User login
Anemia tied to cognitive impairment

A population-based study conducted in Germany has suggested a link between anemia and mild cognitive impairment (MCI).
Researchers found that subjects with anemia, defined as hemoglobin <13 g/dL in men and <12 g/dL in women, performed worse on cognitive tests than their nonanemic peers.
And MCI occurred almost twice as often in subjects with anemia than in subjects with normal hemoglobin levels.
This study was published in the Journal of Alzheimer’s Disease.
About MCI
MCI represents an intermediate and possibly modifiable stage between normal cognitive aging and dementia. Although individuals with MCI have an increased risk of developing dementia or Alzheimer’s disease, they can also remain stable for many years or even revert to a cognitively normal state over time. This modifiable characteristic makes the concept of MCI a promising target in the prevention of dementia.
The following 4 criteria are used to diagnose MCI. First, subjects must report a decline in cognitive performance over the past 2 years. Second, they must show a cognitive impairment in objective cognitive tasks that is greater than one would expect taking their age and education into consideration.
Third, the impairment must not be as pronounced as in demented individuals since people with MCI can perform normal daily living activities or are only slightly impaired in carrying out complex instrumental functions. Fourth, the cognitive impairment has to be insufficient to fulfil criteria for dementia.
The concept of MCI distinguishes between amnestic MCI (aMCI) and non-amnestic MCI (naMCI). In the former, impairment in the memory domain is evident, most likely reflecting Alzheimer’s disease pathology. In the latter, impairment in non-memory domains is present, mainly reflecting vascular pathology but also frontotemporal dementia or dementia with Lewy bodies.
Study details
The Heinz Nixdorf Recall study is an observational, population-based, prospective study in which researchers examined 4814 subjects between 2000 and 2003. Subjects were 50 to 80 years of age and lived in the metropolitan Ruhr Area. Both genders were equally represented.
After 5 years, the researchers conducted a second examination with 92% of the subjects taking part. The publication includes cross-sectional results of the second examination.
First, 163 subjects with anemia and 3870 without anemia were included to compare their performance in all cognitive subtests.
The subjects took verbal memory tests, which were used to gauge immediate recall and delayed recall. They were also tested on executive functioning, which included problem-solving/speed of processing, verbal fluency, visual spatial organization, and the clock drawing test.
In the initial analysis, anemic subjects showed more pronounced cardiovascular risk profiles and lower cognitive performance in all administered cognitive subtests. After adjusting for age, anemic subjects showed a significantly lower performance in the immediate recall task (P=0.009) and the verbal fluency task (P=0.004).
Next, the researchers compared 579 subjects diagnosed with MCI—299 with aMCI and 280 with naMCI—to 1438 cognitively normal subjects to determine the association between anemia at follow-up and MCI.
The team found that MCI occurred more often in anemic than non-anemic subjects. The unadjusted odds ratio (OR) was 2.59 (P<0.001). The OR after adjustment for age, gender, and years of education was 2.15 (P=0.002).
In a third analysis, the researchers adjusted for the aforementioned variables as well as body mass index, high-sensitivity C-reactive protein, glomerular filtration rate, cholesterol, serum iron, hypertension, diabetes mellitus, history of coronary heart disease, history of stroke, history of cancer, APOE4, smoking, severe depressive symptoms, and use of antidepressants. The OR after adjustment for these factors was 1.92 (P=0.04).
Similar results were found for aMCI and naMCI. The researchers said this suggests that having a low hemoglobin level may contribute to cognitive impairment via different pathways.
The team believes that, overall, their study results indicate that anemia is associated with an increased risk of MCI independent of traditional cardiovascular risk factors. They said the association between anemia and MCI has important clinical relevance because—depending on etiology—anemia can be treated effectively, and this might provide means to prevent or delay cognitive decline. ![]()

A population-based study conducted in Germany has suggested a link between anemia and mild cognitive impairment (MCI).
Researchers found that subjects with anemia, defined as hemoglobin <13 g/dL in men and <12 g/dL in women, performed worse on cognitive tests than their nonanemic peers.
And MCI occurred almost twice as often in subjects with anemia than in subjects with normal hemoglobin levels.
This study was published in the Journal of Alzheimer’s Disease.
About MCI
MCI represents an intermediate and possibly modifiable stage between normal cognitive aging and dementia. Although individuals with MCI have an increased risk of developing dementia or Alzheimer’s disease, they can also remain stable for many years or even revert to a cognitively normal state over time. This modifiable characteristic makes the concept of MCI a promising target in the prevention of dementia.
The following 4 criteria are used to diagnose MCI. First, subjects must report a decline in cognitive performance over the past 2 years. Second, they must show a cognitive impairment in objective cognitive tasks that is greater than one would expect taking their age and education into consideration.
Third, the impairment must not be as pronounced as in demented individuals since people with MCI can perform normal daily living activities or are only slightly impaired in carrying out complex instrumental functions. Fourth, the cognitive impairment has to be insufficient to fulfil criteria for dementia.
The concept of MCI distinguishes between amnestic MCI (aMCI) and non-amnestic MCI (naMCI). In the former, impairment in the memory domain is evident, most likely reflecting Alzheimer’s disease pathology. In the latter, impairment in non-memory domains is present, mainly reflecting vascular pathology but also frontotemporal dementia or dementia with Lewy bodies.
Study details
The Heinz Nixdorf Recall study is an observational, population-based, prospective study in which researchers examined 4814 subjects between 2000 and 2003. Subjects were 50 to 80 years of age and lived in the metropolitan Ruhr Area. Both genders were equally represented.
After 5 years, the researchers conducted a second examination with 92% of the subjects taking part. The publication includes cross-sectional results of the second examination.
First, 163 subjects with anemia and 3870 without anemia were included to compare their performance in all cognitive subtests.
The subjects took verbal memory tests, which were used to gauge immediate recall and delayed recall. They were also tested on executive functioning, which included problem-solving/speed of processing, verbal fluency, visual spatial organization, and the clock drawing test.
In the initial analysis, anemic subjects showed more pronounced cardiovascular risk profiles and lower cognitive performance in all administered cognitive subtests. After adjusting for age, anemic subjects showed a significantly lower performance in the immediate recall task (P=0.009) and the verbal fluency task (P=0.004).
Next, the researchers compared 579 subjects diagnosed with MCI—299 with aMCI and 280 with naMCI—to 1438 cognitively normal subjects to determine the association between anemia at follow-up and MCI.
The team found that MCI occurred more often in anemic than non-anemic subjects. The unadjusted odds ratio (OR) was 2.59 (P<0.001). The OR after adjustment for age, gender, and years of education was 2.15 (P=0.002).
In a third analysis, the researchers adjusted for the aforementioned variables as well as body mass index, high-sensitivity C-reactive protein, glomerular filtration rate, cholesterol, serum iron, hypertension, diabetes mellitus, history of coronary heart disease, history of stroke, history of cancer, APOE4, smoking, severe depressive symptoms, and use of antidepressants. The OR after adjustment for these factors was 1.92 (P=0.04).
Similar results were found for aMCI and naMCI. The researchers said this suggests that having a low hemoglobin level may contribute to cognitive impairment via different pathways.
The team believes that, overall, their study results indicate that anemia is associated with an increased risk of MCI independent of traditional cardiovascular risk factors. They said the association between anemia and MCI has important clinical relevance because—depending on etiology—anemia can be treated effectively, and this might provide means to prevent or delay cognitive decline. ![]()

A population-based study conducted in Germany has suggested a link between anemia and mild cognitive impairment (MCI).
Researchers found that subjects with anemia, defined as hemoglobin <13 g/dL in men and <12 g/dL in women, performed worse on cognitive tests than their nonanemic peers.
And MCI occurred almost twice as often in subjects with anemia than in subjects with normal hemoglobin levels.
This study was published in the Journal of Alzheimer’s Disease.
About MCI
MCI represents an intermediate and possibly modifiable stage between normal cognitive aging and dementia. Although individuals with MCI have an increased risk of developing dementia or Alzheimer’s disease, they can also remain stable for many years or even revert to a cognitively normal state over time. This modifiable characteristic makes the concept of MCI a promising target in the prevention of dementia.
The following 4 criteria are used to diagnose MCI. First, subjects must report a decline in cognitive performance over the past 2 years. Second, they must show a cognitive impairment in objective cognitive tasks that is greater than one would expect taking their age and education into consideration.
Third, the impairment must not be as pronounced as in demented individuals since people with MCI can perform normal daily living activities or are only slightly impaired in carrying out complex instrumental functions. Fourth, the cognitive impairment has to be insufficient to fulfil criteria for dementia.
The concept of MCI distinguishes between amnestic MCI (aMCI) and non-amnestic MCI (naMCI). In the former, impairment in the memory domain is evident, most likely reflecting Alzheimer’s disease pathology. In the latter, impairment in non-memory domains is present, mainly reflecting vascular pathology but also frontotemporal dementia or dementia with Lewy bodies.
Study details
The Heinz Nixdorf Recall study is an observational, population-based, prospective study in which researchers examined 4814 subjects between 2000 and 2003. Subjects were 50 to 80 years of age and lived in the metropolitan Ruhr Area. Both genders were equally represented.
After 5 years, the researchers conducted a second examination with 92% of the subjects taking part. The publication includes cross-sectional results of the second examination.
First, 163 subjects with anemia and 3870 without anemia were included to compare their performance in all cognitive subtests.
The subjects took verbal memory tests, which were used to gauge immediate recall and delayed recall. They were also tested on executive functioning, which included problem-solving/speed of processing, verbal fluency, visual spatial organization, and the clock drawing test.
In the initial analysis, anemic subjects showed more pronounced cardiovascular risk profiles and lower cognitive performance in all administered cognitive subtests. After adjusting for age, anemic subjects showed a significantly lower performance in the immediate recall task (P=0.009) and the verbal fluency task (P=0.004).
Next, the researchers compared 579 subjects diagnosed with MCI—299 with aMCI and 280 with naMCI—to 1438 cognitively normal subjects to determine the association between anemia at follow-up and MCI.
The team found that MCI occurred more often in anemic than non-anemic subjects. The unadjusted odds ratio (OR) was 2.59 (P<0.001). The OR after adjustment for age, gender, and years of education was 2.15 (P=0.002).
In a third analysis, the researchers adjusted for the aforementioned variables as well as body mass index, high-sensitivity C-reactive protein, glomerular filtration rate, cholesterol, serum iron, hypertension, diabetes mellitus, history of coronary heart disease, history of stroke, history of cancer, APOE4, smoking, severe depressive symptoms, and use of antidepressants. The OR after adjustment for these factors was 1.92 (P=0.04).
Similar results were found for aMCI and naMCI. The researchers said this suggests that having a low hemoglobin level may contribute to cognitive impairment via different pathways.
The team believes that, overall, their study results indicate that anemia is associated with an increased risk of MCI independent of traditional cardiovascular risk factors. They said the association between anemia and MCI has important clinical relevance because—depending on etiology—anemia can be treated effectively, and this might provide means to prevent or delay cognitive decline. ![]()
The Social Worker’s Role in Delirium Care for Hospitalized Veterans
Delirium, or the state of mental confusion that may occur with physical or mental illness, is common, morbid, and costly; however, of the diagnosed cases, delirium is mentioned in hospital discharge summaries only 16% to 55% of the time.1-3
Social workers often coordinate care transitions for hospitalized older veterans. They serve as interdisciplinary team members who communicate with VA medical staff as well as with the patient and family. This position, in addition to their training in communication and advocacy, primes social workers for a role in delirium care and provides the needed support for veterans who experience delirium and their families.
Background
Delirium is a sudden disturbance of attention with reduced awareness of the environment. Because attention is impaired, other changes in cognition are common, including perceptual and thought disturbances. Additionally, delirium includes fluctuations in consciousness over the course of a day. The acute development of these cognitive disturbances is distinct from a preexisting chronic cognitive impairment, such as dementia. Delirium is a direct consequence of underlying medical conditions, such as infections, polypharmacy, dehydration, and surgery.4
Delirium subtypes all have inattention as a core symptom. In half of the cases, patients are hypoactive and will not awaken easily or participate in daily care plans readily.4 Hyperactive delirium occurs in a quarter of cases. In the remaining mixed delirium cases patients fluctuate between the 2 states.4
Delirium is often falsely mistaken for dementia. Although delirium and dementia can present similarly, delirium has a sudden onset, which can alert health care professionals (HCPs) to the likelihood of delirium. Another important distinction is that delirium is typically reversible. Symptom manifestations of delirium may also be confused with depression.
Related: Delirium in the Cardiac ICU
Preventing delirium is important due to its many negative health outcomes. Older adults who develop delirium are more likely to die sooner. In a Canadian study of hospitalized patients aged ≥ 65 years, 41.6% of the delirium cohort and 14.4% of the control group died within 12 months of hospital admission.5 The death rate predicted by delirium in the Canadian study was comparable to the death rate of those who experience other serious medical conditions, such as sepsis or a heart attack.6
Those who survive delirium experience other serious outcomes, such as a negative impact on function and cognition and an increase in long-term care placement.7 Even when the condition resolves quickly, lasting functional impairment may be evident without return to baseline functioning.8 Hospitalized veterans are generally older, making them susceptible to developing delirium.9
Prevalence
Delirium can result from multiple medical conditions and develops in up to 50% of patients after general surgery and up to 80% of patients in the intensive care unit.10,11 From 20% to 40% of hospitalized older adults and from 50% to 89% of patients with preexisting Alzheimer disease may develop delirium.12-15 The increasing number of aging adults who will be hospitalized may also result in an increased prevalence of delirium.1,16
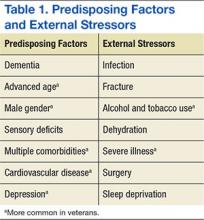
Delirium is a result of various predisposing and precipitating factors.1 Predisposing vulnerabilities are intrinsic to the individual, whereas precipitating external stressors are found in the environment. External stressors may trigger delirium in an individual who is vulnerable due to predisposing risk. The primary risk factors for delirium include dementia, advanced age, sensory impairment, fracture, infection, and dehydration (Table 1).12
Predisposing factors for delirium, such as age and sex, lifestyle choices (alcohol, tobacco), and chronic conditions (atherosclerosis, depression, prior stroke/transient ischemic attack) are more prevalent in the veteran population.9,17-20 In 2011, the median age for male veterans was 64 and the median age for male nonveterans was 41. Of male veterans, 49.9% are aged ≥ 65 years in comparison with 10.5% of the nonveteran male population.21 Veterans also have higher rates of comorbidities; a significant risk factor for delirium.20 A study by Agha and colleagues found that veterans were 14 times more likely to have 5 or more medical conditions than that of the general population.9 In a study comparing veterans aged ≥ 65 years with their age matched nonveteran peers, the health status of the veterans was poorer overall.22 Veterans are more likely to have posttraumatic stress disorder, which can increase the risk of postsurgery delirium and dementia, a primary risk factor for delirium.23-26
Delirium Intervention
Up to 40% of delirium cases can be prevented.27 But delirium may remain undetected in older veterans because its symptoms are sometimes thought to be the unavoidable consequences of aging, dementia, preexisting mental health conditions, substance abuse, a disease process, or the hospital environment.28 Therefore, to avoid the negative consequences of delirium, prevention is critical.28
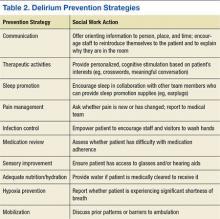
The goals of delirium treatment are to identify and reverse its underlying cause(s).29 Because delirium is typically multifactorial, an HCP must carefully consider the various sources that could have initiated a change in mental status. Delirium may be prevented if HCPs can reduce patient risk factors. The 2010 National Institute for Health and Clinical Excellence (NICE) Delirium Guideline recommended a set of prevention strategies to address delirium risk factors (Table 2).12
As a member of the health care team, social workers can help prevent delirium through attention to pain management, infection control, medication review, sensory improvement, adequate nutrition and hydration, hypoxia prevention, and mobilization.12No pharmacologic approach has been approved for the treatment of delirium.30 Drugs may manage symptoms associated with delirium, but they do not treat the disease and could be associated with toxicity in high-risk patients. However, there are a variety of nonpharmacologic preventative measures that have proven effective. Environmental interventions to prevent delirium include orientation, cognitive stimulation, and sensory aids. A 2013 meta-analysis of 19 delirium prevention programs found that most were effective in preventing delirium in patients at risk during hospitalization.31 This review found that the most successful programs included multidisciplinary teams providing staff education and therapeutic cognitive activities.31 Social workers can encourage and directly provide such services. The Delirium Toolbox is a delirium risk modification program that was piloted with frontline staff, including social workers, at the VA Boston Healthcare System in West Roxbury, Massachusetts, and has been associated with restraint reduction, shortened length of stay (LOS), and lower variable direct costs.32
Social Worker Role
Several studies, both national and international, have indicated that little has been done over the past 2 decades to increase the diagnosis of delirium, because only 12% to 35% of delirium cases are clinically detected within the emergency department and in acute care settings.33-37 Patients may hesitate to report their experience due to a sense of embarrassment or because of an inability to describe it.38
Social workers are skilled at helping individuals feel more at ease when disclosing distressing experiences. Delirium is relevant to HCPs and hospital social workers with care transition responsibilities, because delirium detection should impact discharge planning.1,39 Delirium education needs to be included in efforts to improve transitions from intensive care settings to lower levels of care and from lower levels of care to discharge.40 Hospital social workers are in a position to offer additional support because they see patients at a critical juncture in their care and can take steps to improve postdischarge outcomes.41
Prior to Onset
Social workers can play an important role prior to delirium onset.42 Patient education on delirium needs to be provided during the routine hospital intake assessment. Informing patients in advance that delirium is common, based on their risk factors, as well as what to expect if delirium is experienced has been found to provide comfort.38 Families who anticipated possible delirium-related confusion reported that they experienced less distress.38
Related: Baseball Reminiscence Therapy for Cognitively Impaired Veterans
During hospitalization, social workers can ascertain from families whether an alteration in mental status is a rapid change, possibly indicating delirium, or a gradual dementia onset. The social work skills of advocacy and education can be used to support delirium-risk identification to avoid adverse outcomes.43 When no family caregiver is present to provide a history of the individual’s cognitive function prior to hospitalization, the social worker may be the first to notice an acute change in cognitive status and can report this to the medical team.
During Delirium
Lack of patient responsiveness and difficulty following a conversation are possible signs of delirium. This situation should be reported to the medical team for further delirium assessment and diagnosis.4 The social worker can also attempt to determine whether a patient’s presentation is unusual by contacting the family. Social work training recognizes the important role of the family.44 Social workers often interact with families at the critical period between acute onset of delirium in the hospital and discharge.42 Studies have shown that delirium causes stress for the patient’s loved ones. Moreover, caregivers of patients who experience the syndrome are at a 12 times increased risk of meeting the criteria for generalized anxiety disorder.30 In one study, delirium was rated as more distressing for the caregivers who witnessed it than for the patients who experienced it.38 Education has been shown to reduce delirium-related distress.30
In cases where delirium is irreversible, such as during the active dying process, social workers can serve in a palliative role to ease family confusion and provide comfort.30 The presence of family and other familiar people are considered part of the nonpharmacologic management of delirium.28
Posthospitalization
Delirium complicates physical aspects of care for families, as their loved one may need direct care in areas where they were previously independent due to a loss of function. Logistic considerations such as increased supervision may be necessary due to delirium, and the patient’s condition may be upsetting and confusing for family members, triggering the need for emotional support. During the discharge process, social workers can provide support and education to family members or placement facilities.38
Social workers in the hospital setting are often responsible for discharge planning, including the reduction of extended LOS and unnecessary readmissions to the hospital.45 Increased LOS and hospital readmissions are 2 of the primary negative outcomes associated with delirium. Delirium can persist for months beyond hospitalization, making it a relevant issue at the time of discharge and beyond.46 Distress related to delirium has been documented up to 2 years after onset, due to manifestations of anxiety and depression.38
Distress impacts patients as well as caregivers who witness the delirium and provide care to the patient afterward.38 Long-term changes in mood in addition to loss of function as a result of delirium can lead to an increase in stress for both patients and their caregivers.30 The social work emphasis on counseling and family dynamics as well as the common role of coordinating post-discharge arrangements makes the profession uniquely suited for delirium care.
Barriers
Social workers can play a key role in delirium risk identification and coordination of care but face substantial barriers. Delirium assessments are complex and require training and education in the features of delirium and cognitive assessment.47 To date, social workers receive limited education about delirium and typically do not make deliberate efforts in prevention, support, and follow-up care.
Conclusion
Social workers will encounter delirium, and their training makes them particularly suited to address this health concern. An understanding of the larger ecologic system is a foundational aspect of social work and an essential component of delirium prevention and care.41 The multipathway nature of delirium as well as the importance of prevention suggests that multiple disciplines, including social work, should be involved.1 The American Delirium Society and the European Delirium Association both recognize the need for all HCPs to be engaged in delirium care.1,48
Related: Sharing Alzheimer Research, FasterSharing Alzheimer Research, Faster
Social workers in the hospital setting provide communication, advocacy, and education to other HCPs, as well as to patients and families (Figure). Because delirium directly impacts the emotional and logistic needs of patients and their families, it would be advantageous for social workers to take a more active role in delirium risk identification, prevention, and care. Fortunately, the nonpharmacologic approaches that social workers are skilled in providing (eg, education and emotional support) have been shown to benefit patients with delirium and their families.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Rudolph JL, Boustani M, Kamholz B, Shaughnessey M, Shay K; American Delirium Society. Delirium: a strategic plan to bring an ancient disease into the 21st century. J Am Geriatr Soc. 2011;59(suppl 2):S237-S240.
2. Hope C, Estrada N, Weir C, Teng CC, Damal K, Sauer BC. Documentation of delirium in the VA electronic health record. BMC Res Notes. 2014;7:208.
3. van Zyl LT, Davidson PR. Delirium in hospital: an underreported event at discharge. Can J Psychiatry. 2003;48(8):555-560.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
5. McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457-463.
6. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
7. McCusker J, Cole M, Dendukuri N, Belzile E, Primeau F. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ. 2001;165(5):575-583.
8. Quinlan N, Rudolph JL. Postoperative delirium and functional decline after noncardiac surgery. J Am Geriatr Soc. 2011;59(suppl 2):S301-S304.
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
10. Marcantonio ER, Simon SE, Bergmann MA, Jones RN, Murphy KM, Morris JN. Delirium symptoms in post-acute care: prevalent, persistent, and associated with poor functional recovery. J Am Geriatr Soc. 2003;51(1):4-9.
11. McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598.
12. National Institute for Health and Clinical Excellence. Delirium: Diagnosis, Prevention and Management. National Institute for Health and Clinical Excellence Website. https://www.nice.org.uk/guidance/cg103/resources/delirium-174507018181. Published July 2010.
13. Fick D, Foreman M. Consequences of not recognizing delirium superimposed on dementia in hospitalized elderly individuals. J Gerontol Nurs. 2000;26(1):30-40.
14. Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50(10):1723-1732.
15. Edlund A, Lundström M, Brännström B, Bucht G, Gustafson Y. Delirium before and after operation for femoral neck fracture. J Am Geriatr Soc. 2001;49(10):1335-1340.
16. Popejoy LL, Galambos C, Moylan K, Madsen R. Challenges to hospital discharge planning for older adults. Clin Nurs Res. 2012;21(4):431-449.
17. Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271(2):134-139.
18. Rudolph JL, Jones RN, Rasmussen LS, Silverstein JH, Inouye SK, Marcantonio ER. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. 2007;120(9):807-813.
19. Rudolph JL, Babikian VL, Birjiniuk V, et al. Atherosclerosis is associated with delirium after coronary artery bypass graft surgery. J Am Geriatr Soc. 2005;53(3):462-466.
20. Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119(2):229-236.
21. U.S. Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of Veterans: 2013 Data from the American Community Survey. U.S. Department of Veterans Affairs Website. http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2013.pdf. Accessed November 14, 2015.
22. Selim AJ, Berlowitz DR, Fincke G, et al. The health status of elderly veteran enrollees in the Veterans Health Administration. J Am Geriatr Soc. 2004;52(8):1271-1276.
23. McGuire JM. The incidence of and risk factors for emergence delirium in U.S. military combat veterans. J Perianesth Nurs. 2012;27(4):236-245.
