User login
VIDEO: MS stem cell therapy research progresses, including oligodendrocyte progenitor trial
NEW ORLEANS – Stem cell therapy for progressive multiple sclerosis is an intriguing and controversial topic, and the state of the related science was addressed during a session on “the treatment pipeline” at a meeting sponsored by the Americas Committee for Treatment and Research in Multiple Sclerosis.
In a video interview at the meeting, session chair Dr. Mark Freedman of the University of Ottawa (Ont.) discussed the status of autologous hematopoietic stem cell transplantation; how mesenchymal stem cells are thought to be a potential source for immune system repair; and the intriguing potential for remyelinating therapy with human oligodendrocyte progenitor cells. Research is in the “very preliminary stage” on human oligodendrocyte progenitor cells, but “enticing news” of a safety trial set to begin in North America was presented during the session, he said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Stem cell therapy for progressive multiple sclerosis is an intriguing and controversial topic, and the state of the related science was addressed during a session on “the treatment pipeline” at a meeting sponsored by the Americas Committee for Treatment and Research in Multiple Sclerosis.
In a video interview at the meeting, session chair Dr. Mark Freedman of the University of Ottawa (Ont.) discussed the status of autologous hematopoietic stem cell transplantation; how mesenchymal stem cells are thought to be a potential source for immune system repair; and the intriguing potential for remyelinating therapy with human oligodendrocyte progenitor cells. Research is in the “very preliminary stage” on human oligodendrocyte progenitor cells, but “enticing news” of a safety trial set to begin in North America was presented during the session, he said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – Stem cell therapy for progressive multiple sclerosis is an intriguing and controversial topic, and the state of the related science was addressed during a session on “the treatment pipeline” at a meeting sponsored by the Americas Committee for Treatment and Research in Multiple Sclerosis.
In a video interview at the meeting, session chair Dr. Mark Freedman of the University of Ottawa (Ont.) discussed the status of autologous hematopoietic stem cell transplantation; how mesenchymal stem cells are thought to be a potential source for immune system repair; and the intriguing potential for remyelinating therapy with human oligodendrocyte progenitor cells. Research is in the “very preliminary stage” on human oligodendrocyte progenitor cells, but “enticing news” of a safety trial set to begin in North America was presented during the session, he said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM ACTRIMS FORUM 2016
Ocrelizumab subanalysis hints at better efficacy with active inflammatory lesions
NEW ORLEANS – The greatest effect of the investigational, B-cell–targeting humanized monoclonal antibody ocrelizumab in treating primary progressive multiple sclerosis (PPMS) may be in patients with T1 gadolinium-positive (Gd+) lesions at baseline, which are indicative of an ongoing or recent MS relapse, according to a subgroup analysis of the randomized, double-blind, placebo-controlled ORATORIO trial.
The analysis hinted at the possibility that ocrelizumab may reduce the risk of confirmed disability progression (CDP) at 12 weeks or 24 weeks to a slightly higher degree among PPMS patients with T1 Gd+ lesions than in those without such lesions, although the differences did not reach statistical significance. The findings await further study because ORATORIO was not powered to demonstrate efficacy differences between the subgroups, Dr. Jerry Wolinsky of the University of Texas Health Science Center at Houston noted in a presentation at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
In ORATORIO, ocrelizumab (600 mg intravenous infused every 6 months as two 300-mg infusions given 2 weeks apart) was compared with placebo in 732 people with PPMS in a 120-week blinded treatment. The number of patients with T1 Gd+ lesions at baseline was similar in the placebo arm (60/244, 24.7%) and the ocrelizumab arm (133/488, 27.5%). T1 Gd-negative (Gd-) lesions, which are likely older and indicative of the absence of a relapse, were identified in 183 and 350 of the patients in the placebo and ocrelizumab arms, respectively.
Time to onset of 12-week confirmed disability progression (CDP) was delayed in a quarter of the overall population (hazard ratio, 0.76; 95% confidence interval, 0.59-0.98). Risk reduction was greater in the presence of T1 Gd+ lesions at baseline (HR, 0.65; 95% CI, 0.40-1.06), compared with patients harboring T1 Gd- lesions at baseline (HR, 0.84; 95% CI, 0.62-1.13). A similar pattern was evident for time to onset of 24-week CDP.
The changes in timed 25-foot walk from baseline to week 20, and in brain T2 hyperintense lesion volume from baseline to week 120 were significantly reduced overall by treatment. Reductions in walk time and lesion volume were apparent in the T1 Gd+ and Gd- lesion subgroups, with similar percentage change. In the overall study, ocrelizumab significantly slowed decline in brain volume from weeks 24 to 120, and slowed declines were also evident in the T1 Gd+ and Gd- lesion subgroups, compared with placebo.
The findings warrant further studies powered to assess the apparent treatment benefit in patients with Gd+ lesions – who are likely relapsing – at baseline. If the findings hold, stratifying patients prior to treatment based on MRI of T1 Gd+ lesions could help to guide ocrelizumab treatment.
Gadolinium normally cannot pass from the bloodstream into the brain or spinal cord because of the presence of the blood-brain barrier. Active inflammation in the brain or spinal cord during a MS relapse disrupts the barrier. Gadolinium enters the brain or spinal cord and permeates into MS lesions, which appear bright on MRI.
The study was funded by Hoffmann-La Roche/Genentech. Dr. Wolinsky disclosed receiving consulting fees; compensation for service on steering committees or data monitoring boards; and/or research support from many companies that market MS drugs, including Hoffmann-La Roche/Genentech.
NEW ORLEANS – The greatest effect of the investigational, B-cell–targeting humanized monoclonal antibody ocrelizumab in treating primary progressive multiple sclerosis (PPMS) may be in patients with T1 gadolinium-positive (Gd+) lesions at baseline, which are indicative of an ongoing or recent MS relapse, according to a subgroup analysis of the randomized, double-blind, placebo-controlled ORATORIO trial.
The analysis hinted at the possibility that ocrelizumab may reduce the risk of confirmed disability progression (CDP) at 12 weeks or 24 weeks to a slightly higher degree among PPMS patients with T1 Gd+ lesions than in those without such lesions, although the differences did not reach statistical significance. The findings await further study because ORATORIO was not powered to demonstrate efficacy differences between the subgroups, Dr. Jerry Wolinsky of the University of Texas Health Science Center at Houston noted in a presentation at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
In ORATORIO, ocrelizumab (600 mg intravenous infused every 6 months as two 300-mg infusions given 2 weeks apart) was compared with placebo in 732 people with PPMS in a 120-week blinded treatment. The number of patients with T1 Gd+ lesions at baseline was similar in the placebo arm (60/244, 24.7%) and the ocrelizumab arm (133/488, 27.5%). T1 Gd-negative (Gd-) lesions, which are likely older and indicative of the absence of a relapse, were identified in 183 and 350 of the patients in the placebo and ocrelizumab arms, respectively.
Time to onset of 12-week confirmed disability progression (CDP) was delayed in a quarter of the overall population (hazard ratio, 0.76; 95% confidence interval, 0.59-0.98). Risk reduction was greater in the presence of T1 Gd+ lesions at baseline (HR, 0.65; 95% CI, 0.40-1.06), compared with patients harboring T1 Gd- lesions at baseline (HR, 0.84; 95% CI, 0.62-1.13). A similar pattern was evident for time to onset of 24-week CDP.
The changes in timed 25-foot walk from baseline to week 20, and in brain T2 hyperintense lesion volume from baseline to week 120 were significantly reduced overall by treatment. Reductions in walk time and lesion volume were apparent in the T1 Gd+ and Gd- lesion subgroups, with similar percentage change. In the overall study, ocrelizumab significantly slowed decline in brain volume from weeks 24 to 120, and slowed declines were also evident in the T1 Gd+ and Gd- lesion subgroups, compared with placebo.
The findings warrant further studies powered to assess the apparent treatment benefit in patients with Gd+ lesions – who are likely relapsing – at baseline. If the findings hold, stratifying patients prior to treatment based on MRI of T1 Gd+ lesions could help to guide ocrelizumab treatment.
Gadolinium normally cannot pass from the bloodstream into the brain or spinal cord because of the presence of the blood-brain barrier. Active inflammation in the brain or spinal cord during a MS relapse disrupts the barrier. Gadolinium enters the brain or spinal cord and permeates into MS lesions, which appear bright on MRI.
The study was funded by Hoffmann-La Roche/Genentech. Dr. Wolinsky disclosed receiving consulting fees; compensation for service on steering committees or data monitoring boards; and/or research support from many companies that market MS drugs, including Hoffmann-La Roche/Genentech.
NEW ORLEANS – The greatest effect of the investigational, B-cell–targeting humanized monoclonal antibody ocrelizumab in treating primary progressive multiple sclerosis (PPMS) may be in patients with T1 gadolinium-positive (Gd+) lesions at baseline, which are indicative of an ongoing or recent MS relapse, according to a subgroup analysis of the randomized, double-blind, placebo-controlled ORATORIO trial.
The analysis hinted at the possibility that ocrelizumab may reduce the risk of confirmed disability progression (CDP) at 12 weeks or 24 weeks to a slightly higher degree among PPMS patients with T1 Gd+ lesions than in those without such lesions, although the differences did not reach statistical significance. The findings await further study because ORATORIO was not powered to demonstrate efficacy differences between the subgroups, Dr. Jerry Wolinsky of the University of Texas Health Science Center at Houston noted in a presentation at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
In ORATORIO, ocrelizumab (600 mg intravenous infused every 6 months as two 300-mg infusions given 2 weeks apart) was compared with placebo in 732 people with PPMS in a 120-week blinded treatment. The number of patients with T1 Gd+ lesions at baseline was similar in the placebo arm (60/244, 24.7%) and the ocrelizumab arm (133/488, 27.5%). T1 Gd-negative (Gd-) lesions, which are likely older and indicative of the absence of a relapse, were identified in 183 and 350 of the patients in the placebo and ocrelizumab arms, respectively.
Time to onset of 12-week confirmed disability progression (CDP) was delayed in a quarter of the overall population (hazard ratio, 0.76; 95% confidence interval, 0.59-0.98). Risk reduction was greater in the presence of T1 Gd+ lesions at baseline (HR, 0.65; 95% CI, 0.40-1.06), compared with patients harboring T1 Gd- lesions at baseline (HR, 0.84; 95% CI, 0.62-1.13). A similar pattern was evident for time to onset of 24-week CDP.
The changes in timed 25-foot walk from baseline to week 20, and in brain T2 hyperintense lesion volume from baseline to week 120 were significantly reduced overall by treatment. Reductions in walk time and lesion volume were apparent in the T1 Gd+ and Gd- lesion subgroups, with similar percentage change. In the overall study, ocrelizumab significantly slowed decline in brain volume from weeks 24 to 120, and slowed declines were also evident in the T1 Gd+ and Gd- lesion subgroups, compared with placebo.
The findings warrant further studies powered to assess the apparent treatment benefit in patients with Gd+ lesions – who are likely relapsing – at baseline. If the findings hold, stratifying patients prior to treatment based on MRI of T1 Gd+ lesions could help to guide ocrelizumab treatment.
Gadolinium normally cannot pass from the bloodstream into the brain or spinal cord because of the presence of the blood-brain barrier. Active inflammation in the brain or spinal cord during a MS relapse disrupts the barrier. Gadolinium enters the brain or spinal cord and permeates into MS lesions, which appear bright on MRI.
The study was funded by Hoffmann-La Roche/Genentech. Dr. Wolinsky disclosed receiving consulting fees; compensation for service on steering committees or data monitoring boards; and/or research support from many companies that market MS drugs, including Hoffmann-La Roche/Genentech.
AT ACTRIMS FORUM 2016
Key clinical point: Certain subgroups of patients may respond better to treatment
Major finding: Reduction in the risk of confirmed disability progression at 12 weeks was greater in the presence of T1 Gd+ lesions at baseline (HR, 0.65; 95% CI, 0.40-1.06), compared with patients harboring T1 Gd- lesions at baseline (HR, 0.84; 95% CI, 0.62-1.13).
Data source: The phase III, randomized, double-blind, placebo-controlled ORATORIO trial.
Disclosures: The study was funded by Hoffmann-La Roche/Genentech. Dr. Wolinsky disclosed receiving consulting fees; compensation for service on steering committees or data monitoring boards; and/or research support from many companies that market MS drugs, including Hoffmann-La Roche/Genentech.
Long-term PPI use linked to increased risk of dementia
Long-term use of proton pump inhibitors was significantly associated with later diagnoses of dementia in adults aged 75 years and older in a prospective cohort study of more than 73,000 individuals. The findings were published online Feb. 15 in JAMA Neurology.
