User login
CDC quantifies threat of resistant HAIs in US
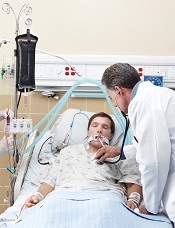
in the intensive care unit
New data from the US Centers for Disease Control and Prevention (CDC) suggest the incidence of certain healthcare-associated infections (HAIs) has fallen in recent years, but antibiotic-resistant bacteria remain a threat.
Therefore, the CDC is advising healthcare workers to use a combination of infection control recommendations to better protect patients from these infections.
“New data show that far too many patients are getting infected with dangerous, drug-resistant bacteria in healthcare settings,” said CDC Director Tom Frieden, MD.
The facts and figures are available in the CDC’s latest Vital Signs report and the agency’s annual progress report on HAI prevention.
The data indicate that 1 in 7 catheter- and surgery-related HAIs in acute care hospitals can be caused by 6 types of antibiotic-resistant bacteria. That number increases to 1 in 4 infections in long-term acute care hospitals.
The 6 antibiotic-resistant threats are carbapenem-resistant Enterobacteriaceae (CRE), methicillin-resistant Staphylococcus aureus (MRSA), ESBL-producing Enterobacteriaceae (extended-spectrum β-lactamases), vancomycin-resistant Enterococcus (VRE), multidrug-resistant Pseudomonas aeruginosa, and multidrug-resistant Acinetobacter.
Prevention and resistance
According to the CDC’s data, acute care hospitals saw a 50% decrease in central line-associated bloodstream infections between 2008 and 2014. But 1 in 6 remaining central line-associated bloodstream infections is caused by urgent or serious antibiotic-resistant bacteria.
Between 2008 and 2014, acute care hospitals saw a 17% decrease in surgical site infections related to 10 procedures that were tracked in previous HAI progress reports. One in 7 remaining surgical site infections is caused by urgent or serious antibiotic-resistant bacteria.
Acute care hospitals saw no change in the incidence of catheter-associated urinary tract infections (CAUTIs) between 2009 and 2014. However, there was a reduction in CAUTIs between 2013 and 2014. One in 10 CAUTIs is caused by urgent or serious antibiotic-resistant bacteria.
The Vital Signs report also examines Clostridium difficile, which caused almost half a million infections in the US in 2011 alone. The CDC’s annual progress report shows that hospital-onset C difficile infections decreased by 8% between 2011 and 2014.
In addition to the reports, the CDC has released a web app with interactive data on HAIs caused by antibiotic-resistant bacteria.
The tool, known as the Antibiotic Resistance Patient Safety Atlas, provides national, regional, and state map views of superbug/drug combinations showing percent resistance over time. The Atlas uses data reported to the CDC’s National Healthcare Safety Network from 2011 to 2014 from more than 4000 healthcare facilities. ![]()

in the intensive care unit
New data from the US Centers for Disease Control and Prevention (CDC) suggest the incidence of certain healthcare-associated infections (HAIs) has fallen in recent years, but antibiotic-resistant bacteria remain a threat.
Therefore, the CDC is advising healthcare workers to use a combination of infection control recommendations to better protect patients from these infections.
“New data show that far too many patients are getting infected with dangerous, drug-resistant bacteria in healthcare settings,” said CDC Director Tom Frieden, MD.
The facts and figures are available in the CDC’s latest Vital Signs report and the agency’s annual progress report on HAI prevention.
The data indicate that 1 in 7 catheter- and surgery-related HAIs in acute care hospitals can be caused by 6 types of antibiotic-resistant bacteria. That number increases to 1 in 4 infections in long-term acute care hospitals.
The 6 antibiotic-resistant threats are carbapenem-resistant Enterobacteriaceae (CRE), methicillin-resistant Staphylococcus aureus (MRSA), ESBL-producing Enterobacteriaceae (extended-spectrum β-lactamases), vancomycin-resistant Enterococcus (VRE), multidrug-resistant Pseudomonas aeruginosa, and multidrug-resistant Acinetobacter.
Prevention and resistance
According to the CDC’s data, acute care hospitals saw a 50% decrease in central line-associated bloodstream infections between 2008 and 2014. But 1 in 6 remaining central line-associated bloodstream infections is caused by urgent or serious antibiotic-resistant bacteria.
Between 2008 and 2014, acute care hospitals saw a 17% decrease in surgical site infections related to 10 procedures that were tracked in previous HAI progress reports. One in 7 remaining surgical site infections is caused by urgent or serious antibiotic-resistant bacteria.
Acute care hospitals saw no change in the incidence of catheter-associated urinary tract infections (CAUTIs) between 2009 and 2014. However, there was a reduction in CAUTIs between 2013 and 2014. One in 10 CAUTIs is caused by urgent or serious antibiotic-resistant bacteria.
The Vital Signs report also examines Clostridium difficile, which caused almost half a million infections in the US in 2011 alone. The CDC’s annual progress report shows that hospital-onset C difficile infections decreased by 8% between 2011 and 2014.
In addition to the reports, the CDC has released a web app with interactive data on HAIs caused by antibiotic-resistant bacteria.
The tool, known as the Antibiotic Resistance Patient Safety Atlas, provides national, regional, and state map views of superbug/drug combinations showing percent resistance over time. The Atlas uses data reported to the CDC’s National Healthcare Safety Network from 2011 to 2014 from more than 4000 healthcare facilities. ![]()

in the intensive care unit
New data from the US Centers for Disease Control and Prevention (CDC) suggest the incidence of certain healthcare-associated infections (HAIs) has fallen in recent years, but antibiotic-resistant bacteria remain a threat.
Therefore, the CDC is advising healthcare workers to use a combination of infection control recommendations to better protect patients from these infections.
“New data show that far too many patients are getting infected with dangerous, drug-resistant bacteria in healthcare settings,” said CDC Director Tom Frieden, MD.
The facts and figures are available in the CDC’s latest Vital Signs report and the agency’s annual progress report on HAI prevention.
The data indicate that 1 in 7 catheter- and surgery-related HAIs in acute care hospitals can be caused by 6 types of antibiotic-resistant bacteria. That number increases to 1 in 4 infections in long-term acute care hospitals.
The 6 antibiotic-resistant threats are carbapenem-resistant Enterobacteriaceae (CRE), methicillin-resistant Staphylococcus aureus (MRSA), ESBL-producing Enterobacteriaceae (extended-spectrum β-lactamases), vancomycin-resistant Enterococcus (VRE), multidrug-resistant Pseudomonas aeruginosa, and multidrug-resistant Acinetobacter.
Prevention and resistance
According to the CDC’s data, acute care hospitals saw a 50% decrease in central line-associated bloodstream infections between 2008 and 2014. But 1 in 6 remaining central line-associated bloodstream infections is caused by urgent or serious antibiotic-resistant bacteria.
Between 2008 and 2014, acute care hospitals saw a 17% decrease in surgical site infections related to 10 procedures that were tracked in previous HAI progress reports. One in 7 remaining surgical site infections is caused by urgent or serious antibiotic-resistant bacteria.
Acute care hospitals saw no change in the incidence of catheter-associated urinary tract infections (CAUTIs) between 2009 and 2014. However, there was a reduction in CAUTIs between 2013 and 2014. One in 10 CAUTIs is caused by urgent or serious antibiotic-resistant bacteria.
The Vital Signs report also examines Clostridium difficile, which caused almost half a million infections in the US in 2011 alone. The CDC’s annual progress report shows that hospital-onset C difficile infections decreased by 8% between 2011 and 2014.
In addition to the reports, the CDC has released a web app with interactive data on HAIs caused by antibiotic-resistant bacteria.
The tool, known as the Antibiotic Resistance Patient Safety Atlas, provides national, regional, and state map views of superbug/drug combinations showing percent resistance over time. The Atlas uses data reported to the CDC’s National Healthcare Safety Network from 2011 to 2014 from more than 4000 healthcare facilities. ![]()
Inhibitor exhibits activity against myeloma

A dual kinase inhibitor has shown early promise as a potential treatment for multiple myeloma (MM), according to investigators.
The inhibitor, ON123300, targets both AMPK-related protein kinase 5 (ARK5) and cyclin-dependent kinase 4 (CDK4), proteins responsible for maintaining energy balance within the cell.
Investigators found that ON123300 can induce apoptosis in MM cells in vitro and halt tumor growth in mouse models of MM.
The team reported these findings in Cancer Research.
“ARK5 is critical for myeloma survival, and this study suggests a novel function for ARK5 in bridging the mTOR and MYC pathways,” said study author Deepak Perumal, PhD, of the Icahn School of Medicine at Mount Sinai in New York, New York.
“Given that MYC is critically overexpressed in myeloma, we sought to determine whether selective inhibition of ARK5 and CDK4 could be an effective way to target MYC-driven proliferation in myeloma.”
The team at Mount Sinai developed ON123300 in collaboration with Onconova Therapeutics, Inc. And they tested the drug in MM cell lines and primary samples from patients with recurring MM, as well as human HS-5 bone marrow stromal cells.
ON123300 induced rapid cell-cycle arrest and apoptosis in MM cells but was not toxic to normal peripheral blood cells.
In mouse models of MM, ON123300 decreased tumor growth without causing significant toxicity.
The investigators also discovered that MM cells that are sensitive to ON123300 have a unique genomic signature, which could guide the clinical development of the drug.
“Our study results show that ON123300 induces cell death and negatively regulates key oncogenic pathways in multiple myeloma cells,” said Samir Parekh, MD, also of the Icahn School of Medicine at Mount Sinai.
“This is the first report showing potent cytotoxicity of CDK4/ARK5 inhibition in MM and provides the foundation for further clinical trials using CDK4/ARK5 inhibitors to improve outcomes for MM patients.” ![]()

A dual kinase inhibitor has shown early promise as a potential treatment for multiple myeloma (MM), according to investigators.
The inhibitor, ON123300, targets both AMPK-related protein kinase 5 (ARK5) and cyclin-dependent kinase 4 (CDK4), proteins responsible for maintaining energy balance within the cell.
Investigators found that ON123300 can induce apoptosis in MM cells in vitro and halt tumor growth in mouse models of MM.
The team reported these findings in Cancer Research.
“ARK5 is critical for myeloma survival, and this study suggests a novel function for ARK5 in bridging the mTOR and MYC pathways,” said study author Deepak Perumal, PhD, of the Icahn School of Medicine at Mount Sinai in New York, New York.
“Given that MYC is critically overexpressed in myeloma, we sought to determine whether selective inhibition of ARK5 and CDK4 could be an effective way to target MYC-driven proliferation in myeloma.”
The team at Mount Sinai developed ON123300 in collaboration with Onconova Therapeutics, Inc. And they tested the drug in MM cell lines and primary samples from patients with recurring MM, as well as human HS-5 bone marrow stromal cells.
ON123300 induced rapid cell-cycle arrest and apoptosis in MM cells but was not toxic to normal peripheral blood cells.
In mouse models of MM, ON123300 decreased tumor growth without causing significant toxicity.
The investigators also discovered that MM cells that are sensitive to ON123300 have a unique genomic signature, which could guide the clinical development of the drug.
“Our study results show that ON123300 induces cell death and negatively regulates key oncogenic pathways in multiple myeloma cells,” said Samir Parekh, MD, also of the Icahn School of Medicine at Mount Sinai.
“This is the first report showing potent cytotoxicity of CDK4/ARK5 inhibition in MM and provides the foundation for further clinical trials using CDK4/ARK5 inhibitors to improve outcomes for MM patients.” ![]()

A dual kinase inhibitor has shown early promise as a potential treatment for multiple myeloma (MM), according to investigators.
The inhibitor, ON123300, targets both AMPK-related protein kinase 5 (ARK5) and cyclin-dependent kinase 4 (CDK4), proteins responsible for maintaining energy balance within the cell.
Investigators found that ON123300 can induce apoptosis in MM cells in vitro and halt tumor growth in mouse models of MM.
The team reported these findings in Cancer Research.
“ARK5 is critical for myeloma survival, and this study suggests a novel function for ARK5 in bridging the mTOR and MYC pathways,” said study author Deepak Perumal, PhD, of the Icahn School of Medicine at Mount Sinai in New York, New York.
“Given that MYC is critically overexpressed in myeloma, we sought to determine whether selective inhibition of ARK5 and CDK4 could be an effective way to target MYC-driven proliferation in myeloma.”
The team at Mount Sinai developed ON123300 in collaboration with Onconova Therapeutics, Inc. And they tested the drug in MM cell lines and primary samples from patients with recurring MM, as well as human HS-5 bone marrow stromal cells.
ON123300 induced rapid cell-cycle arrest and apoptosis in MM cells but was not toxic to normal peripheral blood cells.
In mouse models of MM, ON123300 decreased tumor growth without causing significant toxicity.
The investigators also discovered that MM cells that are sensitive to ON123300 have a unique genomic signature, which could guide the clinical development of the drug.
“Our study results show that ON123300 induces cell death and negatively regulates key oncogenic pathways in multiple myeloma cells,” said Samir Parekh, MD, also of the Icahn School of Medicine at Mount Sinai.
“This is the first report showing potent cytotoxicity of CDK4/ARK5 inhibition in MM and provides the foundation for further clinical trials using CDK4/ARK5 inhibitors to improve outcomes for MM patients.” ![]()
System could aid assessment of sickle cell disease

and a normal one
Image by Betty Pace
A microfluidic system can measure the deformability and adhesion of red blood cells (RBCs) in samples from patients with sickle cell disease (SCD), according to research published in Technology.
The researchers noted that RBC deformability has been associated with vaso-occlusion in SCD, but we have limited knowledge on deformation characteristics of RBCs adhered to endothelium-associated proteins in microphysiological fluid flow conditions.
In the past, various approaches have been used to measure RBC deformability, including optical tweezers, micropipette aspiration, and atomic force microscopy. These methods have enabled sensitive and controlled measurement of RBC mechanical properties, but they are typically performed in open environments without fluid flow.
“Microfluidic techniques allow incorporation of physiological flow conditions, as well as biologically relevant adhesion surfaces in a closed setting, which better mimic the natural physiological environment of the RBCs in blood flow,” said study author Umut Gurkan, PhD, of the Case Western Reserve University in Cleveland, Ohio.
For their system, Dr Gurkan and his colleagues integrated a microfluidic approach with a cell-dimensioning algorithm.
They introduced a new parameter to assess the deformability of RBCs. It is known as the dynamic deformability index (DDI), which they defined as the time-dependent change of the cell’s aspect ratio in response to fluid flow shear stress.
The researchers assessed the deformability and adhesion of RBCs containing healthy hemoglobin A (HbA) and homozygous sickle hemoglobin (HbS). And they found the DDI of HbS-containing RBCs was significantly lower than the DDI of HbA-containing RBCs.
The team also found they could divide HbS-containing RBCs into 2 groups—deformable and non-deformable RBCs.
“We report, for the first time, on the subpopulations of RBCs in terms of dynamic deformation characteristics in SCD: deformable and non-deformable RBCs,” said Yunus Alapan, a PhD candidate at Case Western Reserve University.
“Furthermore, we analyzed adhesion of non-deformable RBCs, in comparison to deformable RBCs, quantitatively at physiological and above physiological flow shear stresses in blood samples obtained from SCD patients.”
“We observed significantly greater numbers of adhered non-deformable sickle RBCs than deformable sickle RBCs at flow shear stresses well above the physiological range, suggesting an interplay between dynamic deformability and increased adhesion of RBCs in vaso-occlusive events.”
Now, the researchers are working to further characterize deformability and adhesion of RBCs in a greater number of SCD patients to analyze their associations with clinical phenotypes and complications.
The team said their system may provide important biophysical insights into disease pathophysiology when widely applied in SCD.
They also believe the microfluidic platform has the potential to be used as an in vitro assay for monitoring disease activity at baseline, during clinical flux after treatment, during painful episodes, and in association with long-term complications. ![]()

and a normal one
Image by Betty Pace
A microfluidic system can measure the deformability and adhesion of red blood cells (RBCs) in samples from patients with sickle cell disease (SCD), according to research published in Technology.
The researchers noted that RBC deformability has been associated with vaso-occlusion in SCD, but we have limited knowledge on deformation characteristics of RBCs adhered to endothelium-associated proteins in microphysiological fluid flow conditions.
In the past, various approaches have been used to measure RBC deformability, including optical tweezers, micropipette aspiration, and atomic force microscopy. These methods have enabled sensitive and controlled measurement of RBC mechanical properties, but they are typically performed in open environments without fluid flow.
“Microfluidic techniques allow incorporation of physiological flow conditions, as well as biologically relevant adhesion surfaces in a closed setting, which better mimic the natural physiological environment of the RBCs in blood flow,” said study author Umut Gurkan, PhD, of the Case Western Reserve University in Cleveland, Ohio.
For their system, Dr Gurkan and his colleagues integrated a microfluidic approach with a cell-dimensioning algorithm.
They introduced a new parameter to assess the deformability of RBCs. It is known as the dynamic deformability index (DDI), which they defined as the time-dependent change of the cell’s aspect ratio in response to fluid flow shear stress.
The researchers assessed the deformability and adhesion of RBCs containing healthy hemoglobin A (HbA) and homozygous sickle hemoglobin (HbS). And they found the DDI of HbS-containing RBCs was significantly lower than the DDI of HbA-containing RBCs.
The team also found they could divide HbS-containing RBCs into 2 groups—deformable and non-deformable RBCs.
“We report, for the first time, on the subpopulations of RBCs in terms of dynamic deformation characteristics in SCD: deformable and non-deformable RBCs,” said Yunus Alapan, a PhD candidate at Case Western Reserve University.
“Furthermore, we analyzed adhesion of non-deformable RBCs, in comparison to deformable RBCs, quantitatively at physiological and above physiological flow shear stresses in blood samples obtained from SCD patients.”
“We observed significantly greater numbers of adhered non-deformable sickle RBCs than deformable sickle RBCs at flow shear stresses well above the physiological range, suggesting an interplay between dynamic deformability and increased adhesion of RBCs in vaso-occlusive events.”
Now, the researchers are working to further characterize deformability and adhesion of RBCs in a greater number of SCD patients to analyze their associations with clinical phenotypes and complications.
The team said their system may provide important biophysical insights into disease pathophysiology when widely applied in SCD.
They also believe the microfluidic platform has the potential to be used as an in vitro assay for monitoring disease activity at baseline, during clinical flux after treatment, during painful episodes, and in association with long-term complications. ![]()

