User login
Postpartum life-threatening strep infection
Postpartum life-threatening strep infection
A pregnant woman received prenatal care from a midwifery practice. A week before her scheduled delivery, the patient became ill with fever and vomiting and visited her midwife. While tests were still pending, the midwife decided to admit the mother to the hospital for induction of labor. The baby was born by vaginal delivery under the midwife’s care. The mother remained in the hospital for observation.
Two days after delivery, the mother began to have nausea, vomiting, and a low-grade fever. The nurse called the midwife, who ordered acetaminophen (Tylenol) but did not come to examine the patient. Two hours later, the nurse notified the midwife that the patient’s condition had worsened and that she was experiencing abdominal pain; the midwife ordered oxycodone. Over the next few hours, the midwife was apprised of the patient’s condition several times by telephone, but she never came to examine the patient nor did she ask her supervising ObGyn to examine the patient.
The next morning, a second midwife noted that the patient was experiencing an itchy rash on her extremities and abdomen. A complete blood count (CBC) showed a “critical lab value” of 44% band neutrophils (normal, 0% to 10% for the hospital laboratory). The second midwife and nurse told the supervising ObGyn that the patient otherwise looked well; he discharged the patient.
At home, the patient’s condition worsened. Her husband called the ObGyn several times and took her to the emergency department (ED) that evening. Her condition deteriorated and she was transferred to another facility where she was diagnosed with a life-threatening Group A Streptococcus (GAS) infection. After weeks of treatment for sepsis, the patient’s foot was amputated.
Patient's claim: The first midwife was negligent in her postpartum treatment of the patient; she should have come to the hospital to examine the patient or have requested that the supervising ObGyn examine the patient. The rash and CBC test results should have initiated further treatment and investigation; the patient should not have been discharged. GAS was not found or treated in a timely manner, resulting in sepsis and amputation.
Defendants' defense: The case was settled during the trial.
Verdict: A $2,500,000 Massachusetts settlement was reached with the midwife, her practice, and the ObGyn.
Failure to follow-up on abnormal Pap
A woman in her 50s reported abnormal bleeding to her gynecologist. Results of an endometrial biopsy were negative for cancer; the gynecologist prescribed hormone therapy. The patient continued to bleed until she entered menopause.
Ten years later, the bleeding returned. Results of a Pap test indicated atypical endometrial cells; an ultrasound showed a markedly abnormal endometrium. The gynecologist recommended a hysteroscopic dilation and curettage (D&C). When he attempted the procedure it ended prematurely because he was unable to enter the patient’s endometrium. The patient’s discharge instructions indicated that she should call the physician for follow up. In a letter to the patient written a month later, the physician discussed the abnormal Pap test results and indicated that the patient had 2 options: another D&C under ultrasound guidance or hysterectomy. He also noted that he would contact the patient’s primary care physician (PCP) for input.
Two years later, the patient returned to the gynecologist because the bleeding, which had never stopped, had increased in intensity. Endometrial cancer was diagnosed.
Patient's claim: The gynecologist never followed up with the patient or her PCP after the incomplete D&C. There is no record that communication ever occurred between the gynecologist and PCP. Lack of follow-up and treatment resulted in progression of the cancer from stage 1 to stage 3C, with a 5-year survivability of 47% (stage 1 survivability is 83%).
Physician's defense: The gynecologist was surprised that no one had ever followed up with the patient. The patient was comparatively negligent for failing to seek medical care for the 2-year period.
Verdict: A $430,000 Minnesota settlement was reached at mediation.
LIVER DISEASE LED TO STILLBIRTH
A 37-year-old woman reported nausea, vomiting, headaches, heartburn, and upper abdominal pain to her ObGyn several times during her third trimester. She had been pregnant before and knew that this pregnancy “felt” different. She went to the ED 1 week before the birth of her child, but she was discharged. The child was stillborn.
Parent's claim: Neither the ObGyn who provided prenatal care nor the on-call ED ObGyn ordered laboratory testing, which would have revealed a rare disease: acute fatty liver of pregnancy. Action could have saved the life of her child.
The patient’s ObGyn disregarded the patient’s reported symptoms; no blood work or liver testing was done. The ObGyn should have recognized the symptoms of liver disease that presented during the third trimester. A diagnosis of liver disease would have initiated induction of labor.
The patient’s expert witness noted that the severity of the third trimester symptoms warranted follow-up testing; the patient should not have had all of those symptoms so late in pregnancy. Testing would have revealed that, by not functioning properly, the liver was creating a toxic environment for the fetus. Labor should have been induced at 36 weeks when the fetal heart testing was still normal.
The ED nurses contacted the on-call ObGyn by telephone to discuss the patient’s symptoms; the ObGyn did not come to the ED to examine the patient or order testing.
The patient suffered emotional distress as a result of the loss of her child.
Defendants' defense: The medical center and the on-call ObGyn settled prior to trial.
The ObGyn claimed that the patient’s symptoms were common for pregnancy and that the disease could not be diagnosed based on the presented symptoms. It was not a violation of the standard of care for the extremely rare liver disease to not be diagnosed. The defense’s expert claimed that the symptoms reported by the patient did not warrant follow-up blood work. There was no way to determine whether or not the fetus died as a result of the mother’s liver disease or nuchal cord involvement.
A placental pathologist noted that the placenta was injured by thrombosis; the fetus’ death was most likely idiopathic. He later acknowledged that thrombosis can be related to liver disease.
Verdict: Jurors were instructed to consider this a personal injury case for the mother due to an unborn fetus’ lacks standing for injury or death under California law. A $160,090 California verdict was returned against the ObGyn who provided prenatal care.
Postpartum life-threatening strep infection
A pregnant woman received prenatal care from a midwifery practice. A week before her scheduled delivery, the patient became ill with fever and vomiting and visited her midwife. While tests were still pending, the midwife decided to admit the mother to the hospital for induction of labor. The baby was born by vaginal delivery under the midwife’s care. The mother remained in the hospital for observation.
Two days after delivery, the mother began to have nausea, vomiting, and a low-grade fever. The nurse called the midwife, who ordered acetaminophen (Tylenol) but did not come to examine the patient. Two hours later, the nurse notified the midwife that the patient’s condition had worsened and that she was experiencing abdominal pain; the midwife ordered oxycodone. Over the next few hours, the midwife was apprised of the patient’s condition several times by telephone, but she never came to examine the patient nor did she ask her supervising ObGyn to examine the patient.
The next morning, a second midwife noted that the patient was experiencing an itchy rash on her extremities and abdomen. A complete blood count (CBC) showed a “critical lab value” of 44% band neutrophils (normal, 0% to 10% for the hospital laboratory). The second midwife and nurse told the supervising ObGyn that the patient otherwise looked well; he discharged the patient.
At home, the patient’s condition worsened. Her husband called the ObGyn several times and took her to the emergency department (ED) that evening. Her condition deteriorated and she was transferred to another facility where she was diagnosed with a life-threatening Group A Streptococcus (GAS) infection. After weeks of treatment for sepsis, the patient’s foot was amputated.
Patient's claim: The first midwife was negligent in her postpartum treatment of the patient; she should have come to the hospital to examine the patient or have requested that the supervising ObGyn examine the patient. The rash and CBC test results should have initiated further treatment and investigation; the patient should not have been discharged. GAS was not found or treated in a timely manner, resulting in sepsis and amputation.
Defendants' defense: The case was settled during the trial.
Verdict: A $2,500,000 Massachusetts settlement was reached with the midwife, her practice, and the ObGyn.
Failure to follow-up on abnormal Pap
A woman in her 50s reported abnormal bleeding to her gynecologist. Results of an endometrial biopsy were negative for cancer; the gynecologist prescribed hormone therapy. The patient continued to bleed until she entered menopause.
Ten years later, the bleeding returned. Results of a Pap test indicated atypical endometrial cells; an ultrasound showed a markedly abnormal endometrium. The gynecologist recommended a hysteroscopic dilation and curettage (D&C). When he attempted the procedure it ended prematurely because he was unable to enter the patient’s endometrium. The patient’s discharge instructions indicated that she should call the physician for follow up. In a letter to the patient written a month later, the physician discussed the abnormal Pap test results and indicated that the patient had 2 options: another D&C under ultrasound guidance or hysterectomy. He also noted that he would contact the patient’s primary care physician (PCP) for input.
Two years later, the patient returned to the gynecologist because the bleeding, which had never stopped, had increased in intensity. Endometrial cancer was diagnosed.
Patient's claim: The gynecologist never followed up with the patient or her PCP after the incomplete D&C. There is no record that communication ever occurred between the gynecologist and PCP. Lack of follow-up and treatment resulted in progression of the cancer from stage 1 to stage 3C, with a 5-year survivability of 47% (stage 1 survivability is 83%).
Physician's defense: The gynecologist was surprised that no one had ever followed up with the patient. The patient was comparatively negligent for failing to seek medical care for the 2-year period.
Verdict: A $430,000 Minnesota settlement was reached at mediation.
LIVER DISEASE LED TO STILLBIRTH
A 37-year-old woman reported nausea, vomiting, headaches, heartburn, and upper abdominal pain to her ObGyn several times during her third trimester. She had been pregnant before and knew that this pregnancy “felt” different. She went to the ED 1 week before the birth of her child, but she was discharged. The child was stillborn.
Parent's claim: Neither the ObGyn who provided prenatal care nor the on-call ED ObGyn ordered laboratory testing, which would have revealed a rare disease: acute fatty liver of pregnancy. Action could have saved the life of her child.
The patient’s ObGyn disregarded the patient’s reported symptoms; no blood work or liver testing was done. The ObGyn should have recognized the symptoms of liver disease that presented during the third trimester. A diagnosis of liver disease would have initiated induction of labor.
The patient’s expert witness noted that the severity of the third trimester symptoms warranted follow-up testing; the patient should not have had all of those symptoms so late in pregnancy. Testing would have revealed that, by not functioning properly, the liver was creating a toxic environment for the fetus. Labor should have been induced at 36 weeks when the fetal heart testing was still normal.
The ED nurses contacted the on-call ObGyn by telephone to discuss the patient’s symptoms; the ObGyn did not come to the ED to examine the patient or order testing.
The patient suffered emotional distress as a result of the loss of her child.
Defendants' defense: The medical center and the on-call ObGyn settled prior to trial.
The ObGyn claimed that the patient’s symptoms were common for pregnancy and that the disease could not be diagnosed based on the presented symptoms. It was not a violation of the standard of care for the extremely rare liver disease to not be diagnosed. The defense’s expert claimed that the symptoms reported by the patient did not warrant follow-up blood work. There was no way to determine whether or not the fetus died as a result of the mother’s liver disease or nuchal cord involvement.
A placental pathologist noted that the placenta was injured by thrombosis; the fetus’ death was most likely idiopathic. He later acknowledged that thrombosis can be related to liver disease.
Verdict: Jurors were instructed to consider this a personal injury case for the mother due to an unborn fetus’ lacks standing for injury or death under California law. A $160,090 California verdict was returned against the ObGyn who provided prenatal care.
Postpartum life-threatening strep infection
A pregnant woman received prenatal care from a midwifery practice. A week before her scheduled delivery, the patient became ill with fever and vomiting and visited her midwife. While tests were still pending, the midwife decided to admit the mother to the hospital for induction of labor. The baby was born by vaginal delivery under the midwife’s care. The mother remained in the hospital for observation.
