User login
Chutzpah
Chutzpah is a Yiddish word that entered American English, joining bagel and nosh. The usual translations of chutzpah – “nerve” or “effrontery” – are correct enough, but leave out the zest implied by chutzpah’s classic case: a man who kills his parents and throws himself on the mercy of the court because he is an orphan.
We all meet patients with chutzpah, which can be amusing, impressive – even breathtaking.
Take for instance the woman who paged me one evening last month. “I visit your nurse for cosmetic stuff,” she said when I called her back. “Your prices for laser hair removal were high, though, so I went to a spa where I could use a Groupon.”
How nice, I thought.
“Anyhow,” she continued, “I went for a treatment at the spa today, and now I have little red bumps all over my thighs. I thought it might be a reaction, and since you are my dermatologist I called to ask what to do.”
Good to be needed.
Then the next week I got another call, this time from a man I hadn’t seen in a long time. “I really like you as a dermatologist,” he began.
“Thank you,” I murmured.
“I saw this spot on my leg that worried me,” he said. “I was going to show it to you, but your office is in an old building, and old buildings don’t agree with me.”
As I scratched my head, he went on. “So I went to another dermatologist who works in a newer building. He did a biopsy and told me I have skin cancer. He said I should have surgery to take it off. I consider you my dermatologist, though, so I called to ask whether you think surgery is a good idea.”
I said I thought it was. I did not add that he should look for an old surgeon in a new building.
These patients are fresh in my mind, but it doesn’t take much effort to come up with others.
“Mr. Skillman wants a refill on his steroid cream,” says my secretary.
“Sure,” I tell her. “E-scribe it over.”
“No,” she says. “He wants a hard copy mailed to him.”
“Does he have one of those mail order pharmacies that requires a written script?”
“No.”
“But it’s so much simpler to call it in or do it by computer. Why does he have to have a hard copy?”
“I don’t know. But he insists on having one.”
I could go on and on. So could you, I’m sure.
When confronted with chutzpah, you have two options: challenge the person showing it and refuse to go along with his demands, or just sigh, comply, and move on. In general, I go with option #2.
First of all, anyone pushy enough to act this way will not react well to being pushed back. (“What’s your problem? Are you too busy to write a prescription? Too stingy to mail it?”)
Second, and perhaps more to the point, many people who display chutzpah don’t know that’s what they are doing. The woman who went for laser at the Groupon spa really has no idea I’d think it odd for her to call me about a complication instead of the spa personnel who lasered her legs. On some level, she figures that they probably don’t know (look how cheap they are), and thinks I should be flattered to be asked. After all, I’m her dermatologist.
Some people with chutzpah are aggressive and difficult and don’t care if they’re being offensive. A lot more are just clueless. The fellow who bores the daylights out of everyone at dinner parties with long, pointless stories doesn’t know he’s being tedious. He just doesn’t pick up social cues.
Most patients, like most people, are polite and deferential. The rest, though, are more memorable.
My building is indeed old. One hundred years ago it was the swankiest apartment house around. Every flat had rooms for a butler, a maid, and a chauffeur for their Packard motorcar. Then the builder went belly-up during the Depression, and the new owner converted it to medical offices. Downward mobility works for me.
Faced with chutzpah, I shrug, smile, and get on with it. Enough people can still tolerate old buildings, and old dermatologists.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. Write to him at [email protected].
Chutzpah is a Yiddish word that entered American English, joining bagel and nosh. The usual translations of chutzpah – “nerve” or “effrontery” – are correct enough, but leave out the zest implied by chutzpah’s classic case: a man who kills his parents and throws himself on the mercy of the court because he is an orphan.
We all meet patients with chutzpah, which can be amusing, impressive – even breathtaking.
Take for instance the woman who paged me one evening last month. “I visit your nurse for cosmetic stuff,” she said when I called her back. “Your prices for laser hair removal were high, though, so I went to a spa where I could use a Groupon.”
How nice, I thought.
“Anyhow,” she continued, “I went for a treatment at the spa today, and now I have little red bumps all over my thighs. I thought it might be a reaction, and since you are my dermatologist I called to ask what to do.”
Good to be needed.
Then the next week I got another call, this time from a man I hadn’t seen in a long time. “I really like you as a dermatologist,” he began.
“Thank you,” I murmured.
“I saw this spot on my leg that worried me,” he said. “I was going to show it to you, but your office is in an old building, and old buildings don’t agree with me.”
As I scratched my head, he went on. “So I went to another dermatologist who works in a newer building. He did a biopsy and told me I have skin cancer. He said I should have surgery to take it off. I consider you my dermatologist, though, so I called to ask whether you think surgery is a good idea.”
I said I thought it was. I did not add that he should look for an old surgeon in a new building.
These patients are fresh in my mind, but it doesn’t take much effort to come up with others.
“Mr. Skillman wants a refill on his steroid cream,” says my secretary.
“Sure,” I tell her. “E-scribe it over.”
“No,” she says. “He wants a hard copy mailed to him.”
“Does he have one of those mail order pharmacies that requires a written script?”
“No.”
“But it’s so much simpler to call it in or do it by computer. Why does he have to have a hard copy?”
“I don’t know. But he insists on having one.”
I could go on and on. So could you, I’m sure.
When confronted with chutzpah, you have two options: challenge the person showing it and refuse to go along with his demands, or just sigh, comply, and move on. In general, I go with option #2.
First of all, anyone pushy enough to act this way will not react well to being pushed back. (“What’s your problem? Are you too busy to write a prescription? Too stingy to mail it?”)
Second, and perhaps more to the point, many people who display chutzpah don’t know that’s what they are doing. The woman who went for laser at the Groupon spa really has no idea I’d think it odd for her to call me about a complication instead of the spa personnel who lasered her legs. On some level, she figures that they probably don’t know (look how cheap they are), and thinks I should be flattered to be asked. After all, I’m her dermatologist.
Some people with chutzpah are aggressive and difficult and don’t care if they’re being offensive. A lot more are just clueless. The fellow who bores the daylights out of everyone at dinner parties with long, pointless stories doesn’t know he’s being tedious. He just doesn’t pick up social cues.
Most patients, like most people, are polite and deferential. The rest, though, are more memorable.
My building is indeed old. One hundred years ago it was the swankiest apartment house around. Every flat had rooms for a butler, a maid, and a chauffeur for their Packard motorcar. Then the builder went belly-up during the Depression, and the new owner converted it to medical offices. Downward mobility works for me.
Faced with chutzpah, I shrug, smile, and get on with it. Enough people can still tolerate old buildings, and old dermatologists.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. Write to him at [email protected].
Chutzpah is a Yiddish word that entered American English, joining bagel and nosh. The usual translations of chutzpah – “nerve” or “effrontery” – are correct enough, but leave out the zest implied by chutzpah’s classic case: a man who kills his parents and throws himself on the mercy of the court because he is an orphan.
We all meet patients with chutzpah, which can be amusing, impressive – even breathtaking.
Take for instance the woman who paged me one evening last month. “I visit your nurse for cosmetic stuff,” she said when I called her back. “Your prices for laser hair removal were high, though, so I went to a spa where I could use a Groupon.”
How nice, I thought.
“Anyhow,” she continued, “I went for a treatment at the spa today, and now I have little red bumps all over my thighs. I thought it might be a reaction, and since you are my dermatologist I called to ask what to do.”
Good to be needed.
Then the next week I got another call, this time from a man I hadn’t seen in a long time. “I really like you as a dermatologist,” he began.
“Thank you,” I murmured.
“I saw this spot on my leg that worried me,” he said. “I was going to show it to you, but your office is in an old building, and old buildings don’t agree with me.”
As I scratched my head, he went on. “So I went to another dermatologist who works in a newer building. He did a biopsy and told me I have skin cancer. He said I should have surgery to take it off. I consider you my dermatologist, though, so I called to ask whether you think surgery is a good idea.”
I said I thought it was. I did not add that he should look for an old surgeon in a new building.
These patients are fresh in my mind, but it doesn’t take much effort to come up with others.
“Mr. Skillman wants a refill on his steroid cream,” says my secretary.
“Sure,” I tell her. “E-scribe it over.”
“No,” she says. “He wants a hard copy mailed to him.”
“Does he have one of those mail order pharmacies that requires a written script?”
“No.”
“But it’s so much simpler to call it in or do it by computer. Why does he have to have a hard copy?”
“I don’t know. But he insists on having one.”
I could go on and on. So could you, I’m sure.
When confronted with chutzpah, you have two options: challenge the person showing it and refuse to go along with his demands, or just sigh, comply, and move on. In general, I go with option #2.
First of all, anyone pushy enough to act this way will not react well to being pushed back. (“What’s your problem? Are you too busy to write a prescription? Too stingy to mail it?”)
Second, and perhaps more to the point, many people who display chutzpah don’t know that’s what they are doing. The woman who went for laser at the Groupon spa really has no idea I’d think it odd for her to call me about a complication instead of the spa personnel who lasered her legs. On some level, she figures that they probably don’t know (look how cheap they are), and thinks I should be flattered to be asked. After all, I’m her dermatologist.
Some people with chutzpah are aggressive and difficult and don’t care if they’re being offensive. A lot more are just clueless. The fellow who bores the daylights out of everyone at dinner parties with long, pointless stories doesn’t know he’s being tedious. He just doesn’t pick up social cues.
Most patients, like most people, are polite and deferential. The rest, though, are more memorable.
My building is indeed old. One hundred years ago it was the swankiest apartment house around. Every flat had rooms for a butler, a maid, and a chauffeur for their Packard motorcar. Then the builder went belly-up during the Depression, and the new owner converted it to medical offices. Downward mobility works for me.
Faced with chutzpah, I shrug, smile, and get on with it. Enough people can still tolerate old buildings, and old dermatologists.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. Write to him at [email protected].
Female Athletes: Unique Challenges Facing Women Warriors
Since Title IX passed in 1972, women have become exponentially more involved in competitive sports, from high school to professional levels. With more women engaging in serious athletics, the specific challenges they face have come to the forefront of sports medicine. These problems include the female athlete triad, concussions, exercise safety in pregnancy, anterior cruciate ligament (ACL) injuries, and continued sex discrimination and social injustice. Orthopedists treating female athletes should be aware of these problems, each of which is discussed in this review.
1. Female athlete triad
In 1992, the term female athlete triad was coined to describe 3 problems that often coexist in high-intensity female athletes.1 Since then, the definition has evolved, but the problem has remained essentially the same. The modern definition incorporates menstrual abnormalities, low energy availability with or without disordered eating, and decreased bone mineral density (BMD).2
With intense exercise and weight loss comes a variety of menstrual disturbances.3 In affected athletes, the hypothalamus is underactivated, and changes in gonadotropin-releasing hormone and luteinizing hormone lead to decreased estrogen production. Research suggests abnormal menses result from having inadequate energy and insufficient caloric intake to support extensive exercise.1 This phenomenon can occur in athletes in any sport but is most prevalent in lean-body sports, such as swimming, gymnastics, and ballet. The incidence of abnormal menses is as high as 79% in ballet dancers but only 5% in the general population.3 Menstrual abnormalities indicate hormonal abnormalities that can interfere with growth and maturation in young athletes.
Although full-blown eating disorders are uncommon among female athletes, disordered eating patterns are often found among women in competitive sports. Disordered eating can involve a spectrum of inadequate caloric intake and purging behavior, such as vomiting or laxative abuse, and has been reported in up to 25% of collegiate female athletes.4 Physicians must recognize these conditions and initiate counseling and treatment when appropriate. Women with disordered eating are at risk for developing electrolyte imbalances, malnutrition syndromes, and osteopenia.
Although careful evaluation and counseling are important, physicians must note that, in most cases, athletics participation may also protect against disordered eating and body image difficulties. A study of 146 college-age women found better body satisfaction among athletes than among nonathletes.5 Lean-sport athletes (eg, swimmers, gymnasts) were at higher risk for disordered eating and body image problems than other athletes were. Similarly, other studies have found that a majority of athletes have healthy eating habits.4
For poorly nourished and hormonally imbalanced female athletes, decreased BMD poses substantial risk. One study found a significant difference in BMD between athletes with amenorrhea and athletes with normal menses.6 In a cohort of female Navy recruits, those with amenorrhea were at 91% higher risk for stress fractures; calcium and vitamin D supplementation reduced risk by 20%.7 Osteopenia may be a special problem for prepubescent athletes. Girls who engage in intense exercise and have delayed menarche may have a low estrogen state, predisposing them to low BMD.3 Osteopenia and osteoporosis are difficult to reverse and can put these athletes at risk for stress fractures the rest of their lives. If unrecognized, stress fractures can end an athlete’s career.
Recommendations for dual-energy X-ray absorptiometry (DXA) include testing female athletes who have a diagnosed eating disorder, body mass index under 17.5, history of delayed menarche, oligomenorrhea, 2 prior stress fractures, or prior abnormal DXA scan. Complete testing recommendations appear in the 2014 consensus statement on the female athlete triad and return to sport.2,8
Orthopedists performing physical examinations for sports participation can screen for the female athlete triad through thoughtful questioning about menstrual history, nutrition habits, and stress fracture symptoms. Best treatment for a diagnosed case of the triad is multidisciplinary care with strong social support. When abnormal menses are an issue, referral to a gynecologist or endocrinologist and consideration of estrogen replacement should be discussed. Some cases require a psychiatrist’s assistance in treating disordered eating. Athletic trainers, coaches, and parents should be involved over the treatment course.1 Orthopedists must counsel women with osteopenia and osteoporosis about decreasing exercise to a safe level, improving nutritional intake, and supplementing with calcium (1200-1500 mg/d) and vitamin D (600-800 IU/d).3,7
2. Concussions
Increasing awareness of males’ sport-related concussions, particularly of concussions that occur during National Football League practice and games, has made physicians and researchers more aware of the rate of concussion in female athletes. That rate has increased, and, according to some reports, the risk for sport-related injury is higher for female athletes.9 A study of high school athletes found that the rate of concussion in girl’s soccer was second only to that in football.10
Concussions are categorized as mild traumatic brain injuries, and manifestations of the diagnosis are divided into physical, emotional, cognitive, and observed symptoms. The spectrum of symptoms is wide, ranging from difficulty concentrating and thinking clearly to headaches and dizziness.11 Compared with male athletes who sustain a concussion, female athletes report more of these concussive symptoms and have worse visual memory scores.12
Efforts to change sports at the player level have been resisted. Helmets have been proposed for field hockey and lacrosse but have not passed stringent concussion testing. In soccer, which has a high rate of concussion, a reform to eliminate heading the ball has been considered. Resistance to these suggestions stems from the thought that changes could alter the traditions of the games. Some individuals have indicated that helmets may give players a false sense of security and thereby cause them to play more aggressively.
Orthopedic surgeons must be aware of concussion symptoms. Multiple concussions may have a cumulative effect on functional ability and emotional well-being and may lead to chronic traumatic encephalopathy.13 Concern about the long-term effects of concussion has led to the implementation of universal “return to play” laws. These laws vary by state but have 3 steps in common: Educate coaches, players, and athletes; remove athletes from play; and obtain health care professionals’ permission to return to play.14 These guidelines set up an action plan for treating an athlete who has sustained a concussion.
Encouraging results of educating coaches have been noted. Coaches who were given Centers for Disease Control and Prevention–sponsored material on preventing, recognizing, and responding to concussions were able to effectively address concussions; 6 months later, 63% were better able to appreciate the severity of concussions.15 Continued education of athletic communities should help bring this injury to the attention of those treating female athletes.
3. Exercise safety in pregnancy
Women in sports can continue their athletic regimens during pregnancy. It is important to address challenges to the pregnant woman and to the fetus when assessing the risks of exercise.
The physiologic changes that occur during pregnancy may affect how a pregnant athlete responds to stress. Plasma volume, red blood cell volume, and cardiac function and output all increase during normal pregnancy.3,16 Abnormal heart rate during pregnancy can adversely affect the fetus. During and after exercise, fetal bradycardia can occur. Therefore, recommendations should include not exceeding pre-pregnancy activity levels.3 Careful monitoring of exercise intensity is recommended by the American College of Obstetrics and Gynecology; the guideline is to maintain less than 70% of maximal heart rate.17,18
The negative effects of exercise on the pregnant athlete are limited, but it is important to educate patients and to consider preventive strategies. One physiologic change that occurs during pregnancy is ligamentous laxity, which is caused by the hormone relaxin.16 Ligamentous laxity has the potential to put pregnant athletes at risk for soft-tissue and bony injury during impact sports. However, the positive effects of exercise during pregnancy include improved appetite, sleep, and emotional health.19 Aerobic exercise during pregnancy may reverse insulin resistance as demonstrated in animal studies; though this outcome has not been demonstrated in human studies,20 women should be reassured that moderate exercise has overall beneficial effects.
Some research suggests that exercise may expose the fetus to hyperthermia, blood sugar changes, physical injury, and premature labor.16 Typically, fetal heat is dissipated from the mother. After intense exercise, maternal body temperature rises and leads to some degree of fetal hyperthermia.16 Animal model studies have suggested that hyperthermia may result in a slightly higher rate of congenital abnormalities. Pregnant women should keep their exercise routines to less than 60 minutes, should exercise in a thermally regulated environment, and should keep themselves hydrated to avoid fetal hyperthermia.18
Reduced blood flow, accompanied by a deficit of oxygen to the uterus and the developing fetus, is another concern for pregnant athletes. During exercise, when more blood is flowing to the muscles, less is flowing to the uterus.16 Furthermore, during the third trimester, women should avoid supine exercise, as venous outflow is poor with the body in that position.21
Elite athletes who continue training during pregnancy should be carefully counseled about adjusting their training regimens. Because of increased cardiac output and blood volume, the heart rate will be lower than usual, demanding an adjustment in interpretation. Blood cell counts do not increase as much as plasma volume does—often leading to relative anemia. For elite athletes, this means iron supplementation is crucial.22 Thermal regulation may be more difficult, as training regimens may demand prolonged exercise. Physicians should recommend adequate hydration for these athletes.18
Although continued exercise is generally safe for a pregnant athlete and her fetus, caution is required when there is increased risk for premature delivery, or other special conditions exist. Multiple gestation, placenta previa, history of early labor or premature births, and incompetent cervix all contraindicate aerobic exercise during pregnancy.18 With these exceptions in mind, physicians can safely counsel pregnant women to do moderate exercise 30 minutes every day.17,18 Other recommendations are listed at the American College of Obstetricians and Gynecologists website.23
4. Anterior cruciate ligament injuries
ACL injuries affect a staggering number of athletes. In the United States, approximately 100,000 people sustain these injuries annually.24 As they occur up to 8 times more often in women than in men, ACL injuries are a top concern for physicians treating female athletes.
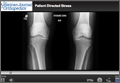
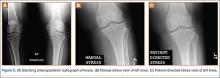
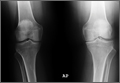
This disproportionate injury rate is influenced by differences between male and female anatomy. The width and shape of the femoral intercondylar notch have been studied as potential variables influencing the risk for ACL injury. Analysis of notch-view radiographs revealed a significant inverse relationship between notch width and ACL injury.25 A-shaped notches, notches with a significantly larger base and a narrowed roof, were more prevalent in women but did not correlate with increased risk for ACL injury. Studies have shown that female athletes with a noncontact ACL injury have a higher lateral tibial plateau posterior slope; this slope is associated with increased peak anteromedial ACL strain, which may contribute to injury.26 An analysis of magnetic resonance imaging scans in patients with and without ACL injury revealed that, for female patients, decreased femoral intercondylar notch width at the anterior outlet combined with increased lateral compartment posterior slope correlated best with risk for ACL injury.27
Although static anatomical factors contribute to ACL injuries in female athletes, dynamic neuromuscular influences are potential opportunities for intervention. Female athletes with high relative quadriceps strength and weak hamstring strength may be at increased risk for ACL injury.28 This “quadriceps dominance” becomes important in sports involving high-risk activities, such as running, cutting, pivoting, and jumping. In addition, compared with male athletes, female athletes demonstrate increased lateral trunk motion and knee valgus torque while landing during noncontact ACL tears, making core stability a factor in ACL injury.29
The collaborative efforts of physicians, physical therapists, athletic trainers, and coaches have yielded multifactorial neuromuscular training programs for the prevention of noncontact ACL injuries. Ideal ACL prevention protocols involve sessions that last for at least 10 minutes and take place 3 times a week. At these sessions, exercises are focused on strengthening, balance, and proprioceptive training.30 The programs last about 8 weeks, but sustained benefits require maintenance after the program has been completed and during the off-season. Program adherence must be encouraged and can be facilitated by varying workouts and raising risk awareness. The most effective programs have reduced the relative risk of noncontact ACL injuries by 75% to 100%.31 These promising results have led to increased focus on program implementation in an effort to prevent ACL injury.
5. Continued sex discrimination and social injustice
In 1972, Title IX was passed as part of the Education Amendments Act. Title IX states, “No person in the United States shall, on the basis of sex, be excluded from participation in, be denied the benefits of, or be subjected to discrimination under any educational program or activity receiving Federal financial assistance.” Passage of this law, which has implications outside of athletic participation, marked an important turning point in women’s ability to participate equally in college sports.32,33 The Civil Rights Restoration Act, passed in 1988, strengthened Title IX and made it applicable to all institutions receiving federal funding.34 Before the 1970s, women typically were restricted to club sports, and funding and participation opportunities were weighted heavily toward men. Over the past 40 years, women’s participation in high school, college, and professional sports has taken a huge leap forward.32 For example, the number of women participating in high school sports increased from 294,000 (7.4% of all athletes) in 1972 to 3.4 million (>41% of all athletes) in 2014.
Despite advances in women’s civil rights, examples of inequality in US schools remain, particularly in the distribution of funding, which still strongly favors men’s football.32 Men’s sports receive 90% of media coverage.33 In 2002, women represented 55% of college students but only 42% of varsity athletes.34 The schools that have complied the least with Title IX are schools in the Midwest and the South and those with football teams.34 Women are underrepresented as coaches, and funding continues to be disproportionately spent on men’s sports.
For women, the benefits of participating in sports are far-reaching and significant. These benefits include improvements in academic success, mental health, and responsible behavior.33 Women’s gaining acceptance and respect throughout the athletic world seems to have carried over elsewhere. Although many institutions remain noncompliant with Title IX, efforts continue to have a strongly positive effect on gender equality in the United States.
1. Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867-1882.
2. De Souza MJ, Nattiv A, Joy E, et al; Expert Panel. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad: 1st international conference held in San Francisco, California, May 2012 and 2nd international conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014;48(4):289.
