User login
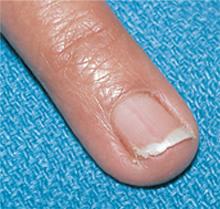
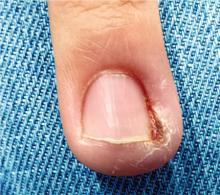
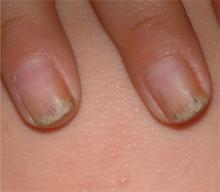
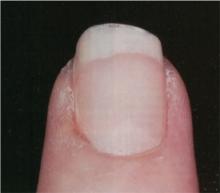
What treatments best prevent chronic tension headaches?
Biofeedback and tricyclic antidepressants appear to be effective as prophylactic treatment for chronic tension headaches, although tricyclic antidepressants have more adverse effects (strength of recommendation [SOR]: B, meta-analysis of randomized controlled trials [RCTs] and pre-post trials).
Acupuncture shows limited evidence for effectiveness after 20 to 25 weeks of treatment (SOR: B, meta-analysis of RCTs).
Biofeedback gets results without adverse effects
A 2008 meta-analysis of 53 trials (32 RCTs, 21 pre-post; 1532 patients, 72% female, average age 36 years) examined the effectiveness of biofeedback for tension-type headaches (TTH) with a mean duration of headache symptoms of 14 years.1 The mean duration of treatment was fewer than 10 hours over 11 sessions. Control groups included placebo, relaxation, pharmacotherapy, cognitive therapy, and physical therapy.
Biofeedback reduced headache pain (measured in structured headache diaries by frequency, duration, and intensity) more than controls (weighted mean difference [WMD]=0.73; 95% confidence interval [CI], 0.61-0.84; WMD effect sizes >0.8 are considered large, 0.6-0.8 are moderate, and 0.2-0.6 are small).
Eighteen trials (15 RCTs, 3 pre-post; 736 patients) included follow-up analysis ranging from 3 to 60 months (mean 15 months), during which biofeedback showed a moderate effect size compared with controls (WMD=0.62; 95% CI, 0.53-0.72). Biofeedback plus relaxation produced a larger effect than controls (6 RCTs, 3 pre-post; 124 patients; WMD=0.98; 95% CI, 0.69-1.3). No adverse effects were reported.
Tricyclics help, too, but have adverse effects
A 2010 meta-analysis of 37 RCTs examined the effectiveness of tricyclic antidepressants as prophylactic treatment for headaches in 3176 patients.2 Seventeen RCTs (1275 patients, 73% women, average age 40 years) evaluated tension headaches, and 15 of them included only patients who had headaches for more than 14 days per month (the other 20 RCTs, with 1901 patients, studied migraine headaches).
Control groups included placebo, selective serotonin reuptake inhibitors (SSRIs), tetracyclics, topiramate, dihydroergotamine, spinal manipulation, and cognitive behavioral therapy, but only placebo (8 RCTs included) and SSRIs (4 RCTs included) had more than 2 RCTs evaluated. Variables included headache days per month, headache intensity (measured by scales that varied among trials), and headache index (calculated by multiplying intensity by frequency).
In 8 trials with 574 patients, the standard mean difference (SMD) headache index improved for tricyclics compared with placebo over a treatment duration averaging 10 weeks (WMD=−0.99; 95% CI, −1.7 to −0.32). However, the tricyclic arm reported more adverse effects such as drowsiness, dizziness, dry mouth, and abdominal complaints (relative risk [RR]=1.9; 95% CI, 1.2-3.0).
Tricyclics and SSRIs reduced the frequency of TTH equally (4 trials; N=479; standardized mean difference=−0.80; 95% CI, −1.63 to 0.02) but tricyclics were more likely to reduce intensity by 50% (RR=1.7; 95% CI, 1.3-2.2).
Headaches decline after acupuncture, not during
A 2008 meta-analysis of 5 RCTs (838 patients, 68% female) evaluated the effectiveness of acupuncture compared with sham acupuncture for treating episodic and chronic TTH.3 Separate data on efficacy for each subtype was not provided. Selection of acupuncture points varied among the trials, and treatment durations ranged from 3 to 8 weeks.
Headache days per month didn’t decline during treatment (WMD=−2.9; 95% CI, −7.5 to 1.6), but were significantly decreased on follow-up at 20 to 25 weeks (WMD=−1.8; 95% CI, −3.0 to −0.64).
1. Netoriuc Y, Rief W, Martin A. Meta-analysis of biofeedback for tension-type headache: efficacy, specificity and treatment moderators. J Consult Clin Psychol. 2008;76:379-396.
2. Jackson JL, Shimeall W, Sessums L, et al. Tricyclic antidepressants and headaches: systematic review and meta-analysis. BMJ. 2010;341:c5222.
3. Davis MA, Kononowech RW, Rolin SA, et al. Acupuncture for tension-type headache: a meta-analysis of randomized, controlled trials. J Pain. 2008;9:667-677.
Biofeedback and tricyclic antidepressants appear to be effective as prophylactic treatment for chronic tension headaches, although tricyclic antidepressants have more adverse effects (strength of recommendation [SOR]: B, meta-analysis of randomized controlled trials [RCTs] and pre-post trials).
Acupuncture shows limited evidence for effectiveness after 20 to 25 weeks of treatment (SOR: B, meta-analysis of RCTs).
Biofeedback gets results without adverse effects
A 2008 meta-analysis of 53 trials (32 RCTs, 21 pre-post; 1532 patients, 72% female, average age 36 years) examined the effectiveness of biofeedback for tension-type headaches (TTH) with a mean duration of headache symptoms of 14 years.1 The mean duration of treatment was fewer than 10 hours over 11 sessions. Control groups included placebo, relaxation, pharmacotherapy, cognitive therapy, and physical therapy.
Biofeedback reduced headache pain (measured in structured headache diaries by frequency, duration, and intensity) more than controls (weighted mean difference [WMD]=0.73; 95% confidence interval [CI], 0.61-0.84; WMD effect sizes >0.8 are considered large, 0.6-0.8 are moderate, and 0.2-0.6 are small).
Eighteen trials (15 RCTs, 3 pre-post; 736 patients) included follow-up analysis ranging from 3 to 60 months (mean 15 months), during which biofeedback showed a moderate effect size compared with controls (WMD=0.62; 95% CI, 0.53-0.72). Biofeedback plus relaxation produced a larger effect than controls (6 RCTs, 3 pre-post; 124 patients; WMD=0.98; 95% CI, 0.69-1.3). No adverse effects were reported.
Tricyclics help, too, but have adverse effects
A 2010 meta-analysis of 37 RCTs examined the effectiveness of tricyclic antidepressants as prophylactic treatment for headaches in 3176 patients.2 Seventeen RCTs (1275 patients, 73% women, average age 40 years) evaluated tension headaches, and 15 of them included only patients who had headaches for more than 14 days per month (the other 20 RCTs, with 1901 patients, studied migraine headaches).
Control groups included placebo, selective serotonin reuptake inhibitors (SSRIs), tetracyclics, topiramate, dihydroergotamine, spinal manipulation, and cognitive behavioral therapy, but only placebo (8 RCTs included) and SSRIs (4 RCTs included) had more than 2 RCTs evaluated. Variables included headache days per month, headache intensity (measured by scales that varied among trials), and headache index (calculated by multiplying intensity by frequency).
In 8 trials with 574 patients, the standard mean difference (SMD) headache index improved for tricyclics compared with placebo over a treatment duration averaging 10 weeks (WMD=−0.99; 95% CI, −1.7 to −0.32). However, the tricyclic arm reported more adverse effects such as drowsiness, dizziness, dry mouth, and abdominal complaints (relative risk [RR]=1.9; 95% CI, 1.2-3.0).
Tricyclics and SSRIs reduced the frequency of TTH equally (4 trials; N=479; standardized mean difference=−0.80; 95% CI, −1.63 to 0.02) but tricyclics were more likely to reduce intensity by 50% (RR=1.7; 95% CI, 1.3-2.2).
Headaches decline after acupuncture, not during
A 2008 meta-analysis of 5 RCTs (838 patients, 68% female) evaluated the effectiveness of acupuncture compared with sham acupuncture for treating episodic and chronic TTH.3 Separate data on efficacy for each subtype was not provided. Selection of acupuncture points varied among the trials, and treatment durations ranged from 3 to 8 weeks.
Headache days per month didn’t decline during treatment (WMD=−2.9; 95% CI, −7.5 to 1.6), but were significantly decreased on follow-up at 20 to 25 weeks (WMD=−1.8; 95% CI, −3.0 to −0.64).
Biofeedback and tricyclic antidepressants appear to be effective as prophylactic treatment for chronic tension headaches, although tricyclic antidepressants have more adverse effects (strength of recommendation [SOR]: B, meta-analysis of randomized controlled trials [RCTs] and pre-post trials).
Acupuncture shows limited evidence for effectiveness after 20 to 25 weeks of treatment (SOR: B, meta-analysis of RCTs).
Biofeedback gets results without adverse effects
A 2008 meta-analysis of 53 trials (32 RCTs, 21 pre-post; 1532 patients, 72% female, average age 36 years) examined the effectiveness of biofeedback for tension-type headaches (TTH) with a mean duration of headache symptoms of 14 years.1 The mean duration of treatment was fewer than 10 hours over 11 sessions. Control groups included placebo, relaxation, pharmacotherapy, cognitive therapy, and physical therapy.
Biofeedback reduced headache pain (measured in structured headache diaries by frequency, duration, and intensity) more than controls (weighted mean difference [WMD]=0.73; 95% confidence interval [CI], 0.61-0.84; WMD effect sizes >0.8 are considered large, 0.6-0.8 are moderate, and 0.2-0.6 are small).
Eighteen trials (15 RCTs, 3 pre-post; 736 patients) included follow-up analysis ranging from 3 to 60 months (mean 15 months), during which biofeedback showed a moderate effect size compared with controls (WMD=0.62; 95% CI, 0.53-0.72). Biofeedback plus relaxation produced a larger effect than controls (6 RCTs, 3 pre-post; 124 patients; WMD=0.98; 95% CI, 0.69-1.3). No adverse effects were reported.
Tricyclics help, too, but have adverse effects
A 2010 meta-analysis of 37 RCTs examined the effectiveness of tricyclic antidepressants as prophylactic treatment for headaches in 3176 patients.2 Seventeen RCTs (1275 patients, 73% women, average age 40 years) evaluated tension headaches, and 15 of them included only patients who had headaches for more than 14 days per month (the other 20 RCTs, with 1901 patients, studied migraine headaches).
Control groups included placebo, selective serotonin reuptake inhibitors (SSRIs), tetracyclics, topiramate, dihydroergotamine, spinal manipulation, and cognitive behavioral therapy, but only placebo (8 RCTs included) and SSRIs (4 RCTs included) had more than 2 RCTs evaluated. Variables included headache days per month, headache intensity (measured by scales that varied among trials), and headache index (calculated by multiplying intensity by frequency).
In 8 trials with 574 patients, the standard mean difference (SMD) headache index improved for tricyclics compared with placebo over a treatment duration averaging 10 weeks (WMD=−0.99; 95% CI, −1.7 to −0.32). However, the tricyclic arm reported more adverse effects such as drowsiness, dizziness, dry mouth, and abdominal complaints (relative risk [RR]=1.9; 95% CI, 1.2-3.0).
Tricyclics and SSRIs reduced the frequency of TTH equally (4 trials; N=479; standardized mean difference=−0.80; 95% CI, −1.63 to 0.02) but tricyclics were more likely to reduce intensity by 50% (RR=1.7; 95% CI, 1.3-2.2).
Headaches decline after acupuncture, not during
A 2008 meta-analysis of 5 RCTs (838 patients, 68% female) evaluated the effectiveness of acupuncture compared with sham acupuncture for treating episodic and chronic TTH.3 Separate data on efficacy for each subtype was not provided. Selection of acupuncture points varied among the trials, and treatment durations ranged from 3 to 8 weeks.
Headache days per month didn’t decline during treatment (WMD=−2.9; 95% CI, −7.5 to 1.6), but were significantly decreased on follow-up at 20 to 25 weeks (WMD=−1.8; 95% CI, −3.0 to −0.64).
1. Netoriuc Y, Rief W, Martin A. Meta-analysis of biofeedback for tension-type headache: efficacy, specificity and treatment moderators. J Consult Clin Psychol. 2008;76:379-396.
2. Jackson JL, Shimeall W, Sessums L, et al. Tricyclic antidepressants and headaches: systematic review and meta-analysis. BMJ. 2010;341:c5222.
3. Davis MA, Kononowech RW, Rolin SA, et al. Acupuncture for tension-type headache: a meta-analysis of randomized, controlled trials. J Pain. 2008;9:667-677.
1. Netoriuc Y, Rief W, Martin A. Meta-analysis of biofeedback for tension-type headache: efficacy, specificity and treatment moderators. J Consult Clin Psychol. 2008;76:379-396.
2. Jackson JL, Shimeall W, Sessums L, et al. Tricyclic antidepressants and headaches: systematic review and meta-analysis. BMJ. 2010;341:c5222.
3. Davis MA, Kononowech RW, Rolin SA, et al. Acupuncture for tension-type headache: a meta-analysis of randomized, controlled trials. J Pain. 2008;9:667-677.
Evidence-based answers from the Family Physicians Inquiries Network
Addressing the Sexual Health Concerns of Women with Gynecologic Cancer: Guidance for Primary Care Physicians
From the Yale School of Medicine, New Haven, CT.
Abstract
- Objective: To review the sexual health concerns of women with gynecologic cancer and provide guidance for primary care physicians.
- Methods: Review of the literature.
- Results: Issues of sexuality and intimacy are known to significantly impact the quality of life of patients following diagnosis and treatment of gynecologic cancers. At the time of diagnosis, women should be informed of the potential physiologic, hormonal, and psychosocial effects of gynecologic cancer on sexuality. Many providers fail to address these issues given time constraints and patients’ trepidation in alerting their providers to their concerns. While systemic hormone therapy directly addresses these symptoms, its use remains controversial due to potential cancer recurrence risks. Thus, treatment centers around therapeutic alternatives. For vasomotor symptoms, selective serotonin reuptake inhibitors have shown effectiveness and are typically well tolerated, and antiepileptics such as gabapentin have shown promise. There is promising but limited data employing pelvic floor physical therapy as a tool to aid in addressing pelvic floor symptoms. Psychological care and the involvement of the partner are also part of managing the sexual health concerns of these patients.
- Conclusion: Sexual morbidity is a distressing and undertreated problem among gynecologic cancer survivors. Successful treatment requires the provider’s appreciation of the problem and willingness to address it.
Issues of sexuality and intimacy are known to significantly impact the lives of patients following diagnosis and treatment of gynecologic cancers. [1,2] Treatment of gynecologic malignancy is highly dependent on pathology and stage, with some patients receiving small excisional procedures while others subject to extensive surgeries, chemotherapy, and radiation treatment, and it is difficult to predict how each individual’s sexual health will be impacted. However, the evidence suggests that at least half of women treated for gynecologic cancer will experience sexual dysfunction [3]. Although the impact of gynecologic cancer and treatment can be profound, providers often do not address their patients’ sexual concerns [4,5], yet most patients have indicated that they would like these issues to be addressed [6].
Female Sexual Dysfunction
Sexual dysfunction is multifactorial and involves physical, social, and psychological dimensions. It is common in the general population, with rates ranging from 25-63% [7]. The DSM-IV [8] defines female sexual dysfunction as a disturbance in or pain during the sexual response, which can be further classified as hypoactive sexual disorder, orgasmic disorder, sexual pain disorder, or sexual arousal disorder [9]. It should be noted that women who have been treated for gynecologic cancers may have a premorbid history of sexual dysfunction. Assessment of a woman’s sexual function prior to her cancer diagnosis can help establish which sexuality changes are due to the cancer treatment and may allow the provider to tailor interventions accordingly.
Sexual Dysfunction and Quality of Life
As treatments for gynecologic cancers improve, toxicity of treatment decreases, and survival increases, quality of life for survivors has become as become an increasingly important health issue. Several studies have examined patient-reported quality of life in short- and long-term cancer survivors and report overall significant alteration in quality of life over many aspects of health and psychological well-being [10]. It is well established that health-related quality of life and sexual functioning are closely associated [11].
In gynecologic cancer patients, sexual and quality of life outcomes can vary. For example, Kim el al recently compared quality of life and sexual functioning in ovarian cancer survivors with no evidence of disease after primary treatment and a cohort of health women. Sexuality, both in terms of desire, arousal, lubrication, orgasm, satisfaction, and pain and in terms of interest in sex, sexual activity, and enjoyment of sex were similar between the groups; however social functioning deteriorated in cancer patients [12]. Other women report different experiences. Women with a history of vulvar, vaginal, or cervical cancer who may have had extensive pelvic surgery, lymphadenectomy, and radiation treatment to the pelvis typically report greater alterations in quality of life and sexual activity depending on the extent of their treatment [13].
Patients undergoing chemotherapy often report significant changes in quality of life due to the physical symptoms of fatigue, nausea, hair loss, diarrhea, etc. as well the psychological effects of cancer diagnosis, changes in body image, and poor coping [14]. Sexual side effects are common due to alterations in the HPA axis, direct gonadal toxicity and neuropathies [15].
Impact of Gynecologic Cancer on Sexuality
Gynecologic cancer and treatment can alter sexuality through a variety of effects. The impact of anatomical changes may alter a women’s self-esteem, body image, and sense of femininity, resulting in a reluctance to engage in intimate behaviors. Furthermore, if the partnership is disrupted by changes in roles in the household or within the relationship, relationships dynamics may be tested and result in mental distress for the patient, thus effecting sexuality and intimacy.
Surgical or chemical withdrawal of sex hormones, new medications, and postoperative sequelae can contribute to sexual arousal problems. The emotional and psychological components of a cancer diagnosis also can hinder the sexual response [16]. Depression and anxiety, the rates of which increase with cancer diagnosis, can also significantly affect sexual function [17].
Sexual pain is common in this population due to hormonal considerations as well as post-treatment side effects and a frequent cause of sexual dysfunction for these patients. Pelvic radiation may result in adverse physical changes to the vagina including vaginal stenosis, thinning of vaginal mucosa, loss of lubrication, and loss of elasticity [18,19]. A recent review of 20 studies of cervical cancer survivors found that patients were at risk for lack of lubrication and had high rates of dyspareunia [20]. Psychosocial factors have been found to be important predictors of sexual desire and more important than hormones in predicting low sexual desire in middle age [21]. These factors include emotional and physical closeness to the partner, satisfactory communication, and a positive relation to one’s own body [16].
Effects on Relationships and Partners
Women whose sexual capacity is compromised may be worried about their partners’ quality of life and overall well-being. Indeed, the partners of women with gynecologic cancer are also impacted by the changes to sexual function and loss of sexuality and intimacy. Hawkins et al found that cessation or decreased frequency of sex and intimacy was reported in 79% of male partners of women affected by cancer. Among partners of the persons with cancer in this study, changes to sexuality were associated with feelings of self-blame, reflection, sadness, anger and lack of sexual fulfillment [22]. Further, male partners of women diagnosed with gynecological cancer often express conflicting emotional states including feeling worried about their significant other’s health, having the desire to engage in sexual activity, and feeling guilty about wanting to increase sexual intimacy. These feelings, in turn, can lead to resentment and withdrawal from their partner and overall relationship discord [23].
Evidence suggests partners of cancer patients greatly benefit from increased social support even years after an apparent cure [24]. Specifically, male partners who take on a new role as caregiver in the relationship experience difficulties with emotional changes, challenges to their masculinity, and new stressors [25]. These new roles and feelings also contribute to changes in sexuality and intimacy in relationships, making support for partners all the more necessary.
Menopausal Symptoms Following Cancer Treatment
Gynecologic cancer treatment invariably affects the female hormonal balance, sometimes suddenly with surgical excision of the gonads or via radiation treatment or chemotherapy. It is well known that the sudden withdrawal of estrogen and testosterone, especially in the premenopausal postoperative population, can lead to significant acute menopausal symptoms. Given the many emotional and physical issues affecting patients during treatment, it can be difficult to delineate what proportion of sexual problems are caused by or enhanced by vasomotor symptoms, sleep disorders, and vaginal atrophy.
In general, premenopausal women who experience abrupt surgical menopause may often have immediate severe symptoms. Many agree that the younger a woman is when going through this process, the more severe her symptoms may be. Although the average age of endometrial cancer diagnosis is 67, approximately 25% of women who are diagnosed are premenopausal, and 5% of cases occur in women under 40. As the obesity epidemic worsens and more women are exposed to higher levels of estrogen at younger ages, it is expected that the number of premenopausal women who are diagnosed will continue to rise. The average age of diagnosis for ovarian cancer is 63, however, there is a large cohort of women diagnosed with borderline and malignant tumors in the premenopausal period [26]. These patients often experience vasomotor symptoms in the hours to days following surgery.
Interventions for Survivors of Gynecologic Cancer with Sexual Dysfunction
Systemic Hormone Therapy
Systemic hormonal therapy to treat menopausal symptoms remains controversial following the release of findings from the Women’s Health Initiative, which showed a number of adverse effects, including an increased risk of breast cancer in healthy postmenopausal women who received systemic hormonal therapy for menopausal symptoms. While views have changed since that time, providers are often reluctant to prescribe hormonal therapy, and patients are reticent to take it, due to fears of cancer recurrence. However, there is no evidence showing hormones negatively influence survival after treatment for epithelial ovarian, squamous cervical, or vulvar cancer [27]. With the exception of endometrial cancer, there is no biological evidence that HRT may increase recurrence risk [28]. Approach to clinical decision making should be individualized, taking into consideration the patients’ symptoms, quality of life, tumor histology, and overall prognosis.
Cervical cancer, vulvar cancer, and vaginal cancer are not considered hormonally responsive tumors. While the data are limited, a study published in 1987 of cervical cancer survivors treated with systemic hormone replacement therapy showed no increase in relapse rates and showed an increase in quality of life [29]. There are no studies regarding systemic HRT in patients with vulvar or vaginal cancer though it is generally accepted they can be treated with HRT. No significant data exists for cervical adenocarcinoma patients, and most oncologists suggest treating these patients the same as those with endometrial cancer primary.
There is limited data on the use of HRT in women with ovarian cancer but available studies do not show any difference in overall or disease-free survival between HRT groups and controls [27,30–32]. Many studies report a significant increase in quality of life with HRT. A 2013 retrospective chart review of 77 patients with epithelial ovarian cancer who received postoperative HRT showed no significant difference in progression-free survival [33].
The most controversy surrounds the use of HRT in patients with endometrial cancer [34]. The only major data available come from the Gynecologic Oncology Group’s truncated study, which was a large prospective randomized controlled trial. Over 1200 women treated for stage I and II endometrial cancer were enrolled between 1997 and 2003, and before the study was terminated these patients were followed for a median of 3 years following initiation of therapy. The recurrence rate of malignancy was low, 2.1, and an insignificant risk of recurrence of 1.27 was noted between with HRT and placebo groups (80% CI, 0.916-1.77) [35]. Women included in this study were between the ages of 26 and 91, and their indications for therapy included vasomotor symptoms, vaginal atrophy, as well as osteoporosis risk and cardiovascular disease risk. Gynecologist oncologists often use estrogen therapy to treat symptomatic women with early stage endometrial cancer given the very low risk of recurrence.
Tibolone is a synthetic steroid with activity on estrogen and progesterone receptors, and mainly acts as an agonist at estrogen receptors. It is prescribed outside the United States for osteoporosis and is being investigated as treatment for female sexual dysfunction. Efficacy on vasomotor symptoms has been positive thus far. One case-control study confirmed tibolone’s safety for endometrial cancer survivors, with no adverse effects on disease-free or overall survival [36]. One group recently examined tibolone in the setting of breast cancer patients in a prospective randomized controlled trial and reported an increased risk of breast cancer recurrences in women receiving tibolone for HT [37]. That study reported no increase in risk of gynecologic cancers and did report favorable outcomes for patients in terms of osteoporosis and vasomotor symptoms.
Topical Therapy
Women who are concerned about systemic HRT but who have been treated with radiation in addition to their surgery may be interested in topical estrogen therapy. These women may have vaginal stenosis and atrophic symptoms, and for them topical estrogen therapy can be helpful [38]. Many formulations of topical estrogen are available, including creams, tablets, and rings. Using vaginal estrogens and dilators can be useful to help with eventual resumption of sexual activity once healing has taken place and can help to avoid dyspareunia. Combination topical and systemic therapy can also be useful to relieve symptoms. Women concerned about absorption with topical therapy can be advised that while there is some absorption initially, absorption is reduced as atrophy is improved with treatment.
One recent study examined the use of alpha-tocopherol to reduce acute vaginal complications in women with endometrial and cervical cancer undergoing radiation treatment. The treatment group experienced reduced vaginal toxicity and pain, although vaginal secretion was not significantly different in the 2 groups studied [39]. No adverse effects were noted. This compound has not been studied further but may be beneficial in the future.
