User login
Inpatient Hospital Pain Management
Pain management is an integral component of patient‐centered medical care and is a major concern for patients who are hospitalized.[1] Patient‐reported ratings of pain management are highly correlated with overall satisfaction with healthcare delivery.[2] Current research indicates that patient satisfaction with pain management may be improving[3]; however, there may be structural and county‐level disparities in these improvements in satisfaction. Although patient satisfaction with pain management increased from 2008 to 2012, a discrepancy in patient satisfaction with pain management has emerged between 3 different hospital systems (safety net, acute care, critical access hospitals)[3] Specifically, acute care hospitals provide less satisfactory pain management as compared to critical access hospitals.[3] Although patients' perception of pain management is an integral part of delivering patient‐centered care, prior research indicates that there may not be a simple inverse association between pain intensity score and patient satisfaction.[4] The management of pain in hospitals continues to be problematic, perhaps, for instance, due to discrepancies in understanding the relationship between patient satisfaction and pain management. Certainly for this reason and many others, satisfaction with pain management is now one of the dimensions assessed by the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey, which is a global measure of patient satisfaction.
The HCAHPS survey is utilized by 85% of all US‐based hospitals and gathers patient satisfaction information pertaining to 10 dimensions, including pain management. Patient satisfaction scores (via HCAHPS) now constitute 30% of Hospital Value‐Based Purchasing (HVBP), which makes up 2% of at‐risk reimbursements by the Centers for Medicare and Medicaid Services (CMS) as put forth by the Affordable Care Act (ACA) of 2010.[5] The ACA mandates that payments to hospitals must partly depend on metrics that assess patient satisfaction, as broadly measured by the HCAHPS, which are completed by patients upon hospital discharge.[5, 6] Therefore, patient satisfaction, as measured by patients, now directly affects CMS payments for over 3000 hospitals across the United States. This constitutes a large amount of money for most hospitals that operate on high revenue but have low profit margins. As such, the 2% at‐risk reimbursement may place many hospitals at financial risk that could be ameliorated with effective inpatient pain management.
In addition to its critical role in reimbursement to hospitals, patient satisfaction with pain management is also integrally related to providing patient‐centered care. As such, patient satisfaction with pain management is considered a critical element of various models of the patient‐centered approach to providing medical care. Although a medical inpatient team can assess objective signs of pain, patient‐centric pain measurements are paramount in understanding the pain experience of patients and providing adequate pain management care. Moreover, patients, doctors, payers of medical services, and now CMS increasingly regard a patient‐centered approach to medical care as crucial for the delivery of high‐quality care.
HCAHPS survey sampling represents an excellent opportunity to help assess current gaps in patient‐centered clinical care. However, ecological factors, such as county‐level demographics and hospital size (eg, bed number), are known to influence health outcomes but have not been adequately studied in pain management patient satisfaction.[7] Hospital and county‐level factors may influence the degree to which patients experience patient‐centered pain management care. For instance, most patient satisfaction scores are worse in urban areas.[8, 9] These disparities in patient satisfaction scores could be associated with population density, greater ethnic diversity or nonEnglish‐speaking individuals, or number of hospital beds.
The US Census demographics and hospital‐bed number provide a concurrent measure that can be used across the country to estimate hospital ecology. This study evaluated the influence of county‐level demographic and structural factors (ie, hospital beds) on patient satisfaction with hospital pain management in all HCAHPS‐participating hospitals across the United States. We hypothesized that demographic diversity, higher population density, and higher numbers of hospital beds would predict lower levels of patient satisfaction with inpatient pain management.
METHODS
Data Collection: County‐Level Predictors
Publically available data were obtained from the American Hospital Directory[10] and United States Census Bureau[11] websites. Twenty US Census data categories were selected a priori by their clinical relevance to influence pain management perception out of the 50 publically reported US Census categories. Final variables utilized in regression modeling are listed under the Variable column in Table 1. Covariate correlation coefficients were all under 0.7, indicating a lack of significant colinearity.
| Variable | Median Value (SD) | Range | Regression Coefficient (SE) | t Value |
|---|---|---|---|---|
| ||||
| African American alone, % | 5.6% (13.8%) | 0%85.4% | 0.02 (00) | 3.609* |
| White alone, % | 86.2% (15.8%) | 5.3%99.0% | 0.06 (0.01) | 6.661* |
| Per capita income | $24,499 ($6,419) | $7,887$61,290 | 0.00 (0.00) | 7.561* |
| With bachelor's degree, % | 22.0% (10.1%) | 6.3%70.7% | 0.06 (0.01) | 7.348* |
| Population 18 years of age, % | 23.2% (3.1%) | 8.3%40.6% | 0.18 (0.05) | 3.498* |
| With a high school degree, % | 86.0% (6.4%) | 46.3%98.6% | 0.02 (0.01) | 1.424 |
| Population change over 1 year, % | 0.7% (2.2%) | 18.1%25.6% | 0.25 (0.04) | 5.645* |
| Same house over 1 year, % | 85.4% (4.2%) | 57.1%98.0% | 0.01 (0.02) | 0.493 |
| White alone (not Hispanic), % | 75.2% (21.8%) | 3.2%98.4% | 0.05(0.00) | 12.077* |
| Household size | 2.52 (0.3) | 1.924.77 | 2.266 (0.36) | 6.283* |
| Population county | 105,937 (1,524,223) | 1,1609,818,605 | 0.00 (0.00) | 13.117* |
| Average travel time to work, min | 23 (5.0) | 642.5 | 0.21 (0.02) | 11.071* |
| NonEnglish speaking, % | 8.6% (15.1%) | 0.2%95.9% | 0.08 (0.01) | 13.843* |
| Total female, % | 50.7% (1.6%) | 34.4%57.0% | 0.44 (0.06) | 7.489* |
| Population 65 years old, % | 14.7% (4.1%) | 5.8%49.3% | 0.06 (0.02) | 2.697 |
| Population in poverty, % | 14.7% (5.6%) | 5.8%49.3% | 0.02 (0.02) | 1.01 |
| Population density | 138.7 (4,534) | 0.369,467 | 0.73 (0.05) | 15.734* |
| Foreign born, % | 4.9% (9.3%) | 0%51.2% | 0.15 (0.01) | 16.775* |
| Median household income | $46,880 ($12,868) | $20,206$120,096 | 0.00 (0.00) | 6.052* |
| No. of hospital beds | 103 (193) | 22,259 | 0.01 (0.00) | 15.403* |
Data Collection: Patient Satisfaction With Pain Management
Pain management was measured using the HCAHPS survey pain management dimension by calculating the percentage of patient responders who said their pain was always controlled. HCAHPS data are publically available on the CMS Hospital Compare website.[6] It contains 32 questions that comprise 10 evaluative measures. It is provided to a random sample of patients across the United States throughout the year at 48 hours to 6 weeks after discharge from the hospital.
Analytic Plan
HCAHPS and US Census datasets were analyzed to assess their distribution curves. The population density variable was converted to a logarithmic scale to account for its skewed distribution and long tail in the area of low population density. Data were subsequently merged into an Excel (Microsoft Corp., Redmond, WA) spreadsheet using the VLOOKUP function such that relevant 2010 census county data were added to each hospital's HCAHPS data.
Bivariate analyses were conducted to determine which US Census categories were significant predictors for patient satisfaction with pain management. All significant predictors were then included in a multivariate model, which predicted for patient satisfaction with pain management. All analyses were 2‐tailed, and statistical significance was set at = 0.05.
RESULTS
Complete HCAHPS scores were obtained from 3907 hospitals out of a total of 4621 US hospitals (85%). The majority of hospitals (73.8%, n = 2884) collected over 300 surveys, fewer (n = 696) collected 100 to 299 surveys, and a small number of hospitals (n = 327) collected less than 100 surveys. Based on the most conservative estimate, results were available from at least 934,800 individual surveys. Missing HCAHPS hospital data averaged 13.4 (standard deviation [SD] = 12.2) hospitals per state. County‐level data were obtained from all 3144 county or county equivalents across the United States (100%).
Bivariate Analyses
Univariate regression indicated a significant association between pain management patient satisfaction and most county‐level demographic variables and number of hospital beds.
Multivariate Analyses
A multivariate linear regression model was run in which 20 county‐level demographic and hospital factors were examined as predictors of patient satisfaction with pain management. The model, which examined county‐level predictors of pain management, explained 12% of the variability in patients' ratings of pain management (R2 = 0.124, P 0.0001). A total of 8 out of the 20 US Census variables were statistically significant predictors of pain management (Table 2). African American and white race were most strongly associated with higher ratings of patient satisfaction with pain management (ie, by partial coefficient and statistical significance). Number of hospital beds, percent foreign born, population density, and female gender were most strongly related to lower ratings of patient satisfaction with pain management.
| Variable | Median Value (SD) | Range | Regression Coefficient (SE) | t Value | |
|---|---|---|---|---|---|
| |||||
| African American alone, % | 5.6% (13.8%) | 0%85.4% | 0.07 (0.01) | 0.23 | 7.104* |
| White alone, % | 86.2% (15.8%) | 5.3%99.0% | 0.08 (0.01) | 0.23 | 6.953* |
| Per capita income | $24,499 ($6,419) | $7,887$61,290 | 0.00 (0.00) | 0.22 | 2.885 |
| With bachelor's degree, % | 22.0% (10.1%) | 6.3%70.7% | 0.03 (0.02) | 0.10 | 1.401 |
| Population 18 years old, % | 23.2% (3.1%) | 8.3%40.6% | 0.18 (0.05) | 0.08 | 3.498* |
| With a high school degree, % | 86.0% (6.4%) | 46.3%98.6% | 0.02 (0.01) | 0.02 | 1.424 |
| Population change over 1 year, % | 0.7% (2.2%) | 18.1%25.6% | 0.11 (0.06) | 0.01 | 1.986 |
| Same house over 1 year, % | 85.4% (4.2%) | 57.1%98.0% | 0.01 (0.02) | 0.01 | 0.493 |
| White alone (not Hispanic), % | 75.2% (21.8%) | 3.2%98.4% | 0.02(0.00) | 0.01 | 0.740 |
| Household size | 2.52 (0.3) | 1.924.77 | 0.92 (0.80) | 0.03 | 1.145 |
| Population county | 105,937 (1,524,223) | 1,1609,818,605 | 0.00 (0.00) | 0.03 | 1.495 |
| Average travel time to work, min | 23 (5.0) | 642.5 | 0.06 (0.02) | 0.06 | 3.054 |
| NonEnglish speaking, % | 8.6% (15.1%) | 0.2%95.9% | 0.00 (0.03) | 0.06 | 0.028 |
| Total female, % | 50.7% (1.6%) | 34.4%57.0% | 0.23 (0.07) | 0.06 | 3.158 |
| Population 65 years old, % | 14.7% (4.1%) | 5.8%49.3% | 0.10 (0.04) | 0.07 | 2.411 |
| Population in poverty, % | 14.7% (5.6%) | 5.8%49.3% | 0.02 (0.02) | 0.08 | 1.01 |
| Population density | 138.7 (4,534) | 0.369,467 | 0.24 (0.09) | 0.08 | 2.823 |
| Foreign born, % | 4.9% (9.3%) | 0%51.2% | 0.07 (0.02) | 0.12 | 4.906* |
| Median household income | $46,880 ($12,868) | $20,206‐$120,096 | 0.00 (0.00) | 0.16 | 2.599 |
| No. of hospital beds | 103 (193) | 22,259 | 0.00 (0.00) | 0.16 | 9.167* |
| Model statistics | F(1, 9) = 62.222, P 0.001 | ||||
| Adjusted R2 | 0.124 | ||||
DISCUSSION
By utilizing county‐level demographic data and the HCAHPS survey measures from across the United States, this study provides a representative sample of US hospitals that can be used to define ecological trends in patient satisfaction with pain management. This statistical model demonstrates the nonrandom variability of pain management satisfaction across the United States, even after CMS patient‐mix adjustment. Although the quality of pain management may be increasing by some reports, our present results indicate that pain management satisfaction is not equitable with the rest of the country among select groups of patients (eg, foreign born, female gender, areas of long travel times to work) or in certain care settings (eg, larger hospitals, population dense areas). These data suggest that areas of pain management may lack in quality compared to pain management across the entire US as a whole. This is consistent with the increasingly recognized contribution of multiple nonmedical determinates to health outcomes.[12] These results demonstrate the overall magnitude of healthcare disparity in the United States, and are particularly concerning because African Americans and Hispanics tend to rate overall satisfaction higher than Caucasians in other studies.[13, 14] The same minority reporting bias may be reflected in HCAHPS results. These patients may be reporting higher pain management satisfaction that is not consistent with the level of care they received, as studies have consistently indicated worse pain management delivery for racial and ethnic minorities.[15]
The present findings reveal structural (eg, hospital beds) and demographic (eg, population density, foreign born) gaps in satisfaction with pain management. An effort to improve pain management for all people in the heterogeneous makeup of the United States is an enormous challenge. However, change may be forthcoming, as Hospital Value‐Based Purchasing draws attention pain practice inequities in real time. Although several of the significant explanatory variables cannot be modified (eg, size of hospital, urban setting, patients served), pain management delivery should receive extra attention in hospitals with those characteristics. Pain management delivery in large, urban hospitals that serve foreign‐born patients may be improved with focused multilevel interventions. Future research should examine these inequities further and develop multilevel interventions that target hospitals in at‐risk areas with the aim of lessening disparities in hospital‐based pain management.
Disclosure
Nothing to report.
- , , , et al. Interventions for providers to promote a patient‐centred approach in clinical consultations. Cochrane Database Syst Rev. 2012;12:CD003267.
- , , , . Patient perception of pain care in hospitals in the United States. J Pain Res. 2009;2:157–164.
- , , , , . Patient perception of pain care in the United States: a 5‐year comparative analysis of hospital consumer assessment of health care providers and systems. Pain Physician. 2014;17(5):369–377.
- , , , , . Assessing the relationship between the level of pain control and patient satisfaction. J Pain Res. 2013;6:683–689.
- H.R.3590—Patient Protection and Affordable Care Act 2010. Available at: https://www.congress.gov/bill/111th‐congress/house‐bill/3590. Accessed December 1, 2013.
- Centers for Medicare 55(1):125–139.
- , . Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342–343.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- American Hospital Directory. Hospital statistics by state. Available at: http://www.ahd.com/state_statistics.html. Accessed December 1, 2013.
- United States Census Bureau. Download center. Available at: http://factfinder.census.gov/faces/nav/jsf/pages/download_center.xhtml. Accessed December 1, 2013.
- Health policy brief: the relative contribution of multiple determinants to health outcomes. Health Affairs website. Available at: http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=123. Accessed December 1, 2013.
- , , , , . Racial and ethnic differences in patient assessments of interactions with providers: disparities or measurement biases? Am J Med Qual. 2006;21(2):109–114.
- , , , , . Survey response style and differential use of CAHPS rating scales by Hispanics. Med Care. 2008;46(9):963–968.
- Institute of Medicine. Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
Pain management is an integral component of patient‐centered medical care and is a major concern for patients who are hospitalized.[1] Patient‐reported ratings of pain management are highly correlated with overall satisfaction with healthcare delivery.[2] Current research indicates that patient satisfaction with pain management may be improving[3]; however, there may be structural and county‐level disparities in these improvements in satisfaction. Although patient satisfaction with pain management increased from 2008 to 2012, a discrepancy in patient satisfaction with pain management has emerged between 3 different hospital systems (safety net, acute care, critical access hospitals)[3] Specifically, acute care hospitals provide less satisfactory pain management as compared to critical access hospitals.[3] Although patients' perception of pain management is an integral part of delivering patient‐centered care, prior research indicates that there may not be a simple inverse association between pain intensity score and patient satisfaction.[4] The management of pain in hospitals continues to be problematic, perhaps, for instance, due to discrepancies in understanding the relationship between patient satisfaction and pain management. Certainly for this reason and many others, satisfaction with pain management is now one of the dimensions assessed by the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey, which is a global measure of patient satisfaction.
The HCAHPS survey is utilized by 85% of all US‐based hospitals and gathers patient satisfaction information pertaining to 10 dimensions, including pain management. Patient satisfaction scores (via HCAHPS) now constitute 30% of Hospital Value‐Based Purchasing (HVBP), which makes up 2% of at‐risk reimbursements by the Centers for Medicare and Medicaid Services (CMS) as put forth by the Affordable Care Act (ACA) of 2010.[5] The ACA mandates that payments to hospitals must partly depend on metrics that assess patient satisfaction, as broadly measured by the HCAHPS, which are completed by patients upon hospital discharge.[5, 6] Therefore, patient satisfaction, as measured by patients, now directly affects CMS payments for over 3000 hospitals across the United States. This constitutes a large amount of money for most hospitals that operate on high revenue but have low profit margins. As such, the 2% at‐risk reimbursement may place many hospitals at financial risk that could be ameliorated with effective inpatient pain management.
In addition to its critical role in reimbursement to hospitals, patient satisfaction with pain management is also integrally related to providing patient‐centered care. As such, patient satisfaction with pain management is considered a critical element of various models of the patient‐centered approach to providing medical care. Although a medical inpatient team can assess objective signs of pain, patient‐centric pain measurements are paramount in understanding the pain experience of patients and providing adequate pain management care. Moreover, patients, doctors, payers of medical services, and now CMS increasingly regard a patient‐centered approach to medical care as crucial for the delivery of high‐quality care.
HCAHPS survey sampling represents an excellent opportunity to help assess current gaps in patient‐centered clinical care. However, ecological factors, such as county‐level demographics and hospital size (eg, bed number), are known to influence health outcomes but have not been adequately studied in pain management patient satisfaction.[7] Hospital and county‐level factors may influence the degree to which patients experience patient‐centered pain management care. For instance, most patient satisfaction scores are worse in urban areas.[8, 9] These disparities in patient satisfaction scores could be associated with population density, greater ethnic diversity or nonEnglish‐speaking individuals, or number of hospital beds.
The US Census demographics and hospital‐bed number provide a concurrent measure that can be used across the country to estimate hospital ecology. This study evaluated the influence of county‐level demographic and structural factors (ie, hospital beds) on patient satisfaction with hospital pain management in all HCAHPS‐participating hospitals across the United States. We hypothesized that demographic diversity, higher population density, and higher numbers of hospital beds would predict lower levels of patient satisfaction with inpatient pain management.
METHODS
Data Collection: County‐Level Predictors
Publically available data were obtained from the American Hospital Directory[10] and United States Census Bureau[11] websites. Twenty US Census data categories were selected a priori by their clinical relevance to influence pain management perception out of the 50 publically reported US Census categories. Final variables utilized in regression modeling are listed under the Variable column in Table 1. Covariate correlation coefficients were all under 0.7, indicating a lack of significant colinearity.
| Variable | Median Value (SD) | Range | Regression Coefficient (SE) | t Value |
|---|---|---|---|---|
| ||||
| African American alone, % | 5.6% (13.8%) | 0%85.4% | 0.02 (00) | 3.609* |
| White alone, % | 86.2% (15.8%) | 5.3%99.0% | 0.06 (0.01) | 6.661* |
| Per capita income | $24,499 ($6,419) | $7,887$61,290 | 0.00 (0.00) | 7.561* |
| With bachelor's degree, % | 22.0% (10.1%) | 6.3%70.7% | 0.06 (0.01) | 7.348* |
| Population 18 years of age, % | 23.2% (3.1%) | 8.3%40.6% | 0.18 (0.05) | 3.498* |
| With a high school degree, % | 86.0% (6.4%) | 46.3%98.6% | 0.02 (0.01) | 1.424 |
| Population change over 1 year, % | 0.7% (2.2%) | 18.1%25.6% | 0.25 (0.04) | 5.645* |
| Same house over 1 year, % | 85.4% (4.2%) | 57.1%98.0% | 0.01 (0.02) | 0.493 |
| White alone (not Hispanic), % | 75.2% (21.8%) | 3.2%98.4% | 0.05(0.00) | 12.077* |
| Household size | 2.52 (0.3) | 1.924.77 | 2.266 (0.36) | 6.283* |
| Population county | 105,937 (1,524,223) | 1,1609,818,605 | 0.00 (0.00) | 13.117* |
| Average travel time to work, min | 23 (5.0) | 642.5 | 0.21 (0.02) | 11.071* |
| NonEnglish speaking, % | 8.6% (15.1%) | 0.2%95.9% | 0.08 (0.01) | 13.843* |
| Total female, % | 50.7% (1.6%) | 34.4%57.0% | 0.44 (0.06) | 7.489* |
| Population 65 years old, % | 14.7% (4.1%) | 5.8%49.3% | 0.06 (0.02) | 2.697 |
| Population in poverty, % | 14.7% (5.6%) | 5.8%49.3% | 0.02 (0.02) | 1.01 |
| Population density | 138.7 (4,534) | 0.369,467 | 0.73 (0.05) | 15.734* |
| Foreign born, % | 4.9% (9.3%) | 0%51.2% | 0.15 (0.01) | 16.775* |
| Median household income | $46,880 ($12,868) | $20,206$120,096 | 0.00 (0.00) | 6.052* |
| No. of hospital beds | 103 (193) | 22,259 | 0.01 (0.00) | 15.403* |
Data Collection: Patient Satisfaction With Pain Management
Pain management was measured using the HCAHPS survey pain management dimension by calculating the percentage of patient responders who said their pain was always controlled. HCAHPS data are publically available on the CMS Hospital Compare website.[6] It contains 32 questions that comprise 10 evaluative measures. It is provided to a random sample of patients across the United States throughout the year at 48 hours to 6 weeks after discharge from the hospital.
Analytic Plan
HCAHPS and US Census datasets were analyzed to assess their distribution curves. The population density variable was converted to a logarithmic scale to account for its skewed distribution and long tail in the area of low population density. Data were subsequently merged into an Excel (Microsoft Corp., Redmond, WA) spreadsheet using the VLOOKUP function such that relevant 2010 census county data were added to each hospital's HCAHPS data.
Bivariate analyses were conducted to determine which US Census categories were significant predictors for patient satisfaction with pain management. All significant predictors were then included in a multivariate model, which predicted for patient satisfaction with pain management. All analyses were 2‐tailed, and statistical significance was set at = 0.05.
RESULTS
Complete HCAHPS scores were obtained from 3907 hospitals out of a total of 4621 US hospitals (85%). The majority of hospitals (73.8%, n = 2884) collected over 300 surveys, fewer (n = 696) collected 100 to 299 surveys, and a small number of hospitals (n = 327) collected less than 100 surveys. Based on the most conservative estimate, results were available from at least 934,800 individual surveys. Missing HCAHPS hospital data averaged 13.4 (standard deviation [SD] = 12.2) hospitals per state. County‐level data were obtained from all 3144 county or county equivalents across the United States (100%).
Bivariate Analyses
Univariate regression indicated a significant association between pain management patient satisfaction and most county‐level demographic variables and number of hospital beds.
Multivariate Analyses
A multivariate linear regression model was run in which 20 county‐level demographic and hospital factors were examined as predictors of patient satisfaction with pain management. The model, which examined county‐level predictors of pain management, explained 12% of the variability in patients' ratings of pain management (R2 = 0.124, P 0.0001). A total of 8 out of the 20 US Census variables were statistically significant predictors of pain management (Table 2). African American and white race were most strongly associated with higher ratings of patient satisfaction with pain management (ie, by partial coefficient and statistical significance). Number of hospital beds, percent foreign born, population density, and female gender were most strongly related to lower ratings of patient satisfaction with pain management.
| Variable | Median Value (SD) | Range | Regression Coefficient (SE) | t Value | |
|---|---|---|---|---|---|
| |||||
| African American alone, % | 5.6% (13.8%) | 0%85.4% | 0.07 (0.01) | 0.23 | 7.104* |
| White alone, % | 86.2% (15.8%) | 5.3%99.0% | 0.08 (0.01) | 0.23 | 6.953* |
| Per capita income | $24,499 ($6,419) | $7,887$61,290 | 0.00 (0.00) | 0.22 | 2.885 |
| With bachelor's degree, % | 22.0% (10.1%) | 6.3%70.7% | 0.03 (0.02) | 0.10 | 1.401 |
| Population 18 years old, % | 23.2% (3.1%) | 8.3%40.6% | 0.18 (0.05) | 0.08 | 3.498* |
| With a high school degree, % | 86.0% (6.4%) | 46.3%98.6% | 0.02 (0.01) | 0.02 | 1.424 |
| Population change over 1 year, % | 0.7% (2.2%) | 18.1%25.6% | 0.11 (0.06) | 0.01 | 1.986 |
| Same house over 1 year, % | 85.4% (4.2%) | 57.1%98.0% | 0.01 (0.02) | 0.01 | 0.493 |
| White alone (not Hispanic), % | 75.2% (21.8%) | 3.2%98.4% | 0.02(0.00) | 0.01 | 0.740 |
| Household size | 2.52 (0.3) | 1.924.77 | 0.92 (0.80) | 0.03 | 1.145 |
| Population county | 105,937 (1,524,223) | 1,1609,818,605 | 0.00 (0.00) | 0.03 | 1.495 |
| Average travel time to work, min | 23 (5.0) | 642.5 | 0.06 (0.02) | 0.06 | 3.054 |
| NonEnglish speaking, % | 8.6% (15.1%) | 0.2%95.9% | 0.00 (0.03) | 0.06 | 0.028 |
| Total female, % | 50.7% (1.6%) | 34.4%57.0% | 0.23 (0.07) | 0.06 | 3.158 |
| Population 65 years old, % | 14.7% (4.1%) | 5.8%49.3% | 0.10 (0.04) | 0.07 | 2.411 |
| Population in poverty, % | 14.7% (5.6%) | 5.8%49.3% | 0.02 (0.02) | 0.08 | 1.01 |
| Population density | 138.7 (4,534) | 0.369,467 | 0.24 (0.09) | 0.08 | 2.823 |
| Foreign born, % | 4.9% (9.3%) | 0%51.2% | 0.07 (0.02) | 0.12 | 4.906* |
| Median household income | $46,880 ($12,868) | $20,206‐$120,096 | 0.00 (0.00) | 0.16 | 2.599 |
| No. of hospital beds | 103 (193) | 22,259 | 0.00 (0.00) | 0.16 | 9.167* |
| Model statistics | F(1, 9) = 62.222, P 0.001 | ||||
| Adjusted R2 | 0.124 | ||||
DISCUSSION
By utilizing county‐level demographic data and the HCAHPS survey measures from across the United States, this study provides a representative sample of US hospitals that can be used to define ecological trends in patient satisfaction with pain management. This statistical model demonstrates the nonrandom variability of pain management satisfaction across the United States, even after CMS patient‐mix adjustment. Although the quality of pain management may be increasing by some reports, our present results indicate that pain management satisfaction is not equitable with the rest of the country among select groups of patients (eg, foreign born, female gender, areas of long travel times to work) or in certain care settings (eg, larger hospitals, population dense areas). These data suggest that areas of pain management may lack in quality compared to pain management across the entire US as a whole. This is consistent with the increasingly recognized contribution of multiple nonmedical determinates to health outcomes.[12] These results demonstrate the overall magnitude of healthcare disparity in the United States, and are particularly concerning because African Americans and Hispanics tend to rate overall satisfaction higher than Caucasians in other studies.[13, 14] The same minority reporting bias may be reflected in HCAHPS results. These patients may be reporting higher pain management satisfaction that is not consistent with the level of care they received, as studies have consistently indicated worse pain management delivery for racial and ethnic minorities.[15]
The present findings reveal structural (eg, hospital beds) and demographic (eg, population density, foreign born) gaps in satisfaction with pain management. An effort to improve pain management for all people in the heterogeneous makeup of the United States is an enormous challenge. However, change may be forthcoming, as Hospital Value‐Based Purchasing draws attention pain practice inequities in real time. Although several of the significant explanatory variables cannot be modified (eg, size of hospital, urban setting, patients served), pain management delivery should receive extra attention in hospitals with those characteristics. Pain management delivery in large, urban hospitals that serve foreign‐born patients may be improved with focused multilevel interventions. Future research should examine these inequities further and develop multilevel interventions that target hospitals in at‐risk areas with the aim of lessening disparities in hospital‐based pain management.
Disclosure
Nothing to report.
