User login
Stomach pain chalked up to flu; patient suffers fatal cardiac event ... More
Stomach pain chalked up to flu; patient suffers fatal cardiac event
A 40-YEAR-OLD MAN went to the emergency department (ED) after 2 days of stomach discomfort. The ED physician who evaluated him released him after 4 or 5 hours without testing for levels of troponin or other cardiac enzymes. The patient’s discomfort continued, and about 3 days later, he told his wife to call 911. He was transported to the ED but did not survive.
PLAINTIFF’S CLAIM The decedent had been suffering from an acute cardiac event during the first ED visit. Testing to rule out cardiac problems should have been performed.
THE DEFENSE The patient had been suffering from a stomach flu during his initial ED visit. Any testing performed at that time would have been normal. The patient’s death was unrelated to the symptoms he was experiencing when he was first seen.
VERDICT $4 million Alabama verdict.
COMMENT Many questions come to mind with this case: How careful was the history? Did the patient’s discomfort get worse with activity? What were the characteristics of his pain? What were the patient’s cardiac risk factors? A colleague of mine missed a very similar case several years ago in a 67-year-old. The patient even had vomiting and diarrhea, but clearly had a myocardial infarction when diagnosed a few days later.
Follow-up failure on PSA results costs patient valuable Tx time
A PATIENT AT A GROUP PRACTICE underwent prostate specific antigen (PSA) screening, which revealed an abnormal result (4.1 ng/mL). The physician circled this value on the lab report, wrote, “Discuss next visit,” and placed the report in the patient’s chart. However, the patient switched to another physician in the group and was not told of the abnormal result for more than 2 years. When the patient went to a medical center for back pain, magnetic resonance imaging of his spine revealed the presence of cancer in his spine, shoulder blades, pelvis, and ribs. A PSA test performed at that time came back at 100 ng/mL. Two days later, a biopsy confirmed the diagnosis of prostate cancer (Gleason score, 9).
PLAINTIFF’S CLAIM In addition to failing to inform the patient of his abnormal PSA test result, the physician did not perform digital rectal exams.
THE DEFENSE Earlier treatment would not have made a difference in the outcome.
VERDICT $934,000 Florida verdict.
COMMENT If you order a PSA, you must follow up on it. When a patient transfers to your care, be sure to obtain and review past testing and provide follow-up on abnormal results. We now send all test results directly to patients so they can serve as a safety check for their own care. Despite fears of being inundated with calls, most organizations that have instituted such a policy have not turned back.
Stomach pain chalked up to flu; patient suffers fatal cardiac event
A 40-YEAR-OLD MAN went to the emergency department (ED) after 2 days of stomach discomfort. The ED physician who evaluated him released him after 4 or 5 hours without testing for levels of troponin or other cardiac enzymes. The patient’s discomfort continued, and about 3 days later, he told his wife to call 911. He was transported to the ED but did not survive.
PLAINTIFF’S CLAIM The decedent had been suffering from an acute cardiac event during the first ED visit. Testing to rule out cardiac problems should have been performed.
THE DEFENSE The patient had been suffering from a stomach flu during his initial ED visit. Any testing performed at that time would have been normal. The patient’s death was unrelated to the symptoms he was experiencing when he was first seen.
VERDICT $4 million Alabama verdict.
COMMENT Many questions come to mind with this case: How careful was the history? Did the patient’s discomfort get worse with activity? What were the characteristics of his pain? What were the patient’s cardiac risk factors? A colleague of mine missed a very similar case several years ago in a 67-year-old. The patient even had vomiting and diarrhea, but clearly had a myocardial infarction when diagnosed a few days later.
Follow-up failure on PSA results costs patient valuable Tx time
A PATIENT AT A GROUP PRACTICE underwent prostate specific antigen (PSA) screening, which revealed an abnormal result (4.1 ng/mL). The physician circled this value on the lab report, wrote, “Discuss next visit,” and placed the report in the patient’s chart. However, the patient switched to another physician in the group and was not told of the abnormal result for more than 2 years. When the patient went to a medical center for back pain, magnetic resonance imaging of his spine revealed the presence of cancer in his spine, shoulder blades, pelvis, and ribs. A PSA test performed at that time came back at 100 ng/mL. Two days later, a biopsy confirmed the diagnosis of prostate cancer (Gleason score, 9).
PLAINTIFF’S CLAIM In addition to failing to inform the patient of his abnormal PSA test result, the physician did not perform digital rectal exams.
THE DEFENSE Earlier treatment would not have made a difference in the outcome.
VERDICT $934,000 Florida verdict.
COMMENT If you order a PSA, you must follow up on it. When a patient transfers to your care, be sure to obtain and review past testing and provide follow-up on abnormal results. We now send all test results directly to patients so they can serve as a safety check for their own care. Despite fears of being inundated with calls, most organizations that have instituted such a policy have not turned back.
Stomach pain chalked up to flu; patient suffers fatal cardiac event
A 40-YEAR-OLD MAN went to the emergency department (ED) after 2 days of stomach discomfort. The ED physician who evaluated him released him after 4 or 5 hours without testing for levels of troponin or other cardiac enzymes. The patient’s discomfort continued, and about 3 days later, he told his wife to call 911. He was transported to the ED but did not survive.
PLAINTIFF’S CLAIM The decedent had been suffering from an acute cardiac event during the first ED visit. Testing to rule out cardiac problems should have been performed.
THE DEFENSE The patient had been suffering from a stomach flu during his initial ED visit. Any testing performed at that time would have been normal. The patient’s death was unrelated to the symptoms he was experiencing when he was first seen.
VERDICT $4 million Alabama verdict.
COMMENT Many questions come to mind with this case: How careful was the history? Did the patient’s discomfort get worse with activity? What were the characteristics of his pain? What were the patient’s cardiac risk factors? A colleague of mine missed a very similar case several years ago in a 67-year-old. The patient even had vomiting and diarrhea, but clearly had a myocardial infarction when diagnosed a few days later.
Follow-up failure on PSA results costs patient valuable Tx time
A PATIENT AT A GROUP PRACTICE underwent prostate specific antigen (PSA) screening, which revealed an abnormal result (4.1 ng/mL). The physician circled this value on the lab report, wrote, “Discuss next visit,” and placed the report in the patient’s chart. However, the patient switched to another physician in the group and was not told of the abnormal result for more than 2 years. When the patient went to a medical center for back pain, magnetic resonance imaging of his spine revealed the presence of cancer in his spine, shoulder blades, pelvis, and ribs. A PSA test performed at that time came back at 100 ng/mL. Two days later, a biopsy confirmed the diagnosis of prostate cancer (Gleason score, 9).
PLAINTIFF’S CLAIM In addition to failing to inform the patient of his abnormal PSA test result, the physician did not perform digital rectal exams.
THE DEFENSE Earlier treatment would not have made a difference in the outcome.
VERDICT $934,000 Florida verdict.
COMMENT If you order a PSA, you must follow up on it. When a patient transfers to your care, be sure to obtain and review past testing and provide follow-up on abnormal results. We now send all test results directly to patients so they can serve as a safety check for their own care. Despite fears of being inundated with calls, most organizations that have instituted such a policy have not turned back.
What is the most effective topical treatment for allergic conjunctivitis?
Topical antihistamines and topical mast cell stabilizers appear to reduce conjunctival injection and itching effectively. Topical nonsteroidal anti-inflammatory drugs (NSAIDs) are also effective, but may sting on application (strength of recommendation: B, meta-analysis of randomized controlled trials [RCTs]).
Both of these treatments relieve redness and itching
A 2004 systematic review of 40 RCTs (total N not provided) assessed the efficacy of topical treatment with mast cell stabilizers and antihistamines, comparing each with the other and placebo.1 Eleven trials that included 899 children and adults compared mast cell stabilizers (sodium cromoglycate, nedocromil, and lodoxamide tromethamine) with placebo. Follow-up periods ranged from 4 to 9 weeks.
Because of study heterogeneity, a random-effects model was used and showed that topical mast cell stabilizers relieved symptoms (ocular itching, burning, and lacrimation) 4.9 times more effectively than placebo (95% confidence interval [CI], 2.5-9.6). Possible publication bias was cited as a limitation.
In the same systematic review, 9 RCTs with 313 patients compared topical antihistamines (levocabastine, azelastine hydrochloride, emedastine, and antazoline phosphate) with placebo. Signs and symptoms (itching, redness, burning, and swelling) were graded using symptom severity scales. Follow-up ranged from 30 minutes to 24 hours. A meta-analysis wasn’t possible because most studies didn’t tabulate the mean scores and error associated with these scores. Most individual studies, however, showed improvement in the cardinal symptom of itchiness.
Finally, 8 RCTs compared topical mast cell stabilizers (sodium cromoglycate, lodoxamide, and nedocromil sodium) with levocabastine, a topical antihistamine. Two RCTs with 74 patients had follow-up periods of 15 minutes to 4 hours; the remaining 6 RCTs with 473 patients had follow-up periods of 14 days to 4 months. Subjective scoring of symptoms was done in 7 of the 8 studies.
Scores between treatment groups were reported as not statistically significant in the 6 longer-term studies. Meta-analysis wasn’t possible because most studies didn’t tabulate the mean scores and error associated with measures. The 2 short-term studies reported a statistically significant reduction in itching and redness (P<.05) in patients treated with the antihistamine (data not provided).
NSAIDs relieve itching but may sting when applied
A 2007 meta-analysis of 8 RCTs compared topical NSAIDs (ketorolac, diclofenac, aspirin, or steroid) with placebo for treating isolated allergic conjunctivitis in 712 children and adults.2 Primary outcomes were measured as subjective reductions in conjunctival injection and itching measured at 2 to 6 weeks using a 0-to-3 severity scale.
Topical NSAIDs produced significantly greater relief of conjunctival itching (4 trials, N=231; mean difference [MD]=-0.54; 95% CI, -0.84 to -0.24) and conjunctival injection (4 trials, N=208; MD=-0.51; 95% CI, -0.97 to -0.05). NSAIDs weren’t superior to placebo in treating other ocular symptoms of eyelid swelling, ocular burning, photophobia, or foreign body sensation, and they had a higher rate of stinging on application (odds ratio=4.0; 95% CI, 2.7-5.9).
Guideline recommends topical antihistamines or mast cell stabilizers
The American Academy of Ophthalmology’s 2012 evidence-based guideline recommends treating allergic conjunctivitis with topical antihistamines (Level A-1 evidence, defined as important evidence supported by at least one RCT or a meta-analysis) and using topical mast cell stabilizers if the condition is recurrent.3
1. Owen CG, Shah A, Henshaw K, et al. Topical treatments for seasonal allergic conjunctivitis: systematic review and meta-analysis of efficacy and effectiveness. Br J Gen Pract. 2004;54:451-456.
2. Swamy BN, Chilov M, McClellan K, et al. Topical non-steroidal anti-inflammatory drugs in allergic conjunctivitis: meta-analysis of randomized trial data. Ophthalmic Epidemiol. 2007;14:311–319.
3. American Academy of Ophthalmology. Conjunctivitis Summary Benchmarks for Preferred Practice Pattern Guidelines. American Academy of Ophthalmology Web site. Available at: http://one.aao.org/summary-benchmark-detail/conjunctivitis-summary-benchmark--october-2012. Accessed October 18, 2013.
Topical antihistamines and topical mast cell stabilizers appear to reduce conjunctival injection and itching effectively. Topical nonsteroidal anti-inflammatory drugs (NSAIDs) are also effective, but may sting on application (strength of recommendation: B, meta-analysis of randomized controlled trials [RCTs]).
Both of these treatments relieve redness and itching
A 2004 systematic review of 40 RCTs (total N not provided) assessed the efficacy of topical treatment with mast cell stabilizers and antihistamines, comparing each with the other and placebo.1 Eleven trials that included 899 children and adults compared mast cell stabilizers (sodium cromoglycate, nedocromil, and lodoxamide tromethamine) with placebo. Follow-up periods ranged from 4 to 9 weeks.
Because of study heterogeneity, a random-effects model was used and showed that topical mast cell stabilizers relieved symptoms (ocular itching, burning, and lacrimation) 4.9 times more effectively than placebo (95% confidence interval [CI], 2.5-9.6). Possible publication bias was cited as a limitation.
In the same systematic review, 9 RCTs with 313 patients compared topical antihistamines (levocabastine, azelastine hydrochloride, emedastine, and antazoline phosphate) with placebo. Signs and symptoms (itching, redness, burning, and swelling) were graded using symptom severity scales. Follow-up ranged from 30 minutes to 24 hours. A meta-analysis wasn’t possible because most studies didn’t tabulate the mean scores and error associated with these scores. Most individual studies, however, showed improvement in the cardinal symptom of itchiness.
Finally, 8 RCTs compared topical mast cell stabilizers (sodium cromoglycate, lodoxamide, and nedocromil sodium) with levocabastine, a topical antihistamine. Two RCTs with 74 patients had follow-up periods of 15 minutes to 4 hours; the remaining 6 RCTs with 473 patients had follow-up periods of 14 days to 4 months. Subjective scoring of symptoms was done in 7 of the 8 studies.
Scores between treatment groups were reported as not statistically significant in the 6 longer-term studies. Meta-analysis wasn’t possible because most studies didn’t tabulate the mean scores and error associated with measures. The 2 short-term studies reported a statistically significant reduction in itching and redness (P<.05) in patients treated with the antihistamine (data not provided).
NSAIDs relieve itching but may sting when applied
A 2007 meta-analysis of 8 RCTs compared topical NSAIDs (ketorolac, diclofenac, aspirin, or steroid) with placebo for treating isolated allergic conjunctivitis in 712 children and adults.2 Primary outcomes were measured as subjective reductions in conjunctival injection and itching measured at 2 to 6 weeks using a 0-to-3 severity scale.
Topical NSAIDs produced significantly greater relief of conjunctival itching (4 trials, N=231; mean difference [MD]=-0.54; 95% CI, -0.84 to -0.24) and conjunctival injection (4 trials, N=208; MD=-0.51; 95% CI, -0.97 to -0.05). NSAIDs weren’t superior to placebo in treating other ocular symptoms of eyelid swelling, ocular burning, photophobia, or foreign body sensation, and they had a higher rate of stinging on application (odds ratio=4.0; 95% CI, 2.7-5.9).
Guideline recommends topical antihistamines or mast cell stabilizers
The American Academy of Ophthalmology’s 2012 evidence-based guideline recommends treating allergic conjunctivitis with topical antihistamines (Level A-1 evidence, defined as important evidence supported by at least one RCT or a meta-analysis) and using topical mast cell stabilizers if the condition is recurrent.3
Topical antihistamines and topical mast cell stabilizers appear to reduce conjunctival injection and itching effectively. Topical nonsteroidal anti-inflammatory drugs (NSAIDs) are also effective, but may sting on application (strength of recommendation: B, meta-analysis of randomized controlled trials [RCTs]).
Both of these treatments relieve redness and itching
A 2004 systematic review of 40 RCTs (total N not provided) assessed the efficacy of topical treatment with mast cell stabilizers and antihistamines, comparing each with the other and placebo.1 Eleven trials that included 899 children and adults compared mast cell stabilizers (sodium cromoglycate, nedocromil, and lodoxamide tromethamine) with placebo. Follow-up periods ranged from 4 to 9 weeks.