3. Warren MP, Shantha S. The female athlete. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14(1):37-53.
4. Greenleaf C, Petrie TA, Carter J, Reel JJ. Female collegiate athletes: prevalence of eating disorders and disordered eating behaviors. J Am Coll Health. 2009;57(5):489-495.
5. Reinking MF, Alexander LE. Prevalence of disordered-eating behaviors in undergraduate female collegiate athletes and nonathletes. J Athl Train. 2005;40(1):47-51.
6. Rencken ML, Chesnut CH 3rd, Drinkwater BL. Bone density at multiple skeletal sites in amenorrheic athletes. JAMA. 1996;276(3):238-240.
7. Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female Navy recruits. J Bone Miner Res. 2008;23(5):741-749.
8. De Souza MJ. 2014 Female athlete triad consensus statement on guidelines for treatment and return to play. National Collegiate Athletic Association (NCAA) website. http://www.ncaa.org/health-and-safety/nutrition-and-performance/2014-female-athlete-triad-consensus-statement-guidelines. Accessed November 24, 2015.
9. Preiss-Farzanegan SJ, Chapman B, Wong TM, Wu J, Bazarian JJ. The relationship between gender and postconcussion symptoms after sport-related mild traumatic brain injury. PM R. 2009;1(3):245-253.
10. Marar M, McIlvain NM, Fields SK, Comstock RD. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40(4):747-755.
11. Uhl RL, Rosenbaum AJ, Czajka C, Mulligan M, King C. Minor traumatic brain injury: a primer for the orthopaedic surgeon. J Am Acad Orthop Surg. 2013;21(10):624-631.
12. Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312.
13. Covassin T, Moran R, Wilhelm K. Concussion symptoms and neurocognitive performance of high school and college athletes who incur multiple concussions. Am J Sports Med. 2013;41(12):2885-2889.
14. Sports concussion policies and laws: information for parents, coaches, and school & sports professionals. Centers for Disease Control and Prevention website. http://www.cdc.gov/headsup/policy/index.html. Updated February 16, 2015. Accessed November 24, 2015.
15. Covassin T, Elbin RJ, Sarmiento K. Educating coaches about concussion in sports: evaluation of the CDC’s “Heads Up: concussion in youth sports” initiative. J Sch Health. 2012;82(5):233-238.
16. Lumbers ER. Exercise in pregnancy: physiological basis of exercise prescription for the pregnant woman. J Sci Med Sport. 2002;5(1):20-31.
17. ACOG Committee Obstetric Practice. ACOG Committee opinion. Number 267, January 2002: exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002;99(1):171-173.
18. Artal R, O’Toole M. Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br J Sports Med. 2003;37(1):6-12.
19. Kramer MS. Regular aerobic exercise during pregnancy. Cochrane Database Syst Rev. 2000;(2):CD000180. Update in: Cochrane Database Syst Rev. 2002;(2):CD000180.
20. Stafne SN, Salvesen KA, Romundstad PR, Stuge B, Morkved S. Does regular exercise during pregnancy influence lumbopelvic pain? A randomized controlled trial. Acta Obstet Gynecol Scand. 2012;91(5):552-559.
21. Nascimento SL, Surita FG, Cecatti JG. Physical exercise during pregnancy: a systematic review. Curr Opin Obstet Gynecol. 2012;24(6):387-394.
22. Hale RW, Milne L. The elite athlete and exercise in pregnancy. Semin Perinatol. 1996;20(4):277-284.
23. Exercise during pregnancy. American College of Obstetricians and Gynecologists website. http://www.acog.org/Patients/FAQs/Exercise-During-Pregnancy. Published August 2011. Accessed November 24, 2015.
24. Giugliano DN, Solomon JL. ACL tears in female athletes. Phys Med Rehabil Clin North Am. 2007;18(3):417-438, viii.
25. Ireland ML, Ballantyne BT, Little K, McClay IS. A radiographic analysis of the relationship between the size and shape of the intercondylar notch and anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):200-205.
26. Lipps DB, Oh YK, Ashton-Miller JA, Wojtys EM. Morphologic characteristics help explain the gender difference in peak anterior cruciate ligament strain during a simulated pivot landing. Am J Sports Med. 2012;40(1):32-40.
27. Sturnick DR, Vacek PM, DeSarno MJ, et al. Combined anatomic factors predicting risk of anterior cruciate ligament injury for males and females. Am J Sports Med. 2015;43(4):839-847.
28. Myer GD, Ford KR, Barber Foss KD, Liu C, Nick TG, Hewett TE. The relationship of hamstrings and quadriceps strength to anterior cruciate ligament injury in female athletes. Clin J Sport Med. 2009;19(1):3-8.
29. Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43(6):417-422.
30. Sutton KM, Bullock JM. Anterior cruciate ligament rupture: differences between males and females. J Am Acad Orthop Surg. 2013;21(1):41-50.
31. Noyes FR, Barber-Westin SD. Neuromuscular retraining intervention programs: do they reduce noncontact anterior cruciate ligament injury rates in adolescent female athletes? Arthroscopy. 2014;30(2):245-255.
32. Ladd AL. The sports bra, the ACL, and Title IX—the game in play. Clin Orthop Relat Res. 2014;472(6):1681-1684.
33. Lopiano DA. Modern history of women in sports. Twenty-five years of Title IX. Clin Sports Med. 2000;19(2):163-173, vii.
34. Anderson DJ, Cheslock JJ, Ehrenberg RG. Gender equity in intercollegiate athletics: determinants of Title IX compliance. J High Educ. 2006;77(2):225-250.
Since Title IX passed in 1972, women have become exponentially more involved in competitive sports, from high school to professional levels. With more women engaging in serious athletics, the specific challenges they face have come to the forefront of sports medicine. These problems include the female athlete triad, concussions, exercise safety in pregnancy, anterior cruciate ligament (ACL) injuries, and continued sex discrimination and social injustice. Orthopedists treating female athletes should be aware of these problems, each of which is discussed in this review.
1. Female athlete triad
In 1992, the term female athlete triad was coined to describe 3 problems that often coexist in high-intensity female athletes.1 Since then, the definition has evolved, but the problem has remained essentially the same. The modern definition incorporates menstrual abnormalities, low energy availability with or without disordered eating, and decreased bone mineral density (BMD).2
With intense exercise and weight loss comes a variety of menstrual disturbances.3 In affected athletes, the hypothalamus is underactivated, and changes in gonadotropin-releasing hormone and luteinizing hormone lead to decreased estrogen production. Research suggests abnormal menses result from having inadequate energy and insufficient caloric intake to support extensive exercise.1 This phenomenon can occur in athletes in any sport but is most prevalent in lean-body sports, such as swimming, gymnastics, and ballet. The incidence of abnormal menses is as high as 79% in ballet dancers but only 5% in the general population.3 Menstrual abnormalities indicate hormonal abnormalities that can interfere with growth and maturation in young athletes.
Although full-blown eating disorders are uncommon among female athletes, disordered eating patterns are often found among women in competitive sports. Disordered eating can involve a spectrum of inadequate caloric intake and purging behavior, such as vomiting or laxative abuse, and has been reported in up to 25% of collegiate female athletes.4 Physicians must recognize these conditions and initiate counseling and treatment when appropriate. Women with disordered eating are at risk for developing electrolyte imbalances, malnutrition syndromes, and osteopenia.
Although careful evaluation and counseling are important, physicians must note that, in most cases, athletics participation may also protect against disordered eating and body image difficulties. A study of 146 college-age women found better body satisfaction among athletes than among nonathletes.5 Lean-sport athletes (eg, swimmers, gymnasts) were at higher risk for disordered eating and body image problems than other athletes were. Similarly, other studies have found that a majority of athletes have healthy eating habits.4
For poorly nourished and hormonally imbalanced female athletes, decreased BMD poses substantial risk. One study found a significant difference in BMD between athletes with amenorrhea and athletes with normal menses.6 In a cohort of female Navy recruits, those with amenorrhea were at 91% higher risk for stress fractures; calcium and vitamin D supplementation reduced risk by 20%.7 Osteopenia may be a special problem for prepubescent athletes. Girls who engage in intense exercise and have delayed menarche may have a low estrogen state, predisposing them to low BMD.3 Osteopenia and osteoporosis are difficult to reverse and can put these athletes at risk for stress fractures the rest of their lives. If unrecognized, stress fractures can end an athlete’s career.
Recommendations for dual-energy X-ray absorptiometry (DXA) include testing female athletes who have a diagnosed eating disorder, body mass index under 17.5, history of delayed menarche, oligomenorrhea, 2 prior stress fractures, or prior abnormal DXA scan. Complete testing recommendations appear in the 2014 consensus statement on the female athlete triad and return to sport.2,8
Orthopedists performing physical examinations for sports participation can screen for the female athlete triad through thoughtful questioning about menstrual history, nutrition habits, and stress fracture symptoms. Best treatment for a diagnosed case of the triad is multidisciplinary care with strong social support. When abnormal menses are an issue, referral to a gynecologist or endocrinologist and consideration of estrogen replacement should be discussed. Some cases require a psychiatrist’s assistance in treating disordered eating. Athletic trainers, coaches, and parents should be involved over the treatment course.1 Orthopedists must counsel women with osteopenia and osteoporosis about decreasing exercise to a safe level, improving nutritional intake, and supplementing with calcium (1200-1500 mg/d) and vitamin D (600-800 IU/d).3,7
2. Concussions
Increasing awareness of males’ sport-related concussions, particularly of concussions that occur during National Football League practice and games, has made physicians and researchers more aware of the rate of concussion in female athletes. That rate has increased, and, according to some reports, the risk for sport-related injury is higher for female athletes.9 A study of high school athletes found that the rate of concussion in girl’s soccer was second only to that in football.10
Concussions are categorized as mild traumatic brain injuries, and manifestations of the diagnosis are divided into physical, emotional, cognitive, and observed symptoms. The spectrum of symptoms is wide, ranging from difficulty concentrating and thinking clearly to headaches and dizziness.11 Compared with male athletes who sustain a concussion, female athletes report more of these concussive symptoms and have worse visual memory scores.12
Efforts to change sports at the player level have been resisted. Helmets have been proposed for field hockey and lacrosse but have not passed stringent concussion testing. In soccer, which has a high rate of concussion, a reform to eliminate heading the ball has been considered. Resistance to these suggestions stems from the thought that changes could alter the traditions of the games. Some individuals have indicated that helmets may give players a false sense of security and thereby cause them to play more aggressively.
Orthopedic surgeons must be aware of concussion symptoms. Multiple concussions may have a cumulative effect on functional ability and emotional well-being and may lead to chronic traumatic encephalopathy.13 Concern about the long-term effects of concussion has led to the implementation of universal “return to play” laws. These laws vary by state but have 3 steps in common: Educate coaches, players, and athletes; remove athletes from play; and obtain health care professionals’ permission to return to play.14 These guidelines set up an action plan for treating an athlete who has sustained a concussion.
Encouraging results of educating coaches have been noted. Coaches who were given Centers for Disease Control and Prevention–sponsored material on preventing, recognizing, and responding to concussions were able to effectively address concussions; 6 months later, 63% were better able to appreciate the severity of concussions.15 Continued education of athletic communities should help bring this injury to the attention of those treating female athletes.
3. Exercise safety in pregnancy
Women in sports can continue their athletic regimens during pregnancy. It is important to address challenges to the pregnant woman and to the fetus when assessing the risks of exercise.
The physiologic changes that occur during pregnancy may affect how a pregnant athlete responds to stress. Plasma volume, red blood cell volume, and cardiac function and output all increase during normal pregnancy.3,16 Abnormal heart rate during pregnancy can adversely affect the fetus. During and after exercise, fetal bradycardia can occur. Therefore, recommendations should include not exceeding pre-pregnancy activity levels.3 Careful monitoring of exercise intensity is recommended by the American College of Obstetrics and Gynecology; the guideline is to maintain less than 70% of maximal heart rate.17,18
The negative effects of exercise on the pregnant athlete are limited, but it is important to educate patients and to consider preventive strategies. One physiologic change that occurs during pregnancy is ligamentous laxity, which is caused by the hormone relaxin.16 Ligamentous laxity has the potential to put pregnant athletes at risk for soft-tissue and bony injury during impact sports. However, the positive effects of exercise during pregnancy include improved appetite, sleep, and emotional health.19 Aerobic exercise during pregnancy may reverse insulin resistance as demonstrated in animal studies; though this outcome has not been demonstrated in human studies,20 women should be reassured that moderate exercise has overall beneficial effects.
Some research suggests that exercise may expose the fetus to hyperthermia, blood sugar changes, physical injury, and premature labor.16 Typically, fetal heat is dissipated from the mother. After intense exercise, maternal body temperature rises and leads to some degree of fetal hyperthermia.16 Animal model studies have suggested that hyperthermia may result in a slightly higher rate of congenital abnormalities. Pregnant women should keep their exercise routines to less than 60 minutes, should exercise in a thermally regulated environment, and should keep themselves hydrated to avoid fetal hyperthermia.18
Reduced blood flow, accompanied by a deficit of oxygen to the uterus and the developing fetus, is another concern for pregnant athletes. During exercise, when more blood is flowing to the muscles, less is flowing to the uterus.16 Furthermore, during the third trimester, women should avoid supine exercise, as venous outflow is poor with the body in that position.21
Elite athletes who continue training during pregnancy should be carefully counseled about adjusting their training regimens. Because of increased cardiac output and blood volume, the heart rate will be lower than usual, demanding an adjustment in interpretation. Blood cell counts do not increase as much as plasma volume does—often leading to relative anemia. For elite athletes, this means iron supplementation is crucial.22 Thermal regulation may be more difficult, as training regimens may demand prolonged exercise. Physicians should recommend adequate hydration for these athletes.18
Although continued exercise is generally safe for a pregnant athlete and her fetus, caution is required when there is increased risk for premature delivery, or other special conditions exist. Multiple gestation, placenta previa, history of early labor or premature births, and incompetent cervix all contraindicate aerobic exercise during pregnancy.18 With these exceptions in mind, physicians can safely counsel pregnant women to do moderate exercise 30 minutes every day.17,18 Other recommendations are listed at the American College of Obstetricians and Gynecologists website.23
4. Anterior cruciate ligament injuries
ACL injuries affect a staggering number of athletes. In the United States, approximately 100,000 people sustain these injuries annually.24 As they occur up to 8 times more often in women than in men, ACL injuries are a top concern for physicians treating female athletes.
This disproportionate injury rate is influenced by differences between male and female anatomy. The width and shape of the femoral intercondylar notch have been studied as potential variables influencing the risk for ACL injury. Analysis of notch-view radiographs revealed a significant inverse relationship between notch width and ACL injury.25 A-shaped notches, notches with a significantly larger base and a narrowed roof, were more prevalent in women but did not correlate with increased risk for ACL injury. Studies have shown that female athletes with a noncontact ACL injury have a higher lateral tibial plateau posterior slope; this slope is associated with increased peak anteromedial ACL strain, which may contribute to injury.26 An analysis of magnetic resonance imaging scans in patients with and without ACL injury revealed that, for female patients, decreased femoral intercondylar notch width at the anterior outlet combined with increased lateral compartment posterior slope correlated best with risk for ACL injury.27
Although static anatomical factors contribute to ACL injuries in female athletes, dynamic neuromuscular influences are potential opportunities for intervention. Female athletes with high relative quadriceps strength and weak hamstring strength may be at increased risk for ACL injury.28 This “quadriceps dominance” becomes important in sports involving high-risk activities, such as running, cutting, pivoting, and jumping. In addition, compared with male athletes, female athletes demonstrate increased lateral trunk motion and knee valgus torque while landing during noncontact ACL tears, making core stability a factor in ACL injury.29
The collaborative efforts of physicians, physical therapists, athletic trainers, and coaches have yielded multifactorial neuromuscular training programs for the prevention of noncontact ACL injuries. Ideal ACL prevention protocols involve sessions that last for at least 10 minutes and take place 3 times a week. At these sessions, exercises are focused on strengthening, balance, and proprioceptive training.30 The programs last about 8 weeks, but sustained benefits require maintenance after the program has been completed and during the off-season. Program adherence must be encouraged and can be facilitated by varying workouts and raising risk awareness. The most effective programs have reduced the relative risk of noncontact ACL injuries by 75% to 100%.31 These promising results have led to increased focus on program implementation in an effort to prevent ACL injury.
5. Continued sex discrimination and social injustice
In 1972, Title IX was passed as part of the Education Amendments Act. Title IX states, “No person in the United States shall, on the basis of sex, be excluded from participation in, be denied the benefits of, or be subjected to discrimination under any educational program or activity receiving Federal financial assistance.” Passage of this law, which has implications outside of athletic participation, marked an important turning point in women’s ability to participate equally in college sports.32,33 The Civil Rights Restoration Act, passed in 1988, strengthened Title IX and made it applicable to all institutions receiving federal funding.34 Before the 1970s, women typically were restricted to club sports, and funding and participation opportunities were weighted heavily toward men. Over the past 40 years, women’s participation in high school, college, and professional sports has taken a huge leap forward.32 For example, the number of women participating in high school sports increased from 294,000 (7.4% of all athletes) in 1972 to 3.4 million (>41% of all athletes) in 2014.
Despite advances in women’s civil rights, examples of inequality in US schools remain, particularly in the distribution of funding, which still strongly favors men’s football.32 Men’s sports receive 90% of media coverage.33 In 2002, women represented 55% of college students but only 42% of varsity athletes.34 The schools that have complied the least with Title IX are schools in the Midwest and the South and those with football teams.34 Women are underrepresented as coaches, and funding continues to be disproportionately spent on men’s sports.
For women, the benefits of participating in sports are far-reaching and significant. These benefits include improvements in academic success, mental health, and responsible behavior.33 Women’s gaining acceptance and respect throughout the athletic world seems to have carried over elsewhere. Although many institutions remain noncompliant with Title IX, efforts continue to have a strongly positive effect on gender equality in the United States.
Since Title IX passed in 1972, women have become exponentially more involved in competitive sports, from high school to professional levels. With more women engaging in serious athletics, the specific challenges they face have come to the forefront of sports medicine. These problems include the female athlete triad, concussions, exercise safety in pregnancy, anterior cruciate ligament (ACL) injuries, and continued sex discrimination and social injustice. Orthopedists treating female athletes should be aware of these problems, each of which is discussed in this review.
1. Female athlete triad
In 1992, the term female athlete triad was coined to describe 3 problems that often coexist in high-intensity female athletes.1 Since then, the definition has evolved, but the problem has remained essentially the same. The modern definition incorporates menstrual abnormalities, low energy availability with or without disordered eating, and decreased bone mineral density (BMD).2
With intense exercise and weight loss comes a variety of menstrual disturbances.3 In affected athletes, the hypothalamus is underactivated, and changes in gonadotropin-releasing hormone and luteinizing hormone lead to decreased estrogen production. Research suggests abnormal menses result from having inadequate energy and insufficient caloric intake to support extensive exercise.1 This phenomenon can occur in athletes in any sport but is most prevalent in lean-body sports, such as swimming, gymnastics, and ballet. The incidence of abnormal menses is as high as 79% in ballet dancers but only 5% in the general population.3 Menstrual abnormalities indicate hormonal abnormalities that can interfere with growth and maturation in young athletes.
Although full-blown eating disorders are uncommon among female athletes, disordered eating patterns are often found among women in competitive sports. Disordered eating can involve a spectrum of inadequate caloric intake and purging behavior, such as vomiting or laxative abuse, and has been reported in up to 25% of collegiate female athletes.4 Physicians must recognize these conditions and initiate counseling and treatment when appropriate. Women with disordered eating are at risk for developing electrolyte imbalances, malnutrition syndromes, and osteopenia.
Although careful evaluation and counseling are important, physicians must note that, in most cases, athletics participation may also protect against disordered eating and body image difficulties. A study of 146 college-age women found better body satisfaction among athletes than among nonathletes.5 Lean-sport athletes (eg, swimmers, gymnasts) were at higher risk for disordered eating and body image problems than other athletes were. Similarly, other studies have found that a majority of athletes have healthy eating habits.4
For poorly nourished and hormonally imbalanced female athletes, decreased BMD poses substantial risk. One study found a significant difference in BMD between athletes with amenorrhea and athletes with normal menses.6 In a cohort of female Navy recruits, those with amenorrhea were at 91% higher risk for stress fractures; calcium and vitamin D supplementation reduced risk by 20%.7 Osteopenia may be a special problem for prepubescent athletes. Girls who engage in intense exercise and have delayed menarche may have a low estrogen state, predisposing them to low BMD.3 Osteopenia and osteoporosis are difficult to reverse and can put these athletes at risk for stress fractures the rest of their lives. If unrecognized, stress fractures can end an athlete’s career.
Recommendations for dual-energy X-ray absorptiometry (DXA) include testing female athletes who have a diagnosed eating disorder, body mass index under 17.5, history of delayed menarche, oligomenorrhea, 2 prior stress fractures, or prior abnormal DXA scan. Complete testing recommendations appear in the 2014 consensus statement on the female athlete triad and return to sport.2,8
Orthopedists performing physical examinations for sports participation can screen for the female athlete triad through thoughtful questioning about menstrual history, nutrition habits, and stress fracture symptoms. Best treatment for a diagnosed case of the triad is multidisciplinary care with strong social support. When abnormal menses are an issue, referral to a gynecologist or endocrinologist and consideration of estrogen replacement should be discussed. Some cases require a psychiatrist’s assistance in treating disordered eating. Athletic trainers, coaches, and parents should be involved over the treatment course.1 Orthopedists must counsel women with osteopenia and osteoporosis about decreasing exercise to a safe level, improving nutritional intake, and supplementing with calcium (1200-1500 mg/d) and vitamin D (600-800 IU/d).3,7
2. Concussions
Increasing awareness of males’ sport-related concussions, particularly of concussions that occur during National Football League practice and games, has made physicians and researchers more aware of the rate of concussion in female athletes. That rate has increased, and, according to some reports, the risk for sport-related injury is higher for female athletes.9 A study of high school athletes found that the rate of concussion in girl’s soccer was second only to that in football.10
Concussions are categorized as mild traumatic brain injuries, and manifestations of the diagnosis are divided into physical, emotional, cognitive, and observed symptoms. The spectrum of symptoms is wide, ranging from difficulty concentrating and thinking clearly to headaches and dizziness.11 Compared with male athletes who sustain a concussion, female athletes report more of these concussive symptoms and have worse visual memory scores.12
Efforts to change sports at the player level have been resisted. Helmets have been proposed for field hockey and lacrosse but have not passed stringent concussion testing. In soccer, which has a high rate of concussion, a reform to eliminate heading the ball has been considered. Resistance to these suggestions stems from the thought that changes could alter the traditions of the games. Some individuals have indicated that helmets may give players a false sense of security and thereby cause them to play more aggressively.
