User login
De novo mutation linked to FA subtype
with Fanconi anemia
Researchers say they have established the cause of a subtype of Fanconi anemia (FA)—a de novo mutation in 1 allele of RAD51, a gene responsible for repairing DNA damage.
The team made this discovery in a child with an FA-like syndrome who has healthy parents and a healthy sister.
“The particular mutation in this patient was a surprise to us,” said Patrick May, PhD, of the University of Luxembourg.
“It occurred only in 1 of the 2 RAD51 gene copies, which every person carries in the genome, but every RAD51 gene copy was normal in the child’s parents.”
Dr May and his colleagues described the mutation in Nature Communications.
Specifically, the researchers found a de novo g.41022153G>A; p.Ala293Thr (NM_002875) missense mutation in 1 allele of RAD51.
They said this heterozygous mutation causes a novel FA subtype, dubbed “FA-R,” which appears to be the first subtype of FA caused by a dominant-negative mutation.
The patient the researchers analyzed has microcephaly and mental retardation but has reached adulthood without the bone marrow failure and pediatric cancers typically observed in patients with FA.
Until this case, researchers believed that mutations leading to FA showed recessive inheritance and therefore had to be derived from both parents to lead to FA. Spontaneous mutations of the RAD51 gene like in this case had not been observed.
Dr May and his colleagues said their finding has implications for genetic counseling of families with a high risk for FA. Previously, people who wanted to have children but had relatives suffering from FA were screened for mutations in 1 of the 17 genes connected with the disease. Now, the risk of having a child with FA has to be recalculated.
“Furthermore, understanding this mutation teaches us more about how the RAD51 gene product protects the DNA and how disruptions of DNA repair may lead to leukemia and solid tumors,” Dr May said. “Of course, understanding the origins of human cancer will help us diagnose it with more confidence earlier and devise new therapies to prevent or mitigate it.” ![]()

with Fanconi anemia
Researchers say they have established the cause of a subtype of Fanconi anemia (FA)—a de novo mutation in 1 allele of RAD51, a gene responsible for repairing DNA damage.
The team made this discovery in a child with an FA-like syndrome who has healthy parents and a healthy sister.
“The particular mutation in this patient was a surprise to us,” said Patrick May, PhD, of the University of Luxembourg.
“It occurred only in 1 of the 2 RAD51 gene copies, which every person carries in the genome, but every RAD51 gene copy was normal in the child’s parents.”
Dr May and his colleagues described the mutation in Nature Communications.
Specifically, the researchers found a de novo g.41022153G>A; p.Ala293Thr (NM_002875) missense mutation in 1 allele of RAD51.
They said this heterozygous mutation causes a novel FA subtype, dubbed “FA-R,” which appears to be the first subtype of FA caused by a dominant-negative mutation.
The patient the researchers analyzed has microcephaly and mental retardation but has reached adulthood without the bone marrow failure and pediatric cancers typically observed in patients with FA.
Until this case, researchers believed that mutations leading to FA showed recessive inheritance and therefore had to be derived from both parents to lead to FA. Spontaneous mutations of the RAD51 gene like in this case had not been observed.
Dr May and his colleagues said their finding has implications for genetic counseling of families with a high risk for FA. Previously, people who wanted to have children but had relatives suffering from FA were screened for mutations in 1 of the 17 genes connected with the disease. Now, the risk of having a child with FA has to be recalculated.
“Furthermore, understanding this mutation teaches us more about how the RAD51 gene product protects the DNA and how disruptions of DNA repair may lead to leukemia and solid tumors,” Dr May said. “Of course, understanding the origins of human cancer will help us diagnose it with more confidence earlier and devise new therapies to prevent or mitigate it.” ![]()

with Fanconi anemia
Researchers say they have established the cause of a subtype of Fanconi anemia (FA)—a de novo mutation in 1 allele of RAD51, a gene responsible for repairing DNA damage.
The team made this discovery in a child with an FA-like syndrome who has healthy parents and a healthy sister.
“The particular mutation in this patient was a surprise to us,” said Patrick May, PhD, of the University of Luxembourg.
“It occurred only in 1 of the 2 RAD51 gene copies, which every person carries in the genome, but every RAD51 gene copy was normal in the child’s parents.”
Dr May and his colleagues described the mutation in Nature Communications.
Specifically, the researchers found a de novo g.41022153G>A; p.Ala293Thr (NM_002875) missense mutation in 1 allele of RAD51.
They said this heterozygous mutation causes a novel FA subtype, dubbed “FA-R,” which appears to be the first subtype of FA caused by a dominant-negative mutation.
The patient the researchers analyzed has microcephaly and mental retardation but has reached adulthood without the bone marrow failure and pediatric cancers typically observed in patients with FA.
Until this case, researchers believed that mutations leading to FA showed recessive inheritance and therefore had to be derived from both parents to lead to FA. Spontaneous mutations of the RAD51 gene like in this case had not been observed.
Dr May and his colleagues said their finding has implications for genetic counseling of families with a high risk for FA. Previously, people who wanted to have children but had relatives suffering from FA were screened for mutations in 1 of the 17 genes connected with the disease. Now, the risk of having a child with FA has to be recalculated.
“Furthermore, understanding this mutation teaches us more about how the RAD51 gene product protects the DNA and how disruptions of DNA repair may lead to leukemia and solid tumors,” Dr May said. “Of course, understanding the origins of human cancer will help us diagnose it with more confidence earlier and devise new therapies to prevent or mitigate it.” ![]()
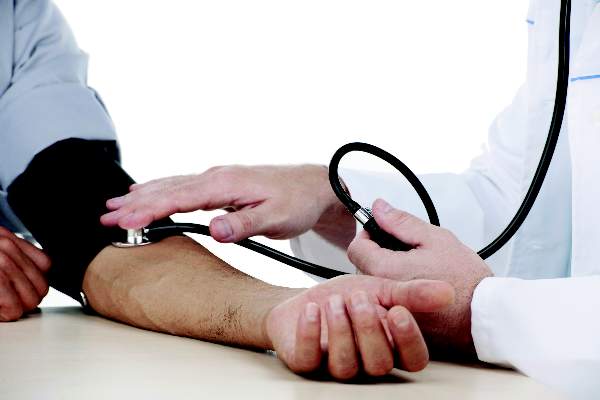
Women can take anticoagulants and hormones safely, study suggests

A study published in Blood indicates that women on anticoagulants can take estrogen-containing contraception or hormone replacement therapy
without an increased risk of venous thromboembolism (VTE) or uterine bleeding.
If a woman is diagnosed with VTE, she is often advised to stop hormone therapy, even while receiving anticoagulant therapy, because she is thought to have an increased risk of VTE recurrence.
However, this practice is based on the known association between hormone therapy and increased clotting risk in the absence of anticoagulants. The safety of the concurrent use of these medications had not been previously explored.
“While it has been common practice among healthcare providers to avoid prescribing hormone therapy and anticoagulants at the same time, there has been no evidence to support this decision,” said study author Ida Martinelli, MD, of the A. Bianchi Bonomi Hemophilia and Thrombosis Center in Milan, Italy.
“We conducted this study to address the fear felt by both the physician and patient when making the decision to stop or continue hormone therapy in this setting.”
The researchers compared the incidences of recurrent VTE and abnormal uterine bleeding in 1888 women who received anticoagulants with or without concurrent hormone therapy.
The team analyzed data from the EINSTEIN DVT and PE study, which was performed to evaluate the safety and efficacy of 2 anticoagulants—the direct oral anticoagulant rivaroxaban and the current standard of care, a low-molecular-weight heparin (enoxaparin) followed by a vitamin K antagonist (VKA).
Women of child-bearing potential were advised to use adequate methods of contraception to avoid birth defects.
Of all the women in the study, 475 used hormone therapy during the analysis period. Medications used included estrogen-only pills, combined estrogen-progestogen contraceptives, and progestin-only contraceptives.
Participants were questioned about symptoms or signs of recurrent VTE and bleeding, including uterine bleeding, during each follow-up visit.
Seven recurrent VTEs occurred while patients were using hormone therapy, while 38 events occurred during a period when patients were not using hormone therapy.
Based on this analysis, the researchers concluded that women on anticoagulants and hormone therapy experienced recurrent VTE at a rate of 3.7% per year. In contrast, those not on hormone therapy had a recurrence rate of 4.7% per year.
The incidence of abnormal uterine bleeding in patients on hormonal therapy was 22.5%, compared to 21.4% for women not on hormone therapy.
According to the study authors, the similar incidence of VTE and abnormal uterine bleeding in women who did and did not receive hormone therapy suggest that combined use of these therapies is safe.
The study also showed that abnormal uterine bleeding occurred more frequently with rivaroxaban than with enoxaparin/VKA. The bleeding rate was estimated at 29.8% per year for patients on rivaroxaban and 15.5% per year in the enoxaparin/VKA group.
The researchers said this outcome suggests the need for further studies on rivaroxaban, which may be preferred for its convenience over enoxaparin/VKA.
“For the first time, we demonstrate that women suffering from blood clots can safely take hormone-containing contraceptives or hormone replacement therapy with anticoagulants, providing women the freedom to choose the method of birth control and other hormone-containing medications they prefer,” Dr Martinelli said.
“While further investigation is needed to evaluate the inconvenience of abnormal uterine bleeding with rivaroxaban and the other direct oral anticoagulants, these results dispel former misconceptions and should allow clinicians to confidently treat their patients who take blood thinners and hormones concurrently.” ![]()

A study published in Blood indicates that women on anticoagulants can take estrogen-containing contraception or hormone replacement therapy
without an increased risk of venous thromboembolism (VTE) or uterine bleeding.
If a woman is diagnosed with VTE, she is often advised to stop hormone therapy, even while receiving anticoagulant therapy, because she is thought to have an increased risk of VTE recurrence.
However, this practice is based on the known association between hormone therapy and increased clotting risk in the absence of anticoagulants. The safety of the concurrent use of these medications had not been previously explored.
“While it has been common practice among healthcare providers to avoid prescribing hormone therapy and anticoagulants at the same time, there has been no evidence to support this decision,” said study author Ida Martinelli, MD, of the A. Bianchi Bonomi Hemophilia and Thrombosis Center in Milan, Italy.
“We conducted this study to address the fear felt by both the physician and patient when making the decision to stop or continue hormone therapy in this setting.”
The researchers compared the incidences of recurrent VTE and abnormal uterine bleeding in 1888 women who received anticoagulants with or without concurrent hormone therapy.
The team analyzed data from the EINSTEIN DVT and PE study, which was performed to evaluate the safety and efficacy of 2 anticoagulants—the direct oral anticoagulant rivaroxaban and the current standard of care, a low-molecular-weight heparin (enoxaparin) followed by a vitamin K antagonist (VKA).
Women of child-bearing potential were advised to use adequate methods of contraception to avoid birth defects.
Of all the women in the study, 475 used hormone therapy during the analysis period. Medications used included estrogen-only pills, combined estrogen-progestogen contraceptives, and progestin-only contraceptives.
Participants were questioned about symptoms or signs of recurrent VTE and bleeding, including uterine bleeding, during each follow-up visit.
Seven recurrent VTEs occurred while patients were using hormone therapy, while 38 events occurred during a period when patients were not using hormone therapy.
Based on this analysis, the researchers concluded that women on anticoagulants and hormone therapy experienced recurrent VTE at a rate of 3.7% per year. In contrast, those not on hormone therapy had a recurrence rate of 4.7% per year.
The incidence of abnormal uterine bleeding in patients on hormonal therapy was 22.5%, compared to 21.4% for women not on hormone therapy.
According to the study authors, the similar incidence of VTE and abnormal uterine bleeding in women who did and did not receive hormone therapy suggest that combined use of these therapies is safe.
The study also showed that abnormal uterine bleeding occurred more frequently with rivaroxaban than with enoxaparin/VKA. The bleeding rate was estimated at 29.8% per year for patients on rivaroxaban and 15.5% per year in the enoxaparin/VKA group.
The researchers said this outcome suggests the need for further studies on rivaroxaban, which may be preferred for its convenience over enoxaparin/VKA.
“For the first time, we demonstrate that women suffering from blood clots can safely take hormone-containing contraceptives or hormone replacement therapy with anticoagulants, providing women the freedom to choose the method of birth control and other hormone-containing medications they prefer,” Dr Martinelli said.
“While further investigation is needed to evaluate the inconvenience of abnormal uterine bleeding with rivaroxaban and the other direct oral anticoagulants, these results dispel former misconceptions and should allow clinicians to confidently treat their patients who take blood thinners and hormones concurrently.” ![]()

A study published in Blood indicates that women on anticoagulants can take estrogen-containing contraception or hormone replacement therapy
without an increased risk of venous thromboembolism (VTE) or uterine bleeding.
If a woman is diagnosed with VTE, she is often advised to stop hormone therapy, even while receiving anticoagulant therapy, because she is thought to have an increased risk of VTE recurrence.
However, this practice is based on the known association between hormone therapy and increased clotting risk in the absence of anticoagulants. The safety of the concurrent use of these medications had not been previously explored.
“While it has been common practice among healthcare providers to avoid prescribing hormone therapy and anticoagulants at the same time, there has been no evidence to support this decision,” said study author Ida Martinelli, MD, of the A. Bianchi Bonomi Hemophilia and Thrombosis Center in Milan, Italy.
“We conducted this study to address the fear felt by both the physician and patient when making the decision to stop or continue hormone therapy in this setting.”
The researchers compared the incidences of recurrent VTE and abnormal uterine bleeding in 1888 women who received anticoagulants with or without concurrent hormone therapy.
The team analyzed data from the EINSTEIN DVT and PE study, which was performed to evaluate the safety and efficacy of 2 anticoagulants—the direct oral anticoagulant rivaroxaban and the current standard of care, a low-molecular-weight heparin (enoxaparin) followed by a vitamin K antagonist (VKA).
Women of child-bearing potential were advised to use adequate methods of contraception to avoid birth defects.
Of all the women in the study, 475 used hormone therapy during the analysis period. Medications used included estrogen-only pills, combined estrogen-progestogen contraceptives, and progestin-only contraceptives.
Participants were questioned about symptoms or signs of recurrent VTE and bleeding, including uterine bleeding, during each follow-up visit.
Seven recurrent VTEs occurred while patients were using hormone therapy, while 38 events occurred during a period when patients were not using hormone therapy.
