User login
Was this patient's transdermal Tx making her dog sick?
THE CASE
A 56-year-old postmenopausal woman with a history of anxiety, depression, alcohol abuse, fatigue, insomnia, and mental fogginess presented to the family medicine clinic with concerns about her companion animal because of symptoms possibly associated with the patient’s medication. Of note, the patient’s physical exam was unremarkable.
The patient noticed that her 5-year-old, 4.5-lb spayed female Chihuahua dog was exhibiting peculiar behaviors, including excessive licking of the abdomen, nipples, and vulvar areas and straining with urination. The dog’s symptoms had started 1 week after the patient began using estradiol transdermal spray (Evamist) for her menopause symptoms. The patient’s menopause symptoms included hot flushes, insomnia, and mental fogginess.
The patient had been applying the estradiol transdermal spray on her inner forearm twice daily, in the morning and at bedtime. She would let the applied medication dry for approximately 2 hours before allowing her arm to come in contact with other items. She worried that some of the hormone may have wiped off onto her couch, pillows, blankets, and other surfaces. In addition, she often cradled the dog in her arms, which allowed the canine’s back to come in contact with her inner forearms. To her knowledge, the dog did not lick or ingest the medication.
The patient had taken the dog to her veterinarian. On physical exam, the veterinarian noted that the dog had nipple and vulvar enlargement but no vaginal discharge, vaginal bleeding, skin changes, or urine abnormalities.
THE (PET’S) DIAGNOSIS, THE PATIENT’S Rx
The veterinarian diagnosed the Chihuahua with vaginal hyperplasia and vulvar enlargement secondary to hyperestrogenism. The animal’s symptoms were likely caused by exposure to the owner’s hormone replacement therapy (HRT) medication—the estradiol spray. The veterinarian advised the woman to return to her family physician to discuss her use of the topical estrogen.
The patient asked her physician (SS) to change her HRT formulation. She was given a prescription for an estradiol 0.05 mg/24-hour transdermal patch to be placed on her abdomen twice weekly. After 2 weeks of using the patch therapy, the patient’s menopausal symptoms were reported to be well controlled. In addition, the companion animal’s breast and vulvar changes resolved, as did the dog’s licking behavior.
DISCUSSION
Estrogen therapy, with or without progesterone, is the most effective treatment for postmenopausal vasomotor symptoms.1 Given the concerns raised in the Women’s Health Initiative (WHI) and other clinical trials regarding hormone therapy and cardiovascular and breast cancer findings, many clinicians look to alternative, nonoral dosage forms to improve the safety profile.
Continue to: Safety of nonroal estrogen therapy
Safety of nonoral estrogen therapy. Administration of nonoral estrogen is associated with avoidance of hepatic first-pass metabolism and a resulting lower impact on hepatic proteins. Thus, data indicate a potentially lower risk for venous thromboembolic events with transdermal estrogen compared to oral estrogen.1 Since the publication of the results of the WHI trials, prescribing patterns in the United States indicate a general decline in the proportion of oral hormones, while transdermal prescription volume has remained steady, and the use of vaginal formulations has increased.2
Topical estrogen formulations. Transdermal or topical delivery of estrogen can be achieved through various formulations, including patches, gels, and a spray. While patches are simple to use, some women display hypersensitivity to the adhesive. Use of gel and spray formulations avoids exposure to adhesives, but these pose a risk of transfer of hormonal ingredients that are not covered by a patch. This risk is amplified by the relative accessibility of the product-specific application sites, which include the arms or thighs. Each manufacturer recommends careful handwashing after handling the product, a specific drying time before the user covers the site with clothing, and avoidance of contact with the application site for a prescribed period of time, usually at least 1 to 2 hours.3-6
Our patient. This case illustrates the importance of discussing the risk of medication transfer to both humans and animals when prescribing individualized hormone therapy. While the Evamist prescribing information specifically addresses the risk of unintentional medication transfer to children, it does not discuss other contact risks.6 In the literature, there have been a limited number of reports on the adverse effects from transdermal or topical human medication transfer to pets. Notably, the American Pet Products Association estimates that in the United States, approximately 90 million dogs and 94 million cats are owned as a pet in 67% of households.7
THE TAKEAWAY
Use of HRT, including transdermal or topical estrogen formulations, is common. Given the large number of companion animals in the United States, physicians should consider that all members of a patient’s household—including pets—may be subject to unintentional secondary exposure to topical estrogen formulations and that they may experience adverse effects. This presents an opportunity for patient education, which can have a larger impact on all occupants of the home.
CORRESPONDENCE
Shannon Scott, DO, FACOFP, Clinical Associate Professor, Arizona College of Osteopathic Medicine, 19389 North 59th Avenue, Glendale, AZ 85308; [email protected].
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. Steinkellner AR, Denison SE, Eldridge SL, et al. A decade of postmenopausal hormone therapy prescribing in the United States: long-term effects of the Women’s Health Initiative. Menopause. 2012;19:616-621.
