User login
Effectiveness of Antipsychotics in Treatment of Delirium
Clinical questions: Are antipsychotics for the treatment of delirium safe and effective? Does efficacy differ between ICU and non-ICU settings? Does efficacy differ between first- and second-generation antipsychotics (SGA)?
Background: Delirium is common in hospitalized patients. Data are mixed about the use of antipsychotics for treatment of delirium, and safety concerns are well founded. A 2007 Cochrane review failed to show compelling evidence for their efficacy, yet they remain widely used for this purpose.
Study design: Systematic review and meta-analysis.
Setting: Fifteen RCTs of adults with delirium.
Synopsis: The primary outcome measure was response rate at the study endpoint, defined using severity of delirium and global scales.
In a comparison of pooled or individual antipsychotics vs. placebo or usual care (UC), antipsychotics were found to be superior, with a response rate of 0.22 (95% CI, 0.15-0.34, P<.00001), NNT=2. Subgroup analysis revealed this superiority to be greater in non-ICU settings, with ICU antipsychotic use only marginally better than UC. Antipsychotics were superior in time to response (TTR). Mortality rates were no different.
There were no differences between chlorpromazine and haloperidol in any outcomes. Among head-to-head comparisons of SGAs, no differences were found. Pooled or individual SGAs, however, had the same overall efficacy as haloperidol but shorter TTR and fewer extrapyramidal side effects. Subgroup analysis showed a small but significant advantage in the use of SGAs over haloperidol in the ICU.
Bottom line: Antipsychotics are more effective than placebo or usual care in the treatment of delirium. There appears to be a benefit to using second-generation antipsychotics over haloperidol.
Citation: Kishi T, Hirota T, Matsunaga S, Iwata N. Antipsychotic medications for the treatment of delirium: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry. 2015;0:1-8.
Clinical questions: Are antipsychotics for the treatment of delirium safe and effective? Does efficacy differ between ICU and non-ICU settings? Does efficacy differ between first- and second-generation antipsychotics (SGA)?
Background: Delirium is common in hospitalized patients. Data are mixed about the use of antipsychotics for treatment of delirium, and safety concerns are well founded. A 2007 Cochrane review failed to show compelling evidence for their efficacy, yet they remain widely used for this purpose.
Study design: Systematic review and meta-analysis.
Setting: Fifteen RCTs of adults with delirium.
Synopsis: The primary outcome measure was response rate at the study endpoint, defined using severity of delirium and global scales.
In a comparison of pooled or individual antipsychotics vs. placebo or usual care (UC), antipsychotics were found to be superior, with a response rate of 0.22 (95% CI, 0.15-0.34, P<.00001), NNT=2. Subgroup analysis revealed this superiority to be greater in non-ICU settings, with ICU antipsychotic use only marginally better than UC. Antipsychotics were superior in time to response (TTR). Mortality rates were no different.
There were no differences between chlorpromazine and haloperidol in any outcomes. Among head-to-head comparisons of SGAs, no differences were found. Pooled or individual SGAs, however, had the same overall efficacy as haloperidol but shorter TTR and fewer extrapyramidal side effects. Subgroup analysis showed a small but significant advantage in the use of SGAs over haloperidol in the ICU.
Bottom line: Antipsychotics are more effective than placebo or usual care in the treatment of delirium. There appears to be a benefit to using second-generation antipsychotics over haloperidol.
Citation: Kishi T, Hirota T, Matsunaga S, Iwata N. Antipsychotic medications for the treatment of delirium: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry. 2015;0:1-8.
Clinical questions: Are antipsychotics for the treatment of delirium safe and effective? Does efficacy differ between ICU and non-ICU settings? Does efficacy differ between first- and second-generation antipsychotics (SGA)?
Background: Delirium is common in hospitalized patients. Data are mixed about the use of antipsychotics for treatment of delirium, and safety concerns are well founded. A 2007 Cochrane review failed to show compelling evidence for their efficacy, yet they remain widely used for this purpose.
Study design: Systematic review and meta-analysis.
Setting: Fifteen RCTs of adults with delirium.
Synopsis: The primary outcome measure was response rate at the study endpoint, defined using severity of delirium and global scales.
In a comparison of pooled or individual antipsychotics vs. placebo or usual care (UC), antipsychotics were found to be superior, with a response rate of 0.22 (95% CI, 0.15-0.34, P<.00001), NNT=2. Subgroup analysis revealed this superiority to be greater in non-ICU settings, with ICU antipsychotic use only marginally better than UC. Antipsychotics were superior in time to response (TTR). Mortality rates were no different.
There were no differences between chlorpromazine and haloperidol in any outcomes. Among head-to-head comparisons of SGAs, no differences were found. Pooled or individual SGAs, however, had the same overall efficacy as haloperidol but shorter TTR and fewer extrapyramidal side effects. Subgroup analysis showed a small but significant advantage in the use of SGAs over haloperidol in the ICU.
Bottom line: Antipsychotics are more effective than placebo or usual care in the treatment of delirium. There appears to be a benefit to using second-generation antipsychotics over haloperidol.
Citation: Kishi T, Hirota T, Matsunaga S, Iwata N. Antipsychotic medications for the treatment of delirium: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry. 2015;0:1-8.
Potential new alternative in CML when TKI therapy fails

ASH Annual Meeting
Photo courtesy of ASH
ORLANDO, FL—ABL001, an allosteric inhibitor of BCR-ABL1, has shown early evidence of single-agent activity in a multicenter, first-in-human, first-in-class trial of heavily treated patients with chronic myeloid leukemia (CML) that is resistant to or intolerant of prior tyrosine kinase inhibitors (TKIs), even at the lowest dose evaluated.
ABL001 and classical TKIs exhibit complementary mutation profiles, with ABL001 showing activity against TKI resistance mutations.
When combined with nilotinib in a mouse model of CML, ABL001 prevented the emergence of resistant disease even after treatment was discontinued.
“This produces a new therapeutic concept—that of allosteric inhibition,” said Oliver G. Ottmann, MD, of Cardiff University in the UK.
The ABL001 binding site is located in a region remote from the kinase domain and has the potential to combine with TKIs for greater pharmacologic control of BCR-ABL1.
“This obviously has the opportunity both for combining different treatments and for overcoming resistance to one or the other,” Dr Ottmann added.
Based on preliminary pharmacokinetic data and preclinical evidence, investigators proceeded to evaluate ABL001 in a phase 1 dose-escalation and dose-expansion study.
Their primary objective was to determine the maximum tolerated dose (MTD) in humans and the recommended dose for expansion (RDE). Secondary objectives were to evaluate the safety, tolerability, preliminary anti-CML activity, and pharmacokinetic and pharmacodynamic profile.
Dr Ottmann presented the findings during the 2015 ASH Annual Meeting as abstract 138.*
Study design
Patients received ABL001 orally as a single agent twice a day (BID) continuously until disease progression, unacceptable toxicity, consent withdrawal, or death.
The dose-escalation schema followed a Bayesian logistic regression model based on dose-limiting toxicities during cycle 1. Doses ranged from 10 mg to 200 mg BID.
A subsequent dose-expansion phase was planned to augment the data generated in the dose-escalation phase and to include patients with Ph-positive acute lymphoblastic leukemia resistant or intolerant to prior TKI therapy.
Dr Ottmann noted that there were 2 protocol amendments. The first amendment was made to include a once-daily (QD) dosing of ABL001 at 120 mg and 200 mg. The second amendment was made to evaluate the combination of 40 mg of ABL001 BID with nilotinib at 300 mg BID.
Inclusion/exclusion criteria
Patients had to be at least 18 years old with CML in chronic or accelerated phase. They had to have failed at least 2 prior TKIs or be intolerant of TKIs. Their performance status had to be 0–2.
Patients were excluded from the trial if they had an absolute neutrophil count less than 500/mm3, a platelet count less than 50,000/mm3, bilirubin level more than 1.5 x the upper limit of normal (ULN) or more than 3.0 x ULN in patients with Gilberts syndrome.
Their aspartate aminotransferase or alanine aminotransferase could not be above 3 x ULN, and creatinine could not be above 1.5 x ULN.
Patients were also excluded if they needed treatment with strong inhibitors or inducers of CYP3A4 or its substrates with narrow therapeutic index.
Patient demographics
Fifty-nine patients were enrolled on the trial at the time of the presentation and they had “typical” characteristics of patients at this stage, Dr Ottmann said.
Their median age was 56 (range, 23–78). Almost two-thirds (61%) were male and 39% female.
All but 1 patient had an ECOG performance status of 0, and patients had a median of 3.5 (range, 2–5) prior lines of therapy. Twenty-four patients (41%) had 2 prior TKIs, and 35 (59%) had 3 or more TKIs. Forty-five patients (76%) were resistant and 14 (24%) were intolerant to their prior TKI.
All but 1 patient had chronic phase CML, 18 (31%) were TKD nonmutated, 14 (24%) were mutated, and 17 (46%) were not evaluable.
Patient disposition
Of the 43 patients in the monotherapy BID cohort, 1 was treated at the 10 mg dose level, 5 at the 20 mg level, 12 at the 40 mg level, 12 at the 80 mg level, 8 at the 150 mg level, and 5 at the 200 mg level. They had a median duration of drug exposure ranging from 25 weeks to 67 weeks.
Of the 11 patients in the monotherapy QD group, 5 were treated at the 120 mg dose level and 6 at the 200 mg level. Their drug exposure was a median of 26 weeks for those receiving 120 mg and 9.8 weeks for those receiving the 200 mg dose.
And the 5 patients in the ABL001-plus-nilotinib group had a median of 6.3 weeks of drug exposure.
“We had a remarkably low rate of discontinuation to date,” Dr Ottmann pointed out.
Ten patients discontinued therapy, all in the monotherapy BID group, 1 at 10 mg, 2 at 40 mg, 2 at 80 mg, 3 at 150 mg, and 2 at 200 mg.
Seven patients discontinued for adverse events. Two patients withdrew consent, and 1 patient in the 40 mg group had disease progression, which is “quite remarkable in a phase 1,” Dr Ottmann said.
Pharmacokinetic profile
ABL001 is rapidly absorbed in a median of 2 to 3 hours, and there is a dose-proportional increase in exposure following single and repeated dosing.
The drug has an approximately 2-fold or lower accumulation on repeated dosing and a short elimination half-life of 5 to 6 hours.
Safety
“We have excellent tolerability,” Dr Ottmann said, with a small number of grade 3/4 adverse events (AEs).
Grade 3/4 AEs considered to be drug-related were mostly associated with hematologic suppression. Four patients (7%) had thrombocytopenia, 4 (7%) neutropenia, 3 (5%) anemia, 4 (7%) lipase increase, and 1 (2%) hypercholesterolemia.
AEs of all grades suspected of being related to the study drug and occurring in 5% or more of patients included thrombocytopenia (19%), neutropenia (15%), anemia (10%), nausea/vomiting/diarrhea (29%), arthralgia/myalgia (20%), rash (17%), fatigue (15%), lipase increase (14%), headache (14%), pruritus (10%), dry skin (7%), hypophosphatemia (7%), and acute pancreatitis (5%).
“The pancreatitis was reversible upon interruption or discontinuation of the drug,” Dr Ottmann explained.
There were 5 dose-limiting toxicities. Two patients had grade 3 lipase elevation in the 40 mg BID and 200 mg QD cohorts. One patient had grade 2 myalgia/arthralgia at 80 mg BID, 1 patient had a grade 3 acute coronary event at 150 mg BID, and 1 patient had a grade 3 bronchospasm at 200 mg BID.
No deaths occurred on the study, and the dose escalation is still ongoing.
Response
Twenty-nine patients with 3 months or more of follow-up were evaluable for response.
Twelve patients, who at baseline had hematologic relapse, achieved complete hematologic response within 2 months, and 8 who had cytogenetic relapse at baseline achieved a complete cytogenetic response within 3 to 6 months.
Of the 29 patients who had molecular relapse at baseline, 10 (34.5%) achieved a molecular response within 6 months, 7 (24.1%) had 1 log or more reduction in BCR-ABL1, 9 (31.0%) had less than a log reduction, and 3 (10.3%) had no reduction.
“The obvious question from the preclinical data,” Dr Ottmann said, “is do the mutations respond?”
And ABL001 has shown clinical activity across TKI-resistant mutations, such as V299L, F317L, and Y253H.
“So to conclude,” he said, “we have a new class, a new therapeutic category of drug, ABL001, which is quite well tolerated in extremely heavily treated patients with CML. We do consider this a promising approach.”
The trial was sponsored by Novartis. ![]()
*Data in the abstract differ from the presentation.

ASH Annual Meeting
Photo courtesy of ASH
ORLANDO, FL—ABL001, an allosteric inhibitor of BCR-ABL1, has shown early evidence of single-agent activity in a multicenter, first-in-human, first-in-class trial of heavily treated patients with chronic myeloid leukemia (CML) that is resistant to or intolerant of prior tyrosine kinase inhibitors (TKIs), even at the lowest dose evaluated.
ABL001 and classical TKIs exhibit complementary mutation profiles, with ABL001 showing activity against TKI resistance mutations.
When combined with nilotinib in a mouse model of CML, ABL001 prevented the emergence of resistant disease even after treatment was discontinued.