Some women may prefer to avoid hormones altogether. Over-the-counter vaginal moisturizers and lubricants can be recommended to women to help with intercourse and atrophic symptoms, with or without estrogen therapy.
Physical Therapy
Pelvic floor physical therapy has become an increasingly popular modality for treatment of many aspects pelvic floor dysfunction, the symptoms of which include defecatory dysfunction, constipation, bladder dysfunction, painful urination, dyspareunia, pelvic pain, and low back pain [40]. Pelvic floor physical therapy is well studied and utilized frequently for urinary incontinence and pelvic organ prolapse, but is understudied for sexual dysfunction [41,42].
Promising areas of study include educational sessions, cognitive behavioral therapy, vaginal dilator therapy, pelvic floor muscle strengthening and relaxation techniques with biofeedback, stretching and massage [40]. Biofeedback specifically has been studied in controlled studies for treatment of sexual dysfunction specifically in the setting of vulvar pain syndromes [43]. A small randomized control trial evaluated the use of pelvic floor muscle training in gynecologic cancer survivors and found that the intervention group reported improved sexual function and improved quality of life [44]. Given the few side effects of this therapy, it may be a helpful addition to a multidisciplinary approach to treating pelvic floor dysfunction in gynecologic cancer patients.
For cervical and endometrial cancer patients, vaginal dilators are often prescribed following radiation therapy to minimize vaginal stenosis. Literature suggests that after radiotherapy dilation therapy is effective when used as directed, typically 3 times per week for 10 minutes to mechanically separate the vaginal walls and increase elasticity [45]. Adherence to recommendations for vaginal dilator use is low in this population due to factors such as aversion to the practice and intrusiveness of the mechanism [46,47]. Acknowledgment of apprehension regarding dilator use should be part of the counseling prior to initiation of treatment; interventions designed to educate women about dilator use benefit may increase adherence [48].
Psychological Therapies
Survivors of gynecological cancer with sexual dysfunction may experience the psychological symptoms in the context of the other emotional challenges of a cancer diagnosis [49]. Changes in body image, physical appearance, and feelings of well-being can significantly affect sexuality and intimacy, while anxiety and negative feelings about a diagnosis can cause further sexual dysfunction. Conversely, sexual morbidity in these patients predicts worsening of general psychological symptoms, including depressive symptoms and overall quality of life [13–15].
While there are limited studies directly assessing the effect of psychological therapies for the treatment of sexual dysfunction in this population, the existing literature shows enduring symptomatic improvement following brief interventions. In women for whom emotional distress, depression, and anxiety appear to be a significant aspect of their concern regarding intimacy and sexuality, these interventions can be particularly helpful.
Cognitive behavioral therapy (CBT), which focuses on mindfulness and the relationships between maladaptive thoughts and how they impact behavior, has been shown to have efficacy in treating the broader psycho-social concerns of gynecological cancer patients [50,51]. In a recent study Brotto and colleagues randomized 31 survivors of gynecological cancer with self-reported sexual dysfunction to either three 90-minute CBT sessions or a waitlist control. Patients who underwent the intervention reported significant improvements in sexual arousal and desire both immediately post-therapy and at 6-month follow-up while patients in the waitlist arm experienced no significant changes in symptomatology [52].
Psychoeducational interventions are another promising avenue for addressing sexual dysfunction in this population [53]. In a 2008 therapeutic trial, Brotto and colleagues combined elements of CBT with education in 3 one-hour sessions featuring written materials on sexuality and relationships. The intervention enrolled 22 women with early stage gynecologic cancer who met criteria for female sexual arousal disorder. The psychoeducational intervention was associated with positive effect on sexual desire, arousal, orgasm, satisfaction, sexual distress, depression, and overall well-being [16]. Psychoeducation can also be used to augment other therapeutic modalities. Robinson and colleagues used such an intervention to improve adherence to vaginal dilator use in 32 women with early stage endometrial cancer who were being treated with radiotherapy [48].
Some gynecologic cancer patients may have significant alterations in their anatomy and thus penetrative intercourse may not be possible. In patients even without these physical changes, some may prefer to avoid intercourse due to pain or anxiety. Thus patients can have significant benefits from discussing their concerns with a therapist specializing in these issues as many patients are concerned about their ability to engage in intimate behavior. Therapy can assist with the changing sexual relationship and assist the partners in different ways of engaging in intimate acts. It is important to avoid stressing penetrative intercourse as the goal for sexual function with these or any patients with anxiety relating to their disease as there are many ways to engage in mutually pleasurable experiences for both partners, thus removing anxiety about inability to resume vaginal intercourse post-treatment. Discussing this with patients can be challenging but can often reduce anxiety surrounding body image issues following treatment.
Studies have shown that cancer care providers often do not adequately address sexual concerns [54] but that when these concerns are appropriately managed, patient satisfaction and quality of life significantly improve [55]. Several studies have focused on how providers can incorporate the approaches of CBT and psychoeducation to better address the sexual concerns of patients without requiring external psychiatric care [56,57]. Barbera et al have described a successful model in which multidisciplinary care teams provide education and counseling for gynecological cancer patients in a sexuality clinic [58]. patients had a significant symptom burden, including menopausal symptoms, the effects of radiation therapy, chemotherapy, and surgical operation as well as psychological responses to cancer, and reported high levels of satisfaction with their experience at the clinic.
Involvement of the partner in interventions has not been well studied; however, involving the partner in in psychological therapies to address sexual dysfunction should be beneficial.
Alternative Therapies for Vasomotor Symptoms
Gynecologic cancer patients suffering prominent vasomotor symptoms have limited alternatives to hormone therapy. Clinicians must balance potential medication benefit with potential exacerbation of other medical and psychological issues, including sexual dysfunction.
Antidepressants
The use of SSRIs and SNRIs for vasomotor symptoms was pioneered by medical oncologists for men with hot flashes secondary to GnRH agonist therapy for prostate cancer and women with breast cancer [59,60]. Limited studies have shown that antidepressant medications do not increase cancer recurrence risk in ovarian cancer patients and are relatively well tolerated [61]. However, these medications are known to have partial efficacy in improving vasomotor symptoms and may worsen sexual symptoms, a well-known side effect of antidepressants. There is variation of the reported rates of sexual dysfunction associated with various antidepressants and clinicians may take the likelihood of sexual side effects into account when prescribing SSRIs or SNRIS [62]. More recently developed SSRIS, such as citalopram and its enantiomer escitalopram, have shown significant improvements in vasomotor symptoms and were better tolerated than venlafaxine and fluoxetine [63,64]. Additionally, limited uncontrolled studies of mirtazipine, a structurally unique SSRI, and bupropion, which acts on dopamine and norepinephrine, have shown significant decreases in hot flash symptoms and are less associated with sexual side effects than SSRIS/SNRIS [65,66].
Other Agents
Other pharmaceutical options for menopausal vasomotor symptoms include gabapentin and adrenergic agonists. Gabapentin can yield impressive reductions in vasomotor symptoms. A recent double-blind randomized trial in 50 patients revealed a 60% reduction in hot flashes as 12 weeks and an 80% reduction in self-reported composite symptom scores [67]. However, side effects such as palpitations, edema, and fatigue, lead to high study withdrawal rates and limit its widespread clinical use for this indication [68]. Clonidine has been assessed versus venlafaxine in several clinical trials with breast cancer patients. These trials have shown mixed results, with findings of both inferiority and superiority to venlafaxine, but with consistent significant improvement in symptoms over placebo. Side effects, such insomnia, constipation and dry mouth, occurred but did not lead to higher dropout rates than venlafaxine [69,70].
Long-Term Sexual Outcomes
For women treated for gynecological cancers, alterations in sexual function may persist in the long term. A study following cervical cancer patients managed with radical hysterectomy up to 2 years post treatment showed they had more sexual dysfunction compared with healthy controls, although at rates similar to those who underwent radical hysterectomy for benign disease [71]. A 2007 review of quality of life studies revealed that although ovarian cancer survivors 5 years past diagnosis had excellent overall quality of life, sexual symptoms persisted, with as many as 57% of patients reporting a decline in sexual function due to their cancer [72].
Studies show some differences in outcomes based on treatment modality. A recent review of cervical cancer outcomes revealed that women who received radiotherapy as a component of their treatment have a higher risk of long-term sexual side effects [73]. In contrast, a study assessing endometrial cancer patients 5 years after initial diagnosis between those patients who had received surgery alone and those who had received surgery and vaginal brachytherapy. There was no significant difference in any measures of quality of life and sexual function between these 2 groups [74].
Age appears to play a role in long-term sexual outcomes regardless of diagnosis. Bifulco and colleagues assessed quality of life in survivors of gynecological cancer, comparing women under age 45 to those over 45 after nearly 3 years of survival. After controlling for age and other factors, younger patients were found to have worse sexual activity, including significantly higher rates of poor body image, perceived worse sexual vaginal function, and more severe menopausal symptoms, probably related to the effects of surgical menopause [75].
Despite enduring sexual dysfunction, symptoms tend to improve over time. A cohort study of 103 gynecological cancer patients undergoing radiation therapy were followed for 3 years. Patients were offered standard interventions for sexual dysfunction, including vaginal lubricants, dilators, and menopausal symptomatic therapy, although adherence to these measures was not assessed. Three years after initial therapy, the percentage of sexually active women increased from 21.5% to 44.2% [76]. In the subset of patients who successfully return to sexual activity, outcomes can be comparable to healthy peers. Kim and colleagues compared disease-free sexually active ovarian cancer patients with demographically matched healthy controls on standardized self-report measures. Sexual functioning did not differ between the 2 groups, despite lower social functioning in cancer survivors [12].
Conclusion
Sexuality and intimacy can be greatly affected by the diagnosis and treatment of gynecologic malignancies. It is important to routinely discuss sexuality and sexual functioning with patients from diagnosis onward. Reassuring patients, acknowledging the importance of their concerns, and validating their desire to enjoy improved intimacy should be considered part of the clinician’s role. Valuable information sources that may aid discussions are available on the internet. Oncolink (www.oncolink.org), a large cancer information website maintained by University of Pennsylvania Cancer Center, offers a plethora of information for patients and health care professionals. In addition, the American Cancer Society offers a detailed guide, “Sexuality for the Woman with Cancer” [77]. Treatment is available, and improvement in outcomes is possible. Further prospective studies are needed to clearly delineate risks and benefits of hormone replacement therapy in patients with gynecologic cancers.
Corresponding author: Elena S. Ratner, MD, PO Box 208063, New Haven, CT 06520, [email protected].
1. Juraskova I, Butow P, Robertson R, et al. Post-treatment sexual adjustment following cervical and endometrial cancer: a qualitative insight. Psychooncology 2003;12:267–79.
2. Bodurka DC, Sun CC. Sexual function after gynecologic cancer. Obstet Gynecol Clin North Am 2006;33:621–30, ix.
3. Andersen BL, Woods XA, Copeland LJ. Sexual self-schema and sexual morbidity among gynecologic cancer survivors. J Consult Clin Psychol 1997;65:221.
4. Park ER, Norris RL, Bober SL. Sexual health communication during cancer care: barriers and recommendations. Cancer J 2009;15:74–7.
5. Stead ML, Brown JM, Fallowfield L, et al. Lack of communication between healthcare professionals and women with ovarian cancer about sexual issues. Br J Cancer 2003;88:666–71.
6. Stead ML, Fallowfield L, Brown JM, et al. Communication about sexual problems and sexual concerns in ovarian cancer: qualitative study. BMJ 2001;323:836–7.
7. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537–44.
8. American Psychiatric Association. Diagnostic and statistical manual of mental health disorders. 4th ed. 2000.
9. Elder JA, Braver Y. Female sexual dysfunction. In: Current clinical medicine. Elsevier; 2010:1222–6.
10. Ferrell BR, Dow KH, Leigh S, et al. Quality of life in long-term cancer survivors. Oncol Nurs Forum 1995;22:915–22.
11. Shamspour N, Assari S, Moghana Lankarani M. Relation between sexuality and health-related quality of life. In: Handbook of disease burdens and quality of life measures. New York: Springer 2010;3457–73.
12. Kim SI, Lee Y, Lim MC, et al. Quality of life and sexuality comparison between sexually active ovarian cancer survivors and healthy women. J Gynecol Oncol 2015;26:148–54.
13. Levin AO, Carpenter KM, Fowler JM, et al. Sexual morbidity associated with poorer psychological adjustment among gynecological cancer survivors. Int J Gynecol Cancer 2010;20:461–70.
14. Lutgendorf SK, Anderson B, Rothrock N, et al. Quality of life and mood in women receiving extensive chemotherapy for gynecologic cancer. Cancer 2000;89:1402–11.
15. Tierney DK. Sexuality: a quality-of-life issue for cancer survivors. Semin Oncol Nurs 2008;24:71–9.
16. Brotto LA, Heiman JR, Goff B, et al. A psychoeducational intervention for sexual dysfunction in women with gynecologic cancer. Arch Sex Behav 2008;37:317–29.
17. Hollingsworth M, Berman J. The role of androgens in female sexual dysfunction. Sex Reprod Menopause 2006;4:27–32.
18. Brand AH, Bull CA, Cakir B. Vaginal stenosis in patients treated with radiotherapy for carcinoma of the cervix. Int J Gynecol Cancer 2006;16:288–93.
19. Lancaster L. Preventing vaginal stenosis after brachytherapy for gynaecological cancer: an overview of Australian practices. Eur J Oncol Nurs 2004;8:30–9.
20. Ponto JA, Barton D. Husbands’ perspective of living with wives’ ovarian cancer. Psychooncology 2008;17:1225–31.
21. Hartmann U, Philippsohn S, Heiser K, et al. Low sexual desire in midlife and older women: personality factors, psychosocial development, present sexuality. Menopause 2004;11:726–40.
22. Hawkins Y, Ussher J, Gilbert E, et al. Changes in sexuality and intimacy after the diagnosis and treatment of cancer: the experience of partners in a sexual relationship with a person with cancer. Cancer Nurs 2009;32:271–80.
23. Gilbert E, Ussher JM, Hawkins Y. Accounts of disruptions to sexuality following cancer: the perspective of informal carers who are partners of a person with cancer. Health 2009;13:523–41.
24. Hodgkinson K, Butow P, Hunt GE, et al. Life after cancer: couples’ and partners’ psychological adjustment and supportive care needs. Support Care Cancer 2007;15:405–15.
25. Lopez V, Copp G, Molassiotis A. Male caregivers of patients with breast and gynecologic cancer: experiences from caring for their spouses and partners. Cancer Nurs 2012;35:402–10.
26. American Cancer Society. Facts and figures 2015.
27. Michaelson-Cohen R, Beller U. Managing menopausal symptoms after gynecological cancer. Curr Opin Oncol 2009;21:407–11.
28. Biglia N Gadducci A, Ponzone R. Hormone replacement therapy in cancer survivors. Maturitas 2004;48:333–46.
29. Ploch E. Hormonal replacement therapy in patients after cervical cancer treatment. Gynecol Oncol 1987;26:169–77.
30. Guidozzi F, Daponte A. Estrogen replacement therapy for ovarian carcinoma survivors. Cancer 1999;86:1013–8.
31. Creasman WT. Hormone replacement therapy after cancers. Curr Opin Oncol 2005;17:493–9.
32. Bebar S, Ursic-Vrscaj M. Hormone replacement therapy after epithelial ovarian cancer treatment. Eur J Gynaecol Oncol 2000;21:192–6.
33. Wen Y, Huang H, Huang H, et al. The safety of postoperative hormone replacement therapy in epithelial ovarian cancer patients in China. Climacteric 2013;16:673–81.
34. Creasman WT, Henderson D, Hinshaw W, et al. Estrogen replacement therapy in the patient treated for endometrial cancer. Obstet Gynecol 1986;67:326–30.
35. Barakat RR, Bundy BN, Spirtos NM, et al. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2006;24:587–92.
36. Lee K-B, Lee J-M, Lee J-K, et al. Endometrial cancer patients and tibolone: A matched case–control study. Maturitas 2006;55:264–9.
37. Kenemans P, Bundred NJ, Foidart J-M, et al. Safety and efficacy of tibolone in breast-cancer patients with vasomotor symptoms: a double-blind, randomised, non-inferiority trial. Lancet Oncol 2009;10:135–46.
38. Al-Baghdadi O, Ewies AAA. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric 2009;12:91–105.
39. Galuppi A, Perrone AM, La Macchia M, et al. Local α-tocopherol for acute and short-term vaginal toxicity prevention in patients treated with radiotherapy for gynecologic tumors. Int J Gynecol Cancer 2011;21:1708–11.
40. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010;29:4–20.
41. Bø K. Pelvic floor muscle training in treatment of female stress urinary incontinence, pelvic organ prolapse and sexual dysfunction. World J Urol 2012;30:437–43.
42. Dumoulin C, Hay-Smith EJC, Mac Habée-Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2014;5:CD005654.
43. Rosenbaum TY. Pelvic floor involvement in male and female sexual dysfunction and the role of pelvic floor rehabilitation in treatment: A literature review. J Sex Med 2007;4:4–13.
44. Yang EJ, Lim J-Y, Rah UW, et al. Effect of a pelvic floor muscle training program on gynecologic cancer survivors with pelvic floor dysfunction: a randomized controlled trial. Gynecol Oncol 2012;125:705–11.
45. Johnson N, Miles TP, Cornes P. Dilating the vagina to prevent damage from radiotherapy: systematic review of the literature. BJOG 2010;117:522–31.
46. Friedman LC, Abdallah R, Schluchter M, et al. Adherence to vaginal dilation following high dose rate brachytherapy for endometrial cancer. Int J Radiat Oncol Biol Phys 2011;80:751–7.
47. Cullen K, Fergus K, DasGupta T, et al. From “sex toy” to intrusive omposition: a qualitative examination of women’s experiences with vaginal dilator use following treatment for gynecological cancer. J Sex Med 2012;9:1162–73.
48. Robinson JW, Faris PD, Scott CB. Psychoeducational group increases vaginal dilation for younger women and reduces sexual fears for women of all ages with gynecological carcinoma treated with radiotherapy. Int J Radial Oncol Biol Phys 1999;44:497–506.
49. Stavraka C, Ford A, Ghaem-Maghami S, et al. A study of symptoms described by ovarian cancer survivors. Gynecol Oncol 2012;125:59–64.
50. Moonsammy SH, Guglietti CL, Mina DS, et al. A pilot study of an exercise & cognitive behavioral therapy intervention for epithelial ovarian cancer patients. J Ovarian Res 2013;6:21.
51. Goerling U, Jaeger C, Walz A, et al. The efficacy of short-term psycho-oncological interventions for women with gynaecological cancer: a randomized study. Oncology 2014;87:114–24.
52. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol 2012;125:320–5.
53. Cleary V, McCarthy G, Hegarty J. Development of an educational intervention focused on sexuality for women with gynecological cancer. J Psychosoc Oncol 2012;30:535–55.
54. Miller BE, Pittman B, Strong C. Gynecologic cancer patients’ psychosocial needs and their views on the physician›s role in meeting those needs. Int J Gynecol Cancer 2003;13:111–9.
55. Fang P, Tan K, Grover S, et al. Psychosocial encounters correlates with higher patient-reported functional quality of life in gynecological cancer patients receiving radiotherapy. Radiat Oncol 2015;10:34.
56. Hordern A, Grainger M, Hegarty S, et al. Discussing sexuality in the clinical setting: The impact of a brief training program for oncology health professionals to enhance communication about sexuality. Asia Pac J Clin Oncol 2009;5:270–7.
57. Simonelli LE, Pasipanodya E. Health disparities in unmet support needs of women with gynecologic cancer: an exploratory study. J Psychosoc Oncol 2014;32:727–34.
58. Barbera L, Fitch M, Adams L, et al. Improving care for women after gynecological cancer: the development of a sexuality clinic. Menopause 2011;18:1327–33.
59. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20:1578–83.
60. Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 2000;356:2059–63.
61. Wu C-S, Lu M-L, Liao Y-T, et al. Ovarian cancer and antidepressants. Psychooncology 2015;24:579–84.
62. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol 2009;29:259–66.
63. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA 2011;305:267–74.
64. Reed SD, Guthrie KA, Joffe H, et al. Sexual function in nondepressed women using escitalopram for vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2012;119:527–38.
65. Pérez DG, Loprinzi CL, Barton DL, et al. Pilot evaluation of mirtazapine for the treatment of hot flashes. J Support Oncol 2004;2:50–6.
66. Pérez DG, Loprinzi CL, Sloan J, et al. Pilot evaluation of bupropion for the treatment of hot flashes. J Palliat Med 2006;9:631–7.
67. Agarwal N, Singh S, Kriplani A, et al. Evaluation of gabapentin in management of hot flushes in postmenopausal women. Post Reprod Health 2014;20:36–8.
68. Guttuso T Jr, Kurlan R, McDermott MP, et al. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101:337–45.
69. Boekhout AH, Vincent AD, Dalesio OB, et al. Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2011;29:3862–8.
70. Loibl S, Schwedler K, von Minckwitz G, et al. Venlafaxine is superior to clonidine as treatment of hot flashes in breast cancer patients--a double-blind, randomized study. Ann Oncol 2007;18:689–93.
71. Aerts L, Enzlin P, Verhaeghe J, et al. Long-term sexual functioning in women after surgical treatment of cervical cancer stages IA to IB: a prospective controlled study. Int J Gynecol Cancer 2014;24:1527–34.
72. Bloom JR, Petersen DM, Kang SH. Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psychooncology 2007;16:691–706.
73. Pfaendler KS, Wenzel L, Mechanic MB, et al. Cervical cancer survivorship: long-term quality of life and social support. Clin Ther 2015;37:39–48.
74. Becker M, Malafy T, Bossart M, et al. Quality of life and sexual functioning in endometrial cancer survivors. Gynecol Oncol 2011;121:169–73.
75. Bifulco G, De Rosa N, Tornesello ML, et al. Quality of life, lifestyle behavior and employment experience: a comparison between young and midlife survivors of gynecology early stage cancers. Gynecol Oncol 2012;124:444–51.
76. Vaz AF, Pinto-Neto AM, Conde DM, et al. Quality of life and menopausal and sexual symptoms in gynecologic cancer survivors: a cohort study. Menopause 2011;18:662–9.
77. American Cancer Society. Sexuality for the woman with cancer. 2013. Available at www.cancer.org/treatment/treatmentsandsideeffects/physicalsideeffects/sexualsideeffectsinwomen/sexualityforthewoman/index.
From the Yale School of Medicine, New Haven, CT.
Abstract
- Objective: To review the sexual health concerns of women with gynecologic cancer and provide guidance for primary care physicians.
- Methods: Review of the literature.
- Results: Issues of sexuality and intimacy are known to significantly impact the quality of life of patients following diagnosis and treatment of gynecologic cancers. At the time of diagnosis, women should be informed of the potential physiologic, hormonal, and psychosocial effects of gynecologic cancer on sexuality. Many providers fail to address these issues given time constraints and patients’ trepidation in alerting their providers to their concerns. While systemic hormone therapy directly addresses these symptoms, its use remains controversial due to potential cancer recurrence risks. Thus, treatment centers around therapeutic alternatives. For vasomotor symptoms, selective serotonin reuptake inhibitors have shown effectiveness and are typically well tolerated, and antiepileptics such as gabapentin have shown promise. There is promising but limited data employing pelvic floor physical therapy as a tool to aid in addressing pelvic floor symptoms. Psychological care and the involvement of the partner are also part of managing the sexual health concerns of these patients.
- Conclusion: Sexual morbidity is a distressing and undertreated problem among gynecologic cancer survivors. Successful treatment requires the provider’s appreciation of the problem and willingness to address it.
Issues of sexuality and intimacy are known to significantly impact the lives of patients following diagnosis and treatment of gynecologic cancers. [1,2] Treatment of gynecologic malignancy is highly dependent on pathology and stage, with some patients receiving small excisional procedures while others subject to extensive surgeries, chemotherapy, and radiation treatment, and it is difficult to predict how each individual’s sexual health will be impacted. However, the evidence suggests that at least half of women treated for gynecologic cancer will experience sexual dysfunction [3]. Although the impact of gynecologic cancer and treatment can be profound, providers often do not address their patients’ sexual concerns [4,5], yet most patients have indicated that they would like these issues to be addressed [6].
Female Sexual Dysfunction
Sexual dysfunction is multifactorial and involves physical, social, and psychological dimensions. It is common in the general population, with rates ranging from 25-63% [7]. The DSM-IV [8] defines female sexual dysfunction as a disturbance in or pain during the sexual response, which can be further classified as hypoactive sexual disorder, orgasmic disorder, sexual pain disorder, or sexual arousal disorder [9]. It should be noted that women who have been treated for gynecologic cancers may have a premorbid history of sexual dysfunction. Assessment of a woman’s sexual function prior to her cancer diagnosis can help establish which sexuality changes are due to the cancer treatment and may allow the provider to tailor interventions accordingly.
