User login
Sweet Syndrome Presenting With an Unusual Morphology
To the Editor:
Sweet syndrome is a neutrophilic dermatosis that typically presents as an acute onset of multiple, painful, sharply demarcated, small (measuring a few centimeters), raised, red plaques that occasionally present with superimposed pustules, vesicles, or bullae on the face, neck, upper chest, back, and extremities. Patients are often febrile and may have mucosal and systemic involvement.1 Although 71% of cases are idiopathic, others are associated with malignancy; autoimmune disorders; infections; pregnancy; and rarely medications, especially all-trans-retinoic acid, granulocyte colony-stimulating factor, vaccines, and antibiotics.1,2 We present a case of Sweet syndrome induced by trimethoprim-sulfamethoxazole (TMP-SMX) with an unusual clinical presentation.
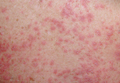
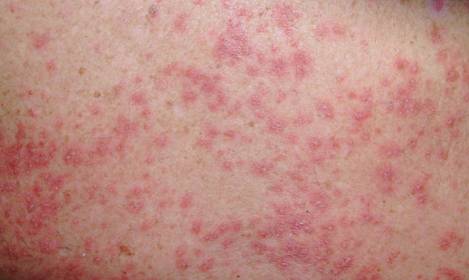
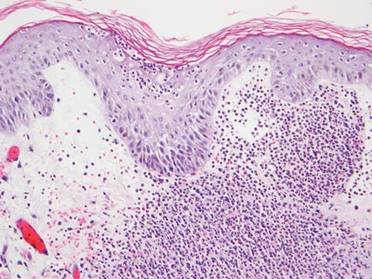
A 71-year-old man with a medical history of nonmelanoma skin cancer initiated a course of TMP-SMX for a wound infection of the lower leg following Mohs micrographic surgery. Eight days later, he developed a painful eruption preceded by 1 day of fever, malaise, blurry vision, and myalgia. Trimethoprim-sulfamethoxazole was discontinued. Physical examination revealed ill-defined, discrete and coalescing, 1- to 6-mm edematous erythematous papules studded with pustules involving the scalp, face, neck, back (Figure 1), and extremities. The patient also had conjunctival erythema and an elevated temperature (38.3°C). Laboratory workup revealed an elevated white blood cell count (11,300/mL [reference range, 4500–11,000/µL]), blood urea nitrogen level (33 mg/µL [reference range, 7–20 mg/dL]), and creatinine level (2.00 mg/dL [reference range, 0.6–1.2 mg/dL]). Liver function tests were normal. A biopsy demonstrated marked papillary dermal edema with a dense, bandlike, superficial dermal neutrophilic infiltrate (Figure 2). A few neutrophils were present in the epidermis with formation of minute intraepidermal pustules. The patient was diagnosed with Sweet syndrome and treated with intravenous methylprednisolone 60 mg 3 times daily (1.5 mg/kg body weight) tapered over 17 days and triamcinolone acetonide ointment 0.1% twice daily. His fever and leukocytosis resolved within 1 day and the eruption improved within 2 days with residual desquamation that cleared by 3 weeks.
| 
|
Morphologically, our case resembled acute generalized exanthematous pustulosis (AGEP), which presents with edematous erythema studded with pustules.3 Although fever and leukocytosis are often present in both AGEP and Sweet syndrome, our patient’s pain, malaise, and myalgia favored Sweet syndrome, as did his conjunctivitis, which is unusual in AGEP.1,3 Histologically, our case was characteristic for Sweet syndrome, which presents with marked papillary dermal edema and a dense neutrophilic dermal infiltrate with neutrophil exocytosis and spongiform pustules in 21% of cases.1 Acute generalized exanthematous pustulosis, characterized by spongiform pustules and a perivascular neutrophilic infiltrate, does not exhibit the dense dermal neutrophilic infiltrate of Sweet syndrome.3 Mecca et al4 also reported a case displaying overlapping features of Sweet syndrome and AGEP. The patient presented with photodistributed papules and pinpoint pustules on an erythematous base favoring a diagnosis of AGEP with histologic findings compatible with Sweet syndrome. The authors suggested a clinicopathologic continuum may exist among drug-related neutrophilic dermatoses.4
In conclusion, we present a case of TMP-SMX–induced Sweet syndrome that morphologically resembled AGEP. It is important to recognize that Sweet syndrome may present in this unusual manner, as it may have notable internal involvement, and responds rapidly to systemic steroids, whereas AGEP has minimal systemic involvement and clears spontaneously.
1. von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556.
2. Kluger N, Marque M, Stoebner PE, et al. Possible drug-induced Sweet’s syndrome due to trimethoprim-sulfamethoxazole. Acta Derm Venereol. 2008;88:637-638.
3. Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
4. Mecca P, Tobin E, Andrew Carlson J. Photo-distributed neutrophilic drug eruption and adult respiratory distress syndrome associated with antidepressant therapy. J Cutan Pathol. 2004;31:189-194.
To the Editor:
Sweet syndrome is a neutrophilic dermatosis that typically presents as an acute onset of multiple, painful, sharply demarcated, small (measuring a few centimeters), raised, red plaques that occasionally present with superimposed pustules, vesicles, or bullae on the face, neck, upper chest, back, and extremities. Patients are often febrile and may have mucosal and systemic involvement.1 Although 71% of cases are idiopathic, others are associated with malignancy; autoimmune disorders; infections; pregnancy; and rarely medications, especially all-trans-retinoic acid, granulocyte colony-stimulating factor, vaccines, and antibiotics.1,2 We present a case of Sweet syndrome induced by trimethoprim-sulfamethoxazole (TMP-SMX) with an unusual clinical presentation.
A 71-year-old man with a medical history of nonmelanoma skin cancer initiated a course of TMP-SMX for a wound infection of the lower leg following Mohs micrographic surgery. Eight days later, he developed a painful eruption preceded by 1 day of fever, malaise, blurry vision, and myalgia. Trimethoprim-sulfamethoxazole was discontinued. Physical examination revealed ill-defined, discrete and coalescing, 1- to 6-mm edematous erythematous papules studded with pustules involving the scalp, face, neck, back (Figure 1), and extremities. The patient also had conjunctival erythema and an elevated temperature (38.3°C). Laboratory workup revealed an elevated white blood cell count (11,300/mL [reference range, 4500–11,000/µL]), blood urea nitrogen level (33 mg/µL [reference range, 7–20 mg/dL]), and creatinine level (2.00 mg/dL [reference range, 0.6–1.2 mg/dL]). Liver function tests were normal. A biopsy demonstrated marked papillary dermal edema with a dense, bandlike, superficial dermal neutrophilic infiltrate (Figure 2). A few neutrophils were present in the epidermis with formation of minute intraepidermal pustules. The patient was diagnosed with Sweet syndrome and treated with intravenous methylprednisolone 60 mg 3 times daily (1.5 mg/kg body weight) tapered over 17 days and triamcinolone acetonide ointment 0.1% twice daily. His fever and leukocytosis resolved within 1 day and the eruption improved within 2 days with residual desquamation that cleared by 3 weeks.

| 
|
Morphologically, our case resembled acute generalized exanthematous pustulosis (AGEP), which presents with edematous erythema studded with pustules.3 Although fever and leukocytosis are often present in both AGEP and Sweet syndrome, our patient’s pain, malaise, and myalgia favored Sweet syndrome, as did his conjunctivitis, which is unusual in AGEP.1,3 Histologically, our case was characteristic for Sweet syndrome, which presents with marked papillary dermal edema and a dense neutrophilic dermal infiltrate with neutrophil exocytosis and spongiform pustules in 21% of cases.1 Acute generalized exanthematous pustulosis, characterized by spongiform pustules and a perivascular neutrophilic infiltrate, does not exhibit the dense dermal neutrophilic infiltrate of Sweet syndrome.3 Mecca et al4 also reported a case displaying overlapping features of Sweet syndrome and AGEP. The patient presented with photodistributed papules and pinpoint pustules on an erythematous base favoring a diagnosis of AGEP with histologic findings compatible with Sweet syndrome. The authors suggested a clinicopathologic continuum may exist among drug-related neutrophilic dermatoses.4
In conclusion, we present a case of TMP-SMX–induced Sweet syndrome that morphologically resembled AGEP. It is important to recognize that Sweet syndrome may present in this unusual manner, as it may have notable internal involvement, and responds rapidly to systemic steroids, whereas AGEP has minimal systemic involvement and clears spontaneously.
To the Editor:
Sweet syndrome is a neutrophilic dermatosis that typically presents as an acute onset of multiple, painful, sharply demarcated, small (measuring a few centimeters), raised, red plaques that occasionally present with superimposed pustules, vesicles, or bullae on the face, neck, upper chest, back, and extremities. Patients are often febrile and may have mucosal and systemic involvement.1 Although 71% of cases are idiopathic, others are associated with malignancy; autoimmune disorders; infections; pregnancy; and rarely medications, especially all-trans-retinoic acid, granulocyte colony-stimulating factor, vaccines, and antibiotics.1,2 We present a case of Sweet syndrome induced by trimethoprim-sulfamethoxazole (TMP-SMX) with an unusual clinical presentation.
A 71-year-old man with a medical history of nonmelanoma skin cancer initiated a course of TMP-SMX for a wound infection of the lower leg following Mohs micrographic surgery. Eight days later, he developed a painful eruption preceded by 1 day of fever, malaise, blurry vision, and myalgia. Trimethoprim-sulfamethoxazole was discontinued. Physical examination revealed ill-defined, discrete and coalescing, 1- to 6-mm edematous erythematous papules studded with pustules involving the scalp, face, neck, back (Figure 1), and extremities. The patient also had conjunctival erythema and an elevated temperature (38.3°C). Laboratory workup revealed an elevated white blood cell count (11,300/mL [reference range, 4500–11,000/µL]), blood urea nitrogen level (33 mg/µL [reference range, 7–20 mg/dL]), and creatinine level (2.00 mg/dL [reference range, 0.6–1.2 mg/dL]). Liver function tests were normal. A biopsy demonstrated marked papillary dermal edema with a dense, bandlike, superficial dermal neutrophilic infiltrate (Figure 2). A few neutrophils were present in the epidermis with formation of minute intraepidermal pustules. The patient was diagnosed with Sweet syndrome and treated with intravenous methylprednisolone 60 mg 3 times daily (1.5 mg/kg body weight) tapered over 17 days and triamcinolone acetonide ointment 0.1% twice daily. His fever and leukocytosis resolved within 1 day and the eruption improved within 2 days with residual desquamation that cleared by 3 weeks.

| 
|
Morphologically, our case resembled acute generalized exanthematous pustulosis (AGEP), which presents with edematous erythema studded with pustules.3 Although fever and leukocytosis are often present in both AGEP and Sweet syndrome, our patient’s pain, malaise, and myalgia favored Sweet syndrome, as did his conjunctivitis, which is unusual in AGEP.1,3 Histologically, our case was characteristic for Sweet syndrome, which presents with marked papillary dermal edema and a dense neutrophilic dermal infiltrate with neutrophil exocytosis and spongiform pustules in 21% of cases.1 Acute generalized exanthematous pustulosis, characterized by spongiform pustules and a perivascular neutrophilic infiltrate, does not exhibit the dense dermal neutrophilic infiltrate of Sweet syndrome.3 Mecca et al4 also reported a case displaying overlapping features of Sweet syndrome and AGEP. The patient presented with photodistributed papules and pinpoint pustules on an erythematous base favoring a diagnosis of AGEP with histologic findings compatible with Sweet syndrome. The authors suggested a clinicopathologic continuum may exist among drug-related neutrophilic dermatoses.4
In conclusion, we present a case of TMP-SMX–induced Sweet syndrome that morphologically resembled AGEP. It is important to recognize that Sweet syndrome may present in this unusual manner, as it may have notable internal involvement, and responds rapidly to systemic steroids, whereas AGEP has minimal systemic involvement and clears spontaneously.
1. von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556.
2. Kluger N, Marque M, Stoebner PE, et al. Possible drug-induced Sweet’s syndrome due to trimethoprim-sulfamethoxazole. Acta Derm Venereol. 2008;88:637-638.
3. Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
4. Mecca P, Tobin E, Andrew Carlson J. Photo-distributed neutrophilic drug eruption and adult respiratory distress syndrome associated with antidepressant therapy. J Cutan Pathol. 2004;31:189-194.
1. von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556.
2. Kluger N, Marque M, Stoebner PE, et al. Possible drug-induced Sweet’s syndrome due to trimethoprim-sulfamethoxazole. Acta Derm Venereol. 2008;88:637-638.
3. Sidoroff A, Halevy S, Bavinck JN, et al. Acute generalized exanthematous pustulosis (AGEP)—a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
4. Mecca P, Tobin E, Andrew Carlson J. Photo-distributed neutrophilic drug eruption and adult respiratory distress syndrome associated with antidepressant therapy. J Cutan Pathol. 2004;31:189-194.
LISTEN NOW: Scott Sears, MD, MBA, Explains How GME Programs Could Be Better Aligned
SCOTT SEARS, MD, MBA, chief clinical officer of Tacoma, Wash.-based Sound Physicians, discusses how
GME programs could be better aligned with the shifting reality in medicine.
SCOTT SEARS, MD, MBA, chief clinical officer of Tacoma, Wash.-based Sound Physicians, discusses how
GME programs could be better aligned with the shifting reality in medicine.
SCOTT SEARS, MD, MBA, chief clinical officer of Tacoma, Wash.-based Sound Physicians, discusses how
GME programs could be better aligned with the shifting reality in medicine.
LISTEN NOW: Ruth Ann Crystal, MD, Pursues Documentary Film "Kitchen Table Deliveries"
Excerpts of The Hospitalist's interview with Dr. Ruth Ann Crystal, who is attempting to create a website with videos of historical medical practice.
Excerpts of The Hospitalist's interview with Dr. Ruth Ann Crystal, who is attempting to create a website with videos of historical medical practice.
Excerpts of The Hospitalist's interview with Dr. Ruth Ann Crystal, who is attempting to create a website with videos of historical medical practice.
Education doesn’t ensure appropriate use of VTE prophylaxis

Photo courtesy of the CDC
In a single-center study, researchers found that educating healthcare providers about the need for venous thromboembolism (VTE) prophylaxis did not ensure that patients received appropriate treatment.
Before the educational program was introduced, 36% of patients who were at risk of VTE were not receiving VTE prophylaxis. After the program, 26% of at-risk patients were not receiving prophylaxis.
The researchers reported these findings in the Canadian Journal of Cardiology.
The team carried out chart reviews of patients in a university-affiliated, tertiary care cardiology center, which included a clinical teaching unit and a coronary care unit.
Audits were conducted 3 and 5 months before the introduction of an educational program on VTE prophylaxis protocol, followed by a second series of audits 3 and 5 months after protocol initiation.
In each set of audits, conducted over 2 months, 3 independent groups consisting of a physician and a nonphysician healthcare provider (nursing, pharmacy) each reviewed the data. Discrepancies were settled by the senior investigators.
In the first set of audits, 173 charts for patients considered at high risk for VTE were evaluated. The second set of audits included 247 patients.
Prior to the educational program, including a guideline-based protocol, 36% of all patients who were considered at risk for VTE did not receive prophylaxis.
Three months after the program was initiated, 21% of patients were still not being treated according to the recommended guidelines, and that percentage rose to 28% at 5 months post-protocol.
“Awareness and education surrounding VTE prophylaxis is challenging in the inpatient teaching unit model due to a number of factors, including the high turnover of senior and junior physicians as well as nursing staff,” said study author Colette Seifer, of the University of Manitoba in Winnipeg, Manitoba, Canada.
“A single time point intervention is unlikely to result in a sustained improvement in VTE prophylaxis rates.”
However, Seifer and her colleagues believe that automated alerts and checklists incorporated into electronic patient records or used via innovative software programs have the potential to improve compliance rates. ![]()

Photo courtesy of the CDC
In a single-center study, researchers found that educating healthcare providers about the need for venous thromboembolism (VTE) prophylaxis did not ensure that patients received appropriate treatment.
Before the educational program was introduced, 36% of patients who were at risk of VTE were not receiving VTE prophylaxis. After the program, 26% of at-risk patients were not receiving prophylaxis.
The researchers reported these findings in the Canadian Journal of Cardiology.
The team carried out chart reviews of patients in a university-affiliated, tertiary care cardiology center, which included a clinical teaching unit and a coronary care unit.
Audits were conducted 3 and 5 months before the introduction of an educational program on VTE prophylaxis protocol, followed by a second series of audits 3 and 5 months after protocol initiation.
In each set of audits, conducted over 2 months, 3 independent groups consisting of a physician and a nonphysician healthcare provider (nursing, pharmacy) each reviewed the data. Discrepancies were settled by the senior investigators.
In the first set of audits, 173 charts for patients considered at high risk for VTE were evaluated. The second set of audits included 247 patients.
Prior to the educational program, including a guideline-based protocol, 36% of all patients who were considered at risk for VTE did not receive prophylaxis.
