User login
Slow and Steady May Not Win the Race for Weight Loss Maintenance
Study Overview
Objective. To compare weight regain after rapid versus slower loss of an equivalent amount of weight.
Study design. Randomized clinical trial.
Setting and participants. This study took place in a single medical center in the Netherlands. Investigators recruited 61 adults (no age range provided) with body mass index (BMI) between 28–35 kg/m2 and at a stable weight (no change of > 3 kg for the past 2 months) to participate in a weight loss study. Individuals with type 2 diabetes, dyslipidemia, uncontrolled hypertension, or liver, heart or kidney disease were excluded, as were those who were currently pregnant or reported consuming more than moderate amounts of alcohol.
Once consented, participants were randomized into one of 2 study arms. The rapid weight loss arm was prescribed a very-low-calorie diet (VLCD) with just 500 kcal/day (43% protein/43% carb/14% fat) for 5 weeks, after which they transitioned to a 4-week “weight stable” period, and then a 9-month follow-up period (overall follow-up time of ~11 months; 10 months after weight loss). In contrast, the slower weight loss arm was prescribed a low-calorie diet (LCD) with 1250 kcal/day (29% protein/48% carb/23% fat) for 12 weeks, after which they also transitioned to a 4-week weight stable period and 9 months of follow-up (overall follow-up time of ~13 months; 10 months after weight loss). VLCD (rapid weight loss) participants received 3 meal replacement shakes per day (totaling 500 kcal) during the weight loss period and were also told they could consume unlimited amounts of low-calorie vegetables. The LCD (slower weight loss) participants received 1 meal replacement shake per day during their 12 weeks of weight loss and were responsible for providing the remainder of their own meals and snacks according to guidelines from a study dietitian. Following active weight loss, both groups then shifted to higher-calorie, food-based diets during a “weight stable” 4-week period and were responsible during this time for providing all of their own food. The researchers do not specify the details of the diet composition for this weight stable period. Exposure to the registered dietitian was the same in both groups, with 5 consultations during weight loss (weekly for VLCD, presumably more spaced out for LCD) and 4 during weight stable period. No further diet advice or meal replacement support was given during the 9-month follow-up period, but participants came in for monthly weigh-ins.
Main outcome measure. The primary outcome measure was change in weight (ie, amount of weight regained) during the 9-month follow-up period, compared between groups using an independent samples t test. Additional biometric measures included change in waist circumference and changes in body composition. For the latter, the researchers used a “Bod Pod” to conduct air-displacement plethysmography and determine what percentage of an individual’s weight was fat mass (FM) versus lean mass/water (FFM [fat-free mass]). They then compared the amount of FFM lost between groups, again using the independent samples t test.
The researchers also collected information on self-reported physical activity (questionnaire) and self-reported history of weight cycling (number of times a participant had previously lost and regained at least 5 kg) prior to this study. These were not outcomes per-se, but were collected so that they could be examined as correlates of the biometric outcomes above, using Pearson and Spearman’s correlation coefficients.
Results. The LCD (n = 29) and VLCD (n = 28) groups were similar at baseline with no significant differences reported. Of the 61 individuals initially enrolled, 57 (93%) completed the study. Summary statistics are reported only for these 57 individuals. No imputation or other methods for handling missing data were used. There were slightly more women than men in the study (53% women); the average (SD) age was 51.8 (1.9) years in the LCD group and 50.7 (1.5) years in the VLCD group. Mean starting BMI was 31 kg/m2 (31.3 [0.5] in LCD, 31.0 [0.4] in VLCD) and both groups had just under 40% body fat at baseline (39.9% [1.8] in LCD, 39.7% [1.5] in VLCD).
After 12 weeks of weight loss for LCD, or 5 weeks of weight loss for VLCD, both groups lost a similar amount of total weight (8.2 [0.5] kg in LCD vs. 9.0 [0.4] kg in VLCD), then had no significant changes in weight during the subsequent 4-week “weight stable” period. However, during the weight stable period VLCD patients had an average 0.8 (0.6) cm increase in waist circumference (a rebounding after a decrease of 7.7 cm during weight loss), while LCD patients on average had a continued decrease of 1.0 (0.5 cm) in waist circumference (P = 0.003).
There was no significant difference between groups for the primary outcome of weight regain during 9-months of follow-up (4.2 [0.6] kg regained for LCD, 4.5 [0.7] for VLCD; P = 0.73). The only significant correlates of weight regain were amount of FFM lost (more lean mass lost predicted more weight regain), and amount of physical activity reported during follow-up (more activity predicted less regain). Participant sex, age, starting BMI, history of weight cycling, and amount of weight lost did not correlate with rate of re-gain.
One area where there was a significant between-group difference, both after initial weight loss and persisting after the weight stable period, was in the amount of FFM lost (a rough approximation of lost lean mass, eg, muscle mass). VLCD participants had more FFM loss (1.6 [0.2] kg) than LCD participants (0.6 [0.2] kg) (P < 0.01) after active weight loss, and continued to have significantly more FFM loss (0.8 [0.2] kg vs. 0.2 [0.2] kg) after the 4-week weight stable period.
There were no between-group differences at the end of weight loss or at the end of follow-up for hip or waist circumference or for blood pressure.
Conclusion. The authors conclude that rate of weight loss does not affect one’s risk of weight regain after a diet, after a similar amount of weight has been lost.
Commentary
The failure of most diets to produce durable weight loss is a frustration for patients, clinicians, and researchers. In general, regardless of the composition of a diet, the majority of patients will regain some or all of their lost weight within several years after completing the diet. The reasons for weight regain are complex, and include reversion to old eating or physical activity behaviors but also a strong physiologic drive by the body to reverse weight loss that it perceives as a threat to health [1].
One area in diet research that has recently generated some controversy is whether or not rate of initial weight loss might impact a patient’s ability to maintain that weight loss, with the conventional wisdom (and national guidelines, in some cases), suggesting that slower weight loss is preferable to rapid weight loss for this reason [2]. A handful of studies have challenged this notion, however, and suggested that rapid weight loss does not necessarily lead to greater weight regain [3,4]. Previous such studies, however, have not generally been designed to compare regain after equal amounts of weight loss, which may make their results more difficult to interpret.
The present study contributes another piece of evidence to the argument that rapid initial weight loss may not increase a patient’s risk of regain. This small randomized trial is timely and has several features that make it a unique contribution. First, the design of the study allowed for both groups, despite losing weight at very different rates, to reach the same amount of total weight loss before being followed forward in time. This made weight regain much easier to compare between groups during follow-up. Second, the study included measurement of changing body composition—ie, what kind of weight was being lost (fat vs. fat-free mass)—rather than just the total amount of weight. This allowed the researchers to present data for an outcome that is mechanistically related to metabolic rate (and therefore weight regain), and one that might have implications for longer-term health after rapid versus more moderate-pace weight loss.
Several aspects of the study design, however, may limit the impact of the findings. For example, in both arms, while a certain type of diet was “prescribed,” there is no comment about assessment of participant fidelity to the prescribed diet, and there is potential for very different levels of adherence between groups, especially in active weight loss, when basically all meals were provided to the VLCD arm, but LCD subjects were responsible for about 90% of their own meals. This could have led to larger discrepancies between prescribed and actual diet in the LCD arm relative to VLCD. Granted, the rate of weight loss was the exposure of interest, and that clearly varied between groups as expected, implying at least moderate fidelity to prescribed caloric content of each diet, but how much protein vs. fat vs. carb was actually consumed by each group is not clear. Additionally, while 9 months of post weight-loss follow-up is certainly a good start in terms of follow-up duration, it may not have been sufficient to observe differences that would later emerge between the groups for weight regain. Other long-term weight loss maintenance studies have followed patients for several years or longer after initial weight loss [5].
Using data from all participants, the researchers reported that the amount of FFM an individual lost was a predictor of weight regain during follow-up. This finding is in keeping with the idea that more lean mass loss leads to lower metabolic rate and predisposes to weight regain (hence the conventional wisdom to avoid rapid weight loss with low-protein diets). In keeping with this theme, VLCD patients, whose protein intakes and activity levels were lower, did lose more FFM (ie, lean mass) than LCD patients. It was therefore surprising that in between-group analyses there was no statistical difference in weight regain. On some level, this raises concerns about the robustness of the overall finding. Perhaps with a larger sample, more precise measures of FFM lost (eg, with DEXA scanning instead of the “bod pod” or longer follow-up, this difference in lost lean mass between groups actually would have predicted greater weight regain for VLCD patients. The researchers attribute some of the FFM loss after the caloric restriction phase to decreased water and glycogen stores, rather than muscle mass, and speculate that this is why no impact on weight regain was seen between groups.
From a generalizability standpoint, there are important safety concerns with the use of VLCDs, aside from subsequent risk of weight regain, that are not addressed with this study. Many patients simply cannot tolerate a 500 kcal per day diet, including those with more severe obesity (who have higher daily energy requirements) or those with complicated chronic medical conditions who might be at higher risk of complications from such low energy intake. Accordingly, these kinds of patients were not included in this study, so it is not clear whether results might generalize to them.
Applications for Clinical Practice
Despite the conventional wisdom that slower weight loss may be more sustainable over time, several recent trials have suggested otherwise. Nonetheless, rapid weight loss produced with the use of VLCDs is not appropriate for every patient and must be carefully overseen by a weight management professional. Furthermore, rapid weight loss may place patients at increased risk of preferentially losing lean mass, which does correlate with risk of weight regain and could set them up for other health problems in the long-term. More studies are needed in this area before a definitive judgment can be made regarding the long term risks and benefits of rapid versus moderate-pace weight loss.
—Kristina Lewis, MD, MPH
1. Anastasiou CA, Karfopoulou E, Yannakoulia M. Weight regaining: From statistics and behaviors to physiology and metabolism. Metabolism 2015;64:1395–407.
2. Casazza K, Brown A, Astrup A, et al. Weighing the evidence of common beliefs in obesity research. Crit Rev Food Sci Nutr 2015;55:2014–53.
3. Purcell K, Sumithran P, Prendergast LA, et al. The effect of rate of weight loss on long-term weight management: a randomised controlled trial. Lancet Diabetes Endocrinol 2014;2:954–62.
4. Toubro S, Astrup A. Randomised comparison of diets for maintaining obese subjects’ weight after major weight loss: ad lib, low fat, high carbohydrate diet v fixed energy intake. BMJ 1997;314:29–34.
5. Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr 2005;82(1 Suppl):222S–225S.
Study Overview
Objective. To compare weight regain after rapid versus slower loss of an equivalent amount of weight.
Study design. Randomized clinical trial.
Setting and participants. This study took place in a single medical center in the Netherlands. Investigators recruited 61 adults (no age range provided) with body mass index (BMI) between 28–35 kg/m2 and at a stable weight (no change of > 3 kg for the past 2 months) to participate in a weight loss study. Individuals with type 2 diabetes, dyslipidemia, uncontrolled hypertension, or liver, heart or kidney disease were excluded, as were those who were currently pregnant or reported consuming more than moderate amounts of alcohol.
Once consented, participants were randomized into one of 2 study arms. The rapid weight loss arm was prescribed a very-low-calorie diet (VLCD) with just 500 kcal/day (43% protein/43% carb/14% fat) for 5 weeks, after which they transitioned to a 4-week “weight stable” period, and then a 9-month follow-up period (overall follow-up time of ~11 months; 10 months after weight loss). In contrast, the slower weight loss arm was prescribed a low-calorie diet (LCD) with 1250 kcal/day (29% protein/48% carb/23% fat) for 12 weeks, after which they also transitioned to a 4-week weight stable period and 9 months of follow-up (overall follow-up time of ~13 months; 10 months after weight loss). VLCD (rapid weight loss) participants received 3 meal replacement shakes per day (totaling 500 kcal) during the weight loss period and were also told they could consume unlimited amounts of low-calorie vegetables. The LCD (slower weight loss) participants received 1 meal replacement shake per day during their 12 weeks of weight loss and were responsible for providing the remainder of their own meals and snacks according to guidelines from a study dietitian. Following active weight loss, both groups then shifted to higher-calorie, food-based diets during a “weight stable” 4-week period and were responsible during this time for providing all of their own food. The researchers do not specify the details of the diet composition for this weight stable period. Exposure to the registered dietitian was the same in both groups, with 5 consultations during weight loss (weekly for VLCD, presumably more spaced out for LCD) and 4 during weight stable period. No further diet advice or meal replacement support was given during the 9-month follow-up period, but participants came in for monthly weigh-ins.
Main outcome measure. The primary outcome measure was change in weight (ie, amount of weight regained) during the 9-month follow-up period, compared between groups using an independent samples t test. Additional biometric measures included change in waist circumference and changes in body composition. For the latter, the researchers used a “Bod Pod” to conduct air-displacement plethysmography and determine what percentage of an individual’s weight was fat mass (FM) versus lean mass/water (FFM [fat-free mass]). They then compared the amount of FFM lost between groups, again using the independent samples t test.
The researchers also collected information on self-reported physical activity (questionnaire) and self-reported history of weight cycling (number of times a participant had previously lost and regained at least 5 kg) prior to this study. These were not outcomes per-se, but were collected so that they could be examined as correlates of the biometric outcomes above, using Pearson and Spearman’s correlation coefficients.
Results. The LCD (n = 29) and VLCD (n = 28) groups were similar at baseline with no significant differences reported. Of the 61 individuals initially enrolled, 57 (93%) completed the study. Summary statistics are reported only for these 57 individuals. No imputation or other methods for handling missing data were used. There were slightly more women than men in the study (53% women); the average (SD) age was 51.8 (1.9) years in the LCD group and 50.7 (1.5) years in the VLCD group. Mean starting BMI was 31 kg/m2 (31.3 [0.5] in LCD, 31.0 [0.4] in VLCD) and both groups had just under 40% body fat at baseline (39.9% [1.8] in LCD, 39.7% [1.5] in VLCD).
After 12 weeks of weight loss for LCD, or 5 weeks of weight loss for VLCD, both groups lost a similar amount of total weight (8.2 [0.5] kg in LCD vs. 9.0 [0.4] kg in VLCD), then had no significant changes in weight during the subsequent 4-week “weight stable” period. However, during the weight stable period VLCD patients had an average 0.8 (0.6) cm increase in waist circumference (a rebounding after a decrease of 7.7 cm during weight loss), while LCD patients on average had a continued decrease of 1.0 (0.5 cm) in waist circumference (P = 0.003).
There was no significant difference between groups for the primary outcome of weight regain during 9-months of follow-up (4.2 [0.6] kg regained for LCD, 4.5 [0.7] for VLCD; P = 0.73). The only significant correlates of weight regain were amount of FFM lost (more lean mass lost predicted more weight regain), and amount of physical activity reported during follow-up (more activity predicted less regain). Participant sex, age, starting BMI, history of weight cycling, and amount of weight lost did not correlate with rate of re-gain.
One area where there was a significant between-group difference, both after initial weight loss and persisting after the weight stable period, was in the amount of FFM lost (a rough approximation of lost lean mass, eg, muscle mass). VLCD participants had more FFM loss (1.6 [0.2] kg) than LCD participants (0.6 [0.2] kg) (P < 0.01) after active weight loss, and continued to have significantly more FFM loss (0.8 [0.2] kg vs. 0.2 [0.2] kg) after the 4-week weight stable period.
There were no between-group differences at the end of weight loss or at the end of follow-up for hip or waist circumference or for blood pressure.
Conclusion. The authors conclude that rate of weight loss does not affect one’s risk of weight regain after a diet, after a similar amount of weight has been lost.
Commentary
The failure of most diets to produce durable weight loss is a frustration for patients, clinicians, and researchers. In general, regardless of the composition of a diet, the majority of patients will regain some or all of their lost weight within several years after completing the diet. The reasons for weight regain are complex, and include reversion to old eating or physical activity behaviors but also a strong physiologic drive by the body to reverse weight loss that it perceives as a threat to health [1].
One area in diet research that has recently generated some controversy is whether or not rate of initial weight loss might impact a patient’s ability to maintain that weight loss, with the conventional wisdom (and national guidelines, in some cases), suggesting that slower weight loss is preferable to rapid weight loss for this reason [2]. A handful of studies have challenged this notion, however, and suggested that rapid weight loss does not necessarily lead to greater weight regain [3,4]. Previous such studies, however, have not generally been designed to compare regain after equal amounts of weight loss, which may make their results more difficult to interpret.
The present study contributes another piece of evidence to the argument that rapid initial weight loss may not increase a patient’s risk of regain. This small randomized trial is timely and has several features that make it a unique contribution. First, the design of the study allowed for both groups, despite losing weight at very different rates, to reach the same amount of total weight loss before being followed forward in time. This made weight regain much easier to compare between groups during follow-up. Second, the study included measurement of changing body composition—ie, what kind of weight was being lost (fat vs. fat-free mass)—rather than just the total amount of weight. This allowed the researchers to present data for an outcome that is mechanistically related to metabolic rate (and therefore weight regain), and one that might have implications for longer-term health after rapid versus more moderate-pace weight loss.
Several aspects of the study design, however, may limit the impact of the findings. For example, in both arms, while a certain type of diet was “prescribed,” there is no comment about assessment of participant fidelity to the prescribed diet, and there is potential for very different levels of adherence between groups, especially in active weight loss, when basically all meals were provided to the VLCD arm, but LCD subjects were responsible for about 90% of their own meals. This could have led to larger discrepancies between prescribed and actual diet in the LCD arm relative to VLCD. Granted, the rate of weight loss was the exposure of interest, and that clearly varied between groups as expected, implying at least moderate fidelity to prescribed caloric content of each diet, but how much protein vs. fat vs. carb was actually consumed by each group is not clear. Additionally, while 9 months of post weight-loss follow-up is certainly a good start in terms of follow-up duration, it may not have been sufficient to observe differences that would later emerge between the groups for weight regain. Other long-term weight loss maintenance studies have followed patients for several years or longer after initial weight loss [5].
Using data from all participants, the researchers reported that the amount of FFM an individual lost was a predictor of weight regain during follow-up. This finding is in keeping with the idea that more lean mass loss leads to lower metabolic rate and predisposes to weight regain (hence the conventional wisdom to avoid rapid weight loss with low-protein diets). In keeping with this theme, VLCD patients, whose protein intakes and activity levels were lower, did lose more FFM (ie, lean mass) than LCD patients. It was therefore surprising that in between-group analyses there was no statistical difference in weight regain. On some level, this raises concerns about the robustness of the overall finding. Perhaps with a larger sample, more precise measures of FFM lost (eg, with DEXA scanning instead of the “bod pod” or longer follow-up, this difference in lost lean mass between groups actually would have predicted greater weight regain for VLCD patients. The researchers attribute some of the FFM loss after the caloric restriction phase to decreased water and glycogen stores, rather than muscle mass, and speculate that this is why no impact on weight regain was seen between groups.
From a generalizability standpoint, there are important safety concerns with the use of VLCDs, aside from subsequent risk of weight regain, that are not addressed with this study. Many patients simply cannot tolerate a 500 kcal per day diet, including those with more severe obesity (who have higher daily energy requirements) or those with complicated chronic medical conditions who might be at higher risk of complications from such low energy intake. Accordingly, these kinds of patients were not included in this study, so it is not clear whether results might generalize to them.
Applications for Clinical Practice
Despite the conventional wisdom that slower weight loss may be more sustainable over time, several recent trials have suggested otherwise. Nonetheless, rapid weight loss produced with the use of VLCDs is not appropriate for every patient and must be carefully overseen by a weight management professional. Furthermore, rapid weight loss may place patients at increased risk of preferentially losing lean mass, which does correlate with risk of weight regain and could set them up for other health problems in the long-term. More studies are needed in this area before a definitive judgment can be made regarding the long term risks and benefits of rapid versus moderate-pace weight loss.
—Kristina Lewis, MD, MPH
Study Overview
Objective. To compare weight regain after rapid versus slower loss of an equivalent amount of weight.
Study design. Randomized clinical trial.
Setting and participants. This study took place in a single medical center in the Netherlands. Investigators recruited 61 adults (no age range provided) with body mass index (BMI) between 28–35 kg/m2 and at a stable weight (no change of > 3 kg for the past 2 months) to participate in a weight loss study. Individuals with type 2 diabetes, dyslipidemia, uncontrolled hypertension, or liver, heart or kidney disease were excluded, as were those who were currently pregnant or reported consuming more than moderate amounts of alcohol.
Once consented, participants were randomized into one of 2 study arms. The rapid weight loss arm was prescribed a very-low-calorie diet (VLCD) with just 500 kcal/day (43% protein/43% carb/14% fat) for 5 weeks, after which they transitioned to a 4-week “weight stable” period, and then a 9-month follow-up period (overall follow-up time of ~11 months; 10 months after weight loss). In contrast, the slower weight loss arm was prescribed a low-calorie diet (LCD) with 1250 kcal/day (29% protein/48% carb/23% fat) for 12 weeks, after which they also transitioned to a 4-week weight stable period and 9 months of follow-up (overall follow-up time of ~13 months; 10 months after weight loss). VLCD (rapid weight loss) participants received 3 meal replacement shakes per day (totaling 500 kcal) during the weight loss period and were also told they could consume unlimited amounts of low-calorie vegetables. The LCD (slower weight loss) participants received 1 meal replacement shake per day during their 12 weeks of weight loss and were responsible for providing the remainder of their own meals and snacks according to guidelines from a study dietitian. Following active weight loss, both groups then shifted to higher-calorie, food-based diets during a “weight stable” 4-week period and were responsible during this time for providing all of their own food. The researchers do not specify the details of the diet composition for this weight stable period. Exposure to the registered dietitian was the same in both groups, with 5 consultations during weight loss (weekly for VLCD, presumably more spaced out for LCD) and 4 during weight stable period. No further diet advice or meal replacement support was given during the 9-month follow-up period, but participants came in for monthly weigh-ins.
Main outcome measure. The primary outcome measure was change in weight (ie, amount of weight regained) during the 9-month follow-up period, compared between groups using an independent samples t test. Additional biometric measures included change in waist circumference and changes in body composition. For the latter, the researchers used a “Bod Pod” to conduct air-displacement plethysmography and determine what percentage of an individual’s weight was fat mass (FM) versus lean mass/water (FFM [fat-free mass]). They then compared the amount of FFM lost between groups, again using the independent samples t test.
The researchers also collected information on self-reported physical activity (questionnaire) and self-reported history of weight cycling (number of times a participant had previously lost and regained at least 5 kg) prior to this study. These were not outcomes per-se, but were collected so that they could be examined as correlates of the biometric outcomes above, using Pearson and Spearman’s correlation coefficients.
Results. The LCD (n = 29) and VLCD (n = 28) groups were similar at baseline with no significant differences reported. Of the 61 individuals initially enrolled, 57 (93%) completed the study. Summary statistics are reported only for these 57 individuals. No imputation or other methods for handling missing data were used. There were slightly more women than men in the study (53% women); the average (SD) age was 51.8 (1.9) years in the LCD group and 50.7 (1.5) years in the VLCD group. Mean starting BMI was 31 kg/m2 (31.3 [0.5] in LCD, 31.0 [0.4] in VLCD) and both groups had just under 40% body fat at baseline (39.9% [1.8] in LCD, 39.7% [1.5] in VLCD).
After 12 weeks of weight loss for LCD, or 5 weeks of weight loss for VLCD, both groups lost a similar amount of total weight (8.2 [0.5] kg in LCD vs. 9.0 [0.4] kg in VLCD), then had no significant changes in weight during the subsequent 4-week “weight stable” period. However, during the weight stable period VLCD patients had an average 0.8 (0.6) cm increase in waist circumference (a rebounding after a decrease of 7.7 cm during weight loss), while LCD patients on average had a continued decrease of 1.0 (0.5 cm) in waist circumference (P = 0.003).
There was no significant difference between groups for the primary outcome of weight regain during 9-months of follow-up (4.2 [0.6] kg regained for LCD, 4.5 [0.7] for VLCD; P = 0.73). The only significant correlates of weight regain were amount of FFM lost (more lean mass lost predicted more weight regain), and amount of physical activity reported during follow-up (more activity predicted less regain). Participant sex, age, starting BMI, history of weight cycling, and amount of weight lost did not correlate with rate of re-gain.
One area where there was a significant between-group difference, both after initial weight loss and persisting after the weight stable period, was in the amount of FFM lost (a rough approximation of lost lean mass, eg, muscle mass). VLCD participants had more FFM loss (1.6 [0.2] kg) than LCD participants (0.6 [0.2] kg) (P < 0.01) after active weight loss, and continued to have significantly more FFM loss (0.8 [0.2] kg vs. 0.2 [0.2] kg) after the 4-week weight stable period.
There were no between-group differences at the end of weight loss or at the end of follow-up for hip or waist circumference or for blood pressure.
Conclusion. The authors conclude that rate of weight loss does not affect one’s risk of weight regain after a diet, after a similar amount of weight has been lost.
Commentary
The failure of most diets to produce durable weight loss is a frustration for patients, clinicians, and researchers. In general, regardless of the composition of a diet, the majority of patients will regain some or all of their lost weight within several years after completing the diet. The reasons for weight regain are complex, and include reversion to old eating or physical activity behaviors but also a strong physiologic drive by the body to reverse weight loss that it perceives as a threat to health [1].
One area in diet research that has recently generated some controversy is whether or not rate of initial weight loss might impact a patient’s ability to maintain that weight loss, with the conventional wisdom (and national guidelines, in some cases), suggesting that slower weight loss is preferable to rapid weight loss for this reason [2]. A handful of studies have challenged this notion, however, and suggested that rapid weight loss does not necessarily lead to greater weight regain [3,4]. Previous such studies, however, have not generally been designed to compare regain after equal amounts of weight loss, which may make their results more difficult to interpret.
The present study contributes another piece of evidence to the argument that rapid initial weight loss may not increase a patient’s risk of regain. This small randomized trial is timely and has several features that make it a unique contribution. First, the design of the study allowed for both groups, despite losing weight at very different rates, to reach the same amount of total weight loss before being followed forward in time. This made weight regain much easier to compare between groups during follow-up. Second, the study included measurement of changing body composition—ie, what kind of weight was being lost (fat vs. fat-free mass)—rather than just the total amount of weight. This allowed the researchers to present data for an outcome that is mechanistically related to metabolic rate (and therefore weight regain), and one that might have implications for longer-term health after rapid versus more moderate-pace weight loss.
Several aspects of the study design, however, may limit the impact of the findings. For example, in both arms, while a certain type of diet was “prescribed,” there is no comment about assessment of participant fidelity to the prescribed diet, and there is potential for very different levels of adherence between groups, especially in active weight loss, when basically all meals were provided to the VLCD arm, but LCD subjects were responsible for about 90% of their own meals. This could have led to larger discrepancies between prescribed and actual diet in the LCD arm relative to VLCD. Granted, the rate of weight loss was the exposure of interest, and that clearly varied between groups as expected, implying at least moderate fidelity to prescribed caloric content of each diet, but how much protein vs. fat vs. carb was actually consumed by each group is not clear. Additionally, while 9 months of post weight-loss follow-up is certainly a good start in terms of follow-up duration, it may not have been sufficient to observe differences that would later emerge between the groups for weight regain. Other long-term weight loss maintenance studies have followed patients for several years or longer after initial weight loss [5].
Using data from all participants, the researchers reported that the amount of FFM an individual lost was a predictor of weight regain during follow-up. This finding is in keeping with the idea that more lean mass loss leads to lower metabolic rate and predisposes to weight regain (hence the conventional wisdom to avoid rapid weight loss with low-protein diets). In keeping with this theme, VLCD patients, whose protein intakes and activity levels were lower, did lose more FFM (ie, lean mass) than LCD patients. It was therefore surprising that in between-group analyses there was no statistical difference in weight regain. On some level, this raises concerns about the robustness of the overall finding. Perhaps with a larger sample, more precise measures of FFM lost (eg, with DEXA scanning instead of the “bod pod” or longer follow-up, this difference in lost lean mass between groups actually would have predicted greater weight regain for VLCD patients. The researchers attribute some of the FFM loss after the caloric restriction phase to decreased water and glycogen stores, rather than muscle mass, and speculate that this is why no impact on weight regain was seen between groups.
From a generalizability standpoint, there are important safety concerns with the use of VLCDs, aside from subsequent risk of weight regain, that are not addressed with this study. Many patients simply cannot tolerate a 500 kcal per day diet, including those with more severe obesity (who have higher daily energy requirements) or those with complicated chronic medical conditions who might be at higher risk of complications from such low energy intake. Accordingly, these kinds of patients were not included in this study, so it is not clear whether results might generalize to them.
Applications for Clinical Practice
Despite the conventional wisdom that slower weight loss may be more sustainable over time, several recent trials have suggested otherwise. Nonetheless, rapid weight loss produced with the use of VLCDs is not appropriate for every patient and must be carefully overseen by a weight management professional. Furthermore, rapid weight loss may place patients at increased risk of preferentially losing lean mass, which does correlate with risk of weight regain and could set them up for other health problems in the long-term. More studies are needed in this area before a definitive judgment can be made regarding the long term risks and benefits of rapid versus moderate-pace weight loss.
—Kristina Lewis, MD, MPH
1. Anastasiou CA, Karfopoulou E, Yannakoulia M. Weight regaining: From statistics and behaviors to physiology and metabolism. Metabolism 2015;64:1395–407.
2. Casazza K, Brown A, Astrup A, et al. Weighing the evidence of common beliefs in obesity research. Crit Rev Food Sci Nutr 2015;55:2014–53.
3. Purcell K, Sumithran P, Prendergast LA, et al. The effect of rate of weight loss on long-term weight management: a randomised controlled trial. Lancet Diabetes Endocrinol 2014;2:954–62.
4. Toubro S, Astrup A. Randomised comparison of diets for maintaining obese subjects’ weight after major weight loss: ad lib, low fat, high carbohydrate diet v fixed energy intake. BMJ 1997;314:29–34.
5. Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr 2005;82(1 Suppl):222S–225S.
1. Anastasiou CA, Karfopoulou E, Yannakoulia M. Weight regaining: From statistics and behaviors to physiology and metabolism. Metabolism 2015;64:1395–407.
2. Casazza K, Brown A, Astrup A, et al. Weighing the evidence of common beliefs in obesity research. Crit Rev Food Sci Nutr 2015;55:2014–53.
3. Purcell K, Sumithran P, Prendergast LA, et al. The effect of rate of weight loss on long-term weight management: a randomised controlled trial. Lancet Diabetes Endocrinol 2014;2:954–62.
4. Toubro S, Astrup A. Randomised comparison of diets for maintaining obese subjects’ weight after major weight loss: ad lib, low fat, high carbohydrate diet v fixed energy intake. BMJ 1997;314:29–34.
5. Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr 2005;82(1 Suppl):222S–225S.
Delayed Prescriptions for Reducing Antibiotic Use
Study Overview
Objective. To determine the efficacy and safety of delayed antibiotic prescribing strategies in acute uncomplicated respiratory infections.
Design. Randomized, multicenter, open-label clinical trial.
Setting and participants. The setting was 23 primary care centers in Spain. The study recruited patients who were 18 years of age or older with an acute uncomplicated respiratory infection (acute pharyngitis, rhinosinusitis, acute bronchitis, exacerbations of chronic bronchitis or mild to moderate chronic obstructive pulmonary disease). Patients with these infections were included by the physicians as long as they were unsure of whether to use antibiotics or not. The study protocol has been published elsewhere [1].
Intervention. Patients were randomized to 1 of 4 potential prescription strategies: (1) a delayed patient-led prescription strategy where patients were given an antibiotic prescription at first consultation but instructed to fill the prescription only if they felt substantially worse or saw no improvement in symptoms in the first few days after initial consultation; (2) a delayed prescription collection strategy requiring patients to collect their prescription from the primary care center reception desk 3 days after the first consultation; (3) an immediate prescription strategy; or (4) no antibiotic strategy. The patient-led and delayed collection strategies were considered delayed prescription strategies.
Main outcome measures. Duration of symptoms and severity of symptoms. Patients filled out a daily questionnaire for a maximum of 30 days, which listed common symptoms such as fever, discomfort or general pain, cough, difficulty sleeping, and changes in everyday life, and specific symptoms according to condition. Patients assessed severity of their symptoms using 6-point Likert scale, with scores of 1-2 considered mild, 3-4 moderate, and 5-6 severe. Secondary outcomes included antibiotic use, patient satisfaction, patients’ beliefs in the effectiveness of antibiotics, and absenteeism (absence from work or doing their daily activities).
Main results. A total of 405 patients were recruited, 398 of whom were included in the analysis. 136 patients (34.2%) were men. The mean (SD) age was 45 (17) years and 265 patients (72%) had at least a secondary education level. The most common infection was pharyngitis (n = 184; 46.2%), followed by acute bronchitis (n = 128; 32.2%). The mean severity of symptoms ranged from 1.8 to 3.5 points on the Likert scale, and mean (SD) duration of symptoms described on first visit was 6 (6) days. The mean (SD) general health status on first visit was 54 (20) based on a scale with 0 indicating worst health status and 100 indicating best health status. 314 patients (80.1%) were nonsmokers, and 372 patients (93.5%) did not have a respiratory comorbidity. The presence of symptoms on first visit was similar among the 4 groups.
The duration of the common symptoms of fever, discomfort or general pain, and cough was shorter in the immediate prescription group versus the no prescription group (P < 0.05 for all). In the immediate prescription group, the duration of patient symptoms after first visit was significantly different from that of the prescription collection and patient-led prescription groups only for discomfort or general pain. The mean (SD) duration of severe symptoms was 3.6 (3.3) days for the immediate prescription group, 4.0 (4.2) days for the prescription collection group, 5.1 (6.3) days for the patient-led prescription group, and 4.7 (3.6) days for the no prescription group. The median (interquartile range [IQR]) of severe symptoms was 3 (1–4) days for the prescription collection group and 3 (2–6) days for the patient-led prescription group. The median (IQR) of the maximum severity for any symptom was 5 (3–5) for the immediate prescription group and the prescription collection group; 5 (4–5) for the patient-led prescription group; and 5 (4–6) for the no prescription group. Patients randomized to the no prescription strategy or to either of the delayed strategies used fewer antibiotics and less frequently believed in antibiotic effectiveness. Among patients in the immediate prescription group, 91.1% used antibiotics; in the delayed patient-led, delayed collection, and no prescription groups, the rates of antibiotic use were 32.6%, 23.0%, and 12.1%, respectively. There were very few adverse events across groups, although the no prescription group had 3 adverse events compared with 0-1 in the other groups. Satisfaction was similar across groups.
Conclusion. Delayed strategies were associated with slightly greater but clinically similar symptom burden and duration and also with substantially reduced antibiotic use when compared with an immediate strategy.
Commentary
Acute respiratory infections are a common reasons for physician visits. These infections tend to be self-limiting and overuse of antibiotics for these infections is widespread. Approximately 60% of patients with a sore throat and ~70% of patients with acute uncomplicated bronchitis receive antibiotic prescriptions despite the literature suggesting no or limited benefit [2,3].Antibiotic resistance is a growing problem and the main cause of this problem is misuse of antibiotics.
Often physicians feel pressured into prescribing anti-biotics due to patient expectation and patient satisfaction metrics. In the face of the critical need to reduce overuse, delayed antibiotic prescribing strategies offers a compromise between immediate and no prescription [4]. Delayed prescribing strategies have been evaluated previously [5–8], with findings suggesting they do reduce antibiotic use. This study strengthens the evidence base supporting the delayed strategy.
This study has a few limitations. The sample size was small, and symptom data was obtained via patient self-report. In addition, the randomization procedure was not described. However, the investigators were able to achieve good patient retention, with very few patients lost to follow-up. The investigators used an intention to treat analysis; thus, the estimate of treatment effect size can be considered conservative.
In terms of baseline characteristics of the study participants, there was a lower overall education level, fewer smokers, and less respiratory comorbidity (defined as only cardiovascular comorbidity [P = 0.12] and diabetes [P = 0.19]) in the patient-led group. Otherwise, groups were very well-matched. Most patients in the study had pharyngitis and bronchitis, limiting the inferences for patients with rhinosinusitis or exacerbation of mild-to-moderate COPD.
Applications for Clinical Practice
Delayed antibiotic prescribing for acute uncomplicated respiratory infections appears to be an acceptable strategy for reducing the overuse of antibiotics. As patients may lack knowledge of this prescribing strategy [9], clinicians may need to spend time explaining the concept. Using the term “back-up antibiotics” instead of “delayed prescription” [10] may help to increase patients’ understanding and acceptance.
—Ajay Dharod, MD
1. de la Poza Abad M, Mas Dalmau G, Moreno Bakedano M, Get al; Delayed Antibiotic Prescription (DAP) Working Group. Rationale, design and organization of the delayed antibiotic prescription (DAP) trial: a randomized controlled trial of the efficacy and safety of delayed antibiotic prescribing strategies in the non-complicated acute respiratory tract infections in general practice. BMC Fam Pract 2013;14:63.
2. Barnett ML, Linder JA. Antibiotic prescribing to adults with sore throat in the United States, 1997-2010. JAMA Intern Med 2014;174:138–40.
3. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996–2010. JAMA 2014;311:2020–2.
4. McCullough AR, Glasziou PP. Delayed antibiotic prescribing strategies-time to implement? JAMA Intern Med 2016;176:29–30.
5. National Institute for Health and Clinical Excellence. Prescribing of antibiotics for self-limiting respiratory tract infections in adults and children in primary care. Clinical guideline 69. London: NICE; 2008.
6. Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev 2005;(4):CD003539.
7. Arroll B, Kenealy T, Kerse N. Do delayed prescriptions reduce antibiotic use in respiratory tract infections? A systematic review. Br J Gen Pract 2003;53:871–7.
8. Spurling GKP, Del Mar CB, Dooley L, et al. Delayed antibiotics for respiratory infections. Cochrane Database Syst Rev 2013;4:CD004417.
9. McNulty CAM, Lecky DM, Hawking MKD, et al. Delayed/back up antibiotic prescriptions: what do the public think? BMJ Open 2015;5:e009748.
10. Bunten AK, Hawking MKD, McNulty CAM. Patient information can improve appropriate antibiotic prescribing. Nurs Pract 2015;82:61–3.
Study Overview
Objective. To determine the efficacy and safety of delayed antibiotic prescribing strategies in acute uncomplicated respiratory infections.
Design. Randomized, multicenter, open-label clinical trial.
Setting and participants. The setting was 23 primary care centers in Spain. The study recruited patients who were 18 years of age or older with an acute uncomplicated respiratory infection (acute pharyngitis, rhinosinusitis, acute bronchitis, exacerbations of chronic bronchitis or mild to moderate chronic obstructive pulmonary disease). Patients with these infections were included by the physicians as long as they were unsure of whether to use antibiotics or not. The study protocol has been published elsewhere [1].
Intervention. Patients were randomized to 1 of 4 potential prescription strategies: (1) a delayed patient-led prescription strategy where patients were given an antibiotic prescription at first consultation but instructed to fill the prescription only if they felt substantially worse or saw no improvement in symptoms in the first few days after initial consultation; (2) a delayed prescription collection strategy requiring patients to collect their prescription from the primary care center reception desk 3 days after the first consultation; (3) an immediate prescription strategy; or (4) no antibiotic strategy. The patient-led and delayed collection strategies were considered delayed prescription strategies.
Main outcome measures. Duration of symptoms and severity of symptoms. Patients filled out a daily questionnaire for a maximum of 30 days, which listed common symptoms such as fever, discomfort or general pain, cough, difficulty sleeping, and changes in everyday life, and specific symptoms according to condition. Patients assessed severity of their symptoms using 6-point Likert scale, with scores of 1-2 considered mild, 3-4 moderate, and 5-6 severe. Secondary outcomes included antibiotic use, patient satisfaction, patients’ beliefs in the effectiveness of antibiotics, and absenteeism (absence from work or doing their daily activities).
Main results. A total of 405 patients were recruited, 398 of whom were included in the analysis. 136 patients (34.2%) were men. The mean (SD) age was 45 (17) years and 265 patients (72%) had at least a secondary education level. The most common infection was pharyngitis (n = 184; 46.2%), followed by acute bronchitis (n = 128; 32.2%). The mean severity of symptoms ranged from 1.8 to 3.5 points on the Likert scale, and mean (SD) duration of symptoms described on first visit was 6 (6) days. The mean (SD) general health status on first visit was 54 (20) based on a scale with 0 indicating worst health status and 100 indicating best health status. 314 patients (80.1%) were nonsmokers, and 372 patients (93.5%) did not have a respiratory comorbidity. The presence of symptoms on first visit was similar among the 4 groups.
The duration of the common symptoms of fever, discomfort or general pain, and cough was shorter in the immediate prescription group versus the no prescription group (P < 0.05 for all). In the immediate prescription group, the duration of patient symptoms after first visit was significantly different from that of the prescription collection and patient-led prescription groups only for discomfort or general pain. The mean (SD) duration of severe symptoms was 3.6 (3.3) days for the immediate prescription group, 4.0 (4.2) days for the prescription collection group, 5.1 (6.3) days for the patient-led prescription group, and 4.7 (3.6) days for the no prescription group. The median (interquartile range [IQR]) of severe symptoms was 3 (1–4) days for the prescription collection group and 3 (2–6) days for the patient-led prescription group. The median (IQR) of the maximum severity for any symptom was 5 (3–5) for the immediate prescription group and the prescription collection group; 5 (4–5) for the patient-led prescription group; and 5 (4–6) for the no prescription group. Patients randomized to the no prescription strategy or to either of the delayed strategies used fewer antibiotics and less frequently believed in antibiotic effectiveness. Among patients in the immediate prescription group, 91.1% used antibiotics; in the delayed patient-led, delayed collection, and no prescription groups, the rates of antibiotic use were 32.6%, 23.0%, and 12.1%, respectively. There were very few adverse events across groups, although the no prescription group had 3 adverse events compared with 0-1 in the other groups. Satisfaction was similar across groups.
Conclusion. Delayed strategies were associated with slightly greater but clinically similar symptom burden and duration and also with substantially reduced antibiotic use when compared with an immediate strategy.
Commentary
Acute respiratory infections are a common reasons for physician visits. These infections tend to be self-limiting and overuse of antibiotics for these infections is widespread. Approximately 60% of patients with a sore throat and ~70% of patients with acute uncomplicated bronchitis receive antibiotic prescriptions despite the literature suggesting no or limited benefit [2,3].Antibiotic resistance is a growing problem and the main cause of this problem is misuse of antibiotics.
Often physicians feel pressured into prescribing anti-biotics due to patient expectation and patient satisfaction metrics. In the face of the critical need to reduce overuse, delayed antibiotic prescribing strategies offers a compromise between immediate and no prescription [4]. Delayed prescribing strategies have been evaluated previously [5–8], with findings suggesting they do reduce antibiotic use. This study strengthens the evidence base supporting the delayed strategy.
This study has a few limitations. The sample size was small, and symptom data was obtained via patient self-report. In addition, the randomization procedure was not described. However, the investigators were able to achieve good patient retention, with very few patients lost to follow-up. The investigators used an intention to treat analysis; thus, the estimate of treatment effect size can be considered conservative.
In terms of baseline characteristics of the study participants, there was a lower overall education level, fewer smokers, and less respiratory comorbidity (defined as only cardiovascular comorbidity [P = 0.12] and diabetes [P = 0.19]) in the patient-led group. Otherwise, groups were very well-matched. Most patients in the study had pharyngitis and bronchitis, limiting the inferences for patients with rhinosinusitis or exacerbation of mild-to-moderate COPD.
Applications for Clinical Practice
Delayed antibiotic prescribing for acute uncomplicated respiratory infections appears to be an acceptable strategy for reducing the overuse of antibiotics. As patients may lack knowledge of this prescribing strategy [9], clinicians may need to spend time explaining the concept. Using the term “back-up antibiotics” instead of “delayed prescription” [10] may help to increase patients’ understanding and acceptance.
—Ajay Dharod, MD
Study Overview
Objective. To determine the efficacy and safety of delayed antibiotic prescribing strategies in acute uncomplicated respiratory infections.
Design. Randomized, multicenter, open-label clinical trial.
Setting and participants. The setting was 23 primary care centers in Spain. The study recruited patients who were 18 years of age or older with an acute uncomplicated respiratory infection (acute pharyngitis, rhinosinusitis, acute bronchitis, exacerbations of chronic bronchitis or mild to moderate chronic obstructive pulmonary disease). Patients with these infections were included by the physicians as long as they were unsure of whether to use antibiotics or not. The study protocol has been published elsewhere [1].
Intervention. Patients were randomized to 1 of 4 potential prescription strategies: (1) a delayed patient-led prescription strategy where patients were given an antibiotic prescription at first consultation but instructed to fill the prescription only if they felt substantially worse or saw no improvement in symptoms in the first few days after initial consultation; (2) a delayed prescription collection strategy requiring patients to collect their prescription from the primary care center reception desk 3 days after the first consultation; (3) an immediate prescription strategy; or (4) no antibiotic strategy. The patient-led and delayed collection strategies were considered delayed prescription strategies.
Main outcome measures. Duration of symptoms and severity of symptoms. Patients filled out a daily questionnaire for a maximum of 30 days, which listed common symptoms such as fever, discomfort or general pain, cough, difficulty sleeping, and changes in everyday life, and specific symptoms according to condition. Patients assessed severity of their symptoms using 6-point Likert scale, with scores of 1-2 considered mild, 3-4 moderate, and 5-6 severe. Secondary outcomes included antibiotic use, patient satisfaction, patients’ beliefs in the effectiveness of antibiotics, and absenteeism (absence from work or doing their daily activities).
Main results. A total of 405 patients were recruited, 398 of whom were included in the analysis. 136 patients (34.2%) were men. The mean (SD) age was 45 (17) years and 265 patients (72%) had at least a secondary education level. The most common infection was pharyngitis (n = 184; 46.2%), followed by acute bronchitis (n = 128; 32.2%). The mean severity of symptoms ranged from 1.8 to 3.5 points on the Likert scale, and mean (SD) duration of symptoms described on first visit was 6 (6) days. The mean (SD) general health status on first visit was 54 (20) based on a scale with 0 indicating worst health status and 100 indicating best health status. 314 patients (80.1%) were nonsmokers, and 372 patients (93.5%) did not have a respiratory comorbidity. The presence of symptoms on first visit was similar among the 4 groups.
The duration of the common symptoms of fever, discomfort or general pain, and cough was shorter in the immediate prescription group versus the no prescription group (P < 0.05 for all). In the immediate prescription group, the duration of patient symptoms after first visit was significantly different from that of the prescription collection and patient-led prescription groups only for discomfort or general pain. The mean (SD) duration of severe symptoms was 3.6 (3.3) days for the immediate prescription group, 4.0 (4.2) days for the prescription collection group, 5.1 (6.3) days for the patient-led prescription group, and 4.7 (3.6) days for the no prescription group. The median (interquartile range [IQR]) of severe symptoms was 3 (1–4) days for the prescription collection group and 3 (2–6) days for the patient-led prescription group. The median (IQR) of the maximum severity for any symptom was 5 (3–5) for the immediate prescription group and the prescription collection group; 5 (4–5) for the patient-led prescription group; and 5 (4–6) for the no prescription group. Patients randomized to the no prescription strategy or to either of the delayed strategies used fewer antibiotics and less frequently believed in antibiotic effectiveness. Among patients in the immediate prescription group, 91.1% used antibiotics; in the delayed patient-led, delayed collection, and no prescription groups, the rates of antibiotic use were 32.6%, 23.0%, and 12.1%, respectively. There were very few adverse events across groups, although the no prescription group had 3 adverse events compared with 0-1 in the other groups. Satisfaction was similar across groups.
Conclusion. Delayed strategies were associated with slightly greater but clinically similar symptom burden and duration and also with substantially reduced antibiotic use when compared with an immediate strategy.
Commentary
Acute respiratory infections are a common reasons for physician visits. These infections tend to be self-limiting and overuse of antibiotics for these infections is widespread. Approximately 60% of patients with a sore throat and ~70% of patients with acute uncomplicated bronchitis receive antibiotic prescriptions despite the literature suggesting no or limited benefit [2,3].Antibiotic resistance is a growing problem and the main cause of this problem is misuse of antibiotics.
Often physicians feel pressured into prescribing anti-biotics due to patient expectation and patient satisfaction metrics. In the face of the critical need to reduce overuse, delayed antibiotic prescribing strategies offers a compromise between immediate and no prescription [4]. Delayed prescribing strategies have been evaluated previously [5–8], with findings suggesting they do reduce antibiotic use. This study strengthens the evidence base supporting the delayed strategy.
This study has a few limitations. The sample size was small, and symptom data was obtained via patient self-report. In addition, the randomization procedure was not described. However, the investigators were able to achieve good patient retention, with very few patients lost to follow-up. The investigators used an intention to treat analysis; thus, the estimate of treatment effect size can be considered conservative.
In terms of baseline characteristics of the study participants, there was a lower overall education level, fewer smokers, and less respiratory comorbidity (defined as only cardiovascular comorbidity [P = 0.12] and diabetes [P = 0.19]) in the patient-led group. Otherwise, groups were very well-matched. Most patients in the study had pharyngitis and bronchitis, limiting the inferences for patients with rhinosinusitis or exacerbation of mild-to-moderate COPD.
Applications for Clinical Practice
Delayed antibiotic prescribing for acute uncomplicated respiratory infections appears to be an acceptable strategy for reducing the overuse of antibiotics. As patients may lack knowledge of this prescribing strategy [9], clinicians may need to spend time explaining the concept. Using the term “back-up antibiotics” instead of “delayed prescription” [10] may help to increase patients’ understanding and acceptance.
—Ajay Dharod, MD
1. de la Poza Abad M, Mas Dalmau G, Moreno Bakedano M, Get al; Delayed Antibiotic Prescription (DAP) Working Group. Rationale, design and organization of the delayed antibiotic prescription (DAP) trial: a randomized controlled trial of the efficacy and safety of delayed antibiotic prescribing strategies in the non-complicated acute respiratory tract infections in general practice. BMC Fam Pract 2013;14:63.
2. Barnett ML, Linder JA. Antibiotic prescribing to adults with sore throat in the United States, 1997-2010. JAMA Intern Med 2014;174:138–40.
3. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996–2010. JAMA 2014;311:2020–2.
4. McCullough AR, Glasziou PP. Delayed antibiotic prescribing strategies-time to implement? JAMA Intern Med 2016;176:29–30.
5. National Institute for Health and Clinical Excellence. Prescribing of antibiotics for self-limiting respiratory tract infections in adults and children in primary care. Clinical guideline 69. London: NICE; 2008.
6. Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev 2005;(4):CD003539.
7. Arroll B, Kenealy T, Kerse N. Do delayed prescriptions reduce antibiotic use in respiratory tract infections? A systematic review. Br J Gen Pract 2003;53:871–7.
8. Spurling GKP, Del Mar CB, Dooley L, et al. Delayed antibiotics for respiratory infections. Cochrane Database Syst Rev 2013;4:CD004417.
9. McNulty CAM, Lecky DM, Hawking MKD, et al. Delayed/back up antibiotic prescriptions: what do the public think? BMJ Open 2015;5:e009748.
10. Bunten AK, Hawking MKD, McNulty CAM. Patient information can improve appropriate antibiotic prescribing. Nurs Pract 2015;82:61–3.
1. de la Poza Abad M, Mas Dalmau G, Moreno Bakedano M, Get al; Delayed Antibiotic Prescription (DAP) Working Group. Rationale, design and organization of the delayed antibiotic prescription (DAP) trial: a randomized controlled trial of the efficacy and safety of delayed antibiotic prescribing strategies in the non-complicated acute respiratory tract infections in general practice. BMC Fam Pract 2013;14:63.
2. Barnett ML, Linder JA. Antibiotic prescribing to adults with sore throat in the United States, 1997-2010. JAMA Intern Med 2014;174:138–40.
3. Barnett ML, Linder JA. Antibiotic prescribing for adults with acute bronchitis in the United States, 1996–2010. JAMA 2014;311:2020–2.
4. McCullough AR, Glasziou PP. Delayed antibiotic prescribing strategies-time to implement? JAMA Intern Med 2016;176:29–30.
5. National Institute for Health and Clinical Excellence. Prescribing of antibiotics for self-limiting respiratory tract infections in adults and children in primary care. Clinical guideline 69. London: NICE; 2008.
6. Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev 2005;(4):CD003539.
7. Arroll B, Kenealy T, Kerse N. Do delayed prescriptions reduce antibiotic use in respiratory tract infections? A systematic review. Br J Gen Pract 2003;53:871–7.
8. Spurling GKP, Del Mar CB, Dooley L, et al. Delayed antibiotics for respiratory infections. Cochrane Database Syst Rev 2013;4:CD004417.
9. McNulty CAM, Lecky DM, Hawking MKD, et al. Delayed/back up antibiotic prescriptions: what do the public think? BMJ Open 2015;5:e009748.
10. Bunten AK, Hawking MKD, McNulty CAM. Patient information can improve appropriate antibiotic prescribing. Nurs Pract 2015;82:61–3.
NPs, PAs Vital to Hospital Medicine
Yes, it’s time for another “year ahead” type column where the writer attempts to provide clarity on future events. What does “Hospital Medicine 2016” hold for us? I hope by the time Hospital Medicine 2017 rolls around, everyone will have forgotten the wrong predictions and only remember those that reveal my exceptional clairvoyance and prescient knowledge.
NP and PA Practice in Hospital Medicine Will Continue to Grow
Well, it doesn’t take a crystal ball or tarot cards to predict this. One only has to look at the data. The 2012 State of Hospital Medicine report revealed that 51.7% of hospital medicine groups (HMGs) employed nurse practitioners (NPs) and/or physician assistants (PAs) in their practice. Two short years later, the survey showed 83% of HMGs reported having NPs and/or PAs in their groups. That is an astounding amount of growth in a short period of time, which brings me to my next prediction.
HMGs Will Have to Continue to Figure Out How to Hire and Deploy NPs and PAs in Sensible Ways
I know that statement is very controversial. Not. But the true work of utilizing NP and PA providers in hospitalist practice is not in the hiring; it’s how to use these providers in thoughtful, sensible, and cost-effective ways.
A group leader really needs to know and understand the drivers behind the need for these hires as well as understand the financial landscape in the hiring. Are you hiring an NP/PA because you want to reduce your provider workforce cost? Are you hiring to target quality outcomes in a specific patient population? Are you hiring to staff your observation unit, freeing up your physicians for higher-acuity work? Are you hiring to treat and improve physician burnout? Or is this the only carbon-based life form you can attract to the outer boroughs of your northern clime in the deepest, darkest days of January?
All these may or may not be good reasons, but understanding those variables will help you get the right person for the right reason and will help you evaluate the return on investment and the impact on practice.
Diversity Prevents Disease
Much like the potato monoculture of McDonald’s french fries increasing the risk of potato diseases, monoculture in your hospitalist group may breed burnout and bad attitudes. Diversity of experience, perspective, and skill set may inoculate your group, keeping the dreaded crispy coated from complaining about schedule, workload, or acuity or, worse yet, simply leaving.
I don’t have data to support this, but I have heard anecdotally from more than one HMG leader that the addition of NP/PA providers to physician teams has improved physician satisfaction. SHM obviously agrees with this philosophy, as they value and support the value of a “big tent” philosophy. This big tent includes all types of people who contribute to the culture of this organization, making it stronger, more nimble and innovative, and definitely more fun.
Diversity in providers can only have a positive impact on your organization’s culture.
Whatever the Reason You Hire Them, Get Ready for Change
Be prepared for evolution. You may have initially hired an NP or PA simply to do admissions or to see all of your orthopedic co-management patients. But over time, your practice is going to morph and evolve, hopefully, in positive ways. Bring your NP/PA colleagues along for the ride; pull up a chair to the table. They may be able to provide new direction, support, or service lines to your practice in ways you hadn’t considered.
NP/PA providers’ abilities and ambitions will change over time as well. Make sure that change goes both ways. You may find that their influence and impact on your organization’s productivity and growth go beyond their industry. Consider utilizing NP/PA providers in novel ways; maybe they have great onboarding skills, are fabulous at scheduling, or can look at a spreadsheet without going cross-eyed or bald.
Change is growth. And growth is good. Unless you would rather die.
HM Needs to Develop Innovative Care Models; NPs/PAs Provide a Platform for Innovation
Inpatient medicine is changing in a rapid and unpredictable way. Some of the necessity of that work is driven by financial incentives and quality indicators, but necessity is the biggest driver of all. People, patients, and providers are getting old (thank God it’s not just me). There simply are not enough physicians to care for our rapidly aging population, or if there are, they are all employed in sunny Southern California. How we respond to this threat or opportunity is one of our most important charges. We own the inpatient kingdom. We need to lead with benevolence and thoughtfulness. We need to really look ahead and identify new ways to manage the complexity of a system whose complexity continues to mutate like some avian virus. I can’t see a future without a crucial role played by my NP/PA brethren. Can we begin this conversation with the long view in mind and really begin to own this in a true and responsible way?
Thanks for your attention, and remember, in 2017 you will have forgotten all the ways, if any, that I was wrong. TH
Ms. Cardin is a nurse practitioner in the Section of Hospital Medicine at the University of Chicago and is chair of SHM’s NP/PA Committee. She is a newly elected SHM board member.
Yes, it’s time for another “year ahead” type column where the writer attempts to provide clarity on future events. What does “Hospital Medicine 2016” hold for us? I hope by the time Hospital Medicine 2017 rolls around, everyone will have forgotten the wrong predictions and only remember those that reveal my exceptional clairvoyance and prescient knowledge.
NP and PA Practice in Hospital Medicine Will Continue to Grow
Well, it doesn’t take a crystal ball or tarot cards to predict this. One only has to look at the data. The 2012 State of Hospital Medicine report revealed that 51.7% of hospital medicine groups (HMGs) employed nurse practitioners (NPs) and/or physician assistants (PAs) in their practice. Two short years later, the survey showed 83% of HMGs reported having NPs and/or PAs in their groups. That is an astounding amount of growth in a short period of time, which brings me to my next prediction.
HMGs Will Have to Continue to Figure Out How to Hire and Deploy NPs and PAs in Sensible Ways
I know that statement is very controversial. Not. But the true work of utilizing NP and PA providers in hospitalist practice is not in the hiring; it’s how to use these providers in thoughtful, sensible, and cost-effective ways.
A group leader really needs to know and understand the drivers behind the need for these hires as well as understand the financial landscape in the hiring. Are you hiring an NP/PA because you want to reduce your provider workforce cost? Are you hiring to target quality outcomes in a specific patient population? Are you hiring to staff your observation unit, freeing up your physicians for higher-acuity work? Are you hiring to treat and improve physician burnout? Or is this the only carbon-based life form you can attract to the outer boroughs of your northern clime in the deepest, darkest days of January?
All these may or may not be good reasons, but understanding those variables will help you get the right person for the right reason and will help you evaluate the return on investment and the impact on practice.
Diversity Prevents Disease
Much like the potato monoculture of McDonald’s french fries increasing the risk of potato diseases, monoculture in your hospitalist group may breed burnout and bad attitudes. Diversity of experience, perspective, and skill set may inoculate your group, keeping the dreaded crispy coated from complaining about schedule, workload, or acuity or, worse yet, simply leaving.
I don’t have data to support this, but I have heard anecdotally from more than one HMG leader that the addition of NP/PA providers to physician teams has improved physician satisfaction. SHM obviously agrees with this philosophy, as they value and support the value of a “big tent” philosophy. This big tent includes all types of people who contribute to the culture of this organization, making it stronger, more nimble and innovative, and definitely more fun.
Diversity in providers can only have a positive impact on your organization’s culture.
Whatever the Reason You Hire Them, Get Ready for Change
Be prepared for evolution. You may have initially hired an NP or PA simply to do admissions or to see all of your orthopedic co-management patients. But over time, your practice is going to morph and evolve, hopefully, in positive ways. Bring your NP/PA colleagues along for the ride; pull up a chair to the table. They may be able to provide new direction, support, or service lines to your practice in ways you hadn’t considered.
NP/PA providers’ abilities and ambitions will change over time as well. Make sure that change goes both ways. You may find that their influence and impact on your organization’s productivity and growth go beyond their industry. Consider utilizing NP/PA providers in novel ways; maybe they have great onboarding skills, are fabulous at scheduling, or can look at a spreadsheet without going cross-eyed or bald.
Change is growth. And growth is good. Unless you would rather die.
HM Needs to Develop Innovative Care Models; NPs/PAs Provide a Platform for Innovation
Inpatient medicine is changing in a rapid and unpredictable way. Some of the necessity of that work is driven by financial incentives and quality indicators, but necessity is the biggest driver of all. People, patients, and providers are getting old (thank God it’s not just me). There simply are not enough physicians to care for our rapidly aging population, or if there are, they are all employed in sunny Southern California. How we respond to this threat or opportunity is one of our most important charges. We own the inpatient kingdom. We need to lead with benevolence and thoughtfulness. We need to really look ahead and identify new ways to manage the complexity of a system whose complexity continues to mutate like some avian virus. I can’t see a future without a crucial role played by my NP/PA brethren. Can we begin this conversation with the long view in mind and really begin to own this in a true and responsible way?
Thanks for your attention, and remember, in 2017 you will have forgotten all the ways, if any, that I was wrong. TH
Ms. Cardin is a nurse practitioner in the Section of Hospital Medicine at the University of Chicago and is chair of SHM’s NP/PA Committee. She is a newly elected SHM board member.
Yes, it’s time for another “year ahead” type column where the writer attempts to provide clarity on future events. What does “Hospital Medicine 2016” hold for us? I hope by the time Hospital Medicine 2017 rolls around, everyone will have forgotten the wrong predictions and only remember those that reveal my exceptional clairvoyance and prescient knowledge.
NP and PA Practice in Hospital Medicine Will Continue to Grow
Well, it doesn’t take a crystal ball or tarot cards to predict this. One only has to look at the data. The 2012 State of Hospital Medicine report revealed that 51.7% of hospital medicine groups (HMGs) employed nurse practitioners (NPs) and/or physician assistants (PAs) in their practice. Two short years later, the survey showed 83% of HMGs reported having NPs and/or PAs in their groups. That is an astounding amount of growth in a short period of time, which brings me to my next prediction.
HMGs Will Have to Continue to Figure Out How to Hire and Deploy NPs and PAs in Sensible Ways
I know that statement is very controversial. Not. But the true work of utilizing NP and PA providers in hospitalist practice is not in the hiring; it’s how to use these providers in thoughtful, sensible, and cost-effective ways.
A group leader really needs to know and understand the drivers behind the need for these hires as well as understand the financial landscape in the hiring. Are you hiring an NP/PA because you want to reduce your provider workforce cost? Are you hiring to target quality outcomes in a specific patient population? Are you hiring to staff your observation unit, freeing up your physicians for higher-acuity work? Are you hiring to treat and improve physician burnout? Or is this the only carbon-based life form you can attract to the outer boroughs of your northern clime in the deepest, darkest days of January?
All these may or may not be good reasons, but understanding those variables will help you get the right person for the right reason and will help you evaluate the return on investment and the impact on practice.
Diversity Prevents Disease
Much like the potato monoculture of McDonald’s french fries increasing the risk of potato diseases, monoculture in your hospitalist group may breed burnout and bad attitudes. Diversity of experience, perspective, and skill set may inoculate your group, keeping the dreaded crispy coated from complaining about schedule, workload, or acuity or, worse yet, simply leaving.
I don’t have data to support this, but I have heard anecdotally from more than one HMG leader that the addition of NP/PA providers to physician teams has improved physician satisfaction. SHM obviously agrees with this philosophy, as they value and support the value of a “big tent” philosophy. This big tent includes all types of people who contribute to the culture of this organization, making it stronger, more nimble and innovative, and definitely more fun.
Diversity in providers can only have a positive impact on your organization’s culture.
Whatever the Reason You Hire Them, Get Ready for Change
Be prepared for evolution. You may have initially hired an NP or PA simply to do admissions or to see all of your orthopedic co-management patients. But over time, your practice is going to morph and evolve, hopefully, in positive ways. Bring your NP/PA colleagues along for the ride; pull up a chair to the table. They may be able to provide new direction, support, or service lines to your practice in ways you hadn’t considered.
NP/PA providers’ abilities and ambitions will change over time as well. Make sure that change goes both ways. You may find that their influence and impact on your organization’s productivity and growth go beyond their industry. Consider utilizing NP/PA providers in novel ways; maybe they have great onboarding skills, are fabulous at scheduling, or can look at a spreadsheet without going cross-eyed or bald.
Change is growth. And growth is good. Unless you would rather die.
HM Needs to Develop Innovative Care Models; NPs/PAs Provide a Platform for Innovation
Inpatient medicine is changing in a rapid and unpredictable way. Some of the necessity of that work is driven by financial incentives and quality indicators, but necessity is the biggest driver of all. People, patients, and providers are getting old (thank God it’s not just me). There simply are not enough physicians to care for our rapidly aging population, or if there are, they are all employed in sunny Southern California. How we respond to this threat or opportunity is one of our most important charges. We own the inpatient kingdom. We need to lead with benevolence and thoughtfulness. We need to really look ahead and identify new ways to manage the complexity of a system whose complexity continues to mutate like some avian virus. I can’t see a future without a crucial role played by my NP/PA brethren. Can we begin this conversation with the long view in mind and really begin to own this in a true and responsible way?
Thanks for your attention, and remember, in 2017 you will have forgotten all the ways, if any, that I was wrong. TH
Ms. Cardin is a nurse practitioner in the Section of Hospital Medicine at the University of Chicago and is chair of SHM’s NP/PA Committee. She is a newly elected SHM board member.
Not Sleeping Enough Can Cause Serious Health Issues
ATLANTA (Reuters) - Did you get enough sleep last night? If not, you are not alone. More than one out of three American adults do not get enough sleep, according to a study released Thursday from the U.S. Centers for Disease Control and Prevention.
"That's a big problem," says Dr. Nancy Collop, director of the Emory Sleep Center at Emory University School of Medicine in Atlanta, who is familiar with the study. "You don't function as well, your ability to pay attention is reduced, and it can have serious, long term side effects. It can change your metabolism for the worse."
At least seven hours of sleep is considered healthy for an adults aged 18 to 60, according to the American Academy of Sleep Medicine and the Sleep Research Society. The CDC analyzed data from a 2014 survey of 444,306 adults and found roughly 65% of respondents reported getting that amount of
sleep.
"Lifestyle changes such as going to bed at the same time each night; rising at the same time each morning; and turning off or removing televisions, computers, mobile devices from the bedroom, can help people get the healthy sleep they need," said Dr. Wayne Giles, director of the CDC's Division of Population Health, in a statement.
Getting less than seven hours a night is associated with an increased risk of obesity, diabetes, high blood pressure, heart disease, stroke and frequent mental distress, the study shows. Published in the CDC's Morbidity and Mortality Weekly Report, the study is the first of its kind to look at all 50 U.S. states and the District of Columbia.
The study found that among those most likely to get great sleep were married or have a job, with 67% and 65%, respectively saying they get enough. Only 56% of divorced adults said they get enough sleep, and just over half of jobless adults sleep seven hours a night regularly. Among the best sleepers were college graduates, with 72% reporting seven hours or more.
The study found geographical differences as well as ethnic disparities. Hawaiian residents get less sleep than those living in South Dakota, the study found. Non-Hispanic whites sleep better than non-Hispanic black residents, with 67% and 54%, respectively.
ATLANTA (Reuters) - Did you get enough sleep last night? If not, you are not alone. More than one out of three American adults do not get enough sleep, according to a study released Thursday from the U.S. Centers for Disease Control and Prevention.
"That's a big problem," says Dr. Nancy Collop, director of the Emory Sleep Center at Emory University School of Medicine in Atlanta, who is familiar with the study. "You don't function as well, your ability to pay attention is reduced, and it can have serious, long term side effects. It can change your metabolism for the worse."
At least seven hours of sleep is considered healthy for an adults aged 18 to 60, according to the American Academy of Sleep Medicine and the Sleep Research Society. The CDC analyzed data from a 2014 survey of 444,306 adults and found roughly 65% of respondents reported getting that amount of
sleep.
"Lifestyle changes such as going to bed at the same time each night; rising at the same time each morning; and turning off or removing televisions, computers, mobile devices from the bedroom, can help people get the healthy sleep they need," said Dr. Wayne Giles, director of the CDC's Division of Population Health, in a statement.
Getting less than seven hours a night is associated with an increased risk of obesity, diabetes, high blood pressure, heart disease, stroke and frequent mental distress, the study shows. Published in the CDC's Morbidity and Mortality Weekly Report, the study is the first of its kind to look at all 50 U.S. states and the District of Columbia.
The study found that among those most likely to get great sleep were married or have a job, with 67% and 65%, respectively saying they get enough. Only 56% of divorced adults said they get enough sleep, and just over half of jobless adults sleep seven hours a night regularly. Among the best sleepers were college graduates, with 72% reporting seven hours or more.
The study found geographical differences as well as ethnic disparities. Hawaiian residents get less sleep than those living in South Dakota, the study found. Non-Hispanic whites sleep better than non-Hispanic black residents, with 67% and 54%, respectively.
ATLANTA (Reuters) - Did you get enough sleep last night? If not, you are not alone. More than one out of three American adults do not get enough sleep, according to a study released Thursday from the U.S. Centers for Disease Control and Prevention.
"That's a big problem," says Dr. Nancy Collop, director of the Emory Sleep Center at Emory University School of Medicine in Atlanta, who is familiar with the study. "You don't function as well, your ability to pay attention is reduced, and it can have serious, long term side effects. It can change your metabolism for the worse."
At least seven hours of sleep is considered healthy for an adults aged 18 to 60, according to the American Academy of Sleep Medicine and the Sleep Research Society. The CDC analyzed data from a 2014 survey of 444,306 adults and found roughly 65% of respondents reported getting that amount of
sleep.
"Lifestyle changes such as going to bed at the same time each night; rising at the same time each morning; and turning off or removing televisions, computers, mobile devices from the bedroom, can help people get the healthy sleep they need," said Dr. Wayne Giles, director of the CDC's Division of Population Health, in a statement.
Getting less than seven hours a night is associated with an increased risk of obesity, diabetes, high blood pressure, heart disease, stroke and frequent mental distress, the study shows. Published in the CDC's Morbidity and Mortality Weekly Report, the study is the first of its kind to look at all 50 U.S. states and the District of Columbia.
The study found that among those most likely to get great sleep were married or have a job, with 67% and 65%, respectively saying they get enough. Only 56% of divorced adults said they get enough sleep, and just over half of jobless adults sleep seven hours a night regularly. Among the best sleepers were college graduates, with 72% reporting seven hours or more.
The study found geographical differences as well as ethnic disparities. Hawaiian residents get less sleep than those living in South Dakota, the study found. Non-Hispanic whites sleep better than non-Hispanic black residents, with 67% and 54%, respectively.
Targeting a protein to prevent malignancy