Sexual Dysfunction and Quality of Life
As treatments for gynecologic cancers improve, toxicity of treatment decreases, and survival increases, quality of life for survivors has become as become an increasingly important health issue. Several studies have examined patient-reported quality of life in short- and long-term cancer survivors and report overall significant alteration in quality of life over many aspects of health and psychological well-being [10]. It is well established that health-related quality of life and sexual functioning are closely associated [11].
In gynecologic cancer patients, sexual and quality of life outcomes can vary. For example, Kim el al recently compared quality of life and sexual functioning in ovarian cancer survivors with no evidence of disease after primary treatment and a cohort of health women. Sexuality, both in terms of desire, arousal, lubrication, orgasm, satisfaction, and pain and in terms of interest in sex, sexual activity, and enjoyment of sex were similar between the groups; however social functioning deteriorated in cancer patients [12]. Other women report different experiences. Women with a history of vulvar, vaginal, or cervical cancer who may have had extensive pelvic surgery, lymphadenectomy, and radiation treatment to the pelvis typically report greater alterations in quality of life and sexual activity depending on the extent of their treatment [13].
Patients undergoing chemotherapy often report significant changes in quality of life due to the physical symptoms of fatigue, nausea, hair loss, diarrhea, etc. as well the psychological effects of cancer diagnosis, changes in body image, and poor coping [14]. Sexual side effects are common due to alterations in the HPA axis, direct gonadal toxicity and neuropathies [15].
Impact of Gynecologic Cancer on Sexuality
Gynecologic cancer and treatment can alter sexuality through a variety of effects. The impact of anatomical changes may alter a women’s self-esteem, body image, and sense of femininity, resulting in a reluctance to engage in intimate behaviors. Furthermore, if the partnership is disrupted by changes in roles in the household or within the relationship, relationships dynamics may be tested and result in mental distress for the patient, thus effecting sexuality and intimacy.
Surgical or chemical withdrawal of sex hormones, new medications, and postoperative sequelae can contribute to sexual arousal problems. The emotional and psychological components of a cancer diagnosis also can hinder the sexual response [16]. Depression and anxiety, the rates of which increase with cancer diagnosis, can also significantly affect sexual function [17].
Sexual pain is common in this population due to hormonal considerations as well as post-treatment side effects and a frequent cause of sexual dysfunction for these patients. Pelvic radiation may result in adverse physical changes to the vagina including vaginal stenosis, thinning of vaginal mucosa, loss of lubrication, and loss of elasticity [18,19]. A recent review of 20 studies of cervical cancer survivors found that patients were at risk for lack of lubrication and had high rates of dyspareunia [20]. Psychosocial factors have been found to be important predictors of sexual desire and more important than hormones in predicting low sexual desire in middle age [21]. These factors include emotional and physical closeness to the partner, satisfactory communication, and a positive relation to one’s own body [16].
Effects on Relationships and Partners
Women whose sexual capacity is compromised may be worried about their partners’ quality of life and overall well-being. Indeed, the partners of women with gynecologic cancer are also impacted by the changes to sexual function and loss of sexuality and intimacy. Hawkins et al found that cessation or decreased frequency of sex and intimacy was reported in 79% of male partners of women affected by cancer. Among partners of the persons with cancer in this study, changes to sexuality were associated with feelings of self-blame, reflection, sadness, anger and lack of sexual fulfillment [22]. Further, male partners of women diagnosed with gynecological cancer often express conflicting emotional states including feeling worried about their significant other’s health, having the desire to engage in sexual activity, and feeling guilty about wanting to increase sexual intimacy. These feelings, in turn, can lead to resentment and withdrawal from their partner and overall relationship discord [23].
Evidence suggests partners of cancer patients greatly benefit from increased social support even years after an apparent cure [24]. Specifically, male partners who take on a new role as caregiver in the relationship experience difficulties with emotional changes, challenges to their masculinity, and new stressors [25]. These new roles and feelings also contribute to changes in sexuality and intimacy in relationships, making support for partners all the more necessary.
Menopausal Symptoms Following Cancer Treatment
Gynecologic cancer treatment invariably affects the female hormonal balance, sometimes suddenly with surgical excision of the gonads or via radiation treatment or chemotherapy. It is well known that the sudden withdrawal of estrogen and testosterone, especially in the premenopausal postoperative population, can lead to significant acute menopausal symptoms. Given the many emotional and physical issues affecting patients during treatment, it can be difficult to delineate what proportion of sexual problems are caused by or enhanced by vasomotor symptoms, sleep disorders, and vaginal atrophy.
In general, premenopausal women who experience abrupt surgical menopause may often have immediate severe symptoms. Many agree that the younger a woman is when going through this process, the more severe her symptoms may be. Although the average age of endometrial cancer diagnosis is 67, approximately 25% of women who are diagnosed are premenopausal, and 5% of cases occur in women under 40. As the obesity epidemic worsens and more women are exposed to higher levels of estrogen at younger ages, it is expected that the number of premenopausal women who are diagnosed will continue to rise. The average age of diagnosis for ovarian cancer is 63, however, there is a large cohort of women diagnosed with borderline and malignant tumors in the premenopausal period [26]. These patients often experience vasomotor symptoms in the hours to days following surgery.
Interventions for Survivors of Gynecologic Cancer with Sexual Dysfunction
Systemic Hormone Therapy
Systemic hormonal therapy to treat menopausal symptoms remains controversial following the release of findings from the Women’s Health Initiative, which showed a number of adverse effects, including an increased risk of breast cancer in healthy postmenopausal women who received systemic hormonal therapy for menopausal symptoms. While views have changed since that time, providers are often reluctant to prescribe hormonal therapy, and patients are reticent to take it, due to fears of cancer recurrence. However, there is no evidence showing hormones negatively influence survival after treatment for epithelial ovarian, squamous cervical, or vulvar cancer [27]. With the exception of endometrial cancer, there is no biological evidence that HRT may increase recurrence risk [28]. Approach to clinical decision making should be individualized, taking into consideration the patients’ symptoms, quality of life, tumor histology, and overall prognosis.
Cervical cancer, vulvar cancer, and vaginal cancer are not considered hormonally responsive tumors. While the data are limited, a study published in 1987 of cervical cancer survivors treated with systemic hormone replacement therapy showed no increase in relapse rates and showed an increase in quality of life [29]. There are no studies regarding systemic HRT in patients with vulvar or vaginal cancer though it is generally accepted they can be treated with HRT. No significant data exists for cervical adenocarcinoma patients, and most oncologists suggest treating these patients the same as those with endometrial cancer primary.
There is limited data on the use of HRT in women with ovarian cancer but available studies do not show any difference in overall or disease-free survival between HRT groups and controls [27,30–32]. Many studies report a significant increase in quality of life with HRT. A 2013 retrospective chart review of 77 patients with epithelial ovarian cancer who received postoperative HRT showed no significant difference in progression-free survival [33].
The most controversy surrounds the use of HRT in patients with endometrial cancer [34]. The only major data available come from the Gynecologic Oncology Group’s truncated study, which was a large prospective randomized controlled trial. Over 1200 women treated for stage I and II endometrial cancer were enrolled between 1997 and 2003, and before the study was terminated these patients were followed for a median of 3 years following initiation of therapy. The recurrence rate of malignancy was low, 2.1, and an insignificant risk of recurrence of 1.27 was noted between with HRT and placebo groups (80% CI, 0.916-1.77) [35]. Women included in this study were between the ages of 26 and 91, and their indications for therapy included vasomotor symptoms, vaginal atrophy, as well as osteoporosis risk and cardiovascular disease risk. Gynecologist oncologists often use estrogen therapy to treat symptomatic women with early stage endometrial cancer given the very low risk of recurrence.
Tibolone is a synthetic steroid with activity on estrogen and progesterone receptors, and mainly acts as an agonist at estrogen receptors. It is prescribed outside the United States for osteoporosis and is being investigated as treatment for female sexual dysfunction. Efficacy on vasomotor symptoms has been positive thus far. One case-control study confirmed tibolone’s safety for endometrial cancer survivors, with no adverse effects on disease-free or overall survival [36]. One group recently examined tibolone in the setting of breast cancer patients in a prospective randomized controlled trial and reported an increased risk of breast cancer recurrences in women receiving tibolone for HT [37]. That study reported no increase in risk of gynecologic cancers and did report favorable outcomes for patients in terms of osteoporosis and vasomotor symptoms.
Topical Therapy
Women who are concerned about systemic HRT but who have been treated with radiation in addition to their surgery may be interested in topical estrogen therapy. These women may have vaginal stenosis and atrophic symptoms, and for them topical estrogen therapy can be helpful [38]. Many formulations of topical estrogen are available, including creams, tablets, and rings. Using vaginal estrogens and dilators can be useful to help with eventual resumption of sexual activity once healing has taken place and can help to avoid dyspareunia. Combination topical and systemic therapy can also be useful to relieve symptoms. Women concerned about absorption with topical therapy can be advised that while there is some absorption initially, absorption is reduced as atrophy is improved with treatment.
One recent study examined the use of alpha-tocopherol to reduce acute vaginal complications in women with endometrial and cervical cancer undergoing radiation treatment. The treatment group experienced reduced vaginal toxicity and pain, although vaginal secretion was not significantly different in the 2 groups studied [39]. No adverse effects were noted. This compound has not been studied further but may be beneficial in the future.
Some women may prefer to avoid hormones altogether. Over-the-counter vaginal moisturizers and lubricants can be recommended to women to help with intercourse and atrophic symptoms, with or without estrogen therapy.
Physical Therapy
Pelvic floor physical therapy has become an increasingly popular modality for treatment of many aspects pelvic floor dysfunction, the symptoms of which include defecatory dysfunction, constipation, bladder dysfunction, painful urination, dyspareunia, pelvic pain, and low back pain [40]. Pelvic floor physical therapy is well studied and utilized frequently for urinary incontinence and pelvic organ prolapse, but is understudied for sexual dysfunction [41,42].
Promising areas of study include educational sessions, cognitive behavioral therapy, vaginal dilator therapy, pelvic floor muscle strengthening and relaxation techniques with biofeedback, stretching and massage [40]. Biofeedback specifically has been studied in controlled studies for treatment of sexual dysfunction specifically in the setting of vulvar pain syndromes [43]. A small randomized control trial evaluated the use of pelvic floor muscle training in gynecologic cancer survivors and found that the intervention group reported improved sexual function and improved quality of life [44]. Given the few side effects of this therapy, it may be a helpful addition to a multidisciplinary approach to treating pelvic floor dysfunction in gynecologic cancer patients.
For cervical and endometrial cancer patients, vaginal dilators are often prescribed following radiation therapy to minimize vaginal stenosis. Literature suggests that after radiotherapy dilation therapy is effective when used as directed, typically 3 times per week for 10 minutes to mechanically separate the vaginal walls and increase elasticity [45]. Adherence to recommendations for vaginal dilator use is low in this population due to factors such as aversion to the practice and intrusiveness of the mechanism [46,47]. Acknowledgment of apprehension regarding dilator use should be part of the counseling prior to initiation of treatment; interventions designed to educate women about dilator use benefit may increase adherence [48].
Psychological Therapies
Survivors of gynecological cancer with sexual dysfunction may experience the psychological symptoms in the context of the other emotional challenges of a cancer diagnosis [49]. Changes in body image, physical appearance, and feelings of well-being can significantly affect sexuality and intimacy, while anxiety and negative feelings about a diagnosis can cause further sexual dysfunction. Conversely, sexual morbidity in these patients predicts worsening of general psychological symptoms, including depressive symptoms and overall quality of life [13–15].
While there are limited studies directly assessing the effect of psychological therapies for the treatment of sexual dysfunction in this population, the existing literature shows enduring symptomatic improvement following brief interventions. In women for whom emotional distress, depression, and anxiety appear to be a significant aspect of their concern regarding intimacy and sexuality, these interventions can be particularly helpful.
Cognitive behavioral therapy (CBT), which focuses on mindfulness and the relationships between maladaptive thoughts and how they impact behavior, has been shown to have efficacy in treating the broader psycho-social concerns of gynecological cancer patients [50,51]. In a recent study Brotto and colleagues randomized 31 survivors of gynecological cancer with self-reported sexual dysfunction to either three 90-minute CBT sessions or a waitlist control. Patients who underwent the intervention reported significant improvements in sexual arousal and desire both immediately post-therapy and at 6-month follow-up while patients in the waitlist arm experienced no significant changes in symptomatology [52].
Psychoeducational interventions are another promising avenue for addressing sexual dysfunction in this population [53]. In a 2008 therapeutic trial, Brotto and colleagues combined elements of CBT with education in 3 one-hour sessions featuring written materials on sexuality and relationships. The intervention enrolled 22 women with early stage gynecologic cancer who met criteria for female sexual arousal disorder. The psychoeducational intervention was associated with positive effect on sexual desire, arousal, orgasm, satisfaction, sexual distress, depression, and overall well-being [16]. Psychoeducation can also be used to augment other therapeutic modalities. Robinson and colleagues used such an intervention to improve adherence to vaginal dilator use in 32 women with early stage endometrial cancer who were being treated with radiotherapy [48].
Some gynecologic cancer patients may have significant alterations in their anatomy and thus penetrative intercourse may not be possible. In patients even without these physical changes, some may prefer to avoid intercourse due to pain or anxiety. Thus patients can have significant benefits from discussing their concerns with a therapist specializing in these issues as many patients are concerned about their ability to engage in intimate behavior. Therapy can assist with the changing sexual relationship and assist the partners in different ways of engaging in intimate acts. It is important to avoid stressing penetrative intercourse as the goal for sexual function with these or any patients with anxiety relating to their disease as there are many ways to engage in mutually pleasurable experiences for both partners, thus removing anxiety about inability to resume vaginal intercourse post-treatment. Discussing this with patients can be challenging but can often reduce anxiety surrounding body image issues following treatment.
Studies have shown that cancer care providers often do not adequately address sexual concerns [54] but that when these concerns are appropriately managed, patient satisfaction and quality of life significantly improve [55]. Several studies have focused on how providers can incorporate the approaches of CBT and psychoeducation to better address the sexual concerns of patients without requiring external psychiatric care [56,57]. Barbera et al have described a successful model in which multidisciplinary care teams provide education and counseling for gynecological cancer patients in a sexuality clinic [58]. patients had a significant symptom burden, including menopausal symptoms, the effects of radiation therapy, chemotherapy, and surgical operation as well as psychological responses to cancer, and reported high levels of satisfaction with their experience at the clinic.
Involvement of the partner in interventions has not been well studied; however, involving the partner in in psychological therapies to address sexual dysfunction should be beneficial.
Alternative Therapies for Vasomotor Symptoms
Gynecologic cancer patients suffering prominent vasomotor symptoms have limited alternatives to hormone therapy. Clinicians must balance potential medication benefit with potential exacerbation of other medical and psychological issues, including sexual dysfunction.
Antidepressants
The use of SSRIs and SNRIs for vasomotor symptoms was pioneered by medical oncologists for men with hot flashes secondary to GnRH agonist therapy for prostate cancer and women with breast cancer [59,60]. Limited studies have shown that antidepressant medications do not increase cancer recurrence risk in ovarian cancer patients and are relatively well tolerated [61]. However, these medications are known to have partial efficacy in improving vasomotor symptoms and may worsen sexual symptoms, a well-known side effect of antidepressants. There is variation of the reported rates of sexual dysfunction associated with various antidepressants and clinicians may take the likelihood of sexual side effects into account when prescribing SSRIs or SNRIS [62]. More recently developed SSRIS, such as citalopram and its enantiomer escitalopram, have shown significant improvements in vasomotor symptoms and were better tolerated than venlafaxine and fluoxetine [63,64]. Additionally, limited uncontrolled studies of mirtazipine, a structurally unique SSRI, and bupropion, which acts on dopamine and norepinephrine, have shown significant decreases in hot flash symptoms and are less associated with sexual side effects than SSRIS/SNRIS [65,66].
Other Agents
Other pharmaceutical options for menopausal vasomotor symptoms include gabapentin and adrenergic agonists. Gabapentin can yield impressive reductions in vasomotor symptoms. A recent double-blind randomized trial in 50 patients revealed a 60% reduction in hot flashes as 12 weeks and an 80% reduction in self-reported composite symptom scores [67]. However, side effects such as palpitations, edema, and fatigue, lead to high study withdrawal rates and limit its widespread clinical use for this indication [68]. Clonidine has been assessed versus venlafaxine in several clinical trials with breast cancer patients. These trials have shown mixed results, with findings of both inferiority and superiority to venlafaxine, but with consistent significant improvement in symptoms over placebo. Side effects, such insomnia, constipation and dry mouth, occurred but did not lead to higher dropout rates than venlafaxine [69,70].
Long-Term Sexual Outcomes
For women treated for gynecological cancers, alterations in sexual function may persist in the long term. A study following cervical cancer patients managed with radical hysterectomy up to 2 years post treatment showed they had more sexual dysfunction compared with healthy controls, although at rates similar to those who underwent radical hysterectomy for benign disease [71]. A 2007 review of quality of life studies revealed that although ovarian cancer survivors 5 years past diagnosis had excellent overall quality of life, sexual symptoms persisted, with as many as 57% of patients reporting a decline in sexual function due to their cancer [72].
Studies show some differences in outcomes based on treatment modality. A recent review of cervical cancer outcomes revealed that women who received radiotherapy as a component of their treatment have a higher risk of long-term sexual side effects [73]. In contrast, a study assessing endometrial cancer patients 5 years after initial diagnosis between those patients who had received surgery alone and those who had received surgery and vaginal brachytherapy. There was no significant difference in any measures of quality of life and sexual function between these 2 groups [74].
Age appears to play a role in long-term sexual outcomes regardless of diagnosis. Bifulco and colleagues assessed quality of life in survivors of gynecological cancer, comparing women under age 45 to those over 45 after nearly 3 years of survival. After controlling for age and other factors, younger patients were found to have worse sexual activity, including significantly higher rates of poor body image, perceived worse sexual vaginal function, and more severe menopausal symptoms, probably related to the effects of surgical menopause [75].
Despite enduring sexual dysfunction, symptoms tend to improve over time. A cohort study of 103 gynecological cancer patients undergoing radiation therapy were followed for 3 years. Patients were offered standard interventions for sexual dysfunction, including vaginal lubricants, dilators, and menopausal symptomatic therapy, although adherence to these measures was not assessed. Three years after initial therapy, the percentage of sexually active women increased from 21.5% to 44.2% [76]. In the subset of patients who successfully return to sexual activity, outcomes can be comparable to healthy peers. Kim and colleagues compared disease-free sexually active ovarian cancer patients with demographically matched healthy controls on standardized self-report measures. Sexual functioning did not differ between the 2 groups, despite lower social functioning in cancer survivors [12].
Conclusion
Sexuality and intimacy can be greatly affected by the diagnosis and treatment of gynecologic malignancies. It is important to routinely discuss sexuality and sexual functioning with patients from diagnosis onward. Reassuring patients, acknowledging the importance of their concerns, and validating their desire to enjoy improved intimacy should be considered part of the clinician’s role. Valuable information sources that may aid discussions are available on the internet. Oncolink (www.oncolink.org), a large cancer information website maintained by University of Pennsylvania Cancer Center, offers a plethora of information for patients and health care professionals. In addition, the American Cancer Society offers a detailed guide, “Sexuality for the Woman with Cancer” [77]. Treatment is available, and improvement in outcomes is possible. Further prospective studies are needed to clearly delineate risks and benefits of hormone replacement therapy in patients with gynecologic cancers.
Corresponding author: Elena S. Ratner, MD, PO Box 208063, New Haven, CT 06520, [email protected].
From the Yale School of Medicine, New Haven, CT.
Abstract
- Objective: To review the sexual health concerns of women with gynecologic cancer and provide guidance for primary care physicians.
- Methods: Review of the literature.
- Results: Issues of sexuality and intimacy are known to significantly impact the quality of life of patients following diagnosis and treatment of gynecologic cancers. At the time of diagnosis, women should be informed of the potential physiologic, hormonal, and psychosocial effects of gynecologic cancer on sexuality. Many providers fail to address these issues given time constraints and patients’ trepidation in alerting their providers to their concerns. While systemic hormone therapy directly addresses these symptoms, its use remains controversial due to potential cancer recurrence risks. Thus, treatment centers around therapeutic alternatives. For vasomotor symptoms, selective serotonin reuptake inhibitors have shown effectiveness and are typically well tolerated, and antiepileptics such as gabapentin have shown promise. There is promising but limited data employing pelvic floor physical therapy as a tool to aid in addressing pelvic floor symptoms. Psychological care and the involvement of the partner are also part of managing the sexual health concerns of these patients.
- Conclusion: Sexual morbidity is a distressing and undertreated problem among gynecologic cancer survivors. Successful treatment requires the provider’s appreciation of the problem and willingness to address it.
Issues of sexuality and intimacy are known to significantly impact the lives of patients following diagnosis and treatment of gynecologic cancers. [1,2] Treatment of gynecologic malignancy is highly dependent on pathology and stage, with some patients receiving small excisional procedures while others subject to extensive surgeries, chemotherapy, and radiation treatment, and it is difficult to predict how each individual’s sexual health will be impacted. However, the evidence suggests that at least half of women treated for gynecologic cancer will experience sexual dysfunction [3]. Although the impact of gynecologic cancer and treatment can be profound, providers often do not address their patients’ sexual concerns [4,5], yet most patients have indicated that they would like these issues to be addressed [6].
Female Sexual Dysfunction
Sexual dysfunction is multifactorial and involves physical, social, and psychological dimensions. It is common in the general population, with rates ranging from 25-63% [7]. The DSM-IV [8] defines female sexual dysfunction as a disturbance in or pain during the sexual response, which can be further classified as hypoactive sexual disorder, orgasmic disorder, sexual pain disorder, or sexual arousal disorder [9]. It should be noted that women who have been treated for gynecologic cancers may have a premorbid history of sexual dysfunction. Assessment of a woman’s sexual function prior to her cancer diagnosis can help establish which sexuality changes are due to the cancer treatment and may allow the provider to tailor interventions accordingly.
Sexual Dysfunction and Quality of Life
As treatments for gynecologic cancers improve, toxicity of treatment decreases, and survival increases, quality of life for survivors has become as become an increasingly important health issue. Several studies have examined patient-reported quality of life in short- and long-term cancer survivors and report overall significant alteration in quality of life over many aspects of health and psychological well-being [10]. It is well established that health-related quality of life and sexual functioning are closely associated [11].
In gynecologic cancer patients, sexual and quality of life outcomes can vary. For example, Kim el al recently compared quality of life and sexual functioning in ovarian cancer survivors with no evidence of disease after primary treatment and a cohort of health women. Sexuality, both in terms of desire, arousal, lubrication, orgasm, satisfaction, and pain and in terms of interest in sex, sexual activity, and enjoyment of sex were similar between the groups; however social functioning deteriorated in cancer patients [12]. Other women report different experiences. Women with a history of vulvar, vaginal, or cervical cancer who may have had extensive pelvic surgery, lymphadenectomy, and radiation treatment to the pelvis typically report greater alterations in quality of life and sexual activity depending on the extent of their treatment [13].
Patients undergoing chemotherapy often report significant changes in quality of life due to the physical symptoms of fatigue, nausea, hair loss, diarrhea, etc. as well the psychological effects of cancer diagnosis, changes in body image, and poor coping [14]. Sexual side effects are common due to alterations in the HPA axis, direct gonadal toxicity and neuropathies [15].
Impact of Gynecologic Cancer on Sexuality
Gynecologic cancer and treatment can alter sexuality through a variety of effects. The impact of anatomical changes may alter a women’s self-esteem, body image, and sense of femininity, resulting in a reluctance to engage in intimate behaviors. Furthermore, if the partnership is disrupted by changes in roles in the household or within the relationship, relationships dynamics may be tested and result in mental distress for the patient, thus effecting sexuality and intimacy.
Surgical or chemical withdrawal of sex hormones, new medications, and postoperative sequelae can contribute to sexual arousal problems. The emotional and psychological components of a cancer diagnosis also can hinder the sexual response [16]. Depression and anxiety, the rates of which increase with cancer diagnosis, can also significantly affect sexual function [17].
Sexual pain is common in this population due to hormonal considerations as well as post-treatment side effects and a frequent cause of sexual dysfunction for these patients. Pelvic radiation may result in adverse physical changes to the vagina including vaginal stenosis, thinning of vaginal mucosa, loss of lubrication, and loss of elasticity [18,19]. A recent review of 20 studies of cervical cancer survivors found that patients were at risk for lack of lubrication and had high rates of dyspareunia [20]. Psychosocial factors have been found to be important predictors of sexual desire and more important than hormones in predicting low sexual desire in middle age [21]. These factors include emotional and physical closeness to the partner, satisfactory communication, and a positive relation to one’s own body [16].