Because of study heterogeneity, a random-effects model was used and showed that topical mast cell stabilizers relieved symptoms (ocular itching, burning, and lacrimation) 4.9 times more effectively than placebo (95% confidence interval [CI], 2.5-9.6). Possible publication bias was cited as a limitation.
In the same systematic review, 9 RCTs with 313 patients compared topical antihistamines (levocabastine, azelastine hydrochloride, emedastine, and antazoline phosphate) with placebo. Signs and symptoms (itching, redness, burning, and swelling) were graded using symptom severity scales. Follow-up ranged from 30 minutes to 24 hours. A meta-analysis wasn’t possible because most studies didn’t tabulate the mean scores and error associated with these scores. Most individual studies, however, showed improvement in the cardinal symptom of itchiness.
Finally, 8 RCTs compared topical mast cell stabilizers (sodium cromoglycate, lodoxamide, and nedocromil sodium) with levocabastine, a topical antihistamine. Two RCTs with 74 patients had follow-up periods of 15 minutes to 4 hours; the remaining 6 RCTs with 473 patients had follow-up periods of 14 days to 4 months. Subjective scoring of symptoms was done in 7 of the 8 studies.
Scores between treatment groups were reported as not statistically significant in the 6 longer-term studies. Meta-analysis wasn’t possible because most studies didn’t tabulate the mean scores and error associated with measures. The 2 short-term studies reported a statistically significant reduction in itching and redness (P<.05) in patients treated with the antihistamine (data not provided).
NSAIDs relieve itching but may sting when applied
A 2007 meta-analysis of 8 RCTs compared topical NSAIDs (ketorolac, diclofenac, aspirin, or steroid) with placebo for treating isolated allergic conjunctivitis in 712 children and adults.2 Primary outcomes were measured as subjective reductions in conjunctival injection and itching measured at 2 to 6 weeks using a 0-to-3 severity scale.
Topical NSAIDs produced significantly greater relief of conjunctival itching (4 trials, N=231; mean difference [MD]=-0.54; 95% CI, -0.84 to -0.24) and conjunctival injection (4 trials, N=208; MD=-0.51; 95% CI, -0.97 to -0.05). NSAIDs weren’t superior to placebo in treating other ocular symptoms of eyelid swelling, ocular burning, photophobia, or foreign body sensation, and they had a higher rate of stinging on application (odds ratio=4.0; 95% CI, 2.7-5.9).
Guideline recommends topical antihistamines or mast cell stabilizers
The American Academy of Ophthalmology’s 2012 evidence-based guideline recommends treating allergic conjunctivitis with topical antihistamines (Level A-1 evidence, defined as important evidence supported by at least one RCT or a meta-analysis) and using topical mast cell stabilizers if the condition is recurrent.3
1. Owen CG, Shah A, Henshaw K, et al. Topical treatments for seasonal allergic conjunctivitis: systematic review and meta-analysis of efficacy and effectiveness. Br J Gen Pract. 2004;54:451-456.
2. Swamy BN, Chilov M, McClellan K, et al. Topical non-steroidal anti-inflammatory drugs in allergic conjunctivitis: meta-analysis of randomized trial data. Ophthalmic Epidemiol. 2007;14:311–319.
3. American Academy of Ophthalmology. Conjunctivitis Summary Benchmarks for Preferred Practice Pattern Guidelines. American Academy of Ophthalmology Web site. Available at: http://one.aao.org/summary-benchmark-detail/conjunctivitis-summary-benchmark--october-2012. Accessed October 18, 2013.
1. Owen CG, Shah A, Henshaw K, et al. Topical treatments for seasonal allergic conjunctivitis: systematic review and meta-analysis of efficacy and effectiveness. Br J Gen Pract. 2004;54:451-456.
2. Swamy BN, Chilov M, McClellan K, et al. Topical non-steroidal anti-inflammatory drugs in allergic conjunctivitis: meta-analysis of randomized trial data. Ophthalmic Epidemiol. 2007;14:311–319.
3. American Academy of Ophthalmology. Conjunctivitis Summary Benchmarks for Preferred Practice Pattern Guidelines. American Academy of Ophthalmology Web site. Available at: http://one.aao.org/summary-benchmark-detail/conjunctivitis-summary-benchmark--october-2012. Accessed October 18, 2013.
Evidence-based answers from the Family Physicians Inquiries Network
Consider These Medications to Help Patients Stay Sober
PRACTICE CHANGER
Consider prescribing oral naltrexone (50 mg/d) for patients with alcohol use disorder who wish to maintain abstinence after a brief period of detoxification.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of 95 randomized controlled trials.1
ILLUSTRATIVE CASE
Your patient, a 42-year-old man with alcohol use disorder (AUD), detoxifies from alcohol during a recent hospitalization. He doesn’t want to resume drinking but reports frequent cravings. Are there any medications you can prescribe to help prevent relapse?
Excessive alcohol consumption is responsible for one of every 10 deaths among US adults ages 20 to 64.2 About 20% to 36% of patients seen in a primary care office have AUD.3 Up to 70% of people who quit with psychosocial support alone will relapse.3
The US Preventive Services Task Force gives a grade B recommendation to screening all adults for AUD, indicating that clinicians should provide this service.4 For patients with AUD who wish to abstain but struggle with cravings and relapse, the National Institute on Alcohol Abuse and Alcoholism (NIAAA) recommends considering medication as an adjunct to brief behavioral counseling.5
Continue for study summary >>
STUDY SUMMARY
Evidence shows naltrexone can prevent a return to drinking
In a meta-analysis, Jonas et al1 reviewed 123 studies (N = 22,803) of pharmacotherapy for AUD. After excluding 28 studies (seven were the only study of a given drug, one was a prospective cohort, and 20 had insufficient data), 95 randomized controlled trials were included in the analysis. Twenty-two were placebo-controlled for acamprosate (1,000 to 3,000 mg/d), 44 for naltrexone (50 mg/d oral, 100 mg/d oral, or injectable) and four compared the two drugs. Additional studies evaluated disulfiram as well as 23 other off-label medications, such as valproic acid and topiramate.
Two investigators independently reviewed the studies, checking for completeness and accuracy. Studies were also analyzed for bias using predefined criteria; those with high or unclear risk for bias were excluded from the main analysis but included in the sensitivity analysis. Funnel plots showed no evidence of publication bias.
Participants were primarily recruited as inpatients, and in most studies the mean age was in the 40s. Most patients were diagnosed with alcohol dependence based on criteria in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM-IV-TR); this diagnosis translates to likely moderate to severe AUD in DSM-5. Prior to starting medications, participants underwent detoxification or achieved at least three days of sobriety. Most studies included psychosocial intervention in addition to medication, but the types of intervention varied. The duration of the trials ranged from 12 to 52 weeks.
Researchers analyzed five drinking outcomes—return to any drinking, return to heavy drinking (defined as ≥ 4 drinks/d for women and ≥ 5 drinks/d for men), number of drinking days, number of heavy drinking days, and drinks per drinking day. They also evaluated health outcomes (accidents, injuries, quality of life, function, and mortality) and adverse effects.
Acamprosate and oral naltrexone (50 mg/d) significantly decreased return to any drinking, with a number needed to treat (NNT) of 12 for acamprosate and 20 for naltrexone. Oral naltrexone (50 mg/d) also decreased return to heavy drinking (NNT, 12), while acamprosate did not. Neither medication showed a decrease in heavy drinking days.
In a post hoc subgroup analysis of acamprosate for return to any drinking, the drug appeared to be more effective in studies with a higher risk for bias and less effective in studies with a lower risk for bias. The two studies with the lowest risk for bias found no significant effect.
Disulfiram had no effect on any of the outcomes analyzed.
Of the off-label medications, topiramate showed a decrease in drinking days (weighted mean difference [WMD], –6.5%), heavy drinking days (WMD, –9.0%), and drinks per drinking day (WMD, –1.0).
There were no significant differences in health outcomes for any of the medications. Adverse events were greater in treatment groups than placebo groups. Acamprosate was associated with increased risk for diarrhea (number needed to harm [NNH], 11), vomiting (NNH, 42), and anxiety (NNH, 7). Naltrexone was associated with increased risk for nausea (NNH, 9), vomiting (NNH, 24), and dizziness (NNH, 16).
WHAT’S NEW
Consider prescribing naltrexone to prevent relapse
While previous studies suggested that pharmacotherapy could help patients with AUD remain abstinent, this methodologically rigorous meta-analysis compared the efficacy of several commonly used medications and found clear evidence favoring oral naltrexone. Prescribe oral naltrexone (50 mg/d) to help patients with moderate to severe AUD avoid returning to any drinking or heavy drinking after alcohol detoxification. Acamprosate may also decrease return to drinking, although the evidence is not as strong (the studies with low bias showed no effect).
Next page: Caveats >>
CAVEATS
Medication should be used with psychosocial treatments
Pharmacotherapy for AUD should be reserved for patients who want to quit drinking and should be used in conjunction with psychosocial intervention.3 Only one of the studies analyzed by Jonas et al1 was conducted in primary care. That said, many of the psychosocial interventions—such as regular follow-up visits to encourage adherence and monitor for adverse effects, in conjunction with attendance at Alcoholics Anonymous meetings—could be done in primary care settings.
Comorbidities may limit therapy options. Naltrexone is contraindicated in acute hepatitis and liver failure and in combination with opioids.5 Acamprosate is contraindicated in renal disease.5
CHALLENGES TO IMPLEMENTATION
Cost, adherence may be factors for some patients
Perhaps the greatest hurdle in pharmacotherapy for AUD in primary care is a lack of familiarity with these medications. For clinicians who are comfortable with prescribing these medications, implementation may be hindered by a lack of available psychosocial resources for successful abstinence.
Additionally, the medications are expensive. The branded version of naltrexone (50 mg) costs approximately $118 for a 30-day supply,6 and the branded version of acamprosate costs approximately $284 for a 30-day supply.7
As is the case with any chronic medical condition, medication adherence is a challenge. Naltrexone is taken once daily, while acamprosate is taken three times a day. The risk for relapse is high until six to 12 months of sobriety is achieved and then wanes over several years.5 The NIAAA recommends treatment for a minimum of three months.5
REFERENCES
1. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311:1889-1900.
2. CDC. Fact sheets - Alcohol use and your health. www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm. Accessed April 13, 2015.
3. Johnson BA. Pharmacotherapy for alcohol use disorder. UpToDate. www.uptodate.com/contents/pharmacotherapy-for-alcohol-use-disorder. Accessed April 13, 2015.
4. US Preventive Services Task Force. Final recommendation statement: Alcohol misuse: Screening and behavioral counseling interventions in primary care. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/alcohol-misuse-screening-and-behavioral-counseling-interventions-in-primary-care. Accessed April 13, 2015.
5. US Department of Health and Human Services; National Institutes of Health; National Institute on Alcohol Abuse and Alcoholism. Excerpt from Helping Patients Who Drink Too Much: A Clinician’s Guide. http://pubs.niaaa.nih.gov/publications/Practitioner/Clinicians Guide2005/PrescribingMeds.pdf. Accessed April 13, 2015.
6. Drugs.com. Revia prices, coupons and patient assistance programs. www.drugs.com/price-guide/revia. Accessed April 13, 2015.
7. Drugs.com. Campral prices, coupons and patient assistance programs. www.drugs.com/price-guide/campral. Accessed April 13, 2015.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(4):238-240.
PRACTICE CHANGER
Consider prescribing oral naltrexone (50 mg/d) for patients with alcohol use disorder who wish to maintain abstinence after a brief period of detoxification.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of 95 randomized controlled trials.1
ILLUSTRATIVE CASE
Your patient, a 42-year-old man with alcohol use disorder (AUD), detoxifies from alcohol during a recent hospitalization. He doesn’t want to resume drinking but reports frequent cravings. Are there any medications you can prescribe to help prevent relapse?
Excessive alcohol consumption is responsible for one of every 10 deaths among US adults ages 20 to 64.2 About 20% to 36% of patients seen in a primary care office have AUD.3 Up to 70% of people who quit with psychosocial support alone will relapse.3
The US Preventive Services Task Force gives a grade B recommendation to screening all adults for AUD, indicating that clinicians should provide this service.4 For patients with AUD who wish to abstain but struggle with cravings and relapse, the National Institute on Alcohol Abuse and Alcoholism (NIAAA) recommends considering medication as an adjunct to brief behavioral counseling.5
Continue for study summary >>
STUDY SUMMARY
Evidence shows naltrexone can prevent a return to drinking
In a meta-analysis, Jonas et al1 reviewed 123 studies (N = 22,803) of pharmacotherapy for AUD. After excluding 28 studies (seven were the only study of a given drug, one was a prospective cohort, and 20 had insufficient data), 95 randomized controlled trials were included in the analysis. Twenty-two were placebo-controlled for acamprosate (1,000 to 3,000 mg/d), 44 for naltrexone (50 mg/d oral, 100 mg/d oral, or injectable) and four compared the two drugs. Additional studies evaluated disulfiram as well as 23 other off-label medications, such as valproic acid and topiramate.
Two investigators independently reviewed the studies, checking for completeness and accuracy. Studies were also analyzed for bias using predefined criteria; those with high or unclear risk for bias were excluded from the main analysis but included in the sensitivity analysis. Funnel plots showed no evidence of publication bias.
Participants were primarily recruited as inpatients, and in most studies the mean age was in the 40s. Most patients were diagnosed with alcohol dependence based on criteria in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM-IV-TR); this diagnosis translates to likely moderate to severe AUD in DSM-5. Prior to starting medications, participants underwent detoxification or achieved at least three days of sobriety. Most studies included psychosocial intervention in addition to medication, but the types of intervention varied. The duration of the trials ranged from 12 to 52 weeks.
Researchers analyzed five drinking outcomes—return to any drinking, return to heavy drinking (defined as ≥ 4 drinks/d for women and ≥ 5 drinks/d for men), number of drinking days, number of heavy drinking days, and drinks per drinking day. They also evaluated health outcomes (accidents, injuries, quality of life, function, and mortality) and adverse effects.
Acamprosate and oral naltrexone (50 mg/d) significantly decreased return to any drinking, with a number needed to treat (NNT) of 12 for acamprosate and 20 for naltrexone. Oral naltrexone (50 mg/d) also decreased return to heavy drinking (NNT, 12), while acamprosate did not. Neither medication showed a decrease in heavy drinking days.
In a post hoc subgroup analysis of acamprosate for return to any drinking, the drug appeared to be more effective in studies with a higher risk for bias and less effective in studies with a lower risk for bias. The two studies with the lowest risk for bias found no significant effect.
Disulfiram had no effect on any of the outcomes analyzed.
Of the off-label medications, topiramate showed a decrease in drinking days (weighted mean difference [WMD], –6.5%), heavy drinking days (WMD, –9.0%), and drinks per drinking day (WMD, –1.0).