Two days after delivery, the mother began to have nausea, vomiting, and a low-grade fever. The nurse called the midwife, who ordered acetaminophen (Tylenol) but did not come to examine the patient. Two hours later, the nurse notified the midwife that the patient’s condition had worsened and that she was experiencing abdominal pain; the midwife ordered oxycodone. Over the next few hours, the midwife was apprised of the patient’s condition several times by telephone, but she never came to examine the patient nor did she ask her supervising ObGyn to examine the patient.
The next morning, a second midwife noted that the patient was experiencing an itchy rash on her extremities and abdomen. A complete blood count (CBC) showed a “critical lab value” of 44% band neutrophils (normal, 0% to 10% for the hospital laboratory). The second midwife and nurse told the supervising ObGyn that the patient otherwise looked well; he discharged the patient.
At home, the patient’s condition worsened. Her husband called the ObGyn several times and took her to the emergency department (ED) that evening. Her condition deteriorated and she was transferred to another facility where she was diagnosed with a life-threatening Group A Streptococcus (GAS) infection. After weeks of treatment for sepsis, the patient’s foot was amputated.
Patient's claim: The first midwife was negligent in her postpartum treatment of the patient; she should have come to the hospital to examine the patient or have requested that the supervising ObGyn examine the patient. The rash and CBC test results should have initiated further treatment and investigation; the patient should not have been discharged. GAS was not found or treated in a timely manner, resulting in sepsis and amputation.
Defendants' defense: The case was settled during the trial.
Verdict: A $2,500,000 Massachusetts settlement was reached with the midwife, her practice, and the ObGyn.
Failure to follow-up on abnormal Pap
A woman in her 50s reported abnormal bleeding to her gynecologist. Results of an endometrial biopsy were negative for cancer; the gynecologist prescribed hormone therapy. The patient continued to bleed until she entered menopause.
Ten years later, the bleeding returned. Results of a Pap test indicated atypical endometrial cells; an ultrasound showed a markedly abnormal endometrium. The gynecologist recommended a hysteroscopic dilation and curettage (D&C). When he attempted the procedure it ended prematurely because he was unable to enter the patient’s endometrium. The patient’s discharge instructions indicated that she should call the physician for follow up. In a letter to the patient written a month later, the physician discussed the abnormal Pap test results and indicated that the patient had 2 options: another D&C under ultrasound guidance or hysterectomy. He also noted that he would contact the patient’s primary care physician (PCP) for input.
Two years later, the patient returned to the gynecologist because the bleeding, which had never stopped, had increased in intensity. Endometrial cancer was diagnosed.
Patient's claim: The gynecologist never followed up with the patient or her PCP after the incomplete D&C. There is no record that communication ever occurred between the gynecologist and PCP. Lack of follow-up and treatment resulted in progression of the cancer from stage 1 to stage 3C, with a 5-year survivability of 47% (stage 1 survivability is 83%).
Physician's defense: The gynecologist was surprised that no one had ever followed up with the patient. The patient was comparatively negligent for failing to seek medical care for the 2-year period.
Verdict: A $430,000 Minnesota settlement was reached at mediation.
LIVER DISEASE LED TO STILLBIRTH
A 37-year-old woman reported nausea, vomiting, headaches, heartburn, and upper abdominal pain to her ObGyn several times during her third trimester. She had been pregnant before and knew that this pregnancy “felt” different. She went to the ED 1 week before the birth of her child, but she was discharged. The child was stillborn.
Parent's claim: Neither the ObGyn who provided prenatal care nor the on-call ED ObGyn ordered laboratory testing, which would have revealed a rare disease: acute fatty liver of pregnancy. Action could have saved the life of her child.
The patient’s ObGyn disregarded the patient’s reported symptoms; no blood work or liver testing was done. The ObGyn should have recognized the symptoms of liver disease that presented during the third trimester. A diagnosis of liver disease would have initiated induction of labor.
The patient’s expert witness noted that the severity of the third trimester symptoms warranted follow-up testing; the patient should not have had all of those symptoms so late in pregnancy. Testing would have revealed that, by not functioning properly, the liver was creating a toxic environment for the fetus. Labor should have been induced at 36 weeks when the fetal heart testing was still normal.
The ED nurses contacted the on-call ObGyn by telephone to discuss the patient’s symptoms; the ObGyn did not come to the ED to examine the patient or order testing.
The patient suffered emotional distress as a result of the loss of her child.
Defendants' defense: The medical center and the on-call ObGyn settled prior to trial.
The ObGyn claimed that the patient’s symptoms were common for pregnancy and that the disease could not be diagnosed based on the presented symptoms. It was not a violation of the standard of care for the extremely rare liver disease to not be diagnosed. The defense’s expert claimed that the symptoms reported by the patient did not warrant follow-up blood work. There was no way to determine whether or not the fetus died as a result of the mother’s liver disease or nuchal cord involvement.
A placental pathologist noted that the placenta was injured by thrombosis; the fetus’ death was most likely idiopathic. He later acknowledged that thrombosis can be related to liver disease.
Verdict: Jurors were instructed to consider this a personal injury case for the mother due to an unborn fetus’ lacks standing for injury or death under California law. A $160,090 California verdict was returned against the ObGyn who provided prenatal care.
Additional Medical Verdicts
• Failure to follow-up on abnormal Pap
• Liver disease led to stillbirth
HDAC inhibition may reverse anthracycline resistance in patients with sarcoma
In patients with advanced solid tumors, including sarcoma, the combination of panobinostat, a histone deacetylase (HDAC) inhibitor, and the anthracycline epirubicin demonstrated a correlation between neutropenia, peripheral blood mononucleocyte (PBMC) histone acetylation, and clinical benefit. Acquired topoisomerase resistance was reversed in 8 of 14 patients, suggesting HDAC inhibition reverses resistance.
In 37 evaluable patients, 4 (11%) had partial responses and 17 (46%) had stable disease. The median time to progression and median overall survival were 3.1 (95% CI, 1.8 to 4.6) months and 7.3 (5.9 to 10.3) months, respectively. All four patients with objective partial response had progressed on previous topoisomerase II inhibitors.
“The potential for prolonged treatment with an anthracycline in combination with an HDAC inhibitor speaks to the tolerability of this regimen. This study suggests that further investigation of HDAC inhibition in combination with DNA-damaging agents in defined advanced sarcoma subtypes to validate these preliminary findings is warranted,” wrote Dr. Scott Thomas of the Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, and colleagues (Ann Oncol. 2016 Feb 21. doi: 10.1093/annonc/mdw044).
HDACs regulate protein acetylation, thereby modulating protein activity and gene expression. Preclinical studies showed that HDAC inhibitors potentiate DNA damaging activity of anthracyclines in various cancer types, including sarcoma.
The phase I trial enrolled patients with metastatic solid tumors in dose escalation cohorts, and 20 patients with advanced sarcoma in the dose expansion cohort at the maximum tolerated dose of 50 mg/day of panobinostat on days 1, 3, and 5, and 75 mg/m2 of epirubicin on day 5.
In total, 24 patients (60%) had at least one grade 3 or 4 adverse event, including neutropenia (45%), leukopenia (35%), lymphopenia (22.5%), thrombocytopenia (17.5%), anemia (15%), and febrile neutropenia (7.5%). Major nonhematologic toxicities of panobinostat were myelotoxicity, nausea/vomiting, and fatigue, which required dose modification in 26% of patients.
In patients with advanced solid tumors, including sarcoma, the combination of panobinostat, a histone deacetylase (HDAC) inhibitor, and the anthracycline epirubicin demonstrated a correlation between neutropenia, peripheral blood mononucleocyte (PBMC) histone acetylation, and clinical benefit. Acquired topoisomerase resistance was reversed in 8 of 14 patients, suggesting HDAC inhibition reverses resistance.
In 37 evaluable patients, 4 (11%) had partial responses and 17 (46%) had stable disease. The median time to progression and median overall survival were 3.1 (95% CI, 1.8 to 4.6) months and 7.3 (5.9 to 10.3) months, respectively. All four patients with objective partial response had progressed on previous topoisomerase II inhibitors.
“The potential for prolonged treatment with an anthracycline in combination with an HDAC inhibitor speaks to the tolerability of this regimen. This study suggests that further investigation of HDAC inhibition in combination with DNA-damaging agents in defined advanced sarcoma subtypes to validate these preliminary findings is warranted,” wrote Dr. Scott Thomas of the Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, and colleagues (Ann Oncol. 2016 Feb 21. doi: 10.1093/annonc/mdw044).
HDACs regulate protein acetylation, thereby modulating protein activity and gene expression. Preclinical studies showed that HDAC inhibitors potentiate DNA damaging activity of anthracyclines in various cancer types, including sarcoma.
The phase I trial enrolled patients with metastatic solid tumors in dose escalation cohorts, and 20 patients with advanced sarcoma in the dose expansion cohort at the maximum tolerated dose of 50 mg/day of panobinostat on days 1, 3, and 5, and 75 mg/m2 of epirubicin on day 5.
In total, 24 patients (60%) had at least one grade 3 or 4 adverse event, including neutropenia (45%), leukopenia (35%), lymphopenia (22.5%), thrombocytopenia (17.5%), anemia (15%), and febrile neutropenia (7.5%). Major nonhematologic toxicities of panobinostat were myelotoxicity, nausea/vomiting, and fatigue, which required dose modification in 26% of patients.
In patients with advanced solid tumors, including sarcoma, the combination of panobinostat, a histone deacetylase (HDAC) inhibitor, and the anthracycline epirubicin demonstrated a correlation between neutropenia, peripheral blood mononucleocyte (PBMC) histone acetylation, and clinical benefit. Acquired topoisomerase resistance was reversed in 8 of 14 patients, suggesting HDAC inhibition reverses resistance.
In 37 evaluable patients, 4 (11%) had partial responses and 17 (46%) had stable disease. The median time to progression and median overall survival were 3.1 (95% CI, 1.8 to 4.6) months and 7.3 (5.9 to 10.3) months, respectively. All four patients with objective partial response had progressed on previous topoisomerase II inhibitors.
“The potential for prolonged treatment with an anthracycline in combination with an HDAC inhibitor speaks to the tolerability of this regimen. This study suggests that further investigation of HDAC inhibition in combination with DNA-damaging agents in defined advanced sarcoma subtypes to validate these preliminary findings is warranted,” wrote Dr. Scott Thomas of the Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, and colleagues (Ann Oncol. 2016 Feb 21. doi: 10.1093/annonc/mdw044).
HDACs regulate protein acetylation, thereby modulating protein activity and gene expression. Preclinical studies showed that HDAC inhibitors potentiate DNA damaging activity of anthracyclines in various cancer types, including sarcoma.
The phase I trial enrolled patients with metastatic solid tumors in dose escalation cohorts, and 20 patients with advanced sarcoma in the dose expansion cohort at the maximum tolerated dose of 50 mg/day of panobinostat on days 1, 3, and 5, and 75 mg/m2 of epirubicin on day 5.
In total, 24 patients (60%) had at least one grade 3 or 4 adverse event, including neutropenia (45%), leukopenia (35%), lymphopenia (22.5%), thrombocytopenia (17.5%), anemia (15%), and febrile neutropenia (7.5%). Major nonhematologic toxicities of panobinostat were myelotoxicity, nausea/vomiting, and fatigue, which required dose modification in 26% of patients.