24. Lepousé C, Lautner CA, Liu L, Gomis P, Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth. 2006;96(6):747-753.
25. Meziab O, Kirby KA, Williams B, Yaffe K, Byers AL, Barnes DE. Prisoner of war status, posttraumatic stress disorder, and dementia in older veterans. Alzheimers Dement. 2014;10(3)(suppl):S236-S241.
26. Elie M, Cole MG, Primeau FJ, Bellavance F. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med. 1998;13(3):204-212.
27. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220.
28. Conley DM. The gerontological clinical nurse specialist's role in prevention, early recognition, and management of delirium in hospitalized older adults. Urol Nurs. 2011;31(6):337-342.
29. Meagher DJ. Delirium: optimising management. BMJ. 2001;322(7279):144-149.
30. Irwin SA, Pirrello RD, Hirst JM, Buckholz GT, Ferris FD. Clarifying delirium management: practical, evidenced-based, expert recommendations for clinical practice. J Palliat Med. 2013;16(4):423-435.
31. Reston JT, Schoelles KM. In-facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5, pt 2):375-380.
32. Rudolph JL, Archambault E, Kelly B; VA Boston Delirium Task Force. A delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):957.e7-957.e11.
33. Gustafson Y, Brännström B, Norberg A, Bucht G, Winblad B. Underdiagnosis and poor documentation of acute confusional states in elderly hip fracture patients. J Am Geriatr Soc. 1991;39(8):760-765.
34. Hustey FM, Meldon SW. The prevalence and documentation of impaired mental status in elderly emergency department patients. Ann Emerg Med. 2002;39(3):248-253.
35. Kales HC, Kamholz BA, Visnic SG, Blow FC. Recorded delirium in a national sample of elderly inpatients: potential implications for recognition. J Geriatr Psychiatry Neurol. 2003;16(1):32-38.
36. Lemiengre J, Nelis T, Joosten E, et al. Detection of delirium by bedside nurses using the confusion assessment method. J Am Geriatr Soc. 2006;54(4):685-689.
37. Milisen K, Foreman MD, Wouters B, et al. Documentation of delirium in elderly patients with hip fracture. J Gerontol Nurs. 2002;28(11):23-29.
38. Partridge JS, Martin FC, Harari D, Dhesi JK. The delirium experience: what is the effect on patients, relatives and staff and what can be done to modify this? Int J Geriatr Psychiatry. 2013;28(8):804-812.
39. Simons K, Connolly RP, Bonifas R, et al. Psychosocial assessment of nursing home residents via MDS 3.0: recommendations for social service training, staffing, and roles in interdisciplinary care. J Am Med Dir Assoc. 2012;13(2):190.e9-190.e15.
40. Alici Y. Interventions to improve recognition of delirium: a sine qua non for successful transitional care programs. Arch Intern Med. 2012;172(1):80-82.
41. Judd RG, Sheffield S. Hospital social work: contemporary roles and professional activities. Soc Work Health Care. 2010;49(9):856-871.
42. Duffy F, Healy JP. Social work with older people in a hospital setting. Soc Work Health Care. 2011;50(2):109-123.
43. Anderson CP, Ngo LH, Marcantonio ER. Complications in post-acute care are associated with persistent delirium. J Am Geriatr Soc. 2012;60(6):1122-1127.
44. Bauer M, Fitzgerald L, Haesler E, Manfrin M. Hospital discharge planning for frail older people and their family. Are we delivering best practice? A review of the evidence. J Clin Nurs. 2009;18(18):2539-2546.
45. Shepperd S, Lannin NA, Clemson LM, McCluskey A, Cameron ID, Barras SL. Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313.
46. McCusker J, Cole M, Dendukuri N, Han L, Belzile E. The course of delirium in older medical inpatients: A prospective study. J Gen Intern Med. 2003;18(9):696-704.
47. Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM Jr. Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161(20):2467-2473.
48. Teodorczuk A, Reynish E, Milisen K. Improving recognition of delirium in clinical practice: a call for action. BMC Geriatr. 2012;12:55.
Delirium, or the state of mental confusion that may occur with physical or mental illness, is common, morbid, and costly; however, of the diagnosed cases, delirium is mentioned in hospital discharge summaries only 16% to 55% of the time.1-3
Social workers often coordinate care transitions for hospitalized older veterans. They serve as interdisciplinary team members who communicate with VA medical staff as well as with the patient and family. This position, in addition to their training in communication and advocacy, primes social workers for a role in delirium care and provides the needed support for veterans who experience delirium and their families.
Background
Delirium is a sudden disturbance of attention with reduced awareness of the environment. Because attention is impaired, other changes in cognition are common, including perceptual and thought disturbances. Additionally, delirium includes fluctuations in consciousness over the course of a day. The acute development of these cognitive disturbances is distinct from a preexisting chronic cognitive impairment, such as dementia. Delirium is a direct consequence of underlying medical conditions, such as infections, polypharmacy, dehydration, and surgery.4
Delirium subtypes all have inattention as a core symptom. In half of the cases, patients are hypoactive and will not awaken easily or participate in daily care plans readily.4 Hyperactive delirium occurs in a quarter of cases. In the remaining mixed delirium cases patients fluctuate between the 2 states.4
Delirium is often falsely mistaken for dementia. Although delirium and dementia can present similarly, delirium has a sudden onset, which can alert health care professionals (HCPs) to the likelihood of delirium. Another important distinction is that delirium is typically reversible. Symptom manifestations of delirium may also be confused with depression.
Related: Delirium in the Cardiac ICU
Preventing delirium is important due to its many negative health outcomes. Older adults who develop delirium are more likely to die sooner. In a Canadian study of hospitalized patients aged ≥ 65 years, 41.6% of the delirium cohort and 14.4% of the control group died within 12 months of hospital admission.5 The death rate predicted by delirium in the Canadian study was comparable to the death rate of those who experience other serious medical conditions, such as sepsis or a heart attack.6
Those who survive delirium experience other serious outcomes, such as a negative impact on function and cognition and an increase in long-term care placement.7 Even when the condition resolves quickly, lasting functional impairment may be evident without return to baseline functioning.8 Hospitalized veterans are generally older, making them susceptible to developing delirium.9
Prevalence
Delirium can result from multiple medical conditions and develops in up to 50% of patients after general surgery and up to 80% of patients in the intensive care unit.10,11 From 20% to 40% of hospitalized older adults and from 50% to 89% of patients with preexisting Alzheimer disease may develop delirium.12-15 The increasing number of aging adults who will be hospitalized may also result in an increased prevalence of delirium.1,16
Delirium is a result of various predisposing and precipitating factors.1 Predisposing vulnerabilities are intrinsic to the individual, whereas precipitating external stressors are found in the environment. External stressors may trigger delirium in an individual who is vulnerable due to predisposing risk. The primary risk factors for delirium include dementia, advanced age, sensory impairment, fracture, infection, and dehydration (Table 1).12
Predisposing factors for delirium, such as age and sex, lifestyle choices (alcohol, tobacco), and chronic conditions (atherosclerosis, depression, prior stroke/transient ischemic attack) are more prevalent in the veteran population.9,17-20 In 2011, the median age for male veterans was 64 and the median age for male nonveterans was 41. Of male veterans, 49.9% are aged ≥ 65 years in comparison with 10.5% of the nonveteran male population.21 Veterans also have higher rates of comorbidities; a significant risk factor for delirium.20 A study by Agha and colleagues found that veterans were 14 times more likely to have 5 or more medical conditions than that of the general population.9 In a study comparing veterans aged ≥ 65 years with their age matched nonveteran peers, the health status of the veterans was poorer overall.22 Veterans are more likely to have posttraumatic stress disorder, which can increase the risk of postsurgery delirium and dementia, a primary risk factor for delirium.23-26
Delirium Intervention
Up to 40% of delirium cases can be prevented.27 But delirium may remain undetected in older veterans because its symptoms are sometimes thought to be the unavoidable consequences of aging, dementia, preexisting mental health conditions, substance abuse, a disease process, or the hospital environment.28 Therefore, to avoid the negative consequences of delirium, prevention is critical.28
The goals of delirium treatment are to identify and reverse its underlying cause(s).29 Because delirium is typically multifactorial, an HCP must carefully consider the various sources that could have initiated a change in mental status. Delirium may be prevented if HCPs can reduce patient risk factors. The 2010 National Institute for Health and Clinical Excellence (NICE) Delirium Guideline recommended a set of prevention strategies to address delirium risk factors (Table 2).12
As a member of the health care team, social workers can help prevent delirium through attention to pain management, infection control, medication review, sensory improvement, adequate nutrition and hydration, hypoxia prevention, and mobilization.12No pharmacologic approach has been approved for the treatment of delirium.30 Drugs may manage symptoms associated with delirium, but they do not treat the disease and could be associated with toxicity in high-risk patients. However, there are a variety of nonpharmacologic preventative measures that have proven effective. Environmental interventions to prevent delirium include orientation, cognitive stimulation, and sensory aids. A 2013 meta-analysis of 19 delirium prevention programs found that most were effective in preventing delirium in patients at risk during hospitalization.31 This review found that the most successful programs included multidisciplinary teams providing staff education and therapeutic cognitive activities.31 Social workers can encourage and directly provide such services. The Delirium Toolbox is a delirium risk modification program that was piloted with frontline staff, including social workers, at the VA Boston Healthcare System in West Roxbury, Massachusetts, and has been associated with restraint reduction, shortened length of stay (LOS), and lower variable direct costs.32
Social Worker Role
Several studies, both national and international, have indicated that little has been done over the past 2 decades to increase the diagnosis of delirium, because only 12% to 35% of delirium cases are clinically detected within the emergency department and in acute care settings.33-37 Patients may hesitate to report their experience due to a sense of embarrassment or because of an inability to describe it.38
Social workers are skilled at helping individuals feel more at ease when disclosing distressing experiences. Delirium is relevant to HCPs and hospital social workers with care transition responsibilities, because delirium detection should impact discharge planning.1,39 Delirium education needs to be included in efforts to improve transitions from intensive care settings to lower levels of care and from lower levels of care to discharge.40 Hospital social workers are in a position to offer additional support because they see patients at a critical juncture in their care and can take steps to improve postdischarge outcomes.41
Prior to Onset
Social workers can play an important role prior to delirium onset.42 Patient education on delirium needs to be provided during the routine hospital intake assessment. Informing patients in advance that delirium is common, based on their risk factors, as well as what to expect if delirium is experienced has been found to provide comfort.38 Families who anticipated possible delirium-related confusion reported that they experienced less distress.38
Related: Baseball Reminiscence Therapy for Cognitively Impaired Veterans
During hospitalization, social workers can ascertain from families whether an alteration in mental status is a rapid change, possibly indicating delirium, or a gradual dementia onset. The social work skills of advocacy and education can be used to support delirium-risk identification to avoid adverse outcomes.43 When no family caregiver is present to provide a history of the individual’s cognitive function prior to hospitalization, the social worker may be the first to notice an acute change in cognitive status and can report this to the medical team.
During Delirium
Lack of patient responsiveness and difficulty following a conversation are possible signs of delirium. This situation should be reported to the medical team for further delirium assessment and diagnosis.4 The social worker can also attempt to determine whether a patient’s presentation is unusual by contacting the family. Social work training recognizes the important role of the family.44 Social workers often interact with families at the critical period between acute onset of delirium in the hospital and discharge.42 Studies have shown that delirium causes stress for the patient’s loved ones. Moreover, caregivers of patients who experience the syndrome are at a 12 times increased risk of meeting the criteria for generalized anxiety disorder.30 In one study, delirium was rated as more distressing for the caregivers who witnessed it than for the patients who experienced it.38 Education has been shown to reduce delirium-related distress.30
In cases where delirium is irreversible, such as during the active dying process, social workers can serve in a palliative role to ease family confusion and provide comfort.30 The presence of family and other familiar people are considered part of the nonpharmacologic management of delirium.28
Posthospitalization
Delirium complicates physical aspects of care for families, as their loved one may need direct care in areas where they were previously independent due to a loss of function. Logistic considerations such as increased supervision may be necessary due to delirium, and the patient’s condition may be upsetting and confusing for family members, triggering the need for emotional support. During the discharge process, social workers can provide support and education to family members or placement facilities.38
Social workers in the hospital setting are often responsible for discharge planning, including the reduction of extended LOS and unnecessary readmissions to the hospital.45 Increased LOS and hospital readmissions are 2 of the primary negative outcomes associated with delirium. Delirium can persist for months beyond hospitalization, making it a relevant issue at the time of discharge and beyond.46 Distress related to delirium has been documented up to 2 years after onset, due to manifestations of anxiety and depression.38
Distress impacts patients as well as caregivers who witness the delirium and provide care to the patient afterward.38 Long-term changes in mood in addition to loss of function as a result of delirium can lead to an increase in stress for both patients and their caregivers.30 The social work emphasis on counseling and family dynamics as well as the common role of coordinating post-discharge arrangements makes the profession uniquely suited for delirium care.
Barriers
Social workers can play a key role in delirium risk identification and coordination of care but face substantial barriers. Delirium assessments are complex and require training and education in the features of delirium and cognitive assessment.47 To date, social workers receive limited education about delirium and typically do not make deliberate efforts in prevention, support, and follow-up care.
Conclusion
Social workers will encounter delirium, and their training makes them particularly suited to address this health concern. An understanding of the larger ecologic system is a foundational aspect of social work and an essential component of delirium prevention and care.41 The multipathway nature of delirium as well as the importance of prevention suggests that multiple disciplines, including social work, should be involved.1 The American Delirium Society and the European Delirium Association both recognize the need for all HCPs to be engaged in delirium care.1,48
Related: Sharing Alzheimer Research, FasterSharing Alzheimer Research, Faster
Social workers in the hospital setting provide communication, advocacy, and education to other HCPs, as well as to patients and families (Figure). Because delirium directly impacts the emotional and logistic needs of patients and their families, it would be advantageous for social workers to take a more active role in delirium risk identification, prevention, and care. Fortunately, the nonpharmacologic approaches that social workers are skilled in providing (eg, education and emotional support) have been shown to benefit patients with delirium and their families.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Delirium, or the state of mental confusion that may occur with physical or mental illness, is common, morbid, and costly; however, of the diagnosed cases, delirium is mentioned in hospital discharge summaries only 16% to 55% of the time.1-3
Social workers often coordinate care transitions for hospitalized older veterans. They serve as interdisciplinary team members who communicate with VA medical staff as well as with the patient and family. This position, in addition to their training in communication and advocacy, primes social workers for a role in delirium care and provides the needed support for veterans who experience delirium and their families.
Background
Delirium is a sudden disturbance of attention with reduced awareness of the environment. Because attention is impaired, other changes in cognition are common, including perceptual and thought disturbances. Additionally, delirium includes fluctuations in consciousness over the course of a day. The acute development of these cognitive disturbances is distinct from a preexisting chronic cognitive impairment, such as dementia. Delirium is a direct consequence of underlying medical conditions, such as infections, polypharmacy, dehydration, and surgery.4
Delirium subtypes all have inattention as a core symptom. In half of the cases, patients are hypoactive and will not awaken easily or participate in daily care plans readily.4 Hyperactive delirium occurs in a quarter of cases. In the remaining mixed delirium cases patients fluctuate between the 2 states.4
Delirium is often falsely mistaken for dementia. Although delirium and dementia can present similarly, delirium has a sudden onset, which can alert health care professionals (HCPs) to the likelihood of delirium. Another important distinction is that delirium is typically reversible. Symptom manifestations of delirium may also be confused with depression.
Related: Delirium in the Cardiac ICU
Preventing delirium is important due to its many negative health outcomes. Older adults who develop delirium are more likely to die sooner. In a Canadian study of hospitalized patients aged ≥ 65 years, 41.6% of the delirium cohort and 14.4% of the control group died within 12 months of hospital admission.5 The death rate predicted by delirium in the Canadian study was comparable to the death rate of those who experience other serious medical conditions, such as sepsis or a heart attack.6
Those who survive delirium experience other serious outcomes, such as a negative impact on function and cognition and an increase in long-term care placement.7 Even when the condition resolves quickly, lasting functional impairment may be evident without return to baseline functioning.8 Hospitalized veterans are generally older, making them susceptible to developing delirium.9
Prevalence
Delirium can result from multiple medical conditions and develops in up to 50% of patients after general surgery and up to 80% of patients in the intensive care unit.10,11 From 20% to 40% of hospitalized older adults and from 50% to 89% of patients with preexisting Alzheimer disease may develop delirium.12-15 The increasing number of aging adults who will be hospitalized may also result in an increased prevalence of delirium.1,16
Delirium is a result of various predisposing and precipitating factors.1 Predisposing vulnerabilities are intrinsic to the individual, whereas precipitating external stressors are found in the environment. External stressors may trigger delirium in an individual who is vulnerable due to predisposing risk. The primary risk factors for delirium include dementia, advanced age, sensory impairment, fracture, infection, and dehydration (Table 1).12
Predisposing factors for delirium, such as age and sex, lifestyle choices (alcohol, tobacco), and chronic conditions (atherosclerosis, depression, prior stroke/transient ischemic attack) are more prevalent in the veteran population.9,17-20 In 2011, the median age for male veterans was 64 and the median age for male nonveterans was 41. Of male veterans, 49.9% are aged ≥ 65 years in comparison with 10.5% of the nonveteran male population.21 Veterans also have higher rates of comorbidities; a significant risk factor for delirium.20 A study by Agha and colleagues found that veterans were 14 times more likely to have 5 or more medical conditions than that of the general population.9 In a study comparing veterans aged ≥ 65 years with their age matched nonveteran peers, the health status of the veterans was poorer overall.22 Veterans are more likely to have posttraumatic stress disorder, which can increase the risk of postsurgery delirium and dementia, a primary risk factor for delirium.23-26
Delirium Intervention
Up to 40% of delirium cases can be prevented.27 But delirium may remain undetected in older veterans because its symptoms are sometimes thought to be the unavoidable consequences of aging, dementia, preexisting mental health conditions, substance abuse, a disease process, or the hospital environment.28 Therefore, to avoid the negative consequences of delirium, prevention is critical.28
The goals of delirium treatment are to identify and reverse its underlying cause(s).29 Because delirium is typically multifactorial, an HCP must carefully consider the various sources that could have initiated a change in mental status. Delirium may be prevented if HCPs can reduce patient risk factors. The 2010 National Institute for Health and Clinical Excellence (NICE) Delirium Guideline recommended a set of prevention strategies to address delirium risk factors (Table 2).12
As a member of the health care team, social workers can help prevent delirium through attention to pain management, infection control, medication review, sensory improvement, adequate nutrition and hydration, hypoxia prevention, and mobilization.12No pharmacologic approach has been approved for the treatment of delirium.30 Drugs may manage symptoms associated with delirium, but they do not treat the disease and could be associated with toxicity in high-risk patients. However, there are a variety of nonpharmacologic preventative measures that have proven effective. Environmental interventions to prevent delirium include orientation, cognitive stimulation, and sensory aids. A 2013 meta-analysis of 19 delirium prevention programs found that most were effective in preventing delirium in patients at risk during hospitalization.31 This review found that the most successful programs included multidisciplinary teams providing staff education and therapeutic cognitive activities.31 Social workers can encourage and directly provide such services. The Delirium Toolbox is a delirium risk modification program that was piloted with frontline staff, including social workers, at the VA Boston Healthcare System in West Roxbury, Massachusetts, and has been associated with restraint reduction, shortened length of stay (LOS), and lower variable direct costs.32
Social Worker Role
Several studies, both national and international, have indicated that little has been done over the past 2 decades to increase the diagnosis of delirium, because only 12% to 35% of delirium cases are clinically detected within the emergency department and in acute care settings.33-37 Patients may hesitate to report their experience due to a sense of embarrassment or because of an inability to describe it.38
Social workers are skilled at helping individuals feel more at ease when disclosing distressing experiences. Delirium is relevant to HCPs and hospital social workers with care transition responsibilities, because delirium detection should impact discharge planning.1,39 Delirium education needs to be included in efforts to improve transitions from intensive care settings to lower levels of care and from lower levels of care to discharge.40 Hospital social workers are in a position to offer additional support because they see patients at a critical juncture in their care and can take steps to improve postdischarge outcomes.41
Prior to Onset
Social workers can play an important role prior to delirium onset.42 Patient education on delirium needs to be provided during the routine hospital intake assessment. Informing patients in advance that delirium is common, based on their risk factors, as well as what to expect if delirium is experienced has been found to provide comfort.38 Families who anticipated possible delirium-related confusion reported that they experienced less distress.38
Related: Baseball Reminiscence Therapy for Cognitively Impaired Veterans
During hospitalization, social workers can ascertain from families whether an alteration in mental status is a rapid change, possibly indicating delirium, or a gradual dementia onset. The social work skills of advocacy and education can be used to support delirium-risk identification to avoid adverse outcomes.43 When no family caregiver is present to provide a history of the individual’s cognitive function prior to hospitalization, the social worker may be the first to notice an acute change in cognitive status and can report this to the medical team.
During Delirium
Lack of patient responsiveness and difficulty following a conversation are possible signs of delirium. This situation should be reported to the medical team for further delirium assessment and diagnosis.4 The social worker can also attempt to determine whether a patient’s presentation is unusual by contacting the family. Social work training recognizes the important role of the family.44 Social workers often interact with families at the critical period between acute onset of delirium in the hospital and discharge.42 Studies have shown that delirium causes stress for the patient’s loved ones. Moreover, caregivers of patients who experience the syndrome are at a 12 times increased risk of meeting the criteria for generalized anxiety disorder.30 In one study, delirium was rated as more distressing for the caregivers who witnessed it than for the patients who experienced it.38 Education has been shown to reduce delirium-related distress.30
In cases where delirium is irreversible, such as during the active dying process, social workers can serve in a palliative role to ease family confusion and provide comfort.30 The presence of family and other familiar people are considered part of the nonpharmacologic management of delirium.28
Posthospitalization
Delirium complicates physical aspects of care for families, as their loved one may need direct care in areas where they were previously independent due to a loss of function. Logistic considerations such as increased supervision may be necessary due to delirium, and the patient’s condition may be upsetting and confusing for family members, triggering the need for emotional support. During the discharge process, social workers can provide support and education to family members or placement facilities.38
Social workers in the hospital setting are often responsible for discharge planning, including the reduction of extended LOS and unnecessary readmissions to the hospital.45 Increased LOS and hospital readmissions are 2 of the primary negative outcomes associated with delirium. Delirium can persist for months beyond hospitalization, making it a relevant issue at the time of discharge and beyond.46 Distress related to delirium has been documented up to 2 years after onset, due to manifestations of anxiety and depression.38
Distress impacts patients as well as caregivers who witness the delirium and provide care to the patient afterward.38 Long-term changes in mood in addition to loss of function as a result of delirium can lead to an increase in stress for both patients and their caregivers.30 The social work emphasis on counseling and family dynamics as well as the common role of coordinating post-discharge arrangements makes the profession uniquely suited for delirium care.
Barriers
Social workers can play a key role in delirium risk identification and coordination of care but face substantial barriers. Delirium assessments are complex and require training and education in the features of delirium and cognitive assessment.47 To date, social workers receive limited education about delirium and typically do not make deliberate efforts in prevention, support, and follow-up care.
Conclusion
Social workers will encounter delirium, and their training makes them particularly suited to address this health concern. An understanding of the larger ecologic system is a foundational aspect of social work and an essential component of delirium prevention and care.41 The multipathway nature of delirium as well as the importance of prevention suggests that multiple disciplines, including social work, should be involved.1 The American Delirium Society and the European Delirium Association both recognize the need for all HCPs to be engaged in delirium care.1,48
Related: Sharing Alzheimer Research, FasterSharing Alzheimer Research, Faster
Social workers in the hospital setting provide communication, advocacy, and education to other HCPs, as well as to patients and families (Figure). Because delirium directly impacts the emotional and logistic needs of patients and their families, it would be advantageous for social workers to take a more active role in delirium risk identification, prevention, and care. Fortunately, the nonpharmacologic approaches that social workers are skilled in providing (eg, education and emotional support) have been shown to benefit patients with delirium and their families.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Rudolph JL, Boustani M, Kamholz B, Shaughnessey M, Shay K; American Delirium Society. Delirium: a strategic plan to bring an ancient disease into the 21st century. J Am Geriatr Soc. 2011;59(suppl 2):S237-S240.