Three months after the program was initiated, 21% of patients were still not being treated according to the recommended guidelines, and that percentage rose to 28% at 5 months post-protocol.
“Awareness and education surrounding VTE prophylaxis is challenging in the inpatient teaching unit model due to a number of factors, including the high turnover of senior and junior physicians as well as nursing staff,” said study author Colette Seifer, of the University of Manitoba in Winnipeg, Manitoba, Canada.
“A single time point intervention is unlikely to result in a sustained improvement in VTE prophylaxis rates.”
However, Seifer and her colleagues believe that automated alerts and checklists incorporated into electronic patient records or used via innovative software programs have the potential to improve compliance rates. ![]()

Photo courtesy of the CDC
In a single-center study, researchers found that educating healthcare providers about the need for venous thromboembolism (VTE) prophylaxis did not ensure that patients received appropriate treatment.
Before the educational program was introduced, 36% of patients who were at risk of VTE were not receiving VTE prophylaxis. After the program, 26% of at-risk patients were not receiving prophylaxis.
The researchers reported these findings in the Canadian Journal of Cardiology.
The team carried out chart reviews of patients in a university-affiliated, tertiary care cardiology center, which included a clinical teaching unit and a coronary care unit.
Audits were conducted 3 and 5 months before the introduction of an educational program on VTE prophylaxis protocol, followed by a second series of audits 3 and 5 months after protocol initiation.
In each set of audits, conducted over 2 months, 3 independent groups consisting of a physician and a nonphysician healthcare provider (nursing, pharmacy) each reviewed the data. Discrepancies were settled by the senior investigators.
In the first set of audits, 173 charts for patients considered at high risk for VTE were evaluated. The second set of audits included 247 patients.
Prior to the educational program, including a guideline-based protocol, 36% of all patients who were considered at risk for VTE did not receive prophylaxis.
Three months after the program was initiated, 21% of patients were still not being treated according to the recommended guidelines, and that percentage rose to 28% at 5 months post-protocol.
“Awareness and education surrounding VTE prophylaxis is challenging in the inpatient teaching unit model due to a number of factors, including the high turnover of senior and junior physicians as well as nursing staff,” said study author Colette Seifer, of the University of Manitoba in Winnipeg, Manitoba, Canada.
“A single time point intervention is unlikely to result in a sustained improvement in VTE prophylaxis rates.”
However, Seifer and her colleagues believe that automated alerts and checklists incorporated into electronic patient records or used via innovative software programs have the potential to improve compliance rates. ![]()
How religion affects well-being in cancer patients

Photo by Petr Kratochvil
Three meta-analyses shed new light on the role religion and spirituality play in cancer patients’ mental, social, and physical well-being.
The analyses, published in Cancer, indicate that religion and spirituality have significant associations with patients’ health.
But investigators observed wide variability among studies with regard to how different dimensions of religion and spirituality relate to different aspects of health.
In the first analysis, the investigators focused on physical health. Patients reporting greater overall religiousness and spirituality reported better physical health, greater ability to perform their usual daily tasks, and fewer physical symptoms of cancer and treatment.
“These relationships were particularly strong in patients who experienced greater emotional aspects of religion and spirituality, including a sense of meaning and purpose in life as well as a connection to a source larger than oneself,” said study author Heather Jim, PhD, of the Moffitt Cancer Center in Tampa, Florida.
Dr Jim noted that patients who reported greater cognitive aspects of religion and spirituality, such as the ability to integrate the cancer into their religious or spiritual beliefs, also reported better physical health. However, physical health was not related to behavioral aspects of religion and spiritualty, such as church attendance, prayer, or meditation.
In the second analysis, the investigators examined patients’ mental health. The team discovered that emotional aspects of religion and spirituality were more strongly associated with positive mental health than behavioral or cognitive aspects of religion and spirituality.
“Spiritual well-being was, unsurprisingly, associated with less anxiety, depression, or distress,” said study author John Salsman, PhD, of Wake Forest School of Medicine in Winston-Salem, North Carolina.
“Also, greater levels of spiritual distress and a sense of disconnectedness with God or a religious community was associated with greater psychological distress or poorer emotional well-being.”
The third analysis pertained to social health, or patients’ capacity to retain social roles and relationships in the face of illness. Religion and spirituality, as well as each of its dimensions, had modest but reliable links with social health.
“When we took a closer look, we found that patients with stronger spiritual well-being, more benign images of God (such as perceptions of a benevolent God rather than an angry or distant God), or stronger beliefs (such as convictions that a personal God can be called upon for assistance) reported better social health,” said study author Allen Sherman, PhD, of the University of Arkansas for Medical Sciences in Little Rock. “In contrast, those who struggled with their faith fared more poorly.”
The investigators believe future research should focus on how relationships between religious or spiritual involvement and health change over time and whether support services designed to enhance particular aspects of religion and spirituality in interested patients might help improve their well-being.
“In addition, some patients struggle with the religious or spiritual significance of their cancer, which is normal,” Dr Jim said. “How they resolve their struggle may impact their health, but more research is needed to better understand and support these patients.” ![]()

Photo by Petr Kratochvil
Three meta-analyses shed new light on the role religion and spirituality play in cancer patients’ mental, social, and physical well-being.
The analyses, published in Cancer, indicate that religion and spirituality have significant associations with patients’ health.
But investigators observed wide variability among studies with regard to how different dimensions of religion and spirituality relate to different aspects of health.
In the first analysis, the investigators focused on physical health. Patients reporting greater overall religiousness and spirituality reported better physical health, greater ability to perform their usual daily tasks, and fewer physical symptoms of cancer and treatment.
“These relationships were particularly strong in patients who experienced greater emotional aspects of religion and spirituality, including a sense of meaning and purpose in life as well as a connection to a source larger than oneself,” said study author Heather Jim, PhD, of the Moffitt Cancer Center in Tampa, Florida.
Dr Jim noted that patients who reported greater cognitive aspects of religion and spirituality, such as the ability to integrate the cancer into their religious or spiritual beliefs, also reported better physical health. However, physical health was not related to behavioral aspects of religion and spiritualty, such as church attendance, prayer, or meditation.
In the second analysis, the investigators examined patients’ mental health. The team discovered that emotional aspects of religion and spirituality were more strongly associated with positive mental health than behavioral or cognitive aspects of religion and spirituality.
“Spiritual well-being was, unsurprisingly, associated with less anxiety, depression, or distress,” said study author John Salsman, PhD, of Wake Forest School of Medicine in Winston-Salem, North Carolina.
“Also, greater levels of spiritual distress and a sense of disconnectedness with God or a religious community was associated with greater psychological distress or poorer emotional well-being.”
The third analysis pertained to social health, or patients’ capacity to retain social roles and relationships in the face of illness. Religion and spirituality, as well as each of its dimensions, had modest but reliable links with social health.
“When we took a closer look, we found that patients with stronger spiritual well-being, more benign images of God (such as perceptions of a benevolent God rather than an angry or distant God), or stronger beliefs (such as convictions that a personal God can be called upon for assistance) reported better social health,” said study author Allen Sherman, PhD, of the University of Arkansas for Medical Sciences in Little Rock. “In contrast, those who struggled with their faith fared more poorly.”
The investigators believe future research should focus on how relationships between religious or spiritual involvement and health change over time and whether support services designed to enhance particular aspects of religion and spirituality in interested patients might help improve their well-being.
“In addition, some patients struggle with the religious or spiritual significance of their cancer, which is normal,” Dr Jim said. “How they resolve their struggle may impact their health, but more research is needed to better understand and support these patients.” ![]()

Photo by Petr Kratochvil
Three meta-analyses shed new light on the role religion and spirituality play in cancer patients’ mental, social, and physical well-being.
The analyses, published in Cancer, indicate that religion and spirituality have significant associations with patients’ health.
But investigators observed wide variability among studies with regard to how different dimensions of religion and spirituality relate to different aspects of health.
In the first analysis, the investigators focused on physical health. Patients reporting greater overall religiousness and spirituality reported better physical health, greater ability to perform their usual daily tasks, and fewer physical symptoms of cancer and treatment.
“These relationships were particularly strong in patients who experienced greater emotional aspects of religion and spirituality, including a sense of meaning and purpose in life as well as a connection to a source larger than oneself,” said study author Heather Jim, PhD, of the Moffitt Cancer Center in Tampa, Florida.
Dr Jim noted that patients who reported greater cognitive aspects of religion and spirituality, such as the ability to integrate the cancer into their religious or spiritual beliefs, also reported better physical health. However, physical health was not related to behavioral aspects of religion and spiritualty, such as church attendance, prayer, or meditation.
In the second analysis, the investigators examined patients’ mental health. The team discovered that emotional aspects of religion and spirituality were more strongly associated with positive mental health than behavioral or cognitive aspects of religion and spirituality.
“Spiritual well-being was, unsurprisingly, associated with less anxiety, depression, or distress,” said study author John Salsman, PhD, of Wake Forest School of Medicine in Winston-Salem, North Carolina.
“Also, greater levels of spiritual distress and a sense of disconnectedness with God or a religious community was associated with greater psychological distress or poorer emotional well-being.”
The third analysis pertained to social health, or patients’ capacity to retain social roles and relationships in the face of illness. Religion and spirituality, as well as each of its dimensions, had modest but reliable links with social health.
“When we took a closer look, we found that patients with stronger spiritual well-being, more benign images of God (such as perceptions of a benevolent God rather than an angry or distant God), or stronger beliefs (such as convictions that a personal God can be called upon for assistance) reported better social health,” said study author Allen Sherman, PhD, of the University of Arkansas for Medical Sciences in Little Rock. “In contrast, those who struggled with their faith fared more poorly.”
The investigators believe future research should focus on how relationships between religious or spiritual involvement and health change over time and whether support services designed to enhance particular aspects of religion and spirituality in interested patients might help improve their well-being.
“In addition, some patients struggle with the religious or spiritual significance of their cancer, which is normal,” Dr Jim said. “How they resolve their struggle may impact their health, but more research is needed to better understand and support these patients.” ![]()
Impact of an Inpatient PN Program
Inpatient medicine is becoming increasingly complex. A growing number of patients with multiple chronic conditions coupled with mounting care fragmentation leave patients vulnerable to adverse events and readmission to the hospital.[1, 2, 3] Moreover, efforts to minimize hospital length of stay (LOS) have resulted in patients being discharged quicker and sicker than ever before.[4]
A cornerstone of safe and high‐quality healthcare is effective communication.[5] Ineffective communication between and among healthcare providers and patients is a leading cause of medical errors and patient harm. An analysis of sentinel events reported to The Joint Commission revealed that communication failure was the root cause in 59% of these events.[6]
The current climate of increasing healthcare complexity has prompted the need for adaptive innovation.[7] However, there are limited data describing interventions targeting improvements in both communication and transitional care planning. We created a new position, the patient navigator (PN), a dedicated patient‐care facilitator not responsible for clinical care. PNs were integrated into the inpatient multidisciplinary clinical team to facilitate patient and provider navigation through the complexity of a hospital admission by enhancing communication between and among patients and providers. The objective of this study was to determine whether this intervention would reduce hospital LOS and 30‐day unplanned readmissions.
METHODS
Setting
Mount Sinai Hospital is a 446‐bed acute care urban academic health center in Toronto, Ontario, Canada. The general internal medicine service operates as a 90‐bed clinical teaching unit physically distributed over 4 inpatient wards. The service is structurally divided into 4 nongeographically based multidisciplinary care teams (teams A, B, C, and D) comprised of the medical team (attending physician, senior resident physician, 23 junior resident physicians, and 23 medical students), pharmacist, social worker, physiotherapist, occupational therapist, speech and language pathologist, dietician, respiratory therapist, and nursing staff allocated by ward. Each team is on call approximately 1 night in 4 with no night float system. At our institution, attending physicians rotate on a 2‐ or 4‐week schedule, resident physicians rotate on a 1‐ or 2‐month schedule, and medical students rotate on a 2‐month schedule. Preintervention, communication occurred in person and by telephone between members of the medical team. Other members of the multidisciplinary care team communicated with the medical team in person at daily multidisciplinary rounds focused on discharge planning, by pager, or using a Web‐based communication tool.
Intervention
PNs were dedicated patient‐care facilitators not responsible for clinical care. They acted as liaisons between and among providers and patients. Each PN was a fully integrated member of their multidisciplinary care team. With ongoing medical team rotations, the PN was notably the only consistent member on the clinical team. Each patient saw the same PN throughout his or her hospital stay, as both the patient and the PN were team based. The average number of patients for whom each PN was responsible daily was dictated by the patient census for their team. On average, each team had a census between 20 and 30 patients daily. PNs worked during the daytime from Monday to Friday, and did not have any overnight or weekend responsibilities.
A PN's typical day began by reviewing and rounding on overnight admissions as a formal member of the clinical team. This was followed by participating in daily multidisciplinary rounds, then documenting and circulating the resultant action items. Thereafter, they expedited consultations and tests by liaising with departmental staff, and proactively established contact with the patient and their family. They answered simple factual questions related to test scheduling, consultations, diagnosis, medications, and treatments as discussed and outlined by the clinical team, and promptly relayed care questions beyond the scope of their knowledge to the clinical team. They were available to patients, family members, and providers via a dedicated mobile number using phone calls and text messages. If indicated, they assisted in discharge coordination by arranging follow‐up appointments and placing postdischarge phone calls. In addition, they served as primary contact for every patient admitted to their clinical team following discharge to ensure appropriate follow through on discharge plans. There were no set criteria for PNs to disengage from a patient's care. They could always be reached using their dedicated mobile number during business hours, with a voicemail system in place for after‐hours calls.
The role was filled by individuals skilled in communication and/or healthcare, such as registered nurses, a masters degreetrained educator, internationally trained physicians, and professionals from the hospitality and human resources industries. There were no prespecified training or degree requirements. Each PN underwent on‐the‐job training and participated in twice monthly PN meetings for ongoing feedback and education.
Program Implementation
We implemented the PN program on the inpatient general internal medicine service in June 2010 on 2 of 4 multidisciplinary clinical teams. Because a PN became an integrated member of 1 of 4 clinical teams, patient assignment to a PN was determined by the team to which the patient was admitted. On average, each of the 4 teams admitted equally on a daily basis. Initially, there were only sufficient resources to fund 2 PNs. Thus, from June 2010 to May 2011, only teams A and C were assigned PNs. To create fairness between the 4 teams, these 2 PNs moved to teams B and D from June 2011 to November 2011, and then back to teams A and C from December 2011 to April 2012. Following this initial pilot period, the program was allocated further resources, and so expanded to all 4 teams in May 2012. PN salaries were the only program costs. These costs were funded by matching donations from physicians within the Mount Sinai Hospital Department of Medicine and donations to the hospital from community members directed to support the implementation and evaluation of novel care delivery systems.
Study Design
We evaluated the PN program using a retrospective cohort study that included all general medical admissions between July 2010 and March 2014 matched by case mix group, age category, and resource intensity weight (a relative value measuring total patient resource use compared with average typical acute inpatients).[8]
Our primary outcomes were LOS and 30‐day readmission rate. These outcomes were stratified by exposure status to a PN. There were no exclusion criteria for the LOS analysis. Patients who died, were transferred to or from an acute care facility, or signed out against medical advice were excluded from the 30‐day readmission analysis. A secondary analysis restricted the timeframe from July 2010 to April 2011, when only 2 of 4 teams were exposed to PNs.
Average LOS has been observed to be higher in Canadian hospitals as compared to their US counterparts across different admission diagnoses, such as coronary artery bypass graft surgery and heart failure.[9, 10] We hypothesize that these differences are party due to systems‐level differences, including posthospital care. Specifically, the Canadian system does not utilize posthospital acute care, such as skilled nursing facilities, which may in part account for these differences. To help contextualize our data, we standardized LOS using an LOS index called the LOS/expected LOS (ELOS) ratio. It takes the LOS and divides it by the ELOS, a validated estimate of the expected LOS for a given patient generated using a national administrative database for acute hospital care in Canada that takes into account case mix group, age, comorbidity level, and intervention factors.[8]
Additionally, We performed an interrupted time‐series analysis, whereby a log‐linear model was fit on LOS and adjusted for weekly and monthly trends, age category, resource intensity weight, major clinical category (a surrogate for case mix group), admission location, and discharge location. The cohort was divided into 3 groups: before program implementation (July 2009June 2010), after program implementation with PN (July 2010March 2014), and after program implementation without PN (July 2010March 2014).
This study was approved by the research ethics board at Mount Sinai Hospital. No patient consent was deemed necessary. Data were obtained from institutional databases monitored by the hospital's performance measurement office.