Orthopedic surgeons must be aware of concussion symptoms. Multiple concussions may have a cumulative effect on functional ability and emotional well-being and may lead to chronic traumatic encephalopathy.13 Concern about the long-term effects of concussion has led to the implementation of universal “return to play” laws. These laws vary by state but have 3 steps in common: Educate coaches, players, and athletes; remove athletes from play; and obtain health care professionals’ permission to return to play.14 These guidelines set up an action plan for treating an athlete who has sustained a concussion.
Encouraging results of educating coaches have been noted. Coaches who were given Centers for Disease Control and Prevention–sponsored material on preventing, recognizing, and responding to concussions were able to effectively address concussions; 6 months later, 63% were better able to appreciate the severity of concussions.15 Continued education of athletic communities should help bring this injury to the attention of those treating female athletes.
3. Exercise safety in pregnancy
Women in sports can continue their athletic regimens during pregnancy. It is important to address challenges to the pregnant woman and to the fetus when assessing the risks of exercise.
The physiologic changes that occur during pregnancy may affect how a pregnant athlete responds to stress. Plasma volume, red blood cell volume, and cardiac function and output all increase during normal pregnancy.3,16 Abnormal heart rate during pregnancy can adversely affect the fetus. During and after exercise, fetal bradycardia can occur. Therefore, recommendations should include not exceeding pre-pregnancy activity levels.3 Careful monitoring of exercise intensity is recommended by the American College of Obstetrics and Gynecology; the guideline is to maintain less than 70% of maximal heart rate.17,18
The negative effects of exercise on the pregnant athlete are limited, but it is important to educate patients and to consider preventive strategies. One physiologic change that occurs during pregnancy is ligamentous laxity, which is caused by the hormone relaxin.16 Ligamentous laxity has the potential to put pregnant athletes at risk for soft-tissue and bony injury during impact sports. However, the positive effects of exercise during pregnancy include improved appetite, sleep, and emotional health.19 Aerobic exercise during pregnancy may reverse insulin resistance as demonstrated in animal studies; though this outcome has not been demonstrated in human studies,20 women should be reassured that moderate exercise has overall beneficial effects.
Some research suggests that exercise may expose the fetus to hyperthermia, blood sugar changes, physical injury, and premature labor.16 Typically, fetal heat is dissipated from the mother. After intense exercise, maternal body temperature rises and leads to some degree of fetal hyperthermia.16 Animal model studies have suggested that hyperthermia may result in a slightly higher rate of congenital abnormalities. Pregnant women should keep their exercise routines to less than 60 minutes, should exercise in a thermally regulated environment, and should keep themselves hydrated to avoid fetal hyperthermia.18
Reduced blood flow, accompanied by a deficit of oxygen to the uterus and the developing fetus, is another concern for pregnant athletes. During exercise, when more blood is flowing to the muscles, less is flowing to the uterus.16 Furthermore, during the third trimester, women should avoid supine exercise, as venous outflow is poor with the body in that position.21
Elite athletes who continue training during pregnancy should be carefully counseled about adjusting their training regimens. Because of increased cardiac output and blood volume, the heart rate will be lower than usual, demanding an adjustment in interpretation. Blood cell counts do not increase as much as plasma volume does—often leading to relative anemia. For elite athletes, this means iron supplementation is crucial.22 Thermal regulation may be more difficult, as training regimens may demand prolonged exercise. Physicians should recommend adequate hydration for these athletes.18
Although continued exercise is generally safe for a pregnant athlete and her fetus, caution is required when there is increased risk for premature delivery, or other special conditions exist. Multiple gestation, placenta previa, history of early labor or premature births, and incompetent cervix all contraindicate aerobic exercise during pregnancy.18 With these exceptions in mind, physicians can safely counsel pregnant women to do moderate exercise 30 minutes every day.17,18 Other recommendations are listed at the American College of Obstetricians and Gynecologists website.23
4. Anterior cruciate ligament injuries
ACL injuries affect a staggering number of athletes. In the United States, approximately 100,000 people sustain these injuries annually.24 As they occur up to 8 times more often in women than in men, ACL injuries are a top concern for physicians treating female athletes.
This disproportionate injury rate is influenced by differences between male and female anatomy. The width and shape of the femoral intercondylar notch have been studied as potential variables influencing the risk for ACL injury. Analysis of notch-view radiographs revealed a significant inverse relationship between notch width and ACL injury.25 A-shaped notches, notches with a significantly larger base and a narrowed roof, were more prevalent in women but did not correlate with increased risk for ACL injury. Studies have shown that female athletes with a noncontact ACL injury have a higher lateral tibial plateau posterior slope; this slope is associated with increased peak anteromedial ACL strain, which may contribute to injury.26 An analysis of magnetic resonance imaging scans in patients with and without ACL injury revealed that, for female patients, decreased femoral intercondylar notch width at the anterior outlet combined with increased lateral compartment posterior slope correlated best with risk for ACL injury.27
Although static anatomical factors contribute to ACL injuries in female athletes, dynamic neuromuscular influences are potential opportunities for intervention. Female athletes with high relative quadriceps strength and weak hamstring strength may be at increased risk for ACL injury.28 This “quadriceps dominance” becomes important in sports involving high-risk activities, such as running, cutting, pivoting, and jumping. In addition, compared with male athletes, female athletes demonstrate increased lateral trunk motion and knee valgus torque while landing during noncontact ACL tears, making core stability a factor in ACL injury.29
The collaborative efforts of physicians, physical therapists, athletic trainers, and coaches have yielded multifactorial neuromuscular training programs for the prevention of noncontact ACL injuries. Ideal ACL prevention protocols involve sessions that last for at least 10 minutes and take place 3 times a week. At these sessions, exercises are focused on strengthening, balance, and proprioceptive training.30 The programs last about 8 weeks, but sustained benefits require maintenance after the program has been completed and during the off-season. Program adherence must be encouraged and can be facilitated by varying workouts and raising risk awareness. The most effective programs have reduced the relative risk of noncontact ACL injuries by 75% to 100%.31 These promising results have led to increased focus on program implementation in an effort to prevent ACL injury.
5. Continued sex discrimination and social injustice
In 1972, Title IX was passed as part of the Education Amendments Act. Title IX states, “No person in the United States shall, on the basis of sex, be excluded from participation in, be denied the benefits of, or be subjected to discrimination under any educational program or activity receiving Federal financial assistance.” Passage of this law, which has implications outside of athletic participation, marked an important turning point in women’s ability to participate equally in college sports.32,33 The Civil Rights Restoration Act, passed in 1988, strengthened Title IX and made it applicable to all institutions receiving federal funding.34 Before the 1970s, women typically were restricted to club sports, and funding and participation opportunities were weighted heavily toward men. Over the past 40 years, women’s participation in high school, college, and professional sports has taken a huge leap forward.32 For example, the number of women participating in high school sports increased from 294,000 (7.4% of all athletes) in 1972 to 3.4 million (>41% of all athletes) in 2014.
Despite advances in women’s civil rights, examples of inequality in US schools remain, particularly in the distribution of funding, which still strongly favors men’s football.32 Men’s sports receive 90% of media coverage.33 In 2002, women represented 55% of college students but only 42% of varsity athletes.34 The schools that have complied the least with Title IX are schools in the Midwest and the South and those with football teams.34 Women are underrepresented as coaches, and funding continues to be disproportionately spent on men’s sports.
For women, the benefits of participating in sports are far-reaching and significant. These benefits include improvements in academic success, mental health, and responsible behavior.33 Women’s gaining acceptance and respect throughout the athletic world seems to have carried over elsewhere. Although many institutions remain noncompliant with Title IX, efforts continue to have a strongly positive effect on gender equality in the United States.
1. Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867-1882.
2. De Souza MJ, Nattiv A, Joy E, et al; Expert Panel. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad: 1st international conference held in San Francisco, California, May 2012 and 2nd international conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014;48(4):289.
3. Warren MP, Shantha S. The female athlete. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14(1):37-53.
4. Greenleaf C, Petrie TA, Carter J, Reel JJ. Female collegiate athletes: prevalence of eating disorders and disordered eating behaviors. J Am Coll Health. 2009;57(5):489-495.
5. Reinking MF, Alexander LE. Prevalence of disordered-eating behaviors in undergraduate female collegiate athletes and nonathletes. J Athl Train. 2005;40(1):47-51.
6. Rencken ML, Chesnut CH 3rd, Drinkwater BL. Bone density at multiple skeletal sites in amenorrheic athletes. JAMA. 1996;276(3):238-240.
7. Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female Navy recruits. J Bone Miner Res. 2008;23(5):741-749.
8. De Souza MJ. 2014 Female athlete triad consensus statement on guidelines for treatment and return to play. National Collegiate Athletic Association (NCAA) website. http://www.ncaa.org/health-and-safety/nutrition-and-performance/2014-female-athlete-triad-consensus-statement-guidelines. Accessed November 24, 2015.
9. Preiss-Farzanegan SJ, Chapman B, Wong TM, Wu J, Bazarian JJ. The relationship between gender and postconcussion symptoms after sport-related mild traumatic brain injury. PM R. 2009;1(3):245-253.
10. Marar M, McIlvain NM, Fields SK, Comstock RD. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40(4):747-755.
11. Uhl RL, Rosenbaum AJ, Czajka C, Mulligan M, King C. Minor traumatic brain injury: a primer for the orthopaedic surgeon. J Am Acad Orthop Surg. 2013;21(10):624-631.
12. Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312.
13. Covassin T, Moran R, Wilhelm K. Concussion symptoms and neurocognitive performance of high school and college athletes who incur multiple concussions. Am J Sports Med. 2013;41(12):2885-2889.
14. Sports concussion policies and laws: information for parents, coaches, and school & sports professionals. Centers for Disease Control and Prevention website. http://www.cdc.gov/headsup/policy/index.html. Updated February 16, 2015. Accessed November 24, 2015.
15. Covassin T, Elbin RJ, Sarmiento K. Educating coaches about concussion in sports: evaluation of the CDC’s “Heads Up: concussion in youth sports” initiative. J Sch Health. 2012;82(5):233-238.
16. Lumbers ER. Exercise in pregnancy: physiological basis of exercise prescription for the pregnant woman. J Sci Med Sport. 2002;5(1):20-31.
17. ACOG Committee Obstetric Practice. ACOG Committee opinion. Number 267, January 2002: exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002;99(1):171-173.
18. Artal R, O’Toole M. Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br J Sports Med. 2003;37(1):6-12.
19. Kramer MS. Regular aerobic exercise during pregnancy. Cochrane Database Syst Rev. 2000;(2):CD000180. Update in: Cochrane Database Syst Rev. 2002;(2):CD000180.
20. Stafne SN, Salvesen KA, Romundstad PR, Stuge B, Morkved S. Does regular exercise during pregnancy influence lumbopelvic pain? A randomized controlled trial. Acta Obstet Gynecol Scand. 2012;91(5):552-559.
21. Nascimento SL, Surita FG, Cecatti JG. Physical exercise during pregnancy: a systematic review. Curr Opin Obstet Gynecol. 2012;24(6):387-394.
22. Hale RW, Milne L. The elite athlete and exercise in pregnancy. Semin Perinatol. 1996;20(4):277-284.
23. Exercise during pregnancy. American College of Obstetricians and Gynecologists website. http://www.acog.org/Patients/FAQs/Exercise-During-Pregnancy. Published August 2011. Accessed November 24, 2015.
24. Giugliano DN, Solomon JL. ACL tears in female athletes. Phys Med Rehabil Clin North Am. 2007;18(3):417-438, viii.
25. Ireland ML, Ballantyne BT, Little K, McClay IS. A radiographic analysis of the relationship between the size and shape of the intercondylar notch and anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):200-205.
26. Lipps DB, Oh YK, Ashton-Miller JA, Wojtys EM. Morphologic characteristics help explain the gender difference in peak anterior cruciate ligament strain during a simulated pivot landing. Am J Sports Med. 2012;40(1):32-40.
27. Sturnick DR, Vacek PM, DeSarno MJ, et al. Combined anatomic factors predicting risk of anterior cruciate ligament injury for males and females. Am J Sports Med. 2015;43(4):839-847.
28. Myer GD, Ford KR, Barber Foss KD, Liu C, Nick TG, Hewett TE. The relationship of hamstrings and quadriceps strength to anterior cruciate ligament injury in female athletes. Clin J Sport Med. 2009;19(1):3-8.
29. Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43(6):417-422.
30. Sutton KM, Bullock JM. Anterior cruciate ligament rupture: differences between males and females. J Am Acad Orthop Surg. 2013;21(1):41-50.
31. Noyes FR, Barber-Westin SD. Neuromuscular retraining intervention programs: do they reduce noncontact anterior cruciate ligament injury rates in adolescent female athletes? Arthroscopy. 2014;30(2):245-255.
32. Ladd AL. The sports bra, the ACL, and Title IX—the game in play. Clin Orthop Relat Res. 2014;472(6):1681-1684.
33. Lopiano DA. Modern history of women in sports. Twenty-five years of Title IX. Clin Sports Med. 2000;19(2):163-173, vii.
34. Anderson DJ, Cheslock JJ, Ehrenberg RG. Gender equity in intercollegiate athletics: determinants of Title IX compliance. J High Educ. 2006;77(2):225-250.
1. Nattiv A, Loucks AB, Manore MM, Sanborn CF, Sundgot-Borgen J, Warren MP; American College of Sports Medicine. American College of Sports Medicine position stand. The female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867-1882.
2. De Souza MJ, Nattiv A, Joy E, et al; Expert Panel. 2014 Female Athlete Triad Coalition consensus statement on treatment and return to play of the female athlete triad: 1st international conference held in San Francisco, California, May 2012 and 2nd international conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014;48(4):289.
3. Warren MP, Shantha S. The female athlete. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14(1):37-53.
4. Greenleaf C, Petrie TA, Carter J, Reel JJ. Female collegiate athletes: prevalence of eating disorders and disordered eating behaviors. J Am Coll Health. 2009;57(5):489-495.
5. Reinking MF, Alexander LE. Prevalence of disordered-eating behaviors in undergraduate female collegiate athletes and nonathletes. J Athl Train. 2005;40(1):47-51.
6. Rencken ML, Chesnut CH 3rd, Drinkwater BL. Bone density at multiple skeletal sites in amenorrheic athletes. JAMA. 1996;276(3):238-240.
7. Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female Navy recruits. J Bone Miner Res. 2008;23(5):741-749.
8. De Souza MJ. 2014 Female athlete triad consensus statement on guidelines for treatment and return to play. National Collegiate Athletic Association (NCAA) website. http://www.ncaa.org/health-and-safety/nutrition-and-performance/2014-female-athlete-triad-consensus-statement-guidelines. Accessed November 24, 2015.
9. Preiss-Farzanegan SJ, Chapman B, Wong TM, Wu J, Bazarian JJ. The relationship between gender and postconcussion symptoms after sport-related mild traumatic brain injury. PM R. 2009;1(3):245-253.
10. Marar M, McIlvain NM, Fields SK, Comstock RD. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40(4):747-755.
11. Uhl RL, Rosenbaum AJ, Czajka C, Mulligan M, King C. Minor traumatic brain injury: a primer for the orthopaedic surgeon. J Am Acad Orthop Surg. 2013;21(10):624-631.
12. Covassin T, Elbin RJ, Harris W, Parker T, Kontos A. The role of age and sex in symptoms, neurocognitive performance, and postural stability in athletes after concussion. Am J Sports Med. 2012;40(6):1303-1312.
13. Covassin T, Moran R, Wilhelm K. Concussion symptoms and neurocognitive performance of high school and college athletes who incur multiple concussions. Am J Sports Med. 2013;41(12):2885-2889.
14. Sports concussion policies and laws: information for parents, coaches, and school & sports professionals. Centers for Disease Control and Prevention website. http://www.cdc.gov/headsup/policy/index.html. Updated February 16, 2015. Accessed November 24, 2015.
15. Covassin T, Elbin RJ, Sarmiento K. Educating coaches about concussion in sports: evaluation of the CDC’s “Heads Up: concussion in youth sports” initiative. J Sch Health. 2012;82(5):233-238.
16. Lumbers ER. Exercise in pregnancy: physiological basis of exercise prescription for the pregnant woman. J Sci Med Sport. 2002;5(1):20-31.
17. ACOG Committee Obstetric Practice. ACOG Committee opinion. Number 267, January 2002: exercise during pregnancy and the postpartum period. Obstet Gynecol. 2002;99(1):171-173.
18. Artal R, O’Toole M. Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br J Sports Med. 2003;37(1):6-12.
19. Kramer MS. Regular aerobic exercise during pregnancy. Cochrane Database Syst Rev. 2000;(2):CD000180. Update in: Cochrane Database Syst Rev. 2002;(2):CD000180.
20. Stafne SN, Salvesen KA, Romundstad PR, Stuge B, Morkved S. Does regular exercise during pregnancy influence lumbopelvic pain? A randomized controlled trial. Acta Obstet Gynecol Scand. 2012;91(5):552-559.
21. Nascimento SL, Surita FG, Cecatti JG. Physical exercise during pregnancy: a systematic review. Curr Opin Obstet Gynecol. 2012;24(6):387-394.
22. Hale RW, Milne L. The elite athlete and exercise in pregnancy. Semin Perinatol. 1996;20(4):277-284.
23. Exercise during pregnancy. American College of Obstetricians and Gynecologists website. http://www.acog.org/Patients/FAQs/Exercise-During-Pregnancy. Published August 2011. Accessed November 24, 2015.
24. Giugliano DN, Solomon JL. ACL tears in female athletes. Phys Med Rehabil Clin North Am. 2007;18(3):417-438, viii.
25. Ireland ML, Ballantyne BT, Little K, McClay IS. A radiographic analysis of the relationship between the size and shape of the intercondylar notch and anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):200-205.
26. Lipps DB, Oh YK, Ashton-Miller JA, Wojtys EM. Morphologic characteristics help explain the gender difference in peak anterior cruciate ligament strain during a simulated pivot landing. Am J Sports Med. 2012;40(1):32-40.
27. Sturnick DR, Vacek PM, DeSarno MJ, et al. Combined anatomic factors predicting risk of anterior cruciate ligament injury for males and females. Am J Sports Med. 2015;43(4):839-847.
28. Myer GD, Ford KR, Barber Foss KD, Liu C, Nick TG, Hewett TE. The relationship of hamstrings and quadriceps strength to anterior cruciate ligament injury in female athletes. Clin J Sport Med. 2009;19(1):3-8.
29. Hewett TE, Torg JS, Boden BP. Video analysis of trunk and knee motion during non-contact anterior cruciate ligament injury in female athletes: lateral trunk and knee abduction motion are combined components of the injury mechanism. Br J Sports Med. 2009;43(6):417-422.
30. Sutton KM, Bullock JM. Anterior cruciate ligament rupture: differences between males and females. J Am Acad Orthop Surg. 2013;21(1):41-50.
31. Noyes FR, Barber-Westin SD. Neuromuscular retraining intervention programs: do they reduce noncontact anterior cruciate ligament injury rates in adolescent female athletes? Arthroscopy. 2014;30(2):245-255.
32. Ladd AL. The sports bra, the ACL, and Title IX—the game in play. Clin Orthop Relat Res. 2014;472(6):1681-1684.
33. Lopiano DA. Modern history of women in sports. Twenty-five years of Title IX. Clin Sports Med. 2000;19(2):163-173, vii.
34. Anderson DJ, Cheslock JJ, Ehrenberg RG. Gender equity in intercollegiate athletics: determinants of Title IX compliance. J High Educ. 2006;77(2):225-250.
Patient-Directed Valgus Stress Radiograph of the Knee: A New and Novel Technique
A new and novel technique for obtaining the patient-directed valgus stress radiograph of the knee.
To read the authors' full article click here.

A new and novel technique for obtaining the patient-directed valgus stress radiograph of the knee.
To read the authors' full article click here.

A new and novel technique for obtaining the patient-directed valgus stress radiograph of the knee.
To read the authors' full article click here.

Patient-Directed Valgus Stress Radiograph of the Knee: A New and Novel Technique
Medial-compartment partial knee arthroplasty (unicompartmental replacement) is an accepted surgical intervention for anteromedial osteoarthritis of the knee.1 The radiographic investigations required in the workup of these patients should include weight-bearing standing anteroposterior (AP), lateral, and sunrise (Merchant) views, as well as a valgus stress AP radiograph to assess the functionality of the lateral compartment. The method of properly obtaining the valgus stress film has been well described by the Oxford Group.2 Its recommended radiographic technique requires that a surgeon or a radiologic technologist perform the valgus stress maneuver, manually, while another technologist shoots the film. The 2 consequences of this technique are that it requires 2 individuals to obtain the film, and it subjects the individual who is applying the stress to some level of radiation exposure, which is undesirable. Because of this and the time inconvenience, many surgeons omit the valgus stress radiograph, which can lead to the adverse outcome of missing a lateral compartment that is functionally incompetent, resulting in the potential for early lateral compartment progression of osteoarthritis and the need for revision surgery, usually to a total knee arthroplasty.
In an attempt to mitigate these barriers to obtaining the necessary valgus stress radiograph, Dr. Mauerhan’s team developed a technique that could be done with the assistance of the patient and would require only 1 technologist to perform. Additionally, this project was a quality improvement initiative, because it lowered radiation exposure to all personnel involved in obtaining the correct films.
Materials and Methods
We initiated the project using weight-bearing strategies to impart the valgus stress view of the knee. After trying several different wedges and blocks, and varying patient instructions, we realized a different approach to this problem would be required to find an acceptable solution. We redirected our efforts to effectively performing the stress view with the patient in a supine position on the radiograph table. Ultimately, we decided that a much stiffer wedge and a denser object to squeeze would facilitate obtaining a proper film. Considering all available options, a youth size 4 soccer ball (diameter, 11 in) was introduced along with a slightly larger positioning wedge. The soccer ball was wrapped with 4-in Coban wrap (3M) to create a nonslip surface. This change in patient positioning, along with a standardized 7º to 10º cephalic radiographic tube angulation, helped to correct issues with tibial plateau visualization. Once these changes were enacted, we obtained fairly consistent positive results, and we instituted this patient-directed valgus stress view of the knee, along with a manual valgus stress view for comparison.