Overall, the risk of incident dementia was 44% higher among the 2,950 patients who received regular proton pump inhibitors, compared with 70,729 who didn’t receive PPIs (hazard ratio of 1.44), according to Willy Gomm, Ph.D., of the German Center for Neurodegenerative Diseases in Bonn, and his colleagues.
To assess the potential link between PPIs and dementia, the researchers reviewed data from a German insurance database during 2004-2011. The study population included 73,679 community-dwelling adults aged 75 years and older who were free of dementia at the start of the study (JAMA Neurol. 2016 Feb 15. doi: 10.1001/jamaneurol.2015.4791). The patients taking PPIs were slightly but significantly older than those not taking PPIs and had a higher proportion of women (P less than .001 for both). PPI users were also significantly more likely than nonusers to have a history of depression, stroke, coronary disease, and use of polypharmacy (P less than .001 for each).
The risk of incident dementia decreased with age, from 69% for patients aged 75-79 years to 49% among those 80-84-years and 32% among those aged 85 years and older.
In addition, the risk of dementia was not significantly different based on specific drug in a subgroup analysis of the three most often prescribed PPIs, omeprazole, pantoprazole, and esomeprazole, for which the hazard ratios were 1.51, 1.58, and 2.12, respectively.
“If PPIs have adverse effects, it is important to be aware of them,” Dr. Daniel E. Freedberg of Columbia University, New York, said in an interview. “When PPIs are indicated, the preponderance of data indicate that their benefits outweigh their potential risks,” he added. “Clinicians should reassure patients that this was a single study and that previous studies have reached different conclusions. Clinicians should focus on whether or not PPIs are indicated rather than on PPI side effects.”
Dr. Freedberg also noted several key limitations of the study.
“First, the authors were unable to adjust for crucial variables that might explain a noncausal link between PPIs and dementia. For example, lower socioeconomic status is an established predictor of dementia and may also be associated with PPI use. However, the authors could not capture socioeconomic status.
“Second, patients who use PPIs have more frequent and more intensive health care interactions than patients who do not use PPIs. These patients are thus also more likely to be diagnosed with dementia. This is another source for bias that the authors were not able to capture. Third, clinicians should be aware that this study was designed to compare extremes of PPI use,” Dr. Freedberg emphasized.
In addition, “In the primary analysis, patients were classified as exposed to PPIs only if they received at least one PPI prescription every 3 months for an 18-month period. Patients who used occasional PPIs were excluded from the study,” said Dr. Freedberg.
“The present study can only provide a statistical association between PPI use and risk of dementia,” the researchers noted. “The possible underlying causal biological mechanism has to be explored in future studies,” they wrote.
The researchers had no financial conflicts to disclose.
Challenging research lies ahead
The researchers have provided an important and interesting challenge to evaluate the possible association of the use of PPIs and the risk of dementia.

|
Dr. Lewis H. Kuller |
Further determinants of whether PPIs are causal for dementia requires validation in large cohorts and probably in well-designed case-control studies with good measures of long-term PPI use, covariates, and especially methods to measure incidence of dementia.
Dr. Lewis H. Kuller is affiliated with the department of epidemiology at the University of Pittsburgh. He made his remarks in an accompanying editorial and had no financial conflicts to disclose.
PPIs and dementia: More of much ado about nothing?
This study has attracted considerable media attention. Unfortunately, this was somewhat unbalanced. One TV news program stated that there was a “44% increased risk of dementia.” Many of our patients would not be able to interpret that appropriately – let alone weigh the potential risks and benefits of PPI treatment.
The 44% increase is derived from the hazard ratio of 1.44 that the investigators reported. However, to quote Dr. David A. Grimes and Dr. Kenneth F. Schulz, “Any claim coming from an observational study is most likely to be wrong. Of the reported associations that are correct, most are exaggerated” (Obstet Gynecol. 2012;120:920-7).
In the German study, the patients who had been taking PPIs were more likely to have prior diagnoses of depression, stroke, and ischemic heart disease than those not on PPIs. They were also much more likely to be on multiple other medicines. These patients may have been more likely to be given a PPI because of their comorbidity. There may, therefore, be an element of channeling bias or confounding by indication to explain the findings.
However, let us assume that there had been a stronger suggestion of a causal association between PPI use and the risk of developing dementia. It would be important to consider what its underlying biological rationale might be. The investigators state that PPI use “has been shown to be potentially involved in cognitive decline.” The evidence offered in support of this claim comes from a case-control study that did not evaluate cognitive function but reported an increased incidence of vitamin B12 deficiency among adults taking PPIs for 2 or more years [very small odds ratio of 1.65 (JAMA 2013;310:2435-42)]. Low B12 levels were linked to “cognitive deficit” in a population-based study from Norway (Psychosom Med. 2013;75:20-9). So, the proposed biological rationale for any association between PPI use and dementia is tenuous at best.
For PPIs, long-term or indefinite use is appropriate for indications such as erosive esophagitis, esophageal stricture, or the prevention of upper GI tract ulcers and ulcer bleeding in patients taking aspirin or NSAIDs. Patients with a valid indication for PPI treatment should continue to receive it for as long as clinically indicated – and at the lowest effective dose. It would be unfortunate if patients with indications for PPI treatment discontinued it on the basis of this study.
Dr. Colin W. Howden, AGAF, is Hyman Professor of Medicine, chief of the division of gastroenterology, University of Tennessee Health Science Center, Memphis. He is a consultant for Takeda, Otsuka, Ironwood, Allergan, Aralez, and Pfizer Consumer Health.
Challenging research lies ahead
The researchers have provided an important and interesting challenge to evaluate the possible association of the use of PPIs and the risk of dementia.

|
Dr. Lewis H. Kuller |
Further determinants of whether PPIs are causal for dementia requires validation in large cohorts and probably in well-designed case-control studies with good measures of long-term PPI use, covariates, and especially methods to measure incidence of dementia.
Dr. Lewis H. Kuller is affiliated with the department of epidemiology at the University of Pittsburgh. He made his remarks in an accompanying editorial and had no financial conflicts to disclose.
PPIs and dementia: More of much ado about nothing?
This study has attracted considerable media attention. Unfortunately, this was somewhat unbalanced. One TV news program stated that there was a “44% increased risk of dementia.” Many of our patients would not be able to interpret that appropriately – let alone weigh the potential risks and benefits of PPI treatment.
The 44% increase is derived from the hazard ratio of 1.44 that the investigators reported. However, to quote Dr. David A. Grimes and Dr. Kenneth F. Schulz, “Any claim coming from an observational study is most likely to be wrong. Of the reported associations that are correct, most are exaggerated” (Obstet Gynecol. 2012;120:920-7).
In the German study, the patients who had been taking PPIs were more likely to have prior diagnoses of depression, stroke, and ischemic heart disease than those not on PPIs. They were also much more likely to be on multiple other medicines. These patients may have been more likely to be given a PPI because of their comorbidity. There may, therefore, be an element of channeling bias or confounding by indication to explain the findings.
However, let us assume that there had been a stronger suggestion of a causal association between PPI use and the risk of developing dementia. It would be important to consider what its underlying biological rationale might be. The investigators state that PPI use “has been shown to be potentially involved in cognitive decline.” The evidence offered in support of this claim comes from a case-control study that did not evaluate cognitive function but reported an increased incidence of vitamin B12 deficiency among adults taking PPIs for 2 or more years [very small odds ratio of 1.65 (JAMA 2013;310:2435-42)]. Low B12 levels were linked to “cognitive deficit” in a population-based study from Norway (Psychosom Med. 2013;75:20-9). So, the proposed biological rationale for any association between PPI use and dementia is tenuous at best.
For PPIs, long-term or indefinite use is appropriate for indications such as erosive esophagitis, esophageal stricture, or the prevention of upper GI tract ulcers and ulcer bleeding in patients taking aspirin or NSAIDs. Patients with a valid indication for PPI treatment should continue to receive it for as long as clinically indicated – and at the lowest effective dose. It would be unfortunate if patients with indications for PPI treatment discontinued it on the basis of this study.
Dr. Colin W. Howden, AGAF, is Hyman Professor of Medicine, chief of the division of gastroenterology, University of Tennessee Health Science Center, Memphis. He is a consultant for Takeda, Otsuka, Ironwood, Allergan, Aralez, and Pfizer Consumer Health.
Challenging research lies ahead
The researchers have provided an important and interesting challenge to evaluate the possible association of the use of PPIs and the risk of dementia.

|
Dr. Lewis H. Kuller |
Further determinants of whether PPIs are causal for dementia requires validation in large cohorts and probably in well-designed case-control studies with good measures of long-term PPI use, covariates, and especially methods to measure incidence of dementia.
Dr. Lewis H. Kuller is affiliated with the department of epidemiology at the University of Pittsburgh. He made his remarks in an accompanying editorial and had no financial conflicts to disclose.
PPIs and dementia: More of much ado about nothing?
This study has attracted considerable media attention. Unfortunately, this was somewhat unbalanced. One TV news program stated that there was a “44% increased risk of dementia.” Many of our patients would not be able to interpret that appropriately – let alone weigh the potential risks and benefits of PPI treatment.
The 44% increase is derived from the hazard ratio of 1.44 that the investigators reported. However, to quote Dr. David A. Grimes and Dr. Kenneth F. Schulz, “Any claim coming from an observational study is most likely to be wrong. Of the reported associations that are correct, most are exaggerated” (Obstet Gynecol. 2012;120:920-7).
In the German study, the patients who had been taking PPIs were more likely to have prior diagnoses of depression, stroke, and ischemic heart disease than those not on PPIs. They were also much more likely to be on multiple other medicines. These patients may have been more likely to be given a PPI because of their comorbidity. There may, therefore, be an element of channeling bias or confounding by indication to explain the findings.
However, let us assume that there had been a stronger suggestion of a causal association between PPI use and the risk of developing dementia. It would be important to consider what its underlying biological rationale might be. The investigators state that PPI use “has been shown to be potentially involved in cognitive decline.” The evidence offered in support of this claim comes from a case-control study that did not evaluate cognitive function but reported an increased incidence of vitamin B12 deficiency among adults taking PPIs for 2 or more years [very small odds ratio of 1.65 (JAMA 2013;310:2435-42)]. Low B12 levels were linked to “cognitive deficit” in a population-based study from Norway (Psychosom Med. 2013;75:20-9). So, the proposed biological rationale for any association between PPI use and dementia is tenuous at best.
For PPIs, long-term or indefinite use is appropriate for indications such as erosive esophagitis, esophageal stricture, or the prevention of upper GI tract ulcers and ulcer bleeding in patients taking aspirin or NSAIDs. Patients with a valid indication for PPI treatment should continue to receive it for as long as clinically indicated – and at the lowest effective dose. It would be unfortunate if patients with indications for PPI treatment discontinued it on the basis of this study.
Dr. Colin W. Howden, AGAF, is Hyman Professor of Medicine, chief of the division of gastroenterology, University of Tennessee Health Science Center, Memphis. He is a consultant for Takeda, Otsuka, Ironwood, Allergan, Aralez, and Pfizer Consumer Health.
Long-term use of proton pump inhibitors was significantly associated with later diagnoses of dementia in adults aged 75 years and older in a prospective cohort study of more than 73,000 individuals. The findings were published online Feb. 15 in JAMA Neurology.
Overall, the risk of incident dementia was 44% higher among the 2,950 patients who received regular proton pump inhibitors, compared with 70,729 who didn’t receive PPIs (hazard ratio of 1.44), according to Willy Gomm, Ph.D., of the German Center for Neurodegenerative Diseases in Bonn, and his colleagues.
To assess the potential link between PPIs and dementia, the researchers reviewed data from a German insurance database during 2004-2011. The study population included 73,679 community-dwelling adults aged 75 years and older who were free of dementia at the start of the study (JAMA Neurol. 2016 Feb 15. doi: 10.1001/jamaneurol.2015.4791). The patients taking PPIs were slightly but significantly older than those not taking PPIs and had a higher proportion of women (P less than .001 for both). PPI users were also significantly more likely than nonusers to have a history of depression, stroke, coronary disease, and use of polypharmacy (P less than .001 for each).
The risk of incident dementia decreased with age, from 69% for patients aged 75-79 years to 49% among those 80-84-years and 32% among those aged 85 years and older.
In addition, the risk of dementia was not significantly different based on specific drug in a subgroup analysis of the three most often prescribed PPIs, omeprazole, pantoprazole, and esomeprazole, for which the hazard ratios were 1.51, 1.58, and 2.12, respectively.
“If PPIs have adverse effects, it is important to be aware of them,” Dr. Daniel E. Freedberg of Columbia University, New York, said in an interview. “When PPIs are indicated, the preponderance of data indicate that their benefits outweigh their potential risks,” he added. “Clinicians should reassure patients that this was a single study and that previous studies have reached different conclusions. Clinicians should focus on whether or not PPIs are indicated rather than on PPI side effects.”