FROM ANNALS OF ONCOLOGY
Key clinical point: Despite prior exposure to multiple regimens, clinical benefit was observed in several patients with advanced solid tumors, including some with sarcoma, who received panobinostat and epirubicin.
Major finding: In 37 evaluable patients, 4 (11%) had partial responses and 17 (46%) had stable disease; acquired topoisomerase resistance was reversed in 8 of 14 patients.
Data source: Phase I trial of 20 patients with metastatic solid tumors in dose escalation cohorts, and 20 patients with advanced sarcoma in the dose expansion cohort.
Disclosures: Research was supported in part by Novartis International AG. Dr. Thomas reported having no disclosures. Dr. Munster received research support from Novartis for this and other clinical trials.
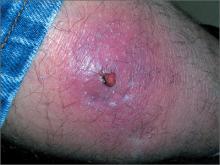
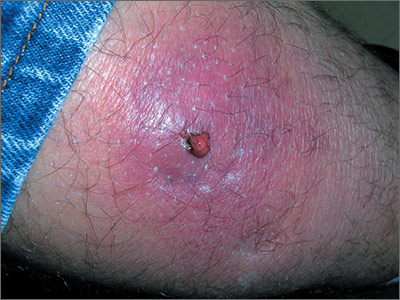
Tender, red thigh
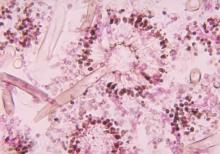
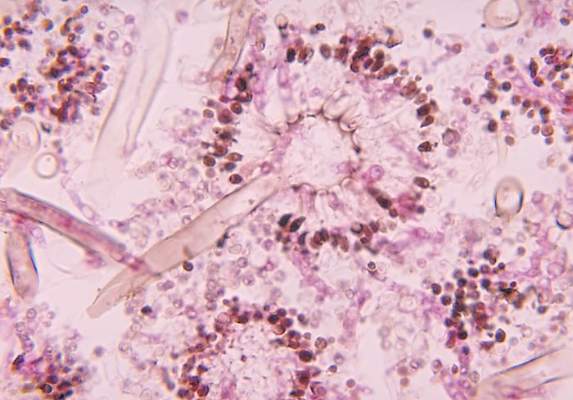
The FP diagnosed an abscess with some surrounding cellulitis. An abscess is a collection of pus in infected tissue. The abscess represents a walled-off infection in which there is a pocket of purulence. In abscesses of the skin, the offending organism is almost always Staphylococcus aureus. In 2004, methicillin-resistant S aureus (MRSA) was the most common identifiable cause of skin and soft-tissue infections among patients presenting to emergency departments in 11 US cities. S aureus was isolated from 76% of these infections and 59% of the infections were community-acquired MRSA.
A clinical cure is often obtained with incision and drainage alone. It is reasonable to obtain wound cultures in high-risk patients, those with signs of systemic infection, and in patients with a history of high recurrence rates.
While the patient’s abscess was beginning to drain spontaneously, it needed to drain some more. The FP performed an incision and drained more than 10 mL of pus. The large abscess cavity was packed with non-iodinated gauze (there is no benefit to iodinated gauze and iodine can be toxic to open tissues). The patient was placed on trimethoprim-sulfamethoxazole DS (double strength) twice daily to cover the surrounding cellulitis. His diabetes medications were renewed and he was reminded of the importance of taking (and not skipping) these important medicines.
When the patient returned 2 days later, the erythema was gone and the packing was removed. The patient was feeling much better and his blood sugar was down to 150 mg/dL. The FP inserted a small amount of packing and told the patient that he could remove it himself in 2 days while in the shower. The patient’s culture grew out MRSA sensitive to trimethoprim-sulfamethoxazole. Two weeks later the patient was fully healed, with only a small scar from the incision remaining.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Abscess. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill;2013:698-701.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed an abscess with some surrounding cellulitis. An abscess is a collection of pus in infected tissue. The abscess represents a walled-off infection in which there is a pocket of purulence. In abscesses of the skin, the offending organism is almost always Staphylococcus aureus. In 2004, methicillin-resistant S aureus (MRSA) was the most common identifiable cause of skin and soft-tissue infections among patients presenting to emergency departments in 11 US cities. S aureus was isolated from 76% of these infections and 59% of the infections were community-acquired MRSA.
A clinical cure is often obtained with incision and drainage alone. It is reasonable to obtain wound cultures in high-risk patients, those with signs of systemic infection, and in patients with a history of high recurrence rates.
While the patient’s abscess was beginning to drain spontaneously, it needed to drain some more. The FP performed an incision and drained more than 10 mL of pus. The large abscess cavity was packed with non-iodinated gauze (there is no benefit to iodinated gauze and iodine can be toxic to open tissues). The patient was placed on trimethoprim-sulfamethoxazole DS (double strength) twice daily to cover the surrounding cellulitis. His diabetes medications were renewed and he was reminded of the importance of taking (and not skipping) these important medicines.
When the patient returned 2 days later, the erythema was gone and the packing was removed. The patient was feeling much better and his blood sugar was down to 150 mg/dL. The FP inserted a small amount of packing and told the patient that he could remove it himself in 2 days while in the shower. The patient’s culture grew out MRSA sensitive to trimethoprim-sulfamethoxazole. Two weeks later the patient was fully healed, with only a small scar from the incision remaining.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Abscess. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill;2013:698-701.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed an abscess with some surrounding cellulitis. An abscess is a collection of pus in infected tissue. The abscess represents a walled-off infection in which there is a pocket of purulence. In abscesses of the skin, the offending organism is almost always Staphylococcus aureus. In 2004, methicillin-resistant S aureus (MRSA) was the most common identifiable cause of skin and soft-tissue infections among patients presenting to emergency departments in 11 US cities. S aureus was isolated from 76% of these infections and 59% of the infections were community-acquired MRSA.
A clinical cure is often obtained with incision and drainage alone. It is reasonable to obtain wound cultures in high-risk patients, those with signs of systemic infection, and in patients with a history of high recurrence rates.
While the patient’s abscess was beginning to drain spontaneously, it needed to drain some more. The FP performed an incision and drained more than 10 mL of pus. The large abscess cavity was packed with non-iodinated gauze (there is no benefit to iodinated gauze and iodine can be toxic to open tissues). The patient was placed on trimethoprim-sulfamethoxazole DS (double strength) twice daily to cover the surrounding cellulitis. His diabetes medications were renewed and he was reminded of the importance of taking (and not skipping) these important medicines.
When the patient returned 2 days later, the erythema was gone and the packing was removed. The patient was feeling much better and his blood sugar was down to 150 mg/dL. The FP inserted a small amount of packing and told the patient that he could remove it himself in 2 days while in the shower. The patient’s culture grew out MRSA sensitive to trimethoprim-sulfamethoxazole. Two weeks later the patient was fully healed, with only a small scar from the incision remaining.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Abscess. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill;2013:698-701.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
New drug comparable to voriconazole for aspergillosis
The broad-spectrum triazole isavuconazole was as effective as voriconazole in patients with suspected invasive mold disease and caused significantly fewer drug-related adverse events, particularly those of the skin, eyes, and hepatobiliary system, a randomized double-blind study of 516 adults has shown.
The findings suggest that the newer agent “could allow safer therapy” for the primary treatment of invasive aspergillosis and other mold disease than standard therapy with voriconazole, researchers for the phase III, industry-sponsored SECURE trial say in a report published in the Lancet.
The researchers assessed the safety and efficacy of isavuconazole versus voriconazole in patients with invasive mold infection. Patients were recruited from 102 centers across 26 countries over a 7-year period and were randomized to receive either drug.
In the study group of 516 adults with suspected invasive mold infection who received at least one dose of either antifungal drug, isavuconazole proved to be noninferior to voriconazole, by the primary endpoint of all-cause mortality at 6 weeks.
All-cause mortality at 6 weeks in this intention-to-treat group, of whom more than 80% had hematologic malignant disease, was 19% in the isavuconazole group (48 of 258) and 20% (52 of 258) in the voriconazole group.
This primary endpoint was chosen because “it provides the most objective and reproducible effect of therapy, and approximates best the attributable mortality, because deaths due to competing causes occur increasingly after 6 weeks,” Dr. Johan A. Maertensof the UZ Leuven (Belgium), and his associates wrote.
Secondary endpoints included overall response at the end of treatment among patients who were determined by an independent review committee to have proven or probable invasive mold disease – the study’s modified intention-to-treat population – as well as all-cause mortality at day 42 and day 84.
All-cause mortality in this modified intention-to-treat group, as well as in the group of patients found to have proven or probable invasive aspergillosis, specifically, supported the study’s primary findings (Lancet 2016 Feb:387:760-9).
Nearly all patients in the study had at least one treatment-emergent adverse event, and the proportion with serious treatment-emergent adverse events was similar between the treatment groups. However, patients treated with isavuconazole had a significantly lower frequency of hepatobiliary disorders, eye disorders, and skin or subcutaneous disorders.
And overall, significantly fewer patients reported drug-related adverse events with isavuconazole (42% of patients) than with voriconazole (60% of patients). Discontinuation from adverse events, moreover, was significantly less common among isavuconazole-treated patients.
Of the 516 patients in the intention-to-treat group, approximately 53% were confirmed to have proven or probable invasive mold disease, and more than 80% of the mycologically documented cases were Aspergillus infections. Enrollment of patients with possible invasive mold disease at the start “reflects the real-life strategy of early initiation of antifungal treatment,” the investigators say.
Isavuconazonium sulfate was approved in 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis.
Voriconazole is the current gold standard for the primary treatment of invasive aspergillosis and is recommended for some other mold infections as well, but it is not active against mucormycosis and has “highly variable nonlinear pharmacokinetics in adults,” which has triggered recommendations for drug monitoring, Dr. Maertens and his associates say.
Therapeutic monitoring aimed at individualizing dosage regimes in order to improve response and prevent adverse events became the standard of care in some institutions during the study period (2007-2013). The study used the labeled dose of voriconazole, however, and did not address the efficacy of either drug with therapeutic drug monitoring.
The study also excluded patients with AIDS, abnormal liver or renal function, and those receiving antifungal prophylaxis with a mold-active triazole – factors that may limit generalizability of the findings, the investigators note.
Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International.
Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer during the study.
The advantages of isavuconazole over voriconazole include its broader spectrum of activity, linear pharmacokinetics, once-daily dosing after the loading dose, and fewer CYP enzyme-mediated drug-drug interactions. This trial represents important progress in widening the therapeutic options for mold infections.
Numerous issues require further evaluation, including the effectiveness of isavuconazole after mold-active triazole prophylaxis, which is a common practice in patients at risk for mold infection, and the agent’s effectiveness against molds other than Aspergillus.
In addition, experience in a more varied patient population will be required to be certain that therapeutic drug monitoring is unnecessary.
Cost-effectiveness must also be explored. Isavuconazole will probably achieve an equivalent recommendation as voriconazole for initial treatment of aspergillosis in clinical guidelines, but voriconazole will soon come off patent in many countries and new formulations of posaconazole are now available.
That the finding that 42-day mortality in both treatment groups (isavuconazole and voriconazole) was no different than the mortality seen in research done 15 years ago on voriconazole treatment of aspergillosis is disappointing and suggests that we need to do better with the prevention and early detection of mold infection in vulnerable patients.
