User login
Compound could treat T-ALL subtype

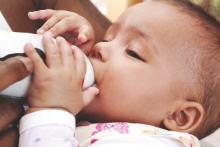
Photo courtesy of
The Ottawa Hospital
An experimental compound is “highly promising” as a potential treatment for a subtype of T-cell acute lymphoblastic leukemia (T-ALL), according to researchers.
The team found the histone demethylase UTX is important for the maintenance of TAL1-positive T-ALL.
Inhibiting UTX with a compound known as GSK-J4 proved toxic to TAL1-positive T-ALL cells in vitro and in vivo, but normal cells were spared.
The researchers reported these findings in Genes & Development.
“It’s very exciting because this is the first time anyone has found a potential personalized treatment for this aggressive disease,” said study author Marjorie Brand, PhD, of The Ottawa Hospital Research Institute in Ontario, Canada.
“Unlike current therapies, ours targets the offending gene without harming the rest of the body.”
Dr Brand and her colleagues decided the best way to find a better treatment for TAL1-positive T-ALL was to investigate exactly how it works at a molecular level. So they analyzed the TAL1 gene, which, in certain circumstances, can transform T-cell precursors into leukemic cells.
They found that TAL1 needs UTX to trigger leukemia production. This was surprising because UTX was previously described as a tumor suppressor in T-ALL.
The team said their work suggests UTX is actually a pro-oncogenic cofactor that is essential for leukemia maintenance in TAL1-positive—but not TAL1-negative—T-ALL.
The researchers found that targeting UTX with the H3K27 demethylase inhibitor GSK-J4 could completely halt the growth of TAL1-positive leukemia cells and induce apoptosis in these cells in vitro. But the compound did not have the same effect in TAL1-negative T-ALL.
The team also tested GSK-J4 in mouse models of T-ALL. After 3 weeks of treatment, there was a “dramatic decrease” in the percentage of leukemic blasts in the bone marrow of mice with TAL1-positive T-ALL. These mice also had a reduction in the infiltration of leukemic cells in the spleen.
However, mice with TAL1-negative T-ALL did not experience the same benefits.
The researchers said GSK-J4 appeared to be well-tolerated. None of the treated mice experienced weight loss or adverse effects in the intestine, spleen, liver, kidney, or hematopoietic system.
“While our study is a proof-of-concept, these promising results might one day lead to a similar targeted treatment for humans,” said study author Carmen Palii, PhD, also of The Ottawa Hospital Research Institute.
In the meantime, the researchers are conducting additional studies in mice, testing higher doses of GSK-J4 and evaluating long-term side effects of the compound. ![]()

Photo courtesy of
The Ottawa Hospital
An experimental compound is “highly promising” as a potential treatment for a subtype of T-cell acute lymphoblastic leukemia (T-ALL), according to researchers.
The team found the histone demethylase UTX is important for the maintenance of TAL1-positive T-ALL.
Inhibiting UTX with a compound known as GSK-J4 proved toxic to TAL1-positive T-ALL cells in vitro and in vivo, but normal cells were spared.
The researchers reported these findings in Genes & Development.
“It’s very exciting because this is the first time anyone has found a potential personalized treatment for this aggressive disease,” said study author Marjorie Brand, PhD, of The Ottawa Hospital Research Institute in Ontario, Canada.
“Unlike current therapies, ours targets the offending gene without harming the rest of the body.”
Dr Brand and her colleagues decided the best way to find a better treatment for TAL1-positive T-ALL was to investigate exactly how it works at a molecular level. So they analyzed the TAL1 gene, which, in certain circumstances, can transform T-cell precursors into leukemic cells.
They found that TAL1 needs UTX to trigger leukemia production. This was surprising because UTX was previously described as a tumor suppressor in T-ALL.
The team said their work suggests UTX is actually a pro-oncogenic cofactor that is essential for leukemia maintenance in TAL1-positive—but not TAL1-negative—T-ALL.
The researchers found that targeting UTX with the H3K27 demethylase inhibitor GSK-J4 could completely halt the growth of TAL1-positive leukemia cells and induce apoptosis in these cells in vitro. But the compound did not have the same effect in TAL1-negative T-ALL.
The team also tested GSK-J4 in mouse models of T-ALL. After 3 weeks of treatment, there was a “dramatic decrease” in the percentage of leukemic blasts in the bone marrow of mice with TAL1-positive T-ALL. These mice also had a reduction in the infiltration of leukemic cells in the spleen.
However, mice with TAL1-negative T-ALL did not experience the same benefits.
The researchers said GSK-J4 appeared to be well-tolerated. None of the treated mice experienced weight loss or adverse effects in the intestine, spleen, liver, kidney, or hematopoietic system.
“While our study is a proof-of-concept, these promising results might one day lead to a similar targeted treatment for humans,” said study author Carmen Palii, PhD, also of The Ottawa Hospital Research Institute.
In the meantime, the researchers are conducting additional studies in mice, testing higher doses of GSK-J4 and evaluating long-term side effects of the compound. ![]()

Photo courtesy of
The Ottawa Hospital
An experimental compound is “highly promising” as a potential treatment for a subtype of T-cell acute lymphoblastic leukemia (T-ALL), according to researchers.
The team found the histone demethylase UTX is important for the maintenance of TAL1-positive T-ALL.
Inhibiting UTX with a compound known as GSK-J4 proved toxic to TAL1-positive T-ALL cells in vitro and in vivo, but normal cells were spared.
The researchers reported these findings in Genes & Development.
“It’s very exciting because this is the first time anyone has found a potential personalized treatment for this aggressive disease,” said study author Marjorie Brand, PhD, of The Ottawa Hospital Research Institute in Ontario, Canada.
“Unlike current therapies, ours targets the offending gene without harming the rest of the body.”
Dr Brand and her colleagues decided the best way to find a better treatment for TAL1-positive T-ALL was to investigate exactly how it works at a molecular level. So they analyzed the TAL1 gene, which, in certain circumstances, can transform T-cell precursors into leukemic cells.
They found that TAL1 needs UTX to trigger leukemia production. This was surprising because UTX was previously described as a tumor suppressor in T-ALL.
The team said their work suggests UTX is actually a pro-oncogenic cofactor that is essential for leukemia maintenance in TAL1-positive—but not TAL1-negative—T-ALL.
The researchers found that targeting UTX with the H3K27 demethylase inhibitor GSK-J4 could completely halt the growth of TAL1-positive leukemia cells and induce apoptosis in these cells in vitro. But the compound did not have the same effect in TAL1-negative T-ALL.
The team also tested GSK-J4 in mouse models of T-ALL. After 3 weeks of treatment, there was a “dramatic decrease” in the percentage of leukemic blasts in the bone marrow of mice with TAL1-positive T-ALL. These mice also had a reduction in the infiltration of leukemic cells in the spleen.
However, mice with TAL1-negative T-ALL did not experience the same benefits.
The researchers said GSK-J4 appeared to be well-tolerated. None of the treated mice experienced weight loss or adverse effects in the intestine, spleen, liver, kidney, or hematopoietic system.
“While our study is a proof-of-concept, these promising results might one day lead to a similar targeted treatment for humans,” said study author Carmen Palii, PhD, also of The Ottawa Hospital Research Institute.