The protocol for obtaining the patient-directed valgus stress view of the knee is as follows: The patient lays supine with a dense 45º spine-positioning wedge (Burlington Medical Supplies) placed under both knees and the patient’s heels on the examining table. The radiographic tube is angled cephalad 7º to 10º centered on the inferior pole of the patella, using a 40-in source to image-receptor distance, collimated to part; the image receptor is placed under the affected knee, below the positioning wedge. The affected knee is rotated to the “true” AP position (the patella will be centered between the femoral condyles on the AP exposure), and the ball is placed between the patient’s legs just above the ankle joint. The technologist demonstrates to the patient how to squeeze the ball while maintaining contact of heels with the table. The technologist can exit the room and obtain the exposure, which is taken while the patient is squeezing the ball, as shown in Figures 1A and 1B. Examples of the standing AP, manual stress, and patient-directed valgus radiographs are shown in Figures 2A-2C. The entire technique is demonstrated in the Video.

Results
During the 9 months of this quality improvement project, 78 examinations were performed. Five studies did not show complete correction of the varus deformity. Of these, 3 showed complete correction on a manual valgus stress radiograph, and 2 did not, contraindicating the use of partial knee replacement. Three patients displayed collapse of the lateral compartment, indicating a nonfunctional lateral compartment, and, therefore, were also a contraindication to partial knee arthroplasty. The remaining 70 patients had identical radiographic results with both the manual and patient-directed valgus stress tests. There was no instance of examination failure or need to repeat as a result of difficulty of the examination for the patient. Repeat films because of positioning errors were very rare, usually early in the learning curve, and no more prevalent than when using the manual stress method. The technique was reproducible and easy to teach and adopt.
Discussion
In total, 73 patients (93.5%) with the patient-directed stress film showed the desired result, either correction of the medial compartment narrowing in conjunction with an intact lateral compartment or narrowing of the lateral compartment. Of the 5 patients (6.5%) whose patient-directed stress films did not show correction of the varus deformity, 3 patients displayed correction with a manually applied stress radiograph and 2 did not. Based on this observation, our recommendation would be for those patients who do not show adequate correction on the patient-directed stress radiograph to have a manual examination to establish the presence or absence of the desired correction.
Performing a valgus stress radiograph is an integral part of the investigation to determine if the patient is an appropriate candidate for partial knee arthroplasty.3 The historical, manually performed valgus stress radiograph requires 2 individuals, 1 to apply the stress with the patient on the table and 1 to shoot the exposure. For the individual or individuals applying this stress, there is an increased radiation exposure that would be undesirable over a long career. The authors developed a new technique using a commercially available spinal positioning wedge and 11-in youth soccer ball wrapped with Coban wrap, as described, which is economical and easy to obtain and use in the clinical setting. We believe this cost-effective method will offer surgeons who perform partial knee arthroplasty a novel method to obtain the important information gleaned from the valgus stress radiograph and to improve surgical outcomes through the preoperative assessment of the lateral compartment. Additionally, as a quality and safety improvement initiative, we believe this technique will reduce radiographic exposure for those performing these studies, and, because the examination can be carried out by a single technologist, it will significantly improve efficiency in the radiology suite.
Conclusion
We have developed a new method of obtaining the important valgus stress radiograph as part of the workup of patients with medial-compartment osteoarthritis of the knee. The technique can be performed with easily obtainable, commercially available products and is reliable 93.5% of the time. It also adds to the efficiency of the radiology suite and reduces radiographic exposure for technologists.
1. White SH, Ludkowski PF, Goodfellow JW. Anteromedial osteoarthritis of the knee. J Bone Joint Surg Br. 1991;73(4):582-586.
2. Goodfellow JW, O’Conner JJ, Dodd CA, Murray DW. Unicompartmental Arthroplasty with the Oxford Knee. Woodeaton, Oxford, England: Goodfellow Publishers Limited; 2006:38-39.
3. Gibson PH, Goodfellow JW. Stress radiography in degenerative arthritis of the knee. J Bone Joint Surg Br. 1986;68(4):608-609.
Medial-compartment partial knee arthroplasty (unicompartmental replacement) is an accepted surgical intervention for anteromedial osteoarthritis of the knee.1 The radiographic investigations required in the workup of these patients should include weight-bearing standing anteroposterior (AP), lateral, and sunrise (Merchant) views, as well as a valgus stress AP radiograph to assess the functionality of the lateral compartment. The method of properly obtaining the valgus stress film has been well described by the Oxford Group.2 Its recommended radiographic technique requires that a surgeon or a radiologic technologist perform the valgus stress maneuver, manually, while another technologist shoots the film. The 2 consequences of this technique are that it requires 2 individuals to obtain the film, and it subjects the individual who is applying the stress to some level of radiation exposure, which is undesirable. Because of this and the time inconvenience, many surgeons omit the valgus stress radiograph, which can lead to the adverse outcome of missing a lateral compartment that is functionally incompetent, resulting in the potential for early lateral compartment progression of osteoarthritis and the need for revision surgery, usually to a total knee arthroplasty.
In an attempt to mitigate these barriers to obtaining the necessary valgus stress radiograph, Dr. Mauerhan’s team developed a technique that could be done with the assistance of the patient and would require only 1 technologist to perform. Additionally, this project was a quality improvement initiative, because it lowered radiation exposure to all personnel involved in obtaining the correct films.
Materials and Methods
We initiated the project using weight-bearing strategies to impart the valgus stress view of the knee. After trying several different wedges and blocks, and varying patient instructions, we realized a different approach to this problem would be required to find an acceptable solution. We redirected our efforts to effectively performing the stress view with the patient in a supine position on the radiograph table. Ultimately, we decided that a much stiffer wedge and a denser object to squeeze would facilitate obtaining a proper film. Considering all available options, a youth size 4 soccer ball (diameter, 11 in) was introduced along with a slightly larger positioning wedge. The soccer ball was wrapped with 4-in Coban wrap (3M) to create a nonslip surface. This change in patient positioning, along with a standardized 7º to 10º cephalic radiographic tube angulation, helped to correct issues with tibial plateau visualization. Once these changes were enacted, we obtained fairly consistent positive results, and we instituted this patient-directed valgus stress view of the knee, along with a manual valgus stress view for comparison.
The protocol for obtaining the patient-directed valgus stress view of the knee is as follows: The patient lays supine with a dense 45º spine-positioning wedge (Burlington Medical Supplies) placed under both knees and the patient’s heels on the examining table. The radiographic tube is angled cephalad 7º to 10º centered on the inferior pole of the patella, using a 40-in source to image-receptor distance, collimated to part; the image receptor is placed under the affected knee, below the positioning wedge. The affected knee is rotated to the “true” AP position (the patella will be centered between the femoral condyles on the AP exposure), and the ball is placed between the patient’s legs just above the ankle joint. The technologist demonstrates to the patient how to squeeze the ball while maintaining contact of heels with the table. The technologist can exit the room and obtain the exposure, which is taken while the patient is squeezing the ball, as shown in Figures 1A and 1B. Examples of the standing AP, manual stress, and patient-directed valgus radiographs are shown in Figures 2A-2C. The entire technique is demonstrated in the Video.

Results
During the 9 months of this quality improvement project, 78 examinations were performed. Five studies did not show complete correction of the varus deformity. Of these, 3 showed complete correction on a manual valgus stress radiograph, and 2 did not, contraindicating the use of partial knee replacement. Three patients displayed collapse of the lateral compartment, indicating a nonfunctional lateral compartment, and, therefore, were also a contraindication to partial knee arthroplasty. The remaining 70 patients had identical radiographic results with both the manual and patient-directed valgus stress tests. There was no instance of examination failure or need to repeat as a result of difficulty of the examination for the patient. Repeat films because of positioning errors were very rare, usually early in the learning curve, and no more prevalent than when using the manual stress method. The technique was reproducible and easy to teach and adopt.
Discussion
In total, 73 patients (93.5%) with the patient-directed stress film showed the desired result, either correction of the medial compartment narrowing in conjunction with an intact lateral compartment or narrowing of the lateral compartment. Of the 5 patients (6.5%) whose patient-directed stress films did not show correction of the varus deformity, 3 patients displayed correction with a manually applied stress radiograph and 2 did not. Based on this observation, our recommendation would be for those patients who do not show adequate correction on the patient-directed stress radiograph to have a manual examination to establish the presence or absence of the desired correction.
Performing a valgus stress radiograph is an integral part of the investigation to determine if the patient is an appropriate candidate for partial knee arthroplasty.3 The historical, manually performed valgus stress radiograph requires 2 individuals, 1 to apply the stress with the patient on the table and 1 to shoot the exposure. For the individual or individuals applying this stress, there is an increased radiation exposure that would be undesirable over a long career. The authors developed a new technique using a commercially available spinal positioning wedge and 11-in youth soccer ball wrapped with Coban wrap, as described, which is economical and easy to obtain and use in the clinical setting. We believe this cost-effective method will offer surgeons who perform partial knee arthroplasty a novel method to obtain the important information gleaned from the valgus stress radiograph and to improve surgical outcomes through the preoperative assessment of the lateral compartment. Additionally, as a quality and safety improvement initiative, we believe this technique will reduce radiographic exposure for those performing these studies, and, because the examination can be carried out by a single technologist, it will significantly improve efficiency in the radiology suite.
Conclusion
We have developed a new method of obtaining the important valgus stress radiograph as part of the workup of patients with medial-compartment osteoarthritis of the knee. The technique can be performed with easily obtainable, commercially available products and is reliable 93.5% of the time. It also adds to the efficiency of the radiology suite and reduces radiographic exposure for technologists.
Medial-compartment partial knee arthroplasty (unicompartmental replacement) is an accepted surgical intervention for anteromedial osteoarthritis of the knee.1 The radiographic investigations required in the workup of these patients should include weight-bearing standing anteroposterior (AP), lateral, and sunrise (Merchant) views, as well as a valgus stress AP radiograph to assess the functionality of the lateral compartment. The method of properly obtaining the valgus stress film has been well described by the Oxford Group.2 Its recommended radiographic technique requires that a surgeon or a radiologic technologist perform the valgus stress maneuver, manually, while another technologist shoots the film. The 2 consequences of this technique are that it requires 2 individuals to obtain the film, and it subjects the individual who is applying the stress to some level of radiation exposure, which is undesirable. Because of this and the time inconvenience, many surgeons omit the valgus stress radiograph, which can lead to the adverse outcome of missing a lateral compartment that is functionally incompetent, resulting in the potential for early lateral compartment progression of osteoarthritis and the need for revision surgery, usually to a total knee arthroplasty.
In an attempt to mitigate these barriers to obtaining the necessary valgus stress radiograph, Dr. Mauerhan’s team developed a technique that could be done with the assistance of the patient and would require only 1 technologist to perform. Additionally, this project was a quality improvement initiative, because it lowered radiation exposure to all personnel involved in obtaining the correct films.
Materials and Methods
We initiated the project using weight-bearing strategies to impart the valgus stress view of the knee. After trying several different wedges and blocks, and varying patient instructions, we realized a different approach to this problem would be required to find an acceptable solution. We redirected our efforts to effectively performing the stress view with the patient in a supine position on the radiograph table. Ultimately, we decided that a much stiffer wedge and a denser object to squeeze would facilitate obtaining a proper film. Considering all available options, a youth size 4 soccer ball (diameter, 11 in) was introduced along with a slightly larger positioning wedge. The soccer ball was wrapped with 4-in Coban wrap (3M) to create a nonslip surface. This change in patient positioning, along with a standardized 7º to 10º cephalic radiographic tube angulation, helped to correct issues with tibial plateau visualization. Once these changes were enacted, we obtained fairly consistent positive results, and we instituted this patient-directed valgus stress view of the knee, along with a manual valgus stress view for comparison.
The protocol for obtaining the patient-directed valgus stress view of the knee is as follows: The patient lays supine with a dense 45º spine-positioning wedge (Burlington Medical Supplies) placed under both knees and the patient’s heels on the examining table. The radiographic tube is angled cephalad 7º to 10º centered on the inferior pole of the patella, using a 40-in source to image-receptor distance, collimated to part; the image receptor is placed under the affected knee, below the positioning wedge. The affected knee is rotated to the “true” AP position (the patella will be centered between the femoral condyles on the AP exposure), and the ball is placed between the patient’s legs just above the ankle joint. The technologist demonstrates to the patient how to squeeze the ball while maintaining contact of heels with the table. The technologist can exit the room and obtain the exposure, which is taken while the patient is squeezing the ball, as shown in Figures 1A and 1B. Examples of the standing AP, manual stress, and patient-directed valgus radiographs are shown in Figures 2A-2C. The entire technique is demonstrated in the Video.

Results
During the 9 months of this quality improvement project, 78 examinations were performed. Five studies did not show complete correction of the varus deformity. Of these, 3 showed complete correction on a manual valgus stress radiograph, and 2 did not, contraindicating the use of partial knee replacement. Three patients displayed collapse of the lateral compartment, indicating a nonfunctional lateral compartment, and, therefore, were also a contraindication to partial knee arthroplasty. The remaining 70 patients had identical radiographic results with both the manual and patient-directed valgus stress tests. There was no instance of examination failure or need to repeat as a result of difficulty of the examination for the patient. Repeat films because of positioning errors were very rare, usually early in the learning curve, and no more prevalent than when using the manual stress method. The technique was reproducible and easy to teach and adopt.
Discussion
In total, 73 patients (93.5%) with the patient-directed stress film showed the desired result, either correction of the medial compartment narrowing in conjunction with an intact lateral compartment or narrowing of the lateral compartment. Of the 5 patients (6.5%) whose patient-directed stress films did not show correction of the varus deformity, 3 patients displayed correction with a manually applied stress radiograph and 2 did not. Based on this observation, our recommendation would be for those patients who do not show adequate correction on the patient-directed stress radiograph to have a manual examination to establish the presence or absence of the desired correction.
Performing a valgus stress radiograph is an integral part of the investigation to determine if the patient is an appropriate candidate for partial knee arthroplasty.3 The historical, manually performed valgus stress radiograph requires 2 individuals, 1 to apply the stress with the patient on the table and 1 to shoot the exposure. For the individual or individuals applying this stress, there is an increased radiation exposure that would be undesirable over a long career. The authors developed a new technique using a commercially available spinal positioning wedge and 11-in youth soccer ball wrapped with Coban wrap, as described, which is economical and easy to obtain and use in the clinical setting. We believe this cost-effective method will offer surgeons who perform partial knee arthroplasty a novel method to obtain the important information gleaned from the valgus stress radiograph and to improve surgical outcomes through the preoperative assessment of the lateral compartment. Additionally, as a quality and safety improvement initiative, we believe this technique will reduce radiographic exposure for those performing these studies, and, because the examination can be carried out by a single technologist, it will significantly improve efficiency in the radiology suite.
Conclusion
We have developed a new method of obtaining the important valgus stress radiograph as part of the workup of patients with medial-compartment osteoarthritis of the knee. The technique can be performed with easily obtainable, commercially available products and is reliable 93.5% of the time. It also adds to the efficiency of the radiology suite and reduces radiographic exposure for technologists.
1. White SH, Ludkowski PF, Goodfellow JW. Anteromedial osteoarthritis of the knee. J Bone Joint Surg Br. 1991;73(4):582-586.
2. Goodfellow JW, O’Conner JJ, Dodd CA, Murray DW. Unicompartmental Arthroplasty with the Oxford Knee. Woodeaton, Oxford, England: Goodfellow Publishers Limited; 2006:38-39.
3. Gibson PH, Goodfellow JW. Stress radiography in degenerative arthritis of the knee. J Bone Joint Surg Br. 1986;68(4):608-609.
1. White SH, Ludkowski PF, Goodfellow JW. Anteromedial osteoarthritis of the knee. J Bone Joint Surg Br. 1991;73(4):582-586.
2. Goodfellow JW, O’Conner JJ, Dodd CA, Murray DW. Unicompartmental Arthroplasty with the Oxford Knee. Woodeaton, Oxford, England: Goodfellow Publishers Limited; 2006:38-39.
3. Gibson PH, Goodfellow JW. Stress radiography in degenerative arthritis of the knee. J Bone Joint Surg Br. 1986;68(4):608-609.
What psychiatrists must know to make the mandated transition to ICD-10
Just as psychiatrists are adapting to DSM-5, they have to cope with implementation of the 10th edition of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). This challenge raises questions: What is the importance of understanding ICD-10? How will it affect the practice of psychiatry?
Furthermore, how does ICD-10 relate to DSM-5 and Current Procedural Terminology (CPT)? How does it differ from ICD-9? What are the ICD-10-Clinical Modification (CM) and ICD-10-Procedures (PCS)?Learning the essence of the changes, and understanding what impact they have on your clinical work, are necessary to ensure that your practice keeps pace with professional and legal standards of care. The effort involved is not onerous, however, and can improve the quality and efficiency of your care and how you document it.
In this article, we provide you with an overview of ICD-10; highlight major changes of the new classification; explain its relevance to clinical practice; and offer guidelines for implementing it effectively. We also emphasize that a good understanding of DSM-5 facilitates appreciation of ICD-10 and makes its implementation fairly easy and straightforward.
To begin, we provide a glossary of ICD-related terms and a review of additional definitions, distinctions, and dates (Box).1-6
Major changes from ICD-9
No question: ICD-10 is going to significantly influence your practice and your reimbursement. Furthermore, a number of revisions in ICD-10 have the potential to meaningfully improve clinical documentation and communication and to enhance your ability to precisely describe the complexity of your patients—with implications for billing.
ICD-10 differs from ICD-9 in organization, structure, code composition, and level of detail. In addition, ICD-10 makes some changes in terminology and definitions, with the goal of improving precision.
ICD-10 also is much larger than ICD-9.The total number of medical diagnostic codes has increased more than 5-fold—from approximately 13,000 to 69,000. This expansion allows for greater specificity in diagnosis and enables differentiation of an initial clinical encounter from a subsequent encounter.
To accommodate the expansion in the number of codes, the 5-digit numeric codes used in ICD-9 have been replaced in ICD-10 by 7-digit alphanumeric codes:
- the first digit always is a letter
- the second and third digits are numbers followed by a decimal point
- the fourth though seventh digits can be letters or numbers
- the first 3 digits denote the diagnostic category
- the fourth through sixth digits provide diagnostic detail
- the seventh digit provides information about the nature of the encounter (eg, initial, subsequent, or sequel, denoted respectively by “A,” “D,” and “S” in the seventh digit).
The number of 3-digit categories for psychiatric disorders has increased from 30 in ICD-9 (290-319) to 100 in ICD-10 (F00-F99). Only the first 5 digits are used for the section on mental disorders in ICD-10, with the first digit always “F” and the second digit a number denoting the broad type of disorders. The second and third digits in conjunction define the major category of the disorder; the fourth and fifth digits provide additional descriptive detail about the disorder (Table).
ICD-9 ‘V’ codes are out
What were called “V” codes in ICD-9—factors that influence health status and contact with health services—have been replaced by “Z” codes in ICD-10. These “Z” codes provide greater detail and precision than “V” codes provided.
Examples of “Z” codes relevant to psychiatry are:
Z00 General psychiatric examination (eg, of a person who does not have a complaint or diagnosis)
Z03 Examination for suspected mental and behavioral disorder
Z04 Examination for medicolegal or other purposes; Z04.8 is relevant laboratory testing, such as drug testing of urine or blood
Z50 Care involving rehabilitation (substance use disorder, etc.)
Z60 Problem related to social environment
Z61 Problem related to negative life events in childhood
Z63 Problem related to primary support group, including family circumstances
Z64-Z65 Problem related to other psychosocial circumstances
Z70-Z71 Condition requiring counseling, not elsewhere classified
Z73 Problem related to difficulty with life management (burnout, stress, role conflict, etc.)
Z75 Problem related to medical facilities and other aspects of health care (eg, awaiting admission)
Z81 Family history of mental or behavioral disorders
Z85-Z91 Personal history of various disorders (must be absent or in full remission at the moment); Z86.51, for example, refers to a history of combat and operational stress reaction.
Greater precision is now possible when coding for treatment-related adverse effects. A particular adverse effect now is coded under the relevant system, along with its attribution to the specific substance. Obesity attributable to antipsychotic treatment,7,8 for example, is coded as E66.1.
Integrating DSM-5 and ICD-10
Because DSM-5 lists corresponding ICD-10-CM codes for all disorders, you will find it much easier than other physicians to implement ICD-10. DSM-5 includes ICD-9-CM and ICD-10-CM codes for each DSM-5 disorder (for example, the ICD-9-CM code for schizophrenia is 295.x; the ICD-10-CM code is F20.9).9
Furthermore, a number of changes from ICD-9-CM to ICD-10-CM enable documentation of greater diagnostic specificity; for example, DSM-5 schizoaffective disorder, bipolar type, and schizoaffective disorder, depressive type, are distinctly coded as F25.0 and F25.1, respectively, in ICD-10-CM, whereas both were coded as 295.7 in ICD-9-CM.10
You will continue to use DSM-5 criteria to guide your diagnostic process, translating the DSM-5 diagnosis (diagnoses) into corresponding ICD-10-CM codes. Experience with DSM-5 substantially simplifies the transition to ICD-10.
Key differences between DSM-5 and ICD-10
There are notable differences in organization and content between DSM-5 and ICD-10.
The 20 chapters in DSM-5 begin with neurodevelopmental disorders; neurocognitive disorders are toward the end (ie, childhood to late life). In contrast, neurocognitive disorders (ie, “dementia”) appear at the beginning of ICD-10; neurodevelopmental disorders are at the end.
Elimination of schizophrenia subtypes in DSM-5 necessitates coding of all schizophrenia as F20.9 in ICD-10-CM because F20.0-F20.8 are specific subtypes. DSM-5 schizophreniform disorder is coded F20.81.
Substance abuse and substance dependence continue to be separate in ICD-10-CM, but they are combined in a single category of substance use disorders in DSM-5. The correct ICD-10-CM code (ie, abuse vs dependence) is determined by the severity of the substance use disorder: “Mild” coding as abuse (F1x.1) and “moderate” and “severe” coding as dependence (F2x.2), with x denoting the substance abused.
There can be multiple applicable diagnoses associated with a clinical encounter, as there was with ICD-9-CM. Give precedence to the diagnosis that best represents the nature of the presenting problem; list other diagnoses in the order of their relevance. DSM-5 and ICD-10-CM are similar in this regard.
