User login
ECT may improve course of bipolar disorder
Electroconvulsive therapy increased illness-free intervals and reduced manic and depressive episodes in bipolar patients, reported Dr. Gian Paolo Minnai of the psychiatry unit at San Martino Hospital in Oristano, Italy, and coauthors.
In a retrospective study of 41 patients with bipolar disorder, the investigators analyzed the number of episodes and admissions 5 years before and after ECT treatment. The duration of free intervals before and after ECT was also studied.
The results showed “significantly longer” free intervals after treatment (13.2 ± 9.0 months before ECT to 25.1 ± 19.1 months after treatment [t = 3.8; P less than .0001]), Dr. Minnai and colleagues reported. In addition, the analysis found “significant reductions” in the number of manic and depressive episodes (5.9 ± 3.0 before ECT to 1.0 ± 1.7 after treatment [t = 9.3; P less than .0001]) and admissions (2.2 ± 1.3 before ECT to 0.2 ± 0.5 after treatment [t = 9.4; P less than .0001]).
The study suggests that “it is plausible that ECT, along with suspending antidepressant treatment, might carry intrinsic stabilizing effect on the course of [bipolar disorder],” the authors concluded.
No financial conflicts of interest were disclosed.
Read the full article in the Journal of Affective Disorders (May 2016;195:180-4).
Electroconvulsive therapy increased illness-free intervals and reduced manic and depressive episodes in bipolar patients, reported Dr. Gian Paolo Minnai of the psychiatry unit at San Martino Hospital in Oristano, Italy, and coauthors.
In a retrospective study of 41 patients with bipolar disorder, the investigators analyzed the number of episodes and admissions 5 years before and after ECT treatment. The duration of free intervals before and after ECT was also studied.
The results showed “significantly longer” free intervals after treatment (13.2 ± 9.0 months before ECT to 25.1 ± 19.1 months after treatment [t = 3.8; P less than .0001]), Dr. Minnai and colleagues reported. In addition, the analysis found “significant reductions” in the number of manic and depressive episodes (5.9 ± 3.0 before ECT to 1.0 ± 1.7 after treatment [t = 9.3; P less than .0001]) and admissions (2.2 ± 1.3 before ECT to 0.2 ± 0.5 after treatment [t = 9.4; P less than .0001]).
The study suggests that “it is plausible that ECT, along with suspending antidepressant treatment, might carry intrinsic stabilizing effect on the course of [bipolar disorder],” the authors concluded.
No financial conflicts of interest were disclosed.
Read the full article in the Journal of Affective Disorders (May 2016;195:180-4).
Electroconvulsive therapy increased illness-free intervals and reduced manic and depressive episodes in bipolar patients, reported Dr. Gian Paolo Minnai of the psychiatry unit at San Martino Hospital in Oristano, Italy, and coauthors.
In a retrospective study of 41 patients with bipolar disorder, the investigators analyzed the number of episodes and admissions 5 years before and after ECT treatment. The duration of free intervals before and after ECT was also studied.
The results showed “significantly longer” free intervals after treatment (13.2 ± 9.0 months before ECT to 25.1 ± 19.1 months after treatment [t = 3.8; P less than .0001]), Dr. Minnai and colleagues reported. In addition, the analysis found “significant reductions” in the number of manic and depressive episodes (5.9 ± 3.0 before ECT to 1.0 ± 1.7 after treatment [t = 9.3; P less than .0001]) and admissions (2.2 ± 1.3 before ECT to 0.2 ± 0.5 after treatment [t = 9.4; P less than .0001]).
The study suggests that “it is plausible that ECT, along with suspending antidepressant treatment, might carry intrinsic stabilizing effect on the course of [bipolar disorder],” the authors concluded.
No financial conflicts of interest were disclosed.
Read the full article in the Journal of Affective Disorders (May 2016;195:180-4).
Practice pathway addresses problem behaviors for children with ASD
A new practice pathway provides primary care providers with evidence-based steps in assessing and addressing irritability and problem behavior in patients at least 3 years old who have been diagnosed with an autism spectrum disorder (ASD).
“As the medical professionals whom children with ASD will probably encounter first and most frequently, pediatric primary care providers need a breadth of knowledge to enable them to identify contributing factors to irritability and problem behavior, decide when and how to initiate treatment, and judge when to refer to specialists,” reported Dr. Kelly McGuire of Columbia University Medical Center and New York State Psychiatric Institute, and her associates (Pediatrics. 2016 Feb;137 Suppl 2:S136-48). “Therefore, this practice pathway can be viewed more as a comprehensive rather than an exhaustive recommendation for [primary care physicians], who must weigh their expertise and resources when addressing the range of issues that a child with ASD and irritability and problem behavior may present.”
The Irritability Workgroup comprised eight child psychiatrists, a developmental pediatrician, and a behavioral psychologist who met regularly to define irritability and problem behavior, and then to review the evidence base on assessing and treating factors that contribute to irritability and problem behavior. They grouped these factors into five domains: “co-occurring medical conditions, lack of functional communication, psychosocial stressors, maladaptive reinforcement patterns, and co-occurring psychiatric conditions.”
Then the group developed a consensus in determining each step in the practice pathway and when it should occur, along with opportunities for a primary care provider to refer the patient to a specialist or to collaborate with school and community providers. The 10 steps in the practice pathway are:
1. Assess the patient for irritability and problem behavior based on the presence of tantrums, meltdowns or rages; property destruction; aggression toward others; and self-injury.
2. Assess the safety of the patient and others in the home, enlisting home-based crisis services or considering hospitalization if safety is threatened.
3. Review the patient’s medical, developmental, communication, environmental, and psychiatric history before and after the problem behavior. Include information on the caretaker’s characteristics, any loss of skills by patient, and any interference occurring with functioning or relationships.
4. Prioritize the behaviors to address based on safety, severity, and impact on daily life.
5. Consider all contributing factors to the problem behavior, including medical problems, difficulties functionally communicating, psychosocial stressors, maladaptive reinforcement patterns (reducing inadvertent triggers), and comorbid psychiatric disorders.
6. Consider possible medication treatments, such as N-acetylcysteine, clonidine, risperidone or aripiprazole, depending on circumstances.
7. Develop an individualized treatment and safety plan.
8. Implement and monitor the treatment plan, setting clear and measurable treatment goals.
9. Follow up at 3 months to assess whether symptoms are continuing and a reassessment is needed.
10. Reevaluate every 3 months.
The article provides a useful and extensive checklist with a variety of specific things to consider when implementing the 10 steps.
“No other provider in the patient’s life combines the medical expertise and first-hand knowledge of the individual patient’s health and development” than the primary care provider, the authors wrote. “The practice pathway is most likely to be efficient and effective in generating a treatment plan if it is systematically followed and the specific combination of individual contributing factors is identified for each patient.”
The research was conducted through the Autism Speaks Autism Treatment Network and funded by the U.S. Department of Health and Human Services with the Autism Intervention Research Network on Physical Health and by Marilyn and James Simons Family Giving. Dr. Jeremy Veenstra-VanderWeele has received research funding from Seaside Therapeutics, Roche, Novartis, Forest, Sunovion, and SynapDx and has consulted with Roche, Novartis and SynapDx.
A new practice pathway provides primary care providers with evidence-based steps in assessing and addressing irritability and problem behavior in patients at least 3 years old who have been diagnosed with an autism spectrum disorder (ASD).
“As the medical professionals whom children with ASD will probably encounter first and most frequently, pediatric primary care providers need a breadth of knowledge to enable them to identify contributing factors to irritability and problem behavior, decide when and how to initiate treatment, and judge when to refer to specialists,” reported Dr. Kelly McGuire of Columbia University Medical Center and New York State Psychiatric Institute, and her associates (Pediatrics. 2016 Feb;137 Suppl 2:S136-48). “Therefore, this practice pathway can be viewed more as a comprehensive rather than an exhaustive recommendation for [primary care physicians], who must weigh their expertise and resources when addressing the range of issues that a child with ASD and irritability and problem behavior may present.”
The Irritability Workgroup comprised eight child psychiatrists, a developmental pediatrician, and a behavioral psychologist who met regularly to define irritability and problem behavior, and then to review the evidence base on assessing and treating factors that contribute to irritability and problem behavior. They grouped these factors into five domains: “co-occurring medical conditions, lack of functional communication, psychosocial stressors, maladaptive reinforcement patterns, and co-occurring psychiatric conditions.”
Then the group developed a consensus in determining each step in the practice pathway and when it should occur, along with opportunities for a primary care provider to refer the patient to a specialist or to collaborate with school and community providers. The 10 steps in the practice pathway are:
1. Assess the patient for irritability and problem behavior based on the presence of tantrums, meltdowns or rages; property destruction; aggression toward others; and self-injury.
2. Assess the safety of the patient and others in the home, enlisting home-based crisis services or considering hospitalization if safety is threatened.
3. Review the patient’s medical, developmental, communication, environmental, and psychiatric history before and after the problem behavior. Include information on the caretaker’s characteristics, any loss of skills by patient, and any interference occurring with functioning or relationships.
4. Prioritize the behaviors to address based on safety, severity, and impact on daily life.
5. Consider all contributing factors to the problem behavior, including medical problems, difficulties functionally communicating, psychosocial stressors, maladaptive reinforcement patterns (reducing inadvertent triggers), and comorbid psychiatric disorders.
6. Consider possible medication treatments, such as N-acetylcysteine, clonidine, risperidone or aripiprazole, depending on circumstances.
7. Develop an individualized treatment and safety plan.
8. Implement and monitor the treatment plan, setting clear and measurable treatment goals.
9. Follow up at 3 months to assess whether symptoms are continuing and a reassessment is needed.
10. Reevaluate every 3 months.
The article provides a useful and extensive checklist with a variety of specific things to consider when implementing the 10 steps.
“No other provider in the patient’s life combines the medical expertise and first-hand knowledge of the individual patient’s health and development” than the primary care provider, the authors wrote. “The practice pathway is most likely to be efficient and effective in generating a treatment plan if it is systematically followed and the specific combination of individual contributing factors is identified for each patient.”
The research was conducted through the Autism Speaks Autism Treatment Network and funded by the U.S. Department of Health and Human Services with the Autism Intervention Research Network on Physical Health and by Marilyn and James Simons Family Giving. Dr. Jeremy Veenstra-VanderWeele has received research funding from Seaside Therapeutics, Roche, Novartis, Forest, Sunovion, and SynapDx and has consulted with Roche, Novartis and SynapDx.
A new practice pathway provides primary care providers with evidence-based steps in assessing and addressing irritability and problem behavior in patients at least 3 years old who have been diagnosed with an autism spectrum disorder (ASD).
“As the medical professionals whom children with ASD will probably encounter first and most frequently, pediatric primary care providers need a breadth of knowledge to enable them to identify contributing factors to irritability and problem behavior, decide when and how to initiate treatment, and judge when to refer to specialists,” reported Dr. Kelly McGuire of Columbia University Medical Center and New York State Psychiatric Institute, and her associates (Pediatrics. 2016 Feb;137 Suppl 2:S136-48). “Therefore, this practice pathway can be viewed more as a comprehensive rather than an exhaustive recommendation for [primary care physicians], who must weigh their expertise and resources when addressing the range of issues that a child with ASD and irritability and problem behavior may present.”
The Irritability Workgroup comprised eight child psychiatrists, a developmental pediatrician, and a behavioral psychologist who met regularly to define irritability and problem behavior, and then to review the evidence base on assessing and treating factors that contribute to irritability and problem behavior. They grouped these factors into five domains: “co-occurring medical conditions, lack of functional communication, psychosocial stressors, maladaptive reinforcement patterns, and co-occurring psychiatric conditions.”
Then the group developed a consensus in determining each step in the practice pathway and when it should occur, along with opportunities for a primary care provider to refer the patient to a specialist or to collaborate with school and community providers. The 10 steps in the practice pathway are:
1. Assess the patient for irritability and problem behavior based on the presence of tantrums, meltdowns or rages; property destruction; aggression toward others; and self-injury.
2. Assess the safety of the patient and others in the home, enlisting home-based crisis services or considering hospitalization if safety is threatened.
3. Review the patient’s medical, developmental, communication, environmental, and psychiatric history before and after the problem behavior. Include information on the caretaker’s characteristics, any loss of skills by patient, and any interference occurring with functioning or relationships.
4. Prioritize the behaviors to address based on safety, severity, and impact on daily life.
5. Consider all contributing factors to the problem behavior, including medical problems, difficulties functionally communicating, psychosocial stressors, maladaptive reinforcement patterns (reducing inadvertent triggers), and comorbid psychiatric disorders.
6. Consider possible medication treatments, such as N-acetylcysteine, clonidine, risperidone or aripiprazole, depending on circumstances.
7. Develop an individualized treatment and safety plan.
8. Implement and monitor the treatment plan, setting clear and measurable treatment goals.
9. Follow up at 3 months to assess whether symptoms are continuing and a reassessment is needed.
10. Reevaluate every 3 months.
The article provides a useful and extensive checklist with a variety of specific things to consider when implementing the 10 steps.
“No other provider in the patient’s life combines the medical expertise and first-hand knowledge of the individual patient’s health and development” than the primary care provider, the authors wrote. “The practice pathway is most likely to be efficient and effective in generating a treatment plan if it is systematically followed and the specific combination of individual contributing factors is identified for each patient.”
The research was conducted through the Autism Speaks Autism Treatment Network and funded by the U.S. Department of Health and Human Services with the Autism Intervention Research Network on Physical Health and by Marilyn and James Simons Family Giving. Dr. Jeremy Veenstra-VanderWeele has received research funding from Seaside Therapeutics, Roche, Novartis, Forest, Sunovion, and SynapDx and has consulted with Roche, Novartis and SynapDx.
FROM PEDIATRICS
Key clinical point: A 10-step practice pathway helps primary care providers address irritability and problem behavior in children with ASD.
Major finding: Ten steps in the pathway include assessment of problems, identification of contributing factors, and formulation of a plan to address the underlying factors and the behavior.
Data source: The pathway is based on a consensus by a 10-member multidisciplinary work group charged with identifying available evidence on effective ways to assess and address irritability and problem behavior in children with ASD.
Disclosures: The research was conducted through the Autism Speaks Autism Treatment Network and funded by the U.S. Department of Health and Human Services with the Autism Intervention Research Network on Physical Health and by Marilyn and James Simons Family Giving. Dr. Jeremy Veenstra-VanderWeele has received research funding from Seaside Therapeutics, Roche, Novartis, Forest, Sunovion and SynapDx and has consulted with Roche, Novartis and SynapDx.
Safety of bioresorbable stents does not match that of metal stents
Bioresorbable vascular scaffold stents are improving rapidly but they are still associated with a higher risk of complications compared with drug-eluting metal stents, according to a meta-analysis of published studies presented at Cardiovascular Research Technologies 2016.
“Bioresorbable stents are clearly an attractive strategy, but our data suggest that physicians and patients should remain aware of the risks,” reported Dr. Alok Saurav of Creighton University Medical Center, Omaha, Neb.
The first bioresorbable vascular scaffold (BVS) device, Synergy, was approved this past October, but this stent, despite bioresorbable struts, still has body parts that are not fully bioresorbable. However, several fully bioresorbable devices have reached late stages of testing and may receive regulatory approval this year.
In the meta-analysis, eight studies – five randomized trials, two studies with propensity matching, and an observational study –the primary goal was to compare BVS to drug eluting metal (DEM) stents for definite stent thrombosis. Secondary outcomes included subacute stent thrombosis within 30 days and within 1 year and cardiac death, all-cause death, MI, and ischemia-driven target vessel revascularization (TVR).
Despite the fact that the mean age and gender distribution was the same when the 2,760 patients receiving BVS stents were compared to the 2,212 receiving DEM stents, and both received comparable antiplatelet regimens after the stent was placed, there was an 80% greater relative risk for definite stent thrombosis in the BVS group. Although this difference fell short of statistical significance (P = .06), Dr. Saurav called it a “strong trend.”
Several of the adverse events that were analyzed as secondary outcomes in this study were less frequent with the BVS, such as cardiac death (relative risk, 0.83) and all-cause death (RR, 0.74), but the statistics did not suggest a trend, so Dr. Saurav characterized these outcomes as similar. MI was an exception. This was more frequent in those received a BVS stent (RR, 1.35; P = .049), and this reached significance.
Most of the studies included in this analysis were conducted with the everolimus-eluting Absorb BVS device, which many are predicting will be the first fully bioresorbable stent to receive regulatory approval.
It is notable that another meta-analysis including some of the same studies and published just weeks prior to the CRT meeting drew the same conclusion about the increased risk of stent thrombosis with BVS relative to DEM stents (Lancet 2016;387:537-44). This meta-analysis was restricted to six trials with 3,738 randomized patients. Unlike the meta-analysis presented at CRT, this study compared the two types of stents for both definite and probable stent thrombosis. For BVS relative to DEM stents, the relative risk for this outcome was 1.99 (P = .05).
“We think our restriction to definite stent thrombosis provides a stricter endpoint, but it’s notable that the results were relatively consistent,” Dr. Saurav reported.
Acknowledging that the increased risk of stent thrombosis appears to be modest for BVS relative to DEM stents, Dr. Saurav emphasized that these data should not discourage further development of bioresorbable stents, which are conceptually attractive.
“We cannot take these bioresorbable devices off the table,” he said. “But we do need more data to evaluate their risks relative to the conventional devices that are now available.”
The meeting was sponsored by the Cardiovascular Research Institute at Washington Hospital Center. Dr. Saurav reported no conflicts of interest.
Bioresorbable vascular scaffold stents are improving rapidly but they are still associated with a higher risk of complications compared with drug-eluting metal stents, according to a meta-analysis of published studies presented at Cardiovascular Research Technologies 2016.
“Bioresorbable stents are clearly an attractive strategy, but our data suggest that physicians and patients should remain aware of the risks,” reported Dr. Alok Saurav of Creighton University Medical Center, Omaha, Neb.
The first bioresorbable vascular scaffold (BVS) device, Synergy, was approved this past October, but this stent, despite bioresorbable struts, still has body parts that are not fully bioresorbable. However, several fully bioresorbable devices have reached late stages of testing and may receive regulatory approval this year.