2. Hope C, Estrada N, Weir C, Teng CC, Damal K, Sauer BC. Documentation of delirium in the VA electronic health record. BMC Res Notes. 2014;7:208.
3. van Zyl LT, Davidson PR. Delirium in hospital: an underreported event at discharge. Can J Psychiatry. 2003;48(8):555-560.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
5. McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457-463.
6. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
7. McCusker J, Cole M, Dendukuri N, Belzile E, Primeau F. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ. 2001;165(5):575-583.
8. Quinlan N, Rudolph JL. Postoperative delirium and functional decline after noncardiac surgery. J Am Geriatr Soc. 2011;59(suppl 2):S301-S304.
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
10. Marcantonio ER, Simon SE, Bergmann MA, Jones RN, Murphy KM, Morris JN. Delirium symptoms in post-acute care: prevalent, persistent, and associated with poor functional recovery. J Am Geriatr Soc. 2003;51(1):4-9.
11. McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598.
12. National Institute for Health and Clinical Excellence. Delirium: Diagnosis, Prevention and Management. National Institute for Health and Clinical Excellence Website. https://www.nice.org.uk/guidance/cg103/resources/delirium-174507018181. Published July 2010.
13. Fick D, Foreman M. Consequences of not recognizing delirium superimposed on dementia in hospitalized elderly individuals. J Gerontol Nurs. 2000;26(1):30-40.
14. Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50(10):1723-1732.
15. Edlund A, Lundström M, Brännström B, Bucht G, Gustafson Y. Delirium before and after operation for femoral neck fracture. J Am Geriatr Soc. 2001;49(10):1335-1340.
16. Popejoy LL, Galambos C, Moylan K, Madsen R. Challenges to hospital discharge planning for older adults. Clin Nurs Res. 2012;21(4):431-449.
17. Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271(2):134-139.
18. Rudolph JL, Jones RN, Rasmussen LS, Silverstein JH, Inouye SK, Marcantonio ER. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. 2007;120(9):807-813.
19. Rudolph JL, Babikian VL, Birjiniuk V, et al. Atherosclerosis is associated with delirium after coronary artery bypass graft surgery. J Am Geriatr Soc. 2005;53(3):462-466.
20. Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119(2):229-236.
21. U.S. Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of Veterans: 2013 Data from the American Community Survey. U.S. Department of Veterans Affairs Website. http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2013.pdf. Accessed November 14, 2015.
22. Selim AJ, Berlowitz DR, Fincke G, et al. The health status of elderly veteran enrollees in the Veterans Health Administration. J Am Geriatr Soc. 2004;52(8):1271-1276.
23. McGuire JM. The incidence of and risk factors for emergence delirium in U.S. military combat veterans. J Perianesth Nurs. 2012;27(4):236-245.
24. Lepousé C, Lautner CA, Liu L, Gomis P, Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth. 2006;96(6):747-753.
25. Meziab O, Kirby KA, Williams B, Yaffe K, Byers AL, Barnes DE. Prisoner of war status, posttraumatic stress disorder, and dementia in older veterans. Alzheimers Dement. 2014;10(3)(suppl):S236-S241.
26. Elie M, Cole MG, Primeau FJ, Bellavance F. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med. 1998;13(3):204-212.
27. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220.
28. Conley DM. The gerontological clinical nurse specialist's role in prevention, early recognition, and management of delirium in hospitalized older adults. Urol Nurs. 2011;31(6):337-342.
29. Meagher DJ. Delirium: optimising management. BMJ. 2001;322(7279):144-149.
30. Irwin SA, Pirrello RD, Hirst JM, Buckholz GT, Ferris FD. Clarifying delirium management: practical, evidenced-based, expert recommendations for clinical practice. J Palliat Med. 2013;16(4):423-435.
31. Reston JT, Schoelles KM. In-facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5, pt 2):375-380.
32. Rudolph JL, Archambault E, Kelly B; VA Boston Delirium Task Force. A delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):957.e7-957.e11.
33. Gustafson Y, Brännström B, Norberg A, Bucht G, Winblad B. Underdiagnosis and poor documentation of acute confusional states in elderly hip fracture patients. J Am Geriatr Soc. 1991;39(8):760-765.
34. Hustey FM, Meldon SW. The prevalence and documentation of impaired mental status in elderly emergency department patients. Ann Emerg Med. 2002;39(3):248-253.
35. Kales HC, Kamholz BA, Visnic SG, Blow FC. Recorded delirium in a national sample of elderly inpatients: potential implications for recognition. J Geriatr Psychiatry Neurol. 2003;16(1):32-38.
36. Lemiengre J, Nelis T, Joosten E, et al. Detection of delirium by bedside nurses using the confusion assessment method. J Am Geriatr Soc. 2006;54(4):685-689.
37. Milisen K, Foreman MD, Wouters B, et al. Documentation of delirium in elderly patients with hip fracture. J Gerontol Nurs. 2002;28(11):23-29.
38. Partridge JS, Martin FC, Harari D, Dhesi JK. The delirium experience: what is the effect on patients, relatives and staff and what can be done to modify this? Int J Geriatr Psychiatry. 2013;28(8):804-812.
39. Simons K, Connolly RP, Bonifas R, et al. Psychosocial assessment of nursing home residents via MDS 3.0: recommendations for social service training, staffing, and roles in interdisciplinary care. J Am Med Dir Assoc. 2012;13(2):190.e9-190.e15.
40. Alici Y. Interventions to improve recognition of delirium: a sine qua non for successful transitional care programs. Arch Intern Med. 2012;172(1):80-82.
41. Judd RG, Sheffield S. Hospital social work: contemporary roles and professional activities. Soc Work Health Care. 2010;49(9):856-871.
42. Duffy F, Healy JP. Social work with older people in a hospital setting. Soc Work Health Care. 2011;50(2):109-123.
43. Anderson CP, Ngo LH, Marcantonio ER. Complications in post-acute care are associated with persistent delirium. J Am Geriatr Soc. 2012;60(6):1122-1127.
44. Bauer M, Fitzgerald L, Haesler E, Manfrin M. Hospital discharge planning for frail older people and their family. Are we delivering best practice? A review of the evidence. J Clin Nurs. 2009;18(18):2539-2546.
45. Shepperd S, Lannin NA, Clemson LM, McCluskey A, Cameron ID, Barras SL. Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313.
46. McCusker J, Cole M, Dendukuri N, Han L, Belzile E. The course of delirium in older medical inpatients: A prospective study. J Gen Intern Med. 2003;18(9):696-704.
47. Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM Jr. Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161(20):2467-2473.
48. Teodorczuk A, Reynish E, Milisen K. Improving recognition of delirium in clinical practice: a call for action. BMC Geriatr. 2012;12:55.
1. Rudolph JL, Boustani M, Kamholz B, Shaughnessey M, Shay K; American Delirium Society. Delirium: a strategic plan to bring an ancient disease into the 21st century. J Am Geriatr Soc. 2011;59(suppl 2):S237-S240.
2. Hope C, Estrada N, Weir C, Teng CC, Damal K, Sauer BC. Documentation of delirium in the VA electronic health record. BMC Res Notes. 2014;7:208.
3. van Zyl LT, Davidson PR. Delirium in hospital: an underreported event at discharge. Can J Psychiatry. 2003;48(8):555-560.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
5. McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457-463.
6. Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
7. McCusker J, Cole M, Dendukuri N, Belzile E, Primeau F. Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ. 2001;165(5):575-583.
8. Quinlan N, Rudolph JL. Postoperative delirium and functional decline after noncardiac surgery. J Am Geriatr Soc. 2011;59(suppl 2):S301-S304.
9. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257.
10. Marcantonio ER, Simon SE, Bergmann MA, Jones RN, Murphy KM, Morris JN. Delirium symptoms in post-acute care: prevalent, persistent, and associated with poor functional recovery. J Am Geriatr Soc. 2003;51(1):4-9.
11. McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591-598.
12. National Institute for Health and Clinical Excellence. Delirium: Diagnosis, Prevention and Management. National Institute for Health and Clinical Excellence Website. https://www.nice.org.uk/guidance/cg103/resources/delirium-174507018181. Published July 2010.
13. Fick D, Foreman M. Consequences of not recognizing delirium superimposed on dementia in hospitalized elderly individuals. J Gerontol Nurs. 2000;26(1):30-40.
14. Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50(10):1723-1732.
15. Edlund A, Lundström M, Brännström B, Bucht G, Gustafson Y. Delirium before and after operation for femoral neck fracture. J Am Geriatr Soc. 2001;49(10):1335-1340.
16. Popejoy LL, Galambos C, Moylan K, Madsen R. Challenges to hospital discharge planning for older adults. Clin Nurs Res. 2012;21(4):431-449.
17. Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;271(2):134-139.
18. Rudolph JL, Jones RN, Rasmussen LS, Silverstein JH, Inouye SK, Marcantonio ER. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. 2007;120(9):807-813.
19. Rudolph JL, Babikian VL, Birjiniuk V, et al. Atherosclerosis is associated with delirium after coronary artery bypass graft surgery. J Am Geriatr Soc. 2005;53(3):462-466.
20. Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119(2):229-236.
21. U.S. Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Profile of Veterans: 2013 Data from the American Community Survey. U.S. Department of Veterans Affairs Website. http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2013.pdf. Accessed November 14, 2015.
22. Selim AJ, Berlowitz DR, Fincke G, et al. The health status of elderly veteran enrollees in the Veterans Health Administration. J Am Geriatr Soc. 2004;52(8):1271-1276.
23. McGuire JM. The incidence of and risk factors for emergence delirium in U.S. military combat veterans. J Perianesth Nurs. 2012;27(4):236-245.
24. Lepousé C, Lautner CA, Liu L, Gomis P, Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth. 2006;96(6):747-753.
25. Meziab O, Kirby KA, Williams B, Yaffe K, Byers AL, Barnes DE. Prisoner of war status, posttraumatic stress disorder, and dementia in older veterans. Alzheimers Dement. 2014;10(3)(suppl):S236-S241.
26. Elie M, Cole MG, Primeau FJ, Bellavance F. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med. 1998;13(3):204-212.
27. Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210-220.
28. Conley DM. The gerontological clinical nurse specialist's role in prevention, early recognition, and management of delirium in hospitalized older adults. Urol Nurs. 2011;31(6):337-342.
29. Meagher DJ. Delirium: optimising management. BMJ. 2001;322(7279):144-149.
30. Irwin SA, Pirrello RD, Hirst JM, Buckholz GT, Ferris FD. Clarifying delirium management: practical, evidenced-based, expert recommendations for clinical practice. J Palliat Med. 2013;16(4):423-435.
31. Reston JT, Schoelles KM. In-facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5, pt 2):375-380.
32. Rudolph JL, Archambault E, Kelly B; VA Boston Delirium Task Force. A delirium risk modification program is associated with hospital outcomes. J Am Med Dir Assoc. 2014;15(12):957.e7-957.e11.
33. Gustafson Y, Brännström B, Norberg A, Bucht G, Winblad B. Underdiagnosis and poor documentation of acute confusional states in elderly hip fracture patients. J Am Geriatr Soc. 1991;39(8):760-765.
34. Hustey FM, Meldon SW. The prevalence and documentation of impaired mental status in elderly emergency department patients. Ann Emerg Med. 2002;39(3):248-253.
35. Kales HC, Kamholz BA, Visnic SG, Blow FC. Recorded delirium in a national sample of elderly inpatients: potential implications for recognition. J Geriatr Psychiatry Neurol. 2003;16(1):32-38.
36. Lemiengre J, Nelis T, Joosten E, et al. Detection of delirium by bedside nurses using the confusion assessment method. J Am Geriatr Soc. 2006;54(4):685-689.
37. Milisen K, Foreman MD, Wouters B, et al. Documentation of delirium in elderly patients with hip fracture. J Gerontol Nurs. 2002;28(11):23-29.
38. Partridge JS, Martin FC, Harari D, Dhesi JK. The delirium experience: what is the effect on patients, relatives and staff and what can be done to modify this? Int J Geriatr Psychiatry. 2013;28(8):804-812.
39. Simons K, Connolly RP, Bonifas R, et al. Psychosocial assessment of nursing home residents via MDS 3.0: recommendations for social service training, staffing, and roles in interdisciplinary care. J Am Med Dir Assoc. 2012;13(2):190.e9-190.e15.
40. Alici Y. Interventions to improve recognition of delirium: a sine qua non for successful transitional care programs. Arch Intern Med. 2012;172(1):80-82.
41. Judd RG, Sheffield S. Hospital social work: contemporary roles and professional activities. Soc Work Health Care. 2010;49(9):856-871.
42. Duffy F, Healy JP. Social work with older people in a hospital setting. Soc Work Health Care. 2011;50(2):109-123.
43. Anderson CP, Ngo LH, Marcantonio ER. Complications in post-acute care are associated with persistent delirium. J Am Geriatr Soc. 2012;60(6):1122-1127.
44. Bauer M, Fitzgerald L, Haesler E, Manfrin M. Hospital discharge planning for frail older people and their family. Are we delivering best practice? A review of the evidence. J Clin Nurs. 2009;18(18):2539-2546.
45. Shepperd S, Lannin NA, Clemson LM, McCluskey A, Cameron ID, Barras SL. Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;1:CD000313.
46. McCusker J, Cole M, Dendukuri N, Han L, Belzile E. The course of delirium in older medical inpatients: A prospective study. J Gen Intern Med. 2003;18(9):696-704.
47. Inouye SK, Foreman MD, Mion LC, Katz KH, Cooney LM Jr. Nurses' recognition of delirium and its symptoms: comparison of nurse and researcher ratings. Arch Intern Med. 2001;161(20):2467-2473.
48. Teodorczuk A, Reynish E, Milisen K. Improving recognition of delirium in clinical practice: a call for action. BMC Geriatr. 2012;12:55.
Urinary Excretion Indices in AKI
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 70‐year‐old woman with a history of diabetes mellitus type 2 and hypertension was admitted with abdominal pain following 2 days of nausea and diarrhea. Initial laboratory studies revealed blood urea nitrogen (BUN) 25 mg/dL and serum creatinine 1.3 mg/dL. Computed tomography of the abdomen and pelvis with nonionic, low osmolar intravenous and oral contrast demonstrated acute diverticulitis with an associated small abscess. She was administered intravenous 0.9% sodium chloride solution and antibiotics. Blood pressure on admission was 92/55 mm Hg, and 24 hours later, her BUN and serum creatinine increased to 33 mg/dL and 1.9 mg/dL, respectively. Her urine output during the preceding 24 hours was 500 mL.
In the evaluation of acute kidney injury (AKI), is the measurement of fractional excretion of sodium (FeNa) and fractional excretion of urea (FeUr) of value?
WHY YOU MIGHT THINK ORDERING FeNa AND/OR FeUr IN THE EVALUATION OF AKI IS HELPFUL
The proper maintenance of sodium balance is paramount to regulating the size of body fluid compartments. Through the interaction of multiple physiologic processes, the kidney regulates tubular reabsorption (or lack thereof) of sodium chloride to match excretion to intake. In normal health, FeNa is typically 1%, although it may vary depending on the dietary sodium intake. The corollary is that 99% of filtered sodium is reabsorbed. Acute tubular injury (ATI) that impairs the tubular resorptive capacity for sodium may increase FeNa to >3%. In addition, during states of water conservation, urea is reabsorbed from the medullary collecting duct, explaining the discrepant rise in BUN relative to creatinine in prerenal azotemia. FeUr falls progressively as water is reabsorbed and urine flow declines, and FeUr less than 35% to 40% may result during prerenal azotemia versus >50% in health or ATI. Theoretically, FeUr is largely unaffected by diuretics, whereas FeNa is increased by diuretics.
In 1976, Espinel reported on the use of FeNa in 17 oliguric patients to discriminate prerenal azotemia from ATI.[1] Establishing what are now familiar indices, FeNa <1% was deemed consistent with prerenal physiology versus >3% indicating ATI. Notably, the study excluded patients who had received diuretics or in whom chronic kidney disease (CKD), glomerulonephritis, or urinary obstruction was suspected.
Given the limitations of FeNa in the context of diuretic use, many physicians instead use FeUr to distinguish prerenal versus ATI causes of AKI. Carvounis et al. reported FeUr and FeNa in 50 patients with prerenal azotemia, 27 with prerenal azotemia receiving diuretics and 25 patients with ATI.[2] Patients with interstitial nephritis, glomerulonephritis, and obstruction were excluded. In the entire cohort, the authors reported sensitivity of 90% and specificity of 96% for FeUr <35% in identifying prerenal azotemia (Table 1). FeNa <1% was slightly less sensitive for prerenal azotemia in the entire cohort at 77%, and this fell to 48% in the presence of diuretics as compared to 89% for FeUr. Naturally, the specificity of FeNa for ATI will fall with the use of diuretics. As shown in Table 1, FeUr <35% has an excellent positive likelihood ratio (LR+) of 22 for prerenal azotemia and a moderate LR+ of 9 for FeUr 35% being consistent with ATI, regardless of the presence of diuretics. This contrasts with FeNa, which if 1% in the presence of diuretics, lacked utility in the diagnosis of ATI. Of note, diuretic use was reported only in the prerenal azotemia group and not specifically in the ATI group. Thus, these comparisons assume diuretics have no effect on test characteristics in ATI. This assumption, however, may not be valid.
| FeUr | FeNa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sens | Spec | PPV | NPV | LR+ | LR | Sens | Spec | PPV | NPV | LR+ | LR | |||
| ||||||||||||||
| Carvounis[2] | Prerenal | Overall | 0.90 | 0.96 | 0.99 | 0.75 | 22.4 | 0.1 | 0.77 | 0.96 | 0.98 | 0.57 | 19.2 | 0.2 |
| No diuretics | 0.90 | 0.96 | 0.98 | 0.83 | 22.5 | 0.1 | 0.92 | 0.96 | 0.98 | 0.86 | 23.0 | 0.1 | ||
| Diuretics | 0.89 | 0.96 | 0.96 | 0.89 | 22.2 | 0.1 | 0.48 | 0.96 | 0.93 | 0.63 | 12.0 | 0.5 | ||
| ATI | Overall | 0.96 | 0.90 | 0.75 | 0.99 | 9.2 | 0.0 | 0.96 | 0.77 | 0.57 | 0.98 | 4.1 | 0.1 | |
| No diuretics* | 0.96 | 0.90 | 0.83 | 0.98 | 9.6 | 0.0 | 0.96 | 0.92 | 0.86 | 0.98 | 12.0 | 0.0 | ||
| Diuretics | 0.96 | 0.89 | 0.89 | 0.96 | 8.6 | 0.0 | 0.96 | 0.48 | 0.63 | 0.93 | 1.9 | 0.1 | ||
| Diskin[8] | Prerenal | Overall | 0.97 | 0.85 | 0.96 | 0.89 | 6.5 | 0.0 | 0.44 | 0.75 | 0.88 | 0.25 | 1.8 | 0.7 |
| No diuretics | 0.91 | 0.89 | 0.95 | 0.80 | 8.2 | 0.1 | 0.83 | 0.67 | 0.86 | 0.60 | 2.5 | 0.3 | ||
| Diuretics | 1.00 | 0.82 | 0.97 | 1.00 | 5.5 | 0.0 | 0.29 | 0.82 | 0.89 | 0.18 | 1.6 | 0.9 | ||
| ATI | Overall | 0.85 | 0.97 | 0.89 | 0.96 | 33.6 | 0.2 | 0.75 | 0.44 | 0.25 | 0.88 | 1.3 | 0.6 | |
| No diuretics | 0.89 | 0.91 | 0.80 | 0.95 | 10.2 | 0.1 | 0.67 | 0.83 | 0.60 | 0.86 | 3.8 | 0.4 | ||
| Diuretics | 0.82 | 1.00 | 1.00 | 0.97 | N/A | 0.2 | 0.82 | 0.29 | 0.18 | 0.89 | 1.1 | 0.6 | ||
WHY THERE IS LITTLE REASON TO ROUTINELY ORDER FeNa AND FeUr IN PATIENTS WITH AKI
The argument against FeNa and FeUr is not primarily financial. FeNa and FeUr testing on all Medicare patients discharged with AKI in 2013 would have cost US$6 million.[3] Although a tiny fraction of annual healthcare expenditure, it would nevertheless be wasteful spending, and its true harm lays in the application of flawed diagnostic reasoning.
That flaw in our conceptual approach to AKI is the broad categorization of patients into either a prerenal or intrinsic etiology of AKI. In reality, renal injury is often multifactorial, and significant prerenal injury may progress to or coexist with intrinsic disease that is commonly ATI. Measurement of a urinary index at a single point in time will often fail to capture this spectrum of causes for AKI. Unfortunately, accurately assessing volume status through physical examination is difficult.[4] Considering FeNa and FeUr may be low in both hemorrhage as well as congestive heart failure, the measurement of these variables adds little to body volume assessment.
It cannot be overemphasized that application of FeNa and FeUr is predicated on the provider already knowing the diagnosis is either prerenal azotemia or ATI. Studies have generally excluded patients >65 years old and those with CKD or notable comorbid renal processes apart from prerenal azotemia or ATI. It is important to recall that a third of kidney biopsies may yield a diagnosis different than the prebiopsy clinical diagnosis, and the gold standard for ATI in studies of FeNa and FeUr was simply a failure of kidney function to improve promptly.[5] Why send a test that is predicated on largely already knowing the answer?
Fractional Excretion of Sodium for Diagnosis
Unfortunately, FeNa is neither sensitive nor specific enough in the general inpatient population to inform important clinical decisions regarding the etiology of AKI. Miller et al. examined 30 patients with oliguric prerenal azotemia, 55 with ATI (oliguric and nonoliguric), 10 with obstructive uropathy, and 7 with glomerulonephritis.[6] None of the patients had received diuretics within 24 hours of study entry. A FeNa <1% was present in 90% of prerenal patients and 4% of oliguric ATI. Importantly, of nonoliguric patients with ATI, 10% had a false positive FeNa <1%. Many subsequent studies have similarly documented the existence of FeNa <1% or otherwise indeterminate in ATI, particularly, but not exclusively, in nonoliguric states.[7] Diskin et al. evaluated FeNa in 100 prospective oliguric AKI patients (80 with prerenal azotemia and 20 with ATI) without CKD, with FeNa <1% being consistent with prerenal azotemia, 1% to 3% indeterminate, and >3% ATI.[8] The derived LR for FeNa for both prerenal azotemia and ATI are unlikely to alter pretest probability (Table 1). In part, this may be due to Diskin et al.'s incorporation of indeterminate FeNa, consistent with clinical reality. Carvounis et al. did not account for indeterminate values, and consequently the LR were likely overinflated in that study. It is now well‐recognized that glomerulonephritis may also result in FeNa <1% despite absence of identifiable prerenal physiology, as can intravenous iodinated contrast administration and rhabdomyolysis. Moreover, diuretic administration, polyuria due to osmotic diuresis, increased excretion of anions such as ketone bodies in diabetic ketoacidosis, the presence of CKD, and increased age, among others, can produce an FeNa that is indeterminate or >3% in the absence of ATI. Regarding diuretics, although the duration of action of furosemide is approximately 6 hours, longer‐acting loop diuretics such as torsemide or thiazide diuretics such as chlorthalidone may result in natriuresis for 24 hours.