In the meantime, the researchers are conducting additional studies in mice, testing higher doses of GSK-J4 and evaluating long-term side effects of the compound. ![]()
Survey shows Clinical Practice in Management of EOS in Newborns Varies
NEW YORK (Reuters Health) - Clinical practice in management of early-onset sepsis (EOS) in newborns varies widely across Europe, North America and Asia, new survey results show.
National guidelines also disagree on when to start antibiotics in low-risk situations, and how to decide to stop therapy in high-risk scenarios, Dr. Wendy van Herk of Erasmus MC University Medical Center-Sophia Children's Hospital in Rotterdam, the Netherlands, and colleagues found."
A discussion leading to terms of a threshold to treat neonates with low infection risk, prospective studies ofstrategies regarding early discontinuation of unnecessary antibiotic therapy with safety endpoints acknowledging different backgrounds of health care systems, and clear and concise guidelines followed by research to study the impact are mandatory to improve management of term and late preterm infants at risk for EOS," they write in their report, online January 13 in The Pediatric Infectious Disease Journal.
Up to 15% of term and late-preterm neonates are evaluated for suspected EOS, and 10% receive intravenous antibiotics within the first three days of life, the researchers note. But the incidence of culture-proven EOS in term and late-preterm newborns is less than 0.1%, they add.
To investigate current management of suspected EOS, the researchers surveyed pediatricians and neonatologists and reviewed guidelines from Canada, the United States, the United Kingdom, Switzerland and Belgium. A total of 439 clinicians responded to the survey.
In response to a question about whether they would start antibiotic treatment in a scenario rated "low risk" for EOS, 29% of physicians said they would, 26% would not, and 45% said they would start treatment if the patients' laboratory markers were abnormal. Nearly all of the respondents (99%) said they would initiate antibiotics in a high-risk scenario.
In the low-risk situation, 89% said they would stop antibiotic treatment before 72 hours. In the high-risk scenario, 35% said they would stop antibiotics before 72 hours, 56% said they would continue treatment for five to seven days, and 9% said they would treat patients for more than seven days.
Overall, 31% of the survey respondents said they would base their decision to start antibiotic treatment on laboratory investigations, while 72% said they would do so when deciding to continue treatment. Most said they would use complete blood count (CBC) and C-reactive protein (CRP), while a small minority said they would use newer inflammation markers including procalcitonin (PCT) and interleukins.
While all the guidelines reviewed recommended treating newborns with clinical signs indicating infection, and re-evaluating whether patients needed more antibiotics at 36 to 48 hours, they did not provide specific advice on treatment when newborns had prolonged clinical signs of infection or high levels of infection markers. All guidelines recommended using CBC or CRP, while only one included PCT.
Dr. van Herk and colleagues also compared the guidelines for each country with the survey responses of physicians from that country, and found most followed national guidelines on when to start or discontinue antibiotics.
"The diversity with regards to duration of antibiotic therapy in higher risk situations raises the question, what are safe strategies to minimize duration of antibiotic therapy without under-treatment of truly septic neonates?" the authors write. "Currently, the duration of antibiotic therapy is controversial even for proven infection. Prospective, international, multicenter trials studying newer infection markers with a safety endpoint may be helpful in answering this question."
NEW YORK (Reuters Health) - Clinical practice in management of early-onset sepsis (EOS) in newborns varies widely across Europe, North America and Asia, new survey results show.
National guidelines also disagree on when to start antibiotics in low-risk situations, and how to decide to stop therapy in high-risk scenarios, Dr. Wendy van Herk of Erasmus MC University Medical Center-Sophia Children's Hospital in Rotterdam, the Netherlands, and colleagues found."
A discussion leading to terms of a threshold to treat neonates with low infection risk, prospective studies ofstrategies regarding early discontinuation of unnecessary antibiotic therapy with safety endpoints acknowledging different backgrounds of health care systems, and clear and concise guidelines followed by research to study the impact are mandatory to improve management of term and late preterm infants at risk for EOS," they write in their report, online January 13 in The Pediatric Infectious Disease Journal.
Up to 15% of term and late-preterm neonates are evaluated for suspected EOS, and 10% receive intravenous antibiotics within the first three days of life, the researchers note. But the incidence of culture-proven EOS in term and late-preterm newborns is less than 0.1%, they add.
To investigate current management of suspected EOS, the researchers surveyed pediatricians and neonatologists and reviewed guidelines from Canada, the United States, the United Kingdom, Switzerland and Belgium. A total of 439 clinicians responded to the survey.
In response to a question about whether they would start antibiotic treatment in a scenario rated "low risk" for EOS, 29% of physicians said they would, 26% would not, and 45% said they would start treatment if the patients' laboratory markers were abnormal. Nearly all of the respondents (99%) said they would initiate antibiotics in a high-risk scenario.
In the low-risk situation, 89% said they would stop antibiotic treatment before 72 hours. In the high-risk scenario, 35% said they would stop antibiotics before 72 hours, 56% said they would continue treatment for five to seven days, and 9% said they would treat patients for more than seven days.
Overall, 31% of the survey respondents said they would base their decision to start antibiotic treatment on laboratory investigations, while 72% said they would do so when deciding to continue treatment. Most said they would use complete blood count (CBC) and C-reactive protein (CRP), while a small minority said they would use newer inflammation markers including procalcitonin (PCT) and interleukins.
While all the guidelines reviewed recommended treating newborns with clinical signs indicating infection, and re-evaluating whether patients needed more antibiotics at 36 to 48 hours, they did not provide specific advice on treatment when newborns had prolonged clinical signs of infection or high levels of infection markers. All guidelines recommended using CBC or CRP, while only one included PCT.
Dr. van Herk and colleagues also compared the guidelines for each country with the survey responses of physicians from that country, and found most followed national guidelines on when to start or discontinue antibiotics.
"The diversity with regards to duration of antibiotic therapy in higher risk situations raises the question, what are safe strategies to minimize duration of antibiotic therapy without under-treatment of truly septic neonates?" the authors write. "Currently, the duration of antibiotic therapy is controversial even for proven infection. Prospective, international, multicenter trials studying newer infection markers with a safety endpoint may be helpful in answering this question."
NEW YORK (Reuters Health) - Clinical practice in management of early-onset sepsis (EOS) in newborns varies widely across Europe, North America and Asia, new survey results show.
National guidelines also disagree on when to start antibiotics in low-risk situations, and how to decide to stop therapy in high-risk scenarios, Dr. Wendy van Herk of Erasmus MC University Medical Center-Sophia Children's Hospital in Rotterdam, the Netherlands, and colleagues found."
A discussion leading to terms of a threshold to treat neonates with low infection risk, prospective studies ofstrategies regarding early discontinuation of unnecessary antibiotic therapy with safety endpoints acknowledging different backgrounds of health care systems, and clear and concise guidelines followed by research to study the impact are mandatory to improve management of term and late preterm infants at risk for EOS," they write in their report, online January 13 in The Pediatric Infectious Disease Journal.
Up to 15% of term and late-preterm neonates are evaluated for suspected EOS, and 10% receive intravenous antibiotics within the first three days of life, the researchers note. But the incidence of culture-proven EOS in term and late-preterm newborns is less than 0.1%, they add.
To investigate current management of suspected EOS, the researchers surveyed pediatricians and neonatologists and reviewed guidelines from Canada, the United States, the United Kingdom, Switzerland and Belgium. A total of 439 clinicians responded to the survey.