and a normal one
Image by Betty Pace
A microfluidic system can measure the deformability and adhesion of red blood cells (RBCs) in samples from patients with sickle cell disease (SCD), according to research published in Technology.
The researchers noted that RBC deformability has been associated with vaso-occlusion in SCD, but we have limited knowledge on deformation characteristics of RBCs adhered to endothelium-associated proteins in microphysiological fluid flow conditions.
In the past, various approaches have been used to measure RBC deformability, including optical tweezers, micropipette aspiration, and atomic force microscopy. These methods have enabled sensitive and controlled measurement of RBC mechanical properties, but they are typically performed in open environments without fluid flow.
“Microfluidic techniques allow incorporation of physiological flow conditions, as well as biologically relevant adhesion surfaces in a closed setting, which better mimic the natural physiological environment of the RBCs in blood flow,” said study author Umut Gurkan, PhD, of the Case Western Reserve University in Cleveland, Ohio.
For their system, Dr Gurkan and his colleagues integrated a microfluidic approach with a cell-dimensioning algorithm.
They introduced a new parameter to assess the deformability of RBCs. It is known as the dynamic deformability index (DDI), which they defined as the time-dependent change of the cell’s aspect ratio in response to fluid flow shear stress.
The researchers assessed the deformability and adhesion of RBCs containing healthy hemoglobin A (HbA) and homozygous sickle hemoglobin (HbS). And they found the DDI of HbS-containing RBCs was significantly lower than the DDI of HbA-containing RBCs.
The team also found they could divide HbS-containing RBCs into 2 groups—deformable and non-deformable RBCs.
“We report, for the first time, on the subpopulations of RBCs in terms of dynamic deformation characteristics in SCD: deformable and non-deformable RBCs,” said Yunus Alapan, a PhD candidate at Case Western Reserve University.
“Furthermore, we analyzed adhesion of non-deformable RBCs, in comparison to deformable RBCs, quantitatively at physiological and above physiological flow shear stresses in blood samples obtained from SCD patients.”
“We observed significantly greater numbers of adhered non-deformable sickle RBCs than deformable sickle RBCs at flow shear stresses well above the physiological range, suggesting an interplay between dynamic deformability and increased adhesion of RBCs in vaso-occlusive events.”
Now, the researchers are working to further characterize deformability and adhesion of RBCs in a greater number of SCD patients to analyze their associations with clinical phenotypes and complications.
The team said their system may provide important biophysical insights into disease pathophysiology when widely applied in SCD.
They also believe the microfluidic platform has the potential to be used as an in vitro assay for monitoring disease activity at baseline, during clinical flux after treatment, during painful episodes, and in association with long-term complications. ![]()
Diet and Atopic Dermatitis
Atopic dermatitis (AD) is the leading diagnosis among pediatric dermatologists,1 and this condition is commonly seen worldwide by dermatologists and allergists.2 There is a widespread misconception held by many patients and their guardians who believe that AD is caused by a food allergy.3 Although AD is related to and part of the atopic complex of disorders associated with food allergies, the role of diet in AD is not well defined. Previously it was recommended to delay early exposure to foods, but now it is recommended to do the opposite in certain situations. In fact, delaying exposure to certain types of foods can increase the likelihood of food allergies (eg, early exposure to peanut butter lowers the statistical chance of developing peanut allergies). This article reviews recent data on the role of diet in AD regarding disease activity as well as new and emerging data on dietary modifications for prevention and intervention. Emerging data on the relationship between AD and food allergies also are presented.
Pathogenesis of AD
The skin barrier plays a vital role in the prevention of pathogens, allergen exposure, and sensitization. There is no solitary root cause of AD, rather it is a combination of inflammation and barrier dysfunction associated with allergic diathesis (eg, atopy). Many patients with AD, especially those with persistent disease, have an intrinsic barrier dysfunction as part of the root cause of their illness, which may be caused by genetically mediated filaggrin defects or alternative barrier dysfunction such as decreased ceramide content that predisposes to percutaneous and mucosal sensitization.4,5 Another source of percutaneous exposure to allergens is macroscopic breaks in the skin caused by scratching, which allows dendritic termini of Langerhans cells to be exposed to percutaneous antigens4,6 through binding to high-affinity IgE receptors.
Langerhans cells exposed to allergens can trigger either an immediate or delayed-type (type I or type II) reaction (sensitization phase) in the lymph node causing inflammatory activation (elicitation). Inflammatory activity in AD is broad and complex and includes the release of IL-4, elevated IgE levels, and eosinophilia, which trigger the helper T cell TH2 and TH17 cascade of cytokines, including IL-2, IL-4, IL-5, IL-8, IL-10, IL-13, IL-17α, tumor necrosis factor α, and IFN-γ,7-9 with the latter worsening barrier defect via downregulation of intercellular substances (eg, filaggrin) and intercellular adhesion expression (eg, claudin 1).6,7,10
Atopic dermatitis does not exist in isolation. The barrier dysfunction associated with AD allows for sensitization to allergens, including those found in food and/or the environment. The atopic march, which occurs via barrier abnormalities facilitating sensitization, can result in further atopy, such as food allergies, environmental allergies, asthma, and eosinophilic esophagitis.11
|
AD and Food Allergies
Many patients and guardians believe AD is caused by a food allergy and that diet restrictions will resolve the disease. Although the latter is not true, in reality many patients with AD do have food allergies. Approximately 40% of infants and young children with moderate to severe AD and 8% of the general population of children will manifest a specific IgE-based food allergy. Food-specific IgE can be triggered or exacerbated by AD through the induction of hives, cutaneous activation of mast cells, increased “spontaneous” basophil histamine release, and food-related lymphocyte-proliferative responses measurable by food patch testing.12 Allergists generally recommend avoidance of or use of heavily denatured food (in the case of a milk/egg allergy) in the setting of documented IgE-mediated allergens.13 Food allergies in AD can manifest with flares, hives, pruritus, and/or other cutaneous symptoms in the absence of flaring AD disease.
Guidelines from the American Academy of Dermatology (AAD)(Table) for the management of AD have recently recommended testing for food allergies in children younger than 5 years who have intractable AD or known food-induced reactions.14 This technique will largely identify children at risk for anaphylaxis but may not yield information contributing to AD improvement. Furthermore, withdrawal of allergens with known IgE-mediated response was classified by the AAD as having consistent good-quality patient-oriented evidence, and asking about allergic reactions as well as acting on a reported allergic history had inconsistent or limited-quality patient-oriented evidence. It is believed that atopy can progress, or march, into a food and/or environmental allergy at any point in life; therefore, testing for a food allergy should be considered in all patients with recent onset of severe and/or persistent AD and/or food-aggravated AD due to a lifetime risk of sensitization.14,15 A food introduction plan may require collaboration with an allergist, especially in high-risk patients (eg, those with known food reactions, family history of food allergies, severe atopy).
Prevention of AD Through Dietary Modification
The National Institute of Allergy and Infectious Diseases consensus group published guidelines on food allergies that affect AD management, including avoidance of proven allergens but not random elimination of food allergens in AD; the group identifies AD and family history of AD as risk factors for food allergies.16 The best data in support of avoidance of documented food allergens to reduce AD severity has been found for egg white allergy and avoidance. Active egg allergy also is linked to staphylococcal superantigen IgE sensitizations,17 but the reason for the link is not yet clear. For the pediatric population, exclusive breastfeeding until 4 to 6 months of age and introduction of solids within the first 4 to 6 months as well as avoidance of maternal dietary restriction during pregnancy and lactation was further endorsed, with use of hydrolyzed formulas as an alternative to exclusive breastfeeding in infants who are not exclusively breastfed (cost permitting).16,18
A Cochrane review of maternal dietary restrictions during pregnancy found no benefit of maternal prenatal dietary restriction on AD prevalence in the first 18 months of life but did note an association with lower mean gestational weight.19
There is currently an effort to produce foods, such as soybeans and corn, that are genetically modified to reduce exposure to the allergenic component, but it is possible that when large-scale challenges occur, these foods also will be allergenic.20,21 In the case of a modified apple, some promising reduction in allergy symptoms has been reported.22 Although genetically modified foods may benefit children with food allergies in the future, they are a source of some controversy.
Complementary and Alternative Medicine
The AAD guidelines do not recommend complementary and alternative medicine (CAM) to treat AD,14 but it remains a commonly used therapy in the United States. A 2014 analysis of data from the 2007 US-based national health interview survey of 9417 children (age range, 0–17 years) demonstrated that 46.9% of children used 1 or more CAM, of which 0.99% used CAM specifically for AD. In this study, herbal therapy, vitamins, homeopathy, diet, and movement techniques were associated with increased prevalence of AD.23 Although some herbals have been shown to be beneficial in AD,24 hepatotoxicity has been reported with some herbal therapies.25 Complementary techniques with evidence-based support include massage therapy,26 relocation to an alternative climate, acupuncture that rivals cetirizine in efficacy, and supportive nutritional advice.24,27
Factors Affecting the Incidence of AD
Atopic dermatitis is of greater prevalence in children in developed wealthy nations such as the United States, supporting the role of enhanced hygiene and overall good health through vaccination as a possible contributor to the rise in AD prevalence in the last 4 decades.28,29 Alternatively, viruses such as respiratory syncytial virus may trigger AD, suggesting vaccination against the virus may reduce the risk for AD.30 Overall, vaccination improves life expectancy and should be conducted on schedule without reservation. Other aspects of hygiene that could conceptually affect prevalence of AD are raw food ingestion and the effects of foodborne microbes on the intestinal microbiome in relationship to AD development. Probiotics have been tested for this purpose.
Probiotics and prebiotics have been theorized to work through a reduction in inflammation; these agents have some evidence in their favor, but they were not endorsed in the AAD guidelines14 despite showing promise in meta-analysis. In particular prenatal and postnatal (maternal and child) supplementation of Lactobacillus rhamnosus shows promise.31-33 Food elimination diets and supplements including vitamin D, selenium, fish oil, borage oil, and zinc were not found to be beneficial and were not recommended in the AAD guidelines.14,34
Percutaneous exposure to peanuts, possibly in household dust, may be the mechanism of peanut sensitization in AD27 via an inherent adjuvant effect of peanut protein.28 The recent LEAP (Learning Early About Peanut Allergy) trial randomized 530 infants aged 4 to 11 months to peanut-avoidant versus peanut-exposed diets for 60 months. The results showed statistically reduced (approximately one-twelfth of the risk) peanut allergy even in infants known to be sensitized (approximately one-third of the risk).35 It is now recommended in countries with a high prevalence of peanut allergies to introduce peanuts to an infant’s diet between 4 and 11 months of age (evidence level 1 [highest level of evidence]), with referral to an allergist for introduction in known sensitization cases and severe AD.36 In the setting of known or documented peanut allergy and for evaluation of potential food allergies, an allergist should be consulted.
Other interventions have been described as promising in mouse models. Those supplements include Lithospermum erythrorhizon,37Platycodon grandiflorus,38Hypsizygus marmoreous,39 fortified ginseng extract,40 polyunsaturated fatty acids,41 and galactooligosaccharide.42 Prebiotic oligosaccharides also are promising for early prevention of AD symptoms in infants, but otherwise these agents have remained largely untested in AD.43 None of these therapies have been endorsed by the AAD, and the long-term safety and efficacy in humans remains to be proven.
Risks of Dietary Restriction
Dietary restrictions in treating AD can have negative consequences, including reduced birth weight when initiated in pregnancy,19 osteomalacia from vitamin D deficiency,44 and nutritional deficiencies (eg, calcium, phosphorus, iron, vitamin K, vitamin D, zinc, vitamin A, B1, B2, B6, niacin, cholesterol, and/or vitamin C deficiencies).45 Excess dietary intake of vegetables in individuals with extensive food allergies can result in carotenemia.46 Protein-restricted diets from use of rice milk or dietary protein restriction can result in kwashiorkorlike protein malnutrition and marasmus.47-49 Nutritional counseling and/or supplementation is recommended for patients with food-restricted diets.
Avoiding Fragrance in Food
Food intolerance often is reported by AD patients. In allergies, food intolerance refers to side effects such as gastrointestinal symptoms; in dermatology, food intolerance can include itching, systemic flares of allergic contact dermatitis (eg, fragrance allergy), or true IgE-mediated allergies such as oral allergy syndrome. Oral allergy syndrome (pollen-food allergy syndrome) is an epitope-spread phenomenon related to an allergy to tree pollen, causing broad allergy to specific groups of fruits and nuts.50 Food triggers in AD include kiwi, milk, apple, tomato, citrus fruits, tree nuts, and peanuts. Oral allergy syndrome is common in food-sensitive AD patients (51.2%) followed by gastrointestinal symptoms (23.5%) and worsening AD (11.4%).51 Sensitization to fragrance can cross-react with foods (eg, balsam of Peru and tomatoes).52 A tomato allergy can be detected either by a skin-prick test or a food patch test in this setting.53 An allergist should be consulted if oral allergy syndrome is suspected.
Conclusion
Food allergies are more common in AD patients and patients should be referred to an allergist for evaluation and management. Strict dietary practice is not recommended, while avoiding proven food allergens in AD could be beneficial. Dermatologists should be aware that patients with dietary restrictions may lack key nutrients, manifesting with nutritional deficiencies in the skin; therefore, nutrition counseling may be needed in the most severe AD/allergy patients. This field is evolving; therefore, ongoing study and evaluation of interventions as they relate to AD will be needed to assess best practices for diet in AD over time.
1. Schachner L, Ling NS, Press S. A statistical analysis of a pediatric dermatology clinic. Pediatr Dermatol. 1983;1:157-164.
2. Kiprono SK, Muchunu JW, Masenga JE. Skin diseases in pediatric patients attending a tertiary dermatology hospital in Northern Tanzania: a cross-sectional study. BMC Dermatol. 2015;15:16.
3. Wensink M, Timmer C, Brand PL. Atopic dermatitis in infants not caused by food allergy [in Dutch]. Ned Tijdschr Geneeskd. 2008;152:4-9.
4. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132(3, pt 2):949-963.
5. Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
6. Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
7. Batista DI, Perez L, Orfali RL, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:1091-1095.
8. Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol. 1998;28:769-779.
9. Correa da Rosa J, Malajian D, Shemer A, et al. Patients with atopic dermatitis have attenuated and distinct contact hypersensitivity responses to common allergens in skin. J Allergy Clin Immunol. 2015;135:712-720.
10. Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
11. Cianferoni A, Spergel J. Eosinophilic esophagitis: a comprehensive review [published online July 22, 2015]. Clin Rev Allergy Immunol. doi:10.1111/all.12846.
12. Sicherer SH, Sampson HA. Food hypersensitivity and atopic dermatitis; pathophysiology, epidemiology, diagnosis, and management. J Allergy Clin Immunol. 1999;104(3, pt 2):S114-S122.
13. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.
14. Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71:1218-1233.
15. Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the epidermal differentiation complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649.
16. Burks AW, Jones SM, Boyce JA, et al. NIAID-sponsored 2010 guidelines for managing food allergy: applications in the pediatric population. Pediatrics. 2011;128:955-965.
17. Ong PY. Association between egg and staphylococcal superantigen IgE sensitizations in atopic dermatitis. Allergy Asthma Proc. 2014;35:346-348.
18. Botteman M, Detzel P. Cost-effectiveness of partially hydrolyzed whey protein formula in the primary prevention of atopic dermatitis in high-risk urban infants in Southeast Asia. Ann Nutr Metab. 2015;66(suppl 1):26-32.
19. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;9:CD000133.
20. Yum HY, Lee SY, Lee KE, et al. Genetically modified and wild soybeans: an immunologic comparison. Allergy Asthma Proc. 2005;26:210-216.
21. Mathur C, Kathuria PC, Dahiya P, et al. Lack of detectable allergenicity in genetically modified maize containing “Cry” proteins as compared to native maize based on in silico & in vitro analysis. PLoS One. 2015;10:e0117340.
22. Dubois AE, Pagliarani G, Brouwer RM, et al. First successful reduction of clinical allergenicity of food by genetic modification: Mal d 1-silenced apples cause fewer allergy symptoms than the wild-type cultivar [published online July 24, 2015]. Allergy. 2015;70:1406-1412.
23. Silverberg JI, Lee-Wong M, Silverberg NB. Complementary and alternative medicines and childhood eczema: a US population-based study. Dermatitis. 2014;25:246-254.
24. Pfab F, Schalock PC, Napadow V, et al. Complementary integrative approach for treating pruritus. Dermatol Ther. 2013;26:149-156.
25. Stickel F, Shouval D. Hepatotoxicity of herbal and dietary supplements: an update. Arch Toxicol. 2015;89:851-865.
26. Schachner L, Field T, Hernandez-Reif M, et al. Atopic dermatitis symptoms decreased in children following massage therapy. Pediatr Dermatol. 1998;15:390-395.
27. Pfab F, Schalock PC, Napadow V, et al. Acupuncture for allergic disease therapy–the current state of evidence. Expert Rev Clin Immunol. 2014;10:831-841.
28. Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
29. Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
30. Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841-846.
31. Foolad N, Brezinski EA, Chase EP, et al. Effect of nutrient supplementation on atopic dermatitis in children: a systematic review of probiotics, prebiotics, formula, and fatty acids. JAMA Dermatol. 2013;149:350-355.
32. Kalliomäki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076-1079.
33. Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184-191.
34. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I: atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.e1-1039.e12.
35. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
36. Fleischer DM, Sicherer S, Greenhawt M, et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants [published online October 2015]. Allergy. 2015;70:1193-1195.
37. Kim J, Cho Y. Gromwell (Lithospermum erythrorhizon) supplementation enhances epidermal levels of cera-mides, glucosylceramides, β-glucocerebrosidase, and acidicsphingomyelinase in NC/Nga mice. J Med Food. 2013;16:927-933.
38. Choi JH, Jin SW, Han EH, et al. Platycodon grandiflorum root-derived saponins attenuate atopic dermatitis-like skin lesions via suppression of NF-κB and STAT1 and activation of Nrf2/ARE-mediated heme oxygenase-1. Phytomedicine. 2014;21:1053-1061.
39. Kim T, Park K, Jung HS, et al. Evaluation of anti-atopic dermatitis activity of Hypsizigus marmoreus extract. Phytother Res. 2014;28:1539-1546.
40. Kim JR, Choi J, Kim J, et al. 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol-fortified ginseng extract attenuates the development of atopic dermatitis-like symptoms in NC/Nga mice. J Ethnopharmacol. 2014;151:365-371.
41. Weise C, Ernst D, van Tol EA, et al. Dietary polyunsaturated fatty acids and non-digestible oligosaccharides reduce dermatitis in mice. Pediatr Allergy Immunol. 2013;24:361-367.
42. Tanabe S, Hochi S. Oral administration of a galactooligosaccharide preparation inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. Int J Mol Med. 2010;25:331-336.
43. Arslanoglu S, Moro GE, Boehm G, et al. Early neutral prebiotic oligosaccharide supplementation reduces the incidence of some allergic manifestations in the first 5 years of life. J Biol Regul Homeost Agents. 2012;26(3 suppl):49-59.
44. Shikino K, Ikusaka M, Yamashita T. Vitamin D-deficient osteomalacia due to excessive self-restrictions for atopic dermatitis [published online July 4, 2014] . BMJ Case Rep.
45. Kim J, Kwon J, Noh G, et al. The effects of elimination diet on nutritional status in subjects with atopic dermatitis. Nutr Res Pract. 2013;7:488-494.
46. Silverberg NB, Lee-Wong M. Generalized yellow discoloration of the skin. Cutis. 2014;93:E11-E12.
47. Hon KL, Nip SY, Cheung KL. A tragic case of atopic eczema: malnutrition and infections despite multivitamins and supplements. Iran J Allergy Asthma Immunol. 2012;11:267-270.
48. Diamanti A, Pedicelli S, D’Argenio P, et al. Iatrogenic kwashiorkor in three infants on a diet of rice beverages. Pediatr Allergy Immunol. 2011;22:878-879.
49. Pillai K, Acharya S. Iatrogenic kwashiorkar. Indian Pediatr. 2010;47:540-541.
50. Price A, Ramachandran S, Smith GP, et al. Oral allergy syndrome (pollen-food allergy syndrome). Dermatitis. 2015;26:78-88.
51. Mattila L, Kilpeläinen M, Terho EO, et al. Food hypersensitivity among Finnish university students: association with atopic diseases. Clin Exp Allergy. 2003;33:600-606.
52. Paulsen E, Christensen LP, Andersen KE. Tomato contact dermatitis. Contact Dermatitis. 2012;67:321-327.
53. Di Leo E, Nettis E, Cardinale F, et al. Tomato atopy patch test in adult atopic dermatitis: diagnostic value and comparison among different methods. Allergy. 2009;64:659-663.
Atopic dermatitis (AD) is the leading diagnosis among pediatric dermatologists,1 and this condition is commonly seen worldwide by dermatologists and allergists.2 There is a widespread misconception held by many patients and their guardians who believe that AD is caused by a food allergy.3 Although AD is related to and part of the atopic complex of disorders associated with food allergies, the role of diet in AD is not well defined. Previously it was recommended to delay early exposure to foods, but now it is recommended to do the opposite in certain situations. In fact, delaying exposure to certain types of foods can increase the likelihood of food allergies (eg, early exposure to peanut butter lowers the statistical chance of developing peanut allergies). This article reviews recent data on the role of diet in AD regarding disease activity as well as new and emerging data on dietary modifications for prevention and intervention. Emerging data on the relationship between AD and food allergies also are presented.
Pathogenesis of AD
The skin barrier plays a vital role in the prevention of pathogens, allergen exposure, and sensitization. There is no solitary root cause of AD, rather it is a combination of inflammation and barrier dysfunction associated with allergic diathesis (eg, atopy). Many patients with AD, especially those with persistent disease, have an intrinsic barrier dysfunction as part of the root cause of their illness, which may be caused by genetically mediated filaggrin defects or alternative barrier dysfunction such as decreased ceramide content that predisposes to percutaneous and mucosal sensitization.4,5 Another source of percutaneous exposure to allergens is macroscopic breaks in the skin caused by scratching, which allows dendritic termini of Langerhans cells to be exposed to percutaneous antigens4,6 through binding to high-affinity IgE receptors.
Langerhans cells exposed to allergens can trigger either an immediate or delayed-type (type I or type II) reaction (sensitization phase) in the lymph node causing inflammatory activation (elicitation). Inflammatory activity in AD is broad and complex and includes the release of IL-4, elevated IgE levels, and eosinophilia, which trigger the helper T cell TH2 and TH17 cascade of cytokines, including IL-2, IL-4, IL-5, IL-8, IL-10, IL-13, IL-17α, tumor necrosis factor α, and IFN-γ,7-9 with the latter worsening barrier defect via downregulation of intercellular substances (eg, filaggrin) and intercellular adhesion expression (eg, claudin 1).6,7,10
Atopic dermatitis does not exist in isolation. The barrier dysfunction associated with AD allows for sensitization to allergens, including those found in food and/or the environment. The atopic march, which occurs via barrier abnormalities facilitating sensitization, can result in further atopy, such as food allergies, environmental allergies, asthma, and eosinophilic esophagitis.11
|
AD and Food Allergies
Many patients and guardians believe AD is caused by a food allergy and that diet restrictions will resolve the disease. Although the latter is not true, in reality many patients with AD do have food allergies. Approximately 40% of infants and young children with moderate to severe AD and 8% of the general population of children will manifest a specific IgE-based food allergy. Food-specific IgE can be triggered or exacerbated by AD through the induction of hives, cutaneous activation of mast cells, increased “spontaneous” basophil histamine release, and food-related lymphocyte-proliferative responses measurable by food patch testing.12 Allergists generally recommend avoidance of or use of heavily denatured food (in the case of a milk/egg allergy) in the setting of documented IgE-mediated allergens.13 Food allergies in AD can manifest with flares, hives, pruritus, and/or other cutaneous symptoms in the absence of flaring AD disease.
Guidelines from the American Academy of Dermatology (AAD)(Table) for the management of AD have recently recommended testing for food allergies in children younger than 5 years who have intractable AD or known food-induced reactions.14 This technique will largely identify children at risk for anaphylaxis but may not yield information contributing to AD improvement. Furthermore, withdrawal of allergens with known IgE-mediated response was classified by the AAD as having consistent good-quality patient-oriented evidence, and asking about allergic reactions as well as acting on a reported allergic history had inconsistent or limited-quality patient-oriented evidence. It is believed that atopy can progress, or march, into a food and/or environmental allergy at any point in life; therefore, testing for a food allergy should be considered in all patients with recent onset of severe and/or persistent AD and/or food-aggravated AD due to a lifetime risk of sensitization.14,15 A food introduction plan may require collaboration with an allergist, especially in high-risk patients (eg, those with known food reactions, family history of food allergies, severe atopy).
Prevention of AD Through Dietary Modification
The National Institute of Allergy and Infectious Diseases consensus group published guidelines on food allergies that affect AD management, including avoidance of proven allergens but not random elimination of food allergens in AD; the group identifies AD and family history of AD as risk factors for food allergies.16 The best data in support of avoidance of documented food allergens to reduce AD severity has been found for egg white allergy and avoidance. Active egg allergy also is linked to staphylococcal superantigen IgE sensitizations,17 but the reason for the link is not yet clear. For the pediatric population, exclusive breastfeeding until 4 to 6 months of age and introduction of solids within the first 4 to 6 months as well as avoidance of maternal dietary restriction during pregnancy and lactation was further endorsed, with use of hydrolyzed formulas as an alternative to exclusive breastfeeding in infants who are not exclusively breastfed (cost permitting).16,18
A Cochrane review of maternal dietary restrictions during pregnancy found no benefit of maternal prenatal dietary restriction on AD prevalence in the first 18 months of life but did note an association with lower mean gestational weight.19
There is currently an effort to produce foods, such as soybeans and corn, that are genetically modified to reduce exposure to the allergenic component, but it is possible that when large-scale challenges occur, these foods also will be allergenic.20,21 In the case of a modified apple, some promising reduction in allergy symptoms has been reported.22 Although genetically modified foods may benefit children with food allergies in the future, they are a source of some controversy.
Complementary and Alternative Medicine
The AAD guidelines do not recommend complementary and alternative medicine (CAM) to treat AD,14 but it remains a commonly used therapy in the United States. A 2014 analysis of data from the 2007 US-based national health interview survey of 9417 children (age range, 0–17 years) demonstrated that 46.9% of children used 1 or more CAM, of which 0.99% used CAM specifically for AD. In this study, herbal therapy, vitamins, homeopathy, diet, and movement techniques were associated with increased prevalence of AD.23 Although some herbals have been shown to be beneficial in AD,24 hepatotoxicity has been reported with some herbal therapies.25 Complementary techniques with evidence-based support include massage therapy,26 relocation to an alternative climate, acupuncture that rivals cetirizine in efficacy, and supportive nutritional advice.24,27
Factors Affecting the Incidence of AD
Atopic dermatitis is of greater prevalence in children in developed wealthy nations such as the United States, supporting the role of enhanced hygiene and overall good health through vaccination as a possible contributor to the rise in AD prevalence in the last 4 decades.28,29 Alternatively, viruses such as respiratory syncytial virus may trigger AD, suggesting vaccination against the virus may reduce the risk for AD.30 Overall, vaccination improves life expectancy and should be conducted on schedule without reservation. Other aspects of hygiene that could conceptually affect prevalence of AD are raw food ingestion and the effects of foodborne microbes on the intestinal microbiome in relationship to AD development. Probiotics have been tested for this purpose.
Probiotics and prebiotics have been theorized to work through a reduction in inflammation; these agents have some evidence in their favor, but they were not endorsed in the AAD guidelines14 despite showing promise in meta-analysis. In particular prenatal and postnatal (maternal and child) supplementation of Lactobacillus rhamnosus shows promise.31-33 Food elimination diets and supplements including vitamin D, selenium, fish oil, borage oil, and zinc were not found to be beneficial and were not recommended in the AAD guidelines.14,34
Percutaneous exposure to peanuts, possibly in household dust, may be the mechanism of peanut sensitization in AD27 via an inherent adjuvant effect of peanut protein.28 The recent LEAP (Learning Early About Peanut Allergy) trial randomized 530 infants aged 4 to 11 months to peanut-avoidant versus peanut-exposed diets for 60 months. The results showed statistically reduced (approximately one-twelfth of the risk) peanut allergy even in infants known to be sensitized (approximately one-third of the risk).35 It is now recommended in countries with a high prevalence of peanut allergies to introduce peanuts to an infant’s diet between 4 and 11 months of age (evidence level 1 [highest level of evidence]), with referral to an allergist for introduction in known sensitization cases and severe AD.36 In the setting of known or documented peanut allergy and for evaluation of potential food allergies, an allergist should be consulted.
Other interventions have been described as promising in mouse models. Those supplements include Lithospermum erythrorhizon,37Platycodon grandiflorus,38Hypsizygus marmoreous,39 fortified ginseng extract,40 polyunsaturated fatty acids,41 and galactooligosaccharide.42 Prebiotic oligosaccharides also are promising for early prevention of AD symptoms in infants, but otherwise these agents have remained largely untested in AD.43 None of these therapies have been endorsed by the AAD, and the long-term safety and efficacy in humans remains to be proven.
Risks of Dietary Restriction
Dietary restrictions in treating AD can have negative consequences, including reduced birth weight when initiated in pregnancy,19 osteomalacia from vitamin D deficiency,44 and nutritional deficiencies (eg, calcium, phosphorus, iron, vitamin K, vitamin D, zinc, vitamin A, B1, B2, B6, niacin, cholesterol, and/or vitamin C deficiencies).45 Excess dietary intake of vegetables in individuals with extensive food allergies can result in carotenemia.46 Protein-restricted diets from use of rice milk or dietary protein restriction can result in kwashiorkorlike protein malnutrition and marasmus.47-49 Nutritional counseling and/or supplementation is recommended for patients with food-restricted diets.
Avoiding Fragrance in Food
Food intolerance often is reported by AD patients. In allergies, food intolerance refers to side effects such as gastrointestinal symptoms; in dermatology, food intolerance can include itching, systemic flares of allergic contact dermatitis (eg, fragrance allergy), or true IgE-mediated allergies such as oral allergy syndrome. Oral allergy syndrome (pollen-food allergy syndrome) is an epitope-spread phenomenon related to an allergy to tree pollen, causing broad allergy to specific groups of fruits and nuts.50 Food triggers in AD include kiwi, milk, apple, tomato, citrus fruits, tree nuts, and peanuts. Oral allergy syndrome is common in food-sensitive AD patients (51.2%) followed by gastrointestinal symptoms (23.5%) and worsening AD (11.4%).51 Sensitization to fragrance can cross-react with foods (eg, balsam of Peru and tomatoes).52 A tomato allergy can be detected either by a skin-prick test or a food patch test in this setting.53 An allergist should be consulted if oral allergy syndrome is suspected.
Conclusion
Food allergies are more common in AD patients and patients should be referred to an allergist for evaluation and management. Strict dietary practice is not recommended, while avoiding proven food allergens in AD could be beneficial. Dermatologists should be aware that patients with dietary restrictions may lack key nutrients, manifesting with nutritional deficiencies in the skin; therefore, nutrition counseling may be needed in the most severe AD/allergy patients. This field is evolving; therefore, ongoing study and evaluation of interventions as they relate to AD will be needed to assess best practices for diet in AD over time.
Atopic dermatitis (AD) is the leading diagnosis among pediatric dermatologists,1 and this condition is commonly seen worldwide by dermatologists and allergists.2 There is a widespread misconception held by many patients and their guardians who believe that AD is caused by a food allergy.3 Although AD is related to and part of the atopic complex of disorders associated with food allergies, the role of diet in AD is not well defined. Previously it was recommended to delay early exposure to foods, but now it is recommended to do the opposite in certain situations. In fact, delaying exposure to certain types of foods can increase the likelihood of food allergies (eg, early exposure to peanut butter lowers the statistical chance of developing peanut allergies). This article reviews recent data on the role of diet in AD regarding disease activity as well as new and emerging data on dietary modifications for prevention and intervention. Emerging data on the relationship between AD and food allergies also are presented.
Pathogenesis of AD
The skin barrier plays a vital role in the prevention of pathogens, allergen exposure, and sensitization. There is no solitary root cause of AD, rather it is a combination of inflammation and barrier dysfunction associated with allergic diathesis (eg, atopy). Many patients with AD, especially those with persistent disease, have an intrinsic barrier dysfunction as part of the root cause of their illness, which may be caused by genetically mediated filaggrin defects or alternative barrier dysfunction such as decreased ceramide content that predisposes to percutaneous and mucosal sensitization.4,5 Another source of percutaneous exposure to allergens is macroscopic breaks in the skin caused by scratching, which allows dendritic termini of Langerhans cells to be exposed to percutaneous antigens4,6 through binding to high-affinity IgE receptors.
Langerhans cells exposed to allergens can trigger either an immediate or delayed-type (type I or type II) reaction (sensitization phase) in the lymph node causing inflammatory activation (elicitation). Inflammatory activity in AD is broad and complex and includes the release of IL-4, elevated IgE levels, and eosinophilia, which trigger the helper T cell TH2 and TH17 cascade of cytokines, including IL-2, IL-4, IL-5, IL-8, IL-10, IL-13, IL-17α, tumor necrosis factor α, and IFN-γ,7-9 with the latter worsening barrier defect via downregulation of intercellular substances (eg, filaggrin) and intercellular adhesion expression (eg, claudin 1).6,7,10
Atopic dermatitis does not exist in isolation. The barrier dysfunction associated with AD allows for sensitization to allergens, including those found in food and/or the environment. The atopic march, which occurs via barrier abnormalities facilitating sensitization, can result in further atopy, such as food allergies, environmental allergies, asthma, and eosinophilic esophagitis.11
|
AD and Food Allergies
Many patients and guardians believe AD is caused by a food allergy and that diet restrictions will resolve the disease. Although the latter is not true, in reality many patients with AD do have food allergies. Approximately 40% of infants and young children with moderate to severe AD and 8% of the general population of children will manifest a specific IgE-based food allergy. Food-specific IgE can be triggered or exacerbated by AD through the induction of hives, cutaneous activation of mast cells, increased “spontaneous” basophil histamine release, and food-related lymphocyte-proliferative responses measurable by food patch testing.12 Allergists generally recommend avoidance of or use of heavily denatured food (in the case of a milk/egg allergy) in the setting of documented IgE-mediated allergens.13 Food allergies in AD can manifest with flares, hives, pruritus, and/or other cutaneous symptoms in the absence of flaring AD disease.
Guidelines from the American Academy of Dermatology (AAD)(Table) for the management of AD have recently recommended testing for food allergies in children younger than 5 years who have intractable AD or known food-induced reactions.14 This technique will largely identify children at risk for anaphylaxis but may not yield information contributing to AD improvement. Furthermore, withdrawal of allergens with known IgE-mediated response was classified by the AAD as having consistent good-quality patient-oriented evidence, and asking about allergic reactions as well as acting on a reported allergic history had inconsistent or limited-quality patient-oriented evidence. It is believed that atopy can progress, or march, into a food and/or environmental allergy at any point in life; therefore, testing for a food allergy should be considered in all patients with recent onset of severe and/or persistent AD and/or food-aggravated AD due to a lifetime risk of sensitization.14,15 A food introduction plan may require collaboration with an allergist, especially in high-risk patients (eg, those with known food reactions, family history of food allergies, severe atopy).
Prevention of AD Through Dietary Modification
The National Institute of Allergy and Infectious Diseases consensus group published guidelines on food allergies that affect AD management, including avoidance of proven allergens but not random elimination of food allergens in AD; the group identifies AD and family history of AD as risk factors for food allergies.16 The best data in support of avoidance of documented food allergens to reduce AD severity has been found for egg white allergy and avoidance. Active egg allergy also is linked to staphylococcal superantigen IgE sensitizations,17 but the reason for the link is not yet clear. For the pediatric population, exclusive breastfeeding until 4 to 6 months of age and introduction of solids within the first 4 to 6 months as well as avoidance of maternal dietary restriction during pregnancy and lactation was further endorsed, with use of hydrolyzed formulas as an alternative to exclusive breastfeeding in infants who are not exclusively breastfed (cost permitting).16,18
A Cochrane review of maternal dietary restrictions during pregnancy found no benefit of maternal prenatal dietary restriction on AD prevalence in the first 18 months of life but did note an association with lower mean gestational weight.19
There is currently an effort to produce foods, such as soybeans and corn, that are genetically modified to reduce exposure to the allergenic component, but it is possible that when large-scale challenges occur, these foods also will be allergenic.20,21 In the case of a modified apple, some promising reduction in allergy symptoms has been reported.22 Although genetically modified foods may benefit children with food allergies in the future, they are a source of some controversy.
Complementary and Alternative Medicine
The AAD guidelines do not recommend complementary and alternative medicine (CAM) to treat AD,14 but it remains a commonly used therapy in the United States. A 2014 analysis of data from the 2007 US-based national health interview survey of 9417 children (age range, 0–17 years) demonstrated that 46.9% of children used 1 or more CAM, of which 0.99% used CAM specifically for AD. In this study, herbal therapy, vitamins, homeopathy, diet, and movement techniques were associated with increased prevalence of AD.23 Although some herbals have been shown to be beneficial in AD,24 hepatotoxicity has been reported with some herbal therapies.25 Complementary techniques with evidence-based support include massage therapy,26 relocation to an alternative climate, acupuncture that rivals cetirizine in efficacy, and supportive nutritional advice.24,27
Factors Affecting the Incidence of AD
Atopic dermatitis is of greater prevalence in children in developed wealthy nations such as the United States, supporting the role of enhanced hygiene and overall good health through vaccination as a possible contributor to the rise in AD prevalence in the last 4 decades.28,29 Alternatively, viruses such as respiratory syncytial virus may trigger AD, suggesting vaccination against the virus may reduce the risk for AD.30 Overall, vaccination improves life expectancy and should be conducted on schedule without reservation. Other aspects of hygiene that could conceptually affect prevalence of AD are raw food ingestion and the effects of foodborne microbes on the intestinal microbiome in relationship to AD development. Probiotics have been tested for this purpose.
Probiotics and prebiotics have been theorized to work through a reduction in inflammation; these agents have some evidence in their favor, but they were not endorsed in the AAD guidelines14 despite showing promise in meta-analysis. In particular prenatal and postnatal (maternal and child) supplementation of Lactobacillus rhamnosus shows promise.31-33 Food elimination diets and supplements including vitamin D, selenium, fish oil, borage oil, and zinc were not found to be beneficial and were not recommended in the AAD guidelines.14,34
Percutaneous exposure to peanuts, possibly in household dust, may be the mechanism of peanut sensitization in AD27 via an inherent adjuvant effect of peanut protein.28 The recent LEAP (Learning Early About Peanut Allergy) trial randomized 530 infants aged 4 to 11 months to peanut-avoidant versus peanut-exposed diets for 60 months. The results showed statistically reduced (approximately one-twelfth of the risk) peanut allergy even in infants known to be sensitized (approximately one-third of the risk).35 It is now recommended in countries with a high prevalence of peanut allergies to introduce peanuts to an infant’s diet between 4 and 11 months of age (evidence level 1 [highest level of evidence]), with referral to an allergist for introduction in known sensitization cases and severe AD.36 In the setting of known or documented peanut allergy and for evaluation of potential food allergies, an allergist should be consulted.
Other interventions have been described as promising in mouse models. Those supplements include Lithospermum erythrorhizon,37Platycodon grandiflorus,38Hypsizygus marmoreous,39 fortified ginseng extract,40 polyunsaturated fatty acids,41 and galactooligosaccharide.42 Prebiotic oligosaccharides also are promising for early prevention of AD symptoms in infants, but otherwise these agents have remained largely untested in AD.43 None of these therapies have been endorsed by the AAD, and the long-term safety and efficacy in humans remains to be proven.
Risks of Dietary Restriction
Dietary restrictions in treating AD can have negative consequences, including reduced birth weight when initiated in pregnancy,19 osteomalacia from vitamin D deficiency,44 and nutritional deficiencies (eg, calcium, phosphorus, iron, vitamin K, vitamin D, zinc, vitamin A, B1, B2, B6, niacin, cholesterol, and/or vitamin C deficiencies).45 Excess dietary intake of vegetables in individuals with extensive food allergies can result in carotenemia.46 Protein-restricted diets from use of rice milk or dietary protein restriction can result in kwashiorkorlike protein malnutrition and marasmus.47-49 Nutritional counseling and/or supplementation is recommended for patients with food-restricted diets.
Avoiding Fragrance in Food
Food intolerance often is reported by AD patients. In allergies, food intolerance refers to side effects such as gastrointestinal symptoms; in dermatology, food intolerance can include itching, systemic flares of allergic contact dermatitis (eg, fragrance allergy), or true IgE-mediated allergies such as oral allergy syndrome. Oral allergy syndrome (pollen-food allergy syndrome) is an epitope-spread phenomenon related to an allergy to tree pollen, causing broad allergy to specific groups of fruits and nuts.50 Food triggers in AD include kiwi, milk, apple, tomato, citrus fruits, tree nuts, and peanuts. Oral allergy syndrome is common in food-sensitive AD patients (51.2%) followed by gastrointestinal symptoms (23.5%) and worsening AD (11.4%).51 Sensitization to fragrance can cross-react with foods (eg, balsam of Peru and tomatoes).52 A tomato allergy can be detected either by a skin-prick test or a food patch test in this setting.53 An allergist should be consulted if oral allergy syndrome is suspected.
Conclusion
Food allergies are more common in AD patients and patients should be referred to an allergist for evaluation and management. Strict dietary practice is not recommended, while avoiding proven food allergens in AD could be beneficial. Dermatologists should be aware that patients with dietary restrictions may lack key nutrients, manifesting with nutritional deficiencies in the skin; therefore, nutrition counseling may be needed in the most severe AD/allergy patients. This field is evolving; therefore, ongoing study and evaluation of interventions as they relate to AD will be needed to assess best practices for diet in AD over time.
1. Schachner L, Ling NS, Press S. A statistical analysis of a pediatric dermatology clinic. Pediatr Dermatol. 1983;1:157-164.
2. Kiprono SK, Muchunu JW, Masenga JE. Skin diseases in pediatric patients attending a tertiary dermatology hospital in Northern Tanzania: a cross-sectional study. BMC Dermatol. 2015;15:16.
3. Wensink M, Timmer C, Brand PL. Atopic dermatitis in infants not caused by food allergy [in Dutch]. Ned Tijdschr Geneeskd. 2008;152:4-9.
4. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132(3, pt 2):949-963.
5. Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
6. Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
7. Batista DI, Perez L, Orfali RL, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:1091-1095.
8. Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol. 1998;28:769-779.
9. Correa da Rosa J, Malajian D, Shemer A, et al. Patients with atopic dermatitis have attenuated and distinct contact hypersensitivity responses to common allergens in skin. J Allergy Clin Immunol. 2015;135:712-720.
10. Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
11. Cianferoni A, Spergel J. Eosinophilic esophagitis: a comprehensive review [published online July 22, 2015]. Clin Rev Allergy Immunol. doi:10.1111/all.12846.
12. Sicherer SH, Sampson HA. Food hypersensitivity and atopic dermatitis; pathophysiology, epidemiology, diagnosis, and management. J Allergy Clin Immunol. 1999;104(3, pt 2):S114-S122.
13. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.
14. Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71:1218-1233.
15. Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the epidermal differentiation complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649.
16. Burks AW, Jones SM, Boyce JA, et al. NIAID-sponsored 2010 guidelines for managing food allergy: applications in the pediatric population. Pediatrics. 2011;128:955-965.
17. Ong PY. Association between egg and staphylococcal superantigen IgE sensitizations in atopic dermatitis. Allergy Asthma Proc. 2014;35:346-348.
18. Botteman M, Detzel P. Cost-effectiveness of partially hydrolyzed whey protein formula in the primary prevention of atopic dermatitis in high-risk urban infants in Southeast Asia. Ann Nutr Metab. 2015;66(suppl 1):26-32.
19. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;9:CD000133.
20. Yum HY, Lee SY, Lee KE, et al. Genetically modified and wild soybeans: an immunologic comparison. Allergy Asthma Proc. 2005;26:210-216.
21. Mathur C, Kathuria PC, Dahiya P, et al. Lack of detectable allergenicity in genetically modified maize containing “Cry” proteins as compared to native maize based on in silico & in vitro analysis. PLoS One. 2015;10:e0117340.
22. Dubois AE, Pagliarani G, Brouwer RM, et al. First successful reduction of clinical allergenicity of food by genetic modification: Mal d 1-silenced apples cause fewer allergy symptoms than the wild-type cultivar [published online July 24, 2015]. Allergy. 2015;70:1406-1412.
23. Silverberg JI, Lee-Wong M, Silverberg NB. Complementary and alternative medicines and childhood eczema: a US population-based study. Dermatitis. 2014;25:246-254.
24. Pfab F, Schalock PC, Napadow V, et al. Complementary integrative approach for treating pruritus. Dermatol Ther. 2013;26:149-156.
25. Stickel F, Shouval D. Hepatotoxicity of herbal and dietary supplements: an update. Arch Toxicol. 2015;89:851-865.
26. Schachner L, Field T, Hernandez-Reif M, et al. Atopic dermatitis symptoms decreased in children following massage therapy. Pediatr Dermatol. 1998;15:390-395.
27. Pfab F, Schalock PC, Napadow V, et al. Acupuncture for allergic disease therapy–the current state of evidence. Expert Rev Clin Immunol. 2014;10:831-841.
28. Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
29. Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
30. Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841-846.
31. Foolad N, Brezinski EA, Chase EP, et al. Effect of nutrient supplementation on atopic dermatitis in children: a systematic review of probiotics, prebiotics, formula, and fatty acids. JAMA Dermatol. 2013;149:350-355.
32. Kalliomäki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076-1079.
33. Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184-191.
34. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I: atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.e1-1039.e12.
35. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
36. Fleischer DM, Sicherer S, Greenhawt M, et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants [published online October 2015]. Allergy. 2015;70:1193-1195.
37. Kim J, Cho Y. Gromwell (Lithospermum erythrorhizon) supplementation enhances epidermal levels of cera-mides, glucosylceramides, β-glucocerebrosidase, and acidicsphingomyelinase in NC/Nga mice. J Med Food. 2013;16:927-933.
38. Choi JH, Jin SW, Han EH, et al. Platycodon grandiflorum root-derived saponins attenuate atopic dermatitis-like skin lesions via suppression of NF-κB and STAT1 and activation of Nrf2/ARE-mediated heme oxygenase-1. Phytomedicine. 2014;21:1053-1061.
39. Kim T, Park K, Jung HS, et al. Evaluation of anti-atopic dermatitis activity of Hypsizigus marmoreus extract. Phytother Res. 2014;28:1539-1546.
40. Kim JR, Choi J, Kim J, et al. 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol-fortified ginseng extract attenuates the development of atopic dermatitis-like symptoms in NC/Nga mice. J Ethnopharmacol. 2014;151:365-371.
41. Weise C, Ernst D, van Tol EA, et al. Dietary polyunsaturated fatty acids and non-digestible oligosaccharides reduce dermatitis in mice. Pediatr Allergy Immunol. 2013;24:361-367.
42. Tanabe S, Hochi S. Oral administration of a galactooligosaccharide preparation inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. Int J Mol Med. 2010;25:331-336.
43. Arslanoglu S, Moro GE, Boehm G, et al. Early neutral prebiotic oligosaccharide supplementation reduces the incidence of some allergic manifestations in the first 5 years of life. J Biol Regul Homeost Agents. 2012;26(3 suppl):49-59.
44. Shikino K, Ikusaka M, Yamashita T. Vitamin D-deficient osteomalacia due to excessive self-restrictions for atopic dermatitis [published online July 4, 2014] . BMJ Case Rep.
45. Kim J, Kwon J, Noh G, et al. The effects of elimination diet on nutritional status in subjects with atopic dermatitis. Nutr Res Pract. 2013;7:488-494.
46. Silverberg NB, Lee-Wong M. Generalized yellow discoloration of the skin. Cutis. 2014;93:E11-E12.
47. Hon KL, Nip SY, Cheung KL. A tragic case of atopic eczema: malnutrition and infections despite multivitamins and supplements. Iran J Allergy Asthma Immunol. 2012;11:267-270.
48. Diamanti A, Pedicelli S, D’Argenio P, et al. Iatrogenic kwashiorkor in three infants on a diet of rice beverages. Pediatr Allergy Immunol. 2011;22:878-879.
49. Pillai K, Acharya S. Iatrogenic kwashiorkar. Indian Pediatr. 2010;47:540-541.
50. Price A, Ramachandran S, Smith GP, et al. Oral allergy syndrome (pollen-food allergy syndrome). Dermatitis. 2015;26:78-88.
51. Mattila L, Kilpeläinen M, Terho EO, et al. Food hypersensitivity among Finnish university students: association with atopic diseases. Clin Exp Allergy. 2003;33:600-606.
52. Paulsen E, Christensen LP, Andersen KE. Tomato contact dermatitis. Contact Dermatitis. 2012;67:321-327.
53. Di Leo E, Nettis E, Cardinale F, et al. Tomato atopy patch test in adult atopic dermatitis: diagnostic value and comparison among different methods. Allergy. 2009;64:659-663.
1. Schachner L, Ling NS, Press S. A statistical analysis of a pediatric dermatology clinic. Pediatr Dermatol. 1983;1:157-164.
2. Kiprono SK, Muchunu JW, Masenga JE. Skin diseases in pediatric patients attending a tertiary dermatology hospital in Northern Tanzania: a cross-sectional study. BMC Dermatol. 2015;15:16.
3. Wensink M, Timmer C, Brand PL. Atopic dermatitis in infants not caused by food allergy [in Dutch]. Ned Tijdschr Geneeskd. 2008;152:4-9.
4. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132(3, pt 2):949-963.
5. Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
6. Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
7. Batista DI, Perez L, Orfali RL, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:1091-1095.
8. Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol. 1998;28:769-779.
9. Correa da Rosa J, Malajian D, Shemer A, et al. Patients with atopic dermatitis have attenuated and distinct contact hypersensitivity responses to common allergens in skin. J Allergy Clin Immunol. 2015;135:712-720.
10. Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
11. Cianferoni A, Spergel J. Eosinophilic esophagitis: a comprehensive review [published online July 22, 2015]. Clin Rev Allergy Immunol. doi:10.1111/all.12846.
12. Sicherer SH, Sampson HA. Food hypersensitivity and atopic dermatitis; pathophysiology, epidemiology, diagnosis, and management. J Allergy Clin Immunol. 1999;104(3, pt 2):S114-S122.
13. Sicherer SH, Sampson HA. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133:291-307.
14. Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014;71:1218-1233.
15. Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the epidermal differentiation complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649.
16. Burks AW, Jones SM, Boyce JA, et al. NIAID-sponsored 2010 guidelines for managing food allergy: applications in the pediatric population. Pediatrics. 2011;128:955-965.
17. Ong PY. Association between egg and staphylococcal superantigen IgE sensitizations in atopic dermatitis. Allergy Asthma Proc. 2014;35:346-348.
18. Botteman M, Detzel P. Cost-effectiveness of partially hydrolyzed whey protein formula in the primary prevention of atopic dermatitis in high-risk urban infants in Southeast Asia. Ann Nutr Metab. 2015;66(suppl 1):26-32.
19. Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;9:CD000133.
20. Yum HY, Lee SY, Lee KE, et al. Genetically modified and wild soybeans: an immunologic comparison. Allergy Asthma Proc. 2005;26:210-216.
21. Mathur C, Kathuria PC, Dahiya P, et al. Lack of detectable allergenicity in genetically modified maize containing “Cry” proteins as compared to native maize based on in silico & in vitro analysis. PLoS One. 2015;10:e0117340.
22. Dubois AE, Pagliarani G, Brouwer RM, et al. First successful reduction of clinical allergenicity of food by genetic modification: Mal d 1-silenced apples cause fewer allergy symptoms than the wild-type cultivar [published online July 24, 2015]. Allergy. 2015;70:1406-1412.
23. Silverberg JI, Lee-Wong M, Silverberg NB. Complementary and alternative medicines and childhood eczema: a US population-based study. Dermatitis. 2014;25:246-254.
24. Pfab F, Schalock PC, Napadow V, et al. Complementary integrative approach for treating pruritus. Dermatol Ther. 2013;26:149-156.
25. Stickel F, Shouval D. Hepatotoxicity of herbal and dietary supplements: an update. Arch Toxicol. 2015;89:851-865.
26. Schachner L, Field T, Hernandez-Reif M, et al. Atopic dermatitis symptoms decreased in children following massage therapy. Pediatr Dermatol. 1998;15:390-395.
27. Pfab F, Schalock PC, Napadow V, et al. Acupuncture for allergic disease therapy–the current state of evidence. Expert Rev Clin Immunol. 2014;10:831-841.
28. Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132:1132-1138.
29. Silverberg JI, Norowitz KB, Kleiman E, et al. Association between varicella zoster virus infection and atopic dermatitis in early and late childhood: a case-control study. J Allergy Clin Immunol. 2010;126:300-305.
30. Welliver RC, Wong DT, Sun M, et al. The development of respiratory syncytial virus-specific IgE and the release of histamine in nasopharyngeal secretions after infection. N Engl J Med. 1981;305:841-846.
31. Foolad N, Brezinski EA, Chase EP, et al. Effect of nutrient supplementation on atopic dermatitis in children: a systematic review of probiotics, prebiotics, formula, and fatty acids. JAMA Dermatol. 2013;149:350-355.
32. Kalliomäki M, Salminen S, Arvilommi H, et al. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076-1079.
33. Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184-191.
34. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I: atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.e1-1039.e12.
35. Du Toit G, Roberts G, Sayre PH, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803-813.
36. Fleischer DM, Sicherer S, Greenhawt M, et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants [published online October 2015]. Allergy. 2015;70:1193-1195.
37. Kim J, Cho Y. Gromwell (Lithospermum erythrorhizon) supplementation enhances epidermal levels of cera-mides, glucosylceramides, β-glucocerebrosidase, and acidicsphingomyelinase in NC/Nga mice. J Med Food. 2013;16:927-933.
38. Choi JH, Jin SW, Han EH, et al. Platycodon grandiflorum root-derived saponins attenuate atopic dermatitis-like skin lesions via suppression of NF-κB and STAT1 and activation of Nrf2/ARE-mediated heme oxygenase-1. Phytomedicine. 2014;21:1053-1061.
39. Kim T, Park K, Jung HS, et al. Evaluation of anti-atopic dermatitis activity of Hypsizigus marmoreus extract. Phytother Res. 2014;28:1539-1546.
40. Kim JR, Choi J, Kim J, et al. 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol-fortified ginseng extract attenuates the development of atopic dermatitis-like symptoms in NC/Nga mice. J Ethnopharmacol. 2014;151:365-371.
41. Weise C, Ernst D, van Tol EA, et al. Dietary polyunsaturated fatty acids and non-digestible oligosaccharides reduce dermatitis in mice. Pediatr Allergy Immunol. 2013;24:361-367.
42. Tanabe S, Hochi S. Oral administration of a galactooligosaccharide preparation inhibits development of atopic dermatitis-like skin lesions in NC/Nga mice. Int J Mol Med. 2010;25:331-336.
43. Arslanoglu S, Moro GE, Boehm G, et al. Early neutral prebiotic oligosaccharide supplementation reduces the incidence of some allergic manifestations in the first 5 years of life. J Biol Regul Homeost Agents. 2012;26(3 suppl):49-59.
44. Shikino K, Ikusaka M, Yamashita T. Vitamin D-deficient osteomalacia due to excessive self-restrictions for atopic dermatitis [published online July 4, 2014] . BMJ Case Rep.
45. Kim J, Kwon J, Noh G, et al. The effects of elimination diet on nutritional status in subjects with atopic dermatitis. Nutr Res Pract. 2013;7:488-494.
46. Silverberg NB, Lee-Wong M. Generalized yellow discoloration of the skin. Cutis. 2014;93:E11-E12.
47. Hon KL, Nip SY, Cheung KL. A tragic case of atopic eczema: malnutrition and infections despite multivitamins and supplements. Iran J Allergy Asthma Immunol. 2012;11:267-270.
48. Diamanti A, Pedicelli S, D’Argenio P, et al. Iatrogenic kwashiorkor in three infants on a diet of rice beverages. Pediatr Allergy Immunol. 2011;22:878-879.
49. Pillai K, Acharya S. Iatrogenic kwashiorkar. Indian Pediatr. 2010;47:540-541.
50. Price A, Ramachandran S, Smith GP, et al. Oral allergy syndrome (pollen-food allergy syndrome). Dermatitis. 2015;26:78-88.
51. Mattila L, Kilpeläinen M, Terho EO, et al. Food hypersensitivity among Finnish university students: association with atopic diseases. Clin Exp Allergy. 2003;33:600-606.
52. Paulsen E, Christensen LP, Andersen KE. Tomato contact dermatitis. Contact Dermatitis. 2012;67:321-327.
53. Di Leo E, Nettis E, Cardinale F, et al. Tomato atopy patch test in adult atopic dermatitis: diagnostic value and comparison among different methods. Allergy. 2009;64:659-663.
Practice Points
- Test children younger than 5 years with moderate to severe atopic dermatitis (AD) for food allergies if they have persistently severe AD or known food-induced reactions.
- Food elimination diets are not recommended for management of AD.
- There is not enough evidence supporting the use of complementary and alternative medicine, probiotics/prebiotics, or supplements for the treatment of AD.
Prolonged Pustular Eruption From Hydroxychloroquine: An Unusual Case of Acute Generalized Exanthematous Pustulosis
Acute generalized exanthematous pustulosis (AGEP) is an uncommon cutaneous eruption characterized by acute, extensive, nonfollicular, sterile pustules accompanied by widespread erythema, fever, and leukocytosis. The clinical hallmark is superficial, sterile, subcorneal pustular dermatosis, which typically starts on the face, axilla, and groin and then progresses to most of the body. Approximately 90% of AGEP cases are due to drug hypersensitivity to a newly initiated medication, while the other 10% are thought to be viral in origin.1 Discontinuation of the offending agent may allow for complete resolution within 15 days. Agents commonly implicated in causing AGEP are antibiotics such as aminopenicillins, macrolides, and cephalosporins.2 Hydroxychloroquine (HCQ) also has been reported to cause AGEP,3-7 with resolution shortly after discontinuation of the drug,4,6 close to the characteristic 15 days of AGEP due to alternate medications.We report an unusual case of HCQ-induced AGEP that lasted far beyond the typical 15 days. We also review other cases of HCQ-induced AGEP and possible mechanisms to explain our patient’s symptoms.
|
|
| Figure 1. Acute generalized exanthematous pustulosis extending to the chest and upper extremities (A) as well as the shoulders and back (B). |
Case Report
A 50-year-old woman who was previously diagnosed with rheumatoid factor seronegative, nonerosive rheumatoid arthritis, which was only moderately controlled with low-dose prednisone (5 mg once daily) after 2 months of treatment, was started on oral HCQ 200 mg twice daily by her rheumatologist. Two weeks after starting HCQ treatment, she developed a pustular exanthem that gradually spread on the back over the next 24 to 48 hours. She described the eruption initially as pruritic, but she then developed painful stinging sensations as the eruption spread. She visited her primary care physician the next day and stopped the HCQ after 14 days following a discussion with the physician. Her prednisone dosage was increased to 50 mg daily for 5 days, but by the fifth day the lesions had spread to the face, full back, shoulders, and upper chest (Figure 1). Morphologically, she presented to the dermatology clinic with innumerable 1- to 2-mm pustules with confluent erythema on the back, extending to the forearms (Figure 2). She also had scattered erythematous macules and papules on the buttocks, legs, and plantar surfaces of the feet. A biopsy taken from the right forearm demonstrated subcorneal pustular dermatosis consistent with AGEP. Prednisone 50 mg once daily was continued. She was scheduled for a follow-up in 3 days but instead went to the emergency department 1 day later due to worsening of the eruption, fever, and malaise. On examination there were multiple discrete and confluent erythematous plaques on the face that extended to the lower extremities. Pustules and scales were noted on the back. New pustules had developed on the hands and feet with intense pruritus.
On admission, her vitals were stable with mild tachycardia. Aggressive intravenous hydration was administered. Her white blood cell count was elevated at 28.3×109/L (reference range, 4.5–10×109/L). She was started on intravenous methylprednisolone 100 mg once daily; topical steroid wet wraps with triamcinolone 0.1% were applied to the trunk, arms, legs, and abdomen twice daily; and hydrocortisone cream 2.5% was applied to the face and intertriginous areas 3 times daily. Over the next 2 days, eruptions continued to persist and the patient reported worsening of pain despite treatment. On day 3, intravenous methylprednisolone 100 mg was switched to oral prednisone 80 mg once daily.
Over the ensuing 5 days, recurrent episodes of erythema on the back had spread to the extremities. After 1 week in the hospital, the diffuse erythema had improved and she had widespread desquamation. She was discharged and prescribed oral prednisone 80 mg once daily and topical therapy twice daily. The patient followed up in the dermatology clinic 4 days after discharge with a mildly pruritic eruption on the trunk and proximal lower extremities but otherwise was doing well. She was instructed to taper the prednisone by 10 mg every 4 days.
At a follow-up 3 weeks later, she had persistent stinging and tingling sensations, widespread xerosis, and diffuse patchy erythema primarily on the back and proximal extremities, which flared over the last week. The patient reported waxing and waning of the erythema and pruritus since being discharged from the hospital. Despite the recent flare, which was her fourth flare of cutaneous eruption, she showed marked improvement since her initial examination and 40 days after discontinuation of HCQ. She was taking prednisone 40 mg once daily and was advised to continue tapering the dose by 2 mg every 6 to 8 days as tolerated. At 81 days after AGEP onset, the eruption had resolved and the patient was back to her baseline prednisone dosage of 5 mg once daily.
Comment
Acute generalized exanthematous pustulosis is characterized by the sudden appearance of erythema and hundreds of sterile nonfollicular pustules, fever, and leukocytosis. Histologically, AGEP is composed of subcorneal and intraepidermal pustules, edema of the papillary dermis, and perivascular infiltrates of neutrophils and possible eosinophils. The pathogenesis of AGEP is thought to be due to the release of increased amounts of IL-8 by T cells, which attract and activate polymorphonuclear neutrophils.1 Psoriasiform changes are uncommon. Clinically, AGEP is similar to pustular psoriasis but has shown to be its own distinct entity. Unlike patients with pustular psoriasis, patients with AGEP lack a personal or family history of psoriasis or arthritis, have a shorter duration of pustules and fever, and have a history of new medication administration. Other conditions to consider in the differential diagnosis include pustular psoriasis, subcorneal pustulosis, IgA pemphigus, drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, Stevens-Johnson syndrome, and acute febrile neutrophilic dermatosis.
In AGEP, the average duration of medication exposure prior to onset varies depending on the causative agent. Antibiotics consistently have been shown to trigger symptoms after 1 day, whereas other medications, including HCQ, averaged closer to 11 days. Hydroxychloroquine is widely used to treat rheumatic and dermatologic diseases and has previously been reported to be a less common cause of AGEP3; however, a EuroSCAR study found that patients treated with HCQ were at a greater risk for AGEP.2 Acute generalized exanthematous pustulosis usually follows a benign self-limiting course. Within days the eruption gradually evolves into superficial desquamation. Characteristically, removal of the offending agent typically leads to spontaneous resolution in less than 15 days. Resolution is generally without complications and, therefore, treatment is not always necessary. Death has been reported in up to 2% of cases.8 There are no known therapies that prevent the spread of lesions or further decline of the patient’s condition. Systemic corticosteroids often are used to treat AGEP with variable results.1,5
Unique to our patient were recurring exacerbations of the cutaneous lesions beyond the typical 15 days for complete resolution. Even up to 40 days after discontinuation of medication, our patient continued to experience cutaneous symptoms. Other reported cases have not described patients with symptoms flaring or continuing for this extended period of time. A review of 7 external AGEP cases caused by HCQ (identified through a PubMed search of articles indexed for MEDLINE using the search terms acute generalized exanthematous pustulosis or eruption with hydroxychloroquine or plaquenil) showed resolution within 8 days to 3 weeks (Table).3-6,8 One case report documented disease exacerbation on day 18 after tapering the methylprednisolone dose. This patient was then treated with cyclosporine and had a prompt recovery.5 One case of AGEP due to terbinafine reported continual symptoms for approximately 4 weeks after terbinafine discontinuation.9 Our patient’s continual symptoms beyond the typical 15 days may be due to the long half-life of HCQ, which is approximately 40 to 50 days. Systemic corticosteroids often are used to control severe eruptions in AGEP and were administered to our patient; however, their utility in shortening the duration or reducing the severity of the eruption has not been proven.
Conclusion
Hydroxychloroquine is a commonly used agent for dermatologic and rheumatologic conditions. The rare but severe acute adverse event of AGEP warrants caution in HCQ use. Correct diagnosis of AGEP with HCQ cessation generally is effective as therapy. Our patient demonstrated that not all cases of AGEP show rapid resolution of cutaneous symptoms after cessation of the drug. Hydroxychloroquine’s extended half-life of 40 to 50 days surpasses that of other medications known to cause AGEP and may explain our patient’s symptoms beyond the usual course.
1. Speeckaert MM, Speeckaert R, Lambert J, et al. Acute generalized exanthematous pustulosis: an overview of the clinical, immunological and diagnostic concepts [published online June 14, 2010]. Eur J Dermatol. 2010;20:425-433.
2. Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) [published online September 13, 2007]. Br J Dermatol. 2007;157:989-996.
3. Park JJ, Yun SJ, Lee JB, et al. A case of hydroxy-chloroquine induced acute generalized exanthematous pustulosis confirmed by accidental oral provocation [published online February 28, 2010]. Ann Dermatol. 2010;22:102-105.
4. Lateef A, Tan KB, Lau TC. Acute generalized exanthematous pustulosis and toxic epidermal necrolysis induced by hydroxychloroquine [published online August 30, 2009]. Clin Rheumatol. 2009;28:1449-1452.
5. Di Lernia V, Grenzi L, Guareschi E, et al. Rapid clearing of acute generalized exanthematous pustulosis after administration of ciclosporin [published online July 29, 2009]. Clin Exp Dermatol. 2009;34:e757-e759.
6. Paradisi A, Bugatti L, Sisto T, et al. Acute generalized exanthematous pustulosis induced by hydroxychloroquine: three cases and a review of the literature. Clin Ther. 2008;30:930-940.
7. Choi MJ, Kim HS, Park HJ, et al. Clinicopathologic manifestations of 36 Korean patients with acute generalized exanthematous pustulosis: a case series and review of the literature [published online May 17, 2010]. Ann Dermatol. 2010;22:163-169.
8. Martins A, Lopes LC, Paiva Lopes MJ, et al. Acute generalized exanthematous pustulosis induced by hydroxychloroquine. Eur J Dermatol. 2006;16:317-318.
9. Lombardo M, Cerati M, Pazzaglia A, et al. Acute generalized exanthematous pustulosis induced by terbinafine. J Am Acad Dermatol. 2003;49:158-159.
Acute generalized exanthematous pustulosis (AGEP) is an uncommon cutaneous eruption characterized by acute, extensive, nonfollicular, sterile pustules accompanied by widespread erythema, fever, and leukocytosis. The clinical hallmark is superficial, sterile, subcorneal pustular dermatosis, which typically starts on the face, axilla, and groin and then progresses to most of the body. Approximately 90% of AGEP cases are due to drug hypersensitivity to a newly initiated medication, while the other 10% are thought to be viral in origin.1 Discontinuation of the offending agent may allow for complete resolution within 15 days. Agents commonly implicated in causing AGEP are antibiotics such as aminopenicillins, macrolides, and cephalosporins.2 Hydroxychloroquine (HCQ) also has been reported to cause AGEP,3-7 with resolution shortly after discontinuation of the drug,4,6 close to the characteristic 15 days of AGEP due to alternate medications.We report an unusual case of HCQ-induced AGEP that lasted far beyond the typical 15 days. We also review other cases of HCQ-induced AGEP and possible mechanisms to explain our patient’s symptoms.
|
|
| Figure 1. Acute generalized exanthematous pustulosis extending to the chest and upper extremities (A) as well as the shoulders and back (B). |
Case Report
A 50-year-old woman who was previously diagnosed with rheumatoid factor seronegative, nonerosive rheumatoid arthritis, which was only moderately controlled with low-dose prednisone (5 mg once daily) after 2 months of treatment, was started on oral HCQ 200 mg twice daily by her rheumatologist. Two weeks after starting HCQ treatment, she developed a pustular exanthem that gradually spread on the back over the next 24 to 48 hours. She described the eruption initially as pruritic, but she then developed painful stinging sensations as the eruption spread. She visited her primary care physician the next day and stopped the HCQ after 14 days following a discussion with the physician. Her prednisone dosage was increased to 50 mg daily for 5 days, but by the fifth day the lesions had spread to the face, full back, shoulders, and upper chest (Figure 1). Morphologically, she presented to the dermatology clinic with innumerable 1- to 2-mm pustules with confluent erythema on the back, extending to the forearms (Figure 2). She also had scattered erythematous macules and papules on the buttocks, legs, and plantar surfaces of the feet. A biopsy taken from the right forearm demonstrated subcorneal pustular dermatosis consistent with AGEP. Prednisone 50 mg once daily was continued. She was scheduled for a follow-up in 3 days but instead went to the emergency department 1 day later due to worsening of the eruption, fever, and malaise. On examination there were multiple discrete and confluent erythematous plaques on the face that extended to the lower extremities. Pustules and scales were noted on the back. New pustules had developed on the hands and feet with intense pruritus.
On admission, her vitals were stable with mild tachycardia. Aggressive intravenous hydration was administered. Her white blood cell count was elevated at 28.3×109/L (reference range, 4.5–10×109/L). She was started on intravenous methylprednisolone 100 mg once daily; topical steroid wet wraps with triamcinolone 0.1% were applied to the trunk, arms, legs, and abdomen twice daily; and hydrocortisone cream 2.5% was applied to the face and intertriginous areas 3 times daily. Over the next 2 days, eruptions continued to persist and the patient reported worsening of pain despite treatment. On day 3, intravenous methylprednisolone 100 mg was switched to oral prednisone 80 mg once daily.
Over the ensuing 5 days, recurrent episodes of erythema on the back had spread to the extremities. After 1 week in the hospital, the diffuse erythema had improved and she had widespread desquamation. She was discharged and prescribed oral prednisone 80 mg once daily and topical therapy twice daily. The patient followed up in the dermatology clinic 4 days after discharge with a mildly pruritic eruption on the trunk and proximal lower extremities but otherwise was doing well. She was instructed to taper the prednisone by 10 mg every 4 days.
At a follow-up 3 weeks later, she had persistent stinging and tingling sensations, widespread xerosis, and diffuse patchy erythema primarily on the back and proximal extremities, which flared over the last week. The patient reported waxing and waning of the erythema and pruritus since being discharged from the hospital. Despite the recent flare, which was her fourth flare of cutaneous eruption, she showed marked improvement since her initial examination and 40 days after discontinuation of HCQ. She was taking prednisone 40 mg once daily and was advised to continue tapering the dose by 2 mg every 6 to 8 days as tolerated. At 81 days after AGEP onset, the eruption had resolved and the patient was back to her baseline prednisone dosage of 5 mg once daily.
Comment
Acute generalized exanthematous pustulosis is characterized by the sudden appearance of erythema and hundreds of sterile nonfollicular pustules, fever, and leukocytosis. Histologically, AGEP is composed of subcorneal and intraepidermal pustules, edema of the papillary dermis, and perivascular infiltrates of neutrophils and possible eosinophils. The pathogenesis of AGEP is thought to be due to the release of increased amounts of IL-8 by T cells, which attract and activate polymorphonuclear neutrophils.1 Psoriasiform changes are uncommon. Clinically, AGEP is similar to pustular psoriasis but has shown to be its own distinct entity. Unlike patients with pustular psoriasis, patients with AGEP lack a personal or family history of psoriasis or arthritis, have a shorter duration of pustules and fever, and have a history of new medication administration. Other conditions to consider in the differential diagnosis include pustular psoriasis, subcorneal pustulosis, IgA pemphigus, drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, Stevens-Johnson syndrome, and acute febrile neutrophilic dermatosis.
In AGEP, the average duration of medication exposure prior to onset varies depending on the causative agent. Antibiotics consistently have been shown to trigger symptoms after 1 day, whereas other medications, including HCQ, averaged closer to 11 days. Hydroxychloroquine is widely used to treat rheumatic and dermatologic diseases and has previously been reported to be a less common cause of AGEP3; however, a EuroSCAR study found that patients treated with HCQ were at a greater risk for AGEP.2 Acute generalized exanthematous pustulosis usually follows a benign self-limiting course. Within days the eruption gradually evolves into superficial desquamation. Characteristically, removal of the offending agent typically leads to spontaneous resolution in less than 15 days. Resolution is generally without complications and, therefore, treatment is not always necessary. Death has been reported in up to 2% of cases.8 There are no known therapies that prevent the spread of lesions or further decline of the patient’s condition. Systemic corticosteroids often are used to treat AGEP with variable results.1,5
Unique to our patient were recurring exacerbations of the cutaneous lesions beyond the typical 15 days for complete resolution. Even up to 40 days after discontinuation of medication, our patient continued to experience cutaneous symptoms. Other reported cases have not described patients with symptoms flaring or continuing for this extended period of time. A review of 7 external AGEP cases caused by HCQ (identified through a PubMed search of articles indexed for MEDLINE using the search terms acute generalized exanthematous pustulosis or eruption with hydroxychloroquine or plaquenil) showed resolution within 8 days to 3 weeks (Table).3-6,8 One case report documented disease exacerbation on day 18 after tapering the methylprednisolone dose. This patient was then treated with cyclosporine and had a prompt recovery.5 One case of AGEP due to terbinafine reported continual symptoms for approximately 4 weeks after terbinafine discontinuation.9 Our patient’s continual symptoms beyond the typical 15 days may be due to the long half-life of HCQ, which is approximately 40 to 50 days. Systemic corticosteroids often are used to control severe eruptions in AGEP and were administered to our patient; however, their utility in shortening the duration or reducing the severity of the eruption has not been proven.
Conclusion
Hydroxychloroquine is a commonly used agent for dermatologic and rheumatologic conditions. The rare but severe acute adverse event of AGEP warrants caution in HCQ use. Correct diagnosis of AGEP with HCQ cessation generally is effective as therapy. Our patient demonstrated that not all cases of AGEP show rapid resolution of cutaneous symptoms after cessation of the drug. Hydroxychloroquine’s extended half-life of 40 to 50 days surpasses that of other medications known to cause AGEP and may explain our patient’s symptoms beyond the usual course.
Acute generalized exanthematous pustulosis (AGEP) is an uncommon cutaneous eruption characterized by acute, extensive, nonfollicular, sterile pustules accompanied by widespread erythema, fever, and leukocytosis. The clinical hallmark is superficial, sterile, subcorneal pustular dermatosis, which typically starts on the face, axilla, and groin and then progresses to most of the body. Approximately 90% of AGEP cases are due to drug hypersensitivity to a newly initiated medication, while the other 10% are thought to be viral in origin.1 Discontinuation of the offending agent may allow for complete resolution within 15 days. Agents commonly implicated in causing AGEP are antibiotics such as aminopenicillins, macrolides, and cephalosporins.2 Hydroxychloroquine (HCQ) also has been reported to cause AGEP,3-7 with resolution shortly after discontinuation of the drug,4,6 close to the characteristic 15 days of AGEP due to alternate medications.We report an unusual case of HCQ-induced AGEP that lasted far beyond the typical 15 days. We also review other cases of HCQ-induced AGEP and possible mechanisms to explain our patient’s symptoms.
|
|
| Figure 1. Acute generalized exanthematous pustulosis extending to the chest and upper extremities (A) as well as the shoulders and back (B). |
Case Report
A 50-year-old woman who was previously diagnosed with rheumatoid factor seronegative, nonerosive rheumatoid arthritis, which was only moderately controlled with low-dose prednisone (5 mg once daily) after 2 months of treatment, was started on oral HCQ 200 mg twice daily by her rheumatologist. Two weeks after starting HCQ treatment, she developed a pustular exanthem that gradually spread on the back over the next 24 to 48 hours. She described the eruption initially as pruritic, but she then developed painful stinging sensations as the eruption spread. She visited her primary care physician the next day and stopped the HCQ after 14 days following a discussion with the physician. Her prednisone dosage was increased to 50 mg daily for 5 days, but by the fifth day the lesions had spread to the face, full back, shoulders, and upper chest (Figure 1). Morphologically, she presented to the dermatology clinic with innumerable 1- to 2-mm pustules with confluent erythema on the back, extending to the forearms (Figure 2). She also had scattered erythematous macules and papules on the buttocks, legs, and plantar surfaces of the feet. A biopsy taken from the right forearm demonstrated subcorneal pustular dermatosis consistent with AGEP. Prednisone 50 mg once daily was continued. She was scheduled for a follow-up in 3 days but instead went to the emergency department 1 day later due to worsening of the eruption, fever, and malaise. On examination there were multiple discrete and confluent erythematous plaques on the face that extended to the lower extremities. Pustules and scales were noted on the back. New pustules had developed on the hands and feet with intense pruritus.
On admission, her vitals were stable with mild tachycardia. Aggressive intravenous hydration was administered. Her white blood cell count was elevated at 28.3×109/L (reference range, 4.5–10×109/L). She was started on intravenous methylprednisolone 100 mg once daily; topical steroid wet wraps with triamcinolone 0.1% were applied to the trunk, arms, legs, and abdomen twice daily; and hydrocortisone cream 2.5% was applied to the face and intertriginous areas 3 times daily. Over the next 2 days, eruptions continued to persist and the patient reported worsening of pain despite treatment. On day 3, intravenous methylprednisolone 100 mg was switched to oral prednisone 80 mg once daily.
Over the ensuing 5 days, recurrent episodes of erythema on the back had spread to the extremities. After 1 week in the hospital, the diffuse erythema had improved and she had widespread desquamation. She was discharged and prescribed oral prednisone 80 mg once daily and topical therapy twice daily. The patient followed up in the dermatology clinic 4 days after discharge with a mildly pruritic eruption on the trunk and proximal lower extremities but otherwise was doing well. She was instructed to taper the prednisone by 10 mg every 4 days.
At a follow-up 3 weeks later, she had persistent stinging and tingling sensations, widespread xerosis, and diffuse patchy erythema primarily on the back and proximal extremities, which flared over the last week. The patient reported waxing and waning of the erythema and pruritus since being discharged from the hospital. Despite the recent flare, which was her fourth flare of cutaneous eruption, she showed marked improvement since her initial examination and 40 days after discontinuation of HCQ. She was taking prednisone 40 mg once daily and was advised to continue tapering the dose by 2 mg every 6 to 8 days as tolerated. At 81 days after AGEP onset, the eruption had resolved and the patient was back to her baseline prednisone dosage of 5 mg once daily.
Comment
Acute generalized exanthematous pustulosis is characterized by the sudden appearance of erythema and hundreds of sterile nonfollicular pustules, fever, and leukocytosis. Histologically, AGEP is composed of subcorneal and intraepidermal pustules, edema of the papillary dermis, and perivascular infiltrates of neutrophils and possible eosinophils. The pathogenesis of AGEP is thought to be due to the release of increased amounts of IL-8 by T cells, which attract and activate polymorphonuclear neutrophils.1 Psoriasiform changes are uncommon. Clinically, AGEP is similar to pustular psoriasis but has shown to be its own distinct entity. Unlike patients with pustular psoriasis, patients with AGEP lack a personal or family history of psoriasis or arthritis, have a shorter duration of pustules and fever, and have a history of new medication administration. Other conditions to consider in the differential diagnosis include pustular psoriasis, subcorneal pustulosis, IgA pemphigus, drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, Stevens-Johnson syndrome, and acute febrile neutrophilic dermatosis.
In AGEP, the average duration of medication exposure prior to onset varies depending on the causative agent. Antibiotics consistently have been shown to trigger symptoms after 1 day, whereas other medications, including HCQ, averaged closer to 11 days. Hydroxychloroquine is widely used to treat rheumatic and dermatologic diseases and has previously been reported to be a less common cause of AGEP3; however, a EuroSCAR study found that patients treated with HCQ were at a greater risk for AGEP.2 Acute generalized exanthematous pustulosis usually follows a benign self-limiting course. Within days the eruption gradually evolves into superficial desquamation. Characteristically, removal of the offending agent typically leads to spontaneous resolution in less than 15 days. Resolution is generally without complications and, therefore, treatment is not always necessary. Death has been reported in up to 2% of cases.8 There are no known therapies that prevent the spread of lesions or further decline of the patient’s condition. Systemic corticosteroids often are used to treat AGEP with variable results.1,5
Unique to our patient were recurring exacerbations of the cutaneous lesions beyond the typical 15 days for complete resolution. Even up to 40 days after discontinuation of medication, our patient continued to experience cutaneous symptoms. Other reported cases have not described patients with symptoms flaring or continuing for this extended period of time. A review of 7 external AGEP cases caused by HCQ (identified through a PubMed search of articles indexed for MEDLINE using the search terms acute generalized exanthematous pustulosis or eruption with hydroxychloroquine or plaquenil) showed resolution within 8 days to 3 weeks (Table).3-6,8 One case report documented disease exacerbation on day 18 after tapering the methylprednisolone dose. This patient was then treated with cyclosporine and had a prompt recovery.5 One case of AGEP due to terbinafine reported continual symptoms for approximately 4 weeks after terbinafine discontinuation.9 Our patient’s continual symptoms beyond the typical 15 days may be due to the long half-life of HCQ, which is approximately 40 to 50 days. Systemic corticosteroids often are used to control severe eruptions in AGEP and were administered to our patient; however, their utility in shortening the duration or reducing the severity of the eruption has not been proven.
Conclusion
Hydroxychloroquine is a commonly used agent for dermatologic and rheumatologic conditions. The rare but severe acute adverse event of AGEP warrants caution in HCQ use. Correct diagnosis of AGEP with HCQ cessation generally is effective as therapy. Our patient demonstrated that not all cases of AGEP show rapid resolution of cutaneous symptoms after cessation of the drug. Hydroxychloroquine’s extended half-life of 40 to 50 days surpasses that of other medications known to cause AGEP and may explain our patient’s symptoms beyond the usual course.
1. Speeckaert MM, Speeckaert R, Lambert J, et al. Acute generalized exanthematous pustulosis: an overview of the clinical, immunological and diagnostic concepts [published online June 14, 2010]. Eur J Dermatol. 2010;20:425-433.
2. Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) [published online September 13, 2007]. Br J Dermatol. 2007;157:989-996.
3. Park JJ, Yun SJ, Lee JB, et al. A case of hydroxy-chloroquine induced acute generalized exanthematous pustulosis confirmed by accidental oral provocation [published online February 28, 2010]. Ann Dermatol. 2010;22:102-105.
4. Lateef A, Tan KB, Lau TC. Acute generalized exanthematous pustulosis and toxic epidermal necrolysis induced by hydroxychloroquine [published online August 30, 2009]. Clin Rheumatol. 2009;28:1449-1452.
5. Di Lernia V, Grenzi L, Guareschi E, et al. Rapid clearing of acute generalized exanthematous pustulosis after administration of ciclosporin [published online July 29, 2009]. Clin Exp Dermatol. 2009;34:e757-e759.
6. Paradisi A, Bugatti L, Sisto T, et al. Acute generalized exanthematous pustulosis induced by hydroxychloroquine: three cases and a review of the literature. Clin Ther. 2008;30:930-940.
7. Choi MJ, Kim HS, Park HJ, et al. Clinicopathologic manifestations of 36 Korean patients with acute generalized exanthematous pustulosis: a case series and review of the literature [published online May 17, 2010]. Ann Dermatol. 2010;22:163-169.
8. Martins A, Lopes LC, Paiva Lopes MJ, et al. Acute generalized exanthematous pustulosis induced by hydroxychloroquine. Eur J Dermatol. 2006;16:317-318.
9. Lombardo M, Cerati M, Pazzaglia A, et al. Acute generalized exanthematous pustulosis induced by terbinafine. J Am Acad Dermatol. 2003;49:158-159.
1. Speeckaert MM, Speeckaert R, Lambert J, et al. Acute generalized exanthematous pustulosis: an overview of the clinical, immunological and diagnostic concepts [published online June 14, 2010]. Eur J Dermatol. 2010;20:425-433.
2. Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) [published online September 13, 2007]. Br J Dermatol. 2007;157:989-996.
3. Park JJ, Yun SJ, Lee JB, et al. A case of hydroxy-chloroquine induced acute generalized exanthematous pustulosis confirmed by accidental oral provocation [published online February 28, 2010]. Ann Dermatol. 2010;22:102-105.
4. Lateef A, Tan KB, Lau TC. Acute generalized exanthematous pustulosis and toxic epidermal necrolysis induced by hydroxychloroquine [published online August 30, 2009]. Clin Rheumatol. 2009;28:1449-1452.
5. Di Lernia V, Grenzi L, Guareschi E, et al. Rapid clearing of acute generalized exanthematous pustulosis after administration of ciclosporin [published online July 29, 2009]. Clin Exp Dermatol. 2009;34:e757-e759.
6. Paradisi A, Bugatti L, Sisto T, et al. Acute generalized exanthematous pustulosis induced by hydroxychloroquine: three cases and a review of the literature. Clin Ther. 2008;30:930-940.
7. Choi MJ, Kim HS, Park HJ, et al. Clinicopathologic manifestations of 36 Korean patients with acute generalized exanthematous pustulosis: a case series and review of the literature [published online May 17, 2010]. Ann Dermatol. 2010;22:163-169.
8. Martins A, Lopes LC, Paiva Lopes MJ, et al. Acute generalized exanthematous pustulosis induced by hydroxychloroquine. Eur J Dermatol. 2006;16:317-318.
9. Lombardo M, Cerati M, Pazzaglia A, et al. Acute generalized exanthematous pustulosis induced by terbinafine. J Am Acad Dermatol. 2003;49:158-159.
Practice Points
- Acute generalized exanthematous pustulosis (AGEP) is most commonly caused by antibiotics (eg, aminopenicillins, macrolides, cephalosporins) followed by calcium channel blockers.
- The main treatment of AGEP is discontinuation of the culprit medication, which typically results in resolution within 2 weeks. Treatment also can symptomatically include topical or systemic corticosteroids and antipyretics.
- Hydroxychloroquine (HCQ) can be a culprit of AGEP with a prolonged recovery course. It is important to inform patients with HCQ-associated AGEP that the clearance of their lesions may take longer than the typical 2 weeks.
Clinical Pearl: The Squeeze Maneuver
Practice Gap
Warts may negatively impact a patient's quality of life, as they may cause not only discomfort and pain but also embarrassment and low self-esteem.1 Moreover, Ciconte et al1 demonstrated that study participants with warts on their feet were more likely to report physical discomfort than those with warts on their hands. Therefore, plantar warts should be diagnosed promptly to allow for proper treatment.
Warts may be identified by viewing the dilated capillaries that lie on their surface, which appear as small black dots to the naked eye.1 The formation of a plantar wart obliterates the normal plantar creases, thereby flattening the skin’s natural markings. However, a plantar wart may appear clinically similar to a callus and both lesions typically form in pressure point areas, warranting the use of a tool that aids in its diagnostic evaluation.1,2
Diagnostic Tools
Dermoscopy, a noninvasive tool that creates a microscopic visualization of lesions, is commonly used to distinguish dermatologic pathology if the clinical presentation overlaps with a similar lesion, such as a callus, corn, or plantar wart.1,3 However, there is another way of differentiating plantar warts from calluses using a simple 2-step clinical maneuver that we learned from Dr. Lewis Kaplan at the University of Miami.
Using the thumb or index finger, apply pressure at a perpendicular angle to the lesion on the sole of the patient’s foot, which will not create substantial discomfort or pain in a patient who has a plantar wart (Figure) but will be painful in a patient who has a callus due to the underlying bony spur. The next step involves applying pressure to the left and right sides of the lesion by squeezing toward the center with the thumb and index finger at a 45° angle. This maneuver will create substantial discomfort and pain in patients with plantar warts, thus helping to confirm the diagnosis.
| 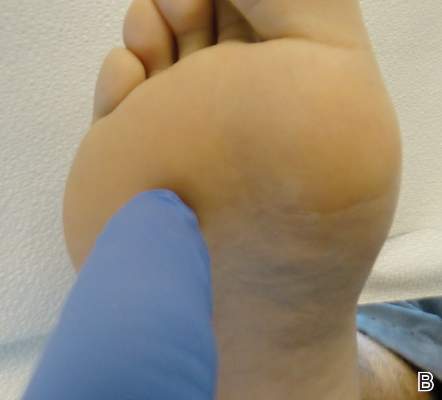
|
| A plantar wart before (A) and after undergoing the squeeze maneuver (B). The patient denied feelings of discomfort or pain. | |
Practice Implications
Rarely, a plantar wart can progress to form a verrucous carcinoma if left untreated.2 Thus, it is important to diagnose and treat plantar warts to avoid pain and potential complications. The technique discussed here, which we are coining as the “squeeze maneuver,” allows for easy diagnosis and negates the need for an expensive diagnostic tool.To submit a clinical pearl, contact our Editorial Office.
- Ciconte A, Campbell J, Tabrizi S, et al. Warts are not merely blemishes on the skin: a study on the morbidity associated with having viral cutaneous warts. Australas J Dermatol. 2003;44:169-173.
- Cardoso J, Calonje E. Cutaneous manifestations of human papillomaviruses: a review. Acta Dermatovenerol. 2011;20:145-154.
- Bae J, Kang H, Kim H, et al. Differential diagnosis of plantar wart from corn, callus and healed wart with the aid of dermoscopy. Br J Dermatol. 2009;160:220-222.
Practice Gap
Warts may negatively impact a patient's quality of life, as they may cause not only discomfort and pain but also embarrassment and low self-esteem.1 Moreover, Ciconte et al1 demonstrated that study participants with warts on their feet were more likely to report physical discomfort than those with warts on their hands. Therefore, plantar warts should be diagnosed promptly to allow for proper treatment.
Warts may be identified by viewing the dilated capillaries that lie on their surface, which appear as small black dots to the naked eye.1 The formation of a plantar wart obliterates the normal plantar creases, thereby flattening the skin’s natural markings. However, a plantar wart may appear clinically similar to a callus and both lesions typically form in pressure point areas, warranting the use of a tool that aids in its diagnostic evaluation.1,2
Diagnostic Tools
Dermoscopy, a noninvasive tool that creates a microscopic visualization of lesions, is commonly used to distinguish dermatologic pathology if the clinical presentation overlaps with a similar lesion, such as a callus, corn, or plantar wart.1,3 However, there is another way of differentiating plantar warts from calluses using a simple 2-step clinical maneuver that we learned from Dr. Lewis Kaplan at the University of Miami.
Using the thumb or index finger, apply pressure at a perpendicular angle to the lesion on the sole of the patient’s foot, which will not create substantial discomfort or pain in a patient who has a plantar wart (Figure) but will be painful in a patient who has a callus due to the underlying bony spur. The next step involves applying pressure to the left and right sides of the lesion by squeezing toward the center with the thumb and index finger at a 45° angle. This maneuver will create substantial discomfort and pain in patients with plantar warts, thus helping to confirm the diagnosis.