Based on this analysis, the researchers concluded that women on anticoagulants and hormone therapy experienced recurrent VTE at a rate of 3.7% per year. In contrast, those not on hormone therapy had a recurrence rate of 4.7% per year.
The incidence of abnormal uterine bleeding in patients on hormonal therapy was 22.5%, compared to 21.4% for women not on hormone therapy.
According to the study authors, the similar incidence of VTE and abnormal uterine bleeding in women who did and did not receive hormone therapy suggest that combined use of these therapies is safe.
The study also showed that abnormal uterine bleeding occurred more frequently with rivaroxaban than with enoxaparin/VKA. The bleeding rate was estimated at 29.8% per year for patients on rivaroxaban and 15.5% per year in the enoxaparin/VKA group.
The researchers said this outcome suggests the need for further studies on rivaroxaban, which may be preferred for its convenience over enoxaparin/VKA.
“For the first time, we demonstrate that women suffering from blood clots can safely take hormone-containing contraceptives or hormone replacement therapy with anticoagulants, providing women the freedom to choose the method of birth control and other hormone-containing medications they prefer,” Dr Martinelli said.
“While further investigation is needed to evaluate the inconvenience of abnormal uterine bleeding with rivaroxaban and the other direct oral anticoagulants, these results dispel former misconceptions and should allow clinicians to confidently treat their patients who take blood thinners and hormones concurrently.” ![]()
ACP Guidelines for Evaluation of Suspected Pulmonary Embolism
Clinical question: What are best practices for evaluating patients with suspected acute pulmonary embolism (PE)?
Background: Use of CT in the evaluation of PE has increased across all clinical settings without improving mortality. Contrast CT carries the risks of radiation exposure, contrast-induced nephropathy, and incidental findings that require further investigation. The authors highlight evidence-based strategies for evaluation of PE, focusing on delivering high-value care.
Study design: Clinical guideline.
Setting: Literature review of studies across all adult clinical settings.
Synopsis: The clinical guidelines committee of the American College of Physicians conducted a literature search surrounding evaluation of suspected acute PE. From their review, they concluded:
- Pretest probability should initially be determined based on validated prediction tools (Wells score, Revised Geneva);
- In patients found to have low pretest probability and meeting the pulmonary embolism rule-out criteria (PERC), clinicians can forego d-dimer testing;
- In those with intermediate pretest probability or those with low pre-test probability who do not pass PERC, d-dimer measurement should be obtained;
- The d-dimer threshold should be age adjusted and imaging should not be pursued in patients whose d-dimer level falls below this cutoff, while those with positive d-dimers should receive CT pulmonary angiography (CTPA); and
- Patients with high pretest probability should undergo CTPA (or V/Q scan if CTPA is contraindicated) without d-dimer testing.
Bottom line: In suspected acute PE, first determine pretest probability using Wells and Revised Geneva, and then use this probability in conjunction with the PERC and d-dimer (as indicated) to guide decisions about imaging.
Citation: Raja AS, Greenberg JO, Qaseem A, Denberg TD, Fitterman N, Schuur JD. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2015;163(9):701-711.
Clinical question: What are best practices for evaluating patients with suspected acute pulmonary embolism (PE)?
Background: Use of CT in the evaluation of PE has increased across all clinical settings without improving mortality. Contrast CT carries the risks of radiation exposure, contrast-induced nephropathy, and incidental findings that require further investigation. The authors highlight evidence-based strategies for evaluation of PE, focusing on delivering high-value care.
Study design: Clinical guideline.
Setting: Literature review of studies across all adult clinical settings.
Synopsis: The clinical guidelines committee of the American College of Physicians conducted a literature search surrounding evaluation of suspected acute PE. From their review, they concluded:
- Pretest probability should initially be determined based on validated prediction tools (Wells score, Revised Geneva);
- In patients found to have low pretest probability and meeting the pulmonary embolism rule-out criteria (PERC), clinicians can forego d-dimer testing;
- In those with intermediate pretest probability or those with low pre-test probability who do not pass PERC, d-dimer measurement should be obtained;
- The d-dimer threshold should be age adjusted and imaging should not be pursued in patients whose d-dimer level falls below this cutoff, while those with positive d-dimers should receive CT pulmonary angiography (CTPA); and
- Patients with high pretest probability should undergo CTPA (or V/Q scan if CTPA is contraindicated) without d-dimer testing.
Bottom line: In suspected acute PE, first determine pretest probability using Wells and Revised Geneva, and then use this probability in conjunction with the PERC and d-dimer (as indicated) to guide decisions about imaging.
Citation: Raja AS, Greenberg JO, Qaseem A, Denberg TD, Fitterman N, Schuur JD. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2015;163(9):701-711.
Clinical question: What are best practices for evaluating patients with suspected acute pulmonary embolism (PE)?
Background: Use of CT in the evaluation of PE has increased across all clinical settings without improving mortality. Contrast CT carries the risks of radiation exposure, contrast-induced nephropathy, and incidental findings that require further investigation. The authors highlight evidence-based strategies for evaluation of PE, focusing on delivering high-value care.
Study design: Clinical guideline.
Setting: Literature review of studies across all adult clinical settings.
Synopsis: The clinical guidelines committee of the American College of Physicians conducted a literature search surrounding evaluation of suspected acute PE. From their review, they concluded:
- Pretest probability should initially be determined based on validated prediction tools (Wells score, Revised Geneva);
- In patients found to have low pretest probability and meeting the pulmonary embolism rule-out criteria (PERC), clinicians can forego d-dimer testing;
- In those with intermediate pretest probability or those with low pre-test probability who do not pass PERC, d-dimer measurement should be obtained;
- The d-dimer threshold should be age adjusted and imaging should not be pursued in patients whose d-dimer level falls below this cutoff, while those with positive d-dimers should receive CT pulmonary angiography (CTPA); and
- Patients with high pretest probability should undergo CTPA (or V/Q scan if CTPA is contraindicated) without d-dimer testing.
Bottom line: In suspected acute PE, first determine pretest probability using Wells and Revised Geneva, and then use this probability in conjunction with the PERC and d-dimer (as indicated) to guide decisions about imaging.
Citation: Raja AS, Greenberg JO, Qaseem A, Denberg TD, Fitterman N, Schuur JD. Evaluation of patients with suspected acute pulmonary embolism: best practice advice from the clinical guidelines committee of the American College of Physicians. Ann Intern Med. 2015;163(9):701-711.
Early Palliative Care Can Save Money
Clinical question: Does time to consult after admission change the effect palliative care consultation has on cost of care?
Background: Studies have shown that early palliative care involvement improves quality of life and survival among cancer patients while reducing the cost of care. Little is known about the optimal timing of palliative care consultation and its effect on cost.
Study design: Prospective, observational study.
Setting: Multi-site, high-volume, tertiary care hospitals with established palliative care teams.
Synopsis: Clinical and cost data were collected for 969 adult patients with advanced cancer admitted to the five participating hospitals. Among those, 256 patients received palliative care consultation and 713 received usual care. Subsamples were created based on time to consultation after admission.
The study found that earlier consultation yielded larger effects on cost savings. There was a 24% reduction in total cost if consultation occurred within two days (95% CI, -$3,438 to -$1,122; P<0.001), with estimated savings of $2,280. For consultation within six days of admission, there was a $1,312 savings (95% CI, -$2,568 to -$ 1,122; P<0.04), consistent with a 14% reduction in total cost.
There are notable limitations to this study. Half of eligible patients were excluded due to incomplete data collection, resulting in a small sample size. Further, these results can be generalized only to inpatients with advanced cancer.
Bottom line: Reducing the time to consultation with palliative care increases cost savings. In advanced cancer patients, a 24% reduction in total costs was realized for consultation within two days following admission.
Citation: May P, Garrido MM, Cassel JB, et al. Prospective cohort study of hospital palliative care teams for inpatients with advanced cancer: earlier consultation is associated with larger cost-saving effect. J Clin Oncol. 2015;33(25):2745-2752.
Clinical question: Does time to consult after admission change the effect palliative care consultation has on cost of care?
Background: Studies have shown that early palliative care involvement improves quality of life and survival among cancer patients while reducing the cost of care. Little is known about the optimal timing of palliative care consultation and its effect on cost.
Study design: Prospective, observational study.
Setting: Multi-site, high-volume, tertiary care hospitals with established palliative care teams.
Synopsis: Clinical and cost data were collected for 969 adult patients with advanced cancer admitted to the five participating hospitals. Among those, 256 patients received palliative care consultation and 713 received usual care. Subsamples were created based on time to consultation after admission.
The study found that earlier consultation yielded larger effects on cost savings. There was a 24% reduction in total cost if consultation occurred within two days (95% CI, -$3,438 to -$1,122; P<0.001), with estimated savings of $2,280. For consultation within six days of admission, there was a $1,312 savings (95% CI, -$2,568 to -$ 1,122; P<0.04), consistent with a 14% reduction in total cost.
There are notable limitations to this study. Half of eligible patients were excluded due to incomplete data collection, resulting in a small sample size. Further, these results can be generalized only to inpatients with advanced cancer.
Bottom line: Reducing the time to consultation with palliative care increases cost savings. In advanced cancer patients, a 24% reduction in total costs was realized for consultation within two days following admission.
Citation: May P, Garrido MM, Cassel JB, et al. Prospective cohort study of hospital palliative care teams for inpatients with advanced cancer: earlier consultation is associated with larger cost-saving effect. J Clin Oncol. 2015;33(25):2745-2752.
Clinical question: Does time to consult after admission change the effect palliative care consultation has on cost of care?
Background: Studies have shown that early palliative care involvement improves quality of life and survival among cancer patients while reducing the cost of care. Little is known about the optimal timing of palliative care consultation and its effect on cost.
Study design: Prospective, observational study.
Setting: Multi-site, high-volume, tertiary care hospitals with established palliative care teams.
Synopsis: Clinical and cost data were collected for 969 adult patients with advanced cancer admitted to the five participating hospitals. Among those, 256 patients received palliative care consultation and 713 received usual care. Subsamples were created based on time to consultation after admission.
The study found that earlier consultation yielded larger effects on cost savings. There was a 24% reduction in total cost if consultation occurred within two days (95% CI, -$3,438 to -$1,122; P<0.001), with estimated savings of $2,280. For consultation within six days of admission, there was a $1,312 savings (95% CI, -$2,568 to -$ 1,122; P<0.04), consistent with a 14% reduction in total cost.
There are notable limitations to this study. Half of eligible patients were excluded due to incomplete data collection, resulting in a small sample size. Further, these results can be generalized only to inpatients with advanced cancer.
Bottom line: Reducing the time to consultation with palliative care increases cost savings. In advanced cancer patients, a 24% reduction in total costs was realized for consultation within two days following admission.
Citation: May P, Garrido MM, Cassel JB, et al. Prospective cohort study of hospital palliative care teams for inpatients with advanced cancer: earlier consultation is associated with larger cost-saving effect. J Clin Oncol. 2015;33(25):2745-2752.
Changes to Healthcare that Hospitalists Should Expect in 2016
On the heels of last year’s repeal of the sustainable growth rate (SGR) formula, 2016 promises to be a year of significant changes for the healthcare system. These changes will require providers to focus not just on the immediate pressures and requirements coming from Medicare, of which there are many, but also to look down the road to how things will change in the coming years.
The final year of reporting on quality measures for the Physician Quality Reporting System (PQRS) is 2016, with performance impacting Medicare payments in 2018. Reporting on quality measures doesn’t end there, however. The Medicare Access and CHIP Reauthorization Act (MACRA) repealed the SGR and created two new pathways for pay-for-performance for physicians and most other providers: the Merit-based Incentive Payment System (MIPS) and alternative payment models. After this year, reporting quality measures becomes one component of the MIPS, a program similar to hospital value-based purchasing, but designed for providers.
Quality measures are here to stay. They form the backbone for evaluating whether healthcare is of value. Under the MIPS, quality measures are combined with cost measures, meaningful use, and clinical performance improvement activities to create an aggregate score for providers. That score will be used to determine payment adjustments for providers starting in 2019.
Also in 2016, the Centers for Medicare and Medicaid Services (CMS) will lay the foundation for the MIPS. It is a completely new program, and although it will build on elements of existing programs like PQRS, meaningful use, and the physician value-based payment modifier, its structure and ramifications are ultimately unknown. CMS has indicated its intention to issue the regulatory backbone of MIPS in just a few months. These regulations will be the new reality of Medicare’s fee-for-service for the foreseeable future.
The ramifications of MIPS cannot be understated. It will apply an adjustment based on performance on all Medicare Part B payments. That adjustment starts at +/- 4.0% in 2019 and rises to +/- 9.0% by 2022, a number that is not as far off as it seems based on how these programs operate.
SHM expects many of the current PQRS policies to be continued under MIPS, which means, unfortunately, that many of the challenges facing hospitalists will continue. Hospitalists do not have many measures to report on; most measures are developed for outpatient practices, are simply not reflective of the variability of hospitalist practice, and, even if specified for inpatient reporting, are not clinically relevant.
To meet the needs of hospitalists, SHM will advocate strongly for CMS to develop more flexible and relevant reporting options. We will work to ensure that hospitalists are not structurally disadvantaged by the policies set in place.
Given these upcoming changes, it is as important as ever for you to stay engaged and informed about the policy changes coming down the road. It might be just the start of the year, but already there’s a lot of critical work to do. To get involved and remain apprised of the changes, join SHM’s grassroots network at www.hospitalmedicine.org/grassroots. TH
Joshua Lapps is SHM’s government relations manager.
On the heels of last year’s repeal of the sustainable growth rate (SGR) formula, 2016 promises to be a year of significant changes for the healthcare system. These changes will require providers to focus not just on the immediate pressures and requirements coming from Medicare, of which there are many, but also to look down the road to how things will change in the coming years.
The final year of reporting on quality measures for the Physician Quality Reporting System (PQRS) is 2016, with performance impacting Medicare payments in 2018. Reporting on quality measures doesn’t end there, however. The Medicare Access and CHIP Reauthorization Act (MACRA) repealed the SGR and created two new pathways for pay-for-performance for physicians and most other providers: the Merit-based Incentive Payment System (MIPS) and alternative payment models. After this year, reporting quality measures becomes one component of the MIPS, a program similar to hospital value-based purchasing, but designed for providers.
