User login
Fracture Blisters After Primary Total Knee Arthroplasty
Fracture blisters are a relatively uncommon complication of high-energy fractures, with an incidence of 2.9%.1 In the lower extremity, fracture blisters almost always occur distal to the knee.1 Histologically, the blisters represent an injury to the dermoepidermal junction.2 On physical examination, there are tense blood- and/or clear fluid–filled bullae overlying markedly swollen and edematous soft tissue,1 resembling a second-degree burn.3 Infection may develop after fracture blisters,1 and this is perhaps the most dreaded complication of total knee arthroplasty (TKA). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 71-year-old man with end-stage osteoarthritis of the right knee underwent an elective TKA with cemented components (Legion PS; Smith & Nephew). His medical history included venous insufficiency, type 2 diabetes mellitus, chronic obstructive sleep apnea, hypertension, morbid obesity (body mass index, 50), and a previous uneventful left TKA. Tourniquet time was 78 minutes and estimated blood loss was 100 mL. An intra-articular drain was used and was removed on the first postoperative day. After wound closure, a soft splint bandage consisting of 2 to 3 layers of cotton and bias wrap was applied. Deep vein thrombosis (DVT) prophylaxis with enoxaparin 40 mg once daily was started on the first postoperative day.
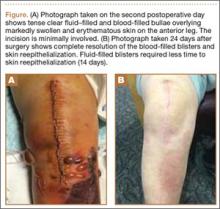
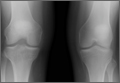
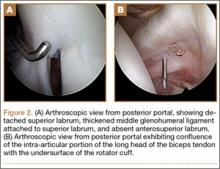
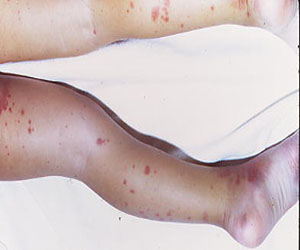
Upon removal of the surgical dressings on the second postoperative day, the anterior leg was found to have a combination of tense clear fluid– and blood-filled blisters on markedly swollen and erythematous skin. The incision was minimally involved (Figure A). There was diffuse 2+ pitting edema with hyperesthesia in the affected skin distal to the knee. Prior to these findings, the patient had complained of increasing pain in his operative leg, but there was no escalation in analgesic requirements. There was no evidence of compartment syndrome on serial examinations. An ultrasound of the lower extremity was negative for DVT. Plain films did not show iatrogenic fractures. There was no intraoperative vascular injury, and the foot pulses remained unchanged between the time the patient was in the preoperative holding unit, the postanesthesia care unit, and the orthopedic ward. The operative leg was treated with elevation and loosely applied Kerlix roll gauze (Kendall, Covidien), but active blister formation continued for another 2 days. A 10-day prophylactic course of trimethoprim/sulfamethoxazole was initiated on the third postoperative day after the blisters started to rupture. The patient was allowed to bear weight as tolerated, but his physical therapy (PT) course was limited by pain and fear “of losing his leg.” He declined several PT sessions and was hesitant to use continuous passive motion. The patient was discharged to a short-term rehabilitation facility with weekly outpatient follow-up. On the second postoperative week, his fluid-filled blisters completely reepithelialized, but the blood-filled blisters required an additional week for reepithelialization (Figure B). While the patient’s knee was stiff because of limited PT participation, it was not until the second postoperative week when most of the fracture blisters had healed that he was able to resume an intensive knee exercise program, avoiding the need for manipulation under anesthesia.
Discussion
Giordano and colleagues2 identified 2 types of fracture blisters: clear fluid– and blood-filled. While both types involved disruption of the dermoepidermal junction, greater disruption and complete absence of dermal epithelial cells was observed in the hemorrhagic type. Clinical follow-up of the patients in the study by Giordano and colleagues2 showed that the mean time for reepithelialization was 12 days for fluid-filled blisters and 16 days for blood-filled blisters. These findings are similar to what we observed in our case report. In particular, the fluid-filled blisters healed in 2 weeks, whereas the blood-filled blisters required 3 weeks to heal.
The etiology of the fracture blisters in this patient is likely multifactorial and related to age, obesity, venous insufficiency, and diabetes mellitus. Farage and colleagues4 described a series of progressive degenerative changes in the aging skin, including vascular atrophy and degradation of dermal connective tissue, leading to compromised skin competence. The integrity of the dermis can be further reduced in patients with diabetes through glycosylation of collagen fibrils.5 Compared with age-matched normal controls, patients with insulin-dependent diabetes have a reduced threshold to suction-induced blister formation.6 Obesity is another potential contributing factor, with multiple studies showing significantly impaired venous flow in obese patients.7,8 Taken together, soft-tissue swelling after surgery in the setting of chronic venous insufficiency and compromised skin due to advanced age and diabetes may lead to markedly elevated interstitial pressure. One mechanism to relieve such abnormally high pressure is the formation of fracture blisters.1
Surgical risk factors that could have contributed to the complication in this case include the surgical skin preparation solution (ChloraPrep; CareFusion), use of adhesive antimicrobial drape (Ioban, 3M), tourniquet time, dressing choice, and DVT prophylaxis regimen. While the skin preparation solution is an unlikely culprit since the presentation is not consistent with contact dermatitis, inappropriate strapping or removal of the adhesive drape could result in stretch injury of the skin, shearing the dermoepidermal junction and causing tension blisters.9 There were no intraoperative complications and the tourniquet time was appropriate (78 minutes). Postoperatively, no compressive or adhesive dressings were used. With regards to DVT prophylaxis, the patient received a single dose of enoxaparin on the first postoperative day. While heparin-induced hemorrhagic blisters have been reported,10 I do not feel that the use of enoxaparin was a contributing factor. Heparin-induced blisters have been described as systemic blisters,10 whereas the blisters in this case were confined to the operative extremity. The patient was not taking any nutritional supplements (eg, fish oil, vitamin E) that could have increased his risk of bleeding. Throughout his hospital stay, he was hemodynamically stable and did not require blood transfusion.
Management of fracture blisters is controversial, and there is no consensus on appropriate soft-tissue handling. In this patient, the blisters were left intact. Blister fluid has been shown to be sterile, containing growth factors, opsonins, and activated neutrophils that aid in healing and infection prevention.1 Giordano and Koval11 found no difference in the outcome of 3 soft-tissue treatment techniques: (1) aspiration of the blister, (2) deroofing of the blister followed by application of a topical antibiotic cream or coverage with nonadherent dressing, or (3) keeping the blister intact and covered with loose dressing or exposed to air. In contrast, Strauss and colleagues12 found that deroofing the fracture blister to healthy tissue followed by twice-daily application of silver sulfadiazine antibiotic cream promoted reepithelialization and resulted in better cosmetic appearance and higher patient satisfaction.
The optimal dressing for fracture blisters remains elusive. Madden and colleagues13 showed that the use of occlusive nonadherent dressing was associated with significantly faster healing and less pain compared with semiocclusive, antibiotic-impregnated dressings. In another study, Varela and colleagues1 found no differences in blister healing between patients treated with either (1) dry dressing and casting, (2) Silvadene dressing (King Pharmaceuticals), or (3) whirlpool débridement and Silvadene dressing.
Infection is perhaps the most dreaded complication of fracture blisters after TKA. Varela and colleagues1 showed that, while the fluid in intact blisters was a sterile transudate, polymicrobial colonization with skin flora often occurred soon after blister rupture and persisted until reepithelialization. Our patient received a 10-day course of prophylactic antibiotics and no superficial or deep infection developed; however, the real contribution of antibiotic prophylaxis to the absence of infection cannot be established based solely on 1 case.
Pain is another concern associated with fracture blisters. Our patient had significant pain that limited his ability to participate in PT, resulting in limited knee range of motion and eventual discharge to a short-term rehabilitation facility. Fortunately, after resolution of the fracture blisters, he was able to participate in an aggressive rehabilitation program. By 6 weeks after surgery, he had significant improvement in his knee motion, avoiding the need for manipulation under anesthesia.
Conclusion
This case represents the first reported fracture blisters after primary TKA. The risk of deep surgical site infection, a devastating complication after TKA, is perhaps the most frightening concern of this rare complication. While the etiology and the management are controversial, there is evidence to recommend prophylactic antibiotics after blister rupture and skin desquamation. The decision to withhold DVT prophylaxis should be based on individual patient risk factors and blister type (blood-filled vs clear fluid–filled). Patients should be encouraged to continue knee exercises during reepithelialization to avoid stiffness.
1. Varela CD, Vaughan TK, Carr JB, Slemmons BK. Fracture blisters: clinical and pathological aspects. J Orthop Trauma. 1993;7(5):417-427.
2. Giordano CP, Koval KJ, Zuckerman JD, Desai P. Fracture blisters. Clin Orthop Relat Res. 1994;(307):214-221.
3. Uebbing CM, Walsh M, Miller JB, Abraham M, Arnold C. Fracture blisters. West J Emerg Med. 2011;12(1):131-133.
4. Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol. 2009;10(2):73-86.
5. Quondamatteo F. Skin and diabetes mellitus: what do we know? Cell Tissue Res. 2014;355(1):1-21.
6. Bernstein JE, Levine LE, Medenica MM, Yung CW, Soltani K. Reduced threshold to suction-induced blister formation in insulin-dependent diabetics. J Am Acad Dermatol. 1983;8(6):790-791.
7. Willenberg T, Schumacher A, Amann-Vesti B, et al. Impact of obesity on venous hemodynamics of the lower limbs. J Vasc Surg. 2010;52(3):664-668.
8. van Rij AM, De Alwis CS, Jiang P, et al. Obesity and impaired venous function. Eur J Vasc Endovasc Surg. 2008;35(6):739-744.
9. Polatsch DB, Baskies MA, Hommen JP, Egol KA, Koval KJ. Tape blisters that develop after hip fracture surgery: a retrospective series and a review of the literature. Am J Orthop. 2004;33(9):452-456.
10. Roux J, Duong TA, Ingen-Housz-Oro S, et al. Heparin-induced hemorrhagic blisters. Eur J Dermatol. 2013;23(1):105-107.
11. Giordano CP, Koval KJ. Treatment of fracture blisters: a prospective study of 53 cases. J Orthop Trauma. 1995;9(2):171-176.
12. Strauss EJ, Petrucelli G, Bong M, Koval KJ, Egol KA. Blisters associated with lower-extremity fracture: results of a prospective treatment protocol. J Orthop Trauma. 2006;20(9):618-622.
13. Madden MR, Nolan E, Finkelstein JL, et al. Comparison of an occlusive and a semi-occlusive dressing and the effect of the wound exudate upon keratinocyte proliferation. J Trauma. 1989;29(7):924-930; discussion 930-931.
Fracture blisters are a relatively uncommon complication of high-energy fractures, with an incidence of 2.9%.1 In the lower extremity, fracture blisters almost always occur distal to the knee.1 Histologically, the blisters represent an injury to the dermoepidermal junction.2 On physical examination, there are tense blood- and/or clear fluid–filled bullae overlying markedly swollen and edematous soft tissue,1 resembling a second-degree burn.3 Infection may develop after fracture blisters,1 and this is perhaps the most dreaded complication of total knee arthroplasty (TKA). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 71-year-old man with end-stage osteoarthritis of the right knee underwent an elective TKA with cemented components (Legion PS; Smith & Nephew). His medical history included venous insufficiency, type 2 diabetes mellitus, chronic obstructive sleep apnea, hypertension, morbid obesity (body mass index, 50), and a previous uneventful left TKA. Tourniquet time was 78 minutes and estimated blood loss was 100 mL. An intra-articular drain was used and was removed on the first postoperative day. After wound closure, a soft splint bandage consisting of 2 to 3 layers of cotton and bias wrap was applied. Deep vein thrombosis (DVT) prophylaxis with enoxaparin 40 mg once daily was started on the first postoperative day.
Upon removal of the surgical dressings on the second postoperative day, the anterior leg was found to have a combination of tense clear fluid– and blood-filled blisters on markedly swollen and erythematous skin. The incision was minimally involved (Figure A). There was diffuse 2+ pitting edema with hyperesthesia in the affected skin distal to the knee. Prior to these findings, the patient had complained of increasing pain in his operative leg, but there was no escalation in analgesic requirements. There was no evidence of compartment syndrome on serial examinations. An ultrasound of the lower extremity was negative for DVT. Plain films did not show iatrogenic fractures. There was no intraoperative vascular injury, and the foot pulses remained unchanged between the time the patient was in the preoperative holding unit, the postanesthesia care unit, and the orthopedic ward. The operative leg was treated with elevation and loosely applied Kerlix roll gauze (Kendall, Covidien), but active blister formation continued for another 2 days. A 10-day prophylactic course of trimethoprim/sulfamethoxazole was initiated on the third postoperative day after the blisters started to rupture. The patient was allowed to bear weight as tolerated, but his physical therapy (PT) course was limited by pain and fear “of losing his leg.” He declined several PT sessions and was hesitant to use continuous passive motion. The patient was discharged to a short-term rehabilitation facility with weekly outpatient follow-up. On the second postoperative week, his fluid-filled blisters completely reepithelialized, but the blood-filled blisters required an additional week for reepithelialization (Figure B). While the patient’s knee was stiff because of limited PT participation, it was not until the second postoperative week when most of the fracture blisters had healed that he was able to resume an intensive knee exercise program, avoiding the need for manipulation under anesthesia.
Discussion
Giordano and colleagues2 identified 2 types of fracture blisters: clear fluid– and blood-filled. While both types involved disruption of the dermoepidermal junction, greater disruption and complete absence of dermal epithelial cells was observed in the hemorrhagic type. Clinical follow-up of the patients in the study by Giordano and colleagues2 showed that the mean time for reepithelialization was 12 days for fluid-filled blisters and 16 days for blood-filled blisters. These findings are similar to what we observed in our case report. In particular, the fluid-filled blisters healed in 2 weeks, whereas the blood-filled blisters required 3 weeks to heal.
The etiology of the fracture blisters in this patient is likely multifactorial and related to age, obesity, venous insufficiency, and diabetes mellitus. Farage and colleagues4 described a series of progressive degenerative changes in the aging skin, including vascular atrophy and degradation of dermal connective tissue, leading to compromised skin competence. The integrity of the dermis can be further reduced in patients with diabetes through glycosylation of collagen fibrils.5 Compared with age-matched normal controls, patients with insulin-dependent diabetes have a reduced threshold to suction-induced blister formation.6 Obesity is another potential contributing factor, with multiple studies showing significantly impaired venous flow in obese patients.7,8 Taken together, soft-tissue swelling after surgery in the setting of chronic venous insufficiency and compromised skin due to advanced age and diabetes may lead to markedly elevated interstitial pressure. One mechanism to relieve such abnormally high pressure is the formation of fracture blisters.1
Surgical risk factors that could have contributed to the complication in this case include the surgical skin preparation solution (ChloraPrep; CareFusion), use of adhesive antimicrobial drape (Ioban, 3M), tourniquet time, dressing choice, and DVT prophylaxis regimen. While the skin preparation solution is an unlikely culprit since the presentation is not consistent with contact dermatitis, inappropriate strapping or removal of the adhesive drape could result in stretch injury of the skin, shearing the dermoepidermal junction and causing tension blisters.9 There were no intraoperative complications and the tourniquet time was appropriate (78 minutes). Postoperatively, no compressive or adhesive dressings were used. With regards to DVT prophylaxis, the patient received a single dose of enoxaparin on the first postoperative day. While heparin-induced hemorrhagic blisters have been reported,10 I do not feel that the use of enoxaparin was a contributing factor. Heparin-induced blisters have been described as systemic blisters,10 whereas the blisters in this case were confined to the operative extremity. The patient was not taking any nutritional supplements (eg, fish oil, vitamin E) that could have increased his risk of bleeding. Throughout his hospital stay, he was hemodynamically stable and did not require blood transfusion.
Management of fracture blisters is controversial, and there is no consensus on appropriate soft-tissue handling. In this patient, the blisters were left intact. Blister fluid has been shown to be sterile, containing growth factors, opsonins, and activated neutrophils that aid in healing and infection prevention.1 Giordano and Koval11 found no difference in the outcome of 3 soft-tissue treatment techniques: (1) aspiration of the blister, (2) deroofing of the blister followed by application of a topical antibiotic cream or coverage with nonadherent dressing, or (3) keeping the blister intact and covered with loose dressing or exposed to air. In contrast, Strauss and colleagues12 found that deroofing the fracture blister to healthy tissue followed by twice-daily application of silver sulfadiazine antibiotic cream promoted reepithelialization and resulted in better cosmetic appearance and higher patient satisfaction.
The optimal dressing for fracture blisters remains elusive. Madden and colleagues13 showed that the use of occlusive nonadherent dressing was associated with significantly faster healing and less pain compared with semiocclusive, antibiotic-impregnated dressings. In another study, Varela and colleagues1 found no differences in blister healing between patients treated with either (1) dry dressing and casting, (2) Silvadene dressing (King Pharmaceuticals), or (3) whirlpool débridement and Silvadene dressing.
Infection is perhaps the most dreaded complication of fracture blisters after TKA. Varela and colleagues1 showed that, while the fluid in intact blisters was a sterile transudate, polymicrobial colonization with skin flora often occurred soon after blister rupture and persisted until reepithelialization. Our patient received a 10-day course of prophylactic antibiotics and no superficial or deep infection developed; however, the real contribution of antibiotic prophylaxis to the absence of infection cannot be established based solely on 1 case.
Pain is another concern associated with fracture blisters. Our patient had significant pain that limited his ability to participate in PT, resulting in limited knee range of motion and eventual discharge to a short-term rehabilitation facility. Fortunately, after resolution of the fracture blisters, he was able to participate in an aggressive rehabilitation program. By 6 weeks after surgery, he had significant improvement in his knee motion, avoiding the need for manipulation under anesthesia.
Conclusion
This case represents the first reported fracture blisters after primary TKA. The risk of deep surgical site infection, a devastating complication after TKA, is perhaps the most frightening concern of this rare complication. While the etiology and the management are controversial, there is evidence to recommend prophylactic antibiotics after blister rupture and skin desquamation. The decision to withhold DVT prophylaxis should be based on individual patient risk factors and blister type (blood-filled vs clear fluid–filled). Patients should be encouraged to continue knee exercises during reepithelialization to avoid stiffness.
Fracture blisters are a relatively uncommon complication of high-energy fractures, with an incidence of 2.9%.1 In the lower extremity, fracture blisters almost always occur distal to the knee.1 Histologically, the blisters represent an injury to the dermoepidermal junction.2 On physical examination, there are tense blood- and/or clear fluid–filled bullae overlying markedly swollen and edematous soft tissue,1 resembling a second-degree burn.3 Infection may develop after fracture blisters,1 and this is perhaps the most dreaded complication of total knee arthroplasty (TKA). The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 71-year-old man with end-stage osteoarthritis of the right knee underwent an elective TKA with cemented components (Legion PS; Smith & Nephew). His medical history included venous insufficiency, type 2 diabetes mellitus, chronic obstructive sleep apnea, hypertension, morbid obesity (body mass index, 50), and a previous uneventful left TKA. Tourniquet time was 78 minutes and estimated blood loss was 100 mL. An intra-articular drain was used and was removed on the first postoperative day. After wound closure, a soft splint bandage consisting of 2 to 3 layers of cotton and bias wrap was applied. Deep vein thrombosis (DVT) prophylaxis with enoxaparin 40 mg once daily was started on the first postoperative day.
Upon removal of the surgical dressings on the second postoperative day, the anterior leg was found to have a combination of tense clear fluid– and blood-filled blisters on markedly swollen and erythematous skin. The incision was minimally involved (Figure A). There was diffuse 2+ pitting edema with hyperesthesia in the affected skin distal to the knee. Prior to these findings, the patient had complained of increasing pain in his operative leg, but there was no escalation in analgesic requirements. There was no evidence of compartment syndrome on serial examinations. An ultrasound of the lower extremity was negative for DVT. Plain films did not show iatrogenic fractures. There was no intraoperative vascular injury, and the foot pulses remained unchanged between the time the patient was in the preoperative holding unit, the postanesthesia care unit, and the orthopedic ward. The operative leg was treated with elevation and loosely applied Kerlix roll gauze (Kendall, Covidien), but active blister formation continued for another 2 days. A 10-day prophylactic course of trimethoprim/sulfamethoxazole was initiated on the third postoperative day after the blisters started to rupture. The patient was allowed to bear weight as tolerated, but his physical therapy (PT) course was limited by pain and fear “of losing his leg.” He declined several PT sessions and was hesitant to use continuous passive motion. The patient was discharged to a short-term rehabilitation facility with weekly outpatient follow-up. On the second postoperative week, his fluid-filled blisters completely reepithelialized, but the blood-filled blisters required an additional week for reepithelialization (Figure B). While the patient’s knee was stiff because of limited PT participation, it was not until the second postoperative week when most of the fracture blisters had healed that he was able to resume an intensive knee exercise program, avoiding the need for manipulation under anesthesia.
Discussion
Giordano and colleagues2 identified 2 types of fracture blisters: clear fluid– and blood-filled. While both types involved disruption of the dermoepidermal junction, greater disruption and complete absence of dermal epithelial cells was observed in the hemorrhagic type. Clinical follow-up of the patients in the study by Giordano and colleagues2 showed that the mean time for reepithelialization was 12 days for fluid-filled blisters and 16 days for blood-filled blisters. These findings are similar to what we observed in our case report. In particular, the fluid-filled blisters healed in 2 weeks, whereas the blood-filled blisters required 3 weeks to heal.
The etiology of the fracture blisters in this patient is likely multifactorial and related to age, obesity, venous insufficiency, and diabetes mellitus. Farage and colleagues4 described a series of progressive degenerative changes in the aging skin, including vascular atrophy and degradation of dermal connective tissue, leading to compromised skin competence. The integrity of the dermis can be further reduced in patients with diabetes through glycosylation of collagen fibrils.5 Compared with age-matched normal controls, patients with insulin-dependent diabetes have a reduced threshold to suction-induced blister formation.6 Obesity is another potential contributing factor, with multiple studies showing significantly impaired venous flow in obese patients.7,8 Taken together, soft-tissue swelling after surgery in the setting of chronic venous insufficiency and compromised skin due to advanced age and diabetes may lead to markedly elevated interstitial pressure. One mechanism to relieve such abnormally high pressure is the formation of fracture blisters.1
Surgical risk factors that could have contributed to the complication in this case include the surgical skin preparation solution (ChloraPrep; CareFusion), use of adhesive antimicrobial drape (Ioban, 3M), tourniquet time, dressing choice, and DVT prophylaxis regimen. While the skin preparation solution is an unlikely culprit since the presentation is not consistent with contact dermatitis, inappropriate strapping or removal of the adhesive drape could result in stretch injury of the skin, shearing the dermoepidermal junction and causing tension blisters.9 There were no intraoperative complications and the tourniquet time was appropriate (78 minutes). Postoperatively, no compressive or adhesive dressings were used. With regards to DVT prophylaxis, the patient received a single dose of enoxaparin on the first postoperative day. While heparin-induced hemorrhagic blisters have been reported,10 I do not feel that the use of enoxaparin was a contributing factor. Heparin-induced blisters have been described as systemic blisters,10 whereas the blisters in this case were confined to the operative extremity. The patient was not taking any nutritional supplements (eg, fish oil, vitamin E) that could have increased his risk of bleeding. Throughout his hospital stay, he was hemodynamically stable and did not require blood transfusion.
Management of fracture blisters is controversial, and there is no consensus on appropriate soft-tissue handling. In this patient, the blisters were left intact. Blister fluid has been shown to be sterile, containing growth factors, opsonins, and activated neutrophils that aid in healing and infection prevention.1 Giordano and Koval11 found no difference in the outcome of 3 soft-tissue treatment techniques: (1) aspiration of the blister, (2) deroofing of the blister followed by application of a topical antibiotic cream or coverage with nonadherent dressing, or (3) keeping the blister intact and covered with loose dressing or exposed to air. In contrast, Strauss and colleagues12 found that deroofing the fracture blister to healthy tissue followed by twice-daily application of silver sulfadiazine antibiotic cream promoted reepithelialization and resulted in better cosmetic appearance and higher patient satisfaction.
The optimal dressing for fracture blisters remains elusive. Madden and colleagues13 showed that the use of occlusive nonadherent dressing was associated with significantly faster healing and less pain compared with semiocclusive, antibiotic-impregnated dressings. In another study, Varela and colleagues1 found no differences in blister healing between patients treated with either (1) dry dressing and casting, (2) Silvadene dressing (King Pharmaceuticals), or (3) whirlpool débridement and Silvadene dressing.
Infection is perhaps the most dreaded complication of fracture blisters after TKA. Varela and colleagues1 showed that, while the fluid in intact blisters was a sterile transudate, polymicrobial colonization with skin flora often occurred soon after blister rupture and persisted until reepithelialization. Our patient received a 10-day course of prophylactic antibiotics and no superficial or deep infection developed; however, the real contribution of antibiotic prophylaxis to the absence of infection cannot be established based solely on 1 case.
Pain is another concern associated with fracture blisters. Our patient had significant pain that limited his ability to participate in PT, resulting in limited knee range of motion and eventual discharge to a short-term rehabilitation facility. Fortunately, after resolution of the fracture blisters, he was able to participate in an aggressive rehabilitation program. By 6 weeks after surgery, he had significant improvement in his knee motion, avoiding the need for manipulation under anesthesia.
