User login
Respiratory artifact: A second vital sign on the electrocardiogram
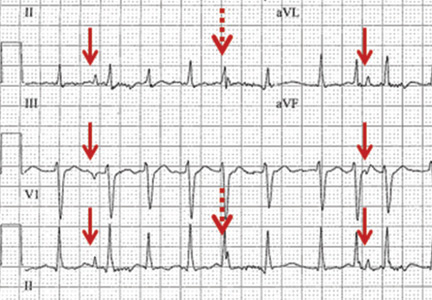
A 57-year-old man hospitalized for treatment of multilobar pneumonia was noted to have a rapid, irregular heart rate on telemetry. He was hypoxemic and appeared to be in respiratory distress. A 12-lead electrocardiogram (ECG) demonstrated atrial fibrillation with rapid ventricular response, as well as what looked like distinct and regular P waves dissociated from the QRS complexes at a rate of about 44/min (Figure 1). What is the explanation and clinical significance of this curious finding?
What appear to be dissociated P waves actually represent respiratory artifact.1–3 The sharp deflections mimicking P waves signify the tonic initiation of inspiratory effort; the subsequent brief periods of low-amplitude, high-frequency micro-oscillations represent surface electrical activity associated with the increased force of the accessory muscles of respiration.1–3
Surface electromyography noninvasively measures muscle activity using electrodes placed on the skin overlying the muscle.4 Using simultaneously recorded mechanical respiratory waveform tracings, we have previously demonstrated that the repetitive pseudo-P waves followed by micro-oscillations have a close temporal relationship with the inspiratory phase of respiration.3 The presence of respiratory artifact indicates a high-risk state frequently necessitating ventilation support.
In addition, when present, respiratory artifact can be viewed as the “second vital sign” on the ECG, the first vital sign being the heart rate. The respiratory rate can be approximated by counting the number of respiratory artifacts in a 10-second recording and multiplying it by 6. A more accurate rate assessment is achieved by measuring 1 or more respiratory artifact cycles in millimeters and then dividing that number into 1,500 or its multiples.3 Based on these calculations, the respiratory rate in this patient was 44/min.
The presence of two atrial rhythms on the same ECG, one not disturbing the other, is consistent with the diagnosis of atrial dissociation.5 Atrial dissociation is a common finding in cardiac transplant recipients in whom the transplantation was performed using atrio-atrial anastomosis.6 Most other cases of apparent atrial dissociation described in the old cardiology and critical care literature probably represented unrecognized respiratory artifact.7,8
An ECG from a different patient (Figure 2) demonstrates rapid respiratory artifact that raised awareness of severe respiratory failure. The respiratory rate calculated from spacing of the pseudo-P waves is 62/min, confirmed by simultaneous respirography.
A FREQUENT FINDING IN SICK HOSPITALIZED PATIENTS
Respiratory artifact is a frequent finding in sick hospitalized patients.3 Most commonly, it manifests as repetitive micro-oscillations.3 Pseudo-P waves, as in this 57-year-old patient, are less often observed; but if their origin is not recognized, the interpretation of the ECG can become puzzling.1–3,7,8
Respiratory artifact is a marker of increased work of breathing and a strong indicator of significant cardiopulmonary compromise. Improvement in the patient’s cardiac or respiratory condition is typically associated with a decrease in the rate or complete elimination of respiratory artifact.3
Recognition of rapid respiratory artifact is less important in critical care units, where patients’ vital signs and cardiorespiratory status are carefully observed. However, in hospital settings where respiratory rate and oxygen saturation are not continuously monitored, recognizing rapid respiratory artifact can help raise awareness of the possibility of severe respiratory distress.
- Higgins TG, Phillips JH Jr, Sumner RG. Atrial dissociation: an electrocardiographic artifact produced by the accessory muscles of respiration. Am J Cardiol 1966; 18:132–139.
- Cheriex EC, Brugada P, Wellens HJ. Pseudo-atrial dissociation: a respiratory artifact. Eur Heart J 1986; 7:357–359.
- Littmann L, Rennyson SL, Wall BP, Parker JM. Significance of respiratory artifact in the electrocardiogram. Am J Cardiol 2008; 102:1090–1096.
- Pullman SL, Goodin DS, Marquinez AI, Tabbal S, Rubin M. Clinical utility of surface EMG: report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology 2000; 55:171–177.
- Chung EK. A reappraisal of atrial dissociation. Am J Cardiol 1971; 28:111–117.
- Stinson EB, Schroeder JS, Griepp RB, Shumway NE, Dong E Jr. Observations on the behavior of recipient atria after cardiac transplantation in man. Am J Cardiol 1972; 30:615–622.
- Cohen J, Scherf D. Complete interatrial and intra-atrial block (atrial dissociation). Am Heart J 1965; 70:23–34.
- Chung KY, Walsh TJ, Massie E. A review of atrial dissociation, with illustrative cases and critical discussion. Am J Med Sci 1965; 250:72–78.
A 57-year-old man hospitalized for treatment of multilobar pneumonia was noted to have a rapid, irregular heart rate on telemetry. He was hypoxemic and appeared to be in respiratory distress. A 12-lead electrocardiogram (ECG) demonstrated atrial fibrillation with rapid ventricular response, as well as what looked like distinct and regular P waves dissociated from the QRS complexes at a rate of about 44/min (Figure 1). What is the explanation and clinical significance of this curious finding?
What appear to be dissociated P waves actually represent respiratory artifact.1–3 The sharp deflections mimicking P waves signify the tonic initiation of inspiratory effort; the subsequent brief periods of low-amplitude, high-frequency micro-oscillations represent surface electrical activity associated with the increased force of the accessory muscles of respiration.1–3
Surface electromyography noninvasively measures muscle activity using electrodes placed on the skin overlying the muscle.4 Using simultaneously recorded mechanical respiratory waveform tracings, we have previously demonstrated that the repetitive pseudo-P waves followed by micro-oscillations have a close temporal relationship with the inspiratory phase of respiration.3 The presence of respiratory artifact indicates a high-risk state frequently necessitating ventilation support.
In addition, when present, respiratory artifact can be viewed as the “second vital sign” on the ECG, the first vital sign being the heart rate. The respiratory rate can be approximated by counting the number of respiratory artifacts in a 10-second recording and multiplying it by 6. A more accurate rate assessment is achieved by measuring 1 or more respiratory artifact cycles in millimeters and then dividing that number into 1,500 or its multiples.3 Based on these calculations, the respiratory rate in this patient was 44/min.
The presence of two atrial rhythms on the same ECG, one not disturbing the other, is consistent with the diagnosis of atrial dissociation.5 Atrial dissociation is a common finding in cardiac transplant recipients in whom the transplantation was performed using atrio-atrial anastomosis.6 Most other cases of apparent atrial dissociation described in the old cardiology and critical care literature probably represented unrecognized respiratory artifact.7,8
An ECG from a different patient (Figure 2) demonstrates rapid respiratory artifact that raised awareness of severe respiratory failure. The respiratory rate calculated from spacing of the pseudo-P waves is 62/min, confirmed by simultaneous respirography.
A FREQUENT FINDING IN SICK HOSPITALIZED PATIENTS
Respiratory artifact is a frequent finding in sick hospitalized patients.3 Most commonly, it manifests as repetitive micro-oscillations.3 Pseudo-P waves, as in this 57-year-old patient, are less often observed; but if their origin is not recognized, the interpretation of the ECG can become puzzling.1–3,7,8
Respiratory artifact is a marker of increased work of breathing and a strong indicator of significant cardiopulmonary compromise. Improvement in the patient’s cardiac or respiratory condition is typically associated with a decrease in the rate or complete elimination of respiratory artifact.3
Recognition of rapid respiratory artifact is less important in critical care units, where patients’ vital signs and cardiorespiratory status are carefully observed. However, in hospital settings where respiratory rate and oxygen saturation are not continuously monitored, recognizing rapid respiratory artifact can help raise awareness of the possibility of severe respiratory distress.
A 57-year-old man hospitalized for treatment of multilobar pneumonia was noted to have a rapid, irregular heart rate on telemetry. He was hypoxemic and appeared to be in respiratory distress. A 12-lead electrocardiogram (ECG) demonstrated atrial fibrillation with rapid ventricular response, as well as what looked like distinct and regular P waves dissociated from the QRS complexes at a rate of about 44/min (Figure 1). What is the explanation and clinical significance of this curious finding?
What appear to be dissociated P waves actually represent respiratory artifact.1–3 The sharp deflections mimicking P waves signify the tonic initiation of inspiratory effort; the subsequent brief periods of low-amplitude, high-frequency micro-oscillations represent surface electrical activity associated with the increased force of the accessory muscles of respiration.1–3
Surface electromyography noninvasively measures muscle activity using electrodes placed on the skin overlying the muscle.4 Using simultaneously recorded mechanical respiratory waveform tracings, we have previously demonstrated that the repetitive pseudo-P waves followed by micro-oscillations have a close temporal relationship with the inspiratory phase of respiration.3 The presence of respiratory artifact indicates a high-risk state frequently necessitating ventilation support.
In addition, when present, respiratory artifact can be viewed as the “second vital sign” on the ECG, the first vital sign being the heart rate. The respiratory rate can be approximated by counting the number of respiratory artifacts in a 10-second recording and multiplying it by 6. A more accurate rate assessment is achieved by measuring 1 or more respiratory artifact cycles in millimeters and then dividing that number into 1,500 or its multiples.3 Based on these calculations, the respiratory rate in this patient was 44/min.
The presence of two atrial rhythms on the same ECG, one not disturbing the other, is consistent with the diagnosis of atrial dissociation.5 Atrial dissociation is a common finding in cardiac transplant recipients in whom the transplantation was performed using atrio-atrial anastomosis.6 Most other cases of apparent atrial dissociation described in the old cardiology and critical care literature probably represented unrecognized respiratory artifact.7,8
An ECG from a different patient (Figure 2) demonstrates rapid respiratory artifact that raised awareness of severe respiratory failure. The respiratory rate calculated from spacing of the pseudo-P waves is 62/min, confirmed by simultaneous respirography.
A FREQUENT FINDING IN SICK HOSPITALIZED PATIENTS
Respiratory artifact is a frequent finding in sick hospitalized patients.3 Most commonly, it manifests as repetitive micro-oscillations.3 Pseudo-P waves, as in this 57-year-old patient, are less often observed; but if their origin is not recognized, the interpretation of the ECG can become puzzling.1–3,7,8
Respiratory artifact is a marker of increased work of breathing and a strong indicator of significant cardiopulmonary compromise. Improvement in the patient’s cardiac or respiratory condition is typically associated with a decrease in the rate or complete elimination of respiratory artifact.3
Recognition of rapid respiratory artifact is less important in critical care units, where patients’ vital signs and cardiorespiratory status are carefully observed. However, in hospital settings where respiratory rate and oxygen saturation are not continuously monitored, recognizing rapid respiratory artifact can help raise awareness of the possibility of severe respiratory distress.
- Higgins TG, Phillips JH Jr, Sumner RG. Atrial dissociation: an electrocardiographic artifact produced by the accessory muscles of respiration. Am J Cardiol 1966; 18:132–139.
- Cheriex EC, Brugada P, Wellens HJ. Pseudo-atrial dissociation: a respiratory artifact. Eur Heart J 1986; 7:357–359.
- Littmann L, Rennyson SL, Wall BP, Parker JM. Significance of respiratory artifact in the electrocardiogram. Am J Cardiol 2008; 102:1090–1096.
- Pullman SL, Goodin DS, Marquinez AI, Tabbal S, Rubin M. Clinical utility of surface EMG: report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology 2000; 55:171–177.
- Chung EK. A reappraisal of atrial dissociation. Am J Cardiol 1971; 28:111–117.
- Stinson EB, Schroeder JS, Griepp RB, Shumway NE, Dong E Jr. Observations on the behavior of recipient atria after cardiac transplantation in man. Am J Cardiol 1972; 30:615–622.
- Cohen J, Scherf D. Complete interatrial and intra-atrial block (atrial dissociation). Am Heart J 1965; 70:23–34.
- Chung KY, Walsh TJ, Massie E. A review of atrial dissociation, with illustrative cases and critical discussion. Am J Med Sci 1965; 250:72–78.
- Higgins TG, Phillips JH Jr, Sumner RG. Atrial dissociation: an electrocardiographic artifact produced by the accessory muscles of respiration. Am J Cardiol 1966; 18:132–139.
- Cheriex EC, Brugada P, Wellens HJ. Pseudo-atrial dissociation: a respiratory artifact. Eur Heart J 1986; 7:357–359.
- Littmann L, Rennyson SL, Wall BP, Parker JM. Significance of respiratory artifact in the electrocardiogram. Am J Cardiol 2008; 102:1090–1096.
- Pullman SL, Goodin DS, Marquinez AI, Tabbal S, Rubin M. Clinical utility of surface EMG: report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology 2000; 55:171–177.
- Chung EK. A reappraisal of atrial dissociation. Am J Cardiol 1971; 28:111–117.
- Stinson EB, Schroeder JS, Griepp RB, Shumway NE, Dong E Jr. Observations on the behavior of recipient atria after cardiac transplantation in man. Am J Cardiol 1972; 30:615–622.
- Cohen J, Scherf D. Complete interatrial and intra-atrial block (atrial dissociation). Am Heart J 1965; 70:23–34.
- Chung KY, Walsh TJ, Massie E. A review of atrial dissociation, with illustrative cases and critical discussion. Am J Med Sci 1965; 250:72–78.
Geriatrics update 2015: Vaccination, frailty, chronic disease guidelines, and cognition
Guidelines for the management of chronic disease are starting to recognize vulnerable elderly patients. The topics in this review, culled from recent studies and recommendations, were chosen because they may change geriatric care. They include the newest influenza and pneumococcal vaccines; recommendations for managing chronic heart failure, cholesterol, and blood pressure; preventing frailty; drug treatments for dementia; and the impact of cognitive impairment on health outcomes.
INFLUENZA VACCINATION: HIGH-DOSE SUPERIOR BUT COSTLIER
Three classes of influenza vaccines have been available for some time:
- The standard-dose, trivalent inactivated injectable vaccine (IIV3-SD) contains H1N1, H3N2, and influenza B strains and is approved for all ages over 6 months.
- The quadrivalent vaccine (available mostly for nasal administration) has the same strains as the trivalent vaccine plus a second, different influenza B strain. The inactivated vaccine is injectable for all persons over the age of 6 months; the live-attenuated vaccine is available as a nasal spray only for ages 2 through 49.
- The high-dose injectable vaccine (IIV3-HD) contains the same strains as the trivalent vaccine plus four times as much hemagglutinin—the influenza virus antigen that stimulates immunity.
Although IIV3-HD has been available since 2010, no clinical data existed until 2014 showing it to be superior to standard-dose vaccine.
DiazGranados et al1 randomized nearly 32,000 adults age 65 and older to receive either the standard-dose or the high-dose vaccine. The primary end point was laboratory-confirmed influenza caused by any influenza viral type or subtype, in association with a protocol-defined influenzalike illness.
The primary end point was reached in 1.4% of those with the high-dose vaccine and 1.9% of those with the standard-dose vaccine (relative efficacy 24.2%, 95% confidence interval 9.7–36.5). There was also a 26% reduction in respiratory illness regardless of laboratory confirmation. Mortality rates were identical and low (0.5%) in both groups. In those without laboratory confirmation of respiratory illness, there was a 26% lower rate of pneumonia but no statistical difference in rates of hospitalization, medication use, routine office visits, and emergency department visits.
These results can be interpreted as meaning that the high-dose vaccine prevented about a quarter of the laboratory-confirmed influenza cases that would have occurred with the standard-dose vaccine. However, due to the low rate of disease in those given the standard-dose vaccine, the number needed to treat to prevent one influenza infection was about 200 with the high-dose vs the standard-dose vaccine; to prevent one case of pneumonia, more than 270 would need to be treated.
The current price differential as well as the high number needed to treat to prevent one infection may discourage the use of the high-dose vaccine. Medicare Part B pays for one dose of either influenza vaccine per season. For patients who paid out of pocket, the 2014–2015 season cost at a typical pharmacy was about $32 for the standard-dose vaccine and $55 for the high-dose.
PNEUMOCOCCAL VACCINATION
Conjugate vaccine now recommended for seniors
The 23-valent polysaccharide vaccine (Pneumovax) has been available since 1983 and is recommended in the United States for all adults age 65 and over. A 13-valent pneumococcal diphtheria conjugate vaccine (Prevnar 13) has been available since 2010. Until recently, the conjugate vaccine was recommended for children; the only adults for whom it was recommended were those age 19 and over who either were immunocompromised or had a cochlear implant, asplenia, a cerebral spinal fluid leak, or renal failure.
The CAPITA trial2 (Community-Acquired Pneumonia Immunisation Trial in Adults) randomized nearly 85,000 people (most 65 and older, and some children) in the Netherlands to receive either the conjugate vaccine or placebo. It found a 46% reduction in community-acquired pneumonia (P = .0006), a 45% reduction in nonbacteremic nonvaccine-type community-acquired pneumonia (P = .0067), and a 75% reduction in vaccine-type invasive pneumococcal disease (P = .0005). Common side effects included pain, swelling at the injection site, limitation of arm movement, fatigue, headache, decreased appetite, chills, and rash.
Based on this one study, the Advisory Committee on Immunization Practices3 recommended that all adults 65 and older receive the conjugate vaccine.
The recommendations for the conjugate vaccine for all ages are complicated. Limited to those age 65 and older, current recommendations are:
- For those who have already received the polysaccharide vaccine: get the conjugated vaccine at least 1 year later
- For those who have never received the polysaccharide vaccine: get the conjugate vaccine now, then the polysaccharide vaccine 6 to 12 months later.
Whether the Netherlands findings fully apply to the United States is under question. At the time of the study, Dutch infants but not adults had received pneumonia conjugate vaccinations since 2002 with a high compliance rate. Unlike in the United States, the polysaccharide pneumococcal vaccine had not been routinely recommended in the Netherlands. There may have been some indirect immunity due to the “herd” effect, but no direct immunity. With a likely higher background immunity to pneumonia in the United States, the dramatic reduction in infection noted in the Netherlands may not be duplicated here.
For those without Medicare coverage, the 2014–2015 winter season cost at a pharmacy was about $95 for the polysaccharide vaccine and about $200 for the conjugate vaccine. As of February 2, 2015, the Centers for Medicare and Medicaid Services are implementing Medicare Part B coverage to allow initial pneumococcal vaccine for Medicare patients who never received a pneumococcal vaccine under Medicare Part B, and then a different, second pneumococcal vaccine, 1 year after the first vaccine was administered.
HEART FAILURE
Eplerenone’s new role in mild heart failure
Aldosterone antagonists have been recommended for moderate to severe heart failure (New York Heart Association [NYHA] classes III and IV) for some time. The 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines also recommend them for mild heart failure (NYHA II).4
The EMPHASIS trial5 (Eplerenone in Patients With Systolic Heart Failure and Mild Symptoms) randomized 2,737 patients, median age 69, with NYHA class II heart failure and an ejection fraction of no more than 35% to receive the aldosterone antagonist eplerenone (up to 50 mg daily) or placebo, in addition to recommended therapy. The trial was stopped early, after a median follow-up of 21 months, when the treatment group was found to have a significantly lower risk of cardiovascular death or hospitalization for heart failure or for any cause.
Of note: hyperkalemia occurred in 11.8% of the eplerenone group vs 7.2% in the placebo group (P < .001). The high frequency of hyperkalemia in the placebo group may have been due to concomitant use of angiotensin-converting enzyme (ACE) inhibitors.
Sodium restriction reasonable
Although sodium restriction has been standard practice in heart failure for decades, restricting sodium in the elderly was given only a IIa (“reasonable”) classification, based on level C (very limited) evidence.4
Strong evidence exists that middle-aged and young older adults with heart failure (with preserved or reduced ejection fraction) should reduce their sodium intake by about 1 g per day or aim for a mean 24-hour urinary sodium excretion of about 2.3 g per day. However, little evidence exists to support a specific long-term target intake, and no evidence exists for “old-old” patients (loosely defined as older than 75 or 80).
Caution with digoxin
Use of digoxin has been recommended in patients with heart failure with reduced ejection fraction to reduce hospitalizations,4 but more recent publications have raised questions regarding its safety and efficacy.
Freeman et al,6 in a prospective study, followed 2,891 patients with newly diagnosed systolic heart failure over 2.5 years, of whom 529 were prescribed digoxin. The digoxin group had a higher rate of death (14.2 vs 11.2 per 100 patient-years) and heart failure-related hospitalization (28.2 vs 24.4 per 100 person-years).
The study was unable to determine if the digoxin level influenced the results, since about 30% of patients had no digoxin level drawn, and an additional 27% had only one level drawn during the study. For those with measured blood levels, the mean digoxin level for men was 0.83 ng/mL and 1.12 ng/mL for women. Risks and benefits of this medication should be weighed carefully.
Simultaneous interventions beneficial
The following evidence-based interventions are recommended for patients with heart failure with reduced ejection fraction:
- Heart failure education
- A beta-blocker
- An ACE inhibitor
- An aldosterone antagonist for NYHA class II–IV symptoms
- Anticoagulation for atrial fibrillation in patients with added risks (eg, hypertension, diabetes, prior transient ischemic attack or cerebrovascular accident, age at least 75)
- An implantable cardioverter-defibrillator and cardiac resynchronization therapy for select patients with symptoms, increased QRS duration, and left bundle branch block.
Fonarow et al7 studied these interventions in an analysis of a prospective study of outpatients with diagnosed heart failure or myocardial infarction and reduced left ventricular ejection fraction. Their nested case-control study compared 1,376 patients, mean age 72, who had died within 24 months and 2,752 propensity-matched controls who survived to 24 months. The survival rate was 37% higher with two simultaneous interventions than with one, and 70% higher with four simultaneous interventions than with one. Benefits plateaued with four to five interventions.
LIPID-LOWERING THERAPY FOR SENIORS
The 2013 ACC/AHA cholesterol guideline8 included new recommendations specifically relevant to the elderly. It advocates using a new cardiovascular disease risk calculator that provides an estimate of 10-year risk of atherosclerotic cardiovascular disease (ASCVD), based on data from multiple community-based populations and applicable to African American and non-Hispanic white men and women ages 40 through 79. Primary prevention with a statin is encouraged for those with a 10-year risk of 7.5% or higher. The tool generated controversy from the moment it was announced and may overestimate ASCVD risk by 67% in women and 86% in men.9
Emphasis on tolerability
The guideline focuses on statins as the main treatment and de-emphasizes the adjunctive use of other drugs to further lower lipids such as niacin, ezetimibe, and fenofibrate.
Statin tolerability is now stressed rather than specific lipid level targets. The guideline recommends reassessing statin choice and intensity according to pain, tenderness, stiffness, cramping, weakness, and fatigue (class IIa recommendation [“reasonable”], level of evidence B [“limited”]). Also recommended is reassessment of statin choice and intensity for patients older than 75 or for those taking multiple medications, drugs that alter metabolism, and conditions requiring complex medications (class IIa, level of evidence C [“very limited”]). For patients with confusion, statin and nonstatin causes should be considered as the source of the problem (class IIb [“consider”], level of evidence C).
Initiating high-intensity statin therapy is not recommended after age 75. However, continuing such treatment is reasonable for patients already receiving and tolerating the therapy for an appropriate indication. Initiation of moderate-intensity statin therapy in this age group is recommended for those with either clinical atherosclerotic cardiovascular disease or a low-density lipoprotein cholesterol (LDL-C) level of at least 190 mg/dL.
No specific guidance is provided for patients older than age 75 without ASCVD, with LDL-C less than 190 mg/dL, or with diabetes. In these groups, statin therapy may be initiated, continued, or intensified (class IIb, level of evidence C).
HYPERTENSION: LESS AGGRESSIVE GOALS FOR ELDERLY
The eighth Joint National Committee (JNC 8)10 made nine recommendations for managing high blood pressure, only one of which specifically addresses people 60 and older.
Drug therapy should be initiated if the blood pressure is 150/90 mm Hg or higher, and the blood pressure should be treated to less than that level (grade A recommendation, ie, strong). If treated systolic blood pressure is less than 140 mm Hg without adverse effects, it should be sustained (grade E recommendation, ie, based on expert opinion).
Tension between guidelines
The higher threshold for hypertension treatment and the lower threshold for statin therapy create tension between guidelines, and between guidelines and epidemiologic data.
For example, in a 67-year-old woman without diabetes and with a favorable lipid profile (eg, total cholesterol 130 mg/dL, high-density lipoprotein cholesterol 55 mg/dL), the ACC/AHA ASCVD risk calculator predicts a 10-year risk of less than 7.5% if her systolic blood pressure is 147 mm Hg. If the patient’s blood pressure were 148 or 149 mm Hg and all the other variables were the same, the JNC 8 would not recommend treatment with antihypertensive medication, but the ACC/AHA guidelines would recommend preventive statin therapy.
Another example is the relationship between heart failure and antihypertensive drugs. Multiple studies11,12 demonstrate a reduction in heart failure incidence with hypertension treatment. A 70-year-old man whose systolic blood pressure is 140 mm Hg has about a 15% lifetime risk of heart failure. If his systolic pressure were 160 mm Hg, his lifetime heart failure risk would be more than 50%.13 If his systolic pressure were 149, his lifetime risk of heart failure would be between 15% and 50%, but the JNC 8 criteria do not recommend antihypertensive therapy.
EXERCISE SLOWS PROGRESSION TO FRAILTY
In the absence of a gold standard, frailty has been operationally defined as meeting three out of five phenotypic criteria: diminished grip strength, low energy, slow gait, low physical activity, and unintentional weight loss. A “prefrail” stage, in which one or two criteria are present, identifies a vulnerable subset at high risk of progression to frailty.
About 42% of older adults in the community are considered vulnerable, or prefrail, and about 11% are frail.14 Interventions at the prefrail stage may prevent progression to frailty, but it is rare, without intervention, for a person to re-achieve the stronger stage once diagnosed with frailty.
Pahor et al15 randomized 1,635 sedentary adults ages 70 to 89 who met the criteria of prefrailty to either a moderate-intensity exercise program (consisting of aerobic, resistance, and flexibility exercises for 150 minutes per week, performed in a center and at home) or to a health education program with workshops on topics relevant to older adults and upper-extremity stretching exercises. Adherence to the exercise program was verified by questionnaire and an accelerometer device. Participants were assessed every 6 months for an average of 2.6 years.
The primary outcome measure was the development of major mobility disability as defined by the loss of ability to walk 400 m without assistance (a cane was acceptable, but not a walker). The primary outcome occurred in 30.1% of those in the exercise group and 35.5% of the health education group (hazard ratio 0.82, P = .03). Those in the exercise group also had one third fewer falls. No differences were found in death rates. The number needed to treat was about 19 to prevent one person from developing major disability. Those most likely to benefit were those who walked slowly at baseline (< 1.8 mph), were more mobility-impaired, and were more cognitively healthy.
SLOWING DEMENTIA IS STILL AN ELUSIVE GOAL
Vitamin E modestly improves cognitive function but may have a cost
Before new information emerged in 2014 regarding vitamin E and dementia, the best data were from a 1997 study16 that randomized patients with moderate dementia to either daily vitamin E 2,000 IU, the monoamine oxidase inhibitor selegiline 10 mg, both, or placebo for 2 years. No benefit of treatment for cognitive function was found. However, after adjusting for the baseline Mini-Mental State Examination score, the investigators found that either treatment was associated with a delay of about 7 months in the primary outcome (death, institutionalization, loss of activities of daily living, or severe dementia).
Unfortunately, selegiline is often poorly tolerated, causing dyskinesia in more than 10% of patients, nausea in 20%, and confusion, hallucinations, and syncope. Although vitamin E is better tolerated, in high doses it can cause fatigue, headache, and bleeding, with increased risk of hemorrhagic stroke. Studies conflict as to whether it increases the risk of death from any cause.17,18
Dysken et al,19 in a study reported in 2014, randomized 613 patients with mild to moderate Alzheimer disease, all of whom were taking an acetylcholinesterase inhibitor, to either daily vitamin E 2,000 IU, memantine 20 mg, both, or placebo. The primary outcome measure was an activities of daily living score (0–78, higher being better), which included the ability to perform such tasks as dressing oneself. Each task was scored from 0 (totally dependent on help) to 4 (able to perform completely independently).
Scores fell in both groups over the mean 2.7 years of the study, but the decrease was slightly slower in the vitamin E group: 3 points less at the end of the study compared with placebo. The groups taking memantine, vitamin E, or both did not differ significantly from one another. No significant differences were found in the secondary outcome of cognitive, neuropsychiatric, functional, and caregiver measures.
Based on the 1997 study, vitamin E may defer the time to important clinical outcomes by 7.5 months over a 2-year period in patients with moderate dementia. Based on the 2014 study, vitamin E may preserve half an activity of daily living over 2.7 years in patients with mild to moderate dementia. On the other hand, high doses of vitamin E may increase the risk of bleeding and falling, and whether they increase the risk of death is unclear.
Antidepressants for behavior issues
Other common problems in patients with major neurocognitive disorders include disturbed perception, thought content, mood, and behavior, collectively called behavioral and psychological symptoms of dementia. No known nondrug intervention is consistently effective for these problems, and no drug approved by the US Food and Drug Administration (FDA), except for a fixed-dose combination of dextromethorphan and quinidine, addresses any specific symptom.
Porsteinsson et al20 randomized 186 patients with Alzheimer disease and agitation to a psychological intervention plus either the selective serotonin reuptake inhibitor citalopram (titrated from 10 to 30 mg per day based on response and tolerability) or placebo. Agitation was reduced with citalopram compared with placebo, based on the agitation subscale of the Neurobehavioral Rating Scale.
Of those taking citalopram, 40% were much or very much improved, compared with 26% of those taking placebo. These results are comparable to or better than those with antipsychotic drugs, which should be avoided for treating dementia-related psychosis in elderly patients because of black-box warnings.
No differences were found in activities of daily living. An interesting finding, not seen in other studies, is that the Mini-Mental Status Examination score declined by 1 point in the treatment group vs no change in the placebo group (P = .03).
Prolonged QTc was found in 12.5% in the citalopram group vs 4.3% in the placebo group (P = .01). The FDA issued a warning in 2012 of a dose-dependent effect of citalopram on QTc and recommended a maximum dose of 20 mg for those over age 60; for “poor metabolizers” of cytochrome P450 2C19 (CYP 2C19); and for those taking medications that inhibit CYP 2C19, including proton pump inhibitors, cimetidine, fluvoxamine, fluoxetine, indomethacin, ketoconazole, modafinil, and probenecid. The United Kingdom has extended this warning to escitalopram. Unfortunately, in the Porsteinsson study, nearly 80% of the treatment group received the 30-mg dose and only 15% received the 20-mg dose, which provided insufficient data for independent analysis.
Possibly, citalopram cannot be administered in a dosage sufficient to produce the benefits seen in the study. Using escitalopram may also be risky. Based on this study, it would be prudent to monitor QTc when using these drugs.