Dr. Freedberg also noted several key limitations of the study.
“First, the authors were unable to adjust for crucial variables that might explain a noncausal link between PPIs and dementia. For example, lower socioeconomic status is an established predictor of dementia and may also be associated with PPI use. However, the authors could not capture socioeconomic status.
“Second, patients who use PPIs have more frequent and more intensive health care interactions than patients who do not use PPIs. These patients are thus also more likely to be diagnosed with dementia. This is another source for bias that the authors were not able to capture. Third, clinicians should be aware that this study was designed to compare extremes of PPI use,” Dr. Freedberg emphasized.
In addition, “In the primary analysis, patients were classified as exposed to PPIs only if they received at least one PPI prescription every 3 months for an 18-month period. Patients who used occasional PPIs were excluded from the study,” said Dr. Freedberg.
“The present study can only provide a statistical association between PPI use and risk of dementia,” the researchers noted. “The possible underlying causal biological mechanism has to be explored in future studies,” they wrote.
The researchers had no financial conflicts to disclose.
Long-term use of proton pump inhibitors was significantly associated with later diagnoses of dementia in adults aged 75 years and older in a prospective cohort study of more than 73,000 individuals. The findings were published online Feb. 15 in JAMA Neurology.
Overall, the risk of incident dementia was 44% higher among the 2,950 patients who received regular proton pump inhibitors, compared with 70,729 who didn’t receive PPIs (hazard ratio of 1.44), according to Willy Gomm, Ph.D., of the German Center for Neurodegenerative Diseases in Bonn, and his colleagues.
To assess the potential link between PPIs and dementia, the researchers reviewed data from a German insurance database during 2004-2011. The study population included 73,679 community-dwelling adults aged 75 years and older who were free of dementia at the start of the study (JAMA Neurol. 2016 Feb 15. doi: 10.1001/jamaneurol.2015.4791). The patients taking PPIs were slightly but significantly older than those not taking PPIs and had a higher proportion of women (P less than .001 for both). PPI users were also significantly more likely than nonusers to have a history of depression, stroke, coronary disease, and use of polypharmacy (P less than .001 for each).
The risk of incident dementia decreased with age, from 69% for patients aged 75-79 years to 49% among those 80-84-years and 32% among those aged 85 years and older.
In addition, the risk of dementia was not significantly different based on specific drug in a subgroup analysis of the three most often prescribed PPIs, omeprazole, pantoprazole, and esomeprazole, for which the hazard ratios were 1.51, 1.58, and 2.12, respectively.
“If PPIs have adverse effects, it is important to be aware of them,” Dr. Daniel E. Freedberg of Columbia University, New York, said in an interview. “When PPIs are indicated, the preponderance of data indicate that their benefits outweigh their potential risks,” he added. “Clinicians should reassure patients that this was a single study and that previous studies have reached different conclusions. Clinicians should focus on whether or not PPIs are indicated rather than on PPI side effects.”
Dr. Freedberg also noted several key limitations of the study.
“First, the authors were unable to adjust for crucial variables that might explain a noncausal link between PPIs and dementia. For example, lower socioeconomic status is an established predictor of dementia and may also be associated with PPI use. However, the authors could not capture socioeconomic status.
“Second, patients who use PPIs have more frequent and more intensive health care interactions than patients who do not use PPIs. These patients are thus also more likely to be diagnosed with dementia. This is another source for bias that the authors were not able to capture. Third, clinicians should be aware that this study was designed to compare extremes of PPI use,” Dr. Freedberg emphasized.
In addition, “In the primary analysis, patients were classified as exposed to PPIs only if they received at least one PPI prescription every 3 months for an 18-month period. Patients who used occasional PPIs were excluded from the study,” said Dr. Freedberg.
“The present study can only provide a statistical association between PPI use and risk of dementia,” the researchers noted. “The possible underlying causal biological mechanism has to be explored in future studies,” they wrote.
The researchers had no financial conflicts to disclose.
FROM JAMA NEUROLOGY
Key clinical point: Proton pump inhibitors may add to the risk of dementia in older adults.
Major finding: The risk of incident dementia was 44% higher in adults who used PPIs long term, compared with those who did not.
Data source: The prospective cohort study included 73,679 adults aged 75 years and older.
Disclosures: The researchers had no financial conflicts to disclose.
ACA accelerated hospital readmission reduction efforts
Hospital readmissions have declined in recent years for three conditions targeted under the Affordable Care Act, with smaller declines for other conditions, according to new research.
The study, published online Feb. 24 in the New England Journal of Medicine, found that 30-day readmission rates declined quickly after the passage of the ACA in 2010 and then slowed at the end of 2012. The researchers also analyzed trends in the use of observation units during the same period and concluded that the drop in readmissions was not being masked by a similar uptick in patients being seen under observation status (N Engl J Med. 2016 Feb 24. doi: 10.1056/NEJMsa1513024).
Under the ACA’s Hospital Readmissions Reduction Program, hospitals are financially penalized if they have higher-than-expected readmission rates for acute myocardial infarction, heart failure, and pneumonia.
The researchers, led by Rachael B. Zuckerman, M.P.H., of the Department of Health & Human Services, examined Medicare data from 3,387 hospitals from October 2007 through May 2015. Overall readmissions for acute myocardial infarction, heart failure, and pneumonia – the three conditions targeted in the readmissions reduction program – dropped from 21.5% to 17.8% during this time period. Readmissions for nontargeted conditions also dropped from 15.3% to 13.1%.
The researchers reported that readmissions for the targeted conditions were already declining before the ACA implementation (slope of monthly rate, –0.017), accelerating between April 2010 and October 2010 (–0.103), then leveling off through 2015 (–0.05). A similar pattern was seen with readmissions for conditions not targeted under the health law, though the declines were less pronounced.
Observation rates for the targeted conditions increased from 2.6% to 4.7% during the study period, while rates for nontargeted conditions rose from 2.5% to 4.2%. The researchers did not observe any significant associations increases in observation-unit stays – which were steady throughout the study period – and the implementation of the ACA.
“It seems likely that the upward trend in observation-service use may be attributable to factors that are largely unrelated to the Hospital Readmissions Reduction Program, such as confusion over whether an inpatient stay would be deemed inappropriate by Medicare recovery audit contractors,” the researchers wrote.
Though the observational design of the study could not confirm a causal link between the ACA penalties and the drop in readmissions, the findings suggest that the declines are not solely a response to the ACA.
The health law likely “catalyzed behavioral change by many hospitals” that was already underway, possibly because of broader concern about readmissions and to earlier Medicare initiatives designed to reduce them. Also, the investigators noted, hospitals may have been helped by other government efforts on the readmission front, including the dissemination of best practices by the Centers for Medicare & Medicaid Services.
The study was funded by HHS and the researchers were agency employees. They reported having no other financial disclosures.
Hospital readmissions have declined in recent years for three conditions targeted under the Affordable Care Act, with smaller declines for other conditions, according to new research.
The study, published online Feb. 24 in the New England Journal of Medicine, found that 30-day readmission rates declined quickly after the passage of the ACA in 2010 and then slowed at the end of 2012. The researchers also analyzed trends in the use of observation units during the same period and concluded that the drop in readmissions was not being masked by a similar uptick in patients being seen under observation status (N Engl J Med. 2016 Feb 24. doi: 10.1056/NEJMsa1513024).
Under the ACA’s Hospital Readmissions Reduction Program, hospitals are financially penalized if they have higher-than-expected readmission rates for acute myocardial infarction, heart failure, and pneumonia.
The researchers, led by Rachael B. Zuckerman, M.P.H., of the Department of Health & Human Services, examined Medicare data from 3,387 hospitals from October 2007 through May 2015. Overall readmissions for acute myocardial infarction, heart failure, and pneumonia – the three conditions targeted in the readmissions reduction program – dropped from 21.5% to 17.8% during this time period. Readmissions for nontargeted conditions also dropped from 15.3% to 13.1%.
The researchers reported that readmissions for the targeted conditions were already declining before the ACA implementation (slope of monthly rate, –0.017), accelerating between April 2010 and October 2010 (–0.103), then leveling off through 2015 (–0.05). A similar pattern was seen with readmissions for conditions not targeted under the health law, though the declines were less pronounced.
Observation rates for the targeted conditions increased from 2.6% to 4.7% during the study period, while rates for nontargeted conditions rose from 2.5% to 4.2%. The researchers did not observe any significant associations increases in observation-unit stays – which were steady throughout the study period – and the implementation of the ACA.
“It seems likely that the upward trend in observation-service use may be attributable to factors that are largely unrelated to the Hospital Readmissions Reduction Program, such as confusion over whether an inpatient stay would be deemed inappropriate by Medicare recovery audit contractors,” the researchers wrote.
Though the observational design of the study could not confirm a causal link between the ACA penalties and the drop in readmissions, the findings suggest that the declines are not solely a response to the ACA.
The health law likely “catalyzed behavioral change by many hospitals” that was already underway, possibly because of broader concern about readmissions and to earlier Medicare initiatives designed to reduce them. Also, the investigators noted, hospitals may have been helped by other government efforts on the readmission front, including the dissemination of best practices by the Centers for Medicare & Medicaid Services.
The study was funded by HHS and the researchers were agency employees. They reported having no other financial disclosures.
Hospital readmissions have declined in recent years for three conditions targeted under the Affordable Care Act, with smaller declines for other conditions, according to new research.
The study, published online Feb. 24 in the New England Journal of Medicine, found that 30-day readmission rates declined quickly after the passage of the ACA in 2010 and then slowed at the end of 2012. The researchers also analyzed trends in the use of observation units during the same period and concluded that the drop in readmissions was not being masked by a similar uptick in patients being seen under observation status (N Engl J Med. 2016 Feb 24. doi: 10.1056/NEJMsa1513024).
Under the ACA’s Hospital Readmissions Reduction Program, hospitals are financially penalized if they have higher-than-expected readmission rates for acute myocardial infarction, heart failure, and pneumonia.
The researchers, led by Rachael B. Zuckerman, M.P.H., of the Department of Health & Human Services, examined Medicare data from 3,387 hospitals from October 2007 through May 2015. Overall readmissions for acute myocardial infarction, heart failure, and pneumonia – the three conditions targeted in the readmissions reduction program – dropped from 21.5% to 17.8% during this time period. Readmissions for nontargeted conditions also dropped from 15.3% to 13.1%.
The researchers reported that readmissions for the targeted conditions were already declining before the ACA implementation (slope of monthly rate, –0.017), accelerating between April 2010 and October 2010 (–0.103), then leveling off through 2015 (–0.05). A similar pattern was seen with readmissions for conditions not targeted under the health law, though the declines were less pronounced.
Observation rates for the targeted conditions increased from 2.6% to 4.7% during the study period, while rates for nontargeted conditions rose from 2.5% to 4.2%. The researchers did not observe any significant associations increases in observation-unit stays – which were steady throughout the study period – and the implementation of the ACA.
“It seems likely that the upward trend in observation-service use may be attributable to factors that are largely unrelated to the Hospital Readmissions Reduction Program, such as confusion over whether an inpatient stay would be deemed inappropriate by Medicare recovery audit contractors,” the researchers wrote.
Though the observational design of the study could not confirm a causal link between the ACA penalties and the drop in readmissions, the findings suggest that the declines are not solely a response to the ACA.
The health law likely “catalyzed behavioral change by many hospitals” that was already underway, possibly because of broader concern about readmissions and to earlier Medicare initiatives designed to reduce them. Also, the investigators noted, hospitals may have been helped by other government efforts on the readmission front, including the dissemination of best practices by the Centers for Medicare & Medicaid Services.
The study was funded by HHS and the researchers were agency employees. They reported having no other financial disclosures.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Hospital readmission rates declined following ACA enactment in 2010, but increased use of observation units did not account for the change.
Major finding: During 2007-2015, 30-day hospital readmissions for three targeted conditions dropped from 21.5% to 17.8%.
Data source: An interrupted time-series analysis of readmission and observation unit stay data of elderly Medicare beneficiaries from nearly 3,400 hospitals from 2007-2015.
Disclosures: The Health and Human Services department funded the study and the researchers were agency employees. They reported having no other financial disclosures.
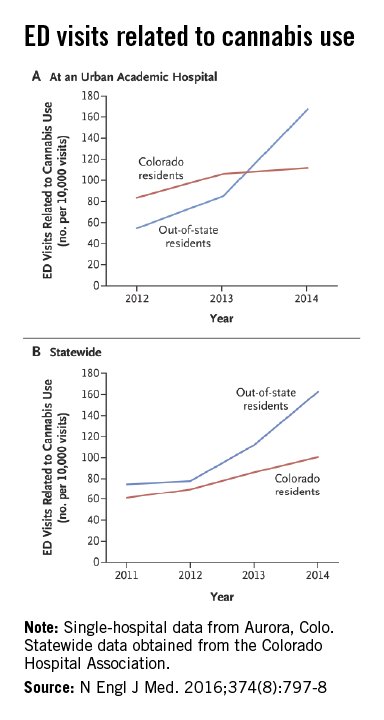
Marijuana tourists also visiting Colorado EDs
Out-of-state residents appear to be driving the recent increases in marijuana-related emergency department visits in Colorado, Dr. Howard S. Kim and his associates reported online Feb. 24 in the New England Journal of Medicine.