In response to a question about whether they would start antibiotic treatment in a scenario rated "low risk" for EOS, 29% of physicians said they would, 26% would not, and 45% said they would start treatment if the patients' laboratory markers were abnormal. Nearly all of the respondents (99%) said they would initiate antibiotics in a high-risk scenario.
In the low-risk situation, 89% said they would stop antibiotic treatment before 72 hours. In the high-risk scenario, 35% said they would stop antibiotics before 72 hours, 56% said they would continue treatment for five to seven days, and 9% said they would treat patients for more than seven days.
Overall, 31% of the survey respondents said they would base their decision to start antibiotic treatment on laboratory investigations, while 72% said they would do so when deciding to continue treatment. Most said they would use complete blood count (CBC) and C-reactive protein (CRP), while a small minority said they would use newer inflammation markers including procalcitonin (PCT) and interleukins.
While all the guidelines reviewed recommended treating newborns with clinical signs indicating infection, and re-evaluating whether patients needed more antibiotics at 36 to 48 hours, they did not provide specific advice on treatment when newborns had prolonged clinical signs of infection or high levels of infection markers. All guidelines recommended using CBC or CRP, while only one included PCT.
Dr. van Herk and colleagues also compared the guidelines for each country with the survey responses of physicians from that country, and found most followed national guidelines on when to start or discontinue antibiotics.
"The diversity with regards to duration of antibiotic therapy in higher risk situations raises the question, what are safe strategies to minimize duration of antibiotic therapy without under-treatment of truly septic neonates?" the authors write. "Currently, the duration of antibiotic therapy is controversial even for proven infection. Prospective, international, multicenter trials studying newer infection markers with a safety endpoint may be helpful in answering this question."
Best Practices: Infant Formula Comparison and Recommendations
A supplement to Pediatric News. This supplement is sponsored by Perrigo Nutritionals.
Topics
- Food Insecurity and Formula Stretching
- Addressing Food Insecurity’s Effects with Store Brand Infant Formula
- Is Switching Formula Safe?
- Conclusion
Faculty/Faculty Disclosure
Jenifer R. Lightdale, MD, MPH
Division Chief, Pediatric Gastroenterology, Hepatology and Nutrition
UMass Memorial Children’s Medical Center
Professor of Pediatrics
University of Massachusetts Medical School
Worcester, MA
Dr. Lightdale reports that she is on the medical advisory board for Perrigo Nutritionals, and is an invited speaker for Mead Johnson & Company, LLC.
A supplement to Pediatric News. This supplement is sponsored by Perrigo Nutritionals.
Topics
- Food Insecurity and Formula Stretching
- Addressing Food Insecurity’s Effects with Store Brand Infant Formula
- Is Switching Formula Safe?
- Conclusion
Faculty/Faculty Disclosure
Jenifer R. Lightdale, MD, MPH
Division Chief, Pediatric Gastroenterology, Hepatology and Nutrition
UMass Memorial Children’s Medical Center
Professor of Pediatrics
University of Massachusetts Medical School
Worcester, MA
Dr. Lightdale reports that she is on the medical advisory board for Perrigo Nutritionals, and is an invited speaker for Mead Johnson & Company, LLC.
A supplement to Pediatric News. This supplement is sponsored by Perrigo Nutritionals.
Topics
- Food Insecurity and Formula Stretching
- Addressing Food Insecurity’s Effects with Store Brand Infant Formula
- Is Switching Formula Safe?
- Conclusion
Faculty/Faculty Disclosure
Jenifer R. Lightdale, MD, MPH
Division Chief, Pediatric Gastroenterology, Hepatology and Nutrition
UMass Memorial Children’s Medical Center
Professor of Pediatrics
University of Massachusetts Medical School
Worcester, MA
Dr. Lightdale reports that she is on the medical advisory board for Perrigo Nutritionals, and is an invited speaker for Mead Johnson & Company, LLC.
No evidence supports hydrolyzed formula over cows’ milk for allergy prevention
Findings on the use of hydrolyzed formula in place of standard cows’ milk formula to prevent allergy in high-risk infants do not support current guidelines, according to Dr. Robert J Boyle of Imperial College London and his associates.
A review and meta-analysis were performed on 28 randomized control trials, 6 quasirandomized trials, and 3 controlled clinical trials describing allergic or autoimmune outcomes, with more than 19,000 participants. Among 13 studies reporting on the risk of food allergy, no significant difference was found in the risk of any food allergy with partially hydrolyzed formula (risk ratio, 1.73; 95% confidence interval, 0.79-3.80) and extensively hydrolyzed formula (RR, 0.86; CI, 0.26-2.82), compared with standard formula at age 0-4 years, and for extensively hydrolyzed formula at age 5-14 years.
The review also examined and found no significant evidence favoring the use of hydrolyzed formula in place of standard cows’ milk formula to avert the risk of eczema, wheeze, allergic rhinitis, or type 1 diabetes mellitus.
The researchers suggest that guidelines be updated and revised to reflect these new findings.
“We found no consistent evidence to support the current recommendations and found evidence of publication bias, methodological biases, and conflict of interest in those studies reporting allergic outcomes,” Dr. Boyle and his associates concluded. “We suggest that any future trials on hydrolyzed formula should be prospectively registered, independently funded, and include adequate oversight to ensure that they do not negatively impact on breastfeeding in study participants”.
Read the full study at the British Medical Journal (doi: 10.1136/bmj.i974)
Findings on the use of hydrolyzed formula in place of standard cows’ milk formula to prevent allergy in high-risk infants do not support current guidelines, according to Dr. Robert J Boyle of Imperial College London and his associates.
A review and meta-analysis were performed on 28 randomized control trials, 6 quasirandomized trials, and 3 controlled clinical trials describing allergic or autoimmune outcomes, with more than 19,000 participants. Among 13 studies reporting on the risk of food allergy, no significant difference was found in the risk of any food allergy with partially hydrolyzed formula (risk ratio, 1.73; 95% confidence interval, 0.79-3.80) and extensively hydrolyzed formula (RR, 0.86; CI, 0.26-2.82), compared with standard formula at age 0-4 years, and for extensively hydrolyzed formula at age 5-14 years.
The review also examined and found no significant evidence favoring the use of hydrolyzed formula in place of standard cows’ milk formula to avert the risk of eczema, wheeze, allergic rhinitis, or type 1 diabetes mellitus.
The researchers suggest that guidelines be updated and revised to reflect these new findings.
“We found no consistent evidence to support the current recommendations and found evidence of publication bias, methodological biases, and conflict of interest in those studies reporting allergic outcomes,” Dr. Boyle and his associates concluded. “We suggest that any future trials on hydrolyzed formula should be prospectively registered, independently funded, and include adequate oversight to ensure that they do not negatively impact on breastfeeding in study participants”.
Read the full study at the British Medical Journal (doi: 10.1136/bmj.i974)
Findings on the use of hydrolyzed formula in place of standard cows’ milk formula to prevent allergy in high-risk infants do not support current guidelines, according to Dr. Robert J Boyle of Imperial College London and his associates.