3. Divigel [package insert]. Bridgewater, NJ: Vertical Pharmaceuticals, LLC; 2014.
4. Elestrin [package insert]. Somerset, NJ: Meda Pharmaceuticals; 2014.
5. Estrogel [package insert]. Herndon, VA: Ascend Therapeutics; 2018.
6. Evamist [package insert]. Minneapolis, MN: Perrigo; 2017.
7. American Pet Products Association. Pet Industry Market Size & Ownership Statistics. www.americanpetproducts.org/press_industrytrends.asp. Accessed November 1, 2019.
THE CASE
A 56-year-old postmenopausal woman with a history of anxiety, depression, alcohol abuse, fatigue, insomnia, and mental fogginess presented to the family medicine clinic with concerns about her companion animal because of symptoms possibly associated with the patient’s medication. Of note, the patient’s physical exam was unremarkable.
The patient noticed that her 5-year-old, 4.5-lb spayed female Chihuahua dog was exhibiting peculiar behaviors, including excessive licking of the abdomen, nipples, and vulvar areas and straining with urination. The dog’s symptoms had started 1 week after the patient began using estradiol transdermal spray (Evamist) for her menopause symptoms. The patient’s menopause symptoms included hot flushes, insomnia, and mental fogginess.
The patient had been applying the estradiol transdermal spray on her inner forearm twice daily, in the morning and at bedtime. She would let the applied medication dry for approximately 2 hours before allowing her arm to come in contact with other items. She worried that some of the hormone may have wiped off onto her couch, pillows, blankets, and other surfaces. In addition, she often cradled the dog in her arms, which allowed the canine’s back to come in contact with her inner forearms. To her knowledge, the dog did not lick or ingest the medication.
The patient had taken the dog to her veterinarian. On physical exam, the veterinarian noted that the dog had nipple and vulvar enlargement but no vaginal discharge, vaginal bleeding, skin changes, or urine abnormalities.
THE (PET’S) DIAGNOSIS, THE PATIENT’S Rx
The veterinarian diagnosed the Chihuahua with vaginal hyperplasia and vulvar enlargement secondary to hyperestrogenism. The animal’s symptoms were likely caused by exposure to the owner’s hormone replacement therapy (HRT) medication—the estradiol spray. The veterinarian advised the woman to return to her family physician to discuss her use of the topical estrogen.
The patient asked her physician (SS) to change her HRT formulation. She was given a prescription for an estradiol 0.05 mg/24-hour transdermal patch to be placed on her abdomen twice weekly. After 2 weeks of using the patch therapy, the patient’s menopausal symptoms were reported to be well controlled. In addition, the companion animal’s breast and vulvar changes resolved, as did the dog’s licking behavior.
DISCUSSION
Estrogen therapy, with or without progesterone, is the most effective treatment for postmenopausal vasomotor symptoms.1 Given the concerns raised in the Women’s Health Initiative (WHI) and other clinical trials regarding hormone therapy and cardiovascular and breast cancer findings, many clinicians look to alternative, nonoral dosage forms to improve the safety profile.
Continue to: Safety of nonroal estrogen therapy
Safety of nonoral estrogen therapy. Administration of nonoral estrogen is associated with avoidance of hepatic first-pass metabolism and a resulting lower impact on hepatic proteins. Thus, data indicate a potentially lower risk for venous thromboembolic events with transdermal estrogen compared to oral estrogen.1 Since the publication of the results of the WHI trials, prescribing patterns in the United States indicate a general decline in the proportion of oral hormones, while transdermal prescription volume has remained steady, and the use of vaginal formulations has increased.2
Topical estrogen formulations. Transdermal or topical delivery of estrogen can be achieved through various formulations, including patches, gels, and a spray. While patches are simple to use, some women display hypersensitivity to the adhesive. Use of gel and spray formulations avoids exposure to adhesives, but these pose a risk of transfer of hormonal ingredients that are not covered by a patch. This risk is amplified by the relative accessibility of the product-specific application sites, which include the arms or thighs. Each manufacturer recommends careful handwashing after handling the product, a specific drying time before the user covers the site with clothing, and avoidance of contact with the application site for a prescribed period of time, usually at least 1 to 2 hours.3-6
Our patient. This case illustrates the importance of discussing the risk of medication transfer to both humans and animals when prescribing individualized hormone therapy. While the Evamist prescribing information specifically addresses the risk of unintentional medication transfer to children, it does not discuss other contact risks.6 In the literature, there have been a limited number of reports on the adverse effects from transdermal or topical human medication transfer to pets. Notably, the American Pet Products Association estimates that in the United States, approximately 90 million dogs and 94 million cats are owned as a pet in 67% of households.7
THE TAKEAWAY
Use of HRT, including transdermal or topical estrogen formulations, is common. Given the large number of companion animals in the United States, physicians should consider that all members of a patient’s household—including pets—may be subject to unintentional secondary exposure to topical estrogen formulations and that they may experience adverse effects. This presents an opportunity for patient education, which can have a larger impact on all occupants of the home.