Pain management is an integral component of patient‐centered medical care and is a major concern for patients who are hospitalized.[1] Patient‐reported ratings of pain management are highly correlated with overall satisfaction with healthcare delivery.[2] Current research indicates that patient satisfaction with pain management may be improving[3]; however, there may be structural and county‐level disparities in these improvements in satisfaction. Although patient satisfaction with pain management increased from 2008 to 2012, a discrepancy in patient satisfaction with pain management has emerged between 3 different hospital systems (safety net, acute care, critical access hospitals)[3] Specifically, acute care hospitals provide less satisfactory pain management as compared to critical access hospitals.[3] Although patients' perception of pain management is an integral part of delivering patient‐centered care, prior research indicates that there may not be a simple inverse association between pain intensity score and patient satisfaction.[4] The management of pain in hospitals continues to be problematic, perhaps, for instance, due to discrepancies in understanding the relationship between patient satisfaction and pain management. Certainly for this reason and many others, satisfaction with pain management is now one of the dimensions assessed by the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey, which is a global measure of patient satisfaction.
The HCAHPS survey is utilized by 85% of all US‐based hospitals and gathers patient satisfaction information pertaining to 10 dimensions, including pain management. Patient satisfaction scores (via HCAHPS) now constitute 30% of Hospital Value‐Based Purchasing (HVBP), which makes up 2% of at‐risk reimbursements by the Centers for Medicare and Medicaid Services (CMS) as put forth by the Affordable Care Act (ACA) of 2010.[5] The ACA mandates that payments to hospitals must partly depend on metrics that assess patient satisfaction, as broadly measured by the HCAHPS, which are completed by patients upon hospital discharge.[5, 6] Therefore, patient satisfaction, as measured by patients, now directly affects CMS payments for over 3000 hospitals across the United States. This constitutes a large amount of money for most hospitals that operate on high revenue but have low profit margins. As such, the 2% at‐risk reimbursement may place many hospitals at financial risk that could be ameliorated with effective inpatient pain management.
In addition to its critical role in reimbursement to hospitals, patient satisfaction with pain management is also integrally related to providing patient‐centered care. As such, patient satisfaction with pain management is considered a critical element of various models of the patient‐centered approach to providing medical care. Although a medical inpatient team can assess objective signs of pain, patient‐centric pain measurements are paramount in understanding the pain experience of patients and providing adequate pain management care. Moreover, patients, doctors, payers of medical services, and now CMS increasingly regard a patient‐centered approach to medical care as crucial for the delivery of high‐quality care.
HCAHPS survey sampling represents an excellent opportunity to help assess current gaps in patient‐centered clinical care. However, ecological factors, such as county‐level demographics and hospital size (eg, bed number), are known to influence health outcomes but have not been adequately studied in pain management patient satisfaction.[7] Hospital and county‐level factors may influence the degree to which patients experience patient‐centered pain management care. For instance, most patient satisfaction scores are worse in urban areas.[8, 9] These disparities in patient satisfaction scores could be associated with population density, greater ethnic diversity or nonEnglish‐speaking individuals, or number of hospital beds.
The US Census demographics and hospital‐bed number provide a concurrent measure that can be used across the country to estimate hospital ecology. This study evaluated the influence of county‐level demographic and structural factors (ie, hospital beds) on patient satisfaction with hospital pain management in all HCAHPS‐participating hospitals across the United States. We hypothesized that demographic diversity, higher population density, and higher numbers of hospital beds would predict lower levels of patient satisfaction with inpatient pain management.
METHODS
Data Collection: County‐Level Predictors
Publically available data were obtained from the American Hospital Directory[10] and United States Census Bureau[11] websites. Twenty US Census data categories were selected a priori by their clinical relevance to influence pain management perception out of the 50 publically reported US Census categories. Final variables utilized in regression modeling are listed under the Variable column in Table 1. Covariate correlation coefficients were all under 0.7, indicating a lack of significant colinearity.
| Variable | Median Value (SD) | Range | Regression Coefficient (SE) | t Value |
|---|---|---|---|---|
| ||||
| African American alone, % | 5.6% (13.8%) | 0%85.4% | 0.02 (00) | 3.609* |
| White alone, % | 86.2% (15.8%) | 5.3%99.0% | 0.06 (0.01) | 6.661* |
| Per capita income | $24,499 ($6,419) | $7,887$61,290 | 0.00 (0.00) | 7.561* |
| With bachelor's degree, % | 22.0% (10.1%) | 6.3%70.7% | 0.06 (0.01) | 7.348* |
| Population 18 years of age, % | 23.2% (3.1%) | 8.3%40.6% | 0.18 (0.05) | 3.498* |
| With a high school degree, % | 86.0% (6.4%) | 46.3%98.6% | 0.02 (0.01) | 1.424 |
| Population change over 1 year, % | 0.7% (2.2%) | 18.1%25.6% | 0.25 (0.04) | 5.645* |
| Same house over 1 year, % | 85.4% (4.2%) | 57.1%98.0% | 0.01 (0.02) | 0.493 |
| White alone (not Hispanic), % | 75.2% (21.8%) | 3.2%98.4% | 0.05(0.00) | 12.077* |
| Household size | 2.52 (0.3) | 1.924.77 | 2.266 (0.36) | 6.283* |
| Population county | 105,937 (1,524,223) | 1,1609,818,605 | 0.00 (0.00) | 13.117* |
| Average travel time to work, min | 23 (5.0) | 642.5 | 0.21 (0.02) | 11.071* |
| NonEnglish speaking, % | 8.6% (15.1%) | 0.2%95.9% | 0.08 (0.01) | 13.843* |
| Total female, % | 50.7% (1.6%) | 34.4%57.0% | 0.44 (0.06) | 7.489* |
| Population 65 years old, % | 14.7% (4.1%) | 5.8%49.3% | 0.06 (0.02) | 2.697 |
| Population in poverty, % | 14.7% (5.6%) | 5.8%49.3% | 0.02 (0.02) | 1.01 |
| Population density | 138.7 (4,534) | 0.369,467 | 0.73 (0.05) | 15.734* |
| Foreign born, % | 4.9% (9.3%) | 0%51.2% | 0.15 (0.01) | 16.775* |
| Median household income | $46,880 ($12,868) | $20,206$120,096 | 0.00 (0.00) | 6.052* |
| No. of hospital beds | 103 (193) | 22,259 | 0.01 (0.00) | 15.403* |
Data Collection: Patient Satisfaction With Pain Management
Pain management was measured using the HCAHPS survey pain management dimension by calculating the percentage of patient responders who said their pain was always controlled. HCAHPS data are publically available on the CMS Hospital Compare website.[6] It contains 32 questions that comprise 10 evaluative measures. It is provided to a random sample of patients across the United States throughout the year at 48 hours to 6 weeks after discharge from the hospital.
Analytic Plan
HCAHPS and US Census datasets were analyzed to assess their distribution curves. The population density variable was converted to a logarithmic scale to account for its skewed distribution and long tail in the area of low population density. Data were subsequently merged into an Excel (Microsoft Corp., Redmond, WA) spreadsheet using the VLOOKUP function such that relevant 2010 census county data were added to each hospital's HCAHPS data.
Bivariate analyses were conducted to determine which US Census categories were significant predictors for patient satisfaction with pain management. All significant predictors were then included in a multivariate model, which predicted for patient satisfaction with pain management. All analyses were 2‐tailed, and statistical significance was set at = 0.05.
RESULTS
Complete HCAHPS scores were obtained from 3907 hospitals out of a total of 4621 US hospitals (85%). The majority of hospitals (73.8%, n = 2884) collected over 300 surveys, fewer (n = 696) collected 100 to 299 surveys, and a small number of hospitals (n = 327) collected less than 100 surveys. Based on the most conservative estimate, results were available from at least 934,800 individual surveys. Missing HCAHPS hospital data averaged 13.4 (standard deviation [SD] = 12.2) hospitals per state. County‐level data were obtained from all 3144 county or county equivalents across the United States (100%).
Bivariate Analyses
Univariate regression indicated a significant association between pain management patient satisfaction and most county‐level demographic variables and number of hospital beds.
Multivariate Analyses
A multivariate linear regression model was run in which 20 county‐level demographic and hospital factors were examined as predictors of patient satisfaction with pain management. The model, which examined county‐level predictors of pain management, explained 12% of the variability in patients' ratings of pain management (R2 = 0.124, P 0.0001). A total of 8 out of the 20 US Census variables were statistically significant predictors of pain management (Table 2). African American and white race were most strongly associated with higher ratings of patient satisfaction with pain management (ie, by partial coefficient and statistical significance). Number of hospital beds, percent foreign born, population density, and female gender were most strongly related to lower ratings of patient satisfaction with pain management.
| Variable | Median Value (SD) | Range | Regression Coefficient (SE) | t Value | |
|---|---|---|---|---|---|
| |||||
| African American alone, % | 5.6% (13.8%) | 0%85.4% | 0.07 (0.01) | 0.23 | 7.104* |
| White alone, % | 86.2% (15.8%) | 5.3%99.0% | 0.08 (0.01) | 0.23 | 6.953* |
| Per capita income | $24,499 ($6,419) | $7,887$61,290 | 0.00 (0.00) | 0.22 | 2.885 |
| With bachelor's degree, % | 22.0% (10.1%) | 6.3%70.7% | 0.03 (0.02) | 0.10 | 1.401 |
| Population 18 years old, % | 23.2% (3.1%) | 8.3%40.6% | 0.18 (0.05) | 0.08 | 3.498* |
| With a high school degree, % | 86.0% (6.4%) | 46.3%98.6% | 0.02 (0.01) | 0.02 | 1.424 |
| Population change over 1 year, % | 0.7% (2.2%) | 18.1%25.6% | 0.11 (0.06) | 0.01 | 1.986 |
| Same house over 1 year, % | 85.4% (4.2%) | 57.1%98.0% | 0.01 (0.02) | 0.01 | 0.493 |
| White alone (not Hispanic), % | 75.2% (21.8%) | 3.2%98.4% | 0.02(0.00) | 0.01 | 0.740 |
| Household size | 2.52 (0.3) | 1.924.77 | 0.92 (0.80) | 0.03 | 1.145 |
| Population county | 105,937 (1,524,223) | 1,1609,818,605 | 0.00 (0.00) | 0.03 | 1.495 |
| Average travel time to work, min | 23 (5.0) | 642.5 | 0.06 (0.02) | 0.06 | 3.054 |
| NonEnglish speaking, % | 8.6% (15.1%) | 0.2%95.9% | 0.00 (0.03) | 0.06 | 0.028 |
| Total female, % | 50.7% (1.6%) | 34.4%57.0% | 0.23 (0.07) | 0.06 | 3.158 |
| Population 65 years old, % | 14.7% (4.1%) | 5.8%49.3% | 0.10 (0.04) | 0.07 | 2.411 |
| Population in poverty, % | 14.7% (5.6%) | 5.8%49.3% | 0.02 (0.02) | 0.08 | 1.01 |
| Population density | 138.7 (4,534) | 0.369,467 | 0.24 (0.09) | 0.08 | 2.823 |
| Foreign born, % | 4.9% (9.3%) | 0%51.2% | 0.07 (0.02) | 0.12 | 4.906* |
| Median household income | $46,880 ($12,868) | $20,206‐$120,096 | 0.00 (0.00) | 0.16 | 2.599 |
| No. of hospital beds | 103 (193) | 22,259 | 0.00 (0.00) | 0.16 | 9.167* |
| Model statistics | F(1, 9) = 62.222, P 0.001 | ||||
| Adjusted R2 | 0.124 | ||||
DISCUSSION
By utilizing county‐level demographic data and the HCAHPS survey measures from across the United States, this study provides a representative sample of US hospitals that can be used to define ecological trends in patient satisfaction with pain management. This statistical model demonstrates the nonrandom variability of pain management satisfaction across the United States, even after CMS patient‐mix adjustment. Although the quality of pain management may be increasing by some reports, our present results indicate that pain management satisfaction is not equitable with the rest of the country among select groups of patients (eg, foreign born, female gender, areas of long travel times to work) or in certain care settings (eg, larger hospitals, population dense areas). These data suggest that areas of pain management may lack in quality compared to pain management across the entire US as a whole. This is consistent with the increasingly recognized contribution of multiple nonmedical determinates to health outcomes.[12] These results demonstrate the overall magnitude of healthcare disparity in the United States, and are particularly concerning because African Americans and Hispanics tend to rate overall satisfaction higher than Caucasians in other studies.[13, 14] The same minority reporting bias may be reflected in HCAHPS results. These patients may be reporting higher pain management satisfaction that is not consistent with the level of care they received, as studies have consistently indicated worse pain management delivery for racial and ethnic minorities.[15]
The present findings reveal structural (eg, hospital beds) and demographic (eg, population density, foreign born) gaps in satisfaction with pain management. An effort to improve pain management for all people in the heterogeneous makeup of the United States is an enormous challenge. However, change may be forthcoming, as Hospital Value‐Based Purchasing draws attention pain practice inequities in real time. Although several of the significant explanatory variables cannot be modified (eg, size of hospital, urban setting, patients served), pain management delivery should receive extra attention in hospitals with those characteristics. Pain management delivery in large, urban hospitals that serve foreign‐born patients may be improved with focused multilevel interventions. Future research should examine these inequities further and develop multilevel interventions that target hospitals in at‐risk areas with the aim of lessening disparities in hospital‐based pain management.
Disclosure
Nothing to report.
- , , , et al. Interventions for providers to promote a patient‐centred approach in clinical consultations. Cochrane Database Syst Rev. 2012;12:CD003267.
- , , , . Patient perception of pain care in hospitals in the United States. J Pain Res. 2009;2:157–164.
- , , , , . Patient perception of pain care in the United States: a 5‐year comparative analysis of hospital consumer assessment of health care providers and systems. Pain Physician. 2014;17(5):369–377.
- , , , , . Assessing the relationship between the level of pain control and patient satisfaction. J Pain Res. 2013;6:683–689.
- H.R.3590—Patient Protection and Affordable Care Act 2010. Available at: https://www.congress.gov/bill/111th‐congress/house‐bill/3590. Accessed December 1, 2013.
- Centers for Medicare 55(1):125–139.
- , . Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342–343.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- American Hospital Directory. Hospital statistics by state. Available at: http://www.ahd.com/state_statistics.html. Accessed December 1, 2013.
- United States Census Bureau. Download center. Available at: http://factfinder.census.gov/faces/nav/jsf/pages/download_center.xhtml. Accessed December 1, 2013.
- Health policy brief: the relative contribution of multiple determinants to health outcomes. Health Affairs website. Available at: http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=123. Accessed December 1, 2013.
- , , , , . Racial and ethnic differences in patient assessments of interactions with providers: disparities or measurement biases? Am J Med Qual. 2006;21(2):109–114.
- , , , , . Survey response style and differential use of CAHPS rating scales by Hispanics. Med Care. 2008;46(9):963–968.
- Institute of Medicine. Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
- , , , et al. Interventions for providers to promote a patient‐centred approach in clinical consultations. Cochrane Database Syst Rev. 2012;12:CD003267.
- , , , . Patient perception of pain care in hospitals in the United States. J Pain Res. 2009;2:157–164.
- , , , , . Patient perception of pain care in the United States: a 5‐year comparative analysis of hospital consumer assessment of health care providers and systems. Pain Physician. 2014;17(5):369–377.
- , , , , . Assessing the relationship between the level of pain control and patient satisfaction. J Pain Res. 2013;6:683–689.
- H.R.3590—Patient Protection and Affordable Care Act 2010. Available at: https://www.congress.gov/bill/111th‐congress/house‐bill/3590. Accessed December 1, 2013.
- Centers for Medicare 55(1):125–139.
- , . Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342–343.
- , , , . Patients' perception of hospital care in the United States. N Engl J Med. 2008;359(18):1921–1931.
- American Hospital Directory. Hospital statistics by state. Available at: http://www.ahd.com/state_statistics.html. Accessed December 1, 2013.
- United States Census Bureau. Download center. Available at: http://factfinder.census.gov/faces/nav/jsf/pages/download_center.xhtml. Accessed December 1, 2013.
- Health policy brief: the relative contribution of multiple determinants to health outcomes. Health Affairs website. Available at: http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=123. Accessed December 1, 2013.
- , , , , . Racial and ethnic differences in patient assessments of interactions with providers: disparities or measurement biases? Am J Med Qual. 2006;21(2):109–114.
- , , , , . Survey response style and differential use of CAHPS rating scales by Hispanics. Med Care. 2008;46(9):963–968.
- Institute of Medicine. Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
Delirium Screening in Older Patients
Delirium is a rapidly developing, fluctuating disturbance in consciousness, caused by a medical condition. The diagnosis of delirium is often missed, potentiating negative outcomes.[1, 2] Regular delirium screening by nurses results in increased recognition and treatment.[3] Although multiple screening tools exist, many are cumbersome to execute. Efforts have been made to shorten them, but although the screening tools may predict adverse outcomes, there are concerns about their specificity.[1, 2, 4, 5, 6] The Delirium Observation Screening Scale[7] (DOS) is a brief screening tool based on observation. It has been validated in several patient populations, but no published studies have taken place in the United States or have focused on an older, general medicine, inpatient population. Given the low numbers of patients in earlier validation studies, the effectiveness of the DOS for screening hospitalized, older patients is not yet fully established.
This study aimed to determine the ability of the DOS to screen hospitalized, older patients for delirium compared to a validated delirium diagnostic tool, the Delirium Rating Scale‐Revised‐98 (DRS‐R‐98).[8] In addition, DOS acceptability, ease of use, and benefit were explored by surveying nurses.
METHODS
Participants
After institutional review board approval, participants were selected by convenience sample from general medicine inpatients at a large, tertiary care, academic hospital. Eligible patients were age 65 years or older, admitted to a medicine inpatient unit, and spoke English. If participants were unable to consent, consent was obtained from the participant's legally authorized representative.
Delirium Observation Screening Scale
The DOS is a 13‐point screen for delirium, based on the Diagnostic and Statistical Manual of Mental Disorders IV delirium criteria, designed to be completed by a nurse (see Supporting Information, Appendix 1, in the online version of this article). Responses are dichotomous. Scores 3 were considered positive delirium screens.[7]
Nurses on medicine units attended educational in‐services on delirium recognition and use of the DOS. The DOS was embedded in the electronic medical record (EMR) and nurses are electronically prompted to chart DOS results every 12 hours for patients, age 65 years or older. Nursing staff utilized the DOS for 1 year prior to study start.
DRS‐R‐98
The DRS‐R‐98 was used as the study reference standard.[8] Scores 15 are indicative of delirium.[9] All assessments were performed by a medical student (K.G.) trained to administer the DRS‐R‐98.
Data Collection
After consent, hospitalized participants were evaluated daily (MondayFriday) using the DRS‐R‐98. Enrollment took place over a 10‐week period. Nurses and researchers were blinded to other delirium assessment results until after participant discharge. Following discharge, additional data were collected from the EMR: age, gender, cognitive comorbidities, and nurse‐charted DOS score. Cognitive comorbidities were classified as no impairment, dementia, or cognitive impairment based on the problem list and admission note. A psychiatrist (M.W.) confirmed questions of cognitive impairment.
The DOS score closest in time, within 24 hours of DRS‐R‐98 assessment, was used for comparison. If a DOS score was not charted within 24 hours of the DRS‐R‐98 evaluation, that assessment was excluded. Partial DRS‐R‐98 assessments were included only if there was enough information to classify a subject as delirious or not.
Nursing Survey
A 13‐question nursing survey was developed and consisted of demographic, Likert‐style, and multiple‐choice questions, with opportunities for open‐ended responses (see Supporting Information, Appendix 2, in the online version of this article). Survey design followed similar surveys investigating staff experiences and clinical functionality of other brief delirium screening tools, such as the Confusion Assessment Method for the Intensive Care Unit.[10, 11] The survey was distributed by e‐mail to 435 nurses on 16 units. Coffee gift cards were raffled as participation incentive.
Statistical Analysis
Statistical analysis was completed using SPSS (IBM, Armonk, NY) and SAS (SAS Institute, Inc., Cary, NC) software. DOS results were compared to the DRS‐R‐98, and validity statistics were calculated for delirium. Confidence intervals were calculated using the Clopper‐Pearson method for binomial data. The Spearman rank correlation coefficient between DOS and DRS‐98 score was calculated. PROC LOGISTIC (SAS Institute, Inc.) modeled the relationship between positive DOS screens and delirium and created a receiver operating characteristic (ROC) curve using continuous DOS score to predict delirium. Because these models did not control for multiple observations per individual, PROC GENMOD (SAS Institute, Inc.) was used to confirm the relationship between a positive DOS screen and delirium using a marginal logistic regression model accounting for repeated measures. In addition, we selected 10 random samples of 1 observation per person, and validity statistics were calculated for each sample.
The nursing survey results were analyzed using descriptive statistics. Open‐ended comments were reviewed in aggregate.
RESULTS
Participant Characteristics
Fifty‐four participants enrolled in the study. Fifty‐three were able to complete 1 DRS‐R‐98 and comprise the study sample (Table 1). Participants completed 1 to 5 daily DRS‐R‐98 assessments (mean, 1.94; standard deviation [SD], 0.90; mean length of admission, 6.06 days). Of the 105 DRS‐R‐98 assessments, 101 were classifiable for delirium. Of the 101 DRS‐R‐98 assessments classifiable for delirium, 100 had a corresponding DOS score within 24 hours. Participant characteristics are listed in Table 1. Eight of the 53 participants (15%) had at least 1 positive DRS‐R‐98. Overall, 10 of the 101 delirium assessments diagnosed delirium (DRS‐R‐98 score 15).
| Characteristic | No Delirium, n = 45 | Delirium, n = 8a |
|---|---|---|
| ||
| Age, y | ||
| 6574, n = 26 | 22 | 4 |
| 7584, n = 15 | 13 | 2 |
| 85+, n = 12 | 10 | 2 |
| Age, y, mean (SD) [range] | 77 (10) [6592] | 76 (8.6) [6592] |
| Gender | ||
| Female, n = 33 | 28 | 5 |
| Male, n = 20 | 17 | 3 |
| Cognitive status per chart | ||
| No impairment, n = 45 | 43 | 2 |
| Cognitive impairment without dementia, n = 5 | 1 | 4 |
| Dementia, n = 3 | 1 | 2 |
DOS Validity
The mean and standard deviation of delirium screening scores are as follows: DRS‐R‐98 (mean, 6.13; SD, 4.74; range, 020) and DOS (mean, 1.22; SD, 2.37; range, 09). The Spearman correlation coefficient between DOS and DRS‐R‐98 scores was 0.58. DOS had a sensitivity of 90% (95% confidence interval [CI]: 56%‐100%) and specificity of 91% (95% CI: 83%‐96%) compared to the DRS‐98‐R standard. There was only 1 false negative DOS screen out of 83 negative assessments (negative predictive value = 99%, 95% CI: 93%‐100%). Out of the 17 positive assessments, 9 were true positives (positive predictive value = 53%, 95% CI: 28%‐77%), and 7 scored in the subsyndromal range for delirium (DRS‐R‐98 score 814).
In analyses using 10 samples, with 1 randomly selected observation per person, the mean sensitivity was 84.6%, ranging from 80% (95% CI: 28%‐99%) to 87.5% (95% CI: 47%‐100%). The mean specificity in these samples was 92%, ranging from 87% (95% CI: 74%‐95%) to 96% (95% CI: 85%‐99%).
Logistic Regression Models
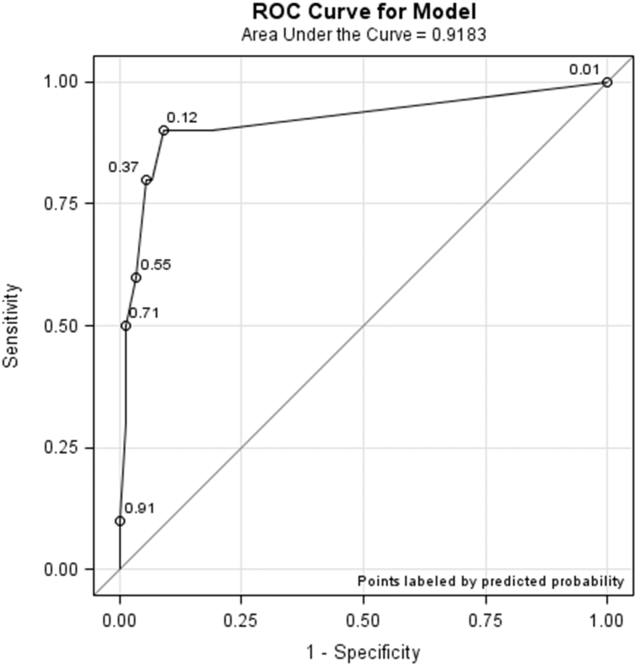
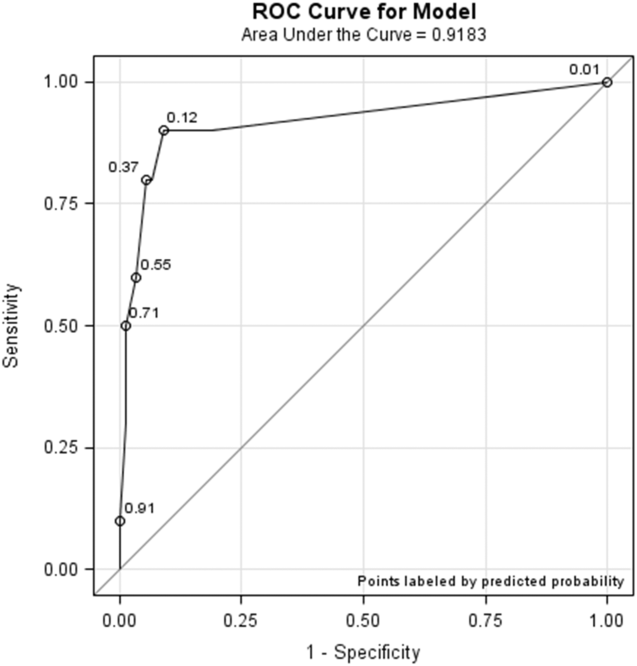
All models confirmed that positive DOS screens significantly predicted delirium. The traditional logistic regression model produced an odds ratio (OR) estimate of 92 (95% CI: 10‐824, P 0.0001) for a positive DOS screen predicting delirium. The marginal logistic regression model accounting for repeated measures produced a consistent estimate (OR: 93, 95% CI: 11‐800, P 0.0001). Continuous DOS scores predicted delirium (OR: 2.1, 95% CI: 1.5‐2.9, P 0.0001), and the ROC curve supported the cutoff of DOS 3, corresponding to a predicted probability of 0.12 (Figure 1).
Nursing Survey
The nursing survey had a response rate of 23% (N = 98). The most robust results related to DOS administration were 87% (N = 83) of nurses were confident in DOS administration, 92% (N = 86) could complete the DOS in under 3 minutes, and 79% (N = 74) agreed that performing the DOS is easy. There was less agreement on the value of the DOS; 37% agreed that the DOS is worth the time to perform, 25% agreed that the DOS enhances patient care, and 36% agreed that the DOS provides valuable information for patient care. Over half the nurses (55%) reported that they perform the DOS 75% to 100% of the prompted times, and 62% stated if the DOS was no longer required, they would not use it. Open‐ended questions generated a wide range of responses, from supportive to critical of delirium screening and the DOS (see Supporting Information, Appendix 3, in the online version of this article).
DISCUSSION
This study demonstrated the effectiveness, efficiency, and ease of use of the DOS as a delirium screening tool. The DOS exhibited high sensitivity (90%) and specificity (91%). Similar to previous findings, the positive predictive value was only 53%, but the negative predictive value was 99%.[12] These results support that the DOS is consistently able to rule out delirium, with only 1 false negative in this study.
Nursing responses regarding user‐friendliness are consistent with other studies; however, there was a knowledge gap related to how positive delirium screens can inform and change care for patients.[7] Education is a known barrier to integrating delirium screening tools secondary to the need for regular and extensive education, frequent reminders to screen, and regular evaluations of assessment quality.[11, 13, 14, 15] Developing guidelines for responding to positive DOS screens and documenting its impact on care may incentivize use.
