User login
Bilateral Auricular Swelling: Marginal Zone Lymphoma With Cutaneous Involvement
To the Editor:
A 66-year-old man with hypertension presented with asymptomatic, edematous, swelling plaques without local heat on the bilateral auricles of 2 months’ duration (Figure 1). Topical corticosteroids and multiple oral antihistamines were prescribed without any improvement. He reported no history of trauma or use of any topical agents except topical corticosteroids. There was no sensory defect or numbness.
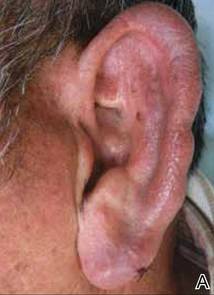
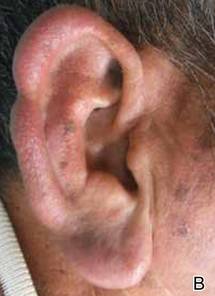
|
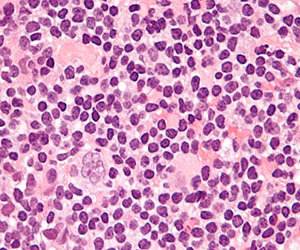
Laboratory results revealed leukocytosis with a white blood cell count of 13,900/mL (reference range, 3500–9900/μL) and 55.8% lymphocytes (reference range, 20%–40%). Biochemistry and tumor markers data were normal. No palpable neck lymphadenopathy was found. A skin biopsy was performed on the left earlobe showing a grenz zone between the tumor infiltrate and epidermis and a dense neoplastic lymphoid proliferation with a bottom-heavy configuration in the reticular dermis (Figure 2A). These lymphoid cells were small to medium sized with indented and irregular nuclei and abundant pale cytoplasm (Figure 2B). Immunohistochemical staining showed positivity for CD20 and BCL2; stains for CD5, CD10, CD23, and BCL6 were negative. Positron emission tomography scan showed bilateral auricular infiltration and bilateral neck lymph node involvement. A bone marrow biopsy was performed during hospitalization and was positive for lymphoma involvement. On the basis of histologic and immunohistochemical findings, a diagnosis of malignant nodal marginal zone lymphoma (MZL) with cutaneous involvement was made. The patient underwent chemotherapy with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone).
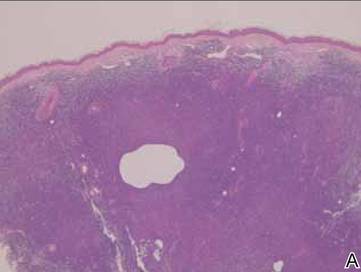
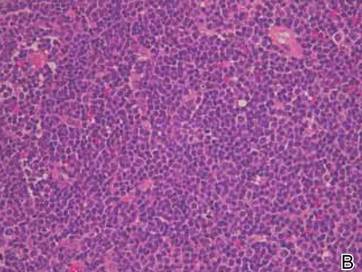
Figure 2. A skin biopsy showed basket weave hyperkeratosis, a grenz zone between the tumor infiltrate and epidermis, and dense lymphoid proliferation with a bottom-heavy configuration in the reticular dermis (A)(H&E, original magnification ×40). Small- to medium-sized lymphoid cells with indented and irregular nuclei and abundant pale cytoplasm were seen (B)(H&E, original magnification ×400). |
Cutaneous MZL may be a primary cutaneous condition or the result of secondary involvement from noncutaneous MZL. The histologic and immunophenotypic changes in skin lesions from secondary cutaneous MZL may be indistinguishable from those in primary cutaneous MZL. Primary cutaneous MZL may be seen in younger patients and favors the trunk and extremities, whereas MZL secondarily involves the skin, favors the head and neck regions, and is limited to older patients.1 Histologic aspects include a dense, nodular, deep-seated infiltrate containing various proportions of small cells displaying a centrocytelike, plasmacytoid, or monocytoid appearance.2 Chronic antigen stimulation is a key player in the pathogenesis and involves deregulation of the nuclear factor κb pathway. While Helicobacter pylori and Epstein-Barr virus do not seem to be implicated in primary cutaneous MZL, the role of Borrelia burgdorferi is still a matter of debate with discordant results.3,4
Treatment may include excision, curative or adjunctive radiotherapy, topical or intralesional corticosteroids, interferon or intralesional rituximab, or systemic therapies such as chemotherapy and/or intravenous rituximab depending on disease stage and tumor burden.5
Cutaneous presentation of MZL as bilateral auricular swelling is unique. Because there may be considerable overlap in the clinical presentations for patients with primary and secondary cutaneous MZL, it is imperative to perform a systemic evaluation. Clinicians should be aware of possible hematologic malignancy in patients with unexplained and refractory bilateral auricular swelling.
1. Gerami P, Wickless SC, Querfeld C, et al. Cutaneous involvement with marginal zone lymphoma [published online ahead of print May 11, 2010]. J Am Acad Dermatol. 2010;63:142-145.
2. de la Fouchardière A, Balme B, Chouvet B, et al. Primary cutaneous marginal zone B-cell lymphoma: a report of 9 cases. J Am Acad Dermatol. 1999;41(2, pt 1):181-188.
3. Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma [published online ahead of print October 12, 2009]. Crit Rev Oncol Hematol. 2010;74:156-162.
4. Li C, Inagaki H, Kuo TT, et al. Primary cutaneous marginal zone B-cell lymphoma: a molecular and clinicopathologic study of 24 Asian cases. Am J Surg Pathol. 2003;27:1061-1069.
5. Grange F, D’Incan M, Ortonne N, et al. Management of cutaneous B-cell lymphoma: recommendations of the French cutaneous lymphoma study group [published online ahead of print June 18, 2010]. Ann Dermatol Venereol. 2010;137:523-531.
To the Editor:
A 66-year-old man with hypertension presented with asymptomatic, edematous, swelling plaques without local heat on the bilateral auricles of 2 months’ duration (Figure 1). Topical corticosteroids and multiple oral antihistamines were prescribed without any improvement. He reported no history of trauma or use of any topical agents except topical corticosteroids. There was no sensory defect or numbness.


|
Laboratory results revealed leukocytosis with a white blood cell count of 13,900/mL (reference range, 3500–9900/μL) and 55.8% lymphocytes (reference range, 20%–40%). Biochemistry and tumor markers data were normal. No palpable neck lymphadenopathy was found. A skin biopsy was performed on the left earlobe showing a grenz zone between the tumor infiltrate and epidermis and a dense neoplastic lymphoid proliferation with a bottom-heavy configuration in the reticular dermis (Figure 2A). These lymphoid cells were small to medium sized with indented and irregular nuclei and abundant pale cytoplasm (Figure 2B). Immunohistochemical staining showed positivity for CD20 and BCL2; stains for CD5, CD10, CD23, and BCL6 were negative. Positron emission tomography scan showed bilateral auricular infiltration and bilateral neck lymph node involvement. A bone marrow biopsy was performed during hospitalization and was positive for lymphoma involvement. On the basis of histologic and immunohistochemical findings, a diagnosis of malignant nodal marginal zone lymphoma (MZL) with cutaneous involvement was made. The patient underwent chemotherapy with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone).


Figure 2. A skin biopsy showed basket weave hyperkeratosis, a grenz zone between the tumor infiltrate and epidermis, and dense lymphoid proliferation with a bottom-heavy configuration in the reticular dermis (A)(H&E, original magnification ×40). Small- to medium-sized lymphoid cells with indented and irregular nuclei and abundant pale cytoplasm were seen (B)(H&E, original magnification ×400). |
Cutaneous MZL may be a primary cutaneous condition or the result of secondary involvement from noncutaneous MZL. The histologic and immunophenotypic changes in skin lesions from secondary cutaneous MZL may be indistinguishable from those in primary cutaneous MZL. Primary cutaneous MZL may be seen in younger patients and favors the trunk and extremities, whereas MZL secondarily involves the skin, favors the head and neck regions, and is limited to older patients.1 Histologic aspects include a dense, nodular, deep-seated infiltrate containing various proportions of small cells displaying a centrocytelike, plasmacytoid, or monocytoid appearance.2 Chronic antigen stimulation is a key player in the pathogenesis and involves deregulation of the nuclear factor κb pathway. While Helicobacter pylori and Epstein-Barr virus do not seem to be implicated in primary cutaneous MZL, the role of Borrelia burgdorferi is still a matter of debate with discordant results.3,4
Treatment may include excision, curative or adjunctive radiotherapy, topical or intralesional corticosteroids, interferon or intralesional rituximab, or systemic therapies such as chemotherapy and/or intravenous rituximab depending on disease stage and tumor burden.5
Cutaneous presentation of MZL as bilateral auricular swelling is unique. Because there may be considerable overlap in the clinical presentations for patients with primary and secondary cutaneous MZL, it is imperative to perform a systemic evaluation. Clinicians should be aware of possible hematologic malignancy in patients with unexplained and refractory bilateral auricular swelling.
To the Editor:
A 66-year-old man with hypertension presented with asymptomatic, edematous, swelling plaques without local heat on the bilateral auricles of 2 months’ duration (Figure 1). Topical corticosteroids and multiple oral antihistamines were prescribed without any improvement. He reported no history of trauma or use of any topical agents except topical corticosteroids. There was no sensory defect or numbness.


|
Laboratory results revealed leukocytosis with a white blood cell count of 13,900/mL (reference range, 3500–9900/μL) and 55.8% lymphocytes (reference range, 20%–40%). Biochemistry and tumor markers data were normal. No palpable neck lymphadenopathy was found. A skin biopsy was performed on the left earlobe showing a grenz zone between the tumor infiltrate and epidermis and a dense neoplastic lymphoid proliferation with a bottom-heavy configuration in the reticular dermis (Figure 2A). These lymphoid cells were small to medium sized with indented and irregular nuclei and abundant pale cytoplasm (Figure 2B). Immunohistochemical staining showed positivity for CD20 and BCL2; stains for CD5, CD10, CD23, and BCL6 were negative. Positron emission tomography scan showed bilateral auricular infiltration and bilateral neck lymph node involvement. A bone marrow biopsy was performed during hospitalization and was positive for lymphoma involvement. On the basis of histologic and immunohistochemical findings, a diagnosis of malignant nodal marginal zone lymphoma (MZL) with cutaneous involvement was made. The patient underwent chemotherapy with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone).


Figure 2. A skin biopsy showed basket weave hyperkeratosis, a grenz zone between the tumor infiltrate and epidermis, and dense lymphoid proliferation with a bottom-heavy configuration in the reticular dermis (A)(H&E, original magnification ×40). Small- to medium-sized lymphoid cells with indented and irregular nuclei and abundant pale cytoplasm were seen (B)(H&E, original magnification ×400). |
Cutaneous MZL may be a primary cutaneous condition or the result of secondary involvement from noncutaneous MZL. The histologic and immunophenotypic changes in skin lesions from secondary cutaneous MZL may be indistinguishable from those in primary cutaneous MZL. Primary cutaneous MZL may be seen in younger patients and favors the trunk and extremities, whereas MZL secondarily involves the skin, favors the head and neck regions, and is limited to older patients.1 Histologic aspects include a dense, nodular, deep-seated infiltrate containing various proportions of small cells displaying a centrocytelike, plasmacytoid, or monocytoid appearance.2 Chronic antigen stimulation is a key player in the pathogenesis and involves deregulation of the nuclear factor κb pathway. While Helicobacter pylori and Epstein-Barr virus do not seem to be implicated in primary cutaneous MZL, the role of Borrelia burgdorferi is still a matter of debate with discordant results.3,4
Treatment may include excision, curative or adjunctive radiotherapy, topical or intralesional corticosteroids, interferon or intralesional rituximab, or systemic therapies such as chemotherapy and/or intravenous rituximab depending on disease stage and tumor burden.5
Cutaneous presentation of MZL as bilateral auricular swelling is unique. Because there may be considerable overlap in the clinical presentations for patients with primary and secondary cutaneous MZL, it is imperative to perform a systemic evaluation. Clinicians should be aware of possible hematologic malignancy in patients with unexplained and refractory bilateral auricular swelling.
1. Gerami P, Wickless SC, Querfeld C, et al. Cutaneous involvement with marginal zone lymphoma [published online ahead of print May 11, 2010]. J Am Acad Dermatol. 2010;63:142-145.
2. de la Fouchardière A, Balme B, Chouvet B, et al. Primary cutaneous marginal zone B-cell lymphoma: a report of 9 cases. J Am Acad Dermatol. 1999;41(2, pt 1):181-188.
3. Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma [published online ahead of print October 12, 2009]. Crit Rev Oncol Hematol. 2010;74:156-162.
4. Li C, Inagaki H, Kuo TT, et al. Primary cutaneous marginal zone B-cell lymphoma: a molecular and clinicopathologic study of 24 Asian cases. Am J Surg Pathol. 2003;27:1061-1069.
5. Grange F, D’Incan M, Ortonne N, et al. Management of cutaneous B-cell lymphoma: recommendations of the French cutaneous lymphoma study group [published online ahead of print June 18, 2010]. Ann Dermatol Venereol. 2010;137:523-531.
1. Gerami P, Wickless SC, Querfeld C, et al. Cutaneous involvement with marginal zone lymphoma [published online ahead of print May 11, 2010]. J Am Acad Dermatol. 2010;63:142-145.
2. de la Fouchardière A, Balme B, Chouvet B, et al. Primary cutaneous marginal zone B-cell lymphoma: a report of 9 cases. J Am Acad Dermatol. 1999;41(2, pt 1):181-188.
3. Dalle S, Thomas L, Balme B, et al. Primary cutaneous marginal zone lymphoma [published online ahead of print October 12, 2009]. Crit Rev Oncol Hematol. 2010;74:156-162.
4. Li C, Inagaki H, Kuo TT, et al. Primary cutaneous marginal zone B-cell lymphoma: a molecular and clinicopathologic study of 24 Asian cases. Am J Surg Pathol. 2003;27:1061-1069.
5. Grange F, D’Incan M, Ortonne N, et al. Management of cutaneous B-cell lymphoma: recommendations of the French cutaneous lymphoma study group [published online ahead of print June 18, 2010]. Ann Dermatol Venereol. 2010;137:523-531.
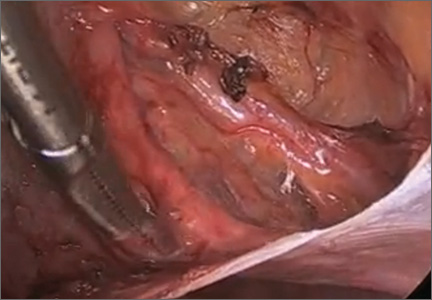
Laparoscopic ureterolysis: Techniques and approaches for ureter identification and dissection
This video was awarded the Eberhard Lotze Award at the 41st Annual Scientific Meeting of the Society of Gynecologic Surgeons.

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: http://sgsonline.org
This video was awarded the Eberhard Lotze Award at the 41st Annual Scientific Meeting of the Society of Gynecologic Surgeons.

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: http://sgsonline.org
This video was awarded the Eberhard Lotze Award at the 41st Annual Scientific Meeting of the Society of Gynecologic Surgeons.