A review and meta-analysis were performed on 28 randomized control trials, 6 quasirandomized trials, and 3 controlled clinical trials describing allergic or autoimmune outcomes, with more than 19,000 participants. Among 13 studies reporting on the risk of food allergy, no significant difference was found in the risk of any food allergy with partially hydrolyzed formula (risk ratio, 1.73; 95% confidence interval, 0.79-3.80) and extensively hydrolyzed formula (RR, 0.86; CI, 0.26-2.82), compared with standard formula at age 0-4 years, and for extensively hydrolyzed formula at age 5-14 years.
The review also examined and found no significant evidence favoring the use of hydrolyzed formula in place of standard cows’ milk formula to avert the risk of eczema, wheeze, allergic rhinitis, or type 1 diabetes mellitus.
The researchers suggest that guidelines be updated and revised to reflect these new findings.
“We found no consistent evidence to support the current recommendations and found evidence of publication bias, methodological biases, and conflict of interest in those studies reporting allergic outcomes,” Dr. Boyle and his associates concluded. “We suggest that any future trials on hydrolyzed formula should be prospectively registered, independently funded, and include adequate oversight to ensure that they do not negatively impact on breastfeeding in study participants”.
Read the full study at the British Medical Journal (doi: 10.1136/bmj.i974)
FROM BRITISH MEDICAL JOURNAL
Cosmetic Corner: Dermatologists Weigh in on Hand Creams
To improve patient care and outcomes, leading dermatologists offered their recommendations on hand creams. Consideration must be given to:
- CeraVe Therapeutic Hand Cream
- Maximum Body Repair
- Neutrogena Norwegian Formula Hand Cream
- O’Keeffe’s Working Hands
Cutis invites readers to send us their recommendations. Scar treatments, body scrubs, and OTC acne treatments will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on hand creams. Consideration must be given to:
- CeraVe Therapeutic Hand Cream
- Maximum Body Repair
- Neutrogena Norwegian Formula Hand Cream
- O’Keeffe’s Working Hands
Cutis invites readers to send us their recommendations. Scar treatments, body scrubs, and OTC acne treatments will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on hand creams. Consideration must be given to:
- CeraVe Therapeutic Hand Cream
- Maximum Body Repair
- Neutrogena Norwegian Formula Hand Cream
- O’Keeffe’s Working Hands
Cutis invites readers to send us their recommendations. Scar treatments, body scrubs, and OTC acne treatments will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Core outcomes needed for endometriosis research
The clinical usefulness of endometriosis research is being hindered by a lack of a uniform core outcomes set in published trials, according to the findings of a systematic review.
Martin Hirsch of Barts and The London School of Medicine and Dentistry and his colleagues reviewed 54 randomized controlled trials with 5,427 participants evaluating a surgical intervention – with or without medical adjuvant therapy – for the treatment of endometriosis symptoms. They included all randomized, controlled trials in the Cochrane Central Register of Controlled Trials, Embase, and MEDLINE from inception to November 2014 and found a wide variation in the outcomes reported.
Across all trials, there were 164 outcomes and 113 outcomes measures reported. The three most commonly reported primary outcomes were dysmenorrhea (23 trials), dyspareunia (21 trials), and pregnancy (26 trials).
This level of variation among trials makes it difficult to make comparisons and synthesize data, according to the researchers. “This limits the usefulness of research to inform clinical practice, enhance patient care, and improve patient outcomes.”
Mr. Hirsch and his colleagues called for an international consensus on a core outcome set for endometriosis trials. Until then, they suggested the use of the three most common pain-related outcomes – dysmenorrhea, dyspareunia, and pelvic pain – as well as subfertility outcomes, measured by pregnancy, miscarriage, and live birth.
Read the full study in the American Journal of Obstetrics & Gynecology (doi: 10.1016/j.ajog.2015.12.039).
On Twitter @maryellenny
The clinical usefulness of endometriosis research is being hindered by a lack of a uniform core outcomes set in published trials, according to the findings of a systematic review.
Martin Hirsch of Barts and The London School of Medicine and Dentistry and his colleagues reviewed 54 randomized controlled trials with 5,427 participants evaluating a surgical intervention – with or without medical adjuvant therapy – for the treatment of endometriosis symptoms. They included all randomized, controlled trials in the Cochrane Central Register of Controlled Trials, Embase, and MEDLINE from inception to November 2014 and found a wide variation in the outcomes reported.
Across all trials, there were 164 outcomes and 113 outcomes measures reported. The three most commonly reported primary outcomes were dysmenorrhea (23 trials), dyspareunia (21 trials), and pregnancy (26 trials).
This level of variation among trials makes it difficult to make comparisons and synthesize data, according to the researchers. “This limits the usefulness of research to inform clinical practice, enhance patient care, and improve patient outcomes.”
Mr. Hirsch and his colleagues called for an international consensus on a core outcome set for endometriosis trials. Until then, they suggested the use of the three most common pain-related outcomes – dysmenorrhea, dyspareunia, and pelvic pain – as well as subfertility outcomes, measured by pregnancy, miscarriage, and live birth.
Read the full study in the American Journal of Obstetrics & Gynecology (doi: 10.1016/j.ajog.2015.12.039).
On Twitter @maryellenny
The clinical usefulness of endometriosis research is being hindered by a lack of a uniform core outcomes set in published trials, according to the findings of a systematic review.
Martin Hirsch of Barts and The London School of Medicine and Dentistry and his colleagues reviewed 54 randomized controlled trials with 5,427 participants evaluating a surgical intervention – with or without medical adjuvant therapy – for the treatment of endometriosis symptoms. They included all randomized, controlled trials in the Cochrane Central Register of Controlled Trials, Embase, and MEDLINE from inception to November 2014 and found a wide variation in the outcomes reported.
Across all trials, there were 164 outcomes and 113 outcomes measures reported. The three most commonly reported primary outcomes were dysmenorrhea (23 trials), dyspareunia (21 trials), and pregnancy (26 trials).
This level of variation among trials makes it difficult to make comparisons and synthesize data, according to the researchers. “This limits the usefulness of research to inform clinical practice, enhance patient care, and improve patient outcomes.”
Mr. Hirsch and his colleagues called for an international consensus on a core outcome set for endometriosis trials. Until then, they suggested the use of the three most common pain-related outcomes – dysmenorrhea, dyspareunia, and pelvic pain – as well as subfertility outcomes, measured by pregnancy, miscarriage, and live birth.
Read the full study in the American Journal of Obstetrics & Gynecology (doi: 10.1016/j.ajog.2015.12.039).
On Twitter @maryellenny
Society of Hospital Medicine Awards Three with Exclusive 'Masters in Hospital Medicine' Designation
SAN DIEGO—The Society of Hospital Medicine (SHM) today named three new Masters in Hospital Medicine at HM16, the largest meeting dedicated to the hospital medicine specialty. The Master in Hospital Medicine (MHM) designation was introduced in 2010 to honor those who have uniquely distinguished themselves in hospital medicine through excellence in the field and healthcare as a whole.
“As hospital medicine continues to evolve, we look to experts in the field to lead us into the future with their innovation and vision,” SHM President Brian Harte, MD, SFHM, says. “These accomplished individuals have played foundational roles in hospital medicine’s growth and success and continue to ensure hospitalists are equipped with the tools and resources they need to provide the highest quality of patient care.”