There were no significant differences in health outcomes for any of the medications. Adverse events were greater in treatment groups than placebo groups. Acamprosate was associated with increased risk for diarrhea (number needed to harm [NNH], 11), vomiting (NNH, 42), and anxiety (NNH, 7). Naltrexone was associated with increased risk for nausea (NNH, 9), vomiting (NNH, 24), and dizziness (NNH, 16).
WHAT’S NEW
Consider prescribing naltrexone to prevent relapse
While previous studies suggested that pharmacotherapy could help patients with AUD remain abstinent, this methodologically rigorous meta-analysis compared the efficacy of several commonly used medications and found clear evidence favoring oral naltrexone. Prescribe oral naltrexone (50 mg/d) to help patients with moderate to severe AUD avoid returning to any drinking or heavy drinking after alcohol detoxification. Acamprosate may also decrease return to drinking, although the evidence is not as strong (the studies with low bias showed no effect).
Next page: Caveats >>
CAVEATS
Medication should be used with psychosocial treatments
Pharmacotherapy for AUD should be reserved for patients who want to quit drinking and should be used in conjunction with psychosocial intervention.3 Only one of the studies analyzed by Jonas et al1 was conducted in primary care. That said, many of the psychosocial interventions—such as regular follow-up visits to encourage adherence and monitor for adverse effects, in conjunction with attendance at Alcoholics Anonymous meetings—could be done in primary care settings.
Comorbidities may limit therapy options. Naltrexone is contraindicated in acute hepatitis and liver failure and in combination with opioids.5 Acamprosate is contraindicated in renal disease.5
CHALLENGES TO IMPLEMENTATION
Cost, adherence may be factors for some patients
Perhaps the greatest hurdle in pharmacotherapy for AUD in primary care is a lack of familiarity with these medications. For clinicians who are comfortable with prescribing these medications, implementation may be hindered by a lack of available psychosocial resources for successful abstinence.
Additionally, the medications are expensive. The branded version of naltrexone (50 mg) costs approximately $118 for a 30-day supply,6 and the branded version of acamprosate costs approximately $284 for a 30-day supply.7
As is the case with any chronic medical condition, medication adherence is a challenge. Naltrexone is taken once daily, while acamprosate is taken three times a day. The risk for relapse is high until six to 12 months of sobriety is achieved and then wanes over several years.5 The NIAAA recommends treatment for a minimum of three months.5
REFERENCES
1. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311:1889-1900.
2. CDC. Fact sheets - Alcohol use and your health. www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm. Accessed April 13, 2015.
3. Johnson BA. Pharmacotherapy for alcohol use disorder. UpToDate. www.uptodate.com/contents/pharmacotherapy-for-alcohol-use-disorder. Accessed April 13, 2015.
4. US Preventive Services Task Force. Final recommendation statement: Alcohol misuse: Screening and behavioral counseling interventions in primary care. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/alcohol-misuse-screening-and-behavioral-counseling-interventions-in-primary-care. Accessed April 13, 2015.
5. US Department of Health and Human Services; National Institutes of Health; National Institute on Alcohol Abuse and Alcoholism. Excerpt from Helping Patients Who Drink Too Much: A Clinician’s Guide. http://pubs.niaaa.nih.gov/publications/Practitioner/Clinicians Guide2005/PrescribingMeds.pdf. Accessed April 13, 2015.
6. Drugs.com. Revia prices, coupons and patient assistance programs. www.drugs.com/price-guide/revia. Accessed April 13, 2015.
7. Drugs.com. Campral prices, coupons and patient assistance programs. www.drugs.com/price-guide/campral. Accessed April 13, 2015.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(4):238-240.
PRACTICE CHANGER
Consider prescribing oral naltrexone (50 mg/d) for patients with alcohol use disorder who wish to maintain abstinence after a brief period of detoxification.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of 95 randomized controlled trials.1
ILLUSTRATIVE CASE
Your patient, a 42-year-old man with alcohol use disorder (AUD), detoxifies from alcohol during a recent hospitalization. He doesn’t want to resume drinking but reports frequent cravings. Are there any medications you can prescribe to help prevent relapse?
Excessive alcohol consumption is responsible for one of every 10 deaths among US adults ages 20 to 64.2 About 20% to 36% of patients seen in a primary care office have AUD.3 Up to 70% of people who quit with psychosocial support alone will relapse.3
The US Preventive Services Task Force gives a grade B recommendation to screening all adults for AUD, indicating that clinicians should provide this service.4 For patients with AUD who wish to abstain but struggle with cravings and relapse, the National Institute on Alcohol Abuse and Alcoholism (NIAAA) recommends considering medication as an adjunct to brief behavioral counseling.5
Continue for study summary >>
STUDY SUMMARY
Evidence shows naltrexone can prevent a return to drinking
In a meta-analysis, Jonas et al1 reviewed 123 studies (N = 22,803) of pharmacotherapy for AUD. After excluding 28 studies (seven were the only study of a given drug, one was a prospective cohort, and 20 had insufficient data), 95 randomized controlled trials were included in the analysis. Twenty-two were placebo-controlled for acamprosate (1,000 to 3,000 mg/d), 44 for naltrexone (50 mg/d oral, 100 mg/d oral, or injectable) and four compared the two drugs. Additional studies evaluated disulfiram as well as 23 other off-label medications, such as valproic acid and topiramate.
Two investigators independently reviewed the studies, checking for completeness and accuracy. Studies were also analyzed for bias using predefined criteria; those with high or unclear risk for bias were excluded from the main analysis but included in the sensitivity analysis. Funnel plots showed no evidence of publication bias.
Participants were primarily recruited as inpatients, and in most studies the mean age was in the 40s. Most patients were diagnosed with alcohol dependence based on criteria in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM-IV-TR); this diagnosis translates to likely moderate to severe AUD in DSM-5. Prior to starting medications, participants underwent detoxification or achieved at least three days of sobriety. Most studies included psychosocial intervention in addition to medication, but the types of intervention varied. The duration of the trials ranged from 12 to 52 weeks.
Researchers analyzed five drinking outcomes—return to any drinking, return to heavy drinking (defined as ≥ 4 drinks/d for women and ≥ 5 drinks/d for men), number of drinking days, number of heavy drinking days, and drinks per drinking day. They also evaluated health outcomes (accidents, injuries, quality of life, function, and mortality) and adverse effects.
Acamprosate and oral naltrexone (50 mg/d) significantly decreased return to any drinking, with a number needed to treat (NNT) of 12 for acamprosate and 20 for naltrexone. Oral naltrexone (50 mg/d) also decreased return to heavy drinking (NNT, 12), while acamprosate did not. Neither medication showed a decrease in heavy drinking days.
In a post hoc subgroup analysis of acamprosate for return to any drinking, the drug appeared to be more effective in studies with a higher risk for bias and less effective in studies with a lower risk for bias. The two studies with the lowest risk for bias found no significant effect.
Disulfiram had no effect on any of the outcomes analyzed.
Of the off-label medications, topiramate showed a decrease in drinking days (weighted mean difference [WMD], –6.5%), heavy drinking days (WMD, –9.0%), and drinks per drinking day (WMD, –1.0).
There were no significant differences in health outcomes for any of the medications. Adverse events were greater in treatment groups than placebo groups. Acamprosate was associated with increased risk for diarrhea (number needed to harm [NNH], 11), vomiting (NNH, 42), and anxiety (NNH, 7). Naltrexone was associated with increased risk for nausea (NNH, 9), vomiting (NNH, 24), and dizziness (NNH, 16).
WHAT’S NEW
Consider prescribing naltrexone to prevent relapse
While previous studies suggested that pharmacotherapy could help patients with AUD remain abstinent, this methodologically rigorous meta-analysis compared the efficacy of several commonly used medications and found clear evidence favoring oral naltrexone. Prescribe oral naltrexone (50 mg/d) to help patients with moderate to severe AUD avoid returning to any drinking or heavy drinking after alcohol detoxification. Acamprosate may also decrease return to drinking, although the evidence is not as strong (the studies with low bias showed no effect).
Next page: Caveats >>
CAVEATS
Medication should be used with psychosocial treatments
Pharmacotherapy for AUD should be reserved for patients who want to quit drinking and should be used in conjunction with psychosocial intervention.3 Only one of the studies analyzed by Jonas et al1 was conducted in primary care. That said, many of the psychosocial interventions—such as regular follow-up visits to encourage adherence and monitor for adverse effects, in conjunction with attendance at Alcoholics Anonymous meetings—could be done in primary care settings.
Comorbidities may limit therapy options. Naltrexone is contraindicated in acute hepatitis and liver failure and in combination with opioids.5 Acamprosate is contraindicated in renal disease.5
CHALLENGES TO IMPLEMENTATION
Cost, adherence may be factors for some patients
Perhaps the greatest hurdle in pharmacotherapy for AUD in primary care is a lack of familiarity with these medications. For clinicians who are comfortable with prescribing these medications, implementation may be hindered by a lack of available psychosocial resources for successful abstinence.
Additionally, the medications are expensive. The branded version of naltrexone (50 mg) costs approximately $118 for a 30-day supply,6 and the branded version of acamprosate costs approximately $284 for a 30-day supply.7
As is the case with any chronic medical condition, medication adherence is a challenge. Naltrexone is taken once daily, while acamprosate is taken three times a day. The risk for relapse is high until six to 12 months of sobriety is achieved and then wanes over several years.5 The NIAAA recommends treatment for a minimum of three months.5
REFERENCES
1. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311:1889-1900.
2. CDC. Fact sheets - Alcohol use and your health. www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm. Accessed April 13, 2015.
3. Johnson BA. Pharmacotherapy for alcohol use disorder. UpToDate. www.uptodate.com/contents/pharmacotherapy-for-alcohol-use-disorder. Accessed April 13, 2015.
4. US Preventive Services Task Force. Final recommendation statement: Alcohol misuse: Screening and behavioral counseling interventions in primary care. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/alcohol-misuse-screening-and-behavioral-counseling-interventions-in-primary-care. Accessed April 13, 2015.
5. US Department of Health and Human Services; National Institutes of Health; National Institute on Alcohol Abuse and Alcoholism. Excerpt from Helping Patients Who Drink Too Much: A Clinician’s Guide. http://pubs.niaaa.nih.gov/publications/Practitioner/Clinicians Guide2005/PrescribingMeds.pdf. Accessed April 13, 2015.
6. Drugs.com. Revia prices, coupons and patient assistance programs. www.drugs.com/price-guide/revia. Accessed April 13, 2015.
7. Drugs.com. Campral prices, coupons and patient assistance programs. www.drugs.com/price-guide/campral. Accessed April 13, 2015.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(4):238-240.
Is nonoperative therapy as effective as surgery for meniscal injuries?
Yes. There is no significant difference in symptom or functional improvement between adult patients with symptomatic meniscal injury who are treated with operative vs nonoperative therapy (strength of recommendation: A, consistent randomized controlled trials [RCTs]).
Both approaches resulted in function and pain improvement
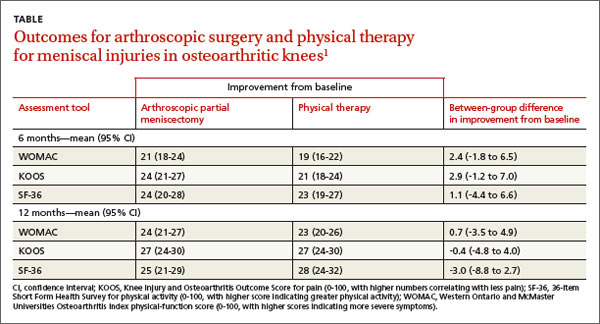
A 2013 multicenter RCT evaluated 351 adults, 45 years and older, with a meniscal tear and mild to moderate osteoarthritis confirmed by imaging, for functional improvement by physical therapy alone compared with arthroscopic partial meniscectomy and physical therapy.1
At the beginning of the study and 6 and 12 months after treatment, researchers assessed symptoms using the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index physical-function score (0-100, with higher scores indicating more severe symptoms), the Knee Injury and Osteoarthritis Outcome Score (KOOS) for pain (0-100, with higher numbers correlating with less pain), and the 36-item Short Form Health Survey (SF-36) for physical activity (0-100, with higher scores indicating greater physical activity).
Modified intention to treat analysis showed no significant difference in function and pain improvement at 6 and 12 months between patients with meniscal injury who underwent arthroscopic repair and physical therapy and patients who underwent physical therapy alone (TABLE1). A limitation of the study was the crossover of 30% of patients from the nonoperative group to the operative group.
No differences found in Tx outcomes for nontraumatic tears
A 2007 prospective RCT evaluated 90 adults ages 45 to 64 with nontraumatic meniscal tears confirmed by magnetic resonance imaging for improvement in knee pain and function with arthroscopic treatment and supervised exercise (AE) or supervised exercise (E) alone.2 Knee pain and function were assessed before intervention, after 8 weeks, and after 6 months of treatment using 3 surveys: the KOOS, the Lysholm Knee Scoring Scale (LKSS; 0-100, with higher scores correlating with good knee function), and the Visual Analogue Scale (VAS) for knee pain (0-10, with 0 indicating no pain and 10 indicating maximum pain).
The KOOS revealed that at 8 weeks and 6 months both groups had significant improvement from the initial evaluation in all subscale scores. In the AE group, the 8-week pain score increased from a baseline of 56 to 89 (P<.001) and remained at 89 at 6 months (P<.001). For the E group, the 8-week pain score improved from a baseline of 62 to 86 (P<.001) and continued at 86 after 6 months (P<.001).
The LKSS score for both groups showed significant improvement from baseline at 8 weeks: 34% of the AE group and 42% of the E group scored higher than 91 (P<.001).
VAS scores showed a significant decrease in pain at 8 weeks for both the AE and E groups: beginning median value for both groups was 5.5 and decreased to 1.0 at 8 weeks and 6 months (P<.001).
The authors concluded that both groups improved significantly from initial evaluation regardless of treatment method and that no statistically significant difference existed between treatment results.
1. Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368:1675-1684.
2. Herrlin S, Hallander M, Wange P, et al. Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomised trial. Knee Surg Sports Traumatol Arthrosc. 2007;15:393-401.
Yes. There is no significant difference in symptom or functional improvement between adult patients with symptomatic meniscal injury who are treated with operative vs nonoperative therapy (strength of recommendation: A, consistent randomized controlled trials [RCTs]).
Both approaches resulted in function and pain improvement
A 2013 multicenter RCT evaluated 351 adults, 45 years and older, with a meniscal tear and mild to moderate osteoarthritis confirmed by imaging, for functional improvement by physical therapy alone compared with arthroscopic partial meniscectomy and physical therapy.1
At the beginning of the study and 6 and 12 months after treatment, researchers assessed symptoms using the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index physical-function score (0-100, with higher scores indicating more severe symptoms), the Knee Injury and Osteoarthritis Outcome Score (KOOS) for pain (0-100, with higher numbers correlating with less pain), and the 36-item Short Form Health Survey (SF-36) for physical activity (0-100, with higher scores indicating greater physical activity).
Modified intention to treat analysis showed no significant difference in function and pain improvement at 6 and 12 months between patients with meniscal injury who underwent arthroscopic repair and physical therapy and patients who underwent physical therapy alone (TABLE1). A limitation of the study was the crossover of 30% of patients from the nonoperative group to the operative group.
