User login
Biomechanical Evaluation of All-Polyethylene Pegged Bony Ingrowth Glenoid Fixation Techniques on Implant Micromotion
Since Neer and colleagues1 first reported in 1982, glenoid loosening persists as a common cause of anatomic total shoulder arthroplasty (TSA) failure.1-4 Currently, cemented, all-polyethylene glenoid components are the gold standard, and minimum clinical survival of 10 to 15 years is expected.3,5 Several clinical studies5-9 and in vitro biomechanical studies10 have suggested an advantage of pegged over keeled glenoid components, but glenoid component loosening remains a frequent complication,11 with the cement–implant interface suggested as the weak link of fixation.10,12 In addition to mechanical loosening, poor cement penetration and heat-induced necrosis have been postulated as contributing to glenoid component loosening.13,14
Because of these potential complications, there is a growing consideration to minimize or abandon cement fixation and rely on biological fixation to polyethylene for long-term component stability.15 A newer pegged glenoid component design consists of traditional, peripherally located pegs designed for cement fixation as well as a central, uncemented, fluted, interference-fit peg that allows for bony ingrowth. Short-term clinical studies have shown that bony ingrowth into the space between the flutes can be achieved with a hybrid cementation technique and that, when that occurs, excellent outcomes are likely.13,16-19 The immediate in vivo stability of this implant design upon initial implantation, before the cement has cured, has prompted some surgeons to consider implanting the device without cement. In a recent series in which this implant design was used without cement, clinical and radiographic results were promising.15
Despite the widespread clinical use, little biomechanical work has been done to characterize initial fixation of all-polyethylene pegged glenoid implants. We conducted a study to compare glenoid micromotion in an all-polyethylene, centrally fluted pegged glenoid component as a function of 3 fixation techniques: cementless interference-fit fixation, hybrid partial cementation based on manufacturer recommendations, and full cementation to simulate a gold-standard, traditional, cemented, pegged design.
Materials and MethodsBiomechanical Testing
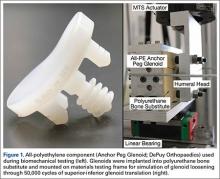
The biomechanical testing methodology used in this study was based on previous studies20-23 and on ASTM standard F2028-1224 using polyurethane bone substitute 0.24 g/cm3 (Pacific Research Laboratories) with ultimate strength of 4.9 MPa and compressive modulus of 123 MPa for component implantation. This material was selected because its mechanical properties are similar to those of cancellous glenoid bone in primary shoulder arthroplasty,25 and it minimizes variability with use of cadaveric specimens. Components were mounted on an MTS 858 Mini-Bionix II materials testing frame (Figure 1). A static compressive load of 756 N (170 lb) was applied via a mass-pulley system simulating the joint compressive force the shoulder is likely to experience during higher load activities.24,26 The glenoid component was positioned on a linear bearing to allow for joint compression.
Test Groups and Cement Fixation Techniques
All-polyethylene pegged glenoid components (Anchor Peg Glenoid, size 44; DePuy Orthopaedics) were used for biomechanical testing (Figure 1). Polyurethane blocks were reamed with a size 44 reamer until the superior-inferior distance reached 33 mm, ensuring complete seating of implant. Three fixation-technique groups were formed: interference-fit, hybrid cement, and fully cemented. Interference-fit fixation was done without polymethylmethacrylate (PMMA) cement. In hybrid fixation, 2 cm3 of PMMA (SpeedSet Cement, Stryker Orthopaedics) was injected (using a catheter tip syringe) into the peripheral peg holes and manually pressurized; the central peg was press-fit into polyurethane bone substitute. In the fully cemented group, both peripheral and central peg holes received PMMA; the peripheral peg holes were cemented as in hybrid fixation, and the central peg hole was injected with 3 cm3 of PMMA, which was then manually pressurized. The humeral head component (Global Advantage, 44×18 mm; DePuy Synthes) was mounted on the test frame actuator and centrally located within the glenoid at the start of the test.
Determination of Humeral Head Translation via Subluxation Testing
Humeral head subluxation distance, simulating a humeral head rim loading event, was calculated on the basis of preliminary tests outlined in the ASTM standard.24 Three glenoids (1 per fixation technique) were mounted on the test frame with a humeral head positioned centrally within the glenoid. After the joint compressive force was applied, the humeral head was translated along the true superior axis of the glenoid at a rate of 50 mm/min. Testing software was used to record humeral head displacement and load data at a frequency of 100 Hz. Humeral head subluxation displacement was determined at the end of the linear region of the force versus displacement response. This distance, averaged from the 3 subluxation tests, was used as the subluxation distance during cyclic testing.
Determination of Glenoid Component Motion via Cyclic Testing
After subluxation displacement was determined, glenoid components were mounted on the test frame (5 per fixation technique) and subjected to 50,000 cycles of humeral head translation at a frequency of 2 Hz. Amplitude of the humeral head displacement against the glenoid component followed a sinusoidal pattern with maxima and minima represented by the subluxation displacement (positive and negative, respectively). Glenoid edge compression/distraction of the superior edge and glenoid inferior/superior translation were monitored with 2 variable resistance reluctance transducers (Microminiature DVRT; 4.5-µm resolution; MicroStrain) secured to the glenoid component and testing fixture.
Microminiature DVRT measurements of glenoid motion were taken for 5 consecutive cycles at cycles 1, 20, 100, 500, 1000, 5000, 10,000, 15,000, 20,000, 30,000, 40,000, and 50,000. Distraction-compression displacement and superior-inferior translation measurements were recorded relative to the glenoid position with the humeral head at the neutral position at a given cycle. Final glenoid micromotion data were calculated from the mean of consecutive cycles at each cycle time point.
Statistical Analysis
Glenoid motion results are reported as means and standard deviations. Comparisons with 2 factors of fixation technique and number of cycles for glenoid distraction, glenoid compression, and absolute glenoid translation were characterized with 2-way analysis of variance (SigmaPlot Version 11.0; Systat Software), with the Holm-Šídák test used for post hoc determination of significant relationships.
Results
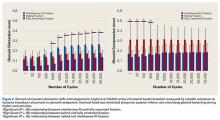
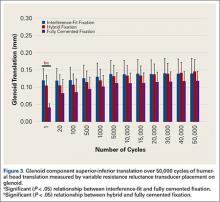
Under subluxation testing, the humeral head translation distance at the end of the linear region was determined to be 0.50 mm. Subsequently for cyclic testing, the humeral head was then translated 0.50 mm from the neutral position of the humeral head along both the superior and inferior axes of the glenoid. All glenoids successfully completed the entire 50,000 cycles of testing. For the glenoid component, Figure 2 depicts distraction and compression, and Figure 3 depicts superior-inferior translation.
Glenoid Component Distraction
Overall, mean (SD) glenoid distraction was significantly higher for interference-fit fixation, 0.21 (0.10) mm, than for hybrid cement fixation, 0.16 (0.05) mm (P < .001), and fully cemented fixation, 0.09 (0.07) mm (P < .001). It was also significantly higher for hybrid fixation than fully cemented fixation (P < .001). From cycle 1000 to cycle 50,000, distraction was significantly higher for interference-fit fixation than for fully cemented fixation at each time point (P < .05).
Glenoid Component Compression
Mean (SD) compression was significantly higher for hybrid cement fixation, 0.31 (0.13) mm, than for interference-fit fixation, 0.17 (0.07) mm (P < .001), and fully cemented fixation, 0.17 (0.08) mm (P < .001). No significant difference was found between interference-fit and fully cemented fixation (P = .793) (Figure 2). At cycles 1, 20, 100, and 500, compression was significantly higher for hybrid fixation than for fully cemented fixation (P < .05). In addition, at cycle 500, it was significantly higher for hybrid fixation than for interference-fit fixation (P < .05).
Glenoid Component Translation
Mean (SD) glenoid translation was significantly lower for fully cemented fixation, 0.10 (0.04) mm, than for interference-fit fixation, 0.13 (0.04) mm (P < .001), and hybrid cement fixation, 0.13 (0.03) mm (P < .001), with all time points considered. There was no significant difference between interference-fit and hybrid fixation (P = .343). Initial translation at cycle 1 was significantly higher for interference-fit and hybid fixation than for fully cemented fixation.
Discussion
Despite advances in glenoid component design, glenoid loosening remains the most common cause of anatomical TSA failure. Recent implants have been designed to take advantage of an all-polyethylene component while providing long-lasting fixation through bony ingrowth into a central peg. In a study of the hybrid cementation technique drescribed here, Groh17 found no glenoid loosening or radiolucent lines but discovered fingerlike projections of bone between the flanges of the implant in 24 (29%) of 83 cases. Churchill and colleagues16 also reported bony ingrowth into the central peg in 15 (75%) of 20 patients. Furthermore, Arnold and colleagues13 reported complete bony ingrowth (6/6 inter-fin compartments) in 23 (71%) of 35 shoulders at a mean of 43 months. Wirth and colleagues19 reported increased radiodensity between the flanges of the central peg in 30 of 44 cases (68%) and osteolysis around the central peg in 3 of 44 cases (7%) at 3 years.
There are also reports of successful bony ingrowth associated with all-polyethylene components implanted without cement. In a canine study using an early ingrowth implant design, Wirth and colleagues27 showed that, though initial fixation was superior with a cemented, keeled implant, pullout strength of the uncemented, pegged implant improved over time and eventually far surpassed that of the cemented, keeled implant owing to both the loosening of the cemented component and the bony ingrowth into the central peg component. Furthermore, Anglin and colleagues10 confirmed that component micromotion was lower with pegged glenoid components than with keeled components in a biomechanical model. De Wilde and colleagues15 recently reported on a series of uncemented, central fluted peg glenoids implanted in 34 patients followed clinically and with computed tomography for a minimum of 24 months. The investigators found bony ingrowth into the central peg in 27 (79%) of 34 patients and no signs of loosening in 30 (88%) of 34 patients. Incomplete lucencies around 1 or 2 peripheral pegs were found in 2 (6%) of 34 patients, and complete lucencies around 2 or more peripheral pegs were found in 2 (6%) of 34 patients. However, there was no statistical difference in clinical outcome between patients with and without loosening.
With this type of implant, initial fixation that provides stability while minimizing micromotion under biomechanical loading likely is crucial for attaining bony growth within the central peg flanges. To our knowledge, this is the first biomechanical study to compare micromotion using 3 different fixation methods with a central fluted peg glenoid component design. Of all these fixation methods, fully cemented fixation yielded the most stable glenoid throughout testing with respect to the evaluated parameters. However, this method is not necessarily clinically applicable, as a fully cemented glenoid would inhibit any bony growth within the central flange, which is necessary for long-term biological fixation. Our data showed that, though glenoid distraction was significantly lower with hybrid cement fixation, this fixation method exhibited significantly higher glenoid compression. In addition, there were no significant differences between glenoid components with hybrid fixation and glenoid components with interference-fit fixation with respect to component translation in the superior-inferior direction. These findings may indicate that initial fixation is not significantly improved by the addition of cement to the peripheral pegs in a glenoid component with a central fluted peg design.
The interference fit of the central peg is primarily responsible for conferring long-term implant stability,13,27 which is ultimately achieved by bony formation between the flutes of the peg. Other authors have reported that, for bony ingrowth to occur, micromotion between the bone–implant interface must not exceed 20 to 150 µm.28-30 Other than for interference-fit distraction at more than 1000 cycles and hybrid cement fixation compression at each time point throughout testing, our data fall within the reported upper limits of micromotion to support bony ingrowth. Increased micromotion in the interference-fit fixation group is seen at later time points and may be caused by the inability to simulate the potential fixation gained from bony ingrowth allowed with this surgical technique. Research is needed to further explain this increase in distraction.
Results from this study must be interpreted with caution because of limitations of the in vitro testing methodology. This biomechanical model using bone substitute characterizes glenoid fixation at time zero, directly after implantation, followed by repetitive cyclic loading simulating 5 years of implant service. This differs from the clinical scenario in which the shoulder undergoes postoperative immobilization or protected motion during which the early phases of bony remodeling are likely occurring. Furthermore, simulation of 5 years of implant service may not be necessary for an implant that is expected to achieve ultimate fixation by bony ingrowth within the first several months after implantation. Use of this implant without cement is classified off-label, and surgeons should take this into consideration during implantation. Last, this study could not simulate the effect of bony ingrowth on fixation, though our experimental technique of cementing the central peg may be a gross approximation of a fully ingrown central peg and its expected rigid fixation.
Fully cemented fixation of a polyethylene glenoid is superior to hybrid cement fixation and interference-fit fixation with respect to early glenoid micromotion. However, the long-term stability of a fully cemented polyethylene glenoid component remains a clinical concern, as fixation is achieved by bony ingrowth around the central fluted peg of the implant. In this study, interference-fit and hybrid fixation had equivocal component micromotion in biomechanical testing. Our findings suggest that cementation of the peripheral pegs confers no additional initial stability over an uncemented interference-fit technique in a biomechanical model. More research is needed to further evaluate interference-fit fixation as a viable option for implantation of a central fluted, all-polyethylene glenoid component.
1. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
2. Sperling JW, Cofield RH, O’Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg. 2000;9(6):507-513.
3. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
4. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
5. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863.
6. Edwards TB, Labriola JE, Stanley RJ, O’Connor DP, Elkousy HA, Gartsman GM. Radiographic comparison of pegged and keeled glenoid components using modern cementing techniques: a prospective randomized study. J Shoulder Elbow Surg. 2010;19(2):251-257.
7. Gartsman GM, Elkousy HA, Warnock KM, Edwards TB, O’Connor DP. Radiographic comparison of pegged and keeled glenoid components. J Shoulder Elbow Surg. 2005;14(3):252-257.
8. Klepps S, Chiang AS, Miller S, Jiang CY, Hazrati Y, Flatow EL. Incidence of early radiolucent glenoid lines in patients having total shoulder replacements. Clin Orthop Relat Res. 2005;(435):118-125.
9. Lazarus MD, Jensen KL, Southworth C, Matsen FA 3rd. The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am. 2002;84(7):1174-1182.
10. Anglin C, Wyss UP, Nyffeler RW, Gerber C. Loosening performance of cemented glenoid prosthesis design pairs. Clin Biomech. 2001;16(2):144-150.
11. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
12. Gregory T, Hansen U, Taillieu F, et al. Glenoid loosening after total shoulder arthroplasty: an in vitro CT-scan study. J Orthop Res. 2009;27(12):1589-1595.
13. Arnold RM, High RR, Grosshans KT, Walker CW, Fehringer EV. Bone presence between the central peg’s radial fins of a partially cemented pegged all poly glenoid component suggest few radiolucencies. J Shoulder Elbow Surg. 2011;20(2):315-321.
14. Churchill RS, Boorman RS, Fehringer EV, Matsen FA 3rd. Glenoid cementing may generate sufficient heat to endanger the surrounding bone. Clin Orthop Relat Res. 2004;(419):76-79.
15. De Wilde L, Dayerizadeh N, De Neve F, Basamania C, Van Tongel A. Fully uncemented glenoid component in total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(10):e1-e7.
16. Churchill RS, Zellmer C, Zimmers HJ, Ruggero R. Clinical and radiographic analysis of a partially cemented glenoid implant: five-year minimum follow-up. J Shoulder Elbow Surg. 2010;19(7):1091-1097.
17. Groh GI. Survival and radiographic analysis of a glenoid component with a cementless fluted central peg. J Shoulder Elbow Surg. 2010;19(8):1265-1268.
18. Vidil A, Valenti P, Guichoux F, Barthas JH. CT scan evaluation of glenoid component fixation: a prospective study of 27 minimally cemented shoulder arthroplasties. Eur J Orthop Surg Traumatol. 2012;23(5):521-525.
19. Wirth MA, Loredo R, Garcia G, Rockwood CA Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94(3):260-267.
20. Anglin C, Wyss UP, Pichora DR. Mechanical testing of shoulder prostheses and recommendations for glenoid design. J Shoulder Elbow Surg. 2000;9(4):323-331.
21. Hoenig MP, Loeffler B, Brown S, et al. Reverse glenoid component fixation: is a posterior screw necessary? J Shoulder Elbow Surg. 2010;19(4):544-549.
22. Sarah J, Sanjay G, Sanjay S, et al. Failure mechanism of the all-polyethylene glenoid implant. J Biomech. 2010;43(4):714-719.
23. Suárez DR, Nerkens W, Valstar ER, Rozing PM, van Keulen F. Interface micromotions increase with less-conforming cementless glenoid components. J Shoulder Elbow Surg. 2012;21(4):474-482.
24. ASTM International. Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation. West Conshocken, PA: ASTM International; 2012. ASTM F2028-08.
25. Anglin C, Tolhurst P, Wyss UP, Pichora DR. Glenoid cancellous bone strength and modulus. J Biomech. 1999;32(10):1091-1097.
26. Anglin C, Wyss U, Pichora D. Glenohumeral contact forces. Proc Inst Mech Eng H. 2000;214(6):637-644.
27. Wirth MA, Korvick DL, Basamania CJ, Toro F, Aufdemorte TB, Rockwood CA Jr. Radiologic, mechanical, and histologic evaluation of 2 glenoid prosthesis designs in a canine model. J Shoulder Elbow Surg. 2001;10(2):140-148.
28. Pilliar RM, Lee JM, Maniatopoulos C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin Orthop Relat Res. 1986;(208):108-113.
29. Ramamurti BS, Orr TE, Bragdon CR, Lowenstein JD, Jasty M, Harris WH. Factors influencing stability at the interface between a porous surface and cancellous bone: a finite element analysis of a canine in vivo micromotion experiment. J Biomed Mater Res. 1997;36(2):274-280.
30. Şahin S, Cehreli MC, Yalçın E. The influence of functional forces on the biomechanics of implant-supported prostheses—a review. J Dent. 2002;30(7-8):271-282.
Since Neer and colleagues1 first reported in 1982, glenoid loosening persists as a common cause of anatomic total shoulder arthroplasty (TSA) failure.1-4 Currently, cemented, all-polyethylene glenoid components are the gold standard, and minimum clinical survival of 10 to 15 years is expected.3,5 Several clinical studies5-9 and in vitro biomechanical studies10 have suggested an advantage of pegged over keeled glenoid components, but glenoid component loosening remains a frequent complication,11 with the cement–implant interface suggested as the weak link of fixation.10,12 In addition to mechanical loosening, poor cement penetration and heat-induced necrosis have been postulated as contributing to glenoid component loosening.13,14
Because of these potential complications, there is a growing consideration to minimize or abandon cement fixation and rely on biological fixation to polyethylene for long-term component stability.15 A newer pegged glenoid component design consists of traditional, peripherally located pegs designed for cement fixation as well as a central, uncemented, fluted, interference-fit peg that allows for bony ingrowth. Short-term clinical studies have shown that bony ingrowth into the space between the flutes can be achieved with a hybrid cementation technique and that, when that occurs, excellent outcomes are likely.13,16-19 The immediate in vivo stability of this implant design upon initial implantation, before the cement has cured, has prompted some surgeons to consider implanting the device without cement. In a recent series in which this implant design was used without cement, clinical and radiographic results were promising.15
Despite the widespread clinical use, little biomechanical work has been done to characterize initial fixation of all-polyethylene pegged glenoid implants. We conducted a study to compare glenoid micromotion in an all-polyethylene, centrally fluted pegged glenoid component as a function of 3 fixation techniques: cementless interference-fit fixation, hybrid partial cementation based on manufacturer recommendations, and full cementation to simulate a gold-standard, traditional, cemented, pegged design.