Dr. Monica A. Slavin and Dr. Karin A. Thursky are affiliated with the Peter MacCallum Cancer Centre, East Melbourne, Australia. Their comments are excerpted from an accompanying editorial in the Lancet. Dr. Slavin reported receiving grants from Merck, Gilead, and Pfizer. Dr. Thursky reported no disclosures.
The advantages of isavuconazole over voriconazole include its broader spectrum of activity, linear pharmacokinetics, once-daily dosing after the loading dose, and fewer CYP enzyme-mediated drug-drug interactions. This trial represents important progress in widening the therapeutic options for mold infections.
Numerous issues require further evaluation, including the effectiveness of isavuconazole after mold-active triazole prophylaxis, which is a common practice in patients at risk for mold infection, and the agent’s effectiveness against molds other than Aspergillus.
In addition, experience in a more varied patient population will be required to be certain that therapeutic drug monitoring is unnecessary.
Cost-effectiveness must also be explored. Isavuconazole will probably achieve an equivalent recommendation as voriconazole for initial treatment of aspergillosis in clinical guidelines, but voriconazole will soon come off patent in many countries and new formulations of posaconazole are now available.
That the finding that 42-day mortality in both treatment groups (isavuconazole and voriconazole) was no different than the mortality seen in research done 15 years ago on voriconazole treatment of aspergillosis is disappointing and suggests that we need to do better with the prevention and early detection of mold infection in vulnerable patients.
Dr. Monica A. Slavin and Dr. Karin A. Thursky are affiliated with the Peter MacCallum Cancer Centre, East Melbourne, Australia. Their comments are excerpted from an accompanying editorial in the Lancet. Dr. Slavin reported receiving grants from Merck, Gilead, and Pfizer. Dr. Thursky reported no disclosures.
The advantages of isavuconazole over voriconazole include its broader spectrum of activity, linear pharmacokinetics, once-daily dosing after the loading dose, and fewer CYP enzyme-mediated drug-drug interactions. This trial represents important progress in widening the therapeutic options for mold infections.
Numerous issues require further evaluation, including the effectiveness of isavuconazole after mold-active triazole prophylaxis, which is a common practice in patients at risk for mold infection, and the agent’s effectiveness against molds other than Aspergillus.
In addition, experience in a more varied patient population will be required to be certain that therapeutic drug monitoring is unnecessary.
Cost-effectiveness must also be explored. Isavuconazole will probably achieve an equivalent recommendation as voriconazole for initial treatment of aspergillosis in clinical guidelines, but voriconazole will soon come off patent in many countries and new formulations of posaconazole are now available.
That the finding that 42-day mortality in both treatment groups (isavuconazole and voriconazole) was no different than the mortality seen in research done 15 years ago on voriconazole treatment of aspergillosis is disappointing and suggests that we need to do better with the prevention and early detection of mold infection in vulnerable patients.
Dr. Monica A. Slavin and Dr. Karin A. Thursky are affiliated with the Peter MacCallum Cancer Centre, East Melbourne, Australia. Their comments are excerpted from an accompanying editorial in the Lancet. Dr. Slavin reported receiving grants from Merck, Gilead, and Pfizer. Dr. Thursky reported no disclosures.
The broad-spectrum triazole isavuconazole was as effective as voriconazole in patients with suspected invasive mold disease and caused significantly fewer drug-related adverse events, particularly those of the skin, eyes, and hepatobiliary system, a randomized double-blind study of 516 adults has shown.
The findings suggest that the newer agent “could allow safer therapy” for the primary treatment of invasive aspergillosis and other mold disease than standard therapy with voriconazole, researchers for the phase III, industry-sponsored SECURE trial say in a report published in the Lancet.
The researchers assessed the safety and efficacy of isavuconazole versus voriconazole in patients with invasive mold infection. Patients were recruited from 102 centers across 26 countries over a 7-year period and were randomized to receive either drug.
In the study group of 516 adults with suspected invasive mold infection who received at least one dose of either antifungal drug, isavuconazole proved to be noninferior to voriconazole, by the primary endpoint of all-cause mortality at 6 weeks.
All-cause mortality at 6 weeks in this intention-to-treat group, of whom more than 80% had hematologic malignant disease, was 19% in the isavuconazole group (48 of 258) and 20% (52 of 258) in the voriconazole group.
This primary endpoint was chosen because “it provides the most objective and reproducible effect of therapy, and approximates best the attributable mortality, because deaths due to competing causes occur increasingly after 6 weeks,” Dr. Johan A. Maertensof the UZ Leuven (Belgium), and his associates wrote.
Secondary endpoints included overall response at the end of treatment among patients who were determined by an independent review committee to have proven or probable invasive mold disease – the study’s modified intention-to-treat population – as well as all-cause mortality at day 42 and day 84.
All-cause mortality in this modified intention-to-treat group, as well as in the group of patients found to have proven or probable invasive aspergillosis, specifically, supported the study’s primary findings (Lancet 2016 Feb:387:760-9).
Nearly all patients in the study had at least one treatment-emergent adverse event, and the proportion with serious treatment-emergent adverse events was similar between the treatment groups. However, patients treated with isavuconazole had a significantly lower frequency of hepatobiliary disorders, eye disorders, and skin or subcutaneous disorders.
And overall, significantly fewer patients reported drug-related adverse events with isavuconazole (42% of patients) than with voriconazole (60% of patients). Discontinuation from adverse events, moreover, was significantly less common among isavuconazole-treated patients.
Of the 516 patients in the intention-to-treat group, approximately 53% were confirmed to have proven or probable invasive mold disease, and more than 80% of the mycologically documented cases were Aspergillus infections. Enrollment of patients with possible invasive mold disease at the start “reflects the real-life strategy of early initiation of antifungal treatment,” the investigators say.
Isavuconazonium sulfate was approved in 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis.
Voriconazole is the current gold standard for the primary treatment of invasive aspergillosis and is recommended for some other mold infections as well, but it is not active against mucormycosis and has “highly variable nonlinear pharmacokinetics in adults,” which has triggered recommendations for drug monitoring, Dr. Maertens and his associates say.
Therapeutic monitoring aimed at individualizing dosage regimes in order to improve response and prevent adverse events became the standard of care in some institutions during the study period (2007-2013). The study used the labeled dose of voriconazole, however, and did not address the efficacy of either drug with therapeutic drug monitoring.
The study also excluded patients with AIDS, abnormal liver or renal function, and those receiving antifungal prophylaxis with a mold-active triazole – factors that may limit generalizability of the findings, the investigators note.
Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International.
Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer during the study.
The broad-spectrum triazole isavuconazole was as effective as voriconazole in patients with suspected invasive mold disease and caused significantly fewer drug-related adverse events, particularly those of the skin, eyes, and hepatobiliary system, a randomized double-blind study of 516 adults has shown.
The findings suggest that the newer agent “could allow safer therapy” for the primary treatment of invasive aspergillosis and other mold disease than standard therapy with voriconazole, researchers for the phase III, industry-sponsored SECURE trial say in a report published in the Lancet.
The researchers assessed the safety and efficacy of isavuconazole versus voriconazole in patients with invasive mold infection. Patients were recruited from 102 centers across 26 countries over a 7-year period and were randomized to receive either drug.
In the study group of 516 adults with suspected invasive mold infection who received at least one dose of either antifungal drug, isavuconazole proved to be noninferior to voriconazole, by the primary endpoint of all-cause mortality at 6 weeks.
All-cause mortality at 6 weeks in this intention-to-treat group, of whom more than 80% had hematologic malignant disease, was 19% in the isavuconazole group (48 of 258) and 20% (52 of 258) in the voriconazole group.
This primary endpoint was chosen because “it provides the most objective and reproducible effect of therapy, and approximates best the attributable mortality, because deaths due to competing causes occur increasingly after 6 weeks,” Dr. Johan A. Maertensof the UZ Leuven (Belgium), and his associates wrote.
Secondary endpoints included overall response at the end of treatment among patients who were determined by an independent review committee to have proven or probable invasive mold disease – the study’s modified intention-to-treat population – as well as all-cause mortality at day 42 and day 84.
All-cause mortality in this modified intention-to-treat group, as well as in the group of patients found to have proven or probable invasive aspergillosis, specifically, supported the study’s primary findings (Lancet 2016 Feb:387:760-9).
Nearly all patients in the study had at least one treatment-emergent adverse event, and the proportion with serious treatment-emergent adverse events was similar between the treatment groups. However, patients treated with isavuconazole had a significantly lower frequency of hepatobiliary disorders, eye disorders, and skin or subcutaneous disorders.
And overall, significantly fewer patients reported drug-related adverse events with isavuconazole (42% of patients) than with voriconazole (60% of patients). Discontinuation from adverse events, moreover, was significantly less common among isavuconazole-treated patients.
Of the 516 patients in the intention-to-treat group, approximately 53% were confirmed to have proven or probable invasive mold disease, and more than 80% of the mycologically documented cases were Aspergillus infections. Enrollment of patients with possible invasive mold disease at the start “reflects the real-life strategy of early initiation of antifungal treatment,” the investigators say.
Isavuconazonium sulfate was approved in 2015 by the FDA for the treatment of invasive aspergillosis and invasive mucormycosis.
Voriconazole is the current gold standard for the primary treatment of invasive aspergillosis and is recommended for some other mold infections as well, but it is not active against mucormycosis and has “highly variable nonlinear pharmacokinetics in adults,” which has triggered recommendations for drug monitoring, Dr. Maertens and his associates say.
Therapeutic monitoring aimed at individualizing dosage regimes in order to improve response and prevent adverse events became the standard of care in some institutions during the study period (2007-2013). The study used the labeled dose of voriconazole, however, and did not address the efficacy of either drug with therapeutic drug monitoring.
The study also excluded patients with AIDS, abnormal liver or renal function, and those receiving antifungal prophylaxis with a mold-active triazole – factors that may limit generalizability of the findings, the investigators note.
Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International.
Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer during the study.
FROM THE LANCET
Key clinical point: Isavuconazole is an appropriate alternative for primary treatment of suspected invasive aspergillosis.
Major finding: All-cause mortality at 6 weeks in the intention-to-treat group of 516 patients was 19% with isavuconazole and 20% with voriconazole. Fewer drug-related adverse events were reported with isavuconazole, however (42% vs. 60% of patients).
Data source: A phase III randomized, double-blind noninferiority trial – the SECURE trial – comparing the safety and efficacy of intravenous and oral formulations of isavuconazole and voriconazole for the primary treatment of invasive aspergillosis and disease caused by other molds.
Disclosures: Funding for the study was provided by Astellas Pharma Global Development and Basilea Pharmaceutica International. Dr. Maertens disclosed receiving grants and fees from Bio-Rad, personal fees and nonfinancial support from Astellas and Basilea, and grants, fees, and support from Gilead Sciences, Merck Sharp and Dohme, and Pfizer, during the study.
Uveitis in juvenile idiopathic arthritis may be preventable
MAUI, HAWAII – Uveitis is a common, highly destructive manifestation of juvenile idiopathic arthritis that is readily treatable when caught early, Dr. Anne M. Stevens said at the 2016 Rheumatology Winter Clinical Symposium.
Moreover, recent evidence from Germany suggests that uveitis may actually be preventable through early aggressive anti-inflammatory therapy, noted Dr. Stevens, a pediatric rheumatologist at Seattle Children’s Hospital and the University of Washington.
