrogen-containing therapy. Patients who develop VTE while taking an oral contraceptive are generally instructed to stop the contraceptive and consider an alternative form of birth control. Although routine screening for thrombophilia is not offered to women before prescribing oral contraceptives, a thorough personal and family history regarding venous and arterial thrombotic events as well as recurrent pregnancy loss in women should be taken to evaluate thromboembolic risk factors. We generally avoid use of oral contraceptives in patients with a known hereditary thrombophilia, and consider screening prior to initiation of therapy in those with a strong family history of VTE.
Superficial VTE
Although the main disorders that comprise VTE are DVT and PE, another common presentation is superficial venous thromboembolism (SVT). The risk factors for developing an SVT are similar to those for DVT. In addition, varicose veins also increase the incidence of developing SVT [117].SVT is not associated with excessive mortality, and the main concern with it is progression to DVT. About 25% of patients diagnosed with SVT may have DVT or PE at the time of diagnosis and about 3% without DVT or PE at time of diagnosis developed one of these complications over the following 3 months; clot propagation is another common complication [118].Ultrasound may be of utility in diagnosing occult DVT in patients who initially diagnosed with SVT [119].
For patients who have only SVT at baseline without concomitant DVT or PE, it is difficult to determine which patients are at risk for developing DVT. Some risk stratification models include clot location. Since SVT clots usually develop in the saphenous vein, the clot would need to either progress from the sapheno-femoral junction to the common femoral vein; thus, any clots located near the sapheno-femoral junction are at risk of progressing into the deep vasculature [120].Clots within 3 cm of the junction may be more likely to progress to DVT [121].Chengelis and colleagues feel that proximal saphenous vein thrombosis should likely be treated with anticoagulation [122].Others have taken a more general approach, stating that all clots above the knee or in the thigh area should be treated aggressively [123].
There are solid data for the use of anticoagulation in SVT. In the STEFLUX (Superficial ThromboEmbolism and Fluxum) study, participants received the LMWH parnaparin at one of 3 doses: 8500 IU once daily for 10 days followed by placebo for 20 days, 8500 IU once daily for 10 days and then 6400 IU once daily for 20 days, or 4250 IU once daily for 30 days. Those who received the intermediate dosing had lower rates of DVT, PE, and relapse/SVT recurrence in the first 33 days [124].In the CALISTO trial, fondaparinux 2.5mg per day for 45 days effectively reduced the risk of symptomatic DVT, PE, or SVT recurrence or extension and was not associated with any increased major bleeding compared to placebo [125].A Cochrane review included 30 studies involving over 6500 participants with SVT of the lower extremities. The treatments used in these studies included fondaparinux, LMWH, UFH, non-steriodal anti-inflammatory agents, topical treatment, and surgery. According to the findings, use of fondaparinux at prophylactic dosing for 6 weeks is considered a valid therapeutic option for SVT [126].It is our practice to consider the use of anticoagulants (generally LMWH or fondaparinux) as part of the treatment regimen for SVT.
Target-Specific Oral Anticoagulants And Treatment of VTE
The direct thrombin inhibitor dabigatran directly binds to thrombin in a concentration-dependent manner [127].Peak plasma concentration is achieved within 0.5 to 2.0 hours after ingestion, and its half-life is 12 to 17 hours. Use of dabigatran in both primary and secondary prevention of VTE has been extensively studied, especially in orthopedic surgery where there have been 4 main trials (RE-MOBILIZE, RE-MODEL, RE-NOVATE, and RE-NOVATE I and II). While RE-MOBILIZE showed that dabigatran 220 mg or 150 mg once daily was inferior to enoxaparin 30 mg twice daily in preventing VTE after total knee arthroplasty, RE-MODEL and RE-NOVATE I and II demonstrated that dabigatran 150 mg or 220 mg once daily was noninferior to enoxaparin 40 mg once daily for prevention of VTE in patients undergoing total knee replacement and hip replacement [128–131].The side effect profile was also promising, with no significant differences in the frequency of major bleeding between dabigatran and enoxaparin. Pooled data and meta-analyses from these trials have demonstrated that for prevention of VTE associated with hip or knee surgery, dabigatran 220 mg or 150 mg once daily is as effective as 40 mg of enoxaparin given daily or 30 mg given twice a day, with a similar bleeding profile [132,133].
More recently, dabigatran been used in the acute treatment and secondary prevention of VTE. In the RE-COVER trial, dabigatran 150 mg twice daily was compared to warfarin (INR 2–3) in the treatment of acute VTE for 6 months, after an initial treatment period of up to 9 days with LMWH or UFH. Dabigatran was noninferior to warfarin with respect to 6-month incidence of recurrent symptomatic objectively confirmed VTE and related deaths, and was not associated with increased bleeding [134].In the RE-MEDY and RE-SONATE trials of extended anticoagulation, dabigatran was as effective as warfarin for prevention of recurrent VTE when continued after 3 months of initial anticoagulation and associated with less bleeding, and was more effective than placebo in preventing recurrent VTE but associated with a higher risk of bleeding [135].Unexpectedly, the risk of acute coronary syndrome was slightly higher in the dabigatran group than the warfarin group, as seen in other studies.
Rivaroxaban, a TSOAC that targets factor Xa, has also shown efficacy in preventing VTE after knee or hip surgery. The RE-CORD 1-4 studies all focused on the use of rivaroxaban in comparison to enoxaparin and found that rivaroxaban 10 mg once daily was superior to enoxaparin 40 mg once daily in prevention of VTE in total knee and total hip arthroplasty [136–138].Meta-analysis of multiple rivaroxaban VTE prophylaxis trials also demonstrated that rivaroxaban significantly lowered the risk of VTE in these surgical patients in comparison to the use of enoxaparin [139].Prophylactic use of rivaroxaban was also studied in acutely ill hospitalized patients in the MAGELLAN trial. Rivaroxaban 10 mg daily for 35 days was compared to enoxaparin 40 mg daily for 10 days followed by placebo and was found to be noninferior to enoxaparin in reduction of VTE risk at day 10 and superior to placebo at day 35 [140].However, the rate of bleeding, although low in both arms, was higher in the rivaroxaban arm.
Rivaroxaban has been studied in randomized clinical trials for acute treatment of DVT and PE and for extended prophylaxis for recurrent VTE (EINSTEIN-DVT, EINSTEIN-PE and EINSTEIN-Extension, respectively). The treatment strategy for use of rivaroxaban differed from that of dabigatran (in the RE-COVER trial), as rivaroxaban was used upfront as initial anticoagulation rather than after an initial period of parenteral therapy with LMWH or UFH. In both the DVT and PE trials, rivaroxaban was noninferior to standard treatment with enoxaparin followed by warfarin therapy, with no significant difference in major bleeding at 6 months of treatment [141,142].The extension trial also demonstrated that use of rivaroxaban in comparison to placebo for an additional 6 or 12 months after standard therapy was associated with significantly fewer recurrent VTE [141]. These studies led to FDA approval for rivaroxaban for primary prevention of VTE in patients undergoing elective total hip or knee repair surgery, for treatment of acute DVT or PE, and for extended prophylaxis in patients following initial treatment.
The anti-factor Xa TSOAC apixaban has been studied in similar fashion as rivaroxaban. In the AMPLIFY study, apixaban was given at a dose of 10 mg twice daily for 7 days followed by 5 mg twice daily for 6 months (as monotherapy, without initial parenteral agent) and compared to enoxaparin followed by warfarin for treatment of acute VTE. Apixaban was as effective as warfarin in terms of recurrent symptomatic VTE or VTE-related death, and was associated with significantly fewer bleeding events [143].Extended-duration apixaban given at treatment dose (5 mg twice daily) or at prophylactic dose (2.5 mg twice daily) for 12 months after completion of treatment-dose apixaban for VTE demonstrated superiority to placebo for extended prophylaxis in AMPLIFY-EXT, and there was no increase in major bleeding compared to placebo [144].Apixaban was recently approved by the FDA for both treatment and secondary prophylaxis of VTE.
More recently, a third anti-factor Xa TSOAC edoxaban demonstrated noninferiority to warfarin in prevention of recurrent symptomatic VTE when administered to patients with DVT or PE at 60 mg once daily for 3 to 12 months [145].Edoxaban also led to significantly less bleeding than warfarin. Edoxaban was recently approved by the FDA for treatment of VTE.
These TSOACs show promise in treatment and prevention of VTE but should be used in patients who meet appropriate criteria for renal function, age, and bleeding risk, as there are currently no available antidotes to reverse their effects. If significant bleeding occurs and cannot be controlled by usual maneuvers such as mechanical compression or surgical intervention, there is little data to guide the use of pharmacologic interventions. Plasma dabigatran levels can be reduced through the use of hemodialysis [146].Antibodies capable of neutralizing dabigatran have been developed, and one specific antibody, idarucizumab, was well-tolerated and showed immediate and complete reversal of dabigatran in subjects of different age and renal function [147,148].Andexanet, a modified recombinant derivative of factor Xa with no catalytic activty, acts as a “decoy receptor” with higher affinity to factor Xa inhibitors than natural factor Xa. Phase II studies in healthy volunteers demonstrated that andexanet immediately reversed the anticoagulation activity of apixaban, rivaroxaban, enoxaparin, and most recently edoxaban without thrombotic consequences [149].Two randomized, double-blind, placebo-controlled phase III studies (ANNEXA-A, looking at the reversal of apixaban, and ANNEXA-R, looking at reversal of rivaroxaban) are underway, and preliminary results show that a single intravenous bolus of andexanet demonstrated almost complete reversal [150].Finally, aripazine (PER977), a synthetic small molecule that binds to heparins as well as all TSOACs, was shown in a phase II trial to decrease blood clotting time to within 10% above baseline value in 10 minutes or less with an effect lasting for 24 hours [151].
Some have advocated for use of prothrombin complex concentrate (PCC) or recombinant factor VIIa for reversal of TSOAC-associated bleeding. Rivaroxaban was demonstrated to be partially reversible by PCC, whereas this approach was not as successful for dabigatran in healthy volunteers [152].In vitro evidence, however, showed that PCC did not significantly change aPTT [153].At present, the use of nonspecific hemostatic agents (including recombinant factor VIIa, 4-factor prothrombin complex concentrate, and activated prothrombin complex concentrates) is suggested for reversal of TSOACs in patients who present with life-threatening bleeding [154,155].
Conclusion
Patients with VTE present with a wide range of findings and factors that impact management. Decision making in VTE management is a fluid process that should be re-evaluated as new data emerge and individual circumstances change. There is more focus on VTE prevention and treatment today than there was even a decade ago. Diagnostic algorithms, identification of new risk factors, refinement in understanding of the pathogenesis of thrombosis, and identification of new anticoagulants with more favorable risk-benefit profiles will all ultimately contribute to improved patient care.
Corresponding author: Jean M. Connors, MD, Brigham and Women's Hospital, 75 Francis St., Boston, MA 02215.
1. White RH. The epidemiology of venous thromboembolism. Circulation 2003;107(Suppl 1):I4-8.
2. Moster KM and Fedullo PF. The diagnosis of deep-vein thrombosis. N Engl J Med 1994;330:863–4.
3. Kearon C. Natural history of venous thromboembolism. Circulation 2003;(Suppl 1):122–30.
4. Heijboer H, Brandjes DP, Buller HR, et al. Deficiencies of coagulation-inhibiting and fibrinolytic proteins in outpatients with deep-vein thrombosis. N Engl J Med 1990;323:1512–6.
5. Mateo J, Oliver A, Borrell M, et al. Laboratory evaluation and clinical characteristics of 2,132 consecutive unselected patients with venous thromboembolism—results of the Spanish Multicentric Study on Thrombophilia (EMET-Study). Thromb Haemost 1997;77:441–51.
6. Lyman GH. Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis. Cancer 2011;117:1334–49.
7. White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost 2003;90:446–55.
8. Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med 2008;359:2025–33.
9. Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 2008;117:93–102.
10. Mahmoodi BK, Gansevoort RT, Naess IA, et al. Association of mild to moderate chronic kidney disease with venous thromboembolism: pooled analysis of five prospective general population cohorts. Circulation 2012;126:1964–71.
11. Landolfi R, Marchioli R, Patrono C. Mechanisms of bleeding and thrombosis in myeloproliferative disorders. Thromb Haemost 1997;78:617–21.
12. Hillmen P, Lewis SM, Bessler M, et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med1995;333:1253–8.
13.Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet 2010;375:657–63.
14. Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost 2008;6:1262–6.
15. Kibbe MR, Ujiki M, Goodwin AL, et al. Iliac vein compression in an aymptomatic patient population. J Vasc Surg 2004;39:937–43.
16. Chee YL, Culligan DJ, Watson HG. Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br J Haematol 2001;114:878–80.
17. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ 2013;347:f5298.
18. Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004;292:1573–80.
19. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 1998;90:1371–88.
20. Glueck CJ, Richardson-Royer C, Schultz R, et al. Testosterone therapy, thrombophilia-hypofibrinolysis, and hospitalization for deep venous thrombosis-pulmonary embolus: an exploratory, hypothesis-generating study. Clin Appl Thromb Hemost 2014;20:244–9.
21. Johannesdottir SA, Horvath-Puho E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med 2013;173:743–52.
22. Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 2006;21:722–7.
23. Ocak G, Vossen CY, Verduijn M, et al. Risk of venous thrombosis in patients with major illnesses: results from the MEGA study. J Thromb Haemost 2013;11:116-23.
24. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107 (Suppl 1): I9–16.
25. Clemetson KJ. Platelet GPIb-V-IX complex. Thromb Haemost 1997;78:266–70.
26. Kroll MH, Schafer AI. Biochemical mechanisms of platelet activation. Blood 1989;74:1181–95.
27. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997;350:1795–8.
28. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients’ probability of pulmonary embolism: increasing the model’s utility with the SimpliRED D-dimer. Thromb Haemost 2000;416–20.
29. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med 2006; 144:165–71.
30. Stein PD, Hull RD, Patel KC, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med 2004;140:589–602.
31. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res 2010;125:e123–27.
32. Crowther MA, Cook DJ, Griffith LE, et al. Neither baseline tests of molecular hypercoagulability nor D-dimer levels predict deep venous thrombosis in critically ill medical-surgical patients. Intensive Care Med 2005;31:48–55.
33. Karami-Djurabi R, Klok FA, Kooiman J, et al. D-dimer testing in patients with suspected pulmonary embolism and impaired renal function. Am J Med 2009;122:1050–3.
34. Chan WS, Chunilal S, Lee A, et al. A red blood cell agglutination D-dimer test to exclude deep venous thrombosis in pregnancy. Ann Intern Med 2007;147:165–70.
35. Righini M, Goehring C, Bounameaux H, Perrier A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med 2000; 109:357–61.
36. Woller SC, Stevens SM, Adams DM, et al. Assessment of the safety and efficiency of using an age-adjusted D-dimer threshold to exclude suspected pulmonary embolism. Chest 2014; 146:1444–51.
37. Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation 2004; 109 (Suppl 1): I9–14.
38. Hirsh J, Lee AY. How we diagnose and treat deep vein thrombosis. Blood 2002;99:3102–10.
39. Tapson VF, Carroll BA, Davidson BL, et al. The diagnostic approach to acute venous thromboembolism. Clinical practice guideline. American Thoracic Society. Am J Respir Crit Care Med 1999;160:1043–66.
40. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e351S–418S.
41. Pezzullo JA, Perkins AB, Cronan JJ. Symptomatic deep vein thrombosis: diagnosis with limited compression US. Radiology 1996;198:67–70.
42. Frederick MG, Hertzberg BS, Kliewer MA, et al. Can the US examination for lower extremity deep venous thrombosis be abbreviated? A prospective study of 755 examinations Radiology 1996;199:45–7.
43. Remy-Jardin M, Remy J, Deschidre F, et al. Diagnosis of pulmonary embolism with spiral CT: comparison with pulmonary angiography and scintigraphy. Radiology 1996;200:699–706.
44. Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:2317–27.
45. Goodacre S. In the clinic. Deep venous thrombosis. Ann Intern Med 2008;149:ITC3–1.
46. Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med 2010;363:266–74.
47. Kucher N. Clinical practice. Deep-vein thrombosis of the upper extremities. N Engl J Med 2011;364:861–9.
48. Constans J, Salmi LR, Sevestre-Pietri MA, et al. A clinical prediction score for upper extremity deep venous thrombosis. Thromb Haemost 2008;99:202–7.
49. Meyer G, Vicault E, Danasys T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402–11.
50. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-bline, placebo-controlled randomized trial. J Thromb Haemost 2014;12:459–68.
51. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012;141(2 Suppl):7S–47S.
52. Bounameaux H. Unfractionated versus low-molecular-weight heparin in the treatment of venous thromboembolism. Vasc Med 1998;3:41–6.
53. Erkens PM, Prins MH. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev 2010;9:CD001100.
54. Buller HR, Davidson BL, Decousus H, et al. Fondaprinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med 2004;140: 867–73.
55. Buller HR, Davidson BL, Decousus H, et al. Subcutaenous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med 2003;349:1695–702.
56. Bates SM, Weitz JI. Coagulation assays. Circulation 2005;112:e53–60.
57. Pon TK, Dager WE, Roberts AJ, White RH. Subcutaneous enoxaparin for therapeutic anticoagulation in hemodialysis patients. Thromb Res 2014;133:1023–8.
58. Speeckaert MM, Devreese KM, Vanholder RC, Dhondt A. Fondaparinux as an alternative to vitamin K antagonists in haemodialysis patients. Nephrol Dial Transplant 2013;28:3090–5.
59. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005;172:1041–6.
60. Jiminez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–9.
61. Schulman S. Advances in the management of venous thromboembolism. Best Pract Res Clin Haematol 2012;25:361–77.
62. Erkens PM, Gandara E, Wells P, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost 2010;8:2412–7.
63. Chan YC, Valenti D, Mansfield AO, Stansby G. Warfarin induced skin necrosis. Br J Surg 2000; 87:266–72.
64. Brandjes DP, Heijboer H, Buller HR, et al. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal-vein thrombosis. N Engl J Med 1992;327:1485–9.
65. Segal JB, Streiff MB, Hofmann LV, et al. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med 2007;146:211–22.
66. Pinede L, Duhaut P, Cucherat M, et al. Comparison of long versus short duration of anticoagulant therapy after a first episode of venous thromboembolism: a meta-analysis of randomized, controlled trials. J Intern Med 2000;247:553–62.
67. Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of anticoagulation trial study group. N Engl J Med 1995;332:1661–5.
68. Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011;342:d3036.
69. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-07.
70. Agnelli G, Prandoni P, Becattini C, et al. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 2003;139:19–25.
71. Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin optimal duration Italian trial investigators. N Engl J Med 2001;345:165–9.
72. Bauer KA. Long-term management of venous thromboembolism: a 61-year old woman with unprovoked venous thromboembolism. JAMA 2011;305:1336–45.
73. Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014;123:
1794–801.
74. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest 2008;133(6 Suppl):257S–298S.
75. Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 1993;95:315–28.
76. Ruiz-Gimenez N, Suarez C, Gonzalez R, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE registry. Thromb Haemost 2008;100:26–31.
77. Kearon C, Akl EA, Comerota AJ. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e419S–94S.
78. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess one-year risk of major bleeding in atrial fibrillation patients: The Euro Heart Survey. Chest 2010;138:1093–1100.
79. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006;151:713–9.
80. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and risk factors in atrial fibrillation) study. J Am Coll Cardiol 2011;58:395–401.
81. Caldeira D, Costa J, Fernandes RM, et al. Performance of the HAS-BLED high bleeding-risk category, compared to ATRIA and HEMORR2HAGES in patients with atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol 2014;40:277–84.
82. Douketis JD, Crowther MA, Foster GA, Ginsberg JS. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med 2001;110:515–9.
83. Palareti G, Legnani C, Cosmi B, et al. Risk of venous thromboembolism recurrence: high negative predictive value of D-dimer performed after oral anticoagulation is stopped. Thromb Haemost 2002; 87:7–12.
84. Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006;355:1780–9.
85. Bruinstroop E, Klok FA, Van De Ree MA, et al. Elevated D-dimer levels predict recurrence in patients with idiopathic venous thromboembolism: a meta-analysis. J Thromb Haemost 2009;611–8.
86. Verhovsek M, Douketis JD, Yi Q, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med 2008;149:481–90.
87. Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012;10:1019–25.
88. Eichinger S, Heinze G, Jandeck LM, et al. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna Prediction Model. Circulation2010; 121:1630–6.
89. Eichinger S, Heinze G, Kyrle PA. D-dimer levels over time and the risk of recurrent venous thromboembolism: an update of the Vienna prediction model. J Am Heart Assoc 2014; 3: e000467.
90. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med 2010;153:523–31.
91. Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002;137:955–60.
92. Prandoni P, Prins MH, Lensing AW, et al. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med 2009;150:577–85.
93. Marcucci M, Iorio A, Douketis J. Management of patients with unprovoked venous thromboembolism: an evidence-based and practical approach. Curr Treat Options Cardiovasc Med 2013;15:224–39.
94. Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003;348:1425–34.
95. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003;349:631–9.
96. Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012;367:1979–87.
97. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012;366:1959–67.
98. Simes J, Becattini C, Agnelli G, et al. Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation 2014;130:1062–71.
99. Makris M. Thrombophilia: grading the risk. Blood 2009;113:5038–9.
100. Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA 2009;30:2472.
101. Ho WK, Hankey GH, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med 2006;166:729–36.
102. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
103. Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on lupus anticoagulant/antiphospholipid antibody of the scientific and standardization committee of the international society on thrombosis and haemostasis. J Thromb Haemost 2009; 7:1737–40.
104. Brandt JT, Barna LK, Triplett DA. Laboratory identification of lupus anticoagulants: results of the Second International Workship for identification of lupus anticoagulants. On behalf of the subcommittee on lupus anticoagulants/antiphospholipid antibodies of the ISTH. Thromb Haemost 1995;74: 1597–603.
105. Gale AJ, Gordon SG. Update on tumor cell procoagulant factors. Acta Haematol 2001;106:25-32.
106. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood 2013;122:1712–23.
107. Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation 2003; 107 (Suppl 1): I17–21.
108. Van Doormaal FF, Terpstra W, Van Der Griend R, et al. Is extensive screening for cancer in idiopathic venous thromboembolism warranted? J Thromb Haemost 2011;9:79–84.
109. Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846–50.
110. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146-53.
111. Streiff MB, Bockenstedt PL, Cataland SR, et al. Venous thromboembolic disease. J Natl Compr Canc Netw 2013;11:1402-29.
112. Elias EG, Sepulveda F, Mink IB. Increasing the efficiency of cancer chemotherapy with heparin: “clinical study.” J Surg Oncol 1973;5:189–93.
113. Lazo-Langner A, Goss GD, Spaans JN, Rodger MA. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J Thromb Haemost 2007;5:729–37.
114. Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol 2003;16:153–68.
115. Gerhardt A, Scharf RE, Beckmann MW, et al. Prothrombin and factor V mutations in women with a history of thrombosis during pregnancy and the puerperium. N Engl J Med 2000;342:374–80.
116. Rott H. Thrombotic risks of oral contraceptives. Curr Opin Obstet Gynecol 2012;24:235-40.
117. Marchiori A, Mosena L, Prandoni P. Superficial vein thrombosis: risk factors, diagnosis, and treatment. Semin Thromb Hemost 2006;32:737–43.
118. Decousus H, Quere I, Presles E, et al. Superficial venous thrombosis and venous thromboembolism: a large, prospective epidemiologic study. Ann Intern Med 2010;152:218–24.
119. Galanaud JP, Genty C, Sevestre MA, et al. Predictive factors for concurrent deep-vein thrombosis and symptomatic venous thromboembolic recurrence in case of superficial venous thrombosis. The OPTIMEV study. Thromb Haemost 2011;105:31–9.
120. Sullivan V, Denk PM, Sonnad SS, et al. Ligation versus anticoagulation: treatment of above-knee superficial thrombophlebitis not involving the deep venous system. J Am Coll Surg 2001;193:556–62.
121. Lohr JM, McDevitt DT, Lutter KS, et al. Operative management of greater saphenous thrombophlebitis involving the saphenofemoral junction. Am J Surg 1992;164:269–75.
122. Chengelis DL, Benedick PJ, Glover JL, et al. Progression of superficial venous thrombosis to deep vein thrombosis. J Vasc Surg 1996;24:745–9.
123. Verlato F, Zuccetta P, Prandoni P, et al. An unexpectedly high rate of pulmonary embolism in patients with superficial thrombophlebitis of the leg. J Vasc Surg 1999;30:1113–5.
124. Cosmi B, Filippini M, Tonti D, et al. A randomized double-blind study of low-molecular-weight heparin (parnaparin) for superficial vein thrombosis: STEFLUX (Superficial ThromboEmbolism and Fluxum). J Thromb Haemost 2012;10:1026–35.
125. Decousus H, Prandoni P, Mismetti P, et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med 2010;363:1222–32.
126. Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev 2013;4:CD004982.
127. Stangier J, Rathgen K, Stahle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007;64:292–303.
128. RE-MOBILIZE Writing Committee, Ginsberg JS, Davidson BL, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009;24:1–9.
129. Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007;5:2178–85.
130. Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomized, double-blind, non-inferiority trial. Lancet 2007;370:949–56.
131. Eriksson BI, Dahl OE, Huo MH, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomized, double-blind, non-inferiority trial. Thromb Haemost2011;105:721–9.
132. Friedman RJ, Dahl OE, Rosencher N, et al. Dabigatran versus enoxaparin for prevention of venous thromboembolism after hip or knee arthroplasty: a pooled analysis of three trials. Thromb Res 2010;126:175–82.
133. Wolowacz SE, Roskell NS, Plumb JM, et al. Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following total hip or knee arthroplasty. A meta-analysis. Thromb Haemost 2009;101:77–85.
134. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;36:2342–52.
135. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013;368:709–18.
136. Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765–75.
137. Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomized controlled trial. Lancet 2008;372:31–9.
138. Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776–86.
139. Cao YB, Zhang JD, Shen H, Jiang YY. Rivaroxaban versus enoxaparin for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 2010;66:1099–108.
140. Cohen AT, Spiro TE, Buller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013;368:513–23.
141. EINSTEIN Investigators, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510.
142. EINSTEIN-PE Investigators, Buller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–97.
143. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799–808.
144. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699–708.
145. Hokusai-VTE investigators, Buller HR, Decousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406–15.
146. Khadzhynov D, Wagner F, Formella S, et al. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost 2013;109:596–605.
147. Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood 2013;121:3554–62.
148. Glund S, Stangier J, Schmohl M, et al. Idarucizumab, a specific antidote for dabigatran: immediate, complete, and sustained reversal of dabigatran induced anticoagulation in elderly and renally impaired subjects. Presented at the American Society of Hematology 2014 Annual Meeting; San Francisco, CA.
149. Crowther MA, Levy GG, Lu G, et al. A phase 2 randomized, double-blind, placebo-controlled trial demonstrating reversal of edoxaban-induced anticoagulation in healthy subjects by andexanet alfa (PRT064445), a universal antidote for factor Xa (fXa) inhibitors. Abstract #4269. Presented at the American Society of Hematology 2014 Annual Meeting, San Francisco, CA.
150. Crowther M, Levy GG, Lu G, et al. ANNEXATM-A: A phase 3 randomized, double-blind, placebo-controlled trial, demonstrating reversal of apixaban-induced anticoagulation in older subjects by andexanet alfa (PRT 064445), a universal antidote for factor Xa (fa) inhibitors. Presented at the American Heart Association 2014 Annual Meeting, Chicago, IL.
151. Ansell JE, Bakhru SH, Lauliche BE, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med 2014;371:2141–2.
152. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011;124:1573–9.
153. Korber MK, Langer E, Ziemer S, et al. Measurement and reversal of prophylactic and therapeutic peak levels of rivaroxaban: an in vitro study. Clin Appl Thromb Hemost 2014;20:735–40.
154. Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013;80:443–51.
155. Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood 2014;123:1152–8.
From the Brigham and Women’s Hospital and Dana-Farber Cancer Institute, Boston, MA.
Abstract
- Objective: To review the diagnosis and management of venous thromboembolism (VTE).
- Methods: Review of the literature.
- Results: VTE and its associated complications account for significant morbidity and mortality. Various imaging modalities can be employed to support a diagnosis of a VTE and are used based on clinical suspicion arising from the presence of signs and symptoms. Clinical decision rules have been developed that can help determine which patients warrant further testing. Anticoagulation, the mainstay of VTE treatment, increases bleeding risk, necessitating tailored treatment strategies that must incorporate etiology, risk, benefit, cost, and patient preference.
- Conclusion: Further study is needed to understand individual patient risks and to identify treatments that will lead to improved patient outcomes.
Venous thromboembolism (VTE) and its associated complications account for significant morbidity and mortality. Each year between 100 and 180 persons per 100,000 in Western countries develop VTE. The majority of VTEs are classified as either pulmonary embolism (PE), which accounts for one third of the events, or deep vein thrombosis (DVT), which is responsible for the remaining two thirds. Between 20% and 30% of those patients diagnosed with thrombotic events will die within the first month after diagnosis [1].PE is a common consequence of DVT; 40% of patients who are diagnosed with DVT will be subsequently found to have PE upon further imaging. This high rate of association is also seen in those who present with PE, 70% of whom will also be found to have concomitant DVT [2,3].
Anatomic risk factors include Paget-Schroetter syndrome (compression of upper extremity veins due to abnormalities at the thoracic outlet), May-Thurner syndrome (significant compression of the left common iliac vein by the right common iliac artery), and abnormalities of the inferior vena cava [14–16].Medications that are associated with increased risk of VTE include but are not limited to estrogen (both in oral contraceptives as well as hormone replacement therapy) [17,18],the selective estrogen receptor modulator tamoxifen [19],testosterone [20],and glucocorticoids [21].It is important to note that many patients with VTE have more than one acquired risk factor for thrombosis [22],and also that acquired risk factors are more likely to lead to VTE in the setting of underlying inherited thrombophilic conditions [23].
Pathogenesis
Abnormalities in both coagulation factors and the vascular bed are at the core of the pathogenesis of VTE. The multifaceted etiology of thrombosis was first described in 1856 by Virchow, who defined a triad of defects in the vessel wall, platelets, and coagulation proteins [24].Usually the vessel wall is lined with endothelial cells that provide a nonthrombotic surface and limit platelet aggregation through release of prostacyclins and nitric oxide. When the endothelial lining is compromised, the homeostatic surveillance system is disturbed and platelet activation and the coagulation system are initiated. Tissue factor exposure in the damaged area of the vessel leads to activation of the coagulation cascade. Collagen that is present in the area of the wound is also exposed and can activate platelets, which provide the phospholipid surface upon which the coagulation cascade occurs. Platelets initially tether to the exposed collagen through binding of glycoprotein Ib-V-IX in association with von Willebrand factor [25].The thrombus is initiated as more platelets are recruited to exposed collagen of the injured endothelium through aggregation in response to the binding of GPIIIb/IIa with fibrinogen. This process is self-perpetuating as these activated platelets release additional proteins such as adenosine diphosphate (ADP), serotonin, and thromboxane A2, all of which fuel the recruitment and activation of additional platelets [26].
Diagnosis
The key to decreasing the morbidity and mortality associated with VTE is timely diagnosis and early initiation of therapy. Various imaging modalities can be employed to support a diagnosis of a VTE and are used based on clinical suspicion arising from the presence of signs and symptoms. DVT is usually associated with pain in calf or thigh, unilateral swelling, tenderness, and redness. PE can present as chest pain, shortness of breath, syncope, hemoptysis, and/or cardiac palpitations.
Decision Rules
Clinical decision rules based on signs, symptoms, and risk factors have been developed to estimate the pretest probability of PE or DVT and to help determine which patients warrant further testing. These clinical decision rules include the Wells criteria (separate rules for DVT and PE) [27,28],as well as the Geneva score [29],which is focused on identifying patients with a likelihood of having a PE. In general, these clinical rules are applied at presentation to predict the risk of VTE, and patients who score high are evaluated by imaging modalities, while those with lower scores should be considered for further stratification based on D-dimer testing. The goal of clinical assessment and use of a decision rule is to identify patients at low risk of VTE to reduce the number of imaging studies performed. Most of the decision rules focus on the use of noninvasive evaluations that are easily implemented, including clinical history and presentation, abnormalities in oxygen saturation, chest radiography findings, and electro-cardiography.
D-Dimer Testing
D-dimer testing is at the core of all predictive models for VTE. D-dimer is a fibrin degradation product that is detectable in the blood during active fibrinolysis and occurs after clot formation. The concentration of D-dimer increases in patients with active clot. D-dimer testing is usually performed as a quantitative ELISA or automated turbidometric assay and is highly sensitive (> 95%) in excluding a diagnosis of VTE if results are in the normal range [30].The presence of a normal D-dimer and a low probability based on clinical assessment criteria can be integrated to determine which patients have a low (generally < 99%) likelihood of having VTE [31].It should be noted that other factors can lead to an increased D-dimer, including malignancy, trauma, critical illness, disseminated intravascular coagulation, pregnancy, infection, and postoperative status, which can produce false-positive results and cloud the utility of the test in excluding those at low risk of VTE from undergoing imaging [32–34].Additionally, D-dimer values naturally increase with age and recent work has shown utility of an age-adjusted D-dimer threshold, though this method is not yet widespread in clinical practice [35,36].
Imaging
After application of a clinical prediction rule, the mainstay of diagnosis of VTE is imaging. For DVT the use of ultrasonography is considered the gold standard, with both high sensitivity (89–100%) and specificity (86–100%), especially when the DVT is located proximally [37–39].We generally recommend compression ultrasound starting with the proximal veins but expanding to include the whole leg if the proximal studies are negative [40–42].Other diagnostic options include computed tomography (CT) venography, which is not first line as it is highly invasive and exposes the patient to iodine-based contrast dyes, and magnetic resonance venography (MRV), which offers superb visualization for diagnosis of pelvic vein thrombosis but is limited because of availability and cost issues.
Helical CT pulmonary angiography (CTPA) is the diagnostic test of choice in PE, with high sensitivity (96%) and specificity (95%), and has replaced conventional ventilation perfusion (VQ) scanning or other methods such as magnetic resonance pulmonary angiography in most settings [43,44].CTPA should be avoided in patients who have severe chronic kidney disease or a contrast allergy, and is often avoided in patients who are pregnant due to potential risk of radiation exposure, and in such situations VQ scanning may be employed.
Algorithmic Approach to Workup
Of note, there are multiple clinical situations in which the application of a clinical prediction rule followed by D-dimer testing and/or imaging cannot be “standardized” with such algorithms. These include situations where D-dimer may be falsely positive (as above), situations in which alternative imaging strategies should be used to avoid contrast exposure in workup of PE (as above), and workup of suspected upper extremity DVT. Upper extremity ultrasound comprises about 10% of all DVT and frequently occurs in the setting of risk factors such as central venous catheters or pacemakers; specific upper-extremity risk-assessment rules have been developed [47,48].
Acute Treatment Options
The first step in treatment is identification of patients who are at high risk of
In standard cases of DVT and PE without hemodynamic compromise, the current standard of care is to initiate parenteral anticoagulation. The immediate goal of therapy is to treat rapidly with anticoagulants to prevent the thrombus from propagating further and to prevent DVT from embolization to the lungs or other vascular beds. The initial treatment of VTE has been extensively discussed and guidelines have been established with recommendations for initiation of anticoagulation; the American College of Chest Physicians (ACCP) released the 9th edition of their guidelines in 2012 based on consensus agreements derived from primary data [51].
