User login
Lower the CT to check the heart for embolic sources in acute stroke
LOS ANGELES – Enlarge the field of CT angiography to include the heart in acute ischemic stroke patients; you’ll quickly identify sources of cardiogenic emboli and other problems that will otherwise be missed, according to investigators from the National University Hospital, Singapore.
It adds only a few seconds to the scan, with no extra contrast or meaningful increase in radiation. There’s no need to gate the heart with beta-blockers.
Among 20 acute ischemic stroke patients presenting within 4.5 hours of symptom onset, Dr. Leonard Yeo and his coinvestigators found one with a localized dissection in the ascending aorta, another with a ventricular thrombus, and a third with an atrial appendage blood clot. Both thrombus cases were confirmed by transesophageal echocardiography and started on anticoagulation the next day. The 2-phase, 64-slice nongated cardiac CT angiographies (CTA) were done in the same sitting as the brain CTA.
“Scans with 1-mm thick slices are best for screening for thrombus and structural abnormalities that cause embolism. Remarkably, [even without gating], the detail is excellent. There’s very little downside [to this, and] it maximizes your return on scans that are already a part of most acute stroke protocols,” said Dr. Yeo, a neurologist at the hospital.
“Since most of our patients get a CTA during acute stroke, it made sense to check the heart for embolic sources.” There isn’t any time to give a beta-blocker, so “these were nongated” scans, Dr. Yeo said during his presentation at the International Stroke Conference, sponsored by the American Heart Association.
If it’s confirmed that nongated heart CTAs provide useful information, “we will probably all be doing this in the future. Everybody does CTs for the head in acute stroke, so all you do is go down a little lower” without any more contrast. “Within an hour of somebody presenting, you know what they have,” said Dr. Robert Hart, a neurology professor at McMaster University in Hamilton, Ont., and co-moderator of Dr. Yeo’s presentation.
In most places, acute ischemic stroke patients only get an ECG. Transesophageal echocardiography (TEE) is also good for checking the heart, but it usually comes later. It “excels at detecting abnormalities with medium embolic risk,” such as patent foramen ovale and septal aneurysm. “However, for these medium-risk cardiac sources of embolism, the optimal choice of therapy is not clear. Unlike high-risk sources which require anticoagulation, TEE does not provide therapeutic gains in terms of clinical decision making,” Dr. Yeo said.
Nongated cardiac CTAs during acute stroke, he added, also check chamber, valve, pericardial, and great vessel morphology, as well as abnormal chambers-vessel communications and “left ventricular aneurysms that can rupture with [tissue plasminogen activator], with catastrophic consequences.”
The mean age in the study was 64 years old, and about 60% of the subjects were men. None of the patients were dead at 3 months, and by then eight (40%) had modified Rankin Scale scores of 0-1. Patients were excluded if they had contraindications to IV contrast, or were unable to provide informed consent. CTA images were read by the treating neurologist and radiologist.
The work was funded by the Singapore Ministry of Health’s National Medical Research Council. The investigators have no relevant disclosures.
LOS ANGELES – Enlarge the field of CT angiography to include the heart in acute ischemic stroke patients; you’ll quickly identify sources of cardiogenic emboli and other problems that will otherwise be missed, according to investigators from the National University Hospital, Singapore.
It adds only a few seconds to the scan, with no extra contrast or meaningful increase in radiation. There’s no need to gate the heart with beta-blockers.
Among 20 acute ischemic stroke patients presenting within 4.5 hours of symptom onset, Dr. Leonard Yeo and his coinvestigators found one with a localized dissection in the ascending aorta, another with a ventricular thrombus, and a third with an atrial appendage blood clot. Both thrombus cases were confirmed by transesophageal echocardiography and started on anticoagulation the next day. The 2-phase, 64-slice nongated cardiac CT angiographies (CTA) were done in the same sitting as the brain CTA.
“Scans with 1-mm thick slices are best for screening for thrombus and structural abnormalities that cause embolism. Remarkably, [even without gating], the detail is excellent. There’s very little downside [to this, and] it maximizes your return on scans that are already a part of most acute stroke protocols,” said Dr. Yeo, a neurologist at the hospital.
“Since most of our patients get a CTA during acute stroke, it made sense to check the heart for embolic sources.” There isn’t any time to give a beta-blocker, so “these were nongated” scans, Dr. Yeo said during his presentation at the International Stroke Conference, sponsored by the American Heart Association.
If it’s confirmed that nongated heart CTAs provide useful information, “we will probably all be doing this in the future. Everybody does CTs for the head in acute stroke, so all you do is go down a little lower” without any more contrast. “Within an hour of somebody presenting, you know what they have,” said Dr. Robert Hart, a neurology professor at McMaster University in Hamilton, Ont., and co-moderator of Dr. Yeo’s presentation.
In most places, acute ischemic stroke patients only get an ECG. Transesophageal echocardiography (TEE) is also good for checking the heart, but it usually comes later. It “excels at detecting abnormalities with medium embolic risk,” such as patent foramen ovale and septal aneurysm. “However, for these medium-risk cardiac sources of embolism, the optimal choice of therapy is not clear. Unlike high-risk sources which require anticoagulation, TEE does not provide therapeutic gains in terms of clinical decision making,” Dr. Yeo said.
Nongated cardiac CTAs during acute stroke, he added, also check chamber, valve, pericardial, and great vessel morphology, as well as abnormal chambers-vessel communications and “left ventricular aneurysms that can rupture with [tissue plasminogen activator], with catastrophic consequences.”
The mean age in the study was 64 years old, and about 60% of the subjects were men. None of the patients were dead at 3 months, and by then eight (40%) had modified Rankin Scale scores of 0-1. Patients were excluded if they had contraindications to IV contrast, or were unable to provide informed consent. CTA images were read by the treating neurologist and radiologist.
The work was funded by the Singapore Ministry of Health’s National Medical Research Council. The investigators have no relevant disclosures.
LOS ANGELES – Enlarge the field of CT angiography to include the heart in acute ischemic stroke patients; you’ll quickly identify sources of cardiogenic emboli and other problems that will otherwise be missed, according to investigators from the National University Hospital, Singapore.
It adds only a few seconds to the scan, with no extra contrast or meaningful increase in radiation. There’s no need to gate the heart with beta-blockers.
Among 20 acute ischemic stroke patients presenting within 4.5 hours of symptom onset, Dr. Leonard Yeo and his coinvestigators found one with a localized dissection in the ascending aorta, another with a ventricular thrombus, and a third with an atrial appendage blood clot. Both thrombus cases were confirmed by transesophageal echocardiography and started on anticoagulation the next day. The 2-phase, 64-slice nongated cardiac CT angiographies (CTA) were done in the same sitting as the brain CTA.
“Scans with 1-mm thick slices are best for screening for thrombus and structural abnormalities that cause embolism. Remarkably, [even without gating], the detail is excellent. There’s very little downside [to this, and] it maximizes your return on scans that are already a part of most acute stroke protocols,” said Dr. Yeo, a neurologist at the hospital.
“Since most of our patients get a CTA during acute stroke, it made sense to check the heart for embolic sources.” There isn’t any time to give a beta-blocker, so “these were nongated” scans, Dr. Yeo said during his presentation at the International Stroke Conference, sponsored by the American Heart Association.
If it’s confirmed that nongated heart CTAs provide useful information, “we will probably all be doing this in the future. Everybody does CTs for the head in acute stroke, so all you do is go down a little lower” without any more contrast. “Within an hour of somebody presenting, you know what they have,” said Dr. Robert Hart, a neurology professor at McMaster University in Hamilton, Ont., and co-moderator of Dr. Yeo’s presentation.
In most places, acute ischemic stroke patients only get an ECG. Transesophageal echocardiography (TEE) is also good for checking the heart, but it usually comes later. It “excels at detecting abnormalities with medium embolic risk,” such as patent foramen ovale and septal aneurysm. “However, for these medium-risk cardiac sources of embolism, the optimal choice of therapy is not clear. Unlike high-risk sources which require anticoagulation, TEE does not provide therapeutic gains in terms of clinical decision making,” Dr. Yeo said.
Nongated cardiac CTAs during acute stroke, he added, also check chamber, valve, pericardial, and great vessel morphology, as well as abnormal chambers-vessel communications and “left ventricular aneurysms that can rupture with [tissue plasminogen activator], with catastrophic consequences.”
The mean age in the study was 64 years old, and about 60% of the subjects were men. None of the patients were dead at 3 months, and by then eight (40%) had modified Rankin Scale scores of 0-1. Patients were excluded if they had contraindications to IV contrast, or were unable to provide informed consent. CTA images were read by the treating neurologist and radiologist.
The work was funded by the Singapore Ministry of Health’s National Medical Research Council. The investigators have no relevant disclosures.
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point: Nongated heart CTAs may provide useful information in acute ischemic stroke.
Major finding: Among 20 acute ischemic stroke patients presenting within 4.5 hours of symptom onset, one had a localized dissection in the ascending aorta, another had a ventricular thrombus, and a third had an atrial appendage blood clot.
Data source: Prospective investigation of 20 patients.
Disclosures: The work was funded by the Singapore Ministry of Health’s National Medical Research Council. The investigators have no relevant disclosures.
Lenalidomide-dexamethasone yields similar PFS as triplet regimens in elderly multiple myeloma patients
A comparison of lenalidomide-based treatments for multiple myeloma patients who were ineligible for stem cell transplantation showed similar progression-free survival (PFS) for two alkylator-containing triplet regimens and an alkylator-free doublet regimen but a higher risk of hematologic toxicity with a melphalan-prednisone-lenalidomide regimen.
For the triplet regimens, melphalan-prednisone-lenalidomide (MPR) and cyclophosphamide-prednisone-lenalidomide (CPR), the median PFS was 22 months, compared with 21 months for the doublet regimen lenalidomide plus low-dose dexamethasone (Rd). The hazard ratio (HR) was 0.906 (95% CI, 0.739-1.11; P = .344). The 4-year overall survival (OS) was 67% with triplet and 58% with doublet regimens (HR, 0.945; 95% CI, 0.700-1.274; P = .709) (Blood. 2016;127[9]:1102-8).
The major safety concern, according to the researchers, was the higher toxicity with MPR compared with CPR and Rd. The most frequent toxicities of grade 3 or more were hematologic, with at least one reported event in 68% of the MPR arm, 32% of CPR, and 29% or Rd patients (P less than .0001). In a post hoc analysis of safety according to patient fitness, the incidence of at least one hematologic adverse event ocurred in 75% of fit patients in the MPR arm occurred, 34% in CPR, and 29% in Rd; the incidence of at least one hematologic adverse event in intermediate fitness patients in the MPR arm was 61%, 33% in CPR, and 25% in Rd; in frail patients, 75% in MPR, 28% in CPR, and 3% in Rd (P = .001 for MPR vs. Rd and MPR vs. CPR). Nonhematologic adverse events were similar for the three groups and less than 10%.
Previous studies, such as the FIRST trial, showed the superiority of lenalidomide-containing regimens over standard treatments, but a question remained over the best drug to combine with lenalidomide – an alkylating agent or steroid. Separate analysis of the three arms further illustrated that the addition of an alkylating agent did not lead to better response or outcome. Median PFS for MPR, CPR, and Rd arms were 24, 20, and 21 months, respectively; 4-year OS rates were 65%, 68%, and 58%, respectively; overall response rates were 71%, 68%, and 74%, respectively.
Compared with the previous FIRST study, the less intense regimen in this study (Rd administered for only 9 months as induction treatment, followed by maintenance with lenalidomide at a lower dose) resulted in less hematological toxicity.
“This suggests that continuous treatment with Rd can be a valuable option for prolonging PFS and achieving a deeper response, and reducing the dose during maintenance can be a valuable strategy for improving tolerability,” wrote Dr. Valeria Magarotto of the myeloma unit in the division of hematology at the University of Torino (Italy), and colleagues. They added, “A more intensive induction treatment with Rd administered for a limited duration (9 months) followed by a less intensive continuous treatment with lenalidomide alone seems to be a sensible and effective choice.”
The phase III trial included 654 patients with newly diagnosed multiple myeloma who were ineligible for stem cell transplantation due to advanced age (65 years and older) or comorbidities. Patients were randomized to receive MPR (n = 217), CPR (n = 220), or Rd (n = 217).
A comparison of lenalidomide-based treatments for multiple myeloma patients who were ineligible for stem cell transplantation showed similar progression-free survival (PFS) for two alkylator-containing triplet regimens and an alkylator-free doublet regimen but a higher risk of hematologic toxicity with a melphalan-prednisone-lenalidomide regimen.
For the triplet regimens, melphalan-prednisone-lenalidomide (MPR) and cyclophosphamide-prednisone-lenalidomide (CPR), the median PFS was 22 months, compared with 21 months for the doublet regimen lenalidomide plus low-dose dexamethasone (Rd). The hazard ratio (HR) was 0.906 (95% CI, 0.739-1.11; P = .344). The 4-year overall survival (OS) was 67% with triplet and 58% with doublet regimens (HR, 0.945; 95% CI, 0.700-1.274; P = .709) (Blood. 2016;127[9]:1102-8).
The major safety concern, according to the researchers, was the higher toxicity with MPR compared with CPR and Rd. The most frequent toxicities of grade 3 or more were hematologic, with at least one reported event in 68% of the MPR arm, 32% of CPR, and 29% or Rd patients (P less than .0001). In a post hoc analysis of safety according to patient fitness, the incidence of at least one hematologic adverse event ocurred in 75% of fit patients in the MPR arm occurred, 34% in CPR, and 29% in Rd; the incidence of at least one hematologic adverse event in intermediate fitness patients in the MPR arm was 61%, 33% in CPR, and 25% in Rd; in frail patients, 75% in MPR, 28% in CPR, and 3% in Rd (P = .001 for MPR vs. Rd and MPR vs. CPR). Nonhematologic adverse events were similar for the three groups and less than 10%.
Previous studies, such as the FIRST trial, showed the superiority of lenalidomide-containing regimens over standard treatments, but a question remained over the best drug to combine with lenalidomide – an alkylating agent or steroid. Separate analysis of the three arms further illustrated that the addition of an alkylating agent did not lead to better response or outcome. Median PFS for MPR, CPR, and Rd arms were 24, 20, and 21 months, respectively; 4-year OS rates were 65%, 68%, and 58%, respectively; overall response rates were 71%, 68%, and 74%, respectively.
Compared with the previous FIRST study, the less intense regimen in this study (Rd administered for only 9 months as induction treatment, followed by maintenance with lenalidomide at a lower dose) resulted in less hematological toxicity.
“This suggests that continuous treatment with Rd can be a valuable option for prolonging PFS and achieving a deeper response, and reducing the dose during maintenance can be a valuable strategy for improving tolerability,” wrote Dr. Valeria Magarotto of the myeloma unit in the division of hematology at the University of Torino (Italy), and colleagues. They added, “A more intensive induction treatment with Rd administered for a limited duration (9 months) followed by a less intensive continuous treatment with lenalidomide alone seems to be a sensible and effective choice.”