She cited a report from investigators participating in the National Pediatric Rheumatological Database in Germany. This large study included 3,512 juvenile idiopathic arthritis (JIA) patients with a mean age at arthritis onset of 7.8 years, a disease duration of less than 12 months at enrollment, and a mean follow-up of 3.6 years.
Uveitis occurred in 5.1% of patients within the first year after onset of JIA, and in another 7.1% following the first year. The key finding in the German study was that aggressive disease-modifying antirheumatic drug (DMARD) therapy during the year prior to uveitis significantly reduced the likelihood of developing this complication. Children on methotrexate during that time period were 37% less likely to develop uveitis than were those not on a DMARD. Moreover, patients placed on methotrexate within the first year after being diagnosed with JIA had a 71% relative risk reduction.
Patients on a tumor necrosis factor inhibitor during the year prior to uveitis had a 44% reduction in the risk of developing the eye complication. Most impressive of all, children on both methotrexate and a TNF inhibitor during the year prior to uveitis had a whopping 90% reduction in the risk of developing uveitis, compared with those not on a DMARD (Arthritis Care Res [Hoboken]. 2016 Jan;68[1]:46-54).
“I was fascinated by this study showing that treatment with two types of therapy may be preventive,” Dr. Stevens commented. “If this is substantiated in another large population-based cohort, it will be interesting to see if practice moves to treating the ANA [antinuclear antibody]–positive oligo JIA patients very early with TNF [tumor necrosis factor] inhibitors and methotrexate to prevent uveitis.”
The logic behind this aggressive preventive therapy lies in the fact that while uveitis occurs in about 20% of patients with oligoarticular JIA overall, roughly 90% of cases involve ANA-positive patients. For this reason, guidelines recommend slit lamp examinations every 3 months for a year in patients with young-onset, ANA-positive JIA.
“Imagine trying to do a slit lamp exam on a 2-year-old. It really helps to send these kids to a pediatric ophthalmologist who’s done a lot of them,” she advised.
The uveitis of JIA is typically anterior, asymptomatic, and low grade. It’s also a leading cause of blindness in childhood. Complications include band keratopathy, glaucomatous optic neuropathy, cataracts, and maculopathy.
“If we catch uveitis early, we can treat this disease really well now,” according to Dr. Stevens.
The initial therapy is short-term topical steroids. It’s important to keep in touch with the ophthalmologist regarding the slit lamp findings, however, because ophthalmologists generally tend to favor longer-term topical steroid therapy, while rheumatologists are appropriately primed to push on to more aggressive systemic therapy very quickly.
The first-line systemic agent for treatment of uveitis in patients with JIA is methotrexate at 1 mg/kg/week. If that doesn’t achieve satisfactory results, pediatric rheumatologists are quick to move on to second-line therapy with cyclosporine, azathioprine, or mycophenolate (CellCept).
“We move on fairly quickly if need be to TNF inhibitors, and we go with very high doses. The literature for infliximab [Remicade] is supportive of 20 mg/kg every 4 weeks. That’s what I use,” she continued.
High-dose adalimumab (Humira) is another option (J Rheumatol. 2013 Jan;40[1]:74-9). However, etanercept (Enbrel) is not effective for this condition. The use of abatacept (Orencia) or rituximab (Rituxan) in refractory patients is supported by favorable case reports.
Dr. Stevens reported having no financial interests relevant to her presentation.
MAUI, HAWAII – Uveitis is a common, highly destructive manifestation of juvenile idiopathic arthritis that is readily treatable when caught early, Dr. Anne M. Stevens said at the 2016 Rheumatology Winter Clinical Symposium.
Moreover, recent evidence from Germany suggests that uveitis may actually be preventable through early aggressive anti-inflammatory therapy, noted Dr. Stevens, a pediatric rheumatologist at Seattle Children’s Hospital and the University of Washington.
She cited a report from investigators participating in the National Pediatric Rheumatological Database in Germany. This large study included 3,512 juvenile idiopathic arthritis (JIA) patients with a mean age at arthritis onset of 7.8 years, a disease duration of less than 12 months at enrollment, and a mean follow-up of 3.6 years.
Uveitis occurred in 5.1% of patients within the first year after onset of JIA, and in another 7.1% following the first year. The key finding in the German study was that aggressive disease-modifying antirheumatic drug (DMARD) therapy during the year prior to uveitis significantly reduced the likelihood of developing this complication. Children on methotrexate during that time period were 37% less likely to develop uveitis than were those not on a DMARD. Moreover, patients placed on methotrexate within the first year after being diagnosed with JIA had a 71% relative risk reduction.
Patients on a tumor necrosis factor inhibitor during the year prior to uveitis had a 44% reduction in the risk of developing the eye complication. Most impressive of all, children on both methotrexate and a TNF inhibitor during the year prior to uveitis had a whopping 90% reduction in the risk of developing uveitis, compared with those not on a DMARD (Arthritis Care Res [Hoboken]. 2016 Jan;68[1]:46-54).
“I was fascinated by this study showing that treatment with two types of therapy may be preventive,” Dr. Stevens commented. “If this is substantiated in another large population-based cohort, it will be interesting to see if practice moves to treating the ANA [antinuclear antibody]–positive oligo JIA patients very early with TNF [tumor necrosis factor] inhibitors and methotrexate to prevent uveitis.”
The logic behind this aggressive preventive therapy lies in the fact that while uveitis occurs in about 20% of patients with oligoarticular JIA overall, roughly 90% of cases involve ANA-positive patients. For this reason, guidelines recommend slit lamp examinations every 3 months for a year in patients with young-onset, ANA-positive JIA.
“Imagine trying to do a slit lamp exam on a 2-year-old. It really helps to send these kids to a pediatric ophthalmologist who’s done a lot of them,” she advised.
The uveitis of JIA is typically anterior, asymptomatic, and low grade. It’s also a leading cause of blindness in childhood. Complications include band keratopathy, glaucomatous optic neuropathy, cataracts, and maculopathy.
“If we catch uveitis early, we can treat this disease really well now,” according to Dr. Stevens.
The initial therapy is short-term topical steroids. It’s important to keep in touch with the ophthalmologist regarding the slit lamp findings, however, because ophthalmologists generally tend to favor longer-term topical steroid therapy, while rheumatologists are appropriately primed to push on to more aggressive systemic therapy very quickly.
The first-line systemic agent for treatment of uveitis in patients with JIA is methotrexate at 1 mg/kg/week. If that doesn’t achieve satisfactory results, pediatric rheumatologists are quick to move on to second-line therapy with cyclosporine, azathioprine, or mycophenolate (CellCept).
“We move on fairly quickly if need be to TNF inhibitors, and we go with very high doses. The literature for infliximab [Remicade] is supportive of 20 mg/kg every 4 weeks. That’s what I use,” she continued.
High-dose adalimumab (Humira) is another option (J Rheumatol. 2013 Jan;40[1]:74-9). However, etanercept (Enbrel) is not effective for this condition. The use of abatacept (Orencia) or rituximab (Rituxan) in refractory patients is supported by favorable case reports.
Dr. Stevens reported having no financial interests relevant to her presentation.
MAUI, HAWAII – Uveitis is a common, highly destructive manifestation of juvenile idiopathic arthritis that is readily treatable when caught early, Dr. Anne M. Stevens said at the 2016 Rheumatology Winter Clinical Symposium.
Moreover, recent evidence from Germany suggests that uveitis may actually be preventable through early aggressive anti-inflammatory therapy, noted Dr. Stevens, a pediatric rheumatologist at Seattle Children’s Hospital and the University of Washington.
She cited a report from investigators participating in the National Pediatric Rheumatological Database in Germany. This large study included 3,512 juvenile idiopathic arthritis (JIA) patients with a mean age at arthritis onset of 7.8 years, a disease duration of less than 12 months at enrollment, and a mean follow-up of 3.6 years.
Uveitis occurred in 5.1% of patients within the first year after onset of JIA, and in another 7.1% following the first year. The key finding in the German study was that aggressive disease-modifying antirheumatic drug (DMARD) therapy during the year prior to uveitis significantly reduced the likelihood of developing this complication. Children on methotrexate during that time period were 37% less likely to develop uveitis than were those not on a DMARD. Moreover, patients placed on methotrexate within the first year after being diagnosed with JIA had a 71% relative risk reduction.
Patients on a tumor necrosis factor inhibitor during the year prior to uveitis had a 44% reduction in the risk of developing the eye complication. Most impressive of all, children on both methotrexate and a TNF inhibitor during the year prior to uveitis had a whopping 90% reduction in the risk of developing uveitis, compared with those not on a DMARD (Arthritis Care Res [Hoboken]. 2016 Jan;68[1]:46-54).
“I was fascinated by this study showing that treatment with two types of therapy may be preventive,” Dr. Stevens commented. “If this is substantiated in another large population-based cohort, it will be interesting to see if practice moves to treating the ANA [antinuclear antibody]–positive oligo JIA patients very early with TNF [tumor necrosis factor] inhibitors and methotrexate to prevent uveitis.”
The logic behind this aggressive preventive therapy lies in the fact that while uveitis occurs in about 20% of patients with oligoarticular JIA overall, roughly 90% of cases involve ANA-positive patients. For this reason, guidelines recommend slit lamp examinations every 3 months for a year in patients with young-onset, ANA-positive JIA.
“Imagine trying to do a slit lamp exam on a 2-year-old. It really helps to send these kids to a pediatric ophthalmologist who’s done a lot of them,” she advised.
The uveitis of JIA is typically anterior, asymptomatic, and low grade. It’s also a leading cause of blindness in childhood. Complications include band keratopathy, glaucomatous optic neuropathy, cataracts, and maculopathy.
“If we catch uveitis early, we can treat this disease really well now,” according to Dr. Stevens.
The initial therapy is short-term topical steroids. It’s important to keep in touch with the ophthalmologist regarding the slit lamp findings, however, because ophthalmologists generally tend to favor longer-term topical steroid therapy, while rheumatologists are appropriately primed to push on to more aggressive systemic therapy very quickly.
The first-line systemic agent for treatment of uveitis in patients with JIA is methotrexate at 1 mg/kg/week. If that doesn’t achieve satisfactory results, pediatric rheumatologists are quick to move on to second-line therapy with cyclosporine, azathioprine, or mycophenolate (CellCept).
“We move on fairly quickly if need be to TNF inhibitors, and we go with very high doses. The literature for infliximab [Remicade] is supportive of 20 mg/kg every 4 weeks. That’s what I use,” she continued.
High-dose adalimumab (Humira) is another option (J Rheumatol. 2013 Jan;40[1]:74-9). However, etanercept (Enbrel) is not effective for this condition. The use of abatacept (Orencia) or rituximab (Rituxan) in refractory patients is supported by favorable case reports.
Dr. Stevens reported having no financial interests relevant to her presentation.
EXPERT ANALYSIS FROM RWCS 2016
Utah Health Sciences Center Realizes Significant Savings from Cost-Analysis Tool
Most healthcare systems don’t know or understand their cost of care at the unit that matters, i.e., the actual cost of care for an individual patient. Knowing this, University of Utah Health Sciences Center (UHSC) sought to develop methodologies using clinical data that would enable it to determine cost of care. Ultimately, a team led by systems, data, and analytics personnel, and including hospitalists, created an analytics framework known as value driven outcomes (VDO) using an agile methodology. Evaluation consisted of measurement against project objectives, including implementation timeliness, system performance, completeness, accuracy, extensibility, adoption, satisfaction, and the ability to support value improvement.1
“The initial results of employing the cost-savings tool have been exciting. For example, a 30% reduction in the cost of an orthopedic joint replacement—an inpatient hospital procedure—was realized,” says Robert C. Pendleton, MD, chief medical quality officer and professor of medicine at the University of Utah in Salt Lake City.