Heparin-based drugs are the mainstay of initial treatment. These drugs act by potentiating antithrombin and therefore inactivating thrombin and other coagulation factors such as Xa. Unfractionated heparin (UFH) can be administered as an initial bolus followed by a continuous infusion with dosing being based on weight and titrated to activated partial thromboplastin time (aPTT) or the anti-factor Xa level. Alternatively, patients may be treated with a low molecular weight heparin (LMWH) administered subcutaneously in fixed weight-adjusted doses, which obviates the need for monitoring in most cases [52].LMWHs work in a similar manner to UFH but have more anti-Xa activity in comparison to anti-thrombin activity. LMWH appears to be more effective than UFH for initial treatment of VTE and has been associated with lower risk of major hemorrhage [53].The options for treatment of VTE have expanded in recent years with the approval of fondparinux, a pentasaccharide specifically targeted to inhibit factor Xa. Fondaparinux has been shown to have similar efficacy to LMWH in patients with DVT [54],and while it has not been evaluated directly against LMWH for initial treatment of PE it has been shown to be at least as effective and safe as UFH [55].
Both LMWH and fondaparinux are cleared renally and therefore have increased bleeding risk in patients with renal impairment. In patients with creatinine clearance of less than 30 mL/min, dose reduction or lengthening of dosing interval may be appropriate. Anti-factor Xa activity can be used as a functional assay to monitor and titrate the level of anticoagulation in patients treated with UFH, LMWH, and fondaparinux. Monitoring is useful in the setting of impaired renal function (as above) in addition to extremes of body weight and pregnancy. When used for monitoring of UFH, the anti-factor Xa activity can be measured at any time during administration with a therapeutic goal range of 0.3–0.7 international units (IU)/mL. When used for LMWH, a “peak” anti-factor Xa should be measured approximately 4 hours after dosing, with therapeutic goals depending on preparation and schedule of treatment but generally between 0.6 to 1.0 IU/mL for twice daily and around 1.0 -2.0 IU/mL for once-daily [56].For patients on dialysis, we generally use intravenous UFH for acute treatment of VTE, though recent work has shown that enoxaparin (doses of 0.4 to 1 mg/kg/day) was as safe as UFH with respect to bleeding and was associated with shorter hospital length of stay [57].For long-term treatment of VTE, warfarin is generally preferred based on clinical experience with this agent, though small studies have suggested that parenteral agents may be useful alternatives to warfarin [58].
In many patients who are clinically stable without significant medical comorbidities, outpatient administration of these medications without hospitalization is considered safe. Patients with DVT are often safe to manage as outpatients unless significant clot burden is present and thrombolysis is being considered. For PE, the pulmonary embolism severity index (PESI) and simplified index (sPESI) may be useful to risk-stratify patients and identify those at low risk of complications who may be suitable for outpatient treatment [59,60].Studies have shown that hemodynamically stable patients who did not require supplemental oxygenation or have contraindications to LMWH therapy were safely managed as outpatients with low risk of recurrent VTE and bleeding [61,62].One exception may be patients with intermediate risk PE, who are hemodynamically stable but have evidence of right ventricular dysfunction and may be better served by an initial in-hospital observation period, especially if thrombolysis is being considered.
Most patients who present with VTE are transitioned to warfarin for long-term therapy. Warfarin can be started on the same day as parenteral anticoagulation. Both drugs are overlapped for at least 5 days, with a target INR of 2.0–3.0. Patients may achieve the target INR level quickly because factor VII has a short half-life and the level drops quickly; however, the overlap of 5 days is essential even when the INR is in the target range because a full anticoagulant affect is not achieved until prothrombin levels decline, and this is a slow process due to the long half-life of prothrombin. Warfarin also causes rapid decrease in levels of natural anticoagulants such as protein C and protein S, which further exacerbates the net hypercoagulable state in the short-term. Warfarin without a bridging parenteral agent carries a risk of warfarin-induced skin necrosis [63]and is not effective as an initial anticoagulant treatment in acute VTE as there is a relatively high risk of symptomatic clot extension or recurrent VTE compared to warfarin with use of a bridging agent [64].In specific cases such as cancer-associated VTE (see discussion below), LMWH is preferred to warfarin for long-term active therapy.
Long-Term Active Therapy After Acute Treatment
Duration of Anticoagulation
Recommended duration of anticoagulation depends on a myriad of factors including severity of VTE, risk of recurrence, bleeding risk, and lifestyle modification issues, as well as on the safety and availability of alternative therapies such as low-intensity warfarin, aspirin, or the new oral anticoagulants. The decision tree for length of treatment starts with whether the VTE was a provoked or a spontaneous event. Provoked events occur when the event is associated with an identifiable risk factor, such as immobilization from prolonged medical illness or surgical intervention, pregnancy or oral contraceptive use, and prolonged air travel.
Consensus guidelines suggest that 3 months of anti-coagulation are generally sufficient treatment for a provoked VTE [51,65,66]. Data from multiple studies and a meta-analysis suggests that less than 3 months of anticoagulation (4 to 6 weeks in most trials) is associated with an approximately 1.5-fold higher risk of recurrent VTE than 3 months [67,68].However, data from this meta-analysis also suggests that anticoagulation for longer than 3 months (6 to 12 months in most trials) is not associated with higher rates of recurrent VTE. We generally anticoagulate for 3 months in patients with provoked VTE.
Determining the duration of anticoagulation is more complex in patients with idiopathic/unprovoked VTE. Kearon and colleagues found that in patients with first idiopathic VTE, patients who were anticoagulated for 24 months versus 3 months had lower risk of recurrent VTE (1.3% per patient-year with 24 months versus 27.4% per patient-year with 3 months) [69].Similar studies and meta-analyses have demonstrated decreased recurrence rates in patients anticoagulated for a prolonged period of time. However, one study of prolonged anticoagulation revealed that at 3 years there was no difference in recurrence rate in patients with PE who were anticoagulated for 6 months versus 1 year [70].The likelihood of recurrent DVT in patients with first episode of idiopathic proximal DVT treated with either 3 months or 12 months of warfarin was similar after treatment was discontinued [71].Prolonged periods of anticoagulation do not directly influence risk of recurrence but instead may only delay occurrence of a second event [72].For that reason, the decision is essentially whether to anticoagulate for 3 months or to continue therapy indefinitely [73]. Current guidelines recommend continuing anticoagulation for 3 months in those at high risk of bleeding, and continuing for an extended duration in those at low or moderate bleeding risk [51]. Patients' values and perferences should be entertained and decisions made on a patient-by-patient basis.
For patients at high risk of recurrent VTE, we generally recommend indefinite anticoagulation unless the patient has a significantly elevated bleeding risk or strongly prefers to discontinue anticoagulation and compliance concerns are evident. High-risk patients are those who have suffered from multiple episodes of recurrent VTE, those who have clotted while being anticoagulated, and those with acquired risk factors, such as antiphospholipid antibodies and malignancy. Other high-risk groups are those with high-risk thrombophilias such as deficiency of protein S, protein C, or antithrombin, homozygous factor V Leiden or prothrombin gene mutations, and compound heterozygous factor V Leiden/prothrombin gene mutation in the setting of an unprovoked event. Further discussion of models for risk assessment of recurrence is provided below.
Assessment of Bleeding Risk
The bleeding risk associated with the use of anticoagulation must be weighed against the risk of clotting events when determining duration of anticoagulation, especially in those patients for whom indefinite anticoagulation is a consideration. Risk of bleeding while on anticoagulation is approximately 1–3% per 100 patient-years [74],but concomitant medical conditions such as renal failure, diabetes-related cerebrovascular disease, malignancy, advanced age, and use of antiplatelet agents all increase the risk of bleeding. Bleeding risk is highest when patients first initiate anticoagulation and is approximately 10 times the risk in the first month of therapy than after the first year of therapy [75].
Risk assessment models such as the RIETE score may be helpful when indefinite anticoagulation is a possibility [76].The RIETE score encompasses 6 risk factors (age > 75 years, recent bleeding, cancer, creatinine level > 1.2 mg/dL, anemia, or PE at baseline) to categorize patients into low risk (0 points, 0.3% risk of bleeding), intermediate risk (1–4 points, 2.6% risk of bleeding) and high risk (> 4 points, 6.2% risk of bleeding) within 3 months of anticoagulant therapy. The ACCP has developed a more extensive list of 17 potential risk factors for bleeding to categorize patients into low risk (no risk factors, 0.8%/year risk of bleeding), intermediate risk (1 risk factor, 1.6%/year risk of bleeding) and high risk (2 or more risk factors, >6.5%/year risk of bleeding) categories [77].The RIETE score is simpler to use but was not developed for assessing risk of bleeding during indefinite therapy, while the ACCP risk categorization predicts a yearly risk and is therefore applicable for long-term risk assessment but is more cumbersome to use. In practice, we generally use a clinical gestalt of a patient’s clinical risk factors (particularly age, renal or hepatic dysfunction, and frequent falls) to assess if they may be at high risk of bleeding and if the risk of indefinite anticoagulation may thus outweigh the potential benefit.
We also note that several scoring systems (HAS-BLED, HEMORR2HAGES, and ATRIA scores) have been developed to predict those at high risk of bleeding on anticoagulation for atrial fibrillation [78–80].These scores generally include similar clinical risk factors to those in the RIETE and ACCP scoring systems. Several studies have compared the HAS-BLED, HEMORR2HAGES, and ATRIA scores and a systematic review and meta-analysis concluded that the HAS-BLED score is recommended, due to increased sensitivity and ease of application [81].However, as these scores have not been validated for anticoagulation in the setting of VTE, we do not use them in this capacity.
Risk Stratification for Recurrent VTE
When predicting risk of recurrent VTE, clinical risk factors including obesity, male gender, and underlying thrombophilia (including the “high risk” inherited thrombophilias identified above) must taken into consideration. Location of the thrombus must also be considered; it has also been demonstrated that patients with DVT involving the iliofemoral veins are at higher risk of recurrence than those without iliac involvement [82].Other factors that may be useful in risk stratification include D-dimer level and ultrasound to search for residual venous thrombosis.
D-dimer Levels
D-dimer levels are one of the more promising methods for assessing the risk of recurrent VTE after cessation of anticoagulation, especially in the case of idiopathic VTE where indefinite anticoagulation should be considered but may pose either risk of bleeding or significant inconvenience to patients. A normal D-dimer measured 1 month after cessation of anticoagulation offers a high negative predictive value for risk of recurrence [83].A number of studies have demonstrated that patients with elevated D-dimer 1 month after anticoagulation cessation are at increased risk for a recurrent event [84–86].Two predictive models that have been developed incorporate D-dimer testing into decision making [87,88].The DASH predictive model relies on the D-dimer result in addition to age, male sex, and use of hormone therapy as a method of risk stratification for recurrent VTE in patients with a first unprovoked event. Using this scoring system, patients with a score of 0 or 1 had a recurrence rate of 3.1%, those with a score of 2 a recurrence rate of 6.4%, and those with a score of 3 or greater a recurrence rate of 12.3%. The authors postulate that by using this assessment scheme they can avoid lifelong anticoagulation in 51% of patients. The Vienna prediction model uses male sex, location of VTE (proximal DVT and PE are at higher risk), and D-dimer level to predict risk of recurrent VTE. This model has recently been updated to include a “dynamic” component to predict risk of recurrence of VTE from multiple random time points [89].
Overall, D-dimer may be useful for risk stratification. We often employ the method of stopping anticoagulation in patients with unprovoked VTE after 3 months (if the patient has no identifiable clinical risk factors that place them at high risk of recurrence) and testing D-dimer 1 month after cessation of anticoagulation. An elevated D-dimer is a solid reason to restart anticoagulation (potentially on an indefinite basis), while a negative D-dimer provides support for withholding further anticoagulation in the absence of other significant risk factors for recurrence. However, lack of agreement regarding assay cut-points as well as multiple reasons other than VTE for D-dimer elevation may limit widespread use of this method. We generally use a cutpoint of 250 ug/L as “negative,” though at least one study showed that cut-points of 250 ug/L versus 500 ug/L did not change the utility of this method [90].In our practice, risk prediction models are most useful to provide patients with additional information and a visual presentation to support our recommendation. This is particularly true of the Vienna prediction rule, which is available in a printable nomogram which can be distributed to patients and completed together during the clinic visit.
Imaging Analysis
Imaging analysis may also assist with risk stratification. Clinical assessment modules have been developed that incorporate repeat imaging studies for assessment of recannulization of affected veins. In patients with residual vein thrombosis (RVT) at the time anticoagulation was stopped, the hazard ratio for recurrence was 2.4 compared to those without RVT [91].There are a number of ways RVT could impact recurrence, including inpaired venous flow leading to stasis and activation of the coagulation cascade. Subsequent studies used serial ultrasound to determine when to stop anticoagulation. In one study, patients were anticoagulated for 3 months and for those that had RVT, anticoagulation was continued for up to 9 months for provoked and 21 months for unprovoked VTE. In comparison to fixed dosing of 6 months of anti-coagulation, those who had their length of anticoagulation tailored to ultrasonography findings had a lower rate of recurrent VTE [92].Limitations to using RVT in clinical decision-making include lack of a standard definition of RVT and variability in both timing of ultrasound (operator variability) and interpretation of results [93].
Other Options
Another option in patients who are being considered for indefinite anticoagulation is to decrease the intensity of anticoagulation. Since this would theoretically lower the risk of bleeding, the perceived benefit of long-term, low-intensity anticoagulation would be reduction in both bleeding and clotting risk. The PREVENT trial randomized patients who had received full-dose anticoagulation for a median of 6.5 months to either low-intensity warfarin (INR goal of 1.5-2.0 instead of 2.0-3.0) or placebo. In the anticoagulation group, there was a 64% risk reduction in recurrent VTE (hazard ratio 0.36, 95% CI 0.19 to 0.67) but an increased risk of bleeding (hazard ratio 1.92, 95% CI 1.26 to 2.93) [94].The ELATE study randomized patients with unprovoked VTE who had completed 3 or more months of full-intensity warfarin therapy (target INR 2.0–3.0) to continue therapy with either low-intensity warfarin (target INR 1.5–2.0) or full-intensity warfarin (target INR 2.0-3.0). Compared to the low-intensity group, the conventional-intensity group had lower rates of recurrent VTE and no increased rates of major bleeding [95].This study, however, has been criticized because of its overall low bleeding rate in both treatment groups.
Aspirin is an option in patients in whom long-term anticoagulation is untenable. The ASPIRE trial demonstrated that in patients with unprovoked VTE who had completed a course of initial anticoagulation, aspirin 100 mg daily reduced the risk of major vascular events compared to placebo with no increase in bleeding [96].However, aspirin was not associated with a significant reduction in risk of VTE alone (only the composite vascular event endpoint). The WARFASA trial, however, demonstrated that aspirin 100 mg daily was associated with a significant reduction in recurrent VTE compared to placebo after 6 to 18 months of anticoagulation without an increase in major bleeding [97].The absolute risk of recurrence was 11% in the placebo group and 5.9% in the aspirin group. More recently, the INSPIRE collaboration analyzed data from both trials and found that aspirin after initial anticoagulation reduced the risk of recurrent VTE by approximately 42% with a low rate of major bleeding [98].The absolute risk reduction was even larger in men and older patients. For this reason, we recommend aspirin to those patients in whom indefinite anticoagulation may be warranted from the standpoint of reducing risk of recurrent VTE but in whom the risk of bleeding precludes its use.
Hypercoagulable States In Specific Populations
Inherited Thrombophilias
Patients with a hereditary thrombophilia are at increased risk for incident VTE [99].These inherited mutations result in either a loss of normal anticoagulant function or gain of a prothrombotic state. Hereditary disorders associated with VTE include deficiency of antithrombin, protein C, or protein S, or the presence of factor V Leiden and/or prothrombin G20210A mutations. Although deficiency of protein C, protein S, or antithrombin is uncommon and affects only 0.5% of the population, these states have been associated with a 10-fold increased risk of thrombosis in comparison to the general population. Factor V Leiden and prothrombin gene mutation are less likely to be associated with incident thrombosis (2 to 5-fold increased risk of VTE) and are more prevalent in the Caucasian population [100].Though these hereditary thrombophilias increase risk of VTE, prophylactic anti-coagulation prior to a first VTE is not generally indicated.
Data regarding the impact of the inherited thrombophilias on risk of recurrent VTE is less well defined. While some data suggest that inherited thrombophilias are associated with increased risk of recurrent VTE, the degree of impact may be clinically modest especially in those with heterozygous factor V Leiden or prothrombin gene mutations [101].Ideally, a clinical trial would be designed to assess whether hereditary thrombophilia testing is beneficial for patients with VTE in decision-making regarding length of anticoagulation, type of anticoagulation, and risk of recurrence. If a patient with a low-risk inherited thrombophilia has a DVT in the setting of an additional provoking risk factor (surgery, pregnancy, etc), a 3-month course of anticoagulation followed by D-dimer assessment as above is reasonable. If a patient with an inherited thrombophilia experiences an idiopathic VTE, or if a patient with a “high-risk” thrombophilia as described above experiences any type of VTE, we generally recommend indefinite anticoagulation in the absence of high bleeding risk, though again this is a very patient-dependent choice.
Acquired Thrombophilias
Antiphospholipid Syndrome
Antibodies directed against proteins that bind phospho-lipids are associated with an acquired hypercoagulable state. The autoantibodies are categorized as antiphospho-lipid antibodies (APLAs), which include anticardiolipin antibodies (IgG and IgM), beta-2 glycoprotein 1 antibodies (anti-B2 GP), and lupus anticoagulant. These antibodies can form autonomously, as seen in primary disorders, or in association with autoimmune disease as a secondary disorder.
Criteria have been developed to distinguish antiphospholipid-associated clotting disorders from other forms of thrombophilia. The updated Sapporo criteria depend on both laboratory and clinical diagnostic criteria [102].The laboratory diagnosis of APLAs requires the presence of lupus anticoagulants, anticardiolipin antibodies, or anti-B2 GP on at least 2 assays at least 12 weeks apart with elevation above the 99th percentile of the testing laboratory’s normal distribution [103].Testing for lupus anticoagulant is based on 3 stages, the first of which is inhibition of phospholipid-dependent coagulation tests with prolonged clotting time (eg, aPTT or dilute Russell’s viper venom time). The diagnosis is confirmed by a secondary test in which excess hexagonal phase phospholipids are added to incubate with the patient’s plasma to absorb the APLA [104].The presence of anticardiolipin antibodies and anti beta-2 GP antibodies is determined using ELISA based immunoassays. Unlike most other thrombophilias, antiphospholipid syndrome is associated with both arterial and venous thromboembolic events and may be an indication for lifelong anticoagulation after a first thrombotic event. We generally recommend indefinite anticoagulation in the absence of significant bleeding risk.
Cancer-Associated Hypercoagulable State
Patients with cancer have a propensity for thromboembolic events. The underlying mechanisms responsible for cancer-associated clotting events are multifactorial and an area of intense research. Tumor cells can initiate activation of the clotting cascade through release of tissue factor and other pro-coagulant molecules [105].Type and stage of cancer impact risk of VTE, and the tumor itself can compress vasculature leading to venous stasis. Furthermore, chemotherapy, hormone therapy, antiangiogenic drugs, erythropoietin agents, and indwelling central venous catheters all are associated with increased risk of thrombotic events. Approximately 25% of all cancer patients will experience a thrombotic event during the course of their disease [106]. In fact, the presence of a spontaneous clot may be a harbinger of underlying malignancy [107].Approximately 10% of patients who present with an idiopathic VTE are diagnosed with cancer in the next 1 to 2 years.
The utility of extensive cancer screening in patients with spontaneous clotting events is often debated. The small studies that have addressed cancer associated clots have not demonstrated any mortality benefit with extensive screening. A prospective cohort study addressed the utility of limited versus extensive screening [108].In this study, all patients underwent a series of basic screening tests such as history taking, physical examination, chest radiograph, and basic laboratory parameters. Approximately half of the patients underwent additional testing (CT of chest and abdomen and mammography for women). Screening did not result in increased survival or fewer cancer-related deaths. 3.5 % of patients in the extensive screening group were diagnosed with malignancy in comparison to 2.4% in the limited screening group. During follow-up, cancer was diagnosed in 3.7% and 5.0% in the extensive and limited screening groups, respectively. The authors concluded that the low yield of extensive screening and lack of survival benefit did not warrant routinely ordering cancer screening tests above and beyond age-appropriate screening in patients with idiopathic VTE. However, it is known that identification of occult malignancy at an earlier stage of disease is beneficial, and cancer diagnosed within one year of an episode of VTE is generally more advanced and associated with a poorer prognosis [109].It is our practice to take a through history from patients with unprovoked clots particularly focusing on symptoms suggestive of an underlying cancer. We recommend that patients be up to date with all age-appropriate cancer screening.
Heparin-based products (rather than warfarin) are recommended for long-term treatment of cancer-associated DVT. Several trials, most prominently the CLOT trial, have demonstrated that LMWH is associated with reduced risk of recurrent VTE compared with warfarin in cancer patients [110].Fondaparinux may be a reasonable alternative if a patient is unable to tolerate a LMWH. In terms of treatment duration, patients with cancer-associated VTE should be anticoagulated indefinitely as long as they continue to have evidence of active malignancy and/or remain on antineoplastic treatment [111].
Heparin has potential anticancer effects beyond its anticoagulation properties. It is believed that heparin use in patients with cancer can influence cancer progression by acting as an antimetastatic agent. The molecular mechanisms underlying this significant observation are not completely understood, although the first documented benefit of these drugs dates back to the 1970s [112].Overall, LMWH have been associated with improved overall survival in cancer patients and this effect appears to be distinct from its ability to prevent life-threatening VTE episodes [113].
Estrogen-Related Thromboembolic Disease
Pregnancy is a well-established acquired hypercoagulable state, and thromboembolic disease accounts for significant morbidity and mortality in pregnancy and the postpartum period. Approximately 1 in 1000 women will suffer from a thrombotic event during pregnancy or shortly after delivery [8]. The etiology of the tendency to clot during pregnancy is multifactorial and mainly reflects venous stasis due to vasculature compression by the uterus, changes in coagulation factors as the pregnancy progresses, and endothelial damage during delivery, especially Cesarean section. Both factor VIII and von Willebrand factor levels increase, especially in the final months of pregnancy. Simultaneously, levels of the natural anticoagulant protein S diminish, leading to an acquired resistance to activated protein C which results in increased thrombin generation and therefore a hypercoagulable state [114].The risk of thrombosis in pregnancy is clearly heightened in women with inherited thrombophilias, especially in the postpartum period [115].
Similarly to pregnancy, hormone-based contraceptive agents and estrogen replacement therapies are also associated with increased thrombotic risk. Over the years, drug manufacturers have tried to mitigate the clotting risk associated with these drugs by reducing the amount of estrogen and altering the type of progesterone used, yet a risk still remains, resulting in a VTE incidence 2 to 7 times higher in this population [116].The risk is highest in the first 4 months of use and is unaffected by duration of use; risk extends for 3 months after cessation of estrogen-containing therapy. Patients who develop VTE while taking an oral contraceptive are generally instructed to stop the contraceptive and consider an alternative form of birth control. Although routine screening for thrombophilia is not offered to women before prescribing oral contraceptives, a thorough personal and family history regarding venous and arterial thrombotic events as well as recurrent pregnancy loss in women should be taken to evaluate thromboembolic risk factors. We generally avoid use of oral contraceptives in patients with a known hereditary thrombophilia, and consider screening prior to initiation of therapy in those with a strong family history of VTE.
Superficial VTE
Although the main disorders that comprise VTE are DVT and PE, another common presentation is superficial venous thromboembolism (SVT). The risk factors for developing an SVT are similar to those for DVT. In addition, varicose veins also increase the incidence of developing SVT [117].SVT is not associated with excessive mortality, and the main concern with it is progression to DVT. About 25% of patients diagnosed with SVT may have DVT or PE at the time of diagnosis and about 3% without DVT or PE at time of diagnosis developed one of these complications over the following 3 months; clot propagation is another common complication [118].Ultrasound may be of utility in diagnosing occult DVT in patients who initially diagnosed with SVT [119].
For patients who have only SVT at baseline without concomitant DVT or PE, it is difficult to determine which patients are at risk for developing DVT. Some risk stratification models include clot location. Since SVT clots usually develop in the saphenous vein, the clot would need to either progress from the sapheno-femoral junction to the common femoral vein; thus, any clots located near the sapheno-femoral junction are at risk of progressing into the deep vasculature [120].Clots within 3 cm of the junction may be more likely to progress to DVT [121].Chengelis and colleagues feel that proximal saphenous vein thrombosis should likely be treated with anticoagulation [122].Others have taken a more general approach, stating that all clots above the knee or in the thigh area should be treated aggressively [123].
There are solid data for the use of anticoagulation in SVT. In the STEFLUX (Superficial ThromboEmbolism and Fluxum) study, participants received the LMWH parnaparin at one of 3 doses: 8500 IU once daily for 10 days followed by placebo for 20 days, 8500 IU once daily for 10 days and then 6400 IU once daily for 20 days, or 4250 IU once daily for 30 days. Those who received the intermediate dosing had lower rates of DVT, PE, and relapse/SVT recurrence in the first 33 days [124].In the CALISTO trial, fondaparinux 2.5mg per day for 45 days effectively reduced the risk of symptomatic DVT, PE, or SVT recurrence or extension and was not associated with any increased major bleeding compared to placebo [125].A Cochrane review included 30 studies involving over 6500 participants with SVT of the lower extremities. The treatments used in these studies included fondaparinux, LMWH, UFH, non-steriodal anti-inflammatory agents, topical treatment, and surgery. According to the findings, use of fondaparinux at prophylactic dosing for 6 weeks is considered a valid therapeutic option for SVT [126].It is our practice to consider the use of anticoagulants (generally LMWH or fondaparinux) as part of the treatment regimen for SVT.
Target-Specific Oral Anticoagulants And Treatment of VTE
The direct thrombin inhibitor dabigatran directly binds to thrombin in a concentration-dependent manner [127].Peak plasma concentration is achieved within 0.5 to 2.0 hours after ingestion, and its half-life is 12 to 17 hours. Use of dabigatran in both primary and secondary prevention of VTE has been extensively studied, especially in orthopedic surgery where there have been 4 main trials (RE-MOBILIZE, RE-MODEL, RE-NOVATE, and RE-NOVATE I and II). While RE-MOBILIZE showed that dabigatran 220 mg or 150 mg once daily was inferior to enoxaparin 30 mg twice daily in preventing VTE after total knee arthroplasty, RE-MODEL and RE-NOVATE I and II demonstrated that dabigatran 150 mg or 220 mg once daily was noninferior to enoxaparin 40 mg once daily for prevention of VTE in patients undergoing total knee replacement and hip replacement [128–131].The side effect profile was also promising, with no significant differences in the frequency of major bleeding between dabigatran and enoxaparin. Pooled data and meta-analyses from these trials have demonstrated that for prevention of VTE associated with hip or knee surgery, dabigatran 220 mg or 150 mg once daily is as effective as 40 mg of enoxaparin given daily or 30 mg given twice a day, with a similar bleeding profile [132,133].
More recently, dabigatran been used in the acute treatment and secondary prevention of VTE. In the RE-COVER trial, dabigatran 150 mg twice daily was compared to warfarin (INR 2–3) in the treatment of acute VTE for 6 months, after an initial treatment period of up to 9 days with LMWH or UFH. Dabigatran was noninferior to warfarin with respect to 6-month incidence of recurrent symptomatic objectively confirmed VTE and related deaths, and was not associated with increased bleeding [134].In the RE-MEDY and RE-SONATE trials of extended anticoagulation, dabigatran was as effective as warfarin for prevention of recurrent VTE when continued after 3 months of initial anticoagulation and associated with less bleeding, and was more effective than placebo in preventing recurrent VTE but associated with a higher risk of bleeding [135].Unexpectedly, the risk of acute coronary syndrome was slightly higher in the dabigatran group than the warfarin group, as seen in other studies.
Rivaroxaban, a TSOAC that targets factor Xa, has also shown efficacy in preventing VTE after knee or hip surgery. The RE-CORD 1-4 studies all focused on the use of rivaroxaban in comparison to enoxaparin and found that rivaroxaban 10 mg once daily was superior to enoxaparin 40 mg once daily in prevention of VTE in total knee and total hip arthroplasty [136–138].Meta-analysis of multiple rivaroxaban VTE prophylaxis trials also demonstrated that rivaroxaban significantly lowered the risk of VTE in these surgical patients in comparison to the use of enoxaparin [139].Prophylactic use of rivaroxaban was also studied in acutely ill hospitalized patients in the MAGELLAN trial. Rivaroxaban 10 mg daily for 35 days was compared to enoxaparin 40 mg daily for 10 days followed by placebo and was found to be noninferior to enoxaparin in reduction of VTE risk at day 10 and superior to placebo at day 35 [140].However, the rate of bleeding, although low in both arms, was higher in the rivaroxaban arm.
Rivaroxaban has been studied in randomized clinical trials for acute treatment of DVT and PE and for extended prophylaxis for recurrent VTE (EINSTEIN-DVT, EINSTEIN-PE and EINSTEIN-Extension, respectively). The treatment strategy for use of rivaroxaban differed from that of dabigatran (in the RE-COVER trial), as rivaroxaban was used upfront as initial anticoagulation rather than after an initial period of parenteral therapy with LMWH or UFH. In both the DVT and PE trials, rivaroxaban was noninferior to standard treatment with enoxaparin followed by warfarin therapy, with no significant difference in major bleeding at 6 months of treatment [141,142].The extension trial also demonstrated that use of rivaroxaban in comparison to placebo for an additional 6 or 12 months after standard therapy was associated with significantly fewer recurrent VTE [141]. These studies led to FDA approval for rivaroxaban for primary prevention of VTE in patients undergoing elective total hip or knee repair surgery, for treatment of acute DVT or PE, and for extended prophylaxis in patients following initial treatment.
The anti-factor Xa TSOAC apixaban has been studied in similar fashion as rivaroxaban. In the AMPLIFY study, apixaban was given at a dose of 10 mg twice daily for 7 days followed by 5 mg twice daily for 6 months (as monotherapy, without initial parenteral agent) and compared to enoxaparin followed by warfarin for treatment of acute VTE. Apixaban was as effective as warfarin in terms of recurrent symptomatic VTE or VTE-related death, and was associated with significantly fewer bleeding events [143].Extended-duration apixaban given at treatment dose (5 mg twice daily) or at prophylactic dose (2.5 mg twice daily) for 12 months after completion of treatment-dose apixaban for VTE demonstrated superiority to placebo for extended prophylaxis in AMPLIFY-EXT, and there was no increase in major bleeding compared to placebo [144].Apixaban was recently approved by the FDA for both treatment and secondary prophylaxis of VTE.
More recently, a third anti-factor Xa TSOAC edoxaban demonstrated noninferiority to warfarin in prevention of recurrent symptomatic VTE when administered to patients with DVT or PE at 60 mg once daily for 3 to 12 months [145].Edoxaban also led to significantly less bleeding than warfarin. Edoxaban was recently approved by the FDA for treatment of VTE.
These TSOACs show promise in treatment and prevention of VTE but should be used in patients who meet appropriate criteria for renal function, age, and bleeding risk, as there are currently no available antidotes to reverse their effects. If significant bleeding occurs and cannot be controlled by usual maneuvers such as mechanical compression or surgical intervention, there is little data to guide the use of pharmacologic interventions. Plasma dabigatran levels can be reduced through the use of hemodialysis [146].Antibodies capable of neutralizing dabigatran have been developed, and one specific antibody, idarucizumab, was well-tolerated and showed immediate and complete reversal of dabigatran in subjects of different age and renal function [147,148].Andexanet, a modified recombinant derivative of factor Xa with no catalytic activty, acts as a “decoy receptor” with higher affinity to factor Xa inhibitors than natural factor Xa. Phase II studies in healthy volunteers demonstrated that andexanet immediately reversed the anticoagulation activity of apixaban, rivaroxaban, enoxaparin, and most recently edoxaban without thrombotic consequences [149].Two randomized, double-blind, placebo-controlled phase III studies (ANNEXA-A, looking at the reversal of apixaban, and ANNEXA-R, looking at reversal of rivaroxaban) are underway, and preliminary results show that a single intravenous bolus of andexanet demonstrated almost complete reversal [150].Finally, aripazine (PER977), a synthetic small molecule that binds to heparins as well as all TSOACs, was shown in a phase II trial to decrease blood clotting time to within 10% above baseline value in 10 minutes or less with an effect lasting for 24 hours [151].
Some have advocated for use of prothrombin complex concentrate (PCC) or recombinant factor VIIa for reversal of TSOAC-associated bleeding. Rivaroxaban was demonstrated to be partially reversible by PCC, whereas this approach was not as successful for dabigatran in healthy volunteers [152].In vitro evidence, however, showed that PCC did not significantly change aPTT [153].At present, the use of nonspecific hemostatic agents (including recombinant factor VIIa, 4-factor prothrombin complex concentrate, and activated prothrombin complex concentrates) is suggested for reversal of TSOACs in patients who present with life-threatening bleeding [154,155].
Conclusion
Patients with VTE present with a wide range of findings and factors that impact management. Decision making in VTE management is a fluid process that should be re-evaluated as new data emerge and individual circumstances change. There is more focus on VTE prevention and treatment today than there was even a decade ago. Diagnostic algorithms, identification of new risk factors, refinement in understanding of the pathogenesis of thrombosis, and identification of new anticoagulants with more favorable risk-benefit profiles will all ultimately contribute to improved patient care.
Corresponding author: Jean M. Connors, MD, Brigham and Women's Hospital, 75 Francis St., Boston, MA 02215.
From the Brigham and Women’s Hospital and Dana-Farber Cancer Institute, Boston, MA.
Abstract
- Objective: To review the diagnosis and management of venous thromboembolism (VTE).
- Methods: Review of the literature.
- Results: VTE and its associated complications account for significant morbidity and mortality. Various imaging modalities can be employed to support a diagnosis of a VTE and are used based on clinical suspicion arising from the presence of signs and symptoms. Clinical decision rules have been developed that can help determine which patients warrant further testing. Anticoagulation, the mainstay of VTE treatment, increases bleeding risk, necessitating tailored treatment strategies that must incorporate etiology, risk, benefit, cost, and patient preference.
- Conclusion: Further study is needed to understand individual patient risks and to identify treatments that will lead to improved patient outcomes.
Venous thromboembolism (VTE) and its associated complications account for significant morbidity and mortality. Each year between 100 and 180 persons per 100,000 in Western countries develop VTE. The majority of VTEs are classified as either pulmonary embolism (PE), which accounts for one third of the events, or deep vein thrombosis (DVT), which is responsible for the remaining two thirds. Between 20% and 30% of those patients diagnosed with thrombotic events will die within the first month after diagnosis [1].PE is a common consequence of DVT; 40% of patients who are diagnosed with DVT will be subsequently found to have PE upon further imaging. This high rate of association is also seen in those who present with PE, 70% of whom will also be found to have concomitant DVT [2,3].
Anatomic risk factors include Paget-Schroetter syndrome (compression of upper extremity veins due to abnormalities at the thoracic outlet), May-Thurner syndrome (significant compression of the left common iliac vein by the right common iliac artery), and abnormalities of the inferior vena cava [14–16].Medications that are associated with increased risk of VTE include but are not limited to estrogen (both in oral contraceptives as well as hormone replacement therapy) [17,18],the selective estrogen receptor modulator tamoxifen [19],testosterone [20],and glucocorticoids [21].It is important to note that many patients with VTE have more than one acquired risk factor for thrombosis [22],and also that acquired risk factors are more likely to lead to VTE in the setting of underlying inherited thrombophilic conditions [23].