In the meta-analysis, eight studies – five randomized trials, two studies with propensity matching, and an observational study –the primary goal was to compare BVS to drug eluting metal (DEM) stents for definite stent thrombosis. Secondary outcomes included subacute stent thrombosis within 30 days and within 1 year and cardiac death, all-cause death, MI, and ischemia-driven target vessel revascularization (TVR).
Despite the fact that the mean age and gender distribution was the same when the 2,760 patients receiving BVS stents were compared to the 2,212 receiving DEM stents, and both received comparable antiplatelet regimens after the stent was placed, there was an 80% greater relative risk for definite stent thrombosis in the BVS group. Although this difference fell short of statistical significance (P = .06), Dr. Saurav called it a “strong trend.”
Several of the adverse events that were analyzed as secondary outcomes in this study were less frequent with the BVS, such as cardiac death (relative risk, 0.83) and all-cause death (RR, 0.74), but the statistics did not suggest a trend, so Dr. Saurav characterized these outcomes as similar. MI was an exception. This was more frequent in those received a BVS stent (RR, 1.35; P = .049), and this reached significance.
Most of the studies included in this analysis were conducted with the everolimus-eluting Absorb BVS device, which many are predicting will be the first fully bioresorbable stent to receive regulatory approval.
It is notable that another meta-analysis including some of the same studies and published just weeks prior to the CRT meeting drew the same conclusion about the increased risk of stent thrombosis with BVS relative to DEM stents (Lancet 2016;387:537-44). This meta-analysis was restricted to six trials with 3,738 randomized patients. Unlike the meta-analysis presented at CRT, this study compared the two types of stents for both definite and probable stent thrombosis. For BVS relative to DEM stents, the relative risk for this outcome was 1.99 (P = .05).
“We think our restriction to definite stent thrombosis provides a stricter endpoint, but it’s notable that the results were relatively consistent,” Dr. Saurav reported.
Acknowledging that the increased risk of stent thrombosis appears to be modest for BVS relative to DEM stents, Dr. Saurav emphasized that these data should not discourage further development of bioresorbable stents, which are conceptually attractive.
“We cannot take these bioresorbable devices off the table,” he said. “But we do need more data to evaluate their risks relative to the conventional devices that are now available.”
The meeting was sponsored by the Cardiovascular Research Institute at Washington Hospital Center. Dr. Saurav reported no conflicts of interest.
Bioresorbable vascular scaffold stents are improving rapidly but they are still associated with a higher risk of complications compared with drug-eluting metal stents, according to a meta-analysis of published studies presented at Cardiovascular Research Technologies 2016.
“Bioresorbable stents are clearly an attractive strategy, but our data suggest that physicians and patients should remain aware of the risks,” reported Dr. Alok Saurav of Creighton University Medical Center, Omaha, Neb.
The first bioresorbable vascular scaffold (BVS) device, Synergy, was approved this past October, but this stent, despite bioresorbable struts, still has body parts that are not fully bioresorbable. However, several fully bioresorbable devices have reached late stages of testing and may receive regulatory approval this year.
In the meta-analysis, eight studies – five randomized trials, two studies with propensity matching, and an observational study –the primary goal was to compare BVS to drug eluting metal (DEM) stents for definite stent thrombosis. Secondary outcomes included subacute stent thrombosis within 30 days and within 1 year and cardiac death, all-cause death, MI, and ischemia-driven target vessel revascularization (TVR).
Despite the fact that the mean age and gender distribution was the same when the 2,760 patients receiving BVS stents were compared to the 2,212 receiving DEM stents, and both received comparable antiplatelet regimens after the stent was placed, there was an 80% greater relative risk for definite stent thrombosis in the BVS group. Although this difference fell short of statistical significance (P = .06), Dr. Saurav called it a “strong trend.”
Several of the adverse events that were analyzed as secondary outcomes in this study were less frequent with the BVS, such as cardiac death (relative risk, 0.83) and all-cause death (RR, 0.74), but the statistics did not suggest a trend, so Dr. Saurav characterized these outcomes as similar. MI was an exception. This was more frequent in those received a BVS stent (RR, 1.35; P = .049), and this reached significance.
Most of the studies included in this analysis were conducted with the everolimus-eluting Absorb BVS device, which many are predicting will be the first fully bioresorbable stent to receive regulatory approval.
It is notable that another meta-analysis including some of the same studies and published just weeks prior to the CRT meeting drew the same conclusion about the increased risk of stent thrombosis with BVS relative to DEM stents (Lancet 2016;387:537-44). This meta-analysis was restricted to six trials with 3,738 randomized patients. Unlike the meta-analysis presented at CRT, this study compared the two types of stents for both definite and probable stent thrombosis. For BVS relative to DEM stents, the relative risk for this outcome was 1.99 (P = .05).
“We think our restriction to definite stent thrombosis provides a stricter endpoint, but it’s notable that the results were relatively consistent,” Dr. Saurav reported.
Acknowledging that the increased risk of stent thrombosis appears to be modest for BVS relative to DEM stents, Dr. Saurav emphasized that these data should not discourage further development of bioresorbable stents, which are conceptually attractive.
“We cannot take these bioresorbable devices off the table,” he said. “But we do need more data to evaluate their risks relative to the conventional devices that are now available.”
The meeting was sponsored by the Cardiovascular Research Institute at Washington Hospital Center. Dr. Saurav reported no conflicts of interest.
AT CARDIOVASCULAR RESEARCH TECHNOLOGIES 2016
Key clinical point: Trial data suggest the risk of thrombosis and other adverse events remains higher with bioresorbable stents than with conventional drug-eluting metal stents.
Major finding: In a meta-analysis, the 80% increased risk of definite stent thrombosis for bioresorbable relative to metal stents fell just short of significance (P = .06) but the 35% increased risk of subsequent MI was significant (P = .049).
Data source: Meta-analysis of eight studies.
Disclosures: Dr. Saurav reported no conflicts of interest.
Limited posttreatment imaging suffices in HPV-positive oropharyngeal cancer
SCOTTSDALE, ARIZ. – Most patients who are treated for human papillomavirus (HPV)–positive oropharyngeal cancer can safely skip routine imaging after a negative 3-month posttreatment scan, suggest results of a retrospective cohort study reported at the Multidisciplinary Head and Neck Cancer Symposium.
Investigators led by Dr. Jessica M. Frakes of the H. Lee Moffitt Cancer Center in Tampa, Fla., studied 246 patients treated nonsurgically between 2006 and 2014 for nonmetastatic HPV-positive disease.
With a median follow-up of 36 months, all local recurrences and the large majority of regional and distant recurrences were detected from symptoms, physical exam, and the 3-month posttreatment PET-CT imaging, according to data reported in a session and related press briefing.
“Routine imaging is not recommended after posttreatment imaging shows a complete response to treatment, unless the patient presents with symptoms or something else that would warrant imaging,” Dr. Frakes commented. “Follow-up should include history and physical examination with direct visualization.”
Currently, the National Comprehensive Cancer Network advises a one-size-fits-all approach to follow-up that does not consider tumor HPV status, she noted. But reducing surveillance imaging for HPV-positive patients would likely have considerable benefit in terms of less stress and anxiety (provided patients are educated about the safety of clinical follow-up) and lower financial burden for both the patient and the health care system as a whole.
Results additionally showed that the majority of recurrences occurred within the first 6 months, a pattern that was consistent whether or not patients had risk factors for recurrence. The 3-year rate of freedom from local failure exceeded 97%, and only 2% of patients had grade 3 or worse toxicity at their last follow-up.
“Our outcomes are excellent with low rates of permanent toxicity, and we think that this partly is due to the fact that they are treated by specialized multidisciplinary team,” Dr. Frakes commented at the meeting.
Press briefing moderator Dr. Christine Gourin of Johns Hopkins University in Baltimore, commented, “This study is one that’s dear to my heart because I think that we probably do too much posttreatment surveillance, and they are exactly right that the NCCN is fairly vague about when to perform imaging.”
“I can tell you that we have stopped routinely imaging patients after 3 months if the PET is negative, and it’s true that we do pick up recurrences more clinically than we do radiologically,” she added. “And of course the false positives are causing much morbidity.”
Introducing the study, Dr. Frakes commented, “Several retrospective and prospective trials have shown increased survivals and decreased toxicity in patients with HPV-associated oropharynx cancer. As the number of patients and survivors grows, so does the need to determine general time to recurrence and the most effective modes of recurrence detection, thereby guiding our standards for optimal follow-up care.”
All patients studied received definitive radiation therapy, and 85% of them also received concurrent chemotherapy.
The patients had a 3-month posttreatment PET-CT scan, plus physical exams every 3 months in the first year post treatment, every 4 months in the second year, every 6 months in the third through fifth years, and annually thereafter.
Results showed that the 3-year rate of local control was 97.8%, and 100% of the local failures were detected by physical exam, including direct visualization or flexible laryngoscopy.
The rate of regional control was 95.3%, and 89% of cases of regional failure were detected through symptoms or the 3-month posttreatment imaging. Risk factors for regional recurrence included involvement of five or more lymph nodes in the neck and involvement of level 4 (low neck) lymph nodes (P less than .05 for each).
The rate of freedom from distant metastases was 91%, and 71% of cases of distant metastases were detected through symptoms or the 3-month posttreatment imaging. Risk factors for distant metastases included tumor in the lymph nodes measuring greater than 6 cm, involvement of bilateral lymph nodes, involvement of five or more lymph nodes in the neck, and involvement of level 4 lymph nodes (P less than .05 for each).
Overall, 9% of patients experienced grade 3 or worse late toxicity (occurring at 3 months or thereafter), consisting of feeding/gastrostomy tube (G-tube) placement, necrosis or ulcer, and tracheostomy. However, these toxicities had resolved as of the last follow-up in most cases, with a final rate of toxicity of only 2%.
The center follows an aggressive approach to preventing and managing toxicity, noted Dr. Frakes.
“Even when the patients have their G-tube in place, we really do encourage p.o. [oral] intake as much as possible with pain medication. And I think that really does make a big difference for our patients,” she said. “They do meet with a speech pathologist and our nutritionist weekly when they are on treatment.” Patients are also allowed to have the G-tube removed by last follow-up, she added.
SCOTTSDALE, ARIZ. – Most patients who are treated for human papillomavirus (HPV)–positive oropharyngeal cancer can safely skip routine imaging after a negative 3-month posttreatment scan, suggest results of a retrospective cohort study reported at the Multidisciplinary Head and Neck Cancer Symposium.
Investigators led by Dr. Jessica M. Frakes of the H. Lee Moffitt Cancer Center in Tampa, Fla., studied 246 patients treated nonsurgically between 2006 and 2014 for nonmetastatic HPV-positive disease.
With a median follow-up of 36 months, all local recurrences and the large majority of regional and distant recurrences were detected from symptoms, physical exam, and the 3-month posttreatment PET-CT imaging, according to data reported in a session and related press briefing.
“Routine imaging is not recommended after posttreatment imaging shows a complete response to treatment, unless the patient presents with symptoms or something else that would warrant imaging,” Dr. Frakes commented. “Follow-up should include history and physical examination with direct visualization.”
Currently, the National Comprehensive Cancer Network advises a one-size-fits-all approach to follow-up that does not consider tumor HPV status, she noted. But reducing surveillance imaging for HPV-positive patients would likely have considerable benefit in terms of less stress and anxiety (provided patients are educated about the safety of clinical follow-up) and lower financial burden for both the patient and the health care system as a whole.
Results additionally showed that the majority of recurrences occurred within the first 6 months, a pattern that was consistent whether or not patients had risk factors for recurrence. The 3-year rate of freedom from local failure exceeded 97%, and only 2% of patients had grade 3 or worse toxicity at their last follow-up.
“Our outcomes are excellent with low rates of permanent toxicity, and we think that this partly is due to the fact that they are treated by specialized multidisciplinary team,” Dr. Frakes commented at the meeting.
Press briefing moderator Dr. Christine Gourin of Johns Hopkins University in Baltimore, commented, “This study is one that’s dear to my heart because I think that we probably do too much posttreatment surveillance, and they are exactly right that the NCCN is fairly vague about when to perform imaging.”
“I can tell you that we have stopped routinely imaging patients after 3 months if the PET is negative, and it’s true that we do pick up recurrences more clinically than we do radiologically,” she added. “And of course the false positives are causing much morbidity.”
Introducing the study, Dr. Frakes commented, “Several retrospective and prospective trials have shown increased survivals and decreased toxicity in patients with HPV-associated oropharynx cancer. As the number of patients and survivors grows, so does the need to determine general time to recurrence and the most effective modes of recurrence detection, thereby guiding our standards for optimal follow-up care.”
All patients studied received definitive radiation therapy, and 85% of them also received concurrent chemotherapy.
The patients had a 3-month posttreatment PET-CT scan, plus physical exams every 3 months in the first year post treatment, every 4 months in the second year, every 6 months in the third through fifth years, and annually thereafter.
Results showed that the 3-year rate of local control was 97.8%, and 100% of the local failures were detected by physical exam, including direct visualization or flexible laryngoscopy.
The rate of regional control was 95.3%, and 89% of cases of regional failure were detected through symptoms or the 3-month posttreatment imaging. Risk factors for regional recurrence included involvement of five or more lymph nodes in the neck and involvement of level 4 (low neck) lymph nodes (P less than .05 for each).
The rate of freedom from distant metastases was 91%, and 71% of cases of distant metastases were detected through symptoms or the 3-month posttreatment imaging. Risk factors for distant metastases included tumor in the lymph nodes measuring greater than 6 cm, involvement of bilateral lymph nodes, involvement of five or more lymph nodes in the neck, and involvement of level 4 lymph nodes (P less than .05 for each).
Overall, 9% of patients experienced grade 3 or worse late toxicity (occurring at 3 months or thereafter), consisting of feeding/gastrostomy tube (G-tube) placement, necrosis or ulcer, and tracheostomy. However, these toxicities had resolved as of the last follow-up in most cases, with a final rate of toxicity of only 2%.
The center follows an aggressive approach to preventing and managing toxicity, noted Dr. Frakes.
“Even when the patients have their G-tube in place, we really do encourage p.o. [oral] intake as much as possible with pain medication. And I think that really does make a big difference for our patients,” she said. “They do meet with a speech pathologist and our nutritionist weekly when they are on treatment.” Patients are also allowed to have the G-tube removed by last follow-up, she added.
SCOTTSDALE, ARIZ. – Most patients who are treated for human papillomavirus (HPV)–positive oropharyngeal cancer can safely skip routine imaging after a negative 3-month posttreatment scan, suggest results of a retrospective cohort study reported at the Multidisciplinary Head and Neck Cancer Symposium.
Investigators led by Dr. Jessica M. Frakes of the H. Lee Moffitt Cancer Center in Tampa, Fla., studied 246 patients treated nonsurgically between 2006 and 2014 for nonmetastatic HPV-positive disease.
With a median follow-up of 36 months, all local recurrences and the large majority of regional and distant recurrences were detected from symptoms, physical exam, and the 3-month posttreatment PET-CT imaging, according to data reported in a session and related press briefing.
“Routine imaging is not recommended after posttreatment imaging shows a complete response to treatment, unless the patient presents with symptoms or something else that would warrant imaging,” Dr. Frakes commented. “Follow-up should include history and physical examination with direct visualization.”
Currently, the National Comprehensive Cancer Network advises a one-size-fits-all approach to follow-up that does not consider tumor HPV status, she noted. But reducing surveillance imaging for HPV-positive patients would likely have considerable benefit in terms of less stress and anxiety (provided patients are educated about the safety of clinical follow-up) and lower financial burden for both the patient and the health care system as a whole.
Results additionally showed that the majority of recurrences occurred within the first 6 months, a pattern that was consistent whether or not patients had risk factors for recurrence. The 3-year rate of freedom from local failure exceeded 97%, and only 2% of patients had grade 3 or worse toxicity at their last follow-up.
“Our outcomes are excellent with low rates of permanent toxicity, and we think that this partly is due to the fact that they are treated by specialized multidisciplinary team,” Dr. Frakes commented at the meeting.
Press briefing moderator Dr. Christine Gourin of Johns Hopkins University in Baltimore, commented, “This study is one that’s dear to my heart because I think that we probably do too much posttreatment surveillance, and they are exactly right that the NCCN is fairly vague about when to perform imaging.”
“I can tell you that we have stopped routinely imaging patients after 3 months if the PET is negative, and it’s true that we do pick up recurrences more clinically than we do radiologically,” she added. “And of course the false positives are causing much morbidity.”
Introducing the study, Dr. Frakes commented, “Several retrospective and prospective trials have shown increased survivals and decreased toxicity in patients with HPV-associated oropharynx cancer. As the number of patients and survivors grows, so does the need to determine general time to recurrence and the most effective modes of recurrence detection, thereby guiding our standards for optimal follow-up care.”
All patients studied received definitive radiation therapy, and 85% of them also received concurrent chemotherapy.
The patients had a 3-month posttreatment PET-CT scan, plus physical exams every 3 months in the first year post treatment, every 4 months in the second year, every 6 months in the third through fifth years, and annually thereafter.
Results showed that the 3-year rate of local control was 97.8%, and 100% of the local failures were detected by physical exam, including direct visualization or flexible laryngoscopy.
The rate of regional control was 95.3%, and 89% of cases of regional failure were detected through symptoms or the 3-month posttreatment imaging. Risk factors for regional recurrence included involvement of five or more lymph nodes in the neck and involvement of level 4 (low neck) lymph nodes (P less than .05 for each).
The rate of freedom from distant metastases was 91%, and 71% of cases of distant metastases were detected through symptoms or the 3-month posttreatment imaging. Risk factors for distant metastases included tumor in the lymph nodes measuring greater than 6 cm, involvement of bilateral lymph nodes, involvement of five or more lymph nodes in the neck, and involvement of level 4 lymph nodes (P less than .05 for each).
Overall, 9% of patients experienced grade 3 or worse late toxicity (occurring at 3 months or thereafter), consisting of feeding/gastrostomy tube (G-tube) placement, necrosis or ulcer, and tracheostomy. However, these toxicities had resolved as of the last follow-up in most cases, with a final rate of toxicity of only 2%.
The center follows an aggressive approach to preventing and managing toxicity, noted Dr. Frakes.