Conclusion
This case represents the first reported fracture blisters after primary TKA. The risk of deep surgical site infection, a devastating complication after TKA, is perhaps the most frightening concern of this rare complication. While the etiology and the management are controversial, there is evidence to recommend prophylactic antibiotics after blister rupture and skin desquamation. The decision to withhold DVT prophylaxis should be based on individual patient risk factors and blister type (blood-filled vs clear fluid–filled). Patients should be encouraged to continue knee exercises during reepithelialization to avoid stiffness.
1. Varela CD, Vaughan TK, Carr JB, Slemmons BK. Fracture blisters: clinical and pathological aspects. J Orthop Trauma. 1993;7(5):417-427.
2. Giordano CP, Koval KJ, Zuckerman JD, Desai P. Fracture blisters. Clin Orthop Relat Res. 1994;(307):214-221.
3. Uebbing CM, Walsh M, Miller JB, Abraham M, Arnold C. Fracture blisters. West J Emerg Med. 2011;12(1):131-133.
4. Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol. 2009;10(2):73-86.
5. Quondamatteo F. Skin and diabetes mellitus: what do we know? Cell Tissue Res. 2014;355(1):1-21.
6. Bernstein JE, Levine LE, Medenica MM, Yung CW, Soltani K. Reduced threshold to suction-induced blister formation in insulin-dependent diabetics. J Am Acad Dermatol. 1983;8(6):790-791.
7. Willenberg T, Schumacher A, Amann-Vesti B, et al. Impact of obesity on venous hemodynamics of the lower limbs. J Vasc Surg. 2010;52(3):664-668.
8. van Rij AM, De Alwis CS, Jiang P, et al. Obesity and impaired venous function. Eur J Vasc Endovasc Surg. 2008;35(6):739-744.
9. Polatsch DB, Baskies MA, Hommen JP, Egol KA, Koval KJ. Tape blisters that develop after hip fracture surgery: a retrospective series and a review of the literature. Am J Orthop. 2004;33(9):452-456.
10. Roux J, Duong TA, Ingen-Housz-Oro S, et al. Heparin-induced hemorrhagic blisters. Eur J Dermatol. 2013;23(1):105-107.
11. Giordano CP, Koval KJ. Treatment of fracture blisters: a prospective study of 53 cases. J Orthop Trauma. 1995;9(2):171-176.
12. Strauss EJ, Petrucelli G, Bong M, Koval KJ, Egol KA. Blisters associated with lower-extremity fracture: results of a prospective treatment protocol. J Orthop Trauma. 2006;20(9):618-622.
13. Madden MR, Nolan E, Finkelstein JL, et al. Comparison of an occlusive and a semi-occlusive dressing and the effect of the wound exudate upon keratinocyte proliferation. J Trauma. 1989;29(7):924-930; discussion 930-931.
1. Varela CD, Vaughan TK, Carr JB, Slemmons BK. Fracture blisters: clinical and pathological aspects. J Orthop Trauma. 1993;7(5):417-427.
2. Giordano CP, Koval KJ, Zuckerman JD, Desai P. Fracture blisters. Clin Orthop Relat Res. 1994;(307):214-221.
3. Uebbing CM, Walsh M, Miller JB, Abraham M, Arnold C. Fracture blisters. West J Emerg Med. 2011;12(1):131-133.
4. Farage MA, Miller KW, Berardesca E, Maibach HI. Clinical implications of aging skin: cutaneous disorders in the elderly. Am J Clin Dermatol. 2009;10(2):73-86.
5. Quondamatteo F. Skin and diabetes mellitus: what do we know? Cell Tissue Res. 2014;355(1):1-21.
6. Bernstein JE, Levine LE, Medenica MM, Yung CW, Soltani K. Reduced threshold to suction-induced blister formation in insulin-dependent diabetics. J Am Acad Dermatol. 1983;8(6):790-791.
7. Willenberg T, Schumacher A, Amann-Vesti B, et al. Impact of obesity on venous hemodynamics of the lower limbs. J Vasc Surg. 2010;52(3):664-668.
8. van Rij AM, De Alwis CS, Jiang P, et al. Obesity and impaired venous function. Eur J Vasc Endovasc Surg. 2008;35(6):739-744.
9. Polatsch DB, Baskies MA, Hommen JP, Egol KA, Koval KJ. Tape blisters that develop after hip fracture surgery: a retrospective series and a review of the literature. Am J Orthop. 2004;33(9):452-456.
10. Roux J, Duong TA, Ingen-Housz-Oro S, et al. Heparin-induced hemorrhagic blisters. Eur J Dermatol. 2013;23(1):105-107.
11. Giordano CP, Koval KJ. Treatment of fracture blisters: a prospective study of 53 cases. J Orthop Trauma. 1995;9(2):171-176.
12. Strauss EJ, Petrucelli G, Bong M, Koval KJ, Egol KA. Blisters associated with lower-extremity fracture: results of a prospective treatment protocol. J Orthop Trauma. 2006;20(9):618-622.
13. Madden MR, Nolan E, Finkelstein JL, et al. Comparison of an occlusive and a semi-occlusive dressing and the effect of the wound exudate upon keratinocyte proliferation. J Trauma. 1989;29(7):924-930; discussion 930-931.
Giant Solitary Synovial Chondromatosis Mimicking Chondrosarcoma: Report of a Rare Histologic Presentation and Literature Review
Synovial chondromatosis (SCM) is a relatively rare benign lesion of the synovium.1 Its pathogenesis has been thought to be a chondral metaplasia of the subintimal layer of the intra- or extra-articular synovium.2 However, evidence supporting a neoplastic cause of the disease is emerging.3 When intra-articular, any joint can be affected, though large joints are more prone to the disease; the knee, hip, and elbow are the most common locations.4 The synovial layer of tendons or bursae can be the origin of extra-articular SCM.5
Synovial chondrosarcoma (SCS), an even rarer pathology, can be caused by malignant transformation of SCM or can appear de novo on a synovial background.6 Histologic differentiation from SCM might be difficult because of the high incidence of hypercellularity, cellular atypia, and binucleated cells.6 Some features, such as presence of a very large mass or erosion of the surrounding bones, have been indicated as possible signs of malignancy.3 An unusual presentation of SCM, giant solitary synovial chondromatosis (GSSCM), can be hard to distinguish from SCS because of the large volume and possible aggressive radiologic findings.7 Some histologic features, such as presence of necrosis and mitotic cells, have been suggested as distinctive criteria for malignancy.8
In this article, we present a case of benign GSSCM with a histologic feature that has not been considered typical for benign SCM. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
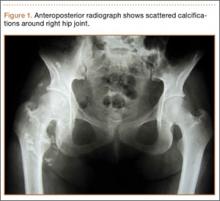
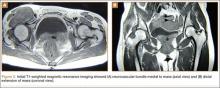
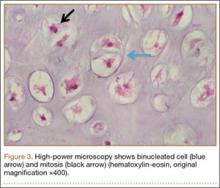
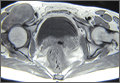
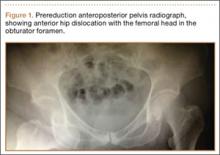
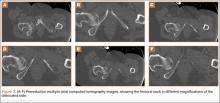
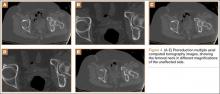
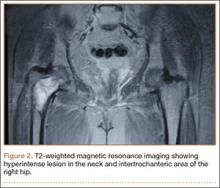
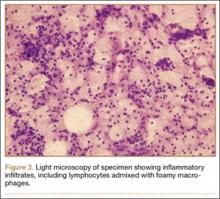
An 18-year-old woman presented with a large mass over the right hip. The mass had been growing slowly for 2 years. One year before presentation, a radiograph showed a large hip mass with fluffy calcification (Figure 1), and magnetic resonance imaging (MRI) showed a large nonhomogeneous mass anterior to the hip capsule and extending into the hip joint back to the posterior part of the joint (Figures 2A, 2B). Open incisional biopsy was performed in a local hospital at the time, and the histologic analysis revealed presence of atypical binucleated cells and pleomorphism, in addition to some mitotic activity (0 to 1 per high-power field) (Figure 3). These findings suggested malignancy. The patient declined surgery up until the time she presented to our hospital, 1 year later.
Clinical examination findings on admission to our hospital were striking. The patient had a large mass in the groin region. It was fairly tender and firm to palpation, immobile, and close to the skin. Hip motion was mildly painful but obviously restricted.
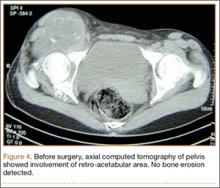
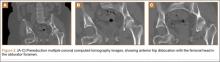
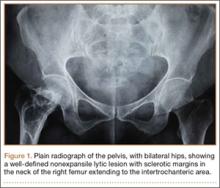
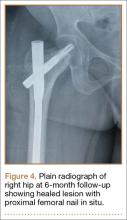
The mass was restaged. New radiographs and MRI did not show any significant changes since the previous year, computed tomography (CT) did not show any bone erosion (Figure 4), and chest radiograph, CT, and whole-body bone scan did not demonstrate any signs of metastasis.
Given the clinical presentation and previous histopathologic findings, a diagnosis of GSSCM with possible malignant transformation was made. The patient was scheduled for surgery. During surgery, the tumor was exposed through the Smith-Petersen approach. The mass was extruding under the fascia between the femoral neurovascular bundle medially and iliopsoas muscle laterally. There was no adhesion of the surrounding structures, including the femoral neurovascular bundle, to the mass. The muscle was sitting on the anterolateral surface of the mass, which was considered located in the iliopsoas bursa but extending to the joint. In the vertical plane, the mass extended down to the subtrochanteric area. The entire solid extra-articular mass was excised en bloc, and hip capsulotomy was performed inferior to the area of emergence of the mass. The joint was occupied by a single solid cartilaginous mass molding around the femoral neck, filling the piriformis fossa and propagating to the posterior joint space. Obtaining enough exposure to the back of the joint required surgical hip dislocation. The visualized acetabular fossa revealed chondral fragments, which were excised. Bone erosion or significant osteoarthritis was not detected in any part of the joint. A nearly total synovectomy was performed, leaving the ascending retinacular vessels intact. Meticulous technique was used to avoid contaminating the extra-articular tissues. The wound was closed in the routine way after hip relocation.
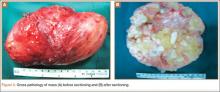
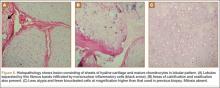
The 16×9.5×9-cm mass (Figure 5A) had a conglomerated internal structure (Figure 5B). Multiple specimens from the intra- and extra-articular portions of the mass were sent for histopathologic analysis, which revealed clusters of mature chondrocytes arranged in a lobular pattern and separated by thin fibrous bands. Areas of calcification and ossification were appreciated as well (Figures 6A-6C). No necrosis, mitosis, or bone permeation was detected. These findings were compatible with typical SCM. Given these pathologic findings and the lack of clinical deterioration over the previous year, a diagnosis of GSSCM with extension along the iliopsoas and obturator externus bursae was made. The already-performed marginal excision was deemed sufficient treatment. At most recent follow-up, 38 months after surgery, the patient was pain-free and had good hip range of motion and no indication of recurrence.
Discussion
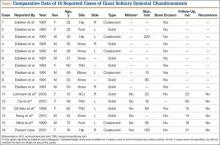
SCM is a benign disorder emerging from the synovium as a result of proliferative changes in the synovial membrane of the joints, tendon sheaths, or bursae, leading to the formation of numerous cartilaginous nodules, usually a few millimeters in diameter.8 In a rare presentation of the disease, the nodules may coalesce to form a large mass, or a single cartilaginous nodule may enlarge to form a mass. Edeiken and colleagues7 named this previously unrecognized SCM feature as GSSCM when there was a major single mass larger than 1 cm in diameter. There have been other SCM cases with multiple giant masses.9,10 In the English-language literature, we found 15 GSSCM cases, which include the first reported, by Edeiken and colleagues7 (Table). However, earlier SCM cases would be reclassified GSSCM according to their definition.11
The present case brings the total to 16. Nine of the 16 patients were male. Mean age at presentation was 41 years (range, 10-80 years). The knee was the most common GSSCM site (6 cases), followed by the temporomandibular and hip joints (3 each). Regarding gross pathology, 10 lesions were solid, and 6 (including the present one) were formed by conglomeration of the chondromatosis nodules. Lesions varied in size (16-200 mm), and 2 were primarily extra-articular (foot). One common issue with most of the cases was the initial diagnosis of chondrosarcoma. The exact surgical technique used was described for 6 cases (cases 11-16); the technique was marginal excision. In no case was recurrence 14 to 60 months after surgery reported.
This chondroproliferative process is potentially a diagnostic challenge, as distinguishing it from a chondrosarcoma, a more common lesion, could be difficult based on clinical and imaging findings, and, as is true for other chondral lesions, even histologic differentiation of the conditions might not be conclusive.12,13 Confusion in diagnosis was almost universal in this series of patients.
One important differentiating feature of benign and malignant skeletal lesions is the time course of the disease. Malignant tumors are expected to demonstrate rapid enlargement and local or systemic spread. Unfortunately, often SCS cannot be distinguished by this characteristic, as grade I or II chondrosarcoma is usually a slow-growing tumor and does not metastasize early.14 Although lack of recurrence is assuring, recurrence is not necessarily a sign of malignancy, as a considerable percentage of benign chondromatosis lesions recur.8
Radiologic differentiation between SCM and SCS is another challenge. Although bone erosion caused by a lesion not originating from bone is usually considered a sign of malignancy, GSSCM was reported as causing bone erosion in 5 of the 16 cases in our literature review.7,15 Our patient did not experience any bone erosion. However, lack of bone erosion is not a reliable criterion for excluding SCS, and bone erosion was noted in only 3 of the 9 SCS cases in the series reported by Bertoni and colleagues.6 Moreover, tumor size and propagation of tumor to surrounding tissue could be surprising in GSSCM. Large size (up to 20 cm) and extra-articular spread of a lesion originating in a joint are common findings.6,16 Our case was an obvious extension of a hip GSSCM to the iliopsoas and obturator externus bursa, which is the most common pattern of extracapsular spread of hip SCM.17 An interesting feature of the present case, however, was the relatively superficial location of the mass immediately under the fascia.
Calcified matrix is key in diagnosing a chondral lesion on imaging studies, but, in some cases, SCM does not demonstrate any radiographically detectable calcification at time of diagnosis.18 However, all the GSSCM cases reported to date had obvious calcified matrix.
The hypercellularity, cellular atypia, binucleated cells, and pleomorphism in the histologic examination of the present case are not features of malignancy in SCM.8 On the contrary, several other characteristics, including qualitative differences in the arrangement of chondrocytes (sheets rather than clusters), myxoid matrix, hypercellularity with crowding and spindling of the nuclei at the periphery, necrosis, and, most important, permeation of the trabecular bone with the filling up of marrow spaces, have been assumed to be indicative of malignancy.8 Furthermore, Davis and colleagues8 found no mitotic activity in the histopathologic investigation of 53 SCM cases. Even in 3 cases that developed malignant transformation to SCS, mitosis was not found in the initial biopsy specimens before transformation. This was compatible with the common opinion that SCM is not a neoplastic, but a metaplastic, process. Histopathologic data were available for only 8 of the previous 15 GSSCM cases. There were no reports of mitosis, and necrosis was found in only 1 case.16 In our patient’s case, however, the first biopsy did show remarkable mitotic activity. This was not the case for the second biopsy, when mature chondrocytes associated with marked calcification and ossification were prominent features (Figures 6A, 6B). We presume that, within a limited period during earlier stages of tissue maturation in SCM, mitotic activity might be a possible finding. Of note, none of the other aforementioned histologic criteria for malignancy was seen in the first or second biopsy in the present case (Figures 3, 6C).
The original idea that SCM originates from a metaplasia in the subintimal layer of the synovium, where the synovium is in direct contact with the articular cartilage, has been challenged. The high incidence of hypercellularity, binucleated cells, and cellular atypia was always an argument against a metaplastic origin for the disease. Evidence of clonal chromosomal changes, like translocation of chromosome 1218 and chromosome 5 and 6 abnormalities,19,20 in addition to other alterations,19,21 provide some evidence supporting a neoplastic rather than a metaplastic origin for SCM. Given the presence of mitosis in the present case, the lack of mitotic activity in SCM, as stated by other authors,22 is not a universal feature and cannot be used as an argument against a neoplastic origin for SCM.
Although mitotic activity is uncommon in SCM, the present case illustrates the possible presence of mitotic activity in GSSCM. The simple presence of mitotic activity, a common finding in some other chondral tumors,23,24 does not preclude the diagnosis of benign SCM, as suggested before,8 and correlation of the clinical and radiologic manifestations with histopathologic findings is crucial for a correct diagnosis.
1. Milgram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59(6):792-801.
2. Trias A, Quintana O. Synovial chondrometaplasia: review of world literature and a study of 18 Canadian cases. Can J Surg. 1976;19(2):151-158.
3. Murphey MD, Vidal JA, Fanburg-Smith JC, Gajewski DA. Imaging of synovial chondromatosis with radiologic-pathologic correlation. Radiographics. 2007;27(5):1465-1488.
4. Milgram JW. Synovial osteochondromatosis in association with Legg-Calve-Perthes disease. Clin Orthop Relat Res. 1979;(145):179-182.
5. Sim FH, Dahlin DC, Ivins JC. Extra-articular synovial chondromatosis. J Bone Joint Surg Am. 1977;59(4):492-495.
6. Bertoni F, Unni KK, Beabout JW, Sim FH. Chondrosarcomas of the synovium. Cancer. 1991;67(1):155-162.
7. Edeiken J, Edeiken BS, Ayala AG, Raymond AK, Murray JA, Guo SQ. Giant solitary synovial chondromatosis. Skeletal Radiol. 1994;23(1):23-29.
8. Davis RI, Hamilton A, Biggart JD. Primary synovial chondromatosis: a clinicopathologic review and assessment of malignant potential. Hum Pathol. 1998;29(7):683-688.
9. Goel A, Cullen C, Paul AS, Freemont AJ. Multiple giant synovial chondromatosis of the knee. Knee. 2001;8(3):243-245.
10. Dogan A, Harman M, Uslu M, Bayram I, Akpinar F. Rocky form giant synovial chondromatosis: a case report. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):465-468.
11. Eisenberg KS, Johnston JO. Synovial chondromatosis of the hip joint presenting as an intrapelvic mass: a case report. J Bone Joint Surg Am. 1972;54(1):176-178.
12. Lohmann CH, Köster G, Klinger HM, Kunze E. Giant synovial osteochondromatosis of the acromio-clavicular joint in a child. A case report and review of the literature. J Pediatr Orthop B. 2005;14(2):126-128.
13. Cai XY, Yang C, Chen MJ, Jiang B, Wang BL. Arthroscopically guided removal of large solitary synovial chondromatosis from the temporomandibular joint. Int J Oral Maxillofac Surg. 2010;39(12):1236-1239.
14. Gil-Salu JL, Lazaro R, Aldasoro J, Gonzalez-Darder JM. Giant solitary synovial chondromatosis of the temporomandibular joint with intracranial extension. Skull Base Surg. 1998;8(2):99-104.
15. Kang CH, Park JH, Lee DH, Kim CH, Park JM, Lee WS. Giant synovial chondromatosis of the knee mimicking a parosteal osteosarcoma: a case report. J Korean Bone Joint Tumor Soc. 2010;16(2):95-98.
16. Nihal A, Read CJ, Henderson DC, Malcolm AJ. Extra-articular giant solitary synovial chondromatosis of the foot: a case report and literature review. Foot Ankle Surg. 1999;5(1):29-32.
17. Robinson P, White LM, Kandel R, Bell RS, Wunder JS. Primary synovial osteochondromatosis of the hip: extracapsular patterns of spread. Skeletal Radiol. 2004;33(4):210-215.
18. Tallini G, Dorfman H, Brys P, et al. Correlation between clinicopathological features and karyotype in 100 cartilaginous and chordoid tumours. A report from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. J Pathol. 2002;196(2):194-203.
19. Sah AP, Geller DS, Mankin HJ, et al. Malignant transformation of synovial chondromatosis of the shoulder to chondrosarcoma. A case report. J Bone Joint Surg Am. 2007;89(6):1321-1328.
20. Buddingh EP, Krallman P, Neff JR, Nelson M, Liu J, Bridge JA. Chromosome 6 abnormalities are recurrent in synovial chondromatosis. Cancer Genet Cytogenet. 2003;140(1):18-22.
21. Rizzo M, Ghert MA, Harrelson JM, Scully SP. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res. 2001;(391):224-233.
22. Davis RI, Foster H, Arthur K, Trewin S, Hamilton PW, Biggart DJ. Cell proliferation studies in primary synovial chondromatosis. J Pathol. 1998;184(1):18-23.
23. Ishikawa E, Tsuboi K, Onizawa K, et al. Chondroblastoma of the temporal base with high mitotic activity. Neurol Med Chir (Tokyo). 2002;42(11):516-520.
24. Kirin I, Jurisic D, Mokrovic H, Stanec Z, Stalekar H. Chondromyxoid fibroma of the second metacarpal bone—a case report. Coll Antropol. 2011;35(3):929-931.
Synovial chondromatosis (SCM) is a relatively rare benign lesion of the synovium.1 Its pathogenesis has been thought to be a chondral metaplasia of the subintimal layer of the intra- or extra-articular synovium.2 However, evidence supporting a neoplastic cause of the disease is emerging.3 When intra-articular, any joint can be affected, though large joints are more prone to the disease; the knee, hip, and elbow are the most common locations.4 The synovial layer of tendons or bursae can be the origin of extra-articular SCM.5
Synovial chondrosarcoma (SCS), an even rarer pathology, can be caused by malignant transformation of SCM or can appear de novo on a synovial background.6 Histologic differentiation from SCM might be difficult because of the high incidence of hypercellularity, cellular atypia, and binucleated cells.6 Some features, such as presence of a very large mass or erosion of the surrounding bones, have been indicated as possible signs of malignancy.3 An unusual presentation of SCM, giant solitary synovial chondromatosis (GSSCM), can be hard to distinguish from SCS because of the large volume and possible aggressive radiologic findings.7 Some histologic features, such as presence of necrosis and mitotic cells, have been suggested as distinctive criteria for malignancy.8
In this article, we present a case of benign GSSCM with a histologic feature that has not been considered typical for benign SCM. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old woman presented with a large mass over the right hip. The mass had been growing slowly for 2 years. One year before presentation, a radiograph showed a large hip mass with fluffy calcification (Figure 1), and magnetic resonance imaging (MRI) showed a large nonhomogeneous mass anterior to the hip capsule and extending into the hip joint back to the posterior part of the joint (Figures 2A, 2B). Open incisional biopsy was performed in a local hospital at the time, and the histologic analysis revealed presence of atypical binucleated cells and pleomorphism, in addition to some mitotic activity (0 to 1 per high-power field) (Figure 3). These findings suggested malignancy. The patient declined surgery up until the time she presented to our hospital, 1 year later.
Clinical examination findings on admission to our hospital were striking. The patient had a large mass in the groin region. It was fairly tender and firm to palpation, immobile, and close to the skin. Hip motion was mildly painful but obviously restricted.
The mass was restaged. New radiographs and MRI did not show any significant changes since the previous year, computed tomography (CT) did not show any bone erosion (Figure 4), and chest radiograph, CT, and whole-body bone scan did not demonstrate any signs of metastasis.
Given the clinical presentation and previous histopathologic findings, a diagnosis of GSSCM with possible malignant transformation was made. The patient was scheduled for surgery. During surgery, the tumor was exposed through the Smith-Petersen approach. The mass was extruding under the fascia between the femoral neurovascular bundle medially and iliopsoas muscle laterally. There was no adhesion of the surrounding structures, including the femoral neurovascular bundle, to the mass. The muscle was sitting on the anterolateral surface of the mass, which was considered located in the iliopsoas bursa but extending to the joint. In the vertical plane, the mass extended down to the subtrochanteric area. The entire solid extra-articular mass was excised en bloc, and hip capsulotomy was performed inferior to the area of emergence of the mass. The joint was occupied by a single solid cartilaginous mass molding around the femoral neck, filling the piriformis fossa and propagating to the posterior joint space. Obtaining enough exposure to the back of the joint required surgical hip dislocation. The visualized acetabular fossa revealed chondral fragments, which were excised. Bone erosion or significant osteoarthritis was not detected in any part of the joint. A nearly total synovectomy was performed, leaving the ascending retinacular vessels intact. Meticulous technique was used to avoid contaminating the extra-articular tissues. The wound was closed in the routine way after hip relocation.
The 16×9.5×9-cm mass (Figure 5A) had a conglomerated internal structure (Figure 5B). Multiple specimens from the intra- and extra-articular portions of the mass were sent for histopathologic analysis, which revealed clusters of mature chondrocytes arranged in a lobular pattern and separated by thin fibrous bands. Areas of calcification and ossification were appreciated as well (Figures 6A-6C). No necrosis, mitosis, or bone permeation was detected. These findings were compatible with typical SCM. Given these pathologic findings and the lack of clinical deterioration over the previous year, a diagnosis of GSSCM with extension along the iliopsoas and obturator externus bursae was made. The already-performed marginal excision was deemed sufficient treatment. At most recent follow-up, 38 months after surgery, the patient was pain-free and had good hip range of motion and no indication of recurrence.