Fractional Excretion of Urea for Diagnosis
Despite the potential superiority of FeUr to FeNa in supporting a diagnosis of prerenal azotemia in the setting of diuretic administration, FeUr nevertheless will only moderately increase the post‐test probability of prerenal azotemia under ideal conditions. In the study by Diskin et al., FeUr <40% was deemed consistent with prerenal azotemia and 40% with ATI. In the diagnosis of prerenal azotemia, the LR+ were 5.5 and 8.2 in the presence and absence of diuretics, respectively. Although the LR+ for the diagnosis of ATI was impressive, this was based on only 9 patients in the ATI‐no diuretic and 11 patients in the ATI‐diuretic groups. Carvounis, moreover, demonstrated considerably lower LR+ of approximately 9 for the diagnosis of ATI, and this study was unable to account for diuretic use specifically within the ATI group. Four of the 5 prerenal patients in Diskin et al.'s study misdiagnosed by FeUr had infection, and each were properly diagnosed by FeNa. Experimental data suggest endotoxemia may downregulate urea transporters as does aging, thereby increasing FeUr in sepsis and the elderly even in times of prerenal azotemia.[9, 10] Moreover, osmotic diuresis, such as with hyperglycemia or sickle cell nephropathy with medullary injury, may result in a falsely negative FeUr during prerenal states. In summary, these data suggest FeUr less than 35% to 40%, with the noted caveats, is most applicable to an oliguric patient in whom the pretest probability of prerenal azotemia is high, and it may be superior in the context of diuretics to the use of FeNa. Nonetheless, the impact on posttest probability is marginal. Of note, the diagnostic categories lack gold standards in these studies, and in the Carvounis study, FeNa (index under study) was 1 of several criteria actually used to categorize patients as either prerenal or ATI (outcomes under study). It is important to recognize these datasets contained very small numbers of patients with ATI, limiting the strength and generalizability of the scientific evidence. Other studies have failed to consistently demonstrate any utility to FeUr, particularly in those with CKD or critical illness.[11, 12, 13, 14, 15]
WHAT YOU SHOULD DO INSTEAD: DECIDE IF VOLUME MANIPULATION IS APPROPRIATE
The gold standard for diagnosis, as in many of the above studies, is the prompt improvement of prerenal azotemia with correction of renal hypoperfusion. Ultimately, the decision to administer intravenous fluids or diuretics in the management of AKI will often be independent of both FeNa and FeUr. In considering, for example, the case described above, it is not possible to realistically dichotomize the patient into either a prerenal or ATI category; both are quite likely present. If the clinical assessment supports a component of prerenal azotemia, a low FeNa and/or FeUr will not change the intervention. An elevated FeNa and/or FeUr, however, has at best moderate and potentially no impact on the likelihood for ATI. A patient, moreover, may still require volume manipulation in the context of established ATI. As such, these indices should not alter therapeutic decisions. There may be value in utilizing and identifying new approaches to determining a priori which patients will be fluid responsive, such as inferior vena cava ultrasound.[16] Lastly, evaluation of the urine sediment is an underutilized tool that may prove more useful in discriminating prerenal azotemia from ATI. It also helps to exclude other etiologies of AKI, such as glomerulonephritis and acute interstitial nephritis, which are typical exclusion criteria in studies of FeNa and FeUr.[17, 18]
WHEN IS FeNa AND/OR FeUr USEFUL IN DEFINING THE ETIOLOGY OF AKI?
FeNa and FeUr at best only support a clinical impression of prerenal azotemia or ATI in oliguric AKI, and the accuracy of these metrics is questionable in the setting of CKD, older age, and a variety of comorbidities. There is, however, a setting in which FeNa may be helpful. In practice, FeNa is useful in the evaluation of hepatorenal syndrome, a disorder characterized by oliguria and intense renal sodium reabsorption with resultant spot urine sodium <10 mEq/L and FeNa <1%.[19]
CONCLUSION
The evidence base supporting the use of FeNa and FeUr is limited and often not generalizable to many patients with AKI. The small sample sizes of the studies do not permit adequate capture of diverse mechanisms for renal injury, and these studies are of patients referred for Nephrology consultation and may not be representative of the larger population of patients with less severe AKI. Ultimately, the true etiology will be proven by time and response to therapy. Apart from a supportive role in the diagnosis of hepatorenal syndrome, there is little practical utility to FeNa and FeUr measurement, and these indices should not alter therapeutic decisions when inconsistent with the clinical impression. The evaluation of AKI requires thoughtful clinical assessment, and the gold standard still remains the judicious decision of when to manipulate the intravascular volume status of a patient. In regard to the presented case, urine chemistries are unhelpful due to the combined vasoconstrictive and tubulotoxic effects of the administered intravenous contrast. The ongoing hypotension further contributes to both pre‐renal as well as ischemic tubular injury.
RECOMMENDATIONS
- FeNa can aid in the diagnosis of hepatorenal syndrome. Otherwise, the routine use of FeNa and FeUr in the diagnosis and management of AKI should be avoided.
- In pre‐renal azotemia, therapeutic intervention is guided by etiology of the disorder (e.g., intravenous crystalloid support based on a history of hypovolemia and ongoing hypoperfusion, diuresis and/or inotropic support in setting of decompensated heart failure, etc.), without regard to baseline FeNa and FeUr.
- In ATI, fluid administration is appropriate if hypovolemia is present. FeNa and FeUr cannot diagnose hypovolemia.
Disclosure
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
- . The FENa test. Use in the differential diagnosis of acute renal failure. JAMA. 1976;236(6):579–581.
- , , . Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 2002;62(6):2223–2229.
- Centers for Medicare 275(8):630–634.
- , , , . Etiologies and outcome of acute renal insufficiency in older adults: a renal biopsy study of 259 cases. Am J Kidney Dis. 2000;35(3):433–447.
- , , , et al. Urinary diagnostic indices in acute renal failure: a prospective study. Ann Intern Med. 1978;89(1):47–50.
- , . Urinary indices and chemistries in the differential diagnosis of prerenal failure and acute tubular necrosis. Semin Nephrol. 1985;5(3):224–233.
- , , , , . The comparative benefits of the fractional excretion of urea and sodium in various azotemic oliguric states. Nephron Clin Pract. 2010;114(2):c145–c150.
- MachasNúñez JF, Cameron JS, Oreopoulos DG, eds. The Aging Kidney in Health and Disease. New York, NY: Springer Science + Business Media, LLC; 2008.
- , , . Cytokine‐mediated regulation of urea transporters during experimental endotoxemia. Am J Physiol Renal Physiol. 2007;292(5):F1479–F1489.
- , , . Urinary biochemistry and microscopy in septic acute renal failure: a systematic review. Am J Kidney Dis. 2006;48(5):695–705.
- , , , et al. Diagnostic performance of fractional excretion of urea in the evaluation of critically ill patients with acute kidney injury: a multicenter cohort study. Crit Care. 2011;15(4):R178.
- , , , et al. Fractional excretion of urea as a diagnostic index in acute kidney injury in intensive care patients. J Crit Care. 2012;27(5):505–510.
- , , , . Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis. 2007;50(4):566–573.
- , , , et al. Transient versus persistent acute kidney injury and the diagnostic performance of fractional excretion of urea in critically ill patients. Nephron Clin Pract. 2014;126(1):8–13.
- , , , , . Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann Emerg Med. 2010;55(3):290–295.
- , , , , , . Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2010;5(3):402–408.
- , , , , . Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2008;3(6):1615–1619.
- , , , et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23(1):164–176.
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 70‐year‐old woman with a history of diabetes mellitus type 2 and hypertension was admitted with abdominal pain following 2 days of nausea and diarrhea. Initial laboratory studies revealed blood urea nitrogen (BUN) 25 mg/dL and serum creatinine 1.3 mg/dL. Computed tomography of the abdomen and pelvis with nonionic, low osmolar intravenous and oral contrast demonstrated acute diverticulitis with an associated small abscess. She was administered intravenous 0.9% sodium chloride solution and antibiotics. Blood pressure on admission was 92/55 mm Hg, and 24 hours later, her BUN and serum creatinine increased to 33 mg/dL and 1.9 mg/dL, respectively. Her urine output during the preceding 24 hours was 500 mL.
In the evaluation of acute kidney injury (AKI), is the measurement of fractional excretion of sodium (FeNa) and fractional excretion of urea (FeUr) of value?
WHY YOU MIGHT THINK ORDERING FeNa AND/OR FeUr IN THE EVALUATION OF AKI IS HELPFUL
The proper maintenance of sodium balance is paramount to regulating the size of body fluid compartments. Through the interaction of multiple physiologic processes, the kidney regulates tubular reabsorption (or lack thereof) of sodium chloride to match excretion to intake. In normal health, FeNa is typically 1%, although it may vary depending on the dietary sodium intake. The corollary is that 99% of filtered sodium is reabsorbed. Acute tubular injury (ATI) that impairs the tubular resorptive capacity for sodium may increase FeNa to >3%. In addition, during states of water conservation, urea is reabsorbed from the medullary collecting duct, explaining the discrepant rise in BUN relative to creatinine in prerenal azotemia. FeUr falls progressively as water is reabsorbed and urine flow declines, and FeUr less than 35% to 40% may result during prerenal azotemia versus >50% in health or ATI. Theoretically, FeUr is largely unaffected by diuretics, whereas FeNa is increased by diuretics.
In 1976, Espinel reported on the use of FeNa in 17 oliguric patients to discriminate prerenal azotemia from ATI.[1] Establishing what are now familiar indices, FeNa <1% was deemed consistent with prerenal physiology versus >3% indicating ATI. Notably, the study excluded patients who had received diuretics or in whom chronic kidney disease (CKD), glomerulonephritis, or urinary obstruction was suspected.
Given the limitations of FeNa in the context of diuretic use, many physicians instead use FeUr to distinguish prerenal versus ATI causes of AKI. Carvounis et al. reported FeUr and FeNa in 50 patients with prerenal azotemia, 27 with prerenal azotemia receiving diuretics and 25 patients with ATI.[2] Patients with interstitial nephritis, glomerulonephritis, and obstruction were excluded. In the entire cohort, the authors reported sensitivity of 90% and specificity of 96% for FeUr <35% in identifying prerenal azotemia (Table 1). FeNa <1% was slightly less sensitive for prerenal azotemia in the entire cohort at 77%, and this fell to 48% in the presence of diuretics as compared to 89% for FeUr. Naturally, the specificity of FeNa for ATI will fall with the use of diuretics. As shown in Table 1, FeUr <35% has an excellent positive likelihood ratio (LR+) of 22 for prerenal azotemia and a moderate LR+ of 9 for FeUr 35% being consistent with ATI, regardless of the presence of diuretics. This contrasts with FeNa, which if 1% in the presence of diuretics, lacked utility in the diagnosis of ATI. Of note, diuretic use was reported only in the prerenal azotemia group and not specifically in the ATI group. Thus, these comparisons assume diuretics have no effect on test characteristics in ATI. This assumption, however, may not be valid.
| FeUr | FeNa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sens | Spec | PPV | NPV | LR+ | LR | Sens | Spec | PPV | NPV | LR+ | LR | |||
| ||||||||||||||
| Carvounis[2] | Prerenal | Overall | 0.90 | 0.96 | 0.99 | 0.75 | 22.4 | 0.1 | 0.77 | 0.96 | 0.98 | 0.57 | 19.2 | 0.2 |
| No diuretics | 0.90 | 0.96 | 0.98 | 0.83 | 22.5 | 0.1 | 0.92 | 0.96 | 0.98 | 0.86 | 23.0 | 0.1 | ||
| Diuretics | 0.89 | 0.96 | 0.96 | 0.89 | 22.2 | 0.1 | 0.48 | 0.96 | 0.93 | 0.63 | 12.0 | 0.5 | ||
| ATI | Overall | 0.96 | 0.90 | 0.75 | 0.99 | 9.2 | 0.0 | 0.96 | 0.77 | 0.57 | 0.98 | 4.1 | 0.1 | |
| No diuretics* | 0.96 | 0.90 | 0.83 | 0.98 | 9.6 | 0.0 | 0.96 | 0.92 | 0.86 | 0.98 | 12.0 | 0.0 | ||
| Diuretics | 0.96 | 0.89 | 0.89 | 0.96 | 8.6 | 0.0 | 0.96 | 0.48 | 0.63 | 0.93 | 1.9 | 0.1 | ||
| Diskin[8] | Prerenal | Overall | 0.97 | 0.85 | 0.96 | 0.89 | 6.5 | 0.0 | 0.44 | 0.75 | 0.88 | 0.25 | 1.8 | 0.7 |
| No diuretics | 0.91 | 0.89 | 0.95 | 0.80 | 8.2 | 0.1 | 0.83 | 0.67 | 0.86 | 0.60 | 2.5 | 0.3 | ||
| Diuretics | 1.00 | 0.82 | 0.97 | 1.00 | 5.5 | 0.0 | 0.29 | 0.82 | 0.89 | 0.18 | 1.6 | 0.9 | ||
| ATI | Overall | 0.85 | 0.97 | 0.89 | 0.96 | 33.6 | 0.2 | 0.75 | 0.44 | 0.25 | 0.88 | 1.3 | 0.6 | |
| No diuretics | 0.89 | 0.91 | 0.80 | 0.95 | 10.2 | 0.1 | 0.67 | 0.83 | 0.60 | 0.86 | 3.8 | 0.4 | ||
| Diuretics | 0.82 | 1.00 | 1.00 | 0.97 | N/A | 0.2 | 0.82 | 0.29 | 0.18 | 0.89 | 1.1 | 0.6 | ||
WHY THERE IS LITTLE REASON TO ROUTINELY ORDER FeNa AND FeUr IN PATIENTS WITH AKI
The argument against FeNa and FeUr is not primarily financial. FeNa and FeUr testing on all Medicare patients discharged with AKI in 2013 would have cost US$6 million.[3] Although a tiny fraction of annual healthcare expenditure, it would nevertheless be wasteful spending, and its true harm lays in the application of flawed diagnostic reasoning.
That flaw in our conceptual approach to AKI is the broad categorization of patients into either a prerenal or intrinsic etiology of AKI. In reality, renal injury is often multifactorial, and significant prerenal injury may progress to or coexist with intrinsic disease that is commonly ATI. Measurement of a urinary index at a single point in time will often fail to capture this spectrum of causes for AKI. Unfortunately, accurately assessing volume status through physical examination is difficult.[4] Considering FeNa and FeUr may be low in both hemorrhage as well as congestive heart failure, the measurement of these variables adds little to body volume assessment.
It cannot be overemphasized that application of FeNa and FeUr is predicated on the provider already knowing the diagnosis is either prerenal azotemia or ATI. Studies have generally excluded patients >65 years old and those with CKD or notable comorbid renal processes apart from prerenal azotemia or ATI. It is important to recall that a third of kidney biopsies may yield a diagnosis different than the prebiopsy clinical diagnosis, and the gold standard for ATI in studies of FeNa and FeUr was simply a failure of kidney function to improve promptly.[5] Why send a test that is predicated on largely already knowing the answer?
Fractional Excretion of Sodium for Diagnosis
Unfortunately, FeNa is neither sensitive nor specific enough in the general inpatient population to inform important clinical decisions regarding the etiology of AKI. Miller et al. examined 30 patients with oliguric prerenal azotemia, 55 with ATI (oliguric and nonoliguric), 10 with obstructive uropathy, and 7 with glomerulonephritis.[6] None of the patients had received diuretics within 24 hours of study entry. A FeNa <1% was present in 90% of prerenal patients and 4% of oliguric ATI. Importantly, of nonoliguric patients with ATI, 10% had a false positive FeNa <1%. Many subsequent studies have similarly documented the existence of FeNa <1% or otherwise indeterminate in ATI, particularly, but not exclusively, in nonoliguric states.[7] Diskin et al. evaluated FeNa in 100 prospective oliguric AKI patients (80 with prerenal azotemia and 20 with ATI) without CKD, with FeNa <1% being consistent with prerenal azotemia, 1% to 3% indeterminate, and >3% ATI.[8] The derived LR for FeNa for both prerenal azotemia and ATI are unlikely to alter pretest probability (Table 1). In part, this may be due to Diskin et al.'s incorporation of indeterminate FeNa, consistent with clinical reality. Carvounis et al. did not account for indeterminate values, and consequently the LR were likely overinflated in that study. It is now well‐recognized that glomerulonephritis may also result in FeNa <1% despite absence of identifiable prerenal physiology, as can intravenous iodinated contrast administration and rhabdomyolysis. Moreover, diuretic administration, polyuria due to osmotic diuresis, increased excretion of anions such as ketone bodies in diabetic ketoacidosis, the presence of CKD, and increased age, among others, can produce an FeNa that is indeterminate or >3% in the absence of ATI. Regarding diuretics, although the duration of action of furosemide is approximately 6 hours, longer‐acting loop diuretics such as torsemide or thiazide diuretics such as chlorthalidone may result in natriuresis for 24 hours.
Fractional Excretion of Urea for Diagnosis
Despite the potential superiority of FeUr to FeNa in supporting a diagnosis of prerenal azotemia in the setting of diuretic administration, FeUr nevertheless will only moderately increase the post‐test probability of prerenal azotemia under ideal conditions. In the study by Diskin et al., FeUr <40% was deemed consistent with prerenal azotemia and 40% with ATI. In the diagnosis of prerenal azotemia, the LR+ were 5.5 and 8.2 in the presence and absence of diuretics, respectively. Although the LR+ for the diagnosis of ATI was impressive, this was based on only 9 patients in the ATI‐no diuretic and 11 patients in the ATI‐diuretic groups. Carvounis, moreover, demonstrated considerably lower LR+ of approximately 9 for the diagnosis of ATI, and this study was unable to account for diuretic use specifically within the ATI group. Four of the 5 prerenal patients in Diskin et al.'s study misdiagnosed by FeUr had infection, and each were properly diagnosed by FeNa. Experimental data suggest endotoxemia may downregulate urea transporters as does aging, thereby increasing FeUr in sepsis and the elderly even in times of prerenal azotemia.[9, 10] Moreover, osmotic diuresis, such as with hyperglycemia or sickle cell nephropathy with medullary injury, may result in a falsely negative FeUr during prerenal states. In summary, these data suggest FeUr less than 35% to 40%, with the noted caveats, is most applicable to an oliguric patient in whom the pretest probability of prerenal azotemia is high, and it may be superior in the context of diuretics to the use of FeNa. Nonetheless, the impact on posttest probability is marginal. Of note, the diagnostic categories lack gold standards in these studies, and in the Carvounis study, FeNa (index under study) was 1 of several criteria actually used to categorize patients as either prerenal or ATI (outcomes under study). It is important to recognize these datasets contained very small numbers of patients with ATI, limiting the strength and generalizability of the scientific evidence. Other studies have failed to consistently demonstrate any utility to FeUr, particularly in those with CKD or critical illness.[11, 12, 13, 14, 15]
WHAT YOU SHOULD DO INSTEAD: DECIDE IF VOLUME MANIPULATION IS APPROPRIATE
The gold standard for diagnosis, as in many of the above studies, is the prompt improvement of prerenal azotemia with correction of renal hypoperfusion. Ultimately, the decision to administer intravenous fluids or diuretics in the management of AKI will often be independent of both FeNa and FeUr. In considering, for example, the case described above, it is not possible to realistically dichotomize the patient into either a prerenal or ATI category; both are quite likely present. If the clinical assessment supports a component of prerenal azotemia, a low FeNa and/or FeUr will not change the intervention. An elevated FeNa and/or FeUr, however, has at best moderate and potentially no impact on the likelihood for ATI. A patient, moreover, may still require volume manipulation in the context of established ATI. As such, these indices should not alter therapeutic decisions. There may be value in utilizing and identifying new approaches to determining a priori which patients will be fluid responsive, such as inferior vena cava ultrasound.[16] Lastly, evaluation of the urine sediment is an underutilized tool that may prove more useful in discriminating prerenal azotemia from ATI. It also helps to exclude other etiologies of AKI, such as glomerulonephritis and acute interstitial nephritis, which are typical exclusion criteria in studies of FeNa and FeUr.[17, 18]
WHEN IS FeNa AND/OR FeUr USEFUL IN DEFINING THE ETIOLOGY OF AKI?
FeNa and FeUr at best only support a clinical impression of prerenal azotemia or ATI in oliguric AKI, and the accuracy of these metrics is questionable in the setting of CKD, older age, and a variety of comorbidities. There is, however, a setting in which FeNa may be helpful. In practice, FeNa is useful in the evaluation of hepatorenal syndrome, a disorder characterized by oliguria and intense renal sodium reabsorption with resultant spot urine sodium <10 mEq/L and FeNa <1%.[19]
CONCLUSION
The evidence base supporting the use of FeNa and FeUr is limited and often not generalizable to many patients with AKI. The small sample sizes of the studies do not permit adequate capture of diverse mechanisms for renal injury, and these studies are of patients referred for Nephrology consultation and may not be representative of the larger population of patients with less severe AKI. Ultimately, the true etiology will be proven by time and response to therapy. Apart from a supportive role in the diagnosis of hepatorenal syndrome, there is little practical utility to FeNa and FeUr measurement, and these indices should not alter therapeutic decisions when inconsistent with the clinical impression. The evaluation of AKI requires thoughtful clinical assessment, and the gold standard still remains the judicious decision of when to manipulate the intravascular volume status of a patient. In regard to the presented case, urine chemistries are unhelpful due to the combined vasoconstrictive and tubulotoxic effects of the administered intravenous contrast. The ongoing hypotension further contributes to both pre‐renal as well as ischemic tubular injury.
RECOMMENDATIONS
- FeNa can aid in the diagnosis of hepatorenal syndrome. Otherwise, the routine use of FeNa and FeUr in the diagnosis and management of AKI should be avoided.
- In pre‐renal azotemia, therapeutic intervention is guided by etiology of the disorder (e.g., intravenous crystalloid support based on a history of hypovolemia and ongoing hypoperfusion, diuresis and/or inotropic support in setting of decompensated heart failure, etc.), without regard to baseline FeNa and FeUr.
- In ATI, fluid administration is appropriate if hypovolemia is present. FeNa and FeUr cannot diagnose hypovolemia.
Disclosure
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 70‐year‐old woman with a history of diabetes mellitus type 2 and hypertension was admitted with abdominal pain following 2 days of nausea and diarrhea. Initial laboratory studies revealed blood urea nitrogen (BUN) 25 mg/dL and serum creatinine 1.3 mg/dL. Computed tomography of the abdomen and pelvis with nonionic, low osmolar intravenous and oral contrast demonstrated acute diverticulitis with an associated small abscess. She was administered intravenous 0.9% sodium chloride solution and antibiotics. Blood pressure on admission was 92/55 mm Hg, and 24 hours later, her BUN and serum creatinine increased to 33 mg/dL and 1.9 mg/dL, respectively. Her urine output during the preceding 24 hours was 500 mL.
In the evaluation of acute kidney injury (AKI), is the measurement of fractional excretion of sodium (FeNa) and fractional excretion of urea (FeUr) of value?
WHY YOU MIGHT THINK ORDERING FeNa AND/OR FeUr IN THE EVALUATION OF AKI IS HELPFUL
The proper maintenance of sodium balance is paramount to regulating the size of body fluid compartments. Through the interaction of multiple physiologic processes, the kidney regulates tubular reabsorption (or lack thereof) of sodium chloride to match excretion to intake. In normal health, FeNa is typically 1%, although it may vary depending on the dietary sodium intake. The corollary is that 99% of filtered sodium is reabsorbed. Acute tubular injury (ATI) that impairs the tubular resorptive capacity for sodium may increase FeNa to >3%. In addition, during states of water conservation, urea is reabsorbed from the medullary collecting duct, explaining the discrepant rise in BUN relative to creatinine in prerenal azotemia. FeUr falls progressively as water is reabsorbed and urine flow declines, and FeUr less than 35% to 40% may result during prerenal azotemia versus >50% in health or ATI. Theoretically, FeUr is largely unaffected by diuretics, whereas FeNa is increased by diuretics.
In 1976, Espinel reported on the use of FeNa in 17 oliguric patients to discriminate prerenal azotemia from ATI.[1] Establishing what are now familiar indices, FeNa <1% was deemed consistent with prerenal physiology versus >3% indicating ATI. Notably, the study excluded patients who had received diuretics or in whom chronic kidney disease (CKD), glomerulonephritis, or urinary obstruction was suspected.
