User login
Judicious Use of Antibiotics in Dermatology
What does your patient need to know at the first visit? Does it apply to patients of all genders, ages, and races?
There are 3 scenarios in which antibiotics are used in dermatology. First, there is the treatment of a bona fide, verified skin infection, which may range from the relatively simple (impetigo) to the complex (botryomycosis) to the exotic (fish tank granuloma). The second scenario is antibiotic administration, often due to ancillary properties such as anti-inflammatory effects, in the management of noninfectious disorders, such as familial benign pemphigus or pityriasis lichenoides et varioliformis acuta. I try hard to avoid antibiotic use in these situations unless all else fails. The third scenario involves use of antibiotics at the patient’s request, usually associated with the phrase “just in case it’s infected.” In my opinion, this practice is completely ill advised.
Male and female patients of all ages, ethnic origins, and socioeconomic backgrounds are woefully uninformed regarding the promise and peril of antibiotics. I want patients to buy into the concept of good antibiotic stewardship. Thus, patients should understand that there must be a specific and justifiable reason for antibiotic use and that the recommended dose and duration of treatment should not be altered. In some situations, antibiotic therapy is intended to be of short duration, while in other situations, such therapy may be quite protracted. Patients also need to know at the outset of treatment when we plan to transition from a short-term, antibiotic-based modality to a long-term nonantibiotic maintenance regimen, which is especially true for acne and rosacea. I try to limit antibiotic use in these disorders to 3 months. Furthermore, patients should always be educated about the potential side effects associated with the particular antibiotic being prescribed. Hoarding and sharing leftover antibiotics should be strongly and explicitly discouraged.
Finally, patients must be educated that taking shortcuts when prescribing antibiotics may lead to therapeutic failure, worsening disease, or serious long-term adverse consequences. For example, rational antibiotic use may require the added expense of an initial and/or subsequent test-of-cure culture and sensitivity. Is that swollen and tender hand following a cat bite due to Pasteurella multocida or methicillin-resistant Staphylococcus aureus? Is that new eruption in an atopic patient due to secondary impetigo or eczema herpeticum? Other laboratory testing also may be required, such as a follow-up serology after treating syphilis. Patients need to know why laboratory tests are being ordered and how the tests complement direct antibiotic intervention.
What are your go-to treatments? What are the side effects?
I am a fan of subantimicrobial-dose doxycycline for both rosacea (on label) and acne (off label). Studies have shown that neither quantitative nor qualitative changes occur in the cutaneous, oral, or gastrointestinal flora. Thus, I avoid contributing to the emerging global crisis of antimicrobial resistance. I am also a proponent of topical antibiotics whenever appropriate and reasonable. Mupirocin and retapamulin, for example, are quite effective for routine cases of impetigo. When incision and drainage alone are insufficient to resolve methicillin-resistant S aureus furunculosis, I prefer either trimethoprim-sulfamethoxazole or doxycycline. Of course, other specific oral and even parenteral antibiotics are appropriate for select disease states.
Although antibiotics generally are well tolerated, there are many possible side effects. Hypersensitivity reactions, ranging from self-limited fixed drug and pruritic maculopapular eruptions through acute urticaria to anaphylaxis, may occur with any antibiotic. Clostridium difficile–associated diarrhea also may occur in conjunction with the use of any antibacterial drug, especially those with a broad spectrum of activity. Nausea and headache are mild but common side effects of these agents. All tetracycline derivatives may be photosensitizers and may provoke intracranial hypertension. Minocycline may lead to hyperpigmentation of skin and teeth, vestibular disturbances (ie, dizziness, ataxia, vertigo, tinnitus) and rarely autoimmune hepatitis. Macrolide antibiotics have been linked to serious cardiotoxicity, and quinolone antibiotics have been linked to tendonitis/tendon rupture, cardiotoxicity, and insomnia. Many antibiotics can result in vaginal yeast infections. There is some evidence that prolonged antibiotic use may precipitate inflammatory bowel disease, especially in those who are genetically predisposed.
Finally, keep in mind that antibiotic administration changes the normal cutaneous flora, which may interfere with the normal antimicrobial and anti-inflammatory homeostatic roles played by resident skin microflora. Antibiotic administration also changes the gut flora and, in this manner, may help promote the development of resistant microbes.
How do you keep patients compliant with treatment?
The most important step to assure adherence is adequate pretreatment education. Whether short-term or long-term antibiotic treatment is anticipated, I always schedule a follow-up office visit in approximately 2 weeks to check on clinical progress and reinforce good habits. Younger patients benefit from periodic reminders using emails, text messages, and tweets.
What do you do if they refuse treatment?
In some instances, antibiotic phobia in patients can be totally accepted and alternative treatments explored. As an example, laser and light therapy, hormonal manipulation, zinc-based nutritional supplements, and intensive nonantibiotic topical combination drugs can supplant antibiotics for the management of acne.
What resources do you recommend to patients for more information?
There are some excellent resources online for patients such as “Using Antibiotics Wisely” and “Get Smart: Know When Antibiotics Work.”
Suggested Readings
Chon SY, Doan HQ, Mays RM. Antibiotic overuse and resistance in dermatology. Dermatol Ther. 2012;25:55-69.
Eichenfield LF, Del Rosso JQ, Mancini AJ, et al. Evolving perspectives on the etiology and pathogenesis of acne vulgaris. J Drugs Dermatol. 2015;14:263-272.
Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol. 2011;131:1974-1980.
Gelband H, Miller-Petrie M, Pant S, et al. The State of the World’s Antibiotics, 2015. Washington, DC: Center for Disease Dynamics, Economics & Policy; 2015. http://cddep.org/publications/state_worlds_antibiotics_2015. Accessed February 11, 2016.
Get smart: know when antibiotics work. Centers for Disease Control and Prevention website. http://www.cdc.gov/getsmart/community/about/index.html. Updated April 17, 2015. Accessed February 11, 2016.
Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: Advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention [published online January 19, 2016]. Ann Intern Med. doi:10.7326/M15-1840.
Kirchner M, Mafura M, Hunt T, et al. Antimicrobial resistance characteristics and fitness of Gram-negative fecal bacteria from volunteers treated with minocycline or amoxicillin. Front Microbiol. 2014;5:722. doi:10.3389/fmicb.2014.00722.
Muhammad M, Rosen T. A controversial proposal: no more antibiotics for acne! Skin Therapy Lett. 2013;18:1-4.
Using antibiotics wisely. WedMD Medical Reference. http://www.webmd.com/a-to-z-guides/using-antibiotics-wisely-topic-overview. Updated November 14, 2014. Accessed February 11, 2016.
What does your patient need to know at the first visit? Does it apply to patients of all genders, ages, and races?
There are 3 scenarios in which antibiotics are used in dermatology. First, there is the treatment of a bona fide, verified skin infection, which may range from the relatively simple (impetigo) to the complex (botryomycosis) to the exotic (fish tank granuloma). The second scenario is antibiotic administration, often due to ancillary properties such as anti-inflammatory effects, in the management of noninfectious disorders, such as familial benign pemphigus or pityriasis lichenoides et varioliformis acuta. I try hard to avoid antibiotic use in these situations unless all else fails. The third scenario involves use of antibiotics at the patient’s request, usually associated with the phrase “just in case it’s infected.” In my opinion, this practice is completely ill advised.
Male and female patients of all ages, ethnic origins, and socioeconomic backgrounds are woefully uninformed regarding the promise and peril of antibiotics. I want patients to buy into the concept of good antibiotic stewardship. Thus, patients should understand that there must be a specific and justifiable reason for antibiotic use and that the recommended dose and duration of treatment should not be altered. In some situations, antibiotic therapy is intended to be of short duration, while in other situations, such therapy may be quite protracted. Patients also need to know at the outset of treatment when we plan to transition from a short-term, antibiotic-based modality to a long-term nonantibiotic maintenance regimen, which is especially true for acne and rosacea. I try to limit antibiotic use in these disorders to 3 months. Furthermore, patients should always be educated about the potential side effects associated with the particular antibiotic being prescribed. Hoarding and sharing leftover antibiotics should be strongly and explicitly discouraged.
Finally, patients must be educated that taking shortcuts when prescribing antibiotics may lead to therapeutic failure, worsening disease, or serious long-term adverse consequences. For example, rational antibiotic use may require the added expense of an initial and/or subsequent test-of-cure culture and sensitivity. Is that swollen and tender hand following a cat bite due to Pasteurella multocida or methicillin-resistant Staphylococcus aureus? Is that new eruption in an atopic patient due to secondary impetigo or eczema herpeticum? Other laboratory testing also may be required, such as a follow-up serology after treating syphilis. Patients need to know why laboratory tests are being ordered and how the tests complement direct antibiotic intervention.
What are your go-to treatments? What are the side effects?
I am a fan of subantimicrobial-dose doxycycline for both rosacea (on label) and acne (off label). Studies have shown that neither quantitative nor qualitative changes occur in the cutaneous, oral, or gastrointestinal flora. Thus, I avoid contributing to the emerging global crisis of antimicrobial resistance. I am also a proponent of topical antibiotics whenever appropriate and reasonable. Mupirocin and retapamulin, for example, are quite effective for routine cases of impetigo. When incision and drainage alone are insufficient to resolve methicillin-resistant S aureus furunculosis, I prefer either trimethoprim-sulfamethoxazole or doxycycline. Of course, other specific oral and even parenteral antibiotics are appropriate for select disease states.
Although antibiotics generally are well tolerated, there are many possible side effects. Hypersensitivity reactions, ranging from self-limited fixed drug and pruritic maculopapular eruptions through acute urticaria to anaphylaxis, may occur with any antibiotic. Clostridium difficile–associated diarrhea also may occur in conjunction with the use of any antibacterial drug, especially those with a broad spectrum of activity. Nausea and headache are mild but common side effects of these agents. All tetracycline derivatives may be photosensitizers and may provoke intracranial hypertension. Minocycline may lead to hyperpigmentation of skin and teeth, vestibular disturbances (ie, dizziness, ataxia, vertigo, tinnitus) and rarely autoimmune hepatitis. Macrolide antibiotics have been linked to serious cardiotoxicity, and quinolone antibiotics have been linked to tendonitis/tendon rupture, cardiotoxicity, and insomnia. Many antibiotics can result in vaginal yeast infections. There is some evidence that prolonged antibiotic use may precipitate inflammatory bowel disease, especially in those who are genetically predisposed.
Finally, keep in mind that antibiotic administration changes the normal cutaneous flora, which may interfere with the normal antimicrobial and anti-inflammatory homeostatic roles played by resident skin microflora. Antibiotic administration also changes the gut flora and, in this manner, may help promote the development of resistant microbes.
How do you keep patients compliant with treatment?
The most important step to assure adherence is adequate pretreatment education. Whether short-term or long-term antibiotic treatment is anticipated, I always schedule a follow-up office visit in approximately 2 weeks to check on clinical progress and reinforce good habits. Younger patients benefit from periodic reminders using emails, text messages, and tweets.
What do you do if they refuse treatment?
In some instances, antibiotic phobia in patients can be totally accepted and alternative treatments explored. As an example, laser and light therapy, hormonal manipulation, zinc-based nutritional supplements, and intensive nonantibiotic topical combination drugs can supplant antibiotics for the management of acne.
What resources do you recommend to patients for more information?
There are some excellent resources online for patients such as “Using Antibiotics Wisely” and “Get Smart: Know When Antibiotics Work.”
What does your patient need to know at the first visit? Does it apply to patients of all genders, ages, and races?
There are 3 scenarios in which antibiotics are used in dermatology. First, there is the treatment of a bona fide, verified skin infection, which may range from the relatively simple (impetigo) to the complex (botryomycosis) to the exotic (fish tank granuloma). The second scenario is antibiotic administration, often due to ancillary properties such as anti-inflammatory effects, in the management of noninfectious disorders, such as familial benign pemphigus or pityriasis lichenoides et varioliformis acuta. I try hard to avoid antibiotic use in these situations unless all else fails. The third scenario involves use of antibiotics at the patient’s request, usually associated with the phrase “just in case it’s infected.” In my opinion, this practice is completely ill advised.
Male and female patients of all ages, ethnic origins, and socioeconomic backgrounds are woefully uninformed regarding the promise and peril of antibiotics. I want patients to buy into the concept of good antibiotic stewardship. Thus, patients should understand that there must be a specific and justifiable reason for antibiotic use and that the recommended dose and duration of treatment should not be altered. In some situations, antibiotic therapy is intended to be of short duration, while in other situations, such therapy may be quite protracted. Patients also need to know at the outset of treatment when we plan to transition from a short-term, antibiotic-based modality to a long-term nonantibiotic maintenance regimen, which is especially true for acne and rosacea. I try to limit antibiotic use in these disorders to 3 months. Furthermore, patients should always be educated about the potential side effects associated with the particular antibiotic being prescribed. Hoarding and sharing leftover antibiotics should be strongly and explicitly discouraged.