“This produces a new therapeutic concept—that of allosteric inhibition,” said Oliver G. Ottmann, MD, of Cardiff University in the UK.
The ABL001 binding site is located in a region remote from the kinase domain and has the potential to combine with TKIs for greater pharmacologic control of BCR-ABL1.
“This obviously has the opportunity both for combining different treatments and for overcoming resistance to one or the other,” Dr Ottmann added.
Based on preliminary pharmacokinetic data and preclinical evidence, investigators proceeded to evaluate ABL001 in a phase 1 dose-escalation and dose-expansion study.
Their primary objective was to determine the maximum tolerated dose (MTD) in humans and the recommended dose for expansion (RDE). Secondary objectives were to evaluate the safety, tolerability, preliminary anti-CML activity, and pharmacokinetic and pharmacodynamic profile.
Dr Ottmann presented the findings during the 2015 ASH Annual Meeting as abstract 138.*
Study design
Patients received ABL001 orally as a single agent twice a day (BID) continuously until disease progression, unacceptable toxicity, consent withdrawal, or death.
The dose-escalation schema followed a Bayesian logistic regression model based on dose-limiting toxicities during cycle 1. Doses ranged from 10 mg to 200 mg BID.
A subsequent dose-expansion phase was planned to augment the data generated in the dose-escalation phase and to include patients with Ph-positive acute lymphoblastic leukemia resistant or intolerant to prior TKI therapy.
Dr Ottmann noted that there were 2 protocol amendments. The first amendment was made to include a once-daily (QD) dosing of ABL001 at 120 mg and 200 mg. The second amendment was made to evaluate the combination of 40 mg of ABL001 BID with nilotinib at 300 mg BID.
Inclusion/exclusion criteria
Patients had to be at least 18 years old with CML in chronic or accelerated phase. They had to have failed at least 2 prior TKIs or be intolerant of TKIs. Their performance status had to be 0–2.
Patients were excluded from the trial if they had an absolute neutrophil count less than 500/mm3, a platelet count less than 50,000/mm3, bilirubin level more than 1.5 x the upper limit of normal (ULN) or more than 3.0 x ULN in patients with Gilberts syndrome.
Their aspartate aminotransferase or alanine aminotransferase could not be above 3 x ULN, and creatinine could not be above 1.5 x ULN.
Patients were also excluded if they needed treatment with strong inhibitors or inducers of CYP3A4 or its substrates with narrow therapeutic index.
Patient demographics
Fifty-nine patients were enrolled on the trial at the time of the presentation and they had “typical” characteristics of patients at this stage, Dr Ottmann said.
Their median age was 56 (range, 23–78). Almost two-thirds (61%) were male and 39% female.
All but 1 patient had an ECOG performance status of 0, and patients had a median of 3.5 (range, 2–5) prior lines of therapy. Twenty-four patients (41%) had 2 prior TKIs, and 35 (59%) had 3 or more TKIs. Forty-five patients (76%) were resistant and 14 (24%) were intolerant to their prior TKI.
All but 1 patient had chronic phase CML, 18 (31%) were TKD nonmutated, 14 (24%) were mutated, and 17 (46%) were not evaluable.
Patient disposition
Of the 43 patients in the monotherapy BID cohort, 1 was treated at the 10 mg dose level, 5 at the 20 mg level, 12 at the 40 mg level, 12 at the 80 mg level, 8 at the 150 mg level, and 5 at the 200 mg level. They had a median duration of drug exposure ranging from 25 weeks to 67 weeks.
Of the 11 patients in the monotherapy QD group, 5 were treated at the 120 mg dose level and 6 at the 200 mg level. Their drug exposure was a median of 26 weeks for those receiving 120 mg and 9.8 weeks for those receiving the 200 mg dose.
And the 5 patients in the ABL001-plus-nilotinib group had a median of 6.3 weeks of drug exposure.
“We had a remarkably low rate of discontinuation to date,” Dr Ottmann pointed out.
Ten patients discontinued therapy, all in the monotherapy BID group, 1 at 10 mg, 2 at 40 mg, 2 at 80 mg, 3 at 150 mg, and 2 at 200 mg.
Seven patients discontinued for adverse events. Two patients withdrew consent, and 1 patient in the 40 mg group had disease progression, which is “quite remarkable in a phase 1,” Dr Ottmann said.
Pharmacokinetic profile
ABL001 is rapidly absorbed in a median of 2 to 3 hours, and there is a dose-proportional increase in exposure following single and repeated dosing.
The drug has an approximately 2-fold or lower accumulation on repeated dosing and a short elimination half-life of 5 to 6 hours.
Safety
“We have excellent tolerability,” Dr Ottmann said, with a small number of grade 3/4 adverse events (AEs).
Grade 3/4 AEs considered to be drug-related were mostly associated with hematologic suppression. Four patients (7%) had thrombocytopenia, 4 (7%) neutropenia, 3 (5%) anemia, 4 (7%) lipase increase, and 1 (2%) hypercholesterolemia.
AEs of all grades suspected of being related to the study drug and occurring in 5% or more of patients included thrombocytopenia (19%), neutropenia (15%), anemia (10%), nausea/vomiting/diarrhea (29%), arthralgia/myalgia (20%), rash (17%), fatigue (15%), lipase increase (14%), headache (14%), pruritus (10%), dry skin (7%), hypophosphatemia (7%), and acute pancreatitis (5%).
“The pancreatitis was reversible upon interruption or discontinuation of the drug,” Dr Ottmann explained.
There were 5 dose-limiting toxicities. Two patients had grade 3 lipase elevation in the 40 mg BID and 200 mg QD cohorts. One patient had grade 2 myalgia/arthralgia at 80 mg BID, 1 patient had a grade 3 acute coronary event at 150 mg BID, and 1 patient had a grade 3 bronchospasm at 200 mg BID.
No deaths occurred on the study, and the dose escalation is still ongoing.
Response
Twenty-nine patients with 3 months or more of follow-up were evaluable for response.
Twelve patients, who at baseline had hematologic relapse, achieved complete hematologic response within 2 months, and 8 who had cytogenetic relapse at baseline achieved a complete cytogenetic response within 3 to 6 months.
Of the 29 patients who had molecular relapse at baseline, 10 (34.5%) achieved a molecular response within 6 months, 7 (24.1%) had 1 log or more reduction in BCR-ABL1, 9 (31.0%) had less than a log reduction, and 3 (10.3%) had no reduction.
“The obvious question from the preclinical data,” Dr Ottmann said, “is do the mutations respond?”
And ABL001 has shown clinical activity across TKI-resistant mutations, such as V299L, F317L, and Y253H.
“So to conclude,” he said, “we have a new class, a new therapeutic category of drug, ABL001, which is quite well tolerated in extremely heavily treated patients with CML. We do consider this a promising approach.”
The trial was sponsored by Novartis. ![]()
*Data in the abstract differ from the presentation.

ASH Annual Meeting
Photo courtesy of ASH
ORLANDO, FL—ABL001, an allosteric inhibitor of BCR-ABL1, has shown early evidence of single-agent activity in a multicenter, first-in-human, first-in-class trial of heavily treated patients with chronic myeloid leukemia (CML) that is resistant to or intolerant of prior tyrosine kinase inhibitors (TKIs), even at the lowest dose evaluated.
ABL001 and classical TKIs exhibit complementary mutation profiles, with ABL001 showing activity against TKI resistance mutations.
When combined with nilotinib in a mouse model of CML, ABL001 prevented the emergence of resistant disease even after treatment was discontinued.
“This produces a new therapeutic concept—that of allosteric inhibition,” said Oliver G. Ottmann, MD, of Cardiff University in the UK.
The ABL001 binding site is located in a region remote from the kinase domain and has the potential to combine with TKIs for greater pharmacologic control of BCR-ABL1.
“This obviously has the opportunity both for combining different treatments and for overcoming resistance to one or the other,” Dr Ottmann added.
Based on preliminary pharmacokinetic data and preclinical evidence, investigators proceeded to evaluate ABL001 in a phase 1 dose-escalation and dose-expansion study.
Their primary objective was to determine the maximum tolerated dose (MTD) in humans and the recommended dose for expansion (RDE). Secondary objectives were to evaluate the safety, tolerability, preliminary anti-CML activity, and pharmacokinetic and pharmacodynamic profile.
Dr Ottmann presented the findings during the 2015 ASH Annual Meeting as abstract 138.*
Study design
Patients received ABL001 orally as a single agent twice a day (BID) continuously until disease progression, unacceptable toxicity, consent withdrawal, or death.
The dose-escalation schema followed a Bayesian logistic regression model based on dose-limiting toxicities during cycle 1. Doses ranged from 10 mg to 200 mg BID.
A subsequent dose-expansion phase was planned to augment the data generated in the dose-escalation phase and to include patients with Ph-positive acute lymphoblastic leukemia resistant or intolerant to prior TKI therapy.
Dr Ottmann noted that there were 2 protocol amendments. The first amendment was made to include a once-daily (QD) dosing of ABL001 at 120 mg and 200 mg. The second amendment was made to evaluate the combination of 40 mg of ABL001 BID with nilotinib at 300 mg BID.
Inclusion/exclusion criteria
Patients had to be at least 18 years old with CML in chronic or accelerated phase. They had to have failed at least 2 prior TKIs or be intolerant of TKIs. Their performance status had to be 0–2.
Patients were excluded from the trial if they had an absolute neutrophil count less than 500/mm3, a platelet count less than 50,000/mm3, bilirubin level more than 1.5 x the upper limit of normal (ULN) or more than 3.0 x ULN in patients with Gilberts syndrome.
Their aspartate aminotransferase or alanine aminotransferase could not be above 3 x ULN, and creatinine could not be above 1.5 x ULN.
Patients were also excluded if they needed treatment with strong inhibitors or inducers of CYP3A4 or its substrates with narrow therapeutic index.
Patient demographics
Fifty-nine patients were enrolled on the trial at the time of the presentation and they had “typical” characteristics of patients at this stage, Dr Ottmann said.
Their median age was 56 (range, 23–78). Almost two-thirds (61%) were male and 39% female.
All but 1 patient had an ECOG performance status of 0, and patients had a median of 3.5 (range, 2–5) prior lines of therapy. Twenty-four patients (41%) had 2 prior TKIs, and 35 (59%) had 3 or more TKIs. Forty-five patients (76%) were resistant and 14 (24%) were intolerant to their prior TKI.
All but 1 patient had chronic phase CML, 18 (31%) were TKD nonmutated, 14 (24%) were mutated, and 17 (46%) were not evaluable.
Patient disposition
Of the 43 patients in the monotherapy BID cohort, 1 was treated at the 10 mg dose level, 5 at the 20 mg level, 12 at the 40 mg level, 12 at the 80 mg level, 8 at the 150 mg level, and 5 at the 200 mg level. They had a median duration of drug exposure ranging from 25 weeks to 67 weeks.
Of the 11 patients in the monotherapy QD group, 5 were treated at the 120 mg dose level and 6 at the 200 mg level. Their drug exposure was a median of 26 weeks for those receiving 120 mg and 9.8 weeks for those receiving the 200 mg dose.
And the 5 patients in the ABL001-plus-nilotinib group had a median of 6.3 weeks of drug exposure.
“We had a remarkably low rate of discontinuation to date,” Dr Ottmann pointed out.
Ten patients discontinued therapy, all in the monotherapy BID group, 1 at 10 mg, 2 at 40 mg, 2 at 80 mg, 3 at 150 mg, and 2 at 200 mg.
Seven patients discontinued for adverse events. Two patients withdrew consent, and 1 patient in the 40 mg group had disease progression, which is “quite remarkable in a phase 1,” Dr Ottmann said.
Pharmacokinetic profile
ABL001 is rapidly absorbed in a median of 2 to 3 hours, and there is a dose-proportional increase in exposure following single and repeated dosing.
The drug has an approximately 2-fold or lower accumulation on repeated dosing and a short elimination half-life of 5 to 6 hours.
Safety
“We have excellent tolerability,” Dr Ottmann said, with a small number of grade 3/4 adverse events (AEs).
Grade 3/4 AEs considered to be drug-related were mostly associated with hematologic suppression. Four patients (7%) had thrombocytopenia, 4 (7%) neutropenia, 3 (5%) anemia, 4 (7%) lipase increase, and 1 (2%) hypercholesterolemia.
AEs of all grades suspected of being related to the study drug and occurring in 5% or more of patients included thrombocytopenia (19%), neutropenia (15%), anemia (10%), nausea/vomiting/diarrhea (29%), arthralgia/myalgia (20%), rash (17%), fatigue (15%), lipase increase (14%), headache (14%), pruritus (10%), dry skin (7%), hypophosphatemia (7%), and acute pancreatitis (5%).
“The pancreatitis was reversible upon interruption or discontinuation of the drug,” Dr Ottmann explained.
There were 5 dose-limiting toxicities. Two patients had grade 3 lipase elevation in the 40 mg BID and 200 mg QD cohorts. One patient had grade 2 myalgia/arthralgia at 80 mg BID, 1 patient had a grade 3 acute coronary event at 150 mg BID, and 1 patient had a grade 3 bronchospasm at 200 mg BID.
No deaths occurred on the study, and the dose escalation is still ongoing.