Photo by Aaron Logan
New research suggests hematologic malignancies driven by MYC might be prevented by lowering levels of another protein, MCL-1.
“Our colleagues had previously discovered that reducing the activity of MCL-1 is a promising strategy to treat malignant MYC-driven cancers,” said Stephanie Grabow, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia.
“We have now shown that the same approach might be able to prevent those cancers from forming in the first place.”
Dr Grabow and her colleagues described this work in Cell Reports.
Previous research indicated that expression from both MCL-1 alleles is essential for the survival of hematopoietic stem and progenitor cells during stress-induced repopulation of the hematopoietic system.
So, with this study, Dr Grabow and her colleagues set out to determine whether reducing MCL-1 protein levels might hinder the development of hematologic malignancies.
In experiments with mice, the investigators found that loss of one MCL-1 allele significantly delayed the development of MYC-driven lymphoma and reduced MYC-driven accumulation of pre-leukemic cancer-initiating cells.
However, loss of one p53 allele accelerated MYC-driven lymphomagenesis even when one MCL-1 allele was deleted. Loss of PUMA accelerated lymphoma development as well, though to a much lesser extent.
Loss of BIM substantially accelerated lymphomagenesis when one MCL-1 allele was deleted, restoring lymphoma-initiating cells and the rate of tumor development.
And loss of one BIM allele overrode the survival defect observed in pre-leukemic Eμ-Myc B-cell progenitors when one MCL-1 allele was deleted.
The investigators noted that loss of one MCL-1 allele did not noticeably impair the survival of normal B lymphoid cells even though it greatly diminished the survival of MYC-overexpressing B-cell progenitors.
“No one had realized just how vulnerable cells undergoing cancerous changes are to a relatively minor reduction in the levels of MCL-1,” Dr Grabow said.
“We found that MCL-1 is critical for keeping developing cancer cells alive through the stressful events that cause the transformation of a healthy cell into a cancerous cell. This result is particularly exciting because MCL-1 inhibitors are already in development as anticancer drugs.”
Study investigator Brandon Aubrey, MBBS, also of the Walter and Eliza Hall Institute, said this research could inform future strategies to prevent cancer.
“Early treatment or even cancer prevention are likely to be a more effective way to fight cancer than treating an established cancer after it has already formed and made a person sick,” he said. ”Our research has suggested that dependency on MCL-1 could be a key vulnerability of many developing cancers.”
“In the future, MCL-1 inhibitors might have potential benefit for treating the very early stages of MYC-driven cancers, or we may even be able use these agents to prevent people from getting cancer in the first place.” ![]()