Statistical Analysis
In Tables 1, 2, mean values were compared using a 2‐tailed t test, and the relationship between categorical groups was determined using a 2 test. For the interrupted time‐series analysis, 2‐tailed t tests were used to test null hypotheses of no association between the parameter value and the outcome, and 2 tests were used to test for the equivalence of 2 given parameters. P0.05 indicated statistical significance for all comparisons and analyses. All data were analyzed using Stata version 13 (StataCorp, College Station, TX) or R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
| With PN, n = 5,628 | Without PN, n = 2,213 | |
|---|---|---|
| ||
| Age, y, mean (SD)* | 69 (20) | 68 (20) |
| Female sex, n (%) | 3,018 (53.6) | 1,196 (54.0) |
| Most responsible diagnosis, n (%) | ||
| Pneumonia | 374 (6.6) | 135 (6.1) |
| Chronic obstructive pulmonary disease | 271 (4.8) | 88 (4.0) |
| Congestive heart failure | 217 (3.9) | 87 (3.9) |
| Admission location, n (%) | ||
| Home | 4,665 (82.9) | 1,943 (87.8) |
| Long‐term care* | 524 (9.3) | 158 (7.1) |
| Other* | 439 (7.8) | 112 (5.1) |
| Discharge location, n (%) | ||
| Home | 3,824 (67.9) | 1,578 (71.3) |
| Long‐term care | 779 (13.8) | 267 (12.1) |
| Other | 1,025 (18.3) | 368 (16.6) |
RESULTS
Our matched cohort included 7841 admissions (6141 patients), with 5628 admissions (4592 patients) exposed and 2213 admissions (1920 patients) not exposed to PNs. The discrepancy between the total number of patients and the sum of exposed and nonexposed patients is resultant from patients admitted more than once over the study period, as patients admitted to at least 1 team staffed with a PN and another team not staffed with a PN over the study period were counted in both groups. The 2 groups were similar with respect to several characteristics (Table 1). However, the 2 groups were significantly different for age (P = 0.046) and admissions from long‐term care (P < 0.01) and other facilities (P < 0.01).
Admissions with PNs were 1.3 days (21%) shorter than admission without PNs (6.2 vs 7.5 days, P < 0.001). Moreover, admissions with PNs had a smaller mean LOS/ELOS ratio compared to admissions without PNs (0.93 vs 1.05, P < 0.001). The restricted analysis found a 1.2‐day (18%) lower LOS (6.4 vs 7.6 days, P < 0.05) and a smaller mean LOS/ELOS ratio (0.91 vs 1.06, P < 0.001). Thirty‐day readmission rate was not different between the 2 groups (13.1 vs 13.8%, P = 0.48) or in the restricted analysis (12.0 vs 13.5%, P = 0.40) (Table 2).
| With PN | Without PN | P Value | |
|---|---|---|---|
| |||
| July 2010‐March 2014 | |||
| LOS, d (95% confidence interval) [n] | 6.2 (6.06.4) [5,628] | 7.5 (7.17.9) [2,213] | <0.001 |
| LOS/ELOS ratio (95% confidence interval) [n] | 0.93 (0.910.95) [5,628] | 1.05 (1.001.09) [2,213] | <0.001 |
| 30‐day readmission rate, % [n] | 13.1 [5,055] | 13.8 [2,012] | 0.48 |
| July 2010 to April 2011 | |||
| LOS, d (95% confidence interval) [n] | 6.4 (5.87.0) [713] | 7.6 (6.88.3) [753) | <0.05 |
| LOS/ELOS ratio (95% confidence interval) [n] | 0.91 (0.850.96) [713] | 1.06 (1.001.11) [753] | <0.001 |
| 30‐day readmission rate, % [n] | 12.0 [627] | 13.5 [681] | 0.40 |
In the interrupted time‐series analysis, prior to the implementation of the PN program, there was a positive relationship between LOS and time. After the implementation of the program, this relationship became inverse, meaning the curve plotting LOS against time had a negative slope. Furthermore, there was a statistically significant drop in LOS at the time of program implementation (P < 0.05). However, there was no difference in slope between the groups with and without PN after program implementation.
DISCUSSION
We describe an innovative inpatient intervention featuring an integrated patient‐care facilitator not responsible for clinical care charged with enhancing communication between and among patients and providers. Data from the almost 4‐year period demonstrated that implementation was associated with a 21% reduction in hospital LOS, with no difference in 30‐day readmission rates.
The patient navigator was first conceptualized in 1990 to help African American women in Harlem with breast cancer negotiate the complex world of oncology.[11] It was later implemented by the National Cancer Institute as an outpatient intervention spanning the continuum of cancer care. This concept has since expanded to other domains of complex single disease outpatient care, including asthma and fertility.[12, 13] To our knowledge, there has been limited evidence in the literature describing implementation of such programs in the inpatient general medical setting.
This study contributes to the growing literature on interventions targeting improvements in transitional care, such as transition coaches and discharge advocates.[14, 15] Balaban et al. recently described a PN intervention in the safety‐net population.[16] A common theme to these interventions was the prioritization of safe care transitions. However, this goal was achieved using related, yet different approaches: transition coaches focused on encouraging the patient and caregiver to assert a more active role,[14] discharge advocates focused on providing a comprehensive discharge plan for patients,[15] PNs from Balaban's study focused on coaching and assistance in navigating patients through the transition from hospital to home, and our study's PNs focused on enhancing communication between and among patients and providers. Additionally, unlike transition coaches and discharge advocates, who were nurses by training, and PNs from Balaban's study, who were community health workers, our PNs did not have any prespecified training or degree requirements.
Patients are at risk of being inadequately informed about important issues related to their care, such as hospital medications, diagnoses, and treatment plans during their hospital stay.[17, 18] Furthermore, we know that ineffective communication is a common cause of poor patient outcomes in hospital‐based care.[6] This phenomenon can be amplified from external pressures to maximize productivity. For example, Elliott and colleagues found that increasing hospitalist workload is associated with higher hospital LOS and cost.[19] PNs may offload care demands by enhancing communication for providers and patients.
Our study has several strengths. By matching admissions by case mix group, age category, and resource intensity weight, we aimed to reduce potential bias contributed by these covariates. Moreover, a staged rollout of the intervention, whereby over a 10‐month period, 2 of the multidisciplinary care teams were assigned PNs, while the remaining 2 were not, enabled contemporaneous comparison. Our study had few exclusion criteria, thus making it potentially generalizable to other inpatient general medicine settings of a similar nature. The relative simplicity of this intervention makes it amenable to scalability. Of note, the intervention has been deemed to show great promise at our institution, and has currently expanded to the cardiology, gastroenterology, and surgical oncology units.
Our study's limitations include a single‐center design. Moreover, although we demonstrate similarity in the majority of measurable covariates between the groups, we cannot exclude the existence of unmeasured confounders. Of the covariates that were found to be different between the groups, we suspect the difference in admissions from long‐term care and other facilities did not largely influence our study's main findings. Furthermore, though age was found to be statistically different between the groups, we postulate that the 1‐year difference between the groups is not particularly relevant clinically. Additionally, 30‐day readmission rates were only captured for our institution. However, the vast majority of readmissions in our region are to the index facility, and are unlikely to differ between the 2 groups.[20]
There may have been secular trends at play. In the interrupted time‐series analysis, there was a statistically significant drop in LOS at the time of program implementation. There was however, no difference in slope between the groups with and without PNs after program implementation. There are some plausible explanations for this lack of difference in slope. The study may not have been powered to detect such a difference, as this analysis was not prespecified. Furthermore, there may have been a spillover effect of the program, such that PNs may have improved efficiency for the teams to which they were assigned, thereby improving the efficiency of the other members of the multidisciplinary team, many of whom cared for patients assigned and not assigned a PN. Additionally, we measured the LOS in a preintervention control group between July 2009 and June 2010 using the same inclusion criteria as the matched cohort. It was found to be 8.5 days, which suggests a secular trend toward improvement in LOS over time at our institution. We are, however, reassured that our restricted analysis enabling contemporaneous comparison between patients exposed and not exposed to PNs was still found to be significant.
The implementation of this intervention could have implications for policymakers‐at‐large. Establishment of criteria for qualifications and a clear educational curriculum to train future PNs is needed, especially in the context of ongoing program expansion. These initiatives are currently underway at our institution. Furthermore, evaluation of the program's operating cost and calculation of its return on investment should include balanced metrics incorporating patient‐, provider‐, organizational‐, and system‐level measures. The current cost to the hospital per PN is approximately $73,800 CAD ($58,700 USD), which covers 1 PN's annual salary and benefits. Thus, the implementation of 4 PNs for each of the 4 multidisciplinary teams costs the hospital approximately $295,000 CAD ($234,700 USD) per year. Although the details of our preliminary calculations are outside the scope of this report, it suggests that the savings incurred from shorter LOS outweigh program costs.
We found that implementation of this innovative inpatient intervention targeting improvements in communication was associated with a reduction in LOS without an increase in 30‐day readmission. Our experience shows promise and may inform others considering similar interventions. Patient and provider experience and generalizability should be evaluated in future work.
Acknowledgements
The authors thank Dr. Allan Detsky and David Wells for their review of the manuscript. They are also grateful to Chin‐Chin Chua, Ningmei Wang, and Joann Bon in the Office of Quality and Performance Measurement for their help with data collection, and John Matelski for his help with data analysis.
Disclosure: This program was funded by matched donations from physicians in the Mount Sinai Hospital Department of Medicine and donations to Mount Sinai Hospital from community members directed to support the implementation and evaluation of novel care delivery systems. The authors report no conflicts of interest. Preliminary abstracts of this study were presented in the online forum, Leading Health Care Innovation, November 12, 2013 (
- , , , et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(S3):391–395.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Prospective payment system and impairment at discharge. The “quicker‐and‐sicker” story revisited. JAMA. 1990;264(15):1980–1983.
- . The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13(suppl 1):i85–i90.
- The Joint Commission. Sentinel event data root: causes by event type (2004–June 2014). Available at: http://www.jointcommission.org/assets/1/18/Root_Causes_by_Event_Type_2004‐2014.pdf. Accessed March 12, 2014.
- , . Complexity science: the challenge of complexity in health care. BMJ. 2001;323(7313):625–628.
- Canadian Institute for Health Information. Case mix. Available at: http://www.cihi.ca/CIHI‐ext‐portal/internet/EN/TabbedContent/standards+and+data+submission/standards/case+mix/cihi010690. Accessed April 12, 2015.
- , , , , , . Outcomes and cost of coronary artery bypass graft surgery in the United States and Canada. Arch Intern Med. 2005;165(13):1506–1513.
- , , , et al. Differences in treatment, outcomes, and quality of life among patients with heart failure in Canada and the United States. JACC Heart Fail. 2013;1(6):523–530.
- , , . Expanding access to cancer screening and clinical follow‐up among the medically underserved. Cancer Pract. 1995;3(1):19–30.
- , , , et al. Clearing clinical barriers: enhancing social support using a patient navigator for asthma care. J Asthma. 2010;47(8):913–919.
- . The role of a patient navigator in fertility preservation. In: Cancer Treatment and Research. Vol 156. Boston, MA: Springer US; 2010:469–470.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , , , . A patient navigator intervention to reduce hospital readmissions among high‐risk safety‐net patients: a randomized controlled trial. J Gen Intern Med. 2015;30(7):907–915.
- , , . Lack of patient knowledge regarding hospital medications. J Hosp Med. 2010;5(2):83–86.
- , . Patients' understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80(8):991–994.
- , , , , . Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–e111.
Inpatient medicine is becoming increasingly complex. A growing number of patients with multiple chronic conditions coupled with mounting care fragmentation leave patients vulnerable to adverse events and readmission to the hospital.[1, 2, 3] Moreover, efforts to minimize hospital length of stay (LOS) have resulted in patients being discharged quicker and sicker than ever before.[4]
A cornerstone of safe and high‐quality healthcare is effective communication.[5] Ineffective communication between and among healthcare providers and patients is a leading cause of medical errors and patient harm. An analysis of sentinel events reported to The Joint Commission revealed that communication failure was the root cause in 59% of these events.[6]
The current climate of increasing healthcare complexity has prompted the need for adaptive innovation.[7] However, there are limited data describing interventions targeting improvements in both communication and transitional care planning. We created a new position, the patient navigator (PN), a dedicated patient‐care facilitator not responsible for clinical care. PNs were integrated into the inpatient multidisciplinary clinical team to facilitate patient and provider navigation through the complexity of a hospital admission by enhancing communication between and among patients and providers. The objective of this study was to determine whether this intervention would reduce hospital LOS and 30‐day unplanned readmissions.
METHODS
Setting
Mount Sinai Hospital is a 446‐bed acute care urban academic health center in Toronto, Ontario, Canada. The general internal medicine service operates as a 90‐bed clinical teaching unit physically distributed over 4 inpatient wards. The service is structurally divided into 4 nongeographically based multidisciplinary care teams (teams A, B, C, and D) comprised of the medical team (attending physician, senior resident physician, 23 junior resident physicians, and 23 medical students), pharmacist, social worker, physiotherapist, occupational therapist, speech and language pathologist, dietician, respiratory therapist, and nursing staff allocated by ward. Each team is on call approximately 1 night in 4 with no night float system. At our institution, attending physicians rotate on a 2‐ or 4‐week schedule, resident physicians rotate on a 1‐ or 2‐month schedule, and medical students rotate on a 2‐month schedule. Preintervention, communication occurred in person and by telephone between members of the medical team. Other members of the multidisciplinary care team communicated with the medical team in person at daily multidisciplinary rounds focused on discharge planning, by pager, or using a Web‐based communication tool.
Intervention
PNs were dedicated patient‐care facilitators not responsible for clinical care. They acted as liaisons between and among providers and patients. Each PN was a fully integrated member of their multidisciplinary care team. With ongoing medical team rotations, the PN was notably the only consistent member on the clinical team. Each patient saw the same PN throughout his or her hospital stay, as both the patient and the PN were team based. The average number of patients for whom each PN was responsible daily was dictated by the patient census for their team. On average, each team had a census between 20 and 30 patients daily. PNs worked during the daytime from Monday to Friday, and did not have any overnight or weekend responsibilities.
A PN's typical day began by reviewing and rounding on overnight admissions as a formal member of the clinical team. This was followed by participating in daily multidisciplinary rounds, then documenting and circulating the resultant action items. Thereafter, they expedited consultations and tests by liaising with departmental staff, and proactively established contact with the patient and their family. They answered simple factual questions related to test scheduling, consultations, diagnosis, medications, and treatments as discussed and outlined by the clinical team, and promptly relayed care questions beyond the scope of their knowledge to the clinical team. They were available to patients, family members, and providers via a dedicated mobile number using phone calls and text messages. If indicated, they assisted in discharge coordination by arranging follow‐up appointments and placing postdischarge phone calls. In addition, they served as primary contact for every patient admitted to their clinical team following discharge to ensure appropriate follow through on discharge plans. There were no set criteria for PNs to disengage from a patient's care. They could always be reached using their dedicated mobile number during business hours, with a voicemail system in place for after‐hours calls.
The role was filled by individuals skilled in communication and/or healthcare, such as registered nurses, a masters degreetrained educator, internationally trained physicians, and professionals from the hospitality and human resources industries. There were no prespecified training or degree requirements. Each PN underwent on‐the‐job training and participated in twice monthly PN meetings for ongoing feedback and education.
Program Implementation
We implemented the PN program on the inpatient general internal medicine service in June 2010 on 2 of 4 multidisciplinary clinical teams. Because a PN became an integrated member of 1 of 4 clinical teams, patient assignment to a PN was determined by the team to which the patient was admitted. On average, each of the 4 teams admitted equally on a daily basis. Initially, there were only sufficient resources to fund 2 PNs. Thus, from June 2010 to May 2011, only teams A and C were assigned PNs. To create fairness between the 4 teams, these 2 PNs moved to teams B and D from June 2011 to November 2011, and then back to teams A and C from December 2011 to April 2012. Following this initial pilot period, the program was allocated further resources, and so expanded to all 4 teams in May 2012. PN salaries were the only program costs. These costs were funded by matching donations from physicians within the Mount Sinai Hospital Department of Medicine and donations to the hospital from community members directed to support the implementation and evaluation of novel care delivery systems.
Study Design
We evaluated the PN program using a retrospective cohort study that included all general medical admissions between July 2010 and March 2014 matched by case mix group, age category, and resource intensity weight (a relative value measuring total patient resource use compared with average typical acute inpatients).[8]
Our primary outcomes were LOS and 30‐day readmission rate. These outcomes were stratified by exposure status to a PN. There were no exclusion criteria for the LOS analysis. Patients who died, were transferred to or from an acute care facility, or signed out against medical advice were excluded from the 30‐day readmission analysis. A secondary analysis restricted the timeframe from July 2010 to April 2011, when only 2 of 4 teams were exposed to PNs.
Average LOS has been observed to be higher in Canadian hospitals as compared to their US counterparts across different admission diagnoses, such as coronary artery bypass graft surgery and heart failure.[9, 10] We hypothesize that these differences are party due to systems‐level differences, including posthospital care. Specifically, the Canadian system does not utilize posthospital acute care, such as skilled nursing facilities, which may in part account for these differences. To help contextualize our data, we standardized LOS using an LOS index called the LOS/expected LOS (ELOS) ratio. It takes the LOS and divides it by the ELOS, a validated estimate of the expected LOS for a given patient generated using a national administrative database for acute hospital care in Canada that takes into account case mix group, age, comorbidity level, and intervention factors.[8]
Additionally, We performed an interrupted time‐series analysis, whereby a log‐linear model was fit on LOS and adjusted for weekly and monthly trends, age category, resource intensity weight, major clinical category (a surrogate for case mix group), admission location, and discharge location. The cohort was divided into 3 groups: before program implementation (July 2009June 2010), after program implementation with PN (July 2010March 2014), and after program implementation without PN (July 2010March 2014).