Dextromethorphan and quinidine
A fixed-dose combination of dextromethorphan and quinidine (Nuedexta) was recently approved by the FDA for treatment of pseudobulbar affect in individuals with stroke, traumatic brain injury, or dementia. Pseudobulbar affect has been defined as a condition of contextually inappropriate or exaggerated emotional expression that often occurs in adults with neurologic damage.
Using a 20/10-mg dose combination, a small 12-week noncomparative trial demonstrated measurable improvement in pseudobulbar symptoms after 30 days (as measured by the Center for Neurologic Study-Lability Scale).21 Though individuals enrolled in this trial appeared to tolerate this dose, additional trials still need to be conducted to more clearly determine its long-term safety and efficacy. It is not approved for dementia with agitation, but a phase 2 trial suggests a benefit compared with placebo in reducing agitation and caregiver burden.22
RISKS OF MILD COGNITIVE IMPAIRMENT
The spectrum of cognitive impairment ranges from mild cognitive impairment (MCI), in which deficits are evident on neuropsychological testing but the person maintains overall function, to the different stages of dementia (mild, moderate, and severe). MCI was documented in the Cardiovascular Health Study in 22% of adults 75 and older.23
Despite presenting with apparently normal function, elderly people with MCI have a higher risk of falls, rehospitalization, and delirium. Screening is not typically performed for MCI in primary care. No study has compared clinical outcomes after screening vs not screening for cognitive impairment (whether MCI or dementia), and the US Preventive Services Task Force maintains that there is insufficient evidence for screening.24
Unrecognized cognitive impairment affects discharge outcomes
Nazir et al,25 in a 1-year longitudinal study, compared 976 patients age 65 and older who upon admission to a public hospital were either diagnosed with cognitive impairment (defined as scoring 7 or less on the 10-question Short Portable Mental Status Questionnaire) or not. They found that 42.5% were cognitively impaired on admission. Overall, 36.5% of patients were discharged to a facility rather than home; those who were cognitively impaired, older, and sicker were more likely to be discharged to a facility.
Interestingly, among those discharged to a facility, patients with cognitive impairment were less likely to be subsequently rehospitalized or die within 30 days of hospital discharge than those without cognitive impairment. Whether this can be explained by differences in comorbidities between the groups was not explored. Those discharged home had similar rates of death and rehospitalization whether or not they were cognitively impaired.
Patel et al26 screened 720 older patients upon discharge after hospitalization for heart failure with the Mini-Cog (a 3-minute test that consists of recall of three words and the ability to draw a clock face). About a quarter of patients were diagnosed with cognitive impairment based on this test.
Among those discharged home (about two-thirds of the group overall), patients were much more likely to be rehospitalized or die within 30 days if they were cognitively impaired. Among those discharged to a facility, the rates between the two groups were similar for the first 20 days; after that, people in the cognitively impaired group were much more likely to die or be readmitted to a hospital.
Dodson et al,27 in a study of 282 hospitalized patients with heart failure (mean age 80), identified 47% as having cognitive impairment at the time of hospitalization based on a score of less than 25 on the Mini-Mental State Examination. Of those found to have mild cognitive impairment (score 21–24), only 11% had documentation of a cognitive deficit in the medical record, and 39% of those found to have moderate to severe cognitive impairment (score < 21) had documentation of it in the medical record. Those with unrecognized impairment were 1.5 times more likely to die or be rehospitalized within 6 months than those with documented impairment.
Do interventions help?
It is unclear whether developing specific interventions tailored to cognitive impairment improves outcomes.
Davis et al28 studied 125 patients hospitalized for heart failure who were identified as having mild cognitive impairment based on a Montreal Cognitive Assessment score of 17 to 25 (out of 30) points. Patients were randomly assigned to either a targeted self-care teaching intervention or usual discharge care. The intervention included education and customized instruction on self-care tasks such as managing symptoms, organizing medications, and measuring fluid and sodium intake.
Thirty days after discharge, the intervention group had greater knowledge about heart failure than the control group, but no significant difference was found in ability to care for themselves or in readmission rates.
Interventions that target the patient-caregiver dyad may have more success. A pilot project in Indiana29 that developed an integrative care model for older people with mild cognitive impairment, dementia, or depression that targeted patients as well as their caregivers found that compared with patients from area primary care clinics, their patients had lower rehospitalization rates within 30 days of discharge (11% vs 20%) and higher rates of achieving a hemoglobin A1c of less than 8% (78% vs 51%). Results of an expanded innovations demonstration project awarded by the Centers for Medicare and Medicaid Services are pending.
The following more recently published data show promise for prevention of dementia through nonpharmacologic interventions.
The FINGER trial30 (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability) screened 2,654 Finnish individuals ages 60 to 77 using the Cardiovascular Risk Factors, Aging and Dementia risk tool, identifying 1,260 individuals with higher levels of cognitive impairment and randomizing them to a 2-year intervention consisting of exercise, cognitive training, and vascular risk monitoring (n = 631), or a control group provided with general health advice only (n = 629). Neuropsychological testing was conducted to measure differences between the groups, and at the end of the study, the mean Z-score difference in the total testing score between the intervention and control group was 0.22 (P = .30). This trial demonstrated that if cognitive impairment were identified, a multimodal intervention could improve or maintain cognitive function in at-risk elderly individuals.
- DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635–645.
- Bonten M, Bolkenbaas M, Huijts S, et al. Community acquired pneumonia immunisation trial in adults (CAPITA) (abstract). Pneumonia 2014; 3:95. Presented at 9th International Symposium on Pneumococci and Pneumococcal Diseases, 2014. Abstract 0541.
- Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2014; 63:822–825.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF-AHA guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Freeman JV, Yang J, Sung SH, Hlatky MA, Go AS. Effectiveness and safety of digoxin among contemporary adults with incident systolic heart failure. Circ Cardiovasc Qual Outcomes 2013; 6:525–533.
- Fonarow GC, Albert NM, Curtis AB, et al. Incremental reduction in risk of death associated with use of guideline-recommended therapies in patients with heart failure: a nested case-control analysis of IMPROVE-HF. J Am Heart Assoc 2012; 1:16–26.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S1–S45.
- DeFilippis AP, Young R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med 2015; 162:266–275.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Kostis JB, Davis BR, Cutler J, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA 1997; 278:212–216.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Lloyd-Jones DM, Larson MG, Leip EP, et al; Framingham Heart Study. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 2002; 106;3068–3072.
- Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012; 60:1487–1492.
- Pahor M, Guralnik JM, Ambrosius WT, et al; LIFE study investigators. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 2014; 311:2387–2396.
- Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med 1997; 336:1216–1222.
- Miller ER 3rd, Pastor-Barriuso R, Dala D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005; 142:37–46.
- Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci 2011; 4:158–170.
- Dysken MW, Sano M, Asthana S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 2014; 311:33–44.
- Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA 2014; 311:682–691.
- Yang LP, Deeks ED. Dextromethorphan/quinidine: a review of its use in adults with pseudobulbar affect. Drugs 2015; 75:83–90.
- Cummings J, Lyketsos C, Tariot P, et al. Dextromethorphan/quinidine (AVP-923) efficacy and safety for treatment of agitation in persons with Alzheimer’s disease: results from a phase 2 study (NCT01584440) (S16.007). Neurology 2015; 84:S16.007.
- Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol 2003; 60:1385–1389.
- Moyer VA; US Preventive Services Task Force. Screening for cognitive impairment in older adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160:791–797.
- Nazir A, LaMantia M, Chodosh J, et al. Interaction between cognitive impairment and discharge destination and its effect on rehospitalization. J Am Geriatr Soc 2013; 61:1958–1963.
- Patel A, Parikh R, Howell E, Hsich E, Gorodeski E. Mini-Cog performance: a novel marker of risk among patients hospitalized for heart failure. J Am Coll Cardiol 4014; 63:A755.
- Dodson JA, Truong TT, Towle VR, Kerins G, Chaudhry SI. Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. Am J Med 2013; 126:120–126.
- Davis KK, Mintzer M, Dennison Himmelfarb CR, Hayat MJ, Rotman S, Allen J. Targeted intervention improves knowledge but not self-care or readmissions in heart failure patients with mild cognitive impairment. Eur J Heart Fail 2012; 14:1041–1049.
- Boustani MA, Sachs GA, Alder CA, et al. Implementing innovative models of dementia care: The Healthy Aging Brain Center. Aging Ment Health 2011; 15:13–22.
- Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385:2255–2263.
Guidelines for the management of chronic disease are starting to recognize vulnerable elderly patients. The topics in this review, culled from recent studies and recommendations, were chosen because they may change geriatric care. They include the newest influenza and pneumococcal vaccines; recommendations for managing chronic heart failure, cholesterol, and blood pressure; preventing frailty; drug treatments for dementia; and the impact of cognitive impairment on health outcomes.
INFLUENZA VACCINATION: HIGH-DOSE SUPERIOR BUT COSTLIER
Three classes of influenza vaccines have been available for some time:
- The standard-dose, trivalent inactivated injectable vaccine (IIV3-SD) contains H1N1, H3N2, and influenza B strains and is approved for all ages over 6 months.
- The quadrivalent vaccine (available mostly for nasal administration) has the same strains as the trivalent vaccine plus a second, different influenza B strain. The inactivated vaccine is injectable for all persons over the age of 6 months; the live-attenuated vaccine is available as a nasal spray only for ages 2 through 49.
- The high-dose injectable vaccine (IIV3-HD) contains the same strains as the trivalent vaccine plus four times as much hemagglutinin—the influenza virus antigen that stimulates immunity.
Although IIV3-HD has been available since 2010, no clinical data existed until 2014 showing it to be superior to standard-dose vaccine.
DiazGranados et al1 randomized nearly 32,000 adults age 65 and older to receive either the standard-dose or the high-dose vaccine. The primary end point was laboratory-confirmed influenza caused by any influenza viral type or subtype, in association with a protocol-defined influenzalike illness.
The primary end point was reached in 1.4% of those with the high-dose vaccine and 1.9% of those with the standard-dose vaccine (relative efficacy 24.2%, 95% confidence interval 9.7–36.5). There was also a 26% reduction in respiratory illness regardless of laboratory confirmation. Mortality rates were identical and low (0.5%) in both groups. In those without laboratory confirmation of respiratory illness, there was a 26% lower rate of pneumonia but no statistical difference in rates of hospitalization, medication use, routine office visits, and emergency department visits.
These results can be interpreted as meaning that the high-dose vaccine prevented about a quarter of the laboratory-confirmed influenza cases that would have occurred with the standard-dose vaccine. However, due to the low rate of disease in those given the standard-dose vaccine, the number needed to treat to prevent one influenza infection was about 200 with the high-dose vs the standard-dose vaccine; to prevent one case of pneumonia, more than 270 would need to be treated.
The current price differential as well as the high number needed to treat to prevent one infection may discourage the use of the high-dose vaccine. Medicare Part B pays for one dose of either influenza vaccine per season. For patients who paid out of pocket, the 2014–2015 season cost at a typical pharmacy was about $32 for the standard-dose vaccine and $55 for the high-dose.
PNEUMOCOCCAL VACCINATION
Conjugate vaccine now recommended for seniors
The 23-valent polysaccharide vaccine (Pneumovax) has been available since 1983 and is recommended in the United States for all adults age 65 and over. A 13-valent pneumococcal diphtheria conjugate vaccine (Prevnar 13) has been available since 2010. Until recently, the conjugate vaccine was recommended for children; the only adults for whom it was recommended were those age 19 and over who either were immunocompromised or had a cochlear implant, asplenia, a cerebral spinal fluid leak, or renal failure.
The CAPITA trial2 (Community-Acquired Pneumonia Immunisation Trial in Adults) randomized nearly 85,000 people (most 65 and older, and some children) in the Netherlands to receive either the conjugate vaccine or placebo. It found a 46% reduction in community-acquired pneumonia (P = .0006), a 45% reduction in nonbacteremic nonvaccine-type community-acquired pneumonia (P = .0067), and a 75% reduction in vaccine-type invasive pneumococcal disease (P = .0005). Common side effects included pain, swelling at the injection site, limitation of arm movement, fatigue, headache, decreased appetite, chills, and rash.
Based on this one study, the Advisory Committee on Immunization Practices3 recommended that all adults 65 and older receive the conjugate vaccine.
The recommendations for the conjugate vaccine for all ages are complicated. Limited to those age 65 and older, current recommendations are:
- For those who have already received the polysaccharide vaccine: get the conjugated vaccine at least 1 year later
- For those who have never received the polysaccharide vaccine: get the conjugate vaccine now, then the polysaccharide vaccine 6 to 12 months later.
Whether the Netherlands findings fully apply to the United States is under question. At the time of the study, Dutch infants but not adults had received pneumonia conjugate vaccinations since 2002 with a high compliance rate. Unlike in the United States, the polysaccharide pneumococcal vaccine had not been routinely recommended in the Netherlands. There may have been some indirect immunity due to the “herd” effect, but no direct immunity. With a likely higher background immunity to pneumonia in the United States, the dramatic reduction in infection noted in the Netherlands may not be duplicated here.
For those without Medicare coverage, the 2014–2015 winter season cost at a pharmacy was about $95 for the polysaccharide vaccine and about $200 for the conjugate vaccine. As of February 2, 2015, the Centers for Medicare and Medicaid Services are implementing Medicare Part B coverage to allow initial pneumococcal vaccine for Medicare patients who never received a pneumococcal vaccine under Medicare Part B, and then a different, second pneumococcal vaccine, 1 year after the first vaccine was administered.
HEART FAILURE
Eplerenone’s new role in mild heart failure
Aldosterone antagonists have been recommended for moderate to severe heart failure (New York Heart Association [NYHA] classes III and IV) for some time. The 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines also recommend them for mild heart failure (NYHA II).4
The EMPHASIS trial5 (Eplerenone in Patients With Systolic Heart Failure and Mild Symptoms) randomized 2,737 patients, median age 69, with NYHA class II heart failure and an ejection fraction of no more than 35% to receive the aldosterone antagonist eplerenone (up to 50 mg daily) or placebo, in addition to recommended therapy. The trial was stopped early, after a median follow-up of 21 months, when the treatment group was found to have a significantly lower risk of cardiovascular death or hospitalization for heart failure or for any cause.
Of note: hyperkalemia occurred in 11.8% of the eplerenone group vs 7.2% in the placebo group (P < .001). The high frequency of hyperkalemia in the placebo group may have been due to concomitant use of angiotensin-converting enzyme (ACE) inhibitors.
Sodium restriction reasonable
Although sodium restriction has been standard practice in heart failure for decades, restricting sodium in the elderly was given only a IIa (“reasonable”) classification, based on level C (very limited) evidence.4
Strong evidence exists that middle-aged and young older adults with heart failure (with preserved or reduced ejection fraction) should reduce their sodium intake by about 1 g per day or aim for a mean 24-hour urinary sodium excretion of about 2.3 g per day. However, little evidence exists to support a specific long-term target intake, and no evidence exists for “old-old” patients (loosely defined as older than 75 or 80).
Caution with digoxin
Use of digoxin has been recommended in patients with heart failure with reduced ejection fraction to reduce hospitalizations,4 but more recent publications have raised questions regarding its safety and efficacy.
Freeman et al,6 in a prospective study, followed 2,891 patients with newly diagnosed systolic heart failure over 2.5 years, of whom 529 were prescribed digoxin. The digoxin group had a higher rate of death (14.2 vs 11.2 per 100 patient-years) and heart failure-related hospitalization (28.2 vs 24.4 per 100 person-years).
The study was unable to determine if the digoxin level influenced the results, since about 30% of patients had no digoxin level drawn, and an additional 27% had only one level drawn during the study. For those with measured blood levels, the mean digoxin level for men was 0.83 ng/mL and 1.12 ng/mL for women. Risks and benefits of this medication should be weighed carefully.
Simultaneous interventions beneficial
The following evidence-based interventions are recommended for patients with heart failure with reduced ejection fraction:
- Heart failure education
- A beta-blocker
- An ACE inhibitor
- An aldosterone antagonist for NYHA class II–IV symptoms
- Anticoagulation for atrial fibrillation in patients with added risks (eg, hypertension, diabetes, prior transient ischemic attack or cerebrovascular accident, age at least 75)
- An implantable cardioverter-defibrillator and cardiac resynchronization therapy for select patients with symptoms, increased QRS duration, and left bundle branch block.
Fonarow et al7 studied these interventions in an analysis of a prospective study of outpatients with diagnosed heart failure or myocardial infarction and reduced left ventricular ejection fraction. Their nested case-control study compared 1,376 patients, mean age 72, who had died within 24 months and 2,752 propensity-matched controls who survived to 24 months. The survival rate was 37% higher with two simultaneous interventions than with one, and 70% higher with four simultaneous interventions than with one. Benefits plateaued with four to five interventions.
LIPID-LOWERING THERAPY FOR SENIORS
The 2013 ACC/AHA cholesterol guideline8 included new recommendations specifically relevant to the elderly. It advocates using a new cardiovascular disease risk calculator that provides an estimate of 10-year risk of atherosclerotic cardiovascular disease (ASCVD), based on data from multiple community-based populations and applicable to African American and non-Hispanic white men and women ages 40 through 79. Primary prevention with a statin is encouraged for those with a 10-year risk of 7.5% or higher. The tool generated controversy from the moment it was announced and may overestimate ASCVD risk by 67% in women and 86% in men.9
Emphasis on tolerability
The guideline focuses on statins as the main treatment and de-emphasizes the adjunctive use of other drugs to further lower lipids such as niacin, ezetimibe, and fenofibrate.
Statin tolerability is now stressed rather than specific lipid level targets. The guideline recommends reassessing statin choice and intensity according to pain, tenderness, stiffness, cramping, weakness, and fatigue (class IIa recommendation [“reasonable”], level of evidence B [“limited”]). Also recommended is reassessment of statin choice and intensity for patients older than 75 or for those taking multiple medications, drugs that alter metabolism, and conditions requiring complex medications (class IIa, level of evidence C [“very limited”]). For patients with confusion, statin and nonstatin causes should be considered as the source of the problem (class IIb [“consider”], level of evidence C).
Initiating high-intensity statin therapy is not recommended after age 75. However, continuing such treatment is reasonable for patients already receiving and tolerating the therapy for an appropriate indication. Initiation of moderate-intensity statin therapy in this age group is recommended for those with either clinical atherosclerotic cardiovascular disease or a low-density lipoprotein cholesterol (LDL-C) level of at least 190 mg/dL.
No specific guidance is provided for patients older than age 75 without ASCVD, with LDL-C less than 190 mg/dL, or with diabetes. In these groups, statin therapy may be initiated, continued, or intensified (class IIb, level of evidence C).
HYPERTENSION: LESS AGGRESSIVE GOALS FOR ELDERLY
The eighth Joint National Committee (JNC 8)10 made nine recommendations for managing high blood pressure, only one of which specifically addresses people 60 and older.
Drug therapy should be initiated if the blood pressure is 150/90 mm Hg or higher, and the blood pressure should be treated to less than that level (grade A recommendation, ie, strong). If treated systolic blood pressure is less than 140 mm Hg without adverse effects, it should be sustained (grade E recommendation, ie, based on expert opinion).
Tension between guidelines
The higher threshold for hypertension treatment and the lower threshold for statin therapy create tension between guidelines, and between guidelines and epidemiologic data.
For example, in a 67-year-old woman without diabetes and with a favorable lipid profile (eg, total cholesterol 130 mg/dL, high-density lipoprotein cholesterol 55 mg/dL), the ACC/AHA ASCVD risk calculator predicts a 10-year risk of less than 7.5% if her systolic blood pressure is 147 mm Hg. If the patient’s blood pressure were 148 or 149 mm Hg and all the other variables were the same, the JNC 8 would not recommend treatment with antihypertensive medication, but the ACC/AHA guidelines would recommend preventive statin therapy.
Another example is the relationship between heart failure and antihypertensive drugs. Multiple studies11,12 demonstrate a reduction in heart failure incidence with hypertension treatment. A 70-year-old man whose systolic blood pressure is 140 mm Hg has about a 15% lifetime risk of heart failure. If his systolic pressure were 160 mm Hg, his lifetime heart failure risk would be more than 50%.13 If his systolic pressure were 149, his lifetime risk of heart failure would be between 15% and 50%, but the JNC 8 criteria do not recommend antihypertensive therapy.
EXERCISE SLOWS PROGRESSION TO FRAILTY
In the absence of a gold standard, frailty has been operationally defined as meeting three out of five phenotypic criteria: diminished grip strength, low energy, slow gait, low physical activity, and unintentional weight loss. A “prefrail” stage, in which one or two criteria are present, identifies a vulnerable subset at high risk of progression to frailty.
About 42% of older adults in the community are considered vulnerable, or prefrail, and about 11% are frail.14 Interventions at the prefrail stage may prevent progression to frailty, but it is rare, without intervention, for a person to re-achieve the stronger stage once diagnosed with frailty.
Pahor et al15 randomized 1,635 sedentary adults ages 70 to 89 who met the criteria of prefrailty to either a moderate-intensity exercise program (consisting of aerobic, resistance, and flexibility exercises for 150 minutes per week, performed in a center and at home) or to a health education program with workshops on topics relevant to older adults and upper-extremity stretching exercises. Adherence to the exercise program was verified by questionnaire and an accelerometer device. Participants were assessed every 6 months for an average of 2.6 years.
The primary outcome measure was the development of major mobility disability as defined by the loss of ability to walk 400 m without assistance (a cane was acceptable, but not a walker). The primary outcome occurred in 30.1% of those in the exercise group and 35.5% of the health education group (hazard ratio 0.82, P = .03). Those in the exercise group also had one third fewer falls. No differences were found in death rates. The number needed to treat was about 19 to prevent one person from developing major disability. Those most likely to benefit were those who walked slowly at baseline (< 1.8 mph), were more mobility-impaired, and were more cognitively healthy.
SLOWING DEMENTIA IS STILL AN ELUSIVE GOAL
Vitamin E modestly improves cognitive function but may have a cost
Before new information emerged in 2014 regarding vitamin E and dementia, the best data were from a 1997 study16 that randomized patients with moderate dementia to either daily vitamin E 2,000 IU, the monoamine oxidase inhibitor selegiline 10 mg, both, or placebo for 2 years. No benefit of treatment for cognitive function was found. However, after adjusting for the baseline Mini-Mental State Examination score, the investigators found that either treatment was associated with a delay of about 7 months in the primary outcome (death, institutionalization, loss of activities of daily living, or severe dementia).
Unfortunately, selegiline is often poorly tolerated, causing dyskinesia in more than 10% of patients, nausea in 20%, and confusion, hallucinations, and syncope. Although vitamin E is better tolerated, in high doses it can cause fatigue, headache, and bleeding, with increased risk of hemorrhagic stroke. Studies conflict as to whether it increases the risk of death from any cause.17,18
Dysken et al,19 in a study reported in 2014, randomized 613 patients with mild to moderate Alzheimer disease, all of whom were taking an acetylcholinesterase inhibitor, to either daily vitamin E 2,000 IU, memantine 20 mg, both, or placebo. The primary outcome measure was an activities of daily living score (0–78, higher being better), which included the ability to perform such tasks as dressing oneself. Each task was scored from 0 (totally dependent on help) to 4 (able to perform completely independently).
Scores fell in both groups over the mean 2.7 years of the study, but the decrease was slightly slower in the vitamin E group: 3 points less at the end of the study compared with placebo. The groups taking memantine, vitamin E, or both did not differ significantly from one another. No significant differences were found in the secondary outcome of cognitive, neuropsychiatric, functional, and caregiver measures.
Based on the 1997 study, vitamin E may defer the time to important clinical outcomes by 7.5 months over a 2-year period in patients with moderate dementia. Based on the 2014 study, vitamin E may preserve half an activity of daily living over 2.7 years in patients with mild to moderate dementia. On the other hand, high doses of vitamin E may increase the risk of bleeding and falling, and whether they increase the risk of death is unclear.
Antidepressants for behavior issues
Other common problems in patients with major neurocognitive disorders include disturbed perception, thought content, mood, and behavior, collectively called behavioral and psychological symptoms of dementia. No known nondrug intervention is consistently effective for these problems, and no drug approved by the US Food and Drug Administration (FDA), except for a fixed-dose combination of dextromethorphan and quinidine, addresses any specific symptom.
Porsteinsson et al20 randomized 186 patients with Alzheimer disease and agitation to a psychological intervention plus either the selective serotonin reuptake inhibitor citalopram (titrated from 10 to 30 mg per day based on response and tolerability) or placebo. Agitation was reduced with citalopram compared with placebo, based on the agitation subscale of the Neurobehavioral Rating Scale.
Of those taking citalopram, 40% were much or very much improved, compared with 26% of those taking placebo. These results are comparable to or better than those with antipsychotic drugs, which should be avoided for treating dementia-related psychosis in elderly patients because of black-box warnings.
No differences were found in activities of daily living. An interesting finding, not seen in other studies, is that the Mini-Mental Status Examination score declined by 1 point in the treatment group vs no change in the placebo group (P = .03).
Prolonged QTc was found in 12.5% in the citalopram group vs 4.3% in the placebo group (P = .01). The FDA issued a warning in 2012 of a dose-dependent effect of citalopram on QTc and recommended a maximum dose of 20 mg for those over age 60; for “poor metabolizers” of cytochrome P450 2C19 (CYP 2C19); and for those taking medications that inhibit CYP 2C19, including proton pump inhibitors, cimetidine, fluvoxamine, fluoxetine, indomethacin, ketoconazole, modafinil, and probenecid. The United Kingdom has extended this warning to escitalopram. Unfortunately, in the Porsteinsson study, nearly 80% of the treatment group received the 30-mg dose and only 15% received the 20-mg dose, which provided insufficient data for independent analysis.
Possibly, citalopram cannot be administered in a dosage sufficient to produce the benefits seen in the study. Using escitalopram may also be risky. Based on this study, it would be prudent to monitor QTc when using these drugs.
Dextromethorphan and quinidine
A fixed-dose combination of dextromethorphan and quinidine (Nuedexta) was recently approved by the FDA for treatment of pseudobulbar affect in individuals with stroke, traumatic brain injury, or dementia. Pseudobulbar affect has been defined as a condition of contextually inappropriate or exaggerated emotional expression that often occurs in adults with neurologic damage.
Using a 20/10-mg dose combination, a small 12-week noncomparative trial demonstrated measurable improvement in pseudobulbar symptoms after 30 days (as measured by the Center for Neurologic Study-Lability Scale).21 Though individuals enrolled in this trial appeared to tolerate this dose, additional trials still need to be conducted to more clearly determine its long-term safety and efficacy. It is not approved for dementia with agitation, but a phase 2 trial suggests a benefit compared with placebo in reducing agitation and caregiver burden.22
RISKS OF MILD COGNITIVE IMPAIRMENT
The spectrum of cognitive impairment ranges from mild cognitive impairment (MCI), in which deficits are evident on neuropsychological testing but the person maintains overall function, to the different stages of dementia (mild, moderate, and severe). MCI was documented in the Cardiovascular Health Study in 22% of adults 75 and older.23
Despite presenting with apparently normal function, elderly people with MCI have a higher risk of falls, rehospitalization, and delirium. Screening is not typically performed for MCI in primary care. No study has compared clinical outcomes after screening vs not screening for cognitive impairment (whether MCI or dementia), and the US Preventive Services Task Force maintains that there is insufficient evidence for screening.24
Unrecognized cognitive impairment affects discharge outcomes
Nazir et al,25 in a 1-year longitudinal study, compared 976 patients age 65 and older who upon admission to a public hospital were either diagnosed with cognitive impairment (defined as scoring 7 or less on the 10-question Short Portable Mental Status Questionnaire) or not. They found that 42.5% were cognitively impaired on admission. Overall, 36.5% of patients were discharged to a facility rather than home; those who were cognitively impaired, older, and sicker were more likely to be discharged to a facility.
Interestingly, among those discharged to a facility, patients with cognitive impairment were less likely to be subsequently rehospitalized or die within 30 days of hospital discharge than those without cognitive impairment. Whether this can be explained by differences in comorbidities between the groups was not explored. Those discharged home had similar rates of death and rehospitalization whether or not they were cognitively impaired.
Patel et al26 screened 720 older patients upon discharge after hospitalization for heart failure with the Mini-Cog (a 3-minute test that consists of recall of three words and the ability to draw a clock face). About a quarter of patients were diagnosed with cognitive impairment based on this test.
Among those discharged home (about two-thirds of the group overall), patients were much more likely to be rehospitalized or die within 30 days if they were cognitively impaired. Among those discharged to a facility, the rates between the two groups were similar for the first 20 days; after that, people in the cognitively impaired group were much more likely to die or be readmitted to a hospital.
Dodson et al,27 in a study of 282 hospitalized patients with heart failure (mean age 80), identified 47% as having cognitive impairment at the time of hospitalization based on a score of less than 25 on the Mini-Mental State Examination. Of those found to have mild cognitive impairment (score 21–24), only 11% had documentation of a cognitive deficit in the medical record, and 39% of those found to have moderate to severe cognitive impairment (score < 21) had documentation of it in the medical record. Those with unrecognized impairment were 1.5 times more likely to die or be rehospitalized within 6 months than those with documented impairment.
Do interventions help?
It is unclear whether developing specific interventions tailored to cognitive impairment improves outcomes.
Davis et al28 studied 125 patients hospitalized for heart failure who were identified as having mild cognitive impairment based on a Montreal Cognitive Assessment score of 17 to 25 (out of 30) points. Patients were randomly assigned to either a targeted self-care teaching intervention or usual discharge care. The intervention included education and customized instruction on self-care tasks such as managing symptoms, organizing medications, and measuring fluid and sodium intake.
Thirty days after discharge, the intervention group had greater knowledge about heart failure than the control group, but no significant difference was found in ability to care for themselves or in readmission rates.
Interventions that target the patient-caregiver dyad may have more success. A pilot project in Indiana29 that developed an integrative care model for older people with mild cognitive impairment, dementia, or depression that targeted patients as well as their caregivers found that compared with patients from area primary care clinics, their patients had lower rehospitalization rates within 30 days of discharge (11% vs 20%) and higher rates of achieving a hemoglobin A1c of less than 8% (78% vs 51%). Results of an expanded innovations demonstration project awarded by the Centers for Medicare and Medicaid Services are pending.
The following more recently published data show promise for prevention of dementia through nonpharmacologic interventions.
The FINGER trial30 (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability) screened 2,654 Finnish individuals ages 60 to 77 using the Cardiovascular Risk Factors, Aging and Dementia risk tool, identifying 1,260 individuals with higher levels of cognitive impairment and randomizing them to a 2-year intervention consisting of exercise, cognitive training, and vascular risk monitoring (n = 631), or a control group provided with general health advice only (n = 629). Neuropsychological testing was conducted to measure differences between the groups, and at the end of the study, the mean Z-score difference in the total testing score between the intervention and control group was 0.22 (P = .30). This trial demonstrated that if cognitive impairment were identified, a multimodal intervention could improve or maintain cognitive function in at-risk elderly individuals.