Photo by Aaron Logan
New research suggests hematologic malignancies driven by MYC might be prevented by lowering levels of another protein, MCL-1.
“Our colleagues had previously discovered that reducing the activity of MCL-1 is a promising strategy to treat malignant MYC-driven cancers,” said Stephanie Grabow, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia.
“We have now shown that the same approach might be able to prevent those cancers from forming in the first place.”
Dr Grabow and her colleagues described this work in Cell Reports.
Previous research indicated that expression from both MCL-1 alleles is essential for the survival of hematopoietic stem and progenitor cells during stress-induced repopulation of the hematopoietic system.
So, with this study, Dr Grabow and her colleagues set out to determine whether reducing MCL-1 protein levels might hinder the development of hematologic malignancies.
In experiments with mice, the investigators found that loss of one MCL-1 allele significantly delayed the development of MYC-driven lymphoma and reduced MYC-driven accumulation of pre-leukemic cancer-initiating cells.
However, loss of one p53 allele accelerated MYC-driven lymphomagenesis even when one MCL-1 allele was deleted. Loss of PUMA accelerated lymphoma development as well, though to a much lesser extent.
Loss of BIM substantially accelerated lymphomagenesis when one MCL-1 allele was deleted, restoring lymphoma-initiating cells and the rate of tumor development.
And loss of one BIM allele overrode the survival defect observed in pre-leukemic Eμ-Myc B-cell progenitors when one MCL-1 allele was deleted.
The investigators noted that loss of one MCL-1 allele did not noticeably impair the survival of normal B lymphoid cells even though it greatly diminished the survival of MYC-overexpressing B-cell progenitors.
“No one had realized just how vulnerable cells undergoing cancerous changes are to a relatively minor reduction in the levels of MCL-1,” Dr Grabow said.
“We found that MCL-1 is critical for keeping developing cancer cells alive through the stressful events that cause the transformation of a healthy cell into a cancerous cell. This result is particularly exciting because MCL-1 inhibitors are already in development as anticancer drugs.”
Study investigator Brandon Aubrey, MBBS, also of the Walter and Eliza Hall Institute, said this research could inform future strategies to prevent cancer.
“Early treatment or even cancer prevention are likely to be a more effective way to fight cancer than treating an established cancer after it has already formed and made a person sick,” he said. ”Our research has suggested that dependency on MCL-1 could be a key vulnerability of many developing cancers.”
“In the future, MCL-1 inhibitors might have potential benefit for treating the very early stages of MYC-driven cancers, or we may even be able use these agents to prevent people from getting cancer in the first place.” ![]()

Photo by Aaron Logan
New research suggests hematologic malignancies driven by MYC might be prevented by lowering levels of another protein, MCL-1.
“Our colleagues had previously discovered that reducing the activity of MCL-1 is a promising strategy to treat malignant MYC-driven cancers,” said Stephanie Grabow, PhD, of the Walter and Eliza Hall Institute of Medical Research in Parkville, Victoria, Australia.
“We have now shown that the same approach might be able to prevent those cancers from forming in the first place.”
Dr Grabow and her colleagues described this work in Cell Reports.
Previous research indicated that expression from both MCL-1 alleles is essential for the survival of hematopoietic stem and progenitor cells during stress-induced repopulation of the hematopoietic system.
So, with this study, Dr Grabow and her colleagues set out to determine whether reducing MCL-1 protein levels might hinder the development of hematologic malignancies.
In experiments with mice, the investigators found that loss of one MCL-1 allele significantly delayed the development of MYC-driven lymphoma and reduced MYC-driven accumulation of pre-leukemic cancer-initiating cells.
However, loss of one p53 allele accelerated MYC-driven lymphomagenesis even when one MCL-1 allele was deleted. Loss of PUMA accelerated lymphoma development as well, though to a much lesser extent.
Loss of BIM substantially accelerated lymphomagenesis when one MCL-1 allele was deleted, restoring lymphoma-initiating cells and the rate of tumor development.
And loss of one BIM allele overrode the survival defect observed in pre-leukemic Eμ-Myc B-cell progenitors when one MCL-1 allele was deleted.
The investigators noted that loss of one MCL-1 allele did not noticeably impair the survival of normal B lymphoid cells even though it greatly diminished the survival of MYC-overexpressing B-cell progenitors.
“No one had realized just how vulnerable cells undergoing cancerous changes are to a relatively minor reduction in the levels of MCL-1,” Dr Grabow said.
“We found that MCL-1 is critical for keeping developing cancer cells alive through the stressful events that cause the transformation of a healthy cell into a cancerous cell. This result is particularly exciting because MCL-1 inhibitors are already in development as anticancer drugs.”
Study investigator Brandon Aubrey, MBBS, also of the Walter and Eliza Hall Institute, said this research could inform future strategies to prevent cancer.
“Early treatment or even cancer prevention are likely to be a more effective way to fight cancer than treating an established cancer after it has already formed and made a person sick,” he said. ”Our research has suggested that dependency on MCL-1 could be a key vulnerability of many developing cancers.”
“In the future, MCL-1 inhibitors might have potential benefit for treating the very early stages of MYC-driven cancers, or we may even be able use these agents to prevent people from getting cancer in the first place.” ![]()
Study sheds new light on blood clot structure

Photo by Vera Kratochvil
Researchers say they have discovered significant differences between blood clot structure in adults and newborns, a finding that could help us better understand the challenges in addressing post-operative bleeding in neonatal patients.
The researchers also found evidence to suggest the current standard of care for treating post-operative bleeding may pose an increased risk of thrombosis in newborns as compared to adults.
The team reported these findings in Anesthesiology.
“We knew that neonates—infants less than 1 month old—are more likely than adults to suffer from severe bleeding after heart surgery, which poses a variety of health risks,” said study author Ashley Brown, PhD, of the University of North Carolina at Chapel Hill.
“The current standard of care is to give neonatal patients blood products . . . derived from adult blood, but neonatal blood and adult blood aren’t the same. Many of the components involved in clotting in newborns have differing levels of activity, or effectiveness, compared to the same components in adults. Our goal was to better understand how clotting in neonates differs from that in adults so that we can move closer to developing more effective treatment strategies for these infants.”
The researchers’ hypothesis was that fibrinogen from neonates would form clots that are different from those formed by adult fibrinogen, and this proved correct. However, the team was surprised to find that fibrinogen from adults did not integrate well with fibrinogen from neonates.
To test their hypothesis, the researchers took samples of neonate fibrinogen and adult fibrinogen and compared clot formation. The team looked at clots formed solely of adult fibrinogen, clots formed solely of neonate fibrinogen, and clots made from a mixture of the two.
Neonate fibrinogen formed less dense, more fragile clots than adult fibrinogen. Likewise, a mixture of adult and neonate fibrinogen formed clots that were fragile and less dense, even if there was relatively little neonate fibrinogen in the mixture.
The researchers also evaluated how long it took these clots to dissolve. Clots of neonate fibrinogen dissolved about twice as quickly as clots formed from adult fibrinogen.
Clots formed from an adult and neonate fibrinogen mixture dissolved at approximately the same rate as adult-only clots, regardless of the percentage of neonate fibrinogen in the mixture.
“This suggests that using adult fibrinogen in neonatal patients may pose an increased risk of embolism or other adverse thrombotic events,” said study author Nina Guzzetta, MD, of the Emory University School of Medicine in Atlanta, Georgia.
“This work drives home that newborns are not just small adults, and we still have much to learn about clotting in neonates. It also tells us that there is a great deal of room for improvement in the current standard of care for post-operative bleeding in neonates.”
“We are investigating several approaches that may help address this problem, evaluating various modes of action,” Dr Brown added. “It is possible that we can use various external factors that promote clotting to stimulate the fibrinogen in neonates to form a denser clot.”
“We are investigating possible alternatives to help neonates form a better clot after major surgery without having to use adult fibrinogen. For example, we are investigating the use of synthetic platelet-like particles developed by our team to augment hemostasis . . . in blood samples collected from these patients.” ![]()

Photo by Vera Kratochvil
Researchers say they have discovered significant differences between blood clot structure in adults and newborns, a finding that could help us better understand the challenges in addressing post-operative bleeding in neonatal patients.
The researchers also found evidence to suggest the current standard of care for treating post-operative bleeding may pose an increased risk of thrombosis in newborns as compared to adults.
The team reported these findings in Anesthesiology.
“We knew that neonates—infants less than 1 month old—are more likely than adults to suffer from severe bleeding after heart surgery, which poses a variety of health risks,” said study author Ashley Brown, PhD, of the University of North Carolina at Chapel Hill.
“The current standard of care is to give neonatal patients blood products . . . derived from adult blood, but neonatal blood and adult blood aren’t the same. Many of the components involved in clotting in newborns have differing levels of activity, or effectiveness, compared to the same components in adults. Our goal was to better understand how clotting in neonates differs from that in adults so that we can move closer to developing more effective treatment strategies for these infants.”
The researchers’ hypothesis was that fibrinogen from neonates would form clots that are different from those formed by adult fibrinogen, and this proved correct. However, the team was surprised to find that fibrinogen from adults did not integrate well with fibrinogen from neonates.
To test their hypothesis, the researchers took samples of neonate fibrinogen and adult fibrinogen and compared clot formation. The team looked at clots formed solely of adult fibrinogen, clots formed solely of neonate fibrinogen, and clots made from a mixture of the two.
Neonate fibrinogen formed less dense, more fragile clots than adult fibrinogen. Likewise, a mixture of adult and neonate fibrinogen formed clots that were fragile and less dense, even if there was relatively little neonate fibrinogen in the mixture.
The researchers also evaluated how long it took these clots to dissolve. Clots of neonate fibrinogen dissolved about twice as quickly as clots formed from adult fibrinogen.
Clots formed from an adult and neonate fibrinogen mixture dissolved at approximately the same rate as adult-only clots, regardless of the percentage of neonate fibrinogen in the mixture.
“This suggests that using adult fibrinogen in neonatal patients may pose an increased risk of embolism or other adverse thrombotic events,” said study author Nina Guzzetta, MD, of the Emory University School of Medicine in Atlanta, Georgia.
“This work drives home that newborns are not just small adults, and we still have much to learn about clotting in neonates. It also tells us that there is a great deal of room for improvement in the current standard of care for post-operative bleeding in neonates.”
“We are investigating several approaches that may help address this problem, evaluating various modes of action,” Dr Brown added. “It is possible that we can use various external factors that promote clotting to stimulate the fibrinogen in neonates to form a denser clot.”
“We are investigating possible alternatives to help neonates form a better clot after major surgery without having to use adult fibrinogen. For example, we are investigating the use of synthetic platelet-like particles developed by our team to augment hemostasis . . . in blood samples collected from these patients.” ![]()