This study was approved by the research ethics board at Mount Sinai Hospital. No patient consent was deemed necessary. Data were obtained from institutional databases monitored by the hospital's performance measurement office.
Statistical Analysis
In Tables 1, 2, mean values were compared using a 2‐tailed t test, and the relationship between categorical groups was determined using a 2 test. For the interrupted time‐series analysis, 2‐tailed t tests were used to test null hypotheses of no association between the parameter value and the outcome, and 2 tests were used to test for the equivalence of 2 given parameters. P0.05 indicated statistical significance for all comparisons and analyses. All data were analyzed using Stata version 13 (StataCorp, College Station, TX) or R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
| With PN, n = 5,628 | Without PN, n = 2,213 | |
|---|---|---|
| ||
| Age, y, mean (SD)* | 69 (20) | 68 (20) |
| Female sex, n (%) | 3,018 (53.6) | 1,196 (54.0) |
| Most responsible diagnosis, n (%) | ||
| Pneumonia | 374 (6.6) | 135 (6.1) |
| Chronic obstructive pulmonary disease | 271 (4.8) | 88 (4.0) |
| Congestive heart failure | 217 (3.9) | 87 (3.9) |
| Admission location, n (%) | ||
| Home | 4,665 (82.9) | 1,943 (87.8) |
| Long‐term care* | 524 (9.3) | 158 (7.1) |
| Other* | 439 (7.8) | 112 (5.1) |
| Discharge location, n (%) | ||
| Home | 3,824 (67.9) | 1,578 (71.3) |
| Long‐term care | 779 (13.8) | 267 (12.1) |
| Other | 1,025 (18.3) | 368 (16.6) |
RESULTS
Our matched cohort included 7841 admissions (6141 patients), with 5628 admissions (4592 patients) exposed and 2213 admissions (1920 patients) not exposed to PNs. The discrepancy between the total number of patients and the sum of exposed and nonexposed patients is resultant from patients admitted more than once over the study period, as patients admitted to at least 1 team staffed with a PN and another team not staffed with a PN over the study period were counted in both groups. The 2 groups were similar with respect to several characteristics (Table 1). However, the 2 groups were significantly different for age (P = 0.046) and admissions from long‐term care (P < 0.01) and other facilities (P < 0.01).
Admissions with PNs were 1.3 days (21%) shorter than admission without PNs (6.2 vs 7.5 days, P < 0.001). Moreover, admissions with PNs had a smaller mean LOS/ELOS ratio compared to admissions without PNs (0.93 vs 1.05, P < 0.001). The restricted analysis found a 1.2‐day (18%) lower LOS (6.4 vs 7.6 days, P < 0.05) and a smaller mean LOS/ELOS ratio (0.91 vs 1.06, P < 0.001). Thirty‐day readmission rate was not different between the 2 groups (13.1 vs 13.8%, P = 0.48) or in the restricted analysis (12.0 vs 13.5%, P = 0.40) (Table 2).
| With PN | Without PN | P Value | |
|---|---|---|---|
| |||
| July 2010‐March 2014 | |||
| LOS, d (95% confidence interval) [n] | 6.2 (6.06.4) [5,628] | 7.5 (7.17.9) [2,213] | <0.001 |
| LOS/ELOS ratio (95% confidence interval) [n] | 0.93 (0.910.95) [5,628] | 1.05 (1.001.09) [2,213] | <0.001 |
| 30‐day readmission rate, % [n] | 13.1 [5,055] | 13.8 [2,012] | 0.48 |
| July 2010 to April 2011 | |||
| LOS, d (95% confidence interval) [n] | 6.4 (5.87.0) [713] | 7.6 (6.88.3) [753) | <0.05 |
| LOS/ELOS ratio (95% confidence interval) [n] | 0.91 (0.850.96) [713] | 1.06 (1.001.11) [753] | <0.001 |
| 30‐day readmission rate, % [n] | 12.0 [627] | 13.5 [681] | 0.40 |
In the interrupted time‐series analysis, prior to the implementation of the PN program, there was a positive relationship between LOS and time. After the implementation of the program, this relationship became inverse, meaning the curve plotting LOS against time had a negative slope. Furthermore, there was a statistically significant drop in LOS at the time of program implementation (P < 0.05). However, there was no difference in slope between the groups with and without PN after program implementation.
DISCUSSION
We describe an innovative inpatient intervention featuring an integrated patient‐care facilitator not responsible for clinical care charged with enhancing communication between and among patients and providers. Data from the almost 4‐year period demonstrated that implementation was associated with a 21% reduction in hospital LOS, with no difference in 30‐day readmission rates.
The patient navigator was first conceptualized in 1990 to help African American women in Harlem with breast cancer negotiate the complex world of oncology.[11] It was later implemented by the National Cancer Institute as an outpatient intervention spanning the continuum of cancer care. This concept has since expanded to other domains of complex single disease outpatient care, including asthma and fertility.[12, 13] To our knowledge, there has been limited evidence in the literature describing implementation of such programs in the inpatient general medical setting.
This study contributes to the growing literature on interventions targeting improvements in transitional care, such as transition coaches and discharge advocates.[14, 15] Balaban et al. recently described a PN intervention in the safety‐net population.[16] A common theme to these interventions was the prioritization of safe care transitions. However, this goal was achieved using related, yet different approaches: transition coaches focused on encouraging the patient and caregiver to assert a more active role,[14] discharge advocates focused on providing a comprehensive discharge plan for patients,[15] PNs from Balaban's study focused on coaching and assistance in navigating patients through the transition from hospital to home, and our study's PNs focused on enhancing communication between and among patients and providers. Additionally, unlike transition coaches and discharge advocates, who were nurses by training, and PNs from Balaban's study, who were community health workers, our PNs did not have any prespecified training or degree requirements.
Patients are at risk of being inadequately informed about important issues related to their care, such as hospital medications, diagnoses, and treatment plans during their hospital stay.[17, 18] Furthermore, we know that ineffective communication is a common cause of poor patient outcomes in hospital‐based care.[6] This phenomenon can be amplified from external pressures to maximize productivity. For example, Elliott and colleagues found that increasing hospitalist workload is associated with higher hospital LOS and cost.[19] PNs may offload care demands by enhancing communication for providers and patients.
Our study has several strengths. By matching admissions by case mix group, age category, and resource intensity weight, we aimed to reduce potential bias contributed by these covariates. Moreover, a staged rollout of the intervention, whereby over a 10‐month period, 2 of the multidisciplinary care teams were assigned PNs, while the remaining 2 were not, enabled contemporaneous comparison. Our study had few exclusion criteria, thus making it potentially generalizable to other inpatient general medicine settings of a similar nature. The relative simplicity of this intervention makes it amenable to scalability. Of note, the intervention has been deemed to show great promise at our institution, and has currently expanded to the cardiology, gastroenterology, and surgical oncology units.
Our study's limitations include a single‐center design. Moreover, although we demonstrate similarity in the majority of measurable covariates between the groups, we cannot exclude the existence of unmeasured confounders. Of the covariates that were found to be different between the groups, we suspect the difference in admissions from long‐term care and other facilities did not largely influence our study's main findings. Furthermore, though age was found to be statistically different between the groups, we postulate that the 1‐year difference between the groups is not particularly relevant clinically. Additionally, 30‐day readmission rates were only captured for our institution. However, the vast majority of readmissions in our region are to the index facility, and are unlikely to differ between the 2 groups.[20]
There may have been secular trends at play. In the interrupted time‐series analysis, there was a statistically significant drop in LOS at the time of program implementation. There was however, no difference in slope between the groups with and without PNs after program implementation. There are some plausible explanations for this lack of difference in slope. The study may not have been powered to detect such a difference, as this analysis was not prespecified. Furthermore, there may have been a spillover effect of the program, such that PNs may have improved efficiency for the teams to which they were assigned, thereby improving the efficiency of the other members of the multidisciplinary team, many of whom cared for patients assigned and not assigned a PN. Additionally, we measured the LOS in a preintervention control group between July 2009 and June 2010 using the same inclusion criteria as the matched cohort. It was found to be 8.5 days, which suggests a secular trend toward improvement in LOS over time at our institution. We are, however, reassured that our restricted analysis enabling contemporaneous comparison between patients exposed and not exposed to PNs was still found to be significant.
The implementation of this intervention could have implications for policymakers‐at‐large. Establishment of criteria for qualifications and a clear educational curriculum to train future PNs is needed, especially in the context of ongoing program expansion. These initiatives are currently underway at our institution. Furthermore, evaluation of the program's operating cost and calculation of its return on investment should include balanced metrics incorporating patient‐, provider‐, organizational‐, and system‐level measures. The current cost to the hospital per PN is approximately $73,800 CAD ($58,700 USD), which covers 1 PN's annual salary and benefits. Thus, the implementation of 4 PNs for each of the 4 multidisciplinary teams costs the hospital approximately $295,000 CAD ($234,700 USD) per year. Although the details of our preliminary calculations are outside the scope of this report, it suggests that the savings incurred from shorter LOS outweigh program costs.
We found that implementation of this innovative inpatient intervention targeting improvements in communication was associated with a reduction in LOS without an increase in 30‐day readmission. Our experience shows promise and may inform others considering similar interventions. Patient and provider experience and generalizability should be evaluated in future work.
Acknowledgements
The authors thank Dr. Allan Detsky and David Wells for their review of the manuscript. They are also grateful to Chin‐Chin Chua, Ningmei Wang, and Joann Bon in the Office of Quality and Performance Measurement for their help with data collection, and John Matelski for his help with data analysis.
Disclosure: This program was funded by matched donations from physicians in the Mount Sinai Hospital Department of Medicine and donations to Mount Sinai Hospital from community members directed to support the implementation and evaluation of novel care delivery systems. The authors report no conflicts of interest. Preliminary abstracts of this study were presented in the online forum, Leading Health Care Innovation, November 12, 2013 (
Inpatient medicine is becoming increasingly complex. A growing number of patients with multiple chronic conditions coupled with mounting care fragmentation leave patients vulnerable to adverse events and readmission to the hospital.[1, 2, 3] Moreover, efforts to minimize hospital length of stay (LOS) have resulted in patients being discharged quicker and sicker than ever before.[4]
A cornerstone of safe and high‐quality healthcare is effective communication.[5] Ineffective communication between and among healthcare providers and patients is a leading cause of medical errors and patient harm. An analysis of sentinel events reported to The Joint Commission revealed that communication failure was the root cause in 59% of these events.[6]
The current climate of increasing healthcare complexity has prompted the need for adaptive innovation.[7] However, there are limited data describing interventions targeting improvements in both communication and transitional care planning. We created a new position, the patient navigator (PN), a dedicated patient‐care facilitator not responsible for clinical care. PNs were integrated into the inpatient multidisciplinary clinical team to facilitate patient and provider navigation through the complexity of a hospital admission by enhancing communication between and among patients and providers. The objective of this study was to determine whether this intervention would reduce hospital LOS and 30‐day unplanned readmissions.
METHODS
Setting
Mount Sinai Hospital is a 446‐bed acute care urban academic health center in Toronto, Ontario, Canada. The general internal medicine service operates as a 90‐bed clinical teaching unit physically distributed over 4 inpatient wards. The service is structurally divided into 4 nongeographically based multidisciplinary care teams (teams A, B, C, and D) comprised of the medical team (attending physician, senior resident physician, 23 junior resident physicians, and 23 medical students), pharmacist, social worker, physiotherapist, occupational therapist, speech and language pathologist, dietician, respiratory therapist, and nursing staff allocated by ward. Each team is on call approximately 1 night in 4 with no night float system. At our institution, attending physicians rotate on a 2‐ or 4‐week schedule, resident physicians rotate on a 1‐ or 2‐month schedule, and medical students rotate on a 2‐month schedule. Preintervention, communication occurred in person and by telephone between members of the medical team. Other members of the multidisciplinary care team communicated with the medical team in person at daily multidisciplinary rounds focused on discharge planning, by pager, or using a Web‐based communication tool.
Intervention
PNs were dedicated patient‐care facilitators not responsible for clinical care. They acted as liaisons between and among providers and patients. Each PN was a fully integrated member of their multidisciplinary care team. With ongoing medical team rotations, the PN was notably the only consistent member on the clinical team. Each patient saw the same PN throughout his or her hospital stay, as both the patient and the PN were team based. The average number of patients for whom each PN was responsible daily was dictated by the patient census for their team. On average, each team had a census between 20 and 30 patients daily. PNs worked during the daytime from Monday to Friday, and did not have any overnight or weekend responsibilities.
A PN's typical day began by reviewing and rounding on overnight admissions as a formal member of the clinical team. This was followed by participating in daily multidisciplinary rounds, then documenting and circulating the resultant action items. Thereafter, they expedited consultations and tests by liaising with departmental staff, and proactively established contact with the patient and their family. They answered simple factual questions related to test scheduling, consultations, diagnosis, medications, and treatments as discussed and outlined by the clinical team, and promptly relayed care questions beyond the scope of their knowledge to the clinical team. They were available to patients, family members, and providers via a dedicated mobile number using phone calls and text messages. If indicated, they assisted in discharge coordination by arranging follow‐up appointments and placing postdischarge phone calls. In addition, they served as primary contact for every patient admitted to their clinical team following discharge to ensure appropriate follow through on discharge plans. There were no set criteria for PNs to disengage from a patient's care. They could always be reached using their dedicated mobile number during business hours, with a voicemail system in place for after‐hours calls.
The role was filled by individuals skilled in communication and/or healthcare, such as registered nurses, a masters degreetrained educator, internationally trained physicians, and professionals from the hospitality and human resources industries. There were no prespecified training or degree requirements. Each PN underwent on‐the‐job training and participated in twice monthly PN meetings for ongoing feedback and education.
Program Implementation
We implemented the PN program on the inpatient general internal medicine service in June 2010 on 2 of 4 multidisciplinary clinical teams. Because a PN became an integrated member of 1 of 4 clinical teams, patient assignment to a PN was determined by the team to which the patient was admitted. On average, each of the 4 teams admitted equally on a daily basis. Initially, there were only sufficient resources to fund 2 PNs. Thus, from June 2010 to May 2011, only teams A and C were assigned PNs. To create fairness between the 4 teams, these 2 PNs moved to teams B and D from June 2011 to November 2011, and then back to teams A and C from December 2011 to April 2012. Following this initial pilot period, the program was allocated further resources, and so expanded to all 4 teams in May 2012. PN salaries were the only program costs. These costs were funded by matching donations from physicians within the Mount Sinai Hospital Department of Medicine and donations to the hospital from community members directed to support the implementation and evaluation of novel care delivery systems.
Study Design
We evaluated the PN program using a retrospective cohort study that included all general medical admissions between July 2010 and March 2014 matched by case mix group, age category, and resource intensity weight (a relative value measuring total patient resource use compared with average typical acute inpatients).[8]
Our primary outcomes were LOS and 30‐day readmission rate. These outcomes were stratified by exposure status to a PN. There were no exclusion criteria for the LOS analysis. Patients who died, were transferred to or from an acute care facility, or signed out against medical advice were excluded from the 30‐day readmission analysis. A secondary analysis restricted the timeframe from July 2010 to April 2011, when only 2 of 4 teams were exposed to PNs.
Average LOS has been observed to be higher in Canadian hospitals as compared to their US counterparts across different admission diagnoses, such as coronary artery bypass graft surgery and heart failure.[9, 10] We hypothesize that these differences are party due to systems‐level differences, including posthospital care. Specifically, the Canadian system does not utilize posthospital acute care, such as skilled nursing facilities, which may in part account for these differences. To help contextualize our data, we standardized LOS using an LOS index called the LOS/expected LOS (ELOS) ratio. It takes the LOS and divides it by the ELOS, a validated estimate of the expected LOS for a given patient generated using a national administrative database for acute hospital care in Canada that takes into account case mix group, age, comorbidity level, and intervention factors.[8]
Additionally, We performed an interrupted time‐series analysis, whereby a log‐linear model was fit on LOS and adjusted for weekly and monthly trends, age category, resource intensity weight, major clinical category (a surrogate for case mix group), admission location, and discharge location. The cohort was divided into 3 groups: before program implementation (July 2009June 2010), after program implementation with PN (July 2010March 2014), and after program implementation without PN (July 2010March 2014).
This study was approved by the research ethics board at Mount Sinai Hospital. No patient consent was deemed necessary. Data were obtained from institutional databases monitored by the hospital's performance measurement office.