The phase III trial included 654 patients with newly diagnosed multiple myeloma who were ineligible for stem cell transplantation due to advanced age (65 years and older) or comorbidities. Patients were randomized to receive MPR (n = 217), CPR (n = 220), or Rd (n = 217).
A comparison of lenalidomide-based treatments for multiple myeloma patients who were ineligible for stem cell transplantation showed similar progression-free survival (PFS) for two alkylator-containing triplet regimens and an alkylator-free doublet regimen but a higher risk of hematologic toxicity with a melphalan-prednisone-lenalidomide regimen.
For the triplet regimens, melphalan-prednisone-lenalidomide (MPR) and cyclophosphamide-prednisone-lenalidomide (CPR), the median PFS was 22 months, compared with 21 months for the doublet regimen lenalidomide plus low-dose dexamethasone (Rd). The hazard ratio (HR) was 0.906 (95% CI, 0.739-1.11; P = .344). The 4-year overall survival (OS) was 67% with triplet and 58% with doublet regimens (HR, 0.945; 95% CI, 0.700-1.274; P = .709) (Blood. 2016;127[9]:1102-8).
The major safety concern, according to the researchers, was the higher toxicity with MPR compared with CPR and Rd. The most frequent toxicities of grade 3 or more were hematologic, with at least one reported event in 68% of the MPR arm, 32% of CPR, and 29% or Rd patients (P less than .0001). In a post hoc analysis of safety according to patient fitness, the incidence of at least one hematologic adverse event ocurred in 75% of fit patients in the MPR arm occurred, 34% in CPR, and 29% in Rd; the incidence of at least one hematologic adverse event in intermediate fitness patients in the MPR arm was 61%, 33% in CPR, and 25% in Rd; in frail patients, 75% in MPR, 28% in CPR, and 3% in Rd (P = .001 for MPR vs. Rd and MPR vs. CPR). Nonhematologic adverse events were similar for the three groups and less than 10%.
Previous studies, such as the FIRST trial, showed the superiority of lenalidomide-containing regimens over standard treatments, but a question remained over the best drug to combine with lenalidomide – an alkylating agent or steroid. Separate analysis of the three arms further illustrated that the addition of an alkylating agent did not lead to better response or outcome. Median PFS for MPR, CPR, and Rd arms were 24, 20, and 21 months, respectively; 4-year OS rates were 65%, 68%, and 58%, respectively; overall response rates were 71%, 68%, and 74%, respectively.
Compared with the previous FIRST study, the less intense regimen in this study (Rd administered for only 9 months as induction treatment, followed by maintenance with lenalidomide at a lower dose) resulted in less hematological toxicity.
“This suggests that continuous treatment with Rd can be a valuable option for prolonging PFS and achieving a deeper response, and reducing the dose during maintenance can be a valuable strategy for improving tolerability,” wrote Dr. Valeria Magarotto of the myeloma unit in the division of hematology at the University of Torino (Italy), and colleagues. They added, “A more intensive induction treatment with Rd administered for a limited duration (9 months) followed by a less intensive continuous treatment with lenalidomide alone seems to be a sensible and effective choice.”
The phase III trial included 654 patients with newly diagnosed multiple myeloma who were ineligible for stem cell transplantation due to advanced age (65 years and older) or comorbidities. Patients were randomized to receive MPR (n = 217), CPR (n = 220), or Rd (n = 217).
FROM BLOOD
Key clinical point: In elderly patients with newly diagnosed multiple myeloma, progression-free survival was similar for alkylator-containing triplet regimens and an alkylator-free doublet regimen, but the doublet resulted in less hematologic toxicity.
Major finding: Median PFS for MPR, CPR and Rd arms were 24, 20, and 21 months, respectively; 4-year OS rates were 65%, 68%, and 58%, respectively.
Data sources: Phase III trial of 654 patients randomized to receive melphalan-prednisone-lenalidomide (n = 217), cyclophosphamide-prednisone-lenalidomide (n = 220), or lenalidomide plus low-dose dexamethasone (n = 217).
Disclosures: Dr. Magarotto reported having no disclosures. Several of her coauthors reported financial ties to industry sources.
Expert examines secukinumab’s role in ankylosing spondylitis treatment strategies
MAUI, HAWAII – The most important development within the past year in the treatment of ankylosing spondylitis was the Food and Drug Administration approval of secukinumab (Cosentyx) as the first non-tumor necrosis factor inhibitor biologic for this condition – but the interleukin-17A inhibitor is not going to immediately step into a role as a first-line therapy, Dr. Eric M. Ruderman predicted at the 2016 Rheumatology Winter Clinical Symposium.
“In all likelihood nobody’s going to use this as a first-line drug right out of the gate. It’s a drug you’re going to potentially go to in people who haven’t responded to the things that you’ve been comfortable using for the last 10 or 15 years. So the big practical issue becomes, ‘How does secukinumab perform in TNF inhibitor-naive patients versus prior TNF inhibitor inadequate responders?’ ” according to the rheumatologist, who is professor of medicine at Northwestern University in Chicago.
This question has been addressed in secondary analyses of the pivotal phase III MEASURE 1 and MEASURE 2 trials which have been presented at the annual European League Against Rheumatism and American College of Rheumatology meetings. The bottom line was that the therapeutic response rate in both trials was markedly lower in TNF inhibitor inadequate responders than in TNF inhibitor-naive subjects.
“But there still is a significant response rate in the inadequate responders. It’s clearly better than placebo. So this is a drug that may have a role in your practice at the point where patients have failed on one or two anti-TNF biologics,” according to Dr. Ruderman.
The difference between MEASURE 1 and MEASURE 2 is that MEASURE 1 entailed three intravenous loading doses of the biologic at 2-week intervals before switching to monthly subcutaneous dosing, while MEASURE 2 featured subcutaneous loading doses given weekly for 4 weeks before moving to monthly administration. Interestingly, the FDA approval of secukinumab at 150 mg doesn’t call for a loading dose, even though both pivotal trials relied on them, the rheumatologist observed.
At 16 weeks in MEASURE 1, 66% of TNF inhibitor-naive subjects on secukinumab 150 mg had at least a 20% improvement from baseline in ankylosing spondylitis signs and symptoms, or Assessment of Spondyloarthritis International Society (ASAS) 20, compared with 46% of TNF inhibitor inadequate responders. The week 16 ASAS 20 rate in MEASURE 2 was 68% in TNF inhibitor-naive patients and 50% in those with a prior inadequate response to TNF inhibitor therapy.
How should rheumatologists expect secukinumab to perform in daily clinical practice? In the 181 ankylosing spoindylitis patients who completed 52 weeks in the MEASURE 2 extension study, 74% of those on secukinumab at 150 mg had an ASAS 20 response. In both trials, the secukinumab side effect profile was “reasonably clean,” in Dr. Ruderman’s view, with serious adverse events that were similar to placebo.
Serial MRI scans showed rapid resolution of bone marrow edema and inflammation by 16 weeks, an effect sustained through 52 weeks.
The big unanswered question is whether secukinumab prevents radiographic progression of the disease. Serial cervical and spinal X-rays rated using the modified Stoke Ankylosing Spondylitis Spinal Score showed a mean increase of just 0.30 points at 2 years from a baseline of 10.22, with 80% of patients demonstrating no change over time. But there were no untreated controls for comparison in this analysis, so it’s not possible to say whether the drug actually slowed disease progression or that’s the natural history of disease in those subjects, Dr. Ruderman noted.
Effect of NSAID dosing frequency on progression
On the topic of preventing radiographic progression in ankylosing spondylitis, the rheumatologist highlighted a prospective study presented at last year’s EULAR meeting and published online last summer (Ann Rheum Dis. 2015 Aug 4. doi: 10.1136/annrheumdis-2015-207897) that demonstrated that continuous use of diclofenac didn’t do any better at preventing radiographic spinal disease progression than on-demand use of the nonsteroidal anti-inflammatory drug (NSAID) over the course of 2 years.
“There’s been a lot of noise in the ankylosing spondylitis community about the potential benefit of NSAIDs in preventing structural progression. Previous information suggested that staying on them continuously actually reduced radiographic progression. This diclofenac study has shaken things up a little. It raises the question of whether there is any added benefit for NSAIDs in terms of structural progression,” he commented.
Current ACR/SAA/SPARTAN guidelines, which predate the study, feature a conditional recommendation that patients with active ankylosing spondylitis stay on continuous NSAID therapy.
Secukinumab is also approved for treatment of psoriasis and psoriatic arthritis.
Dr. Ruderman reported serving as a consultant to and/or receiving research grants from numerous pharmaceutical companies, including Novartis, which markets secukinumab.
MAUI, HAWAII – The most important development within the past year in the treatment of ankylosing spondylitis was the Food and Drug Administration approval of secukinumab (Cosentyx) as the first non-tumor necrosis factor inhibitor biologic for this condition – but the interleukin-17A inhibitor is not going to immediately step into a role as a first-line therapy, Dr. Eric M. Ruderman predicted at the 2016 Rheumatology Winter Clinical Symposium.
“In all likelihood nobody’s going to use this as a first-line drug right out of the gate. It’s a drug you’re going to potentially go to in people who haven’t responded to the things that you’ve been comfortable using for the last 10 or 15 years. So the big practical issue becomes, ‘How does secukinumab perform in TNF inhibitor-naive patients versus prior TNF inhibitor inadequate responders?’ ” according to the rheumatologist, who is professor of medicine at Northwestern University in Chicago.
This question has been addressed in secondary analyses of the pivotal phase III MEASURE 1 and MEASURE 2 trials which have been presented at the annual European League Against Rheumatism and American College of Rheumatology meetings. The bottom line was that the therapeutic response rate in both trials was markedly lower in TNF inhibitor inadequate responders than in TNF inhibitor-naive subjects.
“But there still is a significant response rate in the inadequate responders. It’s clearly better than placebo. So this is a drug that may have a role in your practice at the point where patients have failed on one or two anti-TNF biologics,” according to Dr. Ruderman.
The difference between MEASURE 1 and MEASURE 2 is that MEASURE 1 entailed three intravenous loading doses of the biologic at 2-week intervals before switching to monthly subcutaneous dosing, while MEASURE 2 featured subcutaneous loading doses given weekly for 4 weeks before moving to monthly administration. Interestingly, the FDA approval of secukinumab at 150 mg doesn’t call for a loading dose, even though both pivotal trials relied on them, the rheumatologist observed.
At 16 weeks in MEASURE 1, 66% of TNF inhibitor-naive subjects on secukinumab 150 mg had at least a 20% improvement from baseline in ankylosing spondylitis signs and symptoms, or Assessment of Spondyloarthritis International Society (ASAS) 20, compared with 46% of TNF inhibitor inadequate responders. The week 16 ASAS 20 rate in MEASURE 2 was 68% in TNF inhibitor-naive patients and 50% in those with a prior inadequate response to TNF inhibitor therapy.
How should rheumatologists expect secukinumab to perform in daily clinical practice? In the 181 ankylosing spoindylitis patients who completed 52 weeks in the MEASURE 2 extension study, 74% of those on secukinumab at 150 mg had an ASAS 20 response. In both trials, the secukinumab side effect profile was “reasonably clean,” in Dr. Ruderman’s view, with serious adverse events that were similar to placebo.
Serial MRI scans showed rapid resolution of bone marrow edema and inflammation by 16 weeks, an effect sustained through 52 weeks.
The big unanswered question is whether secukinumab prevents radiographic progression of the disease. Serial cervical and spinal X-rays rated using the modified Stoke Ankylosing Spondylitis Spinal Score showed a mean increase of just 0.30 points at 2 years from a baseline of 10.22, with 80% of patients demonstrating no change over time. But there were no untreated controls for comparison in this analysis, so it’s not possible to say whether the drug actually slowed disease progression or that’s the natural history of disease in those subjects, Dr. Ruderman noted.
Effect of NSAID dosing frequency on progression
On the topic of preventing radiographic progression in ankylosing spondylitis, the rheumatologist highlighted a prospective study presented at last year’s EULAR meeting and published online last summer (Ann Rheum Dis. 2015 Aug 4. doi: 10.1136/annrheumdis-2015-207897) that demonstrated that continuous use of diclofenac didn’t do any better at preventing radiographic spinal disease progression than on-demand use of the nonsteroidal anti-inflammatory drug (NSAID) over the course of 2 years.
“There’s been a lot of noise in the ankylosing spondylitis community about the potential benefit of NSAIDs in preventing structural progression. Previous information suggested that staying on them continuously actually reduced radiographic progression. This diclofenac study has shaken things up a little. It raises the question of whether there is any added benefit for NSAIDs in terms of structural progression,” he commented.
Current ACR/SAA/SPARTAN guidelines, which predate the study, feature a conditional recommendation that patients with active ankylosing spondylitis stay on continuous NSAID therapy.
Secukinumab is also approved for treatment of psoriasis and psoriatic arthritis.
Dr. Ruderman reported serving as a consultant to and/or receiving research grants from numerous pharmaceutical companies, including Novartis, which markets secukinumab.
MAUI, HAWAII – The most important development within the past year in the treatment of ankylosing spondylitis was the Food and Drug Administration approval of secukinumab (Cosentyx) as the first non-tumor necrosis factor inhibitor biologic for this condition – but the interleukin-17A inhibitor is not going to immediately step into a role as a first-line therapy, Dr. Eric M. Ruderman predicted at the 2016 Rheumatology Winter Clinical Symposium.
“In all likelihood nobody’s going to use this as a first-line drug right out of the gate. It’s a drug you’re going to potentially go to in people who haven’t responded to the things that you’ve been comfortable using for the last 10 or 15 years. So the big practical issue becomes, ‘How does secukinumab perform in TNF inhibitor-naive patients versus prior TNF inhibitor inadequate responders?’ ” according to the rheumatologist, who is professor of medicine at Northwestern University in Chicago.
This question has been addressed in secondary analyses of the pivotal phase III MEASURE 1 and MEASURE 2 trials which have been presented at the annual European League Against Rheumatism and American College of Rheumatology meetings. The bottom line was that the therapeutic response rate in both trials was markedly lower in TNF inhibitor inadequate responders than in TNF inhibitor-naive subjects.
“But there still is a significant response rate in the inadequate responders. It’s clearly better than placebo. So this is a drug that may have a role in your practice at the point where patients have failed on one or two anti-TNF biologics,” according to Dr. Ruderman.
The difference between MEASURE 1 and MEASURE 2 is that MEASURE 1 entailed three intravenous loading doses of the biologic at 2-week intervals before switching to monthly subcutaneous dosing, while MEASURE 2 featured subcutaneous loading doses given weekly for 4 weeks before moving to monthly administration. Interestingly, the FDA approval of secukinumab at 150 mg doesn’t call for a loading dose, even though both pivotal trials relied on them, the rheumatologist observed.