Using statewide data from the Colorado Hospital Association, they found that ED visits related to cannabis by out-of-state residents rose from 78 per 10,000 ED visits in 2012 to 163 per 10,000 in 2014, an increase of 109%. For Colorado residents, cannabis-related ED admissions over that same time period went up 44% – from 70 per 10,000 to 101, said Dr. Kim of Northwestern University, Chicago, and his associates (N Engl J Med. 2016 Feb 24;374[8]:797-8. doi:10.1056/NEJMc1515009).

The investigators also looked at a single urban academic hospital in Aurora, Colo., and found that cannabis-related ED visits there for out-of-state residents went from 85 per 10,000 visits in 2013 to 168 per 10,000 in 2014, compared with respective rates of 106 and 112 for Colorado residents.
“The flattening of the rates of ED visits possibly related to cannabis use among Colorado residents in an urban hospital may represent a learning curve during the period when marijuana was potentially available to Colorado residents for medical use (medical marijuana period) but was largely inaccessible to out-of-state residents,” they suggested.
Out-of-state residents appear to be driving the recent increases in marijuana-related emergency department visits in Colorado, Dr. Howard S. Kim and his associates reported online Feb. 24 in the New England Journal of Medicine.
Using statewide data from the Colorado Hospital Association, they found that ED visits related to cannabis by out-of-state residents rose from 78 per 10,000 ED visits in 2012 to 163 per 10,000 in 2014, an increase of 109%. For Colorado residents, cannabis-related ED admissions over that same time period went up 44% – from 70 per 10,000 to 101, said Dr. Kim of Northwestern University, Chicago, and his associates (N Engl J Med. 2016 Feb 24;374[8]:797-8. doi:10.1056/NEJMc1515009).

The investigators also looked at a single urban academic hospital in Aurora, Colo., and found that cannabis-related ED visits there for out-of-state residents went from 85 per 10,000 visits in 2013 to 168 per 10,000 in 2014, compared with respective rates of 106 and 112 for Colorado residents.
“The flattening of the rates of ED visits possibly related to cannabis use among Colorado residents in an urban hospital may represent a learning curve during the period when marijuana was potentially available to Colorado residents for medical use (medical marijuana period) but was largely inaccessible to out-of-state residents,” they suggested.
Out-of-state residents appear to be driving the recent increases in marijuana-related emergency department visits in Colorado, Dr. Howard S. Kim and his associates reported online Feb. 24 in the New England Journal of Medicine.
Using statewide data from the Colorado Hospital Association, they found that ED visits related to cannabis by out-of-state residents rose from 78 per 10,000 ED visits in 2012 to 163 per 10,000 in 2014, an increase of 109%. For Colorado residents, cannabis-related ED admissions over that same time period went up 44% – from 70 per 10,000 to 101, said Dr. Kim of Northwestern University, Chicago, and his associates (N Engl J Med. 2016 Feb 24;374[8]:797-8. doi:10.1056/NEJMc1515009).

The investigators also looked at a single urban academic hospital in Aurora, Colo., and found that cannabis-related ED visits there for out-of-state residents went from 85 per 10,000 visits in 2013 to 168 per 10,000 in 2014, compared with respective rates of 106 and 112 for Colorado residents.
“The flattening of the rates of ED visits possibly related to cannabis use among Colorado residents in an urban hospital may represent a learning curve during the period when marijuana was potentially available to Colorado residents for medical use (medical marijuana period) but was largely inaccessible to out-of-state residents,” they suggested.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Robotic PCI succeeds in patients with acute MI
WASHINGTON – Robotic angioplasty may deliver the same advantages when percutaneous coronary intervention is indicated for acute myocardial infarction as currently claimed for an elective PCI, according to results from a proof-of-principle study.
The first robotic PCI system, CorPath 200, was approved by the U.S. Food and Drug Administration in 2012, but the registration trial, called PRECISE (Percutaneous Robotically Enhanced Coronary Intervention), excluded patients with coronary thrombosis, according to a team of investigators at the Frederik Meijer Cardiovascular Institute, Grand Rapids, Mich. The current study focused exclusively on this population.
In “an initial experience” with robotic PCI in 17 acute MI patients led by Dr. Ryan D. Madder, an interventional cardiologist, “technical success” was achieved in 100% of patients with no repeat revascularizations in follow-up so far.
“These preliminary observations support the performance of larger studies to determine the role of robotic PCI in the treatment of acute MI,” said Andrew O’Brien, a medical student at Michigan State University, Ann Arbor, who presented the data at the meeting, sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
The major proven advantage of robotic PCI is that it reduces radiation exposure to the operator, Mr. O’Brien said. While the procedure was performed in this study behind a lead-lined cockpit, it was noted that the operator works solely on the basis of imaging and could be anywhere, including at another facility. In addition, robotic PCI has at least a theoretical advantage of greater precision relative to conventional PCI.
In this study, technical success was defined as less than 30% residual stenosis after PCI in the absence of a PCI-associated death or the need for a repeat revascularization prior to hospital discharge. The median age of the 17 acute MI patients was 59 years with a range of about 10 years younger or older. Most (71%) were male. Radial arterial access was used in all cases.
Only 23% of the patients met criteria for relatively simple lesions (class A or B1). Just over half (53%) had class B2 lesions, which require at least two complicating characteristics, such as moderate tortuosity, irregular contour, or moderate to heavy calcification, and the remainder had class C lesions. In 10 cases (59%), an angiographic filling defect consistent with thrombus was present at the culprit lesion site.
In addition to achieving the goal reduction in residual stenosis without major adverse cardiovascular events in all patients, the investigators reported that procedural time, which averaged 69 minutes from the time of sheath insertion to removal of the guide catheter, was “acceptable.” The longest procedural time was under 100 minutes.
“We used the same criteria for evaluating outcomes as employed in the original PRECISE study,” Mr. O’Brien said. He explained that acute MI patients were excluded in the published PRECISE trial data because there was no protocol at that time for converting to a conventional procedure on an urgent basis in the case of unexpected problems. With more experience, there was greater confidence that urgent complications could be addressed.
In the multicenter PRECISE trial, which led to approval of the robotic system, 164 candidates for elective PCI were enrolled (J Am Coll Cardiol. 2013;61[15]:1596-1600). Technical success was 98.8%. Although there was no control arm, radiation exposure was reported to be 95% lower for operators participating in that study than levels found at the traditional table position.
Data from the current study provide preliminary evidence that robotic PCI is feasible for management of acute MI. While confirmatory studies are needed for this indication, the Michigan investigators also advocated more studies to evaluate whether robotic PCI improves outcome. They cited the potential for more precise placement of stents to reduce the risk of complications.
“Robotic PCI is still not very widely performed,” said coinvestigator Andrew LaCombe, who suggested that its potential advantages deserve broader evaluation.
Dr. Madder disclosed a financial relationship with InfraRedx.
WASHINGTON – Robotic angioplasty may deliver the same advantages when percutaneous coronary intervention is indicated for acute myocardial infarction as currently claimed for an elective PCI, according to results from a proof-of-principle study.
The first robotic PCI system, CorPath 200, was approved by the U.S. Food and Drug Administration in 2012, but the registration trial, called PRECISE (Percutaneous Robotically Enhanced Coronary Intervention), excluded patients with coronary thrombosis, according to a team of investigators at the Frederik Meijer Cardiovascular Institute, Grand Rapids, Mich. The current study focused exclusively on this population.
In “an initial experience” with robotic PCI in 17 acute MI patients led by Dr. Ryan D. Madder, an interventional cardiologist, “technical success” was achieved in 100% of patients with no repeat revascularizations in follow-up so far.
“These preliminary observations support the performance of larger studies to determine the role of robotic PCI in the treatment of acute MI,” said Andrew O’Brien, a medical student at Michigan State University, Ann Arbor, who presented the data at the meeting, sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
The major proven advantage of robotic PCI is that it reduces radiation exposure to the operator, Mr. O’Brien said. While the procedure was performed in this study behind a lead-lined cockpit, it was noted that the operator works solely on the basis of imaging and could be anywhere, including at another facility. In addition, robotic PCI has at least a theoretical advantage of greater precision relative to conventional PCI.
In this study, technical success was defined as less than 30% residual stenosis after PCI in the absence of a PCI-associated death or the need for a repeat revascularization prior to hospital discharge. The median age of the 17 acute MI patients was 59 years with a range of about 10 years younger or older. Most (71%) were male. Radial arterial access was used in all cases.
Only 23% of the patients met criteria for relatively simple lesions (class A or B1). Just over half (53%) had class B2 lesions, which require at least two complicating characteristics, such as moderate tortuosity, irregular contour, or moderate to heavy calcification, and the remainder had class C lesions. In 10 cases (59%), an angiographic filling defect consistent with thrombus was present at the culprit lesion site.
In addition to achieving the goal reduction in residual stenosis without major adverse cardiovascular events in all patients, the investigators reported that procedural time, which averaged 69 minutes from the time of sheath insertion to removal of the guide catheter, was “acceptable.” The longest procedural time was under 100 minutes.
“We used the same criteria for evaluating outcomes as employed in the original PRECISE study,” Mr. O’Brien said. He explained that acute MI patients were excluded in the published PRECISE trial data because there was no protocol at that time for converting to a conventional procedure on an urgent basis in the case of unexpected problems. With more experience, there was greater confidence that urgent complications could be addressed.
In the multicenter PRECISE trial, which led to approval of the robotic system, 164 candidates for elective PCI were enrolled (J Am Coll Cardiol. 2013;61[15]:1596-1600). Technical success was 98.8%. Although there was no control arm, radiation exposure was reported to be 95% lower for operators participating in that study than levels found at the traditional table position.
Data from the current study provide preliminary evidence that robotic PCI is feasible for management of acute MI. While confirmatory studies are needed for this indication, the Michigan investigators also advocated more studies to evaluate whether robotic PCI improves outcome. They cited the potential for more precise placement of stents to reduce the risk of complications.
“Robotic PCI is still not very widely performed,” said coinvestigator Andrew LaCombe, who suggested that its potential advantages deserve broader evaluation.
Dr. Madder disclosed a financial relationship with InfraRedx.
WASHINGTON – Robotic angioplasty may deliver the same advantages when percutaneous coronary intervention is indicated for acute myocardial infarction as currently claimed for an elective PCI, according to results from a proof-of-principle study.
The first robotic PCI system, CorPath 200, was approved by the U.S. Food and Drug Administration in 2012, but the registration trial, called PRECISE (Percutaneous Robotically Enhanced Coronary Intervention), excluded patients with coronary thrombosis, according to a team of investigators at the Frederik Meijer Cardiovascular Institute, Grand Rapids, Mich. The current study focused exclusively on this population.
In “an initial experience” with robotic PCI in 17 acute MI patients led by Dr. Ryan D. Madder, an interventional cardiologist, “technical success” was achieved in 100% of patients with no repeat revascularizations in follow-up so far.
“These preliminary observations support the performance of larger studies to determine the role of robotic PCI in the treatment of acute MI,” said Andrew O’Brien, a medical student at Michigan State University, Ann Arbor, who presented the data at the meeting, sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
The major proven advantage of robotic PCI is that it reduces radiation exposure to the operator, Mr. O’Brien said. While the procedure was performed in this study behind a lead-lined cockpit, it was noted that the operator works solely on the basis of imaging and could be anywhere, including at another facility. In addition, robotic PCI has at least a theoretical advantage of greater precision relative to conventional PCI.
In this study, technical success was defined as less than 30% residual stenosis after PCI in the absence of a PCI-associated death or the need for a repeat revascularization prior to hospital discharge. The median age of the 17 acute MI patients was 59 years with a range of about 10 years younger or older. Most (71%) were male. Radial arterial access was used in all cases.
Only 23% of the patients met criteria for relatively simple lesions (class A or B1). Just over half (53%) had class B2 lesions, which require at least two complicating characteristics, such as moderate tortuosity, irregular contour, or moderate to heavy calcification, and the remainder had class C lesions. In 10 cases (59%), an angiographic filling defect consistent with thrombus was present at the culprit lesion site.
In addition to achieving the goal reduction in residual stenosis without major adverse cardiovascular events in all patients, the investigators reported that procedural time, which averaged 69 minutes from the time of sheath insertion to removal of the guide catheter, was “acceptable.” The longest procedural time was under 100 minutes.