SHM is proud to announce the following MHMs for their outstanding contributions:
- Tina L. Budnitz, MPH, MHM, for her leadership in advancing the hospital medicine movement and SHM. Throughout her time with SHM, Budnitz has led the development of the Core Competencies for Hospital Medicine to define the skills of a practicing hospitalist, launched Project BOOST, a mentored implementation program to improve care transitions that was recognized with the 2012 John M. Eisenberg Patient Safety and Quality Award and assisted in the development of SHM’s Leadership Academy and Certificate of Leadership Program, designed to build the healthcare leaders of tomorrow.
- Eric E. Howell, MD, MHM, Chief of the Division of Hospital Medicine at Johns Hopkins Bayview Medical Center in Baltimore, MD, in recognition of his foundational leadership in the hospital medicine movement and SHM. Dr. Howell has been instrumental in managing and implementing change in the hospital, including conducting research on the relationship between the emergency department and medicine floors as well as improving communication, throughput and patient outcomes. A past President of SHM, Dr. Howell serves as the Senior Physician Advisor to SHM’s Center for Hospital Innovation and Improvement and has mentored numerous hospitals and hospital medicine programs in more than six countries.
- Gregory Maynard, MD, MSc, MHM, Chief Quality Officer at the University of California Davis Medical Center in Sacramento, CA, in honor of his leadership in local, regional and national quality improvement efforts and related contributions to SHM’s quality improvement programs. A longtime member of the SHM Hospital Quality and Patient Safety Committee, Dr. Maynard played an integral role in creating and leading SHM’s mentored implementation programs to enhance care transitions, improve glycemic control and prevent venous thromboembolism (VTE). Dr. Maynard is nationally recognized in each of these areas, making significant contributions to the medical community.
For details on SHM’s fellowship designations, visit www.hospitalmedicine.org/fellows.
SAN DIEGO—The Society of Hospital Medicine (SHM) today named three new Masters in Hospital Medicine at HM16, the largest meeting dedicated to the hospital medicine specialty. The Master in Hospital Medicine (MHM) designation was introduced in 2010 to honor those who have uniquely distinguished themselves in hospital medicine through excellence in the field and healthcare as a whole.
“As hospital medicine continues to evolve, we look to experts in the field to lead us into the future with their innovation and vision,” SHM President Brian Harte, MD, SFHM, says. “These accomplished individuals have played foundational roles in hospital medicine’s growth and success and continue to ensure hospitalists are equipped with the tools and resources they need to provide the highest quality of patient care.”
SHM is proud to announce the following MHMs for their outstanding contributions:
- Tina L. Budnitz, MPH, MHM, for her leadership in advancing the hospital medicine movement and SHM. Throughout her time with SHM, Budnitz has led the development of the Core Competencies for Hospital Medicine to define the skills of a practicing hospitalist, launched Project BOOST, a mentored implementation program to improve care transitions that was recognized with the 2012 John M. Eisenberg Patient Safety and Quality Award and assisted in the development of SHM’s Leadership Academy and Certificate of Leadership Program, designed to build the healthcare leaders of tomorrow.
- Eric E. Howell, MD, MHM, Chief of the Division of Hospital Medicine at Johns Hopkins Bayview Medical Center in Baltimore, MD, in recognition of his foundational leadership in the hospital medicine movement and SHM. Dr. Howell has been instrumental in managing and implementing change in the hospital, including conducting research on the relationship between the emergency department and medicine floors as well as improving communication, throughput and patient outcomes. A past President of SHM, Dr. Howell serves as the Senior Physician Advisor to SHM’s Center for Hospital Innovation and Improvement and has mentored numerous hospitals and hospital medicine programs in more than six countries.
- Gregory Maynard, MD, MSc, MHM, Chief Quality Officer at the University of California Davis Medical Center in Sacramento, CA, in honor of his leadership in local, regional and national quality improvement efforts and related contributions to SHM’s quality improvement programs. A longtime member of the SHM Hospital Quality and Patient Safety Committee, Dr. Maynard played an integral role in creating and leading SHM’s mentored implementation programs to enhance care transitions, improve glycemic control and prevent venous thromboembolism (VTE). Dr. Maynard is nationally recognized in each of these areas, making significant contributions to the medical community.
For details on SHM’s fellowship designations, visit www.hospitalmedicine.org/fellows.
SAN DIEGO—The Society of Hospital Medicine (SHM) today named three new Masters in Hospital Medicine at HM16, the largest meeting dedicated to the hospital medicine specialty. The Master in Hospital Medicine (MHM) designation was introduced in 2010 to honor those who have uniquely distinguished themselves in hospital medicine through excellence in the field and healthcare as a whole.
“As hospital medicine continues to evolve, we look to experts in the field to lead us into the future with their innovation and vision,” SHM President Brian Harte, MD, SFHM, says. “These accomplished individuals have played foundational roles in hospital medicine’s growth and success and continue to ensure hospitalists are equipped with the tools and resources they need to provide the highest quality of patient care.”
SHM is proud to announce the following MHMs for their outstanding contributions:
- Tina L. Budnitz, MPH, MHM, for her leadership in advancing the hospital medicine movement and SHM. Throughout her time with SHM, Budnitz has led the development of the Core Competencies for Hospital Medicine to define the skills of a practicing hospitalist, launched Project BOOST, a mentored implementation program to improve care transitions that was recognized with the 2012 John M. Eisenberg Patient Safety and Quality Award and assisted in the development of SHM’s Leadership Academy and Certificate of Leadership Program, designed to build the healthcare leaders of tomorrow.
- Eric E. Howell, MD, MHM, Chief of the Division of Hospital Medicine at Johns Hopkins Bayview Medical Center in Baltimore, MD, in recognition of his foundational leadership in the hospital medicine movement and SHM. Dr. Howell has been instrumental in managing and implementing change in the hospital, including conducting research on the relationship between the emergency department and medicine floors as well as improving communication, throughput and patient outcomes. A past President of SHM, Dr. Howell serves as the Senior Physician Advisor to SHM’s Center for Hospital Innovation and Improvement and has mentored numerous hospitals and hospital medicine programs in more than six countries.
- Gregory Maynard, MD, MSc, MHM, Chief Quality Officer at the University of California Davis Medical Center in Sacramento, CA, in honor of his leadership in local, regional and national quality improvement efforts and related contributions to SHM’s quality improvement programs. A longtime member of the SHM Hospital Quality and Patient Safety Committee, Dr. Maynard played an integral role in creating and leading SHM’s mentored implementation programs to enhance care transitions, improve glycemic control and prevent venous thromboembolism (VTE). Dr. Maynard is nationally recognized in each of these areas, making significant contributions to the medical community.
For details on SHM’s fellowship designations, visit www.hospitalmedicine.org/fellows.
The problem of office theft
Why do people steal stuff from my office?
I’m not talking about pens. I’ve unintentionally walked off with more pens then I can count over the years, and never realized that I did until later. I figure others do the same with mine.
In the last few years, I’ve had a Far Side cartoon book stolen from the lobby, a stapler off my secretary’s desk, a roll of medical tape from my EMG cart, and a few other items.
Most recently, my secretary bought a candy dish at the store. It was nothing fancy, just a few bucks, but she liked it. She set it out on the front desk with some Jolly Ranchers.
A few days later, she left her desk to refill her coffee cup. While in back she heard the front door of the office open and close. When she returned up front, the dish (and candy) were gone.
