User login
Psoriatic flare assessment tool in validation stage
MIAMI – Reaching a consensus on measurement and assessment of flare in psoriatic arthritis remains challenging, especially with no widely accepted definition and differing opinions among patients and physicians. But getting standard evaluation of flare under control is critical for both clinical and research outcomes.
Through a series of patient interviews, physician surveys, and lessons learned in rheumatoid arthritis, the GRAPPA Flare Project is close to a validated flare instrument. A 10-question flare assessment tool is now in the final validation stage, according to a presentation at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“Flare is a clearly personal experience and varies from patient to patient. It’s clear that physicians and patients have clearly different ideas of what flare is. Bringing those two worlds together was a challenge,” Philip Helliwell, MD, of Leeds (England) University and GRAPPA president, said at the meeting.
“Some societies – I’m not going to mention any names – have gone down the route of a definition of flare linked to disease activity measures. We have not done this,” Dr. Helliwell said.
Instead, the Flare Project is taking a patient-driven and physician-reviewed approach. Investigators cast a wide net, identifying 79 factors important to patients in six major domains: skin, joint, emotional, participation, fatigue, and unclassified. The results were published in 2015 (Rheumatology [Oxford]. 2015 Aug;54[8]:1448-53). Through a Delphi survey, physicians reviewed these considerations. Then GRAPPA identified items important to both patients and physicians and developed a preliminary flare instrument.
Unlike most assessment tools for psoriatic disease, the new instrument includes patient-reported emotional well-being. Niti Goel, MD, of Duke University, Durham, N.C., said that physicians do not always ask patients about the psychological impact of their disease, so the tool “could provide additional information.”
Another goal of the project is to provide standard answers to some of the common questions related to psoriatic arthritis, including: What exactly is a flare? Does the definition truly capture the worsening of disease? Are there different types of flares? Is flare different if you start from a point of high disease activity? What self-management and other interventions are effective for flare?
GRAPPA adopted a definition of flare developed from rheumatoid arthritis, stating that flare is any worsening of disease activity that would, if persistent, in most cases lead to initiation or change of therapy (J Rheumatol. 2009 Oct;36[10]:2335-41).
“It took [them] some time to get to that definition,” Dr. Helliwell said. “We know from rheumatoid arthritis that there is a lot more to a flare than joint swelling and pain.”
The GRAPPA flare tool is now in the validation stage, Dr. Helliwell said, and will be tested in several studies, including a prospective multicenter study where the 10-item questionnaire will be administered to patients.
A meeting attendee commented that the patient and physician global scales are sufficient and asked: “Is there really a need for a flare instrument?”
“I accept what you are saying, but I would also retort that knowledge is power, and the more information we get, the more we know about what we are doing,” Dr. Helliwell said. “We are going to collect all our information – physician global, patient global, composite disease measures – everything within that study.”
Dr. Helliwell and Dr. Goel reported having no relevant financial disclosures.
MIAMI – Reaching a consensus on measurement and assessment of flare in psoriatic arthritis remains challenging, especially with no widely accepted definition and differing opinions among patients and physicians. But getting standard evaluation of flare under control is critical for both clinical and research outcomes.
Through a series of patient interviews, physician surveys, and lessons learned in rheumatoid arthritis, the GRAPPA Flare Project is close to a validated flare instrument. A 10-question flare assessment tool is now in the final validation stage, according to a presentation at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“Flare is a clearly personal experience and varies from patient to patient. It’s clear that physicians and patients have clearly different ideas of what flare is. Bringing those two worlds together was a challenge,” Philip Helliwell, MD, of Leeds (England) University and GRAPPA president, said at the meeting.
“Some societies – I’m not going to mention any names – have gone down the route of a definition of flare linked to disease activity measures. We have not done this,” Dr. Helliwell said.
Instead, the Flare Project is taking a patient-driven and physician-reviewed approach. Investigators cast a wide net, identifying 79 factors important to patients in six major domains: skin, joint, emotional, participation, fatigue, and unclassified. The results were published in 2015 (Rheumatology [Oxford]. 2015 Aug;54[8]:1448-53). Through a Delphi survey, physicians reviewed these considerations. Then GRAPPA identified items important to both patients and physicians and developed a preliminary flare instrument.
Unlike most assessment tools for psoriatic disease, the new instrument includes patient-reported emotional well-being. Niti Goel, MD, of Duke University, Durham, N.C., said that physicians do not always ask patients about the psychological impact of their disease, so the tool “could provide additional information.”
Another goal of the project is to provide standard answers to some of the common questions related to psoriatic arthritis, including: What exactly is a flare? Does the definition truly capture the worsening of disease? Are there different types of flares? Is flare different if you start from a point of high disease activity? What self-management and other interventions are effective for flare?
GRAPPA adopted a definition of flare developed from rheumatoid arthritis, stating that flare is any worsening of disease activity that would, if persistent, in most cases lead to initiation or change of therapy (J Rheumatol. 2009 Oct;36[10]:2335-41).
“It took [them] some time to get to that definition,” Dr. Helliwell said. “We know from rheumatoid arthritis that there is a lot more to a flare than joint swelling and pain.”
The GRAPPA flare tool is now in the validation stage, Dr. Helliwell said, and will be tested in several studies, including a prospective multicenter study where the 10-item questionnaire will be administered to patients.
A meeting attendee commented that the patient and physician global scales are sufficient and asked: “Is there really a need for a flare instrument?”
“I accept what you are saying, but I would also retort that knowledge is power, and the more information we get, the more we know about what we are doing,” Dr. Helliwell said. “We are going to collect all our information – physician global, patient global, composite disease measures – everything within that study.”
Dr. Helliwell and Dr. Goel reported having no relevant financial disclosures.
MIAMI – Reaching a consensus on measurement and assessment of flare in psoriatic arthritis remains challenging, especially with no widely accepted definition and differing opinions among patients and physicians. But getting standard evaluation of flare under control is critical for both clinical and research outcomes.
Through a series of patient interviews, physician surveys, and lessons learned in rheumatoid arthritis, the GRAPPA Flare Project is close to a validated flare instrument. A 10-question flare assessment tool is now in the final validation stage, according to a presentation at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
“Flare is a clearly personal experience and varies from patient to patient. It’s clear that physicians and patients have clearly different ideas of what flare is. Bringing those two worlds together was a challenge,” Philip Helliwell, MD, of Leeds (England) University and GRAPPA president, said at the meeting.
“Some societies – I’m not going to mention any names – have gone down the route of a definition of flare linked to disease activity measures. We have not done this,” Dr. Helliwell said.
Instead, the Flare Project is taking a patient-driven and physician-reviewed approach. Investigators cast a wide net, identifying 79 factors important to patients in six major domains: skin, joint, emotional, participation, fatigue, and unclassified. The results were published in 2015 (Rheumatology [Oxford]. 2015 Aug;54[8]:1448-53). Through a Delphi survey, physicians reviewed these considerations. Then GRAPPA identified items important to both patients and physicians and developed a preliminary flare instrument.
Unlike most assessment tools for psoriatic disease, the new instrument includes patient-reported emotional well-being. Niti Goel, MD, of Duke University, Durham, N.C., said that physicians do not always ask patients about the psychological impact of their disease, so the tool “could provide additional information.”