Given the limitations of FeNa in the context of diuretic use, many physicians instead use FeUr to distinguish prerenal versus ATI causes of AKI. Carvounis et al. reported FeUr and FeNa in 50 patients with prerenal azotemia, 27 with prerenal azotemia receiving diuretics and 25 patients with ATI.[2] Patients with interstitial nephritis, glomerulonephritis, and obstruction were excluded. In the entire cohort, the authors reported sensitivity of 90% and specificity of 96% for FeUr <35% in identifying prerenal azotemia (Table 1). FeNa <1% was slightly less sensitive for prerenal azotemia in the entire cohort at 77%, and this fell to 48% in the presence of diuretics as compared to 89% for FeUr. Naturally, the specificity of FeNa for ATI will fall with the use of diuretics. As shown in Table 1, FeUr <35% has an excellent positive likelihood ratio (LR+) of 22 for prerenal azotemia and a moderate LR+ of 9 for FeUr 35% being consistent with ATI, regardless of the presence of diuretics. This contrasts with FeNa, which if 1% in the presence of diuretics, lacked utility in the diagnosis of ATI. Of note, diuretic use was reported only in the prerenal azotemia group and not specifically in the ATI group. Thus, these comparisons assume diuretics have no effect on test characteristics in ATI. This assumption, however, may not be valid.
| FeUr | FeNa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sens | Spec | PPV | NPV | LR+ | LR | Sens | Spec | PPV | NPV | LR+ | LR | |||
| ||||||||||||||
| Carvounis[2] | Prerenal | Overall | 0.90 | 0.96 | 0.99 | 0.75 | 22.4 | 0.1 | 0.77 | 0.96 | 0.98 | 0.57 | 19.2 | 0.2 |
| No diuretics | 0.90 | 0.96 | 0.98 | 0.83 | 22.5 | 0.1 | 0.92 | 0.96 | 0.98 | 0.86 | 23.0 | 0.1 | ||
| Diuretics | 0.89 | 0.96 | 0.96 | 0.89 | 22.2 | 0.1 | 0.48 | 0.96 | 0.93 | 0.63 | 12.0 | 0.5 | ||
| ATI | Overall | 0.96 | 0.90 | 0.75 | 0.99 | 9.2 | 0.0 | 0.96 | 0.77 | 0.57 | 0.98 | 4.1 | 0.1 | |
| No diuretics* | 0.96 | 0.90 | 0.83 | 0.98 | 9.6 | 0.0 | 0.96 | 0.92 | 0.86 | 0.98 | 12.0 | 0.0 | ||
| Diuretics | 0.96 | 0.89 | 0.89 | 0.96 | 8.6 | 0.0 | 0.96 | 0.48 | 0.63 | 0.93 | 1.9 | 0.1 | ||
| Diskin[8] | Prerenal | Overall | 0.97 | 0.85 | 0.96 | 0.89 | 6.5 | 0.0 | 0.44 | 0.75 | 0.88 | 0.25 | 1.8 | 0.7 |
| No diuretics | 0.91 | 0.89 | 0.95 | 0.80 | 8.2 | 0.1 | 0.83 | 0.67 | 0.86 | 0.60 | 2.5 | 0.3 | ||
| Diuretics | 1.00 | 0.82 | 0.97 | 1.00 | 5.5 | 0.0 | 0.29 | 0.82 | 0.89 | 0.18 | 1.6 | 0.9 | ||
| ATI | Overall | 0.85 | 0.97 | 0.89 | 0.96 | 33.6 | 0.2 | 0.75 | 0.44 | 0.25 | 0.88 | 1.3 | 0.6 | |
| No diuretics | 0.89 | 0.91 | 0.80 | 0.95 | 10.2 | 0.1 | 0.67 | 0.83 | 0.60 | 0.86 | 3.8 | 0.4 | ||
| Diuretics | 0.82 | 1.00 | 1.00 | 0.97 | N/A | 0.2 | 0.82 | 0.29 | 0.18 | 0.89 | 1.1 | 0.6 | ||
WHY THERE IS LITTLE REASON TO ROUTINELY ORDER FeNa AND FeUr IN PATIENTS WITH AKI
The argument against FeNa and FeUr is not primarily financial. FeNa and FeUr testing on all Medicare patients discharged with AKI in 2013 would have cost US$6 million.[3] Although a tiny fraction of annual healthcare expenditure, it would nevertheless be wasteful spending, and its true harm lays in the application of flawed diagnostic reasoning.
That flaw in our conceptual approach to AKI is the broad categorization of patients into either a prerenal or intrinsic etiology of AKI. In reality, renal injury is often multifactorial, and significant prerenal injury may progress to or coexist with intrinsic disease that is commonly ATI. Measurement of a urinary index at a single point in time will often fail to capture this spectrum of causes for AKI. Unfortunately, accurately assessing volume status through physical examination is difficult.[4] Considering FeNa and FeUr may be low in both hemorrhage as well as congestive heart failure, the measurement of these variables adds little to body volume assessment.
It cannot be overemphasized that application of FeNa and FeUr is predicated on the provider already knowing the diagnosis is either prerenal azotemia or ATI. Studies have generally excluded patients >65 years old and those with CKD or notable comorbid renal processes apart from prerenal azotemia or ATI. It is important to recall that a third of kidney biopsies may yield a diagnosis different than the prebiopsy clinical diagnosis, and the gold standard for ATI in studies of FeNa and FeUr was simply a failure of kidney function to improve promptly.[5] Why send a test that is predicated on largely already knowing the answer?
Fractional Excretion of Sodium for Diagnosis
Unfortunately, FeNa is neither sensitive nor specific enough in the general inpatient population to inform important clinical decisions regarding the etiology of AKI. Miller et al. examined 30 patients with oliguric prerenal azotemia, 55 with ATI (oliguric and nonoliguric), 10 with obstructive uropathy, and 7 with glomerulonephritis.[6] None of the patients had received diuretics within 24 hours of study entry. A FeNa <1% was present in 90% of prerenal patients and 4% of oliguric ATI. Importantly, of nonoliguric patients with ATI, 10% had a false positive FeNa <1%. Many subsequent studies have similarly documented the existence of FeNa <1% or otherwise indeterminate in ATI, particularly, but not exclusively, in nonoliguric states.[7] Diskin et al. evaluated FeNa in 100 prospective oliguric AKI patients (80 with prerenal azotemia and 20 with ATI) without CKD, with FeNa <1% being consistent with prerenal azotemia, 1% to 3% indeterminate, and >3% ATI.[8] The derived LR for FeNa for both prerenal azotemia and ATI are unlikely to alter pretest probability (Table 1). In part, this may be due to Diskin et al.'s incorporation of indeterminate FeNa, consistent with clinical reality. Carvounis et al. did not account for indeterminate values, and consequently the LR were likely overinflated in that study. It is now well‐recognized that glomerulonephritis may also result in FeNa <1% despite absence of identifiable prerenal physiology, as can intravenous iodinated contrast administration and rhabdomyolysis. Moreover, diuretic administration, polyuria due to osmotic diuresis, increased excretion of anions such as ketone bodies in diabetic ketoacidosis, the presence of CKD, and increased age, among others, can produce an FeNa that is indeterminate or >3% in the absence of ATI. Regarding diuretics, although the duration of action of furosemide is approximately 6 hours, longer‐acting loop diuretics such as torsemide or thiazide diuretics such as chlorthalidone may result in natriuresis for 24 hours.
Fractional Excretion of Urea for Diagnosis
Despite the potential superiority of FeUr to FeNa in supporting a diagnosis of prerenal azotemia in the setting of diuretic administration, FeUr nevertheless will only moderately increase the post‐test probability of prerenal azotemia under ideal conditions. In the study by Diskin et al., FeUr <40% was deemed consistent with prerenal azotemia and 40% with ATI. In the diagnosis of prerenal azotemia, the LR+ were 5.5 and 8.2 in the presence and absence of diuretics, respectively. Although the LR+ for the diagnosis of ATI was impressive, this was based on only 9 patients in the ATI‐no diuretic and 11 patients in the ATI‐diuretic groups. Carvounis, moreover, demonstrated considerably lower LR+ of approximately 9 for the diagnosis of ATI, and this study was unable to account for diuretic use specifically within the ATI group. Four of the 5 prerenal patients in Diskin et al.'s study misdiagnosed by FeUr had infection, and each were properly diagnosed by FeNa. Experimental data suggest endotoxemia may downregulate urea transporters as does aging, thereby increasing FeUr in sepsis and the elderly even in times of prerenal azotemia.[9, 10] Moreover, osmotic diuresis, such as with hyperglycemia or sickle cell nephropathy with medullary injury, may result in a falsely negative FeUr during prerenal states. In summary, these data suggest FeUr less than 35% to 40%, with the noted caveats, is most applicable to an oliguric patient in whom the pretest probability of prerenal azotemia is high, and it may be superior in the context of diuretics to the use of FeNa. Nonetheless, the impact on posttest probability is marginal. Of note, the diagnostic categories lack gold standards in these studies, and in the Carvounis study, FeNa (index under study) was 1 of several criteria actually used to categorize patients as either prerenal or ATI (outcomes under study). It is important to recognize these datasets contained very small numbers of patients with ATI, limiting the strength and generalizability of the scientific evidence. Other studies have failed to consistently demonstrate any utility to FeUr, particularly in those with CKD or critical illness.[11, 12, 13, 14, 15]
WHAT YOU SHOULD DO INSTEAD: DECIDE IF VOLUME MANIPULATION IS APPROPRIATE
The gold standard for diagnosis, as in many of the above studies, is the prompt improvement of prerenal azotemia with correction of renal hypoperfusion. Ultimately, the decision to administer intravenous fluids or diuretics in the management of AKI will often be independent of both FeNa and FeUr. In considering, for example, the case described above, it is not possible to realistically dichotomize the patient into either a prerenal or ATI category; both are quite likely present. If the clinical assessment supports a component of prerenal azotemia, a low FeNa and/or FeUr will not change the intervention. An elevated FeNa and/or FeUr, however, has at best moderate and potentially no impact on the likelihood for ATI. A patient, moreover, may still require volume manipulation in the context of established ATI. As such, these indices should not alter therapeutic decisions. There may be value in utilizing and identifying new approaches to determining a priori which patients will be fluid responsive, such as inferior vena cava ultrasound.[16] Lastly, evaluation of the urine sediment is an underutilized tool that may prove more useful in discriminating prerenal azotemia from ATI. It also helps to exclude other etiologies of AKI, such as glomerulonephritis and acute interstitial nephritis, which are typical exclusion criteria in studies of FeNa and FeUr.[17, 18]
WHEN IS FeNa AND/OR FeUr USEFUL IN DEFINING THE ETIOLOGY OF AKI?
FeNa and FeUr at best only support a clinical impression of prerenal azotemia or ATI in oliguric AKI, and the accuracy of these metrics is questionable in the setting of CKD, older age, and a variety of comorbidities. There is, however, a setting in which FeNa may be helpful. In practice, FeNa is useful in the evaluation of hepatorenal syndrome, a disorder characterized by oliguria and intense renal sodium reabsorption with resultant spot urine sodium <10 mEq/L and FeNa <1%.[19]
CONCLUSION
The evidence base supporting the use of FeNa and FeUr is limited and often not generalizable to many patients with AKI. The small sample sizes of the studies do not permit adequate capture of diverse mechanisms for renal injury, and these studies are of patients referred for Nephrology consultation and may not be representative of the larger population of patients with less severe AKI. Ultimately, the true etiology will be proven by time and response to therapy. Apart from a supportive role in the diagnosis of hepatorenal syndrome, there is little practical utility to FeNa and FeUr measurement, and these indices should not alter therapeutic decisions when inconsistent with the clinical impression. The evaluation of AKI requires thoughtful clinical assessment, and the gold standard still remains the judicious decision of when to manipulate the intravascular volume status of a patient. In regard to the presented case, urine chemistries are unhelpful due to the combined vasoconstrictive and tubulotoxic effects of the administered intravenous contrast. The ongoing hypotension further contributes to both pre‐renal as well as ischemic tubular injury.
RECOMMENDATIONS
- FeNa can aid in the diagnosis of hepatorenal syndrome. Otherwise, the routine use of FeNa and FeUr in the diagnosis and management of AKI should be avoided.
- In pre‐renal azotemia, therapeutic intervention is guided by etiology of the disorder (e.g., intravenous crystalloid support based on a history of hypovolemia and ongoing hypoperfusion, diuresis and/or inotropic support in setting of decompensated heart failure, etc.), without regard to baseline FeNa and FeUr.
- In ATI, fluid administration is appropriate if hypovolemia is present. FeNa and FeUr cannot diagnose hypovolemia.
Disclosure
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
- . The FENa test. Use in the differential diagnosis of acute renal failure. JAMA. 1976;236(6):579–581.
- , , . Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 2002;62(6):2223–2229.
- Centers for Medicare 275(8):630–634.
- , , , . Etiologies and outcome of acute renal insufficiency in older adults: a renal biopsy study of 259 cases. Am J Kidney Dis. 2000;35(3):433–447.
- , , , et al. Urinary diagnostic indices in acute renal failure: a prospective study. Ann Intern Med. 1978;89(1):47–50.
- , . Urinary indices and chemistries in the differential diagnosis of prerenal failure and acute tubular necrosis. Semin Nephrol. 1985;5(3):224–233.
- , , , , . The comparative benefits of the fractional excretion of urea and sodium in various azotemic oliguric states. Nephron Clin Pract. 2010;114(2):c145–c150.
- MachasNúñez JF, Cameron JS, Oreopoulos DG, eds. The Aging Kidney in Health and Disease. New York, NY: Springer Science + Business Media, LLC; 2008.
- , , . Cytokine‐mediated regulation of urea transporters during experimental endotoxemia. Am J Physiol Renal Physiol. 2007;292(5):F1479–F1489.
- , , . Urinary biochemistry and microscopy in septic acute renal failure: a systematic review. Am J Kidney Dis. 2006;48(5):695–705.
- , , , et al. Diagnostic performance of fractional excretion of urea in the evaluation of critically ill patients with acute kidney injury: a multicenter cohort study. Crit Care. 2011;15(4):R178.
- , , , et al. Fractional excretion of urea as a diagnostic index in acute kidney injury in intensive care patients. J Crit Care. 2012;27(5):505–510.
- , , , . Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis. 2007;50(4):566–573.
- , , , et al. Transient versus persistent acute kidney injury and the diagnostic performance of fractional excretion of urea in critically ill patients. Nephron Clin Pract. 2014;126(1):8–13.
- , , , , . Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann Emerg Med. 2010;55(3):290–295.
- , , , , , . Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2010;5(3):402–408.
- , , , , . Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2008;3(6):1615–1619.
- , , , et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23(1):164–176.
- . The FENa test. Use in the differential diagnosis of acute renal failure. JAMA. 1976;236(6):579–581.
- , , . Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int. 2002;62(6):2223–2229.
- Centers for Medicare 275(8):630–634.
- , , , . Etiologies and outcome of acute renal insufficiency in older adults: a renal biopsy study of 259 cases. Am J Kidney Dis. 2000;35(3):433–447.
- , , , et al. Urinary diagnostic indices in acute renal failure: a prospective study. Ann Intern Med. 1978;89(1):47–50.
- , . Urinary indices and chemistries in the differential diagnosis of prerenal failure and acute tubular necrosis. Semin Nephrol. 1985;5(3):224–233.
- , , , , . The comparative benefits of the fractional excretion of urea and sodium in various azotemic oliguric states. Nephron Clin Pract. 2010;114(2):c145–c150.
- MachasNúñez JF, Cameron JS, Oreopoulos DG, eds. The Aging Kidney in Health and Disease. New York, NY: Springer Science + Business Media, LLC; 2008.
- , , . Cytokine‐mediated regulation of urea transporters during experimental endotoxemia. Am J Physiol Renal Physiol. 2007;292(5):F1479–F1489.
- , , . Urinary biochemistry and microscopy in septic acute renal failure: a systematic review. Am J Kidney Dis. 2006;48(5):695–705.
- , , , et al. Diagnostic performance of fractional excretion of urea in the evaluation of critically ill patients with acute kidney injury: a multicenter cohort study. Crit Care. 2011;15(4):R178.
- , , , et al. Fractional excretion of urea as a diagnostic index in acute kidney injury in intensive care patients. J Crit Care. 2012;27(5):505–510.
- , , , . Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis. 2007;50(4):566–573.
- , , , et al. Transient versus persistent acute kidney injury and the diagnostic performance of fractional excretion of urea in critically ill patients. Nephron Clin Pract. 2014;126(1):8–13.
- , , , , . Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann Emerg Med. 2010;55(3):290–295.
- , , , , , . Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2010;5(3):402–408.
- , , , , . Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2008;3(6):1615–1619.
- , , , et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23(1):164–176.
© 2015 Society of Hospital Medicine
Say Ahh…
Credit: These cases were adapted from Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013. You can now get the second edition of the Color Atlas of Family Medicine as an app for mobile devices by clicking this link: usatinemedia.com.
1. A painless white, thick lesion with fissuring had been on the side of this 57-year-old man’s tongue for the past 7 months. The patient drinks two to three beers in the evening and smokes one pack of cigarettes a day.
Diagnosis: This patient was given a diagnosis of leukoplakia and the biopsy indicated that the lesion was premalignant. The World Health Organization defines leukoplakia as “white plaques of questionable risk” in cases where other known diseases that don’t carry an increased risk for cancer have been excluded. For all types of leukoplakia, the risk of malignant transformation is approximately 1%, with a much higher risk associated with leukoplakias that contain red spots and/or rough spots.
For more information, read “White patch on tongue.” J Fam Pract. 2014.
For the next photograph, proceed to the next page >>
2. This nonhealing painful lesion on the side of her tongue had increased in size recently, and the patient, a 56-year-old homeless woman, was worried because her dad had died from oral cancer. She had smoked since she was 11 and acknowledged being a heavy drinker.
Diagnosis: A punch biopsy revealed that the patient had a squamous cell carcinoma. Fully two-thirds of oropharyngeal cancers (OPCs) will present with advanced disease at the time of diagnosis. Ninety percent of OPCs are of the squamous cell type. Reports in the literature suggest that clinicians may be missing early disease by not conducting thorough soft-tissue examinations on a routine basis. However, the fact that more than 35% of patients do not see a dentist on a routine basis likely contributes to the diagnostic delay. The 5-year survival rate for OPC is 62% for whites and 42% for blacks.
Tobacco use is the major risk factor for OPC and is implicated in approximately 75% of cases. Alcohol use is also a major risk factor. The combined use of tobacco and alcohol increases the risk of OPC far more than either alone. Human papillomavirus (especially HPV 16) is a newly recognized major risk factor for carcinomas affecting the lingual and palatine tonsils.
For more information, read “Lesion on side of tongue.” J Fam Pract. 2014.
For the next photograph, proceed to the next page >>
3. A 58-year-old man sought care for painful sores that had been in his mouth, on and off, for a year. The ulcers erupted on his tongue, gums, buccal mucosa, and inner lips, making it painful to eat. The patient was not taking any medications.
Diagnosis: The clinician recognized his condition as recurrent aphthous ulcers with giant ulcers. Aphthous ulcers are painful ulcerations in the mouth, which can be single, multiple, occasional, or recurrent. These ulcers can be small or large but are uniformly painful and may interfere with eating, speaking, and swallowing.
For more information, read “Lesions on tongue.” J Fam Pract. 2014.
For the next photograph, proceed to the next page >>
4. A 60-year-old man, smelling of alcohol and tobacco, complained of a black discoloration of his tongue and occasional gagging sensation. He smoked one to 2 packs of cigarettes and drank 6 to 8 beers daily. On physical exam, his teeth were stained and his tongue shows elongated papillae with brown discoloration.
Diagnosis: This patient had a black hairy tongue (BHT), poor oral hygiene, and tobacco and alcohol addiction. BHT is a benign disorder of the tongue characterized by abnormally hypertrophied and elongated filiform papillae on the surface of the tongue. In addition, there is defective desquamation of the papillae on the dorsal tongue, resulting in a hair-like appearance. These papillae, which are normally about 1 mm in length, may become as long as 12 mm. The elongated filiform papillae can then collect debris, bacteria, fungus, or other foreign materials.
For more information, read “Discoloration of the tongue.” J Fam Pract. 2014.
For the next photograph, proceed to the next page >>
5. A 5-year-old girl had a temperature of 102.4°F, a sore throat, and a red tongue with prominent papillae. The posterior pharynx was also erythematous with slight exudate visible. The anterior cervical lymph nodes were mildly tender and somewhat enlarged, and no rashes were noted.
Diagnosis: The child had a strawberry tongue and scarlet fever caused by strep pharyngitis. The tongue had prominent papillae along with erythema, making it resemble a strawberry. Strawberry tongue is most commonly seen in children with scarlet fever or Kawasaki disease. Strawberry tongue usually develops within the first 2 to 3 days of illness. A white or yellowish coating usually precedes the classic red tongue with white papillae.
For more information, read “Papillae on tongue.” J Fam Pract. 2014.
Credit: These cases were adapted from Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013. You can now get the second edition of the Color Atlas of Family Medicine as an app for mobile devices by clicking this link: usatinemedia.com.
1. A painless white, thick lesion with fissuring had been on the side of this 57-year-old man’s tongue for the past 7 months. The patient drinks two to three beers in the evening and smokes one pack of cigarettes a day.
Diagnosis: This patient was given a diagnosis of leukoplakia and the biopsy indicated that the lesion was premalignant. The World Health Organization defines leukoplakia as “white plaques of questionable risk” in cases where other known diseases that don’t carry an increased risk for cancer have been excluded. For all types of leukoplakia, the risk of malignant transformation is approximately 1%, with a much higher risk associated with leukoplakias that contain red spots and/or rough spots.
For more information, read “White patch on tongue.” J Fam Pract. 2014.
For the next photograph, proceed to the next page >>
2. This nonhealing painful lesion on the side of her tongue had increased in size recently, and the patient, a 56-year-old homeless woman, was worried because her dad had died from oral cancer. She had smoked since she was 11 and acknowledged being a heavy drinker.
Diagnosis: A punch biopsy revealed that the patient had a squamous cell carcinoma. Fully two-thirds of oropharyngeal cancers (OPCs) will present with advanced disease at the time of diagnosis. Ninety percent of OPCs are of the squamous cell type. Reports in the literature suggest that clinicians may be missing early disease by not conducting thorough soft-tissue examinations on a routine basis. However, the fact that more than 35% of patients do not see a dentist on a routine basis likely contributes to the diagnostic delay. The 5-year survival rate for OPC is 62% for whites and 42% for blacks.
Tobacco use is the major risk factor for OPC and is implicated in approximately 75% of cases. Alcohol use is also a major risk factor. The combined use of tobacco and alcohol increases the risk of OPC far more than either alone. Human papillomavirus (especially HPV 16) is a newly recognized major risk factor for carcinomas affecting the lingual and palatine tonsils.
For more information, read “Lesion on side of tongue.” J Fam Pract. 2014.
For the next photograph, proceed to the next page >>
3. A 58-year-old man sought care for painful sores that had been in his mouth, on and off, for a year. The ulcers erupted on his tongue, gums, buccal mucosa, and inner lips, making it painful to eat. The patient was not taking any medications.
Diagnosis: The clinician recognized his condition as recurrent aphthous ulcers with giant ulcers. Aphthous ulcers are painful ulcerations in the mouth, which can be single, multiple, occasional, or recurrent. These ulcers can be small or large but are uniformly painful and may interfere with eating, speaking, and swallowing.
For more information, read “Lesions on tongue.” J Fam Pract. 2014.
For the next photograph, proceed to the next page >>
4. A 60-year-old man, smelling of alcohol and tobacco, complained of a black discoloration of his tongue and occasional gagging sensation. He smoked one to 2 packs of cigarettes and drank 6 to 8 beers daily. On physical exam, his teeth were stained and his tongue shows elongated papillae with brown discoloration.
Diagnosis: This patient had a black hairy tongue (BHT), poor oral hygiene, and tobacco and alcohol addiction. BHT is a benign disorder of the tongue characterized by abnormally hypertrophied and elongated filiform papillae on the surface of the tongue. In addition, there is defective desquamation of the papillae on the dorsal tongue, resulting in a hair-like appearance. These papillae, which are normally about 1 mm in length, may become as long as 12 mm. The elongated filiform papillae can then collect debris, bacteria, fungus, or other foreign materials.
For more information, read “Discoloration of the tongue.” J Fam Pract. 2014.
For the next photograph, proceed to the next page >>
5. A 5-year-old girl had a temperature of 102.4°F, a sore throat, and a red tongue with prominent papillae. The posterior pharynx was also erythematous with slight exudate visible. The anterior cervical lymph nodes were mildly tender and somewhat enlarged, and no rashes were noted.
Diagnosis: The child had a strawberry tongue and scarlet fever caused by strep pharyngitis. The tongue had prominent papillae along with erythema, making it resemble a strawberry. Strawberry tongue is most commonly seen in children with scarlet fever or Kawasaki disease. Strawberry tongue usually develops within the first 2 to 3 days of illness. A white or yellowish coating usually precedes the classic red tongue with white papillae.
For more information, read “Papillae on tongue.” J Fam Pract. 2014.