Finally, patients must be educated that taking shortcuts when prescribing antibiotics may lead to therapeutic failure, worsening disease, or serious long-term adverse consequences. For example, rational antibiotic use may require the added expense of an initial and/or subsequent test-of-cure culture and sensitivity. Is that swollen and tender hand following a cat bite due to Pasteurella multocida or methicillin-resistant Staphylococcus aureus? Is that new eruption in an atopic patient due to secondary impetigo or eczema herpeticum? Other laboratory testing also may be required, such as a follow-up serology after treating syphilis. Patients need to know why laboratory tests are being ordered and how the tests complement direct antibiotic intervention.
What are your go-to treatments? What are the side effects?
I am a fan of subantimicrobial-dose doxycycline for both rosacea (on label) and acne (off label). Studies have shown that neither quantitative nor qualitative changes occur in the cutaneous, oral, or gastrointestinal flora. Thus, I avoid contributing to the emerging global crisis of antimicrobial resistance. I am also a proponent of topical antibiotics whenever appropriate and reasonable. Mupirocin and retapamulin, for example, are quite effective for routine cases of impetigo. When incision and drainage alone are insufficient to resolve methicillin-resistant S aureus furunculosis, I prefer either trimethoprim-sulfamethoxazole or doxycycline. Of course, other specific oral and even parenteral antibiotics are appropriate for select disease states.
Although antibiotics generally are well tolerated, there are many possible side effects. Hypersensitivity reactions, ranging from self-limited fixed drug and pruritic maculopapular eruptions through acute urticaria to anaphylaxis, may occur with any antibiotic. Clostridium difficile–associated diarrhea also may occur in conjunction with the use of any antibacterial drug, especially those with a broad spectrum of activity. Nausea and headache are mild but common side effects of these agents. All tetracycline derivatives may be photosensitizers and may provoke intracranial hypertension. Minocycline may lead to hyperpigmentation of skin and teeth, vestibular disturbances (ie, dizziness, ataxia, vertigo, tinnitus) and rarely autoimmune hepatitis. Macrolide antibiotics have been linked to serious cardiotoxicity, and quinolone antibiotics have been linked to tendonitis/tendon rupture, cardiotoxicity, and insomnia. Many antibiotics can result in vaginal yeast infections. There is some evidence that prolonged antibiotic use may precipitate inflammatory bowel disease, especially in those who are genetically predisposed.
Finally, keep in mind that antibiotic administration changes the normal cutaneous flora, which may interfere with the normal antimicrobial and anti-inflammatory homeostatic roles played by resident skin microflora. Antibiotic administration also changes the gut flora and, in this manner, may help promote the development of resistant microbes.
How do you keep patients compliant with treatment?
The most important step to assure adherence is adequate pretreatment education. Whether short-term or long-term antibiotic treatment is anticipated, I always schedule a follow-up office visit in approximately 2 weeks to check on clinical progress and reinforce good habits. Younger patients benefit from periodic reminders using emails, text messages, and tweets.
What do you do if they refuse treatment?
In some instances, antibiotic phobia in patients can be totally accepted and alternative treatments explored. As an example, laser and light therapy, hormonal manipulation, zinc-based nutritional supplements, and intensive nonantibiotic topical combination drugs can supplant antibiotics for the management of acne.
What resources do you recommend to patients for more information?
There are some excellent resources online for patients such as “Using Antibiotics Wisely” and “Get Smart: Know When Antibiotics Work.”
Suggested Readings
Chon SY, Doan HQ, Mays RM. Antibiotic overuse and resistance in dermatology. Dermatol Ther. 2012;25:55-69.
Eichenfield LF, Del Rosso JQ, Mancini AJ, et al. Evolving perspectives on the etiology and pathogenesis of acne vulgaris. J Drugs Dermatol. 2015;14:263-272.
Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol. 2011;131:1974-1980.
Gelband H, Miller-Petrie M, Pant S, et al. The State of the World’s Antibiotics, 2015. Washington, DC: Center for Disease Dynamics, Economics & Policy; 2015. http://cddep.org/publications/state_worlds_antibiotics_2015. Accessed February 11, 2016.
Get smart: know when antibiotics work. Centers for Disease Control and Prevention website. http://www.cdc.gov/getsmart/community/about/index.html. Updated April 17, 2015. Accessed February 11, 2016.
Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: Advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention [published online January 19, 2016]. Ann Intern Med. doi:10.7326/M15-1840.
Kirchner M, Mafura M, Hunt T, et al. Antimicrobial resistance characteristics and fitness of Gram-negative fecal bacteria from volunteers treated with minocycline or amoxicillin. Front Microbiol. 2014;5:722. doi:10.3389/fmicb.2014.00722.
Muhammad M, Rosen T. A controversial proposal: no more antibiotics for acne! Skin Therapy Lett. 2013;18:1-4.
Using antibiotics wisely. WedMD Medical Reference. http://www.webmd.com/a-to-z-guides/using-antibiotics-wisely-topic-overview. Updated November 14, 2014. Accessed February 11, 2016.
Suggested Readings
Chon SY, Doan HQ, Mays RM. Antibiotic overuse and resistance in dermatology. Dermatol Ther. 2012;25:55-69.
Eichenfield LF, Del Rosso JQ, Mancini AJ, et al. Evolving perspectives on the etiology and pathogenesis of acne vulgaris. J Drugs Dermatol. 2015;14:263-272.
Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol. 2011;131:1974-1980.
Gelband H, Miller-Petrie M, Pant S, et al. The State of the World’s Antibiotics, 2015. Washington, DC: Center for Disease Dynamics, Economics & Policy; 2015. http://cddep.org/publications/state_worlds_antibiotics_2015. Accessed February 11, 2016.
Get smart: know when antibiotics work. Centers for Disease Control and Prevention website. http://www.cdc.gov/getsmart/community/about/index.html. Updated April 17, 2015. Accessed February 11, 2016.
Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: Advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention [published online January 19, 2016]. Ann Intern Med. doi:10.7326/M15-1840.
Kirchner M, Mafura M, Hunt T, et al. Antimicrobial resistance characteristics and fitness of Gram-negative fecal bacteria from volunteers treated with minocycline or amoxicillin. Front Microbiol. 2014;5:722. doi:10.3389/fmicb.2014.00722.
Muhammad M, Rosen T. A controversial proposal: no more antibiotics for acne! Skin Therapy Lett. 2013;18:1-4.
Using antibiotics wisely. WedMD Medical Reference. http://www.webmd.com/a-to-z-guides/using-antibiotics-wisely-topic-overview. Updated November 14, 2014. Accessed February 11, 2016.
Non–Drug-Induced Pemphigus Foliaceus in a Patient With Rheumatoid Arthritis
To the Editor:
The term pemphigus describes a group of autoimmune blistering diseases that are histologically characterized by intraepidermal blisters caused by acantholysis. There are several types of pemphigus foliaceus, such as classic and endemic pemphigus foliaceus, pemphigus erythematosus, pemphigus herpetiformis, and drug-induced pemphigus foliaceus.1

|
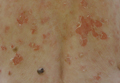
| Figure 1. Multiple erosions and crusted lesions were present on the back. |

|
| Figure 2. Subcorneal bulla containing acantholytic keratinocytes and neutrophils (H&E, original magnification ×100). |
Drug-induced pemphigus foliaceus in patients treated with penicillamine for rheumatoid arthritis (RA) is well documented in the literature.2 An association between pemphigus foliaceus and RA without penicillamine therapy is rare. We present a case of a patient with a history of RA who developed this bullous disease.
A 67-year-old woman with a 15-year history of seropositive RA presented with widespread skin lesions of 4 weeks’ duration. Confluent scaly crusted erosions on an erythematous base were present on the back (Figure 1), chest, and abdomen. There was no alteration of the mucous membranes. Medical treatment consisted of methotrexate (10 mg weekly), folic acid (5 mg twice weekly), prednisolone (5 mg daily), and ketoprofen (50 mg daily). Routine blood analysis was unremarkable, except for a positive rheumatoid factor. Histologic examination showed a subcorneal bulla containing acantholytic keratinocytes and neutrophils. There was a mild lymphocytic and eosinophilic infiltrate in the papillary dermis (Figure 2).
Determination of anti-desmoglein 1 and 3 antibodies was performed by a commercial enzyme-linked immunosorbent assay. Desmoglein 1 antibodies were positive with titers of 30 U/mL (positive, ≥20 U/mL), whereas desmoglein 3 antibody was negative. Thus, a diagnosis of pemphigus foliaceus was established. The polymerase chain reaction ligation-based typing method and the nucleotide sequence was used to examine the protein drought-repressed 4 gene complex, DR4, which tested positive.
Based on a diagnosis of pemphigus foliaceus, the patient’s corticosteroid treatment was changed from 5 mg daily of prednisolone to 40 mg daily of methylprednisolone, leading to marked improvement of the cutaneous lesions. After tapering the steroid dosage over a period of 3 months, no relapse occurred.
Pemphigus foliaceus is a rare autoimmune blistering disease. It can be induced by drugs, such as penicillamine and captopril.2,3 Captopril, an angiotensin-converting enzyme inhibitor, is closely related to penicillamine structurally. Both drugs have highly active thiol groups capable of reducing disulfide bonds and inducing acantholysis.4 The drugs taken by our patient typically are not known to induce pemphigus foliaceus.
The association of pemphigus foliaceus with RA in the absence of penicillamine therapy was first described by Wilkinson et al.4 Since then, additional cases have been published.5,6 Pemphigus foliaceus also has been described with other autoimmune conditions such as autoimmune thyroid disease.7
Rheumatoid arthritis has been genetically linked to the HLA-DR4 gene complex, which also was found in our patient. Patients with pemphigus foliaceus and RA have an increased frequency of the class II major histocompatibility complex, serologically defined HLA-DR4, and HLA-DRw6 haplotypes.4 Therefore, we believe that the association of pemphigus foliaceus and RA in our patient might not be fortuitous.
1. Chams-Davatchi C, Valikhani M, Daneshpazhooh M, et al. Pemphigus: analysis of 1209 cases. Int J Dermatol. 2005;44:470-476.
2. Sugita K, Hirokawa H, Izu K, et al. D-penicillamine-induced pemphigus successfully treated with combination therapy of mizoribine and prednisolone. J Dermatolog Treat. 2004;15:214-217.
3. Kaplan RP, Potter TS, Fox JN. Drug-induced pemphigus related to angiotensin-converting enzyme inhibitors. J Am Acad Dermatol. 1992;26(2, pt 2):364-366.
4. Wilkinson SM, Smith AG, Davis MJ, et al. Rheumatoid arthritis: an association with pemphigus foliaceus. Acta Derm Venereol. 1992;72:289-291.
5. Sáez-de-Ocariz M, Granados J, Yamamoto-Furusho JK, et al. Rheumatoid arthritis associated with pemphigus foliaceus in a patient not taking penicillamine. Skinmed. 2007;6:252-254.
6. Gürcan HM, Ahmed RA. Analysis of current data on the use of methotrexate in the treatment of pemphigus and pemphigoid. Br J Dermatol. 2009;161:723-731.
7. Leshem YA, Katzenelson V, Yosipovitch G, et al. Autoimmune diseases in patients with pemphigus and their first-degree relatives. Int J Dermatol. 2011;50:827-831.
To the Editor:
The term pemphigus describes a group of autoimmune blistering diseases that are histologically characterized by intraepidermal blisters caused by acantholysis. There are several types of pemphigus foliaceus, such as classic and endemic pemphigus foliaceus, pemphigus erythematosus, pemphigus herpetiformis, and drug-induced pemphigus foliaceus.1

|
| Figure 1. Multiple erosions and crusted lesions were present on the back. |

|
| Figure 2. Subcorneal bulla containing acantholytic keratinocytes and neutrophils (H&E, original magnification ×100). |
Drug-induced pemphigus foliaceus in patients treated with penicillamine for rheumatoid arthritis (RA) is well documented in the literature.2 An association between pemphigus foliaceus and RA without penicillamine therapy is rare. We present a case of a patient with a history of RA who developed this bullous disease.
A 67-year-old woman with a 15-year history of seropositive RA presented with widespread skin lesions of 4 weeks’ duration. Confluent scaly crusted erosions on an erythematous base were present on the back (Figure 1), chest, and abdomen. There was no alteration of the mucous membranes. Medical treatment consisted of methotrexate (10 mg weekly), folic acid (5 mg twice weekly), prednisolone (5 mg daily), and ketoprofen (50 mg daily). Routine blood analysis was unremarkable, except for a positive rheumatoid factor. Histologic examination showed a subcorneal bulla containing acantholytic keratinocytes and neutrophils. There was a mild lymphocytic and eosinophilic infiltrate in the papillary dermis (Figure 2).