Pathogenesis
Abnormalities in both coagulation factors and the vascular bed are at the core of the pathogenesis of VTE. The multifaceted etiology of thrombosis was first described in 1856 by Virchow, who defined a triad of defects in the vessel wall, platelets, and coagulation proteins [24].Usually the vessel wall is lined with endothelial cells that provide a nonthrombotic surface and limit platelet aggregation through release of prostacyclins and nitric oxide. When the endothelial lining is compromised, the homeostatic surveillance system is disturbed and platelet activation and the coagulation system are initiated. Tissue factor exposure in the damaged area of the vessel leads to activation of the coagulation cascade. Collagen that is present in the area of the wound is also exposed and can activate platelets, which provide the phospholipid surface upon which the coagulation cascade occurs. Platelets initially tether to the exposed collagen through binding of glycoprotein Ib-V-IX in association with von Willebrand factor [25].The thrombus is initiated as more platelets are recruited to exposed collagen of the injured endothelium through aggregation in response to the binding of GPIIIb/IIa with fibrinogen. This process is self-perpetuating as these activated platelets release additional proteins such as adenosine diphosphate (ADP), serotonin, and thromboxane A2, all of which fuel the recruitment and activation of additional platelets [26].
Diagnosis
The key to decreasing the morbidity and mortality associated with VTE is timely diagnosis and early initiation of therapy. Various imaging modalities can be employed to support a diagnosis of a VTE and are used based on clinical suspicion arising from the presence of signs and symptoms. DVT is usually associated with pain in calf or thigh, unilateral swelling, tenderness, and redness. PE can present as chest pain, shortness of breath, syncope, hemoptysis, and/or cardiac palpitations.
Decision Rules
Clinical decision rules based on signs, symptoms, and risk factors have been developed to estimate the pretest probability of PE or DVT and to help determine which patients warrant further testing. These clinical decision rules include the Wells criteria (separate rules for DVT and PE) [27,28],as well as the Geneva score [29],which is focused on identifying patients with a likelihood of having a PE. In general, these clinical rules are applied at presentation to predict the risk of VTE, and patients who score high are evaluated by imaging modalities, while those with lower scores should be considered for further stratification based on D-dimer testing. The goal of clinical assessment and use of a decision rule is to identify patients at low risk of VTE to reduce the number of imaging studies performed. Most of the decision rules focus on the use of noninvasive evaluations that are easily implemented, including clinical history and presentation, abnormalities in oxygen saturation, chest radiography findings, and electro-cardiography.
D-Dimer Testing
D-dimer testing is at the core of all predictive models for VTE. D-dimer is a fibrin degradation product that is detectable in the blood during active fibrinolysis and occurs after clot formation. The concentration of D-dimer increases in patients with active clot. D-dimer testing is usually performed as a quantitative ELISA or automated turbidometric assay and is highly sensitive (> 95%) in excluding a diagnosis of VTE if results are in the normal range [30].The presence of a normal D-dimer and a low probability based on clinical assessment criteria can be integrated to determine which patients have a low (generally < 99%) likelihood of having VTE [31].It should be noted that other factors can lead to an increased D-dimer, including malignancy, trauma, critical illness, disseminated intravascular coagulation, pregnancy, infection, and postoperative status, which can produce false-positive results and cloud the utility of the test in excluding those at low risk of VTE from undergoing imaging [32–34].Additionally, D-dimer values naturally increase with age and recent work has shown utility of an age-adjusted D-dimer threshold, though this method is not yet widespread in clinical practice [35,36].
Imaging
After application of a clinical prediction rule, the mainstay of diagnosis of VTE is imaging. For DVT the use of ultrasonography is considered the gold standard, with both high sensitivity (89–100%) and specificity (86–100%), especially when the DVT is located proximally [37–39].We generally recommend compression ultrasound starting with the proximal veins but expanding to include the whole leg if the proximal studies are negative [40–42].Other diagnostic options include computed tomography (CT) venography, which is not first line as it is highly invasive and exposes the patient to iodine-based contrast dyes, and magnetic resonance venography (MRV), which offers superb visualization for diagnosis of pelvic vein thrombosis but is limited because of availability and cost issues.
Helical CT pulmonary angiography (CTPA) is the diagnostic test of choice in PE, with high sensitivity (96%) and specificity (95%), and has replaced conventional ventilation perfusion (VQ) scanning or other methods such as magnetic resonance pulmonary angiography in most settings [43,44].CTPA should be avoided in patients who have severe chronic kidney disease or a contrast allergy, and is often avoided in patients who are pregnant due to potential risk of radiation exposure, and in such situations VQ scanning may be employed.
Algorithmic Approach to Workup
Of note, there are multiple clinical situations in which the application of a clinical prediction rule followed by D-dimer testing and/or imaging cannot be “standardized” with such algorithms. These include situations where D-dimer may be falsely positive (as above), situations in which alternative imaging strategies should be used to avoid contrast exposure in workup of PE (as above), and workup of suspected upper extremity DVT. Upper extremity ultrasound comprises about 10% of all DVT and frequently occurs in the setting of risk factors such as central venous catheters or pacemakers; specific upper-extremity risk-assessment rules have been developed [47,48].
Acute Treatment Options
The first step in treatment is identification of patients who are at high risk of
In standard cases of DVT and PE without hemodynamic compromise, the current standard of care is to initiate parenteral anticoagulation. The immediate goal of therapy is to treat rapidly with anticoagulants to prevent the thrombus from propagating further and to prevent DVT from embolization to the lungs or other vascular beds. The initial treatment of VTE has been extensively discussed and guidelines have been established with recommendations for initiation of anticoagulation; the American College of Chest Physicians (ACCP) released the 9th edition of their guidelines in 2012 based on consensus agreements derived from primary data [51].
Heparin-based drugs are the mainstay of initial treatment. These drugs act by potentiating antithrombin and therefore inactivating thrombin and other coagulation factors such as Xa. Unfractionated heparin (UFH) can be administered as an initial bolus followed by a continuous infusion with dosing being based on weight and titrated to activated partial thromboplastin time (aPTT) or the anti-factor Xa level. Alternatively, patients may be treated with a low molecular weight heparin (LMWH) administered subcutaneously in fixed weight-adjusted doses, which obviates the need for monitoring in most cases [52].LMWHs work in a similar manner to UFH but have more anti-Xa activity in comparison to anti-thrombin activity. LMWH appears to be more effective than UFH for initial treatment of VTE and has been associated with lower risk of major hemorrhage [53].The options for treatment of VTE have expanded in recent years with the approval of fondparinux, a pentasaccharide specifically targeted to inhibit factor Xa. Fondaparinux has been shown to have similar efficacy to LMWH in patients with DVT [54],and while it has not been evaluated directly against LMWH for initial treatment of PE it has been shown to be at least as effective and safe as UFH [55].
Both LMWH and fondaparinux are cleared renally and therefore have increased bleeding risk in patients with renal impairment. In patients with creatinine clearance of less than 30 mL/min, dose reduction or lengthening of dosing interval may be appropriate. Anti-factor Xa activity can be used as a functional assay to monitor and titrate the level of anticoagulation in patients treated with UFH, LMWH, and fondaparinux. Monitoring is useful in the setting of impaired renal function (as above) in addition to extremes of body weight and pregnancy. When used for monitoring of UFH, the anti-factor Xa activity can be measured at any time during administration with a therapeutic goal range of 0.3–0.7 international units (IU)/mL. When used for LMWH, a “peak” anti-factor Xa should be measured approximately 4 hours after dosing, with therapeutic goals depending on preparation and schedule of treatment but generally between 0.6 to 1.0 IU/mL for twice daily and around 1.0 -2.0 IU/mL for once-daily [56].For patients on dialysis, we generally use intravenous UFH for acute treatment of VTE, though recent work has shown that enoxaparin (doses of 0.4 to 1 mg/kg/day) was as safe as UFH with respect to bleeding and was associated with shorter hospital length of stay [57].For long-term treatment of VTE, warfarin is generally preferred based on clinical experience with this agent, though small studies have suggested that parenteral agents may be useful alternatives to warfarin [58].
In many patients who are clinically stable without significant medical comorbidities, outpatient administration of these medications without hospitalization is considered safe. Patients with DVT are often safe to manage as outpatients unless significant clot burden is present and thrombolysis is being considered. For PE, the pulmonary embolism severity index (PESI) and simplified index (sPESI) may be useful to risk-stratify patients and identify those at low risk of complications who may be suitable for outpatient treatment [59,60].Studies have shown that hemodynamically stable patients who did not require supplemental oxygenation or have contraindications to LMWH therapy were safely managed as outpatients with low risk of recurrent VTE and bleeding [61,62].One exception may be patients with intermediate risk PE, who are hemodynamically stable but have evidence of right ventricular dysfunction and may be better served by an initial in-hospital observation period, especially if thrombolysis is being considered.
Most patients who present with VTE are transitioned to warfarin for long-term therapy. Warfarin can be started on the same day as parenteral anticoagulation. Both drugs are overlapped for at least 5 days, with a target INR of 2.0–3.0. Patients may achieve the target INR level quickly because factor VII has a short half-life and the level drops quickly; however, the overlap of 5 days is essential even when the INR is in the target range because a full anticoagulant affect is not achieved until prothrombin levels decline, and this is a slow process due to the long half-life of prothrombin. Warfarin also causes rapid decrease in levels of natural anticoagulants such as protein C and protein S, which further exacerbates the net hypercoagulable state in the short-term. Warfarin without a bridging parenteral agent carries a risk of warfarin-induced skin necrosis [63]and is not effective as an initial anticoagulant treatment in acute VTE as there is a relatively high risk of symptomatic clot extension or recurrent VTE compared to warfarin with use of a bridging agent [64].In specific cases such as cancer-associated VTE (see discussion below), LMWH is preferred to warfarin for long-term active therapy.
Long-Term Active Therapy After Acute Treatment
Duration of Anticoagulation
Recommended duration of anticoagulation depends on a myriad of factors including severity of VTE, risk of recurrence, bleeding risk, and lifestyle modification issues, as well as on the safety and availability of alternative therapies such as low-intensity warfarin, aspirin, or the new oral anticoagulants. The decision tree for length of treatment starts with whether the VTE was a provoked or a spontaneous event. Provoked events occur when the event is associated with an identifiable risk factor, such as immobilization from prolonged medical illness or surgical intervention, pregnancy or oral contraceptive use, and prolonged air travel.
Consensus guidelines suggest that 3 months of anti-coagulation are generally sufficient treatment for a provoked VTE [51,65,66]. Data from multiple studies and a meta-analysis suggests that less than 3 months of anticoagulation (4 to 6 weeks in most trials) is associated with an approximately 1.5-fold higher risk of recurrent VTE than 3 months [67,68].However, data from this meta-analysis also suggests that anticoagulation for longer than 3 months (6 to 12 months in most trials) is not associated with higher rates of recurrent VTE. We generally anticoagulate for 3 months in patients with provoked VTE.
Determining the duration of anticoagulation is more complex in patients with idiopathic/unprovoked VTE. Kearon and colleagues found that in patients with first idiopathic VTE, patients who were anticoagulated for 24 months versus 3 months had lower risk of recurrent VTE (1.3% per patient-year with 24 months versus 27.4% per patient-year with 3 months) [69].Similar studies and meta-analyses have demonstrated decreased recurrence rates in patients anticoagulated for a prolonged period of time. However, one study of prolonged anticoagulation revealed that at 3 years there was no difference in recurrence rate in patients with PE who were anticoagulated for 6 months versus 1 year [70].The likelihood of recurrent DVT in patients with first episode of idiopathic proximal DVT treated with either 3 months or 12 months of warfarin was similar after treatment was discontinued [71].Prolonged periods of anticoagulation do not directly influence risk of recurrence but instead may only delay occurrence of a second event [72].For that reason, the decision is essentially whether to anticoagulate for 3 months or to continue therapy indefinitely [73]. Current guidelines recommend continuing anticoagulation for 3 months in those at high risk of bleeding, and continuing for an extended duration in those at low or moderate bleeding risk [51]. Patients' values and perferences should be entertained and decisions made on a patient-by-patient basis.
For patients at high risk of recurrent VTE, we generally recommend indefinite anticoagulation unless the patient has a significantly elevated bleeding risk or strongly prefers to discontinue anticoagulation and compliance concerns are evident. High-risk patients are those who have suffered from multiple episodes of recurrent VTE, those who have clotted while being anticoagulated, and those with acquired risk factors, such as antiphospholipid antibodies and malignancy. Other high-risk groups are those with high-risk thrombophilias such as deficiency of protein S, protein C, or antithrombin, homozygous factor V Leiden or prothrombin gene mutations, and compound heterozygous factor V Leiden/prothrombin gene mutation in the setting of an unprovoked event. Further discussion of models for risk assessment of recurrence is provided below.
Assessment of Bleeding Risk
The bleeding risk associated with the use of anticoagulation must be weighed against the risk of clotting events when determining duration of anticoagulation, especially in those patients for whom indefinite anticoagulation is a consideration. Risk of bleeding while on anticoagulation is approximately 1–3% per 100 patient-years [74],but concomitant medical conditions such as renal failure, diabetes-related cerebrovascular disease, malignancy, advanced age, and use of antiplatelet agents all increase the risk of bleeding. Bleeding risk is highest when patients first initiate anticoagulation and is approximately 10 times the risk in the first month of therapy than after the first year of therapy [75].
Risk assessment models such as the RIETE score may be helpful when indefinite anticoagulation is a possibility [76].The RIETE score encompasses 6 risk factors (age > 75 years, recent bleeding, cancer, creatinine level > 1.2 mg/dL, anemia, or PE at baseline) to categorize patients into low risk (0 points, 0.3% risk of bleeding), intermediate risk (1–4 points, 2.6% risk of bleeding) and high risk (> 4 points, 6.2% risk of bleeding) within 3 months of anticoagulant therapy. The ACCP has developed a more extensive list of 17 potential risk factors for bleeding to categorize patients into low risk (no risk factors, 0.8%/year risk of bleeding), intermediate risk (1 risk factor, 1.6%/year risk of bleeding) and high risk (2 or more risk factors, >6.5%/year risk of bleeding) categories [77].The RIETE score is simpler to use but was not developed for assessing risk of bleeding during indefinite therapy, while the ACCP risk categorization predicts a yearly risk and is therefore applicable for long-term risk assessment but is more cumbersome to use. In practice, we generally use a clinical gestalt of a patient’s clinical risk factors (particularly age, renal or hepatic dysfunction, and frequent falls) to assess if they may be at high risk of bleeding and if the risk of indefinite anticoagulation may thus outweigh the potential benefit.
We also note that several scoring systems (HAS-BLED, HEMORR2HAGES, and ATRIA scores) have been developed to predict those at high risk of bleeding on anticoagulation for atrial fibrillation [78–80].These scores generally include similar clinical risk factors to those in the RIETE and ACCP scoring systems. Several studies have compared the HAS-BLED, HEMORR2HAGES, and ATRIA scores and a systematic review and meta-analysis concluded that the HAS-BLED score is recommended, due to increased sensitivity and ease of application [81].However, as these scores have not been validated for anticoagulation in the setting of VTE, we do not use them in this capacity.
Risk Stratification for Recurrent VTE
When predicting risk of recurrent VTE, clinical risk factors including obesity, male gender, and underlying thrombophilia (including the “high risk” inherited thrombophilias identified above) must taken into consideration. Location of the thrombus must also be considered; it has also been demonstrated that patients with DVT involving the iliofemoral veins are at higher risk of recurrence than those without iliac involvement [82].Other factors that may be useful in risk stratification include D-dimer level and ultrasound to search for residual venous thrombosis.
D-dimer Levels
D-dimer levels are one of the more promising methods for assessing the risk of recurrent VTE after cessation of anticoagulation, especially in the case of idiopathic VTE where indefinite anticoagulation should be considered but may pose either risk of bleeding or significant inconvenience to patients. A normal D-dimer measured 1 month after cessation of anticoagulation offers a high negative predictive value for risk of recurrence [83].A number of studies have demonstrated that patients with elevated D-dimer 1 month after anticoagulation cessation are at increased risk for a recurrent event [84–86].Two predictive models that have been developed incorporate D-dimer testing into decision making [87,88].The DASH predictive model relies on the D-dimer result in addition to age, male sex, and use of hormone therapy as a method of risk stratification for recurrent VTE in patients with a first unprovoked event. Using this scoring system, patients with a score of 0 or 1 had a recurrence rate of 3.1%, those with a score of 2 a recurrence rate of 6.4%, and those with a score of 3 or greater a recurrence rate of 12.3%. The authors postulate that by using this assessment scheme they can avoid lifelong anticoagulation in 51% of patients. The Vienna prediction model uses male sex, location of VTE (proximal DVT and PE are at higher risk), and D-dimer level to predict risk of recurrent VTE. This model has recently been updated to include a “dynamic” component to predict risk of recurrence of VTE from multiple random time points [89].
Overall, D-dimer may be useful for risk stratification. We often employ the method of stopping anticoagulation in patients with unprovoked VTE after 3 months (if the patient has no identifiable clinical risk factors that place them at high risk of recurrence) and testing D-dimer 1 month after cessation of anticoagulation. An elevated D-dimer is a solid reason to restart anticoagulation (potentially on an indefinite basis), while a negative D-dimer provides support for withholding further anticoagulation in the absence of other significant risk factors for recurrence. However, lack of agreement regarding assay cut-points as well as multiple reasons other than VTE for D-dimer elevation may limit widespread use of this method. We generally use a cutpoint of 250 ug/L as “negative,” though at least one study showed that cut-points of 250 ug/L versus 500 ug/L did not change the utility of this method [90].In our practice, risk prediction models are most useful to provide patients with additional information and a visual presentation to support our recommendation. This is particularly true of the Vienna prediction rule, which is available in a printable nomogram which can be distributed to patients and completed together during the clinic visit.
Imaging Analysis
Imaging analysis may also assist with risk stratification. Clinical assessment modules have been developed that incorporate repeat imaging studies for assessment of recannulization of affected veins. In patients with residual vein thrombosis (RVT) at the time anticoagulation was stopped, the hazard ratio for recurrence was 2.4 compared to those without RVT [91].There are a number of ways RVT could impact recurrence, including inpaired venous flow leading to stasis and activation of the coagulation cascade. Subsequent studies used serial ultrasound to determine when to stop anticoagulation. In one study, patients were anticoagulated for 3 months and for those that had RVT, anticoagulation was continued for up to 9 months for provoked and 21 months for unprovoked VTE. In comparison to fixed dosing of 6 months of anti-coagulation, those who had their length of anticoagulation tailored to ultrasonography findings had a lower rate of recurrent VTE [92].Limitations to using RVT in clinical decision-making include lack of a standard definition of RVT and variability in both timing of ultrasound (operator variability) and interpretation of results [93].
Other Options
Another option in patients who are being considered for indefinite anticoagulation is to decrease the intensity of anticoagulation. Since this would theoretically lower the risk of bleeding, the perceived benefit of long-term, low-intensity anticoagulation would be reduction in both bleeding and clotting risk. The PREVENT trial randomized patients who had received full-dose anticoagulation for a median of 6.5 months to either low-intensity warfarin (INR goal of 1.5-2.0 instead of 2.0-3.0) or placebo. In the anticoagulation group, there was a 64% risk reduction in recurrent VTE (hazard ratio 0.36, 95% CI 0.19 to 0.67) but an increased risk of bleeding (hazard ratio 1.92, 95% CI 1.26 to 2.93) [94].The ELATE study randomized patients with unprovoked VTE who had completed 3 or more months of full-intensity warfarin therapy (target INR 2.0–3.0) to continue therapy with either low-intensity warfarin (target INR 1.5–2.0) or full-intensity warfarin (target INR 2.0-3.0). Compared to the low-intensity group, the conventional-intensity group had lower rates of recurrent VTE and no increased rates of major bleeding [95].This study, however, has been criticized because of its overall low bleeding rate in both treatment groups.
Aspirin is an option in patients in whom long-term anticoagulation is untenable. The ASPIRE trial demonstrated that in patients with unprovoked VTE who had completed a course of initial anticoagulation, aspirin 100 mg daily reduced the risk of major vascular events compared to placebo with no increase in bleeding [96].However, aspirin was not associated with a significant reduction in risk of VTE alone (only the composite vascular event endpoint). The WARFASA trial, however, demonstrated that aspirin 100 mg daily was associated with a significant reduction in recurrent VTE compared to placebo after 6 to 18 months of anticoagulation without an increase in major bleeding [97].The absolute risk of recurrence was 11% in the placebo group and 5.9% in the aspirin group. More recently, the INSPIRE collaboration analyzed data from both trials and found that aspirin after initial anticoagulation reduced the risk of recurrent VTE by approximately 42% with a low rate of major bleeding [98].The absolute risk reduction was even larger in men and older patients. For this reason, we recommend aspirin to those patients in whom indefinite anticoagulation may be warranted from the standpoint of reducing risk of recurrent VTE but in whom the risk of bleeding precludes its use.
Hypercoagulable States In Specific Populations
Inherited Thrombophilias
Patients with a hereditary thrombophilia are at increased risk for incident VTE [99].These inherited mutations result in either a loss of normal anticoagulant function or gain of a prothrombotic state. Hereditary disorders associated with VTE include deficiency of antithrombin, protein C, or protein S, or the presence of factor V Leiden and/or prothrombin G20210A mutations. Although deficiency of protein C, protein S, or antithrombin is uncommon and affects only 0.5% of the population, these states have been associated with a 10-fold increased risk of thrombosis in comparison to the general population. Factor V Leiden and prothrombin gene mutation are less likely to be associated with incident thrombosis (2 to 5-fold increased risk of VTE) and are more prevalent in the Caucasian population [100].Though these hereditary thrombophilias increase risk of VTE, prophylactic anti-coagulation prior to a first VTE is not generally indicated.
Data regarding the impact of the inherited thrombophilias on risk of recurrent VTE is less well defined. While some data suggest that inherited thrombophilias are associated with increased risk of recurrent VTE, the degree of impact may be clinically modest especially in those with heterozygous factor V Leiden or prothrombin gene mutations [101].Ideally, a clinical trial would be designed to assess whether hereditary thrombophilia testing is beneficial for patients with VTE in decision-making regarding length of anticoagulation, type of anticoagulation, and risk of recurrence. If a patient with a low-risk inherited thrombophilia has a DVT in the setting of an additional provoking risk factor (surgery, pregnancy, etc), a 3-month course of anticoagulation followed by D-dimer assessment as above is reasonable. If a patient with an inherited thrombophilia experiences an idiopathic VTE, or if a patient with a “high-risk” thrombophilia as described above experiences any type of VTE, we generally recommend indefinite anticoagulation in the absence of high bleeding risk, though again this is a very patient-dependent choice.
Acquired Thrombophilias
Antiphospholipid Syndrome
Antibodies directed against proteins that bind phospho-lipids are associated with an acquired hypercoagulable state. The autoantibodies are categorized as antiphospho-lipid antibodies (APLAs), which include anticardiolipin antibodies (IgG and IgM), beta-2 glycoprotein 1 antibodies (anti-B2 GP), and lupus anticoagulant. These antibodies can form autonomously, as seen in primary disorders, or in association with autoimmune disease as a secondary disorder.
Criteria have been developed to distinguish antiphospholipid-associated clotting disorders from other forms of thrombophilia. The updated Sapporo criteria depend on both laboratory and clinical diagnostic criteria [102].The laboratory diagnosis of APLAs requires the presence of lupus anticoagulants, anticardiolipin antibodies, or anti-B2 GP on at least 2 assays at least 12 weeks apart with elevation above the 99th percentile of the testing laboratory’s normal distribution [103].Testing for lupus anticoagulant is based on 3 stages, the first of which is inhibition of phospholipid-dependent coagulation tests with prolonged clotting time (eg, aPTT or dilute Russell’s viper venom time). The diagnosis is confirmed by a secondary test in which excess hexagonal phase phospholipids are added to incubate with the patient’s plasma to absorb the APLA [104].The presence of anticardiolipin antibodies and anti beta-2 GP antibodies is determined using ELISA based immunoassays. Unlike most other thrombophilias, antiphospholipid syndrome is associated with both arterial and venous thromboembolic events and may be an indication for lifelong anticoagulation after a first thrombotic event. We generally recommend indefinite anticoagulation in the absence of significant bleeding risk.
Cancer-Associated Hypercoagulable State
Patients with cancer have a propensity for thromboembolic events. The underlying mechanisms responsible for cancer-associated clotting events are multifactorial and an area of intense research. Tumor cells can initiate activation of the clotting cascade through release of tissue factor and other pro-coagulant molecules [105].Type and stage of cancer impact risk of VTE, and the tumor itself can compress vasculature leading to venous stasis. Furthermore, chemotherapy, hormone therapy, antiangiogenic drugs, erythropoietin agents, and indwelling central venous catheters all are associated with increased risk of thrombotic events. Approximately 25% of all cancer patients will experience a thrombotic event during the course of their disease [106]. In fact, the presence of a spontaneous clot may be a harbinger of underlying malignancy [107].Approximately 10% of patients who present with an idiopathic VTE are diagnosed with cancer in the next 1 to 2 years.
The utility of extensive cancer screening in patients with spontaneous clotting events is often debated. The small studies that have addressed cancer associated clots have not demonstrated any mortality benefit with extensive screening. A prospective cohort study addressed the utility of limited versus extensive screening [108].In this study, all patients underwent a series of basic screening tests such as history taking, physical examination, chest radiograph, and basic laboratory parameters. Approximately half of the patients underwent additional testing (CT of chest and abdomen and mammography for women). Screening did not result in increased survival or fewer cancer-related deaths. 3.5 % of patients in the extensive screening group were diagnosed with malignancy in comparison to 2.4% in the limited screening group. During follow-up, cancer was diagnosed in 3.7% and 5.0% in the extensive and limited screening groups, respectively. The authors concluded that the low yield of extensive screening and lack of survival benefit did not warrant routinely ordering cancer screening tests above and beyond age-appropriate screening in patients with idiopathic VTE. However, it is known that identification of occult malignancy at an earlier stage of disease is beneficial, and cancer diagnosed within one year of an episode of VTE is generally more advanced and associated with a poorer prognosis [109].It is our practice to take a through history from patients with unprovoked clots particularly focusing on symptoms suggestive of an underlying cancer. We recommend that patients be up to date with all age-appropriate cancer screening.
Heparin-based products (rather than warfarin) are recommended for long-term treatment of cancer-associated DVT. Several trials, most prominently the CLOT trial, have demonstrated that LMWH is associated with reduced risk of recurrent VTE compared with warfarin in cancer patients [110].Fondaparinux may be a reasonable alternative if a patient is unable to tolerate a LMWH. In terms of treatment duration, patients with cancer-associated VTE should be anticoagulated indefinitely as long as they continue to have evidence of active malignancy and/or remain on antineoplastic treatment [111].
Heparin has potential anticancer effects beyond its anticoagulation properties. It is believed that heparin use in patients with cancer can influence cancer progression by acting as an antimetastatic agent. The molecular mechanisms underlying this significant observation are not completely understood, although the first documented benefit of these drugs dates back to the 1970s [112].Overall, LMWH have been associated with improved overall survival in cancer patients and this effect appears to be distinct from its ability to prevent life-threatening VTE episodes [113].
Estrogen-Related Thromboembolic Disease
Pregnancy is a well-established acquired hypercoagulable state, and thromboembolic disease accounts for significant morbidity and mortality in pregnancy and the postpartum period. Approximately 1 in 1000 women will suffer from a thrombotic event during pregnancy or shortly after delivery [8]. The etiology of the tendency to clot during pregnancy is multifactorial and mainly reflects venous stasis due to vasculature compression by the uterus, changes in coagulation factors as the pregnancy progresses, and endothelial damage during delivery, especially Cesarean section. Both factor VIII and von Willebrand factor levels increase, especially in the final months of pregnancy. Simultaneously, levels of the natural anticoagulant protein S diminish, leading to an acquired resistance to activated protein C which results in increased thrombin generation and therefore a hypercoagulable state [114].The risk of thrombosis in pregnancy is clearly heightened in women with inherited thrombophilias, especially in the postpartum period [115].
Similarly to pregnancy, hormone-based contraceptive agents and estrogen replacement therapies are also associated with increased thrombotic risk. Over the years, drug manufacturers have tried to mitigate the clotting risk associated with these drugs by reducing the amount of estrogen and altering the type of progesterone used, yet a risk still remains, resulting in a VTE incidence 2 to 7 times higher in this population [116].The risk is highest in the first 4 months of use and is unaffected by duration of use; risk extends for 3 months after cessation of estrogen-containing therapy. Patients who develop VTE while taking an oral contraceptive are generally instructed to stop the contraceptive and consider an alternative form of birth control. Although routine screening for thrombophilia is not offered to women before prescribing oral contraceptives, a thorough personal and family history regarding venous and arterial thrombotic events as well as recurrent pregnancy loss in women should be taken to evaluate thromboembolic risk factors. We generally avoid use of oral contraceptives in patients with a known hereditary thrombophilia, and consider screening prior to initiation of therapy in those with a strong family history of VTE.
Superficial VTE
Although the main disorders that comprise VTE are DVT and PE, another common presentation is superficial venous thromboembolism (SVT). The risk factors for developing an SVT are similar to those for DVT. In addition, varicose veins also increase the incidence of developing SVT [117].SVT is not associated with excessive mortality, and the main concern with it is progression to DVT. About 25% of patients diagnosed with SVT may have DVT or PE at the time of diagnosis and about 3% without DVT or PE at time of diagnosis developed one of these complications over the following 3 months; clot propagation is another common complication [118].Ultrasound may be of utility in diagnosing occult DVT in patients who initially diagnosed with SVT [119].
For patients who have only SVT at baseline without concomitant DVT or PE, it is difficult to determine which patients are at risk for developing DVT. Some risk stratification models include clot location. Since SVT clots usually develop in the saphenous vein, the clot would need to either progress from the sapheno-femoral junction to the common femoral vein; thus, any clots located near the sapheno-femoral junction are at risk of progressing into the deep vasculature [120].Clots within 3 cm of the junction may be more likely to progress to DVT [121].Chengelis and colleagues feel that proximal saphenous vein thrombosis should likely be treated with anticoagulation [122].Others have taken a more general approach, stating that all clots above the knee or in the thigh area should be treated aggressively [123].
There are solid data for the use of anticoagulation in SVT. In the STEFLUX (Superficial ThromboEmbolism and Fluxum) study, participants received the LMWH parnaparin at one of 3 doses: 8500 IU once daily for 10 days followed by placebo for 20 days, 8500 IU once daily for 10 days and then 6400 IU once daily for 20 days, or 4250 IU once daily for 30 days. Those who received the intermediate dosing had lower rates of DVT, PE, and relapse/SVT recurrence in the first 33 days [124].In the CALISTO trial, fondaparinux 2.5mg per day for 45 days effectively reduced the risk of symptomatic DVT, PE, or SVT recurrence or extension and was not associated with any increased major bleeding compared to placebo [125].A Cochrane review included 30 studies involving over 6500 participants with SVT of the lower extremities. The treatments used in these studies included fondaparinux, LMWH, UFH, non-steriodal anti-inflammatory agents, topical treatment, and surgery. According to the findings, use of fondaparinux at prophylactic dosing for 6 weeks is considered a valid therapeutic option for SVT [126].It is our practice to consider the use of anticoagulants (generally LMWH or fondaparinux) as part of the treatment regimen for SVT.
Target-Specific Oral Anticoagulants And Treatment of VTE
The direct thrombin inhibitor dabigatran directly binds to thrombin in a concentration-dependent manner [127].Peak plasma concentration is achieved within 0.5 to 2.0 hours after ingestion, and its half-life is 12 to 17 hours. Use of dabigatran in both primary and secondary prevention of VTE has been extensively studied, especially in orthopedic surgery where there have been 4 main trials (RE-MOBILIZE, RE-MODEL, RE-NOVATE, and RE-NOVATE I and II). While RE-MOBILIZE showed that dabigatran 220 mg or 150 mg once daily was inferior to enoxaparin 30 mg twice daily in preventing VTE after total knee arthroplasty, RE-MODEL and RE-NOVATE I and II demonstrated that dabigatran 150 mg or 220 mg once daily was noninferior to enoxaparin 40 mg once daily for prevention of VTE in patients undergoing total knee replacement and hip replacement [128–131].The side effect profile was also promising, with no significant differences in the frequency of major bleeding between dabigatran and enoxaparin. Pooled data and meta-analyses from these trials have demonstrated that for prevention of VTE associated with hip or knee surgery, dabigatran 220 mg or 150 mg once daily is as effective as 40 mg of enoxaparin given daily or 30 mg given twice a day, with a similar bleeding profile [132,133].
More recently, dabigatran been used in the acute treatment and secondary prevention of VTE. In the RE-COVER trial, dabigatran 150 mg twice daily was compared to warfarin (INR 2–3) in the treatment of acute VTE for 6 months, after an initial treatment period of up to 9 days with LMWH or UFH. Dabigatran was noninferior to warfarin with respect to 6-month incidence of recurrent symptomatic objectively confirmed VTE and related deaths, and was not associated with increased bleeding [134].In the RE-MEDY and RE-SONATE trials of extended anticoagulation, dabigatran was as effective as warfarin for prevention of recurrent VTE when continued after 3 months of initial anticoagulation and associated with less bleeding, and was more effective than placebo in preventing recurrent VTE but associated with a higher risk of bleeding [135].Unexpectedly, the risk of acute coronary syndrome was slightly higher in the dabigatran group than the warfarin group, as seen in other studies.
Rivaroxaban, a TSOAC that targets factor Xa, has also shown efficacy in preventing VTE after knee or hip surgery. The RE-CORD 1-4 studies all focused on the use of rivaroxaban in comparison to enoxaparin and found that rivaroxaban 10 mg once daily was superior to enoxaparin 40 mg once daily in prevention of VTE in total knee and total hip arthroplasty [136–138].Meta-analysis of multiple rivaroxaban VTE prophylaxis trials also demonstrated that rivaroxaban significantly lowered the risk of VTE in these surgical patients in comparison to the use of enoxaparin [139].Prophylactic use of rivaroxaban was also studied in acutely ill hospitalized patients in the MAGELLAN trial. Rivaroxaban 10 mg daily for 35 days was compared to enoxaparin 40 mg daily for 10 days followed by placebo and was found to be noninferior to enoxaparin in reduction of VTE risk at day 10 and superior to placebo at day 35 [140].However, the rate of bleeding, although low in both arms, was higher in the rivaroxaban arm.
Rivaroxaban has been studied in randomized clinical trials for acute treatment of DVT and PE and for extended prophylaxis for recurrent VTE (EINSTEIN-DVT, EINSTEIN-PE and EINSTEIN-Extension, respectively). The treatment strategy for use of rivaroxaban differed from that of dabigatran (in the RE-COVER trial), as rivaroxaban was used upfront as initial anticoagulation rather than after an initial period of parenteral therapy with LMWH or UFH. In both the DVT and PE trials, rivaroxaban was noninferior to standard treatment with enoxaparin followed by warfarin therapy, with no significant difference in major bleeding at 6 months of treatment [141,142].The extension trial also demonstrated that use of rivaroxaban in comparison to placebo for an additional 6 or 12 months after standard therapy was associated with significantly fewer recurrent VTE [141]. These studies led to FDA approval for rivaroxaban for primary prevention of VTE in patients undergoing elective total hip or knee repair surgery, for treatment of acute DVT or PE, and for extended prophylaxis in patients following initial treatment.
The anti-factor Xa TSOAC apixaban has been studied in similar fashion as rivaroxaban. In the AMPLIFY study, apixaban was given at a dose of 10 mg twice daily for 7 days followed by 5 mg twice daily for 6 months (as monotherapy, without initial parenteral agent) and compared to enoxaparin followed by warfarin for treatment of acute VTE. Apixaban was as effective as warfarin in terms of recurrent symptomatic VTE or VTE-related death, and was associated with significantly fewer bleeding events [143].Extended-duration apixaban given at treatment dose (5 mg twice daily) or at prophylactic dose (2.5 mg twice daily) for 12 months after completion of treatment-dose apixaban for VTE demonstrated superiority to placebo for extended prophylaxis in AMPLIFY-EXT, and there was no increase in major bleeding compared to placebo [144].Apixaban was recently approved by the FDA for both treatment and secondary prophylaxis of VTE.