“We used the same criteria for evaluating outcomes as employed in the original PRECISE study,” Mr. O’Brien said. He explained that acute MI patients were excluded in the published PRECISE trial data because there was no protocol at that time for converting to a conventional procedure on an urgent basis in the case of unexpected problems. With more experience, there was greater confidence that urgent complications could be addressed.
In the multicenter PRECISE trial, which led to approval of the robotic system, 164 candidates for elective PCI were enrolled (J Am Coll Cardiol. 2013;61[15]:1596-1600). Technical success was 98.8%. Although there was no control arm, radiation exposure was reported to be 95% lower for operators participating in that study than levels found at the traditional table position.
Data from the current study provide preliminary evidence that robotic PCI is feasible for management of acute MI. While confirmatory studies are needed for this indication, the Michigan investigators also advocated more studies to evaluate whether robotic PCI improves outcome. They cited the potential for more precise placement of stents to reduce the risk of complications.
“Robotic PCI is still not very widely performed,” said coinvestigator Andrew LaCombe, who suggested that its potential advantages deserve broader evaluation.
Dr. Madder disclosed a financial relationship with InfraRedx.
AT CARDIOVASCULAR RESEARCH TECHNOLOGIES 2016
Key clinical point: A proof-of-principle study suggests that procedural success with robotic angioplasty is at least as good in patients with acute myocardial infarction as previously shown in elective percutaneous coronary intervention.
Major finding: In a series of 17 consecutive patients with acute MI, procedural success was 100%.
Data source: A cohort study.
Disclosures: Dr. Madder disclosed a financial relationship with InfraRedx.
Blood-borne biomarkers of MS relapse identified
NEW ORLEANS – Three potentially useful blood-based biomarkers of relapse and of the response to glatiramer acetate treatment have been identified in patients with relapsing-remitting multiple sclerosis (RRMS).
The study was small, so much more needs to be done to move the findings to the bedside. Nonetheless, the hope is that the findings will someday help guide treatment decisions and outcomes in MS patients, Adam Kruszewski, a medical student at the University of Maryland, Baltimore, commented in a poster presentation at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
The study had its roots in the researcher’s prior findings that the Response Gene to Complement (RGC)–32 is expressed by CD3+ and CD4+ T cells in peripheral blood mononuclear cells (PBMCs) and in brain tissue from RRMS patients. As well, RGC-32 regulates the expressions of Fas ligand (FasL) and interleukin-21 (IL-21), and the activities of CDC2 and Akt.
In the current study, the investigator explored the potential value of RGC-32, FasL, IL-21, CDC2, and Akt as longitudinal biomarkers of relapse and as a way of gauging the response to the treatment of RRMS patients with glatiramer acetate – one of the most common immunomodulatory approaches used in RRMS therapy.
A cohort of 15 glatiramer acetate–treated RRMS patients was enrolled over a 2-year period. The battery of inclusion criteria included an age of 18-65 years; meeting the McDonald criteria for definite MS; a disease course featuring relapses and remissions; newly diagnosed MS or MS not treated with currently used immunomodulatory drugs for 3 months prior to study entry; no exacerbations in the 4 weeks before the study; no steroid therapy for 4 weeks prior to study enrollment; no treatment with natalizumab (Tysabri), fingolimod (Gilenya), dimethyl fumarate (Tecfidera), mitoxantrone, cyclophosphamide, or investigational drugs during the prior year; and an Extended Disability Status Score (EDSS) of 0-5.5. Exclusion criteria were a history of autoimmune disorders, vascular disease, or active acute or chronic infections; use of antibiotics in the last 30 days; a history of intracranial or intraspinal tumor or metabolic myelopathy; or a history of alcohol or drug abuse.
The 15 patients were clinically monitored and PBMCs were collected at 0, 3, 6, and 12 months. mRNA expression of the five candidate biomarkers in the PBMCs was determined using real-time quantitative polymerase chain reaction testing. Nonresponders to glatiramer acetate treatment were defined as patients who exhibited two or more relapse events following the initiation of the treatment.
Acute relapse was associated with decreased expression of RGC-32 and FasL (both P less than .0001) and increased expression of IL-21 (P = .04). No relapse-associated changes in CDC2 or AKT were evident. Compared with those who did not respond to glatiramer acetate, responders displayed an increased expression of RGC-32 and FasL (both P less than .0001), and a decreased expression of IL-21 (P = .02).
Receiver operating characteristic analysis was done to find the cutoff values that best distinguished treatment responders from nonresponders for each candidate biomarker. The probability of accurately detecting relapse was 90% for RGC-32, 88% for FasL, and 75% for IL-21. The probability of accurately detecting response to glatiramer acetate was 85%, 90%, and 85% in the same respective order.
“Right now, there is no test that can help identify a responder or nonresponder in MS therapy. Often, treatment is started and then a trial and error process with time determines the treatment course,” said Mr. Kruszewski during an interview at the poster.
The aim is to expand these results with RCG-32, FasL, and IL-21 to a randomized, controlled trial with the goal of tailoring treatment at the early stage of MS.
NEW ORLEANS – Three potentially useful blood-based biomarkers of relapse and of the response to glatiramer acetate treatment have been identified in patients with relapsing-remitting multiple sclerosis (RRMS).
The study was small, so much more needs to be done to move the findings to the bedside. Nonetheless, the hope is that the findings will someday help guide treatment decisions and outcomes in MS patients, Adam Kruszewski, a medical student at the University of Maryland, Baltimore, commented in a poster presentation at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
The study had its roots in the researcher’s prior findings that the Response Gene to Complement (RGC)–32 is expressed by CD3+ and CD4+ T cells in peripheral blood mononuclear cells (PBMCs) and in brain tissue from RRMS patients. As well, RGC-32 regulates the expressions of Fas ligand (FasL) and interleukin-21 (IL-21), and the activities of CDC2 and Akt.
In the current study, the investigator explored the potential value of RGC-32, FasL, IL-21, CDC2, and Akt as longitudinal biomarkers of relapse and as a way of gauging the response to the treatment of RRMS patients with glatiramer acetate – one of the most common immunomodulatory approaches used in RRMS therapy.
A cohort of 15 glatiramer acetate–treated RRMS patients was enrolled over a 2-year period. The battery of inclusion criteria included an age of 18-65 years; meeting the McDonald criteria for definite MS; a disease course featuring relapses and remissions; newly diagnosed MS or MS not treated with currently used immunomodulatory drugs for 3 months prior to study entry; no exacerbations in the 4 weeks before the study; no steroid therapy for 4 weeks prior to study enrollment; no treatment with natalizumab (Tysabri), fingolimod (Gilenya), dimethyl fumarate (Tecfidera), mitoxantrone, cyclophosphamide, or investigational drugs during the prior year; and an Extended Disability Status Score (EDSS) of 0-5.5. Exclusion criteria were a history of autoimmune disorders, vascular disease, or active acute or chronic infections; use of antibiotics in the last 30 days; a history of intracranial or intraspinal tumor or metabolic myelopathy; or a history of alcohol or drug abuse.
The 15 patients were clinically monitored and PBMCs were collected at 0, 3, 6, and 12 months. mRNA expression of the five candidate biomarkers in the PBMCs was determined using real-time quantitative polymerase chain reaction testing. Nonresponders to glatiramer acetate treatment were defined as patients who exhibited two or more relapse events following the initiation of the treatment.
Acute relapse was associated with decreased expression of RGC-32 and FasL (both P less than .0001) and increased expression of IL-21 (P = .04). No relapse-associated changes in CDC2 or AKT were evident. Compared with those who did not respond to glatiramer acetate, responders displayed an increased expression of RGC-32 and FasL (both P less than .0001), and a decreased expression of IL-21 (P = .02).
Receiver operating characteristic analysis was done to find the cutoff values that best distinguished treatment responders from nonresponders for each candidate biomarker. The probability of accurately detecting relapse was 90% for RGC-32, 88% for FasL, and 75% for IL-21. The probability of accurately detecting response to glatiramer acetate was 85%, 90%, and 85% in the same respective order.
“Right now, there is no test that can help identify a responder or nonresponder in MS therapy. Often, treatment is started and then a trial and error process with time determines the treatment course,” said Mr. Kruszewski during an interview at the poster.
The aim is to expand these results with RCG-32, FasL, and IL-21 to a randomized, controlled trial with the goal of tailoring treatment at the early stage of MS.
NEW ORLEANS – Three potentially useful blood-based biomarkers of relapse and of the response to glatiramer acetate treatment have been identified in patients with relapsing-remitting multiple sclerosis (RRMS).
The study was small, so much more needs to be done to move the findings to the bedside. Nonetheless, the hope is that the findings will someday help guide treatment decisions and outcomes in MS patients, Adam Kruszewski, a medical student at the University of Maryland, Baltimore, commented in a poster presentation at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
The study had its roots in the researcher’s prior findings that the Response Gene to Complement (RGC)–32 is expressed by CD3+ and CD4+ T cells in peripheral blood mononuclear cells (PBMCs) and in brain tissue from RRMS patients. As well, RGC-32 regulates the expressions of Fas ligand (FasL) and interleukin-21 (IL-21), and the activities of CDC2 and Akt.
In the current study, the investigator explored the potential value of RGC-32, FasL, IL-21, CDC2, and Akt as longitudinal biomarkers of relapse and as a way of gauging the response to the treatment of RRMS patients with glatiramer acetate – one of the most common immunomodulatory approaches used in RRMS therapy.
A cohort of 15 glatiramer acetate–treated RRMS patients was enrolled over a 2-year period. The battery of inclusion criteria included an age of 18-65 years; meeting the McDonald criteria for definite MS; a disease course featuring relapses and remissions; newly diagnosed MS or MS not treated with currently used immunomodulatory drugs for 3 months prior to study entry; no exacerbations in the 4 weeks before the study; no steroid therapy for 4 weeks prior to study enrollment; no treatment with natalizumab (Tysabri), fingolimod (Gilenya), dimethyl fumarate (Tecfidera), mitoxantrone, cyclophosphamide, or investigational drugs during the prior year; and an Extended Disability Status Score (EDSS) of 0-5.5. Exclusion criteria were a history of autoimmune disorders, vascular disease, or active acute or chronic infections; use of antibiotics in the last 30 days; a history of intracranial or intraspinal tumor or metabolic myelopathy; or a history of alcohol or drug abuse.
The 15 patients were clinically monitored and PBMCs were collected at 0, 3, 6, and 12 months. mRNA expression of the five candidate biomarkers in the PBMCs was determined using real-time quantitative polymerase chain reaction testing. Nonresponders to glatiramer acetate treatment were defined as patients who exhibited two or more relapse events following the initiation of the treatment.
Acute relapse was associated with decreased expression of RGC-32 and FasL (both P less than .0001) and increased expression of IL-21 (P = .04). No relapse-associated changes in CDC2 or AKT were evident. Compared with those who did not respond to glatiramer acetate, responders displayed an increased expression of RGC-32 and FasL (both P less than .0001), and a decreased expression of IL-21 (P = .02).
Receiver operating characteristic analysis was done to find the cutoff values that best distinguished treatment responders from nonresponders for each candidate biomarker. The probability of accurately detecting relapse was 90% for RGC-32, 88% for FasL, and 75% for IL-21. The probability of accurately detecting response to glatiramer acetate was 85%, 90%, and 85% in the same respective order.
“Right now, there is no test that can help identify a responder or nonresponder in MS therapy. Often, treatment is started and then a trial and error process with time determines the treatment course,” said Mr. Kruszewski during an interview at the poster.
The aim is to expand these results with RCG-32, FasL, and IL-21 to a randomized, controlled trial with the goal of tailoring treatment at the early stage of MS.
AT ACTRIMS FORUM 2016
Key clinical point: Blood-borne biomarkers of MS relapse and treatment efficacy were identified.
Major finding: RGC-32, FasL, and IL-21 are potential biomarkers of response to glatiramer acetate therapy and treatment relapse.
Data source: A single-center study of 15 patients with relapsing-remitting multiple sclerosis.
Disclosures: Funding for the study was provided by a grant from Teva Neuroscience and the U.S. Veterans Administration. Mr. Kruszewski had no relevant financial disclosures.
Treatment justified even for moderately advanced MS disability
NEW ORLEANS – Research from Australia shows that disability in multiple sclerosis (MS) is independent of past damage, even in people whose disease is moderately advanced, with further disease progression varying widely in severity.
The findings should prompt a rethink of the policy of ceasing treatment when MS-related disability becomes more advanced.