Materials and MethodsBiomechanical Testing
The biomechanical testing methodology used in this study was based on previous studies20-23 and on ASTM standard F2028-1224 using polyurethane bone substitute 0.24 g/cm3 (Pacific Research Laboratories) with ultimate strength of 4.9 MPa and compressive modulus of 123 MPa for component implantation. This material was selected because its mechanical properties are similar to those of cancellous glenoid bone in primary shoulder arthroplasty,25 and it minimizes variability with use of cadaveric specimens. Components were mounted on an MTS 858 Mini-Bionix II materials testing frame (Figure 1). A static compressive load of 756 N (170 lb) was applied via a mass-pulley system simulating the joint compressive force the shoulder is likely to experience during higher load activities.24,26 The glenoid component was positioned on a linear bearing to allow for joint compression.
Test Groups and Cement Fixation Techniques
All-polyethylene pegged glenoid components (Anchor Peg Glenoid, size 44; DePuy Orthopaedics) were used for biomechanical testing (Figure 1). Polyurethane blocks were reamed with a size 44 reamer until the superior-inferior distance reached 33 mm, ensuring complete seating of implant. Three fixation-technique groups were formed: interference-fit, hybrid cement, and fully cemented. Interference-fit fixation was done without polymethylmethacrylate (PMMA) cement. In hybrid fixation, 2 cm3 of PMMA (SpeedSet Cement, Stryker Orthopaedics) was injected (using a catheter tip syringe) into the peripheral peg holes and manually pressurized; the central peg was press-fit into polyurethane bone substitute. In the fully cemented group, both peripheral and central peg holes received PMMA; the peripheral peg holes were cemented as in hybrid fixation, and the central peg hole was injected with 3 cm3 of PMMA, which was then manually pressurized. The humeral head component (Global Advantage, 44×18 mm; DePuy Synthes) was mounted on the test frame actuator and centrally located within the glenoid at the start of the test.
Determination of Humeral Head Translation via Subluxation Testing
Humeral head subluxation distance, simulating a humeral head rim loading event, was calculated on the basis of preliminary tests outlined in the ASTM standard.24 Three glenoids (1 per fixation technique) were mounted on the test frame with a humeral head positioned centrally within the glenoid. After the joint compressive force was applied, the humeral head was translated along the true superior axis of the glenoid at a rate of 50 mm/min. Testing software was used to record humeral head displacement and load data at a frequency of 100 Hz. Humeral head subluxation displacement was determined at the end of the linear region of the force versus displacement response. This distance, averaged from the 3 subluxation tests, was used as the subluxation distance during cyclic testing.
Determination of Glenoid Component Motion via Cyclic Testing
After subluxation displacement was determined, glenoid components were mounted on the test frame (5 per fixation technique) and subjected to 50,000 cycles of humeral head translation at a frequency of 2 Hz. Amplitude of the humeral head displacement against the glenoid component followed a sinusoidal pattern with maxima and minima represented by the subluxation displacement (positive and negative, respectively). Glenoid edge compression/distraction of the superior edge and glenoid inferior/superior translation were monitored with 2 variable resistance reluctance transducers (Microminiature DVRT; 4.5-µm resolution; MicroStrain) secured to the glenoid component and testing fixture.
Microminiature DVRT measurements of glenoid motion were taken for 5 consecutive cycles at cycles 1, 20, 100, 500, 1000, 5000, 10,000, 15,000, 20,000, 30,000, 40,000, and 50,000. Distraction-compression displacement and superior-inferior translation measurements were recorded relative to the glenoid position with the humeral head at the neutral position at a given cycle. Final glenoid micromotion data were calculated from the mean of consecutive cycles at each cycle time point.
Statistical Analysis
Glenoid motion results are reported as means and standard deviations. Comparisons with 2 factors of fixation technique and number of cycles for glenoid distraction, glenoid compression, and absolute glenoid translation were characterized with 2-way analysis of variance (SigmaPlot Version 11.0; Systat Software), with the Holm-Šídák test used for post hoc determination of significant relationships.
Results
Under subluxation testing, the humeral head translation distance at the end of the linear region was determined to be 0.50 mm. Subsequently for cyclic testing, the humeral head was then translated 0.50 mm from the neutral position of the humeral head along both the superior and inferior axes of the glenoid. All glenoids successfully completed the entire 50,000 cycles of testing. For the glenoid component, Figure 2 depicts distraction and compression, and Figure 3 depicts superior-inferior translation.
Glenoid Component Distraction
Overall, mean (SD) glenoid distraction was significantly higher for interference-fit fixation, 0.21 (0.10) mm, than for hybrid cement fixation, 0.16 (0.05) mm (P < .001), and fully cemented fixation, 0.09 (0.07) mm (P < .001). It was also significantly higher for hybrid fixation than fully cemented fixation (P < .001). From cycle 1000 to cycle 50,000, distraction was significantly higher for interference-fit fixation than for fully cemented fixation at each time point (P < .05).
Glenoid Component Compression
Mean (SD) compression was significantly higher for hybrid cement fixation, 0.31 (0.13) mm, than for interference-fit fixation, 0.17 (0.07) mm (P < .001), and fully cemented fixation, 0.17 (0.08) mm (P < .001). No significant difference was found between interference-fit and fully cemented fixation (P = .793) (Figure 2). At cycles 1, 20, 100, and 500, compression was significantly higher for hybrid fixation than for fully cemented fixation (P < .05). In addition, at cycle 500, it was significantly higher for hybrid fixation than for interference-fit fixation (P < .05).
Glenoid Component Translation
Mean (SD) glenoid translation was significantly lower for fully cemented fixation, 0.10 (0.04) mm, than for interference-fit fixation, 0.13 (0.04) mm (P < .001), and hybrid cement fixation, 0.13 (0.03) mm (P < .001), with all time points considered. There was no significant difference between interference-fit and hybrid fixation (P = .343). Initial translation at cycle 1 was significantly higher for interference-fit and hybid fixation than for fully cemented fixation.
Discussion
Despite advances in glenoid component design, glenoid loosening remains the most common cause of anatomical TSA failure. Recent implants have been designed to take advantage of an all-polyethylene component while providing long-lasting fixation through bony ingrowth into a central peg. In a study of the hybrid cementation technique drescribed here, Groh17 found no glenoid loosening or radiolucent lines but discovered fingerlike projections of bone between the flanges of the implant in 24 (29%) of 83 cases. Churchill and colleagues16 also reported bony ingrowth into the central peg in 15 (75%) of 20 patients. Furthermore, Arnold and colleagues13 reported complete bony ingrowth (6/6 inter-fin compartments) in 23 (71%) of 35 shoulders at a mean of 43 months. Wirth and colleagues19 reported increased radiodensity between the flanges of the central peg in 30 of 44 cases (68%) and osteolysis around the central peg in 3 of 44 cases (7%) at 3 years.
There are also reports of successful bony ingrowth associated with all-polyethylene components implanted without cement. In a canine study using an early ingrowth implant design, Wirth and colleagues27 showed that, though initial fixation was superior with a cemented, keeled implant, pullout strength of the uncemented, pegged implant improved over time and eventually far surpassed that of the cemented, keeled implant owing to both the loosening of the cemented component and the bony ingrowth into the central peg component. Furthermore, Anglin and colleagues10 confirmed that component micromotion was lower with pegged glenoid components than with keeled components in a biomechanical model. De Wilde and colleagues15 recently reported on a series of uncemented, central fluted peg glenoids implanted in 34 patients followed clinically and with computed tomography for a minimum of 24 months. The investigators found bony ingrowth into the central peg in 27 (79%) of 34 patients and no signs of loosening in 30 (88%) of 34 patients. Incomplete lucencies around 1 or 2 peripheral pegs were found in 2 (6%) of 34 patients, and complete lucencies around 2 or more peripheral pegs were found in 2 (6%) of 34 patients. However, there was no statistical difference in clinical outcome between patients with and without loosening.
With this type of implant, initial fixation that provides stability while minimizing micromotion under biomechanical loading likely is crucial for attaining bony growth within the central peg flanges. To our knowledge, this is the first biomechanical study to compare micromotion using 3 different fixation methods with a central fluted peg glenoid component design. Of all these fixation methods, fully cemented fixation yielded the most stable glenoid throughout testing with respect to the evaluated parameters. However, this method is not necessarily clinically applicable, as a fully cemented glenoid would inhibit any bony growth within the central flange, which is necessary for long-term biological fixation. Our data showed that, though glenoid distraction was significantly lower with hybrid cement fixation, this fixation method exhibited significantly higher glenoid compression. In addition, there were no significant differences between glenoid components with hybrid fixation and glenoid components with interference-fit fixation with respect to component translation in the superior-inferior direction. These findings may indicate that initial fixation is not significantly improved by the addition of cement to the peripheral pegs in a glenoid component with a central fluted peg design.
The interference fit of the central peg is primarily responsible for conferring long-term implant stability,13,27 which is ultimately achieved by bony formation between the flutes of the peg. Other authors have reported that, for bony ingrowth to occur, micromotion between the bone–implant interface must not exceed 20 to 150 µm.28-30 Other than for interference-fit distraction at more than 1000 cycles and hybrid cement fixation compression at each time point throughout testing, our data fall within the reported upper limits of micromotion to support bony ingrowth. Increased micromotion in the interference-fit fixation group is seen at later time points and may be caused by the inability to simulate the potential fixation gained from bony ingrowth allowed with this surgical technique. Research is needed to further explain this increase in distraction.
Results from this study must be interpreted with caution because of limitations of the in vitro testing methodology. This biomechanical model using bone substitute characterizes glenoid fixation at time zero, directly after implantation, followed by repetitive cyclic loading simulating 5 years of implant service. This differs from the clinical scenario in which the shoulder undergoes postoperative immobilization or protected motion during which the early phases of bony remodeling are likely occurring. Furthermore, simulation of 5 years of implant service may not be necessary for an implant that is expected to achieve ultimate fixation by bony ingrowth within the first several months after implantation. Use of this implant without cement is classified off-label, and surgeons should take this into consideration during implantation. Last, this study could not simulate the effect of bony ingrowth on fixation, though our experimental technique of cementing the central peg may be a gross approximation of a fully ingrown central peg and its expected rigid fixation.
Fully cemented fixation of a polyethylene glenoid is superior to hybrid cement fixation and interference-fit fixation with respect to early glenoid micromotion. However, the long-term stability of a fully cemented polyethylene glenoid component remains a clinical concern, as fixation is achieved by bony ingrowth around the central fluted peg of the implant. In this study, interference-fit and hybrid fixation had equivocal component micromotion in biomechanical testing. Our findings suggest that cementation of the peripheral pegs confers no additional initial stability over an uncemented interference-fit technique in a biomechanical model. More research is needed to further evaluate interference-fit fixation as a viable option for implantation of a central fluted, all-polyethylene glenoid component.
Since Neer and colleagues1 first reported in 1982, glenoid loosening persists as a common cause of anatomic total shoulder arthroplasty (TSA) failure.1-4 Currently, cemented, all-polyethylene glenoid components are the gold standard, and minimum clinical survival of 10 to 15 years is expected.3,5 Several clinical studies5-9 and in vitro biomechanical studies10 have suggested an advantage of pegged over keeled glenoid components, but glenoid component loosening remains a frequent complication,11 with the cement–implant interface suggested as the weak link of fixation.10,12 In addition to mechanical loosening, poor cement penetration and heat-induced necrosis have been postulated as contributing to glenoid component loosening.13,14
Because of these potential complications, there is a growing consideration to minimize or abandon cement fixation and rely on biological fixation to polyethylene for long-term component stability.15 A newer pegged glenoid component design consists of traditional, peripherally located pegs designed for cement fixation as well as a central, uncemented, fluted, interference-fit peg that allows for bony ingrowth. Short-term clinical studies have shown that bony ingrowth into the space between the flutes can be achieved with a hybrid cementation technique and that, when that occurs, excellent outcomes are likely.13,16-19 The immediate in vivo stability of this implant design upon initial implantation, before the cement has cured, has prompted some surgeons to consider implanting the device without cement. In a recent series in which this implant design was used without cement, clinical and radiographic results were promising.15
Despite the widespread clinical use, little biomechanical work has been done to characterize initial fixation of all-polyethylene pegged glenoid implants. We conducted a study to compare glenoid micromotion in an all-polyethylene, centrally fluted pegged glenoid component as a function of 3 fixation techniques: cementless interference-fit fixation, hybrid partial cementation based on manufacturer recommendations, and full cementation to simulate a gold-standard, traditional, cemented, pegged design.
Materials and MethodsBiomechanical Testing
The biomechanical testing methodology used in this study was based on previous studies20-23 and on ASTM standard F2028-1224 using polyurethane bone substitute 0.24 g/cm3 (Pacific Research Laboratories) with ultimate strength of 4.9 MPa and compressive modulus of 123 MPa for component implantation. This material was selected because its mechanical properties are similar to those of cancellous glenoid bone in primary shoulder arthroplasty,25 and it minimizes variability with use of cadaveric specimens. Components were mounted on an MTS 858 Mini-Bionix II materials testing frame (Figure 1). A static compressive load of 756 N (170 lb) was applied via a mass-pulley system simulating the joint compressive force the shoulder is likely to experience during higher load activities.24,26 The glenoid component was positioned on a linear bearing to allow for joint compression.
Test Groups and Cement Fixation Techniques
All-polyethylene pegged glenoid components (Anchor Peg Glenoid, size 44; DePuy Orthopaedics) were used for biomechanical testing (Figure 1). Polyurethane blocks were reamed with a size 44 reamer until the superior-inferior distance reached 33 mm, ensuring complete seating of implant. Three fixation-technique groups were formed: interference-fit, hybrid cement, and fully cemented. Interference-fit fixation was done without polymethylmethacrylate (PMMA) cement. In hybrid fixation, 2 cm3 of PMMA (SpeedSet Cement, Stryker Orthopaedics) was injected (using a catheter tip syringe) into the peripheral peg holes and manually pressurized; the central peg was press-fit into polyurethane bone substitute. In the fully cemented group, both peripheral and central peg holes received PMMA; the peripheral peg holes were cemented as in hybrid fixation, and the central peg hole was injected with 3 cm3 of PMMA, which was then manually pressurized. The humeral head component (Global Advantage, 44×18 mm; DePuy Synthes) was mounted on the test frame actuator and centrally located within the glenoid at the start of the test.
Determination of Humeral Head Translation via Subluxation Testing
Humeral head subluxation distance, simulating a humeral head rim loading event, was calculated on the basis of preliminary tests outlined in the ASTM standard.24 Three glenoids (1 per fixation technique) were mounted on the test frame with a humeral head positioned centrally within the glenoid. After the joint compressive force was applied, the humeral head was translated along the true superior axis of the glenoid at a rate of 50 mm/min. Testing software was used to record humeral head displacement and load data at a frequency of 100 Hz. Humeral head subluxation displacement was determined at the end of the linear region of the force versus displacement response. This distance, averaged from the 3 subluxation tests, was used as the subluxation distance during cyclic testing.
Determination of Glenoid Component Motion via Cyclic Testing
After subluxation displacement was determined, glenoid components were mounted on the test frame (5 per fixation technique) and subjected to 50,000 cycles of humeral head translation at a frequency of 2 Hz. Amplitude of the humeral head displacement against the glenoid component followed a sinusoidal pattern with maxima and minima represented by the subluxation displacement (positive and negative, respectively). Glenoid edge compression/distraction of the superior edge and glenoid inferior/superior translation were monitored with 2 variable resistance reluctance transducers (Microminiature DVRT; 4.5-µm resolution; MicroStrain) secured to the glenoid component and testing fixture.
Microminiature DVRT measurements of glenoid motion were taken for 5 consecutive cycles at cycles 1, 20, 100, 500, 1000, 5000, 10,000, 15,000, 20,000, 30,000, 40,000, and 50,000. Distraction-compression displacement and superior-inferior translation measurements were recorded relative to the glenoid position with the humeral head at the neutral position at a given cycle. Final glenoid micromotion data were calculated from the mean of consecutive cycles at each cycle time point.
Statistical Analysis
Glenoid motion results are reported as means and standard deviations. Comparisons with 2 factors of fixation technique and number of cycles for glenoid distraction, glenoid compression, and absolute glenoid translation were characterized with 2-way analysis of variance (SigmaPlot Version 11.0; Systat Software), with the Holm-Šídák test used for post hoc determination of significant relationships.
Results
Under subluxation testing, the humeral head translation distance at the end of the linear region was determined to be 0.50 mm. Subsequently for cyclic testing, the humeral head was then translated 0.50 mm from the neutral position of the humeral head along both the superior and inferior axes of the glenoid. All glenoids successfully completed the entire 50,000 cycles of testing. For the glenoid component, Figure 2 depicts distraction and compression, and Figure 3 depicts superior-inferior translation.
Glenoid Component Distraction
Overall, mean (SD) glenoid distraction was significantly higher for interference-fit fixation, 0.21 (0.10) mm, than for hybrid cement fixation, 0.16 (0.05) mm (P < .001), and fully cemented fixation, 0.09 (0.07) mm (P < .001). It was also significantly higher for hybrid fixation than fully cemented fixation (P < .001). From cycle 1000 to cycle 50,000, distraction was significantly higher for interference-fit fixation than for fully cemented fixation at each time point (P < .05).
Glenoid Component Compression
Mean (SD) compression was significantly higher for hybrid cement fixation, 0.31 (0.13) mm, than for interference-fit fixation, 0.17 (0.07) mm (P < .001), and fully cemented fixation, 0.17 (0.08) mm (P < .001). No significant difference was found between interference-fit and fully cemented fixation (P = .793) (Figure 2). At cycles 1, 20, 100, and 500, compression was significantly higher for hybrid fixation than for fully cemented fixation (P < .05). In addition, at cycle 500, it was significantly higher for hybrid fixation than for interference-fit fixation (P < .05).