| 
|
| A plantar wart before (A) and after undergoing the squeeze maneuver (B). The patient denied feelings of discomfort or pain. | |
Practice Implications
Rarely, a plantar wart can progress to form a verrucous carcinoma if left untreated.2 Thus, it is important to diagnose and treat plantar warts to avoid pain and potential complications. The technique discussed here, which we are coining as the “squeeze maneuver,” allows for easy diagnosis and negates the need for an expensive diagnostic tool.To submit a clinical pearl, contact our Editorial Office.
Practice Gap
Warts may negatively impact a patient's quality of life, as they may cause not only discomfort and pain but also embarrassment and low self-esteem.1 Moreover, Ciconte et al1 demonstrated that study participants with warts on their feet were more likely to report physical discomfort than those with warts on their hands. Therefore, plantar warts should be diagnosed promptly to allow for proper treatment.
Warts may be identified by viewing the dilated capillaries that lie on their surface, which appear as small black dots to the naked eye.1 The formation of a plantar wart obliterates the normal plantar creases, thereby flattening the skin’s natural markings. However, a plantar wart may appear clinically similar to a callus and both lesions typically form in pressure point areas, warranting the use of a tool that aids in its diagnostic evaluation.1,2
Diagnostic Tools
Dermoscopy, a noninvasive tool that creates a microscopic visualization of lesions, is commonly used to distinguish dermatologic pathology if the clinical presentation overlaps with a similar lesion, such as a callus, corn, or plantar wart.1,3 However, there is another way of differentiating plantar warts from calluses using a simple 2-step clinical maneuver that we learned from Dr. Lewis Kaplan at the University of Miami.
Using the thumb or index finger, apply pressure at a perpendicular angle to the lesion on the sole of the patient’s foot, which will not create substantial discomfort or pain in a patient who has a plantar wart (Figure) but will be painful in a patient who has a callus due to the underlying bony spur. The next step involves applying pressure to the left and right sides of the lesion by squeezing toward the center with the thumb and index finger at a 45° angle. This maneuver will create substantial discomfort and pain in patients with plantar warts, thus helping to confirm the diagnosis.