ICD-10-CM uses only subtypes, in contrast to the use of subtypes and specifiers in DSM-5 to describe variability in disorders across patients. It is possible, however, to code certain DSM-5 specifiers in ICD-10-CM. (This is discussed in the “Recording Procedures” section of the DSM-5 text and summarized at the beginning of the manual, and appears in the “Appendix.”) To code the catatonia specifier in the context of schizoaffective disorder, depressive type, for example, use ICD-10-CM code F25.1 for the disorder and add code F06.1 for the catatonia specifier.11
How will ICD-10 affect your practice?
As of October 1, 2015, all health care facilities were to have become ICD-10 compliant. Furthermore, any Health Insurance Portability and Accountability Act-covered entity must use ICD-10-CM codes if it expects to be reimbursed for health care services.
Mental health practitioners might think that the transition from ICD-9-CM to ICD-10-CM involves only billers and coders, not them. They are wrong. All clinicians are responsible for documenting their diagnostic and treatment services properly. Medical records must contain adequate information to support any diagnostic (ICD-10-CM) and treatment (CPT) codes that are applied to a given clinical encounter.
The greater detail and specificity that are provided by ICD-10-CM allow more accurate recording of clinical complexity, which, in turn, influences reimbursement. However, good documentation is necessary for proper coding. Because clinicians are ultimately responsible for proper diagnostic coding, good understanding of ICD-10-CM is essential to be able to code properly.
Similar to the expansion of ICD-10-CM (from volumes 1 and 2 of ICD-9-CM), ICD-10-PCS has undergone similar expansion (from volume 3 of ICD-9-CM), with a corresponding increase in specificity. For example, there are now 5 distinct codes for electroconvulsive therapy (GZB0ZZZ-GZB4ZZZ) that distinguish unilateral from bilateral electrode placement and single from multiple stimulations.
DSM-5 will continue to be the frameworkfor psychiatric assessment and diagnosis. ICD-10-CM will be the coding system to accurately denote DSM-5 diagnoses. The Centers for Medicare and Medicaid Services (CMS) and the National Center for Health Statistics recognize DSM-5 as the means to identify proper ICD-10-CM codes for mental disorders. CMS also has announced that, although ICD-10-CM codes are necessary for reimbursement, use of an incorrect code will not be the basis for denying a Medicare claim for 1 year.
Making ICD-10 part of practice
Here are several keys to implementing ICD-10 with minimum pain and maximum benefit.
Multiple diagnosis codes should be listed in the order of their relevance to the clinical encounter.
Visit type. The seventh character of the ICD-10-CM code denotes the type of visit (initial, subsequent, or sequela) and must be provided:
- An initial encounter is one in which the patient first receives active treatment.
- A subsequent encounter refers to a follow-up visit in which the patient receives routine care during the healing or recovery phase.
- A sequel encounter is one in which a patient receives treatment for complications or conditions that arise as a direct result of the initial condition.
The transition to ICD-10 should be facilitated by adoption of DSM-5. Continue using DSM-5 to determine the correct diagnosis or diagnoses of the mental disorder, then apply the corresponding ICD-10-CM code(s). The better you understand and apply DSM-5, the more precise you can be in utilizing the greater specificity and accuracy afforded by ICD-10-CM coding.
Document well. Good understanding of the structure and organization of ICD-10-CM facilitates efficient, comprehensive documentation. This, in turn, will foster better clinical communication and appropriate reimbursement.
Know your payers—in particular, their policies regarding differential reimbursement for clinical complexity (based on ICD-10-CM/PCS). Medical practices that are part of an accountable care organization, and those that have risk-adjusted contracts must pay special attention to documenting clinical complexity when coding.
Know your electronic health care record, understand what tools it offers to efficiently translate DSM-5 diagnoses into appropriate ICD-10-CM codes, and use those tools efficiently.
Review your medical record documentation for the top 20 conditions in your practice, in the context of their definition in ICD-10-CM.
If you have coders who do ICD-10-CM coding for you, review a few patient charts with them to compare your sense of the patient’s clinical complexity and their coding based on your documentation.
Changes in DSM-5 have encouraged clinicians to improve their assessment of patients and provide measurement-based care. The significant changes in ICD-10-CM should provide the impetus for you to hone your ability to provide documentation. Sufficient flexibility exists within guidelines to permit individualization of the style of documentation.
Because all DSM-5 diagnoses map to appropriate ICD-10-CM codes, effective use of DSM-5 should make the transition to ICD-10 easy.
Bottom Line
Compared with ICD-9, definitions of mental health diagnoses have been improved in ICD-10, and more elaborate code descriptions in ICD-10-CM provide for greater precision when you report a diagnosis. The result? More accurate and efficient documentation of the care you provide and better reimbursement. Understanding what impact the changes in ICD-10 will have on your clinical work will ensure that your practice keeps pace with professional and legal standards of care.
Related Resources
• Blue Cross Blue Shield of Michigan ICD-10 update: mental and behavioral health ICD-10-CM codes. http://www.bcbsm.com/content/dam/public/Providers/Documents/help/faqs/icd10-update-mentalhealth.pdf.
• American Psychiatric Association ICD-10 tutorial. http://www.psychiatry.org/psychiatrists/practice/dsm/icd-10.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 5th edition. Washington DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioral disorders: clinical descriptions and diagnostic guidelines. Geneva, Switzerland: World Health Organization; 1992.
3. American Medical Association. ICD-10-CM 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
4. American Medical Association. CPT-2016, professional edition. Chicago, IL: American Medical Association; 2015.
5. American Medical Association. ICD-10-CM expert for physicians 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
6. American Medical Association. ICD-10-PCS mapping to ICD-9-CM volume 3. Chicago, IL: American Medical Association; 2015.
7. Tandon R, Halbreich U. The second-generation ‘atypical’ antipsychotics: similar efficacy but different neuroendocrine side-effects. Psychoneuroendocrinology. 2003;28(suppl 1):1-7.
8. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(suppl 1):4-8.
9. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10.
10. Malaspina D, Owens MJ, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150(1):21-25.
11. Tandon R, Heckers S, Bustillo J, et al. Catatonia in DSM-5. Schizophr Res. 2013;150(1):26-30.
Just as psychiatrists are adapting to DSM-5, they have to cope with implementation of the 10th edition of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). This challenge raises questions: What is the importance of understanding ICD-10? How will it affect the practice of psychiatry?
Furthermore, how does ICD-10 relate to DSM-5 and Current Procedural Terminology (CPT)? How does it differ from ICD-9? What are the ICD-10-Clinical Modification (CM) and ICD-10-Procedures (PCS)?Learning the essence of the changes, and understanding what impact they have on your clinical work, are necessary to ensure that your practice keeps pace with professional and legal standards of care. The effort involved is not onerous, however, and can improve the quality and efficiency of your care and how you document it.
In this article, we provide you with an overview of ICD-10; highlight major changes of the new classification; explain its relevance to clinical practice; and offer guidelines for implementing it effectively. We also emphasize that a good understanding of DSM-5 facilitates appreciation of ICD-10 and makes its implementation fairly easy and straightforward.
To begin, we provide a glossary of ICD-related terms and a review of additional definitions, distinctions, and dates (Box).1-6
Major changes from ICD-9
No question: ICD-10 is going to significantly influence your practice and your reimbursement. Furthermore, a number of revisions in ICD-10 have the potential to meaningfully improve clinical documentation and communication and to enhance your ability to precisely describe the complexity of your patients—with implications for billing.
ICD-10 differs from ICD-9 in organization, structure, code composition, and level of detail. In addition, ICD-10 makes some changes in terminology and definitions, with the goal of improving precision.
ICD-10 also is much larger than ICD-9.The total number of medical diagnostic codes has increased more than 5-fold—from approximately 13,000 to 69,000. This expansion allows for greater specificity in diagnosis and enables differentiation of an initial clinical encounter from a subsequent encounter.
To accommodate the expansion in the number of codes, the 5-digit numeric codes used in ICD-9 have been replaced in ICD-10 by 7-digit alphanumeric codes:
- the first digit always is a letter
- the second and third digits are numbers followed by a decimal point
- the fourth though seventh digits can be letters or numbers
- the first 3 digits denote the diagnostic category
- the fourth through sixth digits provide diagnostic detail
- the seventh digit provides information about the nature of the encounter (eg, initial, subsequent, or sequel, denoted respectively by “A,” “D,” and “S” in the seventh digit).
The number of 3-digit categories for psychiatric disorders has increased from 30 in ICD-9 (290-319) to 100 in ICD-10 (F00-F99). Only the first 5 digits are used for the section on mental disorders in ICD-10, with the first digit always “F” and the second digit a number denoting the broad type of disorders. The second and third digits in conjunction define the major category of the disorder; the fourth and fifth digits provide additional descriptive detail about the disorder (Table).
ICD-9 ‘V’ codes are out
What were called “V” codes in ICD-9—factors that influence health status and contact with health services—have been replaced by “Z” codes in ICD-10. These “Z” codes provide greater detail and precision than “V” codes provided.
Examples of “Z” codes relevant to psychiatry are:
Z00 General psychiatric examination (eg, of a person who does not have a complaint or diagnosis)
Z03 Examination for suspected mental and behavioral disorder
Z04 Examination for medicolegal or other purposes; Z04.8 is relevant laboratory testing, such as drug testing of urine or blood
Z50 Care involving rehabilitation (substance use disorder, etc.)
Z60 Problem related to social environment
Z61 Problem related to negative life events in childhood
Z63 Problem related to primary support group, including family circumstances
Z64-Z65 Problem related to other psychosocial circumstances
Z70-Z71 Condition requiring counseling, not elsewhere classified
Z73 Problem related to difficulty with life management (burnout, stress, role conflict, etc.)
Z75 Problem related to medical facilities and other aspects of health care (eg, awaiting admission)
Z81 Family history of mental or behavioral disorders
Z85-Z91 Personal history of various disorders (must be absent or in full remission at the moment); Z86.51, for example, refers to a history of combat and operational stress reaction.
Greater precision is now possible when coding for treatment-related adverse effects. A particular adverse effect now is coded under the relevant system, along with its attribution to the specific substance. Obesity attributable to antipsychotic treatment,7,8 for example, is coded as E66.1.
Integrating DSM-5 and ICD-10
Because DSM-5 lists corresponding ICD-10-CM codes for all disorders, you will find it much easier than other physicians to implement ICD-10. DSM-5 includes ICD-9-CM and ICD-10-CM codes for each DSM-5 disorder (for example, the ICD-9-CM code for schizophrenia is 295.x; the ICD-10-CM code is F20.9).9
Furthermore, a number of changes from ICD-9-CM to ICD-10-CM enable documentation of greater diagnostic specificity; for example, DSM-5 schizoaffective disorder, bipolar type, and schizoaffective disorder, depressive type, are distinctly coded as F25.0 and F25.1, respectively, in ICD-10-CM, whereas both were coded as 295.7 in ICD-9-CM.10
You will continue to use DSM-5 criteria to guide your diagnostic process, translating the DSM-5 diagnosis (diagnoses) into corresponding ICD-10-CM codes. Experience with DSM-5 substantially simplifies the transition to ICD-10.
Key differences between DSM-5 and ICD-10
There are notable differences in organization and content between DSM-5 and ICD-10.
The 20 chapters in DSM-5 begin with neurodevelopmental disorders; neurocognitive disorders are toward the end (ie, childhood to late life). In contrast, neurocognitive disorders (ie, “dementia”) appear at the beginning of ICD-10; neurodevelopmental disorders are at the end.
Elimination of schizophrenia subtypes in DSM-5 necessitates coding of all schizophrenia as F20.9 in ICD-10-CM because F20.0-F20.8 are specific subtypes. DSM-5 schizophreniform disorder is coded F20.81.
Substance abuse and substance dependence continue to be separate in ICD-10-CM, but they are combined in a single category of substance use disorders in DSM-5. The correct ICD-10-CM code (ie, abuse vs dependence) is determined by the severity of the substance use disorder: “Mild” coding as abuse (F1x.1) and “moderate” and “severe” coding as dependence (F2x.2), with x denoting the substance abused.
There can be multiple applicable diagnoses associated with a clinical encounter, as there was with ICD-9-CM. Give precedence to the diagnosis that best represents the nature of the presenting problem; list other diagnoses in the order of their relevance. DSM-5 and ICD-10-CM are similar in this regard.
ICD-10-CM uses only subtypes, in contrast to the use of subtypes and specifiers in DSM-5 to describe variability in disorders across patients. It is possible, however, to code certain DSM-5 specifiers in ICD-10-CM. (This is discussed in the “Recording Procedures” section of the DSM-5 text and summarized at the beginning of the manual, and appears in the “Appendix.”) To code the catatonia specifier in the context of schizoaffective disorder, depressive type, for example, use ICD-10-CM code F25.1 for the disorder and add code F06.1 for the catatonia specifier.11
How will ICD-10 affect your practice?
As of October 1, 2015, all health care facilities were to have become ICD-10 compliant. Furthermore, any Health Insurance Portability and Accountability Act-covered entity must use ICD-10-CM codes if it expects to be reimbursed for health care services.
Mental health practitioners might think that the transition from ICD-9-CM to ICD-10-CM involves only billers and coders, not them. They are wrong. All clinicians are responsible for documenting their diagnostic and treatment services properly. Medical records must contain adequate information to support any diagnostic (ICD-10-CM) and treatment (CPT) codes that are applied to a given clinical encounter.
The greater detail and specificity that are provided by ICD-10-CM allow more accurate recording of clinical complexity, which, in turn, influences reimbursement. However, good documentation is necessary for proper coding. Because clinicians are ultimately responsible for proper diagnostic coding, good understanding of ICD-10-CM is essential to be able to code properly.
Similar to the expansion of ICD-10-CM (from volumes 1 and 2 of ICD-9-CM), ICD-10-PCS has undergone similar expansion (from volume 3 of ICD-9-CM), with a corresponding increase in specificity. For example, there are now 5 distinct codes for electroconvulsive therapy (GZB0ZZZ-GZB4ZZZ) that distinguish unilateral from bilateral electrode placement and single from multiple stimulations.
DSM-5 will continue to be the frameworkfor psychiatric assessment and diagnosis. ICD-10-CM will be the coding system to accurately denote DSM-5 diagnoses. The Centers for Medicare and Medicaid Services (CMS) and the National Center for Health Statistics recognize DSM-5 as the means to identify proper ICD-10-CM codes for mental disorders. CMS also has announced that, although ICD-10-CM codes are necessary for reimbursement, use of an incorrect code will not be the basis for denying a Medicare claim for 1 year.
Making ICD-10 part of practice
Here are several keys to implementing ICD-10 with minimum pain and maximum benefit.
Multiple diagnosis codes should be listed in the order of their relevance to the clinical encounter.
Visit type. The seventh character of the ICD-10-CM code denotes the type of visit (initial, subsequent, or sequela) and must be provided:
- An initial encounter is one in which the patient first receives active treatment.
- A subsequent encounter refers to a follow-up visit in which the patient receives routine care during the healing or recovery phase.
- A sequel encounter is one in which a patient receives treatment for complications or conditions that arise as a direct result of the initial condition.
The transition to ICD-10 should be facilitated by adoption of DSM-5. Continue using DSM-5 to determine the correct diagnosis or diagnoses of the mental disorder, then apply the corresponding ICD-10-CM code(s). The better you understand and apply DSM-5, the more precise you can be in utilizing the greater specificity and accuracy afforded by ICD-10-CM coding.
Document well. Good understanding of the structure and organization of ICD-10-CM facilitates efficient, comprehensive documentation. This, in turn, will foster better clinical communication and appropriate reimbursement.
Know your payers—in particular, their policies regarding differential reimbursement for clinical complexity (based on ICD-10-CM/PCS). Medical practices that are part of an accountable care organization, and those that have risk-adjusted contracts must pay special attention to documenting clinical complexity when coding.
Know your electronic health care record, understand what tools it offers to efficiently translate DSM-5 diagnoses into appropriate ICD-10-CM codes, and use those tools efficiently.
Review your medical record documentation for the top 20 conditions in your practice, in the context of their definition in ICD-10-CM.
If you have coders who do ICD-10-CM coding for you, review a few patient charts with them to compare your sense of the patient’s clinical complexity and their coding based on your documentation.
Changes in DSM-5 have encouraged clinicians to improve their assessment of patients and provide measurement-based care. The significant changes in ICD-10-CM should provide the impetus for you to hone your ability to provide documentation. Sufficient flexibility exists within guidelines to permit individualization of the style of documentation.
Because all DSM-5 diagnoses map to appropriate ICD-10-CM codes, effective use of DSM-5 should make the transition to ICD-10 easy.
Bottom Line
Compared with ICD-9, definitions of mental health diagnoses have been improved in ICD-10, and more elaborate code descriptions in ICD-10-CM provide for greater precision when you report a diagnosis. The result? More accurate and efficient documentation of the care you provide and better reimbursement. Understanding what impact the changes in ICD-10 will have on your clinical work will ensure that your practice keeps pace with professional and legal standards of care.
Related Resources
• Blue Cross Blue Shield of Michigan ICD-10 update: mental and behavioral health ICD-10-CM codes. http://www.bcbsm.com/content/dam/public/Providers/Documents/help/faqs/icd10-update-mentalhealth.pdf.
• American Psychiatric Association ICD-10 tutorial. http://www.psychiatry.org/psychiatrists/practice/dsm/icd-10.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Just as psychiatrists are adapting to DSM-5, they have to cope with implementation of the 10th edition of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). This challenge raises questions: What is the importance of understanding ICD-10? How will it affect the practice of psychiatry?
Furthermore, how does ICD-10 relate to DSM-5 and Current Procedural Terminology (CPT)? How does it differ from ICD-9? What are the ICD-10-Clinical Modification (CM) and ICD-10-Procedures (PCS)?Learning the essence of the changes, and understanding what impact they have on your clinical work, are necessary to ensure that your practice keeps pace with professional and legal standards of care. The effort involved is not onerous, however, and can improve the quality and efficiency of your care and how you document it.
In this article, we provide you with an overview of ICD-10; highlight major changes of the new classification; explain its relevance to clinical practice; and offer guidelines for implementing it effectively. We also emphasize that a good understanding of DSM-5 facilitates appreciation of ICD-10 and makes its implementation fairly easy and straightforward.
To begin, we provide a glossary of ICD-related terms and a review of additional definitions, distinctions, and dates (Box).1-6
Major changes from ICD-9
No question: ICD-10 is going to significantly influence your practice and your reimbursement. Furthermore, a number of revisions in ICD-10 have the potential to meaningfully improve clinical documentation and communication and to enhance your ability to precisely describe the complexity of your patients—with implications for billing.
ICD-10 differs from ICD-9 in organization, structure, code composition, and level of detail. In addition, ICD-10 makes some changes in terminology and definitions, with the goal of improving precision.
ICD-10 also is much larger than ICD-9.The total number of medical diagnostic codes has increased more than 5-fold—from approximately 13,000 to 69,000. This expansion allows for greater specificity in diagnosis and enables differentiation of an initial clinical encounter from a subsequent encounter.
To accommodate the expansion in the number of codes, the 5-digit numeric codes used in ICD-9 have been replaced in ICD-10 by 7-digit alphanumeric codes:
- the first digit always is a letter
- the second and third digits are numbers followed by a decimal point
- the fourth though seventh digits can be letters or numbers
- the first 3 digits denote the diagnostic category
- the fourth through sixth digits provide diagnostic detail
- the seventh digit provides information about the nature of the encounter (eg, initial, subsequent, or sequel, denoted respectively by “A,” “D,” and “S” in the seventh digit).
The number of 3-digit categories for psychiatric disorders has increased from 30 in ICD-9 (290-319) to 100 in ICD-10 (F00-F99). Only the first 5 digits are used for the section on mental disorders in ICD-10, with the first digit always “F” and the second digit a number denoting the broad type of disorders. The second and third digits in conjunction define the major category of the disorder; the fourth and fifth digits provide additional descriptive detail about the disorder (Table).
ICD-9 ‘V’ codes are out
What were called “V” codes in ICD-9—factors that influence health status and contact with health services—have been replaced by “Z” codes in ICD-10. These “Z” codes provide greater detail and precision than “V” codes provided.
Examples of “Z” codes relevant to psychiatry are:
Z00 General psychiatric examination (eg, of a person who does not have a complaint or diagnosis)
Z03 Examination for suspected mental and behavioral disorder
Z04 Examination for medicolegal or other purposes; Z04.8 is relevant laboratory testing, such as drug testing of urine or blood
Z50 Care involving rehabilitation (substance use disorder, etc.)
Z60 Problem related to social environment
Z61 Problem related to negative life events in childhood
Z63 Problem related to primary support group, including family circumstances
Z64-Z65 Problem related to other psychosocial circumstances
Z70-Z71 Condition requiring counseling, not elsewhere classified
Z73 Problem related to difficulty with life management (burnout, stress, role conflict, etc.)
Z75 Problem related to medical facilities and other aspects of health care (eg, awaiting admission)
Z81 Family history of mental or behavioral disorders
Z85-Z91 Personal history of various disorders (must be absent or in full remission at the moment); Z86.51, for example, refers to a history of combat and operational stress reaction.
Greater precision is now possible when coding for treatment-related adverse effects. A particular adverse effect now is coded under the relevant system, along with its attribution to the specific substance. Obesity attributable to antipsychotic treatment,7,8 for example, is coded as E66.1.
Integrating DSM-5 and ICD-10
Because DSM-5 lists corresponding ICD-10-CM codes for all disorders, you will find it much easier than other physicians to implement ICD-10. DSM-5 includes ICD-9-CM and ICD-10-CM codes for each DSM-5 disorder (for example, the ICD-9-CM code for schizophrenia is 295.x; the ICD-10-CM code is F20.9).9
Furthermore, a number of changes from ICD-9-CM to ICD-10-CM enable documentation of greater diagnostic specificity; for example, DSM-5 schizoaffective disorder, bipolar type, and schizoaffective disorder, depressive type, are distinctly coded as F25.0 and F25.1, respectively, in ICD-10-CM, whereas both were coded as 295.7 in ICD-9-CM.10
You will continue to use DSM-5 criteria to guide your diagnostic process, translating the DSM-5 diagnosis (diagnoses) into corresponding ICD-10-CM codes. Experience with DSM-5 substantially simplifies the transition to ICD-10.
Key differences between DSM-5 and ICD-10
There are notable differences in organization and content between DSM-5 and ICD-10.
The 20 chapters in DSM-5 begin with neurodevelopmental disorders; neurocognitive disorders are toward the end (ie, childhood to late life). In contrast, neurocognitive disorders (ie, “dementia”) appear at the beginning of ICD-10; neurodevelopmental disorders are at the end.
Elimination of schizophrenia subtypes in DSM-5 necessitates coding of all schizophrenia as F20.9 in ICD-10-CM because F20.0-F20.8 are specific subtypes. DSM-5 schizophreniform disorder is coded F20.81.