Photo by Vera Kratochvil
Researchers say they have discovered significant differences between blood clot structure in adults and newborns, a finding that could help us better understand the challenges in addressing post-operative bleeding in neonatal patients.
The researchers also found evidence to suggest the current standard of care for treating post-operative bleeding may pose an increased risk of thrombosis in newborns as compared to adults.
The team reported these findings in Anesthesiology.
“We knew that neonates—infants less than 1 month old—are more likely than adults to suffer from severe bleeding after heart surgery, which poses a variety of health risks,” said study author Ashley Brown, PhD, of the University of North Carolina at Chapel Hill.
“The current standard of care is to give neonatal patients blood products . . . derived from adult blood, but neonatal blood and adult blood aren’t the same. Many of the components involved in clotting in newborns have differing levels of activity, or effectiveness, compared to the same components in adults. Our goal was to better understand how clotting in neonates differs from that in adults so that we can move closer to developing more effective treatment strategies for these infants.”
The researchers’ hypothesis was that fibrinogen from neonates would form clots that are different from those formed by adult fibrinogen, and this proved correct. However, the team was surprised to find that fibrinogen from adults did not integrate well with fibrinogen from neonates.
To test their hypothesis, the researchers took samples of neonate fibrinogen and adult fibrinogen and compared clot formation. The team looked at clots formed solely of adult fibrinogen, clots formed solely of neonate fibrinogen, and clots made from a mixture of the two.
Neonate fibrinogen formed less dense, more fragile clots than adult fibrinogen. Likewise, a mixture of adult and neonate fibrinogen formed clots that were fragile and less dense, even if there was relatively little neonate fibrinogen in the mixture.
The researchers also evaluated how long it took these clots to dissolve. Clots of neonate fibrinogen dissolved about twice as quickly as clots formed from adult fibrinogen.
Clots formed from an adult and neonate fibrinogen mixture dissolved at approximately the same rate as adult-only clots, regardless of the percentage of neonate fibrinogen in the mixture.
“This suggests that using adult fibrinogen in neonatal patients may pose an increased risk of embolism or other adverse thrombotic events,” said study author Nina Guzzetta, MD, of the Emory University School of Medicine in Atlanta, Georgia.
“This work drives home that newborns are not just small adults, and we still have much to learn about clotting in neonates. It also tells us that there is a great deal of room for improvement in the current standard of care for post-operative bleeding in neonates.”
“We are investigating several approaches that may help address this problem, evaluating various modes of action,” Dr Brown added. “It is possible that we can use various external factors that promote clotting to stimulate the fibrinogen in neonates to form a denser clot.”
“We are investigating possible alternatives to help neonates form a better clot after major surgery without having to use adult fibrinogen. For example, we are investigating the use of synthetic platelet-like particles developed by our team to augment hemostasis . . . in blood samples collected from these patients.” ![]()
HE for the Hospitalist
Reversible impairment of brain function in the setting of cirrhosis defines hepatic encephalopathy (HE). HE is associated with significantly decreased survival,[1] and patients with HE have poor outcomes whether HE occurs in isolation or in conjunction with acute‐on‐chronic liver failure.[2] A large multicenter study comparing cirrhotics with and without HE also found that those with a history of HE were hospitalized more frequently.[2]
The presentation of HE is variable, and diagnosis remains clinical. Subtle manifestations of HE persist between episodes, even if gross cognitive function normalizes.[3] Retrospective data suggest the effects of serial bouts of HE may be cumulative, because even with appropriate treatment, the severity of impairment correlates with the number of prior episodes.[3] Even minimal manifestations of hepatic encephalopathy correlate with reduced quality of life.[4]
The West Haven score is the most validated scoring system.[5] Higher grades of HE correlate with significantly increased mortality,[2] but due to difficulties differentiating stages 0 and 1, these criteria remain somewhat controversial. The Spectrum of Neurocognitive Impairment in Cirrhosis (SONIC) has been proposed as an alternate conceptualization of HE as a continuous spectrum rather than discrete stages.[6] Table 1 shows findings associated with various West Haven and SONIC stages. Both systems include covert and overt encephalopathy. Covert correlates with West Haven grades 0 to 1, and consists mainly of subtle findings that require specialized psychometric testing to detect. The SONIC system terms demonstrable but subclinical manifestations minimal HE.[6] Overt HE includes West Haven grades 2 through 4, and refers to objective findings that can be reliably detected on clinical evaluation.[7] Whereas specific numeric scores are used largely for research purposes, classifying HE as covert or overt is clinically useful.
| West Haven Grade | SONIC Classification | Neurologic Changes | Asterixis |
|---|---|---|---|
| |||
| 0 | Normal | None | None |
| Minimal HE | Requires specialized psychometric testing | ||
| 1 | Overt | Decreased attention span, hypersomnia/emnsomnia | Detectable |
| 2 | Lethargy, disorientation | Obvious | |
| 3 | Semistupor or stupor | None | |
| 4 | Coma | None | |
Although blood ammonia levels correlate well across populations, they are not diagnostically useful for individuals, because considerable overlap exists between patients with no HE and those with severe encephalopathy.[8] Ammonia levels also do not predict HE development.[9] Brain imaging is of limited utility, but may be prudent with abrupt decompensation, focal neurologic findings, or poor response to therapy.[10] A recent single‐center review of head computed tomography in cirrhotic patients presenting with altered level of consciousness found a low incidence of intracranial hemorrhage (ICH).[11] The number needed to scan was 293 patients to detect a single ICH. Only 1 patient out of 316 had ICH when fever, trauma, and focal neurological findings were excluded. The presence of acute ICH was not associated with platelet count, coagulopathy, creatinine, or Model for End‐Stage Liver Disease score.
PRECIPITANTS
Initial evaluation of patients with suspected HE must confirm the presence of HE and identify potentially reversible precipitants. Infection, bleeding, and metabolic derangements (including renal injury, hypovolemia, and hyponatremia) are common precipitants.[12] Searching for precipitants is heavily stressed in the 4‐pronged approach recommended by the American Association for the Study of Liver Disease,[7] as summarized in Table 2. Common precipitants are grouped into episodic and recurrent causes. Episodic causes are those that represent discrete insults with specific, short‐term treatments. Recurrent causes are those that are likely to require active management over time. These distinctions may help inform different approaches for initial or recurrent episodes of HE; in practice, much overlap exists.
| |
| 1. Initiate care for cirrhotic patients with altered consciousness | |
| 2. Seek and treat alternative causes of altered mental status if present | |
| 3. Identify and treat precipitating factors: | |
| Episodic | Recurrent |
| Infection | Electrolyte derangement |
| Gastrointestinal bleeding | Infection |
| Hypovolemia | Constipation |
| Electrolyte derangement | Hypovolemia |
| Constipation | Gastrointestinal bleeding |
| 4. Commence empiric HE treatment | |
Diuretic use has been clearly correlated with incidence of HE.[2] Although diuretic usage may be an indicator of more advanced liver disease, their use can also contribute to HE via increased risk of hypovolemia and dysnatremia.[2] Accordingly, caution is necessary when using diuretics to manage patients with HE and refractory ascites. These findings have led some to suggest serial paracentesis may be preferable to diuretics in this population.[2]
MANAGEMENT
The mainstay of HE treatment is administration of the nonabsorbable disaccharide lactulose. Lactulose is part of nearly all regimens because it is effective, easily titrated, and inexpensive.[13] It is efficacious orally or as an enema.[14] Lactulose increases both cognitive function and quality of life,[15] and is effective for prophylaxis and treatment of all stages of HE.[16, 17]
Rifaximin is often used as an adjunct to lactulose, particularly in cases of recurrent HE. Small trials have associated rifaximin with increased quality of life[18] and cognitive function.[19] The largest randomized trial of rifaximin was a double‐blind, placebo‐controlled trial in patients with multiple episodes of overt HE during the prior 6 months.[20] Lactulose was used concomitantly in approximately 91% of patients. At the end of the 6‐month study, rifaximin was associated with a 58% relative risk reduction in overt HE recurrence and roughly 50% reduction in HE‐related hospitalization. The numbers needed to treat were 4 patients to prevent 1 overt HE episode and 9 to prevent 1 HE‐related hospitalization.[20]
A meta‐analysis of 264 patients included in published, high‐quality trials found rifaximin monotherapy to be similar to nonabsorbable disaccharides in both efficacy and incidence of diarrhea, but with significantly less abdominal pain.[21] This analysis was limited by significant heterogeneity among trials. A larger, more recent systematic review and meta‐analysis of 19 studies (both published and unpublished) found rifaximin to be effective for treatment, secondary prophylaxis, and possibly decreased mortality.[22] Of note, this meta‐analysis included placebo studies as well as studies using varying doses of lactulose or other antibiotics as controls. Despite this variability, the authors concluded that the control used in the individual trials did not significantly affect the aggregate results.[22] In the largest individual study to show a mortality benefit, improvement seemed to be driven by decreased rates of sepsis when rifaximin was used as an adjunct to lactulose.[23] Cost is a barrier to use, as rifaximin has not proven to be cost‐effective as monotherapy instead of lactulose.[24] Many insurers will facilitate adjunctive rifaximin with prior authorization, and the manufacturer offers assistance programs.[25]
Other adjuncts, including laxatives,[26] antibiotics,[12] branched‐chain aminoacids,[27] and acarbose[28] have far less evidentiary support and require further study prior to incorporation into clinical practice.[26] A recent study showed polyethylene glycol to perform similar to lactulose, but the studied volume of 4 L daily may make routine use impractical.[29] Dietary protein restriction has been shown in a prospective randomized controlled trial to accelerate body muscle breakdown without affecting HE,[30] so is best avoided.
ISSUES PERTINENT TO HOSPITAL MANAGEMENT
Concurrent HE frequently complicates inpatient management of acute pain. Acetaminophen below 3 g daily for short‐term use is safe,[31] but may be insufficient. Non‐steroidal anti‐inflammatory agents are best avoided given risks for renal dysfunction and bleeding.[32] Although a direct connection between opiate use and HE remains unproven, these agents are problematic because they can cause both sedation and constipation. Nonetheless, they are often needed for pain control. Oxycodone has a more desirable side effect profile than other narcotics. We often prescribe doses every 6 hours initially to account for decreased hepatic metabolism. Morphine has active metabolites that can accumulate in cirrhotics, so morphine use is best avoided.[32] Fluctuations in cognition may help distinguish narcosis from HE; specifically, narcosis causes chronic somnolence worst shortly after an opiate dose, whereas HE causes alterations in sleep‐wake cycles including insomnia.[32] Frequent adjustment of opiate dose and frequency may be required to balance analgesia with unwanted sedation and constipation.
Decisional capacity frequently complicates care of patients with cirrhosis. Patients may decline therapy because of dissatisfaction with bowel frequency, but such lapses in adherence likely contribute to HE recurrence. Patients with overt HE are often incapable of making decisions based on informed consent. If such patients have inadequate social support to ensure medical attention if symptoms progress, then mandatory treatment is reasonable. This may include involuntary administration of medications via rectal or nasogastric tube. Once cognition improves enough that he or she can reliably articulate risks, benefits, and alternatives of declining therapy, then it is reasonable to allow them to do. Subspecialty consultation with psychiatry or ethics may be useful in such situations.
For cirrhotics admitted for management of nonhepatic issues (particularly operations or invasive procedures), vigilance is needed to monitor for HE during hospitalization. Patients with HE have increased risk of falls and impaired driving, which may lead to admission onto surgical services.[4] Changes in diet, medications, bowel function, and environment may all contribute to encephalopathy. HE occurring during admission for other diagnoses still requires prompt titration of lactulose. Routine inquiry about bowel function and sleep quality are likely to help identify trouble early.
Placement of transvenous intrahepatic portosystemic shunt (TIPS) increases the risk for HE via introduction of neurotoxins directly into the systemic circulation. These patients can typically be treated medically,[33] but are likely to require increased lactulose dosage. TIPS revision may be necessary for patients with treatment‐refractory HE, but retrospective evidence suggests this is rarely necessary.[33] In that study, only a single patient out of 81 with post‐TIPS HE required TIPS closure.
Under the International Classification of Disease, 10th Revision, a diagnosis of HE is often most consistent with metabolic encephalopathy (G93.41).[34] It may also be coded as chronic hepatic failure without coma (K7210) or chronic hepatic failure with coma (K7211).[35] Whenever possible, specifying the underlying liver disease (eg, hepatitis C virus, alcohol) is preferable.
TRANSITIONING TO OUTPATIENT CARE
HE patients are usually ready for community living once their cognition has improved enough to reliably take medications. Key aspects of HE management need to be communicated clearly to patients and caregivers. Barriers to optimal outpatient care mostly relate to lactulose adherence. Stressing the direct correlation between insufficient bowel movements and HE progression may enhance adherence. All patients need a lactulose titration plan including when doses can be skipped and when additional doses are needed. Even minimal symptoms of HE need to be addressed,[36] and specific vigilance for alterations in sleep‐wake cycles needs to be adopted. Table 3 is an example of a lactulose titration plan that can be used at discharge. These plans should be included in discharge documents and within communication to outpatient healthcare providers. Close follow‐up with a hepatology specialist is ideal to ensure appropriate lactulose use, answer questions that arise upon return home, and address other concerns related to cirrhosis.
|
| Your dose of lactulose is 30 mL (1 tbsp) 3 times daily with meals. |
| If you have fewer than 3 BMs in any day, take an additional dose of lactulose at bedtime. |
| If you begin to experience difficulty sleeping at night, excessive drowsiness during the day, or confusion, take 2 doses of lactulose with each meal to ensure 3 or more BMs daily. |
| If you have more than 4 BMs in any 24 hour period and are not having any of the symptoms mentioned above, skip a single dose of lactulose then resume your usual schedule. |
Although specific interventions to decrease readmission have not been studied in this population, best practices from other populations (such as medication self‐management, follow‐up plans, and red flags to be on watch for[37]) likely apply. Defining optimal strategies to decrease readmission is an opportunity for hospitalists to contribute to standardization of care for these patients.
CONCLUSIONS
HE is a common but very treatable complication of cirrhosis. Various metabolic insults may precipitate HE, and hospitalists should seek to reverse contributing factors whenever possible. Lactulose titrated to ensure adequate bowel output is the cornerstone of both therapy and prevention for HE. Adjunctive use of rifaximin improves many outcomes. Patient education about manifestations of HE and medication titration is crucial to achieving smooth transition to the outpatient setting.
Disclosure
Nothing to report.
- , , , et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30(5):890–895.
- , , , et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute‐on‐chronic liver failure (ACLF). J Hepatol. 2014;60(2):275–281.
- , , , et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138(7):2332–2340.
- , , . Minimal hepatic encephalopathy impairs quality of life. J Clin Exp Hepatol. 2015;5(suppl 1):S42–S48.
- , ; Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol. 2001;96(7):1968–1976.
- , , . Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology. 2009;50(6):2014–2021.
- , , , et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–735.
- , , , et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114(3):188–193.
- , . Serum ammonia level for the evaluation of hepatic encephalopathy. JAMA. 2014;312(6):643–644.
- , , . Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute‐on‐chronic liver failure. J Hepatol. 2015;62(2):437–447.
- , , , et al. Low Likelihood of intracranial hemorrhage in patients with cirrhosis and altered mental status. Clin Gastroenterol Hepatol. 2015;13(1):165–169.
- , . The management of hospitalized patients with cirrhosis: the Mount Sinai experience and a guide for hospitalists. Dig Dis Sci. 2011;56(5):1266–1281.
- , , . Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004(2):CD003044.
- , , , et al. Acidifying enemas (lactitol and lactose) vs. nonacidifying enemas (tap water) to treat acute portal‐systemic encephalopathy: a double‐blind, randomized clinical trial. Hepatology. 1987;7(4):639–643.
- , . Disaccharides in the treatment of hepatic encephalopathy. Metab Brain Dis. 2013;28(2):313–320.
- , , , . Secondary prophylaxis of hepatic encephalopathy: an open‐label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137(3):885–891, 91.e1.
- , , , , , . Efficacy of lactulose in cirrhotic patients with subclinical hepatic encephalopathy. Dig Dis Sci. 2000;45(8):1549–1552.
- , , , et al. Randomised clinical trial: rifaximin improves health‐related quality of life in cirrhotic patients with hepatic encephalopathy—a double‐blind placebo‐controlled study. Aliment Pharmacol Ther. 2011;34(8):853–861.
- , , , , , . Rifaximin improves psychometric performance and health‐related quality of life in patients with minimal hepatic encephalopathy (the RIME Trial). Am J Gastroenterol. 2011;106(2):307–316.
- , , , et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071–1081.
- , , , , , . Rifaximin versus nonabsorbable disaccharides in the management of hepatic encephalopathy: a meta‐analysis. Eur J Gastroenterol Hepatol. 2008;20(11):1064–1070.
- , , , , . Systematic review with meta‐analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther. 2014;40(2):123–132.
- , , , , , . A randomized, double‐blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108(9):1458–1463.
- , , . The cost‐effectiveness and budget impact of competing therapies in hepatic encephalopathy—a decision analysis. Aliment Pharmacol Ther. 2007;26(8):1147–1161.
- Salix Pharmaceuticals. Patient assistance program. Available at: http://www.salix.com/about‐us/corporate‐responsibility/patient‐medication‐assistance. Accessed October 24, 2015.
- , . Management of overt hepatic encephalopathy. J Clin Exp Hepatol. 2015;5(suppl 1):S82–S87.
- , , , . Parenteral nutrition with branched‐chain amino acids in hepatic encephalopathy. A meta‐analysis. Gastroenterology. 1989;97(4):1033–1042.
- , , , et al. A randomized controlled trial of acarbose in hepatic encephalopathy. Clin Gastroenterol Hepatol. 2005;3(2):184–191.
- , , , . Lactulose vs polyethylene glycol 3350‐‐electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomized clinical trial. JAMA Intern Med. 2014;174(11):1727–1733.
- , , , et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41(1):38–43.
- , , . The therapeutic use of acetaminophen in patients with liver disease. Am J Ther. 2005;12(2):133–141.
- , . Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc. 2010;85(5):451–458.
- , , , et al. Clearing the confusion over hepatic encephalopathy after TIPS creation: incidence, prognostic factors, and clinical outcomes. Dig Dis Sci. 2015;60(4):1059–66.
- Centers for Medicare and Medicaid Services. ICD‐10 code lookup: encephalopathy. Available at: https://www.cms.gov/medicare‐coverage‐database/staticpages/icd‐10‐code‐lookup.aspx?KeyWord=encephalopathy5(suppl 1):S75–S81.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828.
Reversible impairment of brain function in the setting of cirrhosis defines hepatic encephalopathy (HE). HE is associated with significantly decreased survival,[1] and patients with HE have poor outcomes whether HE occurs in isolation or in conjunction with acute‐on‐chronic liver failure.[2] A large multicenter study comparing cirrhotics with and without HE also found that those with a history of HE were hospitalized more frequently.[2]
The presentation of HE is variable, and diagnosis remains clinical. Subtle manifestations of HE persist between episodes, even if gross cognitive function normalizes.[3] Retrospective data suggest the effects of serial bouts of HE may be cumulative, because even with appropriate treatment, the severity of impairment correlates with the number of prior episodes.[3] Even minimal manifestations of hepatic encephalopathy correlate with reduced quality of life.[4]
The West Haven score is the most validated scoring system.[5] Higher grades of HE correlate with significantly increased mortality,[2] but due to difficulties differentiating stages 0 and 1, these criteria remain somewhat controversial. The Spectrum of Neurocognitive Impairment in Cirrhosis (SONIC) has been proposed as an alternate conceptualization of HE as a continuous spectrum rather than discrete stages.[6] Table 1 shows findings associated with various West Haven and SONIC stages. Both systems include covert and overt encephalopathy. Covert correlates with West Haven grades 0 to 1, and consists mainly of subtle findings that require specialized psychometric testing to detect. The SONIC system terms demonstrable but subclinical manifestations minimal HE.[6] Overt HE includes West Haven grades 2 through 4, and refers to objective findings that can be reliably detected on clinical evaluation.[7] Whereas specific numeric scores are used largely for research purposes, classifying HE as covert or overt is clinically useful.
| West Haven Grade | SONIC Classification | Neurologic Changes | Asterixis |
|---|---|---|---|
| |||
| 0 | Normal | None | None |
| Minimal HE | Requires specialized psychometric testing | ||
| 1 | Overt | Decreased attention span, hypersomnia/emnsomnia | Detectable |
| 2 | Lethargy, disorientation | Obvious | |
| 3 | Semistupor or stupor | None | |
| 4 | Coma | None | |
Although blood ammonia levels correlate well across populations, they are not diagnostically useful for individuals, because considerable overlap exists between patients with no HE and those with severe encephalopathy.[8] Ammonia levels also do not predict HE development.[9] Brain imaging is of limited utility, but may be prudent with abrupt decompensation, focal neurologic findings, or poor response to therapy.[10] A recent single‐center review of head computed tomography in cirrhotic patients presenting with altered level of consciousness found a low incidence of intracranial hemorrhage (ICH).[11] The number needed to scan was 293 patients to detect a single ICH. Only 1 patient out of 316 had ICH when fever, trauma, and focal neurological findings were excluded. The presence of acute ICH was not associated with platelet count, coagulopathy, creatinine, or Model for End‐Stage Liver Disease score.
PRECIPITANTS
Initial evaluation of patients with suspected HE must confirm the presence of HE and identify potentially reversible precipitants. Infection, bleeding, and metabolic derangements (including renal injury, hypovolemia, and hyponatremia) are common precipitants.[12] Searching for precipitants is heavily stressed in the 4‐pronged approach recommended by the American Association for the Study of Liver Disease,[7] as summarized in Table 2. Common precipitants are grouped into episodic and recurrent causes. Episodic causes are those that represent discrete insults with specific, short‐term treatments. Recurrent causes are those that are likely to require active management over time. These distinctions may help inform different approaches for initial or recurrent episodes of HE; in practice, much overlap exists.
| |
| 1. Initiate care for cirrhotic patients with altered consciousness | |
| 2. Seek and treat alternative causes of altered mental status if present | |
| 3. Identify and treat precipitating factors: | |
| Episodic | Recurrent |
| Infection | Electrolyte derangement |
| Gastrointestinal bleeding | Infection |
| Hypovolemia | Constipation |
| Electrolyte derangement | Hypovolemia |
| Constipation | Gastrointestinal bleeding |
| 4. Commence empiric HE treatment | |
Diuretic use has been clearly correlated with incidence of HE.[2] Although diuretic usage may be an indicator of more advanced liver disease, their use can also contribute to HE via increased risk of hypovolemia and dysnatremia.[2] Accordingly, caution is necessary when using diuretics to manage patients with HE and refractory ascites. These findings have led some to suggest serial paracentesis may be preferable to diuretics in this population.[2]
MANAGEMENT
The mainstay of HE treatment is administration of the nonabsorbable disaccharide lactulose. Lactulose is part of nearly all regimens because it is effective, easily titrated, and inexpensive.[13] It is efficacious orally or as an enema.[14] Lactulose increases both cognitive function and quality of life,[15] and is effective for prophylaxis and treatment of all stages of HE.[16, 17]
Rifaximin is often used as an adjunct to lactulose, particularly in cases of recurrent HE. Small trials have associated rifaximin with increased quality of life[18] and cognitive function.[19] The largest randomized trial of rifaximin was a double‐blind, placebo‐controlled trial in patients with multiple episodes of overt HE during the prior 6 months.[20] Lactulose was used concomitantly in approximately 91% of patients. At the end of the 6‐month study, rifaximin was associated with a 58% relative risk reduction in overt HE recurrence and roughly 50% reduction in HE‐related hospitalization. The numbers needed to treat were 4 patients to prevent 1 overt HE episode and 9 to prevent 1 HE‐related hospitalization.[20]
A meta‐analysis of 264 patients included in published, high‐quality trials found rifaximin monotherapy to be similar to nonabsorbable disaccharides in both efficacy and incidence of diarrhea, but with significantly less abdominal pain.[21] This analysis was limited by significant heterogeneity among trials. A larger, more recent systematic review and meta‐analysis of 19 studies (both published and unpublished) found rifaximin to be effective for treatment, secondary prophylaxis, and possibly decreased mortality.[22] Of note, this meta‐analysis included placebo studies as well as studies using varying doses of lactulose or other antibiotics as controls. Despite this variability, the authors concluded that the control used in the individual trials did not significantly affect the aggregate results.[22] In the largest individual study to show a mortality benefit, improvement seemed to be driven by decreased rates of sepsis when rifaximin was used as an adjunct to lactulose.[23] Cost is a barrier to use, as rifaximin has not proven to be cost‐effective as monotherapy instead of lactulose.[24] Many insurers will facilitate adjunctive rifaximin with prior authorization, and the manufacturer offers assistance programs.[25]
Other adjuncts, including laxatives,[26] antibiotics,[12] branched‐chain aminoacids,[27] and acarbose[28] have far less evidentiary support and require further study prior to incorporation into clinical practice.[26] A recent study showed polyethylene glycol to perform similar to lactulose, but the studied volume of 4 L daily may make routine use impractical.[29] Dietary protein restriction has been shown in a prospective randomized controlled trial to accelerate body muscle breakdown without affecting HE,[30] so is best avoided.
ISSUES PERTINENT TO HOSPITAL MANAGEMENT
Concurrent HE frequently complicates inpatient management of acute pain. Acetaminophen below 3 g daily for short‐term use is safe,[31] but may be insufficient. Non‐steroidal anti‐inflammatory agents are best avoided given risks for renal dysfunction and bleeding.[32] Although a direct connection between opiate use and HE remains unproven, these agents are problematic because they can cause both sedation and constipation. Nonetheless, they are often needed for pain control. Oxycodone has a more desirable side effect profile than other narcotics. We often prescribe doses every 6 hours initially to account for decreased hepatic metabolism. Morphine has active metabolites that can accumulate in cirrhotics, so morphine use is best avoided.[32] Fluctuations in cognition may help distinguish narcosis from HE; specifically, narcosis causes chronic somnolence worst shortly after an opiate dose, whereas HE causes alterations in sleep‐wake cycles including insomnia.[32] Frequent adjustment of opiate dose and frequency may be required to balance analgesia with unwanted sedation and constipation.
Decisional capacity frequently complicates care of patients with cirrhosis. Patients may decline therapy because of dissatisfaction with bowel frequency, but such lapses in adherence likely contribute to HE recurrence. Patients with overt HE are often incapable of making decisions based on informed consent. If such patients have inadequate social support to ensure medical attention if symptoms progress, then mandatory treatment is reasonable. This may include involuntary administration of medications via rectal or nasogastric tube. Once cognition improves enough that he or she can reliably articulate risks, benefits, and alternatives of declining therapy, then it is reasonable to allow them to do. Subspecialty consultation with psychiatry or ethics may be useful in such situations.
For cirrhotics admitted for management of nonhepatic issues (particularly operations or invasive procedures), vigilance is needed to monitor for HE during hospitalization. Patients with HE have increased risk of falls and impaired driving, which may lead to admission onto surgical services.[4] Changes in diet, medications, bowel function, and environment may all contribute to encephalopathy. HE occurring during admission for other diagnoses still requires prompt titration of lactulose. Routine inquiry about bowel function and sleep quality are likely to help identify trouble early.
Placement of transvenous intrahepatic portosystemic shunt (TIPS) increases the risk for HE via introduction of neurotoxins directly into the systemic circulation. These patients can typically be treated medically,[33] but are likely to require increased lactulose dosage. TIPS revision may be necessary for patients with treatment‐refractory HE, but retrospective evidence suggests this is rarely necessary.[33] In that study, only a single patient out of 81 with post‐TIPS HE required TIPS closure.
Under the International Classification of Disease, 10th Revision, a diagnosis of HE is often most consistent with metabolic encephalopathy (G93.41).[34] It may also be coded as chronic hepatic failure without coma (K7210) or chronic hepatic failure with coma (K7211).[35] Whenever possible, specifying the underlying liver disease (eg, hepatitis C virus, alcohol) is preferable.
TRANSITIONING TO OUTPATIENT CARE
HE patients are usually ready for community living once their cognition has improved enough to reliably take medications. Key aspects of HE management need to be communicated clearly to patients and caregivers. Barriers to optimal outpatient care mostly relate to lactulose adherence. Stressing the direct correlation between insufficient bowel movements and HE progression may enhance adherence. All patients need a lactulose titration plan including when doses can be skipped and when additional doses are needed. Even minimal symptoms of HE need to be addressed,[36] and specific vigilance for alterations in sleep‐wake cycles needs to be adopted. Table 3 is an example of a lactulose titration plan that can be used at discharge. These plans should be included in discharge documents and within communication to outpatient healthcare providers. Close follow‐up with a hepatology specialist is ideal to ensure appropriate lactulose use, answer questions that arise upon return home, and address other concerns related to cirrhosis.
|
| Your dose of lactulose is 30 mL (1 tbsp) 3 times daily with meals. |
| If you have fewer than 3 BMs in any day, take an additional dose of lactulose at bedtime. |
| If you begin to experience difficulty sleeping at night, excessive drowsiness during the day, or confusion, take 2 doses of lactulose with each meal to ensure 3 or more BMs daily. |
| If you have more than 4 BMs in any 24 hour period and are not having any of the symptoms mentioned above, skip a single dose of lactulose then resume your usual schedule. |
Although specific interventions to decrease readmission have not been studied in this population, best practices from other populations (such as medication self‐management, follow‐up plans, and red flags to be on watch for[37]) likely apply. Defining optimal strategies to decrease readmission is an opportunity for hospitalists to contribute to standardization of care for these patients.
CONCLUSIONS
HE is a common but very treatable complication of cirrhosis. Various metabolic insults may precipitate HE, and hospitalists should seek to reverse contributing factors whenever possible. Lactulose titrated to ensure adequate bowel output is the cornerstone of both therapy and prevention for HE. Adjunctive use of rifaximin improves many outcomes. Patient education about manifestations of HE and medication titration is crucial to achieving smooth transition to the outpatient setting.
Disclosure
Nothing to report.
Reversible impairment of brain function in the setting of cirrhosis defines hepatic encephalopathy (HE). HE is associated with significantly decreased survival,[1] and patients with HE have poor outcomes whether HE occurs in isolation or in conjunction with acute‐on‐chronic liver failure.[2] A large multicenter study comparing cirrhotics with and without HE also found that those with a history of HE were hospitalized more frequently.[2]
The presentation of HE is variable, and diagnosis remains clinical. Subtle manifestations of HE persist between episodes, even if gross cognitive function normalizes.[3] Retrospective data suggest the effects of serial bouts of HE may be cumulative, because even with appropriate treatment, the severity of impairment correlates with the number of prior episodes.[3] Even minimal manifestations of hepatic encephalopathy correlate with reduced quality of life.[4]
The West Haven score is the most validated scoring system.[5] Higher grades of HE correlate with significantly increased mortality,[2] but due to difficulties differentiating stages 0 and 1, these criteria remain somewhat controversial. The Spectrum of Neurocognitive Impairment in Cirrhosis (SONIC) has been proposed as an alternate conceptualization of HE as a continuous spectrum rather than discrete stages.[6] Table 1 shows findings associated with various West Haven and SONIC stages. Both systems include covert and overt encephalopathy. Covert correlates with West Haven grades 0 to 1, and consists mainly of subtle findings that require specialized psychometric testing to detect. The SONIC system terms demonstrable but subclinical manifestations minimal HE.[6] Overt HE includes West Haven grades 2 through 4, and refers to objective findings that can be reliably detected on clinical evaluation.[7] Whereas specific numeric scores are used largely for research purposes, classifying HE as covert or overt is clinically useful.
| West Haven Grade | SONIC Classification | Neurologic Changes | Asterixis |
|---|---|---|---|
| |||
| 0 | Normal | None | None |
| Minimal HE | Requires specialized psychometric testing | ||
| 1 | Overt | Decreased attention span, hypersomnia/emnsomnia | Detectable |
| 2 | Lethargy, disorientation | Obvious | |
| 3 | Semistupor or stupor | None | |
| 4 | Coma | None | |
Although blood ammonia levels correlate well across populations, they are not diagnostically useful for individuals, because considerable overlap exists between patients with no HE and those with severe encephalopathy.[8] Ammonia levels also do not predict HE development.[9] Brain imaging is of limited utility, but may be prudent with abrupt decompensation, focal neurologic findings, or poor response to therapy.[10] A recent single‐center review of head computed tomography in cirrhotic patients presenting with altered level of consciousness found a low incidence of intracranial hemorrhage (ICH).[11] The number needed to scan was 293 patients to detect a single ICH. Only 1 patient out of 316 had ICH when fever, trauma, and focal neurological findings were excluded. The presence of acute ICH was not associated with platelet count, coagulopathy, creatinine, or Model for End‐Stage Liver Disease score.
PRECIPITANTS
Initial evaluation of patients with suspected HE must confirm the presence of HE and identify potentially reversible precipitants. Infection, bleeding, and metabolic derangements (including renal injury, hypovolemia, and hyponatremia) are common precipitants.[12] Searching for precipitants is heavily stressed in the 4‐pronged approach recommended by the American Association for the Study of Liver Disease,[7] as summarized in Table 2. Common precipitants are grouped into episodic and recurrent causes. Episodic causes are those that represent discrete insults with specific, short‐term treatments. Recurrent causes are those that are likely to require active management over time. These distinctions may help inform different approaches for initial or recurrent episodes of HE; in practice, much overlap exists.
| |
| 1. Initiate care for cirrhotic patients with altered consciousness | |
| 2. Seek and treat alternative causes of altered mental status if present | |
| 3. Identify and treat precipitating factors: | |
| Episodic | Recurrent |
| Infection | Electrolyte derangement |
| Gastrointestinal bleeding | Infection |
| Hypovolemia | Constipation |
| Electrolyte derangement | Hypovolemia |
| Constipation | Gastrointestinal bleeding |
| 4. Commence empiric HE treatment | |
Diuretic use has been clearly correlated with incidence of HE.[2] Although diuretic usage may be an indicator of more advanced liver disease, their use can also contribute to HE via increased risk of hypovolemia and dysnatremia.[2] Accordingly, caution is necessary when using diuretics to manage patients with HE and refractory ascites. These findings have led some to suggest serial paracentesis may be preferable to diuretics in this population.[2]
MANAGEMENT
The mainstay of HE treatment is administration of the nonabsorbable disaccharide lactulose. Lactulose is part of nearly all regimens because it is effective, easily titrated, and inexpensive.[13] It is efficacious orally or as an enema.[14] Lactulose increases both cognitive function and quality of life,[15] and is effective for prophylaxis and treatment of all stages of HE.[16, 17]
Rifaximin is often used as an adjunct to lactulose, particularly in cases of recurrent HE. Small trials have associated rifaximin with increased quality of life[18] and cognitive function.[19] The largest randomized trial of rifaximin was a double‐blind, placebo‐controlled trial in patients with multiple episodes of overt HE during the prior 6 months.[20] Lactulose was used concomitantly in approximately 91% of patients. At the end of the 6‐month study, rifaximin was associated with a 58% relative risk reduction in overt HE recurrence and roughly 50% reduction in HE‐related hospitalization. The numbers needed to treat were 4 patients to prevent 1 overt HE episode and 9 to prevent 1 HE‐related hospitalization.[20]
A meta‐analysis of 264 patients included in published, high‐quality trials found rifaximin monotherapy to be similar to nonabsorbable disaccharides in both efficacy and incidence of diarrhea, but with significantly less abdominal pain.[21] This analysis was limited by significant heterogeneity among trials. A larger, more recent systematic review and meta‐analysis of 19 studies (both published and unpublished) found rifaximin to be effective for treatment, secondary prophylaxis, and possibly decreased mortality.[22] Of note, this meta‐analysis included placebo studies as well as studies using varying doses of lactulose or other antibiotics as controls. Despite this variability, the authors concluded that the control used in the individual trials did not significantly affect the aggregate results.[22] In the largest individual study to show a mortality benefit, improvement seemed to be driven by decreased rates of sepsis when rifaximin was used as an adjunct to lactulose.[23] Cost is a barrier to use, as rifaximin has not proven to be cost‐effective as monotherapy instead of lactulose.[24] Many insurers will facilitate adjunctive rifaximin with prior authorization, and the manufacturer offers assistance programs.[25]
Other adjuncts, including laxatives,[26] antibiotics,[12] branched‐chain aminoacids,[27] and acarbose[28] have far less evidentiary support and require further study prior to incorporation into clinical practice.[26] A recent study showed polyethylene glycol to perform similar to lactulose, but the studied volume of 4 L daily may make routine use impractical.[29] Dietary protein restriction has been shown in a prospective randomized controlled trial to accelerate body muscle breakdown without affecting HE,[30] so is best avoided.
ISSUES PERTINENT TO HOSPITAL MANAGEMENT
Concurrent HE frequently complicates inpatient management of acute pain. Acetaminophen below 3 g daily for short‐term use is safe,[31] but may be insufficient. Non‐steroidal anti‐inflammatory agents are best avoided given risks for renal dysfunction and bleeding.[32] Although a direct connection between opiate use and HE remains unproven, these agents are problematic because they can cause both sedation and constipation. Nonetheless, they are often needed for pain control. Oxycodone has a more desirable side effect profile than other narcotics. We often prescribe doses every 6 hours initially to account for decreased hepatic metabolism. Morphine has active metabolites that can accumulate in cirrhotics, so morphine use is best avoided.[32] Fluctuations in cognition may help distinguish narcosis from HE; specifically, narcosis causes chronic somnolence worst shortly after an opiate dose, whereas HE causes alterations in sleep‐wake cycles including insomnia.[32] Frequent adjustment of opiate dose and frequency may be required to balance analgesia with unwanted sedation and constipation.
Decisional capacity frequently complicates care of patients with cirrhosis. Patients may decline therapy because of dissatisfaction with bowel frequency, but such lapses in adherence likely contribute to HE recurrence. Patients with overt HE are often incapable of making decisions based on informed consent. If such patients have inadequate social support to ensure medical attention if symptoms progress, then mandatory treatment is reasonable. This may include involuntary administration of medications via rectal or nasogastric tube. Once cognition improves enough that he or she can reliably articulate risks, benefits, and alternatives of declining therapy, then it is reasonable to allow them to do. Subspecialty consultation with psychiatry or ethics may be useful in such situations.
For cirrhotics admitted for management of nonhepatic issues (particularly operations or invasive procedures), vigilance is needed to monitor for HE during hospitalization. Patients with HE have increased risk of falls and impaired driving, which may lead to admission onto surgical services.[4] Changes in diet, medications, bowel function, and environment may all contribute to encephalopathy. HE occurring during admission for other diagnoses still requires prompt titration of lactulose. Routine inquiry about bowel function and sleep quality are likely to help identify trouble early.
Placement of transvenous intrahepatic portosystemic shunt (TIPS) increases the risk for HE via introduction of neurotoxins directly into the systemic circulation. These patients can typically be treated medically,[33] but are likely to require increased lactulose dosage. TIPS revision may be necessary for patients with treatment‐refractory HE, but retrospective evidence suggests this is rarely necessary.[33] In that study, only a single patient out of 81 with post‐TIPS HE required TIPS closure.
Under the International Classification of Disease, 10th Revision, a diagnosis of HE is often most consistent with metabolic encephalopathy (G93.41).[34] It may also be coded as chronic hepatic failure without coma (K7210) or chronic hepatic failure with coma (K7211).[35] Whenever possible, specifying the underlying liver disease (eg, hepatitis C virus, alcohol) is preferable.
TRANSITIONING TO OUTPATIENT CARE
HE patients are usually ready for community living once their cognition has improved enough to reliably take medications. Key aspects of HE management need to be communicated clearly to patients and caregivers. Barriers to optimal outpatient care mostly relate to lactulose adherence. Stressing the direct correlation between insufficient bowel movements and HE progression may enhance adherence. All patients need a lactulose titration plan including when doses can be skipped and when additional doses are needed. Even minimal symptoms of HE need to be addressed,[36] and specific vigilance for alterations in sleep‐wake cycles needs to be adopted. Table 3 is an example of a lactulose titration plan that can be used at discharge. These plans should be included in discharge documents and within communication to outpatient healthcare providers. Close follow‐up with a hepatology specialist is ideal to ensure appropriate lactulose use, answer questions that arise upon return home, and address other concerns related to cirrhosis.
|
| Your dose of lactulose is 30 mL (1 tbsp) 3 times daily with meals. |
| If you have fewer than 3 BMs in any day, take an additional dose of lactulose at bedtime. |
| If you begin to experience difficulty sleeping at night, excessive drowsiness during the day, or confusion, take 2 doses of lactulose with each meal to ensure 3 or more BMs daily. |
| If you have more than 4 BMs in any 24 hour period and are not having any of the symptoms mentioned above, skip a single dose of lactulose then resume your usual schedule. |
Although specific interventions to decrease readmission have not been studied in this population, best practices from other populations (such as medication self‐management, follow‐up plans, and red flags to be on watch for[37]) likely apply. Defining optimal strategies to decrease readmission is an opportunity for hospitalists to contribute to standardization of care for these patients.
CONCLUSIONS
HE is a common but very treatable complication of cirrhosis. Various metabolic insults may precipitate HE, and hospitalists should seek to reverse contributing factors whenever possible. Lactulose titrated to ensure adequate bowel output is the cornerstone of both therapy and prevention for HE. Adjunctive use of rifaximin improves many outcomes. Patient education about manifestations of HE and medication titration is crucial to achieving smooth transition to the outpatient setting.
Disclosure
Nothing to report.
- , , , et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30(5):890–895.
- , , , et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute‐on‐chronic liver failure (ACLF). J Hepatol. 2014;60(2):275–281.
- , , , et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138(7):2332–2340.
- , , . Minimal hepatic encephalopathy impairs quality of life. J Clin Exp Hepatol. 2015;5(suppl 1):S42–S48.
- , ; Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol. 2001;96(7):1968–1976.
- , , . Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology. 2009;50(6):2014–2021.
- , , , et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–735.
- , , , et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114(3):188–193.
- , . Serum ammonia level for the evaluation of hepatic encephalopathy. JAMA. 2014;312(6):643–644.
- , , . Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute‐on‐chronic liver failure. J Hepatol. 2015;62(2):437–447.
- , , , et al. Low Likelihood of intracranial hemorrhage in patients with cirrhosis and altered mental status. Clin Gastroenterol Hepatol. 2015;13(1):165–169.
- , . The management of hospitalized patients with cirrhosis: the Mount Sinai experience and a guide for hospitalists. Dig Dis Sci. 2011;56(5):1266–1281.
- , , . Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004(2):CD003044.
- , , , et al. Acidifying enemas (lactitol and lactose) vs. nonacidifying enemas (tap water) to treat acute portal‐systemic encephalopathy: a double‐blind, randomized clinical trial. Hepatology. 1987;7(4):639–643.
- , . Disaccharides in the treatment of hepatic encephalopathy. Metab Brain Dis. 2013;28(2):313–320.
- , , , . Secondary prophylaxis of hepatic encephalopathy: an open‐label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137(3):885–891, 91.e1.
- , , , , , . Efficacy of lactulose in cirrhotic patients with subclinical hepatic encephalopathy. Dig Dis Sci. 2000;45(8):1549–1552.
- , , , et al. Randomised clinical trial: rifaximin improves health‐related quality of life in cirrhotic patients with hepatic encephalopathy—a double‐blind placebo‐controlled study. Aliment Pharmacol Ther. 2011;34(8):853–861.
- , , , , , . Rifaximin improves psychometric performance and health‐related quality of life in patients with minimal hepatic encephalopathy (the RIME Trial). Am J Gastroenterol. 2011;106(2):307–316.
- , , , et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071–1081.
- , , , , , . Rifaximin versus nonabsorbable disaccharides in the management of hepatic encephalopathy: a meta‐analysis. Eur J Gastroenterol Hepatol. 2008;20(11):1064–1070.
- , , , , . Systematic review with meta‐analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther. 2014;40(2):123–132.
- , , , , , . A randomized, double‐blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108(9):1458–1463.
- , , . The cost‐effectiveness and budget impact of competing therapies in hepatic encephalopathy—a decision analysis. Aliment Pharmacol Ther. 2007;26(8):1147–1161.
- Salix Pharmaceuticals. Patient assistance program. Available at: http://www.salix.com/about‐us/corporate‐responsibility/patient‐medication‐assistance. Accessed October 24, 2015.
- , . Management of overt hepatic encephalopathy. J Clin Exp Hepatol. 2015;5(suppl 1):S82–S87.
- , , , . Parenteral nutrition with branched‐chain amino acids in hepatic encephalopathy. A meta‐analysis. Gastroenterology. 1989;97(4):1033–1042.
- , , , et al. A randomized controlled trial of acarbose in hepatic encephalopathy. Clin Gastroenterol Hepatol. 2005;3(2):184–191.
- , , , . Lactulose vs polyethylene glycol 3350‐‐electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomized clinical trial. JAMA Intern Med. 2014;174(11):1727–1733.
- , , , et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41(1):38–43.
- , , . The therapeutic use of acetaminophen in patients with liver disease. Am J Ther. 2005;12(2):133–141.
- , . Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc. 2010;85(5):451–458.
- , , , et al. Clearing the confusion over hepatic encephalopathy after TIPS creation: incidence, prognostic factors, and clinical outcomes. Dig Dis Sci. 2015;60(4):1059–66.
- Centers for Medicare and Medicaid Services. ICD‐10 code lookup: encephalopathy. Available at: https://www.cms.gov/medicare‐coverage‐database/staticpages/icd‐10‐code‐lookup.aspx?KeyWord=encephalopathy5(suppl 1):S75–S81.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828.
- , , , et al. Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol. 1999;30(5):890–895.
- , , , et al. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute‐on‐chronic liver failure (ACLF). J Hepatol. 2014;60(2):275–281.
- , , , et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138(7):2332–2340.
- , , . Minimal hepatic encephalopathy impairs quality of life. J Clin Exp Hepatol. 2015;5(suppl 1):S42–S48.
- , ; Practice Parameters Committee of the American College of Gastroenterology. Hepatic encephalopathy. Am J Gastroenterol. 2001;96(7):1968–1976.
- , , . Spectrum of neurocognitive impairment in cirrhosis: Implications for the assessment of hepatic encephalopathy. Hepatology. 2009;50(6):2014–2021.
- , , , et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–735.
- , , , et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med. 2003;114(3):188–193.
- , . Serum ammonia level for the evaluation of hepatic encephalopathy. JAMA. 2014;312(6):643–644.
- , , . Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute‐on‐chronic liver failure. J Hepatol. 2015;62(2):437–447.
- , , , et al. Low Likelihood of intracranial hemorrhage in patients with cirrhosis and altered mental status. Clin Gastroenterol Hepatol. 2015;13(1):165–169.
- , . The management of hospitalized patients with cirrhosis: the Mount Sinai experience and a guide for hospitalists. Dig Dis Sci. 2011;56(5):1266–1281.
- , , . Nonabsorbable disaccharides for hepatic encephalopathy. Cochrane Database Syst Rev. 2004(2):CD003044.
- , , , et al. Acidifying enemas (lactitol and lactose) vs. nonacidifying enemas (tap water) to treat acute portal‐systemic encephalopathy: a double‐blind, randomized clinical trial. Hepatology. 1987;7(4):639–643.
- , . Disaccharides in the treatment of hepatic encephalopathy. Metab Brain Dis. 2013;28(2):313–320.
- , , , . Secondary prophylaxis of hepatic encephalopathy: an open‐label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137(3):885–891, 91.e1.
- , , , , , . Efficacy of lactulose in cirrhotic patients with subclinical hepatic encephalopathy. Dig Dis Sci. 2000;45(8):1549–1552.
- , , , et al. Randomised clinical trial: rifaximin improves health‐related quality of life in cirrhotic patients with hepatic encephalopathy—a double‐blind placebo‐controlled study. Aliment Pharmacol Ther. 2011;34(8):853–861.
- , , , , , . Rifaximin improves psychometric performance and health‐related quality of life in patients with minimal hepatic encephalopathy (the RIME Trial). Am J Gastroenterol. 2011;106(2):307–316.
- , , , et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071–1081.
- , , , , , . Rifaximin versus nonabsorbable disaccharides in the management of hepatic encephalopathy: a meta‐analysis. Eur J Gastroenterol Hepatol. 2008;20(11):1064–1070.
- , , , , . Systematic review with meta‐analysis: the effects of rifaximin in hepatic encephalopathy. Aliment Pharmacol Ther. 2014;40(2):123–132.
- , , , , , . A randomized, double‐blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108(9):1458–1463.
- , , . The cost‐effectiveness and budget impact of competing therapies in hepatic encephalopathy—a decision analysis. Aliment Pharmacol Ther. 2007;26(8):1147–1161.
- Salix Pharmaceuticals. Patient assistance program. Available at: http://www.salix.com/about‐us/corporate‐responsibility/patient‐medication‐assistance. Accessed October 24, 2015.
- , . Management of overt hepatic encephalopathy. J Clin Exp Hepatol. 2015;5(suppl 1):S82–S87.
- , , , . Parenteral nutrition with branched‐chain amino acids in hepatic encephalopathy. A meta‐analysis. Gastroenterology. 1989;97(4):1033–1042.
- , , , et al. A randomized controlled trial of acarbose in hepatic encephalopathy. Clin Gastroenterol Hepatol. 2005;3(2):184–191.
- , , , . Lactulose vs polyethylene glycol 3350‐‐electrolyte solution for treatment of overt hepatic encephalopathy: the HELP randomized clinical trial. JAMA Intern Med. 2014;174(11):1727–1733.
- , , , et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41(1):38–43.
- , , . The therapeutic use of acetaminophen in patients with liver disease. Am J Ther. 2005;12(2):133–141.
- , . Pain management in the cirrhotic patient: the clinical challenge. Mayo Clin Proc. 2010;85(5):451–458.