CORRESPONDENCE
Shannon Scott, DO, FACOFP, Clinical Associate Professor, Arizona College of Osteopathic Medicine, 19389 North 59th Avenue, Glendale, AZ 85308; [email protected].
THE CASE
A 56-year-old postmenopausal woman with a history of anxiety, depression, alcohol abuse, fatigue, insomnia, and mental fogginess presented to the family medicine clinic with concerns about her companion animal because of symptoms possibly associated with the patient’s medication. Of note, the patient’s physical exam was unremarkable.
The patient noticed that her 5-year-old, 4.5-lb spayed female Chihuahua dog was exhibiting peculiar behaviors, including excessive licking of the abdomen, nipples, and vulvar areas and straining with urination. The dog’s symptoms had started 1 week after the patient began using estradiol transdermal spray (Evamist) for her menopause symptoms. The patient’s menopause symptoms included hot flushes, insomnia, and mental fogginess.
The patient had been applying the estradiol transdermal spray on her inner forearm twice daily, in the morning and at bedtime. She would let the applied medication dry for approximately 2 hours before allowing her arm to come in contact with other items. She worried that some of the hormone may have wiped off onto her couch, pillows, blankets, and other surfaces. In addition, she often cradled the dog in her arms, which allowed the canine’s back to come in contact with her inner forearms. To her knowledge, the dog did not lick or ingest the medication.
The patient had taken the dog to her veterinarian. On physical exam, the veterinarian noted that the dog had nipple and vulvar enlargement but no vaginal discharge, vaginal bleeding, skin changes, or urine abnormalities.
THE (PET’S) DIAGNOSIS, THE PATIENT’S Rx
The veterinarian diagnosed the Chihuahua with vaginal hyperplasia and vulvar enlargement secondary to hyperestrogenism. The animal’s symptoms were likely caused by exposure to the owner’s hormone replacement therapy (HRT) medication—the estradiol spray. The veterinarian advised the woman to return to her family physician to discuss her use of the topical estrogen.
The patient asked her physician (SS) to change her HRT formulation. She was given a prescription for an estradiol 0.05 mg/24-hour transdermal patch to be placed on her abdomen twice weekly. After 2 weeks of using the patch therapy, the patient’s menopausal symptoms were reported to be well controlled. In addition, the companion animal’s breast and vulvar changes resolved, as did the dog’s licking behavior.
DISCUSSION
Estrogen therapy, with or without progesterone, is the most effective treatment for postmenopausal vasomotor symptoms.1 Given the concerns raised in the Women’s Health Initiative (WHI) and other clinical trials regarding hormone therapy and cardiovascular and breast cancer findings, many clinicians look to alternative, nonoral dosage forms to improve the safety profile.
Continue to: Safety of nonroal estrogen therapy
Safety of nonoral estrogen therapy. Administration of nonoral estrogen is associated with avoidance of hepatic first-pass metabolism and a resulting lower impact on hepatic proteins. Thus, data indicate a potentially lower risk for venous thromboembolic events with transdermal estrogen compared to oral estrogen.1 Since the publication of the results of the WHI trials, prescribing patterns in the United States indicate a general decline in the proportion of oral hormones, while transdermal prescription volume has remained steady, and the use of vaginal formulations has increased.2
Topical estrogen formulations. Transdermal or topical delivery of estrogen can be achieved through various formulations, including patches, gels, and a spray. While patches are simple to use, some women display hypersensitivity to the adhesive. Use of gel and spray formulations avoids exposure to adhesives, but these pose a risk of transfer of hormonal ingredients that are not covered by a patch. This risk is amplified by the relative accessibility of the product-specific application sites, which include the arms or thighs. Each manufacturer recommends careful handwashing after handling the product, a specific drying time before the user covers the site with clothing, and avoidance of contact with the application site for a prescribed period of time, usually at least 1 to 2 hours.3-6
Our patient. This case illustrates the importance of discussing the risk of medication transfer to both humans and animals when prescribing individualized hormone therapy. While the Evamist prescribing information specifically addresses the risk of unintentional medication transfer to children, it does not discuss other contact risks.6 In the literature, there have been a limited number of reports on the adverse effects from transdermal or topical human medication transfer to pets. Notably, the American Pet Products Association estimates that in the United States, approximately 90 million dogs and 94 million cats are owned as a pet in 67% of households.7
THE TAKEAWAY
Use of HRT, including transdermal or topical estrogen formulations, is common. Given the large number of companion animals in the United States, physicians should consider that all members of a patient’s household—including pets—may be subject to unintentional secondary exposure to topical estrogen formulations and that they may experience adverse effects. This presents an opportunity for patient education, which can have a larger impact on all occupants of the home.
CORRESPONDENCE
Shannon Scott, DO, FACOFP, Clinical Associate Professor, Arizona College of Osteopathic Medicine, 19389 North 59th Avenue, Glendale, AZ 85308; [email protected].
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. Steinkellner AR, Denison SE, Eldridge SL, et al. A decade of postmenopausal hormone therapy prescribing in the United States: long-term effects of the Women’s Health Initiative. Menopause. 2012;19:616-621.