None of these are a major financial loss, maybe adding up to $15-$20 a year at most. But it’s irritating to have someone steal something minor from my office.
Taking pens, or even magazines, is perhaps understandable, at times unintentional. But to reach over a desk and grab a stapler, or to walk in, grab a candy dish, and leave, are volitional and just wrong. I don’t understand this. Do people feel that, because I’m a doctor (and therefore stereotyped as rich), I can afford it? Do they do it because, since they’re giving me a copay and letting me bill their insurance, they feel entitled to something back? Or are they angry at me over something, and this is a passive-aggressive way to get even?
I don’t know. Admittedly, it’s a tiny minority who do such things. The vast majority of people wouldn’t dream of stealing a $3 candy dish from an office. But still, it points to a sad level of dishonesty among a few of the routine people I see day in and day out. I’m pretty sure they aren’t so hard up that they need to steal such petty items, either. I imagine the black market value of a used stapler is pretty low.
P.S. If someone out there is willing to return the candy dish or the Beyond The Far Side cartoon book, no questions will be asked.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Why do people steal stuff from my office?
I’m not talking about pens. I’ve unintentionally walked off with more pens then I can count over the years, and never realized that I did until later. I figure others do the same with mine.
In the last few years, I’ve had a Far Side cartoon book stolen from the lobby, a stapler off my secretary’s desk, a roll of medical tape from my EMG cart, and a few other items.
Most recently, my secretary bought a candy dish at the store. It was nothing fancy, just a few bucks, but she liked it. She set it out on the front desk with some Jolly Ranchers.
A few days later, she left her desk to refill her coffee cup. While in back she heard the front door of the office open and close. When she returned up front, the dish (and candy) were gone.
None of these are a major financial loss, maybe adding up to $15-$20 a year at most. But it’s irritating to have someone steal something minor from my office.
Taking pens, or even magazines, is perhaps understandable, at times unintentional. But to reach over a desk and grab a stapler, or to walk in, grab a candy dish, and leave, are volitional and just wrong. I don’t understand this. Do people feel that, because I’m a doctor (and therefore stereotyped as rich), I can afford it? Do they do it because, since they’re giving me a copay and letting me bill their insurance, they feel entitled to something back? Or are they angry at me over something, and this is a passive-aggressive way to get even?
I don’t know. Admittedly, it’s a tiny minority who do such things. The vast majority of people wouldn’t dream of stealing a $3 candy dish from an office. But still, it points to a sad level of dishonesty among a few of the routine people I see day in and day out. I’m pretty sure they aren’t so hard up that they need to steal such petty items, either. I imagine the black market value of a used stapler is pretty low.
P.S. If someone out there is willing to return the candy dish or the Beyond The Far Side cartoon book, no questions will be asked.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Why do people steal stuff from my office?
I’m not talking about pens. I’ve unintentionally walked off with more pens then I can count over the years, and never realized that I did until later. I figure others do the same with mine.
In the last few years, I’ve had a Far Side cartoon book stolen from the lobby, a stapler off my secretary’s desk, a roll of medical tape from my EMG cart, and a few other items.
Most recently, my secretary bought a candy dish at the store. It was nothing fancy, just a few bucks, but she liked it. She set it out on the front desk with some Jolly Ranchers.
A few days later, she left her desk to refill her coffee cup. While in back she heard the front door of the office open and close. When she returned up front, the dish (and candy) were gone.
None of these are a major financial loss, maybe adding up to $15-$20 a year at most. But it’s irritating to have someone steal something minor from my office.
Taking pens, or even magazines, is perhaps understandable, at times unintentional. But to reach over a desk and grab a stapler, or to walk in, grab a candy dish, and leave, are volitional and just wrong. I don’t understand this. Do people feel that, because I’m a doctor (and therefore stereotyped as rich), I can afford it? Do they do it because, since they’re giving me a copay and letting me bill their insurance, they feel entitled to something back? Or are they angry at me over something, and this is a passive-aggressive way to get even?
I don’t know. Admittedly, it’s a tiny minority who do such things. The vast majority of people wouldn’t dream of stealing a $3 candy dish from an office. But still, it points to a sad level of dishonesty among a few of the routine people I see day in and day out. I’m pretty sure they aren’t so hard up that they need to steal such petty items, either. I imagine the black market value of a used stapler is pretty low.
P.S. If someone out there is willing to return the candy dish or the Beyond The Far Side cartoon book, no questions will be asked.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
HM16 Session Analysis: Physician Engagement in Quality Improvement
Presenter: Jordan Messler, MD, SHFM
Summary: The main objective of this lecture was to understand the culture that often limits physician engagement. It also offered insights on how to best understand motivators for engagement, and tried to focus on strategies to improve and create an environment for physician engagement.
Despite strong evidence, there remains a refusal to confront healthcare provider’s severe quality problems. There is a high rate of failure, considering that 80% of major initiatives don’t meet their objectives. Dr. Messler pointed out that the second principle of the "Key Characteristics of an Effective Hospital Medicine Group" is an engaged hospitalist. To make this more complicated, 40% of hospitalists report inpatient census that exceed safe levels at least once a month; and 52% of hospitalists have signs of burnout.
Dr. Messler explained are intrinsic and extrinsic motivators: the culture of the group will impact the extrinsic motivation factors when it tries to encourage physician to do their work because they expected of themselves, not because some else is looking over their shoulders. Among intrinsic motivators: a sense of autonomy, mastery and purpose drives a culture of not only do the work but also improve it. Some hospitalist groups are trying to explore new approaches, including protective time for physicians to allow them to get involved in committee and QI projects.
Applying behavioral economics concepts (science of human motivation) can help HMGs design incentives among such domains as inertia (by simplifying processes), immediacy (giving bonus right after achieving goals), mental accounting (using paper checks for rewards).
HM Takeaways:
- There is lack of awareness of physician disengagement.
- Burn out is the opposite of engagement and affects patient quality.
- There are intrinsic and extrinsic factors that drives engagement.
- By creating a culture of ownership, mastery, autonomy, and rediscovery of purpose and right mix of incentives physicians can engage more.
- SHM has an Engagement Survey that can help get to know baseline motivators driving among specific groups.
Dr. Villagra is a hospitalist in Batesville, Ark., and a member of Team Hospitalist.
Presenter: Jordan Messler, MD, SHFM
Summary: The main objective of this lecture was to understand the culture that often limits physician engagement. It also offered insights on how to best understand motivators for engagement, and tried to focus on strategies to improve and create an environment for physician engagement.
Despite strong evidence, there remains a refusal to confront healthcare provider’s severe quality problems. There is a high rate of failure, considering that 80% of major initiatives don’t meet their objectives. Dr. Messler pointed out that the second principle of the "Key Characteristics of an Effective Hospital Medicine Group" is an engaged hospitalist. To make this more complicated, 40% of hospitalists report inpatient census that exceed safe levels at least once a month; and 52% of hospitalists have signs of burnout.
Dr. Messler explained are intrinsic and extrinsic motivators: the culture of the group will impact the extrinsic motivation factors when it tries to encourage physician to do their work because they expected of themselves, not because some else is looking over their shoulders. Among intrinsic motivators: a sense of autonomy, mastery and purpose drives a culture of not only do the work but also improve it. Some hospitalist groups are trying to explore new approaches, including protective time for physicians to allow them to get involved in committee and QI projects.