No differences found in Tx outcomes for nontraumatic tears
A 2007 prospective RCT evaluated 90 adults ages 45 to 64 with nontraumatic meniscal tears confirmed by magnetic resonance imaging for improvement in knee pain and function with arthroscopic treatment and supervised exercise (AE) or supervised exercise (E) alone.2 Knee pain and function were assessed before intervention, after 8 weeks, and after 6 months of treatment using 3 surveys: the KOOS, the Lysholm Knee Scoring Scale (LKSS; 0-100, with higher scores correlating with good knee function), and the Visual Analogue Scale (VAS) for knee pain (0-10, with 0 indicating no pain and 10 indicating maximum pain).

The KOOS revealed that at 8 weeks and 6 months both groups had significant improvement from the initial evaluation in all subscale scores. In the AE group, the 8-week pain score increased from a baseline of 56 to 89 (P<.001) and remained at 89 at 6 months (P<.001). For the E group, the 8-week pain score improved from a baseline of 62 to 86 (P<.001) and continued at 86 after 6 months (P<.001).
The LKSS score for both groups showed significant improvement from baseline at 8 weeks: 34% of the AE group and 42% of the E group scored higher than 91 (P<.001).
VAS scores showed a significant decrease in pain at 8 weeks for both the AE and E groups: beginning median value for both groups was 5.5 and decreased to 1.0 at 8 weeks and 6 months (P<.001).
The authors concluded that both groups improved significantly from initial evaluation regardless of treatment method and that no statistically significant difference existed between treatment results.
Yes. There is no significant difference in symptom or functional improvement between adult patients with symptomatic meniscal injury who are treated with operative vs nonoperative therapy (strength of recommendation: A, consistent randomized controlled trials [RCTs]).
Both approaches resulted in function and pain improvement
A 2013 multicenter RCT evaluated 351 adults, 45 years and older, with a meniscal tear and mild to moderate osteoarthritis confirmed by imaging, for functional improvement by physical therapy alone compared with arthroscopic partial meniscectomy and physical therapy.1
At the beginning of the study and 6 and 12 months after treatment, researchers assessed symptoms using the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index physical-function score (0-100, with higher scores indicating more severe symptoms), the Knee Injury and Osteoarthritis Outcome Score (KOOS) for pain (0-100, with higher numbers correlating with less pain), and the 36-item Short Form Health Survey (SF-36) for physical activity (0-100, with higher scores indicating greater physical activity).
Modified intention to treat analysis showed no significant difference in function and pain improvement at 6 and 12 months between patients with meniscal injury who underwent arthroscopic repair and physical therapy and patients who underwent physical therapy alone (TABLE1). A limitation of the study was the crossover of 30% of patients from the nonoperative group to the operative group.
No differences found in Tx outcomes for nontraumatic tears
A 2007 prospective RCT evaluated 90 adults ages 45 to 64 with nontraumatic meniscal tears confirmed by magnetic resonance imaging for improvement in knee pain and function with arthroscopic treatment and supervised exercise (AE) or supervised exercise (E) alone.2 Knee pain and function were assessed before intervention, after 8 weeks, and after 6 months of treatment using 3 surveys: the KOOS, the Lysholm Knee Scoring Scale (LKSS; 0-100, with higher scores correlating with good knee function), and the Visual Analogue Scale (VAS) for knee pain (0-10, with 0 indicating no pain and 10 indicating maximum pain).

The KOOS revealed that at 8 weeks and 6 months both groups had significant improvement from the initial evaluation in all subscale scores. In the AE group, the 8-week pain score increased from a baseline of 56 to 89 (P<.001) and remained at 89 at 6 months (P<.001). For the E group, the 8-week pain score improved from a baseline of 62 to 86 (P<.001) and continued at 86 after 6 months (P<.001).
The LKSS score for both groups showed significant improvement from baseline at 8 weeks: 34% of the AE group and 42% of the E group scored higher than 91 (P<.001).
VAS scores showed a significant decrease in pain at 8 weeks for both the AE and E groups: beginning median value for both groups was 5.5 and decreased to 1.0 at 8 weeks and 6 months (P<.001).
The authors concluded that both groups improved significantly from initial evaluation regardless of treatment method and that no statistically significant difference existed between treatment results.
1. Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368:1675-1684.
2. Herrlin S, Hallander M, Wange P, et al. Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomised trial. Knee Surg Sports Traumatol Arthrosc. 2007;15:393-401.
1. Katz JN, Brophy RH, Chaisson CE, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368:1675-1684.
2. Herrlin S, Hallander M, Wange P, et al. Arthroscopic or conservative treatment of degenerative medial meniscal tears: a prospective randomised trial. Knee Surg Sports Traumatol Arthrosc. 2007;15:393-401.
Evidence-based answers from the Family Physicians Inquiries Network
Child With “Distressing” Problem
ANSWER
The correct answer is nevus sebaceous (choice “a”). This benign hamartomatous lesion is derived from local tissue and grows at the same rate.
It differs considerably from the other items in the differential, including aplasia cutis congenita (choice “b”). In this condition, a focal area of epidermis simply fails to develop, leaving a permanent hairless scar that contrasts sharply with the raised, mammillated plaque of nevus sebaceous.
Epidermal nevus (choice “c”) is usually a collection of tan to brown superficial nevoid papules that can be linear, agminated, or plaque-like. These lesions lack the color and mammillated surface of those seen in nevus sebaceous.
Neonatal lupus (choice “d”) can present at birth with hairless, cicatricial inflamed lesions. However, these tend to resolve quickly, often leaving focal scarring alopecia but no plaque formation.
DISCUSSION
Nevus sebaceous (NS), first described by Jadassohn in 1895, has long been recognized as an unusual but by no means rare congenital lesion. Occurring equally in both sexes and comprising sebaceous glands in a nevoid morphologic context, NS is considered a variant of sebaceous nevi and verrucous epidermal nevi in some circles. All three are derived from overgrowth of local, normal tissues that typically grow at the same rate as surrounding structures.
The vast majority of NS lesions are found in the scalp, although they can also develop on the ear or neck and, rarely, elsewhere on the body. This patient’s plaque—with its uniform surface; tiny, smooth, shiny papules; and (perhaps most important) total lack of hair—is typical. Other classic features are congenital onset and permanent nature, which distinguish them from the rest of the differential.
Focal malignant transformation of NS lesions has been reported—in fact, this author has seen two such cases in 30 years. Both were small basal cell carcinomas, although cases of melanoma and other malignancies have been reported.
Such changes are rare enough that most experts consider prophylactic removal to be unwarranted. Watching the lesions for change over the years is certainly reasonable, as is protecting them from sun exposure.
Surgical removal—usually performed by a plastic surgeon—is occasionally necessary for cosmetic reasons. This is particularly so when NS covers a portion of the face, or when the cosmetic implications of having a hairless plaque in the scalp are sufficiently distressing.
This patient and her parents were educated about the nature of the diagnosis and apprised of their options.
Editor's note: For a similar presentation with a very different diagnosis, see the March 2015 DermaDiagnosis case (http://bit.ly/1ye69Ym).
ANSWER
The correct answer is nevus sebaceous (choice “a”). This benign hamartomatous lesion is derived from local tissue and grows at the same rate.
It differs considerably from the other items in the differential, including aplasia cutis congenita (choice “b”). In this condition, a focal area of epidermis simply fails to develop, leaving a permanent hairless scar that contrasts sharply with the raised, mammillated plaque of nevus sebaceous.
Epidermal nevus (choice “c”) is usually a collection of tan to brown superficial nevoid papules that can be linear, agminated, or plaque-like. These lesions lack the color and mammillated surface of those seen in nevus sebaceous.
Neonatal lupus (choice “d”) can present at birth with hairless, cicatricial inflamed lesions. However, these tend to resolve quickly, often leaving focal scarring alopecia but no plaque formation.
DISCUSSION
Nevus sebaceous (NS), first described by Jadassohn in 1895, has long been recognized as an unusual but by no means rare congenital lesion. Occurring equally in both sexes and comprising sebaceous glands in a nevoid morphologic context, NS is considered a variant of sebaceous nevi and verrucous epidermal nevi in some circles. All three are derived from overgrowth of local, normal tissues that typically grow at the same rate as surrounding structures.
The vast majority of NS lesions are found in the scalp, although they can also develop on the ear or neck and, rarely, elsewhere on the body. This patient’s plaque—with its uniform surface; tiny, smooth, shiny papules; and (perhaps most important) total lack of hair—is typical. Other classic features are congenital onset and permanent nature, which distinguish them from the rest of the differential.
Focal malignant transformation of NS lesions has been reported—in fact, this author has seen two such cases in 30 years. Both were small basal cell carcinomas, although cases of melanoma and other malignancies have been reported.
Such changes are rare enough that most experts consider prophylactic removal to be unwarranted. Watching the lesions for change over the years is certainly reasonable, as is protecting them from sun exposure.
Surgical removal—usually performed by a plastic surgeon—is occasionally necessary for cosmetic reasons. This is particularly so when NS covers a portion of the face, or when the cosmetic implications of having a hairless plaque in the scalp are sufficiently distressing.
This patient and her parents were educated about the nature of the diagnosis and apprised of their options.
Editor's note: For a similar presentation with a very different diagnosis, see the March 2015 DermaDiagnosis case (http://bit.ly/1ye69Ym).
ANSWER
The correct answer is nevus sebaceous (choice “a”). This benign hamartomatous lesion is derived from local tissue and grows at the same rate.
It differs considerably from the other items in the differential, including aplasia cutis congenita (choice “b”). In this condition, a focal area of epidermis simply fails to develop, leaving a permanent hairless scar that contrasts sharply with the raised, mammillated plaque of nevus sebaceous.
Epidermal nevus (choice “c”) is usually a collection of tan to brown superficial nevoid papules that can be linear, agminated, or plaque-like. These lesions lack the color and mammillated surface of those seen in nevus sebaceous.
Neonatal lupus (choice “d”) can present at birth with hairless, cicatricial inflamed lesions. However, these tend to resolve quickly, often leaving focal scarring alopecia but no plaque formation.
DISCUSSION
Nevus sebaceous (NS), first described by Jadassohn in 1895, has long been recognized as an unusual but by no means rare congenital lesion. Occurring equally in both sexes and comprising sebaceous glands in a nevoid morphologic context, NS is considered a variant of sebaceous nevi and verrucous epidermal nevi in some circles. All three are derived from overgrowth of local, normal tissues that typically grow at the same rate as surrounding structures.
The vast majority of NS lesions are found in the scalp, although they can also develop on the ear or neck and, rarely, elsewhere on the body. This patient’s plaque—with its uniform surface; tiny, smooth, shiny papules; and (perhaps most important) total lack of hair—is typical. Other classic features are congenital onset and permanent nature, which distinguish them from the rest of the differential.
Focal malignant transformation of NS lesions has been reported—in fact, this author has seen two such cases in 30 years. Both were small basal cell carcinomas, although cases of melanoma and other malignancies have been reported.
Such changes are rare enough that most experts consider prophylactic removal to be unwarranted. Watching the lesions for change over the years is certainly reasonable, as is protecting them from sun exposure.
Surgical removal—usually performed by a plastic surgeon—is occasionally necessary for cosmetic reasons. This is particularly so when NS covers a portion of the face, or when the cosmetic implications of having a hairless plaque in the scalp are sufficiently distressing.
This patient and her parents were educated about the nature of the diagnosis and apprised of their options.
Editor's note: For a similar presentation with a very different diagnosis, see the March 2015 DermaDiagnosis case (http://bit.ly/1ye69Ym).
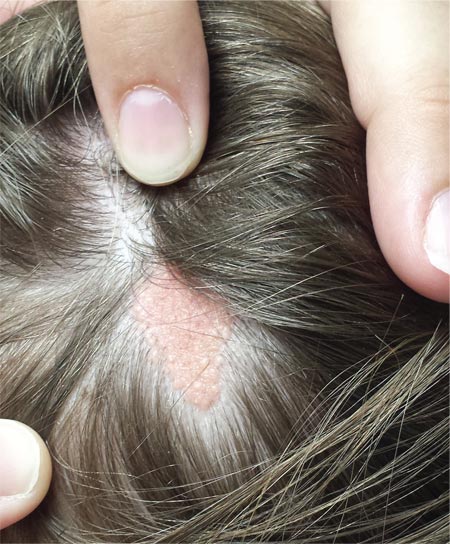
A “bald spot” is the chief complaint of a 12-year-old girl brought for evaluation by her mother. The lesion in her left parietal scalp has been there since birth, slowly growing but producing no symptoms. Although the child’s primary care provider has reassured the family that the “birthmark” is benign, they remain concerned. Furthermore, the patient has become increasingly distressed by the hairlessness. The child is otherwise healthy. There is no history of excessive sun exposure. The lesion is a roughly oval, uniformly pink, hairless 3.6-cm plaque with a faintly mammillated surface and well-defined margins. It is only visible when the surrounding hair is parted sufficiently to reveal it. Examination of the rest of the patient’s skin is unremarkable.
What’s the best test for underlying osteomyelitis in patients with diabetic foot ulcers?
Magnetic resonance imaging (MRI) has a higher sensitivity and specificity (90% and 79%) than plain radiography (54% and 68%) for diagnosing diabetic foot osteomyelitis. MRI performs somewhat better than any of several common tests—probe to bone (PTB), erythrocyte sedimentation rate (ESR) >70 mm/hr, C-reactive protein (CRP) >14 mg/L, procalcitonin >0.3 ng/mL, and ulcer size >2 cm2—although PTB has the highest specificity of any test and is commonly used together with MRI. No studies have directly compared MRI with a combination of these tests, which may assist in diagnosis (strength of recommendation [SOR]: B, meta-analysis of cohort trials and individual cohort and case control trial).
Experts recommend obtaining plain films when considering diabetic foot ulcers to evaluate for bony abnormalities, soft tissue gas, and foreign body; MRI should be considered in most situations when infection is suspected (SOR: B, evidence-based guidelines).
EVIDENCE SUMMARY
One-fifth of patients with diabetes who have foot ulcerations will develop osteomyelitis.1,2 Most cases of diabetic foot osteomyelitis result from the spread of a foot infection to underlying bone.2
MRI has highest sensitivity, probe to bone test is most specific
A meta-analysis3 of 9 cohort trials (8 prospective, 1 retrospective) of 612 patients with diabetes and a foot ulcer examined the accuracy of diagnostic methods for osteomyelitis (TABLE3,4). MRI had the highest sensitivity (90%), followed by bone scan (81%). Bone scan was the least specific (28%), however. Plain film radiography had the lowest sensitivity (54%). A PTB test was highly specific (91%) but had moderate sensitivity (60%). (PTB involves inserting a sterile, blunt stainless steel probe into an ulcerated lesion. If the probe comes to a hard stop, considered to be bone, the test is positive.)