Study strengths include strong evaluator consistency, blinding of evaluator and nurses, and responses from a broad range of nurses (14 of 16 units represented). Additionally, this study demonstrated the efficacy and ease of use of an EMR‐prompted delirium screen. However, this study had several limitations, including a small sample size and a low incidence of delirium. The lower incidence is likely secondary to selection bias that resulted from difficulty consenting delirious subjects. The discordant time between DOS and DRS‐R‐98 assessments may have also influenced results; however, inclusion of data from the previous 8 to 24 hours in both tools makes the temporal separation of assessments less impactful.
The ability of the DOS to accurately identify patients at high risk of delirium is useful for healthcare staff. Future work will include nurse and physician education to emphasize delirium understanding, the importance of regular screening, and the use of nonpharmacological interventions. Additional studies will include examination of the interventions and outcomes of patients who screen positive for delirium to determine the long‐term impact of delirium screening.
Acknowledgements
The study authors would like to thank the University of Iowa Hospitals and Clinics, the Department of Family Medicine, and the University of Iowa Hospitals and Clinics nursing managers and nursing staff.
Disclosures
This study was funded by the Summer Research Fellowship program sponsored by the University of Iowa Carver College of Medicine. Drs. Weckmann and Carnahan were supported by the Health Resources and Services Administration, Iowa Geriatric Education Center (UB4 HP19054) as well as the US Department of Health and Human Services, Agency for Healthcare Research and Quality (AHRQ 1 R18 HS022666‐01).
- , , . An intervention to reduce delirium in care homes. Nurs Older People. 2010;22(4):16–21.
- , , , et al. Assessment of delirium in the intensive care unit: nursing practices and perceptions. Am J Crit Care. 2008;17(6):555–565.
- , , , et al. Delirium and sedation recognition using validated instruments: reliability of bedside intensive care unit nursing assessments from 2007 to 2010. J Am Geriatr Soc. 2011;59(suppl 2):S249–S255.
- , , , et al. Preliminary development of an ultrabrief two‐item bedside test for delirium. J Hosp Med. 2015;10(10):645–650.
- , , , et al. The association between an ultrabrief cognitive screening in older adults and hospital outcomes. J Hosp Med. 2015;10(10):651–657.
- , , , et al. Comparison of mental‐status scales for predicting mortality on the general wards. J Hosp Med. 2015;10(10):658–663.
- , , . The Delirium Observation Screening Scale: a screening instrument for delirium. Res Theory Nurs Pract. 2003;17(1):31–50.
- . Validation of the Delirium Rating Scale‐Revised‐98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229–242.
- , , , et al. Factor analysis of the Colombian translation of the Delirium Rating Scale (DRS), Revised‐98. Psychosomatics. 2009;50(3):255–262.
- , , , et al. Implementation, reliability testing, and compliance monitoring of the Confusion Assessment Method for the Intensive Care Unit in trauma patients. Intensive Care Med. 2008;34(7):1263–1268.
- , , , et al. Limitations and practicalities of CAM‐ICU implementation, a delirium scoring system, in a Dutch intensive care unit. Intensive Crit Care Nurs. 2009;25(5):242–249.
- , . The Neecham Confusion Scale and the Delirium Observation Screening Scale: capacity to discriminate and ease of use in clinical practice. BMC Nurs. 2007;6:3.
- , , . Early recognition of delirium: review of the literature. J Clin Nurs. 2001;10(6):721–729.
- , , , et al. Impact of a delirium screening tool and multifaceted education on nurses' knowledge of delirium and ability to evaluate it correctly. Am J Crit Care. 2012;21(1):e1–e11.
- , , . Optimising the recognition of delirium in the intensive care unit. Best Pract Res Clin Anaesthesiol. 2012;26(3):385–393.
Delirium is a rapidly developing, fluctuating disturbance in consciousness, caused by a medical condition. The diagnosis of delirium is often missed, potentiating negative outcomes.[1, 2] Regular delirium screening by nurses results in increased recognition and treatment.[3] Although multiple screening tools exist, many are cumbersome to execute. Efforts have been made to shorten them, but although the screening tools may predict adverse outcomes, there are concerns about their specificity.[1, 2, 4, 5, 6] The Delirium Observation Screening Scale[7] (DOS) is a brief screening tool based on observation. It has been validated in several patient populations, but no published studies have taken place in the United States or have focused on an older, general medicine, inpatient population. Given the low numbers of patients in earlier validation studies, the effectiveness of the DOS for screening hospitalized, older patients is not yet fully established.
This study aimed to determine the ability of the DOS to screen hospitalized, older patients for delirium compared to a validated delirium diagnostic tool, the Delirium Rating Scale‐Revised‐98 (DRS‐R‐98).[8] In addition, DOS acceptability, ease of use, and benefit were explored by surveying nurses.
METHODS
Participants
After institutional review board approval, participants were selected by convenience sample from general medicine inpatients at a large, tertiary care, academic hospital. Eligible patients were age 65 years or older, admitted to a medicine inpatient unit, and spoke English. If participants were unable to consent, consent was obtained from the participant's legally authorized representative.
Delirium Observation Screening Scale
The DOS is a 13‐point screen for delirium, based on the Diagnostic and Statistical Manual of Mental Disorders IV delirium criteria, designed to be completed by a nurse (see Supporting Information, Appendix 1, in the online version of this article). Responses are dichotomous. Scores 3 were considered positive delirium screens.[7]
Nurses on medicine units attended educational in‐services on delirium recognition and use of the DOS. The DOS was embedded in the electronic medical record (EMR) and nurses are electronically prompted to chart DOS results every 12 hours for patients, age 65 years or older. Nursing staff utilized the DOS for 1 year prior to study start.
DRS‐R‐98
The DRS‐R‐98 was used as the study reference standard.[8] Scores 15 are indicative of delirium.[9] All assessments were performed by a medical student (K.G.) trained to administer the DRS‐R‐98.
Data Collection
After consent, hospitalized participants were evaluated daily (MondayFriday) using the DRS‐R‐98. Enrollment took place over a 10‐week period. Nurses and researchers were blinded to other delirium assessment results until after participant discharge. Following discharge, additional data were collected from the EMR: age, gender, cognitive comorbidities, and nurse‐charted DOS score. Cognitive comorbidities were classified as no impairment, dementia, or cognitive impairment based on the problem list and admission note. A psychiatrist (M.W.) confirmed questions of cognitive impairment.
The DOS score closest in time, within 24 hours of DRS‐R‐98 assessment, was used for comparison. If a DOS score was not charted within 24 hours of the DRS‐R‐98 evaluation, that assessment was excluded. Partial DRS‐R‐98 assessments were included only if there was enough information to classify a subject as delirious or not.
Nursing Survey
A 13‐question nursing survey was developed and consisted of demographic, Likert‐style, and multiple‐choice questions, with opportunities for open‐ended responses (see Supporting Information, Appendix 2, in the online version of this article). Survey design followed similar surveys investigating staff experiences and clinical functionality of other brief delirium screening tools, such as the Confusion Assessment Method for the Intensive Care Unit.[10, 11] The survey was distributed by e‐mail to 435 nurses on 16 units. Coffee gift cards were raffled as participation incentive.
Statistical Analysis
Statistical analysis was completed using SPSS (IBM, Armonk, NY) and SAS (SAS Institute, Inc., Cary, NC) software. DOS results were compared to the DRS‐R‐98, and validity statistics were calculated for delirium. Confidence intervals were calculated using the Clopper‐Pearson method for binomial data. The Spearman rank correlation coefficient between DOS and DRS‐98 score was calculated. PROC LOGISTIC (SAS Institute, Inc.) modeled the relationship between positive DOS screens and delirium and created a receiver operating characteristic (ROC) curve using continuous DOS score to predict delirium. Because these models did not control for multiple observations per individual, PROC GENMOD (SAS Institute, Inc.) was used to confirm the relationship between a positive DOS screen and delirium using a marginal logistic regression model accounting for repeated measures. In addition, we selected 10 random samples of 1 observation per person, and validity statistics were calculated for each sample.
The nursing survey results were analyzed using descriptive statistics. Open‐ended comments were reviewed in aggregate.
RESULTS
Participant Characteristics
Fifty‐four participants enrolled in the study. Fifty‐three were able to complete 1 DRS‐R‐98 and comprise the study sample (Table 1). Participants completed 1 to 5 daily DRS‐R‐98 assessments (mean, 1.94; standard deviation [SD], 0.90; mean length of admission, 6.06 days). Of the 105 DRS‐R‐98 assessments, 101 were classifiable for delirium. Of the 101 DRS‐R‐98 assessments classifiable for delirium, 100 had a corresponding DOS score within 24 hours. Participant characteristics are listed in Table 1. Eight of the 53 participants (15%) had at least 1 positive DRS‐R‐98. Overall, 10 of the 101 delirium assessments diagnosed delirium (DRS‐R‐98 score 15).
| Characteristic | No Delirium, n = 45 | Delirium, n = 8a |
|---|---|---|
| ||
| Age, y | ||
| 6574, n = 26 | 22 | 4 |
| 7584, n = 15 | 13 | 2 |
| 85+, n = 12 | 10 | 2 |
| Age, y, mean (SD) [range] | 77 (10) [6592] | 76 (8.6) [6592] |
| Gender | ||
| Female, n = 33 | 28 | 5 |
| Male, n = 20 | 17 | 3 |
| Cognitive status per chart | ||
| No impairment, n = 45 | 43 | 2 |
| Cognitive impairment without dementia, n = 5 | 1 | 4 |
| Dementia, n = 3 | 1 | 2 |
DOS Validity
The mean and standard deviation of delirium screening scores are as follows: DRS‐R‐98 (mean, 6.13; SD, 4.74; range, 020) and DOS (mean, 1.22; SD, 2.37; range, 09). The Spearman correlation coefficient between DOS and DRS‐R‐98 scores was 0.58. DOS had a sensitivity of 90% (95% confidence interval [CI]: 56%‐100%) and specificity of 91% (95% CI: 83%‐96%) compared to the DRS‐98‐R standard. There was only 1 false negative DOS screen out of 83 negative assessments (negative predictive value = 99%, 95% CI: 93%‐100%). Out of the 17 positive assessments, 9 were true positives (positive predictive value = 53%, 95% CI: 28%‐77%), and 7 scored in the subsyndromal range for delirium (DRS‐R‐98 score 814).
In analyses using 10 samples, with 1 randomly selected observation per person, the mean sensitivity was 84.6%, ranging from 80% (95% CI: 28%‐99%) to 87.5% (95% CI: 47%‐100%). The mean specificity in these samples was 92%, ranging from 87% (95% CI: 74%‐95%) to 96% (95% CI: 85%‐99%).
Logistic Regression Models
All models confirmed that positive DOS screens significantly predicted delirium. The traditional logistic regression model produced an odds ratio (OR) estimate of 92 (95% CI: 10‐824, P 0.0001) for a positive DOS screen predicting delirium. The marginal logistic regression model accounting for repeated measures produced a consistent estimate (OR: 93, 95% CI: 11‐800, P 0.0001). Continuous DOS scores predicted delirium (OR: 2.1, 95% CI: 1.5‐2.9, P 0.0001), and the ROC curve supported the cutoff of DOS 3, corresponding to a predicted probability of 0.12 (Figure 1).

Nursing Survey
The nursing survey had a response rate of 23% (N = 98). The most robust results related to DOS administration were 87% (N = 83) of nurses were confident in DOS administration, 92% (N = 86) could complete the DOS in under 3 minutes, and 79% (N = 74) agreed that performing the DOS is easy. There was less agreement on the value of the DOS; 37% agreed that the DOS is worth the time to perform, 25% agreed that the DOS enhances patient care, and 36% agreed that the DOS provides valuable information for patient care. Over half the nurses (55%) reported that they perform the DOS 75% to 100% of the prompted times, and 62% stated if the DOS was no longer required, they would not use it. Open‐ended questions generated a wide range of responses, from supportive to critical of delirium screening and the DOS (see Supporting Information, Appendix 3, in the online version of this article).
DISCUSSION
This study demonstrated the effectiveness, efficiency, and ease of use of the DOS as a delirium screening tool. The DOS exhibited high sensitivity (90%) and specificity (91%). Similar to previous findings, the positive predictive value was only 53%, but the negative predictive value was 99%.[12] These results support that the DOS is consistently able to rule out delirium, with only 1 false negative in this study.
Nursing responses regarding user‐friendliness are consistent with other studies; however, there was a knowledge gap related to how positive delirium screens can inform and change care for patients.[7] Education is a known barrier to integrating delirium screening tools secondary to the need for regular and extensive education, frequent reminders to screen, and regular evaluations of assessment quality.[11, 13, 14, 15] Developing guidelines for responding to positive DOS screens and documenting its impact on care may incentivize use.
Study strengths include strong evaluator consistency, blinding of evaluator and nurses, and responses from a broad range of nurses (14 of 16 units represented). Additionally, this study demonstrated the efficacy and ease of use of an EMR‐prompted delirium screen. However, this study had several limitations, including a small sample size and a low incidence of delirium. The lower incidence is likely secondary to selection bias that resulted from difficulty consenting delirious subjects. The discordant time between DOS and DRS‐R‐98 assessments may have also influenced results; however, inclusion of data from the previous 8 to 24 hours in both tools makes the temporal separation of assessments less impactful.
The ability of the DOS to accurately identify patients at high risk of delirium is useful for healthcare staff. Future work will include nurse and physician education to emphasize delirium understanding, the importance of regular screening, and the use of nonpharmacological interventions. Additional studies will include examination of the interventions and outcomes of patients who screen positive for delirium to determine the long‐term impact of delirium screening.
Acknowledgements
The study authors would like to thank the University of Iowa Hospitals and Clinics, the Department of Family Medicine, and the University of Iowa Hospitals and Clinics nursing managers and nursing staff.
Disclosures
This study was funded by the Summer Research Fellowship program sponsored by the University of Iowa Carver College of Medicine. Drs. Weckmann and Carnahan were supported by the Health Resources and Services Administration, Iowa Geriatric Education Center (UB4 HP19054) as well as the US Department of Health and Human Services, Agency for Healthcare Research and Quality (AHRQ 1 R18 HS022666‐01).
Delirium is a rapidly developing, fluctuating disturbance in consciousness, caused by a medical condition. The diagnosis of delirium is often missed, potentiating negative outcomes.[1, 2] Regular delirium screening by nurses results in increased recognition and treatment.[3] Although multiple screening tools exist, many are cumbersome to execute. Efforts have been made to shorten them, but although the screening tools may predict adverse outcomes, there are concerns about their specificity.[1, 2, 4, 5, 6] The Delirium Observation Screening Scale[7] (DOS) is a brief screening tool based on observation. It has been validated in several patient populations, but no published studies have taken place in the United States or have focused on an older, general medicine, inpatient population. Given the low numbers of patients in earlier validation studies, the effectiveness of the DOS for screening hospitalized, older patients is not yet fully established.
This study aimed to determine the ability of the DOS to screen hospitalized, older patients for delirium compared to a validated delirium diagnostic tool, the Delirium Rating Scale‐Revised‐98 (DRS‐R‐98).[8] In addition, DOS acceptability, ease of use, and benefit were explored by surveying nurses.
METHODS
Participants
After institutional review board approval, participants were selected by convenience sample from general medicine inpatients at a large, tertiary care, academic hospital. Eligible patients were age 65 years or older, admitted to a medicine inpatient unit, and spoke English. If participants were unable to consent, consent was obtained from the participant's legally authorized representative.
Delirium Observation Screening Scale
The DOS is a 13‐point screen for delirium, based on the Diagnostic and Statistical Manual of Mental Disorders IV delirium criteria, designed to be completed by a nurse (see Supporting Information, Appendix 1, in the online version of this article). Responses are dichotomous. Scores 3 were considered positive delirium screens.[7]
Nurses on medicine units attended educational in‐services on delirium recognition and use of the DOS. The DOS was embedded in the electronic medical record (EMR) and nurses are electronically prompted to chart DOS results every 12 hours for patients, age 65 years or older. Nursing staff utilized the DOS for 1 year prior to study start.
DRS‐R‐98
The DRS‐R‐98 was used as the study reference standard.[8] Scores 15 are indicative of delirium.[9] All assessments were performed by a medical student (K.G.) trained to administer the DRS‐R‐98.
Data Collection
After consent, hospitalized participants were evaluated daily (MondayFriday) using the DRS‐R‐98. Enrollment took place over a 10‐week period. Nurses and researchers were blinded to other delirium assessment results until after participant discharge. Following discharge, additional data were collected from the EMR: age, gender, cognitive comorbidities, and nurse‐charted DOS score. Cognitive comorbidities were classified as no impairment, dementia, or cognitive impairment based on the problem list and admission note. A psychiatrist (M.W.) confirmed questions of cognitive impairment.
The DOS score closest in time, within 24 hours of DRS‐R‐98 assessment, was used for comparison. If a DOS score was not charted within 24 hours of the DRS‐R‐98 evaluation, that assessment was excluded. Partial DRS‐R‐98 assessments were included only if there was enough information to classify a subject as delirious or not.
Nursing Survey
A 13‐question nursing survey was developed and consisted of demographic, Likert‐style, and multiple‐choice questions, with opportunities for open‐ended responses (see Supporting Information, Appendix 2, in the online version of this article). Survey design followed similar surveys investigating staff experiences and clinical functionality of other brief delirium screening tools, such as the Confusion Assessment Method for the Intensive Care Unit.[10, 11] The survey was distributed by e‐mail to 435 nurses on 16 units. Coffee gift cards were raffled as participation incentive.
Statistical Analysis
Statistical analysis was completed using SPSS (IBM, Armonk, NY) and SAS (SAS Institute, Inc., Cary, NC) software. DOS results were compared to the DRS‐R‐98, and validity statistics were calculated for delirium. Confidence intervals were calculated using the Clopper‐Pearson method for binomial data. The Spearman rank correlation coefficient between DOS and DRS‐98 score was calculated. PROC LOGISTIC (SAS Institute, Inc.) modeled the relationship between positive DOS screens and delirium and created a receiver operating characteristic (ROC) curve using continuous DOS score to predict delirium. Because these models did not control for multiple observations per individual, PROC GENMOD (SAS Institute, Inc.) was used to confirm the relationship between a positive DOS screen and delirium using a marginal logistic regression model accounting for repeated measures. In addition, we selected 10 random samples of 1 observation per person, and validity statistics were calculated for each sample.
The nursing survey results were analyzed using descriptive statistics. Open‐ended comments were reviewed in aggregate.
RESULTS
Participant Characteristics
Fifty‐four participants enrolled in the study. Fifty‐three were able to complete 1 DRS‐R‐98 and comprise the study sample (Table 1). Participants completed 1 to 5 daily DRS‐R‐98 assessments (mean, 1.94; standard deviation [SD], 0.90; mean length of admission, 6.06 days). Of the 105 DRS‐R‐98 assessments, 101 were classifiable for delirium. Of the 101 DRS‐R‐98 assessments classifiable for delirium, 100 had a corresponding DOS score within 24 hours. Participant characteristics are listed in Table 1. Eight of the 53 participants (15%) had at least 1 positive DRS‐R‐98. Overall, 10 of the 101 delirium assessments diagnosed delirium (DRS‐R‐98 score 15).
| Characteristic | No Delirium, n = 45 | Delirium, n = 8a |
|---|---|---|
| ||
| Age, y | ||
| 6574, n = 26 | 22 | 4 |
| 7584, n = 15 | 13 | 2 |
| 85+, n = 12 | 10 | 2 |
| Age, y, mean (SD) [range] | 77 (10) [6592] | 76 (8.6) [6592] |
| Gender | ||
| Female, n = 33 | 28 | 5 |
| Male, n = 20 | 17 | 3 |
| Cognitive status per chart | ||
| No impairment, n = 45 | 43 | 2 |
| Cognitive impairment without dementia, n = 5 | 1 | 4 |
| Dementia, n = 3 | 1 | 2 |
DOS Validity
The mean and standard deviation of delirium screening scores are as follows: DRS‐R‐98 (mean, 6.13; SD, 4.74; range, 020) and DOS (mean, 1.22; SD, 2.37; range, 09). The Spearman correlation coefficient between DOS and DRS‐R‐98 scores was 0.58. DOS had a sensitivity of 90% (95% confidence interval [CI]: 56%‐100%) and specificity of 91% (95% CI: 83%‐96%) compared to the DRS‐98‐R standard. There was only 1 false negative DOS screen out of 83 negative assessments (negative predictive value = 99%, 95% CI: 93%‐100%). Out of the 17 positive assessments, 9 were true positives (positive predictive value = 53%, 95% CI: 28%‐77%), and 7 scored in the subsyndromal range for delirium (DRS‐R‐98 score 814).
In analyses using 10 samples, with 1 randomly selected observation per person, the mean sensitivity was 84.6%, ranging from 80% (95% CI: 28%‐99%) to 87.5% (95% CI: 47%‐100%). The mean specificity in these samples was 92%, ranging from 87% (95% CI: 74%‐95%) to 96% (95% CI: 85%‐99%).
Logistic Regression Models
All models confirmed that positive DOS screens significantly predicted delirium. The traditional logistic regression model produced an odds ratio (OR) estimate of 92 (95% CI: 10‐824, P 0.0001) for a positive DOS screen predicting delirium. The marginal logistic regression model accounting for repeated measures produced a consistent estimate (OR: 93, 95% CI: 11‐800, P 0.0001). Continuous DOS scores predicted delirium (OR: 2.1, 95% CI: 1.5‐2.9, P 0.0001), and the ROC curve supported the cutoff of DOS 3, corresponding to a predicted probability of 0.12 (Figure 1).

Nursing Survey
The nursing survey had a response rate of 23% (N = 98). The most robust results related to DOS administration were 87% (N = 83) of nurses were confident in DOS administration, 92% (N = 86) could complete the DOS in under 3 minutes, and 79% (N = 74) agreed that performing the DOS is easy. There was less agreement on the value of the DOS; 37% agreed that the DOS is worth the time to perform, 25% agreed that the DOS enhances patient care, and 36% agreed that the DOS provides valuable information for patient care. Over half the nurses (55%) reported that they perform the DOS 75% to 100% of the prompted times, and 62% stated if the DOS was no longer required, they would not use it. Open‐ended questions generated a wide range of responses, from supportive to critical of delirium screening and the DOS (see Supporting Information, Appendix 3, in the online version of this article).
DISCUSSION
This study demonstrated the effectiveness, efficiency, and ease of use of the DOS as a delirium screening tool. The DOS exhibited high sensitivity (90%) and specificity (91%). Similar to previous findings, the positive predictive value was only 53%, but the negative predictive value was 99%.[12] These results support that the DOS is consistently able to rule out delirium, with only 1 false negative in this study.
Nursing responses regarding user‐friendliness are consistent with other studies; however, there was a knowledge gap related to how positive delirium screens can inform and change care for patients.[7] Education is a known barrier to integrating delirium screening tools secondary to the need for regular and extensive education, frequent reminders to screen, and regular evaluations of assessment quality.[11, 13, 14, 15] Developing guidelines for responding to positive DOS screens and documenting its impact on care may incentivize use.
Study strengths include strong evaluator consistency, blinding of evaluator and nurses, and responses from a broad range of nurses (14 of 16 units represented). Additionally, this study demonstrated the efficacy and ease of use of an EMR‐prompted delirium screen. However, this study had several limitations, including a small sample size and a low incidence of delirium. The lower incidence is likely secondary to selection bias that resulted from difficulty consenting delirious subjects. The discordant time between DOS and DRS‐R‐98 assessments may have also influenced results; however, inclusion of data from the previous 8 to 24 hours in both tools makes the temporal separation of assessments less impactful.
The ability of the DOS to accurately identify patients at high risk of delirium is useful for healthcare staff. Future work will include nurse and physician education to emphasize delirium understanding, the importance of regular screening, and the use of nonpharmacological interventions. Additional studies will include examination of the interventions and outcomes of patients who screen positive for delirium to determine the long‐term impact of delirium screening.
Acknowledgements
The study authors would like to thank the University of Iowa Hospitals and Clinics, the Department of Family Medicine, and the University of Iowa Hospitals and Clinics nursing managers and nursing staff.
Disclosures
This study was funded by the Summer Research Fellowship program sponsored by the University of Iowa Carver College of Medicine. Drs. Weckmann and Carnahan were supported by the Health Resources and Services Administration, Iowa Geriatric Education Center (UB4 HP19054) as well as the US Department of Health and Human Services, Agency for Healthcare Research and Quality (AHRQ 1 R18 HS022666‐01).
- , , . An intervention to reduce delirium in care homes. Nurs Older People. 2010;22(4):16–21.
- , , , et al. Assessment of delirium in the intensive care unit: nursing practices and perceptions. Am J Crit Care. 2008;17(6):555–565.
- , , , et al. Delirium and sedation recognition using validated instruments: reliability of bedside intensive care unit nursing assessments from 2007 to 2010. J Am Geriatr Soc. 2011;59(suppl 2):S249–S255.
- , , , et al. Preliminary development of an ultrabrief two‐item bedside test for delirium. J Hosp Med. 2015;10(10):645–650.
- , , , et al. The association between an ultrabrief cognitive screening in older adults and hospital outcomes. J Hosp Med. 2015;10(10):651–657.
- , , , et al. Comparison of mental‐status scales for predicting mortality on the general wards. J Hosp Med. 2015;10(10):658–663.
- , , . The Delirium Observation Screening Scale: a screening instrument for delirium. Res Theory Nurs Pract. 2003;17(1):31–50.
- . Validation of the Delirium Rating Scale‐Revised‐98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229–242.
- , , , et al. Factor analysis of the Colombian translation of the Delirium Rating Scale (DRS), Revised‐98. Psychosomatics. 2009;50(3):255–262.
- , , , et al. Implementation, reliability testing, and compliance monitoring of the Confusion Assessment Method for the Intensive Care Unit in trauma patients. Intensive Care Med. 2008;34(7):1263–1268.
- , , , et al. Limitations and practicalities of CAM‐ICU implementation, a delirium scoring system, in a Dutch intensive care unit. Intensive Crit Care Nurs. 2009;25(5):242–249.
- , . The Neecham Confusion Scale and the Delirium Observation Screening Scale: capacity to discriminate and ease of use in clinical practice. BMC Nurs. 2007;6:3.
- , , . Early recognition of delirium: review of the literature. J Clin Nurs. 2001;10(6):721–729.
- , , , et al. Impact of a delirium screening tool and multifaceted education on nurses' knowledge of delirium and ability to evaluate it correctly. Am J Crit Care. 2012;21(1):e1–e11.
- , , . Optimising the recognition of delirium in the intensive care unit. Best Pract Res Clin Anaesthesiol. 2012;26(3):385–393.
- , , . An intervention to reduce delirium in care homes. Nurs Older People. 2010;22(4):16–21.
- , , , et al. Assessment of delirium in the intensive care unit: nursing practices and perceptions. Am J Crit Care. 2008;17(6):555–565.
- , , , et al. Delirium and sedation recognition using validated instruments: reliability of bedside intensive care unit nursing assessments from 2007 to 2010. J Am Geriatr Soc. 2011;59(suppl 2):S249–S255.
- , , , et al. Preliminary development of an ultrabrief two‐item bedside test for delirium. J Hosp Med. 2015;10(10):645–650.
- , , , et al. The association between an ultrabrief cognitive screening in older adults and hospital outcomes. J Hosp Med. 2015;10(10):651–657.
- , , , et al. Comparison of mental‐status scales for predicting mortality on the general wards. J Hosp Med. 2015;10(10):658–663.
- , , . The Delirium Observation Screening Scale: a screening instrument for delirium. Res Theory Nurs Pract. 2003;17(1):31–50.
- . Validation of the Delirium Rating Scale‐Revised‐98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229–242.
- , , , et al. Factor analysis of the Colombian translation of the Delirium Rating Scale (DRS), Revised‐98. Psychosomatics. 2009;50(3):255–262.
- , , , et al. Implementation, reliability testing, and compliance monitoring of the Confusion Assessment Method for the Intensive Care Unit in trauma patients. Intensive Care Med. 2008;34(7):1263–1268.
- , , , et al. Limitations and practicalities of CAM‐ICU implementation, a delirium scoring system, in a Dutch intensive care unit. Intensive Crit Care Nurs. 2009;25(5):242–249.
- , . The Neecham Confusion Scale and the Delirium Observation Screening Scale: capacity to discriminate and ease of use in clinical practice. BMC Nurs. 2007;6:3.