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: http://sgsonline.org
This video is brought to you by ![]()
Hospitalization Rates Higher in Young Adult Cancer Survivors
NEW YORK (Reuters Health) - Young adult cancer survivors will continue to have high hospitalization rates over time, a Canadian study shows.
In five-year cancer survivors diagnosed between ages 20 and 44, hospitalization rates were elevated for at least 20 years, compared to rates in age- and sex-matched controls, according to Dr. Nancy N. Baxter at St. Michael's Hospital in Toronto and colleagues.
For all malignancies except melanoma and testicular cancer, the adjusted relative rate (ARR) of hospitalizations was significantly higher among survivors than controls.
"Late effects and complications of cancer treatments are experienced by many survivors for the rest of their lives," Dr. Baxter told Reuters Health in an e-mail.
The patients in this population-based study were treated from 1992-1999.
"Therapies have changed, she said. "In some cases there may be fewer late effects, but in others, they may be worse."
The study cohort included 20,275 survivors of young adult cancers who were recurrence-free for at least five years, and 101,344 controls. The authors observed survivors for a median of 9.93 years (range 0-16 years), according to their report online July 13 in the Journal of Clinical Oncology.
During this period, 34.3% had at least one hospitalization, vs. 27.3% for controls. The rate per 100 person-years was similar between male and female survivors.
Overall, the ARR of hospitalization in survivors compared with controls was 1.51.
At all time periods, survivors were more likely to be hospitalized than controls. The rate of hospitalization (per 100-person years) among survivors was 0.22 during years 5 to 8, 9 to11, and 12 to14. It decreased significantly during years 15 to 17 and 18 to 20, falling to 0.17 and 0.15, respectively (p
Among controls, the hospitalization rate was relatively constant during all time periods, ranging from 0.13 at 5 to 8 years to 0.12 at years 18 to 20.
The ARR of hospitalizations in survivors compared with controls was also relatively constant during for the first three3 time periods: 1.67, 1.55, and 1.57 at years 5 to 8, 9 to 11, and 12 to 14, respectively. It decreased to 1.36 at 15 to 17 years and 1.22 at years 18 to 20.
Those who survived gastrointestinal, urologic, colorectal, or brain cancers, or leukemia or lymphoma, had an ARR of hospitalization at least twice that of controls.
"We only looked at hospital admissions, not visits to the family doctor or medical conditions and disabilities that didn't require inpatient care," Dr. Baxter said, explaining that this likely underestimated the long-term impact of intense treatments that include surgery, chemotherapy, radiation, and hormonal therapy.
Lillie D. Shockney, Director of Cancer Survivorship Programs at the Sidney Kimmel Cancer Center at Johns Hopkins in Baltimore, said in an e-mail to Reuters Health that physical symptoms can be "guilty by association."
"If a patient had cancer, more tests, including inpatient procedures, might be done to rule out recurrence or the presence of a new malignancy," she said.
Studies such as this one could pave the way for more detailed research on the risk of treatment-related conditions that lead to more medical care, she said.
Shockney also said the report raises awareness of the need to pay special attention to cancer survivors; to consider survivorship as we would a chronic illness.
"Understanding the late effects of cancer treatment will help us design better treatments, counsel patients, and improve symptom management," said Dr. Baxter.
NEW YORK (Reuters Health) - Young adult cancer survivors will continue to have high hospitalization rates over time, a Canadian study shows.
In five-year cancer survivors diagnosed between ages 20 and 44, hospitalization rates were elevated for at least 20 years, compared to rates in age- and sex-matched controls, according to Dr. Nancy N. Baxter at St. Michael's Hospital in Toronto and colleagues.
For all malignancies except melanoma and testicular cancer, the adjusted relative rate (ARR) of hospitalizations was significantly higher among survivors than controls.
"Late effects and complications of cancer treatments are experienced by many survivors for the rest of their lives," Dr. Baxter told Reuters Health in an e-mail.
The patients in this population-based study were treated from 1992-1999.
"Therapies have changed, she said. "In some cases there may be fewer late effects, but in others, they may be worse."
The study cohort included 20,275 survivors of young adult cancers who were recurrence-free for at least five years, and 101,344 controls. The authors observed survivors for a median of 9.93 years (range 0-16 years), according to their report online July 13 in the Journal of Clinical Oncology.
During this period, 34.3% had at least one hospitalization, vs. 27.3% for controls. The rate per 100 person-years was similar between male and female survivors.
Overall, the ARR of hospitalization in survivors compared with controls was 1.51.
At all time periods, survivors were more likely to be hospitalized than controls. The rate of hospitalization (per 100-person years) among survivors was 0.22 during years 5 to 8, 9 to11, and 12 to14. It decreased significantly during years 15 to 17 and 18 to 20, falling to 0.17 and 0.15, respectively (p
Among controls, the hospitalization rate was relatively constant during all time periods, ranging from 0.13 at 5 to 8 years to 0.12 at years 18 to 20.
The ARR of hospitalizations in survivors compared with controls was also relatively constant during for the first three3 time periods: 1.67, 1.55, and 1.57 at years 5 to 8, 9 to 11, and 12 to 14, respectively. It decreased to 1.36 at 15 to 17 years and 1.22 at years 18 to 20.
Those who survived gastrointestinal, urologic, colorectal, or brain cancers, or leukemia or lymphoma, had an ARR of hospitalization at least twice that of controls.
"We only looked at hospital admissions, not visits to the family doctor or medical conditions and disabilities that didn't require inpatient care," Dr. Baxter said, explaining that this likely underestimated the long-term impact of intense treatments that include surgery, chemotherapy, radiation, and hormonal therapy.
Lillie D. Shockney, Director of Cancer Survivorship Programs at the Sidney Kimmel Cancer Center at Johns Hopkins in Baltimore, said in an e-mail to Reuters Health that physical symptoms can be "guilty by association."
"If a patient had cancer, more tests, including inpatient procedures, might be done to rule out recurrence or the presence of a new malignancy," she said.
Studies such as this one could pave the way for more detailed research on the risk of treatment-related conditions that lead to more medical care, she said.
Shockney also said the report raises awareness of the need to pay special attention to cancer survivors; to consider survivorship as we would a chronic illness.
"Understanding the late effects of cancer treatment will help us design better treatments, counsel patients, and improve symptom management," said Dr. Baxter.
NEW YORK (Reuters Health) - Young adult cancer survivors will continue to have high hospitalization rates over time, a Canadian study shows.
In five-year cancer survivors diagnosed between ages 20 and 44, hospitalization rates were elevated for at least 20 years, compared to rates in age- and sex-matched controls, according to Dr. Nancy N. Baxter at St. Michael's Hospital in Toronto and colleagues.
For all malignancies except melanoma and testicular cancer, the adjusted relative rate (ARR) of hospitalizations was significantly higher among survivors than controls.
"Late effects and complications of cancer treatments are experienced by many survivors for the rest of their lives," Dr. Baxter told Reuters Health in an e-mail.
The patients in this population-based study were treated from 1992-1999.
"Therapies have changed, she said. "In some cases there may be fewer late effects, but in others, they may be worse."
The study cohort included 20,275 survivors of young adult cancers who were recurrence-free for at least five years, and 101,344 controls. The authors observed survivors for a median of 9.93 years (range 0-16 years), according to their report online July 13 in the Journal of Clinical Oncology.
During this period, 34.3% had at least one hospitalization, vs. 27.3% for controls. The rate per 100 person-years was similar between male and female survivors.
Overall, the ARR of hospitalization in survivors compared with controls was 1.51.
At all time periods, survivors were more likely to be hospitalized than controls. The rate of hospitalization (per 100-person years) among survivors was 0.22 during years 5 to 8, 9 to11, and 12 to14. It decreased significantly during years 15 to 17 and 18 to 20, falling to 0.17 and 0.15, respectively (p
Among controls, the hospitalization rate was relatively constant during all time periods, ranging from 0.13 at 5 to 8 years to 0.12 at years 18 to 20.
The ARR of hospitalizations in survivors compared with controls was also relatively constant during for the first three3 time periods: 1.67, 1.55, and 1.57 at years 5 to 8, 9 to 11, and 12 to 14, respectively. It decreased to 1.36 at 15 to 17 years and 1.22 at years 18 to 20.
Those who survived gastrointestinal, urologic, colorectal, or brain cancers, or leukemia or lymphoma, had an ARR of hospitalization at least twice that of controls.
"We only looked at hospital admissions, not visits to the family doctor or medical conditions and disabilities that didn't require inpatient care," Dr. Baxter said, explaining that this likely underestimated the long-term impact of intense treatments that include surgery, chemotherapy, radiation, and hormonal therapy.
Lillie D. Shockney, Director of Cancer Survivorship Programs at the Sidney Kimmel Cancer Center at Johns Hopkins in Baltimore, said in an e-mail to Reuters Health that physical symptoms can be "guilty by association."
"If a patient had cancer, more tests, including inpatient procedures, might be done to rule out recurrence or the presence of a new malignancy," she said.
Studies such as this one could pave the way for more detailed research on the risk of treatment-related conditions that lead to more medical care, she said.
Shockney also said the report raises awareness of the need to pay special attention to cancer survivors; to consider survivorship as we would a chronic illness.
"Understanding the late effects of cancer treatment will help us design better treatments, counsel patients, and improve symptom management," said Dr. Baxter.
Group proposes revised staging system for MM

Photo by Juan D. Alfonso
Researchers from the International Myeloma Working Group (IMWG) have proposed revising the International Staging System (ISS) used to stratify patients with newly diagnosed multiple myeloma (MM).
The group’s revised ISS (R-ISS) combines the current ISS with tests for chromosomal abnormalities (CAs) and serum lactate dehydrogenase (LDH) in an attempt to refine the system’s prognostic value.
IMWG researchers assessed the R-ISS in more than 3000 newly diagnosed MM patients and found that patients with R-ISS stage I disease had better overall survival (OS) and progression-free survival (PFS) than patients with stage I disease according to the ISS.
And patients with R-ISS stage III disease had worse survival rates than patients with stage III disease according to the ISS. But PFS and OS numbers for stage II disease were the same with both systems.
The researchers reported these results in the Journal of Clinical Oncology.
They noted that the existing ISS relies on tests for serum β2-microglobulin and serum albumin to divide patients into 3 risk-factor stages. But the R-ISS adds interphase fluorescence in situ hybridization to check for CAs, along with separate tests for heightened LDH.
The researchers define the 3 R-ISS groups as follows:
- R-ISS I includes patients with ISS stage I disease (serum β2-microglobulin level < 3.5 mg/L and serum albumin level ≥ 3.5 g/dL), no high-risk CAs (del[17p] and/or t[4;14] and/or t[14;16]), and normal LDH levels (less than the upper limit of normal range).
- R-ISS III includes patients with ISS stage III disease (serum β2-microglobulin level > 5.5 mg/L) and high-risk CAs or high LDH levels.
- R-ISS II includes patients with all other possible combinations.
To evaluate the prognostic value of the R-ISS, the researchers analyzed data from 4445 newly diagnosed MM patients who were enrolled in 11 completed trials. ISS, CA, and LDH data were available for 3060 patients.
At a median follow-up of 46 months, the 5-year OS rate was 82% in the R-ISS I group (n=871), 62% in the R-ISS II group (n=1894), and 40% in the R-ISS III group (n=295). The 5-year PFS rates were 55%, 36%, and 24%, respectively.
In comparison, the 5-year OS rate was 77% for patients with ISS stage I disease (n=1615), 62% for ISS stage II (n=1630), and 47% for ISS stage III (n=987). The 5-year PFS rates were 49%, 36%, and 30%, respectively.
Based on this work, the researchers said the R-ISS is a simple but powerful prognostic staging system, and they recommend its use in future studies to stratify newly diagnosed MM patients effectively.
“The revised staging system can be used by doctors to discuss prognostic results very carefully with individual patients,” added Brian G.M. Durie, MD, chairman of the IMWG.
“It’s helpful to know the expectations and consider how treatments can be modified based on the new ISS system.” ![]()

Photo by Juan D. Alfonso
Researchers from the International Myeloma Working Group (IMWG) have proposed revising the International Staging System (ISS) used to stratify patients with newly diagnosed multiple myeloma (MM).
The group’s revised ISS (R-ISS) combines the current ISS with tests for chromosomal abnormalities (CAs) and serum lactate dehydrogenase (LDH) in an attempt to refine the system’s prognostic value.
IMWG researchers assessed the R-ISS in more than 3000 newly diagnosed MM patients and found that patients with R-ISS stage I disease had better overall survival (OS) and progression-free survival (PFS) than patients with stage I disease according to the ISS.
And patients with R-ISS stage III disease had worse survival rates than patients with stage III disease according to the ISS. But PFS and OS numbers for stage II disease were the same with both systems.
The researchers reported these results in the Journal of Clinical Oncology.
They noted that the existing ISS relies on tests for serum β2-microglobulin and serum albumin to divide patients into 3 risk-factor stages. But the R-ISS adds interphase fluorescence in situ hybridization to check for CAs, along with separate tests for heightened LDH.
The researchers define the 3 R-ISS groups as follows:
- R-ISS I includes patients with ISS stage I disease (serum β2-microglobulin level < 3.5 mg/L and serum albumin level ≥ 3.5 g/dL), no high-risk CAs (del[17p] and/or t[4;14] and/or t[14;16]), and normal LDH levels (less than the upper limit of normal range).
- R-ISS III includes patients with ISS stage III disease (serum β2-microglobulin level > 5.5 mg/L) and high-risk CAs or high LDH levels.
- R-ISS II includes patients with all other possible combinations.
To evaluate the prognostic value of the R-ISS, the researchers analyzed data from 4445 newly diagnosed MM patients who were enrolled in 11 completed trials. ISS, CA, and LDH data were available for 3060 patients.
At a median follow-up of 46 months, the 5-year OS rate was 82% in the R-ISS I group (n=871), 62% in the R-ISS II group (n=1894), and 40% in the R-ISS III group (n=295). The 5-year PFS rates were 55%, 36%, and 24%, respectively.
In comparison, the 5-year OS rate was 77% for patients with ISS stage I disease (n=1615), 62% for ISS stage II (n=1630), and 47% for ISS stage III (n=987). The 5-year PFS rates were 49%, 36%, and 30%, respectively.
Based on this work, the researchers said the R-ISS is a simple but powerful prognostic staging system, and they recommend its use in future studies to stratify newly diagnosed MM patients effectively.
“The revised staging system can be used by doctors to discuss prognostic results very carefully with individual patients,” added Brian G.M. Durie, MD, chairman of the IMWG.
“It’s helpful to know the expectations and consider how treatments can be modified based on the new ISS system.” ![]()

Photo by Juan D. Alfonso
Researchers from the International Myeloma Working Group (IMWG) have proposed revising the International Staging System (ISS) used to stratify patients with newly diagnosed multiple myeloma (MM).
The group’s revised ISS (R-ISS) combines the current ISS with tests for chromosomal abnormalities (CAs) and serum lactate dehydrogenase (LDH) in an attempt to refine the system’s prognostic value.
IMWG researchers assessed the R-ISS in more than 3000 newly diagnosed MM patients and found that patients with R-ISS stage I disease had better overall survival (OS) and progression-free survival (PFS) than patients with stage I disease according to the ISS.
And patients with R-ISS stage III disease had worse survival rates than patients with stage III disease according to the ISS. But PFS and OS numbers for stage II disease were the same with both systems.
The researchers reported these results in the Journal of Clinical Oncology.
They noted that the existing ISS relies on tests for serum β2-microglobulin and serum albumin to divide patients into 3 risk-factor stages. But the R-ISS adds interphase fluorescence in situ hybridization to check for CAs, along with separate tests for heightened LDH.
The researchers define the 3 R-ISS groups as follows:
- R-ISS I includes patients with ISS stage I disease (serum β2-microglobulin level < 3.5 mg/L and serum albumin level ≥ 3.5 g/dL), no high-risk CAs (del[17p] and/or t[4;14] and/or t[14;16]), and normal LDH levels (less than the upper limit of normal range).
- R-ISS III includes patients with ISS stage III disease (serum β2-microglobulin level > 5.5 mg/L) and high-risk CAs or high LDH levels.
- R-ISS II includes patients with all other possible combinations.
To evaluate the prognostic value of the R-ISS, the researchers analyzed data from 4445 newly diagnosed MM patients who were enrolled in 11 completed trials. ISS, CA, and LDH data were available for 3060 patients.
At a median follow-up of 46 months, the 5-year OS rate was 82% in the R-ISS I group (n=871), 62% in the R-ISS II group (n=1894), and 40% in the R-ISS III group (n=295). The 5-year PFS rates were 55%, 36%, and 24%, respectively.
In comparison, the 5-year OS rate was 77% for patients with ISS stage I disease (n=1615), 62% for ISS stage II (n=1630), and 47% for ISS stage III (n=987). The 5-year PFS rates were 49%, 36%, and 30%, respectively.
Based on this work, the researchers said the R-ISS is a simple but powerful prognostic staging system, and they recommend its use in future studies to stratify newly diagnosed MM patients effectively.
“The revised staging system can be used by doctors to discuss prognostic results very carefully with individual patients,” added Brian G.M. Durie, MD, chairman of the IMWG.
“It’s helpful to know the expectations and consider how treatments can be modified based on the new ISS system.” ![]()
FDA grants drug orphan designation for ITP

Photo by Linda Bartlett
The US Food and Drug Administration (FDA) has granted orphan designation to veltuzumab for the treatment of immune thrombocytopenia (ITP).
Veltuzumab is a 2nd-generation, humanized monoclonal antibody targeting CD20. The drug is being developed by Immunomedics as a treatment for ITP, other autoimmune diseases, and non-Hodgkin lymphoma.
Veltuzumab was considered active and well-tolerated in a phase 1 study of adults with ITP. The drug produced responses in about half of patients, with some responses lasting more than 4 years.
The study included 50 patients with primary ITP who had failed 1 or more types of standard therapy, had platelet levels of 30,000/μL or less, and did not have major bleeding. The patients’ median age was 54, and most were female (n=31). Eight patients had undergone splenectomy.
Patients were a median of 2 years from diagnosis. Fourteen had been diagnosed with ITP for a year or less and had received corticosteroids and/or immunoglobulins.
Thirty-six patients had chronic ITP and had received azathioprine or danazol (n=15), thrombopoietin-receptor agonists (n=10), rituximab (n=7), platelets (n=5), and/or chemotherapy (n=4).
The 34 patients assigned to cohort 1 received 2 doses of subcutaneous veltuzumab at 80 mg, 160 mg, or 320 mg, 2 weeks apart (total doses of 160 mg, 320 mg, and 640 mg, respectively). The 18 patients in cohort 2 (which included 2 rollovers) received once-weekly doses at 320 mg for 4 weeks (total dose of 1280 mg).
The researchers said veltuzumab was well tolerated. The only adverse events were grade 1-2, transient injection reactions.
Forty-seven patients were evaluable for response. Forty-seven percent (n=22) had objective responses (ORs), and 28% (n=13) had complete responses (CRs).
Responses did not differ much according to disease duration. Patients with chronic ITP had an OR rate of 42% and a CR rate of 27%. Patients who had ITP for a year or less had an OR rate of 51% and a CR rate of 29%.
The median time to relapse (TTR) did not differ much between patients with CRs and those with partial responses, but there was a sizable difference between patients with chronic ITP and those with newly diagnosed ITP.
The median TTR was 7.9 months for patients with a CR and 7.6 months for patients with a partial response. The median TTR was 6.9 months for patients with chronic ITP and 14.4 months for patients who had ITP for a year or less.
The phase 2 expansion trial of veltuzumab in ITP has completed accrual, and patients are being followed for up to 5 years.
About orphan designation
The FDA grants orphan designation to drugs that are intended to treat diseases or conditions affecting fewer than 200,000 patients in the US. Orphan designation provides the sponsor of a drug with various development incentives.
The orphan designation for veltuzumab provides Immunomedics with opportunities to apply for research-related tax credits and grant funding, assistance in designing clinical trials, 7 years of US marketing exclusivity if the drug is approved, and other benefits. ![]()

Photo by Linda Bartlett
The US Food and Drug Administration (FDA) has granted orphan designation to veltuzumab for the treatment of immune thrombocytopenia (ITP).
Veltuzumab is a 2nd-generation, humanized monoclonal antibody targeting CD20. The drug is being developed by Immunomedics as a treatment for ITP, other autoimmune diseases, and non-Hodgkin lymphoma.
Veltuzumab was considered active and well-tolerated in a phase 1 study of adults with ITP. The drug produced responses in about half of patients, with some responses lasting more than 4 years.
The study included 50 patients with primary ITP who had failed 1 or more types of standard therapy, had platelet levels of 30,000/μL or less, and did not have major bleeding. The patients’ median age was 54, and most were female (n=31). Eight patients had undergone splenectomy.
Patients were a median of 2 years from diagnosis. Fourteen had been diagnosed with ITP for a year or less and had received corticosteroids and/or immunoglobulins.
Thirty-six patients had chronic ITP and had received azathioprine or danazol (n=15), thrombopoietin-receptor agonists (n=10), rituximab (n=7), platelets (n=5), and/or chemotherapy (n=4).
The 34 patients assigned to cohort 1 received 2 doses of subcutaneous veltuzumab at 80 mg, 160 mg, or 320 mg, 2 weeks apart (total doses of 160 mg, 320 mg, and 640 mg, respectively). The 18 patients in cohort 2 (which included 2 rollovers) received once-weekly doses at 320 mg for 4 weeks (total dose of 1280 mg).
The researchers said veltuzumab was well tolerated. The only adverse events were grade 1-2, transient injection reactions.
Forty-seven patients were evaluable for response. Forty-seven percent (n=22) had objective responses (ORs), and 28% (n=13) had complete responses (CRs).
Responses did not differ much according to disease duration. Patients with chronic ITP had an OR rate of 42% and a CR rate of 27%. Patients who had ITP for a year or less had an OR rate of 51% and a CR rate of 29%.
The median time to relapse (TTR) did not differ much between patients with CRs and those with partial responses, but there was a sizable difference between patients with chronic ITP and those with newly diagnosed ITP.
The median TTR was 7.9 months for patients with a CR and 7.6 months for patients with a partial response. The median TTR was 6.9 months for patients with chronic ITP and 14.4 months for patients who had ITP for a year or less.
The phase 2 expansion trial of veltuzumab in ITP has completed accrual, and patients are being followed for up to 5 years.
About orphan designation
The FDA grants orphan designation to drugs that are intended to treat diseases or conditions affecting fewer than 200,000 patients in the US. Orphan designation provides the sponsor of a drug with various development incentives.
The orphan designation for veltuzumab provides Immunomedics with opportunities to apply for research-related tax credits and grant funding, assistance in designing clinical trials, 7 years of US marketing exclusivity if the drug is approved, and other benefits. ![]()