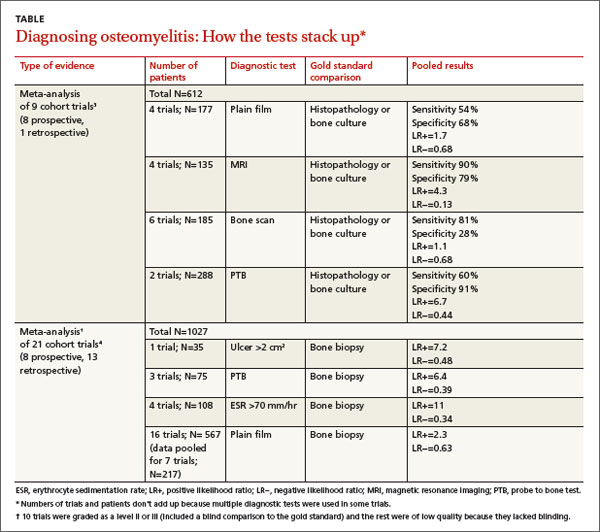
A meta-analysis of 21 prospective and retrospective trials with 1027 diabetic patients with foot ulcers or suspected osteomyelitis found that ulcer size >2 cm2, PTB, and ESR >70 mm/hr were helpful in making the diagnosis.4
Combining ESR with ulcer size increases specificity
A prospective trial of 46 diabetic patients hospitalized with a foot infection examined the accuracy of a combination of clinical and laboratory diagnostic features in patients with diabetic foot osteomyelitis that had been diagnosed by MRI or histopathology.5 (Twenty-four patients had osteomyelitis, and 22 didn’t.)
ESR >70 mm/hr had a sensitivity of 83% and specificity of 77% (positive likelihood ratio [LR+]=3.6; negative likelihood ratio [LR−]=0.22). Ulcer size >2 cm2 had a sensitivity of 88% and specificity of 77% (LR+=3.8; LR−=0.16). Combined, an ESR >70 mm/hr and ulcer size >2cm2 had a slightly better specificity than either finding alone, 82%, but a lower sensitivity of 79% (LR+=4.4; LR−= 0.26).
Serum markers accurately distinguish osteomyelitis from infection
An individual prospective cohort trial of 61 adult patients with diabetes and a foot infection, published after the meta-analysis4 described previously, examined the accuracy of serum markers (ESR, CRP, procalcitonin) for diagnosing osteomyelitis.6 A positive PTB test and imaging study (plain film, MRI, or nuclear scintigraphy) were used as the diagnostic gold standard.
Thirty-four patients had a soft tissue infection and 27 had osteomyelitis. All markers were higher in patients with osteomyelitis than in patients with a soft tissue infection (ESR=76 mm/hr vs 66 mm/hr; P<.001; CRP=25 mg/L vs 8.7 mg/L; P<.001; procalcitonin=2.4 ng/mL vs 0.71 ng/mL; P<.001). The sensitivity and specificity for each marker at its optimum points were: ESR >67 mm/hr (sensitivity 84%; specificity 75%; LR+=3.4; LR−=0.21); CRP >14 mg/L (sensitivity 85%; specificity 83%; LR+=5; LR−=0.18); and procalcitonin >0.3 ng/mL (sensitivity 81%; specificity 71%; LR+=2.8; LR−=0.27).
RECOMMENDATIONS
The Infectious Diseases Society of America (IDSA) recommends performing the PTB test on any diabetic foot infection with an open wound (level of evidence: strong moderate).7 It also recommends performing plain radiography on all patients presenting with a new infection to evaluate for bony abnormalities, soft tissue gas, and foreign bodies (level of evidence: strong moderate).
The IDSA, the American College of Radiology diagnostic imaging expert panel, and the National Institute for Health and Clinical Excellence recommend using MRI in most clinical scenarios when osteomyelitis is suspected (level of evidence: strong moderate).8,9
1. Gemechu FW, Seemant F, Curley CA. Diabetic foot infections. Am Fam Physician. 2013;88:177-184.
2. Lavery LA, Armstrong DG, Peters EJ, et al. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care. 2007;30:270-274.
3. Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis. 2008;47:519-527.
4. Butalia S, Palda VA, Sargeant RJ, et al. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA. 2008;299:806-813.
5. Ertugrul BM, Savk O, Ozturk B, et al. The diagnosis of diabetic foot osteomyelitis: examination findings and laboratory values. Med Sci Monit. 2009;15:CR307-CR312.
6. Michail M, Jude E, Liaskos C, et al. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Low Extrem Wounds. 2013;12:94-99.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173.
8. Schweitzer ME, Daffner RH, Weissman BN, et al. ACR Appropriateness Criteria on suspected osteomyelitis in patients with diabetes mellitus. J Am Coll Radiol. 2008;5:881-886.
9. Tan T, Shaw EJ, Siddiqui F, et al; Guideline Development Group. Inpatient management of diabetic foot problems: summary of NICE guidance. BMJ. 2011;342:d1280.
Magnetic resonance imaging (MRI) has a higher sensitivity and specificity (90% and 79%) than plain radiography (54% and 68%) for diagnosing diabetic foot osteomyelitis. MRI performs somewhat better than any of several common tests—probe to bone (PTB), erythrocyte sedimentation rate (ESR) >70 mm/hr, C-reactive protein (CRP) >14 mg/L, procalcitonin >0.3 ng/mL, and ulcer size >2 cm2—although PTB has the highest specificity of any test and is commonly used together with MRI. No studies have directly compared MRI with a combination of these tests, which may assist in diagnosis (strength of recommendation [SOR]: B, meta-analysis of cohort trials and individual cohort and case control trial).
Experts recommend obtaining plain films when considering diabetic foot ulcers to evaluate for bony abnormalities, soft tissue gas, and foreign body; MRI should be considered in most situations when infection is suspected (SOR: B, evidence-based guidelines).
EVIDENCE SUMMARY
One-fifth of patients with diabetes who have foot ulcerations will develop osteomyelitis.1,2 Most cases of diabetic foot osteomyelitis result from the spread of a foot infection to underlying bone.2
MRI has highest sensitivity, probe to bone test is most specific
A meta-analysis3 of 9 cohort trials (8 prospective, 1 retrospective) of 612 patients with diabetes and a foot ulcer examined the accuracy of diagnostic methods for osteomyelitis (TABLE3,4). MRI had the highest sensitivity (90%), followed by bone scan (81%). Bone scan was the least specific (28%), however. Plain film radiography had the lowest sensitivity (54%). A PTB test was highly specific (91%) but had moderate sensitivity (60%). (PTB involves inserting a sterile, blunt stainless steel probe into an ulcerated lesion. If the probe comes to a hard stop, considered to be bone, the test is positive.)

A meta-analysis of 21 prospective and retrospective trials with 1027 diabetic patients with foot ulcers or suspected osteomyelitis found that ulcer size >2 cm2, PTB, and ESR >70 mm/hr were helpful in making the diagnosis.4
Combining ESR with ulcer size increases specificity
A prospective trial of 46 diabetic patients hospitalized with a foot infection examined the accuracy of a combination of clinical and laboratory diagnostic features in patients with diabetic foot osteomyelitis that had been diagnosed by MRI or histopathology.5 (Twenty-four patients had osteomyelitis, and 22 didn’t.)
ESR >70 mm/hr had a sensitivity of 83% and specificity of 77% (positive likelihood ratio [LR+]=3.6; negative likelihood ratio [LR−]=0.22). Ulcer size >2 cm2 had a sensitivity of 88% and specificity of 77% (LR+=3.8; LR−=0.16). Combined, an ESR >70 mm/hr and ulcer size >2cm2 had a slightly better specificity than either finding alone, 82%, but a lower sensitivity of 79% (LR+=4.4; LR−= 0.26).
Serum markers accurately distinguish osteomyelitis from infection
An individual prospective cohort trial of 61 adult patients with diabetes and a foot infection, published after the meta-analysis4 described previously, examined the accuracy of serum markers (ESR, CRP, procalcitonin) for diagnosing osteomyelitis.6 A positive PTB test and imaging study (plain film, MRI, or nuclear scintigraphy) were used as the diagnostic gold standard.
Thirty-four patients had a soft tissue infection and 27 had osteomyelitis. All markers were higher in patients with osteomyelitis than in patients with a soft tissue infection (ESR=76 mm/hr vs 66 mm/hr; P<.001; CRP=25 mg/L vs 8.7 mg/L; P<.001; procalcitonin=2.4 ng/mL vs 0.71 ng/mL; P<.001). The sensitivity and specificity for each marker at its optimum points were: ESR >67 mm/hr (sensitivity 84%; specificity 75%; LR+=3.4; LR−=0.21); CRP >14 mg/L (sensitivity 85%; specificity 83%; LR+=5; LR−=0.18); and procalcitonin >0.3 ng/mL (sensitivity 81%; specificity 71%; LR+=2.8; LR−=0.27).
RECOMMENDATIONS
The Infectious Diseases Society of America (IDSA) recommends performing the PTB test on any diabetic foot infection with an open wound (level of evidence: strong moderate).7 It also recommends performing plain radiography on all patients presenting with a new infection to evaluate for bony abnormalities, soft tissue gas, and foreign bodies (level of evidence: strong moderate).
The IDSA, the American College of Radiology diagnostic imaging expert panel, and the National Institute for Health and Clinical Excellence recommend using MRI in most clinical scenarios when osteomyelitis is suspected (level of evidence: strong moderate).8,9
Magnetic resonance imaging (MRI) has a higher sensitivity and specificity (90% and 79%) than plain radiography (54% and 68%) for diagnosing diabetic foot osteomyelitis. MRI performs somewhat better than any of several common tests—probe to bone (PTB), erythrocyte sedimentation rate (ESR) >70 mm/hr, C-reactive protein (CRP) >14 mg/L, procalcitonin >0.3 ng/mL, and ulcer size >2 cm2—although PTB has the highest specificity of any test and is commonly used together with MRI. No studies have directly compared MRI with a combination of these tests, which may assist in diagnosis (strength of recommendation [SOR]: B, meta-analysis of cohort trials and individual cohort and case control trial).
Experts recommend obtaining plain films when considering diabetic foot ulcers to evaluate for bony abnormalities, soft tissue gas, and foreign body; MRI should be considered in most situations when infection is suspected (SOR: B, evidence-based guidelines).
EVIDENCE SUMMARY
One-fifth of patients with diabetes who have foot ulcerations will develop osteomyelitis.1,2 Most cases of diabetic foot osteomyelitis result from the spread of a foot infection to underlying bone.2
MRI has highest sensitivity, probe to bone test is most specific
A meta-analysis3 of 9 cohort trials (8 prospective, 1 retrospective) of 612 patients with diabetes and a foot ulcer examined the accuracy of diagnostic methods for osteomyelitis (TABLE3,4). MRI had the highest sensitivity (90%), followed by bone scan (81%). Bone scan was the least specific (28%), however. Plain film radiography had the lowest sensitivity (54%). A PTB test was highly specific (91%) but had moderate sensitivity (60%). (PTB involves inserting a sterile, blunt stainless steel probe into an ulcerated lesion. If the probe comes to a hard stop, considered to be bone, the test is positive.)

A meta-analysis of 21 prospective and retrospective trials with 1027 diabetic patients with foot ulcers or suspected osteomyelitis found that ulcer size >2 cm2, PTB, and ESR >70 mm/hr were helpful in making the diagnosis.4
Combining ESR with ulcer size increases specificity
A prospective trial of 46 diabetic patients hospitalized with a foot infection examined the accuracy of a combination of clinical and laboratory diagnostic features in patients with diabetic foot osteomyelitis that had been diagnosed by MRI or histopathology.5 (Twenty-four patients had osteomyelitis, and 22 didn’t.)
ESR >70 mm/hr had a sensitivity of 83% and specificity of 77% (positive likelihood ratio [LR+]=3.6; negative likelihood ratio [LR−]=0.22). Ulcer size >2 cm2 had a sensitivity of 88% and specificity of 77% (LR+=3.8; LR−=0.16). Combined, an ESR >70 mm/hr and ulcer size >2cm2 had a slightly better specificity than either finding alone, 82%, but a lower sensitivity of 79% (LR+=4.4; LR−= 0.26).
Serum markers accurately distinguish osteomyelitis from infection
An individual prospective cohort trial of 61 adult patients with diabetes and a foot infection, published after the meta-analysis4 described previously, examined the accuracy of serum markers (ESR, CRP, procalcitonin) for diagnosing osteomyelitis.6 A positive PTB test and imaging study (plain film, MRI, or nuclear scintigraphy) were used as the diagnostic gold standard.
Thirty-four patients had a soft tissue infection and 27 had osteomyelitis. All markers were higher in patients with osteomyelitis than in patients with a soft tissue infection (ESR=76 mm/hr vs 66 mm/hr; P<.001; CRP=25 mg/L vs 8.7 mg/L; P<.001; procalcitonin=2.4 ng/mL vs 0.71 ng/mL; P<.001). The sensitivity and specificity for each marker at its optimum points were: ESR >67 mm/hr (sensitivity 84%; specificity 75%; LR+=3.4; LR−=0.21); CRP >14 mg/L (sensitivity 85%; specificity 83%; LR+=5; LR−=0.18); and procalcitonin >0.3 ng/mL (sensitivity 81%; specificity 71%; LR+=2.8; LR−=0.27).
RECOMMENDATIONS
The Infectious Diseases Society of America (IDSA) recommends performing the PTB test on any diabetic foot infection with an open wound (level of evidence: strong moderate).7 It also recommends performing plain radiography on all patients presenting with a new infection to evaluate for bony abnormalities, soft tissue gas, and foreign bodies (level of evidence: strong moderate).
The IDSA, the American College of Radiology diagnostic imaging expert panel, and the National Institute for Health and Clinical Excellence recommend using MRI in most clinical scenarios when osteomyelitis is suspected (level of evidence: strong moderate).8,9
1. Gemechu FW, Seemant F, Curley CA. Diabetic foot infections. Am Fam Physician. 2013;88:177-184.
2. Lavery LA, Armstrong DG, Peters EJ, et al. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care. 2007;30:270-274.
3. Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis. 2008;47:519-527.
4. Butalia S, Palda VA, Sargeant RJ, et al. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA. 2008;299:806-813.
5. Ertugrul BM, Savk O, Ozturk B, et al. The diagnosis of diabetic foot osteomyelitis: examination findings and laboratory values. Med Sci Monit. 2009;15:CR307-CR312.
6. Michail M, Jude E, Liaskos C, et al. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Low Extrem Wounds. 2013;12:94-99.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173.
8. Schweitzer ME, Daffner RH, Weissman BN, et al. ACR Appropriateness Criteria on suspected osteomyelitis in patients with diabetes mellitus. J Am Coll Radiol. 2008;5:881-886.
9. Tan T, Shaw EJ, Siddiqui F, et al; Guideline Development Group. Inpatient management of diabetic foot problems: summary of NICE guidance. BMJ. 2011;342:d1280.
1. Gemechu FW, Seemant F, Curley CA. Diabetic foot infections. Am Fam Physician. 2013;88:177-184.
2. Lavery LA, Armstrong DG, Peters EJ, et al. Probe-to-bone test for diagnosing diabetic foot osteomyelitis: reliable or relic? Diabetes Care. 2007;30:270-274.
3. Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis. 2008;47:519-527.
4. Butalia S, Palda VA, Sargeant RJ, et al. Does this patient with diabetes have osteomyelitis of the lower extremity? JAMA. 2008;299:806-813.