Discussion
SCM is a benign disorder emerging from the synovium as a result of proliferative changes in the synovial membrane of the joints, tendon sheaths, or bursae, leading to the formation of numerous cartilaginous nodules, usually a few millimeters in diameter.8 In a rare presentation of the disease, the nodules may coalesce to form a large mass, or a single cartilaginous nodule may enlarge to form a mass. Edeiken and colleagues7 named this previously unrecognized SCM feature as GSSCM when there was a major single mass larger than 1 cm in diameter. There have been other SCM cases with multiple giant masses.9,10 In the English-language literature, we found 15 GSSCM cases, which include the first reported, by Edeiken and colleagues7 (Table). However, earlier SCM cases would be reclassified GSSCM according to their definition.11
The present case brings the total to 16. Nine of the 16 patients were male. Mean age at presentation was 41 years (range, 10-80 years). The knee was the most common GSSCM site (6 cases), followed by the temporomandibular and hip joints (3 each). Regarding gross pathology, 10 lesions were solid, and 6 (including the present one) were formed by conglomeration of the chondromatosis nodules. Lesions varied in size (16-200 mm), and 2 were primarily extra-articular (foot). One common issue with most of the cases was the initial diagnosis of chondrosarcoma. The exact surgical technique used was described for 6 cases (cases 11-16); the technique was marginal excision. In no case was recurrence 14 to 60 months after surgery reported.
This chondroproliferative process is potentially a diagnostic challenge, as distinguishing it from a chondrosarcoma, a more common lesion, could be difficult based on clinical and imaging findings, and, as is true for other chondral lesions, even histologic differentiation of the conditions might not be conclusive.12,13 Confusion in diagnosis was almost universal in this series of patients.
One important differentiating feature of benign and malignant skeletal lesions is the time course of the disease. Malignant tumors are expected to demonstrate rapid enlargement and local or systemic spread. Unfortunately, often SCS cannot be distinguished by this characteristic, as grade I or II chondrosarcoma is usually a slow-growing tumor and does not metastasize early.14 Although lack of recurrence is assuring, recurrence is not necessarily a sign of malignancy, as a considerable percentage of benign chondromatosis lesions recur.8
Radiologic differentiation between SCM and SCS is another challenge. Although bone erosion caused by a lesion not originating from bone is usually considered a sign of malignancy, GSSCM was reported as causing bone erosion in 5 of the 16 cases in our literature review.7,15 Our patient did not experience any bone erosion. However, lack of bone erosion is not a reliable criterion for excluding SCS, and bone erosion was noted in only 3 of the 9 SCS cases in the series reported by Bertoni and colleagues.6 Moreover, tumor size and propagation of tumor to surrounding tissue could be surprising in GSSCM. Large size (up to 20 cm) and extra-articular spread of a lesion originating in a joint are common findings.6,16 Our case was an obvious extension of a hip GSSCM to the iliopsoas and obturator externus bursa, which is the most common pattern of extracapsular spread of hip SCM.17 An interesting feature of the present case, however, was the relatively superficial location of the mass immediately under the fascia.
Calcified matrix is key in diagnosing a chondral lesion on imaging studies, but, in some cases, SCM does not demonstrate any radiographically detectable calcification at time of diagnosis.18 However, all the GSSCM cases reported to date had obvious calcified matrix.
The hypercellularity, cellular atypia, binucleated cells, and pleomorphism in the histologic examination of the present case are not features of malignancy in SCM.8 On the contrary, several other characteristics, including qualitative differences in the arrangement of chondrocytes (sheets rather than clusters), myxoid matrix, hypercellularity with crowding and spindling of the nuclei at the periphery, necrosis, and, most important, permeation of the trabecular bone with the filling up of marrow spaces, have been assumed to be indicative of malignancy.8 Furthermore, Davis and colleagues8 found no mitotic activity in the histopathologic investigation of 53 SCM cases. Even in 3 cases that developed malignant transformation to SCS, mitosis was not found in the initial biopsy specimens before transformation. This was compatible with the common opinion that SCM is not a neoplastic, but a metaplastic, process. Histopathologic data were available for only 8 of the previous 15 GSSCM cases. There were no reports of mitosis, and necrosis was found in only 1 case.16 In our patient’s case, however, the first biopsy did show remarkable mitotic activity. This was not the case for the second biopsy, when mature chondrocytes associated with marked calcification and ossification were prominent features (Figures 6A, 6B). We presume that, within a limited period during earlier stages of tissue maturation in SCM, mitotic activity might be a possible finding. Of note, none of the other aforementioned histologic criteria for malignancy was seen in the first or second biopsy in the present case (Figures 3, 6C).
The original idea that SCM originates from a metaplasia in the subintimal layer of the synovium, where the synovium is in direct contact with the articular cartilage, has been challenged. The high incidence of hypercellularity, binucleated cells, and cellular atypia was always an argument against a metaplastic origin for the disease. Evidence of clonal chromosomal changes, like translocation of chromosome 1218 and chromosome 5 and 6 abnormalities,19,20 in addition to other alterations,19,21 provide some evidence supporting a neoplastic rather than a metaplastic origin for SCM. Given the presence of mitosis in the present case, the lack of mitotic activity in SCM, as stated by other authors,22 is not a universal feature and cannot be used as an argument against a neoplastic origin for SCM.
Although mitotic activity is uncommon in SCM, the present case illustrates the possible presence of mitotic activity in GSSCM. The simple presence of mitotic activity, a common finding in some other chondral tumors,23,24 does not preclude the diagnosis of benign SCM, as suggested before,8 and correlation of the clinical and radiologic manifestations with histopathologic findings is crucial for a correct diagnosis.
Synovial chondromatosis (SCM) is a relatively rare benign lesion of the synovium.1 Its pathogenesis has been thought to be a chondral metaplasia of the subintimal layer of the intra- or extra-articular synovium.2 However, evidence supporting a neoplastic cause of the disease is emerging.3 When intra-articular, any joint can be affected, though large joints are more prone to the disease; the knee, hip, and elbow are the most common locations.4 The synovial layer of tendons or bursae can be the origin of extra-articular SCM.5
Synovial chondrosarcoma (SCS), an even rarer pathology, can be caused by malignant transformation of SCM or can appear de novo on a synovial background.6 Histologic differentiation from SCM might be difficult because of the high incidence of hypercellularity, cellular atypia, and binucleated cells.6 Some features, such as presence of a very large mass or erosion of the surrounding bones, have been indicated as possible signs of malignancy.3 An unusual presentation of SCM, giant solitary synovial chondromatosis (GSSCM), can be hard to distinguish from SCS because of the large volume and possible aggressive radiologic findings.7 Some histologic features, such as presence of necrosis and mitotic cells, have been suggested as distinctive criteria for malignancy.8
In this article, we present a case of benign GSSCM with a histologic feature that has not been considered typical for benign SCM. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old woman presented with a large mass over the right hip. The mass had been growing slowly for 2 years. One year before presentation, a radiograph showed a large hip mass with fluffy calcification (Figure 1), and magnetic resonance imaging (MRI) showed a large nonhomogeneous mass anterior to the hip capsule and extending into the hip joint back to the posterior part of the joint (Figures 2A, 2B). Open incisional biopsy was performed in a local hospital at the time, and the histologic analysis revealed presence of atypical binucleated cells and pleomorphism, in addition to some mitotic activity (0 to 1 per high-power field) (Figure 3). These findings suggested malignancy. The patient declined surgery up until the time she presented to our hospital, 1 year later.
Clinical examination findings on admission to our hospital were striking. The patient had a large mass in the groin region. It was fairly tender and firm to palpation, immobile, and close to the skin. Hip motion was mildly painful but obviously restricted.
The mass was restaged. New radiographs and MRI did not show any significant changes since the previous year, computed tomography (CT) did not show any bone erosion (Figure 4), and chest radiograph, CT, and whole-body bone scan did not demonstrate any signs of metastasis.
Given the clinical presentation and previous histopathologic findings, a diagnosis of GSSCM with possible malignant transformation was made. The patient was scheduled for surgery. During surgery, the tumor was exposed through the Smith-Petersen approach. The mass was extruding under the fascia between the femoral neurovascular bundle medially and iliopsoas muscle laterally. There was no adhesion of the surrounding structures, including the femoral neurovascular bundle, to the mass. The muscle was sitting on the anterolateral surface of the mass, which was considered located in the iliopsoas bursa but extending to the joint. In the vertical plane, the mass extended down to the subtrochanteric area. The entire solid extra-articular mass was excised en bloc, and hip capsulotomy was performed inferior to the area of emergence of the mass. The joint was occupied by a single solid cartilaginous mass molding around the femoral neck, filling the piriformis fossa and propagating to the posterior joint space. Obtaining enough exposure to the back of the joint required surgical hip dislocation. The visualized acetabular fossa revealed chondral fragments, which were excised. Bone erosion or significant osteoarthritis was not detected in any part of the joint. A nearly total synovectomy was performed, leaving the ascending retinacular vessels intact. Meticulous technique was used to avoid contaminating the extra-articular tissues. The wound was closed in the routine way after hip relocation.
The 16×9.5×9-cm mass (Figure 5A) had a conglomerated internal structure (Figure 5B). Multiple specimens from the intra- and extra-articular portions of the mass were sent for histopathologic analysis, which revealed clusters of mature chondrocytes arranged in a lobular pattern and separated by thin fibrous bands. Areas of calcification and ossification were appreciated as well (Figures 6A-6C). No necrosis, mitosis, or bone permeation was detected. These findings were compatible with typical SCM. Given these pathologic findings and the lack of clinical deterioration over the previous year, a diagnosis of GSSCM with extension along the iliopsoas and obturator externus bursae was made. The already-performed marginal excision was deemed sufficient treatment. At most recent follow-up, 38 months after surgery, the patient was pain-free and had good hip range of motion and no indication of recurrence.
Discussion
SCM is a benign disorder emerging from the synovium as a result of proliferative changes in the synovial membrane of the joints, tendon sheaths, or bursae, leading to the formation of numerous cartilaginous nodules, usually a few millimeters in diameter.8 In a rare presentation of the disease, the nodules may coalesce to form a large mass, or a single cartilaginous nodule may enlarge to form a mass. Edeiken and colleagues7 named this previously unrecognized SCM feature as GSSCM when there was a major single mass larger than 1 cm in diameter. There have been other SCM cases with multiple giant masses.9,10 In the English-language literature, we found 15 GSSCM cases, which include the first reported, by Edeiken and colleagues7 (Table). However, earlier SCM cases would be reclassified GSSCM according to their definition.11
The present case brings the total to 16. Nine of the 16 patients were male. Mean age at presentation was 41 years (range, 10-80 years). The knee was the most common GSSCM site (6 cases), followed by the temporomandibular and hip joints (3 each). Regarding gross pathology, 10 lesions were solid, and 6 (including the present one) were formed by conglomeration of the chondromatosis nodules. Lesions varied in size (16-200 mm), and 2 were primarily extra-articular (foot). One common issue with most of the cases was the initial diagnosis of chondrosarcoma. The exact surgical technique used was described for 6 cases (cases 11-16); the technique was marginal excision. In no case was recurrence 14 to 60 months after surgery reported.
This chondroproliferative process is potentially a diagnostic challenge, as distinguishing it from a chondrosarcoma, a more common lesion, could be difficult based on clinical and imaging findings, and, as is true for other chondral lesions, even histologic differentiation of the conditions might not be conclusive.12,13 Confusion in diagnosis was almost universal in this series of patients.
One important differentiating feature of benign and malignant skeletal lesions is the time course of the disease. Malignant tumors are expected to demonstrate rapid enlargement and local or systemic spread. Unfortunately, often SCS cannot be distinguished by this characteristic, as grade I or II chondrosarcoma is usually a slow-growing tumor and does not metastasize early.14 Although lack of recurrence is assuring, recurrence is not necessarily a sign of malignancy, as a considerable percentage of benign chondromatosis lesions recur.8
Radiologic differentiation between SCM and SCS is another challenge. Although bone erosion caused by a lesion not originating from bone is usually considered a sign of malignancy, GSSCM was reported as causing bone erosion in 5 of the 16 cases in our literature review.7,15 Our patient did not experience any bone erosion. However, lack of bone erosion is not a reliable criterion for excluding SCS, and bone erosion was noted in only 3 of the 9 SCS cases in the series reported by Bertoni and colleagues.6 Moreover, tumor size and propagation of tumor to surrounding tissue could be surprising in GSSCM. Large size (up to 20 cm) and extra-articular spread of a lesion originating in a joint are common findings.6,16 Our case was an obvious extension of a hip GSSCM to the iliopsoas and obturator externus bursa, which is the most common pattern of extracapsular spread of hip SCM.17 An interesting feature of the present case, however, was the relatively superficial location of the mass immediately under the fascia.
Calcified matrix is key in diagnosing a chondral lesion on imaging studies, but, in some cases, SCM does not demonstrate any radiographically detectable calcification at time of diagnosis.18 However, all the GSSCM cases reported to date had obvious calcified matrix.
The hypercellularity, cellular atypia, binucleated cells, and pleomorphism in the histologic examination of the present case are not features of malignancy in SCM.8 On the contrary, several other characteristics, including qualitative differences in the arrangement of chondrocytes (sheets rather than clusters), myxoid matrix, hypercellularity with crowding and spindling of the nuclei at the periphery, necrosis, and, most important, permeation of the trabecular bone with the filling up of marrow spaces, have been assumed to be indicative of malignancy.8 Furthermore, Davis and colleagues8 found no mitotic activity in the histopathologic investigation of 53 SCM cases. Even in 3 cases that developed malignant transformation to SCS, mitosis was not found in the initial biopsy specimens before transformation. This was compatible with the common opinion that SCM is not a neoplastic, but a metaplastic, process. Histopathologic data were available for only 8 of the previous 15 GSSCM cases. There were no reports of mitosis, and necrosis was found in only 1 case.16 In our patient’s case, however, the first biopsy did show remarkable mitotic activity. This was not the case for the second biopsy, when mature chondrocytes associated with marked calcification and ossification were prominent features (Figures 6A, 6B). We presume that, within a limited period during earlier stages of tissue maturation in SCM, mitotic activity might be a possible finding. Of note, none of the other aforementioned histologic criteria for malignancy was seen in the first or second biopsy in the present case (Figures 3, 6C).
The original idea that SCM originates from a metaplasia in the subintimal layer of the synovium, where the synovium is in direct contact with the articular cartilage, has been challenged. The high incidence of hypercellularity, binucleated cells, and cellular atypia was always an argument against a metaplastic origin for the disease. Evidence of clonal chromosomal changes, like translocation of chromosome 1218 and chromosome 5 and 6 abnormalities,19,20 in addition to other alterations,19,21 provide some evidence supporting a neoplastic rather than a metaplastic origin for SCM. Given the presence of mitosis in the present case, the lack of mitotic activity in SCM, as stated by other authors,22 is not a universal feature and cannot be used as an argument against a neoplastic origin for SCM.
Although mitotic activity is uncommon in SCM, the present case illustrates the possible presence of mitotic activity in GSSCM. The simple presence of mitotic activity, a common finding in some other chondral tumors,23,24 does not preclude the diagnosis of benign SCM, as suggested before,8 and correlation of the clinical and radiologic manifestations with histopathologic findings is crucial for a correct diagnosis.
1. Milgram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59(6):792-801.
2. Trias A, Quintana O. Synovial chondrometaplasia: review of world literature and a study of 18 Canadian cases. Can J Surg. 1976;19(2):151-158.
3. Murphey MD, Vidal JA, Fanburg-Smith JC, Gajewski DA. Imaging of synovial chondromatosis with radiologic-pathologic correlation. Radiographics. 2007;27(5):1465-1488.
4. Milgram JW. Synovial osteochondromatosis in association with Legg-Calve-Perthes disease. Clin Orthop Relat Res. 1979;(145):179-182.
5. Sim FH, Dahlin DC, Ivins JC. Extra-articular synovial chondromatosis. J Bone Joint Surg Am. 1977;59(4):492-495.
6. Bertoni F, Unni KK, Beabout JW, Sim FH. Chondrosarcomas of the synovium. Cancer. 1991;67(1):155-162.
7. Edeiken J, Edeiken BS, Ayala AG, Raymond AK, Murray JA, Guo SQ. Giant solitary synovial chondromatosis. Skeletal Radiol. 1994;23(1):23-29.
8. Davis RI, Hamilton A, Biggart JD. Primary synovial chondromatosis: a clinicopathologic review and assessment of malignant potential. Hum Pathol. 1998;29(7):683-688.
9. Goel A, Cullen C, Paul AS, Freemont AJ. Multiple giant synovial chondromatosis of the knee. Knee. 2001;8(3):243-245.
10. Dogan A, Harman M, Uslu M, Bayram I, Akpinar F. Rocky form giant synovial chondromatosis: a case report. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):465-468.
11. Eisenberg KS, Johnston JO. Synovial chondromatosis of the hip joint presenting as an intrapelvic mass: a case report. J Bone Joint Surg Am. 1972;54(1):176-178.
12. Lohmann CH, Köster G, Klinger HM, Kunze E. Giant synovial osteochondromatosis of the acromio-clavicular joint in a child. A case report and review of the literature. J Pediatr Orthop B. 2005;14(2):126-128.
13. Cai XY, Yang C, Chen MJ, Jiang B, Wang BL. Arthroscopically guided removal of large solitary synovial chondromatosis from the temporomandibular joint. Int J Oral Maxillofac Surg. 2010;39(12):1236-1239.
14. Gil-Salu JL, Lazaro R, Aldasoro J, Gonzalez-Darder JM. Giant solitary synovial chondromatosis of the temporomandibular joint with intracranial extension. Skull Base Surg. 1998;8(2):99-104.
15. Kang CH, Park JH, Lee DH, Kim CH, Park JM, Lee WS. Giant synovial chondromatosis of the knee mimicking a parosteal osteosarcoma: a case report. J Korean Bone Joint Tumor Soc. 2010;16(2):95-98.
16. Nihal A, Read CJ, Henderson DC, Malcolm AJ. Extra-articular giant solitary synovial chondromatosis of the foot: a case report and literature review. Foot Ankle Surg. 1999;5(1):29-32.
17. Robinson P, White LM, Kandel R, Bell RS, Wunder JS. Primary synovial osteochondromatosis of the hip: extracapsular patterns of spread. Skeletal Radiol. 2004;33(4):210-215.
18. Tallini G, Dorfman H, Brys P, et al. Correlation between clinicopathological features and karyotype in 100 cartilaginous and chordoid tumours. A report from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. J Pathol. 2002;196(2):194-203.
19. Sah AP, Geller DS, Mankin HJ, et al. Malignant transformation of synovial chondromatosis of the shoulder to chondrosarcoma. A case report. J Bone Joint Surg Am. 2007;89(6):1321-1328.
20. Buddingh EP, Krallman P, Neff JR, Nelson M, Liu J, Bridge JA. Chromosome 6 abnormalities are recurrent in synovial chondromatosis. Cancer Genet Cytogenet. 2003;140(1):18-22.
21. Rizzo M, Ghert MA, Harrelson JM, Scully SP. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res. 2001;(391):224-233.
22. Davis RI, Foster H, Arthur K, Trewin S, Hamilton PW, Biggart DJ. Cell proliferation studies in primary synovial chondromatosis. J Pathol. 1998;184(1):18-23.
23. Ishikawa E, Tsuboi K, Onizawa K, et al. Chondroblastoma of the temporal base with high mitotic activity. Neurol Med Chir (Tokyo). 2002;42(11):516-520.
24. Kirin I, Jurisic D, Mokrovic H, Stanec Z, Stalekar H. Chondromyxoid fibroma of the second metacarpal bone—a case report. Coll Antropol. 2011;35(3):929-931.
1. Milgram JW. Synovial osteochondromatosis: a histopathological study of thirty cases. J Bone Joint Surg Am. 1977;59(6):792-801.
2. Trias A, Quintana O. Synovial chondrometaplasia: review of world literature and a study of 18 Canadian cases. Can J Surg. 1976;19(2):151-158.
3. Murphey MD, Vidal JA, Fanburg-Smith JC, Gajewski DA. Imaging of synovial chondromatosis with radiologic-pathologic correlation. Radiographics. 2007;27(5):1465-1488.
4. Milgram JW. Synovial osteochondromatosis in association with Legg-Calve-Perthes disease. Clin Orthop Relat Res. 1979;(145):179-182.
5. Sim FH, Dahlin DC, Ivins JC. Extra-articular synovial chondromatosis. J Bone Joint Surg Am. 1977;59(4):492-495.
6. Bertoni F, Unni KK, Beabout JW, Sim FH. Chondrosarcomas of the synovium. Cancer. 1991;67(1):155-162.
7. Edeiken J, Edeiken BS, Ayala AG, Raymond AK, Murray JA, Guo SQ. Giant solitary synovial chondromatosis. Skeletal Radiol. 1994;23(1):23-29.
8. Davis RI, Hamilton A, Biggart JD. Primary synovial chondromatosis: a clinicopathologic review and assessment of malignant potential. Hum Pathol. 1998;29(7):683-688.
9. Goel A, Cullen C, Paul AS, Freemont AJ. Multiple giant synovial chondromatosis of the knee. Knee. 2001;8(3):243-245.
10. Dogan A, Harman M, Uslu M, Bayram I, Akpinar F. Rocky form giant synovial chondromatosis: a case report. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):465-468.
11. Eisenberg KS, Johnston JO. Synovial chondromatosis of the hip joint presenting as an intrapelvic mass: a case report. J Bone Joint Surg Am. 1972;54(1):176-178.
12. Lohmann CH, Köster G, Klinger HM, Kunze E. Giant synovial osteochondromatosis of the acromio-clavicular joint in a child. A case report and review of the literature. J Pediatr Orthop B. 2005;14(2):126-128.
13. Cai XY, Yang C, Chen MJ, Jiang B, Wang BL. Arthroscopically guided removal of large solitary synovial chondromatosis from the temporomandibular joint. Int J Oral Maxillofac Surg. 2010;39(12):1236-1239.
14. Gil-Salu JL, Lazaro R, Aldasoro J, Gonzalez-Darder JM. Giant solitary synovial chondromatosis of the temporomandibular joint with intracranial extension. Skull Base Surg. 1998;8(2):99-104.
15. Kang CH, Park JH, Lee DH, Kim CH, Park JM, Lee WS. Giant synovial chondromatosis of the knee mimicking a parosteal osteosarcoma: a case report. J Korean Bone Joint Tumor Soc. 2010;16(2):95-98.
16. Nihal A, Read CJ, Henderson DC, Malcolm AJ. Extra-articular giant solitary synovial chondromatosis of the foot: a case report and literature review. Foot Ankle Surg. 1999;5(1):29-32.
17. Robinson P, White LM, Kandel R, Bell RS, Wunder JS. Primary synovial osteochondromatosis of the hip: extracapsular patterns of spread. Skeletal Radiol. 2004;33(4):210-215.
18. Tallini G, Dorfman H, Brys P, et al. Correlation between clinicopathological features and karyotype in 100 cartilaginous and chordoid tumours. A report from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. J Pathol. 2002;196(2):194-203.
19. Sah AP, Geller DS, Mankin HJ, et al. Malignant transformation of synovial chondromatosis of the shoulder to chondrosarcoma. A case report. J Bone Joint Surg Am. 2007;89(6):1321-1328.
20. Buddingh EP, Krallman P, Neff JR, Nelson M, Liu J, Bridge JA. Chromosome 6 abnormalities are recurrent in synovial chondromatosis. Cancer Genet Cytogenet. 2003;140(1):18-22.
21. Rizzo M, Ghert MA, Harrelson JM, Scully SP. Chondrosarcoma of bone: analysis of 108 cases and evaluation for predictors of outcome. Clin Orthop Relat Res. 2001;(391):224-233.
22. Davis RI, Foster H, Arthur K, Trewin S, Hamilton PW, Biggart DJ. Cell proliferation studies in primary synovial chondromatosis. J Pathol. 1998;184(1):18-23.
23. Ishikawa E, Tsuboi K, Onizawa K, et al. Chondroblastoma of the temporal base with high mitotic activity. Neurol Med Chir (Tokyo). 2002;42(11):516-520.
24. Kirin I, Jurisic D, Mokrovic H, Stanec Z, Stalekar H. Chondromyxoid fibroma of the second metacarpal bone—a case report. Coll Antropol. 2011;35(3):929-931.
Congenital Absence of the Anterior Cruciate Ligament
Congenital absence of the anterior cruciate ligament (ACL) is a rare occurrence and has been seen most often in conjunction with conditions such as knee dislocation, knee dysplasia, proximal focal femoral deficiency, and fibular hemimelia.
We report on the incidental finding of ACL aplasia in a patient with a medial meniscal tear and history of leg-length discrepancy. Similar to earlier cases, this patient had hypertrophy of the meniscofemoral ligament of Humphrey, which likely provided stability. This case report emphasizes the importance of distinguishing between a stable and an unstable knee in congenital absence of the ACL. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 20-year-old woman presented for orthopedic evaluation with worsening medial left knee pain. Her pain was intermittent in nature, occurring about every 1 to 2 months and of 1 to 2 days’ duration. Onset was while using the elliptical machine, walking on uneven ground, or navigating stairs. She denied any buckling, catching, locking, instability, or swelling.
Her history was significant for a breech delivery and leg anisomelia, for which she had a contralateral distal femoral and proximal tibial percutaneous epiphysiodesis performed at age 10 years. Family history was negative for limb deformities.
Physical examination was notable for absence of global ligamentous laxity, overall valgus alignment of the left lower extremity, minimally decreased motion, trace effusion, positive medial joint line tenderness, positive McMurray test, and 1+ Lachman test with guarding on pivot shift testing.