- , , , et al. Clearing the confusion over hepatic encephalopathy after TIPS creation: incidence, prognostic factors, and clinical outcomes. Dig Dis Sci. 2015;60(4):1059–66.
- Centers for Medicare and Medicaid Services. ICD‐10 code lookup: encephalopathy. Available at: https://www.cms.gov/medicare‐coverage‐database/staticpages/icd‐10‐code‐lookup.aspx?KeyWord=encephalopathy5(suppl 1):S75–S81.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828.
Therapies to Improve the Cosmetic Symptoms of Atopic Dermatitis
Atopic dermatitis (AD), more commonly referred to as eczema, is a chronic pruritic inflammatory skin disease that frequently affects both children and adults. Atopic dermatitis is most common in urban and developed countries, with a prevalence of approximately 11% in the United States.1 The pathophysiology of AD is complex and not fully understood, despite the increasing incidence of the disease.2 A myriad of factors, including genetics, defects in the innate and adaptive immune response, and skin barrier abnormalities all contribute to the pathogenesis.3,4 As a result of these abnormalities, patients with AD are more prone to damage from environmental irritants and allergens.
The diagnosis of AD is made clinically based on patient history and visual assessment of the skin.5 Atopic dermatitis follows a chronic and relapsing course characterized by severe pruritus and visible skin changes including xerosis, redness, blistering, oozing, crusting, scaling, thickening, and color change.6,7 Due to the genetic predisposition to make IgE antibodies in response to common environmental and food antigens, patients also may develop allergic rhinitis, asthma, and food-induced anaphylaxis.8,9 Patients also are susceptible to cutaneous viral, fungal, and bacterial infections, the most common of which is an infection with Staphylococcus aureus.10
Atopic dermatitis can have a substantial impact on quality of life, which has been revealed in studies linking chronic skin conditions to depression, impairment of self-esteem, and financial hardship.11 Because skin appearance impacts how a person is initially perceived by others, patients often report feeling self-conscious about their disease and experience teasing or bullying.12 To improve their physical appearance, patients may incur considerable medical expenses. According to 2 population-based studies comprising more than 60,000 adults aged 18 to 85 years, individuals with AD face substantial financial burdens and utilize the health care system more than those without the disease. On average, patients with AD spend $371 to $489 per year on costly out-of-pocket medical expenses and report more absences from work.13
Although there currently is no cure for AD, treatment is aimed at relieving its symptoms and preventing acute exacerbations as well as improving cosmetic appearance to enhance quality of life. Treatment must follow a stepwise approach, which focuses on hydrating the skin, repairing the dysfunctional epithelial barrier, and controlling inflammation. Thus, the standard of care focuses on avoiding skin irritants and triggers along with the use of moisturizers and topical corticosteroids (TCs). In patients with recurring severe disease, topical calcineurin inhibitors, phototherapy, and systemic agents also may be utilized.14
Avoiding Irritants and Triggers
Atopic dermatitis is worsened by skin contact with physical and chemical irritants. Exacerbating factors in AD include exposure to food allergens, dust, emotional stress, detergents, fragranced soaps, textiles, and ingredients in cosmetic products. Patients should be advised to use mild detergents and fragrance-free soaps and to avoid harsh materials such as wool. However, avoidance of specific ingredients in cosmetic products is not as straightforward because manufacturers are not required to disclose certain ingredients. In general, fragrances such as balsam of Peru and cinnamaldehyde, as well as preservatives such as parabens, isothiazolinones, and formaldehyde, should be avoided when selecting cosmetic products. Patients with AD should purchase fragrance-free products that are specifically formulated for sensitive skin. Additionally, patients should not apply makeup if their skin is irritated or oozing, as the flare may worsen.15
Moisturizers
Due to the impaired skin barrier function in patients with AD, regular application of fragrance-free moisturizers is essential to maintain hydration and to reduce xerosis. Various classes of moisturizers may be prescribed (eg, lotions, creams, gels, ointments) based on disease severity and patient preference. Light preparations such as lotions, creams, and gels have a high water content and generally are more appealing from a cosmetic standpoint because they do not create any residue on the skin. However, these options may require more frequent application because they are absorbed quickly. Heavy preparations such as ointments have longer-lasting effects due to their high oil content but tend to be less cosmetically appealing because of their greasiness.16
Although the amount and frequency of application of moisturizers has not been defined, liberal application several times daily is generally advised to minimize xerosis.17 Most physicians recommend applying moisturizer to the skin immediately after bathing to seal in moisture. Some patients prefer to use lotions and creams during the day because these products make the skin feel smooth and reserve the greasier ointments for nighttime application.
Topical Corticosteroids
Prescribed in conjunction with moisturizers, TCs are the mainstay of anti-inflammatory therapy in AD. Topical corticosteroids are classified into 7 groups based on potency, ranging from superpotent (class 1) to least potent (class 7). For acute AD flares, TCs should be applied daily for up to several weeks. Once the inflammation has resolved, it is recommended to apply TCs once to twice weekly to reduce the rate of relapse.18 Despite their effectiveness in the treatment of acute AD flares, TCs have a considerable side-effect profile. Potential adverse effects include skin atrophy, striae, telangiectasia, hypopigmentation, increased hair growth, steroid acne, growth retardation, and Cushing syndrome. Skin atrophy, which is the most common complication associated with TCs, results in shiny transparent skin, allowing for visualization of veins.19,20 Although many of these side effects will resolve after discontinuing the TCs, they are aesthetically displeasing during treatment, making it crucial for physicians to educate their patients on the proper usage of TCs to prevent negative outcomes.
Topical Calcineurin Inhibitors
Topical calcineurin inhibitors (TCIs) are a class of anti-inflammatories that are used to overcome the adverse effects of TCs. They are approved as alternatives to TCs in patients who have failed to respond to other topical treatments as well as those who have developed cutaneous atrophy from the use of TCs or have AD in sensitive areas such as the face, neck, and/or skin folds. Unlike TCs, TCIs do not cause atrophy, striae, or discoloration of the skin, which makes them more desirable from a cosmetic perspective. Their mechanism of action is distinct from TCs in that they inhibit calcineurin-dependent T-cell activation, thus preventing the transcription of inflammatory cytokines.21 Two TCIs are currently available: tacrolimus ointment 0.03% and 0.1% concentrations for moderate to severe AD and pimecrolimus cream 1% for mild to moderate AD.22 Twice-daily application of TCIs is recommended to decrease inflammation and pruritus associated with AD. Studies also have shown that intermittent use of TCIs 3 times weekly can aid in reducing relapses.23-25
The results from clinical trials demonstrate the rapid and continuous effects of both pimecrolimus and tacrolimus. In a controlled long-term study of adults, pimecrolimus provided significant relief of pruritus as soon as day 3 (P<.001).26,27 Pimecrolimus also provides long-term relief by preventing disease progression to flares, which was exemplified in a study (N=713) with no flares in 51% of pimecrolimus patients at 12 months versus 28% in the conventional treatment group (P<.001).28 Similarly, long-term studies of tacrolimus demonstrated an improvement of all symptoms of AD after 1 week of treatment. Maximal improvement was achieved with continued use of tacrolimus, and up to 1 year of tacrolimus use was found to be safe and effective.29,30 Thus, TCIs have been proven to be an effective choice in maintenance therapy for AD and have a good safety profile. The most common adverse effects of TCIs are local skin reactions, such as stinging and burning at the site of application. Rare cases of skin cancer and lymphoma have been reported; however, a causal relationship has yet to be established.31,32
Additional Therapies
Wet wrap therapy is effective for rapid control of flares and in controlling recalcitrant AD. Wet wraps function via several mechanisms; they provide a mechanical barrier against scratching, increase moisture and soften the skin, and enhance absorption of topical medications.33,34 The following method is employed when using wet wraps: an emollient or TC is applied to the area, a tubular bandage soaked in warm water is wrapped over the area, and dry bandages are used to form the outermost layer. Although wet wrap therapy is beneficial in treating AD, it is labor intensive and may require the expertise of a nurse. Thus, unlike other therapies, which patients can easily apply without interfering with their day, wet wraps must be applied at home or in a hospital setting.
Light therapy is another effective method of controlling AD. Although multiple forms of UV phototherapy are beneficial for symptom control in AD, there is no definitive recommendation regarding the specific type of light therapy due to a lack of comparative studies. Natural sunlight, narrowband UVB, broadband UVB, UVA, oral or topical psoralen plus UVA, as well as UVA and UVB can all be utilized in the treatment of AD. However, similar to natural sunlight, artificial light therapy can cause burning, blistering, hyperpigmentation, dark spots, and wrinkles. Because society places a large emphasis on maintaining a youthful appearance, patients may be hesitant to use a treatment that could potentially advance the skin’s aging process. Thus, it is important that this therapy is properly controlled to prevent further skin damage.35-37
When optimal topical regimens and phototherapy have failed to control AD, systemic immunomodulation therapies may be used. Currently, the most commonly used medications are cyclosporine 150 to 300 mg daily, methotrexate 7.5 to 25 mg weekly, mycophenolate mofetil 0.5 to 3 g daily, and azathioprine 1 to 3 mg/kg daily.38,39 Decisions regarding the specific class of drugs should be based on the patient’s AD status, comorbidities, and personal preference.
Conclusion
Atopic dermatitis is a common chronic condition that can occur at any age and cause substantial physical, psychological, social, and/or emotional stress for patients and their families. Although TCs have been the standard of treatment for many years, ongoing concerns regarding their safety have led to the use of TCIs, which overcome some of the drawbacks of steroid therapy. Phototherapy and systemic immunosuppressant therapy are reserved for patients who have not responded to optimal topical therapies. Although several therapeutic avenues exist for patients, there is a need for the development of more effective and safer drugs. Furthermore, cosmetic products created specifically for patients with AD would be beneficial, as patients often struggle to select products that do not cause more harm than good. Given the complexity of the pathogenesis of AD, further research must focus on defining the specific pathways involved in the disease and targeting these pathways with therapies.
1. Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
2. Deckers IA, McLean S, Linssen S, et al. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS One. 2012;7:e39803.
3. Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233-246.
4. Peate I. Eczema: causes, symptoms and treatment in the community. Br J Community Nurs. 2011;16:324, 326-331.
5. Williams HC, Burney PG, Pembroke AC, et al. The U.K. Working Party’s diagnostic criteria for atopic dermatitis. III. independent hospital validation. Br J Dermatol. 1994;131:406-416.
6. Magin P, Adams J, Heading G, et al. Experiences of appearance-related teasing and bullying in skin diseases and their psychological sequelae: results of a qualitative study. Scand J Caring Sci. 2008;22:430-436.
7. Beattie P, Lewis-Jones M. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155:145-151.
8. Spergel JM. From atopic dermatitis to asthma: the atopic march [published online January 22, 2010]. Ann Allergy Asthma Immunol. 2010;105:99-106; quiz 107-109, 117.
9. Leung DY. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int. 2013;62:151-161.
10. Balma-Mena A, Lara-Corrales I, Zeller J, et al. Colonization with community-acquired methicillin-resistant Staphylococcus aureus in children with atopic dermatitis: a cross-sectional study. Int J Dermatol. 2011;50:682-688.
11. Strawser MS, Storch EA, Roberti JW. The Teasing Questionnaire-Revised: measurement of childhood teasing in adults. J Anxiety Disord. 2005;19:780-792.
12. Magin P, Adams J, Heading G, et al. Experiences of appearance-related teasing and bullying in skin diseases and their psychological sequelae: results of a qualitative study. Scand J Caring Sci. 2008;22:430-436.
13. Silverberg J. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
14. Ellis C, Luger T, Abeck D, et al. International Consensus Conference on Atopic Dermatitis II (ICCAD II): clinical update and current treatment strategies. Br J Dermatol. 2003;148(suppl 63):3-10.
15. Kim K. Influences of environmental chemicals on atopic dermatitis. Toxicol Res. 2015;31:89-96.
16. Ridd M, Redmond N, Hollinghurst S, et al. Choice of Moisturiser for Eczema Treatment (COMET): study protocol for a randomized controlled trial. Trials. 2015;16:304.
17. Hon KL, Ching GK, Leung TF, et al. Estimating emollient usage in patients with eczema. Clin Exp Dermatol. 2010;35:22-26.
18. Hanifin J, Gupta AK, Rajagopalan R. Intermittent dosing of fluticasone propionate cream for reducing the risk of relapse in atopic dermatitis patients. Br J Dermatol. 2002;147:528-537.
19. Hill CJ, Rostenberg A Jr. Adverse effects from topical steroids. Cutis. 1978;21:624-628.
20. Ruiz-Maldonado R, Zapata G, Lourdes T, et al. Cushing’s syndrome after topical application of corticosteroids. Am J Dis Child. 1982;136:274-275.
21. Grassberger M, Baumruker T, Enz A, et al. A novel anti-inflammatory drug, SDZ ASM 981, for the treatment of skin diseases: in vitro pharmacology. Br J Dermatol. 1999;141:264-273.
22. Eichenfield L, Wynnis T, Berger T. Guidelines of care for the management of atopic dermatitis: management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
23. Reitamo S, Harper J, Bos JD, et al. 0.03% Tacrolimus ointment applied once or twice daily is more efficacious than 1% hydrocortisone acetate in children with moderate to severe atopic dermatitis: results of a randomized double-blind controlled trial. Br J Dermatol. 2004;150:554-562.
24. Ruer-Mulard M, Aberer W, Gunstone A, et al. Twice-daily versus once-daily applications of pimecrolimus cream 1% for the prevention of disease relapse in pediatric patients with atopic dermatitis. Pediatr Dermatol. 2009;26:551-558.
25. Breneman D, Fleischer AB Jr, Abramovits W, et al. Intermittent therapy for flare prevention and long-term disease control in stabilized atopic dermatitis: a randomized comparison of 3-times-weekly applications of tacrolimus ointment versus vehicle. J Am Acad Dermatol. 2008;58:990-999.
26. Meurer M, Fölster-Holst R, Wozel G, et al. Pimecrolimus cream 1% (Elidel) provides significant and rapid relief of pruritus and improves disease control and quality of life in atopic dermatitis in adults. J Invest Dermatol. 2002;119:350.
27. Meurer M, Fölster-Holst R, Wozel G, et al. Pimecrolimus cream in the long-term management of atopic dermatitis in adults: a six-month study. Dermatology. 2002;205:271-277.
28. Wahn U, Bos JD, Goodfield M, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics. 2002;110(1, pt 1):e2.
29. Kang S, Lucky AW, Pariser D, et al. Long-term safety and efficacy of tacrolimus ointment for the treatment of atopic dermatitis in children. J Am Acad Dermatol. 2001;44(suppl 1):S58-S64.
30. Reitamo S, Wollenberg A, Schöpf E, et al. Safety and efficacy of 1 year of tacrolimus ointment monotherapy in adults with atopic dermatitis. the European Tacrolimus Ointment Study Group. Arch Dermatol. 2000;136:999-1006.
31. Frankel HC, Qureshi AA. Comparative effectiveness of topical calcineurin inhibitors in adult patients with atopic dermatitis. Am J Clin Dermatol. 2012;13:113-123.
32. Tennis P, Gelfand JM, Rothman KJ. Evaluation of cancer risk related to atopic dermatitis and use of topical calcineurin inhibitors. Br J Dermatol. 2011;165:465-473.
33. Dabade TS, Davis DM, Wetter DA, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2012;67:100-106.
34. Devillers AC, Oranje AP. Efficacy and safety of ‘wet-wrap’ dressings as an intervention treatment in children with severe and/or refractory atopic dermatitis: a critical review of the literature. Br J Dermatol. 2006;154:579-585.
35. Meduri NB, Vandergriff T, Rasmussen H, et al. Phototherapy in the management of atopic dermatitis: a systematic review. Photodermatol Photoimmunol Photomed. 2007;23:106-112.
36. Clayton TH, Clark SM, Turner D, et al. The treatment of severe atopic dermatitis in childhood with narrowband ultraviolet B phototherapy. Clin Exp Dermatol. 2007;32:28-33.
37. Jekler J, Larko O. UVB phototherapy of atopic dermatitis. Br J Dermatol. 1988;119:697-705.
38. Roekevisch E, Spuls PI, Kuester D, et al. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133:429-438.39. Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess. 2000;4:1-191.
39. Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess. 2000;4:1-191.
Atopic dermatitis (AD), more commonly referred to as eczema, is a chronic pruritic inflammatory skin disease that frequently affects both children and adults. Atopic dermatitis is most common in urban and developed countries, with a prevalence of approximately 11% in the United States.1 The pathophysiology of AD is complex and not fully understood, despite the increasing incidence of the disease.2 A myriad of factors, including genetics, defects in the innate and adaptive immune response, and skin barrier abnormalities all contribute to the pathogenesis.3,4 As a result of these abnormalities, patients with AD are more prone to damage from environmental irritants and allergens.
The diagnosis of AD is made clinically based on patient history and visual assessment of the skin.5 Atopic dermatitis follows a chronic and relapsing course characterized by severe pruritus and visible skin changes including xerosis, redness, blistering, oozing, crusting, scaling, thickening, and color change.6,7 Due to the genetic predisposition to make IgE antibodies in response to common environmental and food antigens, patients also may develop allergic rhinitis, asthma, and food-induced anaphylaxis.8,9 Patients also are susceptible to cutaneous viral, fungal, and bacterial infections, the most common of which is an infection with Staphylococcus aureus.10
Atopic dermatitis can have a substantial impact on quality of life, which has been revealed in studies linking chronic skin conditions to depression, impairment of self-esteem, and financial hardship.11 Because skin appearance impacts how a person is initially perceived by others, patients often report feeling self-conscious about their disease and experience teasing or bullying.12 To improve their physical appearance, patients may incur considerable medical expenses. According to 2 population-based studies comprising more than 60,000 adults aged 18 to 85 years, individuals with AD face substantial financial burdens and utilize the health care system more than those without the disease. On average, patients with AD spend $371 to $489 per year on costly out-of-pocket medical expenses and report more absences from work.13
Although there currently is no cure for AD, treatment is aimed at relieving its symptoms and preventing acute exacerbations as well as improving cosmetic appearance to enhance quality of life. Treatment must follow a stepwise approach, which focuses on hydrating the skin, repairing the dysfunctional epithelial barrier, and controlling inflammation. Thus, the standard of care focuses on avoiding skin irritants and triggers along with the use of moisturizers and topical corticosteroids (TCs). In patients with recurring severe disease, topical calcineurin inhibitors, phototherapy, and systemic agents also may be utilized.14
Avoiding Irritants and Triggers
Atopic dermatitis is worsened by skin contact with physical and chemical irritants. Exacerbating factors in AD include exposure to food allergens, dust, emotional stress, detergents, fragranced soaps, textiles, and ingredients in cosmetic products. Patients should be advised to use mild detergents and fragrance-free soaps and to avoid harsh materials such as wool. However, avoidance of specific ingredients in cosmetic products is not as straightforward because manufacturers are not required to disclose certain ingredients. In general, fragrances such as balsam of Peru and cinnamaldehyde, as well as preservatives such as parabens, isothiazolinones, and formaldehyde, should be avoided when selecting cosmetic products. Patients with AD should purchase fragrance-free products that are specifically formulated for sensitive skin. Additionally, patients should not apply makeup if their skin is irritated or oozing, as the flare may worsen.15
Moisturizers
Due to the impaired skin barrier function in patients with AD, regular application of fragrance-free moisturizers is essential to maintain hydration and to reduce xerosis. Various classes of moisturizers may be prescribed (eg, lotions, creams, gels, ointments) based on disease severity and patient preference. Light preparations such as lotions, creams, and gels have a high water content and generally are more appealing from a cosmetic standpoint because they do not create any residue on the skin. However, these options may require more frequent application because they are absorbed quickly. Heavy preparations such as ointments have longer-lasting effects due to their high oil content but tend to be less cosmetically appealing because of their greasiness.16
Although the amount and frequency of application of moisturizers has not been defined, liberal application several times daily is generally advised to minimize xerosis.17 Most physicians recommend applying moisturizer to the skin immediately after bathing to seal in moisture. Some patients prefer to use lotions and creams during the day because these products make the skin feel smooth and reserve the greasier ointments for nighttime application.
Topical Corticosteroids
Prescribed in conjunction with moisturizers, TCs are the mainstay of anti-inflammatory therapy in AD. Topical corticosteroids are classified into 7 groups based on potency, ranging from superpotent (class 1) to least potent (class 7). For acute AD flares, TCs should be applied daily for up to several weeks. Once the inflammation has resolved, it is recommended to apply TCs once to twice weekly to reduce the rate of relapse.18 Despite their effectiveness in the treatment of acute AD flares, TCs have a considerable side-effect profile. Potential adverse effects include skin atrophy, striae, telangiectasia, hypopigmentation, increased hair growth, steroid acne, growth retardation, and Cushing syndrome. Skin atrophy, which is the most common complication associated with TCs, results in shiny transparent skin, allowing for visualization of veins.19,20 Although many of these side effects will resolve after discontinuing the TCs, they are aesthetically displeasing during treatment, making it crucial for physicians to educate their patients on the proper usage of TCs to prevent negative outcomes.
Topical Calcineurin Inhibitors
Topical calcineurin inhibitors (TCIs) are a class of anti-inflammatories that are used to overcome the adverse effects of TCs. They are approved as alternatives to TCs in patients who have failed to respond to other topical treatments as well as those who have developed cutaneous atrophy from the use of TCs or have AD in sensitive areas such as the face, neck, and/or skin folds. Unlike TCs, TCIs do not cause atrophy, striae, or discoloration of the skin, which makes them more desirable from a cosmetic perspective. Their mechanism of action is distinct from TCs in that they inhibit calcineurin-dependent T-cell activation, thus preventing the transcription of inflammatory cytokines.21 Two TCIs are currently available: tacrolimus ointment 0.03% and 0.1% concentrations for moderate to severe AD and pimecrolimus cream 1% for mild to moderate AD.22 Twice-daily application of TCIs is recommended to decrease inflammation and pruritus associated with AD. Studies also have shown that intermittent use of TCIs 3 times weekly can aid in reducing relapses.23-25
The results from clinical trials demonstrate the rapid and continuous effects of both pimecrolimus and tacrolimus. In a controlled long-term study of adults, pimecrolimus provided significant relief of pruritus as soon as day 3 (P<.001).26,27 Pimecrolimus also provides long-term relief by preventing disease progression to flares, which was exemplified in a study (N=713) with no flares in 51% of pimecrolimus patients at 12 months versus 28% in the conventional treatment group (P<.001).28 Similarly, long-term studies of tacrolimus demonstrated an improvement of all symptoms of AD after 1 week of treatment. Maximal improvement was achieved with continued use of tacrolimus, and up to 1 year of tacrolimus use was found to be safe and effective.29,30 Thus, TCIs have been proven to be an effective choice in maintenance therapy for AD and have a good safety profile. The most common adverse effects of TCIs are local skin reactions, such as stinging and burning at the site of application. Rare cases of skin cancer and lymphoma have been reported; however, a causal relationship has yet to be established.31,32
Additional Therapies
Wet wrap therapy is effective for rapid control of flares and in controlling recalcitrant AD. Wet wraps function via several mechanisms; they provide a mechanical barrier against scratching, increase moisture and soften the skin, and enhance absorption of topical medications.33,34 The following method is employed when using wet wraps: an emollient or TC is applied to the area, a tubular bandage soaked in warm water is wrapped over the area, and dry bandages are used to form the outermost layer. Although wet wrap therapy is beneficial in treating AD, it is labor intensive and may require the expertise of a nurse. Thus, unlike other therapies, which patients can easily apply without interfering with their day, wet wraps must be applied at home or in a hospital setting.
Light therapy is another effective method of controlling AD. Although multiple forms of UV phototherapy are beneficial for symptom control in AD, there is no definitive recommendation regarding the specific type of light therapy due to a lack of comparative studies. Natural sunlight, narrowband UVB, broadband UVB, UVA, oral or topical psoralen plus UVA, as well as UVA and UVB can all be utilized in the treatment of AD. However, similar to natural sunlight, artificial light therapy can cause burning, blistering, hyperpigmentation, dark spots, and wrinkles. Because society places a large emphasis on maintaining a youthful appearance, patients may be hesitant to use a treatment that could potentially advance the skin’s aging process. Thus, it is important that this therapy is properly controlled to prevent further skin damage.35-37
When optimal topical regimens and phototherapy have failed to control AD, systemic immunomodulation therapies may be used. Currently, the most commonly used medications are cyclosporine 150 to 300 mg daily, methotrexate 7.5 to 25 mg weekly, mycophenolate mofetil 0.5 to 3 g daily, and azathioprine 1 to 3 mg/kg daily.38,39 Decisions regarding the specific class of drugs should be based on the patient’s AD status, comorbidities, and personal preference.
Conclusion
Atopic dermatitis is a common chronic condition that can occur at any age and cause substantial physical, psychological, social, and/or emotional stress for patients and their families. Although TCs have been the standard of treatment for many years, ongoing concerns regarding their safety have led to the use of TCIs, which overcome some of the drawbacks of steroid therapy. Phototherapy and systemic immunosuppressant therapy are reserved for patients who have not responded to optimal topical therapies. Although several therapeutic avenues exist for patients, there is a need for the development of more effective and safer drugs. Furthermore, cosmetic products created specifically for patients with AD would be beneficial, as patients often struggle to select products that do not cause more harm than good. Given the complexity of the pathogenesis of AD, further research must focus on defining the specific pathways involved in the disease and targeting these pathways with therapies.
Atopic dermatitis (AD), more commonly referred to as eczema, is a chronic pruritic inflammatory skin disease that frequently affects both children and adults. Atopic dermatitis is most common in urban and developed countries, with a prevalence of approximately 11% in the United States.1 The pathophysiology of AD is complex and not fully understood, despite the increasing incidence of the disease.2 A myriad of factors, including genetics, defects in the innate and adaptive immune response, and skin barrier abnormalities all contribute to the pathogenesis.3,4 As a result of these abnormalities, patients with AD are more prone to damage from environmental irritants and allergens.
The diagnosis of AD is made clinically based on patient history and visual assessment of the skin.5 Atopic dermatitis follows a chronic and relapsing course characterized by severe pruritus and visible skin changes including xerosis, redness, blistering, oozing, crusting, scaling, thickening, and color change.6,7 Due to the genetic predisposition to make IgE antibodies in response to common environmental and food antigens, patients also may develop allergic rhinitis, asthma, and food-induced anaphylaxis.8,9 Patients also are susceptible to cutaneous viral, fungal, and bacterial infections, the most common of which is an infection with Staphylococcus aureus.10
Atopic dermatitis can have a substantial impact on quality of life, which has been revealed in studies linking chronic skin conditions to depression, impairment of self-esteem, and financial hardship.11 Because skin appearance impacts how a person is initially perceived by others, patients often report feeling self-conscious about their disease and experience teasing or bullying.12 To improve their physical appearance, patients may incur considerable medical expenses. According to 2 population-based studies comprising more than 60,000 adults aged 18 to 85 years, individuals with AD face substantial financial burdens and utilize the health care system more than those without the disease. On average, patients with AD spend $371 to $489 per year on costly out-of-pocket medical expenses and report more absences from work.13
Although there currently is no cure for AD, treatment is aimed at relieving its symptoms and preventing acute exacerbations as well as improving cosmetic appearance to enhance quality of life. Treatment must follow a stepwise approach, which focuses on hydrating the skin, repairing the dysfunctional epithelial barrier, and controlling inflammation. Thus, the standard of care focuses on avoiding skin irritants and triggers along with the use of moisturizers and topical corticosteroids (TCs). In patients with recurring severe disease, topical calcineurin inhibitors, phototherapy, and systemic agents also may be utilized.14
Avoiding Irritants and Triggers
Atopic dermatitis is worsened by skin contact with physical and chemical irritants. Exacerbating factors in AD include exposure to food allergens, dust, emotional stress, detergents, fragranced soaps, textiles, and ingredients in cosmetic products. Patients should be advised to use mild detergents and fragrance-free soaps and to avoid harsh materials such as wool. However, avoidance of specific ingredients in cosmetic products is not as straightforward because manufacturers are not required to disclose certain ingredients. In general, fragrances such as balsam of Peru and cinnamaldehyde, as well as preservatives such as parabens, isothiazolinones, and formaldehyde, should be avoided when selecting cosmetic products. Patients with AD should purchase fragrance-free products that are specifically formulated for sensitive skin. Additionally, patients should not apply makeup if their skin is irritated or oozing, as the flare may worsen.15
Moisturizers
Due to the impaired skin barrier function in patients with AD, regular application of fragrance-free moisturizers is essential to maintain hydration and to reduce xerosis. Various classes of moisturizers may be prescribed (eg, lotions, creams, gels, ointments) based on disease severity and patient preference. Light preparations such as lotions, creams, and gels have a high water content and generally are more appealing from a cosmetic standpoint because they do not create any residue on the skin. However, these options may require more frequent application because they are absorbed quickly. Heavy preparations such as ointments have longer-lasting effects due to their high oil content but tend to be less cosmetically appealing because of their greasiness.16
Although the amount and frequency of application of moisturizers has not been defined, liberal application several times daily is generally advised to minimize xerosis.17 Most physicians recommend applying moisturizer to the skin immediately after bathing to seal in moisture. Some patients prefer to use lotions and creams during the day because these products make the skin feel smooth and reserve the greasier ointments for nighttime application.
Topical Corticosteroids
Prescribed in conjunction with moisturizers, TCs are the mainstay of anti-inflammatory therapy in AD. Topical corticosteroids are classified into 7 groups based on potency, ranging from superpotent (class 1) to least potent (class 7). For acute AD flares, TCs should be applied daily for up to several weeks. Once the inflammation has resolved, it is recommended to apply TCs once to twice weekly to reduce the rate of relapse.18 Despite their effectiveness in the treatment of acute AD flares, TCs have a considerable side-effect profile. Potential adverse effects include skin atrophy, striae, telangiectasia, hypopigmentation, increased hair growth, steroid acne, growth retardation, and Cushing syndrome. Skin atrophy, which is the most common complication associated with TCs, results in shiny transparent skin, allowing for visualization of veins.19,20 Although many of these side effects will resolve after discontinuing the TCs, they are aesthetically displeasing during treatment, making it crucial for physicians to educate their patients on the proper usage of TCs to prevent negative outcomes.
Topical Calcineurin Inhibitors
Topical calcineurin inhibitors (TCIs) are a class of anti-inflammatories that are used to overcome the adverse effects of TCs. They are approved as alternatives to TCs in patients who have failed to respond to other topical treatments as well as those who have developed cutaneous atrophy from the use of TCs or have AD in sensitive areas such as the face, neck, and/or skin folds. Unlike TCs, TCIs do not cause atrophy, striae, or discoloration of the skin, which makes them more desirable from a cosmetic perspective. Their mechanism of action is distinct from TCs in that they inhibit calcineurin-dependent T-cell activation, thus preventing the transcription of inflammatory cytokines.21 Two TCIs are currently available: tacrolimus ointment 0.03% and 0.1% concentrations for moderate to severe AD and pimecrolimus cream 1% for mild to moderate AD.22 Twice-daily application of TCIs is recommended to decrease inflammation and pruritus associated with AD. Studies also have shown that intermittent use of TCIs 3 times weekly can aid in reducing relapses.23-25
The results from clinical trials demonstrate the rapid and continuous effects of both pimecrolimus and tacrolimus. In a controlled long-term study of adults, pimecrolimus provided significant relief of pruritus as soon as day 3 (P<.001).26,27 Pimecrolimus also provides long-term relief by preventing disease progression to flares, which was exemplified in a study (N=713) with no flares in 51% of pimecrolimus patients at 12 months versus 28% in the conventional treatment group (P<.001).28 Similarly, long-term studies of tacrolimus demonstrated an improvement of all symptoms of AD after 1 week of treatment. Maximal improvement was achieved with continued use of tacrolimus, and up to 1 year of tacrolimus use was found to be safe and effective.29,30 Thus, TCIs have been proven to be an effective choice in maintenance therapy for AD and have a good safety profile. The most common adverse effects of TCIs are local skin reactions, such as stinging and burning at the site of application. Rare cases of skin cancer and lymphoma have been reported; however, a causal relationship has yet to be established.31,32
Additional Therapies
Wet wrap therapy is effective for rapid control of flares and in controlling recalcitrant AD. Wet wraps function via several mechanisms; they provide a mechanical barrier against scratching, increase moisture and soften the skin, and enhance absorption of topical medications.33,34 The following method is employed when using wet wraps: an emollient or TC is applied to the area, a tubular bandage soaked in warm water is wrapped over the area, and dry bandages are used to form the outermost layer. Although wet wrap therapy is beneficial in treating AD, it is labor intensive and may require the expertise of a nurse. Thus, unlike other therapies, which patients can easily apply without interfering with their day, wet wraps must be applied at home or in a hospital setting.
Light therapy is another effective method of controlling AD. Although multiple forms of UV phototherapy are beneficial for symptom control in AD, there is no definitive recommendation regarding the specific type of light therapy due to a lack of comparative studies. Natural sunlight, narrowband UVB, broadband UVB, UVA, oral or topical psoralen plus UVA, as well as UVA and UVB can all be utilized in the treatment of AD. However, similar to natural sunlight, artificial light therapy can cause burning, blistering, hyperpigmentation, dark spots, and wrinkles. Because society places a large emphasis on maintaining a youthful appearance, patients may be hesitant to use a treatment that could potentially advance the skin’s aging process. Thus, it is important that this therapy is properly controlled to prevent further skin damage.35-37
When optimal topical regimens and phototherapy have failed to control AD, systemic immunomodulation therapies may be used. Currently, the most commonly used medications are cyclosporine 150 to 300 mg daily, methotrexate 7.5 to 25 mg weekly, mycophenolate mofetil 0.5 to 3 g daily, and azathioprine 1 to 3 mg/kg daily.38,39 Decisions regarding the specific class of drugs should be based on the patient’s AD status, comorbidities, and personal preference.
Conclusion
Atopic dermatitis is a common chronic condition that can occur at any age and cause substantial physical, psychological, social, and/or emotional stress for patients and their families. Although TCs have been the standard of treatment for many years, ongoing concerns regarding their safety have led to the use of TCIs, which overcome some of the drawbacks of steroid therapy. Phototherapy and systemic immunosuppressant therapy are reserved for patients who have not responded to optimal topical therapies. Although several therapeutic avenues exist for patients, there is a need for the development of more effective and safer drugs. Furthermore, cosmetic products created specifically for patients with AD would be beneficial, as patients often struggle to select products that do not cause more harm than good. Given the complexity of the pathogenesis of AD, further research must focus on defining the specific pathways involved in the disease and targeting these pathways with therapies.
1. Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
2. Deckers IA, McLean S, Linssen S, et al. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS One. 2012;7:e39803.
3. Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233-246.
4. Peate I. Eczema: causes, symptoms and treatment in the community. Br J Community Nurs. 2011;16:324, 326-331.
5. Williams HC, Burney PG, Pembroke AC, et al. The U.K. Working Party’s diagnostic criteria for atopic dermatitis. III. independent hospital validation. Br J Dermatol. 1994;131:406-416.
6. Magin P, Adams J, Heading G, et al. Experiences of appearance-related teasing and bullying in skin diseases and their psychological sequelae: results of a qualitative study. Scand J Caring Sci. 2008;22:430-436.
7. Beattie P, Lewis-Jones M. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155:145-151.
8. Spergel JM. From atopic dermatitis to asthma: the atopic march [published online January 22, 2010]. Ann Allergy Asthma Immunol. 2010;105:99-106; quiz 107-109, 117.
9. Leung DY. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int. 2013;62:151-161.
10. Balma-Mena A, Lara-Corrales I, Zeller J, et al. Colonization with community-acquired methicillin-resistant Staphylococcus aureus in children with atopic dermatitis: a cross-sectional study. Int J Dermatol. 2011;50:682-688.
11. Strawser MS, Storch EA, Roberti JW. The Teasing Questionnaire-Revised: measurement of childhood teasing in adults. J Anxiety Disord. 2005;19:780-792.
12. Magin P, Adams J, Heading G, et al. Experiences of appearance-related teasing and bullying in skin diseases and their psychological sequelae: results of a qualitative study. Scand J Caring Sci. 2008;22:430-436.
13. Silverberg J. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
14. Ellis C, Luger T, Abeck D, et al. International Consensus Conference on Atopic Dermatitis II (ICCAD II): clinical update and current treatment strategies. Br J Dermatol. 2003;148(suppl 63):3-10.
15. Kim K. Influences of environmental chemicals on atopic dermatitis. Toxicol Res. 2015;31:89-96.
16. Ridd M, Redmond N, Hollinghurst S, et al. Choice of Moisturiser for Eczema Treatment (COMET): study protocol for a randomized controlled trial. Trials. 2015;16:304.
17. Hon KL, Ching GK, Leung TF, et al. Estimating emollient usage in patients with eczema. Clin Exp Dermatol. 2010;35:22-26.
18. Hanifin J, Gupta AK, Rajagopalan R. Intermittent dosing of fluticasone propionate cream for reducing the risk of relapse in atopic dermatitis patients. Br J Dermatol. 2002;147:528-537.
19. Hill CJ, Rostenberg A Jr. Adverse effects from topical steroids. Cutis. 1978;21:624-628.
20. Ruiz-Maldonado R, Zapata G, Lourdes T, et al. Cushing’s syndrome after topical application of corticosteroids. Am J Dis Child. 1982;136:274-275.
21. Grassberger M, Baumruker T, Enz A, et al. A novel anti-inflammatory drug, SDZ ASM 981, for the treatment of skin diseases: in vitro pharmacology. Br J Dermatol. 1999;141:264-273.
22. Eichenfield L, Wynnis T, Berger T. Guidelines of care for the management of atopic dermatitis: management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
23. Reitamo S, Harper J, Bos JD, et al. 0.03% Tacrolimus ointment applied once or twice daily is more efficacious than 1% hydrocortisone acetate in children with moderate to severe atopic dermatitis: results of a randomized double-blind controlled trial. Br J Dermatol. 2004;150:554-562.
24. Ruer-Mulard M, Aberer W, Gunstone A, et al. Twice-daily versus once-daily applications of pimecrolimus cream 1% for the prevention of disease relapse in pediatric patients with atopic dermatitis. Pediatr Dermatol. 2009;26:551-558.
25. Breneman D, Fleischer AB Jr, Abramovits W, et al. Intermittent therapy for flare prevention and long-term disease control in stabilized atopic dermatitis: a randomized comparison of 3-times-weekly applications of tacrolimus ointment versus vehicle. J Am Acad Dermatol. 2008;58:990-999.
26. Meurer M, Fölster-Holst R, Wozel G, et al. Pimecrolimus cream 1% (Elidel) provides significant and rapid relief of pruritus and improves disease control and quality of life in atopic dermatitis in adults. J Invest Dermatol. 2002;119:350.
27. Meurer M, Fölster-Holst R, Wozel G, et al. Pimecrolimus cream in the long-term management of atopic dermatitis in adults: a six-month study. Dermatology. 2002;205:271-277.
28. Wahn U, Bos JD, Goodfield M, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics. 2002;110(1, pt 1):e2.
29. Kang S, Lucky AW, Pariser D, et al. Long-term safety and efficacy of tacrolimus ointment for the treatment of atopic dermatitis in children. J Am Acad Dermatol. 2001;44(suppl 1):S58-S64.
30. Reitamo S, Wollenberg A, Schöpf E, et al. Safety and efficacy of 1 year of tacrolimus ointment monotherapy in adults with atopic dermatitis. the European Tacrolimus Ointment Study Group. Arch Dermatol. 2000;136:999-1006.
31. Frankel HC, Qureshi AA. Comparative effectiveness of topical calcineurin inhibitors in adult patients with atopic dermatitis. Am J Clin Dermatol. 2012;13:113-123.
32. Tennis P, Gelfand JM, Rothman KJ. Evaluation of cancer risk related to atopic dermatitis and use of topical calcineurin inhibitors. Br J Dermatol. 2011;165:465-473.
33. Dabade TS, Davis DM, Wetter DA, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2012;67:100-106.
34. Devillers AC, Oranje AP. Efficacy and safety of ‘wet-wrap’ dressings as an intervention treatment in children with severe and/or refractory atopic dermatitis: a critical review of the literature. Br J Dermatol. 2006;154:579-585.
35. Meduri NB, Vandergriff T, Rasmussen H, et al. Phototherapy in the management of atopic dermatitis: a systematic review. Photodermatol Photoimmunol Photomed. 2007;23:106-112.
36. Clayton TH, Clark SM, Turner D, et al. The treatment of severe atopic dermatitis in childhood with narrowband ultraviolet B phototherapy. Clin Exp Dermatol. 2007;32:28-33.
37. Jekler J, Larko O. UVB phototherapy of atopic dermatitis. Br J Dermatol. 1988;119:697-705.
38. Roekevisch E, Spuls PI, Kuester D, et al. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133:429-438.39. Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess. 2000;4:1-191.
39. Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess. 2000;4:1-191.
1. Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
2. Deckers IA, McLean S, Linssen S, et al. Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies. PLoS One. 2012;7:e39803.
3. Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233-246.
4. Peate I. Eczema: causes, symptoms and treatment in the community. Br J Community Nurs. 2011;16:324, 326-331.
5. Williams HC, Burney PG, Pembroke AC, et al. The U.K. Working Party’s diagnostic criteria for atopic dermatitis. III. independent hospital validation. Br J Dermatol. 1994;131:406-416.
6. Magin P, Adams J, Heading G, et al. Experiences of appearance-related teasing and bullying in skin diseases and their psychological sequelae: results of a qualitative study. Scand J Caring Sci. 2008;22:430-436.
7. Beattie P, Lewis-Jones M. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155:145-151.
8. Spergel JM. From atopic dermatitis to asthma: the atopic march [published online January 22, 2010]. Ann Allergy Asthma Immunol. 2010;105:99-106; quiz 107-109, 117.
9. Leung DY. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int. 2013;62:151-161.
10. Balma-Mena A, Lara-Corrales I, Zeller J, et al. Colonization with community-acquired methicillin-resistant Staphylococcus aureus in children with atopic dermatitis: a cross-sectional study. Int J Dermatol. 2011;50:682-688.
11. Strawser MS, Storch EA, Roberti JW. The Teasing Questionnaire-Revised: measurement of childhood teasing in adults. J Anxiety Disord. 2005;19:780-792.
12. Magin P, Adams J, Heading G, et al. Experiences of appearance-related teasing and bullying in skin diseases and their psychological sequelae: results of a qualitative study. Scand J Caring Sci. 2008;22:430-436.
13. Silverberg J. Health care utilization, patient costs, and access to care in US adults with eczema: a population-based study. JAMA Dermatol. 2015;151:743-752.
14. Ellis C, Luger T, Abeck D, et al. International Consensus Conference on Atopic Dermatitis II (ICCAD II): clinical update and current treatment strategies. Br J Dermatol. 2003;148(suppl 63):3-10.
15. Kim K. Influences of environmental chemicals on atopic dermatitis. Toxicol Res. 2015;31:89-96.
16. Ridd M, Redmond N, Hollinghurst S, et al. Choice of Moisturiser for Eczema Treatment (COMET): study protocol for a randomized controlled trial. Trials. 2015;16:304.
17. Hon KL, Ching GK, Leung TF, et al. Estimating emollient usage in patients with eczema. Clin Exp Dermatol. 2010;35:22-26.
18. Hanifin J, Gupta AK, Rajagopalan R. Intermittent dosing of fluticasone propionate cream for reducing the risk of relapse in atopic dermatitis patients. Br J Dermatol. 2002;147:528-537.
19. Hill CJ, Rostenberg A Jr. Adverse effects from topical steroids. Cutis. 1978;21:624-628.
20. Ruiz-Maldonado R, Zapata G, Lourdes T, et al. Cushing’s syndrome after topical application of corticosteroids. Am J Dis Child. 1982;136:274-275.
21. Grassberger M, Baumruker T, Enz A, et al. A novel anti-inflammatory drug, SDZ ASM 981, for the treatment of skin diseases: in vitro pharmacology. Br J Dermatol. 1999;141:264-273.
22. Eichenfield L, Wynnis T, Berger T. Guidelines of care for the management of atopic dermatitis: management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
23. Reitamo S, Harper J, Bos JD, et al. 0.03% Tacrolimus ointment applied once or twice daily is more efficacious than 1% hydrocortisone acetate in children with moderate to severe atopic dermatitis: results of a randomized double-blind controlled trial. Br J Dermatol. 2004;150:554-562.
24. Ruer-Mulard M, Aberer W, Gunstone A, et al. Twice-daily versus once-daily applications of pimecrolimus cream 1% for the prevention of disease relapse in pediatric patients with atopic dermatitis. Pediatr Dermatol. 2009;26:551-558.
25. Breneman D, Fleischer AB Jr, Abramovits W, et al. Intermittent therapy for flare prevention and long-term disease control in stabilized atopic dermatitis: a randomized comparison of 3-times-weekly applications of tacrolimus ointment versus vehicle. J Am Acad Dermatol. 2008;58:990-999.
26. Meurer M, Fölster-Holst R, Wozel G, et al. Pimecrolimus cream 1% (Elidel) provides significant and rapid relief of pruritus and improves disease control and quality of life in atopic dermatitis in adults. J Invest Dermatol. 2002;119:350.
27. Meurer M, Fölster-Holst R, Wozel G, et al. Pimecrolimus cream in the long-term management of atopic dermatitis in adults: a six-month study. Dermatology. 2002;205:271-277.
28. Wahn U, Bos JD, Goodfield M, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics. 2002;110(1, pt 1):e2.
29. Kang S, Lucky AW, Pariser D, et al. Long-term safety and efficacy of tacrolimus ointment for the treatment of atopic dermatitis in children. J Am Acad Dermatol. 2001;44(suppl 1):S58-S64.
30. Reitamo S, Wollenberg A, Schöpf E, et al. Safety and efficacy of 1 year of tacrolimus ointment monotherapy in adults with atopic dermatitis. the European Tacrolimus Ointment Study Group. Arch Dermatol. 2000;136:999-1006.
31. Frankel HC, Qureshi AA. Comparative effectiveness of topical calcineurin inhibitors in adult patients with atopic dermatitis. Am J Clin Dermatol. 2012;13:113-123.
32. Tennis P, Gelfand JM, Rothman KJ. Evaluation of cancer risk related to atopic dermatitis and use of topical calcineurin inhibitors. Br J Dermatol. 2011;165:465-473.
33. Dabade TS, Davis DM, Wetter DA, et al. Wet dressing therapy in conjunction with topical corticosteroids is effective for rapid control of severe pediatric atopic dermatitis: experience with 218 patients over 30 years at Mayo Clinic. J Am Acad Dermatol. 2012;67:100-106.
34. Devillers AC, Oranje AP. Efficacy and safety of ‘wet-wrap’ dressings as an intervention treatment in children with severe and/or refractory atopic dermatitis: a critical review of the literature. Br J Dermatol. 2006;154:579-585.
35. Meduri NB, Vandergriff T, Rasmussen H, et al. Phototherapy in the management of atopic dermatitis: a systematic review. Photodermatol Photoimmunol Photomed. 2007;23:106-112.
36. Clayton TH, Clark SM, Turner D, et al. The treatment of severe atopic dermatitis in childhood with narrowband ultraviolet B phototherapy. Clin Exp Dermatol. 2007;32:28-33.
37. Jekler J, Larko O. UVB phototherapy of atopic dermatitis. Br J Dermatol. 1988;119:697-705.
38. Roekevisch E, Spuls PI, Kuester D, et al. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133:429-438.39. Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess. 2000;4:1-191.
39. Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess. 2000;4:1-191.
Practice Points
- Cosmetic symptoms of atopic dermatitis can have a serious impact on the patient’s quality of life.
- Avoidance of flares and prevention of triggers is an important aspect of care.
- Treatment options range from optimized skin care to topical prescription therapies to systemic medications.
Brown Macule on the Waist
The best diagnosis is:
a. granular cell tumor
b. intradermal nevus
c. Langerhans cell disease
d. mastocytosis
e. multicentric reticulohistiocytosis
|
| Monomorphic cell infiltrate in the upper dermis (H&E, original magnification ×100). |
|
| A closer view reveals cuboidal or spindle cells with basal hyperpigmentation (H&E, original magnification ×200). |
Continue to the next page for the diagnosis >>
Mastocytosis
Mastocytosis is a clonal proliferation of mast cells in the skin and various systems of the body including the bone marrow, liver, lymph nodes, and gastrointestinal tract.1,2 Mast cell proliferation is closely associated with germline and acquired activating KIT mutations.3-5 Adult-onset mastocytosis is likely to involve several organs, whereas pediatric mastocytosis usually affects only the skin and is self-limiting. Patients with profound mast cell infiltration in the skin or other organs are likely to have attacks of flushing, palpitation, or diarrhea resulting from the degranulation of mast cells and release of histamine.6,7 In a majority of patients with advanced systemic mastocytosis, mast cells are positive for the Ki-1 antigen (CD30), whereas in most patients with indolent systemic mastocytosis, only a few mast cells are positive for CD30.8 Recently, CD30 was reported as a new drug target in patients with CD30+ advanced systemic mastocytosis.9 Because the skin frequently is involved and easily accessible in comparison with other organs, skin biopsy often is performed to establish a diagnosis of mastocytosis. Cutaneous mastocytosis comprises urticaria pigmentosa, solitary mastocytoma, diffuse cutaneous mastocytosis, and telangiectasia macularis eruptiva perstans; approximately 80% of all cases have urticaria pigmentosa.10-12 In cutaneous mastocytosis, skin biopsy typically shows monomorphous mast cell infiltrate mostly in the upper third of the dermis. The density of mast cells varies according to the clinical variant. For example, a lesion of telangiectasia macularis eruptiva perstans has only a perivascular mast cell infiltrate, whereas a solitary mastocytoma has sheets of mast cells in the dermis, sometimes extending into the subcutis. A skin biopsy of the brown macule on the waist showed a number of cuboidal or spindle mast cells in the upper dermis with occasional eosinophils. These mast cells are monomorphous, and no mitotic figures, necrotic cells, or atypical cells are seen. Mast cells have metachromatic granules in the cytoplasm, which can be seen with toluidine blue or Giemsa stain. CD117 (c-kit) also is positive. Mast cells in urticaria pigmentosa easily may be mistaken for nevus cells. Hyperpigmentation of the basal layer, a characteristic feature seen in urticaria pigmentosa, also may erroneously suggest a diagnosis of a melanocytic nevus.
Granular cell tumors predominantly affect the oral cavity, but the skin also can be involved. It comprises a fascicular infiltrate of large and polygonal cells with characteristic eosinophilic granular cytoplasm in the dermis (Figure 1).13 Cell membranes are not always distinct. Although the nuclei usually are small and centrally located, irregular and plump nuclei with distinct nucleoli also may be seen. The overlying epidermis tends to be hyperplastic. Granular cell tumor is considered a group of lesions of varying histogenesis. Cases in which tumors originated from a neural crest–derived peripheral nerve–related cell as well as a Schwann cell have been reported.14,15 The origin of granular cell tumors is controversial.
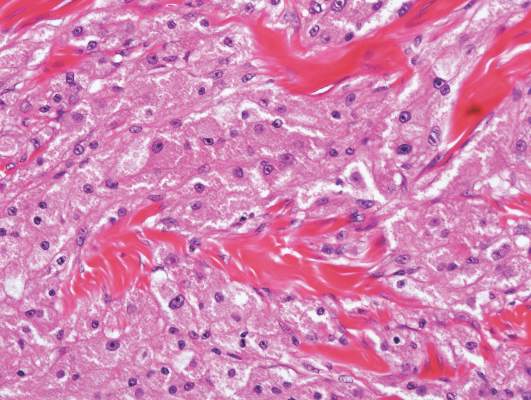
|
| Figure 1. Granular cell tumor showing fascicles of large and polygonal cells with characteristic eosinophilic granular cytoplasm in the dermis (H&E, original magnification ×200). |