Quality measures are here to stay. They form the backbone for evaluating whether healthcare is of value. Under the MIPS, quality measures are combined with cost measures, meaningful use, and clinical performance improvement activities to create an aggregate score for providers. That score will be used to determine payment adjustments for providers starting in 2019.
Also in 2016, the Centers for Medicare and Medicaid Services (CMS) will lay the foundation for the MIPS. It is a completely new program, and although it will build on elements of existing programs like PQRS, meaningful use, and the physician value-based payment modifier, its structure and ramifications are ultimately unknown. CMS has indicated its intention to issue the regulatory backbone of MIPS in just a few months. These regulations will be the new reality of Medicare’s fee-for-service for the foreseeable future.
The ramifications of MIPS cannot be understated. It will apply an adjustment based on performance on all Medicare Part B payments. That adjustment starts at +/- 4.0% in 2019 and rises to +/- 9.0% by 2022, a number that is not as far off as it seems based on how these programs operate.
SHM expects many of the current PQRS policies to be continued under MIPS, which means, unfortunately, that many of the challenges facing hospitalists will continue. Hospitalists do not have many measures to report on; most measures are developed for outpatient practices, are simply not reflective of the variability of hospitalist practice, and, even if specified for inpatient reporting, are not clinically relevant.
To meet the needs of hospitalists, SHM will advocate strongly for CMS to develop more flexible and relevant reporting options. We will work to ensure that hospitalists are not structurally disadvantaged by the policies set in place.
Given these upcoming changes, it is as important as ever for you to stay engaged and informed about the policy changes coming down the road. It might be just the start of the year, but already there’s a lot of critical work to do. To get involved and remain apprised of the changes, join SHM’s grassroots network at www.hospitalmedicine.org/grassroots. TH
Joshua Lapps is SHM’s government relations manager.
On the heels of last year’s repeal of the sustainable growth rate (SGR) formula, 2016 promises to be a year of significant changes for the healthcare system. These changes will require providers to focus not just on the immediate pressures and requirements coming from Medicare, of which there are many, but also to look down the road to how things will change in the coming years.
The final year of reporting on quality measures for the Physician Quality Reporting System (PQRS) is 2016, with performance impacting Medicare payments in 2018. Reporting on quality measures doesn’t end there, however. The Medicare Access and CHIP Reauthorization Act (MACRA) repealed the SGR and created two new pathways for pay-for-performance for physicians and most other providers: the Merit-based Incentive Payment System (MIPS) and alternative payment models. After this year, reporting quality measures becomes one component of the MIPS, a program similar to hospital value-based purchasing, but designed for providers.
Quality measures are here to stay. They form the backbone for evaluating whether healthcare is of value. Under the MIPS, quality measures are combined with cost measures, meaningful use, and clinical performance improvement activities to create an aggregate score for providers. That score will be used to determine payment adjustments for providers starting in 2019.
Also in 2016, the Centers for Medicare and Medicaid Services (CMS) will lay the foundation for the MIPS. It is a completely new program, and although it will build on elements of existing programs like PQRS, meaningful use, and the physician value-based payment modifier, its structure and ramifications are ultimately unknown. CMS has indicated its intention to issue the regulatory backbone of MIPS in just a few months. These regulations will be the new reality of Medicare’s fee-for-service for the foreseeable future.
The ramifications of MIPS cannot be understated. It will apply an adjustment based on performance on all Medicare Part B payments. That adjustment starts at +/- 4.0% in 2019 and rises to +/- 9.0% by 2022, a number that is not as far off as it seems based on how these programs operate.
SHM expects many of the current PQRS policies to be continued under MIPS, which means, unfortunately, that many of the challenges facing hospitalists will continue. Hospitalists do not have many measures to report on; most measures are developed for outpatient practices, are simply not reflective of the variability of hospitalist practice, and, even if specified for inpatient reporting, are not clinically relevant.
To meet the needs of hospitalists, SHM will advocate strongly for CMS to develop more flexible and relevant reporting options. We will work to ensure that hospitalists are not structurally disadvantaged by the policies set in place.
Given these upcoming changes, it is as important as ever for you to stay engaged and informed about the policy changes coming down the road. It might be just the start of the year, but already there’s a lot of critical work to do. To get involved and remain apprised of the changes, join SHM’s grassroots network at www.hospitalmedicine.org/grassroots. TH
Joshua Lapps is SHM’s government relations manager.