“Even when the patients have their G-tube in place, we really do encourage p.o. [oral] intake as much as possible with pain medication. And I think that really does make a big difference for our patients,” she said. “They do meet with a speech pathologist and our nutritionist weekly when they are on treatment.” Patients are also allowed to have the G-tube removed by last follow-up, she added.
AT THE HEAD AND NECK CANCER SYMPOSIUM
Key clinical point: Most patients with HPV-positive oropharyngeal cancer do not need routine imaging after a negative 3-month posttreatment scan.
Major finding: Overall, 100%, 89%, and 71% of local, regional, and distant recurrences, respectively, were detected from symptoms, physical exam, and 3-month posttreatment imaging.
Data source: A retrospective cohort study of 246 patients treated for HPV-positive oropharyngeal cancer.
Disclosures: Dr. Frakes disclosed that she had no relevant conflicts of interest.
Childhood maltreatment tied to lifetime anxiety disorders in bipolar
Childhood maltreatment is associated with lifetime anxiety among people with bipolar disorder, Barbara Pavlova, Ph.D., and her associates reported.
The researchers recruited 174 adult outpatients with a diagnosis of bipolar disorder I or bipolar disorder II, of whom 29% had one anxiety disorder and 20% had two or more. More than half (56%) of the patients were female, and their median age was 42. The types of anxiety disorders among the patients ranged from generalized anxiety disorder (28%) to obsessive-compulsive disorder (4%).
Dr. Pavlova and her associates assessed the patients’ history of maltreatment in childhood using the Childhood Trauma Questionnaire (CTQ), a 28-item self-report measure that asks about emotional, physical, and sexual abuse and about emotional and physical neglect. Anxiety disorders were assessed using the Mini-International Neuropsychiatric Interview (MINI), wrote Dr. Pavlova of the psychiatry department at Dalhousie University, Halifax, N.S.
They found that childhood maltreatment, indexed by higher CTQ total scores, was linked to a higher number of lifetime anxiety disorders (odds ratio, 1.5; 95% confidence interval, 1.01-2.14; P = .04). In addition, panic disorder was most strongly tied to childhood maltreatment (OR, 2.27; 95% CI, 1.28-4.02; P = .01).
The results suggest “that bipolar disorder with comorbid anxiety constitutes an [etiologic] subtype shaped to a greater extent by early environment,” the investigators wrote.
Read the full study here: (J Affect Dis. 2016 Mar 1;192:22-7).
On Twittter @ginalhenderson
Childhood maltreatment is associated with lifetime anxiety among people with bipolar disorder, Barbara Pavlova, Ph.D., and her associates reported.
The researchers recruited 174 adult outpatients with a diagnosis of bipolar disorder I or bipolar disorder II, of whom 29% had one anxiety disorder and 20% had two or more. More than half (56%) of the patients were female, and their median age was 42. The types of anxiety disorders among the patients ranged from generalized anxiety disorder (28%) to obsessive-compulsive disorder (4%).
Dr. Pavlova and her associates assessed the patients’ history of maltreatment in childhood using the Childhood Trauma Questionnaire (CTQ), a 28-item self-report measure that asks about emotional, physical, and sexual abuse and about emotional and physical neglect. Anxiety disorders were assessed using the Mini-International Neuropsychiatric Interview (MINI), wrote Dr. Pavlova of the psychiatry department at Dalhousie University, Halifax, N.S.
They found that childhood maltreatment, indexed by higher CTQ total scores, was linked to a higher number of lifetime anxiety disorders (odds ratio, 1.5; 95% confidence interval, 1.01-2.14; P = .04). In addition, panic disorder was most strongly tied to childhood maltreatment (OR, 2.27; 95% CI, 1.28-4.02; P = .01).
The results suggest “that bipolar disorder with comorbid anxiety constitutes an [etiologic] subtype shaped to a greater extent by early environment,” the investigators wrote.
Read the full study here: (J Affect Dis. 2016 Mar 1;192:22-7).
On Twittter @ginalhenderson
Childhood maltreatment is associated with lifetime anxiety among people with bipolar disorder, Barbara Pavlova, Ph.D., and her associates reported.
The researchers recruited 174 adult outpatients with a diagnosis of bipolar disorder I or bipolar disorder II, of whom 29% had one anxiety disorder and 20% had two or more. More than half (56%) of the patients were female, and their median age was 42. The types of anxiety disorders among the patients ranged from generalized anxiety disorder (28%) to obsessive-compulsive disorder (4%).
Dr. Pavlova and her associates assessed the patients’ history of maltreatment in childhood using the Childhood Trauma Questionnaire (CTQ), a 28-item self-report measure that asks about emotional, physical, and sexual abuse and about emotional and physical neglect. Anxiety disorders were assessed using the Mini-International Neuropsychiatric Interview (MINI), wrote Dr. Pavlova of the psychiatry department at Dalhousie University, Halifax, N.S.
They found that childhood maltreatment, indexed by higher CTQ total scores, was linked to a higher number of lifetime anxiety disorders (odds ratio, 1.5; 95% confidence interval, 1.01-2.14; P = .04). In addition, panic disorder was most strongly tied to childhood maltreatment (OR, 2.27; 95% CI, 1.28-4.02; P = .01).
The results suggest “that bipolar disorder with comorbid anxiety constitutes an [etiologic] subtype shaped to a greater extent by early environment,” the investigators wrote.
Read the full study here: (J Affect Dis. 2016 Mar 1;192:22-7).
On Twittter @ginalhenderson
FROM THE JOURNAL OF AFFECTIVE DISORDERS
What Matters: What’s the magic behind successful bariatric patients?
A fair number of my patients have had or are undergoing bariatric surgery. Disconcertingly, a not insignificant number of them are regaining the weight after surgery. Weight regain will occur in 20% of patients undergoing bariatric surgery after initial weight loss.
When this occurs, not only do we have a patient with an altered gut putting them at risk for nutritional deficiencies if we are not fastidious in our follow-up, but they are discouraged and overweight again.
Add this to the concern that bariatric surgery has been associated with an increase in suicides (2.33-3.63 per 1000 patient-years), and we may have some cause for alarm.
So, what predicts success – and can we facilitate it?
Several factors have been shown to predict successful weight loss after bariatric surgery. An “active coping style” (that is, planning vs. denial) and adherence to follow-up after bariatric surgery have both been shown to be associated with a higher percentage of excess weight loss. Interestingly, psychological burden and motivation have not been associated with weight loss.
In a recent article, Lori Liebl, Ph.D., and her colleagues conducted a qualitative study of the experiences of adults who successfully maintained weight loss after bariatric surgery (J Clin Nurs. 2016 Feb 23. doi: 10.1111/jocn.13129). Success was defined as 50% or more of the excessive weight loss 24 months after bariatric surgery.
The voice of the successful bariatric patient is an interesting and important one. Several themes were identified: 1) taking life back (“I did it for myself”); 2) a new lease on life (“There are things I can do now that I am not exhausted”); 3) the importance of social support; 4) avoiding the negative (terminating unhealthy relationships in which “food is love”); 5) the void (food addiction and sense of loss); 6) fighting food demons; 7) finding the happy weight; and 8) a ripple effect (that is, if you don’t eat it, the rest of family doesn’t, either).
I was left wondering how I can best help my patients using this information.
First, I think the themes can mature our empathy for the struggles that these patients face, and perhaps help us combat bias. Second, I think this knowledge can inform early discussions around what sorts of things need to be lined up for after the procedure, such as social support.
Finally, I think the themes can be universalized and help us counsel patients who may be struggling with weight, but who are otherwise not candidates for bariatric surgery.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
A fair number of my patients have had or are undergoing bariatric surgery. Disconcertingly, a not insignificant number of them are regaining the weight after surgery. Weight regain will occur in 20% of patients undergoing bariatric surgery after initial weight loss.
When this occurs, not only do we have a patient with an altered gut putting them at risk for nutritional deficiencies if we are not fastidious in our follow-up, but they are discouraged and overweight again.
Add this to the concern that bariatric surgery has been associated with an increase in suicides (2.33-3.63 per 1000 patient-years), and we may have some cause for alarm.
So, what predicts success – and can we facilitate it?
Several factors have been shown to predict successful weight loss after bariatric surgery. An “active coping style” (that is, planning vs. denial) and adherence to follow-up after bariatric surgery have both been shown to be associated with a higher percentage of excess weight loss. Interestingly, psychological burden and motivation have not been associated with weight loss.
In a recent article, Lori Liebl, Ph.D., and her colleagues conducted a qualitative study of the experiences of adults who successfully maintained weight loss after bariatric surgery (J Clin Nurs. 2016 Feb 23. doi: 10.1111/jocn.13129). Success was defined as 50% or more of the excessive weight loss 24 months after bariatric surgery.
The voice of the successful bariatric patient is an interesting and important one. Several themes were identified: 1) taking life back (“I did it for myself”); 2) a new lease on life (“There are things I can do now that I am not exhausted”); 3) the importance of social support; 4) avoiding the negative (terminating unhealthy relationships in which “food is love”); 5) the void (food addiction and sense of loss); 6) fighting food demons; 7) finding the happy weight; and 8) a ripple effect (that is, if you don’t eat it, the rest of family doesn’t, either).
I was left wondering how I can best help my patients using this information.
First, I think the themes can mature our empathy for the struggles that these patients face, and perhaps help us combat bias. Second, I think this knowledge can inform early discussions around what sorts of things need to be lined up for after the procedure, such as social support.
Finally, I think the themes can be universalized and help us counsel patients who may be struggling with weight, but who are otherwise not candidates for bariatric surgery.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
A fair number of my patients have had or are undergoing bariatric surgery. Disconcertingly, a not insignificant number of them are regaining the weight after surgery. Weight regain will occur in 20% of patients undergoing bariatric surgery after initial weight loss.
When this occurs, not only do we have a patient with an altered gut putting them at risk for nutritional deficiencies if we are not fastidious in our follow-up, but they are discouraged and overweight again.
Add this to the concern that bariatric surgery has been associated with an increase in suicides (2.33-3.63 per 1000 patient-years), and we may have some cause for alarm.
So, what predicts success – and can we facilitate it?
Several factors have been shown to predict successful weight loss after bariatric surgery. An “active coping style” (that is, planning vs. denial) and adherence to follow-up after bariatric surgery have both been shown to be associated with a higher percentage of excess weight loss. Interestingly, psychological burden and motivation have not been associated with weight loss.
In a recent article, Lori Liebl, Ph.D., and her colleagues conducted a qualitative study of the experiences of adults who successfully maintained weight loss after bariatric surgery (J Clin Nurs. 2016 Feb 23. doi: 10.1111/jocn.13129). Success was defined as 50% or more of the excessive weight loss 24 months after bariatric surgery.
The voice of the successful bariatric patient is an interesting and important one. Several themes were identified: 1) taking life back (“I did it for myself”); 2) a new lease on life (“There are things I can do now that I am not exhausted”); 3) the importance of social support; 4) avoiding the negative (terminating unhealthy relationships in which “food is love”); 5) the void (food addiction and sense of loss); 6) fighting food demons; 7) finding the happy weight; and 8) a ripple effect (that is, if you don’t eat it, the rest of family doesn’t, either).
I was left wondering how I can best help my patients using this information.
First, I think the themes can mature our empathy for the struggles that these patients face, and perhaps help us combat bias. Second, I think this knowledge can inform early discussions around what sorts of things need to be lined up for after the procedure, such as social support.
Finally, I think the themes can be universalized and help us counsel patients who may be struggling with weight, but who are otherwise not candidates for bariatric surgery.
Dr. Ebbert is professor of medicine, a general internist at the Mayo Clinic in Rochester, Minn., and a diplomate of the American Board of Addiction Medicine. The opinions expressed are those of the author and do not necessarily represent the views and opinions of the Mayo Clinic. The opinions expressed in this article should not be used to diagnose or treat any medical condition nor should they be used as a substitute for medical advice from a qualified, board-certified practicing clinician. Dr. Ebbert has no relevant financial disclosures about this article.
$1.8 Billion Wasted With Single Dose Chemotherapy Vials
The use of single dose vials for chemotherapy leads to significant wastage and unnecessary expense, according to a study published in BMJ. Researchers, led by Dr. Peter B. Bach, director of the Center for Health Policy and Outcomes at Memorial Sloan Kettering Cancer Center, examined the top 20 cancer drugs based on sales projections for 2016 that are packaged in single dose vials and dosed by body size, which often results in leftover and unused medication. According to the authors, as much as 10% of the drugs were not used. However, hospital systems, including the VA and the DoD, pay for the entire dose, “making wasted drug a source of unnecessary spending,” the researchers note. The authors estimate the cost for this waste could reach $1.8 billion.
Related:FDA Approves Rescue Drug for Chemotherapy Overdose
Currently, safety standards from the U.S. Pharmacopeial Convention only permit sharing if a leftover drug is used within 6 hours, and only in specialized pharmacies. “Policy makers should also revisit the current FDA guidance on the appropriate packaging of infused drugs in single dose vials and encourage the FDA, Centers for Drug Control and Prevention, Centers for Medicare and Medicaid Services, and US Pharmacopeial Convention to reconcile their views on vial contents and vial sharing,” the authors urged. “Such steps could lead to savings for our health care system without sacrificing health outcomes. Opportunities to eradicate waste of this kind are rare.”.
Related: DoD Proposed 2017 Budget Include Cost Hikes For Military Retirees
To measure the waste the researchers estimated Medicare claims records to determine how frequently vial sharing occurred. They then calculated the most efficient way to combine available vial sizes to achieve the lowest FDA-approved dose in a representative sample of the US population derived from the National Health and Nutrition Examination Survey with adjustments for the cancer patient population.
Related: The Cost of Oncology Drugs: A Pharmacy Perspective, Part I
To reduce waste, the authors suggest that manufacturers be required to package drugs in quantities that allow better matching with required doses or enable virtual return of leftover drug.
The use of single dose vials for chemotherapy leads to significant wastage and unnecessary expense, according to a study published in BMJ. Researchers, led by Dr. Peter B. Bach, director of the Center for Health Policy and Outcomes at Memorial Sloan Kettering Cancer Center, examined the top 20 cancer drugs based on sales projections for 2016 that are packaged in single dose vials and dosed by body size, which often results in leftover and unused medication. According to the authors, as much as 10% of the drugs were not used. However, hospital systems, including the VA and the DoD, pay for the entire dose, “making wasted drug a source of unnecessary spending,” the researchers note. The authors estimate the cost for this waste could reach $1.8 billion.
Related:FDA Approves Rescue Drug for Chemotherapy Overdose
Currently, safety standards from the U.S. Pharmacopeial Convention only permit sharing if a leftover drug is used within 6 hours, and only in specialized pharmacies. “Policy makers should also revisit the current FDA guidance on the appropriate packaging of infused drugs in single dose vials and encourage the FDA, Centers for Drug Control and Prevention, Centers for Medicare and Medicaid Services, and US Pharmacopeial Convention to reconcile their views on vial contents and vial sharing,” the authors urged. “Such steps could lead to savings for our health care system without sacrificing health outcomes. Opportunities to eradicate waste of this kind are rare.”.
Related: DoD Proposed 2017 Budget Include Cost Hikes For Military Retirees
To measure the waste the researchers estimated Medicare claims records to determine how frequently vial sharing occurred. They then calculated the most efficient way to combine available vial sizes to achieve the lowest FDA-approved dose in a representative sample of the US population derived from the National Health and Nutrition Examination Survey with adjustments for the cancer patient population.
Related: The Cost of Oncology Drugs: A Pharmacy Perspective, Part I
To reduce waste, the authors suggest that manufacturers be required to package drugs in quantities that allow better matching with required doses or enable virtual return of leftover drug.
The use of single dose vials for chemotherapy leads to significant wastage and unnecessary expense, according to a study published in BMJ. Researchers, led by Dr. Peter B. Bach, director of the Center for Health Policy and Outcomes at Memorial Sloan Kettering Cancer Center, examined the top 20 cancer drugs based on sales projections for 2016 that are packaged in single dose vials and dosed by body size, which often results in leftover and unused medication. According to the authors, as much as 10% of the drugs were not used. However, hospital systems, including the VA and the DoD, pay for the entire dose, “making wasted drug a source of unnecessary spending,” the researchers note. The authors estimate the cost for this waste could reach $1.8 billion.
Related:FDA Approves Rescue Drug for Chemotherapy Overdose
Currently, safety standards from the U.S. Pharmacopeial Convention only permit sharing if a leftover drug is used within 6 hours, and only in specialized pharmacies. “Policy makers should also revisit the current FDA guidance on the appropriate packaging of infused drugs in single dose vials and encourage the FDA, Centers for Drug Control and Prevention, Centers for Medicare and Medicaid Services, and US Pharmacopeial Convention to reconcile their views on vial contents and vial sharing,” the authors urged. “Such steps could lead to savings for our health care system without sacrificing health outcomes. Opportunities to eradicate waste of this kind are rare.”.
Related: DoD Proposed 2017 Budget Include Cost Hikes For Military Retirees
To measure the waste the researchers estimated Medicare claims records to determine how frequently vial sharing occurred. They then calculated the most efficient way to combine available vial sizes to achieve the lowest FDA-approved dose in a representative sample of the US population derived from the National Health and Nutrition Examination Survey with adjustments for the cancer patient population.
Related: The Cost of Oncology Drugs: A Pharmacy Perspective, Part I
To reduce waste, the authors suggest that manufacturers be required to package drugs in quantities that allow better matching with required doses or enable virtual return of leftover drug.
Intranasal Drug Delivery Bypasses the Blood–Brain Barrier
LAS VEGAS—The nasal mucosa in the upper third of the nasal cavity provides a direct pathway from the external environment to the brain and, according to William H. Frey II, PhD, that pathway can be used to noninvasively deliver therapeutics into the brain. This pathway effectively bypasses the blood–brain barrier and avoids the systemic exposure and side effects associated with therapeutics that enter the bloodstream. At the 19th Annual Meeting of the North American Neuromodulation Society, Dr. Frey presented an in-depth look at intranasal delivery of therapeutics to the brain.
“We have learned from experience that therapeutics sprayed into the nose or even given as nose drops can travel extracellularly and paracellularly along the olfactory axon bundles and along the trigeminal nerve pathway from the nose to the brain,” said Dr. Frey, who is Founder and Codirector of the Alzheimer’s Research Center at Regions Hospital and Senior Director of HealthPartners Neuroscience Research in St. Paul.