More recently, a third anti-factor Xa TSOAC edoxaban demonstrated noninferiority to warfarin in prevention of recurrent symptomatic VTE when administered to patients with DVT or PE at 60 mg once daily for 3 to 12 months [145].Edoxaban also led to significantly less bleeding than warfarin. Edoxaban was recently approved by the FDA for treatment of VTE.
These TSOACs show promise in treatment and prevention of VTE but should be used in patients who meet appropriate criteria for renal function, age, and bleeding risk, as there are currently no available antidotes to reverse their effects. If significant bleeding occurs and cannot be controlled by usual maneuvers such as mechanical compression or surgical intervention, there is little data to guide the use of pharmacologic interventions. Plasma dabigatran levels can be reduced through the use of hemodialysis [146].Antibodies capable of neutralizing dabigatran have been developed, and one specific antibody, idarucizumab, was well-tolerated and showed immediate and complete reversal of dabigatran in subjects of different age and renal function [147,148].Andexanet, a modified recombinant derivative of factor Xa with no catalytic activty, acts as a “decoy receptor” with higher affinity to factor Xa inhibitors than natural factor Xa. Phase II studies in healthy volunteers demonstrated that andexanet immediately reversed the anticoagulation activity of apixaban, rivaroxaban, enoxaparin, and most recently edoxaban without thrombotic consequences [149].Two randomized, double-blind, placebo-controlled phase III studies (ANNEXA-A, looking at the reversal of apixaban, and ANNEXA-R, looking at reversal of rivaroxaban) are underway, and preliminary results show that a single intravenous bolus of andexanet demonstrated almost complete reversal [150].Finally, aripazine (PER977), a synthetic small molecule that binds to heparins as well as all TSOACs, was shown in a phase II trial to decrease blood clotting time to within 10% above baseline value in 10 minutes or less with an effect lasting for 24 hours [151].
Some have advocated for use of prothrombin complex concentrate (PCC) or recombinant factor VIIa for reversal of TSOAC-associated bleeding. Rivaroxaban was demonstrated to be partially reversible by PCC, whereas this approach was not as successful for dabigatran in healthy volunteers [152].In vitro evidence, however, showed that PCC did not significantly change aPTT [153].At present, the use of nonspecific hemostatic agents (including recombinant factor VIIa, 4-factor prothrombin complex concentrate, and activated prothrombin complex concentrates) is suggested for reversal of TSOACs in patients who present with life-threatening bleeding [154,155].
Conclusion
Patients with VTE present with a wide range of findings and factors that impact management. Decision making in VTE management is a fluid process that should be re-evaluated as new data emerge and individual circumstances change. There is more focus on VTE prevention and treatment today than there was even a decade ago. Diagnostic algorithms, identification of new risk factors, refinement in understanding of the pathogenesis of thrombosis, and identification of new anticoagulants with more favorable risk-benefit profiles will all ultimately contribute to improved patient care.
Corresponding author: Jean M. Connors, MD, Brigham and Women's Hospital, 75 Francis St., Boston, MA 02215.
1. White RH. The epidemiology of venous thromboembolism. Circulation 2003;107(Suppl 1):I4-8.
2. Moster KM and Fedullo PF. The diagnosis of deep-vein thrombosis. N Engl J Med 1994;330:863–4.
3. Kearon C. Natural history of venous thromboembolism. Circulation 2003;(Suppl 1):122–30.
4. Heijboer H, Brandjes DP, Buller HR, et al. Deficiencies of coagulation-inhibiting and fibrinolytic proteins in outpatients with deep-vein thrombosis. N Engl J Med 1990;323:1512–6.
5. Mateo J, Oliver A, Borrell M, et al. Laboratory evaluation and clinical characteristics of 2,132 consecutive unselected patients with venous thromboembolism—results of the Spanish Multicentric Study on Thrombophilia (EMET-Study). Thromb Haemost 1997;77:441–51.
6. Lyman GH. Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis. Cancer 2011;117:1334–49.
7. White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost 2003;90:446–55.
8. Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med 2008;359:2025–33.
9. Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 2008;117:93–102.
10. Mahmoodi BK, Gansevoort RT, Naess IA, et al. Association of mild to moderate chronic kidney disease with venous thromboembolism: pooled analysis of five prospective general population cohorts. Circulation 2012;126:1964–71.
11. Landolfi R, Marchioli R, Patrono C. Mechanisms of bleeding and thrombosis in myeloproliferative disorders. Thromb Haemost 1997;78:617–21.
12. Hillmen P, Lewis SM, Bessler M, et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med1995;333:1253–8.
13.Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet 2010;375:657–63.
14. Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost 2008;6:1262–6.
15. Kibbe MR, Ujiki M, Goodwin AL, et al. Iliac vein compression in an aymptomatic patient population. J Vasc Surg 2004;39:937–43.
16. Chee YL, Culligan DJ, Watson HG. Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br J Haematol 2001;114:878–80.
17. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ 2013;347:f5298.
18. Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004;292:1573–80.
19. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 1998;90:1371–88.
20. Glueck CJ, Richardson-Royer C, Schultz R, et al. Testosterone therapy, thrombophilia-hypofibrinolysis, and hospitalization for deep venous thrombosis-pulmonary embolus: an exploratory, hypothesis-generating study. Clin Appl Thromb Hemost 2014;20:244–9.
21. Johannesdottir SA, Horvath-Puho E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med 2013;173:743–52.
22. Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 2006;21:722–7.
23. Ocak G, Vossen CY, Verduijn M, et al. Risk of venous thrombosis in patients with major illnesses: results from the MEGA study. J Thromb Haemost 2013;11:116-23.
24. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107 (Suppl 1): I9–16.
25. Clemetson KJ. Platelet GPIb-V-IX complex. Thromb Haemost 1997;78:266–70.
26. Kroll MH, Schafer AI. Biochemical mechanisms of platelet activation. Blood 1989;74:1181–95.
27. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997;350:1795–8.
28. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients’ probability of pulmonary embolism: increasing the model’s utility with the SimpliRED D-dimer. Thromb Haemost 2000;416–20.
29. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med 2006; 144:165–71.
30. Stein PD, Hull RD, Patel KC, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med 2004;140:589–602.
31. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res 2010;125:e123–27.
32. Crowther MA, Cook DJ, Griffith LE, et al. Neither baseline tests of molecular hypercoagulability nor D-dimer levels predict deep venous thrombosis in critically ill medical-surgical patients. Intensive Care Med 2005;31:48–55.
33. Karami-Djurabi R, Klok FA, Kooiman J, et al. D-dimer testing in patients with suspected pulmonary embolism and impaired renal function. Am J Med 2009;122:1050–3.
34. Chan WS, Chunilal S, Lee A, et al. A red blood cell agglutination D-dimer test to exclude deep venous thrombosis in pregnancy. Ann Intern Med 2007;147:165–70.
35. Righini M, Goehring C, Bounameaux H, Perrier A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med 2000; 109:357–61.
36. Woller SC, Stevens SM, Adams DM, et al. Assessment of the safety and efficiency of using an age-adjusted D-dimer threshold to exclude suspected pulmonary embolism. Chest 2014; 146:1444–51.
37. Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation 2004; 109 (Suppl 1): I9–14.
38. Hirsh J, Lee AY. How we diagnose and treat deep vein thrombosis. Blood 2002;99:3102–10.
39. Tapson VF, Carroll BA, Davidson BL, et al. The diagnostic approach to acute venous thromboembolism. Clinical practice guideline. American Thoracic Society. Am J Respir Crit Care Med 1999;160:1043–66.
40. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e351S–418S.
41. Pezzullo JA, Perkins AB, Cronan JJ. Symptomatic deep vein thrombosis: diagnosis with limited compression US. Radiology 1996;198:67–70.
42. Frederick MG, Hertzberg BS, Kliewer MA, et al. Can the US examination for lower extremity deep venous thrombosis be abbreviated? A prospective study of 755 examinations Radiology 1996;199:45–7.
43. Remy-Jardin M, Remy J, Deschidre F, et al. Diagnosis of pulmonary embolism with spiral CT: comparison with pulmonary angiography and scintigraphy. Radiology 1996;200:699–706.
44. Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:2317–27.
45. Goodacre S. In the clinic. Deep venous thrombosis. Ann Intern Med 2008;149:ITC3–1.
46. Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med 2010;363:266–74.
47. Kucher N. Clinical practice. Deep-vein thrombosis of the upper extremities. N Engl J Med 2011;364:861–9.
48. Constans J, Salmi LR, Sevestre-Pietri MA, et al. A clinical prediction score for upper extremity deep venous thrombosis. Thromb Haemost 2008;99:202–7.
49. Meyer G, Vicault E, Danasys T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402–11.
50. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-bline, placebo-controlled randomized trial. J Thromb Haemost 2014;12:459–68.
51. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012;141(2 Suppl):7S–47S.
52. Bounameaux H. Unfractionated versus low-molecular-weight heparin in the treatment of venous thromboembolism. Vasc Med 1998;3:41–6.
53. Erkens PM, Prins MH. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev 2010;9:CD001100.
54. Buller HR, Davidson BL, Decousus H, et al. Fondaprinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med 2004;140: 867–73.
55. Buller HR, Davidson BL, Decousus H, et al. Subcutaenous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med 2003;349:1695–702.
56. Bates SM, Weitz JI. Coagulation assays. Circulation 2005;112:e53–60.
57. Pon TK, Dager WE, Roberts AJ, White RH. Subcutaneous enoxaparin for therapeutic anticoagulation in hemodialysis patients. Thromb Res 2014;133:1023–8.
58. Speeckaert MM, Devreese KM, Vanholder RC, Dhondt A. Fondaparinux as an alternative to vitamin K antagonists in haemodialysis patients. Nephrol Dial Transplant 2013;28:3090–5.
59. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005;172:1041–6.
60. Jiminez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–9.
61. Schulman S. Advances in the management of venous thromboembolism. Best Pract Res Clin Haematol 2012;25:361–77.
62. Erkens PM, Gandara E, Wells P, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost 2010;8:2412–7.
63. Chan YC, Valenti D, Mansfield AO, Stansby G. Warfarin induced skin necrosis. Br J Surg 2000; 87:266–72.
64. Brandjes DP, Heijboer H, Buller HR, et al. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal-vein thrombosis. N Engl J Med 1992;327:1485–9.
65. Segal JB, Streiff MB, Hofmann LV, et al. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med 2007;146:211–22.
66. Pinede L, Duhaut P, Cucherat M, et al. Comparison of long versus short duration of anticoagulant therapy after a first episode of venous thromboembolism: a meta-analysis of randomized, controlled trials. J Intern Med 2000;247:553–62.
67. Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of anticoagulation trial study group. N Engl J Med 1995;332:1661–5.
68. Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011;342:d3036.
69. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-07.
70. Agnelli G, Prandoni P, Becattini C, et al. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 2003;139:19–25.
71. Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin optimal duration Italian trial investigators. N Engl J Med 2001;345:165–9.
72. Bauer KA. Long-term management of venous thromboembolism: a 61-year old woman with unprovoked venous thromboembolism. JAMA 2011;305:1336–45.
73. Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014;123:
1794–801.
74. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest 2008;133(6 Suppl):257S–298S.
75. Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 1993;95:315–28.
76. Ruiz-Gimenez N, Suarez C, Gonzalez R, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE registry. Thromb Haemost 2008;100:26–31.
77. Kearon C, Akl EA, Comerota AJ. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e419S–94S.
78. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess one-year risk of major bleeding in atrial fibrillation patients: The Euro Heart Survey. Chest 2010;138:1093–1100.
79. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006;151:713–9.
80. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and risk factors in atrial fibrillation) study. J Am Coll Cardiol 2011;58:395–401.
81. Caldeira D, Costa J, Fernandes RM, et al. Performance of the HAS-BLED high bleeding-risk category, compared to ATRIA and HEMORR2HAGES in patients with atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol 2014;40:277–84.
82. Douketis JD, Crowther MA, Foster GA, Ginsberg JS. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med 2001;110:515–9.
83. Palareti G, Legnani C, Cosmi B, et al. Risk of venous thromboembolism recurrence: high negative predictive value of D-dimer performed after oral anticoagulation is stopped. Thromb Haemost 2002; 87:7–12.
84. Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006;355:1780–9.
85. Bruinstroop E, Klok FA, Van De Ree MA, et al. Elevated D-dimer levels predict recurrence in patients with idiopathic venous thromboembolism: a meta-analysis. J Thromb Haemost 2009;611–8.
86. Verhovsek M, Douketis JD, Yi Q, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med 2008;149:481–90.
87. Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012;10:1019–25.
88. Eichinger S, Heinze G, Jandeck LM, et al. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna Prediction Model. Circulation2010; 121:1630–6.
89. Eichinger S, Heinze G, Kyrle PA. D-dimer levels over time and the risk of recurrent venous thromboembolism: an update of the Vienna prediction model. J Am Heart Assoc 2014; 3: e000467.
90. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med 2010;153:523–31.
91. Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002;137:955–60.
92. Prandoni P, Prins MH, Lensing AW, et al. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med 2009;150:577–85.
93. Marcucci M, Iorio A, Douketis J. Management of patients with unprovoked venous thromboembolism: an evidence-based and practical approach. Curr Treat Options Cardiovasc Med 2013;15:224–39.
94. Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003;348:1425–34.
95. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003;349:631–9.
96. Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012;367:1979–87.
97. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012;366:1959–67.
98. Simes J, Becattini C, Agnelli G, et al. Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation 2014;130:1062–71.
99. Makris M. Thrombophilia: grading the risk. Blood 2009;113:5038–9.
100. Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA 2009;30:2472.
101. Ho WK, Hankey GH, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med 2006;166:729–36.
102. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
103. Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on lupus anticoagulant/antiphospholipid antibody of the scientific and standardization committee of the international society on thrombosis and haemostasis. J Thromb Haemost 2009; 7:1737–40.
104. Brandt JT, Barna LK, Triplett DA. Laboratory identification of lupus anticoagulants: results of the Second International Workship for identification of lupus anticoagulants. On behalf of the subcommittee on lupus anticoagulants/antiphospholipid antibodies of the ISTH. Thromb Haemost 1995;74: 1597–603.
105. Gale AJ, Gordon SG. Update on tumor cell procoagulant factors. Acta Haematol 2001;106:25-32.
106. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood 2013;122:1712–23.
107. Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation 2003; 107 (Suppl 1): I17–21.
108. Van Doormaal FF, Terpstra W, Van Der Griend R, et al. Is extensive screening for cancer in idiopathic venous thromboembolism warranted? J Thromb Haemost 2011;9:79–84.
109. Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846–50.
110. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146-53.
111. Streiff MB, Bockenstedt PL, Cataland SR, et al. Venous thromboembolic disease. J Natl Compr Canc Netw 2013;11:1402-29.
112. Elias EG, Sepulveda F, Mink IB. Increasing the efficiency of cancer chemotherapy with heparin: “clinical study.” J Surg Oncol 1973;5:189–93.
113. Lazo-Langner A, Goss GD, Spaans JN, Rodger MA. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J Thromb Haemost 2007;5:729–37.
114. Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol 2003;16:153–68.
115. Gerhardt A, Scharf RE, Beckmann MW, et al. Prothrombin and factor V mutations in women with a history of thrombosis during pregnancy and the puerperium. N Engl J Med 2000;342:374–80.
116. Rott H. Thrombotic risks of oral contraceptives. Curr Opin Obstet Gynecol 2012;24:235-40.
117. Marchiori A, Mosena L, Prandoni P. Superficial vein thrombosis: risk factors, diagnosis, and treatment. Semin Thromb Hemost 2006;32:737–43.
118. Decousus H, Quere I, Presles E, et al. Superficial venous thrombosis and venous thromboembolism: a large, prospective epidemiologic study. Ann Intern Med 2010;152:218–24.
119. Galanaud JP, Genty C, Sevestre MA, et al. Predictive factors for concurrent deep-vein thrombosis and symptomatic venous thromboembolic recurrence in case of superficial venous thrombosis. The OPTIMEV study. Thromb Haemost 2011;105:31–9.
120. Sullivan V, Denk PM, Sonnad SS, et al. Ligation versus anticoagulation: treatment of above-knee superficial thrombophlebitis not involving the deep venous system. J Am Coll Surg 2001;193:556–62.
121. Lohr JM, McDevitt DT, Lutter KS, et al. Operative management of greater saphenous thrombophlebitis involving the saphenofemoral junction. Am J Surg 1992;164:269–75.
122. Chengelis DL, Benedick PJ, Glover JL, et al. Progression of superficial venous thrombosis to deep vein thrombosis. J Vasc Surg 1996;24:745–9.
123. Verlato F, Zuccetta P, Prandoni P, et al. An unexpectedly high rate of pulmonary embolism in patients with superficial thrombophlebitis of the leg. J Vasc Surg 1999;30:1113–5.
124. Cosmi B, Filippini M, Tonti D, et al. A randomized double-blind study of low-molecular-weight heparin (parnaparin) for superficial vein thrombosis: STEFLUX (Superficial ThromboEmbolism and Fluxum). J Thromb Haemost 2012;10:1026–35.
125. Decousus H, Prandoni P, Mismetti P, et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med 2010;363:1222–32.
126. Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev 2013;4:CD004982.
127. Stangier J, Rathgen K, Stahle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007;64:292–303.
128. RE-MOBILIZE Writing Committee, Ginsberg JS, Davidson BL, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009;24:1–9.
129. Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007;5:2178–85.
130. Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomized, double-blind, non-inferiority trial. Lancet 2007;370:949–56.
131. Eriksson BI, Dahl OE, Huo MH, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomized, double-blind, non-inferiority trial. Thromb Haemost2011;105:721–9.
132. Friedman RJ, Dahl OE, Rosencher N, et al. Dabigatran versus enoxaparin for prevention of venous thromboembolism after hip or knee arthroplasty: a pooled analysis of three trials. Thromb Res 2010;126:175–82.
133. Wolowacz SE, Roskell NS, Plumb JM, et al. Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following total hip or knee arthroplasty. A meta-analysis. Thromb Haemost 2009;101:77–85.
134. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;36:2342–52.
135. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013;368:709–18.
136. Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765–75.
137. Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomized controlled trial. Lancet 2008;372:31–9.
138. Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776–86.
139. Cao YB, Zhang JD, Shen H, Jiang YY. Rivaroxaban versus enoxaparin for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 2010;66:1099–108.
140. Cohen AT, Spiro TE, Buller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013;368:513–23.
141. EINSTEIN Investigators, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510.
142. EINSTEIN-PE Investigators, Buller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–97.
143. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799–808.
144. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699–708.
145. Hokusai-VTE investigators, Buller HR, Decousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406–15.
146. Khadzhynov D, Wagner F, Formella S, et al. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost 2013;109:596–605.
147. Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood 2013;121:3554–62.
148. Glund S, Stangier J, Schmohl M, et al. Idarucizumab, a specific antidote for dabigatran: immediate, complete, and sustained reversal of dabigatran induced anticoagulation in elderly and renally impaired subjects. Presented at the American Society of Hematology 2014 Annual Meeting; San Francisco, CA.
149. Crowther MA, Levy GG, Lu G, et al. A phase 2 randomized, double-blind, placebo-controlled trial demonstrating reversal of edoxaban-induced anticoagulation in healthy subjects by andexanet alfa (PRT064445), a universal antidote for factor Xa (fXa) inhibitors. Abstract #4269. Presented at the American Society of Hematology 2014 Annual Meeting, San Francisco, CA.
150. Crowther M, Levy GG, Lu G, et al. ANNEXATM-A: A phase 3 randomized, double-blind, placebo-controlled trial, demonstrating reversal of apixaban-induced anticoagulation in older subjects by andexanet alfa (PRT 064445), a universal antidote for factor Xa (fa) inhibitors. Presented at the American Heart Association 2014 Annual Meeting, Chicago, IL.
151. Ansell JE, Bakhru SH, Lauliche BE, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med 2014;371:2141–2.
152. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011;124:1573–9.
153. Korber MK, Langer E, Ziemer S, et al. Measurement and reversal of prophylactic and therapeutic peak levels of rivaroxaban: an in vitro study. Clin Appl Thromb Hemost 2014;20:735–40.
154. Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013;80:443–51.
155. Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood 2014;123:1152–8.
1. White RH. The epidemiology of venous thromboembolism. Circulation 2003;107(Suppl 1):I4-8.
2. Moster KM and Fedullo PF. The diagnosis of deep-vein thrombosis. N Engl J Med 1994;330:863–4.
3. Kearon C. Natural history of venous thromboembolism. Circulation 2003;(Suppl 1):122–30.
4. Heijboer H, Brandjes DP, Buller HR, et al. Deficiencies of coagulation-inhibiting and fibrinolytic proteins in outpatients with deep-vein thrombosis. N Engl J Med 1990;323:1512–6.
5. Mateo J, Oliver A, Borrell M, et al. Laboratory evaluation and clinical characteristics of 2,132 consecutive unselected patients with venous thromboembolism—results of the Spanish Multicentric Study on Thrombophilia (EMET-Study). Thromb Haemost 1997;77:441–51.
6. Lyman GH. Venous thromboembolism in the patient with cancer: focus on burden of disease and benefits of thromboprophylaxis. Cancer 2011;117:1334–49.
7. White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost 2003;90:446–55.
8. Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med 2008;359:2025–33.
9. Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 2008;117:93–102.
10. Mahmoodi BK, Gansevoort RT, Naess IA, et al. Association of mild to moderate chronic kidney disease with venous thromboembolism: pooled analysis of five prospective general population cohorts. Circulation 2012;126:1964–71.
11. Landolfi R, Marchioli R, Patrono C. Mechanisms of bleeding and thrombosis in myeloproliferative disorders. Thromb Haemost 1997;78:617–21.
12. Hillmen P, Lewis SM, Bessler M, et al. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med1995;333:1253–8.
13.Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet 2010;375:657–63.
14. Flinterman LE, Van Der Meer FJ, Rosendaal FR, Doggen CJ. Current perspective of venous thrombosis in the upper extremity. J Thromb Haemost 2008;6:1262–6.
15. Kibbe MR, Ujiki M, Goodwin AL, et al. Iliac vein compression in an aymptomatic patient population. J Vasc Surg 2004;39:937–43.
16. Chee YL, Culligan DJ, Watson HG. Inferior vena cava malformation as a risk factor for deep venous thrombosis in the young. Br J Haematol 2001;114:878–80.
17. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ 2013;347:f5298.
18. Cushman M, Kuller LH, Prentice R, et al. Estrogen plus progestin and risk of venous thrombosis. JAMA 2004;292:1573–80.
19. Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 1998;90:1371–88.
20. Glueck CJ, Richardson-Royer C, Schultz R, et al. Testosterone therapy, thrombophilia-hypofibrinolysis, and hospitalization for deep venous thrombosis-pulmonary embolus: an exploratory, hypothesis-generating study. Clin Appl Thromb Hemost 2014;20:244–9.
21. Johannesdottir SA, Horvath-Puho E, Dekkers OM, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. JAMA Intern Med 2013;173:743–52.
22. Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 2006;21:722–7.
23. Ocak G, Vossen CY, Verduijn M, et al. Risk of venous thrombosis in patients with major illnesses: results from the MEGA study. J Thromb Haemost 2013;11:116-23.
24. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107 (Suppl 1): I9–16.
25. Clemetson KJ. Platelet GPIb-V-IX complex. Thromb Haemost 1997;78:266–70.
26. Kroll MH, Schafer AI. Biochemical mechanisms of platelet activation. Blood 1989;74:1181–95.
27. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997;350:1795–8.
28. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients’ probability of pulmonary embolism: increasing the model’s utility with the SimpliRED D-dimer. Thromb Haemost 2000;416–20.
29. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med 2006; 144:165–71.
30. Stein PD, Hull RD, Patel KC, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med 2004;140:589–602.
31. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res 2010;125:e123–27.
32. Crowther MA, Cook DJ, Griffith LE, et al. Neither baseline tests of molecular hypercoagulability nor D-dimer levels predict deep venous thrombosis in critically ill medical-surgical patients. Intensive Care Med 2005;31:48–55.
33. Karami-Djurabi R, Klok FA, Kooiman J, et al. D-dimer testing in patients with suspected pulmonary embolism and impaired renal function. Am J Med 2009;122:1050–3.
34. Chan WS, Chunilal S, Lee A, et al. A red blood cell agglutination D-dimer test to exclude deep venous thrombosis in pregnancy. Ann Intern Med 2007;147:165–70.
35. Righini M, Goehring C, Bounameaux H, Perrier A. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med 2000; 109:357–61.
36. Woller SC, Stevens SM, Adams DM, et al. Assessment of the safety and efficiency of using an age-adjusted D-dimer threshold to exclude suspected pulmonary embolism. Chest 2014; 146:1444–51.
37. Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation 2004; 109 (Suppl 1): I9–14.
38. Hirsh J, Lee AY. How we diagnose and treat deep vein thrombosis. Blood 2002;99:3102–10.
39. Tapson VF, Carroll BA, Davidson BL, et al. The diagnostic approach to acute venous thromboembolism. Clinical practice guideline. American Thoracic Society. Am J Respir Crit Care Med 1999;160:1043–66.
40. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e351S–418S.
41. Pezzullo JA, Perkins AB, Cronan JJ. Symptomatic deep vein thrombosis: diagnosis with limited compression US. Radiology 1996;198:67–70.
42. Frederick MG, Hertzberg BS, Kliewer MA, et al. Can the US examination for lower extremity deep venous thrombosis be abbreviated? A prospective study of 755 examinations Radiology 1996;199:45–7.
43. Remy-Jardin M, Remy J, Deschidre F, et al. Diagnosis of pulmonary embolism with spiral CT: comparison with pulmonary angiography and scintigraphy. Radiology 1996;200:699–706.
44. Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006;354:2317–27.
45. Goodacre S. In the clinic. Deep venous thrombosis. Ann Intern Med 2008;149:ITC3–1.
46. Agnelli G, Becattini C. Acute pulmonary embolism. N Engl J Med 2010;363:266–74.
47. Kucher N. Clinical practice. Deep-vein thrombosis of the upper extremities. N Engl J Med 2011;364:861–9.
48. Constans J, Salmi LR, Sevestre-Pietri MA, et al. A clinical prediction score for upper extremity deep venous thrombosis. Thromb Haemost 2008;99:202–7.
49. Meyer G, Vicault E, Danasys T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 2014;370:1402–11.
50. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-bline, placebo-controlled randomized trial. J Thromb Haemost 2014;12:459–68.
51. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012;141(2 Suppl):7S–47S.
52. Bounameaux H. Unfractionated versus low-molecular-weight heparin in the treatment of venous thromboembolism. Vasc Med 1998;3:41–6.
53. Erkens PM, Prins MH. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev 2010;9:CD001100.
54. Buller HR, Davidson BL, Decousus H, et al. Fondaprinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med 2004;140: 867–73.
55. Buller HR, Davidson BL, Decousus H, et al. Subcutaenous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med 2003;349:1695–702.
56. Bates SM, Weitz JI. Coagulation assays. Circulation 2005;112:e53–60.
57. Pon TK, Dager WE, Roberts AJ, White RH. Subcutaneous enoxaparin for therapeutic anticoagulation in hemodialysis patients. Thromb Res 2014;133:1023–8.
58. Speeckaert MM, Devreese KM, Vanholder RC, Dhondt A. Fondaparinux as an alternative to vitamin K antagonists in haemodialysis patients. Nephrol Dial Transplant 2013;28:3090–5.
59. Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005;172:1041–6.
60. Jiminez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–9.
61. Schulman S. Advances in the management of venous thromboembolism. Best Pract Res Clin Haematol 2012;25:361–77.
62. Erkens PM, Gandara E, Wells P, et al. Safety of outpatient treatment in acute pulmonary embolism. J Thromb Haemost 2010;8:2412–7.
63. Chan YC, Valenti D, Mansfield AO, Stansby G. Warfarin induced skin necrosis. Br J Surg 2000; 87:266–72.
64. Brandjes DP, Heijboer H, Buller HR, et al. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal-vein thrombosis. N Engl J Med 1992;327:1485–9.
65. Segal JB, Streiff MB, Hofmann LV, et al. Management of venous thromboembolism: a systematic review for a practice guideline. Ann Intern Med 2007;146:211–22.
66. Pinede L, Duhaut P, Cucherat M, et al. Comparison of long versus short duration of anticoagulant therapy after a first episode of venous thromboembolism: a meta-analysis of randomized, controlled trials. J Intern Med 2000;247:553–62.
67. Schulman S, Rhedin AS, Lindmarker P, et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of anticoagulation trial study group. N Engl J Med 1995;332:1661–5.
68. Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants’ data from seven trials. BMJ 2011;342:d3036.
69. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-07.
70. Agnelli G, Prandoni P, Becattini C, et al. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 2003;139:19–25.
71. Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin optimal duration Italian trial investigators. N Engl J Med 2001;345:165–9.
72. Bauer KA. Long-term management of venous thromboembolism: a 61-year old woman with unprovoked venous thromboembolism. JAMA 2011;305:1336–45.
73. Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014;123:
1794–801.
74. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American college of chest physicians evidence-based clinical practice guidelines (8th edition). Chest 2008;133(6 Suppl):257S–298S.
75. Landefeld CS, Beyth RJ. Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 1993;95:315–28.
76. Ruiz-Gimenez N, Suarez C, Gonzalez R, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE registry. Thromb Haemost 2008;100:26–31.
77. Kearon C, Akl EA, Comerota AJ. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012; 141 (2 Suppl): e419S–94S.
78. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess one-year risk of major bleeding in atrial fibrillation patients: The Euro Heart Survey. Chest 2010;138:1093–1100.
79. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006;151:713–9.
80. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and risk factors in atrial fibrillation) study. J Am Coll Cardiol 2011;58:395–401.
81. Caldeira D, Costa J, Fernandes RM, et al. Performance of the HAS-BLED high bleeding-risk category, compared to ATRIA and HEMORR2HAGES in patients with atrial fibrillation: a systematic review and meta-analysis. J Interv Card Electrophysiol 2014;40:277–84.
82. Douketis JD, Crowther MA, Foster GA, Ginsberg JS. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis? Am J Med 2001;110:515–9.
83. Palareti G, Legnani C, Cosmi B, et al. Risk of venous thromboembolism recurrence: high negative predictive value of D-dimer performed after oral anticoagulation is stopped. Thromb Haemost 2002; 87:7–12.
84. Palareti G, Cosmi B, Legnani C, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006;355:1780–9.
85. Bruinstroop E, Klok FA, Van De Ree MA, et al. Elevated D-dimer levels predict recurrence in patients with idiopathic venous thromboembolism: a meta-analysis. J Thromb Haemost 2009;611–8.
86. Verhovsek M, Douketis JD, Yi Q, et al. Systematic review: D-dimer to predict recurrent disease after stopping anticoagulant therapy for unprovoked venous thromboembolism. Ann Intern Med 2008;149:481–90.
87. Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost 2012;10:1019–25.
88. Eichinger S, Heinze G, Jandeck LM, et al. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna Prediction Model. Circulation2010; 121:1630–6.
89. Eichinger S, Heinze G, Kyrle PA. D-dimer levels over time and the risk of recurrent venous thromboembolism: an update of the Vienna prediction model. J Am Heart Assoc 2014; 3: e000467.
90. Douketis J, Tosetto A, Marcucci M, et al. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Ann Intern Med 2010;153:523–31.
91. Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002;137:955–60.
92. Prandoni P, Prins MH, Lensing AW, et al. Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med 2009;150:577–85.
93. Marcucci M, Iorio A, Douketis J. Management of patients with unprovoked venous thromboembolism: an evidence-based and practical approach. Curr Treat Options Cardiovasc Med 2013;15:224–39.
94. Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003;348:1425–34.
95. Kearon C, Ginsberg JS, Kovacs MJ, et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003;349:631–9.
96. Brighton TA, Eikelboom JW, Mann K, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012;367:1979–87.
97. Becattini C, Agnelli G, Schenone A, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012;366:1959–67.
98. Simes J, Becattini C, Agnelli G, et al. Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation 2014;130:1062–71.
99. Makris M. Thrombophilia: grading the risk. Blood 2009;113:5038–9.
100. Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA 2009;30:2472.
101. Ho WK, Hankey GH, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med 2006;166:729–36.
102. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306.
103. Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on lupus anticoagulant/antiphospholipid antibody of the scientific and standardization committee of the international society on thrombosis and haemostasis. J Thromb Haemost 2009; 7:1737–40.
104. Brandt JT, Barna LK, Triplett DA. Laboratory identification of lupus anticoagulants: results of the Second International Workship for identification of lupus anticoagulants. On behalf of the subcommittee on lupus anticoagulants/antiphospholipid antibodies of the ISTH. Thromb Haemost 1995;74: 1597–603.
105. Gale AJ, Gordon SG. Update on tumor cell procoagulant factors. Acta Haematol 2001;106:25-32.
106. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood 2013;122:1712–23.
107. Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation 2003; 107 (Suppl 1): I17–21.
108. Van Doormaal FF, Terpstra W, Van Der Griend R, et al. Is extensive screening for cancer in idiopathic venous thromboembolism warranted? J Thromb Haemost 2011;9:79–84.
109. Sorensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343:1846–50.
110. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146-53.
111. Streiff MB, Bockenstedt PL, Cataland SR, et al. Venous thromboembolic disease. J Natl Compr Canc Netw 2013;11:1402-29.
112. Elias EG, Sepulveda F, Mink IB. Increasing the efficiency of cancer chemotherapy with heparin: “clinical study.” J Surg Oncol 1973;5:189–93.
113. Lazo-Langner A, Goss GD, Spaans JN, Rodger MA. The effect of low-molecular-weight heparin on cancer survival. A systematic review and meta-analysis of randomized trials. J Thromb Haemost 2007;5:729–37.
114. Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol 2003;16:153–68.
115. Gerhardt A, Scharf RE, Beckmann MW, et al. Prothrombin and factor V mutations in women with a history of thrombosis during pregnancy and the puerperium. N Engl J Med 2000;342:374–80.
116. Rott H. Thrombotic risks of oral contraceptives. Curr Opin Obstet Gynecol 2012;24:235-40.
117. Marchiori A, Mosena L, Prandoni P. Superficial vein thrombosis: risk factors, diagnosis, and treatment. Semin Thromb Hemost 2006;32:737–43.
118. Decousus H, Quere I, Presles E, et al. Superficial venous thrombosis and venous thromboembolism: a large, prospective epidemiologic study. Ann Intern Med 2010;152:218–24.
119. Galanaud JP, Genty C, Sevestre MA, et al. Predictive factors for concurrent deep-vein thrombosis and symptomatic venous thromboembolic recurrence in case of superficial venous thrombosis. The OPTIMEV study. Thromb Haemost 2011;105:31–9.
120. Sullivan V, Denk PM, Sonnad SS, et al. Ligation versus anticoagulation: treatment of above-knee superficial thrombophlebitis not involving the deep venous system. J Am Coll Surg 2001;193:556–62.
121. Lohr JM, McDevitt DT, Lutter KS, et al. Operative management of greater saphenous thrombophlebitis involving the saphenofemoral junction. Am J Surg 1992;164:269–75.
122. Chengelis DL, Benedick PJ, Glover JL, et al. Progression of superficial venous thrombosis to deep vein thrombosis. J Vasc Surg 1996;24:745–9.
123. Verlato F, Zuccetta P, Prandoni P, et al. An unexpectedly high rate of pulmonary embolism in patients with superficial thrombophlebitis of the leg. J Vasc Surg 1999;30:1113–5.
124. Cosmi B, Filippini M, Tonti D, et al. A randomized double-blind study of low-molecular-weight heparin (parnaparin) for superficial vein thrombosis: STEFLUX (Superficial ThromboEmbolism and Fluxum). J Thromb Haemost 2012;10:1026–35.