Another goal of the project is to provide standard answers to some of the common questions related to psoriatic arthritis, including: What exactly is a flare? Does the definition truly capture the worsening of disease? Are there different types of flares? Is flare different if you start from a point of high disease activity? What self-management and other interventions are effective for flare?
GRAPPA adopted a definition of flare developed from rheumatoid arthritis, stating that flare is any worsening of disease activity that would, if persistent, in most cases lead to initiation or change of therapy (J Rheumatol. 2009 Oct;36[10]:2335-41).
“It took [them] some time to get to that definition,” Dr. Helliwell said. “We know from rheumatoid arthritis that there is a lot more to a flare than joint swelling and pain.”
The GRAPPA flare tool is now in the validation stage, Dr. Helliwell said, and will be tested in several studies, including a prospective multicenter study where the 10-item questionnaire will be administered to patients.
A meeting attendee commented that the patient and physician global scales are sufficient and asked: “Is there really a need for a flare instrument?”
“I accept what you are saying, but I would also retort that knowledge is power, and the more information we get, the more we know about what we are doing,” Dr. Helliwell said. “We are going to collect all our information – physician global, patient global, composite disease measures – everything within that study.”
Dr. Helliwell and Dr. Goel reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM 2016 GRAPPA ANNUAL MEETING
Meningococcal B vaccine less protective than expected during outbreak
One-third of the individuals who received a multicomponent meningococcal B vaccine during an outbreak did not show a protective response against the outbreak strain, though they also did not contract the disease, a study showed.
“This level of seropositivity was lower than expected, given the antigenic similarity between the outbreak strain and the components of the vaccine and given that the Meningococcal Antigen Typing System predicted that 4CMenB would induce responses against the outbreak strain,” Nicole E. Basta, PhD, of the University of Minnesota, Minneapolis, and her associates wrote in the New England Journal of Medicine (2016;375:220-8. doi:10.1056/NEJMoa1514866).
“Our results indicate that knowledge of [human complement serum bactericidal antibodies] immunity against the vaccine reference strains is not sufficient to predict individual-level immunity against an outbreak strain, even when the strain expresses one or more antigens that are closely related to the vaccine antigens,” the investigators added.
Amidst an outbreak of meningococcal B at Princeton (N.J.) University in the winter of 2013, the Food and Drug Administration allowed use of the meningococcal serogroup B (4CMenB) vaccine before its licensure, in an attempt to control the outbreak. Two strains of meningitis were used in developing this vaccine: 5/99 strain, which was not at all similar to the outbreak strain, and 44/76-SL, which was 96% genetically similar to the outbreak strain.
Among 535 people who completed the study, 499 individuals received two doses of 4CMenB 10 weeks apart, 17 received one dose of the vaccine, and 19 were unvaccinated. When the fully vaccinated individuals were tested for titers 8 weeks after their second dose, two-thirds (66.1%) had low-level seropositivity for the outbreak strain, with a geometric mean titer (GMT) of 7.6. One in five of the unvaccinated people (21.1%) had low seropositivity for the outbreak strain (GMT, 2.8), and 58.8% of the partly vaccinated individuals had seropositivity with a GMT of 5.4.
The researchers then assessed titers for the two strains used in developing 4CMenB in 61 fully vaccinated individuals, all randomly selected from the one-third of fully vaccinated people who did not have a detectable response to the outbreak strain. Most of them (86.9%) were seropositive for the 44/76-SL strain with a GMT of 17.4, and all of them were seropositive for the 5/99 strain, with a considerably larger GMT of 256.3.
Among those fully vaccinated who responded to the outbreak strain with titers above 8, all had seropositivity for 44/76-SL (GMT, 178.8), and 96.7% had seropositivity for the 5/99 strain (GMT, 214.2). Among the 18 unvaccinated individuals, just 1 had very low seropositivity to the 5/99 strain (GMT, 1.2), and 6 of them (33.3%) responded to the 44/76-SL strain (GMT, 3.2).
“These findings have implications for vaccination policies aimed at preventing and controlling meningococcal B disease,” the authors wrote.
The research was funded by Princeton University, the National Institutes of Health, and the Department of Homeland Security. Dr. Bai, Dr. Borrow, and Dr. Findlow reported having previously received research funding, unrelated to this study, from GlaxoSmithKline, Novartis, Pfizer, and Sanofi Pasteur. Dr. Johnsen has been reimbursed for travel expenses from Pfizer for a presentation. No other authors had financial disclosures.
Proving the clinical efficacy of Neisseria meningitidis serogroup B (MenB) vaccines has been difficult. There is substantial genetic (and corresponding antigenic) diversity, and serogroup B meningococcal disease is both uncommon and in decline in countries where the burden is well understood. The Advisory Committee on Immunization Practices reviewed available data and concluded that a public health recommendation for vaccination of all adolescents could not be supported but that sufficient evidence existed to support individual decision making by clinicians with regard to patients between 16 and 23 years of age. Vaccination of all adolescents would prevent 15-29 cases and five to nine deaths annually in the United States. The effect of vaccinating college students is correspondingly smaller – preventing perhaps nine cases and one death, at an estimated cost per quality-adjusted life-year of $9.4 million.
The Centers for Disease Control and Prevention notes that the MenB vaccines approved in the United States should provide “protection against most, but not all, serogroup B strains” and represent an important contribution to the prevention of serogroup B meningococcal disease. More than 60,000 persons have received at least one vaccination with 4CMenB in studies conducted worldwide, including studies at universities in the United States and Canada, and the safety profile for 4CMenB has been good. For a relatively uncommon but devastating infectious disease, the regulatory approval of a vaccine in the absence of ideal data may be necessary and appropriate if the vaccine is deployed in the context of a systematic public health response and if there is a commitment to the generation of additional information necessary for determining recommendations for use. The regulatory approval and clinical use of vaccines for pathogens that cause outbreaks will remain challenging.
Jerome H. Kim, MD, is with the International Vaccine Institute in Seoul, South Korea. He reported having no relevant financial disclosures. These comments were excerpted from the editorial accompanying the study (doi:10.1056/NEJMe1606015).
Proving the clinical efficacy of Neisseria meningitidis serogroup B (MenB) vaccines has been difficult. There is substantial genetic (and corresponding antigenic) diversity, and serogroup B meningococcal disease is both uncommon and in decline in countries where the burden is well understood. The Advisory Committee on Immunization Practices reviewed available data and concluded that a public health recommendation for vaccination of all adolescents could not be supported but that sufficient evidence existed to support individual decision making by clinicians with regard to patients between 16 and 23 years of age. Vaccination of all adolescents would prevent 15-29 cases and five to nine deaths annually in the United States. The effect of vaccinating college students is correspondingly smaller – preventing perhaps nine cases and one death, at an estimated cost per quality-adjusted life-year of $9.4 million.
The Centers for Disease Control and Prevention notes that the MenB vaccines approved in the United States should provide “protection against most, but not all, serogroup B strains” and represent an important contribution to the prevention of serogroup B meningococcal disease. More than 60,000 persons have received at least one vaccination with 4CMenB in studies conducted worldwide, including studies at universities in the United States and Canada, and the safety profile for 4CMenB has been good. For a relatively uncommon but devastating infectious disease, the regulatory approval of a vaccine in the absence of ideal data may be necessary and appropriate if the vaccine is deployed in the context of a systematic public health response and if there is a commitment to the generation of additional information necessary for determining recommendations for use. The regulatory approval and clinical use of vaccines for pathogens that cause outbreaks will remain challenging.