Plain films showed valgus alignment with narrowing of the lateral compartment, narrow intercondylar notch, and hypoplasia of the tibial eminences and lateral femoral condyle (Figure 1). Magnetic resonance imaging showed a large tear in the posterior horn of the medial meniscus, hypertrophy of the meniscofemoral ligament of Humphrey (Figure 2A), and nonvisualization of the ACL with a small remnant (Figure 2B).
Arthroscopy showed complete absence of fibers of the ACL, hypertrophy of the meniscofemoral ligament of Humphrey, and a large posterior horn medial meniscal tear. A partial medial meniscectomy was performed. More than 2 years after surgery, the patient was doing very well without pain or instability, and was exercising regularly without difficulty.
Discussion
Our patient had left-sided congenital absence of the ACL with associated limb-length discrepancy of more than 2.5 cm. Isolated absence of the ACL has been described in a few case reports in the literature. Congenital ACL absence has most often been found in association with conditions such as knee dislocation (occurring with a frequency of .017/1000 births),1 knee dysplasia,2,3 fibular hemimelia,4 and proximal focal femoral deficiency.5 Johansson and Aparisi5,6 linked the finding of ACL absence with instability in those patients with known limb-length discrepancy and symptomatic instability. This report presents a patient who has congenital absence of the ACL in a foreshortened limb and torn medial meniscus. The classification of the patient’s cruciate dysplasia would be type I, as described by Manner and colleagues.7 The incidence of meniscal tears in association with congenital ACL absence is unknown. There have been reports of absence of the ACL associated with a ring meniscus,8 absence of both cruciate ligaments and menisci,9 and a bucket-handle tear of the medial meniscus.10
Gabos and colleagues4 recommend reconstructive surgery for patients with congenital absence of the ACL and symptomatic knee instability. Limb lengthening/shortening and realignment procedures have allowed patients such as ours to have functionally anatomic limbs and high activity levels. Surgical treatment is pursued to restore mechanical alignment and stability. Our patient had no symptoms of instability.
Similar to 3 of the 4 patients presented by Gabos and colleagues,4 our patient had marked hypertrophy of the meniscofemoral ligament of Humphrey. The report by Gabos and colleagues4 of this finding was the first in the literature. The hypertrophy of this ligament suggests it has a role in stabilizing the knee with a congenitally absent ACL. Our patient had no instability in her left knee but presented because of episodes of pain.
Of significant concern is the long-term outcome of patients with congenital ACL aplasia. Crawford and colleagues11 reported 11 patients with ACL deficiency and fibular hemimelia at a mean age of 37 years, showing similar functional outcomes to age-matched controls. However, there was no radiographic follow-up reported in regard to the development of osteoarthritis. To our knowledge, there have been no series published comparing surgical and nonsurgical treatment of congenital absence of the ACL. In the study by Gabos and colleagues,4 all patients were treated with reconstruction because these patients had symptomatic instability.
Conclusion
This report presents a patient whose symptoms improved after resection of her medial meniscal tear. This patient will be followed long-term to delineate her clinical course and to monitor for instability and/or development of osteoarthritis. Future studies should compare the treatment of congenital absence of the ACL with reconstruction and with conservative management.
1. Tachdjian MO. Pediatric Orthopedics. 2nd ed. Philadelphia: Saunders; 1990.
2. Thomas NP, Jackson AM, Aichroth PM. Congenital absence of the anterior cruciate ligament: A common component of knee dysplasia. J Bone Joint Surg Br. 1985;67(4):572-575.
3. Hejgaard N, Kjaerulff H. Congenital aplasia of the anterior cruciate ligament. Report of a case in a seven-year-old girl. Int Orthop. 1987;11(3):223-225.
4. Gabos PG, El Rassi G, Pahys J. Knee reconstruction in syndromes with congenital absence of the anterior cruciate ligament. J Pediatr Orthop. 2005;25(2):210-214.
5. Johansson E, Aparisi T. Missing cruciate ligament in congenital short femur. J Bone Joint Surg Am. 1983;65(8):1109-1115.
6. Johannson E, Aparisi T. Congenital absence of the cruciate ligaments. A case report and review of the literature. Clin Orthop Relat Res. 1982;162:108-111.
7. Manner HM, Radler C, Ganger R, Grill F. Dysplasia of the cruciate ligaments: radiographic assessment and classification. J Bone Joint Surg Am. 2006;88(1):130-137.
8. Noble J. Congenital absence of the anterior cruciate ligament associated with a ring meniscus. J Bone Joint Surg Am. 1975;57(8):1165-1166.
9. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
10. Kaelin A, Hulin PH, Carlioz H. Congenital aplasia of the cruciate ligaments. A report of six cases. J Bone Joint Surg Br. 1986;68(5):827-828.
11. Crawford DA, Tompkins BJ, Baird GO, Caskey PM. The long term function of the knee in patients with fibular hemimelia and anterior cruciate ligament deficiency. J Bone Joint Surg Br. 2012;94(3):328-333.
Congenital absence of the anterior cruciate ligament (ACL) is a rare occurrence and has been seen most often in conjunction with conditions such as knee dislocation, knee dysplasia, proximal focal femoral deficiency, and fibular hemimelia.
We report on the incidental finding of ACL aplasia in a patient with a medial meniscal tear and history of leg-length discrepancy. Similar to earlier cases, this patient had hypertrophy of the meniscofemoral ligament of Humphrey, which likely provided stability. This case report emphasizes the importance of distinguishing between a stable and an unstable knee in congenital absence of the ACL. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 20-year-old woman presented for orthopedic evaluation with worsening medial left knee pain. Her pain was intermittent in nature, occurring about every 1 to 2 months and of 1 to 2 days’ duration. Onset was while using the elliptical machine, walking on uneven ground, or navigating stairs. She denied any buckling, catching, locking, instability, or swelling.
Her history was significant for a breech delivery and leg anisomelia, for which she had a contralateral distal femoral and proximal tibial percutaneous epiphysiodesis performed at age 10 years. Family history was negative for limb deformities.
Physical examination was notable for absence of global ligamentous laxity, overall valgus alignment of the left lower extremity, minimally decreased motion, trace effusion, positive medial joint line tenderness, positive McMurray test, and 1+ Lachman test with guarding on pivot shift testing.
Plain films showed valgus alignment with narrowing of the lateral compartment, narrow intercondylar notch, and hypoplasia of the tibial eminences and lateral femoral condyle (Figure 1). Magnetic resonance imaging showed a large tear in the posterior horn of the medial meniscus, hypertrophy of the meniscofemoral ligament of Humphrey (Figure 2A), and nonvisualization of the ACL with a small remnant (Figure 2B).
Arthroscopy showed complete absence of fibers of the ACL, hypertrophy of the meniscofemoral ligament of Humphrey, and a large posterior horn medial meniscal tear. A partial medial meniscectomy was performed. More than 2 years after surgery, the patient was doing very well without pain or instability, and was exercising regularly without difficulty.
Discussion
Our patient had left-sided congenital absence of the ACL with associated limb-length discrepancy of more than 2.5 cm. Isolated absence of the ACL has been described in a few case reports in the literature. Congenital ACL absence has most often been found in association with conditions such as knee dislocation (occurring with a frequency of .017/1000 births),1 knee dysplasia,2,3 fibular hemimelia,4 and proximal focal femoral deficiency.5 Johansson and Aparisi5,6 linked the finding of ACL absence with instability in those patients with known limb-length discrepancy and symptomatic instability. This report presents a patient who has congenital absence of the ACL in a foreshortened limb and torn medial meniscus. The classification of the patient’s cruciate dysplasia would be type I, as described by Manner and colleagues.7 The incidence of meniscal tears in association with congenital ACL absence is unknown. There have been reports of absence of the ACL associated with a ring meniscus,8 absence of both cruciate ligaments and menisci,9 and a bucket-handle tear of the medial meniscus.10
Gabos and colleagues4 recommend reconstructive surgery for patients with congenital absence of the ACL and symptomatic knee instability. Limb lengthening/shortening and realignment procedures have allowed patients such as ours to have functionally anatomic limbs and high activity levels. Surgical treatment is pursued to restore mechanical alignment and stability. Our patient had no symptoms of instability.
Similar to 3 of the 4 patients presented by Gabos and colleagues,4 our patient had marked hypertrophy of the meniscofemoral ligament of Humphrey. The report by Gabos and colleagues4 of this finding was the first in the literature. The hypertrophy of this ligament suggests it has a role in stabilizing the knee with a congenitally absent ACL. Our patient had no instability in her left knee but presented because of episodes of pain.
Of significant concern is the long-term outcome of patients with congenital ACL aplasia. Crawford and colleagues11 reported 11 patients with ACL deficiency and fibular hemimelia at a mean age of 37 years, showing similar functional outcomes to age-matched controls. However, there was no radiographic follow-up reported in regard to the development of osteoarthritis. To our knowledge, there have been no series published comparing surgical and nonsurgical treatment of congenital absence of the ACL. In the study by Gabos and colleagues,4 all patients were treated with reconstruction because these patients had symptomatic instability.
Conclusion
This report presents a patient whose symptoms improved after resection of her medial meniscal tear. This patient will be followed long-term to delineate her clinical course and to monitor for instability and/or development of osteoarthritis. Future studies should compare the treatment of congenital absence of the ACL with reconstruction and with conservative management.
Congenital absence of the anterior cruciate ligament (ACL) is a rare occurrence and has been seen most often in conjunction with conditions such as knee dislocation, knee dysplasia, proximal focal femoral deficiency, and fibular hemimelia.
We report on the incidental finding of ACL aplasia in a patient with a medial meniscal tear and history of leg-length discrepancy. Similar to earlier cases, this patient had hypertrophy of the meniscofemoral ligament of Humphrey, which likely provided stability. This case report emphasizes the importance of distinguishing between a stable and an unstable knee in congenital absence of the ACL. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 20-year-old woman presented for orthopedic evaluation with worsening medial left knee pain. Her pain was intermittent in nature, occurring about every 1 to 2 months and of 1 to 2 days’ duration. Onset was while using the elliptical machine, walking on uneven ground, or navigating stairs. She denied any buckling, catching, locking, instability, or swelling.
Her history was significant for a breech delivery and leg anisomelia, for which she had a contralateral distal femoral and proximal tibial percutaneous epiphysiodesis performed at age 10 years. Family history was negative for limb deformities.
Physical examination was notable for absence of global ligamentous laxity, overall valgus alignment of the left lower extremity, minimally decreased motion, trace effusion, positive medial joint line tenderness, positive McMurray test, and 1+ Lachman test with guarding on pivot shift testing.
Plain films showed valgus alignment with narrowing of the lateral compartment, narrow intercondylar notch, and hypoplasia of the tibial eminences and lateral femoral condyle (Figure 1). Magnetic resonance imaging showed a large tear in the posterior horn of the medial meniscus, hypertrophy of the meniscofemoral ligament of Humphrey (Figure 2A), and nonvisualization of the ACL with a small remnant (Figure 2B).
Arthroscopy showed complete absence of fibers of the ACL, hypertrophy of the meniscofemoral ligament of Humphrey, and a large posterior horn medial meniscal tear. A partial medial meniscectomy was performed. More than 2 years after surgery, the patient was doing very well without pain or instability, and was exercising regularly without difficulty.
Discussion
Our patient had left-sided congenital absence of the ACL with associated limb-length discrepancy of more than 2.5 cm. Isolated absence of the ACL has been described in a few case reports in the literature. Congenital ACL absence has most often been found in association with conditions such as knee dislocation (occurring with a frequency of .017/1000 births),1 knee dysplasia,2,3 fibular hemimelia,4 and proximal focal femoral deficiency.5 Johansson and Aparisi5,6 linked the finding of ACL absence with instability in those patients with known limb-length discrepancy and symptomatic instability. This report presents a patient who has congenital absence of the ACL in a foreshortened limb and torn medial meniscus. The classification of the patient’s cruciate dysplasia would be type I, as described by Manner and colleagues.7 The incidence of meniscal tears in association with congenital ACL absence is unknown. There have been reports of absence of the ACL associated with a ring meniscus,8 absence of both cruciate ligaments and menisci,9 and a bucket-handle tear of the medial meniscus.10
Gabos and colleagues4 recommend reconstructive surgery for patients with congenital absence of the ACL and symptomatic knee instability. Limb lengthening/shortening and realignment procedures have allowed patients such as ours to have functionally anatomic limbs and high activity levels. Surgical treatment is pursued to restore mechanical alignment and stability. Our patient had no symptoms of instability.
Similar to 3 of the 4 patients presented by Gabos and colleagues,4 our patient had marked hypertrophy of the meniscofemoral ligament of Humphrey. The report by Gabos and colleagues4 of this finding was the first in the literature. The hypertrophy of this ligament suggests it has a role in stabilizing the knee with a congenitally absent ACL. Our patient had no instability in her left knee but presented because of episodes of pain.
Of significant concern is the long-term outcome of patients with congenital ACL aplasia. Crawford and colleagues11 reported 11 patients with ACL deficiency and fibular hemimelia at a mean age of 37 years, showing similar functional outcomes to age-matched controls. However, there was no radiographic follow-up reported in regard to the development of osteoarthritis. To our knowledge, there have been no series published comparing surgical and nonsurgical treatment of congenital absence of the ACL. In the study by Gabos and colleagues,4 all patients were treated with reconstruction because these patients had symptomatic instability.
Conclusion
This report presents a patient whose symptoms improved after resection of her medial meniscal tear. This patient will be followed long-term to delineate her clinical course and to monitor for instability and/or development of osteoarthritis. Future studies should compare the treatment of congenital absence of the ACL with reconstruction and with conservative management.
1. Tachdjian MO. Pediatric Orthopedics. 2nd ed. Philadelphia: Saunders; 1990.
2. Thomas NP, Jackson AM, Aichroth PM. Congenital absence of the anterior cruciate ligament: A common component of knee dysplasia. J Bone Joint Surg Br. 1985;67(4):572-575.
3. Hejgaard N, Kjaerulff H. Congenital aplasia of the anterior cruciate ligament. Report of a case in a seven-year-old girl. Int Orthop. 1987;11(3):223-225.
4. Gabos PG, El Rassi G, Pahys J. Knee reconstruction in syndromes with congenital absence of the anterior cruciate ligament. J Pediatr Orthop. 2005;25(2):210-214.
5. Johansson E, Aparisi T. Missing cruciate ligament in congenital short femur. J Bone Joint Surg Am. 1983;65(8):1109-1115.
6. Johannson E, Aparisi T. Congenital absence of the cruciate ligaments. A case report and review of the literature. Clin Orthop Relat Res. 1982;162:108-111.
7. Manner HM, Radler C, Ganger R, Grill F. Dysplasia of the cruciate ligaments: radiographic assessment and classification. J Bone Joint Surg Am. 2006;88(1):130-137.
8. Noble J. Congenital absence of the anterior cruciate ligament associated with a ring meniscus. J Bone Joint Surg Am. 1975;57(8):1165-1166.
9. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
10. Kaelin A, Hulin PH, Carlioz H. Congenital aplasia of the cruciate ligaments. A report of six cases. J Bone Joint Surg Br. 1986;68(5):827-828.
11. Crawford DA, Tompkins BJ, Baird GO, Caskey PM. The long term function of the knee in patients with fibular hemimelia and anterior cruciate ligament deficiency. J Bone Joint Surg Br. 2012;94(3):328-333.
1. Tachdjian MO. Pediatric Orthopedics. 2nd ed. Philadelphia: Saunders; 1990.
2. Thomas NP, Jackson AM, Aichroth PM. Congenital absence of the anterior cruciate ligament: A common component of knee dysplasia. J Bone Joint Surg Br. 1985;67(4):572-575.
3. Hejgaard N, Kjaerulff H. Congenital aplasia of the anterior cruciate ligament. Report of a case in a seven-year-old girl. Int Orthop. 1987;11(3):223-225.
4. Gabos PG, El Rassi G, Pahys J. Knee reconstruction in syndromes with congenital absence of the anterior cruciate ligament. J Pediatr Orthop. 2005;25(2):210-214.
5. Johansson E, Aparisi T. Missing cruciate ligament in congenital short femur. J Bone Joint Surg Am. 1983;65(8):1109-1115.
6. Johannson E, Aparisi T. Congenital absence of the cruciate ligaments. A case report and review of the literature. Clin Orthop Relat Res. 1982;162:108-111.
7. Manner HM, Radler C, Ganger R, Grill F. Dysplasia of the cruciate ligaments: radiographic assessment and classification. J Bone Joint Surg Am. 2006;88(1):130-137.
8. Noble J. Congenital absence of the anterior cruciate ligament associated with a ring meniscus. J Bone Joint Surg Am. 1975;57(8):1165-1166.
9. Tolo VT. Congenital absence of the menisci and cruciate ligaments of the knee. A case report. J Bone Joint Surg Am. 1981;63(6):1022-1024.
10. Kaelin A, Hulin PH, Carlioz H. Congenital aplasia of the cruciate ligaments. A report of six cases. J Bone Joint Surg Br. 1986;68(5):827-828.
11. Crawford DA, Tompkins BJ, Baird GO, Caskey PM. The long term function of the knee in patients with fibular hemimelia and anterior cruciate ligament deficiency. J Bone Joint Surg Br. 2012;94(3):328-333.
Iatrogenic Femoral Neck Fracture After Closed Reduction of Anterior Hip Dislocation in the Emergency Department
Anterior hip dislocations have been reported to account for approximately 5% to 10% of all hip dislocations.1 Epstein and Wiss2 originally divided anterior hip dislocations into superior (type I, including pubic or subspinous) and inferior (type II, including obturator and perineal) dislocations. This classification was further subdivided based on the presence of either no associated fracture (type A), fracture of the femoral head or neck (FNF; type B), or fracture of the acetabulum (type C).3 Of all anterior hip dislocations, it has been reported that the inferior or obturator type of dislocation is more common, constituting approximately 70% of all anterior dislocations.4 In 1943, Pringle5 described the mechanism of obturator dislocation as simultaneous abduction, flexion, and external rotation of the hip. Our literature search found only 2 case reports in non-English-language journals of a complete FNF associated with an attempted reduction of an anterior hip dislocation.6,7 Indentation fractures of the femoral head have been more commonly reported than FNFs, with a reported incidence of 35% to 55% after anterior dislocation.4,8 DeLee and colleagues8 also found that those patients with indentation fractures were at a higher risk for developing avascular necrosis of the femoral head in addition to being more likely to report poor or fair function of the hip 2 years after reduction.
There have been a number of different reduction maneuvers for anterior dislocation of hips published in the literature. Epstein and Harvey9 advocated reduction by traction in the line of the femur with the hip flexed and in gentle internal rotation and abduction while the patient was under general anesthesia. Toms and Williams,10 however, recommended adduction with gradual release of the longitudinal traction. Polesky and Polesky11 described a reduction method involving sharp internal rotation, which was found to be associated with FNF. The patient provided written informed consent for print and electronic publication of this case report, and approval was obtained from the Emory University Institutional Review Board.
Case Report
The patient was a 73-year-old woman, an independent ambulator with minimal antecedent hip pain, who, as a pedestrian, was struck by a heavy-duty pickup truck at low velocity. She was flown to our level I trauma center from an outside hospital. The patient arrived hemodynamically stable, with a Glasgow Coma Scale score of 15 and with major complaints of right shoulder and right hip pain. She had a positive Focused Assessment with Sonography for Trauma (FAST), and underwent a subsequent urgent chest, abdomen, and pelvis computed tomography (CT) scan for further investigation. CT showed a grade 1 liver laceration. Her anteroposterior (AP) pelvic radiograph and pelvic CT scan showed an anterior hip dislocation with the femoral head located adjacent to the obturator foramen (Figures 1, 2). The AP pelvic radiograph and pelvic CT scan were scrutinized extensively before reduction to rule out a possible FNF. Comparing the right and left femoral necks through multiple axial CT images showed no obvious differences between the 2 sides (Figures 3, 4). Her only other orthopedic injury was an inferior shoulder dislocation. It is not routine for the general surgery trauma team to obtain a pelvic CT scan prior to involvement of the orthopedic service and prompt reduction of a hip dislocation. Upon initial examination of her right hip, it was fixed in slight flexion and external rotation; she was neurovascularly intact.
After being cleared by the trauma service, the patient provided informed consent for closed reduction of the hip and shoulder under conscious sedation, performed by the emergency department (ED) staff. She received intravenous fentanyl and midazolam, and the reduction was attempted. The reduction maneuver was performed with gentle inline traction, adduction, and internal rotation and extension. There was an audible clunk, and the hip was thought to be reduced and stable. The right leg lower extremity was placed into a knee immobilizer and she remained neurovascularly intact. The shoulder was reduced. After the procedure, the patient had an episode of hypoxia requiring oxygenation via a bag valve mask by the ED staff. Postreduction radiographs confirmed reduction of the right shoulder; however, they also showed a FNF with the femoral head retained near the obturator foramen (Figures 5, 6). The patient and her family were informed of the fracture, and a total hip arthroplasty (THA) was recommended, given her pre-injury mild symptomatic osteoarthritis in the hip and her age. The patient was admitted to the intensive care unit for cardiopulmonary monitoring and was found to have a troponin leak on hospital day 1. She was evaluated by the cardiology service; serial electrocardiograms and troponins ruled out acute myocardial infarction. The patient was cleared for surgery on hospital day 4.
On hospital day 5, she underwent a right THA via a Kocher-Langenbeck approach. The patient’s femoral head was found to be anterior and laterally adjacent to her ischial tuberosity with an indentation fracture. The sciatic nerve was identified and found to be intact. A metal-on-polyethylene Stryker Accolade femoral component and Trident acetabular shell were implanted, and a posterior capsular repair was performed (Figure 7).
The patient tolerated the procedure well, and her postoperative course was uneventful. She was discharged to a subacute rehabilitation facility on postoperative day 3. The patient returned for her 2-week postoperative visit ambulating without assistance. At her last follow-up visit, approximately 6 weeks after surgery, she was a functionally independent community ambulator. Phone conversations with her private orthopedist at 6 months confirmed continued ambulation without problems.
Discussion
This case report of a complication that occurred in our institution has resulted in a change in our protocol for treatment of geriatric anterior hip dislocations. Our institution is a level I trauma center, and traumatic hip dislocations are relatively common, occurring usually in young patients with high-energy trauma. Although somewhat controversial, it is generally assumed that the incidence of avascular necrosis of the femoral head after dislocation of the hip is correlated with the time interval from dislocation to reduction of the hip. Therefore, our protocol for hip dislocations of the hip in young trauma patients is urgent reduction in the ED under appropriate analgesia and muscle relaxation.
In this case report, the patient was older than 65 years with radiographic evidence of possible impingement and postsurgical evidence of impingement of the femoral head in the obturator foremen (Figures 1, 2, 8). In addition, the patient was significantly osteopenic radiographically. An attempted reduction in the ED resulted in FNF requiring THA (Figures 5, 6, 9). After discussion of this complication in our institution’s morbidity and mortality conference, we have developed a protocol for the geriatric patient (older than 65 years) with a traumatic hip dislocation. These patients will undergo attempted reduction under controlled analgesia and muscle relaxation in the operating room (OR) with an attending surgeon present, ideally, an attending surgeon comfortable with arthroplasty in a terminally cleaned OR room. Our institution’s surgical site infection rate after total joint arthroplasty has significantly decreased with improved patient selection and the use of terminally cleaned OR rooms. Because our policy is to perform closed reduction of dislocated hips in an urgent manner, if there is not a terminally clean room or an arthroplasty-trained attending orthopedic surgeon available, then informed consent with discussion of the possibility of fracture requiring a subsequent arthroplasty should be obtained from the patient before the attempted reduction.
After review of the available literature, we believe that this case highlights some of the important treatment principles when treating anterior hip dislocations in the ED. The relatively high incidence of indentation fractures of the femoral head with obturator dislocations puts these fractures at higher risk for possible impingement around the obturator ring. This impingement, coupled with preexisting osteopenia, can predispose these dislocations to FNF, if appropriate analgesia and sedation are not obtained and gentle reduction is not performed. In addition, while it may not be time- or cost-effective to perform closed reduction on every hip dislocation, we bring geriatric patients with radiographic osteopenia to the OR for more controlled reductions. In the informed consent discussion, the possibility of FNF is mentioned, and the patient and family are told that an elective total hip replacement will be performed if this complication occurs.
We consider the following to be risk factors for closed reductions of anterior hip dislocations: (1) preexisting osteopenia on plain films, (2) age greater than 65 years, and (3) radiographic femoral head impingement on the surrounding bony pelvis. We continue to consider closed reduction of both anterior and posterior hip dislocations as urgent (within 6 hours from time of dislocation). This case adds to the existing literature on the risk of FNF with closed reduction of obturator hip dislocations, and we hope that it will encourage further study into the safest and most cost-effective reduction protocol.
1. Amihood, S. Anterior dislocation of the hip. Injury. 1975;7(2):107-110.
2. Epstein HC, Wiss DA. Traumatic anterior dislocation of the hip. Orthopedics. 1985;8(1):130, 132-134.
3. Epstein HC. Traumatic dislocations of the hip. Clin Orthop Relat Res. 1973(92):116-142.
4. Erb RE, Steele JR, Nance EP Jr, Edwards JR. Traumatic anterior dislocation of the hip: spectrum of plain film and CT findings. AJR Am J Roentgenol. 1995;165(5):1215-1219.