125. Decousus H, Prandoni P, Mismetti P, et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med 2010;363:1222–32.
126. Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev 2013;4:CD004982.
127. Stangier J, Rathgen K, Stahle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007;64:292–303.
128. RE-MOBILIZE Writing Committee, Ginsberg JS, Davidson BL, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009;24:1–9.
129. Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007;5:2178–85.
130. Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomized, double-blind, non-inferiority trial. Lancet 2007;370:949–56.
131. Eriksson BI, Dahl OE, Huo MH, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomized, double-blind, non-inferiority trial. Thromb Haemost2011;105:721–9.
132. Friedman RJ, Dahl OE, Rosencher N, et al. Dabigatran versus enoxaparin for prevention of venous thromboembolism after hip or knee arthroplasty: a pooled analysis of three trials. Thromb Res 2010;126:175–82.
133. Wolowacz SE, Roskell NS, Plumb JM, et al. Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following total hip or knee arthroplasty. A meta-analysis. Thromb Haemost 2009;101:77–85.
134. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;36:2342–52.
135. Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013;368:709–18.
136. Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765–75.
137. Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomized controlled trial. Lancet 2008;372:31–9.
138. Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776–86.
139. Cao YB, Zhang JD, Shen H, Jiang YY. Rivaroxaban versus enoxaparin for thromboprophylaxis after total hip or knee arthroplasty: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 2010;66:1099–108.
140. Cohen AT, Spiro TE, Buller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013;368:513–23.
141. EINSTEIN Investigators, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510.
142. EINSTEIN-PE Investigators, Buller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–97.
143. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799–808.
144. Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699–708.
145. Hokusai-VTE investigators, Buller HR, Decousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406–15.
146. Khadzhynov D, Wagner F, Formella S, et al. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost 2013;109:596–605.
147. Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood 2013;121:3554–62.
148. Glund S, Stangier J, Schmohl M, et al. Idarucizumab, a specific antidote for dabigatran: immediate, complete, and sustained reversal of dabigatran induced anticoagulation in elderly and renally impaired subjects. Presented at the American Society of Hematology 2014 Annual Meeting; San Francisco, CA.
149. Crowther MA, Levy GG, Lu G, et al. A phase 2 randomized, double-blind, placebo-controlled trial demonstrating reversal of edoxaban-induced anticoagulation in healthy subjects by andexanet alfa (PRT064445), a universal antidote for factor Xa (fXa) inhibitors. Abstract #4269. Presented at the American Society of Hematology 2014 Annual Meeting, San Francisco, CA.
150. Crowther M, Levy GG, Lu G, et al. ANNEXATM-A: A phase 3 randomized, double-blind, placebo-controlled trial, demonstrating reversal of apixaban-induced anticoagulation in older subjects by andexanet alfa (PRT 064445), a universal antidote for factor Xa (fa) inhibitors. Presented at the American Heart Association 2014 Annual Meeting, Chicago, IL.
151. Ansell JE, Bakhru SH, Lauliche BE, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med 2014;371:2141–2.
152. Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011;124:1573–9.
153. Korber MK, Langer E, Ziemer S, et al. Measurement and reversal of prophylactic and therapeutic peak levels of rivaroxaban: an in vitro study. Clin Appl Thromb Hemost 2014;20:735–40.
154. Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013;80:443–51.
155. Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated bleeding. Blood 2014;123:1152–8.
When Should Hypopituitarism Be Suspected?
Case
A 53-year-old woman with a history of a suprasellar meningioma resected nine years ago with recurrence of a 4.5x2 cm mass one year ago and recent ventriculoperitoneal (VP) shunt placement for hydrocephalus presented with altered mental status (AMS) and hallucinations. She was admitted for radiation therapy to the mass. The patient had little improvement in her mental status four weeks into a six-week, 4860 cGy course of photon therapy.
The internal medicine service was consulted for new onset tachycardia (103), hypotension (83/55), and fever (38.6 C). Laboratory data revealed a white blood cell count 4.8 x 109 cells/L, sodium 137 mmol/L, potassium 4.1 mmol/L, chloride 110 mmol/L, bicarbonate 28 mmol/L, blood urea nitrogen 3 mg/dl, creatinine 0.6 mg/dl, and glucose 91 mg/dl. Thyroid-stimulating hormone (TSH) was low at 0.38 mIU/mL. Urine specific gravity was 1.006. Workups for infectious and thromboembolic diseases were unremarkable.
Discussion
Hypopituitarism is a disorder of impaired hormone production from the anterior and, less commonly, posterior pituitary gland. The condition can originate from several broad categories of diseases affecting the hypothalamus, pituitary stalk, or pituitary gland. In adults, the etiology is often from the mass effect of tumors or from treatment with surgery or radiotherapy. Other causes include vascular, infectious, infiltrative, inflammatory, and idiopathic. Well-substantiated data on the incidence and prevalence of hypopituitarism is sparse. It has an estimated prevalence of 45.5 cases per 100,000 and incidence of 4.2 cases per 100,000 per year.1
Clinical manifestations of hypopituitarism depend on the type and severity of hormone deficiency. The consequences of adrenal insufficiency (AI) range from smoldering and nonspecific findings (e.g. fatigue, lethargy, indistinct gastrointestinal symptoms, eosinophilia, fever) to full-fledged crisis (e.g. AMS, severe electrolyte abnormalities, hemodynamic compromise, shock). The presentation of central AI (i.e., arising from hypothalamic or pituitary pathology) is often more subtle than primary AI. In central AI, only glucocorticoid (GC) function is disrupted, leaving the renin-angiotensin-aldosterone system and mineralocorticoid (MC) function intact. This is in stark contrast to primary AI resulting from direct adrenal gland injury, which nearly always disrupts both GC and MC function, leading to more profound circulatory collapse and electrolyte disturbance.2
Aside from orthostatic blood pressure or possible low-grade fever, few physical exam features are associated with central AI. Hyperpigmentation is not seen due to the lack of anterior pituitary-derived melanocortins that stimulate melanocytes and induce pigmentation. As for laboratory findings, hyperkalemia is a feature of primary AI (due to hypoaldosteronism) but is not seen in central AI. Hyponatremia occurs in both types of AI and is vasopressin-mediated. Hyponatremia is more common in primary AI, resulting from appropriate vasopressin release that occurs due to hypotension. Hyponatremia also occurs in secondary AI because of increased vasopressin secretion mediated directly by hypocortisolemia.3,4
In summary, hyperpigmentation and the electrolyte pattern of hyponatremia and hyperkalemia are distinguishing clinical characteristics of primary AI, occurring in up to 90% of cases, but these features would not be expected with central AI.5
In the hospitalized patient with multiple active acute illnesses and infectious risk factors, it can be difficult to recognize the diagnosis of AI or hypopituitarism. Not only do signs and symptoms frequently overlap, but concomitant acute illness is usually a triggering event. Crisis should be suspected in the setting of unexplained fever, dehydration, or shock out of proportion to severity of current illness.5
Not surprisingly, high rates of partial or complete hypopituitarism are seen in patients following surgical removal of pituitary tumors or nearby neoplasms (e.g. craniopharyngiomas). Both surgery and radiotherapy for non-pituitary brain tumors are also major risk factors for development of hypopituitarism, occurring in up to 38% and 41% of patients, respectively.6 The strongest predictors of hormone failure are higher radiation doses, proximity to the pituitary-hypothalamus, and longer time interval after completion of radiotherapy. Within 10 years after a median dose of 5000 rad (50Gy) directed at the skull base, nasopharynx, or cranium, up to three-fourths of patients will develop some degree of pituitary insufficiency. Later onset of hormone failure usually reflects hypothalamic injury, whereas higher irradiation doses can lead to earlier onset pituitary damage.5
Not all hormone-secreting cells of the hypothalamus or pituitary are equally susceptible to injury; there is a characteristic sequence of hormonal failure. The typical order of hormone deficiency from pituitary compression or destruction is as follows: growth hormone (GH) > follicle-stimulating hormone (FSH) > luteinizing hormone (LH) > TSH > adrenocorticotropic hormone (ACTH) > vasopressin. A similar pattern is seen following brain irradiation: GH > FSH and LH > ACTH > TSH. A recent systematic review of 18 studies with 813 patients receiving cranial radiotherapy for non-pituitary tumors found pituitary dysfunction was 45% for GH deficiency, compared to 22% for ACTH deficiency.7
Biochemical diagnosis of hypopituitarism consists of measuring the various pituitary and target hormone levels as well as provocation testing. When interpreting these tests, whether to identify excess or deficient states, it is important to remember the individual values are part of the broader hypothalamic-pituitary axis feedback loops. Thus, it can be more useful designating if a high or low test value is appropriately or inappropriately high or low. In the presented case, low TSH level could be misinterpreted as excess thyroid hormone supplementation. An appropriately elevated free T4 level would confirm this, but an inappropriately low free T4 would raise suspicion of central hypothalamic-pituitary dysfunction.
With high enough clinical suspicion of hypopituitarism, empiric treatment with thyroid supplementation and corticosteroids should be started before confirmation of the diagnosis, to prevent secondary organ dysfunction and improve morbidity and mortality.2 Rapid administration with intravenous levothyroxine can be given in severe hypothyroidism or myxedema.
“Stress-dose” steroids are generally recommended for patients who are also administered levothyroxine, as the desired increased in metabolic rate can deplete existing pituitary-adrenocortical hormone reserves, precipitating adrenal crisis.5 Stress-dose corticosteroids also ensure recruitment of a mineralocorticoid response. Cortisol has both GC and MC stimulating effects but is rapidly metabolized to cortisone, which lacks MC stimulating effects. Thus, high doses overwhelm this conversion step and allow remaining cortisol to stimulate MC receptors.2 These high doses may not be necessary in secondary AI (i.e., preserved MC function) but would be reasonable in an unstable patient or until confirmation is made with an inappropriately low ACTH.
Back to the Case
Morning cortisol returned undetectable, and ACTH was 14 pg/mL (6-58). Past records revealed a down-trending TSH from 1.12 to 0.38 mIU/mL, which had inappropriately prompted a levothyroxine dose reduction from 50 mcg to 25 mcg. A free thyroxine (T4) was low at 0.67 ng/dL (0.89-1.76). Estradiol, FSH, and LH were undetectable. Prolactin was 23 ng/mL (3-27). She was started on prednisone, 5 mg daily, and her levothyroxine was adjusted to a weight-based dose. Her fever resolved with the initiation of prednisone, and all cultures remained negative. Over two weeks, she improved back to her baseline, was discharged to a rehabilitation center, and eventually returned home.
Dr. Inman is a hospitalist at St. Mary’s Hospital and Regional Medical Center in Grand Junction, Colo. Dr. Bridenstine is an endocrinologist at the University of Colorado Denver. Dr. Cumbler is a hospitalist at the University of Colorado Denver.
References
- Regal M, Pàramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol. 2001;55(6):735-740.
- Bouillon R. Acute adrenal insufficiency. Endocrinol Metab Clin North Am. 2006;35(4):767-75, ix.
- Raff H. Glucocorticoid inhibition of neurohypophysial vasopressin secretion. Am J Physiol. 1987;252(4 Pt 2):R635-644.
- Erkut ZA, Pool C, Swaab DF. Glucocorticoids suppress corticotropin-releasing hormone and vasopressin expression in human hypothalamic neurons. J Clin Endocrinol Metab. 1998;83(6):2066-2073.
- Melmed S, Polonski KS, Reed Larsen P, Kronenberg HM. Williams Textbook of Endocrinology. 12th ed. Philadelphia, Pa.: Saunders/Elsevier; 2012.
- Schneider HJ, Aimaretti G, Kreitschmann-Andermahr I, Stalla GK, Ghigo E. Hypopituitarism. Lancet. 2007;369(9571):1461-1470.
- Appelman-Dijkstra NM, Kokshoorn NE, Dekkers OM, et al. Pituitary dysfunction in adult patients after cranial radiotherapy: systematic review and meta-analysis. J Clin Endocrinol Metabol. 2011;96(8):2330-2340.
Case
A 53-year-old woman with a history of a suprasellar meningioma resected nine years ago with recurrence of a 4.5x2 cm mass one year ago and recent ventriculoperitoneal (VP) shunt placement for hydrocephalus presented with altered mental status (AMS) and hallucinations. She was admitted for radiation therapy to the mass. The patient had little improvement in her mental status four weeks into a six-week, 4860 cGy course of photon therapy.
The internal medicine service was consulted for new onset tachycardia (103), hypotension (83/55), and fever (38.6 C). Laboratory data revealed a white blood cell count 4.8 x 109 cells/L, sodium 137 mmol/L, potassium 4.1 mmol/L, chloride 110 mmol/L, bicarbonate 28 mmol/L, blood urea nitrogen 3 mg/dl, creatinine 0.6 mg/dl, and glucose 91 mg/dl. Thyroid-stimulating hormone (TSH) was low at 0.38 mIU/mL. Urine specific gravity was 1.006. Workups for infectious and thromboembolic diseases were unremarkable.
Discussion
Hypopituitarism is a disorder of impaired hormone production from the anterior and, less commonly, posterior pituitary gland. The condition can originate from several broad categories of diseases affecting the hypothalamus, pituitary stalk, or pituitary gland. In adults, the etiology is often from the mass effect of tumors or from treatment with surgery or radiotherapy. Other causes include vascular, infectious, infiltrative, inflammatory, and idiopathic. Well-substantiated data on the incidence and prevalence of hypopituitarism is sparse. It has an estimated prevalence of 45.5 cases per 100,000 and incidence of 4.2 cases per 100,000 per year.1
Clinical manifestations of hypopituitarism depend on the type and severity of hormone deficiency. The consequences of adrenal insufficiency (AI) range from smoldering and nonspecific findings (e.g. fatigue, lethargy, indistinct gastrointestinal symptoms, eosinophilia, fever) to full-fledged crisis (e.g. AMS, severe electrolyte abnormalities, hemodynamic compromise, shock). The presentation of central AI (i.e., arising from hypothalamic or pituitary pathology) is often more subtle than primary AI. In central AI, only glucocorticoid (GC) function is disrupted, leaving the renin-angiotensin-aldosterone system and mineralocorticoid (MC) function intact. This is in stark contrast to primary AI resulting from direct adrenal gland injury, which nearly always disrupts both GC and MC function, leading to more profound circulatory collapse and electrolyte disturbance.2
Aside from orthostatic blood pressure or possible low-grade fever, few physical exam features are associated with central AI. Hyperpigmentation is not seen due to the lack of anterior pituitary-derived melanocortins that stimulate melanocytes and induce pigmentation. As for laboratory findings, hyperkalemia is a feature of primary AI (due to hypoaldosteronism) but is not seen in central AI. Hyponatremia occurs in both types of AI and is vasopressin-mediated. Hyponatremia is more common in primary AI, resulting from appropriate vasopressin release that occurs due to hypotension. Hyponatremia also occurs in secondary AI because of increased vasopressin secretion mediated directly by hypocortisolemia.3,4
In summary, hyperpigmentation and the electrolyte pattern of hyponatremia and hyperkalemia are distinguishing clinical characteristics of primary AI, occurring in up to 90% of cases, but these features would not be expected with central AI.5
In the hospitalized patient with multiple active acute illnesses and infectious risk factors, it can be difficult to recognize the diagnosis of AI or hypopituitarism. Not only do signs and symptoms frequently overlap, but concomitant acute illness is usually a triggering event. Crisis should be suspected in the setting of unexplained fever, dehydration, or shock out of proportion to severity of current illness.5
Not surprisingly, high rates of partial or complete hypopituitarism are seen in patients following surgical removal of pituitary tumors or nearby neoplasms (e.g. craniopharyngiomas). Both surgery and radiotherapy for non-pituitary brain tumors are also major risk factors for development of hypopituitarism, occurring in up to 38% and 41% of patients, respectively.6 The strongest predictors of hormone failure are higher radiation doses, proximity to the pituitary-hypothalamus, and longer time interval after completion of radiotherapy. Within 10 years after a median dose of 5000 rad (50Gy) directed at the skull base, nasopharynx, or cranium, up to three-fourths of patients will develop some degree of pituitary insufficiency. Later onset of hormone failure usually reflects hypothalamic injury, whereas higher irradiation doses can lead to earlier onset pituitary damage.5
Not all hormone-secreting cells of the hypothalamus or pituitary are equally susceptible to injury; there is a characteristic sequence of hormonal failure. The typical order of hormone deficiency from pituitary compression or destruction is as follows: growth hormone (GH) > follicle-stimulating hormone (FSH) > luteinizing hormone (LH) > TSH > adrenocorticotropic hormone (ACTH) > vasopressin. A similar pattern is seen following brain irradiation: GH > FSH and LH > ACTH > TSH. A recent systematic review of 18 studies with 813 patients receiving cranial radiotherapy for non-pituitary tumors found pituitary dysfunction was 45% for GH deficiency, compared to 22% for ACTH deficiency.7
Biochemical diagnosis of hypopituitarism consists of measuring the various pituitary and target hormone levels as well as provocation testing. When interpreting these tests, whether to identify excess or deficient states, it is important to remember the individual values are part of the broader hypothalamic-pituitary axis feedback loops. Thus, it can be more useful designating if a high or low test value is appropriately or inappropriately high or low. In the presented case, low TSH level could be misinterpreted as excess thyroid hormone supplementation. An appropriately elevated free T4 level would confirm this, but an inappropriately low free T4 would raise suspicion of central hypothalamic-pituitary dysfunction.
With high enough clinical suspicion of hypopituitarism, empiric treatment with thyroid supplementation and corticosteroids should be started before confirmation of the diagnosis, to prevent secondary organ dysfunction and improve morbidity and mortality.2 Rapid administration with intravenous levothyroxine can be given in severe hypothyroidism or myxedema.
“Stress-dose” steroids are generally recommended for patients who are also administered levothyroxine, as the desired increased in metabolic rate can deplete existing pituitary-adrenocortical hormone reserves, precipitating adrenal crisis.5 Stress-dose corticosteroids also ensure recruitment of a mineralocorticoid response. Cortisol has both GC and MC stimulating effects but is rapidly metabolized to cortisone, which lacks MC stimulating effects. Thus, high doses overwhelm this conversion step and allow remaining cortisol to stimulate MC receptors.2 These high doses may not be necessary in secondary AI (i.e., preserved MC function) but would be reasonable in an unstable patient or until confirmation is made with an inappropriately low ACTH.
Back to the Case
Morning cortisol returned undetectable, and ACTH was 14 pg/mL (6-58). Past records revealed a down-trending TSH from 1.12 to 0.38 mIU/mL, which had inappropriately prompted a levothyroxine dose reduction from 50 mcg to 25 mcg. A free thyroxine (T4) was low at 0.67 ng/dL (0.89-1.76). Estradiol, FSH, and LH were undetectable. Prolactin was 23 ng/mL (3-27). She was started on prednisone, 5 mg daily, and her levothyroxine was adjusted to a weight-based dose. Her fever resolved with the initiation of prednisone, and all cultures remained negative. Over two weeks, she improved back to her baseline, was discharged to a rehabilitation center, and eventually returned home.
Dr. Inman is a hospitalist at St. Mary’s Hospital and Regional Medical Center in Grand Junction, Colo. Dr. Bridenstine is an endocrinologist at the University of Colorado Denver. Dr. Cumbler is a hospitalist at the University of Colorado Denver.
References
- Regal M, Pàramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol. 2001;55(6):735-740.
- Bouillon R. Acute adrenal insufficiency. Endocrinol Metab Clin North Am. 2006;35(4):767-75, ix.
- Raff H. Glucocorticoid inhibition of neurohypophysial vasopressin secretion. Am J Physiol. 1987;252(4 Pt 2):R635-644.
- Erkut ZA, Pool C, Swaab DF. Glucocorticoids suppress corticotropin-releasing hormone and vasopressin expression in human hypothalamic neurons. J Clin Endocrinol Metab. 1998;83(6):2066-2073.
- Melmed S, Polonski KS, Reed Larsen P, Kronenberg HM. Williams Textbook of Endocrinology. 12th ed. Philadelphia, Pa.: Saunders/Elsevier; 2012.
- Schneider HJ, Aimaretti G, Kreitschmann-Andermahr I, Stalla GK, Ghigo E. Hypopituitarism. Lancet. 2007;369(9571):1461-1470.
- Appelman-Dijkstra NM, Kokshoorn NE, Dekkers OM, et al. Pituitary dysfunction in adult patients after cranial radiotherapy: systematic review and meta-analysis. J Clin Endocrinol Metabol. 2011;96(8):2330-2340.
Case
A 53-year-old woman with a history of a suprasellar meningioma resected nine years ago with recurrence of a 4.5x2 cm mass one year ago and recent ventriculoperitoneal (VP) shunt placement for hydrocephalus presented with altered mental status (AMS) and hallucinations. She was admitted for radiation therapy to the mass. The patient had little improvement in her mental status four weeks into a six-week, 4860 cGy course of photon therapy.
The internal medicine service was consulted for new onset tachycardia (103), hypotension (83/55), and fever (38.6 C). Laboratory data revealed a white blood cell count 4.8 x 109 cells/L, sodium 137 mmol/L, potassium 4.1 mmol/L, chloride 110 mmol/L, bicarbonate 28 mmol/L, blood urea nitrogen 3 mg/dl, creatinine 0.6 mg/dl, and glucose 91 mg/dl. Thyroid-stimulating hormone (TSH) was low at 0.38 mIU/mL. Urine specific gravity was 1.006. Workups for infectious and thromboembolic diseases were unremarkable.
Discussion
Hypopituitarism is a disorder of impaired hormone production from the anterior and, less commonly, posterior pituitary gland. The condition can originate from several broad categories of diseases affecting the hypothalamus, pituitary stalk, or pituitary gland. In adults, the etiology is often from the mass effect of tumors or from treatment with surgery or radiotherapy. Other causes include vascular, infectious, infiltrative, inflammatory, and idiopathic. Well-substantiated data on the incidence and prevalence of hypopituitarism is sparse. It has an estimated prevalence of 45.5 cases per 100,000 and incidence of 4.2 cases per 100,000 per year.1
Clinical manifestations of hypopituitarism depend on the type and severity of hormone deficiency. The consequences of adrenal insufficiency (AI) range from smoldering and nonspecific findings (e.g. fatigue, lethargy, indistinct gastrointestinal symptoms, eosinophilia, fever) to full-fledged crisis (e.g. AMS, severe electrolyte abnormalities, hemodynamic compromise, shock). The presentation of central AI (i.e., arising from hypothalamic or pituitary pathology) is often more subtle than primary AI. In central AI, only glucocorticoid (GC) function is disrupted, leaving the renin-angiotensin-aldosterone system and mineralocorticoid (MC) function intact. This is in stark contrast to primary AI resulting from direct adrenal gland injury, which nearly always disrupts both GC and MC function, leading to more profound circulatory collapse and electrolyte disturbance.2
Aside from orthostatic blood pressure or possible low-grade fever, few physical exam features are associated with central AI. Hyperpigmentation is not seen due to the lack of anterior pituitary-derived melanocortins that stimulate melanocytes and induce pigmentation. As for laboratory findings, hyperkalemia is a feature of primary AI (due to hypoaldosteronism) but is not seen in central AI. Hyponatremia occurs in both types of AI and is vasopressin-mediated. Hyponatremia is more common in primary AI, resulting from appropriate vasopressin release that occurs due to hypotension. Hyponatremia also occurs in secondary AI because of increased vasopressin secretion mediated directly by hypocortisolemia.3,4
In summary, hyperpigmentation and the electrolyte pattern of hyponatremia and hyperkalemia are distinguishing clinical characteristics of primary AI, occurring in up to 90% of cases, but these features would not be expected with central AI.5
In the hospitalized patient with multiple active acute illnesses and infectious risk factors, it can be difficult to recognize the diagnosis of AI or hypopituitarism. Not only do signs and symptoms frequently overlap, but concomitant acute illness is usually a triggering event. Crisis should be suspected in the setting of unexplained fever, dehydration, or shock out of proportion to severity of current illness.5
Not surprisingly, high rates of partial or complete hypopituitarism are seen in patients following surgical removal of pituitary tumors or nearby neoplasms (e.g. craniopharyngiomas). Both surgery and radiotherapy for non-pituitary brain tumors are also major risk factors for development of hypopituitarism, occurring in up to 38% and 41% of patients, respectively.6 The strongest predictors of hormone failure are higher radiation doses, proximity to the pituitary-hypothalamus, and longer time interval after completion of radiotherapy. Within 10 years after a median dose of 5000 rad (50Gy) directed at the skull base, nasopharynx, or cranium, up to three-fourths of patients will develop some degree of pituitary insufficiency. Later onset of hormone failure usually reflects hypothalamic injury, whereas higher irradiation doses can lead to earlier onset pituitary damage.5
Not all hormone-secreting cells of the hypothalamus or pituitary are equally susceptible to injury; there is a characteristic sequence of hormonal failure. The typical order of hormone deficiency from pituitary compression or destruction is as follows: growth hormone (GH) > follicle-stimulating hormone (FSH) > luteinizing hormone (LH) > TSH > adrenocorticotropic hormone (ACTH) > vasopressin. A similar pattern is seen following brain irradiation: GH > FSH and LH > ACTH > TSH. A recent systematic review of 18 studies with 813 patients receiving cranial radiotherapy for non-pituitary tumors found pituitary dysfunction was 45% for GH deficiency, compared to 22% for ACTH deficiency.7
Biochemical diagnosis of hypopituitarism consists of measuring the various pituitary and target hormone levels as well as provocation testing. When interpreting these tests, whether to identify excess or deficient states, it is important to remember the individual values are part of the broader hypothalamic-pituitary axis feedback loops. Thus, it can be more useful designating if a high or low test value is appropriately or inappropriately high or low. In the presented case, low TSH level could be misinterpreted as excess thyroid hormone supplementation. An appropriately elevated free T4 level would confirm this, but an inappropriately low free T4 would raise suspicion of central hypothalamic-pituitary dysfunction.
With high enough clinical suspicion of hypopituitarism, empiric treatment with thyroid supplementation and corticosteroids should be started before confirmation of the diagnosis, to prevent secondary organ dysfunction and improve morbidity and mortality.2 Rapid administration with intravenous levothyroxine can be given in severe hypothyroidism or myxedema.
“Stress-dose” steroids are generally recommended for patients who are also administered levothyroxine, as the desired increased in metabolic rate can deplete existing pituitary-adrenocortical hormone reserves, precipitating adrenal crisis.5 Stress-dose corticosteroids also ensure recruitment of a mineralocorticoid response. Cortisol has both GC and MC stimulating effects but is rapidly metabolized to cortisone, which lacks MC stimulating effects. Thus, high doses overwhelm this conversion step and allow remaining cortisol to stimulate MC receptors.2 These high doses may not be necessary in secondary AI (i.e., preserved MC function) but would be reasonable in an unstable patient or until confirmation is made with an inappropriately low ACTH.
Back to the Case
Morning cortisol returned undetectable, and ACTH was 14 pg/mL (6-58). Past records revealed a down-trending TSH from 1.12 to 0.38 mIU/mL, which had inappropriately prompted a levothyroxine dose reduction from 50 mcg to 25 mcg. A free thyroxine (T4) was low at 0.67 ng/dL (0.89-1.76). Estradiol, FSH, and LH were undetectable. Prolactin was 23 ng/mL (3-27). She was started on prednisone, 5 mg daily, and her levothyroxine was adjusted to a weight-based dose. Her fever resolved with the initiation of prednisone, and all cultures remained negative. Over two weeks, she improved back to her baseline, was discharged to a rehabilitation center, and eventually returned home.
Dr. Inman is a hospitalist at St. Mary’s Hospital and Regional Medical Center in Grand Junction, Colo. Dr. Bridenstine is an endocrinologist at the University of Colorado Denver. Dr. Cumbler is a hospitalist at the University of Colorado Denver.
References
- Regal M, Pàramo C, Sierra SM, Garcia-Mayor RV. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin Endocrinol. 2001;55(6):735-740.
- Bouillon R. Acute adrenal insufficiency. Endocrinol Metab Clin North Am. 2006;35(4):767-75, ix.
- Raff H. Glucocorticoid inhibition of neurohypophysial vasopressin secretion. Am J Physiol. 1987;252(4 Pt 2):R635-644.
- Erkut ZA, Pool C, Swaab DF. Glucocorticoids suppress corticotropin-releasing hormone and vasopressin expression in human hypothalamic neurons. J Clin Endocrinol Metab. 1998;83(6):2066-2073.
- Melmed S, Polonski KS, Reed Larsen P, Kronenberg HM. Williams Textbook of Endocrinology. 12th ed. Philadelphia, Pa.: Saunders/Elsevier; 2012.
- Schneider HJ, Aimaretti G, Kreitschmann-Andermahr I, Stalla GK, Ghigo E. Hypopituitarism. Lancet. 2007;369(9571):1461-1470.
- Appelman-Dijkstra NM, Kokshoorn NE, Dekkers OM, et al. Pituitary dysfunction in adult patients after cranial radiotherapy: systematic review and meta-analysis. J Clin Endocrinol Metabol. 2011;96(8):2330-2340.
Impact of a Community Health Worker–Led Diabetes Education Program on Hospital and Emergency Department Utilization and Costs
From Baylor Scott & White Health.
Abstract
- Objective: To assess the impact of a community health worker–led diabetes education program (DEP) on hospital utilization trends and to evaluate the return on investment.
- Methods: A retrospective, pre-post study design was used to examine differences in inpatient and emergency department (ED) utilization and costs for DEP patients in the year prior to and following enrollment. Patients with diabetes who received care at the same clinics but who were not enrolled in DEP served as a control group. Analysis of covariance was used to test for differences in utilization outcomes between DEP patients and controls, controlling for age, sex, and ethnicity.
- Results: DEP patients had a significant reduction in mean inpatient encounters (0.08 vs. 0.18), LOS per inpatient encounter (0.28 vs. 0.67), and inpatient cost per patient ($406 vs. $902) following DEP enrollment. Patients in the control group also experienced a significant reduction in inpatient LOS and costs. Neither group experienced a significant difference in ED utilization or costs. Return on investment for DEP was –66%, as the annual cost savings generated per patient from reduced utilization ($137.19) were less than the annual DEP costs (investment) per patient ($402.80).
- Conclusion: CHW-led diabetes education programs like DEP may provide additional benefits than can be gained from access to primary care alone in terms of avoidance of costly hospitalizations for diabetes-related complications. Although the DEP did not generate an overall cost savings for the health care system in the short term, additional savings may be generated in the long term through reductions of diabetes-related complications.
Diabetes poses a significant burden on the population of the United States in terms of morbidity, mortality, and costs of care. Currently, 29.1 million people in the United States have diabetes [1], and it is expected that 1 in 3 people could have diabetes by the year 2050 [2]. The economic impact of diabetes is also significant; as of 2012, 1 in 5 health care dollars went towards diabetes care [3], and the total cost of diabetes was estimated to be $245 billion [1]. This rapid increase in the incidence of diabetes and associated costs emphasizes the need for cost-effective strategies to prevent and manage diabetes within the U.S. population.
One strategy that has shown success in improving diabetes care and outcomes is the use of community health workers (CHWs) to deliver disease education and management programs. A CHW is a front-line public health worker who is often a trusted member of the community and has the capacity to influence patient understanding, enhance patient compliance, and promote more equitable practices in patient care management [4–6]. Several studies have shown that CHW-led interventions in diabetes management can help patients achieve control over their disease and improved health indicators such as HbA1c, blood pressure, lipid levels, and weight [7–17]. In addition, a few studies have shown that CHW-led interventions for patients with diabetes can help these patients avoid costly health care utilization in the form of emergency department (ED) visits and hospitalizations. Fedder et al found that diabetes patients on Medicaid who worked closely with CHWs experienced a decrease in ED visits (38%), hospitalizations (30%), and hospital admissions from ED (53%) [11]. This CHW intervention was associated with improved patient quality of life and a cost-savings of $2245 per patient per year for a total savings of $262,080 for 117 patients. More recently, a 2010 randomized controlled trial showed that African-American patients with diabetes who worked with a nurse case manager and a CHW were 23% less likely to make ED visits than those who just had a nurse case manager [18].
Despite the documented successes of CHW programs, the CHW model has not been widely adopted within integrated health care systems. A major barrier to adoption of CHW programs has been the lack of sustained funding for CHW services [17,19]. Historically, many CHW programs were supported by grants, as most payers were unwilling to fund these initiatives [4,17,19.] In 2008, the Centers for Medicare and Medicaid Services provided a mechanism to support CHW activities by approving a Medicaid state plan amendment authorizing payment for CHWs who worked under Medicaid-approved providers, such as physicians and nurses [19]. However, the lack of data available regarding the costs, cost-effectiveness, and potential costs savings of CHW programs continues to serve as a barrier to adoption [13,20].
Baylor Scott & White Health North (BSWH), formerly Baylor Health Care System in Dallas, Texas, created the CHW-led Diabetes Equity Project, a 5-year program supported with funding from Merck Foundation’s Alliance to Reduce Disparities in Diabetes, with the goal of reducing observed disparities in diabetes care and outcomes in the medically underserved, predominantly Hispanic communities surrounding BSWH hospitals [16,21,22]. The program featured specially trained, bilingual CHWs who served as members of primary care teams in 5 community clinics and delivered a culturally relevant diabetes self-management and education curriculum (DSME) targeting barriers to diabetes management commonly experienced by Hispanics. The objective of this study was to assess the impact of a CHW-led diabetes educucation program (DEP) on hospital utilization trends and to evaluate the return on investment of the program.
Methods
Setting
BSWH is one of the largest nonprofit health care systems in the United States and includes 46 hospitals, > 800 patient care sites, > 6000 affiliated physicians, 35,000 employees, and an accountable care organization. This study was conducted in 5 community clinics located in the Dallas metroplex surrounding BSWH North hospitals. The community clinics serve low-income, uninsured, and chronically ill patients. The study was approved by the Baylor Research Institute institutional review board.
DEP Intervention
The DEP program consisted of 2 initial 60-minute educational sessions and quarterly clinical assessments scheduled for 30 to 60 minutes for a maximum of 6 patient-contact hours over 12 consecutive months. The DSME curriculum for DEP was adapted from CoDE, a pilot program implemented in a Dallas clinic serving a largely uninsured Mexican American population [23]. Patients who participated in CoDE for 12 months experienced a significant reduction in HbA1c [23,24]. During the 2 educational sessions, the CHWs educated DEP participants about diabetes and the importance of blood glucose control, medication adherence, diet, and exercise. In addition to the educational sessions, CHWs performed quarterly clinical assessments of HbA1c, blood pressure, weight, and foot condition (visual and monofilament assessment). They also assessed self-management behaviors and facilitated goal setting at each visit. The CHWs documented patient visits in the electronic health record and contacted the patient’s primary care provider immediately if the patient was symptomatic or had critical blood glucose or blood pressure measurements as defined by program protocol.
Participants
Study participants were recruited from the clinics or referred by Baylor care coordinators following hospital visits related to uncontrolled diabetes from September 2009 to July 2013. Participants had to be 18 years or older with a diagnosis of type 2 diabetes and be uninsured or underinsured. Although the program targeted Hispanic patients, all patients who met the inclusion criteria were eligible to participate. To control for internal threats to validity such as history and maturation biases, we created a control group consisting of clinic patients who had a diagnosis of diabetes and met the DEP inclusion criteria but who did not enroll in DEP.
Assessment
We used a retrospective pre-post study design to assess the impact of the DEP on hospital utilization trends and the program’s return on investment (ROI). The primary outcomes were number of hospital encounters, length of stay (LOS) per encounter, and direct cost per patient. The pre-enrollment period was defined as the year priod to the day of the initial DEP visit (the date of enrollment for DEP patients) or the initial clinic visit (the date of “enrollment” for control patients). All study participants were included in the analysis regardless of their number of inpatient or ED encounters. If a participant did not have a documented encounter during the analysis time period, encounters, LOS, and medical costs were set to zero. Patients with at least 2 HbA1c measurements taken during the first year post enrollment were included in the analysis.