“Our main findings were that moderately advanced multiple sclerosis is indeed independent of previous disease, but still extremely variable. Higher relapse rates later in disease increased the risk of disability worsening. More promisingly, more time spent on therapy later in disease lowered the risk of disability progression to EDSS [Expanded Disability Status Scale] 6 or 6.5,” Nathaniel Lizak said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
The study sought to identify factors that modified progression in early and moderately advanced MS. Three prior cohort studies had not shed light on the variability in disease trajectory during moderately advanced MS.
“We wanted to examine how much variability there was later in disease, what determined this variability, and to confirm whether later disease was really independent of previous disease, as was suggested in previous studies,” said Mr. Lizak of Monash University, Clayton, Australia, and the University of Melbourne. The influences of relapse rate and proportion of time treated prior to and during each disability time period, age, and disease duration at baseline on the progression to moderate MS disability (EDSS 6 or 6.5) were assessed. The hypothesis was that, even at a later stage of MS, disease progression varies from patient to patient, and that some patients can benefit from immunomodulatory therapy.
The time between EDSS steps 3-6 (n = 1,560, 71% female, 40.9±9.9 years old at baseline), 4-6 (n = 1,504, 69% female, 43.0±9.6 years old at baseline), and 6-6.5 (n = 1,231, 67% female, 46.5±10.2 years old at baseline) were analyzed for the 32,336 patients included in the MSBase large, international, observational MS cohort study who met the inclusion criteria. Median disease duration at baseline was 9.4, 11.1, and 14.0 years in the same respective order.
Pre- and postbaseline disability trajectories showed large coefficients of variance and did not correlate. The probability of reaching the outcome EDSS was independent of prebaseline variables, but was increased if relapse during any particular EDSS was more frequent (hazard ratios, 1.58-3.07; P less than .001). At each measured stage of MS, higher-efficacy therapies were beneficial and lowered the risk of progression (HR, 0.27-0.68; P less than .02).
Some countries, such as New Zealand, by policy don’t allow patients to receive treatments if they display moderate or significant disability. In other places this can be common practice because of concerns with treatment-related side effects or affordability. The main driver of this practice is that the clinical trials of most MS therapies that have validated these medications specifically recruited patients with low disability scores. So, according to Mr. Lizak, the evidence exists only for therapies at lower disability scores.
“These results are important as they show that there is still hope. Reaching disability landmarks of EDSS 3, 4, and 6 does not guarantee continuous worsening of disability. Doctors still have the ability to reduce their patients’ risk of attaining further disability, even at later stages of disease,” said Mr. Lizak.
The hope is that the data will change clinical practice to encourage more therapy even when substantial disability has accumulated.
“Translating results into everyday practice is difficult, and doctors must always weigh the risks and benefits. We hope that our work will influence the clinical decision to, where in doubt, favor high-efficacy treatments at later disease stages. That’s not to say that patients should be blindly treated, as side effects always merit consideration. But we hope our work will inform neurologists that there is evidence supporting treating patients with more advanced disability. Where, by practice or policy, treatments are not provided to patients who have accumulated significant disability, we hope to change such clinical practice,” said Mr. Lizak.
The study did not require funding. Mr. Lizak had no disclosures.
NEW ORLEANS – Research from Australia shows that disability in multiple sclerosis (MS) is independent of past damage, even in people whose disease is moderately advanced, with further disease progression varying widely in severity.
The findings should prompt a rethink of the policy of ceasing treatment when MS-related disability becomes more advanced.
“Our main findings were that moderately advanced multiple sclerosis is indeed independent of previous disease, but still extremely variable. Higher relapse rates later in disease increased the risk of disability worsening. More promisingly, more time spent on therapy later in disease lowered the risk of disability progression to EDSS [Expanded Disability Status Scale] 6 or 6.5,” Nathaniel Lizak said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
The study sought to identify factors that modified progression in early and moderately advanced MS. Three prior cohort studies had not shed light on the variability in disease trajectory during moderately advanced MS.
“We wanted to examine how much variability there was later in disease, what determined this variability, and to confirm whether later disease was really independent of previous disease, as was suggested in previous studies,” said Mr. Lizak of Monash University, Clayton, Australia, and the University of Melbourne. The influences of relapse rate and proportion of time treated prior to and during each disability time period, age, and disease duration at baseline on the progression to moderate MS disability (EDSS 6 or 6.5) were assessed. The hypothesis was that, even at a later stage of MS, disease progression varies from patient to patient, and that some patients can benefit from immunomodulatory therapy.
The time between EDSS steps 3-6 (n = 1,560, 71% female, 40.9±9.9 years old at baseline), 4-6 (n = 1,504, 69% female, 43.0±9.6 years old at baseline), and 6-6.5 (n = 1,231, 67% female, 46.5±10.2 years old at baseline) were analyzed for the 32,336 patients included in the MSBase large, international, observational MS cohort study who met the inclusion criteria. Median disease duration at baseline was 9.4, 11.1, and 14.0 years in the same respective order.
Pre- and postbaseline disability trajectories showed large coefficients of variance and did not correlate. The probability of reaching the outcome EDSS was independent of prebaseline variables, but was increased if relapse during any particular EDSS was more frequent (hazard ratios, 1.58-3.07; P less than .001). At each measured stage of MS, higher-efficacy therapies were beneficial and lowered the risk of progression (HR, 0.27-0.68; P less than .02).
Some countries, such as New Zealand, by policy don’t allow patients to receive treatments if they display moderate or significant disability. In other places this can be common practice because of concerns with treatment-related side effects or affordability. The main driver of this practice is that the clinical trials of most MS therapies that have validated these medications specifically recruited patients with low disability scores. So, according to Mr. Lizak, the evidence exists only for therapies at lower disability scores.
“These results are important as they show that there is still hope. Reaching disability landmarks of EDSS 3, 4, and 6 does not guarantee continuous worsening of disability. Doctors still have the ability to reduce their patients’ risk of attaining further disability, even at later stages of disease,” said Mr. Lizak.
The hope is that the data will change clinical practice to encourage more therapy even when substantial disability has accumulated.
“Translating results into everyday practice is difficult, and doctors must always weigh the risks and benefits. We hope that our work will influence the clinical decision to, where in doubt, favor high-efficacy treatments at later disease stages. That’s not to say that patients should be blindly treated, as side effects always merit consideration. But we hope our work will inform neurologists that there is evidence supporting treating patients with more advanced disability. Where, by practice or policy, treatments are not provided to patients who have accumulated significant disability, we hope to change such clinical practice,” said Mr. Lizak.
The study did not require funding. Mr. Lizak had no disclosures.
NEW ORLEANS – Research from Australia shows that disability in multiple sclerosis (MS) is independent of past damage, even in people whose disease is moderately advanced, with further disease progression varying widely in severity.
The findings should prompt a rethink of the policy of ceasing treatment when MS-related disability becomes more advanced.
“Our main findings were that moderately advanced multiple sclerosis is indeed independent of previous disease, but still extremely variable. Higher relapse rates later in disease increased the risk of disability worsening. More promisingly, more time spent on therapy later in disease lowered the risk of disability progression to EDSS [Expanded Disability Status Scale] 6 or 6.5,” Nathaniel Lizak said in an interview at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
The study sought to identify factors that modified progression in early and moderately advanced MS. Three prior cohort studies had not shed light on the variability in disease trajectory during moderately advanced MS.
“We wanted to examine how much variability there was later in disease, what determined this variability, and to confirm whether later disease was really independent of previous disease, as was suggested in previous studies,” said Mr. Lizak of Monash University, Clayton, Australia, and the University of Melbourne. The influences of relapse rate and proportion of time treated prior to and during each disability time period, age, and disease duration at baseline on the progression to moderate MS disability (EDSS 6 or 6.5) were assessed. The hypothesis was that, even at a later stage of MS, disease progression varies from patient to patient, and that some patients can benefit from immunomodulatory therapy.
The time between EDSS steps 3-6 (n = 1,560, 71% female, 40.9±9.9 years old at baseline), 4-6 (n = 1,504, 69% female, 43.0±9.6 years old at baseline), and 6-6.5 (n = 1,231, 67% female, 46.5±10.2 years old at baseline) were analyzed for the 32,336 patients included in the MSBase large, international, observational MS cohort study who met the inclusion criteria. Median disease duration at baseline was 9.4, 11.1, and 14.0 years in the same respective order.
Pre- and postbaseline disability trajectories showed large coefficients of variance and did not correlate. The probability of reaching the outcome EDSS was independent of prebaseline variables, but was increased if relapse during any particular EDSS was more frequent (hazard ratios, 1.58-3.07; P less than .001). At each measured stage of MS, higher-efficacy therapies were beneficial and lowered the risk of progression (HR, 0.27-0.68; P less than .02).
Some countries, such as New Zealand, by policy don’t allow patients to receive treatments if they display moderate or significant disability. In other places this can be common practice because of concerns with treatment-related side effects or affordability. The main driver of this practice is that the clinical trials of most MS therapies that have validated these medications specifically recruited patients with low disability scores. So, according to Mr. Lizak, the evidence exists only for therapies at lower disability scores.
“These results are important as they show that there is still hope. Reaching disability landmarks of EDSS 3, 4, and 6 does not guarantee continuous worsening of disability. Doctors still have the ability to reduce their patients’ risk of attaining further disability, even at later stages of disease,” said Mr. Lizak.
The hope is that the data will change clinical practice to encourage more therapy even when substantial disability has accumulated.
“Translating results into everyday practice is difficult, and doctors must always weigh the risks and benefits. We hope that our work will influence the clinical decision to, where in doubt, favor high-efficacy treatments at later disease stages. That’s not to say that patients should be blindly treated, as side effects always merit consideration. But we hope our work will inform neurologists that there is evidence supporting treating patients with more advanced disability. Where, by practice or policy, treatments are not provided to patients who have accumulated significant disability, we hope to change such clinical practice,” said Mr. Lizak.
The study did not require funding. Mr. Lizak had no disclosures.
AT ACTRIMS FORUM 2016
Key clinical point:Treatment for MS should continue even when disability is advanced.
Major finding: Disease progression in moderately advanced MS is not influenced by prior disease activity.
Data source: Large, international, observational MS cohort study.
Disclosures: The study did not require funding. Mr. Lizak had no disclosures.
Loss of pulmonary vascular distensibility precedes PH
Loss of distensibility of the pulmonary vasculature may be a marker that allows earlier detection of impending pulmonary hypertension, based on hemodynamic data from the medical records of 90 patients across the spectrum of pulmonary vascular disease.
Normal pulmonary circulation is distensible, allowing distension and recruitment of the pulmonary vasculature during exertion that in turn reduces pulmonary vascular resistance. Loss of this distensibility increases resistance and thus pulmonary arterial pressure, and is a characteristic of mild pulmonary vascular disease. Such disease is a precursor of full-blown pulmonary hypertension (PH), which “is a relatively late hemodynamic event in the evolution of pulmonary vascular disease,” said Dr. Edmund M. T. Lau of Université Paris-Sud, and his associates.
The percentage change in vascular diameter per mm Hg increase in distending pressure has been proposed for estimating the distensibility of resistive pulmonary vessels. This “distensibility value” has been assessed in animal studies and in healthy human subjects, but has not yet been assessed as a possible marker of mild pulmonary vascular disease or PH.
The researchers assessed this distensibility value in 31 patients with PH, 33 with mild pulmonary vascular disease but no PH as yet, and 26 control subjects with no pulmonary vascular disease. The data were obtained from the medical records of these patients, who had undergone right-sided heart catheterization, both at rest and during exercise, over a 6-year period.
The percentage change in vascular diameter per mm Hg increase in distending pressure was “strikingly reduced” (0.45%/mm Hg) in the mild pulmonary vascular disease group compared with the control group (1.4%/mm Hg). As expected, the group with PH had the lowest distensibility value, at 0.25%/mm Hg.
Using a cutoff value of 0.76%/mm Hg allowed the researchers to distinguish control subjects from patients with mild disease with a sensitivity of 88% and a specificity of 100%. “To our knowledge, this is the first study to validate the fit of [this] model in subjects with pulmonary vascular disease and to demonstrate that [percentage change in vascular diameter per mm Hg increase in distending pressure] is dramatically reduced in patients who have mild pulmonary vascular disease without manifest PH.
“Taken together, our findings suggest that vascular distensibility is markedly attenuated prior to the development of PH and that [this value] may serve as a useful vascular index in the setting of early disease detection,” Dr. Lau and his associates said (CHEST 2016;149:353-61).
The distensibility value calculated for this study’s control group (1.4%/mm Hg) was slightly lower than that reported in the literature for normal, healthy subjects and in vitro animal vessels (2%/mm Hg). That is likely because the control participants were older than the subjects in previous studies, and vascular distensibility is known to decrease with increasing age, the researchers said.
They added that it might be useful to calculate the distensibility value when patients suspected of having pulmonary vascular disease undergo invasive pulmonary hemodynamic evaluations. “It would be of particular interest to assess [it] in populations at a high risk of developing PH, such as carriers of the BMPR2 mutation and patients with systemic sclerosis.”