Therapeutics that can be delivered intranasally include proteins like insulin, small molecules, charged molecules, oligonucleotides, therapeutic cells like stem cells and Treg cells, nanoparticles, and microparticles. “You do not have to modify your drug or therapeutic in any way in order to do this, but this method only works for really potent therapeutics that are active in the picomolar, nanomolar, or very low micromolar concentration range,” Dr. Frey said.
This technique is being investigated in various disorders. “Most of the studies have been done in animal models, but the Alzheimer’s work has also been done in humans,” Dr. Frey said.
The Neuroanatomy of Intranasal Delivery
The cribriform plate of the skull separates the upper part of the nasal cavity from the brain. The primary olfactory nerves are located in the roof of the nasal cavity under the cribriform plate and include the olfactory sensory neurons and odorant receptors. Sniffing brings molecules into the nose, thus allowing them to bind to odorant receptors and send a signal. Intranasal delivery of therapeutics involves spraying therapeutics into the upper part of the nasal cavity to enable them to follow these olfactory axon bundles directly into the brain through foramena in the cribriform plate. Once across the cribriform plate, the therapeutics penetrate the subarachnoid space and enter the perivascular spaces of the brain’s blood vessels.
When the heart pumps, a corresponding pulsation in the cerebrovasculature creates a perivascular pumping mechanism that moves the therapeutics throughout the brain. “They are near the blood vessels, but on the brain side of the blood–brain barrier throughout the brain,” Dr. Frey explained. Drugs also follow the trigeminal nerves that innervate the entire nasal mucosa and follow the trigeminal neural pathway through the trigeminal ganglion and into the brain and upper spinal cord.
“[This method] results in rapid delivery—within 10 minutes in mice, rats, and monkeys—to the brain and upper spinal cord,” Dr. Frey said. In humans, intranasal neuropeptides reach the CSF within 10 minutes.
Stroke
Preclinical studies have examined intranasal therapy for stroke. Researchers gave rats a stroke by occluding the middle cerebral artery. Two hours of occlusion were followed by reperfusion. Ten minutes after the reperfusion was initiated, investigators administered nose drops containing insulin-like growth factor 1—a 7,600-Da neurotrophic protein naturally found in humans. Compared with controls, rats that received 150 mg of this peptide intranasally had an infarct volume or amount of brain damage that was reduced by 63%. Benefit was also seen when treatment was delayed for two or four hours.
Brain Tumors
A different intranasal treatment uses GRN163, a polynucleotide that inhibits the enzyme telomerase. Telomerase is expressed highly in brain tumors and is required for the brain tumor cells to keep dividing. Investigators tagged the negatively charged large polynucleotide with a fluorescent label and administered it. They observed that GRN163 accumulated in the brain tumor over a period of four hours but did not accumulate in the normal brain. After 24 hours, GRN163 was completely cleared from the brain. Survival time was doubled following treatment with the intranasal polynucleotide.
Neurodegenerative Disease
Iron accumulates abnormally in the brain in all of the neurodegenerative disorders. “Obviously, our bodies need iron, but the abnormal accumulation of free iron is damaging because it is a strong promoter of free-radical damage,” Dr. Frey said. Data also indicate that the key receptor for memory, the human brain muscarinic cholinergic receptor, is rapidly inactivated by free iron or free heme, which are present at increased levels in the brains of people with Alzheimer’s disease. “We have a potent iron chelator, deferoxamine mesylate, that has been around for about 40 years. It has a high affinity for iron and it is a generic drug. It has been used to treat beta thalassemia, sickle cell anemia, and various conditions where too much iron is accumulated in the blood. When given intramuscularly over a period of two years to patients with Alzheimer’s disease, it reduced their cognitive decline by 50%. That’s a far bigger benefit than any drug on the market today for Alzheimer’s disease,” Dr. Frey noted. But there were significant side effects at the injection site, and deferoxamine does not cross the blood–brain barrier well. “Consequently, we’ve been developing and have patented intranasal deferoxamine to treat Parkinson’s disease, Alzheimer’s disease, stroke, and traumatic brain injury,” Dr. Frey said.
“We’ve shown that intranasal deferoxamine protects dopamine brain cells and improves movement in animals with Parkinson’s disease… We’ve shown that just a few nose drops of deferoxamine given before or after a stroke reduce brain damage in rats by 55%. We’ve shown that even in normal mice, it improves memory when given intranasally. And it also improves or reduces memory loss in Alzheimer transgenic mice.”
Alzheimer’s Disease
Fludeoxyglucose (18F) PET scans reveal adequate uptake and utilization of glucose, the main energy source for brain cells, in the brains of healthy elderly controls. But the brains of patients with Alzheimer’s disease do not take up glucose normally, and their brain cells consequently have less energy. “A number of areas of the brain require insulin to take up glucose, and the hippocampus is one of those areas,” Dr. Frey explained. “Insulin signaling is reduced in the brains of patients with Alzheimer’s disease, causing what some have called type 3 diabetes, or diabetes of the brain, which leaves these brain cells starved for energy and not able to function normally.”
Dr. Frey obtained several patents on the direct intranasal delivery of therapeutics, including insulin, to the brain, and various clinical trials have been conducted. “Four trials in patients with Alzheimer’s disease and five trials in normal, healthy adults have demonstrated improved memory following intranasal insulin treatment, with no change in the blood levels of insulin or glucose,” Dr. Frey reported.
In one of the first trials, a single intranasal insulin treatment improved verbal memory for individuals with Alzheimer’s disease within 15 minutes. In a three-week trial, intranasal insulin enhanced memory, compared with placebo, and significantly improved attention and functional status in patients with Alzheimer’s disease. However, patients who carried the APOE ε4 gene allele were not improved with intranasal regular insulin. “Only long-acting insulin detemir, given intranasally, has been shown to improve memory in patients who have the APOE ε4 gene allele,”Dr. Frey said.
The longest completed trial lasted for four months and showed improved memory and function in patients who were given insulin twice per day in a nasal spray. It also showed that the treatment reduced the loss of glucose uptake and utilization in key brain regions, as seen in PET scans. A new six-month treatment trial is now underway at the HealthPartners Center for Memory & Aging in St. Paul.
Mechanism of Action
One open question is whether intranasal insulin only provides symptomatic treatment (ie, improved memory and functioning in patients with Alzheimer’s disease) or also has the potential to change the underlying disease process. “We know it can provide energy to prevent brain cells from degenerating and allow the cells to produce materials to replace worn-out parts,” said Dr. Frey. “That result has been shown in humans using P-31 MRI. After administration of intranasal insulin, levels of brain cell adenosine triphosphate (ATP) and brain cell phosphocreatine increase significantly. We know that insulin, after it causes signaling, and glucose uptake occurs, causes the production of insulin-degrading enzyme to reduce the insulin signal so that the next time the signal comes in, it can be easily detected. That turns out to be the enzyme that degrades beta amyloid, which accumulates abnormally in the brains of individuals with Alzheimer disease. So, if you don’t have insulin signaling, you don’t make insulin-degrading enzyme, and you accumulate beta amyloid. Insulin also inhibits glycogen synthase kinase 3 beta that phosphorylates tau to form Alzheimer neurofibrillary tangles. Insulin is also needed to maintain synaptic density, so it is possible that if humans were given intranasal insulin at the first sign of an insulin-signaling deficiency or a decrease in glucose uptake in the brain, it might be possible to delay, or maybe even prevent, the onset of this disease,” Dr. Frey said.
Stem Cells
Dr. Frey and research collaborators in Germany, including Lusine Danielyan MD, discovered and patented that intranasal stem cells bypass the blood–brain barrier to reach the brain and treat Parkinson’s disease in rats. Adult bone marrow–derived stem cells have anti-inflammatory and neurotrophic properties. “After cell treatment, the proinflammatory cytokines in this inflammatory brain disease go down to normal levels. Our study showed highly significant improvement, compared with placebo, in motor function or movement,” Dr. Frey said.
Researchers in the Netherlands demonstrated that intranasal adult stem cells treat neonatal ischemia and neonatal brain damage. Other researchers at Emory University reported treatment of stroke with adult stem cells from bone marrow. Swedish researchers reported that intranasal Treg cells treat multiple sclerosis (MS). “These studies are all in animals,” Dr. Frey noted. Other researchers reported that neuronal stem cells induce recovery and remyelination in an animal model of MS. Brain tumors have also been treated in animals with intranasal stem cells. Recently, intranasal stem cell therapy was also reported to improve motor function and reduce lesion size in spinal cord injury in animals. “Noninvasive intranasal delivery can target therapeutics to the brain while reducing systemic exposure to facilitate the treatment of brain disorders,” said Dr. Frey.
—Glenn S. Williams
Suggested Reading
Danielyan L, Schäfer R, von Ameln-Mayerhofer A, et al. Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson’s disease. Rejuvenation Res. 2011;14(1):3-16.
Danielyan L, Beer-Hammer S, Stolzing A, et al. Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer’s and Parkinson’s disease. Cell Transplant. 2014;23(suppl 1):S123-S139.
Frey WH 2nd. Noninvasive intranasal stem cells bypass the blood-brain barrier to target the brain to treat Parkinson’s disease, stroke, MS, brain tumors, cerebral ischemia, Alzheimer’s and other CNS disorders. J Nat Sci. 2015;1(1):e23.
Lochhead JJ, Wolak DJ, Pizzo ME, Thorne RG. Rapid transport within cerebral perivascular spaces underlies widespread tracer distribution in the brain after intranasal administration. J Cereb Blood Flow Metab. 2015;35(3):371-381.
LAS VEGAS—The nasal mucosa in the upper third of the nasal cavity provides a direct pathway from the external environment to the brain and, according to William H. Frey II, PhD, that pathway can be used to noninvasively deliver therapeutics into the brain. This pathway effectively bypasses the blood–brain barrier and avoids the systemic exposure and side effects associated with therapeutics that enter the bloodstream. At the 19th Annual Meeting of the North American Neuromodulation Society, Dr. Frey presented an in-depth look at intranasal delivery of therapeutics to the brain.
“We have learned from experience that therapeutics sprayed into the nose or even given as nose drops can travel extracellularly and paracellularly along the olfactory axon bundles and along the trigeminal nerve pathway from the nose to the brain,” said Dr. Frey, who is Founder and Codirector of the Alzheimer’s Research Center at Regions Hospital and Senior Director of HealthPartners Neuroscience Research in St. Paul.
Therapeutics that can be delivered intranasally include proteins like insulin, small molecules, charged molecules, oligonucleotides, therapeutic cells like stem cells and Treg cells, nanoparticles, and microparticles. “You do not have to modify your drug or therapeutic in any way in order to do this, but this method only works for really potent therapeutics that are active in the picomolar, nanomolar, or very low micromolar concentration range,” Dr. Frey said.
This technique is being investigated in various disorders. “Most of the studies have been done in animal models, but the Alzheimer’s work has also been done in humans,” Dr. Frey said.
The Neuroanatomy of Intranasal Delivery
The cribriform plate of the skull separates the upper part of the nasal cavity from the brain. The primary olfactory nerves are located in the roof of the nasal cavity under the cribriform plate and include the olfactory sensory neurons and odorant receptors. Sniffing brings molecules into the nose, thus allowing them to bind to odorant receptors and send a signal. Intranasal delivery of therapeutics involves spraying therapeutics into the upper part of the nasal cavity to enable them to follow these olfactory axon bundles directly into the brain through foramena in the cribriform plate. Once across the cribriform plate, the therapeutics penetrate the subarachnoid space and enter the perivascular spaces of the brain’s blood vessels.
When the heart pumps, a corresponding pulsation in the cerebrovasculature creates a perivascular pumping mechanism that moves the therapeutics throughout the brain. “They are near the blood vessels, but on the brain side of the blood–brain barrier throughout the brain,” Dr. Frey explained. Drugs also follow the trigeminal nerves that innervate the entire nasal mucosa and follow the trigeminal neural pathway through the trigeminal ganglion and into the brain and upper spinal cord.
“[This method] results in rapid delivery—within 10 minutes in mice, rats, and monkeys—to the brain and upper spinal cord,” Dr. Frey said. In humans, intranasal neuropeptides reach the CSF within 10 minutes.
Stroke
Preclinical studies have examined intranasal therapy for stroke. Researchers gave rats a stroke by occluding the middle cerebral artery. Two hours of occlusion were followed by reperfusion. Ten minutes after the reperfusion was initiated, investigators administered nose drops containing insulin-like growth factor 1—a 7,600-Da neurotrophic protein naturally found in humans. Compared with controls, rats that received 150 mg of this peptide intranasally had an infarct volume or amount of brain damage that was reduced by 63%. Benefit was also seen when treatment was delayed for two or four hours.
Brain Tumors
A different intranasal treatment uses GRN163, a polynucleotide that inhibits the enzyme telomerase. Telomerase is expressed highly in brain tumors and is required for the brain tumor cells to keep dividing. Investigators tagged the negatively charged large polynucleotide with a fluorescent label and administered it. They observed that GRN163 accumulated in the brain tumor over a period of four hours but did not accumulate in the normal brain. After 24 hours, GRN163 was completely cleared from the brain. Survival time was doubled following treatment with the intranasal polynucleotide.
Neurodegenerative Disease
Iron accumulates abnormally in the brain in all of the neurodegenerative disorders. “Obviously, our bodies need iron, but the abnormal accumulation of free iron is damaging because it is a strong promoter of free-radical damage,” Dr. Frey said. Data also indicate that the key receptor for memory, the human brain muscarinic cholinergic receptor, is rapidly inactivated by free iron or free heme, which are present at increased levels in the brains of people with Alzheimer’s disease. “We have a potent iron chelator, deferoxamine mesylate, that has been around for about 40 years. It has a high affinity for iron and it is a generic drug. It has been used to treat beta thalassemia, sickle cell anemia, and various conditions where too much iron is accumulated in the blood. When given intramuscularly over a period of two years to patients with Alzheimer’s disease, it reduced their cognitive decline by 50%. That’s a far bigger benefit than any drug on the market today for Alzheimer’s disease,” Dr. Frey noted. But there were significant side effects at the injection site, and deferoxamine does not cross the blood–brain barrier well. “Consequently, we’ve been developing and have patented intranasal deferoxamine to treat Parkinson’s disease, Alzheimer’s disease, stroke, and traumatic brain injury,” Dr. Frey said.
“We’ve shown that intranasal deferoxamine protects dopamine brain cells and improves movement in animals with Parkinson’s disease… We’ve shown that just a few nose drops of deferoxamine given before or after a stroke reduce brain damage in rats by 55%. We’ve shown that even in normal mice, it improves memory when given intranasally. And it also improves or reduces memory loss in Alzheimer transgenic mice.”
Alzheimer’s Disease
Fludeoxyglucose (18F) PET scans reveal adequate uptake and utilization of glucose, the main energy source for brain cells, in the brains of healthy elderly controls. But the brains of patients with Alzheimer’s disease do not take up glucose normally, and their brain cells consequently have less energy. “A number of areas of the brain require insulin to take up glucose, and the hippocampus is one of those areas,” Dr. Frey explained. “Insulin signaling is reduced in the brains of patients with Alzheimer’s disease, causing what some have called type 3 diabetes, or diabetes of the brain, which leaves these brain cells starved for energy and not able to function normally.”
Dr. Frey obtained several patents on the direct intranasal delivery of therapeutics, including insulin, to the brain, and various clinical trials have been conducted. “Four trials in patients with Alzheimer’s disease and five trials in normal, healthy adults have demonstrated improved memory following intranasal insulin treatment, with no change in the blood levels of insulin or glucose,” Dr. Frey reported.
In one of the first trials, a single intranasal insulin treatment improved verbal memory for individuals with Alzheimer’s disease within 15 minutes. In a three-week trial, intranasal insulin enhanced memory, compared with placebo, and significantly improved attention and functional status in patients with Alzheimer’s disease. However, patients who carried the APOE ε4 gene allele were not improved with intranasal regular insulin. “Only long-acting insulin detemir, given intranasally, has been shown to improve memory in patients who have the APOE ε4 gene allele,”Dr. Frey said.
The longest completed trial lasted for four months and showed improved memory and function in patients who were given insulin twice per day in a nasal spray. It also showed that the treatment reduced the loss of glucose uptake and utilization in key brain regions, as seen in PET scans. A new six-month treatment trial is now underway at the HealthPartners Center for Memory & Aging in St. Paul.
Mechanism of Action
One open question is whether intranasal insulin only provides symptomatic treatment (ie, improved memory and functioning in patients with Alzheimer’s disease) or also has the potential to change the underlying disease process. “We know it can provide energy to prevent brain cells from degenerating and allow the cells to produce materials to replace worn-out parts,” said Dr. Frey. “That result has been shown in humans using P-31 MRI. After administration of intranasal insulin, levels of brain cell adenosine triphosphate (ATP) and brain cell phosphocreatine increase significantly. We know that insulin, after it causes signaling, and glucose uptake occurs, causes the production of insulin-degrading enzyme to reduce the insulin signal so that the next time the signal comes in, it can be easily detected. That turns out to be the enzyme that degrades beta amyloid, which accumulates abnormally in the brains of individuals with Alzheimer disease. So, if you don’t have insulin signaling, you don’t make insulin-degrading enzyme, and you accumulate beta amyloid. Insulin also inhibits glycogen synthase kinase 3 beta that phosphorylates tau to form Alzheimer neurofibrillary tangles. Insulin is also needed to maintain synaptic density, so it is possible that if humans were given intranasal insulin at the first sign of an insulin-signaling deficiency or a decrease in glucose uptake in the brain, it might be possible to delay, or maybe even prevent, the onset of this disease,” Dr. Frey said.
Stem Cells
Dr. Frey and research collaborators in Germany, including Lusine Danielyan MD, discovered and patented that intranasal stem cells bypass the blood–brain barrier to reach the brain and treat Parkinson’s disease in rats. Adult bone marrow–derived stem cells have anti-inflammatory and neurotrophic properties. “After cell treatment, the proinflammatory cytokines in this inflammatory brain disease go down to normal levels. Our study showed highly significant improvement, compared with placebo, in motor function or movement,” Dr. Frey said.