Determination of anti-desmoglein 1 and 3 antibodies was performed by a commercial enzyme-linked immunosorbent assay. Desmoglein 1 antibodies were positive with titers of 30 U/mL (positive, ≥20 U/mL), whereas desmoglein 3 antibody was negative. Thus, a diagnosis of pemphigus foliaceus was established. The polymerase chain reaction ligation-based typing method and the nucleotide sequence was used to examine the protein drought-repressed 4 gene complex, DR4, which tested positive.
Based on a diagnosis of pemphigus foliaceus, the patient’s corticosteroid treatment was changed from 5 mg daily of prednisolone to 40 mg daily of methylprednisolone, leading to marked improvement of the cutaneous lesions. After tapering the steroid dosage over a period of 3 months, no relapse occurred.
Pemphigus foliaceus is a rare autoimmune blistering disease. It can be induced by drugs, such as penicillamine and captopril.2,3 Captopril, an angiotensin-converting enzyme inhibitor, is closely related to penicillamine structurally. Both drugs have highly active thiol groups capable of reducing disulfide bonds and inducing acantholysis.4 The drugs taken by our patient typically are not known to induce pemphigus foliaceus.
The association of pemphigus foliaceus with RA in the absence of penicillamine therapy was first described by Wilkinson et al.4 Since then, additional cases have been published.5,6 Pemphigus foliaceus also has been described with other autoimmune conditions such as autoimmune thyroid disease.7
Rheumatoid arthritis has been genetically linked to the HLA-DR4 gene complex, which also was found in our patient. Patients with pemphigus foliaceus and RA have an increased frequency of the class II major histocompatibility complex, serologically defined HLA-DR4, and HLA-DRw6 haplotypes.4 Therefore, we believe that the association of pemphigus foliaceus and RA in our patient might not be fortuitous.
To the Editor:
The term pemphigus describes a group of autoimmune blistering diseases that are histologically characterized by intraepidermal blisters caused by acantholysis. There are several types of pemphigus foliaceus, such as classic and endemic pemphigus foliaceus, pemphigus erythematosus, pemphigus herpetiformis, and drug-induced pemphigus foliaceus.1

|
| Figure 1. Multiple erosions and crusted lesions were present on the back. |

|
| Figure 2. Subcorneal bulla containing acantholytic keratinocytes and neutrophils (H&E, original magnification ×100). |
Drug-induced pemphigus foliaceus in patients treated with penicillamine for rheumatoid arthritis (RA) is well documented in the literature.2 An association between pemphigus foliaceus and RA without penicillamine therapy is rare. We present a case of a patient with a history of RA who developed this bullous disease.
A 67-year-old woman with a 15-year history of seropositive RA presented with widespread skin lesions of 4 weeks’ duration. Confluent scaly crusted erosions on an erythematous base were present on the back (Figure 1), chest, and abdomen. There was no alteration of the mucous membranes. Medical treatment consisted of methotrexate (10 mg weekly), folic acid (5 mg twice weekly), prednisolone (5 mg daily), and ketoprofen (50 mg daily). Routine blood analysis was unremarkable, except for a positive rheumatoid factor. Histologic examination showed a subcorneal bulla containing acantholytic keratinocytes and neutrophils. There was a mild lymphocytic and eosinophilic infiltrate in the papillary dermis (Figure 2).
Determination of anti-desmoglein 1 and 3 antibodies was performed by a commercial enzyme-linked immunosorbent assay. Desmoglein 1 antibodies were positive with titers of 30 U/mL (positive, ≥20 U/mL), whereas desmoglein 3 antibody was negative. Thus, a diagnosis of pemphigus foliaceus was established. The polymerase chain reaction ligation-based typing method and the nucleotide sequence was used to examine the protein drought-repressed 4 gene complex, DR4, which tested positive.
Based on a diagnosis of pemphigus foliaceus, the patient’s corticosteroid treatment was changed from 5 mg daily of prednisolone to 40 mg daily of methylprednisolone, leading to marked improvement of the cutaneous lesions. After tapering the steroid dosage over a period of 3 months, no relapse occurred.
Pemphigus foliaceus is a rare autoimmune blistering disease. It can be induced by drugs, such as penicillamine and captopril.2,3 Captopril, an angiotensin-converting enzyme inhibitor, is closely related to penicillamine structurally. Both drugs have highly active thiol groups capable of reducing disulfide bonds and inducing acantholysis.4 The drugs taken by our patient typically are not known to induce pemphigus foliaceus.
The association of pemphigus foliaceus with RA in the absence of penicillamine therapy was first described by Wilkinson et al.4 Since then, additional cases have been published.5,6 Pemphigus foliaceus also has been described with other autoimmune conditions such as autoimmune thyroid disease.7
Rheumatoid arthritis has been genetically linked to the HLA-DR4 gene complex, which also was found in our patient. Patients with pemphigus foliaceus and RA have an increased frequency of the class II major histocompatibility complex, serologically defined HLA-DR4, and HLA-DRw6 haplotypes.4 Therefore, we believe that the association of pemphigus foliaceus and RA in our patient might not be fortuitous.
1. Chams-Davatchi C, Valikhani M, Daneshpazhooh M, et al. Pemphigus: analysis of 1209 cases. Int J Dermatol. 2005;44:470-476.
2. Sugita K, Hirokawa H, Izu K, et al. D-penicillamine-induced pemphigus successfully treated with combination therapy of mizoribine and prednisolone. J Dermatolog Treat. 2004;15:214-217.
3. Kaplan RP, Potter TS, Fox JN. Drug-induced pemphigus related to angiotensin-converting enzyme inhibitors. J Am Acad Dermatol. 1992;26(2, pt 2):364-366.
4. Wilkinson SM, Smith AG, Davis MJ, et al. Rheumatoid arthritis: an association with pemphigus foliaceus. Acta Derm Venereol. 1992;72:289-291.
5. Sáez-de-Ocariz M, Granados J, Yamamoto-Furusho JK, et al. Rheumatoid arthritis associated with pemphigus foliaceus in a patient not taking penicillamine. Skinmed. 2007;6:252-254.
6. Gürcan HM, Ahmed RA. Analysis of current data on the use of methotrexate in the treatment of pemphigus and pemphigoid. Br J Dermatol. 2009;161:723-731.
7. Leshem YA, Katzenelson V, Yosipovitch G, et al. Autoimmune diseases in patients with pemphigus and their first-degree relatives. Int J Dermatol. 2011;50:827-831.
1. Chams-Davatchi C, Valikhani M, Daneshpazhooh M, et al. Pemphigus: analysis of 1209 cases. Int J Dermatol. 2005;44:470-476.
2. Sugita K, Hirokawa H, Izu K, et al. D-penicillamine-induced pemphigus successfully treated with combination therapy of mizoribine and prednisolone. J Dermatolog Treat. 2004;15:214-217.
3. Kaplan RP, Potter TS, Fox JN. Drug-induced pemphigus related to angiotensin-converting enzyme inhibitors. J Am Acad Dermatol. 1992;26(2, pt 2):364-366.
4. Wilkinson SM, Smith AG, Davis MJ, et al. Rheumatoid arthritis: an association with pemphigus foliaceus. Acta Derm Venereol. 1992;72:289-291.
5. Sáez-de-Ocariz M, Granados J, Yamamoto-Furusho JK, et al. Rheumatoid arthritis associated with pemphigus foliaceus in a patient not taking penicillamine. Skinmed. 2007;6:252-254.
6. Gürcan HM, Ahmed RA. Analysis of current data on the use of methotrexate in the treatment of pemphigus and pemphigoid. Br J Dermatol. 2009;161:723-731.
7. Leshem YA, Katzenelson V, Yosipovitch G, et al. Autoimmune diseases in patients with pemphigus and their first-degree relatives. Int J Dermatol. 2011;50:827-831.
Practice Points
- Physicians should consider pemphigus foliaceus in the differential diagnosis in patients with rheumatoid arthritis and blistering eruptions.
- Appropriate analyses should be performed, including skin biopsy for histologic and immunohistochemical examination as well as search for circulating antibodies.
VIDEO – Survey: Isolation, rejection is real for acne patients
WASHINGTON – Acne patients are telling the truth when they describe feeling isolated, rejected, and stigmatized – and now there are data to prove it.
Dr. Alexa B. Kimball, professor of dermatology at Harvard Medical School, Boston, found that almost 70% of people surveyed believe that those with acne are unattractive and hesitate to be seen with them. Her survey of 56 people also found that they harbor fears that acne is infectious and can be transmitted, that it’s caused by poor hygiene and diet.
“The widespread misconceptions about acne contribute to negative perceptions, which can affect patients’ quality of life and social interaction,” Dr. Kimball said. “When our patients describe these feelings, they are describing their real, day-to-day life experiences.”
See more of her comments on treating patients with acne in this video.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Acne patients are telling the truth when they describe feeling isolated, rejected, and stigmatized – and now there are data to prove it.
Dr. Alexa B. Kimball, professor of dermatology at Harvard Medical School, Boston, found that almost 70% of people surveyed believe that those with acne are unattractive and hesitate to be seen with them. Her survey of 56 people also found that they harbor fears that acne is infectious and can be transmitted, that it’s caused by poor hygiene and diet.
“The widespread misconceptions about acne contribute to negative perceptions, which can affect patients’ quality of life and social interaction,” Dr. Kimball said. “When our patients describe these feelings, they are describing their real, day-to-day life experiences.”
See more of her comments on treating patients with acne in this video.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Acne patients are telling the truth when they describe feeling isolated, rejected, and stigmatized – and now there are data to prove it.
Dr. Alexa B. Kimball, professor of dermatology at Harvard Medical School, Boston, found that almost 70% of people surveyed believe that those with acne are unattractive and hesitate to be seen with them. Her survey of 56 people also found that they harbor fears that acne is infectious and can be transmitted, that it’s caused by poor hygiene and diet.
“The widespread misconceptions about acne contribute to negative perceptions, which can affect patients’ quality of life and social interaction,” Dr. Kimball said. “When our patients describe these feelings, they are describing their real, day-to-day life experiences.”