- , , . Early recognition of delirium: review of the literature. J Clin Nurs. 2001;10(6):721–729.
- , , , et al. Impact of a delirium screening tool and multifaceted education on nurses' knowledge of delirium and ability to evaluate it correctly. Am J Crit Care. 2012;21(1):e1–e11.
- , , . Optimising the recognition of delirium in the intensive care unit. Best Pract Res Clin Anaesthesiol. 2012;26(3):385–393.
Patients' Sleep Quality and Duration
Approximately 70 million adults within the United States have sleep disorders,[1] and up to 30% of adults report sleeping less than 6 hours per night.[2] Poor sleep has been associated with undesirable health outcomes.[1] Suboptimal sleep duration and sleep quality has been associated with a higher prevalence of chronic health conditions including hypertension, type 2 diabetes, coronary artery disease, stroke, and obesity, as well as increased overall mortality.[3, 4, 5, 6, 7]
Sleep plays an important role in restoration of wellness. Poor sleep is associated with physiological disturbances that may result in poor healing.[8, 9, 10] In the literature, prevalence of insomnia among elderly hospitalized patients was 36.7%,[11] whereas in younger hospitalized patients it was 50%.[12] Hospitalized patients frequently cite their acute illness, hospital‐related environmental factors, and disruptions that are part of routine care as causes for poor sleep during hospitalization.[13, 14, 15] Although the pervasiveness of poor sleep among hospitalized patients is high, interventions that prioritize sleep optimization as routine care, are uncommon. Few studies have reviewed the effect of sleep‐promoting measures on both sleep quality and sleep duration among patients hospitalized on general medicine units.
In this study, we aimed to assess the feasibility of incorporating sleep‐promoting interventions on a general medicine unit. We sought to identify differences in sleep measures between intervention and control groups. The primary outcome that we hoped to influence and lengthen in the intervention group was sleep duration. This outcome was measured both by sleep diary and with actigraphy. Secondary outcomes that we hypothesized should improve in the intervention group included feeling more refreshed in the mornings, sleep efficiency, and fewer sleep disruptions. As a feasibility pilot, we also wanted to explore the ease or difficulty with which sleep‐promoting interventions could be incorporated to the team's workflow.
METHODS
Study Design
A quasi‐experimental prospective pilot study was conducted at a single academic center, the Johns Hopkins Bayview Medical Center. Participants included adult patients admitted to the general medicine ward from July 2013 through January 2014. Patients with dementia; inability to complete survey questionnaires due to delirium, disability, or a language barrier; active withdrawal from alcohol or controlled substances; or acute psychiatric illness were excluded in this study.
The medicine ward at our medical center is comprised of 2 structurally identical units that admit patients with similar diagnoses, disease severity, and case‐mix disease groups. Nursing and support staff are unit specific. Pertaining to the sleep environment, the units both have semiprivate and private rooms. Visitors are encouraged to leave by 10 pm. Patients admitted from the emergency room to the medicine ward are assigned haphazardly to either unit based on bed availability. For the purpose of this study, we selected 1 unit to be a control unit and identified the other as the sleep‐promoting intervention unit.
Study Procedure
Upon arrival to the medicine unit, the research team approached all patients who met study eligibility criteria for study participation. Patients were provided full disclosure of the study using institutional research guidelines, and those interested in participating were consented. Participants were not explicitly told about their group assignment. This study was approved by the Johns Hopkins Institutional Review Board for human subject research.
In this study, the control group participants received standard of care as it pertains to sleep promotion. No additional sleep‐promoting measures were implemented to routine medical care, medication administration, nursing care, and overnight monitoring. Patients who used sleep medications at home, prior to admission, had those medicines continued only if they requested them and they were not contraindicated given their acute illness. Participants on the intervention unit were exposed to a nurse‐delivered sleep‐promoting protocol aimed at transforming the culture of care such that helping patients to sleep soundly was made a top priority. Environmental changes included unit‐wide efforts to minimize light and noise disturbances by dimming hallway lights, turning off room lights, and encouraging care teams to be as quiet as possible. Other strategies focused largely on minimizing care‐related disruptions. These included, when appropriate, administering nighttime medications in the early evening, minimizing fluids overnight, and closing patient room doors where appropriate. Further, patients were offered the following sleep‐promoting items to choose from: ear plugs, eye masks, warm blankets, and relaxation music. The final component of our intervention was 30‐minute sleep hygiene education taught by a physician. It highlighted basic sleep physiology and healthy sleep behavior adapted from Buysse.[16] Patients learned the role of behaviors such as reducing time lying awake in bed, setting standard wake‐up time and sleep time, and going to bed only when sleepy. This behavioral education was supplemented by a handout with sleep‐promoting suggestions.
The care team on the intervention unit received comprehensive study‐focused training in which night nursing teams were familiarized with the sleep‐promoting protocol through in‐service sessions facilitated by 1 of the authors (E.W.G.). To further promote study implementation, sleep‐promoting procedures were supported and encouraged by supervising nurses who made daily reminders to the intervention unit night care team of the goals of the sleep‐promoting study during evening huddles performed at the beginning of each shift. To assess the adherence of the sleep protocol, the nursing staff completed a daily checklist of elements within the protocol that were employed .
Data Collection and Measures
Baseline Measures
At the time of enrollment, study patients' demographic information, including use of chronic sleep medication prior to admission, was collected. Participants were assessed for baseline sleep disturbance prior to admission using standardized, validated sleep assessment tools: Pittsburgh Sleep Quality Index (PSQI), the Insomnia Severity Index (ISI), and the Epworth Sleepiness Scale (ESS). PSQI, a 19‐item tool, assessed self‐rated sleep quality measured over the prior month; a score of 5 or greater indicated poor sleep.[17] ISI, a 7‐item tool, identified the presence, rated the severity, and described the impact of insomnia; a score of 10 or greater indicated insomnia.[18] ESS, an 8‐item self‐rated tool, evaluated the impact of perceived sleepiness on daily functioning in 8 different environments; a score of 9 or greater was linked to burden of sleepiness. Participants were also screened for both obstructive sleep apnea (using the Berlin Sleep Apnea Index) and clinical depression (using Center for Epidemiologic Studies‐Depression 10‐point scale), as these conditions affect sleep patterns. These data are shown in Table 1.
| Intervention, n = 48 | Control, n = 64 | P Value | |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 58.2 (16) | 56.9 (17) | 0.69 |
| Female, n (%) | 26 (54.2) | 36 (56.3) | 0.83 |
| Race, n (%) | |||
| Caucasian | 33 (68.8) | 46 (71.9) | 0.92 |
| African American | 13 (27.1) | 16 (25.0) | |
| Other | 2 (4.2) | 2 (3.1) | |
| BMI, mean (SD) | 32.1 (9.2) | 31.8 (9.3) | 0.85 |
| Admitting service, n (%) | |||
| Teaching | 21 (43.8) | 18 (28.1) | 0.09 |
| Nonteaching | 27 (56.3) | 46 (71.9) | |
| Sleep medication prior to admission, n (%) | 7 (14.9) | 21 (32.8) | 0.03 |
| Length of stay, d, mean (SD) | 4.9 (3) | 5.8 (3.9) | 0.19 |
| Number of sleep diaries per participant, mean (SD) | 2.2 (0.8) | 2.6 (0.9) | 0.02 |
| Proportion of hospital days with sleep diaries per participant, (SD) | 0.6 (0.2) | 0.5 (0.2) | 0.71 |
| Number of nights with actigraphy per participant, mean (SD) | 1.2 (0.7) | 1.4 (0.8) | 0.16 |
| Proportion of hospital nights with actigraphy per participant (SD) | 0.3 (0.2) | 0.3 (0.1) | 0.91 |
| Baseline sleep measures | |||
| PSQI, mean (SD) | 9.9 (4.6) | 9.1 (4.5) | 0.39 |
| ESS, mean (SD) | 7.4 (4.2) | 7.7 (4.8) | 0.79 |
| ISI, mean (SD) | 11.9 (7.6) | 10.8 (7.4) | 0.44 |
| CESD‐10, mean (SD) | 12.2 (7.2) | 12.8 (7.6) | 0.69 |
| Berlin Sleep Apnea, mean (SD) | 0.63 (0.5) | 0.61 (0.5) | 0.87 |
Sleep Diary Measures
A sleep diary completed each morning assessed the outcome measures, perceived sleep quality, how refreshing sleep was, and sleep durations. The diary employed a 5‐point Likert rating scale ranging from poor (1) to excellent (5). Perceived sleep duration was calculated from patients' reported time in bed, time to fall asleep, wake time, and number and duration of awakenings after sleep onset on their sleep diary. These data were used to compute total sleep time (TST) and sleep efficiency (SE). The sleep diary also included other pertinent sleep‐related measures including use of sleep medication the night prior and specific sleep disruptions from the prior night. To measure the impact of disruptions due to disturbances the prior night, we created a summed scale score of 4 items that negatively interfered with sleep (light, temperature, noise, and interruptions; 5 point scales from 1 = not at all to 5 = significant). Analysis of principal axis factors with varimax rotation yielded 1 disruption factor accounting for 55% of the variance, and Cronbach's was 0.73.
Actigraphy Measures
Actigraphy outcomes of sleep were recorded using the actigraphy wrist watch (ActiSleep Plus (GT3X+); ActiGraph, Pensacola, FL). Participants wore the monitor from the day of enrollment throughout the hospital stay or until transfer out of the unit. Objective data were analyzed and scored using ActiLife 6 data analysis software (version 6.10.1; Actigraph). Time in bed, given the unique inpatient setting, was calculated using sleep diary responses as the interval between sleep time and reported wake up time. These were entered into the Actilife 6 software for the sleep scoring analysis using a validated algorithm, Cole‐Kripke, to calculate actigraphy TST and SE.
Statistical Analysis
Descriptive and inferential statistics were computed using Statistical Package for the Social Sciences version 22 (IBM, Armonk, NY). We computed means, proportions, and measures of dispersion for all study variables. To test differences in sleep diary and actigraphy outcomes between the intervention and control arms, we used linear mixed models with full maximum likelihood estimation to model each of the 7 continuous sleep outcomes. These statistical methods are appropriate to account for the nonindependence of continuous repeated observations within hospital patients.[19] For all outcomes, the unit of analysis was nightly observations nested within patient‐ level characteristics. The use of full maximum likelihood estimation is a robust and preferred method for handling values missing at random in longitudinal datasets.[20]
To model repeated observations, mixed models included a term representing time in days. For each outcome, we specified unconditional growth models to examine the variability between and within patients by computing intraclass correlations and inspecting variance components. We used model fit indices (‐2LL deviance, Akaike's information criterion, and Schwartz's Bayesian criterion) as appropriate to determine best fitting model specifications in terms of random effects and covariance structure.[21, 22]
We tested the main effect of the intervention on sleep outcomes and the interactive effect of group (intervention vs control) by hospital day, to test whether there were group differences in slopes representing average change in sleep outcomes over hospital days. All models adjusted for age, body mass index, depression, and baseline sleep quality (PSQI) as time‐invariant covariates, and whether participants had taken a sleep medication the day before, as a time‐varying covariate. Adjustment for prehospitalization sleep quality was a matter of particular importance. We used the PSQI to control for sleep quality because it is both a well‐validated, multidimensional measure, and it includes prehospital use of sleep medications. In a series of sensitivity analyses, we also explored whether the dichotomous self‐reported measure of whether or not participants regularly took sleep medications prior to hospitalization, rather than the PSQI, would change our substantive findings. All covariates were centered at the grand‐mean following guidelines for appropriate interpretation of regression coefficients.[23]
RESULTS
Of the 112 study patients, 48 were in the intervention unit and 64 in the control unit. Eighty‐five percent of study participants endorsed poor sleep prior to hospital admission on the PSQI sleep quality measure, which was similar in both groups (Table 1).
Participants completed 1 to 8 sleep diary entries (mean = 2.5, standard deviation = 1.1). Because only 6 participants completed 5 or more diaries, we constrained the number of diaries included in the inferential analysis to 4 to avoid influential outliers identified by scatterplots. Fifty‐seven percent of participants had 1 night of valid actigraphy data (n = 64); 29%, 2 nights (n = 32), 8% had 3 or 4 nights, and 9 participants did not have any usable actigraphy data. The extent to which the intervention was accepted by patients in the intervention group was highly variable. Unit‐wide patient adherence with the 10 pm lights off, telephone off, and TV off policy was 87%, 67%, and 64% of intervention patients, respectively. Uptake of sleep menu items was also highly variable, and not a single element was used by more than half of patients (acceptance rates ranged from 11% to 44%). Eye masks (44%) and ear plugs (32%) were the most commonly utilized items.
A greater proportion of patients in the control arm (33%) had been taking sleep medications prior to hospitalization compared to the intervention arm (15%; 2 = 4.6, P 0.05). However, hypnotic medication use in the hospital was similar across the both groups (intervention unit patients: 25% and controls: 21%, P = 0.49).
Intraclass correlations for the 7 sleep outcomes ranged from 0.59 to 0.76 on sleep diary outcomes, and from 0.61 to 0.85 on actigraphy. Dependency of sleep measures within patients accounted for 59% to 85% of variance in sleep outcomes. The best‐fit mixed models included random intercepts only. The results of mixed models testing the main effect of intervention versus comparison arm on sleep outcome measures, adjusted for covariates, are presented in Table 2. Total sleep time was the only outcome that was significantly different between groups; the average total sleep time, calculated from sleep diary data, was longer in the intervention group by 49 minutes.
| Intervention, n = 48 | Control, n = 64 | P Value | |
|---|---|---|---|
| |||
| Sleep diary outcomes | |||
| Sleep quality, mean (SE) | 3.14 (0.16) | 3.08 (0.13) | 0.79 |
| Refreshed sleep, mean (SE) | 2.94 (0.17) | 2.74 (0.14) | 0.38 |
| Negative impact of sleep disruptions, mean (SE) | 4.39 (0.58) | 4.81 (0.48) | 0.58 |
| Total sleep time, min, mean (SE) | 422 (16.2) | 373 (13.2) | 0.02 |
| Sleep efficiency, %, mean (SE) | 83.5 (2.3) | 82.1 (1.9) | 0.65 |
| Actigraphy outcomes | |||
| Total sleep time, min, mean (SE) | 377 (16.8) | 356 (13.2) | 0.32 |
| Sleep efficiency, %, mean (SE) | 72.7 (2.2) | 74.8 (1.8) | 0.45 |
Table 3 lists slopes representing average change in sleep measures over hospital days in both groups. The P values represent z tests of interaction terms in mixed models, after adjustment for covariates, testing whether slopes significantly differed between groups. Of the 7 outcomes, 3 sleep diary measures had significant interaction terms. For ratings of sleep quality, refreshing sleep, and sleep disruptions, slopes in the control group were flat, whereas slopes in the intervention group demonstrated improvements in ratings of sleep quality and refreshed sleep, and a decrease in the impact of sleep disruptions over the course of subsequent nights in the hospital. Figure 1 illustrates a plot of the adjusted average slopes for the refreshed sleep score across hospital days in intervention and control groups.
| Intervention, Slope (SE), n = 48 | Control, Slope (SE), n = 64 | P Value | |
|---|---|---|---|
| |||
| Refreshed sleep rating | 0.55 (0.18) | 0.03 (0.13) | 0.006 |
| Sleep quality rating | 0.52 (0.16) | 0.02 (0.11) | 0.012 |
| Negative impact of sleep interruptions | 1.65 (0.48) | 0.05 (0.32) | 0.006 |
| Total sleep time, diary | 11.2 (18.1) | 6.3 (13.0) | 0.44 |
| Total sleep time, actigraphy | 7.3 (25.5) | 1.0 (15.3) | 0.83 |
| Sleep efficiency, diary | 1.1 (2.3) | 1.5 (1.6) | 0.89 |
| Sleep efficiency, actigraphy | 0.9 (4.0) | 0.7 (2.4) | 0.74 |
DISCUSSION
Poor sleep is common among hospitalized adults, both at home prior to the admission and especially when in the hospital. This pilot study demonstrated the feasibility of rolling out a sleep‐promoting intervention on a hospital's general medicine unit. Although participants on the intervention unit reported improved sleep quality and feeling more refreshed, this was not supported by actigraphy data (such as sleep time or sleep efficiency). Although care team engagement and implementation of unit‐wide interventions were high, patient use of individual components was imperfect. Of particular interest, however, the intervention group actually began to have improved sleep quality and fewer disruptions with subsequent nights sleeping in the hospital.
Our findings of the high prevalence of poor sleep among hospitalized patients is congruent with prior studies and supports the great need to screen for and address poor sleep within the hospital setting.[24, 25, 26] Attempts to promote sleep among hospitalized patients may be effective. Prior literature on sleep‐promoting intervention studies demonstrated relaxation techniques improved sleep quality by almost 38%,[27] and ear plugs and eye masks showed some benefit in promoting sleep within the hospital.[28] Our study's multicomponent intervention that attempted to minimize disruptions led to improvement in sleep quality, more restorative sleep, and decreased report of sleep disruptions, especially among patients who had a longer length of stay. As suggested by Thomas et al.[29] and seen in our data, this temporal relationship with improvement across subsequent nights suggests there may be an adaptation to the new environment and that it may take time for the sleep intervention to work.
Hospitalized patients often fail to reclaim the much‐needed restorative sleep at the time when they are most vulnerable. Patients cite routine care as the primary cause of sleep disruption, and often recognize the way that the hospital environment interferes with their ability to sleep.[30, 31, 32] The sleep‐promoting interventions used in our study would be characterized by most as low effort[33] and a potential for high yield, even though our patients only appreciated modest improvements in sleep outcomes.
Several limitations of this study should be considered. First, although we had hoped to collect substantial amounts of objective data, the average time of actigraphy observation was less than 48 hours. This may have constrained the group by time interaction analysis with actigraphy data, as studies have shown increased accuracy in actigraphy measures with longer wear.[34] By contrast, the sleep diary survey collected throughout hospitalization yielded significant improvements in consecutive daily measurements. Second, the proximity of the study units raised concern for study contamination, which could have reduced the differences in the outcome measures that may have been observed. Although the physicians work on both units, the nursing and support care teams are distinct and unit dependent. Finally, this was not a randomized trial. Patient assignment to the treatment arms was haphazard and occurred within the hospital's admitting strategy. Allocation of patients to either the intervention or the control group was based on bed availability at the time of admission. Although both groups were similar in most characteristics, more of the control participants reported taking more sleep medications prior to admission as compared to the intervention participants. Fortunately, hypnotic use was not different between groups during the admission, the time when sleep data were being captured.
Overall, this pilot study suggests that patients admitted to general medical ward fail to realize sufficient restorative sleep when they are in the hospital. Sleep disruption is rather frequent. This study demonstrates the opportunity for and feasibility of sleep‐promoting interventions where facilitating sleep is considered to be a top priority and vital component of the healthcare delivery. When trying to improve patients' sleep in the hospital, it may take several consecutive nights to realize a return on investment.
Acknowledgements
The authors acknowledge the Department of Nursing, Johns Hopkins Bayview Medical Center, and care teams of the Zieve Medicine Units, and the Center for Child and Community Health Research Biostatistics, Epidemiology and Data Management (BEAD) Core group.
Disclosures: Dr. Wright is a Miller‐Coulson Family Scholar and is supported through the Johns Hopkins Center for Innovative Medicine. Dr. Howell is the chief of the Division of Hospital Medicine at Johns Hopkins Bayview Medical Center and associate professor at Johns Hopkins School of Medicine. He served as the president of the Society of Hospital Medicine (SHM) in 2013 and currently serves as a board member. He is also a senior physician advisor for SHM. He is a coinvestigator grant recipient on an Agency for Healthcare Research and Quality grant on medication reconciliation funded through Baylor University. He was previously a coinvestigator grant recipient of Center for Medicare and Medicaid Innovations grant that ended in June 2015.
- Institute of Medicine (US) Committee on Sleep Medicine and Research. Sleep disorders and sleep deprivation: an unmet public health problem. Washington, DC: National Academies Press; 2006. Available at: http://www.ncbi.nlm.nih.gov/books/NBK19960. Accessed September 16, 2014.
- , . Health behaviors of adults: United States, 2005–2007. Vital Health Stat 10. 2010;245:1–132.
- , , . High incidence of diabetes in men with sleep complaints or short sleep duration: a 12‐year follow‐up study of a middle‐aged population. Diabetes Care. 2005;28:2762–2767.
- , , , et al. Linking sleep duration and obesity among black and white US adults. Clin Pract (Lond). 2013;10(5):661–667.
- , , , et al. Gender‐specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700.
- , , , , , . The joint effect of sleep duration and disturbed sleep on cause‐specific mortality: results from the Whitehall II cohort study. PLoS One. 2014;9(4):e91965.
- , , , , , . Poor self‐reported sleep quality predicts mortality within one year of inpatient post‐acute rehabilitation among older adults. Sleep. 2011;34(12):1715–1721.
- , , , , . The effects of sleep deprivation on symptoms of psychopathology in healthy adults. Sleep Med. 2007;8(3):215–221.
- , , , , . Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762.
- , , , . The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–178.
- , , , et al. Insomnia among hospitalized elderly patients: prevalence, clinical characteristics and risk factors. Arch Gerontol Geriatr. 2011;52:133–137.
- , , , et al. Is insomnia a marker for psychiatric disorders in general hospitals? Sleep Med. 2005;6:549–553.
- , , , , , . Perceived control and sleep in hospitalized older adults: a sound hypothesis? J Hosp Med. 2013;8:184–190.
- , , , et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med. 2012;157:170–179.
- . Sleep in acute care settings: an integrative review. J Nurs Scholarsh. 2000;32(1):31–38.
- . Physical health as it relates to insomnia. Talk presented at: Center for Behavior and Health, Lecture Series in Johns Hopkins Bayview Medical Center; July 17, 2012; Baltimore, MD.
- , , , , . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
- , . Measures of sleep: The Insomnia Severity Index, Medical Outcomes Study (MOS) Sleep Scale, Pittsburgh Sleep Diary (PSD), and Pittsburgh Sleep Quality Index (PSQI). Arthritis Rheumatol. 2003;49:S184–S196.
- , . Applied Mixed Models in Medicine. 3rd ed. Somerset, NJ: Wiley; 2014:539.
- , , , Applying mixed regression models to the analysis of repeated‐measures data in psychosomatic medicine. Psychosom Med. 2006;68(6):870–878.
- , . Using the SPSS mixed procedure to fit cross‐sectional and longitudinal multilevel models. Educ Psychol Meas. 2005;65(5):717–741.
- , . Introduction to estimation issues in multilevel modeling. New Dir Inst Res. 2012;2012(154):23–39.
- , . Centering predictor variables in cross‐sectional multilevel models: a new look at an old issue. Psychol Methods. 2007;12(2):121–138.
- , . Sleep quality in adult hospitalized patients with infection: an observational study. Am J Med Sci. 2015;349(1):56–60.
- , , , et al. Risk of sleep apnea in hospitalized older patients. J Clin Sleep Med. 2014;10:1061–1066.
- , , . Hospital ward policy and patients' sleep patterns: a multiple baseline study. Rehabil Psychol. 1989;34(1):43–50.
- , , . Non‐pharmacologic interventions to improve the sleep of hospitalized patients: a systematic review. J Gen Intern Med. 2014;29:788–795.
- , , , , , Earplugs and eye masks vs routine care prevent sleep impairment in post‐anaesthesia care unit: a randomized study. Br J Anaesth. 2014;112(1):89–95.
- , , , et al. Sleep rounds: a multidisciplinary approach to optimize sleep quality and satisfaction in hospitalized patients. J Hosp Med. 2012;7:508–512.
- , , , , . Factors affecting sleep quality of patients in intensive care unit. J Clin Sleep Med. 2012;8(3):301–307.
- . Insomnia among hospitalized older persons. Clin Geriatr Med. 2008;24(1):51–67.
- , , , . A nonpharmacological sleep protocol for hospitalized older patients. J Am Geriatr Soc. 1998;46(6):700–705.
- The Action Priority Matrix: making the most of your opportunities. TimeAnalyzer website. Available at: http://www.timeanalyzer.com/lib/priority.htm. Published 2006. Accessed July 10, 2015.
- , , , et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36(11):1747–1755.
Approximately 70 million adults within the United States have sleep disorders,[1] and up to 30% of adults report sleeping less than 6 hours per night.[2] Poor sleep has been associated with undesirable health outcomes.[1] Suboptimal sleep duration and sleep quality has been associated with a higher prevalence of chronic health conditions including hypertension, type 2 diabetes, coronary artery disease, stroke, and obesity, as well as increased overall mortality.[3, 4, 5, 6, 7]
Sleep plays an important role in restoration of wellness. Poor sleep is associated with physiological disturbances that may result in poor healing.[8, 9, 10] In the literature, prevalence of insomnia among elderly hospitalized patients was 36.7%,[11] whereas in younger hospitalized patients it was 50%.[12] Hospitalized patients frequently cite their acute illness, hospital‐related environmental factors, and disruptions that are part of routine care as causes for poor sleep during hospitalization.[13, 14, 15] Although the pervasiveness of poor sleep among hospitalized patients is high, interventions that prioritize sleep optimization as routine care, are uncommon. Few studies have reviewed the effect of sleep‐promoting measures on both sleep quality and sleep duration among patients hospitalized on general medicine units.
In this study, we aimed to assess the feasibility of incorporating sleep‐promoting interventions on a general medicine unit. We sought to identify differences in sleep measures between intervention and control groups. The primary outcome that we hoped to influence and lengthen in the intervention group was sleep duration. This outcome was measured both by sleep diary and with actigraphy. Secondary outcomes that we hypothesized should improve in the intervention group included feeling more refreshed in the mornings, sleep efficiency, and fewer sleep disruptions. As a feasibility pilot, we also wanted to explore the ease or difficulty with which sleep‐promoting interventions could be incorporated to the team's workflow.
METHODS
Study Design
A quasi‐experimental prospective pilot study was conducted at a single academic center, the Johns Hopkins Bayview Medical Center. Participants included adult patients admitted to the general medicine ward from July 2013 through January 2014. Patients with dementia; inability to complete survey questionnaires due to delirium, disability, or a language barrier; active withdrawal from alcohol or controlled substances; or acute psychiatric illness were excluded in this study.
The medicine ward at our medical center is comprised of 2 structurally identical units that admit patients with similar diagnoses, disease severity, and case‐mix disease groups. Nursing and support staff are unit specific. Pertaining to the sleep environment, the units both have semiprivate and private rooms. Visitors are encouraged to leave by 10 pm. Patients admitted from the emergency room to the medicine ward are assigned haphazardly to either unit based on bed availability. For the purpose of this study, we selected 1 unit to be a control unit and identified the other as the sleep‐promoting intervention unit.
Study Procedure
Upon arrival to the medicine unit, the research team approached all patients who met study eligibility criteria for study participation. Patients were provided full disclosure of the study using institutional research guidelines, and those interested in participating were consented. Participants were not explicitly told about their group assignment. This study was approved by the Johns Hopkins Institutional Review Board for human subject research.
In this study, the control group participants received standard of care as it pertains to sleep promotion. No additional sleep‐promoting measures were implemented to routine medical care, medication administration, nursing care, and overnight monitoring. Patients who used sleep medications at home, prior to admission, had those medicines continued only if they requested them and they were not contraindicated given their acute illness. Participants on the intervention unit were exposed to a nurse‐delivered sleep‐promoting protocol aimed at transforming the culture of care such that helping patients to sleep soundly was made a top priority. Environmental changes included unit‐wide efforts to minimize light and noise disturbances by dimming hallway lights, turning off room lights, and encouraging care teams to be as quiet as possible. Other strategies focused largely on minimizing care‐related disruptions. These included, when appropriate, administering nighttime medications in the early evening, minimizing fluids overnight, and closing patient room doors where appropriate. Further, patients were offered the following sleep‐promoting items to choose from: ear plugs, eye masks, warm blankets, and relaxation music. The final component of our intervention was 30‐minute sleep hygiene education taught by a physician. It highlighted basic sleep physiology and healthy sleep behavior adapted from Buysse.[16] Patients learned the role of behaviors such as reducing time lying awake in bed, setting standard wake‐up time and sleep time, and going to bed only when sleepy. This behavioral education was supplemented by a handout with sleep‐promoting suggestions.