Guidelines for the management of chronic disease are starting to recognize vulnerable elderly patients. The topics in this review, culled from recent studies and recommendations, were chosen because they may change geriatric care. They include the newest influenza and pneumococcal vaccines; recommendations for managing chronic heart failure, cholesterol, and blood pressure; preventing frailty; drug treatments for dementia; and the impact of cognitive impairment on health outcomes.
INFLUENZA VACCINATION: HIGH-DOSE SUPERIOR BUT COSTLIER
Three classes of influenza vaccines have been available for some time:
- The standard-dose, trivalent inactivated injectable vaccine (IIV3-SD) contains H1N1, H3N2, and influenza B strains and is approved for all ages over 6 months.
- The quadrivalent vaccine (available mostly for nasal administration) has the same strains as the trivalent vaccine plus a second, different influenza B strain. The inactivated vaccine is injectable for all persons over the age of 6 months; the live-attenuated vaccine is available as a nasal spray only for ages 2 through 49.
- The high-dose injectable vaccine (IIV3-HD) contains the same strains as the trivalent vaccine plus four times as much hemagglutinin—the influenza virus antigen that stimulates immunity.
Although IIV3-HD has been available since 2010, no clinical data existed until 2014 showing it to be superior to standard-dose vaccine.
DiazGranados et al1 randomized nearly 32,000 adults age 65 and older to receive either the standard-dose or the high-dose vaccine. The primary end point was laboratory-confirmed influenza caused by any influenza viral type or subtype, in association with a protocol-defined influenzalike illness.
The primary end point was reached in 1.4% of those with the high-dose vaccine and 1.9% of those with the standard-dose vaccine (relative efficacy 24.2%, 95% confidence interval 9.7–36.5). There was also a 26% reduction in respiratory illness regardless of laboratory confirmation. Mortality rates were identical and low (0.5%) in both groups. In those without laboratory confirmation of respiratory illness, there was a 26% lower rate of pneumonia but no statistical difference in rates of hospitalization, medication use, routine office visits, and emergency department visits.
These results can be interpreted as meaning that the high-dose vaccine prevented about a quarter of the laboratory-confirmed influenza cases that would have occurred with the standard-dose vaccine. However, due to the low rate of disease in those given the standard-dose vaccine, the number needed to treat to prevent one influenza infection was about 200 with the high-dose vs the standard-dose vaccine; to prevent one case of pneumonia, more than 270 would need to be treated.
The current price differential as well as the high number needed to treat to prevent one infection may discourage the use of the high-dose vaccine. Medicare Part B pays for one dose of either influenza vaccine per season. For patients who paid out of pocket, the 2014–2015 season cost at a typical pharmacy was about $32 for the standard-dose vaccine and $55 for the high-dose.
PNEUMOCOCCAL VACCINATION
Conjugate vaccine now recommended for seniors
The 23-valent polysaccharide vaccine (Pneumovax) has been available since 1983 and is recommended in the United States for all adults age 65 and over. A 13-valent pneumococcal diphtheria conjugate vaccine (Prevnar 13) has been available since 2010. Until recently, the conjugate vaccine was recommended for children; the only adults for whom it was recommended were those age 19 and over who either were immunocompromised or had a cochlear implant, asplenia, a cerebral spinal fluid leak, or renal failure.
The CAPITA trial2 (Community-Acquired Pneumonia Immunisation Trial in Adults) randomized nearly 85,000 people (most 65 and older, and some children) in the Netherlands to receive either the conjugate vaccine or placebo. It found a 46% reduction in community-acquired pneumonia (P = .0006), a 45% reduction in nonbacteremic nonvaccine-type community-acquired pneumonia (P = .0067), and a 75% reduction in vaccine-type invasive pneumococcal disease (P = .0005). Common side effects included pain, swelling at the injection site, limitation of arm movement, fatigue, headache, decreased appetite, chills, and rash.
Based on this one study, the Advisory Committee on Immunization Practices3 recommended that all adults 65 and older receive the conjugate vaccine.
The recommendations for the conjugate vaccine for all ages are complicated. Limited to those age 65 and older, current recommendations are:
- For those who have already received the polysaccharide vaccine: get the conjugated vaccine at least 1 year later
- For those who have never received the polysaccharide vaccine: get the conjugate vaccine now, then the polysaccharide vaccine 6 to 12 months later.
Whether the Netherlands findings fully apply to the United States is under question. At the time of the study, Dutch infants but not adults had received pneumonia conjugate vaccinations since 2002 with a high compliance rate. Unlike in the United States, the polysaccharide pneumococcal vaccine had not been routinely recommended in the Netherlands. There may have been some indirect immunity due to the “herd” effect, but no direct immunity. With a likely higher background immunity to pneumonia in the United States, the dramatic reduction in infection noted in the Netherlands may not be duplicated here.
For those without Medicare coverage, the 2014–2015 winter season cost at a pharmacy was about $95 for the polysaccharide vaccine and about $200 for the conjugate vaccine. As of February 2, 2015, the Centers for Medicare and Medicaid Services are implementing Medicare Part B coverage to allow initial pneumococcal vaccine for Medicare patients who never received a pneumococcal vaccine under Medicare Part B, and then a different, second pneumococcal vaccine, 1 year after the first vaccine was administered.
HEART FAILURE
Eplerenone’s new role in mild heart failure
Aldosterone antagonists have been recommended for moderate to severe heart failure (New York Heart Association [NYHA] classes III and IV) for some time. The 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines also recommend them for mild heart failure (NYHA II).4
The EMPHASIS trial5 (Eplerenone in Patients With Systolic Heart Failure and Mild Symptoms) randomized 2,737 patients, median age 69, with NYHA class II heart failure and an ejection fraction of no more than 35% to receive the aldosterone antagonist eplerenone (up to 50 mg daily) or placebo, in addition to recommended therapy. The trial was stopped early, after a median follow-up of 21 months, when the treatment group was found to have a significantly lower risk of cardiovascular death or hospitalization for heart failure or for any cause.
Of note: hyperkalemia occurred in 11.8% of the eplerenone group vs 7.2% in the placebo group (P < .001). The high frequency of hyperkalemia in the placebo group may have been due to concomitant use of angiotensin-converting enzyme (ACE) inhibitors.
Sodium restriction reasonable
Although sodium restriction has been standard practice in heart failure for decades, restricting sodium in the elderly was given only a IIa (“reasonable”) classification, based on level C (very limited) evidence.4
Strong evidence exists that middle-aged and young older adults with heart failure (with preserved or reduced ejection fraction) should reduce their sodium intake by about 1 g per day or aim for a mean 24-hour urinary sodium excretion of about 2.3 g per day. However, little evidence exists to support a specific long-term target intake, and no evidence exists for “old-old” patients (loosely defined as older than 75 or 80).
Caution with digoxin
Use of digoxin has been recommended in patients with heart failure with reduced ejection fraction to reduce hospitalizations,4 but more recent publications have raised questions regarding its safety and efficacy.
Freeman et al,6 in a prospective study, followed 2,891 patients with newly diagnosed systolic heart failure over 2.5 years, of whom 529 were prescribed digoxin. The digoxin group had a higher rate of death (14.2 vs 11.2 per 100 patient-years) and heart failure-related hospitalization (28.2 vs 24.4 per 100 person-years).
The study was unable to determine if the digoxin level influenced the results, since about 30% of patients had no digoxin level drawn, and an additional 27% had only one level drawn during the study. For those with measured blood levels, the mean digoxin level for men was 0.83 ng/mL and 1.12 ng/mL for women. Risks and benefits of this medication should be weighed carefully.
Simultaneous interventions beneficial
The following evidence-based interventions are recommended for patients with heart failure with reduced ejection fraction:
- Heart failure education
- A beta-blocker
- An ACE inhibitor
- An aldosterone antagonist for NYHA class II–IV symptoms
- Anticoagulation for atrial fibrillation in patients with added risks (eg, hypertension, diabetes, prior transient ischemic attack or cerebrovascular accident, age at least 75)
- An implantable cardioverter-defibrillator and cardiac resynchronization therapy for select patients with symptoms, increased QRS duration, and left bundle branch block.
Fonarow et al7 studied these interventions in an analysis of a prospective study of outpatients with diagnosed heart failure or myocardial infarction and reduced left ventricular ejection fraction. Their nested case-control study compared 1,376 patients, mean age 72, who had died within 24 months and 2,752 propensity-matched controls who survived to 24 months. The survival rate was 37% higher with two simultaneous interventions than with one, and 70% higher with four simultaneous interventions than with one. Benefits plateaued with four to five interventions.
LIPID-LOWERING THERAPY FOR SENIORS
The 2013 ACC/AHA cholesterol guideline8 included new recommendations specifically relevant to the elderly. It advocates using a new cardiovascular disease risk calculator that provides an estimate of 10-year risk of atherosclerotic cardiovascular disease (ASCVD), based on data from multiple community-based populations and applicable to African American and non-Hispanic white men and women ages 40 through 79. Primary prevention with a statin is encouraged for those with a 10-year risk of 7.5% or higher. The tool generated controversy from the moment it was announced and may overestimate ASCVD risk by 67% in women and 86% in men.9
Emphasis on tolerability
The guideline focuses on statins as the main treatment and de-emphasizes the adjunctive use of other drugs to further lower lipids such as niacin, ezetimibe, and fenofibrate.
Statin tolerability is now stressed rather than specific lipid level targets. The guideline recommends reassessing statin choice and intensity according to pain, tenderness, stiffness, cramping, weakness, and fatigue (class IIa recommendation [“reasonable”], level of evidence B [“limited”]). Also recommended is reassessment of statin choice and intensity for patients older than 75 or for those taking multiple medications, drugs that alter metabolism, and conditions requiring complex medications (class IIa, level of evidence C [“very limited”]). For patients with confusion, statin and nonstatin causes should be considered as the source of the problem (class IIb [“consider”], level of evidence C).
Initiating high-intensity statin therapy is not recommended after age 75. However, continuing such treatment is reasonable for patients already receiving and tolerating the therapy for an appropriate indication. Initiation of moderate-intensity statin therapy in this age group is recommended for those with either clinical atherosclerotic cardiovascular disease or a low-density lipoprotein cholesterol (LDL-C) level of at least 190 mg/dL.
No specific guidance is provided for patients older than age 75 without ASCVD, with LDL-C less than 190 mg/dL, or with diabetes. In these groups, statin therapy may be initiated, continued, or intensified (class IIb, level of evidence C).
HYPERTENSION: LESS AGGRESSIVE GOALS FOR ELDERLY
The eighth Joint National Committee (JNC 8)10 made nine recommendations for managing high blood pressure, only one of which specifically addresses people 60 and older.
Drug therapy should be initiated if the blood pressure is 150/90 mm Hg or higher, and the blood pressure should be treated to less than that level (grade A recommendation, ie, strong). If treated systolic blood pressure is less than 140 mm Hg without adverse effects, it should be sustained (grade E recommendation, ie, based on expert opinion).
Tension between guidelines
The higher threshold for hypertension treatment and the lower threshold for statin therapy create tension between guidelines, and between guidelines and epidemiologic data.
For example, in a 67-year-old woman without diabetes and with a favorable lipid profile (eg, total cholesterol 130 mg/dL, high-density lipoprotein cholesterol 55 mg/dL), the ACC/AHA ASCVD risk calculator predicts a 10-year risk of less than 7.5% if her systolic blood pressure is 147 mm Hg. If the patient’s blood pressure were 148 or 149 mm Hg and all the other variables were the same, the JNC 8 would not recommend treatment with antihypertensive medication, but the ACC/AHA guidelines would recommend preventive statin therapy.
Another example is the relationship between heart failure and antihypertensive drugs. Multiple studies11,12 demonstrate a reduction in heart failure incidence with hypertension treatment. A 70-year-old man whose systolic blood pressure is 140 mm Hg has about a 15% lifetime risk of heart failure. If his systolic pressure were 160 mm Hg, his lifetime heart failure risk would be more than 50%.13 If his systolic pressure were 149, his lifetime risk of heart failure would be between 15% and 50%, but the JNC 8 criteria do not recommend antihypertensive therapy.
EXERCISE SLOWS PROGRESSION TO FRAILTY
In the absence of a gold standard, frailty has been operationally defined as meeting three out of five phenotypic criteria: diminished grip strength, low energy, slow gait, low physical activity, and unintentional weight loss. A “prefrail” stage, in which one or two criteria are present, identifies a vulnerable subset at high risk of progression to frailty.
About 42% of older adults in the community are considered vulnerable, or prefrail, and about 11% are frail.14 Interventions at the prefrail stage may prevent progression to frailty, but it is rare, without intervention, for a person to re-achieve the stronger stage once diagnosed with frailty.
Pahor et al15 randomized 1,635 sedentary adults ages 70 to 89 who met the criteria of prefrailty to either a moderate-intensity exercise program (consisting of aerobic, resistance, and flexibility exercises for 150 minutes per week, performed in a center and at home) or to a health education program with workshops on topics relevant to older adults and upper-extremity stretching exercises. Adherence to the exercise program was verified by questionnaire and an accelerometer device. Participants were assessed every 6 months for an average of 2.6 years.
The primary outcome measure was the development of major mobility disability as defined by the loss of ability to walk 400 m without assistance (a cane was acceptable, but not a walker). The primary outcome occurred in 30.1% of those in the exercise group and 35.5% of the health education group (hazard ratio 0.82, P = .03). Those in the exercise group also had one third fewer falls. No differences were found in death rates. The number needed to treat was about 19 to prevent one person from developing major disability. Those most likely to benefit were those who walked slowly at baseline (< 1.8 mph), were more mobility-impaired, and were more cognitively healthy.
SLOWING DEMENTIA IS STILL AN ELUSIVE GOAL
Vitamin E modestly improves cognitive function but may have a cost
Before new information emerged in 2014 regarding vitamin E and dementia, the best data were from a 1997 study16 that randomized patients with moderate dementia to either daily vitamin E 2,000 IU, the monoamine oxidase inhibitor selegiline 10 mg, both, or placebo for 2 years. No benefit of treatment for cognitive function was found. However, after adjusting for the baseline Mini-Mental State Examination score, the investigators found that either treatment was associated with a delay of about 7 months in the primary outcome (death, institutionalization, loss of activities of daily living, or severe dementia).
Unfortunately, selegiline is often poorly tolerated, causing dyskinesia in more than 10% of patients, nausea in 20%, and confusion, hallucinations, and syncope. Although vitamin E is better tolerated, in high doses it can cause fatigue, headache, and bleeding, with increased risk of hemorrhagic stroke. Studies conflict as to whether it increases the risk of death from any cause.17,18
Dysken et al,19 in a study reported in 2014, randomized 613 patients with mild to moderate Alzheimer disease, all of whom were taking an acetylcholinesterase inhibitor, to either daily vitamin E 2,000 IU, memantine 20 mg, both, or placebo. The primary outcome measure was an activities of daily living score (0–78, higher being better), which included the ability to perform such tasks as dressing oneself. Each task was scored from 0 (totally dependent on help) to 4 (able to perform completely independently).
Scores fell in both groups over the mean 2.7 years of the study, but the decrease was slightly slower in the vitamin E group: 3 points less at the end of the study compared with placebo. The groups taking memantine, vitamin E, or both did not differ significantly from one another. No significant differences were found in the secondary outcome of cognitive, neuropsychiatric, functional, and caregiver measures.
Based on the 1997 study, vitamin E may defer the time to important clinical outcomes by 7.5 months over a 2-year period in patients with moderate dementia. Based on the 2014 study, vitamin E may preserve half an activity of daily living over 2.7 years in patients with mild to moderate dementia. On the other hand, high doses of vitamin E may increase the risk of bleeding and falling, and whether they increase the risk of death is unclear.
Antidepressants for behavior issues
Other common problems in patients with major neurocognitive disorders include disturbed perception, thought content, mood, and behavior, collectively called behavioral and psychological symptoms of dementia. No known nondrug intervention is consistently effective for these problems, and no drug approved by the US Food and Drug Administration (FDA), except for a fixed-dose combination of dextromethorphan and quinidine, addresses any specific symptom.
Porsteinsson et al20 randomized 186 patients with Alzheimer disease and agitation to a psychological intervention plus either the selective serotonin reuptake inhibitor citalopram (titrated from 10 to 30 mg per day based on response and tolerability) or placebo. Agitation was reduced with citalopram compared with placebo, based on the agitation subscale of the Neurobehavioral Rating Scale.
Of those taking citalopram, 40% were much or very much improved, compared with 26% of those taking placebo. These results are comparable to or better than those with antipsychotic drugs, which should be avoided for treating dementia-related psychosis in elderly patients because of black-box warnings.
No differences were found in activities of daily living. An interesting finding, not seen in other studies, is that the Mini-Mental Status Examination score declined by 1 point in the treatment group vs no change in the placebo group (P = .03).
Prolonged QTc was found in 12.5% in the citalopram group vs 4.3% in the placebo group (P = .01). The FDA issued a warning in 2012 of a dose-dependent effect of citalopram on QTc and recommended a maximum dose of 20 mg for those over age 60; for “poor metabolizers” of cytochrome P450 2C19 (CYP 2C19); and for those taking medications that inhibit CYP 2C19, including proton pump inhibitors, cimetidine, fluvoxamine, fluoxetine, indomethacin, ketoconazole, modafinil, and probenecid. The United Kingdom has extended this warning to escitalopram. Unfortunately, in the Porsteinsson study, nearly 80% of the treatment group received the 30-mg dose and only 15% received the 20-mg dose, which provided insufficient data for independent analysis.
Possibly, citalopram cannot be administered in a dosage sufficient to produce the benefits seen in the study. Using escitalopram may also be risky. Based on this study, it would be prudent to monitor QTc when using these drugs.
Dextromethorphan and quinidine
A fixed-dose combination of dextromethorphan and quinidine (Nuedexta) was recently approved by the FDA for treatment of pseudobulbar affect in individuals with stroke, traumatic brain injury, or dementia. Pseudobulbar affect has been defined as a condition of contextually inappropriate or exaggerated emotional expression that often occurs in adults with neurologic damage.
Using a 20/10-mg dose combination, a small 12-week noncomparative trial demonstrated measurable improvement in pseudobulbar symptoms after 30 days (as measured by the Center for Neurologic Study-Lability Scale).21 Though individuals enrolled in this trial appeared to tolerate this dose, additional trials still need to be conducted to more clearly determine its long-term safety and efficacy. It is not approved for dementia with agitation, but a phase 2 trial suggests a benefit compared with placebo in reducing agitation and caregiver burden.22
RISKS OF MILD COGNITIVE IMPAIRMENT
The spectrum of cognitive impairment ranges from mild cognitive impairment (MCI), in which deficits are evident on neuropsychological testing but the person maintains overall function, to the different stages of dementia (mild, moderate, and severe). MCI was documented in the Cardiovascular Health Study in 22% of adults 75 and older.23
Despite presenting with apparently normal function, elderly people with MCI have a higher risk of falls, rehospitalization, and delirium. Screening is not typically performed for MCI in primary care. No study has compared clinical outcomes after screening vs not screening for cognitive impairment (whether MCI or dementia), and the US Preventive Services Task Force maintains that there is insufficient evidence for screening.24
Unrecognized cognitive impairment affects discharge outcomes
Nazir et al,25 in a 1-year longitudinal study, compared 976 patients age 65 and older who upon admission to a public hospital were either diagnosed with cognitive impairment (defined as scoring 7 or less on the 10-question Short Portable Mental Status Questionnaire) or not. They found that 42.5% were cognitively impaired on admission. Overall, 36.5% of patients were discharged to a facility rather than home; those who were cognitively impaired, older, and sicker were more likely to be discharged to a facility.
Interestingly, among those discharged to a facility, patients with cognitive impairment were less likely to be subsequently rehospitalized or die within 30 days of hospital discharge than those without cognitive impairment. Whether this can be explained by differences in comorbidities between the groups was not explored. Those discharged home had similar rates of death and rehospitalization whether or not they were cognitively impaired.
Patel et al26 screened 720 older patients upon discharge after hospitalization for heart failure with the Mini-Cog (a 3-minute test that consists of recall of three words and the ability to draw a clock face). About a quarter of patients were diagnosed with cognitive impairment based on this test.
Among those discharged home (about two-thirds of the group overall), patients were much more likely to be rehospitalized or die within 30 days if they were cognitively impaired. Among those discharged to a facility, the rates between the two groups were similar for the first 20 days; after that, people in the cognitively impaired group were much more likely to die or be readmitted to a hospital.
Dodson et al,27 in a study of 282 hospitalized patients with heart failure (mean age 80), identified 47% as having cognitive impairment at the time of hospitalization based on a score of less than 25 on the Mini-Mental State Examination. Of those found to have mild cognitive impairment (score 21–24), only 11% had documentation of a cognitive deficit in the medical record, and 39% of those found to have moderate to severe cognitive impairment (score < 21) had documentation of it in the medical record. Those with unrecognized impairment were 1.5 times more likely to die or be rehospitalized within 6 months than those with documented impairment.
Do interventions help?
It is unclear whether developing specific interventions tailored to cognitive impairment improves outcomes.
Davis et al28 studied 125 patients hospitalized for heart failure who were identified as having mild cognitive impairment based on a Montreal Cognitive Assessment score of 17 to 25 (out of 30) points. Patients were randomly assigned to either a targeted self-care teaching intervention or usual discharge care. The intervention included education and customized instruction on self-care tasks such as managing symptoms, organizing medications, and measuring fluid and sodium intake.
Thirty days after discharge, the intervention group had greater knowledge about heart failure than the control group, but no significant difference was found in ability to care for themselves or in readmission rates.
Interventions that target the patient-caregiver dyad may have more success. A pilot project in Indiana29 that developed an integrative care model for older people with mild cognitive impairment, dementia, or depression that targeted patients as well as their caregivers found that compared with patients from area primary care clinics, their patients had lower rehospitalization rates within 30 days of discharge (11% vs 20%) and higher rates of achieving a hemoglobin A1c of less than 8% (78% vs 51%). Results of an expanded innovations demonstration project awarded by the Centers for Medicare and Medicaid Services are pending.
The following more recently published data show promise for prevention of dementia through nonpharmacologic interventions.
The FINGER trial30 (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability) screened 2,654 Finnish individuals ages 60 to 77 using the Cardiovascular Risk Factors, Aging and Dementia risk tool, identifying 1,260 individuals with higher levels of cognitive impairment and randomizing them to a 2-year intervention consisting of exercise, cognitive training, and vascular risk monitoring (n = 631), or a control group provided with general health advice only (n = 629). Neuropsychological testing was conducted to measure differences between the groups, and at the end of the study, the mean Z-score difference in the total testing score between the intervention and control group was 0.22 (P = .30). This trial demonstrated that if cognitive impairment were identified, a multimodal intervention could improve or maintain cognitive function in at-risk elderly individuals.
- DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635–645.
- Bonten M, Bolkenbaas M, Huijts S, et al. Community acquired pneumonia immunisation trial in adults (CAPITA) (abstract). Pneumonia 2014; 3:95. Presented at 9th International Symposium on Pneumococci and Pneumococcal Diseases, 2014. Abstract 0541.
- Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2014; 63:822–825.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF-AHA guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Freeman JV, Yang J, Sung SH, Hlatky MA, Go AS. Effectiveness and safety of digoxin among contemporary adults with incident systolic heart failure. Circ Cardiovasc Qual Outcomes 2013; 6:525–533.
- Fonarow GC, Albert NM, Curtis AB, et al. Incremental reduction in risk of death associated with use of guideline-recommended therapies in patients with heart failure: a nested case-control analysis of IMPROVE-HF. J Am Heart Assoc 2012; 1:16–26.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S1–S45.
- DeFilippis AP, Young R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med 2015; 162:266–275.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Kostis JB, Davis BR, Cutler J, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA 1997; 278:212–216.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Lloyd-Jones DM, Larson MG, Leip EP, et al; Framingham Heart Study. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 2002; 106;3068–3072.
- Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012; 60:1487–1492.
- Pahor M, Guralnik JM, Ambrosius WT, et al; LIFE study investigators. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 2014; 311:2387–2396.
- Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med 1997; 336:1216–1222.
- Miller ER 3rd, Pastor-Barriuso R, Dala D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005; 142:37–46.
- Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci 2011; 4:158–170.
- Dysken MW, Sano M, Asthana S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 2014; 311:33–44.
- Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA 2014; 311:682–691.
- Yang LP, Deeks ED. Dextromethorphan/quinidine: a review of its use in adults with pseudobulbar affect. Drugs 2015; 75:83–90.
- Cummings J, Lyketsos C, Tariot P, et al. Dextromethorphan/quinidine (AVP-923) efficacy and safety for treatment of agitation in persons with Alzheimer’s disease: results from a phase 2 study (NCT01584440) (S16.007). Neurology 2015; 84:S16.007.
- Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol 2003; 60:1385–1389.
- Moyer VA; US Preventive Services Task Force. Screening for cognitive impairment in older adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160:791–797.
- Nazir A, LaMantia M, Chodosh J, et al. Interaction between cognitive impairment and discharge destination and its effect on rehospitalization. J Am Geriatr Soc 2013; 61:1958–1963.
- Patel A, Parikh R, Howell E, Hsich E, Gorodeski E. Mini-Cog performance: a novel marker of risk among patients hospitalized for heart failure. J Am Coll Cardiol 4014; 63:A755.
- Dodson JA, Truong TT, Towle VR, Kerins G, Chaudhry SI. Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. Am J Med 2013; 126:120–126.
- Davis KK, Mintzer M, Dennison Himmelfarb CR, Hayat MJ, Rotman S, Allen J. Targeted intervention improves knowledge but not self-care or readmissions in heart failure patients with mild cognitive impairment. Eur J Heart Fail 2012; 14:1041–1049.
- Boustani MA, Sachs GA, Alder CA, et al. Implementing innovative models of dementia care: The Healthy Aging Brain Center. Aging Ment Health 2011; 15:13–22.
- Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385:2255–2263.
- DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014; 371:635–645.
- Bonten M, Bolkenbaas M, Huijts S, et al. Community acquired pneumonia immunisation trial in adults (CAPITA) (abstract). Pneumonia 2014; 3:95. Presented at 9th International Symposium on Pneumococci and Pneumococcal Diseases, 2014. Abstract 0541.
- Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2014; 63:822–825.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF-AHA guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Freeman JV, Yang J, Sung SH, Hlatky MA, Go AS. Effectiveness and safety of digoxin among contemporary adults with incident systolic heart failure. Circ Cardiovasc Qual Outcomes 2013; 6:525–533.
- Fonarow GC, Albert NM, Curtis AB, et al. Incremental reduction in risk of death associated with use of guideline-recommended therapies in patients with heart failure: a nested case-control analysis of IMPROVE-HF. J Am Heart Assoc 2012; 1:16–26.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S1–S45.
- DeFilippis AP, Young R, Carrubba CJ, et al. An analysis of calibration and discrimination among multiple cardiovascular risk scores in a modern multiethnic cohort. Ann Intern Med 2015; 162:266–275.
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Kostis JB, Davis BR, Cutler J, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA 1997; 278:212–216.
- Beckett NS, Peters R, Fletcher AE, et al; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med 2008; 358:1887–1898.
- Lloyd-Jones DM, Larson MG, Leip EP, et al; Framingham Heart Study. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation 2002; 106;3068–3072.
- Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012; 60:1487–1492.
- Pahor M, Guralnik JM, Ambrosius WT, et al; LIFE study investigators. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 2014; 311:2387–2396.
- Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med 1997; 336:1216–1222.
- Miller ER 3rd, Pastor-Barriuso R, Dala D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005; 142:37–46.
- Abner EL, Schmitt FA, Mendiondo MS, Marcum JL, Kryscio RJ. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci 2011; 4:158–170.
- Dysken MW, Sano M, Asthana S, et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: the TEAM-AD VA cooperative randomized trial. JAMA 2014; 311:33–44.
- Porsteinsson AP, Drye LT, Pollock BG, et al; CitAD Research group. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA 2014; 311:682–691.
- Yang LP, Deeks ED. Dextromethorphan/quinidine: a review of its use in adults with pseudobulbar affect. Drugs 2015; 75:83–90.
- Cummings J, Lyketsos C, Tariot P, et al. Dextromethorphan/quinidine (AVP-923) efficacy and safety for treatment of agitation in persons with Alzheimer’s disease: results from a phase 2 study (NCT01584440) (S16.007). Neurology 2015; 84:S16.007.
- Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol 2003; 60:1385–1389.
- Moyer VA; US Preventive Services Task Force. Screening for cognitive impairment in older adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160:791–797.
- Nazir A, LaMantia M, Chodosh J, et al. Interaction between cognitive impairment and discharge destination and its effect on rehospitalization. J Am Geriatr Soc 2013; 61:1958–1963.
- Patel A, Parikh R, Howell E, Hsich E, Gorodeski E. Mini-Cog performance: a novel marker of risk among patients hospitalized for heart failure. J Am Coll Cardiol 4014; 63:A755.
- Dodson JA, Truong TT, Towle VR, Kerins G, Chaudhry SI. Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. Am J Med 2013; 126:120–126.
- Davis KK, Mintzer M, Dennison Himmelfarb CR, Hayat MJ, Rotman S, Allen J. Targeted intervention improves knowledge but not self-care or readmissions in heart failure patients with mild cognitive impairment. Eur J Heart Fail 2012; 14:1041–1049.
- Boustani MA, Sachs GA, Alder CA, et al. Implementing innovative models of dementia care: The Healthy Aging Brain Center. Aging Ment Health 2011; 15:13–22.
- Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385:2255–2263.
KEY POINTS
- Vaccination costs will increase—with unclear added value—with new guidelines for influenza and pneumococcal vaccines.
- Multiple simultaneous interventions for heart failure have additive value. These are education, a beta-blocker, an angiotensin-converting enzyme inhibitor, and, in some, an aldosterone antagonist, anticoagulation for atrial fibrillation, and an implantable cardioverter-defibrillator or cardiac resynchronization therapy.
- Statin therapy should be intensified with an eye to goals of care and tolerability rather than a specific lipid goal.
- Exercise improves physical and mental health in all, including the elderly.
- Dementia still has no magic bullet. Selective serotonin reuptake inhibitors might help behavior issues, and vitamin E might bring modest cognitive improvement but with possible risk.
Does stenting of severe renal artery stenosis improve outomes compared with medical therapy alone?
No. In patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting offers no additional benefit when added to comprehensive medical therapy.
In these patients, renal artery stenting in addition to antihypertensive drug therapy can improve blood pressure control modestly but has no significant effect on outcomes such as adverse cardiovascular events and death. And because renal artery stenting carries a risk of complications, medical management should continue to be the first-line therapy.
RENAL ARTERY STENOSIS
Renal artery stenosis is a common form of peripheral artery disease. Atherosclerosis is the most common cause, but it can also be caused by fibromuscular dysplasia or vasculitis (eg, Takayasu arteritis). It is most often unilateral, but bilateral disease has also been reported.