Effects on Relationships and Partners
Women whose sexual capacity is compromised may be worried about their partners’ quality of life and overall well-being. Indeed, the partners of women with gynecologic cancer are also impacted by the changes to sexual function and loss of sexuality and intimacy. Hawkins et al found that cessation or decreased frequency of sex and intimacy was reported in 79% of male partners of women affected by cancer. Among partners of the persons with cancer in this study, changes to sexuality were associated with feelings of self-blame, reflection, sadness, anger and lack of sexual fulfillment [22]. Further, male partners of women diagnosed with gynecological cancer often express conflicting emotional states including feeling worried about their significant other’s health, having the desire to engage in sexual activity, and feeling guilty about wanting to increase sexual intimacy. These feelings, in turn, can lead to resentment and withdrawal from their partner and overall relationship discord [23].
Evidence suggests partners of cancer patients greatly benefit from increased social support even years after an apparent cure [24]. Specifically, male partners who take on a new role as caregiver in the relationship experience difficulties with emotional changes, challenges to their masculinity, and new stressors [25]. These new roles and feelings also contribute to changes in sexuality and intimacy in relationships, making support for partners all the more necessary.
Menopausal Symptoms Following Cancer Treatment
Gynecologic cancer treatment invariably affects the female hormonal balance, sometimes suddenly with surgical excision of the gonads or via radiation treatment or chemotherapy. It is well known that the sudden withdrawal of estrogen and testosterone, especially in the premenopausal postoperative population, can lead to significant acute menopausal symptoms. Given the many emotional and physical issues affecting patients during treatment, it can be difficult to delineate what proportion of sexual problems are caused by or enhanced by vasomotor symptoms, sleep disorders, and vaginal atrophy.
In general, premenopausal women who experience abrupt surgical menopause may often have immediate severe symptoms. Many agree that the younger a woman is when going through this process, the more severe her symptoms may be. Although the average age of endometrial cancer diagnosis is 67, approximately 25% of women who are diagnosed are premenopausal, and 5% of cases occur in women under 40. As the obesity epidemic worsens and more women are exposed to higher levels of estrogen at younger ages, it is expected that the number of premenopausal women who are diagnosed will continue to rise. The average age of diagnosis for ovarian cancer is 63, however, there is a large cohort of women diagnosed with borderline and malignant tumors in the premenopausal period [26]. These patients often experience vasomotor symptoms in the hours to days following surgery.
Interventions for Survivors of Gynecologic Cancer with Sexual Dysfunction
Systemic Hormone Therapy
Systemic hormonal therapy to treat menopausal symptoms remains controversial following the release of findings from the Women’s Health Initiative, which showed a number of adverse effects, including an increased risk of breast cancer in healthy postmenopausal women who received systemic hormonal therapy for menopausal symptoms. While views have changed since that time, providers are often reluctant to prescribe hormonal therapy, and patients are reticent to take it, due to fears of cancer recurrence. However, there is no evidence showing hormones negatively influence survival after treatment for epithelial ovarian, squamous cervical, or vulvar cancer [27]. With the exception of endometrial cancer, there is no biological evidence that HRT may increase recurrence risk [28]. Approach to clinical decision making should be individualized, taking into consideration the patients’ symptoms, quality of life, tumor histology, and overall prognosis.
Cervical cancer, vulvar cancer, and vaginal cancer are not considered hormonally responsive tumors. While the data are limited, a study published in 1987 of cervical cancer survivors treated with systemic hormone replacement therapy showed no increase in relapse rates and showed an increase in quality of life [29]. There are no studies regarding systemic HRT in patients with vulvar or vaginal cancer though it is generally accepted they can be treated with HRT. No significant data exists for cervical adenocarcinoma patients, and most oncologists suggest treating these patients the same as those with endometrial cancer primary.
There is limited data on the use of HRT in women with ovarian cancer but available studies do not show any difference in overall or disease-free survival between HRT groups and controls [27,30–32]. Many studies report a significant increase in quality of life with HRT. A 2013 retrospective chart review of 77 patients with epithelial ovarian cancer who received postoperative HRT showed no significant difference in progression-free survival [33].
The most controversy surrounds the use of HRT in patients with endometrial cancer [34]. The only major data available come from the Gynecologic Oncology Group’s truncated study, which was a large prospective randomized controlled trial. Over 1200 women treated for stage I and II endometrial cancer were enrolled between 1997 and 2003, and before the study was terminated these patients were followed for a median of 3 years following initiation of therapy. The recurrence rate of malignancy was low, 2.1, and an insignificant risk of recurrence of 1.27 was noted between with HRT and placebo groups (80% CI, 0.916-1.77) [35]. Women included in this study were between the ages of 26 and 91, and their indications for therapy included vasomotor symptoms, vaginal atrophy, as well as osteoporosis risk and cardiovascular disease risk. Gynecologist oncologists often use estrogen therapy to treat symptomatic women with early stage endometrial cancer given the very low risk of recurrence.
Tibolone is a synthetic steroid with activity on estrogen and progesterone receptors, and mainly acts as an agonist at estrogen receptors. It is prescribed outside the United States for osteoporosis and is being investigated as treatment for female sexual dysfunction. Efficacy on vasomotor symptoms has been positive thus far. One case-control study confirmed tibolone’s safety for endometrial cancer survivors, with no adverse effects on disease-free or overall survival [36]. One group recently examined tibolone in the setting of breast cancer patients in a prospective randomized controlled trial and reported an increased risk of breast cancer recurrences in women receiving tibolone for HT [37]. That study reported no increase in risk of gynecologic cancers and did report favorable outcomes for patients in terms of osteoporosis and vasomotor symptoms.
Topical Therapy
Women who are concerned about systemic HRT but who have been treated with radiation in addition to their surgery may be interested in topical estrogen therapy. These women may have vaginal stenosis and atrophic symptoms, and for them topical estrogen therapy can be helpful [38]. Many formulations of topical estrogen are available, including creams, tablets, and rings. Using vaginal estrogens and dilators can be useful to help with eventual resumption of sexual activity once healing has taken place and can help to avoid dyspareunia. Combination topical and systemic therapy can also be useful to relieve symptoms. Women concerned about absorption with topical therapy can be advised that while there is some absorption initially, absorption is reduced as atrophy is improved with treatment.
One recent study examined the use of alpha-tocopherol to reduce acute vaginal complications in women with endometrial and cervical cancer undergoing radiation treatment. The treatment group experienced reduced vaginal toxicity and pain, although vaginal secretion was not significantly different in the 2 groups studied [39]. No adverse effects were noted. This compound has not been studied further but may be beneficial in the future.
Some women may prefer to avoid hormones altogether. Over-the-counter vaginal moisturizers and lubricants can be recommended to women to help with intercourse and atrophic symptoms, with or without estrogen therapy.
Physical Therapy
Pelvic floor physical therapy has become an increasingly popular modality for treatment of many aspects pelvic floor dysfunction, the symptoms of which include defecatory dysfunction, constipation, bladder dysfunction, painful urination, dyspareunia, pelvic pain, and low back pain [40]. Pelvic floor physical therapy is well studied and utilized frequently for urinary incontinence and pelvic organ prolapse, but is understudied for sexual dysfunction [41,42].
Promising areas of study include educational sessions, cognitive behavioral therapy, vaginal dilator therapy, pelvic floor muscle strengthening and relaxation techniques with biofeedback, stretching and massage [40]. Biofeedback specifically has been studied in controlled studies for treatment of sexual dysfunction specifically in the setting of vulvar pain syndromes [43]. A small randomized control trial evaluated the use of pelvic floor muscle training in gynecologic cancer survivors and found that the intervention group reported improved sexual function and improved quality of life [44]. Given the few side effects of this therapy, it may be a helpful addition to a multidisciplinary approach to treating pelvic floor dysfunction in gynecologic cancer patients.
For cervical and endometrial cancer patients, vaginal dilators are often prescribed following radiation therapy to minimize vaginal stenosis. Literature suggests that after radiotherapy dilation therapy is effective when used as directed, typically 3 times per week for 10 minutes to mechanically separate the vaginal walls and increase elasticity [45]. Adherence to recommendations for vaginal dilator use is low in this population due to factors such as aversion to the practice and intrusiveness of the mechanism [46,47]. Acknowledgment of apprehension regarding dilator use should be part of the counseling prior to initiation of treatment; interventions designed to educate women about dilator use benefit may increase adherence [48].
Psychological Therapies
Survivors of gynecological cancer with sexual dysfunction may experience the psychological symptoms in the context of the other emotional challenges of a cancer diagnosis [49]. Changes in body image, physical appearance, and feelings of well-being can significantly affect sexuality and intimacy, while anxiety and negative feelings about a diagnosis can cause further sexual dysfunction. Conversely, sexual morbidity in these patients predicts worsening of general psychological symptoms, including depressive symptoms and overall quality of life [13–15].
While there are limited studies directly assessing the effect of psychological therapies for the treatment of sexual dysfunction in this population, the existing literature shows enduring symptomatic improvement following brief interventions. In women for whom emotional distress, depression, and anxiety appear to be a significant aspect of their concern regarding intimacy and sexuality, these interventions can be particularly helpful.
Cognitive behavioral therapy (CBT), which focuses on mindfulness and the relationships between maladaptive thoughts and how they impact behavior, has been shown to have efficacy in treating the broader psycho-social concerns of gynecological cancer patients [50,51]. In a recent study Brotto and colleagues randomized 31 survivors of gynecological cancer with self-reported sexual dysfunction to either three 90-minute CBT sessions or a waitlist control. Patients who underwent the intervention reported significant improvements in sexual arousal and desire both immediately post-therapy and at 6-month follow-up while patients in the waitlist arm experienced no significant changes in symptomatology [52].
Psychoeducational interventions are another promising avenue for addressing sexual dysfunction in this population [53]. In a 2008 therapeutic trial, Brotto and colleagues combined elements of CBT with education in 3 one-hour sessions featuring written materials on sexuality and relationships. The intervention enrolled 22 women with early stage gynecologic cancer who met criteria for female sexual arousal disorder. The psychoeducational intervention was associated with positive effect on sexual desire, arousal, orgasm, satisfaction, sexual distress, depression, and overall well-being [16]. Psychoeducation can also be used to augment other therapeutic modalities. Robinson and colleagues used such an intervention to improve adherence to vaginal dilator use in 32 women with early stage endometrial cancer who were being treated with radiotherapy [48].
Some gynecologic cancer patients may have significant alterations in their anatomy and thus penetrative intercourse may not be possible. In patients even without these physical changes, some may prefer to avoid intercourse due to pain or anxiety. Thus patients can have significant benefits from discussing their concerns with a therapist specializing in these issues as many patients are concerned about their ability to engage in intimate behavior. Therapy can assist with the changing sexual relationship and assist the partners in different ways of engaging in intimate acts. It is important to avoid stressing penetrative intercourse as the goal for sexual function with these or any patients with anxiety relating to their disease as there are many ways to engage in mutually pleasurable experiences for both partners, thus removing anxiety about inability to resume vaginal intercourse post-treatment. Discussing this with patients can be challenging but can often reduce anxiety surrounding body image issues following treatment.
Studies have shown that cancer care providers often do not adequately address sexual concerns [54] but that when these concerns are appropriately managed, patient satisfaction and quality of life significantly improve [55]. Several studies have focused on how providers can incorporate the approaches of CBT and psychoeducation to better address the sexual concerns of patients without requiring external psychiatric care [56,57]. Barbera et al have described a successful model in which multidisciplinary care teams provide education and counseling for gynecological cancer patients in a sexuality clinic [58]. patients had a significant symptom burden, including menopausal symptoms, the effects of radiation therapy, chemotherapy, and surgical operation as well as psychological responses to cancer, and reported high levels of satisfaction with their experience at the clinic.
Involvement of the partner in interventions has not been well studied; however, involving the partner in in psychological therapies to address sexual dysfunction should be beneficial.
Alternative Therapies for Vasomotor Symptoms
Gynecologic cancer patients suffering prominent vasomotor symptoms have limited alternatives to hormone therapy. Clinicians must balance potential medication benefit with potential exacerbation of other medical and psychological issues, including sexual dysfunction.
Antidepressants
The use of SSRIs and SNRIs for vasomotor symptoms was pioneered by medical oncologists for men with hot flashes secondary to GnRH agonist therapy for prostate cancer and women with breast cancer [59,60]. Limited studies have shown that antidepressant medications do not increase cancer recurrence risk in ovarian cancer patients and are relatively well tolerated [61]. However, these medications are known to have partial efficacy in improving vasomotor symptoms and may worsen sexual symptoms, a well-known side effect of antidepressants. There is variation of the reported rates of sexual dysfunction associated with various antidepressants and clinicians may take the likelihood of sexual side effects into account when prescribing SSRIs or SNRIS [62]. More recently developed SSRIS, such as citalopram and its enantiomer escitalopram, have shown significant improvements in vasomotor symptoms and were better tolerated than venlafaxine and fluoxetine [63,64]. Additionally, limited uncontrolled studies of mirtazipine, a structurally unique SSRI, and bupropion, which acts on dopamine and norepinephrine, have shown significant decreases in hot flash symptoms and are less associated with sexual side effects than SSRIS/SNRIS [65,66].
Other Agents
Other pharmaceutical options for menopausal vasomotor symptoms include gabapentin and adrenergic agonists. Gabapentin can yield impressive reductions in vasomotor symptoms. A recent double-blind randomized trial in 50 patients revealed a 60% reduction in hot flashes as 12 weeks and an 80% reduction in self-reported composite symptom scores [67]. However, side effects such as palpitations, edema, and fatigue, lead to high study withdrawal rates and limit its widespread clinical use for this indication [68]. Clonidine has been assessed versus venlafaxine in several clinical trials with breast cancer patients. These trials have shown mixed results, with findings of both inferiority and superiority to venlafaxine, but with consistent significant improvement in symptoms over placebo. Side effects, such insomnia, constipation and dry mouth, occurred but did not lead to higher dropout rates than venlafaxine [69,70].
Long-Term Sexual Outcomes
For women treated for gynecological cancers, alterations in sexual function may persist in the long term. A study following cervical cancer patients managed with radical hysterectomy up to 2 years post treatment showed they had more sexual dysfunction compared with healthy controls, although at rates similar to those who underwent radical hysterectomy for benign disease [71]. A 2007 review of quality of life studies revealed that although ovarian cancer survivors 5 years past diagnosis had excellent overall quality of life, sexual symptoms persisted, with as many as 57% of patients reporting a decline in sexual function due to their cancer [72].
Studies show some differences in outcomes based on treatment modality. A recent review of cervical cancer outcomes revealed that women who received radiotherapy as a component of their treatment have a higher risk of long-term sexual side effects [73]. In contrast, a study assessing endometrial cancer patients 5 years after initial diagnosis between those patients who had received surgery alone and those who had received surgery and vaginal brachytherapy. There was no significant difference in any measures of quality of life and sexual function between these 2 groups [74].
Age appears to play a role in long-term sexual outcomes regardless of diagnosis. Bifulco and colleagues assessed quality of life in survivors of gynecological cancer, comparing women under age 45 to those over 45 after nearly 3 years of survival. After controlling for age and other factors, younger patients were found to have worse sexual activity, including significantly higher rates of poor body image, perceived worse sexual vaginal function, and more severe menopausal symptoms, probably related to the effects of surgical menopause [75].
Despite enduring sexual dysfunction, symptoms tend to improve over time. A cohort study of 103 gynecological cancer patients undergoing radiation therapy were followed for 3 years. Patients were offered standard interventions for sexual dysfunction, including vaginal lubricants, dilators, and menopausal symptomatic therapy, although adherence to these measures was not assessed. Three years after initial therapy, the percentage of sexually active women increased from 21.5% to 44.2% [76]. In the subset of patients who successfully return to sexual activity, outcomes can be comparable to healthy peers. Kim and colleagues compared disease-free sexually active ovarian cancer patients with demographically matched healthy controls on standardized self-report measures. Sexual functioning did not differ between the 2 groups, despite lower social functioning in cancer survivors [12].
Conclusion
Sexuality and intimacy can be greatly affected by the diagnosis and treatment of gynecologic malignancies. It is important to routinely discuss sexuality and sexual functioning with patients from diagnosis onward. Reassuring patients, acknowledging the importance of their concerns, and validating their desire to enjoy improved intimacy should be considered part of the clinician’s role. Valuable information sources that may aid discussions are available on the internet. Oncolink (www.oncolink.org), a large cancer information website maintained by University of Pennsylvania Cancer Center, offers a plethora of information for patients and health care professionals. In addition, the American Cancer Society offers a detailed guide, “Sexuality for the Woman with Cancer” [77]. Treatment is available, and improvement in outcomes is possible. Further prospective studies are needed to clearly delineate risks and benefits of hormone replacement therapy in patients with gynecologic cancers.
Corresponding author: Elena S. Ratner, MD, PO Box 208063, New Haven, CT 06520, [email protected].
1. Juraskova I, Butow P, Robertson R, et al. Post-treatment sexual adjustment following cervical and endometrial cancer: a qualitative insight. Psychooncology 2003;12:267–79.
2. Bodurka DC, Sun CC. Sexual function after gynecologic cancer. Obstet Gynecol Clin North Am 2006;33:621–30, ix.
3. Andersen BL, Woods XA, Copeland LJ. Sexual self-schema and sexual morbidity among gynecologic cancer survivors. J Consult Clin Psychol 1997;65:221.
4. Park ER, Norris RL, Bober SL. Sexual health communication during cancer care: barriers and recommendations. Cancer J 2009;15:74–7.
5. Stead ML, Brown JM, Fallowfield L, et al. Lack of communication between healthcare professionals and women with ovarian cancer about sexual issues. Br J Cancer 2003;88:666–71.
6. Stead ML, Fallowfield L, Brown JM, et al. Communication about sexual problems and sexual concerns in ovarian cancer: qualitative study. BMJ 2001;323:836–7.
7. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537–44.
8. American Psychiatric Association. Diagnostic and statistical manual of mental health disorders. 4th ed. 2000.
9. Elder JA, Braver Y. Female sexual dysfunction. In: Current clinical medicine. Elsevier; 2010:1222–6.
10. Ferrell BR, Dow KH, Leigh S, et al. Quality of life in long-term cancer survivors. Oncol Nurs Forum 1995;22:915–22.
11. Shamspour N, Assari S, Moghana Lankarani M. Relation between sexuality and health-related quality of life. In: Handbook of disease burdens and quality of life measures. New York: Springer 2010;3457–73.
12. Kim SI, Lee Y, Lim MC, et al. Quality of life and sexuality comparison between sexually active ovarian cancer survivors and healthy women. J Gynecol Oncol 2015;26:148–54.
13. Levin AO, Carpenter KM, Fowler JM, et al. Sexual morbidity associated with poorer psychological adjustment among gynecological cancer survivors. Int J Gynecol Cancer 2010;20:461–70.
14. Lutgendorf SK, Anderson B, Rothrock N, et al. Quality of life and mood in women receiving extensive chemotherapy for gynecologic cancer. Cancer 2000;89:1402–11.
15. Tierney DK. Sexuality: a quality-of-life issue for cancer survivors. Semin Oncol Nurs 2008;24:71–9.
16. Brotto LA, Heiman JR, Goff B, et al. A psychoeducational intervention for sexual dysfunction in women with gynecologic cancer. Arch Sex Behav 2008;37:317–29.
17. Hollingsworth M, Berman J. The role of androgens in female sexual dysfunction. Sex Reprod Menopause 2006;4:27–32.
18. Brand AH, Bull CA, Cakir B. Vaginal stenosis in patients treated with radiotherapy for carcinoma of the cervix. Int J Gynecol Cancer 2006;16:288–93.
19. Lancaster L. Preventing vaginal stenosis after brachytherapy for gynaecological cancer: an overview of Australian practices. Eur J Oncol Nurs 2004;8:30–9.
20. Ponto JA, Barton D. Husbands’ perspective of living with wives’ ovarian cancer. Psychooncology 2008;17:1225–31.
21. Hartmann U, Philippsohn S, Heiser K, et al. Low sexual desire in midlife and older women: personality factors, psychosocial development, present sexuality. Menopause 2004;11:726–40.
22. Hawkins Y, Ussher J, Gilbert E, et al. Changes in sexuality and intimacy after the diagnosis and treatment of cancer: the experience of partners in a sexual relationship with a person with cancer. Cancer Nurs 2009;32:271–80.
23. Gilbert E, Ussher JM, Hawkins Y. Accounts of disruptions to sexuality following cancer: the perspective of informal carers who are partners of a person with cancer. Health 2009;13:523–41.
24. Hodgkinson K, Butow P, Hunt GE, et al. Life after cancer: couples’ and partners’ psychological adjustment and supportive care needs. Support Care Cancer 2007;15:405–15.
25. Lopez V, Copp G, Molassiotis A. Male caregivers of patients with breast and gynecologic cancer: experiences from caring for their spouses and partners. Cancer Nurs 2012;35:402–10.
26. American Cancer Society. Facts and figures 2015.
27. Michaelson-Cohen R, Beller U. Managing menopausal symptoms after gynecological cancer. Curr Opin Oncol 2009;21:407–11.
28. Biglia N Gadducci A, Ponzone R. Hormone replacement therapy in cancer survivors. Maturitas 2004;48:333–46.
29. Ploch E. Hormonal replacement therapy in patients after cervical cancer treatment. Gynecol Oncol 1987;26:169–77.
30. Guidozzi F, Daponte A. Estrogen replacement therapy for ovarian carcinoma survivors. Cancer 1999;86:1013–8.
31. Creasman WT. Hormone replacement therapy after cancers. Curr Opin Oncol 2005;17:493–9.
32. Bebar S, Ursic-Vrscaj M. Hormone replacement therapy after epithelial ovarian cancer treatment. Eur J Gynaecol Oncol 2000;21:192–6.
33. Wen Y, Huang H, Huang H, et al. The safety of postoperative hormone replacement therapy in epithelial ovarian cancer patients in China. Climacteric 2013;16:673–81.
34. Creasman WT, Henderson D, Hinshaw W, et al. Estrogen replacement therapy in the patient treated for endometrial cancer. Obstet Gynecol 1986;67:326–30.
35. Barakat RR, Bundy BN, Spirtos NM, et al. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2006;24:587–92.
36. Lee K-B, Lee J-M, Lee J-K, et al. Endometrial cancer patients and tibolone: A matched case–control study. Maturitas 2006;55:264–9.
37. Kenemans P, Bundred NJ, Foidart J-M, et al. Safety and efficacy of tibolone in breast-cancer patients with vasomotor symptoms: a double-blind, randomised, non-inferiority trial. Lancet Oncol 2009;10:135–46.
38. Al-Baghdadi O, Ewies AAA. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric 2009;12:91–105.
39. Galuppi A, Perrone AM, La Macchia M, et al. Local α-tocopherol for acute and short-term vaginal toxicity prevention in patients treated with radiotherapy for gynecologic tumors. Int J Gynecol Cancer 2011;21:1708–11.
40. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010;29:4–20.
41. Bø K. Pelvic floor muscle training in treatment of female stress urinary incontinence, pelvic organ prolapse and sexual dysfunction. World J Urol 2012;30:437–43.
42. Dumoulin C, Hay-Smith EJC, Mac Habée-Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2014;5:CD005654.
43. Rosenbaum TY. Pelvic floor involvement in male and female sexual dysfunction and the role of pelvic floor rehabilitation in treatment: A literature review. J Sex Med 2007;4:4–13.
44. Yang EJ, Lim J-Y, Rah UW, et al. Effect of a pelvic floor muscle training program on gynecologic cancer survivors with pelvic floor dysfunction: a randomized controlled trial. Gynecol Oncol 2012;125:705–11.
45. Johnson N, Miles TP, Cornes P. Dilating the vagina to prevent damage from radiotherapy: systematic review of the literature. BJOG 2010;117:522–31.
46. Friedman LC, Abdallah R, Schluchter M, et al. Adherence to vaginal dilation following high dose rate brachytherapy for endometrial cancer. Int J Radiat Oncol Biol Phys 2011;80:751–7.
47. Cullen K, Fergus K, DasGupta T, et al. From “sex toy” to intrusive omposition: a qualitative examination of women’s experiences with vaginal dilator use following treatment for gynecological cancer. J Sex Med 2012;9:1162–73.
48. Robinson JW, Faris PD, Scott CB. Psychoeducational group increases vaginal dilation for younger women and reduces sexual fears for women of all ages with gynecological carcinoma treated with radiotherapy. Int J Radial Oncol Biol Phys 1999;44:497–506.
49. Stavraka C, Ford A, Ghaem-Maghami S, et al. A study of symptoms described by ovarian cancer survivors. Gynecol Oncol 2012;125:59–64.
50. Moonsammy SH, Guglietti CL, Mina DS, et al. A pilot study of an exercise & cognitive behavioral therapy intervention for epithelial ovarian cancer patients. J Ovarian Res 2013;6:21.