| 
|
| A plantar wart before (A) and after undergoing the squeeze maneuver (B). The patient denied feelings of discomfort or pain. | |
Practice Implications
Rarely, a plantar wart can progress to form a verrucous carcinoma if left untreated.2 Thus, it is important to diagnose and treat plantar warts to avoid pain and potential complications. The technique discussed here, which we are coining as the “squeeze maneuver,” allows for easy diagnosis and negates the need for an expensive diagnostic tool.To submit a clinical pearl, contact our Editorial Office.
- Ciconte A, Campbell J, Tabrizi S, et al. Warts are not merely blemishes on the skin: a study on the morbidity associated with having viral cutaneous warts. Australas J Dermatol. 2003;44:169-173.
- Cardoso J, Calonje E. Cutaneous manifestations of human papillomaviruses: a review. Acta Dermatovenerol. 2011;20:145-154.
- Bae J, Kang H, Kim H, et al. Differential diagnosis of plantar wart from corn, callus and healed wart with the aid of dermoscopy. Br J Dermatol. 2009;160:220-222.
- Ciconte A, Campbell J, Tabrizi S, et al. Warts are not merely blemishes on the skin: a study on the morbidity associated with having viral cutaneous warts. Australas J Dermatol. 2003;44:169-173.
- Cardoso J, Calonje E. Cutaneous manifestations of human papillomaviruses: a review. Acta Dermatovenerol. 2011;20:145-154.
- Bae J, Kang H, Kim H, et al. Differential diagnosis of plantar wart from corn, callus and healed wart with the aid of dermoscopy. Br J Dermatol. 2009;160:220-222.
Don’t Get Hung Up on Fishhooks: A Guide to Fishhook Removal
Fishing is one of the world’s most beloved activities, enjoyed as a sport or a leisure activity. However, a common injury from fishing is embedment of the fishhook in the cutaneous tissue. Barbed fishhooks are used for their effectiveness in maintaining the fish on the hook once it is caught, but when implanted in the hand of a fisherman or fisherwoman, barbs can pose problems for removal without exacerbating internal tissue injury. Nevertheless, dermatologists should not shy away from removal of barbed fishhooks, as there are several simple methods that can be easily utilized in the outpatient setting.
Case Report
A 68-year-old man presented to an outpatient dermatology clinic after sustaining a barbed fishhook injury while fishing. The fishhook was firmly inserted into the ventral side of the third digit of the right hand (Figure 1).
Prior to presenting to dermatology, the patient went to 2 urgent care clinics the same day seeking treatment. He reported that practitioners at the first clinic were not able to remove the fishhook because they did not have pliers in stock. At the second clinic he was told the fishhook might be embedded in deeper tissues and was advised to go to the emergency department at the local hospital. When he arrived at the emergency department, a 6-hour wait time prompted him to see a local dermatologist instead.
To remove the fishhook, the area was cleaned and prepared first; lidocaine 2% was administered for local anesthesia. An 18-gauge needle was then advanced through the puncture site parallel to the fishhook’s inner shaft on the same side as the barb, which could be successfully palpated using the tip of the 18-gauge needle. The tip of the needle was then used to cap the barb beneath the skin. This technique allowed for the hook to be easily extracted in a retrograde manner without causing further destruction to the surrounding tissue. The patient then was started on prophylaxis cephalexin 500 mg 3 times daily for 3 days.
Comment
The hand is the most common site of fishhook injury, followed closely by the head and eyes.1 Barbless fishhooks usually can be removed by pushing the hook in a retrograde manner along the path of insertion. This method is simple and rarely results in complications. However, there are no guidelines for removal of barbed fishhooks. Furthermore, removing a barbed fishhook in the same retrograde manner would result in extensive internal tissue destruction and increased complications. Due to the popularity of the sport of fishing, fishhook injuries, depending on geographical location, are not uncommon.2 For this reason, trauma and emergency practitioners have become well versed in safe methods for barbed fishhook removal. However, patients are not always able or willing to seek medical care in emergency departments and may opt to seek treatment in outpatient settings, such as in our case. As a result, dermatologists should familiarize themselves with safe and effective fishhook removal methods, as they are not time consuming and do not require complex equipment. Failure to treat the patient may lead to further patient discomfort and increased risk for complications. Additionally, many of the techniques for removal may be useful with other foreign bodies embedded in cutaneous tissue (eg, splinters).
There are a number of safe and effective techniques for removing barbed fishhooks from cutaneous tissue, including the advance-and-cut method, the cut-it-out technique, the string-pull method, and the needle cover technique.1-3 The method chosen to remove the fishhook is dependent on a variety of factors, such as anatomic location, tissue depth, and provider comfort.
With the advance-and-cut method (Figure 2), the affected area is anesthetized and a small incision in the skin is created to expose the barb. The fishhook is then advanced through the incision, providing visibility of the barb and thus allowing the practitioner to cut the barbed tip without creating further damage to the surrounding tissue. The shaft of the fishhook can subsequently be removed in a retrograde fashion. The advantages of this technique include that it may be successfully used in all types of barbed fishhooks and it provides the practitioner with direct visibility of the barb, thus minimizing risk for neurovascular injury during removal.1 However, the primary disadvantage is that a second cutaneous wound is created in exposing the barb.
| 
| |
| Figure 2. The advance-and-cut method for fishhook removal. | Figure 3. The cut-it-out method for fishhook removal. |
|
The cut-it-out technique (Figure 3) is similar to the advance-and-cut method in that they both require anesthesia along with creating an incision. With this method, a scalpel is used to create a small linear incision originating at the fishhook entrance site and ending at the approximated location of the fishhook’s tip. The fishhook then is simply lifted superiorly in a retrograde fashion.
|
The string-pull method (Figure 4) has been credited to fishermen in South Australia and was first described by Cooke2 in 1961. This method is relatively painless, does not require anesthesia, and has a high success rate when properly administered. However, it does require rapid and confident motions (ie, without hesitation) by the practitioner and should not be performed on free-moving areas of the body (eg, earlobe).3 With this technique, a sturdy piece of suture (eg, 2/0 or 3/0 strength silk) is looped around the hook and is extended away from the practitioner at a 30° angle. The free end of the suture is then securely fastened around the index finger of the practitioner’s dominant hand. The index finger of the nondominant hand should apply a downward pressure to the hook shaft to disengage the barb from the tissue. Simultaneously and rather quickly and forcefully the practitioner must pull the dominant index finger with the string attached in a superior and lateral direction, as depicted by the long arrow in Figure 4. If successful, the barbed hook will pull out of the entrance site. The use of string in pulling the fishhook parallel to the site of injury is helpful for smaller fishhooks that may be difficult to grab with fingers alone. However, with larger fishhooks, the string may not be required so long as the practitioner is able to obtain a secure grasp on the fishhook shaft. The string-pull method becomes particularly useful when anesthesia is unavailable or when the barb of the hook is embedded too deeply for safe advancement through tissue to visualize and cut the barb.
|
Lastly, the needle cover technique (Figure 5) is another simple method that does not require the creation of a secondary wound. An 18-gauge needle is simply inserted parallel to the fishhook curvature into the site of entry. By using the needle to slide along the fishhook’s curve, the practitioner is able to follow its pathway while in the tissue. The tip of the 18-gauge needle is then used to cap or cover the barb, thus allowing the fishhook to be removed in a retrograde fashion from the wound. In an outpatient setting, this technique does not require the creation of additional tissue damage and practitioners who are inexperienced with fishhook removal may proceed through the motions more slowly and methodically than the string-pull method permits.
Wound care following fishhook removal should involve adequate flushing of the wound with normal saline along with the application of topical antibiotics and a simple dressing and adhesive bandage. Oral prophylactic antibiotics typically are not required for shallow cutaneous injuries unless the fishhook is dirty, the patient is immunocompromised, or the patient has a condition lending to poor wound healing (eg, diabetes mellitus, peripheral vascular disease).3 When deciding on antibiotics, it is important to note that fishhook injuries while saltwater fishing are associated with Vibrio infection, while injuries sustained during freshwater fishing are associated with gram-negative bacteria (eg, Pseudomonas and Aeromonas species).3 Lastly, it is essential to find out the immunization status of the patient, and tetanus immune globulin should be provided if necessary.
| 
| |
| Figure 4. The string-pull method for fishhook removal. | Figure 5. The needle cover technique for fishhook removal. |
Conclusion
Although guidelines for barbed fishhook removal are not available, outpatient physicians, including dermatologists, should not fear removal procedures. There are many safe and effective fishhook removal methods that are not time consuming and do not require complex equipment. Furthermore, familiarization with these same techniques may be useful for removal of other foreign bodies embedded in cutaneous tissue.
1. Khan HA, Kamal Y, Lone AU. Fish hook injury: removal by “push through and cut off” technique: a case report and brief literature review [published online March 24, 2014]. Trauma Mon. 2014;19:e17728.
2. Cooke T. How to remove fish-hooks with a bit of string. Med J Aust. 1961;48:815-816.
3. Thommasen HV, Thommasen A. The occasional removal of an embedded fish hook. Can J Rural Med. 2005;10:255-259.
Fishing is one of the world’s most beloved activities, enjoyed as a sport or a leisure activity. However, a common injury from fishing is embedment of the fishhook in the cutaneous tissue. Barbed fishhooks are used for their effectiveness in maintaining the fish on the hook once it is caught, but when implanted in the hand of a fisherman or fisherwoman, barbs can pose problems for removal without exacerbating internal tissue injury. Nevertheless, dermatologists should not shy away from removal of barbed fishhooks, as there are several simple methods that can be easily utilized in the outpatient setting.
Case Report
A 68-year-old man presented to an outpatient dermatology clinic after sustaining a barbed fishhook injury while fishing. The fishhook was firmly inserted into the ventral side of the third digit of the right hand (Figure 1).
Prior to presenting to dermatology, the patient went to 2 urgent care clinics the same day seeking treatment. He reported that practitioners at the first clinic were not able to remove the fishhook because they did not have pliers in stock. At the second clinic he was told the fishhook might be embedded in deeper tissues and was advised to go to the emergency department at the local hospital. When he arrived at the emergency department, a 6-hour wait time prompted him to see a local dermatologist instead.
To remove the fishhook, the area was cleaned and prepared first; lidocaine 2% was administered for local anesthesia. An 18-gauge needle was then advanced through the puncture site parallel to the fishhook’s inner shaft on the same side as the barb, which could be successfully palpated using the tip of the 18-gauge needle. The tip of the needle was then used to cap the barb beneath the skin. This technique allowed for the hook to be easily extracted in a retrograde manner without causing further destruction to the surrounding tissue. The patient then was started on prophylaxis cephalexin 500 mg 3 times daily for 3 days.
Comment
The hand is the most common site of fishhook injury, followed closely by the head and eyes.1 Barbless fishhooks usually can be removed by pushing the hook in a retrograde manner along the path of insertion. This method is simple and rarely results in complications. However, there are no guidelines for removal of barbed fishhooks. Furthermore, removing a barbed fishhook in the same retrograde manner would result in extensive internal tissue destruction and increased complications. Due to the popularity of the sport of fishing, fishhook injuries, depending on geographical location, are not uncommon.2 For this reason, trauma and emergency practitioners have become well versed in safe methods for barbed fishhook removal. However, patients are not always able or willing to seek medical care in emergency departments and may opt to seek treatment in outpatient settings, such as in our case. As a result, dermatologists should familiarize themselves with safe and effective fishhook removal methods, as they are not time consuming and do not require complex equipment. Failure to treat the patient may lead to further patient discomfort and increased risk for complications. Additionally, many of the techniques for removal may be useful with other foreign bodies embedded in cutaneous tissue (eg, splinters).
There are a number of safe and effective techniques for removing barbed fishhooks from cutaneous tissue, including the advance-and-cut method, the cut-it-out technique, the string-pull method, and the needle cover technique.1-3 The method chosen to remove the fishhook is dependent on a variety of factors, such as anatomic location, tissue depth, and provider comfort.
With the advance-and-cut method (Figure 2), the affected area is anesthetized and a small incision in the skin is created to expose the barb. The fishhook is then advanced through the incision, providing visibility of the barb and thus allowing the practitioner to cut the barbed tip without creating further damage to the surrounding tissue. The shaft of the fishhook can subsequently be removed in a retrograde fashion. The advantages of this technique include that it may be successfully used in all types of barbed fishhooks and it provides the practitioner with direct visibility of the barb, thus minimizing risk for neurovascular injury during removal.1 However, the primary disadvantage is that a second cutaneous wound is created in exposing the barb.