See more of her comments on treating patients with acne in this video.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT AAD 16
New SHM Members – March 2016
D. Holt, Arkansas
S. Bhansali, MD, California
E. Bhansin, BS, DO, California
A. Bogart, MD, California
D. Donald, MD, California
S. Evans, FNP, California
R. Godbout, California
E. Gustafson, MD, California
M. Hasan, MD, California
M. Kantor, MD, California
M. McClellan, California
A. Milin, MD, California
E. Nguyen, California
J. Young, BSN, RN, California
M. Hicks, MD, Colorado
J. Tyler, DO, Colorado
P. Sharma, MHA, Connecticut
J. Meyer, DO, Florida
M. Prabhu, ACMPE, Florida
K. Slazinski, MD, MA, Florida
P. Zipper, Florida
S. Gayle, MD, Georgia
N. Palmer, DO, Georgia
R. Schaefer, MD, Hawaii
E. Diehl, MD, Iowa
C. Kuehn, MD, Iowa
A. Buchwach, MD, Illinois
F. Evangelista, MD, Illinois
S. Kurup, Illinois
J. Shanahan, Illinois
U. Tekin, MD, Illinois
C. Dennis, Massachusetts
D. Gewanter, Massachusetts
S. Master, MD, Massachusetts
C. Mathew, MD, Massachusetts
M. Sakr, MD, Massachusetts
M. Mason, PA-C, Maryland
M. Kowalczyk, ACNP, Minnesota
M. Doose, MD, Minnesota
F. Abualrub, Missouri
R. Adkison, DO, Missouri
S. Katukoori, MD, Missouri
A. Persaud, PA-C, Missouri
L. Gerstle, NP, Montana
S. Brown, MD, North Carolina
S. Okorie, New Jersey
P. Mathew, MD, New Mexico
A. Turney, DO, Nevada
J. Chester, MD, New York
C. Flynn, DO, New York
M. Hoefer, PMGR, New York
M. Islam, MD, New York
C. Karno, MD, New York
S. Mir, New York
P. Nadkarni, MD, New York
A. Rana, New York
G. Rubinfeld, New York
A. Black, MBA, Ohio
R. Cartabuke, MHA, Ohio
P. He, DO, Ohio
R. Rivero, DO, Ohio
K. Welch, Ohio
S. Parker, APRN-BC, Oklahoma
T. Basra, MD, MBBS, Oregon
B. Baxter, Oregon
S. Mehta, MD, Oregon
V. Karper, ACNP, Pennsylvania
D. Messner, MSN, NP, Pennsylvania
M. Scoulos-Hanson, Pennsylvania
K. Willoughby, MD, Pennsylvania
R. Yazdanfar, MD, Pennsylvania
J. Hennessey, CCFP, Canada
J. Brown, MD, South Carolina
K. Medlin, RN, South Carolina
K. Kays, Tennessee
M. Begum, MD, Texas
S. Blinchevsky, MD, Texas
C. Ciborowski, Texas
K. Gupta, MD, Texas
H. Lam, MD, Texas
A. Owens, Texas
K. Salciccioli, BSE, Texas
D. Lundberg, Wisconsin
H. Peto, Wisconsin
B. Quinn, MD, Wisconsin
N. Ros, PhD, Spain
S. Takeuchi, MD, Japan
D. Holt, Arkansas
S. Bhansali, MD, California
E. Bhansin, BS, DO, California
A. Bogart, MD, California
D. Donald, MD, California
S. Evans, FNP, California
R. Godbout, California
E. Gustafson, MD, California
M. Hasan, MD, California
M. Kantor, MD, California
M. McClellan, California
A. Milin, MD, California
E. Nguyen, California
J. Young, BSN, RN, California
M. Hicks, MD, Colorado
J. Tyler, DO, Colorado
P. Sharma, MHA, Connecticut
J. Meyer, DO, Florida
M. Prabhu, ACMPE, Florida
K. Slazinski, MD, MA, Florida
P. Zipper, Florida
S. Gayle, MD, Georgia
N. Palmer, DO, Georgia
R. Schaefer, MD, Hawaii
E. Diehl, MD, Iowa
C. Kuehn, MD, Iowa
A. Buchwach, MD, Illinois
F. Evangelista, MD, Illinois
S. Kurup, Illinois
J. Shanahan, Illinois
U. Tekin, MD, Illinois
C. Dennis, Massachusetts
D. Gewanter, Massachusetts
S. Master, MD, Massachusetts
C. Mathew, MD, Massachusetts
M. Sakr, MD, Massachusetts
M. Mason, PA-C, Maryland
M. Kowalczyk, ACNP, Minnesota
M. Doose, MD, Minnesota
F. Abualrub, Missouri
R. Adkison, DO, Missouri
S. Katukoori, MD, Missouri
A. Persaud, PA-C, Missouri
L. Gerstle, NP, Montana
S. Brown, MD, North Carolina
S. Okorie, New Jersey
P. Mathew, MD, New Mexico
A. Turney, DO, Nevada
J. Chester, MD, New York
C. Flynn, DO, New York
M. Hoefer, PMGR, New York
M. Islam, MD, New York
C. Karno, MD, New York
S. Mir, New York
P. Nadkarni, MD, New York
A. Rana, New York
G. Rubinfeld, New York
A. Black, MBA, Ohio
R. Cartabuke, MHA, Ohio
P. He, DO, Ohio
R. Rivero, DO, Ohio
K. Welch, Ohio
S. Parker, APRN-BC, Oklahoma
T. Basra, MD, MBBS, Oregon
B. Baxter, Oregon
S. Mehta, MD, Oregon
V. Karper, ACNP, Pennsylvania
D. Messner, MSN, NP, Pennsylvania
M. Scoulos-Hanson, Pennsylvania
K. Willoughby, MD, Pennsylvania
R. Yazdanfar, MD, Pennsylvania
J. Hennessey, CCFP, Canada
J. Brown, MD, South Carolina
K. Medlin, RN, South Carolina
K. Kays, Tennessee
M. Begum, MD, Texas
S. Blinchevsky, MD, Texas
C. Ciborowski, Texas
K. Gupta, MD, Texas
H. Lam, MD, Texas
A. Owens, Texas
K. Salciccioli, BSE, Texas
D. Lundberg, Wisconsin
H. Peto, Wisconsin
B. Quinn, MD, Wisconsin
N. Ros, PhD, Spain
S. Takeuchi, MD, Japan
D. Holt, Arkansas
S. Bhansali, MD, California
E. Bhansin, BS, DO, California
A. Bogart, MD, California
D. Donald, MD, California
S. Evans, FNP, California
R. Godbout, California
E. Gustafson, MD, California
M. Hasan, MD, California
M. Kantor, MD, California
M. McClellan, California
A. Milin, MD, California
E. Nguyen, California
J. Young, BSN, RN, California
M. Hicks, MD, Colorado
J. Tyler, DO, Colorado
P. Sharma, MHA, Connecticut
J. Meyer, DO, Florida
M. Prabhu, ACMPE, Florida
K. Slazinski, MD, MA, Florida
P. Zipper, Florida
S. Gayle, MD, Georgia
N. Palmer, DO, Georgia
R. Schaefer, MD, Hawaii
E. Diehl, MD, Iowa
C. Kuehn, MD, Iowa
A. Buchwach, MD, Illinois
F. Evangelista, MD, Illinois
S. Kurup, Illinois
J. Shanahan, Illinois
U. Tekin, MD, Illinois
C. Dennis, Massachusetts
D. Gewanter, Massachusetts
S. Master, MD, Massachusetts
C. Mathew, MD, Massachusetts
M. Sakr, MD, Massachusetts
M. Mason, PA-C, Maryland
M. Kowalczyk, ACNP, Minnesota
M. Doose, MD, Minnesota
F. Abualrub, Missouri
R. Adkison, DO, Missouri
S. Katukoori, MD, Missouri
A. Persaud, PA-C, Missouri
L. Gerstle, NP, Montana
S. Brown, MD, North Carolina
S. Okorie, New Jersey
P. Mathew, MD, New Mexico
A. Turney, DO, Nevada
J. Chester, MD, New York
C. Flynn, DO, New York
M. Hoefer, PMGR, New York
M. Islam, MD, New York
C. Karno, MD, New York
S. Mir, New York
P. Nadkarni, MD, New York
A. Rana, New York
G. Rubinfeld, New York
A. Black, MBA, Ohio
R. Cartabuke, MHA, Ohio
P. He, DO, Ohio
R. Rivero, DO, Ohio
K. Welch, Ohio
S. Parker, APRN-BC, Oklahoma
T. Basra, MD, MBBS, Oregon
B. Baxter, Oregon
S. Mehta, MD, Oregon
V. Karper, ACNP, Pennsylvania
D. Messner, MSN, NP, Pennsylvania
M. Scoulos-Hanson, Pennsylvania
K. Willoughby, MD, Pennsylvania
R. Yazdanfar, MD, Pennsylvania
J. Hennessey, CCFP, Canada
J. Brown, MD, South Carolina
K. Medlin, RN, South Carolina
K. Kays, Tennessee
M. Begum, MD, Texas
S. Blinchevsky, MD, Texas
C. Ciborowski, Texas
K. Gupta, MD, Texas
H. Lam, MD, Texas
A. Owens, Texas
K. Salciccioli, BSE, Texas
D. Lundberg, Wisconsin
H. Peto, Wisconsin
B. Quinn, MD, Wisconsin
N. Ros, PhD, Spain
S. Takeuchi, MD, Japan
FDA approves long-acting hemophilia B therapy
The US Food and Drug Administration (FDA) has approved Idelvion (Coagulation Factor IX [Recombinant], Albumin Fusion Protein) for the treatment of hemophilia B.
The product—a fusion protein linking recombinant coagulation factor IX with recombinant albumin—is intended for use in children and adults for routine prophylaxis to prevent or reduce the frequency of bleeding episodes, for on-demand control and prevention of bleeding episodes, and for the perioperative management of bleeding.
Appropriate patients age 12 and older can go up to 14 days between Idelvion infusions. This dosing interval has been achieved while maintaining high levels of factor IX activity—above 5% over 14 days at 75 IU/kg.
Idelvion is the first FDA-approved recombinant factor IX therapy that can extend the dosing interval up to 14 days.
Idelvion is expected to be available in the US later this month. The product is being developed by CSL Behring. For more details on the drug, see the full prescribing information.
Phase 3 trial
The FDA approved Idelvion based on results of the PROLONG-9FP clinical development program. PROLONG-9FP includes phase 1, 2, and 3 studies evaluating the safety and efficacy of Idelvion in adults and children (ages 1 to 61) with hemophilia B.
Data from the phase 3 study were recently published in Blood. This study included 63 previously treated male patients with severe hemophilia B. They had a mean age of 33 (range, 12 to 61).
The patients were divided into 2 groups. Group 1 (n=40) received routine prophylaxis with Idelvion once every 7 days for 26 weeks, followed by a 7-, 10- or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively.
Group 2 received on-demand treatment with Idelvion for bleeding episodes for 26 weeks (n=23) and then switched to a 7-day prophylaxis regimen for a mean of 45 weeks (n=19).
The median annualized bleeding rate (ABR) was 2.0 in the prophylaxis arm (group 1) and 23.5 in the on-demand treatment arm (group 2). The median spontaneous ABRs were 0.0 and 17.0, respectively.
For patients in group 2, there was a significant reduction in median ABRs when patients switched from on-demand treatment to prophylaxis—19.22 and 1.58, respectively (P<0.0001). And there was a significant reduction in median spontaneous ABRs—15.43 and 0.00, respectively (P<0.0001).
Overall, 98.6% of bleeding episodes were treated successfully, including 93.6% that were treated with a single injection of Idelvion.
None of the patients developed inhibitors or experienced thromboembolic events, anaphylaxis, or life-threatening adverse events (AEs).
There were 347 treatment-emergent AEs reported in 54 (85.7%) patients. The most common were nasopharyngitis (25.4%), headache (23.8%), arthralgia (4.3%), and influenza (11.1%).
Eleven mild/moderate AEs in 5 patients (7.9%) were considered possibly related to Idelvion. Two patients discontinued treatment due to AEs—1 with hypersensitivity and 1 with headache. ![]()
The US Food and Drug Administration (FDA) has approved Idelvion (Coagulation Factor IX [Recombinant], Albumin Fusion Protein) for the treatment of hemophilia B.
The product—a fusion protein linking recombinant coagulation factor IX with recombinant albumin—is intended for use in children and adults for routine prophylaxis to prevent or reduce the frequency of bleeding episodes, for on-demand control and prevention of bleeding episodes, and for the perioperative management of bleeding.
Appropriate patients age 12 and older can go up to 14 days between Idelvion infusions. This dosing interval has been achieved while maintaining high levels of factor IX activity—above 5% over 14 days at 75 IU/kg.
Idelvion is the first FDA-approved recombinant factor IX therapy that can extend the dosing interval up to 14 days.
Idelvion is expected to be available in the US later this month. The product is being developed by CSL Behring. For more details on the drug, see the full prescribing information.
Phase 3 trial
The FDA approved Idelvion based on results of the PROLONG-9FP clinical development program. PROLONG-9FP includes phase 1, 2, and 3 studies evaluating the safety and efficacy of Idelvion in adults and children (ages 1 to 61) with hemophilia B.
Data from the phase 3 study were recently published in Blood. This study included 63 previously treated male patients with severe hemophilia B. They had a mean age of 33 (range, 12 to 61).
The patients were divided into 2 groups. Group 1 (n=40) received routine prophylaxis with Idelvion once every 7 days for 26 weeks, followed by a 7-, 10- or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively.
Group 2 received on-demand treatment with Idelvion for bleeding episodes for 26 weeks (n=23) and then switched to a 7-day prophylaxis regimen for a mean of 45 weeks (n=19).
The median annualized bleeding rate (ABR) was 2.0 in the prophylaxis arm (group 1) and 23.5 in the on-demand treatment arm (group 2). The median spontaneous ABRs were 0.0 and 17.0, respectively.
For patients in group 2, there was a significant reduction in median ABRs when patients switched from on-demand treatment to prophylaxis—19.22 and 1.58, respectively (P<0.0001). And there was a significant reduction in median spontaneous ABRs—15.43 and 0.00, respectively (P<0.0001).
Overall, 98.6% of bleeding episodes were treated successfully, including 93.6% that were treated with a single injection of Idelvion.
None of the patients developed inhibitors or experienced thromboembolic events, anaphylaxis, or life-threatening adverse events (AEs).
There were 347 treatment-emergent AEs reported in 54 (85.7%) patients. The most common were nasopharyngitis (25.4%), headache (23.8%), arthralgia (4.3%), and influenza (11.1%).
Eleven mild/moderate AEs in 5 patients (7.9%) were considered possibly related to Idelvion. Two patients discontinued treatment due to AEs—1 with hypersensitivity and 1 with headache. ![]()
The US Food and Drug Administration (FDA) has approved Idelvion (Coagulation Factor IX [Recombinant], Albumin Fusion Protein) for the treatment of hemophilia B.
The product—a fusion protein linking recombinant coagulation factor IX with recombinant albumin—is intended for use in children and adults for routine prophylaxis to prevent or reduce the frequency of bleeding episodes, for on-demand control and prevention of bleeding episodes, and for the perioperative management of bleeding.
Appropriate patients age 12 and older can go up to 14 days between Idelvion infusions. This dosing interval has been achieved while maintaining high levels of factor IX activity—above 5% over 14 days at 75 IU/kg.
Idelvion is the first FDA-approved recombinant factor IX therapy that can extend the dosing interval up to 14 days.
Idelvion is expected to be available in the US later this month. The product is being developed by CSL Behring. For more details on the drug, see the full prescribing information.
Phase 3 trial
The FDA approved Idelvion based on results of the PROLONG-9FP clinical development program. PROLONG-9FP includes phase 1, 2, and 3 studies evaluating the safety and efficacy of Idelvion in adults and children (ages 1 to 61) with hemophilia B.
Data from the phase 3 study were recently published in Blood. This study included 63 previously treated male patients with severe hemophilia B. They had a mean age of 33 (range, 12 to 61).