The care team on the intervention unit received comprehensive study‐focused training in which night nursing teams were familiarized with the sleep‐promoting protocol through in‐service sessions facilitated by 1 of the authors (E.W.G.). To further promote study implementation, sleep‐promoting procedures were supported and encouraged by supervising nurses who made daily reminders to the intervention unit night care team of the goals of the sleep‐promoting study during evening huddles performed at the beginning of each shift. To assess the adherence of the sleep protocol, the nursing staff completed a daily checklist of elements within the protocol that were employed .
Data Collection and Measures
Baseline Measures
At the time of enrollment, study patients' demographic information, including use of chronic sleep medication prior to admission, was collected. Participants were assessed for baseline sleep disturbance prior to admission using standardized, validated sleep assessment tools: Pittsburgh Sleep Quality Index (PSQI), the Insomnia Severity Index (ISI), and the Epworth Sleepiness Scale (ESS). PSQI, a 19‐item tool, assessed self‐rated sleep quality measured over the prior month; a score of 5 or greater indicated poor sleep.[17] ISI, a 7‐item tool, identified the presence, rated the severity, and described the impact of insomnia; a score of 10 or greater indicated insomnia.[18] ESS, an 8‐item self‐rated tool, evaluated the impact of perceived sleepiness on daily functioning in 8 different environments; a score of 9 or greater was linked to burden of sleepiness. Participants were also screened for both obstructive sleep apnea (using the Berlin Sleep Apnea Index) and clinical depression (using Center for Epidemiologic Studies‐Depression 10‐point scale), as these conditions affect sleep patterns. These data are shown in Table 1.
| Intervention, n = 48 | Control, n = 64 | P Value | |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 58.2 (16) | 56.9 (17) | 0.69 |
| Female, n (%) | 26 (54.2) | 36 (56.3) | 0.83 |
| Race, n (%) | |||
| Caucasian | 33 (68.8) | 46 (71.9) | 0.92 |
| African American | 13 (27.1) | 16 (25.0) | |
| Other | 2 (4.2) | 2 (3.1) | |
| BMI, mean (SD) | 32.1 (9.2) | 31.8 (9.3) | 0.85 |
| Admitting service, n (%) | |||
| Teaching | 21 (43.8) | 18 (28.1) | 0.09 |
| Nonteaching | 27 (56.3) | 46 (71.9) | |
| Sleep medication prior to admission, n (%) | 7 (14.9) | 21 (32.8) | 0.03 |
| Length of stay, d, mean (SD) | 4.9 (3) | 5.8 (3.9) | 0.19 |
| Number of sleep diaries per participant, mean (SD) | 2.2 (0.8) | 2.6 (0.9) | 0.02 |
| Proportion of hospital days with sleep diaries per participant, (SD) | 0.6 (0.2) | 0.5 (0.2) | 0.71 |
| Number of nights with actigraphy per participant, mean (SD) | 1.2 (0.7) | 1.4 (0.8) | 0.16 |
| Proportion of hospital nights with actigraphy per participant (SD) | 0.3 (0.2) | 0.3 (0.1) | 0.91 |
| Baseline sleep measures | |||
| PSQI, mean (SD) | 9.9 (4.6) | 9.1 (4.5) | 0.39 |
| ESS, mean (SD) | 7.4 (4.2) | 7.7 (4.8) | 0.79 |
| ISI, mean (SD) | 11.9 (7.6) | 10.8 (7.4) | 0.44 |
| CESD‐10, mean (SD) | 12.2 (7.2) | 12.8 (7.6) | 0.69 |
| Berlin Sleep Apnea, mean (SD) | 0.63 (0.5) | 0.61 (0.5) | 0.87 |
Sleep Diary Measures
A sleep diary completed each morning assessed the outcome measures, perceived sleep quality, how refreshing sleep was, and sleep durations. The diary employed a 5‐point Likert rating scale ranging from poor (1) to excellent (5). Perceived sleep duration was calculated from patients' reported time in bed, time to fall asleep, wake time, and number and duration of awakenings after sleep onset on their sleep diary. These data were used to compute total sleep time (TST) and sleep efficiency (SE). The sleep diary also included other pertinent sleep‐related measures including use of sleep medication the night prior and specific sleep disruptions from the prior night. To measure the impact of disruptions due to disturbances the prior night, we created a summed scale score of 4 items that negatively interfered with sleep (light, temperature, noise, and interruptions; 5 point scales from 1 = not at all to 5 = significant). Analysis of principal axis factors with varimax rotation yielded 1 disruption factor accounting for 55% of the variance, and Cronbach's was 0.73.
Actigraphy Measures
Actigraphy outcomes of sleep were recorded using the actigraphy wrist watch (ActiSleep Plus (GT3X+); ActiGraph, Pensacola, FL). Participants wore the monitor from the day of enrollment throughout the hospital stay or until transfer out of the unit. Objective data were analyzed and scored using ActiLife 6 data analysis software (version 6.10.1; Actigraph). Time in bed, given the unique inpatient setting, was calculated using sleep diary responses as the interval between sleep time and reported wake up time. These were entered into the Actilife 6 software for the sleep scoring analysis using a validated algorithm, Cole‐Kripke, to calculate actigraphy TST and SE.
Statistical Analysis
Descriptive and inferential statistics were computed using Statistical Package for the Social Sciences version 22 (IBM, Armonk, NY). We computed means, proportions, and measures of dispersion for all study variables. To test differences in sleep diary and actigraphy outcomes between the intervention and control arms, we used linear mixed models with full maximum likelihood estimation to model each of the 7 continuous sleep outcomes. These statistical methods are appropriate to account for the nonindependence of continuous repeated observations within hospital patients.[19] For all outcomes, the unit of analysis was nightly observations nested within patient‐ level characteristics. The use of full maximum likelihood estimation is a robust and preferred method for handling values missing at random in longitudinal datasets.[20]
To model repeated observations, mixed models included a term representing time in days. For each outcome, we specified unconditional growth models to examine the variability between and within patients by computing intraclass correlations and inspecting variance components. We used model fit indices (‐2LL deviance, Akaike's information criterion, and Schwartz's Bayesian criterion) as appropriate to determine best fitting model specifications in terms of random effects and covariance structure.[21, 22]
We tested the main effect of the intervention on sleep outcomes and the interactive effect of group (intervention vs control) by hospital day, to test whether there were group differences in slopes representing average change in sleep outcomes over hospital days. All models adjusted for age, body mass index, depression, and baseline sleep quality (PSQI) as time‐invariant covariates, and whether participants had taken a sleep medication the day before, as a time‐varying covariate. Adjustment for prehospitalization sleep quality was a matter of particular importance. We used the PSQI to control for sleep quality because it is both a well‐validated, multidimensional measure, and it includes prehospital use of sleep medications. In a series of sensitivity analyses, we also explored whether the dichotomous self‐reported measure of whether or not participants regularly took sleep medications prior to hospitalization, rather than the PSQI, would change our substantive findings. All covariates were centered at the grand‐mean following guidelines for appropriate interpretation of regression coefficients.[23]
RESULTS
Of the 112 study patients, 48 were in the intervention unit and 64 in the control unit. Eighty‐five percent of study participants endorsed poor sleep prior to hospital admission on the PSQI sleep quality measure, which was similar in both groups (Table 1).
Participants completed 1 to 8 sleep diary entries (mean = 2.5, standard deviation = 1.1). Because only 6 participants completed 5 or more diaries, we constrained the number of diaries included in the inferential analysis to 4 to avoid influential outliers identified by scatterplots. Fifty‐seven percent of participants had 1 night of valid actigraphy data (n = 64); 29%, 2 nights (n = 32), 8% had 3 or 4 nights, and 9 participants did not have any usable actigraphy data. The extent to which the intervention was accepted by patients in the intervention group was highly variable. Unit‐wide patient adherence with the 10 pm lights off, telephone off, and TV off policy was 87%, 67%, and 64% of intervention patients, respectively. Uptake of sleep menu items was also highly variable, and not a single element was used by more than half of patients (acceptance rates ranged from 11% to 44%). Eye masks (44%) and ear plugs (32%) were the most commonly utilized items.
A greater proportion of patients in the control arm (33%) had been taking sleep medications prior to hospitalization compared to the intervention arm (15%; 2 = 4.6, P 0.05). However, hypnotic medication use in the hospital was similar across the both groups (intervention unit patients: 25% and controls: 21%, P = 0.49).
Intraclass correlations for the 7 sleep outcomes ranged from 0.59 to 0.76 on sleep diary outcomes, and from 0.61 to 0.85 on actigraphy. Dependency of sleep measures within patients accounted for 59% to 85% of variance in sleep outcomes. The best‐fit mixed models included random intercepts only. The results of mixed models testing the main effect of intervention versus comparison arm on sleep outcome measures, adjusted for covariates, are presented in Table 2. Total sleep time was the only outcome that was significantly different between groups; the average total sleep time, calculated from sleep diary data, was longer in the intervention group by 49 minutes.
| Intervention, n = 48 | Control, n = 64 | P Value | |
|---|---|---|---|
| |||
| Sleep diary outcomes | |||
| Sleep quality, mean (SE) | 3.14 (0.16) | 3.08 (0.13) | 0.79 |
| Refreshed sleep, mean (SE) | 2.94 (0.17) | 2.74 (0.14) | 0.38 |
| Negative impact of sleep disruptions, mean (SE) | 4.39 (0.58) | 4.81 (0.48) | 0.58 |
| Total sleep time, min, mean (SE) | 422 (16.2) | 373 (13.2) | 0.02 |
| Sleep efficiency, %, mean (SE) | 83.5 (2.3) | 82.1 (1.9) | 0.65 |
| Actigraphy outcomes | |||
| Total sleep time, min, mean (SE) | 377 (16.8) | 356 (13.2) | 0.32 |
| Sleep efficiency, %, mean (SE) | 72.7 (2.2) | 74.8 (1.8) | 0.45 |
Table 3 lists slopes representing average change in sleep measures over hospital days in both groups. The P values represent z tests of interaction terms in mixed models, after adjustment for covariates, testing whether slopes significantly differed between groups. Of the 7 outcomes, 3 sleep diary measures had significant interaction terms. For ratings of sleep quality, refreshing sleep, and sleep disruptions, slopes in the control group were flat, whereas slopes in the intervention group demonstrated improvements in ratings of sleep quality and refreshed sleep, and a decrease in the impact of sleep disruptions over the course of subsequent nights in the hospital. Figure 1 illustrates a plot of the adjusted average slopes for the refreshed sleep score across hospital days in intervention and control groups.
| Intervention, Slope (SE), n = 48 | Control, Slope (SE), n = 64 | P Value | |
|---|---|---|---|
| |||
| Refreshed sleep rating | 0.55 (0.18) | 0.03 (0.13) | 0.006 |
| Sleep quality rating | 0.52 (0.16) | 0.02 (0.11) | 0.012 |
| Negative impact of sleep interruptions | 1.65 (0.48) | 0.05 (0.32) | 0.006 |
| Total sleep time, diary | 11.2 (18.1) | 6.3 (13.0) | 0.44 |
| Total sleep time, actigraphy | 7.3 (25.5) | 1.0 (15.3) | 0.83 |
| Sleep efficiency, diary | 1.1 (2.3) | 1.5 (1.6) | 0.89 |
| Sleep efficiency, actigraphy | 0.9 (4.0) | 0.7 (2.4) | 0.74 |

DISCUSSION
Poor sleep is common among hospitalized adults, both at home prior to the admission and especially when in the hospital. This pilot study demonstrated the feasibility of rolling out a sleep‐promoting intervention on a hospital's general medicine unit. Although participants on the intervention unit reported improved sleep quality and feeling more refreshed, this was not supported by actigraphy data (such as sleep time or sleep efficiency). Although care team engagement and implementation of unit‐wide interventions were high, patient use of individual components was imperfect. Of particular interest, however, the intervention group actually began to have improved sleep quality and fewer disruptions with subsequent nights sleeping in the hospital.
Our findings of the high prevalence of poor sleep among hospitalized patients is congruent with prior studies and supports the great need to screen for and address poor sleep within the hospital setting.[24, 25, 26] Attempts to promote sleep among hospitalized patients may be effective. Prior literature on sleep‐promoting intervention studies demonstrated relaxation techniques improved sleep quality by almost 38%,[27] and ear plugs and eye masks showed some benefit in promoting sleep within the hospital.[28] Our study's multicomponent intervention that attempted to minimize disruptions led to improvement in sleep quality, more restorative sleep, and decreased report of sleep disruptions, especially among patients who had a longer length of stay. As suggested by Thomas et al.[29] and seen in our data, this temporal relationship with improvement across subsequent nights suggests there may be an adaptation to the new environment and that it may take time for the sleep intervention to work.
Hospitalized patients often fail to reclaim the much‐needed restorative sleep at the time when they are most vulnerable. Patients cite routine care as the primary cause of sleep disruption, and often recognize the way that the hospital environment interferes with their ability to sleep.[30, 31, 32] The sleep‐promoting interventions used in our study would be characterized by most as low effort[33] and a potential for high yield, even though our patients only appreciated modest improvements in sleep outcomes.
Several limitations of this study should be considered. First, although we had hoped to collect substantial amounts of objective data, the average time of actigraphy observation was less than 48 hours. This may have constrained the group by time interaction analysis with actigraphy data, as studies have shown increased accuracy in actigraphy measures with longer wear.[34] By contrast, the sleep diary survey collected throughout hospitalization yielded significant improvements in consecutive daily measurements. Second, the proximity of the study units raised concern for study contamination, which could have reduced the differences in the outcome measures that may have been observed. Although the physicians work on both units, the nursing and support care teams are distinct and unit dependent. Finally, this was not a randomized trial. Patient assignment to the treatment arms was haphazard and occurred within the hospital's admitting strategy. Allocation of patients to either the intervention or the control group was based on bed availability at the time of admission. Although both groups were similar in most characteristics, more of the control participants reported taking more sleep medications prior to admission as compared to the intervention participants. Fortunately, hypnotic use was not different between groups during the admission, the time when sleep data were being captured.
Overall, this pilot study suggests that patients admitted to general medical ward fail to realize sufficient restorative sleep when they are in the hospital. Sleep disruption is rather frequent. This study demonstrates the opportunity for and feasibility of sleep‐promoting interventions where facilitating sleep is considered to be a top priority and vital component of the healthcare delivery. When trying to improve patients' sleep in the hospital, it may take several consecutive nights to realize a return on investment.
Acknowledgements
The authors acknowledge the Department of Nursing, Johns Hopkins Bayview Medical Center, and care teams of the Zieve Medicine Units, and the Center for Child and Community Health Research Biostatistics, Epidemiology and Data Management (BEAD) Core group.
Disclosures: Dr. Wright is a Miller‐Coulson Family Scholar and is supported through the Johns Hopkins Center for Innovative Medicine. Dr. Howell is the chief of the Division of Hospital Medicine at Johns Hopkins Bayview Medical Center and associate professor at Johns Hopkins School of Medicine. He served as the president of the Society of Hospital Medicine (SHM) in 2013 and currently serves as a board member. He is also a senior physician advisor for SHM. He is a coinvestigator grant recipient on an Agency for Healthcare Research and Quality grant on medication reconciliation funded through Baylor University. He was previously a coinvestigator grant recipient of Center for Medicare and Medicaid Innovations grant that ended in June 2015.
Approximately 70 million adults within the United States have sleep disorders,[1] and up to 30% of adults report sleeping less than 6 hours per night.[2] Poor sleep has been associated with undesirable health outcomes.[1] Suboptimal sleep duration and sleep quality has been associated with a higher prevalence of chronic health conditions including hypertension, type 2 diabetes, coronary artery disease, stroke, and obesity, as well as increased overall mortality.[3, 4, 5, 6, 7]
Sleep plays an important role in restoration of wellness. Poor sleep is associated with physiological disturbances that may result in poor healing.[8, 9, 10] In the literature, prevalence of insomnia among elderly hospitalized patients was 36.7%,[11] whereas in younger hospitalized patients it was 50%.[12] Hospitalized patients frequently cite their acute illness, hospital‐related environmental factors, and disruptions that are part of routine care as causes for poor sleep during hospitalization.[13, 14, 15] Although the pervasiveness of poor sleep among hospitalized patients is high, interventions that prioritize sleep optimization as routine care, are uncommon. Few studies have reviewed the effect of sleep‐promoting measures on both sleep quality and sleep duration among patients hospitalized on general medicine units.
In this study, we aimed to assess the feasibility of incorporating sleep‐promoting interventions on a general medicine unit. We sought to identify differences in sleep measures between intervention and control groups. The primary outcome that we hoped to influence and lengthen in the intervention group was sleep duration. This outcome was measured both by sleep diary and with actigraphy. Secondary outcomes that we hypothesized should improve in the intervention group included feeling more refreshed in the mornings, sleep efficiency, and fewer sleep disruptions. As a feasibility pilot, we also wanted to explore the ease or difficulty with which sleep‐promoting interventions could be incorporated to the team's workflow.
METHODS
Study Design
A quasi‐experimental prospective pilot study was conducted at a single academic center, the Johns Hopkins Bayview Medical Center. Participants included adult patients admitted to the general medicine ward from July 2013 through January 2014. Patients with dementia; inability to complete survey questionnaires due to delirium, disability, or a language barrier; active withdrawal from alcohol or controlled substances; or acute psychiatric illness were excluded in this study.
The medicine ward at our medical center is comprised of 2 structurally identical units that admit patients with similar diagnoses, disease severity, and case‐mix disease groups. Nursing and support staff are unit specific. Pertaining to the sleep environment, the units both have semiprivate and private rooms. Visitors are encouraged to leave by 10 pm. Patients admitted from the emergency room to the medicine ward are assigned haphazardly to either unit based on bed availability. For the purpose of this study, we selected 1 unit to be a control unit and identified the other as the sleep‐promoting intervention unit.
Study Procedure
Upon arrival to the medicine unit, the research team approached all patients who met study eligibility criteria for study participation. Patients were provided full disclosure of the study using institutional research guidelines, and those interested in participating were consented. Participants were not explicitly told about their group assignment. This study was approved by the Johns Hopkins Institutional Review Board for human subject research.
In this study, the control group participants received standard of care as it pertains to sleep promotion. No additional sleep‐promoting measures were implemented to routine medical care, medication administration, nursing care, and overnight monitoring. Patients who used sleep medications at home, prior to admission, had those medicines continued only if they requested them and they were not contraindicated given their acute illness. Participants on the intervention unit were exposed to a nurse‐delivered sleep‐promoting protocol aimed at transforming the culture of care such that helping patients to sleep soundly was made a top priority. Environmental changes included unit‐wide efforts to minimize light and noise disturbances by dimming hallway lights, turning off room lights, and encouraging care teams to be as quiet as possible. Other strategies focused largely on minimizing care‐related disruptions. These included, when appropriate, administering nighttime medications in the early evening, minimizing fluids overnight, and closing patient room doors where appropriate. Further, patients were offered the following sleep‐promoting items to choose from: ear plugs, eye masks, warm blankets, and relaxation music. The final component of our intervention was 30‐minute sleep hygiene education taught by a physician. It highlighted basic sleep physiology and healthy sleep behavior adapted from Buysse.[16] Patients learned the role of behaviors such as reducing time lying awake in bed, setting standard wake‐up time and sleep time, and going to bed only when sleepy. This behavioral education was supplemented by a handout with sleep‐promoting suggestions.
The care team on the intervention unit received comprehensive study‐focused training in which night nursing teams were familiarized with the sleep‐promoting protocol through in‐service sessions facilitated by 1 of the authors (E.W.G.). To further promote study implementation, sleep‐promoting procedures were supported and encouraged by supervising nurses who made daily reminders to the intervention unit night care team of the goals of the sleep‐promoting study during evening huddles performed at the beginning of each shift. To assess the adherence of the sleep protocol, the nursing staff completed a daily checklist of elements within the protocol that were employed .
Data Collection and Measures
Baseline Measures
At the time of enrollment, study patients' demographic information, including use of chronic sleep medication prior to admission, was collected. Participants were assessed for baseline sleep disturbance prior to admission using standardized, validated sleep assessment tools: Pittsburgh Sleep Quality Index (PSQI), the Insomnia Severity Index (ISI), and the Epworth Sleepiness Scale (ESS). PSQI, a 19‐item tool, assessed self‐rated sleep quality measured over the prior month; a score of 5 or greater indicated poor sleep.[17] ISI, a 7‐item tool, identified the presence, rated the severity, and described the impact of insomnia; a score of 10 or greater indicated insomnia.[18] ESS, an 8‐item self‐rated tool, evaluated the impact of perceived sleepiness on daily functioning in 8 different environments; a score of 9 or greater was linked to burden of sleepiness. Participants were also screened for both obstructive sleep apnea (using the Berlin Sleep Apnea Index) and clinical depression (using Center for Epidemiologic Studies‐Depression 10‐point scale), as these conditions affect sleep patterns. These data are shown in Table 1.
| Intervention, n = 48 | Control, n = 64 | P Value | |
|---|---|---|---|
| |||
| Age, y, mean (SD) | 58.2 (16) | 56.9 (17) | 0.69 |
| Female, n (%) | 26 (54.2) | 36 (56.3) | 0.83 |
| Race, n (%) | |||
| Caucasian | 33 (68.8) | 46 (71.9) | 0.92 |
| African American | 13 (27.1) | 16 (25.0) | |
| Other | 2 (4.2) | 2 (3.1) | |
| BMI, mean (SD) | 32.1 (9.2) | 31.8 (9.3) | 0.85 |
| Admitting service, n (%) | |||
| Teaching | 21 (43.8) | 18 (28.1) | 0.09 |
| Nonteaching | 27 (56.3) | 46 (71.9) | |
| Sleep medication prior to admission, n (%) | 7 (14.9) | 21 (32.8) | 0.03 |
| Length of stay, d, mean (SD) | 4.9 (3) | 5.8 (3.9) | 0.19 |
| Number of sleep diaries per participant, mean (SD) | 2.2 (0.8) | 2.6 (0.9) | 0.02 |
| Proportion of hospital days with sleep diaries per participant, (SD) | 0.6 (0.2) | 0.5 (0.2) | 0.71 |
| Number of nights with actigraphy per participant, mean (SD) | 1.2 (0.7) | 1.4 (0.8) | 0.16 |
| Proportion of hospital nights with actigraphy per participant (SD) | 0.3 (0.2) | 0.3 (0.1) | 0.91 |
| Baseline sleep measures | |||
| PSQI, mean (SD) | 9.9 (4.6) | 9.1 (4.5) | 0.39 |
| ESS, mean (SD) | 7.4 (4.2) | 7.7 (4.8) | 0.79 |
| ISI, mean (SD) | 11.9 (7.6) | 10.8 (7.4) | 0.44 |
| CESD‐10, mean (SD) | 12.2 (7.2) | 12.8 (7.6) | 0.69 |
| Berlin Sleep Apnea, mean (SD) | 0.63 (0.5) | 0.61 (0.5) | 0.87 |
Sleep Diary Measures
A sleep diary completed each morning assessed the outcome measures, perceived sleep quality, how refreshing sleep was, and sleep durations. The diary employed a 5‐point Likert rating scale ranging from poor (1) to excellent (5). Perceived sleep duration was calculated from patients' reported time in bed, time to fall asleep, wake time, and number and duration of awakenings after sleep onset on their sleep diary. These data were used to compute total sleep time (TST) and sleep efficiency (SE). The sleep diary also included other pertinent sleep‐related measures including use of sleep medication the night prior and specific sleep disruptions from the prior night. To measure the impact of disruptions due to disturbances the prior night, we created a summed scale score of 4 items that negatively interfered with sleep (light, temperature, noise, and interruptions; 5 point scales from 1 = not at all to 5 = significant). Analysis of principal axis factors with varimax rotation yielded 1 disruption factor accounting for 55% of the variance, and Cronbach's was 0.73.
Actigraphy Measures
Actigraphy outcomes of sleep were recorded using the actigraphy wrist watch (ActiSleep Plus (GT3X+); ActiGraph, Pensacola, FL). Participants wore the monitor from the day of enrollment throughout the hospital stay or until transfer out of the unit. Objective data were analyzed and scored using ActiLife 6 data analysis software (version 6.10.1; Actigraph). Time in bed, given the unique inpatient setting, was calculated using sleep diary responses as the interval between sleep time and reported wake up time. These were entered into the Actilife 6 software for the sleep scoring analysis using a validated algorithm, Cole‐Kripke, to calculate actigraphy TST and SE.
Statistical Analysis
Descriptive and inferential statistics were computed using Statistical Package for the Social Sciences version 22 (IBM, Armonk, NY). We computed means, proportions, and measures of dispersion for all study variables. To test differences in sleep diary and actigraphy outcomes between the intervention and control arms, we used linear mixed models with full maximum likelihood estimation to model each of the 7 continuous sleep outcomes. These statistical methods are appropriate to account for the nonindependence of continuous repeated observations within hospital patients.[19] For all outcomes, the unit of analysis was nightly observations nested within patient‐ level characteristics. The use of full maximum likelihood estimation is a robust and preferred method for handling values missing at random in longitudinal datasets.[20]
To model repeated observations, mixed models included a term representing time in days. For each outcome, we specified unconditional growth models to examine the variability between and within patients by computing intraclass correlations and inspecting variance components. We used model fit indices (‐2LL deviance, Akaike's information criterion, and Schwartz's Bayesian criterion) as appropriate to determine best fitting model specifications in terms of random effects and covariance structure.[21, 22]
We tested the main effect of the intervention on sleep outcomes and the interactive effect of group (intervention vs control) by hospital day, to test whether there were group differences in slopes representing average change in sleep outcomes over hospital days. All models adjusted for age, body mass index, depression, and baseline sleep quality (PSQI) as time‐invariant covariates, and whether participants had taken a sleep medication the day before, as a time‐varying covariate. Adjustment for prehospitalization sleep quality was a matter of particular importance. We used the PSQI to control for sleep quality because it is both a well‐validated, multidimensional measure, and it includes prehospital use of sleep medications. In a series of sensitivity analyses, we also explored whether the dichotomous self‐reported measure of whether or not participants regularly took sleep medications prior to hospitalization, rather than the PSQI, would change our substantive findings. All covariates were centered at the grand‐mean following guidelines for appropriate interpretation of regression coefficients.[23]
RESULTS
Of the 112 study patients, 48 were in the intervention unit and 64 in the control unit. Eighty‐five percent of study participants endorsed poor sleep prior to hospital admission on the PSQI sleep quality measure, which was similar in both groups (Table 1).
Participants completed 1 to 8 sleep diary entries (mean = 2.5, standard deviation = 1.1). Because only 6 participants completed 5 or more diaries, we constrained the number of diaries included in the inferential analysis to 4 to avoid influential outliers identified by scatterplots. Fifty‐seven percent of participants had 1 night of valid actigraphy data (n = 64); 29%, 2 nights (n = 32), 8% had 3 or 4 nights, and 9 participants did not have any usable actigraphy data. The extent to which the intervention was accepted by patients in the intervention group was highly variable. Unit‐wide patient adherence with the 10 pm lights off, telephone off, and TV off policy was 87%, 67%, and 64% of intervention patients, respectively. Uptake of sleep menu items was also highly variable, and not a single element was used by more than half of patients (acceptance rates ranged from 11% to 44%). Eye masks (44%) and ear plugs (32%) were the most commonly utilized items.
A greater proportion of patients in the control arm (33%) had been taking sleep medications prior to hospitalization compared to the intervention arm (15%; 2 = 4.6, P 0.05). However, hypnotic medication use in the hospital was similar across the both groups (intervention unit patients: 25% and controls: 21%, P = 0.49).