New SHM Members – January 2016
S. Godfrey, Alabama
W. Mohamed, MD, Alabama
S. Paladugu, MBBS, Alabama
E. Razzouk, Alabama
S. Bommena, MD, Arizona
L. Ledbetter, NP, Arizona
R. Nambusi, MD, Arkansas
S. Asarch, California
J. Barber, California
M. Bikhchandani, California
B. Boesch, DO, California
C. Brown, California
A. Bui, California
E. Collier, California
L. Demyan, California
S. Dowlatshahi, California
M. Edmunds, California
A. Eniasivam, MD, California
Z. Fernandez, California
S. George, MD, California
E. Granflor, ACNP, MSN, RN, California
V. Guitierrez, California
M. Incze, California
B. Jones-Linares, California
S. Judon, California
L. Khuu, MD, California
T. Kim, MD, California
A. Lakhanpal, California
B. Lee, California
E. Li, California
E. Liaw, California
V. Lieu, California
S. Lim, California
B. Lin, California
B. Lizarraga, California
J. Martinez-Cuellar, MD, California
M. Militante-Miller, DO, California
H. Montoya, California
D. Moon, California
L. Mukdad, California
N. Nardoni, California
K. Nguyen, California
B. Ramirez, California
R. Ramos, California
A. Reyes, California
W. Schlesinger, California
B. Scott, California
S. Singh, DO, California
C. Su, California
A. Tavakoli, California
O. Viramontes, California
J. Wassei, MD, California
R. Weiss, MD, California
J. Yuan, MD, California
W. Zellalem, DO, California
Y. Zheng, California
P. Filipowski, MD, Colorado
T. Guns, BHA, Colorado
A. Koch, DO, Colorado
N. Matthews, MD, Colorado
G. McGuire, MD, Colorado
M. Prakash, MBBS, Colorado
L. Stiff, MD, Colorado
J. Garcia, MD, Connecticut
L. Haut, Connecticut
O. Aly, MD, Washington, D.C.
C. Cole, MBA, Washington, D.C.
K. Allen, DO, Florida
S. Andrews, ANP, MS, Florida
G. Clayton, MD, Florida
P. Dubon, MD, Florida
S. Jadonath, MD, Florida
F. Keen, FACP, MD, Florida
A. Khanna, MD, Florida
J. Morrison, MD, PhD, Florida
K. Myint, MBBS, Florida
C. Riccard, MD, Florida
P. Russoniello, ARNP, RN, Florida
L. Staat, ARNP, Florida
K. Tamar, FACS, Florida
R. Torres, MD, Florida
M. Klimenko, MD, Georgia
S. Kommidi, MD, Georgia
H. Patel, MD, Georgia
T. Agni, Illinois
O. Al-Heeti, MD, Illinois
M. Allen, Illinois
C. Brines, Illinois
C. Campbell, Illinois
J. Cho, Illinois
A. Cordasco, Illinois
K. Cramer, Illinois
K. Crawford, Illinois
L. Crawford, Illinois
J. Dale, Illinois
R. Davidov, Illinois
O. Doolittle, Illinois
A. Fuller, Illinois
L. Garland, MD, Illinois
S. Godbois, Illinois
E. Gonzales, Illinois
S. Gupta, MD, Illinois
R. Hameeduddin, DO, Illinois
K. Hayes, Illinois
C. Hill, Illinois
M. Jackson, Illinois
S. Jackson, Illinois
H. Jang, Illinois
M. Keegan, Illinois
E. Kimmie, Illinois
T. Lombardo, Illinois
S. McGowan, Illinois
M. Megaly, Illinois
A. Morker, Illinois
L. Moyar, Illinois
V. Patel, Illinois
C. Pena, Illinois
C. Pinotti, Illinois
W. Poisson, Illinois
K. Puleo, Illinois
R. Schmidgall, Illinois
B. Segel, MD, Illinois
S. Teshale, MD, Illinois
N. Velasquez, Illinois
S. Yeom, Illinois
M. Deb Roy, MD, Indiana
N. Delecaris, MD, Indiana
J. Gilbert, MD, Indiana
M. Ali, Iowa
S. Patel, MD, Kentucky
H. Shah, DO, Kentucky
S. Abraham, MD, Louisiana
M. Bergstedt, MD, Louisiana
J. Burtch, Louisiana
S. Chaney, MD, Louisiana
P. Karam, Louisiana
D. Kim, Louisiana
J. Leong, Louisiana
A. Sheeder, MD, Louisiana
C. Yeh, Louisiana
M. Cunanan-Bush, Maryland
K. Gottlieb, MD, MBA, MS, Maryland
T. Halley, FAAP, Maryland
E. Sholder, PA-C, Maryland
S. Sumner, DO, Maryland
A. Diranian, PA-C, Massachusetts
M. Hunt, DO, Massachusetts
S. Sasidharan, Massachusetts
A. Abdulrazzak, MD, FACP, Michigan
M. Antonishen, Michigan
A. Dhaliwal, MD, Michigan
A. Drummond, MD, Michigan
K. Fitzgerald, MD, Michigan
J. Greenberg, MD, Michigan
C. Lang, MD, Michigan
S. McGinnis, DO, Michigan
L. McMann, Michigan
A. Uwaje, FACP, MD, Michigan
E. Wisniewski, MSN, RN, Michigan
J. Benson, DO, Minnesota
V. Chaudhary, MD, Minnesota
T. Wood, Minnesota
C. Yarke, MD, Minnesota
D. Phillippi, MD, Mississippi
D. Loa, Missouri
J. Loa, Missouri
N. Patel, MD, Missouri
E. Sauer, Missouri
K. Tompkins, MD, FAAP, Missouri
J. Price, FAAFP, Montana
J. Codjoe, MD, New Jersey
I. Khan, MD, New Jersey
E. Merrill, MD, New Jersey
S. Park, DO, New Jersey
T. Ronan, MD, New Mexico
N. Varvaresou, ACNP, New Mexico
E. Ahn, MD, New York
S. Anandan, New York
D. Buff, MD, New York
B. Kranitzky, MD, New York
E. Levine, MHS, MD, New York
J. Noworyta, PA-C, New York
M. Padial, New York
J. Tucker, PA-C, New York
A. Vien, New York
J. DeCoster, MD, MPH, North Carolina
P. Gambrell, NP-C, North Carolina
D. Shah, NP, North Carolina
L. Tlhabano, MD, North Carolina
O. Aduroja, MD, Ohio
A. Ahsan, MD, Ohio
A. Belagavi, Ohio
R. Carletti, Ohio
C. Cox, RN, BSN, Ohio
G. Farkas, Ohio
M. Lileas, MD, DO, FACP, Ohio
A. Lopez, MD, Ohio
S. Mall, Ohio
A. Moren, MD, Ohio
B. Sanaullah, MD, MBBS, Ohio
A. Singh, MBBS, MD, Ohio
A. Thakur, MBBS, Ohio
T. Klimenko, ACNP, Oklahoma
L. Van Dyke, ACNP, Oklahoma
K. Gandhi, Oregon
R. Petersen, Oregon
J. Pruett, MD, Oregon
C. Cobb, MSN, NP, CRNP, FNP-C, Pennsylvania
J. Hickland, Pennsylvania
O. Kufile, MD, Pennsylvania
E. McCullough, MPH, PA-C, Pennsylvania
M. McFall, Pennsylvania
S. Nazir, Pennsylvania
A. Puri, MD, Pennsylvania
W. Romeo, MS, Pennsylvania
J. Gelzhiser, MD, Rhode Island
S. Kim, BA, Rhode Island
K. Cooley, South Carolina
J. Katchman, South Carolina
T. Phillips, South Carolina
J. Oakley, PA, South Dakota
J. Douglass, DO, Tennessee
B. Herron, Tennessee
M. McCain, LPN, FNP, Tennessee
K. Zaman, MD, Tennessee
R. Desai, DO, Texas
P. LeGros, Texas
O. Nguyen, MS, Texas
S. Papineni, MD, Texas
B. Rhinehart, PA-C, Texas
C. Szych, MD, Texas
D. Allred, APRN, Utah
G. Price, Utah
K. Leonard, MD, FAAP, Vermont
D. Rand, DO, Vermont
G. Cabrera, MD, MBA, Virginia
M. Stanton, PA-C, Virginia
J. Voss, Virginia
J. Cameron, MD, Washington
K. Chaganur, MBBS, Washington
G. Dalmacion, MD, Washington
M. Lo, Washington
M. Mahal, BS, MD, Washington
D. Newton, MD, Washington
H. Bertelson, Wisconsin
E. Kitchin, MD, Wisconsin
S. Patel, MD, Wisconsin
E. Yanke, MD, Wisconsin
S. Negrete, BSC, CCFP, MD, Canada
C. Chu, MBBS, MRCP, China
J. Chan, China
N. Pillai, MBBS, MACP, Malaysia
J. Gonzalez Moreno, MD, Mexico
S. Godfrey, Alabama
W. Mohamed, MD, Alabama
S. Paladugu, MBBS, Alabama
E. Razzouk, Alabama
S. Bommena, MD, Arizona
L. Ledbetter, NP, Arizona
R. Nambusi, MD, Arkansas
S. Asarch, California
J. Barber, California
M. Bikhchandani, California
B. Boesch, DO, California
C. Brown, California
A. Bui, California
E. Collier, California
L. Demyan, California
S. Dowlatshahi, California
M. Edmunds, California
A. Eniasivam, MD, California
Z. Fernandez, California
S. George, MD, California
E. Granflor, ACNP, MSN, RN, California
V. Guitierrez, California
M. Incze, California
B. Jones-Linares, California
S. Judon, California
L. Khuu, MD, California
T. Kim, MD, California
A. Lakhanpal, California
B. Lee, California
E. Li, California
E. Liaw, California
V. Lieu, California
S. Lim, California
B. Lin, California
B. Lizarraga, California
J. Martinez-Cuellar, MD, California
M. Militante-Miller, DO, California
H. Montoya, California
D. Moon, California
L. Mukdad, California
N. Nardoni, California
K. Nguyen, California
B. Ramirez, California
R. Ramos, California
A. Reyes, California
W. Schlesinger, California
B. Scott, California
S. Singh, DO, California
C. Su, California
A. Tavakoli, California
O. Viramontes, California
J. Wassei, MD, California
R. Weiss, MD, California
J. Yuan, MD, California
W. Zellalem, DO, California
Y. Zheng, California
P. Filipowski, MD, Colorado
T. Guns, BHA, Colorado
A. Koch, DO, Colorado
N. Matthews, MD, Colorado
G. McGuire, MD, Colorado
M. Prakash, MBBS, Colorado
L. Stiff, MD, Colorado
J. Garcia, MD, Connecticut
L. Haut, Connecticut
O. Aly, MD, Washington, D.C.
C. Cole, MBA, Washington, D.C.
K. Allen, DO, Florida
S. Andrews, ANP, MS, Florida
G. Clayton, MD, Florida
P. Dubon, MD, Florida
S. Jadonath, MD, Florida
F. Keen, FACP, MD, Florida
A. Khanna, MD, Florida
J. Morrison, MD, PhD, Florida
K. Myint, MBBS, Florida
C. Riccard, MD, Florida
P. Russoniello, ARNP, RN, Florida
L. Staat, ARNP, Florida
K. Tamar, FACS, Florida
R. Torres, MD, Florida
M. Klimenko, MD, Georgia
S. Kommidi, MD, Georgia
H. Patel, MD, Georgia
T. Agni, Illinois
O. Al-Heeti, MD, Illinois
M. Allen, Illinois
C. Brines, Illinois
C. Campbell, Illinois
J. Cho, Illinois
A. Cordasco, Illinois
K. Cramer, Illinois
K. Crawford, Illinois
L. Crawford, Illinois
J. Dale, Illinois
R. Davidov, Illinois
O. Doolittle, Illinois
A. Fuller, Illinois
L. Garland, MD, Illinois
S. Godbois, Illinois
E. Gonzales, Illinois
S. Gupta, MD, Illinois
R. Hameeduddin, DO, Illinois
K. Hayes, Illinois
C. Hill, Illinois
M. Jackson, Illinois
S. Jackson, Illinois
H. Jang, Illinois
M. Keegan, Illinois
E. Kimmie, Illinois
T. Lombardo, Illinois
S. McGowan, Illinois
M. Megaly, Illinois
A. Morker, Illinois
L. Moyar, Illinois
V. Patel, Illinois
C. Pena, Illinois
C. Pinotti, Illinois
W. Poisson, Illinois
K. Puleo, Illinois
R. Schmidgall, Illinois
B. Segel, MD, Illinois
S. Teshale, MD, Illinois
N. Velasquez, Illinois
S. Yeom, Illinois
M. Deb Roy, MD, Indiana
N. Delecaris, MD, Indiana
J. Gilbert, MD, Indiana
M. Ali, Iowa
S. Patel, MD, Kentucky
H. Shah, DO, Kentucky
S. Abraham, MD, Louisiana
M. Bergstedt, MD, Louisiana
J. Burtch, Louisiana
S. Chaney, MD, Louisiana
P. Karam, Louisiana
D. Kim, Louisiana
J. Leong, Louisiana
A. Sheeder, MD, Louisiana
C. Yeh, Louisiana
M. Cunanan-Bush, Maryland
K. Gottlieb, MD, MBA, MS, Maryland
T. Halley, FAAP, Maryland
E. Sholder, PA-C, Maryland
S. Sumner, DO, Maryland
A. Diranian, PA-C, Massachusetts
M. Hunt, DO, Massachusetts
S. Sasidharan, Massachusetts
A. Abdulrazzak, MD, FACP, Michigan
M. Antonishen, Michigan
A. Dhaliwal, MD, Michigan
A. Drummond, MD, Michigan
K. Fitzgerald, MD, Michigan
J. Greenberg, MD, Michigan
C. Lang, MD, Michigan
S. McGinnis, DO, Michigan
L. McMann, Michigan
A. Uwaje, FACP, MD, Michigan
E. Wisniewski, MSN, RN, Michigan
J. Benson, DO, Minnesota
V. Chaudhary, MD, Minnesota
T. Wood, Minnesota
C. Yarke, MD, Minnesota
D. Phillippi, MD, Mississippi
D. Loa, Missouri
J. Loa, Missouri
N. Patel, MD, Missouri
E. Sauer, Missouri
K. Tompkins, MD, FAAP, Missouri
J. Price, FAAFP, Montana
J. Codjoe, MD, New Jersey
I. Khan, MD, New Jersey
E. Merrill, MD, New Jersey
S. Park, DO, New Jersey
T. Ronan, MD, New Mexico
N. Varvaresou, ACNP, New Mexico
E. Ahn, MD, New York
S. Anandan, New York
D. Buff, MD, New York
B. Kranitzky, MD, New York
E. Levine, MHS, MD, New York
J. Noworyta, PA-C, New York
M. Padial, New York
J. Tucker, PA-C, New York
A. Vien, New York
J. DeCoster, MD, MPH, North Carolina
P. Gambrell, NP-C, North Carolina
D. Shah, NP, North Carolina
L. Tlhabano, MD, North Carolina
O. Aduroja, MD, Ohio
A. Ahsan, MD, Ohio
A. Belagavi, Ohio
R. Carletti, Ohio
C. Cox, RN, BSN, Ohio
G. Farkas, Ohio
M. Lileas, MD, DO, FACP, Ohio
A. Lopez, MD, Ohio
S. Mall, Ohio
A. Moren, MD, Ohio
B. Sanaullah, MD, MBBS, Ohio
A. Singh, MBBS, MD, Ohio
A. Thakur, MBBS, Ohio
T. Klimenko, ACNP, Oklahoma
L. Van Dyke, ACNP, Oklahoma
K. Gandhi, Oregon
R. Petersen, Oregon
J. Pruett, MD, Oregon
C. Cobb, MSN, NP, CRNP, FNP-C, Pennsylvania
J. Hickland, Pennsylvania
O. Kufile, MD, Pennsylvania
E. McCullough, MPH, PA-C, Pennsylvania
M. McFall, Pennsylvania
S. Nazir, Pennsylvania
A. Puri, MD, Pennsylvania
W. Romeo, MS, Pennsylvania
J. Gelzhiser, MD, Rhode Island
S. Kim, BA, Rhode Island
K. Cooley, South Carolina
J. Katchman, South Carolina
T. Phillips, South Carolina
J. Oakley, PA, South Dakota
J. Douglass, DO, Tennessee
B. Herron, Tennessee
M. McCain, LPN, FNP, Tennessee
K. Zaman, MD, Tennessee
R. Desai, DO, Texas
P. LeGros, Texas
O. Nguyen, MS, Texas
S. Papineni, MD, Texas
B. Rhinehart, PA-C, Texas
C. Szych, MD, Texas
D. Allred, APRN, Utah
G. Price, Utah
K. Leonard, MD, FAAP, Vermont
D. Rand, DO, Vermont
G. Cabrera, MD, MBA, Virginia
M. Stanton, PA-C, Virginia
J. Voss, Virginia
J. Cameron, MD, Washington
K. Chaganur, MBBS, Washington
G. Dalmacion, MD, Washington
M. Lo, Washington
M. Mahal, BS, MD, Washington
D. Newton, MD, Washington
H. Bertelson, Wisconsin
E. Kitchin, MD, Wisconsin
S. Patel, MD, Wisconsin
E. Yanke, MD, Wisconsin
S. Negrete, BSC, CCFP, MD, Canada
C. Chu, MBBS, MRCP, China
J. Chan, China
N. Pillai, MBBS, MACP, Malaysia
J. Gonzalez Moreno, MD, Mexico
S. Godfrey, Alabama
W. Mohamed, MD, Alabama
S. Paladugu, MBBS, Alabama
E. Razzouk, Alabama
S. Bommena, MD, Arizona
L. Ledbetter, NP, Arizona
R. Nambusi, MD, Arkansas
S. Asarch, California
J. Barber, California
M. Bikhchandani, California
B. Boesch, DO, California
C. Brown, California
A. Bui, California
E. Collier, California
L. Demyan, California
S. Dowlatshahi, California
M. Edmunds, California
A. Eniasivam, MD, California
Z. Fernandez, California
S. George, MD, California
E. Granflor, ACNP, MSN, RN, California
V. Guitierrez, California
M. Incze, California
B. Jones-Linares, California
S. Judon, California
L. Khuu, MD, California
T. Kim, MD, California
A. Lakhanpal, California
B. Lee, California
E. Li, California
E. Liaw, California
V. Lieu, California
S. Lim, California
B. Lin, California
B. Lizarraga, California
J. Martinez-Cuellar, MD, California
M. Militante-Miller, DO, California
H. Montoya, California
D. Moon, California
L. Mukdad, California
N. Nardoni, California
K. Nguyen, California
B. Ramirez, California
R. Ramos, California
A. Reyes, California
W. Schlesinger, California
B. Scott, California
S. Singh, DO, California
C. Su, California
A. Tavakoli, California
O. Viramontes, California
J. Wassei, MD, California
R. Weiss, MD, California
J. Yuan, MD, California
W. Zellalem, DO, California
Y. Zheng, California
P. Filipowski, MD, Colorado
T. Guns, BHA, Colorado
A. Koch, DO, Colorado
N. Matthews, MD, Colorado
G. McGuire, MD, Colorado
M. Prakash, MBBS, Colorado
L. Stiff, MD, Colorado
J. Garcia, MD, Connecticut
L. Haut, Connecticut
O. Aly, MD, Washington, D.C.
C. Cole, MBA, Washington, D.C.
K. Allen, DO, Florida
S. Andrews, ANP, MS, Florida
G. Clayton, MD, Florida
P. Dubon, MD, Florida
S. Jadonath, MD, Florida
F. Keen, FACP, MD, Florida
A. Khanna, MD, Florida
J. Morrison, MD, PhD, Florida
K. Myint, MBBS, Florida
C. Riccard, MD, Florida
P. Russoniello, ARNP, RN, Florida
L. Staat, ARNP, Florida
K. Tamar, FACS, Florida
R. Torres, MD, Florida
M. Klimenko, MD, Georgia
S. Kommidi, MD, Georgia
H. Patel, MD, Georgia
T. Agni, Illinois
O. Al-Heeti, MD, Illinois
M. Allen, Illinois
C. Brines, Illinois
C. Campbell, Illinois
J. Cho, Illinois
A. Cordasco, Illinois
K. Cramer, Illinois
K. Crawford, Illinois
L. Crawford, Illinois
J. Dale, Illinois
R. Davidov, Illinois
O. Doolittle, Illinois
A. Fuller, Illinois
L. Garland, MD, Illinois
S. Godbois, Illinois
E. Gonzales, Illinois
S. Gupta, MD, Illinois
R. Hameeduddin, DO, Illinois
K. Hayes, Illinois
C. Hill, Illinois
M. Jackson, Illinois
S. Jackson, Illinois
H. Jang, Illinois
M. Keegan, Illinois
E. Kimmie, Illinois
T. Lombardo, Illinois
S. McGowan, Illinois
M. Megaly, Illinois
A. Morker, Illinois
L. Moyar, Illinois
V. Patel, Illinois
C. Pena, Illinois
C. Pinotti, Illinois
W. Poisson, Illinois
K. Puleo, Illinois
R. Schmidgall, Illinois
B. Segel, MD, Illinois
S. Teshale, MD, Illinois
N. Velasquez, Illinois
S. Yeom, Illinois
M. Deb Roy, MD, Indiana
N. Delecaris, MD, Indiana
J. Gilbert, MD, Indiana
M. Ali, Iowa
S. Patel, MD, Kentucky
H. Shah, DO, Kentucky
S. Abraham, MD, Louisiana
M. Bergstedt, MD, Louisiana
J. Burtch, Louisiana
S. Chaney, MD, Louisiana
P. Karam, Louisiana
D. Kim, Louisiana
J. Leong, Louisiana
A. Sheeder, MD, Louisiana
C. Yeh, Louisiana
M. Cunanan-Bush, Maryland
K. Gottlieb, MD, MBA, MS, Maryland
T. Halley, FAAP, Maryland
E. Sholder, PA-C, Maryland
S. Sumner, DO, Maryland
A. Diranian, PA-C, Massachusetts
M. Hunt, DO, Massachusetts
S. Sasidharan, Massachusetts
A. Abdulrazzak, MD, FACP, Michigan
M. Antonishen, Michigan
A. Dhaliwal, MD, Michigan
A. Drummond, MD, Michigan
K. Fitzgerald, MD, Michigan
J. Greenberg, MD, Michigan
C. Lang, MD, Michigan
S. McGinnis, DO, Michigan
L. McMann, Michigan
A. Uwaje, FACP, MD, Michigan
E. Wisniewski, MSN, RN, Michigan
J. Benson, DO, Minnesota
V. Chaudhary, MD, Minnesota
T. Wood, Minnesota
C. Yarke, MD, Minnesota
D. Phillippi, MD, Mississippi
D. Loa, Missouri
J. Loa, Missouri
N. Patel, MD, Missouri
E. Sauer, Missouri
K. Tompkins, MD, FAAP, Missouri
J. Price, FAAFP, Montana
J. Codjoe, MD, New Jersey
I. Khan, MD, New Jersey
E. Merrill, MD, New Jersey
S. Park, DO, New Jersey
T. Ronan, MD, New Mexico
N. Varvaresou, ACNP, New Mexico
E. Ahn, MD, New York
S. Anandan, New York
D. Buff, MD, New York
B. Kranitzky, MD, New York
E. Levine, MHS, MD, New York
J. Noworyta, PA-C, New York
M. Padial, New York
J. Tucker, PA-C, New York
A. Vien, New York
J. DeCoster, MD, MPH, North Carolina
P. Gambrell, NP-C, North Carolina
D. Shah, NP, North Carolina
L. Tlhabano, MD, North Carolina
O. Aduroja, MD, Ohio
A. Ahsan, MD, Ohio
A. Belagavi, Ohio
R. Carletti, Ohio
C. Cox, RN, BSN, Ohio
G. Farkas, Ohio
M. Lileas, MD, DO, FACP, Ohio
A. Lopez, MD, Ohio
S. Mall, Ohio
A. Moren, MD, Ohio
B. Sanaullah, MD, MBBS, Ohio
A. Singh, MBBS, MD, Ohio
A. Thakur, MBBS, Ohio
T. Klimenko, ACNP, Oklahoma
L. Van Dyke, ACNP, Oklahoma
K. Gandhi, Oregon
R. Petersen, Oregon
J. Pruett, MD, Oregon
C. Cobb, MSN, NP, CRNP, FNP-C, Pennsylvania
J. Hickland, Pennsylvania
O. Kufile, MD, Pennsylvania
E. McCullough, MPH, PA-C, Pennsylvania
M. McFall, Pennsylvania
S. Nazir, Pennsylvania
A. Puri, MD, Pennsylvania
W. Romeo, MS, Pennsylvania
J. Gelzhiser, MD, Rhode Island
S. Kim, BA, Rhode Island
K. Cooley, South Carolina
J. Katchman, South Carolina
T. Phillips, South Carolina
J. Oakley, PA, South Dakota
J. Douglass, DO, Tennessee
B. Herron, Tennessee
M. McCain, LPN, FNP, Tennessee
K. Zaman, MD, Tennessee
R. Desai, DO, Texas
P. LeGros, Texas
O. Nguyen, MS, Texas
S. Papineni, MD, Texas
B. Rhinehart, PA-C, Texas
C. Szych, MD, Texas
D. Allred, APRN, Utah
G. Price, Utah
K. Leonard, MD, FAAP, Vermont
D. Rand, DO, Vermont
G. Cabrera, MD, MBA, Virginia
M. Stanton, PA-C, Virginia
J. Voss, Virginia
J. Cameron, MD, Washington
K. Chaganur, MBBS, Washington
G. Dalmacion, MD, Washington
M. Lo, Washington
M. Mahal, BS, MD, Washington
D. Newton, MD, Washington
H. Bertelson, Wisconsin
E. Kitchin, MD, Wisconsin
S. Patel, MD, Wisconsin
E. Yanke, MD, Wisconsin
S. Negrete, BSC, CCFP, MD, Canada
C. Chu, MBBS, MRCP, China
J. Chan, China
N. Pillai, MBBS, MACP, Malaysia
J. Gonzalez Moreno, MD, Mexico
Combo prolongs PFS in phase 3 MM trial

Photo by Bill Branson
Results of the phase 3 ENDEAVOR trial suggest combination carfilzomib and dexamethasone prolongs progression-free survival (PFS) in patients with relapsed or refractory multiple myeloma (MM), when compared to bortezomib plus dexamethasone.