Glenoid Component Translation
Mean (SD) glenoid translation was significantly lower for fully cemented fixation, 0.10 (0.04) mm, than for interference-fit fixation, 0.13 (0.04) mm (P < .001), and hybrid cement fixation, 0.13 (0.03) mm (P < .001), with all time points considered. There was no significant difference between interference-fit and hybrid fixation (P = .343). Initial translation at cycle 1 was significantly higher for interference-fit and hybid fixation than for fully cemented fixation.
Discussion
Despite advances in glenoid component design, glenoid loosening remains the most common cause of anatomical TSA failure. Recent implants have been designed to take advantage of an all-polyethylene component while providing long-lasting fixation through bony ingrowth into a central peg. In a study of the hybrid cementation technique drescribed here, Groh17 found no glenoid loosening or radiolucent lines but discovered fingerlike projections of bone between the flanges of the implant in 24 (29%) of 83 cases. Churchill and colleagues16 also reported bony ingrowth into the central peg in 15 (75%) of 20 patients. Furthermore, Arnold and colleagues13 reported complete bony ingrowth (6/6 inter-fin compartments) in 23 (71%) of 35 shoulders at a mean of 43 months. Wirth and colleagues19 reported increased radiodensity between the flanges of the central peg in 30 of 44 cases (68%) and osteolysis around the central peg in 3 of 44 cases (7%) at 3 years.
There are also reports of successful bony ingrowth associated with all-polyethylene components implanted without cement. In a canine study using an early ingrowth implant design, Wirth and colleagues27 showed that, though initial fixation was superior with a cemented, keeled implant, pullout strength of the uncemented, pegged implant improved over time and eventually far surpassed that of the cemented, keeled implant owing to both the loosening of the cemented component and the bony ingrowth into the central peg component. Furthermore, Anglin and colleagues10 confirmed that component micromotion was lower with pegged glenoid components than with keeled components in a biomechanical model. De Wilde and colleagues15 recently reported on a series of uncemented, central fluted peg glenoids implanted in 34 patients followed clinically and with computed tomography for a minimum of 24 months. The investigators found bony ingrowth into the central peg in 27 (79%) of 34 patients and no signs of loosening in 30 (88%) of 34 patients. Incomplete lucencies around 1 or 2 peripheral pegs were found in 2 (6%) of 34 patients, and complete lucencies around 2 or more peripheral pegs were found in 2 (6%) of 34 patients. However, there was no statistical difference in clinical outcome between patients with and without loosening.
With this type of implant, initial fixation that provides stability while minimizing micromotion under biomechanical loading likely is crucial for attaining bony growth within the central peg flanges. To our knowledge, this is the first biomechanical study to compare micromotion using 3 different fixation methods with a central fluted peg glenoid component design. Of all these fixation methods, fully cemented fixation yielded the most stable glenoid throughout testing with respect to the evaluated parameters. However, this method is not necessarily clinically applicable, as a fully cemented glenoid would inhibit any bony growth within the central flange, which is necessary for long-term biological fixation. Our data showed that, though glenoid distraction was significantly lower with hybrid cement fixation, this fixation method exhibited significantly higher glenoid compression. In addition, there were no significant differences between glenoid components with hybrid fixation and glenoid components with interference-fit fixation with respect to component translation in the superior-inferior direction. These findings may indicate that initial fixation is not significantly improved by the addition of cement to the peripheral pegs in a glenoid component with a central fluted peg design.
The interference fit of the central peg is primarily responsible for conferring long-term implant stability,13,27 which is ultimately achieved by bony formation between the flutes of the peg. Other authors have reported that, for bony ingrowth to occur, micromotion between the bone–implant interface must not exceed 20 to 150 µm.28-30 Other than for interference-fit distraction at more than 1000 cycles and hybrid cement fixation compression at each time point throughout testing, our data fall within the reported upper limits of micromotion to support bony ingrowth. Increased micromotion in the interference-fit fixation group is seen at later time points and may be caused by the inability to simulate the potential fixation gained from bony ingrowth allowed with this surgical technique. Research is needed to further explain this increase in distraction.
Results from this study must be interpreted with caution because of limitations of the in vitro testing methodology. This biomechanical model using bone substitute characterizes glenoid fixation at time zero, directly after implantation, followed by repetitive cyclic loading simulating 5 years of implant service. This differs from the clinical scenario in which the shoulder undergoes postoperative immobilization or protected motion during which the early phases of bony remodeling are likely occurring. Furthermore, simulation of 5 years of implant service may not be necessary for an implant that is expected to achieve ultimate fixation by bony ingrowth within the first several months after implantation. Use of this implant without cement is classified off-label, and surgeons should take this into consideration during implantation. Last, this study could not simulate the effect of bony ingrowth on fixation, though our experimental technique of cementing the central peg may be a gross approximation of a fully ingrown central peg and its expected rigid fixation.
Fully cemented fixation of a polyethylene glenoid is superior to hybrid cement fixation and interference-fit fixation with respect to early glenoid micromotion. However, the long-term stability of a fully cemented polyethylene glenoid component remains a clinical concern, as fixation is achieved by bony ingrowth around the central fluted peg of the implant. In this study, interference-fit and hybrid fixation had equivocal component micromotion in biomechanical testing. Our findings suggest that cementation of the peripheral pegs confers no additional initial stability over an uncemented interference-fit technique in a biomechanical model. More research is needed to further evaluate interference-fit fixation as a viable option for implantation of a central fluted, all-polyethylene glenoid component.
1. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
2. Sperling JW, Cofield RH, O’Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg. 2000;9(6):507-513.
3. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
4. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
5. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863.
6. Edwards TB, Labriola JE, Stanley RJ, O’Connor DP, Elkousy HA, Gartsman GM. Radiographic comparison of pegged and keeled glenoid components using modern cementing techniques: a prospective randomized study. J Shoulder Elbow Surg. 2010;19(2):251-257.
7. Gartsman GM, Elkousy HA, Warnock KM, Edwards TB, O’Connor DP. Radiographic comparison of pegged and keeled glenoid components. J Shoulder Elbow Surg. 2005;14(3):252-257.
8. Klepps S, Chiang AS, Miller S, Jiang CY, Hazrati Y, Flatow EL. Incidence of early radiolucent glenoid lines in patients having total shoulder replacements. Clin Orthop Relat Res. 2005;(435):118-125.
9. Lazarus MD, Jensen KL, Southworth C, Matsen FA 3rd. The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am. 2002;84(7):1174-1182.
10. Anglin C, Wyss UP, Nyffeler RW, Gerber C. Loosening performance of cemented glenoid prosthesis design pairs. Clin Biomech. 2001;16(2):144-150.
11. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
12. Gregory T, Hansen U, Taillieu F, et al. Glenoid loosening after total shoulder arthroplasty: an in vitro CT-scan study. J Orthop Res. 2009;27(12):1589-1595.
13. Arnold RM, High RR, Grosshans KT, Walker CW, Fehringer EV. Bone presence between the central peg’s radial fins of a partially cemented pegged all poly glenoid component suggest few radiolucencies. J Shoulder Elbow Surg. 2011;20(2):315-321.
14. Churchill RS, Boorman RS, Fehringer EV, Matsen FA 3rd. Glenoid cementing may generate sufficient heat to endanger the surrounding bone. Clin Orthop Relat Res. 2004;(419):76-79.
15. De Wilde L, Dayerizadeh N, De Neve F, Basamania C, Van Tongel A. Fully uncemented glenoid component in total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(10):e1-e7.
16. Churchill RS, Zellmer C, Zimmers HJ, Ruggero R. Clinical and radiographic analysis of a partially cemented glenoid implant: five-year minimum follow-up. J Shoulder Elbow Surg. 2010;19(7):1091-1097.
17. Groh GI. Survival and radiographic analysis of a glenoid component with a cementless fluted central peg. J Shoulder Elbow Surg. 2010;19(8):1265-1268.
18. Vidil A, Valenti P, Guichoux F, Barthas JH. CT scan evaluation of glenoid component fixation: a prospective study of 27 minimally cemented shoulder arthroplasties. Eur J Orthop Surg Traumatol. 2012;23(5):521-525.
19. Wirth MA, Loredo R, Garcia G, Rockwood CA Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94(3):260-267.
20. Anglin C, Wyss UP, Pichora DR. Mechanical testing of shoulder prostheses and recommendations for glenoid design. J Shoulder Elbow Surg. 2000;9(4):323-331.
21. Hoenig MP, Loeffler B, Brown S, et al. Reverse glenoid component fixation: is a posterior screw necessary? J Shoulder Elbow Surg. 2010;19(4):544-549.
22. Sarah J, Sanjay G, Sanjay S, et al. Failure mechanism of the all-polyethylene glenoid implant. J Biomech. 2010;43(4):714-719.
23. Suárez DR, Nerkens W, Valstar ER, Rozing PM, van Keulen F. Interface micromotions increase with less-conforming cementless glenoid components. J Shoulder Elbow Surg. 2012;21(4):474-482.
24. ASTM International. Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation. West Conshocken, PA: ASTM International; 2012. ASTM F2028-08.
25. Anglin C, Tolhurst P, Wyss UP, Pichora DR. Glenoid cancellous bone strength and modulus. J Biomech. 1999;32(10):1091-1097.
26. Anglin C, Wyss U, Pichora D. Glenohumeral contact forces. Proc Inst Mech Eng H. 2000;214(6):637-644.
27. Wirth MA, Korvick DL, Basamania CJ, Toro F, Aufdemorte TB, Rockwood CA Jr. Radiologic, mechanical, and histologic evaluation of 2 glenoid prosthesis designs in a canine model. J Shoulder Elbow Surg. 2001;10(2):140-148.
28. Pilliar RM, Lee JM, Maniatopoulos C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin Orthop Relat Res. 1986;(208):108-113.
29. Ramamurti BS, Orr TE, Bragdon CR, Lowenstein JD, Jasty M, Harris WH. Factors influencing stability at the interface between a porous surface and cancellous bone: a finite element analysis of a canine in vivo micromotion experiment. J Biomed Mater Res. 1997;36(2):274-280.
30. Şahin S, Cehreli MC, Yalçın E. The influence of functional forces on the biomechanics of implant-supported prostheses—a review. J Dent. 2002;30(7-8):271-282.
1. Neer CS 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64(3):319-337.
2. Sperling JW, Cofield RH, O’Driscoll SW, Torchia ME, Rowland CM. Radiographic assessment of ingrowth total shoulder arthroplasty. J Shoulder Elbow Surg. 2000;9(6):507-513.
3. Torchia ME, Cofield RH, Settergren CR. Total shoulder arthroplasty with the Neer prosthesis: long-term results. J Shoulder Elbow Surg. 1997;6(6):495-505.
4. Wirth MA, Rockwood CA Jr. Complications of total shoulder-replacement arthroplasty. J Bone Joint Surg Am. 1996;78(4):603-616.
5. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863.
6. Edwards TB, Labriola JE, Stanley RJ, O’Connor DP, Elkousy HA, Gartsman GM. Radiographic comparison of pegged and keeled glenoid components using modern cementing techniques: a prospective randomized study. J Shoulder Elbow Surg. 2010;19(2):251-257.
7. Gartsman GM, Elkousy HA, Warnock KM, Edwards TB, O’Connor DP. Radiographic comparison of pegged and keeled glenoid components. J Shoulder Elbow Surg. 2005;14(3):252-257.
8. Klepps S, Chiang AS, Miller S, Jiang CY, Hazrati Y, Flatow EL. Incidence of early radiolucent glenoid lines in patients having total shoulder replacements. Clin Orthop Relat Res. 2005;(435):118-125.
9. Lazarus MD, Jensen KL, Southworth C, Matsen FA 3rd. The radiographic evaluation of keeled and pegged glenoid component insertion. J Bone Joint Surg Am. 2002;84(7):1174-1182.
10. Anglin C, Wyss UP, Nyffeler RW, Gerber C. Loosening performance of cemented glenoid prosthesis design pairs. Clin Biomech. 2001;16(2):144-150.
11. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
12. Gregory T, Hansen U, Taillieu F, et al. Glenoid loosening after total shoulder arthroplasty: an in vitro CT-scan study. J Orthop Res. 2009;27(12):1589-1595.
13. Arnold RM, High RR, Grosshans KT, Walker CW, Fehringer EV. Bone presence between the central peg’s radial fins of a partially cemented pegged all poly glenoid component suggest few radiolucencies. J Shoulder Elbow Surg. 2011;20(2):315-321.
14. Churchill RS, Boorman RS, Fehringer EV, Matsen FA 3rd. Glenoid cementing may generate sufficient heat to endanger the surrounding bone. Clin Orthop Relat Res. 2004;(419):76-79.
15. De Wilde L, Dayerizadeh N, De Neve F, Basamania C, Van Tongel A. Fully uncemented glenoid component in total shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(10):e1-e7.
16. Churchill RS, Zellmer C, Zimmers HJ, Ruggero R. Clinical and radiographic analysis of a partially cemented glenoid implant: five-year minimum follow-up. J Shoulder Elbow Surg. 2010;19(7):1091-1097.
17. Groh GI. Survival and radiographic analysis of a glenoid component with a cementless fluted central peg. J Shoulder Elbow Surg. 2010;19(8):1265-1268.
18. Vidil A, Valenti P, Guichoux F, Barthas JH. CT scan evaluation of glenoid component fixation: a prospective study of 27 minimally cemented shoulder arthroplasties. Eur J Orthop Surg Traumatol. 2012;23(5):521-525.
19. Wirth MA, Loredo R, Garcia G, Rockwood CA Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94(3):260-267.
20. Anglin C, Wyss UP, Pichora DR. Mechanical testing of shoulder prostheses and recommendations for glenoid design. J Shoulder Elbow Surg. 2000;9(4):323-331.
21. Hoenig MP, Loeffler B, Brown S, et al. Reverse glenoid component fixation: is a posterior screw necessary? J Shoulder Elbow Surg. 2010;19(4):544-549.
22. Sarah J, Sanjay G, Sanjay S, et al. Failure mechanism of the all-polyethylene glenoid implant. J Biomech. 2010;43(4):714-719.
23. Suárez DR, Nerkens W, Valstar ER, Rozing PM, van Keulen F. Interface micromotions increase with less-conforming cementless glenoid components. J Shoulder Elbow Surg. 2012;21(4):474-482.
24. ASTM International. Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation. West Conshocken, PA: ASTM International; 2012. ASTM F2028-08.
25. Anglin C, Tolhurst P, Wyss UP, Pichora DR. Glenoid cancellous bone strength and modulus. J Biomech. 1999;32(10):1091-1097.
26. Anglin C, Wyss U, Pichora D. Glenohumeral contact forces. Proc Inst Mech Eng H. 2000;214(6):637-644.
27. Wirth MA, Korvick DL, Basamania CJ, Toro F, Aufdemorte TB, Rockwood CA Jr. Radiologic, mechanical, and histologic evaluation of 2 glenoid prosthesis designs in a canine model. J Shoulder Elbow Surg. 2001;10(2):140-148.
28. Pilliar RM, Lee JM, Maniatopoulos C. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin Orthop Relat Res. 1986;(208):108-113.
29. Ramamurti BS, Orr TE, Bragdon CR, Lowenstein JD, Jasty M, Harris WH. Factors influencing stability at the interface between a porous surface and cancellous bone: a finite element analysis of a canine in vivo micromotion experiment. J Biomed Mater Res. 1997;36(2):274-280.
30. Şahin S, Cehreli MC, Yalçın E. The influence of functional forces on the biomechanics of implant-supported prostheses—a review. J Dent. 2002;30(7-8):271-282.
No OS benefit with tasquinimod in mCRPC
The oral immunotherapy tasquinimod improved radiographic progression-free survival (rPFS) in men with metastatic castration-resistant prostate cancer (mCRPC), but the drug failed to improve overall survival (OS), according to results from a large, multinational phase III trial.
Median rPFS was 7.0 months (95% CI, 5.8-8.2 months) for the tasquinimod group and 4.4 months (95% CI, 3.5-5.5 months) for placebo (HR, 0.64; 95% CI, 0.54-0.75; P less than .001). However, median OS was similar for the two groups: 21.3 months (19.5-23.0) for tasquinimod and 24.0 months (21.4-26.9) for placebo (HR, 1.10; 95% CI, 0.94 to 1.28; P = .25). At a median follow-up of 30 months, 96% of patients had discontinued treatment, most commonly because of progression (radiographic and symptomatic) and adverse events (J Clin Oncol. 2016 June 13. doi: 10.1200/JCO.2016.66.9697).
The 36% reduced risk of progression with tasquinimod versus placebo confirmed the phase II trial results, but the significant rPFS benefit did not translate to improved OS. The authors note that among one of several explanations for the lack of OS benefit is the availability of effective salvage therapies, many of which were not available during the phase II study.
“The current availability of such agents (e.g., abiraterone and enzalutamide) may have had an impact on the course of disease because patients in the placebo group gained access before those in the tasquinimod group on account of their earlier withdrawal from study treatment. Indeed, posttreatment use of abiraterone and enzalutamide was more common among patients in the placebo group,” wrote Dr. Cora Sternberg, chair of the department of medical oncology at San Camillo Forlanini Hospital, Italy, and colleagues.
The randomized, double-blind, placebo-controlled phase III study enrolled 1,245 patients from 241 sites in 37 countries. Patients with prostate adenocarcinoma with evidence of bone metastasis who had not received cytotoxic chemotherapy for 2 years were randomly assigned 2:1 to receive tasquinimod (n = 832) or placebo (n = 413).
Radiographic- and PSA-based secondary outcomes favored tasquinimod over placebo. By contrast, symptomatically assessed outcomes, such as time to symptomatic progression, time to opiate use, and deterioration of QoL, favored placebo. A greater proportion of the tasquinimod group discontinued treatment because of adverse events (17.7% vs. 10.2%), mainly decreased appetite, fatigue, asthenia, or nausea.
Tasquinimod affects the tumor microenvironment to counteract tumor growth. Preclinical evidence suggests it has an inhibitory effect on myeloid-derived suppressive cells and M2-polarized tumor-associated macrophages. Identification of immunologic biomarkers may help patient selection and determination of a rational combination strategy, according to the authors. Due to the lack of OS benefit, further clinical development of tasquinimod in this patient population was not pursued.
Dr. Sternberg reported having financial ties to Pfizer, Novartis, Janssen Pharmaceuticals, Sanofi, GlaxoSmithKline, Bristol-Myers Squibb, Bayer HealthCare Pharmaceuticals, Astellas Pharma, Eisai, Exelixis, Medivation, Active Biotech, and Genentech. Several of her coauthors reported ties to industry sources.
The oral immunotherapy tasquinimod improved radiographic progression-free survival (rPFS) in men with metastatic castration-resistant prostate cancer (mCRPC), but the drug failed to improve overall survival (OS), according to results from a large, multinational phase III trial.