Response
Twenty-nine patients with 3 months or more of follow-up were evaluable for response.
Twelve patients, who at baseline had hematologic relapse, achieved complete hematologic response within 2 months, and 8 who had cytogenetic relapse at baseline achieved a complete cytogenetic response within 3 to 6 months.
Of the 29 patients who had molecular relapse at baseline, 10 (34.5%) achieved a molecular response within 6 months, 7 (24.1%) had 1 log or more reduction in BCR-ABL1, 9 (31.0%) had less than a log reduction, and 3 (10.3%) had no reduction.
“The obvious question from the preclinical data,” Dr Ottmann said, “is do the mutations respond?”
And ABL001 has shown clinical activity across TKI-resistant mutations, such as V299L, F317L, and Y253H.
“So to conclude,” he said, “we have a new class, a new therapeutic category of drug, ABL001, which is quite well tolerated in extremely heavily treated patients with CML. We do consider this a promising approach.”
The trial was sponsored by Novartis. ![]()
*Data in the abstract differ from the presentation.
CHMP recommends FVIII product for hemophilia A

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended BAY 81-8973, a recombinant factor VIII (FVIII) compound, for approval in the European Union.
BAY 81-8973 is an unmodified, full-length, recombinant FVIII compound intended for the treatment and prophylaxis of bleeding in hemophilia A patients of all ages.
The CHMP’s recommendation has been relayed to the European Commission, which is expected to make a decision regarding BAY 81-8973 in the coming weeks.
If approved, BAY 81-8973 will be marketed for the aforementioned indication in the 28 member countries of the European Union, as well as Iceland, Liechtenstein, and Norway.
The CHMP’s recommendation is based on results from the LEOPOLD trials, which were presented at the National Hemophilia Foundation’s 67th Annual Meeting last August.
LEOPOLD trials
The LEOPOLD Clinical Development Program consists of 3 multinational clinical trials designed to evaluate the pharmacokinetics, efficacy, and safety of BAY 81-8973 in subjects with severe hemophilia A (<1% FVIII:C).
LEOPOLD I is an open-label, cross-over, phase 3 study of males ages 12 to 65. The objectives were to demonstrate the efficacy and safety of BAY 81-8973 when used as prophylaxis, for the treatment of bleeding episodes, and for maintaining hemostasis during surgery. In LEOPOLD I, investigators assigned subjects to either the 2- or 3-times-weekly dosing regimens based on each patient’s phenotype, prior bleeding history and other factors.
LEOPOLD II is a randomized, cross-over, open-label trial conducted in male subjects ages 12 to 65. In this phase 3 study, 80 subjects were randomized to receive BAY 81-8973 either as a low-dose prophylaxis regimen (20-30 IU/kg; n=28) twice per week, high-dose prophylaxis (30-40 IU/kg; n=31) 3 times a week, or on-demand (n=21).
The primary objective was to demonstrate the superiority of prophylaxis over on-demand therapy, with the primary endpoint being bleeding frequency at 12 months.
LEOPOLD Kids is an open-label, non-randomized, phase 3 study. Part A is designed to evaluate the efficacy and safety of BAY 81-8973 for prophylaxis, treatment of bleeds, and surgical management in previously treated children at least 12 years of age with twice or 3 times per week or every other day prophylaxis regimens. Part B of the study, which involves previously untreated patients, is ongoing.
Bayer HealthCare AG has submitted marketing applications for BAY 81-8973 in the US and several other countries and is pursuing regulatory approvals worldwide. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended BAY 81-8973, a recombinant factor VIII (FVIII) compound, for approval in the European Union.
BAY 81-8973 is an unmodified, full-length, recombinant FVIII compound intended for the treatment and prophylaxis of bleeding in hemophilia A patients of all ages.
The CHMP’s recommendation has been relayed to the European Commission, which is expected to make a decision regarding BAY 81-8973 in the coming weeks.
If approved, BAY 81-8973 will be marketed for the aforementioned indication in the 28 member countries of the European Union, as well as Iceland, Liechtenstein, and Norway.
The CHMP’s recommendation is based on results from the LEOPOLD trials, which were presented at the National Hemophilia Foundation’s 67th Annual Meeting last August.
LEOPOLD trials
The LEOPOLD Clinical Development Program consists of 3 multinational clinical trials designed to evaluate the pharmacokinetics, efficacy, and safety of BAY 81-8973 in subjects with severe hemophilia A (<1% FVIII:C).
LEOPOLD I is an open-label, cross-over, phase 3 study of males ages 12 to 65. The objectives were to demonstrate the efficacy and safety of BAY 81-8973 when used as prophylaxis, for the treatment of bleeding episodes, and for maintaining hemostasis during surgery. In LEOPOLD I, investigators assigned subjects to either the 2- or 3-times-weekly dosing regimens based on each patient’s phenotype, prior bleeding history and other factors.
LEOPOLD II is a randomized, cross-over, open-label trial conducted in male subjects ages 12 to 65. In this phase 3 study, 80 subjects were randomized to receive BAY 81-8973 either as a low-dose prophylaxis regimen (20-30 IU/kg; n=28) twice per week, high-dose prophylaxis (30-40 IU/kg; n=31) 3 times a week, or on-demand (n=21).
The primary objective was to demonstrate the superiority of prophylaxis over on-demand therapy, with the primary endpoint being bleeding frequency at 12 months.
LEOPOLD Kids is an open-label, non-randomized, phase 3 study. Part A is designed to evaluate the efficacy and safety of BAY 81-8973 for prophylaxis, treatment of bleeds, and surgical management in previously treated children at least 12 years of age with twice or 3 times per week or every other day prophylaxis regimens. Part B of the study, which involves previously untreated patients, is ongoing.
Bayer HealthCare AG has submitted marketing applications for BAY 81-8973 in the US and several other countries and is pursuing regulatory approvals worldwide. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended BAY 81-8973, a recombinant factor VIII (FVIII) compound, for approval in the European Union.
BAY 81-8973 is an unmodified, full-length, recombinant FVIII compound intended for the treatment and prophylaxis of bleeding in hemophilia A patients of all ages.
The CHMP’s recommendation has been relayed to the European Commission, which is expected to make a decision regarding BAY 81-8973 in the coming weeks.
If approved, BAY 81-8973 will be marketed for the aforementioned indication in the 28 member countries of the European Union, as well as Iceland, Liechtenstein, and Norway.
The CHMP’s recommendation is based on results from the LEOPOLD trials, which were presented at the National Hemophilia Foundation’s 67th Annual Meeting last August.
LEOPOLD trials
The LEOPOLD Clinical Development Program consists of 3 multinational clinical trials designed to evaluate the pharmacokinetics, efficacy, and safety of BAY 81-8973 in subjects with severe hemophilia A (<1% FVIII:C).
LEOPOLD I is an open-label, cross-over, phase 3 study of males ages 12 to 65. The objectives were to demonstrate the efficacy and safety of BAY 81-8973 when used as prophylaxis, for the treatment of bleeding episodes, and for maintaining hemostasis during surgery. In LEOPOLD I, investigators assigned subjects to either the 2- or 3-times-weekly dosing regimens based on each patient’s phenotype, prior bleeding history and other factors.
LEOPOLD II is a randomized, cross-over, open-label trial conducted in male subjects ages 12 to 65. In this phase 3 study, 80 subjects were randomized to receive BAY 81-8973 either as a low-dose prophylaxis regimen (20-30 IU/kg; n=28) twice per week, high-dose prophylaxis (30-40 IU/kg; n=31) 3 times a week, or on-demand (n=21).
The primary objective was to demonstrate the superiority of prophylaxis over on-demand therapy, with the primary endpoint being bleeding frequency at 12 months.
LEOPOLD Kids is an open-label, non-randomized, phase 3 study. Part A is designed to evaluate the efficacy and safety of BAY 81-8973 for prophylaxis, treatment of bleeds, and surgical management in previously treated children at least 12 years of age with twice or 3 times per week or every other day prophylaxis regimens. Part B of the study, which involves previously untreated patients, is ongoing.
Bayer HealthCare AG has submitted marketing applications for BAY 81-8973 in the US and several other countries and is pursuing regulatory approvals worldwide. ![]()
Six factors predicted poor surgical outcomes in toxic colitis
Older age, female sex, preoperative chronic steroid use, azotemia, respiratory insufficiency, and coagulopathy all predicted death within a month of colectomy in patients with toxic colitis in the National Surgical Quality Improvement Project database.
The study is the largest to evaluate patients undergoing colectomy for toxic colitis, according to lead investigator Dr. Anand Dayama of the University of California, Davis, and his associates.
“In a case such as multiorgan failure with toxic colitis, the decision whether ... to operate can be an immensely difficult one,” Dr. Dayama and his colleagues wrote. “This study can help in making informed decisions in order to avoid the medicolegal ramifications of either performing an unnecessary procedure or withholding a lifesaving one,” they wrote in the American Journal of Surgery.
Surgical salvage remains the preferred treatment for patients with medically refractory toxic colitis. To assess outcomes in these patients, the researchers queried the National Surgical Quality Improvement Project database for relevant International Classification of Diseases, Ninth Revision codes between 2005 and 2012 (Am J Surg. 2015 Nov;210[5]:852-8).
The results underscored the severity of toxic colitis, they said. Of 189 patients, more than 26% died within 30 days after surgery, one in five developed postsurgical sepsis, about 17% had cardiovascular complications, 15% had wound complications, and 13% had renal complications. Furthermore, patients who were 70-80 years old had 3.5 times greater odds of dying, compared with younger patients (95% confidence interval, 1.0-12.8), and the increased likelihood of death rose by 22.2 when patients were older than 80 years (95% CI, 5.7-86.4).
Other baseline predictors of 30-day mortality included female sex (odds ratio, 4.1), blood urea nitrogen levels above 40 mg/dL (OR, 4.1), an international normalized ratio exceeding 2 (OR, 7.7), preoperative respiratory insufficiency (OR, 2.73), and a history of chronic steroid use (OR, 3.9), the researchers said. In addition, patients who died within 30 days after surgery were more likely than survivors to have undergone prolonged mechanical ventilation (56% vs. 27%), to have returned to the operating room (18% vs. 14%), to have acute renal failure (28% vs. 6%), or to have suffered a cardiac arrest that required cardiopulmonary resuscitation (18% vs. 7%). Survivors averaged about 2 fewer days in the hospital, compared with patients who died after surgery.
“The high morbidity and mortality of toxic colitis requires early and intensive medical management with IV [intravenous] steroids, antibiotics, decompressive maneuvers, and other resuscitative measures to treat the underlying cause,” they emphasized. “If there is no sign of improvement within 7 days or if there are any signs of deterioration, urgent surgical intervention should be considered.”
The link between female sex and mortality might reflect hormonal changes associated with menopause, but the study did not assess hormonal status or use of hormone therapy, the investigators noted. The association between chronic steroid use and postoperative death “is highly relevant” because long-term steroids are so often used in inflammatory bowel disease, they added. Clinical guidelines (Am J Gastroenterol. 2012 Feb;107:179-94) recommend that patients with acute severe ulcerative colitis proceed to second-line therapy or surgery if they do not respond to 3 days of intravenous steroids, because unnecessary delays can increase the risk of postoperative complications, they added.
The researchers reported no funding sources and had no disclosures.
Older age, female sex, preoperative chronic steroid use, azotemia, respiratory insufficiency, and coagulopathy all predicted death within a month of colectomy in patients with toxic colitis in the National Surgical Quality Improvement Project database.
The study is the largest to evaluate patients undergoing colectomy for toxic colitis, according to lead investigator Dr. Anand Dayama of the University of California, Davis, and his associates.
“In a case such as multiorgan failure with toxic colitis, the decision whether ... to operate can be an immensely difficult one,” Dr. Dayama and his colleagues wrote. “This study can help in making informed decisions in order to avoid the medicolegal ramifications of either performing an unnecessary procedure or withholding a lifesaving one,” they wrote in the American Journal of Surgery.
Surgical salvage remains the preferred treatment for patients with medically refractory toxic colitis. To assess outcomes in these patients, the researchers queried the National Surgical Quality Improvement Project database for relevant International Classification of Diseases, Ninth Revision codes between 2005 and 2012 (Am J Surg. 2015 Nov;210[5]:852-8).
The results underscored the severity of toxic colitis, they said. Of 189 patients, more than 26% died within 30 days after surgery, one in five developed postsurgical sepsis, about 17% had cardiovascular complications, 15% had wound complications, and 13% had renal complications. Furthermore, patients who were 70-80 years old had 3.5 times greater odds of dying, compared with younger patients (95% confidence interval, 1.0-12.8), and the increased likelihood of death rose by 22.2 when patients were older than 80 years (95% CI, 5.7-86.4).
Other baseline predictors of 30-day mortality included female sex (odds ratio, 4.1), blood urea nitrogen levels above 40 mg/dL (OR, 4.1), an international normalized ratio exceeding 2 (OR, 7.7), preoperative respiratory insufficiency (OR, 2.73), and a history of chronic steroid use (OR, 3.9), the researchers said. In addition, patients who died within 30 days after surgery were more likely than survivors to have undergone prolonged mechanical ventilation (56% vs. 27%), to have returned to the operating room (18% vs. 14%), to have acute renal failure (28% vs. 6%), or to have suffered a cardiac arrest that required cardiopulmonary resuscitation (18% vs. 7%). Survivors averaged about 2 fewer days in the hospital, compared with patients who died after surgery.