The patients were divided into 2 groups. Group 1 (n=40) received routine prophylaxis with Idelvion once every 7 days for 26 weeks, followed by a 7-, 10- or 14-day prophylaxis regimen for a mean of 50, 38, or 51 weeks, respectively.
Group 2 received on-demand treatment with Idelvion for bleeding episodes for 26 weeks (n=23) and then switched to a 7-day prophylaxis regimen for a mean of 45 weeks (n=19).
The median annualized bleeding rate (ABR) was 2.0 in the prophylaxis arm (group 1) and 23.5 in the on-demand treatment arm (group 2). The median spontaneous ABRs were 0.0 and 17.0, respectively.
For patients in group 2, there was a significant reduction in median ABRs when patients switched from on-demand treatment to prophylaxis—19.22 and 1.58, respectively (P<0.0001). And there was a significant reduction in median spontaneous ABRs—15.43 and 0.00, respectively (P<0.0001).
Overall, 98.6% of bleeding episodes were treated successfully, including 93.6% that were treated with a single injection of Idelvion.
None of the patients developed inhibitors or experienced thromboembolic events, anaphylaxis, or life-threatening adverse events (AEs).
There were 347 treatment-emergent AEs reported in 54 (85.7%) patients. The most common were nasopharyngitis (25.4%), headache (23.8%), arthralgia (4.3%), and influenza (11.1%).
Eleven mild/moderate AEs in 5 patients (7.9%) were considered possibly related to Idelvion. Two patients discontinued treatment due to AEs—1 with hypersensitivity and 1 with headache. ![]()
U.S. flu activity falls for first time since early January
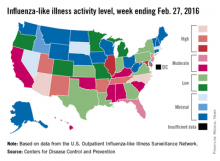
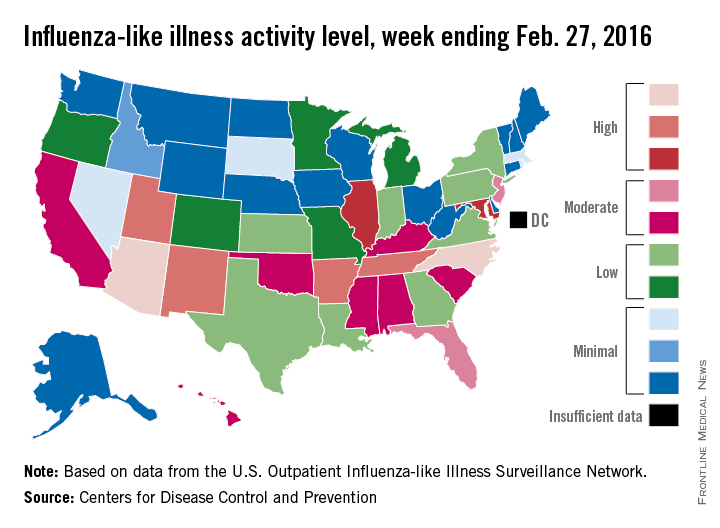
Influenza-like illness (ILI) activity in the 2015-2016 U.S. flu season declined for the first time since early January, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for ILI was reported at 3.2% for the week ending Feb. 20, but the CDC has adjusted that figure to 3.3%, which makes the 3.2% reported for this most recent week (week 20 of the season, ending Feb. 27, 2016) a decrease from the week before.
Despite that drop, two states were at level 10 on the CDC’s 1-10 scale of ILI activity for the first time this season. Arizona had already reached level 10, and joining it there last week was North Carolina, moving up from level 8 the week before. Other states in the “high” range of activity were Arkansas, New Mexico, Tennessee, and Utah at level 9, and Illinois and Maryland at level 8, the CDC reported March 4. Puerto Rico, which had been at level 10 for several weeks, moved down to level 8.
States in the “moderate” range of activity for the week ending Feb. 27 were Florida and New Jersey at level 7 and Alabama, California, Hawaii, Kentucky, Mississippi, Oklahoma, and South Carolina at level 6. Altogether, there were 35 states at level 2 or higher, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
Four pediatric ILI-related deaths were reported to the CDC during week 20, but three actually occurred during week 19. There have been 18 ILI-related deaths so far during the 2015-2016 season, the CDC said.
Influenza-like illness (ILI) activity in the 2015-2016 U.S. flu season declined for the first time since early January, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for ILI was reported at 3.2% for the week ending Feb. 20, but the CDC has adjusted that figure to 3.3%, which makes the 3.2% reported for this most recent week (week 20 of the season, ending Feb. 27, 2016) a decrease from the week before.
Despite that drop, two states were at level 10 on the CDC’s 1-10 scale of ILI activity for the first time this season. Arizona had already reached level 10, and joining it there last week was North Carolina, moving up from level 8 the week before. Other states in the “high” range of activity were Arkansas, New Mexico, Tennessee, and Utah at level 9, and Illinois and Maryland at level 8, the CDC reported March 4. Puerto Rico, which had been at level 10 for several weeks, moved down to level 8.
States in the “moderate” range of activity for the week ending Feb. 27 were Florida and New Jersey at level 7 and Alabama, California, Hawaii, Kentucky, Mississippi, Oklahoma, and South Carolina at level 6. Altogether, there were 35 states at level 2 or higher, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
Four pediatric ILI-related deaths were reported to the CDC during week 20, but three actually occurred during week 19. There have been 18 ILI-related deaths so far during the 2015-2016 season, the CDC said.
Influenza-like illness (ILI) activity in the 2015-2016 U.S. flu season declined for the first time since early January, according to the Centers for Disease Control and Prevention.
The proportion of outpatient visits for ILI was reported at 3.2% for the week ending Feb. 20, but the CDC has adjusted that figure to 3.3%, which makes the 3.2% reported for this most recent week (week 20 of the season, ending Feb. 27, 2016) a decrease from the week before.
Despite that drop, two states were at level 10 on the CDC’s 1-10 scale of ILI activity for the first time this season. Arizona had already reached level 10, and joining it there last week was North Carolina, moving up from level 8 the week before. Other states in the “high” range of activity were Arkansas, New Mexico, Tennessee, and Utah at level 9, and Illinois and Maryland at level 8, the CDC reported March 4. Puerto Rico, which had been at level 10 for several weeks, moved down to level 8.
States in the “moderate” range of activity for the week ending Feb. 27 were Florida and New Jersey at level 7 and Alabama, California, Hawaii, Kentucky, Mississippi, Oklahoma, and South Carolina at level 6. Altogether, there were 35 states at level 2 or higher, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
Four pediatric ILI-related deaths were reported to the CDC during week 20, but three actually occurred during week 19. There have been 18 ILI-related deaths so far during the 2015-2016 season, the CDC said.
Guidelines: Combine topical, oral therapy for most effective acne treatment
WASHINGTON – Monotherapy is not recommended in treating moderate-severe acne, and antibiotics should always be coupled with topical therapy, according to the latest guidelines from the American Academy of Dermatology.
And although it may be hard – even nearly impossible – to discontinue antibiotics completely, patients should be reevaluated every 3-4 months to determine whether reducing the dosage may be possible while maintaining effectiveness, the document says.
AAD published the guideline on Feb 17. At the academy’s annual meeting, a panel met to discuss its practical application.
Topical therapy
Benzoyl peroxide is a first-line agent that not only effectively fights Propionibacterium acnes, but also discourages the development of antibiotic resistance. Topical antibiotics also decrease P. acnes populations and exert a mild anti-inflammatory effect; however, monotherapy with a topical antibiotic is strongly discouraged. These should be used in combination with another agent such as a retinoid, benzoyl peroxide, adapalene, azelaic acid, or dapsone. This approach decreases the chance of antibiotic resistance, attacks the acne on several fronts, and provides for a maintenance transition.
Systemic antibiotics
Tetracycline-class antibiotics are still the best option for moderate-severe acne. A Cochrane review found that minocycline and doxycycline are equally effective (Cochrane Skin Group Nov 2011. doi: 10.1002/14651858.CD002086.pub2).
The incidence of adverse events associated with each is low, although minocycline may be marginally more troublesome. Low doses seem to be as effective as traditional doses, but pulsed therapy is inadequate. To prevent antibiotic resistance, limit both dose and length of therapy as much as possible. This can best be accomplished by adding a topical agent – either benzoyl peroxide or a retinoid – to the regimen.
“This is critical,” Dr. Jonette E. Keri of the University of Miami said at the meeting. “When antibiotics are eventually discontinued, the retinoid will fulfill the need for maintenance therapy.”
Hormonal agents
Four combination oral contraceptives are Food and Drug Administration–approved for acne treatment. Each of them decreases androgens by interrupting the pathway of testosterone production. There are no data suggesting that one is better than the other; patient preferences and their individual clinical picture should drive choice. Because of the cardiovascular risks associated with these combination OCs, they should not be prescribed for anyone with a personal or family history of clotting disorders or thromboembolic events. Smoking should also be a contraindication.
Oral contraceptives can be tried alone or as part of a comprehensive treatment regimen, including one containing antibiotics. Rifampin and griseofulvin are the only antibiotics known to decrease the contraceptive effect of the medications.
The tincture of time is an important part of this therapy, said Dr. Diane M. Thiboutot, professor of dermatology at Pennsylvania State University, Hershey. “You can’t rush it. It may take three cycles to see any real improvement in acne, and patients should be aware of this.”
Isotretinoin
Oral isotretinoin is a highly effective treatment for severe, recalcitrant acne. It decreases sebum production, acne lesion count, and scarring. Despite concerns about depression and suicidality, isotretinoin treatment can actually improve mood in most patients, said Dr. Megha M. Tollefson of the Mayo Clinic, Rochester, Minn.
“A very well-done Swedish study published in 2010 in BMJ found a slightly increased risk of suicide in the first 6 months after treatment started, but that risk was already rising before treatment started, so it could [be unrelated] to the drug,” she said. “And, in those who got isotretinoin, the [suicide] rate after that was actually decreased, compared to the general population.”
Female patients need education on isotretinoin’s teratogenic potential. After discussions, they should sign the SMART or iPLEDGE agreements about using effective birth control while taking the drug. Unfortunately, Dr. Tollefson said, “We continue to see hundreds of isotretinoin-exposed pregnancies each year.”
A recent study found that up to 30% of women did not comply with the birth control measures they agreed to while taking the drug (J Am Acad Dermatol. 2011 Oct. doi: 10.1016/j.jaad.2013.08.034).
The link between isotretinoin and inflammatory bowel disease is not well founded, Dr. Tollefson said. Studies have been contradictory, and most evidence is based on case report and association studies. There is, however, some evidence suggesting an innate connection between acne and inflammatory bowel disease, she noted.
Diet
Emerging evidence suggests that high glycemic diets may be associated with acne, but these studies are small. However, those randomized to a low glycemic index diet showed decreased sebum production and inflammation.
A small case-control study in 2012 suggested a link between milk and acne. AAD makes no recommendation based on this. Milk remains an important source of calcium and vitamin D for Americans, especially children, the panel said.
Dr. Tollefson had no financial disclosures. Dr. Keri said she has been a consultant for Hoffmann-LaRoche.
WASHINGTON – Monotherapy is not recommended in treating moderate-severe acne, and antibiotics should always be coupled with topical therapy, according to the latest guidelines from the American Academy of Dermatology.
And although it may be hard – even nearly impossible – to discontinue antibiotics completely, patients should be reevaluated every 3-4 months to determine whether reducing the dosage may be possible while maintaining effectiveness, the document says.
AAD published the guideline on Feb 17. At the academy’s annual meeting, a panel met to discuss its practical application.
Topical therapy
Benzoyl peroxide is a first-line agent that not only effectively fights Propionibacterium acnes, but also discourages the development of antibiotic resistance. Topical antibiotics also decrease P. acnes populations and exert a mild anti-inflammatory effect; however, monotherapy with a topical antibiotic is strongly discouraged. These should be used in combination with another agent such as a retinoid, benzoyl peroxide, adapalene, azelaic acid, or dapsone. This approach decreases the chance of antibiotic resistance, attacks the acne on several fronts, and provides for a maintenance transition.
Systemic antibiotics
Tetracycline-class antibiotics are still the best option for moderate-severe acne. A Cochrane review found that minocycline and doxycycline are equally effective (Cochrane Skin Group Nov 2011. doi: 10.1002/14651858.CD002086.pub2).
The incidence of adverse events associated with each is low, although minocycline may be marginally more troublesome. Low doses seem to be as effective as traditional doses, but pulsed therapy is inadequate. To prevent antibiotic resistance, limit both dose and length of therapy as much as possible. This can best be accomplished by adding a topical agent – either benzoyl peroxide or a retinoid – to the regimen.
“This is critical,” Dr. Jonette E. Keri of the University of Miami said at the meeting. “When antibiotics are eventually discontinued, the retinoid will fulfill the need for maintenance therapy.”