Jerome H. Kim, MD, is with the International Vaccine Institute in Seoul, South Korea. He reported having no relevant financial disclosures. These comments were excerpted from the editorial accompanying the study (doi:10.1056/NEJMe1606015).
Proving the clinical efficacy of Neisseria meningitidis serogroup B (MenB) vaccines has been difficult. There is substantial genetic (and corresponding antigenic) diversity, and serogroup B meningococcal disease is both uncommon and in decline in countries where the burden is well understood. The Advisory Committee on Immunization Practices reviewed available data and concluded that a public health recommendation for vaccination of all adolescents could not be supported but that sufficient evidence existed to support individual decision making by clinicians with regard to patients between 16 and 23 years of age. Vaccination of all adolescents would prevent 15-29 cases and five to nine deaths annually in the United States. The effect of vaccinating college students is correspondingly smaller – preventing perhaps nine cases and one death, at an estimated cost per quality-adjusted life-year of $9.4 million.
The Centers for Disease Control and Prevention notes that the MenB vaccines approved in the United States should provide “protection against most, but not all, serogroup B strains” and represent an important contribution to the prevention of serogroup B meningococcal disease. More than 60,000 persons have received at least one vaccination with 4CMenB in studies conducted worldwide, including studies at universities in the United States and Canada, and the safety profile for 4CMenB has been good. For a relatively uncommon but devastating infectious disease, the regulatory approval of a vaccine in the absence of ideal data may be necessary and appropriate if the vaccine is deployed in the context of a systematic public health response and if there is a commitment to the generation of additional information necessary for determining recommendations for use. The regulatory approval and clinical use of vaccines for pathogens that cause outbreaks will remain challenging.
Jerome H. Kim, MD, is with the International Vaccine Institute in Seoul, South Korea. He reported having no relevant financial disclosures. These comments were excerpted from the editorial accompanying the study (doi:10.1056/NEJMe1606015).
One-third of the individuals who received a multicomponent meningococcal B vaccine during an outbreak did not show a protective response against the outbreak strain, though they also did not contract the disease, a study showed.
“This level of seropositivity was lower than expected, given the antigenic similarity between the outbreak strain and the components of the vaccine and given that the Meningococcal Antigen Typing System predicted that 4CMenB would induce responses against the outbreak strain,” Nicole E. Basta, PhD, of the University of Minnesota, Minneapolis, and her associates wrote in the New England Journal of Medicine (2016;375:220-8. doi:10.1056/NEJMoa1514866).
“Our results indicate that knowledge of [human complement serum bactericidal antibodies] immunity against the vaccine reference strains is not sufficient to predict individual-level immunity against an outbreak strain, even when the strain expresses one or more antigens that are closely related to the vaccine antigens,” the investigators added.
Amidst an outbreak of meningococcal B at Princeton (N.J.) University in the winter of 2013, the Food and Drug Administration allowed use of the meningococcal serogroup B (4CMenB) vaccine before its licensure, in an attempt to control the outbreak. Two strains of meningitis were used in developing this vaccine: 5/99 strain, which was not at all similar to the outbreak strain, and 44/76-SL, which was 96% genetically similar to the outbreak strain.
Among 535 people who completed the study, 499 individuals received two doses of 4CMenB 10 weeks apart, 17 received one dose of the vaccine, and 19 were unvaccinated. When the fully vaccinated individuals were tested for titers 8 weeks after their second dose, two-thirds (66.1%) had low-level seropositivity for the outbreak strain, with a geometric mean titer (GMT) of 7.6. One in five of the unvaccinated people (21.1%) had low seropositivity for the outbreak strain (GMT, 2.8), and 58.8% of the partly vaccinated individuals had seropositivity with a GMT of 5.4.
The researchers then assessed titers for the two strains used in developing 4CMenB in 61 fully vaccinated individuals, all randomly selected from the one-third of fully vaccinated people who did not have a detectable response to the outbreak strain. Most of them (86.9%) were seropositive for the 44/76-SL strain with a GMT of 17.4, and all of them were seropositive for the 5/99 strain, with a considerably larger GMT of 256.3.
Among those fully vaccinated who responded to the outbreak strain with titers above 8, all had seropositivity for 44/76-SL (GMT, 178.8), and 96.7% had seropositivity for the 5/99 strain (GMT, 214.2). Among the 18 unvaccinated individuals, just 1 had very low seropositivity to the 5/99 strain (GMT, 1.2), and 6 of them (33.3%) responded to the 44/76-SL strain (GMT, 3.2).
“These findings have implications for vaccination policies aimed at preventing and controlling meningococcal B disease,” the authors wrote.
The research was funded by Princeton University, the National Institutes of Health, and the Department of Homeland Security. Dr. Bai, Dr. Borrow, and Dr. Findlow reported having previously received research funding, unrelated to this study, from GlaxoSmithKline, Novartis, Pfizer, and Sanofi Pasteur. Dr. Johnsen has been reimbursed for travel expenses from Pfizer for a presentation. No other authors had financial disclosures.
One-third of the individuals who received a multicomponent meningococcal B vaccine during an outbreak did not show a protective response against the outbreak strain, though they also did not contract the disease, a study showed.
“This level of seropositivity was lower than expected, given the antigenic similarity between the outbreak strain and the components of the vaccine and given that the Meningococcal Antigen Typing System predicted that 4CMenB would induce responses against the outbreak strain,” Nicole E. Basta, PhD, of the University of Minnesota, Minneapolis, and her associates wrote in the New England Journal of Medicine (2016;375:220-8. doi:10.1056/NEJMoa1514866).
“Our results indicate that knowledge of [human complement serum bactericidal antibodies] immunity against the vaccine reference strains is not sufficient to predict individual-level immunity against an outbreak strain, even when the strain expresses one or more antigens that are closely related to the vaccine antigens,” the investigators added.
Amidst an outbreak of meningococcal B at Princeton (N.J.) University in the winter of 2013, the Food and Drug Administration allowed use of the meningococcal serogroup B (4CMenB) vaccine before its licensure, in an attempt to control the outbreak. Two strains of meningitis were used in developing this vaccine: 5/99 strain, which was not at all similar to the outbreak strain, and 44/76-SL, which was 96% genetically similar to the outbreak strain.
Among 535 people who completed the study, 499 individuals received two doses of 4CMenB 10 weeks apart, 17 received one dose of the vaccine, and 19 were unvaccinated. When the fully vaccinated individuals were tested for titers 8 weeks after their second dose, two-thirds (66.1%) had low-level seropositivity for the outbreak strain, with a geometric mean titer (GMT) of 7.6. One in five of the unvaccinated people (21.1%) had low seropositivity for the outbreak strain (GMT, 2.8), and 58.8% of the partly vaccinated individuals had seropositivity with a GMT of 5.4.
The researchers then assessed titers for the two strains used in developing 4CMenB in 61 fully vaccinated individuals, all randomly selected from the one-third of fully vaccinated people who did not have a detectable response to the outbreak strain. Most of them (86.9%) were seropositive for the 44/76-SL strain with a GMT of 17.4, and all of them were seropositive for the 5/99 strain, with a considerably larger GMT of 256.3.