Researchers in the Netherlands demonstrated that intranasal adult stem cells treat neonatal ischemia and neonatal brain damage. Other researchers at Emory University reported treatment of stroke with adult stem cells from bone marrow. Swedish researchers reported that intranasal Treg cells treat multiple sclerosis (MS). “These studies are all in animals,” Dr. Frey noted. Other researchers reported that neuronal stem cells induce recovery and remyelination in an animal model of MS. Brain tumors have also been treated in animals with intranasal stem cells. Recently, intranasal stem cell therapy was also reported to improve motor function and reduce lesion size in spinal cord injury in animals. “Noninvasive intranasal delivery can target therapeutics to the brain while reducing systemic exposure to facilitate the treatment of brain disorders,” said Dr. Frey.
—Glenn S. Williams
LAS VEGAS—The nasal mucosa in the upper third of the nasal cavity provides a direct pathway from the external environment to the brain and, according to William H. Frey II, PhD, that pathway can be used to noninvasively deliver therapeutics into the brain. This pathway effectively bypasses the blood–brain barrier and avoids the systemic exposure and side effects associated with therapeutics that enter the bloodstream. At the 19th Annual Meeting of the North American Neuromodulation Society, Dr. Frey presented an in-depth look at intranasal delivery of therapeutics to the brain.
“We have learned from experience that therapeutics sprayed into the nose or even given as nose drops can travel extracellularly and paracellularly along the olfactory axon bundles and along the trigeminal nerve pathway from the nose to the brain,” said Dr. Frey, who is Founder and Codirector of the Alzheimer’s Research Center at Regions Hospital and Senior Director of HealthPartners Neuroscience Research in St. Paul.
Therapeutics that can be delivered intranasally include proteins like insulin, small molecules, charged molecules, oligonucleotides, therapeutic cells like stem cells and Treg cells, nanoparticles, and microparticles. “You do not have to modify your drug or therapeutic in any way in order to do this, but this method only works for really potent therapeutics that are active in the picomolar, nanomolar, or very low micromolar concentration range,” Dr. Frey said.
This technique is being investigated in various disorders. “Most of the studies have been done in animal models, but the Alzheimer’s work has also been done in humans,” Dr. Frey said.
The Neuroanatomy of Intranasal Delivery
The cribriform plate of the skull separates the upper part of the nasal cavity from the brain. The primary olfactory nerves are located in the roof of the nasal cavity under the cribriform plate and include the olfactory sensory neurons and odorant receptors. Sniffing brings molecules into the nose, thus allowing them to bind to odorant receptors and send a signal. Intranasal delivery of therapeutics involves spraying therapeutics into the upper part of the nasal cavity to enable them to follow these olfactory axon bundles directly into the brain through foramena in the cribriform plate. Once across the cribriform plate, the therapeutics penetrate the subarachnoid space and enter the perivascular spaces of the brain’s blood vessels.
When the heart pumps, a corresponding pulsation in the cerebrovasculature creates a perivascular pumping mechanism that moves the therapeutics throughout the brain. “They are near the blood vessels, but on the brain side of the blood–brain barrier throughout the brain,” Dr. Frey explained. Drugs also follow the trigeminal nerves that innervate the entire nasal mucosa and follow the trigeminal neural pathway through the trigeminal ganglion and into the brain and upper spinal cord.
“[This method] results in rapid delivery—within 10 minutes in mice, rats, and monkeys—to the brain and upper spinal cord,” Dr. Frey said. In humans, intranasal neuropeptides reach the CSF within 10 minutes.
Stroke
Preclinical studies have examined intranasal therapy for stroke. Researchers gave rats a stroke by occluding the middle cerebral artery. Two hours of occlusion were followed by reperfusion. Ten minutes after the reperfusion was initiated, investigators administered nose drops containing insulin-like growth factor 1—a 7,600-Da neurotrophic protein naturally found in humans. Compared with controls, rats that received 150 mg of this peptide intranasally had an infarct volume or amount of brain damage that was reduced by 63%. Benefit was also seen when treatment was delayed for two or four hours.
Brain Tumors
A different intranasal treatment uses GRN163, a polynucleotide that inhibits the enzyme telomerase. Telomerase is expressed highly in brain tumors and is required for the brain tumor cells to keep dividing. Investigators tagged the negatively charged large polynucleotide with a fluorescent label and administered it. They observed that GRN163 accumulated in the brain tumor over a period of four hours but did not accumulate in the normal brain. After 24 hours, GRN163 was completely cleared from the brain. Survival time was doubled following treatment with the intranasal polynucleotide.
Neurodegenerative Disease
Iron accumulates abnormally in the brain in all of the neurodegenerative disorders. “Obviously, our bodies need iron, but the abnormal accumulation of free iron is damaging because it is a strong promoter of free-radical damage,” Dr. Frey said. Data also indicate that the key receptor for memory, the human brain muscarinic cholinergic receptor, is rapidly inactivated by free iron or free heme, which are present at increased levels in the brains of people with Alzheimer’s disease. “We have a potent iron chelator, deferoxamine mesylate, that has been around for about 40 years. It has a high affinity for iron and it is a generic drug. It has been used to treat beta thalassemia, sickle cell anemia, and various conditions where too much iron is accumulated in the blood. When given intramuscularly over a period of two years to patients with Alzheimer’s disease, it reduced their cognitive decline by 50%. That’s a far bigger benefit than any drug on the market today for Alzheimer’s disease,” Dr. Frey noted. But there were significant side effects at the injection site, and deferoxamine does not cross the blood–brain barrier well. “Consequently, we’ve been developing and have patented intranasal deferoxamine to treat Parkinson’s disease, Alzheimer’s disease, stroke, and traumatic brain injury,” Dr. Frey said.
“We’ve shown that intranasal deferoxamine protects dopamine brain cells and improves movement in animals with Parkinson’s disease… We’ve shown that just a few nose drops of deferoxamine given before or after a stroke reduce brain damage in rats by 55%. We’ve shown that even in normal mice, it improves memory when given intranasally. And it also improves or reduces memory loss in Alzheimer transgenic mice.”
Alzheimer’s Disease
Fludeoxyglucose (18F) PET scans reveal adequate uptake and utilization of glucose, the main energy source for brain cells, in the brains of healthy elderly controls. But the brains of patients with Alzheimer’s disease do not take up glucose normally, and their brain cells consequently have less energy. “A number of areas of the brain require insulin to take up glucose, and the hippocampus is one of those areas,” Dr. Frey explained. “Insulin signaling is reduced in the brains of patients with Alzheimer’s disease, causing what some have called type 3 diabetes, or diabetes of the brain, which leaves these brain cells starved for energy and not able to function normally.”
Dr. Frey obtained several patents on the direct intranasal delivery of therapeutics, including insulin, to the brain, and various clinical trials have been conducted. “Four trials in patients with Alzheimer’s disease and five trials in normal, healthy adults have demonstrated improved memory following intranasal insulin treatment, with no change in the blood levels of insulin or glucose,” Dr. Frey reported.
In one of the first trials, a single intranasal insulin treatment improved verbal memory for individuals with Alzheimer’s disease within 15 minutes. In a three-week trial, intranasal insulin enhanced memory, compared with placebo, and significantly improved attention and functional status in patients with Alzheimer’s disease. However, patients who carried the APOE ε4 gene allele were not improved with intranasal regular insulin. “Only long-acting insulin detemir, given intranasally, has been shown to improve memory in patients who have the APOE ε4 gene allele,”Dr. Frey said.
The longest completed trial lasted for four months and showed improved memory and function in patients who were given insulin twice per day in a nasal spray. It also showed that the treatment reduced the loss of glucose uptake and utilization in key brain regions, as seen in PET scans. A new six-month treatment trial is now underway at the HealthPartners Center for Memory & Aging in St. Paul.
Mechanism of Action
One open question is whether intranasal insulin only provides symptomatic treatment (ie, improved memory and functioning in patients with Alzheimer’s disease) or also has the potential to change the underlying disease process. “We know it can provide energy to prevent brain cells from degenerating and allow the cells to produce materials to replace worn-out parts,” said Dr. Frey. “That result has been shown in humans using P-31 MRI. After administration of intranasal insulin, levels of brain cell adenosine triphosphate (ATP) and brain cell phosphocreatine increase significantly. We know that insulin, after it causes signaling, and glucose uptake occurs, causes the production of insulin-degrading enzyme to reduce the insulin signal so that the next time the signal comes in, it can be easily detected. That turns out to be the enzyme that degrades beta amyloid, which accumulates abnormally in the brains of individuals with Alzheimer disease. So, if you don’t have insulin signaling, you don’t make insulin-degrading enzyme, and you accumulate beta amyloid. Insulin also inhibits glycogen synthase kinase 3 beta that phosphorylates tau to form Alzheimer neurofibrillary tangles. Insulin is also needed to maintain synaptic density, so it is possible that if humans were given intranasal insulin at the first sign of an insulin-signaling deficiency or a decrease in glucose uptake in the brain, it might be possible to delay, or maybe even prevent, the onset of this disease,” Dr. Frey said.
Stem Cells
Dr. Frey and research collaborators in Germany, including Lusine Danielyan MD, discovered and patented that intranasal stem cells bypass the blood–brain barrier to reach the brain and treat Parkinson’s disease in rats. Adult bone marrow–derived stem cells have anti-inflammatory and neurotrophic properties. “After cell treatment, the proinflammatory cytokines in this inflammatory brain disease go down to normal levels. Our study showed highly significant improvement, compared with placebo, in motor function or movement,” Dr. Frey said.
Researchers in the Netherlands demonstrated that intranasal adult stem cells treat neonatal ischemia and neonatal brain damage. Other researchers at Emory University reported treatment of stroke with adult stem cells from bone marrow. Swedish researchers reported that intranasal Treg cells treat multiple sclerosis (MS). “These studies are all in animals,” Dr. Frey noted. Other researchers reported that neuronal stem cells induce recovery and remyelination in an animal model of MS. Brain tumors have also been treated in animals with intranasal stem cells. Recently, intranasal stem cell therapy was also reported to improve motor function and reduce lesion size in spinal cord injury in animals. “Noninvasive intranasal delivery can target therapeutics to the brain while reducing systemic exposure to facilitate the treatment of brain disorders,” said Dr. Frey.
—Glenn S. Williams
Suggested Reading
Danielyan L, Schäfer R, von Ameln-Mayerhofer A, et al. Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson’s disease. Rejuvenation Res. 2011;14(1):3-16.
Danielyan L, Beer-Hammer S, Stolzing A, et al. Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer’s and Parkinson’s disease. Cell Transplant. 2014;23(suppl 1):S123-S139.
Frey WH 2nd. Noninvasive intranasal stem cells bypass the blood-brain barrier to target the brain to treat Parkinson’s disease, stroke, MS, brain tumors, cerebral ischemia, Alzheimer’s and other CNS disorders. J Nat Sci. 2015;1(1):e23.
Lochhead JJ, Wolak DJ, Pizzo ME, Thorne RG. Rapid transport within cerebral perivascular spaces underlies widespread tracer distribution in the brain after intranasal administration. J Cereb Blood Flow Metab. 2015;35(3):371-381.
Suggested Reading
Danielyan L, Schäfer R, von Ameln-Mayerhofer A, et al. Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson’s disease. Rejuvenation Res. 2011;14(1):3-16.
Danielyan L, Beer-Hammer S, Stolzing A, et al. Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer’s and Parkinson’s disease. Cell Transplant. 2014;23(suppl 1):S123-S139.
Frey WH 2nd. Noninvasive intranasal stem cells bypass the blood-brain barrier to target the brain to treat Parkinson’s disease, stroke, MS, brain tumors, cerebral ischemia, Alzheimer’s and other CNS disorders. J Nat Sci. 2015;1(1):e23.
Lochhead JJ, Wolak DJ, Pizzo ME, Thorne RG. Rapid transport within cerebral perivascular spaces underlies widespread tracer distribution in the brain after intranasal administration. J Cereb Blood Flow Metab. 2015;35(3):371-381.
MRSA incidence decreased in children as clindamycin resistance increased
The incidence of methicillin-resistant Staphylococcus aureus (MRSA) infections has decreased in children in recent years, but resistance to clindamycin has increased over the same period, a study showed.
“The epidemic of skin and soft tissue infections and invasive MRSA led to modifications of antimicrobial prescribing practices for suspected S. aureus infections,” reported Dr. Deena E. Sutter of the San Antonio Military Medical Center in Fort Sam Houston, Tex., and her associates. “Over the study period, erythromycin susceptibility among methicillin-susceptible S. aureus (MSSA) remained stable, suggesting that declining clindamycin susceptibility is a result of an increase in inducible resistance.”
The steady decline in clindamycin susceptibility “may lead to some concern about the continued reliance on clindamycin for the empirical treatment of presumptive S. aureus infections, although it is probably premature to abandon this effective antibiotic choice,” they wrote (Pediatrics. 2016 Mar. 1. doi: 10.1542/peds.2015-3099). “It is crucial that clinicians remain knowledgeable about local susceptibility rates as it would be prudent to consider [alternative] antimicrobial agents for empirical use when the local clindamycin susceptibility rate drops below 85%.”
The researchers retrospectively analyzed lab results from 39,209 patients under age 18 who were treated for S. aureus infections at one of the 266 U.S. facilities of the Military Health System from 2005 to 2014. The data included 41,745 S. aureus isolates, classified as MRSA if found resistant to cefoxitin, methicillin, or oxacillin and as methicillin susceptible (MSSA) if susceptible to those antimicrobials. The isolates had also been tested for susceptibility to ciprofloxacin, clindamycin, erythromycin, gentamicin, oxacillin, penicillin, rifampin, tetracycline, and trimethoprim/sulfamethoxazole (TMP/SMX).
During that decade, overall S. aureus susceptibility to clindamycin, ciprofloxacin, and TMP/SMX decreased – although susceptibility to TMP/SMX in 2014 stayed high at 98% – while overall susceptibility to erythromycin, gentamicin, and oxacillin increased. Specifically, 59% of S. aureus isolates were susceptible to oxacillin in 2005, which dropped briefly to 54% in 2007 before climbing to the 2014 rate of 68%.
Meanwhile, overall susceptibility to clindamycin dropped from 91% in 2005 to 86% in 2014, and MSSA susceptibility to clindamycin dropped from 91% in 2005 to 84% in 2014. “Erythromycin susceptibility remained stable among MSSA isolates throughout the study period at 63.5%, whereas MRSA susceptibility to erythromycin increased from 12.1% to 20.5%,” Dr. Sutter and her associates reported. “Ciprofloxacin susceptibility significantly decreased overall, although an initial decrease of 10.6% over the first 7 years of the study was subsequently followed by an increase of 6% between 2011 and 2014.”
Most of the isolates came from patients with skin and soft tissue infections, which were less likely to be susceptible to oxacillin than were other infections. Infections in children aged 1-5 years also were less likely to be susceptible to oxacillin, compared with infections in children of other age groups.
If the local clindamycin susceptibility rate falls below 85%, “beta-lactams, TMP/SMX, or tetracyclines may be used for less severe infections with intravenous vancomycin employed in severe cases,” the investigators said. “If overall MRSA rates continue to decline and clindamycin resistance among MSSA continues to increase, we may see a return to antistaphylococcal beta-lactam antimicrobial agents such as oxacillin or first-generation cephalosporins as preferred empirical therapy for presumed S. aureus infections.”
The research did not use external funding, and the authors reported no relevant financial disclosures.
Staphylococcus aureus is one of the most common organisms isolated from children with health care–associated infections, regardless of whether these infections had their onset in the community or were acquired in the hospital. Thus, the initial empiric treatment of a skin or soft tissue infection or invasive infection in a child almost always includes an antibiotic effective against S. aureus.
However, over the years, clindamycin susceptibility among S. aureus isolates has declined, likely related to the increased use of this agent for empiric as well as definitive treatment of community-acquired (CA) MRSA infections, encouraging the transmission of the genes associated with clindamycin resistance.
What are the implications of the findings from the report by Sutter et al. with respect to the selection of empiric antibiotics for children with suspected S. aureus infections? Currently, considering the still substantial MRSA resistance rates that exceed the 10%-15% level suggested by many experts as the threshold above which agents effective against CA-MRSA isolates should be administered for empiric treatment, changes in the selection of empiric antibiotics are not warranted. If rates of MRSA among S. aureus isolates from otherwise normal children are documented to drop below the 10%-15% threshold in different communities, a modification of current recommendations should be considered. It would also be important to understand why methicillin resistance is declining among S. aureus isolates from CA infections; this information may provide clues for preventing CA-MRSA infections with the use of vaccines or other means. The epidemiology of S. aureus infections in children has been changing over the past 2 decades, which is why it is critical to keep a very close eye on this common pathogen.
These comments were excerpted from an accompanying commentary by Dr. Sheldon L. Kaplan of the infectious disease service at Texas Children’s Hospital in Houston (Pediatrics. 2016 Mar 1. doi: 10.1542/peds.2016-0101). Dr. Kaplan has received research funds from Pfizer, Forest Laboratories, and Cubist.
Staphylococcus aureus is one of the most common organisms isolated from children with health care–associated infections, regardless of whether these infections had their onset in the community or were acquired in the hospital. Thus, the initial empiric treatment of a skin or soft tissue infection or invasive infection in a child almost always includes an antibiotic effective against S. aureus.
However, over the years, clindamycin susceptibility among S. aureus isolates has declined, likely related to the increased use of this agent for empiric as well as definitive treatment of community-acquired (CA) MRSA infections, encouraging the transmission of the genes associated with clindamycin resistance.
What are the implications of the findings from the report by Sutter et al. with respect to the selection of empiric antibiotics for children with suspected S. aureus infections? Currently, considering the still substantial MRSA resistance rates that exceed the 10%-15% level suggested by many experts as the threshold above which agents effective against CA-MRSA isolates should be administered for empiric treatment, changes in the selection of empiric antibiotics are not warranted. If rates of MRSA among S. aureus isolates from otherwise normal children are documented to drop below the 10%-15% threshold in different communities, a modification of current recommendations should be considered. It would also be important to understand why methicillin resistance is declining among S. aureus isolates from CA infections; this information may provide clues for preventing CA-MRSA infections with the use of vaccines or other means. The epidemiology of S. aureus infections in children has been changing over the past 2 decades, which is why it is critical to keep a very close eye on this common pathogen.