Hormonal agents
Four combination oral contraceptives are Food and Drug Administration–approved for acne treatment. Each of them decreases androgens by interrupting the pathway of testosterone production. There are no data suggesting that one is better than the other; patient preferences and their individual clinical picture should drive choice. Because of the cardiovascular risks associated with these combination OCs, they should not be prescribed for anyone with a personal or family history of clotting disorders or thromboembolic events. Smoking should also be a contraindication.
Oral contraceptives can be tried alone or as part of a comprehensive treatment regimen, including one containing antibiotics. Rifampin and griseofulvin are the only antibiotics known to decrease the contraceptive effect of the medications.
The tincture of time is an important part of this therapy, said Dr. Diane M. Thiboutot, professor of dermatology at Pennsylvania State University, Hershey. “You can’t rush it. It may take three cycles to see any real improvement in acne, and patients should be aware of this.”
Isotretinoin
Oral isotretinoin is a highly effective treatment for severe, recalcitrant acne. It decreases sebum production, acne lesion count, and scarring. Despite concerns about depression and suicidality, isotretinoin treatment can actually improve mood in most patients, said Dr. Megha M. Tollefson of the Mayo Clinic, Rochester, Minn.
“A very well-done Swedish study published in 2010 in BMJ found a slightly increased risk of suicide in the first 6 months after treatment started, but that risk was already rising before treatment started, so it could [be unrelated] to the drug,” she said. “And, in those who got isotretinoin, the [suicide] rate after that was actually decreased, compared to the general population.”
Female patients need education on isotretinoin’s teratogenic potential. After discussions, they should sign the SMART or iPLEDGE agreements about using effective birth control while taking the drug. Unfortunately, Dr. Tollefson said, “We continue to see hundreds of isotretinoin-exposed pregnancies each year.”
A recent study found that up to 30% of women did not comply with the birth control measures they agreed to while taking the drug (J Am Acad Dermatol. 2011 Oct. doi: 10.1016/j.jaad.2013.08.034).
The link between isotretinoin and inflammatory bowel disease is not well founded, Dr. Tollefson said. Studies have been contradictory, and most evidence is based on case report and association studies. There is, however, some evidence suggesting an innate connection between acne and inflammatory bowel disease, she noted.
Diet
Emerging evidence suggests that high glycemic diets may be associated with acne, but these studies are small. However, those randomized to a low glycemic index diet showed decreased sebum production and inflammation.
A small case-control study in 2012 suggested a link between milk and acne. AAD makes no recommendation based on this. Milk remains an important source of calcium and vitamin D for Americans, especially children, the panel said.
Dr. Tollefson had no financial disclosures. Dr. Keri said she has been a consultant for Hoffmann-LaRoche.
WASHINGTON – Monotherapy is not recommended in treating moderate-severe acne, and antibiotics should always be coupled with topical therapy, according to the latest guidelines from the American Academy of Dermatology.
And although it may be hard – even nearly impossible – to discontinue antibiotics completely, patients should be reevaluated every 3-4 months to determine whether reducing the dosage may be possible while maintaining effectiveness, the document says.
AAD published the guideline on Feb 17. At the academy’s annual meeting, a panel met to discuss its practical application.
Topical therapy
Benzoyl peroxide is a first-line agent that not only effectively fights Propionibacterium acnes, but also discourages the development of antibiotic resistance. Topical antibiotics also decrease P. acnes populations and exert a mild anti-inflammatory effect; however, monotherapy with a topical antibiotic is strongly discouraged. These should be used in combination with another agent such as a retinoid, benzoyl peroxide, adapalene, azelaic acid, or dapsone. This approach decreases the chance of antibiotic resistance, attacks the acne on several fronts, and provides for a maintenance transition.
Systemic antibiotics
Tetracycline-class antibiotics are still the best option for moderate-severe acne. A Cochrane review found that minocycline and doxycycline are equally effective (Cochrane Skin Group Nov 2011. doi: 10.1002/14651858.CD002086.pub2).
The incidence of adverse events associated with each is low, although minocycline may be marginally more troublesome. Low doses seem to be as effective as traditional doses, but pulsed therapy is inadequate. To prevent antibiotic resistance, limit both dose and length of therapy as much as possible. This can best be accomplished by adding a topical agent – either benzoyl peroxide or a retinoid – to the regimen.
“This is critical,” Dr. Jonette E. Keri of the University of Miami said at the meeting. “When antibiotics are eventually discontinued, the retinoid will fulfill the need for maintenance therapy.”
Hormonal agents
Four combination oral contraceptives are Food and Drug Administration–approved for acne treatment. Each of them decreases androgens by interrupting the pathway of testosterone production. There are no data suggesting that one is better than the other; patient preferences and their individual clinical picture should drive choice. Because of the cardiovascular risks associated with these combination OCs, they should not be prescribed for anyone with a personal or family history of clotting disorders or thromboembolic events. Smoking should also be a contraindication.
Oral contraceptives can be tried alone or as part of a comprehensive treatment regimen, including one containing antibiotics. Rifampin and griseofulvin are the only antibiotics known to decrease the contraceptive effect of the medications.
The tincture of time is an important part of this therapy, said Dr. Diane M. Thiboutot, professor of dermatology at Pennsylvania State University, Hershey. “You can’t rush it. It may take three cycles to see any real improvement in acne, and patients should be aware of this.”
Isotretinoin
Oral isotretinoin is a highly effective treatment for severe, recalcitrant acne. It decreases sebum production, acne lesion count, and scarring. Despite concerns about depression and suicidality, isotretinoin treatment can actually improve mood in most patients, said Dr. Megha M. Tollefson of the Mayo Clinic, Rochester, Minn.
“A very well-done Swedish study published in 2010 in BMJ found a slightly increased risk of suicide in the first 6 months after treatment started, but that risk was already rising before treatment started, so it could [be unrelated] to the drug,” she said. “And, in those who got isotretinoin, the [suicide] rate after that was actually decreased, compared to the general population.”
Female patients need education on isotretinoin’s teratogenic potential. After discussions, they should sign the SMART or iPLEDGE agreements about using effective birth control while taking the drug. Unfortunately, Dr. Tollefson said, “We continue to see hundreds of isotretinoin-exposed pregnancies each year.”
A recent study found that up to 30% of women did not comply with the birth control measures they agreed to while taking the drug (J Am Acad Dermatol. 2011 Oct. doi: 10.1016/j.jaad.2013.08.034).
The link between isotretinoin and inflammatory bowel disease is not well founded, Dr. Tollefson said. Studies have been contradictory, and most evidence is based on case report and association studies. There is, however, some evidence suggesting an innate connection between acne and inflammatory bowel disease, she noted.
Diet
Emerging evidence suggests that high glycemic diets may be associated with acne, but these studies are small. However, those randomized to a low glycemic index diet showed decreased sebum production and inflammation.
A small case-control study in 2012 suggested a link between milk and acne. AAD makes no recommendation based on this. Milk remains an important source of calcium and vitamin D for Americans, especially children, the panel said.
Dr. Tollefson had no financial disclosures. Dr. Keri said she has been a consultant for Hoffmann-LaRoche.
AT AAD 2016
FDA approves ibrutinib as first-line CLL therapy

Photo courtesy of Janssen
The US Food and Drug Administration (FDA) has approved the BTK inhibitor ibrutinib (Imbruvica) as a first-line treatment for patients with chronic lymphocytic leukemia (CLL).
This means ibrutinib is now FDA-approved to treat CLL patients regardless of their treatment history, including patients with 17p deletion.
Ibrutinib is also FDA-approved to treat Waldenström’s macroglobulinemia, and the drug was granted accelerated approval to treat patients with mantle cell lymphoma who have received at least 1 prior therapy.
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc. For more details on the drug, see the full prescribing information, available at imbruvica.com.
RESONATE-2 trial
The latest FDA approval for ibrutinib is based on results from the phase 3 RESONATE-2 trial (PCYC-1115), which were presented at the 2015 ASH Annual Meeting and simultaneously published in NEJM.
RESONATE-2 enrolled 269 treatment-naïve patients with CLL or small lymphocytic lymphoma who were 65 or older.
Patients were randomized to receive ibrutinib (n=136) at 420 mg once a day until progression or unacceptable toxicity, or chlorambucil (n=133) on days 1 and 15 of each 28-day cycle for up to 12 cycles. The starting dose for chlorambucil in cycle 1 was 0.5 mg/kg and was increased based on tolerability in cycle 2 by increments of 0.1 mg/kg to a maximum of 0.8 mg/kg.
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC) according to the International Workshop on Chronic Lymphocytic Leukemia (iWCLL) 2008 criteria, with modification for treatment-related lymphocytosis.
Key secondary endpoints included overall response rate (based on the same iWCLL criteria), overall survival (OS), and safety.
Ibrutinib significantly prolonged PFS, as determined by the IRC, reducing the risk of progression or death by 84% compared to chlorambucil. The hazard ratio was 0.16 (P<0.001). The median PFS was not reached in the ibrutinib arm but was 18.9 months for the chlorambucil arm.
Ibrutinib significantly prolonged OS as well, although the median OS was not reached in either treatment arm. The OS rate at 24 months was 98% with ibrutinib and 85% with chlorambucil. The relative risk of death with ibrutinib was 84% lower than that with chlorambucil. The hazard ratio was 0.16 (P=0.001).
Ibrutinib was associated with a significantly higher IRC-assessed overall response rate compared to chlorambucil—82% and 35%, respectively (P<0.0001). Five patients (4%) in the ibrutinib arm achieved a complete response, as did 2 patients (2%) in the chlorambucil arm.
The median duration of treatment was 17.4 months in the ibrutinib arm and 7.1 months in the chlorambucil arm.
The most common adverse events of any grade—in the ibrutinib and chlorambucil arms, respectively—were diarrhea (42% and 17%), fatigue (30% and 38%), cough (22% and 15%), nausea (22% and 39%), peripheral edema (19% and 9%), dry eye (17% and 5%), arthralgia (16% and 7%), neutropenia (16% and 23%), and vomiting (13% and 20%).
Adverse events of grade 3 or higher—in the ibrutinib and chlorambucil arms, respectively—were neutropenia (10% and 18%), anemia (6% and 8%), hypertension (4% and 0%), pneumonia (4% and 2%), diarrhea (4% and 0%), maculopapular rash (3% and 2%), decreased platelet count (3% and 1%), abdominal pain (3% and 1%), hyponatremia (3% and 0%), thrombocytopenia (2% and 6%), febrile neutropenia (2% and 2%), upper respiratory tract infection (2% and 2%), pleural effusion (2% and 1%), cellulitis (2% and 0%), fatigue (1% and 5%), syncope (1% and 2%), and hemolytic anemia (0% and 2%). ![]()

Photo courtesy of Janssen
The US Food and Drug Administration (FDA) has approved the BTK inhibitor ibrutinib (Imbruvica) as a first-line treatment for patients with chronic lymphocytic leukemia (CLL).
This means ibrutinib is now FDA-approved to treat CLL patients regardless of their treatment history, including patients with 17p deletion.
Ibrutinib is also FDA-approved to treat Waldenström’s macroglobulinemia, and the drug was granted accelerated approval to treat patients with mantle cell lymphoma who have received at least 1 prior therapy.
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc. For more details on the drug, see the full prescribing information, available at imbruvica.com.
RESONATE-2 trial
The latest FDA approval for ibrutinib is based on results from the phase 3 RESONATE-2 trial (PCYC-1115), which were presented at the 2015 ASH Annual Meeting and simultaneously published in NEJM.
RESONATE-2 enrolled 269 treatment-naïve patients with CLL or small lymphocytic lymphoma who were 65 or older.
Patients were randomized to receive ibrutinib (n=136) at 420 mg once a day until progression or unacceptable toxicity, or chlorambucil (n=133) on days 1 and 15 of each 28-day cycle for up to 12 cycles. The starting dose for chlorambucil in cycle 1 was 0.5 mg/kg and was increased based on tolerability in cycle 2 by increments of 0.1 mg/kg to a maximum of 0.8 mg/kg.
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC) according to the International Workshop on Chronic Lymphocytic Leukemia (iWCLL) 2008 criteria, with modification for treatment-related lymphocytosis.
Key secondary endpoints included overall response rate (based on the same iWCLL criteria), overall survival (OS), and safety.
Ibrutinib significantly prolonged PFS, as determined by the IRC, reducing the risk of progression or death by 84% compared to chlorambucil. The hazard ratio was 0.16 (P<0.001). The median PFS was not reached in the ibrutinib arm but was 18.9 months for the chlorambucil arm.
Ibrutinib significantly prolonged OS as well, although the median OS was not reached in either treatment arm. The OS rate at 24 months was 98% with ibrutinib and 85% with chlorambucil. The relative risk of death with ibrutinib was 84% lower than that with chlorambucil. The hazard ratio was 0.16 (P=0.001).
Ibrutinib was associated with a significantly higher IRC-assessed overall response rate compared to chlorambucil—82% and 35%, respectively (P<0.0001). Five patients (4%) in the ibrutinib arm achieved a complete response, as did 2 patients (2%) in the chlorambucil arm.