5. Pringle JH. Traumatic dislocation at the hip joint. An experimental study in the cadaver. Glasgow Med J. 1943;21:25-40.
6. Esenkaya I, Görgeç M. Traumatic anterior dislocation of the hip associated with ipsilateral femoral neck fracture: a case report. Acta Orthop Traumatol Turc. 2002;36(4):366-368.
7. Sadler AH, DiStefano M. Anterior dislocation of the hip with ipsilateral basicervical fracture. A case report. J Bone Joint Surg Am. 1985;67(2):326-329.
8. DeLee JC, Evans JA, Thomas J. Anterior dislocation of the hip and associated femoral-head fractures. J Bone Joint Surg Am. 1980;62(6):960-964.
9. Epstein HC, Harvey JP Jr. Traumatic anterior dislocations of the hip: management and results. An analysis of fifty-five cases. J Bone Joint Surg Am. 1972;54(7):1561-1562.
10. Toms AD, Williams S, White SH. Obturator dislocation of the hip. J Bone Joint Surg Br. 2001;83(1):113-115.
11. Polesky RE, Polesky FA. Intrapelvic dislocation of the femoral head following anterior dislocation of the hip. A case report. J Bone Joint Surg Am. 1972;54(5):1097-1098.
Anterior hip dislocations have been reported to account for approximately 5% to 10% of all hip dislocations.1 Epstein and Wiss2 originally divided anterior hip dislocations into superior (type I, including pubic or subspinous) and inferior (type II, including obturator and perineal) dislocations. This classification was further subdivided based on the presence of either no associated fracture (type A), fracture of the femoral head or neck (FNF; type B), or fracture of the acetabulum (type C).3 Of all anterior hip dislocations, it has been reported that the inferior or obturator type of dislocation is more common, constituting approximately 70% of all anterior dislocations.4 In 1943, Pringle5 described the mechanism of obturator dislocation as simultaneous abduction, flexion, and external rotation of the hip. Our literature search found only 2 case reports in non-English-language journals of a complete FNF associated with an attempted reduction of an anterior hip dislocation.6,7 Indentation fractures of the femoral head have been more commonly reported than FNFs, with a reported incidence of 35% to 55% after anterior dislocation.4,8 DeLee and colleagues8 also found that those patients with indentation fractures were at a higher risk for developing avascular necrosis of the femoral head in addition to being more likely to report poor or fair function of the hip 2 years after reduction.
There have been a number of different reduction maneuvers for anterior dislocation of hips published in the literature. Epstein and Harvey9 advocated reduction by traction in the line of the femur with the hip flexed and in gentle internal rotation and abduction while the patient was under general anesthesia. Toms and Williams,10 however, recommended adduction with gradual release of the longitudinal traction. Polesky and Polesky11 described a reduction method involving sharp internal rotation, which was found to be associated with FNF. The patient provided written informed consent for print and electronic publication of this case report, and approval was obtained from the Emory University Institutional Review Board.
Case Report
The patient was a 73-year-old woman, an independent ambulator with minimal antecedent hip pain, who, as a pedestrian, was struck by a heavy-duty pickup truck at low velocity. She was flown to our level I trauma center from an outside hospital. The patient arrived hemodynamically stable, with a Glasgow Coma Scale score of 15 and with major complaints of right shoulder and right hip pain. She had a positive Focused Assessment with Sonography for Trauma (FAST), and underwent a subsequent urgent chest, abdomen, and pelvis computed tomography (CT) scan for further investigation. CT showed a grade 1 liver laceration. Her anteroposterior (AP) pelvic radiograph and pelvic CT scan showed an anterior hip dislocation with the femoral head located adjacent to the obturator foramen (Figures 1, 2). The AP pelvic radiograph and pelvic CT scan were scrutinized extensively before reduction to rule out a possible FNF. Comparing the right and left femoral necks through multiple axial CT images showed no obvious differences between the 2 sides (Figures 3, 4). Her only other orthopedic injury was an inferior shoulder dislocation. It is not routine for the general surgery trauma team to obtain a pelvic CT scan prior to involvement of the orthopedic service and prompt reduction of a hip dislocation. Upon initial examination of her right hip, it was fixed in slight flexion and external rotation; she was neurovascularly intact.
After being cleared by the trauma service, the patient provided informed consent for closed reduction of the hip and shoulder under conscious sedation, performed by the emergency department (ED) staff. She received intravenous fentanyl and midazolam, and the reduction was attempted. The reduction maneuver was performed with gentle inline traction, adduction, and internal rotation and extension. There was an audible clunk, and the hip was thought to be reduced and stable. The right leg lower extremity was placed into a knee immobilizer and she remained neurovascularly intact. The shoulder was reduced. After the procedure, the patient had an episode of hypoxia requiring oxygenation via a bag valve mask by the ED staff. Postreduction radiographs confirmed reduction of the right shoulder; however, they also showed a FNF with the femoral head retained near the obturator foramen (Figures 5, 6). The patient and her family were informed of the fracture, and a total hip arthroplasty (THA) was recommended, given her pre-injury mild symptomatic osteoarthritis in the hip and her age. The patient was admitted to the intensive care unit for cardiopulmonary monitoring and was found to have a troponin leak on hospital day 1. She was evaluated by the cardiology service; serial electrocardiograms and troponins ruled out acute myocardial infarction. The patient was cleared for surgery on hospital day 4.
On hospital day 5, she underwent a right THA via a Kocher-Langenbeck approach. The patient’s femoral head was found to be anterior and laterally adjacent to her ischial tuberosity with an indentation fracture. The sciatic nerve was identified and found to be intact. A metal-on-polyethylene Stryker Accolade femoral component and Trident acetabular shell were implanted, and a posterior capsular repair was performed (Figure 7).
The patient tolerated the procedure well, and her postoperative course was uneventful. She was discharged to a subacute rehabilitation facility on postoperative day 3. The patient returned for her 2-week postoperative visit ambulating without assistance. At her last follow-up visit, approximately 6 weeks after surgery, she was a functionally independent community ambulator. Phone conversations with her private orthopedist at 6 months confirmed continued ambulation without problems.
Discussion
This case report of a complication that occurred in our institution has resulted in a change in our protocol for treatment of geriatric anterior hip dislocations. Our institution is a level I trauma center, and traumatic hip dislocations are relatively common, occurring usually in young patients with high-energy trauma. Although somewhat controversial, it is generally assumed that the incidence of avascular necrosis of the femoral head after dislocation of the hip is correlated with the time interval from dislocation to reduction of the hip. Therefore, our protocol for hip dislocations of the hip in young trauma patients is urgent reduction in the ED under appropriate analgesia and muscle relaxation.
In this case report, the patient was older than 65 years with radiographic evidence of possible impingement and postsurgical evidence of impingement of the femoral head in the obturator foremen (Figures 1, 2, 8). In addition, the patient was significantly osteopenic radiographically. An attempted reduction in the ED resulted in FNF requiring THA (Figures 5, 6, 9). After discussion of this complication in our institution’s morbidity and mortality conference, we have developed a protocol for the geriatric patient (older than 65 years) with a traumatic hip dislocation. These patients will undergo attempted reduction under controlled analgesia and muscle relaxation in the operating room (OR) with an attending surgeon present, ideally, an attending surgeon comfortable with arthroplasty in a terminally cleaned OR room. Our institution’s surgical site infection rate after total joint arthroplasty has significantly decreased with improved patient selection and the use of terminally cleaned OR rooms. Because our policy is to perform closed reduction of dislocated hips in an urgent manner, if there is not a terminally clean room or an arthroplasty-trained attending orthopedic surgeon available, then informed consent with discussion of the possibility of fracture requiring a subsequent arthroplasty should be obtained from the patient before the attempted reduction.
After review of the available literature, we believe that this case highlights some of the important treatment principles when treating anterior hip dislocations in the ED. The relatively high incidence of indentation fractures of the femoral head with obturator dislocations puts these fractures at higher risk for possible impingement around the obturator ring. This impingement, coupled with preexisting osteopenia, can predispose these dislocations to FNF, if appropriate analgesia and sedation are not obtained and gentle reduction is not performed. In addition, while it may not be time- or cost-effective to perform closed reduction on every hip dislocation, we bring geriatric patients with radiographic osteopenia to the OR for more controlled reductions. In the informed consent discussion, the possibility of FNF is mentioned, and the patient and family are told that an elective total hip replacement will be performed if this complication occurs.
We consider the following to be risk factors for closed reductions of anterior hip dislocations: (1) preexisting osteopenia on plain films, (2) age greater than 65 years, and (3) radiographic femoral head impingement on the surrounding bony pelvis. We continue to consider closed reduction of both anterior and posterior hip dislocations as urgent (within 6 hours from time of dislocation). This case adds to the existing literature on the risk of FNF with closed reduction of obturator hip dislocations, and we hope that it will encourage further study into the safest and most cost-effective reduction protocol.
Anterior hip dislocations have been reported to account for approximately 5% to 10% of all hip dislocations.1 Epstein and Wiss2 originally divided anterior hip dislocations into superior (type I, including pubic or subspinous) and inferior (type II, including obturator and perineal) dislocations. This classification was further subdivided based on the presence of either no associated fracture (type A), fracture of the femoral head or neck (FNF; type B), or fracture of the acetabulum (type C).3 Of all anterior hip dislocations, it has been reported that the inferior or obturator type of dislocation is more common, constituting approximately 70% of all anterior dislocations.4 In 1943, Pringle5 described the mechanism of obturator dislocation as simultaneous abduction, flexion, and external rotation of the hip. Our literature search found only 2 case reports in non-English-language journals of a complete FNF associated with an attempted reduction of an anterior hip dislocation.6,7 Indentation fractures of the femoral head have been more commonly reported than FNFs, with a reported incidence of 35% to 55% after anterior dislocation.4,8 DeLee and colleagues8 also found that those patients with indentation fractures were at a higher risk for developing avascular necrosis of the femoral head in addition to being more likely to report poor or fair function of the hip 2 years after reduction.
There have been a number of different reduction maneuvers for anterior dislocation of hips published in the literature. Epstein and Harvey9 advocated reduction by traction in the line of the femur with the hip flexed and in gentle internal rotation and abduction while the patient was under general anesthesia. Toms and Williams,10 however, recommended adduction with gradual release of the longitudinal traction. Polesky and Polesky11 described a reduction method involving sharp internal rotation, which was found to be associated with FNF. The patient provided written informed consent for print and electronic publication of this case report, and approval was obtained from the Emory University Institutional Review Board.
Case Report
The patient was a 73-year-old woman, an independent ambulator with minimal antecedent hip pain, who, as a pedestrian, was struck by a heavy-duty pickup truck at low velocity. She was flown to our level I trauma center from an outside hospital. The patient arrived hemodynamically stable, with a Glasgow Coma Scale score of 15 and with major complaints of right shoulder and right hip pain. She had a positive Focused Assessment with Sonography for Trauma (FAST), and underwent a subsequent urgent chest, abdomen, and pelvis computed tomography (CT) scan for further investigation. CT showed a grade 1 liver laceration. Her anteroposterior (AP) pelvic radiograph and pelvic CT scan showed an anterior hip dislocation with the femoral head located adjacent to the obturator foramen (Figures 1, 2). The AP pelvic radiograph and pelvic CT scan were scrutinized extensively before reduction to rule out a possible FNF. Comparing the right and left femoral necks through multiple axial CT images showed no obvious differences between the 2 sides (Figures 3, 4). Her only other orthopedic injury was an inferior shoulder dislocation. It is not routine for the general surgery trauma team to obtain a pelvic CT scan prior to involvement of the orthopedic service and prompt reduction of a hip dislocation. Upon initial examination of her right hip, it was fixed in slight flexion and external rotation; she was neurovascularly intact.
After being cleared by the trauma service, the patient provided informed consent for closed reduction of the hip and shoulder under conscious sedation, performed by the emergency department (ED) staff. She received intravenous fentanyl and midazolam, and the reduction was attempted. The reduction maneuver was performed with gentle inline traction, adduction, and internal rotation and extension. There was an audible clunk, and the hip was thought to be reduced and stable. The right leg lower extremity was placed into a knee immobilizer and she remained neurovascularly intact. The shoulder was reduced. After the procedure, the patient had an episode of hypoxia requiring oxygenation via a bag valve mask by the ED staff. Postreduction radiographs confirmed reduction of the right shoulder; however, they also showed a FNF with the femoral head retained near the obturator foramen (Figures 5, 6). The patient and her family were informed of the fracture, and a total hip arthroplasty (THA) was recommended, given her pre-injury mild symptomatic osteoarthritis in the hip and her age. The patient was admitted to the intensive care unit for cardiopulmonary monitoring and was found to have a troponin leak on hospital day 1. She was evaluated by the cardiology service; serial electrocardiograms and troponins ruled out acute myocardial infarction. The patient was cleared for surgery on hospital day 4.
On hospital day 5, she underwent a right THA via a Kocher-Langenbeck approach. The patient’s femoral head was found to be anterior and laterally adjacent to her ischial tuberosity with an indentation fracture. The sciatic nerve was identified and found to be intact. A metal-on-polyethylene Stryker Accolade femoral component and Trident acetabular shell were implanted, and a posterior capsular repair was performed (Figure 7).
The patient tolerated the procedure well, and her postoperative course was uneventful. She was discharged to a subacute rehabilitation facility on postoperative day 3. The patient returned for her 2-week postoperative visit ambulating without assistance. At her last follow-up visit, approximately 6 weeks after surgery, she was a functionally independent community ambulator. Phone conversations with her private orthopedist at 6 months confirmed continued ambulation without problems.
Discussion
This case report of a complication that occurred in our institution has resulted in a change in our protocol for treatment of geriatric anterior hip dislocations. Our institution is a level I trauma center, and traumatic hip dislocations are relatively common, occurring usually in young patients with high-energy trauma. Although somewhat controversial, it is generally assumed that the incidence of avascular necrosis of the femoral head after dislocation of the hip is correlated with the time interval from dislocation to reduction of the hip. Therefore, our protocol for hip dislocations of the hip in young trauma patients is urgent reduction in the ED under appropriate analgesia and muscle relaxation.
In this case report, the patient was older than 65 years with radiographic evidence of possible impingement and postsurgical evidence of impingement of the femoral head in the obturator foremen (Figures 1, 2, 8). In addition, the patient was significantly osteopenic radiographically. An attempted reduction in the ED resulted in FNF requiring THA (Figures 5, 6, 9). After discussion of this complication in our institution’s morbidity and mortality conference, we have developed a protocol for the geriatric patient (older than 65 years) with a traumatic hip dislocation. These patients will undergo attempted reduction under controlled analgesia and muscle relaxation in the operating room (OR) with an attending surgeon present, ideally, an attending surgeon comfortable with arthroplasty in a terminally cleaned OR room. Our institution’s surgical site infection rate after total joint arthroplasty has significantly decreased with improved patient selection and the use of terminally cleaned OR rooms. Because our policy is to perform closed reduction of dislocated hips in an urgent manner, if there is not a terminally clean room or an arthroplasty-trained attending orthopedic surgeon available, then informed consent with discussion of the possibility of fracture requiring a subsequent arthroplasty should be obtained from the patient before the attempted reduction.
After review of the available literature, we believe that this case highlights some of the important treatment principles when treating anterior hip dislocations in the ED. The relatively high incidence of indentation fractures of the femoral head with obturator dislocations puts these fractures at higher risk for possible impingement around the obturator ring. This impingement, coupled with preexisting osteopenia, can predispose these dislocations to FNF, if appropriate analgesia and sedation are not obtained and gentle reduction is not performed. In addition, while it may not be time- or cost-effective to perform closed reduction on every hip dislocation, we bring geriatric patients with radiographic osteopenia to the OR for more controlled reductions. In the informed consent discussion, the possibility of FNF is mentioned, and the patient and family are told that an elective total hip replacement will be performed if this complication occurs.
We consider the following to be risk factors for closed reductions of anterior hip dislocations: (1) preexisting osteopenia on plain films, (2) age greater than 65 years, and (3) radiographic femoral head impingement on the surrounding bony pelvis. We continue to consider closed reduction of both anterior and posterior hip dislocations as urgent (within 6 hours from time of dislocation). This case adds to the existing literature on the risk of FNF with closed reduction of obturator hip dislocations, and we hope that it will encourage further study into the safest and most cost-effective reduction protocol.
1. Amihood, S. Anterior dislocation of the hip. Injury. 1975;7(2):107-110.
2. Epstein HC, Wiss DA. Traumatic anterior dislocation of the hip. Orthopedics. 1985;8(1):130, 132-134.
3. Epstein HC. Traumatic dislocations of the hip. Clin Orthop Relat Res. 1973(92):116-142.
4. Erb RE, Steele JR, Nance EP Jr, Edwards JR. Traumatic anterior dislocation of the hip: spectrum of plain film and CT findings. AJR Am J Roentgenol. 1995;165(5):1215-1219.
5. Pringle JH. Traumatic dislocation at the hip joint. An experimental study in the cadaver. Glasgow Med J. 1943;21:25-40.
6. Esenkaya I, Görgeç M. Traumatic anterior dislocation of the hip associated with ipsilateral femoral neck fracture: a case report. Acta Orthop Traumatol Turc. 2002;36(4):366-368.
7. Sadler AH, DiStefano M. Anterior dislocation of the hip with ipsilateral basicervical fracture. A case report. J Bone Joint Surg Am. 1985;67(2):326-329.
8. DeLee JC, Evans JA, Thomas J. Anterior dislocation of the hip and associated femoral-head fractures. J Bone Joint Surg Am. 1980;62(6):960-964.
9. Epstein HC, Harvey JP Jr. Traumatic anterior dislocations of the hip: management and results. An analysis of fifty-five cases. J Bone Joint Surg Am. 1972;54(7):1561-1562.
10. Toms AD, Williams S, White SH. Obturator dislocation of the hip. J Bone Joint Surg Br. 2001;83(1):113-115.
11. Polesky RE, Polesky FA. Intrapelvic dislocation of the femoral head following anterior dislocation of the hip. A case report. J Bone Joint Surg Am. 1972;54(5):1097-1098.
1. Amihood, S. Anterior dislocation of the hip. Injury. 1975;7(2):107-110.
2. Epstein HC, Wiss DA. Traumatic anterior dislocation of the hip. Orthopedics. 1985;8(1):130, 132-134.
3. Epstein HC. Traumatic dislocations of the hip. Clin Orthop Relat Res. 1973(92):116-142.
4. Erb RE, Steele JR, Nance EP Jr, Edwards JR. Traumatic anterior dislocation of the hip: spectrum of plain film and CT findings. AJR Am J Roentgenol. 1995;165(5):1215-1219.
5. Pringle JH. Traumatic dislocation at the hip joint. An experimental study in the cadaver. Glasgow Med J. 1943;21:25-40.
6. Esenkaya I, Görgeç M. Traumatic anterior dislocation of the hip associated with ipsilateral femoral neck fracture: a case report. Acta Orthop Traumatol Turc. 2002;36(4):366-368.
7. Sadler AH, DiStefano M. Anterior dislocation of the hip with ipsilateral basicervical fracture. A case report. J Bone Joint Surg Am. 1985;67(2):326-329.
8. DeLee JC, Evans JA, Thomas J. Anterior dislocation of the hip and associated femoral-head fractures. J Bone Joint Surg Am. 1980;62(6):960-964.
9. Epstein HC, Harvey JP Jr. Traumatic anterior dislocations of the hip: management and results. An analysis of fifty-five cases. J Bone Joint Surg Am. 1972;54(7):1561-1562.
10. Toms AD, Williams S, White SH. Obturator dislocation of the hip. J Bone Joint Surg Br. 2001;83(1):113-115.
11. Polesky RE, Polesky FA. Intrapelvic dislocation of the femoral head following anterior dislocation of the hip. A case report. J Bone Joint Surg Am. 1972;54(5):1097-1098.
Bilateral Superior Labrum Anterior to Posterior (SLAP) Tears With Abnormal Anatomy of Biceps Tendon
The biceps brachii derives its name from the 2 heads of the muscle. The short head originates from the coracoid apex, with the coracobrachialis muscle. The long head of the biceps tendon (LHBT) starts within the capsule of the shoulder joint, running from the supraglenoid tubercle or labrum.1 The tendon typically runs free along its intra-articular course, but it is also extrasynovial and ensheathed by a continuation of the synovial lining of the articular capsule that extends to the inferior-most extent of the bicipital groove.2 Congenital anomalies of the LHBT are uncommon, although several atypical forms have been described. A literature search for anomalous LHBT identified several variations in anatomic descriptions, including Y-shaped variant, complete absence of tendon, extra-articular attachment, and a variety of intracapsular attachments. In all, 8 case reports of aberrant intracapsular attachment of LHBT3-12 were identified. These cases presented with a variety of clinical manifestations and pathologic changes. Often, these anatomic variations are considered innocuous, yet some present with pathologic findings.
We present the clinical, magnetic resonance imaging (MRI), and arthroscopic findings of a relatively young athletic patient who was experiencing symptoms of bilateral superior labrum anterior to posterior (SLAP) tears that were unresponsive to conservative management. A unique anatomic variant of the LHBT that involved confluence of the LHBT with the undersurface of the anterosuperior capsule at the rotator interval, as well as a Buford complex anteriorly, was identified and treated. We believe that the tethering of the biceps tendon to the capsule combined with the Buford complex created increased stress on the superior labrum and biceps anchor variant, leading to the development of bilateral symptomatic type II SLAP tears. Knowledge of this variant, though perhaps rare, may be relevant for diagnostic recognition of young athletic patients who present with recalcitrant shoulder symptoms. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
A 15-year-old healthy and active athletic boy presented with pain in the right shoulder without history of trauma. He was active in both swimming and baseball. He complained of pain that was present with activities, such as lifting weights, swimming, and throwing. His treatment prior to the office visit consisted of nonsteroidal anti-inflammatory medication, rest, and a therapy program initiated by his high school athletic trainer.
Physical examination demonstrated tenderness to palpation over the posterior capsule and biceps. Motion was full, cuff strength was normal, and SLAP signs (O’Brien, Speed, and Jobe relocation) were positive. A radiograph showed no sign of fracture or dislocation, and no evidence of bony abnormality.
The patient was sent for an MRI arthrogram, which showed a SLAP tear extending from 1 o’clock anteriorly to 10 o’clock posteriorly without intra-articular displacement. No rotator cuff tear was noted. The biceps tendon was noted to be unremarkable and located within the bicipital groove, although retrospective review of the MRI showed that the intra-articular biceps tendon was somewhat confluent with the adjacent tissues.
The patient underwent right shoulder arthroscopy. The shoulder was stable to ligamentous examination under anesthesia. Arthroscopic evaluation revealed that there was a type II SLAP tear extending from the 11-o’clock to the 2-o’clock positions. The superior glenohumeral ligament was identified as it arose from the upper pole of the glenoid labrum and then ran parallel and inferior to the tendon of the biceps towards the lesser tubercle. Surprisingly, there was a very unusual attachment of the intracapsular LHBT to the undersurface of the rotator interval, which restricted biceps excursion in relation to the rotator cuff. Additionally, there was a thick cord-like middle glenohumeral ligament anteriorly that lacked the normal glenoid attachments, thus representing a Buford complex. Interestingly, the labral tear could not only be displaced with a probe, but placing the shoulder through a range of motion also led to increased displacement of the labrum from the glenoid, likely because the biceps tendon was tethered to the undersurface of the capsule.
At the time of arthroscopy, the LHBT was released from its attachment to the capsule at the rotator interval with a radiofrequency wand and shaver. A labral repair was performed using three 2.9-mm bioabsorbable suture anchors, placing 2 posterior and 1 anterior to the biceps tendon. The integrity of the labral repair was observed while placing the shoulder through range of motion.
Postoperatively, the patient was kept in a sling for 5 weeks. Home exercises were initiated at 2 weeks, and outpatient physical therapy was implemented at 4 weeks. The patient resumed swimming, throwing, and other activities—with minimal discomfort—at 6 months postoperatively.
Three years after his initial visit, the patient returned to the office with a similar complaint of pain and limitation of function in his left shoulder after returning to full athletic competition. Once again, there was no history of injury, and history, physical examination, and MRI arthrogram (Figures 1A, 1B) evaluation proved to be very similar to this young athlete’s right shoulder work-up.
The patient once again underwent shoulder arthroscopy and treatment. Although this was now the left shoulder, the findings were essentially identical to the right shoulder. Once again, the labrum was detached from the 11-o’clock to 2-o’clock positions, and a Buford complex was present anteriorly (Figure 2A). The labral tear was easily displaceable from the glenoid with a probe, and placing the shoulder through a range of motion led to increased displacement of the labrum from the glenoid. There was also confluence of the intra-articular LHBT with the undersurface of the capsule within the rotator interval (Figure 2B). A radiofrequency wand, shaver, and elevator were used to define the biceps tendon and separate it from the undersurface of the capsule. The SLAP repair was performed using three 2.9-mm absorbable suture anchors with 2 posterior and 1 anterior to the biceps tendon insertion. The labral repair was observed while placing the shoulder through range of motion and the shoulder was seen to be free of any undue tension on the labrum.
Postoperatively, the patient’s sling and rehabilitation protocol was identical to that of the right shoulder. The patient progressed well, was released to full activity at 6 months, and has not returned with any further complaints of left or right shoulder pain. Approximately 3 years after treatment the patient was contacted via phone and asked about symptoms, pain, and activity. He denies current symptoms of clicking or instability and has no pain that he can identify as being related to previous pathology or treatment. Since the surgery, he has ceased competitive sports and weight lifting, which he attributes to deconditioning associated with postsurgical immobilization and lack of motivation.