Photo by Linda Bartlett
The US Food and Drug Administration (FDA) has granted orphan designation to veltuzumab for the treatment of immune thrombocytopenia (ITP).
Veltuzumab is a 2nd-generation, humanized monoclonal antibody targeting CD20. The drug is being developed by Immunomedics as a treatment for ITP, other autoimmune diseases, and non-Hodgkin lymphoma.
Veltuzumab was considered active and well-tolerated in a phase 1 study of adults with ITP. The drug produced responses in about half of patients, with some responses lasting more than 4 years.
The study included 50 patients with primary ITP who had failed 1 or more types of standard therapy, had platelet levels of 30,000/μL or less, and did not have major bleeding. The patients’ median age was 54, and most were female (n=31). Eight patients had undergone splenectomy.
Patients were a median of 2 years from diagnosis. Fourteen had been diagnosed with ITP for a year or less and had received corticosteroids and/or immunoglobulins.
Thirty-six patients had chronic ITP and had received azathioprine or danazol (n=15), thrombopoietin-receptor agonists (n=10), rituximab (n=7), platelets (n=5), and/or chemotherapy (n=4).
The 34 patients assigned to cohort 1 received 2 doses of subcutaneous veltuzumab at 80 mg, 160 mg, or 320 mg, 2 weeks apart (total doses of 160 mg, 320 mg, and 640 mg, respectively). The 18 patients in cohort 2 (which included 2 rollovers) received once-weekly doses at 320 mg for 4 weeks (total dose of 1280 mg).
The researchers said veltuzumab was well tolerated. The only adverse events were grade 1-2, transient injection reactions.
Forty-seven patients were evaluable for response. Forty-seven percent (n=22) had objective responses (ORs), and 28% (n=13) had complete responses (CRs).
Responses did not differ much according to disease duration. Patients with chronic ITP had an OR rate of 42% and a CR rate of 27%. Patients who had ITP for a year or less had an OR rate of 51% and a CR rate of 29%.
The median time to relapse (TTR) did not differ much between patients with CRs and those with partial responses, but there was a sizable difference between patients with chronic ITP and those with newly diagnosed ITP.
The median TTR was 7.9 months for patients with a CR and 7.6 months for patients with a partial response. The median TTR was 6.9 months for patients with chronic ITP and 14.4 months for patients who had ITP for a year or less.
The phase 2 expansion trial of veltuzumab in ITP has completed accrual, and patients are being followed for up to 5 years.
About orphan designation
The FDA grants orphan designation to drugs that are intended to treat diseases or conditions affecting fewer than 200,000 patients in the US. Orphan designation provides the sponsor of a drug with various development incentives.
The orphan designation for veltuzumab provides Immunomedics with opportunities to apply for research-related tax credits and grant funding, assistance in designing clinical trials, 7 years of US marketing exclusivity if the drug is approved, and other benefits. ![]()
Reporting requirements may affect trial outcomes

Photo by Esther Dyson
The reporting requirements developed to increase transparency in US medical research may lead to fewer positive trial outcomes, according to a study published in PLOS ONE.
Researchers analyzed data from large-budget trials funded by the National Heart, Lung and Blood Institute (NHLBI).
And they found evidence suggesting the reporting requirements may have contributed to a significant reduction in studies with positive findings.
The reporting standards were phased in around 2000. They require researchers conducting drug or dietary supplement trials using human subjects to identify
projected outcomes and register their trials on ClinicalTrials.gov before they begin to collect data.
When entering their trial into the database, researchers are required to state the specific outcome on which they will focus. In the past, a researcher might have published an aspect of a study that was successful, even if the study overall did not produce the expected results.
But the new requirements mean researchers are less likely to change their analysis plan to consider another outcome that may have shown a positive result, said Veronica L. Irvin, PhD, of Oregon State University in Corvallis.
Dr Irvin began working on this project with the study’s lead author, Robert M. Kaplan, PhD, of the Agency for Healthcare Research and Quality in Rockville,
Maryland, while the two worked together in the National Institutes of Health’s Office of Behavior and Social Science Research.
The pair reviewed all large-budget clinical trials evaluating drugs or dietary supplements for the treatment or prevention of cardiovascular disease that had received funding from the NHLBI between 1970 and 2012.
They chose large-budget, NHLBI-funded trials in part because outcomes from the trials were more likely to be published, even if they did not produce the expected result.
Fifty-five studies were included in the research. Thirty were published prior to the reporting changes in 2000 (1970 to 1999), and 25 were published after the changes (2000 to 2012).
Of the studies published after 2000, only 2 (8%) showed positive outcomes, while 17 (57%) of the studies published before 2000 showed positive results.
Drs Kaplan and Irvin acknowledged that factors other than the reporting requirements may be contributing to the decline in positive outcomes, but they were unable to identify other compelling explanations.
For example, one suggestion was that older trials were more likely to compare new treatments to placebos, while newer trials were more likely to compare new
treatments to established treatments.
But when Drs Kaplan and Irvin examined the data, they found that 60% of trials published before 2000 used placebo comparators and nearly the same amount, 64%, of trials published after 2000 used placebos.
The researchers noted that although this work focused on clinical trials related to cardiovascular health, it would be reasonable to see similar changes in results
across other disease types.
“We don’t know if this decrease in positive outcomes also affects drug trials for prevention and treatment of cancer, diabetes, or other diseases,” Dr Irvin said. “But it would not be surprising because they have the same reporting requirements.” ![]()

Photo by Esther Dyson
The reporting requirements developed to increase transparency in US medical research may lead to fewer positive trial outcomes, according to a study published in PLOS ONE.
Researchers analyzed data from large-budget trials funded by the National Heart, Lung and Blood Institute (NHLBI).
And they found evidence suggesting the reporting requirements may have contributed to a significant reduction in studies with positive findings.
The reporting standards were phased in around 2000. They require researchers conducting drug or dietary supplement trials using human subjects to identify
projected outcomes and register their trials on ClinicalTrials.gov before they begin to collect data.
When entering their trial into the database, researchers are required to state the specific outcome on which they will focus. In the past, a researcher might have published an aspect of a study that was successful, even if the study overall did not produce the expected results.
But the new requirements mean researchers are less likely to change their analysis plan to consider another outcome that may have shown a positive result, said Veronica L. Irvin, PhD, of Oregon State University in Corvallis.
Dr Irvin began working on this project with the study’s lead author, Robert M. Kaplan, PhD, of the Agency for Healthcare Research and Quality in Rockville,
Maryland, while the two worked together in the National Institutes of Health’s Office of Behavior and Social Science Research.
The pair reviewed all large-budget clinical trials evaluating drugs or dietary supplements for the treatment or prevention of cardiovascular disease that had received funding from the NHLBI between 1970 and 2012.
They chose large-budget, NHLBI-funded trials in part because outcomes from the trials were more likely to be published, even if they did not produce the expected result.
Fifty-five studies were included in the research. Thirty were published prior to the reporting changes in 2000 (1970 to 1999), and 25 were published after the changes (2000 to 2012).
Of the studies published after 2000, only 2 (8%) showed positive outcomes, while 17 (57%) of the studies published before 2000 showed positive results.
Drs Kaplan and Irvin acknowledged that factors other than the reporting requirements may be contributing to the decline in positive outcomes, but they were unable to identify other compelling explanations.
For example, one suggestion was that older trials were more likely to compare new treatments to placebos, while newer trials were more likely to compare new
treatments to established treatments.
But when Drs Kaplan and Irvin examined the data, they found that 60% of trials published before 2000 used placebo comparators and nearly the same amount, 64%, of trials published after 2000 used placebos.
The researchers noted that although this work focused on clinical trials related to cardiovascular health, it would be reasonable to see similar changes in results
across other disease types.
“We don’t know if this decrease in positive outcomes also affects drug trials for prevention and treatment of cancer, diabetes, or other diseases,” Dr Irvin said. “But it would not be surprising because they have the same reporting requirements.” ![]()

Photo by Esther Dyson
The reporting requirements developed to increase transparency in US medical research may lead to fewer positive trial outcomes, according to a study published in PLOS ONE.
Researchers analyzed data from large-budget trials funded by the National Heart, Lung and Blood Institute (NHLBI).
And they found evidence suggesting the reporting requirements may have contributed to a significant reduction in studies with positive findings.
The reporting standards were phased in around 2000. They require researchers conducting drug or dietary supplement trials using human subjects to identify
projected outcomes and register their trials on ClinicalTrials.gov before they begin to collect data.
When entering their trial into the database, researchers are required to state the specific outcome on which they will focus. In the past, a researcher might have published an aspect of a study that was successful, even if the study overall did not produce the expected results.
But the new requirements mean researchers are less likely to change their analysis plan to consider another outcome that may have shown a positive result, said Veronica L. Irvin, PhD, of Oregon State University in Corvallis.
Dr Irvin began working on this project with the study’s lead author, Robert M. Kaplan, PhD, of the Agency for Healthcare Research and Quality in Rockville,
Maryland, while the two worked together in the National Institutes of Health’s Office of Behavior and Social Science Research.
The pair reviewed all large-budget clinical trials evaluating drugs or dietary supplements for the treatment or prevention of cardiovascular disease that had received funding from the NHLBI between 1970 and 2012.
They chose large-budget, NHLBI-funded trials in part because outcomes from the trials were more likely to be published, even if they did not produce the expected result.
Fifty-five studies were included in the research. Thirty were published prior to the reporting changes in 2000 (1970 to 1999), and 25 were published after the changes (2000 to 2012).
Of the studies published after 2000, only 2 (8%) showed positive outcomes, while 17 (57%) of the studies published before 2000 showed positive results.
Drs Kaplan and Irvin acknowledged that factors other than the reporting requirements may be contributing to the decline in positive outcomes, but they were unable to identify other compelling explanations.
For example, one suggestion was that older trials were more likely to compare new treatments to placebos, while newer trials were more likely to compare new
treatments to established treatments.
But when Drs Kaplan and Irvin examined the data, they found that 60% of trials published before 2000 used placebo comparators and nearly the same amount, 64%, of trials published after 2000 used placebos.
The researchers noted that although this work focused on clinical trials related to cardiovascular health, it would be reasonable to see similar changes in results
across other disease types.
“We don’t know if this decrease in positive outcomes also affects drug trials for prevention and treatment of cancer, diabetes, or other diseases,” Dr Irvin said. “But it would not be surprising because they have the same reporting requirements.” ![]()
Lenalidomide can treat pulmonary sarcoidosis in MDS

Treatment with lenalidomide can have a significant effect on pulmonary sarcoidosis in myelodysplastic syndrome (MDS), according to a case study.
The case was a 71-year-old woman with newly diagnosed 5q-MDS and a long-standing history of refractory pulmonary sarcoidosis.
After 2 cycles of treatment with lenalidomide, the patient had substantial improvements in lung function, fatigue, daily activity, and quality of life.
This case is the first of its kind to show the potential effects of lenalidomide as a therapeutic option in patients with pulmonary sarcoidosis.
Ali Bazargan, MD, of St. Vincent’s Hospital in Melbourne, Victoria, Australia, and his colleagues described this case in CHEST.
The patient had a 12-year history of stage IV pulmonary sarcoidosis with no extrapulmonary organ involvement. She had never smoked but had a history of hypertension that was managed with perindopril.
The patient presented with refractory and worsening dyspnea, despite receiving long-term therapy with methotrexate and inhaled and systemic corticosteroids. Before she began receiving lenalidomide, the patient was taking 15 mg of prednisolone and 400 mg of inhaled budesonide daily.
Blood tests revealed the patient had macrocytic anemia (hemoglobin level, 81 g/L; mean corpuscular volume, 114 fL).
A subsequent bone marrow biopsy revealed hypocellular marrow with trilineage dysplasia consistent with 5q-MDS but no evidence of noncaseating granulomas. So the patient began receiving lenalidomide at 10 mg daily.
While the researchers were trying to establish her diagnosis of 5q-MDS, the patient became transfusion-dependent and experienced severe dyspnea, fatigue, and a considerable decline in quality of life.
A chest CT scan revealed irregular masses in her lung, with bibasal alveolar infiltrates that had developed within a 12-month period.
However, after 2 cycles of lenalidomide, the patient had significant improvements in dyspnea, fatigue, daily activity, and quality of life. Lung function testing showed an increase in vital capacity from 1.73 L to 1.93 L.
And a chest CT scan performed 4 months after the patient began taking lenalidomide showed that the bibasal alveolar infiltrates had completely cleared.
During this period, the patient’s dose of prednisolone was reduced from 15 mg daily to 5 mg on alternate days, but she continues to receive the same dose of lenalidomide. ![]()

Treatment with lenalidomide can have a significant effect on pulmonary sarcoidosis in myelodysplastic syndrome (MDS), according to a case study.
The case was a 71-year-old woman with newly diagnosed 5q-MDS and a long-standing history of refractory pulmonary sarcoidosis.
After 2 cycles of treatment with lenalidomide, the patient had substantial improvements in lung function, fatigue, daily activity, and quality of life.
This case is the first of its kind to show the potential effects of lenalidomide as a therapeutic option in patients with pulmonary sarcoidosis.
Ali Bazargan, MD, of St. Vincent’s Hospital in Melbourne, Victoria, Australia, and his colleagues described this case in CHEST.
The patient had a 12-year history of stage IV pulmonary sarcoidosis with no extrapulmonary organ involvement. She had never smoked but had a history of hypertension that was managed with perindopril.
The patient presented with refractory and worsening dyspnea, despite receiving long-term therapy with methotrexate and inhaled and systemic corticosteroids. Before she began receiving lenalidomide, the patient was taking 15 mg of prednisolone and 400 mg of inhaled budesonide daily.
Blood tests revealed the patient had macrocytic anemia (hemoglobin level, 81 g/L; mean corpuscular volume, 114 fL).
A subsequent bone marrow biopsy revealed hypocellular marrow with trilineage dysplasia consistent with 5q-MDS but no evidence of noncaseating granulomas. So the patient began receiving lenalidomide at 10 mg daily.
While the researchers were trying to establish her diagnosis of 5q-MDS, the patient became transfusion-dependent and experienced severe dyspnea, fatigue, and a considerable decline in quality of life.
A chest CT scan revealed irregular masses in her lung, with bibasal alveolar infiltrates that had developed within a 12-month period.
However, after 2 cycles of lenalidomide, the patient had significant improvements in dyspnea, fatigue, daily activity, and quality of life. Lung function testing showed an increase in vital capacity from 1.73 L to 1.93 L.
And a chest CT scan performed 4 months after the patient began taking lenalidomide showed that the bibasal alveolar infiltrates had completely cleared.
During this period, the patient’s dose of prednisolone was reduced from 15 mg daily to 5 mg on alternate days, but she continues to receive the same dose of lenalidomide. ![]()