51. Goerling U, Jaeger C, Walz A, et al. The efficacy of short-term psycho-oncological interventions for women with gynaecological cancer: a randomized study. Oncology 2014;87:114–24.
52. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol 2012;125:320–5.
53. Cleary V, McCarthy G, Hegarty J. Development of an educational intervention focused on sexuality for women with gynecological cancer. J Psychosoc Oncol 2012;30:535–55.
54. Miller BE, Pittman B, Strong C. Gynecologic cancer patients’ psychosocial needs and their views on the physician›s role in meeting those needs. Int J Gynecol Cancer 2003;13:111–9.
55. Fang P, Tan K, Grover S, et al. Psychosocial encounters correlates with higher patient-reported functional quality of life in gynecological cancer patients receiving radiotherapy. Radiat Oncol 2015;10:34.
56. Hordern A, Grainger M, Hegarty S, et al. Discussing sexuality in the clinical setting: The impact of a brief training program for oncology health professionals to enhance communication about sexuality. Asia Pac J Clin Oncol 2009;5:270–7.
57. Simonelli LE, Pasipanodya E. Health disparities in unmet support needs of women with gynecologic cancer: an exploratory study. J Psychosoc Oncol 2014;32:727–34.
58. Barbera L, Fitch M, Adams L, et al. Improving care for women after gynecological cancer: the development of a sexuality clinic. Menopause 2011;18:1327–33.
59. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20:1578–83.
60. Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 2000;356:2059–63.
61. Wu C-S, Lu M-L, Liao Y-T, et al. Ovarian cancer and antidepressants. Psychooncology 2015;24:579–84.
62. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol 2009;29:259–66.
63. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA 2011;305:267–74.
64. Reed SD, Guthrie KA, Joffe H, et al. Sexual function in nondepressed women using escitalopram for vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2012;119:527–38.
65. Pérez DG, Loprinzi CL, Barton DL, et al. Pilot evaluation of mirtazapine for the treatment of hot flashes. J Support Oncol 2004;2:50–6.
66. Pérez DG, Loprinzi CL, Sloan J, et al. Pilot evaluation of bupropion for the treatment of hot flashes. J Palliat Med 2006;9:631–7.
67. Agarwal N, Singh S, Kriplani A, et al. Evaluation of gabapentin in management of hot flushes in postmenopausal women. Post Reprod Health 2014;20:36–8.
68. Guttuso T Jr, Kurlan R, McDermott MP, et al. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101:337–45.
69. Boekhout AH, Vincent AD, Dalesio OB, et al. Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2011;29:3862–8.
70. Loibl S, Schwedler K, von Minckwitz G, et al. Venlafaxine is superior to clonidine as treatment of hot flashes in breast cancer patients--a double-blind, randomized study. Ann Oncol 2007;18:689–93.
71. Aerts L, Enzlin P, Verhaeghe J, et al. Long-term sexual functioning in women after surgical treatment of cervical cancer stages IA to IB: a prospective controlled study. Int J Gynecol Cancer 2014;24:1527–34.
72. Bloom JR, Petersen DM, Kang SH. Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psychooncology 2007;16:691–706.
73. Pfaendler KS, Wenzel L, Mechanic MB, et al. Cervical cancer survivorship: long-term quality of life and social support. Clin Ther 2015;37:39–48.
74. Becker M, Malafy T, Bossart M, et al. Quality of life and sexual functioning in endometrial cancer survivors. Gynecol Oncol 2011;121:169–73.
75. Bifulco G, De Rosa N, Tornesello ML, et al. Quality of life, lifestyle behavior and employment experience: a comparison between young and midlife survivors of gynecology early stage cancers. Gynecol Oncol 2012;124:444–51.
76. Vaz AF, Pinto-Neto AM, Conde DM, et al. Quality of life and menopausal and sexual symptoms in gynecologic cancer survivors: a cohort study. Menopause 2011;18:662–9.
77. American Cancer Society. Sexuality for the woman with cancer. 2013. Available at www.cancer.org/treatment/treatmentsandsideeffects/physicalsideeffects/sexualsideeffectsinwomen/sexualityforthewoman/index.
1. Juraskova I, Butow P, Robertson R, et al. Post-treatment sexual adjustment following cervical and endometrial cancer: a qualitative insight. Psychooncology 2003;12:267–79.
2. Bodurka DC, Sun CC. Sexual function after gynecologic cancer. Obstet Gynecol Clin North Am 2006;33:621–30, ix.
3. Andersen BL, Woods XA, Copeland LJ. Sexual self-schema and sexual morbidity among gynecologic cancer survivors. J Consult Clin Psychol 1997;65:221.
4. Park ER, Norris RL, Bober SL. Sexual health communication during cancer care: barriers and recommendations. Cancer J 2009;15:74–7.
5. Stead ML, Brown JM, Fallowfield L, et al. Lack of communication between healthcare professionals and women with ovarian cancer about sexual issues. Br J Cancer 2003;88:666–71.
6. Stead ML, Fallowfield L, Brown JM, et al. Communication about sexual problems and sexual concerns in ovarian cancer: qualitative study. BMJ 2001;323:836–7.
7. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537–44.
8. American Psychiatric Association. Diagnostic and statistical manual of mental health disorders. 4th ed. 2000.
9. Elder JA, Braver Y. Female sexual dysfunction. In: Current clinical medicine. Elsevier; 2010:1222–6.
10. Ferrell BR, Dow KH, Leigh S, et al. Quality of life in long-term cancer survivors. Oncol Nurs Forum 1995;22:915–22.
11. Shamspour N, Assari S, Moghana Lankarani M. Relation between sexuality and health-related quality of life. In: Handbook of disease burdens and quality of life measures. New York: Springer 2010;3457–73.
12. Kim SI, Lee Y, Lim MC, et al. Quality of life and sexuality comparison between sexually active ovarian cancer survivors and healthy women. J Gynecol Oncol 2015;26:148–54.
13. Levin AO, Carpenter KM, Fowler JM, et al. Sexual morbidity associated with poorer psychological adjustment among gynecological cancer survivors. Int J Gynecol Cancer 2010;20:461–70.
14. Lutgendorf SK, Anderson B, Rothrock N, et al. Quality of life and mood in women receiving extensive chemotherapy for gynecologic cancer. Cancer 2000;89:1402–11.
15. Tierney DK. Sexuality: a quality-of-life issue for cancer survivors. Semin Oncol Nurs 2008;24:71–9.
16. Brotto LA, Heiman JR, Goff B, et al. A psychoeducational intervention for sexual dysfunction in women with gynecologic cancer. Arch Sex Behav 2008;37:317–29.
17. Hollingsworth M, Berman J. The role of androgens in female sexual dysfunction. Sex Reprod Menopause 2006;4:27–32.
18. Brand AH, Bull CA, Cakir B. Vaginal stenosis in patients treated with radiotherapy for carcinoma of the cervix. Int J Gynecol Cancer 2006;16:288–93.
19. Lancaster L. Preventing vaginal stenosis after brachytherapy for gynaecological cancer: an overview of Australian practices. Eur J Oncol Nurs 2004;8:30–9.
20. Ponto JA, Barton D. Husbands’ perspective of living with wives’ ovarian cancer. Psychooncology 2008;17:1225–31.
21. Hartmann U, Philippsohn S, Heiser K, et al. Low sexual desire in midlife and older women: personality factors, psychosocial development, present sexuality. Menopause 2004;11:726–40.
22. Hawkins Y, Ussher J, Gilbert E, et al. Changes in sexuality and intimacy after the diagnosis and treatment of cancer: the experience of partners in a sexual relationship with a person with cancer. Cancer Nurs 2009;32:271–80.
23. Gilbert E, Ussher JM, Hawkins Y. Accounts of disruptions to sexuality following cancer: the perspective of informal carers who are partners of a person with cancer. Health 2009;13:523–41.
24. Hodgkinson K, Butow P, Hunt GE, et al. Life after cancer: couples’ and partners’ psychological adjustment and supportive care needs. Support Care Cancer 2007;15:405–15.
25. Lopez V, Copp G, Molassiotis A. Male caregivers of patients with breast and gynecologic cancer: experiences from caring for their spouses and partners. Cancer Nurs 2012;35:402–10.
26. American Cancer Society. Facts and figures 2015.
27. Michaelson-Cohen R, Beller U. Managing menopausal symptoms after gynecological cancer. Curr Opin Oncol 2009;21:407–11.
28. Biglia N Gadducci A, Ponzone R. Hormone replacement therapy in cancer survivors. Maturitas 2004;48:333–46.
29. Ploch E. Hormonal replacement therapy in patients after cervical cancer treatment. Gynecol Oncol 1987;26:169–77.
30. Guidozzi F, Daponte A. Estrogen replacement therapy for ovarian carcinoma survivors. Cancer 1999;86:1013–8.
31. Creasman WT. Hormone replacement therapy after cancers. Curr Opin Oncol 2005;17:493–9.
32. Bebar S, Ursic-Vrscaj M. Hormone replacement therapy after epithelial ovarian cancer treatment. Eur J Gynaecol Oncol 2000;21:192–6.
33. Wen Y, Huang H, Huang H, et al. The safety of postoperative hormone replacement therapy in epithelial ovarian cancer patients in China. Climacteric 2013;16:673–81.
34. Creasman WT, Henderson D, Hinshaw W, et al. Estrogen replacement therapy in the patient treated for endometrial cancer. Obstet Gynecol 1986;67:326–30.
35. Barakat RR, Bundy BN, Spirtos NM, et al. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study. J Clin Oncol 2006;24:587–92.
36. Lee K-B, Lee J-M, Lee J-K, et al. Endometrial cancer patients and tibolone: A matched case–control study. Maturitas 2006;55:264–9.
37. Kenemans P, Bundred NJ, Foidart J-M, et al. Safety and efficacy of tibolone in breast-cancer patients with vasomotor symptoms: a double-blind, randomised, non-inferiority trial. Lancet Oncol 2009;10:135–46.
38. Al-Baghdadi O, Ewies AAA. Topical estrogen therapy in the management of postmenopausal vaginal atrophy: an up-to-date overview. Climacteric 2009;12:91–105.
39. Galuppi A, Perrone AM, La Macchia M, et al. Local α-tocopherol for acute and short-term vaginal toxicity prevention in patients treated with radiotherapy for gynecologic tumors. Int J Gynecol Cancer 2011;21:1708–11.
40. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010;29:4–20.
41. Bø K. Pelvic floor muscle training in treatment of female stress urinary incontinence, pelvic organ prolapse and sexual dysfunction. World J Urol 2012;30:437–43.
42. Dumoulin C, Hay-Smith EJC, Mac Habée-Séguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2014;5:CD005654.
43. Rosenbaum TY. Pelvic floor involvement in male and female sexual dysfunction and the role of pelvic floor rehabilitation in treatment: A literature review. J Sex Med 2007;4:4–13.
44. Yang EJ, Lim J-Y, Rah UW, et al. Effect of a pelvic floor muscle training program on gynecologic cancer survivors with pelvic floor dysfunction: a randomized controlled trial. Gynecol Oncol 2012;125:705–11.
45. Johnson N, Miles TP, Cornes P. Dilating the vagina to prevent damage from radiotherapy: systematic review of the literature. BJOG 2010;117:522–31.
46. Friedman LC, Abdallah R, Schluchter M, et al. Adherence to vaginal dilation following high dose rate brachytherapy for endometrial cancer. Int J Radiat Oncol Biol Phys 2011;80:751–7.
47. Cullen K, Fergus K, DasGupta T, et al. From “sex toy” to intrusive omposition: a qualitative examination of women’s experiences with vaginal dilator use following treatment for gynecological cancer. J Sex Med 2012;9:1162–73.
48. Robinson JW, Faris PD, Scott CB. Psychoeducational group increases vaginal dilation for younger women and reduces sexual fears for women of all ages with gynecological carcinoma treated with radiotherapy. Int J Radial Oncol Biol Phys 1999;44:497–506.
49. Stavraka C, Ford A, Ghaem-Maghami S, et al. A study of symptoms described by ovarian cancer survivors. Gynecol Oncol 2012;125:59–64.
50. Moonsammy SH, Guglietti CL, Mina DS, et al. A pilot study of an exercise & cognitive behavioral therapy intervention for epithelial ovarian cancer patients. J Ovarian Res 2013;6:21.
51. Goerling U, Jaeger C, Walz A, et al. The efficacy of short-term psycho-oncological interventions for women with gynaecological cancer: a randomized study. Oncology 2014;87:114–24.
52. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol 2012;125:320–5.
53. Cleary V, McCarthy G, Hegarty J. Development of an educational intervention focused on sexuality for women with gynecological cancer. J Psychosoc Oncol 2012;30:535–55.
54. Miller BE, Pittman B, Strong C. Gynecologic cancer patients’ psychosocial needs and their views on the physician›s role in meeting those needs. Int J Gynecol Cancer 2003;13:111–9.
55. Fang P, Tan K, Grover S, et al. Psychosocial encounters correlates with higher patient-reported functional quality of life in gynecological cancer patients receiving radiotherapy. Radiat Oncol 2015;10:34.
56. Hordern A, Grainger M, Hegarty S, et al. Discussing sexuality in the clinical setting: The impact of a brief training program for oncology health professionals to enhance communication about sexuality. Asia Pac J Clin Oncol 2009;5:270–7.
57. Simonelli LE, Pasipanodya E. Health disparities in unmet support needs of women with gynecologic cancer: an exploratory study. J Psychosoc Oncol 2014;32:727–34.
58. Barbera L, Fitch M, Adams L, et al. Improving care for women after gynecological cancer: the development of a sexuality clinic. Menopause 2011;18:1327–33.
59. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20:1578–83.
60. Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 2000;356:2059–63.
61. Wu C-S, Lu M-L, Liao Y-T, et al. Ovarian cancer and antidepressants. Psychooncology 2015;24:579–84.
62. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol 2009;29:259–66.
63. Freeman EW, Guthrie KA, Caan B, et al. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA 2011;305:267–74.
64. Reed SD, Guthrie KA, Joffe H, et al. Sexual function in nondepressed women using escitalopram for vasomotor symptoms: a randomized controlled trial. Obstet Gynecol 2012;119:527–38.
65. Pérez DG, Loprinzi CL, Barton DL, et al. Pilot evaluation of mirtazapine for the treatment of hot flashes. J Support Oncol 2004;2:50–6.
66. Pérez DG, Loprinzi CL, Sloan J, et al. Pilot evaluation of bupropion for the treatment of hot flashes. J Palliat Med 2006;9:631–7.
67. Agarwal N, Singh S, Kriplani A, et al. Evaluation of gabapentin in management of hot flushes in postmenopausal women. Post Reprod Health 2014;20:36–8.
68. Guttuso T Jr, Kurlan R, McDermott MP, et al. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101:337–45.
69. Boekhout AH, Vincent AD, Dalesio OB, et al. Management of hot flashes in patients who have breast cancer with venlafaxine and clonidine: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2011;29:3862–8.
70. Loibl S, Schwedler K, von Minckwitz G, et al. Venlafaxine is superior to clonidine as treatment of hot flashes in breast cancer patients--a double-blind, randomized study. Ann Oncol 2007;18:689–93.
71. Aerts L, Enzlin P, Verhaeghe J, et al. Long-term sexual functioning in women after surgical treatment of cervical cancer stages IA to IB: a prospective controlled study. Int J Gynecol Cancer 2014;24:1527–34.
72. Bloom JR, Petersen DM, Kang SH. Multi-dimensional quality of life among long-term (5+ years) adult cancer survivors. Psychooncology 2007;16:691–706.
73. Pfaendler KS, Wenzel L, Mechanic MB, et al. Cervical cancer survivorship: long-term quality of life and social support. Clin Ther 2015;37:39–48.
74. Becker M, Malafy T, Bossart M, et al. Quality of life and sexual functioning in endometrial cancer survivors. Gynecol Oncol 2011;121:169–73.
75. Bifulco G, De Rosa N, Tornesello ML, et al. Quality of life, lifestyle behavior and employment experience: a comparison between young and midlife survivors of gynecology early stage cancers. Gynecol Oncol 2012;124:444–51.
76. Vaz AF, Pinto-Neto AM, Conde DM, et al. Quality of life and menopausal and sexual symptoms in gynecologic cancer survivors: a cohort study. Menopause 2011;18:662–9.
77. American Cancer Society. Sexuality for the woman with cancer. 2013. Available at www.cancer.org/treatment/treatmentsandsideeffects/physicalsideeffects/sexualsideeffectsinwomen/sexualityforthewoman/index.
It’s Time to Reconsider Early-morning Testosterone Tests
PRACTICE CHANGER
Early-morning testosterone tests are necessary only for men younger than 45. Because the natural diurnal variation in testosterone levels tends to diminish with age, it is acceptable to test men ages 45 and older before 2 pm.1
STRENGTH OF RECOMMENDATION
B: Based on a retrospective cohort study.1
ILLUSTRATIVE CASE
You are finishing up a visit with a 62-year-old man who has erectile dysfunction (ED), and you want to evaluate for androgen deficiency. It’s already noon. Should you ask him to return for an early-morning visit so you can test his testosterone level?
Increasing public awareness of androgen deficiency has led to more men being tested for testosterone levels. Current Endocrine Society guidelines recommend against routine screening for androgen deficiency in men who do not have symptoms.2 However, for men with classic symptoms of androgen deficiency—such as decreased libido, ED, infertility, depression, osteoporosis, loss of secondary sexual characteristics, or reduced muscle bulk or strength—measurement of total testosterone level is recommended.2
Due to the natural diurnal variation in serum testosterone levels, the guidelines recommend collecting the sample in the early morning.2 This recommendation is based on small observational studies that included mostly men younger than 45, which found a significant difference in testosterone levels between samples drawn early in the morning and in the afternoon.3-5
In recent years, several studies have indicated that this variation declines as men age.4-6 Recently, researchers evaluated the effects of age and time of testing on men’s total testosterone levels.
STUDY SUMMARY
Differences in testosterone levels are significant only in younger men
Welliver et al1 performed a retrospective review of charts from a Minneapolis Veterans Affairs hospital. They identified 2,569 men seen for ED who had total testosterone levels measured between 7 AM and 2 PM in a 15-year period. Men whose total testosterone levels were outside the normal range (> 1,000 or < 50 ng/dL) or who had total testosterone drawn after 2 PM were excluded.
The authors analyzed the results based on age, creating one group for men younger than 40 and five-year age-groups for all other men. Using scatterplot techniques, they separated each age-group into two subgroups based on draw times—7 AM to 9 AM, or 9 AM to 2 PM—and compared the mean total testosterone level for each age and time.
Participants’ mean age was 63. Younger men (< 45) had the largest variation in serum total testosterone, with a large and significant decrease after 9 AM. Only the two youngest groups (ages < 40 and 40 to 44) showed a large decrease in total testosterone in specimens collected after 9 am, compared to those drawn earlier (mean difference, 207 and 149 ng/dL, respectively). This variation was not observed in patients older than 45. Although there was a statistically significant difference between early and later testosterone levels in men ages 70 to 74, the absolute difference—34 ng/dL (452 vs 418 ng/dL)—was unlikely to be clinically significant.
WHAT’S NEW
For older men, later testing will not affect results
This study confirms previous research indicating that the diurnal effect on testosterone levels becomes blunted with increasing age, at least in this group of men with ED. Allowing older men to have their total testosterone levels drawn until 2 PM would allow for greater patient flexibility in draw times, with little change in results.
CAVEATS
Study’s methodology cannot account for several potential confounders
This retrospective study analyzed a single random testosterone measurement from each participant, rather than repeat testosterone levels over the course of a day. However, the study was large (2,569 men) and used mean values, which should at least partially mitigate the effect of having only a single measurement from each participant.
The study measured total testosterone and did not account for potential confounding factors—such as obesity or use of testosterone replacement therapy or androgen deprivation therapy—that could affect sex hormone binding globulin, thus potentially altering total testosterone level. However, the authors estimated that less than 2% of the entire cohort was likely to have unrecognized hormonal manipulation with exogenous gonadotropins.
All of the men in the study were seen for ED, and it is possible that men with ED have more flattening of the diurnal variation than men without ED. However, we are unaware of other data that support this.
Up to 30% of men who have a low early-morning testosterone level may have a normal result when testing is repeated.2,5 Therefore, for all men who have low testosterone level test results, draw a repeat total testosterone level before 9 am to confirm the diagnosis. Also, this study did not evaluate the course of testosterone levels throughout the later afternoon and evening, and it remains unclear whether levels can be drawn even later in the day.
CHALLENGES TO IMPLEMENTATION
Your lab’s policies might require early-morning draws
There will probably be few barriers to implementing this change, unless local laboratory policies are inflexible regarding the timing of testosterone draws.
REFERENCES
1. Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
2. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559.
3. Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf). 1993;39:163-171.
4. Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278-1281.
5. Brambilla DJ, Matsumoto AM, Araujo AB, et al. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907-913.
6. Crawford ED, Barqawi AB, O’Donnell C, et al. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100:509-513.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(7):418-419.
PRACTICE CHANGER
Early-morning testosterone tests are necessary only for men younger than 45. Because the natural diurnal variation in testosterone levels tends to diminish with age, it is acceptable to test men ages 45 and older before 2 pm.1
STRENGTH OF RECOMMENDATION
B: Based on a retrospective cohort study.1
ILLUSTRATIVE CASE
You are finishing up a visit with a 62-year-old man who has erectile dysfunction (ED), and you want to evaluate for androgen deficiency. It’s already noon. Should you ask him to return for an early-morning visit so you can test his testosterone level?
Increasing public awareness of androgen deficiency has led to more men being tested for testosterone levels. Current Endocrine Society guidelines recommend against routine screening for androgen deficiency in men who do not have symptoms.2 However, for men with classic symptoms of androgen deficiency—such as decreased libido, ED, infertility, depression, osteoporosis, loss of secondary sexual characteristics, or reduced muscle bulk or strength—measurement of total testosterone level is recommended.2
Due to the natural diurnal variation in serum testosterone levels, the guidelines recommend collecting the sample in the early morning.2 This recommendation is based on small observational studies that included mostly men younger than 45, which found a significant difference in testosterone levels between samples drawn early in the morning and in the afternoon.3-5
In recent years, several studies have indicated that this variation declines as men age.4-6 Recently, researchers evaluated the effects of age and time of testing on men’s total testosterone levels.
STUDY SUMMARY
Differences in testosterone levels are significant only in younger men
Welliver et al1 performed a retrospective review of charts from a Minneapolis Veterans Affairs hospital. They identified 2,569 men seen for ED who had total testosterone levels measured between 7 AM and 2 PM in a 15-year period. Men whose total testosterone levels were outside the normal range (> 1,000 or < 50 ng/dL) or who had total testosterone drawn after 2 PM were excluded.
The authors analyzed the results based on age, creating one group for men younger than 40 and five-year age-groups for all other men. Using scatterplot techniques, they separated each age-group into two subgroups based on draw times—7 AM to 9 AM, or 9 AM to 2 PM—and compared the mean total testosterone level for each age and time.
Participants’ mean age was 63. Younger men (< 45) had the largest variation in serum total testosterone, with a large and significant decrease after 9 AM. Only the two youngest groups (ages < 40 and 40 to 44) showed a large decrease in total testosterone in specimens collected after 9 am, compared to those drawn earlier (mean difference, 207 and 149 ng/dL, respectively). This variation was not observed in patients older than 45. Although there was a statistically significant difference between early and later testosterone levels in men ages 70 to 74, the absolute difference—34 ng/dL (452 vs 418 ng/dL)—was unlikely to be clinically significant.
WHAT’S NEW
For older men, later testing will not affect results
This study confirms previous research indicating that the diurnal effect on testosterone levels becomes blunted with increasing age, at least in this group of men with ED. Allowing older men to have their total testosterone levels drawn until 2 PM would allow for greater patient flexibility in draw times, with little change in results.
CAVEATS
Study’s methodology cannot account for several potential confounders
This retrospective study analyzed a single random testosterone measurement from each participant, rather than repeat testosterone levels over the course of a day. However, the study was large (2,569 men) and used mean values, which should at least partially mitigate the effect of having only a single measurement from each participant.
The study measured total testosterone and did not account for potential confounding factors—such as obesity or use of testosterone replacement therapy or androgen deprivation therapy—that could affect sex hormone binding globulin, thus potentially altering total testosterone level. However, the authors estimated that less than 2% of the entire cohort was likely to have unrecognized hormonal manipulation with exogenous gonadotropins.
All of the men in the study were seen for ED, and it is possible that men with ED have more flattening of the diurnal variation than men without ED. However, we are unaware of other data that support this.
Up to 30% of men who have a low early-morning testosterone level may have a normal result when testing is repeated.2,5 Therefore, for all men who have low testosterone level test results, draw a repeat total testosterone level before 9 am to confirm the diagnosis. Also, this study did not evaluate the course of testosterone levels throughout the later afternoon and evening, and it remains unclear whether levels can be drawn even later in the day.