Discussion
Of the 8 case reports in the literature that identified variable intra-articular biceps insertional anatomy, only 2 reports represented confluence of the biceps within the rotator interval.7 Interestingly, of the cases identified, the single case that presented a patient with similar pathology of a type II SLAP lesion had an almost identical anatomical variant presentation consisting of both the anomalous insertion of the LHBT into the undersurface of the rotator interval and a Buford variant of the anterosuperior glenohumeral ligament complex. To our knowledge, our bilateral case of an altered intra-articular biceps insertion and a concomitant SLAP tear supports the theory that this pattern of anomalous insertion may very well have altered the biomechanics of the tendon, resulting in acquired pathology to the superior labrum.
The literature reviewed showed the prevalence of anatomic variations of the LHBT ranged from 1.9% to 7.4%.13,14 These variations are generally considered benign; however, in some cases—as in the cases of the young athletes presented by Wahl and MacGillivray7 and in this report—anatomic variation may play an important role in pathogenesis of different injury patterns. The primary function of the LHBT is the stabilization of the glenohumeral joint during abduction and external rotation.15 When the insertion diverges from normal (eg, when the tendon is tethered to the undersurface of the rotator cuff), the biomechanical stresses on the tendon likely change. As a result of the anomalous position of the LHBT origin, there may be a change in the shoulder joint’s biomechanics, with increased strain on the glenohumeral ligament and its attachment onto the glenoid.16
This case report differs from publications on variable superior glenohumeral ligament attachments because a discrete superior glenohumeral ligament structure was isolated from the biceps tendon. Although a larger case series or patient cohort, as well as more involved biomechanical analysis, would certainly be necessary to prove our hypothesis, we believe that this case suggests certain anatomic LHBT and labral variations can contribute to the develop of SLAP tears in younger individuals.
1. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
2. Burkhead WZ Jr. The biceps tendon. In: Rockwood CA Jr, Matsen FA III, eds. The Shoulder. Vol. 2. Philadelphia: WB Saunders; 1990:791-836.
3. Parikh SN, Bonnaig N, Zbojniewicz A. Intracapsular origin of the long head biceps tendon with glenoid avulsion of the glenohumeral ligaments. Orthopedics. 2011;34(11):781-784.
4. Gaskin CM, Golish SR, Blount KJ, Diduch DR. Anomalies of the long head of the biceps brachii tendon: clinical significance, MR arthrographic findings, and arthroscopic correlation in two patients. Skeletal Radiol. 2007;36(8):785-789.
5. Yeh L, Pedowitz R, Kwak S, et al. Intracapsular origin of the long head of the biceps tendon. Skeletal Radiol. 1999;28(3):178-181.
6. Richards DP, Schwartz M. Anomalous intraarticular origin of the long head of the biceps brachii. Clin J Sport Med. 2003;13(2):122-124.
7. Wahl CJ, MacGillivray JD. Three congenital variations in the long head of the biceps tendon: a review of the pathoanatomic considerations and case reports. J Shoulder Elbow Surg. 2007;16(6):e25-e30.I
8. Egea JM, Melguizo C, Prados J, Aránega A. Capsular origin of the long head of the biceps tendon: a clinical case. Rom J Morphol Embryol. 2010;51(2):375-377.
9. Hyman JL, Warren RF. Extra-articular origin of biceps brachii. Arthroscopy. 2001;17(7): E29.
10. Enad JG. Bifurcate origin of the long head of the biceps tendon. Arthroscopy. 2004;20(10):1081-1083.
11. Mariani PP, Bellelli A, Botticella C. Arthroscopic absence of the long head of the biceps tendon. Arthroscopy. 1997;13(4):499-501.
12. Koplas MC, Winalski CS, Ulmer WH Jr, Recht M. Bilateral congenital absence of the long head of the biceps tendon. Skeletal Radiol. 2009;38(7):715-719.
13. Kanatli U, Ozturk BY, Eisen E, Bolukbasi S. Intra-articular variations of the long head of the biceps tendon. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1576-1581.
14. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
15. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
16. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;(330):13-30.
The biceps brachii derives its name from the 2 heads of the muscle. The short head originates from the coracoid apex, with the coracobrachialis muscle. The long head of the biceps tendon (LHBT) starts within the capsule of the shoulder joint, running from the supraglenoid tubercle or labrum.1 The tendon typically runs free along its intra-articular course, but it is also extrasynovial and ensheathed by a continuation of the synovial lining of the articular capsule that extends to the inferior-most extent of the bicipital groove.2 Congenital anomalies of the LHBT are uncommon, although several atypical forms have been described. A literature search for anomalous LHBT identified several variations in anatomic descriptions, including Y-shaped variant, complete absence of tendon, extra-articular attachment, and a variety of intracapsular attachments. In all, 8 case reports of aberrant intracapsular attachment of LHBT3-12 were identified. These cases presented with a variety of clinical manifestations and pathologic changes. Often, these anatomic variations are considered innocuous, yet some present with pathologic findings.
We present the clinical, magnetic resonance imaging (MRI), and arthroscopic findings of a relatively young athletic patient who was experiencing symptoms of bilateral superior labrum anterior to posterior (SLAP) tears that were unresponsive to conservative management. A unique anatomic variant of the LHBT that involved confluence of the LHBT with the undersurface of the anterosuperior capsule at the rotator interval, as well as a Buford complex anteriorly, was identified and treated. We believe that the tethering of the biceps tendon to the capsule combined with the Buford complex created increased stress on the superior labrum and biceps anchor variant, leading to the development of bilateral symptomatic type II SLAP tears. Knowledge of this variant, though perhaps rare, may be relevant for diagnostic recognition of young athletic patients who present with recalcitrant shoulder symptoms. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
A 15-year-old healthy and active athletic boy presented with pain in the right shoulder without history of trauma. He was active in both swimming and baseball. He complained of pain that was present with activities, such as lifting weights, swimming, and throwing. His treatment prior to the office visit consisted of nonsteroidal anti-inflammatory medication, rest, and a therapy program initiated by his high school athletic trainer.
Physical examination demonstrated tenderness to palpation over the posterior capsule and biceps. Motion was full, cuff strength was normal, and SLAP signs (O’Brien, Speed, and Jobe relocation) were positive. A radiograph showed no sign of fracture or dislocation, and no evidence of bony abnormality.
The patient was sent for an MRI arthrogram, which showed a SLAP tear extending from 1 o’clock anteriorly to 10 o’clock posteriorly without intra-articular displacement. No rotator cuff tear was noted. The biceps tendon was noted to be unremarkable and located within the bicipital groove, although retrospective review of the MRI showed that the intra-articular biceps tendon was somewhat confluent with the adjacent tissues.
The patient underwent right shoulder arthroscopy. The shoulder was stable to ligamentous examination under anesthesia. Arthroscopic evaluation revealed that there was a type II SLAP tear extending from the 11-o’clock to the 2-o’clock positions. The superior glenohumeral ligament was identified as it arose from the upper pole of the glenoid labrum and then ran parallel and inferior to the tendon of the biceps towards the lesser tubercle. Surprisingly, there was a very unusual attachment of the intracapsular LHBT to the undersurface of the rotator interval, which restricted biceps excursion in relation to the rotator cuff. Additionally, there was a thick cord-like middle glenohumeral ligament anteriorly that lacked the normal glenoid attachments, thus representing a Buford complex. Interestingly, the labral tear could not only be displaced with a probe, but placing the shoulder through a range of motion also led to increased displacement of the labrum from the glenoid, likely because the biceps tendon was tethered to the undersurface of the capsule.
At the time of arthroscopy, the LHBT was released from its attachment to the capsule at the rotator interval with a radiofrequency wand and shaver. A labral repair was performed using three 2.9-mm bioabsorbable suture anchors, placing 2 posterior and 1 anterior to the biceps tendon. The integrity of the labral repair was observed while placing the shoulder through range of motion.
Postoperatively, the patient was kept in a sling for 5 weeks. Home exercises were initiated at 2 weeks, and outpatient physical therapy was implemented at 4 weeks. The patient resumed swimming, throwing, and other activities—with minimal discomfort—at 6 months postoperatively.
Three years after his initial visit, the patient returned to the office with a similar complaint of pain and limitation of function in his left shoulder after returning to full athletic competition. Once again, there was no history of injury, and history, physical examination, and MRI arthrogram (Figures 1A, 1B) evaluation proved to be very similar to this young athlete’s right shoulder work-up.
The patient once again underwent shoulder arthroscopy and treatment. Although this was now the left shoulder, the findings were essentially identical to the right shoulder. Once again, the labrum was detached from the 11-o’clock to 2-o’clock positions, and a Buford complex was present anteriorly (Figure 2A). The labral tear was easily displaceable from the glenoid with a probe, and placing the shoulder through a range of motion led to increased displacement of the labrum from the glenoid. There was also confluence of the intra-articular LHBT with the undersurface of the capsule within the rotator interval (Figure 2B). A radiofrequency wand, shaver, and elevator were used to define the biceps tendon and separate it from the undersurface of the capsule. The SLAP repair was performed using three 2.9-mm absorbable suture anchors with 2 posterior and 1 anterior to the biceps tendon insertion. The labral repair was observed while placing the shoulder through range of motion and the shoulder was seen to be free of any undue tension on the labrum.
Postoperatively, the patient’s sling and rehabilitation protocol was identical to that of the right shoulder. The patient progressed well, was released to full activity at 6 months, and has not returned with any further complaints of left or right shoulder pain. Approximately 3 years after treatment the patient was contacted via phone and asked about symptoms, pain, and activity. He denies current symptoms of clicking or instability and has no pain that he can identify as being related to previous pathology or treatment. Since the surgery, he has ceased competitive sports and weight lifting, which he attributes to deconditioning associated with postsurgical immobilization and lack of motivation.
Discussion
Of the 8 case reports in the literature that identified variable intra-articular biceps insertional anatomy, only 2 reports represented confluence of the biceps within the rotator interval.7 Interestingly, of the cases identified, the single case that presented a patient with similar pathology of a type II SLAP lesion had an almost identical anatomical variant presentation consisting of both the anomalous insertion of the LHBT into the undersurface of the rotator interval and a Buford variant of the anterosuperior glenohumeral ligament complex. To our knowledge, our bilateral case of an altered intra-articular biceps insertion and a concomitant SLAP tear supports the theory that this pattern of anomalous insertion may very well have altered the biomechanics of the tendon, resulting in acquired pathology to the superior labrum.
The literature reviewed showed the prevalence of anatomic variations of the LHBT ranged from 1.9% to 7.4%.13,14 These variations are generally considered benign; however, in some cases—as in the cases of the young athletes presented by Wahl and MacGillivray7 and in this report—anatomic variation may play an important role in pathogenesis of different injury patterns. The primary function of the LHBT is the stabilization of the glenohumeral joint during abduction and external rotation.15 When the insertion diverges from normal (eg, when the tendon is tethered to the undersurface of the rotator cuff), the biomechanical stresses on the tendon likely change. As a result of the anomalous position of the LHBT origin, there may be a change in the shoulder joint’s biomechanics, with increased strain on the glenohumeral ligament and its attachment onto the glenoid.16
This case report differs from publications on variable superior glenohumeral ligament attachments because a discrete superior glenohumeral ligament structure was isolated from the biceps tendon. Although a larger case series or patient cohort, as well as more involved biomechanical analysis, would certainly be necessary to prove our hypothesis, we believe that this case suggests certain anatomic LHBT and labral variations can contribute to the develop of SLAP tears in younger individuals.
The biceps brachii derives its name from the 2 heads of the muscle. The short head originates from the coracoid apex, with the coracobrachialis muscle. The long head of the biceps tendon (LHBT) starts within the capsule of the shoulder joint, running from the supraglenoid tubercle or labrum.1 The tendon typically runs free along its intra-articular course, but it is also extrasynovial and ensheathed by a continuation of the synovial lining of the articular capsule that extends to the inferior-most extent of the bicipital groove.2 Congenital anomalies of the LHBT are uncommon, although several atypical forms have been described. A literature search for anomalous LHBT identified several variations in anatomic descriptions, including Y-shaped variant, complete absence of tendon, extra-articular attachment, and a variety of intracapsular attachments. In all, 8 case reports of aberrant intracapsular attachment of LHBT3-12 were identified. These cases presented with a variety of clinical manifestations and pathologic changes. Often, these anatomic variations are considered innocuous, yet some present with pathologic findings.
We present the clinical, magnetic resonance imaging (MRI), and arthroscopic findings of a relatively young athletic patient who was experiencing symptoms of bilateral superior labrum anterior to posterior (SLAP) tears that were unresponsive to conservative management. A unique anatomic variant of the LHBT that involved confluence of the LHBT with the undersurface of the anterosuperior capsule at the rotator interval, as well as a Buford complex anteriorly, was identified and treated. We believe that the tethering of the biceps tendon to the capsule combined with the Buford complex created increased stress on the superior labrum and biceps anchor variant, leading to the development of bilateral symptomatic type II SLAP tears. Knowledge of this variant, though perhaps rare, may be relevant for diagnostic recognition of young athletic patients who present with recalcitrant shoulder symptoms. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Case Report
A 15-year-old healthy and active athletic boy presented with pain in the right shoulder without history of trauma. He was active in both swimming and baseball. He complained of pain that was present with activities, such as lifting weights, swimming, and throwing. His treatment prior to the office visit consisted of nonsteroidal anti-inflammatory medication, rest, and a therapy program initiated by his high school athletic trainer.
Physical examination demonstrated tenderness to palpation over the posterior capsule and biceps. Motion was full, cuff strength was normal, and SLAP signs (O’Brien, Speed, and Jobe relocation) were positive. A radiograph showed no sign of fracture or dislocation, and no evidence of bony abnormality.
The patient was sent for an MRI arthrogram, which showed a SLAP tear extending from 1 o’clock anteriorly to 10 o’clock posteriorly without intra-articular displacement. No rotator cuff tear was noted. The biceps tendon was noted to be unremarkable and located within the bicipital groove, although retrospective review of the MRI showed that the intra-articular biceps tendon was somewhat confluent with the adjacent tissues.
The patient underwent right shoulder arthroscopy. The shoulder was stable to ligamentous examination under anesthesia. Arthroscopic evaluation revealed that there was a type II SLAP tear extending from the 11-o’clock to the 2-o’clock positions. The superior glenohumeral ligament was identified as it arose from the upper pole of the glenoid labrum and then ran parallel and inferior to the tendon of the biceps towards the lesser tubercle. Surprisingly, there was a very unusual attachment of the intracapsular LHBT to the undersurface of the rotator interval, which restricted biceps excursion in relation to the rotator cuff. Additionally, there was a thick cord-like middle glenohumeral ligament anteriorly that lacked the normal glenoid attachments, thus representing a Buford complex. Interestingly, the labral tear could not only be displaced with a probe, but placing the shoulder through a range of motion also led to increased displacement of the labrum from the glenoid, likely because the biceps tendon was tethered to the undersurface of the capsule.
At the time of arthroscopy, the LHBT was released from its attachment to the capsule at the rotator interval with a radiofrequency wand and shaver. A labral repair was performed using three 2.9-mm bioabsorbable suture anchors, placing 2 posterior and 1 anterior to the biceps tendon. The integrity of the labral repair was observed while placing the shoulder through range of motion.
Postoperatively, the patient was kept in a sling for 5 weeks. Home exercises were initiated at 2 weeks, and outpatient physical therapy was implemented at 4 weeks. The patient resumed swimming, throwing, and other activities—with minimal discomfort—at 6 months postoperatively.
Three years after his initial visit, the patient returned to the office with a similar complaint of pain and limitation of function in his left shoulder after returning to full athletic competition. Once again, there was no history of injury, and history, physical examination, and MRI arthrogram (Figures 1A, 1B) evaluation proved to be very similar to this young athlete’s right shoulder work-up.
The patient once again underwent shoulder arthroscopy and treatment. Although this was now the left shoulder, the findings were essentially identical to the right shoulder. Once again, the labrum was detached from the 11-o’clock to 2-o’clock positions, and a Buford complex was present anteriorly (Figure 2A). The labral tear was easily displaceable from the glenoid with a probe, and placing the shoulder through a range of motion led to increased displacement of the labrum from the glenoid. There was also confluence of the intra-articular LHBT with the undersurface of the capsule within the rotator interval (Figure 2B). A radiofrequency wand, shaver, and elevator were used to define the biceps tendon and separate it from the undersurface of the capsule. The SLAP repair was performed using three 2.9-mm absorbable suture anchors with 2 posterior and 1 anterior to the biceps tendon insertion. The labral repair was observed while placing the shoulder through range of motion and the shoulder was seen to be free of any undue tension on the labrum.
Postoperatively, the patient’s sling and rehabilitation protocol was identical to that of the right shoulder. The patient progressed well, was released to full activity at 6 months, and has not returned with any further complaints of left or right shoulder pain. Approximately 3 years after treatment the patient was contacted via phone and asked about symptoms, pain, and activity. He denies current symptoms of clicking or instability and has no pain that he can identify as being related to previous pathology or treatment. Since the surgery, he has ceased competitive sports and weight lifting, which he attributes to deconditioning associated with postsurgical immobilization and lack of motivation.
Discussion
Of the 8 case reports in the literature that identified variable intra-articular biceps insertional anatomy, only 2 reports represented confluence of the biceps within the rotator interval.7 Interestingly, of the cases identified, the single case that presented a patient with similar pathology of a type II SLAP lesion had an almost identical anatomical variant presentation consisting of both the anomalous insertion of the LHBT into the undersurface of the rotator interval and a Buford variant of the anterosuperior glenohumeral ligament complex. To our knowledge, our bilateral case of an altered intra-articular biceps insertion and a concomitant SLAP tear supports the theory that this pattern of anomalous insertion may very well have altered the biomechanics of the tendon, resulting in acquired pathology to the superior labrum.
The literature reviewed showed the prevalence of anatomic variations of the LHBT ranged from 1.9% to 7.4%.13,14 These variations are generally considered benign; however, in some cases—as in the cases of the young athletes presented by Wahl and MacGillivray7 and in this report—anatomic variation may play an important role in pathogenesis of different injury patterns. The primary function of the LHBT is the stabilization of the glenohumeral joint during abduction and external rotation.15 When the insertion diverges from normal (eg, when the tendon is tethered to the undersurface of the rotator cuff), the biomechanical stresses on the tendon likely change. As a result of the anomalous position of the LHBT origin, there may be a change in the shoulder joint’s biomechanics, with increased strain on the glenohumeral ligament and its attachment onto the glenoid.16
This case report differs from publications on variable superior glenohumeral ligament attachments because a discrete superior glenohumeral ligament structure was isolated from the biceps tendon. Although a larger case series or patient cohort, as well as more involved biomechanical analysis, would certainly be necessary to prove our hypothesis, we believe that this case suggests certain anatomic LHBT and labral variations can contribute to the develop of SLAP tears in younger individuals.
1. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
2. Burkhead WZ Jr. The biceps tendon. In: Rockwood CA Jr, Matsen FA III, eds. The Shoulder. Vol. 2. Philadelphia: WB Saunders; 1990:791-836.
3. Parikh SN, Bonnaig N, Zbojniewicz A. Intracapsular origin of the long head biceps tendon with glenoid avulsion of the glenohumeral ligaments. Orthopedics. 2011;34(11):781-784.
4. Gaskin CM, Golish SR, Blount KJ, Diduch DR. Anomalies of the long head of the biceps brachii tendon: clinical significance, MR arthrographic findings, and arthroscopic correlation in two patients. Skeletal Radiol. 2007;36(8):785-789.
5. Yeh L, Pedowitz R, Kwak S, et al. Intracapsular origin of the long head of the biceps tendon. Skeletal Radiol. 1999;28(3):178-181.
6. Richards DP, Schwartz M. Anomalous intraarticular origin of the long head of the biceps brachii. Clin J Sport Med. 2003;13(2):122-124.
7. Wahl CJ, MacGillivray JD. Three congenital variations in the long head of the biceps tendon: a review of the pathoanatomic considerations and case reports. J Shoulder Elbow Surg. 2007;16(6):e25-e30.I
8. Egea JM, Melguizo C, Prados J, Aránega A. Capsular origin of the long head of the biceps tendon: a clinical case. Rom J Morphol Embryol. 2010;51(2):375-377.
9. Hyman JL, Warren RF. Extra-articular origin of biceps brachii. Arthroscopy. 2001;17(7): E29.
10. Enad JG. Bifurcate origin of the long head of the biceps tendon. Arthroscopy. 2004;20(10):1081-1083.
11. Mariani PP, Bellelli A, Botticella C. Arthroscopic absence of the long head of the biceps tendon. Arthroscopy. 1997;13(4):499-501.
12. Koplas MC, Winalski CS, Ulmer WH Jr, Recht M. Bilateral congenital absence of the long head of the biceps tendon. Skeletal Radiol. 2009;38(7):715-719.
13. Kanatli U, Ozturk BY, Eisen E, Bolukbasi S. Intra-articular variations of the long head of the biceps tendon. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1576-1581.
14. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
15. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
16. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;(330):13-30.
1. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994;76(6):951-954.
2. Burkhead WZ Jr. The biceps tendon. In: Rockwood CA Jr, Matsen FA III, eds. The Shoulder. Vol. 2. Philadelphia: WB Saunders; 1990:791-836.
3. Parikh SN, Bonnaig N, Zbojniewicz A. Intracapsular origin of the long head biceps tendon with glenoid avulsion of the glenohumeral ligaments. Orthopedics. 2011;34(11):781-784.
4. Gaskin CM, Golish SR, Blount KJ, Diduch DR. Anomalies of the long head of the biceps brachii tendon: clinical significance, MR arthrographic findings, and arthroscopic correlation in two patients. Skeletal Radiol. 2007;36(8):785-789.
5. Yeh L, Pedowitz R, Kwak S, et al. Intracapsular origin of the long head of the biceps tendon. Skeletal Radiol. 1999;28(3):178-181.
6. Richards DP, Schwartz M. Anomalous intraarticular origin of the long head of the biceps brachii. Clin J Sport Med. 2003;13(2):122-124.
7. Wahl CJ, MacGillivray JD. Three congenital variations in the long head of the biceps tendon: a review of the pathoanatomic considerations and case reports. J Shoulder Elbow Surg. 2007;16(6):e25-e30.I
8. Egea JM, Melguizo C, Prados J, Aránega A. Capsular origin of the long head of the biceps tendon: a clinical case. Rom J Morphol Embryol. 2010;51(2):375-377.
9. Hyman JL, Warren RF. Extra-articular origin of biceps brachii. Arthroscopy. 2001;17(7): E29.
10. Enad JG. Bifurcate origin of the long head of the biceps tendon. Arthroscopy. 2004;20(10):1081-1083.
11. Mariani PP, Bellelli A, Botticella C. Arthroscopic absence of the long head of the biceps tendon. Arthroscopy. 1997;13(4):499-501.
12. Koplas MC, Winalski CS, Ulmer WH Jr, Recht M. Bilateral congenital absence of the long head of the biceps tendon. Skeletal Radiol. 2009;38(7):715-719.
13. Kanatli U, Ozturk BY, Eisen E, Bolukbasi S. Intra-articular variations of the long head of the biceps tendon. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1576-1581.
14. Dierickx C, Ceccarelli E, Conti M, Vanlommel J, Castagna A. Variations of the intra-articular portion of the long head of the biceps tendon: a classification of embryologically explained variations. J Shoulder Elbow Surg. 2009;18(4):556-565.
15. Rodosky MW, Harner CD, Fu FH. The role of the long head of the biceps muscle and superior glenoid labrum in anterior stability of the shoulder. Am J Sports Med. 1994;22(1):121-130.
16. Bigliani LU, Kelkar R, Flatow EL, Pollock RG, Mow VC. Glenohumeral stability. Biomechanical properties of passive and active stabilizers. Clin Orthop Relat Res. 1996;(330):13-30.
Xanthogranulomatous Osteomyelitis of Proximal Femur Masquerading as Benign Bone Tumor
Xanthogranulomatous osteomyelitis (XO) is a type of chronic inflammatory process that is characterized by the collection of foamy macrophages along with mononuclear cells in the tissue.1 Xanthogranulomatous osteomyelitis is characterized by the presence of granular, eosinophilic, periodic acid–Schiff–positive histiocytes in the initial stages, followed by the mixture of foamy macrophages and activated plasma cells and, last, by the presence of suppurative foci and hemorrhage. This is an uncommon process best known to occur in the gallbladder, kidney, urinary bladder, fallopian tube, ovary, vagina, prostate, testis, epididymis, colon, and appendix.2-4 Very rarely, it can affect lungs, brain, or bone. Only 5 cases of XO have been reported in the literature.5-8
We report XO of the proximal femur in a 65-year-old woman who initially had a clinical and radiologic diagnosis of aneurysmal bone cyst; however, histopathologic examination confirmed the diagnosis of XO. Xanthogranulomatous osteomyelitis mimics a neoplastic pathology in gallbladder, kidney, and prostrate on gross clinical and radiologic examination.9 The pathogenesis of XO is best characterized by a delayed type of hypersensitivity reaction.10 The differential diagnosis includes chronic recurrent multifocal osteomyelitis, xanthoma, infiltrative storage disorder, malakoplakia, Langerhans cell histiocytosis, fibrohistiocytic tumor, Erdheim-Chester disease, and metastatic renal cell carcinoma.11-14 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 65-year-old hypertensive woman presented with complaints of pain in the right hip for a duration of 6 months. Pain was radiating from the right hip region to the anteromedial aspect of the knee and progressively increasing, with a history of pain at rest suggestive of a nonmechanical pathology in the hip. There was no history of fever, weight loss, loss of appetite, pain in any other joint, or morning stiffness. The patient was mobile without support and was able to squat and sit cross-legged; however, the stance phase on the right side was less than on the left side, suggestive of an antalgic component in the gait.