3. Divigel [package insert]. Bridgewater, NJ: Vertical Pharmaceuticals, LLC; 2014.
4. Elestrin [package insert]. Somerset, NJ: Meda Pharmaceuticals; 2014.
5. Estrogel [package insert]. Herndon, VA: Ascend Therapeutics; 2018.
6. Evamist [package insert]. Minneapolis, MN: Perrigo; 2017.
7. American Pet Products Association. Pet Industry Market Size & Ownership Statistics. www.americanpetproducts.org/press_industrytrends.asp. Accessed November 1, 2019.
1. The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24:728-753.
2. Steinkellner AR, Denison SE, Eldridge SL, et al. A decade of postmenopausal hormone therapy prescribing in the United States: long-term effects of the Women’s Health Initiative. Menopause. 2012;19:616-621.
3. Divigel [package insert]. Bridgewater, NJ: Vertical Pharmaceuticals, LLC; 2014.
4. Elestrin [package insert]. Somerset, NJ: Meda Pharmaceuticals; 2014.
5. Estrogel [package insert]. Herndon, VA: Ascend Therapeutics; 2018.
6. Evamist [package insert]. Minneapolis, MN: Perrigo; 2017.
7. American Pet Products Association. Pet Industry Market Size & Ownership Statistics. www.americanpetproducts.org/press_industrytrends.asp. Accessed November 1, 2019.
Swollen lymph nodes • patient is otherwise "healthy" • Dx?
THE CASE
A 52-year-old woman presented to our family clinic for a well woman exam. The only complaints she had were fatigue, which she attributed to a work day that began at 4 am, and hot flashes. She denied fever, weight loss, abdominal pain, medication use, or recent foreign travel. She had a history of hyperlipidemia and surgical removal of a cutaneous melanoma at age 12.
Her vital signs and physical exam were normal with the exception of 3 enlarged left inguinal lymph nodes and approximately 5 enlarged right inguinal lymph nodes. The nodes were freely moveable and non-tender. No additional lymphadenopathy or splenomegaly was found.
THE DIAGNOSIS
The patient’s work-up included a Pap smear, complete blood count (CBC), comprehensive metabolic panel (CMP), and pelvic and inguinal ultrasound. All tests were normal, except the ultrasound, which revealed 3 solid left inguinal lymph nodes measuring 1.2 to 1.6 cm and 6 solid right inguinal lymph nodes measuring 1.1 to 1.8 cm. An abdominal and pelvic computed tomography (CT) scan with contrast identified nonspecific mesenteric, inguinal, retrocrural, and retroperitoneal adenopathy. An open biopsy of the largest inguinal lymph node revealed follicular lymphoma, a form of non-Hodgkin’s lymphoma. (Hodgkin’s and non-Hodgkin’s lymphoma (NHL) are uncommon causes of inguinal lymphadenopathy.1)
We consulted Oncology and they recommended a positron emission tomography (PET)/CT scan, which showed widespread lymphadenopathy. A bone marrow biopsy confirmed follicular lymphoma grade II, Ann Arbor stage III.
DISCUSSION
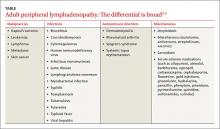
Generalized lymphadenopathy involves lymph node enlargement in more than one region of the body. Lymph nodes >1 cm in adults are considered abnormal and the differential diagnosis is broad (TABLE2-5). A patient’s age is a significant factor in the evaluation of peripheral lymphadenopathy.2-5 Results from one study of 628 patients who underwent nodal biopsy for peripheral lymphadenopathy revealed approximately 80% of nodes in patients under age 30 were noncancerous and likely had an infectious cause.3 However, among patients over age 50, only 40% were noncancerous.3
Node enlargement can be palpated in the head, neck, axilla, inguinal, and popliteal areas. Inguinal lymph nodes up to 2 cm in size may be palpable in healthy patients who spend time barefoot outdoors, have chronic leg trauma or infections, or have sexually transmitted infections.6 However, any lymph node >1 cm in adults should be considered abnormal.2-5
Method of diagnosis depends on malignancy risk
A definitive diagnosis in patients with lymph nodes >1 cm can be made by open lymph node biopsy (the gold standard) or fine needle aspiration (FNA); however, these procedures are rarely needed if malignancy risk is low.
Data on the prevalence of malignant peripheral lymphadenopathy is limited.4 Fijten et al reported that among 2556 patients who presented to a family medicine clinic with unexplained lymphadenopathy, the prevalence of malignancy was as low as 1.1%.7 However, the prevalence of malignant lymph nodes among patients referred to a surgical center for biopsy by primary care physicians was approximately 40% to 60%.3 This highlights the importance of a thorough history, physical exam, and referral when appropriate to increase the yield of diagnostic biopsies.