The median PFS was 18.7 months in the carfilzomib arm and 9.4 months in the bortezomib arm.
It is not clear whether this translates to an improvement in overall survival, as those data are not yet mature.
Treatment discontinuation due to adverse events (AEs) and on-study deaths were comparable between the treatment arms, although there were several AEs that occurred more frequently in the carfilzomib arm than the bortezomib arm.
These results were published in The Lancet Oncology. The trial was funded by Onyx Pharmaceuticals, Inc., a subsidiary of Amgen.
Patient treatment and characteristics
The ENDEAVOR trial included 929 patients with relapsed/refractory MM who had received 1 to 3 prior therapeutic regimens. They were randomized to receive carfilzomib in combination with low-dose dexamethasone (n=464) or bortezomib with low-dose dexamethasone (n=465) until progression.
Patients received carfilzomib as a 30-minute infusion on days 1, 2, 8, 9, 15, and 16 of 28-day treatment cycles, along with 20 mg of dexamethasone. For cycle 1 only, carfilzomib was administered at 20 mg/m2 on days 1 and 2, followed by escalation to 56 mg/m2 from day 8. Patients who tolerated 56 mg/m2 in cycle 1 were kept at this dose for subsequent cycles.
Patients who received bortezomib (1.3 mg/m2) with low-dose dexamethasone (20 mg) were given bortezomib subcutaneously or intravenously at the discretion of the investigator. More than 75% of the patients received bortezomib subcutaneously.
Baseline characteristics were generally balanced between the treatment arms. Both arms had a median age of 65 (overall range, 30-89), and about half were male. Three-quarters of patients in both arms were white, a little over 10% were Asian, 2% were black, and about 10% did not report race/ethnicity.
A majority of patients in both arms (more than 90%) had an ECOG score of 0 or 1. Most patients (more than 60%) had standard-risk cytogenetics.
The median number of prior treatment regimens was 2 in both arms. Patients in both arms had received prior lenalidomide (38% in both arms), thalidomide (45% in the carfilzomib arm and 53% in the bortezomib arm), bortezomib (54% in both arms), and carfilzomib (<1% in both arms).
Results
The median PFS in the carfilzomib arm was roughly double that of the bortezomib arm—18.7 months and 9.4 months, respectively. The hazard ratio was 0.53 (P<0.0001).
Overall survival data are not mature and are still being monitored.
The overall response rate was 77% in the carfilzomib arm and 63% in the bortezomib arm (P<0.0001). The duration of response was 21.3 months and 10.4 months, respectively.
The proportion of patients achieving a very good partial response or better was 54.3% in the carfilzomib arm and 29% in the bortezomib arm (P<0.0001). The complete response rates were 13% and 6%, respectively (P<0.001).
Treatment discontinuation due to AEs and on-study deaths were comparable between the arms. There were 75 deaths in the carfilzomib arm and 88 deaths in the bortezomib arm.
Of the 263 patients in the carfilzomib arm who discontinued treatment, 65 did so because of AEs. Of the 351 patients in the bortezomib arm who discontinued treatment, 73 did so because of AEs.
A number of known AEs were reported at a higher rate in the carfilzomib arm than the bortezomib arm, including any-grade dyspnea (28% vs 13%), hypertension (25% vs 3%), pyrexia (27% vs 14%), cough (25% vs 15%), cardiac failure (8% vs 3%), and acute renal failure (8% vs 5%).
Rates of grade 3 or higher AEs were 73% in the carfilzomib arm and 67% in the bortezomib arm. Grade 3 or higher AEs of interest in the carfilzomib and bortezomib arms, respectively, were hypertension (9% vs 3%), dyspnea (5% vs 2%), cardiac failure (5% vs 2%), acute renal failure (4% vs 3%), ischemic heart disease (2% vs 2%), and pulmonary hypertension (0.6% vs 0.2%). ![]()

Photo by Bill Branson
Results of the phase 3 ENDEAVOR trial suggest combination carfilzomib and dexamethasone prolongs progression-free survival (PFS) in patients with relapsed or refractory multiple myeloma (MM), when compared to bortezomib plus dexamethasone.
The median PFS was 18.7 months in the carfilzomib arm and 9.4 months in the bortezomib arm.
It is not clear whether this translates to an improvement in overall survival, as those data are not yet mature.
Treatment discontinuation due to adverse events (AEs) and on-study deaths were comparable between the treatment arms, although there were several AEs that occurred more frequently in the carfilzomib arm than the bortezomib arm.
These results were published in The Lancet Oncology. The trial was funded by Onyx Pharmaceuticals, Inc., a subsidiary of Amgen.
Patient treatment and characteristics
The ENDEAVOR trial included 929 patients with relapsed/refractory MM who had received 1 to 3 prior therapeutic regimens. They were randomized to receive carfilzomib in combination with low-dose dexamethasone (n=464) or bortezomib with low-dose dexamethasone (n=465) until progression.
Patients received carfilzomib as a 30-minute infusion on days 1, 2, 8, 9, 15, and 16 of 28-day treatment cycles, along with 20 mg of dexamethasone. For cycle 1 only, carfilzomib was administered at 20 mg/m2 on days 1 and 2, followed by escalation to 56 mg/m2 from day 8. Patients who tolerated 56 mg/m2 in cycle 1 were kept at this dose for subsequent cycles.
Patients who received bortezomib (1.3 mg/m2) with low-dose dexamethasone (20 mg) were given bortezomib subcutaneously or intravenously at the discretion of the investigator. More than 75% of the patients received bortezomib subcutaneously.
Baseline characteristics were generally balanced between the treatment arms. Both arms had a median age of 65 (overall range, 30-89), and about half were male. Three-quarters of patients in both arms were white, a little over 10% were Asian, 2% were black, and about 10% did not report race/ethnicity.
A majority of patients in both arms (more than 90%) had an ECOG score of 0 or 1. Most patients (more than 60%) had standard-risk cytogenetics.
The median number of prior treatment regimens was 2 in both arms. Patients in both arms had received prior lenalidomide (38% in both arms), thalidomide (45% in the carfilzomib arm and 53% in the bortezomib arm), bortezomib (54% in both arms), and carfilzomib (<1% in both arms).
Results
The median PFS in the carfilzomib arm was roughly double that of the bortezomib arm—18.7 months and 9.4 months, respectively. The hazard ratio was 0.53 (P<0.0001).
Overall survival data are not mature and are still being monitored.
The overall response rate was 77% in the carfilzomib arm and 63% in the bortezomib arm (P<0.0001). The duration of response was 21.3 months and 10.4 months, respectively.
The proportion of patients achieving a very good partial response or better was 54.3% in the carfilzomib arm and 29% in the bortezomib arm (P<0.0001). The complete response rates were 13% and 6%, respectively (P<0.001).
Treatment discontinuation due to AEs and on-study deaths were comparable between the arms. There were 75 deaths in the carfilzomib arm and 88 deaths in the bortezomib arm.
Of the 263 patients in the carfilzomib arm who discontinued treatment, 65 did so because of AEs. Of the 351 patients in the bortezomib arm who discontinued treatment, 73 did so because of AEs.
A number of known AEs were reported at a higher rate in the carfilzomib arm than the bortezomib arm, including any-grade dyspnea (28% vs 13%), hypertension (25% vs 3%), pyrexia (27% vs 14%), cough (25% vs 15%), cardiac failure (8% vs 3%), and acute renal failure (8% vs 5%).
Rates of grade 3 or higher AEs were 73% in the carfilzomib arm and 67% in the bortezomib arm. Grade 3 or higher AEs of interest in the carfilzomib and bortezomib arms, respectively, were hypertension (9% vs 3%), dyspnea (5% vs 2%), cardiac failure (5% vs 2%), acute renal failure (4% vs 3%), ischemic heart disease (2% vs 2%), and pulmonary hypertension (0.6% vs 0.2%). ![]()

Photo by Bill Branson
Results of the phase 3 ENDEAVOR trial suggest combination carfilzomib and dexamethasone prolongs progression-free survival (PFS) in patients with relapsed or refractory multiple myeloma (MM), when compared to bortezomib plus dexamethasone.
The median PFS was 18.7 months in the carfilzomib arm and 9.4 months in the bortezomib arm.
It is not clear whether this translates to an improvement in overall survival, as those data are not yet mature.
Treatment discontinuation due to adverse events (AEs) and on-study deaths were comparable between the treatment arms, although there were several AEs that occurred more frequently in the carfilzomib arm than the bortezomib arm.
These results were published in The Lancet Oncology. The trial was funded by Onyx Pharmaceuticals, Inc., a subsidiary of Amgen.
Patient treatment and characteristics
The ENDEAVOR trial included 929 patients with relapsed/refractory MM who had received 1 to 3 prior therapeutic regimens. They were randomized to receive carfilzomib in combination with low-dose dexamethasone (n=464) or bortezomib with low-dose dexamethasone (n=465) until progression.
Patients received carfilzomib as a 30-minute infusion on days 1, 2, 8, 9, 15, and 16 of 28-day treatment cycles, along with 20 mg of dexamethasone. For cycle 1 only, carfilzomib was administered at 20 mg/m2 on days 1 and 2, followed by escalation to 56 mg/m2 from day 8. Patients who tolerated 56 mg/m2 in cycle 1 were kept at this dose for subsequent cycles.
Patients who received bortezomib (1.3 mg/m2) with low-dose dexamethasone (20 mg) were given bortezomib subcutaneously or intravenously at the discretion of the investigator. More than 75% of the patients received bortezomib subcutaneously.
Baseline characteristics were generally balanced between the treatment arms. Both arms had a median age of 65 (overall range, 30-89), and about half were male. Three-quarters of patients in both arms were white, a little over 10% were Asian, 2% were black, and about 10% did not report race/ethnicity.
A majority of patients in both arms (more than 90%) had an ECOG score of 0 or 1. Most patients (more than 60%) had standard-risk cytogenetics.
The median number of prior treatment regimens was 2 in both arms. Patients in both arms had received prior lenalidomide (38% in both arms), thalidomide (45% in the carfilzomib arm and 53% in the bortezomib arm), bortezomib (54% in both arms), and carfilzomib (<1% in both arms).
Results
The median PFS in the carfilzomib arm was roughly double that of the bortezomib arm—18.7 months and 9.4 months, respectively. The hazard ratio was 0.53 (P<0.0001).
Overall survival data are not mature and are still being monitored.
The overall response rate was 77% in the carfilzomib arm and 63% in the bortezomib arm (P<0.0001). The duration of response was 21.3 months and 10.4 months, respectively.
The proportion of patients achieving a very good partial response or better was 54.3% in the carfilzomib arm and 29% in the bortezomib arm (P<0.0001). The complete response rates were 13% and 6%, respectively (P<0.001).
Treatment discontinuation due to AEs and on-study deaths were comparable between the arms. There were 75 deaths in the carfilzomib arm and 88 deaths in the bortezomib arm.
Of the 263 patients in the carfilzomib arm who discontinued treatment, 65 did so because of AEs. Of the 351 patients in the bortezomib arm who discontinued treatment, 73 did so because of AEs.
A number of known AEs were reported at a higher rate in the carfilzomib arm than the bortezomib arm, including any-grade dyspnea (28% vs 13%), hypertension (25% vs 3%), pyrexia (27% vs 14%), cough (25% vs 15%), cardiac failure (8% vs 3%), and acute renal failure (8% vs 5%).
Rates of grade 3 or higher AEs were 73% in the carfilzomib arm and 67% in the bortezomib arm. Grade 3 or higher AEs of interest in the carfilzomib and bortezomib arms, respectively, were hypertension (9% vs 3%), dyspnea (5% vs 2%), cardiac failure (5% vs 2%), acute renal failure (4% vs 3%), ischemic heart disease (2% vs 2%), and pulmonary hypertension (0.6% vs 0.2%). ![]()
Meta-analysis backs SPRINT findings, argues for lower BP targets
In high-risk patients, blood pressure lowering is associated with significant reductions in vascular events for a range of comorbidities and baseline blood pressures, said the authors of a meta-analysis of 123 randomized controlled trials published in the last 50 years.