Among those fully vaccinated who responded to the outbreak strain with titers above 8, all had seropositivity for 44/76-SL (GMT, 178.8), and 96.7% had seropositivity for the 5/99 strain (GMT, 214.2). Among the 18 unvaccinated individuals, just 1 had very low seropositivity to the 5/99 strain (GMT, 1.2), and 6 of them (33.3%) responded to the 44/76-SL strain (GMT, 3.2).
“These findings have implications for vaccination policies aimed at preventing and controlling meningococcal B disease,” the authors wrote.
The research was funded by Princeton University, the National Institutes of Health, and the Department of Homeland Security. Dr. Bai, Dr. Borrow, and Dr. Findlow reported having previously received research funding, unrelated to this study, from GlaxoSmithKline, Novartis, Pfizer, and Sanofi Pasteur. Dr. Johnsen has been reimbursed for travel expenses from Pfizer for a presentation. No other authors had financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: The 4CMenB vaccine provided less protection than anticipated during an outbreak.
Major finding: A total of 66.1% of vaccinated individuals had seropositivity against the meningococcal B outbreak strain.
Data source: The findings are based on the immunogenicity of 4CMenB against a meningococcal B outbreak strain in 535 individuals during an outbreak at a New Jersey university in the winter of 2013.
Disclosures: The research was funded by Princeton University, the National Institutes of Health, and the Department of Homeland Security. Dr. Bai, Dr. Borrow, and Dr. Findlow reported having previously received research funding, unrelated to this study, from GlaxoSmithKline, Novartis, Pfizer, and Sanofi Pasteur. Dr. Johnsen has been reimbursed for travel expenses from Pfizer for a presentation. No other authors had disclosures.
Candida colonization raises risk of Acinetobacter-based VAP
Acinetobacter baumanii was the most common cause of ventilator-associated pneumonia in ICU patients, and the risk of A. baumannii infection was significantly higher when airways were colonized with Candida species, based on data from 618 adults.
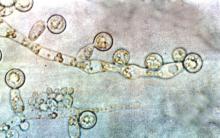
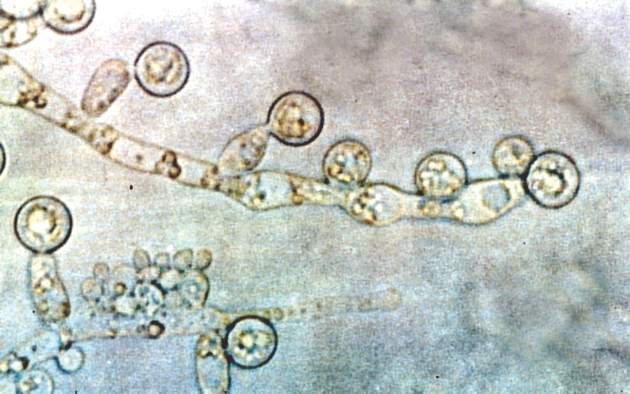
A. baumannii is a frequent cause of ventilator-associated pneumonia (VAP) in ICU patients, but its potential interactions with Candida species have not been well studied, wrote Dr. Xiaojiang Tan of Southern Medical University, Guangzhou, China, and colleagues (Med Mycol. 2016 Aug 1;54[6]:557-66. doi: 10.1093/mmy/myw009). The researchers reviewed data from 264 ICU patients on mechanical ventilation who had Candida species airway colonization and 354 who did not.
Overall, Candida was an independent risk factor for A. baumannii VAP; patients with Candida were significantly more likely than those without Candida to develop A. baumannii (23% vs. 15%). Other independent risk factors for A. baumannii VAP included the use of a central venous catheter and the use of mechanical ventilation for at least 7 days. Among patients on mechanical ventilation for at least 48 hours, Candida airway colonization occurred in 43%, and A. baumannii VAP occurred in 18%.
Candida albicans showed an especially strong association with A. baumannii VAP; it was identified in 38% of cases, compared with 21% caused by non-albicans species.
No significant differences in hospital stay or in-hospital mortality were noted between Candida-colonized and noncolonized patients, and antifungal treatment had no apparent impact on the development of A. baumannii VAP, but antifungals were associated with higher in-hospital mortality (53% vs. 39%, P = .037).
The results were limited by the retrospective nature of the study and the use of data from a single center; thus, the relationship between Candida and A. baumannii VAP may not be generalizable, the researchers noted. However, “the strong independent association between the two suggests that Candida spp. growth from the lower respiratory tract in intubated patients could be an important indicator of the risk for VAP, and even that C. albicans airway colonization may play a role in subsequent development of A. baumannii VAP,” they wrote.
The researchers reported having no relevant financial conflicts.
Acinetobacter baumanii was the most common cause of ventilator-associated pneumonia in ICU patients, and the risk of A. baumannii infection was significantly higher when airways were colonized with Candida species, based on data from 618 adults.
A. baumannii is a frequent cause of ventilator-associated pneumonia (VAP) in ICU patients, but its potential interactions with Candida species have not been well studied, wrote Dr. Xiaojiang Tan of Southern Medical University, Guangzhou, China, and colleagues (Med Mycol. 2016 Aug 1;54[6]:557-66. doi: 10.1093/mmy/myw009). The researchers reviewed data from 264 ICU patients on mechanical ventilation who had Candida species airway colonization and 354 who did not.
Overall, Candida was an independent risk factor for A. baumannii VAP; patients with Candida were significantly more likely than those without Candida to develop A. baumannii (23% vs. 15%). Other independent risk factors for A. baumannii VAP included the use of a central venous catheter and the use of mechanical ventilation for at least 7 days. Among patients on mechanical ventilation for at least 48 hours, Candida airway colonization occurred in 43%, and A. baumannii VAP occurred in 18%.
Candida albicans showed an especially strong association with A. baumannii VAP; it was identified in 38% of cases, compared with 21% caused by non-albicans species.
No significant differences in hospital stay or in-hospital mortality were noted between Candida-colonized and noncolonized patients, and antifungal treatment had no apparent impact on the development of A. baumannii VAP, but antifungals were associated with higher in-hospital mortality (53% vs. 39%, P = .037).
The results were limited by the retrospective nature of the study and the use of data from a single center; thus, the relationship between Candida and A. baumannii VAP may not be generalizable, the researchers noted. However, “the strong independent association between the two suggests that Candida spp. growth from the lower respiratory tract in intubated patients could be an important indicator of the risk for VAP, and even that C. albicans airway colonization may play a role in subsequent development of A. baumannii VAP,” they wrote.
The researchers reported having no relevant financial conflicts.
Acinetobacter baumanii was the most common cause of ventilator-associated pneumonia in ICU patients, and the risk of A. baumannii infection was significantly higher when airways were colonized with Candida species, based on data from 618 adults.
A. baumannii is a frequent cause of ventilator-associated pneumonia (VAP) in ICU patients, but its potential interactions with Candida species have not been well studied, wrote Dr. Xiaojiang Tan of Southern Medical University, Guangzhou, China, and colleagues (Med Mycol. 2016 Aug 1;54[6]:557-66. doi: 10.1093/mmy/myw009). The researchers reviewed data from 264 ICU patients on mechanical ventilation who had Candida species airway colonization and 354 who did not.
Overall, Candida was an independent risk factor for A. baumannii VAP; patients with Candida were significantly more likely than those without Candida to develop A. baumannii (23% vs. 15%). Other independent risk factors for A. baumannii VAP included the use of a central venous catheter and the use of mechanical ventilation for at least 7 days. Among patients on mechanical ventilation for at least 48 hours, Candida airway colonization occurred in 43%, and A. baumannii VAP occurred in 18%.