Low risk for malignancy is suggested when lymphadenopathy is present for less than 2 weeks or persists for more than one year with no increase in size.2 Benign causes such as sexually transmitted infections, Epstein-Barr virus, or medications should be treated appropriately. With no cause identified, 4 weeks of observation is recommended before biopsy.2,4,5,8 CT, PET, and biopsy should be considered early for large, concerning masses. No evidence supports empiric antibiotic use for unknown causes.2,5
High risk for malignancy is suggested in patients who are ≥50 years, present with constitutional symptoms, have lymphadenopathy >1 cm in >2 regions of the body, history of cancer, or have nodes that are rapidly enlarging, firm, fixed, or painless.2,3,5,7,9 Supraclavicular lymphadenopathy has the highest risk for malignancy, especially in patients ≥40 years.7 Enlarged iliac, popliteal, epitrochlear, and umbilical lymph nodes are never normal.2,4,5,7,10 Biopsy should be considered early in these patients.2-4,7 FNA or core needle biopsy is acceptable for an initial diagnosis, but negative results may require open biopsy.1,5,8 Prior to biopsy, imaging with ultrasound is recommended.1,2,8,11
Our patient was offered rituximab alone or rituximab in addition to cyclophosphamide, hydroxydoxorubicin, vincristine, and prednisone (R-CHOP). The patient chose rituximab alone, which resulted in a 30% reduction in the size of her intra-abdominal disease. At this point, the patient and her oncologist chose to stop treatment and monitor her clinically.
Three months later, the patient returned to our family clinic complaining of postnasal drip, throat pain, and neck fullness that she’d had for one month that weren’t responsive to over-the-counter remedies and antibiotics. A supervised osteopathic medical student’s exam revealed right tonsillar enlargement (grade 3+) with minimal erythema and no exudates. A neck CT confirmed right tonsillar enlargement. The patient was referred to Otolaryngology, and the surgeon performed a tonsillectomy that demonstrated disease progression to follicular lymphoma grade IIIa. Given the new findings, Oncology recommended R-CHOP and the patient agreed.
The patient completed R-CHOP and her cancer was in remission one year later.
THE TAKEAWAY
Peripheral lymphadenopathy presents a diagnostic challenge that requires a thorough history and physical exam. General wellness exams should incorporate a comprehensive physical that includes the palpation of lymph nodes. Exam challenges include distinguishing benign lymphadenopathy (reactive lymphadenitis) from malignant lymphadenopathy.
In patients with low risk for malignancy, a period of 4 weeks of observation is reasonable. Biopsy should be considered early for risk factors including patient’s age ≥50, constitutional symptoms, lymphadenopathy >1 cm in >2 regions of the body, history of cancer, or rapidly enlarging nodes.
1. Metzgeroth G, Schneider S, Walz C, et al. Fine needle aspiration and core needle biopsy in the diagnosis of lymphadenopathy of unknown aetiology. Ann Hematol. 2012;91:1477-1484.
2. Bazemore AW, Smucker DR. Lymphadenopathy and malignancy. Am Fam Physician. 2002;66:2103-2110.
3. Lee Y, Terry R, Lukes RJ. Lymph node biopsy for diagnosis: a statistical study. J Surg Oncol. 1980;14:53-60.
4. Ferrer R. Lymphadenopathy: differential diagnosis and evaluation. Am Fam Physician. 1998;58:1313-1320.
5. Motyckova G, Steensma DP. Why does my patient have lymphadenopathy or splenomegaly? Hematol Oncol Clin North Am. 2012;26:395-408.
6. Habermann TM, Steensma DP. Lymphadenopathy. Mayo Clin Proc. 2000;75:723-732.
7. Fijten GH, Blijham GH. Unexplained lymphadenopathy in family practice. An evaluation of the probability of malignant causes and the effectiveness of physicians’ workup. J Fam Pract. 1988;27:373-376.
8. Chau I, Kelleher MT, Cunningham D, et al. Rapid access multidisciplinary lymph node diagnostic clinic: analysis of 550 patients. Br J Cancer. 2003;88:354-361.
9. Vassilakopoulos TP, Pangalis GA. Application of a prediction rule to select which patients presenting with lymphadenopathy should undergo a lymph node biopsy. Medicine (Baltimore). 2000;79:338-347.
10. Dar IH, Kamili MA, Dar SH, et al. Sister Mary Joseph nodule-A case report with review of literature. J Res Med Sci. 2009;14:385-387.
11. Cui XW, Jenssen C, Saftoiu A, et al. New ultrasound techniques for lymph node evaluation. World J Gastroenterol. 2013;19:4850-4860.
THE CASE
A 52-year-old woman presented to our family clinic for a well woman exam. The only complaints she had were fatigue, which she attributed to a work day that began at 4 am, and hot flashes. She denied fever, weight loss, abdominal pain, medication use, or recent foreign travel. She had a history of hyperlipidemia and surgical removal of a cutaneous melanoma at age 12.
Her vital signs and physical exam were normal with the exception of 3 enlarged left inguinal lymph nodes and approximately 5 enlarged right inguinal lymph nodes. The nodes were freely moveable and non-tender. No additional lymphadenopathy or splenomegaly was found.