5. Ertugrul BM, Savk O, Ozturk B, et al. The diagnosis of diabetic foot osteomyelitis: examination findings and laboratory values. Med Sci Monit. 2009;15:CR307-CR312.
6. Michail M, Jude E, Liaskos C, et al. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Low Extrem Wounds. 2013;12:94-99.
7. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132-e173.
8. Schweitzer ME, Daffner RH, Weissman BN, et al. ACR Appropriateness Criteria on suspected osteomyelitis in patients with diabetes mellitus. J Am Coll Radiol. 2008;5:881-886.
9. Tan T, Shaw EJ, Siddiqui F, et al; Guideline Development Group. Inpatient management of diabetic foot problems: summary of NICE guidance. BMJ. 2011;342:d1280.
Evidence-based answers from the Family Physicians Inquiries Network
VIDEO: Larger lentigo maligna lesions increase risk
ASHEVILLE, N.C. – What are the risk factors for invasive melanoma in patients with lentigo maligna? Size, for one thing, according to Dr. Suzanne M. Olbricht.
In an interview at the annual meeting of the Noah Worcester Dermatological Society, Dr. Olbricht of the Lahey Hospital and Medical Center in Burlington, Mass., reviewed evidence suggesting that the recurrence rate is highest for large lesions. “This is important information that helps us think about the treatments we can use,” she said.
Dr. Olbricht had no financial conflicts to disclose.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ASHEVILLE, N.C. – What are the risk factors for invasive melanoma in patients with lentigo maligna? Size, for one thing, according to Dr. Suzanne M. Olbricht.
In an interview at the annual meeting of the Noah Worcester Dermatological Society, Dr. Olbricht of the Lahey Hospital and Medical Center in Burlington, Mass., reviewed evidence suggesting that the recurrence rate is highest for large lesions. “This is important information that helps us think about the treatments we can use,” she said.
Dr. Olbricht had no financial conflicts to disclose.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ASHEVILLE, N.C. – What are the risk factors for invasive melanoma in patients with lentigo maligna? Size, for one thing, according to Dr. Suzanne M. Olbricht.
In an interview at the annual meeting of the Noah Worcester Dermatological Society, Dr. Olbricht of the Lahey Hospital and Medical Center in Burlington, Mass., reviewed evidence suggesting that the recurrence rate is highest for large lesions. “This is important information that helps us think about the treatments we can use,” she said.
Dr. Olbricht had no financial conflicts to disclose.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT NOAH 57
Pleuritic chest pain and globus pharyngeus
A 22-year-old woman with a history of attention-deficit/hyperactivity disorder and childhood asthma came to the emergency department (ED) for treatment of a cramping, substernal, pleuritic chest pain she’d had for a week and the feeling of a “lump in her throat” that made it difficult and painful for her to swallow. The patient’s vital signs were normal and her substernal chest pain was reproducible with palpation. An anteroposterior (AP) chest x-ray (CXR) was unremarkable.
A “GI cocktail” (lidocaine, Mylanta and Donnatal), ketorolac, morphine, and lorazepam were administered in the ED, but did not provide the patient with any relief. She was admitted to the hospital to rule out acute coronary syndrome and was kept NPO overnight. A repeat CXR with posteroanterior (PA) and lateral views was also obtained (FIGURE 1A AND 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pneumomediastinum
The PA and lateral view CXRs revealed the presence of retrosternal air, suggesting the patient had pneumomediastinum. A computed tomography (CT) scan of the chest also showed retrosternal air (FIGURE 2A AND 2B, arrows) and confirmed this diagnosis. To rule out esophageal perforation, the team ordered Gastrografin and barium swallow studies. The patient was kept NPO until both studies were confirmed to be negative.
Pneumomediastinum—the presence of free air in the mediastinum—can develop spontaneously (as was the case with our patient) or in response to trauma. Common causes include respiratory diseases such as asthma, and trauma to the esophagus secondary to mechanical ventilation, endoscopy, and excessive vomiting.1 Other possible causes include respiratory infections, foreign body aspiration, recent dental extraction, diabetic ketoacidosis, esophageal perforation, barotrauma (due to activities such as flying or scuba diving), and use of illicit drugs.1
Patients with pneumomediastinum often complain of retrosternal, pleuritic pain that radiates to their back, shoulders, and arms. They may also have difficulty swallowing (globus pharyngeus), a nasal voice, and/or dyspnea. Physical findings can include subcutaneous emphysema in the neck and supraclavicular fossa as manifested by Hamman’s sign (a precordial “crunching” sound heard during systole), a fever, and distended neck veins.1
Differential diagnosis includes inflammatory conditions
The differential diagnosis for pneumomediastinum includes pericarditis, mediastinitis, Boerhaave syndrome, and acute coronary syndrome.
Pericarditis. In a patient with inflammation of the pericardium, you would hear reduced heart sounds and observe electrocardiogram (EKG) changes (eg, diffuse ST elevation in acute pericarditis). These signs typically would not be present in a patient with pneumomediastinum.1
Mediastinitis. Patients with mediastinitis—inflammation of the mediastinum—are more likely to have hypotension and shock.1
Boerhaave syndrome, or spontaneous esophageal perforation, has a similar presentation to pneumomediastinum but is more likely to be accompanied by hypotension and shock. Additionally, there would be extravasation of the contrast agent during swallow studies.2
Acute coronary syndrome is also part of the differential. However, in ACS, you would see ST changes on the patient’s EKG and elevated cardiac enzymes.1
Lateral x-rays are especially useful in making the diagnosis
Diagnosis is made by CXR and/or chest CT. On a CXR, retrosternal air is best seen in the lateral projection. Small amounts of air can appear as linear lucencies outlining mediastinal contours. This air can be seen under the skin, surrounding the pericardium, around the pulmonary and/or aortic vasculature, and/or between the parietal pleura and diaphragm.2 A pleural effusion—particularly on the patient’s left side—should raise concern for esophageal perforation.
For most patients, rest and pain control are key
Because pneumomediastinum is generally a self-limiting condition, patients who don’t have severe symptoms, such as respiratory distress or signs of inflammation, should be observed for 2 days, managed with rest and pain control, and discharged home.
If severe symptoms or inflammatory signs are present, a Gastrografin swallow study is recommended to rule out esophageal perforation. If the result of this test is abnormal, a follow-up study with barium is recommended.3 Gastrografin swallow studies are the preferred initial study.3 A barium swallow study is more sensitive, but has a higher risk of causing pneumomediastinitis if an esophageal perforation is present.2
If the swallow study reveals a perforation, surgical decompression and antibiotics may be necessary.1,4,5
Our patient received subsequent serial CXRs that showed improvement in pneumomediastinum. Once our patient’s pain was well controlled with oral nonsteroidal anti-inflammatory drugs, she was discharged home after a 3-day hospitalization with close follow-up. One week later, she had no further complaints and her pain had almost entirely resolved.
CORRESPONDENCE
Breanna Gawrys, DO, Fort Belvoir Community Hospital Family Medicine Residency, 9300 DeWitt Loop, Fort Belvoir, VA 22060; [email protected]
1. Park DE, Vallieres E. Pneumomediastinum and mediastinitis. In: Mason R, Broaddus V, Murray J, et al. Murray and Nadel’s Textbook of Respiratory Medicine. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2005:2039–2068.
2. Zylak CM, Standen JR, Barnes GR, et al. Pneumomediastinum revisited. Radiographics. 2000;20:1043-1057.
3. Takada K, Matsumoto S, Hiramatsu T, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med. 2008;102:1329-1334.
4. Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg. 2007;31:1110-1114.
5. Chalumeau M, Le Clainche L, Sayeg N, et al. Spontaneous pneumomediastinum in children. Pediatr Pulmonol. 2001;31:67-75.
A 22-year-old woman with a history of attention-deficit/hyperactivity disorder and childhood asthma came to the emergency department (ED) for treatment of a cramping, substernal, pleuritic chest pain she’d had for a week and the feeling of a “lump in her throat” that made it difficult and painful for her to swallow. The patient’s vital signs were normal and her substernal chest pain was reproducible with palpation. An anteroposterior (AP) chest x-ray (CXR) was unremarkable.
A “GI cocktail” (lidocaine, Mylanta and Donnatal), ketorolac, morphine, and lorazepam were administered in the ED, but did not provide the patient with any relief. She was admitted to the hospital to rule out acute coronary syndrome and was kept NPO overnight. A repeat CXR with posteroanterior (PA) and lateral views was also obtained (FIGURE 1A AND 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pneumomediastinum
The PA and lateral view CXRs revealed the presence of retrosternal air, suggesting the patient had pneumomediastinum. A computed tomography (CT) scan of the chest also showed retrosternal air (FIGURE 2A AND 2B, arrows) and confirmed this diagnosis. To rule out esophageal perforation, the team ordered Gastrografin and barium swallow studies. The patient was kept NPO until both studies were confirmed to be negative.
Pneumomediastinum—the presence of free air in the mediastinum—can develop spontaneously (as was the case with our patient) or in response to trauma. Common causes include respiratory diseases such as asthma, and trauma to the esophagus secondary to mechanical ventilation, endoscopy, and excessive vomiting.1 Other possible causes include respiratory infections, foreign body aspiration, recent dental extraction, diabetic ketoacidosis, esophageal perforation, barotrauma (due to activities such as flying or scuba diving), and use of illicit drugs.1
Patients with pneumomediastinum often complain of retrosternal, pleuritic pain that radiates to their back, shoulders, and arms. They may also have difficulty swallowing (globus pharyngeus), a nasal voice, and/or dyspnea. Physical findings can include subcutaneous emphysema in the neck and supraclavicular fossa as manifested by Hamman’s sign (a precordial “crunching” sound heard during systole), a fever, and distended neck veins.1
Differential diagnosis includes inflammatory conditions
The differential diagnosis for pneumomediastinum includes pericarditis, mediastinitis, Boerhaave syndrome, and acute coronary syndrome.
Pericarditis. In a patient with inflammation of the pericardium, you would hear reduced heart sounds and observe electrocardiogram (EKG) changes (eg, diffuse ST elevation in acute pericarditis). These signs typically would not be present in a patient with pneumomediastinum.1
Mediastinitis. Patients with mediastinitis—inflammation of the mediastinum—are more likely to have hypotension and shock.1
Boerhaave syndrome, or spontaneous esophageal perforation, has a similar presentation to pneumomediastinum but is more likely to be accompanied by hypotension and shock. Additionally, there would be extravasation of the contrast agent during swallow studies.2
Acute coronary syndrome is also part of the differential. However, in ACS, you would see ST changes on the patient’s EKG and elevated cardiac enzymes.1
Lateral x-rays are especially useful in making the diagnosis
Diagnosis is made by CXR and/or chest CT. On a CXR, retrosternal air is best seen in the lateral projection. Small amounts of air can appear as linear lucencies outlining mediastinal contours. This air can be seen under the skin, surrounding the pericardium, around the pulmonary and/or aortic vasculature, and/or between the parietal pleura and diaphragm.2 A pleural effusion—particularly on the patient’s left side—should raise concern for esophageal perforation.
For most patients, rest and pain control are key
Because pneumomediastinum is generally a self-limiting condition, patients who don’t have severe symptoms, such as respiratory distress or signs of inflammation, should be observed for 2 days, managed with rest and pain control, and discharged home.
If severe symptoms or inflammatory signs are present, a Gastrografin swallow study is recommended to rule out esophageal perforation. If the result of this test is abnormal, a follow-up study with barium is recommended.3 Gastrografin swallow studies are the preferred initial study.3 A barium swallow study is more sensitive, but has a higher risk of causing pneumomediastinitis if an esophageal perforation is present.2
If the swallow study reveals a perforation, surgical decompression and antibiotics may be necessary.1,4,5
Our patient received subsequent serial CXRs that showed improvement in pneumomediastinum. Once our patient’s pain was well controlled with oral nonsteroidal anti-inflammatory drugs, she was discharged home after a 3-day hospitalization with close follow-up. One week later, she had no further complaints and her pain had almost entirely resolved.
CORRESPONDENCE
Breanna Gawrys, DO, Fort Belvoir Community Hospital Family Medicine Residency, 9300 DeWitt Loop, Fort Belvoir, VA 22060; [email protected]
A 22-year-old woman with a history of attention-deficit/hyperactivity disorder and childhood asthma came to the emergency department (ED) for treatment of a cramping, substernal, pleuritic chest pain she’d had for a week and the feeling of a “lump in her throat” that made it difficult and painful for her to swallow. The patient’s vital signs were normal and her substernal chest pain was reproducible with palpation. An anteroposterior (AP) chest x-ray (CXR) was unremarkable.
A “GI cocktail” (lidocaine, Mylanta and Donnatal), ketorolac, morphine, and lorazepam were administered in the ED, but did not provide the patient with any relief. She was admitted to the hospital to rule out acute coronary syndrome and was kept NPO overnight. A repeat CXR with posteroanterior (PA) and lateral views was also obtained (FIGURE 1A AND 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Pneumomediastinum
The PA and lateral view CXRs revealed the presence of retrosternal air, suggesting the patient had pneumomediastinum. A computed tomography (CT) scan of the chest also showed retrosternal air (FIGURE 2A AND 2B, arrows) and confirmed this diagnosis. To rule out esophageal perforation, the team ordered Gastrografin and barium swallow studies. The patient was kept NPO until both studies were confirmed to be negative.
Pneumomediastinum—the presence of free air in the mediastinum—can develop spontaneously (as was the case with our patient) or in response to trauma. Common causes include respiratory diseases such as asthma, and trauma to the esophagus secondary to mechanical ventilation, endoscopy, and excessive vomiting.1 Other possible causes include respiratory infections, foreign body aspiration, recent dental extraction, diabetic ketoacidosis, esophageal perforation, barotrauma (due to activities such as flying or scuba diving), and use of illicit drugs.1
Patients with pneumomediastinum often complain of retrosternal, pleuritic pain that radiates to their back, shoulders, and arms. They may also have difficulty swallowing (globus pharyngeus), a nasal voice, and/or dyspnea. Physical findings can include subcutaneous emphysema in the neck and supraclavicular fossa as manifested by Hamman’s sign (a precordial “crunching” sound heard during systole), a fever, and distended neck veins.1
Differential diagnosis includes inflammatory conditions
The differential diagnosis for pneumomediastinum includes pericarditis, mediastinitis, Boerhaave syndrome, and acute coronary syndrome.
Pericarditis. In a patient with inflammation of the pericardium, you would hear reduced heart sounds and observe electrocardiogram (EKG) changes (eg, diffuse ST elevation in acute pericarditis). These signs typically would not be present in a patient with pneumomediastinum.1
Mediastinitis. Patients with mediastinitis—inflammation of the mediastinum—are more likely to have hypotension and shock.1
Boerhaave syndrome, or spontaneous esophageal perforation, has a similar presentation to pneumomediastinum but is more likely to be accompanied by hypotension and shock. Additionally, there would be extravasation of the contrast agent during swallow studies.2
Acute coronary syndrome is also part of the differential. However, in ACS, you would see ST changes on the patient’s EKG and elevated cardiac enzymes.1
Lateral x-rays are especially useful in making the diagnosis
Diagnosis is made by CXR and/or chest CT. On a CXR, retrosternal air is best seen in the lateral projection. Small amounts of air can appear as linear lucencies outlining mediastinal contours. This air can be seen under the skin, surrounding the pericardium, around the pulmonary and/or aortic vasculature, and/or between the parietal pleura and diaphragm.2 A pleural effusion—particularly on the patient’s left side—should raise concern for esophageal perforation.