| 
| |
| Figure 2. The advance-and-cut method for fishhook removal. | Figure 3. The cut-it-out method for fishhook removal. |
|
The cut-it-out technique (Figure 3) is similar to the advance-and-cut method in that they both require anesthesia along with creating an incision. With this method, a scalpel is used to create a small linear incision originating at the fishhook entrance site and ending at the approximated location of the fishhook’s tip. The fishhook then is simply lifted superiorly in a retrograde fashion.
|
The string-pull method (Figure 4) has been credited to fishermen in South Australia and was first described by Cooke2 in 1961. This method is relatively painless, does not require anesthesia, and has a high success rate when properly administered. However, it does require rapid and confident motions (ie, without hesitation) by the practitioner and should not be performed on free-moving areas of the body (eg, earlobe).3 With this technique, a sturdy piece of suture (eg, 2/0 or 3/0 strength silk) is looped around the hook and is extended away from the practitioner at a 30° angle. The free end of the suture is then securely fastened around the index finger of the practitioner’s dominant hand. The index finger of the nondominant hand should apply a downward pressure to the hook shaft to disengage the barb from the tissue. Simultaneously and rather quickly and forcefully the practitioner must pull the dominant index finger with the string attached in a superior and lateral direction, as depicted by the long arrow in Figure 4. If successful, the barbed hook will pull out of the entrance site. The use of string in pulling the fishhook parallel to the site of injury is helpful for smaller fishhooks that may be difficult to grab with fingers alone. However, with larger fishhooks, the string may not be required so long as the practitioner is able to obtain a secure grasp on the fishhook shaft. The string-pull method becomes particularly useful when anesthesia is unavailable or when the barb of the hook is embedded too deeply for safe advancement through tissue to visualize and cut the barb.
|
Lastly, the needle cover technique (Figure 5) is another simple method that does not require the creation of a secondary wound. An 18-gauge needle is simply inserted parallel to the fishhook curvature into the site of entry. By using the needle to slide along the fishhook’s curve, the practitioner is able to follow its pathway while in the tissue. The tip of the 18-gauge needle is then used to cap or cover the barb, thus allowing the fishhook to be removed in a retrograde fashion from the wound. In an outpatient setting, this technique does not require the creation of additional tissue damage and practitioners who are inexperienced with fishhook removal may proceed through the motions more slowly and methodically than the string-pull method permits.
Wound care following fishhook removal should involve adequate flushing of the wound with normal saline along with the application of topical antibiotics and a simple dressing and adhesive bandage. Oral prophylactic antibiotics typically are not required for shallow cutaneous injuries unless the fishhook is dirty, the patient is immunocompromised, or the patient has a condition lending to poor wound healing (eg, diabetes mellitus, peripheral vascular disease).3 When deciding on antibiotics, it is important to note that fishhook injuries while saltwater fishing are associated with Vibrio infection, while injuries sustained during freshwater fishing are associated with gram-negative bacteria (eg, Pseudomonas and Aeromonas species).3 Lastly, it is essential to find out the immunization status of the patient, and tetanus immune globulin should be provided if necessary.