In another instance, hospitalists at UHSC led an initiative to reduce the use of unnecessary laboratory tests. That resulted in $400,000 in cost savings in the first year.
Another UHSC hospitalist used data to reduce unnecessary telemetry utilization by 60% on the hospitalist service.
“The accumulative impact of these initiatives, among others, will be a savings of millions of dollars annually by making process changes,” says Dr. Pendleton, a hospitalist who served on the executive steering committee that designed the web-based tool. “Our early experience shows that there is a lot of waste in the healthcare system.”
Along with the costing tool, quality and outcome measures were integrated so that the actual value of care (best outcomes at the lowest cost) could be assessed and incrementally improved.
Widespread Use?
Dr. Pendleton believes the tool could become popular among hospitalists, explaining that the field is known as the “leading system thinkers in healthcare.”
“By their nature, if you give them meaningful data and they can compare themselves to their peers, they are highly engaged to determine where waste in their hospitals exists and to lead improvement efforts,” he says.
UHSC is currently in the process of rolling out the VDO tool systemwide to its 1,200 faculty members. The rollout will take six to 12 months. In the next year, it’s also working to define and integrate outcome measures for the top 50 medical conditions it treats, which will allow it to robustly assess the value of care delivery.
“Success will equally be to increase quality without an increase in cost,” he says.
What’s more, Dr. Pendleton says efforts are in the works to make the self-intuitive system available to institutions nationwide. For example, the center is working to address technical complexities resulting from the use of multiple electronic health record vendors.
“The goal is to make it usable in every environment so we can support other organizations in the most effective ways,” he says.
Although the tool is very easy to use, stakeholders are providing feedback that allows for continuous improvements in how data are presented.
“Anyone with basic computer skills and the ability to interpret bar charts and basic graphs can use it,” Dr. Pendleton says. The center also offers a 15-minute, confidential, training module and a one-hour, one-on-one training session with an analyst. TH
Karen Appold is a freelance author in Pennsylvania.
Reference
- Kawamoto K, Martin CJ, Williams K, et al. Value driven outcomes (VDO): a pragmatic, modular, and extensible software framework for understanding and improving health care costs and outcomes. J Am Med Inform Assoc. 2015;22(1):223-235.
Most healthcare systems don’t know or understand their cost of care at the unit that matters, i.e., the actual cost of care for an individual patient. Knowing this, University of Utah Health Sciences Center (UHSC) sought to develop methodologies using clinical data that would enable it to determine cost of care. Ultimately, a team led by systems, data, and analytics personnel, and including hospitalists, created an analytics framework known as value driven outcomes (VDO) using an agile methodology. Evaluation consisted of measurement against project objectives, including implementation timeliness, system performance, completeness, accuracy, extensibility, adoption, satisfaction, and the ability to support value improvement.1
“The initial results of employing the cost-savings tool have been exciting. For example, a 30% reduction in the cost of an orthopedic joint replacement—an inpatient hospital procedure—was realized,” says Robert C. Pendleton, MD, chief medical quality officer and professor of medicine at the University of Utah in Salt Lake City.
In another instance, hospitalists at UHSC led an initiative to reduce the use of unnecessary laboratory tests. That resulted in $400,000 in cost savings in the first year.
Another UHSC hospitalist used data to reduce unnecessary telemetry utilization by 60% on the hospitalist service.
“The accumulative impact of these initiatives, among others, will be a savings of millions of dollars annually by making process changes,” says Dr. Pendleton, a hospitalist who served on the executive steering committee that designed the web-based tool. “Our early experience shows that there is a lot of waste in the healthcare system.”
Along with the costing tool, quality and outcome measures were integrated so that the actual value of care (best outcomes at the lowest cost) could be assessed and incrementally improved.
Widespread Use?
Dr. Pendleton believes the tool could become popular among hospitalists, explaining that the field is known as the “leading system thinkers in healthcare.”
“By their nature, if you give them meaningful data and they can compare themselves to their peers, they are highly engaged to determine where waste in their hospitals exists and to lead improvement efforts,” he says.
UHSC is currently in the process of rolling out the VDO tool systemwide to its 1,200 faculty members. The rollout will take six to 12 months. In the next year, it’s also working to define and integrate outcome measures for the top 50 medical conditions it treats, which will allow it to robustly assess the value of care delivery.
“Success will equally be to increase quality without an increase in cost,” he says.
What’s more, Dr. Pendleton says efforts are in the works to make the self-intuitive system available to institutions nationwide. For example, the center is working to address technical complexities resulting from the use of multiple electronic health record vendors.
“The goal is to make it usable in every environment so we can support other organizations in the most effective ways,” he says.
Although the tool is very easy to use, stakeholders are providing feedback that allows for continuous improvements in how data are presented.
“Anyone with basic computer skills and the ability to interpret bar charts and basic graphs can use it,” Dr. Pendleton says. The center also offers a 15-minute, confidential, training module and a one-hour, one-on-one training session with an analyst. TH
Karen Appold is a freelance author in Pennsylvania.
Reference
- Kawamoto K, Martin CJ, Williams K, et al. Value driven outcomes (VDO): a pragmatic, modular, and extensible software framework for understanding and improving health care costs and outcomes. J Am Med Inform Assoc. 2015;22(1):223-235.
Most healthcare systems don’t know or understand their cost of care at the unit that matters, i.e., the actual cost of care for an individual patient. Knowing this, University of Utah Health Sciences Center (UHSC) sought to develop methodologies using clinical data that would enable it to determine cost of care. Ultimately, a team led by systems, data, and analytics personnel, and including hospitalists, created an analytics framework known as value driven outcomes (VDO) using an agile methodology. Evaluation consisted of measurement against project objectives, including implementation timeliness, system performance, completeness, accuracy, extensibility, adoption, satisfaction, and the ability to support value improvement.1
“The initial results of employing the cost-savings tool have been exciting. For example, a 30% reduction in the cost of an orthopedic joint replacement—an inpatient hospital procedure—was realized,” says Robert C. Pendleton, MD, chief medical quality officer and professor of medicine at the University of Utah in Salt Lake City.
In another instance, hospitalists at UHSC led an initiative to reduce the use of unnecessary laboratory tests. That resulted in $400,000 in cost savings in the first year.
Another UHSC hospitalist used data to reduce unnecessary telemetry utilization by 60% on the hospitalist service.
“The accumulative impact of these initiatives, among others, will be a savings of millions of dollars annually by making process changes,” says Dr. Pendleton, a hospitalist who served on the executive steering committee that designed the web-based tool. “Our early experience shows that there is a lot of waste in the healthcare system.”
Along with the costing tool, quality and outcome measures were integrated so that the actual value of care (best outcomes at the lowest cost) could be assessed and incrementally improved.
Widespread Use?
Dr. Pendleton believes the tool could become popular among hospitalists, explaining that the field is known as the “leading system thinkers in healthcare.”
“By their nature, if you give them meaningful data and they can compare themselves to their peers, they are highly engaged to determine where waste in their hospitals exists and to lead improvement efforts,” he says.
UHSC is currently in the process of rolling out the VDO tool systemwide to its 1,200 faculty members. The rollout will take six to 12 months. In the next year, it’s also working to define and integrate outcome measures for the top 50 medical conditions it treats, which will allow it to robustly assess the value of care delivery.
“Success will equally be to increase quality without an increase in cost,” he says.
What’s more, Dr. Pendleton says efforts are in the works to make the self-intuitive system available to institutions nationwide. For example, the center is working to address technical complexities resulting from the use of multiple electronic health record vendors.
“The goal is to make it usable in every environment so we can support other organizations in the most effective ways,” he says.
Although the tool is very easy to use, stakeholders are providing feedback that allows for continuous improvements in how data are presented.
“Anyone with basic computer skills and the ability to interpret bar charts and basic graphs can use it,” Dr. Pendleton says. The center also offers a 15-minute, confidential, training module and a one-hour, one-on-one training session with an analyst. TH
Karen Appold is a freelance author in Pennsylvania.
Reference
- Kawamoto K, Martin CJ, Williams K, et al. Value driven outcomes (VDO): a pragmatic, modular, and extensible software framework for understanding and improving health care costs and outcomes. J Am Med Inform Assoc. 2015;22(1):223-235.
Risk of Chronic Kidney Disease is Increased If You Have Sleep Apnea
(Reuters Health) - Having sleep apnea may increase the risk of chronic kidney disease, according to a report from Taiwan.
Researchers analyzed data from 2000 through 2010 on 8,600 adults diagnosed with sleep apnea and four times as many adults of similar age, sex and monthly income without sleep apnea, using Taiwan's National Health Insurance Research Database.
They found 157 new cases of chronic kidney disease among people with sleep apnea and 298 cases in the comparison group, according to Yung-Tai Chen of Taipei City Hospital Heping Fuyou Branch in Taiwan and coauthors.
After taking other health factors into account, sleep apnea increased the risk of kidney disease by 58%. By comparison, hypertension (a known risk factor for kidney disease) increased the risk by 17%. Diabetes was a stronger predictor than both other factors, more than doubling the risk of kidney disease, the research team reported online February 1 in Respirology.
Intermittent low oxygen levels during the night and fragmented sleep patterns may activate higher blood pressure, which would damage the kidneys and could make individuals more susceptible to chronic kidney disease, said Tetyana Kendzerska of the University of Toronto Institute for Clinical Evaluative Sciences, who was not part of the study in Taiwan.
But, "the findings from this study are limited by lack of information on sleep apnea and chronic kidney disease severity given that these conditions were defined through the health administrative data," Kendzerska said.
Factors like obesity and smoking status are also important for kidney risk but were not included in the assessment, she told Reuters Health by email.
"So, instead of concluding that sleep apnea has the same impact as high blood pressure on the kidney, I would rather conclude that this study suggests that the association between sleep apnea and chronic kidney disease may exist," and should be validated with more powerful studies, she said.
Moderate to severe obstructive sleep apnea can be treated with continuous positive airway pressure (CPAP) at night which may decrease high blood pressure and mitigate kidney risk, Kendzerska said.
"These findings raise the issue of whether the relationship between sleep apnea and chronic kidney disease is unidirectional or bidirectional," she said. "If the importance of sleep apnea and preventive effect of treatment will be confirmed in further studies, sleep apnea should be added to the list of modifiable risk factors considered in (chronic kidney disease) risk assessment."
The authors of the study did not respond to a request for comment.
(Reuters Health) - Having sleep apnea may increase the risk of chronic kidney disease, according to a report from Taiwan.
Researchers analyzed data from 2000 through 2010 on 8,600 adults diagnosed with sleep apnea and four times as many adults of similar age, sex and monthly income without sleep apnea, using Taiwan's National Health Insurance Research Database.
They found 157 new cases of chronic kidney disease among people with sleep apnea and 298 cases in the comparison group, according to Yung-Tai Chen of Taipei City Hospital Heping Fuyou Branch in Taiwan and coauthors.