The prevalence of atherosclerotic renal vascular disease in the US Medicare population is 0.5%, and 5.5% in those with chronic kidney disease.1 Furthermore, renal artery stenosis is found in 6.8% of adults over age 65.2 The prevalence increases with age and is higher in patients with hyperlipidemia, peripheral arterial disease, and hypertension. The prevalence of renal artery stenosis in patients with atherosclerotic disease and renal dysfunction is as high as 50%.3
Patients with peripheral artery disease may be five times more likely to develop renal artery stenosis than people without peripheral artery disease.4 Significant stenosis can result in resistant arterial hypertension, renal insufficiency, left ventricular hypertrophy, and congestive heart failure.5
Nephropathy due to renal artery stenosis is complex and is caused by hypoperfusion and chronic microatheroembolism. Renal artery stenosis leads to oxidative stress, inflammation, fibrosis in the stenotic kidney, and, over time, loss of kidney function. Hypoperfusion also leads to activation of the renin-angiotensin-aldosterone system, which plays a role in development of left ventricular hypertrophy.5,6
Adequate blood pressure control, goal-directed lipid-lowering therapy, smoking cessation, and other preventive measures are the foundation of management.
RENAL ARTERY STENOSIS AND HYPERTENSION
Renal artery stenosis is a cause of secondary hypertension. The stenosis decreases renal perfusion pressure, activating the release of renin and the production of angiotensin II, which in turn raises the blood pressure by two mechanisms (Figure 1): directly, by causing generalized vasoconstriction, and indirectly, by stimulating the release of aldosterone, which in turn increases the reabsorption of sodium and causes hypervolemia. These two mechanisms play a major role in renal vascular hypertension when renal artery stenosis is bilateral. In unilateral renal artery stenosis, pressure diuresis in the unaffected kidney compensates for the reabsorption of sodium in the affected kidney, keeping the blood pressure down. However, with time, the unaffected kidney will develop hypertensive nephropathy, and pressure diuresis will be lost.7,8 In addition, the activation of the renin-angiotensin-aldosterone system results in structural heart disease, such as left ventricular hypertrophy,5 and may shorten survival.
STENTING PLUS ANTIHYPERTENSIVE DRUG THERAPY
Because observational studies showed improvement in blood pressure control after endovascular stenting of atherosclerotic renal artery stenosis,9,10 this approach became a treatment option for uncontrolled hypertension in these patients. The 2005 joint guidelines of the American College of Cardiology and the American Heart Association11 considered percutaneous revascularization a reasonable option (level of evidence B) for patients who meet one of the following criteria:
- Hemodynamically significant stenosis and accelerated, resistant, or malignant hypertension, hypertension with an unexplained unilateral small kidney, or hypertension with intolerance to medication
- Renal artery stenosis and progressive chronic kidney disease with bilateral stenosis or stenosis in a solitary functioning kidney
- Hemodynamically significant stenosis and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema or unstable angina.11
However, no randomized study has shown a direct benefit of renal artery stenting on rates of cardiovascular events or renal function compared with drug therapy alone.
TRIALS OF STENTING VS MEDICAL THERAPY ALONE
Technical improvements have led to more widespread use of diagnostic and interventional endovascular tools for renal artery revascularization. Studies over the past 10 years examined the impact of stenting in patients with uncontrolled hypertension.
The STAR trial
In the Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial,9 patients with creatinine clearance less than 80 mL/min/1.73 m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy or medical therapy alone. The authors concluded that stenting had no effect on the progression of renal dysfunction but led to a small number of significant, procedure-related complications. The study was criticized for including patients with mild stenosis (< 50% stenosis) and for being underpowered for the primary end point.
The ASTRAL study
The Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) study10 was a similar comparison with similar results, showing no benefit from stenting with respect to renal function, systolic blood pressure control, cardiovascular events, or death.
HERCULES
The Herculink Elite Cobalt Chromium Renal Stent Trial to Demonstrate Efficacy and Safety (HERCULES)12 was a prospective multicenter study of the effects of renal artery stenting in 202 patients with significant renal artery stenosis and uncontrolled hypertension. It showed a reduction in systolic blood pressure from baseline (P < .0001). However, follow-up was only 9 months, which was insufficient to show a significant effect on long-term cardiovascular and cerebrovascular outcomes.
The CORAL trial
The Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial13 used more stringent definitions and longer follow-up. It randomized 947 patients to either stenting plus medical therapy or medical therapy alone. Patients had atherosclerotic renal artery stenosis, defined as stenosis of at least 80% or stenosis of 60% to 80% with a gradient of at least 20 mm Hg in the systolic pressure), and either systolic hypertension while taking two or more antihypertensive drugs or stage 3 or higher chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2 as calculated by the Modification of Diet in Renal Disease formula).
Participants were followed for 43 months to detect the occurrence of adverse cardiovascular and renal events. There was no significant difference in primary outcome between stenting plus drug therapy and drug therapy alone (35.1% and 35.8%, respectively; P = .58). However, stenting plus drug therapy was associated with modestly lower systolic pressures compared with drug therapy alone (−2.3 mm Hg, 95% confidence interval −4.4 to −0.2 mm Hg, P = .03).13 This study provided strong evidence that renal artery stenting offers no significant benefit to patients with moderately severe atherosclerotic renal artery stenosis, and that stenting may actually pose an unnecessary risk.
COMPLICATIONS OF RENAL ARTERY STENTING
Complications of renal artery stenting are a limiting factor compared with drug therapy alone, especially since the procedure offers no significant benefit in outcome. Procedural complication rates of 10% to 15% have been reported.9,10,12 The CORAL trial reported arterial dissection in 2.2%, branch-vessel occlusion in 1.2%, and distal embolization in 1.2% of patients undergoing stenting.13 Other reported complications have included stent misplacement requiring an additional stent, access-vessel damage, stent embolization, renal artery thrombosis or occlusion, and death.10,12
- Kalra PA, Guo H, Kausz AT, et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 68:293–301.
- Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451.
- Uzu T, Takeji M, Yamada N, et al. Prevalence and outcome of renal artery stenosis in atherosclerotic patients with renal dysfunction. Hypertens Res 2002; 25:537–542.
- Benjamin MM, Fazel P, Filardo G, Choi JW, Stoler RC. Prevalence of and risk factors of renal artery stenosis in patients with resistant hypertension. Am J Cardiol 2014; 113:687–690.
- Wu S, Polavarapu N, Stouffer GA. Left ventricular hypertrophy in patients with renal artery stenosis. Am J Med Sci 2006; 332:334–338.
- Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis 2009; 52:196–203.
- Black HR, Glickman MG, Schiff M Jr, Pingoud EG. Renovascular hypertension: pathophysiology, diagnosis, and treatment. Yale J Biol Med 1978; 51:635–654.
- Tobe SW, Burgess E, Lebel M. Atherosclerotic renovascular disease. Can J Cardiol 2006; 22:623–628.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of stent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by atherosclerotic ostial stenosis of the renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
No. In patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting offers no additional benefit when added to comprehensive medical therapy.
In these patients, renal artery stenting in addition to antihypertensive drug therapy can improve blood pressure control modestly but has no significant effect on outcomes such as adverse cardiovascular events and death. And because renal artery stenting carries a risk of complications, medical management should continue to be the first-line therapy.
RENAL ARTERY STENOSIS
Renal artery stenosis is a common form of peripheral artery disease. Atherosclerosis is the most common cause, but it can also be caused by fibromuscular dysplasia or vasculitis (eg, Takayasu arteritis). It is most often unilateral, but bilateral disease has also been reported.
The prevalence of atherosclerotic renal vascular disease in the US Medicare population is 0.5%, and 5.5% in those with chronic kidney disease.1 Furthermore, renal artery stenosis is found in 6.8% of adults over age 65.2 The prevalence increases with age and is higher in patients with hyperlipidemia, peripheral arterial disease, and hypertension. The prevalence of renal artery stenosis in patients with atherosclerotic disease and renal dysfunction is as high as 50%.3
Patients with peripheral artery disease may be five times more likely to develop renal artery stenosis than people without peripheral artery disease.4 Significant stenosis can result in resistant arterial hypertension, renal insufficiency, left ventricular hypertrophy, and congestive heart failure.5
Nephropathy due to renal artery stenosis is complex and is caused by hypoperfusion and chronic microatheroembolism. Renal artery stenosis leads to oxidative stress, inflammation, fibrosis in the stenotic kidney, and, over time, loss of kidney function. Hypoperfusion also leads to activation of the renin-angiotensin-aldosterone system, which plays a role in development of left ventricular hypertrophy.5,6
Adequate blood pressure control, goal-directed lipid-lowering therapy, smoking cessation, and other preventive measures are the foundation of management.
RENAL ARTERY STENOSIS AND HYPERTENSION
Renal artery stenosis is a cause of secondary hypertension. The stenosis decreases renal perfusion pressure, activating the release of renin and the production of angiotensin II, which in turn raises the blood pressure by two mechanisms (Figure 1): directly, by causing generalized vasoconstriction, and indirectly, by stimulating the release of aldosterone, which in turn increases the reabsorption of sodium and causes hypervolemia. These two mechanisms play a major role in renal vascular hypertension when renal artery stenosis is bilateral. In unilateral renal artery stenosis, pressure diuresis in the unaffected kidney compensates for the reabsorption of sodium in the affected kidney, keeping the blood pressure down. However, with time, the unaffected kidney will develop hypertensive nephropathy, and pressure diuresis will be lost.7,8 In addition, the activation of the renin-angiotensin-aldosterone system results in structural heart disease, such as left ventricular hypertrophy,5 and may shorten survival.
STENTING PLUS ANTIHYPERTENSIVE DRUG THERAPY
Because observational studies showed improvement in blood pressure control after endovascular stenting of atherosclerotic renal artery stenosis,9,10 this approach became a treatment option for uncontrolled hypertension in these patients. The 2005 joint guidelines of the American College of Cardiology and the American Heart Association11 considered percutaneous revascularization a reasonable option (level of evidence B) for patients who meet one of the following criteria:
- Hemodynamically significant stenosis and accelerated, resistant, or malignant hypertension, hypertension with an unexplained unilateral small kidney, or hypertension with intolerance to medication
- Renal artery stenosis and progressive chronic kidney disease with bilateral stenosis or stenosis in a solitary functioning kidney
- Hemodynamically significant stenosis and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema or unstable angina.11
However, no randomized study has shown a direct benefit of renal artery stenting on rates of cardiovascular events or renal function compared with drug therapy alone.
TRIALS OF STENTING VS MEDICAL THERAPY ALONE
Technical improvements have led to more widespread use of diagnostic and interventional endovascular tools for renal artery revascularization. Studies over the past 10 years examined the impact of stenting in patients with uncontrolled hypertension.
The STAR trial
In the Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial,9 patients with creatinine clearance less than 80 mL/min/1.73 m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy or medical therapy alone. The authors concluded that stenting had no effect on the progression of renal dysfunction but led to a small number of significant, procedure-related complications. The study was criticized for including patients with mild stenosis (< 50% stenosis) and for being underpowered for the primary end point.
The ASTRAL study
The Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) study10 was a similar comparison with similar results, showing no benefit from stenting with respect to renal function, systolic blood pressure control, cardiovascular events, or death.
HERCULES
The Herculink Elite Cobalt Chromium Renal Stent Trial to Demonstrate Efficacy and Safety (HERCULES)12 was a prospective multicenter study of the effects of renal artery stenting in 202 patients with significant renal artery stenosis and uncontrolled hypertension. It showed a reduction in systolic blood pressure from baseline (P < .0001). However, follow-up was only 9 months, which was insufficient to show a significant effect on long-term cardiovascular and cerebrovascular outcomes.
The CORAL trial
The Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial13 used more stringent definitions and longer follow-up. It randomized 947 patients to either stenting plus medical therapy or medical therapy alone. Patients had atherosclerotic renal artery stenosis, defined as stenosis of at least 80% or stenosis of 60% to 80% with a gradient of at least 20 mm Hg in the systolic pressure), and either systolic hypertension while taking two or more antihypertensive drugs or stage 3 or higher chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2 as calculated by the Modification of Diet in Renal Disease formula).
Participants were followed for 43 months to detect the occurrence of adverse cardiovascular and renal events. There was no significant difference in primary outcome between stenting plus drug therapy and drug therapy alone (35.1% and 35.8%, respectively; P = .58). However, stenting plus drug therapy was associated with modestly lower systolic pressures compared with drug therapy alone (−2.3 mm Hg, 95% confidence interval −4.4 to −0.2 mm Hg, P = .03).13 This study provided strong evidence that renal artery stenting offers no significant benefit to patients with moderately severe atherosclerotic renal artery stenosis, and that stenting may actually pose an unnecessary risk.
COMPLICATIONS OF RENAL ARTERY STENTING
Complications of renal artery stenting are a limiting factor compared with drug therapy alone, especially since the procedure offers no significant benefit in outcome. Procedural complication rates of 10% to 15% have been reported.9,10,12 The CORAL trial reported arterial dissection in 2.2%, branch-vessel occlusion in 1.2%, and distal embolization in 1.2% of patients undergoing stenting.13 Other reported complications have included stent misplacement requiring an additional stent, access-vessel damage, stent embolization, renal artery thrombosis or occlusion, and death.10,12
No. In patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting offers no additional benefit when added to comprehensive medical therapy.
In these patients, renal artery stenting in addition to antihypertensive drug therapy can improve blood pressure control modestly but has no significant effect on outcomes such as adverse cardiovascular events and death. And because renal artery stenting carries a risk of complications, medical management should continue to be the first-line therapy.
RENAL ARTERY STENOSIS
Renal artery stenosis is a common form of peripheral artery disease. Atherosclerosis is the most common cause, but it can also be caused by fibromuscular dysplasia or vasculitis (eg, Takayasu arteritis). It is most often unilateral, but bilateral disease has also been reported.
The prevalence of atherosclerotic renal vascular disease in the US Medicare population is 0.5%, and 5.5% in those with chronic kidney disease.1 Furthermore, renal artery stenosis is found in 6.8% of adults over age 65.2 The prevalence increases with age and is higher in patients with hyperlipidemia, peripheral arterial disease, and hypertension. The prevalence of renal artery stenosis in patients with atherosclerotic disease and renal dysfunction is as high as 50%.3
Patients with peripheral artery disease may be five times more likely to develop renal artery stenosis than people without peripheral artery disease.4 Significant stenosis can result in resistant arterial hypertension, renal insufficiency, left ventricular hypertrophy, and congestive heart failure.5
Nephropathy due to renal artery stenosis is complex and is caused by hypoperfusion and chronic microatheroembolism. Renal artery stenosis leads to oxidative stress, inflammation, fibrosis in the stenotic kidney, and, over time, loss of kidney function. Hypoperfusion also leads to activation of the renin-angiotensin-aldosterone system, which plays a role in development of left ventricular hypertrophy.5,6
Adequate blood pressure control, goal-directed lipid-lowering therapy, smoking cessation, and other preventive measures are the foundation of management.
RENAL ARTERY STENOSIS AND HYPERTENSION
Renal artery stenosis is a cause of secondary hypertension. The stenosis decreases renal perfusion pressure, activating the release of renin and the production of angiotensin II, which in turn raises the blood pressure by two mechanisms (Figure 1): directly, by causing generalized vasoconstriction, and indirectly, by stimulating the release of aldosterone, which in turn increases the reabsorption of sodium and causes hypervolemia. These two mechanisms play a major role in renal vascular hypertension when renal artery stenosis is bilateral. In unilateral renal artery stenosis, pressure diuresis in the unaffected kidney compensates for the reabsorption of sodium in the affected kidney, keeping the blood pressure down. However, with time, the unaffected kidney will develop hypertensive nephropathy, and pressure diuresis will be lost.7,8 In addition, the activation of the renin-angiotensin-aldosterone system results in structural heart disease, such as left ventricular hypertrophy,5 and may shorten survival.
STENTING PLUS ANTIHYPERTENSIVE DRUG THERAPY
Because observational studies showed improvement in blood pressure control after endovascular stenting of atherosclerotic renal artery stenosis,9,10 this approach became a treatment option for uncontrolled hypertension in these patients. The 2005 joint guidelines of the American College of Cardiology and the American Heart Association11 considered percutaneous revascularization a reasonable option (level of evidence B) for patients who meet one of the following criteria:
- Hemodynamically significant stenosis and accelerated, resistant, or malignant hypertension, hypertension with an unexplained unilateral small kidney, or hypertension with intolerance to medication
- Renal artery stenosis and progressive chronic kidney disease with bilateral stenosis or stenosis in a solitary functioning kidney
- Hemodynamically significant stenosis and recurrent, unexplained congestive heart failure or sudden, unexplained pulmonary edema or unstable angina.11
However, no randomized study has shown a direct benefit of renal artery stenting on rates of cardiovascular events or renal function compared with drug therapy alone.
TRIALS OF STENTING VS MEDICAL THERAPY ALONE
Technical improvements have led to more widespread use of diagnostic and interventional endovascular tools for renal artery revascularization. Studies over the past 10 years examined the impact of stenting in patients with uncontrolled hypertension.
The STAR trial
In the Stent Placement and Blood Pressure and Lipid-lowering for the Prevention of Progression of Renal Dysfunction Caused by Atherosclerotic Ostial Stenosis of the Renal Artery (STAR) trial,9 patients with creatinine clearance less than 80 mL/min/1.73 m2, renal artery stenosis greater than 50%, and well-controlled blood pressure were randomized to either renal artery stenting plus medical therapy or medical therapy alone. The authors concluded that stenting had no effect on the progression of renal dysfunction but led to a small number of significant, procedure-related complications. The study was criticized for including patients with mild stenosis (< 50% stenosis) and for being underpowered for the primary end point.
The ASTRAL study
The Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) study10 was a similar comparison with similar results, showing no benefit from stenting with respect to renal function, systolic blood pressure control, cardiovascular events, or death.
HERCULES
The Herculink Elite Cobalt Chromium Renal Stent Trial to Demonstrate Efficacy and Safety (HERCULES)12 was a prospective multicenter study of the effects of renal artery stenting in 202 patients with significant renal artery stenosis and uncontrolled hypertension. It showed a reduction in systolic blood pressure from baseline (P < .0001). However, follow-up was only 9 months, which was insufficient to show a significant effect on long-term cardiovascular and cerebrovascular outcomes.
The CORAL trial
The Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial13 used more stringent definitions and longer follow-up. It randomized 947 patients to either stenting plus medical therapy or medical therapy alone. Patients had atherosclerotic renal artery stenosis, defined as stenosis of at least 80% or stenosis of 60% to 80% with a gradient of at least 20 mm Hg in the systolic pressure), and either systolic hypertension while taking two or more antihypertensive drugs or stage 3 or higher chronic kidney disease (glomerular filtration rate < 60 mL/min/1.73 m2 as calculated by the Modification of Diet in Renal Disease formula).
Participants were followed for 43 months to detect the occurrence of adverse cardiovascular and renal events. There was no significant difference in primary outcome between stenting plus drug therapy and drug therapy alone (35.1% and 35.8%, respectively; P = .58). However, stenting plus drug therapy was associated with modestly lower systolic pressures compared with drug therapy alone (−2.3 mm Hg, 95% confidence interval −4.4 to −0.2 mm Hg, P = .03).13 This study provided strong evidence that renal artery stenting offers no significant benefit to patients with moderately severe atherosclerotic renal artery stenosis, and that stenting may actually pose an unnecessary risk.
COMPLICATIONS OF RENAL ARTERY STENTING
Complications of renal artery stenting are a limiting factor compared with drug therapy alone, especially since the procedure offers no significant benefit in outcome. Procedural complication rates of 10% to 15% have been reported.9,10,12 The CORAL trial reported arterial dissection in 2.2%, branch-vessel occlusion in 1.2%, and distal embolization in 1.2% of patients undergoing stenting.13 Other reported complications have included stent misplacement requiring an additional stent, access-vessel damage, stent embolization, renal artery thrombosis or occlusion, and death.10,12
- Kalra PA, Guo H, Kausz AT, et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 68:293–301.
- Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451.
- Uzu T, Takeji M, Yamada N, et al. Prevalence and outcome of renal artery stenosis in atherosclerotic patients with renal dysfunction. Hypertens Res 2002; 25:537–542.
- Benjamin MM, Fazel P, Filardo G, Choi JW, Stoler RC. Prevalence of and risk factors of renal artery stenosis in patients with resistant hypertension. Am J Cardiol 2014; 113:687–690.
- Wu S, Polavarapu N, Stouffer GA. Left ventricular hypertrophy in patients with renal artery stenosis. Am J Med Sci 2006; 332:334–338.
- Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis 2009; 52:196–203.
- Black HR, Glickman MG, Schiff M Jr, Pingoud EG. Renovascular hypertension: pathophysiology, diagnosis, and treatment. Yale J Biol Med 1978; 51:635–654.
- Tobe SW, Burgess E, Lebel M. Atherosclerotic renovascular disease. Can J Cardiol 2006; 22:623–628.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of stent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by atherosclerotic ostial stenosis of the renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
- Kalra PA, Guo H, Kausz AT, et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int 2005; 68:293–301.
- Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg 2002; 36:443–451.
- Uzu T, Takeji M, Yamada N, et al. Prevalence and outcome of renal artery stenosis in atherosclerotic patients with renal dysfunction. Hypertens Res 2002; 25:537–542.
- Benjamin MM, Fazel P, Filardo G, Choi JW, Stoler RC. Prevalence of and risk factors of renal artery stenosis in patients with resistant hypertension. Am J Cardiol 2014; 113:687–690.
- Wu S, Polavarapu N, Stouffer GA. Left ventricular hypertrophy in patients with renal artery stenosis. Am J Med Sci 2006; 332:334–338.
- Lerman LO, Textor SC, Grande JP. Mechanisms of tissue injury in renal artery stenosis: ischemia and beyond. Prog Cardiovasc Dis 2009; 52:196–203.
- Black HR, Glickman MG, Schiff M Jr, Pingoud EG. Renovascular hypertension: pathophysiology, diagnosis, and treatment. Yale J Biol Med 1978; 51:635–654.
- Tobe SW, Burgess E, Lebel M. Atherosclerotic renovascular disease. Can J Cardiol 2006; 22:623–628.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of stent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by atherosclerotic ostial stenosis of the renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
Stenting may benefit select patients with severe renal artery stenosis
In their article in this issue of the Cleveland Clinic Journal of Medicine, Kabach et al answer no to the question of whether stenting of severe renal artery stenosis improves outcomes compared with medical therapy alone.1 They review the findings of four key studies2–5 published between 2003 and 2014 and conclude that, in patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting with medical therapy can improve blood pressure control but has no significant impact on cardiovascular or mortality outcomes.1
Furthermore, the authors state that in view of the risk of complications associated with stenting, medical management should continue to be the first-line therapy.1 Indeed, the ASTRAL study (Angioplasty and Stenting for Renal Artery Lesions) investigators found substantial risks without evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease.3
Nevertheless, I believe that this procedure may benefit certain patients.
MAYO CLINIC COHORT STUDY
In 2008, our group at Mayo Clinic Health system in Eau Claire, Wisconsin, published the results of a prospective cohort study in 26 patients with renal artery stenosis and chronic kidney disease who presented with rapidly worsening renal failure (defined as an increase in serum creatinine of ≥ 25%) while receiving an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB).6,7
These drugs—inhibitors of the renin-angiotensin-aldosterone system—slow the progression of chronic kidney disease but can acutely worsen renal function, especially in patients with renal artery stenosis, and withdrawing them in this situation was the focus of our study.
The patients (10 men and 16 women) ranged in age from 63 to 87 (mean age 75.3).
At enrollment, the ACE inhibitors and ARBs were discontinued, standard nephrologic care was applied, and the glomerular filtration rate (estimated by the Modification of Diet in Renal Disease Study equation) was monitored. After at least 2 weeks, percutaneous renal angioplasty with stent placement was considered if the patient met any of the following criteria:
- Persistence of renal failure
- Flash pulmonary edema
- Uncontrolled hypertension despite the use of at least three antihypertensive medications.
Nine patients underwent percutaneous angioplasty and stenting and 17 did not. The procedure was done on one renal artery in 8 patients and both renal arteries in 1. Indications for the procedure were recent worsening of renal failure in 8 patients and recent worsening renal failure together with symptomatic flash pulmonary edema in 1 patient. (Flash pulmonary edema is the only class I recommendation for percutaneous renal angioplasty in the 2006 joint guidelines of the American College of Cardiology and the American Heart Association.8) As noted above, all the patients were experiencing acute exacerbation of chronic kidney disease at the time.
We found clear evidence of additional improved and sustained renal function in the patients who underwent percutaneous renal angioplasty and stenting compared with the patients who did not (Figures 1 and 2).6,7
In an editorial9 following publication of the ASTRAL study, our group reported a subsequent 82-month analysis of our 26-patient cohort completed in June 2009. In the 7 surviving patients who had undergone percutaneous renal angioplasty and stenting, the estimated glomerular filtration rate had increased from 27.4 mL/min/1.73 m2 (± 12.7, range 11–47) at baseline to 50.3 (± 21.7, range 23–68) (P = .018) after 46.9 months.
PATIENT NUMBER 13
To illustrate how percutaneous renal angioplasty and stenting can reverse recently worsening renal failure in renal artery stenosis, I would like to discuss in greater detail a patient from our two previous reports.6,7
Patient 13, a 67-year-old woman with hypertension, was referred to our nephrology service in September 2004 to consider starting hemodialysis for symptomatic renal failure. Her serum creatinine had increased to 3.4 mg/dL from a previous baseline level of 2.0 mg/dL, and she also had worsening anemia and hyperkalemia. She had been taking lisinopril 10 mg/day for the last 12 months. Magnetic resonance angiography revealed high-grade (> 95%) bilateral renal artery stenosis with an atrophic left kidney.
Lisinopril was promptly discontinued, and within 2 weeks her serum creatinine level had decreased by more than 0.5 mg/dL. In mid-November 2004, she underwent right renal artery angioplasty with stent placement. After that, her serum creatinine decreased further, and 3 months later it had dropped to 1.6 mg/dL. The value continued to improve, with the lowest measurement of 1.1 mg/dL, equivalent to an estimated glomerular filtration rate of 51 mL/min/1.73 m2. This was in May 2006, 19 months after stopping lisinopril and 17 months after angioplasty and stenting. The last available serum creatinine level (August 2006) was 1.2 mg/dL, equivalent to an estimated glomerular filtration rate of 45 mL/min/1.73 m2 (Figure 1). Unfortunately, the patient died of metastatic lung cancer in December of that year.
Also of note, the patient who underwent angioplasty because of recurrent flash pulmonary edema had no recurrences of it afterward.
We concluded that, in patients with hemodynamically significant renal artery stenosis presenting with acutely worsening renal failure, renal angioplasty with stenting has the potential to reverse renal failure, improve blood pressure control, and stop flash pulmonary edema.6–8
Notably, all patients in our study who underwent renal angioplasty with stenting had new-onset acute renal injury as defined by an increase in the baseline serum creatinine of more than 25% during the 3 months before stent placement.6–8 Patients in the STAR,2 HERCULES,4 and CORAL5 studies had renal artery stenosis but otherwise stable chronic kidney disease at the time of enrollment. In the ASTRAL study,3 12% of the patients had acute kidney injury on study enrollment, defined as an increase in the serum creatinine level of more than 20% or of more than 1.13 mg/dL.3
While we strongly agree with aggressive yet monitored medical therapy for patients with hemodynamically significant renal artery stenosis, I posit that selected patients do indeed derive significant clinical benefits from renal angioplasty and stenting. Our group’s prospective individual-patient-level data support the paradigm that angioplasty with stenting is useful in patients with renal artery stenosis who experience “acute-on-chronic” kidney disease.
The pathophysiology of renal artery stenosis and the progression of chronic kidney disease are complex, and many factors affect patient outcomes and response to treatment. Thus, the message is that treatment of severe renal artery stenosis must be individualized.9–11 No one treatment fits all.10,11
- Kabach A, Agha OQ, Baibars M, Alraiyes AH, Alraies MC. Does stenting of severe renal artery stenosis improve outcomes compared with medical therapy alone? Cleve Clin J Med 2015; 82:491–494.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of STent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by Atherosclerotic ostial stenosis of the Renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Jaff MR, Bates M, Sullivan T, et al; HERCULES Investigators. Significant reduction in systolic blood pressure following renal artery stenting in patients with uncontrolled hypertension: results from the HERCULES trial. Catheter Cardiovasc Interv 2012; 80:343–350.
- Cooper CJ, Murphy TP, Cutlip DE, et al; CORAL Investigators. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014; 370:13–22.
- Onuigbo MAC, Onuigbo NTC. Worsening renal failure in older chronic kidney disease patients with renal artery stenosis concurrently on renin angiotensin aldosterone system blockade: a prospective 50-month Mayo Health System clinic analysis. QJM 2008; 101:519–527.
- Onuigbo MA, Onuigbo NT. Renal failure and concurrent RAAS blockade in older CKD patients with renal artery stenosis: an extended Mayo Clinic prospective 63-month experience. Ren Fail 2008; 30:363–371.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
- Onuigbo M, Frenandes R, Nijhawan V. The ASTRAL trial results revisited—to stent or not to stent in renal artery stenosis? QJM 2010; 103:357.
- Singh M, Kovacs DF, Singh A, Dhaliwal P, Khosla S. ACE inhibition and renal artery stenosis: what lessons have we learnt? A 21st century perspective. In: Onuigbo MAC, ed. ACE inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. Volume 2. New York, NY: NOVA Publishers; 2013:203–218.
- Onuigbo MA, Onuigbo C, Onuigbo V, et al. The CKD enigma, reengineering CKD care, narrowing asymmetric information and confronting ethicomedicinomics in CKD care: the introduction of the new 'CKD express©' IT software program. In: Onuigbo MAC, ed. ACE Inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. Volume 1. New York, NY: NOVA Publishers; 2013: 41–56.
In their article in this issue of the Cleveland Clinic Journal of Medicine, Kabach et al answer no to the question of whether stenting of severe renal artery stenosis improves outcomes compared with medical therapy alone.1 They review the findings of four key studies2–5 published between 2003 and 2014 and conclude that, in patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting with medical therapy can improve blood pressure control but has no significant impact on cardiovascular or mortality outcomes.1
Furthermore, the authors state that in view of the risk of complications associated with stenting, medical management should continue to be the first-line therapy.1 Indeed, the ASTRAL study (Angioplasty and Stenting for Renal Artery Lesions) investigators found substantial risks without evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease.3
Nevertheless, I believe that this procedure may benefit certain patients.
MAYO CLINIC COHORT STUDY
In 2008, our group at Mayo Clinic Health system in Eau Claire, Wisconsin, published the results of a prospective cohort study in 26 patients with renal artery stenosis and chronic kidney disease who presented with rapidly worsening renal failure (defined as an increase in serum creatinine of ≥ 25%) while receiving an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB).6,7
These drugs—inhibitors of the renin-angiotensin-aldosterone system—slow the progression of chronic kidney disease but can acutely worsen renal function, especially in patients with renal artery stenosis, and withdrawing them in this situation was the focus of our study.
The patients (10 men and 16 women) ranged in age from 63 to 87 (mean age 75.3).