| 
| |
| Figure 4. The string-pull method for fishhook removal. | Figure 5. The needle cover technique for fishhook removal. |
Conclusion
Although guidelines for barbed fishhook removal are not available, outpatient physicians, including dermatologists, should not fear removal procedures. There are many safe and effective fishhook removal methods that are not time consuming and do not require complex equipment. Furthermore, familiarization with these same techniques may be useful for removal of other foreign bodies embedded in cutaneous tissue.
Fishing is one of the world’s most beloved activities, enjoyed as a sport or a leisure activity. However, a common injury from fishing is embedment of the fishhook in the cutaneous tissue. Barbed fishhooks are used for their effectiveness in maintaining the fish on the hook once it is caught, but when implanted in the hand of a fisherman or fisherwoman, barbs can pose problems for removal without exacerbating internal tissue injury. Nevertheless, dermatologists should not shy away from removal of barbed fishhooks, as there are several simple methods that can be easily utilized in the outpatient setting.
Case Report
A 68-year-old man presented to an outpatient dermatology clinic after sustaining a barbed fishhook injury while fishing. The fishhook was firmly inserted into the ventral side of the third digit of the right hand (Figure 1).
Prior to presenting to dermatology, the patient went to 2 urgent care clinics the same day seeking treatment. He reported that practitioners at the first clinic were not able to remove the fishhook because they did not have pliers in stock. At the second clinic he was told the fishhook might be embedded in deeper tissues and was advised to go to the emergency department at the local hospital. When he arrived at the emergency department, a 6-hour wait time prompted him to see a local dermatologist instead.
To remove the fishhook, the area was cleaned and prepared first; lidocaine 2% was administered for local anesthesia. An 18-gauge needle was then advanced through the puncture site parallel to the fishhook’s inner shaft on the same side as the barb, which could be successfully palpated using the tip of the 18-gauge needle. The tip of the needle was then used to cap the barb beneath the skin. This technique allowed for the hook to be easily extracted in a retrograde manner without causing further destruction to the surrounding tissue. The patient then was started on prophylaxis cephalexin 500 mg 3 times daily for 3 days.
Comment
The hand is the most common site of fishhook injury, followed closely by the head and eyes.1 Barbless fishhooks usually can be removed by pushing the hook in a retrograde manner along the path of insertion. This method is simple and rarely results in complications. However, there are no guidelines for removal of barbed fishhooks. Furthermore, removing a barbed fishhook in the same retrograde manner would result in extensive internal tissue destruction and increased complications. Due to the popularity of the sport of fishing, fishhook injuries, depending on geographical location, are not uncommon.2 For this reason, trauma and emergency practitioners have become well versed in safe methods for barbed fishhook removal. However, patients are not always able or willing to seek medical care in emergency departments and may opt to seek treatment in outpatient settings, such as in our case. As a result, dermatologists should familiarize themselves with safe and effective fishhook removal methods, as they are not time consuming and do not require complex equipment. Failure to treat the patient may lead to further patient discomfort and increased risk for complications. Additionally, many of the techniques for removal may be useful with other foreign bodies embedded in cutaneous tissue (eg, splinters).
There are a number of safe and effective techniques for removing barbed fishhooks from cutaneous tissue, including the advance-and-cut method, the cut-it-out technique, the string-pull method, and the needle cover technique.1-3 The method chosen to remove the fishhook is dependent on a variety of factors, such as anatomic location, tissue depth, and provider comfort.
With the advance-and-cut method (Figure 2), the affected area is anesthetized and a small incision in the skin is created to expose the barb. The fishhook is then advanced through the incision, providing visibility of the barb and thus allowing the practitioner to cut the barbed tip without creating further damage to the surrounding tissue. The shaft of the fishhook can subsequently be removed in a retrograde fashion. The advantages of this technique include that it may be successfully used in all types of barbed fishhooks and it provides the practitioner with direct visibility of the barb, thus minimizing risk for neurovascular injury during removal.1 However, the primary disadvantage is that a second cutaneous wound is created in exposing the barb.