Median rPFS was 7.0 months (95% CI, 5.8-8.2 months) for the tasquinimod group and 4.4 months (95% CI, 3.5-5.5 months) for placebo (HR, 0.64; 95% CI, 0.54-0.75; P less than .001). However, median OS was similar for the two groups: 21.3 months (19.5-23.0) for tasquinimod and 24.0 months (21.4-26.9) for placebo (HR, 1.10; 95% CI, 0.94 to 1.28; P = .25). At a median follow-up of 30 months, 96% of patients had discontinued treatment, most commonly because of progression (radiographic and symptomatic) and adverse events (J Clin Oncol. 2016 June 13. doi: 10.1200/JCO.2016.66.9697).
The 36% reduced risk of progression with tasquinimod versus placebo confirmed the phase II trial results, but the significant rPFS benefit did not translate to improved OS. The authors note that among one of several explanations for the lack of OS benefit is the availability of effective salvage therapies, many of which were not available during the phase II study.
“The current availability of such agents (e.g., abiraterone and enzalutamide) may have had an impact on the course of disease because patients in the placebo group gained access before those in the tasquinimod group on account of their earlier withdrawal from study treatment. Indeed, posttreatment use of abiraterone and enzalutamide was more common among patients in the placebo group,” wrote Dr. Cora Sternberg, chair of the department of medical oncology at San Camillo Forlanini Hospital, Italy, and colleagues.
The randomized, double-blind, placebo-controlled phase III study enrolled 1,245 patients from 241 sites in 37 countries. Patients with prostate adenocarcinoma with evidence of bone metastasis who had not received cytotoxic chemotherapy for 2 years were randomly assigned 2:1 to receive tasquinimod (n = 832) or placebo (n = 413).
Radiographic- and PSA-based secondary outcomes favored tasquinimod over placebo. By contrast, symptomatically assessed outcomes, such as time to symptomatic progression, time to opiate use, and deterioration of QoL, favored placebo. A greater proportion of the tasquinimod group discontinued treatment because of adverse events (17.7% vs. 10.2%), mainly decreased appetite, fatigue, asthenia, or nausea.
Tasquinimod affects the tumor microenvironment to counteract tumor growth. Preclinical evidence suggests it has an inhibitory effect on myeloid-derived suppressive cells and M2-polarized tumor-associated macrophages. Identification of immunologic biomarkers may help patient selection and determination of a rational combination strategy, according to the authors. Due to the lack of OS benefit, further clinical development of tasquinimod in this patient population was not pursued.
Dr. Sternberg reported having financial ties to Pfizer, Novartis, Janssen Pharmaceuticals, Sanofi, GlaxoSmithKline, Bristol-Myers Squibb, Bayer HealthCare Pharmaceuticals, Astellas Pharma, Eisai, Exelixis, Medivation, Active Biotech, and Genentech. Several of her coauthors reported ties to industry sources.
The oral immunotherapy tasquinimod improved radiographic progression-free survival (rPFS) in men with metastatic castration-resistant prostate cancer (mCRPC), but the drug failed to improve overall survival (OS), according to results from a large, multinational phase III trial.
Median rPFS was 7.0 months (95% CI, 5.8-8.2 months) for the tasquinimod group and 4.4 months (95% CI, 3.5-5.5 months) for placebo (HR, 0.64; 95% CI, 0.54-0.75; P less than .001). However, median OS was similar for the two groups: 21.3 months (19.5-23.0) for tasquinimod and 24.0 months (21.4-26.9) for placebo (HR, 1.10; 95% CI, 0.94 to 1.28; P = .25). At a median follow-up of 30 months, 96% of patients had discontinued treatment, most commonly because of progression (radiographic and symptomatic) and adverse events (J Clin Oncol. 2016 June 13. doi: 10.1200/JCO.2016.66.9697).
The 36% reduced risk of progression with tasquinimod versus placebo confirmed the phase II trial results, but the significant rPFS benefit did not translate to improved OS. The authors note that among one of several explanations for the lack of OS benefit is the availability of effective salvage therapies, many of which were not available during the phase II study.
“The current availability of such agents (e.g., abiraterone and enzalutamide) may have had an impact on the course of disease because patients in the placebo group gained access before those in the tasquinimod group on account of their earlier withdrawal from study treatment. Indeed, posttreatment use of abiraterone and enzalutamide was more common among patients in the placebo group,” wrote Dr. Cora Sternberg, chair of the department of medical oncology at San Camillo Forlanini Hospital, Italy, and colleagues.
The randomized, double-blind, placebo-controlled phase III study enrolled 1,245 patients from 241 sites in 37 countries. Patients with prostate adenocarcinoma with evidence of bone metastasis who had not received cytotoxic chemotherapy for 2 years were randomly assigned 2:1 to receive tasquinimod (n = 832) or placebo (n = 413).
Radiographic- and PSA-based secondary outcomes favored tasquinimod over placebo. By contrast, symptomatically assessed outcomes, such as time to symptomatic progression, time to opiate use, and deterioration of QoL, favored placebo. A greater proportion of the tasquinimod group discontinued treatment because of adverse events (17.7% vs. 10.2%), mainly decreased appetite, fatigue, asthenia, or nausea.
Tasquinimod affects the tumor microenvironment to counteract tumor growth. Preclinical evidence suggests it has an inhibitory effect on myeloid-derived suppressive cells and M2-polarized tumor-associated macrophages. Identification of immunologic biomarkers may help patient selection and determination of a rational combination strategy, according to the authors. Due to the lack of OS benefit, further clinical development of tasquinimod in this patient population was not pursued.
Dr. Sternberg reported having financial ties to Pfizer, Novartis, Janssen Pharmaceuticals, Sanofi, GlaxoSmithKline, Bristol-Myers Squibb, Bayer HealthCare Pharmaceuticals, Astellas Pharma, Eisai, Exelixis, Medivation, Active Biotech, and Genentech. Several of her coauthors reported ties to industry sources.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Tasquinimod improved progression-free survival (PFS) but not overall survival (OS) in men with metastatic castration-resistant prostate cancer (mCRPC).
Major finding: Median radiographic PFS was 7.0 months (95% CI, 5.8-8.2 months) for the tasquinimod group and 4.4 months (95% CI, 3.5-5.5) months for placebo (HR, 0.64; 95% CI, 0.54 to 0.75; P less than .001). Median OS was similar for the two groups: 21.3 and 24.0 months, respectively (HR, 1.10; 95% CI, 0.94-1.28; P = .25).
Data sources: A randomized, double-blind, placebo-controlled phase III study conducted at 241 sites in 37 countries, comprising 832 patients who received tasquinimod and 413 who received placebo.
Disclosures: Dr. Sternberg reported having financial ties to Pfizer, Novartis, Janssen Pharmaceuticals, Sanofi, GlaxoSmithKline, Bristol-Myers Squibb, Bayer HealthCare Pharmaceuticals, Astellas Pharma, Eisai, Exelixis, Medivation, Active Biotech, and Genentech. Several of her coauthors reported ties to industry sources.
Baseline PSA at midlife predicts lethal prostate cancer
A single, baseline prostate-specific antigen (PSA) level measured at midlife predicted risk of lethal prostate cancer over a 30-year follow-up, according to a nested, case-control study among men who participated in the Physicians’ Health Study.
PSA levels at the 90th percentile and above, compared with levels at the median and lower, were associated with increased risk of lethal prostate cancer (PCa) across all age groups: for men aged 40-49 years, the odds ratio was 8.7 (95% confidence interval, 1.0-78.2), for 50-54 years, 12.6 (1.4-110.4), and for 55-59 years, 6.9 (2.5-19.1). PSA levels above the median were associated with increased risk of all PCa: odds ratios were 7.3 (95% CI, 2.4-21.8) for 40-49 years, 7.6 (3.4-17.2) for 50-54 years, and 10.1 (5.2-19.6) for 55-59 years.
“These data identify subgroups of men, on the basis of their PSA levels at a given age, with widely divergent lifetime risk of PCa death, who therefore could benefit from screening intervals tailored to their actual magnitude of risk,” wrote Dr. Mark Preston of Brigham and Women’s Hospital, Boston, and colleagues (J Clin Oncol. 2016 Jun 13. doi: 10.1200/JCO.2016.66.7527).
The investigators noted that one of seven men with PSA greater than 2.1 mg/mL at 55-59 years and one of 12 men with PSA greater than 2.1 ng/mL at 50-54 years died as a result of PCa within 30 years.
“These findings do not necessarily imply that prostate biopsy or definitive treatment is immediately required in younger men with higher PSA levels at baseline, because this could lead to overdiagnosis, but only that they undergo more intensive PSA screening to enable earlier identification of cancer and potential cure while still possible,” the investigators wrote.
As a subset of the Physicians’ Health Study, a randomized, placebo-controlled trial of aspirin and beta-carotene, 14,916 men aged 40-84 years provided a blood sample during 1982-1984. Total PSA was determined from stored specimens, and self-reported incident PCa cases from 1982 to 2012 were confirmed through medical records.
In answer to the question of whether a low PSA level at 40-49 years might safely exempt men from further screening, results showed that for PSA levels below the 25th percentile, cumulative incidence of lethal PCa at 30 years was 0.37% (0.05-1.70) for men 40-44 years and 0.97% (0.30-2.49) for men 45-49 years. Because a small risk remains even with an exceptionally low first measure, another PSA test during the lifetime of men 40-49 is prudent, according to the researchers. At age 60 years, men with PSA below the median are unlikely to develop lethal PCa, based on the analysis.
A single, baseline prostate-specific antigen (PSA) level measured at midlife predicted risk of lethal prostate cancer over a 30-year follow-up, according to a nested, case-control study among men who participated in the Physicians’ Health Study.
PSA levels at the 90th percentile and above, compared with levels at the median and lower, were associated with increased risk of lethal prostate cancer (PCa) across all age groups: for men aged 40-49 years, the odds ratio was 8.7 (95% confidence interval, 1.0-78.2), for 50-54 years, 12.6 (1.4-110.4), and for 55-59 years, 6.9 (2.5-19.1). PSA levels above the median were associated with increased risk of all PCa: odds ratios were 7.3 (95% CI, 2.4-21.8) for 40-49 years, 7.6 (3.4-17.2) for 50-54 years, and 10.1 (5.2-19.6) for 55-59 years.
“These data identify subgroups of men, on the basis of their PSA levels at a given age, with widely divergent lifetime risk of PCa death, who therefore could benefit from screening intervals tailored to their actual magnitude of risk,” wrote Dr. Mark Preston of Brigham and Women’s Hospital, Boston, and colleagues (J Clin Oncol. 2016 Jun 13. doi: 10.1200/JCO.2016.66.7527).
The investigators noted that one of seven men with PSA greater than 2.1 mg/mL at 55-59 years and one of 12 men with PSA greater than 2.1 ng/mL at 50-54 years died as a result of PCa within 30 years.
“These findings do not necessarily imply that prostate biopsy or definitive treatment is immediately required in younger men with higher PSA levels at baseline, because this could lead to overdiagnosis, but only that they undergo more intensive PSA screening to enable earlier identification of cancer and potential cure while still possible,” the investigators wrote.
As a subset of the Physicians’ Health Study, a randomized, placebo-controlled trial of aspirin and beta-carotene, 14,916 men aged 40-84 years provided a blood sample during 1982-1984. Total PSA was determined from stored specimens, and self-reported incident PCa cases from 1982 to 2012 were confirmed through medical records.
In answer to the question of whether a low PSA level at 40-49 years might safely exempt men from further screening, results showed that for PSA levels below the 25th percentile, cumulative incidence of lethal PCa at 30 years was 0.37% (0.05-1.70) for men 40-44 years and 0.97% (0.30-2.49) for men 45-49 years. Because a small risk remains even with an exceptionally low first measure, another PSA test during the lifetime of men 40-49 is prudent, according to the researchers. At age 60 years, men with PSA below the median are unlikely to develop lethal PCa, based on the analysis.
A single, baseline prostate-specific antigen (PSA) level measured at midlife predicted risk of lethal prostate cancer over a 30-year follow-up, according to a nested, case-control study among men who participated in the Physicians’ Health Study.
PSA levels at the 90th percentile and above, compared with levels at the median and lower, were associated with increased risk of lethal prostate cancer (PCa) across all age groups: for men aged 40-49 years, the odds ratio was 8.7 (95% confidence interval, 1.0-78.2), for 50-54 years, 12.6 (1.4-110.4), and for 55-59 years, 6.9 (2.5-19.1). PSA levels above the median were associated with increased risk of all PCa: odds ratios were 7.3 (95% CI, 2.4-21.8) for 40-49 years, 7.6 (3.4-17.2) for 50-54 years, and 10.1 (5.2-19.6) for 55-59 years.
“These data identify subgroups of men, on the basis of their PSA levels at a given age, with widely divergent lifetime risk of PCa death, who therefore could benefit from screening intervals tailored to their actual magnitude of risk,” wrote Dr. Mark Preston of Brigham and Women’s Hospital, Boston, and colleagues (J Clin Oncol. 2016 Jun 13. doi: 10.1200/JCO.2016.66.7527).
The investigators noted that one of seven men with PSA greater than 2.1 mg/mL at 55-59 years and one of 12 men with PSA greater than 2.1 ng/mL at 50-54 years died as a result of PCa within 30 years.
“These findings do not necessarily imply that prostate biopsy or definitive treatment is immediately required in younger men with higher PSA levels at baseline, because this could lead to overdiagnosis, but only that they undergo more intensive PSA screening to enable earlier identification of cancer and potential cure while still possible,” the investigators wrote.
As a subset of the Physicians’ Health Study, a randomized, placebo-controlled trial of aspirin and beta-carotene, 14,916 men aged 40-84 years provided a blood sample during 1982-1984. Total PSA was determined from stored specimens, and self-reported incident PCa cases from 1982 to 2012 were confirmed through medical records.
In answer to the question of whether a low PSA level at 40-49 years might safely exempt men from further screening, results showed that for PSA levels below the 25th percentile, cumulative incidence of lethal PCa at 30 years was 0.37% (0.05-1.70) for men 40-44 years and 0.97% (0.30-2.49) for men 45-49 years. Because a small risk remains even with an exceptionally low first measure, another PSA test during the lifetime of men 40-49 is prudent, according to the researchers. At age 60 years, men with PSA below the median are unlikely to develop lethal PCa, based on the analysis.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Prostate-specific antigen levels at midlife predicted subsequent lethal prostate cancer in men who participated in the Physicians’ Health Study and underwent opportunistic screening.
Major finding: PSA levels at the 90th percentile and above, compared with levels at the median and lower, were associated with increased risk of lethal PCa across all age groups: For men 40-49 years, the OR was 8.7 (95% CI, 1.0-78.2), for 50-54 years, 12.6 (1.4-110.4), and for 55-59 years, 6.9 (2.5-19.1).
Data sources: In the Physicians’ Health Study, 14,916 men aged 40-84 years provided a blood sample used for total PSA determination, and self-reported incident PCa cases from 1982 to 2012 were confirmed through medical records.
Disclosures: Dr. Preston reported having no disclosures. Several of his coauthors reported ties to industry sources.
Atezolizumab has good showing as first-line therapy in urothelial cancer
CHICAGO – The immune checkpoint inhibitor atezolizumab is efficacious when used as first-line therapy for advanced urothelial carcinoma, according to a study reported at the annual meeting of the American Society of Clinical Oncology.
The study – cohort 1 of the IMvigor210 trial – was conducted among 119 cisplatin-ineligible patients with metastatic or locally advanced disease. All were treated with the antibody atezolizumab, which targets PD-L1 (programmed death–ligand 1), a negative regulator of the immune system, and thereby promotes the antitumor immune response.
Nearly a quarter of patients had a tumor response to atezolizumab, and median overall survival approached 15 months, first author Dr. Arjun V. Balar of the New York University Langone Medical Center and director of genitourinary medical oncology at the NYU Perlmutter Cancer Center, New York, reported in a session and press briefing.
“Overall, this therapy was efficacious and also very well tolerated,” he commented. “These data make a compelling argument for atezolizumab to be a potential new standard of care in patients with cisplatin-ineligible metastatic urothelial cancer. However, moreover, they could represent the beginning of a seismic shift in our treatment approach to all patients with metastatic disease, irrespective of their eligibility for cisplatin.”
Positive findings from the trial’s cohort 2, patients who had already received platinum-based chemotherapy for advanced disease, recently led to the agent’s approval by the Food and Drug Administration for use in that population.
Cohort 1 was initially set up as an exploratory study but was expanded, Dr. Balar explained. “I do think that there is a benefit there, but until we have comparative data, it’s going to be really hard to hold that against immunotherapy necessarily,” he acknowledged. “That being said, do I envision a future where there is PD-L1 and PD-1 targeted therapy as a front-line therapy? Yes, absolutely, I think we are headed in that direction. We just need the trials to show it.”
ASCO expert Dr. Charles Ryan, professor of clinical medicine and urology program leader, genitourinary medical oncology, at the University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center, concurred, saying, “I think it is safe at this point to envision a future where PD-L1 therapy could be used in the front line, but we do need to do those confirmatory studies.
“I would just underscore that in this study, the importance is that this is cisplatin ineligible as opposed to carboplatin treated,” Dr. Ryan added. “Cisplatin is the only platinum in bladder cancer that is associated with a survival benefit, so this is a very significant point to make. A very substantial part of the bladder cancer population, many patients out there, are cisplatin ineligible due to a variety of reasons, because organ dysfunction is quite common in advanced urothelial cancer.”
Patients were entered into IMVigor210’s cohort 1 if they had impaired kidney function, peripheral neuropathy, moderate to severe hearing loss, or poor performance status, precluding the use of cisplatin.
All were treated with atezolizumab (Tecentriq) every 3 weeks until investigator-defined progression. To evaluate a potential biomarker for benefit, the investigators assessed PD-L1 expression on tumor-infiltrating immune cells by immunohistochemistry in archival tissue.
Study results showed that with a median follow-up of 14.4 months, the centrally confirmed overall response rate, the study’s primary endpoint, was 24% (7% of patients had a complete response and 17% had a partial response), Dr. Balar reported.
Complete responses were seen in all subgroups of patients stratified by PD-L1 expression. Fully 75% of all responses were still ongoing at the time of data cutoff, and the median duration of response has not yet been reached in any of the subgroups.
The median duration of overall survival was 14.8 months, and the 1-year rate of overall survival was 57%, although data for that endpoint are still immature. Survival also appeared to be similar regardless of PD-L1 expression.
Taken together, these efficacy findings compare favorably with those seen historically in similar patients treated with other agents in trials and in real-world settings, according to Dr. Balar.
Atezolizumab was well tolerated, with only 6% of patients experiencing an adverse event leading to trial discontinuation. Most events seen were of grade 1 or 2 severity; a single patient had a grade 5 event (sepsis).
About 15% of patients had treatment-related grade 3 or 4 adverse events, about the same as the rate seen in cohort 2. The most common were fatigue and an increase in liver enzymes.