Each 10–mm Hg reduction in systolic blood pressure was associated with a 20% reduction in major cardiovascular disease events (95% confidence interval, 0.77-0.83), a 17% reduction in coronary heart disease (95% CI, 0.78-0.88), a 27% reduction in stroke (95% CI, 0.68-0.77), and a 28% reduction in heart failure (95% CI, 0.67-0.78), based on the meta-analysis published Dec. 23 by the Lancet.
The exception was a lack of overall benefit of blood pressure lowering for renal failure events, a finding consistent with a previous meta-analysis of moderate versus intensive blood pressure reduction.
“Lowering of blood pressure into what has been regarded the normotensive range should therefore be routinely considered for the prevention of cardiovascular disease among those deemed to be of sufficient absolute risk,” wrote Dena Ettehad of the George Institute for Global Health, Oxford, and coauthors.
“Revision is urgently needed to recent blood pressure lowering guidelines that have relaxed the blood pressure lowering thresholds,” they added.
The researchers conducted a meta-analysis of blood pressure lowering treatment, involving a total of 613,815 participants and a minimum of 1,000 patient-years of follow-up in each study arm.
The analysis indicated that a 10–mm Hg reduction in systolic blood pressure achieved an overall 13% reduction in all-cause mortality (95% CI, 0.84-0.91) but had no significant impact on the risk of renal failure events.
These effects remained similar even when the effects were compared between strata of mean baseline systolic blood pressure, baseline coronary heart disease, or baseline cardiovascular disease (Lancet 2015 Dec 23. doi: 10.1016/S0140-6736(15)01225-8).
“In stratified analyses, we saw no strong evidence that proportional effects were diminished in trials that included people with lower baseline systolic blood pressure (less than 130 mm Hg), and major cardiovascular events were clearly reduced in high-risk patients with various baseline comorbidities,” the investigators wrote.
“Both of these major findings – the efficacy of blood pressure lowering below 130 mm Hg and the similar proportional effects in high-risk populations – are consistent with and extend the findings of the SPRINT trial,” they said.
The authors did note greater proportional reductions in the risk of stroke in populations without a history of cerebrovascular disease, compared with those with a history.
Populations without diabetes had significantly greater proportional reductions in risk, compared with those with diabetes, while populations without chronic kidney disease had greater proportional reductions in the risk of major cardiovascular disease events, compared with those with chronic kidney disease.
The five classes of antihypertensives were generally as effective as each other in reducing the risk of major outcomes.
The authors noted that, while there were small but significant differences between drug classes for outcomes, these effects may have been the result of differences in control regimens or the concurrent use of multiple drug classes in many trials.
Two authors were supported by the National Institute of Health Research, one by the Clarendon Fund, and one by the Rhodes Trust. The George Institute is supported by the Oxford Martin School. Two authors declared grants from Servier, and one also declared investments for the development of a polypill. No other conflicts of interest were declared.
The finding from this meta-analysis that there is no increased risk of any outcome with systolic blood pressure lowering shows that a J-shaped relationship could not be substantiated and that the treatment effects were unlikely to be attenuated in trials that included participants with low systolic blood pressures at baseline, particularly those with less than 130 mm Hg.
Since data are accumulating against the J-shaped relationship, and because energetic lowering of blood pressure seems safe and beneficial to patients, there is no reason not to apply this approach to high-risk patients.
Dr. Stéphane Laurent and Dr. Pierre Boutouyrie are with the department of pharmacology at European Georges Pompidou Hospital, Paris. These comments were taken from an accompanying editorial (Lancet 2015 Dec 23. doi: 10.1016/S0140-6736(15)01344-6). Dr. Boutouyrie declared grants and personal fees from Servier. Dr. Laurent had no conflicts of interest to declare.
The finding from this meta-analysis that there is no increased risk of any outcome with systolic blood pressure lowering shows that a J-shaped relationship could not be substantiated and that the treatment effects were unlikely to be attenuated in trials that included participants with low systolic blood pressures at baseline, particularly those with less than 130 mm Hg.
Since data are accumulating against the J-shaped relationship, and because energetic lowering of blood pressure seems safe and beneficial to patients, there is no reason not to apply this approach to high-risk patients.
Dr. Stéphane Laurent and Dr. Pierre Boutouyrie are with the department of pharmacology at European Georges Pompidou Hospital, Paris. These comments were taken from an accompanying editorial (Lancet 2015 Dec 23. doi: 10.1016/S0140-6736(15)01344-6). Dr. Boutouyrie declared grants and personal fees from Servier. Dr. Laurent had no conflicts of interest to declare.
The finding from this meta-analysis that there is no increased risk of any outcome with systolic blood pressure lowering shows that a J-shaped relationship could not be substantiated and that the treatment effects were unlikely to be attenuated in trials that included participants with low systolic blood pressures at baseline, particularly those with less than 130 mm Hg.
Since data are accumulating against the J-shaped relationship, and because energetic lowering of blood pressure seems safe and beneficial to patients, there is no reason not to apply this approach to high-risk patients.
Dr. Stéphane Laurent and Dr. Pierre Boutouyrie are with the department of pharmacology at European Georges Pompidou Hospital, Paris. These comments were taken from an accompanying editorial (Lancet 2015 Dec 23. doi: 10.1016/S0140-6736(15)01344-6). Dr. Boutouyrie declared grants and personal fees from Servier. Dr. Laurent had no conflicts of interest to declare.
In high-risk patients, blood pressure lowering is associated with significant reductions in vascular events for a range of comorbidities and baseline blood pressures, said the authors of a meta-analysis of 123 randomized controlled trials published in the last 50 years.
Each 10–mm Hg reduction in systolic blood pressure was associated with a 20% reduction in major cardiovascular disease events (95% confidence interval, 0.77-0.83), a 17% reduction in coronary heart disease (95% CI, 0.78-0.88), a 27% reduction in stroke (95% CI, 0.68-0.77), and a 28% reduction in heart failure (95% CI, 0.67-0.78), based on the meta-analysis published Dec. 23 by the Lancet.
The exception was a lack of overall benefit of blood pressure lowering for renal failure events, a finding consistent with a previous meta-analysis of moderate versus intensive blood pressure reduction.
“Lowering of blood pressure into what has been regarded the normotensive range should therefore be routinely considered for the prevention of cardiovascular disease among those deemed to be of sufficient absolute risk,” wrote Dena Ettehad of the George Institute for Global Health, Oxford, and coauthors.
“Revision is urgently needed to recent blood pressure lowering guidelines that have relaxed the blood pressure lowering thresholds,” they added.
The researchers conducted a meta-analysis of blood pressure lowering treatment, involving a total of 613,815 participants and a minimum of 1,000 patient-years of follow-up in each study arm.
The analysis indicated that a 10–mm Hg reduction in systolic blood pressure achieved an overall 13% reduction in all-cause mortality (95% CI, 0.84-0.91) but had no significant impact on the risk of renal failure events.
These effects remained similar even when the effects were compared between strata of mean baseline systolic blood pressure, baseline coronary heart disease, or baseline cardiovascular disease (Lancet 2015 Dec 23. doi: 10.1016/S0140-6736(15)01225-8).
“In stratified analyses, we saw no strong evidence that proportional effects were diminished in trials that included people with lower baseline systolic blood pressure (less than 130 mm Hg), and major cardiovascular events were clearly reduced in high-risk patients with various baseline comorbidities,” the investigators wrote.
“Both of these major findings – the efficacy of blood pressure lowering below 130 mm Hg and the similar proportional effects in high-risk populations – are consistent with and extend the findings of the SPRINT trial,” they said.
The authors did note greater proportional reductions in the risk of stroke in populations without a history of cerebrovascular disease, compared with those with a history.
Populations without diabetes had significantly greater proportional reductions in risk, compared with those with diabetes, while populations without chronic kidney disease had greater proportional reductions in the risk of major cardiovascular disease events, compared with those with chronic kidney disease.
The five classes of antihypertensives were generally as effective as each other in reducing the risk of major outcomes.
The authors noted that, while there were small but significant differences between drug classes for outcomes, these effects may have been the result of differences in control regimens or the concurrent use of multiple drug classes in many trials.
Two authors were supported by the National Institute of Health Research, one by the Clarendon Fund, and one by the Rhodes Trust. The George Institute is supported by the Oxford Martin School. Two authors declared grants from Servier, and one also declared investments for the development of a polypill. No other conflicts of interest were declared.
In high-risk patients, blood pressure lowering is associated with significant reductions in vascular events for a range of comorbidities and baseline blood pressures, said the authors of a meta-analysis of 123 randomized controlled trials published in the last 50 years.
Each 10–mm Hg reduction in systolic blood pressure was associated with a 20% reduction in major cardiovascular disease events (95% confidence interval, 0.77-0.83), a 17% reduction in coronary heart disease (95% CI, 0.78-0.88), a 27% reduction in stroke (95% CI, 0.68-0.77), and a 28% reduction in heart failure (95% CI, 0.67-0.78), based on the meta-analysis published Dec. 23 by the Lancet.
The exception was a lack of overall benefit of blood pressure lowering for renal failure events, a finding consistent with a previous meta-analysis of moderate versus intensive blood pressure reduction.
“Lowering of blood pressure into what has been regarded the normotensive range should therefore be routinely considered for the prevention of cardiovascular disease among those deemed to be of sufficient absolute risk,” wrote Dena Ettehad of the George Institute for Global Health, Oxford, and coauthors.
“Revision is urgently needed to recent blood pressure lowering guidelines that have relaxed the blood pressure lowering thresholds,” they added.
The researchers conducted a meta-analysis of blood pressure lowering treatment, involving a total of 613,815 participants and a minimum of 1,000 patient-years of follow-up in each study arm.
The analysis indicated that a 10–mm Hg reduction in systolic blood pressure achieved an overall 13% reduction in all-cause mortality (95% CI, 0.84-0.91) but had no significant impact on the risk of renal failure events.
These effects remained similar even when the effects were compared between strata of mean baseline systolic blood pressure, baseline coronary heart disease, or baseline cardiovascular disease (Lancet 2015 Dec 23. doi: 10.1016/S0140-6736(15)01225-8).
“In stratified analyses, we saw no strong evidence that proportional effects were diminished in trials that included people with lower baseline systolic blood pressure (less than 130 mm Hg), and major cardiovascular events were clearly reduced in high-risk patients with various baseline comorbidities,” the investigators wrote.
“Both of these major findings – the efficacy of blood pressure lowering below 130 mm Hg and the similar proportional effects in high-risk populations – are consistent with and extend the findings of the SPRINT trial,” they said.
The authors did note greater proportional reductions in the risk of stroke in populations without a history of cerebrovascular disease, compared with those with a history.
Populations without diabetes had significantly greater proportional reductions in risk, compared with those with diabetes, while populations without chronic kidney disease had greater proportional reductions in the risk of major cardiovascular disease events, compared with those with chronic kidney disease.
The five classes of antihypertensives were generally as effective as each other in reducing the risk of major outcomes.
The authors noted that, while there were small but significant differences between drug classes for outcomes, these effects may have been the result of differences in control regimens or the concurrent use of multiple drug classes in many trials.
Two authors were supported by the National Institute of Health Research, one by the Clarendon Fund, and one by the Rhodes Trust. The George Institute is supported by the Oxford Martin School. Two authors declared grants from Servier, and one also declared investments for the development of a polypill. No other conflicts of interest were declared.
FROM THE LANCET
Key clinical point: Blood pressure lowering is associated with significant reductions in vascular events in patients with a range of comorbidities and baseline blood pressures.
Major finding: Each 10–mm Hg reduction in systolic blood pressure is associated with a 20% reduction in major cardiovascular disease events.
Data source: A meta-analysis of 123 randomized controlled trials of blood pressure lowering treatment, involving a total of 613,815 participants.
Disclosures: Two authors were supported by the National Institute of Health Research, one by the Clarendon Fund, and one by the Rhodes Trust. The George Institute is supported by the Oxford Martin School. Two authors declared grants from Servier, and one also declared investments for the development of a polypill. No other conflicts of interest were declared.
Secukinumab cut ankylosing spondylitis symptoms in MEASURE trials
Secukinumab, an interleukin 17-A inhibitor approved for the treatment of moderate to severe psoriasis, significantly reduced the signs and symptoms of ankylosing spondylitis in two phase III trials, researchers reported Dec. 23 in the New England Journal of Medicine.
The results of the double-blind MEASURE 1 and MEASURE 2 trials extend the positive results of the phase II study, according to Dr. Dominique Baeten of the Academic Medical Center at the University of Amsterdam and his colleagues (N Engl J Med. 2015 Dec 23. doi: 10.1056/NEJMoa1505066).
“Although head-to-head trials would be required to fully assess the efficacy and safety of secukinumab versus TNF-inhibitors, the [20% improvement in Assessment of Spondyloarthritis International Society (ASAS20) response criteria] response rates achieved with secukinumab at week 16 in our studies were similar to those reported in phase III studies of anti-TNF agents in which most of the patients had not received previous anti-TNF therapy (response rates of 58% to 64% at weeks 12 to 24), even though 30% to 40% of the patients in our studies had had no response to previous anti-TNF treatment,” the authors wrote.
“Thus, secukinumab not only is effective in patients who have not received TNF agents previously but also may be effective in patients in whom previous anti-TNF treatment failed,” they added.
In MEASURE 1, 371 patients received intravenous secukinumab (10 mg/kg of body weight) or matched placebo at weeks 0, 2, and 4, followed by subcutaneous secukinumab (150 mg or 75 mg) or matched placebo every 4 weeks starting at week 8.
The study’s primary endpoint of ASAS20 response rates at week 16 were 61%, 60%, and 29% for subcutaneous secukinumab at doses of 150 mg and 75 mg and for placebo, respectively, (P less than .001 for both comparisons with placebo).
In MEASURE 2, 219 patients received subcutaneous secukinumab (150 mg or 75 mg) or matched placebo at baseline; at weeks 1, 2, and 3; and every 4 weeks starting at week 4.
At week 16, patients in the placebo group were randomly reassigned to subcutaneous secukinumab at a dose of 150 mg or 75 mg.
In this trial, ASAS20 rates were 61%, 41%, and 28% for subcutaneous secukinumab at doses of 150 mg and 75 mg and for placebo, respectively (P less than .001 for the 150-mg dose and P = .10 for the 75-mg dose).