These comments were excerpted from an accompanying commentary by Dr. Sheldon L. Kaplan of the infectious disease service at Texas Children’s Hospital in Houston (Pediatrics. 2016 Mar 1. doi: 10.1542/peds.2016-0101). Dr. Kaplan has received research funds from Pfizer, Forest Laboratories, and Cubist.
Staphylococcus aureus is one of the most common organisms isolated from children with health care–associated infections, regardless of whether these infections had their onset in the community or were acquired in the hospital. Thus, the initial empiric treatment of a skin or soft tissue infection or invasive infection in a child almost always includes an antibiotic effective against S. aureus.
However, over the years, clindamycin susceptibility among S. aureus isolates has declined, likely related to the increased use of this agent for empiric as well as definitive treatment of community-acquired (CA) MRSA infections, encouraging the transmission of the genes associated with clindamycin resistance.
What are the implications of the findings from the report by Sutter et al. with respect to the selection of empiric antibiotics for children with suspected S. aureus infections? Currently, considering the still substantial MRSA resistance rates that exceed the 10%-15% level suggested by many experts as the threshold above which agents effective against CA-MRSA isolates should be administered for empiric treatment, changes in the selection of empiric antibiotics are not warranted. If rates of MRSA among S. aureus isolates from otherwise normal children are documented to drop below the 10%-15% threshold in different communities, a modification of current recommendations should be considered. It would also be important to understand why methicillin resistance is declining among S. aureus isolates from CA infections; this information may provide clues for preventing CA-MRSA infections with the use of vaccines or other means. The epidemiology of S. aureus infections in children has been changing over the past 2 decades, which is why it is critical to keep a very close eye on this common pathogen.
These comments were excerpted from an accompanying commentary by Dr. Sheldon L. Kaplan of the infectious disease service at Texas Children’s Hospital in Houston (Pediatrics. 2016 Mar 1. doi: 10.1542/peds.2016-0101). Dr. Kaplan has received research funds from Pfizer, Forest Laboratories, and Cubist.
The incidence of methicillin-resistant Staphylococcus aureus (MRSA) infections has decreased in children in recent years, but resistance to clindamycin has increased over the same period, a study showed.
“The epidemic of skin and soft tissue infections and invasive MRSA led to modifications of antimicrobial prescribing practices for suspected S. aureus infections,” reported Dr. Deena E. Sutter of the San Antonio Military Medical Center in Fort Sam Houston, Tex., and her associates. “Over the study period, erythromycin susceptibility among methicillin-susceptible S. aureus (MSSA) remained stable, suggesting that declining clindamycin susceptibility is a result of an increase in inducible resistance.”
The steady decline in clindamycin susceptibility “may lead to some concern about the continued reliance on clindamycin for the empirical treatment of presumptive S. aureus infections, although it is probably premature to abandon this effective antibiotic choice,” they wrote (Pediatrics. 2016 Mar. 1. doi: 10.1542/peds.2015-3099). “It is crucial that clinicians remain knowledgeable about local susceptibility rates as it would be prudent to consider [alternative] antimicrobial agents for empirical use when the local clindamycin susceptibility rate drops below 85%.”
The researchers retrospectively analyzed lab results from 39,209 patients under age 18 who were treated for S. aureus infections at one of the 266 U.S. facilities of the Military Health System from 2005 to 2014. The data included 41,745 S. aureus isolates, classified as MRSA if found resistant to cefoxitin, methicillin, or oxacillin and as methicillin susceptible (MSSA) if susceptible to those antimicrobials. The isolates had also been tested for susceptibility to ciprofloxacin, clindamycin, erythromycin, gentamicin, oxacillin, penicillin, rifampin, tetracycline, and trimethoprim/sulfamethoxazole (TMP/SMX).
During that decade, overall S. aureus susceptibility to clindamycin, ciprofloxacin, and TMP/SMX decreased – although susceptibility to TMP/SMX in 2014 stayed high at 98% – while overall susceptibility to erythromycin, gentamicin, and oxacillin increased. Specifically, 59% of S. aureus isolates were susceptible to oxacillin in 2005, which dropped briefly to 54% in 2007 before climbing to the 2014 rate of 68%.
Meanwhile, overall susceptibility to clindamycin dropped from 91% in 2005 to 86% in 2014, and MSSA susceptibility to clindamycin dropped from 91% in 2005 to 84% in 2014. “Erythromycin susceptibility remained stable among MSSA isolates throughout the study period at 63.5%, whereas MRSA susceptibility to erythromycin increased from 12.1% to 20.5%,” Dr. Sutter and her associates reported. “Ciprofloxacin susceptibility significantly decreased overall, although an initial decrease of 10.6% over the first 7 years of the study was subsequently followed by an increase of 6% between 2011 and 2014.”
Most of the isolates came from patients with skin and soft tissue infections, which were less likely to be susceptible to oxacillin than were other infections. Infections in children aged 1-5 years also were less likely to be susceptible to oxacillin, compared with infections in children of other age groups.
If the local clindamycin susceptibility rate falls below 85%, “beta-lactams, TMP/SMX, or tetracyclines may be used for less severe infections with intravenous vancomycin employed in severe cases,” the investigators said. “If overall MRSA rates continue to decline and clindamycin resistance among MSSA continues to increase, we may see a return to antistaphylococcal beta-lactam antimicrobial agents such as oxacillin or first-generation cephalosporins as preferred empirical therapy for presumed S. aureus infections.”
The research did not use external funding, and the authors reported no relevant financial disclosures.
The incidence of methicillin-resistant Staphylococcus aureus (MRSA) infections has decreased in children in recent years, but resistance to clindamycin has increased over the same period, a study showed.
“The epidemic of skin and soft tissue infections and invasive MRSA led to modifications of antimicrobial prescribing practices for suspected S. aureus infections,” reported Dr. Deena E. Sutter of the San Antonio Military Medical Center in Fort Sam Houston, Tex., and her associates. “Over the study period, erythromycin susceptibility among methicillin-susceptible S. aureus (MSSA) remained stable, suggesting that declining clindamycin susceptibility is a result of an increase in inducible resistance.”
The steady decline in clindamycin susceptibility “may lead to some concern about the continued reliance on clindamycin for the empirical treatment of presumptive S. aureus infections, although it is probably premature to abandon this effective antibiotic choice,” they wrote (Pediatrics. 2016 Mar. 1. doi: 10.1542/peds.2015-3099). “It is crucial that clinicians remain knowledgeable about local susceptibility rates as it would be prudent to consider [alternative] antimicrobial agents for empirical use when the local clindamycin susceptibility rate drops below 85%.”
The researchers retrospectively analyzed lab results from 39,209 patients under age 18 who were treated for S. aureus infections at one of the 266 U.S. facilities of the Military Health System from 2005 to 2014. The data included 41,745 S. aureus isolates, classified as MRSA if found resistant to cefoxitin, methicillin, or oxacillin and as methicillin susceptible (MSSA) if susceptible to those antimicrobials. The isolates had also been tested for susceptibility to ciprofloxacin, clindamycin, erythromycin, gentamicin, oxacillin, penicillin, rifampin, tetracycline, and trimethoprim/sulfamethoxazole (TMP/SMX).
During that decade, overall S. aureus susceptibility to clindamycin, ciprofloxacin, and TMP/SMX decreased – although susceptibility to TMP/SMX in 2014 stayed high at 98% – while overall susceptibility to erythromycin, gentamicin, and oxacillin increased. Specifically, 59% of S. aureus isolates were susceptible to oxacillin in 2005, which dropped briefly to 54% in 2007 before climbing to the 2014 rate of 68%.
Meanwhile, overall susceptibility to clindamycin dropped from 91% in 2005 to 86% in 2014, and MSSA susceptibility to clindamycin dropped from 91% in 2005 to 84% in 2014. “Erythromycin susceptibility remained stable among MSSA isolates throughout the study period at 63.5%, whereas MRSA susceptibility to erythromycin increased from 12.1% to 20.5%,” Dr. Sutter and her associates reported. “Ciprofloxacin susceptibility significantly decreased overall, although an initial decrease of 10.6% over the first 7 years of the study was subsequently followed by an increase of 6% between 2011 and 2014.”
Most of the isolates came from patients with skin and soft tissue infections, which were less likely to be susceptible to oxacillin than were other infections. Infections in children aged 1-5 years also were less likely to be susceptible to oxacillin, compared with infections in children of other age groups.
If the local clindamycin susceptibility rate falls below 85%, “beta-lactams, TMP/SMX, or tetracyclines may be used for less severe infections with intravenous vancomycin employed in severe cases,” the investigators said. “If overall MRSA rates continue to decline and clindamycin resistance among MSSA continues to increase, we may see a return to antistaphylococcal beta-lactam antimicrobial agents such as oxacillin or first-generation cephalosporins as preferred empirical therapy for presumed S. aureus infections.”
The research did not use external funding, and the authors reported no relevant financial disclosures.
FROM PEDIATRICS
Key clinical point: The incidence of methicillin-resistant Staphylococcus aureus infections has decreased in children in recent years while resistance to clindamycin has increased.
Major finding: MRSA susceptibility to oxacillin increased to 68.4% in 2014, and susceptibility dropped to 86% for clindamycin.
Data source: A retrospective analysis of 41,745 S. aureus isolates from 39,209 patients under age 18 years in the U.S. Military Health System between 2005 and 2014.
Disclosures: The research did not use external funding, and the authors reported no relevant financial disclosures.
The medicolegal considerations of interacting with your patients online
CASE: Patient discloses personal information in electronic communication. How to respond and what’s at stake?
Your nurse comes to you with a dilemma. Last Friday she received an email from a patient, sent to the nurse’s personal email account (G-mail) that conveyed information regarding the patient’s recent treatment for a herpetic vulvar lesion. The text details presumed exposure, date and time, number of sexual partners, concernfor “spread of disease,” and the patient’s desire to have a comprehensive sexually transmitted infection screening as soon as possible.
Your nurse has years of professional experience, but she is perhaps not the most savvy with regard to current information technology and social media. Nonetheless, she knows it is best not to immediately respond to the patient’s email without checking with you. She tracks you down on Monday morning to review the email and the dilemma she feels she has been placed in. What’s the best next step?
While discussing the general question with the staff, another nurse notes that there have been some reviews of the office on social media. It seems that this second nurse tweets and texts with patients all the time. The office manager strongly suggests that the office “join the 21st Century” by setting up a Facebook page and using their webpage to attract new patients and communicate with current patients.
How do you prepare for this? Is your staff knowledgeable about the dos and don’ts of social media?
The use of social media by health care providers has been growing for several years. Back in 2011 a large survey by QuantiaMD revealed that 87% of physicians used social media for personal reasons, and 67% of them used it professionally.1 How they used it for professional purposes also was explored in 2011, with almost 3 of 4 physicians using it for social networking and more than half engaging with their own institution’s social media (FIGURE).2 In 2013, 53% of physicians indicated that their practice had a Facebook platform, 28% had a presence on LinkedIn, and 21% were on Twitter.3 Not surprisingly, social media use is higher among younger physicians4; the 2016 equivalents to these percentages most likely are higher.
| Health providers’ use of social media for professional reasons2 |
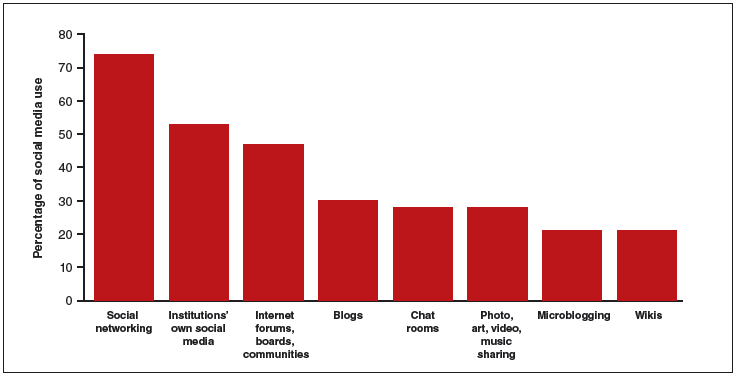
|
In 2011, a survey found that most health providers used social networking, their institutions’ own social media, and Internet forums, boards, and communities for professional reasons. |
Patients’ outreach through social media regarding health care information continues to grow, with 33.8% asking for health advice using social media.5 While email and other social media open the possibility of improved communication with patients, they also present a number of important professional and legal issues that deserve special consideration.6 Each medium presents its own challenges, but there are 4 categories of concern related to basic values and rights that we consider important to review:
- confidentiality
- dual relationships and conflicts of interest
- quality of care and advice
- general professionalism (including advertising).
Confidentiality
Few values of the medical profession are of longer standing than the commitment to maintain patient privacy. Fifth Century BC obligations continue to apply to the technology of the 21st Century AD. And the challenges are significant.
Email is not secure
In the opening case, the choice to email her clinician was apparently the patient’s. She probably does not realize that email is not very confidential, although it is undoubtedly in the Terms of Service Agreement she clicked through. Her email was likely scanned by her email service provider—Google, in this case—as well as the nurse. If, however, the physician’s office responds by email, it may well compound the confidentiality problem by further distributing the information through yet another email provider.
If, as a physician, you encourage email communication by your patients, a smart approach is to emphasize that such communications are not very confidential. At a minimum, until a secure email system can be established, it is best not to transmit medical information via email and to inform patients of the risk of such communication. In the case above, the nurse who received the email should respond to the patient by telephone (much more secure). Or she can respond to the patient by email (not including the patient’s message in the return), writing that, because email communications are inherently not confidential, she suggests a phone call or personal visit.
This case also notes that the patient sent the email to the nurse’s personal account, not to an office email account. Sending medical emails to an employee’s personal account raises additional problems of confidentiality and appropriate controls. It should be made clear that employees should not be discussing private medical matters via their own email accounts.
Other forms of social media are also not secure
Similar concerns arise about texting and using Twitter by the second nurse. These activities apparently had been unknown to the physician, but the practice still may be responsible for her actions. These are insecure forms of communication and raise serious ethical and legal concerns.
Other social media pose confidentiality risks as well. For example, a physician was dismissed from a position and reprimanded by the medical board for posting patient information on Facebook,7 and an ObGyn caused problems by posting a nasty note about a patient who showed up late for an appointment.8 Too many patients may not understand that posting on social media is the equivalent of standing on a street corner yelling private information. Social media sites that invite the discussion of personal matters are an invitation to trouble.
Physicians are ethically obliged to protect confidentiality
Professional standards place significant ethical obligations on physicians to protect patient confidentiality. The American Medical Association (AMA) has an ethics opinion on professionalism with social media,9 as does the American College of Obstetricians and Gynecologists (ACOG).10 Another excellent discussion of ethical and practical issues is a joint position paper by the American College of Physicians and the Federation of State Medical Boards.11 Both documents focus attention on issues of confidentiality.
Physicians are legally obliged to protect confidentiality
There are many legal protections for confidentiality that can be implicated by electronic communications and social media. All states provide protection for unwarranted disclosure of private patient information. Such disclosures made electronically are included.12 Indeed, because electronic disclosures may be broadcast more widely, they may be especially dangerous. The misuse of social media may result in license discipline by the state board, regulatory sanctions, or civil liability (rare, but criminal sanctions are a possibility in extreme circumstances).
In addition to state laws regarding confidentiality, there are a number of federal laws that cover confidential medical information. None is more important than the Health Insurance Portability and Accountability Act (HIPAA) and the more recent HITECH amendments (Health Information Technology for Economic and Clinical Health).13 These laws have both privacy provisions and security (including “encryption”) requirements. These are complicated laws but at their core are the notions that health care providers and some others:
- are responsible for maintaining the security and privacy of health information
- may not transmit (even unintentionally) such information to others without patient permission or legal authority.14
- may not transmit (even unintentionally) such information to others without patient permission or legal authority.
A good source of step-by-step information about these laws is “Health information privacy: Covered entities and business associates,” on the US Health and Human Services website.14
HITECH also provides for notice to patients when health information is inappropriately transmitted. Thus, a missing USB flash drive with patient information may require notification to thousands of patients.15 Any consideration of the use of email or social media in medical practice must take into account the HIPAA/HITECH obligations to protect the security of patient health information. There can be serious professional consequences for failing to follow the HIPAA requirements.16
Dual relationships and conflicts of interest
In our hypothetical case, the office manager’s suggestion that the office use Facebook and their website to attract new patients also may raise confidentiality problems. The Facebook suggestion especially needs to be considered carefully. Facebook use is estimated to be 63% to 96% among students and 13% to 47% among health care professionals.17 Facebook is most often seen as an interactive social site; it risks blurring the lines between personal and professional relationships.9 There is a consensus that a physician should not “friend” patients on Facebook. The AMA ethics opinion notes that “physicians must maintain appropriate boundaries of the patient-physician relationship in accordance with professional ethical guidelines, just as they would in any other context.”9
Separate personal and professional contacts
Difficulties with interactive social media are not limited to the physicians in a practice. The problems increase with the number of staff members who post or respond on social media. Control of social media is essential. The practice must ensure that staff members do not slip into inappropriate personal comments and relationships. Staff should understand (and be reminded of) the necessity of separating personal and professional contacts.
Avoid misunderstandings
In addition, whatever the intent of the physician and staff may be, it is essentially impossible to know how patients will interpret interactions on these social media. The very informal, off-the-cuff, chatty way in which Facebook and similar sites are used invites misunderstandings, and maintaining professional boundaries is necessary.
Ground rules
All of this is not to say that professionals should never use Facebook or similar sites. Rather, if used, ground rules need to be established.
Social media communications must:
- be professional and not related to personal matters
- not be used to give medical advice
- be controlled by high level staff
- be reviewed periodically.
Staff training
Particularly for interactive social media (email, texts, Twitter, Facebook, etc), it is essential that there be both clear policies and good staff training (TABLE).9–11,18 There really should be no “making it up as we go along.” Staff on a social media lark of their own can be disastrous for the practice. Policies need to be updated frequently, and staff training reinforced and repeated periodically.
Quality of care and advice
Start with your website
Institutions’ websites are major sources of health care information: Nearly 32% of US adults would be very likely to prefer a hospital based on its website.5 Your website can be an important face of your practice to the community—for good or for bad. On one hand, the practice can control what is on a website and, unlike some social media, it will not be directed to individual patients. Done well, it “provides golden opportunities for marketing physician services, as well as for contributing to public health by providing high-quality online content that is both accurate and understandable to laypeople.”19 Done badly, it can convey incorrect and harmful information and discredit the medical practice that established it.