Obviously, estimating the distensibility value using noninvasive evaluation would be preferable, the researchers noted. Preliminary studies of healthy control subjects and carriers of the BMPR2 mutation undergoing stress ECG testing have shown that calculating the distensibility value is feasible using Doppler echocardiography data, they added.
This study was supported by Fonds de Dotation Recherche en Santé Respiratoire, Fondation du Souffle, and the INSERM–University of Sydney Exchange Grant. Dr. Lau reported having no relevant financial disclosures; his associates reported ties to Actelion, Aires, Bayer, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and United Therapeutics Corporation.
These findings have the potential to completely revamp screening for pulmonary vascular disease, but first they must be validated in further research.
It will also be important to determine whether, as the authors suggest, a noninvasive method to determine vascular distensibility can be developed, perhaps using stress echocardiography or stress cardiac magnetic resonance testing. Only then can this measure – the percentage change in vascular diameter per mm Hg increase in distending pressure – translate from the realm of novel research into real-world clinical practice.
Dr. Richa Agarwal is in the department of medicine at Temple University, Philadelphia, and at the Cardiovascular Institute at Allegheny General Hospital, Pittsburgh. Dr. Mardi Gomberg-Maitland is in the department of medicine at the University of Chicago. Dr. Agarwal reported having no relevant financial disclosures; Dr. Gomberg-Maitland reported ties to Actelion, Bayer, GeNo, Gilead, Medtronic, Novartis, Lung Biotechnology, Reata, Bellerophon, United Therapeutics, Medscape, and ABComm. Dr. Agarwal and Dr. Gomberg-Maitland made these remarks in an editorial accompanying Dr. Lau’s report (Chest 2016;149:295-7).
These findings have the potential to completely revamp screening for pulmonary vascular disease, but first they must be validated in further research.
It will also be important to determine whether, as the authors suggest, a noninvasive method to determine vascular distensibility can be developed, perhaps using stress echocardiography or stress cardiac magnetic resonance testing. Only then can this measure – the percentage change in vascular diameter per mm Hg increase in distending pressure – translate from the realm of novel research into real-world clinical practice.
Dr. Richa Agarwal is in the department of medicine at Temple University, Philadelphia, and at the Cardiovascular Institute at Allegheny General Hospital, Pittsburgh. Dr. Mardi Gomberg-Maitland is in the department of medicine at the University of Chicago. Dr. Agarwal reported having no relevant financial disclosures; Dr. Gomberg-Maitland reported ties to Actelion, Bayer, GeNo, Gilead, Medtronic, Novartis, Lung Biotechnology, Reata, Bellerophon, United Therapeutics, Medscape, and ABComm. Dr. Agarwal and Dr. Gomberg-Maitland made these remarks in an editorial accompanying Dr. Lau’s report (Chest 2016;149:295-7).
These findings have the potential to completely revamp screening for pulmonary vascular disease, but first they must be validated in further research.
It will also be important to determine whether, as the authors suggest, a noninvasive method to determine vascular distensibility can be developed, perhaps using stress echocardiography or stress cardiac magnetic resonance testing. Only then can this measure – the percentage change in vascular diameter per mm Hg increase in distending pressure – translate from the realm of novel research into real-world clinical practice.
Dr. Richa Agarwal is in the department of medicine at Temple University, Philadelphia, and at the Cardiovascular Institute at Allegheny General Hospital, Pittsburgh. Dr. Mardi Gomberg-Maitland is in the department of medicine at the University of Chicago. Dr. Agarwal reported having no relevant financial disclosures; Dr. Gomberg-Maitland reported ties to Actelion, Bayer, GeNo, Gilead, Medtronic, Novartis, Lung Biotechnology, Reata, Bellerophon, United Therapeutics, Medscape, and ABComm. Dr. Agarwal and Dr. Gomberg-Maitland made these remarks in an editorial accompanying Dr. Lau’s report (Chest 2016;149:295-7).
Loss of distensibility of the pulmonary vasculature may be a marker that allows earlier detection of impending pulmonary hypertension, based on hemodynamic data from the medical records of 90 patients across the spectrum of pulmonary vascular disease.
Normal pulmonary circulation is distensible, allowing distension and recruitment of the pulmonary vasculature during exertion that in turn reduces pulmonary vascular resistance. Loss of this distensibility increases resistance and thus pulmonary arterial pressure, and is a characteristic of mild pulmonary vascular disease. Such disease is a precursor of full-blown pulmonary hypertension (PH), which “is a relatively late hemodynamic event in the evolution of pulmonary vascular disease,” said Dr. Edmund M. T. Lau of Université Paris-Sud, and his associates.
The percentage change in vascular diameter per mm Hg increase in distending pressure has been proposed for estimating the distensibility of resistive pulmonary vessels. This “distensibility value” has been assessed in animal studies and in healthy human subjects, but has not yet been assessed as a possible marker of mild pulmonary vascular disease or PH.
The researchers assessed this distensibility value in 31 patients with PH, 33 with mild pulmonary vascular disease but no PH as yet, and 26 control subjects with no pulmonary vascular disease. The data were obtained from the medical records of these patients, who had undergone right-sided heart catheterization, both at rest and during exercise, over a 6-year period.
The percentage change in vascular diameter per mm Hg increase in distending pressure was “strikingly reduced” (0.45%/mm Hg) in the mild pulmonary vascular disease group compared with the control group (1.4%/mm Hg). As expected, the group with PH had the lowest distensibility value, at 0.25%/mm Hg.
Using a cutoff value of 0.76%/mm Hg allowed the researchers to distinguish control subjects from patients with mild disease with a sensitivity of 88% and a specificity of 100%. “To our knowledge, this is the first study to validate the fit of [this] model in subjects with pulmonary vascular disease and to demonstrate that [percentage change in vascular diameter per mm Hg increase in distending pressure] is dramatically reduced in patients who have mild pulmonary vascular disease without manifest PH.
“Taken together, our findings suggest that vascular distensibility is markedly attenuated prior to the development of PH and that [this value] may serve as a useful vascular index in the setting of early disease detection,” Dr. Lau and his associates said (CHEST 2016;149:353-61).
The distensibility value calculated for this study’s control group (1.4%/mm Hg) was slightly lower than that reported in the literature for normal, healthy subjects and in vitro animal vessels (2%/mm Hg). That is likely because the control participants were older than the subjects in previous studies, and vascular distensibility is known to decrease with increasing age, the researchers said.
They added that it might be useful to calculate the distensibility value when patients suspected of having pulmonary vascular disease undergo invasive pulmonary hemodynamic evaluations. “It would be of particular interest to assess [it] in populations at a high risk of developing PH, such as carriers of the BMPR2 mutation and patients with systemic sclerosis.”
Obviously, estimating the distensibility value using noninvasive evaluation would be preferable, the researchers noted. Preliminary studies of healthy control subjects and carriers of the BMPR2 mutation undergoing stress ECG testing have shown that calculating the distensibility value is feasible using Doppler echocardiography data, they added.
This study was supported by Fonds de Dotation Recherche en Santé Respiratoire, Fondation du Souffle, and the INSERM–University of Sydney Exchange Grant. Dr. Lau reported having no relevant financial disclosures; his associates reported ties to Actelion, Aires, Bayer, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and United Therapeutics Corporation.
Loss of distensibility of the pulmonary vasculature may be a marker that allows earlier detection of impending pulmonary hypertension, based on hemodynamic data from the medical records of 90 patients across the spectrum of pulmonary vascular disease.
Normal pulmonary circulation is distensible, allowing distension and recruitment of the pulmonary vasculature during exertion that in turn reduces pulmonary vascular resistance. Loss of this distensibility increases resistance and thus pulmonary arterial pressure, and is a characteristic of mild pulmonary vascular disease. Such disease is a precursor of full-blown pulmonary hypertension (PH), which “is a relatively late hemodynamic event in the evolution of pulmonary vascular disease,” said Dr. Edmund M. T. Lau of Université Paris-Sud, and his associates.
The percentage change in vascular diameter per mm Hg increase in distending pressure has been proposed for estimating the distensibility of resistive pulmonary vessels. This “distensibility value” has been assessed in animal studies and in healthy human subjects, but has not yet been assessed as a possible marker of mild pulmonary vascular disease or PH.
The researchers assessed this distensibility value in 31 patients with PH, 33 with mild pulmonary vascular disease but no PH as yet, and 26 control subjects with no pulmonary vascular disease. The data were obtained from the medical records of these patients, who had undergone right-sided heart catheterization, both at rest and during exercise, over a 6-year period.
The percentage change in vascular diameter per mm Hg increase in distending pressure was “strikingly reduced” (0.45%/mm Hg) in the mild pulmonary vascular disease group compared with the control group (1.4%/mm Hg). As expected, the group with PH had the lowest distensibility value, at 0.25%/mm Hg.
Using a cutoff value of 0.76%/mm Hg allowed the researchers to distinguish control subjects from patients with mild disease with a sensitivity of 88% and a specificity of 100%. “To our knowledge, this is the first study to validate the fit of [this] model in subjects with pulmonary vascular disease and to demonstrate that [percentage change in vascular diameter per mm Hg increase in distending pressure] is dramatically reduced in patients who have mild pulmonary vascular disease without manifest PH.
“Taken together, our findings suggest that vascular distensibility is markedly attenuated prior to the development of PH and that [this value] may serve as a useful vascular index in the setting of early disease detection,” Dr. Lau and his associates said (CHEST 2016;149:353-61).
The distensibility value calculated for this study’s control group (1.4%/mm Hg) was slightly lower than that reported in the literature for normal, healthy subjects and in vitro animal vessels (2%/mm Hg). That is likely because the control participants were older than the subjects in previous studies, and vascular distensibility is known to decrease with increasing age, the researchers said.
They added that it might be useful to calculate the distensibility value when patients suspected of having pulmonary vascular disease undergo invasive pulmonary hemodynamic evaluations. “It would be of particular interest to assess [it] in populations at a high risk of developing PH, such as carriers of the BMPR2 mutation and patients with systemic sclerosis.”
Obviously, estimating the distensibility value using noninvasive evaluation would be preferable, the researchers noted. Preliminary studies of healthy control subjects and carriers of the BMPR2 mutation undergoing stress ECG testing have shown that calculating the distensibility value is feasible using Doppler echocardiography data, they added.
This study was supported by Fonds de Dotation Recherche en Santé Respiratoire, Fondation du Souffle, and the INSERM–University of Sydney Exchange Grant. Dr. Lau reported having no relevant financial disclosures; his associates reported ties to Actelion, Aires, Bayer, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and United Therapeutics Corporation.
FROM CHEST
Key clinical point: Loss of distensibility of the pulmonary vasculature precedes the development of pulmonary hypertension and may serve as a marker allowing earlier detection of the disorder.
Major finding: During exercise, the percentage change in vascular diameter per mm Hg increase in distending pressure was “strikingly reduced” (0.45%/mm Hg) in the mild pulmonary vascular disease group compared with the control group (1.4%/mm Hg).
Data source: A retrospective single-center comparison of pulmonary vascular distensibility in 31 patients with PH, 33 with mild pulmonary vascular disease but no PH as yet, and 26 control subjects.
Disclosures: This study was supported by Fonds de Dotation Recherche en Santé Respiratoire, Fondation du Souffle, and the INSERM–University of Sydney Exchange Grant. Dr. Lau reported having no relevant financial disclosures; his associates reported ties to Actelion, Aires, Bayer, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and United Therapeutics Corporation.
Disability Insurance: What Dermatology Residents Need to Know
Several older physicians have emphasized to me the importance of choosing an excellent disability insurance policy during residency. However, choosing the right policy can be a difficult task. The policy definitions are complicated, and there is a lot of fine print. To understand this confusing topic, start with answers to these 3 questions: What is my most valuable asset? When is the best point in my career to purchase disability insurance? What would I do if I were no longer able to perform the material and substantial duties of my occupation as a dermatologist?
A Resident’s Assets
In the world of disability insurance, your most valuable assets are your education and your ability to earn an income in the future.1,2 A resident’s ability to earn an income in the future reflects a massive investment of time, cost of education, and postponed accruement of wealth due to time spent in training. Any negative impact on your health (eg, back injury, vision loss, hand injury) can jeopardize these assets. Purchasing disability insurance while still in dermatology residency will protect this investment; it also will ensure that you obtain a policy while you are still healthy.1,2
Choosing a Policy
Disability insurance comes in 2 main forms: individual or group. Individual insurance may be slightly more expensive but may offer better coverage than group insurance. Group insurance often is offered through a large medical association such as the American Academy of Dermatology. Group insurance may be less expensive but often has more limits to coverage. A definite must-have in a disability insurance policy is one that has guaranteed renewal and is noncancellable.1-3
Interestingly, women are considered a higher risk for disability, and many insurance policies will charge a higher monthly rate for women than men because women are slightly more likely than men to develop a disability, and women are more likely to develop a disability that prevents them from being able to work.4 Some insurance companies do offer a unisex policy, which does not discriminate.