The researchers noted that the ineffectiveness of the 75-mg dose in MEASURE 2 suggests that the efficacy of secukinumab at the 75-mg dose in MEASURE 1 may have been due to the greater exposure at week 16 as a result of the intravenous loading regimen, not to the 75-mg subcutaneous maintenance dose.
The safety profile of secukinumab in the present studies was consistent with previous studies of secukinumab for ankylosing spondylitis and moderate-to-severe plaque psoriasis, Dr. Baeten and his associates said.
During the entire treatment period, pooled exposure-adjusted incidence rates of grade 3 or 4 neutropenia, candida infections, and Crohn’s disease were 0.7, 0.9, and 0.7 cases per 100 patient-years, respectively, in secukinumab-treated patients.
Overall, the results suggest that interleukin-17A plays a role in the pathogenesis of ankylosing spondylitis, and they validate inhibition of this cytokine as a potential therapeutic approach, the study authors concluded.
The study was sponsored by Novartis Pharma. Dr. Baeten has received a grant from Novartis to study the impact of IL-17A blockade in experimental models of spondyloarthritis. He also has been a consultant for Novartis for the design and conduct of the secukinumab program in ankylosing spondylitis and psoriatic arthritis.
Secukinumab, an interleukin 17-A inhibitor approved for the treatment of moderate to severe psoriasis, significantly reduced the signs and symptoms of ankylosing spondylitis in two phase III trials, researchers reported Dec. 23 in the New England Journal of Medicine.
The results of the double-blind MEASURE 1 and MEASURE 2 trials extend the positive results of the phase II study, according to Dr. Dominique Baeten of the Academic Medical Center at the University of Amsterdam and his colleagues (N Engl J Med. 2015 Dec 23. doi: 10.1056/NEJMoa1505066).
“Although head-to-head trials would be required to fully assess the efficacy and safety of secukinumab versus TNF-inhibitors, the [20% improvement in Assessment of Spondyloarthritis International Society (ASAS20) response criteria] response rates achieved with secukinumab at week 16 in our studies were similar to those reported in phase III studies of anti-TNF agents in which most of the patients had not received previous anti-TNF therapy (response rates of 58% to 64% at weeks 12 to 24), even though 30% to 40% of the patients in our studies had had no response to previous anti-TNF treatment,” the authors wrote.
“Thus, secukinumab not only is effective in patients who have not received TNF agents previously but also may be effective in patients in whom previous anti-TNF treatment failed,” they added.
In MEASURE 1, 371 patients received intravenous secukinumab (10 mg/kg of body weight) or matched placebo at weeks 0, 2, and 4, followed by subcutaneous secukinumab (150 mg or 75 mg) or matched placebo every 4 weeks starting at week 8.
The study’s primary endpoint of ASAS20 response rates at week 16 were 61%, 60%, and 29% for subcutaneous secukinumab at doses of 150 mg and 75 mg and for placebo, respectively, (P less than .001 for both comparisons with placebo).
In MEASURE 2, 219 patients received subcutaneous secukinumab (150 mg or 75 mg) or matched placebo at baseline; at weeks 1, 2, and 3; and every 4 weeks starting at week 4.
At week 16, patients in the placebo group were randomly reassigned to subcutaneous secukinumab at a dose of 150 mg or 75 mg.
In this trial, ASAS20 rates were 61%, 41%, and 28% for subcutaneous secukinumab at doses of 150 mg and 75 mg and for placebo, respectively (P less than .001 for the 150-mg dose and P = .10 for the 75-mg dose).
The researchers noted that the ineffectiveness of the 75-mg dose in MEASURE 2 suggests that the efficacy of secukinumab at the 75-mg dose in MEASURE 1 may have been due to the greater exposure at week 16 as a result of the intravenous loading regimen, not to the 75-mg subcutaneous maintenance dose.
The safety profile of secukinumab in the present studies was consistent with previous studies of secukinumab for ankylosing spondylitis and moderate-to-severe plaque psoriasis, Dr. Baeten and his associates said.
During the entire treatment period, pooled exposure-adjusted incidence rates of grade 3 or 4 neutropenia, candida infections, and Crohn’s disease were 0.7, 0.9, and 0.7 cases per 100 patient-years, respectively, in secukinumab-treated patients.
Overall, the results suggest that interleukin-17A plays a role in the pathogenesis of ankylosing spondylitis, and they validate inhibition of this cytokine as a potential therapeutic approach, the study authors concluded.
The study was sponsored by Novartis Pharma. Dr. Baeten has received a grant from Novartis to study the impact of IL-17A blockade in experimental models of spondyloarthritis. He also has been a consultant for Novartis for the design and conduct of the secukinumab program in ankylosing spondylitis and psoriatic arthritis.
Secukinumab, an interleukin 17-A inhibitor approved for the treatment of moderate to severe psoriasis, significantly reduced the signs and symptoms of ankylosing spondylitis in two phase III trials, researchers reported Dec. 23 in the New England Journal of Medicine.
The results of the double-blind MEASURE 1 and MEASURE 2 trials extend the positive results of the phase II study, according to Dr. Dominique Baeten of the Academic Medical Center at the University of Amsterdam and his colleagues (N Engl J Med. 2015 Dec 23. doi: 10.1056/NEJMoa1505066).
“Although head-to-head trials would be required to fully assess the efficacy and safety of secukinumab versus TNF-inhibitors, the [20% improvement in Assessment of Spondyloarthritis International Society (ASAS20) response criteria] response rates achieved with secukinumab at week 16 in our studies were similar to those reported in phase III studies of anti-TNF agents in which most of the patients had not received previous anti-TNF therapy (response rates of 58% to 64% at weeks 12 to 24), even though 30% to 40% of the patients in our studies had had no response to previous anti-TNF treatment,” the authors wrote.
“Thus, secukinumab not only is effective in patients who have not received TNF agents previously but also may be effective in patients in whom previous anti-TNF treatment failed,” they added.
In MEASURE 1, 371 patients received intravenous secukinumab (10 mg/kg of body weight) or matched placebo at weeks 0, 2, and 4, followed by subcutaneous secukinumab (150 mg or 75 mg) or matched placebo every 4 weeks starting at week 8.
The study’s primary endpoint of ASAS20 response rates at week 16 were 61%, 60%, and 29% for subcutaneous secukinumab at doses of 150 mg and 75 mg and for placebo, respectively, (P less than .001 for both comparisons with placebo).
In MEASURE 2, 219 patients received subcutaneous secukinumab (150 mg or 75 mg) or matched placebo at baseline; at weeks 1, 2, and 3; and every 4 weeks starting at week 4.
At week 16, patients in the placebo group were randomly reassigned to subcutaneous secukinumab at a dose of 150 mg or 75 mg.
In this trial, ASAS20 rates were 61%, 41%, and 28% for subcutaneous secukinumab at doses of 150 mg and 75 mg and for placebo, respectively (P less than .001 for the 150-mg dose and P = .10 for the 75-mg dose).
The researchers noted that the ineffectiveness of the 75-mg dose in MEASURE 2 suggests that the efficacy of secukinumab at the 75-mg dose in MEASURE 1 may have been due to the greater exposure at week 16 as a result of the intravenous loading regimen, not to the 75-mg subcutaneous maintenance dose.
The safety profile of secukinumab in the present studies was consistent with previous studies of secukinumab for ankylosing spondylitis and moderate-to-severe plaque psoriasis, Dr. Baeten and his associates said.
During the entire treatment period, pooled exposure-adjusted incidence rates of grade 3 or 4 neutropenia, candida infections, and Crohn’s disease were 0.7, 0.9, and 0.7 cases per 100 patient-years, respectively, in secukinumab-treated patients.
Overall, the results suggest that interleukin-17A plays a role in the pathogenesis of ankylosing spondylitis, and they validate inhibition of this cytokine as a potential therapeutic approach, the study authors concluded.
The study was sponsored by Novartis Pharma. Dr. Baeten has received a grant from Novartis to study the impact of IL-17A blockade in experimental models of spondyloarthritis. He also has been a consultant for Novartis for the design and conduct of the secukinumab program in ankylosing spondylitis and psoriatic arthritis.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Interleukin-17A may play a role in ankylosing spondylitis, and secukinumab may prove to be an effective therapy for these patients.
Major finding: The primary endpoint of Assessment of Spondyloarthritis International Society (ASAS20) response rates at week 16 was met in both secukinumab groups in MEASURE 1 and in the group that received 150 mg of secukinumab subcutaneously in MEASURE 2.
Data source: Two double-blind, phase III studies: MEASURE 1 involving 371 patients with AS and MEASURE 2 involving 371 patients.
Disclosures: The studies were funded by Novartis Pharma. Dr. Baeten has received a grant from Novartis to study the impact of IL-17A blockade in experimental models of spondyloarthritis. He also has been a consultant for Novartis for the design and conduct of the secukinumab program in ankylosing spondylitis and psoriatic arthritis.
European societies issue aspergillosis diagnosis, management guidelines
European respiratory disease and infectious disease specialists have banded together to issue new clinical guidelines on the diagnosis and management of an uncommon but serious problem: chronic pulmonary aspergillosis (CPA).
Pulmonary infections with Aspergillus species, although uncommon, are a complicating factor in several lung diseases, especially tuberculosis, and aspergillosis is a serious, often fatal opportunistic infection in transplant recipients who are on chronic immunosuppression or patients who are immunocompromised from disease or cytotoxic chemotherapy.
Approximately 240,000 people in Europe and 3 million people worldwide have chronic pulmonary aspergillosis (CPA). The Centers for Disease Control and Prevention notes that because aspergillosis is not classified as a reportable disease, data on the actual incidence of infections in the United States are hard to come by.
“You don’t see this every day, whether you’re an infectious disease specialist or pulmonologist, so you really can’t rely on your experience to guide you in managing these cases, which is why guidelines such as this can be very helpful,” commented Dr. Norman Edelman, a pulmonologist and senior consultant for scientific affairs for the American Lung Association.
The guidelines, issued by the European Society for Clinical Microbiology and Infectious Diseases in cooperation with the European Confederation of Medical Mycology and the European Respiratory Society, are an attempt to provide clinicians with the best possible evidence-based guidance on managing patients with aspergillosis, primarily those with CPA (Eur Respir J. 2015. doi: 10.1183/13993003.00583-2015).
Dr. Edelman noted that the most frequent presentation he sees – and that very infrequently – is allergic bronchopulmonary aspergillosis in patients with asthma.
The most recent U.S. guidelines, issued under the aegis of the Infectious Diseases Society of America (IDSA) in 2000 and revised in 2008 (CID 2008;46:327-360), differ from the European recommendations in their level of detail, explained Prof. David W. Denning, professor of infectious diseases in global health at the University of Manchester (England) and lead author of the European guidelines.
“The IDSA guidelines assume that you know how to make the diagnosis, but actually for chronic pulmonary aspergillosis that’s not so easy with some patients,” he said in an interview.
“The European ones go into in great detail the diagnosis, the radiology, whether this test is better than that test, how they all add up, and all that sort of stuff,” he said,
The European guidelines also make recommendations for duration of therapy and comment on the use of steroids and immunotherapy with interferon-gamma, Dr. Denning noted.
Diagnostic criteria
The European guidelines categorize Aspergillus infections according to differences in clinical management:
• Simple aspergilloma. A single pulmonary cavity containing a fungal ball, supported by serologic or microbiologic evidence of infections with Aspergillus species in patients who are not immunocompromised and are asymptomatic or have only minor symptoms and no radiographic evidence of progression for at least 3 months.
• Chronic cavitary pulmonary aspergillosis (CCPA). The presence of one or more pulmonary cavities that may contain one or more aspergillomas or irregular intraluminal material, evidence of Aspergillus species, significant pulmonary/systemic symptoms, and overt progression on radiography over 3 or more months of observations.
• Chronic fibrosing pulmonary aspergillosis (CFPA). Severe, fibrotic destruction of at least two lung lobes as a complication of CCPA, causing a major loss of lung function. The guidelines note that destruction of a single lobe is designated as CCPA of that lobe.
• Aspergillus nodules. This unusual presentation is marked by the presence of one or more nodules that may or may not cavitate. The nodules may resemble tuberculoma, carcinoma of the lung, or coccidioidomycosis; histology is required to make an accurate diagnosis.
• Subacute invasive aspergillosis (SAIA). This can occur over the course of 1-3 months in patients who are mildly immunocompromised. Radiologic features can vary, and may include cavitation, the presence of nodules, and progressive consolidation with the appearance of abscess formation. Fungal hyphae (filaments) can be seen in biopsied lung tissues, and there may be evidence of Aspergillus galactomannan antigen in respiratory fluids or blood.
Treatment
The guidelines note that most of the evidence for managing CPA are based on cohort studies and case reports rather than randomized clinical trials, and that there have been no head-to-head trials comparing oral triazole agents.
For treatment of CPA, the European guidelines recommend:
• Itraconazole 200 mg twice daily, with therapeutic drug monitoring and dose adjustment as necessary (Grade A [strong] recommendation).
• Voriconazole 150-200 mg twice daily, with monitoring and dose adjustment. The guidelines recommend lower doses for patients older than 70 years, those with low body weight, significant liver disease, and/or those of Northeast Asian descent, who may be genetically inclined to slow drug metabolism (Grade A).
• Posaconazole liquid 400 mg twice daily, or tablets 300 mg once daily (Grade B [moderate] recommendation].
In general, the recommended duration of therapy for control of infection in patients with CPA or curative intent for patients with SAIA or chronic necrotizing pulmonary aspergillosis is 6 months or more, depending on patient status and drug tolerance.
For patients with CPA with progressive disease, those whom therapy has failed, or those who are intolerant of or have disease resistant to triazoles, intravenous therapy with micafungin, 150 mg day (Grade B); amphotericin B deoxycholate, 0.7-1.0 mg/kg per day (Grade C [marginal] recommendation); liposomal amphotericin B, 3 mg/kg per day (Grade B); or caspofungin, 50-70 mg/day (Grade C) are recommended.
The guidelines also recommend surgical excision of simple aspergilloma, preferably by a video-assisted thoracic surgery technique, if technically feasible.
“In my own experience, we resort to surgery very infrequently,” Dr. Edelman said.
He noted that it would be helpful if the guidelines had also allergic bronchopulmonary aspergillosis as a separate entity.