Substance abuse and substance dependence continue to be separate in ICD-10-CM, but they are combined in a single category of substance use disorders in DSM-5. The correct ICD-10-CM code (ie, abuse vs dependence) is determined by the severity of the substance use disorder: “Mild” coding as abuse (F1x.1) and “moderate” and “severe” coding as dependence (F2x.2), with x denoting the substance abused.
There can be multiple applicable diagnoses associated with a clinical encounter, as there was with ICD-9-CM. Give precedence to the diagnosis that best represents the nature of the presenting problem; list other diagnoses in the order of their relevance. DSM-5 and ICD-10-CM are similar in this regard.
ICD-10-CM uses only subtypes, in contrast to the use of subtypes and specifiers in DSM-5 to describe variability in disorders across patients. It is possible, however, to code certain DSM-5 specifiers in ICD-10-CM. (This is discussed in the “Recording Procedures” section of the DSM-5 text and summarized at the beginning of the manual, and appears in the “Appendix.”) To code the catatonia specifier in the context of schizoaffective disorder, depressive type, for example, use ICD-10-CM code F25.1 for the disorder and add code F06.1 for the catatonia specifier.11
How will ICD-10 affect your practice?
As of October 1, 2015, all health care facilities were to have become ICD-10 compliant. Furthermore, any Health Insurance Portability and Accountability Act-covered entity must use ICD-10-CM codes if it expects to be reimbursed for health care services.
Mental health practitioners might think that the transition from ICD-9-CM to ICD-10-CM involves only billers and coders, not them. They are wrong. All clinicians are responsible for documenting their diagnostic and treatment services properly. Medical records must contain adequate information to support any diagnostic (ICD-10-CM) and treatment (CPT) codes that are applied to a given clinical encounter.
The greater detail and specificity that are provided by ICD-10-CM allow more accurate recording of clinical complexity, which, in turn, influences reimbursement. However, good documentation is necessary for proper coding. Because clinicians are ultimately responsible for proper diagnostic coding, good understanding of ICD-10-CM is essential to be able to code properly.
Similar to the expansion of ICD-10-CM (from volumes 1 and 2 of ICD-9-CM), ICD-10-PCS has undergone similar expansion (from volume 3 of ICD-9-CM), with a corresponding increase in specificity. For example, there are now 5 distinct codes for electroconvulsive therapy (GZB0ZZZ-GZB4ZZZ) that distinguish unilateral from bilateral electrode placement and single from multiple stimulations.
DSM-5 will continue to be the frameworkfor psychiatric assessment and diagnosis. ICD-10-CM will be the coding system to accurately denote DSM-5 diagnoses. The Centers for Medicare and Medicaid Services (CMS) and the National Center for Health Statistics recognize DSM-5 as the means to identify proper ICD-10-CM codes for mental disorders. CMS also has announced that, although ICD-10-CM codes are necessary for reimbursement, use of an incorrect code will not be the basis for denying a Medicare claim for 1 year.
Making ICD-10 part of practice
Here are several keys to implementing ICD-10 with minimum pain and maximum benefit.
Multiple diagnosis codes should be listed in the order of their relevance to the clinical encounter.
Visit type. The seventh character of the ICD-10-CM code denotes the type of visit (initial, subsequent, or sequela) and must be provided:
- An initial encounter is one in which the patient first receives active treatment.
- A subsequent encounter refers to a follow-up visit in which the patient receives routine care during the healing or recovery phase.
- A sequel encounter is one in which a patient receives treatment for complications or conditions that arise as a direct result of the initial condition.
The transition to ICD-10 should be facilitated by adoption of DSM-5. Continue using DSM-5 to determine the correct diagnosis or diagnoses of the mental disorder, then apply the corresponding ICD-10-CM code(s). The better you understand and apply DSM-5, the more precise you can be in utilizing the greater specificity and accuracy afforded by ICD-10-CM coding.
Document well. Good understanding of the structure and organization of ICD-10-CM facilitates efficient, comprehensive documentation. This, in turn, will foster better clinical communication and appropriate reimbursement.
Know your payers—in particular, their policies regarding differential reimbursement for clinical complexity (based on ICD-10-CM/PCS). Medical practices that are part of an accountable care organization, and those that have risk-adjusted contracts must pay special attention to documenting clinical complexity when coding.
Know your electronic health care record, understand what tools it offers to efficiently translate DSM-5 diagnoses into appropriate ICD-10-CM codes, and use those tools efficiently.
Review your medical record documentation for the top 20 conditions in your practice, in the context of their definition in ICD-10-CM.
If you have coders who do ICD-10-CM coding for you, review a few patient charts with them to compare your sense of the patient’s clinical complexity and their coding based on your documentation.
Changes in DSM-5 have encouraged clinicians to improve their assessment of patients and provide measurement-based care. The significant changes in ICD-10-CM should provide the impetus for you to hone your ability to provide documentation. Sufficient flexibility exists within guidelines to permit individualization of the style of documentation.
Because all DSM-5 diagnoses map to appropriate ICD-10-CM codes, effective use of DSM-5 should make the transition to ICD-10 easy.
Bottom Line
Compared with ICD-9, definitions of mental health diagnoses have been improved in ICD-10, and more elaborate code descriptions in ICD-10-CM provide for greater precision when you report a diagnosis. The result? More accurate and efficient documentation of the care you provide and better reimbursement. Understanding what impact the changes in ICD-10 will have on your clinical work will ensure that your practice keeps pace with professional and legal standards of care.
Related Resources
• Blue Cross Blue Shield of Michigan ICD-10 update: mental and behavioral health ICD-10-CM codes. http://www.bcbsm.com/content/dam/public/Providers/Documents/help/faqs/icd10-update-mentalhealth.pdf.
• American Psychiatric Association ICD-10 tutorial. http://www.psychiatry.org/psychiatrists/practice/dsm/icd-10.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 5th edition. Washington DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioral disorders: clinical descriptions and diagnostic guidelines. Geneva, Switzerland: World Health Organization; 1992.
3. American Medical Association. ICD-10-CM 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
4. American Medical Association. CPT-2016, professional edition. Chicago, IL: American Medical Association; 2015.
5. American Medical Association. ICD-10-CM expert for physicians 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
6. American Medical Association. ICD-10-PCS mapping to ICD-9-CM volume 3. Chicago, IL: American Medical Association; 2015.
7. Tandon R, Halbreich U. The second-generation ‘atypical’ antipsychotics: similar efficacy but different neuroendocrine side-effects. Psychoneuroendocrinology. 2003;28(suppl 1):1-7.
8. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(suppl 1):4-8.
9. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10.
10. Malaspina D, Owens MJ, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150(1):21-25.
11. Tandon R, Heckers S, Bustillo J, et al. Catatonia in DSM-5. Schizophr Res. 2013;150(1):26-30.
1. Diagnostic and statistical manual of mental disorders, 5th edition. Washington DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioral disorders: clinical descriptions and diagnostic guidelines. Geneva, Switzerland: World Health Organization; 1992.
3. American Medical Association. ICD-10-CM 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
4. American Medical Association. CPT-2016, professional edition. Chicago, IL: American Medical Association; 2015.
5. American Medical Association. ICD-10-CM expert for physicians 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
6. American Medical Association. ICD-10-PCS mapping to ICD-9-CM volume 3. Chicago, IL: American Medical Association; 2015.
7. Tandon R, Halbreich U. The second-generation ‘atypical’ antipsychotics: similar efficacy but different neuroendocrine side-effects. Psychoneuroendocrinology. 2003;28(suppl 1):1-7.
8. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(suppl 1):4-8.
9. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10.
10. Malaspina D, Owens MJ, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150(1):21-25.
11. Tandon R, Heckers S, Bustillo J, et al. Catatonia in DSM-5. Schizophr Res. 2013;150(1):26-30.
Isolated Brachialis Muscle Atrophy
Isolated brachialis muscle atrophy has been rarely reported. Among the few cases in the literature, 1 was attributed to a presumed compartment syndrome,1 1 to a displaced clavicle fracture,2 and 3 to neuralgic amyotrophy.3,4 We present a case of isolated brachialis muscle atrophy of unknown etiology, the presentation of which is consistent with neuralgic amyotrophy, also known as Parsonage-Turner syndrome or brachial plexitis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 37-year-old right-handed highway worker presented for evaluation of right-arm muscle atrophy. One year earlier, while lifting heavy bags at work, he felt a painful strain in his right arm, although there was no bruising or swelling. Approximately 4 weeks after this incident, he developed right shoulder pain and began to notice a slight decrease in the muscle mass of his right anterior arm. On evaluation at an outside facility, the physician noted some brachialis muscle atrophy. His shoulder pain was attributed to acromioclavicular joint problems. After an initial trial of physical therapy that did not alleviate this joint pain, an acromioclavicular joint resection was performed, and his pain improved. The brachialis muscle atrophy continued to progress, however. Over the course of the next 6 months, the patient noticed a continually decreasing muscle mass in his right arm, as well as arm fatigue with routine recreational activities. On follow-up, again at an outside institution, the treating physicians noted continued atrophy of the distal arm corresponding to the region of the brachialis musculature. Magnetic resonance imaging showed continuity of the brachialis muscle and tendon, with muscle atrophy. The patient was able to return to work, although with a subjective decrease in right elbow flexion strength.
On presentation at our institution, the patient complained of right arm weakness with heavy use but did not have pain or sensory complaints. His medical history was otherwise unremarkable. Physical examination revealed obvious wasting of the right brachialis muscle, most notable on the lateral aspect of the distal arm (Figures 1, 2A, 2B). His biceps muscle was functioning with full strength and had a normal bulk. He had a normal range of active and passive motion, including full extension and flexion of both elbows, as well as complete pronosupination of the forearms. There was no focal tenderness. Manual muscle testing of both upper extremities was completely normal except for 4/5 flexion strength of the right elbow. Neurovascular examination also revealed normal findings, including intact sensation over the radiolateral forearm. A second magnetic resonance image showed that the brachialis muscle had completely atrophied. Because the clinical examination and imaging studies both indicated isolated brachialis atrophy without deficit elsewhere along the musculocutaneous nerve, electromyography was not performed. The patient was fully functional and working at his usual occupation, and no further intervention was recommended.
Discussion
Isolated wasting of the brachialis muscle is extremely rare with few reports in the literature. Farmer and colleagues1 reported a case of brachialis atrophy that was presumed to have resulted from exercise-induced chronic compartment syndrome. In that case, the patient developed a prodrome of arm pain followed by brachialis muscle atrophy. This patient was treated with oral anti-inflammatory agents with improvement in pain but without recovery of the brachialis muscle. While this case was attributed to compartment syndrome, it is likely that it represented neuralgic amyotrophy because there was no evidence of elbow flexion contracture, which would have accompanied true necrosis of the brachialis muscle as seen in compartment syndrome. However, acute compartment syndrome of the brachialis muscle after minor trauma has been reported.5 In that case, full-scale compartment syndrome was treated with rapid fasciotomy, with complete recovery of the brachialis.
Isolated brachialis atrophy has also been described in the setting of a displaced midshaft clavicle fracture in an elite athlete.2 Two fracture fragments were thought to have injured the brachial plexus, separately causing brachialis atrophy and altered sensation over the clavicular head of the deltoid muscle. Atrophy remained 1 year after injury.
Although it had been occasionally reported, the first large series of patients with sporadic neuralgic amyotrophy in the upper extremity was reported by Parsonage and Turner6 in 1948. They described 136 patients who developed flaccid paralysis and atrophy of various muscles of the shoulder girdle and/or upper extremity. This was generally preceded by acute pain in the shoulder girdle, often associated with antecedent viral infection, stress, illness, or other precipitating factors.
To our knowledge, there have been 3 other reported cases of neuralgic amyotrophy of the brachialis muscle. Watson and colleagues3 presented 2 patients with nonspecific, neurogenic shoulder pain after which an indolent, progressive atrophy of the brachialis muscle ensued.3 Van Tongel and colleagues4 described a more traditional case of Parsonage-Turner syndrome, with bilateral wasting of the shoulder girdle that also exhibited unilateral brachialis atrophy without affecting other muscles in the arm.4 Our case, with shoulder pain followed by muscle atrophy, fits the pattern of neuralgic amyotrophy.
Others have similarly described isolated wasting of 1 muscle with the sparing of other muscles with a common innervation. Isolated atrophy of the extensor or flexor pollicis longus has been reported as variants of either posterior or anterior interosseous neuropathy, respectively.7,8 Nerve fibers in the brachial plexus destined to innervate muscles supplied by the anterior interosseous nerve may be the cause of the motor deficit in cases of anterior interosseous nerve palsy, which seem to be associated with brachial plexitis.9
We present a case of isolated brachialis muscle atrophy after a minor trauma that may have resulted from Parsonage-Turner syndrome or a variant of brachial plexitis. The constellation of shoulder and arm pain, with subsequent muscle atrophy, makes this diagnosis likely.
1. Farmer KW, McFarland EG, Sonin A, Cosgarea AJ, Roehrig GJ. Isolated necrosis of the brachialis muscle due to exercise. Orthopedics. 2002;25(6):682-684.
2. Rüst CA, Knechtle B, Knechtle P, Rosemann T. Atrophy of the brachialis muscle after a displaced clavicle fracture in an Ironman triathlete: case report. J Brachial Plex Periph Nerve Inj. 2011;6(1):e44-e47.
3. Watson BV, Rose-Innes A, Engstrom JW, Brown JD. Isolated brachialis wasting: an unusual presentation of neuralgic amyotrophy. Muscle Nerve. 2001;24(12):1699-1702.
4. Van Tongel A, Schreurs M, Bruyninckx F, Debeer P. Bilateral Parsonage-Turner syndrome with unilateral brachialis muscle wasting: a case report. J Shoulder Elbow Surg. 2010;19(8):e14-e16.
5. Jenkins NH, Mintowt-Czyz WJ. Compression of the biceps-brachialis compartment after trivial trauma. J Bone Joint Surg Br. 1986;68(3):374.
6. Parsonage MJ, Turner JW. Neuralgic amyotrophy; the shoulder-girdle syndrome. Lancet. 1948;1(6513):973-978.
7. Horton TC. Isolated paralysis of the extensor pollicis longus muscle: a further variation of posterior interosseous nerve palsy. J Hand Surg Br. 2000;25(2):225-226.
8. Hill NA, Howard FM, Huffer BR. The incomplete anterior interosseous nerve syndrome. J Hand Surg Am. 1985;10(1):4-16.
9. Rennels GD, Ochoa J. Neuralgic amyotrophy manifesting as anterior interosseous nerve palsy. Muscle Nerve. 1980;3(2):160-164.
Isolated brachialis muscle atrophy has been rarely reported. Among the few cases in the literature, 1 was attributed to a presumed compartment syndrome,1 1 to a displaced clavicle fracture,2 and 3 to neuralgic amyotrophy.3,4 We present a case of isolated brachialis muscle atrophy of unknown etiology, the presentation of which is consistent with neuralgic amyotrophy, also known as Parsonage-Turner syndrome or brachial plexitis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 37-year-old right-handed highway worker presented for evaluation of right-arm muscle atrophy. One year earlier, while lifting heavy bags at work, he felt a painful strain in his right arm, although there was no bruising or swelling. Approximately 4 weeks after this incident, he developed right shoulder pain and began to notice a slight decrease in the muscle mass of his right anterior arm. On evaluation at an outside facility, the physician noted some brachialis muscle atrophy. His shoulder pain was attributed to acromioclavicular joint problems. After an initial trial of physical therapy that did not alleviate this joint pain, an acromioclavicular joint resection was performed, and his pain improved. The brachialis muscle atrophy continued to progress, however. Over the course of the next 6 months, the patient noticed a continually decreasing muscle mass in his right arm, as well as arm fatigue with routine recreational activities. On follow-up, again at an outside institution, the treating physicians noted continued atrophy of the distal arm corresponding to the region of the brachialis musculature. Magnetic resonance imaging showed continuity of the brachialis muscle and tendon, with muscle atrophy. The patient was able to return to work, although with a subjective decrease in right elbow flexion strength.
On presentation at our institution, the patient complained of right arm weakness with heavy use but did not have pain or sensory complaints. His medical history was otherwise unremarkable. Physical examination revealed obvious wasting of the right brachialis muscle, most notable on the lateral aspect of the distal arm (Figures 1, 2A, 2B). His biceps muscle was functioning with full strength and had a normal bulk. He had a normal range of active and passive motion, including full extension and flexion of both elbows, as well as complete pronosupination of the forearms. There was no focal tenderness. Manual muscle testing of both upper extremities was completely normal except for 4/5 flexion strength of the right elbow. Neurovascular examination also revealed normal findings, including intact sensation over the radiolateral forearm. A second magnetic resonance image showed that the brachialis muscle had completely atrophied. Because the clinical examination and imaging studies both indicated isolated brachialis atrophy without deficit elsewhere along the musculocutaneous nerve, electromyography was not performed. The patient was fully functional and working at his usual occupation, and no further intervention was recommended.
Discussion
Isolated wasting of the brachialis muscle is extremely rare with few reports in the literature. Farmer and colleagues1 reported a case of brachialis atrophy that was presumed to have resulted from exercise-induced chronic compartment syndrome. In that case, the patient developed a prodrome of arm pain followed by brachialis muscle atrophy. This patient was treated with oral anti-inflammatory agents with improvement in pain but without recovery of the brachialis muscle. While this case was attributed to compartment syndrome, it is likely that it represented neuralgic amyotrophy because there was no evidence of elbow flexion contracture, which would have accompanied true necrosis of the brachialis muscle as seen in compartment syndrome. However, acute compartment syndrome of the brachialis muscle after minor trauma has been reported.5 In that case, full-scale compartment syndrome was treated with rapid fasciotomy, with complete recovery of the brachialis.
Isolated brachialis atrophy has also been described in the setting of a displaced midshaft clavicle fracture in an elite athlete.2 Two fracture fragments were thought to have injured the brachial plexus, separately causing brachialis atrophy and altered sensation over the clavicular head of the deltoid muscle. Atrophy remained 1 year after injury.
Although it had been occasionally reported, the first large series of patients with sporadic neuralgic amyotrophy in the upper extremity was reported by Parsonage and Turner6 in 1948. They described 136 patients who developed flaccid paralysis and atrophy of various muscles of the shoulder girdle and/or upper extremity. This was generally preceded by acute pain in the shoulder girdle, often associated with antecedent viral infection, stress, illness, or other precipitating factors.
To our knowledge, there have been 3 other reported cases of neuralgic amyotrophy of the brachialis muscle. Watson and colleagues3 presented 2 patients with nonspecific, neurogenic shoulder pain after which an indolent, progressive atrophy of the brachialis muscle ensued.3 Van Tongel and colleagues4 described a more traditional case of Parsonage-Turner syndrome, with bilateral wasting of the shoulder girdle that also exhibited unilateral brachialis atrophy without affecting other muscles in the arm.4 Our case, with shoulder pain followed by muscle atrophy, fits the pattern of neuralgic amyotrophy.
Others have similarly described isolated wasting of 1 muscle with the sparing of other muscles with a common innervation. Isolated atrophy of the extensor or flexor pollicis longus has been reported as variants of either posterior or anterior interosseous neuropathy, respectively.7,8 Nerve fibers in the brachial plexus destined to innervate muscles supplied by the anterior interosseous nerve may be the cause of the motor deficit in cases of anterior interosseous nerve palsy, which seem to be associated with brachial plexitis.9
We present a case of isolated brachialis muscle atrophy after a minor trauma that may have resulted from Parsonage-Turner syndrome or a variant of brachial plexitis. The constellation of shoulder and arm pain, with subsequent muscle atrophy, makes this diagnosis likely.
Isolated brachialis muscle atrophy has been rarely reported. Among the few cases in the literature, 1 was attributed to a presumed compartment syndrome,1 1 to a displaced clavicle fracture,2 and 3 to neuralgic amyotrophy.3,4 We present a case of isolated brachialis muscle atrophy of unknown etiology, the presentation of which is consistent with neuralgic amyotrophy, also known as Parsonage-Turner syndrome or brachial plexitis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 37-year-old right-handed highway worker presented for evaluation of right-arm muscle atrophy. One year earlier, while lifting heavy bags at work, he felt a painful strain in his right arm, although there was no bruising or swelling. Approximately 4 weeks after this incident, he developed right shoulder pain and began to notice a slight decrease in the muscle mass of his right anterior arm. On evaluation at an outside facility, the physician noted some brachialis muscle atrophy. His shoulder pain was attributed to acromioclavicular joint problems. After an initial trial of physical therapy that did not alleviate this joint pain, an acromioclavicular joint resection was performed, and his pain improved. The brachialis muscle atrophy continued to progress, however. Over the course of the next 6 months, the patient noticed a continually decreasing muscle mass in his right arm, as well as arm fatigue with routine recreational activities. On follow-up, again at an outside institution, the treating physicians noted continued atrophy of the distal arm corresponding to the region of the brachialis musculature. Magnetic resonance imaging showed continuity of the brachialis muscle and tendon, with muscle atrophy. The patient was able to return to work, although with a subjective decrease in right elbow flexion strength.
On presentation at our institution, the patient complained of right arm weakness with heavy use but did not have pain or sensory complaints. His medical history was otherwise unremarkable. Physical examination revealed obvious wasting of the right brachialis muscle, most notable on the lateral aspect of the distal arm (Figures 1, 2A, 2B). His biceps muscle was functioning with full strength and had a normal bulk. He had a normal range of active and passive motion, including full extension and flexion of both elbows, as well as complete pronosupination of the forearms. There was no focal tenderness. Manual muscle testing of both upper extremities was completely normal except for 4/5 flexion strength of the right elbow. Neurovascular examination also revealed normal findings, including intact sensation over the radiolateral forearm. A second magnetic resonance image showed that the brachialis muscle had completely atrophied. Because the clinical examination and imaging studies both indicated isolated brachialis atrophy without deficit elsewhere along the musculocutaneous nerve, electromyography was not performed. The patient was fully functional and working at his usual occupation, and no further intervention was recommended.
Discussion
Isolated wasting of the brachialis muscle is extremely rare with few reports in the literature. Farmer and colleagues1 reported a case of brachialis atrophy that was presumed to have resulted from exercise-induced chronic compartment syndrome. In that case, the patient developed a prodrome of arm pain followed by brachialis muscle atrophy. This patient was treated with oral anti-inflammatory agents with improvement in pain but without recovery of the brachialis muscle. While this case was attributed to compartment syndrome, it is likely that it represented neuralgic amyotrophy because there was no evidence of elbow flexion contracture, which would have accompanied true necrosis of the brachialis muscle as seen in compartment syndrome. However, acute compartment syndrome of the brachialis muscle after minor trauma has been reported.5 In that case, full-scale compartment syndrome was treated with rapid fasciotomy, with complete recovery of the brachialis.