At enrollment, the ACE inhibitors and ARBs were discontinued, standard nephrologic care was applied, and the glomerular filtration rate (estimated by the Modification of Diet in Renal Disease Study equation) was monitored. After at least 2 weeks, percutaneous renal angioplasty with stent placement was considered if the patient met any of the following criteria:
- Persistence of renal failure
- Flash pulmonary edema
- Uncontrolled hypertension despite the use of at least three antihypertensive medications.
Nine patients underwent percutaneous angioplasty and stenting and 17 did not. The procedure was done on one renal artery in 8 patients and both renal arteries in 1. Indications for the procedure were recent worsening of renal failure in 8 patients and recent worsening renal failure together with symptomatic flash pulmonary edema in 1 patient. (Flash pulmonary edema is the only class I recommendation for percutaneous renal angioplasty in the 2006 joint guidelines of the American College of Cardiology and the American Heart Association.8) As noted above, all the patients were experiencing acute exacerbation of chronic kidney disease at the time.
We found clear evidence of additional improved and sustained renal function in the patients who underwent percutaneous renal angioplasty and stenting compared with the patients who did not (Figures 1 and 2).6,7
In an editorial9 following publication of the ASTRAL study, our group reported a subsequent 82-month analysis of our 26-patient cohort completed in June 2009. In the 7 surviving patients who had undergone percutaneous renal angioplasty and stenting, the estimated glomerular filtration rate had increased from 27.4 mL/min/1.73 m2 (± 12.7, range 11–47) at baseline to 50.3 (± 21.7, range 23–68) (P = .018) after 46.9 months.
PATIENT NUMBER 13
To illustrate how percutaneous renal angioplasty and stenting can reverse recently worsening renal failure in renal artery stenosis, I would like to discuss in greater detail a patient from our two previous reports.6,7
Patient 13, a 67-year-old woman with hypertension, was referred to our nephrology service in September 2004 to consider starting hemodialysis for symptomatic renal failure. Her serum creatinine had increased to 3.4 mg/dL from a previous baseline level of 2.0 mg/dL, and she also had worsening anemia and hyperkalemia. She had been taking lisinopril 10 mg/day for the last 12 months. Magnetic resonance angiography revealed high-grade (> 95%) bilateral renal artery stenosis with an atrophic left kidney.
Lisinopril was promptly discontinued, and within 2 weeks her serum creatinine level had decreased by more than 0.5 mg/dL. In mid-November 2004, she underwent right renal artery angioplasty with stent placement. After that, her serum creatinine decreased further, and 3 months later it had dropped to 1.6 mg/dL. The value continued to improve, with the lowest measurement of 1.1 mg/dL, equivalent to an estimated glomerular filtration rate of 51 mL/min/1.73 m2. This was in May 2006, 19 months after stopping lisinopril and 17 months after angioplasty and stenting. The last available serum creatinine level (August 2006) was 1.2 mg/dL, equivalent to an estimated glomerular filtration rate of 45 mL/min/1.73 m2 (Figure 1). Unfortunately, the patient died of metastatic lung cancer in December of that year.
Also of note, the patient who underwent angioplasty because of recurrent flash pulmonary edema had no recurrences of it afterward.
We concluded that, in patients with hemodynamically significant renal artery stenosis presenting with acutely worsening renal failure, renal angioplasty with stenting has the potential to reverse renal failure, improve blood pressure control, and stop flash pulmonary edema.6–8
Notably, all patients in our study who underwent renal angioplasty with stenting had new-onset acute renal injury as defined by an increase in the baseline serum creatinine of more than 25% during the 3 months before stent placement.6–8 Patients in the STAR,2 HERCULES,4 and CORAL5 studies had renal artery stenosis but otherwise stable chronic kidney disease at the time of enrollment. In the ASTRAL study,3 12% of the patients had acute kidney injury on study enrollment, defined as an increase in the serum creatinine level of more than 20% or of more than 1.13 mg/dL.3
While we strongly agree with aggressive yet monitored medical therapy for patients with hemodynamically significant renal artery stenosis, I posit that selected patients do indeed derive significant clinical benefits from renal angioplasty and stenting. Our group’s prospective individual-patient-level data support the paradigm that angioplasty with stenting is useful in patients with renal artery stenosis who experience “acute-on-chronic” kidney disease.
The pathophysiology of renal artery stenosis and the progression of chronic kidney disease are complex, and many factors affect patient outcomes and response to treatment. Thus, the message is that treatment of severe renal artery stenosis must be individualized.9–11 No one treatment fits all.10,11
In their article in this issue of the Cleveland Clinic Journal of Medicine, Kabach et al answer no to the question of whether stenting of severe renal artery stenosis improves outcomes compared with medical therapy alone.1 They review the findings of four key studies2–5 published between 2003 and 2014 and conclude that, in patients with severe atherosclerotic renal artery stenosis and hypertension or chronic kidney disease, renal artery stenting with medical therapy can improve blood pressure control but has no significant impact on cardiovascular or mortality outcomes.1
Furthermore, the authors state that in view of the risk of complications associated with stenting, medical management should continue to be the first-line therapy.1 Indeed, the ASTRAL study (Angioplasty and Stenting for Renal Artery Lesions) investigators found substantial risks without evidence of a worthwhile clinical benefit from revascularization in patients with atherosclerotic renovascular disease.3
Nevertheless, I believe that this procedure may benefit certain patients.
MAYO CLINIC COHORT STUDY
In 2008, our group at Mayo Clinic Health system in Eau Claire, Wisconsin, published the results of a prospective cohort study in 26 patients with renal artery stenosis and chronic kidney disease who presented with rapidly worsening renal failure (defined as an increase in serum creatinine of ≥ 25%) while receiving an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin II receptor blocker (ARB).6,7
These drugs—inhibitors of the renin-angiotensin-aldosterone system—slow the progression of chronic kidney disease but can acutely worsen renal function, especially in patients with renal artery stenosis, and withdrawing them in this situation was the focus of our study.
The patients (10 men and 16 women) ranged in age from 63 to 87 (mean age 75.3).
At enrollment, the ACE inhibitors and ARBs were discontinued, standard nephrologic care was applied, and the glomerular filtration rate (estimated by the Modification of Diet in Renal Disease Study equation) was monitored. After at least 2 weeks, percutaneous renal angioplasty with stent placement was considered if the patient met any of the following criteria:
- Persistence of renal failure
- Flash pulmonary edema
- Uncontrolled hypertension despite the use of at least three antihypertensive medications.
Nine patients underwent percutaneous angioplasty and stenting and 17 did not. The procedure was done on one renal artery in 8 patients and both renal arteries in 1. Indications for the procedure were recent worsening of renal failure in 8 patients and recent worsening renal failure together with symptomatic flash pulmonary edema in 1 patient. (Flash pulmonary edema is the only class I recommendation for percutaneous renal angioplasty in the 2006 joint guidelines of the American College of Cardiology and the American Heart Association.8) As noted above, all the patients were experiencing acute exacerbation of chronic kidney disease at the time.
We found clear evidence of additional improved and sustained renal function in the patients who underwent percutaneous renal angioplasty and stenting compared with the patients who did not (Figures 1 and 2).6,7
In an editorial9 following publication of the ASTRAL study, our group reported a subsequent 82-month analysis of our 26-patient cohort completed in June 2009. In the 7 surviving patients who had undergone percutaneous renal angioplasty and stenting, the estimated glomerular filtration rate had increased from 27.4 mL/min/1.73 m2 (± 12.7, range 11–47) at baseline to 50.3 (± 21.7, range 23–68) (P = .018) after 46.9 months.
PATIENT NUMBER 13
To illustrate how percutaneous renal angioplasty and stenting can reverse recently worsening renal failure in renal artery stenosis, I would like to discuss in greater detail a patient from our two previous reports.6,7
Patient 13, a 67-year-old woman with hypertension, was referred to our nephrology service in September 2004 to consider starting hemodialysis for symptomatic renal failure. Her serum creatinine had increased to 3.4 mg/dL from a previous baseline level of 2.0 mg/dL, and she also had worsening anemia and hyperkalemia. She had been taking lisinopril 10 mg/day for the last 12 months. Magnetic resonance angiography revealed high-grade (> 95%) bilateral renal artery stenosis with an atrophic left kidney.
Lisinopril was promptly discontinued, and within 2 weeks her serum creatinine level had decreased by more than 0.5 mg/dL. In mid-November 2004, she underwent right renal artery angioplasty with stent placement. After that, her serum creatinine decreased further, and 3 months later it had dropped to 1.6 mg/dL. The value continued to improve, with the lowest measurement of 1.1 mg/dL, equivalent to an estimated glomerular filtration rate of 51 mL/min/1.73 m2. This was in May 2006, 19 months after stopping lisinopril and 17 months after angioplasty and stenting. The last available serum creatinine level (August 2006) was 1.2 mg/dL, equivalent to an estimated glomerular filtration rate of 45 mL/min/1.73 m2 (Figure 1). Unfortunately, the patient died of metastatic lung cancer in December of that year.
Also of note, the patient who underwent angioplasty because of recurrent flash pulmonary edema had no recurrences of it afterward.
We concluded that, in patients with hemodynamically significant renal artery stenosis presenting with acutely worsening renal failure, renal angioplasty with stenting has the potential to reverse renal failure, improve blood pressure control, and stop flash pulmonary edema.6–8
Notably, all patients in our study who underwent renal angioplasty with stenting had new-onset acute renal injury as defined by an increase in the baseline serum creatinine of more than 25% during the 3 months before stent placement.6–8 Patients in the STAR,2 HERCULES,4 and CORAL5 studies had renal artery stenosis but otherwise stable chronic kidney disease at the time of enrollment. In the ASTRAL study,3 12% of the patients had acute kidney injury on study enrollment, defined as an increase in the serum creatinine level of more than 20% or of more than 1.13 mg/dL.3
While we strongly agree with aggressive yet monitored medical therapy for patients with hemodynamically significant renal artery stenosis, I posit that selected patients do indeed derive significant clinical benefits from renal angioplasty and stenting. Our group’s prospective individual-patient-level data support the paradigm that angioplasty with stenting is useful in patients with renal artery stenosis who experience “acute-on-chronic” kidney disease.
The pathophysiology of renal artery stenosis and the progression of chronic kidney disease are complex, and many factors affect patient outcomes and response to treatment. Thus, the message is that treatment of severe renal artery stenosis must be individualized.9–11 No one treatment fits all.10,11
- Kabach A, Agha OQ, Baibars M, Alraiyes AH, Alraies MC. Does stenting of severe renal artery stenosis improve outcomes compared with medical therapy alone? Cleve Clin J Med 2015; 82:491–494.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of STent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by Atherosclerotic ostial stenosis of the Renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Jaff MR, Bates M, Sullivan T, et al; HERCULES Investigators. Significant reduction in systolic blood pressure following renal artery stenting in patients with uncontrolled hypertension: results from the HERCULES trial. Catheter Cardiovasc Interv 2012; 80:343–350.
- Cooper CJ, Murphy TP, Cutlip DE, et al; CORAL Investigators. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014; 370:13–22.
- Onuigbo MAC, Onuigbo NTC. Worsening renal failure in older chronic kidney disease patients with renal artery stenosis concurrently on renin angiotensin aldosterone system blockade: a prospective 50-month Mayo Health System clinic analysis. QJM 2008; 101:519–527.
- Onuigbo MA, Onuigbo NT. Renal failure and concurrent RAAS blockade in older CKD patients with renal artery stenosis: an extended Mayo Clinic prospective 63-month experience. Ren Fail 2008; 30:363–371.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
- Onuigbo M, Frenandes R, Nijhawan V. The ASTRAL trial results revisited—to stent or not to stent in renal artery stenosis? QJM 2010; 103:357.
- Singh M, Kovacs DF, Singh A, Dhaliwal P, Khosla S. ACE inhibition and renal artery stenosis: what lessons have we learnt? A 21st century perspective. In: Onuigbo MAC, ed. ACE inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. Volume 2. New York, NY: NOVA Publishers; 2013:203–218.
- Onuigbo MA, Onuigbo C, Onuigbo V, et al. The CKD enigma, reengineering CKD care, narrowing asymmetric information and confronting ethicomedicinomics in CKD care: the introduction of the new 'CKD express©' IT software program. In: Onuigbo MAC, ed. ACE Inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. Volume 1. New York, NY: NOVA Publishers; 2013: 41–56.
- Kabach A, Agha OQ, Baibars M, Alraiyes AH, Alraies MC. Does stenting of severe renal artery stenosis improve outcomes compared with medical therapy alone? Cleve Clin J Med 2015; 82:491–494.
- Bax L, Mali WP, Buskens E, et al; STAR Study Group. The benefit of STent placement and blood pressure and lipid-lowering for the prevention of progression of renal dysfunction caused by Atherosclerotic ostial stenosis of the Renal artery. The STAR-study: rationale and study design. J Nephrol 2003; 16:807–812.
- ASTRAL Investigators; Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med 2009; 361:1953–1962.
- Jaff MR, Bates M, Sullivan T, et al; HERCULES Investigators. Significant reduction in systolic blood pressure following renal artery stenting in patients with uncontrolled hypertension: results from the HERCULES trial. Catheter Cardiovasc Interv 2012; 80:343–350.
- Cooper CJ, Murphy TP, Cutlip DE, et al; CORAL Investigators. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014; 370:13–22.
- Onuigbo MAC, Onuigbo NTC. Worsening renal failure in older chronic kidney disease patients with renal artery stenosis concurrently on renin angiotensin aldosterone system blockade: a prospective 50-month Mayo Health System clinic analysis. QJM 2008; 101:519–527.
- Onuigbo MA, Onuigbo NT. Renal failure and concurrent RAAS blockade in older CKD patients with renal artery stenosis: an extended Mayo Clinic prospective 63-month experience. Ren Fail 2008; 30:363–371.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol 2006; 47:1239–1312.
- Onuigbo M, Frenandes R, Nijhawan V. The ASTRAL trial results revisited—to stent or not to stent in renal artery stenosis? QJM 2010; 103:357.
- Singh M, Kovacs DF, Singh A, Dhaliwal P, Khosla S. ACE inhibition and renal artery stenosis: what lessons have we learnt? A 21st century perspective. In: Onuigbo MAC, ed. ACE inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. Volume 2. New York, NY: NOVA Publishers; 2013:203–218.
- Onuigbo MA, Onuigbo C, Onuigbo V, et al. The CKD enigma, reengineering CKD care, narrowing asymmetric information and confronting ethicomedicinomics in CKD care: the introduction of the new 'CKD express©' IT software program. In: Onuigbo MAC, ed. ACE Inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. Volume 1. New York, NY: NOVA Publishers; 2013: 41–56.
Parvovirus mimicking acute HIV infection
A 25-year-old Jamaican man presented to the emergency department for evaluation of a rash on his face, back, and hands (Figure 1). He recalled a puncture injury after handling garbage at work. He denied recent travel, blood transfusions, or sick contacts. He was not aware of any recent arthropod bites. All standard vaccines including a tetanus booster were up to date.
Examination revealed edema of the hands and uvula. He was discharged with diphenhydramine and a short course of a systemic corticosteroid for presumed contact dermatitis.
He returned 4 days later with new symptoms, including sore throat, fever with a temperature of 103°F (39.4°C), oropharyngeal pain, dysphagia, dysuria, and purpura on the hands, abdomen, and legs. He was admitted to the hospital.
Examination revealed bright red confluent erythema of both legs extending to the lower abdomen, with petechiae on the palms (Figure 2), soles, toes, and fingers. Several small scrotal ulcers with well-defined borders were noted. Oral examination revealed white-yellow adherent plaques on the tongue and similar small ulcers on the lower lip and soft and hard palates.
A complete blood cell count revealed absolute lymphopenia, with a white blood cell count of 0.64 × 109/L (reference range 1.0–4.8) and a neutrophil percentage of 78.9% (39%–68%). Other values were within normal limits, with a red blood cell count of 5.3 × 1012/L (3.9–5.5) and a platelet count of 161 × 109/L (150–350). Serum liver enzymes were also within normal limits.
Treatment with intravenous fluids and intramuscular penicillin G was started empirically, pending a workup for infectious disease. Tests for syphilis immunoglobulin G (IgG), streptococci, anti-streptolysin O, Epstein-Barr virus, and human immunodeficiency virus (HIV) 1 and 2 were negative. The scrotal ulcers were swabbed, and culture and direct fluorescent antibody testing for cytomegalovirus and herpes simplex virus were negative. Urine testing for gonococcal and chlamydial infection was negative.
On the fifth day of hospitalization, the patient’s condition was improving, but there was still no definitive diagnosis. Consultation with the inpatient dermatology team prompted testing for parvovirus B19 infection, based on the gloves-and-socks distribution of the purpura. Testing revealed a slightly elevated parvovirus B19 IgG titer (2.61) and a significantly elevated parvovirus B19 IgM titer (12.74), which confirmed acute parvovirus infection.
The patient’s condition improved over several days with fluid administration, and he was discharged in good condition. He returned 1 week later for a follow-up appointment, at which time only superficial desquamation was noted in the areas previously affected by purpura.
PARVOVIRUS B19: NOT ONLY IN CHILDREN
Parvovirus B19 is responsible for the common childhood viral exanthem known as fifth disease.1 However, although much less common, the virus can also affect young adults, precipitating a dermatosis referred to as gloves-and-socks syndrome characterized by purpura on the hands and feet,2,3 and with a higher incidence in the spring and summer.1
Although papular-purpuric gloves-and- socks syndrome is characterized by purpura on the hands and feet, the cheeks, oral mucosa, inner thighs, buttocks, and genitalia are affected in about 50% of patients.2 In one report, in two-thirds of adult patients the presentation was caused by parvovirus B19 infection,4 but the syndrome has also been associated with Epstein-Barr virus, cytomegalovirus, human herpesvirus types 6 and 7, hepatitis B virus, rubella virus, and varicella zoster virus.4
Parvovirus B19 infection is commonly associated with systemic manifestations such as fever, fatigue, and lymphadenopathy, as well as swelling of the lips, cutaneous and mucosal ulcerations, polyarthritis, and petechiae involving the hard palate, the soft palate, or both.1
The syndrome is self-limited and resolves within 1 to 2 weeks.1
THE DIAGNOSTIC CHALLENGE
The differential diagnosis of the syndrome’s gloves-and-socks presentation includes hand-foot-mouth disease, erythema multiforme, Henoch-Schönlein purpura, and Kawasaki disease,4 in addition to viral exanthems and sexually transmitted diseases. Our patient’s fever, rash, and absolute lymphopenia focused attention on possible HIV infection, which caused the patient significant anxiety while awaiting the results of HIV testing. Heightened awareness of the cutaneous presentation of parvovirus B19 infection can help avoid unnecessary hospitalization and patient anxiety.
- Smith PT, Landry ML, Carey H, Krasnoff J, Cooney E. Papular-purpuric gloves and socks syndrome associated with acute parvovirus B19 infection: case report and review. Clin Infect Dis 1998; 27:164–168.
- Harms M, Feldmann R, Saurat JH. Papular-purpuric “gloves and socks” syndrome. J Am Acad Dermatol 1990; 23:850–854.
- Bagot M, Revuz J. Papular-purpuric “gloves and socks” syndrome: primary infection with parvovirus B19? J Am Acad Dermatol 1991; 25:341–342.
- Gutermuth J, Nadas K, Zirbs M, et al. Papular-purpuric gloves and socks syndrome. Lancet 2011; 378:198.
A 25-year-old Jamaican man presented to the emergency department for evaluation of a rash on his face, back, and hands (Figure 1). He recalled a puncture injury after handling garbage at work. He denied recent travel, blood transfusions, or sick contacts. He was not aware of any recent arthropod bites. All standard vaccines including a tetanus booster were up to date.
Examination revealed edema of the hands and uvula. He was discharged with diphenhydramine and a short course of a systemic corticosteroid for presumed contact dermatitis.
He returned 4 days later with new symptoms, including sore throat, fever with a temperature of 103°F (39.4°C), oropharyngeal pain, dysphagia, dysuria, and purpura on the hands, abdomen, and legs. He was admitted to the hospital.
Examination revealed bright red confluent erythema of both legs extending to the lower abdomen, with petechiae on the palms (Figure 2), soles, toes, and fingers. Several small scrotal ulcers with well-defined borders were noted. Oral examination revealed white-yellow adherent plaques on the tongue and similar small ulcers on the lower lip and soft and hard palates.
A complete blood cell count revealed absolute lymphopenia, with a white blood cell count of 0.64 × 109/L (reference range 1.0–4.8) and a neutrophil percentage of 78.9% (39%–68%). Other values were within normal limits, with a red blood cell count of 5.3 × 1012/L (3.9–5.5) and a platelet count of 161 × 109/L (150–350). Serum liver enzymes were also within normal limits.
Treatment with intravenous fluids and intramuscular penicillin G was started empirically, pending a workup for infectious disease. Tests for syphilis immunoglobulin G (IgG), streptococci, anti-streptolysin O, Epstein-Barr virus, and human immunodeficiency virus (HIV) 1 and 2 were negative. The scrotal ulcers were swabbed, and culture and direct fluorescent antibody testing for cytomegalovirus and herpes simplex virus were negative. Urine testing for gonococcal and chlamydial infection was negative.
On the fifth day of hospitalization, the patient’s condition was improving, but there was still no definitive diagnosis. Consultation with the inpatient dermatology team prompted testing for parvovirus B19 infection, based on the gloves-and-socks distribution of the purpura. Testing revealed a slightly elevated parvovirus B19 IgG titer (2.61) and a significantly elevated parvovirus B19 IgM titer (12.74), which confirmed acute parvovirus infection.
The patient’s condition improved over several days with fluid administration, and he was discharged in good condition. He returned 1 week later for a follow-up appointment, at which time only superficial desquamation was noted in the areas previously affected by purpura.
PARVOVIRUS B19: NOT ONLY IN CHILDREN
Parvovirus B19 is responsible for the common childhood viral exanthem known as fifth disease.1 However, although much less common, the virus can also affect young adults, precipitating a dermatosis referred to as gloves-and-socks syndrome characterized by purpura on the hands and feet,2,3 and with a higher incidence in the spring and summer.1
Although papular-purpuric gloves-and- socks syndrome is characterized by purpura on the hands and feet, the cheeks, oral mucosa, inner thighs, buttocks, and genitalia are affected in about 50% of patients.2 In one report, in two-thirds of adult patients the presentation was caused by parvovirus B19 infection,4 but the syndrome has also been associated with Epstein-Barr virus, cytomegalovirus, human herpesvirus types 6 and 7, hepatitis B virus, rubella virus, and varicella zoster virus.4
Parvovirus B19 infection is commonly associated with systemic manifestations such as fever, fatigue, and lymphadenopathy, as well as swelling of the lips, cutaneous and mucosal ulcerations, polyarthritis, and petechiae involving the hard palate, the soft palate, or both.1
The syndrome is self-limited and resolves within 1 to 2 weeks.1
THE DIAGNOSTIC CHALLENGE
The differential diagnosis of the syndrome’s gloves-and-socks presentation includes hand-foot-mouth disease, erythema multiforme, Henoch-Schönlein purpura, and Kawasaki disease,4 in addition to viral exanthems and sexually transmitted diseases. Our patient’s fever, rash, and absolute lymphopenia focused attention on possible HIV infection, which caused the patient significant anxiety while awaiting the results of HIV testing. Heightened awareness of the cutaneous presentation of parvovirus B19 infection can help avoid unnecessary hospitalization and patient anxiety.
A 25-year-old Jamaican man presented to the emergency department for evaluation of a rash on his face, back, and hands (Figure 1). He recalled a puncture injury after handling garbage at work. He denied recent travel, blood transfusions, or sick contacts. He was not aware of any recent arthropod bites. All standard vaccines including a tetanus booster were up to date.
Examination revealed edema of the hands and uvula. He was discharged with diphenhydramine and a short course of a systemic corticosteroid for presumed contact dermatitis.
He returned 4 days later with new symptoms, including sore throat, fever with a temperature of 103°F (39.4°C), oropharyngeal pain, dysphagia, dysuria, and purpura on the hands, abdomen, and legs. He was admitted to the hospital.
Examination revealed bright red confluent erythema of both legs extending to the lower abdomen, with petechiae on the palms (Figure 2), soles, toes, and fingers. Several small scrotal ulcers with well-defined borders were noted. Oral examination revealed white-yellow adherent plaques on the tongue and similar small ulcers on the lower lip and soft and hard palates.
A complete blood cell count revealed absolute lymphopenia, with a white blood cell count of 0.64 × 109/L (reference range 1.0–4.8) and a neutrophil percentage of 78.9% (39%–68%). Other values were within normal limits, with a red blood cell count of 5.3 × 1012/L (3.9–5.5) and a platelet count of 161 × 109/L (150–350). Serum liver enzymes were also within normal limits.
Treatment with intravenous fluids and intramuscular penicillin G was started empirically, pending a workup for infectious disease. Tests for syphilis immunoglobulin G (IgG), streptococci, anti-streptolysin O, Epstein-Barr virus, and human immunodeficiency virus (HIV) 1 and 2 were negative. The scrotal ulcers were swabbed, and culture and direct fluorescent antibody testing for cytomegalovirus and herpes simplex virus were negative. Urine testing for gonococcal and chlamydial infection was negative.
On the fifth day of hospitalization, the patient’s condition was improving, but there was still no definitive diagnosis. Consultation with the inpatient dermatology team prompted testing for parvovirus B19 infection, based on the gloves-and-socks distribution of the purpura. Testing revealed a slightly elevated parvovirus B19 IgG titer (2.61) and a significantly elevated parvovirus B19 IgM titer (12.74), which confirmed acute parvovirus infection.
The patient’s condition improved over several days with fluid administration, and he was discharged in good condition. He returned 1 week later for a follow-up appointment, at which time only superficial desquamation was noted in the areas previously affected by purpura.
PARVOVIRUS B19: NOT ONLY IN CHILDREN
Parvovirus B19 is responsible for the common childhood viral exanthem known as fifth disease.1 However, although much less common, the virus can also affect young adults, precipitating a dermatosis referred to as gloves-and-socks syndrome characterized by purpura on the hands and feet,2,3 and with a higher incidence in the spring and summer.1
Although papular-purpuric gloves-and- socks syndrome is characterized by purpura on the hands and feet, the cheeks, oral mucosa, inner thighs, buttocks, and genitalia are affected in about 50% of patients.2 In one report, in two-thirds of adult patients the presentation was caused by parvovirus B19 infection,4 but the syndrome has also been associated with Epstein-Barr virus, cytomegalovirus, human herpesvirus types 6 and 7, hepatitis B virus, rubella virus, and varicella zoster virus.4
Parvovirus B19 infection is commonly associated with systemic manifestations such as fever, fatigue, and lymphadenopathy, as well as swelling of the lips, cutaneous and mucosal ulcerations, polyarthritis, and petechiae involving the hard palate, the soft palate, or both.1
The syndrome is self-limited and resolves within 1 to 2 weeks.1
THE DIAGNOSTIC CHALLENGE
The differential diagnosis of the syndrome’s gloves-and-socks presentation includes hand-foot-mouth disease, erythema multiforme, Henoch-Schönlein purpura, and Kawasaki disease,4 in addition to viral exanthems and sexually transmitted diseases. Our patient’s fever, rash, and absolute lymphopenia focused attention on possible HIV infection, which caused the patient significant anxiety while awaiting the results of HIV testing. Heightened awareness of the cutaneous presentation of parvovirus B19 infection can help avoid unnecessary hospitalization and patient anxiety.
- Smith PT, Landry ML, Carey H, Krasnoff J, Cooney E. Papular-purpuric gloves and socks syndrome associated with acute parvovirus B19 infection: case report and review. Clin Infect Dis 1998; 27:164–168.
- Harms M, Feldmann R, Saurat JH. Papular-purpuric “gloves and socks” syndrome. J Am Acad Dermatol 1990; 23:850–854.
- Bagot M, Revuz J. Papular-purpuric “gloves and socks” syndrome: primary infection with parvovirus B19? J Am Acad Dermatol 1991; 25:341–342.
- Gutermuth J, Nadas K, Zirbs M, et al. Papular-purpuric gloves and socks syndrome. Lancet 2011; 378:198.
- Smith PT, Landry ML, Carey H, Krasnoff J, Cooney E. Papular-purpuric gloves and socks syndrome associated with acute parvovirus B19 infection: case report and review. Clin Infect Dis 1998; 27:164–168.
- Harms M, Feldmann R, Saurat JH. Papular-purpuric “gloves and socks” syndrome. J Am Acad Dermatol 1990; 23:850–854.
- Bagot M, Revuz J. Papular-purpuric “gloves and socks” syndrome: primary infection with parvovirus B19? J Am Acad Dermatol 1991; 25:341–342.
- Gutermuth J, Nadas K, Zirbs M, et al. Papular-purpuric gloves and socks syndrome. Lancet 2011; 378:198.
Remembering that old dogs can still do tricks
More and more we are realizing that we need trials that use hard clinical end points to inform our clinical practice. Several things we used to do based on observational studies have fallen from grace after being evaluated in interventional trials. And faced with the US Food and Drug Administration’s mandate to demonstrate clinical impact, pharmaceutical companies can rarely count on using even well-accepted biomarkers instead of clinical outcomes when trying to bring new drugs to market.
This atmosphere often makes us a bit uncomfortable when prescribing older drugs that have passed the test of time and collective anecdotal experience but not rigorous clinical testing. In some cases this is good, and robust evaluation provides greater confidence in our choice of therapy: witness the demise of digoxin for heart failure.
Many older drugs have never been compared with newer drugs in well-designed trials using hard clinical outcomes and likely never will, owing to cost, marketing, and logistic reasons. But sometimes these trials are done, and the results are surprising. For instance, methotrexate in appropriate doses may actually be comparable to newer and far more expensive tumor necrosis factor inhibitors when used to treat rheumatoid arthritis.
Should we be willing to sometimes accept data on surrogate markers (eg, low-density lipoprotein cholesterol levels, blood pressure, hemoglobin A1c ) or even extensive clinical experience in the absence of hard outcome data when using older, tried-and-true drugs? Markers can mislead: consider the higher number of deaths recorded in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial in the group receiving more aggressive control of their glucose levels.
So we should not be totally sanguine when using older drugs instead of newer ones. But some drugs may have slipped out of our mental formularies yet still have real value in niche or even common settings. Methyldopa remains an effective antihypertensive drug and may be especially useful in peripartum patients. Yet relatively few young physicians know the drug.
And so it may be with chlorthalidone. In this issue of the Journal, Cooney et al remind us not only that this drug is still around, but that it has proven efficacy and, compared with its more popular cousin hydrochlorothiazide, favorable pharmacokinetic properties such as longer action. Not to mention that it was a comparator drug in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack (ALLHAT) trial.
In our current cost-saving environment, we should remember that some old dogs can still do good tricks.
More and more we are realizing that we need trials that use hard clinical end points to inform our clinical practice. Several things we used to do based on observational studies have fallen from grace after being evaluated in interventional trials. And faced with the US Food and Drug Administration’s mandate to demonstrate clinical impact, pharmaceutical companies can rarely count on using even well-accepted biomarkers instead of clinical outcomes when trying to bring new drugs to market.