THE DIAGNOSIS
The patient’s work-up included a Pap smear, complete blood count (CBC), comprehensive metabolic panel (CMP), and pelvic and inguinal ultrasound. All tests were normal, except the ultrasound, which revealed 3 solid left inguinal lymph nodes measuring 1.2 to 1.6 cm and 6 solid right inguinal lymph nodes measuring 1.1 to 1.8 cm. An abdominal and pelvic computed tomography (CT) scan with contrast identified nonspecific mesenteric, inguinal, retrocrural, and retroperitoneal adenopathy. An open biopsy of the largest inguinal lymph node revealed follicular lymphoma, a form of non-Hodgkin’s lymphoma. (Hodgkin’s and non-Hodgkin’s lymphoma (NHL) are uncommon causes of inguinal lymphadenopathy.1)
We consulted Oncology and they recommended a positron emission tomography (PET)/CT scan, which showed widespread lymphadenopathy. A bone marrow biopsy confirmed follicular lymphoma grade II, Ann Arbor stage III.
DISCUSSION
Generalized lymphadenopathy involves lymph node enlargement in more than one region of the body. Lymph nodes >1 cm in adults are considered abnormal and the differential diagnosis is broad (TABLE2-5). A patient’s age is a significant factor in the evaluation of peripheral lymphadenopathy.2-5 Results from one study of 628 patients who underwent nodal biopsy for peripheral lymphadenopathy revealed approximately 80% of nodes in patients under age 30 were noncancerous and likely had an infectious cause.3 However, among patients over age 50, only 40% were noncancerous.3
Node enlargement can be palpated in the head, neck, axilla, inguinal, and popliteal areas. Inguinal lymph nodes up to 2 cm in size may be palpable in healthy patients who spend time barefoot outdoors, have chronic leg trauma or infections, or have sexually transmitted infections.6 However, any lymph node >1 cm in adults should be considered abnormal.2-5
Method of diagnosis depends on malignancy risk
A definitive diagnosis in patients with lymph nodes >1 cm can be made by open lymph node biopsy (the gold standard) or fine needle aspiration (FNA); however, these procedures are rarely needed if malignancy risk is low.
Data on the prevalence of malignant peripheral lymphadenopathy is limited.4 Fijten et al reported that among 2556 patients who presented to a family medicine clinic with unexplained lymphadenopathy, the prevalence of malignancy was as low as 1.1%.7 However, the prevalence of malignant lymph nodes among patients referred to a surgical center for biopsy by primary care physicians was approximately 40% to 60%.3 This highlights the importance of a thorough history, physical exam, and referral when appropriate to increase the yield of diagnostic biopsies.
Low risk for malignancy is suggested when lymphadenopathy is present for less than 2 weeks or persists for more than one year with no increase in size.2 Benign causes such as sexually transmitted infections, Epstein-Barr virus, or medications should be treated appropriately. With no cause identified, 4 weeks of observation is recommended before biopsy.2,4,5,8 CT, PET, and biopsy should be considered early for large, concerning masses. No evidence supports empiric antibiotic use for unknown causes.2,5
High risk for malignancy is suggested in patients who are ≥50 years, present with constitutional symptoms, have lymphadenopathy >1 cm in >2 regions of the body, history of cancer, or have nodes that are rapidly enlarging, firm, fixed, or painless.2,3,5,7,9 Supraclavicular lymphadenopathy has the highest risk for malignancy, especially in patients ≥40 years.7 Enlarged iliac, popliteal, epitrochlear, and umbilical lymph nodes are never normal.2,4,5,7,10 Biopsy should be considered early in these patients.2-4,7 FNA or core needle biopsy is acceptable for an initial diagnosis, but negative results may require open biopsy.1,5,8 Prior to biopsy, imaging with ultrasound is recommended.1,2,8,11
Our patient was offered rituximab alone or rituximab in addition to cyclophosphamide, hydroxydoxorubicin, vincristine, and prednisone (R-CHOP). The patient chose rituximab alone, which resulted in a 30% reduction in the size of her intra-abdominal disease. At this point, the patient and her oncologist chose to stop treatment and monitor her clinically.
Three months later, the patient returned to our family clinic complaining of postnasal drip, throat pain, and neck fullness that she’d had for one month that weren’t responsive to over-the-counter remedies and antibiotics. A supervised osteopathic medical student’s exam revealed right tonsillar enlargement (grade 3+) with minimal erythema and no exudates. A neck CT confirmed right tonsillar enlargement. The patient was referred to Otolaryngology, and the surgeon performed a tonsillectomy that demonstrated disease progression to follicular lymphoma grade IIIa. Given the new findings, Oncology recommended R-CHOP and the patient agreed.
The patient completed R-CHOP and her cancer was in remission one year later.
THE TAKEAWAY
Peripheral lymphadenopathy presents a diagnostic challenge that requires a thorough history and physical exam. General wellness exams should incorporate a comprehensive physical that includes the palpation of lymph nodes. Exam challenges include distinguishing benign lymphadenopathy (reactive lymphadenitis) from malignant lymphadenopathy.