Data Sources
Inpatient and emergency department (ED) utilization data were obtained from the Dallas Fort-Worth Hospital Council (DFWHC) database. The DFWHC captures administrative data from over 80 participating hospital systems and 9 million unique patients in North Texas. The DFWHC applied a matching algorithm using first and last name and date of birth to match study participants with encounter and length of stay detail for all hospitalizations across all DFWHC member hospitals. We obtained direct medical costs for patients treated at BSWH facilities from the BSWH Trendstar administrative database. Direct medical cost was not available for encounters at non-BSWH facilities so cost was estimated for these patients based on BSWH costs using a prediction model that accounted for LOS, primary ICD-9 diagnosis, patient age, sex, and race. The model explained approximately 66% of the variation in direct costs (R2 = 0.6581).
Statistical Analysis
All analyses were performed in SAS V9.3 using an α level of 0.05. A 2-tailed independent samples t test was used to test the mean differences in utilization outcomes one year prior to and post program enrollment. An analysis of covariance (ANCOVA) was used to assess whether the mean change in utilization outcomes was greater for DEP patients than the control group, , after controlling for age, sex, and ethnicity. The gamma distribution with a log link function was used to model direct cost and the negative binomial distribution was used to model hospital encounters and LOS.
The ROI calculation included 2 components: DEP investment cost and risk-adjusted direct medical cost savings 1 year post program enrollment. DEP investment cost included the average yearly costs per community health worker, the fixed one-time start-up costs distributed across the length of the program (4 years), and the salaries of the 5 community health workers employed for the duration of the program. These costs were divided by the number of patients who enrolled in the DEP to calculate the per patient cost (investment). Direct medical cost savings were calculated as the mean reduction in hospitalization and ED costs per patient adjusted for age, sex, and ethnicity.
To account for DEP participants without encounter data, we estimated average cost savings in a 4-step process using a propensity score adjustment approach. We stratified encounters based on admission type (inpatient vs. ED) and combined the average cost savings calculated for both admission types to calculate mean total medical cost savings.
In the first step, direct cost was modeled on DEP participants with an encounter to obtain parameter estimates for pre- and post- program enrollment, age, sex, and ethnicity. The model was based on the log-link function with the gamma distribution. In the second step, we ran a propensity score logistic regression model to estimate the probability of any encounter being identified in the DFWHC database, adjusted for age, sex, ethnicity, and DEP clinic. In the third step, we generated 10,000 bootstrap samples, with replacement, to estimate the DEP population’s median expected change in cost. In the bootstrap sample, the parameter estimates from step 1 were used to predict the cost pre- and post- program enrollment for each patient. The expected change in cost per participant was then calculated, while adjusting for the propensity to have an encounter, estimated in step 2. The formula for calculating change in cost was Expected change in cost = [pre probability*pre cost – post probability*post cost].
In the final step, we retained the median cost savings from each bootstrap sample. Inpatient and ED cost savings were combined to produce a total cost savings across the patient population. The ROI formula was Financial gain (utilization cost savings – DEP investment)/DEP investment.
Results
Participant Demographics
Of the 1140 study participants, 878 were DEP patients and 262 were controls (Table 1). The majority of DEP patients were Hispanic females older than 40 years while the majority of the the control group was non-Hispanic males older than 40. Table 2 and Table 3 display the top 5 primary ICD-9 diagnoses by intervention group and pre/post enrollment status. Type 2 diabetes was the top diagnosis among DEP participants and controls with a pre-enrollment encounter.
Inpatient Encounters
Fourteen percent of the DEP participants and 37% of control group patients matched to an inpatient record during the pre-enrollment time period. The number of mean inpatient encounters for DEP participants decreased from 0.18 to 0.08 (P < 0.001) in the post period
Emergency Department Encounters
Eight percent of the DEP participants and 30% of controls matched to an ED record for the year prior to program enrollment. Neither DEP patients nor patients in the control group had a significant reduction in mean ED encounters (P = 0.88 and 0.74, respectively) or costs (P = 0.76 and 0.12, respectively) post enrollment.
The cost savings per DEP patient are shown in Table 5. The annual cost for a CHW to educate 1 DEP patient was $403. The combined inpatient and ED cost savings for DEP patients post-program enrollment was $137 per patient. ROI for the DEP was –66%, indicating that DEP investment costs were greater than the savings achieved through the reduction of inpatient and ED costs for DEP patients.
Discussion
In our examination of the impact of the DEP on inpatient and ED utilization, we found that DEP patients experienced a significant decrease in inpatient visits, LOS, and direct costs in the year following DEP enrollment. In comparison, a control group of patients who were treated at the same clinics as DEP patients also experienced significant decreases in inpatient LOS and direct costs. No significant differences in ED visits, LOS, or direct costs were observed for either DEP patients or control patients in the post period.
The reduction in inpatient visits and LOS for DEP patients in the year following DEP enrollment indicates that the DEP helped patients achieve improved health and avoid costly hospitalizations. The control group did experience greater reductions in inpatient utilization, LOS, and direct costs. However, because this was not designed as a controlled trial, we utilized a nonequivalent control group and inherent differences between the DEP and control patients and utilization patterns made it difficult to draw unbiased comparisons between the groups. For instance, the number of inpatient encounters, LOS, and costs were 2 to 3 times higher in the pre-period for the control group. The mean number of inpatient encounters for DEP patients prior to DEP participation was 0.18, and the smaller change observed in utilization for DEP patients is likely due to a floor effect.
Despite the observed reductions for both DEP patients and the control group in inpatient utilization, neither group had significant reductions in ED visits or costs per patient in the post- period. The majority of ED use in the pre-period may have been due to diabetes-related complications that are difficult to prevent even with improved diabetes care or for emergencies not related to diabetes, as we included all ED admissions regardless of admitting diagnosis. In addition, similar to observed trends in inpatient utilization, ED use in the pre-period was relatively low for DEP patients (0.16), and the lack of observed changes in ED utilization for DEP patients was also likely due to a floor effect.
The DEP generated a negative ROI (–66%) in the short term as the annual cost savings generated per patient from reduced utilization ($137) were less than the annual DEP costs (investment) per patient ($403). This finding is not surprising, as it is difficult to achieve cost savings for interventions designed to increase access to health care in underserved populations.[15] Although Fedder et al. observed an average savings of $2245 per patient per year in Medicaid reimbursements for a CHW-led outreach program for patients on Medicaid with diabetes, patients who participated in this program had much higher inpatient encounters (0.95 vs. 0.18) and ED utilization (1.49 vs. 0.16) at baseline compared to DEP patients. DEP patients may not have been as sick as these patients or have been more reluctant to seek medical care due to their lack of insurance. In addition, Fedder et al did not factor in the costs of the CHW program in the cost savings calculation. However, these costs were likely to be much lower than the costs of the DEP, as the program relied on volunteer CHWs instead of paid CHWs who were also certified medical assistants.
Several studies have evaluated the cost-effectiveness of CHW-led diabetes management programs rather than the ROI as these types of programs are often associated with improved health outcomes but also increased health care costs as the result of expanding health care access and services to underserved populations [15,25–29]. Most of these evaluations modeled the long-term cost-effectiveness, as the majority of cost savings from diabetes management programs are likely to accrue in the long run as a result of the prevention of diabetes-related complications such as amputations, blindness, kidney failure, coronary heart disease, and stroke [15,25]. Although these CHW-led interventions for diabetes differed in scope, the incremental cost effectiveness ratios for these interventions were less than the common willingness-to-pay threshold of $50,000 per quality-adjusted life year (QALY). These studies may have underestimated the societal benefits of these programs as they did not incorporate non-medical cost savings such as those that may be attributed to gains in productivity [26].
A recent cost-effectiveness analysis of the DEP and the 4 other programs that were part of the Alliance to Reduce Disparities in Diabetes found that these programs spent an average of $975 per patient in the first year and additional $520 per patient in subsequent years to improve care for diabetes patients [15]. Based on improvements in health indicators such as HbA1c, systolic blood pressure, and total cholesterol observed for participants in Alliance programs, the incremental cost-effectiveness ratio of these programs was $23,161 per QALY given the optimistic assumption that the observed improvements in health indicators were all attributable to the interventions. DEP patients achieved a 1% average reduction in mean HbA1c and this improvement was sustained over the course of the program. The UK Prospective Diabetes Study Group found that every 1% reduction in HbA1C reduces a patient’s risk of developing eye, kidney, and nerve disease by 40% and the risk of heart attack by 14% [30]. Thus, if DEP patients sustain improvements gained as a result of program participation, they may avoid serious and expensive medical complications in the future.
Ultimately, the goal of the DEP was to provide patients with improved access to diabetes care and to help them achieve improved glucose control. Improving access to chronic disease care is costly. However, employment of CHWs is a less costly alternative to employing additional clinicians, and CHWs may be more effective in assisting patients with chronic disease management particularly those from underserved and vulnerable populations. Although we did not generate a positive ROI for the DEP in this short-term analysis, the program is likely cost-effective when the low cost of the program is compared with the improvements in health outcomes we have observed.
This study had several limitations. There were observed differences in gender and ethnicity between the intervention and control groups and it is likely that risk-adjustment (including propensity scoring) did not fully accountfor underlying differences between the populations. The observed reductions in inpatient utilization and costs in the intervention group may have been due to other factors besides the DEP as the control group also achieved reductions in inpatient LOS and costs. The control group did contain a few outliers in terms of high pre-period medical costs which were retained in the analysis and these outliers may have caused reductions in costs for this group to be overstated. The observed outcomes may also be biased due to intervention contamination. Patients in the control group attended the same clinics as DEP patients and were treated by the same primary care physicians. The primary care physicians likely applied the knowledge gained from working with DEP patients and the CHWs, including how to identify and help patients address the specific barriers to diabetes self-management faced by clinic patients, to the treatment of patients who were not enrolled in the DEP. The fact that health care utilization and costs were much higher for patients in the control group in the pre-period indicates that these patients had greater severity of illness and additional comorbidities, and the observed reductions in utilization and costs for these patients may have been the result of obtaining access to a regular source of primary care through the clinics. However, the observed decrease in inpatient encounters from .18 to .08 in the post period for DEP patients as well as the observed decreases in inpatient LOS and direct costs indicate that the DEP may provide additional benefits compared to access to primary care alone in terms of avoidance costly hospitalizations for diabetes-related complications.
Conclusion
From the health care system perspective, CHW-led diabetes education programs like the DEP may provide additional benefits than can be gained from access to primary care alone in terms of avoidance of costly hospitalizations for diabetes-related complications. Although the costs of the DEP were greater than the savings it generated through reduced inpatient utilization and costs in the short term, additional savings to the health care system or society may be generated in the long term through reductions of diabetes-related complications in patients who were able to achieve improved glycemic control through program participation. More importantly, the improvements in glycemic control achieved by DEP patients can lead to both short and long term gains in overall health and quality of life.
Corresponding author: Ashley Collinsworth, ScD, MPH, Scott & White Health, Center for Clinical Effectiveness, Dallas, TX 75206, [email protected].
Funding/support: This program/initiative was supported by a grant from the Merck Company Foundation through its Merck Alliance to Reduce Disparities in Diabetes program.
1. Centers for Disease Control and Prevention, National Diabetes Statistics Report: estimates of diabetes and its burden in the United States, 2014. US Department of Health and Human Services: Atlanta, GA.
2. Centers for Disease Control and Prevention, Diabetes Report Card 2012. US Department of Health and Human Services: Atlanta, GA.
3. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46.
4. Balcazar H, Rosenthal EL, Brownstein JN, et al. Community health workers can be a public health force for change in the United States: three actions for a new paradigm. Am J Public Health 2011;101:2199–203.
5. Rosenthal EL, Brownstein JN, Rush CH, et al. Community health workers: part of the solution. Health Aff (Millwood) 2010;29:1338–42.
6. Viswanathan M, Kraschnewski JL, Nishikawa B, et al. Outcomes and costs of community health worker interventions: a systematic review. Med Care 2010;48:792–808.
7. Babamoto KS, Sey KA, Camilleri AJ, et al. Improving diabetes care and health measures among hispanics using community health workers: results from a randomized controlled trial. Health Educ Behav 2009;36:113–26.
8. Brown SA, Garcia AA, Kouzekanani K, Hanis CL. Culturally competent diabetes self-management education for Mexican Americans: the Starr County border health initiative. Diabetes Care 2002;25:259–68.
9. Prezio EA, Cheng D, Balasubramanian BA, et al. Community Diabetes Education (CoDE) for uninsured Mexican Americans: a randomized controlled trial of a culturally tailored diabetes education and management program led by a community health worker. Diabetes Res Clin Pract 2013;100:19–28.
10. Spencer MS, Rosland AM, Kieffer EC, et al. Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: a randomized controlled trial. Am J Public Health 2011;101:2253–60.
11. Fedder DO, Chang RJ, Curry S, Nichols G. The effectiveness of a community health worker outreach program on healthcare utilization of west Baltimore City Medicaid patients with diabetes, with or without hypertension. Ethn Dis 2003;13:22–7.
12. Lorig KR, Ritter P, Stewart AL, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care 2001;39:1217–23.
13. Whitley EM, Everhart RM, Wright RA. Measuring return on investment of outreach by community health workers. J Health Care Poor Underserved 2006;17(1 Suppl):6–15.
14. Skelly AH. Culturally tailored intervention for African Americans with type 2 diabetes administered by a nurse case manager and community health worker reduces emergency room visits. Evid Based Nurs 2010;13:51–2.
15. Lewis MA, Bann CM, Karns SA, et al. Cross-site evaluation of the Alliance to Reduce Disparities in Diabetes: clinical and patient-report
ed outcomes. Health Promot Pract 2014;15(2 Suppl):92S–102S.
16. Collinsworth AW, Vulimiri M, Schmidt KL, Snead CA. Effectiveness of a community health worker-led diabetes self-management education program and implications for CHW involvement in care coordination strategies. Diabetes Educ 2013;39:792–9.
17. Shah M, Kaselitz E, Heisler M. The role of community health workers in diabetes: update on current literature. Curr Diab Rep 2013;13:163–71.
18. Gary TL, Batts-Turner M, Yeh HC, et al. The effects of a nurse case manager and a community health worker team on diabetic control, emergency department visits, and hospitalizations among urban African Americans with type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med 2009;169:1788–94.
19. Martinez J, Ro M, Villa NW, et al. Transforming the delivery of care in the post-health reform era: what role will community health workers play? Am J Public Health 2011;101:e1–5.
20. Rush CH. Return on investment from employment of community health workers. J Ambul Care Manage 2012;35:133–7.
21. Collinsworth A, Vulimiri M, Snead C, Walton J. Community health workers in primary care practice: redesigning health care delivery systems to extend and improve diabetes care in underserved populations. Health Promot Pract 2014;15(2 Suppl):51S–61S.
22. Walton JW, Snead CA, Collinsworth AW, Schmidt KL. Reducing diabetes disparities through the implementation of a community health worker-led diabetes self-management education program. Fam Community Health 2012;35:161–71.
23. Culica D, Walton JW, Prezio EA. CoDE: Community Diabetes Education for uninsured Mexican Americans. Proc (Bayl Univ Med Cent) 2007;20:111–7.
24. Culica D, Walton JW, Harker K, Prezio EA. Effectiveness of a community health worker as sole diabetes educator: comparison of CoDE with similar culturally appropriate interventions. J Health Care Poor Underserved 2008;19:1076–95.
25. Brownson CA, Hoerger TJ, Fisher EB, Kilpatrick KE. Cost-effectiveness of diabetes self-management programs in community primary care settings. Diabetes Educ 2009;35:761–9.
26. Gilmer TP, Roze S, Valentine WJ, et al. Cost-effectiveness of diabetes case management for low-income populations. Health Serv Res 2007;42:1943–59.
27. Brown HS 3rd, Wilson KJ, Pagán JA, et al. Cost-effectiveness analysis of a community health worker intervention for low-income Hispanic adults with diabetes. Prev Chronic Dis 2012;9:E140.
28. Ryabov I. Cost-effectiveness of community health workers in controlling diabetes epidemic on the US-Mexico border. Public Health 2014;128:636–42.
29. Prezio EA, Pagán JA, Shuval K, Culica D. The Community Diabetes Education (CoDE) program: cost-effectiveness and health outcomes. Am J Prev Med 2014;47:771–9.
30. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–53.
From Baylor Scott & White Health.
Abstract
- Objective: To assess the impact of a community health worker–led diabetes education program (DEP) on hospital utilization trends and to evaluate the return on investment.
- Methods: A retrospective, pre-post study design was used to examine differences in inpatient and emergency department (ED) utilization and costs for DEP patients in the year prior to and following enrollment. Patients with diabetes who received care at the same clinics but who were not enrolled in DEP served as a control group. Analysis of covariance was used to test for differences in utilization outcomes between DEP patients and controls, controlling for age, sex, and ethnicity.
- Results: DEP patients had a significant reduction in mean inpatient encounters (0.08 vs. 0.18), LOS per inpatient encounter (0.28 vs. 0.67), and inpatient cost per patient ($406 vs. $902) following DEP enrollment. Patients in the control group also experienced a significant reduction in inpatient LOS and costs. Neither group experienced a significant difference in ED utilization or costs. Return on investment for DEP was –66%, as the annual cost savings generated per patient from reduced utilization ($137.19) were less than the annual DEP costs (investment) per patient ($402.80).
- Conclusion: CHW-led diabetes education programs like DEP may provide additional benefits than can be gained from access to primary care alone in terms of avoidance of costly hospitalizations for diabetes-related complications. Although the DEP did not generate an overall cost savings for the health care system in the short term, additional savings may be generated in the long term through reductions of diabetes-related complications.
Diabetes poses a significant burden on the population of the United States in terms of morbidity, mortality, and costs of care. Currently, 29.1 million people in the United States have diabetes [1], and it is expected that 1 in 3 people could have diabetes by the year 2050 [2]. The economic impact of diabetes is also significant; as of 2012, 1 in 5 health care dollars went towards diabetes care [3], and the total cost of diabetes was estimated to be $245 billion [1]. This rapid increase in the incidence of diabetes and associated costs emphasizes the need for cost-effective strategies to prevent and manage diabetes within the U.S. population.
One strategy that has shown success in improving diabetes care and outcomes is the use of community health workers (CHWs) to deliver disease education and management programs. A CHW is a front-line public health worker who is often a trusted member of the community and has the capacity to influence patient understanding, enhance patient compliance, and promote more equitable practices in patient care management [4–6]. Several studies have shown that CHW-led interventions in diabetes management can help patients achieve control over their disease and improved health indicators such as HbA1c, blood pressure, lipid levels, and weight [7–17]. In addition, a few studies have shown that CHW-led interventions for patients with diabetes can help these patients avoid costly health care utilization in the form of emergency department (ED) visits and hospitalizations. Fedder et al found that diabetes patients on Medicaid who worked closely with CHWs experienced a decrease in ED visits (38%), hospitalizations (30%), and hospital admissions from ED (53%) [11]. This CHW intervention was associated with improved patient quality of life and a cost-savings of $2245 per patient per year for a total savings of $262,080 for 117 patients. More recently, a 2010 randomized controlled trial showed that African-American patients with diabetes who worked with a nurse case manager and a CHW were 23% less likely to make ED visits than those who just had a nurse case manager [18].
Despite the documented successes of CHW programs, the CHW model has not been widely adopted within integrated health care systems. A major barrier to adoption of CHW programs has been the lack of sustained funding for CHW services [17,19]. Historically, many CHW programs were supported by grants, as most payers were unwilling to fund these initiatives [4,17,19.] In 2008, the Centers for Medicare and Medicaid Services provided a mechanism to support CHW activities by approving a Medicaid state plan amendment authorizing payment for CHWs who worked under Medicaid-approved providers, such as physicians and nurses [19]. However, the lack of data available regarding the costs, cost-effectiveness, and potential costs savings of CHW programs continues to serve as a barrier to adoption [13,20].
Baylor Scott & White Health North (BSWH), formerly Baylor Health Care System in Dallas, Texas, created the CHW-led Diabetes Equity Project, a 5-year program supported with funding from Merck Foundation’s Alliance to Reduce Disparities in Diabetes, with the goal of reducing observed disparities in diabetes care and outcomes in the medically underserved, predominantly Hispanic communities surrounding BSWH hospitals [16,21,22]. The program featured specially trained, bilingual CHWs who served as members of primary care teams in 5 community clinics and delivered a culturally relevant diabetes self-management and education curriculum (DSME) targeting barriers to diabetes management commonly experienced by Hispanics. The objective of this study was to assess the impact of a CHW-led diabetes educucation program (DEP) on hospital utilization trends and to evaluate the return on investment of the program.
Methods
Setting
BSWH is one of the largest nonprofit health care systems in the United States and includes 46 hospitals, > 800 patient care sites, > 6000 affiliated physicians, 35,000 employees, and an accountable care organization. This study was conducted in 5 community clinics located in the Dallas metroplex surrounding BSWH North hospitals. The community clinics serve low-income, uninsured, and chronically ill patients. The study was approved by the Baylor Research Institute institutional review board.
DEP Intervention
The DEP program consisted of 2 initial 60-minute educational sessions and quarterly clinical assessments scheduled for 30 to 60 minutes for a maximum of 6 patient-contact hours over 12 consecutive months. The DSME curriculum for DEP was adapted from CoDE, a pilot program implemented in a Dallas clinic serving a largely uninsured Mexican American population [23]. Patients who participated in CoDE for 12 months experienced a significant reduction in HbA1c [23,24]. During the 2 educational sessions, the CHWs educated DEP participants about diabetes and the importance of blood glucose control, medication adherence, diet, and exercise. In addition to the educational sessions, CHWs performed quarterly clinical assessments of HbA1c, blood pressure, weight, and foot condition (visual and monofilament assessment). They also assessed self-management behaviors and facilitated goal setting at each visit. The CHWs documented patient visits in the electronic health record and contacted the patient’s primary care provider immediately if the patient was symptomatic or had critical blood glucose or blood pressure measurements as defined by program protocol.
Participants
Study participants were recruited from the clinics or referred by Baylor care coordinators following hospital visits related to uncontrolled diabetes from September 2009 to July 2013. Participants had to be 18 years or older with a diagnosis of type 2 diabetes and be uninsured or underinsured. Although the program targeted Hispanic patients, all patients who met the inclusion criteria were eligible to participate. To control for internal threats to validity such as history and maturation biases, we created a control group consisting of clinic patients who had a diagnosis of diabetes and met the DEP inclusion criteria but who did not enroll in DEP.
Assessment
We used a retrospective pre-post study design to assess the impact of the DEP on hospital utilization trends and the program’s return on investment (ROI). The primary outcomes were number of hospital encounters, length of stay (LOS) per encounter, and direct cost per patient. The pre-enrollment period was defined as the year priod to the day of the initial DEP visit (the date of enrollment for DEP patients) or the initial clinic visit (the date of “enrollment” for control patients). All study participants were included in the analysis regardless of their number of inpatient or ED encounters. If a participant did not have a documented encounter during the analysis time period, encounters, LOS, and medical costs were set to zero. Patients with at least 2 HbA1c measurements taken during the first year post enrollment were included in the analysis.
Data Sources
Inpatient and emergency department (ED) utilization data were obtained from the Dallas Fort-Worth Hospital Council (DFWHC) database. The DFWHC captures administrative data from over 80 participating hospital systems and 9 million unique patients in North Texas. The DFWHC applied a matching algorithm using first and last name and date of birth to match study participants with encounter and length of stay detail for all hospitalizations across all DFWHC member hospitals. We obtained direct medical costs for patients treated at BSWH facilities from the BSWH Trendstar administrative database. Direct medical cost was not available for encounters at non-BSWH facilities so cost was estimated for these patients based on BSWH costs using a prediction model that accounted for LOS, primary ICD-9 diagnosis, patient age, sex, and race. The model explained approximately 66% of the variation in direct costs (R2 = 0.6581).
Statistical Analysis
All analyses were performed in SAS V9.3 using an α level of 0.05. A 2-tailed independent samples t test was used to test the mean differences in utilization outcomes one year prior to and post program enrollment. An analysis of covariance (ANCOVA) was used to assess whether the mean change in utilization outcomes was greater for DEP patients than the control group, , after controlling for age, sex, and ethnicity. The gamma distribution with a log link function was used to model direct cost and the negative binomial distribution was used to model hospital encounters and LOS.
The ROI calculation included 2 components: DEP investment cost and risk-adjusted direct medical cost savings 1 year post program enrollment. DEP investment cost included the average yearly costs per community health worker, the fixed one-time start-up costs distributed across the length of the program (4 years), and the salaries of the 5 community health workers employed for the duration of the program. These costs were divided by the number of patients who enrolled in the DEP to calculate the per patient cost (investment). Direct medical cost savings were calculated as the mean reduction in hospitalization and ED costs per patient adjusted for age, sex, and ethnicity.
To account for DEP participants without encounter data, we estimated average cost savings in a 4-step process using a propensity score adjustment approach. We stratified encounters based on admission type (inpatient vs. ED) and combined the average cost savings calculated for both admission types to calculate mean total medical cost savings.
In the first step, direct cost was modeled on DEP participants with an encounter to obtain parameter estimates for pre- and post- program enrollment, age, sex, and ethnicity. The model was based on the log-link function with the gamma distribution. In the second step, we ran a propensity score logistic regression model to estimate the probability of any encounter being identified in the DFWHC database, adjusted for age, sex, ethnicity, and DEP clinic. In the third step, we generated 10,000 bootstrap samples, with replacement, to estimate the DEP population’s median expected change in cost. In the bootstrap sample, the parameter estimates from step 1 were used to predict the cost pre- and post- program enrollment for each patient. The expected change in cost per participant was then calculated, while adjusting for the propensity to have an encounter, estimated in step 2. The formula for calculating change in cost was Expected change in cost = [pre probability*pre cost – post probability*post cost].
In the final step, we retained the median cost savings from each bootstrap sample. Inpatient and ED cost savings were combined to produce a total cost savings across the patient population. The ROI formula was Financial gain (utilization cost savings – DEP investment)/DEP investment.
Results
Participant Demographics
Of the 1140 study participants, 878 were DEP patients and 262 were controls (Table 1). The majority of DEP patients were Hispanic females older than 40 years while the majority of the the control group was non-Hispanic males older than 40. Table 2 and Table 3 display the top 5 primary ICD-9 diagnoses by intervention group and pre/post enrollment status. Type 2 diabetes was the top diagnosis among DEP participants and controls with a pre-enrollment encounter.
Inpatient Encounters
Fourteen percent of the DEP participants and 37% of control group patients matched to an inpatient record during the pre-enrollment time period. The number of mean inpatient encounters for DEP participants decreased from 0.18 to 0.08 (P < 0.001) in the post period
Emergency Department Encounters
Eight percent of the DEP participants and 30% of controls matched to an ED record for the year prior to program enrollment. Neither DEP patients nor patients in the control group had a significant reduction in mean ED encounters (P = 0.88 and 0.74, respectively) or costs (P = 0.76 and 0.12, respectively) post enrollment.
The cost savings per DEP patient are shown in Table 5. The annual cost for a CHW to educate 1 DEP patient was $403. The combined inpatient and ED cost savings for DEP patients post-program enrollment was $137 per patient. ROI for the DEP was –66%, indicating that DEP investment costs were greater than the savings achieved through the reduction of inpatient and ED costs for DEP patients.
Discussion
In our examination of the impact of the DEP on inpatient and ED utilization, we found that DEP patients experienced a significant decrease in inpatient visits, LOS, and direct costs in the year following DEP enrollment. In comparison, a control group of patients who were treated at the same clinics as DEP patients also experienced significant decreases in inpatient LOS and direct costs. No significant differences in ED visits, LOS, or direct costs were observed for either DEP patients or control patients in the post period.
The reduction in inpatient visits and LOS for DEP patients in the year following DEP enrollment indicates that the DEP helped patients achieve improved health and avoid costly hospitalizations. The control group did experience greater reductions in inpatient utilization, LOS, and direct costs. However, because this was not designed as a controlled trial, we utilized a nonequivalent control group and inherent differences between the DEP and control patients and utilization patterns made it difficult to draw unbiased comparisons between the groups. For instance, the number of inpatient encounters, LOS, and costs were 2 to 3 times higher in the pre-period for the control group. The mean number of inpatient encounters for DEP patients prior to DEP participation was 0.18, and the smaller change observed in utilization for DEP patients is likely due to a floor effect.
Despite the observed reductions for both DEP patients and the control group in inpatient utilization, neither group had significant reductions in ED visits or costs per patient in the post- period. The majority of ED use in the pre-period may have been due to diabetes-related complications that are difficult to prevent even with improved diabetes care or for emergencies not related to diabetes, as we included all ED admissions regardless of admitting diagnosis. In addition, similar to observed trends in inpatient utilization, ED use in the pre-period was relatively low for DEP patients (0.16), and the lack of observed changes in ED utilization for DEP patients was also likely due to a floor effect.
The DEP generated a negative ROI (–66%) in the short term as the annual cost savings generated per patient from reduced utilization ($137) were less than the annual DEP costs (investment) per patient ($403). This finding is not surprising, as it is difficult to achieve cost savings for interventions designed to increase access to health care in underserved populations.[15] Although Fedder et al. observed an average savings of $2245 per patient per year in Medicaid reimbursements for a CHW-led outreach program for patients on Medicaid with diabetes, patients who participated in this program had much higher inpatient encounters (0.95 vs. 0.18) and ED utilization (1.49 vs. 0.16) at baseline compared to DEP patients. DEP patients may not have been as sick as these patients or have been more reluctant to seek medical care due to their lack of insurance. In addition, Fedder et al did not factor in the costs of the CHW program in the cost savings calculation. However, these costs were likely to be much lower than the costs of the DEP, as the program relied on volunteer CHWs instead of paid CHWs who were also certified medical assistants.
Several studies have evaluated the cost-effectiveness of CHW-led diabetes management programs rather than the ROI as these types of programs are often associated with improved health outcomes but also increased health care costs as the result of expanding health care access and services to underserved populations [15,25–29]. Most of these evaluations modeled the long-term cost-effectiveness, as the majority of cost savings from diabetes management programs are likely to accrue in the long run as a result of the prevention of diabetes-related complications such as amputations, blindness, kidney failure, coronary heart disease, and stroke [15,25]. Although these CHW-led interventions for diabetes differed in scope, the incremental cost effectiveness ratios for these interventions were less than the common willingness-to-pay threshold of $50,000 per quality-adjusted life year (QALY). These studies may have underestimated the societal benefits of these programs as they did not incorporate non-medical cost savings such as those that may be attributed to gains in productivity [26].
A recent cost-effectiveness analysis of the DEP and the 4 other programs that were part of the Alliance to Reduce Disparities in Diabetes found that these programs spent an average of $975 per patient in the first year and additional $520 per patient in subsequent years to improve care for diabetes patients [15]. Based on improvements in health indicators such as HbA1c, systolic blood pressure, and total cholesterol observed for participants in Alliance programs, the incremental cost-effectiveness ratio of these programs was $23,161 per QALY given the optimistic assumption that the observed improvements in health indicators were all attributable to the interventions. DEP patients achieved a 1% average reduction in mean HbA1c and this improvement was sustained over the course of the program. The UK Prospective Diabetes Study Group found that every 1% reduction in HbA1C reduces a patient’s risk of developing eye, kidney, and nerve disease by 40% and the risk of heart attack by 14% [30]. Thus, if DEP patients sustain improvements gained as a result of program participation, they may avoid serious and expensive medical complications in the future.
Ultimately, the goal of the DEP was to provide patients with improved access to diabetes care and to help them achieve improved glucose control. Improving access to chronic disease care is costly. However, employment of CHWs is a less costly alternative to employing additional clinicians, and CHWs may be more effective in assisting patients with chronic disease management particularly those from underserved and vulnerable populations. Although we did not generate a positive ROI for the DEP in this short-term analysis, the program is likely cost-effective when the low cost of the program is compared with the improvements in health outcomes we have observed.
This study had several limitations. There were observed differences in gender and ethnicity between the intervention and control groups and it is likely that risk-adjustment (including propensity scoring) did not fully accountfor underlying differences between the populations. The observed reductions in inpatient utilization and costs in the intervention group may have been due to other factors besides the DEP as the control group also achieved reductions in inpatient LOS and costs. The control group did contain a few outliers in terms of high pre-period medical costs which were retained in the analysis and these outliers may have caused reductions in costs for this group to be overstated. The observed outcomes may also be biased due to intervention contamination. Patients in the control group attended the same clinics as DEP patients and were treated by the same primary care physicians. The primary care physicians likely applied the knowledge gained from working with DEP patients and the CHWs, including how to identify and help patients address the specific barriers to diabetes self-management faced by clinic patients, to the treatment of patients who were not enrolled in the DEP. The fact that health care utilization and costs were much higher for patients in the control group in the pre-period indicates that these patients had greater severity of illness and additional comorbidities, and the observed reductions in utilization and costs for these patients may have been the result of obtaining access to a regular source of primary care through the clinics. However, the observed decrease in inpatient encounters from .18 to .08 in the post period for DEP patients as well as the observed decreases in inpatient LOS and direct costs indicate that the DEP may provide additional benefits compared to access to primary care alone in terms of avoidance costly hospitalizations for diabetes-related complications.
Conclusion
From the health care system perspective, CHW-led diabetes education programs like the DEP may provide additional benefits than can be gained from access to primary care alone in terms of avoidance of costly hospitalizations for diabetes-related complications. Although the costs of the DEP were greater than the savings it generated through reduced inpatient utilization and costs in the short term, additional savings to the health care system or society may be generated in the long term through reductions of diabetes-related complications in patients who were able to achieve improved glycemic control through program participation. More importantly, the improvements in glycemic control achieved by DEP patients can lead to both short and long term gains in overall health and quality of life.
Corresponding author: Ashley Collinsworth, ScD, MPH, Scott & White Health, Center for Clinical Effectiveness, Dallas, TX 75206, [email protected].
Funding/support: This program/initiative was supported by a grant from the Merck Company Foundation through its Merck Alliance to Reduce Disparities in Diabetes program.
From Baylor Scott & White Health.
Abstract
- Objective: To assess the impact of a community health worker–led diabetes education program (DEP) on hospital utilization trends and to evaluate the return on investment.
- Methods: A retrospective, pre-post study design was used to examine differences in inpatient and emergency department (ED) utilization and costs for DEP patients in the year prior to and following enrollment. Patients with diabetes who received care at the same clinics but who were not enrolled in DEP served as a control group. Analysis of covariance was used to test for differences in utilization outcomes between DEP patients and controls, controlling for age, sex, and ethnicity.
- Results: DEP patients had a significant reduction in mean inpatient encounters (0.08 vs. 0.18), LOS per inpatient encounter (0.28 vs. 0.67), and inpatient cost per patient ($406 vs. $902) following DEP enrollment. Patients in the control group also experienced a significant reduction in inpatient LOS and costs. Neither group experienced a significant difference in ED utilization or costs. Return on investment for DEP was –66%, as the annual cost savings generated per patient from reduced utilization ($137.19) were less than the annual DEP costs (investment) per patient ($402.80).
- Conclusion: CHW-led diabetes education programs like DEP may provide additional benefits than can be gained from access to primary care alone in terms of avoidance of costly hospitalizations for diabetes-related complications. Although the DEP did not generate an overall cost savings for the health care system in the short term, additional savings may be generated in the long term through reductions of diabetes-related complications.
Diabetes poses a significant burden on the population of the United States in terms of morbidity, mortality, and costs of care. Currently, 29.1 million people in the United States have diabetes [1], and it is expected that 1 in 3 people could have diabetes by the year 2050 [2]. The economic impact of diabetes is also significant; as of 2012, 1 in 5 health care dollars went towards diabetes care [3], and the total cost of diabetes was estimated to be $245 billion [1]. This rapid increase in the incidence of diabetes and associated costs emphasizes the need for cost-effective strategies to prevent and manage diabetes within the U.S. population.