CHALLENGES TO IMPLEMENTATION
Your lab’s policies might require early-morning draws
There will probably be few barriers to implementing this change, unless local laboratory policies are inflexible regarding the timing of testosterone draws.
REFERENCES
1. Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
2. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559.
3. Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf). 1993;39:163-171.
4. Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278-1281.
5. Brambilla DJ, Matsumoto AM, Araujo AB, et al. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907-913.
6. Crawford ED, Barqawi AB, O’Donnell C, et al. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100:509-513.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(7):418-419.
PRACTICE CHANGER
Early-morning testosterone tests are necessary only for men younger than 45. Because the natural diurnal variation in testosterone levels tends to diminish with age, it is acceptable to test men ages 45 and older before 2 pm.1
STRENGTH OF RECOMMENDATION
B: Based on a retrospective cohort study.1
ILLUSTRATIVE CASE
You are finishing up a visit with a 62-year-old man who has erectile dysfunction (ED), and you want to evaluate for androgen deficiency. It’s already noon. Should you ask him to return for an early-morning visit so you can test his testosterone level?
Increasing public awareness of androgen deficiency has led to more men being tested for testosterone levels. Current Endocrine Society guidelines recommend against routine screening for androgen deficiency in men who do not have symptoms.2 However, for men with classic symptoms of androgen deficiency—such as decreased libido, ED, infertility, depression, osteoporosis, loss of secondary sexual characteristics, or reduced muscle bulk or strength—measurement of total testosterone level is recommended.2
Due to the natural diurnal variation in serum testosterone levels, the guidelines recommend collecting the sample in the early morning.2 This recommendation is based on small observational studies that included mostly men younger than 45, which found a significant difference in testosterone levels between samples drawn early in the morning and in the afternoon.3-5
In recent years, several studies have indicated that this variation declines as men age.4-6 Recently, researchers evaluated the effects of age and time of testing on men’s total testosterone levels.
STUDY SUMMARY
Differences in testosterone levels are significant only in younger men
Welliver et al1 performed a retrospective review of charts from a Minneapolis Veterans Affairs hospital. They identified 2,569 men seen for ED who had total testosterone levels measured between 7 AM and 2 PM in a 15-year period. Men whose total testosterone levels were outside the normal range (> 1,000 or < 50 ng/dL) or who had total testosterone drawn after 2 PM were excluded.
The authors analyzed the results based on age, creating one group for men younger than 40 and five-year age-groups for all other men. Using scatterplot techniques, they separated each age-group into two subgroups based on draw times—7 AM to 9 AM, or 9 AM to 2 PM—and compared the mean total testosterone level for each age and time.
Participants’ mean age was 63. Younger men (< 45) had the largest variation in serum total testosterone, with a large and significant decrease after 9 AM. Only the two youngest groups (ages < 40 and 40 to 44) showed a large decrease in total testosterone in specimens collected after 9 am, compared to those drawn earlier (mean difference, 207 and 149 ng/dL, respectively). This variation was not observed in patients older than 45. Although there was a statistically significant difference between early and later testosterone levels in men ages 70 to 74, the absolute difference—34 ng/dL (452 vs 418 ng/dL)—was unlikely to be clinically significant.
WHAT’S NEW
For older men, later testing will not affect results
This study confirms previous research indicating that the diurnal effect on testosterone levels becomes blunted with increasing age, at least in this group of men with ED. Allowing older men to have their total testosterone levels drawn until 2 PM would allow for greater patient flexibility in draw times, with little change in results.
CAVEATS
Study’s methodology cannot account for several potential confounders
This retrospective study analyzed a single random testosterone measurement from each participant, rather than repeat testosterone levels over the course of a day. However, the study was large (2,569 men) and used mean values, which should at least partially mitigate the effect of having only a single measurement from each participant.
The study measured total testosterone and did not account for potential confounding factors—such as obesity or use of testosterone replacement therapy or androgen deprivation therapy—that could affect sex hormone binding globulin, thus potentially altering total testosterone level. However, the authors estimated that less than 2% of the entire cohort was likely to have unrecognized hormonal manipulation with exogenous gonadotropins.
All of the men in the study were seen for ED, and it is possible that men with ED have more flattening of the diurnal variation than men without ED. However, we are unaware of other data that support this.
Up to 30% of men who have a low early-morning testosterone level may have a normal result when testing is repeated.2,5 Therefore, for all men who have low testosterone level test results, draw a repeat total testosterone level before 9 am to confirm the diagnosis. Also, this study did not evaluate the course of testosterone levels throughout the later afternoon and evening, and it remains unclear whether levels can be drawn even later in the day.
CHALLENGES TO IMPLEMENTATION
Your lab’s policies might require early-morning draws
There will probably be few barriers to implementing this change, unless local laboratory policies are inflexible regarding the timing of testosterone draws.
REFERENCES
1. Welliver RC Jr, Wiser HJ, Brannigan RE, et al. Validity of midday total testosterone levels in older men with erectile dysfunction. J Urol. 2014;192:165-169.
2. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536-2559.
3. Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf). 1993;39:163-171.
4. Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278-1281.
5. Brambilla DJ, Matsumoto AM, Araujo AB, et al. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907-913.
6. Crawford ED, Barqawi AB, O’Donnell C, et al. The association of time of day and serum testosterone concentration in a large screening population. BJU Int. 2007;100:509-513.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2015. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2015;64(7):418-419.
The mainstreaming of alternative therapies
In this issue, Dr. Onysko discusses “alternative” pharmacologic approaches to painful peripheral neuropathy. (See “Targeting neuropathic pain: Consider these alternatives.”) Because so many of our patients use alternative therapies, I contend that alternative therapies are no longer “alternative.” Even the federal government has officially recognized the widespread use of alternative therapies by changing the name of the National Center for Complementary and Alternative Medicine in December 2014 to the National Center for Complementary and Integrative Health.
Alternative medicine had a bad name in mainstream medicine until 1991, when the National Institutes of Health established the Office of Alternative Medicine, officially recognizing that some alternative treatments might have a scientific basis and true therapeutic effects beyond the placebo effect. Over the years, hundreds of randomized controlled trials (RCTs) have emerged to investigate the value of various herbal treatments, vitamin therapies, magnet therapy, acupuncture, tai chi, aromatherapy, and other physical medicine and medicinal treatment modalities.
Last spring, as I prepared an evidence-based medicine talk, I was struck by the solid evidence supporting numerous therapies we used to consider alternative. Many trials of acupuncture, for instance, have shown positive effects for various musculoskeletal problems. But acupuncture is also effective for functional dyspepsia, according to a well-designed RCT.1 In addition, it can relieve symptoms of irritable bowel syndrome (IBS), according to a Cochrane meta-analysis of 17 RCTs.2
One of the new kids on the block in alternative medicine is functional medicine, founded by nutritionist/biochemist Jeff Bland. According to the Institute of Functional Medicine Web site, functional medicine is a combination of holistic medicine principles and a belief that we can treat a wide variety of ailments with various dietary treatments, including supplements.3 Although research on the interaction between gut flora and human health is burgeoning, I’m wary of claims of effectiveness until we see evidence of improved patient-oriented outcomes from well-executed RCTs.
I’m keeping an open mind, however, about all forms of complementary and integrative therapies. After all, who would have guessed 30 years ago that peptic ulcer disease could be cured with antibiotics?
1. Ma TT, Yu SY, Li Y, et al. Randomised clinical trial: an assessment of acupuncture on specific meridian or specific acupoint vs. sham acupuncture for treating functional dyspepsia. Aliment Pharmacol Ther. 2012;35:552-561.
2. Manheimer E, Cheng K, Wieland LS, et al. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2012;5:CD005111.
3. Institute of Functional Medicine. What is functional medicine? Institute of Functional Medicine Web site. Available at: https://www.functionalmedicine.org/about/whatisfm/. Accessed July 20, 2015.
In this issue, Dr. Onysko discusses “alternative” pharmacologic approaches to painful peripheral neuropathy. (See “Targeting neuropathic pain: Consider these alternatives.”) Because so many of our patients use alternative therapies, I contend that alternative therapies are no longer “alternative.” Even the federal government has officially recognized the widespread use of alternative therapies by changing the name of the National Center for Complementary and Alternative Medicine in December 2014 to the National Center for Complementary and Integrative Health.
Alternative medicine had a bad name in mainstream medicine until 1991, when the National Institutes of Health established the Office of Alternative Medicine, officially recognizing that some alternative treatments might have a scientific basis and true therapeutic effects beyond the placebo effect. Over the years, hundreds of randomized controlled trials (RCTs) have emerged to investigate the value of various herbal treatments, vitamin therapies, magnet therapy, acupuncture, tai chi, aromatherapy, and other physical medicine and medicinal treatment modalities.
Last spring, as I prepared an evidence-based medicine talk, I was struck by the solid evidence supporting numerous therapies we used to consider alternative. Many trials of acupuncture, for instance, have shown positive effects for various musculoskeletal problems. But acupuncture is also effective for functional dyspepsia, according to a well-designed RCT.1 In addition, it can relieve symptoms of irritable bowel syndrome (IBS), according to a Cochrane meta-analysis of 17 RCTs.2
One of the new kids on the block in alternative medicine is functional medicine, founded by nutritionist/biochemist Jeff Bland. According to the Institute of Functional Medicine Web site, functional medicine is a combination of holistic medicine principles and a belief that we can treat a wide variety of ailments with various dietary treatments, including supplements.3 Although research on the interaction between gut flora and human health is burgeoning, I’m wary of claims of effectiveness until we see evidence of improved patient-oriented outcomes from well-executed RCTs.
I’m keeping an open mind, however, about all forms of complementary and integrative therapies. After all, who would have guessed 30 years ago that peptic ulcer disease could be cured with antibiotics?
In this issue, Dr. Onysko discusses “alternative” pharmacologic approaches to painful peripheral neuropathy. (See “Targeting neuropathic pain: Consider these alternatives.”) Because so many of our patients use alternative therapies, I contend that alternative therapies are no longer “alternative.” Even the federal government has officially recognized the widespread use of alternative therapies by changing the name of the National Center for Complementary and Alternative Medicine in December 2014 to the National Center for Complementary and Integrative Health.
Alternative medicine had a bad name in mainstream medicine until 1991, when the National Institutes of Health established the Office of Alternative Medicine, officially recognizing that some alternative treatments might have a scientific basis and true therapeutic effects beyond the placebo effect. Over the years, hundreds of randomized controlled trials (RCTs) have emerged to investigate the value of various herbal treatments, vitamin therapies, magnet therapy, acupuncture, tai chi, aromatherapy, and other physical medicine and medicinal treatment modalities.
Last spring, as I prepared an evidence-based medicine talk, I was struck by the solid evidence supporting numerous therapies we used to consider alternative. Many trials of acupuncture, for instance, have shown positive effects for various musculoskeletal problems. But acupuncture is also effective for functional dyspepsia, according to a well-designed RCT.1 In addition, it can relieve symptoms of irritable bowel syndrome (IBS), according to a Cochrane meta-analysis of 17 RCTs.2
One of the new kids on the block in alternative medicine is functional medicine, founded by nutritionist/biochemist Jeff Bland. According to the Institute of Functional Medicine Web site, functional medicine is a combination of holistic medicine principles and a belief that we can treat a wide variety of ailments with various dietary treatments, including supplements.3 Although research on the interaction between gut flora and human health is burgeoning, I’m wary of claims of effectiveness until we see evidence of improved patient-oriented outcomes from well-executed RCTs.
I’m keeping an open mind, however, about all forms of complementary and integrative therapies. After all, who would have guessed 30 years ago that peptic ulcer disease could be cured with antibiotics?
1. Ma TT, Yu SY, Li Y, et al. Randomised clinical trial: an assessment of acupuncture on specific meridian or specific acupoint vs. sham acupuncture for treating functional dyspepsia. Aliment Pharmacol Ther. 2012;35:552-561.
2. Manheimer E, Cheng K, Wieland LS, et al. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2012;5:CD005111.
3. Institute of Functional Medicine. What is functional medicine? Institute of Functional Medicine Web site. Available at: https://www.functionalmedicine.org/about/whatisfm/. Accessed July 20, 2015.
1. Ma TT, Yu SY, Li Y, et al. Randomised clinical trial: an assessment of acupuncture on specific meridian or specific acupoint vs. sham acupuncture for treating functional dyspepsia. Aliment Pharmacol Ther. 2012;35:552-561.
2. Manheimer E, Cheng K, Wieland LS, et al. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2012;5:CD005111.
3. Institute of Functional Medicine. What is functional medicine? Institute of Functional Medicine Web site. Available at: https://www.functionalmedicine.org/about/whatisfm/. Accessed July 20, 2015.
Nail the Genetic Disorder
1. The patient has a long-standing pruritic rash. Flat, slightly elevated, greasy brown papules are scattered on the chest, abdomen, and upper back, with mild surrounding erythema. The fingernails are deformed, with longitudinal red streaks and ridges and v-shaped notching of the free margin.
Reprinted with permission from Cutis. 2003;72:124-126.
Diagnosis: Darier disease (DD) or Darier-White disease is a rare genetic skin disorder caused by mutations of the ATP2A2 gene, located on the long arm of chromosome 12 at position 24,11.1,2 This genetic mutation is inherited as an autosomal dominant trait with complete penetrance. DD affects men and women equally, with progressive skin signs of interfamilial and intrafamilial variability.3 Skin manifestations occur from late childhood to early adulthood and are typical during adolescence.3 Acute flare-ups can be triggered by heat, perspiration, sunlight, ultraviolet B exposure, stress, or certain medications (in particular, lithium).2 DD is not contagious.2
1. Creamer D, Barker J, Kerdel FA. Papular and papulosquamous dermatoses. In: Acute Adult Dermatology: Diagnosis and Management (A Colour Handbook). London, UK: Manson Publishing Ltd; 2011:48.
2. Kelly EB. Darier disease (DAR). In: Encyclopedia of Human Genetics and Disease. Santa Barbara, CA: ABC-CLIO; 2013:186-187.
3. Ringpfeil F. Dermatologic disorders. In: NORD Guide to Rare Disorders. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:101.
For more information on this case, see “Man, 45, With Greasy Rash and Deformed Nails.” Clin Rev. 2014;24(1):38,40-41
For the next photograph, proceed to the next page >>
2. Six months ago, this 36-year-old man’s left fourth finger began to bother him. He’s tried topical antibiotics, colloidal silver solution, and two different oral antibiotics. None have relieved the pain, which is severe enough to interfere with daily activity—particularly his job, which requires extensive computer time.
Diagnosis: “Infection” is only one potential cause of redness, swelling, increased warmth, and localized pain. Classically termed rubor, tumor, calor, and dolor, these are indicators of inflammation, which can occur in many conditions besides “infection.” In the case described, a simple hangnail was incompletely removed, leaving a shard of nail that then dug into the perionychial skin as it grew out. This set into motion a healing process that could not proceed to resolution, because the tissue was re-injured every time the finger struck the computer keyboard. This not only caused the wound to get stuck in a certain phase of healing (angioneogenesis) but also prevented completion of the process.
For more information on this case, see “Red and Swollen Doesn’t Mean “Infection.” Clin Rev. 2014;24(6):W1.
For the next photograph, proceed to the next page >>
3. A 25-year-old man presents with what he describes as a “fungal infection” of the fingernails that he’s had since birth. The nails are uniformly thickened and dystrophic, without significant discoloration. The patient’s palms and soles are hyperkeratotic, and the upper anterior legs are covered by a folliculocentric papular hyperkeratosis reminiscent of a coarse keratosis pilaris.
Diagnosis: Pachyonychia congenita (PC) is a rare condition that represents a mutation of keratin genes and is usually of autosomal dominant inheritance. First described by Muller in 1904, it was eventually categorized into one of two types: type I, MIM 167200, also known as Jadassohn-Lewandowsky, the most common type, and type II, MIM 167210, also known as Jackson-Lawler, with slightly different features. Today, a more common view is that no such divisions exist—only variations of PC that exhibit overlapping features.
For more information on this case, see “A fingernail "infection" present since birth.” Clin Rev. 2013;23(4):W6.
For the next photograph, proceed to the next page >>
4. Usually apparent at birth, this disorder may manifest with abnormal or missing nails. Characteristic nail changes include triangular lunulae and, most prominently on the thumb and index fingers, hypoplasia. Also apparent are orthopedic changes (particularly affecting the knees and elbows), renal disease, and glaucoma.
Reprinted with permission from Cutis. 2000;66:71, 75-76.
Diagnosis: The osteo-onychodysplasia, or nail-patella syndrome, is an autosomal dominant disorder. Studies have linked the syndrome to chromosome 9q34 and identified point mutations in the LMX1B gene.4 Other kindreds have been linked to chromosome 17q21-22.5 Prenatal diagnosis is possible, including noninvasive prenatal diagnosis using ultrasonography. Because of the risk for glaucoma, all family members should be screened by an ophthalmologist.6
4. Seri M, Melchionda S, Dreyer S, et al. Identification of LMX1B gene point mutations in Italian patients affected with nail-patella syndrome. Int J Mol Med. 1999;4:285-290.
5. Mangino M, Sanchez O, Torrente I, et al. Localization of a gene for familial patella aplasia-hypoplasia (PTLAH) to chromosome 17q21-22. Am J Hum Genetics. 1999;65:441-447.
6. Lichter PR, Richards JE, Downs CA, et al. Cosegregation of open-angle glaucoma and the nail-patella syndrome. Am J Ophthalmol. 1997;124:506-515.
For more information on this case, see “What Is Your Diagnosis? Nail-Patella Syndrome.” Cutis. 2000;66:71, 75-76.
1. The patient has a long-standing pruritic rash. Flat, slightly elevated, greasy brown papules are scattered on the chest, abdomen, and upper back, with mild surrounding erythema. The fingernails are deformed, with longitudinal red streaks and ridges and v-shaped notching of the free margin.
Reprinted with permission from Cutis. 2003;72:124-126.
Diagnosis: Darier disease (DD) or Darier-White disease is a rare genetic skin disorder caused by mutations of the ATP2A2 gene, located on the long arm of chromosome 12 at position 24,11.1,2 This genetic mutation is inherited as an autosomal dominant trait with complete penetrance. DD affects men and women equally, with progressive skin signs of interfamilial and intrafamilial variability.3 Skin manifestations occur from late childhood to early adulthood and are typical during adolescence.3 Acute flare-ups can be triggered by heat, perspiration, sunlight, ultraviolet B exposure, stress, or certain medications (in particular, lithium).2 DD is not contagious.2
1. Creamer D, Barker J, Kerdel FA. Papular and papulosquamous dermatoses. In: Acute Adult Dermatology: Diagnosis and Management (A Colour Handbook). London, UK: Manson Publishing Ltd; 2011:48.
2. Kelly EB. Darier disease (DAR). In: Encyclopedia of Human Genetics and Disease. Santa Barbara, CA: ABC-CLIO; 2013:186-187.
3. Ringpfeil F. Dermatologic disorders. In: NORD Guide to Rare Disorders. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:101.
For more information on this case, see “Man, 45, With Greasy Rash and Deformed Nails.” Clin Rev. 2014;24(1):38,40-41
For the next photograph, proceed to the next page >>
2. Six months ago, this 36-year-old man’s left fourth finger began to bother him. He’s tried topical antibiotics, colloidal silver solution, and two different oral antibiotics. None have relieved the pain, which is severe enough to interfere with daily activity—particularly his job, which requires extensive computer time.
Diagnosis: “Infection” is only one potential cause of redness, swelling, increased warmth, and localized pain. Classically termed rubor, tumor, calor, and dolor, these are indicators of inflammation, which can occur in many conditions besides “infection.” In the case described, a simple hangnail was incompletely removed, leaving a shard of nail that then dug into the perionychial skin as it grew out. This set into motion a healing process that could not proceed to resolution, because the tissue was re-injured every time the finger struck the computer keyboard. This not only caused the wound to get stuck in a certain phase of healing (angioneogenesis) but also prevented completion of the process.
For more information on this case, see “Red and Swollen Doesn’t Mean “Infection.” Clin Rev. 2014;24(6):W1.
For the next photograph, proceed to the next page >>
3. A 25-year-old man presents with what he describes as a “fungal infection” of the fingernails that he’s had since birth. The nails are uniformly thickened and dystrophic, without significant discoloration. The patient’s palms and soles are hyperkeratotic, and the upper anterior legs are covered by a folliculocentric papular hyperkeratosis reminiscent of a coarse keratosis pilaris.
Diagnosis: Pachyonychia congenita (PC) is a rare condition that represents a mutation of keratin genes and is usually of autosomal dominant inheritance. First described by Muller in 1904, it was eventually categorized into one of two types: type I, MIM 167200, also known as Jadassohn-Lewandowsky, the most common type, and type II, MIM 167210, also known as Jackson-Lawler, with slightly different features. Today, a more common view is that no such divisions exist—only variations of PC that exhibit overlapping features.
For more information on this case, see “A fingernail "infection" present since birth.” Clin Rev. 2013;23(4):W6.
For the next photograph, proceed to the next page >>
4. Usually apparent at birth, this disorder may manifest with abnormal or missing nails. Characteristic nail changes include triangular lunulae and, most prominently on the thumb and index fingers, hypoplasia. Also apparent are orthopedic changes (particularly affecting the knees and elbows), renal disease, and glaucoma.
Reprinted with permission from Cutis. 2000;66:71, 75-76.
Diagnosis: The osteo-onychodysplasia, or nail-patella syndrome, is an autosomal dominant disorder. Studies have linked the syndrome to chromosome 9q34 and identified point mutations in the LMX1B gene.4 Other kindreds have been linked to chromosome 17q21-22.5 Prenatal diagnosis is possible, including noninvasive prenatal diagnosis using ultrasonography. Because of the risk for glaucoma, all family members should be screened by an ophthalmologist.6
4. Seri M, Melchionda S, Dreyer S, et al. Identification of LMX1B gene point mutations in Italian patients affected with nail-patella syndrome. Int J Mol Med. 1999;4:285-290.
5. Mangino M, Sanchez O, Torrente I, et al. Localization of a gene for familial patella aplasia-hypoplasia (PTLAH) to chromosome 17q21-22. Am J Hum Genetics. 1999;65:441-447.
6. Lichter PR, Richards JE, Downs CA, et al. Cosegregation of open-angle glaucoma and the nail-patella syndrome. Am J Ophthalmol. 1997;124:506-515.
For more information on this case, see “What Is Your Diagnosis? Nail-Patella Syndrome.” Cutis. 2000;66:71, 75-76.
1. The patient has a long-standing pruritic rash. Flat, slightly elevated, greasy brown papules are scattered on the chest, abdomen, and upper back, with mild surrounding erythema. The fingernails are deformed, with longitudinal red streaks and ridges and v-shaped notching of the free margin.
Reprinted with permission from Cutis. 2003;72:124-126.
Diagnosis: Darier disease (DD) or Darier-White disease is a rare genetic skin disorder caused by mutations of the ATP2A2 gene, located on the long arm of chromosome 12 at position 24,11.1,2 This genetic mutation is inherited as an autosomal dominant trait with complete penetrance. DD affects men and women equally, with progressive skin signs of interfamilial and intrafamilial variability.3 Skin manifestations occur from late childhood to early adulthood and are typical during adolescence.3 Acute flare-ups can be triggered by heat, perspiration, sunlight, ultraviolet B exposure, stress, or certain medications (in particular, lithium).2 DD is not contagious.2
1. Creamer D, Barker J, Kerdel FA. Papular and papulosquamous dermatoses. In: Acute Adult Dermatology: Diagnosis and Management (A Colour Handbook). London, UK: Manson Publishing Ltd; 2011:48.
2. Kelly EB. Darier disease (DAR). In: Encyclopedia of Human Genetics and Disease. Santa Barbara, CA: ABC-CLIO; 2013:186-187.
3. Ringpfeil F. Dermatologic disorders. In: NORD Guide to Rare Disorders. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:101.
For more information on this case, see “Man, 45, With Greasy Rash and Deformed Nails.” Clin Rev. 2014;24(1):38,40-41
For the next photograph, proceed to the next page >>
2. Six months ago, this 36-year-old man’s left fourth finger began to bother him. He’s tried topical antibiotics, colloidal silver solution, and two different oral antibiotics. None have relieved the pain, which is severe enough to interfere with daily activity—particularly his job, which requires extensive computer time.
Diagnosis: “Infection” is only one potential cause of redness, swelling, increased warmth, and localized pain. Classically termed rubor, tumor, calor, and dolor, these are indicators of inflammation, which can occur in many conditions besides “infection.” In the case described, a simple hangnail was incompletely removed, leaving a shard of nail that then dug into the perionychial skin as it grew out. This set into motion a healing process that could not proceed to resolution, because the tissue was re-injured every time the finger struck the computer keyboard. This not only caused the wound to get stuck in a certain phase of healing (angioneogenesis) but also prevented completion of the process.