Treatment with lenalidomide can have a significant effect on pulmonary sarcoidosis in myelodysplastic syndrome (MDS), according to a case study.
The case was a 71-year-old woman with newly diagnosed 5q-MDS and a long-standing history of refractory pulmonary sarcoidosis.
After 2 cycles of treatment with lenalidomide, the patient had substantial improvements in lung function, fatigue, daily activity, and quality of life.
This case is the first of its kind to show the potential effects of lenalidomide as a therapeutic option in patients with pulmonary sarcoidosis.
Ali Bazargan, MD, of St. Vincent’s Hospital in Melbourne, Victoria, Australia, and his colleagues described this case in CHEST.
The patient had a 12-year history of stage IV pulmonary sarcoidosis with no extrapulmonary organ involvement. She had never smoked but had a history of hypertension that was managed with perindopril.
The patient presented with refractory and worsening dyspnea, despite receiving long-term therapy with methotrexate and inhaled and systemic corticosteroids. Before she began receiving lenalidomide, the patient was taking 15 mg of prednisolone and 400 mg of inhaled budesonide daily.
Blood tests revealed the patient had macrocytic anemia (hemoglobin level, 81 g/L; mean corpuscular volume, 114 fL).
A subsequent bone marrow biopsy revealed hypocellular marrow with trilineage dysplasia consistent with 5q-MDS but no evidence of noncaseating granulomas. So the patient began receiving lenalidomide at 10 mg daily.
While the researchers were trying to establish her diagnosis of 5q-MDS, the patient became transfusion-dependent and experienced severe dyspnea, fatigue, and a considerable decline in quality of life.
A chest CT scan revealed irregular masses in her lung, with bibasal alveolar infiltrates that had developed within a 12-month period.
However, after 2 cycles of lenalidomide, the patient had significant improvements in dyspnea, fatigue, daily activity, and quality of life. Lung function testing showed an increase in vital capacity from 1.73 L to 1.93 L.
And a chest CT scan performed 4 months after the patient began taking lenalidomide showed that the bibasal alveolar infiltrates had completely cleared.
During this period, the patient’s dose of prednisolone was reduced from 15 mg daily to 5 mg on alternate days, but she continues to receive the same dose of lenalidomide. ![]()
Febrile Infant Diagnosis Code Accuracy
Fever is one of the most common reasons for emergency department (ED) evaluation of infants under 90 days of age.[1] Up to 10% to 20% of febrile young infants will have a serious bacterial infection (SBI),[2, 3, 4] but infants with SBI are difficult to distinguish from those without SBI based upon symptoms and physical examination findings alone.[5] Previously developed clinical prediction algorithms can help to identify febrile infants at low risk for SBI, but differ in age range as well as recommendations for testing and empiric treatment.[6, 7, 8] Consequently, there is widespread variation in management of febrile young infants at US children's hospitals,[9, 10, 11] and defining optimal management strategies remains an important issue in pediatric healthcare.[12] Administrative datasets are convenient and inexpensive, and can be used to evaluate practice variation, trends, and outcomes of a large, diverse group of patients within and across institutions.[9, 10] Accurately identifying febrile infants evaluated for suspected SBI in administrative databases would facilitate comparative effectiveness research, quality improvement initiatives, and institutional benchmarking.
Prior studies have validated the accuracy of administrative billing codes for identification of other common childhood illnesses, including urinary tract infection (UTI)[13] and pneumonia.[14] The accuracy of International Classification of Diseases, Ninth Revision (ICD‐9) diagnosis codes in identifying febrile young infants evaluated for SBI is not known. Reliance on administrative ICD‐9 diagnosis codes for patient identification can lead to misclassification of patients due to variable database quality, the validity of the diagnosis codes being utilized, and hospital coding practices.[15] Additionally, fever is a symptom and not a specific diagnosis. If a particular bacterial or viral diagnosis is established (eg, enterovirus meningitis), a discharge diagnosis of fever may not be attributed to the patient encounter. Thus, evaluating the performance characteristics and capture of clinical outcomes of different combinations of ICD‐9 diagnosis codes for identifying febrile infants is necessary for both the conduct and interpretation of studies that utilize administrative databases. The primary objective of this investigation was to identify the most accurate ICD‐9 coding strategies for the identification of febrile infants aged <90 days using administrative data. We also sought to evaluate capture of clinically important outcomes across identification strategies.
METHODS
Study Design and Setting
For this multicenter retrospective study, we used the Pediatric Health Information System (PHIS) database to identify infants <90 days of age[16] who presented between July 1, 2012 and June 30, 2013 to 1 of 8 EDs. We assessed performance characteristics of ICD‐9 diagnosis code case‐identification algorithms by comparing ICD‐9 code combinations to a fever reference standard determined by medical record review. The institutional review board at each participating site approved the study protocol.
Data Source
Data were obtained from 2 sources: the PHIS database and medical record review. We used the PHIS database to identify eligible patients by ICD‐9 diagnosis codes; patient encounters were randomly selected using a random number generator. The PHIS database contains demographic, diagnosis, and billing data from 44 hospitals affiliated with the Children's Hospital Association (Overland Park, Kansas) and represents 85% of freestanding children's hospitals in the United States.[17] Data are deidentified; encrypted unique patient identifiers permit tracking of patients across visits within a site.[18] The Children's Hospital Association and participating hospitals jointly assure the quality and integrity of the data.[19]
For each patient encounter identified in the PHIS database, detailed medical record review was performed by trained investigators at each of the 8 study sites (see Supporting Information, Appendix, in the online version of this article). A standardized data collection instrument was pilot tested by all investigators prior to use. Data were collected and managed using the Research Electronic Data Capture (REDCap) tool hosted at Boston Children's Hospital.[20]
Exclusions
Using PHIS data, prior to medical record review we excluded infants with a complex chronic condition as defined previously[21] and those transferred from another institution, as these infants may warrant a nonstandard evaluation and/or may have incomplete data.
ICD‐9 Diagnosis Code Groups
In the PHIS database, all patients discharged from the hospital (including hospitalized patients as well as patients discharged from the ED) receive 1 or more ICD‐9 discharge diagnosis codes. These diagnosis codes are ascribed after discharge from the hospital, or for ED patients, after ED discharge. Additionally, patients may receive an admission diagnosis, which reflects the diagnosis ascribed at the time of ED discharge or transfer to the inpatient unit.
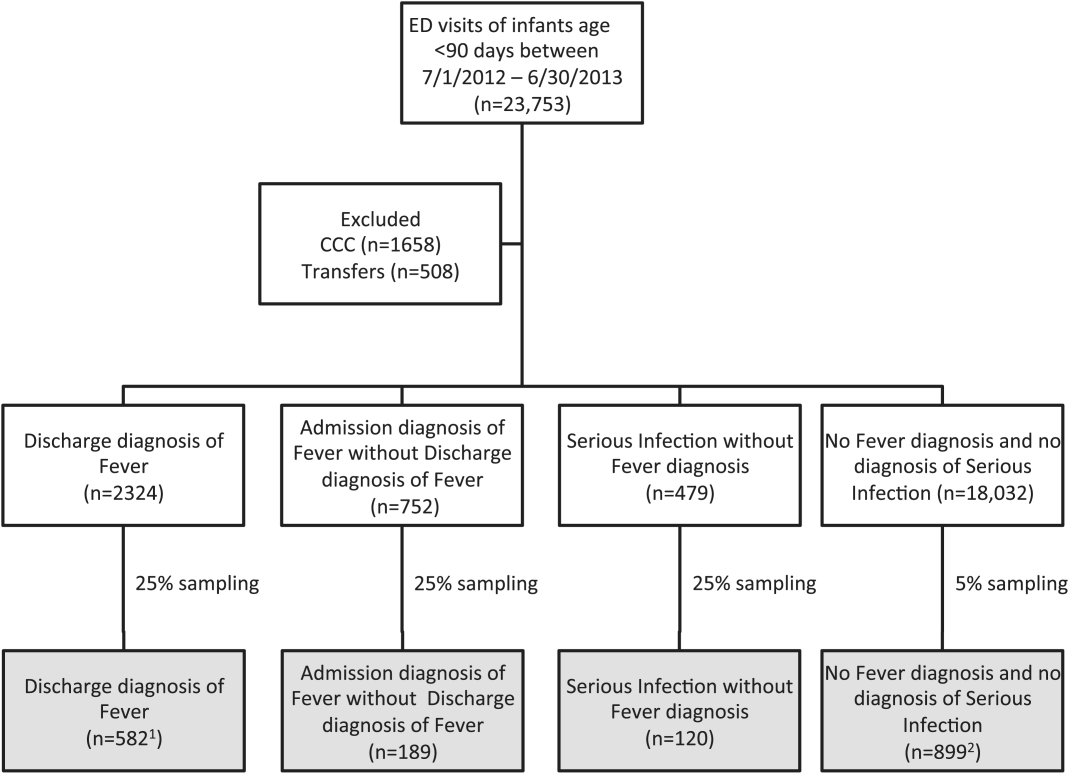
We reviewed medical records of infants selected from the following ICD‐9 diagnosis code groups (Figure 1): (1) discharge diagnosis code of fever (780.6 [fever and other physiologic disturbances of temperature regulation], 778.4 [other disturbances of temperature regulation of newborn], 780.60 [fever, unspecified], or 780.61 [fever presenting with conditions classified elsewhere])[9, 10] regardless of the presence of admission diagnosis of fever or diagnosis of serious infection, (2) admission diagnosis code of fever without associated discharge diagnosis code of fever,[10] (3) discharge diagnosis code of serious infection determined a priori (see Supporting Information, Appendix, in the online version of this article) without discharge or admission diagnosis code of fever, and (4) infants without any diagnosis code of fever or serious infection.
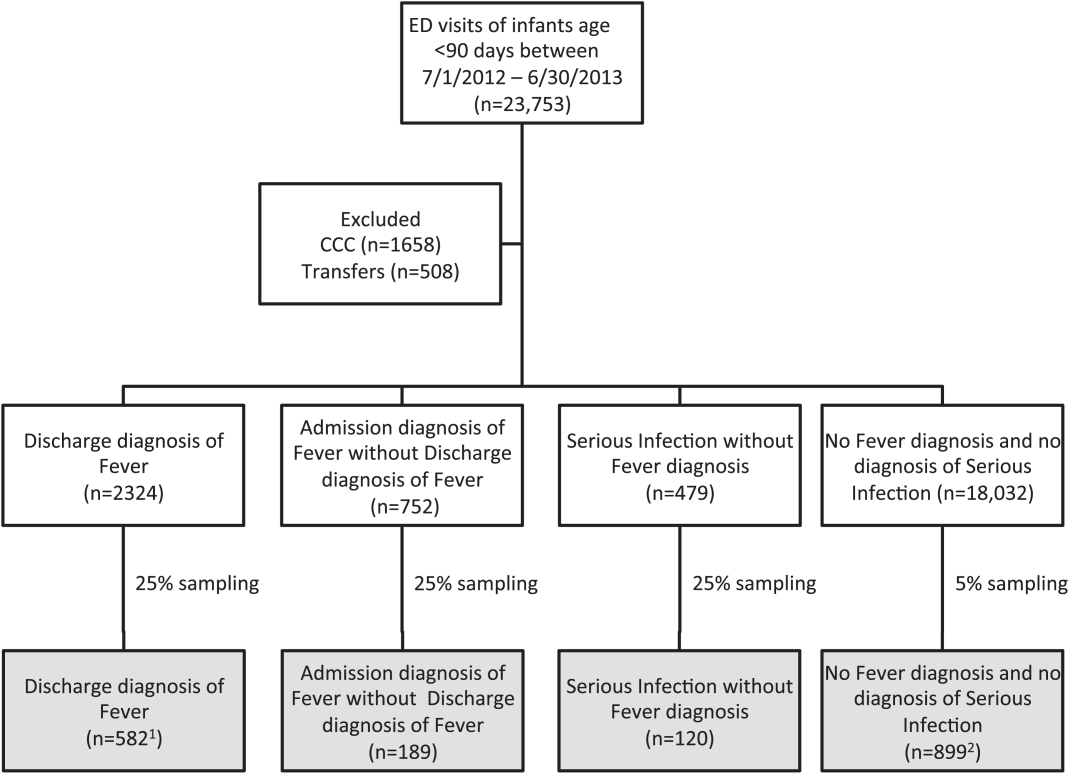
Medical records reviewed in each of the 4 ICD‐9 diagnosis code groups were randomly selected from the overall set of ED encounters in the population of infants <90 days of age evaluated during the study period. Twenty‐five percent population sampling was used for 3 of the ICD‐9 diagnosis code groups, whereas 5% sampling was used for the no fever/no serious infection code group. The number of medical records reviewed in each ICD‐9 diagnosis code group was proportional to the distribution of ICD‐9 codes across the entire population of infants <90 days of age. These records were distributed equally across sites (228 records per site), except for 1 site that does not assign admission diagnoses (201 records).
Investigators were blinded to ICD‐9 diagnosis code groups during medical record review. Infants with multiple visits during the study period were eligible to be included more than once if the visits occurred more than 3 days apart. For infants with more than 1 ED visit on a particular calendar day, investigators were instructed to review the initial visit.
For each encounter, we also abstracted demographic characteristics (gender, race/ethnicity), insurance status, hospital region (using US Census categories[22]), and season from the PHIS database.
Reference Standard
The presence of fever was determined by medical record review. We defined fever as any documented temperature 100.4F (38.0C) at home or in the ED.[16]
ICD‐9 Code Case‐Identification Algorithms
Using the aforementioned ICD‐9 diagnosis code groups individually and in combination, the following 4 case‐identification algorithms, determined from prior study or group consensus, were compared to the reference standard: (1) ICD‐9 discharge diagnosis code of fever,[9] (2) ICD‐9 admission or discharge diagnosis code of fever,[10, 11] (3) ICD‐9 discharge diagnosis code of fever or serious infection, and (4) ICD‐9 discharge or admission diagnosis code of fever or serious infection. Algorithms were compared overall, separately for discharged and hospitalized infants, and across 3 distinct age groups (28 days, 2956 days, and 5789 days).
Patient‐Level Outcomes
To compare differences in outcomes by case‐identification algorithm, from the PHIS database we abstracted hospitalization rates, rates of UTI/pyelonephritis,[13] bacteremia/sepsis, and bacterial meningitis.[19] Severe outcomes were defined as intensive care unit admission, mechanical ventilation, central line placement, receipt of extracorporeal membrane oxygenation, or death. We assessed hospital length of stay for admitted infants and 3‐day revisits,[23, 24] and revisits resulting in hospitalization for infants discharged from the ED at the index visit. Patients billed for observation care were classified as being hospitalized.[25, 26]
Data Analysis
Accuracy of the 4 case‐identification algorithms (compared with the reference standard) was calculated using sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV), along with 95% confidence interval (CI). Prior to analysis, a 5‐fold weighting factor was applied to the no fever/no serious infection group to account for the differential sampling used for this group (5% vs 25% for the other 3 ICD‐9 diagnosis code groups). This weighting was done to approximate the true prevalence of each ICD‐9 code group within the larger population, so that an accurate rate of false negatives (infants with fever who had neither a diagnosis of fever nor serious infection) could be calculated.
We described continuous variables using median and interquartile range or range values and categorical variables using frequencies with 95% CIs. We compared categorical variables using a 2 test. We determined statistical significance as a 2‐tailed P value <0.05. Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Study Patients
During the 1‐year study period, 23,753 ED encounters for infants <90 days of age were identified in the PHIS database at the 8 participating sites. Of these infant encounters, 2166 (9.2%) were excluded (1658 infants who had a complex chronic condition and 508 transferred into the ED), leaving 21,587 infants available for selection. After applying our sampling strategy, we identified 1797 encounters for medical record review. Seven encounters from 3 hospitals with missing medical records were excluded, resulting in a final cohort of 1790 encounters (Figure 1). Among included infants, 552 (30.8%) were 28 days, 743 (41.5%) were 29 to 56 days, and 495 (27.8%) were 57 to 89 days of age; 737 (41.2%) infants were hospitalized. Patients differed in age, race, payer, and season across ICD‐9 diagnosis code groups (see Supporting Information, Table 1, in the online version of this article).
| ICD‐9 Diagnosis Code Algorithm | Overall | |||
|---|---|---|---|---|
| Sensitivity, % (95% CI) | Specificity, % (95% CI) | Negative Predictive Value, % (95% CI) | Positive Predictive Value, % (95% CI) | |
| ||||
| Discharge diagnosis of fever | 53.2 (50.056.4) | 98.2 (97.898.6) | 90.8 (90.091.6) | 86.1 (83.388.9) |
| Hospitalized | 47.3 (43.151.5) | 97.7 (96.998.5) | 80.6 (78.682.6) | 90.2 (86.893.6) |
| Discharged from ED | 61.4 (56.666.2) | 98.4 (98.098.8) | 95.4 (94.796.1) | 82.1 (77.786.5) |
| Discharge or admission diagnosis of Fever | 71.1 (68.274.0) | 97.7 (97.398.1) | 94.1 (93.494.8) | 86.9 (84.589.3) |
| Hospitalized | 72.5 (68.876.2) | 97.1 (96.298.0) | 88.8 (87.190.5) | 91.7 (89.194.3) |
| Discharged from ED | 69.2 (64.773.7) | 98.0 (97.598.5) | 96.3 (95.796.9) | 80.8 (76.685.0) |
| Discharge diagnosis of fever or serious infection | 63.7 (60.666.8) | 96.5 (96.097.0) | 92.6 (91.893.4) | 79.6 (76.782.5) |
| Hospitalized | 63.9 (59.967.9) | 92.5 (91.094.0) | 85.1 (83.287.0) | 79.1 (75.382.9) |
| Discharged from ED | 63.4 (58.768.1) | 98.1 (97.698.6) | 95.6 (94.996.3) | 80.2 (75.884.6) |
| Discharge or admission diagnosis of fever or serious infection | 76.6 (73.979.3) | 96.2 (95.696.8) | 95.1 (94.595.7) | 81.0 (78.483.6) |
| Hospitalized | 80.8 (77.584.1) | 92.1 (90.693.6) | 91.5 (89.993.1) | 82.1 (78.985.3) |
| Discharged from ED | 71.0 (66.575.5) | 97.7 (97.298.2) | 96.5 (95.997.1) | 79.4 (75.283.6) |
Among the 1790 patient encounters reviewed, a total of 766 infants (42.8%) met the reference standard definition for fever in the cohort. An additional 47 infants had abnormal temperature reported (documentation of tactile fever, history of fever without a specific temperature described, or hypothermia) but were classified as having no fever by the reference standard.
ICD‐9 Code Case‐Identification Algorithm Performance
Compared with the reference standard, the 4 case‐identification algorithms demonstrated specificity of 96.2% to 98.2% but lower sensitivity overall (Figure 2). Discharge diagnosis of fever alone demonstrated the lowest sensitivity. The algorithm of discharge or admission diagnosis of fever resulted in increased sensitivity and the highest PPV of all 4 algorithms (86.9%, 95% CI: 84.5‐89.3). Addition of serious infection codes to this algorithm resulted in a marginal increase in sensitivity and a similar decrease in PPV (Table 1). When limited to hospitalized infants, specificity was highest for the case‐identification algorithm of discharge diagnosis of fever and similarly high for discharge or admission diagnosis of fever; sensitivity was highest for the algorithm of discharge or admission diagnosis of fever or diagnosis of serious infection. For infants discharged from the ED, algorithm specificity was 97.7% to 98.4%, with lower sensitivity for all 4 algorithms (Table 1). Inclusion of the 47 infants with abnormal temperature as fever did not materially change algorithm performance (data not shown).
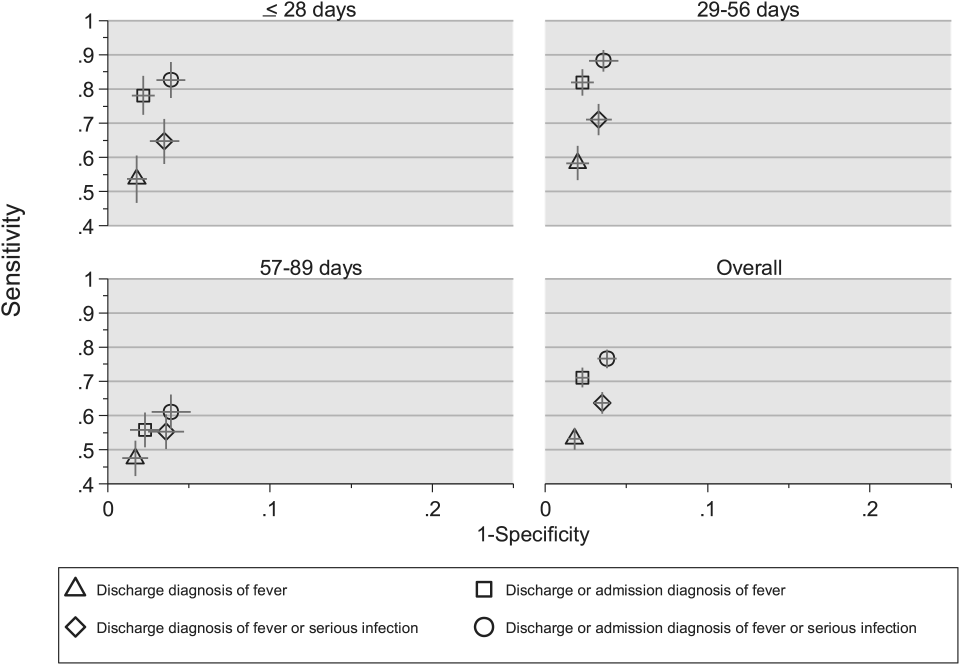
Across all 3 age groups (28 days, 2956 days, and 5789 days), the 4 case‐identification algorithms demonstrated specificity >96%, whereas algorithm sensitivity was highest in the 29‐ to 56‐days‐old age group and lowest among infants 57 to 89 days old across all 4 algorithms (Figure 2). Similar to the overall cohort, an algorithm of discharge or admission diagnosis of fever demonstrated specificity of nearly 98% in all age groups; addition of serious infection codes to this algorithm increased sensitivity, highest in the 29‐ to 56‐days‐old age group (Figure 2; see also Supporting Information, Table 2, in the online version of this article).
| ICD‐9 Diagnosis Code Algorithm | Sensitivity, Median % (Range) | Specificity, Median % (Range) | Negative Predictive Value, Median % (Range) | Positive Predictive Value, Median % (Range) |
|---|---|---|---|---|
| ||||
| Discharge diagnosis of fever | 56.2 (34.681.0) | 98.3 (96.499.1) | 92.1 (83.297.4) | 87.7 (74.093.2) |
| Discharge or Admission diagnosis of Fever | 76.7 (51.385.0) | 97.8 (96.298.7) | 95.6 (86.997.4) | 87.4 (80.092.9) |
| Discharge diagnosis of fever or serious infection | 68.3 (44.287.3) | 96.5 (95.498.0) | 93.6 (85.298.2) | 78.3 (74.289.0) |
| Discharge or admission diagnosis of fever or serious infection | 83.1 (58.390.7) | 95.8 (95.498.0) | 96.5 (88.598.2) | 79.1 (77.490.4) |
Across the 8 study sites, median specificity was 95.8% to 98.3% for the 4 algorithms, with little interhospital variability; however, algorithm sensitivity varied widely by site. Median PPV was highest for discharge diagnosis of fever alone at 87.7% but ranged from 74.0% to 93.2% across sites. Median PPV for an algorithm of discharge or admission diagnosis of fever was similar (87.4%) but with less variation by site (range 80.0%92.9%) (Table 2).
Outcomes by ICD‐9 Diagnosis Code Group and Case‐Identification Algorithm
When compared with discharge diagnosis of fever, adding admission diagnosis of fever captured a higher proportion of hospitalized infants with SBIs (UTI/pyelonephritis, bacteremia/sepsis, or bacterial meningitis). However, median hospital length of stay, severe outcomes, and 3‐day revisits and revisits with hospitalization did not materially differ when including infants with admission diagnosis of fever in addition to discharge diagnosis of fever. Addition of infants with a diagnosis code for serious infection substantially increased the number of infants with SBIs and severe outcomes but did not capture additional 3‐day revisits (Table 3). There were no additional cases of SBI in the no fever/no serious illness diagnosis code group.
| ICD‐9 Diagnosis Code Algorithm | Outcome | 3‐Day Revisit, % (95% CI) | 3‐Day Revisit With Hospitalization, % (95% CI) | |||
|---|---|---|---|---|---|---|
| Hospitalized, % (95% CI) | UTI/Pyelonephritis, Bacteremia/Sepsis, or Bacterial Meningitis, % (95% CI) | Severe Outcome, % (95% CI)* | Length of Stay in Days, Median (IQR) | |||
| ||||||
| Discharge diagnosis of fever | 44.3 (40.348.4) | 3.3 (1.84.7) | 1.4 (0.42.3) | 3 (23) | 11.7 (8.215.2) | 5.9 (3.38.4) |
| Discharge or admission diagnosis of fever | 52.4 (48.955.9) | 6.1 (4.47.8) | 1.9 (1.02.9) | 3 (23) | 10.9 (7.714.1) | 5.4 (3.17.8) |
| Discharge diagnosis of fever or serious infection | 54.0 (50.457.5) | 15.3 (12.717.8) | 3.8 (2.55.2) | 3 (24) | 11.0 (7.714.2) | 5.5 (3.17.9) |
| Discharge or admission diagnosis of fever or serious infection | 56.5 (53.259.7) | 12.9 (10.715.1) | 3.6 (2.44.8) | 3 (24) | 10.3 (7.313.3) | 5.2 (3.07.4) |
Among infants who met the reference standard for fever but did not have a discharge or admission diagnosis of fever (false negatives), 11.8% had a diagnosis of SBI. Overall, 43.2% of febrile infants (and 84.4% of hospitalized infants) with SBI did not have an ICD‐9 discharge or admission diagnosis of fever. Addition of ICD‐9 diagnosis codes of serious infection to the algorithm of discharge or admission diagnosis of fever captured all additional SBIs, and no false negativeinfants missed with this algorithm had an SBI.
DISCUSSION
We described the performance of 4 ICD‐9 diagnosis code case‐identification algorithms for the identification of febrile young infants <90 days of age at US children's hospitals. Although the specificity was high across algorithms and institutions, the sensitivity was relatively low, particularly for discharge diagnosis of fever, and varied by institution. Given the high specificity, ICD‐9 diagnosis code case‐identification algorithms for fever reliably identify febrile infants using administrative data with low rates of inclusion of infants without fever. However, underidentification of patients, particularly those more prone to SBIs and severe outcomes depending on the algorithm utilized, can impact interpretation of comparative effectiveness studies or the quality of care delivered by an institution.
ICD‐9 discharge diagnosis codes are frequently used to identify pediatric patients across a variety of administrative databases, diseases, and symptoms.[19, 27, 28, 29, 30, 31] Although discharge diagnosis of fever is highly specific, sensitivity is substantially lower than other case‐identification algorithms we studied, particularly for hospitalized infants. This may be due to a fever code sometimes being omitted in favor of a more specific diagnosis (eg, bacteremia) prior to hospital discharge. Therefore, case identification relying only on ICD‐9 discharge diagnosis codes for fever may under‐report clinically important SBI or severe outcomes as demonstrated in our study. This is in contrast to ICD‐9 diagnosis code identification strategies for childhood UTI and pneumonia, which largely have higher sensitivity but lower specificity than fever codes.[13, 14]
Admission diagnosis of fever is important for febrile infants as they may not have an explicit diagnosis at the time of disposition from the ED. Addition of admission diagnosis of fever to an algorithm relying on discharge diagnosis code alone increased sensitivity without a demonstrable reduction in specificity and PPV, likely due to capture of infants with a fever diagnosis at presentation before a specific infection was identified. Although using an algorithm of discharge or admission diagnosis of fever captured a higher percentage of hospitalized febrile infants with SBIs, sensitivity was only 71% overall with this algorithm, and 43% of febrile infants with SBI would still have been missed. Importantly, though, addition of various ICD‐9 codes for serious infection to this algorithm resulted in capture of all febrile infants with SBI and should be used as a sensitivity analysis.
The test characteristics of diagnosis codes were highest in the 29‐ to 56‐days‐old age group. Given the differing low‐risk criteria[6, 7, 8] and lack of best practice guidelines[16] in this age group, the use of administrative data may allow for the comparison of testing and treatment strategies across a large cohort of febrile infants aged 29 to 56 days. However, individual hospital coding practices may affect algorithm performance, in particular sensitivity, which varied substantially by hospital. This variation in algorithm sensitivity may impact comparisons of outcomes across institutions. Therefore, when conducting studies of febrile infants using administrative data, sensitivity analyses or use of chart review should be considered to augment the use of ICD‐9 code‐based identification strategies, particularly for comparative benchmarking and outcomes studies. These additional analyses are particularly important for studies of febrile infants >56 days of age, in whom the sensitivity of diagnosis codes is particularly low. We speculate that the lower sensitivity in older febrile infants may relate to a lack of consensus on the clinical significance of fever in this age group and the varying management strategies employed.[10]
Strengths of this study include the assessment of ICD‐9 code algorithms across multiple institutions for identification of fever in young infants, and the patterns of our findings remained robust when comparing median performance characteristics of the algorithms across hospitals to our overall findings. We were also able to accurately estimate PPV and NPV using a case‐identification strategy weighted to the actual population sizes. Although sensitivity and specificity are the primary measures of test performance, predictive values are highly informative for investigators using administrative data. Additionally, our findings may inform public health efforts including disease surveillance, assessment of seasonal variation, and identification and monitoring of healthcare‐associated infections among febrile infants.
Our study has limitations. We did not review all identified records, which raises the possibility that our evaluated cohort may not be representative of the entire febrile infant population. We attempted to mitigate this possibility by using a random sampling strategy for our population selection that was weighted to the actual population sizes. Second, we identified serious infections using ICD‐9 diagnosis codes determined by group consensus, which may not capture all serious infection codes that identify febrile infants whose fever code was omitted. Third, 47 infants had abnormal temperature that did not meet our reference standard criteria for fever and were included in the no fever group. Although there may be disagreement regarding what constitutes a fever, we used a widely accepted reference standard to define fever.[16] Further, inclusion of these 47 infants as fever did not materially change algorithm performance. Last, our study was conducted at 8 large tertiary‐care children's hospitals, and our results may not be generalizable to other children's hospitals and community‐based hospitals.
CONCLUSIONS
Studies of febrile young infants that rely on ICD‐9 discharge diagnosis code of fever for case ascertainment have high specificity but low sensitivity for the identification of febrile infants, particularly among hospitalized patients. A case‐identification strategy that includes discharge or admission diagnosis of fever demonstrated higher sensitivity, and should be considered for studies of febrile infants using administrative data. However, additional strategies such as incorporation of ICD‐9 codes for serious infection should be used when comparing outcomes across institutions.
Acknowledgements
The Febrile Young Infant Research Collaborative includes the following additional collaborators who are acknowledged for their work on this study: Erica DiLeo, MA, Department of Medical Education and Research, Danbury Hospital, Danbury, Connecticut; Janet Flores, BS, Division of Emergency Medicine, Ann and Robert H. Lurie Children's Hospital of Chicago, Chicago, Illinois.
Disclosures: This project funded in part by The Gerber Foundation Novice Researcher Award, (Ref No. 1827‐3835). Dr. Fran Balamuth received career development support from the National Institutes of Health (NHLBI K12‐HL109009). Funders were not involved in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The authors have no conflicts of interest relevant to this article to disclose.
- . The prevalence of serious bacterial infections by age in febrile infants during the first 3 months of life. Pediatr Ann. 1993;22:462–466.
- , , . Performance of low‐risk criteria in the evaluation of young infants with fever: review of the literature. Pediatrics. 2010;125:228–233.
- , , , , , . A week‐by‐week analysis of the low‐risk criteria for serious bacterial infection in febrile neonates. Arch Dis Child. 2009;94:287–292.
- , , , et al. Is 15 days an appropriate cut‐off age for considering serious bacterial infection in the management of febrile infants? Pediatr Infect Dis J. 2012;31:455–458.
- , , . Failure of infant observation scales in detecting serious illness in febrile, 4‐ to 8‐week‐old infants. Pediatrics. 1990;85:1040–1043.
- , , . Outpatient management without antibiotics of fever in selected infants. N Engl J Med. 1993;329:1437–1441.
- , , . Identifying febrile infants at risk for a serious bacterial infection. J Pediatr. 1993;123:489–490.
- , , , et al. Febrile infants at low risk for serious bacterial infection—an appraisal of the Rochester criteria and implications for management. Febrile Infant Collaborative Study Group. Pediatrics. 1994;94:390–396.
- , , , et al. Management of febrile neonates in US pediatric emergency departments. Pediatrics. 2014;133:187–195.
- , , , et al. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134:667–677.
- , , , et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10:358–365.
- , , , et al. Diagnosis and management of febrile infants (0‐3 months). Evid Rep Technol Assess (Full Rep). 2012;(205):1–297.
- , , , et al. Accuracy of administrative billing codes to detect urinary tract infection hospitalizations. Pediatrics. 2011;128:323–330.
- , , , et al. Identifying pediatric community‐acquired pneumonia hospitalizations: accuracy of administrative billing codes. JAMA Pediatr. 2013;167:851–858.
- , , , , , . Development and use of reporting guidelines for assessing the quality of validation studies of health administrative data. J Clin Epidemiol. 2011;64:821–829.
- American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42:530–545.
- , , , , , . Variation in occult injury screening for children with suspected abuse in selected US children's hospitals. Pediatrics. 2012;130:853–860.
- . Achieving data quality. How data from a pediatric health information system earns the trust of its users. J AHIMA. 2004;75:22–26.
- , , , . Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299:2048–2055.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381.
- , , , , , . Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107:E99.
- US Census Bureau. Geographic terms and concepts—census divisions and census regions. Available at: https://www.census.gov/geo/reference/gtc/gtc_census_divreg.html. Accessed October 20, 2014.
- , , , . Initial emergency department diagnosis and return visits: risk versus perception. Ann Emerg Med. 1998;32:569–573.
- , , , , . A national depiction of children with return visits to the emergency department within 72 hours, 2001–2007. Pediatr Emerg Care. 2012;28:606–610.
- , , , et al. Pediatric observation status: are we overlooking a growing population in children's hospitals? J Hosp Med. 2012;7:530–536.
- , , , et al. Differences in designations of observation care in US freestanding children's hospitals: are they virtual or real? J Hosp Med. 2012;7:287–293.
- , , , , . Trends in the management of viral meningitis at United States children's hospitals. Pediatrics. 2013;131:670–676.
- , , , et al. Impact of increasing ondansetron use on clinical outcomes in children with gastroenteritis. JAMA Pediatr. 2014;168:321–329.
- , , , , . Race, otitis media, and antibiotic selection. Pediatrics. 2014;134:1059–1066.
- , , , et al. Establishing benchmarks for the hospitalized care of children with asthma, bronchiolitis, and pneumonia. Pediatrics. 2014;134:555–562.
- , , , , . Diagnostic testing and treatment of pediatric headache in the emergency department. J Pediatr. 2013;163:1634–1637.
Fever is one of the most common reasons for emergency department (ED) evaluation of infants under 90 days of age.[1] Up to 10% to 20% of febrile young infants will have a serious bacterial infection (SBI),[2, 3, 4] but infants with SBI are difficult to distinguish from those without SBI based upon symptoms and physical examination findings alone.[5] Previously developed clinical prediction algorithms can help to identify febrile infants at low risk for SBI, but differ in age range as well as recommendations for testing and empiric treatment.[6, 7, 8] Consequently, there is widespread variation in management of febrile young infants at US children's hospitals,[9, 10, 11] and defining optimal management strategies remains an important issue in pediatric healthcare.[12] Administrative datasets are convenient and inexpensive, and can be used to evaluate practice variation, trends, and outcomes of a large, diverse group of patients within and across institutions.[9, 10] Accurately identifying febrile infants evaluated for suspected SBI in administrative databases would facilitate comparative effectiveness research, quality improvement initiatives, and institutional benchmarking.
Prior studies have validated the accuracy of administrative billing codes for identification of other common childhood illnesses, including urinary tract infection (UTI)[13] and pneumonia.[14] The accuracy of International Classification of Diseases, Ninth Revision (ICD‐9) diagnosis codes in identifying febrile young infants evaluated for SBI is not known. Reliance on administrative ICD‐9 diagnosis codes for patient identification can lead to misclassification of patients due to variable database quality, the validity of the diagnosis codes being utilized, and hospital coding practices.[15] Additionally, fever is a symptom and not a specific diagnosis. If a particular bacterial or viral diagnosis is established (eg, enterovirus meningitis), a discharge diagnosis of fever may not be attributed to the patient encounter. Thus, evaluating the performance characteristics and capture of clinical outcomes of different combinations of ICD‐9 diagnosis codes for identifying febrile infants is necessary for both the conduct and interpretation of studies that utilize administrative databases. The primary objective of this investigation was to identify the most accurate ICD‐9 coding strategies for the identification of febrile infants aged <90 days using administrative data. We also sought to evaluate capture of clinically important outcomes across identification strategies.
METHODS
Study Design and Setting
For this multicenter retrospective study, we used the Pediatric Health Information System (PHIS) database to identify infants <90 days of age[16] who presented between July 1, 2012 and June 30, 2013 to 1 of 8 EDs. We assessed performance characteristics of ICD‐9 diagnosis code case‐identification algorithms by comparing ICD‐9 code combinations to a fever reference standard determined by medical record review. The institutional review board at each participating site approved the study protocol.
Data Source
Data were obtained from 2 sources: the PHIS database and medical record review. We used the PHIS database to identify eligible patients by ICD‐9 diagnosis codes; patient encounters were randomly selected using a random number generator. The PHIS database contains demographic, diagnosis, and billing data from 44 hospitals affiliated with the Children's Hospital Association (Overland Park, Kansas) and represents 85% of freestanding children's hospitals in the United States.[17] Data are deidentified; encrypted unique patient identifiers permit tracking of patients across visits within a site.[18] The Children's Hospital Association and participating hospitals jointly assure the quality and integrity of the data.[19]
For each patient encounter identified in the PHIS database, detailed medical record review was performed by trained investigators at each of the 8 study sites (see Supporting Information, Appendix, in the online version of this article). A standardized data collection instrument was pilot tested by all investigators prior to use. Data were collected and managed using the Research Electronic Data Capture (REDCap) tool hosted at Boston Children's Hospital.[20]
Exclusions
Using PHIS data, prior to medical record review we excluded infants with a complex chronic condition as defined previously[21] and those transferred from another institution, as these infants may warrant a nonstandard evaluation and/or may have incomplete data.
ICD‐9 Diagnosis Code Groups
In the PHIS database, all patients discharged from the hospital (including hospitalized patients as well as patients discharged from the ED) receive 1 or more ICD‐9 discharge diagnosis codes. These diagnosis codes are ascribed after discharge from the hospital, or for ED patients, after ED discharge. Additionally, patients may receive an admission diagnosis, which reflects the diagnosis ascribed at the time of ED discharge or transfer to the inpatient unit.
We reviewed medical records of infants selected from the following ICD‐9 diagnosis code groups (Figure 1): (1) discharge diagnosis code of fever (780.6 [fever and other physiologic disturbances of temperature regulation], 778.4 [other disturbances of temperature regulation of newborn], 780.60 [fever, unspecified], or 780.61 [fever presenting with conditions classified elsewhere])[9, 10] regardless of the presence of admission diagnosis of fever or diagnosis of serious infection, (2) admission diagnosis code of fever without associated discharge diagnosis code of fever,[10] (3) discharge diagnosis code of serious infection determined a priori (see Supporting Information, Appendix, in the online version of this article) without discharge or admission diagnosis code of fever, and (4) infants without any diagnosis code of fever or serious infection.