This atmosphere often makes us a bit uncomfortable when prescribing older drugs that have passed the test of time and collective anecdotal experience but not rigorous clinical testing. In some cases this is good, and robust evaluation provides greater confidence in our choice of therapy: witness the demise of digoxin for heart failure.
Many older drugs have never been compared with newer drugs in well-designed trials using hard clinical outcomes and likely never will, owing to cost, marketing, and logistic reasons. But sometimes these trials are done, and the results are surprising. For instance, methotrexate in appropriate doses may actually be comparable to newer and far more expensive tumor necrosis factor inhibitors when used to treat rheumatoid arthritis.
Should we be willing to sometimes accept data on surrogate markers (eg, low-density lipoprotein cholesterol levels, blood pressure, hemoglobin A1c ) or even extensive clinical experience in the absence of hard outcome data when using older, tried-and-true drugs? Markers can mislead: consider the higher number of deaths recorded in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial in the group receiving more aggressive control of their glucose levels.
So we should not be totally sanguine when using older drugs instead of newer ones. But some drugs may have slipped out of our mental formularies yet still have real value in niche or even common settings. Methyldopa remains an effective antihypertensive drug and may be especially useful in peripartum patients. Yet relatively few young physicians know the drug.
And so it may be with chlorthalidone. In this issue of the Journal, Cooney et al remind us not only that this drug is still around, but that it has proven efficacy and, compared with its more popular cousin hydrochlorothiazide, favorable pharmacokinetic properties such as longer action. Not to mention that it was a comparator drug in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack (ALLHAT) trial.
In our current cost-saving environment, we should remember that some old dogs can still do good tricks.
More and more we are realizing that we need trials that use hard clinical end points to inform our clinical practice. Several things we used to do based on observational studies have fallen from grace after being evaluated in interventional trials. And faced with the US Food and Drug Administration’s mandate to demonstrate clinical impact, pharmaceutical companies can rarely count on using even well-accepted biomarkers instead of clinical outcomes when trying to bring new drugs to market.
This atmosphere often makes us a bit uncomfortable when prescribing older drugs that have passed the test of time and collective anecdotal experience but not rigorous clinical testing. In some cases this is good, and robust evaluation provides greater confidence in our choice of therapy: witness the demise of digoxin for heart failure.
Many older drugs have never been compared with newer drugs in well-designed trials using hard clinical outcomes and likely never will, owing to cost, marketing, and logistic reasons. But sometimes these trials are done, and the results are surprising. For instance, methotrexate in appropriate doses may actually be comparable to newer and far more expensive tumor necrosis factor inhibitors when used to treat rheumatoid arthritis.
Should we be willing to sometimes accept data on surrogate markers (eg, low-density lipoprotein cholesterol levels, blood pressure, hemoglobin A1c ) or even extensive clinical experience in the absence of hard outcome data when using older, tried-and-true drugs? Markers can mislead: consider the higher number of deaths recorded in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial in the group receiving more aggressive control of their glucose levels.
So we should not be totally sanguine when using older drugs instead of newer ones. But some drugs may have slipped out of our mental formularies yet still have real value in niche or even common settings. Methyldopa remains an effective antihypertensive drug and may be especially useful in peripartum patients. Yet relatively few young physicians know the drug.
And so it may be with chlorthalidone. In this issue of the Journal, Cooney et al remind us not only that this drug is still around, but that it has proven efficacy and, compared with its more popular cousin hydrochlorothiazide, favorable pharmacokinetic properties such as longer action. Not to mention that it was a comparator drug in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack (ALLHAT) trial.
In our current cost-saving environment, we should remember that some old dogs can still do good tricks.
Diuretics for hypertension: Hydrochlorothiazide or chlorthalidone?
The thiazide diuretic hydrochlorothiazide and the thiazidelike diuretic chlorthalidone are two old drugs that are still useful. Although similar, they differ in important ways still not fully appreciated more than a half century after they were introduced.
Most hypertension guidelines recommend thiazide diuretics as one of the classes of agents that can be used either as initial antihypertensive drug therapy or as part of combination therapy.1–3
In the United States, hydrochlorothiazide is used more often than chlorthalidone, but many clinical trials of antihypertensive therapy have used chlorthalidone.4,5 In recent years, particularly after the publication of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), interest in chlorthalidone has been increasing, and new data are now available comparing these two diuretics.6 While current US guidelines do not recommend one over the other, British guidelines prefer chlorthalidone.7
This review summarizes the data comparing the two drugs’ pharmacology, antihypertensive effect, and impact on clinical outcomes to help guide clinicians in choosing antihypertensive drug therapy.
PHARMACOLOGY AND MECHANISM OF ACTION
Many of the differences in effectiveness and adverse effects of hydrochlorothiazide and chlorthalidone are thought to be due to their different pharmacodynamic and pharmacokinetic effects.
Pharmacodynamic effects
Hydrochlorothiazide and chlorthalidone differ significantly in chemical structure (Figure 1), but both contain a sulfonamide group that inhibits carbonic anhydrase activity, which may be associated with lower vascular contractility. Both drugs are concentrated in the kidney and secreted into the tubular lumen8; therefore, their therapeutic diuretic effects are often achieved with relatively low plasma concentrations.
Both drugs inhibit the sodium-chloride cotransporter in the luminal membrane of the distal convoluted tubule of the ascending loop of Henle, leading to a modest natriuresis and diuresis. The exact mechanism by which they lower blood pressure is not known: while the initial response is from diuresis and volume changes, long-term reduction in blood pressure is through uncertain mechanisms. In addition, chlorthalidone may have beneficial effects on endothelial function and oxidative stress.9,10
Both drugs also increase secretion of potassium and hydrogen ions and promote increased reabsorption of calcium through increased expression of a sodium-calcium exchange channel.8 Chlorthalidone may cause more inhibition of carbonic anhydrase than hydrochlorothiazide, which can lead to lower intracellular pH and cell volume. This effect may in part explain a pleiotropic effect of chlorthalidone, ie, inhibition of platelet function, which in turn may contribute to this drug’s beneficial effect on cardiovascular outcomes.9
Pharmacokinetic differences
Hydrochlorothiazide and chlorthalidone have important differences in their pharmacokinetic properties (Table 1).11
Hydrochlorothiazide has its onset of action in about 2 hours, and it reaches its peak in 4 to 6 hours. Though its duration of action is short—up to 12 hours—its pharmacodynamic response can be much longer than predicted by its kinetics, allowing once-daily dosing.8
Chlorthalidone has a longer duration of action than hydrochlorothiazide. This may be because it has a very high volume of distribution, since it is taken up into red blood cells and is bound to carbonic anhydrase.12 This may result in a “drug reservoir” that keeps drug levels higher for a longer time.13 Its long duration of action makes it a favorable choice for patients who have difficulty adhering to medication instructions. In addition, a missed dose is unlikely to have a “rebound” effect like that seen with some other antihypertensive agents. However, both chlorthalidone and hydrochlorothiazide are effective if taken once daily.
BLOOD PRESSURE-LOWERING
Both hydrochlorothiazide and chlorthalidone are effective antihypertensive agents. Table 2 summarizes findings from studies that evaluated their blood pressure-lowering effect at various doses.14–33 However, relatively few studies have directly compared these two agents’ effects on blood pressure.
Ernst et al,34 in a small study (but probably the best one to address this issue), compared chlorthalidone 12.5 mg/day (force-titrated to 25 mg/day) and hydrochlorothiazide 25 mg/day (force-titrated to 50 mg/day) in untreated hypertensive patients. After 8 weeks, ambulatory blood pressure monitoring indicated a greater reduction from baseline in systolic blood pressure with chlorthalidone 25 mg/day than with hydrochlorothiazide 50 mg/day (24-hour mean –12.4 vs –7.4 mm Hg, P = .05). Interestingly, the change in nighttime blood pressure was greater in the chlorthalidone group (–13.5 mm Hg) than in the hydrochlorothiazide group (–6.4 mm Hg; P = .009). These data suggest that at the doses studied, chlorthalidone is more effective than hydrochlorothiazide in lowering systolic blood pressure.
Bakris et al,35 using a different study design, compared the single-pill combination of azilsartan medoxomil and chlorthalidone vs coadministration of azilsartan medoxomil and hydrochlorothiazide in participants with stage 2 primary hypertension (≥ 160/100 mm Hg). Systolic blood pressure, as measured in the clinic, declined more with the chlorthalidone combination (–35.1 mm Hg) than with the hydrochlorothiazide combination (–29.5 mm Hg, mean difference –5.6 mm Hg, P < .001).
Meta-analyses also support the conclusion that chlorthalidone is more potent than hydrochlorothiazide in lowering blood pressure.35,36 Several studies have shown that chlorthalidone at the same dose is 1.5 to 2 times as potent as hydrochlorothiazide.33,36,37 Therefore, for clinical purposes, it is reasonable to consider chlorthalidone 12.5 mg daily as similar to 25 mg of hydrochlorothiazide daily.
ADVERSE EFFECTS
Electrolyte disturbances are a common adverse effect of thiazide diuretics.
Hypokalemia. All thiazide diuretics cause potassium wasting. The frequency of hypokalemia depends on the dose, frequency of administration, diet, and other pharmacologic agents used.
Two large clinical trials, the Systolic Hypertension in the Elderly Program and ALLHAT, found that chlorthalidone caused hypokalemia requiring therapy in about 6% to 8% of patients.38,39 Chlorthalidone therapy was associated with a lowering of serum potassium levels of 0.2 to 0.5 mmol/L.36 In ALLHAT, chlorthalidone was associated with a reduction in potassium levels of approximately 0.2 mmol/L after 4 years.38
All diuretics require monitoring of electrolytes, especially during the first 2 weeks of therapy. Once a steady state is reached, patients are not usually at risk of hypokalemia unless the dose is increased, extrarenal losses of potassium increase, or dietary potassium is reduced.
Other electrolyte changes. Thiazide and thiazide-like diuretics can cause other metabolic and endocrine abnormalities such as hypochloremic alkalosis, hyponatremia, and hypercalcemia.40,41 They can also cause photosensitivity and can precipitate gout.42
Observational studies have suggested that metabolic adverse effects such as hypokalemia and hyperuricemia are more common with chlorthalidone than with hydrochlorothiazide.43 It is prudent to monitor laboratory values periodically in patients on diuretic therapy.
DRUG INTERACTIONS
The drug interaction profiles of hydrochlorothiazide and chlorthalidone are also similar. The most common interactions are pharmacodynamic interactions resulting from potassium depletion caused by the diuretics.
Antiarrythymic drugs. Hypokalemia is a risk factor for arrhythmias such as torsades de pointes, and the risk is magnified with concomitant therapy with antiarrhythmic agents that prolong the QT interval independently of serum potassium concentration (eg, sotalol, dronedarone, ibutilide, propafenone). Therefore, combinations of drugs that can cause hypokalemia (eg, diuretics) and antiarrhythmic agents require vigilant monitoring of potassium and appropriate replenishment.44
Dofetilide is a class III antiarrhythmic agent and, like other antiarrhythmic drugs, carries a risk of QT prolongation and torsades de pointes, which is magnified by hypokalemia.45 In addition, dofetilide undergoes active tubular secretion in the kidney via the cation transport system, which is inhibited by hydrochlorothiazide.45 The resulting increase in plasma concentrations of dofetilide may magnify the risk of arrhythmias. Chlorthalidone has not been specifically studied in combination with dofetilide, but thiazide diuretics in general are thought to have a similar effect on tubular secretion and, therefore, should be considered similar to hydrochlorothiazide in this regard.
Digoxin. Similarly, digoxin toxicity may be enhanced in hypokalemia. As with antiarrhythmic agents, serum potassium should be carefully monitored and replenished appropriately when diuretics are used in combination with digoxin.
Lithium is reabsorbed in the proximal tubule along with sodium. Diuretics including hydrochlorothiazide and chlorthalidone that alter sodium reabsorption can also alter lithium absorption.46 When sodium reabsorption is decreased, lithium ion reabsorption is increased and may result in lithium toxicity. Although this combination is not contraindicated, monitoring of serum lithium concentrations and clinical signs and symptoms of lithium toxicity is recommended during initiation and dose adjustments of thiazide diuretics.
Nonsteroidal anti-inflammatory drugs can decrease the natriuretic, diuretic, and antihypertensive effects of both hydrochlorothiazide and chlorthalidone.47
Renin-angiotensin-aldosterone system antagonists, ie, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and the renin inhibitor aliskiren, have potentially beneficial interactions with hydrochlorothiazide and chlorthalidone, producing additive decreases in blood pressure when coadministered with these diuretics. These effects may be particularly potent early in concomitant therapy and allow use of lower doses of diuretics, typically 12.5 mg of hydrochlorothiazide in combination therapy.
LONG-TERM EFFECTS ON CARDIOVASCULAR EVENTS
The long-term goal in treating hypertension is to lower the risk of cardiovascular disease. Therefore, the clinician needs to consider the effect of antihypertensive drug therapy on long-term clinical outcomes.
Antihypertensive drug therapy based on thiazide diuretics has been shown to lower cardiovascular risk when compared with placebo.48 In addition, the effect of chlorthalidone-based antihypertensive therapy was similar to that of other antihypertensive drug classes in preventing most cardiovascular outcomes in ALLHAT.4
However, no study has directly compared hydrochlorothiazide and chlorthalidone with the primary outcome of reduction in long-term cardiovascular events. The data to date come from observational studies and meta-analyses. For example, in a retrospective analysis of the Multiple Risk Factor Intervention Trial, cardiovascular events were significantly fewer in those receiving chlorthalidone vs hydrochlorothiazide (P = .0016).43
In a systematic review and meta-analysis, chlorthalidone was associated with a 23% lower risk of heart failure and a 21% lower risk of all cardiovascular events.49
However, a Canadian observational study of 29,873 patients found no difference in the composite outcome of death or hospitalization for heart failure, stroke, or myocardial infarction between chlorthalidone recipients (3.2 events per 100 person-years) and hydrochlorothiazide recipients (3.4 events per 100 person-years; adjusted hazard ratio 0.93, 95% confidence interval 0.81–1.06).50
In summary, it is unclear whether chlorthalidone or hydrochlorothiazide is superior in preventing cardiovascular events.
SUMMARY
Thiazide and thiazidelike diuretics play an important role in managing hypertension in most patients. The eighth Joint National Committee guidelines do not recommend either hydrochlorothiazide or chlorthalidone over the other. The target dose recommendations are hydrochlorothiazide 25 to 50 mg or chlorthalidone 12.5 to 25 mg daily, with lower doses considered for the elderly.
There are important differences between hydrochlorothiazide and chlorthalidone in pharmacology, potency, and frequency of metabolic side effects. Clinicians should consider these factors to tailor the choice of thiazide diuretic therapy in hypertensive patients.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Dasgupta K, Quinn RR, Zarnke KB, et al; Canadian Hypertension Education Program. The 2014 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol 2014; 30:485–501.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group; The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288:2981–2997.
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265:3255–3264.
- Roush GC, Kaur R, Ernst ME. Diuretics: a review and update. J Cardiovasc Pharmacol Ther 2014; 19:5–13.
- McCormack T, Krause T, O’Flynn N. Management of hypertension in adults in primary care: NICE guideline. Br J Gen Pract 2012; 62:163–164.
- Bhattacharaya M, Alper SL. Pharmacology of volume regulation. In: Golan DE, Tashjian AH Jr, Armstrong EJ, Armstrong AW, editors. Principles of Pharmacology: The pathophysiologic Basis of Drug Therapy. 3rd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012:332–352.
- Woodman R, Brown C, Lockette W. Chlorthalidone decreases platelet aggregation and vascular permeability and promotes angiogenesis. Hypertension 2010; 56:463–470.
- Sato K, Dohi Y, Kojima M, Takase H, Suzuki S, Ito S. Antioxidative effects of thiazide diuretics in refractory hypertensive patients. A randomized crossover trial of chlortalidone and trichlormethiazide. Arzneimittelforschung 2010; 60:612–616.
- US National Library of Medicine. Dailymed. dailymed.nlm.nih.gov. Accessed May 14, 2015.
- Collste P, Garle M, Rawlins MD, Sjöqvist F. Interindividual differences in chlorthalidone concentration in plasma and red cells of man after single and multiple doses. Eur J Clin Pharmacol 1976; 9:319–325.
- Roush GC, Buddharaju V, Ernst ME, Holford TR. Chlorthalidone: mechanisms of action and effect on cardiovascular events. Curr Hypertens Rep 2013; 15:514–521.
- Pool JL, Cushman WC, Saini RK, Nwachuku CE, Battikha JP. Use of the factorial design and quadratic response surface models to evaluate the fosinopril and hydrochlorothiazide combination therapy in hypertension. Am J Hypertens 1997; 10:117–123.
- Pool JL, Glazer R, Weinberger M, Alvarado R, Huang J, Graff A. Comparison of valsartan/hydrochlorothiazide combination therapy at doses up to 320/25 mg versus monotherapy: a double-blind, placebo-controlled study followed by long-term combination therapy in hypertensive adults. Clin Ther 2007; 29:61–73.
- Horie Y, Higaki J, Takeuchi M. Design, statistical analysis and sample size calculation of dose response study of telmisartan and hydrochlorothiazide. Contemp Clin Trials 2007; 28:647–653.
- Chrysant SG. Antihypertensive effectiveness of low-dose lisinopril-hydrochlorothiazide combination. A large multicenter study. Lisinopril-Hydrochlorothiazide Group. Arch Intern Med 1994; 154:737–743.
- Lacourcière Y, Arnott W. Placebo-controlled comparison of the effects of nebivolol and low-dose hydrochlorothiazide as monotherapies and in combination on blood pressure and lipid profile in hypertensive patients. J Hum Hypertens 1994; 8:283–288.
- Villamil A, Chrysant SG, Calhoun D, et al. Renin inhibition with aliskiren provides additive antihypertensive efficacy when used in combination with hydrochlorothiazide. J Hypertens 2007; 25:217–226.
- McGill JB, Reilly PA. Telmisartan plus hydrochlorothiazide versus telmisartan or hydrochlorothiazide monotherapy in patients with mild to moderate hypertension: a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. Clin Ther 2001; 23:833–850.
- Weir MR, Weber MA, Punzi HA, Serfer HM, Rosenblatt S, Cady WJ. A dose escalation trial comparing the combination of diltiazem SR and hydrochlorothiazide with the monotherapies in patients with essential hypertension. J Hum Hypertens 1992; 6:133–138.
- Goldberg MR, Rockhold FW, Offen WW, Dornseif BE. Dose-effect and concentration-effect relationships of pinacidil and hydrochlorothiazide in hypertension. Clin Pharmacol Ther 1989; 46:208–218.
- Papademetriou V, Hainer JW, Sugg J, Munzer D; ATTACH Study Group. Factorial antihypertensive study of an extended-release metoprolol and hydrochlorothiazide combination. Am J Hypertens 2006; 19:1217–1225.
- Chrysant SG, Chrysant GS. Antihypertensive efficacy of olmesartan medoxomil alone and in combination with hydrochlorothiazide. Expert Opin Pharmacother 2004; 5:657–667.
- Kochar M, Guthrie R, Triscari J, Kassler-Taub K, Reeves RA. Matrix study of irbesartan with hydrochlorothiazide in mild-to-moderate hypertension. Am J Hypertens 1999; 12:797–805.
- Benz JR, Black HR, Graff A, Reed A, Fitzsimmons S, Shi Y. Valsartan and hydrochlorothiazide in patients with essential hypertension. A multiple dose, double-blind, placebo controlled trial comparing combination therapy with monotherapy. J Hum Hypertens 1998; 12:861–866.
- Jounela AJ, Lilja M, Lumme J, et al. Relation between low dose of hydrochlorothiazide, antihypertensive effect and adverse effects. Blood Press 1994; 3:231–235.
- Scholze J, Breitstadt A, Cairns V, et al. Short report: ramipril and hydrochlorothiazide combination therapy in hypertension: a clinical trial of factorial design. East Germany Collaborative Trial Group. J Hypertens 1993; 11:217–221.
- Canter D, Frank GJ, Knapp LE, Phelps M, Quade M, Texter M. Quinapril and hydrochlorothiazide combination for control of hypertension: assessment by factorial design. Quinapril Investigator Group. J Hum Hypertens 1994; 8:155–162.
- Vardan S, Mehrotra KG, Mookherjee S, Willsey GA, Gens JD, Green DE. Efficacy and reduced metabolic side effects of a 15-mg chlorthalidone formulation in the treatment of mild hypertension. A multicenter study. JAMA 1987; 258:484–488.
- Materson BJ, Oster JR, Michael UF, et al. Dose response to chlorthalidone in patients with mild hypertension. Efficacy of a lower dose. Clin Pharmacol Ther 1978; 24:192–198.
- Morledge JH, Ettinger B, Aranda J, et al. Isolated systolic hypertension in the elderly. A placebo-controlled, dose-response evaluation of chlorthalidone. J Am Geriatr Soc 1986; 34:199–206.
- Peterzan MA, Hardy R, Chaturvedi N, Hughes AD. Meta-analysis of dose-response relationships for hydrochlorothiazide, chlorthalidone, and bendroflumethiazide on blood pressure, serum potassium, and urate. Hypertension 2012; 59:1104–1109.
- Ernst ME, Carter BL, Goerdt CJ, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension 2006; 47:352–358.
- Bakris GL, Sica D, White WB, et al. Antihypertensive efficacy of hydrochlorothiazide vs chlorthalidone combined with azilsartan medoxomil. Am J Med 2012; 25:1229.e1–1229.e10.
- Ernst ME, Carter BL, Zheng S, Grimm RH Jr. Meta-analysis of dose-response characteristics of hydrochlorothiazide and chlorthalidone: effects on systolic blood pressure and potassium. Am J Hypertens 2010; 23:440–446.
- Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension 2004; 43:4–9.
- Alderman MH, Piller LB, Ford CE, et al; Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Clinical significance of incident hypokalemia and hyperkalemia in treated hypertensive patients in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Hypertension 2012; 59:926–933.
- Franse LV, Pahor M, Di Bari M, Somes GW, Cushman WC, Applegate WB. Hypokalemia associated with diuretic use and cardiovascular events in the Systolic Hypertension in the Elderly Program. Hypertension 2000; 35:1025–1030.
- Egom EE, Chirico D, Clark AL. A review of thiazide-induced hyponatraemia. Clin Med 2011; 11:448–451.
- Palmer BF. Metabolic complications associated with use of diuretics. Semin Nephrol 2011; 31:542–552.
- Hueskes BA, Roovers EA, Mantel-Teeuwisse AK, Janssens HJ, van de Lisdonk EH, Janssen M. Use of diuretics and the risk of gouty arthritis: a systematic review. Semin Arthritis Rheum 2012; 41:879–889.
- Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension 2011; 57:689–694.
- Trinkley KE, Page RL 2nd, Lien H, Yamanouye K, Tisdale JE. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin 2013; 29:1719–1726.
- Crist LW, Dixon DL. Considerations for dofetilide use in the elderly. Consult Pharm 2014; 29:270–274.
- Handler J. Lithium and antihypertensive medication: a potentially dangerous interaction. J Clin Hypertens (Greenwich) 2009; 11:738–742.
- Pavlicević I, Kuzmanić M, Rumboldt M, Rumboldt Z. Interaction between antihypertensives and NSAIDs in primary care: a controlled trial. Can J Clin Pharmacol 2008; 15:e372–e382.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension 2012; 59:1110–1117.
- Dhalla IA, Gomes T, Yao Z, et al. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med 2013; 158:447–455.
The thiazide diuretic hydrochlorothiazide and the thiazidelike diuretic chlorthalidone are two old drugs that are still useful. Although similar, they differ in important ways still not fully appreciated more than a half century after they were introduced.
Most hypertension guidelines recommend thiazide diuretics as one of the classes of agents that can be used either as initial antihypertensive drug therapy or as part of combination therapy.1–3
In the United States, hydrochlorothiazide is used more often than chlorthalidone, but many clinical trials of antihypertensive therapy have used chlorthalidone.4,5 In recent years, particularly after the publication of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), interest in chlorthalidone has been increasing, and new data are now available comparing these two diuretics.6 While current US guidelines do not recommend one over the other, British guidelines prefer chlorthalidone.7
This review summarizes the data comparing the two drugs’ pharmacology, antihypertensive effect, and impact on clinical outcomes to help guide clinicians in choosing antihypertensive drug therapy.
PHARMACOLOGY AND MECHANISM OF ACTION
Many of the differences in effectiveness and adverse effects of hydrochlorothiazide and chlorthalidone are thought to be due to their different pharmacodynamic and pharmacokinetic effects.
Pharmacodynamic effects
Hydrochlorothiazide and chlorthalidone differ significantly in chemical structure (Figure 1), but both contain a sulfonamide group that inhibits carbonic anhydrase activity, which may be associated with lower vascular contractility. Both drugs are concentrated in the kidney and secreted into the tubular lumen8; therefore, their therapeutic diuretic effects are often achieved with relatively low plasma concentrations.
Both drugs inhibit the sodium-chloride cotransporter in the luminal membrane of the distal convoluted tubule of the ascending loop of Henle, leading to a modest natriuresis and diuresis. The exact mechanism by which they lower blood pressure is not known: while the initial response is from diuresis and volume changes, long-term reduction in blood pressure is through uncertain mechanisms. In addition, chlorthalidone may have beneficial effects on endothelial function and oxidative stress.9,10
Both drugs also increase secretion of potassium and hydrogen ions and promote increased reabsorption of calcium through increased expression of a sodium-calcium exchange channel.8 Chlorthalidone may cause more inhibition of carbonic anhydrase than hydrochlorothiazide, which can lead to lower intracellular pH and cell volume. This effect may in part explain a pleiotropic effect of chlorthalidone, ie, inhibition of platelet function, which in turn may contribute to this drug’s beneficial effect on cardiovascular outcomes.9
Pharmacokinetic differences
Hydrochlorothiazide and chlorthalidone have important differences in their pharmacokinetic properties (Table 1).11
Hydrochlorothiazide has its onset of action in about 2 hours, and it reaches its peak in 4 to 6 hours. Though its duration of action is short—up to 12 hours—its pharmacodynamic response can be much longer than predicted by its kinetics, allowing once-daily dosing.8
Chlorthalidone has a longer duration of action than hydrochlorothiazide. This may be because it has a very high volume of distribution, since it is taken up into red blood cells and is bound to carbonic anhydrase.12 This may result in a “drug reservoir” that keeps drug levels higher for a longer time.13 Its long duration of action makes it a favorable choice for patients who have difficulty adhering to medication instructions. In addition, a missed dose is unlikely to have a “rebound” effect like that seen with some other antihypertensive agents. However, both chlorthalidone and hydrochlorothiazide are effective if taken once daily.
BLOOD PRESSURE-LOWERING
Both hydrochlorothiazide and chlorthalidone are effective antihypertensive agents. Table 2 summarizes findings from studies that evaluated their blood pressure-lowering effect at various doses.14–33 However, relatively few studies have directly compared these two agents’ effects on blood pressure.
Ernst et al,34 in a small study (but probably the best one to address this issue), compared chlorthalidone 12.5 mg/day (force-titrated to 25 mg/day) and hydrochlorothiazide 25 mg/day (force-titrated to 50 mg/day) in untreated hypertensive patients. After 8 weeks, ambulatory blood pressure monitoring indicated a greater reduction from baseline in systolic blood pressure with chlorthalidone 25 mg/day than with hydrochlorothiazide 50 mg/day (24-hour mean –12.4 vs –7.4 mm Hg, P = .05). Interestingly, the change in nighttime blood pressure was greater in the chlorthalidone group (–13.5 mm Hg) than in the hydrochlorothiazide group (–6.4 mm Hg; P = .009). These data suggest that at the doses studied, chlorthalidone is more effective than hydrochlorothiazide in lowering systolic blood pressure.
Bakris et al,35 using a different study design, compared the single-pill combination of azilsartan medoxomil and chlorthalidone vs coadministration of azilsartan medoxomil and hydrochlorothiazide in participants with stage 2 primary hypertension (≥ 160/100 mm Hg). Systolic blood pressure, as measured in the clinic, declined more with the chlorthalidone combination (–35.1 mm Hg) than with the hydrochlorothiazide combination (–29.5 mm Hg, mean difference –5.6 mm Hg, P < .001).
Meta-analyses also support the conclusion that chlorthalidone is more potent than hydrochlorothiazide in lowering blood pressure.35,36 Several studies have shown that chlorthalidone at the same dose is 1.5 to 2 times as potent as hydrochlorothiazide.33,36,37 Therefore, for clinical purposes, it is reasonable to consider chlorthalidone 12.5 mg daily as similar to 25 mg of hydrochlorothiazide daily.
ADVERSE EFFECTS
Electrolyte disturbances are a common adverse effect of thiazide diuretics.
Hypokalemia. All thiazide diuretics cause potassium wasting. The frequency of hypokalemia depends on the dose, frequency of administration, diet, and other pharmacologic agents used.
Two large clinical trials, the Systolic Hypertension in the Elderly Program and ALLHAT, found that chlorthalidone caused hypokalemia requiring therapy in about 6% to 8% of patients.38,39 Chlorthalidone therapy was associated with a lowering of serum potassium levels of 0.2 to 0.5 mmol/L.36 In ALLHAT, chlorthalidone was associated with a reduction in potassium levels of approximately 0.2 mmol/L after 4 years.38
All diuretics require monitoring of electrolytes, especially during the first 2 weeks of therapy. Once a steady state is reached, patients are not usually at risk of hypokalemia unless the dose is increased, extrarenal losses of potassium increase, or dietary potassium is reduced.
Other electrolyte changes. Thiazide and thiazide-like diuretics can cause other metabolic and endocrine abnormalities such as hypochloremic alkalosis, hyponatremia, and hypercalcemia.40,41 They can also cause photosensitivity and can precipitate gout.42
Observational studies have suggested that metabolic adverse effects such as hypokalemia and hyperuricemia are more common with chlorthalidone than with hydrochlorothiazide.43 It is prudent to monitor laboratory values periodically in patients on diuretic therapy.
DRUG INTERACTIONS
The drug interaction profiles of hydrochlorothiazide and chlorthalidone are also similar. The most common interactions are pharmacodynamic interactions resulting from potassium depletion caused by the diuretics.
Antiarrythymic drugs. Hypokalemia is a risk factor for arrhythmias such as torsades de pointes, and the risk is magnified with concomitant therapy with antiarrhythmic agents that prolong the QT interval independently of serum potassium concentration (eg, sotalol, dronedarone, ibutilide, propafenone). Therefore, combinations of drugs that can cause hypokalemia (eg, diuretics) and antiarrhythmic agents require vigilant monitoring of potassium and appropriate replenishment.44
Dofetilide is a class III antiarrhythmic agent and, like other antiarrhythmic drugs, carries a risk of QT prolongation and torsades de pointes, which is magnified by hypokalemia.45 In addition, dofetilide undergoes active tubular secretion in the kidney via the cation transport system, which is inhibited by hydrochlorothiazide.45 The resulting increase in plasma concentrations of dofetilide may magnify the risk of arrhythmias. Chlorthalidone has not been specifically studied in combination with dofetilide, but thiazide diuretics in general are thought to have a similar effect on tubular secretion and, therefore, should be considered similar to hydrochlorothiazide in this regard.