|
| Figure 2. Intradermal nevus showing nests with melanin in the uppermost area of the lesion and neurotized nevus cells in the lower part (H&E, original magnification ×100). Pseudovascular spaces are seen on the right side. |
Intradermal nevus usually has nests and cords of nevus cells in the upper dermis. The uppermost melanocytes often contain a moderate amount of melanin, whereas nevus cells in the mid and lower dermis usually do not contain melanin (Figure 2). Shrinkage during tissue processing maycause clefts between nevus cells, resulting in pseudovascular spaces.16 The deeper dermis may have a neuroid appearance with spindle-shaped cells and Meissner corpuscle–like structures.17
Although Langerhans cell disease was formerly known as Langerhans cell histiocytosis and subdivided into several clinical subtypes, including Letterer-Siwe disease, Hand-Schüller-Christian disease, and eosinophilic granuloma, these clinical subtypes commonly overlapped. Langerhans cell disease is now used as a terminology that encompasses all subtypes.18,19 Langerhans cell disease is characterized by a proliferation of Langerhans cells with a variable mixture of other inflammatory cells. The constituent cells are large and ovoid with a distinct folded or lobulated, often kidney-shaped nucleus.20 Langerhans cells usually infiltrate the upper dermis and occasionally the epidermis (Figure 3). CD1a, HLA-DR, S-100 protein, and langerin are positive in Langerhans cells.21