Medical records reviewed in each of the 4 ICD‐9 diagnosis code groups were randomly selected from the overall set of ED encounters in the population of infants <90 days of age evaluated during the study period. Twenty‐five percent population sampling was used for 3 of the ICD‐9 diagnosis code groups, whereas 5% sampling was used for the no fever/no serious infection code group. The number of medical records reviewed in each ICD‐9 diagnosis code group was proportional to the distribution of ICD‐9 codes across the entire population of infants <90 days of age. These records were distributed equally across sites (228 records per site), except for 1 site that does not assign admission diagnoses (201 records).
Investigators were blinded to ICD‐9 diagnosis code groups during medical record review. Infants with multiple visits during the study period were eligible to be included more than once if the visits occurred more than 3 days apart. For infants with more than 1 ED visit on a particular calendar day, investigators were instructed to review the initial visit.
For each encounter, we also abstracted demographic characteristics (gender, race/ethnicity), insurance status, hospital region (using US Census categories[22]), and season from the PHIS database.
Reference Standard
The presence of fever was determined by medical record review. We defined fever as any documented temperature 100.4F (38.0C) at home or in the ED.[16]
ICD‐9 Code Case‐Identification Algorithms
Using the aforementioned ICD‐9 diagnosis code groups individually and in combination, the following 4 case‐identification algorithms, determined from prior study or group consensus, were compared to the reference standard: (1) ICD‐9 discharge diagnosis code of fever,[9] (2) ICD‐9 admission or discharge diagnosis code of fever,[10, 11] (3) ICD‐9 discharge diagnosis code of fever or serious infection, and (4) ICD‐9 discharge or admission diagnosis code of fever or serious infection. Algorithms were compared overall, separately for discharged and hospitalized infants, and across 3 distinct age groups (28 days, 2956 days, and 5789 days).
Patient‐Level Outcomes
To compare differences in outcomes by case‐identification algorithm, from the PHIS database we abstracted hospitalization rates, rates of UTI/pyelonephritis,[13] bacteremia/sepsis, and bacterial meningitis.[19] Severe outcomes were defined as intensive care unit admission, mechanical ventilation, central line placement, receipt of extracorporeal membrane oxygenation, or death. We assessed hospital length of stay for admitted infants and 3‐day revisits,[23, 24] and revisits resulting in hospitalization for infants discharged from the ED at the index visit. Patients billed for observation care were classified as being hospitalized.[25, 26]
Data Analysis
Accuracy of the 4 case‐identification algorithms (compared with the reference standard) was calculated using sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV), along with 95% confidence interval (CI). Prior to analysis, a 5‐fold weighting factor was applied to the no fever/no serious infection group to account for the differential sampling used for this group (5% vs 25% for the other 3 ICD‐9 diagnosis code groups). This weighting was done to approximate the true prevalence of each ICD‐9 code group within the larger population, so that an accurate rate of false negatives (infants with fever who had neither a diagnosis of fever nor serious infection) could be calculated.
We described continuous variables using median and interquartile range or range values and categorical variables using frequencies with 95% CIs. We compared categorical variables using a 2 test. We determined statistical significance as a 2‐tailed P value <0.05. Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Study Patients
During the 1‐year study period, 23,753 ED encounters for infants <90 days of age were identified in the PHIS database at the 8 participating sites. Of these infant encounters, 2166 (9.2%) were excluded (1658 infants who had a complex chronic condition and 508 transferred into the ED), leaving 21,587 infants available for selection. After applying our sampling strategy, we identified 1797 encounters for medical record review. Seven encounters from 3 hospitals with missing medical records were excluded, resulting in a final cohort of 1790 encounters (Figure 1). Among included infants, 552 (30.8%) were 28 days, 743 (41.5%) were 29 to 56 days, and 495 (27.8%) were 57 to 89 days of age; 737 (41.2%) infants were hospitalized. Patients differed in age, race, payer, and season across ICD‐9 diagnosis code groups (see Supporting Information, Table 1, in the online version of this article).
| ICD‐9 Diagnosis Code Algorithm | Overall | |||
|---|---|---|---|---|
| Sensitivity, % (95% CI) | Specificity, % (95% CI) | Negative Predictive Value, % (95% CI) | Positive Predictive Value, % (95% CI) | |
| ||||
| Discharge diagnosis of fever | 53.2 (50.056.4) | 98.2 (97.898.6) | 90.8 (90.091.6) | 86.1 (83.388.9) |
| Hospitalized | 47.3 (43.151.5) | 97.7 (96.998.5) | 80.6 (78.682.6) | 90.2 (86.893.6) |
| Discharged from ED | 61.4 (56.666.2) | 98.4 (98.098.8) | 95.4 (94.796.1) | 82.1 (77.786.5) |
| Discharge or admission diagnosis of Fever | 71.1 (68.274.0) | 97.7 (97.398.1) | 94.1 (93.494.8) | 86.9 (84.589.3) |
| Hospitalized | 72.5 (68.876.2) | 97.1 (96.298.0) | 88.8 (87.190.5) | 91.7 (89.194.3) |
| Discharged from ED | 69.2 (64.773.7) | 98.0 (97.598.5) | 96.3 (95.796.9) | 80.8 (76.685.0) |
| Discharge diagnosis of fever or serious infection | 63.7 (60.666.8) | 96.5 (96.097.0) | 92.6 (91.893.4) | 79.6 (76.782.5) |
| Hospitalized | 63.9 (59.967.9) | 92.5 (91.094.0) | 85.1 (83.287.0) | 79.1 (75.382.9) |
| Discharged from ED | 63.4 (58.768.1) | 98.1 (97.698.6) | 95.6 (94.996.3) | 80.2 (75.884.6) |
| Discharge or admission diagnosis of fever or serious infection | 76.6 (73.979.3) | 96.2 (95.696.8) | 95.1 (94.595.7) | 81.0 (78.483.6) |
| Hospitalized | 80.8 (77.584.1) | 92.1 (90.693.6) | 91.5 (89.993.1) | 82.1 (78.985.3) |
| Discharged from ED | 71.0 (66.575.5) | 97.7 (97.298.2) | 96.5 (95.997.1) | 79.4 (75.283.6) |
Among the 1790 patient encounters reviewed, a total of 766 infants (42.8%) met the reference standard definition for fever in the cohort. An additional 47 infants had abnormal temperature reported (documentation of tactile fever, history of fever without a specific temperature described, or hypothermia) but were classified as having no fever by the reference standard.
ICD‐9 Code Case‐Identification Algorithm Performance
Compared with the reference standard, the 4 case‐identification algorithms demonstrated specificity of 96.2% to 98.2% but lower sensitivity overall (Figure 2). Discharge diagnosis of fever alone demonstrated the lowest sensitivity. The algorithm of discharge or admission diagnosis of fever resulted in increased sensitivity and the highest PPV of all 4 algorithms (86.9%, 95% CI: 84.5‐89.3). Addition of serious infection codes to this algorithm resulted in a marginal increase in sensitivity and a similar decrease in PPV (Table 1). When limited to hospitalized infants, specificity was highest for the case‐identification algorithm of discharge diagnosis of fever and similarly high for discharge or admission diagnosis of fever; sensitivity was highest for the algorithm of discharge or admission diagnosis of fever or diagnosis of serious infection. For infants discharged from the ED, algorithm specificity was 97.7% to 98.4%, with lower sensitivity for all 4 algorithms (Table 1). Inclusion of the 47 infants with abnormal temperature as fever did not materially change algorithm performance (data not shown).