Ideal not always achievable
Prof. Denning points out that the optimum therapies and practices described in the guidelines can’t always be implemented. Worldwide, he said, antifungal therapy is not widely available, with the exception of fluconazole, which has no activity against Aspergillus, and is inferior to itraconazole and other extended azoles for other fungal diseases such as histoplasmosis, blastomycosis, and paracoccidioidomycosis.
The price of antifungal therapies can also be a barrier to effective treatment in many parts of the world.
“If you’re having to pay for your medicines and you’re living on $5 or $10 a day in Kenya, say, you can’t afford to buy them. So even if the drugs are physically there, it may not be really affordable for a course of therapy for these patients, and there’s some advocacy to be done around that for the whole world,” he said.
The guidelines were funded primarily by grants from ESCMID and ERS with additional support from ECMM. Authors’ travel expenses were funded jointly by ESCMID and ERS. Dr. Denning has received grant support and founder shares in F2G, and has received grants from the Fungal Research Trust, Wellcome Trust, Moulton Trust, Medical Research Council, Chronic Granulomatous Disease Research Trust, National Institute of Allergy and Infectious Diseases, National Institute of Health Research and the European Union, and AstraZeneca. Dr. Edelman reported no relevant disclosures.
European respiratory disease and infectious disease specialists have banded together to issue new clinical guidelines on the diagnosis and management of an uncommon but serious problem: chronic pulmonary aspergillosis (CPA).
Pulmonary infections with Aspergillus species, although uncommon, are a complicating factor in several lung diseases, especially tuberculosis, and aspergillosis is a serious, often fatal opportunistic infection in transplant recipients who are on chronic immunosuppression or patients who are immunocompromised from disease or cytotoxic chemotherapy.
Approximately 240,000 people in Europe and 3 million people worldwide have chronic pulmonary aspergillosis (CPA). The Centers for Disease Control and Prevention notes that because aspergillosis is not classified as a reportable disease, data on the actual incidence of infections in the United States are hard to come by.
“You don’t see this every day, whether you’re an infectious disease specialist or pulmonologist, so you really can’t rely on your experience to guide you in managing these cases, which is why guidelines such as this can be very helpful,” commented Dr. Norman Edelman, a pulmonologist and senior consultant for scientific affairs for the American Lung Association.
The guidelines, issued by the European Society for Clinical Microbiology and Infectious Diseases in cooperation with the European Confederation of Medical Mycology and the European Respiratory Society, are an attempt to provide clinicians with the best possible evidence-based guidance on managing patients with aspergillosis, primarily those with CPA (Eur Respir J. 2015. doi: 10.1183/13993003.00583-2015).
Dr. Edelman noted that the most frequent presentation he sees – and that very infrequently – is allergic bronchopulmonary aspergillosis in patients with asthma.
The most recent U.S. guidelines, issued under the aegis of the Infectious Diseases Society of America (IDSA) in 2000 and revised in 2008 (CID 2008;46:327-360), differ from the European recommendations in their level of detail, explained Prof. David W. Denning, professor of infectious diseases in global health at the University of Manchester (England) and lead author of the European guidelines.
“The IDSA guidelines assume that you know how to make the diagnosis, but actually for chronic pulmonary aspergillosis that’s not so easy with some patients,” he said in an interview.
“The European ones go into in great detail the diagnosis, the radiology, whether this test is better than that test, how they all add up, and all that sort of stuff,” he said,
The European guidelines also make recommendations for duration of therapy and comment on the use of steroids and immunotherapy with interferon-gamma, Dr. Denning noted.
Diagnostic criteria
The European guidelines categorize Aspergillus infections according to differences in clinical management:
• Simple aspergilloma. A single pulmonary cavity containing a fungal ball, supported by serologic or microbiologic evidence of infections with Aspergillus species in patients who are not immunocompromised and are asymptomatic or have only minor symptoms and no radiographic evidence of progression for at least 3 months.
• Chronic cavitary pulmonary aspergillosis (CCPA). The presence of one or more pulmonary cavities that may contain one or more aspergillomas or irregular intraluminal material, evidence of Aspergillus species, significant pulmonary/systemic symptoms, and overt progression on radiography over 3 or more months of observations.
• Chronic fibrosing pulmonary aspergillosis (CFPA). Severe, fibrotic destruction of at least two lung lobes as a complication of CCPA, causing a major loss of lung function. The guidelines note that destruction of a single lobe is designated as CCPA of that lobe.
• Aspergillus nodules. This unusual presentation is marked by the presence of one or more nodules that may or may not cavitate. The nodules may resemble tuberculoma, carcinoma of the lung, or coccidioidomycosis; histology is required to make an accurate diagnosis.
• Subacute invasive aspergillosis (SAIA). This can occur over the course of 1-3 months in patients who are mildly immunocompromised. Radiologic features can vary, and may include cavitation, the presence of nodules, and progressive consolidation with the appearance of abscess formation. Fungal hyphae (filaments) can be seen in biopsied lung tissues, and there may be evidence of Aspergillus galactomannan antigen in respiratory fluids or blood.
Treatment
The guidelines note that most of the evidence for managing CPA are based on cohort studies and case reports rather than randomized clinical trials, and that there have been no head-to-head trials comparing oral triazole agents.
For treatment of CPA, the European guidelines recommend:
• Itraconazole 200 mg twice daily, with therapeutic drug monitoring and dose adjustment as necessary (Grade A [strong] recommendation).
• Voriconazole 150-200 mg twice daily, with monitoring and dose adjustment. The guidelines recommend lower doses for patients older than 70 years, those with low body weight, significant liver disease, and/or those of Northeast Asian descent, who may be genetically inclined to slow drug metabolism (Grade A).
• Posaconazole liquid 400 mg twice daily, or tablets 300 mg once daily (Grade B [moderate] recommendation].
In general, the recommended duration of therapy for control of infection in patients with CPA or curative intent for patients with SAIA or chronic necrotizing pulmonary aspergillosis is 6 months or more, depending on patient status and drug tolerance.
For patients with CPA with progressive disease, those whom therapy has failed, or those who are intolerant of or have disease resistant to triazoles, intravenous therapy with micafungin, 150 mg day (Grade B); amphotericin B deoxycholate, 0.7-1.0 mg/kg per day (Grade C [marginal] recommendation); liposomal amphotericin B, 3 mg/kg per day (Grade B); or caspofungin, 50-70 mg/day (Grade C) are recommended.
The guidelines also recommend surgical excision of simple aspergilloma, preferably by a video-assisted thoracic surgery technique, if technically feasible.
“In my own experience, we resort to surgery very infrequently,” Dr. Edelman said.
He noted that it would be helpful if the guidelines had also allergic bronchopulmonary aspergillosis as a separate entity.
Ideal not always achievable
Prof. Denning points out that the optimum therapies and practices described in the guidelines can’t always be implemented. Worldwide, he said, antifungal therapy is not widely available, with the exception of fluconazole, which has no activity against Aspergillus, and is inferior to itraconazole and other extended azoles for other fungal diseases such as histoplasmosis, blastomycosis, and paracoccidioidomycosis.
The price of antifungal therapies can also be a barrier to effective treatment in many parts of the world.
“If you’re having to pay for your medicines and you’re living on $5 or $10 a day in Kenya, say, you can’t afford to buy them. So even if the drugs are physically there, it may not be really affordable for a course of therapy for these patients, and there’s some advocacy to be done around that for the whole world,” he said.
The guidelines were funded primarily by grants from ESCMID and ERS with additional support from ECMM. Authors’ travel expenses were funded jointly by ESCMID and ERS. Dr. Denning has received grant support and founder shares in F2G, and has received grants from the Fungal Research Trust, Wellcome Trust, Moulton Trust, Medical Research Council, Chronic Granulomatous Disease Research Trust, National Institute of Allergy and Infectious Diseases, National Institute of Health Research and the European Union, and AstraZeneca. Dr. Edelman reported no relevant disclosures.
European respiratory disease and infectious disease specialists have banded together to issue new clinical guidelines on the diagnosis and management of an uncommon but serious problem: chronic pulmonary aspergillosis (CPA).
Pulmonary infections with Aspergillus species, although uncommon, are a complicating factor in several lung diseases, especially tuberculosis, and aspergillosis is a serious, often fatal opportunistic infection in transplant recipients who are on chronic immunosuppression or patients who are immunocompromised from disease or cytotoxic chemotherapy.
Approximately 240,000 people in Europe and 3 million people worldwide have chronic pulmonary aspergillosis (CPA). The Centers for Disease Control and Prevention notes that because aspergillosis is not classified as a reportable disease, data on the actual incidence of infections in the United States are hard to come by.
“You don’t see this every day, whether you’re an infectious disease specialist or pulmonologist, so you really can’t rely on your experience to guide you in managing these cases, which is why guidelines such as this can be very helpful,” commented Dr. Norman Edelman, a pulmonologist and senior consultant for scientific affairs for the American Lung Association.
The guidelines, issued by the European Society for Clinical Microbiology and Infectious Diseases in cooperation with the European Confederation of Medical Mycology and the European Respiratory Society, are an attempt to provide clinicians with the best possible evidence-based guidance on managing patients with aspergillosis, primarily those with CPA (Eur Respir J. 2015. doi: 10.1183/13993003.00583-2015).
Dr. Edelman noted that the most frequent presentation he sees – and that very infrequently – is allergic bronchopulmonary aspergillosis in patients with asthma.
The most recent U.S. guidelines, issued under the aegis of the Infectious Diseases Society of America (IDSA) in 2000 and revised in 2008 (CID 2008;46:327-360), differ from the European recommendations in their level of detail, explained Prof. David W. Denning, professor of infectious diseases in global health at the University of Manchester (England) and lead author of the European guidelines.
“The IDSA guidelines assume that you know how to make the diagnosis, but actually for chronic pulmonary aspergillosis that’s not so easy with some patients,” he said in an interview.
“The European ones go into in great detail the diagnosis, the radiology, whether this test is better than that test, how they all add up, and all that sort of stuff,” he said,
The European guidelines also make recommendations for duration of therapy and comment on the use of steroids and immunotherapy with interferon-gamma, Dr. Denning noted.
Diagnostic criteria
The European guidelines categorize Aspergillus infections according to differences in clinical management:
• Simple aspergilloma. A single pulmonary cavity containing a fungal ball, supported by serologic or microbiologic evidence of infections with Aspergillus species in patients who are not immunocompromised and are asymptomatic or have only minor symptoms and no radiographic evidence of progression for at least 3 months.
• Chronic cavitary pulmonary aspergillosis (CCPA). The presence of one or more pulmonary cavities that may contain one or more aspergillomas or irregular intraluminal material, evidence of Aspergillus species, significant pulmonary/systemic symptoms, and overt progression on radiography over 3 or more months of observations.
• Chronic fibrosing pulmonary aspergillosis (CFPA). Severe, fibrotic destruction of at least two lung lobes as a complication of CCPA, causing a major loss of lung function. The guidelines note that destruction of a single lobe is designated as CCPA of that lobe.
• Aspergillus nodules. This unusual presentation is marked by the presence of one or more nodules that may or may not cavitate. The nodules may resemble tuberculoma, carcinoma of the lung, or coccidioidomycosis; histology is required to make an accurate diagnosis.
• Subacute invasive aspergillosis (SAIA). This can occur over the course of 1-3 months in patients who are mildly immunocompromised. Radiologic features can vary, and may include cavitation, the presence of nodules, and progressive consolidation with the appearance of abscess formation. Fungal hyphae (filaments) can be seen in biopsied lung tissues, and there may be evidence of Aspergillus galactomannan antigen in respiratory fluids or blood.
Treatment
The guidelines note that most of the evidence for managing CPA are based on cohort studies and case reports rather than randomized clinical trials, and that there have been no head-to-head trials comparing oral triazole agents.
For treatment of CPA, the European guidelines recommend:
• Itraconazole 200 mg twice daily, with therapeutic drug monitoring and dose adjustment as necessary (Grade A [strong] recommendation).
• Voriconazole 150-200 mg twice daily, with monitoring and dose adjustment. The guidelines recommend lower doses for patients older than 70 years, those with low body weight, significant liver disease, and/or those of Northeast Asian descent, who may be genetically inclined to slow drug metabolism (Grade A).
• Posaconazole liquid 400 mg twice daily, or tablets 300 mg once daily (Grade B [moderate] recommendation].
In general, the recommended duration of therapy for control of infection in patients with CPA or curative intent for patients with SAIA or chronic necrotizing pulmonary aspergillosis is 6 months or more, depending on patient status and drug tolerance.
For patients with CPA with progressive disease, those whom therapy has failed, or those who are intolerant of or have disease resistant to triazoles, intravenous therapy with micafungin, 150 mg day (Grade B); amphotericin B deoxycholate, 0.7-1.0 mg/kg per day (Grade C [marginal] recommendation); liposomal amphotericin B, 3 mg/kg per day (Grade B); or caspofungin, 50-70 mg/day (Grade C) are recommended.
The guidelines also recommend surgical excision of simple aspergilloma, preferably by a video-assisted thoracic surgery technique, if technically feasible.
“In my own experience, we resort to surgery very infrequently,” Dr. Edelman said.
He noted that it would be helpful if the guidelines had also allergic bronchopulmonary aspergillosis as a separate entity.
Ideal not always achievable
Prof. Denning points out that the optimum therapies and practices described in the guidelines can’t always be implemented. Worldwide, he said, antifungal therapy is not widely available, with the exception of fluconazole, which has no activity against Aspergillus, and is inferior to itraconazole and other extended azoles for other fungal diseases such as histoplasmosis, blastomycosis, and paracoccidioidomycosis.
The price of antifungal therapies can also be a barrier to effective treatment in many parts of the world.
“If you’re having to pay for your medicines and you’re living on $5 or $10 a day in Kenya, say, you can’t afford to buy them. So even if the drugs are physically there, it may not be really affordable for a course of therapy for these patients, and there’s some advocacy to be done around that for the whole world,” he said.
The guidelines were funded primarily by grants from ESCMID and ERS with additional support from ECMM. Authors’ travel expenses were funded jointly by ESCMID and ERS. Dr. Denning has received grant support and founder shares in F2G, and has received grants from the Fungal Research Trust, Wellcome Trust, Moulton Trust, Medical Research Council, Chronic Granulomatous Disease Research Trust, National Institute of Allergy and Infectious Diseases, National Institute of Health Research and the European Union, and AstraZeneca. Dr. Edelman reported no relevant disclosures.
FROM JOURNAL OF DRUGS IN DERMATOLOGY