Intraclass correlations for the 7 sleep outcomes ranged from 0.59 to 0.76 on sleep diary outcomes, and from 0.61 to 0.85 on actigraphy. Dependency of sleep measures within patients accounted for 59% to 85% of variance in sleep outcomes. The best‐fit mixed models included random intercepts only. The results of mixed models testing the main effect of intervention versus comparison arm on sleep outcome measures, adjusted for covariates, are presented in Table 2. Total sleep time was the only outcome that was significantly different between groups; the average total sleep time, calculated from sleep diary data, was longer in the intervention group by 49 minutes.
| Intervention, n = 48 | Control, n = 64 | P Value | |
|---|---|---|---|
| |||
| Sleep diary outcomes | |||
| Sleep quality, mean (SE) | 3.14 (0.16) | 3.08 (0.13) | 0.79 |
| Refreshed sleep, mean (SE) | 2.94 (0.17) | 2.74 (0.14) | 0.38 |
| Negative impact of sleep disruptions, mean (SE) | 4.39 (0.58) | 4.81 (0.48) | 0.58 |
| Total sleep time, min, mean (SE) | 422 (16.2) | 373 (13.2) | 0.02 |
| Sleep efficiency, %, mean (SE) | 83.5 (2.3) | 82.1 (1.9) | 0.65 |
| Actigraphy outcomes | |||
| Total sleep time, min, mean (SE) | 377 (16.8) | 356 (13.2) | 0.32 |
| Sleep efficiency, %, mean (SE) | 72.7 (2.2) | 74.8 (1.8) | 0.45 |
Table 3 lists slopes representing average change in sleep measures over hospital days in both groups. The P values represent z tests of interaction terms in mixed models, after adjustment for covariates, testing whether slopes significantly differed between groups. Of the 7 outcomes, 3 sleep diary measures had significant interaction terms. For ratings of sleep quality, refreshing sleep, and sleep disruptions, slopes in the control group were flat, whereas slopes in the intervention group demonstrated improvements in ratings of sleep quality and refreshed sleep, and a decrease in the impact of sleep disruptions over the course of subsequent nights in the hospital. Figure 1 illustrates a plot of the adjusted average slopes for the refreshed sleep score across hospital days in intervention and control groups.
| Intervention, Slope (SE), n = 48 | Control, Slope (SE), n = 64 | P Value | |
|---|---|---|---|
| |||
| Refreshed sleep rating | 0.55 (0.18) | 0.03 (0.13) | 0.006 |
| Sleep quality rating | 0.52 (0.16) | 0.02 (0.11) | 0.012 |
| Negative impact of sleep interruptions | 1.65 (0.48) | 0.05 (0.32) | 0.006 |
| Total sleep time, diary | 11.2 (18.1) | 6.3 (13.0) | 0.44 |
| Total sleep time, actigraphy | 7.3 (25.5) | 1.0 (15.3) | 0.83 |
| Sleep efficiency, diary | 1.1 (2.3) | 1.5 (1.6) | 0.89 |
| Sleep efficiency, actigraphy | 0.9 (4.0) | 0.7 (2.4) | 0.74 |

DISCUSSION
Poor sleep is common among hospitalized adults, both at home prior to the admission and especially when in the hospital. This pilot study demonstrated the feasibility of rolling out a sleep‐promoting intervention on a hospital's general medicine unit. Although participants on the intervention unit reported improved sleep quality and feeling more refreshed, this was not supported by actigraphy data (such as sleep time or sleep efficiency). Although care team engagement and implementation of unit‐wide interventions were high, patient use of individual components was imperfect. Of particular interest, however, the intervention group actually began to have improved sleep quality and fewer disruptions with subsequent nights sleeping in the hospital.
Our findings of the high prevalence of poor sleep among hospitalized patients is congruent with prior studies and supports the great need to screen for and address poor sleep within the hospital setting.[24, 25, 26] Attempts to promote sleep among hospitalized patients may be effective. Prior literature on sleep‐promoting intervention studies demonstrated relaxation techniques improved sleep quality by almost 38%,[27] and ear plugs and eye masks showed some benefit in promoting sleep within the hospital.[28] Our study's multicomponent intervention that attempted to minimize disruptions led to improvement in sleep quality, more restorative sleep, and decreased report of sleep disruptions, especially among patients who had a longer length of stay. As suggested by Thomas et al.[29] and seen in our data, this temporal relationship with improvement across subsequent nights suggests there may be an adaptation to the new environment and that it may take time for the sleep intervention to work.
Hospitalized patients often fail to reclaim the much‐needed restorative sleep at the time when they are most vulnerable. Patients cite routine care as the primary cause of sleep disruption, and often recognize the way that the hospital environment interferes with their ability to sleep.[30, 31, 32] The sleep‐promoting interventions used in our study would be characterized by most as low effort[33] and a potential for high yield, even though our patients only appreciated modest improvements in sleep outcomes.
Several limitations of this study should be considered. First, although we had hoped to collect substantial amounts of objective data, the average time of actigraphy observation was less than 48 hours. This may have constrained the group by time interaction analysis with actigraphy data, as studies have shown increased accuracy in actigraphy measures with longer wear.[34] By contrast, the sleep diary survey collected throughout hospitalization yielded significant improvements in consecutive daily measurements. Second, the proximity of the study units raised concern for study contamination, which could have reduced the differences in the outcome measures that may have been observed. Although the physicians work on both units, the nursing and support care teams are distinct and unit dependent. Finally, this was not a randomized trial. Patient assignment to the treatment arms was haphazard and occurred within the hospital's admitting strategy. Allocation of patients to either the intervention or the control group was based on bed availability at the time of admission. Although both groups were similar in most characteristics, more of the control participants reported taking more sleep medications prior to admission as compared to the intervention participants. Fortunately, hypnotic use was not different between groups during the admission, the time when sleep data were being captured.
Overall, this pilot study suggests that patients admitted to general medical ward fail to realize sufficient restorative sleep when they are in the hospital. Sleep disruption is rather frequent. This study demonstrates the opportunity for and feasibility of sleep‐promoting interventions where facilitating sleep is considered to be a top priority and vital component of the healthcare delivery. When trying to improve patients' sleep in the hospital, it may take several consecutive nights to realize a return on investment.
Acknowledgements
The authors acknowledge the Department of Nursing, Johns Hopkins Bayview Medical Center, and care teams of the Zieve Medicine Units, and the Center for Child and Community Health Research Biostatistics, Epidemiology and Data Management (BEAD) Core group.
Disclosures: Dr. Wright is a Miller‐Coulson Family Scholar and is supported through the Johns Hopkins Center for Innovative Medicine. Dr. Howell is the chief of the Division of Hospital Medicine at Johns Hopkins Bayview Medical Center and associate professor at Johns Hopkins School of Medicine. He served as the president of the Society of Hospital Medicine (SHM) in 2013 and currently serves as a board member. He is also a senior physician advisor for SHM. He is a coinvestigator grant recipient on an Agency for Healthcare Research and Quality grant on medication reconciliation funded through Baylor University. He was previously a coinvestigator grant recipient of Center for Medicare and Medicaid Innovations grant that ended in June 2015.
- Institute of Medicine (US) Committee on Sleep Medicine and Research. Sleep disorders and sleep deprivation: an unmet public health problem. Washington, DC: National Academies Press; 2006. Available at: http://www.ncbi.nlm.nih.gov/books/NBK19960. Accessed September 16, 2014.
- , . Health behaviors of adults: United States, 2005–2007. Vital Health Stat 10. 2010;245:1–132.
- , , . High incidence of diabetes in men with sleep complaints or short sleep duration: a 12‐year follow‐up study of a middle‐aged population. Diabetes Care. 2005;28:2762–2767.
- , , , et al. Linking sleep duration and obesity among black and white US adults. Clin Pract (Lond). 2013;10(5):661–667.
- , , , et al. Gender‐specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700.
- , , , , , . The joint effect of sleep duration and disturbed sleep on cause‐specific mortality: results from the Whitehall II cohort study. PLoS One. 2014;9(4):e91965.
- , , , , , . Poor self‐reported sleep quality predicts mortality within one year of inpatient post‐acute rehabilitation among older adults. Sleep. 2011;34(12):1715–1721.
- , , , , . The effects of sleep deprivation on symptoms of psychopathology in healthy adults. Sleep Med. 2007;8(3):215–221.
- , , , , . Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762.
- , , , . The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–178.
- , , , et al. Insomnia among hospitalized elderly patients: prevalence, clinical characteristics and risk factors. Arch Gerontol Geriatr. 2011;52:133–137.
- , , , et al. Is insomnia a marker for psychiatric disorders in general hospitals? Sleep Med. 2005;6:549–553.
- , , , , , . Perceived control and sleep in hospitalized older adults: a sound hypothesis? J Hosp Med. 2013;8:184–190.
- , , , et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med. 2012;157:170–179.
- . Sleep in acute care settings: an integrative review. J Nurs Scholarsh. 2000;32(1):31–38.
- . Physical health as it relates to insomnia. Talk presented at: Center for Behavior and Health, Lecture Series in Johns Hopkins Bayview Medical Center; July 17, 2012; Baltimore, MD.
- , , , , . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
- , . Measures of sleep: The Insomnia Severity Index, Medical Outcomes Study (MOS) Sleep Scale, Pittsburgh Sleep Diary (PSD), and Pittsburgh Sleep Quality Index (PSQI). Arthritis Rheumatol. 2003;49:S184–S196.
- , . Applied Mixed Models in Medicine. 3rd ed. Somerset, NJ: Wiley; 2014:539.
- , , , Applying mixed regression models to the analysis of repeated‐measures data in psychosomatic medicine. Psychosom Med. 2006;68(6):870–878.
- , . Using the SPSS mixed procedure to fit cross‐sectional and longitudinal multilevel models. Educ Psychol Meas. 2005;65(5):717–741.
- , . Introduction to estimation issues in multilevel modeling. New Dir Inst Res. 2012;2012(154):23–39.
- , . Centering predictor variables in cross‐sectional multilevel models: a new look at an old issue. Psychol Methods. 2007;12(2):121–138.
- , . Sleep quality in adult hospitalized patients with infection: an observational study. Am J Med Sci. 2015;349(1):56–60.
- , , , et al. Risk of sleep apnea in hospitalized older patients. J Clin Sleep Med. 2014;10:1061–1066.
- , , . Hospital ward policy and patients' sleep patterns: a multiple baseline study. Rehabil Psychol. 1989;34(1):43–50.
- , , . Non‐pharmacologic interventions to improve the sleep of hospitalized patients: a systematic review. J Gen Intern Med. 2014;29:788–795.
- , , , , , Earplugs and eye masks vs routine care prevent sleep impairment in post‐anaesthesia care unit: a randomized study. Br J Anaesth. 2014;112(1):89–95.
- , , , et al. Sleep rounds: a multidisciplinary approach to optimize sleep quality and satisfaction in hospitalized patients. J Hosp Med. 2012;7:508–512.
- , , , , . Factors affecting sleep quality of patients in intensive care unit. J Clin Sleep Med. 2012;8(3):301–307.
- . Insomnia among hospitalized older persons. Clin Geriatr Med. 2008;24(1):51–67.
- , , , . A nonpharmacological sleep protocol for hospitalized older patients. J Am Geriatr Soc. 1998;46(6):700–705.
- The Action Priority Matrix: making the most of your opportunities. TimeAnalyzer website. Available at: http://www.timeanalyzer.com/lib/priority.htm. Published 2006. Accessed July 10, 2015.
- , , , et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36(11):1747–1755.
- Institute of Medicine (US) Committee on Sleep Medicine and Research. Sleep disorders and sleep deprivation: an unmet public health problem. Washington, DC: National Academies Press; 2006. Available at: http://www.ncbi.nlm.nih.gov/books/NBK19960. Accessed September 16, 2014.
- , . Health behaviors of adults: United States, 2005–2007. Vital Health Stat 10. 2010;245:1–132.
- , , . High incidence of diabetes in men with sleep complaints or short sleep duration: a 12‐year follow‐up study of a middle‐aged population. Diabetes Care. 2005;28:2762–2767.
- , , , et al. Linking sleep duration and obesity among black and white US adults. Clin Pract (Lond). 2013;10(5):661–667.
- , , , et al. Gender‐specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700.
- , , , , , . The joint effect of sleep duration and disturbed sleep on cause‐specific mortality: results from the Whitehall II cohort study. PLoS One. 2014;9(4):e91965.
- , , , , , . Poor self‐reported sleep quality predicts mortality within one year of inpatient post‐acute rehabilitation among older adults. Sleep. 2011;34(12):1715–1721.
- , , , , . The effects of sleep deprivation on symptoms of psychopathology in healthy adults. Sleep Med. 2007;8(3):215–221.
- , , , , . Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762.
- , , , . The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–178.
- , , , et al. Insomnia among hospitalized elderly patients: prevalence, clinical characteristics and risk factors. Arch Gerontol Geriatr. 2011;52:133–137.
- , , , et al. Is insomnia a marker for psychiatric disorders in general hospitals? Sleep Med. 2005;6:549–553.
- , , , , , . Perceived control and sleep in hospitalized older adults: a sound hypothesis? J Hosp Med. 2013;8:184–190.
- , , , et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med. 2012;157:170–179.
- . Sleep in acute care settings: an integrative review. J Nurs Scholarsh. 2000;32(1):31–38.
- . Physical health as it relates to insomnia. Talk presented at: Center for Behavior and Health, Lecture Series in Johns Hopkins Bayview Medical Center; July 17, 2012; Baltimore, MD.
- , , , , . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213.
- , . Measures of sleep: The Insomnia Severity Index, Medical Outcomes Study (MOS) Sleep Scale, Pittsburgh Sleep Diary (PSD), and Pittsburgh Sleep Quality Index (PSQI). Arthritis Rheumatol. 2003;49:S184–S196.
- , . Applied Mixed Models in Medicine. 3rd ed. Somerset, NJ: Wiley; 2014:539.
- , , , Applying mixed regression models to the analysis of repeated‐measures data in psychosomatic medicine. Psychosom Med. 2006;68(6):870–878.
- , . Using the SPSS mixed procedure to fit cross‐sectional and longitudinal multilevel models. Educ Psychol Meas. 2005;65(5):717–741.
- , . Introduction to estimation issues in multilevel modeling. New Dir Inst Res. 2012;2012(154):23–39.
- , . Centering predictor variables in cross‐sectional multilevel models: a new look at an old issue. Psychol Methods. 2007;12(2):121–138.
- , . Sleep quality in adult hospitalized patients with infection: an observational study. Am J Med Sci. 2015;349(1):56–60.
- , , , et al. Risk of sleep apnea in hospitalized older patients. J Clin Sleep Med. 2014;10:1061–1066.
- , , . Hospital ward policy and patients' sleep patterns: a multiple baseline study. Rehabil Psychol. 1989;34(1):43–50.
- , , . Non‐pharmacologic interventions to improve the sleep of hospitalized patients: a systematic review. J Gen Intern Med. 2014;29:788–795.
- , , , , , Earplugs and eye masks vs routine care prevent sleep impairment in post‐anaesthesia care unit: a randomized study. Br J Anaesth. 2014;112(1):89–95.
- , , , et al. Sleep rounds: a multidisciplinary approach to optimize sleep quality and satisfaction in hospitalized patients. J Hosp Med. 2012;7:508–512.
- , , , , . Factors affecting sleep quality of patients in intensive care unit. J Clin Sleep Med. 2012;8(3):301–307.
- . Insomnia among hospitalized older persons. Clin Geriatr Med. 2008;24(1):51–67.
- , , , . A nonpharmacological sleep protocol for hospitalized older patients. J Am Geriatr Soc. 1998;46(6):700–705.
- The Action Priority Matrix: making the most of your opportunities. TimeAnalyzer website. Available at: http://www.timeanalyzer.com/lib/priority.htm. Published 2006. Accessed July 10, 2015.
- , , , et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36(11):1747–1755.
HM16 Session Analysis: Nonpharmacological Treatment Approach Better for Neonatal Abstinence Syndrome
Presenter: Matthew Grossman, MD, FAAP
Summary: Treating Neonatal Abstinence Syndrome (NAS) traditionally has followed a standardized approach using the Finnegan Scoring System in which if there were three consecutive scores > 8 or two scores > 12, medications would be started. Common medications included tincture of opium or morphine. Medication doses would be adjusted or weaned, typically every other day, by Finnegan scoring.
A better approach is indicated with the 2012 AAP guidelines that indicate the first-line approach to NAS should be nonpharmacological. The approach should be that used for any crying baby, i.e., holding, swaddling, on-demand feeding, and parents rooming in with the infant. NAS infants without significant other medical problems are best cared for in a regular nursery or hospital unit rather than a NICU. With these simple interventions, some NAS infants may not need medications, and if they do, may be weaned sooner.
Additionally, medication management can be more successful if using combinations of a narcotic plus an additional agent such as clonidine or phenobarbital. Medications may be safely weaned more quickly than every other day. Using such a combined approach, the Yale New Haven Hospital has significantly reduced NAS infant LOS, total narcotic dose, and cost while increasing rates of breast feeding.
Key Takeaways
- Treat NAS first by providing high quality nursing care with infants out of an ICU, swaddled, fed and held when first exhibiting withdrawal symptoms.
- Use combination narcotic and other medication if pharmacologic treatment is needed.
- Wean aggressively by symptoms. TH
Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
Presenter: Matthew Grossman, MD, FAAP
Summary: Treating Neonatal Abstinence Syndrome (NAS) traditionally has followed a standardized approach using the Finnegan Scoring System in which if there were three consecutive scores > 8 or two scores > 12, medications would be started. Common medications included tincture of opium or morphine. Medication doses would be adjusted or weaned, typically every other day, by Finnegan scoring.
A better approach is indicated with the 2012 AAP guidelines that indicate the first-line approach to NAS should be nonpharmacological. The approach should be that used for any crying baby, i.e., holding, swaddling, on-demand feeding, and parents rooming in with the infant. NAS infants without significant other medical problems are best cared for in a regular nursery or hospital unit rather than a NICU. With these simple interventions, some NAS infants may not need medications, and if they do, may be weaned sooner.
Additionally, medication management can be more successful if using combinations of a narcotic plus an additional agent such as clonidine or phenobarbital. Medications may be safely weaned more quickly than every other day. Using such a combined approach, the Yale New Haven Hospital has significantly reduced NAS infant LOS, total narcotic dose, and cost while increasing rates of breast feeding.
Key Takeaways
- Treat NAS first by providing high quality nursing care with infants out of an ICU, swaddled, fed and held when first exhibiting withdrawal symptoms.
- Use combination narcotic and other medication if pharmacologic treatment is needed.
- Wean aggressively by symptoms. TH
Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
Presenter: Matthew Grossman, MD, FAAP
Summary: Treating Neonatal Abstinence Syndrome (NAS) traditionally has followed a standardized approach using the Finnegan Scoring System in which if there were three consecutive scores > 8 or two scores > 12, medications would be started. Common medications included tincture of opium or morphine. Medication doses would be adjusted or weaned, typically every other day, by Finnegan scoring.
A better approach is indicated with the 2012 AAP guidelines that indicate the first-line approach to NAS should be nonpharmacological. The approach should be that used for any crying baby, i.e., holding, swaddling, on-demand feeding, and parents rooming in with the infant. NAS infants without significant other medical problems are best cared for in a regular nursery or hospital unit rather than a NICU. With these simple interventions, some NAS infants may not need medications, and if they do, may be weaned sooner.
Additionally, medication management can be more successful if using combinations of a narcotic plus an additional agent such as clonidine or phenobarbital. Medications may be safely weaned more quickly than every other day. Using such a combined approach, the Yale New Haven Hospital has significantly reduced NAS infant LOS, total narcotic dose, and cost while increasing rates of breast feeding.
Key Takeaways
- Treat NAS first by providing high quality nursing care with infants out of an ICU, swaddled, fed and held when first exhibiting withdrawal symptoms.
- Use combination narcotic and other medication if pharmacologic treatment is needed.
- Wean aggressively by symptoms. TH
Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
HM16 Session Analysis: Stay Calm, Safe During Inpatient Behavioral Emergencies
Presenters: David Pressel, MD, PhD, FAAP, FHM, Emily Fingado, MD, FAAP, and Jessica Tomaszewski, MD, FAAP
Summary: Patients may engage in violent behaviors that pose a danger to themselves or others. Behavioral emergencies may be rare, can be dangerous, and staff may feel ill-trained to respond appropriately. Patients with ingestions, or underlying psychiatric or developmental difficulties, are at highest risk for developing a behavioral emergency.
The first strategy in handling a potentially violent patient is de-escalation, i.e., trying to identify and rectify the behavioral trigger. If de-escalation is not successful, personal safety is paramount. Get away from the patient and get help. If a patient needs to be physically restrained, minimally there should be one staff member per limb. Various physical devices, including soft restraints, four-point leathers, hand mittens, and spit hoods may be used to control a violent patient. A violent restraint is characterized by the indication, not the device. Medications may be used to treat the underlying mental health issue and should not be used as PRN chemical restraints.
After a violent patient is safely restrained, further steps need to be taken, including notification of the attending or legal guardian if a minor; documentation of the event, including a debrief of what occurred; a room sweep to ensure securing any dangerous items (metal eating utensils); and modification of the care plan to strategize on removal of the restraints as soon as is safe.
Hospitals should view behavioral emergencies similarly to a Code Blue. Have a specialized team that responds and undergoes regular training.
Key Takeaways
- Behavioral emergencies occur when a patient becomes violent.
- De-escalation is the best response.
- If not successful, maintain personal safety, control and medicate the patient as appropriate, and document clearly. TH
Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
Presenters: David Pressel, MD, PhD, FAAP, FHM, Emily Fingado, MD, FAAP, and Jessica Tomaszewski, MD, FAAP
Summary: Patients may engage in violent behaviors that pose a danger to themselves or others. Behavioral emergencies may be rare, can be dangerous, and staff may feel ill-trained to respond appropriately. Patients with ingestions, or underlying psychiatric or developmental difficulties, are at highest risk for developing a behavioral emergency.
The first strategy in handling a potentially violent patient is de-escalation, i.e., trying to identify and rectify the behavioral trigger. If de-escalation is not successful, personal safety is paramount. Get away from the patient and get help. If a patient needs to be physically restrained, minimally there should be one staff member per limb. Various physical devices, including soft restraints, four-point leathers, hand mittens, and spit hoods may be used to control a violent patient. A violent restraint is characterized by the indication, not the device. Medications may be used to treat the underlying mental health issue and should not be used as PRN chemical restraints.
After a violent patient is safely restrained, further steps need to be taken, including notification of the attending or legal guardian if a minor; documentation of the event, including a debrief of what occurred; a room sweep to ensure securing any dangerous items (metal eating utensils); and modification of the care plan to strategize on removal of the restraints as soon as is safe.
Hospitals should view behavioral emergencies similarly to a Code Blue. Have a specialized team that responds and undergoes regular training.
Key Takeaways
- Behavioral emergencies occur when a patient becomes violent.
- De-escalation is the best response.
- If not successful, maintain personal safety, control and medicate the patient as appropriate, and document clearly. TH
Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
Presenters: David Pressel, MD, PhD, FAAP, FHM, Emily Fingado, MD, FAAP, and Jessica Tomaszewski, MD, FAAP
Summary: Patients may engage in violent behaviors that pose a danger to themselves or others. Behavioral emergencies may be rare, can be dangerous, and staff may feel ill-trained to respond appropriately. Patients with ingestions, or underlying psychiatric or developmental difficulties, are at highest risk for developing a behavioral emergency.
The first strategy in handling a potentially violent patient is de-escalation, i.e., trying to identify and rectify the behavioral trigger. If de-escalation is not successful, personal safety is paramount. Get away from the patient and get help. If a patient needs to be physically restrained, minimally there should be one staff member per limb. Various physical devices, including soft restraints, four-point leathers, hand mittens, and spit hoods may be used to control a violent patient. A violent restraint is characterized by the indication, not the device. Medications may be used to treat the underlying mental health issue and should not be used as PRN chemical restraints.
After a violent patient is safely restrained, further steps need to be taken, including notification of the attending or legal guardian if a minor; documentation of the event, including a debrief of what occurred; a room sweep to ensure securing any dangerous items (metal eating utensils); and modification of the care plan to strategize on removal of the restraints as soon as is safe.
Hospitals should view behavioral emergencies similarly to a Code Blue. Have a specialized team that responds and undergoes regular training.
Key Takeaways
- Behavioral emergencies occur when a patient becomes violent.
- De-escalation is the best response.
- If not successful, maintain personal safety, control and medicate the patient as appropriate, and document clearly. TH
Dr. Pressel is a pediatric hospitalist and inpatient medical director at Nemours/Alfred I. duPont Hospital for Children in Wilmington, Del., and a member of Team Hospitalist.
When toenail onychomycosis can turn deadly
WAIKOLOA, HAWAII – Toenail onychomycosis is a common condition in the general population, but it’s three- to fourfold more prevalent in certain at risk populations where it can have serious and even life-threatening consequences, Dr. Theodore Rosen observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
He cited a recent systematic review led by Dr. Aditya K. Gupta, professor of dermatology at the University of Toronto, whom Dr. Rosen hailed as one of the world’s great fungal disease authorities. Dr. Gupta and coworkers concluded that while the prevalence of dermatophyte toenail onychomycosis is 3.2% worldwide in the general population, it climbs to 8.8% in diabetics, 10.2% in psoriatics, 10.3% in the elderly, 11.9% in dialysis patients, 5.2% in renal transplant recipients, and 10.4% in HIV-positive individuals. The highest prevalence of onychomycosis due to non-dermatophyte molds was seen in psoriasis patients, at 2.5%, while elderly patients had the highest prevalence of onychomycosis caused by yeasts, at 6.1% (J Eur Acad Dermatol Venereol. 2015 Jun;29[6]:1039-44).
“Onychomycosis is especially important in those who are immunocompromised and immunosuppressed, for two reasons. One is that really odd organisms that aren’t Trichophyton rubrum or T. interdigitale can be involved: saprophytes like Scopulariopsis, Acremonium, Aspergillus, and Paecilomyces. And some of these saprophytes, like Fusarium, can get from the nail and nail bed into the bloodstream and can kill,” explained Dr. Rosen, professor of dermatology at Baylor College of Medicine in Houston.
“Onychomycosis, aside from the fact that it looks bad and often leads to pain, can also lead to breaks in the skin which then result in secondary bacterial infections. In fact, after motor vehicle accidents, onychomycosis and tinea pedis combined are the most common cause of lower extremity cellulitis leading to hospitalization in the United States,” he continued.
The go-to treatments for onychomycosis in patients with a bad prognostic factor are oral itraconazole (Sporanox) and terbinafine. Don’t be unduly swayed by the complete cure rates reported in clinical trials and cited in the product package inserts; they don’t tell the full story because of important differences in study design, according to Dr. Rosen.
He recommended that physicians familiarize themselves with posaconazole (Noxafil) as an antifungal to consider for second-line therapy in difficult-to-cure cases of onychomycosis in immunosuppressed patients. This is off-label therapy. The approved indications for this triazole antifungal agent are prophylaxis of invasive Aspergillus and Candida infections in severely immunocompromised patients, as well as treatment of oropharyngeal candidiasis. But this is a potent agent that provides broad-spectrum coverage coupled with a favorable safety profile. It performed well in a phase IIb randomized, placebo- and active-controlled, multicenter, investigator-blinded study of 218 adults with toenail onychomycosis (Br J Dermatol. 2012 Feb;166[2]:389-98).
Dr. Rosen reported serving on scientific advisory boards for Anacor, Merz, and Valeant.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Toenail onychomycosis is a common condition in the general population, but it’s three- to fourfold more prevalent in certain at risk populations where it can have serious and even life-threatening consequences, Dr. Theodore Rosen observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
He cited a recent systematic review led by Dr. Aditya K. Gupta, professor of dermatology at the University of Toronto, whom Dr. Rosen hailed as one of the world’s great fungal disease authorities. Dr. Gupta and coworkers concluded that while the prevalence of dermatophyte toenail onychomycosis is 3.2% worldwide in the general population, it climbs to 8.8% in diabetics, 10.2% in psoriatics, 10.3% in the elderly, 11.9% in dialysis patients, 5.2% in renal transplant recipients, and 10.4% in HIV-positive individuals. The highest prevalence of onychomycosis due to non-dermatophyte molds was seen in psoriasis patients, at 2.5%, while elderly patients had the highest prevalence of onychomycosis caused by yeasts, at 6.1% (J Eur Acad Dermatol Venereol. 2015 Jun;29[6]:1039-44).