Digoxin. Similarly, digoxin toxicity may be enhanced in hypokalemia. As with antiarrhythmic agents, serum potassium should be carefully monitored and replenished appropriately when diuretics are used in combination with digoxin.
Lithium is reabsorbed in the proximal tubule along with sodium. Diuretics including hydrochlorothiazide and chlorthalidone that alter sodium reabsorption can also alter lithium absorption.46 When sodium reabsorption is decreased, lithium ion reabsorption is increased and may result in lithium toxicity. Although this combination is not contraindicated, monitoring of serum lithium concentrations and clinical signs and symptoms of lithium toxicity is recommended during initiation and dose adjustments of thiazide diuretics.
Nonsteroidal anti-inflammatory drugs can decrease the natriuretic, diuretic, and antihypertensive effects of both hydrochlorothiazide and chlorthalidone.47
Renin-angiotensin-aldosterone system antagonists, ie, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and the renin inhibitor aliskiren, have potentially beneficial interactions with hydrochlorothiazide and chlorthalidone, producing additive decreases in blood pressure when coadministered with these diuretics. These effects may be particularly potent early in concomitant therapy and allow use of lower doses of diuretics, typically 12.5 mg of hydrochlorothiazide in combination therapy.
LONG-TERM EFFECTS ON CARDIOVASCULAR EVENTS
The long-term goal in treating hypertension is to lower the risk of cardiovascular disease. Therefore, the clinician needs to consider the effect of antihypertensive drug therapy on long-term clinical outcomes.
Antihypertensive drug therapy based on thiazide diuretics has been shown to lower cardiovascular risk when compared with placebo.48 In addition, the effect of chlorthalidone-based antihypertensive therapy was similar to that of other antihypertensive drug classes in preventing most cardiovascular outcomes in ALLHAT.4
However, no study has directly compared hydrochlorothiazide and chlorthalidone with the primary outcome of reduction in long-term cardiovascular events. The data to date come from observational studies and meta-analyses. For example, in a retrospective analysis of the Multiple Risk Factor Intervention Trial, cardiovascular events were significantly fewer in those receiving chlorthalidone vs hydrochlorothiazide (P = .0016).43
In a systematic review and meta-analysis, chlorthalidone was associated with a 23% lower risk of heart failure and a 21% lower risk of all cardiovascular events.49
However, a Canadian observational study of 29,873 patients found no difference in the composite outcome of death or hospitalization for heart failure, stroke, or myocardial infarction between chlorthalidone recipients (3.2 events per 100 person-years) and hydrochlorothiazide recipients (3.4 events per 100 person-years; adjusted hazard ratio 0.93, 95% confidence interval 0.81–1.06).50
In summary, it is unclear whether chlorthalidone or hydrochlorothiazide is superior in preventing cardiovascular events.
SUMMARY
Thiazide and thiazidelike diuretics play an important role in managing hypertension in most patients. The eighth Joint National Committee guidelines do not recommend either hydrochlorothiazide or chlorthalidone over the other. The target dose recommendations are hydrochlorothiazide 25 to 50 mg or chlorthalidone 12.5 to 25 mg daily, with lower doses considered for the elderly.
There are important differences between hydrochlorothiazide and chlorthalidone in pharmacology, potency, and frequency of metabolic side effects. Clinicians should consider these factors to tailor the choice of thiazide diuretic therapy in hypertensive patients.
The thiazide diuretic hydrochlorothiazide and the thiazidelike diuretic chlorthalidone are two old drugs that are still useful. Although similar, they differ in important ways still not fully appreciated more than a half century after they were introduced.
Most hypertension guidelines recommend thiazide diuretics as one of the classes of agents that can be used either as initial antihypertensive drug therapy or as part of combination therapy.1–3
In the United States, hydrochlorothiazide is used more often than chlorthalidone, but many clinical trials of antihypertensive therapy have used chlorthalidone.4,5 In recent years, particularly after the publication of the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), interest in chlorthalidone has been increasing, and new data are now available comparing these two diuretics.6 While current US guidelines do not recommend one over the other, British guidelines prefer chlorthalidone.7
This review summarizes the data comparing the two drugs’ pharmacology, antihypertensive effect, and impact on clinical outcomes to help guide clinicians in choosing antihypertensive drug therapy.
PHARMACOLOGY AND MECHANISM OF ACTION
Many of the differences in effectiveness and adverse effects of hydrochlorothiazide and chlorthalidone are thought to be due to their different pharmacodynamic and pharmacokinetic effects.
Pharmacodynamic effects
Hydrochlorothiazide and chlorthalidone differ significantly in chemical structure (Figure 1), but both contain a sulfonamide group that inhibits carbonic anhydrase activity, which may be associated with lower vascular contractility. Both drugs are concentrated in the kidney and secreted into the tubular lumen8; therefore, their therapeutic diuretic effects are often achieved with relatively low plasma concentrations.
Both drugs inhibit the sodium-chloride cotransporter in the luminal membrane of the distal convoluted tubule of the ascending loop of Henle, leading to a modest natriuresis and diuresis. The exact mechanism by which they lower blood pressure is not known: while the initial response is from diuresis and volume changes, long-term reduction in blood pressure is through uncertain mechanisms. In addition, chlorthalidone may have beneficial effects on endothelial function and oxidative stress.9,10
Both drugs also increase secretion of potassium and hydrogen ions and promote increased reabsorption of calcium through increased expression of a sodium-calcium exchange channel.8 Chlorthalidone may cause more inhibition of carbonic anhydrase than hydrochlorothiazide, which can lead to lower intracellular pH and cell volume. This effect may in part explain a pleiotropic effect of chlorthalidone, ie, inhibition of platelet function, which in turn may contribute to this drug’s beneficial effect on cardiovascular outcomes.9
Pharmacokinetic differences
Hydrochlorothiazide and chlorthalidone have important differences in their pharmacokinetic properties (Table 1).11
Hydrochlorothiazide has its onset of action in about 2 hours, and it reaches its peak in 4 to 6 hours. Though its duration of action is short—up to 12 hours—its pharmacodynamic response can be much longer than predicted by its kinetics, allowing once-daily dosing.8
Chlorthalidone has a longer duration of action than hydrochlorothiazide. This may be because it has a very high volume of distribution, since it is taken up into red blood cells and is bound to carbonic anhydrase.12 This may result in a “drug reservoir” that keeps drug levels higher for a longer time.13 Its long duration of action makes it a favorable choice for patients who have difficulty adhering to medication instructions. In addition, a missed dose is unlikely to have a “rebound” effect like that seen with some other antihypertensive agents. However, both chlorthalidone and hydrochlorothiazide are effective if taken once daily.
BLOOD PRESSURE-LOWERING
Both hydrochlorothiazide and chlorthalidone are effective antihypertensive agents. Table 2 summarizes findings from studies that evaluated their blood pressure-lowering effect at various doses.14–33 However, relatively few studies have directly compared these two agents’ effects on blood pressure.
Ernst et al,34 in a small study (but probably the best one to address this issue), compared chlorthalidone 12.5 mg/day (force-titrated to 25 mg/day) and hydrochlorothiazide 25 mg/day (force-titrated to 50 mg/day) in untreated hypertensive patients. After 8 weeks, ambulatory blood pressure monitoring indicated a greater reduction from baseline in systolic blood pressure with chlorthalidone 25 mg/day than with hydrochlorothiazide 50 mg/day (24-hour mean –12.4 vs –7.4 mm Hg, P = .05). Interestingly, the change in nighttime blood pressure was greater in the chlorthalidone group (–13.5 mm Hg) than in the hydrochlorothiazide group (–6.4 mm Hg; P = .009). These data suggest that at the doses studied, chlorthalidone is more effective than hydrochlorothiazide in lowering systolic blood pressure.
Bakris et al,35 using a different study design, compared the single-pill combination of azilsartan medoxomil and chlorthalidone vs coadministration of azilsartan medoxomil and hydrochlorothiazide in participants with stage 2 primary hypertension (≥ 160/100 mm Hg). Systolic blood pressure, as measured in the clinic, declined more with the chlorthalidone combination (–35.1 mm Hg) than with the hydrochlorothiazide combination (–29.5 mm Hg, mean difference –5.6 mm Hg, P < .001).
Meta-analyses also support the conclusion that chlorthalidone is more potent than hydrochlorothiazide in lowering blood pressure.35,36 Several studies have shown that chlorthalidone at the same dose is 1.5 to 2 times as potent as hydrochlorothiazide.33,36,37 Therefore, for clinical purposes, it is reasonable to consider chlorthalidone 12.5 mg daily as similar to 25 mg of hydrochlorothiazide daily.
ADVERSE EFFECTS
Electrolyte disturbances are a common adverse effect of thiazide diuretics.
Hypokalemia. All thiazide diuretics cause potassium wasting. The frequency of hypokalemia depends on the dose, frequency of administration, diet, and other pharmacologic agents used.
Two large clinical trials, the Systolic Hypertension in the Elderly Program and ALLHAT, found that chlorthalidone caused hypokalemia requiring therapy in about 6% to 8% of patients.38,39 Chlorthalidone therapy was associated with a lowering of serum potassium levels of 0.2 to 0.5 mmol/L.36 In ALLHAT, chlorthalidone was associated with a reduction in potassium levels of approximately 0.2 mmol/L after 4 years.38
All diuretics require monitoring of electrolytes, especially during the first 2 weeks of therapy. Once a steady state is reached, patients are not usually at risk of hypokalemia unless the dose is increased, extrarenal losses of potassium increase, or dietary potassium is reduced.
Other electrolyte changes. Thiazide and thiazide-like diuretics can cause other metabolic and endocrine abnormalities such as hypochloremic alkalosis, hyponatremia, and hypercalcemia.40,41 They can also cause photosensitivity and can precipitate gout.42
Observational studies have suggested that metabolic adverse effects such as hypokalemia and hyperuricemia are more common with chlorthalidone than with hydrochlorothiazide.43 It is prudent to monitor laboratory values periodically in patients on diuretic therapy.
DRUG INTERACTIONS
The drug interaction profiles of hydrochlorothiazide and chlorthalidone are also similar. The most common interactions are pharmacodynamic interactions resulting from potassium depletion caused by the diuretics.
Antiarrythymic drugs. Hypokalemia is a risk factor for arrhythmias such as torsades de pointes, and the risk is magnified with concomitant therapy with antiarrhythmic agents that prolong the QT interval independently of serum potassium concentration (eg, sotalol, dronedarone, ibutilide, propafenone). Therefore, combinations of drugs that can cause hypokalemia (eg, diuretics) and antiarrhythmic agents require vigilant monitoring of potassium and appropriate replenishment.44
Dofetilide is a class III antiarrhythmic agent and, like other antiarrhythmic drugs, carries a risk of QT prolongation and torsades de pointes, which is magnified by hypokalemia.45 In addition, dofetilide undergoes active tubular secretion in the kidney via the cation transport system, which is inhibited by hydrochlorothiazide.45 The resulting increase in plasma concentrations of dofetilide may magnify the risk of arrhythmias. Chlorthalidone has not been specifically studied in combination with dofetilide, but thiazide diuretics in general are thought to have a similar effect on tubular secretion and, therefore, should be considered similar to hydrochlorothiazide in this regard.
Digoxin. Similarly, digoxin toxicity may be enhanced in hypokalemia. As with antiarrhythmic agents, serum potassium should be carefully monitored and replenished appropriately when diuretics are used in combination with digoxin.
Lithium is reabsorbed in the proximal tubule along with sodium. Diuretics including hydrochlorothiazide and chlorthalidone that alter sodium reabsorption can also alter lithium absorption.46 When sodium reabsorption is decreased, lithium ion reabsorption is increased and may result in lithium toxicity. Although this combination is not contraindicated, monitoring of serum lithium concentrations and clinical signs and symptoms of lithium toxicity is recommended during initiation and dose adjustments of thiazide diuretics.
Nonsteroidal anti-inflammatory drugs can decrease the natriuretic, diuretic, and antihypertensive effects of both hydrochlorothiazide and chlorthalidone.47
Renin-angiotensin-aldosterone system antagonists, ie, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and the renin inhibitor aliskiren, have potentially beneficial interactions with hydrochlorothiazide and chlorthalidone, producing additive decreases in blood pressure when coadministered with these diuretics. These effects may be particularly potent early in concomitant therapy and allow use of lower doses of diuretics, typically 12.5 mg of hydrochlorothiazide in combination therapy.
LONG-TERM EFFECTS ON CARDIOVASCULAR EVENTS
The long-term goal in treating hypertension is to lower the risk of cardiovascular disease. Therefore, the clinician needs to consider the effect of antihypertensive drug therapy on long-term clinical outcomes.
Antihypertensive drug therapy based on thiazide diuretics has been shown to lower cardiovascular risk when compared with placebo.48 In addition, the effect of chlorthalidone-based antihypertensive therapy was similar to that of other antihypertensive drug classes in preventing most cardiovascular outcomes in ALLHAT.4
However, no study has directly compared hydrochlorothiazide and chlorthalidone with the primary outcome of reduction in long-term cardiovascular events. The data to date come from observational studies and meta-analyses. For example, in a retrospective analysis of the Multiple Risk Factor Intervention Trial, cardiovascular events were significantly fewer in those receiving chlorthalidone vs hydrochlorothiazide (P = .0016).43
In a systematic review and meta-analysis, chlorthalidone was associated with a 23% lower risk of heart failure and a 21% lower risk of all cardiovascular events.49
However, a Canadian observational study of 29,873 patients found no difference in the composite outcome of death or hospitalization for heart failure, stroke, or myocardial infarction between chlorthalidone recipients (3.2 events per 100 person-years) and hydrochlorothiazide recipients (3.4 events per 100 person-years; adjusted hazard ratio 0.93, 95% confidence interval 0.81–1.06).50
In summary, it is unclear whether chlorthalidone or hydrochlorothiazide is superior in preventing cardiovascular events.
SUMMARY
Thiazide and thiazidelike diuretics play an important role in managing hypertension in most patients. The eighth Joint National Committee guidelines do not recommend either hydrochlorothiazide or chlorthalidone over the other. The target dose recommendations are hydrochlorothiazide 25 to 50 mg or chlorthalidone 12.5 to 25 mg daily, with lower doses considered for the elderly.
There are important differences between hydrochlorothiazide and chlorthalidone in pharmacology, potency, and frequency of metabolic side effects. Clinicians should consider these factors to tailor the choice of thiazide diuretic therapy in hypertensive patients.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Dasgupta K, Quinn RR, Zarnke KB, et al; Canadian Hypertension Education Program. The 2014 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol 2014; 30:485–501.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group; The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288:2981–2997.
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265:3255–3264.
- Roush GC, Kaur R, Ernst ME. Diuretics: a review and update. J Cardiovasc Pharmacol Ther 2014; 19:5–13.
- McCormack T, Krause T, O’Flynn N. Management of hypertension in adults in primary care: NICE guideline. Br J Gen Pract 2012; 62:163–164.
- Bhattacharaya M, Alper SL. Pharmacology of volume regulation. In: Golan DE, Tashjian AH Jr, Armstrong EJ, Armstrong AW, editors. Principles of Pharmacology: The pathophysiologic Basis of Drug Therapy. 3rd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012:332–352.
- Woodman R, Brown C, Lockette W. Chlorthalidone decreases platelet aggregation and vascular permeability and promotes angiogenesis. Hypertension 2010; 56:463–470.
- Sato K, Dohi Y, Kojima M, Takase H, Suzuki S, Ito S. Antioxidative effects of thiazide diuretics in refractory hypertensive patients. A randomized crossover trial of chlortalidone and trichlormethiazide. Arzneimittelforschung 2010; 60:612–616.
- US National Library of Medicine. Dailymed. dailymed.nlm.nih.gov. Accessed May 14, 2015.
- Collste P, Garle M, Rawlins MD, Sjöqvist F. Interindividual differences in chlorthalidone concentration in plasma and red cells of man after single and multiple doses. Eur J Clin Pharmacol 1976; 9:319–325.
- Roush GC, Buddharaju V, Ernst ME, Holford TR. Chlorthalidone: mechanisms of action and effect on cardiovascular events. Curr Hypertens Rep 2013; 15:514–521.
- Pool JL, Cushman WC, Saini RK, Nwachuku CE, Battikha JP. Use of the factorial design and quadratic response surface models to evaluate the fosinopril and hydrochlorothiazide combination therapy in hypertension. Am J Hypertens 1997; 10:117–123.
- Pool JL, Glazer R, Weinberger M, Alvarado R, Huang J, Graff A. Comparison of valsartan/hydrochlorothiazide combination therapy at doses up to 320/25 mg versus monotherapy: a double-blind, placebo-controlled study followed by long-term combination therapy in hypertensive adults. Clin Ther 2007; 29:61–73.
- Horie Y, Higaki J, Takeuchi M. Design, statistical analysis and sample size calculation of dose response study of telmisartan and hydrochlorothiazide. Contemp Clin Trials 2007; 28:647–653.
- Chrysant SG. Antihypertensive effectiveness of low-dose lisinopril-hydrochlorothiazide combination. A large multicenter study. Lisinopril-Hydrochlorothiazide Group. Arch Intern Med 1994; 154:737–743.
- Lacourcière Y, Arnott W. Placebo-controlled comparison of the effects of nebivolol and low-dose hydrochlorothiazide as monotherapies and in combination on blood pressure and lipid profile in hypertensive patients. J Hum Hypertens 1994; 8:283–288.
- Villamil A, Chrysant SG, Calhoun D, et al. Renin inhibition with aliskiren provides additive antihypertensive efficacy when used in combination with hydrochlorothiazide. J Hypertens 2007; 25:217–226.
- McGill JB, Reilly PA. Telmisartan plus hydrochlorothiazide versus telmisartan or hydrochlorothiazide monotherapy in patients with mild to moderate hypertension: a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. Clin Ther 2001; 23:833–850.
- Weir MR, Weber MA, Punzi HA, Serfer HM, Rosenblatt S, Cady WJ. A dose escalation trial comparing the combination of diltiazem SR and hydrochlorothiazide with the monotherapies in patients with essential hypertension. J Hum Hypertens 1992; 6:133–138.
- Goldberg MR, Rockhold FW, Offen WW, Dornseif BE. Dose-effect and concentration-effect relationships of pinacidil and hydrochlorothiazide in hypertension. Clin Pharmacol Ther 1989; 46:208–218.
- Papademetriou V, Hainer JW, Sugg J, Munzer D; ATTACH Study Group. Factorial antihypertensive study of an extended-release metoprolol and hydrochlorothiazide combination. Am J Hypertens 2006; 19:1217–1225.
- Chrysant SG, Chrysant GS. Antihypertensive efficacy of olmesartan medoxomil alone and in combination with hydrochlorothiazide. Expert Opin Pharmacother 2004; 5:657–667.
- Kochar M, Guthrie R, Triscari J, Kassler-Taub K, Reeves RA. Matrix study of irbesartan with hydrochlorothiazide in mild-to-moderate hypertension. Am J Hypertens 1999; 12:797–805.
- Benz JR, Black HR, Graff A, Reed A, Fitzsimmons S, Shi Y. Valsartan and hydrochlorothiazide in patients with essential hypertension. A multiple dose, double-blind, placebo controlled trial comparing combination therapy with monotherapy. J Hum Hypertens 1998; 12:861–866.
- Jounela AJ, Lilja M, Lumme J, et al. Relation between low dose of hydrochlorothiazide, antihypertensive effect and adverse effects. Blood Press 1994; 3:231–235.
- Scholze J, Breitstadt A, Cairns V, et al. Short report: ramipril and hydrochlorothiazide combination therapy in hypertension: a clinical trial of factorial design. East Germany Collaborative Trial Group. J Hypertens 1993; 11:217–221.
- Canter D, Frank GJ, Knapp LE, Phelps M, Quade M, Texter M. Quinapril and hydrochlorothiazide combination for control of hypertension: assessment by factorial design. Quinapril Investigator Group. J Hum Hypertens 1994; 8:155–162.
- Vardan S, Mehrotra KG, Mookherjee S, Willsey GA, Gens JD, Green DE. Efficacy and reduced metabolic side effects of a 15-mg chlorthalidone formulation in the treatment of mild hypertension. A multicenter study. JAMA 1987; 258:484–488.
- Materson BJ, Oster JR, Michael UF, et al. Dose response to chlorthalidone in patients with mild hypertension. Efficacy of a lower dose. Clin Pharmacol Ther 1978; 24:192–198.
- Morledge JH, Ettinger B, Aranda J, et al. Isolated systolic hypertension in the elderly. A placebo-controlled, dose-response evaluation of chlorthalidone. J Am Geriatr Soc 1986; 34:199–206.
- Peterzan MA, Hardy R, Chaturvedi N, Hughes AD. Meta-analysis of dose-response relationships for hydrochlorothiazide, chlorthalidone, and bendroflumethiazide on blood pressure, serum potassium, and urate. Hypertension 2012; 59:1104–1109.
- Ernst ME, Carter BL, Goerdt CJ, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension 2006; 47:352–358.
- Bakris GL, Sica D, White WB, et al. Antihypertensive efficacy of hydrochlorothiazide vs chlorthalidone combined with azilsartan medoxomil. Am J Med 2012; 25:1229.e1–1229.e10.
- Ernst ME, Carter BL, Zheng S, Grimm RH Jr. Meta-analysis of dose-response characteristics of hydrochlorothiazide and chlorthalidone: effects on systolic blood pressure and potassium. Am J Hypertens 2010; 23:440–446.
- Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension 2004; 43:4–9.
- Alderman MH, Piller LB, Ford CE, et al; Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Clinical significance of incident hypokalemia and hyperkalemia in treated hypertensive patients in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Hypertension 2012; 59:926–933.
- Franse LV, Pahor M, Di Bari M, Somes GW, Cushman WC, Applegate WB. Hypokalemia associated with diuretic use and cardiovascular events in the Systolic Hypertension in the Elderly Program. Hypertension 2000; 35:1025–1030.
- Egom EE, Chirico D, Clark AL. A review of thiazide-induced hyponatraemia. Clin Med 2011; 11:448–451.
- Palmer BF. Metabolic complications associated with use of diuretics. Semin Nephrol 2011; 31:542–552.
- Hueskes BA, Roovers EA, Mantel-Teeuwisse AK, Janssens HJ, van de Lisdonk EH, Janssen M. Use of diuretics and the risk of gouty arthritis: a systematic review. Semin Arthritis Rheum 2012; 41:879–889.
- Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension 2011; 57:689–694.
- Trinkley KE, Page RL 2nd, Lien H, Yamanouye K, Tisdale JE. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin 2013; 29:1719–1726.
- Crist LW, Dixon DL. Considerations for dofetilide use in the elderly. Consult Pharm 2014; 29:270–274.
- Handler J. Lithium and antihypertensive medication: a potentially dangerous interaction. J Clin Hypertens (Greenwich) 2009; 11:738–742.
- Pavlicević I, Kuzmanić M, Rumboldt M, Rumboldt Z. Interaction between antihypertensives and NSAIDs in primary care: a controlled trial. Can J Clin Pharmacol 2008; 15:e372–e382.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension 2012; 59:1110–1117.
- Dhalla IA, Gomes T, Yao Z, et al. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med 2013; 158:447–455.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520.
- Dasgupta K, Quinn RR, Zarnke KB, et al; Canadian Hypertension Education Program. The 2014 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol 2014; 30:485–501.
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219.
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group; The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002; 288:2981–2997.
- Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA 1991; 265:3255–3264.
- Roush GC, Kaur R, Ernst ME. Diuretics: a review and update. J Cardiovasc Pharmacol Ther 2014; 19:5–13.
- McCormack T, Krause T, O’Flynn N. Management of hypertension in adults in primary care: NICE guideline. Br J Gen Pract 2012; 62:163–164.
- Bhattacharaya M, Alper SL. Pharmacology of volume regulation. In: Golan DE, Tashjian AH Jr, Armstrong EJ, Armstrong AW, editors. Principles of Pharmacology: The pathophysiologic Basis of Drug Therapy. 3rd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012:332–352.
- Woodman R, Brown C, Lockette W. Chlorthalidone decreases platelet aggregation and vascular permeability and promotes angiogenesis. Hypertension 2010; 56:463–470.
- Sato K, Dohi Y, Kojima M, Takase H, Suzuki S, Ito S. Antioxidative effects of thiazide diuretics in refractory hypertensive patients. A randomized crossover trial of chlortalidone and trichlormethiazide. Arzneimittelforschung 2010; 60:612–616.
- US National Library of Medicine. Dailymed. dailymed.nlm.nih.gov. Accessed May 14, 2015.
- Collste P, Garle M, Rawlins MD, Sjöqvist F. Interindividual differences in chlorthalidone concentration in plasma and red cells of man after single and multiple doses. Eur J Clin Pharmacol 1976; 9:319–325.
- Roush GC, Buddharaju V, Ernst ME, Holford TR. Chlorthalidone: mechanisms of action and effect on cardiovascular events. Curr Hypertens Rep 2013; 15:514–521.
- Pool JL, Cushman WC, Saini RK, Nwachuku CE, Battikha JP. Use of the factorial design and quadratic response surface models to evaluate the fosinopril and hydrochlorothiazide combination therapy in hypertension. Am J Hypertens 1997; 10:117–123.
- Pool JL, Glazer R, Weinberger M, Alvarado R, Huang J, Graff A. Comparison of valsartan/hydrochlorothiazide combination therapy at doses up to 320/25 mg versus monotherapy: a double-blind, placebo-controlled study followed by long-term combination therapy in hypertensive adults. Clin Ther 2007; 29:61–73.
- Horie Y, Higaki J, Takeuchi M. Design, statistical analysis and sample size calculation of dose response study of telmisartan and hydrochlorothiazide. Contemp Clin Trials 2007; 28:647–653.
- Chrysant SG. Antihypertensive effectiveness of low-dose lisinopril-hydrochlorothiazide combination. A large multicenter study. Lisinopril-Hydrochlorothiazide Group. Arch Intern Med 1994; 154:737–743.
- Lacourcière Y, Arnott W. Placebo-controlled comparison of the effects of nebivolol and low-dose hydrochlorothiazide as monotherapies and in combination on blood pressure and lipid profile in hypertensive patients. J Hum Hypertens 1994; 8:283–288.
- Villamil A, Chrysant SG, Calhoun D, et al. Renin inhibition with aliskiren provides additive antihypertensive efficacy when used in combination with hydrochlorothiazide. J Hypertens 2007; 25:217–226.
- McGill JB, Reilly PA. Telmisartan plus hydrochlorothiazide versus telmisartan or hydrochlorothiazide monotherapy in patients with mild to moderate hypertension: a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial. Clin Ther 2001; 23:833–850.
- Weir MR, Weber MA, Punzi HA, Serfer HM, Rosenblatt S, Cady WJ. A dose escalation trial comparing the combination of diltiazem SR and hydrochlorothiazide with the monotherapies in patients with essential hypertension. J Hum Hypertens 1992; 6:133–138.
- Goldberg MR, Rockhold FW, Offen WW, Dornseif BE. Dose-effect and concentration-effect relationships of pinacidil and hydrochlorothiazide in hypertension. Clin Pharmacol Ther 1989; 46:208–218.
- Papademetriou V, Hainer JW, Sugg J, Munzer D; ATTACH Study Group. Factorial antihypertensive study of an extended-release metoprolol and hydrochlorothiazide combination. Am J Hypertens 2006; 19:1217–1225.
- Chrysant SG, Chrysant GS. Antihypertensive efficacy of olmesartan medoxomil alone and in combination with hydrochlorothiazide. Expert Opin Pharmacother 2004; 5:657–667.
- Kochar M, Guthrie R, Triscari J, Kassler-Taub K, Reeves RA. Matrix study of irbesartan with hydrochlorothiazide in mild-to-moderate hypertension. Am J Hypertens 1999; 12:797–805.
- Benz JR, Black HR, Graff A, Reed A, Fitzsimmons S, Shi Y. Valsartan and hydrochlorothiazide in patients with essential hypertension. A multiple dose, double-blind, placebo controlled trial comparing combination therapy with monotherapy. J Hum Hypertens 1998; 12:861–866.
- Jounela AJ, Lilja M, Lumme J, et al. Relation between low dose of hydrochlorothiazide, antihypertensive effect and adverse effects. Blood Press 1994; 3:231–235.
- Scholze J, Breitstadt A, Cairns V, et al. Short report: ramipril and hydrochlorothiazide combination therapy in hypertension: a clinical trial of factorial design. East Germany Collaborative Trial Group. J Hypertens 1993; 11:217–221.
- Canter D, Frank GJ, Knapp LE, Phelps M, Quade M, Texter M. Quinapril and hydrochlorothiazide combination for control of hypertension: assessment by factorial design. Quinapril Investigator Group. J Hum Hypertens 1994; 8:155–162.
- Vardan S, Mehrotra KG, Mookherjee S, Willsey GA, Gens JD, Green DE. Efficacy and reduced metabolic side effects of a 15-mg chlorthalidone formulation in the treatment of mild hypertension. A multicenter study. JAMA 1987; 258:484–488.
- Materson BJ, Oster JR, Michael UF, et al. Dose response to chlorthalidone in patients with mild hypertension. Efficacy of a lower dose. Clin Pharmacol Ther 1978; 24:192–198.
- Morledge JH, Ettinger B, Aranda J, et al. Isolated systolic hypertension in the elderly. A placebo-controlled, dose-response evaluation of chlorthalidone. J Am Geriatr Soc 1986; 34:199–206.
- Peterzan MA, Hardy R, Chaturvedi N, Hughes AD. Meta-analysis of dose-response relationships for hydrochlorothiazide, chlorthalidone, and bendroflumethiazide on blood pressure, serum potassium, and urate. Hypertension 2012; 59:1104–1109.
- Ernst ME, Carter BL, Goerdt CJ, et al. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension 2006; 47:352–358.
- Bakris GL, Sica D, White WB, et al. Antihypertensive efficacy of hydrochlorothiazide vs chlorthalidone combined with azilsartan medoxomil. Am J Med 2012; 25:1229.e1–1229.e10.
- Ernst ME, Carter BL, Zheng S, Grimm RH Jr. Meta-analysis of dose-response characteristics of hydrochlorothiazide and chlorthalidone: effects on systolic blood pressure and potassium. Am J Hypertens 2010; 23:440–446.
- Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension 2004; 43:4–9.
- Alderman MH, Piller LB, Ford CE, et al; Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Clinical significance of incident hypokalemia and hyperkalemia in treated hypertensive patients in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Hypertension 2012; 59:926–933.
- Franse LV, Pahor M, Di Bari M, Somes GW, Cushman WC, Applegate WB. Hypokalemia associated with diuretic use and cardiovascular events in the Systolic Hypertension in the Elderly Program. Hypertension 2000; 35:1025–1030.