Your website introduces the practice and settings, but it will serve another purpose to thousands of people who likely will see it over time as a source of credible health information. The importance of ensuring that your website is carefully constructed to provide, or link to, good medical advice that contributes to quality of care cannot be overstated.
A good website begins with a clear statement of the reasons and goals for having the site. Professional design assistance generally is used to create the site, but that design process needs to be overseen by a medical professional to ensure that it conveys the sense of the practice and provides completely accurate information. A homepage of dancing clowns with stethoscopes may seem good to a 20-something-year-old designer, but it is not appropriate for a physician. It will be the practice, not the designer, who is held accountable for the site content. Links to other sites need to be vetted and used with care. Patients and other members of the public may well take the links as carrying the endorsement of the practice and its physicians.
Perhaps the greatest risk of a website is that it will not be kept current. Unfortunately, they do not update themselves. Some knowledgeable staff member must frequently review it to update everything from office hours and personnel to links to other sites. In addition, the physicians periodically must review it to ensure that all medical information is up to date and accurate. Old, outdated information about the office can put off potential patients. Outdated medical information may be harmful to patients who rely on it.
Any professional website should include disclaimers informing users that the site is not intended to establish a professional relationship or to give professional advice. The nature and extent of the disclaimer will depend on the type of information on the site. An example of a particularly thorough disclaimer is the Mayo Clinic disclaimer and terms of use (http://www.mayoclinic.org/about-this-site/terms-conditions-use-policy).
General professionalism
At the end of the day, social media are an outreach from a medical practice and from the profession to the public.20 Failure to treat these platforms with appropriate professional standards may result in professional discipline, damages, or civil penalties. Almost all of the reviews of social media use in health care practice note that the risks of inappropriate use are not only to the individual physician but also to the general medical profession, which may be undermined. Consider posting policies of the relevent state medical boards, the AMA, and ACOG in your office after you have had a discussion with your staff about them.21
The AMA statement includes a provision that a physician seeing unprofessional social media conduct by a colleague has the responsibility to bring that to the attention of the colleague. If the colleague does not correct a significant problem, “the physician should report the matter to appropriate authorities.”9
Bottom line
Any practitioner considering the use of social media must view it as a major step that requires caution, expert assistance, and constant attention to potential privacy, quality, and professionalism issues. If you are considering it, ensure that all staff associated with the practice understand and agree to the established limits on social media use.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Modahl M, Tompsett L, Moorhead T. Doctors, patients, & social media. Quantia MD website. http://www.quantiamd.com/q-qcp/DoctorsPatientSocialMedia.pdf. Published September 2011. Accessed February 18, 2016.
- Kuberacka A, Wengrojj J, Fabozzi N. Social media use in U.S. healthcare provider institutions: Insights from Frost & Sullivan and iHT2 survey. Frost and Sullivan website. http://ihealthtran.com/pdf/frostiht2survey.pdf. Published August 30, 2011. Accessed February 18, 2016.
- O’Connor ME. How do tech savvy physicians use health technology and social media? Health Care Social Media website. http://hcsmmonitor.com/2014/01/08/how-do-tech-savvy-physicians-use-health-technology-and-social-media/. Published January 8, 2014. Accessed February 18, 2016.
- American Medical Association (AMA) Insurance. 2014 work/life profiles of today’s U.S. physician. AMA Insurance website. https://www.amainsure.com/work-life-profiles-of-todays-us-physician.html. Published April 2014. Accessed February 18, 2016.
- National Research Corporation. 2013 National Market Insights Survey: Health care social media website. https://healthcaresocialmedia.files.wordpress.com/2014/04/nrc-infographiclong.jpg. Accessed February 18, 2016.
- Suby C. Social media in health care: benefits, concerns and guidelines for use. Creat Nurs. 2013;19(3):140–147.
- Conaboy C. For doctors, social media a tricky case. Boston Globe. http://www.boston.com/lifestyle/health/articles/2011/04/20/for_doctors_social_media_a_tricky_case/?page=full. Published April 20, 2011. Accessed February 18, 2016.
- Matyszczyk C. Outcry as ob-gyn uses Facebook to complain about patient. CNET. http://www.cnet.com/news/outcry-as-ob-gyn-uses-facebook-to-complain-about-patient/Minion Pro. Published February 9, 2013. Accessed February 18, 2016.
- American Medical Association (AMA). Opinion 9.124: Professionalism in the use of social media. AMA website. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion9124.page? Published June 2011. Accessed February 18, 2016.
- American College of Obstetricians and Gynecologists (ACOG) Committee on Professional Liability. ACOG Committee Opinion No. 622: professional use of digital and social media. Obstet Gynecol. 2015;125(2):516-520.
- Farnan JM, Sulmasy LS, Worster BK, et al. Online medical professionalism: patient and public relationships: Policy Statement From the American College of Physicians and the Federation of State Medical Boards. Ann Intern Med. 2013;158(8):620–627.
- Hader A, Drown E. Patient privacy and social media. AANA J. 2010;78(4):270–274.
- Kavoussi SC, Huang JJ, Tsai JC, Kempton JE. HIPAA for physicians in the information age. Conn Med. 2014;78(7):425–427.
- U.S. Department of Health & Human Services (HHS). Health information privacy: Covered entities and business associates. HHS website. http://www.hhs.gov/ocr/privacy/hipaa/understanding/coveredentities/. Published March 14, 2012. Accessed February 18, 2016.
- Perna G. Breach report: lost flash drive at Kaiser Permanente affects 49,000 patients. Healthcare Informatics website. http://www.healthcare-informatics.com/news-item/breach-report-lost-flash-drive-kaiser-permanente-affects-49000-patients. Published December 11, 2013. Accessed February 18, 2016.
- McBride M. How to ensure your social media efforts are HIPAA-compliant. Med Econ. 2012;89:70–74.
- Von Muhlen M, Ohno-Machado L. Reviewing social media use by clinicians. J Am Med Inform Assoc. 2012;19(5):777–781.
- Omurtag K, Turek P. Incorporating social media into practice: a blueprint for reproductive health providers. Clin Obstet Gynecol. 2013;56(3):463–470.
- Radmanesh A, Duszak R, Fitzgerald R. Social media and public outreach: a physician primer. Am J Neuroradiol. 2015;36(7):1223–1224.
- Grajales FJ 3rd, Sheps S, Ho K, Novak-Lauscher H, Eysenbach G. Social media: a review and tutorial of applications in medicine and health care. J Med Internet Res. 2014;16(2):e13.
- ACOG Today. Social media guide: how to comment with patients and spread women’s health messages. American Congress of Obstetricians and Gynecologists website. http://www.acog.org/-/media/ACOG-Today/acogToday201211.pdf. Published November 2012. Accessed February 18, 2016.
CASE: Patient discloses personal information in electronic communication. How to respond and what’s at stake?
Your nurse comes to you with a dilemma. Last Friday she received an email from a patient, sent to the nurse’s personal email account (G-mail) that conveyed information regarding the patient’s recent treatment for a herpetic vulvar lesion. The text details presumed exposure, date and time, number of sexual partners, concernfor “spread of disease,” and the patient’s desire to have a comprehensive sexually transmitted infection screening as soon as possible.
Your nurse has years of professional experience, but she is perhaps not the most savvy with regard to current information technology and social media. Nonetheless, she knows it is best not to immediately respond to the patient’s email without checking with you. She tracks you down on Monday morning to review the email and the dilemma she feels she has been placed in. What’s the best next step?
While discussing the general question with the staff, another nurse notes that there have been some reviews of the office on social media. It seems that this second nurse tweets and texts with patients all the time. The office manager strongly suggests that the office “join the 21st Century” by setting up a Facebook page and using their webpage to attract new patients and communicate with current patients.
How do you prepare for this? Is your staff knowledgeable about the dos and don’ts of social media?
The use of social media by health care providers has been growing for several years. Back in 2011 a large survey by QuantiaMD revealed that 87% of physicians used social media for personal reasons, and 67% of them used it professionally.1 How they used it for professional purposes also was explored in 2011, with almost 3 of 4 physicians using it for social networking and more than half engaging with their own institution’s social media (FIGURE).2 In 2013, 53% of physicians indicated that their practice had a Facebook platform, 28% had a presence on LinkedIn, and 21% were on Twitter.3 Not surprisingly, social media use is higher among younger physicians4; the 2016 equivalents to these percentages most likely are higher.
| Health providers’ use of social media for professional reasons2 |

|
In 2011, a survey found that most health providers used social networking, their institutions’ own social media, and Internet forums, boards, and communities for professional reasons. |
Patients’ outreach through social media regarding health care information continues to grow, with 33.8% asking for health advice using social media.5 While email and other social media open the possibility of improved communication with patients, they also present a number of important professional and legal issues that deserve special consideration.6 Each medium presents its own challenges, but there are 4 categories of concern related to basic values and rights that we consider important to review:
- confidentiality
- dual relationships and conflicts of interest
- quality of care and advice
- general professionalism (including advertising).
Confidentiality
Few values of the medical profession are of longer standing than the commitment to maintain patient privacy. Fifth Century BC obligations continue to apply to the technology of the 21st Century AD. And the challenges are significant.
Email is not secure
In the opening case, the choice to email her clinician was apparently the patient’s. She probably does not realize that email is not very confidential, although it is undoubtedly in the Terms of Service Agreement she clicked through. Her email was likely scanned by her email service provider—Google, in this case—as well as the nurse. If, however, the physician’s office responds by email, it may well compound the confidentiality problem by further distributing the information through yet another email provider.
If, as a physician, you encourage email communication by your patients, a smart approach is to emphasize that such communications are not very confidential. At a minimum, until a secure email system can be established, it is best not to transmit medical information via email and to inform patients of the risk of such communication. In the case above, the nurse who received the email should respond to the patient by telephone (much more secure). Or she can respond to the patient by email (not including the patient’s message in the return), writing that, because email communications are inherently not confidential, she suggests a phone call or personal visit.
This case also notes that the patient sent the email to the nurse’s personal account, not to an office email account. Sending medical emails to an employee’s personal account raises additional problems of confidentiality and appropriate controls. It should be made clear that employees should not be discussing private medical matters via their own email accounts.
Other forms of social media are also not secure
Similar concerns arise about texting and using Twitter by the second nurse. These activities apparently had been unknown to the physician, but the practice still may be responsible for her actions. These are insecure forms of communication and raise serious ethical and legal concerns.
Other social media pose confidentiality risks as well. For example, a physician was dismissed from a position and reprimanded by the medical board for posting patient information on Facebook,7 and an ObGyn caused problems by posting a nasty note about a patient who showed up late for an appointment.8 Too many patients may not understand that posting on social media is the equivalent of standing on a street corner yelling private information. Social media sites that invite the discussion of personal matters are an invitation to trouble.
Physicians are ethically obliged to protect confidentiality
Professional standards place significant ethical obligations on physicians to protect patient confidentiality. The American Medical Association (AMA) has an ethics opinion on professionalism with social media,9 as does the American College of Obstetricians and Gynecologists (ACOG).10 Another excellent discussion of ethical and practical issues is a joint position paper by the American College of Physicians and the Federation of State Medical Boards.11 Both documents focus attention on issues of confidentiality.
Physicians are legally obliged to protect confidentiality
There are many legal protections for confidentiality that can be implicated by electronic communications and social media. All states provide protection for unwarranted disclosure of private patient information. Such disclosures made electronically are included.12 Indeed, because electronic disclosures may be broadcast more widely, they may be especially dangerous. The misuse of social media may result in license discipline by the state board, regulatory sanctions, or civil liability (rare, but criminal sanctions are a possibility in extreme circumstances).
In addition to state laws regarding confidentiality, there are a number of federal laws that cover confidential medical information. None is more important than the Health Insurance Portability and Accountability Act (HIPAA) and the more recent HITECH amendments (Health Information Technology for Economic and Clinical Health).13 These laws have both privacy provisions and security (including “encryption”) requirements. These are complicated laws but at their core are the notions that health care providers and some others:
- are responsible for maintaining the security and privacy of health information
- may not transmit (even unintentionally) such information to others without patient permission or legal authority.14
- may not transmit (even unintentionally) such information to others without patient permission or legal authority.
A good source of step-by-step information about these laws is “Health information privacy: Covered entities and business associates,” on the US Health and Human Services website.14
HITECH also provides for notice to patients when health information is inappropriately transmitted. Thus, a missing USB flash drive with patient information may require notification to thousands of patients.15 Any consideration of the use of email or social media in medical practice must take into account the HIPAA/HITECH obligations to protect the security of patient health information. There can be serious professional consequences for failing to follow the HIPAA requirements.16
Dual relationships and conflicts of interest
In our hypothetical case, the office manager’s suggestion that the office use Facebook and their website to attract new patients also may raise confidentiality problems. The Facebook suggestion especially needs to be considered carefully. Facebook use is estimated to be 63% to 96% among students and 13% to 47% among health care professionals.17 Facebook is most often seen as an interactive social site; it risks blurring the lines between personal and professional relationships.9 There is a consensus that a physician should not “friend” patients on Facebook. The AMA ethics opinion notes that “physicians must maintain appropriate boundaries of the patient-physician relationship in accordance with professional ethical guidelines, just as they would in any other context.”9
Separate personal and professional contacts
Difficulties with interactive social media are not limited to the physicians in a practice. The problems increase with the number of staff members who post or respond on social media. Control of social media is essential. The practice must ensure that staff members do not slip into inappropriate personal comments and relationships. Staff should understand (and be reminded of) the necessity of separating personal and professional contacts.
Avoid misunderstandings
In addition, whatever the intent of the physician and staff may be, it is essentially impossible to know how patients will interpret interactions on these social media. The very informal, off-the-cuff, chatty way in which Facebook and similar sites are used invites misunderstandings, and maintaining professional boundaries is necessary.
Ground rules
All of this is not to say that professionals should never use Facebook or similar sites. Rather, if used, ground rules need to be established.
Social media communications must:
- be professional and not related to personal matters
- not be used to give medical advice
- be controlled by high level staff
- be reviewed periodically.
Staff training
Particularly for interactive social media (email, texts, Twitter, Facebook, etc), it is essential that there be both clear policies and good staff training (TABLE).9–11,18 There really should be no “making it up as we go along.” Staff on a social media lark of their own can be disastrous for the practice. Policies need to be updated frequently, and staff training reinforced and repeated periodically.
Quality of care and advice
Start with your website
Institutions’ websites are major sources of health care information: Nearly 32% of US adults would be very likely to prefer a hospital based on its website.5 Your website can be an important face of your practice to the community—for good or for bad. On one hand, the practice can control what is on a website and, unlike some social media, it will not be directed to individual patients. Done well, it “provides golden opportunities for marketing physician services, as well as for contributing to public health by providing high-quality online content that is both accurate and understandable to laypeople.”19 Done badly, it can convey incorrect and harmful information and discredit the medical practice that established it.
Your website introduces the practice and settings, but it will serve another purpose to thousands of people who likely will see it over time as a source of credible health information. The importance of ensuring that your website is carefully constructed to provide, or link to, good medical advice that contributes to quality of care cannot be overstated.
A good website begins with a clear statement of the reasons and goals for having the site. Professional design assistance generally is used to create the site, but that design process needs to be overseen by a medical professional to ensure that it conveys the sense of the practice and provides completely accurate information. A homepage of dancing clowns with stethoscopes may seem good to a 20-something-year-old designer, but it is not appropriate for a physician. It will be the practice, not the designer, who is held accountable for the site content. Links to other sites need to be vetted and used with care. Patients and other members of the public may well take the links as carrying the endorsement of the practice and its physicians.
Perhaps the greatest risk of a website is that it will not be kept current. Unfortunately, they do not update themselves. Some knowledgeable staff member must frequently review it to update everything from office hours and personnel to links to other sites. In addition, the physicians periodically must review it to ensure that all medical information is up to date and accurate. Old, outdated information about the office can put off potential patients. Outdated medical information may be harmful to patients who rely on it.
Any professional website should include disclaimers informing users that the site is not intended to establish a professional relationship or to give professional advice. The nature and extent of the disclaimer will depend on the type of information on the site. An example of a particularly thorough disclaimer is the Mayo Clinic disclaimer and terms of use (http://www.mayoclinic.org/about-this-site/terms-conditions-use-policy).
General professionalism
At the end of the day, social media are an outreach from a medical practice and from the profession to the public.20 Failure to treat these platforms with appropriate professional standards may result in professional discipline, damages, or civil penalties. Almost all of the reviews of social media use in health care practice note that the risks of inappropriate use are not only to the individual physician but also to the general medical profession, which may be undermined. Consider posting policies of the relevent state medical boards, the AMA, and ACOG in your office after you have had a discussion with your staff about them.21
The AMA statement includes a provision that a physician seeing unprofessional social media conduct by a colleague has the responsibility to bring that to the attention of the colleague. If the colleague does not correct a significant problem, “the physician should report the matter to appropriate authorities.”9
Bottom line
Any practitioner considering the use of social media must view it as a major step that requires caution, expert assistance, and constant attention to potential privacy, quality, and professionalism issues. If you are considering it, ensure that all staff associated with the practice understand and agree to the established limits on social media use.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE: Patient discloses personal information in electronic communication. How to respond and what’s at stake?
Your nurse comes to you with a dilemma. Last Friday she received an email from a patient, sent to the nurse’s personal email account (G-mail) that conveyed information regarding the patient’s recent treatment for a herpetic vulvar lesion. The text details presumed exposure, date and time, number of sexual partners, concernfor “spread of disease,” and the patient’s desire to have a comprehensive sexually transmitted infection screening as soon as possible.
Your nurse has years of professional experience, but she is perhaps not the most savvy with regard to current information technology and social media. Nonetheless, she knows it is best not to immediately respond to the patient’s email without checking with you. She tracks you down on Monday morning to review the email and the dilemma she feels she has been placed in. What’s the best next step?
While discussing the general question with the staff, another nurse notes that there have been some reviews of the office on social media. It seems that this second nurse tweets and texts with patients all the time. The office manager strongly suggests that the office “join the 21st Century” by setting up a Facebook page and using their webpage to attract new patients and communicate with current patients.