Overall, 14% of patients had an immune-mediated adverse event requiring corticosteroid treatment. “Notably, no patients required any other immunosuppression beyond steroids for the management of an immune-related adverse event,” he reported
The PD-L1 analyses in the trial had some limitations, Dr. Balar said. “PD-L1 testing continues to be the most hotly contested issue,” he said. “Obviously, the immune system is very dynamic, and we were testing something in archival specimens, in a static environment, so there are obviously all the caveats there.”
Some data have suggested that mutational burden may help identify the patient subset who will benefit. However, “to be able to make your decision in the clinic, those types of readouts need to be timely …, and I think that’s the gap,” he commented. “So in the future, is the right biomarker PD-1 or PD-L1? My hunch is no, that is probably not the right biomarker, there are probably better ones, and those are being worked on.”
Dr. Balar disclosed that he has a consulting or advisory role with Cerulean Pharma, Dendreon, Pfizer, and Roche/Genentech. The trial was sponsored by Hoffmann-La Roche. Ventana Medical Systems assisted with PD-L1 testing.
CHICAGO – The immune checkpoint inhibitor atezolizumab is efficacious when used as first-line therapy for advanced urothelial carcinoma, according to a study reported at the annual meeting of the American Society of Clinical Oncology.
The study – cohort 1 of the IMvigor210 trial – was conducted among 119 cisplatin-ineligible patients with metastatic or locally advanced disease. All were treated with the antibody atezolizumab, which targets PD-L1 (programmed death–ligand 1), a negative regulator of the immune system, and thereby promotes the antitumor immune response.
Nearly a quarter of patients had a tumor response to atezolizumab, and median overall survival approached 15 months, first author Dr. Arjun V. Balar of the New York University Langone Medical Center and director of genitourinary medical oncology at the NYU Perlmutter Cancer Center, New York, reported in a session and press briefing.
“Overall, this therapy was efficacious and also very well tolerated,” he commented. “These data make a compelling argument for atezolizumab to be a potential new standard of care in patients with cisplatin-ineligible metastatic urothelial cancer. However, moreover, they could represent the beginning of a seismic shift in our treatment approach to all patients with metastatic disease, irrespective of their eligibility for cisplatin.”
Positive findings from the trial’s cohort 2, patients who had already received platinum-based chemotherapy for advanced disease, recently led to the agent’s approval by the Food and Drug Administration for use in that population.
Cohort 1 was initially set up as an exploratory study but was expanded, Dr. Balar explained. “I do think that there is a benefit there, but until we have comparative data, it’s going to be really hard to hold that against immunotherapy necessarily,” he acknowledged. “That being said, do I envision a future where there is PD-L1 and PD-1 targeted therapy as a front-line therapy? Yes, absolutely, I think we are headed in that direction. We just need the trials to show it.”
ASCO expert Dr. Charles Ryan, professor of clinical medicine and urology program leader, genitourinary medical oncology, at the University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center, concurred, saying, “I think it is safe at this point to envision a future where PD-L1 therapy could be used in the front line, but we do need to do those confirmatory studies.
“I would just underscore that in this study, the importance is that this is cisplatin ineligible as opposed to carboplatin treated,” Dr. Ryan added. “Cisplatin is the only platinum in bladder cancer that is associated with a survival benefit, so this is a very significant point to make. A very substantial part of the bladder cancer population, many patients out there, are cisplatin ineligible due to a variety of reasons, because organ dysfunction is quite common in advanced urothelial cancer.”
Patients were entered into IMVigor210’s cohort 1 if they had impaired kidney function, peripheral neuropathy, moderate to severe hearing loss, or poor performance status, precluding the use of cisplatin.
All were treated with atezolizumab (Tecentriq) every 3 weeks until investigator-defined progression. To evaluate a potential biomarker for benefit, the investigators assessed PD-L1 expression on tumor-infiltrating immune cells by immunohistochemistry in archival tissue.
Study results showed that with a median follow-up of 14.4 months, the centrally confirmed overall response rate, the study’s primary endpoint, was 24% (7% of patients had a complete response and 17% had a partial response), Dr. Balar reported.
Complete responses were seen in all subgroups of patients stratified by PD-L1 expression. Fully 75% of all responses were still ongoing at the time of data cutoff, and the median duration of response has not yet been reached in any of the subgroups.
The median duration of overall survival was 14.8 months, and the 1-year rate of overall survival was 57%, although data for that endpoint are still immature. Survival also appeared to be similar regardless of PD-L1 expression.
Taken together, these efficacy findings compare favorably with those seen historically in similar patients treated with other agents in trials and in real-world settings, according to Dr. Balar.
Atezolizumab was well tolerated, with only 6% of patients experiencing an adverse event leading to trial discontinuation. Most events seen were of grade 1 or 2 severity; a single patient had a grade 5 event (sepsis).
About 15% of patients had treatment-related grade 3 or 4 adverse events, about the same as the rate seen in cohort 2. The most common were fatigue and an increase in liver enzymes.
Overall, 14% of patients had an immune-mediated adverse event requiring corticosteroid treatment. “Notably, no patients required any other immunosuppression beyond steroids for the management of an immune-related adverse event,” he reported
The PD-L1 analyses in the trial had some limitations, Dr. Balar said. “PD-L1 testing continues to be the most hotly contested issue,” he said. “Obviously, the immune system is very dynamic, and we were testing something in archival specimens, in a static environment, so there are obviously all the caveats there.”
Some data have suggested that mutational burden may help identify the patient subset who will benefit. However, “to be able to make your decision in the clinic, those types of readouts need to be timely …, and I think that’s the gap,” he commented. “So in the future, is the right biomarker PD-1 or PD-L1? My hunch is no, that is probably not the right biomarker, there are probably better ones, and those are being worked on.”
Dr. Balar disclosed that he has a consulting or advisory role with Cerulean Pharma, Dendreon, Pfizer, and Roche/Genentech. The trial was sponsored by Hoffmann-La Roche. Ventana Medical Systems assisted with PD-L1 testing.
CHICAGO – The immune checkpoint inhibitor atezolizumab is efficacious when used as first-line therapy for advanced urothelial carcinoma, according to a study reported at the annual meeting of the American Society of Clinical Oncology.
The study – cohort 1 of the IMvigor210 trial – was conducted among 119 cisplatin-ineligible patients with metastatic or locally advanced disease. All were treated with the antibody atezolizumab, which targets PD-L1 (programmed death–ligand 1), a negative regulator of the immune system, and thereby promotes the antitumor immune response.
Nearly a quarter of patients had a tumor response to atezolizumab, and median overall survival approached 15 months, first author Dr. Arjun V. Balar of the New York University Langone Medical Center and director of genitourinary medical oncology at the NYU Perlmutter Cancer Center, New York, reported in a session and press briefing.
“Overall, this therapy was efficacious and also very well tolerated,” he commented. “These data make a compelling argument for atezolizumab to be a potential new standard of care in patients with cisplatin-ineligible metastatic urothelial cancer. However, moreover, they could represent the beginning of a seismic shift in our treatment approach to all patients with metastatic disease, irrespective of their eligibility for cisplatin.”
Positive findings from the trial’s cohort 2, patients who had already received platinum-based chemotherapy for advanced disease, recently led to the agent’s approval by the Food and Drug Administration for use in that population.
Cohort 1 was initially set up as an exploratory study but was expanded, Dr. Balar explained. “I do think that there is a benefit there, but until we have comparative data, it’s going to be really hard to hold that against immunotherapy necessarily,” he acknowledged. “That being said, do I envision a future where there is PD-L1 and PD-1 targeted therapy as a front-line therapy? Yes, absolutely, I think we are headed in that direction. We just need the trials to show it.”
ASCO expert Dr. Charles Ryan, professor of clinical medicine and urology program leader, genitourinary medical oncology, at the University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center, concurred, saying, “I think it is safe at this point to envision a future where PD-L1 therapy could be used in the front line, but we do need to do those confirmatory studies.
“I would just underscore that in this study, the importance is that this is cisplatin ineligible as opposed to carboplatin treated,” Dr. Ryan added. “Cisplatin is the only platinum in bladder cancer that is associated with a survival benefit, so this is a very significant point to make. A very substantial part of the bladder cancer population, many patients out there, are cisplatin ineligible due to a variety of reasons, because organ dysfunction is quite common in advanced urothelial cancer.”
Patients were entered into IMVigor210’s cohort 1 if they had impaired kidney function, peripheral neuropathy, moderate to severe hearing loss, or poor performance status, precluding the use of cisplatin.
All were treated with atezolizumab (Tecentriq) every 3 weeks until investigator-defined progression. To evaluate a potential biomarker for benefit, the investigators assessed PD-L1 expression on tumor-infiltrating immune cells by immunohistochemistry in archival tissue.
Study results showed that with a median follow-up of 14.4 months, the centrally confirmed overall response rate, the study’s primary endpoint, was 24% (7% of patients had a complete response and 17% had a partial response), Dr. Balar reported.
Complete responses were seen in all subgroups of patients stratified by PD-L1 expression. Fully 75% of all responses were still ongoing at the time of data cutoff, and the median duration of response has not yet been reached in any of the subgroups.
The median duration of overall survival was 14.8 months, and the 1-year rate of overall survival was 57%, although data for that endpoint are still immature. Survival also appeared to be similar regardless of PD-L1 expression.
Taken together, these efficacy findings compare favorably with those seen historically in similar patients treated with other agents in trials and in real-world settings, according to Dr. Balar.
Atezolizumab was well tolerated, with only 6% of patients experiencing an adverse event leading to trial discontinuation. Most events seen were of grade 1 or 2 severity; a single patient had a grade 5 event (sepsis).
About 15% of patients had treatment-related grade 3 or 4 adverse events, about the same as the rate seen in cohort 2. The most common were fatigue and an increase in liver enzymes.
Overall, 14% of patients had an immune-mediated adverse event requiring corticosteroid treatment. “Notably, no patients required any other immunosuppression beyond steroids for the management of an immune-related adverse event,” he reported
The PD-L1 analyses in the trial had some limitations, Dr. Balar said. “PD-L1 testing continues to be the most hotly contested issue,” he said. “Obviously, the immune system is very dynamic, and we were testing something in archival specimens, in a static environment, so there are obviously all the caveats there.”
Some data have suggested that mutational burden may help identify the patient subset who will benefit. However, “to be able to make your decision in the clinic, those types of readouts need to be timely …, and I think that’s the gap,” he commented. “So in the future, is the right biomarker PD-1 or PD-L1? My hunch is no, that is probably not the right biomarker, there are probably better ones, and those are being worked on.”
Dr. Balar disclosed that he has a consulting or advisory role with Cerulean Pharma, Dendreon, Pfizer, and Roche/Genentech. The trial was sponsored by Hoffmann-La Roche. Ventana Medical Systems assisted with PD-L1 testing.
AT THE 2016 ASCO ANNUAL MEETING
Key clinical point: Atezolizumab is efficacious when used as first-line therapy in advanced urothelial cancer.
Major finding: The overall response rate was 24%, and the median duration of overall survival was 14.8 months.
Data source: A study of cisplatin-ineligible patients with locally advanced or metastatic urothelial carcinoma from a single-arm phase II trial (IMvigor210 trial cohort 1).
Disclosures: Dr. Balar disclosed that he has a consulting or advisory role with Cerulean Pharma, Dendreon, Pfizer, and Roche/Genentech. The trial was sponsored by Hoffmann-La Roche. Ventana Medical Systems assisted with PD-L1 testing.
‘Meticulous’ surgical procedure best defense against rectal cancer recurrence
LOS ANGELES – In the clinical experience of Dr. Ian C. Lavery, prevention efforts are the best defense against local recurrence of rectal cancer.
“This means adjuvant treatment, if necessary, neoadjuvant treatment, and a meticulous surgical operation,” Dr. Lavery of the department of colorectal surgery at the Cleveland Clinic said at the annual meeting of the American Society of Colon and Rectal Surgeons. “If the circumferential resection margin is negative, the local recurrence rate is 10% or less. If it’s positive, local recurrence goes up to 78%. Even when we attempt to do the perfect total mesorectal excision, local recurrence is in the order of 4%.”
Selective use of radiotherapy in the neoadjuvant setting appears to be reducing the incidence of local recurrence, “certainly in the short term,” he added. “In the long term, I’m not sure we know the true answer to that yet. Using other techniques like washing the rectal stump out, the use of stapling, and en-bloc resection if necessary [can help prevention efforts].”
The incidence of local rectal cancer recurrence is reported to be between 3% and 50%, but neither curative nor palliative treatment is standardized. “When you get local recurrence after a rectal cancer operation, it’s a disaster,” Dr. Lavery said. “It may cause intractable pain, bleeding, perforation, obstruction, and sepsis – all incredibly difficult things to manage.”
Patients who develop a local recurrence of rectal cancer are often asymptomatic. A digital rectal exam (DRE) may or may not identify a recurrence and carcinoembryonic antigen levels are helpful on some occasions. According to Dr. Lavery, optimal surveillance consists of a clinical examination including DRE, endoscopy, blood tests, CT scans, MRI, and PET scans. “If they were all to be done routinely it would increase the detection earlier rather than later,” he said.
CT and MRI appear to be about 85% accurate but both modalities are “very poor at detecting peritoneal disease,” he said. PET scans for recurrent carcinoma have been shown to change the management in 20%-56% of cases (Ann Surg Oncol. 1997 Dec; 4:613-20).
While follow-up of patients who have undergone surgery for local rectal cancer is generally favored, there is no consensus on what the ideal follow-up timeline should be. “In my opinion, the more intensive follow-up is going to be better than the cursory conventional follow-up examination,” Dr. Lavery said. “One of the big reasons for that is the vast majority of recurrences are extraluminal so they may be difficult to feel. Doing endoscopy, you can’t see them if they’re extraluminal.”
The goal in treating recurrent rectal cancer is to improve quality of life, he continued, as the common symptoms include obstruction, pain, bleeding, bowel discharge, or perforation/abscess. Optimal treatment involves striving for tumor-free margins after the operation. “This may require en bloc resection of an adjacent prostate, bladder, lateral pelvic wall,” he said. “But clinically and radiologically, it’s very difficult to identify those patients that have a potentially R0 resection.”
Curative treatment is possible if the recurrence is locally resectable and the patient has minimal comorbidities. “The potential morbidity after the surgery has to be acceptable, considering the severity of the problem that we’re dealing with,” Dr. Lavery noted. “Distant disease also complicates the issue.”
Reasons to avoid resection include rigid tumor fixation, leg lymphedema, major vessel encasement, bilateral ureteric involvement, extensive para-aortic lymph node involvement, and radicular pain. “If you embark on one of these cases, you want to make it at least the first if not the only case of the day,” Dr. Lavery advised. “Anticipate the need for assistance during the operation, but above all, make sure you have optimal exposure to do the surgery.” He reported having no financial disclosures.
LOS ANGELES – In the clinical experience of Dr. Ian C. Lavery, prevention efforts are the best defense against local recurrence of rectal cancer.
“This means adjuvant treatment, if necessary, neoadjuvant treatment, and a meticulous surgical operation,” Dr. Lavery of the department of colorectal surgery at the Cleveland Clinic said at the annual meeting of the American Society of Colon and Rectal Surgeons. “If the circumferential resection margin is negative, the local recurrence rate is 10% or less. If it’s positive, local recurrence goes up to 78%. Even when we attempt to do the perfect total mesorectal excision, local recurrence is in the order of 4%.”
Selective use of radiotherapy in the neoadjuvant setting appears to be reducing the incidence of local recurrence, “certainly in the short term,” he added. “In the long term, I’m not sure we know the true answer to that yet. Using other techniques like washing the rectal stump out, the use of stapling, and en-bloc resection if necessary [can help prevention efforts].”
The incidence of local rectal cancer recurrence is reported to be between 3% and 50%, but neither curative nor palliative treatment is standardized. “When you get local recurrence after a rectal cancer operation, it’s a disaster,” Dr. Lavery said. “It may cause intractable pain, bleeding, perforation, obstruction, and sepsis – all incredibly difficult things to manage.”
Patients who develop a local recurrence of rectal cancer are often asymptomatic. A digital rectal exam (DRE) may or may not identify a recurrence and carcinoembryonic antigen levels are helpful on some occasions. According to Dr. Lavery, optimal surveillance consists of a clinical examination including DRE, endoscopy, blood tests, CT scans, MRI, and PET scans. “If they were all to be done routinely it would increase the detection earlier rather than later,” he said.
CT and MRI appear to be about 85% accurate but both modalities are “very poor at detecting peritoneal disease,” he said. PET scans for recurrent carcinoma have been shown to change the management in 20%-56% of cases (Ann Surg Oncol. 1997 Dec; 4:613-20).
While follow-up of patients who have undergone surgery for local rectal cancer is generally favored, there is no consensus on what the ideal follow-up timeline should be. “In my opinion, the more intensive follow-up is going to be better than the cursory conventional follow-up examination,” Dr. Lavery said. “One of the big reasons for that is the vast majority of recurrences are extraluminal so they may be difficult to feel. Doing endoscopy, you can’t see them if they’re extraluminal.”
The goal in treating recurrent rectal cancer is to improve quality of life, he continued, as the common symptoms include obstruction, pain, bleeding, bowel discharge, or perforation/abscess. Optimal treatment involves striving for tumor-free margins after the operation. “This may require en bloc resection of an adjacent prostate, bladder, lateral pelvic wall,” he said. “But clinically and radiologically, it’s very difficult to identify those patients that have a potentially R0 resection.”
Curative treatment is possible if the recurrence is locally resectable and the patient has minimal comorbidities. “The potential morbidity after the surgery has to be acceptable, considering the severity of the problem that we’re dealing with,” Dr. Lavery noted. “Distant disease also complicates the issue.”
Reasons to avoid resection include rigid tumor fixation, leg lymphedema, major vessel encasement, bilateral ureteric involvement, extensive para-aortic lymph node involvement, and radicular pain. “If you embark on one of these cases, you want to make it at least the first if not the only case of the day,” Dr. Lavery advised. “Anticipate the need for assistance during the operation, but above all, make sure you have optimal exposure to do the surgery.” He reported having no financial disclosures.
LOS ANGELES – In the clinical experience of Dr. Ian C. Lavery, prevention efforts are the best defense against local recurrence of rectal cancer.
“This means adjuvant treatment, if necessary, neoadjuvant treatment, and a meticulous surgical operation,” Dr. Lavery of the department of colorectal surgery at the Cleveland Clinic said at the annual meeting of the American Society of Colon and Rectal Surgeons. “If the circumferential resection margin is negative, the local recurrence rate is 10% or less. If it’s positive, local recurrence goes up to 78%. Even when we attempt to do the perfect total mesorectal excision, local recurrence is in the order of 4%.”