Statistical Analysis
In Tables 1, 2, mean values were compared using a 2‐tailed t test, and the relationship between categorical groups was determined using a 2 test. For the interrupted time‐series analysis, 2‐tailed t tests were used to test null hypotheses of no association between the parameter value and the outcome, and 2 tests were used to test for the equivalence of 2 given parameters. P0.05 indicated statistical significance for all comparisons and analyses. All data were analyzed using Stata version 13 (StataCorp, College Station, TX) or R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
| With PN, n = 5,628 | Without PN, n = 2,213 | |
|---|---|---|
| ||
| Age, y, mean (SD)* | 69 (20) | 68 (20) |
| Female sex, n (%) | 3,018 (53.6) | 1,196 (54.0) |
| Most responsible diagnosis, n (%) | ||
| Pneumonia | 374 (6.6) | 135 (6.1) |
| Chronic obstructive pulmonary disease | 271 (4.8) | 88 (4.0) |
| Congestive heart failure | 217 (3.9) | 87 (3.9) |
| Admission location, n (%) | ||
| Home | 4,665 (82.9) | 1,943 (87.8) |
| Long‐term care* | 524 (9.3) | 158 (7.1) |
| Other* | 439 (7.8) | 112 (5.1) |
| Discharge location, n (%) | ||
| Home | 3,824 (67.9) | 1,578 (71.3) |
| Long‐term care | 779 (13.8) | 267 (12.1) |
| Other | 1,025 (18.3) | 368 (16.6) |
RESULTS
Our matched cohort included 7841 admissions (6141 patients), with 5628 admissions (4592 patients) exposed and 2213 admissions (1920 patients) not exposed to PNs. The discrepancy between the total number of patients and the sum of exposed and nonexposed patients is resultant from patients admitted more than once over the study period, as patients admitted to at least 1 team staffed with a PN and another team not staffed with a PN over the study period were counted in both groups. The 2 groups were similar with respect to several characteristics (Table 1). However, the 2 groups were significantly different for age (P = 0.046) and admissions from long‐term care (P < 0.01) and other facilities (P < 0.01).
Admissions with PNs were 1.3 days (21%) shorter than admission without PNs (6.2 vs 7.5 days, P < 0.001). Moreover, admissions with PNs had a smaller mean LOS/ELOS ratio compared to admissions without PNs (0.93 vs 1.05, P < 0.001). The restricted analysis found a 1.2‐day (18%) lower LOS (6.4 vs 7.6 days, P < 0.05) and a smaller mean LOS/ELOS ratio (0.91 vs 1.06, P < 0.001). Thirty‐day readmission rate was not different between the 2 groups (13.1 vs 13.8%, P = 0.48) or in the restricted analysis (12.0 vs 13.5%, P = 0.40) (Table 2).
| With PN | Without PN | P Value | |
|---|---|---|---|
| |||
| July 2010‐March 2014 | |||
| LOS, d (95% confidence interval) [n] | 6.2 (6.06.4) [5,628] | 7.5 (7.17.9) [2,213] | <0.001 |
| LOS/ELOS ratio (95% confidence interval) [n] | 0.93 (0.910.95) [5,628] | 1.05 (1.001.09) [2,213] | <0.001 |
| 30‐day readmission rate, % [n] | 13.1 [5,055] | 13.8 [2,012] | 0.48 |
| July 2010 to April 2011 | |||
| LOS, d (95% confidence interval) [n] | 6.4 (5.87.0) [713] | 7.6 (6.88.3) [753) | <0.05 |
| LOS/ELOS ratio (95% confidence interval) [n] | 0.91 (0.850.96) [713] | 1.06 (1.001.11) [753] | <0.001 |
| 30‐day readmission rate, % [n] | 12.0 [627] | 13.5 [681] | 0.40 |
In the interrupted time‐series analysis, prior to the implementation of the PN program, there was a positive relationship between LOS and time. After the implementation of the program, this relationship became inverse, meaning the curve plotting LOS against time had a negative slope. Furthermore, there was a statistically significant drop in LOS at the time of program implementation (P < 0.05). However, there was no difference in slope between the groups with and without PN after program implementation.
DISCUSSION
We describe an innovative inpatient intervention featuring an integrated patient‐care facilitator not responsible for clinical care charged with enhancing communication between and among patients and providers. Data from the almost 4‐year period demonstrated that implementation was associated with a 21% reduction in hospital LOS, with no difference in 30‐day readmission rates.
The patient navigator was first conceptualized in 1990 to help African American women in Harlem with breast cancer negotiate the complex world of oncology.[11] It was later implemented by the National Cancer Institute as an outpatient intervention spanning the continuum of cancer care. This concept has since expanded to other domains of complex single disease outpatient care, including asthma and fertility.[12, 13] To our knowledge, there has been limited evidence in the literature describing implementation of such programs in the inpatient general medical setting.
This study contributes to the growing literature on interventions targeting improvements in transitional care, such as transition coaches and discharge advocates.[14, 15] Balaban et al. recently described a PN intervention in the safety‐net population.[16] A common theme to these interventions was the prioritization of safe care transitions. However, this goal was achieved using related, yet different approaches: transition coaches focused on encouraging the patient and caregiver to assert a more active role,[14] discharge advocates focused on providing a comprehensive discharge plan for patients,[15] PNs from Balaban's study focused on coaching and assistance in navigating patients through the transition from hospital to home, and our study's PNs focused on enhancing communication between and among patients and providers. Additionally, unlike transition coaches and discharge advocates, who were nurses by training, and PNs from Balaban's study, who were community health workers, our PNs did not have any prespecified training or degree requirements.
Patients are at risk of being inadequately informed about important issues related to their care, such as hospital medications, diagnoses, and treatment plans during their hospital stay.[17, 18] Furthermore, we know that ineffective communication is a common cause of poor patient outcomes in hospital‐based care.[6] This phenomenon can be amplified from external pressures to maximize productivity. For example, Elliott and colleagues found that increasing hospitalist workload is associated with higher hospital LOS and cost.[19] PNs may offload care demands by enhancing communication for providers and patients.
Our study has several strengths. By matching admissions by case mix group, age category, and resource intensity weight, we aimed to reduce potential bias contributed by these covariates. Moreover, a staged rollout of the intervention, whereby over a 10‐month period, 2 of the multidisciplinary care teams were assigned PNs, while the remaining 2 were not, enabled contemporaneous comparison. Our study had few exclusion criteria, thus making it potentially generalizable to other inpatient general medicine settings of a similar nature. The relative simplicity of this intervention makes it amenable to scalability. Of note, the intervention has been deemed to show great promise at our institution, and has currently expanded to the cardiology, gastroenterology, and surgical oncology units.
Our study's limitations include a single‐center design. Moreover, although we demonstrate similarity in the majority of measurable covariates between the groups, we cannot exclude the existence of unmeasured confounders. Of the covariates that were found to be different between the groups, we suspect the difference in admissions from long‐term care and other facilities did not largely influence our study's main findings. Furthermore, though age was found to be statistically different between the groups, we postulate that the 1‐year difference between the groups is not particularly relevant clinically. Additionally, 30‐day readmission rates were only captured for our institution. However, the vast majority of readmissions in our region are to the index facility, and are unlikely to differ between the 2 groups.[20]
There may have been secular trends at play. In the interrupted time‐series analysis, there was a statistically significant drop in LOS at the time of program implementation. There was however, no difference in slope between the groups with and without PNs after program implementation. There are some plausible explanations for this lack of difference in slope. The study may not have been powered to detect such a difference, as this analysis was not prespecified. Furthermore, there may have been a spillover effect of the program, such that PNs may have improved efficiency for the teams to which they were assigned, thereby improving the efficiency of the other members of the multidisciplinary team, many of whom cared for patients assigned and not assigned a PN. Additionally, we measured the LOS in a preintervention control group between July 2009 and June 2010 using the same inclusion criteria as the matched cohort. It was found to be 8.5 days, which suggests a secular trend toward improvement in LOS over time at our institution. We are, however, reassured that our restricted analysis enabling contemporaneous comparison between patients exposed and not exposed to PNs was still found to be significant.
The implementation of this intervention could have implications for policymakers‐at‐large. Establishment of criteria for qualifications and a clear educational curriculum to train future PNs is needed, especially in the context of ongoing program expansion. These initiatives are currently underway at our institution. Furthermore, evaluation of the program's operating cost and calculation of its return on investment should include balanced metrics incorporating patient‐, provider‐, organizational‐, and system‐level measures. The current cost to the hospital per PN is approximately $73,800 CAD ($58,700 USD), which covers 1 PN's annual salary and benefits. Thus, the implementation of 4 PNs for each of the 4 multidisciplinary teams costs the hospital approximately $295,000 CAD ($234,700 USD) per year. Although the details of our preliminary calculations are outside the scope of this report, it suggests that the savings incurred from shorter LOS outweigh program costs.
We found that implementation of this innovative inpatient intervention targeting improvements in communication was associated with a reduction in LOS without an increase in 30‐day readmission. Our experience shows promise and may inform others considering similar interventions. Patient and provider experience and generalizability should be evaluated in future work.
Acknowledgements
The authors thank Dr. Allan Detsky and David Wells for their review of the manuscript. They are also grateful to Chin‐Chin Chua, Ningmei Wang, and Joann Bon in the Office of Quality and Performance Measurement for their help with data collection, and John Matelski for his help with data analysis.
Disclosure: This program was funded by matched donations from physicians in the Mount Sinai Hospital Department of Medicine and donations to Mount Sinai Hospital from community members directed to support the implementation and evaluation of novel care delivery systems. The authors report no conflicts of interest. Preliminary abstracts of this study were presented in the online forum, Leading Health Care Innovation, November 12, 2013 (
- , , , et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(S3):391–395.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Prospective payment system and impairment at discharge. The “quicker‐and‐sicker” story revisited. JAMA. 1990;264(15):1980–1983.
- . The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13(suppl 1):i85–i90.
- The Joint Commission. Sentinel event data root: causes by event type (2004–June 2014). Available at: http://www.jointcommission.org/assets/1/18/Root_Causes_by_Event_Type_2004‐2014.pdf. Accessed March 12, 2014.
- , . Complexity science: the challenge of complexity in health care. BMJ. 2001;323(7313):625–628.
- Canadian Institute for Health Information. Case mix. Available at: http://www.cihi.ca/CIHI‐ext‐portal/internet/EN/TabbedContent/standards+and+data+submission/standards/case+mix/cihi010690. Accessed April 12, 2015.
- , , , , , . Outcomes and cost of coronary artery bypass graft surgery in the United States and Canada. Arch Intern Med. 2005;165(13):1506–1513.
- , , , et al. Differences in treatment, outcomes, and quality of life among patients with heart failure in Canada and the United States. JACC Heart Fail. 2013;1(6):523–530.
- , , . Expanding access to cancer screening and clinical follow‐up among the medically underserved. Cancer Pract. 1995;3(1):19–30.
- , , , et al. Clearing clinical barriers: enhancing social support using a patient navigator for asthma care. J Asthma. 2010;47(8):913–919.
- . The role of a patient navigator in fertility preservation. In: Cancer Treatment and Research. Vol 156. Boston, MA: Springer US; 2010:469–470.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , , , . A patient navigator intervention to reduce hospital readmissions among high‐risk safety‐net patients: a randomized controlled trial. J Gen Intern Med. 2015;30(7):907–915.
- , , . Lack of patient knowledge regarding hospital medications. J Hosp Med. 2010;5(2):83–86.
- , . Patients' understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80(8):991–994.
- , , , , . Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–e111.
- , , , et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22(S3):391–395.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- , , , et al. Prospective payment system and impairment at discharge. The “quicker‐and‐sicker” story revisited. JAMA. 1990;264(15):1980–1983.
- . The human factor: the critical importance of effective teamwork and communication in providing safe care. Qual Saf Health Care. 2004;13(suppl 1):i85–i90.
- The Joint Commission. Sentinel event data root: causes by event type (2004–June 2014). Available at: http://www.jointcommission.org/assets/1/18/Root_Causes_by_Event_Type_2004‐2014.pdf. Accessed March 12, 2014.
- , . Complexity science: the challenge of complexity in health care. BMJ. 2001;323(7313):625–628.
- Canadian Institute for Health Information. Case mix. Available at: http://www.cihi.ca/CIHI‐ext‐portal/internet/EN/TabbedContent/standards+and+data+submission/standards/case+mix/cihi010690. Accessed April 12, 2015.
- , , , , , . Outcomes and cost of coronary artery bypass graft surgery in the United States and Canada. Arch Intern Med. 2005;165(13):1506–1513.
- , , , et al. Differences in treatment, outcomes, and quality of life among patients with heart failure in Canada and the United States. JACC Heart Fail. 2013;1(6):523–530.
- , , . Expanding access to cancer screening and clinical follow‐up among the medically underserved. Cancer Pract. 1995;3(1):19–30.
- , , , et al. Clearing clinical barriers: enhancing social support using a patient navigator for asthma care. J Asthma. 2010;47(8):913–919.
- . The role of a patient navigator in fertility preservation. In: Cancer Treatment and Research. Vol 156. Boston, MA: Springer US; 2010:469–470.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , , , . A patient navigator intervention to reduce hospital readmissions among high‐risk safety‐net patients: a randomized controlled trial. J Gen Intern Med. 2015;30(7):907–915.
- , , . Lack of patient knowledge regarding hospital medications. J Hosp Med. 2010;5(2):83–86.
- , . Patients' understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc. 2005;80(8):991–994.
- , , , , . Effect of Hospitalist Workload on the Quality and Efficiency of Care. JAMA Intern Med. 2014;174(5):786.
- , , , et al. Unplanned readmissions after hospital discharge among patients identified as being at high risk for readmission using a validated predictive algorithm. Open Med. 2011;5(2):e104–e111.
© 2015 Society of Hospital Medicine
Blood Cultures Contribute Little to Uncomplicated SSTI Treatment
SAN ANTONIO – Despite mounting evidence that blood cultures don’t contribute to the care of immunocompetent children admitted to the hospital with uncomplicated skin and soft tissue infections (SSTIs), they continue to be performed routinely in some hospitals, according to a study presented at the Pediatric Hospital Medicine 2015 meeting.
Current practice guidelines recommend against routine use of blood cultures in uncomplicated SSTIs (Clin Infect Dis. 2014;59(2):e10-52).
A 2013 study of children admitted for uncomplicated SSTIs (n = 482) found no positive blood cultures in the cohort, and cultures were also associated with a significantly longer length of stay. More than half of those children, however, had received antibiotics before their blood cultures, leaving open the possibility that some negative results were the result of treatment with antibiotics (Pediatrics. 2013;132(3):454-9).
Dr. Claudette Gonzalez and her colleagues at Nicklaus Children’s Hospital, Miami, presented findings from a study that used a cohort of otherwise healthy infants and children (n = 176) admitted from the emergency department with uncomplicated SSTIs.
Dr. Gonzalez and her associates sought to strengthen the evidence against routine use of cultures by excluding children who had received antibiotics within 2 weeks of presenting to the hospital.Dr. Gonzalez noted that, despite guidelines, blood cultures remained a routine part of the workup at her hospital, with 66% of the study sample receiving cultures (n = 116). Of febrile patients, 80% received cultures; of nonfebrile patients, 59% received cultures. Patients who had a blood culture drawn were significantly more likely to have had fever (P < .01). They also were more likely to have higher white blood cell and C-reactive protein (CRP) counts (P > .05 for both), but despite this, the rate of positive blood cultures was still less than 1%.
Of the 116 blood cultures drawn, 5 grew contaminants and only 1 was a true positive, for methicillin-susceptible Staphylococcus aureus (MSSA).
The study, unlike most previous studies, enrolled patients younger than 1 year of age (n = 28), but Dr. Gonzalez said that “we don’t have a big enough sample to really make conclusions about that age group.” Also in contrast to some previous studies, Dr. Gonzalez and her associates did not find a statistically significant difference in length of stay between the patients who had received cultures and those that did not (mean 3.62 vs. 3.4 days, P > .05).The one patient in the study with true bacteremia was a 1.4-year-old child presenting with no fever, cellulitis of hands and feet, no lymphangitis, and a white blood count of 8.5 × 103/L and a CRP of less than 0.5 mg/dL. “The WBC count was within normal range and the CRP was not elevated, so you wouldn’t have necessarily picked this kid out to say he needs a blood culture,” Dr. Gonzalez said at the meeting, which was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, the AAP Section on Hospital Medicine, and the Academic Pediatric Association.
Still, she said, the study strengthens the evidence base against use of blood cultures in this population. “For children 1 year and older I think it’s very clear,” she said. The investigators are now proceeding with an implementation study to determine whether guidance against routine blood cultures should be put into practice at their institution.
Although the initial study revealed that 66% of children with uncomplicated SSTIs were receiving cultures, preliminary unpublished results show “it’s now at about 44%,” she said, following education of residents, fellows, and ED clinicians.
In addition to communicating with the ED to reduce use of blood cultures in this population, Dr. Gonzalez said, “we’re getting guidelines plugged into our order set in the [electronic medical record], so that’s a second reminder not to draw blood cultures. And we’re measuring to see if our rates improve further.”
Dr. Gonzalez received no outside funding for her study and disclosed no conflicts of interest.
