User login
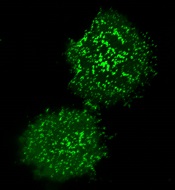
plasma membrane upon T-cell
activation. Image courtesy
of the Salk Institute
New research suggests that, contrary to previous assumptions, T-cell activation is a fluid process that relies on a dynamic protein network at the cell surface.
Previously, scientists thought T-cell activation was a static process in which molecules assemble within a T cell.
Instead, investigators discovered that molecules rapidly come and go, and the times at which these molecules arrive and depart affect the immune response.
“This is a completely new principle for how T-cell activity is controlled—whether it ignores or responds to a threat,” said Björn Lillemeier, PhD, of the Salk Institute in La Jolla, California.
He and his colleagues described this discovery in Nature Immunology.
They noted that the protein kinase ZAP-70 plays a central role in T-cell activation. Until now, scientists assumed that a silent form of ZAP-70 floats around inside the T cell until a threat is detected. The threat recruits ZAP-70 to the cell surface and activates it.
By analyzing mutant forms of ZAP-70, Dr Lillemeier’s group discovered that, instead of ZAP-70 binding the T-cell receptor firmly, it comes in contact with the receptor sporadically. Each time this happens, ZAP-70 has to adopt an unfavorable shape that forces it back inside the cell.
This cycle continues until a second molecule, Lck, helps ZAP-70 remain with the T-cell receptor. The prolonged stay at the cell surface activates ZAP-70 and prompts the T cell to attack invaders and diseased cells.
The investigators said this study suggests the steps underlying T-cell activation are more dynamic than the less mobile modes scientists had suspected before. And the research highlights how ZAP-70 and other molecules communicate in space and time, which is crucial for controlling the ultimate activity of a T cell.
By understanding this process, Dr Lillemeier said, “We might be able to encourage the immune system to be a little more sensitive in order to recognize and eliminate diseases.”
His team is now working to identify new principles that determine if T cells respond to a threat or stay quiet. They are also testing whether their findings could be applied across additional processes in T cells and other immune cells.
Because proteins have many of the same modular building blocks, Dr Lillemeier said that, in principle, any protein with structural characteristics comparable to those of ZAP-70 could be controlled by similar mechanisms. ![]()

plasma membrane upon T-cell
activation. Image courtesy
of the Salk Institute
New research suggests that, contrary to previous assumptions, T-cell activation is a fluid process that relies on a dynamic protein network at the cell surface.
Previously, scientists thought T-cell activation was a static process in which molecules assemble within a T cell.
Instead, investigators discovered that molecules rapidly come and go, and the times at which these molecules arrive and depart affect the immune response.
“This is a completely new principle for how T-cell activity is controlled—whether it ignores or responds to a threat,” said Björn Lillemeier, PhD, of the Salk Institute in La Jolla, California.
He and his colleagues described this discovery in Nature Immunology.
They noted that the protein kinase ZAP-70 plays a central role in T-cell activation. Until now, scientists assumed that a silent form of ZAP-70 floats around inside the T cell until a threat is detected. The threat recruits ZAP-70 to the cell surface and activates it.
By analyzing mutant forms of ZAP-70, Dr Lillemeier’s group discovered that, instead of ZAP-70 binding the T-cell receptor firmly, it comes in contact with the receptor sporadically. Each time this happens, ZAP-70 has to adopt an unfavorable shape that forces it back inside the cell.
This cycle continues until a second molecule, Lck, helps ZAP-70 remain with the T-cell receptor. The prolonged stay at the cell surface activates ZAP-70 and prompts the T cell to attack invaders and diseased cells.
The investigators said this study suggests the steps underlying T-cell activation are more dynamic than the less mobile modes scientists had suspected before. And the research highlights how ZAP-70 and other molecules communicate in space and time, which is crucial for controlling the ultimate activity of a T cell.
By understanding this process, Dr Lillemeier said, “We might be able to encourage the immune system to be a little more sensitive in order to recognize and eliminate diseases.”
His team is now working to identify new principles that determine if T cells respond to a threat or stay quiet. They are also testing whether their findings could be applied across additional processes in T cells and other immune cells.
Because proteins have many of the same modular building blocks, Dr Lillemeier said that, in principle, any protein with structural characteristics comparable to those of ZAP-70 could be controlled by similar mechanisms. ![]()

plasma membrane upon T-cell
activation. Image courtesy
of the Salk Institute
New research suggests that, contrary to previous assumptions, T-cell activation is a fluid process that relies on a dynamic protein network at the cell surface.
Previously, scientists thought T-cell activation was a static process in which molecules assemble within a T cell.
Instead, investigators discovered that molecules rapidly come and go, and the times at which these molecules arrive and depart affect the immune response.
“This is a completely new principle for how T-cell activity is controlled—whether it ignores or responds to a threat,” said Björn Lillemeier, PhD, of the Salk Institute in La Jolla, California.
He and his colleagues described this discovery in Nature Immunology.
They noted that the protein kinase ZAP-70 plays a central role in T-cell activation. Until now, scientists assumed that a silent form of ZAP-70 floats around inside the T cell until a threat is detected. The threat recruits ZAP-70 to the cell surface and activates it.
By analyzing mutant forms of ZAP-70, Dr Lillemeier’s group discovered that, instead of ZAP-70 binding the T-cell receptor firmly, it comes in contact with the receptor sporadically. Each time this happens, ZAP-70 has to adopt an unfavorable shape that forces it back inside the cell.
This cycle continues until a second molecule, Lck, helps ZAP-70 remain with the T-cell receptor. The prolonged stay at the cell surface activates ZAP-70 and prompts the T cell to attack invaders and diseased cells.
The investigators said this study suggests the steps underlying T-cell activation are more dynamic than the less mobile modes scientists had suspected before. And the research highlights how ZAP-70 and other molecules communicate in space and time, which is crucial for controlling the ultimate activity of a T cell.
By understanding this process, Dr Lillemeier said, “We might be able to encourage the immune system to be a little more sensitive in order to recognize and eliminate diseases.”
His team is now working to identify new principles that determine if T cells respond to a threat or stay quiet. They are also testing whether their findings could be applied across additional processes in T cells and other immune cells.
Because proteins have many of the same modular building blocks, Dr Lillemeier said that, in principle, any protein with structural characteristics comparable to those of ZAP-70 could be controlled by similar mechanisms. ![]()