Candida albicans showed an especially strong association with A. baumannii VAP; it was identified in 38% of cases, compared with 21% caused by non-albicans species.
No significant differences in hospital stay or in-hospital mortality were noted between Candida-colonized and noncolonized patients, and antifungal treatment had no apparent impact on the development of A. baumannii VAP, but antifungals were associated with higher in-hospital mortality (53% vs. 39%, P = .037).
The results were limited by the retrospective nature of the study and the use of data from a single center; thus, the relationship between Candida and A. baumannii VAP may not be generalizable, the researchers noted. However, “the strong independent association between the two suggests that Candida spp. growth from the lower respiratory tract in intubated patients could be an important indicator of the risk for VAP, and even that C. albicans airway colonization may play a role in subsequent development of A. baumannii VAP,” they wrote.
The researchers reported having no relevant financial conflicts.
FROM MEDICAL MYCOLOGY
Key clinical point: Candida species colonization was an independent risk factor for ventilator-associated pneumonia caused by Acinetobacter baumannii among ICU patients.
Major finding: Hospitalized patients with Candida species airway colonization were significantly more likely to develop A. baumannii infection than were those without Candida (23% vs. 15%).
Data source: A retrospective case-control study of 618 ICU patients.
Disclosures: The researchers reported having no relevant financial conflicts.
CHMP recommends enoxaparin biosimilars

Image by Andre E.X. Brown
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for 2 biosimilars of the low-molecular-weight heparin enoxaparin.
Both of the agents, Inhixa and Thorinane, are intended to prevent and treat thrombosis-related disorders in adults.
The CHMP’s recommendations will be reviewed by the European Commission.
If the recommendations are formally adopted, Inhixa and Thorinane will be approved for use in the European Union as well as Norway, Liechtenstein, and Iceland.
Both Inhixa and Thorinane are indicated for:
- Prophylaxis of venous thromboembolism (VTE), particularly in patients undergoing orthopedic, general, or oncological surgery.
- VTE prophylaxis in patients bedridden due to acute illnesses, including acute heart failure, acute respiratory failure, severe infections, and exacerbation of rheumatic diseases causing immobilization of the patient (applies to strengths of 40 mg/0.4 mL).
- Treatment of deep vein thrombosis, complicated or uncomplicated by pulmonary embolism.
- Treatment of unstable angina and non-Q wave myocardial infarction, in combination with acetylsalicylic acid.
- Treatment of acute ST segment elevation myocardial infarction, including patients who will be treated conservatively or who will later undergo percutaneous coronary angioplasty (applies to strengths of 60 mg/0.6 mL, 80 mg/0.8 mL, and 100 mg/1 mL).
- VTE prevention in the extracorporeal circulation during hemodialysis.
If approved, Inhixa will be available as a solution for injection—2000 IU (20 mg) in 0.2 mL, 4000 IU (40 mg) in 0.4 mL, 6000 IU (60 mg) in 0.6 mL, 8000 IU (80 mg) in 0.8 mL, and 10,000 IU (100 mg) in 1 mL.
And Thorinane will be available as a solution for injection—2000 IU (20 mg) in 0.2 mL, 4000 IU (40 mg) in 0.4 mL, 6000 IU (60 mg) in 0.6 mL, 8000 IU (80 mg) in 0.8 mL, and 10,000 IU (100 mg) in 1 mL.
Inhixa is being developed by Techdow Europe AB, and Thorinane is being developed by Pharmathen S.A. ![]()

Image by Andre E.X. Brown
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for 2 biosimilars of the low-molecular-weight heparin enoxaparin.
Both of the agents, Inhixa and Thorinane, are intended to prevent and treat thrombosis-related disorders in adults.
The CHMP’s recommendations will be reviewed by the European Commission.
If the recommendations are formally adopted, Inhixa and Thorinane will be approved for use in the European Union as well as Norway, Liechtenstein, and Iceland.
Both Inhixa and Thorinane are indicated for:
- Prophylaxis of venous thromboembolism (VTE), particularly in patients undergoing orthopedic, general, or oncological surgery.
- VTE prophylaxis in patients bedridden due to acute illnesses, including acute heart failure, acute respiratory failure, severe infections, and exacerbation of rheumatic diseases causing immobilization of the patient (applies to strengths of 40 mg/0.4 mL).
- Treatment of deep vein thrombosis, complicated or uncomplicated by pulmonary embolism.
- Treatment of unstable angina and non-Q wave myocardial infarction, in combination with acetylsalicylic acid.
- Treatment of acute ST segment elevation myocardial infarction, including patients who will be treated conservatively or who will later undergo percutaneous coronary angioplasty (applies to strengths of 60 mg/0.6 mL, 80 mg/0.8 mL, and 100 mg/1 mL).
- VTE prevention in the extracorporeal circulation during hemodialysis.
If approved, Inhixa will be available as a solution for injection—2000 IU (20 mg) in 0.2 mL, 4000 IU (40 mg) in 0.4 mL, 6000 IU (60 mg) in 0.6 mL, 8000 IU (80 mg) in 0.8 mL, and 10,000 IU (100 mg) in 1 mL.
And Thorinane will be available as a solution for injection—2000 IU (20 mg) in 0.2 mL, 4000 IU (40 mg) in 0.4 mL, 6000 IU (60 mg) in 0.6 mL, 8000 IU (80 mg) in 0.8 mL, and 10,000 IU (100 mg) in 1 mL.
Inhixa is being developed by Techdow Europe AB, and Thorinane is being developed by Pharmathen S.A. ![]()

Image by Andre E.X. Brown
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval for 2 biosimilars of the low-molecular-weight heparin enoxaparin.
Both of the agents, Inhixa and Thorinane, are intended to prevent and treat thrombosis-related disorders in adults.
The CHMP’s recommendations will be reviewed by the European Commission.
If the recommendations are formally adopted, Inhixa and Thorinane will be approved for use in the European Union as well as Norway, Liechtenstein, and Iceland.
Both Inhixa and Thorinane are indicated for:
- Prophylaxis of venous thromboembolism (VTE), particularly in patients undergoing orthopedic, general, or oncological surgery.
- VTE prophylaxis in patients bedridden due to acute illnesses, including acute heart failure, acute respiratory failure, severe infections, and exacerbation of rheumatic diseases causing immobilization of the patient (applies to strengths of 40 mg/0.4 mL).
- Treatment of deep vein thrombosis, complicated or uncomplicated by pulmonary embolism.
- Treatment of unstable angina and non-Q wave myocardial infarction, in combination with acetylsalicylic acid.
- Treatment of acute ST segment elevation myocardial infarction, including patients who will be treated conservatively or who will later undergo percutaneous coronary angioplasty (applies to strengths of 60 mg/0.6 mL, 80 mg/0.8 mL, and 100 mg/1 mL).
- VTE prevention in the extracorporeal circulation during hemodialysis.
If approved, Inhixa will be available as a solution for injection—2000 IU (20 mg) in 0.2 mL, 4000 IU (40 mg) in 0.4 mL, 6000 IU (60 mg) in 0.6 mL, 8000 IU (80 mg) in 0.8 mL, and 10,000 IU (100 mg) in 1 mL.