Across all 3 age groups (28 days, 2956 days, and 5789 days), the 4 case‐identification algorithms demonstrated specificity >96%, whereas algorithm sensitivity was highest in the 29‐ to 56‐days‐old age group and lowest among infants 57 to 89 days old across all 4 algorithms (Figure 2). Similar to the overall cohort, an algorithm of discharge or admission diagnosis of fever demonstrated specificity of nearly 98% in all age groups; addition of serious infection codes to this algorithm increased sensitivity, highest in the 29‐ to 56‐days‐old age group (Figure 2; see also Supporting Information, Table 2, in the online version of this article).
| ICD‐9 Diagnosis Code Algorithm | Sensitivity, Median % (Range) | Specificity, Median % (Range) | Negative Predictive Value, Median % (Range) | Positive Predictive Value, Median % (Range) |
|---|---|---|---|---|
| ||||
| Discharge diagnosis of fever | 56.2 (34.681.0) | 98.3 (96.499.1) | 92.1 (83.297.4) | 87.7 (74.093.2) |
| Discharge or Admission diagnosis of Fever | 76.7 (51.385.0) | 97.8 (96.298.7) | 95.6 (86.997.4) | 87.4 (80.092.9) |
| Discharge diagnosis of fever or serious infection | 68.3 (44.287.3) | 96.5 (95.498.0) | 93.6 (85.298.2) | 78.3 (74.289.0) |
| Discharge or admission diagnosis of fever or serious infection | 83.1 (58.390.7) | 95.8 (95.498.0) | 96.5 (88.598.2) | 79.1 (77.490.4) |
Across the 8 study sites, median specificity was 95.8% to 98.3% for the 4 algorithms, with little interhospital variability; however, algorithm sensitivity varied widely by site. Median PPV was highest for discharge diagnosis of fever alone at 87.7% but ranged from 74.0% to 93.2% across sites. Median PPV for an algorithm of discharge or admission diagnosis of fever was similar (87.4%) but with less variation by site (range 80.0%92.9%) (Table 2).
Outcomes by ICD‐9 Diagnosis Code Group and Case‐Identification Algorithm
When compared with discharge diagnosis of fever, adding admission diagnosis of fever captured a higher proportion of hospitalized infants with SBIs (UTI/pyelonephritis, bacteremia/sepsis, or bacterial meningitis). However, median hospital length of stay, severe outcomes, and 3‐day revisits and revisits with hospitalization did not materially differ when including infants with admission diagnosis of fever in addition to discharge diagnosis of fever. Addition of infants with a diagnosis code for serious infection substantially increased the number of infants with SBIs and severe outcomes but did not capture additional 3‐day revisits (Table 3). There were no additional cases of SBI in the no fever/no serious illness diagnosis code group.
| ICD‐9 Diagnosis Code Algorithm | Outcome | 3‐Day Revisit, % (95% CI) | 3‐Day Revisit With Hospitalization, % (95% CI) | |||
|---|---|---|---|---|---|---|
| Hospitalized, % (95% CI) | UTI/Pyelonephritis, Bacteremia/Sepsis, or Bacterial Meningitis, % (95% CI) | Severe Outcome, % (95% CI)* | Length of Stay in Days, Median (IQR) | |||
| ||||||
| Discharge diagnosis of fever | 44.3 (40.348.4) | 3.3 (1.84.7) | 1.4 (0.42.3) | 3 (23) | 11.7 (8.215.2) | 5.9 (3.38.4) |
| Discharge or admission diagnosis of fever | 52.4 (48.955.9) | 6.1 (4.47.8) | 1.9 (1.02.9) | 3 (23) | 10.9 (7.714.1) | 5.4 (3.17.8) |
| Discharge diagnosis of fever or serious infection | 54.0 (50.457.5) | 15.3 (12.717.8) | 3.8 (2.55.2) | 3 (24) | 11.0 (7.714.2) | 5.5 (3.17.9) |
| Discharge or admission diagnosis of fever or serious infection | 56.5 (53.259.7) | 12.9 (10.715.1) | 3.6 (2.44.8) | 3 (24) | 10.3 (7.313.3) | 5.2 (3.07.4) |
Among infants who met the reference standard for fever but did not have a discharge or admission diagnosis of fever (false negatives), 11.8% had a diagnosis of SBI. Overall, 43.2% of febrile infants (and 84.4% of hospitalized infants) with SBI did not have an ICD‐9 discharge or admission diagnosis of fever. Addition of ICD‐9 diagnosis codes of serious infection to the algorithm of discharge or admission diagnosis of fever captured all additional SBIs, and no false negativeinfants missed with this algorithm had an SBI.
DISCUSSION
We described the performance of 4 ICD‐9 diagnosis code case‐identification algorithms for the identification of febrile young infants <90 days of age at US children's hospitals. Although the specificity was high across algorithms and institutions, the sensitivity was relatively low, particularly for discharge diagnosis of fever, and varied by institution. Given the high specificity, ICD‐9 diagnosis code case‐identification algorithms for fever reliably identify febrile infants using administrative data with low rates of inclusion of infants without fever. However, underidentification of patients, particularly those more prone to SBIs and severe outcomes depending on the algorithm utilized, can impact interpretation of comparative effectiveness studies or the quality of care delivered by an institution.
ICD‐9 discharge diagnosis codes are frequently used to identify pediatric patients across a variety of administrative databases, diseases, and symptoms.[19, 27, 28, 29, 30, 31] Although discharge diagnosis of fever is highly specific, sensitivity is substantially lower than other case‐identification algorithms we studied, particularly for hospitalized infants. This may be due to a fever code sometimes being omitted in favor of a more specific diagnosis (eg, bacteremia) prior to hospital discharge. Therefore, case identification relying only on ICD‐9 discharge diagnosis codes for fever may under‐report clinically important SBI or severe outcomes as demonstrated in our study. This is in contrast to ICD‐9 diagnosis code identification strategies for childhood UTI and pneumonia, which largely have higher sensitivity but lower specificity than fever codes.[13, 14]
Admission diagnosis of fever is important for febrile infants as they may not have an explicit diagnosis at the time of disposition from the ED. Addition of admission diagnosis of fever to an algorithm relying on discharge diagnosis code alone increased sensitivity without a demonstrable reduction in specificity and PPV, likely due to capture of infants with a fever diagnosis at presentation before a specific infection was identified. Although using an algorithm of discharge or admission diagnosis of fever captured a higher percentage of hospitalized febrile infants with SBIs, sensitivity was only 71% overall with this algorithm, and 43% of febrile infants with SBI would still have been missed. Importantly, though, addition of various ICD‐9 codes for serious infection to this algorithm resulted in capture of all febrile infants with SBI and should be used as a sensitivity analysis.
The test characteristics of diagnosis codes were highest in the 29‐ to 56‐days‐old age group. Given the differing low‐risk criteria[6, 7, 8] and lack of best practice guidelines[16] in this age group, the use of administrative data may allow for the comparison of testing and treatment strategies across a large cohort of febrile infants aged 29 to 56 days. However, individual hospital coding practices may affect algorithm performance, in particular sensitivity, which varied substantially by hospital. This variation in algorithm sensitivity may impact comparisons of outcomes across institutions. Therefore, when conducting studies of febrile infants using administrative data, sensitivity analyses or use of chart review should be considered to augment the use of ICD‐9 code‐based identification strategies, particularly for comparative benchmarking and outcomes studies. These additional analyses are particularly important for studies of febrile infants >56 days of age, in whom the sensitivity of diagnosis codes is particularly low. We speculate that the lower sensitivity in older febrile infants may relate to a lack of consensus on the clinical significance of fever in this age group and the varying management strategies employed.[10]
Strengths of this study include the assessment of ICD‐9 code algorithms across multiple institutions for identification of fever in young infants, and the patterns of our findings remained robust when comparing median performance characteristics of the algorithms across hospitals to our overall findings. We were also able to accurately estimate PPV and NPV using a case‐identification strategy weighted to the actual population sizes. Although sensitivity and specificity are the primary measures of test performance, predictive values are highly informative for investigators using administrative data. Additionally, our findings may inform public health efforts including disease surveillance, assessment of seasonal variation, and identification and monitoring of healthcare‐associated infections among febrile infants.
Our study has limitations. We did not review all identified records, which raises the possibility that our evaluated cohort may not be representative of the entire febrile infant population. We attempted to mitigate this possibility by using a random sampling strategy for our population selection that was weighted to the actual population sizes. Second, we identified serious infections using ICD‐9 diagnosis codes determined by group consensus, which may not capture all serious infection codes that identify febrile infants whose fever code was omitted. Third, 47 infants had abnormal temperature that did not meet our reference standard criteria for fever and were included in the no fever group. Although there may be disagreement regarding what constitutes a fever, we used a widely accepted reference standard to define fever.[16] Further, inclusion of these 47 infants as fever did not materially change algorithm performance. Last, our study was conducted at 8 large tertiary‐care children's hospitals, and our results may not be generalizable to other children's hospitals and community‐based hospitals.
CONCLUSIONS
Studies of febrile young infants that rely on ICD‐9 discharge diagnosis code of fever for case ascertainment have high specificity but low sensitivity for the identification of febrile infants, particularly among hospitalized patients. A case‐identification strategy that includes discharge or admission diagnosis of fever demonstrated higher sensitivity, and should be considered for studies of febrile infants using administrative data. However, additional strategies such as incorporation of ICD‐9 codes for serious infection should be used when comparing outcomes across institutions.
Acknowledgements
The Febrile Young Infant Research Collaborative includes the following additional collaborators who are acknowledged for their work on this study: Erica DiLeo, MA, Department of Medical Education and Research, Danbury Hospital, Danbury, Connecticut; Janet Flores, BS, Division of Emergency Medicine, Ann and Robert H. Lurie Children's Hospital of Chicago, Chicago, Illinois.
Disclosures: This project funded in part by The Gerber Foundation Novice Researcher Award, (Ref No. 1827‐3835). Dr. Fran Balamuth received career development support from the National Institutes of Health (NHLBI K12‐HL109009). Funders were not involved in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The authors have no conflicts of interest relevant to this article to disclose.
Fever is one of the most common reasons for emergency department (ED) evaluation of infants under 90 days of age.[1] Up to 10% to 20% of febrile young infants will have a serious bacterial infection (SBI),[2, 3, 4] but infants with SBI are difficult to distinguish from those without SBI based upon symptoms and physical examination findings alone.[5] Previously developed clinical prediction algorithms can help to identify febrile infants at low risk for SBI, but differ in age range as well as recommendations for testing and empiric treatment.[6, 7, 8] Consequently, there is widespread variation in management of febrile young infants at US children's hospitals,[9, 10, 11] and defining optimal management strategies remains an important issue in pediatric healthcare.[12] Administrative datasets are convenient and inexpensive, and can be used to evaluate practice variation, trends, and outcomes of a large, diverse group of patients within and across institutions.[9, 10] Accurately identifying febrile infants evaluated for suspected SBI in administrative databases would facilitate comparative effectiveness research, quality improvement initiatives, and institutional benchmarking.
Prior studies have validated the accuracy of administrative billing codes for identification of other common childhood illnesses, including urinary tract infection (UTI)[13] and pneumonia.[14] The accuracy of International Classification of Diseases, Ninth Revision (ICD‐9) diagnosis codes in identifying febrile young infants evaluated for SBI is not known. Reliance on administrative ICD‐9 diagnosis codes for patient identification can lead to misclassification of patients due to variable database quality, the validity of the diagnosis codes being utilized, and hospital coding practices.[15] Additionally, fever is a symptom and not a specific diagnosis. If a particular bacterial or viral diagnosis is established (eg, enterovirus meningitis), a discharge diagnosis of fever may not be attributed to the patient encounter. Thus, evaluating the performance characteristics and capture of clinical outcomes of different combinations of ICD‐9 diagnosis codes for identifying febrile infants is necessary for both the conduct and interpretation of studies that utilize administrative databases. The primary objective of this investigation was to identify the most accurate ICD‐9 coding strategies for the identification of febrile infants aged <90 days using administrative data. We also sought to evaluate capture of clinically important outcomes across identification strategies.
METHODS
Study Design and Setting
For this multicenter retrospective study, we used the Pediatric Health Information System (PHIS) database to identify infants <90 days of age[16] who presented between July 1, 2012 and June 30, 2013 to 1 of 8 EDs. We assessed performance characteristics of ICD‐9 diagnosis code case‐identification algorithms by comparing ICD‐9 code combinations to a fever reference standard determined by medical record review. The institutional review board at each participating site approved the study protocol.
Data Source
Data were obtained from 2 sources: the PHIS database and medical record review. We used the PHIS database to identify eligible patients by ICD‐9 diagnosis codes; patient encounters were randomly selected using a random number generator. The PHIS database contains demographic, diagnosis, and billing data from 44 hospitals affiliated with the Children's Hospital Association (Overland Park, Kansas) and represents 85% of freestanding children's hospitals in the United States.[17] Data are deidentified; encrypted unique patient identifiers permit tracking of patients across visits within a site.[18] The Children's Hospital Association and participating hospitals jointly assure the quality and integrity of the data.[19]
For each patient encounter identified in the PHIS database, detailed medical record review was performed by trained investigators at each of the 8 study sites (see Supporting Information, Appendix, in the online version of this article). A standardized data collection instrument was pilot tested by all investigators prior to use. Data were collected and managed using the Research Electronic Data Capture (REDCap) tool hosted at Boston Children's Hospital.[20]
Exclusions
Using PHIS data, prior to medical record review we excluded infants with a complex chronic condition as defined previously[21] and those transferred from another institution, as these infants may warrant a nonstandard evaluation and/or may have incomplete data.
ICD‐9 Diagnosis Code Groups
In the PHIS database, all patients discharged from the hospital (including hospitalized patients as well as patients discharged from the ED) receive 1 or more ICD‐9 discharge diagnosis codes. These diagnosis codes are ascribed after discharge from the hospital, or for ED patients, after ED discharge. Additionally, patients may receive an admission diagnosis, which reflects the diagnosis ascribed at the time of ED discharge or transfer to the inpatient unit.
We reviewed medical records of infants selected from the following ICD‐9 diagnosis code groups (Figure 1): (1) discharge diagnosis code of fever (780.6 [fever and other physiologic disturbances of temperature regulation], 778.4 [other disturbances of temperature regulation of newborn], 780.60 [fever, unspecified], or 780.61 [fever presenting with conditions classified elsewhere])[9, 10] regardless of the presence of admission diagnosis of fever or diagnosis of serious infection, (2) admission diagnosis code of fever without associated discharge diagnosis code of fever,[10] (3) discharge diagnosis code of serious infection determined a priori (see Supporting Information, Appendix, in the online version of this article) without discharge or admission diagnosis code of fever, and (4) infants without any diagnosis code of fever or serious infection.