Applying behavioral economics concepts (science of human motivation) can help HMGs design incentives among such domains as inertia (by simplifying processes), immediacy (giving bonus right after achieving goals), mental accounting (using paper checks for rewards).
HM Takeaways:
- There is lack of awareness of physician disengagement.
- Burn out is the opposite of engagement and affects patient quality.
- There are intrinsic and extrinsic factors that drives engagement.
- By creating a culture of ownership, mastery, autonomy, and rediscovery of purpose and right mix of incentives physicians can engage more.
- SHM has an Engagement Survey that can help get to know baseline motivators driving among specific groups.
Dr. Villagra is a hospitalist in Batesville, Ark., and a member of Team Hospitalist.
Presenter: Jordan Messler, MD, SHFM
Summary: The main objective of this lecture was to understand the culture that often limits physician engagement. It also offered insights on how to best understand motivators for engagement, and tried to focus on strategies to improve and create an environment for physician engagement.
Despite strong evidence, there remains a refusal to confront healthcare provider’s severe quality problems. There is a high rate of failure, considering that 80% of major initiatives don’t meet their objectives. Dr. Messler pointed out that the second principle of the "Key Characteristics of an Effective Hospital Medicine Group" is an engaged hospitalist. To make this more complicated, 40% of hospitalists report inpatient census that exceed safe levels at least once a month; and 52% of hospitalists have signs of burnout.
Dr. Messler explained are intrinsic and extrinsic motivators: the culture of the group will impact the extrinsic motivation factors when it tries to encourage physician to do their work because they expected of themselves, not because some else is looking over their shoulders. Among intrinsic motivators: a sense of autonomy, mastery and purpose drives a culture of not only do the work but also improve it. Some hospitalist groups are trying to explore new approaches, including protective time for physicians to allow them to get involved in committee and QI projects.
Applying behavioral economics concepts (science of human motivation) can help HMGs design incentives among such domains as inertia (by simplifying processes), immediacy (giving bonus right after achieving goals), mental accounting (using paper checks for rewards).
HM Takeaways:
- There is lack of awareness of physician disengagement.
- Burn out is the opposite of engagement and affects patient quality.
- There are intrinsic and extrinsic factors that drives engagement.
- By creating a culture of ownership, mastery, autonomy, and rediscovery of purpose and right mix of incentives physicians can engage more.
- SHM has an Engagement Survey that can help get to know baseline motivators driving among specific groups.
Dr. Villagra is a hospitalist in Batesville, Ark., and a member of Team Hospitalist.
Data don’t support use of induction chemotherapy in head and neck cancer
SCOTTSDALE, ARIZ. – Patients with head and neck cancer undergoing radiation therapy do not have better survival if given induction chemotherapy instead of concurrent chemotherapy, finds a cohort study reported at the Multidisciplinary Head and Neck Cancer Symposium.
Using the National Cancer Data Base, Dr. Daniel W. Bowles and his colleagues analyzed outcomes for 8,003 patients treated for nonmetastatic but more advanced disease with one of these two approaches.
Results reported in a poster session and related press briefing showed that the patients given induction chemotherapy were more likely to receive radiation doses lower than those recommended in guidelines and lived, on average, about 13 months less.
“The use of induction chemotherapy is not supported by this analysis,” commented Dr. Bowles, director of cancer research at the University of Colorado at Denver, Aurora, and staff physician at the Denver VA Medical Center.
The study – the largest yet to compare the two approaches and specifically in a cohort of patients with more advanced disease – adds to others that have hinted at inferior outcomes with induction chemotherapy as compared with concurrent chemotherapy, the standard of care.
“Recently, there have been several randomized controlled studies that have looked at induction chemotherapy followed by concurrent chemoradiation versus concurrent chemoradiation alone,” he elaborated. “These studies have had somewhat varied results, but have been critiqued as being underpowered and [the possibility] that no survival benefit was seen in the induction chemotherapy arm perhaps because there were too many low-risk cancers that were included in these studies, including patients who have stage III cancer or patients who had N0 to N2a disease.”
Press briefing moderator Dr. Randall J. Kimple of the University of Wisconsin–Madison, commented, “I think this study adds to the growing data that I would say is now nearly overwhelming that induction chemotherapy, other than in maybe very selected cases, has essentially no role in the treatment of head and neck cancer patients in a routine setting and outside of the setting of a clinical trial.”
The investigators included in their analysis patients with stage Tis-T4,N2b-3,M0 squamous cell carcinoma of the oropharynx, hypopharynx, or larynx diagnosed between 2003 and 2011 and treated with external-beam radiation therapy, without surgery.
Analyses were based on 1,907 patients given induction chemotherapy (starting 43 to 98 days before radiation therapy) and 6,086 patients given concurrent chemotherapy (starting within 7 days of radiation therapy).
In univariate analyses, median overall survival was 52.1 months with induction chemotherapy versus 64.9 months with concurrent chemotherapy, reported Dr. Bowles, who disclosed that he had no relevant conflicts of interest. The difference translated to a 14% higher risk of death with the former (hazard ratio, 1.14; P less than .01).
In multivariate analysis, survival did not differ significantly between the two chemotherapy approaches in the cohort as a whole or in various subgroups of patients having especially advanced disease: those with T4 or N3 disease, with N3 disease only, or with T4N3 disease. Repeating analyses after propensity score matching yielded essentially the same results.
“We couldn’t identify any specific subgroups that appeared to benefit with regards to overall survival,” Dr. Bowles commented, while also noting some caveats.
“One potential limitation from looking at the National Cancer Data Base is that they only provide information about overall survival. We don’t know about cancer-specific survival, so you can’t say based on these data whether there is a progression-free survival benefit. The other question is how this affects quality of life,” he elaborated. “Those are important questions, [whether] induction chemotherapy would benefit anyone with regards to those outcomes. We just don’t have that data from the National Cancer Data Base.”
In other study findings, compared with peers given concurrent chemotherapy, patients given induction chemotherapy were more likely to receive a radiation dose less than the minimum of 66 Gy recommended by the National Comprehensive Cancer Network and the American Society for Radiation Oncology (20.9% vs. 14.9%, P less than .01).
In multivariate analyses, patients given induction chemotherapy were still less likely to receive guideline-concordant doses of radiation (odds ratio, 1.42; P less than .01), and receipt of such doses was associated with an increased risk of death (HR, 1.76; P less than .01).
SCOTTSDALE, ARIZ. – Patients with head and neck cancer undergoing radiation therapy do not have better survival if given induction chemotherapy instead of concurrent chemotherapy, finds a cohort study reported at the Multidisciplinary Head and Neck Cancer Symposium.
Using the National Cancer Data Base, Dr. Daniel W. Bowles and his colleagues analyzed outcomes for 8,003 patients treated for nonmetastatic but more advanced disease with one of these two approaches.
Results reported in a poster session and related press briefing showed that the patients given induction chemotherapy were more likely to receive radiation doses lower than those recommended in guidelines and lived, on average, about 13 months less.
“The use of induction chemotherapy is not supported by this analysis,” commented Dr. Bowles, director of cancer research at the University of Colorado at Denver, Aurora, and staff physician at the Denver VA Medical Center.
The study – the largest yet to compare the two approaches and specifically in a cohort of patients with more advanced disease – adds to others that have hinted at inferior outcomes with induction chemotherapy as compared with concurrent chemotherapy, the standard of care.