Selective use of radiotherapy in the neoadjuvant setting appears to be reducing the incidence of local recurrence, “certainly in the short term,” he added. “In the long term, I’m not sure we know the true answer to that yet. Using other techniques like washing the rectal stump out, the use of stapling, and en-bloc resection if necessary [can help prevention efforts].”
The incidence of local rectal cancer recurrence is reported to be between 3% and 50%, but neither curative nor palliative treatment is standardized. “When you get local recurrence after a rectal cancer operation, it’s a disaster,” Dr. Lavery said. “It may cause intractable pain, bleeding, perforation, obstruction, and sepsis – all incredibly difficult things to manage.”
Patients who develop a local recurrence of rectal cancer are often asymptomatic. A digital rectal exam (DRE) may or may not identify a recurrence and carcinoembryonic antigen levels are helpful on some occasions. According to Dr. Lavery, optimal surveillance consists of a clinical examination including DRE, endoscopy, blood tests, CT scans, MRI, and PET scans. “If they were all to be done routinely it would increase the detection earlier rather than later,” he said.
CT and MRI appear to be about 85% accurate but both modalities are “very poor at detecting peritoneal disease,” he said. PET scans for recurrent carcinoma have been shown to change the management in 20%-56% of cases (Ann Surg Oncol. 1997 Dec; 4:613-20).
While follow-up of patients who have undergone surgery for local rectal cancer is generally favored, there is no consensus on what the ideal follow-up timeline should be. “In my opinion, the more intensive follow-up is going to be better than the cursory conventional follow-up examination,” Dr. Lavery said. “One of the big reasons for that is the vast majority of recurrences are extraluminal so they may be difficult to feel. Doing endoscopy, you can’t see them if they’re extraluminal.”
The goal in treating recurrent rectal cancer is to improve quality of life, he continued, as the common symptoms include obstruction, pain, bleeding, bowel discharge, or perforation/abscess. Optimal treatment involves striving for tumor-free margins after the operation. “This may require en bloc resection of an adjacent prostate, bladder, lateral pelvic wall,” he said. “But clinically and radiologically, it’s very difficult to identify those patients that have a potentially R0 resection.”
Curative treatment is possible if the recurrence is locally resectable and the patient has minimal comorbidities. “The potential morbidity after the surgery has to be acceptable, considering the severity of the problem that we’re dealing with,” Dr. Lavery noted. “Distant disease also complicates the issue.”
Reasons to avoid resection include rigid tumor fixation, leg lymphedema, major vessel encasement, bilateral ureteric involvement, extensive para-aortic lymph node involvement, and radicular pain. “If you embark on one of these cases, you want to make it at least the first if not the only case of the day,” Dr. Lavery advised. “Anticipate the need for assistance during the operation, but above all, make sure you have optimal exposure to do the surgery.” He reported having no financial disclosures.
EXPERT ANALYSIS AT THE ASCRS ANNUAL MEETING
Far fewer adults would meet SPRINT than guideline-recommended BP goals
Applying the more stringent SPRINT criteria to a general population of persons with hypertension would yield a significant reduction in the number of people meeting their treatment goals, although those who do would achieve a significant reduction in their risk of cardiovascular disease, a study published June 13 in the Journal of the American College of Cardiology has found.
Min Jung Ko, Ph.D., of the National Evidence-Based Healthcare Collaborating Agency in Seoul, Korea, and coauthors explored the relative impacts of SPRINT target of less than 120 mm Hg for hypertension treatments with the 2014 hypertension recommendations of the Eighth Joint National Committee of less than 140 mm Hg, using data from 13,346 individuals in the Korean National Health and Nutrition Examination Survey of 2008-2013, and 67,965 individuals in the Korean National Health Insurance Service health examinee cohort of 2007.
The investigators found that 11.9% of adults with hypertension would meet the treatment goals of the SPRINT criteria, compared with 70.8% who would meet the 2014 recommendations.
However, the analysis showed that those who met the more aggressive SPRINT treatment goal of systolic BP below 120 mm Hg also had the lowest 10-year risk of a major cardiovascular event (6.2%), compared with 7.7% in those who met the 2014 targets but not the SPRINT targets, and 9.4% in those who failed to meet the 2014 treatment targets (J Am Coll Cardiol. 2016 Jun 13. doi 10.1016/j.jacc.2016.03.572).
After adjustment for factors such as age, diabetes, chronic kidney disease, hyperlipidemia, body mass index, and smoking, the least-controlled group showed a 62% increase in the risk of cardiovascular events, compared with the SPRINT criteria group. Those who met the 2014 criteria had a 17% greater risk than those who met the SPRINT criteria.
“Despite greater cardiovascular protection with intensive BP lowering, achieving SPRINT-defined BP goals might not be easy or practical because the target BP was not met in more than one-half of the participants in the intensive-treatment group,” the authors wrote.
Individuals who were older, female, or had diabetes, chronic kidney disease, or prevalent cardiovascular disease were more likely to meet the stricter goals of SPRINT (Systolic Blood Pressure Intervention Trial), in which combined cardiovascular events occurred in 5.2% of patients treated to a target systolic blood pressure of less than 120 mm Hg and 6.8% of patients treated to a target of less than 140 mm Hg (N Engl J Med. 2015;373:2103-16).
Researchers also noted a significant linear association between lesser blood pressure control and an increased risk of myocardial infarction and stroke, although there was no significant trend seen relating to cardiovascular or all-cause mortality. The authors noted that this was the opposite to what was observed in the original SPRINT trial, where there was a reduction in cardiovascular mortality and heart failure but only a modest, nonsignificant impact on MI or stroke.
“Although the exact reasons remain unclear, this discrepancy might be explained in part by differences in study design, population characteristics, clinical practice pattern, or race or ethnic groups,” they suggested. “The generalizability of the SPRINT experience to multiple groups of various ethnic backgrounds warrants further investigations and is likely to be of considerable interest.”
Unlike the SPRINT trial, the Korean analysis did not look into the potential adverse effects of more aggressive blood pressure–lowering, but the authors noted that the SPRINT trial did see an increased incidence of more serious adverse events, including hypotension, syncope, and acute kidney injury.
“Therefore, beyond the BP target per se, several important factors should be considered for optimal BP management in the contemporary medical setting; for example, an integrated and systematic assessment of combined risk factors and baseline cardiovascular risk, concomitant preventive medical therapies, cost-effectiveness, clinician-patient discussions of the potential benefits and harms, or the clinical judgment of the treating physician.”
The National Evidence-Based Healthcare Collaborating Agency, Seoul, South Korea, funded the study. No conflicts of interest were declared.
How broadly SPRINT findings should be generalized is an important and challenging consideration for clinicians, guideline committees, and policy decision makers.
Changing the target for hypertension treatment to systolic BP below 120 mm Hg for all Korean adults would require considerable effort and would almost certainly result in a substantial reduction in hypertension control rates, but these data suggest that more intensive reduction in systolic BP may also result in substantial reduction in cardiovascular disease risk.
However, the findings of Ko et al. must be interpreted with caution. This study provides CVD event rate estimates based on experience in all Korean adults with hypertension, whereas the SPRINT experience was derived from a much more restricted sample of older U.S. adults with hypertension and a high risk of CVD.
Guideline committees and the practice community must use caution when generalizing SPRINT results to adults with a profile that differs from the participants in the parent study.
Dr. Paul K. Whelton is in the department of epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, and Paul Muntner, Ph.D., is in the department of epidemiology at the University of Alabama at Birmingham. These comments were part of an editorial (JACC 2016 June 21. doi 10.1016/j.jacc.2016.04.010). Dr. Whelton serves as chair of the SPRINT steering committee. Dr. Muntner has received grant support from Amgen unrelated to the topic of the current paper.
How broadly SPRINT findings should be generalized is an important and challenging consideration for clinicians, guideline committees, and policy decision makers.
Changing the target for hypertension treatment to systolic BP below 120 mm Hg for all Korean adults would require considerable effort and would almost certainly result in a substantial reduction in hypertension control rates, but these data suggest that more intensive reduction in systolic BP may also result in substantial reduction in cardiovascular disease risk.
However, the findings of Ko et al. must be interpreted with caution. This study provides CVD event rate estimates based on experience in all Korean adults with hypertension, whereas the SPRINT experience was derived from a much more restricted sample of older U.S. adults with hypertension and a high risk of CVD.
Guideline committees and the practice community must use caution when generalizing SPRINT results to adults with a profile that differs from the participants in the parent study.
Dr. Paul K. Whelton is in the department of epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, and Paul Muntner, Ph.D., is in the department of epidemiology at the University of Alabama at Birmingham. These comments were part of an editorial (JACC 2016 June 21. doi 10.1016/j.jacc.2016.04.010). Dr. Whelton serves as chair of the SPRINT steering committee. Dr. Muntner has received grant support from Amgen unrelated to the topic of the current paper.
How broadly SPRINT findings should be generalized is an important and challenging consideration for clinicians, guideline committees, and policy decision makers.
Changing the target for hypertension treatment to systolic BP below 120 mm Hg for all Korean adults would require considerable effort and would almost certainly result in a substantial reduction in hypertension control rates, but these data suggest that more intensive reduction in systolic BP may also result in substantial reduction in cardiovascular disease risk.
However, the findings of Ko et al. must be interpreted with caution. This study provides CVD event rate estimates based on experience in all Korean adults with hypertension, whereas the SPRINT experience was derived from a much more restricted sample of older U.S. adults with hypertension and a high risk of CVD.
Guideline committees and the practice community must use caution when generalizing SPRINT results to adults with a profile that differs from the participants in the parent study.
Dr. Paul K. Whelton is in the department of epidemiology, Tulane University School of Public Health and Tropical Medicine, New Orleans, and Paul Muntner, Ph.D., is in the department of epidemiology at the University of Alabama at Birmingham. These comments were part of an editorial (JACC 2016 June 21. doi 10.1016/j.jacc.2016.04.010). Dr. Whelton serves as chair of the SPRINT steering committee. Dr. Muntner has received grant support from Amgen unrelated to the topic of the current paper.
Applying the more stringent SPRINT criteria to a general population of persons with hypertension would yield a significant reduction in the number of people meeting their treatment goals, although those who do would achieve a significant reduction in their risk of cardiovascular disease, a study published June 13 in the Journal of the American College of Cardiology has found.
Min Jung Ko, Ph.D., of the National Evidence-Based Healthcare Collaborating Agency in Seoul, Korea, and coauthors explored the relative impacts of SPRINT target of less than 120 mm Hg for hypertension treatments with the 2014 hypertension recommendations of the Eighth Joint National Committee of less than 140 mm Hg, using data from 13,346 individuals in the Korean National Health and Nutrition Examination Survey of 2008-2013, and 67,965 individuals in the Korean National Health Insurance Service health examinee cohort of 2007.
The investigators found that 11.9% of adults with hypertension would meet the treatment goals of the SPRINT criteria, compared with 70.8% who would meet the 2014 recommendations.
However, the analysis showed that those who met the more aggressive SPRINT treatment goal of systolic BP below 120 mm Hg also had the lowest 10-year risk of a major cardiovascular event (6.2%), compared with 7.7% in those who met the 2014 targets but not the SPRINT targets, and 9.4% in those who failed to meet the 2014 treatment targets (J Am Coll Cardiol. 2016 Jun 13. doi 10.1016/j.jacc.2016.03.572).
After adjustment for factors such as age, diabetes, chronic kidney disease, hyperlipidemia, body mass index, and smoking, the least-controlled group showed a 62% increase in the risk of cardiovascular events, compared with the SPRINT criteria group. Those who met the 2014 criteria had a 17% greater risk than those who met the SPRINT criteria.
“Despite greater cardiovascular protection with intensive BP lowering, achieving SPRINT-defined BP goals might not be easy or practical because the target BP was not met in more than one-half of the participants in the intensive-treatment group,” the authors wrote.
Individuals who were older, female, or had diabetes, chronic kidney disease, or prevalent cardiovascular disease were more likely to meet the stricter goals of SPRINT (Systolic Blood Pressure Intervention Trial), in which combined cardiovascular events occurred in 5.2% of patients treated to a target systolic blood pressure of less than 120 mm Hg and 6.8% of patients treated to a target of less than 140 mm Hg (N Engl J Med. 2015;373:2103-16).
Researchers also noted a significant linear association between lesser blood pressure control and an increased risk of myocardial infarction and stroke, although there was no significant trend seen relating to cardiovascular or all-cause mortality. The authors noted that this was the opposite to what was observed in the original SPRINT trial, where there was a reduction in cardiovascular mortality and heart failure but only a modest, nonsignificant impact on MI or stroke.
“Although the exact reasons remain unclear, this discrepancy might be explained in part by differences in study design, population characteristics, clinical practice pattern, or race or ethnic groups,” they suggested. “The generalizability of the SPRINT experience to multiple groups of various ethnic backgrounds warrants further investigations and is likely to be of considerable interest.”
Unlike the SPRINT trial, the Korean analysis did not look into the potential adverse effects of more aggressive blood pressure–lowering, but the authors noted that the SPRINT trial did see an increased incidence of more serious adverse events, including hypotension, syncope, and acute kidney injury.
“Therefore, beyond the BP target per se, several important factors should be considered for optimal BP management in the contemporary medical setting; for example, an integrated and systematic assessment of combined risk factors and baseline cardiovascular risk, concomitant preventive medical therapies, cost-effectiveness, clinician-patient discussions of the potential benefits and harms, or the clinical judgment of the treating physician.”
The National Evidence-Based Healthcare Collaborating Agency, Seoul, South Korea, funded the study. No conflicts of interest were declared.
Applying the more stringent SPRINT criteria to a general population of persons with hypertension would yield a significant reduction in the number of people meeting their treatment goals, although those who do would achieve a significant reduction in their risk of cardiovascular disease, a study published June 13 in the Journal of the American College of Cardiology has found.
Min Jung Ko, Ph.D., of the National Evidence-Based Healthcare Collaborating Agency in Seoul, Korea, and coauthors explored the relative impacts of SPRINT target of less than 120 mm Hg for hypertension treatments with the 2014 hypertension recommendations of the Eighth Joint National Committee of less than 140 mm Hg, using data from 13,346 individuals in the Korean National Health and Nutrition Examination Survey of 2008-2013, and 67,965 individuals in the Korean National Health Insurance Service health examinee cohort of 2007.
The investigators found that 11.9% of adults with hypertension would meet the treatment goals of the SPRINT criteria, compared with 70.8% who would meet the 2014 recommendations.
However, the analysis showed that those who met the more aggressive SPRINT treatment goal of systolic BP below 120 mm Hg also had the lowest 10-year risk of a major cardiovascular event (6.2%), compared with 7.7% in those who met the 2014 targets but not the SPRINT targets, and 9.4% in those who failed to meet the 2014 treatment targets (J Am Coll Cardiol. 2016 Jun 13. doi 10.1016/j.jacc.2016.03.572).
After adjustment for factors such as age, diabetes, chronic kidney disease, hyperlipidemia, body mass index, and smoking, the least-controlled group showed a 62% increase in the risk of cardiovascular events, compared with the SPRINT criteria group. Those who met the 2014 criteria had a 17% greater risk than those who met the SPRINT criteria.
“Despite greater cardiovascular protection with intensive BP lowering, achieving SPRINT-defined BP goals might not be easy or practical because the target BP was not met in more than one-half of the participants in the intensive-treatment group,” the authors wrote.
Individuals who were older, female, or had diabetes, chronic kidney disease, or prevalent cardiovascular disease were more likely to meet the stricter goals of SPRINT (Systolic Blood Pressure Intervention Trial), in which combined cardiovascular events occurred in 5.2% of patients treated to a target systolic blood pressure of less than 120 mm Hg and 6.8% of patients treated to a target of less than 140 mm Hg (N Engl J Med. 2015;373:2103-16).
Researchers also noted a significant linear association between lesser blood pressure control and an increased risk of myocardial infarction and stroke, although there was no significant trend seen relating to cardiovascular or all-cause mortality. The authors noted that this was the opposite to what was observed in the original SPRINT trial, where there was a reduction in cardiovascular mortality and heart failure but only a modest, nonsignificant impact on MI or stroke.
“Although the exact reasons remain unclear, this discrepancy might be explained in part by differences in study design, population characteristics, clinical practice pattern, or race or ethnic groups,” they suggested. “The generalizability of the SPRINT experience to multiple groups of various ethnic backgrounds warrants further investigations and is likely to be of considerable interest.”
Unlike the SPRINT trial, the Korean analysis did not look into the potential adverse effects of more aggressive blood pressure–lowering, but the authors noted that the SPRINT trial did see an increased incidence of more serious adverse events, including hypotension, syncope, and acute kidney injury.
“Therefore, beyond the BP target per se, several important factors should be considered for optimal BP management in the contemporary medical setting; for example, an integrated and systematic assessment of combined risk factors and baseline cardiovascular risk, concomitant preventive medical therapies, cost-effectiveness, clinician-patient discussions of the potential benefits and harms, or the clinical judgment of the treating physician.”
The National Evidence-Based Healthcare Collaborating Agency, Seoul, South Korea, funded the study. No conflicts of interest were declared.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: The more aggressive SPRINT targets for blood pressure lowering reduce major cardiovascular events but significantly fewer people meet the treatment goals, compared with the 2014 recommendations.
Major finding: Only 11.9% of hypertensive adults would meet the treatment goals of the SPRINT criteria compared to 70.8% who would meet the 2014 recommendations.
Data source: Database study in two population-based Korean cohorts comprising 81,311 adults.
Disclosures: The National Evidence-Based Healthcare Collaborating Agency, Seoul, South Korea, funded the study. No conflicts of interest were declared.
Tissue flap reconstruction associated with higher costs, postop complication risk
LOS ANGELES – The use of locoregional tissue flaps in combination with abdominoperineal resection was associated with higher rates of perioperative complications, longer hospital stays, and higher total hospital charges, compared with patients who did not undergo tissue flap reconstruction, an analysis of national data showed.
The findings come at a time when closure of perineal wounds with tissue flaps is an increasingly common approach, especially in academic institutions, Dr. Nicole Lopez said at the annual meeting of the American Society of Colon and Rectal Surgeons. “The role of selection bias in this [study] is difficult to determine, but I think it’s important that we clarify the utility of this technique before more widespread adoption of the approach,” she said.
According to Dr. Lopez of the department of surgery at the University of North Carolina, Chapel Hill, perineal wound complications can occur in 16%-49% of patients undergoing abdominoperineal resection. Contributing factors include noncollapsible dead space, bacterial contamination, wound characteristics, and patient comorbidities.
In an effort to identify national trends in the use of tissue flaps in patients undergoing abdominoperineal resection for rectal or anal cancer, as well as the effect of this approach on perioperative complications, length of stay, and total hospital charges, Dr. Lopez and her associates used the National Inpatient Sample to identify patients aged 18-80 years who were treated between 2000 and 2013. They excluded patients undergoing nonelective procedures or additional pelvic organ resections. Patients who received a tissue flap were compared with those who did not.