And Thorinane will be available as a solution for injection—2000 IU (20 mg) in 0.2 mL, 4000 IU (40 mg) in 0.4 mL, 6000 IU (60 mg) in 0.6 mL, 8000 IU (80 mg) in 0.8 mL, and 10,000 IU (100 mg) in 1 mL.
Inhixa is being developed by Techdow Europe AB, and Thorinane is being developed by Pharmathen S.A. ![]()
Psoriatic Arthritis Patients Face More Endocrine Comorbidities
MIAMI – Diabetes mellitus, hypothyroidism, Cushing’s disease, and osteoporosis occur more frequently in people with psoriatic arthritis than in controls, a large cohort study reveals. Prevalence of these endocrine conditions was greater in a group of 3,161 patients with psoriatic arthritis, compared with 31,610 matched controls.
“We recommend that physicians should be aware of comorbid associations to provide comprehensive medical care to patients with psoriatic arthritis,” said Amir Haddad, MD, of the department of rheumatology at Carmel Medical Center in Haifa, Israel.
Dr. Haddad and his colleagues, however, found no significant differences in the prevalence of hyperthyroidism, hypo- and hyperparathyroidism, hyperprolactinemia, Addison’s disease, diabetes insipidus, pituitary adenoma, or acromegaly between groups in this retrospective, cross-sectional study.
They identified 1,474 men and 1,687 women diagnosed with psoriatic disease from 2000 to 2013 using the Clalit health services database in Israel. This group was a mean of 58 years old and 53% were women. Each patient was matched with 10 age- and gender-matched controls without psoriatic disease for the study.
“This is, to our knowledge, one of the largest real-life cohorts of psoriatic patient registries,” Dr. Haddad said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
In the psoriatic arthritis group versus controls, diabetes mellitus prevalence was 27.9% vs. 20.7%; for hypothyroidism it was 12.7% vs. 8.6%; and for Cushing’s disease it was 0.3% vs. 0.1%. All these differences were statically significant (P less than 0.0001). Osteoporosis prevalence also differed significantly between the psoriatic arthritis and control groups: 13.2% vs. 9.1% (P less than 0.001).
Greater awareness of nonskin and nonjoint comorbidities is important, Dr. Haddad said, because it can influence choice of therapy and management of patients with psoriatic arthritis.
The investigators also conducted univariate and multivariate regression analyses. Compared with controls, the results suggest psoriatic arthritis patients have a higher risk for diabetes mellitus (odds ratio, 1.48), hypothyroidism (OR, 1.56), and osteoporosis (OR, 1.52). The risk for Cushing’s disease was notably higher (OR, 5.31) in the univariate analysis.
Risks for these endocrine conditions remained higher for the psoriatic arthritis patients in a multivariate regression analysis as well. For example, risk for diabetes mellitus (OR, 1.30) remained after adjusting for age, gender, smoking, obesity, and steroid use. Risk of hypothyroidism (OR, 1.61) remained after adjusting for age and gender; risk of osteoporosis (OR, 1.50) after adjusting for age, gender, steroid use, and smoking; and risk of Cushing’s disease (OR, 3.79) after adjustment for age, gender, and steroid use.
The large, population-based cohort is a strength of the study. “We are now going back to see how many of these patients were seen by rheumatologists,” Dr. Haddad said. A lack of association with disease burden is a potential limitation, he added.
Thirty percent of patients were treated with biologics and about 67% with steroids. “That number treated with steroids seems high,” a meeting attendee commented. Dr. Haddad explained that it is the percentage ever treated with steroids, not necessarily currently on steroids.
In a separate session at the GRAPPA meeting addressing psoriatic disease treatment recommendations, an attendee asked about specific recommendations for comorbidities. For now, GRAPPA plans to include comorbidities within its overall recommendations, as it did in its most recent update, released in January 2016. A limited amount of data is a primary reason.
“As the evidence on comorbidities gets better, we may someday have separate recommendations for comorbidities,” said Laura Coates, MD, a clinical lecturer in rheumatology at the University of Leeds (England).
“The comorbidities are very important,” said Arthur F. Kavanaugh, MD, professor of medicine at the University of California, San Diego. “That’s trickier and deals with the international nature of GRAPPA. It’s hard to say, ‘Go see this specialist,’ because that might not be standard of care in that country.”
Dr. Haddad, Dr. Coates, and Dr. Kavanaugh reported having no relevant financial disclosures.
MIAMI – Diabetes mellitus, hypothyroidism, Cushing’s disease, and osteoporosis occur more frequently in people with psoriatic arthritis than in controls, a large cohort study reveals. Prevalence of these endocrine conditions was greater in a group of 3,161 patients with psoriatic arthritis, compared with 31,610 matched controls.
“We recommend that physicians should be aware of comorbid associations to provide comprehensive medical care to patients with psoriatic arthritis,” said Amir Haddad, MD, of the department of rheumatology at Carmel Medical Center in Haifa, Israel.
Dr. Haddad and his colleagues, however, found no significant differences in the prevalence of hyperthyroidism, hypo- and hyperparathyroidism, hyperprolactinemia, Addison’s disease, diabetes insipidus, pituitary adenoma, or acromegaly between groups in this retrospective, cross-sectional study.
They identified 1,474 men and 1,687 women diagnosed with psoriatic disease from 2000 to 2013 using the Clalit health services database in Israel. This group was a mean of 58 years old and 53% were women. Each patient was matched with 10 age- and gender-matched controls without psoriatic disease for the study.
“This is, to our knowledge, one of the largest real-life cohorts of psoriatic patient registries,” Dr. Haddad said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
In the psoriatic arthritis group versus controls, diabetes mellitus prevalence was 27.9% vs. 20.7%; for hypothyroidism it was 12.7% vs. 8.6%; and for Cushing’s disease it was 0.3% vs. 0.1%. All these differences were statically significant (P less than 0.0001). Osteoporosis prevalence also differed significantly between the psoriatic arthritis and control groups: 13.2% vs. 9.1% (P less than 0.001).
Greater awareness of nonskin and nonjoint comorbidities is important, Dr. Haddad said, because it can influence choice of therapy and management of patients with psoriatic arthritis.
The investigators also conducted univariate and multivariate regression analyses. Compared with controls, the results suggest psoriatic arthritis patients have a higher risk for diabetes mellitus (odds ratio, 1.48), hypothyroidism (OR, 1.56), and osteoporosis (OR, 1.52). The risk for Cushing’s disease was notably higher (OR, 5.31) in the univariate analysis.
Risks for these endocrine conditions remained higher for the psoriatic arthritis patients in a multivariate regression analysis as well. For example, risk for diabetes mellitus (OR, 1.30) remained after adjusting for age, gender, smoking, obesity, and steroid use. Risk of hypothyroidism (OR, 1.61) remained after adjusting for age and gender; risk of osteoporosis (OR, 1.50) after adjusting for age, gender, steroid use, and smoking; and risk of Cushing’s disease (OR, 3.79) after adjustment for age, gender, and steroid use.
The large, population-based cohort is a strength of the study. “We are now going back to see how many of these patients were seen by rheumatologists,” Dr. Haddad said. A lack of association with disease burden is a potential limitation, he added.
Thirty percent of patients were treated with biologics and about 67% with steroids. “That number treated with steroids seems high,” a meeting attendee commented. Dr. Haddad explained that it is the percentage ever treated with steroids, not necessarily currently on steroids.