| 
| |
| Figure 2. The advance-and-cut method for fishhook removal. | Figure 3. The cut-it-out method for fishhook removal. |
|
The cut-it-out technique (Figure 3) is similar to the advance-and-cut method in that they both require anesthesia along with creating an incision. With this method, a scalpel is used to create a small linear incision originating at the fishhook entrance site and ending at the approximated location of the fishhook’s tip. The fishhook then is simply lifted superiorly in a retrograde fashion.
|
The string-pull method (Figure 4) has been credited to fishermen in South Australia and was first described by Cooke2 in 1961. This method is relatively painless, does not require anesthesia, and has a high success rate when properly administered. However, it does require rapid and confident motions (ie, without hesitation) by the practitioner and should not be performed on free-moving areas of the body (eg, earlobe).3 With this technique, a sturdy piece of suture (eg, 2/0 or 3/0 strength silk) is looped around the hook and is extended away from the practitioner at a 30° angle. The free end of the suture is then securely fastened around the index finger of the practitioner’s dominant hand. The index finger of the nondominant hand should apply a downward pressure to the hook shaft to disengage the barb from the tissue. Simultaneously and rather quickly and forcefully the practitioner must pull the dominant index finger with the string attached in a superior and lateral direction, as depicted by the long arrow in Figure 4. If successful, the barbed hook will pull out of the entrance site. The use of string in pulling the fishhook parallel to the site of injury is helpful for smaller fishhooks that may be difficult to grab with fingers alone. However, with larger fishhooks, the string may not be required so long as the practitioner is able to obtain a secure grasp on the fishhook shaft. The string-pull method becomes particularly useful when anesthesia is unavailable or when the barb of the hook is embedded too deeply for safe advancement through tissue to visualize and cut the barb.
|
Lastly, the needle cover technique (Figure 5) is another simple method that does not require the creation of a secondary wound. An 18-gauge needle is simply inserted parallel to the fishhook curvature into the site of entry. By using the needle to slide along the fishhook’s curve, the practitioner is able to follow its pathway while in the tissue. The tip of the 18-gauge needle is then used to cap or cover the barb, thus allowing the fishhook to be removed in a retrograde fashion from the wound. In an outpatient setting, this technique does not require the creation of additional tissue damage and practitioners who are inexperienced with fishhook removal may proceed through the motions more slowly and methodically than the string-pull method permits.
Wound care following fishhook removal should involve adequate flushing of the wound with normal saline along with the application of topical antibiotics and a simple dressing and adhesive bandage. Oral prophylactic antibiotics typically are not required for shallow cutaneous injuries unless the fishhook is dirty, the patient is immunocompromised, or the patient has a condition lending to poor wound healing (eg, diabetes mellitus, peripheral vascular disease).3 When deciding on antibiotics, it is important to note that fishhook injuries while saltwater fishing are associated with Vibrio infection, while injuries sustained during freshwater fishing are associated with gram-negative bacteria (eg, Pseudomonas and Aeromonas species).3 Lastly, it is essential to find out the immunization status of the patient, and tetanus immune globulin should be provided if necessary.

| 
| |
| Figure 4. The string-pull method for fishhook removal. | Figure 5. The needle cover technique for fishhook removal. |
Conclusion
Although guidelines for barbed fishhook removal are not available, outpatient physicians, including dermatologists, should not fear removal procedures. There are many safe and effective fishhook removal methods that are not time consuming and do not require complex equipment. Furthermore, familiarization with these same techniques may be useful for removal of other foreign bodies embedded in cutaneous tissue.
1. Khan HA, Kamal Y, Lone AU. Fish hook injury: removal by “push through and cut off” technique: a case report and brief literature review [published online March 24, 2014]. Trauma Mon. 2014;19:e17728.
2. Cooke T. How to remove fish-hooks with a bit of string. Med J Aust. 1961;48:815-816.
3. Thommasen HV, Thommasen A. The occasional removal of an embedded fish hook. Can J Rural Med. 2005;10:255-259.
1. Khan HA, Kamal Y, Lone AU. Fish hook injury: removal by “push through and cut off” technique: a case report and brief literature review [published online March 24, 2014]. Trauma Mon. 2014;19:e17728.
2. Cooke T. How to remove fish-hooks with a bit of string. Med J Aust. 1961;48:815-816.
3. Thommasen HV, Thommasen A. The occasional removal of an embedded fish hook. Can J Rural Med. 2005;10:255-259.
Practice Points
- Barbed fishhooks should never be removed by pushing the hook in a retrograde manner along the path of insertion, as this method may result in extensive internal tissue destruction and increased complications.
- There are a number of safe and effective techniques for removing barbed fishhooks from cutaneous tissue that also may be applicable in removing other foreign bodies embedded in cutaneous tissue (eg, splinters).
Diagnosing Porokeratosis of Mibelli Every Time: A Novel Biopsy Technique to Maximize Histopathologic Confirmation
Porokeratosis of Mibelli (PM) is a lesion characterized by a surrounding cornoid lamella with variable nonspecific findings (eg, atrophy, acanthosis, verrucous hyperplasia) in the center of the lesion that typically presents in infancy to early childhood.1 We report a case of PM in which a prior biopsy from the center of the lesion demonstrated papulosquamous dermatitis. We propose a 3-step technique to ensure proper orientation of a punch biopsy in cases of suspected PM.
Case Report
A 3-year-old girl presented with an erythematous, hypopigmented, scaling plaque on the posterior aspect of the left ankle surrounded by a hard rim. The plaque was first noted at 12 months of age and had slowly enlarged as the patient grew. Six months prior, a biopsy from the center of the lesion performed at another facility demonstrated a papulosquamous dermatitis.
Physical examination revealed a lesion that was 4.2-cm long, 2.2-cm wide at the superior pole, and 3.5-cm wide at the inferior pole (Figure 1). A line was drawn with a skin marker perpendicular to the rim of the lesion (Figure 2A) and a 6-mm punch biopsy was performed, centered at the intersection of the drawn line and the cornoid lamella (Figure 2B). The tissue was then bisected at the bedside along the skin marker line with a #15 blade (Figure 2C) and submitted in formalin for histologic processing. Histologic examination revealed an invagination of the epidermis producing a tier of parakeratotic cells with its apex pointed away from the center of the lesion. Dyskeratotic cells were noted at the base of the parakeratosis (Figure 3). Verrucous hyperplasia was present in the central portion of the specimen adjacent to the cornoid lamella. Based on these histopathologic findings, the correct diagnosis of PM was made.
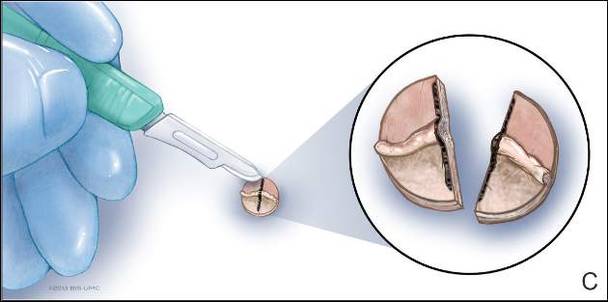
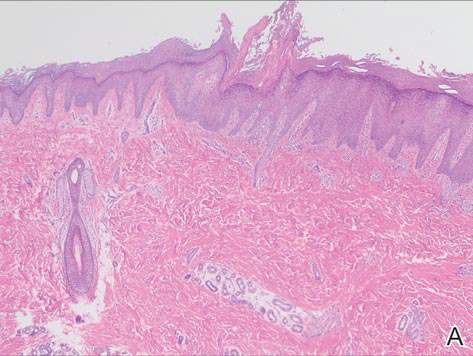
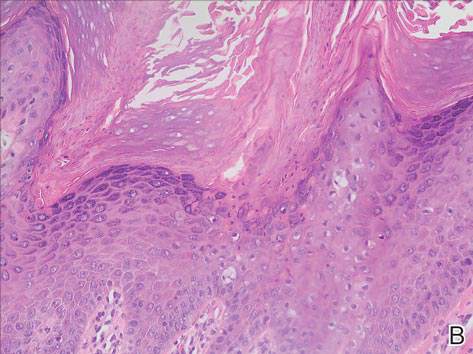
Comment
Porokeratosis of Mibelli is a rare condition that typically presents in infancy to early childhood.1 It may appear as small keratotic papules or larger plaques that reach several centimeters in diameter.2 There is a 7.5% risk for malignant transformation (eg, basal cell carcinoma, squamous cell carcinoma, Bowen disease).3 Variable nonspecific findings (eg, atrophy, acanthosis, verrucous hyperplasia) typically are present in the center of the lesion. In our case, a biopsy from the center of the plaque demonstrated verrucous hyperplasia. The incorrect diagnosis of PM as psoriasis also has been reported.4
We propose a 3-step technique to ensure proper orientation of a punch biopsy in cases of suspected PM. First, draw a line perpendicular to the rim of the lesion to mark the biopsy site (Figure 2A). Second, perform a punch biopsy centered at the intersection of the drawn line and the cornoid lamella (Figure 2B). Third, section the biopsied tissue with a #15 blade along the perpendicular line at the bedside (Figure 2C). The surgical pathology requisition should mention that the specimen has been transected and the cut edges should be placed down in the cassette, ensuring that the cornoid lamella will be present in cross-section on the slides.
If the punch biopsy specimen is not bisected, it can be difficult to orient it in the pathology laboratory, especially if the cornoid lamellae are not prominent. Furthermore, the technician processing the tissue may not be aware of the importance of sectioning the specimen perpendicular to the cornoid lamella. Following this procedure, diagnosis can be confirmed in virtually every case of PM.
- Richard G, Irvine A, Traupe H, et al. Ichthyosis and disorders of other conification. In: Schachner L, Hansen R, Krafchik B, et al, eds. Pediatric Dermatology. Philadelphia, PA: Elsevier Health Sciences; 2011:640-643.
- Pierson D, Bandel C, Ehrig, et al. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo J, Rapini R, et al, eds. Dermatology. 1st ed. Vol 2. Edinburgh, Scotland: Elsevier; 2003:1707-1709.
- Cort DF, Abdel-Aziz AH. Epithelioma arising in porokeratosis of Mibelli. Br J Plast Surg. 1972;25:318-328.
- De Simone C, Paradisi A, Massi G, et al. Giant verrucous porokeratosis of Mibelli mimicking psoriasis in a patient with psoriasis. J Am Acad Dermatol. 2007;57:665-668.
Porokeratosis of Mibelli (PM) is a lesion characterized by a surrounding cornoid lamella with variable nonspecific findings (eg, atrophy, acanthosis, verrucous hyperplasia) in the center of the lesion that typically presents in infancy to early childhood.1 We report a case of PM in which a prior biopsy from the center of the lesion demonstrated papulosquamous dermatitis. We propose a 3-step technique to ensure proper orientation of a punch biopsy in cases of suspected PM.
Case Report
A 3-year-old girl presented with an erythematous, hypopigmented, scaling plaque on the posterior aspect of the left ankle surrounded by a hard rim. The plaque was first noted at 12 months of age and had slowly enlarged as the patient grew. Six months prior, a biopsy from the center of the lesion performed at another facility demonstrated a papulosquamous dermatitis.
Physical examination revealed a lesion that was 4.2-cm long, 2.2-cm wide at the superior pole, and 3.5-cm wide at the inferior pole (Figure 1). A line was drawn with a skin marker perpendicular to the rim of the lesion (Figure 2A) and a 6-mm punch biopsy was performed, centered at the intersection of the drawn line and the cornoid lamella (Figure 2B). The tissue was then bisected at the bedside along the skin marker line with a #15 blade (Figure 2C) and submitted in formalin for histologic processing. Histologic examination revealed an invagination of the epidermis producing a tier of parakeratotic cells with its apex pointed away from the center of the lesion. Dyskeratotic cells were noted at the base of the parakeratosis (Figure 3). Verrucous hyperplasia was present in the central portion of the specimen adjacent to the cornoid lamella. Based on these histopathologic findings, the correct diagnosis of PM was made.