“The high morbidity and mortality of toxic colitis requires early and intensive medical management with IV [intravenous] steroids, antibiotics, decompressive maneuvers, and other resuscitative measures to treat the underlying cause,” they emphasized. “If there is no sign of improvement within 7 days or if there are any signs of deterioration, urgent surgical intervention should be considered.”
The link between female sex and mortality might reflect hormonal changes associated with menopause, but the study did not assess hormonal status or use of hormone therapy, the investigators noted. The association between chronic steroid use and postoperative death “is highly relevant” because long-term steroids are so often used in inflammatory bowel disease, they added. Clinical guidelines (Am J Gastroenterol. 2012 Feb;107:179-94) recommend that patients with acute severe ulcerative colitis proceed to second-line therapy or surgery if they do not respond to 3 days of intravenous steroids, because unnecessary delays can increase the risk of postoperative complications, they added.
The researchers reported no funding sources and had no disclosures.
Older age, female sex, preoperative chronic steroid use, azotemia, respiratory insufficiency, and coagulopathy all predicted death within a month of colectomy in patients with toxic colitis in the National Surgical Quality Improvement Project database.
The study is the largest to evaluate patients undergoing colectomy for toxic colitis, according to lead investigator Dr. Anand Dayama of the University of California, Davis, and his associates.
“In a case such as multiorgan failure with toxic colitis, the decision whether ... to operate can be an immensely difficult one,” Dr. Dayama and his colleagues wrote. “This study can help in making informed decisions in order to avoid the medicolegal ramifications of either performing an unnecessary procedure or withholding a lifesaving one,” they wrote in the American Journal of Surgery.
Surgical salvage remains the preferred treatment for patients with medically refractory toxic colitis. To assess outcomes in these patients, the researchers queried the National Surgical Quality Improvement Project database for relevant International Classification of Diseases, Ninth Revision codes between 2005 and 2012 (Am J Surg. 2015 Nov;210[5]:852-8).
The results underscored the severity of toxic colitis, they said. Of 189 patients, more than 26% died within 30 days after surgery, one in five developed postsurgical sepsis, about 17% had cardiovascular complications, 15% had wound complications, and 13% had renal complications. Furthermore, patients who were 70-80 years old had 3.5 times greater odds of dying, compared with younger patients (95% confidence interval, 1.0-12.8), and the increased likelihood of death rose by 22.2 when patients were older than 80 years (95% CI, 5.7-86.4).
Other baseline predictors of 30-day mortality included female sex (odds ratio, 4.1), blood urea nitrogen levels above 40 mg/dL (OR, 4.1), an international normalized ratio exceeding 2 (OR, 7.7), preoperative respiratory insufficiency (OR, 2.73), and a history of chronic steroid use (OR, 3.9), the researchers said. In addition, patients who died within 30 days after surgery were more likely than survivors to have undergone prolonged mechanical ventilation (56% vs. 27%), to have returned to the operating room (18% vs. 14%), to have acute renal failure (28% vs. 6%), or to have suffered a cardiac arrest that required cardiopulmonary resuscitation (18% vs. 7%). Survivors averaged about 2 fewer days in the hospital, compared with patients who died after surgery.
“The high morbidity and mortality of toxic colitis requires early and intensive medical management with IV [intravenous] steroids, antibiotics, decompressive maneuvers, and other resuscitative measures to treat the underlying cause,” they emphasized. “If there is no sign of improvement within 7 days or if there are any signs of deterioration, urgent surgical intervention should be considered.”
The link between female sex and mortality might reflect hormonal changes associated with menopause, but the study did not assess hormonal status or use of hormone therapy, the investigators noted. The association between chronic steroid use and postoperative death “is highly relevant” because long-term steroids are so often used in inflammatory bowel disease, they added. Clinical guidelines (Am J Gastroenterol. 2012 Feb;107:179-94) recommend that patients with acute severe ulcerative colitis proceed to second-line therapy or surgery if they do not respond to 3 days of intravenous steroids, because unnecessary delays can increase the risk of postoperative complications, they added.
The researchers reported no funding sources and had no disclosures.
FROM THE AMERICAN JOURNAL OF SURGERY
Key clinical point: Older age, female sex, preoperative azotemia, chronic steroid use, preoperative respiratory failure, and coagulopathy predicted adverse surgical outcomes in patients with toxic colitis.
Major finding: Odds ratios for these factors in the multivariate model ranged from 2.7 (preoperative respiratory failure) to 22.2 (age older than 80 years).
Data source: A multicenter prospective analysis of data from the National Surgical Quality Improvement Project.
Disclosures: The investigators reported no funding sources and had no disclosures.
Prescribing a winning home-exercise plan for PAD patients
CHICAGO – Following a few simple steps can help patients with peripheral artery disease (PAD) get off on the right foot with a home-based exercise program.
“There is growing evidence that PAD patients can walk for exercise at home and improve their walking performance,” Dr. Mary McDermott said at a symposium on vascular surgery sponsored by Northwestern University.
Getting them to do so, however, can be challenging. Patients with peripheral artery disease have greater functional impairment and are at higher risk for cardiovascular disease than is the general population. They also frequently limit their activity to avoid leg problems, as their PAD progresses.
“It’s hard enough to get patients without peripheral artery disease to exercise. As a general internist, I’m well aware of that,” she said. “It’s even more difficult when walking is so painful and uncomfortable.”
At present, the American College of Cardiology/American Heart Association practice guidelines for the management of patients with PAD do not recommend advising patients with PAD to go home and walk.
The guidelines were published in 2005, however, and since 2011, three of four randomized clinical trials of home-based exercise programs have shown a significant gain in walking endurance in patients with PAD, Dr. McDermott of Northwestern University in Chicago, observed.
The most hands-off of these trials showed that patients with symptomatic PAD randomized to a supervised exercise program did the best at treadmill walking, but the home-exercise group who received a step monitor and in-person feedback just once per month did significantly better than did controls assigned light resistance training. Moreover, the home-exercise group had greater change in 6-minute walk distances than either of the other groups (J Am Heart Assoc. 2014 Oct;3[5]:e001107).
“I think the reason for this is that the home-based group was walking outside or perhaps in a mall and getting better at walking over ground,” Dr. McDermott. “The treadmill group was walking only on the treadmill.”
Supervised treadmill exercise may seem like an easier prescription to write, but it faces two major barriers, she said. Most medical insurers, including Medicare, do not pay for supervised exercise for people with PAD and intermittent claudication, and most PAD patients don’t participate. The burden of traveling to an exercise center three times weekly, week after week, to participate can be overwhelming.
“For all of these reasons, we really need to develop home-based exercise programs that work,” Dr. McDermott said.
Based on the successful trials, home-based programs should include monitoring, group support, and goal setting. Dr. McDermott and her colleagues added cognitive-behavioral therapy to group support in the Group Oriented Arterial Leg Study (GOALS), resulting in significant improvement in 6-minute walk distance at 6 months, physical activity over 7 days, and self-perception of walking endurance and speed among home walkers (JAMA. 2013 Jul 3;310[1]:57-65).
Before embarking on any home-exercise program, all patients with PAD should undergo a baseline cardiac stress test to rule out coronary artery ischemia, she cautioned. This also serves to identify any coronary ischemia that may develop during the new walking program.
Once this is performed, Dr. McDermott recommends clinicians:
• Advise patients to walk 5 days per week.
“This may seem like a lot, but it’s important to have them see this as part of their daily routine; just something they get in the habit of doing,” she said.
• Start with 10-15 minutes of walking per exercise session. Tailor the program to the individual patient.
• Walk to maximal leg pain or onset of ischemic pain. Stopping to rest is acceptable.
“A lot of patients, I find, have questions, “ ‘I can’t do this.’ ‘How can this be beneficial?’ So just letting them know that if they just walk and stop, walk and stop, and start with just 10 minutes of that and increase this over time, they really can see improvement,” Dr. McDermott said.
• Increase the walk time by 5 minutes each week.
Increase the duration until the patient is walking at least 30 minutes per session and preferably 45-50 minutes per session, excluding rest periods, she said.
• Advise patients to write down their walking goals.
Specify where they will walk, when they will walk, and the duration of walking to improve compliance, which can slip following hospitalizations or when patients experience acute illness.
• Have patients self-monitor, but also check in with someone for support.
“It doesn’t have to be a nurse,” Dr. McDermott said. “In our studies, we’ve used bachelor’s degree-level people, but told them what to look for. They can do this and the patient feels there is someone they’re accountable to.”
Dr. McDermott and her colleagues are testing the boundaries of support in the ongoing HONOR trial, which includes four weekly visits to an exercise center in phase I to meet the telephone coach, learn to use a Fitbit monitor, and learn the behavioral skills necessary for long-term adherence. Phase II, however, is entirely home based and includes only Fitbit self-monitoring, regular telephone calls from the coach for feedback, use of the study website, and optional group telephone calls.
The bottom line with any program is for patients to understand it must be indefinite to maintain improvement.
“Unfortunately, if they don’t stick with it, they will slide back,” she cautioned.
Dr. McDermott reported research funding from the National Institutes of Health, the Patient-Centered Outcomes Research Institute (PCORI), and Novartis.
CHICAGO – Following a few simple steps can help patients with peripheral artery disease (PAD) get off on the right foot with a home-based exercise program.
“There is growing evidence that PAD patients can walk for exercise at home and improve their walking performance,” Dr. Mary McDermott said at a symposium on vascular surgery sponsored by Northwestern University.
Getting them to do so, however, can be challenging. Patients with peripheral artery disease have greater functional impairment and are at higher risk for cardiovascular disease than is the general population. They also frequently limit their activity to avoid leg problems, as their PAD progresses.
“It’s hard enough to get patients without peripheral artery disease to exercise. As a general internist, I’m well aware of that,” she said. “It’s even more difficult when walking is so painful and uncomfortable.”
At present, the American College of Cardiology/American Heart Association practice guidelines for the management of patients with PAD do not recommend advising patients with PAD to go home and walk.
The guidelines were published in 2005, however, and since 2011, three of four randomized clinical trials of home-based exercise programs have shown a significant gain in walking endurance in patients with PAD, Dr. McDermott of Northwestern University in Chicago, observed.
The most hands-off of these trials showed that patients with symptomatic PAD randomized to a supervised exercise program did the best at treadmill walking, but the home-exercise group who received a step monitor and in-person feedback just once per month did significantly better than did controls assigned light resistance training. Moreover, the home-exercise group had greater change in 6-minute walk distances than either of the other groups (J Am Heart Assoc. 2014 Oct;3[5]:e001107).
“I think the reason for this is that the home-based group was walking outside or perhaps in a mall and getting better at walking over ground,” Dr. McDermott. “The treadmill group was walking only on the treadmill.”
Supervised treadmill exercise may seem like an easier prescription to write, but it faces two major barriers, she said. Most medical insurers, including Medicare, do not pay for supervised exercise for people with PAD and intermittent claudication, and most PAD patients don’t participate. The burden of traveling to an exercise center three times weekly, week after week, to participate can be overwhelming.
“For all of these reasons, we really need to develop home-based exercise programs that work,” Dr. McDermott said.
Based on the successful trials, home-based programs should include monitoring, group support, and goal setting. Dr. McDermott and her colleagues added cognitive-behavioral therapy to group support in the Group Oriented Arterial Leg Study (GOALS), resulting in significant improvement in 6-minute walk distance at 6 months, physical activity over 7 days, and self-perception of walking endurance and speed among home walkers (JAMA. 2013 Jul 3;310[1]:57-65).
Before embarking on any home-exercise program, all patients with PAD should undergo a baseline cardiac stress test to rule out coronary artery ischemia, she cautioned. This also serves to identify any coronary ischemia that may develop during the new walking program.
Once this is performed, Dr. McDermott recommends clinicians:
• Advise patients to walk 5 days per week.
“This may seem like a lot, but it’s important to have them see this as part of their daily routine; just something they get in the habit of doing,” she said.
• Start with 10-15 minutes of walking per exercise session. Tailor the program to the individual patient.
• Walk to maximal leg pain or onset of ischemic pain. Stopping to rest is acceptable.
“A lot of patients, I find, have questions, “ ‘I can’t do this.’ ‘How can this be beneficial?’ So just letting them know that if they just walk and stop, walk and stop, and start with just 10 minutes of that and increase this over time, they really can see improvement,” Dr. McDermott said.
• Increase the walk time by 5 minutes each week.
Increase the duration until the patient is walking at least 30 minutes per session and preferably 45-50 minutes per session, excluding rest periods, she said.
• Advise patients to write down their walking goals.
Specify where they will walk, when they will walk, and the duration of walking to improve compliance, which can slip following hospitalizations or when patients experience acute illness.
• Have patients self-monitor, but also check in with someone for support.
“It doesn’t have to be a nurse,” Dr. McDermott said. “In our studies, we’ve used bachelor’s degree-level people, but told them what to look for. They can do this and the patient feels there is someone they’re accountable to.”