On examining the patient, there was anterior hip joint tenderness with no local sign of any infective or inflammatory pathology. Trochanteric tenderness was present, but there was no irregularity, broadening, or thickening of the trochanter. There was no restriction in the range of motion, and no coronal or sagittal plane deformity in the right hip. There was no limb-length discrepancy. However, the patient was not able to raise her leg actively, probably because of pain in the right hip.
On plain radiographs of the pelvis with bilateral hips, a well-defined nonexpansile uniloculated lytic lesion with sclerotic margins was present in the neck of the right femur, extending to the intertrochanteric area (Figure 1). Ground-glass appearance was also noted. Considering the benign nature of the lesion radiologically and clinically, a differential diagnosis of hyperparathyroidism, renal osteodystrophy, multiple myeloma, and fibrous dysplasia was considered. Hematologic investigations, skeletal survey, and magnetic resonance imaging (MRI) of the bilateral hips were performed to rule out the differential diagnosis.
The patient’s hemoglobin level was 11.8 g/dL with total white blood cell count of 10,300/µL. Renal and hepatic functions were within normal limit. Serum erythrocyte sedimentation rate (ESR) was 12 mm/h and C-reactive protein level was normal. Serum parathyroid level was 32 pg/mL, which was within normal limits, with an alkaline phosphatase level of 101 U/L. The skeletal survey showed no other bony lesion in the body. T1-weighted MRI of both hips showed a well-defined hypointense lesion in the neck and intertrochanteric area of the right hip, which was hyperintense on T2-weighted MRI, suggestive of aneurysmal bone cyst (Figure 2).
Normal ESR, hemoglobin, alkaline phosphatase, and serum parathyroid levels and normal skeletal survey almost ruled out multiple myeloma and hyperparathyroidism. Normal renal profile ruled out renal osteodystrophy and the osteitis fibrosa cystica lesion associated with it. We planned for prophylactic internal fixation of the lesion to prevent a pathologic fracture. According to Mirels,15 if there is a lytic lesion covering more than two-thirds of the circumference of the bone in the peritrochanteric area, the chances of a pathologic fracture are high and such fractures should be fixed.
We planned for curettage of the lesion with bone grafting and in situ intramedullary fixation of the lesion. Curettage was done according to the plan and the sample was sent for histopathologic examination. In situ internal fixation and bone grafting were performed by using a proximal femoral intramedullary nail. To our surprise, the biopsy sample was reported as xanthogranuloma, with multiple foamy macrophages mixed with inflammatory cells and aggregates of lymphocytes (Figure 3). Mycobacterial and routine bacterial cultures were reported as negative. The patient was kept on oral antibiotics (cefixime and moxifloxacin) for 6 weeks, and she made an uneventful recovery. At 6-month follow-up, a radiograph of the right hip showed a healed lesion with proximal femoral nail in situ (Figure 4).
Discussion
To the best of our knowledge, a total of 5 cases of XO have been reported in the literature. The earliest of these reports were by Cozzutto and Carbone,1 who reported 2 cases of XO of the first rib and of the epiphysis of the tibia, respectively. The importance of these lesions to diagnosis is their confusion with a neoplastic disease, as XO is itself a benign disorder. These lesions can mimic a neoplastic lesion in clinical and radiologic presentation and the only way to differentiate the lesion from a neoplastic disease is by histopathologic examination of the tissue. Hypothetically, xanthogranulomatous disorders can be related to trauma or infection.
In 2007, Vankalakunti and colleagues6 reported XO of the ulna in a 50-year-old postmenopausal woman. In that case, progressive swelling was present on the extensor aspect of her right forearm for a period of 2 years, for which curettage and bone grafting were performed, using autograft from the ipsilateral iliac crest. The tissue culture was sterile, and XO was diagnosed as a result of the histopathologic examination. In 2009, Cennimo and colleagues7 reported XO of the index finger and wrist of a man complaining of pain and swelling for 1 year, which was unresponsive to antibiotics. The diagnosis of XO was confirmed histopathologically, when the culture of the same tissue grew Mycobacterium marinum. Radical synovectomy of the lesion was performed, after which minocycline, clarithromycin, and ethambutol were administered. In 2012, Borjian and colleagues8 reported a case of XO of the proximal humerus and proximal fibula in a 14-year-old child. The child, who presented with fever, pain, and restriction of shoulder movements, was started on oral antibiotics as the tissue culture grew Staphylococcus aureus; the patient did not complete the course of treatment in the hospital. No surgical intervention was done in this case. The diagnosis of XO was confirmed by microscopic examination of the tissue.
An association between bacterial infection and xanthogranulomatous inflammation has existed in several organs, such as the kidneys, and in the gastrointestinal system, but such an association of the 2 is yet to be determined for bone.5,10,16-19 Because of the paucity of literature on the disease, a management protocol for XO of bone has not been defined, and decisions have to be made considering the natural history of the disease in other organs. We present this case primarily because of its rarity, curability, and its close resemblance to bone tumors. While XO is benign, it can mimic a neoplastic bone lesion in its imaging and clinical manifestations, and appropriate differentiation is crucial. Currently, histopathologic examination of lesions is the most specific and is the gold standard for diagnosis.
Conclusion
Xanthogranulomatous osteomyelitis is a very rare entity, and only a few cases have been reported in the English-language literature. Though rare, XO warrants greater emphasis than it receives in the literature. It is a chronic inflammatory disease having a close resemblance to bone tumors. A high index of suspicion must be practiced to differentiate XO from tumors. Histopathologic examination is mandatory to establish definitive diagnosis and correct treatment.
1. Cozzutto C, Carbone A. The xanthogranulomatous process. Xanthogranulomatous inflammation. Pathol Res Pract. 1988;183(4):395-402.
2. Ladefoged C, Lorentzen M. Xanthogranulomatous cholecystitis. A clinicopathological study of 20 cases and review of the literature. APMIS. 1993;101(11):869-875.
3. Nistal M, Gonzalez-Peramato P, Serrano A, Regadera J. Xanthogranulomatous funiculitis and orchiepididymitis: report of 2 cases with immunohistochemical study and literature review. Arch Pathol Lab Med. 2004;128(8):911-914.
4. Oh YH, Seong SS, Jang KS, et al. Xanthogranulomatous inflammation presenting as a submucosal mass of the sigmoid colon. Pathol Int. 2005;55(7):440-444.
5. Cozzutto C. Xanthogranulomatous osteomyelitis. Arch Pathol Lab Med. 1984;108(12):973-6.
6. Vankalakunti M, Saikia UN, Mathew M, Kang M. Xanthogranulomatous osteomyelitis of ulna mimicking neoplasm. World J Surg Oncol. 2007;30(5):46.
7. Cennimo DJ, Agag R, Fleegler E, et al. Mycobacterium marinum hand infection in a “sushi chef.” Eplasty. 2009;14(9):e43.
8. Borjian A, Rezaei F, Eshaghi MA, Shemshaki H. Xanthogranulomatous osteomyelitis. J Orthop Traumatol. 2012;13(4):217-220.
9. Rafique M, Yaqoob N. Xanthogranulomatous prostatitis: a mimic of carcinoma of prostate. World J Surg Oncol. 2006;4:30.
10. Nakashiro H, Haraoka S, Fujiwara K, Harada S, Hisatsugu T, Watanabe T. Xanthogranulomatous cholecystis. Cell composition and a possible pathogenetic role of cell-mediated immunity. Pathol Res Pract. 1995;191(11):1078-1086.
11. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
12. Kossard S, Chow E, Wilkinson B, Killingsworth M. Lipid and giant cell poor necrobiotic xanthogranuloma. J Cutan Pathol. 2000;27(7):374-378.
13. Girschick HJ, Huppertz HI, Harmsen D, Krauspe R, Müller-Hermelink HK, Papadopoulos T. Chronic recurrent multifocal osteomyelitis in children: diagnostic value of histopathology and microbial testing. Hum Pathol. 1999;30(1):59-65.
14. Kayser R, Mahlfeld K, Grasshoff H. Vertebral Langerhans-cell histiocytosis in childhood – a differential diagnosis of spinal osteomyelitis. Klin Padiatr. 1999;211(5):399-402.
15. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-264.
16. Machiz S, Gordon J, Block N, Politano VA. Salmonella typhosa urinary tract infection and xanthogranulomatous pyelonephritis. Case report and review of literature. J Fla Med Assoc. 1974;61(9):703-705.
17. Gauperaa T, Stalsberg H. Renal endometriosis. A case report. Scand J Urol Nephrol. 1977;11(2):189-191.
18. Goodman M, Curry T, Russell T. Xanthogranulomatous pyelonephritis (XGP): a local disease with systemic manifestations. Report of 23 patients and review of the literature. Medicine. 1979;58(2):171-181.
19. Guarino M, Reale D, Micoli G, Tricomi P, Cristofori E. Xanthogranulomatous gastritis: association with xanthogranulomatous cholecystitis. J Clin Pathol. 1993;46(1):88-90.
Xanthogranulomatous osteomyelitis (XO) is a type of chronic inflammatory process that is characterized by the collection of foamy macrophages along with mononuclear cells in the tissue.1 Xanthogranulomatous osteomyelitis is characterized by the presence of granular, eosinophilic, periodic acid–Schiff–positive histiocytes in the initial stages, followed by the mixture of foamy macrophages and activated plasma cells and, last, by the presence of suppurative foci and hemorrhage. This is an uncommon process best known to occur in the gallbladder, kidney, urinary bladder, fallopian tube, ovary, vagina, prostate, testis, epididymis, colon, and appendix.2-4 Very rarely, it can affect lungs, brain, or bone. Only 5 cases of XO have been reported in the literature.5-8
We report XO of the proximal femur in a 65-year-old woman who initially had a clinical and radiologic diagnosis of aneurysmal bone cyst; however, histopathologic examination confirmed the diagnosis of XO. Xanthogranulomatous osteomyelitis mimics a neoplastic pathology in gallbladder, kidney, and prostrate on gross clinical and radiologic examination.9 The pathogenesis of XO is best characterized by a delayed type of hypersensitivity reaction.10 The differential diagnosis includes chronic recurrent multifocal osteomyelitis, xanthoma, infiltrative storage disorder, malakoplakia, Langerhans cell histiocytosis, fibrohistiocytic tumor, Erdheim-Chester disease, and metastatic renal cell carcinoma.11-14 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 65-year-old hypertensive woman presented with complaints of pain in the right hip for a duration of 6 months. Pain was radiating from the right hip region to the anteromedial aspect of the knee and progressively increasing, with a history of pain at rest suggestive of a nonmechanical pathology in the hip. There was no history of fever, weight loss, loss of appetite, pain in any other joint, or morning stiffness. The patient was mobile without support and was able to squat and sit cross-legged; however, the stance phase on the right side was less than on the left side, suggestive of an antalgic component in the gait.
On examining the patient, there was anterior hip joint tenderness with no local sign of any infective or inflammatory pathology. Trochanteric tenderness was present, but there was no irregularity, broadening, or thickening of the trochanter. There was no restriction in the range of motion, and no coronal or sagittal plane deformity in the right hip. There was no limb-length discrepancy. However, the patient was not able to raise her leg actively, probably because of pain in the right hip.
On plain radiographs of the pelvis with bilateral hips, a well-defined nonexpansile uniloculated lytic lesion with sclerotic margins was present in the neck of the right femur, extending to the intertrochanteric area (Figure 1). Ground-glass appearance was also noted. Considering the benign nature of the lesion radiologically and clinically, a differential diagnosis of hyperparathyroidism, renal osteodystrophy, multiple myeloma, and fibrous dysplasia was considered. Hematologic investigations, skeletal survey, and magnetic resonance imaging (MRI) of the bilateral hips were performed to rule out the differential diagnosis.
The patient’s hemoglobin level was 11.8 g/dL with total white blood cell count of 10,300/µL. Renal and hepatic functions were within normal limit. Serum erythrocyte sedimentation rate (ESR) was 12 mm/h and C-reactive protein level was normal. Serum parathyroid level was 32 pg/mL, which was within normal limits, with an alkaline phosphatase level of 101 U/L. The skeletal survey showed no other bony lesion in the body. T1-weighted MRI of both hips showed a well-defined hypointense lesion in the neck and intertrochanteric area of the right hip, which was hyperintense on T2-weighted MRI, suggestive of aneurysmal bone cyst (Figure 2).
Normal ESR, hemoglobin, alkaline phosphatase, and serum parathyroid levels and normal skeletal survey almost ruled out multiple myeloma and hyperparathyroidism. Normal renal profile ruled out renal osteodystrophy and the osteitis fibrosa cystica lesion associated with it. We planned for prophylactic internal fixation of the lesion to prevent a pathologic fracture. According to Mirels,15 if there is a lytic lesion covering more than two-thirds of the circumference of the bone in the peritrochanteric area, the chances of a pathologic fracture are high and such fractures should be fixed.
We planned for curettage of the lesion with bone grafting and in situ intramedullary fixation of the lesion. Curettage was done according to the plan and the sample was sent for histopathologic examination. In situ internal fixation and bone grafting were performed by using a proximal femoral intramedullary nail. To our surprise, the biopsy sample was reported as xanthogranuloma, with multiple foamy macrophages mixed with inflammatory cells and aggregates of lymphocytes (Figure 3). Mycobacterial and routine bacterial cultures were reported as negative. The patient was kept on oral antibiotics (cefixime and moxifloxacin) for 6 weeks, and she made an uneventful recovery. At 6-month follow-up, a radiograph of the right hip showed a healed lesion with proximal femoral nail in situ (Figure 4).
Discussion
To the best of our knowledge, a total of 5 cases of XO have been reported in the literature. The earliest of these reports were by Cozzutto and Carbone,1 who reported 2 cases of XO of the first rib and of the epiphysis of the tibia, respectively. The importance of these lesions to diagnosis is their confusion with a neoplastic disease, as XO is itself a benign disorder. These lesions can mimic a neoplastic lesion in clinical and radiologic presentation and the only way to differentiate the lesion from a neoplastic disease is by histopathologic examination of the tissue. Hypothetically, xanthogranulomatous disorders can be related to trauma or infection.
In 2007, Vankalakunti and colleagues6 reported XO of the ulna in a 50-year-old postmenopausal woman. In that case, progressive swelling was present on the extensor aspect of her right forearm for a period of 2 years, for which curettage and bone grafting were performed, using autograft from the ipsilateral iliac crest. The tissue culture was sterile, and XO was diagnosed as a result of the histopathologic examination. In 2009, Cennimo and colleagues7 reported XO of the index finger and wrist of a man complaining of pain and swelling for 1 year, which was unresponsive to antibiotics. The diagnosis of XO was confirmed histopathologically, when the culture of the same tissue grew Mycobacterium marinum. Radical synovectomy of the lesion was performed, after which minocycline, clarithromycin, and ethambutol were administered. In 2012, Borjian and colleagues8 reported a case of XO of the proximal humerus and proximal fibula in a 14-year-old child. The child, who presented with fever, pain, and restriction of shoulder movements, was started on oral antibiotics as the tissue culture grew Staphylococcus aureus; the patient did not complete the course of treatment in the hospital. No surgical intervention was done in this case. The diagnosis of XO was confirmed by microscopic examination of the tissue.
An association between bacterial infection and xanthogranulomatous inflammation has existed in several organs, such as the kidneys, and in the gastrointestinal system, but such an association of the 2 is yet to be determined for bone.5,10,16-19 Because of the paucity of literature on the disease, a management protocol for XO of bone has not been defined, and decisions have to be made considering the natural history of the disease in other organs. We present this case primarily because of its rarity, curability, and its close resemblance to bone tumors. While XO is benign, it can mimic a neoplastic bone lesion in its imaging and clinical manifestations, and appropriate differentiation is crucial. Currently, histopathologic examination of lesions is the most specific and is the gold standard for diagnosis.
Conclusion
Xanthogranulomatous osteomyelitis is a very rare entity, and only a few cases have been reported in the English-language literature. Though rare, XO warrants greater emphasis than it receives in the literature. It is a chronic inflammatory disease having a close resemblance to bone tumors. A high index of suspicion must be practiced to differentiate XO from tumors. Histopathologic examination is mandatory to establish definitive diagnosis and correct treatment.
Xanthogranulomatous osteomyelitis (XO) is a type of chronic inflammatory process that is characterized by the collection of foamy macrophages along with mononuclear cells in the tissue.1 Xanthogranulomatous osteomyelitis is characterized by the presence of granular, eosinophilic, periodic acid–Schiff–positive histiocytes in the initial stages, followed by the mixture of foamy macrophages and activated plasma cells and, last, by the presence of suppurative foci and hemorrhage. This is an uncommon process best known to occur in the gallbladder, kidney, urinary bladder, fallopian tube, ovary, vagina, prostate, testis, epididymis, colon, and appendix.2-4 Very rarely, it can affect lungs, brain, or bone. Only 5 cases of XO have been reported in the literature.5-8
We report XO of the proximal femur in a 65-year-old woman who initially had a clinical and radiologic diagnosis of aneurysmal bone cyst; however, histopathologic examination confirmed the diagnosis of XO. Xanthogranulomatous osteomyelitis mimics a neoplastic pathology in gallbladder, kidney, and prostrate on gross clinical and radiologic examination.9 The pathogenesis of XO is best characterized by a delayed type of hypersensitivity reaction.10 The differential diagnosis includes chronic recurrent multifocal osteomyelitis, xanthoma, infiltrative storage disorder, malakoplakia, Langerhans cell histiocytosis, fibrohistiocytic tumor, Erdheim-Chester disease, and metastatic renal cell carcinoma.11-14 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 65-year-old hypertensive woman presented with complaints of pain in the right hip for a duration of 6 months. Pain was radiating from the right hip region to the anteromedial aspect of the knee and progressively increasing, with a history of pain at rest suggestive of a nonmechanical pathology in the hip. There was no history of fever, weight loss, loss of appetite, pain in any other joint, or morning stiffness. The patient was mobile without support and was able to squat and sit cross-legged; however, the stance phase on the right side was less than on the left side, suggestive of an antalgic component in the gait.
On examining the patient, there was anterior hip joint tenderness with no local sign of any infective or inflammatory pathology. Trochanteric tenderness was present, but there was no irregularity, broadening, or thickening of the trochanter. There was no restriction in the range of motion, and no coronal or sagittal plane deformity in the right hip. There was no limb-length discrepancy. However, the patient was not able to raise her leg actively, probably because of pain in the right hip.
On plain radiographs of the pelvis with bilateral hips, a well-defined nonexpansile uniloculated lytic lesion with sclerotic margins was present in the neck of the right femur, extending to the intertrochanteric area (Figure 1). Ground-glass appearance was also noted. Considering the benign nature of the lesion radiologically and clinically, a differential diagnosis of hyperparathyroidism, renal osteodystrophy, multiple myeloma, and fibrous dysplasia was considered. Hematologic investigations, skeletal survey, and magnetic resonance imaging (MRI) of the bilateral hips were performed to rule out the differential diagnosis.
The patient’s hemoglobin level was 11.8 g/dL with total white blood cell count of 10,300/µL. Renal and hepatic functions were within normal limit. Serum erythrocyte sedimentation rate (ESR) was 12 mm/h and C-reactive protein level was normal. Serum parathyroid level was 32 pg/mL, which was within normal limits, with an alkaline phosphatase level of 101 U/L. The skeletal survey showed no other bony lesion in the body. T1-weighted MRI of both hips showed a well-defined hypointense lesion in the neck and intertrochanteric area of the right hip, which was hyperintense on T2-weighted MRI, suggestive of aneurysmal bone cyst (Figure 2).
Normal ESR, hemoglobin, alkaline phosphatase, and serum parathyroid levels and normal skeletal survey almost ruled out multiple myeloma and hyperparathyroidism. Normal renal profile ruled out renal osteodystrophy and the osteitis fibrosa cystica lesion associated with it. We planned for prophylactic internal fixation of the lesion to prevent a pathologic fracture. According to Mirels,15 if there is a lytic lesion covering more than two-thirds of the circumference of the bone in the peritrochanteric area, the chances of a pathologic fracture are high and such fractures should be fixed.
We planned for curettage of the lesion with bone grafting and in situ intramedullary fixation of the lesion. Curettage was done according to the plan and the sample was sent for histopathologic examination. In situ internal fixation and bone grafting were performed by using a proximal femoral intramedullary nail. To our surprise, the biopsy sample was reported as xanthogranuloma, with multiple foamy macrophages mixed with inflammatory cells and aggregates of lymphocytes (Figure 3). Mycobacterial and routine bacterial cultures were reported as negative. The patient was kept on oral antibiotics (cefixime and moxifloxacin) for 6 weeks, and she made an uneventful recovery. At 6-month follow-up, a radiograph of the right hip showed a healed lesion with proximal femoral nail in situ (Figure 4).
Discussion
To the best of our knowledge, a total of 5 cases of XO have been reported in the literature. The earliest of these reports were by Cozzutto and Carbone,1 who reported 2 cases of XO of the first rib and of the epiphysis of the tibia, respectively. The importance of these lesions to diagnosis is their confusion with a neoplastic disease, as XO is itself a benign disorder. These lesions can mimic a neoplastic lesion in clinical and radiologic presentation and the only way to differentiate the lesion from a neoplastic disease is by histopathologic examination of the tissue. Hypothetically, xanthogranulomatous disorders can be related to trauma or infection.
In 2007, Vankalakunti and colleagues6 reported XO of the ulna in a 50-year-old postmenopausal woman. In that case, progressive swelling was present on the extensor aspect of her right forearm for a period of 2 years, for which curettage and bone grafting were performed, using autograft from the ipsilateral iliac crest. The tissue culture was sterile, and XO was diagnosed as a result of the histopathologic examination. In 2009, Cennimo and colleagues7 reported XO of the index finger and wrist of a man complaining of pain and swelling for 1 year, which was unresponsive to antibiotics. The diagnosis of XO was confirmed histopathologically, when the culture of the same tissue grew Mycobacterium marinum. Radical synovectomy of the lesion was performed, after which minocycline, clarithromycin, and ethambutol were administered. In 2012, Borjian and colleagues8 reported a case of XO of the proximal humerus and proximal fibula in a 14-year-old child. The child, who presented with fever, pain, and restriction of shoulder movements, was started on oral antibiotics as the tissue culture grew Staphylococcus aureus; the patient did not complete the course of treatment in the hospital. No surgical intervention was done in this case. The diagnosis of XO was confirmed by microscopic examination of the tissue.
An association between bacterial infection and xanthogranulomatous inflammation has existed in several organs, such as the kidneys, and in the gastrointestinal system, but such an association of the 2 is yet to be determined for bone.5,10,16-19 Because of the paucity of literature on the disease, a management protocol for XO of bone has not been defined, and decisions have to be made considering the natural history of the disease in other organs. We present this case primarily because of its rarity, curability, and its close resemblance to bone tumors. While XO is benign, it can mimic a neoplastic bone lesion in its imaging and clinical manifestations, and appropriate differentiation is crucial. Currently, histopathologic examination of lesions is the most specific and is the gold standard for diagnosis.
Conclusion
Xanthogranulomatous osteomyelitis is a very rare entity, and only a few cases have been reported in the English-language literature. Though rare, XO warrants greater emphasis than it receives in the literature. It is a chronic inflammatory disease having a close resemblance to bone tumors. A high index of suspicion must be practiced to differentiate XO from tumors. Histopathologic examination is mandatory to establish definitive diagnosis and correct treatment.
1. Cozzutto C, Carbone A. The xanthogranulomatous process. Xanthogranulomatous inflammation. Pathol Res Pract. 1988;183(4):395-402.
2. Ladefoged C, Lorentzen M. Xanthogranulomatous cholecystitis. A clinicopathological study of 20 cases and review of the literature. APMIS. 1993;101(11):869-875.
3. Nistal M, Gonzalez-Peramato P, Serrano A, Regadera J. Xanthogranulomatous funiculitis and orchiepididymitis: report of 2 cases with immunohistochemical study and literature review. Arch Pathol Lab Med. 2004;128(8):911-914.
4. Oh YH, Seong SS, Jang KS, et al. Xanthogranulomatous inflammation presenting as a submucosal mass of the sigmoid colon. Pathol Int. 2005;55(7):440-444.
5. Cozzutto C. Xanthogranulomatous osteomyelitis. Arch Pathol Lab Med. 1984;108(12):973-6.
6. Vankalakunti M, Saikia UN, Mathew M, Kang M. Xanthogranulomatous osteomyelitis of ulna mimicking neoplasm. World J Surg Oncol. 2007;30(5):46.
7. Cennimo DJ, Agag R, Fleegler E, et al. Mycobacterium marinum hand infection in a “sushi chef.” Eplasty. 2009;14(9):e43.
8. Borjian A, Rezaei F, Eshaghi MA, Shemshaki H. Xanthogranulomatous osteomyelitis. J Orthop Traumatol. 2012;13(4):217-220.
9. Rafique M, Yaqoob N. Xanthogranulomatous prostatitis: a mimic of carcinoma of prostate. World J Surg Oncol. 2006;4:30.