- Egom EE, Chirico D, Clark AL. A review of thiazide-induced hyponatraemia. Clin Med 2011; 11:448–451.
- Palmer BF. Metabolic complications associated with use of diuretics. Semin Nephrol 2011; 31:542–552.
- Hueskes BA, Roovers EA, Mantel-Teeuwisse AK, Janssens HJ, van de Lisdonk EH, Janssen M. Use of diuretics and the risk of gouty arthritis: a systematic review. Semin Arthritis Rheum 2012; 41:879–889.
- Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension 2011; 57:689–694.
- Trinkley KE, Page RL 2nd, Lien H, Yamanouye K, Tisdale JE. QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin 2013; 29:1719–1726.
- Crist LW, Dixon DL. Considerations for dofetilide use in the elderly. Consult Pharm 2014; 29:270–274.
- Handler J. Lithium and antihypertensive medication: a potentially dangerous interaction. J Clin Hypertens (Greenwich) 2009; 11:738–742.
- Pavlicević I, Kuzmanić M, Rumboldt M, Rumboldt Z. Interaction between antihypertensives and NSAIDs in primary care: a controlled trial. Can J Clin Pharmacol 2008; 15:e372–e382.
- Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA 1997; 277:739–745.
- Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension 2012; 59:1110–1117.
- Dhalla IA, Gomes T, Yao Z, et al. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med 2013; 158:447–455.
KEY POINTS
- Chlorthalidone has a longer duration of action and a longer half-life than hydrochlorothiazide.
- Chlorthalidone may be more potent than hydrochlorothiazide in lowering blood pressure, but it also may be associated with more metabolic adverse effects, such as hypokalemia.
- No study has conclusively shown either drug to be better in preventing adverse clinical outcomes.
- These differences should be considered when making choices about thiazide diuretic therapy for hypertension.
Lifestyle changes, surgical weight loss benefit NAFLD
Losing weight through lifestyle changes can significantly improve several measures of nonalcoholic fatty liver disease, particularly if patients lose at least 10% of their body weight, and bariatric surgery is a valid alternative if diet and exercise fail, according to two studies reported in the August issue of Gastroenterology (2015 Apr. 9 [doi:10.1053/j.gastro.2015.04.005]).
Global rates of nonalcoholic fatty liver disease (NAFLD) are up because of the “parallel epidemics” of obesity and type 2 diabetes mellitus, said Dr. Eduardo Vilar-Gomez of the National Institute of Hepatology in Havana, Cuba, who authored the study of lifestyle changes. There are no approved therapies for the more aggressive form of NAFLD, steatohepatitis, he and his associates said. In past studies, patients who lost about 7%-10% of their body weight substantially improved their NAFLD activity score and had reductions in steatosis, lobular inflammation, and ballooning, but the results did not extend to fibrosis, and prospective studies of the effect of lifestyle changes on histology are lacking, the researchers added .
To fill the gap, they followed 293 adults with histologically confirmed nonalcoholic steatohepatitis who completed a 52-week lifestyle intervention program that included keeping a food diary, restricting saturated fats to less than 10% of total intake, and walking at least 200 minutes a week. Patients had not received hypolipidemic treatment in the preceding 3 months and were not allowed to take insulin sensitizers or vitamin E, both of which are potentially beneficial for nonalcoholic steatohepatitis, the investigators said.
At the end of the yearlong program, steatohepatitis had resolved in 25% of patients, 47% had lower NAFLD activity scores, and 19% had regression of fibrosis, the researchers reported. Although only 30% of participants lost at least 5% of their body weight, weight loss correlated positively with resolution of steatohepatitis and with 2-point reductions in histologic activity scores (P <.001). Among patients who lost at least 10% of their body weight, 90% had resolution of steatohepatitis, 45% had regression of fibrosis, and all had improved histologic activity scores, even if they had negative risk factors such as female sex, a baseline body mass index of at least 35 kg/m2, and a baseline fasting glucose level of at least 5.5 mmol/L, the investigators added. “Our findings support the current recommendation for weight loss using lifestyle modification as the first step in the management of patients with nonalcoholic steatohepatitis,” they wrote.
But what if lifestyle changes fail? The impact of bariatric surgery on nonalcoholic steatohepatitis has not been well studied, said Dr. Guillaume Lassailly at CHRU Lille (France). He and his associates therefore followed 109 morbidly obese patients (BMI ≥40 kg/m2) with biopsy-confirmed nonalcoholic steatohepatitis who underwent bariatric surgery at a single tertiary hospital (Gastroenterology 2015 Apr. 25 [doi:10.1053/j.gastro.2015.04.014]) .
One year after surgery, 85% of patients had achieved disease resolution (95% confidence interval, 75.8%-92.2%), the investigators reported. Stratifying patients by baseline Brunt scores showed that those with milder presurgical disease were more likely to have complete resolution than were patients whose disease was severe (94% vs. 70%; P <.05), the researchers added. Histologic analyses supported the findings, revealing steatosis in 60% of presurgical tissue samples, compared with 10% of samples taken a year after surgery. In addition, average NAFLD disease scores dropped from 5 to 1 (P <.001), hepatocyte ballooning decreased in 84% of samples, lobular inflammation decreased in 67%, and Metavir fibrosis scores dropped in one-third of specimens.
Notably, BMI scores for patients with persistent postsurgical disease dropped by an average of only 9.1, compared with 12.3 for patients whose disease resolved (P = .005), said the researchers. Gastric bypass surgery achieved greater weight loss and improvements in disease status, compared with laparoscopic banding, they added. “The encouraging results of the present study suggest that bariatric surgery should be tested in multicenter, randomized controlled trials in morbidly or severely obese patients with nonalcoholic steatohepatitis who did not respond to lifestyle therapy,” they wrote.
The Cuban National Institute of Gastroenterology and Ministry of Health partially funded the study by Dr. Vilar-Gomez and his associates. The French Ministry of Health and the Conseil Regional Nord-Pas de Calais supported the work by Dr. Lassailly and his colleagues. All investigators declared having no relevant financial conflicts of interest.
Losing weight through lifestyle changes can significantly improve several measures of nonalcoholic fatty liver disease, particularly if patients lose at least 10% of their body weight, and bariatric surgery is a valid alternative if diet and exercise fail, according to two studies reported in the August issue of Gastroenterology (2015 Apr. 9 [doi:10.1053/j.gastro.2015.04.005]).
Global rates of nonalcoholic fatty liver disease (NAFLD) are up because of the “parallel epidemics” of obesity and type 2 diabetes mellitus, said Dr. Eduardo Vilar-Gomez of the National Institute of Hepatology in Havana, Cuba, who authored the study of lifestyle changes. There are no approved therapies for the more aggressive form of NAFLD, steatohepatitis, he and his associates said. In past studies, patients who lost about 7%-10% of their body weight substantially improved their NAFLD activity score and had reductions in steatosis, lobular inflammation, and ballooning, but the results did not extend to fibrosis, and prospective studies of the effect of lifestyle changes on histology are lacking, the researchers added .
To fill the gap, they followed 293 adults with histologically confirmed nonalcoholic steatohepatitis who completed a 52-week lifestyle intervention program that included keeping a food diary, restricting saturated fats to less than 10% of total intake, and walking at least 200 minutes a week. Patients had not received hypolipidemic treatment in the preceding 3 months and were not allowed to take insulin sensitizers or vitamin E, both of which are potentially beneficial for nonalcoholic steatohepatitis, the investigators said.
At the end of the yearlong program, steatohepatitis had resolved in 25% of patients, 47% had lower NAFLD activity scores, and 19% had regression of fibrosis, the researchers reported. Although only 30% of participants lost at least 5% of their body weight, weight loss correlated positively with resolution of steatohepatitis and with 2-point reductions in histologic activity scores (P <.001). Among patients who lost at least 10% of their body weight, 90% had resolution of steatohepatitis, 45% had regression of fibrosis, and all had improved histologic activity scores, even if they had negative risk factors such as female sex, a baseline body mass index of at least 35 kg/m2, and a baseline fasting glucose level of at least 5.5 mmol/L, the investigators added. “Our findings support the current recommendation for weight loss using lifestyle modification as the first step in the management of patients with nonalcoholic steatohepatitis,” they wrote.
But what if lifestyle changes fail? The impact of bariatric surgery on nonalcoholic steatohepatitis has not been well studied, said Dr. Guillaume Lassailly at CHRU Lille (France). He and his associates therefore followed 109 morbidly obese patients (BMI ≥40 kg/m2) with biopsy-confirmed nonalcoholic steatohepatitis who underwent bariatric surgery at a single tertiary hospital (Gastroenterology 2015 Apr. 25 [doi:10.1053/j.gastro.2015.04.014]) .
One year after surgery, 85% of patients had achieved disease resolution (95% confidence interval, 75.8%-92.2%), the investigators reported. Stratifying patients by baseline Brunt scores showed that those with milder presurgical disease were more likely to have complete resolution than were patients whose disease was severe (94% vs. 70%; P <.05), the researchers added. Histologic analyses supported the findings, revealing steatosis in 60% of presurgical tissue samples, compared with 10% of samples taken a year after surgery. In addition, average NAFLD disease scores dropped from 5 to 1 (P <.001), hepatocyte ballooning decreased in 84% of samples, lobular inflammation decreased in 67%, and Metavir fibrosis scores dropped in one-third of specimens.
Notably, BMI scores for patients with persistent postsurgical disease dropped by an average of only 9.1, compared with 12.3 for patients whose disease resolved (P = .005), said the researchers. Gastric bypass surgery achieved greater weight loss and improvements in disease status, compared with laparoscopic banding, they added. “The encouraging results of the present study suggest that bariatric surgery should be tested in multicenter, randomized controlled trials in morbidly or severely obese patients with nonalcoholic steatohepatitis who did not respond to lifestyle therapy,” they wrote.
The Cuban National Institute of Gastroenterology and Ministry of Health partially funded the study by Dr. Vilar-Gomez and his associates. The French Ministry of Health and the Conseil Regional Nord-Pas de Calais supported the work by Dr. Lassailly and his colleagues. All investigators declared having no relevant financial conflicts of interest.
Losing weight through lifestyle changes can significantly improve several measures of nonalcoholic fatty liver disease, particularly if patients lose at least 10% of their body weight, and bariatric surgery is a valid alternative if diet and exercise fail, according to two studies reported in the August issue of Gastroenterology (2015 Apr. 9 [doi:10.1053/j.gastro.2015.04.005]).
Global rates of nonalcoholic fatty liver disease (NAFLD) are up because of the “parallel epidemics” of obesity and type 2 diabetes mellitus, said Dr. Eduardo Vilar-Gomez of the National Institute of Hepatology in Havana, Cuba, who authored the study of lifestyle changes. There are no approved therapies for the more aggressive form of NAFLD, steatohepatitis, he and his associates said. In past studies, patients who lost about 7%-10% of their body weight substantially improved their NAFLD activity score and had reductions in steatosis, lobular inflammation, and ballooning, but the results did not extend to fibrosis, and prospective studies of the effect of lifestyle changes on histology are lacking, the researchers added .
To fill the gap, they followed 293 adults with histologically confirmed nonalcoholic steatohepatitis who completed a 52-week lifestyle intervention program that included keeping a food diary, restricting saturated fats to less than 10% of total intake, and walking at least 200 minutes a week. Patients had not received hypolipidemic treatment in the preceding 3 months and were not allowed to take insulin sensitizers or vitamin E, both of which are potentially beneficial for nonalcoholic steatohepatitis, the investigators said.
At the end of the yearlong program, steatohepatitis had resolved in 25% of patients, 47% had lower NAFLD activity scores, and 19% had regression of fibrosis, the researchers reported. Although only 30% of participants lost at least 5% of their body weight, weight loss correlated positively with resolution of steatohepatitis and with 2-point reductions in histologic activity scores (P <.001). Among patients who lost at least 10% of their body weight, 90% had resolution of steatohepatitis, 45% had regression of fibrosis, and all had improved histologic activity scores, even if they had negative risk factors such as female sex, a baseline body mass index of at least 35 kg/m2, and a baseline fasting glucose level of at least 5.5 mmol/L, the investigators added. “Our findings support the current recommendation for weight loss using lifestyle modification as the first step in the management of patients with nonalcoholic steatohepatitis,” they wrote.
But what if lifestyle changes fail? The impact of bariatric surgery on nonalcoholic steatohepatitis has not been well studied, said Dr. Guillaume Lassailly at CHRU Lille (France). He and his associates therefore followed 109 morbidly obese patients (BMI ≥40 kg/m2) with biopsy-confirmed nonalcoholic steatohepatitis who underwent bariatric surgery at a single tertiary hospital (Gastroenterology 2015 Apr. 25 [doi:10.1053/j.gastro.2015.04.014]) .
One year after surgery, 85% of patients had achieved disease resolution (95% confidence interval, 75.8%-92.2%), the investigators reported. Stratifying patients by baseline Brunt scores showed that those with milder presurgical disease were more likely to have complete resolution than were patients whose disease was severe (94% vs. 70%; P <.05), the researchers added. Histologic analyses supported the findings, revealing steatosis in 60% of presurgical tissue samples, compared with 10% of samples taken a year after surgery. In addition, average NAFLD disease scores dropped from 5 to 1 (P <.001), hepatocyte ballooning decreased in 84% of samples, lobular inflammation decreased in 67%, and Metavir fibrosis scores dropped in one-third of specimens.
Notably, BMI scores for patients with persistent postsurgical disease dropped by an average of only 9.1, compared with 12.3 for patients whose disease resolved (P = .005), said the researchers. Gastric bypass surgery achieved greater weight loss and improvements in disease status, compared with laparoscopic banding, they added. “The encouraging results of the present study suggest that bariatric surgery should be tested in multicenter, randomized controlled trials in morbidly or severely obese patients with nonalcoholic steatohepatitis who did not respond to lifestyle therapy,” they wrote.
The Cuban National Institute of Gastroenterology and Ministry of Health partially funded the study by Dr. Vilar-Gomez and his associates. The French Ministry of Health and the Conseil Regional Nord-Pas de Calais supported the work by Dr. Lassailly and his colleagues. All investigators declared having no relevant financial conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Losing weight through lifestyle changes or bariatric surgery can significantly improve several measures of nonalcoholic steatohepatitis.
Major finding: A yearlong diet and exercise program led to resolution of nonalcoholic steatohepatitis in 25% of patients, while bariatric surgery achieved that outcome for 85% of patients in a separate study.
Data source: Two prospective uncontrolled cohort studies of 402 total adults with nonalcoholic steatohepatitis (the more severe form of nonalcoholic fatty liver disease).
Disclosures: The Cuban National Institute of Gastroenterology and Ministry of Health partially funded the study by Dr. Vilar-Gomez and his associates. The French Ministry of Health and the Conseil Regional Nord-Pas de Calais supported the work by Dr. Lassailly and his colleagues. All investigators declared having no relevant financial conflicts of interest.
FDA approves azelaic acid foam for rosacea
A foam formulation of azelaic acid has been approved for the topical treatment of the inflammatory papules and pustules of mild to moderate rosacea, the manufacturer announced July 31.
Azelaic acid foam, 15%, was compared with a vehicle foam, applied twice a day for 12 weeks, in two studies with a total of 1,362 people with papulopustular rosacea (mean age 50.6 years) and a mean of about 21 inflammatory papules and pustules at baseline. The success rate was defined as a score of clear or minimal with at least a two-step reduction from baseline on the Investigator’s Global Assessment scale.
At 12 weeks, the success rate in the two studies, respectively, was 32% and 43% among those treated with azelaic foam vs. 23% and 32% among controls, according to the Bayer HealthCare press release announcing the approval.
The most common adverse events associated with treatment were pain at the application site in about 6%, pruritus (2.5%), dryness (0.7%), and erythema (0.7%). Warnings in the prescribing information note that treatment has been associated with “isolated” cases of hypopigmentation and can cause eye irritation, and that the contents are flammable. Patients are instructed to avoid “fire, flame, and smoking during and immediately following application.”
Azelaic acid foam is marketed as Finacea Foam by Bayer; it is the first foam formulation of azelaic acid to be approved, and it will be available in September, according to the company. A gel formulation of azelaic acid, 15%, was approved by the FDA in 2002 for topical treatment of inflammatory papules and pustules of mild to moderate rosacea.
Serious adverse events associated with azelaic acid should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch/default.htm.
A foam formulation of azelaic acid has been approved for the topical treatment of the inflammatory papules and pustules of mild to moderate rosacea, the manufacturer announced July 31.
Azelaic acid foam, 15%, was compared with a vehicle foam, applied twice a day for 12 weeks, in two studies with a total of 1,362 people with papulopustular rosacea (mean age 50.6 years) and a mean of about 21 inflammatory papules and pustules at baseline. The success rate was defined as a score of clear or minimal with at least a two-step reduction from baseline on the Investigator’s Global Assessment scale.
At 12 weeks, the success rate in the two studies, respectively, was 32% and 43% among those treated with azelaic foam vs. 23% and 32% among controls, according to the Bayer HealthCare press release announcing the approval.
The most common adverse events associated with treatment were pain at the application site in about 6%, pruritus (2.5%), dryness (0.7%), and erythema (0.7%). Warnings in the prescribing information note that treatment has been associated with “isolated” cases of hypopigmentation and can cause eye irritation, and that the contents are flammable. Patients are instructed to avoid “fire, flame, and smoking during and immediately following application.”
Azelaic acid foam is marketed as Finacea Foam by Bayer; it is the first foam formulation of azelaic acid to be approved, and it will be available in September, according to the company. A gel formulation of azelaic acid, 15%, was approved by the FDA in 2002 for topical treatment of inflammatory papules and pustules of mild to moderate rosacea.
Serious adverse events associated with azelaic acid should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch/default.htm.
A foam formulation of azelaic acid has been approved for the topical treatment of the inflammatory papules and pustules of mild to moderate rosacea, the manufacturer announced July 31.
Azelaic acid foam, 15%, was compared with a vehicle foam, applied twice a day for 12 weeks, in two studies with a total of 1,362 people with papulopustular rosacea (mean age 50.6 years) and a mean of about 21 inflammatory papules and pustules at baseline. The success rate was defined as a score of clear or minimal with at least a two-step reduction from baseline on the Investigator’s Global Assessment scale.
At 12 weeks, the success rate in the two studies, respectively, was 32% and 43% among those treated with azelaic foam vs. 23% and 32% among controls, according to the Bayer HealthCare press release announcing the approval.
The most common adverse events associated with treatment were pain at the application site in about 6%, pruritus (2.5%), dryness (0.7%), and erythema (0.7%). Warnings in the prescribing information note that treatment has been associated with “isolated” cases of hypopigmentation and can cause eye irritation, and that the contents are flammable. Patients are instructed to avoid “fire, flame, and smoking during and immediately following application.”
Azelaic acid foam is marketed as Finacea Foam by Bayer; it is the first foam formulation of azelaic acid to be approved, and it will be available in September, according to the company. A gel formulation of azelaic acid, 15%, was approved by the FDA in 2002 for topical treatment of inflammatory papules and pustules of mild to moderate rosacea.
Serious adverse events associated with azelaic acid should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/Safety/MedWatch/default.htm.
New guidelines stress identifying Lynch syndrome
Clinicians and researchers must get better at identifying Lynch syndrome because the diagnosis is so often missed, according to new AGA clinical guidelines for diagnosing and managing the disorder, published in the September issue of Gastroenterology.
Lynch syndrome, previously known as hereditary nonpolyposis colorectal cancer syndrome (HNPCC), is the most common heritable cause of colorectal cancer and also is associated with cancers of the endometrium, stomach, small intestine, pancreas, biliary tract, ovary, urinary tract, and brain. Lynch syndrome accounts for 2%-3% of all colorectal cancers in the United States, and the estimated prevalence is 1 in 440 in the general population. People with the syndrome are estimated to have a lifetime cumulative incidence of colorectal cancer approaching 80%, and affected women have an estimated 60% lifetime cumulative incidence of endometrial cancer.
The new AGA guidelines focus on identifying Lynch syndrome, both in patients without cancer who have a family history suggestive of the disorder and in all patients who develop colorectal cancer, said Dr. Joel H. Rubenstein and his associates on the clinical guidelines committee (Gastroenterology 2015 Jul. 28 [doi: 10.1053/j.gastro.2015.07.036]).
The guidelines strongly recommend that all colorectal cancers now be tested using immunohistochemistry or assessment of microsatellite instability to identify potential cases of Lynch syndrome. Given the high incidence of colorectal cancer in the United States, this recommendation in particular “may be ripe for consideration as a process measure of quality of care,” they noted.
Until now, older patients with colorectal cancer have not undergone such testing because the yield of positive results was lower than in younger patients, but now there is new appreciation that these results have a significant impact on younger family members, not just the patients themselves. From the perspective of preventing cancer in these relatives, such testing is actually cost effective, said Dr. Rubenstein of the Veterans Affairs Center for Clinical Management Research and the gastroenterology division at the University of Michigan, both in Ann Arbor, and his associates.
At present, the evidence is insufficient to recommend either of these tests above the other for identifying Lynch syndrome. The two have comparable sensitivities and specificities.
The guidelines also recommend that, in people who have no personal history of colorectal or other cancer but who have a family history that suggests Lynch syndrome, risk prediction should be performed, “rather than doing nothing.” If a first-degree relative is known to have a Lynch mutation, people should be offered germline genetic testing for that mutation. Alternatively, “if tumor tissue from an affected relative is available, the screening process should begin with testing on that tumor.”
If none of this information is available, online risk prediction models or free downloadable software incorporating such models can be used to quickly and easily estimate the probability of carrying a Lynch syndrome mutation. This approach is “imperative” to improve case finding, since it is likely that most Lynch syndrome kindreds are undiagnosed, Dr. Rubenstein and his associates said.
Such patients should be offered risk-prediction models rather than proceeding directly to germline genetic testing because of the currently high costs of genetic testing. People without cancer who have a family history suggestive of Lynch syndrome should proceed straight to germline testing if they are considered to be at high risk – for example, if they meet the highly specific Amsterdam criteria.
The AGA guidelines strongly recommend that patients identified as having Lynch syndrome undergo surveillance colonoscopy, as opposed to no surveillance. Good-quality evidence shows that this strategy decreases the overall burden of colorectal cancer and reduces disease-specific mortality. People who carry Lynch syndrome genetic mutations increase their life expectancy by 7 years if they undergo surveillance colonoscopy, and cost-effectiveness analyses indicate that the expense of such screening is lower than the expense of no screening.
The optimal screening interval for such patients has not been determined, but low-quality evidence suggests that undergoing colonoscopy every 1-2 years is the “most prudent” course and is better than doing so at longer intervals.
The guidelines also include a conditional recommendation that people found to have Lynch syndrome should be offered aspirin therapy as cancer prophylaxis. The optimal dose and frequency of aspirin use is not yet known, and there is no evidence that the treatment improves mortality, but some low-quality evidence suggests that aspirin therapy reduces the risk of colorectal and other cancers, and the risk of adverse events is quite low.
Clinicians and researchers must get better at identifying Lynch syndrome because the diagnosis is so often missed, according to new AGA clinical guidelines for diagnosing and managing the disorder, published in the September issue of Gastroenterology.
Lynch syndrome, previously known as hereditary nonpolyposis colorectal cancer syndrome (HNPCC), is the most common heritable cause of colorectal cancer and also is associated with cancers of the endometrium, stomach, small intestine, pancreas, biliary tract, ovary, urinary tract, and brain. Lynch syndrome accounts for 2%-3% of all colorectal cancers in the United States, and the estimated prevalence is 1 in 440 in the general population. People with the syndrome are estimated to have a lifetime cumulative incidence of colorectal cancer approaching 80%, and affected women have an estimated 60% lifetime cumulative incidence of endometrial cancer.
The new AGA guidelines focus on identifying Lynch syndrome, both in patients without cancer who have a family history suggestive of the disorder and in all patients who develop colorectal cancer, said Dr. Joel H. Rubenstein and his associates on the clinical guidelines committee (Gastroenterology 2015 Jul. 28 [doi: 10.1053/j.gastro.2015.07.036]).
The guidelines strongly recommend that all colorectal cancers now be tested using immunohistochemistry or assessment of microsatellite instability to identify potential cases of Lynch syndrome. Given the high incidence of colorectal cancer in the United States, this recommendation in particular “may be ripe for consideration as a process measure of quality of care,” they noted.
Until now, older patients with colorectal cancer have not undergone such testing because the yield of positive results was lower than in younger patients, but now there is new appreciation that these results have a significant impact on younger family members, not just the patients themselves. From the perspective of preventing cancer in these relatives, such testing is actually cost effective, said Dr. Rubenstein of the Veterans Affairs Center for Clinical Management Research and the gastroenterology division at the University of Michigan, both in Ann Arbor, and his associates.
At present, the evidence is insufficient to recommend either of these tests above the other for identifying Lynch syndrome. The two have comparable sensitivities and specificities.
The guidelines also recommend that, in people who have no personal history of colorectal or other cancer but who have a family history that suggests Lynch syndrome, risk prediction should be performed, “rather than doing nothing.” If a first-degree relative is known to have a Lynch mutation, people should be offered germline genetic testing for that mutation. Alternatively, “if tumor tissue from an affected relative is available, the screening process should begin with testing on that tumor.”
If none of this information is available, online risk prediction models or free downloadable software incorporating such models can be used to quickly and easily estimate the probability of carrying a Lynch syndrome mutation. This approach is “imperative” to improve case finding, since it is likely that most Lynch syndrome kindreds are undiagnosed, Dr. Rubenstein and his associates said.
Such patients should be offered risk-prediction models rather than proceeding directly to germline genetic testing because of the currently high costs of genetic testing. People without cancer who have a family history suggestive of Lynch syndrome should proceed straight to germline testing if they are considered to be at high risk – for example, if they meet the highly specific Amsterdam criteria.
The AGA guidelines strongly recommend that patients identified as having Lynch syndrome undergo surveillance colonoscopy, as opposed to no surveillance. Good-quality evidence shows that this strategy decreases the overall burden of colorectal cancer and reduces disease-specific mortality. People who carry Lynch syndrome genetic mutations increase their life expectancy by 7 years if they undergo surveillance colonoscopy, and cost-effectiveness analyses indicate that the expense of such screening is lower than the expense of no screening.
The optimal screening interval for such patients has not been determined, but low-quality evidence suggests that undergoing colonoscopy every 1-2 years is the “most prudent” course and is better than doing so at longer intervals.
The guidelines also include a conditional recommendation that people found to have Lynch syndrome should be offered aspirin therapy as cancer prophylaxis. The optimal dose and frequency of aspirin use is not yet known, and there is no evidence that the treatment improves mortality, but some low-quality evidence suggests that aspirin therapy reduces the risk of colorectal and other cancers, and the risk of adverse events is quite low.
Clinicians and researchers must get better at identifying Lynch syndrome because the diagnosis is so often missed, according to new AGA clinical guidelines for diagnosing and managing the disorder, published in the September issue of Gastroenterology.
Lynch syndrome, previously known as hereditary nonpolyposis colorectal cancer syndrome (HNPCC), is the most common heritable cause of colorectal cancer and also is associated with cancers of the endometrium, stomach, small intestine, pancreas, biliary tract, ovary, urinary tract, and brain. Lynch syndrome accounts for 2%-3% of all colorectal cancers in the United States, and the estimated prevalence is 1 in 440 in the general population. People with the syndrome are estimated to have a lifetime cumulative incidence of colorectal cancer approaching 80%, and affected women have an estimated 60% lifetime cumulative incidence of endometrial cancer.
The new AGA guidelines focus on identifying Lynch syndrome, both in patients without cancer who have a family history suggestive of the disorder and in all patients who develop colorectal cancer, said Dr. Joel H. Rubenstein and his associates on the clinical guidelines committee (Gastroenterology 2015 Jul. 28 [doi: 10.1053/j.gastro.2015.07.036]).
The guidelines strongly recommend that all colorectal cancers now be tested using immunohistochemistry or assessment of microsatellite instability to identify potential cases of Lynch syndrome. Given the high incidence of colorectal cancer in the United States, this recommendation in particular “may be ripe for consideration as a process measure of quality of care,” they noted.
Until now, older patients with colorectal cancer have not undergone such testing because the yield of positive results was lower than in younger patients, but now there is new appreciation that these results have a significant impact on younger family members, not just the patients themselves. From the perspective of preventing cancer in these relatives, such testing is actually cost effective, said Dr. Rubenstein of the Veterans Affairs Center for Clinical Management Research and the gastroenterology division at the University of Michigan, both in Ann Arbor, and his associates.
At present, the evidence is insufficient to recommend either of these tests above the other for identifying Lynch syndrome. The two have comparable sensitivities and specificities.
The guidelines also recommend that, in people who have no personal history of colorectal or other cancer but who have a family history that suggests Lynch syndrome, risk prediction should be performed, “rather than doing nothing.” If a first-degree relative is known to have a Lynch mutation, people should be offered germline genetic testing for that mutation. Alternatively, “if tumor tissue from an affected relative is available, the screening process should begin with testing on that tumor.”
If none of this information is available, online risk prediction models or free downloadable software incorporating such models can be used to quickly and easily estimate the probability of carrying a Lynch syndrome mutation. This approach is “imperative” to improve case finding, since it is likely that most Lynch syndrome kindreds are undiagnosed, Dr. Rubenstein and his associates said.
Such patients should be offered risk-prediction models rather than proceeding directly to germline genetic testing because of the currently high costs of genetic testing. People without cancer who have a family history suggestive of Lynch syndrome should proceed straight to germline testing if they are considered to be at high risk – for example, if they meet the highly specific Amsterdam criteria.
The AGA guidelines strongly recommend that patients identified as having Lynch syndrome undergo surveillance colonoscopy, as opposed to no surveillance. Good-quality evidence shows that this strategy decreases the overall burden of colorectal cancer and reduces disease-specific mortality. People who carry Lynch syndrome genetic mutations increase their life expectancy by 7 years if they undergo surveillance colonoscopy, and cost-effectiveness analyses indicate that the expense of such screening is lower than the expense of no screening.
The optimal screening interval for such patients has not been determined, but low-quality evidence suggests that undergoing colonoscopy every 1-2 years is the “most prudent” course and is better than doing so at longer intervals.
The guidelines also include a conditional recommendation that people found to have Lynch syndrome should be offered aspirin therapy as cancer prophylaxis. The optimal dose and frequency of aspirin use is not yet known, and there is no evidence that the treatment improves mortality, but some low-quality evidence suggests that aspirin therapy reduces the risk of colorectal and other cancers, and the risk of adverse events is quite low.
FROM GASTROENTEROLOGY
Key clinical point: New AGA guidelines stress that identification of Lynch syndrome must improve because it is so underdiagnosed.
Major finding: Lynch syndrome accounts for 2%-3% of all colorectal cancers in the United States, and the estimated prevalence is 1 in 440 members of the general population.
Data source: A review of the literature and compilation of six key recommendations for diagnosing and managing Lynch syndrome.
Disclosures: This work was supported by the AGA Institute. Dr. Rubenstein and his associates on the clinical guidelines committee reported having no relevant financial or professional conflicts of interest.