For most patients, rest and pain control are key
Because pneumomediastinum is generally a self-limiting condition, patients who don’t have severe symptoms, such as respiratory distress or signs of inflammation, should be observed for 2 days, managed with rest and pain control, and discharged home.
If severe symptoms or inflammatory signs are present, a Gastrografin swallow study is recommended to rule out esophageal perforation. If the result of this test is abnormal, a follow-up study with barium is recommended.3 Gastrografin swallow studies are the preferred initial study.3 A barium swallow study is more sensitive, but has a higher risk of causing pneumomediastinitis if an esophageal perforation is present.2
If the swallow study reveals a perforation, surgical decompression and antibiotics may be necessary.1,4,5
Our patient received subsequent serial CXRs that showed improvement in pneumomediastinum. Once our patient’s pain was well controlled with oral nonsteroidal anti-inflammatory drugs, she was discharged home after a 3-day hospitalization with close follow-up. One week later, she had no further complaints and her pain had almost entirely resolved.
CORRESPONDENCE
Breanna Gawrys, DO, Fort Belvoir Community Hospital Family Medicine Residency, 9300 DeWitt Loop, Fort Belvoir, VA 22060; [email protected]
1. Park DE, Vallieres E. Pneumomediastinum and mediastinitis. In: Mason R, Broaddus V, Murray J, et al. Murray and Nadel’s Textbook of Respiratory Medicine. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2005:2039–2068.
2. Zylak CM, Standen JR, Barnes GR, et al. Pneumomediastinum revisited. Radiographics. 2000;20:1043-1057.
3. Takada K, Matsumoto S, Hiramatsu T, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med. 2008;102:1329-1334.
4. Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg. 2007;31:1110-1114.
5. Chalumeau M, Le Clainche L, Sayeg N, et al. Spontaneous pneumomediastinum in children. Pediatr Pulmonol. 2001;31:67-75.
1. Park DE, Vallieres E. Pneumomediastinum and mediastinitis. In: Mason R, Broaddus V, Murray J, et al. Murray and Nadel’s Textbook of Respiratory Medicine. 4th ed. Philadelphia, PA: Elsevier Health Sciences; 2005:2039–2068.
2. Zylak CM, Standen JR, Barnes GR, et al. Pneumomediastinum revisited. Radiographics. 2000;20:1043-1057.
3. Takada K, Matsumoto S, Hiramatsu T, et al. Management of spontaneous pneumomediastinum based on clinical experience of 25 cases. Respir Med. 2008;102:1329-1334.
4. Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardiothorac Surg. 2007;31:1110-1114.
5. Chalumeau M, Le Clainche L, Sayeg N, et al. Spontaneous pneumomediastinum in children. Pediatr Pulmonol. 2001;31:67-75.
Another good reason to recommend low-dose aspirin
Prescribe low-dose aspirin (eg, 81 mg/d) to pregnant women who are at high risk for preeclampsia because it reduces the risk of this complication, as well as preterm birth and intrauterine growth restriction.1
Strength of recommendation
A: Based on a systematic review and meta-analysis of 23 studies, including 21 randomized controlled trials.
Henderson J, Whitlock E, O’Connor E, et al. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:695-703.
Illustrative case
A 22-year-old G2P1 pregnant woman at 18 weeks gestation who has a history of preeclampsia comes to your office for a routine prenatal visit. On exam, her blood pressure continues to be in the 110s/60s, as it has been for several visits. Her history puts her at risk of developing preeclampsia again, and you wonder if anything can be done to prevent this from happening.
The incidence of preeclampsia, which occurs in 2% to 8% of pregnancies worldwide and 3.4% of pregnancies in the United States, appears to be steadily increasing.2,3 Preeclampsia is defined as new-onset hypertension at >20 weeks gestation, plus proteinuria, thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, and/or cerebral or visual symptoms.4 The condition is associated with several adverse maternal and fetal outcomes, including eclampsia, abruption, intrauterine growth restriction (IUGR), preterm birth, stillbirth, and maternal death.2,4 Risk factors for preeclampsia include previous preeclampsia, maternal age ≥40 years, chronic medical conditions, and multi-fetal pregnancy.5
The only effective treatment for preeclampsia is delivery.4 Given the lack of other treatments, strategies for preventing preeclampsia would be highly valuable.
In 1996, the US Preventive Services Task Force (USPSTF) addressed this issue and concluded that there was insufficient evidence to recommend for or against using aspirin to prevent preeclampsia.6 More recently, Henderson et al1 conducted a systematic review and meta-analysis to support the USPSTF in a revision of its earlier recommendation.
STUDY SUMMARY: Aspirin use lowers risk of preeclampsia and preterm birth
Henderson et al1 evaluated the impact of low-dose aspirin on maternal and fetal outcomes among pregnant women at risk for preeclampsia. The review of 23 studies included 21 randomized placebo-controlled trials that evaluated 24,666 patients. Slightly more than half of the studies that evaluated maternal and fetal health benefits were graded as good-quality, and 67% of those that evaluated maternal, perinatal, and developmental harms were rated good-quality.
Most women were white and ages 20 to 33 years. Aspirin doses ranged from 60 mg/d to 150 mg/d; most studies used 60 mg/d or 100 mg/d. Aspirin was initiated between 12 to 36 weeks gestation, with 9 trials initiating aspirin before 16 weeks. In most trials, aspirin was continued until delivery.
Among women at high preeclampsia risk (10 studies), the pooled relative risk (RR) for perinatal death was 0.81 (95% confidence interval [CI], 0.65-1.01) for low-dose aspirin compared to placebo. However, this finding was not statistically significant (P=.78).
Among women who received low-dose aspirin, researchers noted a 14% risk reduction for preterm birth (RR=0.86; 95% CI, 0.76-0.98); a 20% risk reduction for IUGR (RR=0.80; 95% CI, 0.65-0.99), and a 24% risk reduction for preeclampsia (RR=0.76; 95% CI, 0.62-0.95). The absolute risk reduction for preeclampsia was estimated to be 2% to 5%.
While the results for preterm birth, IUGR, and preeclampsia were statistically significant, the authors noted that these results could have been biased by small study effects (the tendency of smaller studies to report positive findings, which in turn can skew the results of a meta-analysis based primarily on such studies). The timing and dosage of aspirin had no significant effect on outcomes.
There was no evidence of increased maternal postpartum hemorrhage with aspirin use (RR=1.02; 95% CI, 0.96-1.09). Aspirin use did not seem to increase perinatal mortality among all risk levels (RR=0.92; 95% CI, 0.76-1.11; P=.65). No differences were noted in the toddlers’ development at 18 months.
WHAT'S NEW: Low-dose aspirin use is now recommended
The 1996 USPSTF recommendation concluded that there was insufficient evidence to recommend aspirin use for preventing preeclampsia. This systematic review and meta-analysis, along with findings from a 2007 Cochrane review7 and a meta-analysis from the PARIS Collaborative Group,8 provide good-quality evidence that aspirin reduces negative maternal and fetal outcomes associated with preeclampsia. In 2014, the USPSTF cited this evidence when it decided to recommend using low-dose aspirin (81 mg/d) to prevent preeclampsia in women who are at high risk for preeclampsia (Grade B).9 (For more on the USPSTF, see “Catching up on the latest USPSTF recommendations”.)
CAVEATS: Much of the data came from small studies
A substantial portion of the data in this systematic review and meta-analysis came from small studies with positive findings. Because small studies with null findings tend to not be published, there is concern that the results reported by Henderson et al1 may be somewhat biased, and that future studies may push the overall observed effect toward a null finding.
Also, the criteria used to define “high risk” for preeclampsia varied by study, so it’s unclear which groups of women would benefit most from aspirin use during pregnancy. Finally, there is a lack of high-quality data on the effects of aspirin use during pregnancy on long-term outcomes in children. Despite these caveats, the cumulative evidence strongly points to greater benefit than harm.
CHALLENGES TO IMPLEMENTATION: You need to determine which patients are at highest risk
The principle challenge lies in identifying which patients are at high risk for preeclampsia, and thus, will likely benefit from this intervention. This systematic review and meta-analysis used a large variety of risk factors to determine whether a woman was high risk. A 2013 American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy report defined high-risk as women with a history of preeclampsia in more than one previous pregnancy or women with a previous preterm delivery due to preeclampsia.4
The updated USPSTF recommendation suggests that women be considered high risk if they have any of the following: 1) previous preeclampsia, 2) multifetal gestation, 3) chronic hypertension, 4) diabetes, 5) renal disease, or 6) autoimmune disease.9 We consider both sets of criteria reasonable for identifying women who may benefit from low-dose aspirin during pregnancy.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Henderson J, Whitlock E, O’Connor E, et al. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:695-703.
2. Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56-59.
3. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564.
4. American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122-1131.
5. Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565.
6. US Preventive Services Task Force. Aspirin prophylaxis in pregnancy. In: Guide to Clinical Preventive Services: Report of the U.S. Preventive Services Task Force. 2nd edition. Washington, DC: US Department of Health and Human Services; 1996.
7. Duley L, Henderson-Smart DJ, Meher S, et al. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007(2):CD004659.
8. Askie LM, Duley L, Henderson-Smart DJ, et al; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369:1791-1798.
9. LeFevre ML; U.S. Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:819-826.
Prescribe low-dose aspirin (eg, 81 mg/d) to pregnant women who are at high risk for preeclampsia because it reduces the risk of this complication, as well as preterm birth and intrauterine growth restriction.1
Strength of recommendation
A: Based on a systematic review and meta-analysis of 23 studies, including 21 randomized controlled trials.
Henderson J, Whitlock E, O’Connor E, et al. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:695-703.
Illustrative case
A 22-year-old G2P1 pregnant woman at 18 weeks gestation who has a history of preeclampsia comes to your office for a routine prenatal visit. On exam, her blood pressure continues to be in the 110s/60s, as it has been for several visits. Her history puts her at risk of developing preeclampsia again, and you wonder if anything can be done to prevent this from happening.
The incidence of preeclampsia, which occurs in 2% to 8% of pregnancies worldwide and 3.4% of pregnancies in the United States, appears to be steadily increasing.2,3 Preeclampsia is defined as new-onset hypertension at >20 weeks gestation, plus proteinuria, thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, and/or cerebral or visual symptoms.4 The condition is associated with several adverse maternal and fetal outcomes, including eclampsia, abruption, intrauterine growth restriction (IUGR), preterm birth, stillbirth, and maternal death.2,4 Risk factors for preeclampsia include previous preeclampsia, maternal age ≥40 years, chronic medical conditions, and multi-fetal pregnancy.5
The only effective treatment for preeclampsia is delivery.4 Given the lack of other treatments, strategies for preventing preeclampsia would be highly valuable.
In 1996, the US Preventive Services Task Force (USPSTF) addressed this issue and concluded that there was insufficient evidence to recommend for or against using aspirin to prevent preeclampsia.6 More recently, Henderson et al1 conducted a systematic review and meta-analysis to support the USPSTF in a revision of its earlier recommendation.
STUDY SUMMARY: Aspirin use lowers risk of preeclampsia and preterm birth
Henderson et al1 evaluated the impact of low-dose aspirin on maternal and fetal outcomes among pregnant women at risk for preeclampsia. The review of 23 studies included 21 randomized placebo-controlled trials that evaluated 24,666 patients. Slightly more than half of the studies that evaluated maternal and fetal health benefits were graded as good-quality, and 67% of those that evaluated maternal, perinatal, and developmental harms were rated good-quality.
Most women were white and ages 20 to 33 years. Aspirin doses ranged from 60 mg/d to 150 mg/d; most studies used 60 mg/d or 100 mg/d. Aspirin was initiated between 12 to 36 weeks gestation, with 9 trials initiating aspirin before 16 weeks. In most trials, aspirin was continued until delivery.
Among women at high preeclampsia risk (10 studies), the pooled relative risk (RR) for perinatal death was 0.81 (95% confidence interval [CI], 0.65-1.01) for low-dose aspirin compared to placebo. However, this finding was not statistically significant (P=.78).
Among women who received low-dose aspirin, researchers noted a 14% risk reduction for preterm birth (RR=0.86; 95% CI, 0.76-0.98); a 20% risk reduction for IUGR (RR=0.80; 95% CI, 0.65-0.99), and a 24% risk reduction for preeclampsia (RR=0.76; 95% CI, 0.62-0.95). The absolute risk reduction for preeclampsia was estimated to be 2% to 5%.
While the results for preterm birth, IUGR, and preeclampsia were statistically significant, the authors noted that these results could have been biased by small study effects (the tendency of smaller studies to report positive findings, which in turn can skew the results of a meta-analysis based primarily on such studies). The timing and dosage of aspirin had no significant effect on outcomes.
There was no evidence of increased maternal postpartum hemorrhage with aspirin use (RR=1.02; 95% CI, 0.96-1.09). Aspirin use did not seem to increase perinatal mortality among all risk levels (RR=0.92; 95% CI, 0.76-1.11; P=.65). No differences were noted in the toddlers’ development at 18 months.
WHAT'S NEW: Low-dose aspirin use is now recommended
The 1996 USPSTF recommendation concluded that there was insufficient evidence to recommend aspirin use for preventing preeclampsia. This systematic review and meta-analysis, along with findings from a 2007 Cochrane review7 and a meta-analysis from the PARIS Collaborative Group,8 provide good-quality evidence that aspirin reduces negative maternal and fetal outcomes associated with preeclampsia. In 2014, the USPSTF cited this evidence when it decided to recommend using low-dose aspirin (81 mg/d) to prevent preeclampsia in women who are at high risk for preeclampsia (Grade B).9 (For more on the USPSTF, see “Catching up on the latest USPSTF recommendations”.)
CAVEATS: Much of the data came from small studies
A substantial portion of the data in this systematic review and meta-analysis came from small studies with positive findings. Because small studies with null findings tend to not be published, there is concern that the results reported by Henderson et al1 may be somewhat biased, and that future studies may push the overall observed effect toward a null finding.
Also, the criteria used to define “high risk” for preeclampsia varied by study, so it’s unclear which groups of women would benefit most from aspirin use during pregnancy. Finally, there is a lack of high-quality data on the effects of aspirin use during pregnancy on long-term outcomes in children. Despite these caveats, the cumulative evidence strongly points to greater benefit than harm.