|
| Figure 3. Langerhans cell disease showing an infiltrate of large and ovoid Langerhans cells with a distinct folded or lobulated, often kidney-shaped nucleus in the upper dermis and epidermis (H&E, original magnification ×200). |

|
| Figure 4. Multicentric reticulohistiocytosis showing a mixture of mononuclear and multinucleate histiocytes with abundant eosinophilic and finely granular cytoplasm (H&E, original magnification ×200). |
Multicentric reticulohistiocytosis is characterized by a combination of papulonodular cutaneous lesions and severe arthropathy.22 An irregular mixture of mononuclear and multinucleate histiocytes showing abundant eosinophilic and finely granular cytoplasm, often with a ground-glass appearance, is seen along with lymphocytic infiltration (Figure 4).23 A few giant cells may be seen in early lesions; older lesions more commonly have giant cells and fibrosis.
1. Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497-516.
2. Pardanani A. Systemic mastocytosis in adults: 2013 update on diagnosis, risk stratification, and management. Am J Hematol. 2013;88:612-624.
3. Orfao A, Garcia-Montero AC, Sanchez L, et al. Recent advances in the understanding of mastocytosis: the role of KIT mutations. Br J Haematol. 2007;138:12-30.
4. Yanagihori H, Oyama N, Nakamura K, et al. c-KIT mutations in patients with childhood-onset mastocytosis and genotype-phenotype correlation. J Mol Diagn. 2005;7:252-257.
5. Bodemer C, Hermine O, Palmérini F, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 2010;130:804-815.
6. Kettelhut BV, Metcalfe DD. Pediatric mastocytosis. Ann Allergy. 1994;73:197-202; quiz 202-207.
7. Longley J, Duffy TP, Kohn S. The mast cell and mast cell disease. J Am Acad Dermatol. 1995;32:545-561; quiz 562-564.
8. Sotlar K, Cerny-Reiterer S, Petat-Dutter K, et al. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011;24:585-595.
9. Blatt K, Cerny-Reiterer S, Schwaab J, et al. Identification of the Ki-1 antigen (CD30) as a novel therapeutic target in systemic mastocytosis [published online October 20, 2015]. Blood. 2015;126:2832-2841.
10. Kiszewski AE, Duran-Mckinster C, Orozco-Covarrubias L, et al. Cutaneous mastocytosis in children: a clinical analysis of 71 cases. J Eur Acad Dermatol Venereol. 2004;18:285-290.
11. Akoglu G, Erkin G, Cakir B, et al. Cutaneous mastocytosis: demographic aspects and clinical features of 55 patients. J Eur Acad Dermatol Venereol. 2006;20:969-973.
12. Sarkany RP, Monk BE, Handfield-Jones SE. Telangiectasia macularis eruptiva perstans: a case report and review of the literature. Clin Exp Dermatol. 1998;23:38-39.
13. Lack EE, Worsham GF, Callihan MD, et al. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Oncol. 1980;13:301-316.
14. Buley ID, Gatter KC, Kelly PM, et al. Granular cell tumours revisited. an immunohistological and ultrastructural study. Histopathology. 1988;12:263-274.
15. Penneys NS, Adachi K, Ziegels-Weissman J, et al. Granular cell tumors of the skin contain myelin basic protein. Arch Pathol Lab Med. 1983;107:302-303.
16. Modlin RL, Gottlieb B, Taylor C, et al. Identification of cells lining pseudovascular spaces of benign pigmented nevi. Am J Dermatopathol. 1984;(suppl 6):25-29.
17. Fullen DR, Reed JA, Finnerty B, et al. S100A6 preferentially labels type C nevus cells and nevic corpuscles: additional support for Schwannian differentiation of intradermal nevi. J Cutan Pathol. 2001;28:393-399.
18. Newman B, Hu W, Nigro K, et al. Aggressive histiocytic disorders that can involve the skin. J Am Acad Dermatol. 2007;56:302-316.
19. Weedon D. Cutaneous infiltrates—non-lymphoid. In: Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Amsterdam, Netherlands: Elsevier; 2010:937-970.
20. Harrist TJ, Bhan AK, Murphy GF, et al. Histiocytosis-X: in situ characterization of cutaneous infiltrates with monoclonal antibodies. Am J Clin Pathol. 1983;79:294-300.
21. Lau SK, Chu PG, Weiss LM. Immunohistochemical expression of langerin in Langerhans cell histiocytosis and non-Langerhans cell histiocytic disorders. Am J Surg Pathol. 2008;32:615-619.
22. Lesher JL Jr, Allen BS. Multicentric reticulohistiocytosis. J Am Acad Dermatol. 1984;11:713-723.
23. Heathcote JG, Guenther LC, Wallace AC. Multicentric reticulohistiocytosis: a report of a case and a review of the pathology. Pathology. 1985;17:601-608.
The best diagnosis is:
a. granular cell tumor
b. intradermal nevus
c. Langerhans cell disease
d. mastocytosis
e. multicentric reticulohistiocytosis

|
| Monomorphic cell infiltrate in the upper dermis (H&E, original magnification ×100). |

|
| A closer view reveals cuboidal or spindle cells with basal hyperpigmentation (H&E, original magnification ×200). |
Continue to the next page for the diagnosis >>
Mastocytosis
Mastocytosis is a clonal proliferation of mast cells in the skin and various systems of the body including the bone marrow, liver, lymph nodes, and gastrointestinal tract.1,2 Mast cell proliferation is closely associated with germline and acquired activating KIT mutations.3-5 Adult-onset mastocytosis is likely to involve several organs, whereas pediatric mastocytosis usually affects only the skin and is self-limiting. Patients with profound mast cell infiltration in the skin or other organs are likely to have attacks of flushing, palpitation, or diarrhea resulting from the degranulation of mast cells and release of histamine.6,7 In a majority of patients with advanced systemic mastocytosis, mast cells are positive for the Ki-1 antigen (CD30), whereas in most patients with indolent systemic mastocytosis, only a few mast cells are positive for CD30.8 Recently, CD30 was reported as a new drug target in patients with CD30+ advanced systemic mastocytosis.9 Because the skin frequently is involved and easily accessible in comparison with other organs, skin biopsy often is performed to establish a diagnosis of mastocytosis. Cutaneous mastocytosis comprises urticaria pigmentosa, solitary mastocytoma, diffuse cutaneous mastocytosis, and telangiectasia macularis eruptiva perstans; approximately 80% of all cases have urticaria pigmentosa.10-12 In cutaneous mastocytosis, skin biopsy typically shows monomorphous mast cell infiltrate mostly in the upper third of the dermis. The density of mast cells varies according to the clinical variant. For example, a lesion of telangiectasia macularis eruptiva perstans has only a perivascular mast cell infiltrate, whereas a solitary mastocytoma has sheets of mast cells in the dermis, sometimes extending into the subcutis. A skin biopsy of the brown macule on the waist showed a number of cuboidal or spindle mast cells in the upper dermis with occasional eosinophils. These mast cells are monomorphous, and no mitotic figures, necrotic cells, or atypical cells are seen. Mast cells have metachromatic granules in the cytoplasm, which can be seen with toluidine blue or Giemsa stain. CD117 (c-kit) also is positive. Mast cells in urticaria pigmentosa easily may be mistaken for nevus cells. Hyperpigmentation of the basal layer, a characteristic feature seen in urticaria pigmentosa, also may erroneously suggest a diagnosis of a melanocytic nevus.
Granular cell tumors predominantly affect the oral cavity, but the skin also can be involved. It comprises a fascicular infiltrate of large and polygonal cells with characteristic eosinophilic granular cytoplasm in the dermis (Figure 1).13 Cell membranes are not always distinct. Although the nuclei usually are small and centrally located, irregular and plump nuclei with distinct nucleoli also may be seen. The overlying epidermis tends to be hyperplastic. Granular cell tumor is considered a group of lesions of varying histogenesis. Cases in which tumors originated from a neural crest–derived peripheral nerve–related cell as well as a Schwann cell have been reported.14,15 The origin of granular cell tumors is controversial.