For more information on this case, see “Red and Swollen Doesn’t Mean “Infection.” Clin Rev. 2014;24(6):W1.
For the next photograph, proceed to the next page >>
3. A 25-year-old man presents with what he describes as a “fungal infection” of the fingernails that he’s had since birth. The nails are uniformly thickened and dystrophic, without significant discoloration. The patient’s palms and soles are hyperkeratotic, and the upper anterior legs are covered by a folliculocentric papular hyperkeratosis reminiscent of a coarse keratosis pilaris.
Diagnosis: Pachyonychia congenita (PC) is a rare condition that represents a mutation of keratin genes and is usually of autosomal dominant inheritance. First described by Muller in 1904, it was eventually categorized into one of two types: type I, MIM 167200, also known as Jadassohn-Lewandowsky, the most common type, and type II, MIM 167210, also known as Jackson-Lawler, with slightly different features. Today, a more common view is that no such divisions exist—only variations of PC that exhibit overlapping features.
For more information on this case, see “A fingernail "infection" present since birth.” Clin Rev. 2013;23(4):W6.
For the next photograph, proceed to the next page >>
4. Usually apparent at birth, this disorder may manifest with abnormal or missing nails. Characteristic nail changes include triangular lunulae and, most prominently on the thumb and index fingers, hypoplasia. Also apparent are orthopedic changes (particularly affecting the knees and elbows), renal disease, and glaucoma.
Reprinted with permission from Cutis. 2000;66:71, 75-76.
Diagnosis: The osteo-onychodysplasia, or nail-patella syndrome, is an autosomal dominant disorder. Studies have linked the syndrome to chromosome 9q34 and identified point mutations in the LMX1B gene.4 Other kindreds have been linked to chromosome 17q21-22.5 Prenatal diagnosis is possible, including noninvasive prenatal diagnosis using ultrasonography. Because of the risk for glaucoma, all family members should be screened by an ophthalmologist.6
4. Seri M, Melchionda S, Dreyer S, et al. Identification of LMX1B gene point mutations in Italian patients affected with nail-patella syndrome. Int J Mol Med. 1999;4:285-290.
5. Mangino M, Sanchez O, Torrente I, et al. Localization of a gene for familial patella aplasia-hypoplasia (PTLAH) to chromosome 17q21-22. Am J Hum Genetics. 1999;65:441-447.
6. Lichter PR, Richards JE, Downs CA, et al. Cosegregation of open-angle glaucoma and the nail-patella syndrome. Am J Ophthalmol. 1997;124:506-515.
For more information on this case, see “What Is Your Diagnosis? Nail-Patella Syndrome.” Cutis. 2000;66:71, 75-76.
Parents Frightened by Son’s Thigh Lesion
ANSWER
The correct answer is lichen striatus (choice “d”), a rare, self-limited condition usually seen on the extremities of children. It will be discussed further in the next section.
The differential for lichen striatus includes psoriasis (choice “a”). However, typical psoriatic lesions would not assume this configuration and would likely manifest with other corroborative areas of involvement.
Eczema (choice “b”) is certainly common in children, but it is unlikely to be relegated to one linear area. The same can be said of pityriasis rosea (choice “c”).
DISCUSSION
Lichen striatus, also known as Blaschko linear acquired skin inflammation, is an unusual self-limited condition of unknown etiology seen mostly on the extremities of children ages 3 to 10. More common on legs than on arms, it can occasionally appear on the face.
Its linear configuration is instantly diagnostic: The lesion typically follows the Blaschko lines, which represent a segmental clone of cutaneous cells deposited at an embryonic stage of development.
As seen in this case, postinflammatory hypopigmentation is common. In a minority of cases, the condition can involve the entire length of the extremity—even the digits, where it can lead to dystrophy of the affected nail. It almost always resolves within a few weeks to months, with or without treatment (which is limited to symptom relief).
Several potential causes of lichen striatus have been posited. The most convincing theory is a possible connection with human herpesviruses 6 and 7, which are also triggers for pityriasis rosea. There have been cases in which both conditions are present, producing somewhat similar microscopic changes in the skin (and both resolving without treatment). Additional items in the differential include lichen planus, lichen nitidus, and warts.
TREATMENT
For most cases of lichen striatus, treatment is supportive: topical steroids for the itching, which is seldom severe. Given the self-limited but prolonged nature of the problem, patient education is the more important component; these days, there are web-based sources to which you can direct your patients for reliable information, including the Mayo Clinic and the American Academy of Dermatology.
ANSWER
The correct answer is lichen striatus (choice “d”), a rare, self-limited condition usually seen on the extremities of children. It will be discussed further in the next section.
The differential for lichen striatus includes psoriasis (choice “a”). However, typical psoriatic lesions would not assume this configuration and would likely manifest with other corroborative areas of involvement.
Eczema (choice “b”) is certainly common in children, but it is unlikely to be relegated to one linear area. The same can be said of pityriasis rosea (choice “c”).
DISCUSSION
Lichen striatus, also known as Blaschko linear acquired skin inflammation, is an unusual self-limited condition of unknown etiology seen mostly on the extremities of children ages 3 to 10. More common on legs than on arms, it can occasionally appear on the face.
Its linear configuration is instantly diagnostic: The lesion typically follows the Blaschko lines, which represent a segmental clone of cutaneous cells deposited at an embryonic stage of development.
As seen in this case, postinflammatory hypopigmentation is common. In a minority of cases, the condition can involve the entire length of the extremity—even the digits, where it can lead to dystrophy of the affected nail. It almost always resolves within a few weeks to months, with or without treatment (which is limited to symptom relief).
Several potential causes of lichen striatus have been posited. The most convincing theory is a possible connection with human herpesviruses 6 and 7, which are also triggers for pityriasis rosea. There have been cases in which both conditions are present, producing somewhat similar microscopic changes in the skin (and both resolving without treatment). Additional items in the differential include lichen planus, lichen nitidus, and warts.
TREATMENT
For most cases of lichen striatus, treatment is supportive: topical steroids for the itching, which is seldom severe. Given the self-limited but prolonged nature of the problem, patient education is the more important component; these days, there are web-based sources to which you can direct your patients for reliable information, including the Mayo Clinic and the American Academy of Dermatology.
ANSWER
The correct answer is lichen striatus (choice “d”), a rare, self-limited condition usually seen on the extremities of children. It will be discussed further in the next section.
The differential for lichen striatus includes psoriasis (choice “a”). However, typical psoriatic lesions would not assume this configuration and would likely manifest with other corroborative areas of involvement.
Eczema (choice “b”) is certainly common in children, but it is unlikely to be relegated to one linear area. The same can be said of pityriasis rosea (choice “c”).
DISCUSSION
Lichen striatus, also known as Blaschko linear acquired skin inflammation, is an unusual self-limited condition of unknown etiology seen mostly on the extremities of children ages 3 to 10. More common on legs than on arms, it can occasionally appear on the face.
Its linear configuration is instantly diagnostic: The lesion typically follows the Blaschko lines, which represent a segmental clone of cutaneous cells deposited at an embryonic stage of development.
As seen in this case, postinflammatory hypopigmentation is common. In a minority of cases, the condition can involve the entire length of the extremity—even the digits, where it can lead to dystrophy of the affected nail. It almost always resolves within a few weeks to months, with or without treatment (which is limited to symptom relief).
Several potential causes of lichen striatus have been posited. The most convincing theory is a possible connection with human herpesviruses 6 and 7, which are also triggers for pityriasis rosea. There have been cases in which both conditions are present, producing somewhat similar microscopic changes in the skin (and both resolving without treatment). Additional items in the differential include lichen planus, lichen nitidus, and warts.
TREATMENT
For most cases of lichen striatus, treatment is supportive: topical steroids for the itching, which is seldom severe. Given the self-limited but prolonged nature of the problem, patient education is the more important component; these days, there are web-based sources to which you can direct your patients for reliable information, including the Mayo Clinic and the American Academy of Dermatology.
A 6-year-old boy is brought in by his mother, referred to dermatology for evaluation of a lesion on his right thigh that manifested three months ago. Although asymptomatic, the lesion has frightened the boy’s parents. They first took him to a local urgent care clinic, where a diagnosis of “probable fungal infection” was made. But twice-daily application of the prescribed ketoconazole cream did not produce results. The boy is otherwise healthy, although he does have seasonal allergies. There is no family history of skin problems. The affected site is a hypopigmented linear patch of skin from the mid-medial right thigh to the mid-medial calf area. The width of the patch varies, from 3 to 5 cm. The margins are somewhat irregular, and in several places, slightly rough, scaly sections are noted. The hypopigmentation, though partial, is evident in the context of the boy’s type IV Hispanic skin. No erythema or edema is noted. Elsewhere, the child’s skin is free of significant changes or lesions.
Interpretation: Humans, 1; Machine, 0
ANSWER
The correct interpretation of this ECG is atrial fibrillation with a rapid ventricular response and aberrantly conducted QRS complexes. The latter were misinterpreted as ventricular tachycardia. Although they represent a wide complex at a rate of more than 100 beats/min, the rhythm is irregular and the intrinsic (initial) inflection of normally conducted and aberrant beats is the same. (See lead I rhythm strip at bottom.)
What is unusual (and doesn’t make sense) regarding this ECG is the machine’s reading of the PR interval (128 ms) and the QRS duration (88 ms). For one thing, there is no measurable PR interval. And for another, the measured QRS duration accounts for the normally conducted complex and not the aberrantly conducted ones.
The technician was reassured, and the patient underwent successful cardioversion back to normal sinus rhythm.
ANSWER
The correct interpretation of this ECG is atrial fibrillation with a rapid ventricular response and aberrantly conducted QRS complexes. The latter were misinterpreted as ventricular tachycardia. Although they represent a wide complex at a rate of more than 100 beats/min, the rhythm is irregular and the intrinsic (initial) inflection of normally conducted and aberrant beats is the same. (See lead I rhythm strip at bottom.)
What is unusual (and doesn’t make sense) regarding this ECG is the machine’s reading of the PR interval (128 ms) and the QRS duration (88 ms). For one thing, there is no measurable PR interval. And for another, the measured QRS duration accounts for the normally conducted complex and not the aberrantly conducted ones.
The technician was reassured, and the patient underwent successful cardioversion back to normal sinus rhythm.
ANSWER
The correct interpretation of this ECG is atrial fibrillation with a rapid ventricular response and aberrantly conducted QRS complexes. The latter were misinterpreted as ventricular tachycardia. Although they represent a wide complex at a rate of more than 100 beats/min, the rhythm is irregular and the intrinsic (initial) inflection of normally conducted and aberrant beats is the same. (See lead I rhythm strip at bottom.)
What is unusual (and doesn’t make sense) regarding this ECG is the machine’s reading of the PR interval (128 ms) and the QRS duration (88 ms). For one thing, there is no measurable PR interval. And for another, the measured QRS duration accounts for the normally conducted complex and not the aberrantly conducted ones.
The technician was reassured, and the patient underwent successful cardioversion back to normal sinus rhythm.
A 72-year-old woman with recurring palpitations and a rapid heart rate presents for evaluation stating that her heart started racing early yesterday morning. It began while she was sleeping, which is normal for her, but while the problem usually resolves within hours, this time it lasted longer. She had an MI about seven years ago, at which time she was told she had atrial fibrillation. Since then, she has had multiple episodes requiring cardioversion and suspects that is what is required this time. She has brought along a copy of her baseline ECG, which shows normal sinus rhythm, an old inferior MI, and an intraventricular conduction delay. Her history includes hypertension, hyperlipidemia, and diabetes. Surgical history is remarkable for coronary stenting (right coronary artery, first diagonal coronary artery, and an obtuse marginal coronary artery). She also has a remote history of hysterectomy and appendectomy. Her current medications include metoprolol, atorvastatin, amiodarone, metformin, and glyburide; she takes an OTC stool softener daily. She is allergic to sulfa. The patient, a retired schoolteacher and the matriarch of her church, is married and has two grown children. She has two siblings, both of whom have diabetes and hypertension. She smoked 1.5 packs of cigarettes per day until her MI, at which point she quit. She does not drink alcohol and has never used recreational drugs. Review of systems is positive for increasingly worsening eyesight, particularly halos around lights at night; she says she was told this might happen when she started taking amiodarone. She has intermittent episodes of diarrhea that she attributes to the stool softener, adding that she considers this consequence “better than the alternative.” The rest of the review is unremarkable. Vital signs include a blood pressure of 148/92 mm Hg; pulse, 140 beats/min and irregular; temperature, 98.4°F; and O2 saturation, 97% on room air. She is 64 in tall and weighs 169 lb. Physical exam reveals a very spry-appearing woman in no distress; in fact, she jokingly complains that she’s too young to have “old people’s diseases” and proudly points out that she has no symptoms of arthritis or dementia. She wears corrective lenses and hearing aids. The exam reveals no thyromegaly or jugular venous distention; clear lung fields; and an irregularly irregular heart rate of 146 beats/min. Her heart rate is too rapid to assess for murmurs or extra heart sounds. The abdomen is benign, and the patient has strong bilateral peripheral pulses in all extremities. The neurologic exam is intact. Suspecting that the patient is in atrial fibrillation, you ask the new ECG technician to obtain a reading. Five minutes later, he calls for help because “the patient is in ventricular tachycardia.” But when you walk into the room, the patient looks quite comfortable on the exam table and exhibits no distress. Reviewing the ECG, you note a ventricular rate of 152 beats/min; PR interval, 128 ms; QRS duration, 88 ms; QT/QTc interval, 280/445 ms; P axis, 27°; R axis, 23°; and T axis, 232°. What is your interpretation of this ECG—and what findings are inconsistent with the machine’s “interpretation”?
Elective Craniotomy Results in Respiratory Distress
ANSWER
The radiograph shows complete opacification of the left hemithorax. The differential includes total atelectasis of the lung, mucus plug within the left bronchus, or possible blood or fluid collection. Of note, the patient has a catheter within the left subclavian vein, the distal tip of which appears to be in an unusual location. In this case, it was determined that the displaced catheter tip, resulting in hemothorax, was the etiology. The line was removed, and urgent cardiothoracic consultation was obtained. A left chest tube was promptly placed, with a resultant 2 L of immediate output. The patient improved clinically as well.
ANSWER
The radiograph shows complete opacification of the left hemithorax. The differential includes total atelectasis of the lung, mucus plug within the left bronchus, or possible blood or fluid collection. Of note, the patient has a catheter within the left subclavian vein, the distal tip of which appears to be in an unusual location. In this case, it was determined that the displaced catheter tip, resulting in hemothorax, was the etiology. The line was removed, and urgent cardiothoracic consultation was obtained. A left chest tube was promptly placed, with a resultant 2 L of immediate output. The patient improved clinically as well.
ANSWER
The radiograph shows complete opacification of the left hemithorax. The differential includes total atelectasis of the lung, mucus plug within the left bronchus, or possible blood or fluid collection. Of note, the patient has a catheter within the left subclavian vein, the distal tip of which appears to be in an unusual location. In this case, it was determined that the displaced catheter tip, resulting in hemothorax, was the etiology. The line was removed, and urgent cardiothoracic consultation was obtained. A left chest tube was promptly placed, with a resultant 2 L of immediate output. The patient improved clinically as well.
A 60-year-old woman undergoes an elective craniotomy for clipping of a nonruptured aneurysm. The perioperative course is uneventful, and the aneurysm is clipped without complication. The patient is extubated and sent to the recovery room. Within 30 minutes, you are notified by the nurse that the patient is experiencing moderate respiratory distress. There are no neurologic deficits. Her medical history includes hypertension, hypercholesterolemia, and coronary artery disease, with previous stenting. Preoperative medical and cardiac clearance for the craniotomy had been obtained. Examination reveals the patient to be in a postanesthetic state, with mild-to-moderate dyspnea and tachypnea. She appears to be moving all of her extremities well. Her O2 saturation is 92% on 100% oxygen via a nonrebreather mask. Her breath sounds are significantly diminished on the left side. A stat portable chest radiograph is obtained. What is your impression?
Be Sure to Look for Secondary Diabetes
A 63-year-old Hispanic woman was referred to endocrinology by her primary care provider for uncontrolled type 2 diabetes mellitus (T2DM), which was diagnosed 16 years ago. Her antidiabetic medications included insulin glargine (55 U bid), metformin (1,000 mg bid), and glipizide (10 mg bid). She also had known dyslipidemia, hypertension, and depression. There was a history of poorly controlled glucose (A1C between 9% and 13% in the past three years).
This was a relatively common new patient consult in our endocrine clinic. Upon entering the room, I was greeted by the patient and two family members. I quickly noticed the patient’s facial plethora and central obesity with comparatively thin extremities. Further inquiry revealed that the greatest challenge for the patient and her family was her bouts of severe depression, during which she would stop caring and cease to take her medications.
During the physical exam, mild but not significant supraclavicular and dorsocervical fat pads were appreciated. The exam was otherwise unremarkable, with no purple striae on the torso, abdomen, breasts, and extremities.
In addition to routine diabetes lab tests (ie, A1C, chemistry panel, lipid panel, urine microalbumin-to-creatinine ratio), an overnight 1-mg oral dexamethasone suppression test was ordered. Results of the latter were abnormal, and further workup confirmed Cushing disease (see Table 1 for results). The patient was referred for neurosurgery.
Continue for Discussion >>
DISCUSSION: SECONDARY DIABETES
It is well known that the prevalence of diabetes is skyrocketing, and medical offices are filled with affected patients. According to a 2011 report from the CDC, 90% to 95% of all diabetes cases are type 2, 5% are type 1 (autoimmune), and the rest (about 1% to 5%) are “other types” of diabetes.3 Due to these disproportionate statistics, clinicians often overlook the possibility of uncommon etiologies and assume all patients with diabetes have type 2—especially when the patient is overweight or obese.
Table 2 lists conditions and medications that may contribute to significant hyperglycemia.4 Some contributors are rather obvious (eg, status post pancreatectomy) or have no impact on treatment strategy (eg, chromosomal defects such as Down or Turner syndrome). However, certain conditions, such as Cushing syndrome, acromegaly, and hemochromatosis, can be relatively hard to recognize due to the variable rate of clinical manifestation, especially in the earlier stages of the disease. Experts have raised concerns that the prevalence of secondary diabetes (1% to 5%) may actually be underestimated due to “misdiagnosis” as T2DM.
Early detection of the underlying disorder, followed by initiation of appropriate treatment, is critical. It will not only improve but also may resolve the patient’s hyperglycemia, and it may also reverse or stop the damage to other vital organs.
The case patient had an unfortunate situation in which her Cushing syndrome was masked by commonly encountered diagnoses of hypertension, T2DM, obesity, and depression. Cushing is an easy diagnosis to miss, since it has an insidious onset and it can take more than five years for some of the physical findings to become evident.
Pancreatic cancer is another uncommon but critical disease worth mentioning. Pancreatic cancer should be in the differential diagnosis for previously euglycemic patients who experience abrupt elevation of glucose or previously well-managed patients whose glucose values quickly get out of control without obvious cause (eg, medication cessation, addition of glucocorticoid therapy, uncontrolled diet).
In our practice, we have encountered three patients with pancreatic cancer in this setting. The only sign was a sudden rise in glucose (300 to 500 mg/dL throughout the day) in patients whose A1C had been low (in the 6% range) with one or two oral medications. Thorough history taking did not reveal any potential causes for sudden hyperglycemia. Only one patient had a palpable mass on abdominal exam and elevated liver enzymes and bilirubin. Unfortunately, that patient died eight months later. The other two had favorable outcomes from surgery and chemotherapy. Early detection was the key for those two patients.
Next page: Conclusion >>
CONCLUSION
Since the majority of patients with diabetes have T2DM, it is easy to “default” and start treating all patients as such, especially if they are overweight or obese. However, up to 5% of patients actually have underlying disease that may cause or worsen their diabetic status. Overlooking these rare conditions can be detrimental to the patient, as it will adversely affect not only glycemic control but more importantly, overall health. Identifying the underlying disease will allow the patient to receive appropriate treatment, which may offload a significant burden on glycemic control and in some cases, cure the hyperglycemia.
REFERENCES
1. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540.
2. Nieman LK. Establishing the cause of Cushing’s syndrome. Up-to-Date. www.uptodate.com/contents/establishing-the-cause-of-cushings-syndrome. Accessed June 24, 2015.
3. CDC. National Diabetes Fact Sheet, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed June 24, 2015.
4. Ganda OP. Prevalence and incidence of secondary and other types of diabetes. In: Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health; 1995:69-84.
A 63-year-old Hispanic woman was referred to endocrinology by her primary care provider for uncontrolled type 2 diabetes mellitus (T2DM), which was diagnosed 16 years ago. Her antidiabetic medications included insulin glargine (55 U bid), metformin (1,000 mg bid), and glipizide (10 mg bid). She also had known dyslipidemia, hypertension, and depression. There was a history of poorly controlled glucose (A1C between 9% and 13% in the past three years).
This was a relatively common new patient consult in our endocrine clinic. Upon entering the room, I was greeted by the patient and two family members. I quickly noticed the patient’s facial plethora and central obesity with comparatively thin extremities. Further inquiry revealed that the greatest challenge for the patient and her family was her bouts of severe depression, during which she would stop caring and cease to take her medications.
During the physical exam, mild but not significant supraclavicular and dorsocervical fat pads were appreciated. The exam was otherwise unremarkable, with no purple striae on the torso, abdomen, breasts, and extremities.
In addition to routine diabetes lab tests (ie, A1C, chemistry panel, lipid panel, urine microalbumin-to-creatinine ratio), an overnight 1-mg oral dexamethasone suppression test was ordered. Results of the latter were abnormal, and further workup confirmed Cushing disease (see Table 1 for results). The patient was referred for neurosurgery.
Continue for Discussion >>
DISCUSSION: SECONDARY DIABETES
It is well known that the prevalence of diabetes is skyrocketing, and medical offices are filled with affected patients. According to a 2011 report from the CDC, 90% to 95% of all diabetes cases are type 2, 5% are type 1 (autoimmune), and the rest (about 1% to 5%) are “other types” of diabetes.3 Due to these disproportionate statistics, clinicians often overlook the possibility of uncommon etiologies and assume all patients with diabetes have type 2—especially when the patient is overweight or obese.
Table 2 lists conditions and medications that may contribute to significant hyperglycemia.4 Some contributors are rather obvious (eg, status post pancreatectomy) or have no impact on treatment strategy (eg, chromosomal defects such as Down or Turner syndrome). However, certain conditions, such as Cushing syndrome, acromegaly, and hemochromatosis, can be relatively hard to recognize due to the variable rate of clinical manifestation, especially in the earlier stages of the disease. Experts have raised concerns that the prevalence of secondary diabetes (1% to 5%) may actually be underestimated due to “misdiagnosis” as T2DM.
Early detection of the underlying disorder, followed by initiation of appropriate treatment, is critical. It will not only improve but also may resolve the patient’s hyperglycemia, and it may also reverse or stop the damage to other vital organs.
The case patient had an unfortunate situation in which her Cushing syndrome was masked by commonly encountered diagnoses of hypertension, T2DM, obesity, and depression. Cushing is an easy diagnosis to miss, since it has an insidious onset and it can take more than five years for some of the physical findings to become evident.
Pancreatic cancer is another uncommon but critical disease worth mentioning. Pancreatic cancer should be in the differential diagnosis for previously euglycemic patients who experience abrupt elevation of glucose or previously well-managed patients whose glucose values quickly get out of control without obvious cause (eg, medication cessation, addition of glucocorticoid therapy, uncontrolled diet).
In our practice, we have encountered three patients with pancreatic cancer in this setting. The only sign was a sudden rise in glucose (300 to 500 mg/dL throughout the day) in patients whose A1C had been low (in the 6% range) with one or two oral medications. Thorough history taking did not reveal any potential causes for sudden hyperglycemia. Only one patient had a palpable mass on abdominal exam and elevated liver enzymes and bilirubin. Unfortunately, that patient died eight months later. The other two had favorable outcomes from surgery and chemotherapy. Early detection was the key for those two patients.
Next page: Conclusion >>
CONCLUSION
Since the majority of patients with diabetes have T2DM, it is easy to “default” and start treating all patients as such, especially if they are overweight or obese. However, up to 5% of patients actually have underlying disease that may cause or worsen their diabetic status. Overlooking these rare conditions can be detrimental to the patient, as it will adversely affect not only glycemic control but more importantly, overall health. Identifying the underlying disease will allow the patient to receive appropriate treatment, which may offload a significant burden on glycemic control and in some cases, cure the hyperglycemia.
REFERENCES
1. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540.
2. Nieman LK. Establishing the cause of Cushing’s syndrome. Up-to-Date. www.uptodate.com/contents/establishing-the-cause-of-cushings-syndrome. Accessed June 24, 2015.
3. CDC. National Diabetes Fact Sheet, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed June 24, 2015.
4. Ganda OP. Prevalence and incidence of secondary and other types of diabetes. In: Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health; 1995:69-84.