Dr. McDermott and her colleagues are testing the boundaries of support in the ongoing HONOR trial, which includes four weekly visits to an exercise center in phase I to meet the telephone coach, learn to use a Fitbit monitor, and learn the behavioral skills necessary for long-term adherence. Phase II, however, is entirely home based and includes only Fitbit self-monitoring, regular telephone calls from the coach for feedback, use of the study website, and optional group telephone calls.
The bottom line with any program is for patients to understand it must be indefinite to maintain improvement.
“Unfortunately, if they don’t stick with it, they will slide back,” she cautioned.
Dr. McDermott reported research funding from the National Institutes of Health, the Patient-Centered Outcomes Research Institute (PCORI), and Novartis.
CHICAGO – Following a few simple steps can help patients with peripheral artery disease (PAD) get off on the right foot with a home-based exercise program.
“There is growing evidence that PAD patients can walk for exercise at home and improve their walking performance,” Dr. Mary McDermott said at a symposium on vascular surgery sponsored by Northwestern University.
Getting them to do so, however, can be challenging. Patients with peripheral artery disease have greater functional impairment and are at higher risk for cardiovascular disease than is the general population. They also frequently limit their activity to avoid leg problems, as their PAD progresses.
“It’s hard enough to get patients without peripheral artery disease to exercise. As a general internist, I’m well aware of that,” she said. “It’s even more difficult when walking is so painful and uncomfortable.”
At present, the American College of Cardiology/American Heart Association practice guidelines for the management of patients with PAD do not recommend advising patients with PAD to go home and walk.
The guidelines were published in 2005, however, and since 2011, three of four randomized clinical trials of home-based exercise programs have shown a significant gain in walking endurance in patients with PAD, Dr. McDermott of Northwestern University in Chicago, observed.
The most hands-off of these trials showed that patients with symptomatic PAD randomized to a supervised exercise program did the best at treadmill walking, but the home-exercise group who received a step monitor and in-person feedback just once per month did significantly better than did controls assigned light resistance training. Moreover, the home-exercise group had greater change in 6-minute walk distances than either of the other groups (J Am Heart Assoc. 2014 Oct;3[5]:e001107).
“I think the reason for this is that the home-based group was walking outside or perhaps in a mall and getting better at walking over ground,” Dr. McDermott. “The treadmill group was walking only on the treadmill.”
Supervised treadmill exercise may seem like an easier prescription to write, but it faces two major barriers, she said. Most medical insurers, including Medicare, do not pay for supervised exercise for people with PAD and intermittent claudication, and most PAD patients don’t participate. The burden of traveling to an exercise center three times weekly, week after week, to participate can be overwhelming.
“For all of these reasons, we really need to develop home-based exercise programs that work,” Dr. McDermott said.
Based on the successful trials, home-based programs should include monitoring, group support, and goal setting. Dr. McDermott and her colleagues added cognitive-behavioral therapy to group support in the Group Oriented Arterial Leg Study (GOALS), resulting in significant improvement in 6-minute walk distance at 6 months, physical activity over 7 days, and self-perception of walking endurance and speed among home walkers (JAMA. 2013 Jul 3;310[1]:57-65).
Before embarking on any home-exercise program, all patients with PAD should undergo a baseline cardiac stress test to rule out coronary artery ischemia, she cautioned. This also serves to identify any coronary ischemia that may develop during the new walking program.
Once this is performed, Dr. McDermott recommends clinicians:
• Advise patients to walk 5 days per week.
“This may seem like a lot, but it’s important to have them see this as part of their daily routine; just something they get in the habit of doing,” she said.
• Start with 10-15 minutes of walking per exercise session. Tailor the program to the individual patient.
• Walk to maximal leg pain or onset of ischemic pain. Stopping to rest is acceptable.
“A lot of patients, I find, have questions, “ ‘I can’t do this.’ ‘How can this be beneficial?’ So just letting them know that if they just walk and stop, walk and stop, and start with just 10 minutes of that and increase this over time, they really can see improvement,” Dr. McDermott said.
• Increase the walk time by 5 minutes each week.
Increase the duration until the patient is walking at least 30 minutes per session and preferably 45-50 minutes per session, excluding rest periods, she said.
• Advise patients to write down their walking goals.
Specify where they will walk, when they will walk, and the duration of walking to improve compliance, which can slip following hospitalizations or when patients experience acute illness.
• Have patients self-monitor, but also check in with someone for support.
“It doesn’t have to be a nurse,” Dr. McDermott said. “In our studies, we’ve used bachelor’s degree-level people, but told them what to look for. They can do this and the patient feels there is someone they’re accountable to.”
Dr. McDermott and her colleagues are testing the boundaries of support in the ongoing HONOR trial, which includes four weekly visits to an exercise center in phase I to meet the telephone coach, learn to use a Fitbit monitor, and learn the behavioral skills necessary for long-term adherence. Phase II, however, is entirely home based and includes only Fitbit self-monitoring, regular telephone calls from the coach for feedback, use of the study website, and optional group telephone calls.
The bottom line with any program is for patients to understand it must be indefinite to maintain improvement.
“Unfortunately, if they don’t stick with it, they will slide back,” she cautioned.
Dr. McDermott reported research funding from the National Institutes of Health, the Patient-Centered Outcomes Research Institute (PCORI), and Novartis.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
A microfluidic biochip for blood cell counts

Researchers say they have created a biosensor capable of counting blood cells electrically using only a drop of blood.
The microfluidic device can measure red blood cell, platelet, and white blood cell counts using as little as 11 µL of blood.
The device electrically counts the different types of blood cells based on their size and membrane properties.
To count leukocyte and its differentials, red blood cells are selectively lysed, and the remaining white blood cells are individually counted. Specific cells like neutrophils can be counted using multi-frequency analysis, which probes the membrane properties of the cells.
For red blood cells and platelets, 1 µL of whole blood is diluted with PBS on-chip, and the cells are counted electrically. The total time for measurement is under 20 minutes.
The researchers described this device in TECHNOLOGY.
“Our biosensor exhibits the potential to improve patient care in a spectrum of settings,” said Rashid Bashir, PhD, of the University of Illinois at Urbana-Champaign.
He noted that the device could be particularly useful in resource-limited settings where laboratory tests are often inaccessible due to costs, poor prevalence of laboratory facilities, and the difficulty of follow-up upon receiving results that take days to process.
“There exists a huge potential to translate our biosensor commercially for blood cell count applications,” added Umer Hassan, PhD, of the University of Illinois at Urbana-Champaign.
“The translation of our technology will result in minimal to no experience requirement for device operation. Even patients can perform the test at the comfort of their home and share the results with their primary care physicians via electronic means too.”
“The technology is scalable, and, in future, we plan to apply it to many other potential applications in the areas of animal diagnostics, blood transfusion analysis, ER/ICU applications, and blood cell counting for chemotherapy management,” Dr Bashir said.
The researchers are now working to further develop a portable prototype of the cell counter.
“The cartridges will be disposable and the size of a credit card,” Dr Umer said. “The base unit or the reader will be portable and possibly hand-held. Our technology has the potential to reduce the cost of the test to less than $10, as compared to $100 or more currently charged.” ![]()

Researchers say they have created a biosensor capable of counting blood cells electrically using only a drop of blood.
The microfluidic device can measure red blood cell, platelet, and white blood cell counts using as little as 11 µL of blood.
The device electrically counts the different types of blood cells based on their size and membrane properties.
To count leukocyte and its differentials, red blood cells are selectively lysed, and the remaining white blood cells are individually counted. Specific cells like neutrophils can be counted using multi-frequency analysis, which probes the membrane properties of the cells.
For red blood cells and platelets, 1 µL of whole blood is diluted with PBS on-chip, and the cells are counted electrically. The total time for measurement is under 20 minutes.
The researchers described this device in TECHNOLOGY.
“Our biosensor exhibits the potential to improve patient care in a spectrum of settings,” said Rashid Bashir, PhD, of the University of Illinois at Urbana-Champaign.
He noted that the device could be particularly useful in resource-limited settings where laboratory tests are often inaccessible due to costs, poor prevalence of laboratory facilities, and the difficulty of follow-up upon receiving results that take days to process.
“There exists a huge potential to translate our biosensor commercially for blood cell count applications,” added Umer Hassan, PhD, of the University of Illinois at Urbana-Champaign.
“The translation of our technology will result in minimal to no experience requirement for device operation. Even patients can perform the test at the comfort of their home and share the results with their primary care physicians via electronic means too.”
“The technology is scalable, and, in future, we plan to apply it to many other potential applications in the areas of animal diagnostics, blood transfusion analysis, ER/ICU applications, and blood cell counting for chemotherapy management,” Dr Bashir said.
The researchers are now working to further develop a portable prototype of the cell counter.
“The cartridges will be disposable and the size of a credit card,” Dr Umer said. “The base unit or the reader will be portable and possibly hand-held. Our technology has the potential to reduce the cost of the test to less than $10, as compared to $100 or more currently charged.” ![]()

Researchers say they have created a biosensor capable of counting blood cells electrically using only a drop of blood.
The microfluidic device can measure red blood cell, platelet, and white blood cell counts using as little as 11 µL of blood.
The device electrically counts the different types of blood cells based on their size and membrane properties.
To count leukocyte and its differentials, red blood cells are selectively lysed, and the remaining white blood cells are individually counted. Specific cells like neutrophils can be counted using multi-frequency analysis, which probes the membrane properties of the cells.
For red blood cells and platelets, 1 µL of whole blood is diluted with PBS on-chip, and the cells are counted electrically. The total time for measurement is under 20 minutes.
The researchers described this device in TECHNOLOGY.
“Our biosensor exhibits the potential to improve patient care in a spectrum of settings,” said Rashid Bashir, PhD, of the University of Illinois at Urbana-Champaign.
He noted that the device could be particularly useful in resource-limited settings where laboratory tests are often inaccessible due to costs, poor prevalence of laboratory facilities, and the difficulty of follow-up upon receiving results that take days to process.
“There exists a huge potential to translate our biosensor commercially for blood cell count applications,” added Umer Hassan, PhD, of the University of Illinois at Urbana-Champaign.
“The translation of our technology will result in minimal to no experience requirement for device operation. Even patients can perform the test at the comfort of their home and share the results with their primary care physicians via electronic means too.”
“The technology is scalable, and, in future, we plan to apply it to many other potential applications in the areas of animal diagnostics, blood transfusion analysis, ER/ICU applications, and blood cell counting for chemotherapy management,” Dr Bashir said.
The researchers are now working to further develop a portable prototype of the cell counter.
“The cartridges will be disposable and the size of a credit card,” Dr Umer said. “The base unit or the reader will be portable and possibly hand-held. Our technology has the potential to reduce the cost of the test to less than $10, as compared to $100 or more currently charged.” ![]()
Myeloma drug could treat sickle cell disease, team says

and a normal one
Image by Betty Pace
Preclinical research suggests a drug used to treat multiple myeloma (MM) might also prove effective in the treatment of sickle cell disease (SCD).
Researchers say this study is the first to reveal how the drug, pomalidomide, increases the production of fetal hemoglobin, which is known to interfere with the sickling of red blood cells.
“We knew the drug would make fetal hemoglobin, but we didn’t know to what extent or how,” said Lionel Blanc, PhD, of the Feinstein Institute for Medical Research in Manhasset, New York.
He and his colleagues reported their findings in Blood.
The researchers’ in vitro experiments revealed that pomalidomide reverses γ-globin silencing during adult erythropoiesis, and the drug delays the maturation of early erythroid precursors without impairing terminal differentiation.
The team also found that pomalidomide selectively targets BCL11A and SOX6 to induce γ-globin synthesis, the drug’s mechanism of action during erythropoiesis is independent of IKZF1 degradation, and pomalidomide partially reprograms adult erythroid progenitors to a fetal-like state.
Finally, the researchers discovered that pomalidomide’s mechanism of action is conserved in cells from SCD patients, and treatment with pomalidomide leads to γ-globin production in MM patients.
“We can also say something else—that hydroxyurea, the only FDA-approved drug for sickle cell anemia, was less effective than pomalidomide and appeared to act through a different mechanism of action,” Dr Blanc said.
“The current therapy is good, but not everyone responds equally to hydroxyurea, and what we hope with pomalidomide is to improve this.”
Dr Blanc and his colleagues plan to launch a clinical trial in the near future to test pomalidomide in young adults with SCD. ![]()

and a normal one
Image by Betty Pace
Preclinical research suggests a drug used to treat multiple myeloma (MM) might also prove effective in the treatment of sickle cell disease (SCD).
Researchers say this study is the first to reveal how the drug, pomalidomide, increases the production of fetal hemoglobin, which is known to interfere with the sickling of red blood cells.
“We knew the drug would make fetal hemoglobin, but we didn’t know to what extent or how,” said Lionel Blanc, PhD, of the Feinstein Institute for Medical Research in Manhasset, New York.
He and his colleagues reported their findings in Blood.
The researchers’ in vitro experiments revealed that pomalidomide reverses γ-globin silencing during adult erythropoiesis, and the drug delays the maturation of early erythroid precursors without impairing terminal differentiation.
The team also found that pomalidomide selectively targets BCL11A and SOX6 to induce γ-globin synthesis, the drug’s mechanism of action during erythropoiesis is independent of IKZF1 degradation, and pomalidomide partially reprograms adult erythroid progenitors to a fetal-like state.