CHALLENGES TO IMPLEMENTATION: You need to determine which patients are at highest risk
The principle challenge lies in identifying which patients are at high risk for preeclampsia, and thus, will likely benefit from this intervention. This systematic review and meta-analysis used a large variety of risk factors to determine whether a woman was high risk. A 2013 American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy report defined high-risk as women with a history of preeclampsia in more than one previous pregnancy or women with a previous preterm delivery due to preeclampsia.4
The updated USPSTF recommendation suggests that women be considered high risk if they have any of the following: 1) previous preeclampsia, 2) multifetal gestation, 3) chronic hypertension, 4) diabetes, 5) renal disease, or 6) autoimmune disease.9 We consider both sets of criteria reasonable for identifying women who may benefit from low-dose aspirin during pregnancy.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Prescribe low-dose aspirin (eg, 81 mg/d) to pregnant women who are at high risk for preeclampsia because it reduces the risk of this complication, as well as preterm birth and intrauterine growth restriction.1
Strength of recommendation
A: Based on a systematic review and meta-analysis of 23 studies, including 21 randomized controlled trials.
Henderson J, Whitlock E, O’Connor E, et al. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:695-703.
Illustrative case
A 22-year-old G2P1 pregnant woman at 18 weeks gestation who has a history of preeclampsia comes to your office for a routine prenatal visit. On exam, her blood pressure continues to be in the 110s/60s, as it has been for several visits. Her history puts her at risk of developing preeclampsia again, and you wonder if anything can be done to prevent this from happening.
The incidence of preeclampsia, which occurs in 2% to 8% of pregnancies worldwide and 3.4% of pregnancies in the United States, appears to be steadily increasing.2,3 Preeclampsia is defined as new-onset hypertension at >20 weeks gestation, plus proteinuria, thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, and/or cerebral or visual symptoms.4 The condition is associated with several adverse maternal and fetal outcomes, including eclampsia, abruption, intrauterine growth restriction (IUGR), preterm birth, stillbirth, and maternal death.2,4 Risk factors for preeclampsia include previous preeclampsia, maternal age ≥40 years, chronic medical conditions, and multi-fetal pregnancy.5
The only effective treatment for preeclampsia is delivery.4 Given the lack of other treatments, strategies for preventing preeclampsia would be highly valuable.
In 1996, the US Preventive Services Task Force (USPSTF) addressed this issue and concluded that there was insufficient evidence to recommend for or against using aspirin to prevent preeclampsia.6 More recently, Henderson et al1 conducted a systematic review and meta-analysis to support the USPSTF in a revision of its earlier recommendation.
STUDY SUMMARY: Aspirin use lowers risk of preeclampsia and preterm birth
Henderson et al1 evaluated the impact of low-dose aspirin on maternal and fetal outcomes among pregnant women at risk for preeclampsia. The review of 23 studies included 21 randomized placebo-controlled trials that evaluated 24,666 patients. Slightly more than half of the studies that evaluated maternal and fetal health benefits were graded as good-quality, and 67% of those that evaluated maternal, perinatal, and developmental harms were rated good-quality.
Most women were white and ages 20 to 33 years. Aspirin doses ranged from 60 mg/d to 150 mg/d; most studies used 60 mg/d or 100 mg/d. Aspirin was initiated between 12 to 36 weeks gestation, with 9 trials initiating aspirin before 16 weeks. In most trials, aspirin was continued until delivery.
Among women at high preeclampsia risk (10 studies), the pooled relative risk (RR) for perinatal death was 0.81 (95% confidence interval [CI], 0.65-1.01) for low-dose aspirin compared to placebo. However, this finding was not statistically significant (P=.78).
Among women who received low-dose aspirin, researchers noted a 14% risk reduction for preterm birth (RR=0.86; 95% CI, 0.76-0.98); a 20% risk reduction for IUGR (RR=0.80; 95% CI, 0.65-0.99), and a 24% risk reduction for preeclampsia (RR=0.76; 95% CI, 0.62-0.95). The absolute risk reduction for preeclampsia was estimated to be 2% to 5%.
While the results for preterm birth, IUGR, and preeclampsia were statistically significant, the authors noted that these results could have been biased by small study effects (the tendency of smaller studies to report positive findings, which in turn can skew the results of a meta-analysis based primarily on such studies). The timing and dosage of aspirin had no significant effect on outcomes.
There was no evidence of increased maternal postpartum hemorrhage with aspirin use (RR=1.02; 95% CI, 0.96-1.09). Aspirin use did not seem to increase perinatal mortality among all risk levels (RR=0.92; 95% CI, 0.76-1.11; P=.65). No differences were noted in the toddlers’ development at 18 months.
WHAT'S NEW: Low-dose aspirin use is now recommended
The 1996 USPSTF recommendation concluded that there was insufficient evidence to recommend aspirin use for preventing preeclampsia. This systematic review and meta-analysis, along with findings from a 2007 Cochrane review7 and a meta-analysis from the PARIS Collaborative Group,8 provide good-quality evidence that aspirin reduces negative maternal and fetal outcomes associated with preeclampsia. In 2014, the USPSTF cited this evidence when it decided to recommend using low-dose aspirin (81 mg/d) to prevent preeclampsia in women who are at high risk for preeclampsia (Grade B).9 (For more on the USPSTF, see “Catching up on the latest USPSTF recommendations”.)
CAVEATS: Much of the data came from small studies
A substantial portion of the data in this systematic review and meta-analysis came from small studies with positive findings. Because small studies with null findings tend to not be published, there is concern that the results reported by Henderson et al1 may be somewhat biased, and that future studies may push the overall observed effect toward a null finding.
Also, the criteria used to define “high risk” for preeclampsia varied by study, so it’s unclear which groups of women would benefit most from aspirin use during pregnancy. Finally, there is a lack of high-quality data on the effects of aspirin use during pregnancy on long-term outcomes in children. Despite these caveats, the cumulative evidence strongly points to greater benefit than harm.
CHALLENGES TO IMPLEMENTATION: You need to determine which patients are at highest risk
The principle challenge lies in identifying which patients are at high risk for preeclampsia, and thus, will likely benefit from this intervention. This systematic review and meta-analysis used a large variety of risk factors to determine whether a woman was high risk. A 2013 American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy report defined high-risk as women with a history of preeclampsia in more than one previous pregnancy or women with a previous preterm delivery due to preeclampsia.4
The updated USPSTF recommendation suggests that women be considered high risk if they have any of the following: 1) previous preeclampsia, 2) multifetal gestation, 3) chronic hypertension, 4) diabetes, 5) renal disease, or 6) autoimmune disease.9 We consider both sets of criteria reasonable for identifying women who may benefit from low-dose aspirin during pregnancy.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Henderson J, Whitlock E, O’Connor E, et al. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:695-703.
2. Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56-59.
3. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564.
4. American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122-1131.
5. Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565.
6. US Preventive Services Task Force. Aspirin prophylaxis in pregnancy. In: Guide to Clinical Preventive Services: Report of the U.S. Preventive Services Task Force. 2nd edition. Washington, DC: US Department of Health and Human Services; 1996.
7. Duley L, Henderson-Smart DJ, Meher S, et al. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007(2):CD004659.
8. Askie LM, Duley L, Henderson-Smart DJ, et al; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369:1791-1798.
9. LeFevre ML; U.S. Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:819-826.
1. Henderson J, Whitlock E, O’Connor E, et al. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160:695-703.
2. Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56-59.
3. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564.
4. American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122-1131.
5. Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565.
6. US Preventive Services Task Force. Aspirin prophylaxis in pregnancy. In: Guide to Clinical Preventive Services: Report of the U.S. Preventive Services Task Force. 2nd edition. Washington, DC: US Department of Health and Human Services; 1996.
7. Duley L, Henderson-Smart DJ, Meher S, et al. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007(2):CD004659.
8. Askie LM, Duley L, Henderson-Smart DJ, et al; PARIS Collaborative Group. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369:1791-1798.
9. LeFevre ML; U.S. Preventive Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:819-826.
Copyright © 2015 Family Physicians Inquiries Network. All rights reserved.
Acute Kidney Injury: Magnesium for Protection
Q) Our radiology department is discussing use of IV magnesium for diabetic patients to “protect them from kidney injury.” Is this a standard of care now?
Magnesium, the fourth most abundant cation in the body, plays an important physiologic role. Balance is maintained by renal regulation of magnesium reabsorption, and deficiency occurs when there is increased renal excretion initiated by osmotic diuresis. Clinical manifestations of deficiency include cardiac arrhythmias, neuromuscular hyperexcitability, and biochemical abnormalities of hypocalcaemia and hypokalemia.
Diabetes is one of the leading causes of magnesium deficiency, with incidence ranging from 25% to 39%.1 Fluctuations in serum magnesium concentrations are directly correlated with fasting blood glucose, A1C levels, albumin excretion, and the duration of diabetes. It has been postulated that magnesium depletion, via its effect on inositol transport, is pathogenic in the progression of diabetic complications.
Contrast-induced acute kidney injury (CI-AKI) is a potentially adverse consequence of percutaneous coronary interventions (PCI), particularly in diabetic patients. It results in significant morbidity and mortality and adds to the costs of diagnostic and interventional cardiology procedures. Intravenous (IV) agents used during radiologic imaging are notorious for causing acute kidney injury in diabetic patients. Preprocedural hydration and discontinuation of all nephrotoxic medications have proven beneficial in protecting these patients from CI-AKI.
A recent prospective, randomized, open-label clinical trial looked at the effect of administering IV magnesium prior to PCI.2 The control group underwent standard preprocedural hydration and discontinuation of nephrotoxic medications. The study group added IV magnesium to the standard protocol.
In this single-center study, 26.6% of patients in the control group and 14.5% in the study group sustained CI-AKI, a statistically significant result (P = .01). Neither group experienced mortality or required dialysis.
Although not considered standard of care at this time, prophylactic use of IV magnesium (pending pre-op labs), along with the recognized benefit of preprocedural hydration and discontinuation of nephrotoxic medications, can be supported in primary PCI patients. Your radiology department is on the cutting edge of protecting these very high-risk patients.
Debra L. Coplon, DNP, DCC
City of Memphis Wellness Clinic, Tennessee
REFERENCES
1. Ayuk J, Gittoes N. Contemporary view of the clinical relevance of magnesium homeostasis. Ann Clin Biochem. 2014;51(Pt 2):179-188.
2. Firouzi A, Maadani M, Kiani R, et al. Intravenous magnesium sulfate: new method in prevention of contrast-induced nephropathy in primary percutaneous coronary intervention. Int Urol Nephrol. 2015;47(3):521-525.
Q) Our radiology department is discussing use of IV magnesium for diabetic patients to “protect them from kidney injury.” Is this a standard of care now?
Magnesium, the fourth most abundant cation in the body, plays an important physiologic role. Balance is maintained by renal regulation of magnesium reabsorption, and deficiency occurs when there is increased renal excretion initiated by osmotic diuresis. Clinical manifestations of deficiency include cardiac arrhythmias, neuromuscular hyperexcitability, and biochemical abnormalities of hypocalcaemia and hypokalemia.
Diabetes is one of the leading causes of magnesium deficiency, with incidence ranging from 25% to 39%.1 Fluctuations in serum magnesium concentrations are directly correlated with fasting blood glucose, A1C levels, albumin excretion, and the duration of diabetes. It has been postulated that magnesium depletion, via its effect on inositol transport, is pathogenic in the progression of diabetic complications.
Contrast-induced acute kidney injury (CI-AKI) is a potentially adverse consequence of percutaneous coronary interventions (PCI), particularly in diabetic patients. It results in significant morbidity and mortality and adds to the costs of diagnostic and interventional cardiology procedures. Intravenous (IV) agents used during radiologic imaging are notorious for causing acute kidney injury in diabetic patients. Preprocedural hydration and discontinuation of all nephrotoxic medications have proven beneficial in protecting these patients from CI-AKI.
A recent prospective, randomized, open-label clinical trial looked at the effect of administering IV magnesium prior to PCI.2 The control group underwent standard preprocedural hydration and discontinuation of nephrotoxic medications. The study group added IV magnesium to the standard protocol.
In this single-center study, 26.6% of patients in the control group and 14.5% in the study group sustained CI-AKI, a statistically significant result (P = .01). Neither group experienced mortality or required dialysis.
Although not considered standard of care at this time, prophylactic use of IV magnesium (pending pre-op labs), along with the recognized benefit of preprocedural hydration and discontinuation of nephrotoxic medications, can be supported in primary PCI patients. Your radiology department is on the cutting edge of protecting these very high-risk patients.
Debra L. Coplon, DNP, DCC
City of Memphis Wellness Clinic, Tennessee
REFERENCES
1. Ayuk J, Gittoes N. Contemporary view of the clinical relevance of magnesium homeostasis. Ann Clin Biochem. 2014;51(Pt 2):179-188.
2. Firouzi A, Maadani M, Kiani R, et al. Intravenous magnesium sulfate: new method in prevention of contrast-induced nephropathy in primary percutaneous coronary intervention. Int Urol Nephrol. 2015;47(3):521-525.
Q) Our radiology department is discussing use of IV magnesium for diabetic patients to “protect them from kidney injury.” Is this a standard of care now?
Magnesium, the fourth most abundant cation in the body, plays an important physiologic role. Balance is maintained by renal regulation of magnesium reabsorption, and deficiency occurs when there is increased renal excretion initiated by osmotic diuresis. Clinical manifestations of deficiency include cardiac arrhythmias, neuromuscular hyperexcitability, and biochemical abnormalities of hypocalcaemia and hypokalemia.
Diabetes is one of the leading causes of magnesium deficiency, with incidence ranging from 25% to 39%.1 Fluctuations in serum magnesium concentrations are directly correlated with fasting blood glucose, A1C levels, albumin excretion, and the duration of diabetes. It has been postulated that magnesium depletion, via its effect on inositol transport, is pathogenic in the progression of diabetic complications.
Contrast-induced acute kidney injury (CI-AKI) is a potentially adverse consequence of percutaneous coronary interventions (PCI), particularly in diabetic patients. It results in significant morbidity and mortality and adds to the costs of diagnostic and interventional cardiology procedures. Intravenous (IV) agents used during radiologic imaging are notorious for causing acute kidney injury in diabetic patients. Preprocedural hydration and discontinuation of all nephrotoxic medications have proven beneficial in protecting these patients from CI-AKI.
A recent prospective, randomized, open-label clinical trial looked at the effect of administering IV magnesium prior to PCI.2 The control group underwent standard preprocedural hydration and discontinuation of nephrotoxic medications. The study group added IV magnesium to the standard protocol.
In this single-center study, 26.6% of patients in the control group and 14.5% in the study group sustained CI-AKI, a statistically significant result (P = .01). Neither group experienced mortality or required dialysis.
Although not considered standard of care at this time, prophylactic use of IV magnesium (pending pre-op labs), along with the recognized benefit of preprocedural hydration and discontinuation of nephrotoxic medications, can be supported in primary PCI patients. Your radiology department is on the cutting edge of protecting these very high-risk patients.
Debra L. Coplon, DNP, DCC
City of Memphis Wellness Clinic, Tennessee
REFERENCES
1. Ayuk J, Gittoes N. Contemporary view of the clinical relevance of magnesium homeostasis. Ann Clin Biochem. 2014;51(Pt 2):179-188.
2. Firouzi A, Maadani M, Kiani R, et al. Intravenous magnesium sulfate: new method in prevention of contrast-induced nephropathy in primary percutaneous coronary intervention. Int Urol Nephrol. 2015;47(3):521-525.