Comment
Porokeratosis of Mibelli is a rare condition that typically presents in infancy to early childhood.1 It may appear as small keratotic papules or larger plaques that reach several centimeters in diameter.2 There is a 7.5% risk for malignant transformation (eg, basal cell carcinoma, squamous cell carcinoma, Bowen disease).3 Variable nonspecific findings (eg, atrophy, acanthosis, verrucous hyperplasia) typically are present in the center of the lesion. In our case, a biopsy from the center of the plaque demonstrated verrucous hyperplasia. The incorrect diagnosis of PM as psoriasis also has been reported.4
We propose a 3-step technique to ensure proper orientation of a punch biopsy in cases of suspected PM. First, draw a line perpendicular to the rim of the lesion to mark the biopsy site (Figure 2A). Second, perform a punch biopsy centered at the intersection of the drawn line and the cornoid lamella (Figure 2B). Third, section the biopsied tissue with a #15 blade along the perpendicular line at the bedside (Figure 2C). The surgical pathology requisition should mention that the specimen has been transected and the cut edges should be placed down in the cassette, ensuring that the cornoid lamella will be present in cross-section on the slides.
If the punch biopsy specimen is not bisected, it can be difficult to orient it in the pathology laboratory, especially if the cornoid lamellae are not prominent. Furthermore, the technician processing the tissue may not be aware of the importance of sectioning the specimen perpendicular to the cornoid lamella. Following this procedure, diagnosis can be confirmed in virtually every case of PM.
Porokeratosis of Mibelli (PM) is a lesion characterized by a surrounding cornoid lamella with variable nonspecific findings (eg, atrophy, acanthosis, verrucous hyperplasia) in the center of the lesion that typically presents in infancy to early childhood.1 We report a case of PM in which a prior biopsy from the center of the lesion demonstrated papulosquamous dermatitis. We propose a 3-step technique to ensure proper orientation of a punch biopsy in cases of suspected PM.
Case Report
A 3-year-old girl presented with an erythematous, hypopigmented, scaling plaque on the posterior aspect of the left ankle surrounded by a hard rim. The plaque was first noted at 12 months of age and had slowly enlarged as the patient grew. Six months prior, a biopsy from the center of the lesion performed at another facility demonstrated a papulosquamous dermatitis.
Physical examination revealed a lesion that was 4.2-cm long, 2.2-cm wide at the superior pole, and 3.5-cm wide at the inferior pole (Figure 1). A line was drawn with a skin marker perpendicular to the rim of the lesion (Figure 2A) and a 6-mm punch biopsy was performed, centered at the intersection of the drawn line and the cornoid lamella (Figure 2B). The tissue was then bisected at the bedside along the skin marker line with a #15 blade (Figure 2C) and submitted in formalin for histologic processing. Histologic examination revealed an invagination of the epidermis producing a tier of parakeratotic cells with its apex pointed away from the center of the lesion. Dyskeratotic cells were noted at the base of the parakeratosis (Figure 3). Verrucous hyperplasia was present in the central portion of the specimen adjacent to the cornoid lamella. Based on these histopathologic findings, the correct diagnosis of PM was made.






Comment
Porokeratosis of Mibelli is a rare condition that typically presents in infancy to early childhood.1 It may appear as small keratotic papules or larger plaques that reach several centimeters in diameter.2 There is a 7.5% risk for malignant transformation (eg, basal cell carcinoma, squamous cell carcinoma, Bowen disease).3 Variable nonspecific findings (eg, atrophy, acanthosis, verrucous hyperplasia) typically are present in the center of the lesion. In our case, a biopsy from the center of the plaque demonstrated verrucous hyperplasia. The incorrect diagnosis of PM as psoriasis also has been reported.4
We propose a 3-step technique to ensure proper orientation of a punch biopsy in cases of suspected PM. First, draw a line perpendicular to the rim of the lesion to mark the biopsy site (Figure 2A). Second, perform a punch biopsy centered at the intersection of the drawn line and the cornoid lamella (Figure 2B). Third, section the biopsied tissue with a #15 blade along the perpendicular line at the bedside (Figure 2C). The surgical pathology requisition should mention that the specimen has been transected and the cut edges should be placed down in the cassette, ensuring that the cornoid lamella will be present in cross-section on the slides.
If the punch biopsy specimen is not bisected, it can be difficult to orient it in the pathology laboratory, especially if the cornoid lamellae are not prominent. Furthermore, the technician processing the tissue may not be aware of the importance of sectioning the specimen perpendicular to the cornoid lamella. Following this procedure, diagnosis can be confirmed in virtually every case of PM.
- Richard G, Irvine A, Traupe H, et al. Ichthyosis and disorders of other conification. In: Schachner L, Hansen R, Krafchik B, et al, eds. Pediatric Dermatology. Philadelphia, PA: Elsevier Health Sciences; 2011:640-643.
- Pierson D, Bandel C, Ehrig, et al. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo J, Rapini R, et al, eds. Dermatology. 1st ed. Vol 2. Edinburgh, Scotland: Elsevier; 2003:1707-1709.
- Cort DF, Abdel-Aziz AH. Epithelioma arising in porokeratosis of Mibelli. Br J Plast Surg. 1972;25:318-328.
- De Simone C, Paradisi A, Massi G, et al. Giant verrucous porokeratosis of Mibelli mimicking psoriasis in a patient with psoriasis. J Am Acad Dermatol. 2007;57:665-668.
- Richard G, Irvine A, Traupe H, et al. Ichthyosis and disorders of other conification. In: Schachner L, Hansen R, Krafchik B, et al, eds. Pediatric Dermatology. Philadelphia, PA: Elsevier Health Sciences; 2011:640-643.
- Pierson D, Bandel C, Ehrig, et al. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo J, Rapini R, et al, eds. Dermatology. 1st ed. Vol 2. Edinburgh, Scotland: Elsevier; 2003:1707-1709.
- Cort DF, Abdel-Aziz AH. Epithelioma arising in porokeratosis of Mibelli. Br J Plast Surg. 1972;25:318-328.
- De Simone C, Paradisi A, Massi G, et al. Giant verrucous porokeratosis of Mibelli mimicking psoriasis in a patient with psoriasis. J Am Acad Dermatol. 2007;57:665-668.
Practice Points
- A biopsy from the center of a plaque of porokeratosis will produce nonspecific findings.
- Bisecting the punch specimen at the bedside along a line drawn perpendicular to the cornoid lamella guarantees proper orientation of the specimen.
HM16 Session Analysis: Lead Your Way to Success: Five Key Lessons for Hospitalists
Physicians Nasim Afsar, MD, SFHM, and Eric Howell, MD, SFHM, presented key leadership lessons to a standing-room-only audience at Hospital Medicine 2016, the “Year of the Hospitalist.” The value of leadership and management skills is important in every day decisions from co-management of patients to motivating your teams.
Dr. Afsar and Dr. Howell went into detailed tips for these leadership lessons:
- Decision-making bias. It is important to be aware of bias in decisions. A technique to evaluate a decision and “de-bias” is the WRAP process: Widen your options, Reality-test your assumptions, Attain distance before deciding, and Prepare to be wrong.
- Performance management. Feedback and 360 evaluations are helpful tools in appraising performance.
- Motivation can be intrinsic or extrinsic. Intrinsic motivation is essential for non-routine high level work in medicine. Understanding the motivation of a team member is very useful to the team leader.
- Groups versus teams. The composition of a team is crucial to success. It is also important to be aware of team limitations and plan for these potential limitations.
- Persuasion and influence. Six principles of persuasion are:
- Demonstrate trustworthiness and expertise.
- Social proof. Highlight existing norms or set new norms.
- Highlight similarities.
- A win-win situation with concessions shows willingness to participate.
- Reach agreement.
- An option that appears to be a rare offer is more desirable.
Key Takeaways
- Consistently using a standard decision-making process, such as WRAP, can ensure better decision making.
- Financial compensation can be detrimental to intrinsic motivation and worsen performance.
- Make a conscious decision about when you need a group to help make decisions versus a team to work towards a common goal.
- Set specific goals for performance during feedback: include timeline, particular actions, and results that are expected.
- Social proof can be a powerful tool in persuasion.
- The SHM Leadership Academy is available to hospitalists interested in expanding leadership skills. TH
Dr. Hale is a pediatric hospitalist at Floating Hospital for Children at Tufts University Medical Center in Boston, and a former member of Team Hospitalist.
Physicians Nasim Afsar, MD, SFHM, and Eric Howell, MD, SFHM, presented key leadership lessons to a standing-room-only audience at Hospital Medicine 2016, the “Year of the Hospitalist.” The value of leadership and management skills is important in every day decisions from co-management of patients to motivating your teams.
Dr. Afsar and Dr. Howell went into detailed tips for these leadership lessons:
- Decision-making bias. It is important to be aware of bias in decisions. A technique to evaluate a decision and “de-bias” is the WRAP process: Widen your options, Reality-test your assumptions, Attain distance before deciding, and Prepare to be wrong.
- Performance management. Feedback and 360 evaluations are helpful tools in appraising performance.
- Motivation can be intrinsic or extrinsic. Intrinsic motivation is essential for non-routine high level work in medicine. Understanding the motivation of a team member is very useful to the team leader.
- Groups versus teams. The composition of a team is crucial to success. It is also important to be aware of team limitations and plan for these potential limitations.
- Persuasion and influence. Six principles of persuasion are:
- Demonstrate trustworthiness and expertise.
- Social proof. Highlight existing norms or set new norms.
- Highlight similarities.
- A win-win situation with concessions shows willingness to participate.
- Reach agreement.
- An option that appears to be a rare offer is more desirable.
Key Takeaways
- Consistently using a standard decision-making process, such as WRAP, can ensure better decision making.
- Financial compensation can be detrimental to intrinsic motivation and worsen performance.
- Make a conscious decision about when you need a group to help make decisions versus a team to work towards a common goal.
- Set specific goals for performance during feedback: include timeline, particular actions, and results that are expected.
- Social proof can be a powerful tool in persuasion.
- The SHM Leadership Academy is available to hospitalists interested in expanding leadership skills. TH
Dr. Hale is a pediatric hospitalist at Floating Hospital for Children at Tufts University Medical Center in Boston, and a former member of Team Hospitalist.
Physicians Nasim Afsar, MD, SFHM, and Eric Howell, MD, SFHM, presented key leadership lessons to a standing-room-only audience at Hospital Medicine 2016, the “Year of the Hospitalist.” The value of leadership and management skills is important in every day decisions from co-management of patients to motivating your teams.
Dr. Afsar and Dr. Howell went into detailed tips for these leadership lessons:
- Decision-making bias. It is important to be aware of bias in decisions. A technique to evaluate a decision and “de-bias” is the WRAP process: Widen your options, Reality-test your assumptions, Attain distance before deciding, and Prepare to be wrong.
- Performance management. Feedback and 360 evaluations are helpful tools in appraising performance.
- Motivation can be intrinsic or extrinsic. Intrinsic motivation is essential for non-routine high level work in medicine. Understanding the motivation of a team member is very useful to the team leader.
- Groups versus teams. The composition of a team is crucial to success. It is also important to be aware of team limitations and plan for these potential limitations.
- Persuasion and influence. Six principles of persuasion are:
- Demonstrate trustworthiness and expertise.
- Social proof. Highlight existing norms or set new norms.
- Highlight similarities.
- A win-win situation with concessions shows willingness to participate.
- Reach agreement.
- An option that appears to be a rare offer is more desirable.
Key Takeaways
- Consistently using a standard decision-making process, such as WRAP, can ensure better decision making.
- Financial compensation can be detrimental to intrinsic motivation and worsen performance.
- Make a conscious decision about when you need a group to help make decisions versus a team to work towards a common goal.
- Set specific goals for performance during feedback: include timeline, particular actions, and results that are expected.
- Social proof can be a powerful tool in persuasion.
- The SHM Leadership Academy is available to hospitalists interested in expanding leadership skills. TH
Dr. Hale is a pediatric hospitalist at Floating Hospital for Children at Tufts University Medical Center in Boston, and a former member of Team Hospitalist.
U.S. Surgeon General Vivek Murthy, MD, MBA, Calls for Renewed Commitment to Public Health
Dr. Vivek Murthy delivered an excellent opening address to Hospital Medicine 2016, the “Year of the Hospitalist.” He presented a key message that hospitalists can be major supporters of public health and disease prevention. He described the clean water crisis in Flint, Michigan as a tragedy that should not be occurring in the United States in the year 2016.
We need to renew our commitment to a strong foundation of public health. “Health is the key to opportunity,” Dr. Murthy stated. He reviewed four pillars for the foundation of good public health:
- Make healthy choices a desired choice. We should try to establish exercise and good eating as a part of a normal lifestyle, not something onerous or difficult. Healthy choices can be a source of pleasure.
- Change the environment to make healthy changes sustainable. The environment includes advertising and marketing of good choices, access to healthy foods, and access to increased activity. An example was local government commitments to increased walkable routes and parks will increase activity in a population.
- Focus on the mind and spirit, not just the body.
- Cultivate the ability to give and receive kindness.
Dr. Murthy left the hospitalist with three take-home questions:
- Can a hospitalist leverage leadership to create a culture of healing?
- Can a hospitalist be a force for change outside the hospital setting? Can you assist with nutrition wellness or safety projects outside of the hospital?
- Can we inspire the next generation of physicians to work on public health and preventing illness?
Key Takeaways
- Hospitalists can be major supporters of public health and disease prevention; and
- The foundation of good public health includes the changes to make healthy choices a desired choice, change the environment to make healthy changes sustainable, focus on the mind and spirit, and cultivate the ability to give and receive kindness.
Dr. Vivek Murthy delivered an excellent opening address to Hospital Medicine 2016, the “Year of the Hospitalist.” He presented a key message that hospitalists can be major supporters of public health and disease prevention. He described the clean water crisis in Flint, Michigan as a tragedy that should not be occurring in the United States in the year 2016.
We need to renew our commitment to a strong foundation of public health. “Health is the key to opportunity,” Dr. Murthy stated. He reviewed four pillars for the foundation of good public health:
- Make healthy choices a desired choice. We should try to establish exercise and good eating as a part of a normal lifestyle, not something onerous or difficult. Healthy choices can be a source of pleasure.
- Change the environment to make healthy changes sustainable. The environment includes advertising and marketing of good choices, access to healthy foods, and access to increased activity. An example was local government commitments to increased walkable routes and parks will increase activity in a population.
- Focus on the mind and spirit, not just the body.
- Cultivate the ability to give and receive kindness.
Dr. Murthy left the hospitalist with three take-home questions:
- Can a hospitalist leverage leadership to create a culture of healing?
- Can a hospitalist be a force for change outside the hospital setting? Can you assist with nutrition wellness or safety projects outside of the hospital?
- Can we inspire the next generation of physicians to work on public health and preventing illness?
Key Takeaways
- Hospitalists can be major supporters of public health and disease prevention; and
- The foundation of good public health includes the changes to make healthy choices a desired choice, change the environment to make healthy changes sustainable, focus on the mind and spirit, and cultivate the ability to give and receive kindness.
Dr. Vivek Murthy delivered an excellent opening address to Hospital Medicine 2016, the “Year of the Hospitalist.” He presented a key message that hospitalists can be major supporters of public health and disease prevention. He described the clean water crisis in Flint, Michigan as a tragedy that should not be occurring in the United States in the year 2016.
We need to renew our commitment to a strong foundation of public health. “Health is the key to opportunity,” Dr. Murthy stated. He reviewed four pillars for the foundation of good public health:
- Make healthy choices a desired choice. We should try to establish exercise and good eating as a part of a normal lifestyle, not something onerous or difficult. Healthy choices can be a source of pleasure.
- Change the environment to make healthy changes sustainable. The environment includes advertising and marketing of good choices, access to healthy foods, and access to increased activity. An example was local government commitments to increased walkable routes and parks will increase activity in a population.
- Focus on the mind and spirit, not just the body.
- Cultivate the ability to give and receive kindness.
Dr. Murthy left the hospitalist with three take-home questions:
- Can a hospitalist leverage leadership to create a culture of healing?
- Can a hospitalist be a force for change outside the hospital setting? Can you assist with nutrition wellness or safety projects outside of the hospital?
- Can we inspire the next generation of physicians to work on public health and preventing illness?
Key Takeaways
- Hospitalists can be major supporters of public health and disease prevention; and
- The foundation of good public health includes the changes to make healthy choices a desired choice, change the environment to make healthy changes sustainable, focus on the mind and spirit, and cultivate the ability to give and receive kindness.