Credit: These cases were adapted from Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013. You can now get the second edition of the Color Atlas of Family Medicine as an app for mobile devices by clicking this link: usatinemedia.com.
1. A painless white, thick lesion with fissuring had been on the side of this 57-year-old man’s tongue for the past 7 months. The patient drinks two to three beers in the evening and smokes one pack of cigarettes a day.
Diagnosis: This patient was given a diagnosis of leukoplakia and the biopsy indicated that the lesion was premalignant. The World Health Organization defines leukoplakia as “white plaques of questionable risk” in cases where other known diseases that don’t carry an increased risk for cancer have been excluded. For all types of leukoplakia, the risk of malignant transformation is approximately 1%, with a much higher risk associated with leukoplakias that contain red spots and/or rough spots.
For more information, read “White patch on tongue.” J Fam Pract. 2014.
For the next photograph, proceed to the next page >>
2. This nonhealing painful lesion on the side of her tongue had increased in size recently, and the patient, a 56-year-old homeless woman, was worried because her dad had died from oral cancer. She had smoked since she was 11 and acknowledged being a heavy drinker.
Diagnosis: A punch biopsy revealed that the patient had a squamous cell carcinoma. Fully two-thirds of oropharyngeal cancers (OPCs) will present with advanced disease at the time of diagnosis. Ninety percent of OPCs are of the squamous cell type. Reports in the literature suggest that clinicians may be missing early disease by not conducting thorough soft-tissue examinations on a routine basis. However, the fact that more than 35% of patients do not see a dentist on a routine basis likely contributes to the diagnostic delay. The 5-year survival rate for OPC is 62% for whites and 42% for blacks.
Tobacco use is the major risk factor for OPC and is implicated in approximately 75% of cases. Alcohol use is also a major risk factor. The combined use of tobacco and alcohol increases the risk of OPC far more than either alone. Human papillomavirus (especially HPV 16) is a newly recognized major risk factor for carcinomas affecting the lingual and palatine tonsils.
For more information, read “Lesion on side of tongue.” J Fam Pract. 2014.
For the next photograph, proceed to the next page >>
3. A 58-year-old man sought care for painful sores that had been in his mouth, on and off, for a year. The ulcers erupted on his tongue, gums, buccal mucosa, and inner lips, making it painful to eat. The patient was not taking any medications.
Diagnosis: The clinician recognized his condition as recurrent aphthous ulcers with giant ulcers. Aphthous ulcers are painful ulcerations in the mouth, which can be single, multiple, occasional, or recurrent. These ulcers can be small or large but are uniformly painful and may interfere with eating, speaking, and swallowing.
For more information, read “Lesions on tongue.” J Fam Pract. 2014.
For the next photograph, proceed to the next page >>
4. A 60-year-old man, smelling of alcohol and tobacco, complained of a black discoloration of his tongue and occasional gagging sensation. He smoked one to 2 packs of cigarettes and drank 6 to 8 beers daily. On physical exam, his teeth were stained and his tongue shows elongated papillae with brown discoloration.
Diagnosis: This patient had a black hairy tongue (BHT), poor oral hygiene, and tobacco and alcohol addiction. BHT is a benign disorder of the tongue characterized by abnormally hypertrophied and elongated filiform papillae on the surface of the tongue. In addition, there is defective desquamation of the papillae on the dorsal tongue, resulting in a hair-like appearance. These papillae, which are normally about 1 mm in length, may become as long as 12 mm. The elongated filiform papillae can then collect debris, bacteria, fungus, or other foreign materials.
For more information, read “Discoloration of the tongue.” J Fam Pract. 2014.
For the next photograph, proceed to the next page >>
5. A 5-year-old girl had a temperature of 102.4°F, a sore throat, and a red tongue with prominent papillae. The posterior pharynx was also erythematous with slight exudate visible. The anterior cervical lymph nodes were mildly tender and somewhat enlarged, and no rashes were noted.
Diagnosis: The child had a strawberry tongue and scarlet fever caused by strep pharyngitis. The tongue had prominent papillae along with erythema, making it resemble a strawberry. Strawberry tongue is most commonly seen in children with scarlet fever or Kawasaki disease. Strawberry tongue usually develops within the first 2 to 3 days of illness. A white or yellowish coating usually precedes the classic red tongue with white papillae.
For more information, read “Papillae on tongue.” J Fam Pract. 2014.
Complete Closing Wedge Osteotomy for Correction of Blount Disease (Tibia Vara): A Technique
Blount disease (tibia vara) is an angular tibia deformity that includes varus, increased posterior slope, and internal rotation. This deformity was first described in 1922 by Erlacher1 in Germany. In 1937, Walter Blount2 reported on it in the United States. It is the most common cause of pathologic genu varum in adolescence and childhood.
An oblique incomplete closing wedge osteotomy of the proximal tibial metaphysis was described by Wagner3 for the treatment of unicompartmental osteoarthrosis of the knee in adults. Laurencin and colleagues4 applied this technique to the treatment of pediatric tibia vara with favorable results. They spared the medial cortex of the tibia in their incomplete closing wedge osteotomy technique. In each of the 9 cases we treated and describe here, we accidentally completed the tibial osteotomy when attempting the Laurencin technique. Given that the osteotomy was completed, we modified the Laurencin technique by using a 6-hole, 4.5-mm compression plate rather than a 5-hole semitubular plate, and added a large oblique screw from the medial side to compress the osteotomy site and to protect the plate from fracture. In addition, in 2 patients who weighed more than 250 pounds, we used an external fixator for additional stability. In this article, we report the outcomes of correcting adolescent tibia vara with a complete closing wedge tibial osteotomy and an oblique fibular osteotomy.
Materials and Methods
This study was approved by the Institutional Review Board at Pennsylvania State University. Between 2009 and 2012, we performed 9 complete oblique proximal tibial lateral closing wedge osteotomies on 8 patients (2 girls, 6 boys). In each case, the primary diagnosis was Blount disease. One patient also had renal dysplasia and was receiving dialysis. Mean age at time of operation was 15 years (range, 13-17 years). Mean preoperative weight was 215 pounds (range, 119-317 lb). Mean weight gain at follow-up was 4.39 pounds (range, –10 to 19 lb). Mean body mass index (BMI) was 38 (range, 25-48) (Table). All patients had varus angulation of the proximal tibia before surgery. Mean preoperative varus on standing films was 22° (range, 10°-36°). Because of the patients’ size, we used standing long-leg radiographs, on individual cassettes, for each leg.
Surgical Technique
Before surgery, we use paper cutouts to template the osteotomy wedge. We also use perioperative antibiotics and a standard time-out. For visualization of the entire leg for accurate correction, we prepare and drape the entire leg. A sterile tourniquet is used. At the midshaft of the fibula, a 4-cm incision is made, and dissection is carefully carried down to the fibula. Subperiosteal dissection is performed about the fibula, allowing adequate clearance for an oblique osteotomy. The osteotomy removes about 1 cm of fibula, which is to be used as bone graft for the tibial osteotomy. In addition, a lateral compartment fasciotomy is performed to prevent swelling-related complications. The wound is irrigated and injected with bupivacaine and closed in routine fashion.
We then make an inverted hockey-stick incision over the proximal tibia, centered down to the tibial tubercle. After dissecting down to the anterior compartment, we perform a fasciotomy of about 8 cm to accommodate swelling. Subperiosteal dissection is then performed around the proximal tibia. The medial soft tissues are left attached to increase blood supply and healing. During subperiosteal dissection, soft elevators are used to gently retract the lateral soft tissues along with the inferior and posterior structures. We use fluoroscopic imaging to guide the osteotomy as well as screw and plate placement. We use a 6-hole, 4.5-mm compression plate and screws for fixation. The 2 proximal screws of the plate are predrilled in place to allow for application of the plate after completion of the osteotomy. The plate is then rotated out of position on 1 screw, and the osteotomy is identified under fluoroscopy with the appropriate position distal to the second hole of the 6-hole plate.
An oscillating saw and osteotomes are used to perform the oblique osteotomy. The pre-estimated bone wedge is removed. Wedge size is adjusted, if needed. The bone wedge is morselized for bone graft. The osteotomy is then closed, correcting both varus and internal tibial torsion. Our goal is 5° valgus. After correction is obtained, the plate is placed, and the proximal screw is snugly seated. Three cortical screws are placed distally to hold the plate in place under compression mode, and a cancellous screw is placed superiorly at the proximal portion of the plate for additional fixation. The screw placed proximal to the osteotomy site is a fully threaded cortical screw with excellent compression. Correction and proper placement of hardware are verified with fluoroscopy.
The wound is irrigated and injected with bupivacaine. Bone graft is then placed at the osteotomy site. Additional bone graft is placed posteriorly between the osteotomy site and the muscle mass to stimulate additional healing. Another screw is placed obliquely from the medial side across the osteotomy site to provide additional fixation (Figure 1).
A deep drain is placed and connected to bulb suction for 24 hours after surgery. The wound is then closed in routine fashion. In 2 patients who weighed more than 250 pounds, we used an external fixator for additional stability (Figure 2).
Postoperative Care
The incisions are dressed with antibiotic ointment and 4×4-in bandages and then wrapped with sterile cotton under-cast padding. The leg is placed into a well-padded cylinder cast with the knee flexed 10°. The leg is aligned to about 5° valgus. The cast is then split on the side and spread to allow for swelling and to prevent compartment syndrome.5 We also use a drain hooked to bulb suction, which is removed 24 hours after surgery. Toe-touch weight-bearing with crutches is allowed immediately after surgery. The cast is removed at 6 weeks, and a hinged range-of-motion knee brace is worn for another 6 weeks. All patients are allowed to resume normal activity after 4 months. In our 2 external-fixator cases, a cast was not used, and toe-touch weight-bearing and knee motion were allowed immediately. The external fixators were removed at about 10 weeks.
Results
Mean postoperative mechanical femoral-tibial angle was 3°, and mean correction was 26° (range, 16°-43°) (Table). Lateral distal femoral angle did not show significant femoral deformity in our sample. Mean medial proximal tibial angle was 74° (range, 63°-79°). In each case, the varus deformity was primarily in the tibia. Mean tourniquet time was 88 minutes (range, 50-119 min). Our complication rate was 11% (1 knee). In our first case, in which we did not use an extra medial screw, the 4.5-mm plate fractured at the osteotomy site 2.5 months after surgery. The 250-pound patient subsequently lost 17° of correction, and valgus alignment was not achieved. Preoperative varus was 25°, and postoperative alignment was 8° varus. This plate fracture led us to use an extra medial screw for additional stability in all subsequent cases and to consider using an external fixator for patients weighing more than 250 pounds. After the first case, there were no other plate fractures. A potential problem with closing wedge osteotomy is shortening, but varus correction restores some length. Mean postoperative leg-length difference was 10 mm (range, 0-16 mm). No patient complained of leg-length difference during the postoperative follow-up.
Eight and a half months after surgery, 1 patient had hardware removed, at the family’s request. No patient experienced perioperative infection or neurovascular damage. Our overall patient population was obese—mean BMI was 38 (range, 25-48), and mean postoperative weight was 219 pounds. Three of our 8 patients were overweight (BMI, 25-30), and 5 were obese (BMI, >30). For prevention of plate failure, we recommend using an extra oblique screw in all patients and considering an external fixator for patients who weigh more than 250 pounds.
Discussion
Correction of adolescent tibia vara can be challenging because of patient obesity. The technique described here—a modification of the technique of Laurencin and colleagues4—is practical and reproducible in this population. The goals in performing osteotomy are to correct the deformity, restore joint alignment, preserve leg length, and prevent recurrent deformity and other complications, such as neurovascular injury, nonunion, and infection.3,6-8 Our technique minimizes the risk for these complications. For example, the fasciotomy provides excellent decompression of the anterior and lateral compartments, minimizing neurovascular ischemia and the risk for compartment syndrome. During cast placement, splitting and spreading reduce the risk for compartment syndrome as well.5
Wagner3,9 demonstrated the utility of a closing wedge proximal tibial osteotomy in adults. Laurencin and colleagues4 showed this technique is effective in correcting tibia vara in a pediatric population. However, they did not specify patient weight and used a small semitubular plate for fixation, and some of their patients had infantile Blount disease. We modified the technique in 3 ways. First, we performed a complete osteotomy. Second, because our patients were adolescents and very large, we used a 6-hole, 4.5-mm compression plate and screws. Third, we used an external fixator for increased stability in patients who weighed more than 250 pounds.
The reported technique, using an oblique metaphyseal closing wedge osteotomy with internal fixation in obese patients, is practical, safe, and reliable. This technique is a useful alternative to an external fixator. We used it on 9 knees with tibia vara, and it was completely successful in 8 cases and partially successful in 1 (hardware breakage occurred). An external fixator was used to prevent hardware breakage in 2 patients who weighed more than 250 pounds. This technique is a valuable treatment option for surgical correction, especially in obese patients.
1. Erlacher P. Deformierende Prozesse der Epiphysengegend bei Kindem. Archiv Orthop Unfall-Chir. 1922;20:81-96.
2. Blount WP. Tibia vara. J Bone Joint Surg. 1937;29:1-28.
3. Wagner H. Principles of corrective osteotomies in osteoarthrosis of the knee. In: Weal UH, ed. Joint Preserving Procedures of the Lower Extremity. New York, NY: Springer; 1980:77-102.
4. Laurencin CT, Ferriter PJ, Millis MB. Oblique proximal tibial osteotomy for the correction of tibia vara in the young. Clin Orthop Relat Res. 1996;(327):218-224.
5. Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
6. Mycoskie PJ. Complications of osteotomies about the knee in children. Orthopedics. 1981;4(9):1005-1015.
7. Matsen FA, Staheli LT. Neurovascular complications following tibial osteotomy in children. A case report. Clin Orthop Relat Res. 1975;(110):210-214.
8. Steel HH, Sandrew RE, Sullivan PD. Complications of tibial osteotomy in children for genu varum or valgum. Evidence that neurological changes are due to ischemia. J Bone Joint Surg Am. 1971;53(8):1629-1635.
9. Wagner H. The displacement osteotomy as a correction principle. In: Heirholzer G, Muller KH, eds. Corrective Osteotomies of the Lower Extremity After Trauma. Berlin, Germany: Springer; 1985:141-150.
Blount disease (tibia vara) is an angular tibia deformity that includes varus, increased posterior slope, and internal rotation. This deformity was first described in 1922 by Erlacher1 in Germany. In 1937, Walter Blount2 reported on it in the United States. It is the most common cause of pathologic genu varum in adolescence and childhood.
An oblique incomplete closing wedge osteotomy of the proximal tibial metaphysis was described by Wagner3 for the treatment of unicompartmental osteoarthrosis of the knee in adults. Laurencin and colleagues4 applied this technique to the treatment of pediatric tibia vara with favorable results. They spared the medial cortex of the tibia in their incomplete closing wedge osteotomy technique. In each of the 9 cases we treated and describe here, we accidentally completed the tibial osteotomy when attempting the Laurencin technique. Given that the osteotomy was completed, we modified the Laurencin technique by using a 6-hole, 4.5-mm compression plate rather than a 5-hole semitubular plate, and added a large oblique screw from the medial side to compress the osteotomy site and to protect the plate from fracture. In addition, in 2 patients who weighed more than 250 pounds, we used an external fixator for additional stability. In this article, we report the outcomes of correcting adolescent tibia vara with a complete closing wedge tibial osteotomy and an oblique fibular osteotomy.
Materials and Methods
This study was approved by the Institutional Review Board at Pennsylvania State University. Between 2009 and 2012, we performed 9 complete oblique proximal tibial lateral closing wedge osteotomies on 8 patients (2 girls, 6 boys). In each case, the primary diagnosis was Blount disease. One patient also had renal dysplasia and was receiving dialysis. Mean age at time of operation was 15 years (range, 13-17 years). Mean preoperative weight was 215 pounds (range, 119-317 lb). Mean weight gain at follow-up was 4.39 pounds (range, –10 to 19 lb). Mean body mass index (BMI) was 38 (range, 25-48) (Table). All patients had varus angulation of the proximal tibia before surgery. Mean preoperative varus on standing films was 22° (range, 10°-36°). Because of the patients’ size, we used standing long-leg radiographs, on individual cassettes, for each leg.
Surgical Technique
Before surgery, we use paper cutouts to template the osteotomy wedge. We also use perioperative antibiotics and a standard time-out. For visualization of the entire leg for accurate correction, we prepare and drape the entire leg. A sterile tourniquet is used. At the midshaft of the fibula, a 4-cm incision is made, and dissection is carefully carried down to the fibula. Subperiosteal dissection is performed about the fibula, allowing adequate clearance for an oblique osteotomy. The osteotomy removes about 1 cm of fibula, which is to be used as bone graft for the tibial osteotomy. In addition, a lateral compartment fasciotomy is performed to prevent swelling-related complications. The wound is irrigated and injected with bupivacaine and closed in routine fashion.
We then make an inverted hockey-stick incision over the proximal tibia, centered down to the tibial tubercle. After dissecting down to the anterior compartment, we perform a fasciotomy of about 8 cm to accommodate swelling. Subperiosteal dissection is then performed around the proximal tibia. The medial soft tissues are left attached to increase blood supply and healing. During subperiosteal dissection, soft elevators are used to gently retract the lateral soft tissues along with the inferior and posterior structures. We use fluoroscopic imaging to guide the osteotomy as well as screw and plate placement. We use a 6-hole, 4.5-mm compression plate and screws for fixation. The 2 proximal screws of the plate are predrilled in place to allow for application of the plate after completion of the osteotomy. The plate is then rotated out of position on 1 screw, and the osteotomy is identified under fluoroscopy with the appropriate position distal to the second hole of the 6-hole plate.
An oscillating saw and osteotomes are used to perform the oblique osteotomy. The pre-estimated bone wedge is removed. Wedge size is adjusted, if needed. The bone wedge is morselized for bone graft. The osteotomy is then closed, correcting both varus and internal tibial torsion. Our goal is 5° valgus. After correction is obtained, the plate is placed, and the proximal screw is snugly seated. Three cortical screws are placed distally to hold the plate in place under compression mode, and a cancellous screw is placed superiorly at the proximal portion of the plate for additional fixation. The screw placed proximal to the osteotomy site is a fully threaded cortical screw with excellent compression. Correction and proper placement of hardware are verified with fluoroscopy.
The wound is irrigated and injected with bupivacaine. Bone graft is then placed at the osteotomy site. Additional bone graft is placed posteriorly between the osteotomy site and the muscle mass to stimulate additional healing. Another screw is placed obliquely from the medial side across the osteotomy site to provide additional fixation (Figure 1).
A deep drain is placed and connected to bulb suction for 24 hours after surgery. The wound is then closed in routine fashion. In 2 patients who weighed more than 250 pounds, we used an external fixator for additional stability (Figure 2).
Postoperative Care
The incisions are dressed with antibiotic ointment and 4×4-in bandages and then wrapped with sterile cotton under-cast padding. The leg is placed into a well-padded cylinder cast with the knee flexed 10°. The leg is aligned to about 5° valgus. The cast is then split on the side and spread to allow for swelling and to prevent compartment syndrome.5 We also use a drain hooked to bulb suction, which is removed 24 hours after surgery. Toe-touch weight-bearing with crutches is allowed immediately after surgery. The cast is removed at 6 weeks, and a hinged range-of-motion knee brace is worn for another 6 weeks. All patients are allowed to resume normal activity after 4 months. In our 2 external-fixator cases, a cast was not used, and toe-touch weight-bearing and knee motion were allowed immediately. The external fixators were removed at about 10 weeks.
Results
Mean postoperative mechanical femoral-tibial angle was 3°, and mean correction was 26° (range, 16°-43°) (Table). Lateral distal femoral angle did not show significant femoral deformity in our sample. Mean medial proximal tibial angle was 74° (range, 63°-79°). In each case, the varus deformity was primarily in the tibia. Mean tourniquet time was 88 minutes (range, 50-119 min). Our complication rate was 11% (1 knee). In our first case, in which we did not use an extra medial screw, the 4.5-mm plate fractured at the osteotomy site 2.5 months after surgery. The 250-pound patient subsequently lost 17° of correction, and valgus alignment was not achieved. Preoperative varus was 25°, and postoperative alignment was 8° varus. This plate fracture led us to use an extra medial screw for additional stability in all subsequent cases and to consider using an external fixator for patients weighing more than 250 pounds. After the first case, there were no other plate fractures. A potential problem with closing wedge osteotomy is shortening, but varus correction restores some length. Mean postoperative leg-length difference was 10 mm (range, 0-16 mm). No patient complained of leg-length difference during the postoperative follow-up.
Eight and a half months after surgery, 1 patient had hardware removed, at the family’s request. No patient experienced perioperative infection or neurovascular damage. Our overall patient population was obese—mean BMI was 38 (range, 25-48), and mean postoperative weight was 219 pounds. Three of our 8 patients were overweight (BMI, 25-30), and 5 were obese (BMI, >30). For prevention of plate failure, we recommend using an extra oblique screw in all patients and considering an external fixator for patients who weigh more than 250 pounds.
Discussion
Correction of adolescent tibia vara can be challenging because of patient obesity. The technique described here—a modification of the technique of Laurencin and colleagues4—is practical and reproducible in this population. The goals in performing osteotomy are to correct the deformity, restore joint alignment, preserve leg length, and prevent recurrent deformity and other complications, such as neurovascular injury, nonunion, and infection.3,6-8 Our technique minimizes the risk for these complications. For example, the fasciotomy provides excellent decompression of the anterior and lateral compartments, minimizing neurovascular ischemia and the risk for compartment syndrome. During cast placement, splitting and spreading reduce the risk for compartment syndrome as well.5
Wagner3,9 demonstrated the utility of a closing wedge proximal tibial osteotomy in adults. Laurencin and colleagues4 showed this technique is effective in correcting tibia vara in a pediatric population. However, they did not specify patient weight and used a small semitubular plate for fixation, and some of their patients had infantile Blount disease. We modified the technique in 3 ways. First, we performed a complete osteotomy. Second, because our patients were adolescents and very large, we used a 6-hole, 4.5-mm compression plate and screws. Third, we used an external fixator for increased stability in patients who weighed more than 250 pounds.
The reported technique, using an oblique metaphyseal closing wedge osteotomy with internal fixation in obese patients, is practical, safe, and reliable. This technique is a useful alternative to an external fixator. We used it on 9 knees with tibia vara, and it was completely successful in 8 cases and partially successful in 1 (hardware breakage occurred). An external fixator was used to prevent hardware breakage in 2 patients who weighed more than 250 pounds. This technique is a valuable treatment option for surgical correction, especially in obese patients.
Blount disease (tibia vara) is an angular tibia deformity that includes varus, increased posterior slope, and internal rotation. This deformity was first described in 1922 by Erlacher1 in Germany. In 1937, Walter Blount2 reported on it in the United States. It is the most common cause of pathologic genu varum in adolescence and childhood.
An oblique incomplete closing wedge osteotomy of the proximal tibial metaphysis was described by Wagner3 for the treatment of unicompartmental osteoarthrosis of the knee in adults. Laurencin and colleagues4 applied this technique to the treatment of pediatric tibia vara with favorable results. They spared the medial cortex of the tibia in their incomplete closing wedge osteotomy technique. In each of the 9 cases we treated and describe here, we accidentally completed the tibial osteotomy when attempting the Laurencin technique. Given that the osteotomy was completed, we modified the Laurencin technique by using a 6-hole, 4.5-mm compression plate rather than a 5-hole semitubular plate, and added a large oblique screw from the medial side to compress the osteotomy site and to protect the plate from fracture. In addition, in 2 patients who weighed more than 250 pounds, we used an external fixator for additional stability. In this article, we report the outcomes of correcting adolescent tibia vara with a complete closing wedge tibial osteotomy and an oblique fibular osteotomy.
Materials and Methods
This study was approved by the Institutional Review Board at Pennsylvania State University. Between 2009 and 2012, we performed 9 complete oblique proximal tibial lateral closing wedge osteotomies on 8 patients (2 girls, 6 boys). In each case, the primary diagnosis was Blount disease. One patient also had renal dysplasia and was receiving dialysis. Mean age at time of operation was 15 years (range, 13-17 years). Mean preoperative weight was 215 pounds (range, 119-317 lb). Mean weight gain at follow-up was 4.39 pounds (range, –10 to 19 lb). Mean body mass index (BMI) was 38 (range, 25-48) (Table). All patients had varus angulation of the proximal tibia before surgery. Mean preoperative varus on standing films was 22° (range, 10°-36°). Because of the patients’ size, we used standing long-leg radiographs, on individual cassettes, for each leg.