Finally, the researchers discovered that pomalidomide’s mechanism of action is conserved in cells from SCD patients, and treatment with pomalidomide leads to γ-globin production in MM patients.
“We can also say something else—that hydroxyurea, the only FDA-approved drug for sickle cell anemia, was less effective than pomalidomide and appeared to act through a different mechanism of action,” Dr Blanc said.
“The current therapy is good, but not everyone responds equally to hydroxyurea, and what we hope with pomalidomide is to improve this.”
Dr Blanc and his colleagues plan to launch a clinical trial in the near future to test pomalidomide in young adults with SCD. ![]()

and a normal one
Image by Betty Pace
Preclinical research suggests a drug used to treat multiple myeloma (MM) might also prove effective in the treatment of sickle cell disease (SCD).
Researchers say this study is the first to reveal how the drug, pomalidomide, increases the production of fetal hemoglobin, which is known to interfere with the sickling of red blood cells.
“We knew the drug would make fetal hemoglobin, but we didn’t know to what extent or how,” said Lionel Blanc, PhD, of the Feinstein Institute for Medical Research in Manhasset, New York.
He and his colleagues reported their findings in Blood.
The researchers’ in vitro experiments revealed that pomalidomide reverses γ-globin silencing during adult erythropoiesis, and the drug delays the maturation of early erythroid precursors without impairing terminal differentiation.
The team also found that pomalidomide selectively targets BCL11A and SOX6 to induce γ-globin synthesis, the drug’s mechanism of action during erythropoiesis is independent of IKZF1 degradation, and pomalidomide partially reprograms adult erythroid progenitors to a fetal-like state.
Finally, the researchers discovered that pomalidomide’s mechanism of action is conserved in cells from SCD patients, and treatment with pomalidomide leads to γ-globin production in MM patients.
“We can also say something else—that hydroxyurea, the only FDA-approved drug for sickle cell anemia, was less effective than pomalidomide and appeared to act through a different mechanism of action,” Dr Blanc said.
“The current therapy is good, but not everyone responds equally to hydroxyurea, and what we hope with pomalidomide is to improve this.”
Dr Blanc and his colleagues plan to launch a clinical trial in the near future to test pomalidomide in young adults with SCD. ![]()
2014 sets U.S. record for drug overdose deaths
In 2014, 47,055 people in the United States died from drug overdoses – more deaths than attributed to this cause in any previous year on record, according to data from the National Vital Statistics System.
Opioids, primarily prescription pain relievers and heroin, were the main drugs associated with overdose deaths. In 2014, opioids were involved in 28,647 deaths, or 61% of all drug overdose deaths, Rose A. Rudd of the Centers for Disease Control and Prevention and her colleagues wrote (MMWR. 2015 Dec 18;64[Early release]:1-5).
The rate of opioid overdoses has tripled since 2000; the 15-year trend data implicate prescription opioid pain relievers and a recent surge in illicit opioid overdose deaths, driven largely by heroin.
From 2013 to 2014, synthetic opioids other than methadone (e.g., fentanyl and tramadol) drove the largest increase in the rate of drug overdose deaths. The rate nearly doubled from 1 per 100,000 persons to 1.8 per 100,000 persons. In 2014, the rate of drug overdose deaths involving natural and semisynthetic opioids (for example, morphine, oxycodone, and hydrocodone) was 3.8 per 100,000. The rate of drug overdose deaths involving methadone, a synthetic opioid classified separately from other synthetic opioids, was similar in 2013 and 2014.
The five states with the highest rates of drug overdose deaths in 2014 were West Virginia (35.5 deaths per 100,000), New Mexico (27.3), New Hampshire (26.2), Kentucky (24.7), and Ohio (24.6).
States with statistically significant increases in the rate of drug overdose deaths from 2013 to 2014 included Alabama, Georgia, Illinois, Indiana, Maine, Maryland, Massachusetts, Michigan, New Hampshire, New Mexico, North Dakota, Ohio, Pennsylvania, and Virginia.
The rates were noted in all adult age groups. From 2013 to 2014, statistically significant increases in drug overdose death rates were seen for both males and females, persons aged 25-34 years, 35-44 years, 55-64 years, and 65 years and older. Based on ethnicity, increases were seen in non-Hispanic whites and non-Hispanic blacks. Based on residency, increases were most common in the Northeast, Midwest, and South.
The authors noted three limitations of the data: First, the substances tested for and circumstances under which toxicologic tests are performed vary by jurisdiction; in 2013 and 2014, 22% and 19% of drug overdose deaths, respectively, did not include information on the death certificate about the specific types of drugs involved, and the percent of overdose deaths with specific drugs identified on the death certificate varies widely by state. Second, an increase from 2013 to 2014 in reporting of specific drugs involved in drug overdose deaths might have contributed to some of the observed increases in drug overdose death rates involving different types of opioids. Finally, some heroin deaths might be misclassified or underreported because morphine and heroin are similarly metabolized.
Efforts to encourage safer prescribing of opioid pain relievers should be strengthened, according to the authors. CDC has developed a draft guideline for the prescribing of opioids for chronic pain to address this need. The guideline is available at www.cdc.gov/drugoverdose/prescribing/guideline.html.
On Twitter @maryjodales
In 2014, 47,055 people in the United States died from drug overdoses – more deaths than attributed to this cause in any previous year on record, according to data from the National Vital Statistics System.
Opioids, primarily prescription pain relievers and heroin, were the main drugs associated with overdose deaths. In 2014, opioids were involved in 28,647 deaths, or 61% of all drug overdose deaths, Rose A. Rudd of the Centers for Disease Control and Prevention and her colleagues wrote (MMWR. 2015 Dec 18;64[Early release]:1-5).
The rate of opioid overdoses has tripled since 2000; the 15-year trend data implicate prescription opioid pain relievers and a recent surge in illicit opioid overdose deaths, driven largely by heroin.
From 2013 to 2014, synthetic opioids other than methadone (e.g., fentanyl and tramadol) drove the largest increase in the rate of drug overdose deaths. The rate nearly doubled from 1 per 100,000 persons to 1.8 per 100,000 persons. In 2014, the rate of drug overdose deaths involving natural and semisynthetic opioids (for example, morphine, oxycodone, and hydrocodone) was 3.8 per 100,000. The rate of drug overdose deaths involving methadone, a synthetic opioid classified separately from other synthetic opioids, was similar in 2013 and 2014.
The five states with the highest rates of drug overdose deaths in 2014 were West Virginia (35.5 deaths per 100,000), New Mexico (27.3), New Hampshire (26.2), Kentucky (24.7), and Ohio (24.6).
States with statistically significant increases in the rate of drug overdose deaths from 2013 to 2014 included Alabama, Georgia, Illinois, Indiana, Maine, Maryland, Massachusetts, Michigan, New Hampshire, New Mexico, North Dakota, Ohio, Pennsylvania, and Virginia.
The rates were noted in all adult age groups. From 2013 to 2014, statistically significant increases in drug overdose death rates were seen for both males and females, persons aged 25-34 years, 35-44 years, 55-64 years, and 65 years and older. Based on ethnicity, increases were seen in non-Hispanic whites and non-Hispanic blacks. Based on residency, increases were most common in the Northeast, Midwest, and South.
The authors noted three limitations of the data: First, the substances tested for and circumstances under which toxicologic tests are performed vary by jurisdiction; in 2013 and 2014, 22% and 19% of drug overdose deaths, respectively, did not include information on the death certificate about the specific types of drugs involved, and the percent of overdose deaths with specific drugs identified on the death certificate varies widely by state. Second, an increase from 2013 to 2014 in reporting of specific drugs involved in drug overdose deaths might have contributed to some of the observed increases in drug overdose death rates involving different types of opioids. Finally, some heroin deaths might be misclassified or underreported because morphine and heroin are similarly metabolized.
Efforts to encourage safer prescribing of opioid pain relievers should be strengthened, according to the authors. CDC has developed a draft guideline for the prescribing of opioids for chronic pain to address this need. The guideline is available at www.cdc.gov/drugoverdose/prescribing/guideline.html.
On Twitter @maryjodales
In 2014, 47,055 people in the United States died from drug overdoses – more deaths than attributed to this cause in any previous year on record, according to data from the National Vital Statistics System.
Opioids, primarily prescription pain relievers and heroin, were the main drugs associated with overdose deaths. In 2014, opioids were involved in 28,647 deaths, or 61% of all drug overdose deaths, Rose A. Rudd of the Centers for Disease Control and Prevention and her colleagues wrote (MMWR. 2015 Dec 18;64[Early release]:1-5).
The rate of opioid overdoses has tripled since 2000; the 15-year trend data implicate prescription opioid pain relievers and a recent surge in illicit opioid overdose deaths, driven largely by heroin.
From 2013 to 2014, synthetic opioids other than methadone (e.g., fentanyl and tramadol) drove the largest increase in the rate of drug overdose deaths. The rate nearly doubled from 1 per 100,000 persons to 1.8 per 100,000 persons. In 2014, the rate of drug overdose deaths involving natural and semisynthetic opioids (for example, morphine, oxycodone, and hydrocodone) was 3.8 per 100,000. The rate of drug overdose deaths involving methadone, a synthetic opioid classified separately from other synthetic opioids, was similar in 2013 and 2014.
The five states with the highest rates of drug overdose deaths in 2014 were West Virginia (35.5 deaths per 100,000), New Mexico (27.3), New Hampshire (26.2), Kentucky (24.7), and Ohio (24.6).
States with statistically significant increases in the rate of drug overdose deaths from 2013 to 2014 included Alabama, Georgia, Illinois, Indiana, Maine, Maryland, Massachusetts, Michigan, New Hampshire, New Mexico, North Dakota, Ohio, Pennsylvania, and Virginia.
The rates were noted in all adult age groups. From 2013 to 2014, statistically significant increases in drug overdose death rates were seen for both males and females, persons aged 25-34 years, 35-44 years, 55-64 years, and 65 years and older. Based on ethnicity, increases were seen in non-Hispanic whites and non-Hispanic blacks. Based on residency, increases were most common in the Northeast, Midwest, and South.
The authors noted three limitations of the data: First, the substances tested for and circumstances under which toxicologic tests are performed vary by jurisdiction; in 2013 and 2014, 22% and 19% of drug overdose deaths, respectively, did not include information on the death certificate about the specific types of drugs involved, and the percent of overdose deaths with specific drugs identified on the death certificate varies widely by state. Second, an increase from 2013 to 2014 in reporting of specific drugs involved in drug overdose deaths might have contributed to some of the observed increases in drug overdose death rates involving different types of opioids. Finally, some heroin deaths might be misclassified or underreported because morphine and heroin are similarly metabolized.
Efforts to encourage safer prescribing of opioid pain relievers should be strengthened, according to the authors. CDC has developed a draft guideline for the prescribing of opioids for chronic pain to address this need. The guideline is available at www.cdc.gov/drugoverdose/prescribing/guideline.html.
On Twitter @maryjodales
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Key clinical point: Efforts to encourage safer prescribing of opioid pain relievers should be strengthened.
Major finding: In 2014, opioids were involved in 28,647 deaths, or 61% of all drug overdose deaths.
Data source: The National Vital Statistics System multiple cause-of-death mortality files.
Disclosures: The authors had no relevant financial disclosures.
Flu activity increases slightly across U.S.
Influenza activity dropped slightly in South Carolina, but remained high enough to make it the nation’s hot spot during week 9 of the 2015-2016 U.S. flu season, the Centers for Disease Control and Prevention reported Dec. 18.
The activity of influenza-like illness (ILI) in South Carolina went from level 9 down to level 8 for the week ending Dec. 12 (week 9), but that kept it in the “high” range, according to the CDC report.
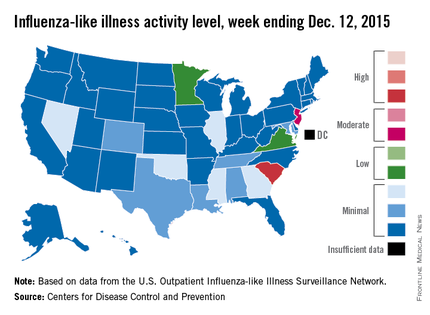
The rest of the United States saw a slight increase in activity, with 15 states at level 2 or higher, compared with 13 the week before. New Jersey had the next-highest level of activity after South Carolina, rising from level 5 last week to level 6, which moved it into the “moderate” range.
Minnesota had the largest increase in ILI activity from the previous week, going from level 1 to level 4, and other states with increased activity were Alabama, Colorado, Georgia, Illinois, Nevada, Tennessee, and Virginia. States besides South Carolina with decreased activity were Arizona, Hawaii, Louisiana, Mississippi, and Texas, the CDC data show.
The proportion of outpatient visits nationwide for ILI – defined as a temperature of 100° F or greater and cough and/or sore throat – was 1.9%, continuing to stay below the national baseline of 2.1%, the CDC said.
Outside of the fifty states, Guam reported widespread activity, Puerto Rico reported “moderate” (level 7) activity, and the District of Columbia and the U.S. Virgin Islands reported sporadic activity, the CDC noted.
Influenza activity dropped slightly in South Carolina, but remained high enough to make it the nation’s hot spot during week 9 of the 2015-2016 U.S. flu season, the Centers for Disease Control and Prevention reported Dec. 18.