Dr. Lopez reported results from 298 patients who received a tissue flap graft and 12,107 who did not. Variables significantly associated with receiving a tissue flap, compared with not receiving one, were being male (73% vs. 66%, respectively; P =. 01), having anal cancer (32% vs. 11%; P less than .0001), being a smoker (34% vs. 23%; P less than .0001), undergoing the procedure in a large hospital (75% vs. 67%; P = .003), and undergoing the procedure in an urban teaching hospital (89% vs. 53%; P less than .0001).
The researchers also found that the number of concurrent tissue flaps performed rose significantly during the study period, from 0.4% in 2000 to 6% in 2013 (P less than .0001). “This was most noted in teaching institutions, compared with nonteaching institutions,” Dr. Lopez said.
Bivariate analysis revealed that, compared with patients who did not receive tissue flaps, those who did had higher rates of postoperative complications (43% vs. 33%, respectively; P less than .0001), a longer hospital stay (mean of 9 vs. 7 days; P less than .001), and higher total hospital charges (mean of $67,200 vs. $42,300; P less than .001). These trends persisted on multivariate analysis. Specifically, patients who received tissue flaps were 4.14 times more likely to have wound complications, had a length of stay that averaged an additional 2.78 days, and had $28,000 more in total hospital charges.
“The extended duration of the study enables evaluation of trends over time, and this is the first study that analyzes the costs associated with these procedures,” Dr. Lopez said. She acknowledged certain limitations of the study, including its retrospective, nonrandomized design and the potential for selection bias. In addition, the National Inpatient Sample “is susceptible to coding errors, a lack of patient-specific oncologic history, and the inability to assess postdischarge occurrences, since this only looks at inpatient stays.”
Dr. Lopez reported having no financial disclosures.
LOS ANGELES – The use of locoregional tissue flaps in combination with abdominoperineal resection was associated with higher rates of perioperative complications, longer hospital stays, and higher total hospital charges, compared with patients who did not undergo tissue flap reconstruction, an analysis of national data showed.
The findings come at a time when closure of perineal wounds with tissue flaps is an increasingly common approach, especially in academic institutions, Dr. Nicole Lopez said at the annual meeting of the American Society of Colon and Rectal Surgeons. “The role of selection bias in this [study] is difficult to determine, but I think it’s important that we clarify the utility of this technique before more widespread adoption of the approach,” she said.
According to Dr. Lopez of the department of surgery at the University of North Carolina, Chapel Hill, perineal wound complications can occur in 16%-49% of patients undergoing abdominoperineal resection. Contributing factors include noncollapsible dead space, bacterial contamination, wound characteristics, and patient comorbidities.
In an effort to identify national trends in the use of tissue flaps in patients undergoing abdominoperineal resection for rectal or anal cancer, as well as the effect of this approach on perioperative complications, length of stay, and total hospital charges, Dr. Lopez and her associates used the National Inpatient Sample to identify patients aged 18-80 years who were treated between 2000 and 2013. They excluded patients undergoing nonelective procedures or additional pelvic organ resections. Patients who received a tissue flap were compared with those who did not.
Dr. Lopez reported results from 298 patients who received a tissue flap graft and 12,107 who did not. Variables significantly associated with receiving a tissue flap, compared with not receiving one, were being male (73% vs. 66%, respectively; P =. 01), having anal cancer (32% vs. 11%; P less than .0001), being a smoker (34% vs. 23%; P less than .0001), undergoing the procedure in a large hospital (75% vs. 67%; P = .003), and undergoing the procedure in an urban teaching hospital (89% vs. 53%; P less than .0001).
The researchers also found that the number of concurrent tissue flaps performed rose significantly during the study period, from 0.4% in 2000 to 6% in 2013 (P less than .0001). “This was most noted in teaching institutions, compared with nonteaching institutions,” Dr. Lopez said.
Bivariate analysis revealed that, compared with patients who did not receive tissue flaps, those who did had higher rates of postoperative complications (43% vs. 33%, respectively; P less than .0001), a longer hospital stay (mean of 9 vs. 7 days; P less than .001), and higher total hospital charges (mean of $67,200 vs. $42,300; P less than .001). These trends persisted on multivariate analysis. Specifically, patients who received tissue flaps were 4.14 times more likely to have wound complications, had a length of stay that averaged an additional 2.78 days, and had $28,000 more in total hospital charges.
“The extended duration of the study enables evaluation of trends over time, and this is the first study that analyzes the costs associated with these procedures,” Dr. Lopez said. She acknowledged certain limitations of the study, including its retrospective, nonrandomized design and the potential for selection bias. In addition, the National Inpatient Sample “is susceptible to coding errors, a lack of patient-specific oncologic history, and the inability to assess postdischarge occurrences, since this only looks at inpatient stays.”
Dr. Lopez reported having no financial disclosures.
LOS ANGELES – The use of locoregional tissue flaps in combination with abdominoperineal resection was associated with higher rates of perioperative complications, longer hospital stays, and higher total hospital charges, compared with patients who did not undergo tissue flap reconstruction, an analysis of national data showed.
The findings come at a time when closure of perineal wounds with tissue flaps is an increasingly common approach, especially in academic institutions, Dr. Nicole Lopez said at the annual meeting of the American Society of Colon and Rectal Surgeons. “The role of selection bias in this [study] is difficult to determine, but I think it’s important that we clarify the utility of this technique before more widespread adoption of the approach,” she said.
According to Dr. Lopez of the department of surgery at the University of North Carolina, Chapel Hill, perineal wound complications can occur in 16%-49% of patients undergoing abdominoperineal resection. Contributing factors include noncollapsible dead space, bacterial contamination, wound characteristics, and patient comorbidities.
In an effort to identify national trends in the use of tissue flaps in patients undergoing abdominoperineal resection for rectal or anal cancer, as well as the effect of this approach on perioperative complications, length of stay, and total hospital charges, Dr. Lopez and her associates used the National Inpatient Sample to identify patients aged 18-80 years who were treated between 2000 and 2013. They excluded patients undergoing nonelective procedures or additional pelvic organ resections. Patients who received a tissue flap were compared with those who did not.
Dr. Lopez reported results from 298 patients who received a tissue flap graft and 12,107 who did not. Variables significantly associated with receiving a tissue flap, compared with not receiving one, were being male (73% vs. 66%, respectively; P =. 01), having anal cancer (32% vs. 11%; P less than .0001), being a smoker (34% vs. 23%; P less than .0001), undergoing the procedure in a large hospital (75% vs. 67%; P = .003), and undergoing the procedure in an urban teaching hospital (89% vs. 53%; P less than .0001).
The researchers also found that the number of concurrent tissue flaps performed rose significantly during the study period, from 0.4% in 2000 to 6% in 2013 (P less than .0001). “This was most noted in teaching institutions, compared with nonteaching institutions,” Dr. Lopez said.
Bivariate analysis revealed that, compared with patients who did not receive tissue flaps, those who did had higher rates of postoperative complications (43% vs. 33%, respectively; P less than .0001), a longer hospital stay (mean of 9 vs. 7 days; P less than .001), and higher total hospital charges (mean of $67,200 vs. $42,300; P less than .001). These trends persisted on multivariate analysis. Specifically, patients who received tissue flaps were 4.14 times more likely to have wound complications, had a length of stay that averaged an additional 2.78 days, and had $28,000 more in total hospital charges.
“The extended duration of the study enables evaluation of trends over time, and this is the first study that analyzes the costs associated with these procedures,” Dr. Lopez said. She acknowledged certain limitations of the study, including its retrospective, nonrandomized design and the potential for selection bias. In addition, the National Inpatient Sample “is susceptible to coding errors, a lack of patient-specific oncologic history, and the inability to assess postdischarge occurrences, since this only looks at inpatient stays.”
Dr. Lopez reported having no financial disclosures.
AT THE ASCRS ANNUAL MEETING
Key clinical point: Complications occurred more often in patients who underwent concurrent tissue flap reconstruction during abdominoperineal resection, compared with those who did not.
Major finding: Compared with patients who did not receive tissue flaps, those who did were 4.14 times more likely to have wound complications, had a length of stay that averaged an additional 2.78 days, and had $28,000 more in total hospital charges.
Data source: A study of 12,405 patients aged 18-80 years from the National Inpatient Sample who underwent abdominoperineal resection for rectal or anal cancer between 2000 and 2013.
Disclosures: Dr. Lopez reported having no financial disclosures.
Check out our Vascular Annual Meeting Coverage Online
For individual stories every day and to see the complete daily issues of Vascular Connections, the official on-site newspaper of the Vascular Annual Meeting, check us out at our website.
Featuring the President’s Address, the E. Stanley Crawford Critical Issues Forum and ‘person on the street’ interviews, as well as plenary talks and breakfast sessions, it is your one stop shopping for coverage.
And be sure to follow our continuing advanced coverage of the Vascular Annual Meeting in future issues of Vascular Specialist.
For individual stories every day and to see the complete daily issues of Vascular Connections, the official on-site newspaper of the Vascular Annual Meeting, check us out at our website.
Featuring the President’s Address, the E. Stanley Crawford Critical Issues Forum and ‘person on the street’ interviews, as well as plenary talks and breakfast sessions, it is your one stop shopping for coverage.
And be sure to follow our continuing advanced coverage of the Vascular Annual Meeting in future issues of Vascular Specialist.
For individual stories every day and to see the complete daily issues of Vascular Connections, the official on-site newspaper of the Vascular Annual Meeting, check us out at our website.
Featuring the President’s Address, the E. Stanley Crawford Critical Issues Forum and ‘person on the street’ interviews, as well as plenary talks and breakfast sessions, it is your one stop shopping for coverage.
And be sure to follow our continuing advanced coverage of the Vascular Annual Meeting in future issues of Vascular Specialist.
VIDEO: ASD doesn’t appear any more prevalent in children with type 1 diabetes
NEW ORLEANS – The prevalence of autism spectrum disorder in children and adolescents with type 1 diabetes appears to be similar to that in the general pediatric population, according to a study conducted in Colorado.
“There is no known literature on management of patients with autism spectrum disorder and type 1 diabetes to assess if management is different in this population,” Dr. Shideh Majidi said in an interview at the annual scientific sessions of the American Diabetes Association.
In what she said is the first study of its kind conducted in the United States, Dr. Majidi and her associates investigated the prevalence of autism spectrum disorder (ASD) in a large diabetes center to better understand the diabetes characteristics and management of those with both type 1 diabetes and ASD. The researchers evaluated 2,360 patients aged 18 months to 18 years cared for at the Barbara Davis Center for Childhood Diabetes at the University of Colorado, Aurora. Of the 2,360 patients, 30 (28 males and 2 females) had ASD, for a prevalence of 1 in 87 (1.15%). This was similar to the prevalence of ASD in the general Colorado population, which is estimated to be 1 in 85 (1.18%).
Patients with type 1 diabetes and ASD had a mean age of 12.9 years and had the disease for a mean of 5 years. There were fewer females with type 1 diabetes and ASD, compared with those who had type 1 diabetes only (7% vs. 48%, respectively; P less than .001).
Compared with patients who had type 1 diabetes, those with type 1 diabetes and ASD had similar hemoglobin A1c levels (a median of 8.2% vs. 8.8%, P = .17) and number of blood glucose tests per day (a median of 5.1 vs. 4.9, P = .32), but were less likely to be on an insulin pump (43.3% vs. 57%, P = .14).
The overall findings suggest that management in patients with ASD and type 1 diabetes does not necessarily need to differ from those without ASD. “For instance, it is possible for ASD patients to do well on an insulin pump,” said Dr. Majidi, who is a pediatric endocrinologist at the Barbara Davis Center for Childhood Diabetes. “Also, A1c and blood sugar checks are similar between those with and without ASD, and thus similar intensive management can be recommended for this group. So just like in patients without ASD, diabetes should be managed on an individual basis, looking at individual needs, but having a diagnosis of ASD does not have to limit our views as providers of what types of management we can offer for ideal diabetes management.”
She acknowledged certain limitations of the study, including its single-center design and relatively small sample size. “It would be beneficial to obtain larger numbers of patients with ASD and type 1 diabetes via multicenter studies in order to get a larger group of patients with both diagnoses, in order to see if our results remain when looking on a larger scale.”
In a video interview at the meeting, Dr. Majidi and Dr. Kelly Stanek of the Barbara Davis Center for Childhood Diabetes discussed the study's findings and the next steps for research, including a closer examination of the challenges parents face in caring for children with type 1 diabetes and ASD.
Dr. Majidi and Dr. Stanek reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – The prevalence of autism spectrum disorder in children and adolescents with type 1 diabetes appears to be similar to that in the general pediatric population, according to a study conducted in Colorado.
“There is no known literature on management of patients with autism spectrum disorder and type 1 diabetes to assess if management is different in this population,” Dr. Shideh Majidi said in an interview at the annual scientific sessions of the American Diabetes Association.
In what she said is the first study of its kind conducted in the United States, Dr. Majidi and her associates investigated the prevalence of autism spectrum disorder (ASD) in a large diabetes center to better understand the diabetes characteristics and management of those with both type 1 diabetes and ASD. The researchers evaluated 2,360 patients aged 18 months to 18 years cared for at the Barbara Davis Center for Childhood Diabetes at the University of Colorado, Aurora. Of the 2,360 patients, 30 (28 males and 2 females) had ASD, for a prevalence of 1 in 87 (1.15%). This was similar to the prevalence of ASD in the general Colorado population, which is estimated to be 1 in 85 (1.18%).
Patients with type 1 diabetes and ASD had a mean age of 12.9 years and had the disease for a mean of 5 years. There were fewer females with type 1 diabetes and ASD, compared with those who had type 1 diabetes only (7% vs. 48%, respectively; P less than .001).
Compared with patients who had type 1 diabetes, those with type 1 diabetes and ASD had similar hemoglobin A1c levels (a median of 8.2% vs. 8.8%, P = .17) and number of blood glucose tests per day (a median of 5.1 vs. 4.9, P = .32), but were less likely to be on an insulin pump (43.3% vs. 57%, P = .14).
The overall findings suggest that management in patients with ASD and type 1 diabetes does not necessarily need to differ from those without ASD. “For instance, it is possible for ASD patients to do well on an insulin pump,” said Dr. Majidi, who is a pediatric endocrinologist at the Barbara Davis Center for Childhood Diabetes. “Also, A1c and blood sugar checks are similar between those with and without ASD, and thus similar intensive management can be recommended for this group. So just like in patients without ASD, diabetes should be managed on an individual basis, looking at individual needs, but having a diagnosis of ASD does not have to limit our views as providers of what types of management we can offer for ideal diabetes management.”
She acknowledged certain limitations of the study, including its single-center design and relatively small sample size. “It would be beneficial to obtain larger numbers of patients with ASD and type 1 diabetes via multicenter studies in order to get a larger group of patients with both diagnoses, in order to see if our results remain when looking on a larger scale.”
In a video interview at the meeting, Dr. Majidi and Dr. Kelly Stanek of the Barbara Davis Center for Childhood Diabetes discussed the study's findings and the next steps for research, including a closer examination of the challenges parents face in caring for children with type 1 diabetes and ASD.
Dr. Majidi and Dr. Stanek reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – The prevalence of autism spectrum disorder in children and adolescents with type 1 diabetes appears to be similar to that in the general pediatric population, according to a study conducted in Colorado.
“There is no known literature on management of patients with autism spectrum disorder and type 1 diabetes to assess if management is different in this population,” Dr. Shideh Majidi said in an interview at the annual scientific sessions of the American Diabetes Association.
In what she said is the first study of its kind conducted in the United States, Dr. Majidi and her associates investigated the prevalence of autism spectrum disorder (ASD) in a large diabetes center to better understand the diabetes characteristics and management of those with both type 1 diabetes and ASD. The researchers evaluated 2,360 patients aged 18 months to 18 years cared for at the Barbara Davis Center for Childhood Diabetes at the University of Colorado, Aurora. Of the 2,360 patients, 30 (28 males and 2 females) had ASD, for a prevalence of 1 in 87 (1.15%). This was similar to the prevalence of ASD in the general Colorado population, which is estimated to be 1 in 85 (1.18%).
Patients with type 1 diabetes and ASD had a mean age of 12.9 years and had the disease for a mean of 5 years. There were fewer females with type 1 diabetes and ASD, compared with those who had type 1 diabetes only (7% vs. 48%, respectively; P less than .001).
Compared with patients who had type 1 diabetes, those with type 1 diabetes and ASD had similar hemoglobin A1c levels (a median of 8.2% vs. 8.8%, P = .17) and number of blood glucose tests per day (a median of 5.1 vs. 4.9, P = .32), but were less likely to be on an insulin pump (43.3% vs. 57%, P = .14).
The overall findings suggest that management in patients with ASD and type 1 diabetes does not necessarily need to differ from those without ASD. “For instance, it is possible for ASD patients to do well on an insulin pump,” said Dr. Majidi, who is a pediatric endocrinologist at the Barbara Davis Center for Childhood Diabetes. “Also, A1c and blood sugar checks are similar between those with and without ASD, and thus similar intensive management can be recommended for this group. So just like in patients without ASD, diabetes should be managed on an individual basis, looking at individual needs, but having a diagnosis of ASD does not have to limit our views as providers of what types of management we can offer for ideal diabetes management.”
She acknowledged certain limitations of the study, including its single-center design and relatively small sample size. “It would be beneficial to obtain larger numbers of patients with ASD and type 1 diabetes via multicenter studies in order to get a larger group of patients with both diagnoses, in order to see if our results remain when looking on a larger scale.”
In a video interview at the meeting, Dr. Majidi and Dr. Kelly Stanek of the Barbara Davis Center for Childhood Diabetes discussed the study's findings and the next steps for research, including a closer examination of the challenges parents face in caring for children with type 1 diabetes and ASD.
Dr. Majidi and Dr. Stanek reported having no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ADA SCIENTIFIC SESSIONS
Key clinical point: Overall, the prevalence of autism spectrum disorder among Colorado youth with type 1 diabetes is similar to the prevalence of ASD in the general Colorado pediatric population.
Major finding: The prevalence of pediatric patients in Colorado with type 1 diabetes and ASD was 1 in 87 (1.15%), which was similar to the prevalence of ASD in the general Colorado pediatric population, 1 in 85 (1.18%).
Data source: An analysis of 2,360 patients with type 1 diabetes aged 18 months to 18 years old who were cared for at a single center in Colorado.
Disclosures: Dr. Majidi and Dr. Stanek reported having no relevant financial disclosures.
Transgender youth can successfully transition to adulthood
ORLANDO – In the case of transgender youth and adults – those with what is now called gender dysphoria – physicians are faced with treating individuals who generally have no physical disease or abnormalities.