In a separate session at the GRAPPA meeting addressing psoriatic disease treatment recommendations, an attendee asked about specific recommendations for comorbidities. For now, GRAPPA plans to include comorbidities within its overall recommendations, as it did in its most recent update, released in January 2016. A limited amount of data is a primary reason.
“As the evidence on comorbidities gets better, we may someday have separate recommendations for comorbidities,” said Laura Coates, MD, a clinical lecturer in rheumatology at the University of Leeds (England).
“The comorbidities are very important,” said Arthur F. Kavanaugh, MD, professor of medicine at the University of California, San Diego. “That’s trickier and deals with the international nature of GRAPPA. It’s hard to say, ‘Go see this specialist,’ because that might not be standard of care in that country.”
Dr. Haddad, Dr. Coates, and Dr. Kavanaugh reported having no relevant financial disclosures.
MIAMI – Diabetes mellitus, hypothyroidism, Cushing’s disease, and osteoporosis occur more frequently in people with psoriatic arthritis than in controls, a large cohort study reveals. Prevalence of these endocrine conditions was greater in a group of 3,161 patients with psoriatic arthritis, compared with 31,610 matched controls.
“We recommend that physicians should be aware of comorbid associations to provide comprehensive medical care to patients with psoriatic arthritis,” said Amir Haddad, MD, of the department of rheumatology at Carmel Medical Center in Haifa, Israel.
Dr. Haddad and his colleagues, however, found no significant differences in the prevalence of hyperthyroidism, hypo- and hyperparathyroidism, hyperprolactinemia, Addison’s disease, diabetes insipidus, pituitary adenoma, or acromegaly between groups in this retrospective, cross-sectional study.
They identified 1,474 men and 1,687 women diagnosed with psoriatic disease from 2000 to 2013 using the Clalit health services database in Israel. This group was a mean of 58 years old and 53% were women. Each patient was matched with 10 age- and gender-matched controls without psoriatic disease for the study.
“This is, to our knowledge, one of the largest real-life cohorts of psoriatic patient registries,” Dr. Haddad said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
In the psoriatic arthritis group versus controls, diabetes mellitus prevalence was 27.9% vs. 20.7%; for hypothyroidism it was 12.7% vs. 8.6%; and for Cushing’s disease it was 0.3% vs. 0.1%. All these differences were statically significant (P less than 0.0001). Osteoporosis prevalence also differed significantly between the psoriatic arthritis and control groups: 13.2% vs. 9.1% (P less than 0.001).
Greater awareness of nonskin and nonjoint comorbidities is important, Dr. Haddad said, because it can influence choice of therapy and management of patients with psoriatic arthritis.
The investigators also conducted univariate and multivariate regression analyses. Compared with controls, the results suggest psoriatic arthritis patients have a higher risk for diabetes mellitus (odds ratio, 1.48), hypothyroidism (OR, 1.56), and osteoporosis (OR, 1.52). The risk for Cushing’s disease was notably higher (OR, 5.31) in the univariate analysis.
Risks for these endocrine conditions remained higher for the psoriatic arthritis patients in a multivariate regression analysis as well. For example, risk for diabetes mellitus (OR, 1.30) remained after adjusting for age, gender, smoking, obesity, and steroid use. Risk of hypothyroidism (OR, 1.61) remained after adjusting for age and gender; risk of osteoporosis (OR, 1.50) after adjusting for age, gender, steroid use, and smoking; and risk of Cushing’s disease (OR, 3.79) after adjustment for age, gender, and steroid use.
The large, population-based cohort is a strength of the study. “We are now going back to see how many of these patients were seen by rheumatologists,” Dr. Haddad said. A lack of association with disease burden is a potential limitation, he added.
Thirty percent of patients were treated with biologics and about 67% with steroids. “That number treated with steroids seems high,” a meeting attendee commented. Dr. Haddad explained that it is the percentage ever treated with steroids, not necessarily currently on steroids.
In a separate session at the GRAPPA meeting addressing psoriatic disease treatment recommendations, an attendee asked about specific recommendations for comorbidities. For now, GRAPPA plans to include comorbidities within its overall recommendations, as it did in its most recent update, released in January 2016. A limited amount of data is a primary reason.
“As the evidence on comorbidities gets better, we may someday have separate recommendations for comorbidities,” said Laura Coates, MD, a clinical lecturer in rheumatology at the University of Leeds (England).
“The comorbidities are very important,” said Arthur F. Kavanaugh, MD, professor of medicine at the University of California, San Diego. “That’s trickier and deals with the international nature of GRAPPA. It’s hard to say, ‘Go see this specialist,’ because that might not be standard of care in that country.”
Dr. Haddad, Dr. Coates, and Dr. Kavanaugh reported having no relevant financial disclosures.
AT 2016 GRAPPA ANNUAL MEETING
Psoriatic arthritis patients face more endocrine comorbidities
MIAMI – Diabetes mellitus, hypothyroidism, Cushing’s disease, and osteoporosis occur more frequently in people with psoriatic arthritis than in controls, a large cohort study reveals. Prevalence of these endocrine conditions was greater in a group of 3,161 patients with psoriatic arthritis, compared with 31,610 matched controls.
“We recommend that physicians should be aware of comorbid associations to provide comprehensive medical care to patients with psoriatic arthritis,” said Amir Haddad, MD, of the department of rheumatology at Carmel Medical Center in Haifa, Israel.
Dr. Haddad and his colleagues, however, found no significant differences in the prevalence of hyperthyroidism, hypo- and hyperparathyroidism, hyperprolactinemia, Addison’s disease, diabetes insipidus, pituitary adenoma, or acromegaly between groups in this retrospective, cross-sectional study.
They identified 1,474 men and 1,687 women diagnosed with psoriatic disease from 2000 to 2013 using the Clalit health services database in Israel. This group was a mean of 58 years old and 53% were women. Each patient was matched with 10 age- and gender-matched controls without psoriatic disease for the study.
“This is, to our knowledge, one of the largest real-life cohorts of psoriatic patient registries,” Dr. Haddad said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
In the psoriatic arthritis group versus controls, diabetes mellitus prevalence was 27.9% vs. 20.7%; for hypothyroidism it was 12.7% vs. 8.6%; and for Cushing’s disease it was 0.3% vs. 0.1%. All these differences were statically significant (P less than 0.0001). Osteoporosis prevalence also differed significantly between the psoriatic arthritis and control groups: 13.2% vs. 9.1% (P less than 0.001).
Greater awareness of nonskin and nonjoint comorbidities is important, Dr. Haddad said, because it can influence choice of therapy and management of patients with psoriatic arthritis.
The investigators also conducted univariate and multivariate regression analyses. Compared with controls, the results suggest psoriatic arthritis patients have a higher risk for diabetes mellitus (odds ratio, 1.48), hypothyroidism (OR, 1.56), and osteoporosis (OR, 1.52). The risk for Cushing’s disease was notably higher (OR, 5.31) in the univariate analysis.
Risks for these endocrine conditions remained higher for the psoriatic arthritis patients in a multivariate regression analysis as well. For example, risk for diabetes mellitus (OR, 1.30) remained after adjusting for age, gender, smoking, obesity, and steroid use. Risk of hypothyroidism (OR, 1.61) remained after adjusting for age and gender; risk of osteoporosis (OR, 1.50) after adjusting for age, gender, steroid use, and smoking; and risk of Cushing’s disease (OR, 3.79) after adjustment for age, gender, and steroid use.