Surgical Technique
Before surgery, we use paper cutouts to template the osteotomy wedge. We also use perioperative antibiotics and a standard time-out. For visualization of the entire leg for accurate correction, we prepare and drape the entire leg. A sterile tourniquet is used. At the midshaft of the fibula, a 4-cm incision is made, and dissection is carefully carried down to the fibula. Subperiosteal dissection is performed about the fibula, allowing adequate clearance for an oblique osteotomy. The osteotomy removes about 1 cm of fibula, which is to be used as bone graft for the tibial osteotomy. In addition, a lateral compartment fasciotomy is performed to prevent swelling-related complications. The wound is irrigated and injected with bupivacaine and closed in routine fashion.
We then make an inverted hockey-stick incision over the proximal tibia, centered down to the tibial tubercle. After dissecting down to the anterior compartment, we perform a fasciotomy of about 8 cm to accommodate swelling. Subperiosteal dissection is then performed around the proximal tibia. The medial soft tissues are left attached to increase blood supply and healing. During subperiosteal dissection, soft elevators are used to gently retract the lateral soft tissues along with the inferior and posterior structures. We use fluoroscopic imaging to guide the osteotomy as well as screw and plate placement. We use a 6-hole, 4.5-mm compression plate and screws for fixation. The 2 proximal screws of the plate are predrilled in place to allow for application of the plate after completion of the osteotomy. The plate is then rotated out of position on 1 screw, and the osteotomy is identified under fluoroscopy with the appropriate position distal to the second hole of the 6-hole plate.
An oscillating saw and osteotomes are used to perform the oblique osteotomy. The pre-estimated bone wedge is removed. Wedge size is adjusted, if needed. The bone wedge is morselized for bone graft. The osteotomy is then closed, correcting both varus and internal tibial torsion. Our goal is 5° valgus. After correction is obtained, the plate is placed, and the proximal screw is snugly seated. Three cortical screws are placed distally to hold the plate in place under compression mode, and a cancellous screw is placed superiorly at the proximal portion of the plate for additional fixation. The screw placed proximal to the osteotomy site is a fully threaded cortical screw with excellent compression. Correction and proper placement of hardware are verified with fluoroscopy.
The wound is irrigated and injected with bupivacaine. Bone graft is then placed at the osteotomy site. Additional bone graft is placed posteriorly between the osteotomy site and the muscle mass to stimulate additional healing. Another screw is placed obliquely from the medial side across the osteotomy site to provide additional fixation (Figure 1).
A deep drain is placed and connected to bulb suction for 24 hours after surgery. The wound is then closed in routine fashion. In 2 patients who weighed more than 250 pounds, we used an external fixator for additional stability (Figure 2).
Postoperative Care
The incisions are dressed with antibiotic ointment and 4×4-in bandages and then wrapped with sterile cotton under-cast padding. The leg is placed into a well-padded cylinder cast with the knee flexed 10°. The leg is aligned to about 5° valgus. The cast is then split on the side and spread to allow for swelling and to prevent compartment syndrome.5 We also use a drain hooked to bulb suction, which is removed 24 hours after surgery. Toe-touch weight-bearing with crutches is allowed immediately after surgery. The cast is removed at 6 weeks, and a hinged range-of-motion knee brace is worn for another 6 weeks. All patients are allowed to resume normal activity after 4 months. In our 2 external-fixator cases, a cast was not used, and toe-touch weight-bearing and knee motion were allowed immediately. The external fixators were removed at about 10 weeks.
Results
Mean postoperative mechanical femoral-tibial angle was 3°, and mean correction was 26° (range, 16°-43°) (Table). Lateral distal femoral angle did not show significant femoral deformity in our sample. Mean medial proximal tibial angle was 74° (range, 63°-79°). In each case, the varus deformity was primarily in the tibia. Mean tourniquet time was 88 minutes (range, 50-119 min). Our complication rate was 11% (1 knee). In our first case, in which we did not use an extra medial screw, the 4.5-mm plate fractured at the osteotomy site 2.5 months after surgery. The 250-pound patient subsequently lost 17° of correction, and valgus alignment was not achieved. Preoperative varus was 25°, and postoperative alignment was 8° varus. This plate fracture led us to use an extra medial screw for additional stability in all subsequent cases and to consider using an external fixator for patients weighing more than 250 pounds. After the first case, there were no other plate fractures. A potential problem with closing wedge osteotomy is shortening, but varus correction restores some length. Mean postoperative leg-length difference was 10 mm (range, 0-16 mm). No patient complained of leg-length difference during the postoperative follow-up.
Eight and a half months after surgery, 1 patient had hardware removed, at the family’s request. No patient experienced perioperative infection or neurovascular damage. Our overall patient population was obese—mean BMI was 38 (range, 25-48), and mean postoperative weight was 219 pounds. Three of our 8 patients were overweight (BMI, 25-30), and 5 were obese (BMI, >30). For prevention of plate failure, we recommend using an extra oblique screw in all patients and considering an external fixator for patients who weigh more than 250 pounds.
Discussion
Correction of adolescent tibia vara can be challenging because of patient obesity. The technique described here—a modification of the technique of Laurencin and colleagues4—is practical and reproducible in this population. The goals in performing osteotomy are to correct the deformity, restore joint alignment, preserve leg length, and prevent recurrent deformity and other complications, such as neurovascular injury, nonunion, and infection.3,6-8 Our technique minimizes the risk for these complications. For example, the fasciotomy provides excellent decompression of the anterior and lateral compartments, minimizing neurovascular ischemia and the risk for compartment syndrome. During cast placement, splitting and spreading reduce the risk for compartment syndrome as well.5
Wagner3,9 demonstrated the utility of a closing wedge proximal tibial osteotomy in adults. Laurencin and colleagues4 showed this technique is effective in correcting tibia vara in a pediatric population. However, they did not specify patient weight and used a small semitubular plate for fixation, and some of their patients had infantile Blount disease. We modified the technique in 3 ways. First, we performed a complete osteotomy. Second, because our patients were adolescents and very large, we used a 6-hole, 4.5-mm compression plate and screws. Third, we used an external fixator for increased stability in patients who weighed more than 250 pounds.
The reported technique, using an oblique metaphyseal closing wedge osteotomy with internal fixation in obese patients, is practical, safe, and reliable. This technique is a useful alternative to an external fixator. We used it on 9 knees with tibia vara, and it was completely successful in 8 cases and partially successful in 1 (hardware breakage occurred). An external fixator was used to prevent hardware breakage in 2 patients who weighed more than 250 pounds. This technique is a valuable treatment option for surgical correction, especially in obese patients.
1. Erlacher P. Deformierende Prozesse der Epiphysengegend bei Kindem. Archiv Orthop Unfall-Chir. 1922;20:81-96.
2. Blount WP. Tibia vara. J Bone Joint Surg. 1937;29:1-28.
3. Wagner H. Principles of corrective osteotomies in osteoarthrosis of the knee. In: Weal UH, ed. Joint Preserving Procedures of the Lower Extremity. New York, NY: Springer; 1980:77-102.
4. Laurencin CT, Ferriter PJ, Millis MB. Oblique proximal tibial osteotomy for the correction of tibia vara in the young. Clin Orthop Relat Res. 1996;(327):218-224.
5. Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
6. Mycoskie PJ. Complications of osteotomies about the knee in children. Orthopedics. 1981;4(9):1005-1015.
7. Matsen FA, Staheli LT. Neurovascular complications following tibial osteotomy in children. A case report. Clin Orthop Relat Res. 1975;(110):210-214.
8. Steel HH, Sandrew RE, Sullivan PD. Complications of tibial osteotomy in children for genu varum or valgum. Evidence that neurological changes are due to ischemia. J Bone Joint Surg Am. 1971;53(8):1629-1635.
9. Wagner H. The displacement osteotomy as a correction principle. In: Heirholzer G, Muller KH, eds. Corrective Osteotomies of the Lower Extremity After Trauma. Berlin, Germany: Springer; 1985:141-150.
1. Erlacher P. Deformierende Prozesse der Epiphysengegend bei Kindem. Archiv Orthop Unfall-Chir. 1922;20:81-96.
2. Blount WP. Tibia vara. J Bone Joint Surg. 1937;29:1-28.
3. Wagner H. Principles of corrective osteotomies in osteoarthrosis of the knee. In: Weal UH, ed. Joint Preserving Procedures of the Lower Extremity. New York, NY: Springer; 1980:77-102.
4. Laurencin CT, Ferriter PJ, Millis MB. Oblique proximal tibial osteotomy for the correction of tibia vara in the young. Clin Orthop Relat Res. 1996;(327):218-224.
5. Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
6. Mycoskie PJ. Complications of osteotomies about the knee in children. Orthopedics. 1981;4(9):1005-1015.
7. Matsen FA, Staheli LT. Neurovascular complications following tibial osteotomy in children. A case report. Clin Orthop Relat Res. 1975;(110):210-214.
8. Steel HH, Sandrew RE, Sullivan PD. Complications of tibial osteotomy in children for genu varum or valgum. Evidence that neurological changes are due to ischemia. J Bone Joint Surg Am. 1971;53(8):1629-1635.
9. Wagner H. The displacement osteotomy as a correction principle. In: Heirholzer G, Muller KH, eds. Corrective Osteotomies of the Lower Extremity After Trauma. Berlin, Germany: Springer; 1985:141-150.
Wide variation in clinical management of thyroid nodules seen in first-ever survey
LAKE BUENA VISTA, FLA. – When making diagnostic and treatment decisions about thyroid nodules, many endocrinologists are not following current clinical practice guidelines, according to a recent survey. Further, according to a recent international survey of endocrinologists, there is wide regional variation in the use of molecular testing and calcitonin levels.
Dr. Nicole Vietor of the department of endocrinology at Walter Reed National Military Medical Center, Bethesda, Md., and her collaborators contacted members of the American Thyroid Association (ATA), the Endocrine Society (TES), and the American Association of Clinical Endocrinologists (AACE). Members of these societies were contacted directly by investigators and asked to complete a web-based survey regarding their practices for diagnosing and managing thyroid nodules.
The survey consisted of 36 questions, with an index case with variations presented to respondents, who then answered questions about diagnostic and management decisionmaking practices. The hypothetical index patient was a 52 year old woman with an incidental finding of a 1.5 cm right thyroid nodule. The patient was healthy and without risk factors; the patient’s nodule was not palpable on physical exam and she had no cervical lymphadenopathy.
Almost all respondents (99.4%) would order a thyroid-stimulating hormone for initial lab testing. Other commonly ordered exams included free T4 levels and thyroid peroxidase antibody requested by 41.5% and 24.3% of respondents, respectively. Fewer than 15% of respondents would have ordered any further lab exams.
All but 1.5% of respondents would order an anatomic or functional test for this index patient, with 57.2% ordering a thyroid ultrasound to be performed in radiology and 52.1% ordering an ultrasound in clinic (multiple responses were permitted). Cervical lymph nodes were included in the initial ultrasound assessment by 68.5% of respondents. Overall, more than half (56.6%) of thyroid ultrasounds were performed by endocrinologists, and about a third (31.9%) done by radiologists.
Practice variation from guidelines became apparent when respondents were asked how various nodule characteristics affected the decision to perform a fine needle aspiration (FNA). For a 1.5-cm solid hypoechoic nodule, 93.8% of respondents would perform FNA, while two thirds (67.0%) would perform an FNA for a 0.7-cm hypoechoic nodule with microcalcifications.
When performing FNAs, 83.3% of respondents use ultrasound to guide the biopsy, with most operators performing two (19.2%), three (28.5%), or four (23.0%) passes per nodule.
Of the 897 respondents, 80.5% were TES members, 56.5% were AACE members, and 44.5% were ATA members; most respondents belonged to more than one society. Almost two thirds of respondents (63.0%) were from North America, while 12.2% were from Europe, 10.8% were from Latin America, 6.5% were from Asia, 5.6% were from the Middle East or Africa, and just 1.9% were from Oceania. More men (60.2%) than women responded.
The AACE issued clinical practice guidelines for the management of thyroid nodules in 2010, as did the ATA in 2009 and 2015.
“In summary, management of a thyroid nodule is highly variable and differs from societal guidelines in multiple areas,” wrote Dr. Vietor and her colleagues in the presentation abstract.
On Twitter @karioakes
LAKE BUENA VISTA, FLA. – When making diagnostic and treatment decisions about thyroid nodules, many endocrinologists are not following current clinical practice guidelines, according to a recent survey. Further, according to a recent international survey of endocrinologists, there is wide regional variation in the use of molecular testing and calcitonin levels.
Dr. Nicole Vietor of the department of endocrinology at Walter Reed National Military Medical Center, Bethesda, Md., and her collaborators contacted members of the American Thyroid Association (ATA), the Endocrine Society (TES), and the American Association of Clinical Endocrinologists (AACE). Members of these societies were contacted directly by investigators and asked to complete a web-based survey regarding their practices for diagnosing and managing thyroid nodules.
The survey consisted of 36 questions, with an index case with variations presented to respondents, who then answered questions about diagnostic and management decisionmaking practices. The hypothetical index patient was a 52 year old woman with an incidental finding of a 1.5 cm right thyroid nodule. The patient was healthy and without risk factors; the patient’s nodule was not palpable on physical exam and she had no cervical lymphadenopathy.
Almost all respondents (99.4%) would order a thyroid-stimulating hormone for initial lab testing. Other commonly ordered exams included free T4 levels and thyroid peroxidase antibody requested by 41.5% and 24.3% of respondents, respectively. Fewer than 15% of respondents would have ordered any further lab exams.
All but 1.5% of respondents would order an anatomic or functional test for this index patient, with 57.2% ordering a thyroid ultrasound to be performed in radiology and 52.1% ordering an ultrasound in clinic (multiple responses were permitted). Cervical lymph nodes were included in the initial ultrasound assessment by 68.5% of respondents. Overall, more than half (56.6%) of thyroid ultrasounds were performed by endocrinologists, and about a third (31.9%) done by radiologists.
Practice variation from guidelines became apparent when respondents were asked how various nodule characteristics affected the decision to perform a fine needle aspiration (FNA). For a 1.5-cm solid hypoechoic nodule, 93.8% of respondents would perform FNA, while two thirds (67.0%) would perform an FNA for a 0.7-cm hypoechoic nodule with microcalcifications.
When performing FNAs, 83.3% of respondents use ultrasound to guide the biopsy, with most operators performing two (19.2%), three (28.5%), or four (23.0%) passes per nodule.
Of the 897 respondents, 80.5% were TES members, 56.5% were AACE members, and 44.5% were ATA members; most respondents belonged to more than one society. Almost two thirds of respondents (63.0%) were from North America, while 12.2% were from Europe, 10.8% were from Latin America, 6.5% were from Asia, 5.6% were from the Middle East or Africa, and just 1.9% were from Oceania. More men (60.2%) than women responded.
The AACE issued clinical practice guidelines for the management of thyroid nodules in 2010, as did the ATA in 2009 and 2015.
“In summary, management of a thyroid nodule is highly variable and differs from societal guidelines in multiple areas,” wrote Dr. Vietor and her colleagues in the presentation abstract.
On Twitter @karioakes
LAKE BUENA VISTA, FLA. – When making diagnostic and treatment decisions about thyroid nodules, many endocrinologists are not following current clinical practice guidelines, according to a recent survey. Further, according to a recent international survey of endocrinologists, there is wide regional variation in the use of molecular testing and calcitonin levels.
Dr. Nicole Vietor of the department of endocrinology at Walter Reed National Military Medical Center, Bethesda, Md., and her collaborators contacted members of the American Thyroid Association (ATA), the Endocrine Society (TES), and the American Association of Clinical Endocrinologists (AACE). Members of these societies were contacted directly by investigators and asked to complete a web-based survey regarding their practices for diagnosing and managing thyroid nodules.
The survey consisted of 36 questions, with an index case with variations presented to respondents, who then answered questions about diagnostic and management decisionmaking practices. The hypothetical index patient was a 52 year old woman with an incidental finding of a 1.5 cm right thyroid nodule. The patient was healthy and without risk factors; the patient’s nodule was not palpable on physical exam and she had no cervical lymphadenopathy.
Almost all respondents (99.4%) would order a thyroid-stimulating hormone for initial lab testing. Other commonly ordered exams included free T4 levels and thyroid peroxidase antibody requested by 41.5% and 24.3% of respondents, respectively. Fewer than 15% of respondents would have ordered any further lab exams.
All but 1.5% of respondents would order an anatomic or functional test for this index patient, with 57.2% ordering a thyroid ultrasound to be performed in radiology and 52.1% ordering an ultrasound in clinic (multiple responses were permitted). Cervical lymph nodes were included in the initial ultrasound assessment by 68.5% of respondents. Overall, more than half (56.6%) of thyroid ultrasounds were performed by endocrinologists, and about a third (31.9%) done by radiologists.
Practice variation from guidelines became apparent when respondents were asked how various nodule characteristics affected the decision to perform a fine needle aspiration (FNA). For a 1.5-cm solid hypoechoic nodule, 93.8% of respondents would perform FNA, while two thirds (67.0%) would perform an FNA for a 0.7-cm hypoechoic nodule with microcalcifications.
When performing FNAs, 83.3% of respondents use ultrasound to guide the biopsy, with most operators performing two (19.2%), three (28.5%), or four (23.0%) passes per nodule.
Of the 897 respondents, 80.5% were TES members, 56.5% were AACE members, and 44.5% were ATA members; most respondents belonged to more than one society. Almost two thirds of respondents (63.0%) were from North America, while 12.2% were from Europe, 10.8% were from Latin America, 6.5% were from Asia, 5.6% were from the Middle East or Africa, and just 1.9% were from Oceania. More men (60.2%) than women responded.
The AACE issued clinical practice guidelines for the management of thyroid nodules in 2010, as did the ATA in 2009 and 2015.
“In summary, management of a thyroid nodule is highly variable and differs from societal guidelines in multiple areas,” wrote Dr. Vietor and her colleagues in the presentation abstract.
On Twitter @karioakes
AT THE 15TH INTERNATIONAL THYROID CONGRESS
Key clinical point: A first-ever international survey of endocrinologists showed wide variation in clinical management of thyroid nodules.
Major finding: Respondents reported performing more fine needle aspiration (FNA) biopsies of thyroid nodules than recommended by practice guidelines.
Data source: Web-based survey of 897 members of three different professional organizations.
Disclosures: No disclosures were identified.
FDA finalizes 1-year blood donor deferral for gay, bisexual men
The Food and Drug Administration announced finalized blood-donor deferral guidance that includes cutting the deferral for men who have sex with men from indefinitely to 1 year from the most recent sexual contact of this type.
The finalized guidance, which had been in development since its initial announcement in December 2014, contains 10 recommended questions that the blood industry should pose to potential donors to exclude those at greatest risk for potentially transmitting HIV in their blood.
In addition to reducing the deferral period for men who have had sex with men, the finalized guidance also cuts the deferral period for a woman who has had sex with a man who had sex with men from indefinitely to 12 months, and also reduces the deferral period for any person who has had sex with someone with a history of a positive test for HIV from indefinitely to 12 months.
The new guidance continues to recommend indefinite deferral for people who have ever tested positive for HIV, those who have exchanged sex for money or drugs, and those with a history of nonprescription injection drug use.
The FDA’s guidance document highlights that these are recommended steps for the blood products industry to take and are not required by law or legally enforceable.
The finalized guidance, which followed a public comment period on essentially unchanged draft guidance issued last May, received quick support in a statement from Dr. Andrew W. Gurman, president-elect of the American Medical Association.
“The American Medical Association commends the U.S. Food and Drug Administration for ending the lifetime ban that prohibits men who have had sex with men from donating blood,” Dr. Gurman said. “The AMA has been a strong advocate for eliminating public policies that do not align with scientific evidence and best ethical practices in public policy. The FDA’s final guidance takes important steps to improve the balance among ensuring health equity, engaging with high-risk populations, and protecting the safety of the national blood supply.”
However, a highly critical comment came from Daniel Bruner, senior director of policy for Whitman-Walker Health, a Washington-based health center focused on the LGBT community and HIV care.
“Although some may argue that a 12-month ban is better than a grossly outdated lifetime ban, the updated policy is still discriminatory and not rooted in the reality of HIV testing today,” Mr. Bruner said in a statement. “As we called for in our recommendations, the deferral period should be no longer than 30 days, given that with current testing technology an HIV infection can be detected in donated blood within several weeks of exposure.
“And even then, those that would be subject to the deferral period should be able to donate blood if they agree to return for an HIV test 30 days after donating,” Mr. Bruner said. “Sadly, and also not in line with modern science, the new guidelines continue the discriminatory lifetime bans on individuals who have ever engaged in sex work or ever used nonprescription injection drugs.”
Mr. Bruner added, “We are also disheartened that the FDA has failed to give clear guidance to prevent discrimination against transgender individuals, which occurs too often at blood donation centers. The FDA must do better than this slow chipping away at antiquated bans.”
On Twitter @mitchelzoler
The Food and Drug Administration announced finalized blood-donor deferral guidance that includes cutting the deferral for men who have sex with men from indefinitely to 1 year from the most recent sexual contact of this type.
The finalized guidance, which had been in development since its initial announcement in December 2014, contains 10 recommended questions that the blood industry should pose to potential donors to exclude those at greatest risk for potentially transmitting HIV in their blood.
In addition to reducing the deferral period for men who have had sex with men, the finalized guidance also cuts the deferral period for a woman who has had sex with a man who had sex with men from indefinitely to 12 months, and also reduces the deferral period for any person who has had sex with someone with a history of a positive test for HIV from indefinitely to 12 months.
The new guidance continues to recommend indefinite deferral for people who have ever tested positive for HIV, those who have exchanged sex for money or drugs, and those with a history of nonprescription injection drug use.
The FDA’s guidance document highlights that these are recommended steps for the blood products industry to take and are not required by law or legally enforceable.
The finalized guidance, which followed a public comment period on essentially unchanged draft guidance issued last May, received quick support in a statement from Dr. Andrew W. Gurman, president-elect of the American Medical Association.
“The American Medical Association commends the U.S. Food and Drug Administration for ending the lifetime ban that prohibits men who have had sex with men from donating blood,” Dr. Gurman said. “The AMA has been a strong advocate for eliminating public policies that do not align with scientific evidence and best ethical practices in public policy. The FDA’s final guidance takes important steps to improve the balance among ensuring health equity, engaging with high-risk populations, and protecting the safety of the national blood supply.”
However, a highly critical comment came from Daniel Bruner, senior director of policy for Whitman-Walker Health, a Washington-based health center focused on the LGBT community and HIV care.
“Although some may argue that a 12-month ban is better than a grossly outdated lifetime ban, the updated policy is still discriminatory and not rooted in the reality of HIV testing today,” Mr. Bruner said in a statement. “As we called for in our recommendations, the deferral period should be no longer than 30 days, given that with current testing technology an HIV infection can be detected in donated blood within several weeks of exposure.
“And even then, those that would be subject to the deferral period should be able to donate blood if they agree to return for an HIV test 30 days after donating,” Mr. Bruner said. “Sadly, and also not in line with modern science, the new guidelines continue the discriminatory lifetime bans on individuals who have ever engaged in sex work or ever used nonprescription injection drugs.”
Mr. Bruner added, “We are also disheartened that the FDA has failed to give clear guidance to prevent discrimination against transgender individuals, which occurs too often at blood donation centers. The FDA must do better than this slow chipping away at antiquated bans.”
On Twitter @mitchelzoler
The Food and Drug Administration announced finalized blood-donor deferral guidance that includes cutting the deferral for men who have sex with men from indefinitely to 1 year from the most recent sexual contact of this type.
The finalized guidance, which had been in development since its initial announcement in December 2014, contains 10 recommended questions that the blood industry should pose to potential donors to exclude those at greatest risk for potentially transmitting HIV in their blood.
In addition to reducing the deferral period for men who have had sex with men, the finalized guidance also cuts the deferral period for a woman who has had sex with a man who had sex with men from indefinitely to 12 months, and also reduces the deferral period for any person who has had sex with someone with a history of a positive test for HIV from indefinitely to 12 months.