At 16 weeks in MEASURE 1, 66% of TNF inhibitor-naive subjects on secukinumab 150 mg had at least a 20% improvement from baseline in ankylosing spondylitis signs and symptoms, or Assessment of Spondyloarthritis International Society (ASAS) 20, compared with 46% of TNF inhibitor inadequate responders. The week 16 ASAS 20 rate in MEASURE 2 was 68% in TNF inhibitor-naive patients and 50% in those with a prior inadequate response to TNF inhibitor therapy.
How should rheumatologists expect secukinumab to perform in daily clinical practice? In the 181 ankylosing spoindylitis patients who completed 52 weeks in the MEASURE 2 extension study, 74% of those on secukinumab at 150 mg had an ASAS 20 response. In both trials, the secukinumab side effect profile was “reasonably clean,” in Dr. Ruderman’s view, with serious adverse events that were similar to placebo.
Serial MRI scans showed rapid resolution of bone marrow edema and inflammation by 16 weeks, an effect sustained through 52 weeks.
The big unanswered question is whether secukinumab prevents radiographic progression of the disease. Serial cervical and spinal X-rays rated using the modified Stoke Ankylosing Spondylitis Spinal Score showed a mean increase of just 0.30 points at 2 years from a baseline of 10.22, with 80% of patients demonstrating no change over time. But there were no untreated controls for comparison in this analysis, so it’s not possible to say whether the drug actually slowed disease progression or that’s the natural history of disease in those subjects, Dr. Ruderman noted.
Effect of NSAID dosing frequency on progression
On the topic of preventing radiographic progression in ankylosing spondylitis, the rheumatologist highlighted a prospective study presented at last year’s EULAR meeting and published online last summer (Ann Rheum Dis. 2015 Aug 4. doi: 10.1136/annrheumdis-2015-207897) that demonstrated that continuous use of diclofenac didn’t do any better at preventing radiographic spinal disease progression than on-demand use of the nonsteroidal anti-inflammatory drug (NSAID) over the course of 2 years.
“There’s been a lot of noise in the ankylosing spondylitis community about the potential benefit of NSAIDs in preventing structural progression. Previous information suggested that staying on them continuously actually reduced radiographic progression. This diclofenac study has shaken things up a little. It raises the question of whether there is any added benefit for NSAIDs in terms of structural progression,” he commented.
Current ACR/SAA/SPARTAN guidelines, which predate the study, feature a conditional recommendation that patients with active ankylosing spondylitis stay on continuous NSAID therapy.
Secukinumab is also approved for treatment of psoriasis and psoriatic arthritis.
Dr. Ruderman reported serving as a consultant to and/or receiving research grants from numerous pharmaceutical companies, including Novartis, which markets secukinumab.
EXPERT ANALYSIS FROM RWCS 2016
Benefits of Medicaid Expansion for Hospitalists
By January 2016, 31 states and the District of Columbia had embraced the Medicaid expansion brought to bear by the Affordable Care Act. Three states had not expanded but were “in active discussion,” while 16 states continued to opt out.1
The impacts of those decisions—on hospitals, on patients, and on physicians—are now beginning to be emerge. Several early studies, published toward the end of 2015 and in early 2016, show how the choice to expand or not expand impacted payor mix, patient access to quality healthcare, and physician reimbursement.
A study published in Health Affairs found states that expanded Medicaid in 2014, including Minnesota, Kentucky, and Arizona, saw a dramatic decrease in uninsured hospital stays and a significant increase in Medicaid stays. In six states that did not expand that year, including Florida, Georgia, and Missouri, there was no significant change in payor mix.2
“What a lot of these early studies are saying is that when you expand Medicaid, people get on Medicaid, and that’s exactly what you hope will happen when you do a major public coverage expansion,” says study lead author Sayeh Nikpay, PhD, MPH, assistant professor of health policy at Vanderbilt University School of Medicine in Nashville, Tenn. “Physicians are grappling with payment issues, and it should be quite a relief that people are coming in the door with some kind of insurance rather than uninsured.”
Instant Impact
Dr. Nikpay and the research team at the University of Michigan Institute for Healthcare Policy & Innovation (where she was previously a postdoctoral researcher) utilized a free online tool, HCUP Fast Stats (Healthcare Cost and Utilization Project), from the Agency for Healthcare Research and Quality. They examined adult discharges by quarter in 2013 and 2014 in each state in the study, controlling for demographic and economic characteristics.
Expansion states, the team learned, experienced a seven percentage point rise in Medicaid shares and a six percentage point drop in uninsured shares, reflecting a respective 20% increase in Medicaid discharges and 50% decrease in uninsured discharges. The effect was particularly profound in Kentucky, which saw a 13.5% drop in uninsured shares.
This underscores the “significant benefits of Medicaid expansion for low-income adults and for the hospitals that serve them,” the study authors concluded.
With positive data from this study and others—and the federal government willing to work with states on alternative expansion models, like in Arkansas, which is using Medicaid dollars to subsidize private insurance for recipients—Colleen M. Grogan, professor in the School of Social Service Administration at The University of Chicago, says the remaining states may feel more pressure to expand.
They are “getting pressure from hospitals and the business sector,” Grogan says. “It has an enormous impact on the economy. I don’t think any state is exempt from economic impact when they give up an infusion of federal funds.”
The federal government currently pays 100% of state Medicaid costs for the newly eligible upon expansion, eventually dropping to 90% by 2020.
A January 2016 Health Affairs study from researchers at Harvard University and Brigham and Women’s Hospital in Boston showed that traditional expansion in Kentucky and the “private option” expansion adopted in Arkansas both led to a decrease in the number of uninsured patients, an increase in access to healthcare, and fewer patients skipping medications or experiencing trouble paying medical bills between 2013 and expansion in 2014. This contrasted with the results in Texas, which has not expanded.3
Hospitalist Concerns
Patrick Cawley, MD, MBA, MHM, is CEO of the Medical University of South Carolina, previously practiced as a hospitalist, and is a past president of the Society of Hospital Medicine. For now, South Carolina is, like Texas, a non-expansion state. Dr. Cawley is concerned for the future of his hospital, an 800-bed academic, tertiary, safety-net hospital in Charleston, because payments to hospitals like his ultimately will drop.
Before a Supreme Court decision that ruled states were not compelled to expand Medicaid, the Affordable Care Act provided for a reduction in payments to safety-net hospitals. This was motivated by the notion that all hospitals would see a significant decrease in uncompensated care. The reduction has been delayed but is still scheduled to start in 2017.
“We couldn’t survive if disproportionate share goes away and something didn’t replace it, like Medicaid expansion,” Dr. Cawley says. But, he adds, over time he expects all or nearly all states will expand.
“When Medicaid first rolled out, it took 10 to 12 years before all states took it. I think expansion is the same way,” he says. “It’s one of those things that probably does work out, but what’s the transition going to be like, and how long is that transition going to last?” TH
Kelly April Tyrrell is a freelance writer in Madison, Wis.
References
- Status of state action on the Medicaid expansion decision. Kaiser Family Foundation website. http://kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/. Updated January 12, 2016. Accessed January 14, 2016.
- Nikpay S, Buchmueller T, Levy HG. Affordable Care Act Medicaid expansion reduced uninsured hospital stays in 2014. Health Aff. 2016;35(1):106-110. doi:10.1377/hlthaff.2015.1144.
- Sommers BD, Blendon RJ, Orav EJ. Both the ‘private option’ and traditional Medicaid expansions improved access to care for low-income adults. Health Aff. 2016;35(1):96-105. doi:10.1377/hlthaff.2015.0917.
- Jones CD, Scott SJ, Anoff DL, Pierce RG, Glasheen JJ. Changes in payer mix and physician reimbursement after the Affordable Care Act and Medicaid expansion. Inquiry. 2015;52. doi:10.1177/0046958015602464.
By January 2016, 31 states and the District of Columbia had embraced the Medicaid expansion brought to bear by the Affordable Care Act. Three states had not expanded but were “in active discussion,” while 16 states continued to opt out.1
The impacts of those decisions—on hospitals, on patients, and on physicians—are now beginning to be emerge. Several early studies, published toward the end of 2015 and in early 2016, show how the choice to expand or not expand impacted payor mix, patient access to quality healthcare, and physician reimbursement.
A study published in Health Affairs found states that expanded Medicaid in 2014, including Minnesota, Kentucky, and Arizona, saw a dramatic decrease in uninsured hospital stays and a significant increase in Medicaid stays. In six states that did not expand that year, including Florida, Georgia, and Missouri, there was no significant change in payor mix.2
“What a lot of these early studies are saying is that when you expand Medicaid, people get on Medicaid, and that’s exactly what you hope will happen when you do a major public coverage expansion,” says study lead author Sayeh Nikpay, PhD, MPH, assistant professor of health policy at Vanderbilt University School of Medicine in Nashville, Tenn. “Physicians are grappling with payment issues, and it should be quite a relief that people are coming in the door with some kind of insurance rather than uninsured.”
Instant Impact
Dr. Nikpay and the research team at the University of Michigan Institute for Healthcare Policy & Innovation (where she was previously a postdoctoral researcher) utilized a free online tool, HCUP Fast Stats (Healthcare Cost and Utilization Project), from the Agency for Healthcare Research and Quality. They examined adult discharges by quarter in 2013 and 2014 in each state in the study, controlling for demographic and economic characteristics.
Expansion states, the team learned, experienced a seven percentage point rise in Medicaid shares and a six percentage point drop in uninsured shares, reflecting a respective 20% increase in Medicaid discharges and 50% decrease in uninsured discharges. The effect was particularly profound in Kentucky, which saw a 13.5% drop in uninsured shares.
This underscores the “significant benefits of Medicaid expansion for low-income adults and for the hospitals that serve them,” the study authors concluded.
With positive data from this study and others—and the federal government willing to work with states on alternative expansion models, like in Arkansas, which is using Medicaid dollars to subsidize private insurance for recipients—Colleen M. Grogan, professor in the School of Social Service Administration at The University of Chicago, says the remaining states may feel more pressure to expand.
They are “getting pressure from hospitals and the business sector,” Grogan says. “It has an enormous impact on the economy. I don’t think any state is exempt from economic impact when they give up an infusion of federal funds.”
The federal government currently pays 100% of state Medicaid costs for the newly eligible upon expansion, eventually dropping to 90% by 2020.
A January 2016 Health Affairs study from researchers at Harvard University and Brigham and Women’s Hospital in Boston showed that traditional expansion in Kentucky and the “private option” expansion adopted in Arkansas both led to a decrease in the number of uninsured patients, an increase in access to healthcare, and fewer patients skipping medications or experiencing trouble paying medical bills between 2013 and expansion in 2014. This contrasted with the results in Texas, which has not expanded.3
Hospitalist Concerns
Patrick Cawley, MD, MBA, MHM, is CEO of the Medical University of South Carolina, previously practiced as a hospitalist, and is a past president of the Society of Hospital Medicine. For now, South Carolina is, like Texas, a non-expansion state. Dr. Cawley is concerned for the future of his hospital, an 800-bed academic, tertiary, safety-net hospital in Charleston, because payments to hospitals like his ultimately will drop.
Before a Supreme Court decision that ruled states were not compelled to expand Medicaid, the Affordable Care Act provided for a reduction in payments to safety-net hospitals. This was motivated by the notion that all hospitals would see a significant decrease in uncompensated care. The reduction has been delayed but is still scheduled to start in 2017.
“We couldn’t survive if disproportionate share goes away and something didn’t replace it, like Medicaid expansion,” Dr. Cawley says. But, he adds, over time he expects all or nearly all states will expand.
“When Medicaid first rolled out, it took 10 to 12 years before all states took it. I think expansion is the same way,” he says. “It’s one of those things that probably does work out, but what’s the transition going to be like, and how long is that transition going to last?” TH
Kelly April Tyrrell is a freelance writer in Madison, Wis.
References
- Status of state action on the Medicaid expansion decision. Kaiser Family Foundation website. http://kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/. Updated January 12, 2016. Accessed January 14, 2016.
- Nikpay S, Buchmueller T, Levy HG. Affordable Care Act Medicaid expansion reduced uninsured hospital stays in 2014. Health Aff. 2016;35(1):106-110. doi:10.1377/hlthaff.2015.1144.
- Sommers BD, Blendon RJ, Orav EJ. Both the ‘private option’ and traditional Medicaid expansions improved access to care for low-income adults. Health Aff. 2016;35(1):96-105. doi:10.1377/hlthaff.2015.0917.
- Jones CD, Scott SJ, Anoff DL, Pierce RG, Glasheen JJ. Changes in payer mix and physician reimbursement after the Affordable Care Act and Medicaid expansion. Inquiry. 2015;52. doi:10.1177/0046958015602464.
By January 2016, 31 states and the District of Columbia had embraced the Medicaid expansion brought to bear by the Affordable Care Act. Three states had not expanded but were “in active discussion,” while 16 states continued to opt out.1
The impacts of those decisions—on hospitals, on patients, and on physicians—are now beginning to be emerge. Several early studies, published toward the end of 2015 and in early 2016, show how the choice to expand or not expand impacted payor mix, patient access to quality healthcare, and physician reimbursement.
A study published in Health Affairs found states that expanded Medicaid in 2014, including Minnesota, Kentucky, and Arizona, saw a dramatic decrease in uninsured hospital stays and a significant increase in Medicaid stays. In six states that did not expand that year, including Florida, Georgia, and Missouri, there was no significant change in payor mix.2
“What a lot of these early studies are saying is that when you expand Medicaid, people get on Medicaid, and that’s exactly what you hope will happen when you do a major public coverage expansion,” says study lead author Sayeh Nikpay, PhD, MPH, assistant professor of health policy at Vanderbilt University School of Medicine in Nashville, Tenn. “Physicians are grappling with payment issues, and it should be quite a relief that people are coming in the door with some kind of insurance rather than uninsured.”
Instant Impact
Dr. Nikpay and the research team at the University of Michigan Institute for Healthcare Policy & Innovation (where she was previously a postdoctoral researcher) utilized a free online tool, HCUP Fast Stats (Healthcare Cost and Utilization Project), from the Agency for Healthcare Research and Quality. They examined adult discharges by quarter in 2013 and 2014 in each state in the study, controlling for demographic and economic characteristics.
Expansion states, the team learned, experienced a seven percentage point rise in Medicaid shares and a six percentage point drop in uninsured shares, reflecting a respective 20% increase in Medicaid discharges and 50% decrease in uninsured discharges. The effect was particularly profound in Kentucky, which saw a 13.5% drop in uninsured shares.
This underscores the “significant benefits of Medicaid expansion for low-income adults and for the hospitals that serve them,” the study authors concluded.
With positive data from this study and others—and the federal government willing to work with states on alternative expansion models, like in Arkansas, which is using Medicaid dollars to subsidize private insurance for recipients—Colleen M. Grogan, professor in the School of Social Service Administration at The University of Chicago, says the remaining states may feel more pressure to expand.
They are “getting pressure from hospitals and the business sector,” Grogan says. “It has an enormous impact on the economy. I don’t think any state is exempt from economic impact when they give up an infusion of federal funds.”
The federal government currently pays 100% of state Medicaid costs for the newly eligible upon expansion, eventually dropping to 90% by 2020.
A January 2016 Health Affairs study from researchers at Harvard University and Brigham and Women’s Hospital in Boston showed that traditional expansion in Kentucky and the “private option” expansion adopted in Arkansas both led to a decrease in the number of uninsured patients, an increase in access to healthcare, and fewer patients skipping medications or experiencing trouble paying medical bills between 2013 and expansion in 2014. This contrasted with the results in Texas, which has not expanded.3
Hospitalist Concerns
Patrick Cawley, MD, MBA, MHM, is CEO of the Medical University of South Carolina, previously practiced as a hospitalist, and is a past president of the Society of Hospital Medicine. For now, South Carolina is, like Texas, a non-expansion state. Dr. Cawley is concerned for the future of his hospital, an 800-bed academic, tertiary, safety-net hospital in Charleston, because payments to hospitals like his ultimately will drop.
Before a Supreme Court decision that ruled states were not compelled to expand Medicaid, the Affordable Care Act provided for a reduction in payments to safety-net hospitals. This was motivated by the notion that all hospitals would see a significant decrease in uncompensated care. The reduction has been delayed but is still scheduled to start in 2017.
“We couldn’t survive if disproportionate share goes away and something didn’t replace it, like Medicaid expansion,” Dr. Cawley says. But, he adds, over time he expects all or nearly all states will expand.
“When Medicaid first rolled out, it took 10 to 12 years before all states took it. I think expansion is the same way,” he says. “It’s one of those things that probably does work out, but what’s the transition going to be like, and how long is that transition going to last?” TH
Kelly April Tyrrell is a freelance writer in Madison, Wis.
References
- Status of state action on the Medicaid expansion decision. Kaiser Family Foundation website. http://kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/. Updated January 12, 2016. Accessed January 14, 2016.
- Nikpay S, Buchmueller T, Levy HG. Affordable Care Act Medicaid expansion reduced uninsured hospital stays in 2014. Health Aff. 2016;35(1):106-110. doi:10.1377/hlthaff.2015.1144.
- Sommers BD, Blendon RJ, Orav EJ. Both the ‘private option’ and traditional Medicaid expansions improved access to care for low-income adults. Health Aff. 2016;35(1):96-105. doi:10.1377/hlthaff.2015.0917.
- Jones CD, Scott SJ, Anoff DL, Pierce RG, Glasheen JJ. Changes in payer mix and physician reimbursement after the Affordable Care Act and Medicaid expansion. Inquiry. 2015;52. doi:10.1177/0046958015602464.
Class of drugs could treat B-cell malignancies
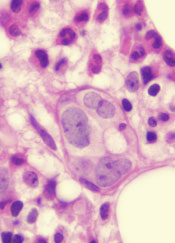
A class of drugs targeting a protein found in the endoplasmic reticulum could be effective against B-cell malignancies, according to a study published in Cancer Research.
The protein, STING, plays a critical role in producing type I interferons that help regulate the immune system.
Previous research suggested that STING agonists can improve immune responses when used in cancer immunotherapy or as vaccine adjuvants.
However, the way B cells respond to STING agonists was not well understood.
Chih-Chi Andrew Hu, PhD, of The Wistar Institute in Philadelphia, Pennsylvania, and his colleagues conducted a study to gain some insight.
The researchers found that normal B cells respond to STING agonists by undergoing mitochondria-mediated apoptosis, and STING agonists induce apoptosis in
malignant B cells through binding to STING.
STING agonists proved cytotoxic to B-cell leukemia, lymphoma, and multiple myeloma in vitro. But the drugs did not induce apoptosis in solid tumor malignancies or normal T cells.
The research also revealed that the IRE-1/XBP-1 stress response pathway is required for normal STING function. And B-cell leukemia, lymphoma, and myeloma require the IRE-1/XBP-1 pathway to be activated for survival.
Stimulation by STING agonists suppressed the IRE-1/XBP-1 pathway, which increased the level of apoptosis in malignant B cells.
The researchers confirmed these results in animal models, as treatment with STING agonists led to regression of chronic lymphocytic leukemia and multiple myeloma in mice.
“This specific cytotoxicity toward B cells strongly supports the use of STING agonists in the treatment of B-cell hematologic malignancies,” said Chih-Hang Anthony Tang, MD, PhD, of The Wistar Institute.
“We also believe that cytotoxicity in normal B cells can be managed with the administration of intravenous immunoglobulin that can help maintain normal levels of antibodies while treatment is being administered. This is something we plan on studying further.”
The Wistar Institute’s business development team is looking for a development partner for the advancement of novel STING agonists in treating B-cell hematologic malignancies. ![]()

A class of drugs targeting a protein found in the endoplasmic reticulum could be effective against B-cell malignancies, according to a study published in Cancer Research.
The protein, STING, plays a critical role in producing type I interferons that help regulate the immune system.
Previous research suggested that STING agonists can improve immune responses when used in cancer immunotherapy or as vaccine adjuvants.
However, the way B cells respond to STING agonists was not well understood.
Chih-Chi Andrew Hu, PhD, of The Wistar Institute in Philadelphia, Pennsylvania, and his colleagues conducted a study to gain some insight.
The researchers found that normal B cells respond to STING agonists by undergoing mitochondria-mediated apoptosis, and STING agonists induce apoptosis in
malignant B cells through binding to STING.
STING agonists proved cytotoxic to B-cell leukemia, lymphoma, and multiple myeloma in vitro. But the drugs did not induce apoptosis in solid tumor malignancies or normal T cells.
The research also revealed that the IRE-1/XBP-1 stress response pathway is required for normal STING function. And B-cell leukemia, lymphoma, and myeloma require the IRE-1/XBP-1 pathway to be activated for survival.
Stimulation by STING agonists suppressed the IRE-1/XBP-1 pathway, which increased the level of apoptosis in malignant B cells.
The researchers confirmed these results in animal models, as treatment with STING agonists led to regression of chronic lymphocytic leukemia and multiple myeloma in mice.
“This specific cytotoxicity toward B cells strongly supports the use of STING agonists in the treatment of B-cell hematologic malignancies,” said Chih-Hang Anthony Tang, MD, PhD, of The Wistar Institute.
“We also believe that cytotoxicity in normal B cells can be managed with the administration of intravenous immunoglobulin that can help maintain normal levels of antibodies while treatment is being administered. This is something we plan on studying further.”
The Wistar Institute’s business development team is looking for a development partner for the advancement of novel STING agonists in treating B-cell hematologic malignancies. ![]()

A class of drugs targeting a protein found in the endoplasmic reticulum could be effective against B-cell malignancies, according to a study published in Cancer Research.
The protein, STING, plays a critical role in producing type I interferons that help regulate the immune system.
Previous research suggested that STING agonists can improve immune responses when used in cancer immunotherapy or as vaccine adjuvants.
However, the way B cells respond to STING agonists was not well understood.
Chih-Chi Andrew Hu, PhD, of The Wistar Institute in Philadelphia, Pennsylvania, and his colleagues conducted a study to gain some insight.
The researchers found that normal B cells respond to STING agonists by undergoing mitochondria-mediated apoptosis, and STING agonists induce apoptosis in
malignant B cells through binding to STING.
STING agonists proved cytotoxic to B-cell leukemia, lymphoma, and multiple myeloma in vitro. But the drugs did not induce apoptosis in solid tumor malignancies or normal T cells.
The research also revealed that the IRE-1/XBP-1 stress response pathway is required for normal STING function. And B-cell leukemia, lymphoma, and myeloma require the IRE-1/XBP-1 pathway to be activated for survival.
Stimulation by STING agonists suppressed the IRE-1/XBP-1 pathway, which increased the level of apoptosis in malignant B cells.
The researchers confirmed these results in animal models, as treatment with STING agonists led to regression of chronic lymphocytic leukemia and multiple myeloma in mice.
“This specific cytotoxicity toward B cells strongly supports the use of STING agonists in the treatment of B-cell hematologic malignancies,” said Chih-Hang Anthony Tang, MD, PhD, of The Wistar Institute.
“We also believe that cytotoxicity in normal B cells can be managed with the administration of intravenous immunoglobulin that can help maintain normal levels of antibodies while treatment is being administered. This is something we plan on studying further.”
The Wistar Institute’s business development team is looking for a development partner for the advancement of novel STING agonists in treating B-cell hematologic malignancies. ![]()
Flu activity reaches another new season high
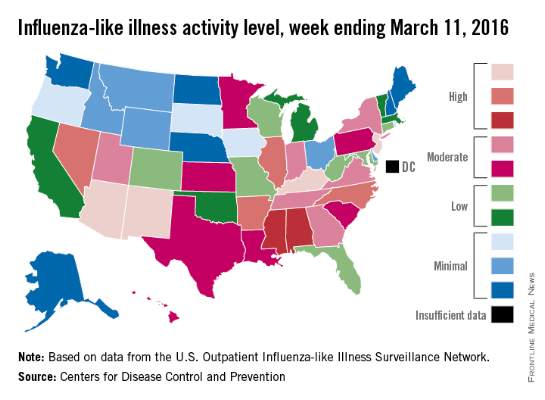
There were four states at the highest level of influenza-like illness (ILI) activity for the week, more than any other week of the 2015-2016 flu season, according to the Centers for Disease Control and Prevention.
Arizona, Kentucky, New Jersey, and New Mexico, along with Puerto Rico, were all at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending March 5, 2016. Other states in the “high” range were Arkansas, Illinois, Nevada, and North Carolina at level 9 and Alabama and Mississippi at level 8, the CDC reported.
The proportion of outpatient visits for ILI was 3.5% for the week, the highest of the season so far and well above the national baseline of 2.1%. In addition to the 10 states and Puerto Rico with ILI levels in the high range, there were 13 states in the “moderate” range (6 or 7 on the 1-10 scale) and 12 states in the “low” range (4 or 5), with a total of 44 states at level 2 or higher, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network (ILINet) show.
Two flu-related pediatric deaths were reported during week 21 of the flu season, although both occurred earlier: one during the week ending Feb. 13 and one during the week ending Feb. 27. There have been a total of 20 pediatric deaths reported for the 2015-2016 season, with Florida (four deaths), California, (three), Arizona (two), and Nevada (two) the only states reporting more than one, the CDC report noted.
The continued rise in ILI activity is somewhat unusual. The only season out of the previous 10 with a peak later than the current one was 2011-2012, which peaked at only 2.4% on the week ending March 17, 2012. The earliest of the bunch came during the 2009-2010 season, which peaked at 7.7% during the week ending Oct. 24, 2009, according to ILINet data.
There were four states at the highest level of influenza-like illness (ILI) activity for the week, more than any other week of the 2015-2016 flu season, according to the Centers for Disease Control and Prevention.
Arizona, Kentucky, New Jersey, and New Mexico, along with Puerto Rico, were all at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending March 5, 2016. Other states in the “high” range were Arkansas, Illinois, Nevada, and North Carolina at level 9 and Alabama and Mississippi at level 8, the CDC reported.
The proportion of outpatient visits for ILI was 3.5% for the week, the highest of the season so far and well above the national baseline of 2.1%. In addition to the 10 states and Puerto Rico with ILI levels in the high range, there were 13 states in the “moderate” range (6 or 7 on the 1-10 scale) and 12 states in the “low” range (4 or 5), with a total of 44 states at level 2 or higher, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network (ILINet) show.
Two flu-related pediatric deaths were reported during week 21 of the flu season, although both occurred earlier: one during the week ending Feb. 13 and one during the week ending Feb. 27. There have been a total of 20 pediatric deaths reported for the 2015-2016 season, with Florida (four deaths), California, (three), Arizona (two), and Nevada (two) the only states reporting more than one, the CDC report noted.
The continued rise in ILI activity is somewhat unusual. The only season out of the previous 10 with a peak later than the current one was 2011-2012, which peaked at only 2.4% on the week ending March 17, 2012. The earliest of the bunch came during the 2009-2010 season, which peaked at 7.7% during the week ending Oct. 24, 2009, according to ILINet data.
There were four states at the highest level of influenza-like illness (ILI) activity for the week, more than any other week of the 2015-2016 flu season, according to the Centers for Disease Control and Prevention.
Arizona, Kentucky, New Jersey, and New Mexico, along with Puerto Rico, were all at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending March 5, 2016. Other states in the “high” range were Arkansas, Illinois, Nevada, and North Carolina at level 9 and Alabama and Mississippi at level 8, the CDC reported.
The proportion of outpatient visits for ILI was 3.5% for the week, the highest of the season so far and well above the national baseline of 2.1%. In addition to the 10 states and Puerto Rico with ILI levels in the high range, there were 13 states in the “moderate” range (6 or 7 on the 1-10 scale) and 12 states in the “low” range (4 or 5), with a total of 44 states at level 2 or higher, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network (ILINet) show.
Two flu-related pediatric deaths were reported during week 21 of the flu season, although both occurred earlier: one during the week ending Feb. 13 and one during the week ending Feb. 27. There have been a total of 20 pediatric deaths reported for the 2015-2016 season, with Florida (four deaths), California, (three), Arizona (two), and Nevada (two) the only states reporting more than one, the CDC report noted.
The continued rise in ILI activity is somewhat unusual. The only season out of the previous 10 with a peak later than the current one was 2011-2012, which peaked at only 2.4% on the week ending March 17, 2012. The earliest of the bunch came during the 2009-2010 season, which peaked at 7.7% during the week ending Oct. 24, 2009, according to ILINet data.
Late-week discharges to home after CRC surgery prone to readmission
BOSTON – The day of the week a patient is discharged from the hospital may have an impact the likelihood of readmission.
Patients discharged home from the hospital on a Thursday after colorectal cancer surgery are more likely to be readmitted within 30 days than those discharged on any other day of the week, investigators found.
In contrast, there were no significant day-dependent differences in readmission rates among patients discharged to a skilled nursing facility or acute rehabilitation program, although patients admitted to clinical facilities had higher overall readmission rates, reported Anna Gustin and coinvestigators at the Levine Cancer Institute at the Carolinas Medical Center in Charlotte, N.C.
“For a patient discharged on a Thursday, if you’re going to get an infection, it’s going to be probably during the weekend, when it’s difficult to contact your primary physician, and when other resources are not as readily available,” said Ms. Gustin, who conducts epidemiologic research at Levine Cancer Center and is also a pre-med student and Japanese major at Wake Forest University in Winston-Salem, N.C.
In a study presented in a poster session at the annual Society of Surgical Oncology Cancer Symposium, Ms. Gustin and her coauthors looked at factors influencing readmission rates among patients undergoing surgery for primary, nonmetastatic colorectal cancer resections.
They drew on the to evaluate outcomes for 93,04 SEER-(Surveillance, Epidemiology, and End Results) Medicare database seven patients aged 66 years and older treated for primary colorectal cancer from 1998 through 2009.
They looked at potential contributing factors such as patient demographics, socioeconomic status, length of stay, days of admission and discharge, and discharge setting (home or clinical facility).
They use multivariate logistic regression models to analyze readmission rates at 14 and 30 days after initial discharge.
Focusing on home discharges, they found that as the week progressed, there was a significant likelihood that a patient discharged home would be readmitted (P less then .001 by chi-square and Cochran-Armitage tests). As noted before, the highest rate of readmission was for patients discharged on Thursday, at 12.4%, compared with 10.1% for patients discharged on Sunday, the discharge day least likely to be associated with rehospitalization.
In multivariate analysis, factors significantly associated with risk for 30-day readmission included male vs. female (hazard ratio, 1.16), black vs. other race (HR, 1.22), length of stay 5, 6-7, or 8-10 vs. 12 or more days (HR, 0.48, 0.59, 0.77, respectively), Charlson comorbidity index score 0, 1 or 3 vs. 3 (HR, 0.59, 0.73, 0.82, respectively), and home discharge vs. other (HR, 0.66; all above comparisons significant as shown by 95% confidence intervals).
The authors concluded that although home discharge itself reduces the likelihood of readmission, “improvements in preparing patients for discharge to home are needed. Additional outpatient interventions could rescue patients from readmission.”
They also suggested reexamining staffing policies and weekend availability of resources for patients, and call for addressing disparities in readmissions based on race, sex, length of stay, and comorbidities.
The study was internally supported. The authors reported having no relevant disclosures.
BOSTON – The day of the week a patient is discharged from the hospital may have an impact the likelihood of readmission.
Patients discharged home from the hospital on a Thursday after colorectal cancer surgery are more likely to be readmitted within 30 days than those discharged on any other day of the week, investigators found.
In contrast, there were no significant day-dependent differences in readmission rates among patients discharged to a skilled nursing facility or acute rehabilitation program, although patients admitted to clinical facilities had higher overall readmission rates, reported Anna Gustin and coinvestigators at the Levine Cancer Institute at the Carolinas Medical Center in Charlotte, N.C.
“For a patient discharged on a Thursday, if you’re going to get an infection, it’s going to be probably during the weekend, when it’s difficult to contact your primary physician, and when other resources are not as readily available,” said Ms. Gustin, who conducts epidemiologic research at Levine Cancer Center and is also a pre-med student and Japanese major at Wake Forest University in Winston-Salem, N.C.
In a study presented in a poster session at the annual Society of Surgical Oncology Cancer Symposium, Ms. Gustin and her coauthors looked at factors influencing readmission rates among patients undergoing surgery for primary, nonmetastatic colorectal cancer resections.
They drew on the to evaluate outcomes for 93,04 SEER-(Surveillance, Epidemiology, and End Results) Medicare database seven patients aged 66 years and older treated for primary colorectal cancer from 1998 through 2009.
They looked at potential contributing factors such as patient demographics, socioeconomic status, length of stay, days of admission and discharge, and discharge setting (home or clinical facility).
They use multivariate logistic regression models to analyze readmission rates at 14 and 30 days after initial discharge.
Focusing on home discharges, they found that as the week progressed, there was a significant likelihood that a patient discharged home would be readmitted (P less then .001 by chi-square and Cochran-Armitage tests). As noted before, the highest rate of readmission was for patients discharged on Thursday, at 12.4%, compared with 10.1% for patients discharged on Sunday, the discharge day least likely to be associated with rehospitalization.
In multivariate analysis, factors significantly associated with risk for 30-day readmission included male vs. female (hazard ratio, 1.16), black vs. other race (HR, 1.22), length of stay 5, 6-7, or 8-10 vs. 12 or more days (HR, 0.48, 0.59, 0.77, respectively), Charlson comorbidity index score 0, 1 or 3 vs. 3 (HR, 0.59, 0.73, 0.82, respectively), and home discharge vs. other (HR, 0.66; all above comparisons significant as shown by 95% confidence intervals).
The authors concluded that although home discharge itself reduces the likelihood of readmission, “improvements in preparing patients for discharge to home are needed. Additional outpatient interventions could rescue patients from readmission.”
They also suggested reexamining staffing policies and weekend availability of resources for patients, and call for addressing disparities in readmissions based on race, sex, length of stay, and comorbidities.
The study was internally supported. The authors reported having no relevant disclosures.
BOSTON – The day of the week a patient is discharged from the hospital may have an impact the likelihood of readmission.
Patients discharged home from the hospital on a Thursday after colorectal cancer surgery are more likely to be readmitted within 30 days than those discharged on any other day of the week, investigators found.
In contrast, there were no significant day-dependent differences in readmission rates among patients discharged to a skilled nursing facility or acute rehabilitation program, although patients admitted to clinical facilities had higher overall readmission rates, reported Anna Gustin and coinvestigators at the Levine Cancer Institute at the Carolinas Medical Center in Charlotte, N.C.
“For a patient discharged on a Thursday, if you’re going to get an infection, it’s going to be probably during the weekend, when it’s difficult to contact your primary physician, and when other resources are not as readily available,” said Ms. Gustin, who conducts epidemiologic research at Levine Cancer Center and is also a pre-med student and Japanese major at Wake Forest University in Winston-Salem, N.C.
In a study presented in a poster session at the annual Society of Surgical Oncology Cancer Symposium, Ms. Gustin and her coauthors looked at factors influencing readmission rates among patients undergoing surgery for primary, nonmetastatic colorectal cancer resections.
They drew on the to evaluate outcomes for 93,04 SEER-(Surveillance, Epidemiology, and End Results) Medicare database seven patients aged 66 years and older treated for primary colorectal cancer from 1998 through 2009.
They looked at potential contributing factors such as patient demographics, socioeconomic status, length of stay, days of admission and discharge, and discharge setting (home or clinical facility).
They use multivariate logistic regression models to analyze readmission rates at 14 and 30 days after initial discharge.
Focusing on home discharges, they found that as the week progressed, there was a significant likelihood that a patient discharged home would be readmitted (P less then .001 by chi-square and Cochran-Armitage tests). As noted before, the highest rate of readmission was for patients discharged on Thursday, at 12.4%, compared with 10.1% for patients discharged on Sunday, the discharge day least likely to be associated with rehospitalization.
In multivariate analysis, factors significantly associated with risk for 30-day readmission included male vs. female (hazard ratio, 1.16), black vs. other race (HR, 1.22), length of stay 5, 6-7, or 8-10 vs. 12 or more days (HR, 0.48, 0.59, 0.77, respectively), Charlson comorbidity index score 0, 1 or 3 vs. 3 (HR, 0.59, 0.73, 0.82, respectively), and home discharge vs. other (HR, 0.66; all above comparisons significant as shown by 95% confidence intervals).
The authors concluded that although home discharge itself reduces the likelihood of readmission, “improvements in preparing patients for discharge to home are needed. Additional outpatient interventions could rescue patients from readmission.”
They also suggested reexamining staffing policies and weekend availability of resources for patients, and call for addressing disparities in readmissions based on race, sex, length of stay, and comorbidities.
The study was internally supported. The authors reported having no relevant disclosures.
Key clinical point: Patients discharged home on a Thursday following surgery for primary colorectal cancer are more likely to be readmitted with 30 days than are patients discharged home on any other day of the week.
Major finding: The highest rate of readmission was for patients discharged on Thursday, at 12.4%, compared with lowest rate of 10.1% for patients discharged on Sunday.
Data source: Retrospective SEER-Medicare database review of records on 93,047 patients treated for colorectal cancer.
Disclosures: The study was internally supported. The authors reported having no relevant disclosures.
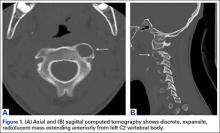
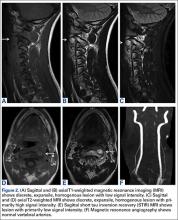
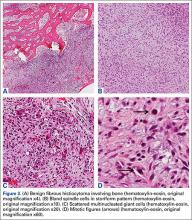
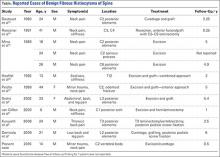
14-Year-Old Boy With Mild Antecedent Neck Pain in Setting of Acute Trauma: A Rare Case of Benign Fibrous Histiocytoma of the Spine
Benign fibrous histiocytoma (BFH) is a rare, well-recognized, primary skeletal tumor accounting for approximately 1% of all benign bone tumors. Spinal involvement is exceedingly rare with only 11 cases reported in the literature.1,2 We present a case of BFH located in the cervical spine of a pediatric patient that was successfully treated with curretage through an anterior surgical approach, along with a review of the literature and appropriate management concerning BFH of the spine.
Case Report
A 14-year-old boy was tackled while playing football and noticed immediate neck pain and subjective paresthesia in the upper extremities. Examination revealed a nontender spine (cervical, thoracic, lumbar) and normal strength and range of motion in all extremities. Sensation was diffusely intact, long tract signs were absent, and gait was normal. On questioning, the patient endorsed mild antecedent neck pain but denied prior history of any trauma. Neck pain did not radiate and was slightly worsened by activity but was mostly intermittent and random. As the neck pain was very mild and was not interfering with daily activities, the patient had not sought care before presenting to the emergency department. He had no pertinent past medical or surgical history.
The patient presented with a computed tomography (CT) scan of his head and cervical spine and a magnetic resonance imaging (MRI) scan of the cervical spine. A magnetic resonance angiography (MRA) scan of the neck was ordered after his arrival.
Axial and sagittal CT (Figures 1A, 1B) showed a 1×1.2-cm discrete, expansile, lytic, radiolucent mass extending anterior from the left C2 vertebral body. The mass appeared to abut the left vertebral artery foramen. The cortical bone surrounding the lesion was thin but uniform. Sagittal and axial T1-weighted MRI (Figures 2A, 2B) showed the discrete, expansile, homogenous lesion with the same intensity as normal bone marrow. Sagittal and axial T2-weighted MRI (Figures 2C, 2D) showed a discrete, expansile, homogenous lesion with primarily high signal intensity. Sagittal short tau inversion recovery (STIR) MRI (Figure 2E) again showed the lesion with primarily low intensity. Given the close proximity of the lesion to the vertebral foramen, MRA was ordered; it showed the lesion was not interfering with the vertebral artery (Figure 2F).
The tumor’s location, in the left anterior aspect of the C2 vertebral body, was not conducive to percutaneous biopsy for establishing tissue diagnosis, so the decision was made to surgically excise the lesion. A left-sided anterior incision was made 2 fingerbreadths inferior to the jaw line in a neck crease. A head and neck surgeon assisted with dissection. Dissection was carried down through the skin, subcutaneous tissue, and platysma on to the anterior part of the spine medial to the carotid sheath. Superior thyroid nerve and vessels and superior laryngeal nerve were identified and preserved. Fluoroscopy confirmed correct location at C2. The tumor was easily visualized, and the outer shell broke easily with palpation. Gentle curettage was necessary when removing the tumor off the vertebral artery. A portion of the specimen was sent during surgery for frozen section, which showed infrequent mitotic figures and no other findings concerning for malignancy. No instability was created after curettage and excision of the tumor, so no grafting or instrumentation was necessary.
Grossly, the tumor was pale tan and firm. Histologic examination with hematoxylin-eosin staining revealed a bland spindle-cell neoplasm that focally involved bone. A storiform pattern was present. The cells had scant cytoplasm and oval to elongate nuclei with tapered ends. Significant nuclear pleomorphism was not seen. The stroma was loose, with focal myxoid change. Benign multinucleated giant cells were present. Mitotic activity was infrequent (Figures 3A–3D). Two attending pathologists reviewed the case material and the frozen and formalin-fixed specimens independently and concurred with the diagnosis of BFH. In addition, the case was reviewed at the surgical pathology consensus conference; the reviewers agreed on BFH, and additional studies were deemed unnecessary.
Given the patient’s complete clinical picture, the differential diagnosis included nonossifying fibroma (NOF), eosinophilic granuloma (EG), BFH, fibrous dysplasia, giant cell tumor (GCT), aneurysmal bone cyst (ABC), and osteoblastoma (OB).
Discussion
BFH is an extremely rare bone lesion, accounting for only 1% of all surgically managed bone tumors; not counting the present case, only 11 spine cases have been reported in the literature.1,2 BFH of the spine traditionally causes nonspecific, poorly localized pain. The Table lists the reported cases of spinal BFH and their presenting symptoms, location, and treatment. BFH usually occurs in young adults, but the age range is 5 to 75 years.2-4 Mean age of the 12 patients with spinal BFH in the literature (including ours) is 25 years.1 In addition, spinal BFH appears to have no predilection for sex.
Skeletal BFH presents as a discrete, well-defined, osteolytic lesion with sharp borders and potentially a sclerotic rim.4-6 Cortical expansion and even cortical disruption with invasion into adjacent tissue have occurred in flat bones.7 Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in storiform pattern.6
BFH shares many of its radiologic and histologic characteristics and clinical symptoms with other benign bone lesions (the tumors listed above). Therefore, accurate diagnosis of BFH requires appropriate correlation of clinical, radiographic, and histologic data.2,3,8 Below is a comparison of BFH with related bone lesions.
Spinal BFH causes a nonspecific, poorly localized pain similar to that of EG, ABC, GCT, and OB.3,9 NOF and fibrous dysplasia generally do not cause pain, unless these lesions are discovered secondary to a pathologic fracture.8,10,11 Our patient had minor antecedent neck pain, which was brought to light by his football accident. ABC and OB are more locally aggressive than BFH and can cause neurologic symptoms by mass effect and spinal cord or nerve root compression.1,8 In this case and in the 6 other cases of BFH of the cervical spine, there were no neurologic changes.4,10
Of the tumors mentioned, NOF and EG almost always occur in children. However, NOF usually occurs in the metaphyseal region of long bones, and EG is usually accompanied by systemic symptoms, such as lymphadenopathy, hepatomegaly, and increased inflammatory markers.1,8 Fibrous dysplasia usually presents in childhood but does not become symptomatic until adulthood. GCTs and OB predominantly occur in adulthood.12,13 Our patient’s age and lack of other systemic symptoms supported the diagnosis of BFH.
Appearance on MRI is reported less with BFH than with other tumors, but heterogenous signal intensity similar to that of skeletal muscle on T1-weighted images and high signal intensity on T2-weighted images is typically reported.8,14 NOF and fibrous dysplasia do not disrupt the bony cortex unless a pathologic fracture has occurred.4 GCTs are more aggressive lytic lesions with more aggressive radiologic features. GCTs generally cause cortical expansion/attenuation, and lack a sclerotic rim. GCTs also have a heterogenous appearance on MRI and give a low to intermediate signal on both T1- and T2-weighted images.12,15 The appearance of EG is similar to that of BFH as an osteolytic lesion with a sclerotic rim, though EGs typically break through the cortex and acquire a “punched-out” look.1,8 ABC typically is described as an expansile osteolytic lesion with a “soap-bubble” appearance on radiographs; periosteal elevation and cortical attenuation can also be visualized. MRI shows the typical multilobular appearance of the lesion with fluid levels.13
OB appears as a radiolucent lesion, with or without calcifications, surrounded by a thin margin of reactive bone.14,16 A distinguishing characteristic of OB was thought to be intense radioisotope uptake on bone scintigraphy, but recently a bony BFH demonstrated intense uptake.17 OBs typically demonstrate nonspecific MRI results similar to those of BFH: low to intermediate signal on T1-weighted images and intermediate to high signal on T2-weighted images.13 In our patient’s case, the radiographic appearance and lack of specific radiographic findings consistent with the other tumors supported the diagnosis of BFH.
Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in a storiform pattern6 which was demonstrated in our patient’s case. In addition, significant nuclear pleomorphism, mitotic activity, and necrosis were absent—a difference between BFH and malignant fibrous histiocytoma.4,15 The microscopic characteristics of BFH readily differentiate it from OB, ABC, EG, and GCT, but not from NOF on microscopic appearance alone. Clinical and radiographic findings must be consistent, as mentioned.7,18
Complete surgical excision is the reported treatment for BFH. Prognosis after resection or curettage is usually good, and recurrences have been rare.1,2 Depending on the intraspinous location of BFH, stabilization after resection or curettage may be necessary to prevent residual instability. Three of the 11 reported cases of spinal BFH required stabilization by anterior fusion or posterior pedicle screw fixation after resection.1,2 The other 8 cases underwent excision alone or excision and grafting. All 11 patients were disease-free at a mean follow-up of 3.5 years.1 In nonspinal BFH, however, both local recurrence and lung metastasis have been reported.2,5,9,19 Clarke and colleagues9 reported local recurrences in 3 of 8 cases. These recurrences involved BFH in long bones of the leg, which had been treated with curettage and grafting. There has been no reliable report of a malignant change in BFH.2,9 The only case of lung metastasis, reported by Unni and Dahlin6 in their study of 10 cases, occurred 2 years after local recurrence in the distal femur.Our patient was doing well at most recent follow-up, 6 months after surgery. He had no pain and had returned to normal activities. Although there are no reported cases of spinal BFH recurrence, we will follow this patient with imaging on an annual basis. His case is of particular interest to orthopedic surgeons because they encounter benign bone lesions every day, and many of these lesions are in difficult anatomical locations. Knowing the characteristics, differential diagnoses, and appropriate diagnostic workups for benign bone lesions is important for optimal and timely patient care.
1. Demiralp B, Kose O, Oguz E, Sanal T, Ozcan A, Sehirlioglu A. Benign fibrous histiocytoma of the lumbar vertebrae. Skeletal Radiol. 2009;38(2):187-191.
2. Kuruvath S, O’Donovan DG, Aspoas AR, David KM. Benign fibrous histiocytoma of the thoracic spine: case report and review of the literature. J Neurosurg Spine. 2006;4(3):260-264.
3. Ceroni D, Dayer R, De Coulon G, Kaelin A. Benign fibrous histiocytoma of bone in a paediatric population: a report of 6 cases. Musculoskelet Surg. 2011;95(2):107-114.
4. Dorfman HD, Czerniak B. Bone Tumors. St. Louis, MO: Mosby; 1998.
5. Grohs JG, Nicolakis M, Kainberger F, Lang S, Kotz R. Benign fibrous histiocytoma of bone: a report of ten cases and review of literature. Wien Klin Wochenschr. 2002;114(1-2):56-63.
6. Unni KK, Dahlin DC. Dahlin’s Bone Tumors. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
7. Balasubramanian C, Rajaraman G, Singh CS, Baliga DK. Benign fibrous histiocytoma of the sacrum—diagnostic difficulties facing this rare bone tumor. Pediatr Neurosurg. 2005;41(5):253-257.
8. van Giffen NH, van Rhijn LW, van Ooij A, et al. Benign fibrous histiocytoma of the posterior arch of C1 in a 6-year old boy: a case report. Spine. 2003;28(18):E359-E363.
9. Clarke BE, Xipell JM, Thomas DP. Benign fibrous histiocytoma of bone. Am J Surg Pathol. 1985;9(11):806-815.
10. Peicha G, Siebert FJ, Bratschitsch G, Fankhauser F, Grechenig W. Pathologic odontoid fracture and benign fibrous histiocytoma of bone. Eur Spine J. 1999;8(2):161-163.
11. Unni KK, Inwards CY, Bridge JA, Kindblom LG, Wold LE. Tumors of the Bones and Joints (AFIP Atlas of Tumor Pathology Series IV). Annapolis Junction, MD: American Registry of Pathology Press; 2005.
12. Dee R. Principles of Orthopaedic Practice. 2nd ed. New York, NY: McGraw-Hill; 1997.
13. Murphey M, Andrews C, Flemming D, Temple HT, Smith WS, Smirniotopoulos JG. Primary tumors of the spine: radiologic–pathologic correlation. Radiographics. 1996;16(5):1131-1158.
14. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
15. Mirra JM, Picci P, Gold RH. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Vol 1. Philadelphia, PA: Lea & Febiger; 1989.
16. Theodorou DJ, Theodorou SJ, Sartoris DJ. An imaging overview of primary tumors of the spine: part 1. Benign tumors. Clin Imaging. 2008;32(3):196-203.
17. Li X, Meng Z, Li D, Tan J, Song X. Benign fibrous histiocytoma of a rib. Clin Nucl Med. 2014;39(9): 837-841.
18. Roessner A, Immenkamp M, Weidner A, Hobik HP, Grundmann E. Benign fibrous histiocytoma of bone. Light- and electron-microscopic observations. J Cancer Res Clin Oncol. 1981;101(2):191-202.
19. Destouet JM, Kyriakos M, Gilula LA. Fibrous histiocytoma (fibroxanthoma) of a cervical vertebra. A report with a review of the literature. Skeletal Radiol. 1980;5(4):241-246.
20. Hoeffel JC, Bomand-Ferrand F, Tachet F, Lascombes P, Czorny A, Bernard C. So-called benign fibrous histiocytoma: report of a case. J Pediatr Surg. 1992;27(5):672-674.
Benign fibrous histiocytoma (BFH) is a rare, well-recognized, primary skeletal tumor accounting for approximately 1% of all benign bone tumors. Spinal involvement is exceedingly rare with only 11 cases reported in the literature.1,2 We present a case of BFH located in the cervical spine of a pediatric patient that was successfully treated with curretage through an anterior surgical approach, along with a review of the literature and appropriate management concerning BFH of the spine.
Case Report
A 14-year-old boy was tackled while playing football and noticed immediate neck pain and subjective paresthesia in the upper extremities. Examination revealed a nontender spine (cervical, thoracic, lumbar) and normal strength and range of motion in all extremities. Sensation was diffusely intact, long tract signs were absent, and gait was normal. On questioning, the patient endorsed mild antecedent neck pain but denied prior history of any trauma. Neck pain did not radiate and was slightly worsened by activity but was mostly intermittent and random. As the neck pain was very mild and was not interfering with daily activities, the patient had not sought care before presenting to the emergency department. He had no pertinent past medical or surgical history.
The patient presented with a computed tomography (CT) scan of his head and cervical spine and a magnetic resonance imaging (MRI) scan of the cervical spine. A magnetic resonance angiography (MRA) scan of the neck was ordered after his arrival.
Axial and sagittal CT (Figures 1A, 1B) showed a 1×1.2-cm discrete, expansile, lytic, radiolucent mass extending anterior from the left C2 vertebral body. The mass appeared to abut the left vertebral artery foramen. The cortical bone surrounding the lesion was thin but uniform. Sagittal and axial T1-weighted MRI (Figures 2A, 2B) showed the discrete, expansile, homogenous lesion with the same intensity as normal bone marrow. Sagittal and axial T2-weighted MRI (Figures 2C, 2D) showed a discrete, expansile, homogenous lesion with primarily high signal intensity. Sagittal short tau inversion recovery (STIR) MRI (Figure 2E) again showed the lesion with primarily low intensity. Given the close proximity of the lesion to the vertebral foramen, MRA was ordered; it showed the lesion was not interfering with the vertebral artery (Figure 2F).
The tumor’s location, in the left anterior aspect of the C2 vertebral body, was not conducive to percutaneous biopsy for establishing tissue diagnosis, so the decision was made to surgically excise the lesion. A left-sided anterior incision was made 2 fingerbreadths inferior to the jaw line in a neck crease. A head and neck surgeon assisted with dissection. Dissection was carried down through the skin, subcutaneous tissue, and platysma on to the anterior part of the spine medial to the carotid sheath. Superior thyroid nerve and vessels and superior laryngeal nerve were identified and preserved. Fluoroscopy confirmed correct location at C2. The tumor was easily visualized, and the outer shell broke easily with palpation. Gentle curettage was necessary when removing the tumor off the vertebral artery. A portion of the specimen was sent during surgery for frozen section, which showed infrequent mitotic figures and no other findings concerning for malignancy. No instability was created after curettage and excision of the tumor, so no grafting or instrumentation was necessary.
Grossly, the tumor was pale tan and firm. Histologic examination with hematoxylin-eosin staining revealed a bland spindle-cell neoplasm that focally involved bone. A storiform pattern was present. The cells had scant cytoplasm and oval to elongate nuclei with tapered ends. Significant nuclear pleomorphism was not seen. The stroma was loose, with focal myxoid change. Benign multinucleated giant cells were present. Mitotic activity was infrequent (Figures 3A–3D). Two attending pathologists reviewed the case material and the frozen and formalin-fixed specimens independently and concurred with the diagnosis of BFH. In addition, the case was reviewed at the surgical pathology consensus conference; the reviewers agreed on BFH, and additional studies were deemed unnecessary.
Given the patient’s complete clinical picture, the differential diagnosis included nonossifying fibroma (NOF), eosinophilic granuloma (EG), BFH, fibrous dysplasia, giant cell tumor (GCT), aneurysmal bone cyst (ABC), and osteoblastoma (OB).
Discussion
BFH is an extremely rare bone lesion, accounting for only 1% of all surgically managed bone tumors; not counting the present case, only 11 spine cases have been reported in the literature.1,2 BFH of the spine traditionally causes nonspecific, poorly localized pain. The Table lists the reported cases of spinal BFH and their presenting symptoms, location, and treatment. BFH usually occurs in young adults, but the age range is 5 to 75 years.2-4 Mean age of the 12 patients with spinal BFH in the literature (including ours) is 25 years.1 In addition, spinal BFH appears to have no predilection for sex.
Skeletal BFH presents as a discrete, well-defined, osteolytic lesion with sharp borders and potentially a sclerotic rim.4-6 Cortical expansion and even cortical disruption with invasion into adjacent tissue have occurred in flat bones.7 Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in storiform pattern.6
BFH shares many of its radiologic and histologic characteristics and clinical symptoms with other benign bone lesions (the tumors listed above). Therefore, accurate diagnosis of BFH requires appropriate correlation of clinical, radiographic, and histologic data.2,3,8 Below is a comparison of BFH with related bone lesions.
Spinal BFH causes a nonspecific, poorly localized pain similar to that of EG, ABC, GCT, and OB.3,9 NOF and fibrous dysplasia generally do not cause pain, unless these lesions are discovered secondary to a pathologic fracture.8,10,11 Our patient had minor antecedent neck pain, which was brought to light by his football accident. ABC and OB are more locally aggressive than BFH and can cause neurologic symptoms by mass effect and spinal cord or nerve root compression.1,8 In this case and in the 6 other cases of BFH of the cervical spine, there were no neurologic changes.4,10
Of the tumors mentioned, NOF and EG almost always occur in children. However, NOF usually occurs in the metaphyseal region of long bones, and EG is usually accompanied by systemic symptoms, such as lymphadenopathy, hepatomegaly, and increased inflammatory markers.1,8 Fibrous dysplasia usually presents in childhood but does not become symptomatic until adulthood. GCTs and OB predominantly occur in adulthood.12,13 Our patient’s age and lack of other systemic symptoms supported the diagnosis of BFH.
Appearance on MRI is reported less with BFH than with other tumors, but heterogenous signal intensity similar to that of skeletal muscle on T1-weighted images and high signal intensity on T2-weighted images is typically reported.8,14 NOF and fibrous dysplasia do not disrupt the bony cortex unless a pathologic fracture has occurred.4 GCTs are more aggressive lytic lesions with more aggressive radiologic features. GCTs generally cause cortical expansion/attenuation, and lack a sclerotic rim. GCTs also have a heterogenous appearance on MRI and give a low to intermediate signal on both T1- and T2-weighted images.12,15 The appearance of EG is similar to that of BFH as an osteolytic lesion with a sclerotic rim, though EGs typically break through the cortex and acquire a “punched-out” look.1,8 ABC typically is described as an expansile osteolytic lesion with a “soap-bubble” appearance on radiographs; periosteal elevation and cortical attenuation can also be visualized. MRI shows the typical multilobular appearance of the lesion with fluid levels.13
OB appears as a radiolucent lesion, with or without calcifications, surrounded by a thin margin of reactive bone.14,16 A distinguishing characteristic of OB was thought to be intense radioisotope uptake on bone scintigraphy, but recently a bony BFH demonstrated intense uptake.17 OBs typically demonstrate nonspecific MRI results similar to those of BFH: low to intermediate signal on T1-weighted images and intermediate to high signal on T2-weighted images.13 In our patient’s case, the radiographic appearance and lack of specific radiographic findings consistent with the other tumors supported the diagnosis of BFH.
Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in a storiform pattern6 which was demonstrated in our patient’s case. In addition, significant nuclear pleomorphism, mitotic activity, and necrosis were absent—a difference between BFH and malignant fibrous histiocytoma.4,15 The microscopic characteristics of BFH readily differentiate it from OB, ABC, EG, and GCT, but not from NOF on microscopic appearance alone. Clinical and radiographic findings must be consistent, as mentioned.7,18
Complete surgical excision is the reported treatment for BFH. Prognosis after resection or curettage is usually good, and recurrences have been rare.1,2 Depending on the intraspinous location of BFH, stabilization after resection or curettage may be necessary to prevent residual instability. Three of the 11 reported cases of spinal BFH required stabilization by anterior fusion or posterior pedicle screw fixation after resection.1,2 The other 8 cases underwent excision alone or excision and grafting. All 11 patients were disease-free at a mean follow-up of 3.5 years.1 In nonspinal BFH, however, both local recurrence and lung metastasis have been reported.2,5,9,19 Clarke and colleagues9 reported local recurrences in 3 of 8 cases. These recurrences involved BFH in long bones of the leg, which had been treated with curettage and grafting. There has been no reliable report of a malignant change in BFH.2,9 The only case of lung metastasis, reported by Unni and Dahlin6 in their study of 10 cases, occurred 2 years after local recurrence in the distal femur.Our patient was doing well at most recent follow-up, 6 months after surgery. He had no pain and had returned to normal activities. Although there are no reported cases of spinal BFH recurrence, we will follow this patient with imaging on an annual basis. His case is of particular interest to orthopedic surgeons because they encounter benign bone lesions every day, and many of these lesions are in difficult anatomical locations. Knowing the characteristics, differential diagnoses, and appropriate diagnostic workups for benign bone lesions is important for optimal and timely patient care.
Benign fibrous histiocytoma (BFH) is a rare, well-recognized, primary skeletal tumor accounting for approximately 1% of all benign bone tumors. Spinal involvement is exceedingly rare with only 11 cases reported in the literature.1,2 We present a case of BFH located in the cervical spine of a pediatric patient that was successfully treated with curretage through an anterior surgical approach, along with a review of the literature and appropriate management concerning BFH of the spine.
Case Report
A 14-year-old boy was tackled while playing football and noticed immediate neck pain and subjective paresthesia in the upper extremities. Examination revealed a nontender spine (cervical, thoracic, lumbar) and normal strength and range of motion in all extremities. Sensation was diffusely intact, long tract signs were absent, and gait was normal. On questioning, the patient endorsed mild antecedent neck pain but denied prior history of any trauma. Neck pain did not radiate and was slightly worsened by activity but was mostly intermittent and random. As the neck pain was very mild and was not interfering with daily activities, the patient had not sought care before presenting to the emergency department. He had no pertinent past medical or surgical history.
The patient presented with a computed tomography (CT) scan of his head and cervical spine and a magnetic resonance imaging (MRI) scan of the cervical spine. A magnetic resonance angiography (MRA) scan of the neck was ordered after his arrival.
Axial and sagittal CT (Figures 1A, 1B) showed a 1×1.2-cm discrete, expansile, lytic, radiolucent mass extending anterior from the left C2 vertebral body. The mass appeared to abut the left vertebral artery foramen. The cortical bone surrounding the lesion was thin but uniform. Sagittal and axial T1-weighted MRI (Figures 2A, 2B) showed the discrete, expansile, homogenous lesion with the same intensity as normal bone marrow. Sagittal and axial T2-weighted MRI (Figures 2C, 2D) showed a discrete, expansile, homogenous lesion with primarily high signal intensity. Sagittal short tau inversion recovery (STIR) MRI (Figure 2E) again showed the lesion with primarily low intensity. Given the close proximity of the lesion to the vertebral foramen, MRA was ordered; it showed the lesion was not interfering with the vertebral artery (Figure 2F).
The tumor’s location, in the left anterior aspect of the C2 vertebral body, was not conducive to percutaneous biopsy for establishing tissue diagnosis, so the decision was made to surgically excise the lesion. A left-sided anterior incision was made 2 fingerbreadths inferior to the jaw line in a neck crease. A head and neck surgeon assisted with dissection. Dissection was carried down through the skin, subcutaneous tissue, and platysma on to the anterior part of the spine medial to the carotid sheath. Superior thyroid nerve and vessels and superior laryngeal nerve were identified and preserved. Fluoroscopy confirmed correct location at C2. The tumor was easily visualized, and the outer shell broke easily with palpation. Gentle curettage was necessary when removing the tumor off the vertebral artery. A portion of the specimen was sent during surgery for frozen section, which showed infrequent mitotic figures and no other findings concerning for malignancy. No instability was created after curettage and excision of the tumor, so no grafting or instrumentation was necessary.
Grossly, the tumor was pale tan and firm. Histologic examination with hematoxylin-eosin staining revealed a bland spindle-cell neoplasm that focally involved bone. A storiform pattern was present. The cells had scant cytoplasm and oval to elongate nuclei with tapered ends. Significant nuclear pleomorphism was not seen. The stroma was loose, with focal myxoid change. Benign multinucleated giant cells were present. Mitotic activity was infrequent (Figures 3A–3D). Two attending pathologists reviewed the case material and the frozen and formalin-fixed specimens independently and concurred with the diagnosis of BFH. In addition, the case was reviewed at the surgical pathology consensus conference; the reviewers agreed on BFH, and additional studies were deemed unnecessary.
Given the patient’s complete clinical picture, the differential diagnosis included nonossifying fibroma (NOF), eosinophilic granuloma (EG), BFH, fibrous dysplasia, giant cell tumor (GCT), aneurysmal bone cyst (ABC), and osteoblastoma (OB).
Discussion
BFH is an extremely rare bone lesion, accounting for only 1% of all surgically managed bone tumors; not counting the present case, only 11 spine cases have been reported in the literature.1,2 BFH of the spine traditionally causes nonspecific, poorly localized pain. The Table lists the reported cases of spinal BFH and their presenting symptoms, location, and treatment. BFH usually occurs in young adults, but the age range is 5 to 75 years.2-4 Mean age of the 12 patients with spinal BFH in the literature (including ours) is 25 years.1 In addition, spinal BFH appears to have no predilection for sex.
Skeletal BFH presents as a discrete, well-defined, osteolytic lesion with sharp borders and potentially a sclerotic rim.4-6 Cortical expansion and even cortical disruption with invasion into adjacent tissue have occurred in flat bones.7 Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in storiform pattern.6
BFH shares many of its radiologic and histologic characteristics and clinical symptoms with other benign bone lesions (the tumors listed above). Therefore, accurate diagnosis of BFH requires appropriate correlation of clinical, radiographic, and histologic data.2,3,8 Below is a comparison of BFH with related bone lesions.
Spinal BFH causes a nonspecific, poorly localized pain similar to that of EG, ABC, GCT, and OB.3,9 NOF and fibrous dysplasia generally do not cause pain, unless these lesions are discovered secondary to a pathologic fracture.8,10,11 Our patient had minor antecedent neck pain, which was brought to light by his football accident. ABC and OB are more locally aggressive than BFH and can cause neurologic symptoms by mass effect and spinal cord or nerve root compression.1,8 In this case and in the 6 other cases of BFH of the cervical spine, there were no neurologic changes.4,10
Of the tumors mentioned, NOF and EG almost always occur in children. However, NOF usually occurs in the metaphyseal region of long bones, and EG is usually accompanied by systemic symptoms, such as lymphadenopathy, hepatomegaly, and increased inflammatory markers.1,8 Fibrous dysplasia usually presents in childhood but does not become symptomatic until adulthood. GCTs and OB predominantly occur in adulthood.12,13 Our patient’s age and lack of other systemic symptoms supported the diagnosis of BFH.
Appearance on MRI is reported less with BFH than with other tumors, but heterogenous signal intensity similar to that of skeletal muscle on T1-weighted images and high signal intensity on T2-weighted images is typically reported.8,14 NOF and fibrous dysplasia do not disrupt the bony cortex unless a pathologic fracture has occurred.4 GCTs are more aggressive lytic lesions with more aggressive radiologic features. GCTs generally cause cortical expansion/attenuation, and lack a sclerotic rim. GCTs also have a heterogenous appearance on MRI and give a low to intermediate signal on both T1- and T2-weighted images.12,15 The appearance of EG is similar to that of BFH as an osteolytic lesion with a sclerotic rim, though EGs typically break through the cortex and acquire a “punched-out” look.1,8 ABC typically is described as an expansile osteolytic lesion with a “soap-bubble” appearance on radiographs; periosteal elevation and cortical attenuation can also be visualized. MRI shows the typical multilobular appearance of the lesion with fluid levels.13
OB appears as a radiolucent lesion, with or without calcifications, surrounded by a thin margin of reactive bone.14,16 A distinguishing characteristic of OB was thought to be intense radioisotope uptake on bone scintigraphy, but recently a bony BFH demonstrated intense uptake.17 OBs typically demonstrate nonspecific MRI results similar to those of BFH: low to intermediate signal on T1-weighted images and intermediate to high signal on T2-weighted images.13 In our patient’s case, the radiographic appearance and lack of specific radiographic findings consistent with the other tumors supported the diagnosis of BFH.
Histologically, BFHs contain spindle cells, multinucleated giant cells, and foam cells in a storiform pattern6 which was demonstrated in our patient’s case. In addition, significant nuclear pleomorphism, mitotic activity, and necrosis were absent—a difference between BFH and malignant fibrous histiocytoma.4,15 The microscopic characteristics of BFH readily differentiate it from OB, ABC, EG, and GCT, but not from NOF on microscopic appearance alone. Clinical and radiographic findings must be consistent, as mentioned.7,18
Complete surgical excision is the reported treatment for BFH. Prognosis after resection or curettage is usually good, and recurrences have been rare.1,2 Depending on the intraspinous location of BFH, stabilization after resection or curettage may be necessary to prevent residual instability. Three of the 11 reported cases of spinal BFH required stabilization by anterior fusion or posterior pedicle screw fixation after resection.1,2 The other 8 cases underwent excision alone or excision and grafting. All 11 patients were disease-free at a mean follow-up of 3.5 years.1 In nonspinal BFH, however, both local recurrence and lung metastasis have been reported.2,5,9,19 Clarke and colleagues9 reported local recurrences in 3 of 8 cases. These recurrences involved BFH in long bones of the leg, which had been treated with curettage and grafting. There has been no reliable report of a malignant change in BFH.2,9 The only case of lung metastasis, reported by Unni and Dahlin6 in their study of 10 cases, occurred 2 years after local recurrence in the distal femur.Our patient was doing well at most recent follow-up, 6 months after surgery. He had no pain and had returned to normal activities. Although there are no reported cases of spinal BFH recurrence, we will follow this patient with imaging on an annual basis. His case is of particular interest to orthopedic surgeons because they encounter benign bone lesions every day, and many of these lesions are in difficult anatomical locations. Knowing the characteristics, differential diagnoses, and appropriate diagnostic workups for benign bone lesions is important for optimal and timely patient care.
1. Demiralp B, Kose O, Oguz E, Sanal T, Ozcan A, Sehirlioglu A. Benign fibrous histiocytoma of the lumbar vertebrae. Skeletal Radiol. 2009;38(2):187-191.
2. Kuruvath S, O’Donovan DG, Aspoas AR, David KM. Benign fibrous histiocytoma of the thoracic spine: case report and review of the literature. J Neurosurg Spine. 2006;4(3):260-264.
3. Ceroni D, Dayer R, De Coulon G, Kaelin A. Benign fibrous histiocytoma of bone in a paediatric population: a report of 6 cases. Musculoskelet Surg. 2011;95(2):107-114.
4. Dorfman HD, Czerniak B. Bone Tumors. St. Louis, MO: Mosby; 1998.
5. Grohs JG, Nicolakis M, Kainberger F, Lang S, Kotz R. Benign fibrous histiocytoma of bone: a report of ten cases and review of literature. Wien Klin Wochenschr. 2002;114(1-2):56-63.
6. Unni KK, Dahlin DC. Dahlin’s Bone Tumors. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
7. Balasubramanian C, Rajaraman G, Singh CS, Baliga DK. Benign fibrous histiocytoma of the sacrum—diagnostic difficulties facing this rare bone tumor. Pediatr Neurosurg. 2005;41(5):253-257.
8. van Giffen NH, van Rhijn LW, van Ooij A, et al. Benign fibrous histiocytoma of the posterior arch of C1 in a 6-year old boy: a case report. Spine. 2003;28(18):E359-E363.
9. Clarke BE, Xipell JM, Thomas DP. Benign fibrous histiocytoma of bone. Am J Surg Pathol. 1985;9(11):806-815.
10. Peicha G, Siebert FJ, Bratschitsch G, Fankhauser F, Grechenig W. Pathologic odontoid fracture and benign fibrous histiocytoma of bone. Eur Spine J. 1999;8(2):161-163.
11. Unni KK, Inwards CY, Bridge JA, Kindblom LG, Wold LE. Tumors of the Bones and Joints (AFIP Atlas of Tumor Pathology Series IV). Annapolis Junction, MD: American Registry of Pathology Press; 2005.
12. Dee R. Principles of Orthopaedic Practice. 2nd ed. New York, NY: McGraw-Hill; 1997.
13. Murphey M, Andrews C, Flemming D, Temple HT, Smith WS, Smirniotopoulos JG. Primary tumors of the spine: radiologic–pathologic correlation. Radiographics. 1996;16(5):1131-1158.
14. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
15. Mirra JM, Picci P, Gold RH. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Vol 1. Philadelphia, PA: Lea & Febiger; 1989.
16. Theodorou DJ, Theodorou SJ, Sartoris DJ. An imaging overview of primary tumors of the spine: part 1. Benign tumors. Clin Imaging. 2008;32(3):196-203.
17. Li X, Meng Z, Li D, Tan J, Song X. Benign fibrous histiocytoma of a rib. Clin Nucl Med. 2014;39(9): 837-841.
18. Roessner A, Immenkamp M, Weidner A, Hobik HP, Grundmann E. Benign fibrous histiocytoma of bone. Light- and electron-microscopic observations. J Cancer Res Clin Oncol. 1981;101(2):191-202.
19. Destouet JM, Kyriakos M, Gilula LA. Fibrous histiocytoma (fibroxanthoma) of a cervical vertebra. A report with a review of the literature. Skeletal Radiol. 1980;5(4):241-246.
20. Hoeffel JC, Bomand-Ferrand F, Tachet F, Lascombes P, Czorny A, Bernard C. So-called benign fibrous histiocytoma: report of a case. J Pediatr Surg. 1992;27(5):672-674.
1. Demiralp B, Kose O, Oguz E, Sanal T, Ozcan A, Sehirlioglu A. Benign fibrous histiocytoma of the lumbar vertebrae. Skeletal Radiol. 2009;38(2):187-191.
2. Kuruvath S, O’Donovan DG, Aspoas AR, David KM. Benign fibrous histiocytoma of the thoracic spine: case report and review of the literature. J Neurosurg Spine. 2006;4(3):260-264.
3. Ceroni D, Dayer R, De Coulon G, Kaelin A. Benign fibrous histiocytoma of bone in a paediatric population: a report of 6 cases. Musculoskelet Surg. 2011;95(2):107-114.
4. Dorfman HD, Czerniak B. Bone Tumors. St. Louis, MO: Mosby; 1998.
5. Grohs JG, Nicolakis M, Kainberger F, Lang S, Kotz R. Benign fibrous histiocytoma of bone: a report of ten cases and review of literature. Wien Klin Wochenschr. 2002;114(1-2):56-63.
6. Unni KK, Dahlin DC. Dahlin’s Bone Tumors. 5th ed. Philadelphia, PA: Lippincott-Raven; 1996.
7. Balasubramanian C, Rajaraman G, Singh CS, Baliga DK. Benign fibrous histiocytoma of the sacrum—diagnostic difficulties facing this rare bone tumor. Pediatr Neurosurg. 2005;41(5):253-257.