Endocrinologists are the professionals who are often tasked with the medical aspects of treating gender dysphoria. In an effort to help them understand the underpinnings and aspects of the conditions, Dr. Stephen Rosenthal, professor of pediatrics and medical director of the Child and Adolescent Medical Gender Center at the University of California, San Francisco, reviewed current knowledge about the biological basis for gender identity, current treatment models, and barriers to care of patients with gender dysphoria.
Speaking at the annual meeting of the American Association of Clinical Endocrinologists, Dr. Rosenthal said the dysphoria derives from the significant emotional distress that may be associated with a transgender identity, essentially from the social and psychological pressures of being born and living in a body (the “natal sex”) that does not match an individual’s gender identity, defined as one’s fundamental sense of self as male or female. “It’s not always limited to those two choices, and it’s not always binary,” he said, since individuals may identify with aspects of both or, at times, neither gender.
He defined some terms, such as transgender, which refers to a transient or persistent identification with gender different from the one others assume based on physical sex characteristics at birth. That gender becomes the one of rearing, which may not be how the individual feels growing up. Gender identity should not be confused with sexual identity or orientation because people of any gender can have any sexual orientation.
“Gender Identity Disorder,” a term used in the DSM IV (Diagnostic and Statistical Manual of Mental Disorders IV) has been replaced by “Gender Dysphoria in Children” in DSM 5. And even that term may be revised since transgender identity in itself is not a pathology.
In one survey of 28,176 people, 0.5% self-identified as transgender. Another survey showed statistically significant risks associated with being transgender. Comparing 180 transgender youth with 180 non–transgender youth (average age, 19.6 years; range, 12-29 years), researchers found a two- to threefold increased risk of depression, anxiety disorder, suicidal ideation, suicide attempt, and self- harm without lethal intent among the transgender youths. Parental support helped alleviate some of these risks, especially suicide attempts, but did not eliminate them entirely; that support also contributed to better mental and physical health, improved self-esteem, and even adequate food and housing for transgender adolescents.
Clues to biological influences
A complex interplay of biological, environmental, and cultural factors affect the determination of gender identity. Evidence points to the role of biology in gender identity development through studies of genetics, hormones, and the brain, but none of these is a “litmus test” for gender identity, Dr. Rosenthal said.
A study of 23 monozygotic twin pairs, 21 same-sex dizygotic twin pairs, and 7 opposite-sex twin pairs showed a 39.1% concordance for gender dysphoria among the monozygotic twins but none for the other sets.
Most transgender individuals do not have any obvious disorder of sexual development, but that is not to rule out a role of prenatal or postnatal androgens (specifically enzymes of the steroid pathways), androgen insensitivity, or extragonadal sources of androgen, as in congenital adrenal hyperplasia (CAH). Among 250 46XX females with CAH raised as female, 5.2% had male gender identity or gender dysphoria (a 10- to 20-fold increased risk vs. controls), suggesting a possible role for prenatal androgens in gender identity development.
A neurobiological basis for transgender is supported by some studies of sexually dimorphic brain structures but is by no means conclusive. Numerous studies of gray and white matter showed that sexually dimorphic structures are more closely aligned with gender identity than with physical sex (even before cross-sex hormones have been applied). But morphometry on areas of the brain that show the largest sex differences found that variability was more prevalent than was consistency in the 1,400 brains studied.
Tests of “functional sexual dimorphism” used PET or MRI to measure changes in regional blood flow in the anterior hypothalamus when control adolescent girls or boys or those with gender dysphoria were asked to smell substances containing pheromones of the opposite sex (for girls: androstadienone in a mixture of male sweat and semen; for boys: estrogen-like compounds in urine of pregnant women). Both girls and boys with gender dysphoria had responses significantly different from those of their respective controls.
Natural history of gender dysphoria
Dr. Rosenthal said symptoms of gender dysphoria in prepubertal children decrease or disappear in 70%-95% of cases, but if they persist into early puberty, the individual is likely to be transgender as an adult. Children with more intense gender dysphoria and those who believed they “were” the opposite sex were more likely to have persistent gender dysphoria as adults. In a study based on parents’ completed measures, prepubescent transgender boys and girls who have socially transitioned had depression scores no higher than those of matched nontransgender controls. They had much lower anxiety and depression, compared with non–socially transitioned transgender historical control children.
Medically induced sexual transitioning
For pediatric and adolescent transsexual patients who express a desire to transition to the opposite sex, an Endocrine Society clinical practice guideline on endocrine treatment recommends that a mental health professional make the diagnosis of gender dysphoria. Then the medical provider needs to ensure that the patient understands the consequences of hormone suppression and cross-sex hormone therapy prior to beginning treatments. Only after early puberty has begun should gonadotropin-releasing hormone (GnRH) agonists be used to suppress pubertal hormones. At about age 16 years, cross-sex hormone treatments can begin, with surgery deferred at least until age 18 years if the patient desires full transitioning.
A Dutch study (Pediatrics. 2014 Oct. 134:696-704) showed that after gender reassignment, in young adulthood, gender dysphoria “was alleviated and psychological functioning had steadily improved. Well-being was similar to or better than same-age young adults from the general population.” No patients reported any regret during any stages of the sex-reassignment protocol.
There is some concern about adverse effects of the GnRH agonists, such as on bone mass and health, the brain, and fertility. But no detrimental effects were observed in a study on executive functioning, which undergoes significant development during puberty, in either male-to-female or female-to-male individuals.
Future parenthood may be an option if the patient is old enough. “We always encourage them to either freeze sperm, or we can potentially freeze eggs before embarking on phenotypic transition,” Dr. Rosenthal said. But allowing a patient to get to a stage of spermatogenesis or egg production would allow puberty to proceed to a significant degree. “So one of the exciting areas of research is actually taking prepubertal tissue … [in mice] they took neonatal testicular tissue and they basically showed you could take it all the way through the steps of full maturation and get progeny that were reproductively competent,” he said. Similar studies are being done in humans, mainly because there is interest in preserving fertility of children undergoing cancer treatments.
Barriers to care for transgender youth include limited access to medications, including off-label use, great expense, and insurance company denials of reimbursement. There are also relatively few clinical programs and a lack of training for health care professionals, as well as prejudice and misunderstanding, even among professionals.
ORLANDO – In the case of transgender youth and adults – those with what is now called gender dysphoria – physicians are faced with treating individuals who generally have no physical disease or abnormalities.
Endocrinologists are the professionals who are often tasked with the medical aspects of treating gender dysphoria. In an effort to help them understand the underpinnings and aspects of the conditions, Dr. Stephen Rosenthal, professor of pediatrics and medical director of the Child and Adolescent Medical Gender Center at the University of California, San Francisco, reviewed current knowledge about the biological basis for gender identity, current treatment models, and barriers to care of patients with gender dysphoria.
Speaking at the annual meeting of the American Association of Clinical Endocrinologists, Dr. Rosenthal said the dysphoria derives from the significant emotional distress that may be associated with a transgender identity, essentially from the social and psychological pressures of being born and living in a body (the “natal sex”) that does not match an individual’s gender identity, defined as one’s fundamental sense of self as male or female. “It’s not always limited to those two choices, and it’s not always binary,” he said, since individuals may identify with aspects of both or, at times, neither gender.
He defined some terms, such as transgender, which refers to a transient or persistent identification with gender different from the one others assume based on physical sex characteristics at birth. That gender becomes the one of rearing, which may not be how the individual feels growing up. Gender identity should not be confused with sexual identity or orientation because people of any gender can have any sexual orientation.
“Gender Identity Disorder,” a term used in the DSM IV (Diagnostic and Statistical Manual of Mental Disorders IV) has been replaced by “Gender Dysphoria in Children” in DSM 5. And even that term may be revised since transgender identity in itself is not a pathology.
In one survey of 28,176 people, 0.5% self-identified as transgender. Another survey showed statistically significant risks associated with being transgender. Comparing 180 transgender youth with 180 non–transgender youth (average age, 19.6 years; range, 12-29 years), researchers found a two- to threefold increased risk of depression, anxiety disorder, suicidal ideation, suicide attempt, and self- harm without lethal intent among the transgender youths. Parental support helped alleviate some of these risks, especially suicide attempts, but did not eliminate them entirely; that support also contributed to better mental and physical health, improved self-esteem, and even adequate food and housing for transgender adolescents.
Clues to biological influences
A complex interplay of biological, environmental, and cultural factors affect the determination of gender identity. Evidence points to the role of biology in gender identity development through studies of genetics, hormones, and the brain, but none of these is a “litmus test” for gender identity, Dr. Rosenthal said.
A study of 23 monozygotic twin pairs, 21 same-sex dizygotic twin pairs, and 7 opposite-sex twin pairs showed a 39.1% concordance for gender dysphoria among the monozygotic twins but none for the other sets.
Most transgender individuals do not have any obvious disorder of sexual development, but that is not to rule out a role of prenatal or postnatal androgens (specifically enzymes of the steroid pathways), androgen insensitivity, or extragonadal sources of androgen, as in congenital adrenal hyperplasia (CAH). Among 250 46XX females with CAH raised as female, 5.2% had male gender identity or gender dysphoria (a 10- to 20-fold increased risk vs. controls), suggesting a possible role for prenatal androgens in gender identity development.
A neurobiological basis for transgender is supported by some studies of sexually dimorphic brain structures but is by no means conclusive. Numerous studies of gray and white matter showed that sexually dimorphic structures are more closely aligned with gender identity than with physical sex (even before cross-sex hormones have been applied). But morphometry on areas of the brain that show the largest sex differences found that variability was more prevalent than was consistency in the 1,400 brains studied.
Tests of “functional sexual dimorphism” used PET or MRI to measure changes in regional blood flow in the anterior hypothalamus when control adolescent girls or boys or those with gender dysphoria were asked to smell substances containing pheromones of the opposite sex (for girls: androstadienone in a mixture of male sweat and semen; for boys: estrogen-like compounds in urine of pregnant women). Both girls and boys with gender dysphoria had responses significantly different from those of their respective controls.
Natural history of gender dysphoria
Dr. Rosenthal said symptoms of gender dysphoria in prepubertal children decrease or disappear in 70%-95% of cases, but if they persist into early puberty, the individual is likely to be transgender as an adult. Children with more intense gender dysphoria and those who believed they “were” the opposite sex were more likely to have persistent gender dysphoria as adults. In a study based on parents’ completed measures, prepubescent transgender boys and girls who have socially transitioned had depression scores no higher than those of matched nontransgender controls. They had much lower anxiety and depression, compared with non–socially transitioned transgender historical control children.
Medically induced sexual transitioning
For pediatric and adolescent transsexual patients who express a desire to transition to the opposite sex, an Endocrine Society clinical practice guideline on endocrine treatment recommends that a mental health professional make the diagnosis of gender dysphoria. Then the medical provider needs to ensure that the patient understands the consequences of hormone suppression and cross-sex hormone therapy prior to beginning treatments. Only after early puberty has begun should gonadotropin-releasing hormone (GnRH) agonists be used to suppress pubertal hormones. At about age 16 years, cross-sex hormone treatments can begin, with surgery deferred at least until age 18 years if the patient desires full transitioning.
A Dutch study (Pediatrics. 2014 Oct. 134:696-704) showed that after gender reassignment, in young adulthood, gender dysphoria “was alleviated and psychological functioning had steadily improved. Well-being was similar to or better than same-age young adults from the general population.” No patients reported any regret during any stages of the sex-reassignment protocol.
There is some concern about adverse effects of the GnRH agonists, such as on bone mass and health, the brain, and fertility. But no detrimental effects were observed in a study on executive functioning, which undergoes significant development during puberty, in either male-to-female or female-to-male individuals.
Future parenthood may be an option if the patient is old enough. “We always encourage them to either freeze sperm, or we can potentially freeze eggs before embarking on phenotypic transition,” Dr. Rosenthal said. But allowing a patient to get to a stage of spermatogenesis or egg production would allow puberty to proceed to a significant degree. “So one of the exciting areas of research is actually taking prepubertal tissue … [in mice] they took neonatal testicular tissue and they basically showed you could take it all the way through the steps of full maturation and get progeny that were reproductively competent,” he said. Similar studies are being done in humans, mainly because there is interest in preserving fertility of children undergoing cancer treatments.
Barriers to care for transgender youth include limited access to medications, including off-label use, great expense, and insurance company denials of reimbursement. There are also relatively few clinical programs and a lack of training for health care professionals, as well as prejudice and misunderstanding, even among professionals.
ORLANDO – In the case of transgender youth and adults – those with what is now called gender dysphoria – physicians are faced with treating individuals who generally have no physical disease or abnormalities.
Endocrinologists are the professionals who are often tasked with the medical aspects of treating gender dysphoria. In an effort to help them understand the underpinnings and aspects of the conditions, Dr. Stephen Rosenthal, professor of pediatrics and medical director of the Child and Adolescent Medical Gender Center at the University of California, San Francisco, reviewed current knowledge about the biological basis for gender identity, current treatment models, and barriers to care of patients with gender dysphoria.
Speaking at the annual meeting of the American Association of Clinical Endocrinologists, Dr. Rosenthal said the dysphoria derives from the significant emotional distress that may be associated with a transgender identity, essentially from the social and psychological pressures of being born and living in a body (the “natal sex”) that does not match an individual’s gender identity, defined as one’s fundamental sense of self as male or female. “It’s not always limited to those two choices, and it’s not always binary,” he said, since individuals may identify with aspects of both or, at times, neither gender.
He defined some terms, such as transgender, which refers to a transient or persistent identification with gender different from the one others assume based on physical sex characteristics at birth. That gender becomes the one of rearing, which may not be how the individual feels growing up. Gender identity should not be confused with sexual identity or orientation because people of any gender can have any sexual orientation.
“Gender Identity Disorder,” a term used in the DSM IV (Diagnostic and Statistical Manual of Mental Disorders IV) has been replaced by “Gender Dysphoria in Children” in DSM 5. And even that term may be revised since transgender identity in itself is not a pathology.
In one survey of 28,176 people, 0.5% self-identified as transgender. Another survey showed statistically significant risks associated with being transgender. Comparing 180 transgender youth with 180 non–transgender youth (average age, 19.6 years; range, 12-29 years), researchers found a two- to threefold increased risk of depression, anxiety disorder, suicidal ideation, suicide attempt, and self- harm without lethal intent among the transgender youths. Parental support helped alleviate some of these risks, especially suicide attempts, but did not eliminate them entirely; that support also contributed to better mental and physical health, improved self-esteem, and even adequate food and housing for transgender adolescents.
Clues to biological influences
A complex interplay of biological, environmental, and cultural factors affect the determination of gender identity. Evidence points to the role of biology in gender identity development through studies of genetics, hormones, and the brain, but none of these is a “litmus test” for gender identity, Dr. Rosenthal said.
A study of 23 monozygotic twin pairs, 21 same-sex dizygotic twin pairs, and 7 opposite-sex twin pairs showed a 39.1% concordance for gender dysphoria among the monozygotic twins but none for the other sets.
Most transgender individuals do not have any obvious disorder of sexual development, but that is not to rule out a role of prenatal or postnatal androgens (specifically enzymes of the steroid pathways), androgen insensitivity, or extragonadal sources of androgen, as in congenital adrenal hyperplasia (CAH). Among 250 46XX females with CAH raised as female, 5.2% had male gender identity or gender dysphoria (a 10- to 20-fold increased risk vs. controls), suggesting a possible role for prenatal androgens in gender identity development.
A neurobiological basis for transgender is supported by some studies of sexually dimorphic brain structures but is by no means conclusive. Numerous studies of gray and white matter showed that sexually dimorphic structures are more closely aligned with gender identity than with physical sex (even before cross-sex hormones have been applied). But morphometry on areas of the brain that show the largest sex differences found that variability was more prevalent than was consistency in the 1,400 brains studied.
Tests of “functional sexual dimorphism” used PET or MRI to measure changes in regional blood flow in the anterior hypothalamus when control adolescent girls or boys or those with gender dysphoria were asked to smell substances containing pheromones of the opposite sex (for girls: androstadienone in a mixture of male sweat and semen; for boys: estrogen-like compounds in urine of pregnant women). Both girls and boys with gender dysphoria had responses significantly different from those of their respective controls.
Natural history of gender dysphoria
Dr. Rosenthal said symptoms of gender dysphoria in prepubertal children decrease or disappear in 70%-95% of cases, but if they persist into early puberty, the individual is likely to be transgender as an adult. Children with more intense gender dysphoria and those who believed they “were” the opposite sex were more likely to have persistent gender dysphoria as adults. In a study based on parents’ completed measures, prepubescent transgender boys and girls who have socially transitioned had depression scores no higher than those of matched nontransgender controls. They had much lower anxiety and depression, compared with non–socially transitioned transgender historical control children.
Medically induced sexual transitioning
For pediatric and adolescent transsexual patients who express a desire to transition to the opposite sex, an Endocrine Society clinical practice guideline on endocrine treatment recommends that a mental health professional make the diagnosis of gender dysphoria. Then the medical provider needs to ensure that the patient understands the consequences of hormone suppression and cross-sex hormone therapy prior to beginning treatments. Only after early puberty has begun should gonadotropin-releasing hormone (GnRH) agonists be used to suppress pubertal hormones. At about age 16 years, cross-sex hormone treatments can begin, with surgery deferred at least until age 18 years if the patient desires full transitioning.
A Dutch study (Pediatrics. 2014 Oct. 134:696-704) showed that after gender reassignment, in young adulthood, gender dysphoria “was alleviated and psychological functioning had steadily improved. Well-being was similar to or better than same-age young adults from the general population.” No patients reported any regret during any stages of the sex-reassignment protocol.
There is some concern about adverse effects of the GnRH agonists, such as on bone mass and health, the brain, and fertility. But no detrimental effects were observed in a study on executive functioning, which undergoes significant development during puberty, in either male-to-female or female-to-male individuals.
Future parenthood may be an option if the patient is old enough. “We always encourage them to either freeze sperm, or we can potentially freeze eggs before embarking on phenotypic transition,” Dr. Rosenthal said. But allowing a patient to get to a stage of spermatogenesis or egg production would allow puberty to proceed to a significant degree. “So one of the exciting areas of research is actually taking prepubertal tissue … [in mice] they took neonatal testicular tissue and they basically showed you could take it all the way through the steps of full maturation and get progeny that were reproductively competent,” he said. Similar studies are being done in humans, mainly because there is interest in preserving fertility of children undergoing cancer treatments.
Barriers to care for transgender youth include limited access to medications, including off-label use, great expense, and insurance company denials of reimbursement. There are also relatively few clinical programs and a lack of training for health care professionals, as well as prejudice and misunderstanding, even among professionals.
EXPERT ANALYSIS FROM AACE 2016