Isolated brachialis atrophy has also been described in the setting of a displaced midshaft clavicle fracture in an elite athlete.2 Two fracture fragments were thought to have injured the brachial plexus, separately causing brachialis atrophy and altered sensation over the clavicular head of the deltoid muscle. Atrophy remained 1 year after injury.
Although it had been occasionally reported, the first large series of patients with sporadic neuralgic amyotrophy in the upper extremity was reported by Parsonage and Turner6 in 1948. They described 136 patients who developed flaccid paralysis and atrophy of various muscles of the shoulder girdle and/or upper extremity. This was generally preceded by acute pain in the shoulder girdle, often associated with antecedent viral infection, stress, illness, or other precipitating factors.
To our knowledge, there have been 3 other reported cases of neuralgic amyotrophy of the brachialis muscle. Watson and colleagues3 presented 2 patients with nonspecific, neurogenic shoulder pain after which an indolent, progressive atrophy of the brachialis muscle ensued.3 Van Tongel and colleagues4 described a more traditional case of Parsonage-Turner syndrome, with bilateral wasting of the shoulder girdle that also exhibited unilateral brachialis atrophy without affecting other muscles in the arm.4 Our case, with shoulder pain followed by muscle atrophy, fits the pattern of neuralgic amyotrophy.
Others have similarly described isolated wasting of 1 muscle with the sparing of other muscles with a common innervation. Isolated atrophy of the extensor or flexor pollicis longus has been reported as variants of either posterior or anterior interosseous neuropathy, respectively.7,8 Nerve fibers in the brachial plexus destined to innervate muscles supplied by the anterior interosseous nerve may be the cause of the motor deficit in cases of anterior interosseous nerve palsy, which seem to be associated with brachial plexitis.9
We present a case of isolated brachialis muscle atrophy after a minor trauma that may have resulted from Parsonage-Turner syndrome or a variant of brachial plexitis. The constellation of shoulder and arm pain, with subsequent muscle atrophy, makes this diagnosis likely.
1. Farmer KW, McFarland EG, Sonin A, Cosgarea AJ, Roehrig GJ. Isolated necrosis of the brachialis muscle due to exercise. Orthopedics. 2002;25(6):682-684.
2. Rüst CA, Knechtle B, Knechtle P, Rosemann T. Atrophy of the brachialis muscle after a displaced clavicle fracture in an Ironman triathlete: case report. J Brachial Plex Periph Nerve Inj. 2011;6(1):e44-e47.
3. Watson BV, Rose-Innes A, Engstrom JW, Brown JD. Isolated brachialis wasting: an unusual presentation of neuralgic amyotrophy. Muscle Nerve. 2001;24(12):1699-1702.
4. Van Tongel A, Schreurs M, Bruyninckx F, Debeer P. Bilateral Parsonage-Turner syndrome with unilateral brachialis muscle wasting: a case report. J Shoulder Elbow Surg. 2010;19(8):e14-e16.
5. Jenkins NH, Mintowt-Czyz WJ. Compression of the biceps-brachialis compartment after trivial trauma. J Bone Joint Surg Br. 1986;68(3):374.
6. Parsonage MJ, Turner JW. Neuralgic amyotrophy; the shoulder-girdle syndrome. Lancet. 1948;1(6513):973-978.
7. Horton TC. Isolated paralysis of the extensor pollicis longus muscle: a further variation of posterior interosseous nerve palsy. J Hand Surg Br. 2000;25(2):225-226.
8. Hill NA, Howard FM, Huffer BR. The incomplete anterior interosseous nerve syndrome. J Hand Surg Am. 1985;10(1):4-16.
9. Rennels GD, Ochoa J. Neuralgic amyotrophy manifesting as anterior interosseous nerve palsy. Muscle Nerve. 1980;3(2):160-164.
1. Farmer KW, McFarland EG, Sonin A, Cosgarea AJ, Roehrig GJ. Isolated necrosis of the brachialis muscle due to exercise. Orthopedics. 2002;25(6):682-684.
2. Rüst CA, Knechtle B, Knechtle P, Rosemann T. Atrophy of the brachialis muscle after a displaced clavicle fracture in an Ironman triathlete: case report. J Brachial Plex Periph Nerve Inj. 2011;6(1):e44-e47.
3. Watson BV, Rose-Innes A, Engstrom JW, Brown JD. Isolated brachialis wasting: an unusual presentation of neuralgic amyotrophy. Muscle Nerve. 2001;24(12):1699-1702.
4. Van Tongel A, Schreurs M, Bruyninckx F, Debeer P. Bilateral Parsonage-Turner syndrome with unilateral brachialis muscle wasting: a case report. J Shoulder Elbow Surg. 2010;19(8):e14-e16.
5. Jenkins NH, Mintowt-Czyz WJ. Compression of the biceps-brachialis compartment after trivial trauma. J Bone Joint Surg Br. 1986;68(3):374.
6. Parsonage MJ, Turner JW. Neuralgic amyotrophy; the shoulder-girdle syndrome. Lancet. 1948;1(6513):973-978.
7. Horton TC. Isolated paralysis of the extensor pollicis longus muscle: a further variation of posterior interosseous nerve palsy. J Hand Surg Br. 2000;25(2):225-226.
8. Hill NA, Howard FM, Huffer BR. The incomplete anterior interosseous nerve syndrome. J Hand Surg Am. 1985;10(1):4-16.
9. Rennels GD, Ochoa J. Neuralgic amyotrophy manifesting as anterior interosseous nerve palsy. Muscle Nerve. 1980;3(2):160-164.
FDA approves lesinurad for uric acid lowering in gout
Lesinurad (Zurampic) has been approved to treat hyperuricemia associated with gout, when used in combination with a xanthine oxidase inhibitor, the Food and Drug Administration announced on Dec. 22.
Lesinurad promotes uric acid excretion by inhibiting the function of transporter proteins involved in uric acid reabsorption in the kidney.
“Zurampic provides a new treatment option for the millions of people who may develop gout over their lifetimes,” said Dr. Badrul Chowdhury, director of the Division of Pulmonary, Allergy, and Rheumatology Products in the FDA’s Center for Drug Evaluation and Research.
The drug’s safety and efficacy were evaluated in 1,537 participants in three randomized, placebo-controlled studies of its use in combination with a xanthine oxidase inhibitor. Participants treated for up to 12 months with lesinurad experienced reduced serum uric acid levels, compared with participants given placebo.
The most common adverse reactions in the clinical trials were headache, influenza, increased blood creatinine, and gastroesophageal reflux disease. Lesinurad has a boxed warning that provides important safety information, including the risk for acute renal failure, which is more common when lesinurad is used without a xanthine oxidase inhibitor and with higher-than-approved doses.
The FDA also said in its statement that the agency is requiring a postmarketing study to further evaluate the renal and cardiovascular safety of lesinurad.
Zurampic is manufactured by AstraZeneca Pharmaceuticals.
On Twitter @maryjodales
Lesinurad (Zurampic) has been approved to treat hyperuricemia associated with gout, when used in combination with a xanthine oxidase inhibitor, the Food and Drug Administration announced on Dec. 22.
Lesinurad promotes uric acid excretion by inhibiting the function of transporter proteins involved in uric acid reabsorption in the kidney.
“Zurampic provides a new treatment option for the millions of people who may develop gout over their lifetimes,” said Dr. Badrul Chowdhury, director of the Division of Pulmonary, Allergy, and Rheumatology Products in the FDA’s Center for Drug Evaluation and Research.
The drug’s safety and efficacy were evaluated in 1,537 participants in three randomized, placebo-controlled studies of its use in combination with a xanthine oxidase inhibitor. Participants treated for up to 12 months with lesinurad experienced reduced serum uric acid levels, compared with participants given placebo.
The most common adverse reactions in the clinical trials were headache, influenza, increased blood creatinine, and gastroesophageal reflux disease. Lesinurad has a boxed warning that provides important safety information, including the risk for acute renal failure, which is more common when lesinurad is used without a xanthine oxidase inhibitor and with higher-than-approved doses.
The FDA also said in its statement that the agency is requiring a postmarketing study to further evaluate the renal and cardiovascular safety of lesinurad.
Zurampic is manufactured by AstraZeneca Pharmaceuticals.
On Twitter @maryjodales
Lesinurad (Zurampic) has been approved to treat hyperuricemia associated with gout, when used in combination with a xanthine oxidase inhibitor, the Food and Drug Administration announced on Dec. 22.
Lesinurad promotes uric acid excretion by inhibiting the function of transporter proteins involved in uric acid reabsorption in the kidney.
“Zurampic provides a new treatment option for the millions of people who may develop gout over their lifetimes,” said Dr. Badrul Chowdhury, director of the Division of Pulmonary, Allergy, and Rheumatology Products in the FDA’s Center for Drug Evaluation and Research.
The drug’s safety and efficacy were evaluated in 1,537 participants in three randomized, placebo-controlled studies of its use in combination with a xanthine oxidase inhibitor. Participants treated for up to 12 months with lesinurad experienced reduced serum uric acid levels, compared with participants given placebo.
The most common adverse reactions in the clinical trials were headache, influenza, increased blood creatinine, and gastroesophageal reflux disease. Lesinurad has a boxed warning that provides important safety information, including the risk for acute renal failure, which is more common when lesinurad is used without a xanthine oxidase inhibitor and with higher-than-approved doses.
The FDA also said in its statement that the agency is requiring a postmarketing study to further evaluate the renal and cardiovascular safety of lesinurad.
Zurampic is manufactured by AstraZeneca Pharmaceuticals.
On Twitter @maryjodales
16 New Year’s resolutions for psychiatrists in 2016
Such decisions can be made at any time, but the dawn of a year is a powerful signal of a new beginning—another lease on life, a potential turning point. Imbedded in those resolutions is a subliminal sense of urgency to correct one’s long-neglected shortcomings as the calendar ruthlessly points to inevitable aging and the relentless march of time.
A psychiatric perspective
For psychiatrists, New Year’s resolutions transcend the (often ephemeral) impulse to go on a diet or buy a membership at the local gym. We have a unique perspective on the challenges that our patients face every day as they cope with the complex demands of life despite their anxiety, depression, or psychosis.
We are aware of the many unmet needs in managing complex neuropsychiatric brain disorders and the major challenges of erasing the burdensome stigma that engulfs our patients and the practice of psychiatry itself—despite its noble mission of repairing fractured brains, mending tortured souls, and restoring peace of mind and wellness. We are proud of our clinical and scientific accomplishments but are painfully cognizant of our limitations and the huge chasm between what we know and what we will eventually know once the brain reveals its glorious mysteries through neuroscientific research.
What can you resolve?
Here is my proposed list of pragmatic resolutions that most psychiatrists would regard as part of a perpetual to-do list—a must-do bucket of cherished goals and brave new horizons to bring complete mental health for our patients and immeasurable gratification for us, who dream of cures for brain disorders that trigger various ailments of the mind.
- Practice like a physician to emphasize the medical foundation of psychiatry: Always check on a patient’s physical health, and monitor his (her) cardiometabolic status. Wear the symbolic white coat that often enhances the physician−patient relationship.
- Dedicate a significant percentage of your practice to the sickest patients. There are enough non-physician mental health professionals to handle the walking wounded and worried well.
- Advocate relentlessly throughout your sphere of influence, and publicly, for true parity between psychiatric and non-mental medical disorders—not only for insurance coverage but for overall societal acceptance and compassion as well.
- Lobby vigorously for hospitalization instead of imprisonment of the seriously mentally ill because psychosis is a brain disease, not a criminal offense.
- Adopt evidence-based psychiatric practice whenever possible to achieve the best outcomes. Judiciously implement off-label practices, however, if no evidence-based treatments exist for a suffering patient.
- Avoid senseless and irrational polypharmacy but do not hesitate to use rational, beneficial combination therapy.
- Provide 1 hour a week of pro bono psychiatric work for the indigent and underserved. The rewards of giving what amounts to 1 week a year are immeasurably more gratifying than a few more dollars in your bank account.
- Resist calling an ill person a ‘client’ or ‘consumer’—at least until oncologists and cardiologists start doing so. Refuse to give up your medical identify in the many de-medicalized mental health clinics.
- Never let a patient leave your office without some psychotherapy, even as part of a 15-minute med-check.
- Stay current and on the cutting edge of evolving psychiatric practice by logging into PubMed every day (even briefly) to read a few abstracts of the latest studies related to patients you saw that day.
- Think like a neurologist by identifying the neural circuits of psychiatric symptoms. Act like a cardiologist by doing everything medically possible to prevent recurrence of psychotic, manic, or depressive episodes because they damage brain tissue just as a myocardial infarction damages the heart.
- Support research with words, money, and passion. Psychiatric neuroscientific breakthroughs generate superior treatments, erase stigma, and advance the quality of life for patients. Donate annually to the researchers of your choice, at the medical school where you were trained, or at a nonprofit research institute.
- Make time to write for publication, annually, at least 1 case report or a letter to the editor about observations from your practice. You can contribute immensely to the discovery process by sharing novel clinical insights.
- Never give up on any patient or set expectations too low, regardless of the diagnosis or severity of illness. Giving up destroys hope and ushers in despondency. Get a second opinion if you run out of options for a patient.
- Always set remission followed by recovery as the therapeutic goal for every patient. Let the patient know this and ask him (her) commit to that goal with you.
- Be genuinely proud to be a psychiatrist. You assess and rectify disorders of the mind, the most complex and magical product of the human brain that determines who we are and how we think, emote, communicate, verbalize, empathize, love, hate, remember, plan, problem-solve, and, of course, make resolutions.
Back to diet and exercise—for our patients and for us!
It’s OK to include, among your New Year’s resolutions, a pledge to strongly encourage patients to diet and exercise. Given the tendency of many of them to gain weight and die prematurely as a consequence of obesity-related cardiometabolic risk factors, you should urge them to eat healthy and exercise every time you see them, not only on New Year’s Day.
Such decisions can be made at any time, but the dawn of a year is a powerful signal of a new beginning—another lease on life, a potential turning point. Imbedded in those resolutions is a subliminal sense of urgency to correct one’s long-neglected shortcomings as the calendar ruthlessly points to inevitable aging and the relentless march of time.
A psychiatric perspective
For psychiatrists, New Year’s resolutions transcend the (often ephemeral) impulse to go on a diet or buy a membership at the local gym. We have a unique perspective on the challenges that our patients face every day as they cope with the complex demands of life despite their anxiety, depression, or psychosis.
We are aware of the many unmet needs in managing complex neuropsychiatric brain disorders and the major challenges of erasing the burdensome stigma that engulfs our patients and the practice of psychiatry itself—despite its noble mission of repairing fractured brains, mending tortured souls, and restoring peace of mind and wellness. We are proud of our clinical and scientific accomplishments but are painfully cognizant of our limitations and the huge chasm between what we know and what we will eventually know once the brain reveals its glorious mysteries through neuroscientific research.
What can you resolve?
Here is my proposed list of pragmatic resolutions that most psychiatrists would regard as part of a perpetual to-do list—a must-do bucket of cherished goals and brave new horizons to bring complete mental health for our patients and immeasurable gratification for us, who dream of cures for brain disorders that trigger various ailments of the mind.
- Practice like a physician to emphasize the medical foundation of psychiatry: Always check on a patient’s physical health, and monitor his (her) cardiometabolic status. Wear the symbolic white coat that often enhances the physician−patient relationship.
- Dedicate a significant percentage of your practice to the sickest patients. There are enough non-physician mental health professionals to handle the walking wounded and worried well.
- Advocate relentlessly throughout your sphere of influence, and publicly, for true parity between psychiatric and non-mental medical disorders—not only for insurance coverage but for overall societal acceptance and compassion as well.
- Lobby vigorously for hospitalization instead of imprisonment of the seriously mentally ill because psychosis is a brain disease, not a criminal offense.
- Adopt evidence-based psychiatric practice whenever possible to achieve the best outcomes. Judiciously implement off-label practices, however, if no evidence-based treatments exist for a suffering patient.
- Avoid senseless and irrational polypharmacy but do not hesitate to use rational, beneficial combination therapy.
- Provide 1 hour a week of pro bono psychiatric work for the indigent and underserved. The rewards of giving what amounts to 1 week a year are immeasurably more gratifying than a few more dollars in your bank account.
- Resist calling an ill person a ‘client’ or ‘consumer’—at least until oncologists and cardiologists start doing so. Refuse to give up your medical identify in the many de-medicalized mental health clinics.
- Never let a patient leave your office without some psychotherapy, even as part of a 15-minute med-check.
- Stay current and on the cutting edge of evolving psychiatric practice by logging into PubMed every day (even briefly) to read a few abstracts of the latest studies related to patients you saw that day.
- Think like a neurologist by identifying the neural circuits of psychiatric symptoms. Act like a cardiologist by doing everything medically possible to prevent recurrence of psychotic, manic, or depressive episodes because they damage brain tissue just as a myocardial infarction damages the heart.
- Support research with words, money, and passion. Psychiatric neuroscientific breakthroughs generate superior treatments, erase stigma, and advance the quality of life for patients. Donate annually to the researchers of your choice, at the medical school where you were trained, or at a nonprofit research institute.
- Make time to write for publication, annually, at least 1 case report or a letter to the editor about observations from your practice. You can contribute immensely to the discovery process by sharing novel clinical insights.
- Never give up on any patient or set expectations too low, regardless of the diagnosis or severity of illness. Giving up destroys hope and ushers in despondency. Get a second opinion if you run out of options for a patient.
- Always set remission followed by recovery as the therapeutic goal for every patient. Let the patient know this and ask him (her) commit to that goal with you.
- Be genuinely proud to be a psychiatrist. You assess and rectify disorders of the mind, the most complex and magical product of the human brain that determines who we are and how we think, emote, communicate, verbalize, empathize, love, hate, remember, plan, problem-solve, and, of course, make resolutions.
Back to diet and exercise—for our patients and for us!
It’s OK to include, among your New Year’s resolutions, a pledge to strongly encourage patients to diet and exercise. Given the tendency of many of them to gain weight and die prematurely as a consequence of obesity-related cardiometabolic risk factors, you should urge them to eat healthy and exercise every time you see them, not only on New Year’s Day.
Such decisions can be made at any time, but the dawn of a year is a powerful signal of a new beginning—another lease on life, a potential turning point. Imbedded in those resolutions is a subliminal sense of urgency to correct one’s long-neglected shortcomings as the calendar ruthlessly points to inevitable aging and the relentless march of time.
A psychiatric perspective
For psychiatrists, New Year’s resolutions transcend the (often ephemeral) impulse to go on a diet or buy a membership at the local gym. We have a unique perspective on the challenges that our patients face every day as they cope with the complex demands of life despite their anxiety, depression, or psychosis.
We are aware of the many unmet needs in managing complex neuropsychiatric brain disorders and the major challenges of erasing the burdensome stigma that engulfs our patients and the practice of psychiatry itself—despite its noble mission of repairing fractured brains, mending tortured souls, and restoring peace of mind and wellness. We are proud of our clinical and scientific accomplishments but are painfully cognizant of our limitations and the huge chasm between what we know and what we will eventually know once the brain reveals its glorious mysteries through neuroscientific research.
What can you resolve?
Here is my proposed list of pragmatic resolutions that most psychiatrists would regard as part of a perpetual to-do list—a must-do bucket of cherished goals and brave new horizons to bring complete mental health for our patients and immeasurable gratification for us, who dream of cures for brain disorders that trigger various ailments of the mind.
- Practice like a physician to emphasize the medical foundation of psychiatry: Always check on a patient’s physical health, and monitor his (her) cardiometabolic status. Wear the symbolic white coat that often enhances the physician−patient relationship.
- Dedicate a significant percentage of your practice to the sickest patients. There are enough non-physician mental health professionals to handle the walking wounded and worried well.
- Advocate relentlessly throughout your sphere of influence, and publicly, for true parity between psychiatric and non-mental medical disorders—not only for insurance coverage but for overall societal acceptance and compassion as well.
- Lobby vigorously for hospitalization instead of imprisonment of the seriously mentally ill because psychosis is a brain disease, not a criminal offense.
- Adopt evidence-based psychiatric practice whenever possible to achieve the best outcomes. Judiciously implement off-label practices, however, if no evidence-based treatments exist for a suffering patient.
- Avoid senseless and irrational polypharmacy but do not hesitate to use rational, beneficial combination therapy.
- Provide 1 hour a week of pro bono psychiatric work for the indigent and underserved. The rewards of giving what amounts to 1 week a year are immeasurably more gratifying than a few more dollars in your bank account.
- Resist calling an ill person a ‘client’ or ‘consumer’—at least until oncologists and cardiologists start doing so. Refuse to give up your medical identify in the many de-medicalized mental health clinics.
- Never let a patient leave your office without some psychotherapy, even as part of a 15-minute med-check.
- Stay current and on the cutting edge of evolving psychiatric practice by logging into PubMed every day (even briefly) to read a few abstracts of the latest studies related to patients you saw that day.
- Think like a neurologist by identifying the neural circuits of psychiatric symptoms. Act like a cardiologist by doing everything medically possible to prevent recurrence of psychotic, manic, or depressive episodes because they damage brain tissue just as a myocardial infarction damages the heart.
- Support research with words, money, and passion. Psychiatric neuroscientific breakthroughs generate superior treatments, erase stigma, and advance the quality of life for patients. Donate annually to the researchers of your choice, at the medical school where you were trained, or at a nonprofit research institute.
- Make time to write for publication, annually, at least 1 case report or a letter to the editor about observations from your practice. You can contribute immensely to the discovery process by sharing novel clinical insights.
- Never give up on any patient or set expectations too low, regardless of the diagnosis or severity of illness. Giving up destroys hope and ushers in despondency. Get a second opinion if you run out of options for a patient.
- Always set remission followed by recovery as the therapeutic goal for every patient. Let the patient know this and ask him (her) commit to that goal with you.
- Be genuinely proud to be a psychiatrist. You assess and rectify disorders of the mind, the most complex and magical product of the human brain that determines who we are and how we think, emote, communicate, verbalize, empathize, love, hate, remember, plan, problem-solve, and, of course, make resolutions.
Back to diet and exercise—for our patients and for us!