10. Nakashiro H, Haraoka S, Fujiwara K, Harada S, Hisatsugu T, Watanabe T. Xanthogranulomatous cholecystis. Cell composition and a possible pathogenetic role of cell-mediated immunity. Pathol Res Pract. 1995;191(11):1078-1086.
11. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
12. Kossard S, Chow E, Wilkinson B, Killingsworth M. Lipid and giant cell poor necrobiotic xanthogranuloma. J Cutan Pathol. 2000;27(7):374-378.
13. Girschick HJ, Huppertz HI, Harmsen D, Krauspe R, Müller-Hermelink HK, Papadopoulos T. Chronic recurrent multifocal osteomyelitis in children: diagnostic value of histopathology and microbial testing. Hum Pathol. 1999;30(1):59-65.
14. Kayser R, Mahlfeld K, Grasshoff H. Vertebral Langerhans-cell histiocytosis in childhood – a differential diagnosis of spinal osteomyelitis. Klin Padiatr. 1999;211(5):399-402.
15. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-264.
16. Machiz S, Gordon J, Block N, Politano VA. Salmonella typhosa urinary tract infection and xanthogranulomatous pyelonephritis. Case report and review of literature. J Fla Med Assoc. 1974;61(9):703-705.
17. Gauperaa T, Stalsberg H. Renal endometriosis. A case report. Scand J Urol Nephrol. 1977;11(2):189-191.
18. Goodman M, Curry T, Russell T. Xanthogranulomatous pyelonephritis (XGP): a local disease with systemic manifestations. Report of 23 patients and review of the literature. Medicine. 1979;58(2):171-181.
19. Guarino M, Reale D, Micoli G, Tricomi P, Cristofori E. Xanthogranulomatous gastritis: association with xanthogranulomatous cholecystitis. J Clin Pathol. 1993;46(1):88-90.
1. Cozzutto C, Carbone A. The xanthogranulomatous process. Xanthogranulomatous inflammation. Pathol Res Pract. 1988;183(4):395-402.
2. Ladefoged C, Lorentzen M. Xanthogranulomatous cholecystitis. A clinicopathological study of 20 cases and review of the literature. APMIS. 1993;101(11):869-875.
3. Nistal M, Gonzalez-Peramato P, Serrano A, Regadera J. Xanthogranulomatous funiculitis and orchiepididymitis: report of 2 cases with immunohistochemical study and literature review. Arch Pathol Lab Med. 2004;128(8):911-914.
4. Oh YH, Seong SS, Jang KS, et al. Xanthogranulomatous inflammation presenting as a submucosal mass of the sigmoid colon. Pathol Int. 2005;55(7):440-444.
5. Cozzutto C. Xanthogranulomatous osteomyelitis. Arch Pathol Lab Med. 1984;108(12):973-6.
6. Vankalakunti M, Saikia UN, Mathew M, Kang M. Xanthogranulomatous osteomyelitis of ulna mimicking neoplasm. World J Surg Oncol. 2007;30(5):46.
7. Cennimo DJ, Agag R, Fleegler E, et al. Mycobacterium marinum hand infection in a “sushi chef.” Eplasty. 2009;14(9):e43.
8. Borjian A, Rezaei F, Eshaghi MA, Shemshaki H. Xanthogranulomatous osteomyelitis. J Orthop Traumatol. 2012;13(4):217-220.
9. Rafique M, Yaqoob N. Xanthogranulomatous prostatitis: a mimic of carcinoma of prostate. World J Surg Oncol. 2006;4:30.
10. Nakashiro H, Haraoka S, Fujiwara K, Harada S, Hisatsugu T, Watanabe T. Xanthogranulomatous cholecystis. Cell composition and a possible pathogenetic role of cell-mediated immunity. Pathol Res Pract. 1995;191(11):1078-1086.
11. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
12. Kossard S, Chow E, Wilkinson B, Killingsworth M. Lipid and giant cell poor necrobiotic xanthogranuloma. J Cutan Pathol. 2000;27(7):374-378.
13. Girschick HJ, Huppertz HI, Harmsen D, Krauspe R, Müller-Hermelink HK, Papadopoulos T. Chronic recurrent multifocal osteomyelitis in children: diagnostic value of histopathology and microbial testing. Hum Pathol. 1999;30(1):59-65.
14. Kayser R, Mahlfeld K, Grasshoff H. Vertebral Langerhans-cell histiocytosis in childhood – a differential diagnosis of spinal osteomyelitis. Klin Padiatr. 1999;211(5):399-402.
15. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-264.
16. Machiz S, Gordon J, Block N, Politano VA. Salmonella typhosa urinary tract infection and xanthogranulomatous pyelonephritis. Case report and review of literature. J Fla Med Assoc. 1974;61(9):703-705.
17. Gauperaa T, Stalsberg H. Renal endometriosis. A case report. Scand J Urol Nephrol. 1977;11(2):189-191.
18. Goodman M, Curry T, Russell T. Xanthogranulomatous pyelonephritis (XGP): a local disease with systemic manifestations. Report of 23 patients and review of the literature. Medicine. 1979;58(2):171-181.
19. Guarino M, Reale D, Micoli G, Tricomi P, Cristofori E. Xanthogranulomatous gastritis: association with xanthogranulomatous cholecystitis. J Clin Pathol. 1993;46(1):88-90.
Listen Now: Highlights of the August 2015 Issue of The Hospitalist
This issue of The Hospitalist, features perspectives on medical education, hospitalist consults, and the latest in hospital medicine clinical literature.
[audio mp3="http://www.the-hospitalist.org/wp-content/uploads/2015/08/2015-August-Hospitalist-Highlights.mp3"][/audio]
This issue of The Hospitalist, features perspectives on medical education, hospitalist consults, and the latest in hospital medicine clinical literature.
[audio mp3="http://www.the-hospitalist.org/wp-content/uploads/2015/08/2015-August-Hospitalist-Highlights.mp3"][/audio]
This issue of The Hospitalist, features perspectives on medical education, hospitalist consults, and the latest in hospital medicine clinical literature.
[audio mp3="http://www.the-hospitalist.org/wp-content/uploads/2015/08/2015-August-Hospitalist-Highlights.mp3"][/audio]
PHM15: Writing and Publishing Quality Improvement (QI)
Presenters: Dr. Patrick Brady, Dr. Michele Saysana, Dr. Christine White, and Dr. Mark Shen.
Session analysis:
QI is about making positive changes in the delivery of healthcare. Multiple QI interventions are been implemented daily throughout our hospitals. Some of those interventions result in positive changes and affect specific outcomes the way we want. It is our job, as hospitalists, to share them with our colleagues so patients can benefit from them.
Some of the barriers to publishing QI as identified by the group are: lack of time, resources available and administrative support, lack of mentorship, and unrecognized value of QI in the academic world. The group also identified some strategies to be successful at writing and publishing QI, including: blocking time in the schedule and labeling it "writing days," joining a collaborative, reaching out to Journal editors and becoming familiar with the SQUIRE guidelines. Some key points as discussed by the experts that will aid during the process of writing QI are:
- A specific goal/aim statement needs to be identified,
- The measurement needs to match your goal/aim,
- Always start with writing your methods since you know exactly what you did,
- Plot data over time using a run chart, and
- Keep a notebook with documentation of dates all interventions started.
It is also important for everyone to know there are multiple quality and safety journals willing to review QI manuscripts for publication.
Presenters: Dr. Patrick Brady, Dr. Michele Saysana, Dr. Christine White, and Dr. Mark Shen.
Session analysis:
QI is about making positive changes in the delivery of healthcare. Multiple QI interventions are been implemented daily throughout our hospitals. Some of those interventions result in positive changes and affect specific outcomes the way we want. It is our job, as hospitalists, to share them with our colleagues so patients can benefit from them.
Some of the barriers to publishing QI as identified by the group are: lack of time, resources available and administrative support, lack of mentorship, and unrecognized value of QI in the academic world. The group also identified some strategies to be successful at writing and publishing QI, including: blocking time in the schedule and labeling it "writing days," joining a collaborative, reaching out to Journal editors and becoming familiar with the SQUIRE guidelines. Some key points as discussed by the experts that will aid during the process of writing QI are:
- A specific goal/aim statement needs to be identified,
- The measurement needs to match your goal/aim,
- Always start with writing your methods since you know exactly what you did,
- Plot data over time using a run chart, and
- Keep a notebook with documentation of dates all interventions started.
It is also important for everyone to know there are multiple quality and safety journals willing to review QI manuscripts for publication.
Presenters: Dr. Patrick Brady, Dr. Michele Saysana, Dr. Christine White, and Dr. Mark Shen.
Session analysis:
QI is about making positive changes in the delivery of healthcare. Multiple QI interventions are been implemented daily throughout our hospitals. Some of those interventions result in positive changes and affect specific outcomes the way we want. It is our job, as hospitalists, to share them with our colleagues so patients can benefit from them.
Some of the barriers to publishing QI as identified by the group are: lack of time, resources available and administrative support, lack of mentorship, and unrecognized value of QI in the academic world. The group also identified some strategies to be successful at writing and publishing QI, including: blocking time in the schedule and labeling it "writing days," joining a collaborative, reaching out to Journal editors and becoming familiar with the SQUIRE guidelines. Some key points as discussed by the experts that will aid during the process of writing QI are:
- A specific goal/aim statement needs to be identified,
- The measurement needs to match your goal/aim,
- Always start with writing your methods since you know exactly what you did,
- Plot data over time using a run chart, and
- Keep a notebook with documentation of dates all interventions started.
It is also important for everyone to know there are multiple quality and safety journals willing to review QI manuscripts for publication.
Eltrombopag yields 40% response rate in pediatric immune thrombocytopenia
Treatment with the thrombopoietin receptor agonist eltrombopag led to a sustained platelet response in 40% of children and adolescents with chronic immune thrombocytopenia, compared with only 3% of the placebo group, according to a randomized multicenter trial published online in The Lancet.
Eltrombopag is approved in the United States for adults with chronic immune thrombocytopenia (CIT) who have not responded adequately to corticosteroids, immunoglobulins, or splenectomy, but few trials have assessed CIT therapies in children, said Dr. John Grainger of the Royal Manchester Children’s Hospital and the University of Manchester (England) and his associates.
Their multicenter, international study included 92 patients up to age 17 with CIT. During the 13-week double-blinded period of the study, patients received once-daily placebo or eltrombopag dosed at 0.89-1.2 mg/kg for patients aged 1-5 years and at 25-50 mg for patients aged 6-17 years. Dosing ranges were adjusted for ethnicity as well as body weight because east Asians have higher eltrombopag exposures and need lower starting doses, the investigators noted. After the double-blinded period, all patients entered 24 weeks of open-label treatment with eltrombopag (Lancet. 2015 Jul 29. doi: 10.1016/S0140-6736(15)61107-2.).
A total of 25 (40%) patients who received eltrombopag achieved platelet counts of at least 50 × 10⁹ per L for at least 6 of the last 8 weeks of the double-blinded period, compared with only one patient (3%) on placebo (odds ratio, 18; 95% confidence interval, 2.3-140.9; P = .0004), said the researchers. Based on the World Health Organization bleeding scale, the percentage of patients who experienced grade 1-4 bleeding events fell from 63% at the start of the open-label period to 24% at the end, and clinically significant (grade 2-4) bleeding events dropped from 20% to 6%. Seven of 87 patients were able to stop all other drugs they were taking for CIT without needing rescue therapy during open-label treatment, the researchers said.
Two patients stopped eltrombopag because of elevated liver aminotransferases. Rates of nasopharyngitis, rhinitis, upper respiratory tract infection, and cough were more common for eltrombopag than placebo, the investigators reported. However, serious adverse events were more common with placebo (14% vs. 8%), and there were no deaths, thrombotic events, or malignancies. Safety trends were similar during the double-blinded and open-label study periods, they added.
GlaxoSmithKline funded the study. Dr. Grainger reported receiving honoraria from GlaxoSmithKline, Amgen, and Baxter. Eleven coauthors reported financial conflicts of interest with GlaxoSmithKline and a number of other pharmaceutical companies.
Treatment with the thrombopoietin receptor agonist eltrombopag led to a sustained platelet response in 40% of children and adolescents with chronic immune thrombocytopenia, compared with only 3% of the placebo group, according to a randomized multicenter trial published online in The Lancet.
Eltrombopag is approved in the United States for adults with chronic immune thrombocytopenia (CIT) who have not responded adequately to corticosteroids, immunoglobulins, or splenectomy, but few trials have assessed CIT therapies in children, said Dr. John Grainger of the Royal Manchester Children’s Hospital and the University of Manchester (England) and his associates.
Their multicenter, international study included 92 patients up to age 17 with CIT. During the 13-week double-blinded period of the study, patients received once-daily placebo or eltrombopag dosed at 0.89-1.2 mg/kg for patients aged 1-5 years and at 25-50 mg for patients aged 6-17 years. Dosing ranges were adjusted for ethnicity as well as body weight because east Asians have higher eltrombopag exposures and need lower starting doses, the investigators noted. After the double-blinded period, all patients entered 24 weeks of open-label treatment with eltrombopag (Lancet. 2015 Jul 29. doi: 10.1016/S0140-6736(15)61107-2.).
A total of 25 (40%) patients who received eltrombopag achieved platelet counts of at least 50 × 10⁹ per L for at least 6 of the last 8 weeks of the double-blinded period, compared with only one patient (3%) on placebo (odds ratio, 18; 95% confidence interval, 2.3-140.9; P = .0004), said the researchers. Based on the World Health Organization bleeding scale, the percentage of patients who experienced grade 1-4 bleeding events fell from 63% at the start of the open-label period to 24% at the end, and clinically significant (grade 2-4) bleeding events dropped from 20% to 6%. Seven of 87 patients were able to stop all other drugs they were taking for CIT without needing rescue therapy during open-label treatment, the researchers said.
Two patients stopped eltrombopag because of elevated liver aminotransferases. Rates of nasopharyngitis, rhinitis, upper respiratory tract infection, and cough were more common for eltrombopag than placebo, the investigators reported. However, serious adverse events were more common with placebo (14% vs. 8%), and there were no deaths, thrombotic events, or malignancies. Safety trends were similar during the double-blinded and open-label study periods, they added.
GlaxoSmithKline funded the study. Dr. Grainger reported receiving honoraria from GlaxoSmithKline, Amgen, and Baxter. Eleven coauthors reported financial conflicts of interest with GlaxoSmithKline and a number of other pharmaceutical companies.
Treatment with the thrombopoietin receptor agonist eltrombopag led to a sustained platelet response in 40% of children and adolescents with chronic immune thrombocytopenia, compared with only 3% of the placebo group, according to a randomized multicenter trial published online in The Lancet.
Eltrombopag is approved in the United States for adults with chronic immune thrombocytopenia (CIT) who have not responded adequately to corticosteroids, immunoglobulins, or splenectomy, but few trials have assessed CIT therapies in children, said Dr. John Grainger of the Royal Manchester Children’s Hospital and the University of Manchester (England) and his associates.
Their multicenter, international study included 92 patients up to age 17 with CIT. During the 13-week double-blinded period of the study, patients received once-daily placebo or eltrombopag dosed at 0.89-1.2 mg/kg for patients aged 1-5 years and at 25-50 mg for patients aged 6-17 years. Dosing ranges were adjusted for ethnicity as well as body weight because east Asians have higher eltrombopag exposures and need lower starting doses, the investigators noted. After the double-blinded period, all patients entered 24 weeks of open-label treatment with eltrombopag (Lancet. 2015 Jul 29. doi: 10.1016/S0140-6736(15)61107-2.).
A total of 25 (40%) patients who received eltrombopag achieved platelet counts of at least 50 × 10⁹ per L for at least 6 of the last 8 weeks of the double-blinded period, compared with only one patient (3%) on placebo (odds ratio, 18; 95% confidence interval, 2.3-140.9; P = .0004), said the researchers. Based on the World Health Organization bleeding scale, the percentage of patients who experienced grade 1-4 bleeding events fell from 63% at the start of the open-label period to 24% at the end, and clinically significant (grade 2-4) bleeding events dropped from 20% to 6%. Seven of 87 patients were able to stop all other drugs they were taking for CIT without needing rescue therapy during open-label treatment, the researchers said.
Two patients stopped eltrombopag because of elevated liver aminotransferases. Rates of nasopharyngitis, rhinitis, upper respiratory tract infection, and cough were more common for eltrombopag than placebo, the investigators reported. However, serious adverse events were more common with placebo (14% vs. 8%), and there were no deaths, thrombotic events, or malignancies. Safety trends were similar during the double-blinded and open-label study periods, they added.
GlaxoSmithKline funded the study. Dr. Grainger reported receiving honoraria from GlaxoSmithKline, Amgen, and Baxter. Eleven coauthors reported financial conflicts of interest with GlaxoSmithKline and a number of other pharmaceutical companies.
FROM THE LANCET
Key clinical point: Eltrombopag markedly outperformed placebo and had no new safety signals in children and adolescents with chronic immune thrombocytopenia.
Major finding: Forty percent of patients achieved sustained platelet response on eltrombopag, compared with 3% of the placebo group (OR, 18.0; P = .0004).
Data source: Randomized, double-blinded, multicenter, international trial of 92 patients aged 1-17 years.
Disclosures: GlaxoSmithKline funded the study. Dr. Grainger reported receiving honoraria from GlaxoSmithKline, Amgen, and Baxter. Eleven coauthors reported financial conflicts of interest with GlaxoSmithKline and a number of other pharmaceutical companies.
PHM15: Teaching, Supervising Pediatric Hospitalist Fellows
Session: “Teaching and Supervising a PHM Fellow: The Transition from Learner to Instructor."
Analysis: The session was led by Sarah Denniston, MD, FAAP, of Children’s Hospital of San Antonio/Baylor College of Medicine, and a collaboration of presenters ranging from fellowship directors to new pediatric hospitalist fellows. It was a very interactive discussion that prompted shared stories and encouraged changes in the way hospital medicine views a fellow. Presenters reviewed current evidence-based approaches to effective teaching, while supervision of senior residents and fellow-level trainees rounded out the discussion.
As the number of pediatric hospital medicine fellowships increases, so does the variability in each program. This session focused on the unique challenges PHM fellowships face, a balance of supervision and autonomy, specifically:
- Limited faculty development
- Resident exposure to fundamental PHM core competencies
- No national standards
- Resident transition directly into PHM attending role
Looking at survey responses from fellowship programs, Dr. Denniston described multiple program variables in defining the role of the PHM fellow, specifically if they were billable providers, what type of supervision they have, and what training is provided to the faculty who work with the PHM fellows.
Supervision was the hottest topic. Many of the fellows in the audience were able to share their thoughts and encourage session attendees to discuss with their home institutions the idea of autonomy. The billing provides a challenge, but ways to provide increased autonomy as a fellow progresses in the fellowship were addressed. From independent rounding to “running the list” as a daily check in, this can be accomplished even if the fellow is not able to provide billing. If at least two hospitalist teams are within the structure of the daily rounding, the discussion encouraged independent rounding and check in with a senior hospitalist.
Other hot topics were faculty development and senior hospitalist leadership. Many programs have a large enough staff pool to allow for more senior hospitalists to be the “supervisor” for the fellow, reporting that from a fellow standpoint they valued the mentorship and guidance elicited from the experience of a senior hospitalist. The junior or newer hospitalists also agreed that it is a challenge to feel adequate in providing the education and mentorship to a fellow.
Overall, faculty development is a necessary engagement process in creating a strong PHM fellowship, and both attendees and presenterd cited the need for clear expectations—not only for the faculty but the fellows as well.
Rounding out the session was a short didactic on principles of learning and understanding the generational differences in learners. It is important for all educators to recognize different teaching methods as ways to promote autonomy and enhance not only fellow experience, but senior resident as well.
Setting expectations was a theme, encouraging personal and program responsibility to education.
Key Takeaways:
- PHM fellows need autonomy as they progress towards the role of an independent attending.
- PHM fellowship programs need to establish very clear faculty and fellow expectations, noting the need for senior hospitalist experience.
- Educators need to be aware of generational differences in learning and utilize different learning styles.
Dr. Pestak is a pediatric hospitalist and associate program director for the pediatric residency program at Cleveland Clinic Children’s.
Session: “Teaching and Supervising a PHM Fellow: The Transition from Learner to Instructor."
Analysis: The session was led by Sarah Denniston, MD, FAAP, of Children’s Hospital of San Antonio/Baylor College of Medicine, and a collaboration of presenters ranging from fellowship directors to new pediatric hospitalist fellows. It was a very interactive discussion that prompted shared stories and encouraged changes in the way hospital medicine views a fellow. Presenters reviewed current evidence-based approaches to effective teaching, while supervision of senior residents and fellow-level trainees rounded out the discussion.
As the number of pediatric hospital medicine fellowships increases, so does the variability in each program. This session focused on the unique challenges PHM fellowships face, a balance of supervision and autonomy, specifically:
- Limited faculty development
- Resident exposure to fundamental PHM core competencies
- No national standards
- Resident transition directly into PHM attending role
Looking at survey responses from fellowship programs, Dr. Denniston described multiple program variables in defining the role of the PHM fellow, specifically if they were billable providers, what type of supervision they have, and what training is provided to the faculty who work with the PHM fellows.
Supervision was the hottest topic. Many of the fellows in the audience were able to share their thoughts and encourage session attendees to discuss with their home institutions the idea of autonomy. The billing provides a challenge, but ways to provide increased autonomy as a fellow progresses in the fellowship were addressed. From independent rounding to “running the list” as a daily check in, this can be accomplished even if the fellow is not able to provide billing. If at least two hospitalist teams are within the structure of the daily rounding, the discussion encouraged independent rounding and check in with a senior hospitalist.
Other hot topics were faculty development and senior hospitalist leadership. Many programs have a large enough staff pool to allow for more senior hospitalists to be the “supervisor” for the fellow, reporting that from a fellow standpoint they valued the mentorship and guidance elicited from the experience of a senior hospitalist. The junior or newer hospitalists also agreed that it is a challenge to feel adequate in providing the education and mentorship to a fellow.
Overall, faculty development is a necessary engagement process in creating a strong PHM fellowship, and both attendees and presenterd cited the need for clear expectations—not only for the faculty but the fellows as well.
Rounding out the session was a short didactic on principles of learning and understanding the generational differences in learners. It is important for all educators to recognize different teaching methods as ways to promote autonomy and enhance not only fellow experience, but senior resident as well.
Setting expectations was a theme, encouraging personal and program responsibility to education.
Key Takeaways:
- PHM fellows need autonomy as they progress towards the role of an independent attending.
- PHM fellowship programs need to establish very clear faculty and fellow expectations, noting the need for senior hospitalist experience.
- Educators need to be aware of generational differences in learning and utilize different learning styles.
Dr. Pestak is a pediatric hospitalist and associate program director for the pediatric residency program at Cleveland Clinic Children’s.
Session: “Teaching and Supervising a PHM Fellow: The Transition from Learner to Instructor."
Analysis: The session was led by Sarah Denniston, MD, FAAP, of Children’s Hospital of San Antonio/Baylor College of Medicine, and a collaboration of presenters ranging from fellowship directors to new pediatric hospitalist fellows. It was a very interactive discussion that prompted shared stories and encouraged changes in the way hospital medicine views a fellow. Presenters reviewed current evidence-based approaches to effective teaching, while supervision of senior residents and fellow-level trainees rounded out the discussion.
As the number of pediatric hospital medicine fellowships increases, so does the variability in each program. This session focused on the unique challenges PHM fellowships face, a balance of supervision and autonomy, specifically:
- Limited faculty development
- Resident exposure to fundamental PHM core competencies
- No national standards
- Resident transition directly into PHM attending role
Looking at survey responses from fellowship programs, Dr. Denniston described multiple program variables in defining the role of the PHM fellow, specifically if they were billable providers, what type of supervision they have, and what training is provided to the faculty who work with the PHM fellows.
Supervision was the hottest topic. Many of the fellows in the audience were able to share their thoughts and encourage session attendees to discuss with their home institutions the idea of autonomy. The billing provides a challenge, but ways to provide increased autonomy as a fellow progresses in the fellowship were addressed. From independent rounding to “running the list” as a daily check in, this can be accomplished even if the fellow is not able to provide billing. If at least two hospitalist teams are within the structure of the daily rounding, the discussion encouraged independent rounding and check in with a senior hospitalist.
Other hot topics were faculty development and senior hospitalist leadership. Many programs have a large enough staff pool to allow for more senior hospitalists to be the “supervisor” for the fellow, reporting that from a fellow standpoint they valued the mentorship and guidance elicited from the experience of a senior hospitalist. The junior or newer hospitalists also agreed that it is a challenge to feel adequate in providing the education and mentorship to a fellow.
Overall, faculty development is a necessary engagement process in creating a strong PHM fellowship, and both attendees and presenterd cited the need for clear expectations—not only for the faculty but the fellows as well.
Rounding out the session was a short didactic on principles of learning and understanding the generational differences in learners. It is important for all educators to recognize different teaching methods as ways to promote autonomy and enhance not only fellow experience, but senior resident as well.
Setting expectations was a theme, encouraging personal and program responsibility to education.
Key Takeaways:
- PHM fellows need autonomy as they progress towards the role of an independent attending.
- PHM fellowship programs need to establish very clear faculty and fellow expectations, noting the need for senior hospitalist experience.
- Educators need to be aware of generational differences in learning and utilize different learning styles.
Dr. Pestak is a pediatric hospitalist and associate program director for the pediatric residency program at Cleveland Clinic Children’s.