A 63-year-old Hispanic woman was referred to endocrinology by her primary care provider for uncontrolled type 2 diabetes mellitus (T2DM), which was diagnosed 16 years ago. Her antidiabetic medications included insulin glargine (55 U bid), metformin (1,000 mg bid), and glipizide (10 mg bid). She also had known dyslipidemia, hypertension, and depression. There was a history of poorly controlled glucose (A1C between 9% and 13% in the past three years).
This was a relatively common new patient consult in our endocrine clinic. Upon entering the room, I was greeted by the patient and two family members. I quickly noticed the patient’s facial plethora and central obesity with comparatively thin extremities. Further inquiry revealed that the greatest challenge for the patient and her family was her bouts of severe depression, during which she would stop caring and cease to take her medications.
During the physical exam, mild but not significant supraclavicular and dorsocervical fat pads were appreciated. The exam was otherwise unremarkable, with no purple striae on the torso, abdomen, breasts, and extremities.
In addition to routine diabetes lab tests (ie, A1C, chemistry panel, lipid panel, urine microalbumin-to-creatinine ratio), an overnight 1-mg oral dexamethasone suppression test was ordered. Results of the latter were abnormal, and further workup confirmed Cushing disease (see Table 1 for results). The patient was referred for neurosurgery.
Continue for Discussion >>
DISCUSSION: SECONDARY DIABETES
It is well known that the prevalence of diabetes is skyrocketing, and medical offices are filled with affected patients. According to a 2011 report from the CDC, 90% to 95% of all diabetes cases are type 2, 5% are type 1 (autoimmune), and the rest (about 1% to 5%) are “other types” of diabetes.3 Due to these disproportionate statistics, clinicians often overlook the possibility of uncommon etiologies and assume all patients with diabetes have type 2—especially when the patient is overweight or obese.
Table 2 lists conditions and medications that may contribute to significant hyperglycemia.4 Some contributors are rather obvious (eg, status post pancreatectomy) or have no impact on treatment strategy (eg, chromosomal defects such as Down or Turner syndrome). However, certain conditions, such as Cushing syndrome, acromegaly, and hemochromatosis, can be relatively hard to recognize due to the variable rate of clinical manifestation, especially in the earlier stages of the disease. Experts have raised concerns that the prevalence of secondary diabetes (1% to 5%) may actually be underestimated due to “misdiagnosis” as T2DM.
Early detection of the underlying disorder, followed by initiation of appropriate treatment, is critical. It will not only improve but also may resolve the patient’s hyperglycemia, and it may also reverse or stop the damage to other vital organs.
The case patient had an unfortunate situation in which her Cushing syndrome was masked by commonly encountered diagnoses of hypertension, T2DM, obesity, and depression. Cushing is an easy diagnosis to miss, since it has an insidious onset and it can take more than five years for some of the physical findings to become evident.
Pancreatic cancer is another uncommon but critical disease worth mentioning. Pancreatic cancer should be in the differential diagnosis for previously euglycemic patients who experience abrupt elevation of glucose or previously well-managed patients whose glucose values quickly get out of control without obvious cause (eg, medication cessation, addition of glucocorticoid therapy, uncontrolled diet).
In our practice, we have encountered three patients with pancreatic cancer in this setting. The only sign was a sudden rise in glucose (300 to 500 mg/dL throughout the day) in patients whose A1C had been low (in the 6% range) with one or two oral medications. Thorough history taking did not reveal any potential causes for sudden hyperglycemia. Only one patient had a palpable mass on abdominal exam and elevated liver enzymes and bilirubin. Unfortunately, that patient died eight months later. The other two had favorable outcomes from surgery and chemotherapy. Early detection was the key for those two patients.
Next page: Conclusion >>
CONCLUSION
Since the majority of patients with diabetes have T2DM, it is easy to “default” and start treating all patients as such, especially if they are overweight or obese. However, up to 5% of patients actually have underlying disease that may cause or worsen their diabetic status. Overlooking these rare conditions can be detrimental to the patient, as it will adversely affect not only glycemic control but more importantly, overall health. Identifying the underlying disease will allow the patient to receive appropriate treatment, which may offload a significant burden on glycemic control and in some cases, cure the hyperglycemia.
REFERENCES
1. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526-1540.
2. Nieman LK. Establishing the cause of Cushing’s syndrome. Up-to-Date. www.uptodate.com/contents/establishing-the-cause-of-cushings-syndrome. Accessed June 24, 2015.
3. CDC. National Diabetes Fact Sheet, 2011. www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. Accessed June 24, 2015.
4. Ganda OP. Prevalence and incidence of secondary and other types of diabetes. In: Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health; 1995:69-84.
The Failure to Deliver as Promised
In March 2009, a 63-year-old man was diagnosed with stage IV gastric carcinoma with metastasis to the liver. His treating oncologist gave him a prognosis of about 10 months’ life expectancy with chemotherapy. The patient’s family searched for alternative treatment options and found a natural alternative treatment center claiming the ability to cure the patient.
The patient and his family decided to defer chemotherapy, and he was admitted to the alternative treatment center for three to four weeks of inpatient care. The treatment consisted of “colonic hydrotherapy,” supplements designed to cleanse the body, and a diet restricted to seed milk, vegetable juice, and spinach soup.
After six days, the patient developed severe diarrhea, confusion, and profound weakness. He was taken to a local hospital and admitted with a diagnosis of acute renal failure. Dialysis attempts were unsuccessful, and the man died of respiratory distress secondary to acute renal failure a week later.
The plaintiff claimed that the treatment provided by the defendants was contraindicated and caused acute renal failure, noting that the patient’s kidney function had been relatively normal when he entered the treatment facility. The plaintiff claimed that defendant Dr N., a chiropractor, never reviewed any of the decedent’s medical records, did not discuss the proposed treatment plan with his treating physicians, and failed to properly monitor the patient’s condition, notice his deterioration, and provide timely transfer to a hospital.
The defendant claimed that the treatment given had no adverse effects on the decedent and that the acute renal failure was due to hepatorenal syndrome due to his advanced metastatic liver cancer.
What was the outcome? >>
OUTCOME
A $2.5 million verdict was returned. An appeal was pending.
COMMENT
This is a case against a chiropractor, so why discuss it in a journal dedicated to NP and PA practice? Because it involves scope of practice, alternative medicine, the safety of “natural” treatments, and the ethical and legal problems of making unsupportable promises to patients.
Know your scope of practice, and don’t overextend. Clinicians trained as specialists (eg, in pediatrics, midwifery, or anesthesia) should use caution departing from that area. Those trained as “generalists” need to be careful as well; even if you were trained in a family practice program, if you are a PA who has worked in dermatology for the past 10 years, think twice about giving treatment or advice to your friend with a neurologic complaint. In the event of a lawsuit, the plaintiff will spend a great deal of time building your resume as an expert in your discipline, only to attack you as inexperienced and unqualified in the case in which you extended yourself.
Here a chiropractor, without ever examining the patient, directed the treatment of a very sick man in an area in which he was not qualified. While chiropractors may claim the ability to treat outside their traditional scope, the jury’s verdict in this case proves that they were not persuaded he was right to do so. The chiropractor, Dr N., eventually lost his license, based in part on the false promises he made about his ability to cure patients of “any and all diseases, including cancer, by restoring the body to its natural state ….” This opportunistic preying upon the most ill and vulnerable in our society likely irked the jurors, who returned a substantial award, considering that the patient’s short life expectancy was uncontested.
Handle alternative medicine with particular care, because an alternative treatment may not qualify as “medicine” at all. If we define medicine as the application of scientific principles to health care, an alternative that is unproven, unstudied, and unknown does not qualify. Rather, it is guesswork—with potentially devastating consequences.
In this case, through his company, the chiropractor based his treatment plan on guesswork that colonic hydrotherapy and severe dietary restrictions would help a patient with stage IV metastatic gastric carcinoma. He was wrong, and the jury concluded that these alternatives injured the patient and hastened his death.
Certainly, Western medicine has been rightly and fairly criticized for failing to promote wellness through a healthy lifestyle, including diet, exercise, safety, emotional well-being, and stress management. However, when venturing from generally accepted health promotion strategies to a specific recommendation that an alternative agent “is good for” a specific problem, be careful. You may believe lavender oil is an effective antibiotic—but can you prove it?
If you choose lavender oil over a demonstrably effective antibiotic to treat pneumonia, and the patient deteriorates, you will be held accountable. The plaintiff will demand answers, and the jury will await your explanation. Reliance on vague concepts, not generally accepted in the literature (eg, “energy management,” detoxifying, unblocking “clogged” nervous systems), will be ridiculed by the plaintiff’s experts, and you will be skewered on cross-examination. It is not enough to personally “believe” in the alternative; you must be able to support your treatment decisions through the best evidence possible.
To be fair, this cuts both ways: Some Western medical practices are based on anecdotal evidence with minimal scientific support. There was a time when a corneal abrasion was patched, a fractured clavicle was stabilized with a figure-of-eight dressing, and narcotics were withheld from a suffering patient with acute abdomen because it would “mask signs.” Our “Western” system is not immune from the impact of poor research, group-think, dogmas leading to inappropriate practice, and other sources of logical fallacy.
As NPs and PAs, we will be held to a scientific evidentiary standard. The standard of care will be based upon the care a reasonably prudent clinician would deliver in a similar situation. At trial, you will be confronted with a PA or NP on the stand testifying against you regarding what is reasonably prudent, acceptable care. Make sure your actions are scientifically defensible.
Interestingly, the standard for admitting a scientific opinion as expert testimony has changed. In 1923, Frye v United States1 established that, for an expert opinion to be admissible, the testimony had to be based on what is “generally accepted in the scientific community.” In 1993, the Supreme Court case Daubert v Merrell Dow Pharmaceuticals2 determined that the opinion need not be “generally accepted” but must be based on scientific method and must be relevant to the case; the judge serves as a “gatekeeper” to be sure the opinions flow from “scientific knowledge.”
Medical malpractice cases are based on state law. Some follow Frye, some Daubert. The latter is a more relaxed standard, but even in states following Daubert, an expert witness who purports to testify on an alternative treatment must follow the scientific method. For example, the webpage of the defendant chiropractor’s institute (still in business) currently claims that “Heart/Brain Entrainment Therapy balances frequencies of organs/glands/tissues. Everything in the universe resonates at a particular frequency—light, sound, and every cell, organ, gland, and tissue in you.”3
So, whatever Heart/Brain Entrainment Therapy is, for that theory to be admissible in a Frye jurisdiction it would likely have to be “generally accepted” in the medical community. To be admissible in a Daubert jurisdiction, proponents of the testimony would have to show evidence of a scientific methodology supporting the theory before the jury could hear any testimony about it. In either case, strategically, the defense attorney would likely file a motion to block either certain parts of the testimony or the testimony entirely.
IN SUM
Jurors expect sound scientific methodology supporting medical decisions; use care when selecting treatment for patients. Robustly adopt health promotion and general wellness strategies. However, if you use alternatives directed toward a specific therapy solving a specific problem, use them cautiously and with an awareness that the indication for the therapy should be scientifically defensible. —DML
REFERENCES
1. Frye v United States, 293 F. 1013 (D.C. Cir. 1923).
2. Daubert v Merrell Dow Pharmaceuticals, 509 U.S. 579 (1993).
3. Total Health Institute. Bioelectrical Energy, Quantum Frequency Resonance. www.totalhealthinstitute.com/about. Accessed July 14, 2015.
In March 2009, a 63-year-old man was diagnosed with stage IV gastric carcinoma with metastasis to the liver. His treating oncologist gave him a prognosis of about 10 months’ life expectancy with chemotherapy. The patient’s family searched for alternative treatment options and found a natural alternative treatment center claiming the ability to cure the patient.
The patient and his family decided to defer chemotherapy, and he was admitted to the alternative treatment center for three to four weeks of inpatient care. The treatment consisted of “colonic hydrotherapy,” supplements designed to cleanse the body, and a diet restricted to seed milk, vegetable juice, and spinach soup.
After six days, the patient developed severe diarrhea, confusion, and profound weakness. He was taken to a local hospital and admitted with a diagnosis of acute renal failure. Dialysis attempts were unsuccessful, and the man died of respiratory distress secondary to acute renal failure a week later.
The plaintiff claimed that the treatment provided by the defendants was contraindicated and caused acute renal failure, noting that the patient’s kidney function had been relatively normal when he entered the treatment facility. The plaintiff claimed that defendant Dr N., a chiropractor, never reviewed any of the decedent’s medical records, did not discuss the proposed treatment plan with his treating physicians, and failed to properly monitor the patient’s condition, notice his deterioration, and provide timely transfer to a hospital.
The defendant claimed that the treatment given had no adverse effects on the decedent and that the acute renal failure was due to hepatorenal syndrome due to his advanced metastatic liver cancer.
What was the outcome? >>
OUTCOME
A $2.5 million verdict was returned. An appeal was pending.
COMMENT
This is a case against a chiropractor, so why discuss it in a journal dedicated to NP and PA practice? Because it involves scope of practice, alternative medicine, the safety of “natural” treatments, and the ethical and legal problems of making unsupportable promises to patients.
Know your scope of practice, and don’t overextend. Clinicians trained as specialists (eg, in pediatrics, midwifery, or anesthesia) should use caution departing from that area. Those trained as “generalists” need to be careful as well; even if you were trained in a family practice program, if you are a PA who has worked in dermatology for the past 10 years, think twice about giving treatment or advice to your friend with a neurologic complaint. In the event of a lawsuit, the plaintiff will spend a great deal of time building your resume as an expert in your discipline, only to attack you as inexperienced and unqualified in the case in which you extended yourself.
Here a chiropractor, without ever examining the patient, directed the treatment of a very sick man in an area in which he was not qualified. While chiropractors may claim the ability to treat outside their traditional scope, the jury’s verdict in this case proves that they were not persuaded he was right to do so. The chiropractor, Dr N., eventually lost his license, based in part on the false promises he made about his ability to cure patients of “any and all diseases, including cancer, by restoring the body to its natural state ….” This opportunistic preying upon the most ill and vulnerable in our society likely irked the jurors, who returned a substantial award, considering that the patient’s short life expectancy was uncontested.
Handle alternative medicine with particular care, because an alternative treatment may not qualify as “medicine” at all. If we define medicine as the application of scientific principles to health care, an alternative that is unproven, unstudied, and unknown does not qualify. Rather, it is guesswork—with potentially devastating consequences.
In this case, through his company, the chiropractor based his treatment plan on guesswork that colonic hydrotherapy and severe dietary restrictions would help a patient with stage IV metastatic gastric carcinoma. He was wrong, and the jury concluded that these alternatives injured the patient and hastened his death.
Certainly, Western medicine has been rightly and fairly criticized for failing to promote wellness through a healthy lifestyle, including diet, exercise, safety, emotional well-being, and stress management. However, when venturing from generally accepted health promotion strategies to a specific recommendation that an alternative agent “is good for” a specific problem, be careful. You may believe lavender oil is an effective antibiotic—but can you prove it?
If you choose lavender oil over a demonstrably effective antibiotic to treat pneumonia, and the patient deteriorates, you will be held accountable. The plaintiff will demand answers, and the jury will await your explanation. Reliance on vague concepts, not generally accepted in the literature (eg, “energy management,” detoxifying, unblocking “clogged” nervous systems), will be ridiculed by the plaintiff’s experts, and you will be skewered on cross-examination. It is not enough to personally “believe” in the alternative; you must be able to support your treatment decisions through the best evidence possible.
To be fair, this cuts both ways: Some Western medical practices are based on anecdotal evidence with minimal scientific support. There was a time when a corneal abrasion was patched, a fractured clavicle was stabilized with a figure-of-eight dressing, and narcotics were withheld from a suffering patient with acute abdomen because it would “mask signs.” Our “Western” system is not immune from the impact of poor research, group-think, dogmas leading to inappropriate practice, and other sources of logical fallacy.
As NPs and PAs, we will be held to a scientific evidentiary standard. The standard of care will be based upon the care a reasonably prudent clinician would deliver in a similar situation. At trial, you will be confronted with a PA or NP on the stand testifying against you regarding what is reasonably prudent, acceptable care. Make sure your actions are scientifically defensible.
Interestingly, the standard for admitting a scientific opinion as expert testimony has changed. In 1923, Frye v United States1 established that, for an expert opinion to be admissible, the testimony had to be based on what is “generally accepted in the scientific community.” In 1993, the Supreme Court case Daubert v Merrell Dow Pharmaceuticals2 determined that the opinion need not be “generally accepted” but must be based on scientific method and must be relevant to the case; the judge serves as a “gatekeeper” to be sure the opinions flow from “scientific knowledge.”
Medical malpractice cases are based on state law. Some follow Frye, some Daubert. The latter is a more relaxed standard, but even in states following Daubert, an expert witness who purports to testify on an alternative treatment must follow the scientific method. For example, the webpage of the defendant chiropractor’s institute (still in business) currently claims that “Heart/Brain Entrainment Therapy balances frequencies of organs/glands/tissues. Everything in the universe resonates at a particular frequency—light, sound, and every cell, organ, gland, and tissue in you.”3
So, whatever Heart/Brain Entrainment Therapy is, for that theory to be admissible in a Frye jurisdiction it would likely have to be “generally accepted” in the medical community. To be admissible in a Daubert jurisdiction, proponents of the testimony would have to show evidence of a scientific methodology supporting the theory before the jury could hear any testimony about it. In either case, strategically, the defense attorney would likely file a motion to block either certain parts of the testimony or the testimony entirely.
IN SUM
Jurors expect sound scientific methodology supporting medical decisions; use care when selecting treatment for patients. Robustly adopt health promotion and general wellness strategies. However, if you use alternatives directed toward a specific therapy solving a specific problem, use them cautiously and with an awareness that the indication for the therapy should be scientifically defensible. —DML
REFERENCES
1. Frye v United States, 293 F. 1013 (D.C. Cir. 1923).
2. Daubert v Merrell Dow Pharmaceuticals, 509 U.S. 579 (1993).
3. Total Health Institute. Bioelectrical Energy, Quantum Frequency Resonance. www.totalhealthinstitute.com/about. Accessed July 14, 2015.
In March 2009, a 63-year-old man was diagnosed with stage IV gastric carcinoma with metastasis to the liver. His treating oncologist gave him a prognosis of about 10 months’ life expectancy with chemotherapy. The patient’s family searched for alternative treatment options and found a natural alternative treatment center claiming the ability to cure the patient.
The patient and his family decided to defer chemotherapy, and he was admitted to the alternative treatment center for three to four weeks of inpatient care. The treatment consisted of “colonic hydrotherapy,” supplements designed to cleanse the body, and a diet restricted to seed milk, vegetable juice, and spinach soup.
After six days, the patient developed severe diarrhea, confusion, and profound weakness. He was taken to a local hospital and admitted with a diagnosis of acute renal failure. Dialysis attempts were unsuccessful, and the man died of respiratory distress secondary to acute renal failure a week later.
The plaintiff claimed that the treatment provided by the defendants was contraindicated and caused acute renal failure, noting that the patient’s kidney function had been relatively normal when he entered the treatment facility. The plaintiff claimed that defendant Dr N., a chiropractor, never reviewed any of the decedent’s medical records, did not discuss the proposed treatment plan with his treating physicians, and failed to properly monitor the patient’s condition, notice his deterioration, and provide timely transfer to a hospital.
The defendant claimed that the treatment given had no adverse effects on the decedent and that the acute renal failure was due to hepatorenal syndrome due to his advanced metastatic liver cancer.
What was the outcome? >>
OUTCOME
A $2.5 million verdict was returned. An appeal was pending.
COMMENT
This is a case against a chiropractor, so why discuss it in a journal dedicated to NP and PA practice? Because it involves scope of practice, alternative medicine, the safety of “natural” treatments, and the ethical and legal problems of making unsupportable promises to patients.
Know your scope of practice, and don’t overextend. Clinicians trained as specialists (eg, in pediatrics, midwifery, or anesthesia) should use caution departing from that area. Those trained as “generalists” need to be careful as well; even if you were trained in a family practice program, if you are a PA who has worked in dermatology for the past 10 years, think twice about giving treatment or advice to your friend with a neurologic complaint. In the event of a lawsuit, the plaintiff will spend a great deal of time building your resume as an expert in your discipline, only to attack you as inexperienced and unqualified in the case in which you extended yourself.
Here a chiropractor, without ever examining the patient, directed the treatment of a very sick man in an area in which he was not qualified. While chiropractors may claim the ability to treat outside their traditional scope, the jury’s verdict in this case proves that they were not persuaded he was right to do so. The chiropractor, Dr N., eventually lost his license, based in part on the false promises he made about his ability to cure patients of “any and all diseases, including cancer, by restoring the body to its natural state ….” This opportunistic preying upon the most ill and vulnerable in our society likely irked the jurors, who returned a substantial award, considering that the patient’s short life expectancy was uncontested.
Handle alternative medicine with particular care, because an alternative treatment may not qualify as “medicine” at all. If we define medicine as the application of scientific principles to health care, an alternative that is unproven, unstudied, and unknown does not qualify. Rather, it is guesswork—with potentially devastating consequences.
In this case, through his company, the chiropractor based his treatment plan on guesswork that colonic hydrotherapy and severe dietary restrictions would help a patient with stage IV metastatic gastric carcinoma. He was wrong, and the jury concluded that these alternatives injured the patient and hastened his death.
Certainly, Western medicine has been rightly and fairly criticized for failing to promote wellness through a healthy lifestyle, including diet, exercise, safety, emotional well-being, and stress management. However, when venturing from generally accepted health promotion strategies to a specific recommendation that an alternative agent “is good for” a specific problem, be careful. You may believe lavender oil is an effective antibiotic—but can you prove it?
If you choose lavender oil over a demonstrably effective antibiotic to treat pneumonia, and the patient deteriorates, you will be held accountable. The plaintiff will demand answers, and the jury will await your explanation. Reliance on vague concepts, not generally accepted in the literature (eg, “energy management,” detoxifying, unblocking “clogged” nervous systems), will be ridiculed by the plaintiff’s experts, and you will be skewered on cross-examination. It is not enough to personally “believe” in the alternative; you must be able to support your treatment decisions through the best evidence possible.
To be fair, this cuts both ways: Some Western medical practices are based on anecdotal evidence with minimal scientific support. There was a time when a corneal abrasion was patched, a fractured clavicle was stabilized with a figure-of-eight dressing, and narcotics were withheld from a suffering patient with acute abdomen because it would “mask signs.” Our “Western” system is not immune from the impact of poor research, group-think, dogmas leading to inappropriate practice, and other sources of logical fallacy.
As NPs and PAs, we will be held to a scientific evidentiary standard. The standard of care will be based upon the care a reasonably prudent clinician would deliver in a similar situation. At trial, you will be confronted with a PA or NP on the stand testifying against you regarding what is reasonably prudent, acceptable care. Make sure your actions are scientifically defensible.
Interestingly, the standard for admitting a scientific opinion as expert testimony has changed. In 1923, Frye v United States1 established that, for an expert opinion to be admissible, the testimony had to be based on what is “generally accepted in the scientific community.” In 1993, the Supreme Court case Daubert v Merrell Dow Pharmaceuticals2 determined that the opinion need not be “generally accepted” but must be based on scientific method and must be relevant to the case; the judge serves as a “gatekeeper” to be sure the opinions flow from “scientific knowledge.”
Medical malpractice cases are based on state law. Some follow Frye, some Daubert. The latter is a more relaxed standard, but even in states following Daubert, an expert witness who purports to testify on an alternative treatment must follow the scientific method. For example, the webpage of the defendant chiropractor’s institute (still in business) currently claims that “Heart/Brain Entrainment Therapy balances frequencies of organs/glands/tissues. Everything in the universe resonates at a particular frequency—light, sound, and every cell, organ, gland, and tissue in you.”3
So, whatever Heart/Brain Entrainment Therapy is, for that theory to be admissible in a Frye jurisdiction it would likely have to be “generally accepted” in the medical community. To be admissible in a Daubert jurisdiction, proponents of the testimony would have to show evidence of a scientific methodology supporting the theory before the jury could hear any testimony about it. In either case, strategically, the defense attorney would likely file a motion to block either certain parts of the testimony or the testimony entirely.
IN SUM
Jurors expect sound scientific methodology supporting medical decisions; use care when selecting treatment for patients. Robustly adopt health promotion and general wellness strategies. However, if you use alternatives directed toward a specific therapy solving a specific problem, use them cautiously and with an awareness that the indication for the therapy should be scientifically defensible. —DML
REFERENCES
1. Frye v United States, 293 F. 1013 (D.C. Cir. 1923).
2. Daubert v Merrell Dow Pharmaceuticals, 509 U.S. 579 (1993).
3. Total Health Institute. Bioelectrical Energy, Quantum Frequency Resonance. www.totalhealthinstitute.com/about. Accessed July 14, 2015.