When choosing a policy, you want to carefully read the vendor’s definition of disability. The best definition of the term disability is going to be one that includes phrases such as “unable to perform the material and substantial duties of your [own] occupation . . . even if you are gainfully employed in another occupation.”1-3,5,6 This definition of disability is the least restrictive and would allow you to receive full benefits even if you are able to work in another capacity or occupation.1-3,5,6 The benefit period of the policy also is something to choose carefully. It is recommended to choose a benefit period that extends to at least 65 years of age.1-3,5 It is important to remember that the devil is in the details; for example, some disability insurance policies with more restrictive definitions will not pay you benefits if you are working in another capacity (eg, a physician who develops an injury that prohibits working with patients and then chooses to work in another capacity).
Some policies will only pay benefits if you become totally disabled. Shy away from these more restrictive policies; instead, look for a policy that has a liberal definition of what constitutes disability and allows you the option to add in a future purchase option rider. It is important that your policy includes a future purchase option rider, which means that as your income increases you have the option to purchase an increase in your disability coverage.1-3,5 Look for a policy that allows you to be insured without penalizing you for preexisting conditions; during residency is one of the few times some policy vendors will do it, as they assume residents are generally young and healthy.1-3,5,7
Final Thoughts
When you choose your policy, read the details carefully. Finally, remember that other physicians in the community are available as resources; they can be a wealth of information on different policies. There are many websites available to read more on this topic. Often, your training institution will offer a disability policy for the duration of your residency. Many residents choose to purchase their postresidency policies while in their third or fourth year of training. Take the time to choose a good policy now; you will be glad you did.
- Relvas M. Must-know disability insurance policy features. MR Insurance Consultants website. https://www.mr-disability-insurance.com/Policy-Information.php. Accessed January 25, 2016.
- Keller L. Disability insurance: what you need to know before you buy. Dermatology Resident Roundup. 2003:4-5.
- Dahle JM, Relvas MR. 4 critical steps in purchasing resident disability insurance. Physician’s Money Digest website. http://www.hcplive.com/physicians-money-digest/personal-finance/dahle-4-critical-steps-in-purchasing-resident-disability-insurance. Published March 22, 2014. Accessed January 25, 2016.
- Schneider L, Quist-Newens M. Women and the risk of disability. insights from a landmark study by the State Farm Center for women and financial services at The American College. The American College of Financial Services web site. http://womenscenter.theamericancollege.edu/uploads/documents/Women-and-the-Risk-of-Disability-Study-5-4-12-v1a.pdf. Published May 7, 2012. Accessed February 16, 2016.
- Hill J. Consider buying disability insurance during residency.” Medical Economics website. http://medicaleconomics.modernmedicine.com/medical-economics/news/modernmedicine/modern-medicine-now/consider-buying-disability-insurance-durin. Published August 10, 2011. Accessed January 25, 2016.
- Walters C. What is own occupation disability insurance? Policy Genius. https://www.policygenius.com/blog/own-occupation-disability-insurance/. Published October 20, 2014. Accessed February 12, 2016.
- The five big money items you should do as a resident. The White Coat Investor website. http://whitecoatinvestor.com/the-five-big-money-items-you-should-do-as-a-resident/. Published July 7, 2011. Accessed January 25, 2016.
Several older physicians have emphasized to me the importance of choosing an excellent disability insurance policy during residency. However, choosing the right policy can be a difficult task. The policy definitions are complicated, and there is a lot of fine print. To understand this confusing topic, start with answers to these 3 questions: What is my most valuable asset? When is the best point in my career to purchase disability insurance? What would I do if I were no longer able to perform the material and substantial duties of my occupation as a dermatologist?
A Resident’s Assets
In the world of disability insurance, your most valuable assets are your education and your ability to earn an income in the future.1,2 A resident’s ability to earn an income in the future reflects a massive investment of time, cost of education, and postponed accruement of wealth due to time spent in training. Any negative impact on your health (eg, back injury, vision loss, hand injury) can jeopardize these assets. Purchasing disability insurance while still in dermatology residency will protect this investment; it also will ensure that you obtain a policy while you are still healthy.1,2
Choosing a Policy
Disability insurance comes in 2 main forms: individual or group. Individual insurance may be slightly more expensive but may offer better coverage than group insurance. Group insurance often is offered through a large medical association such as the American Academy of Dermatology. Group insurance may be less expensive but often has more limits to coverage. A definite must-have in a disability insurance policy is one that has guaranteed renewal and is noncancellable.1-3
Interestingly, women are considered a higher risk for disability, and many insurance policies will charge a higher monthly rate for women than men because women are slightly more likely than men to develop a disability, and women are more likely to develop a disability that prevents them from being able to work.4 Some insurance companies do offer a unisex policy, which does not discriminate.
When choosing a policy, you want to carefully read the vendor’s definition of disability. The best definition of the term disability is going to be one that includes phrases such as “unable to perform the material and substantial duties of your [own] occupation . . . even if you are gainfully employed in another occupation.”1-3,5,6 This definition of disability is the least restrictive and would allow you to receive full benefits even if you are able to work in another capacity or occupation.1-3,5,6 The benefit period of the policy also is something to choose carefully. It is recommended to choose a benefit period that extends to at least 65 years of age.1-3,5 It is important to remember that the devil is in the details; for example, some disability insurance policies with more restrictive definitions will not pay you benefits if you are working in another capacity (eg, a physician who develops an injury that prohibits working with patients and then chooses to work in another capacity).
Some policies will only pay benefits if you become totally disabled. Shy away from these more restrictive policies; instead, look for a policy that has a liberal definition of what constitutes disability and allows you the option to add in a future purchase option rider. It is important that your policy includes a future purchase option rider, which means that as your income increases you have the option to purchase an increase in your disability coverage.1-3,5 Look for a policy that allows you to be insured without penalizing you for preexisting conditions; during residency is one of the few times some policy vendors will do it, as they assume residents are generally young and healthy.1-3,5,7
Final Thoughts
When you choose your policy, read the details carefully. Finally, remember that other physicians in the community are available as resources; they can be a wealth of information on different policies. There are many websites available to read more on this topic. Often, your training institution will offer a disability policy for the duration of your residency. Many residents choose to purchase their postresidency policies while in their third or fourth year of training. Take the time to choose a good policy now; you will be glad you did.
Several older physicians have emphasized to me the importance of choosing an excellent disability insurance policy during residency. However, choosing the right policy can be a difficult task. The policy definitions are complicated, and there is a lot of fine print. To understand this confusing topic, start with answers to these 3 questions: What is my most valuable asset? When is the best point in my career to purchase disability insurance? What would I do if I were no longer able to perform the material and substantial duties of my occupation as a dermatologist?
A Resident’s Assets
In the world of disability insurance, your most valuable assets are your education and your ability to earn an income in the future.1,2 A resident’s ability to earn an income in the future reflects a massive investment of time, cost of education, and postponed accruement of wealth due to time spent in training. Any negative impact on your health (eg, back injury, vision loss, hand injury) can jeopardize these assets. Purchasing disability insurance while still in dermatology residency will protect this investment; it also will ensure that you obtain a policy while you are still healthy.1,2
Choosing a Policy
Disability insurance comes in 2 main forms: individual or group. Individual insurance may be slightly more expensive but may offer better coverage than group insurance. Group insurance often is offered through a large medical association such as the American Academy of Dermatology. Group insurance may be less expensive but often has more limits to coverage. A definite must-have in a disability insurance policy is one that has guaranteed renewal and is noncancellable.1-3
Interestingly, women are considered a higher risk for disability, and many insurance policies will charge a higher monthly rate for women than men because women are slightly more likely than men to develop a disability, and women are more likely to develop a disability that prevents them from being able to work.4 Some insurance companies do offer a unisex policy, which does not discriminate.
When choosing a policy, you want to carefully read the vendor’s definition of disability. The best definition of the term disability is going to be one that includes phrases such as “unable to perform the material and substantial duties of your [own] occupation . . . even if you are gainfully employed in another occupation.”1-3,5,6 This definition of disability is the least restrictive and would allow you to receive full benefits even if you are able to work in another capacity or occupation.1-3,5,6 The benefit period of the policy also is something to choose carefully. It is recommended to choose a benefit period that extends to at least 65 years of age.1-3,5 It is important to remember that the devil is in the details; for example, some disability insurance policies with more restrictive definitions will not pay you benefits if you are working in another capacity (eg, a physician who develops an injury that prohibits working with patients and then chooses to work in another capacity).
Some policies will only pay benefits if you become totally disabled. Shy away from these more restrictive policies; instead, look for a policy that has a liberal definition of what constitutes disability and allows you the option to add in a future purchase option rider. It is important that your policy includes a future purchase option rider, which means that as your income increases you have the option to purchase an increase in your disability coverage.1-3,5 Look for a policy that allows you to be insured without penalizing you for preexisting conditions; during residency is one of the few times some policy vendors will do it, as they assume residents are generally young and healthy.1-3,5,7
Final Thoughts
When you choose your policy, read the details carefully. Finally, remember that other physicians in the community are available as resources; they can be a wealth of information on different policies. There are many websites available to read more on this topic. Often, your training institution will offer a disability policy for the duration of your residency. Many residents choose to purchase their postresidency policies while in their third or fourth year of training. Take the time to choose a good policy now; you will be glad you did.
- Relvas M. Must-know disability insurance policy features. MR Insurance Consultants website. https://www.mr-disability-insurance.com/Policy-Information.php. Accessed January 25, 2016.
- Keller L. Disability insurance: what you need to know before you buy. Dermatology Resident Roundup. 2003:4-5.
- Dahle JM, Relvas MR. 4 critical steps in purchasing resident disability insurance. Physician’s Money Digest website. http://www.hcplive.com/physicians-money-digest/personal-finance/dahle-4-critical-steps-in-purchasing-resident-disability-insurance. Published March 22, 2014. Accessed January 25, 2016.
- Schneider L, Quist-Newens M. Women and the risk of disability. insights from a landmark study by the State Farm Center for women and financial services at The American College. The American College of Financial Services web site. http://womenscenter.theamericancollege.edu/uploads/documents/Women-and-the-Risk-of-Disability-Study-5-4-12-v1a.pdf. Published May 7, 2012. Accessed February 16, 2016.
- Hill J. Consider buying disability insurance during residency.” Medical Economics website. http://medicaleconomics.modernmedicine.com/medical-economics/news/modernmedicine/modern-medicine-now/consider-buying-disability-insurance-durin. Published August 10, 2011. Accessed January 25, 2016.
- Walters C. What is own occupation disability insurance? Policy Genius. https://www.policygenius.com/blog/own-occupation-disability-insurance/. Published October 20, 2014. Accessed February 12, 2016.
- The five big money items you should do as a resident. The White Coat Investor website. http://whitecoatinvestor.com/the-five-big-money-items-you-should-do-as-a-resident/. Published July 7, 2011. Accessed January 25, 2016.
- Relvas M. Must-know disability insurance policy features. MR Insurance Consultants website. https://www.mr-disability-insurance.com/Policy-Information.php. Accessed January 25, 2016.
- Keller L. Disability insurance: what you need to know before you buy. Dermatology Resident Roundup. 2003:4-5.
- Dahle JM, Relvas MR. 4 critical steps in purchasing resident disability insurance. Physician’s Money Digest website. http://www.hcplive.com/physicians-money-digest/personal-finance/dahle-4-critical-steps-in-purchasing-resident-disability-insurance. Published March 22, 2014. Accessed January 25, 2016.
- Schneider L, Quist-Newens M. Women and the risk of disability. insights from a landmark study by the State Farm Center for women and financial services at The American College. The American College of Financial Services web site. http://womenscenter.theamericancollege.edu/uploads/documents/Women-and-the-Risk-of-Disability-Study-5-4-12-v1a.pdf. Published May 7, 2012. Accessed February 16, 2016.
- Hill J. Consider buying disability insurance during residency.” Medical Economics website. http://medicaleconomics.modernmedicine.com/medical-economics/news/modernmedicine/modern-medicine-now/consider-buying-disability-insurance-durin. Published August 10, 2011. Accessed January 25, 2016.
- Walters C. What is own occupation disability insurance? Policy Genius. https://www.policygenius.com/blog/own-occupation-disability-insurance/. Published October 20, 2014. Accessed February 12, 2016.
- The five big money items you should do as a resident. The White Coat Investor website. http://whitecoatinvestor.com/the-five-big-money-items-you-should-do-as-a-resident/. Published July 7, 2011. Accessed January 25, 2016.