SAN ANTONIO – Despite mounting evidence that blood cultures don’t contribute to the care of immunocompetent children admitted to the hospital with uncomplicated skin and soft tissue infections (SSTIs), they continue to be performed routinely in some hospitals, according to a study presented at the Pediatric Hospital Medicine 2015 meeting.
Current practice guidelines recommend against routine use of blood cultures in uncomplicated SSTIs (Clin Infect Dis. 2014;59(2):e10-52).
A 2013 study of children admitted for uncomplicated SSTIs (n = 482) found no positive blood cultures in the cohort, and cultures were also associated with a significantly longer length of stay. More than half of those children, however, had received antibiotics before their blood cultures, leaving open the possibility that some negative results were the result of treatment with antibiotics (Pediatrics. 2013;132(3):454-9).
Dr. Claudette Gonzalez and her colleagues at Nicklaus Children’s Hospital, Miami, presented findings from a study that used a cohort of otherwise healthy infants and children (n = 176) admitted from the emergency department with uncomplicated SSTIs.
Dr. Gonzalez and her associates sought to strengthen the evidence against routine use of cultures by excluding children who had received antibiotics within 2 weeks of presenting to the hospital.Dr. Gonzalez noted that, despite guidelines, blood cultures remained a routine part of the workup at her hospital, with 66% of the study sample receiving cultures (n = 116). Of febrile patients, 80% received cultures; of nonfebrile patients, 59% received cultures. Patients who had a blood culture drawn were significantly more likely to have had fever (P < .01). They also were more likely to have higher white blood cell and C-reactive protein (CRP) counts (P > .05 for both), but despite this, the rate of positive blood cultures was still less than 1%.
Of the 116 blood cultures drawn, 5 grew contaminants and only 1 was a true positive, for methicillin-susceptible Staphylococcus aureus (MSSA).
The study, unlike most previous studies, enrolled patients younger than 1 year of age (n = 28), but Dr. Gonzalez said that “we don’t have a big enough sample to really make conclusions about that age group.” Also in contrast to some previous studies, Dr. Gonzalez and her associates did not find a statistically significant difference in length of stay between the patients who had received cultures and those that did not (mean 3.62 vs. 3.4 days, P > .05).The one patient in the study with true bacteremia was a 1.4-year-old child presenting with no fever, cellulitis of hands and feet, no lymphangitis, and a white blood count of 8.5 × 103/L and a CRP of less than 0.5 mg/dL. “The WBC count was within normal range and the CRP was not elevated, so you wouldn’t have necessarily picked this kid out to say he needs a blood culture,” Dr. Gonzalez said at the meeting, which was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, the AAP Section on Hospital Medicine, and the Academic Pediatric Association.
Still, she said, the study strengthens the evidence base against use of blood cultures in this population. “For children 1 year and older I think it’s very clear,” she said. The investigators are now proceeding with an implementation study to determine whether guidance against routine blood cultures should be put into practice at their institution.
Although the initial study revealed that 66% of children with uncomplicated SSTIs were receiving cultures, preliminary unpublished results show “it’s now at about 44%,” she said, following education of residents, fellows, and ED clinicians.
In addition to communicating with the ED to reduce use of blood cultures in this population, Dr. Gonzalez said, “we’re getting guidelines plugged into our order set in the [electronic medical record], so that’s a second reminder not to draw blood cultures. And we’re measuring to see if our rates improve further.”
Dr. Gonzalez received no outside funding for her study and disclosed no conflicts of interest.
SAN ANTONIO – Despite mounting evidence that blood cultures don’t contribute to the care of immunocompetent children admitted to the hospital with uncomplicated skin and soft tissue infections (SSTIs), they continue to be performed routinely in some hospitals, according to a study presented at the Pediatric Hospital Medicine 2015 meeting.
Current practice guidelines recommend against routine use of blood cultures in uncomplicated SSTIs (Clin Infect Dis. 2014;59(2):e10-52).
A 2013 study of children admitted for uncomplicated SSTIs (n = 482) found no positive blood cultures in the cohort, and cultures were also associated with a significantly longer length of stay. More than half of those children, however, had received antibiotics before their blood cultures, leaving open the possibility that some negative results were the result of treatment with antibiotics (Pediatrics. 2013;132(3):454-9).
Dr. Claudette Gonzalez and her colleagues at Nicklaus Children’s Hospital, Miami, presented findings from a study that used a cohort of otherwise healthy infants and children (n = 176) admitted from the emergency department with uncomplicated SSTIs.
Dr. Gonzalez and her associates sought to strengthen the evidence against routine use of cultures by excluding children who had received antibiotics within 2 weeks of presenting to the hospital.Dr. Gonzalez noted that, despite guidelines, blood cultures remained a routine part of the workup at her hospital, with 66% of the study sample receiving cultures (n = 116). Of febrile patients, 80% received cultures; of nonfebrile patients, 59% received cultures. Patients who had a blood culture drawn were significantly more likely to have had fever (P < .01). They also were more likely to have higher white blood cell and C-reactive protein (CRP) counts (P > .05 for both), but despite this, the rate of positive blood cultures was still less than 1%.
Of the 116 blood cultures drawn, 5 grew contaminants and only 1 was a true positive, for methicillin-susceptible Staphylococcus aureus (MSSA).
The study, unlike most previous studies, enrolled patients younger than 1 year of age (n = 28), but Dr. Gonzalez said that “we don’t have a big enough sample to really make conclusions about that age group.” Also in contrast to some previous studies, Dr. Gonzalez and her associates did not find a statistically significant difference in length of stay between the patients who had received cultures and those that did not (mean 3.62 vs. 3.4 days, P > .05).The one patient in the study with true bacteremia was a 1.4-year-old child presenting with no fever, cellulitis of hands and feet, no lymphangitis, and a white blood count of 8.5 × 103/L and a CRP of less than 0.5 mg/dL. “The WBC count was within normal range and the CRP was not elevated, so you wouldn’t have necessarily picked this kid out to say he needs a blood culture,” Dr. Gonzalez said at the meeting, which was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, the AAP Section on Hospital Medicine, and the Academic Pediatric Association.
Still, she said, the study strengthens the evidence base against use of blood cultures in this population. “For children 1 year and older I think it’s very clear,” she said. The investigators are now proceeding with an implementation study to determine whether guidance against routine blood cultures should be put into practice at their institution.
Although the initial study revealed that 66% of children with uncomplicated SSTIs were receiving cultures, preliminary unpublished results show “it’s now at about 44%,” she said, following education of residents, fellows, and ED clinicians.
In addition to communicating with the ED to reduce use of blood cultures in this population, Dr. Gonzalez said, “we’re getting guidelines plugged into our order set in the [electronic medical record], so that’s a second reminder not to draw blood cultures. And we’re measuring to see if our rates improve further.”
Dr. Gonzalez received no outside funding for her study and disclosed no conflicts of interest.
AT PEDIATRIC HOSPITAL MEDICINE 2015
Mutations linked to Fanconi anemia, repair pathway

Photo courtesy of NIGMS
Investigators say they have identified mutations that cause Fanconi anemia and, in the process, gained new insight into interstrand crosslink (ICL) repair.
The researchers studied two patients who had Fanconi anemia with no known genetic cause.
Genomic sequencing revealed that one patient had a mutation in RAD51, and the other had mutations in UBE2T.
These genes—and others linked to Fanconi anemia in previous studies—contribute to ICL repair, which fixes a misplaced attachment between two strands of DNA.
Caused by chemical agents, ICLs block the replication of DNA, making it impossible for cells to accurately copy their genomes as they divide. The ICL repair process uses multiple enzymes that cut away the connection between the DNA strands, freeing them up and allowing the cells to grow.
The genome is at constant risk of forming ICLs, and defects in the ICL repair pathway can produce a constellation of symptoms associated with Fanconi anemia—a predisposition to cancer, bone marrow failure, infertility, and developmental defects.
Via two different studies, Agata Smogorzewska, MD, PhD, of The Rockefeller University in New York, New York, and her colleagues unearthed new discoveries relating to the disease and the pathway.
“Our work began, as it often does, with samples and histories from patients,” Dr Smogorzewska said. “In these cases, we had two patients who each represented a sort of mystery. They had symptoms of Fanconi anemia but no genetic cause yet identified.”
With the RAD51 research, which was published in Molecular Cell, the investigators set out to determine the cause of the Fanconi anemia-like symptoms in a girl in the university’s International Fanconi Anemia Registry.
When they sequenced the protein-coding genes in her genome, the researchers found a mutation in one of two copies of the RAD51 gene.
The RAD1 protein was already known to be important for another DNA repair process—homologous recombination, in which a missing section of DNA is replaced using its sister strand as a template. Homologous recombination is thought to be used during the last step of ICL repair, after the crosslink has been cut.
Because only one copy of the RAD51 gene was partially defective, the patient’s cells could still perform homologous recombination but not ICL repair.
To show that the defective copy of the RAD51 gene was indeed responsible for the patient’s symptoms, the investigators genetically engineered the girl’s own cells to remove the defect, which restored their ability to fix ICLs.
Further experiments on the patient’s cells led the researchers to suspect that RAD51 plays a role outside of homologous recombination, by tamping down the activity of two enzymes that degrade the DNA at the ICL. When RAD51 is defective, these enzymes—DNA2 and WRN—become overly destructive.
With the UBE2T study, published in Cell Reports, the investigators analyzed genomic data from another patient in the International Fanconi Anemia Registry.
They found compound heterozygous mutations in UBE2T—a large genomic deletion in the paternally derived allele and a large duplication in the maternally derived allele.
While it was already known that UBE2T is involved in activating ICL repair, the researchers said the discovery that these mutations could produce Fanconi anemia revealed that UBE2T is an irreplaceable player in the pathway.
“Although we have discovered new causes for this devastating but very rare genetic disease, the implications of this work go much further,” Dr Smogorzewska said.
“By identifying new disruptions to this repair pathway, we can better understand the mechanisms of an event that is crucial to every cell division—a process that occurs constantly within the human body throughout a lifetime.” ![]()

Photo courtesy of NIGMS
Investigators say they have identified mutations that cause Fanconi anemia and, in the process, gained new insight into interstrand crosslink (ICL) repair.
The researchers studied two patients who had Fanconi anemia with no known genetic cause.
Genomic sequencing revealed that one patient had a mutation in RAD51, and the other had mutations in UBE2T.
These genes—and others linked to Fanconi anemia in previous studies—contribute to ICL repair, which fixes a misplaced attachment between two strands of DNA.
Caused by chemical agents, ICLs block the replication of DNA, making it impossible for cells to accurately copy their genomes as they divide. The ICL repair process uses multiple enzymes that cut away the connection between the DNA strands, freeing them up and allowing the cells to grow.
The genome is at constant risk of forming ICLs, and defects in the ICL repair pathway can produce a constellation of symptoms associated with Fanconi anemia—a predisposition to cancer, bone marrow failure, infertility, and developmental defects.
Via two different studies, Agata Smogorzewska, MD, PhD, of The Rockefeller University in New York, New York, and her colleagues unearthed new discoveries relating to the disease and the pathway.
“Our work began, as it often does, with samples and histories from patients,” Dr Smogorzewska said. “In these cases, we had two patients who each represented a sort of mystery. They had symptoms of Fanconi anemia but no genetic cause yet identified.”
With the RAD51 research, which was published in Molecular Cell, the investigators set out to determine the cause of the Fanconi anemia-like symptoms in a girl in the university’s International Fanconi Anemia Registry.
When they sequenced the protein-coding genes in her genome, the researchers found a mutation in one of two copies of the RAD51 gene.
The RAD1 protein was already known to be important for another DNA repair process—homologous recombination, in which a missing section of DNA is replaced using its sister strand as a template. Homologous recombination is thought to be used during the last step of ICL repair, after the crosslink has been cut.
Because only one copy of the RAD51 gene was partially defective, the patient’s cells could still perform homologous recombination but not ICL repair.
To show that the defective copy of the RAD51 gene was indeed responsible for the patient’s symptoms, the investigators genetically engineered the girl’s own cells to remove the defect, which restored their ability to fix ICLs.
Further experiments on the patient’s cells led the researchers to suspect that RAD51 plays a role outside of homologous recombination, by tamping down the activity of two enzymes that degrade the DNA at the ICL. When RAD51 is defective, these enzymes—DNA2 and WRN—become overly destructive.
With the UBE2T study, published in Cell Reports, the investigators analyzed genomic data from another patient in the International Fanconi Anemia Registry.
They found compound heterozygous mutations in UBE2T—a large genomic deletion in the paternally derived allele and a large duplication in the maternally derived allele.
While it was already known that UBE2T is involved in activating ICL repair, the researchers said the discovery that these mutations could produce Fanconi anemia revealed that UBE2T is an irreplaceable player in the pathway.
“Although we have discovered new causes for this devastating but very rare genetic disease, the implications of this work go much further,” Dr Smogorzewska said.
“By identifying new disruptions to this repair pathway, we can better understand the mechanisms of an event that is crucial to every cell division—a process that occurs constantly within the human body throughout a lifetime.” ![]()

Photo courtesy of NIGMS
Investigators say they have identified mutations that cause Fanconi anemia and, in the process, gained new insight into interstrand crosslink (ICL) repair.
The researchers studied two patients who had Fanconi anemia with no known genetic cause.
Genomic sequencing revealed that one patient had a mutation in RAD51, and the other had mutations in UBE2T.
These genes—and others linked to Fanconi anemia in previous studies—contribute to ICL repair, which fixes a misplaced attachment between two strands of DNA.
Caused by chemical agents, ICLs block the replication of DNA, making it impossible for cells to accurately copy their genomes as they divide. The ICL repair process uses multiple enzymes that cut away the connection between the DNA strands, freeing them up and allowing the cells to grow.
The genome is at constant risk of forming ICLs, and defects in the ICL repair pathway can produce a constellation of symptoms associated with Fanconi anemia—a predisposition to cancer, bone marrow failure, infertility, and developmental defects.
Via two different studies, Agata Smogorzewska, MD, PhD, of The Rockefeller University in New York, New York, and her colleagues unearthed new discoveries relating to the disease and the pathway.
“Our work began, as it often does, with samples and histories from patients,” Dr Smogorzewska said. “In these cases, we had two patients who each represented a sort of mystery. They had symptoms of Fanconi anemia but no genetic cause yet identified.”
With the RAD51 research, which was published in Molecular Cell, the investigators set out to determine the cause of the Fanconi anemia-like symptoms in a girl in the university’s International Fanconi Anemia Registry.
When they sequenced the protein-coding genes in her genome, the researchers found a mutation in one of two copies of the RAD51 gene.
The RAD1 protein was already known to be important for another DNA repair process—homologous recombination, in which a missing section of DNA is replaced using its sister strand as a template. Homologous recombination is thought to be used during the last step of ICL repair, after the crosslink has been cut.
Because only one copy of the RAD51 gene was partially defective, the patient’s cells could still perform homologous recombination but not ICL repair.
To show that the defective copy of the RAD51 gene was indeed responsible for the patient’s symptoms, the investigators genetically engineered the girl’s own cells to remove the defect, which restored their ability to fix ICLs.
Further experiments on the patient’s cells led the researchers to suspect that RAD51 plays a role outside of homologous recombination, by tamping down the activity of two enzymes that degrade the DNA at the ICL. When RAD51 is defective, these enzymes—DNA2 and WRN—become overly destructive.
With the UBE2T study, published in Cell Reports, the investigators analyzed genomic data from another patient in the International Fanconi Anemia Registry.
They found compound heterozygous mutations in UBE2T—a large genomic deletion in the paternally derived allele and a large duplication in the maternally derived allele.
While it was already known that UBE2T is involved in activating ICL repair, the researchers said the discovery that these mutations could produce Fanconi anemia revealed that UBE2T is an irreplaceable player in the pathway.
“Although we have discovered new causes for this devastating but very rare genetic disease, the implications of this work go much further,” Dr Smogorzewska said.
“By identifying new disruptions to this repair pathway, we can better understand the mechanisms of an event that is crucial to every cell division—a process that occurs constantly within the human body throughout a lifetime.” ![]()
Lymph2Cx assay identifies outcomes in DLBCL
In patients with diffuse large B-cell lymphoma (DLBCL) treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), the Lymph2Cx assay was able to separate the cohort into groups with significantly different outcomes based on cell of origin (COO), investigators reported online in the Journal of Clinical Oncology.
In pairwise multivariable analyses, COO was associated with outcomes that were independent of International Prognostic Index score (IPI) and MYC/BCL2 immunohistochemistry (IHC).
Gene expression profiling of DLBCL has provided classification into two distinct subtypes: germinal center B-cell–like (GCB) and activated B-cell–like (ABC) subtypes. Using the cell of origin classification can define subgroups with distinct biology ad pathogenesis, as well as identify patient groups with different outcomes after treatment. Improvements in technology have allowed for the use of formalin-fixed paraffin-embedded tissue (FFPET) biopsies for more reliable gene expression profiling.