The activity of influenza-like illness (ILI) in South Carolina went from level 9 down to level 8 for the week ending Dec. 12 (week 9), but that kept it in the “high” range, according to the CDC report.

The rest of the United States saw a slight increase in activity, with 15 states at level 2 or higher, compared with 13 the week before. New Jersey had the next-highest level of activity after South Carolina, rising from level 5 last week to level 6, which moved it into the “moderate” range.
Minnesota had the largest increase in ILI activity from the previous week, going from level 1 to level 4, and other states with increased activity were Alabama, Colorado, Georgia, Illinois, Nevada, Tennessee, and Virginia. States besides South Carolina with decreased activity were Arizona, Hawaii, Louisiana, Mississippi, and Texas, the CDC data show.
The proportion of outpatient visits nationwide for ILI – defined as a temperature of 100° F or greater and cough and/or sore throat – was 1.9%, continuing to stay below the national baseline of 2.1%, the CDC said.
Outside of the fifty states, Guam reported widespread activity, Puerto Rico reported “moderate” (level 7) activity, and the District of Columbia and the U.S. Virgin Islands reported sporadic activity, the CDC noted.
Influenza activity dropped slightly in South Carolina, but remained high enough to make it the nation’s hot spot during week 9 of the 2015-2016 U.S. flu season, the Centers for Disease Control and Prevention reported Dec. 18.
The activity of influenza-like illness (ILI) in South Carolina went from level 9 down to level 8 for the week ending Dec. 12 (week 9), but that kept it in the “high” range, according to the CDC report.

The rest of the United States saw a slight increase in activity, with 15 states at level 2 or higher, compared with 13 the week before. New Jersey had the next-highest level of activity after South Carolina, rising from level 5 last week to level 6, which moved it into the “moderate” range.
Minnesota had the largest increase in ILI activity from the previous week, going from level 1 to level 4, and other states with increased activity were Alabama, Colorado, Georgia, Illinois, Nevada, Tennessee, and Virginia. States besides South Carolina with decreased activity were Arizona, Hawaii, Louisiana, Mississippi, and Texas, the CDC data show.
The proportion of outpatient visits nationwide for ILI – defined as a temperature of 100° F or greater and cough and/or sore throat – was 1.9%, continuing to stay below the national baseline of 2.1%, the CDC said.
Outside of the fifty states, Guam reported widespread activity, Puerto Rico reported “moderate” (level 7) activity, and the District of Columbia and the U.S. Virgin Islands reported sporadic activity, the CDC noted.
New agents effectively target CLL’s molecular Achilles
SAN FRANCISCO – Novel targeted agents offer more options for treating chronic lymphocytic leukemia (CLL), and if properly leveraged, may be able to shorten the time on treatment, improving acceptability to patients and possibly reducing treatment costs, according to Dr. William G. Wierda.
BTK inhibitors
Agents that inhibit Bruton tyrosine kinase (BTK) block signaling through the B-cell receptor in CLL, triggering apoptosis, said Dr. Wierda, professor and medical director, department of leukemia, division of cancer medicine, at the University of Texas MD Anderson Cancer Center in Houston. One such agent, ibrutinib (Imbruvica), is approved by the Food and Drug Administration for treatment of relapsed CLL and for treatment of newly diagnosed CLL having 17p deletion, a high-risk factor.
Results from RESONATE-2, a randomized trial comparing ibrutinib with chlorambucil as frontline therapy in older adults with CLL or small lymphocytic lymphoma, will be reported later this year. “We don’t know the details of that publicly yet, but we do know from a press release that it is a positive trial and showed improvement in outcomes for ibrutinib-treated patients. With that data, we will likely have an expanded label for ibrutinib into the frontline setting, at least for the elderly population,” he said at the NCCN Annual Congress: Hematologic Malignancies.
Longer-term data, collected 3 years after patients started ibrutinib monotherapy, have been very good, with overall response rates of 90% in those with relapsed or refractory disease and 87% in those with treatment-naive disease (ASCO 2014. Abstract 7014). “The last time I saw these data updated, the complete remission portions have increased. So as patients remain on the treatment, responses do improve,” Dr. Wierda noted. Complete remission rates now are about 14% and 24%, respectively. Median progression-free survival has not been reached in either group.
Data from the randomized RESONATE trial, which led to ibrutinib’s approval in relapsed CLL, showed benefit relative to ofatumumab across subgroups, including patients who had disease that was refractory to purine analogs, who had the 17p deletion and who had received at least three prior regimens (ASCO 2014. Abstract LBA7008).
The main grade 3 or 4 toxicity of ibrutinib in patients with CLL is infections, but atrial fibrillation and bleeding/hemorrhage are each seen in about 5% of patients. “The trials all excluded patients on warfarin, so we do not recommend treating patients with ibrutinib who are on warfarin,” Dr. Wierda commented. “If patients are anticoagulated on warfarin and we want to put them on ibrutinib, I will usually switch them over to something like Xarelto [rivaroxaban],” he said. Toxicity generally declines with longer treatment.
Discontinuations because of toxicity or Richter transformation usually occur within the first 18 months (JAMA Oncol. 2015;1[1]:80-7). “The concerning [point] for me though is the patients who develop refractory disease. … The incidence starts to go up significantly as you go out beyond 30 or 36 months,” he said. “We are reviewing our data right now to see if we make a similar observation. But that suggests to me that the longer the patients stay on treatment, the more at risk they are for progressing and developing refractory disease.”
The HELIOS trial tested addition of ibrutinib to bendamustine (Treanda) and rituximab (Rituxan) (ASCO 2015. Abstract LBA7005). Results showed superior progression-free survival with the three-drug combination. “But the question that always comes up when this data is presented is, well, how would it compare with ibrutinib monotherapy? Until that question for me is adequately addressed … I would probably be inclined to give patients ibrutinib monotherapy over the combination,” Dr. Wierda said.
Trials are testing a wide range of other combinations. “To me, this suggests that we really don’t have a direction or a rational strategy for combinations with these agents. … Right now, I’m excited about combining ibrutinib with venetoclax. … They seem clinically complementary, and there is some laboratory data that suggests as well that there will be a complementary mechanism of action.”
PI3 kinase inhibitors
Inhibitors of PI3 kinase also block signaling through the B-cell receptor. In this drug class, idelalisib (Zydelig) is approved for treating relapsed CLL in combination with rituximab.
The phase III trial establishing efficacy of this combination showed that it improved both progression-free and overall survival over rituximab alone (ASH 2014. Abstract 330). Median progression-free survival was 19.4 months. There was similar benefit across various subgroups, including patients with 17p deletion or an unmutated IGHV gene, another high-risk factor.
One of the main toxicities of idelalisib, elevation of liver function test results, typically occurs early and is usually not treatment limiting. Colitis occurs with two predominant patterns: early onset and late onset. “The early colitis in my experience hasn’t necessarily been treatment limiting. We can usually get those patients through their diarrhea [by] withholding the drug; sometimes we’ll give budesonide, and can restart the drug at a lower dose,” Dr. Wierda said. “It’s the late colitis that we have difficulty with – colitis that occurs after patients have been on 3 months, 6 months. And in my experience, those patients have had more severe colitis, and it’s been more treatment limiting.”
Ongoing trials are testing idelalisib in combinations for CLL as well. “Certainly, there’s a number of strategies, and as with ibrutinib, it’s difficult for me to identify a rational combination or a clear combination that I think is going to be superior or a significant advance,” he said. Trials are also testing other PI3 kinase inhibitors, such as duvelisib (IPI-145), now in a phase III registration trial in patients with relapsed or refractory disease.
BCL-2 inhibitors
The investigational agent venetoclax (formerly ABT-199/GDC-199) inhibits BCL-2, which is overexpressed in CLL and renders the cells resistant to apoptosis. It has advanced to a pair of phase III trials, one testing it when combined with rituximab and the other when combined with obinutuzumab (Gazyva).
When used as monotherapy for patients with relapsed disease, venetoclax achieved an overall response rate of 77% and a complete response rate of 23% (EHA 2014. Abstract S702). Benefit was similar among high-risk groups, including patients with the 17p deletion or fludarabine-refractory disease. With a median follow-up of 5.3 months, median progression-free survival for patients treated at the full dose has not been reached.
In earlier trials, venetoclax was associated with a problematic tumor lysis syndrome, according to Dr. Wierda. But that issue has largely been resolved by starting at a low dose and escalating gradually to a full dose; in the trial, it was seen in 7% of patients. The most common grade 3 or 4 adverse event was neutropenia, seen in 33% of patients; however, this toxicity can usually be managed with growth factors and dose reduction, he said.
The combination of venetoclax with rituximab in relapsed CLL yields an 88% overall response rate and a 31% complete response rate (ASH 2014. Abstract 325). Respective values were 78% and 22% in patients with 17p deletion. Moreover, some patients were found to have become negative for minimal residual disease on the combination, although it was not systematically assessed, Dr. Wierda noted.
“Venetoclax is a drug we will hear more about. … It has activity. I think it has a future in treating CLL, and it will be approved in time,” he said.
Leveraging targeted therapies
These new targeted agents, and others in the pipeline, could potentially be leveraged in several ways to improve CLL treatment strategies, according to Dr. Wierda.
Importantly, if ibrutinib becomes approved for universal frontline therapy, a large share of patients are likely to achieve partial remission. “We know if patients are in partial remission, you can’t really stop their treatment on ibrutinib; they will progress. And there was some data reported at ASH [American Society of Hematology] this past year that patients who were on a lower dose or patients who had dose interruption did poorer,” he said. Furthermore, most patients don’t like to be on treatment indefinitely.
“So we’re working on trials to expand our options for consolidation strategies in patients who have been on ibrutinib. We are trying to push them over into a complete remission by adding additional agents,” he explained.
For example, an ongoing trial is testing addition of nivolumab (Opdivo), an immune checkpoint inhibitor, in patients who have been on ibrutinib for at least 9 months and still have a partial remission. “The strategy with that is to try to consolidate them and to get them into a deep remission, where we can have a discussion about holding their treatment or stopping their treatment, or at least to the point where we are comfortable doing that,” he said.
Dr. Wierda disclosed that he has various relationships with AbbVie, Ascerta, Celgene, Emergent BioSolutions, Genentech, Genzyme, Gilead Sciences, GlaxoSmithKline, Juno Therapeutics, Karyopharm, Kite Pharma, Merck, Novartis Pharmaceuticals, Pharmacyclics, Roche Laboratories, and Sanofi-Aventis U.S.
SAN FRANCISCO – Novel targeted agents offer more options for treating chronic lymphocytic leukemia (CLL), and if properly leveraged, may be able to shorten the time on treatment, improving acceptability to patients and possibly reducing treatment costs, according to Dr. William G. Wierda.
BTK inhibitors
Agents that inhibit Bruton tyrosine kinase (BTK) block signaling through the B-cell receptor in CLL, triggering apoptosis, said Dr. Wierda, professor and medical director, department of leukemia, division of cancer medicine, at the University of Texas MD Anderson Cancer Center in Houston. One such agent, ibrutinib (Imbruvica), is approved by the Food and Drug Administration for treatment of relapsed CLL and for treatment of newly diagnosed CLL having 17p deletion, a high-risk factor.
Results from RESONATE-2, a randomized trial comparing ibrutinib with chlorambucil as frontline therapy in older adults with CLL or small lymphocytic lymphoma, will be reported later this year. “We don’t know the details of that publicly yet, but we do know from a press release that it is a positive trial and showed improvement in outcomes for ibrutinib-treated patients. With that data, we will likely have an expanded label for ibrutinib into the frontline setting, at least for the elderly population,” he said at the NCCN Annual Congress: Hematologic Malignancies.
Longer-term data, collected 3 years after patients started ibrutinib monotherapy, have been very good, with overall response rates of 90% in those with relapsed or refractory disease and 87% in those with treatment-naive disease (ASCO 2014. Abstract 7014). “The last time I saw these data updated, the complete remission portions have increased. So as patients remain on the treatment, responses do improve,” Dr. Wierda noted. Complete remission rates now are about 14% and 24%, respectively. Median progression-free survival has not been reached in either group.
Data from the randomized RESONATE trial, which led to ibrutinib’s approval in relapsed CLL, showed benefit relative to ofatumumab across subgroups, including patients who had disease that was refractory to purine analogs, who had the 17p deletion and who had received at least three prior regimens (ASCO 2014. Abstract LBA7008).
The main grade 3 or 4 toxicity of ibrutinib in patients with CLL is infections, but atrial fibrillation and bleeding/hemorrhage are each seen in about 5% of patients. “The trials all excluded patients on warfarin, so we do not recommend treating patients with ibrutinib who are on warfarin,” Dr. Wierda commented. “If patients are anticoagulated on warfarin and we want to put them on ibrutinib, I will usually switch them over to something like Xarelto [rivaroxaban],” he said. Toxicity generally declines with longer treatment.
Discontinuations because of toxicity or Richter transformation usually occur within the first 18 months (JAMA Oncol. 2015;1[1]:80-7). “The concerning [point] for me though is the patients who develop refractory disease. … The incidence starts to go up significantly as you go out beyond 30 or 36 months,” he said. “We are reviewing our data right now to see if we make a similar observation. But that suggests to me that the longer the patients stay on treatment, the more at risk they are for progressing and developing refractory disease.”