One strategy that has shown success in improving diabetes care and outcomes is the use of community health workers (CHWs) to deliver disease education and management programs. A CHW is a front-line public health worker who is often a trusted member of the community and has the capacity to influence patient understanding, enhance patient compliance, and promote more equitable practices in patient care management [4–6]. Several studies have shown that CHW-led interventions in diabetes management can help patients achieve control over their disease and improved health indicators such as HbA1c, blood pressure, lipid levels, and weight [7–17]. In addition, a few studies have shown that CHW-led interventions for patients with diabetes can help these patients avoid costly health care utilization in the form of emergency department (ED) visits and hospitalizations. Fedder et al found that diabetes patients on Medicaid who worked closely with CHWs experienced a decrease in ED visits (38%), hospitalizations (30%), and hospital admissions from ED (53%) [11]. This CHW intervention was associated with improved patient quality of life and a cost-savings of $2245 per patient per year for a total savings of $262,080 for 117 patients. More recently, a 2010 randomized controlled trial showed that African-American patients with diabetes who worked with a nurse case manager and a CHW were 23% less likely to make ED visits than those who just had a nurse case manager [18].
Despite the documented successes of CHW programs, the CHW model has not been widely adopted within integrated health care systems. A major barrier to adoption of CHW programs has been the lack of sustained funding for CHW services [17,19]. Historically, many CHW programs were supported by grants, as most payers were unwilling to fund these initiatives [4,17,19.] In 2008, the Centers for Medicare and Medicaid Services provided a mechanism to support CHW activities by approving a Medicaid state plan amendment authorizing payment for CHWs who worked under Medicaid-approved providers, such as physicians and nurses [19]. However, the lack of data available regarding the costs, cost-effectiveness, and potential costs savings of CHW programs continues to serve as a barrier to adoption [13,20].
Baylor Scott & White Health North (BSWH), formerly Baylor Health Care System in Dallas, Texas, created the CHW-led Diabetes Equity Project, a 5-year program supported with funding from Merck Foundation’s Alliance to Reduce Disparities in Diabetes, with the goal of reducing observed disparities in diabetes care and outcomes in the medically underserved, predominantly Hispanic communities surrounding BSWH hospitals [16,21,22]. The program featured specially trained, bilingual CHWs who served as members of primary care teams in 5 community clinics and delivered a culturally relevant diabetes self-management and education curriculum (DSME) targeting barriers to diabetes management commonly experienced by Hispanics. The objective of this study was to assess the impact of a CHW-led diabetes educucation program (DEP) on hospital utilization trends and to evaluate the return on investment of the program.
Methods
Setting
BSWH is one of the largest nonprofit health care systems in the United States and includes 46 hospitals, > 800 patient care sites, > 6000 affiliated physicians, 35,000 employees, and an accountable care organization. This study was conducted in 5 community clinics located in the Dallas metroplex surrounding BSWH North hospitals. The community clinics serve low-income, uninsured, and chronically ill patients. The study was approved by the Baylor Research Institute institutional review board.
DEP Intervention
The DEP program consisted of 2 initial 60-minute educational sessions and quarterly clinical assessments scheduled for 30 to 60 minutes for a maximum of 6 patient-contact hours over 12 consecutive months. The DSME curriculum for DEP was adapted from CoDE, a pilot program implemented in a Dallas clinic serving a largely uninsured Mexican American population [23]. Patients who participated in CoDE for 12 months experienced a significant reduction in HbA1c [23,24]. During the 2 educational sessions, the CHWs educated DEP participants about diabetes and the importance of blood glucose control, medication adherence, diet, and exercise. In addition to the educational sessions, CHWs performed quarterly clinical assessments of HbA1c, blood pressure, weight, and foot condition (visual and monofilament assessment). They also assessed self-management behaviors and facilitated goal setting at each visit. The CHWs documented patient visits in the electronic health record and contacted the patient’s primary care provider immediately if the patient was symptomatic or had critical blood glucose or blood pressure measurements as defined by program protocol.
Participants
Study participants were recruited from the clinics or referred by Baylor care coordinators following hospital visits related to uncontrolled diabetes from September 2009 to July 2013. Participants had to be 18 years or older with a diagnosis of type 2 diabetes and be uninsured or underinsured. Although the program targeted Hispanic patients, all patients who met the inclusion criteria were eligible to participate. To control for internal threats to validity such as history and maturation biases, we created a control group consisting of clinic patients who had a diagnosis of diabetes and met the DEP inclusion criteria but who did not enroll in DEP.
Assessment
We used a retrospective pre-post study design to assess the impact of the DEP on hospital utilization trends and the program’s return on investment (ROI). The primary outcomes were number of hospital encounters, length of stay (LOS) per encounter, and direct cost per patient. The pre-enrollment period was defined as the year priod to the day of the initial DEP visit (the date of enrollment for DEP patients) or the initial clinic visit (the date of “enrollment” for control patients). All study participants were included in the analysis regardless of their number of inpatient or ED encounters. If a participant did not have a documented encounter during the analysis time period, encounters, LOS, and medical costs were set to zero. Patients with at least 2 HbA1c measurements taken during the first year post enrollment were included in the analysis.
Data Sources
Inpatient and emergency department (ED) utilization data were obtained from the Dallas Fort-Worth Hospital Council (DFWHC) database. The DFWHC captures administrative data from over 80 participating hospital systems and 9 million unique patients in North Texas. The DFWHC applied a matching algorithm using first and last name and date of birth to match study participants with encounter and length of stay detail for all hospitalizations across all DFWHC member hospitals. We obtained direct medical costs for patients treated at BSWH facilities from the BSWH Trendstar administrative database. Direct medical cost was not available for encounters at non-BSWH facilities so cost was estimated for these patients based on BSWH costs using a prediction model that accounted for LOS, primary ICD-9 diagnosis, patient age, sex, and race. The model explained approximately 66% of the variation in direct costs (R2 = 0.6581).
Statistical Analysis
All analyses were performed in SAS V9.3 using an α level of 0.05. A 2-tailed independent samples t test was used to test the mean differences in utilization outcomes one year prior to and post program enrollment. An analysis of covariance (ANCOVA) was used to assess whether the mean change in utilization outcomes was greater for DEP patients than the control group, , after controlling for age, sex, and ethnicity. The gamma distribution with a log link function was used to model direct cost and the negative binomial distribution was used to model hospital encounters and LOS.
The ROI calculation included 2 components: DEP investment cost and risk-adjusted direct medical cost savings 1 year post program enrollment. DEP investment cost included the average yearly costs per community health worker, the fixed one-time start-up costs distributed across the length of the program (4 years), and the salaries of the 5 community health workers employed for the duration of the program. These costs were divided by the number of patients who enrolled in the DEP to calculate the per patient cost (investment). Direct medical cost savings were calculated as the mean reduction in hospitalization and ED costs per patient adjusted for age, sex, and ethnicity.
To account for DEP participants without encounter data, we estimated average cost savings in a 4-step process using a propensity score adjustment approach. We stratified encounters based on admission type (inpatient vs. ED) and combined the average cost savings calculated for both admission types to calculate mean total medical cost savings.
In the first step, direct cost was modeled on DEP participants with an encounter to obtain parameter estimates for pre- and post- program enrollment, age, sex, and ethnicity. The model was based on the log-link function with the gamma distribution. In the second step, we ran a propensity score logistic regression model to estimate the probability of any encounter being identified in the DFWHC database, adjusted for age, sex, ethnicity, and DEP clinic. In the third step, we generated 10,000 bootstrap samples, with replacement, to estimate the DEP population’s median expected change in cost. In the bootstrap sample, the parameter estimates from step 1 were used to predict the cost pre- and post- program enrollment for each patient. The expected change in cost per participant was then calculated, while adjusting for the propensity to have an encounter, estimated in step 2. The formula for calculating change in cost was Expected change in cost = [pre probability*pre cost – post probability*post cost].
In the final step, we retained the median cost savings from each bootstrap sample. Inpatient and ED cost savings were combined to produce a total cost savings across the patient population. The ROI formula was Financial gain (utilization cost savings – DEP investment)/DEP investment.
Results
Participant Demographics
Of the 1140 study participants, 878 were DEP patients and 262 were controls (Table 1). The majority of DEP patients were Hispanic females older than 40 years while the majority of the the control group was non-Hispanic males older than 40. Table 2 and Table 3 display the top 5 primary ICD-9 diagnoses by intervention group and pre/post enrollment status. Type 2 diabetes was the top diagnosis among DEP participants and controls with a pre-enrollment encounter.
Inpatient Encounters
Fourteen percent of the DEP participants and 37% of control group patients matched to an inpatient record during the pre-enrollment time period. The number of mean inpatient encounters for DEP participants decreased from 0.18 to 0.08 (P < 0.001) in the post period
Emergency Department Encounters
Eight percent of the DEP participants and 30% of controls matched to an ED record for the year prior to program enrollment. Neither DEP patients nor patients in the control group had a significant reduction in mean ED encounters (P = 0.88 and 0.74, respectively) or costs (P = 0.76 and 0.12, respectively) post enrollment.
The cost savings per DEP patient are shown in Table 5. The annual cost for a CHW to educate 1 DEP patient was $403. The combined inpatient and ED cost savings for DEP patients post-program enrollment was $137 per patient. ROI for the DEP was –66%, indicating that DEP investment costs were greater than the savings achieved through the reduction of inpatient and ED costs for DEP patients.
Discussion
In our examination of the impact of the DEP on inpatient and ED utilization, we found that DEP patients experienced a significant decrease in inpatient visits, LOS, and direct costs in the year following DEP enrollment. In comparison, a control group of patients who were treated at the same clinics as DEP patients also experienced significant decreases in inpatient LOS and direct costs. No significant differences in ED visits, LOS, or direct costs were observed for either DEP patients or control patients in the post period.
The reduction in inpatient visits and LOS for DEP patients in the year following DEP enrollment indicates that the DEP helped patients achieve improved health and avoid costly hospitalizations. The control group did experience greater reductions in inpatient utilization, LOS, and direct costs. However, because this was not designed as a controlled trial, we utilized a nonequivalent control group and inherent differences between the DEP and control patients and utilization patterns made it difficult to draw unbiased comparisons between the groups. For instance, the number of inpatient encounters, LOS, and costs were 2 to 3 times higher in the pre-period for the control group. The mean number of inpatient encounters for DEP patients prior to DEP participation was 0.18, and the smaller change observed in utilization for DEP patients is likely due to a floor effect.
Despite the observed reductions for both DEP patients and the control group in inpatient utilization, neither group had significant reductions in ED visits or costs per patient in the post- period. The majority of ED use in the pre-period may have been due to diabetes-related complications that are difficult to prevent even with improved diabetes care or for emergencies not related to diabetes, as we included all ED admissions regardless of admitting diagnosis. In addition, similar to observed trends in inpatient utilization, ED use in the pre-period was relatively low for DEP patients (0.16), and the lack of observed changes in ED utilization for DEP patients was also likely due to a floor effect.
The DEP generated a negative ROI (–66%) in the short term as the annual cost savings generated per patient from reduced utilization ($137) were less than the annual DEP costs (investment) per patient ($403). This finding is not surprising, as it is difficult to achieve cost savings for interventions designed to increase access to health care in underserved populations.[15] Although Fedder et al. observed an average savings of $2245 per patient per year in Medicaid reimbursements for a CHW-led outreach program for patients on Medicaid with diabetes, patients who participated in this program had much higher inpatient encounters (0.95 vs. 0.18) and ED utilization (1.49 vs. 0.16) at baseline compared to DEP patients. DEP patients may not have been as sick as these patients or have been more reluctant to seek medical care due to their lack of insurance. In addition, Fedder et al did not factor in the costs of the CHW program in the cost savings calculation. However, these costs were likely to be much lower than the costs of the DEP, as the program relied on volunteer CHWs instead of paid CHWs who were also certified medical assistants.
Several studies have evaluated the cost-effectiveness of CHW-led diabetes management programs rather than the ROI as these types of programs are often associated with improved health outcomes but also increased health care costs as the result of expanding health care access and services to underserved populations [15,25–29]. Most of these evaluations modeled the long-term cost-effectiveness, as the majority of cost savings from diabetes management programs are likely to accrue in the long run as a result of the prevention of diabetes-related complications such as amputations, blindness, kidney failure, coronary heart disease, and stroke [15,25]. Although these CHW-led interventions for diabetes differed in scope, the incremental cost effectiveness ratios for these interventions were less than the common willingness-to-pay threshold of $50,000 per quality-adjusted life year (QALY). These studies may have underestimated the societal benefits of these programs as they did not incorporate non-medical cost savings such as those that may be attributed to gains in productivity [26].
A recent cost-effectiveness analysis of the DEP and the 4 other programs that were part of the Alliance to Reduce Disparities in Diabetes found that these programs spent an average of $975 per patient in the first year and additional $520 per patient in subsequent years to improve care for diabetes patients [15]. Based on improvements in health indicators such as HbA1c, systolic blood pressure, and total cholesterol observed for participants in Alliance programs, the incremental cost-effectiveness ratio of these programs was $23,161 per QALY given the optimistic assumption that the observed improvements in health indicators were all attributable to the interventions. DEP patients achieved a 1% average reduction in mean HbA1c and this improvement was sustained over the course of the program. The UK Prospective Diabetes Study Group found that every 1% reduction in HbA1C reduces a patient’s risk of developing eye, kidney, and nerve disease by 40% and the risk of heart attack by 14% [30]. Thus, if DEP patients sustain improvements gained as a result of program participation, they may avoid serious and expensive medical complications in the future.
Ultimately, the goal of the DEP was to provide patients with improved access to diabetes care and to help them achieve improved glucose control. Improving access to chronic disease care is costly. However, employment of CHWs is a less costly alternative to employing additional clinicians, and CHWs may be more effective in assisting patients with chronic disease management particularly those from underserved and vulnerable populations. Although we did not generate a positive ROI for the DEP in this short-term analysis, the program is likely cost-effective when the low cost of the program is compared with the improvements in health outcomes we have observed.
This study had several limitations. There were observed differences in gender and ethnicity between the intervention and control groups and it is likely that risk-adjustment (including propensity scoring) did not fully accountfor underlying differences between the populations. The observed reductions in inpatient utilization and costs in the intervention group may have been due to other factors besides the DEP as the control group also achieved reductions in inpatient LOS and costs. The control group did contain a few outliers in terms of high pre-period medical costs which were retained in the analysis and these outliers may have caused reductions in costs for this group to be overstated. The observed outcomes may also be biased due to intervention contamination. Patients in the control group attended the same clinics as DEP patients and were treated by the same primary care physicians. The primary care physicians likely applied the knowledge gained from working with DEP patients and the CHWs, including how to identify and help patients address the specific barriers to diabetes self-management faced by clinic patients, to the treatment of patients who were not enrolled in the DEP. The fact that health care utilization and costs were much higher for patients in the control group in the pre-period indicates that these patients had greater severity of illness and additional comorbidities, and the observed reductions in utilization and costs for these patients may have been the result of obtaining access to a regular source of primary care through the clinics. However, the observed decrease in inpatient encounters from .18 to .08 in the post period for DEP patients as well as the observed decreases in inpatient LOS and direct costs indicate that the DEP may provide additional benefits compared to access to primary care alone in terms of avoidance costly hospitalizations for diabetes-related complications.
Conclusion
From the health care system perspective, CHW-led diabetes education programs like the DEP may provide additional benefits than can be gained from access to primary care alone in terms of avoidance of costly hospitalizations for diabetes-related complications. Although the costs of the DEP were greater than the savings it generated through reduced inpatient utilization and costs in the short term, additional savings to the health care system or society may be generated in the long term through reductions of diabetes-related complications in patients who were able to achieve improved glycemic control through program participation. More importantly, the improvements in glycemic control achieved by DEP patients can lead to both short and long term gains in overall health and quality of life.
Corresponding author: Ashley Collinsworth, ScD, MPH, Scott & White Health, Center for Clinical Effectiveness, Dallas, TX 75206, [email protected].
Funding/support: This program/initiative was supported by a grant from the Merck Company Foundation through its Merck Alliance to Reduce Disparities in Diabetes program.
1. Centers for Disease Control and Prevention, National Diabetes Statistics Report: estimates of diabetes and its burden in the United States, 2014. US Department of Health and Human Services: Atlanta, GA.
2. Centers for Disease Control and Prevention, Diabetes Report Card 2012. US Department of Health and Human Services: Atlanta, GA.
3. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46.
4. Balcazar H, Rosenthal EL, Brownstein JN, et al. Community health workers can be a public health force for change in the United States: three actions for a new paradigm. Am J Public Health 2011;101:2199–203.
5. Rosenthal EL, Brownstein JN, Rush CH, et al. Community health workers: part of the solution. Health Aff (Millwood) 2010;29:1338–42.
6. Viswanathan M, Kraschnewski JL, Nishikawa B, et al. Outcomes and costs of community health worker interventions: a systematic review. Med Care 2010;48:792–808.
7. Babamoto KS, Sey KA, Camilleri AJ, et al. Improving diabetes care and health measures among hispanics using community health workers: results from a randomized controlled trial. Health Educ Behav 2009;36:113–26.
8. Brown SA, Garcia AA, Kouzekanani K, Hanis CL. Culturally competent diabetes self-management education for Mexican Americans: the Starr County border health initiative. Diabetes Care 2002;25:259–68.
9. Prezio EA, Cheng D, Balasubramanian BA, et al. Community Diabetes Education (CoDE) for uninsured Mexican Americans: a randomized controlled trial of a culturally tailored diabetes education and management program led by a community health worker. Diabetes Res Clin Pract 2013;100:19–28.
10. Spencer MS, Rosland AM, Kieffer EC, et al. Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: a randomized controlled trial. Am J Public Health 2011;101:2253–60.
11. Fedder DO, Chang RJ, Curry S, Nichols G. The effectiveness of a community health worker outreach program on healthcare utilization of west Baltimore City Medicaid patients with diabetes, with or without hypertension. Ethn Dis 2003;13:22–7.
12. Lorig KR, Ritter P, Stewart AL, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care 2001;39:1217–23.
13. Whitley EM, Everhart RM, Wright RA. Measuring return on investment of outreach by community health workers. J Health Care Poor Underserved 2006;17(1 Suppl):6–15.
14. Skelly AH. Culturally tailored intervention for African Americans with type 2 diabetes administered by a nurse case manager and community health worker reduces emergency room visits. Evid Based Nurs 2010;13:51–2.
15. Lewis MA, Bann CM, Karns SA, et al. Cross-site evaluation of the Alliance to Reduce Disparities in Diabetes: clinical and patient-report
ed outcomes. Health Promot Pract 2014;15(2 Suppl):92S–102S.
16. Collinsworth AW, Vulimiri M, Schmidt KL, Snead CA. Effectiveness of a community health worker-led diabetes self-management education program and implications for CHW involvement in care coordination strategies. Diabetes Educ 2013;39:792–9.
17. Shah M, Kaselitz E, Heisler M. The role of community health workers in diabetes: update on current literature. Curr Diab Rep 2013;13:163–71.
18. Gary TL, Batts-Turner M, Yeh HC, et al. The effects of a nurse case manager and a community health worker team on diabetic control, emergency department visits, and hospitalizations among urban African Americans with type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med 2009;169:1788–94.
19. Martinez J, Ro M, Villa NW, et al. Transforming the delivery of care in the post-health reform era: what role will community health workers play? Am J Public Health 2011;101:e1–5.
20. Rush CH. Return on investment from employment of community health workers. J Ambul Care Manage 2012;35:133–7.
21. Collinsworth A, Vulimiri M, Snead C, Walton J. Community health workers in primary care practice: redesigning health care delivery systems to extend and improve diabetes care in underserved populations. Health Promot Pract 2014;15(2 Suppl):51S–61S.
22. Walton JW, Snead CA, Collinsworth AW, Schmidt KL. Reducing diabetes disparities through the implementation of a community health worker-led diabetes self-management education program. Fam Community Health 2012;35:161–71.
23. Culica D, Walton JW, Prezio EA. CoDE: Community Diabetes Education for uninsured Mexican Americans. Proc (Bayl Univ Med Cent) 2007;20:111–7.
24. Culica D, Walton JW, Harker K, Prezio EA. Effectiveness of a community health worker as sole diabetes educator: comparison of CoDE with similar culturally appropriate interventions. J Health Care Poor Underserved 2008;19:1076–95.
25. Brownson CA, Hoerger TJ, Fisher EB, Kilpatrick KE. Cost-effectiveness of diabetes self-management programs in community primary care settings. Diabetes Educ 2009;35:761–9.
26. Gilmer TP, Roze S, Valentine WJ, et al. Cost-effectiveness of diabetes case management for low-income populations. Health Serv Res 2007;42:1943–59.
27. Brown HS 3rd, Wilson KJ, Pagán JA, et al. Cost-effectiveness analysis of a community health worker intervention for low-income Hispanic adults with diabetes. Prev Chronic Dis 2012;9:E140.
28. Ryabov I. Cost-effectiveness of community health workers in controlling diabetes epidemic on the US-Mexico border. Public Health 2014;128:636–42.
29. Prezio EA, Pagán JA, Shuval K, Culica D. The Community Diabetes Education (CoDE) program: cost-effectiveness and health outcomes. Am J Prev Med 2014;47:771–9.
30. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–53.
1. Centers for Disease Control and Prevention, National Diabetes Statistics Report: estimates of diabetes and its burden in the United States, 2014. US Department of Health and Human Services: Atlanta, GA.
2. Centers for Disease Control and Prevention, Diabetes Report Card 2012. US Department of Health and Human Services: Atlanta, GA.
3. American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–46.
4. Balcazar H, Rosenthal EL, Brownstein JN, et al. Community health workers can be a public health force for change in the United States: three actions for a new paradigm. Am J Public Health 2011;101:2199–203.
5. Rosenthal EL, Brownstein JN, Rush CH, et al. Community health workers: part of the solution. Health Aff (Millwood) 2010;29:1338–42.
6. Viswanathan M, Kraschnewski JL, Nishikawa B, et al. Outcomes and costs of community health worker interventions: a systematic review. Med Care 2010;48:792–808.
7. Babamoto KS, Sey KA, Camilleri AJ, et al. Improving diabetes care and health measures among hispanics using community health workers: results from a randomized controlled trial. Health Educ Behav 2009;36:113–26.
8. Brown SA, Garcia AA, Kouzekanani K, Hanis CL. Culturally competent diabetes self-management education for Mexican Americans: the Starr County border health initiative. Diabetes Care 2002;25:259–68.
9. Prezio EA, Cheng D, Balasubramanian BA, et al. Community Diabetes Education (CoDE) for uninsured Mexican Americans: a randomized controlled trial of a culturally tailored diabetes education and management program led by a community health worker. Diabetes Res Clin Pract 2013;100:19–28.
10. Spencer MS, Rosland AM, Kieffer EC, et al. Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: a randomized controlled trial. Am J Public Health 2011;101:2253–60.
11. Fedder DO, Chang RJ, Curry S, Nichols G. The effectiveness of a community health worker outreach program on healthcare utilization of west Baltimore City Medicaid patients with diabetes, with or without hypertension. Ethn Dis 2003;13:22–7.
12. Lorig KR, Ritter P, Stewart AL, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care 2001;39:1217–23.
13. Whitley EM, Everhart RM, Wright RA. Measuring return on investment of outreach by community health workers. J Health Care Poor Underserved 2006;17(1 Suppl):6–15.
14. Skelly AH. Culturally tailored intervention for African Americans with type 2 diabetes administered by a nurse case manager and community health worker reduces emergency room visits. Evid Based Nurs 2010;13:51–2.
15. Lewis MA, Bann CM, Karns SA, et al. Cross-site evaluation of the Alliance to Reduce Disparities in Diabetes: clinical and patient-report
ed outcomes. Health Promot Pract 2014;15(2 Suppl):92S–102S.
16. Collinsworth AW, Vulimiri M, Schmidt KL, Snead CA. Effectiveness of a community health worker-led diabetes self-management education program and implications for CHW involvement in care coordination strategies. Diabetes Educ 2013;39:792–9.
17. Shah M, Kaselitz E, Heisler M. The role of community health workers in diabetes: update on current literature. Curr Diab Rep 2013;13:163–71.
18. Gary TL, Batts-Turner M, Yeh HC, et al. The effects of a nurse case manager and a community health worker team on diabetic control, emergency department visits, and hospitalizations among urban African Americans with type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med 2009;169:1788–94.
19. Martinez J, Ro M, Villa NW, et al. Transforming the delivery of care in the post-health reform era: what role will community health workers play? Am J Public Health 2011;101:e1–5.
20. Rush CH. Return on investment from employment of community health workers. J Ambul Care Manage 2012;35:133–7.
21. Collinsworth A, Vulimiri M, Snead C, Walton J. Community health workers in primary care practice: redesigning health care delivery systems to extend and improve diabetes care in underserved populations. Health Promot Pract 2014;15(2 Suppl):51S–61S.
22. Walton JW, Snead CA, Collinsworth AW, Schmidt KL. Reducing diabetes disparities through the implementation of a community health worker-led diabetes self-management education program. Fam Community Health 2012;35:161–71.
23. Culica D, Walton JW, Prezio EA. CoDE: Community Diabetes Education for uninsured Mexican Americans. Proc (Bayl Univ Med Cent) 2007;20:111–7.
24. Culica D, Walton JW, Harker K, Prezio EA. Effectiveness of a community health worker as sole diabetes educator: comparison of CoDE with similar culturally appropriate interventions. J Health Care Poor Underserved 2008;19:1076–95.
25. Brownson CA, Hoerger TJ, Fisher EB, Kilpatrick KE. Cost-effectiveness of diabetes self-management programs in community primary care settings. Diabetes Educ 2009;35:761–9.
26. Gilmer TP, Roze S, Valentine WJ, et al. Cost-effectiveness of diabetes case management for low-income populations. Health Serv Res 2007;42:1943–59.
27. Brown HS 3rd, Wilson KJ, Pagán JA, et al. Cost-effectiveness analysis of a community health worker intervention for low-income Hispanic adults with diabetes. Prev Chronic Dis 2012;9:E140.
28. Ryabov I. Cost-effectiveness of community health workers in controlling diabetes epidemic on the US-Mexico border. Public Health 2014;128:636–42.
29. Prezio EA, Pagán JA, Shuval K, Culica D. The Community Diabetes Education (CoDE) program: cost-effectiveness and health outcomes. Am J Prev Med 2014;47:771–9.
30. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837–53.
NASPAG: Teens largely support OTC oral contraceptive access
ORLANDO – Most adolescents expressed interest in and support for access to over-the-counter oral contraceptives, a survey found.
Data from a separate cross-sectional study showed that adolescents are skilled at self-screening for contraindications to combined OCs, demonstrating preliminary support for the safety of over-the-counter access.
Of 348 girls aged 14-17 years who completed the survey in the first study, 73% reported supporting over-the-counter (OTC) OC access for teens, and 61% reported being likely to use OCs if they were available OTC. Significant associations were found between support for OTC access and having used birth control and having had sex. An association was also found between the likelihood of OTC use and having used birth control, having had sex, and being white, Ruth Manski of the Jane Fonda Center for Adolescent Reproductive Health, Atlanta, reported at the annual meeting of the North American Society for Pediatric and Adolescent Gynecology.
Those who had never been tested for sexually transmitted infections were more likely to support OTC access (91% vs. 76%).
Support for, and the likelihood of, using OTC OCs were not influenced by age, geographic region, rural/urban location, health insurance status, or pregnancy history. Race played a factor in that white participants were more likely than nonwhite participants to be interested in OTC access. The study also showed that participants understood an average of 7.1 of 8 key product-labeling concepts, and that most would be willing to pay up to $20 per month for OTC OCs, with the largest percentage (42%) willing to pay between $11 and $20. About a third said they would pay $21 or more.
Survey respondents were girls recruited via Facebook advertisements in 2014. Nearly a third (32%) were aged 17 years, 31% were aged 16 years, 24% were aged 15, and 13% were aged 14. Most (79%) were white, Ms. Manski said, adding that the respondents represented 44 states, 53% lived in a suburban area, 41% had private health insurance, and 33% had public health insurance.
About 90% reported having used contraception, and 44% reported having had sex. Of those who had sex, 60% reported having had unprotected sex, 58% used OCs, and 12% reported having been pregnant.
“So results from this study show that participants are supportive of over-the-counter access and that they’re interested in obtaining oral contraceptives over the counter. The findings also suggest that teenagers understand how to use oral contraceptives based on independent label review, which offers some evidence in response to concerns raised in other studies about teenagers’ ability to understand how to use over-the-counter products,” Ms. Manski said.
The findings suggest that while cost is a concern for teens and could impact their contraceptive choices, making OCs available OTC without age restrictions may help increase adolescents’ contraceptive access and use, she said, noting that this is important given the unique barriers that adolescents face with respect to accessing contraception.
The high levels of interest, and the perception of benefit, highlight the potential of this strategy to increase contraceptive access and reduce unintended pregnancy, she added.
Although some respondents expressed concerns about safety and the potential impact on sexual behavior, the evidence with respect to currently available OTC emergency contraception demonstrates that easier access does not increase sexual risk taking, and that teens can safely use OTC emergency contraception, she said.
However, the behavioral effects of OTC OC availability are not known and should be evaluated in actual use studies, Ms. Manski added.
Concerns about the safety of OTC OC access were specifically addressed in the second study, which sought to determine whether adolescents are capable of self-screening for contraindications to OC use.
Prior studies have shown that adults are able to self-screen adequately, but little is known about adolescents’ ability to do so, Dr. Rebekah L. Williams of Riley Hospital for Children, Indianapolis, said at the meeting.
Findings in the first 61 teens aged 14-21 years enrolled in the study showed that the teens were actually more likely than their providers to report potential contraindications.
The teens completed screening questionnaires prior to their office visit, and the provider completed the medical history questionnaire after the visit.
Perfectly concordant responses between providers and patients were seen for six potential contraindications: diabetes (which was present in two cases) and heavy smoking, breastfeeding, wheelchair use, surgery within 4 weeks, and current HIV medication use (which were not present in any cases). Discordant responses were seen for the remaining potential contraindications, including breast cancer, liver disease, medication interactions, smoking, migraine, hypertension, gallbladder disease, thromboembolism/heart disease, having a first-degree relative with thromboembolism, weight over 200 pounds, and having been told/having the perception that OCs should not be used.
Potential contraindications reported only by the participant were breast cancer, liver disease, medication interaction, any smoking, hypertension, personal history of thromboembolism or heart disease, and having a first-degree relative with thromboembolism.
Participants were adolescents with a mean age of 17 years from a primary care medicine clinic in a Midwest urban setting. Of the 61 subjects, 62% were black, 62% reported having ever used contraceptives, 44% were currently using contraceptives, and 62% said they would be interested in OTC OC access. Among the 38 who reported having penile/vaginal sex, 87% had ever used OCs, and 60% were currently using them.
“In summary, adolescent women are interested in over-the-counter access to combined oral contraceptive pills, they’re skilled at self-screening, and they’re mostly more likely than providers to report potential contraindications. So these data really provide some preliminary support for the over-the-counter provision of oral contraceptive pills to adolescents,” Dr. Williams said, noting that additional research is needed to assess adolescents’ ability to use combined oral contraceptive pills correctly.
In addition, larger studies in groups with a higher prevalence of contraindications are needed, as are studies with recruitment from nonclinical settings and more diverse populations, she said.
Ms. Manski and Dr. Williams both reported having no relevant financial disclosures.
ORLANDO – Most adolescents expressed interest in and support for access to over-the-counter oral contraceptives, a survey found.
Data from a separate cross-sectional study showed that adolescents are skilled at self-screening for contraindications to combined OCs, demonstrating preliminary support for the safety of over-the-counter access.
Of 348 girls aged 14-17 years who completed the survey in the first study, 73% reported supporting over-the-counter (OTC) OC access for teens, and 61% reported being likely to use OCs if they were available OTC. Significant associations were found between support for OTC access and having used birth control and having had sex. An association was also found between the likelihood of OTC use and having used birth control, having had sex, and being white, Ruth Manski of the Jane Fonda Center for Adolescent Reproductive Health, Atlanta, reported at the annual meeting of the North American Society for Pediatric and Adolescent Gynecology.
Those who had never been tested for sexually transmitted infections were more likely to support OTC access (91% vs. 76%).
Support for, and the likelihood of, using OTC OCs were not influenced by age, geographic region, rural/urban location, health insurance status, or pregnancy history. Race played a factor in that white participants were more likely than nonwhite participants to be interested in OTC access. The study also showed that participants understood an average of 7.1 of 8 key product-labeling concepts, and that most would be willing to pay up to $20 per month for OTC OCs, with the largest percentage (42%) willing to pay between $11 and $20. About a third said they would pay $21 or more.
Survey respondents were girls recruited via Facebook advertisements in 2014. Nearly a third (32%) were aged 17 years, 31% were aged 16 years, 24% were aged 15, and 13% were aged 14. Most (79%) were white, Ms. Manski said, adding that the respondents represented 44 states, 53% lived in a suburban area, 41% had private health insurance, and 33% had public health insurance.
About 90% reported having used contraception, and 44% reported having had sex. Of those who had sex, 60% reported having had unprotected sex, 58% used OCs, and 12% reported having been pregnant.
“So results from this study show that participants are supportive of over-the-counter access and that they’re interested in obtaining oral contraceptives over the counter. The findings also suggest that teenagers understand how to use oral contraceptives based on independent label review, which offers some evidence in response to concerns raised in other studies about teenagers’ ability to understand how to use over-the-counter products,” Ms. Manski said.
The findings suggest that while cost is a concern for teens and could impact their contraceptive choices, making OCs available OTC without age restrictions may help increase adolescents’ contraceptive access and use, she said, noting that this is important given the unique barriers that adolescents face with respect to accessing contraception.
The high levels of interest, and the perception of benefit, highlight the potential of this strategy to increase contraceptive access and reduce unintended pregnancy, she added.
Although some respondents expressed concerns about safety and the potential impact on sexual behavior, the evidence with respect to currently available OTC emergency contraception demonstrates that easier access does not increase sexual risk taking, and that teens can safely use OTC emergency contraception, she said.
However, the behavioral effects of OTC OC availability are not known and should be evaluated in actual use studies, Ms. Manski added.
Concerns about the safety of OTC OC access were specifically addressed in the second study, which sought to determine whether adolescents are capable of self-screening for contraindications to OC use.
Prior studies have shown that adults are able to self-screen adequately, but little is known about adolescents’ ability to do so, Dr. Rebekah L. Williams of Riley Hospital for Children, Indianapolis, said at the meeting.
Findings in the first 61 teens aged 14-21 years enrolled in the study showed that the teens were actually more likely than their providers to report potential contraindications.
The teens completed screening questionnaires prior to their office visit, and the provider completed the medical history questionnaire after the visit.
Perfectly concordant responses between providers and patients were seen for six potential contraindications: diabetes (which was present in two cases) and heavy smoking, breastfeeding, wheelchair use, surgery within 4 weeks, and current HIV medication use (which were not present in any cases). Discordant responses were seen for the remaining potential contraindications, including breast cancer, liver disease, medication interactions, smoking, migraine, hypertension, gallbladder disease, thromboembolism/heart disease, having a first-degree relative with thromboembolism, weight over 200 pounds, and having been told/having the perception that OCs should not be used.
Potential contraindications reported only by the participant were breast cancer, liver disease, medication interaction, any smoking, hypertension, personal history of thromboembolism or heart disease, and having a first-degree relative with thromboembolism.