“Onychomycosis is especially important in those who are immunocompromised and immunosuppressed, for two reasons. One is that really odd organisms that aren’t Trichophyton rubrum or T. interdigitale can be involved: saprophytes like Scopulariopsis, Acremonium, Aspergillus, and Paecilomyces. And some of these saprophytes, like Fusarium, can get from the nail and nail bed into the bloodstream and can kill,” explained Dr. Rosen, professor of dermatology at Baylor College of Medicine in Houston.
“Onychomycosis, aside from the fact that it looks bad and often leads to pain, can also lead to breaks in the skin which then result in secondary bacterial infections. In fact, after motor vehicle accidents, onychomycosis and tinea pedis combined are the most common cause of lower extremity cellulitis leading to hospitalization in the United States,” he continued.
The go-to treatments for onychomycosis in patients with a bad prognostic factor are oral itraconazole (Sporanox) and terbinafine. Don’t be unduly swayed by the complete cure rates reported in clinical trials and cited in the product package inserts; they don’t tell the full story because of important differences in study design, according to Dr. Rosen.
He recommended that physicians familiarize themselves with posaconazole (Noxafil) as an antifungal to consider for second-line therapy in difficult-to-cure cases of onychomycosis in immunosuppressed patients. This is off-label therapy. The approved indications for this triazole antifungal agent are prophylaxis of invasive Aspergillus and Candida infections in severely immunocompromised patients, as well as treatment of oropharyngeal candidiasis. But this is a potent agent that provides broad-spectrum coverage coupled with a favorable safety profile. It performed well in a phase IIb randomized, placebo- and active-controlled, multicenter, investigator-blinded study of 218 adults with toenail onychomycosis (Br J Dermatol. 2012 Feb;166[2]:389-98).
Dr. Rosen reported serving on scientific advisory boards for Anacor, Merz, and Valeant.
SDEF and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Toenail onychomycosis is a common condition in the general population, but it’s three- to fourfold more prevalent in certain at risk populations where it can have serious and even life-threatening consequences, Dr. Theodore Rosen observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
He cited a recent systematic review led by Dr. Aditya K. Gupta, professor of dermatology at the University of Toronto, whom Dr. Rosen hailed as one of the world’s great fungal disease authorities. Dr. Gupta and coworkers concluded that while the prevalence of dermatophyte toenail onychomycosis is 3.2% worldwide in the general population, it climbs to 8.8% in diabetics, 10.2% in psoriatics, 10.3% in the elderly, 11.9% in dialysis patients, 5.2% in renal transplant recipients, and 10.4% in HIV-positive individuals. The highest prevalence of onychomycosis due to non-dermatophyte molds was seen in psoriasis patients, at 2.5%, while elderly patients had the highest prevalence of onychomycosis caused by yeasts, at 6.1% (J Eur Acad Dermatol Venereol. 2015 Jun;29[6]:1039-44).
“Onychomycosis is especially important in those who are immunocompromised and immunosuppressed, for two reasons. One is that really odd organisms that aren’t Trichophyton rubrum or T. interdigitale can be involved: saprophytes like Scopulariopsis, Acremonium, Aspergillus, and Paecilomyces. And some of these saprophytes, like Fusarium, can get from the nail and nail bed into the bloodstream and can kill,” explained Dr. Rosen, professor of dermatology at Baylor College of Medicine in Houston.
“Onychomycosis, aside from the fact that it looks bad and often leads to pain, can also lead to breaks in the skin which then result in secondary bacterial infections. In fact, after motor vehicle accidents, onychomycosis and tinea pedis combined are the most common cause of lower extremity cellulitis leading to hospitalization in the United States,” he continued.
The go-to treatments for onychomycosis in patients with a bad prognostic factor are oral itraconazole (Sporanox) and terbinafine. Don’t be unduly swayed by the complete cure rates reported in clinical trials and cited in the product package inserts; they don’t tell the full story because of important differences in study design, according to Dr. Rosen.
He recommended that physicians familiarize themselves with posaconazole (Noxafil) as an antifungal to consider for second-line therapy in difficult-to-cure cases of onychomycosis in immunosuppressed patients. This is off-label therapy. The approved indications for this triazole antifungal agent are prophylaxis of invasive Aspergillus and Candida infections in severely immunocompromised patients, as well as treatment of oropharyngeal candidiasis. But this is a potent agent that provides broad-spectrum coverage coupled with a favorable safety profile. It performed well in a phase IIb randomized, placebo- and active-controlled, multicenter, investigator-blinded study of 218 adults with toenail onychomycosis (Br J Dermatol. 2012 Feb;166[2]:389-98).
Dr. Rosen reported serving on scientific advisory boards for Anacor, Merz, and Valeant.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Identifying and Managing Abscess Formation Related to Soft-Tissue Fillers
Injectable soft-tissue fillers continue to be popular in the cosmetic arena. In the United States there are many fillers currently on the market and many more coming through the pipeline. A multitude of products are available outside the United States. As with any procedure, the more fillers we inject, the more complications we are bound to see.
Conrad et al (Modern Plastic Surgery. 2015;5:14-18) performed a retrospective analysis of patients treated over a 10-year period with soft-tissue injections (1559 patients) looking for cases complicated by abscess formation. Four patients were identified (0.3% of total patients). The authors discussed the 4 cases, the patients’ medical history and experience with other injectable agents, and the management of each complication.
Case 1 was a 52-year-old woman with systemic lupus erythematosus on a low-dose steroid who presented with an inflammatory response in the lower lip 7 days following injection with a hyaluronic acid (HA)–based gel filler in 2011. Her history was notable for prior HA filler in 2008 and polyacrylamide filler in 2009 and 2010. She was treated with 4 sessions of incision and drainage (I&D) and systemic clindamycin. Most of the cultures were negative, but one showed streptococci.
Case 2 was a 56-year-old woman treated in the nasolabial fold with HA in 2009. She developed inflammation shortly after and an abscess at the site a month later. She was treated with clindamycin both times, though cultures were negative. Furthermore, the abscess was treated with I&D and an intralesional steroid. She was a smoker and had been treated with a polymethyl methacrylate filler in 2002 and subsequently in 2013 with no issues.
Case 3 was a 39-year-old woman injected with an HA filler in the upper and lower lips in 2011. One month later she developed abscesses in both areas that were treated twice with I&D. Cultures were negative. She had a history of polyacrylamide injections of the nasolabial fold in 2009. The patient’s medical history was notable for scleroderma.
Case 4 was a 58-year-old woman injected with an HA filler in 2009 in the prejowl sulcus and nasolabial fold. She developed recurrent sterile abscesses in the areas 8 months after treatment that were managed by drainage of the areas and intralesional steroid injections over the ensuing 6 months. The scars were then excised, lasered 6 weeks later, and then filled in with expanded polytetrafluoroethylene implants, followed by 1 more session of laser resurfacing. She had a history of polymethyl methacrylate filler in 2002.
All patients eventually recovered. The authors stressed 3 important factors in managing dermal filler complications: (1) identifying the causative pathogen, (2) choosing the appropriate treatment of delayed-onset abscess formation, and (3) identifying the risk factors for patients at risk for abscess formation.
The issue of biofilms complicates the ability to identify the bacterial agent, yet biofilms are becoming recognized as the causative factors in what were previously thought of as sterile abscesses. The authors suggested using a peptide nucleic acid fluorescent in situ hybridization test to identify the biofilm bacteria. Conrad et el also discussed the development of slippery liquid-infused porous surfaces technology to coat the inside of syringes to help prevent biofilm formation.
The management of these patients is tricky because it is difficult to differentiate between a biofilm abscess and a hypersensitivity reaction. For this reason, the authors advocated using hyaluronidase versus intralesional steroids in the initial management to make the area more susceptible to antibiotics and to avoid promoting the growth of bacteria with the use of steroids. For patient risk factors, the authors focused on the fact that 2 of 4 patients had concomitant autoimmune disorders—scleroderma and systemic lupus erythematosus—that may have predisposed them to infection. Lastly, 2 patients had prior polyacrylamide injections and the authors also speculated if the positive charge of this filler attracted bacteria.
What’s the issue?
The use of fillers will continue to increase as there are more fillers with novel properties entering the market. As with new technology, only time will tell if we will see any particular type of reaction or risk for infection with them. The issue of biofilm bacterial contamination is real. It is recognized as one of the causes of capsular contraction following breast implant surgery. The etiology may not be from contamination during production but from contamination of the filler after injection due to any transient bacteremia that the patient may experience. A concern is that dental manipulation (eg, dental cleaning, filling of dental caries, periodontal surgery) during the 2- to 4-week postfiller period may “seed” bacteria into the area and cause the bacteria to settle and grow on the foreign substance. For patients who have semipermanent or permanent fillers such as polyacrylamide, polymethyl methacrylate beads, or poly-L-lactic acid, biofilm risk is greater and can occur months to years after the procedure. I have personally seen 2 cases of poly-L-lactic acid filler develop red, tender, sterile abscesses 1 year after placement in the tissue. Both cases responded to prolonged clarithromycin use (2 months). However, these cases highlight the fact that the fillers persist long after we place them, and any bacteremia, even mild, can cause an unsightly reaction.
Have you seen delayed soft-tissue filler reactions in your practice? Given this information, will you change the way you advise patients on dental procedures in the 2- to 4-week postfiller period?
Injectable soft-tissue fillers continue to be popular in the cosmetic arena. In the United States there are many fillers currently on the market and many more coming through the pipeline. A multitude of products are available outside the United States. As with any procedure, the more fillers we inject, the more complications we are bound to see.
Conrad et al (Modern Plastic Surgery. 2015;5:14-18) performed a retrospective analysis of patients treated over a 10-year period with soft-tissue injections (1559 patients) looking for cases complicated by abscess formation. Four patients were identified (0.3% of total patients). The authors discussed the 4 cases, the patients’ medical history and experience with other injectable agents, and the management of each complication.
Case 1 was a 52-year-old woman with systemic lupus erythematosus on a low-dose steroid who presented with an inflammatory response in the lower lip 7 days following injection with a hyaluronic acid (HA)–based gel filler in 2011. Her history was notable for prior HA filler in 2008 and polyacrylamide filler in 2009 and 2010. She was treated with 4 sessions of incision and drainage (I&D) and systemic clindamycin. Most of the cultures were negative, but one showed streptococci.
Case 2 was a 56-year-old woman treated in the nasolabial fold with HA in 2009. She developed inflammation shortly after and an abscess at the site a month later. She was treated with clindamycin both times, though cultures were negative. Furthermore, the abscess was treated with I&D and an intralesional steroid. She was a smoker and had been treated with a polymethyl methacrylate filler in 2002 and subsequently in 2013 with no issues.
Case 3 was a 39-year-old woman injected with an HA filler in the upper and lower lips in 2011. One month later she developed abscesses in both areas that were treated twice with I&D. Cultures were negative. She had a history of polyacrylamide injections of the nasolabial fold in 2009. The patient’s medical history was notable for scleroderma.
Case 4 was a 58-year-old woman injected with an HA filler in 2009 in the prejowl sulcus and nasolabial fold. She developed recurrent sterile abscesses in the areas 8 months after treatment that were managed by drainage of the areas and intralesional steroid injections over the ensuing 6 months. The scars were then excised, lasered 6 weeks later, and then filled in with expanded polytetrafluoroethylene implants, followed by 1 more session of laser resurfacing. She had a history of polymethyl methacrylate filler in 2002.
All patients eventually recovered. The authors stressed 3 important factors in managing dermal filler complications: (1) identifying the causative pathogen, (2) choosing the appropriate treatment of delayed-onset abscess formation, and (3) identifying the risk factors for patients at risk for abscess formation.
The issue of biofilms complicates the ability to identify the bacterial agent, yet biofilms are becoming recognized as the causative factors in what were previously thought of as sterile abscesses. The authors suggested using a peptide nucleic acid fluorescent in situ hybridization test to identify the biofilm bacteria. Conrad et el also discussed the development of slippery liquid-infused porous surfaces technology to coat the inside of syringes to help prevent biofilm formation.
The management of these patients is tricky because it is difficult to differentiate between a biofilm abscess and a hypersensitivity reaction. For this reason, the authors advocated using hyaluronidase versus intralesional steroids in the initial management to make the area more susceptible to antibiotics and to avoid promoting the growth of bacteria with the use of steroids. For patient risk factors, the authors focused on the fact that 2 of 4 patients had concomitant autoimmune disorders—scleroderma and systemic lupus erythematosus—that may have predisposed them to infection. Lastly, 2 patients had prior polyacrylamide injections and the authors also speculated if the positive charge of this filler attracted bacteria.
What’s the issue?
The use of fillers will continue to increase as there are more fillers with novel properties entering the market. As with new technology, only time will tell if we will see any particular type of reaction or risk for infection with them. The issue of biofilm bacterial contamination is real. It is recognized as one of the causes of capsular contraction following breast implant surgery. The etiology may not be from contamination during production but from contamination of the filler after injection due to any transient bacteremia that the patient may experience. A concern is that dental manipulation (eg, dental cleaning, filling of dental caries, periodontal surgery) during the 2- to 4-week postfiller period may “seed” bacteria into the area and cause the bacteria to settle and grow on the foreign substance. For patients who have semipermanent or permanent fillers such as polyacrylamide, polymethyl methacrylate beads, or poly-L-lactic acid, biofilm risk is greater and can occur months to years after the procedure. I have personally seen 2 cases of poly-L-lactic acid filler develop red, tender, sterile abscesses 1 year after placement in the tissue. Both cases responded to prolonged clarithromycin use (2 months). However, these cases highlight the fact that the fillers persist long after we place them, and any bacteremia, even mild, can cause an unsightly reaction.
Have you seen delayed soft-tissue filler reactions in your practice? Given this information, will you change the way you advise patients on dental procedures in the 2- to 4-week postfiller period?
Injectable soft-tissue fillers continue to be popular in the cosmetic arena. In the United States there are many fillers currently on the market and many more coming through the pipeline. A multitude of products are available outside the United States. As with any procedure, the more fillers we inject, the more complications we are bound to see.
Conrad et al (Modern Plastic Surgery. 2015;5:14-18) performed a retrospective analysis of patients treated over a 10-year period with soft-tissue injections (1559 patients) looking for cases complicated by abscess formation. Four patients were identified (0.3% of total patients). The authors discussed the 4 cases, the patients’ medical history and experience with other injectable agents, and the management of each complication.
Case 1 was a 52-year-old woman with systemic lupus erythematosus on a low-dose steroid who presented with an inflammatory response in the lower lip 7 days following injection with a hyaluronic acid (HA)–based gel filler in 2011. Her history was notable for prior HA filler in 2008 and polyacrylamide filler in 2009 and 2010. She was treated with 4 sessions of incision and drainage (I&D) and systemic clindamycin. Most of the cultures were negative, but one showed streptococci.
Case 2 was a 56-year-old woman treated in the nasolabial fold with HA in 2009. She developed inflammation shortly after and an abscess at the site a month later. She was treated with clindamycin both times, though cultures were negative. Furthermore, the abscess was treated with I&D and an intralesional steroid. She was a smoker and had been treated with a polymethyl methacrylate filler in 2002 and subsequently in 2013 with no issues.
Case 3 was a 39-year-old woman injected with an HA filler in the upper and lower lips in 2011. One month later she developed abscesses in both areas that were treated twice with I&D. Cultures were negative. She had a history of polyacrylamide injections of the nasolabial fold in 2009. The patient’s medical history was notable for scleroderma.
Case 4 was a 58-year-old woman injected with an HA filler in 2009 in the prejowl sulcus and nasolabial fold. She developed recurrent sterile abscesses in the areas 8 months after treatment that were managed by drainage of the areas and intralesional steroid injections over the ensuing 6 months. The scars were then excised, lasered 6 weeks later, and then filled in with expanded polytetrafluoroethylene implants, followed by 1 more session of laser resurfacing. She had a history of polymethyl methacrylate filler in 2002.
All patients eventually recovered. The authors stressed 3 important factors in managing dermal filler complications: (1) identifying the causative pathogen, (2) choosing the appropriate treatment of delayed-onset abscess formation, and (3) identifying the risk factors for patients at risk for abscess formation.
The issue of biofilms complicates the ability to identify the bacterial agent, yet biofilms are becoming recognized as the causative factors in what were previously thought of as sterile abscesses. The authors suggested using a peptide nucleic acid fluorescent in situ hybridization test to identify the biofilm bacteria. Conrad et el also discussed the development of slippery liquid-infused porous surfaces technology to coat the inside of syringes to help prevent biofilm formation.
The management of these patients is tricky because it is difficult to differentiate between a biofilm abscess and a hypersensitivity reaction. For this reason, the authors advocated using hyaluronidase versus intralesional steroids in the initial management to make the area more susceptible to antibiotics and to avoid promoting the growth of bacteria with the use of steroids. For patient risk factors, the authors focused on the fact that 2 of 4 patients had concomitant autoimmune disorders—scleroderma and systemic lupus erythematosus—that may have predisposed them to infection. Lastly, 2 patients had prior polyacrylamide injections and the authors also speculated if the positive charge of this filler attracted bacteria.
What’s the issue?
The use of fillers will continue to increase as there are more fillers with novel properties entering the market. As with new technology, only time will tell if we will see any particular type of reaction or risk for infection with them. The issue of biofilm bacterial contamination is real. It is recognized as one of the causes of capsular contraction following breast implant surgery. The etiology may not be from contamination during production but from contamination of the filler after injection due to any transient bacteremia that the patient may experience. A concern is that dental manipulation (eg, dental cleaning, filling of dental caries, periodontal surgery) during the 2- to 4-week postfiller period may “seed” bacteria into the area and cause the bacteria to settle and grow on the foreign substance. For patients who have semipermanent or permanent fillers such as polyacrylamide, polymethyl methacrylate beads, or poly-L-lactic acid, biofilm risk is greater and can occur months to years after the procedure. I have personally seen 2 cases of poly-L-lactic acid filler develop red, tender, sterile abscesses 1 year after placement in the tissue. Both cases responded to prolonged clarithromycin use (2 months). However, these cases highlight the fact that the fillers persist long after we place them, and any bacteremia, even mild, can cause an unsightly reaction.
Have you seen delayed soft-tissue filler reactions in your practice? Given this information, will you change the way you advise patients on dental procedures in the 2- to 4-week postfiller period?
Follicular lymphoma: Quantitative PET/CT measures for detecting bone marrow involvement
Quantifying bone marrow uptake of FDG (18fluorodeoxyglucose) improved the diagnostic accuracy of PET/CT for predicting bone marrow involvement in patients with follicular lymphoma, based on the results of a retrospective study.
Visual evidence of focal increased uptake on PET/CT indicates marrow involvement in follicular lymphoma; however, diffuse uptake is a nonspecific finding. Measuring the mean bone marrow standardized uptake value (BM SUV mean) improves PET/CT diagnostic accuracy, Dr. Chava Perry and his colleagues at Tel Aviv Sourasky Medical Center reported in Medicine [(Baltimore). 2016 Mar;95(9):e2910].
The researchers evaluated 68 consecutive patients with follicular lymphoma; 16 had bone marrow involvement – 13 had biopsy-proven involvement and 3 had a negative biopsy with increased medullary uptake that normalized after treatment. BM FDG uptake was diffuse in 8 of them and focal in the other 8.
While focal increased uptake is indicative of bone marrow involvement, diffuse uptake can be associated with false-positive results, as it was in the case of 17 patients (32.7% of those with diffuse uptake). Overall, visual assessment of scan results had a negative predictive value of 100% and a positive predictive value (PPV) of 48.5%.
On a quantitative assessment, however, BM SUV mean was significantly higher in patients with bone marrow involvement (SUV mean of 3.7 [1.7-6] vs. 1.4 [0.4-2.65]; P less than .001). On the receiver operator curve (ROC) analysis, a BM SUV mean exceeding 2.7 had a positive predictive value of 100% for bone marrow involvement (sensitivity of 68%). A BM SUV mean less than 1.7 had an negative predictive value of 100% (specificity of 73%).
A mean standardized uptake value (BM SUV mean) below 1.7 may spare the need for bone marrow biopsy while a BM SUV mean above 2.7 is compatible with bone marrow involvement, although biopsy may still be recommended to exclude large cell transformation, the researchers concluded.
On Twitter @maryjodales
Quantifying bone marrow uptake of FDG (18fluorodeoxyglucose) improved the diagnostic accuracy of PET/CT for predicting bone marrow involvement in patients with follicular lymphoma, based on the results of a retrospective study.
Visual evidence of focal increased uptake on PET/CT indicates marrow involvement in follicular lymphoma; however, diffuse uptake is a nonspecific finding. Measuring the mean bone marrow standardized uptake value (BM SUV mean) improves PET/CT diagnostic accuracy, Dr. Chava Perry and his colleagues at Tel Aviv Sourasky Medical Center reported in Medicine [(Baltimore). 2016 Mar;95(9):e2910].
The researchers evaluated 68 consecutive patients with follicular lymphoma; 16 had bone marrow involvement – 13 had biopsy-proven involvement and 3 had a negative biopsy with increased medullary uptake that normalized after treatment. BM FDG uptake was diffuse in 8 of them and focal in the other 8.
While focal increased uptake is indicative of bone marrow involvement, diffuse uptake can be associated with false-positive results, as it was in the case of 17 patients (32.7% of those with diffuse uptake). Overall, visual assessment of scan results had a negative predictive value of 100% and a positive predictive value (PPV) of 48.5%.
On a quantitative assessment, however, BM SUV mean was significantly higher in patients with bone marrow involvement (SUV mean of 3.7 [1.7-6] vs. 1.4 [0.4-2.65]; P less than .001). On the receiver operator curve (ROC) analysis, a BM SUV mean exceeding 2.7 had a positive predictive value of 100% for bone marrow involvement (sensitivity of 68%). A BM SUV mean less than 1.7 had an negative predictive value of 100% (specificity of 73%).
A mean standardized uptake value (BM SUV mean) below 1.7 may spare the need for bone marrow biopsy while a BM SUV mean above 2.7 is compatible with bone marrow involvement, although biopsy may still be recommended to exclude large cell transformation, the researchers concluded.
On Twitter @maryjodales
Quantifying bone marrow uptake of FDG (18fluorodeoxyglucose) improved the diagnostic accuracy of PET/CT for predicting bone marrow involvement in patients with follicular lymphoma, based on the results of a retrospective study.
Visual evidence of focal increased uptake on PET/CT indicates marrow involvement in follicular lymphoma; however, diffuse uptake is a nonspecific finding. Measuring the mean bone marrow standardized uptake value (BM SUV mean) improves PET/CT diagnostic accuracy, Dr. Chava Perry and his colleagues at Tel Aviv Sourasky Medical Center reported in Medicine [(Baltimore). 2016 Mar;95(9):e2910].
The researchers evaluated 68 consecutive patients with follicular lymphoma; 16 had bone marrow involvement – 13 had biopsy-proven involvement and 3 had a negative biopsy with increased medullary uptake that normalized after treatment. BM FDG uptake was diffuse in 8 of them and focal in the other 8.
While focal increased uptake is indicative of bone marrow involvement, diffuse uptake can be associated with false-positive results, as it was in the case of 17 patients (32.7% of those with diffuse uptake). Overall, visual assessment of scan results had a negative predictive value of 100% and a positive predictive value (PPV) of 48.5%.
On a quantitative assessment, however, BM SUV mean was significantly higher in patients with bone marrow involvement (SUV mean of 3.7 [1.7-6] vs. 1.4 [0.4-2.65]; P less than .001). On the receiver operator curve (ROC) analysis, a BM SUV mean exceeding 2.7 had a positive predictive value of 100% for bone marrow involvement (sensitivity of 68%). A BM SUV mean less than 1.7 had an negative predictive value of 100% (specificity of 73%).
A mean standardized uptake value (BM SUV mean) below 1.7 may spare the need for bone marrow biopsy while a BM SUV mean above 2.7 is compatible with bone marrow involvement, although biopsy may still be recommended to exclude large cell transformation, the researchers concluded.
On Twitter @maryjodales
FROM MEDICINE
Key clinical point: Measuring the mean standardized uptake value of 18fluorodeoxyglucose in the bone marrow of patients with follicular lymphoma improves the diagnostic accuracy of PET/CT.
Major finding: In this study, diffuse uptake was associated with 17 (32.7%) false positive cases.
Data source: Retrospective study of 68 consecutive patients with follicular lymphoma.
Disclosures: The authors had no funding and conflicts of interest to disclose.
Infant egg introduction can prevent sensitization at 12 months
LOS ANGELES – Among infants at risk for allergic disease, egg introduction at 4 months cuts the risk of egg sensitization at 12 months by about half, according a randomized, placebo-controlled, double blind trial from Australia.
“This is what we hoped to find.” Introducing egg early “is certainly safe, and it may promote tolerance,” said senior investigator Dr. Dianne Campbell, professor and chair of pediatric allergy and clinical immunology at the Children’s Hospital at Westmead, which is affiliated with the University of Sydney.
Four-month-old children were randomized to 350 mg of pasteurized raw whole egg powder or – as a control – rice powder sprinkled once daily on their weaning food until month 8, at which time parents in both groups were encouraged to add eggs to their children’s diets. At least one of each child’s parents had a history of atopic disease, including asthma, eczema, hay fever, or food allergy. Even so, all of the infants had negative (less than 2 mm) skin prick tests (SPTs) at baseline. Compliance by parent diary was 89% in the rice and 81% in the egg groups.
At 12 months, SPTs were positive (3 mm or more) for whole egg in 25 of 122 (20%) children in the rice group, but only 13 of 122 (11%) in the egg group (odds ratio, 0.46; 95% confidence interval, 0.22-0.95; P = .03). Whole egg IgG4 and IgG4/IgE ratios to egg, ovalbumin, and ovomucoid were also higher in the egg group, indicating developing tolerance (P less than .0001 for each).
About 10% of the children originally in the egg group broke out in hives after their first few doses, and were withdrawn from the study. “This intervention may not be for everybody. There will be individuals who react” and it’s impossible, at this point, to predict who they will be. “We cannot prevent allergy in everyone,” said lead investigator Dr. John Tan, a pediatric immunologist at the hospital.
Overall, however, early introduction was safe. There was no anaphylaxis in the trial, and no cardiovascular or respiratory complications. Rates of eczema and peanut allergy were similar at 12 months between the two groups, meaning that early egg introduction did not increase the risk of atopy.
The findings echo results from several recent pediatric egg allergy studies, as well as findings from recent peanut trials. Slowly, it’s becoming clear that delaying the introduction of at least some allergenic foods – a common practice for years – doesn’t prevent allergies and may, in fact, promote them.
Despite those findings, there remains “a big disconnect between the [new] research and what we [still] recommend” in Australia, the United States, and elsewhere. Delaying food introductions was medical “dogma for 20 years, from highly esteemed societies,” and it corresponded with a marked increase in food allergies, but “it’s very hard to turn these things around,” Dr. Campbell said at the American Academy of Allergy, Asthma, and Immunology annual meeting.
The Australian government is reworking its infant feeding guidelines to incorporate the new evidence. “Our revised guidelines will say that there’s strong evidence for peanut and moderate evidence for egg” in favor of early introduction in children who are not sensitized by 4 or so months old, she said.
The trial was powered to detect differences in SPT, not actual egg allergies, which were diagnosed in 13 children (11%) in the rice group and eight (7%) in the egg group; the difference was not statistically significant. “Not all kids who are sensitized will be allergic,” she noted.
The study groups were well matched; there were about equal numbers of boys and girls in each, and, in both groups, about 15% of children were exposed to second hand smoke at home and almost all were breastfed.
The work was funded by the Australian government, among others. The investigators have no disclosures.
LOS ANGELES – Among infants at risk for allergic disease, egg introduction at 4 months cuts the risk of egg sensitization at 12 months by about half, according a randomized, placebo-controlled, double blind trial from Australia.
“This is what we hoped to find.” Introducing egg early “is certainly safe, and it may promote tolerance,” said senior investigator Dr. Dianne Campbell, professor and chair of pediatric allergy and clinical immunology at the Children’s Hospital at Westmead, which is affiliated with the University of Sydney.
Four-month-old children were randomized to 350 mg of pasteurized raw whole egg powder or – as a control – rice powder sprinkled once daily on their weaning food until month 8, at which time parents in both groups were encouraged to add eggs to their children’s diets. At least one of each child’s parents had a history of atopic disease, including asthma, eczema, hay fever, or food allergy. Even so, all of the infants had negative (less than 2 mm) skin prick tests (SPTs) at baseline. Compliance by parent diary was 89% in the rice and 81% in the egg groups.