“The size of the study cohort allowed exploration of the prognostic value of COO in comparison with other prognostic tools,” wrote Dr. David W. Scott of the British Columbia Cancer Agency, Vancouver, and his colleagues (J Clin Oncol. 2015 Aug 3. doi: 10.1200/JCO.2014.60.2383).“Although the IPI remains the most powerful tool for risk stratification, COO assignment provides additional prognostic information, particularly evident in the intermediate IPI score group,” the authors noted.
The consistency and reproducibility of COO assignment using the Lymph2Cx assay was evaluated in a large patient cohort treated with R-CHOP therapy, and the relationship between COO, MYC/BCL2 dual expression, and IPI score with respect to defining prognosis in patients with DLBCL was also investigated.
Reproducibility of COO assignment using the Lymph2Cx assay was tested using repeated sampling within tumor biopsies and changes in reagent lots, and concordance of COO calls across the two reagent lots was 100%.
The COO was then determined in 344 patients with de novo DLBCL who were treated with R-CHOP at a single center. MYC and BCL2 protein expression was assessed using immunohistochemistry on tissue microarrays, and the median follow-up of living patients was 6.5 years (range, 0.75-13.2 years).
COO was a prognostic biomarker in the patient cohort. Those with activated B-cell–like DLBCL had significantly inferior outcomes as compared to patients with germinal center B-cell–like DLBCL (log-rank P less than .001 for time to progression, progression-free survival, disease-specific survival, and overall survival).
When reviewing the relationship between COO, IPI score, and MYC/BCL2 IHC, pairwise multivariable analyses demonstrated that the prognostic impact of COO is independent of IPI score. The prognostic value added by COO was most notable when patients with intermediate IPI scores were evaluated (ABC vs. GCB: 5-year time to progression, 53% v 74%; log-rank P = .003).
Both COO and MYC/BCL2 IHC identified high-risk groups that were similar in size (32% vs. 31%) with comparable outcomes (5-year time to progression, 51% vs. 51%). In pairwise multivariable analyses that included COO and MYC/BCL2 IHC as variables, COO remained significant, thus showing that COO had prognostic power “beyond that conferred merely by enrichment of MYC-positive/BCL2-positive patient cases of the ABC subtype,” wrote the authors.
When COO, IPI, and MYC/BCL2 IHC were all included in multivariable analyses, COO remained significantly associated with time to progression and progression-free survival.
“We anticipate that over the next few years, with the emergence of agents with selective activity in ABC or GCB DLBCL, the determination of COO will become part of the foundation for optimal patient care,” the authors concluded.
In patients with diffuse large B-cell lymphoma (DLBCL) treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), the Lymph2Cx assay was able to separate the cohort into groups with significantly different outcomes based on cell of origin (COO), investigators reported online in the Journal of Clinical Oncology.
In pairwise multivariable analyses, COO was associated with outcomes that were independent of International Prognostic Index score (IPI) and MYC/BCL2 immunohistochemistry (IHC).
Gene expression profiling of DLBCL has provided classification into two distinct subtypes: germinal center B-cell–like (GCB) and activated B-cell–like (ABC) subtypes. Using the cell of origin classification can define subgroups with distinct biology ad pathogenesis, as well as identify patient groups with different outcomes after treatment. Improvements in technology have allowed for the use of formalin-fixed paraffin-embedded tissue (FFPET) biopsies for more reliable gene expression profiling.
“The size of the study cohort allowed exploration of the prognostic value of COO in comparison with other prognostic tools,” wrote Dr. David W. Scott of the British Columbia Cancer Agency, Vancouver, and his colleagues (J Clin Oncol. 2015 Aug 3. doi: 10.1200/JCO.2014.60.2383).“Although the IPI remains the most powerful tool for risk stratification, COO assignment provides additional prognostic information, particularly evident in the intermediate IPI score group,” the authors noted.
The consistency and reproducibility of COO assignment using the Lymph2Cx assay was evaluated in a large patient cohort treated with R-CHOP therapy, and the relationship between COO, MYC/BCL2 dual expression, and IPI score with respect to defining prognosis in patients with DLBCL was also investigated.
Reproducibility of COO assignment using the Lymph2Cx assay was tested using repeated sampling within tumor biopsies and changes in reagent lots, and concordance of COO calls across the two reagent lots was 100%.
The COO was then determined in 344 patients with de novo DLBCL who were treated with R-CHOP at a single center. MYC and BCL2 protein expression was assessed using immunohistochemistry on tissue microarrays, and the median follow-up of living patients was 6.5 years (range, 0.75-13.2 years).
COO was a prognostic biomarker in the patient cohort. Those with activated B-cell–like DLBCL had significantly inferior outcomes as compared to patients with germinal center B-cell–like DLBCL (log-rank P less than .001 for time to progression, progression-free survival, disease-specific survival, and overall survival).
When reviewing the relationship between COO, IPI score, and MYC/BCL2 IHC, pairwise multivariable analyses demonstrated that the prognostic impact of COO is independent of IPI score. The prognostic value added by COO was most notable when patients with intermediate IPI scores were evaluated (ABC vs. GCB: 5-year time to progression, 53% v 74%; log-rank P = .003).
Both COO and MYC/BCL2 IHC identified high-risk groups that were similar in size (32% vs. 31%) with comparable outcomes (5-year time to progression, 51% vs. 51%). In pairwise multivariable analyses that included COO and MYC/BCL2 IHC as variables, COO remained significant, thus showing that COO had prognostic power “beyond that conferred merely by enrichment of MYC-positive/BCL2-positive patient cases of the ABC subtype,” wrote the authors.
When COO, IPI, and MYC/BCL2 IHC were all included in multivariable analyses, COO remained significantly associated with time to progression and progression-free survival.
“We anticipate that over the next few years, with the emergence of agents with selective activity in ABC or GCB DLBCL, the determination of COO will become part of the foundation for optimal patient care,” the authors concluded.
In patients with diffuse large B-cell lymphoma (DLBCL) treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), the Lymph2Cx assay was able to separate the cohort into groups with significantly different outcomes based on cell of origin (COO), investigators reported online in the Journal of Clinical Oncology.
In pairwise multivariable analyses, COO was associated with outcomes that were independent of International Prognostic Index score (IPI) and MYC/BCL2 immunohistochemistry (IHC).
Gene expression profiling of DLBCL has provided classification into two distinct subtypes: germinal center B-cell–like (GCB) and activated B-cell–like (ABC) subtypes. Using the cell of origin classification can define subgroups with distinct biology ad pathogenesis, as well as identify patient groups with different outcomes after treatment. Improvements in technology have allowed for the use of formalin-fixed paraffin-embedded tissue (FFPET) biopsies for more reliable gene expression profiling.
“The size of the study cohort allowed exploration of the prognostic value of COO in comparison with other prognostic tools,” wrote Dr. David W. Scott of the British Columbia Cancer Agency, Vancouver, and his colleagues (J Clin Oncol. 2015 Aug 3. doi: 10.1200/JCO.2014.60.2383).“Although the IPI remains the most powerful tool for risk stratification, COO assignment provides additional prognostic information, particularly evident in the intermediate IPI score group,” the authors noted.
The consistency and reproducibility of COO assignment using the Lymph2Cx assay was evaluated in a large patient cohort treated with R-CHOP therapy, and the relationship between COO, MYC/BCL2 dual expression, and IPI score with respect to defining prognosis in patients with DLBCL was also investigated.
Reproducibility of COO assignment using the Lymph2Cx assay was tested using repeated sampling within tumor biopsies and changes in reagent lots, and concordance of COO calls across the two reagent lots was 100%.
The COO was then determined in 344 patients with de novo DLBCL who were treated with R-CHOP at a single center. MYC and BCL2 protein expression was assessed using immunohistochemistry on tissue microarrays, and the median follow-up of living patients was 6.5 years (range, 0.75-13.2 years).
COO was a prognostic biomarker in the patient cohort. Those with activated B-cell–like DLBCL had significantly inferior outcomes as compared to patients with germinal center B-cell–like DLBCL (log-rank P less than .001 for time to progression, progression-free survival, disease-specific survival, and overall survival).
When reviewing the relationship between COO, IPI score, and MYC/BCL2 IHC, pairwise multivariable analyses demonstrated that the prognostic impact of COO is independent of IPI score. The prognostic value added by COO was most notable when patients with intermediate IPI scores were evaluated (ABC vs. GCB: 5-year time to progression, 53% v 74%; log-rank P = .003).
Both COO and MYC/BCL2 IHC identified high-risk groups that were similar in size (32% vs. 31%) with comparable outcomes (5-year time to progression, 51% vs. 51%). In pairwise multivariable analyses that included COO and MYC/BCL2 IHC as variables, COO remained significant, thus showing that COO had prognostic power “beyond that conferred merely by enrichment of MYC-positive/BCL2-positive patient cases of the ABC subtype,” wrote the authors.
When COO, IPI, and MYC/BCL2 IHC were all included in multivariable analyses, COO remained significantly associated with time to progression and progression-free survival.
“We anticipate that over the next few years, with the emergence of agents with selective activity in ABC or GCB DLBCL, the determination of COO will become part of the foundation for optimal patient care,” the authors concluded.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The Lymph2Cx is an accurate assay for DLBCL cell of origin assignment, and identified groups with significantly different outcomes after treatment with R-CHOP.
Major finding: The Lymph2Cx assay provided concordant COO calls in 100% of 83 formalin-fixed paraffin-embedded tissue biopsies, and COO was associated with significantly different outcomes independent of IPI score and MYC/BCL2 immunohistochemistry
Data source: The Lymph2Cx assay was compared with the IPI) score and MYC/BCL2 coexpression status in FFPET biopsies from 344 patients with DLBCL uniformly treated with R-CHOP.
Disclosures: The study was supported by the Terry Fox Foundation and by the British Columbia Cancer Foundation. Dr. Scott is potentially named inventor on pending patent on use of gene expression profiling to assign COO in DLBCL. Dr. Scott and several coauthors also report financial relationships with industry.
Black tea
Camellia sinensis, an evergreen tree belonging to the Theaceae family and used by human beings for approximately 4,000 years, is the source of the beverage tea, which is popular throughout the world, especially in Asia.1 Of the four main true teas (that is, derived from the tea plant C. sinensis), green and white are unfermented, black tea is fermented, and oolong tea is semifermented.2,3
Polyphenols, many of which act as strong antioxidants, are a diverse family of thousands of chemical substances found in plants. Theaflavins are black tea polyphenols with well-documented tumor-suppressing activity.4 In fact, they are thought to be the primary constituents of black tea responsible for conferring chemoprotection against cancer.5 Black tea, through oral administration and topical application, has been shown in the laboratory setting to protect skin from UV-induced erythema, premature aging, and cancer.6
Halder et al. have found that theaflavins and thearubigins, another key class of black tea polyphenols, can suppress A431 (human epidermoid carcinoma) and A375 (human malignant melanoma) cell proliferation without adversely impacting normal human epidermal keratinocytes. The researchers concluded that theaflavins and thearubigins appear to impart chemopreventive activity via cell cycle arrest and promotion of apoptosis in human skin cancer cells through a mitochondrial death cascade.7
In a 2005 English-language literature review, Thornfeldt cited green and black tea, as well as pomegranate, as the only ingredients supported by clinical trial evidence for effectiveness in treating extrinsic aging.2
Oral administration findings in animals and humans
More than 2 decades ago, Wang et al. found that the effects of orally administered black tea were comparable to those of green tea in suppressing UVB-induced skin carcinogenesis in 7,12-dimethylbenz[a]anthracene (DMBA)-initiated SKH-1 mice.8
In 1997, Lu et al. found that orally administered black tea inhibited the proliferation of skin tumors and enhanced apoptosis in nonmalignant and malignant skin tumors in female CD-1 mice with tumors initiated by the application of DMBA and promoted with 12-O-tetradecanoylphorbol-13-acetate (TPA).9 Record et al. reported in 1998 that black tea may confer greater protection than green tea against simulated solar irradiation.10
Hakim and Harris conducted a population-based case-control study in 2001 to assess the effects of the consumption of citrus peel and black tea on squamous cell skin cancer. They found that participants who reported intake of hot black tea and citrus peel had a significant reduction in the risk of squamous cell carcinoma. Further, they concluded that hot black tea and citrus peel displayed independent potential protection against SCC.11
Two years earlier, Zhao et al. used cultured keratinocytes and mouse and human skin to evaluate the effect of both orally and topically administered standardized black tea extract and its two major polyphenolic subfractions against UVB-induced photodamage. Topical pretreatment with the extract on SKH-1 hairless mice significantly lowered the incidence and severity of erythema and diminished skinfold thickness, compared with UVB-exposed nontreated mice. The black tea extract was similarly effective in human subjects. UVB-induced inflammation in murine as well as human skin also was reduced when the standardized extract was administered 5 minutes after UVB exposure. The investigators suggested that their findings indicated that black tea extracts have the capacity to mitigate UVB-generated erythema in human and murine skin.12
In 2011, George et al. assessed the chemopreventive effects of topical resveratrol and oral black tea polyphenols in blocking skin carcinogenesis in a two-stage mouse model initiated and promoted by DMBA and TPA, respectively. The combined treatment was found to reduce tumor incidence by approximately 89% (resveratrol alone, approximately 67%; black tea polyphenols alone, approximately 75%). Tumor volume and number also were significantly diminished by the synergistic combination, which, histologically, was noted for suppressing cellular proliferation and inducing apoptosis. The investigators concluded that oral black tea polyphenols combined with topical resveratrol exert greater chemopreventive activity than either compound alone and warrant study in trials for treating skin and other cancers.13
Animal studies on topical application
In 1997, Katiyar et al. investigated the anti-inflammatory effects of topically applied black tea polyphenols, primarily theaflavin gallates and (-)-epigallocatechin-3-gallate (EGCG), against TPA-induced inflammatory responses in murine skin. Significant inhibition against TPA-promoted induction of epidermal edema, hyperplasia, leukocyte infiltration, and proinflammatory cytokine expression was rendered by the preapplication of black tea polyphenols prior to TPA exposure. The investigators concluded that black tea polyphenols may be effective against human cutaneous inflammatory responses.14
Just over a decade later, Patel et al. investigated the in vivo antitumor-promoting effects of the most plentiful polymeric black tea polyphenols (thearubigins) in mice exposed to tumor-initiating DMBA and tumor-promoting TPA over a 40-week period. Pretreatment with topical thearubigins resulted in antipromoting effects in terms of latency, multiplicity, and incidence of skin papillomas. The black tea polyphenols also were found to reduce TPA-induced cell proliferation and epidermal cell apoptosis. The researchers attributed the protective effects of these compounds to their inhibitory impact on TPA-induced cellular proliferation.15
In 2011, Choi and Kim assessed the whitening effect of black tea water extract topically applied twice daily (6 days a week for 4 weeks) to UVB-induced hyperpigmented spots on the backs of brown guinea pigs. Treatment was divided into control (UVB and saline), vehicle control (UVB, propylene glycol, ethanol, and water), positive control (UVB and 2% hydroquinone), and two experimental groups (UVB and 1% black tea; UVB and 2% black tea). The investigators observed that the hyperpigmented spots treated with hydroquinone and black tea were clearly lighter than those treated by the control or vehicle-control groups. Histologic examination revealed that melanin pigmentation, melanocyte proliferation, and melanin production were significantly diminished in the groups treated with hydroquinone and both concentrations of black tea. The authors concluded that black tea suppresses melanocyte proliferation and melanosome synthesis in vivo, thus displaying the capacity to whiten skin in brown guinea pigs.16
In 2013, Yeh et al. found in nude mouse skin in vitro that niosomes appear to be feasible as a delivery vehicle for the dermal administration of black tea extracts as a sunscreen agent.1
Topical studies in humans
Building on findings 3 years earlier18, Türkoglu et al., in 2010, assessed the photoprotective effects of dermal gels produced from green and black tea aqueous extracts tested in vivo in the forearms of six volunteers exposed to artificial UV light (200-400 nm). In addition to the green tea and black tea gels, a 0.3% caffeine gel, a carbomer gel base, and a control were tested. The investigators reported no eruptions of UV-induced erythema in any of the black and green tea gel sites, but erythema was present to varying degrees at the areas treated with caffeine gel, carbomer gel, and control. The investigators concluded that the black and green tea extracts exhibited potent UV absorbance and that the formulated gels were effective in protecting the skin against UV-induced erythema. Further, the investigators suggested that these agents have the potential to protect against other harm caused by UV radiation, including photoaging.19
Conclusion
Though not as widely investigated as green tea, the therapeutic potential of black tea is of great interest. Although an abundance of laboratory evidence has emerged, clinical evidence is sparse. Nevertheless, laboratory data suggest the potential uses of black tea in the dermatologic realm and justify more human trials.