The HELIOS trial tested addition of ibrutinib to bendamustine (Treanda) and rituximab (Rituxan) (ASCO 2015. Abstract LBA7005). Results showed superior progression-free survival with the three-drug combination. “But the question that always comes up when this data is presented is, well, how would it compare with ibrutinib monotherapy? Until that question for me is adequately addressed … I would probably be inclined to give patients ibrutinib monotherapy over the combination,” Dr. Wierda said.
Trials are testing a wide range of other combinations. “To me, this suggests that we really don’t have a direction or a rational strategy for combinations with these agents. … Right now, I’m excited about combining ibrutinib with venetoclax. … They seem clinically complementary, and there is some laboratory data that suggests as well that there will be a complementary mechanism of action.”
PI3 kinase inhibitors
Inhibitors of PI3 kinase also block signaling through the B-cell receptor. In this drug class, idelalisib (Zydelig) is approved for treating relapsed CLL in combination with rituximab.
The phase III trial establishing efficacy of this combination showed that it improved both progression-free and overall survival over rituximab alone (ASH 2014. Abstract 330). Median progression-free survival was 19.4 months. There was similar benefit across various subgroups, including patients with 17p deletion or an unmutated IGHV gene, another high-risk factor.
One of the main toxicities of idelalisib, elevation of liver function test results, typically occurs early and is usually not treatment limiting. Colitis occurs with two predominant patterns: early onset and late onset. “The early colitis in my experience hasn’t necessarily been treatment limiting. We can usually get those patients through their diarrhea [by] withholding the drug; sometimes we’ll give budesonide, and can restart the drug at a lower dose,” Dr. Wierda said. “It’s the late colitis that we have difficulty with – colitis that occurs after patients have been on 3 months, 6 months. And in my experience, those patients have had more severe colitis, and it’s been more treatment limiting.”
Ongoing trials are testing idelalisib in combinations for CLL as well. “Certainly, there’s a number of strategies, and as with ibrutinib, it’s difficult for me to identify a rational combination or a clear combination that I think is going to be superior or a significant advance,” he said. Trials are also testing other PI3 kinase inhibitors, such as duvelisib (IPI-145), now in a phase III registration trial in patients with relapsed or refractory disease.
BCL-2 inhibitors
The investigational agent venetoclax (formerly ABT-199/GDC-199) inhibits BCL-2, which is overexpressed in CLL and renders the cells resistant to apoptosis. It has advanced to a pair of phase III trials, one testing it when combined with rituximab and the other when combined with obinutuzumab (Gazyva).
When used as monotherapy for patients with relapsed disease, venetoclax achieved an overall response rate of 77% and a complete response rate of 23% (EHA 2014. Abstract S702). Benefit was similar among high-risk groups, including patients with the 17p deletion or fludarabine-refractory disease. With a median follow-up of 5.3 months, median progression-free survival for patients treated at the full dose has not been reached.
In earlier trials, venetoclax was associated with a problematic tumor lysis syndrome, according to Dr. Wierda. But that issue has largely been resolved by starting at a low dose and escalating gradually to a full dose; in the trial, it was seen in 7% of patients. The most common grade 3 or 4 adverse event was neutropenia, seen in 33% of patients; however, this toxicity can usually be managed with growth factors and dose reduction, he said.
The combination of venetoclax with rituximab in relapsed CLL yields an 88% overall response rate and a 31% complete response rate (ASH 2014. Abstract 325). Respective values were 78% and 22% in patients with 17p deletion. Moreover, some patients were found to have become negative for minimal residual disease on the combination, although it was not systematically assessed, Dr. Wierda noted.
“Venetoclax is a drug we will hear more about. … It has activity. I think it has a future in treating CLL, and it will be approved in time,” he said.
Leveraging targeted therapies
These new targeted agents, and others in the pipeline, could potentially be leveraged in several ways to improve CLL treatment strategies, according to Dr. Wierda.
Importantly, if ibrutinib becomes approved for universal frontline therapy, a large share of patients are likely to achieve partial remission. “We know if patients are in partial remission, you can’t really stop their treatment on ibrutinib; they will progress. And there was some data reported at ASH [American Society of Hematology] this past year that patients who were on a lower dose or patients who had dose interruption did poorer,” he said. Furthermore, most patients don’t like to be on treatment indefinitely.
“So we’re working on trials to expand our options for consolidation strategies in patients who have been on ibrutinib. We are trying to push them over into a complete remission by adding additional agents,” he explained.
For example, an ongoing trial is testing addition of nivolumab (Opdivo), an immune checkpoint inhibitor, in patients who have been on ibrutinib for at least 9 months and still have a partial remission. “The strategy with that is to try to consolidate them and to get them into a deep remission, where we can have a discussion about holding their treatment or stopping their treatment, or at least to the point where we are comfortable doing that,” he said.
Dr. Wierda disclosed that he has various relationships with AbbVie, Ascerta, Celgene, Emergent BioSolutions, Genentech, Genzyme, Gilead Sciences, GlaxoSmithKline, Juno Therapeutics, Karyopharm, Kite Pharma, Merck, Novartis Pharmaceuticals, Pharmacyclics, Roche Laboratories, and Sanofi-Aventis U.S.
SAN FRANCISCO – Novel targeted agents offer more options for treating chronic lymphocytic leukemia (CLL), and if properly leveraged, may be able to shorten the time on treatment, improving acceptability to patients and possibly reducing treatment costs, according to Dr. William G. Wierda.
BTK inhibitors
Agents that inhibit Bruton tyrosine kinase (BTK) block signaling through the B-cell receptor in CLL, triggering apoptosis, said Dr. Wierda, professor and medical director, department of leukemia, division of cancer medicine, at the University of Texas MD Anderson Cancer Center in Houston. One such agent, ibrutinib (Imbruvica), is approved by the Food and Drug Administration for treatment of relapsed CLL and for treatment of newly diagnosed CLL having 17p deletion, a high-risk factor.
Results from RESONATE-2, a randomized trial comparing ibrutinib with chlorambucil as frontline therapy in older adults with CLL or small lymphocytic lymphoma, will be reported later this year. “We don’t know the details of that publicly yet, but we do know from a press release that it is a positive trial and showed improvement in outcomes for ibrutinib-treated patients. With that data, we will likely have an expanded label for ibrutinib into the frontline setting, at least for the elderly population,” he said at the NCCN Annual Congress: Hematologic Malignancies.
Longer-term data, collected 3 years after patients started ibrutinib monotherapy, have been very good, with overall response rates of 90% in those with relapsed or refractory disease and 87% in those with treatment-naive disease (ASCO 2014. Abstract 7014). “The last time I saw these data updated, the complete remission portions have increased. So as patients remain on the treatment, responses do improve,” Dr. Wierda noted. Complete remission rates now are about 14% and 24%, respectively. Median progression-free survival has not been reached in either group.
Data from the randomized RESONATE trial, which led to ibrutinib’s approval in relapsed CLL, showed benefit relative to ofatumumab across subgroups, including patients who had disease that was refractory to purine analogs, who had the 17p deletion and who had received at least three prior regimens (ASCO 2014. Abstract LBA7008).
The main grade 3 or 4 toxicity of ibrutinib in patients with CLL is infections, but atrial fibrillation and bleeding/hemorrhage are each seen in about 5% of patients. “The trials all excluded patients on warfarin, so we do not recommend treating patients with ibrutinib who are on warfarin,” Dr. Wierda commented. “If patients are anticoagulated on warfarin and we want to put them on ibrutinib, I will usually switch them over to something like Xarelto [rivaroxaban],” he said. Toxicity generally declines with longer treatment.
Discontinuations because of toxicity or Richter transformation usually occur within the first 18 months (JAMA Oncol. 2015;1[1]:80-7). “The concerning [point] for me though is the patients who develop refractory disease. … The incidence starts to go up significantly as you go out beyond 30 or 36 months,” he said. “We are reviewing our data right now to see if we make a similar observation. But that suggests to me that the longer the patients stay on treatment, the more at risk they are for progressing and developing refractory disease.”
The HELIOS trial tested addition of ibrutinib to bendamustine (Treanda) and rituximab (Rituxan) (ASCO 2015. Abstract LBA7005). Results showed superior progression-free survival with the three-drug combination. “But the question that always comes up when this data is presented is, well, how would it compare with ibrutinib monotherapy? Until that question for me is adequately addressed … I would probably be inclined to give patients ibrutinib monotherapy over the combination,” Dr. Wierda said.
Trials are testing a wide range of other combinations. “To me, this suggests that we really don’t have a direction or a rational strategy for combinations with these agents. … Right now, I’m excited about combining ibrutinib with venetoclax. … They seem clinically complementary, and there is some laboratory data that suggests as well that there will be a complementary mechanism of action.”
PI3 kinase inhibitors
Inhibitors of PI3 kinase also block signaling through the B-cell receptor. In this drug class, idelalisib (Zydelig) is approved for treating relapsed CLL in combination with rituximab.
The phase III trial establishing efficacy of this combination showed that it improved both progression-free and overall survival over rituximab alone (ASH 2014. Abstract 330). Median progression-free survival was 19.4 months. There was similar benefit across various subgroups, including patients with 17p deletion or an unmutated IGHV gene, another high-risk factor.
One of the main toxicities of idelalisib, elevation of liver function test results, typically occurs early and is usually not treatment limiting. Colitis occurs with two predominant patterns: early onset and late onset. “The early colitis in my experience hasn’t necessarily been treatment limiting. We can usually get those patients through their diarrhea [by] withholding the drug; sometimes we’ll give budesonide, and can restart the drug at a lower dose,” Dr. Wierda said. “It’s the late colitis that we have difficulty with – colitis that occurs after patients have been on 3 months, 6 months. And in my experience, those patients have had more severe colitis, and it’s been more treatment limiting.”
Ongoing trials are testing idelalisib in combinations for CLL as well. “Certainly, there’s a number of strategies, and as with ibrutinib, it’s difficult for me to identify a rational combination or a clear combination that I think is going to be superior or a significant advance,” he said. Trials are also testing other PI3 kinase inhibitors, such as duvelisib (IPI-145), now in a phase III registration trial in patients with relapsed or refractory disease.
BCL-2 inhibitors
The investigational agent venetoclax (formerly ABT-199/GDC-199) inhibits BCL-2, which is overexpressed in CLL and renders the cells resistant to apoptosis. It has advanced to a pair of phase III trials, one testing it when combined with rituximab and the other when combined with obinutuzumab (Gazyva).
When used as monotherapy for patients with relapsed disease, venetoclax achieved an overall response rate of 77% and a complete response rate of 23% (EHA 2014. Abstract S702). Benefit was similar among high-risk groups, including patients with the 17p deletion or fludarabine-refractory disease. With a median follow-up of 5.3 months, median progression-free survival for patients treated at the full dose has not been reached.
In earlier trials, venetoclax was associated with a problematic tumor lysis syndrome, according to Dr. Wierda. But that issue has largely been resolved by starting at a low dose and escalating gradually to a full dose; in the trial, it was seen in 7% of patients. The most common grade 3 or 4 adverse event was neutropenia, seen in 33% of patients; however, this toxicity can usually be managed with growth factors and dose reduction, he said.
The combination of venetoclax with rituximab in relapsed CLL yields an 88% overall response rate and a 31% complete response rate (ASH 2014. Abstract 325). Respective values were 78% and 22% in patients with 17p deletion. Moreover, some patients were found to have become negative for minimal residual disease on the combination, although it was not systematically assessed, Dr. Wierda noted.
“Venetoclax is a drug we will hear more about. … It has activity. I think it has a future in treating CLL, and it will be approved in time,” he said.
Leveraging targeted therapies
These new targeted agents, and others in the pipeline, could potentially be leveraged in several ways to improve CLL treatment strategies, according to Dr. Wierda.
Importantly, if ibrutinib becomes approved for universal frontline therapy, a large share of patients are likely to achieve partial remission. “We know if patients are in partial remission, you can’t really stop their treatment on ibrutinib; they will progress. And there was some data reported at ASH [American Society of Hematology] this past year that patients who were on a lower dose or patients who had dose interruption did poorer,” he said. Furthermore, most patients don’t like to be on treatment indefinitely.
“So we’re working on trials to expand our options for consolidation strategies in patients who have been on ibrutinib. We are trying to push them over into a complete remission by adding additional agents,” he explained.
For example, an ongoing trial is testing addition of nivolumab (Opdivo), an immune checkpoint inhibitor, in patients who have been on ibrutinib for at least 9 months and still have a partial remission. “The strategy with that is to try to consolidate them and to get them into a deep remission, where we can have a discussion about holding their treatment or stopping their treatment, or at least to the point where we are comfortable doing that,” he said.
Dr. Wierda disclosed that he has various relationships with AbbVie, Ascerta, Celgene, Emergent BioSolutions, Genentech, Genzyme, Gilead Sciences, GlaxoSmithKline, Juno Therapeutics, Karyopharm, Kite Pharma, Merck, Novartis Pharmaceuticals, Pharmacyclics, Roche Laboratories, and Sanofi-Aventis U.S.
EXPERT ANALYSIS FROM NCCN ANNUAL CONGRESS: HEMATOLOGIC MALIGNANCIES