“Recently, there have been several randomized controlled studies that have looked at induction chemotherapy followed by concurrent chemoradiation versus concurrent chemoradiation alone,” he elaborated. “These studies have had somewhat varied results, but have been critiqued as being underpowered and [the possibility] that no survival benefit was seen in the induction chemotherapy arm perhaps because there were too many low-risk cancers that were included in these studies, including patients who have stage III cancer or patients who had N0 to N2a disease.”
Press briefing moderator Dr. Randall J. Kimple of the University of Wisconsin–Madison, commented, “I think this study adds to the growing data that I would say is now nearly overwhelming that induction chemotherapy, other than in maybe very selected cases, has essentially no role in the treatment of head and neck cancer patients in a routine setting and outside of the setting of a clinical trial.”
The investigators included in their analysis patients with stage Tis-T4,N2b-3,M0 squamous cell carcinoma of the oropharynx, hypopharynx, or larynx diagnosed between 2003 and 2011 and treated with external-beam radiation therapy, without surgery.
Analyses were based on 1,907 patients given induction chemotherapy (starting 43 to 98 days before radiation therapy) and 6,086 patients given concurrent chemotherapy (starting within 7 days of radiation therapy).
In univariate analyses, median overall survival was 52.1 months with induction chemotherapy versus 64.9 months with concurrent chemotherapy, reported Dr. Bowles, who disclosed that he had no relevant conflicts of interest. The difference translated to a 14% higher risk of death with the former (hazard ratio, 1.14; P less than .01).
In multivariate analysis, survival did not differ significantly between the two chemotherapy approaches in the cohort as a whole or in various subgroups of patients having especially advanced disease: those with T4 or N3 disease, with N3 disease only, or with T4N3 disease. Repeating analyses after propensity score matching yielded essentially the same results.
“We couldn’t identify any specific subgroups that appeared to benefit with regards to overall survival,” Dr. Bowles commented, while also noting some caveats.
“One potential limitation from looking at the National Cancer Data Base is that they only provide information about overall survival. We don’t know about cancer-specific survival, so you can’t say based on these data whether there is a progression-free survival benefit. The other question is how this affects quality of life,” he elaborated. “Those are important questions, [whether] induction chemotherapy would benefit anyone with regards to those outcomes. We just don’t have that data from the National Cancer Data Base.”
In other study findings, compared with peers given concurrent chemotherapy, patients given induction chemotherapy were more likely to receive a radiation dose less than the minimum of 66 Gy recommended by the National Comprehensive Cancer Network and the American Society for Radiation Oncology (20.9% vs. 14.9%, P less than .01).
In multivariate analyses, patients given induction chemotherapy were still less likely to receive guideline-concordant doses of radiation (odds ratio, 1.42; P less than .01), and receipt of such doses was associated with an increased risk of death (HR, 1.76; P less than .01).
SCOTTSDALE, ARIZ. – Patients with head and neck cancer undergoing radiation therapy do not have better survival if given induction chemotherapy instead of concurrent chemotherapy, finds a cohort study reported at the Multidisciplinary Head and Neck Cancer Symposium.
Using the National Cancer Data Base, Dr. Daniel W. Bowles and his colleagues analyzed outcomes for 8,003 patients treated for nonmetastatic but more advanced disease with one of these two approaches.
Results reported in a poster session and related press briefing showed that the patients given induction chemotherapy were more likely to receive radiation doses lower than those recommended in guidelines and lived, on average, about 13 months less.
“The use of induction chemotherapy is not supported by this analysis,” commented Dr. Bowles, director of cancer research at the University of Colorado at Denver, Aurora, and staff physician at the Denver VA Medical Center.
The study – the largest yet to compare the two approaches and specifically in a cohort of patients with more advanced disease – adds to others that have hinted at inferior outcomes with induction chemotherapy as compared with concurrent chemotherapy, the standard of care.
“Recently, there have been several randomized controlled studies that have looked at induction chemotherapy followed by concurrent chemoradiation versus concurrent chemoradiation alone,” he elaborated. “These studies have had somewhat varied results, but have been critiqued as being underpowered and [the possibility] that no survival benefit was seen in the induction chemotherapy arm perhaps because there were too many low-risk cancers that were included in these studies, including patients who have stage III cancer or patients who had N0 to N2a disease.”
Press briefing moderator Dr. Randall J. Kimple of the University of Wisconsin–Madison, commented, “I think this study adds to the growing data that I would say is now nearly overwhelming that induction chemotherapy, other than in maybe very selected cases, has essentially no role in the treatment of head and neck cancer patients in a routine setting and outside of the setting of a clinical trial.”
The investigators included in their analysis patients with stage Tis-T4,N2b-3,M0 squamous cell carcinoma of the oropharynx, hypopharynx, or larynx diagnosed between 2003 and 2011 and treated with external-beam radiation therapy, without surgery.
Analyses were based on 1,907 patients given induction chemotherapy (starting 43 to 98 days before radiation therapy) and 6,086 patients given concurrent chemotherapy (starting within 7 days of radiation therapy).
In univariate analyses, median overall survival was 52.1 months with induction chemotherapy versus 64.9 months with concurrent chemotherapy, reported Dr. Bowles, who disclosed that he had no relevant conflicts of interest. The difference translated to a 14% higher risk of death with the former (hazard ratio, 1.14; P less than .01).
In multivariate analysis, survival did not differ significantly between the two chemotherapy approaches in the cohort as a whole or in various subgroups of patients having especially advanced disease: those with T4 or N3 disease, with N3 disease only, or with T4N3 disease. Repeating analyses after propensity score matching yielded essentially the same results.
“We couldn’t identify any specific subgroups that appeared to benefit with regards to overall survival,” Dr. Bowles commented, while also noting some caveats.
“One potential limitation from looking at the National Cancer Data Base is that they only provide information about overall survival. We don’t know about cancer-specific survival, so you can’t say based on these data whether there is a progression-free survival benefit. The other question is how this affects quality of life,” he elaborated. “Those are important questions, [whether] induction chemotherapy would benefit anyone with regards to those outcomes. We just don’t have that data from the National Cancer Data Base.”
In other study findings, compared with peers given concurrent chemotherapy, patients given induction chemotherapy were more likely to receive a radiation dose less than the minimum of 66 Gy recommended by the National Comprehensive Cancer Network and the American Society for Radiation Oncology (20.9% vs. 14.9%, P less than .01).
In multivariate analyses, patients given induction chemotherapy were still less likely to receive guideline-concordant doses of radiation (odds ratio, 1.42; P less than .01), and receipt of such doses was associated with an increased risk of death (HR, 1.76; P less than .01).
AT THE MULTIDISCIPLINARY HEAD AND NECK CANCER SYMPOSIUM
Key clinical point: Induction chemotherapy has no survival advantage, compared with concurrent chemotherapy in patients with head and neck cancer.
Major finding: Median overall survival was 52.1 months with induction chemotherapy versus 64.9 months with concurrent chemotherapy.
Data source: A cohort study of 8,003 patients with more advanced head and neck cancer given radiation therapy plus either induction or concurrent chemotherapy (National Cancer Data Base).
Disclosures: Dr. Bowles disclosed that he had no relevant conflicts of interest.