Medical records reviewed in each of the 4 ICD‐9 diagnosis code groups were randomly selected from the overall set of ED encounters in the population of infants <90 days of age evaluated during the study period. Twenty‐five percent population sampling was used for 3 of the ICD‐9 diagnosis code groups, whereas 5% sampling was used for the no fever/no serious infection code group. The number of medical records reviewed in each ICD‐9 diagnosis code group was proportional to the distribution of ICD‐9 codes across the entire population of infants <90 days of age. These records were distributed equally across sites (228 records per site), except for 1 site that does not assign admission diagnoses (201 records).
Investigators were blinded to ICD‐9 diagnosis code groups during medical record review. Infants with multiple visits during the study period were eligible to be included more than once if the visits occurred more than 3 days apart. For infants with more than 1 ED visit on a particular calendar day, investigators were instructed to review the initial visit.
For each encounter, we also abstracted demographic characteristics (gender, race/ethnicity), insurance status, hospital region (using US Census categories[22]), and season from the PHIS database.
Reference Standard
The presence of fever was determined by medical record review. We defined fever as any documented temperature 100.4F (38.0C) at home or in the ED.[16]
ICD‐9 Code Case‐Identification Algorithms
Using the aforementioned ICD‐9 diagnosis code groups individually and in combination, the following 4 case‐identification algorithms, determined from prior study or group consensus, were compared to the reference standard: (1) ICD‐9 discharge diagnosis code of fever,[9] (2) ICD‐9 admission or discharge diagnosis code of fever,[10, 11] (3) ICD‐9 discharge diagnosis code of fever or serious infection, and (4) ICD‐9 discharge or admission diagnosis code of fever or serious infection. Algorithms were compared overall, separately for discharged and hospitalized infants, and across 3 distinct age groups (28 days, 2956 days, and 5789 days).
Patient‐Level Outcomes
To compare differences in outcomes by case‐identification algorithm, from the PHIS database we abstracted hospitalization rates, rates of UTI/pyelonephritis,[13] bacteremia/sepsis, and bacterial meningitis.[19] Severe outcomes were defined as intensive care unit admission, mechanical ventilation, central line placement, receipt of extracorporeal membrane oxygenation, or death. We assessed hospital length of stay for admitted infants and 3‐day revisits,[23, 24] and revisits resulting in hospitalization for infants discharged from the ED at the index visit. Patients billed for observation care were classified as being hospitalized.[25, 26]
Data Analysis
Accuracy of the 4 case‐identification algorithms (compared with the reference standard) was calculated using sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV), along with 95% confidence interval (CI). Prior to analysis, a 5‐fold weighting factor was applied to the no fever/no serious infection group to account for the differential sampling used for this group (5% vs 25% for the other 3 ICD‐9 diagnosis code groups). This weighting was done to approximate the true prevalence of each ICD‐9 code group within the larger population, so that an accurate rate of false negatives (infants with fever who had neither a diagnosis of fever nor serious infection) could be calculated.
We described continuous variables using median and interquartile range or range values and categorical variables using frequencies with 95% CIs. We compared categorical variables using a 2 test. We determined statistical significance as a 2‐tailed P value <0.05. Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Study Patients
During the 1‐year study period, 23,753 ED encounters for infants <90 days of age were identified in the PHIS database at the 8 participating sites. Of these infant encounters, 2166 (9.2%) were excluded (1658 infants who had a complex chronic condition and 508 transferred into the ED), leaving 21,587 infants available for selection. After applying our sampling strategy, we identified 1797 encounters for medical record review. Seven encounters from 3 hospitals with missing medical records were excluded, resulting in a final cohort of 1790 encounters (Figure 1). Among included infants, 552 (30.8%) were 28 days, 743 (41.5%) were 29 to 56 days, and 495 (27.8%) were 57 to 89 days of age; 737 (41.2%) infants were hospitalized. Patients differed in age, race, payer, and season across ICD‐9 diagnosis code groups (see Supporting Information, Table 1, in the online version of this article).
| ICD‐9 Diagnosis Code Algorithm | Overall | |||
|---|---|---|---|---|
| Sensitivity, % (95% CI) | Specificity, % (95% CI) | Negative Predictive Value, % (95% CI) | Positive Predictive Value, % (95% CI) | |
| ||||
| Discharge diagnosis of fever | 53.2 (50.056.4) | 98.2 (97.898.6) | 90.8 (90.091.6) | 86.1 (83.388.9) |
| Hospitalized | 47.3 (43.151.5) | 97.7 (96.998.5) | 80.6 (78.682.6) | 90.2 (86.893.6) |
| Discharged from ED | 61.4 (56.666.2) | 98.4 (98.098.8) | 95.4 (94.796.1) | 82.1 (77.786.5) |
| Discharge or admission diagnosis of Fever | 71.1 (68.274.0) | 97.7 (97.398.1) | 94.1 (93.494.8) | 86.9 (84.589.3) |
| Hospitalized | 72.5 (68.876.2) | 97.1 (96.298.0) | 88.8 (87.190.5) | 91.7 (89.194.3) |
| Discharged from ED | 69.2 (64.773.7) | 98.0 (97.598.5) | 96.3 (95.796.9) | 80.8 (76.685.0) |
| Discharge diagnosis of fever or serious infection | 63.7 (60.666.8) | 96.5 (96.097.0) | 92.6 (91.893.4) | 79.6 (76.782.5) |
| Hospitalized | 63.9 (59.967.9) | 92.5 (91.094.0) | 85.1 (83.287.0) | 79.1 (75.382.9) |
| Discharged from ED | 63.4 (58.768.1) | 98.1 (97.698.6) | 95.6 (94.996.3) | 80.2 (75.884.6) |
| Discharge or admission diagnosis of fever or serious infection | 76.6 (73.979.3) | 96.2 (95.696.8) | 95.1 (94.595.7) | 81.0 (78.483.6) |
| Hospitalized | 80.8 (77.584.1) | 92.1 (90.693.6) | 91.5 (89.993.1) | 82.1 (78.985.3) |
| Discharged from ED | 71.0 (66.575.5) | 97.7 (97.298.2) | 96.5 (95.997.1) | 79.4 (75.283.6) |
Among the 1790 patient encounters reviewed, a total of 766 infants (42.8%) met the reference standard definition for fever in the cohort. An additional 47 infants had abnormal temperature reported (documentation of tactile fever, history of fever without a specific temperature described, or hypothermia) but were classified as having no fever by the reference standard.
ICD‐9 Code Case‐Identification Algorithm Performance
Compared with the reference standard, the 4 case‐identification algorithms demonstrated specificity of 96.2% to 98.2% but lower sensitivity overall (Figure 2). Discharge diagnosis of fever alone demonstrated the lowest sensitivity. The algorithm of discharge or admission diagnosis of fever resulted in increased sensitivity and the highest PPV of all 4 algorithms (86.9%, 95% CI: 84.5‐89.3). Addition of serious infection codes to this algorithm resulted in a marginal increase in sensitivity and a similar decrease in PPV (Table 1). When limited to hospitalized infants, specificity was highest for the case‐identification algorithm of discharge diagnosis of fever and similarly high for discharge or admission diagnosis of fever; sensitivity was highest for the algorithm of discharge or admission diagnosis of fever or diagnosis of serious infection. For infants discharged from the ED, algorithm specificity was 97.7% to 98.4%, with lower sensitivity for all 4 algorithms (Table 1). Inclusion of the 47 infants with abnormal temperature as fever did not materially change algorithm performance (data not shown).