After taking other health factors into account, sleep apnea increased the risk of kidney disease by 58%. By comparison, hypertension (a known risk factor for kidney disease) increased the risk by 17%. Diabetes was a stronger predictor than both other factors, more than doubling the risk of kidney disease, the research team reported online February 1 in Respirology.
Intermittent low oxygen levels during the night and fragmented sleep patterns may activate higher blood pressure, which would damage the kidneys and could make individuals more susceptible to chronic kidney disease, said Tetyana Kendzerska of the University of Toronto Institute for Clinical Evaluative Sciences, who was not part of the study in Taiwan.
But, "the findings from this study are limited by lack of information on sleep apnea and chronic kidney disease severity given that these conditions were defined through the health administrative data," Kendzerska said.
Factors like obesity and smoking status are also important for kidney risk but were not included in the assessment, she told Reuters Health by email.
"So, instead of concluding that sleep apnea has the same impact as high blood pressure on the kidney, I would rather conclude that this study suggests that the association between sleep apnea and chronic kidney disease may exist," and should be validated with more powerful studies, she said.
Moderate to severe obstructive sleep apnea can be treated with continuous positive airway pressure (CPAP) at night which may decrease high blood pressure and mitigate kidney risk, Kendzerska said.
"These findings raise the issue of whether the relationship between sleep apnea and chronic kidney disease is unidirectional or bidirectional," she said. "If the importance of sleep apnea and preventive effect of treatment will be confirmed in further studies, sleep apnea should be added to the list of modifiable risk factors considered in (chronic kidney disease) risk assessment."
The authors of the study did not respond to a request for comment.
(Reuters Health) - Having sleep apnea may increase the risk of chronic kidney disease, according to a report from Taiwan.
Researchers analyzed data from 2000 through 2010 on 8,600 adults diagnosed with sleep apnea and four times as many adults of similar age, sex and monthly income without sleep apnea, using Taiwan's National Health Insurance Research Database.
They found 157 new cases of chronic kidney disease among people with sleep apnea and 298 cases in the comparison group, according to Yung-Tai Chen of Taipei City Hospital Heping Fuyou Branch in Taiwan and coauthors.
After taking other health factors into account, sleep apnea increased the risk of kidney disease by 58%. By comparison, hypertension (a known risk factor for kidney disease) increased the risk by 17%. Diabetes was a stronger predictor than both other factors, more than doubling the risk of kidney disease, the research team reported online February 1 in Respirology.
Intermittent low oxygen levels during the night and fragmented sleep patterns may activate higher blood pressure, which would damage the kidneys and could make individuals more susceptible to chronic kidney disease, said Tetyana Kendzerska of the University of Toronto Institute for Clinical Evaluative Sciences, who was not part of the study in Taiwan.
But, "the findings from this study are limited by lack of information on sleep apnea and chronic kidney disease severity given that these conditions were defined through the health administrative data," Kendzerska said.
Factors like obesity and smoking status are also important for kidney risk but were not included in the assessment, she told Reuters Health by email.
"So, instead of concluding that sleep apnea has the same impact as high blood pressure on the kidney, I would rather conclude that this study suggests that the association between sleep apnea and chronic kidney disease may exist," and should be validated with more powerful studies, she said.
Moderate to severe obstructive sleep apnea can be treated with continuous positive airway pressure (CPAP) at night which may decrease high blood pressure and mitigate kidney risk, Kendzerska said.
"These findings raise the issue of whether the relationship between sleep apnea and chronic kidney disease is unidirectional or bidirectional," she said. "If the importance of sleep apnea and preventive effect of treatment will be confirmed in further studies, sleep apnea should be added to the list of modifiable risk factors considered in (chronic kidney disease) risk assessment."
The authors of the study did not respond to a request for comment.
FDA issues new guidance on Zika virus transmission

Photo courtesy of NHS
The US Food and Drug Administration (FDA) has issued a guidance document intended to help reduce the risk of Zika virus transmission via human cells, tissues, and cellular and tissue-based products (HCT/Ps).
The guidance addresses donation of HCT/Ps from both living and deceased donors, including donors of umbilical cord blood and hematopoietic stem/progenitor cells.
In this document, the FDA recommends a 6-month deferral period for most HCT/P donors who may have been exposed to the Zika virus.
The exception is donors of gestational tissues, who should be considered ineligible if they were at risk of contracting the virus at any time during their pregnancy.
The new guidance is a part of the FDA’s ongoing efforts to protect HCT/Ps and blood products from Zika virus transmission. Last month, the agency issued recommendations for reducing the risk of Zika virus transmission via blood transfusion.
There is a potential risk that Zika virus can be transmitted by HCT/Ps used as part of a medical, surgical, or reproductive procedure. HCT/Ps include products such as corneas, bone, skin, heart valves, hematopoietic stem/progenitor cells, gestational tissues such as amniotic membrane, and reproductive tissues such as semen and oocytes.
According to the Centers for Disease Control and Prevention, Zika virus can be spread by a man to his sexual partners. To date, there have been several cases of sexual transmission in the US.
Current information about Zika virus detection in semen suggests the period of deferral for HCT/P donors should be longer than the period recommended for donors of whole blood and blood components.
Therefore, the FDA has recommended that living donors of HCT/Ps be considered ineligible if they were diagnosed with Zika virus infection, were in an area with active Zika virus transmission, or had sex with a male with either of those risk factors within the past 6 months.
Donors of umbilical cord blood, placenta, or other gestational tissues should be considered ineligible if they have had any of the above risk factors at any point during their pregnancy.
Deceased donors should be considered ineligible if they were diagnosed with Zika virus infection in the past 6 months.
A deferral period of 6 months was chosen because of the limited data available on the length of time the virus can persist in all tissues. Zika virus has been detected in tissues and body fluids after the virus is no longer detectable in the blood stream and has been detected in semen possibly up to 10 weeks after the onset of symptoms.
Less evidence exists regarding the potential for transmission of Zika virus by HCT/Ps typically recovered from deceased donors. As more information becomes available, the understanding of the risks to recipients of HCT/Ps, including HCT/Ps recovered from deceased donors, may evolve.
The FDA said it will continue to monitor the situation and evaluate new information regarding the associated risks as it becomes available.
In addition, the FDA said it is prioritizing the development of blood donor screening and diagnostic tests that may be useful for identifying the presence of or recent infection with the Zika virus. The agency is prepared to evaluate investigational vaccines and therapeutics that might be developed and review technology that may help suppress populations of the mosquitoes that can spread the virus. ![]()

Photo courtesy of NHS
The US Food and Drug Administration (FDA) has issued a guidance document intended to help reduce the risk of Zika virus transmission via human cells, tissues, and cellular and tissue-based products (HCT/Ps).
The guidance addresses donation of HCT/Ps from both living and deceased donors, including donors of umbilical cord blood and hematopoietic stem/progenitor cells.
In this document, the FDA recommends a 6-month deferral period for most HCT/P donors who may have been exposed to the Zika virus.
The exception is donors of gestational tissues, who should be considered ineligible if they were at risk of contracting the virus at any time during their pregnancy.
The new guidance is a part of the FDA’s ongoing efforts to protect HCT/Ps and blood products from Zika virus transmission. Last month, the agency issued recommendations for reducing the risk of Zika virus transmission via blood transfusion.
There is a potential risk that Zika virus can be transmitted by HCT/Ps used as part of a medical, surgical, or reproductive procedure. HCT/Ps include products such as corneas, bone, skin, heart valves, hematopoietic stem/progenitor cells, gestational tissues such as amniotic membrane, and reproductive tissues such as semen and oocytes.
According to the Centers for Disease Control and Prevention, Zika virus can be spread by a man to his sexual partners. To date, there have been several cases of sexual transmission in the US.
Current information about Zika virus detection in semen suggests the period of deferral for HCT/P donors should be longer than the period recommended for donors of whole blood and blood components.
Therefore, the FDA has recommended that living donors of HCT/Ps be considered ineligible if they were diagnosed with Zika virus infection, were in an area with active Zika virus transmission, or had sex with a male with either of those risk factors within the past 6 months.
Donors of umbilical cord blood, placenta, or other gestational tissues should be considered ineligible if they have had any of the above risk factors at any point during their pregnancy.
Deceased donors should be considered ineligible if they were diagnosed with Zika virus infection in the past 6 months.
A deferral period of 6 months was chosen because of the limited data available on the length of time the virus can persist in all tissues. Zika virus has been detected in tissues and body fluids after the virus is no longer detectable in the blood stream and has been detected in semen possibly up to 10 weeks after the onset of symptoms.
Less evidence exists regarding the potential for transmission of Zika virus by HCT/Ps typically recovered from deceased donors. As more information becomes available, the understanding of the risks to recipients of HCT/Ps, including HCT/Ps recovered from deceased donors, may evolve.
The FDA said it will continue to monitor the situation and evaluate new information regarding the associated risks as it becomes available.
In addition, the FDA said it is prioritizing the development of blood donor screening and diagnostic tests that may be useful for identifying the presence of or recent infection with the Zika virus. The agency is prepared to evaluate investigational vaccines and therapeutics that might be developed and review technology that may help suppress populations of the mosquitoes that can spread the virus. ![]()

Photo courtesy of NHS
The US Food and Drug Administration (FDA) has issued a guidance document intended to help reduce the risk of Zika virus transmission via human cells, tissues, and cellular and tissue-based products (HCT/Ps).
The guidance addresses donation of HCT/Ps from both living and deceased donors, including donors of umbilical cord blood and hematopoietic stem/progenitor cells.
In this document, the FDA recommends a 6-month deferral period for most HCT/P donors who may have been exposed to the Zika virus.
The exception is donors of gestational tissues, who should be considered ineligible if they were at risk of contracting the virus at any time during their pregnancy.
The new guidance is a part of the FDA’s ongoing efforts to protect HCT/Ps and blood products from Zika virus transmission. Last month, the agency issued recommendations for reducing the risk of Zika virus transmission via blood transfusion.
There is a potential risk that Zika virus can be transmitted by HCT/Ps used as part of a medical, surgical, or reproductive procedure. HCT/Ps include products such as corneas, bone, skin, heart valves, hematopoietic stem/progenitor cells, gestational tissues such as amniotic membrane, and reproductive tissues such as semen and oocytes.
According to the Centers for Disease Control and Prevention, Zika virus can be spread by a man to his sexual partners. To date, there have been several cases of sexual transmission in the US.
Current information about Zika virus detection in semen suggests the period of deferral for HCT/P donors should be longer than the period recommended for donors of whole blood and blood components.
Therefore, the FDA has recommended that living donors of HCT/Ps be considered ineligible if they were diagnosed with Zika virus infection, were in an area with active Zika virus transmission, or had sex with a male with either of those risk factors within the past 6 months.
Donors of umbilical cord blood, placenta, or other gestational tissues should be considered ineligible if they have had any of the above risk factors at any point during their pregnancy.
Deceased donors should be considered ineligible if they were diagnosed with Zika virus infection in the past 6 months.
A deferral period of 6 months was chosen because of the limited data available on the length of time the virus can persist in all tissues. Zika virus has been detected in tissues and body fluids after the virus is no longer detectable in the blood stream and has been detected in semen possibly up to 10 weeks after the onset of symptoms.