In patients with low risk for malignancy, a period of 4 weeks of observation is reasonable. Biopsy should be considered early for risk factors including patient’s age ≥50, constitutional symptoms, lymphadenopathy >1 cm in >2 regions of the body, history of cancer, or rapidly enlarging nodes.
THE CASE
A 52-year-old woman presented to our family clinic for a well woman exam. The only complaints she had were fatigue, which she attributed to a work day that began at 4 am, and hot flashes. She denied fever, weight loss, abdominal pain, medication use, or recent foreign travel. She had a history of hyperlipidemia and surgical removal of a cutaneous melanoma at age 12.
Her vital signs and physical exam were normal with the exception of 3 enlarged left inguinal lymph nodes and approximately 5 enlarged right inguinal lymph nodes. The nodes were freely moveable and non-tender. No additional lymphadenopathy or splenomegaly was found.
THE DIAGNOSIS
The patient’s work-up included a Pap smear, complete blood count (CBC), comprehensive metabolic panel (CMP), and pelvic and inguinal ultrasound. All tests were normal, except the ultrasound, which revealed 3 solid left inguinal lymph nodes measuring 1.2 to 1.6 cm and 6 solid right inguinal lymph nodes measuring 1.1 to 1.8 cm. An abdominal and pelvic computed tomography (CT) scan with contrast identified nonspecific mesenteric, inguinal, retrocrural, and retroperitoneal adenopathy. An open biopsy of the largest inguinal lymph node revealed follicular lymphoma, a form of non-Hodgkin’s lymphoma. (Hodgkin’s and non-Hodgkin’s lymphoma (NHL) are uncommon causes of inguinal lymphadenopathy.1)
We consulted Oncology and they recommended a positron emission tomography (PET)/CT scan, which showed widespread lymphadenopathy. A bone marrow biopsy confirmed follicular lymphoma grade II, Ann Arbor stage III.
DISCUSSION
Generalized lymphadenopathy involves lymph node enlargement in more than one region of the body. Lymph nodes >1 cm in adults are considered abnormal and the differential diagnosis is broad (TABLE2-5). A patient’s age is a significant factor in the evaluation of peripheral lymphadenopathy.2-5 Results from one study of 628 patients who underwent nodal biopsy for peripheral lymphadenopathy revealed approximately 80% of nodes in patients under age 30 were noncancerous and likely had an infectious cause.3 However, among patients over age 50, only 40% were noncancerous.3
Node enlargement can be palpated in the head, neck, axilla, inguinal, and popliteal areas. Inguinal lymph nodes up to 2 cm in size may be palpable in healthy patients who spend time barefoot outdoors, have chronic leg trauma or infections, or have sexually transmitted infections.6 However, any lymph node >1 cm in adults should be considered abnormal.2-5
Method of diagnosis depends on malignancy risk
A definitive diagnosis in patients with lymph nodes >1 cm can be made by open lymph node biopsy (the gold standard) or fine needle aspiration (FNA); however, these procedures are rarely needed if malignancy risk is low.
Data on the prevalence of malignant peripheral lymphadenopathy is limited.4 Fijten et al reported that among 2556 patients who presented to a family medicine clinic with unexplained lymphadenopathy, the prevalence of malignancy was as low as 1.1%.7 However, the prevalence of malignant lymph nodes among patients referred to a surgical center for biopsy by primary care physicians was approximately 40% to 60%.3 This highlights the importance of a thorough history, physical exam, and referral when appropriate to increase the yield of diagnostic biopsies.
Low risk for malignancy is suggested when lymphadenopathy is present for less than 2 weeks or persists for more than one year with no increase in size.2 Benign causes such as sexually transmitted infections, Epstein-Barr virus, or medications should be treated appropriately. With no cause identified, 4 weeks of observation is recommended before biopsy.2,4,5,8 CT, PET, and biopsy should be considered early for large, concerning masses. No evidence supports empiric antibiotic use for unknown causes.2,5
High risk for malignancy is suggested in patients who are ≥50 years, present with constitutional symptoms, have lymphadenopathy >1 cm in >2 regions of the body, history of cancer, or have nodes that are rapidly enlarging, firm, fixed, or painless.2,3,5,7,9 Supraclavicular lymphadenopathy has the highest risk for malignancy, especially in patients ≥40 years.7 Enlarged iliac, popliteal, epitrochlear, and umbilical lymph nodes are never normal.2,4,5,7,10 Biopsy should be considered early in these patients.2-4,7 FNA or core needle biopsy is acceptable for an initial diagnosis, but negative results may require open biopsy.1,5,8 Prior to biopsy, imaging with ultrasound is recommended.1,2,8,11
Our patient was offered rituximab alone or rituximab in addition to cyclophosphamide, hydroxydoxorubicin, vincristine, and prednisone (R-CHOP). The patient chose rituximab alone, which resulted in a 30% reduction in the size of her intra-abdominal disease. At this point, the patient and her oncologist chose to stop treatment and monitor her clinically.