The new guidance continues to recommend indefinite deferral for people who have ever tested positive for HIV, those who have exchanged sex for money or drugs, and those with a history of nonprescription injection drug use.
The FDA’s guidance document highlights that these are recommended steps for the blood products industry to take and are not required by law or legally enforceable.
The finalized guidance, which followed a public comment period on essentially unchanged draft guidance issued last May, received quick support in a statement from Dr. Andrew W. Gurman, president-elect of the American Medical Association.
“The American Medical Association commends the U.S. Food and Drug Administration for ending the lifetime ban that prohibits men who have had sex with men from donating blood,” Dr. Gurman said. “The AMA has been a strong advocate for eliminating public policies that do not align with scientific evidence and best ethical practices in public policy. The FDA’s final guidance takes important steps to improve the balance among ensuring health equity, engaging with high-risk populations, and protecting the safety of the national blood supply.”
However, a highly critical comment came from Daniel Bruner, senior director of policy for Whitman-Walker Health, a Washington-based health center focused on the LGBT community and HIV care.
“Although some may argue that a 12-month ban is better than a grossly outdated lifetime ban, the updated policy is still discriminatory and not rooted in the reality of HIV testing today,” Mr. Bruner said in a statement. “As we called for in our recommendations, the deferral period should be no longer than 30 days, given that with current testing technology an HIV infection can be detected in donated blood within several weeks of exposure.
“And even then, those that would be subject to the deferral period should be able to donate blood if they agree to return for an HIV test 30 days after donating,” Mr. Bruner said. “Sadly, and also not in line with modern science, the new guidelines continue the discriminatory lifetime bans on individuals who have ever engaged in sex work or ever used nonprescription injection drugs.”
Mr. Bruner added, “We are also disheartened that the FDA has failed to give clear guidance to prevent discrimination against transgender individuals, which occurs too often at blood donation centers. The FDA must do better than this slow chipping away at antiquated bans.”
On Twitter @mitchelzoler
SF-6D best quality of life measure in cervical spine patients
SAN DIEGO – Among patients undergoing elective surgical spine procedures, the Short Form–6D derived from the Neck Disability Index was more valid and a better responsive measure of general health and quality of life, compared with the Short Form–6D derived from the Short Form–12 or the EuroQol-5D, results from a single-center study showed.
For such quality of life measures to be useful and meaningful, they “should be reproducible, responsive, economical, easy to use, and sensitive to responder burden,” Dr. John A. Sielatycki said at the annual meeting of the Cervical Spine Research Society.
“The EQ-5D is well established and commonly used in many of these studies, as is SF-6D, which in some cases has been shown to be more sensitive in certain disease states,” explained Dr. Sielatycki, a resident in the department of orthopedics at Vanderbilt University, Nashville, Tenn. “The differences between SF-6D and EQ-5D have been studied in a wide variety of disease conditions, but to our knowledge few have looked at this specifically in the setting of cervical spine operations.”
To analyze the validity and responsiveness of the SF-6D (derived from both the SF-12 and the NDI) and the EQ-5D in determining overall health and quality of life following elective cervical spine procedures, Dr. Sielatycki and his associates compared the three tools in 420 consecutive patients who presented over the course of 2 years. Trauma and workers’ compensation cases were excluded from the study, as were patients who had a tumor or an infection.
The researchers collected outcome measures at baseline, 3 months, 6 months, 12 months, and yearly thereafter, and defined meaningful improvement as having a North American Spine Society patient satisfaction score of 1, indicating the procedure “met the patient’s expectations.” Next, they generated receiver operating characteristic curves to discriminate between meaningful and nonmeaningful improvement.
The SF-6D (NDI) was a more valid discriminator of meaningful improvement, compared with the SF-6D (SF-12) or the EQ-5D (area under the curve of .69, .65, and .62, respectively). It was also a more responsive measure, compared with the SF-6D (SF-12) and the EQ-5D (standardized response means difference of .66, .48, and .44, respectively).
“Surgeons, outcomes researchers, and payers should use health metrics that are most responsive to changes in the particular disease in question,” Dr. Sielatycki said. “Based on this analysis, SF-6D derived from NDI may be a more valid and responsive measure of improvement in patients undergoing cervical procedures. We suggest that this metric be used in cost-effectiveness analysis and in calculating quality-adjusted life years for cervical spine patients.”
Dr. Sielatycki acknowledged certain limitations of the study, including the fact that it “should have some external validation done to further corroborate our findings. Our gold standard of meaningful improvement has not been established.”
Dr. Sielatycki reported having no financial disclosures.
SAN DIEGO – Among patients undergoing elective surgical spine procedures, the Short Form–6D derived from the Neck Disability Index was more valid and a better responsive measure of general health and quality of life, compared with the Short Form–6D derived from the Short Form–12 or the EuroQol-5D, results from a single-center study showed.
For such quality of life measures to be useful and meaningful, they “should be reproducible, responsive, economical, easy to use, and sensitive to responder burden,” Dr. John A. Sielatycki said at the annual meeting of the Cervical Spine Research Society.
“The EQ-5D is well established and commonly used in many of these studies, as is SF-6D, which in some cases has been shown to be more sensitive in certain disease states,” explained Dr. Sielatycki, a resident in the department of orthopedics at Vanderbilt University, Nashville, Tenn. “The differences between SF-6D and EQ-5D have been studied in a wide variety of disease conditions, but to our knowledge few have looked at this specifically in the setting of cervical spine operations.”
To analyze the validity and responsiveness of the SF-6D (derived from both the SF-12 and the NDI) and the EQ-5D in determining overall health and quality of life following elective cervical spine procedures, Dr. Sielatycki and his associates compared the three tools in 420 consecutive patients who presented over the course of 2 years. Trauma and workers’ compensation cases were excluded from the study, as were patients who had a tumor or an infection.
The researchers collected outcome measures at baseline, 3 months, 6 months, 12 months, and yearly thereafter, and defined meaningful improvement as having a North American Spine Society patient satisfaction score of 1, indicating the procedure “met the patient’s expectations.” Next, they generated receiver operating characteristic curves to discriminate between meaningful and nonmeaningful improvement.
The SF-6D (NDI) was a more valid discriminator of meaningful improvement, compared with the SF-6D (SF-12) or the EQ-5D (area under the curve of .69, .65, and .62, respectively). It was also a more responsive measure, compared with the SF-6D (SF-12) and the EQ-5D (standardized response means difference of .66, .48, and .44, respectively).
“Surgeons, outcomes researchers, and payers should use health metrics that are most responsive to changes in the particular disease in question,” Dr. Sielatycki said. “Based on this analysis, SF-6D derived from NDI may be a more valid and responsive measure of improvement in patients undergoing cervical procedures. We suggest that this metric be used in cost-effectiveness analysis and in calculating quality-adjusted life years for cervical spine patients.”
Dr. Sielatycki acknowledged certain limitations of the study, including the fact that it “should have some external validation done to further corroborate our findings. Our gold standard of meaningful improvement has not been established.”
Dr. Sielatycki reported having no financial disclosures.
SAN DIEGO – Among patients undergoing elective surgical spine procedures, the Short Form–6D derived from the Neck Disability Index was more valid and a better responsive measure of general health and quality of life, compared with the Short Form–6D derived from the Short Form–12 or the EuroQol-5D, results from a single-center study showed.
For such quality of life measures to be useful and meaningful, they “should be reproducible, responsive, economical, easy to use, and sensitive to responder burden,” Dr. John A. Sielatycki said at the annual meeting of the Cervical Spine Research Society.
“The EQ-5D is well established and commonly used in many of these studies, as is SF-6D, which in some cases has been shown to be more sensitive in certain disease states,” explained Dr. Sielatycki, a resident in the department of orthopedics at Vanderbilt University, Nashville, Tenn. “The differences between SF-6D and EQ-5D have been studied in a wide variety of disease conditions, but to our knowledge few have looked at this specifically in the setting of cervical spine operations.”
To analyze the validity and responsiveness of the SF-6D (derived from both the SF-12 and the NDI) and the EQ-5D in determining overall health and quality of life following elective cervical spine procedures, Dr. Sielatycki and his associates compared the three tools in 420 consecutive patients who presented over the course of 2 years. Trauma and workers’ compensation cases were excluded from the study, as were patients who had a tumor or an infection.
The researchers collected outcome measures at baseline, 3 months, 6 months, 12 months, and yearly thereafter, and defined meaningful improvement as having a North American Spine Society patient satisfaction score of 1, indicating the procedure “met the patient’s expectations.” Next, they generated receiver operating characteristic curves to discriminate between meaningful and nonmeaningful improvement.
The SF-6D (NDI) was a more valid discriminator of meaningful improvement, compared with the SF-6D (SF-12) or the EQ-5D (area under the curve of .69, .65, and .62, respectively). It was also a more responsive measure, compared with the SF-6D (SF-12) and the EQ-5D (standardized response means difference of .66, .48, and .44, respectively).
“Surgeons, outcomes researchers, and payers should use health metrics that are most responsive to changes in the particular disease in question,” Dr. Sielatycki said. “Based on this analysis, SF-6D derived from NDI may be a more valid and responsive measure of improvement in patients undergoing cervical procedures. We suggest that this metric be used in cost-effectiveness analysis and in calculating quality-adjusted life years for cervical spine patients.”
Dr. Sielatycki acknowledged certain limitations of the study, including the fact that it “should have some external validation done to further corroborate our findings. Our gold standard of meaningful improvement has not been established.”
Dr. Sielatycki reported having no financial disclosures.
AT CSRS 2015
Key clinical point: The Short Form–6D derived from the Neck Disability Index is an effective measure of outcomes in cervical spine patients.
Major finding: The Short Form–6D derived from the Neck Disability Index was a more valid discriminator of meaningful improvement, compared with the Short Form–6D derived from the Short Form–12 or the EuroQol-5D (AUC of .69, .65, and .62, respectively).
Data source: A single-center study that compared three quality of life measures in 420 patients presenting for elective surgical spine procedures.
Disclosures: Dr. Sielatycki reported having no financial disclosures.
‘Hot’ Joints May Predict RA Joint Damage
Recording the temperature of skin over an inflamed joint may identify rheumatoid arthritis patients at high risk of joint damage, an exploratory study suggested.
Dermal joint temperature could become a screening test to “quickly and accurately” identify individual RA patients at high risk for radiographic damage and those who may benefit from biologic therapy, said Dr. Maria Greenwald, a rheumatologist in group practice in Palm Desert, Calif., and her colleagues.
During 2009-2014, the investigators enrolled seropositive RA patients who were on stable doses of methotrexate (20-25 mg/wk) for the past 3 months and did not use biologics or other disease-modifying antirheumatic drugs. It took 9 months to enroll 104 patients with cool joints and 42 months to enroll 104 patients with hot joints, suggesting “that at a single office visit, RA patients on methotrexate are five times more likely to have cool joints than hot joints,” the researchers reported.
The results showed that patients in the hot-joint cohort had a nearly fourfold higher risk of x-ray damage at 1-year follow-up, compared with those with cool joints (change in modified van der Heijde total Sharp score [mTSS]: 8.7 vs. 2.5; P less than .001). Patients with hot joints had an average joint temperature exceeding central forehead body temperature by 1.1° F, whereas those with cool joints had an average joint temperature of 4.3° F below central forehead body temperature.
In the cohort of patients with hot joints, 74% had clear x-ray evidence of new joint damage (mTSS of 5 or greater), compared with 7% of cold-joint cohort patients at 1-year follow-up. Joint temperature at the hand or wrist predicted x-ray damage in the next year with 92% sensitivity and 78% specificity(Arthritis Care Res. 2015 Dec 14. doi: 10.1002/acr.22813).
Patients in the hot-joint cohort were younger, had more recent onset of RA, and had a significantly higher Westergren erythrocyte sedimentation rate than patients in the cool-joint cohort, the investigators noted.
They suggested a future study might define a hot-joint cohort as RA patients with a joint that measures over a set point such as 98° F. “Such a cutoff would make assessment very simple and would maintain the specificity and sensitivity of the model,” they said.
No conflicts of interest were disclosed.
Recording the temperature of skin over an inflamed joint may identify rheumatoid arthritis patients at high risk of joint damage, an exploratory study suggested.
Dermal joint temperature could become a screening test to “quickly and accurately” identify individual RA patients at high risk for radiographic damage and those who may benefit from biologic therapy, said Dr. Maria Greenwald, a rheumatologist in group practice in Palm Desert, Calif., and her colleagues.
During 2009-2014, the investigators enrolled seropositive RA patients who were on stable doses of methotrexate (20-25 mg/wk) for the past 3 months and did not use biologics or other disease-modifying antirheumatic drugs. It took 9 months to enroll 104 patients with cool joints and 42 months to enroll 104 patients with hot joints, suggesting “that at a single office visit, RA patients on methotrexate are five times more likely to have cool joints than hot joints,” the researchers reported.
The results showed that patients in the hot-joint cohort had a nearly fourfold higher risk of x-ray damage at 1-year follow-up, compared with those with cool joints (change in modified van der Heijde total Sharp score [mTSS]: 8.7 vs. 2.5; P less than .001). Patients with hot joints had an average joint temperature exceeding central forehead body temperature by 1.1° F, whereas those with cool joints had an average joint temperature of 4.3° F below central forehead body temperature.
In the cohort of patients with hot joints, 74% had clear x-ray evidence of new joint damage (mTSS of 5 or greater), compared with 7% of cold-joint cohort patients at 1-year follow-up. Joint temperature at the hand or wrist predicted x-ray damage in the next year with 92% sensitivity and 78% specificity(Arthritis Care Res. 2015 Dec 14. doi: 10.1002/acr.22813).
Patients in the hot-joint cohort were younger, had more recent onset of RA, and had a significantly higher Westergren erythrocyte sedimentation rate than patients in the cool-joint cohort, the investigators noted.
They suggested a future study might define a hot-joint cohort as RA patients with a joint that measures over a set point such as 98° F. “Such a cutoff would make assessment very simple and would maintain the specificity and sensitivity of the model,” they said.
No conflicts of interest were disclosed.
Recording the temperature of skin over an inflamed joint may identify rheumatoid arthritis patients at high risk of joint damage, an exploratory study suggested.
Dermal joint temperature could become a screening test to “quickly and accurately” identify individual RA patients at high risk for radiographic damage and those who may benefit from biologic therapy, said Dr. Maria Greenwald, a rheumatologist in group practice in Palm Desert, Calif., and her colleagues.
During 2009-2014, the investigators enrolled seropositive RA patients who were on stable doses of methotrexate (20-25 mg/wk) for the past 3 months and did not use biologics or other disease-modifying antirheumatic drugs. It took 9 months to enroll 104 patients with cool joints and 42 months to enroll 104 patients with hot joints, suggesting “that at a single office visit, RA patients on methotrexate are five times more likely to have cool joints than hot joints,” the researchers reported.
The results showed that patients in the hot-joint cohort had a nearly fourfold higher risk of x-ray damage at 1-year follow-up, compared with those with cool joints (change in modified van der Heijde total Sharp score [mTSS]: 8.7 vs. 2.5; P less than .001). Patients with hot joints had an average joint temperature exceeding central forehead body temperature by 1.1° F, whereas those with cool joints had an average joint temperature of 4.3° F below central forehead body temperature.
In the cohort of patients with hot joints, 74% had clear x-ray evidence of new joint damage (mTSS of 5 or greater), compared with 7% of cold-joint cohort patients at 1-year follow-up. Joint temperature at the hand or wrist predicted x-ray damage in the next year with 92% sensitivity and 78% specificity(Arthritis Care Res. 2015 Dec 14. doi: 10.1002/acr.22813).
Patients in the hot-joint cohort were younger, had more recent onset of RA, and had a significantly higher Westergren erythrocyte sedimentation rate than patients in the cool-joint cohort, the investigators noted.
They suggested a future study might define a hot-joint cohort as RA patients with a joint that measures over a set point such as 98° F. “Such a cutoff would make assessment very simple and would maintain the specificity and sensitivity of the model,” they said.
No conflicts of interest were disclosed.
FROM ARTHRITIS CARE & RESEARCH
Thigh muscle weakness a risk factor for knee replacement in women
Women with knee osteoarthritis who had low thigh muscle strength were more likely to need a knee replacement in a case-control study of participants in the Osteoarthritis Initiative (OAI).
In particular, predictors of knee replacement included knee extensor weakness in the year prior to knee replacement and longitudinal deterioration in knee extensor strength over a 2-year observation period prior to surgery. Measurement of knee extensor strength in women with knee osteoarthritis may then indicate who could benefit from weight training exercises to potentially delay or prevent the need for knee replacement surgery, said the researchers, led by Dr. Adam Culvenor of Paracelsus Medical University in Salzburg, Austria (Arthritis Rheumatol. 2015 Dec 14. doi: 10.1002/art.39540).
The optimal knee extensor strength threshold for differentiating those with and without knee replacement risk was approximately 200 N or 0.9 Nm/kg; or prevention of any loss of knee extensor strength over 2 years.
“There appears to be a considerable window for women below this threshold to obtain realistic strength gains and potentially lower the risk of knee replacement,” the study authors concluded.
In the multicenter, longitudinal, case-control study of 4,796 participants in the OAI (60% of whom were women), the investigators identified 136 participants who had received a knee replacement and matched them with controls who had not received a knee replacement and were similar in age, body mass index (BMI), and radiographic stage. The mean age of the women was 65 years and the mean BMI was 29 kg/m2.
The results showed that knee extensor strength at the examination prior to knee replacement (time T0), which occurred 2 years or less before surgery, was significantly lower in females who had received a knee replacement than in matched controls (pain-adjusted odds ratio, 1.72; 95% confidence interval, 1.16-2.56; P = .007). Measurement of the longitudinal change in knee extensor and flexor strength between T0 and 2 years prior to T0 (T-2) also provided similar results (pain-adjusted OR, 4.30; 95% CI, 1.34-13.79; P = .014). The findings were independent of age, BMI, and radiographic disease severity, the researchers noted.
The investigators found no relationship between knee extensor or flexor muscle strength in men and subsequent need for knee replacement surgery. The relationship between thigh muscle strength and knee replacement for women did not extend to measurements made at T-2 or T-4 or the change in thigh muscle strength between T-2 and T-4.
The OAI receives funding from the National Institutes of Health, Merck Research Laboratories, Novartis, GlaxoSmithKline, and Pfizer. The work was also funded by a grant from the European Union Seventh Framework Programme. One author disclosed consulting or preparing educational sessions for pharmaceutical companies and for receiving research support. Two authors reported being employees of Chondrometrics GmbH, a company providing MR image analysis services to academic researchers and to industry.
Women with knee osteoarthritis who had low thigh muscle strength were more likely to need a knee replacement in a case-control study of participants in the Osteoarthritis Initiative (OAI).
In particular, predictors of knee replacement included knee extensor weakness in the year prior to knee replacement and longitudinal deterioration in knee extensor strength over a 2-year observation period prior to surgery. Measurement of knee extensor strength in women with knee osteoarthritis may then indicate who could benefit from weight training exercises to potentially delay or prevent the need for knee replacement surgery, said the researchers, led by Dr. Adam Culvenor of Paracelsus Medical University in Salzburg, Austria (Arthritis Rheumatol. 2015 Dec 14. doi: 10.1002/art.39540).
The optimal knee extensor strength threshold for differentiating those with and without knee replacement risk was approximately 200 N or 0.9 Nm/kg; or prevention of any loss of knee extensor strength over 2 years.
“There appears to be a considerable window for women below this threshold to obtain realistic strength gains and potentially lower the risk of knee replacement,” the study authors concluded.
In the multicenter, longitudinal, case-control study of 4,796 participants in the OAI (60% of whom were women), the investigators identified 136 participants who had received a knee replacement and matched them with controls who had not received a knee replacement and were similar in age, body mass index (BMI), and radiographic stage. The mean age of the women was 65 years and the mean BMI was 29 kg/m2.
The results showed that knee extensor strength at the examination prior to knee replacement (time T0), which occurred 2 years or less before surgery, was significantly lower in females who had received a knee replacement than in matched controls (pain-adjusted odds ratio, 1.72; 95% confidence interval, 1.16-2.56; P = .007). Measurement of the longitudinal change in knee extensor and flexor strength between T0 and 2 years prior to T0 (T-2) also provided similar results (pain-adjusted OR, 4.30; 95% CI, 1.34-13.79; P = .014). The findings were independent of age, BMI, and radiographic disease severity, the researchers noted.
The investigators found no relationship between knee extensor or flexor muscle strength in men and subsequent need for knee replacement surgery. The relationship between thigh muscle strength and knee replacement for women did not extend to measurements made at T-2 or T-4 or the change in thigh muscle strength between T-2 and T-4.
The OAI receives funding from the National Institutes of Health, Merck Research Laboratories, Novartis, GlaxoSmithKline, and Pfizer. The work was also funded by a grant from the European Union Seventh Framework Programme. One author disclosed consulting or preparing educational sessions for pharmaceutical companies and for receiving research support. Two authors reported being employees of Chondrometrics GmbH, a company providing MR image analysis services to academic researchers and to industry.
Women with knee osteoarthritis who had low thigh muscle strength were more likely to need a knee replacement in a case-control study of participants in the Osteoarthritis Initiative (OAI).
In particular, predictors of knee replacement included knee extensor weakness in the year prior to knee replacement and longitudinal deterioration in knee extensor strength over a 2-year observation period prior to surgery. Measurement of knee extensor strength in women with knee osteoarthritis may then indicate who could benefit from weight training exercises to potentially delay or prevent the need for knee replacement surgery, said the researchers, led by Dr. Adam Culvenor of Paracelsus Medical University in Salzburg, Austria (Arthritis Rheumatol. 2015 Dec 14. doi: 10.1002/art.39540).
The optimal knee extensor strength threshold for differentiating those with and without knee replacement risk was approximately 200 N or 0.9 Nm/kg; or prevention of any loss of knee extensor strength over 2 years.
“There appears to be a considerable window for women below this threshold to obtain realistic strength gains and potentially lower the risk of knee replacement,” the study authors concluded.
In the multicenter, longitudinal, case-control study of 4,796 participants in the OAI (60% of whom were women), the investigators identified 136 participants who had received a knee replacement and matched them with controls who had not received a knee replacement and were similar in age, body mass index (BMI), and radiographic stage. The mean age of the women was 65 years and the mean BMI was 29 kg/m2.
The results showed that knee extensor strength at the examination prior to knee replacement (time T0), which occurred 2 years or less before surgery, was significantly lower in females who had received a knee replacement than in matched controls (pain-adjusted odds ratio, 1.72; 95% confidence interval, 1.16-2.56; P = .007). Measurement of the longitudinal change in knee extensor and flexor strength between T0 and 2 years prior to T0 (T-2) also provided similar results (pain-adjusted OR, 4.30; 95% CI, 1.34-13.79; P = .014). The findings were independent of age, BMI, and radiographic disease severity, the researchers noted.
The investigators found no relationship between knee extensor or flexor muscle strength in men and subsequent need for knee replacement surgery. The relationship between thigh muscle strength and knee replacement for women did not extend to measurements made at T-2 or T-4 or the change in thigh muscle strength between T-2 and T-4.
The OAI receives funding from the National Institutes of Health, Merck Research Laboratories, Novartis, GlaxoSmithKline, and Pfizer. The work was also funded by a grant from the European Union Seventh Framework Programme. One author disclosed consulting or preparing educational sessions for pharmaceutical companies and for receiving research support. Two authors reported being employees of Chondrometrics GmbH, a company providing MR image analysis services to academic researchers and to industry.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: A window of opportunity exists for women with knee osteoarthritis and low thigh muscle strength to potentially lower their risk of knee replacement.
Major finding: Women who underwent knee replacement had significantly lower thigh muscle strength compared to matched controls who had not had knee surgery.
Data source: A multicenter longitudinal case control study involving 4,796 participants in the Osteoarthritis Initiative (OAI).
Disclosures: The OAI receives funding from the National Institutes of Health, Merck Research Laboratories, Novartis, GlaxoSmithKline, and Pfizer. The work was also funded by a grant from the European Union Seventh Framework Programme. One author disclosed consulting or preparing educational sessions for pharmaceutical companies and for receiving research support. Two authors reported being employees of Chondrometrics GmbH, a company providing MR image analysis services to academic researchers and to industry.