Less evidence exists regarding the potential for transmission of Zika virus by HCT/Ps typically recovered from deceased donors. As more information becomes available, the understanding of the risks to recipients of HCT/Ps, including HCT/Ps recovered from deceased donors, may evolve.
The FDA said it will continue to monitor the situation and evaluate new information regarding the associated risks as it becomes available.
In addition, the FDA said it is prioritizing the development of blood donor screening and diagnostic tests that may be useful for identifying the presence of or recent infection with the Zika virus. The agency is prepared to evaluate investigational vaccines and therapeutics that might be developed and review technology that may help suppress populations of the mosquitoes that can spread the virus. ![]()
Factors driving blood cell development
Researchers believe they have identified key factors that drive hematopoietic specification and differentiation.
The team performed detailed analyses on cells at 6 consecutive stages of development—starting with embryonic stem cells and ending with macrophages.
They said this revealed the complete set of regulatory elements driving differential gene expression during these stages of development.
The researchers described this work in Developmental Cell.
The team studied hematopoietic specification and differentiation by looking at 6 stages of cell development—embryonic stem cells, mesoderm cells, hemangioblasts, hemogenic endothelium, hematopoietic precursors, and macrophages.
“We examined how embryonic cells develop towards blood cells by collecting multi-omics data from measuring gene activity, changes in chromosome structure, and the interaction of regulatory factors with the genes themselves,” explained study author Constanze Bonifer, PhD, of the University of Birmingham in the UK.
“Our research shows, in unprecedented detail, how a vast network of interacting genes control blood cell development. It also shows how we can use such data to enhance our knowledge of this process.”
The researchers said their findings help explain how regulatory elements in the DNA work together, driving gene expression and the switch from one developmental stage to another.
The team believes the work also revealed the minimum requirements for generating blood cells from an unrelated, cultured cell type.
The researchers have made their findings available to the public on the following website: http://www.haemopoiesis.leeds.ac.uk/data_analysis/. ![]()

Researchers believe they have identified key factors that drive hematopoietic specification and differentiation.
The team performed detailed analyses on cells at 6 consecutive stages of development—starting with embryonic stem cells and ending with macrophages.
They said this revealed the complete set of regulatory elements driving differential gene expression during these stages of development.
The researchers described this work in Developmental Cell.
The team studied hematopoietic specification and differentiation by looking at 6 stages of cell development—embryonic stem cells, mesoderm cells, hemangioblasts, hemogenic endothelium, hematopoietic precursors, and macrophages.
“We examined how embryonic cells develop towards blood cells by collecting multi-omics data from measuring gene activity, changes in chromosome structure, and the interaction of regulatory factors with the genes themselves,” explained study author Constanze Bonifer, PhD, of the University of Birmingham in the UK.
“Our research shows, in unprecedented detail, how a vast network of interacting genes control blood cell development. It also shows how we can use such data to enhance our knowledge of this process.”
The researchers said their findings help explain how regulatory elements in the DNA work together, driving gene expression and the switch from one developmental stage to another.
The team believes the work also revealed the minimum requirements for generating blood cells from an unrelated, cultured cell type.
The researchers have made their findings available to the public on the following website: http://www.haemopoiesis.leeds.ac.uk/data_analysis/. ![]()

Researchers believe they have identified key factors that drive hematopoietic specification and differentiation.
The team performed detailed analyses on cells at 6 consecutive stages of development—starting with embryonic stem cells and ending with macrophages.
They said this revealed the complete set of regulatory elements driving differential gene expression during these stages of development.
The researchers described this work in Developmental Cell.
The team studied hematopoietic specification and differentiation by looking at 6 stages of cell development—embryonic stem cells, mesoderm cells, hemangioblasts, hemogenic endothelium, hematopoietic precursors, and macrophages.
“We examined how embryonic cells develop towards blood cells by collecting multi-omics data from measuring gene activity, changes in chromosome structure, and the interaction of regulatory factors with the genes themselves,” explained study author Constanze Bonifer, PhD, of the University of Birmingham in the UK.
“Our research shows, in unprecedented detail, how a vast network of interacting genes control blood cell development. It also shows how we can use such data to enhance our knowledge of this process.”
The researchers said their findings help explain how regulatory elements in the DNA work together, driving gene expression and the switch from one developmental stage to another.
The team believes the work also revealed the minimum requirements for generating blood cells from an unrelated, cultured cell type.
The researchers have made their findings available to the public on the following website: http://www.haemopoiesis.leeds.ac.uk/data_analysis/. ![]()
Study results support screening of childhood HL survivors

A new study indicates that early screening can reduce the risk of death from breast cancer among female survivors of childhood Hodgkin lymphoma (HL) who received chest radiation.
Researchers found evidence to suggest that starting mammograms at age 25 can reduce the risk of breast cancer death among these patients, but using MRI can reduce the risk further.
Unfortunately, both methods come with a risk of false-positive results.
David Hodgson, MD, of the University of Toronto in Canada, and his colleagues reported these findings in the Journal of the National Cancer Institute.
Dr Hodgson estimates there are thousands of HL survivors in North America treated throughout the 1990s and later who received chest radiation and are unaware they are at risk of breast cancer and eligible for early screening.
“Many of these are women who received radiotherapy to more normal tissue or at higher doses than are used currently,” he said. “But even for more recently treated patients, screening should reduce the risk of breast cancer death.”
For this study, Dr Hodgson and his colleagues gathered published information from dozens of studies about the risk of developing breast cancer in childhood HL survivors, the accuracy of different forms of breast cancer screening, and the rates at which women agree to be screened when asked.
Using mathematical models, the researchers used these data to quantify the effectiveness of starting screening early—at age 25.
The team found that, using mammography, about 260 survivors of childhood HL would need to be invited to have early breast cancer screening to prevent 1 breast cancer death.
However, the use of MRI for screening improved the effectiveness considerably. It reduced the number of women needing screening to prevent 1 breast cancer death to less than 80.
For HL survivors treated at age 15, the absolute risk of breast cancer mortality by age 75 was predicted to decrease from 16.65% with no early screening to 16.28% with annual mammography, 15.40% with annual MRI, 15.38% with same-day annual mammography and MRI, and 15.37% with alternating mammography and MRI every 6 months.
Dr Hodgson cautioned that there is a risk of false-positive screening results, particuarly with MRI, given that the method can detect many changes in breast tissue, most of which are not cancer.
The data suggested that, from age 25 to 75, at least one false-positive result would occur in 48% of women screened with mammography, 74% screened with MRI alone, and 79% screened with both methods.
The number of false-positives per 1000 screens would be 29.98 with mammography, 71.71 with MRI, and 99.52 with either same-day mammography and MRI or alternating mammography and MRI.
“So this is important for patients to know and for physicians to counsel patients about because it’s stressful for a patient to be called back about suspicious findings,” Dr Hodgson said. ![]()

A new study indicates that early screening can reduce the risk of death from breast cancer among female survivors of childhood Hodgkin lymphoma (HL) who received chest radiation.
Researchers found evidence to suggest that starting mammograms at age 25 can reduce the risk of breast cancer death among these patients, but using MRI can reduce the risk further.
Unfortunately, both methods come with a risk of false-positive results.
David Hodgson, MD, of the University of Toronto in Canada, and his colleagues reported these findings in the Journal of the National Cancer Institute.
Dr Hodgson estimates there are thousands of HL survivors in North America treated throughout the 1990s and later who received chest radiation and are unaware they are at risk of breast cancer and eligible for early screening.
“Many of these are women who received radiotherapy to more normal tissue or at higher doses than are used currently,” he said. “But even for more recently treated patients, screening should reduce the risk of breast cancer death.”
For this study, Dr Hodgson and his colleagues gathered published information from dozens of studies about the risk of developing breast cancer in childhood HL survivors, the accuracy of different forms of breast cancer screening, and the rates at which women agree to be screened when asked.
Using mathematical models, the researchers used these data to quantify the effectiveness of starting screening early—at age 25.
The team found that, using mammography, about 260 survivors of childhood HL would need to be invited to have early breast cancer screening to prevent 1 breast cancer death.
However, the use of MRI for screening improved the effectiveness considerably. It reduced the number of women needing screening to prevent 1 breast cancer death to less than 80.
For HL survivors treated at age 15, the absolute risk of breast cancer mortality by age 75 was predicted to decrease from 16.65% with no early screening to 16.28% with annual mammography, 15.40% with annual MRI, 15.38% with same-day annual mammography and MRI, and 15.37% with alternating mammography and MRI every 6 months.
Dr Hodgson cautioned that there is a risk of false-positive screening results, particuarly with MRI, given that the method can detect many changes in breast tissue, most of which are not cancer.
The data suggested that, from age 25 to 75, at least one false-positive result would occur in 48% of women screened with mammography, 74% screened with MRI alone, and 79% screened with both methods.
The number of false-positives per 1000 screens would be 29.98 with mammography, 71.71 with MRI, and 99.52 with either same-day mammography and MRI or alternating mammography and MRI.
“So this is important for patients to know and for physicians to counsel patients about because it’s stressful for a patient to be called back about suspicious findings,” Dr Hodgson said. ![]()

A new study indicates that early screening can reduce the risk of death from breast cancer among female survivors of childhood Hodgkin lymphoma (HL) who received chest radiation.
Researchers found evidence to suggest that starting mammograms at age 25 can reduce the risk of breast cancer death among these patients, but using MRI can reduce the risk further.
Unfortunately, both methods come with a risk of false-positive results.
David Hodgson, MD, of the University of Toronto in Canada, and his colleagues reported these findings in the Journal of the National Cancer Institute.
Dr Hodgson estimates there are thousands of HL survivors in North America treated throughout the 1990s and later who received chest radiation and are unaware they are at risk of breast cancer and eligible for early screening.
“Many of these are women who received radiotherapy to more normal tissue or at higher doses than are used currently,” he said. “But even for more recently treated patients, screening should reduce the risk of breast cancer death.”
For this study, Dr Hodgson and his colleagues gathered published information from dozens of studies about the risk of developing breast cancer in childhood HL survivors, the accuracy of different forms of breast cancer screening, and the rates at which women agree to be screened when asked.
Using mathematical models, the researchers used these data to quantify the effectiveness of starting screening early—at age 25.
The team found that, using mammography, about 260 survivors of childhood HL would need to be invited to have early breast cancer screening to prevent 1 breast cancer death.
However, the use of MRI for screening improved the effectiveness considerably. It reduced the number of women needing screening to prevent 1 breast cancer death to less than 80.
For HL survivors treated at age 15, the absolute risk of breast cancer mortality by age 75 was predicted to decrease from 16.65% with no early screening to 16.28% with annual mammography, 15.40% with annual MRI, 15.38% with same-day annual mammography and MRI, and 15.37% with alternating mammography and MRI every 6 months.
Dr Hodgson cautioned that there is a risk of false-positive screening results, particuarly with MRI, given that the method can detect many changes in breast tissue, most of which are not cancer.
The data suggested that, from age 25 to 75, at least one false-positive result would occur in 48% of women screened with mammography, 74% screened with MRI alone, and 79% screened with both methods.
The number of false-positives per 1000 screens would be 29.98 with mammography, 71.71 with MRI, and 99.52 with either same-day mammography and MRI or alternating mammography and MRI.
“So this is important for patients to know and for physicians to counsel patients about because it’s stressful for a patient to be called back about suspicious findings,” Dr Hodgson said. ![]()