The median duration of treatment was 17.4 months in the ibrutinib arm and 7.1 months in the chlorambucil arm.
The most common adverse events of any grade—in the ibrutinib and chlorambucil arms, respectively—were diarrhea (42% and 17%), fatigue (30% and 38%), cough (22% and 15%), nausea (22% and 39%), peripheral edema (19% and 9%), dry eye (17% and 5%), arthralgia (16% and 7%), neutropenia (16% and 23%), and vomiting (13% and 20%).
Adverse events of grade 3 or higher—in the ibrutinib and chlorambucil arms, respectively—were neutropenia (10% and 18%), anemia (6% and 8%), hypertension (4% and 0%), pneumonia (4% and 2%), diarrhea (4% and 0%), maculopapular rash (3% and 2%), decreased platelet count (3% and 1%), abdominal pain (3% and 1%), hyponatremia (3% and 0%), thrombocytopenia (2% and 6%), febrile neutropenia (2% and 2%), upper respiratory tract infection (2% and 2%), pleural effusion (2% and 1%), cellulitis (2% and 0%), fatigue (1% and 5%), syncope (1% and 2%), and hemolytic anemia (0% and 2%). ![]()

Photo courtesy of Janssen
The US Food and Drug Administration (FDA) has approved the BTK inhibitor ibrutinib (Imbruvica) as a first-line treatment for patients with chronic lymphocytic leukemia (CLL).
This means ibrutinib is now FDA-approved to treat CLL patients regardless of their treatment history, including patients with 17p deletion.
Ibrutinib is also FDA-approved to treat Waldenström’s macroglobulinemia, and the drug was granted accelerated approval to treat patients with mantle cell lymphoma who have received at least 1 prior therapy.
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc. For more details on the drug, see the full prescribing information, available at imbruvica.com.
RESONATE-2 trial
The latest FDA approval for ibrutinib is based on results from the phase 3 RESONATE-2 trial (PCYC-1115), which were presented at the 2015 ASH Annual Meeting and simultaneously published in NEJM.
RESONATE-2 enrolled 269 treatment-naïve patients with CLL or small lymphocytic lymphoma who were 65 or older.
Patients were randomized to receive ibrutinib (n=136) at 420 mg once a day until progression or unacceptable toxicity, or chlorambucil (n=133) on days 1 and 15 of each 28-day cycle for up to 12 cycles. The starting dose for chlorambucil in cycle 1 was 0.5 mg/kg and was increased based on tolerability in cycle 2 by increments of 0.1 mg/kg to a maximum of 0.8 mg/kg.
The primary endpoint of the study was progression-free survival (PFS), as assessed by an independent review committee (IRC) according to the International Workshop on Chronic Lymphocytic Leukemia (iWCLL) 2008 criteria, with modification for treatment-related lymphocytosis.
Key secondary endpoints included overall response rate (based on the same iWCLL criteria), overall survival (OS), and safety.
Ibrutinib significantly prolonged PFS, as determined by the IRC, reducing the risk of progression or death by 84% compared to chlorambucil. The hazard ratio was 0.16 (P<0.001). The median PFS was not reached in the ibrutinib arm but was 18.9 months for the chlorambucil arm.
Ibrutinib significantly prolonged OS as well, although the median OS was not reached in either treatment arm. The OS rate at 24 months was 98% with ibrutinib and 85% with chlorambucil. The relative risk of death with ibrutinib was 84% lower than that with chlorambucil. The hazard ratio was 0.16 (P=0.001).
Ibrutinib was associated with a significantly higher IRC-assessed overall response rate compared to chlorambucil—82% and 35%, respectively (P<0.0001). Five patients (4%) in the ibrutinib arm achieved a complete response, as did 2 patients (2%) in the chlorambucil arm.
The median duration of treatment was 17.4 months in the ibrutinib arm and 7.1 months in the chlorambucil arm.
The most common adverse events of any grade—in the ibrutinib and chlorambucil arms, respectively—were diarrhea (42% and 17%), fatigue (30% and 38%), cough (22% and 15%), nausea (22% and 39%), peripheral edema (19% and 9%), dry eye (17% and 5%), arthralgia (16% and 7%), neutropenia (16% and 23%), and vomiting (13% and 20%).
Adverse events of grade 3 or higher—in the ibrutinib and chlorambucil arms, respectively—were neutropenia (10% and 18%), anemia (6% and 8%), hypertension (4% and 0%), pneumonia (4% and 2%), diarrhea (4% and 0%), maculopapular rash (3% and 2%), decreased platelet count (3% and 1%), abdominal pain (3% and 1%), hyponatremia (3% and 0%), thrombocytopenia (2% and 6%), febrile neutropenia (2% and 2%), upper respiratory tract infection (2% and 2%), pleural effusion (2% and 1%), cellulitis (2% and 0%), fatigue (1% and 5%), syncope (1% and 2%), and hemolytic anemia (0% and 2%). ![]()
CMS Introduces Billing Code for Hospitalists: What You Need to Know
The Centers for Medicare & Medicaid Services (CMS) recently announced the approval of a dedicated specialty billing code for hospitalists that will soon be ready for official use. This is a monumental step for hospital medicine, which continues to be the fastest growing medical specialty in the U.S., with more than 48,000 practitioners identifying as hospitalists.
The Hospitalist recently discussed the implications of this decision with Ron Greeno, MD, MHM, chief strategy officer for IPC Healthcare and chair of SHM’s Public Policy Committee (PPC), and Josh Boswell, director of government relations at SHM, to answer questions raised by SHM members.
Question: What are the benefits to hospitalists using the code?
Dr. Greeno: As we transition from fee-for-service to quality-based payment models, using this code will become critical to ensure hospitalists are reimbursed and evaluated fairly. Under the current code structure, hospitalists are missing opportunities to be rewarded and may be penalized unnecessarily because they are required to identify with internal medicine, family medicine, or another specialty that most closely resembles their daily practice. What current measures do not account for is that hospitalists’ patients are inherently more complex than those seen by practitioners in these other—most often outpatient—specialties. We as hospitalists face unique challenges and work with patients from all demographics, often with severe illnesses, making it nearly impossible to rely on benchmarks used for these other specialties.
There are a few prime examples of this that illustrate the need for the new code. Under the current system, some quality-based patient satisfaction measures under MACRA, on which hospitalists are being evaluated, pertain to the outpatient setting, including waiting room quality and office staff–irrelevant measurements for hospitalists. Hospitalists are also often incorrectly penalized under meaningful use due to complications brought on by observation status and its classification as an outpatient stay. This can cause both quality and cost measures to be extremely flawed and can misrepresent the performance and cost of hospitalists and hospital medicine groups. In the current billing structure, there is no way to accurately identify hospitalists and enable a definite fix to these problems.
To get what we want (fair measurement using relevant metrics), we must be able to identify as a separate group, and fortunately, now we can. There will be benefits we don’t even know about yet. We have to wait and see how healthcare policy continues to evolve and change moving forward. What we do know is that having this code will help us shape MACRA and future healthcare policy so that it works better for hospitalists as the specialty continues to grow in scope and impact.
Q: When will the new code go into effect?
Boswell: While there is not a set date at this time, CMS has reported that it can take up to a year, mostly due to technical changes that need to be made within their own systems. The code has already been officially approved; we just need to wait a bit longer to actually use it.
Q: What happens to hospitalists if they do not use the code?
Dr. Greeno: Some hospitalists might be nervous about the change after having billed a certain way for so long. While there is no absolute requirement for hospitalists to use the new code, the bottom line is that if hospitalists do not adopt the new code, they risk not receiving fair evaluations. Using this code should provide hospitalists with greater insight into their own performance—the data will be much more accurate and meaningful. This will allow hospitalists to hone in on areas needing improvement and provide them with more confidence that they are being compared using accurate benchmarks.
I want to stress that hospitalists, or in some cases their hospital medicine groups, will need to physically change their specialty affiliation when the code becomes effective. Otherwise, they risk not reaping the benefits associated with the new code and will continue to be evaluated using less-than-optimal benchmarks. The ball is in their court to make the change when the code is available, and SHM will serve as a resource to help ensure they know what to do and when.
Q: Where can someone go to find the code? Will it be available on the CMS website?
Boswell: When the code does become available for use, it will be communicated through various channels at SHM and also through the Medicare Learning Network, the site that houses education, information, and resources for healthcare professionals. It will also likely be distributed through additional Medicare circulars and newsletters.
As more details from CMS become available, we will have more specific information to share with members, including information on our website, webinars with billing and coding experts, email communication, and more. Continue to watch your email and social media channels for the latest updates and information.
Q: What role did SHM play in bringing this code to fruition?
Boswell: We can say with confidence that this effort was driven entirely by SHM. To start, a formal application needs to be filed in order for a code to even be considered. After determining that the benefits associated with this code far outweighed the costs and then receiving the support of our board of directors, SHM’s staff and PPC members collaborated to draft a brief and made the argument for the addition of a hospitalist billing code based on the individual elements CMS requires for consideration.
Due to the fact hospital medicine doesn’t have a board certification, while solid, our argument was far from a slam dunk. After submitting the application, SHM continuously followed up with and pressured CMS through various channels and utilized our grassroots network of hospitalists on the Hill to put this code on legislators’ radars—the result was pressure getting applied from interested members of Congress as well. If it weren’t for the persistent advocacy efforts of SHM and its members over the past several years, this code would not have even been considered, let alone approved.
This is a significant development—to our knowledge, this is the first medical specialty to be granted a code without also having a board certification. We’re thrilled that what we have been advocating for on behalf of our members is now a reality!
For the latest information on the new hospitalist billing code and other important healthcare policy updates, continue to check for SHM emails and follow SHM’s social media channels, including @SHMLive and @SHMAdvocacy on Twitter.
Sign up for the network to get the latest news in healthcare policy and discover opportunities to advocate for yourself and fellow hospitalists. TH
Brett Radler is SHM’s communications coordinator.
The Centers for Medicare & Medicaid Services (CMS) recently announced the approval of a dedicated specialty billing code for hospitalists that will soon be ready for official use. This is a monumental step for hospital medicine, which continues to be the fastest growing medical specialty in the U.S., with more than 48,000 practitioners identifying as hospitalists.
The Hospitalist recently discussed the implications of this decision with Ron Greeno, MD, MHM, chief strategy officer for IPC Healthcare and chair of SHM’s Public Policy Committee (PPC), and Josh Boswell, director of government relations at SHM, to answer questions raised by SHM members.
Question: What are the benefits to hospitalists using the code?
Dr. Greeno: As we transition from fee-for-service to quality-based payment models, using this code will become critical to ensure hospitalists are reimbursed and evaluated fairly. Under the current code structure, hospitalists are missing opportunities to be rewarded and may be penalized unnecessarily because they are required to identify with internal medicine, family medicine, or another specialty that most closely resembles their daily practice. What current measures do not account for is that hospitalists’ patients are inherently more complex than those seen by practitioners in these other—most often outpatient—specialties. We as hospitalists face unique challenges and work with patients from all demographics, often with severe illnesses, making it nearly impossible to rely on benchmarks used for these other specialties.
There are a few prime examples of this that illustrate the need for the new code. Under the current system, some quality-based patient satisfaction measures under MACRA, on which hospitalists are being evaluated, pertain to the outpatient setting, including waiting room quality and office staff–irrelevant measurements for hospitalists. Hospitalists are also often incorrectly penalized under meaningful use due to complications brought on by observation status and its classification as an outpatient stay. This can cause both quality and cost measures to be extremely flawed and can misrepresent the performance and cost of hospitalists and hospital medicine groups. In the current billing structure, there is no way to accurately identify hospitalists and enable a definite fix to these problems.
To get what we want (fair measurement using relevant metrics), we must be able to identify as a separate group, and fortunately, now we can. There will be benefits we don’t even know about yet. We have to wait and see how healthcare policy continues to evolve and change moving forward. What we do know is that having this code will help us shape MACRA and future healthcare policy so that it works better for hospitalists as the specialty continues to grow in scope and impact.
Q: When will the new code go into effect?
Boswell: While there is not a set date at this time, CMS has reported that it can take up to a year, mostly due to technical changes that need to be made within their own systems. The code has already been officially approved; we just need to wait a bit longer to actually use it.
Q: What happens to hospitalists if they do not use the code?
Dr. Greeno: Some hospitalists might be nervous about the change after having billed a certain way for so long. While there is no absolute requirement for hospitalists to use the new code, the bottom line is that if hospitalists do not adopt the new code, they risk not receiving fair evaluations. Using this code should provide hospitalists with greater insight into their own performance—the data will be much more accurate and meaningful. This will allow hospitalists to hone in on areas needing improvement and provide them with more confidence that they are being compared using accurate benchmarks.