References
1. Cancer Lett. 1997 Mar 19;114(1-2):315-7.
2. Dermatol. Surg. 2005;31(7 Pt 2):873-80.
3. Oxid Med Cell Longev. 2012:2012:560682.
4. J Environ Pathol Toxicol Oncol. 2010;29(1):55-68.
5. Mol Carcinog. 2000 Jul;28(3):148-55.
6. Am J Clin Dermatol. 2010;11(4):247-67.
7. Carcinogenesis. 2008 Jan;29(1):129-38.
8. Cancer Res. 1994 Jul 1;54(13):3428-35.
9. Carcinogenesis. 1997 Nov;18(11):2163-9.
10. Mutat Res. 1998 Nov 9;422(1):191-9.
12. Photochem Photobiol. 1999 Oct;70(4):637-44.
13. PLoS One. 2011;6(8):e23395.
14. Carcinogenesis. 1997 Oct;18(10):1911-6.
15. Cell Prolif. 2008 Jun;41(3):532-53.
16. Toxicol Res. 2011 Sep;27(3):153-60.
17. Int J Dermatol. 2013 Feb;52(2):239-45.
18. Int J Cosmet Sci. 2007 Dec;29(6):437-42.
19. Drug Discov Ther. 2010 Oct;4(5):362-7.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Camellia sinensis, an evergreen tree belonging to the Theaceae family and used by human beings for approximately 4,000 years, is the source of the beverage tea, which is popular throughout the world, especially in Asia.1 Of the four main true teas (that is, derived from the tea plant C. sinensis), green and white are unfermented, black tea is fermented, and oolong tea is semifermented.2,3
Polyphenols, many of which act as strong antioxidants, are a diverse family of thousands of chemical substances found in plants. Theaflavins are black tea polyphenols with well-documented tumor-suppressing activity.4 In fact, they are thought to be the primary constituents of black tea responsible for conferring chemoprotection against cancer.5 Black tea, through oral administration and topical application, has been shown in the laboratory setting to protect skin from UV-induced erythema, premature aging, and cancer.6
Halder et al. have found that theaflavins and thearubigins, another key class of black tea polyphenols, can suppress A431 (human epidermoid carcinoma) and A375 (human malignant melanoma) cell proliferation without adversely impacting normal human epidermal keratinocytes. The researchers concluded that theaflavins and thearubigins appear to impart chemopreventive activity via cell cycle arrest and promotion of apoptosis in human skin cancer cells through a mitochondrial death cascade.7
In a 2005 English-language literature review, Thornfeldt cited green and black tea, as well as pomegranate, as the only ingredients supported by clinical trial evidence for effectiveness in treating extrinsic aging.2
Oral administration findings in animals and humans
More than 2 decades ago, Wang et al. found that the effects of orally administered black tea were comparable to those of green tea in suppressing UVB-induced skin carcinogenesis in 7,12-dimethylbenz[a]anthracene (DMBA)-initiated SKH-1 mice.8
In 1997, Lu et al. found that orally administered black tea inhibited the proliferation of skin tumors and enhanced apoptosis in nonmalignant and malignant skin tumors in female CD-1 mice with tumors initiated by the application of DMBA and promoted with 12-O-tetradecanoylphorbol-13-acetate (TPA).9 Record et al. reported in 1998 that black tea may confer greater protection than green tea against simulated solar irradiation.10
Hakim and Harris conducted a population-based case-control study in 2001 to assess the effects of the consumption of citrus peel and black tea on squamous cell skin cancer. They found that participants who reported intake of hot black tea and citrus peel had a significant reduction in the risk of squamous cell carcinoma. Further, they concluded that hot black tea and citrus peel displayed independent potential protection against SCC.11
Two years earlier, Zhao et al. used cultured keratinocytes and mouse and human skin to evaluate the effect of both orally and topically administered standardized black tea extract and its two major polyphenolic subfractions against UVB-induced photodamage. Topical pretreatment with the extract on SKH-1 hairless mice significantly lowered the incidence and severity of erythema and diminished skinfold thickness, compared with UVB-exposed nontreated mice. The black tea extract was similarly effective in human subjects. UVB-induced inflammation in murine as well as human skin also was reduced when the standardized extract was administered 5 minutes after UVB exposure. The investigators suggested that their findings indicated that black tea extracts have the capacity to mitigate UVB-generated erythema in human and murine skin.12
In 2011, George et al. assessed the chemopreventive effects of topical resveratrol and oral black tea polyphenols in blocking skin carcinogenesis in a two-stage mouse model initiated and promoted by DMBA and TPA, respectively. The combined treatment was found to reduce tumor incidence by approximately 89% (resveratrol alone, approximately 67%; black tea polyphenols alone, approximately 75%). Tumor volume and number also were significantly diminished by the synergistic combination, which, histologically, was noted for suppressing cellular proliferation and inducing apoptosis. The investigators concluded that oral black tea polyphenols combined with topical resveratrol exert greater chemopreventive activity than either compound alone and warrant study in trials for treating skin and other cancers.13
Animal studies on topical application
In 1997, Katiyar et al. investigated the anti-inflammatory effects of topically applied black tea polyphenols, primarily theaflavin gallates and (-)-epigallocatechin-3-gallate (EGCG), against TPA-induced inflammatory responses in murine skin. Significant inhibition against TPA-promoted induction of epidermal edema, hyperplasia, leukocyte infiltration, and proinflammatory cytokine expression was rendered by the preapplication of black tea polyphenols prior to TPA exposure. The investigators concluded that black tea polyphenols may be effective against human cutaneous inflammatory responses.14
Just over a decade later, Patel et al. investigated the in vivo antitumor-promoting effects of the most plentiful polymeric black tea polyphenols (thearubigins) in mice exposed to tumor-initiating DMBA and tumor-promoting TPA over a 40-week period. Pretreatment with topical thearubigins resulted in antipromoting effects in terms of latency, multiplicity, and incidence of skin papillomas. The black tea polyphenols also were found to reduce TPA-induced cell proliferation and epidermal cell apoptosis. The researchers attributed the protective effects of these compounds to their inhibitory impact on TPA-induced cellular proliferation.15
In 2011, Choi and Kim assessed the whitening effect of black tea water extract topically applied twice daily (6 days a week for 4 weeks) to UVB-induced hyperpigmented spots on the backs of brown guinea pigs. Treatment was divided into control (UVB and saline), vehicle control (UVB, propylene glycol, ethanol, and water), positive control (UVB and 2% hydroquinone), and two experimental groups (UVB and 1% black tea; UVB and 2% black tea). The investigators observed that the hyperpigmented spots treated with hydroquinone and black tea were clearly lighter than those treated by the control or vehicle-control groups. Histologic examination revealed that melanin pigmentation, melanocyte proliferation, and melanin production were significantly diminished in the groups treated with hydroquinone and both concentrations of black tea. The authors concluded that black tea suppresses melanocyte proliferation and melanosome synthesis in vivo, thus displaying the capacity to whiten skin in brown guinea pigs.16
In 2013, Yeh et al. found in nude mouse skin in vitro that niosomes appear to be feasible as a delivery vehicle for the dermal administration of black tea extracts as a sunscreen agent.1
Topical studies in humans
Building on findings 3 years earlier18, Türkoglu et al., in 2010, assessed the photoprotective effects of dermal gels produced from green and black tea aqueous extracts tested in vivo in the forearms of six volunteers exposed to artificial UV light (200-400 nm). In addition to the green tea and black tea gels, a 0.3% caffeine gel, a carbomer gel base, and a control were tested. The investigators reported no eruptions of UV-induced erythema in any of the black and green tea gel sites, but erythema was present to varying degrees at the areas treated with caffeine gel, carbomer gel, and control. The investigators concluded that the black and green tea extracts exhibited potent UV absorbance and that the formulated gels were effective in protecting the skin against UV-induced erythema. Further, the investigators suggested that these agents have the potential to protect against other harm caused by UV radiation, including photoaging.19
Conclusion
Though not as widely investigated as green tea, the therapeutic potential of black tea is of great interest. Although an abundance of laboratory evidence has emerged, clinical evidence is sparse. Nevertheless, laboratory data suggest the potential uses of black tea in the dermatologic realm and justify more human trials.
References
1. Cancer Lett. 1997 Mar 19;114(1-2):315-7.
2. Dermatol. Surg. 2005;31(7 Pt 2):873-80.
3. Oxid Med Cell Longev. 2012:2012:560682.
4. J Environ Pathol Toxicol Oncol. 2010;29(1):55-68.
5. Mol Carcinog. 2000 Jul;28(3):148-55.
6. Am J Clin Dermatol. 2010;11(4):247-67.
7. Carcinogenesis. 2008 Jan;29(1):129-38.
8. Cancer Res. 1994 Jul 1;54(13):3428-35.
9. Carcinogenesis. 1997 Nov;18(11):2163-9.
10. Mutat Res. 1998 Nov 9;422(1):191-9.
12. Photochem Photobiol. 1999 Oct;70(4):637-44.
13. PLoS One. 2011;6(8):e23395.
14. Carcinogenesis. 1997 Oct;18(10):1911-6.
15. Cell Prolif. 2008 Jun;41(3):532-53.
16. Toxicol Res. 2011 Sep;27(3):153-60.
17. Int J Dermatol. 2013 Feb;52(2):239-45.
18. Int J Cosmet Sci. 2007 Dec;29(6):437-42.
19. Drug Discov Ther. 2010 Oct;4(5):362-7.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.
Camellia sinensis, an evergreen tree belonging to the Theaceae family and used by human beings for approximately 4,000 years, is the source of the beverage tea, which is popular throughout the world, especially in Asia.1 Of the four main true teas (that is, derived from the tea plant C. sinensis), green and white are unfermented, black tea is fermented, and oolong tea is semifermented.2,3
Polyphenols, many of which act as strong antioxidants, are a diverse family of thousands of chemical substances found in plants. Theaflavins are black tea polyphenols with well-documented tumor-suppressing activity.4 In fact, they are thought to be the primary constituents of black tea responsible for conferring chemoprotection against cancer.5 Black tea, through oral administration and topical application, has been shown in the laboratory setting to protect skin from UV-induced erythema, premature aging, and cancer.6
Halder et al. have found that theaflavins and thearubigins, another key class of black tea polyphenols, can suppress A431 (human epidermoid carcinoma) and A375 (human malignant melanoma) cell proliferation without adversely impacting normal human epidermal keratinocytes. The researchers concluded that theaflavins and thearubigins appear to impart chemopreventive activity via cell cycle arrest and promotion of apoptosis in human skin cancer cells through a mitochondrial death cascade.7
In a 2005 English-language literature review, Thornfeldt cited green and black tea, as well as pomegranate, as the only ingredients supported by clinical trial evidence for effectiveness in treating extrinsic aging.2
Oral administration findings in animals and humans
More than 2 decades ago, Wang et al. found that the effects of orally administered black tea were comparable to those of green tea in suppressing UVB-induced skin carcinogenesis in 7,12-dimethylbenz[a]anthracene (DMBA)-initiated SKH-1 mice.8
In 1997, Lu et al. found that orally administered black tea inhibited the proliferation of skin tumors and enhanced apoptosis in nonmalignant and malignant skin tumors in female CD-1 mice with tumors initiated by the application of DMBA and promoted with 12-O-tetradecanoylphorbol-13-acetate (TPA).9 Record et al. reported in 1998 that black tea may confer greater protection than green tea against simulated solar irradiation.10
Hakim and Harris conducted a population-based case-control study in 2001 to assess the effects of the consumption of citrus peel and black tea on squamous cell skin cancer. They found that participants who reported intake of hot black tea and citrus peel had a significant reduction in the risk of squamous cell carcinoma. Further, they concluded that hot black tea and citrus peel displayed independent potential protection against SCC.11
Two years earlier, Zhao et al. used cultured keratinocytes and mouse and human skin to evaluate the effect of both orally and topically administered standardized black tea extract and its two major polyphenolic subfractions against UVB-induced photodamage. Topical pretreatment with the extract on SKH-1 hairless mice significantly lowered the incidence and severity of erythema and diminished skinfold thickness, compared with UVB-exposed nontreated mice. The black tea extract was similarly effective in human subjects. UVB-induced inflammation in murine as well as human skin also was reduced when the standardized extract was administered 5 minutes after UVB exposure. The investigators suggested that their findings indicated that black tea extracts have the capacity to mitigate UVB-generated erythema in human and murine skin.12
In 2011, George et al. assessed the chemopreventive effects of topical resveratrol and oral black tea polyphenols in blocking skin carcinogenesis in a two-stage mouse model initiated and promoted by DMBA and TPA, respectively. The combined treatment was found to reduce tumor incidence by approximately 89% (resveratrol alone, approximately 67%; black tea polyphenols alone, approximately 75%). Tumor volume and number also were significantly diminished by the synergistic combination, which, histologically, was noted for suppressing cellular proliferation and inducing apoptosis. The investigators concluded that oral black tea polyphenols combined with topical resveratrol exert greater chemopreventive activity than either compound alone and warrant study in trials for treating skin and other cancers.13
Animal studies on topical application
In 1997, Katiyar et al. investigated the anti-inflammatory effects of topically applied black tea polyphenols, primarily theaflavin gallates and (-)-epigallocatechin-3-gallate (EGCG), against TPA-induced inflammatory responses in murine skin. Significant inhibition against TPA-promoted induction of epidermal edema, hyperplasia, leukocyte infiltration, and proinflammatory cytokine expression was rendered by the preapplication of black tea polyphenols prior to TPA exposure. The investigators concluded that black tea polyphenols may be effective against human cutaneous inflammatory responses.14
Just over a decade later, Patel et al. investigated the in vivo antitumor-promoting effects of the most plentiful polymeric black tea polyphenols (thearubigins) in mice exposed to tumor-initiating DMBA and tumor-promoting TPA over a 40-week period. Pretreatment with topical thearubigins resulted in antipromoting effects in terms of latency, multiplicity, and incidence of skin papillomas. The black tea polyphenols also were found to reduce TPA-induced cell proliferation and epidermal cell apoptosis. The researchers attributed the protective effects of these compounds to their inhibitory impact on TPA-induced cellular proliferation.15
In 2011, Choi and Kim assessed the whitening effect of black tea water extract topically applied twice daily (6 days a week for 4 weeks) to UVB-induced hyperpigmented spots on the backs of brown guinea pigs. Treatment was divided into control (UVB and saline), vehicle control (UVB, propylene glycol, ethanol, and water), positive control (UVB and 2% hydroquinone), and two experimental groups (UVB and 1% black tea; UVB and 2% black tea). The investigators observed that the hyperpigmented spots treated with hydroquinone and black tea were clearly lighter than those treated by the control or vehicle-control groups. Histologic examination revealed that melanin pigmentation, melanocyte proliferation, and melanin production were significantly diminished in the groups treated with hydroquinone and both concentrations of black tea. The authors concluded that black tea suppresses melanocyte proliferation and melanosome synthesis in vivo, thus displaying the capacity to whiten skin in brown guinea pigs.16
In 2013, Yeh et al. found in nude mouse skin in vitro that niosomes appear to be feasible as a delivery vehicle for the dermal administration of black tea extracts as a sunscreen agent.1
Topical studies in humans
Building on findings 3 years earlier18, Türkoglu et al., in 2010, assessed the photoprotective effects of dermal gels produced from green and black tea aqueous extracts tested in vivo in the forearms of six volunteers exposed to artificial UV light (200-400 nm). In addition to the green tea and black tea gels, a 0.3% caffeine gel, a carbomer gel base, and a control were tested. The investigators reported no eruptions of UV-induced erythema in any of the black and green tea gel sites, but erythema was present to varying degrees at the areas treated with caffeine gel, carbomer gel, and control. The investigators concluded that the black and green tea extracts exhibited potent UV absorbance and that the formulated gels were effective in protecting the skin against UV-induced erythema. Further, the investigators suggested that these agents have the potential to protect against other harm caused by UV radiation, including photoaging.19
Conclusion
Though not as widely investigated as green tea, the therapeutic potential of black tea is of great interest. Although an abundance of laboratory evidence has emerged, clinical evidence is sparse. Nevertheless, laboratory data suggest the potential uses of black tea in the dermatologic realm and justify more human trials.
References
1. Cancer Lett. 1997 Mar 19;114(1-2):315-7.
2. Dermatol. Surg. 2005;31(7 Pt 2):873-80.
3. Oxid Med Cell Longev. 2012:2012:560682.
4. J Environ Pathol Toxicol Oncol. 2010;29(1):55-68.
5. Mol Carcinog. 2000 Jul;28(3):148-55.
6. Am J Clin Dermatol. 2010;11(4):247-67.
7. Carcinogenesis. 2008 Jan;29(1):129-38.
8. Cancer Res. 1994 Jul 1;54(13):3428-35.
9. Carcinogenesis. 1997 Nov;18(11):2163-9.
10. Mutat Res. 1998 Nov 9;422(1):191-9.
12. Photochem Photobiol. 1999 Oct;70(4):637-44.
13. PLoS One. 2011;6(8):e23395.
14. Carcinogenesis. 1997 Oct;18(10):1911-6.
15. Cell Prolif. 2008 Jun;41(3):532-53.
16. Toxicol Res. 2011 Sep;27(3):153-60.
17. Int J Dermatol. 2013 Feb;52(2):239-45.
18. Int J Cosmet Sci. 2007 Dec;29(6):437-42.
19. Drug Discov Ther. 2010 Oct;4(5):362-7.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). She has contributed to the Cosmeceutical Critique column in Dermatology News since January 2001. Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever.