User login
Study: TNF inhibitors improve extraintestinal IBD manifestations
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
SAN DIEGO – Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease (IBD) among more than half of affected patients, according to a national cohort study.
“The best response rates were for psoriasis, aphthous stomatitis, uveitis, and peripheral arthritis,” said Dr. Thomas Greuter of University Hospital in Zürich. Patients responded similarly whether they received oral infliximab or subcutaneous adalimumab or certolizumab, he noted.
IBD often is associated with debilitating disorders of the skin, joints, eyes, and hepatobiliary tract, but “due to the lack of randomized, controlled trials, the therapy of extraintestinal manifestations remains rather empirical,” Dr. Greuter said at the annual Digestive Disease Week.
To study the role of anti-TNF agents in treating these disorders, he and his associates analyzed data for 1,249 patients from the national Swiss IBD Cohort Study between 2006 and 2010. Patients were typically in their mid-30s and had lived with IBD for about 9 years, he said.
A total of 366 patients (29%) had at least one extraintestinal manifestation of IBD – most commonly peripheral arthritis (75%), followed by aphthous stomatitis (24%), and ankylosing spondylitis (22%). In all, 213 (58%) patients received at least one anti-TNF agent, and 40% received the prescription specifically for extraintestinal manifestations. Nearly two-thirds of the patients received infliximab, while 22% received adalimumab and 15% received certolizumab.
About 55% of patients improved on anti-TNF therapy over an average of 7 years of follow-up, Dr. Greuter and his associates reported. Among all three anti-TNF agents, response rates ranged from 100% for psoriasis, to 80% for erythema nodosum and stomatitis, to 73% for arthritis and uveitis, to 50% for pyoderma granulosum. Overall rates of improvement were slightly higher for infliximab than for the other two drugs, but “adalimumab and certolizumab were used mostly as a second or a third-line anti-TNF agent, and the response rate to a second or third-line treatment was lower than for the first one,” Dr. Greuter said. Some patients also received corticosteroids and immunomodulators, but excluding this subgroup had little effect on rates of response to anti-TNF therapy, he added.
Dr. Greuter also reported that 11 patients (about 5% of the cohort) developed 14 new extraintestinal manifestations after starting anti-TNF agents – usually peripheral arthritis, but also pyoderma granulosum, aphthous stomatitis, psoriasis, and uveitis. “We cannot say if this was primary, or a side effect of treatment,” he said. These disorders usually improved if patients stayed on their anti-TNF agent, he added.
About two-thirds of patients in the cohort were female, more than three-quarters had Crohn’s disease, 19% had ulcerative colitis, and 3% had indeterminate colitis, he noted.
A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
AT DDW® 2016
Key clinical point: Tumor necrosis factor inhibitors improved the extraintestinal manifestations of inflammatory bowel disease among more than half of affected patients.
Major finding: About 55% of patients who received infliximab, adalimumab, or certolizumab had a clinical response.
Data source: A study of 1,249 patients with IBD from a national cohort.
Disclosures: A research grant from the Swiss National Science Foundation funded the study. Dr. Greuter had no disclosures.
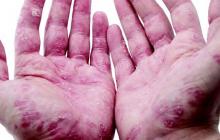
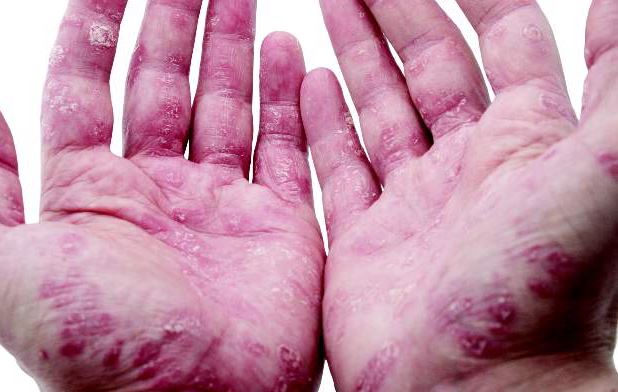
TB still a risk in psoriasis patients taking TNF blockers
Despite following guidelines aimed at reducing the likelihood of tuberculosis infection in patients with psoriasis treated with tumor necrosis factor antagonists, a French study has identified a number of active TB cases in this patient group.
The nationwide retrospective study identified 12 cases of tuberculosis in individuals with psoriasis during treatment with a TNF-antagonist between 2006 and 2014 – 9 men and 3 women, whose mean age was 49 years – according to Dr. E. Guinard of the dermatology service, CHU Toulouse Larrey at Paul Sabatier University, Toulouse, France, and associates (J Eur Acad Dermatol Venereol. 2016 Jun 3. doi: 10.1111/jdv.13633).
The investigators identified the cases from a national pharmacosurveillance database and by contacting members of the psoriasis research group in France’s national dermatology society and the national college of French dermatology professors.
Before starting anti-TNF therapy, all 12 patients had been screened for latent TB based on French guidelines: three patients were tested with the tuberculosis skin test alone, six were tested with QuantiFERON-TB Gold test, and three patients had both the skin test and QuantiFERON-TB. Each had a pretreatment chest x-ray.
Based on this testing, three were diagnosed with latent TB and received chemoprophylaxis; the other nine patients tested negative.
Information on TB risk factors was not available for all 12 cases, but three people had had contact with someone with TB during treatment. Three of the individuals had traveled to or were born in areas where TB was endemic, and one patient had had a case of TB in the family in the year before starting anti-TNF therapy. Nine patients had a known history of BCG vaccination, the investigators said.
The time between starting anti-TNF therapy and the appearance of TB symptoms ranged from 2 to 176 weeks (mean, 23 weeks), and diagnosis was based on clinical signs – such as fever, night sweats, and cough – and on imaging and laboratory investigations. Most (10) of the cases were extrapulmonary presentations, and two were pleuropulmonary. When diagnosed with tuberculosis, seven of the patients were being treated with infliximab, four were being treated with adalimumab, and one was being treated with certolizumab. All stopped anti-TNF therapy once they were diagnosed with tuberculosis.
Dr. Guinard and associates noted that the low sensitivity of Mycobacterium tuberculosis diagnostic tests was “a striking feature” of these cases. Only one-third of the patients showed a positive direct Ziehl-Neelsen stain for acid-fast bacilli and half had a positive culture.
“According to this study and published experience, in a patient with suggestive symptoms, a negative Mycobacterium tuberculosis test should not delay the decision to initiate anti-tuberculosis treatment,” they wrote. “This is particularly important considering the high mortality of tuberculosis in patients treated with TNF antagonists,” which ranges from 6%-17%, they added.
Two of the twelve patients – aged 77 and 32 years – died of disseminated tuberculosis disease during treatment for TB.
“This is the largest study of tuberculosis in psoriasis patients treated with TNF antagonists,” according to the authors.
No conflicts of interest were declared, and the study had no funding source.
Despite following guidelines aimed at reducing the likelihood of tuberculosis infection in patients with psoriasis treated with tumor necrosis factor antagonists, a French study has identified a number of active TB cases in this patient group.
The nationwide retrospective study identified 12 cases of tuberculosis in individuals with psoriasis during treatment with a TNF-antagonist between 2006 and 2014 – 9 men and 3 women, whose mean age was 49 years – according to Dr. E. Guinard of the dermatology service, CHU Toulouse Larrey at Paul Sabatier University, Toulouse, France, and associates (J Eur Acad Dermatol Venereol. 2016 Jun 3. doi: 10.1111/jdv.13633).
The investigators identified the cases from a national pharmacosurveillance database and by contacting members of the psoriasis research group in France’s national dermatology society and the national college of French dermatology professors.
Before starting anti-TNF therapy, all 12 patients had been screened for latent TB based on French guidelines: three patients were tested with the tuberculosis skin test alone, six were tested with QuantiFERON-TB Gold test, and three patients had both the skin test and QuantiFERON-TB. Each had a pretreatment chest x-ray.
Based on this testing, three were diagnosed with latent TB and received chemoprophylaxis; the other nine patients tested negative.
Information on TB risk factors was not available for all 12 cases, but three people had had contact with someone with TB during treatment. Three of the individuals had traveled to or were born in areas where TB was endemic, and one patient had had a case of TB in the family in the year before starting anti-TNF therapy. Nine patients had a known history of BCG vaccination, the investigators said.
The time between starting anti-TNF therapy and the appearance of TB symptoms ranged from 2 to 176 weeks (mean, 23 weeks), and diagnosis was based on clinical signs – such as fever, night sweats, and cough – and on imaging and laboratory investigations. Most (10) of the cases were extrapulmonary presentations, and two were pleuropulmonary. When diagnosed with tuberculosis, seven of the patients were being treated with infliximab, four were being treated with adalimumab, and one was being treated with certolizumab. All stopped anti-TNF therapy once they were diagnosed with tuberculosis.
Dr. Guinard and associates noted that the low sensitivity of Mycobacterium tuberculosis diagnostic tests was “a striking feature” of these cases. Only one-third of the patients showed a positive direct Ziehl-Neelsen stain for acid-fast bacilli and half had a positive culture.
“According to this study and published experience, in a patient with suggestive symptoms, a negative Mycobacterium tuberculosis test should not delay the decision to initiate anti-tuberculosis treatment,” they wrote. “This is particularly important considering the high mortality of tuberculosis in patients treated with TNF antagonists,” which ranges from 6%-17%, they added.
Two of the twelve patients – aged 77 and 32 years – died of disseminated tuberculosis disease during treatment for TB.
“This is the largest study of tuberculosis in psoriasis patients treated with TNF antagonists,” according to the authors.
No conflicts of interest were declared, and the study had no funding source.
Despite following guidelines aimed at reducing the likelihood of tuberculosis infection in patients with psoriasis treated with tumor necrosis factor antagonists, a French study has identified a number of active TB cases in this patient group.
The nationwide retrospective study identified 12 cases of tuberculosis in individuals with psoriasis during treatment with a TNF-antagonist between 2006 and 2014 – 9 men and 3 women, whose mean age was 49 years – according to Dr. E. Guinard of the dermatology service, CHU Toulouse Larrey at Paul Sabatier University, Toulouse, France, and associates (J Eur Acad Dermatol Venereol. 2016 Jun 3. doi: 10.1111/jdv.13633).
The investigators identified the cases from a national pharmacosurveillance database and by contacting members of the psoriasis research group in France’s national dermatology society and the national college of French dermatology professors.
Before starting anti-TNF therapy, all 12 patients had been screened for latent TB based on French guidelines: three patients were tested with the tuberculosis skin test alone, six were tested with QuantiFERON-TB Gold test, and three patients had both the skin test and QuantiFERON-TB. Each had a pretreatment chest x-ray.
Based on this testing, three were diagnosed with latent TB and received chemoprophylaxis; the other nine patients tested negative.
Information on TB risk factors was not available for all 12 cases, but three people had had contact with someone with TB during treatment. Three of the individuals had traveled to or were born in areas where TB was endemic, and one patient had had a case of TB in the family in the year before starting anti-TNF therapy. Nine patients had a known history of BCG vaccination, the investigators said.
The time between starting anti-TNF therapy and the appearance of TB symptoms ranged from 2 to 176 weeks (mean, 23 weeks), and diagnosis was based on clinical signs – such as fever, night sweats, and cough – and on imaging and laboratory investigations. Most (10) of the cases were extrapulmonary presentations, and two were pleuropulmonary. When diagnosed with tuberculosis, seven of the patients were being treated with infliximab, four were being treated with adalimumab, and one was being treated with certolizumab. All stopped anti-TNF therapy once they were diagnosed with tuberculosis.
Dr. Guinard and associates noted that the low sensitivity of Mycobacterium tuberculosis diagnostic tests was “a striking feature” of these cases. Only one-third of the patients showed a positive direct Ziehl-Neelsen stain for acid-fast bacilli and half had a positive culture.
“According to this study and published experience, in a patient with suggestive symptoms, a negative Mycobacterium tuberculosis test should not delay the decision to initiate anti-tuberculosis treatment,” they wrote. “This is particularly important considering the high mortality of tuberculosis in patients treated with TNF antagonists,” which ranges from 6%-17%, they added.
Two of the twelve patients – aged 77 and 32 years – died of disseminated tuberculosis disease during treatment for TB.
“This is the largest study of tuberculosis in psoriasis patients treated with TNF antagonists,” according to the authors.
No conflicts of interest were declared, and the study had no funding source.
FROM THE JOURNAL OF THE EADV
Key clinical point: Individuals with psoriasis treated with tumor necrosis factor inhibitors may still present with tuberculosis, despite guidelines to reduce the likelihood of developing the disease.
Major finding: Twelve individuals with psoriasis were diagnosed with TB while on anti-TNF therapy, although TB prevention guidelines had been employed.
Data source: The cases were identified from a national pharmacosurveillance database in France and from members of the psoriasis research group in France’s national dermatology society and the national college of French dermatology professors.
Disclosures: No funding or conflicts of interest were declared.
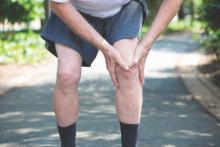
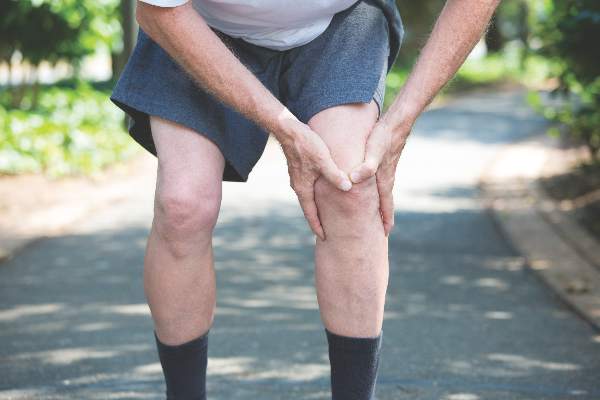
Jury still out on mortality benefits of knee replacement in OA
People with osteoarthritis who go on to have a total or partial knee replacement do not appear to have an increased risk of all-cause mortality, but the jury is still out on whether they gain any improvement, a study showed.
In their research published in the Annals of the Rheumatic Diseases [2016 May 17. doi: 10.1136/annrheumdis-2016-209167], Dr. Devyani Misra of Boston University and colleagues noted that knee replacement (KR) was thought to decrease long-term mortality risk because of the relief from pain and improvement in function that typically comes with surgery. However, studies on the topic had been conflicting, largely because of the challenges associated with studying mortality with KR surgery in observational settings.
In the current study the research team sought to evaluate the relation of KR to the risk of all-cause mortality among subjects with knee OA, while at the same time giving particular attention to “potential sources of confounding bias that may account for [the] effect of KR on mortality.”
Using patient data from the U.K. primary care electronic database THIN, the investigators compared the risk of mortality among 14,042 subjects who had OA, were aged 50-89 years old, and had had or had not had KR.
They discovered a strong protective effect of KR on all-cause long-term mortality risk, particularly among the adults over 63 years of age.
For example, people who had undergone KR had a 28% lower risk of mortality than did non-KR subjects (hazard ratio, 0.72; 95% confidence interval, 0.66-0.78).
In the overall propensity score–matched study sample, crude mortality per 1,000 person-years (total person-years) for the KR and non-KR cohorts were 19 (61,015) and 25 (58,294), respectively.
However, despite their best efforts, the researchers said the results showed evidence of residual confounding.
“For example, the observation of improved survival immediately after KR, despite the expectation of potential short-term increased postoperative mortality risk supports the presence of residual confounding,” they wrote.
Another finding suggestive of confounding was that the protective effect was seen only in older patients (over 63) when the authors stratified study participants by age.
“While it is possible that survival benefit seen in older patients with KR is a true effect because it is in this group that greater physical activity is particularly important to survival, more likely it is a result of residual confounding because subject selection is rigorous in this age group due to vulnerability,” the authors wrote.
They concluded that knee replacement “did not appear to be associated with an increased risk of all-cause mortality.”
“While we cannot rule out that KR may potentially reduce the risk of mortality over the long term, the true extent of that potential benefit is difficult to discern due to confounding by indication in observational studies using administrative data or electronic health records,” they added.
This study was funded by the Arthritis Foundation Postdoctoral Fellowship Award, the ACR Rheumatology Research Foundation Investigator Award, and a Boston University scholarship grant.
People with osteoarthritis who go on to have a total or partial knee replacement do not appear to have an increased risk of all-cause mortality, but the jury is still out on whether they gain any improvement, a study showed.
In their research published in the Annals of the Rheumatic Diseases [2016 May 17. doi: 10.1136/annrheumdis-2016-209167], Dr. Devyani Misra of Boston University and colleagues noted that knee replacement (KR) was thought to decrease long-term mortality risk because of the relief from pain and improvement in function that typically comes with surgery. However, studies on the topic had been conflicting, largely because of the challenges associated with studying mortality with KR surgery in observational settings.
In the current study the research team sought to evaluate the relation of KR to the risk of all-cause mortality among subjects with knee OA, while at the same time giving particular attention to “potential sources of confounding bias that may account for [the] effect of KR on mortality.”
Using patient data from the U.K. primary care electronic database THIN, the investigators compared the risk of mortality among 14,042 subjects who had OA, were aged 50-89 years old, and had had or had not had KR.
They discovered a strong protective effect of KR on all-cause long-term mortality risk, particularly among the adults over 63 years of age.
For example, people who had undergone KR had a 28% lower risk of mortality than did non-KR subjects (hazard ratio, 0.72; 95% confidence interval, 0.66-0.78).
In the overall propensity score–matched study sample, crude mortality per 1,000 person-years (total person-years) for the KR and non-KR cohorts were 19 (61,015) and 25 (58,294), respectively.
However, despite their best efforts, the researchers said the results showed evidence of residual confounding.
“For example, the observation of improved survival immediately after KR, despite the expectation of potential short-term increased postoperative mortality risk supports the presence of residual confounding,” they wrote.
Another finding suggestive of confounding was that the protective effect was seen only in older patients (over 63) when the authors stratified study participants by age.
“While it is possible that survival benefit seen in older patients with KR is a true effect because it is in this group that greater physical activity is particularly important to survival, more likely it is a result of residual confounding because subject selection is rigorous in this age group due to vulnerability,” the authors wrote.
They concluded that knee replacement “did not appear to be associated with an increased risk of all-cause mortality.”
“While we cannot rule out that KR may potentially reduce the risk of mortality over the long term, the true extent of that potential benefit is difficult to discern due to confounding by indication in observational studies using administrative data or electronic health records,” they added.
This study was funded by the Arthritis Foundation Postdoctoral Fellowship Award, the ACR Rheumatology Research Foundation Investigator Award, and a Boston University scholarship grant.
People with osteoarthritis who go on to have a total or partial knee replacement do not appear to have an increased risk of all-cause mortality, but the jury is still out on whether they gain any improvement, a study showed.
In their research published in the Annals of the Rheumatic Diseases [2016 May 17. doi: 10.1136/annrheumdis-2016-209167], Dr. Devyani Misra of Boston University and colleagues noted that knee replacement (KR) was thought to decrease long-term mortality risk because of the relief from pain and improvement in function that typically comes with surgery. However, studies on the topic had been conflicting, largely because of the challenges associated with studying mortality with KR surgery in observational settings.
In the current study the research team sought to evaluate the relation of KR to the risk of all-cause mortality among subjects with knee OA, while at the same time giving particular attention to “potential sources of confounding bias that may account for [the] effect of KR on mortality.”
Using patient data from the U.K. primary care electronic database THIN, the investigators compared the risk of mortality among 14,042 subjects who had OA, were aged 50-89 years old, and had had or had not had KR.
They discovered a strong protective effect of KR on all-cause long-term mortality risk, particularly among the adults over 63 years of age.
For example, people who had undergone KR had a 28% lower risk of mortality than did non-KR subjects (hazard ratio, 0.72; 95% confidence interval, 0.66-0.78).
In the overall propensity score–matched study sample, crude mortality per 1,000 person-years (total person-years) for the KR and non-KR cohorts were 19 (61,015) and 25 (58,294), respectively.
However, despite their best efforts, the researchers said the results showed evidence of residual confounding.
“For example, the observation of improved survival immediately after KR, despite the expectation of potential short-term increased postoperative mortality risk supports the presence of residual confounding,” they wrote.
Another finding suggestive of confounding was that the protective effect was seen only in older patients (over 63) when the authors stratified study participants by age.
“While it is possible that survival benefit seen in older patients with KR is a true effect because it is in this group that greater physical activity is particularly important to survival, more likely it is a result of residual confounding because subject selection is rigorous in this age group due to vulnerability,” the authors wrote.
They concluded that knee replacement “did not appear to be associated with an increased risk of all-cause mortality.”
“While we cannot rule out that KR may potentially reduce the risk of mortality over the long term, the true extent of that potential benefit is difficult to discern due to confounding by indication in observational studies using administrative data or electronic health records,” they added.
This study was funded by the Arthritis Foundation Postdoctoral Fellowship Award, the ACR Rheumatology Research Foundation Investigator Award, and a Boston University scholarship grant.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:Knee replacement surgery in people with OA showed a protective effect on mortality, but residual confounding in the study makes it challenging to definitively conclude whether the surgery conferred a long-term mortality benefit.
Major finding: Subjects who had undergone a knee replacement had a 28% lower risk of mortality than non-KR subjects (HR, 0.72; 95% CI, 0.66-0.78).
Data source: Population-based time-varying propensity score–matched cohort of 14,042 subjects with OA aged 50-89 years with and without knee replacement.
Disclosures: This study was funded by the Arthritis Foundation Postdoctoral Fellowship Award, the ACR Rheumatology Research Foundation Investigator Award, and a Boston University scholarship grant.
FDA Strengthens Kidney Warnings on Two Diabetes Drugs
The Food and Drug Administration has revised warnings on certain medications for type 2 diabetes mellitus based on recent reports of kidney injury. The warning labels on canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR) now include recommendations on ways to minimize the risk and more information on kidney injury.
“From March 2013, when canagliflozin was approved, to October 2015, FDA received reports of 101 confirmable cases of acute kidney injury, some requiring hospitalization and dialysis, with canagliflozin or dapagliflozin use,” the FDA statement said.
The FDA went on to note: “Health care professionals should consider factors that may predispose patients to acute kidney injury prior to starting them on canagliflozin or dapagliflozin. These include decreased blood volume; chronic kidney insufficiency; congestive heart failure; and taking other medications such as diuretics, blood pressure medicines called angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), and nonsteroidal anti-inflammatory drugs (NSAIDs). Assess kidney function prior to starting canagliflozin or dapagliflozin and monitor periodically thereafter. If acute kidney injury occurs, promptly discontinue the drug and treat the kidney impairment.”
The Food and Drug Administration has revised warnings on certain medications for type 2 diabetes mellitus based on recent reports of kidney injury. The warning labels on canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR) now include recommendations on ways to minimize the risk and more information on kidney injury.
“From March 2013, when canagliflozin was approved, to October 2015, FDA received reports of 101 confirmable cases of acute kidney injury, some requiring hospitalization and dialysis, with canagliflozin or dapagliflozin use,” the FDA statement said.
The FDA went on to note: “Health care professionals should consider factors that may predispose patients to acute kidney injury prior to starting them on canagliflozin or dapagliflozin. These include decreased blood volume; chronic kidney insufficiency; congestive heart failure; and taking other medications such as diuretics, blood pressure medicines called angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), and nonsteroidal anti-inflammatory drugs (NSAIDs). Assess kidney function prior to starting canagliflozin or dapagliflozin and monitor periodically thereafter. If acute kidney injury occurs, promptly discontinue the drug and treat the kidney impairment.”
The Food and Drug Administration has revised warnings on certain medications for type 2 diabetes mellitus based on recent reports of kidney injury. The warning labels on canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR) now include recommendations on ways to minimize the risk and more information on kidney injury.
“From March 2013, when canagliflozin was approved, to October 2015, FDA received reports of 101 confirmable cases of acute kidney injury, some requiring hospitalization and dialysis, with canagliflozin or dapagliflozin use,” the FDA statement said.
The FDA went on to note: “Health care professionals should consider factors that may predispose patients to acute kidney injury prior to starting them on canagliflozin or dapagliflozin. These include decreased blood volume; chronic kidney insufficiency; congestive heart failure; and taking other medications such as diuretics, blood pressure medicines called angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), and nonsteroidal anti-inflammatory drugs (NSAIDs). Assess kidney function prior to starting canagliflozin or dapagliflozin and monitor periodically thereafter. If acute kidney injury occurs, promptly discontinue the drug and treat the kidney impairment.”
FDA strengthens kidney warnings on two diabetes drugs
The Food and Drug Administration has revised warnings on certain medications for type 2 diabetes mellitus based on recent reports of kidney injury. The warning labels on canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR) now include recommendations on ways to minimize the risk and more information on kidney injury.
“From March 2013, when canagliflozin was approved, to October 2015, FDA received reports of 101 confirmable cases of acute kidney injury, some requiring hospitalization and dialysis, with canagliflozin or dapagliflozin use,” the FDA statement said.
The FDA went on to note: “Health care professionals should consider factors that may predispose patients to acute kidney injury prior to starting them on canagliflozin or dapagliflozin. These include decreased blood volume; chronic kidney insufficiency; congestive heart failure; and taking other medications such as diuretics, blood pressure medicines called angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), and nonsteroidal anti-inflammatory drugs (NSAIDs). Assess kidney function prior to starting canagliflozin or dapagliflozin and monitor periodically thereafter. If acute kidney injury occurs, promptly discontinue the drug and treat the kidney impairment.”
The Food and Drug Administration has revised warnings on certain medications for type 2 diabetes mellitus based on recent reports of kidney injury. The warning labels on canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR) now include recommendations on ways to minimize the risk and more information on kidney injury.
“From March 2013, when canagliflozin was approved, to October 2015, FDA received reports of 101 confirmable cases of acute kidney injury, some requiring hospitalization and dialysis, with canagliflozin or dapagliflozin use,” the FDA statement said.
The FDA went on to note: “Health care professionals should consider factors that may predispose patients to acute kidney injury prior to starting them on canagliflozin or dapagliflozin. These include decreased blood volume; chronic kidney insufficiency; congestive heart failure; and taking other medications such as diuretics, blood pressure medicines called angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), and nonsteroidal anti-inflammatory drugs (NSAIDs). Assess kidney function prior to starting canagliflozin or dapagliflozin and monitor periodically thereafter. If acute kidney injury occurs, promptly discontinue the drug and treat the kidney impairment.”
The Food and Drug Administration has revised warnings on certain medications for type 2 diabetes mellitus based on recent reports of kidney injury. The warning labels on canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR) now include recommendations on ways to minimize the risk and more information on kidney injury.
“From March 2013, when canagliflozin was approved, to October 2015, FDA received reports of 101 confirmable cases of acute kidney injury, some requiring hospitalization and dialysis, with canagliflozin or dapagliflozin use,” the FDA statement said.
The FDA went on to note: “Health care professionals should consider factors that may predispose patients to acute kidney injury prior to starting them on canagliflozin or dapagliflozin. These include decreased blood volume; chronic kidney insufficiency; congestive heart failure; and taking other medications such as diuretics, blood pressure medicines called angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), and nonsteroidal anti-inflammatory drugs (NSAIDs). Assess kidney function prior to starting canagliflozin or dapagliflozin and monitor periodically thereafter. If acute kidney injury occurs, promptly discontinue the drug and treat the kidney impairment.”
Women underrepresented as GI division chiefs
SAN DIEGO – Despite an increase in women entering the gastroenterology field, a new study finds that they are severely underrepresented in academia as fellowship program directors and division chiefs.
In addition, most female program directors have lower academic ranks than did their male counterparts.
“A lack of female leadership can detract future women from entering the field,” said study lead author Dr. Sonali Paul, a gastroenterologist and research fellow in medicine at Massachusetts General Hospital in Boston. “Further research is needed to understand these barriers, whether they’re due to personal choice or more systemic issues. Understanding the challenges women face in academic promotions will allow for policy changes.”
According to statistics quoted in the study, women made up more than a third of gastroenterology fellowship positions and 18% of the academic workforce in 2013. At the same time, more than half of medical graduates are women.
“The role of women in GI is important, especially given that studies have shown that women prefer female endoscopists,” Dr. Paul said. And women make up significant portions of the patient load in GI clinical practices, she said.
But it hasn’t been clear if the increase in fellowship positions has corresponded to a higher number of women in positions of academic leadership.
To better understand the situation, Dr. Paul and her associates analyzed the gender and academic rank of GI fellowship program directors and division chiefs in 2015. The researchers analyzed websites and social media to gather the data and also made direct contacts, Dr. Paul said.
At 165 academic GI programs, 85% of these positions were held by men. Of program directors, 81% were male; 89% of division chiefs were male.
Of the men who hold these positions, half were program directors and half were division chiefs. Among women, however, 67% were program directors and 33% were division chiefs.
There was also a disparity in terms of academic experience. Female division chiefs were less likely to be professors (46%), compared with male division chiefs (82%).
Why the disparity? One possible explanation is a “leaky pipeline,” Dr. Paul said. This refers to the fact that women leave academic medicine at higher rates than men do.
Family obligations are one explanation, she said, “but I don’t think that explains everything in the process. A lot of women have felt that promotion was of no benefit to them and that there was a lack of encouragement.”
Dr. Paul notes that the study has limitations. It’s cross sectional, and misclassification errors are possible.
During a discussion period, a questioner noted that it takes time for an academic physician to move up: “Shouldn’t we look at the fraction of graduates in 1996 who were female and now are professors? It’s unusual for someone at less than a professor rank to be division chief, as compared with program director.” Dr. Paul agreed that this would be a good avenue of research.
In addition, she said, “it will be important to examine if these gender disparities are unique to gastroenterology or if they extend into other specialties that are more female dominant, such as rheumatology or geriatrics.”
SAN DIEGO – Despite an increase in women entering the gastroenterology field, a new study finds that they are severely underrepresented in academia as fellowship program directors and division chiefs.
In addition, most female program directors have lower academic ranks than did their male counterparts.
“A lack of female leadership can detract future women from entering the field,” said study lead author Dr. Sonali Paul, a gastroenterologist and research fellow in medicine at Massachusetts General Hospital in Boston. “Further research is needed to understand these barriers, whether they’re due to personal choice or more systemic issues. Understanding the challenges women face in academic promotions will allow for policy changes.”
According to statistics quoted in the study, women made up more than a third of gastroenterology fellowship positions and 18% of the academic workforce in 2013. At the same time, more than half of medical graduates are women.
“The role of women in GI is important, especially given that studies have shown that women prefer female endoscopists,” Dr. Paul said. And women make up significant portions of the patient load in GI clinical practices, she said.
But it hasn’t been clear if the increase in fellowship positions has corresponded to a higher number of women in positions of academic leadership.
To better understand the situation, Dr. Paul and her associates analyzed the gender and academic rank of GI fellowship program directors and division chiefs in 2015. The researchers analyzed websites and social media to gather the data and also made direct contacts, Dr. Paul said.
At 165 academic GI programs, 85% of these positions were held by men. Of program directors, 81% were male; 89% of division chiefs were male.
Of the men who hold these positions, half were program directors and half were division chiefs. Among women, however, 67% were program directors and 33% were division chiefs.
There was also a disparity in terms of academic experience. Female division chiefs were less likely to be professors (46%), compared with male division chiefs (82%).
Why the disparity? One possible explanation is a “leaky pipeline,” Dr. Paul said. This refers to the fact that women leave academic medicine at higher rates than men do.
Family obligations are one explanation, she said, “but I don’t think that explains everything in the process. A lot of women have felt that promotion was of no benefit to them and that there was a lack of encouragement.”
Dr. Paul notes that the study has limitations. It’s cross sectional, and misclassification errors are possible.
During a discussion period, a questioner noted that it takes time for an academic physician to move up: “Shouldn’t we look at the fraction of graduates in 1996 who were female and now are professors? It’s unusual for someone at less than a professor rank to be division chief, as compared with program director.” Dr. Paul agreed that this would be a good avenue of research.
In addition, she said, “it will be important to examine if these gender disparities are unique to gastroenterology or if they extend into other specialties that are more female dominant, such as rheumatology or geriatrics.”
SAN DIEGO – Despite an increase in women entering the gastroenterology field, a new study finds that they are severely underrepresented in academia as fellowship program directors and division chiefs.
In addition, most female program directors have lower academic ranks than did their male counterparts.
“A lack of female leadership can detract future women from entering the field,” said study lead author Dr. Sonali Paul, a gastroenterologist and research fellow in medicine at Massachusetts General Hospital in Boston. “Further research is needed to understand these barriers, whether they’re due to personal choice or more systemic issues. Understanding the challenges women face in academic promotions will allow for policy changes.”
According to statistics quoted in the study, women made up more than a third of gastroenterology fellowship positions and 18% of the academic workforce in 2013. At the same time, more than half of medical graduates are women.
“The role of women in GI is important, especially given that studies have shown that women prefer female endoscopists,” Dr. Paul said. And women make up significant portions of the patient load in GI clinical practices, she said.
But it hasn’t been clear if the increase in fellowship positions has corresponded to a higher number of women in positions of academic leadership.
To better understand the situation, Dr. Paul and her associates analyzed the gender and academic rank of GI fellowship program directors and division chiefs in 2015. The researchers analyzed websites and social media to gather the data and also made direct contacts, Dr. Paul said.
At 165 academic GI programs, 85% of these positions were held by men. Of program directors, 81% were male; 89% of division chiefs were male.
Of the men who hold these positions, half were program directors and half were division chiefs. Among women, however, 67% were program directors and 33% were division chiefs.
There was also a disparity in terms of academic experience. Female division chiefs were less likely to be professors (46%), compared with male division chiefs (82%).
Why the disparity? One possible explanation is a “leaky pipeline,” Dr. Paul said. This refers to the fact that women leave academic medicine at higher rates than men do.
Family obligations are one explanation, she said, “but I don’t think that explains everything in the process. A lot of women have felt that promotion was of no benefit to them and that there was a lack of encouragement.”
Dr. Paul notes that the study has limitations. It’s cross sectional, and misclassification errors are possible.
During a discussion period, a questioner noted that it takes time for an academic physician to move up: “Shouldn’t we look at the fraction of graduates in 1996 who were female and now are professors? It’s unusual for someone at less than a professor rank to be division chief, as compared with program director.” Dr. Paul agreed that this would be a good avenue of research.
In addition, she said, “it will be important to examine if these gender disparities are unique to gastroenterology or if they extend into other specialties that are more female dominant, such as rheumatology or geriatrics.”
AT DDW® 2016
Delaying renal replacement therapy in critically ill patients has advantages
SAN FRANCISCO – Delaying initiation of renal replacement therapy in critically ill patients with severe acute kidney injury appears to be not only safe but beneficial, according to a randomized controlled trial conducted in France.
The trial, known as Artificial Kidney Initiation in Kidney Injury (AKIKI), was conducted among 620 adult patients from 31 intensive care units. The investigators were led by Dr. Stéphane Gaudry of Assistance Publique–Hôpitaux de Paris.
The death rate did not differ significantly between groups assigned to an early versus a late initiation strategy, according to results presented at an international conference of the American Thoracic Society and simultaneously published (N Engl J Med. 2016 May 15. doi: 10.1056/NEJMoa1603017).
Moreover, nearly half of the patients in the delayed initiation group were able to avoid renal replacement therapy. And they were less likely to develop bloodstream infections and had more rapid onset of diuresis (heralding recovery of renal function) than did peers in whom the therapy was initiated early.
“Our study should not be interpreted as suggesting that a ‘wait and see’ approach is safe for all patients. Indeed, careful surveillance is mandatory when deciding to delay renal-replacement therapy in patients with severe acute kidney injury so that any complications will be detected and renal-replacement therapy initiated without delay,” the investigators concluded. “In our trial, delaying the initiation of therapy allowed many patients to recover from acute kidney injury without embarking on such a treatment course.”
Further, the “findings may not be generalizable, because more than 50% of the patients in our trial received intermittent hemodialysis as the first method of therapy and only 30% of the patients received continuous renal-replacement therapy as the sole method (with no intermittent dialysis at any time).”
The author of an accompanying editorial, Dr. Ravindra L. Mehta of the University of California, San Diego, lists some caveats in interpreting the trial’s findings as support for the delayed initiation strategy.
For example, he notes, the longer time to initiation with the delayed strategy contributed to worsening of metabolic and clinical status in the patients who ultimately did need therapy; the study did not assess the development of chronic kidney disease; and the types of renal replacement therapy selected for patients seem “surprising” as the majority put on this therapy needed vasopressors.
“The findings highlight a need for dynamic risk-stratification tools to identify patients who will not need renal-replacement therapy for management of their acute kidney injury,” Dr. Mehta concluded, noting that ongoing studies should help inform management in this area. “Meanwhile, we should focus on the timely application of renal-replacement therapy while considering individual patient characteristics, process-of-care elements, and logistics to achieve therapeutic goals …”
Patients were eligible for the trial if they had severe acute kidney injury, defined as Kidney Disease: Improving Global Outcomes (KDIGO) stage 3; required mechanical ventilation, catecholamine infusion, or both; and did not have a potentially life-threatening complication directly related to renal failure.
In those assigned to the early strategy, renal replacement therapy was started immediately after randomization. In those assigned to the delayed strategy, it was started if any of several criteria was met: severe hyperkalemia, metabolic acidosis, pulmonary edema, blood urea nitrogen level higher than 112 mg/dL, or oliguria for more than 72 hours after randomization. The specific type of renal replacement therapy was left up to each study site.
The median time between randomization and initiation of renal replacement therapy was 2 hours in the early strategy group and 57 hours in the delayed strategy group.
The estimated 60-day mortality rate – the trial’s primary outcome – was 48.5% with early initiation of therapy and 49.7% with delayed initiation, a nonsignificant difference.
Fully 49% of patients in the delayed strategy group never received renal replacement therapy. In addition, patients in this group were half as likely as were peers in the early initiation group to develop a bloodstream infection (5% vs. 10%), and they had more rapid onset of diuresis (P less than .001).
The groups were essentially the same with respect to the rate of gastrointestinal bleeding and the lengths of stay in the intensive care unit and in the hospital.
Dr. Gaudry disclosed that he received grant support from the French Ministry of Health during the study, and from XENIOS France outside the research. The trial was supported by a grant from Programme Hospitalier de Recherche Clinique National, 2012 (AOM12456), funded by the French Ministry of Health..
SAN FRANCISCO – Delaying initiation of renal replacement therapy in critically ill patients with severe acute kidney injury appears to be not only safe but beneficial, according to a randomized controlled trial conducted in France.
The trial, known as Artificial Kidney Initiation in Kidney Injury (AKIKI), was conducted among 620 adult patients from 31 intensive care units. The investigators were led by Dr. Stéphane Gaudry of Assistance Publique–Hôpitaux de Paris.
The death rate did not differ significantly between groups assigned to an early versus a late initiation strategy, according to results presented at an international conference of the American Thoracic Society and simultaneously published (N Engl J Med. 2016 May 15. doi: 10.1056/NEJMoa1603017).
Moreover, nearly half of the patients in the delayed initiation group were able to avoid renal replacement therapy. And they were less likely to develop bloodstream infections and had more rapid onset of diuresis (heralding recovery of renal function) than did peers in whom the therapy was initiated early.
“Our study should not be interpreted as suggesting that a ‘wait and see’ approach is safe for all patients. Indeed, careful surveillance is mandatory when deciding to delay renal-replacement therapy in patients with severe acute kidney injury so that any complications will be detected and renal-replacement therapy initiated without delay,” the investigators concluded. “In our trial, delaying the initiation of therapy allowed many patients to recover from acute kidney injury without embarking on such a treatment course.”
Further, the “findings may not be generalizable, because more than 50% of the patients in our trial received intermittent hemodialysis as the first method of therapy and only 30% of the patients received continuous renal-replacement therapy as the sole method (with no intermittent dialysis at any time).”
The author of an accompanying editorial, Dr. Ravindra L. Mehta of the University of California, San Diego, lists some caveats in interpreting the trial’s findings as support for the delayed initiation strategy.
For example, he notes, the longer time to initiation with the delayed strategy contributed to worsening of metabolic and clinical status in the patients who ultimately did need therapy; the study did not assess the development of chronic kidney disease; and the types of renal replacement therapy selected for patients seem “surprising” as the majority put on this therapy needed vasopressors.
“The findings highlight a need for dynamic risk-stratification tools to identify patients who will not need renal-replacement therapy for management of their acute kidney injury,” Dr. Mehta concluded, noting that ongoing studies should help inform management in this area. “Meanwhile, we should focus on the timely application of renal-replacement therapy while considering individual patient characteristics, process-of-care elements, and logistics to achieve therapeutic goals …”
Patients were eligible for the trial if they had severe acute kidney injury, defined as Kidney Disease: Improving Global Outcomes (KDIGO) stage 3; required mechanical ventilation, catecholamine infusion, or both; and did not have a potentially life-threatening complication directly related to renal failure.
In those assigned to the early strategy, renal replacement therapy was started immediately after randomization. In those assigned to the delayed strategy, it was started if any of several criteria was met: severe hyperkalemia, metabolic acidosis, pulmonary edema, blood urea nitrogen level higher than 112 mg/dL, or oliguria for more than 72 hours after randomization. The specific type of renal replacement therapy was left up to each study site.
The median time between randomization and initiation of renal replacement therapy was 2 hours in the early strategy group and 57 hours in the delayed strategy group.
The estimated 60-day mortality rate – the trial’s primary outcome – was 48.5% with early initiation of therapy and 49.7% with delayed initiation, a nonsignificant difference.
Fully 49% of patients in the delayed strategy group never received renal replacement therapy. In addition, patients in this group were half as likely as were peers in the early initiation group to develop a bloodstream infection (5% vs. 10%), and they had more rapid onset of diuresis (P less than .001).
The groups were essentially the same with respect to the rate of gastrointestinal bleeding and the lengths of stay in the intensive care unit and in the hospital.
Dr. Gaudry disclosed that he received grant support from the French Ministry of Health during the study, and from XENIOS France outside the research. The trial was supported by a grant from Programme Hospitalier de Recherche Clinique National, 2012 (AOM12456), funded by the French Ministry of Health..
SAN FRANCISCO – Delaying initiation of renal replacement therapy in critically ill patients with severe acute kidney injury appears to be not only safe but beneficial, according to a randomized controlled trial conducted in France.
The trial, known as Artificial Kidney Initiation in Kidney Injury (AKIKI), was conducted among 620 adult patients from 31 intensive care units. The investigators were led by Dr. Stéphane Gaudry of Assistance Publique–Hôpitaux de Paris.
The death rate did not differ significantly between groups assigned to an early versus a late initiation strategy, according to results presented at an international conference of the American Thoracic Society and simultaneously published (N Engl J Med. 2016 May 15. doi: 10.1056/NEJMoa1603017).
Moreover, nearly half of the patients in the delayed initiation group were able to avoid renal replacement therapy. And they were less likely to develop bloodstream infections and had more rapid onset of diuresis (heralding recovery of renal function) than did peers in whom the therapy was initiated early.
“Our study should not be interpreted as suggesting that a ‘wait and see’ approach is safe for all patients. Indeed, careful surveillance is mandatory when deciding to delay renal-replacement therapy in patients with severe acute kidney injury so that any complications will be detected and renal-replacement therapy initiated without delay,” the investigators concluded. “In our trial, delaying the initiation of therapy allowed many patients to recover from acute kidney injury without embarking on such a treatment course.”
Further, the “findings may not be generalizable, because more than 50% of the patients in our trial received intermittent hemodialysis as the first method of therapy and only 30% of the patients received continuous renal-replacement therapy as the sole method (with no intermittent dialysis at any time).”
The author of an accompanying editorial, Dr. Ravindra L. Mehta of the University of California, San Diego, lists some caveats in interpreting the trial’s findings as support for the delayed initiation strategy.
For example, he notes, the longer time to initiation with the delayed strategy contributed to worsening of metabolic and clinical status in the patients who ultimately did need therapy; the study did not assess the development of chronic kidney disease; and the types of renal replacement therapy selected for patients seem “surprising” as the majority put on this therapy needed vasopressors.
“The findings highlight a need for dynamic risk-stratification tools to identify patients who will not need renal-replacement therapy for management of their acute kidney injury,” Dr. Mehta concluded, noting that ongoing studies should help inform management in this area. “Meanwhile, we should focus on the timely application of renal-replacement therapy while considering individual patient characteristics, process-of-care elements, and logistics to achieve therapeutic goals …”
Patients were eligible for the trial if they had severe acute kidney injury, defined as Kidney Disease: Improving Global Outcomes (KDIGO) stage 3; required mechanical ventilation, catecholamine infusion, or both; and did not have a potentially life-threatening complication directly related to renal failure.
In those assigned to the early strategy, renal replacement therapy was started immediately after randomization. In those assigned to the delayed strategy, it was started if any of several criteria was met: severe hyperkalemia, metabolic acidosis, pulmonary edema, blood urea nitrogen level higher than 112 mg/dL, or oliguria for more than 72 hours after randomization. The specific type of renal replacement therapy was left up to each study site.
The median time between randomization and initiation of renal replacement therapy was 2 hours in the early strategy group and 57 hours in the delayed strategy group.
The estimated 60-day mortality rate – the trial’s primary outcome – was 48.5% with early initiation of therapy and 49.7% with delayed initiation, a nonsignificant difference.
Fully 49% of patients in the delayed strategy group never received renal replacement therapy. In addition, patients in this group were half as likely as were peers in the early initiation group to develop a bloodstream infection (5% vs. 10%), and they had more rapid onset of diuresis (P less than .001).
The groups were essentially the same with respect to the rate of gastrointestinal bleeding and the lengths of stay in the intensive care unit and in the hospital.
Dr. Gaudry disclosed that he received grant support from the French Ministry of Health during the study, and from XENIOS France outside the research. The trial was supported by a grant from Programme Hospitalier de Recherche Clinique National, 2012 (AOM12456), funded by the French Ministry of Health..
AT ATS 2016
Key clinical point: Delaying initiation of renal replacement therapy in critically ill patients with kidney injury appears safe and beneficial.
Major finding: The estimated 60-day mortality rate did not differ significantly between the early and delayed initiation strategies (48.5% vs. 49.7%).
Data source: A randomized controlled trial of 620 critically ill patients with severe acute kidney injury.
Disclosures: Dr. Gaudry disclosed that he received grant support from the French Ministry of Health during the study, and from XENIOS France outside the research. The trial was supported by a grant from Programme Hospitalier de Recherche Clinique National, 2012 (AOM12456), funded by the French Ministry of Health.
Driverless health care
Health care is changing. For some, this is cause for celebration: A safer, convenient, more efficient system is upon us. For others, the end is nigh: impossibly demanding patients, crushing bureaucracy, and a once sacred relationship desecrated by invasive technology.
The ascent of digital health technologies is an important driver of health care change, yet its impact is still indeterminate. Any technology that empowers patients as well as physicians will improve patients’ health outcomes. Or so it might seem. The truth is more nuanced: Some services will improve outcomes, others won’t. Fitting Fitbits into our current system is like inserting the wrong key into a lock: It might go in, but it doesn’t open anything. Fortunately, some keys are opening doors to better care – doors that that we’ve never entered – but finding the right ones is laborious. Dr. Joe Kvedar is here to help.
As a physician leader who straddles the gap between physician-centered and consumer-centered health care, Dr. Kvedar has spent his career leveraging technology to improve care delivery for both. In his new book, “The Internet of Healthy Things,” he shares what he has learned. He uses numerous examples from his experience as a physician and pioneer in digital health care with Partners HealthCare, Boston, delving deeply into the business of health care and the behavioral habits of patients.
As he notes, Partners was “prescient” in the health care landscape, introducing video conferencing in the 1990s, second opinions on the Internet in 2001, and texting as a tool for health messaging in 2008. He asks now: “What are the connected health devices and applications that our clinicians will be using in 5-10 years?” Then he uses his acumen and research to answer his own question. The ensuing chapters are more prescriptive than predictive, however. None of us knows where health care will be in 10 years, but we should think about where it ought to be.
Whether you chart on an Apple Watch or on paper, this discussion is important to you. Physicians are key players in determining where and how medicine is practiced, and we need to understand relevant risks and benefits to make the right decisions.
Confusing the matter is that desired outcomes are not absolute but relative. It depends on the frame of reference. Patients measure outcomes with service, payers with cost, and we physicians with quality. Which measurement is correct? How can we know if a remote monitoring device is worthwhile if we can’t agree on what it delivers? Is it simply sending home “the sicker even quicker?” Does a Big Pharma beyond-the-pill app really only increase consumption of the costliest medications or create more affordable alternatives?
Technologies that increase access to services such as live chat, messaging, and monitoring may be preferred by patients, but physicians see them as piling on to backbreaking loads. More artificial intelligence is needed to enable these services without requiring physician work. We need driverless health care.
Keeping patients involved has a whole other set of requirements. The tools must be easy, the information personal, the data actionable, and its use Candy-Crush-Saga addictive. This is no small feat, but there is hope.
Partner’s Text 2 Move program, which Dr. Kvedar describes as the “gold standard of what learning about your consumer means,” showed that highly personalized, targeted text messages could have a significant impact on patients’ behavior and health. It is just this type of technology that many are relentlessly pursuing to deliver care more effectively.
Dr. Kvedar devotes significant attention to the patient/consumer experience in a thoughtful, complex manner. Rather than elevate or denigrate the rise of the engaged patient, he examines this phenomenon through several lenses, addressing equally the concerns of practicing physicians and health care entrepreneurs. Nearly 20 years ago, Regina Herzlinger of Harvard Business School, Boston, predicted the rise of health care consumerism. Retail clinics, direct-to-consumer services, and the “Yelpification” of health care are signals that we’ve arrived.
It’s tough to make predictions, especially about the future, Yogi Berra warned us. The book’s mention of Theranos, the failing pinprick blood lab company founded by celebrity Stanford student Elizabeth Holmes, is an example of how risky it is to place bets on where we are headed and how quickly we will get there. DIY at-home labs are further off than they appeared, and that is the hazard of any such books: it is difficult to see more than what’s just in front of us.
Ultimately, Dr. Kvedar’s message is as realistic as it is optimistic: “The same information that could help drive healthcare costs down can be used to create highly individualized programs that will help people stay healthier and happier.” But we should resist the urge to ask “Are we there yet?” No. We’ve a ways to go.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Health care is changing. For some, this is cause for celebration: A safer, convenient, more efficient system is upon us. For others, the end is nigh: impossibly demanding patients, crushing bureaucracy, and a once sacred relationship desecrated by invasive technology.
The ascent of digital health technologies is an important driver of health care change, yet its impact is still indeterminate. Any technology that empowers patients as well as physicians will improve patients’ health outcomes. Or so it might seem. The truth is more nuanced: Some services will improve outcomes, others won’t. Fitting Fitbits into our current system is like inserting the wrong key into a lock: It might go in, but it doesn’t open anything. Fortunately, some keys are opening doors to better care – doors that that we’ve never entered – but finding the right ones is laborious. Dr. Joe Kvedar is here to help.
As a physician leader who straddles the gap between physician-centered and consumer-centered health care, Dr. Kvedar has spent his career leveraging technology to improve care delivery for both. In his new book, “The Internet of Healthy Things,” he shares what he has learned. He uses numerous examples from his experience as a physician and pioneer in digital health care with Partners HealthCare, Boston, delving deeply into the business of health care and the behavioral habits of patients.
As he notes, Partners was “prescient” in the health care landscape, introducing video conferencing in the 1990s, second opinions on the Internet in 2001, and texting as a tool for health messaging in 2008. He asks now: “What are the connected health devices and applications that our clinicians will be using in 5-10 years?” Then he uses his acumen and research to answer his own question. The ensuing chapters are more prescriptive than predictive, however. None of us knows where health care will be in 10 years, but we should think about where it ought to be.
Whether you chart on an Apple Watch or on paper, this discussion is important to you. Physicians are key players in determining where and how medicine is practiced, and we need to understand relevant risks and benefits to make the right decisions.
Confusing the matter is that desired outcomes are not absolute but relative. It depends on the frame of reference. Patients measure outcomes with service, payers with cost, and we physicians with quality. Which measurement is correct? How can we know if a remote monitoring device is worthwhile if we can’t agree on what it delivers? Is it simply sending home “the sicker even quicker?” Does a Big Pharma beyond-the-pill app really only increase consumption of the costliest medications or create more affordable alternatives?
Technologies that increase access to services such as live chat, messaging, and monitoring may be preferred by patients, but physicians see them as piling on to backbreaking loads. More artificial intelligence is needed to enable these services without requiring physician work. We need driverless health care.
Keeping patients involved has a whole other set of requirements. The tools must be easy, the information personal, the data actionable, and its use Candy-Crush-Saga addictive. This is no small feat, but there is hope.
Partner’s Text 2 Move program, which Dr. Kvedar describes as the “gold standard of what learning about your consumer means,” showed that highly personalized, targeted text messages could have a significant impact on patients’ behavior and health. It is just this type of technology that many are relentlessly pursuing to deliver care more effectively.
Dr. Kvedar devotes significant attention to the patient/consumer experience in a thoughtful, complex manner. Rather than elevate or denigrate the rise of the engaged patient, he examines this phenomenon through several lenses, addressing equally the concerns of practicing physicians and health care entrepreneurs. Nearly 20 years ago, Regina Herzlinger of Harvard Business School, Boston, predicted the rise of health care consumerism. Retail clinics, direct-to-consumer services, and the “Yelpification” of health care are signals that we’ve arrived.
It’s tough to make predictions, especially about the future, Yogi Berra warned us. The book’s mention of Theranos, the failing pinprick blood lab company founded by celebrity Stanford student Elizabeth Holmes, is an example of how risky it is to place bets on where we are headed and how quickly we will get there. DIY at-home labs are further off than they appeared, and that is the hazard of any such books: it is difficult to see more than what’s just in front of us.
Ultimately, Dr. Kvedar’s message is as realistic as it is optimistic: “The same information that could help drive healthcare costs down can be used to create highly individualized programs that will help people stay healthier and happier.” But we should resist the urge to ask “Are we there yet?” No. We’ve a ways to go.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Health care is changing. For some, this is cause for celebration: A safer, convenient, more efficient system is upon us. For others, the end is nigh: impossibly demanding patients, crushing bureaucracy, and a once sacred relationship desecrated by invasive technology.
The ascent of digital health technologies is an important driver of health care change, yet its impact is still indeterminate. Any technology that empowers patients as well as physicians will improve patients’ health outcomes. Or so it might seem. The truth is more nuanced: Some services will improve outcomes, others won’t. Fitting Fitbits into our current system is like inserting the wrong key into a lock: It might go in, but it doesn’t open anything. Fortunately, some keys are opening doors to better care – doors that that we’ve never entered – but finding the right ones is laborious. Dr. Joe Kvedar is here to help.
As a physician leader who straddles the gap between physician-centered and consumer-centered health care, Dr. Kvedar has spent his career leveraging technology to improve care delivery for both. In his new book, “The Internet of Healthy Things,” he shares what he has learned. He uses numerous examples from his experience as a physician and pioneer in digital health care with Partners HealthCare, Boston, delving deeply into the business of health care and the behavioral habits of patients.
As he notes, Partners was “prescient” in the health care landscape, introducing video conferencing in the 1990s, second opinions on the Internet in 2001, and texting as a tool for health messaging in 2008. He asks now: “What are the connected health devices and applications that our clinicians will be using in 5-10 years?” Then he uses his acumen and research to answer his own question. The ensuing chapters are more prescriptive than predictive, however. None of us knows where health care will be in 10 years, but we should think about where it ought to be.
Whether you chart on an Apple Watch or on paper, this discussion is important to you. Physicians are key players in determining where and how medicine is practiced, and we need to understand relevant risks and benefits to make the right decisions.
Confusing the matter is that desired outcomes are not absolute but relative. It depends on the frame of reference. Patients measure outcomes with service, payers with cost, and we physicians with quality. Which measurement is correct? How can we know if a remote monitoring device is worthwhile if we can’t agree on what it delivers? Is it simply sending home “the sicker even quicker?” Does a Big Pharma beyond-the-pill app really only increase consumption of the costliest medications or create more affordable alternatives?
Technologies that increase access to services such as live chat, messaging, and monitoring may be preferred by patients, but physicians see them as piling on to backbreaking loads. More artificial intelligence is needed to enable these services without requiring physician work. We need driverless health care.
Keeping patients involved has a whole other set of requirements. The tools must be easy, the information personal, the data actionable, and its use Candy-Crush-Saga addictive. This is no small feat, but there is hope.
Partner’s Text 2 Move program, which Dr. Kvedar describes as the “gold standard of what learning about your consumer means,” showed that highly personalized, targeted text messages could have a significant impact on patients’ behavior and health. It is just this type of technology that many are relentlessly pursuing to deliver care more effectively.
Dr. Kvedar devotes significant attention to the patient/consumer experience in a thoughtful, complex manner. Rather than elevate or denigrate the rise of the engaged patient, he examines this phenomenon through several lenses, addressing equally the concerns of practicing physicians and health care entrepreneurs. Nearly 20 years ago, Regina Herzlinger of Harvard Business School, Boston, predicted the rise of health care consumerism. Retail clinics, direct-to-consumer services, and the “Yelpification” of health care are signals that we’ve arrived.
It’s tough to make predictions, especially about the future, Yogi Berra warned us. The book’s mention of Theranos, the failing pinprick blood lab company founded by celebrity Stanford student Elizabeth Holmes, is an example of how risky it is to place bets on where we are headed and how quickly we will get there. DIY at-home labs are further off than they appeared, and that is the hazard of any such books: it is difficult to see more than what’s just in front of us.
Ultimately, Dr. Kvedar’s message is as realistic as it is optimistic: “The same information that could help drive healthcare costs down can be used to create highly individualized programs that will help people stay healthier and happier.” But we should resist the urge to ask “Are we there yet?” No. We’ve a ways to go.
Dr. Benabio is a partner physician in the department of dermatology of the Southern California Permanente Group in San Diego and a volunteer clinical assistant professor at the University of California, San Diego. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
A new standard of care for relapsed/refractory MM?

COPENHAGEN—The combination of daratumumab, lenalidomide, and dexamethasone (DRd) could become a new standard of care for patients with relapsed or refractory multiple myeloma (MM), according to a speaker at the 21st Congress of the European Hematology Association.
In the phase 3 POLLUX study, DRd conferred the highest response rate reported to date in the treatment of relapsed/refractory MM.
DRd also significantly improved progression free survival (PFS) when compared to treatment with lenalidomide and dexamethasone (Rd).
In addition, the safety profile of DRd was manageable and consistent with results observed in previous studies, according to Meletios A. Dimopoulos, MD, of the National and Kapodistrian University of Athens in Greece.
Dr Dimopoulos presented these results at the congress as abstract LB2238. The POLLUX study was funded by Janssen Research & Development, LLC.
Treatment and patients
POLLUX was a randomized, double-blind study that enrolled 569 patients with relapsed or refractory MM. Patients had received 1 or more prior lines of therapy and had progressive disease.
The patients were randomized to receive DRd (n=286) or Rd (n=283). All patients received lenalidomide at 25 mg on days 1-21 of each cycle until disease progression. They also received dexamethasone at 40 mg weekly until disease progression.
Patients in the daratumumab arm also received daratumumab at 16 mg/kg once a week in cycles 1-2, every other week in cycles 3-6, and once every 4 weeks until disease progression.
Dr Dimopoulos noted that patient and disease features were equally distributed between the treatment arms. The median age was 65 in both arms (overall range, 34-89). About half of patients in each arm had ISS stage I disease, and patients were, roughly, a median of 4 years from diagnosis.
In both arms, patients had a median of 1 prior lines of therapy (overall range, 1-11). Roughly 60% of patients in both arms had received a prior transplant, 86% had received a proteasome inhibitor, 55% had received an immunomodulatory agent, 18% had prior lenalidomide, and 44% had received a proteasome inhibitor and an immunomodulatory agent.
Roughly 28% of patients in each arm were refractory to a proteasome inhibitor, and nearly 30% were refractory to their last line of therapy.
Results
At a median follow-up of 13.5 months, about 35% of patients had discontinued treatment—23% in the DRd arm and 47% in the Rd arm.
Reasons for discontinuation included disease progression (14% and 34%, respectively), adverse events (7% and 8%, respectively), non-compliance with study drug (0.4% and 2%, respectively), withdrawal by patient (0.4% and 2%, respectively), physician decision (1% and 0.7%, respectively), and death (0.7% and 0.4%, respectively).
The study’s primary endpoint was PFS. The median PFS has not been reached in the DRd arm and was 18.4 months in the Rd arm. The 12-month PFS was 83% and 60%, respectively, and the 18-month PFS was 78% and 52%, respectively.
“There is an unprecedented improvement of progression-free survival in favor of daratumumab with lenalidomide and dexamethasone, with a hazard ratio of 0.37 [95% CI, 0.27-0.52; P<0.0001], which corresponds to a 63% reduction in the risk of progressive disease or death in favor of DRd,” Dr Dimopoulos said.
He also pointed out that the improvement in PFS for the DRd arm was seen across all patient subgroups, regardless of age, ISS stage, prior treatment, and type of MM.
Furthermore, response rates were significantly higher in the DRd arm than in the Rd arm. The overall response rates were 93% and 76%, respectively (P<0.0001). And the complete response rates were 43% and 19%, respectively (P<0.0001).
“This trial was associated with the highest response ever reported—so far, at least—in the treatment of relapsed/refractory myeloma,” Dr Dimopoulos noted.
The median duration of response was not reached in the DRd arm and was 17.4 months in the Rd arm.
In addition, there was an overall survival advantage with DRd. The 18-month overall survival was 86% in the DRd arm and 76% in the Rd arm. The hazard ratio was 0.64 (95% CI, 0.40-1.01; P=0.0534).
As for safety, the most common hematologic adverse events (in the DRd and Rd arms, respectively) were neutropenia (59% and 43%), anemia (31% and 35%), thrombocytopenia (27% for both), lymphopenia (6% and 5%), and febrile neutropenia (6% and 3%).
The most common non-hematologic adverse events (in the DRd and Rd arms, respectively) were diarrhea (43% and 25%), fatigue (35% and 28%), upper respiratory tract infection (32% and 21%), constipation (29% and 25%), cough (29% and 13%), muscle spasms (26% and 19%), and pneumonia (14% and 13%).
“DRd has a manageable safety profile consistent with the known safety profile of daratumumab or Rd alone,” Dr Dimopoulos said. “And we believe the combination of daratumumab with lenalidomide and dexamethasone potentially represents a new standard of care for myeloma patients after 1 or more prior lines of therapy.” ![]()

COPENHAGEN—The combination of daratumumab, lenalidomide, and dexamethasone (DRd) could become a new standard of care for patients with relapsed or refractory multiple myeloma (MM), according to a speaker at the 21st Congress of the European Hematology Association.
In the phase 3 POLLUX study, DRd conferred the highest response rate reported to date in the treatment of relapsed/refractory MM.
DRd also significantly improved progression free survival (PFS) when compared to treatment with lenalidomide and dexamethasone (Rd).
In addition, the safety profile of DRd was manageable and consistent with results observed in previous studies, according to Meletios A. Dimopoulos, MD, of the National and Kapodistrian University of Athens in Greece.
Dr Dimopoulos presented these results at the congress as abstract LB2238. The POLLUX study was funded by Janssen Research & Development, LLC.
Treatment and patients
POLLUX was a randomized, double-blind study that enrolled 569 patients with relapsed or refractory MM. Patients had received 1 or more prior lines of therapy and had progressive disease.
The patients were randomized to receive DRd (n=286) or Rd (n=283). All patients received lenalidomide at 25 mg on days 1-21 of each cycle until disease progression. They also received dexamethasone at 40 mg weekly until disease progression.
Patients in the daratumumab arm also received daratumumab at 16 mg/kg once a week in cycles 1-2, every other week in cycles 3-6, and once every 4 weeks until disease progression.
Dr Dimopoulos noted that patient and disease features were equally distributed between the treatment arms. The median age was 65 in both arms (overall range, 34-89). About half of patients in each arm had ISS stage I disease, and patients were, roughly, a median of 4 years from diagnosis.
In both arms, patients had a median of 1 prior lines of therapy (overall range, 1-11). Roughly 60% of patients in both arms had received a prior transplant, 86% had received a proteasome inhibitor, 55% had received an immunomodulatory agent, 18% had prior lenalidomide, and 44% had received a proteasome inhibitor and an immunomodulatory agent.
Roughly 28% of patients in each arm were refractory to a proteasome inhibitor, and nearly 30% were refractory to their last line of therapy.
Results
At a median follow-up of 13.5 months, about 35% of patients had discontinued treatment—23% in the DRd arm and 47% in the Rd arm.
Reasons for discontinuation included disease progression (14% and 34%, respectively), adverse events (7% and 8%, respectively), non-compliance with study drug (0.4% and 2%, respectively), withdrawal by patient (0.4% and 2%, respectively), physician decision (1% and 0.7%, respectively), and death (0.7% and 0.4%, respectively).
The study’s primary endpoint was PFS. The median PFS has not been reached in the DRd arm and was 18.4 months in the Rd arm. The 12-month PFS was 83% and 60%, respectively, and the 18-month PFS was 78% and 52%, respectively.
“There is an unprecedented improvement of progression-free survival in favor of daratumumab with lenalidomide and dexamethasone, with a hazard ratio of 0.37 [95% CI, 0.27-0.52; P<0.0001], which corresponds to a 63% reduction in the risk of progressive disease or death in favor of DRd,” Dr Dimopoulos said.
He also pointed out that the improvement in PFS for the DRd arm was seen across all patient subgroups, regardless of age, ISS stage, prior treatment, and type of MM.
Furthermore, response rates were significantly higher in the DRd arm than in the Rd arm. The overall response rates were 93% and 76%, respectively (P<0.0001). And the complete response rates were 43% and 19%, respectively (P<0.0001).
“This trial was associated with the highest response ever reported—so far, at least—in the treatment of relapsed/refractory myeloma,” Dr Dimopoulos noted.
The median duration of response was not reached in the DRd arm and was 17.4 months in the Rd arm.
In addition, there was an overall survival advantage with DRd. The 18-month overall survival was 86% in the DRd arm and 76% in the Rd arm. The hazard ratio was 0.64 (95% CI, 0.40-1.01; P=0.0534).
As for safety, the most common hematologic adverse events (in the DRd and Rd arms, respectively) were neutropenia (59% and 43%), anemia (31% and 35%), thrombocytopenia (27% for both), lymphopenia (6% and 5%), and febrile neutropenia (6% and 3%).
The most common non-hematologic adverse events (in the DRd and Rd arms, respectively) were diarrhea (43% and 25%), fatigue (35% and 28%), upper respiratory tract infection (32% and 21%), constipation (29% and 25%), cough (29% and 13%), muscle spasms (26% and 19%), and pneumonia (14% and 13%).
“DRd has a manageable safety profile consistent with the known safety profile of daratumumab or Rd alone,” Dr Dimopoulos said. “And we believe the combination of daratumumab with lenalidomide and dexamethasone potentially represents a new standard of care for myeloma patients after 1 or more prior lines of therapy.” ![]()

COPENHAGEN—The combination of daratumumab, lenalidomide, and dexamethasone (DRd) could become a new standard of care for patients with relapsed or refractory multiple myeloma (MM), according to a speaker at the 21st Congress of the European Hematology Association.
In the phase 3 POLLUX study, DRd conferred the highest response rate reported to date in the treatment of relapsed/refractory MM.
DRd also significantly improved progression free survival (PFS) when compared to treatment with lenalidomide and dexamethasone (Rd).
In addition, the safety profile of DRd was manageable and consistent with results observed in previous studies, according to Meletios A. Dimopoulos, MD, of the National and Kapodistrian University of Athens in Greece.
Dr Dimopoulos presented these results at the congress as abstract LB2238. The POLLUX study was funded by Janssen Research & Development, LLC.
Treatment and patients
POLLUX was a randomized, double-blind study that enrolled 569 patients with relapsed or refractory MM. Patients had received 1 or more prior lines of therapy and had progressive disease.
The patients were randomized to receive DRd (n=286) or Rd (n=283). All patients received lenalidomide at 25 mg on days 1-21 of each cycle until disease progression. They also received dexamethasone at 40 mg weekly until disease progression.
Patients in the daratumumab arm also received daratumumab at 16 mg/kg once a week in cycles 1-2, every other week in cycles 3-6, and once every 4 weeks until disease progression.
Dr Dimopoulos noted that patient and disease features were equally distributed between the treatment arms. The median age was 65 in both arms (overall range, 34-89). About half of patients in each arm had ISS stage I disease, and patients were, roughly, a median of 4 years from diagnosis.
In both arms, patients had a median of 1 prior lines of therapy (overall range, 1-11). Roughly 60% of patients in both arms had received a prior transplant, 86% had received a proteasome inhibitor, 55% had received an immunomodulatory agent, 18% had prior lenalidomide, and 44% had received a proteasome inhibitor and an immunomodulatory agent.
Roughly 28% of patients in each arm were refractory to a proteasome inhibitor, and nearly 30% were refractory to their last line of therapy.
Results
At a median follow-up of 13.5 months, about 35% of patients had discontinued treatment—23% in the DRd arm and 47% in the Rd arm.
Reasons for discontinuation included disease progression (14% and 34%, respectively), adverse events (7% and 8%, respectively), non-compliance with study drug (0.4% and 2%, respectively), withdrawal by patient (0.4% and 2%, respectively), physician decision (1% and 0.7%, respectively), and death (0.7% and 0.4%, respectively).
The study’s primary endpoint was PFS. The median PFS has not been reached in the DRd arm and was 18.4 months in the Rd arm. The 12-month PFS was 83% and 60%, respectively, and the 18-month PFS was 78% and 52%, respectively.
“There is an unprecedented improvement of progression-free survival in favor of daratumumab with lenalidomide and dexamethasone, with a hazard ratio of 0.37 [95% CI, 0.27-0.52; P<0.0001], which corresponds to a 63% reduction in the risk of progressive disease or death in favor of DRd,” Dr Dimopoulos said.
He also pointed out that the improvement in PFS for the DRd arm was seen across all patient subgroups, regardless of age, ISS stage, prior treatment, and type of MM.
Furthermore, response rates were significantly higher in the DRd arm than in the Rd arm. The overall response rates were 93% and 76%, respectively (P<0.0001). And the complete response rates were 43% and 19%, respectively (P<0.0001).
“This trial was associated with the highest response ever reported—so far, at least—in the treatment of relapsed/refractory myeloma,” Dr Dimopoulos noted.
The median duration of response was not reached in the DRd arm and was 17.4 months in the Rd arm.
In addition, there was an overall survival advantage with DRd. The 18-month overall survival was 86% in the DRd arm and 76% in the Rd arm. The hazard ratio was 0.64 (95% CI, 0.40-1.01; P=0.0534).
As for safety, the most common hematologic adverse events (in the DRd and Rd arms, respectively) were neutropenia (59% and 43%), anemia (31% and 35%), thrombocytopenia (27% for both), lymphopenia (6% and 5%), and febrile neutropenia (6% and 3%).
The most common non-hematologic adverse events (in the DRd and Rd arms, respectively) were diarrhea (43% and 25%), fatigue (35% and 28%), upper respiratory tract infection (32% and 21%), constipation (29% and 25%), cough (29% and 13%), muscle spasms (26% and 19%), and pneumonia (14% and 13%).
“DRd has a manageable safety profile consistent with the known safety profile of daratumumab or Rd alone,” Dr Dimopoulos said. “And we believe the combination of daratumumab with lenalidomide and dexamethasone potentially represents a new standard of care for myeloma patients after 1 or more prior lines of therapy.” ![]()
Researchers identify 11 subgroups of AML

Photo courtesy of NIGMS
Using patient samples from 3 prospective multicenter clinical trials of the German-Austrian AML Study Group, researchers have identified 5234 driver mutations involving 76 genes or regions in 1540 patients with acute myeloid leukemia (AML).
They say the sample size afforded a more comprehensive analysis than previously conducted, and as a result, they found patients were divided into at least 11 subgroups of AML.
They also identified 3 genomic categories beyond the existing World Health Organization (WHO) subgroups. These genomic categories are chromatin–spliceosome mutations, TP53–aneuploidy, and provisionally, IDH2R172 mutations.
Their study, led by scientists at the Wellcome Trust Sanger Institute and international collaborators, could have an impact on clinical trial design and improve the way patients are diagnosed and treated in the future.
The scientists stated in their published paper that 736 patients (48%) in their study would not have fit into the molecular groups included in the 2008 WHO classification of adult AML. This prompted them to reevaluate genomic classification of AML from the beginning.
“We have shown that AML is an umbrella term for a group of at least 11 different types of leukemia,” said Peter Campbell, MBChB, PhD, co-leader of the study. “We can now start to decode these genetics to shape clinical trials and develop diagnostics.”
The scientists found NPM1-mutated AML to be the largest class in their cohort, accounting for 27% of the patients.
The second largest subgroup--the chromatin-spliceosome group—accounted for 18% of patients, the TP53-aneuploidy group accounted for 13%, and the IDH2R172 mutations for 1%.
The study also showed that most patients had a unique combination of genetic changes driving their leukemia. This genetic complexity helps explain why AML shows such variability in survival rates among patients.
Under their new schema, the scientists were able to unambiguously classify 80% of the 1540 patients with driver mutations in a single subgroup. Fifty-six of the patients (4%) met criteria for 2 or more categories, primarily in the TP53-aneuploidy and chromatin-spliceosome groups.
They were not able to classify 11% of patients with driver mutations. They explained that these patients might have had mutations in drivers that were either not sequenced or had mutations that were missed.
The scientists applied their classification schema to an independent cohort from the Cancer Genome Atlas (TCGA). The new schema was able to replicate the absence of overlap among subgroups, and relative frequencies of the mutations were equivalent to those in the AML cohort.
“For the first time we untangled the genetic complexity seen in most AML cancer genomes into distinct evolutionary paths that lead to AML,” joint first author Elli Papaemmanuil, PhD, of Memorial Sloan Kettering Cancer Center in New York, said.
“By understanding these paths, we can help develop more appropriate treatments for individual patients with AML. We are now extending such studies across other leukemias."
The investigators recommend prospective clinical trials to further validate the schema.
The work was supported by the Wellcome Trust, Bundesministerium fur Bildung und Forschung, Deutsche Krebshilfe and Deutsche Forschungsgemeinschaft, the European Hematology Association, Amgen and the Kay Kendall Leukaemia Fund. ![]()

Photo courtesy of NIGMS
Using patient samples from 3 prospective multicenter clinical trials of the German-Austrian AML Study Group, researchers have identified 5234 driver mutations involving 76 genes or regions in 1540 patients with acute myeloid leukemia (AML).
They say the sample size afforded a more comprehensive analysis than previously conducted, and as a result, they found patients were divided into at least 11 subgroups of AML.
They also identified 3 genomic categories beyond the existing World Health Organization (WHO) subgroups. These genomic categories are chromatin–spliceosome mutations, TP53–aneuploidy, and provisionally, IDH2R172 mutations.
Their study, led by scientists at the Wellcome Trust Sanger Institute and international collaborators, could have an impact on clinical trial design and improve the way patients are diagnosed and treated in the future.
The scientists stated in their published paper that 736 patients (48%) in their study would not have fit into the molecular groups included in the 2008 WHO classification of adult AML. This prompted them to reevaluate genomic classification of AML from the beginning.
“We have shown that AML is an umbrella term for a group of at least 11 different types of leukemia,” said Peter Campbell, MBChB, PhD, co-leader of the study. “We can now start to decode these genetics to shape clinical trials and develop diagnostics.”
The scientists found NPM1-mutated AML to be the largest class in their cohort, accounting for 27% of the patients.
The second largest subgroup--the chromatin-spliceosome group—accounted for 18% of patients, the TP53-aneuploidy group accounted for 13%, and the IDH2R172 mutations for 1%.
The study also showed that most patients had a unique combination of genetic changes driving their leukemia. This genetic complexity helps explain why AML shows such variability in survival rates among patients.
Under their new schema, the scientists were able to unambiguously classify 80% of the 1540 patients with driver mutations in a single subgroup. Fifty-six of the patients (4%) met criteria for 2 or more categories, primarily in the TP53-aneuploidy and chromatin-spliceosome groups.
They were not able to classify 11% of patients with driver mutations. They explained that these patients might have had mutations in drivers that were either not sequenced or had mutations that were missed.
The scientists applied their classification schema to an independent cohort from the Cancer Genome Atlas (TCGA). The new schema was able to replicate the absence of overlap among subgroups, and relative frequencies of the mutations were equivalent to those in the AML cohort.
“For the first time we untangled the genetic complexity seen in most AML cancer genomes into distinct evolutionary paths that lead to AML,” joint first author Elli Papaemmanuil, PhD, of Memorial Sloan Kettering Cancer Center in New York, said.
“By understanding these paths, we can help develop more appropriate treatments for individual patients with AML. We are now extending such studies across other leukemias."
The investigators recommend prospective clinical trials to further validate the schema.
The work was supported by the Wellcome Trust, Bundesministerium fur Bildung und Forschung, Deutsche Krebshilfe and Deutsche Forschungsgemeinschaft, the European Hematology Association, Amgen and the Kay Kendall Leukaemia Fund. ![]()

Photo courtesy of NIGMS
Using patient samples from 3 prospective multicenter clinical trials of the German-Austrian AML Study Group, researchers have identified 5234 driver mutations involving 76 genes or regions in 1540 patients with acute myeloid leukemia (AML).
They say the sample size afforded a more comprehensive analysis than previously conducted, and as a result, they found patients were divided into at least 11 subgroups of AML.
They also identified 3 genomic categories beyond the existing World Health Organization (WHO) subgroups. These genomic categories are chromatin–spliceosome mutations, TP53–aneuploidy, and provisionally, IDH2R172 mutations.
Their study, led by scientists at the Wellcome Trust Sanger Institute and international collaborators, could have an impact on clinical trial design and improve the way patients are diagnosed and treated in the future.
The scientists stated in their published paper that 736 patients (48%) in their study would not have fit into the molecular groups included in the 2008 WHO classification of adult AML. This prompted them to reevaluate genomic classification of AML from the beginning.
“We have shown that AML is an umbrella term for a group of at least 11 different types of leukemia,” said Peter Campbell, MBChB, PhD, co-leader of the study. “We can now start to decode these genetics to shape clinical trials and develop diagnostics.”
The scientists found NPM1-mutated AML to be the largest class in their cohort, accounting for 27% of the patients.
The second largest subgroup--the chromatin-spliceosome group—accounted for 18% of patients, the TP53-aneuploidy group accounted for 13%, and the IDH2R172 mutations for 1%.
The study also showed that most patients had a unique combination of genetic changes driving their leukemia. This genetic complexity helps explain why AML shows such variability in survival rates among patients.
Under their new schema, the scientists were able to unambiguously classify 80% of the 1540 patients with driver mutations in a single subgroup. Fifty-six of the patients (4%) met criteria for 2 or more categories, primarily in the TP53-aneuploidy and chromatin-spliceosome groups.
They were not able to classify 11% of patients with driver mutations. They explained that these patients might have had mutations in drivers that were either not sequenced or had mutations that were missed.
The scientists applied their classification schema to an independent cohort from the Cancer Genome Atlas (TCGA). The new schema was able to replicate the absence of overlap among subgroups, and relative frequencies of the mutations were equivalent to those in the AML cohort.
“For the first time we untangled the genetic complexity seen in most AML cancer genomes into distinct evolutionary paths that lead to AML,” joint first author Elli Papaemmanuil, PhD, of Memorial Sloan Kettering Cancer Center in New York, said.
“By understanding these paths, we can help develop more appropriate treatments for individual patients with AML. We are now extending such studies across other leukemias."
The investigators recommend prospective clinical trials to further validate the schema.
The work was supported by the Wellcome Trust, Bundesministerium fur Bildung und Forschung, Deutsche Krebshilfe and Deutsche Forschungsgemeinschaft, the European Hematology Association, Amgen and the Kay Kendall Leukaemia Fund. ![]()