|
| Figure 1. Granular cell tumor showing fascicles of large and polygonal cells with characteristic eosinophilic granular cytoplasm in the dermis (H&E, original magnification ×200). |

|
| Figure 2. Intradermal nevus showing nests with melanin in the uppermost area of the lesion and neurotized nevus cells in the lower part (H&E, original magnification ×100). Pseudovascular spaces are seen on the right side. |
Intradermal nevus usually has nests and cords of nevus cells in the upper dermis. The uppermost melanocytes often contain a moderate amount of melanin, whereas nevus cells in the mid and lower dermis usually do not contain melanin (Figure 2). Shrinkage during tissue processing maycause clefts between nevus cells, resulting in pseudovascular spaces.16 The deeper dermis may have a neuroid appearance with spindle-shaped cells and Meissner corpuscle–like structures.17
Although Langerhans cell disease was formerly known as Langerhans cell histiocytosis and subdivided into several clinical subtypes, including Letterer-Siwe disease, Hand-Schüller-Christian disease, and eosinophilic granuloma, these clinical subtypes commonly overlapped. Langerhans cell disease is now used as a terminology that encompasses all subtypes.18,19 Langerhans cell disease is characterized by a proliferation of Langerhans cells with a variable mixture of other inflammatory cells. The constituent cells are large and ovoid with a distinct folded or lobulated, often kidney-shaped nucleus.20 Langerhans cells usually infiltrate the upper dermis and occasionally the epidermis (Figure 3). CD1a, HLA-DR, S-100 protein, and langerin are positive in Langerhans cells.21

|
| Figure 3. Langerhans cell disease showing an infiltrate of large and ovoid Langerhans cells with a distinct folded or lobulated, often kidney-shaped nucleus in the upper dermis and epidermis (H&E, original magnification ×200). |

|
| Figure 4. Multicentric reticulohistiocytosis showing a mixture of mononuclear and multinucleate histiocytes with abundant eosinophilic and finely granular cytoplasm (H&E, original magnification ×200). |
Multicentric reticulohistiocytosis is characterized by a combination of papulonodular cutaneous lesions and severe arthropathy.22 An irregular mixture of mononuclear and multinucleate histiocytes showing abundant eosinophilic and finely granular cytoplasm, often with a ground-glass appearance, is seen along with lymphocytic infiltration (Figure 4).23 A few giant cells may be seen in early lesions; older lesions more commonly have giant cells and fibrosis.
The best diagnosis is:
a. granular cell tumor
b. intradermal nevus
c. Langerhans cell disease
d. mastocytosis
e. multicentric reticulohistiocytosis

|
| Monomorphic cell infiltrate in the upper dermis (H&E, original magnification ×100). |

|
| A closer view reveals cuboidal or spindle cells with basal hyperpigmentation (H&E, original magnification ×200). |
Continue to the next page for the diagnosis >>
Mastocytosis
Mastocytosis is a clonal proliferation of mast cells in the skin and various systems of the body including the bone marrow, liver, lymph nodes, and gastrointestinal tract.1,2 Mast cell proliferation is closely associated with germline and acquired activating KIT mutations.3-5 Adult-onset mastocytosis is likely to involve several organs, whereas pediatric mastocytosis usually affects only the skin and is self-limiting. Patients with profound mast cell infiltration in the skin or other organs are likely to have attacks of flushing, palpitation, or diarrhea resulting from the degranulation of mast cells and release of histamine.6,7 In a majority of patients with advanced systemic mastocytosis, mast cells are positive for the Ki-1 antigen (CD30), whereas in most patients with indolent systemic mastocytosis, only a few mast cells are positive for CD30.8 Recently, CD30 was reported as a new drug target in patients with CD30+ advanced systemic mastocytosis.9 Because the skin frequently is involved and easily accessible in comparison with other organs, skin biopsy often is performed to establish a diagnosis of mastocytosis. Cutaneous mastocytosis comprises urticaria pigmentosa, solitary mastocytoma, diffuse cutaneous mastocytosis, and telangiectasia macularis eruptiva perstans; approximately 80% of all cases have urticaria pigmentosa.10-12 In cutaneous mastocytosis, skin biopsy typically shows monomorphous mast cell infiltrate mostly in the upper third of the dermis. The density of mast cells varies according to the clinical variant. For example, a lesion of telangiectasia macularis eruptiva perstans has only a perivascular mast cell infiltrate, whereas a solitary mastocytoma has sheets of mast cells in the dermis, sometimes extending into the subcutis. A skin biopsy of the brown macule on the waist showed a number of cuboidal or spindle mast cells in the upper dermis with occasional eosinophils. These mast cells are monomorphous, and no mitotic figures, necrotic cells, or atypical cells are seen. Mast cells have metachromatic granules in the cytoplasm, which can be seen with toluidine blue or Giemsa stain. CD117 (c-kit) also is positive. Mast cells in urticaria pigmentosa easily may be mistaken for nevus cells. Hyperpigmentation of the basal layer, a characteristic feature seen in urticaria pigmentosa, also may erroneously suggest a diagnosis of a melanocytic nevus.
Granular cell tumors predominantly affect the oral cavity, but the skin also can be involved. It comprises a fascicular infiltrate of large and polygonal cells with characteristic eosinophilic granular cytoplasm in the dermis (Figure 1).13 Cell membranes are not always distinct. Although the nuclei usually are small and centrally located, irregular and plump nuclei with distinct nucleoli also may be seen. The overlying epidermis tends to be hyperplastic. Granular cell tumor is considered a group of lesions of varying histogenesis. Cases in which tumors originated from a neural crest–derived peripheral nerve–related cell as well as a Schwann cell have been reported.14,15 The origin of granular cell tumors is controversial.

|
| Figure 1. Granular cell tumor showing fascicles of large and polygonal cells with characteristic eosinophilic granular cytoplasm in the dermis (H&E, original magnification ×200). |

|
| Figure 2. Intradermal nevus showing nests with melanin in the uppermost area of the lesion and neurotized nevus cells in the lower part (H&E, original magnification ×100). Pseudovascular spaces are seen on the right side. |
Intradermal nevus usually has nests and cords of nevus cells in the upper dermis. The uppermost melanocytes often contain a moderate amount of melanin, whereas nevus cells in the mid and lower dermis usually do not contain melanin (Figure 2). Shrinkage during tissue processing maycause clefts between nevus cells, resulting in pseudovascular spaces.16 The deeper dermis may have a neuroid appearance with spindle-shaped cells and Meissner corpuscle–like structures.17
Although Langerhans cell disease was formerly known as Langerhans cell histiocytosis and subdivided into several clinical subtypes, including Letterer-Siwe disease, Hand-Schüller-Christian disease, and eosinophilic granuloma, these clinical subtypes commonly overlapped. Langerhans cell disease is now used as a terminology that encompasses all subtypes.18,19 Langerhans cell disease is characterized by a proliferation of Langerhans cells with a variable mixture of other inflammatory cells. The constituent cells are large and ovoid with a distinct folded or lobulated, often kidney-shaped nucleus.20 Langerhans cells usually infiltrate the upper dermis and occasionally the epidermis (Figure 3). CD1a, HLA-DR, S-100 protein, and langerin are positive in Langerhans cells.21

|
| Figure 3. Langerhans cell disease showing an infiltrate of large and ovoid Langerhans cells with a distinct folded or lobulated, often kidney-shaped nucleus in the upper dermis and epidermis (H&E, original magnification ×200). |

|
| Figure 4. Multicentric reticulohistiocytosis showing a mixture of mononuclear and multinucleate histiocytes with abundant eosinophilic and finely granular cytoplasm (H&E, original magnification ×200). |
Multicentric reticulohistiocytosis is characterized by a combination of papulonodular cutaneous lesions and severe arthropathy.22 An irregular mixture of mononuclear and multinucleate histiocytes showing abundant eosinophilic and finely granular cytoplasm, often with a ground-glass appearance, is seen along with lymphocytic infiltration (Figure 4).23 A few giant cells may be seen in early lesions; older lesions more commonly have giant cells and fibrosis.
1. Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497-516.
2. Pardanani A. Systemic mastocytosis in adults: 2013 update on diagnosis, risk stratification, and management. Am J Hematol. 2013;88:612-624.
3. Orfao A, Garcia-Montero AC, Sanchez L, et al. Recent advances in the understanding of mastocytosis: the role of KIT mutations. Br J Haematol. 2007;138:12-30.
4. Yanagihori H, Oyama N, Nakamura K, et al. c-KIT mutations in patients with childhood-onset mastocytosis and genotype-phenotype correlation. J Mol Diagn. 2005;7:252-257.
5. Bodemer C, Hermine O, Palmérini F, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 2010;130:804-815.
6. Kettelhut BV, Metcalfe DD. Pediatric mastocytosis. Ann Allergy. 1994;73:197-202; quiz 202-207.
7. Longley J, Duffy TP, Kohn S. The mast cell and mast cell disease. J Am Acad Dermatol. 1995;32:545-561; quiz 562-564.
8. Sotlar K, Cerny-Reiterer S, Petat-Dutter K, et al. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011;24:585-595.
9. Blatt K, Cerny-Reiterer S, Schwaab J, et al. Identification of the Ki-1 antigen (CD30) as a novel therapeutic target in systemic mastocytosis [published online October 20, 2015]. Blood. 2015;126:2832-2841.
10. Kiszewski AE, Duran-Mckinster C, Orozco-Covarrubias L, et al. Cutaneous mastocytosis in children: a clinical analysis of 71 cases. J Eur Acad Dermatol Venereol. 2004;18:285-290.
11. Akoglu G, Erkin G, Cakir B, et al. Cutaneous mastocytosis: demographic aspects and clinical features of 55 patients. J Eur Acad Dermatol Venereol. 2006;20:969-973.
12. Sarkany RP, Monk BE, Handfield-Jones SE. Telangiectasia macularis eruptiva perstans: a case report and review of the literature. Clin Exp Dermatol. 1998;23:38-39.
13. Lack EE, Worsham GF, Callihan MD, et al. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Oncol. 1980;13:301-316.
14. Buley ID, Gatter KC, Kelly PM, et al. Granular cell tumours revisited. an immunohistological and ultrastructural study. Histopathology. 1988;12:263-274.
15. Penneys NS, Adachi K, Ziegels-Weissman J, et al. Granular cell tumors of the skin contain myelin basic protein. Arch Pathol Lab Med. 1983;107:302-303.
16. Modlin RL, Gottlieb B, Taylor C, et al. Identification of cells lining pseudovascular spaces of benign pigmented nevi. Am J Dermatopathol. 1984;(suppl 6):25-29.
17. Fullen DR, Reed JA, Finnerty B, et al. S100A6 preferentially labels type C nevus cells and nevic corpuscles: additional support for Schwannian differentiation of intradermal nevi. J Cutan Pathol. 2001;28:393-399.
18. Newman B, Hu W, Nigro K, et al. Aggressive histiocytic disorders that can involve the skin. J Am Acad Dermatol. 2007;56:302-316.
19. Weedon D. Cutaneous infiltrates—non-lymphoid. In: Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Amsterdam, Netherlands: Elsevier; 2010:937-970.
20. Harrist TJ, Bhan AK, Murphy GF, et al. Histiocytosis-X: in situ characterization of cutaneous infiltrates with monoclonal antibodies. Am J Clin Pathol. 1983;79:294-300.
21. Lau SK, Chu PG, Weiss LM. Immunohistochemical expression of langerin in Langerhans cell histiocytosis and non-Langerhans cell histiocytic disorders. Am J Surg Pathol. 2008;32:615-619.
22. Lesher JL Jr, Allen BS. Multicentric reticulohistiocytosis. J Am Acad Dermatol. 1984;11:713-723.
23. Heathcote JG, Guenther LC, Wallace AC. Multicentric reticulohistiocytosis: a report of a case and a review of the pathology. Pathology. 1985;17:601-608.
1. Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497-516.
2. Pardanani A. Systemic mastocytosis in adults: 2013 update on diagnosis, risk stratification, and management. Am J Hematol. 2013;88:612-624.
3. Orfao A, Garcia-Montero AC, Sanchez L, et al. Recent advances in the understanding of mastocytosis: the role of KIT mutations. Br J Haematol. 2007;138:12-30.
4. Yanagihori H, Oyama N, Nakamura K, et al. c-KIT mutations in patients with childhood-onset mastocytosis and genotype-phenotype correlation. J Mol Diagn. 2005;7:252-257.
5. Bodemer C, Hermine O, Palmérini F, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 2010;130:804-815.
6. Kettelhut BV, Metcalfe DD. Pediatric mastocytosis. Ann Allergy. 1994;73:197-202; quiz 202-207.
7. Longley J, Duffy TP, Kohn S. The mast cell and mast cell disease. J Am Acad Dermatol. 1995;32:545-561; quiz 562-564.
8. Sotlar K, Cerny-Reiterer S, Petat-Dutter K, et al. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011;24:585-595.
9. Blatt K, Cerny-Reiterer S, Schwaab J, et al. Identification of the Ki-1 antigen (CD30) as a novel therapeutic target in systemic mastocytosis [published online October 20, 2015]. Blood. 2015;126:2832-2841.
10. Kiszewski AE, Duran-Mckinster C, Orozco-Covarrubias L, et al. Cutaneous mastocytosis in children: a clinical analysis of 71 cases. J Eur Acad Dermatol Venereol. 2004;18:285-290.
11. Akoglu G, Erkin G, Cakir B, et al. Cutaneous mastocytosis: demographic aspects and clinical features of 55 patients. J Eur Acad Dermatol Venereol. 2006;20:969-973.
12. Sarkany RP, Monk BE, Handfield-Jones SE. Telangiectasia macularis eruptiva perstans: a case report and review of the literature. Clin Exp Dermatol. 1998;23:38-39.
13. Lack EE, Worsham GF, Callihan MD, et al. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Oncol. 1980;13:301-316.
14. Buley ID, Gatter KC, Kelly PM, et al. Granular cell tumours revisited. an immunohistological and ultrastructural study. Histopathology. 1988;12:263-274.
15. Penneys NS, Adachi K, Ziegels-Weissman J, et al. Granular cell tumors of the skin contain myelin basic protein. Arch Pathol Lab Med. 1983;107:302-303.
16. Modlin RL, Gottlieb B, Taylor C, et al. Identification of cells lining pseudovascular spaces of benign pigmented nevi. Am J Dermatopathol. 1984;(suppl 6):25-29.
17. Fullen DR, Reed JA, Finnerty B, et al. S100A6 preferentially labels type C nevus cells and nevic corpuscles: additional support for Schwannian differentiation of intradermal nevi. J Cutan Pathol. 2001;28:393-399.
18. Newman B, Hu W, Nigro K, et al. Aggressive histiocytic disorders that can involve the skin. J Am Acad Dermatol. 2007;56:302-316.
19. Weedon D. Cutaneous infiltrates—non-lymphoid. In: Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Amsterdam, Netherlands: Elsevier; 2010:937-970.
20. Harrist TJ, Bhan AK, Murphy GF, et al. Histiocytosis-X: in situ characterization of cutaneous infiltrates with monoclonal antibodies. Am J Clin Pathol. 1983;79:294-300.
21. Lau SK, Chu PG, Weiss LM. Immunohistochemical expression of langerin in Langerhans cell histiocytosis and non-Langerhans cell histiocytic disorders. Am J Surg Pathol. 2008;32:615-619.
22. Lesher JL Jr, Allen BS. Multicentric reticulohistiocytosis. J Am Acad Dermatol. 1984;11:713-723.
23. Heathcote JG, Guenther LC, Wallace AC. Multicentric reticulohistiocytosis: a report of a case and a review of the pathology. Pathology. 1985;17:601-608.
Multiple Superficial White Nodules on the Bilateral Helical Rims
The Diagnosis: Bilateral Auricular Tophaceous Gout
Histopathologic evaluation with hematoxylin and eosin staining demonstrated clusters of abundant granular amorphous material within the subcutaneous tissue (Figure 1). The overlying epidermis and dermis were unremarkable. The granular amorphous material demonstrated numerous monosodium urate crystals under polarized light (Figure 2). At a return visit following the biopsy results, the patient reported a history of a single episode of monoarticular gouty arthritis involving the right hallux approximately 6 months after the onset of the skin lesions. With the added clinical history and the biopsy results, his serum uric acid level was obtained and was found to be elevated at 9.2 mg/dL (reference range, 3.5–8 mg/dL).
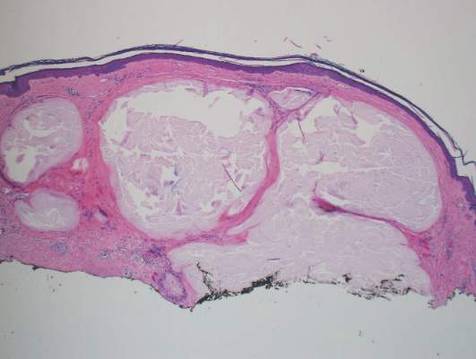
|
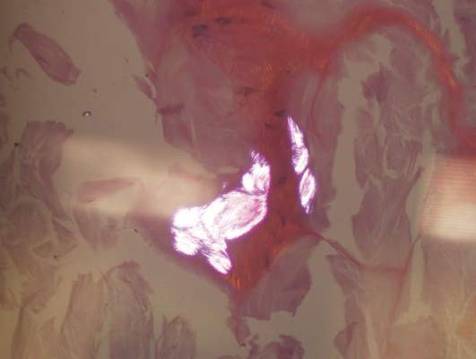
|
In our patient, the clinical differential diagnosis included calcium deposits, weathering nodules, and tophaceous gout. The differential diagnosis of auricular lesions is broad, and benign lesions may mimic cancerous entities such as basal cell carcinoma and squamous cell carcinoma.1 Therefore a detailed history, thorough physical examination, and tissue sampling are key to establishing the correct diagnosis. Our patient’s history of monoarticular gouty arthritis was only elucidated after a diagnosis of bilateral auricular tophaceous gout was made based on the biopsy results.
Subcutaneous tophi represent a chronic state of hyperuricemia and tend to manifest after long-standing polyarthritis and repeated acute gout attacks.2-5 These lesions develop in approximately 50% of gout patients and usually occur an average of 11.6 years after the onset of disease.2 There is a subset of individuals that are at higher risk for developing tophi, including elderly and female patients, diuretic and chronic nonsteroidal anti-inflammatory drug users, patients with a history of cyclosporine therapy, and patients with underlying chronic renal insufficiency.2,6,7 The most commonly affected tissues are those with poor blood supply and lower temperatures, such as the ear helix and first metacarpal joint.4 The auricle is the most common site of tophi on the head and neck. Tophi of the helices are generally asymptomatic and nontender; however, tophi can become large, inflamed, and ulcerated, causing pressure and discomfort.2 Combination treatment with dietary modification and antihyperuricemic therapy (eg, allopurinol) has been shown to reduce the size of lesions and prevent future tophi formation. However, these results may take months, warranting excision of large and symptomatic lesions.4,8
Our case is unusual in that the onset of the auricular lesions predated the articular gout by 6 months. Gouty tophi as the initial presentation of hyperuricemia is rare; however, tophi formation without concomitant arthritis has been reported.2,3,7,9 Wernick et al7 described 6 patients presenting with tophi before the onset of inflammatory arthritis that they attributed to changes in active inflammation by age (eg, elderly patients were more commonly immunosuppressed), chronic illnesses, and anti-inflammatory medications (eg, nonsteroidal anti-inflammatory drugs). Another possible explanation for this atypical presentation is misdiagnosis caused by other forms of arthritis (eg, rheumatoid arthritis, osteoarthritis) masking acute gout episodes. It also has been reported that monosodium urate crystals can be found in synovial fluid with no inflammation and therefore no symptoms.7
Tophi, although rare, may be the sole clinical manifestation of underlying gouty disease. It is important to be aware of this atypical presentation to prevent misdiagnosis and provide appropriate treatment.
- Dompmartin A. Nodules of the external ear [in French]. Ann Dermatol Venereol. 1999;126:261-266.
- Griffin G, Munns J, Fullen D, et al. Auricular tophi as the initial presentation of gout. Otolaryngol Head Neck Surg. 2009;141:153-154.
- Koley S, Salodkar A, Choudhary S, et al. Tophi as first manifestation of gout. Indian J Dermatol Venerol. 2010;76:393-393-396.
- Moriwaki Y. Tophaceous gout [in Japanese]. Nihon Rinsho. 2008;66:711-716.
- Eggebeen AT. Gout: an update. Am Fam Physician. 2007;76:801-808.
- Hollingworth P, Scott JT, Burry HC. Nonarticular gout: hyperuricemia and tophus formation without gouty arthritis. Arthritis Rheum. 1983;26:98-101.
- Wernick R, Winkler C, Campbell S. Tophi as the initial manifestation of gout. report of six cases and review of the literature. Arch Intern Med. 1992;152:873-876.
- Caldas CA, Fuller R. Excellent response to the clinical treatment of tophaceous gout. Clin Rheumatol. 2009;26:1553-1555.
- Iglesias A, Londono JC, Saaibi DL, et al. Gout nodulosis: widespread subcutaneous deposits without gout. Arthritis Care Res. 1996;9:74-77.
The Diagnosis: Bilateral Auricular Tophaceous Gout
Histopathologic evaluation with hematoxylin and eosin staining demonstrated clusters of abundant granular amorphous material within the subcutaneous tissue (Figure 1). The overlying epidermis and dermis were unremarkable. The granular amorphous material demonstrated numerous monosodium urate crystals under polarized light (Figure 2). At a return visit following the biopsy results, the patient reported a history of a single episode of monoarticular gouty arthritis involving the right hallux approximately 6 months after the onset of the skin lesions. With the added clinical history and the biopsy results, his serum uric acid level was obtained and was found to be elevated at 9.2 mg/dL (reference range, 3.5–8 mg/dL).

|

|
In our patient, the clinical differential diagnosis included calcium deposits, weathering nodules, and tophaceous gout. The differential diagnosis of auricular lesions is broad, and benign lesions may mimic cancerous entities such as basal cell carcinoma and squamous cell carcinoma.1 Therefore a detailed history, thorough physical examination, and tissue sampling are key to establishing the correct diagnosis. Our patient’s history of monoarticular gouty arthritis was only elucidated after a diagnosis of bilateral auricular tophaceous gout was made based on the biopsy results.
Subcutaneous tophi represent a chronic state of hyperuricemia and tend to manifest after long-standing polyarthritis and repeated acute gout attacks.2-5 These lesions develop in approximately 50% of gout patients and usually occur an average of 11.6 years after the onset of disease.2 There is a subset of individuals that are at higher risk for developing tophi, including elderly and female patients, diuretic and chronic nonsteroidal anti-inflammatory drug users, patients with a history of cyclosporine therapy, and patients with underlying chronic renal insufficiency.2,6,7 The most commonly affected tissues are those with poor blood supply and lower temperatures, such as the ear helix and first metacarpal joint.4 The auricle is the most common site of tophi on the head and neck. Tophi of the helices are generally asymptomatic and nontender; however, tophi can become large, inflamed, and ulcerated, causing pressure and discomfort.2 Combination treatment with dietary modification and antihyperuricemic therapy (eg, allopurinol) has been shown to reduce the size of lesions and prevent future tophi formation. However, these results may take months, warranting excision of large and symptomatic lesions.4,8
Our case is unusual in that the onset of the auricular lesions predated the articular gout by 6 months. Gouty tophi as the initial presentation of hyperuricemia is rare; however, tophi formation without concomitant arthritis has been reported.2,3,7,9 Wernick et al7 described 6 patients presenting with tophi before the onset of inflammatory arthritis that they attributed to changes in active inflammation by age (eg, elderly patients were more commonly immunosuppressed), chronic illnesses, and anti-inflammatory medications (eg, nonsteroidal anti-inflammatory drugs). Another possible explanation for this atypical presentation is misdiagnosis caused by other forms of arthritis (eg, rheumatoid arthritis, osteoarthritis) masking acute gout episodes. It also has been reported that monosodium urate crystals can be found in synovial fluid with no inflammation and therefore no symptoms.7
Tophi, although rare, may be the sole clinical manifestation of underlying gouty disease. It is important to be aware of this atypical presentation to prevent misdiagnosis and provide appropriate treatment.
The Diagnosis: Bilateral Auricular Tophaceous Gout
Histopathologic evaluation with hematoxylin and eosin staining demonstrated clusters of abundant granular amorphous material within the subcutaneous tissue (Figure 1). The overlying epidermis and dermis were unremarkable. The granular amorphous material demonstrated numerous monosodium urate crystals under polarized light (Figure 2). At a return visit following the biopsy results, the patient reported a history of a single episode of monoarticular gouty arthritis involving the right hallux approximately 6 months after the onset of the skin lesions. With the added clinical history and the biopsy results, his serum uric acid level was obtained and was found to be elevated at 9.2 mg/dL (reference range, 3.5–8 mg/dL).

|

|
In our patient, the clinical differential diagnosis included calcium deposits, weathering nodules, and tophaceous gout. The differential diagnosis of auricular lesions is broad, and benign lesions may mimic cancerous entities such as basal cell carcinoma and squamous cell carcinoma.1 Therefore a detailed history, thorough physical examination, and tissue sampling are key to establishing the correct diagnosis. Our patient’s history of monoarticular gouty arthritis was only elucidated after a diagnosis of bilateral auricular tophaceous gout was made based on the biopsy results.
Subcutaneous tophi represent a chronic state of hyperuricemia and tend to manifest after long-standing polyarthritis and repeated acute gout attacks.2-5 These lesions develop in approximately 50% of gout patients and usually occur an average of 11.6 years after the onset of disease.2 There is a subset of individuals that are at higher risk for developing tophi, including elderly and female patients, diuretic and chronic nonsteroidal anti-inflammatory drug users, patients with a history of cyclosporine therapy, and patients with underlying chronic renal insufficiency.2,6,7 The most commonly affected tissues are those with poor blood supply and lower temperatures, such as the ear helix and first metacarpal joint.4 The auricle is the most common site of tophi on the head and neck. Tophi of the helices are generally asymptomatic and nontender; however, tophi can become large, inflamed, and ulcerated, causing pressure and discomfort.2 Combination treatment with dietary modification and antihyperuricemic therapy (eg, allopurinol) has been shown to reduce the size of lesions and prevent future tophi formation. However, these results may take months, warranting excision of large and symptomatic lesions.4,8
Our case is unusual in that the onset of the auricular lesions predated the articular gout by 6 months. Gouty tophi as the initial presentation of hyperuricemia is rare; however, tophi formation without concomitant arthritis has been reported.2,3,7,9 Wernick et al7 described 6 patients presenting with tophi before the onset of inflammatory arthritis that they attributed to changes in active inflammation by age (eg, elderly patients were more commonly immunosuppressed), chronic illnesses, and anti-inflammatory medications (eg, nonsteroidal anti-inflammatory drugs). Another possible explanation for this atypical presentation is misdiagnosis caused by other forms of arthritis (eg, rheumatoid arthritis, osteoarthritis) masking acute gout episodes. It also has been reported that monosodium urate crystals can be found in synovial fluid with no inflammation and therefore no symptoms.7
Tophi, although rare, may be the sole clinical manifestation of underlying gouty disease. It is important to be aware of this atypical presentation to prevent misdiagnosis and provide appropriate treatment.
- Dompmartin A. Nodules of the external ear [in French]. Ann Dermatol Venereol. 1999;126:261-266.
- Griffin G, Munns J, Fullen D, et al. Auricular tophi as the initial presentation of gout. Otolaryngol Head Neck Surg. 2009;141:153-154.
- Koley S, Salodkar A, Choudhary S, et al. Tophi as first manifestation of gout. Indian J Dermatol Venerol. 2010;76:393-393-396.
- Moriwaki Y. Tophaceous gout [in Japanese]. Nihon Rinsho. 2008;66:711-716.
- Eggebeen AT. Gout: an update. Am Fam Physician. 2007;76:801-808.
- Hollingworth P, Scott JT, Burry HC. Nonarticular gout: hyperuricemia and tophus formation without gouty arthritis. Arthritis Rheum. 1983;26:98-101.
- Wernick R, Winkler C, Campbell S. Tophi as the initial manifestation of gout. report of six cases and review of the literature. Arch Intern Med. 1992;152:873-876.
- Caldas CA, Fuller R. Excellent response to the clinical treatment of tophaceous gout. Clin Rheumatol. 2009;26:1553-1555.
- Iglesias A, Londono JC, Saaibi DL, et al. Gout nodulosis: widespread subcutaneous deposits without gout. Arthritis Care Res. 1996;9:74-77.
- Dompmartin A. Nodules of the external ear [in French]. Ann Dermatol Venereol. 1999;126:261-266.
- Griffin G, Munns J, Fullen D, et al. Auricular tophi as the initial presentation of gout. Otolaryngol Head Neck Surg. 2009;141:153-154.
- Koley S, Salodkar A, Choudhary S, et al. Tophi as first manifestation of gout. Indian J Dermatol Venerol. 2010;76:393-393-396.
- Moriwaki Y. Tophaceous gout [in Japanese]. Nihon Rinsho. 2008;66:711-716.
- Eggebeen AT. Gout: an update. Am Fam Physician. 2007;76:801-808.
- Hollingworth P, Scott JT, Burry HC. Nonarticular gout: hyperuricemia and tophus formation without gouty arthritis. Arthritis Rheum. 1983;26:98-101.
- Wernick R, Winkler C, Campbell S. Tophi as the initial manifestation of gout. report of six cases and review of the literature. Arch Intern Med. 1992;152:873-876.
- Caldas CA, Fuller R. Excellent response to the clinical treatment of tophaceous gout. Clin Rheumatol. 2009;26:1553-1555.
- Iglesias A, Londono JC, Saaibi DL, et al. Gout nodulosis: widespread subcutaneous deposits without gout. Arthritis Care Res. 1996;9:74-77.
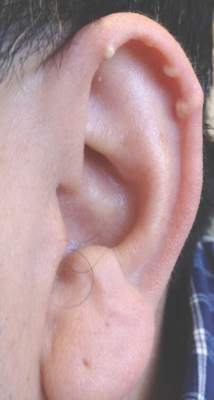
A 40-year-old man presented for evaluation of multiple small nodules on the bilateral auricles primarily involving the helices of 1 year’s duration. The lesions were nontender with no associated bleeding, burning, or pruritus. He denied any trauma to these sites and denied any systemic symptoms including fever, chills, joint pain, or weight loss. His medical history was remarkable for type 2 diabetes mellitus. He had no history of similar skin lesions or renal disease and denied any alcohol intake. He also denied taking any over-the-counter or prescription medications. Physical examination revealed several 1- to 4-mm superficial white dermal nodules located on the bilateral helical rims. The lesions were firm and well circumscribed and the surrounding skin showed mild erythema. Shave biopsies of the nodules were performed.