It’s OK to include, among your New Year’s resolutions, a pledge to strongly encourage patients to diet and exercise. Given the tendency of many of them to gain weight and die prematurely as a consequence of obesity-related cardiometabolic risk factors, you should urge them to eat healthy and exercise every time you see them, not only on New Year’s Day.
A checklist of approaches for alleviating behavioral problems in dementia
Dementia—“major neurocognitive disorder” in DSM-5—manifests as progressive decline in cognitive function.In tandem with that decline, approximately 80% of nursing home patients with dementia exhibit behavioral disturbances,1 including irritability, insomnia, wandering, and repetitive questioning.1,2 These disturbances can erode their quality of life and can frustrate caregivers and providers.3
Causative pathology
Before designing a therapeutic intervention for cognitively impaired people with behavioral disturbances, a precise diagnosis of the causative pathology must be determined. This affords therapies that specifically address the patient’s problems. Other related and unrelated somatic or mental health concerns should be identified to specify the optimal approach.
Patients in whom dementia is suspected require that a thorough medical, psychiatric, substance use, and family history be taken to identify predisposing factors for their illness2; exhaustive review of the history might reveal drug interactions or polypharmacy that can cause or exacerbate symptoms, including behavioral manifestations. Physical examination, cognitive function testing, laboratory tests, and neuroimaging also help reveal the etiologic diagnosis of the dementia.1,3
Identifying the diagnosis directs the treatment; for example, a behaviorally discontrolled person with a cognitive, stroke-induced encephalopathy requires an entirely different regimen than a comparatively compromised individual with Alzheimer’s disease or frontotemporal dementia. Early detection of dementia also is helpful for managing its cognitive and behavioral problems more effectively.1Once a diagnosis of dementia is established, it might be behavioral symptoms and poor insight that become more worrisome to the patient’s caregivers and providers than cognitive deficits. Your task is then to apply behavioral approaches to management, with consistency, to maximize, at all times, the patient’s safety and comfort.4
How you approach behavioral management is important
Consider these interventions:
- Ensure that you appropriately treat associated depression, pain, and somatic illness—whether related or unrelated to dementia.
- Offer caregivers and staff a plan for attending to supportive measures, including nutrition, hydration, and socialization.
- Provide family and caregivers with disease education, social support, and management tips1,2; be respectful to family members in all interactions.3
- Offer caregivers and staff a plan for attending to supportive measures, including nutrition, hydration, and socialization.
Minimize psychosocial and environmental stressors
- Avoid unnecessary environmental changes, such as rearranging or refurbishing the patient’s living space.1
- As noted, ensure that the patient is comfortable and safe in his (her) surroundings, such as providing wall-mounted handrails and other aids for ambulation.
- Provide access to television, proper lighting, and other indicated life-enhancing devices.1,2
- Consider a pet for the patient; pets can be an important adjunct in providing comfort.
- Provide music to reduce agitation and anxiety.4
- Appeal to institutional administration to provide a higher staff−patient ratio for comfort and security.2,5
- Because social contact is helpful to build a pleasant environment, preserve opportunities for the patient to communicate with others, and facilitate socialization by encouraging friendly interactions.1
- Provide stimulation and diversion with social activities, support programs, and physical exercise—sources of interaction that can promote health and improve sleep.
- Redirection and validation are helpful to divert a patient’s attention from stressful situations and keep him (her) calm.2,5
- Pharmacotherapy should be implemented if psychosocial methods of behavioral management fail or the patient’s behavior becomes threatening.1
- Provide access to television, proper lighting, and other indicated life-enhancing devices.Provide music to reduce agitation and anxiety.Redirection and validation are helpful to divert a patient’s attention from stressful situations and keep him (her) calm.Pharmacotherapy should be implemented if psychosocial methods of behavioral management fail or the patient’s behavior becomes threatening.
Other considerations
- Identify and treat primary and secondary causes of the underlying major neurocognitive disorder.
- Use an integrative, multidisciplinary approach to manage behavioral problems in dementia.
- Utilize a social worker’s expertise to faciliate family, financial, or related social issues and better cooperation. This promotes comfort for patients, families, and staff.
- Physical therapy aids in maintaining physical function, especially preservation of gait, balance, and range of motion. Thus, with greater stability avoiding a fall can be a life-saving event.
- Socialization, mental outlook, and emotional health are improved by occupational therapist interventions.
- Individual psychotherapy helps to improve self-esteem and personal adjustment. Group activities reinforces interpersonal connections.
- Refer the family and caregivers for supportive therapy and education on dementia; such resources help minimize deleterious effects of the patient’s behavioral problems on those key people.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tampi RR, Williamson D, Muralee S, et al. Behavioral and psychological symptoms of dementia: part I—epidemiology, neurobiology, heritability, and evaluation. Clinical Geriatrics. 2011;19:41-46.
2. Hulme C, Wright J, Crocker T, et al. Non-pharmacological approaches for dementia that informal carers might try or access: a systematic review. Int J Geriatr Psychiatry. 2010;25(7):756-763.
3. Perkins R. Evidence-based practice interventions for managing behavioral and psychological symptoms of dementia in nursing home residents. Ann Longterm Care. 2012;20(12):24.
4. Desai AK, Grossberg GT. Recognition and management of behavioral disturbances in dementia. Prim Care Companion J Clin Psychiatry. 2001;3(3):93-109.
5. Douglas S, James I, Ballard C. Non-pharmacological interventions in dementia. Advances in Psychiatric Treatment. 2004;10(3):171-177.
Dementia—“major neurocognitive disorder” in DSM-5—manifests as progressive decline in cognitive function.In tandem with that decline, approximately 80% of nursing home patients with dementia exhibit behavioral disturbances,1 including irritability, insomnia, wandering, and repetitive questioning.1,2 These disturbances can erode their quality of life and can frustrate caregivers and providers.3
Causative pathology
Before designing a therapeutic intervention for cognitively impaired people with behavioral disturbances, a precise diagnosis of the causative pathology must be determined. This affords therapies that specifically address the patient’s problems. Other related and unrelated somatic or mental health concerns should be identified to specify the optimal approach.
Patients in whom dementia is suspected require that a thorough medical, psychiatric, substance use, and family history be taken to identify predisposing factors for their illness2; exhaustive review of the history might reveal drug interactions or polypharmacy that can cause or exacerbate symptoms, including behavioral manifestations. Physical examination, cognitive function testing, laboratory tests, and neuroimaging also help reveal the etiologic diagnosis of the dementia.1,3
Identifying the diagnosis directs the treatment; for example, a behaviorally discontrolled person with a cognitive, stroke-induced encephalopathy requires an entirely different regimen than a comparatively compromised individual with Alzheimer’s disease or frontotemporal dementia. Early detection of dementia also is helpful for managing its cognitive and behavioral problems more effectively.1Once a diagnosis of dementia is established, it might be behavioral symptoms and poor insight that become more worrisome to the patient’s caregivers and providers than cognitive deficits. Your task is then to apply behavioral approaches to management, with consistency, to maximize, at all times, the patient’s safety and comfort.4
How you approach behavioral management is important
Consider these interventions:
- Ensure that you appropriately treat associated depression, pain, and somatic illness—whether related or unrelated to dementia.
- Offer caregivers and staff a plan for attending to supportive measures, including nutrition, hydration, and socialization.
- Provide family and caregivers with disease education, social support, and management tips1,2; be respectful to family members in all interactions.3
- Offer caregivers and staff a plan for attending to supportive measures, including nutrition, hydration, and socialization.
Minimize psychosocial and environmental stressors
- Avoid unnecessary environmental changes, such as rearranging or refurbishing the patient’s living space.1
- As noted, ensure that the patient is comfortable and safe in his (her) surroundings, such as providing wall-mounted handrails and other aids for ambulation.
- Provide access to television, proper lighting, and other indicated life-enhancing devices.1,2
- Consider a pet for the patient; pets can be an important adjunct in providing comfort.
- Provide music to reduce agitation and anxiety.4
- Appeal to institutional administration to provide a higher staff−patient ratio for comfort and security.2,5
- Because social contact is helpful to build a pleasant environment, preserve opportunities for the patient to communicate with others, and facilitate socialization by encouraging friendly interactions.1
- Provide stimulation and diversion with social activities, support programs, and physical exercise—sources of interaction that can promote health and improve sleep.
- Redirection and validation are helpful to divert a patient’s attention from stressful situations and keep him (her) calm.2,5
- Pharmacotherapy should be implemented if psychosocial methods of behavioral management fail or the patient’s behavior becomes threatening.1
- Provide access to television, proper lighting, and other indicated life-enhancing devices.Provide music to reduce agitation and anxiety.Redirection and validation are helpful to divert a patient’s attention from stressful situations and keep him (her) calm.Pharmacotherapy should be implemented if psychosocial methods of behavioral management fail or the patient’s behavior becomes threatening.
Other considerations
- Identify and treat primary and secondary causes of the underlying major neurocognitive disorder.
- Use an integrative, multidisciplinary approach to manage behavioral problems in dementia.
- Utilize a social worker’s expertise to faciliate family, financial, or related social issues and better cooperation. This promotes comfort for patients, families, and staff.
- Physical therapy aids in maintaining physical function, especially preservation of gait, balance, and range of motion. Thus, with greater stability avoiding a fall can be a life-saving event.
- Socialization, mental outlook, and emotional health are improved by occupational therapist interventions.
- Individual psychotherapy helps to improve self-esteem and personal adjustment. Group activities reinforces interpersonal connections.
- Refer the family and caregivers for supportive therapy and education on dementia; such resources help minimize deleterious effects of the patient’s behavioral problems on those key people.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dementia—“major neurocognitive disorder” in DSM-5—manifests as progressive decline in cognitive function.In tandem with that decline, approximately 80% of nursing home patients with dementia exhibit behavioral disturbances,1 including irritability, insomnia, wandering, and repetitive questioning.1,2 These disturbances can erode their quality of life and can frustrate caregivers and providers.3
Causative pathology
Before designing a therapeutic intervention for cognitively impaired people with behavioral disturbances, a precise diagnosis of the causative pathology must be determined. This affords therapies that specifically address the patient’s problems. Other related and unrelated somatic or mental health concerns should be identified to specify the optimal approach.
Patients in whom dementia is suspected require that a thorough medical, psychiatric, substance use, and family history be taken to identify predisposing factors for their illness2; exhaustive review of the history might reveal drug interactions or polypharmacy that can cause or exacerbate symptoms, including behavioral manifestations. Physical examination, cognitive function testing, laboratory tests, and neuroimaging also help reveal the etiologic diagnosis of the dementia.1,3
Identifying the diagnosis directs the treatment; for example, a behaviorally discontrolled person with a cognitive, stroke-induced encephalopathy requires an entirely different regimen than a comparatively compromised individual with Alzheimer’s disease or frontotemporal dementia. Early detection of dementia also is helpful for managing its cognitive and behavioral problems more effectively.1Once a diagnosis of dementia is established, it might be behavioral symptoms and poor insight that become more worrisome to the patient’s caregivers and providers than cognitive deficits. Your task is then to apply behavioral approaches to management, with consistency, to maximize, at all times, the patient’s safety and comfort.4
How you approach behavioral management is important
Consider these interventions:
- Ensure that you appropriately treat associated depression, pain, and somatic illness—whether related or unrelated to dementia.
- Offer caregivers and staff a plan for attending to supportive measures, including nutrition, hydration, and socialization.
- Provide family and caregivers with disease education, social support, and management tips1,2; be respectful to family members in all interactions.3
- Offer caregivers and staff a plan for attending to supportive measures, including nutrition, hydration, and socialization.
Minimize psychosocial and environmental stressors
- Avoid unnecessary environmental changes, such as rearranging or refurbishing the patient’s living space.1
- As noted, ensure that the patient is comfortable and safe in his (her) surroundings, such as providing wall-mounted handrails and other aids for ambulation.
- Provide access to television, proper lighting, and other indicated life-enhancing devices.1,2
- Consider a pet for the patient; pets can be an important adjunct in providing comfort.
- Provide music to reduce agitation and anxiety.4
- Appeal to institutional administration to provide a higher staff−patient ratio for comfort and security.2,5
- Because social contact is helpful to build a pleasant environment, preserve opportunities for the patient to communicate with others, and facilitate socialization by encouraging friendly interactions.1
- Provide stimulation and diversion with social activities, support programs, and physical exercise—sources of interaction that can promote health and improve sleep.
- Redirection and validation are helpful to divert a patient’s attention from stressful situations and keep him (her) calm.2,5
- Pharmacotherapy should be implemented if psychosocial methods of behavioral management fail or the patient’s behavior becomes threatening.1
- Provide access to television, proper lighting, and other indicated life-enhancing devices.Provide music to reduce agitation and anxiety.Redirection and validation are helpful to divert a patient’s attention from stressful situations and keep him (her) calm.Pharmacotherapy should be implemented if psychosocial methods of behavioral management fail or the patient’s behavior becomes threatening.
Other considerations
- Identify and treat primary and secondary causes of the underlying major neurocognitive disorder.
- Use an integrative, multidisciplinary approach to manage behavioral problems in dementia.
- Utilize a social worker’s expertise to faciliate family, financial, or related social issues and better cooperation. This promotes comfort for patients, families, and staff.
- Physical therapy aids in maintaining physical function, especially preservation of gait, balance, and range of motion. Thus, with greater stability avoiding a fall can be a life-saving event.
- Socialization, mental outlook, and emotional health are improved by occupational therapist interventions.
- Individual psychotherapy helps to improve self-esteem and personal adjustment. Group activities reinforces interpersonal connections.
- Refer the family and caregivers for supportive therapy and education on dementia; such resources help minimize deleterious effects of the patient’s behavioral problems on those key people.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Tampi RR, Williamson D, Muralee S, et al. Behavioral and psychological symptoms of dementia: part I—epidemiology, neurobiology, heritability, and evaluation. Clinical Geriatrics. 2011;19:41-46.
2. Hulme C, Wright J, Crocker T, et al. Non-pharmacological approaches for dementia that informal carers might try or access: a systematic review. Int J Geriatr Psychiatry. 2010;25(7):756-763.
3. Perkins R. Evidence-based practice interventions for managing behavioral and psychological symptoms of dementia in nursing home residents. Ann Longterm Care. 2012;20(12):24.
4. Desai AK, Grossberg GT. Recognition and management of behavioral disturbances in dementia. Prim Care Companion J Clin Psychiatry. 2001;3(3):93-109.
5. Douglas S, James I, Ballard C. Non-pharmacological interventions in dementia. Advances in Psychiatric Treatment. 2004;10(3):171-177.
1. Tampi RR, Williamson D, Muralee S, et al. Behavioral and psychological symptoms of dementia: part I—epidemiology, neurobiology, heritability, and evaluation. Clinical Geriatrics. 2011;19:41-46.
2. Hulme C, Wright J, Crocker T, et al. Non-pharmacological approaches for dementia that informal carers might try or access: a systematic review. Int J Geriatr Psychiatry. 2010;25(7):756-763.
3. Perkins R. Evidence-based practice interventions for managing behavioral and psychological symptoms of dementia in nursing home residents. Ann Longterm Care. 2012;20(12):24.
4. Desai AK, Grossberg GT. Recognition and management of behavioral disturbances in dementia. Prim Care Companion J Clin Psychiatry. 2001;3(3):93-109.
5. Douglas S, James I, Ballard C. Non-pharmacological interventions in dementia. Advances in Psychiatric Treatment. 2004;10(3):171-177.
Self-directed learning
Never before in history has medicine progressed as quickly as it does today. The half-life of knowledge and practices is shortening, and the ocean of literature continues to amass every day. In this context, it is simply not possible for training programs to teach in didactics everything residents must know to become competent, much less excellent, doctors. Self-directed learning has become a critical part of residents’ education.
How can we make self-directed learning a more successful process? Attending physicians are likely to answer with the old saying, ‘You can lead a horse to water, but you can’t make it drink!’ While this saying points to the fact that self-directed learning requires a thirsty horse, it takes for granted the role of the guide in showing where water is plentiful. We argue that residents’ self-directed learning can be made more successful by recognizing the role of attendings in this process.
In an era of infinite resources, the limiting factor to learning has become time. The more we learn, the more humbled we are by the vastness of what we don’t know. Self-directed learners must be smart in deciding what should be learned. Herein lies the value of attendings, who, whether we are aware or not, shape our learning simply by virtue of their example. We would do well to pay closer attention to them. No textbook can replace their vast experience, which allows them to hone in on relevant details, to quickly develop comprehensive differentials, or revise plans.
But this learning cannot be based on simply observing and blindly emulating our teachers. We refer to Dr. Bloom’s taxonomy for levels of cognitive learning, in saying that these steps will only get us to the most basic levels of learning, which is “knowing” a disease to the extent that we can apply that knowledge in patient care. These can be acquired without significant mental effort; just by listening to morning reports, reading quick tidbits in between taking care of patients, etc. The goal, however, should be utilizing this basic knowledge as a foundation to develop higher levels of learning, namely Analysis, Synthesis, and Evaluation.
An example for analysis would be quickly going over each of the differentials in a disease and learning what distinguishes them. Synthesis is integrating different ideas and creating a customized plan for the particular patient that is found in no book. Lastly, evaluation is the level of cognition needed to be able to appraise and critique the large volume of opinion that we come across, establish our own opinion, and be able to defend it.
Here again our attendings are valuable resources who can guide us in reaching each of these levels. We must be willing to challenge ourselves by challenging our attendings when things do not make sense. It means always questioning why your attending physician made one medical decision versus another. It means also to challenge what we think we know, in order to discover what we don’t know. … Returning to the old adage, perhaps the key to self-directed learning is for the horse to learn his masters’ ways to the well, so he may adapt to an ever-changing environment.
Dr. Hung and Dr. Ramakrishna are pediatric residents at the Metrohealth Medical Center in Cleveland, Ohio. Email them at [email protected].
Never before in history has medicine progressed as quickly as it does today. The half-life of knowledge and practices is shortening, and the ocean of literature continues to amass every day. In this context, it is simply not possible for training programs to teach in didactics everything residents must know to become competent, much less excellent, doctors. Self-directed learning has become a critical part of residents’ education.
How can we make self-directed learning a more successful process? Attending physicians are likely to answer with the old saying, ‘You can lead a horse to water, but you can’t make it drink!’ While this saying points to the fact that self-directed learning requires a thirsty horse, it takes for granted the role of the guide in showing where water is plentiful. We argue that residents’ self-directed learning can be made more successful by recognizing the role of attendings in this process.
In an era of infinite resources, the limiting factor to learning has become time. The more we learn, the more humbled we are by the vastness of what we don’t know. Self-directed learners must be smart in deciding what should be learned. Herein lies the value of attendings, who, whether we are aware or not, shape our learning simply by virtue of their example. We would do well to pay closer attention to them. No textbook can replace their vast experience, which allows them to hone in on relevant details, to quickly develop comprehensive differentials, or revise plans.
But this learning cannot be based on simply observing and blindly emulating our teachers. We refer to Dr. Bloom’s taxonomy for levels of cognitive learning, in saying that these steps will only get us to the most basic levels of learning, which is “knowing” a disease to the extent that we can apply that knowledge in patient care. These can be acquired without significant mental effort; just by listening to morning reports, reading quick tidbits in between taking care of patients, etc. The goal, however, should be utilizing this basic knowledge as a foundation to develop higher levels of learning, namely Analysis, Synthesis, and Evaluation.
An example for analysis would be quickly going over each of the differentials in a disease and learning what distinguishes them. Synthesis is integrating different ideas and creating a customized plan for the particular patient that is found in no book. Lastly, evaluation is the level of cognition needed to be able to appraise and critique the large volume of opinion that we come across, establish our own opinion, and be able to defend it.
Here again our attendings are valuable resources who can guide us in reaching each of these levels. We must be willing to challenge ourselves by challenging our attendings when things do not make sense. It means always questioning why your attending physician made one medical decision versus another. It means also to challenge what we think we know, in order to discover what we don’t know. … Returning to the old adage, perhaps the key to self-directed learning is for the horse to learn his masters’ ways to the well, so he may adapt to an ever-changing environment.
Dr. Hung and Dr. Ramakrishna are pediatric residents at the Metrohealth Medical Center in Cleveland, Ohio. Email them at [email protected].
Never before in history has medicine progressed as quickly as it does today. The half-life of knowledge and practices is shortening, and the ocean of literature continues to amass every day. In this context, it is simply not possible for training programs to teach in didactics everything residents must know to become competent, much less excellent, doctors. Self-directed learning has become a critical part of residents’ education.
How can we make self-directed learning a more successful process? Attending physicians are likely to answer with the old saying, ‘You can lead a horse to water, but you can’t make it drink!’ While this saying points to the fact that self-directed learning requires a thirsty horse, it takes for granted the role of the guide in showing where water is plentiful. We argue that residents’ self-directed learning can be made more successful by recognizing the role of attendings in this process.
In an era of infinite resources, the limiting factor to learning has become time. The more we learn, the more humbled we are by the vastness of what we don’t know. Self-directed learners must be smart in deciding what should be learned. Herein lies the value of attendings, who, whether we are aware or not, shape our learning simply by virtue of their example. We would do well to pay closer attention to them. No textbook can replace their vast experience, which allows them to hone in on relevant details, to quickly develop comprehensive differentials, or revise plans.
But this learning cannot be based on simply observing and blindly emulating our teachers. We refer to Dr. Bloom’s taxonomy for levels of cognitive learning, in saying that these steps will only get us to the most basic levels of learning, which is “knowing” a disease to the extent that we can apply that knowledge in patient care. These can be acquired without significant mental effort; just by listening to morning reports, reading quick tidbits in between taking care of patients, etc. The goal, however, should be utilizing this basic knowledge as a foundation to develop higher levels of learning, namely Analysis, Synthesis, and Evaluation.
An example for analysis would be quickly going over each of the differentials in a disease and learning what distinguishes them. Synthesis is integrating different ideas and creating a customized plan for the particular patient that is found in no book. Lastly, evaluation is the level of cognition needed to be able to appraise and critique the large volume of opinion that we come across, establish our own opinion, and be able to defend it.
Here again our attendings are valuable resources who can guide us in reaching each of these levels. We must be willing to challenge ourselves by challenging our attendings when things do not make sense. It means always questioning why your attending physician made one medical decision versus another. It means also to challenge what we think we know, in order to discover what we don’t know. … Returning to the old adage, perhaps the key to self-directed learning is for the horse to learn his masters’ ways to the well, so he may adapt to an ever-changing environment.
Dr. Hung and Dr. Ramakrishna are pediatric residents at the Metrohealth Medical Center in Cleveland, Ohio. Email them at [email protected].