How do you prepare for this? Is your staff knowledgeable about the dos and don’ts of social media?
The use of social media by health care providers has been growing for several years. Back in 2011 a large survey by QuantiaMD revealed that 87% of physicians used social media for personal reasons, and 67% of them used it professionally.1 How they used it for professional purposes also was explored in 2011, with almost 3 of 4 physicians using it for social networking and more than half engaging with their own institution’s social media (FIGURE).2 In 2013, 53% of physicians indicated that their practice had a Facebook platform, 28% had a presence on LinkedIn, and 21% were on Twitter.3 Not surprisingly, social media use is higher among younger physicians4; the 2016 equivalents to these percentages most likely are higher.
| Health providers’ use of social media for professional reasons2 |

|
In 2011, a survey found that most health providers used social networking, their institutions’ own social media, and Internet forums, boards, and communities for professional reasons. |
Patients’ outreach through social media regarding health care information continues to grow, with 33.8% asking for health advice using social media.5 While email and other social media open the possibility of improved communication with patients, they also present a number of important professional and legal issues that deserve special consideration.6 Each medium presents its own challenges, but there are 4 categories of concern related to basic values and rights that we consider important to review:
- confidentiality
- dual relationships and conflicts of interest
- quality of care and advice
- general professionalism (including advertising).
Confidentiality
Few values of the medical profession are of longer standing than the commitment to maintain patient privacy. Fifth Century BC obligations continue to apply to the technology of the 21st Century AD. And the challenges are significant.
Email is not secure
In the opening case, the choice to email her clinician was apparently the patient’s. She probably does not realize that email is not very confidential, although it is undoubtedly in the Terms of Service Agreement she clicked through. Her email was likely scanned by her email service provider—Google, in this case—as well as the nurse. If, however, the physician’s office responds by email, it may well compound the confidentiality problem by further distributing the information through yet another email provider.
If, as a physician, you encourage email communication by your patients, a smart approach is to emphasize that such communications are not very confidential. At a minimum, until a secure email system can be established, it is best not to transmit medical information via email and to inform patients of the risk of such communication. In the case above, the nurse who received the email should respond to the patient by telephone (much more secure). Or she can respond to the patient by email (not including the patient’s message in the return), writing that, because email communications are inherently not confidential, she suggests a phone call or personal visit.
This case also notes that the patient sent the email to the nurse’s personal account, not to an office email account. Sending medical emails to an employee’s personal account raises additional problems of confidentiality and appropriate controls. It should be made clear that employees should not be discussing private medical matters via their own email accounts.
Other forms of social media are also not secure
Similar concerns arise about texting and using Twitter by the second nurse. These activities apparently had been unknown to the physician, but the practice still may be responsible for her actions. These are insecure forms of communication and raise serious ethical and legal concerns.
Other social media pose confidentiality risks as well. For example, a physician was dismissed from a position and reprimanded by the medical board for posting patient information on Facebook,7 and an ObGyn caused problems by posting a nasty note about a patient who showed up late for an appointment.8 Too many patients may not understand that posting on social media is the equivalent of standing on a street corner yelling private information. Social media sites that invite the discussion of personal matters are an invitation to trouble.
Physicians are ethically obliged to protect confidentiality
Professional standards place significant ethical obligations on physicians to protect patient confidentiality. The American Medical Association (AMA) has an ethics opinion on professionalism with social media,9 as does the American College of Obstetricians and Gynecologists (ACOG).10 Another excellent discussion of ethical and practical issues is a joint position paper by the American College of Physicians and the Federation of State Medical Boards.11 Both documents focus attention on issues of confidentiality.
Physicians are legally obliged to protect confidentiality
There are many legal protections for confidentiality that can be implicated by electronic communications and social media. All states provide protection for unwarranted disclosure of private patient information. Such disclosures made electronically are included.12 Indeed, because electronic disclosures may be broadcast more widely, they may be especially dangerous. The misuse of social media may result in license discipline by the state board, regulatory sanctions, or civil liability (rare, but criminal sanctions are a possibility in extreme circumstances).
In addition to state laws regarding confidentiality, there are a number of federal laws that cover confidential medical information. None is more important than the Health Insurance Portability and Accountability Act (HIPAA) and the more recent HITECH amendments (Health Information Technology for Economic and Clinical Health).13 These laws have both privacy provisions and security (including “encryption”) requirements. These are complicated laws but at their core are the notions that health care providers and some others:
- are responsible for maintaining the security and privacy of health information
- may not transmit (even unintentionally) such information to others without patient permission or legal authority.14
- may not transmit (even unintentionally) such information to others without patient permission or legal authority.
A good source of step-by-step information about these laws is “Health information privacy: Covered entities and business associates,” on the US Health and Human Services website.14
HITECH also provides for notice to patients when health information is inappropriately transmitted. Thus, a missing USB flash drive with patient information may require notification to thousands of patients.15 Any consideration of the use of email or social media in medical practice must take into account the HIPAA/HITECH obligations to protect the security of patient health information. There can be serious professional consequences for failing to follow the HIPAA requirements.16
Dual relationships and conflicts of interest
In our hypothetical case, the office manager’s suggestion that the office use Facebook and their website to attract new patients also may raise confidentiality problems. The Facebook suggestion especially needs to be considered carefully. Facebook use is estimated to be 63% to 96% among students and 13% to 47% among health care professionals.17 Facebook is most often seen as an interactive social site; it risks blurring the lines between personal and professional relationships.9 There is a consensus that a physician should not “friend” patients on Facebook. The AMA ethics opinion notes that “physicians must maintain appropriate boundaries of the patient-physician relationship in accordance with professional ethical guidelines, just as they would in any other context.”9
Separate personal and professional contacts
Difficulties with interactive social media are not limited to the physicians in a practice. The problems increase with the number of staff members who post or respond on social media. Control of social media is essential. The practice must ensure that staff members do not slip into inappropriate personal comments and relationships. Staff should understand (and be reminded of) the necessity of separating personal and professional contacts.
Avoid misunderstandings
In addition, whatever the intent of the physician and staff may be, it is essentially impossible to know how patients will interpret interactions on these social media. The very informal, off-the-cuff, chatty way in which Facebook and similar sites are used invites misunderstandings, and maintaining professional boundaries is necessary.
Ground rules
All of this is not to say that professionals should never use Facebook or similar sites. Rather, if used, ground rules need to be established.
Social media communications must:
- be professional and not related to personal matters
- not be used to give medical advice
- be controlled by high level staff
- be reviewed periodically.
Staff training
Particularly for interactive social media (email, texts, Twitter, Facebook, etc), it is essential that there be both clear policies and good staff training (TABLE).9–11,18 There really should be no “making it up as we go along.” Staff on a social media lark of their own can be disastrous for the practice. Policies need to be updated frequently, and staff training reinforced and repeated periodically.
Quality of care and advice
Start with your website
Institutions’ websites are major sources of health care information: Nearly 32% of US adults would be very likely to prefer a hospital based on its website.5 Your website can be an important face of your practice to the community—for good or for bad. On one hand, the practice can control what is on a website and, unlike some social media, it will not be directed to individual patients. Done well, it “provides golden opportunities for marketing physician services, as well as for contributing to public health by providing high-quality online content that is both accurate and understandable to laypeople.”19 Done badly, it can convey incorrect and harmful information and discredit the medical practice that established it.
Your website introduces the practice and settings, but it will serve another purpose to thousands of people who likely will see it over time as a source of credible health information. The importance of ensuring that your website is carefully constructed to provide, or link to, good medical advice that contributes to quality of care cannot be overstated.
A good website begins with a clear statement of the reasons and goals for having the site. Professional design assistance generally is used to create the site, but that design process needs to be overseen by a medical professional to ensure that it conveys the sense of the practice and provides completely accurate information. A homepage of dancing clowns with stethoscopes may seem good to a 20-something-year-old designer, but it is not appropriate for a physician. It will be the practice, not the designer, who is held accountable for the site content. Links to other sites need to be vetted and used with care. Patients and other members of the public may well take the links as carrying the endorsement of the practice and its physicians.
Perhaps the greatest risk of a website is that it will not be kept current. Unfortunately, they do not update themselves. Some knowledgeable staff member must frequently review it to update everything from office hours and personnel to links to other sites. In addition, the physicians periodically must review it to ensure that all medical information is up to date and accurate. Old, outdated information about the office can put off potential patients. Outdated medical information may be harmful to patients who rely on it.
Any professional website should include disclaimers informing users that the site is not intended to establish a professional relationship or to give professional advice. The nature and extent of the disclaimer will depend on the type of information on the site. An example of a particularly thorough disclaimer is the Mayo Clinic disclaimer and terms of use (http://www.mayoclinic.org/about-this-site/terms-conditions-use-policy).
General professionalism
At the end of the day, social media are an outreach from a medical practice and from the profession to the public.20 Failure to treat these platforms with appropriate professional standards may result in professional discipline, damages, or civil penalties. Almost all of the reviews of social media use in health care practice note that the risks of inappropriate use are not only to the individual physician but also to the general medical profession, which may be undermined. Consider posting policies of the relevent state medical boards, the AMA, and ACOG in your office after you have had a discussion with your staff about them.21
The AMA statement includes a provision that a physician seeing unprofessional social media conduct by a colleague has the responsibility to bring that to the attention of the colleague. If the colleague does not correct a significant problem, “the physician should report the matter to appropriate authorities.”9
Bottom line
Any practitioner considering the use of social media must view it as a major step that requires caution, expert assistance, and constant attention to potential privacy, quality, and professionalism issues. If you are considering it, ensure that all staff associated with the practice understand and agree to the established limits on social media use.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Modahl M, Tompsett L, Moorhead T. Doctors, patients, & social media. Quantia MD website. http://www.quantiamd.com/q-qcp/DoctorsPatientSocialMedia.pdf. Published September 2011. Accessed February 18, 2016.
- Kuberacka A, Wengrojj J, Fabozzi N. Social media use in U.S. healthcare provider institutions: Insights from Frost & Sullivan and iHT2 survey. Frost and Sullivan website. http://ihealthtran.com/pdf/frostiht2survey.pdf. Published August 30, 2011. Accessed February 18, 2016.
- O’Connor ME. How do tech savvy physicians use health technology and social media? Health Care Social Media website. http://hcsmmonitor.com/2014/01/08/how-do-tech-savvy-physicians-use-health-technology-and-social-media/. Published January 8, 2014. Accessed February 18, 2016.
- American Medical Association (AMA) Insurance. 2014 work/life profiles of today’s U.S. physician. AMA Insurance website. https://www.amainsure.com/work-life-profiles-of-todays-us-physician.html. Published April 2014. Accessed February 18, 2016.
- National Research Corporation. 2013 National Market Insights Survey: Health care social media website. https://healthcaresocialmedia.files.wordpress.com/2014/04/nrc-infographiclong.jpg. Accessed February 18, 2016.
- Suby C. Social media in health care: benefits, concerns and guidelines for use. Creat Nurs. 2013;19(3):140–147.
- Conaboy C. For doctors, social media a tricky case. Boston Globe. http://www.boston.com/lifestyle/health/articles/2011/04/20/for_doctors_social_media_a_tricky_case/?page=full. Published April 20, 2011. Accessed February 18, 2016.
- Matyszczyk C. Outcry as ob-gyn uses Facebook to complain about patient. CNET. http://www.cnet.com/news/outcry-as-ob-gyn-uses-facebook-to-complain-about-patient/Minion Pro. Published February 9, 2013. Accessed February 18, 2016.
- American Medical Association (AMA). Opinion 9.124: Professionalism in the use of social media. AMA website. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion9124.page? Published June 2011. Accessed February 18, 2016.
- American College of Obstetricians and Gynecologists (ACOG) Committee on Professional Liability. ACOG Committee Opinion No. 622: professional use of digital and social media. Obstet Gynecol. 2015;125(2):516-520.
- Farnan JM, Sulmasy LS, Worster BK, et al. Online medical professionalism: patient and public relationships: Policy Statement From the American College of Physicians and the Federation of State Medical Boards. Ann Intern Med. 2013;158(8):620–627.
- Hader A, Drown E. Patient privacy and social media. AANA J. 2010;78(4):270–274.
- Kavoussi SC, Huang JJ, Tsai JC, Kempton JE. HIPAA for physicians in the information age. Conn Med. 2014;78(7):425–427.
- U.S. Department of Health & Human Services (HHS). Health information privacy: Covered entities and business associates. HHS website. http://www.hhs.gov/ocr/privacy/hipaa/understanding/coveredentities/. Published March 14, 2012. Accessed February 18, 2016.
- Perna G. Breach report: lost flash drive at Kaiser Permanente affects 49,000 patients. Healthcare Informatics website. http://www.healthcare-informatics.com/news-item/breach-report-lost-flash-drive-kaiser-permanente-affects-49000-patients. Published December 11, 2013. Accessed February 18, 2016.
- McBride M. How to ensure your social media efforts are HIPAA-compliant. Med Econ. 2012;89:70–74.
- Von Muhlen M, Ohno-Machado L. Reviewing social media use by clinicians. J Am Med Inform Assoc. 2012;19(5):777–781.
- Omurtag K, Turek P. Incorporating social media into practice: a blueprint for reproductive health providers. Clin Obstet Gynecol. 2013;56(3):463–470.
- Radmanesh A, Duszak R, Fitzgerald R. Social media and public outreach: a physician primer. Am J Neuroradiol. 2015;36(7):1223–1224.
- Grajales FJ 3rd, Sheps S, Ho K, Novak-Lauscher H, Eysenbach G. Social media: a review and tutorial of applications in medicine and health care. J Med Internet Res. 2014;16(2):e13.
- ACOG Today. Social media guide: how to comment with patients and spread women’s health messages. American Congress of Obstetricians and Gynecologists website. http://www.acog.org/-/media/ACOG-Today/acogToday201211.pdf. Published November 2012. Accessed February 18, 2016.
- Modahl M, Tompsett L, Moorhead T. Doctors, patients, & social media. Quantia MD website. http://www.quantiamd.com/q-qcp/DoctorsPatientSocialMedia.pdf. Published September 2011. Accessed February 18, 2016.
- Kuberacka A, Wengrojj J, Fabozzi N. Social media use in U.S. healthcare provider institutions: Insights from Frost & Sullivan and iHT2 survey. Frost and Sullivan website. http://ihealthtran.com/pdf/frostiht2survey.pdf. Published August 30, 2011. Accessed February 18, 2016.
- O’Connor ME. How do tech savvy physicians use health technology and social media? Health Care Social Media website. http://hcsmmonitor.com/2014/01/08/how-do-tech-savvy-physicians-use-health-technology-and-social-media/. Published January 8, 2014. Accessed February 18, 2016.
- American Medical Association (AMA) Insurance. 2014 work/life profiles of today’s U.S. physician. AMA Insurance website. https://www.amainsure.com/work-life-profiles-of-todays-us-physician.html. Published April 2014. Accessed February 18, 2016.
- National Research Corporation. 2013 National Market Insights Survey: Health care social media website. https://healthcaresocialmedia.files.wordpress.com/2014/04/nrc-infographiclong.jpg. Accessed February 18, 2016.
- Suby C. Social media in health care: benefits, concerns and guidelines for use. Creat Nurs. 2013;19(3):140–147.
- Conaboy C. For doctors, social media a tricky case. Boston Globe. http://www.boston.com/lifestyle/health/articles/2011/04/20/for_doctors_social_media_a_tricky_case/?page=full. Published April 20, 2011. Accessed February 18, 2016.
- Matyszczyk C. Outcry as ob-gyn uses Facebook to complain about patient. CNET. http://www.cnet.com/news/outcry-as-ob-gyn-uses-facebook-to-complain-about-patient/Minion Pro. Published February 9, 2013. Accessed February 18, 2016.
- American Medical Association (AMA). Opinion 9.124: Professionalism in the use of social media. AMA website. http://www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion9124.page? Published June 2011. Accessed February 18, 2016.
- American College of Obstetricians and Gynecologists (ACOG) Committee on Professional Liability. ACOG Committee Opinion No. 622: professional use of digital and social media. Obstet Gynecol. 2015;125(2):516-520.
- Farnan JM, Sulmasy LS, Worster BK, et al. Online medical professionalism: patient and public relationships: Policy Statement From the American College of Physicians and the Federation of State Medical Boards. Ann Intern Med. 2013;158(8):620–627.
- Hader A, Drown E. Patient privacy and social media. AANA J. 2010;78(4):270–274.
- Kavoussi SC, Huang JJ, Tsai JC, Kempton JE. HIPAA for physicians in the information age. Conn Med. 2014;78(7):425–427.
- U.S. Department of Health & Human Services (HHS). Health information privacy: Covered entities and business associates. HHS website. http://www.hhs.gov/ocr/privacy/hipaa/understanding/coveredentities/. Published March 14, 2012. Accessed February 18, 2016.
- Perna G. Breach report: lost flash drive at Kaiser Permanente affects 49,000 patients. Healthcare Informatics website. http://www.healthcare-informatics.com/news-item/breach-report-lost-flash-drive-kaiser-permanente-affects-49000-patients. Published December 11, 2013. Accessed February 18, 2016.
- McBride M. How to ensure your social media efforts are HIPAA-compliant. Med Econ. 2012;89:70–74.
- Von Muhlen M, Ohno-Machado L. Reviewing social media use by clinicians. J Am Med Inform Assoc. 2012;19(5):777–781.
- Omurtag K, Turek P. Incorporating social media into practice: a blueprint for reproductive health providers. Clin Obstet Gynecol. 2013;56(3):463–470.
- Radmanesh A, Duszak R, Fitzgerald R. Social media and public outreach: a physician primer. Am J Neuroradiol. 2015;36(7):1223–1224.
- Grajales FJ 3rd, Sheps S, Ho K, Novak-Lauscher H, Eysenbach G. Social media: a review and tutorial of applications in medicine and health care. J Med Internet Res. 2014;16(2):e13.
- ACOG Today. Social media guide: how to comment with patients and spread women’s health messages. American Congress of Obstetricians and Gynecologists website. http://www.acog.org/-/media/ACOG-Today/acogToday201211.pdf. Published November 2012. Accessed February 18, 2016.
In this Article
- Health providers’ use of social media
- Protecting confidentiality
- Creating a social media policy