Participants were adolescents with a mean age of 17 years from a primary care medicine clinic in a Midwest urban setting. Of the 61 subjects, 62% were black, 62% reported having ever used contraceptives, 44% were currently using contraceptives, and 62% said they would be interested in OTC OC access. Among the 38 who reported having penile/vaginal sex, 87% had ever used OCs, and 60% were currently using them.
“In summary, adolescent women are interested in over-the-counter access to combined oral contraceptive pills, they’re skilled at self-screening, and they’re mostly more likely than providers to report potential contraindications. So these data really provide some preliminary support for the over-the-counter provision of oral contraceptive pills to adolescents,” Dr. Williams said, noting that additional research is needed to assess adolescents’ ability to use combined oral contraceptive pills correctly.
In addition, larger studies in groups with a higher prevalence of contraindications are needed, as are studies with recruitment from nonclinical settings and more diverse populations, she said.
Ms. Manski and Dr. Williams both reported having no relevant financial disclosures.
ORLANDO – Most adolescents expressed interest in and support for access to over-the-counter oral contraceptives, a survey found.
Data from a separate cross-sectional study showed that adolescents are skilled at self-screening for contraindications to combined OCs, demonstrating preliminary support for the safety of over-the-counter access.
Of 348 girls aged 14-17 years who completed the survey in the first study, 73% reported supporting over-the-counter (OTC) OC access for teens, and 61% reported being likely to use OCs if they were available OTC. Significant associations were found between support for OTC access and having used birth control and having had sex. An association was also found between the likelihood of OTC use and having used birth control, having had sex, and being white, Ruth Manski of the Jane Fonda Center for Adolescent Reproductive Health, Atlanta, reported at the annual meeting of the North American Society for Pediatric and Adolescent Gynecology.
Those who had never been tested for sexually transmitted infections were more likely to support OTC access (91% vs. 76%).
Support for, and the likelihood of, using OTC OCs were not influenced by age, geographic region, rural/urban location, health insurance status, or pregnancy history. Race played a factor in that white participants were more likely than nonwhite participants to be interested in OTC access. The study also showed that participants understood an average of 7.1 of 8 key product-labeling concepts, and that most would be willing to pay up to $20 per month for OTC OCs, with the largest percentage (42%) willing to pay between $11 and $20. About a third said they would pay $21 or more.
Survey respondents were girls recruited via Facebook advertisements in 2014. Nearly a third (32%) were aged 17 years, 31% were aged 16 years, 24% were aged 15, and 13% were aged 14. Most (79%) were white, Ms. Manski said, adding that the respondents represented 44 states, 53% lived in a suburban area, 41% had private health insurance, and 33% had public health insurance.
About 90% reported having used contraception, and 44% reported having had sex. Of those who had sex, 60% reported having had unprotected sex, 58% used OCs, and 12% reported having been pregnant.
“So results from this study show that participants are supportive of over-the-counter access and that they’re interested in obtaining oral contraceptives over the counter. The findings also suggest that teenagers understand how to use oral contraceptives based on independent label review, which offers some evidence in response to concerns raised in other studies about teenagers’ ability to understand how to use over-the-counter products,” Ms. Manski said.
The findings suggest that while cost is a concern for teens and could impact their contraceptive choices, making OCs available OTC without age restrictions may help increase adolescents’ contraceptive access and use, she said, noting that this is important given the unique barriers that adolescents face with respect to accessing contraception.
The high levels of interest, and the perception of benefit, highlight the potential of this strategy to increase contraceptive access and reduce unintended pregnancy, she added.
Although some respondents expressed concerns about safety and the potential impact on sexual behavior, the evidence with respect to currently available OTC emergency contraception demonstrates that easier access does not increase sexual risk taking, and that teens can safely use OTC emergency contraception, she said.
However, the behavioral effects of OTC OC availability are not known and should be evaluated in actual use studies, Ms. Manski added.
Concerns about the safety of OTC OC access were specifically addressed in the second study, which sought to determine whether adolescents are capable of self-screening for contraindications to OC use.
Prior studies have shown that adults are able to self-screen adequately, but little is known about adolescents’ ability to do so, Dr. Rebekah L. Williams of Riley Hospital for Children, Indianapolis, said at the meeting.
Findings in the first 61 teens aged 14-21 years enrolled in the study showed that the teens were actually more likely than their providers to report potential contraindications.
The teens completed screening questionnaires prior to their office visit, and the provider completed the medical history questionnaire after the visit.
Perfectly concordant responses between providers and patients were seen for six potential contraindications: diabetes (which was present in two cases) and heavy smoking, breastfeeding, wheelchair use, surgery within 4 weeks, and current HIV medication use (which were not present in any cases). Discordant responses were seen for the remaining potential contraindications, including breast cancer, liver disease, medication interactions, smoking, migraine, hypertension, gallbladder disease, thromboembolism/heart disease, having a first-degree relative with thromboembolism, weight over 200 pounds, and having been told/having the perception that OCs should not be used.
Potential contraindications reported only by the participant were breast cancer, liver disease, medication interaction, any smoking, hypertension, personal history of thromboembolism or heart disease, and having a first-degree relative with thromboembolism.
Participants were adolescents with a mean age of 17 years from a primary care medicine clinic in a Midwest urban setting. Of the 61 subjects, 62% were black, 62% reported having ever used contraceptives, 44% were currently using contraceptives, and 62% said they would be interested in OTC OC access. Among the 38 who reported having penile/vaginal sex, 87% had ever used OCs, and 60% were currently using them.
“In summary, adolescent women are interested in over-the-counter access to combined oral contraceptive pills, they’re skilled at self-screening, and they’re mostly more likely than providers to report potential contraindications. So these data really provide some preliminary support for the over-the-counter provision of oral contraceptive pills to adolescents,” Dr. Williams said, noting that additional research is needed to assess adolescents’ ability to use combined oral contraceptive pills correctly.
In addition, larger studies in groups with a higher prevalence of contraindications are needed, as are studies with recruitment from nonclinical settings and more diverse populations, she said.
Ms. Manski and Dr. Williams both reported having no relevant financial disclosures.
AT THE NASPAG ANNUAL MEETING
Key clinical point: Adolescents have an interest in access to over-the-counter OCs and appear capable of using them safely.
Major finding: 73% reported supporting OTC access for teens.
Data source: A survey of 348 subjects and a cross-sectional study involving 61 subjects.
Disclosures: Ms. Manski and Dr. Williams both reported having no relevant financial disclosures.
Cancer rate doubles in HCV patients
tissue with active HCV
Photo: Sutter Health
VIENNA, AUSTRIA—A 5-year retrospective study has shown that the cancer rate in patients with hepatitis C virus (HCV) is about double that for people without HCV, even when liver cancer is excluded.
And when liver cancer is included, the rate increases to 2.5 times higher in people with HCV.
Researchers presented these findings at the International Liver Congress 2015 as abstract 0058.
The team reviewed patient records from 2008 to 2012 at Kaiser Permanente Southern California, recording all cancer diagnoses in patients 18 years or older with or without HCV.
During the 5-year time period, there were 145,210 patient years in the HCV cohort and 13,948,826 patient years in the non-HCV cohort.
The mean age at cancer diagnosis was 61.8 in the HCV cohort and 63.5 in the non-HCV cohort.
Researchers recorded 2213 cancer diagnoses in the HCV cohort (1524/100,000). This number decreased to 1654 when they excluded liver cancer (1139/100,000).
In the non-HCV cohort, they recorded 84,419 cancer diagnoses (605/100,000), which decreased to 83,795 when liver cancer was excluded (601/100,000).
Cancer types known to be associated with HCV include non-Hodgkin lymphoma (NHL), renal and prostate cancers, and liver cancer.
NHL occurred 3.63 times more frequently in patients with HCV than in those without HCV, and myeloma occurred 2.93 times more frequently.
Renal cancer occurred 3.27 times and prostate cancer 1.98 times more frequently in patients with HCV than in those without.
“The results suggest that cancer rates are increased in the cohort of hepatitis C patients versus the non-hepatitis C patients, both including and excluding liver cancers,” said senior study author Lisa Nyberg, MD, MPH, of Kaiser Permanente.
“These findings certainly point to the suggestion that hepatitis C may be associated with an increased risk of cancer.”
However, she added that the findings “must be interpreted with caution, as the study also showed that confounding factors such as alcohol abuse, tobacco, obesity, and diabetes modified the results.” ![]()

tissue with active HCV
Photo: Sutter Health
VIENNA, AUSTRIA—A 5-year retrospective study has shown that the cancer rate in patients with hepatitis C virus (HCV) is about double that for people without HCV, even when liver cancer is excluded.
And when liver cancer is included, the rate increases to 2.5 times higher in people with HCV.
Researchers presented these findings at the International Liver Congress 2015 as abstract 0058.
The team reviewed patient records from 2008 to 2012 at Kaiser Permanente Southern California, recording all cancer diagnoses in patients 18 years or older with or without HCV.
During the 5-year time period, there were 145,210 patient years in the HCV cohort and 13,948,826 patient years in the non-HCV cohort.
The mean age at cancer diagnosis was 61.8 in the HCV cohort and 63.5 in the non-HCV cohort.
Researchers recorded 2213 cancer diagnoses in the HCV cohort (1524/100,000). This number decreased to 1654 when they excluded liver cancer (1139/100,000).
In the non-HCV cohort, they recorded 84,419 cancer diagnoses (605/100,000), which decreased to 83,795 when liver cancer was excluded (601/100,000).
Cancer types known to be associated with HCV include non-Hodgkin lymphoma (NHL), renal and prostate cancers, and liver cancer.
NHL occurred 3.63 times more frequently in patients with HCV than in those without HCV, and myeloma occurred 2.93 times more frequently.
Renal cancer occurred 3.27 times and prostate cancer 1.98 times more frequently in patients with HCV than in those without.
“The results suggest that cancer rates are increased in the cohort of hepatitis C patients versus the non-hepatitis C patients, both including and excluding liver cancers,” said senior study author Lisa Nyberg, MD, MPH, of Kaiser Permanente.
“These findings certainly point to the suggestion that hepatitis C may be associated with an increased risk of cancer.”
However, she added that the findings “must be interpreted with caution, as the study also showed that confounding factors such as alcohol abuse, tobacco, obesity, and diabetes modified the results.” ![]()

tissue with active HCV
Photo: Sutter Health
VIENNA, AUSTRIA—A 5-year retrospective study has shown that the cancer rate in patients with hepatitis C virus (HCV) is about double that for people without HCV, even when liver cancer is excluded.
And when liver cancer is included, the rate increases to 2.5 times higher in people with HCV.
Researchers presented these findings at the International Liver Congress 2015 as abstract 0058.
The team reviewed patient records from 2008 to 2012 at Kaiser Permanente Southern California, recording all cancer diagnoses in patients 18 years or older with or without HCV.
During the 5-year time period, there were 145,210 patient years in the HCV cohort and 13,948,826 patient years in the non-HCV cohort.
The mean age at cancer diagnosis was 61.8 in the HCV cohort and 63.5 in the non-HCV cohort.
Researchers recorded 2213 cancer diagnoses in the HCV cohort (1524/100,000). This number decreased to 1654 when they excluded liver cancer (1139/100,000).
In the non-HCV cohort, they recorded 84,419 cancer diagnoses (605/100,000), which decreased to 83,795 when liver cancer was excluded (601/100,000).
Cancer types known to be associated with HCV include non-Hodgkin lymphoma (NHL), renal and prostate cancers, and liver cancer.
NHL occurred 3.63 times more frequently in patients with HCV than in those without HCV, and myeloma occurred 2.93 times more frequently.
Renal cancer occurred 3.27 times and prostate cancer 1.98 times more frequently in patients with HCV than in those without.
“The results suggest that cancer rates are increased in the cohort of hepatitis C patients versus the non-hepatitis C patients, both including and excluding liver cancers,” said senior study author Lisa Nyberg, MD, MPH, of Kaiser Permanente.
“These findings certainly point to the suggestion that hepatitis C may be associated with an increased risk of cancer.”
However, she added that the findings “must be interpreted with caution, as the study also showed that confounding factors such as alcohol abuse, tobacco, obesity, and diabetes modified the results.” ![]()
Applications expand for vorapaxar in cardiovascular prevention
SAN DIEGO– A fuller picture of the benefits of vorapaxar for secondary cardiovascular prevention emerged from three separate secondary analyses of the pivotal Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P TIMI-50) trial presented at the annual meeting of the American College of Cardiology.
These new reports provided evidence that vorapaxar (Zontivity), a first-in-class, once-daily thrombin inhibitor with rapid onset and a half-life in excess of 7 days, reduces acute limb ischemia and the need for peripheral revascularization in patients with peripheral arterial disease (PAD), and also that the antiplatelet agent is particularly beneficial in patients with a history of coronary artery bypass graft surgery.
That being said, the three secondary analyses were post hoc and as such are inherently exploratory and hypothesis-generating rather than definitive, the investigators noted.
The Food and Drug Administration approved vorapaxar in May 2014 for the reduction of thrombotic cardiovascular events in patients with a history of MI or in patients with PAD in the absence of a previous stroke or TIA. Marketing approval was based chiefly on the results of the landmark 26,449-patient, double-blind, prospective, multinational TIMI 50 trial. In an analysis of the 20,170 randomized patients without a prior stroke or TIA, vorapaxar at 2.5 mg once daily, when added to standard therapy with aspirin and/or clopidogrel, reduced the 3-year rate of a composite outcome – comprised of cardiovascular death, MI, or stroke – by 20% compared with placebo, with a number-needed-to-treat of 63 (J. Am. Heart Assoc. 2015 [doi: 10.1161/JAHA.114.001505]).
At ACC 15, Dr. Ethan C. Kosova presented a subanalysis involving the 2,942 TIMI 50 participants who had undergone CABG surgery prior to the study. The rationale for taking a closer look at this group is that rates of venous graft occlusion and recurrent ischemic events remain high after CABG surgery, so the efficacy and safety of vorapaxar in this population are of particular interest.
Underscoring the high-risk nature of this population, at baseline the patients with a history of CABG were significantly older and had higher rates of hypertension, dyslipidemia, diabetes, PAD, heart failure, and chronic kidney disease than participants without prior CABG who had no history of stroke or TIA, observed Dr. Kosova of Brigham and Women’s Hospital, Boston.
The 3-year composite event rate of cardiovascular death, MI, or stroke occurred in 11.9% of the 1,471 patients with prior CABG who were randomized to vorapaxar compared with 15.6% of those on placebo. That translates into a 29% relative risk reduction and a number-needed-to-treat of 27, an even stronger result than in the general study population.
Moreover, the clinically relevant composite outcome consisting of cardiovascular death or MI occurred in 10.9% of those on vorapaxar compared with 14.3% of controls, for a 29% relative risk reduction and a number-needed-to-treat of 29, he continued.
The 3-year all-cause mortality was 5.8% in patients with prior CABG who received vorapaxar compared to 8% in those on placebo.
Vorapaxar, which inhibits protease-activated receptor-1 on platelets and vascular endothelium, increased the rate of GUSTO moderate-to-severe bleeding: 6.8% compared with 3.7% in controls.
“Putting together the efficacy and safety data to derive the net clinical outcome – the combined endpoint of all-cause mortality, MI, cerebrovascular accident, and GUSTO severe bleeding – the result was a 28% improvement with vorapaxar compared with placebo,” Dr. Kosova said.
In a separate presentation focused on the 3,787 patients who gained entry into the TIMI 50 trial on the basis of symptomatic PAD, Dr. Antonio Gutierrez reported that acute limb ischemia occurred in 109 of them during the trial. Fifty-four percent of these events were due to acute surgical graft thrombosis, 27% to in situ thrombosis of a native vessel, and lesser numbers were due to thromboembolissm or stent thrombosis.
The 3-year acute limb ischemia rate in patients with baseline PAD was 3.9% with placebo compared to 2.3% with vorapaxar, for a 42% relative risk reduction, according to Dr. Gutierrez of Brigham and Women’s Hospital.
Dr. Ian Gilchrist, also from Brigham and Women’s Hospital, reported that in TIMI 50 participants with a history of PAD, vorapaxar resulted in a 16% reduction in the need for peripheral revascularization procedures for claudication, with rates of 9.4% for vorapaxar and 11.6% for placebo. There was also a statistically significant 41% reduction in the rate of surgical peripheral revascularization procedures, with rates of 4.5% for vorapaxar and 7.7% for placebo.
The TIMI 50 trial was funded by Merck. Drs. Kosova, Gutierrez, and Gilchrist reported having no financial conflicts of interest.
SAN DIEGO– A fuller picture of the benefits of vorapaxar for secondary cardiovascular prevention emerged from three separate secondary analyses of the pivotal Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P TIMI-50) trial presented at the annual meeting of the American College of Cardiology.
These new reports provided evidence that vorapaxar (Zontivity), a first-in-class, once-daily thrombin inhibitor with rapid onset and a half-life in excess of 7 days, reduces acute limb ischemia and the need for peripheral revascularization in patients with peripheral arterial disease (PAD), and also that the antiplatelet agent is particularly beneficial in patients with a history of coronary artery bypass graft surgery.
That being said, the three secondary analyses were post hoc and as such are inherently exploratory and hypothesis-generating rather than definitive, the investigators noted.
The Food and Drug Administration approved vorapaxar in May 2014 for the reduction of thrombotic cardiovascular events in patients with a history of MI or in patients with PAD in the absence of a previous stroke or TIA. Marketing approval was based chiefly on the results of the landmark 26,449-patient, double-blind, prospective, multinational TIMI 50 trial. In an analysis of the 20,170 randomized patients without a prior stroke or TIA, vorapaxar at 2.5 mg once daily, when added to standard therapy with aspirin and/or clopidogrel, reduced the 3-year rate of a composite outcome – comprised of cardiovascular death, MI, or stroke – by 20% compared with placebo, with a number-needed-to-treat of 63 (J. Am. Heart Assoc. 2015 [doi: 10.1161/JAHA.114.001505]).
At ACC 15, Dr. Ethan C. Kosova presented a subanalysis involving the 2,942 TIMI 50 participants who had undergone CABG surgery prior to the study. The rationale for taking a closer look at this group is that rates of venous graft occlusion and recurrent ischemic events remain high after CABG surgery, so the efficacy and safety of vorapaxar in this population are of particular interest.
Underscoring the high-risk nature of this population, at baseline the patients with a history of CABG were significantly older and had higher rates of hypertension, dyslipidemia, diabetes, PAD, heart failure, and chronic kidney disease than participants without prior CABG who had no history of stroke or TIA, observed Dr. Kosova of Brigham and Women’s Hospital, Boston.
The 3-year composite event rate of cardiovascular death, MI, or stroke occurred in 11.9% of the 1,471 patients with prior CABG who were randomized to vorapaxar compared with 15.6% of those on placebo. That translates into a 29% relative risk reduction and a number-needed-to-treat of 27, an even stronger result than in the general study population.
Moreover, the clinically relevant composite outcome consisting of cardiovascular death or MI occurred in 10.9% of those on vorapaxar compared with 14.3% of controls, for a 29% relative risk reduction and a number-needed-to-treat of 29, he continued.
The 3-year all-cause mortality was 5.8% in patients with prior CABG who received vorapaxar compared to 8% in those on placebo.
Vorapaxar, which inhibits protease-activated receptor-1 on platelets and vascular endothelium, increased the rate of GUSTO moderate-to-severe bleeding: 6.8% compared with 3.7% in controls.
“Putting together the efficacy and safety data to derive the net clinical outcome – the combined endpoint of all-cause mortality, MI, cerebrovascular accident, and GUSTO severe bleeding – the result was a 28% improvement with vorapaxar compared with placebo,” Dr. Kosova said.
In a separate presentation focused on the 3,787 patients who gained entry into the TIMI 50 trial on the basis of symptomatic PAD, Dr. Antonio Gutierrez reported that acute limb ischemia occurred in 109 of them during the trial. Fifty-four percent of these events were due to acute surgical graft thrombosis, 27% to in situ thrombosis of a native vessel, and lesser numbers were due to thromboembolissm or stent thrombosis.
The 3-year acute limb ischemia rate in patients with baseline PAD was 3.9% with placebo compared to 2.3% with vorapaxar, for a 42% relative risk reduction, according to Dr. Gutierrez of Brigham and Women’s Hospital.
Dr. Ian Gilchrist, also from Brigham and Women’s Hospital, reported that in TIMI 50 participants with a history of PAD, vorapaxar resulted in a 16% reduction in the need for peripheral revascularization procedures for claudication, with rates of 9.4% for vorapaxar and 11.6% for placebo. There was also a statistically significant 41% reduction in the rate of surgical peripheral revascularization procedures, with rates of 4.5% for vorapaxar and 7.7% for placebo.
The TIMI 50 trial was funded by Merck. Drs. Kosova, Gutierrez, and Gilchrist reported having no financial conflicts of interest.
SAN DIEGO– A fuller picture of the benefits of vorapaxar for secondary cardiovascular prevention emerged from three separate secondary analyses of the pivotal Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P TIMI-50) trial presented at the annual meeting of the American College of Cardiology.
These new reports provided evidence that vorapaxar (Zontivity), a first-in-class, once-daily thrombin inhibitor with rapid onset and a half-life in excess of 7 days, reduces acute limb ischemia and the need for peripheral revascularization in patients with peripheral arterial disease (PAD), and also that the antiplatelet agent is particularly beneficial in patients with a history of coronary artery bypass graft surgery.
That being said, the three secondary analyses were post hoc and as such are inherently exploratory and hypothesis-generating rather than definitive, the investigators noted.
The Food and Drug Administration approved vorapaxar in May 2014 for the reduction of thrombotic cardiovascular events in patients with a history of MI or in patients with PAD in the absence of a previous stroke or TIA. Marketing approval was based chiefly on the results of the landmark 26,449-patient, double-blind, prospective, multinational TIMI 50 trial. In an analysis of the 20,170 randomized patients without a prior stroke or TIA, vorapaxar at 2.5 mg once daily, when added to standard therapy with aspirin and/or clopidogrel, reduced the 3-year rate of a composite outcome – comprised of cardiovascular death, MI, or stroke – by 20% compared with placebo, with a number-needed-to-treat of 63 (J. Am. Heart Assoc. 2015 [doi: 10.1161/JAHA.114.001505]).
At ACC 15, Dr. Ethan C. Kosova presented a subanalysis involving the 2,942 TIMI 50 participants who had undergone CABG surgery prior to the study. The rationale for taking a closer look at this group is that rates of venous graft occlusion and recurrent ischemic events remain high after CABG surgery, so the efficacy and safety of vorapaxar in this population are of particular interest.
Underscoring the high-risk nature of this population, at baseline the patients with a history of CABG were significantly older and had higher rates of hypertension, dyslipidemia, diabetes, PAD, heart failure, and chronic kidney disease than participants without prior CABG who had no history of stroke or TIA, observed Dr. Kosova of Brigham and Women’s Hospital, Boston.
The 3-year composite event rate of cardiovascular death, MI, or stroke occurred in 11.9% of the 1,471 patients with prior CABG who were randomized to vorapaxar compared with 15.6% of those on placebo. That translates into a 29% relative risk reduction and a number-needed-to-treat of 27, an even stronger result than in the general study population.
Moreover, the clinically relevant composite outcome consisting of cardiovascular death or MI occurred in 10.9% of those on vorapaxar compared with 14.3% of controls, for a 29% relative risk reduction and a number-needed-to-treat of 29, he continued.
The 3-year all-cause mortality was 5.8% in patients with prior CABG who received vorapaxar compared to 8% in those on placebo.
Vorapaxar, which inhibits protease-activated receptor-1 on platelets and vascular endothelium, increased the rate of GUSTO moderate-to-severe bleeding: 6.8% compared with 3.7% in controls.
“Putting together the efficacy and safety data to derive the net clinical outcome – the combined endpoint of all-cause mortality, MI, cerebrovascular accident, and GUSTO severe bleeding – the result was a 28% improvement with vorapaxar compared with placebo,” Dr. Kosova said.
In a separate presentation focused on the 3,787 patients who gained entry into the TIMI 50 trial on the basis of symptomatic PAD, Dr. Antonio Gutierrez reported that acute limb ischemia occurred in 109 of them during the trial. Fifty-four percent of these events were due to acute surgical graft thrombosis, 27% to in situ thrombosis of a native vessel, and lesser numbers were due to thromboembolissm or stent thrombosis.
The 3-year acute limb ischemia rate in patients with baseline PAD was 3.9% with placebo compared to 2.3% with vorapaxar, for a 42% relative risk reduction, according to Dr. Gutierrez of Brigham and Women’s Hospital.
Dr. Ian Gilchrist, also from Brigham and Women’s Hospital, reported that in TIMI 50 participants with a history of PAD, vorapaxar resulted in a 16% reduction in the need for peripheral revascularization procedures for claudication, with rates of 9.4% for vorapaxar and 11.6% for placebo. There was also a statistically significant 41% reduction in the rate of surgical peripheral revascularization procedures, with rates of 4.5% for vorapaxar and 7.7% for placebo.
The TIMI 50 trial was funded by Merck. Drs. Kosova, Gutierrez, and Gilchrist reported having no financial conflicts of interest.
AT ACC 15
Key clinical point: Vorapaxar reduced the composite outcome of cardiovascular death, MI, or stroke by 29% compared with placebo in patients with a history of MI and/or peripheral arterial disease and prior coronary artery bypass surgery.
Major finding: The novel antiplatelet agent also reduced acute limb ischemia events by 42% in patients with symptomatic peripheral arterial disease.
Data source: These were findings from post hoc, exploratory analyses of a 26,449-patient, randomized, double-blind pivotal trial of vorapaxar for secondary cardiovascular prevention.
Disclosures: The TRA 2°P TIMI-50 trial was sponsored by Merck. The presenters reported having no financial conflicts.
First spray-dried fibrin sealant approved by FDA

Photo by Piotr Bodzek
The US Food and Drug Administration (FDA) has approved the first spray-dried fibrin sealant to help control bleeding during surgery.
The agency approved Raplixa (formerly known as Fibrocaps) for use in adults to control bleeding from small blood vessels when standard surgical techniques are ineffective or impractical.
The standard techniques include suture, ligature, or cautery.
Raplixa contains fibrinogen and thrombin, proteins found in human plasma. When the surgical team applies Raplixa to the bleeding site, the sealant dissolves in the blood, and the fibrinogen and thrombin proteins react, resulting in the formation of blood clots.
The protein components are individually purified using a manufacturing process that includes virus inactivation and removal steps to help reduce the risk for the transmission of blood-borne viruses. The fibrin sealant components are then spray-dried, blended, and packaged in a vial.
Raplixa can be applied directly from the original product vial or by spraying it with a delivery device. Raplixa is used in conjunction with an absorbable gelatin sponge.
“The spray-drying process used to manufacture Raplixa produces dried powders that can be combined into a single vial,” said Karen Midthun, MD, of the FDA’s Center for Biologics Evaluation and Research.
“This eliminates the need to combine the fibrinogen and thrombin before use and allows the product to be stored at room temperature.”
Raplixa was approved for use in the European Union in March, based on the recommendation of the European Medicines Agency’s Committee for Medicinal Products for Human Use.
Phase 3 trial
The FDA approved Raplixa based on data from the FINISH-3 trial, a randomized, single-blind, controlled, phase 3 study of 719 patients undergoing spinal surgery (n=183), hepatic resection (n=180), vascular surgery (n=175), or soft tissue dissection (n=181) in 4 countries over 11 months.
As reported in the Journal of the American College of Surgeons, adults with mild or moderate surgical bleeding were randomized to recieve Raplixa or the gelatin sponge. Researchers recorded the time to hemostasis over 5 minutes within each surgical indication.
Four hundred and eighty patients were treated with Raplixa, and 239 were treated with the gelatin sponge. Surgeons used the spray device in 53% of Raplixa procedures.
Raplixa used in conjunction with the gelatin sponge significantly reduced time to hemostasis compared to the gelatin sponge alone (P<0.001 for each of the 4 sugical indications).
Raplixa also significantly reduced median time to hemostasis for each indication (P<0.0001).
Adverse events were similar between the treatment arms. The most commonly reported adverse reactions were surgical pain, nausea, constipation, fever, and decreased blood pressure.
Two percent of Raplixa-treated patients developed non-neutralizing anti-thrombin antibodies, as did 3% of patients treated with the gelatin sponge.
Raplixa is manufactured by ProFibrix BV, a wholly owned subsidiary of The Medicines Company, based in Parsippany, New Jersey. ![]()

Photo by Piotr Bodzek
The US Food and Drug Administration (FDA) has approved the first spray-dried fibrin sealant to help control bleeding during surgery.
The agency approved Raplixa (formerly known as Fibrocaps) for use in adults to control bleeding from small blood vessels when standard surgical techniques are ineffective or impractical.
The standard techniques include suture, ligature, or cautery.
Raplixa contains fibrinogen and thrombin, proteins found in human plasma. When the surgical team applies Raplixa to the bleeding site, the sealant dissolves in the blood, and the fibrinogen and thrombin proteins react, resulting in the formation of blood clots.
The protein components are individually purified using a manufacturing process that includes virus inactivation and removal steps to help reduce the risk for the transmission of blood-borne viruses. The fibrin sealant components are then spray-dried, blended, and packaged in a vial.
Raplixa can be applied directly from the original product vial or by spraying it with a delivery device. Raplixa is used in conjunction with an absorbable gelatin sponge.
“The spray-drying process used to manufacture Raplixa produces dried powders that can be combined into a single vial,” said Karen Midthun, MD, of the FDA’s Center for Biologics Evaluation and Research.
“This eliminates the need to combine the fibrinogen and thrombin before use and allows the product to be stored at room temperature.”
Raplixa was approved for use in the European Union in March, based on the recommendation of the European Medicines Agency’s Committee for Medicinal Products for Human Use.
Phase 3 trial
The FDA approved Raplixa based on data from the FINISH-3 trial, a randomized, single-blind, controlled, phase 3 study of 719 patients undergoing spinal surgery (n=183), hepatic resection (n=180), vascular surgery (n=175), or soft tissue dissection (n=181) in 4 countries over 11 months.
As reported in the Journal of the American College of Surgeons, adults with mild or moderate surgical bleeding were randomized to recieve Raplixa or the gelatin sponge. Researchers recorded the time to hemostasis over 5 minutes within each surgical indication.
Four hundred and eighty patients were treated with Raplixa, and 239 were treated with the gelatin sponge. Surgeons used the spray device in 53% of Raplixa procedures.
Raplixa used in conjunction with the gelatin sponge significantly reduced time to hemostasis compared to the gelatin sponge alone (P<0.001 for each of the 4 sugical indications).
Raplixa also significantly reduced median time to hemostasis for each indication (P<0.0001).
Adverse events were similar between the treatment arms. The most commonly reported adverse reactions were surgical pain, nausea, constipation, fever, and decreased blood pressure.
Two percent of Raplixa-treated patients developed non-neutralizing anti-thrombin antibodies, as did 3% of patients treated with the gelatin sponge.
Raplixa is manufactured by ProFibrix BV, a wholly owned subsidiary of The Medicines Company, based in Parsippany, New Jersey. ![]()

Photo by Piotr Bodzek
The US Food and Drug Administration (FDA) has approved the first spray-dried fibrin sealant to help control bleeding during surgery.
The agency approved Raplixa (formerly known as Fibrocaps) for use in adults to control bleeding from small blood vessels when standard surgical techniques are ineffective or impractical.
The standard techniques include suture, ligature, or cautery.
Raplixa contains fibrinogen and thrombin, proteins found in human plasma. When the surgical team applies Raplixa to the bleeding site, the sealant dissolves in the blood, and the fibrinogen and thrombin proteins react, resulting in the formation of blood clots.
The protein components are individually purified using a manufacturing process that includes virus inactivation and removal steps to help reduce the risk for the transmission of blood-borne viruses. The fibrin sealant components are then spray-dried, blended, and packaged in a vial.
Raplixa can be applied directly from the original product vial or by spraying it with a delivery device. Raplixa is used in conjunction with an absorbable gelatin sponge.
“The spray-drying process used to manufacture Raplixa produces dried powders that can be combined into a single vial,” said Karen Midthun, MD, of the FDA’s Center for Biologics Evaluation and Research.
“This eliminates the need to combine the fibrinogen and thrombin before use and allows the product to be stored at room temperature.”
Raplixa was approved for use in the European Union in March, based on the recommendation of the European Medicines Agency’s Committee for Medicinal Products for Human Use.
Phase 3 trial
The FDA approved Raplixa based on data from the FINISH-3 trial, a randomized, single-blind, controlled, phase 3 study of 719 patients undergoing spinal surgery (n=183), hepatic resection (n=180), vascular surgery (n=175), or soft tissue dissection (n=181) in 4 countries over 11 months.
As reported in the Journal of the American College of Surgeons, adults with mild or moderate surgical bleeding were randomized to recieve Raplixa or the gelatin sponge. Researchers recorded the time to hemostasis over 5 minutes within each surgical indication.
Four hundred and eighty patients were treated with Raplixa, and 239 were treated with the gelatin sponge. Surgeons used the spray device in 53% of Raplixa procedures.
Raplixa used in conjunction with the gelatin sponge significantly reduced time to hemostasis compared to the gelatin sponge alone (P<0.001 for each of the 4 sugical indications).
Raplixa also significantly reduced median time to hemostasis for each indication (P<0.0001).
Adverse events were similar between the treatment arms. The most commonly reported adverse reactions were surgical pain, nausea, constipation, fever, and decreased blood pressure.
Two percent of Raplixa-treated patients developed non-neutralizing anti-thrombin antibodies, as did 3% of patients treated with the gelatin sponge.
Raplixa is manufactured by ProFibrix BV, a wholly owned subsidiary of The Medicines Company, based in Parsippany, New Jersey. ![]()
Hospital Medicine 2015 Photo Gallery - Day Four
Photographs from Hospital Medicine 2015, which took place March 29-April 1 at the Gaylord National Hotel and Conference Center in National Harbor, Md.
Photos by Manuel Noguera
[gallery ids="9197,9196,9195,9194,9193,9192,9191,9190,9189,9188,9187,9186,9185,9184,9183,9182,9181,9180,9179,9178,9177,9176,9175,9171"]
Photographs from Hospital Medicine 2015, which took place March 29-April 1 at the Gaylord National Hotel and Conference Center in National Harbor, Md.
Photos by Manuel Noguera
[gallery ids="9197,9196,9195,9194,9193,9192,9191,9190,9189,9188,9187,9186,9185,9184,9183,9182,9181,9180,9179,9178,9177,9176,9175,9171"]
Photographs from Hospital Medicine 2015, which took place March 29-April 1 at the Gaylord National Hotel and Conference Center in National Harbor, Md.
Photos by Manuel Noguera
[gallery ids="9197,9196,9195,9194,9193,9192,9191,9190,9189,9188,9187,9186,9185,9184,9183,9182,9181,9180,9179,9178,9177,9176,9175,9171"]
LISTEN NOW: David Weidig, MD, talks about best practices for multi-site hospital medicine
Excerpts from our interview with Team Hospitalist member David Weidig, MD, director of hospital medicine for Aurora Medical Group in West Allis, Wis., about best practices for multi-site hospital medicine.
[audio mp3="http://www.the-hospitalist.org/wp-content/uploads/2015/05/David-Weidig_HM15_FINAL_050215.mp3"][/audio]
Excerpts from our interview with Team Hospitalist member David Weidig, MD, director of hospital medicine for Aurora Medical Group in West Allis, Wis., about best practices for multi-site hospital medicine.
[audio mp3="http://www.the-hospitalist.org/wp-content/uploads/2015/05/David-Weidig_HM15_FINAL_050215.mp3"][/audio]
Excerpts from our interview with Team Hospitalist member David Weidig, MD, director of hospital medicine for Aurora Medical Group in West Allis, Wis., about best practices for multi-site hospital medicine.
[audio mp3="http://www.the-hospitalist.org/wp-content/uploads/2015/05/David-Weidig_HM15_FINAL_050215.mp3"][/audio]