At 12 months, SPTs were positive (3 mm or more) for whole egg in 25 of 122 (20%) children in the rice group, but only 13 of 122 (11%) in the egg group (odds ratio, 0.46; 95% confidence interval, 0.22-0.95; P = .03). Whole egg IgG4 and IgG4/IgE ratios to egg, ovalbumin, and ovomucoid were also higher in the egg group, indicating developing tolerance (P less than .0001 for each).
About 10% of the children originally in the egg group broke out in hives after their first few doses, and were withdrawn from the study. “This intervention may not be for everybody. There will be individuals who react” and it’s impossible, at this point, to predict who they will be. “We cannot prevent allergy in everyone,” said lead investigator Dr. John Tan, a pediatric immunologist at the hospital.
Overall, however, early introduction was safe. There was no anaphylaxis in the trial, and no cardiovascular or respiratory complications. Rates of eczema and peanut allergy were similar at 12 months between the two groups, meaning that early egg introduction did not increase the risk of atopy.
The findings echo results from several recent pediatric egg allergy studies, as well as findings from recent peanut trials. Slowly, it’s becoming clear that delaying the introduction of at least some allergenic foods – a common practice for years – doesn’t prevent allergies and may, in fact, promote them.
Despite those findings, there remains “a big disconnect between the [new] research and what we [still] recommend” in Australia, the United States, and elsewhere. Delaying food introductions was medical “dogma for 20 years, from highly esteemed societies,” and it corresponded with a marked increase in food allergies, but “it’s very hard to turn these things around,” Dr. Campbell said at the American Academy of Allergy, Asthma, and Immunology annual meeting.
The Australian government is reworking its infant feeding guidelines to incorporate the new evidence. “Our revised guidelines will say that there’s strong evidence for peanut and moderate evidence for egg” in favor of early introduction in children who are not sensitized by 4 or so months old, she said.
The trial was powered to detect differences in SPT, not actual egg allergies, which were diagnosed in 13 children (11%) in the rice group and eight (7%) in the egg group; the difference was not statistically significant. “Not all kids who are sensitized will be allergic,” she noted.
The study groups were well matched; there were about equal numbers of boys and girls in each, and, in both groups, about 15% of children were exposed to second hand smoke at home and almost all were breastfed.
The work was funded by the Australian government, among others. The investigators have no disclosures.
LOS ANGELES – Among infants at risk for allergic disease, egg introduction at 4 months cuts the risk of egg sensitization at 12 months by about half, according a randomized, placebo-controlled, double blind trial from Australia.
“This is what we hoped to find.” Introducing egg early “is certainly safe, and it may promote tolerance,” said senior investigator Dr. Dianne Campbell, professor and chair of pediatric allergy and clinical immunology at the Children’s Hospital at Westmead, which is affiliated with the University of Sydney.
Four-month-old children were randomized to 350 mg of pasteurized raw whole egg powder or – as a control – rice powder sprinkled once daily on their weaning food until month 8, at which time parents in both groups were encouraged to add eggs to their children’s diets. At least one of each child’s parents had a history of atopic disease, including asthma, eczema, hay fever, or food allergy. Even so, all of the infants had negative (less than 2 mm) skin prick tests (SPTs) at baseline. Compliance by parent diary was 89% in the rice and 81% in the egg groups.
At 12 months, SPTs were positive (3 mm or more) for whole egg in 25 of 122 (20%) children in the rice group, but only 13 of 122 (11%) in the egg group (odds ratio, 0.46; 95% confidence interval, 0.22-0.95; P = .03). Whole egg IgG4 and IgG4/IgE ratios to egg, ovalbumin, and ovomucoid were also higher in the egg group, indicating developing tolerance (P less than .0001 for each).
About 10% of the children originally in the egg group broke out in hives after their first few doses, and were withdrawn from the study. “This intervention may not be for everybody. There will be individuals who react” and it’s impossible, at this point, to predict who they will be. “We cannot prevent allergy in everyone,” said lead investigator Dr. John Tan, a pediatric immunologist at the hospital.
Overall, however, early introduction was safe. There was no anaphylaxis in the trial, and no cardiovascular or respiratory complications. Rates of eczema and peanut allergy were similar at 12 months between the two groups, meaning that early egg introduction did not increase the risk of atopy.
The findings echo results from several recent pediatric egg allergy studies, as well as findings from recent peanut trials. Slowly, it’s becoming clear that delaying the introduction of at least some allergenic foods – a common practice for years – doesn’t prevent allergies and may, in fact, promote them.
Despite those findings, there remains “a big disconnect between the [new] research and what we [still] recommend” in Australia, the United States, and elsewhere. Delaying food introductions was medical “dogma for 20 years, from highly esteemed societies,” and it corresponded with a marked increase in food allergies, but “it’s very hard to turn these things around,” Dr. Campbell said at the American Academy of Allergy, Asthma, and Immunology annual meeting.
The Australian government is reworking its infant feeding guidelines to incorporate the new evidence. “Our revised guidelines will say that there’s strong evidence for peanut and moderate evidence for egg” in favor of early introduction in children who are not sensitized by 4 or so months old, she said.
The trial was powered to detect differences in SPT, not actual egg allergies, which were diagnosed in 13 children (11%) in the rice group and eight (7%) in the egg group; the difference was not statistically significant. “Not all kids who are sensitized will be allergic,” she noted.
The study groups were well matched; there were about equal numbers of boys and girls in each, and, in both groups, about 15% of children were exposed to second hand smoke at home and almost all were breastfed.
The work was funded by the Australian government, among others. The investigators have no disclosures.
AT 2016 AAAAI ANNUAL MEETING
Key clinical point: If at-risk infants have negative skin prick tests (SPTs) at 4 months, tell their moms to introduce egg into their weaning diets.
Major finding: At 12 months, SPTs were positive (3 mm or more) for whole egg in 25 of 122 children (20%) in the rice group, but only 13 of 122 (11%) in the egg group (odds ratio, 0.46; 95% confidence interval, 0.22-0.95; P = .03).
Data source: Randomized clinical trial or 244 infants at risk for egg allergy.
Disclosures: The work was funded by the Australian government, among others. The investigators have no disclosures.
Sports Medicine Fellowship: What Should I Be Looking For?
The Orthopaedic Sports Medicine Fellowship Match was first established in 2008 as a joint-sponsored venture between the American Orthopaedic Society for Sports Medicine and the Arthroscopy Association of North America to pair applicants with participating training programs.1 Operated under the San Francisco Match,2 the current fellowship match process was adopted to systematically coordinate training appointments and eliminate the role of “exploding offers,” which are pressured early decisions predicated on immediate acceptance. Other advantages of this system include its operation through a central application service to avoid redundancy of submitted paperwork, as well as to create greater awareness and to publicize training options and standardization of the match timeline.1
In its current state, the orthopedic sports medicine match represents 96 programs with 230 positions, accounting for approximately 97% of training programs and fellowship positions.1 While unaccredited options remain available through the Match, many programs have migrated towards American Council for Graduate Medical Education (ACGME) accreditation because of an increased focus on objective learning metrics during fellowship and the requirement for Subspecialty Certification in Orthopaedic Sports Medicine through the American Board of Orthopaedic Surgery.3 However, other programs have also eschewed the increasing constraints and administrative resources associated with ACGME accreditation, particularly among fellowships based at community-based hospitals or private practices that lack formal affiliation with academic institutions or residency training programs.
Along with a greater understanding of the historical background of the match process, fellowship applicants must also appreciate the relative merits of fellowship training. More than 90% of orthopedic surgery residents now pursue further subspecialty fellowship training, with some individuals opting for 2 additional fellowship opportunities.4 As a so-called “nontraditional applicant,” I represent a different demographic, returning to fellowship after years of clinical practice while serving in the military. Individual preferences notwithstanding, I wanted to take the opportunity to emphasize some important considerations in deliberating between different fellowship programs.
- Geography. Your eventual desired practice location may play a role in determining fellowship location or, at least, region of the country. Additionally, this can be an important factor in family happiness. In competitive markets, such as the Northeast or the West Coast, you may make inroads and establish professional connections that result in potential job opportunities. Conversely, other programs may adopt anticompetitive measures to limit local practice options.
- Training setting. Despite the trending consolidation of fellowship training programs in affiliated university and hospital-based teaching systems, many community-based programs and private-practice models thrive, providing an alternative to traditional academic training centers. The latter may provide more in-depth exposure to practice management, billing/coding, and ancillary services. The former typically offer a more structured, academically oriented environment with formal teaching conferences and a broader department hierarchy.
- Program size. Some applicants may prefer a larger, more diverse array of teaching staff or fellows, while others gravitate toward fewer, more personal mentoring relationships that allow more intimate familiarity with practice habits or surgical techniques.
- Associated training programs. Affiliations with a residency or physician-extender training program can offer benefits and drawbacks, including offloading clerical work, shared hands-on experience in the clinic and operating room, and midlevel supervisory responsibilities. This can offer useful opportunities to formulate an individual teaching style and valuable mentoring relationships. However, it can also impose greater time requirements or detract from one-on-one teaching with staff.
- Reputation. Applicants may attach distinction to a well-established regional or national reputation associated with a given training program. Often, certain programs may carry prestige as a result of their academic name, hospital affiliation, or accomplishments. This can offer certain marketing advantages for patient recruitment. However, less renowned programs may provide better training opportunities and confer higher esteem among your professional colleagues. Program reputation can change dramatically with time, so this should be balanced with other potential strengths and overall training experience.
- Practice “niches”/areas of interest. With increasing adoption of arthroscopic techniques among practicing surgeons and a relative excess of sports medicine–trained orthopedists, it is paramount to develop a novel skill set during fellowship to differentiate you from other graduates. I sought a sports medicine fellowship that would offer me a broad-based exposure to arthroscopic and open knee and shoulder reconstruction, chondral restoration techniques, hip arthroscopy and preservation, and shoulder arthroplasty. Opportunities in elbow reconstruction, foot and ankle arthroscopy, and pediatric sports medicine may also be valuable as a distinguishing factor in searching for jobs after training.
- Marketability. Closely intertwined with reputation and scope of practice, an institution’s marketability is another intangible attribute to consider. Professional or collegiate team coverage offers significant market value for patient advertising, and it is frequently publicized by orthopedic practices and hospital systems. Additionally, the importance of ACGME accreditation should also be considered.
- Nonmedical training. This is increasingly important in subsequent subspecialty training. Further education on the business aspects of orthopedic surgery should be emphasized. Additionally, dedicated curricula on professional or leadership development are important for career progression.
- Mentorship. Throughout the interview process, one of my foremost priorities was a strong and enduring pattern of mentorship. Fellowship offers the opportunity to establish 1 or multiple mentors in your subspecialty. These individuals will be instrumental in the development of your early professional career and your approach to clinical practice. From discussions about complicated patients to advice on contract negotiations, your ideal mentor should champion your early successes and work generously on your behalf, even long after fellowship has ended.
- Research opportunities. Given my academic career goals, I actively pursued a program with rich clinical and laboratory resources, and an established infrastructure for accomplishing high-quality, relevant research. Interested individuals should gauge the availability of research support staff, biomechanical or bench-level laboratory collaboration, grant or institutional research funding, cadaveric specimens, or clinical outcomes data for research conducted by fellows. However, not all fellowship applicants have a vested interest in research during fellowship, so I would encourage inquiries regarding core research requirements and expectations.
- Clinical exposure. This encompasses several different and equally important variables, including diversity of clinical or surgical caseloads, case complexity, operative exposure, athletic team coverage, and office or clinical experience. Interestingly, this latter aspect of training is often neglected but cannot be overemphasized. Outpatient clinical evaluation is key to honing important physical examination techniques and critically evaluating patients’ outcomes postoperatively.
- Surgical autonomy. Hands-on operative experience and surgical autonomy vary widely among fellowship programs. Most fellowships advocate for a graduated level of surgical responsibility dependent on individual abilities and staff comfort, while others offer greater potential for independence. Conversely, some programs espouse more of an “observership” model, and arthroscopic simulators and/or cadaveric skills laboratories are designed to complement operative experience. While most fellowship applicants desire maximal case participation, we must also recognize the value in watching talented surgeons performing technically demanding procedures.
- Family. You cannot put a premium on your personal contentment and family’s well-being. Proximity to a support network can be important with the work demands and time constraints of fellowship.
Despite financial obligations and significant time commitments, the fellowship match process offers an incredible range of programs and practice environments. Inevitably, no program can completely fulfill all your criteria, but you should be able to tailor your learning style, professional ambitions, and personal preferences with an excellent training program. For many, fellowship represents the last, and perhaps most integral, stage of formal surgical training. Considering all factors of your chosen fellowship program will ensure a rich and fulfilling educational experience.
1. Sports medicine/arthroscopy fellowship match. American Orthopaedic Society for Sports Medicine website. https://www.sportsmed.org/AOSSMIMIS/Members/Members/Education/Sports_Medicine_Arthroscopy_Fellowship_Match.aspx. Accessed December 21, 2015.
2. Orthopaedic sports medicine fellowship. SF Match website. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=11&typ=1&name=Orthopaedic%20Sports%20Medicine. Accessed December 21, 2015.
3. Orthopaedic sports medicine. American Board of Orthopaedic Surgery website. https://www.abos.org/certification/sports-subspecialty.aspx. Accessed December 21, 2015.
4. Hariri S, York SC, O’Connor MI, Parsley BS, McCarthy JC. Career plans of current orthopaedic residents with a focus on sex-based and generational differences. J Bone Joint Surg Am. 2011;93(5):e16.
The Orthopaedic Sports Medicine Fellowship Match was first established in 2008 as a joint-sponsored venture between the American Orthopaedic Society for Sports Medicine and the Arthroscopy Association of North America to pair applicants with participating training programs.1 Operated under the San Francisco Match,2 the current fellowship match process was adopted to systematically coordinate training appointments and eliminate the role of “exploding offers,” which are pressured early decisions predicated on immediate acceptance. Other advantages of this system include its operation through a central application service to avoid redundancy of submitted paperwork, as well as to create greater awareness and to publicize training options and standardization of the match timeline.1
In its current state, the orthopedic sports medicine match represents 96 programs with 230 positions, accounting for approximately 97% of training programs and fellowship positions.1 While unaccredited options remain available through the Match, many programs have migrated towards American Council for Graduate Medical Education (ACGME) accreditation because of an increased focus on objective learning metrics during fellowship and the requirement for Subspecialty Certification in Orthopaedic Sports Medicine through the American Board of Orthopaedic Surgery.3 However, other programs have also eschewed the increasing constraints and administrative resources associated with ACGME accreditation, particularly among fellowships based at community-based hospitals or private practices that lack formal affiliation with academic institutions or residency training programs.
Along with a greater understanding of the historical background of the match process, fellowship applicants must also appreciate the relative merits of fellowship training. More than 90% of orthopedic surgery residents now pursue further subspecialty fellowship training, with some individuals opting for 2 additional fellowship opportunities.4 As a so-called “nontraditional applicant,” I represent a different demographic, returning to fellowship after years of clinical practice while serving in the military. Individual preferences notwithstanding, I wanted to take the opportunity to emphasize some important considerations in deliberating between different fellowship programs.
- Geography. Your eventual desired practice location may play a role in determining fellowship location or, at least, region of the country. Additionally, this can be an important factor in family happiness. In competitive markets, such as the Northeast or the West Coast, you may make inroads and establish professional connections that result in potential job opportunities. Conversely, other programs may adopt anticompetitive measures to limit local practice options.
- Training setting. Despite the trending consolidation of fellowship training programs in affiliated university and hospital-based teaching systems, many community-based programs and private-practice models thrive, providing an alternative to traditional academic training centers. The latter may provide more in-depth exposure to practice management, billing/coding, and ancillary services. The former typically offer a more structured, academically oriented environment with formal teaching conferences and a broader department hierarchy.
- Program size. Some applicants may prefer a larger, more diverse array of teaching staff or fellows, while others gravitate toward fewer, more personal mentoring relationships that allow more intimate familiarity with practice habits or surgical techniques.
- Associated training programs. Affiliations with a residency or physician-extender training program can offer benefits and drawbacks, including offloading clerical work, shared hands-on experience in the clinic and operating room, and midlevel supervisory responsibilities. This can offer useful opportunities to formulate an individual teaching style and valuable mentoring relationships. However, it can also impose greater time requirements or detract from one-on-one teaching with staff.
- Reputation. Applicants may attach distinction to a well-established regional or national reputation associated with a given training program. Often, certain programs may carry prestige as a result of their academic name, hospital affiliation, or accomplishments. This can offer certain marketing advantages for patient recruitment. However, less renowned programs may provide better training opportunities and confer higher esteem among your professional colleagues. Program reputation can change dramatically with time, so this should be balanced with other potential strengths and overall training experience.
- Practice “niches”/areas of interest. With increasing adoption of arthroscopic techniques among practicing surgeons and a relative excess of sports medicine–trained orthopedists, it is paramount to develop a novel skill set during fellowship to differentiate you from other graduates. I sought a sports medicine fellowship that would offer me a broad-based exposure to arthroscopic and open knee and shoulder reconstruction, chondral restoration techniques, hip arthroscopy and preservation, and shoulder arthroplasty. Opportunities in elbow reconstruction, foot and ankle arthroscopy, and pediatric sports medicine may also be valuable as a distinguishing factor in searching for jobs after training.
- Marketability. Closely intertwined with reputation and scope of practice, an institution’s marketability is another intangible attribute to consider. Professional or collegiate team coverage offers significant market value for patient advertising, and it is frequently publicized by orthopedic practices and hospital systems. Additionally, the importance of ACGME accreditation should also be considered.
- Nonmedical training. This is increasingly important in subsequent subspecialty training. Further education on the business aspects of orthopedic surgery should be emphasized. Additionally, dedicated curricula on professional or leadership development are important for career progression.
- Mentorship. Throughout the interview process, one of my foremost priorities was a strong and enduring pattern of mentorship. Fellowship offers the opportunity to establish 1 or multiple mentors in your subspecialty. These individuals will be instrumental in the development of your early professional career and your approach to clinical practice. From discussions about complicated patients to advice on contract negotiations, your ideal mentor should champion your early successes and work generously on your behalf, even long after fellowship has ended.
- Research opportunities. Given my academic career goals, I actively pursued a program with rich clinical and laboratory resources, and an established infrastructure for accomplishing high-quality, relevant research. Interested individuals should gauge the availability of research support staff, biomechanical or bench-level laboratory collaboration, grant or institutional research funding, cadaveric specimens, or clinical outcomes data for research conducted by fellows. However, not all fellowship applicants have a vested interest in research during fellowship, so I would encourage inquiries regarding core research requirements and expectations.
- Clinical exposure. This encompasses several different and equally important variables, including diversity of clinical or surgical caseloads, case complexity, operative exposure, athletic team coverage, and office or clinical experience. Interestingly, this latter aspect of training is often neglected but cannot be overemphasized. Outpatient clinical evaluation is key to honing important physical examination techniques and critically evaluating patients’ outcomes postoperatively.
- Surgical autonomy. Hands-on operative experience and surgical autonomy vary widely among fellowship programs. Most fellowships advocate for a graduated level of surgical responsibility dependent on individual abilities and staff comfort, while others offer greater potential for independence. Conversely, some programs espouse more of an “observership” model, and arthroscopic simulators and/or cadaveric skills laboratories are designed to complement operative experience. While most fellowship applicants desire maximal case participation, we must also recognize the value in watching talented surgeons performing technically demanding procedures.
- Family. You cannot put a premium on your personal contentment and family’s well-being. Proximity to a support network can be important with the work demands and time constraints of fellowship.
Despite financial obligations and significant time commitments, the fellowship match process offers an incredible range of programs and practice environments. Inevitably, no program can completely fulfill all your criteria, but you should be able to tailor your learning style, professional ambitions, and personal preferences with an excellent training program. For many, fellowship represents the last, and perhaps most integral, stage of formal surgical training. Considering all factors of your chosen fellowship program will ensure a rich and fulfilling educational experience.
The Orthopaedic Sports Medicine Fellowship Match was first established in 2008 as a joint-sponsored venture between the American Orthopaedic Society for Sports Medicine and the Arthroscopy Association of North America to pair applicants with participating training programs.1 Operated under the San Francisco Match,2 the current fellowship match process was adopted to systematically coordinate training appointments and eliminate the role of “exploding offers,” which are pressured early decisions predicated on immediate acceptance. Other advantages of this system include its operation through a central application service to avoid redundancy of submitted paperwork, as well as to create greater awareness and to publicize training options and standardization of the match timeline.1
In its current state, the orthopedic sports medicine match represents 96 programs with 230 positions, accounting for approximately 97% of training programs and fellowship positions.1 While unaccredited options remain available through the Match, many programs have migrated towards American Council for Graduate Medical Education (ACGME) accreditation because of an increased focus on objective learning metrics during fellowship and the requirement for Subspecialty Certification in Orthopaedic Sports Medicine through the American Board of Orthopaedic Surgery.3 However, other programs have also eschewed the increasing constraints and administrative resources associated with ACGME accreditation, particularly among fellowships based at community-based hospitals or private practices that lack formal affiliation with academic institutions or residency training programs.
Along with a greater understanding of the historical background of the match process, fellowship applicants must also appreciate the relative merits of fellowship training. More than 90% of orthopedic surgery residents now pursue further subspecialty fellowship training, with some individuals opting for 2 additional fellowship opportunities.4 As a so-called “nontraditional applicant,” I represent a different demographic, returning to fellowship after years of clinical practice while serving in the military. Individual preferences notwithstanding, I wanted to take the opportunity to emphasize some important considerations in deliberating between different fellowship programs.
- Geography. Your eventual desired practice location may play a role in determining fellowship location or, at least, region of the country. Additionally, this can be an important factor in family happiness. In competitive markets, such as the Northeast or the West Coast, you may make inroads and establish professional connections that result in potential job opportunities. Conversely, other programs may adopt anticompetitive measures to limit local practice options.
- Training setting. Despite the trending consolidation of fellowship training programs in affiliated university and hospital-based teaching systems, many community-based programs and private-practice models thrive, providing an alternative to traditional academic training centers. The latter may provide more in-depth exposure to practice management, billing/coding, and ancillary services. The former typically offer a more structured, academically oriented environment with formal teaching conferences and a broader department hierarchy.
- Program size. Some applicants may prefer a larger, more diverse array of teaching staff or fellows, while others gravitate toward fewer, more personal mentoring relationships that allow more intimate familiarity with practice habits or surgical techniques.
- Associated training programs. Affiliations with a residency or physician-extender training program can offer benefits and drawbacks, including offloading clerical work, shared hands-on experience in the clinic and operating room, and midlevel supervisory responsibilities. This can offer useful opportunities to formulate an individual teaching style and valuable mentoring relationships. However, it can also impose greater time requirements or detract from one-on-one teaching with staff.
- Reputation. Applicants may attach distinction to a well-established regional or national reputation associated with a given training program. Often, certain programs may carry prestige as a result of their academic name, hospital affiliation, or accomplishments. This can offer certain marketing advantages for patient recruitment. However, less renowned programs may provide better training opportunities and confer higher esteem among your professional colleagues. Program reputation can change dramatically with time, so this should be balanced with other potential strengths and overall training experience.
- Practice “niches”/areas of interest. With increasing adoption of arthroscopic techniques among practicing surgeons and a relative excess of sports medicine–trained orthopedists, it is paramount to develop a novel skill set during fellowship to differentiate you from other graduates. I sought a sports medicine fellowship that would offer me a broad-based exposure to arthroscopic and open knee and shoulder reconstruction, chondral restoration techniques, hip arthroscopy and preservation, and shoulder arthroplasty. Opportunities in elbow reconstruction, foot and ankle arthroscopy, and pediatric sports medicine may also be valuable as a distinguishing factor in searching for jobs after training.
- Marketability. Closely intertwined with reputation and scope of practice, an institution’s marketability is another intangible attribute to consider. Professional or collegiate team coverage offers significant market value for patient advertising, and it is frequently publicized by orthopedic practices and hospital systems. Additionally, the importance of ACGME accreditation should also be considered.
- Nonmedical training. This is increasingly important in subsequent subspecialty training. Further education on the business aspects of orthopedic surgery should be emphasized. Additionally, dedicated curricula on professional or leadership development are important for career progression.
- Mentorship. Throughout the interview process, one of my foremost priorities was a strong and enduring pattern of mentorship. Fellowship offers the opportunity to establish 1 or multiple mentors in your subspecialty. These individuals will be instrumental in the development of your early professional career and your approach to clinical practice. From discussions about complicated patients to advice on contract negotiations, your ideal mentor should champion your early successes and work generously on your behalf, even long after fellowship has ended.
- Research opportunities. Given my academic career goals, I actively pursued a program with rich clinical and laboratory resources, and an established infrastructure for accomplishing high-quality, relevant research. Interested individuals should gauge the availability of research support staff, biomechanical or bench-level laboratory collaboration, grant or institutional research funding, cadaveric specimens, or clinical outcomes data for research conducted by fellows. However, not all fellowship applicants have a vested interest in research during fellowship, so I would encourage inquiries regarding core research requirements and expectations.
- Clinical exposure. This encompasses several different and equally important variables, including diversity of clinical or surgical caseloads, case complexity, operative exposure, athletic team coverage, and office or clinical experience. Interestingly, this latter aspect of training is often neglected but cannot be overemphasized. Outpatient clinical evaluation is key to honing important physical examination techniques and critically evaluating patients’ outcomes postoperatively.
- Surgical autonomy. Hands-on operative experience and surgical autonomy vary widely among fellowship programs. Most fellowships advocate for a graduated level of surgical responsibility dependent on individual abilities and staff comfort, while others offer greater potential for independence. Conversely, some programs espouse more of an “observership” model, and arthroscopic simulators and/or cadaveric skills laboratories are designed to complement operative experience. While most fellowship applicants desire maximal case participation, we must also recognize the value in watching talented surgeons performing technically demanding procedures.
- Family. You cannot put a premium on your personal contentment and family’s well-being. Proximity to a support network can be important with the work demands and time constraints of fellowship.
Despite financial obligations and significant time commitments, the fellowship match process offers an incredible range of programs and practice environments. Inevitably, no program can completely fulfill all your criteria, but you should be able to tailor your learning style, professional ambitions, and personal preferences with an excellent training program. For many, fellowship represents the last, and perhaps most integral, stage of formal surgical training. Considering all factors of your chosen fellowship program will ensure a rich and fulfilling educational experience.
1. Sports medicine/arthroscopy fellowship match. American Orthopaedic Society for Sports Medicine website. https://www.sportsmed.org/AOSSMIMIS/Members/Members/Education/Sports_Medicine_Arthroscopy_Fellowship_Match.aspx. Accessed December 21, 2015.
2. Orthopaedic sports medicine fellowship. SF Match website. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=11&typ=1&name=Orthopaedic%20Sports%20Medicine. Accessed December 21, 2015.
3. Orthopaedic sports medicine. American Board of Orthopaedic Surgery website. https://www.abos.org/certification/sports-subspecialty.aspx. Accessed December 21, 2015.
4. Hariri S, York SC, O’Connor MI, Parsley BS, McCarthy JC. Career plans of current orthopaedic residents with a focus on sex-based and generational differences. J Bone Joint Surg Am. 2011;93(5):e16.
1. Sports medicine/arthroscopy fellowship match. American Orthopaedic Society for Sports Medicine website. https://www.sportsmed.org/AOSSMIMIS/Members/Members/Education/Sports_Medicine_Arthroscopy_Fellowship_Match.aspx. Accessed December 21, 2015.
2. Orthopaedic sports medicine fellowship. SF Match website. https://www.sfmatch.org/SpecialtyInsideAll.aspx?id=11&typ=1&name=Orthopaedic%20Sports%20Medicine. Accessed December 21, 2015.
3. Orthopaedic sports medicine. American Board of Orthopaedic Surgery website. https://www.abos.org/certification/sports-subspecialty.aspx. Accessed December 21, 2015.
4. Hariri S, York SC, O’Connor MI, Parsley BS, McCarthy JC. Career plans of current orthopaedic residents with a focus on sex-based and generational differences. J Bone Joint Surg Am. 2011;93(5):e16.