Three months later, the patient returned to our family clinic complaining of postnasal drip, throat pain, and neck fullness that she’d had for one month that weren’t responsive to over-the-counter remedies and antibiotics. A supervised osteopathic medical student’s exam revealed right tonsillar enlargement (grade 3+) with minimal erythema and no exudates. A neck CT confirmed right tonsillar enlargement. The patient was referred to Otolaryngology, and the surgeon performed a tonsillectomy that demonstrated disease progression to follicular lymphoma grade IIIa. Given the new findings, Oncology recommended R-CHOP and the patient agreed.
The patient completed R-CHOP and her cancer was in remission one year later.
THE TAKEAWAY
Peripheral lymphadenopathy presents a diagnostic challenge that requires a thorough history and physical exam. General wellness exams should incorporate a comprehensive physical that includes the palpation of lymph nodes. Exam challenges include distinguishing benign lymphadenopathy (reactive lymphadenitis) from malignant lymphadenopathy.
In patients with low risk for malignancy, a period of 4 weeks of observation is reasonable. Biopsy should be considered early for risk factors including patient’s age ≥50, constitutional symptoms, lymphadenopathy >1 cm in >2 regions of the body, history of cancer, or rapidly enlarging nodes.
1. Metzgeroth G, Schneider S, Walz C, et al. Fine needle aspiration and core needle biopsy in the diagnosis of lymphadenopathy of unknown aetiology. Ann Hematol. 2012;91:1477-1484.
2. Bazemore AW, Smucker DR. Lymphadenopathy and malignancy. Am Fam Physician. 2002;66:2103-2110.
3. Lee Y, Terry R, Lukes RJ. Lymph node biopsy for diagnosis: a statistical study. J Surg Oncol. 1980;14:53-60.
4. Ferrer R. Lymphadenopathy: differential diagnosis and evaluation. Am Fam Physician. 1998;58:1313-1320.
5. Motyckova G, Steensma DP. Why does my patient have lymphadenopathy or splenomegaly? Hematol Oncol Clin North Am. 2012;26:395-408.
6. Habermann TM, Steensma DP. Lymphadenopathy. Mayo Clin Proc. 2000;75:723-732.
7. Fijten GH, Blijham GH. Unexplained lymphadenopathy in family practice. An evaluation of the probability of malignant causes and the effectiveness of physicians’ workup. J Fam Pract. 1988;27:373-376.
8. Chau I, Kelleher MT, Cunningham D, et al. Rapid access multidisciplinary lymph node diagnostic clinic: analysis of 550 patients. Br J Cancer. 2003;88:354-361.
9. Vassilakopoulos TP, Pangalis GA. Application of a prediction rule to select which patients presenting with lymphadenopathy should undergo a lymph node biopsy. Medicine (Baltimore). 2000;79:338-347.
10. Dar IH, Kamili MA, Dar SH, et al. Sister Mary Joseph nodule-A case report with review of literature. J Res Med Sci. 2009;14:385-387.
11. Cui XW, Jenssen C, Saftoiu A, et al. New ultrasound techniques for lymph node evaluation. World J Gastroenterol. 2013;19:4850-4860.
1. Metzgeroth G, Schneider S, Walz C, et al. Fine needle aspiration and core needle biopsy in the diagnosis of lymphadenopathy of unknown aetiology. Ann Hematol. 2012;91:1477-1484.
2. Bazemore AW, Smucker DR. Lymphadenopathy and malignancy. Am Fam Physician. 2002;66:2103-2110.
3. Lee Y, Terry R, Lukes RJ. Lymph node biopsy for diagnosis: a statistical study. J Surg Oncol. 1980;14:53-60.
4. Ferrer R. Lymphadenopathy: differential diagnosis and evaluation. Am Fam Physician. 1998;58:1313-1320.
5. Motyckova G, Steensma DP. Why does my patient have lymphadenopathy or splenomegaly? Hematol Oncol Clin North Am. 2012;26:395-408.
6. Habermann TM, Steensma DP. Lymphadenopathy. Mayo Clin Proc. 2000;75:723-732.
7. Fijten GH, Blijham GH. Unexplained lymphadenopathy in family practice. An evaluation of the probability of malignant causes and the effectiveness of physicians’ workup. J Fam Pract. 1988;27:373-376.
8. Chau I, Kelleher MT, Cunningham D, et al. Rapid access multidisciplinary lymph node diagnostic clinic: analysis of 550 patients. Br J Cancer. 2003;88:354-361.
9. Vassilakopoulos TP, Pangalis GA. Application of a prediction rule to select which patients presenting with lymphadenopathy should undergo a lymph node biopsy. Medicine (Baltimore). 2000;79:338-347.
10. Dar IH, Kamili MA, Dar SH, et al. Sister Mary Joseph nodule-A case report with review of literature. J Res Med Sci. 2009;14:385-387.
11. Cui XW, Jenssen C, Saftoiu A, et al. New ultrasound techniques for lymph node evaluation. World J Gastroenterol. 2013;19:4850-4860.