8. van Giffen NH, van Rhijn LW, van Ooij A, et al. Benign fibrous histiocytoma of the posterior arch of C1 in a 6-year old boy: a case report. Spine. 2003;28(18):E359-E363.
9. Clarke BE, Xipell JM, Thomas DP. Benign fibrous histiocytoma of bone. Am J Surg Pathol. 1985;9(11):806-815.
10. Peicha G, Siebert FJ, Bratschitsch G, Fankhauser F, Grechenig W. Pathologic odontoid fracture and benign fibrous histiocytoma of bone. Eur Spine J. 1999;8(2):161-163.
11. Unni KK, Inwards CY, Bridge JA, Kindblom LG, Wold LE. Tumors of the Bones and Joints (AFIP Atlas of Tumor Pathology Series IV). Annapolis Junction, MD: American Registry of Pathology Press; 2005.
12. Dee R. Principles of Orthopaedic Practice. 2nd ed. New York, NY: McGraw-Hill; 1997.
13. Murphey M, Andrews C, Flemming D, Temple HT, Smith WS, Smirniotopoulos JG. Primary tumors of the spine: radiologic–pathologic correlation. Radiographics. 1996;16(5):1131-1158.
14. Hamada T, Ito H, Araki Y, Fujii K, Inoue M, Ishida O. Benign fibrous histiocytoma of the femur: review of three cases. Skeletal Radiol. 1996;25(1):25-29.
15. Mirra JM, Picci P, Gold RH. Bone Tumors: Clinical, Radiologic, and Pathologic Correlations. Vol 1. Philadelphia, PA: Lea & Febiger; 1989.
16. Theodorou DJ, Theodorou SJ, Sartoris DJ. An imaging overview of primary tumors of the spine: part 1. Benign tumors. Clin Imaging. 2008;32(3):196-203.
17. Li X, Meng Z, Li D, Tan J, Song X. Benign fibrous histiocytoma of a rib. Clin Nucl Med. 2014;39(9): 837-841.
18. Roessner A, Immenkamp M, Weidner A, Hobik HP, Grundmann E. Benign fibrous histiocytoma of bone. Light- and electron-microscopic observations. J Cancer Res Clin Oncol. 1981;101(2):191-202.
19. Destouet JM, Kyriakos M, Gilula LA. Fibrous histiocytoma (fibroxanthoma) of a cervical vertebra. A report with a review of the literature. Skeletal Radiol. 1980;5(4):241-246.
20. Hoeffel JC, Bomand-Ferrand F, Tachet F, Lascombes P, Czorny A, Bernard C. So-called benign fibrous histiocytoma: report of a case. J Pediatr Surg. 1992;27(5):672-674.
Trust
At this ripe point in my career, many new patients come referred by Dr. Google. “I checked the Internet,” they say. “You have great reviews.”
I don’t read my reviews. The abusive ones make me ill. People must filter out the bile and focus on the positives.
Laymen have little sense of how good the professionals they consult really are. Unless I’m audited and lose, how would I know how skillful my accountant is? My urologist is a nice man. How good is he at prostate surgery? I hope not to find out. Nevertheless, reviews are here to stay, as are physician evaluations by insurers and professional agencies.
Some office days highlight the gap, really the chasm, between the truth of the professional matter and what makes patients decide to trust or mistrust us. Last Thursday was one of those days.
Marla brought in her daughter, aged 3 years. Zoe had a scaly rash and some red papules on her arms and legs.
“Did your pediatrician treat this?” I asked.
“No, I came right to you,” said Marla. “You diagnosed her with bedbugs when she was an infant. The pediatricians had no idea what was going on. I trust you.”
That is flattering, but if I were being fully honest, I would tell Marla:
• Bedbug bites are tricky to diagnose. I’ve missed my share.
• What helped me diagnose them in her daughter was that the pediatrician had already tried several remedies that hadn’t helped.
Even if I said these things, though – and why waste all that wonderful transference? – Marla would probably have said, “Maybe so, but you got it right, and I trust you.” Nothing succeeds like success.
The reverse, however, is also true: Nothing fails like failure.
Later the same day Brian brought in Luke, aged 6 years. Luke has severe atopic dermatitis. As usual, he was scratching up a storm. “I think it’s infected,” Brian said. “Shouldn’t he take antibiotics?”
I examined Luke and found subacute eczema. “I don’t think so,” I said. “This is what Luke’s eczema flares look like. Let’s treat him with a topical steroid cream and see how he does.”
“But he had staph last year,” said Brian.
“I recall,” I said, “but most of his outbreaks have not been infected, and it doesn’t look like staph now. Let’s treat it as we usually do and see what happens over the next week.”
Two weeks later Brian brought back Luke, still scratching. There were still no pustules or deeper inflammatory lesions. We started Luke on an antibiotic, and swabbed scratched areas. Two days later the culture grew staph. By the time I called Brian with the results, he had brought Luke to an emergency room. “He has abscesses,” he told me.
The next day the sensitivities were back, confirming staph. I called Brian, who had this to say: “He should have been on antibiotics 2 weeks ago. From now on, whenever he starts scratching, he should be started on them right away. I won’t be bringing him back to your practice. I don’t trust you. I trust the doctors in the ER more.”
Is it really a good idea to start every eczema patient on antibiotics? How about every eczema patient who once had staph? Based on my own clinical experience with both conditions, I would answer both questions in the negative. Others might disagree.
One thing is sure, though: Like most patients, Brian sees the situation not through the eyes of my experience but through his own case series, with an n of 1. But that 1 carries a lot of weight, because the 1 is Luke, his son. It is therefore clear – to him – that his son should be treated preemptively with antibiotics for every eczema flare.
At this point I might too, for Luke, but I will not get the chance. Once trust is gone, the clinical relationship is over. Sometimes it’s one strike and you’re out.
For her part, Marla sees things through her single case report as well, drawing the opposite conclusion: that my success earned me the trust her pediatrician lost.
The subtleties and nuances of such cases, which every clinician knows, are lost in the often black-and-white world of lay reviews and pay-for-performance algorithms. That’s clinical life.
Trust me.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. Write to him at [email protected].
At this ripe point in my career, many new patients come referred by Dr. Google. “I checked the Internet,” they say. “You have great reviews.”
I don’t read my reviews. The abusive ones make me ill. People must filter out the bile and focus on the positives.
Laymen have little sense of how good the professionals they consult really are. Unless I’m audited and lose, how would I know how skillful my accountant is? My urologist is a nice man. How good is he at prostate surgery? I hope not to find out. Nevertheless, reviews are here to stay, as are physician evaluations by insurers and professional agencies.
Some office days highlight the gap, really the chasm, between the truth of the professional matter and what makes patients decide to trust or mistrust us. Last Thursday was one of those days.
Marla brought in her daughter, aged 3 years. Zoe had a scaly rash and some red papules on her arms and legs.
“Did your pediatrician treat this?” I asked.
“No, I came right to you,” said Marla. “You diagnosed her with bedbugs when she was an infant. The pediatricians had no idea what was going on. I trust you.”
That is flattering, but if I were being fully honest, I would tell Marla:
• Bedbug bites are tricky to diagnose. I’ve missed my share.
• What helped me diagnose them in her daughter was that the pediatrician had already tried several remedies that hadn’t helped.
Even if I said these things, though – and why waste all that wonderful transference? – Marla would probably have said, “Maybe so, but you got it right, and I trust you.” Nothing succeeds like success.
The reverse, however, is also true: Nothing fails like failure.
Later the same day Brian brought in Luke, aged 6 years. Luke has severe atopic dermatitis. As usual, he was scratching up a storm. “I think it’s infected,” Brian said. “Shouldn’t he take antibiotics?”
I examined Luke and found subacute eczema. “I don’t think so,” I said. “This is what Luke’s eczema flares look like. Let’s treat him with a topical steroid cream and see how he does.”
“But he had staph last year,” said Brian.
“I recall,” I said, “but most of his outbreaks have not been infected, and it doesn’t look like staph now. Let’s treat it as we usually do and see what happens over the next week.”
Two weeks later Brian brought back Luke, still scratching. There were still no pustules or deeper inflammatory lesions. We started Luke on an antibiotic, and swabbed scratched areas. Two days later the culture grew staph. By the time I called Brian with the results, he had brought Luke to an emergency room. “He has abscesses,” he told me.
The next day the sensitivities were back, confirming staph. I called Brian, who had this to say: “He should have been on antibiotics 2 weeks ago. From now on, whenever he starts scratching, he should be started on them right away. I won’t be bringing him back to your practice. I don’t trust you. I trust the doctors in the ER more.”
Is it really a good idea to start every eczema patient on antibiotics? How about every eczema patient who once had staph? Based on my own clinical experience with both conditions, I would answer both questions in the negative. Others might disagree.
One thing is sure, though: Like most patients, Brian sees the situation not through the eyes of my experience but through his own case series, with an n of 1. But that 1 carries a lot of weight, because the 1 is Luke, his son. It is therefore clear – to him – that his son should be treated preemptively with antibiotics for every eczema flare.
At this point I might too, for Luke, but I will not get the chance. Once trust is gone, the clinical relationship is over. Sometimes it’s one strike and you’re out.
For her part, Marla sees things through her single case report as well, drawing the opposite conclusion: that my success earned me the trust her pediatrician lost.
The subtleties and nuances of such cases, which every clinician knows, are lost in the often black-and-white world of lay reviews and pay-for-performance algorithms. That’s clinical life.
Trust me.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. Write to him at [email protected].
At this ripe point in my career, many new patients come referred by Dr. Google. “I checked the Internet,” they say. “You have great reviews.”
I don’t read my reviews. The abusive ones make me ill. People must filter out the bile and focus on the positives.
Laymen have little sense of how good the professionals they consult really are. Unless I’m audited and lose, how would I know how skillful my accountant is? My urologist is a nice man. How good is he at prostate surgery? I hope not to find out. Nevertheless, reviews are here to stay, as are physician evaluations by insurers and professional agencies.
Some office days highlight the gap, really the chasm, between the truth of the professional matter and what makes patients decide to trust or mistrust us. Last Thursday was one of those days.
Marla brought in her daughter, aged 3 years. Zoe had a scaly rash and some red papules on her arms and legs.
“Did your pediatrician treat this?” I asked.
“No, I came right to you,” said Marla. “You diagnosed her with bedbugs when she was an infant. The pediatricians had no idea what was going on. I trust you.”
That is flattering, but if I were being fully honest, I would tell Marla:
• Bedbug bites are tricky to diagnose. I’ve missed my share.
• What helped me diagnose them in her daughter was that the pediatrician had already tried several remedies that hadn’t helped.
Even if I said these things, though – and why waste all that wonderful transference? – Marla would probably have said, “Maybe so, but you got it right, and I trust you.” Nothing succeeds like success.
The reverse, however, is also true: Nothing fails like failure.
Later the same day Brian brought in Luke, aged 6 years. Luke has severe atopic dermatitis. As usual, he was scratching up a storm. “I think it’s infected,” Brian said. “Shouldn’t he take antibiotics?”
I examined Luke and found subacute eczema. “I don’t think so,” I said. “This is what Luke’s eczema flares look like. Let’s treat him with a topical steroid cream and see how he does.”
“But he had staph last year,” said Brian.
“I recall,” I said, “but most of his outbreaks have not been infected, and it doesn’t look like staph now. Let’s treat it as we usually do and see what happens over the next week.”
Two weeks later Brian brought back Luke, still scratching. There were still no pustules or deeper inflammatory lesions. We started Luke on an antibiotic, and swabbed scratched areas. Two days later the culture grew staph. By the time I called Brian with the results, he had brought Luke to an emergency room. “He has abscesses,” he told me.
The next day the sensitivities were back, confirming staph. I called Brian, who had this to say: “He should have been on antibiotics 2 weeks ago. From now on, whenever he starts scratching, he should be started on them right away. I won’t be bringing him back to your practice. I don’t trust you. I trust the doctors in the ER more.”
Is it really a good idea to start every eczema patient on antibiotics? How about every eczema patient who once had staph? Based on my own clinical experience with both conditions, I would answer both questions in the negative. Others might disagree.
One thing is sure, though: Like most patients, Brian sees the situation not through the eyes of my experience but through his own case series, with an n of 1. But that 1 carries a lot of weight, because the 1 is Luke, his son. It is therefore clear – to him – that his son should be treated preemptively with antibiotics for every eczema flare.
At this point I might too, for Luke, but I will not get the chance. Once trust is gone, the clinical relationship is over. Sometimes it’s one strike and you’re out.
For her part, Marla sees things through her single case report as well, drawing the opposite conclusion: that my success earned me the trust her pediatrician lost.
The subtleties and nuances of such cases, which every clinician knows, are lost in the often black-and-white world of lay reviews and pay-for-performance algorithms. That’s clinical life.
Trust me.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. Write to him at [email protected].
Psychosis: Watch for sudden poor academic performance
When an adolescent presents with a history of sudden decline in academic performance, be sure to consider serious mental illness.
According to Dr. David Pickar, a psychiatrist and former (retired) director of intramural research at the National Institute of Mental Health, 90%of cases – particularly of schizophrenia – occur between the ages of 16 and 24 years.
“As a scientist, I’ve spent my career thinking about that, but for the primary care doc, if the family comes in and reports that their kid was a good student, and he’s now terrible,” first-episode psychosis should be considered, said Dr. Pickar, who produced a documentary short film describing how to recognize schizophrenia and psychosis. In this video, Dr. Pickar also explains how the use of marijuana also can precipitate psychosis in some people with a genetic predisposition to the illness.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
When an adolescent presents with a history of sudden decline in academic performance, be sure to consider serious mental illness.
According to Dr. David Pickar, a psychiatrist and former (retired) director of intramural research at the National Institute of Mental Health, 90%of cases – particularly of schizophrenia – occur between the ages of 16 and 24 years.
“As a scientist, I’ve spent my career thinking about that, but for the primary care doc, if the family comes in and reports that their kid was a good student, and he’s now terrible,” first-episode psychosis should be considered, said Dr. Pickar, who produced a documentary short film describing how to recognize schizophrenia and psychosis. In this video, Dr. Pickar also explains how the use of marijuana also can precipitate psychosis in some people with a genetic predisposition to the illness.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
When an adolescent presents with a history of sudden decline in academic performance, be sure to consider serious mental illness.
According to Dr. David Pickar, a psychiatrist and former (retired) director of intramural research at the National Institute of Mental Health, 90%of cases – particularly of schizophrenia – occur between the ages of 16 and 24 years.
“As a scientist, I’ve spent my career thinking about that, but for the primary care doc, if the family comes in and reports that their kid was a good student, and he’s now terrible,” first-episode psychosis should be considered, said Dr. Pickar, who produced a documentary short film describing how to recognize schizophrenia and psychosis. In this video, Dr. Pickar also explains how the use of marijuana also can precipitate psychosis in some people with a genetic predisposition to the illness.