Across all 3 age groups (28 days, 2956 days, and 5789 days), the 4 case‐identification algorithms demonstrated specificity >96%, whereas algorithm sensitivity was highest in the 29‐ to 56‐days‐old age group and lowest among infants 57 to 89 days old across all 4 algorithms (Figure 2). Similar to the overall cohort, an algorithm of discharge or admission diagnosis of fever demonstrated specificity of nearly 98% in all age groups; addition of serious infection codes to this algorithm increased sensitivity, highest in the 29‐ to 56‐days‐old age group (Figure 2; see also Supporting Information, Table 2, in the online version of this article).
| ICD‐9 Diagnosis Code Algorithm | Sensitivity, Median % (Range) | Specificity, Median % (Range) | Negative Predictive Value, Median % (Range) | Positive Predictive Value, Median % (Range) |
|---|---|---|---|---|
| ||||
| Discharge diagnosis of fever | 56.2 (34.681.0) | 98.3 (96.499.1) | 92.1 (83.297.4) | 87.7 (74.093.2) |
| Discharge or Admission diagnosis of Fever | 76.7 (51.385.0) | 97.8 (96.298.7) | 95.6 (86.997.4) | 87.4 (80.092.9) |
| Discharge diagnosis of fever or serious infection | 68.3 (44.287.3) | 96.5 (95.498.0) | 93.6 (85.298.2) | 78.3 (74.289.0) |
| Discharge or admission diagnosis of fever or serious infection | 83.1 (58.390.7) | 95.8 (95.498.0) | 96.5 (88.598.2) | 79.1 (77.490.4) |
Across the 8 study sites, median specificity was 95.8% to 98.3% for the 4 algorithms, with little interhospital variability; however, algorithm sensitivity varied widely by site. Median PPV was highest for discharge diagnosis of fever alone at 87.7% but ranged from 74.0% to 93.2% across sites. Median PPV for an algorithm of discharge or admission diagnosis of fever was similar (87.4%) but with less variation by site (range 80.0%92.9%) (Table 2).
Outcomes by ICD‐9 Diagnosis Code Group and Case‐Identification Algorithm
When compared with discharge diagnosis of fever, adding admission diagnosis of fever captured a higher proportion of hospitalized infants with SBIs (UTI/pyelonephritis, bacteremia/sepsis, or bacterial meningitis). However, median hospital length of stay, severe outcomes, and 3‐day revisits and revisits with hospitalization did not materially differ when including infants with admission diagnosis of fever in addition to discharge diagnosis of fever. Addition of infants with a diagnosis code for serious infection substantially increased the number of infants with SBIs and severe outcomes but did not capture additional 3‐day revisits (Table 3). There were no additional cases of SBI in the no fever/no serious illness diagnosis code group.
| ICD‐9 Diagnosis Code Algorithm | Outcome | 3‐Day Revisit, % (95% CI) | 3‐Day Revisit With Hospitalization, % (95% CI) | |||
|---|---|---|---|---|---|---|
| Hospitalized, % (95% CI) | UTI/Pyelonephritis, Bacteremia/Sepsis, or Bacterial Meningitis, % (95% CI) | Severe Outcome, % (95% CI)* | Length of Stay in Days, Median (IQR) | |||
| ||||||
| Discharge diagnosis of fever | 44.3 (40.348.4) | 3.3 (1.84.7) | 1.4 (0.42.3) | 3 (23) | 11.7 (8.215.2) | 5.9 (3.38.4) |
| Discharge or admission diagnosis of fever | 52.4 (48.955.9) | 6.1 (4.47.8) | 1.9 (1.02.9) | 3 (23) | 10.9 (7.714.1) | 5.4 (3.17.8) |
| Discharge diagnosis of fever or serious infection | 54.0 (50.457.5) | 15.3 (12.717.8) | 3.8 (2.55.2) | 3 (24) | 11.0 (7.714.2) | 5.5 (3.17.9) |
| Discharge or admission diagnosis of fever or serious infection | 56.5 (53.259.7) | 12.9 (10.715.1) | 3.6 (2.44.8) | 3 (24) | 10.3 (7.313.3) | 5.2 (3.07.4) |
Among infants who met the reference standard for fever but did not have a discharge or admission diagnosis of fever (false negatives), 11.8% had a diagnosis of SBI. Overall, 43.2% of febrile infants (and 84.4% of hospitalized infants) with SBI did not have an ICD‐9 discharge or admission diagnosis of fever. Addition of ICD‐9 diagnosis codes of serious infection to the algorithm of discharge or admission diagnosis of fever captured all additional SBIs, and no false negativeinfants missed with this algorithm had an SBI.
DISCUSSION
We described the performance of 4 ICD‐9 diagnosis code case‐identification algorithms for the identification of febrile young infants <90 days of age at US children's hospitals. Although the specificity was high across algorithms and institutions, the sensitivity was relatively low, particularly for discharge diagnosis of fever, and varied by institution. Given the high specificity, ICD‐9 diagnosis code case‐identification algorithms for fever reliably identify febrile infants using administrative data with low rates of inclusion of infants without fever. However, underidentification of patients, particularly those more prone to SBIs and severe outcomes depending on the algorithm utilized, can impact interpretation of comparative effectiveness studies or the quality of care delivered by an institution.
ICD‐9 discharge diagnosis codes are frequently used to identify pediatric patients across a variety of administrative databases, diseases, and symptoms.[19, 27, 28, 29, 30, 31] Although discharge diagnosis of fever is highly specific, sensitivity is substantially lower than other case‐identification algorithms we studied, particularly for hospitalized infants. This may be due to a fever code sometimes being omitted in favor of a more specific diagnosis (eg, bacteremia) prior to hospital discharge. Therefore, case identification relying only on ICD‐9 discharge diagnosis codes for fever may under‐report clinically important SBI or severe outcomes as demonstrated in our study. This is in contrast to ICD‐9 diagnosis code identification strategies for childhood UTI and pneumonia, which largely have higher sensitivity but lower specificity than fever codes.[13, 14]
Admission diagnosis of fever is important for febrile infants as they may not have an explicit diagnosis at the time of disposition from the ED. Addition of admission diagnosis of fever to an algorithm relying on discharge diagnosis code alone increased sensitivity without a demonstrable reduction in specificity and PPV, likely due to capture of infants with a fever diagnosis at presentation before a specific infection was identified. Although using an algorithm of discharge or admission diagnosis of fever captured a higher percentage of hospitalized febrile infants with SBIs, sensitivity was only 71% overall with this algorithm, and 43% of febrile infants with SBI would still have been missed. Importantly, though, addition of various ICD‐9 codes for serious infection to this algorithm resulted in capture of all febrile infants with SBI and should be used as a sensitivity analysis.
The test characteristics of diagnosis codes were highest in the 29‐ to 56‐days‐old age group. Given the differing low‐risk criteria[6, 7, 8] and lack of best practice guidelines[16] in this age group, the use of administrative data may allow for the comparison of testing and treatment strategies across a large cohort of febrile infants aged 29 to 56 days. However, individual hospital coding practices may affect algorithm performance, in particular sensitivity, which varied substantially by hospital. This variation in algorithm sensitivity may impact comparisons of outcomes across institutions. Therefore, when conducting studies of febrile infants using administrative data, sensitivity analyses or use of chart review should be considered to augment the use of ICD‐9 code‐based identification strategies, particularly for comparative benchmarking and outcomes studies. These additional analyses are particularly important for studies of febrile infants >56 days of age, in whom the sensitivity of diagnosis codes is particularly low. We speculate that the lower sensitivity in older febrile infants may relate to a lack of consensus on the clinical significance of fever in this age group and the varying management strategies employed.[10]
Strengths of this study include the assessment of ICD‐9 code algorithms across multiple institutions for identification of fever in young infants, and the patterns of our findings remained robust when comparing median performance characteristics of the algorithms across hospitals to our overall findings. We were also able to accurately estimate PPV and NPV using a case‐identification strategy weighted to the actual population sizes. Although sensitivity and specificity are the primary measures of test performance, predictive values are highly informative for investigators using administrative data. Additionally, our findings may inform public health efforts including disease surveillance, assessment of seasonal variation, and identification and monitoring of healthcare‐associated infections among febrile infants.
Our study has limitations. We did not review all identified records, which raises the possibility that our evaluated cohort may not be representative of the entire febrile infant population. We attempted to mitigate this possibility by using a random sampling strategy for our population selection that was weighted to the actual population sizes. Second, we identified serious infections using ICD‐9 diagnosis codes determined by group consensus, which may not capture all serious infection codes that identify febrile infants whose fever code was omitted. Third, 47 infants had abnormal temperature that did not meet our reference standard criteria for fever and were included in the no fever group. Although there may be disagreement regarding what constitutes a fever, we used a widely accepted reference standard to define fever.[16] Further, inclusion of these 47 infants as fever did not materially change algorithm performance. Last, our study was conducted at 8 large tertiary‐care children's hospitals, and our results may not be generalizable to other children's hospitals and community‐based hospitals.
CONCLUSIONS
Studies of febrile young infants that rely on ICD‐9 discharge diagnosis code of fever for case ascertainment have high specificity but low sensitivity for the identification of febrile infants, particularly among hospitalized patients. A case‐identification strategy that includes discharge or admission diagnosis of fever demonstrated higher sensitivity, and should be considered for studies of febrile infants using administrative data. However, additional strategies such as incorporation of ICD‐9 codes for serious infection should be used when comparing outcomes across institutions.
Acknowledgements
The Febrile Young Infant Research Collaborative includes the following additional collaborators who are acknowledged for their work on this study: Erica DiLeo, MA, Department of Medical Education and Research, Danbury Hospital, Danbury, Connecticut; Janet Flores, BS, Division of Emergency Medicine, Ann and Robert H. Lurie Children's Hospital of Chicago, Chicago, Illinois.
Disclosures: This project funded in part by The Gerber Foundation Novice Researcher Award, (Ref No. 1827‐3835). Dr. Fran Balamuth received career development support from the National Institutes of Health (NHLBI K12‐HL109009). Funders were not involved in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript. The authors have no conflicts of interest relevant to this article to disclose.
- . The prevalence of serious bacterial infections by age in febrile infants during the first 3 months of life. Pediatr Ann. 1993;22:462–466.
- , , . Performance of low‐risk criteria in the evaluation of young infants with fever: review of the literature. Pediatrics. 2010;125:228–233.
- , , , , , . A week‐by‐week analysis of the low‐risk criteria for serious bacterial infection in febrile neonates. Arch Dis Child. 2009;94:287–292.
- , , , et al. Is 15 days an appropriate cut‐off age for considering serious bacterial infection in the management of febrile infants? Pediatr Infect Dis J. 2012;31:455–458.
- , , . Failure of infant observation scales in detecting serious illness in febrile, 4‐ to 8‐week‐old infants. Pediatrics. 1990;85:1040–1043.
- , , . Outpatient management without antibiotics of fever in selected infants. N Engl J Med. 1993;329:1437–1441.
- , , . Identifying febrile infants at risk for a serious bacterial infection. J Pediatr. 1993;123:489–490.
- , , , et al. Febrile infants at low risk for serious bacterial infection—an appraisal of the Rochester criteria and implications for management. Febrile Infant Collaborative Study Group. Pediatrics. 1994;94:390–396.
- , , , et al. Management of febrile neonates in US pediatric emergency departments. Pediatrics. 2014;133:187–195.
- , , , et al. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134:667–677.
- , , , et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10:358–365.
- , , , et al. Diagnosis and management of febrile infants (0‐3 months). Evid Rep Technol Assess (Full Rep). 2012;(205):1–297.
- , , , et al. Accuracy of administrative billing codes to detect urinary tract infection hospitalizations. Pediatrics. 2011;128:323–330.
- , , , et al. Identifying pediatric community‐acquired pneumonia hospitalizations: accuracy of administrative billing codes. JAMA Pediatr. 2013;167:851–858.
- , , , , , . Development and use of reporting guidelines for assessing the quality of validation studies of health administrative data. J Clin Epidemiol. 2011;64:821–829.
- American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42:530–545.
- , , , , , . Variation in occult injury screening for children with suspected abuse in selected US children's hospitals. Pediatrics. 2012;130:853–860.
- . Achieving data quality. How data from a pediatric health information system earns the trust of its users. J AHIMA. 2004;75:22–26.
- , , , . Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299:2048–2055.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381.
- , , , , , . Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107:E99.
- US Census Bureau. Geographic terms and concepts—census divisions and census regions. Available at: https://www.census.gov/geo/reference/gtc/gtc_census_divreg.html. Accessed October 20, 2014.
- , , , . Initial emergency department diagnosis and return visits: risk versus perception. Ann Emerg Med. 1998;32:569–573.
- , , , , . A national depiction of children with return visits to the emergency department within 72 hours, 2001–2007. Pediatr Emerg Care. 2012;28:606–610.
- , , , et al. Pediatric observation status: are we overlooking a growing population in children's hospitals? J Hosp Med. 2012;7:530–536.
- , , , et al. Differences in designations of observation care in US freestanding children's hospitals: are they virtual or real? J Hosp Med. 2012;7:287–293.
- , , , , . Trends in the management of viral meningitis at United States children's hospitals. Pediatrics. 2013;131:670–676.
- , , , et al. Impact of increasing ondansetron use on clinical outcomes in children with gastroenteritis. JAMA Pediatr. 2014;168:321–329.
- , , , , . Race, otitis media, and antibiotic selection. Pediatrics. 2014;134:1059–1066.
- , , , et al. Establishing benchmarks for the hospitalized care of children with asthma, bronchiolitis, and pneumonia. Pediatrics. 2014;134:555–562.
- , , , , . Diagnostic testing and treatment of pediatric headache in the emergency department. J Pediatr. 2013;163:1634–1637.
- . The prevalence of serious bacterial infections by age in febrile infants during the first 3 months of life. Pediatr Ann. 1993;22:462–466.
- , , . Performance of low‐risk criteria in the evaluation of young infants with fever: review of the literature. Pediatrics. 2010;125:228–233.
- , , , , , . A week‐by‐week analysis of the low‐risk criteria for serious bacterial infection in febrile neonates. Arch Dis Child. 2009;94:287–292.
- , , , et al. Is 15 days an appropriate cut‐off age for considering serious bacterial infection in the management of febrile infants? Pediatr Infect Dis J. 2012;31:455–458.
- , , . Failure of infant observation scales in detecting serious illness in febrile, 4‐ to 8‐week‐old infants. Pediatrics. 1990;85:1040–1043.
- , , . Outpatient management without antibiotics of fever in selected infants. N Engl J Med. 1993;329:1437–1441.
- , , . Identifying febrile infants at risk for a serious bacterial infection. J Pediatr. 1993;123:489–490.
- , , , et al. Febrile infants at low risk for serious bacterial infection—an appraisal of the Rochester criteria and implications for management. Febrile Infant Collaborative Study Group. Pediatrics. 1994;94:390–396.
- , , , et al. Management of febrile neonates in US pediatric emergency departments. Pediatrics. 2014;133:187–195.
- , , , et al. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134:667–677.
- , , , et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10:358–365.
- , , , et al. Diagnosis and management of febrile infants (0‐3 months). Evid Rep Technol Assess (Full Rep). 2012;(205):1–297.
- , , , et al. Accuracy of administrative billing codes to detect urinary tract infection hospitalizations. Pediatrics. 2011;128:323–330.
- , , , et al. Identifying pediatric community‐acquired pneumonia hospitalizations: accuracy of administrative billing codes. JAMA Pediatr. 2013;167:851–858.
- , , , , , . Development and use of reporting guidelines for assessing the quality of validation studies of health administrative data. J Clin Epidemiol. 2011;64:821–829.
- American College of Emergency Physicians Clinical Policies Committee; American College of Emergency Physicians Clinical Policies Subcommittee on Pediatric Fever. Clinical policy for children younger than three years presenting to the emergency department with fever. Ann Emerg Med. 2003;42:530–545.
- , , , , , . Variation in occult injury screening for children with suspected abuse in selected US children's hospitals. Pediatrics. 2012;130:853–860.
- . Achieving data quality. How data from a pediatric health information system earns the trust of its users. J AHIMA. 2004;75:22–26.
- , , , . Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299:2048–2055.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381.
- , , , , , . Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107:E99.
- US Census Bureau. Geographic terms and concepts—census divisions and census regions. Available at: https://www.census.gov/geo/reference/gtc/gtc_census_divreg.html. Accessed October 20, 2014.
- , , , . Initial emergency department diagnosis and return visits: risk versus perception. Ann Emerg Med. 1998;32:569–573.
- , , , , . A national depiction of children with return visits to the emergency department within 72 hours, 2001–2007. Pediatr Emerg Care. 2012;28:606–610.
- , , , et al. Pediatric observation status: are we overlooking a growing population in children's hospitals? J Hosp Med. 2012;7:530–536.
- , , , et al. Differences in designations of observation care in US freestanding children's hospitals: are they virtual or real? J Hosp Med. 2012;7:287–293.
- , , , , . Trends in the management of viral meningitis at United States children's hospitals. Pediatrics. 2013;131:670–676.
- , , , et al. Impact of increasing ondansetron use on clinical outcomes in children with gastroenteritis. JAMA Pediatr. 2014;168:321–329.
- , , , , . Race, otitis media, and antibiotic selection. Pediatrics. 2014;134:1059–1066.
- , , , et al. Establishing benchmarks for the hospitalized care of children with asthma, bronchiolitis, and pneumonia. Pediatrics. 2014;134:555–562.
- , , , , . Diagnostic testing and treatment of pediatric headache in the emergency department. J Pediatr. 2013;163:1634–1637.
© 2015 Society of Hospital Medicine
Warfarin therapy highly unpredictable in real-world practice
Only a minority of patients with atrial fibrillation or venous thromboembolism achieved good anticoagulation control while on warfarin, according to a population-based data analysis by Ana Filipa Macedo and her colleagues at Boehringer Ingelheim in the United Kingdom. In fact, only 44% of 140,078 AF patients and 36% of 70,371 VTE patients assessed had an optimal International Normalized Ratio (INR) time in therapeutic range (TTR) more than 70% of the time while on warfarin.
Patient characteristics associated with a significant increase in time spent above or below the recommended INR range of 2.0-3.0 were current smoking, the use of NSAIDs, age less than 45 years, and a body mass index less than 18.5 kg/m2 in both AF and VTE patients, whereas patients with VTE alone had predictors of poor control that included chronic obstructive pulmonary disease or asthma, heart failure, and active cancer.
“The study provides evidence that in the first 12 months of warfarin use, there is a high amount of unpredictable variability in an individual’s TTR,” the researchers summarized. “These findings confirm the difficulty of achieving high-quality anticoagulation with warfarin in real-world clinical practice and can be used to identify patients who require closer monitoring or innovative management strategies,” the authors added.
More of the study can be read here (Thromb Res. 2015 Aug; 136(2):250-260.).
The study received funding from Boehringer Ingelheim, and the authors disclosed that they were all employees of the company, which makes a variety of cardiovascular therapies, including anticoagulants.
Only a minority of patients with atrial fibrillation or venous thromboembolism achieved good anticoagulation control while on warfarin, according to a population-based data analysis by Ana Filipa Macedo and her colleagues at Boehringer Ingelheim in the United Kingdom. In fact, only 44% of 140,078 AF patients and 36% of 70,371 VTE patients assessed had an optimal International Normalized Ratio (INR) time in therapeutic range (TTR) more than 70% of the time while on warfarin.
Patient characteristics associated with a significant increase in time spent above or below the recommended INR range of 2.0-3.0 were current smoking, the use of NSAIDs, age less than 45 years, and a body mass index less than 18.5 kg/m2 in both AF and VTE patients, whereas patients with VTE alone had predictors of poor control that included chronic obstructive pulmonary disease or asthma, heart failure, and active cancer.
“The study provides evidence that in the first 12 months of warfarin use, there is a high amount of unpredictable variability in an individual’s TTR,” the researchers summarized. “These findings confirm the difficulty of achieving high-quality anticoagulation with warfarin in real-world clinical practice and can be used to identify patients who require closer monitoring or innovative management strategies,” the authors added.
More of the study can be read here (Thromb Res. 2015 Aug; 136(2):250-260.).
The study received funding from Boehringer Ingelheim, and the authors disclosed that they were all employees of the company, which makes a variety of cardiovascular therapies, including anticoagulants.
Only a minority of patients with atrial fibrillation or venous thromboembolism achieved good anticoagulation control while on warfarin, according to a population-based data analysis by Ana Filipa Macedo and her colleagues at Boehringer Ingelheim in the United Kingdom. In fact, only 44% of 140,078 AF patients and 36% of 70,371 VTE patients assessed had an optimal International Normalized Ratio (INR) time in therapeutic range (TTR) more than 70% of the time while on warfarin.
Patient characteristics associated with a significant increase in time spent above or below the recommended INR range of 2.0-3.0 were current smoking, the use of NSAIDs, age less than 45 years, and a body mass index less than 18.5 kg/m2 in both AF and VTE patients, whereas patients with VTE alone had predictors of poor control that included chronic obstructive pulmonary disease or asthma, heart failure, and active cancer.
“The study provides evidence that in the first 12 months of warfarin use, there is a high amount of unpredictable variability in an individual’s TTR,” the researchers summarized. “These findings confirm the difficulty of achieving high-quality anticoagulation with warfarin in real-world clinical practice and can be used to identify patients who require closer monitoring or innovative management strategies,” the authors added.
More of the study can be read here (Thromb Res. 2015 Aug; 136(2):250-260.).
The study received funding from Boehringer Ingelheim, and the authors disclosed that they were all employees of the company, which makes a variety of cardiovascular therapies, including anticoagulants.
FROM THROMBOSIS RESEARCH
Treatment of choice for NLPHL? Involved-field RT alone
Involved-field radiotherapy alone should be the treatment of choice for stage 1A nodular lymphocyte-predominant Hodgkin lymphoma because it yields similar survival outcomes but fewer toxic effects than other therapies, investigators reported online Aug. 3 in Journal of Clinical Oncology.
Few studies have examined the long-term outcomes of patients who have this generally indolent malignancy, even though late treatment-associated effects are their major cause of death, said Dr. Dennis A. Eichenauer and his associates at University Hospital Cologne and University Hospital Münster, both in Germany.
To assess long-term outcomes in nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL), the investigators analyzed data for 256 patients who participated in seven prospective randomized clinical trials comparing various treatments during a 21-year period, who were followed for a median of 91 months. The study participants’ median age at diagnosis was 39 years (range, 16-75 years), and all achieved remission with therapy. They were categorized by treatment type: 108 received involved-field radiotherapy (IF-RT) alone; 49 received extended-field radiotherapy (EF-RT) alone; 72 received combined modality treatment involving various combinations of doxorubicin, bleomycin, vinblastine, dacarbazine, IF-RT, EF-RT, and/or rituximab; and 27 received rituximab alone.
At 8 years, progression-free survival was 91.9% with IF-RT, 84.3% with EF-RT, and 88.5% with combined modalities, which are nonsignificant differences. Overall survival was 99.0%, 95.7%, and 98.6%, respectively, which also are nonsignificant differences. Rituximab alone was markedly less effective than the other treatments, with a 4-year progression-free survival of only 81.0%. “Compared with patients who received IF-RT, patients treated with rituximab had a hazard ratio of 4.99 for relapse,” Dr. Eichenauer and his associates wrote (J Clin Oncol. 2015 Aug 3 [doi: 10.1200/JCO. 2014.60.4363]).
Only 3.7% of patients treated with IF-RT developed a second malignancy during follow-up, compared with 11.1% of those treated with combined modalities, 6.1% of those treated with EF-RT, and 7.4% of those treated with rituximab. The rate of relapse was markedly higher with rituximab than with the other treatments.
The main study findings are twofold. First, IF-RT was at least as effective as other treatments in controlling NLPHL, was less toxic acutely, and carried similar or reduced risks of late adverse effects such as relapse and second malignancies. It should be considered the first-line treatment of choice. Second, rituximab alone should not be used routinely in these patients because it yields poorer survival outcomes and a higher relapse rate. “However, it might represent an option for individual patients, such as young women with abdominal disease, to avoid gonadotoxic effects of radiotherapy,” the investigators said.
Involved-field radiotherapy alone should be the treatment of choice for stage 1A nodular lymphocyte-predominant Hodgkin lymphoma because it yields similar survival outcomes but fewer toxic effects than other therapies, investigators reported online Aug. 3 in Journal of Clinical Oncology.
Few studies have examined the long-term outcomes of patients who have this generally indolent malignancy, even though late treatment-associated effects are their major cause of death, said Dr. Dennis A. Eichenauer and his associates at University Hospital Cologne and University Hospital Münster, both in Germany.
To assess long-term outcomes in nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL), the investigators analyzed data for 256 patients who participated in seven prospective randomized clinical trials comparing various treatments during a 21-year period, who were followed for a median of 91 months. The study participants’ median age at diagnosis was 39 years (range, 16-75 years), and all achieved remission with therapy. They were categorized by treatment type: 108 received involved-field radiotherapy (IF-RT) alone; 49 received extended-field radiotherapy (EF-RT) alone; 72 received combined modality treatment involving various combinations of doxorubicin, bleomycin, vinblastine, dacarbazine, IF-RT, EF-RT, and/or rituximab; and 27 received rituximab alone.
At 8 years, progression-free survival was 91.9% with IF-RT, 84.3% with EF-RT, and 88.5% with combined modalities, which are nonsignificant differences. Overall survival was 99.0%, 95.7%, and 98.6%, respectively, which also are nonsignificant differences. Rituximab alone was markedly less effective than the other treatments, with a 4-year progression-free survival of only 81.0%. “Compared with patients who received IF-RT, patients treated with rituximab had a hazard ratio of 4.99 for relapse,” Dr. Eichenauer and his associates wrote (J Clin Oncol. 2015 Aug 3 [doi: 10.1200/JCO. 2014.60.4363]).
Only 3.7% of patients treated with IF-RT developed a second malignancy during follow-up, compared with 11.1% of those treated with combined modalities, 6.1% of those treated with EF-RT, and 7.4% of those treated with rituximab. The rate of relapse was markedly higher with rituximab than with the other treatments.
The main study findings are twofold. First, IF-RT was at least as effective as other treatments in controlling NLPHL, was less toxic acutely, and carried similar or reduced risks of late adverse effects such as relapse and second malignancies. It should be considered the first-line treatment of choice. Second, rituximab alone should not be used routinely in these patients because it yields poorer survival outcomes and a higher relapse rate. “However, it might represent an option for individual patients, such as young women with abdominal disease, to avoid gonadotoxic effects of radiotherapy,” the investigators said.
Involved-field radiotherapy alone should be the treatment of choice for stage 1A nodular lymphocyte-predominant Hodgkin lymphoma because it yields similar survival outcomes but fewer toxic effects than other therapies, investigators reported online Aug. 3 in Journal of Clinical Oncology.
Few studies have examined the long-term outcomes of patients who have this generally indolent malignancy, even though late treatment-associated effects are their major cause of death, said Dr. Dennis A. Eichenauer and his associates at University Hospital Cologne and University Hospital Münster, both in Germany.
To assess long-term outcomes in nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL), the investigators analyzed data for 256 patients who participated in seven prospective randomized clinical trials comparing various treatments during a 21-year period, who were followed for a median of 91 months. The study participants’ median age at diagnosis was 39 years (range, 16-75 years), and all achieved remission with therapy. They were categorized by treatment type: 108 received involved-field radiotherapy (IF-RT) alone; 49 received extended-field radiotherapy (EF-RT) alone; 72 received combined modality treatment involving various combinations of doxorubicin, bleomycin, vinblastine, dacarbazine, IF-RT, EF-RT, and/or rituximab; and 27 received rituximab alone.
At 8 years, progression-free survival was 91.9% with IF-RT, 84.3% with EF-RT, and 88.5% with combined modalities, which are nonsignificant differences. Overall survival was 99.0%, 95.7%, and 98.6%, respectively, which also are nonsignificant differences. Rituximab alone was markedly less effective than the other treatments, with a 4-year progression-free survival of only 81.0%. “Compared with patients who received IF-RT, patients treated with rituximab had a hazard ratio of 4.99 for relapse,” Dr. Eichenauer and his associates wrote (J Clin Oncol. 2015 Aug 3 [doi: 10.1200/JCO. 2014.60.4363]).
Only 3.7% of patients treated with IF-RT developed a second malignancy during follow-up, compared with 11.1% of those treated with combined modalities, 6.1% of those treated with EF-RT, and 7.4% of those treated with rituximab. The rate of relapse was markedly higher with rituximab than with the other treatments.
The main study findings are twofold. First, IF-RT was at least as effective as other treatments in controlling NLPHL, was less toxic acutely, and carried similar or reduced risks of late adverse effects such as relapse and second malignancies. It should be considered the first-line treatment of choice. Second, rituximab alone should not be used routinely in these patients because it yields poorer survival outcomes and a higher relapse rate. “However, it might represent an option for individual patients, such as young women with abdominal disease, to avoid gonadotoxic effects of radiotherapy,” the investigators said.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Involved-field radiotherapy alone yields similar survival but fewer toxic effects than do other treatments for stage 1A nodular lymphocyte–predominant Hodgkin lymphoma.
Major finding: Progression-free survival was 91.9% with IF-RT, 84.3% with EF-RT, and 88.5% with combined modalities, and overall survival was 99.0%, 95.7%, and 98.6%, respectively.
Data source: A retrospective analysis of outcomes of seven German prospective clinical trials involving 256 patients followed for a median of 91 months.
Disclosures: This study was supported by University Hospital Cologne and University Hospital Münster. Dr. Eichenauer reported having no relevant financial disclosures; his associates reported ties to Amgen, Takeda, Novartis, Gilead, and Takeda/Millennium.