I want to stress that hospitalists, or in some cases their hospital medicine groups, will need to physically change their specialty affiliation when the code becomes effective. Otherwise, they risk not reaping the benefits associated with the new code and will continue to be evaluated using less-than-optimal benchmarks. The ball is in their court to make the change when the code is available, and SHM will serve as a resource to help ensure they know what to do and when.
Q: Where can someone go to find the code? Will it be available on the CMS website?
Boswell: When the code does become available for use, it will be communicated through various channels at SHM and also through the Medicare Learning Network, the site that houses education, information, and resources for healthcare professionals. It will also likely be distributed through additional Medicare circulars and newsletters.
As more details from CMS become available, we will have more specific information to share with members, including information on our website, webinars with billing and coding experts, email communication, and more. Continue to watch your email and social media channels for the latest updates and information.
Q: What role did SHM play in bringing this code to fruition?
Boswell: We can say with confidence that this effort was driven entirely by SHM. To start, a formal application needs to be filed in order for a code to even be considered. After determining that the benefits associated with this code far outweighed the costs and then receiving the support of our board of directors, SHM’s staff and PPC members collaborated to draft a brief and made the argument for the addition of a hospitalist billing code based on the individual elements CMS requires for consideration.
Due to the fact hospital medicine doesn’t have a board certification, while solid, our argument was far from a slam dunk. After submitting the application, SHM continuously followed up with and pressured CMS through various channels and utilized our grassroots network of hospitalists on the Hill to put this code on legislators’ radars—the result was pressure getting applied from interested members of Congress as well. If it weren’t for the persistent advocacy efforts of SHM and its members over the past several years, this code would not have even been considered, let alone approved.
This is a significant development—to our knowledge, this is the first medical specialty to be granted a code without also having a board certification. We’re thrilled that what we have been advocating for on behalf of our members is now a reality!
For the latest information on the new hospitalist billing code and other important healthcare policy updates, continue to check for SHM emails and follow SHM’s social media channels, including @SHMLive and @SHMAdvocacy on Twitter.
Sign up for the network to get the latest news in healthcare policy and discover opportunities to advocate for yourself and fellow hospitalists. TH
Brett Radler is SHM’s communications coordinator.
The Centers for Medicare & Medicaid Services (CMS) recently announced the approval of a dedicated specialty billing code for hospitalists that will soon be ready for official use. This is a monumental step for hospital medicine, which continues to be the fastest growing medical specialty in the U.S., with more than 48,000 practitioners identifying as hospitalists.
The Hospitalist recently discussed the implications of this decision with Ron Greeno, MD, MHM, chief strategy officer for IPC Healthcare and chair of SHM’s Public Policy Committee (PPC), and Josh Boswell, director of government relations at SHM, to answer questions raised by SHM members.
Question: What are the benefits to hospitalists using the code?
Dr. Greeno: As we transition from fee-for-service to quality-based payment models, using this code will become critical to ensure hospitalists are reimbursed and evaluated fairly. Under the current code structure, hospitalists are missing opportunities to be rewarded and may be penalized unnecessarily because they are required to identify with internal medicine, family medicine, or another specialty that most closely resembles their daily practice. What current measures do not account for is that hospitalists’ patients are inherently more complex than those seen by practitioners in these other—most often outpatient—specialties. We as hospitalists face unique challenges and work with patients from all demographics, often with severe illnesses, making it nearly impossible to rely on benchmarks used for these other specialties.
There are a few prime examples of this that illustrate the need for the new code. Under the current system, some quality-based patient satisfaction measures under MACRA, on which hospitalists are being evaluated, pertain to the outpatient setting, including waiting room quality and office staff–irrelevant measurements for hospitalists. Hospitalists are also often incorrectly penalized under meaningful use due to complications brought on by observation status and its classification as an outpatient stay. This can cause both quality and cost measures to be extremely flawed and can misrepresent the performance and cost of hospitalists and hospital medicine groups. In the current billing structure, there is no way to accurately identify hospitalists and enable a definite fix to these problems.
To get what we want (fair measurement using relevant metrics), we must be able to identify as a separate group, and fortunately, now we can. There will be benefits we don’t even know about yet. We have to wait and see how healthcare policy continues to evolve and change moving forward. What we do know is that having this code will help us shape MACRA and future healthcare policy so that it works better for hospitalists as the specialty continues to grow in scope and impact.
Q: When will the new code go into effect?
Boswell: While there is not a set date at this time, CMS has reported that it can take up to a year, mostly due to technical changes that need to be made within their own systems. The code has already been officially approved; we just need to wait a bit longer to actually use it.
Q: What happens to hospitalists if they do not use the code?
Dr. Greeno: Some hospitalists might be nervous about the change after having billed a certain way for so long. While there is no absolute requirement for hospitalists to use the new code, the bottom line is that if hospitalists do not adopt the new code, they risk not receiving fair evaluations. Using this code should provide hospitalists with greater insight into their own performance—the data will be much more accurate and meaningful. This will allow hospitalists to hone in on areas needing improvement and provide them with more confidence that they are being compared using accurate benchmarks.
I want to stress that hospitalists, or in some cases their hospital medicine groups, will need to physically change their specialty affiliation when the code becomes effective. Otherwise, they risk not reaping the benefits associated with the new code and will continue to be evaluated using less-than-optimal benchmarks. The ball is in their court to make the change when the code is available, and SHM will serve as a resource to help ensure they know what to do and when.
Q: Where can someone go to find the code? Will it be available on the CMS website?
Boswell: When the code does become available for use, it will be communicated through various channels at SHM and also through the Medicare Learning Network, the site that houses education, information, and resources for healthcare professionals. It will also likely be distributed through additional Medicare circulars and newsletters.
As more details from CMS become available, we will have more specific information to share with members, including information on our website, webinars with billing and coding experts, email communication, and more. Continue to watch your email and social media channels for the latest updates and information.
Q: What role did SHM play in bringing this code to fruition?
Boswell: We can say with confidence that this effort was driven entirely by SHM. To start, a formal application needs to be filed in order for a code to even be considered. After determining that the benefits associated with this code far outweighed the costs and then receiving the support of our board of directors, SHM’s staff and PPC members collaborated to draft a brief and made the argument for the addition of a hospitalist billing code based on the individual elements CMS requires for consideration.
Due to the fact hospital medicine doesn’t have a board certification, while solid, our argument was far from a slam dunk. After submitting the application, SHM continuously followed up with and pressured CMS through various channels and utilized our grassroots network of hospitalists on the Hill to put this code on legislators’ radars—the result was pressure getting applied from interested members of Congress as well. If it weren’t for the persistent advocacy efforts of SHM and its members over the past several years, this code would not have even been considered, let alone approved.
This is a significant development—to our knowledge, this is the first medical specialty to be granted a code without also having a board certification. We’re thrilled that what we have been advocating for on behalf of our members is now a reality!
For the latest information on the new hospitalist billing code and other important healthcare policy updates, continue to check for SHM emails and follow SHM’s social media channels, including @SHMLive and @SHMAdvocacy on Twitter.
Sign up for the network to get the latest news in healthcare policy and discover opportunities to advocate for yourself and fellow hospitalists. TH
Brett Radler is SHM’s communications coordinator.
Vaccines committee approves recommended influenza strains for 2016-2017 vaccine
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee unanimously approved recommendations regarding the trivalent and quadrivalent influenza vaccines to be distributed during the 2016-2017 flu season.
The 14-member committee voted that the components of the trivalent influenza vaccine for the upcoming flu season should include an A/California/7/2009 (H1N1) pdm09-like virus; an A/Hong Kong/4801/2014 (H3N2)-like virus; and a B/Brisbane/60/2008-like virus of the B/Victoria lineage.
Additionally, the quadrivalent influenza vaccine should include a B/Phuket/3073/2013-like virus of the B/Yamagata lineage as “the second influenza B strain in the vaccine.”
All four components correspond with the recommendations of the World Health Organization, which announced its proposed components for influenza vaccines in the Northern Hemisphere on Feb. 25.
“I’m comfortable trying to follow the footprint of the virus we’ve seen today, quite elegantly put out in front of us,” said committee member Dr. Sarah Long, professor of pediatrics at Drexel University in Philadelphia, adding that she was “very pleased with what happened in the last year” regarding the predictions of dominant virus strains and the effectiveness of the eventual vaccine.
The proposed vaccine for next season differs from the one distributed during the 2015-16 flu season. While both vaccines contain the identical California strain of influenza A and the Phuket strain of influenza B, the 2015-2016 vaccine included an A/Switzerland/9715293/2013 (H3N2)-like virus in its trivalent form, and a B/Brisbane/60/2008-like virus of the B/Victoria lineage in its quadrivalent form.
“Timely vaccine supply requires close collaboration and communication between multiple stakeholders to ensure sufficient provision of [a] well-matched vaccine,” said Matthew Downham, Ph.D., associate director of biopharmaceutical development, AstraZeneca. Timely strain selection will ensure “vaccine availability and usage” for the most widespread and effective coverage.
While the FDA is not obligated to follow the recommendations of the Vaccines and Related Biological Products Advisory Committee, it generally does.
None of the committee members reported any relevant financial disclosures, nor were there any waivers for conflicts of interest.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee unanimously approved recommendations regarding the trivalent and quadrivalent influenza vaccines to be distributed during the 2016-2017 flu season.
The 14-member committee voted that the components of the trivalent influenza vaccine for the upcoming flu season should include an A/California/7/2009 (H1N1) pdm09-like virus; an A/Hong Kong/4801/2014 (H3N2)-like virus; and a B/Brisbane/60/2008-like virus of the B/Victoria lineage.
Additionally, the quadrivalent influenza vaccine should include a B/Phuket/3073/2013-like virus of the B/Yamagata lineage as “the second influenza B strain in the vaccine.”
All four components correspond with the recommendations of the World Health Organization, which announced its proposed components for influenza vaccines in the Northern Hemisphere on Feb. 25.
“I’m comfortable trying to follow the footprint of the virus we’ve seen today, quite elegantly put out in front of us,” said committee member Dr. Sarah Long, professor of pediatrics at Drexel University in Philadelphia, adding that she was “very pleased with what happened in the last year” regarding the predictions of dominant virus strains and the effectiveness of the eventual vaccine.
The proposed vaccine for next season differs from the one distributed during the 2015-16 flu season. While both vaccines contain the identical California strain of influenza A and the Phuket strain of influenza B, the 2015-2016 vaccine included an A/Switzerland/9715293/2013 (H3N2)-like virus in its trivalent form, and a B/Brisbane/60/2008-like virus of the B/Victoria lineage in its quadrivalent form.
“Timely vaccine supply requires close collaboration and communication between multiple stakeholders to ensure sufficient provision of [a] well-matched vaccine,” said Matthew Downham, Ph.D., associate director of biopharmaceutical development, AstraZeneca. Timely strain selection will ensure “vaccine availability and usage” for the most widespread and effective coverage.
While the FDA is not obligated to follow the recommendations of the Vaccines and Related Biological Products Advisory Committee, it generally does.
None of the committee members reported any relevant financial disclosures, nor were there any waivers for conflicts of interest.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee unanimously approved recommendations regarding the trivalent and quadrivalent influenza vaccines to be distributed during the 2016-2017 flu season.
The 14-member committee voted that the components of the trivalent influenza vaccine for the upcoming flu season should include an A/California/7/2009 (H1N1) pdm09-like virus; an A/Hong Kong/4801/2014 (H3N2)-like virus; and a B/Brisbane/60/2008-like virus of the B/Victoria lineage.
Additionally, the quadrivalent influenza vaccine should include a B/Phuket/3073/2013-like virus of the B/Yamagata lineage as “the second influenza B strain in the vaccine.”
All four components correspond with the recommendations of the World Health Organization, which announced its proposed components for influenza vaccines in the Northern Hemisphere on Feb. 25.
“I’m comfortable trying to follow the footprint of the virus we’ve seen today, quite elegantly put out in front of us,” said committee member Dr. Sarah Long, professor of pediatrics at Drexel University in Philadelphia, adding that she was “very pleased with what happened in the last year” regarding the predictions of dominant virus strains and the effectiveness of the eventual vaccine.
The proposed vaccine for next season differs from the one distributed during the 2015-16 flu season. While both vaccines contain the identical California strain of influenza A and the Phuket strain of influenza B, the 2015-2016 vaccine included an A/Switzerland/9715293/2013 (H3N2)-like virus in its trivalent form, and a B/Brisbane/60/2008-like virus of the B/Victoria lineage in its quadrivalent form.
“Timely vaccine supply requires close collaboration and communication between multiple stakeholders to ensure sufficient provision of [a] well-matched vaccine,” said Matthew Downham, Ph.D., associate director of biopharmaceutical development, AstraZeneca. Timely strain selection will ensure “vaccine availability and usage” for the most widespread and effective coverage.
While the FDA is not obligated to follow the recommendations of the Vaccines and Related Biological Products Advisory Committee, it generally does.
None of the committee members reported any relevant financial disclosures, nor were there any waivers for conflicts of interest.
FROM AN FDA ADVISORY COMMITTEE MEETING