The large, population-based cohort is a strength of the study. “We are now going back to see how many of these patients were seen by rheumatologists,” Dr. Haddad said. A lack of association with disease burden is a potential limitation, he added.
Thirty percent of patients were treated with biologics and about 67% with steroids. “That number treated with steroids seems high,” a meeting attendee commented. Dr. Haddad explained that it is the percentage ever treated with steroids, not necessarily currently on steroids.
In a separate session at the GRAPPA meeting addressing psoriatic disease treatment recommendations, an attendee asked about specific recommendations for comorbidities. For now, GRAPPA plans to include comorbidities within its overall recommendations, as it did in its most recent update, released in January 2016. A limited amount of data is a primary reason.
“As the evidence on comorbidities gets better, we may someday have separate recommendations for comorbidities,” said Laura Coates, MD, a clinical lecturer in rheumatology at the University of Leeds (England).
“The comorbidities are very important,” said Arthur F. Kavanaugh, MD, professor of medicine at the University of California, San Diego. “That’s trickier and deals with the international nature of GRAPPA. It’s hard to say, ‘Go see this specialist,’ because that might not be standard of care in that country.”
Dr. Haddad, Dr. Coates, and Dr. Kavanaugh reported having no relevant financial disclosures.
MIAMI – Diabetes mellitus, hypothyroidism, Cushing’s disease, and osteoporosis occur more frequently in people with psoriatic arthritis than in controls, a large cohort study reveals. Prevalence of these endocrine conditions was greater in a group of 3,161 patients with psoriatic arthritis, compared with 31,610 matched controls.
“We recommend that physicians should be aware of comorbid associations to provide comprehensive medical care to patients with psoriatic arthritis,” said Amir Haddad, MD, of the department of rheumatology at Carmel Medical Center in Haifa, Israel.
Dr. Haddad and his colleagues, however, found no significant differences in the prevalence of hyperthyroidism, hypo- and hyperparathyroidism, hyperprolactinemia, Addison’s disease, diabetes insipidus, pituitary adenoma, or acromegaly between groups in this retrospective, cross-sectional study.
They identified 1,474 men and 1,687 women diagnosed with psoriatic disease from 2000 to 2013 using the Clalit health services database in Israel. This group was a mean of 58 years old and 53% were women. Each patient was matched with 10 age- and gender-matched controls without psoriatic disease for the study.
“This is, to our knowledge, one of the largest real-life cohorts of psoriatic patient registries,” Dr. Haddad said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
In the psoriatic arthritis group versus controls, diabetes mellitus prevalence was 27.9% vs. 20.7%; for hypothyroidism it was 12.7% vs. 8.6%; and for Cushing’s disease it was 0.3% vs. 0.1%. All these differences were statically significant (P less than 0.0001). Osteoporosis prevalence also differed significantly between the psoriatic arthritis and control groups: 13.2% vs. 9.1% (P less than 0.001).
Greater awareness of nonskin and nonjoint comorbidities is important, Dr. Haddad said, because it can influence choice of therapy and management of patients with psoriatic arthritis.
The investigators also conducted univariate and multivariate regression analyses. Compared with controls, the results suggest psoriatic arthritis patients have a higher risk for diabetes mellitus (odds ratio, 1.48), hypothyroidism (OR, 1.56), and osteoporosis (OR, 1.52). The risk for Cushing’s disease was notably higher (OR, 5.31) in the univariate analysis.
Risks for these endocrine conditions remained higher for the psoriatic arthritis patients in a multivariate regression analysis as well. For example, risk for diabetes mellitus (OR, 1.30) remained after adjusting for age, gender, smoking, obesity, and steroid use. Risk of hypothyroidism (OR, 1.61) remained after adjusting for age and gender; risk of osteoporosis (OR, 1.50) after adjusting for age, gender, steroid use, and smoking; and risk of Cushing’s disease (OR, 3.79) after adjustment for age, gender, and steroid use.
The large, population-based cohort is a strength of the study. “We are now going back to see how many of these patients were seen by rheumatologists,” Dr. Haddad said. A lack of association with disease burden is a potential limitation, he added.
Thirty percent of patients were treated with biologics and about 67% with steroids. “That number treated with steroids seems high,” a meeting attendee commented. Dr. Haddad explained that it is the percentage ever treated with steroids, not necessarily currently on steroids.
In a separate session at the GRAPPA meeting addressing psoriatic disease treatment recommendations, an attendee asked about specific recommendations for comorbidities. For now, GRAPPA plans to include comorbidities within its overall recommendations, as it did in its most recent update, released in January 2016. A limited amount of data is a primary reason.
“As the evidence on comorbidities gets better, we may someday have separate recommendations for comorbidities,” said Laura Coates, MD, a clinical lecturer in rheumatology at the University of Leeds (England).
“The comorbidities are very important,” said Arthur F. Kavanaugh, MD, professor of medicine at the University of California, San Diego. “That’s trickier and deals with the international nature of GRAPPA. It’s hard to say, ‘Go see this specialist,’ because that might not be standard of care in that country.”
Dr. Haddad, Dr. Coates, and Dr. Kavanaugh reported having no relevant financial disclosures.
MIAMI – Diabetes mellitus, hypothyroidism, Cushing’s disease, and osteoporosis occur more frequently in people with psoriatic arthritis than in controls, a large cohort study reveals. Prevalence of these endocrine conditions was greater in a group of 3,161 patients with psoriatic arthritis, compared with 31,610 matched controls.
“We recommend that physicians should be aware of comorbid associations to provide comprehensive medical care to patients with psoriatic arthritis,” said Amir Haddad, MD, of the department of rheumatology at Carmel Medical Center in Haifa, Israel.
Dr. Haddad and his colleagues, however, found no significant differences in the prevalence of hyperthyroidism, hypo- and hyperparathyroidism, hyperprolactinemia, Addison’s disease, diabetes insipidus, pituitary adenoma, or acromegaly between groups in this retrospective, cross-sectional study.
They identified 1,474 men and 1,687 women diagnosed with psoriatic disease from 2000 to 2013 using the Clalit health services database in Israel. This group was a mean of 58 years old and 53% were women. Each patient was matched with 10 age- and gender-matched controls without psoriatic disease for the study.
“This is, to our knowledge, one of the largest real-life cohorts of psoriatic patient registries,” Dr. Haddad said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA).
In the psoriatic arthritis group versus controls, diabetes mellitus prevalence was 27.9% vs. 20.7%; for hypothyroidism it was 12.7% vs. 8.6%; and for Cushing’s disease it was 0.3% vs. 0.1%. All these differences were statically significant (P less than 0.0001). Osteoporosis prevalence also differed significantly between the psoriatic arthritis and control groups: 13.2% vs. 9.1% (P less than 0.001).
Greater awareness of nonskin and nonjoint comorbidities is important, Dr. Haddad said, because it can influence choice of therapy and management of patients with psoriatic arthritis.
The investigators also conducted univariate and multivariate regression analyses. Compared with controls, the results suggest psoriatic arthritis patients have a higher risk for diabetes mellitus (odds ratio, 1.48), hypothyroidism (OR, 1.56), and osteoporosis (OR, 1.52). The risk for Cushing’s disease was notably higher (OR, 5.31) in the univariate analysis.
Risks for these endocrine conditions remained higher for the psoriatic arthritis patients in a multivariate regression analysis as well. For example, risk for diabetes mellitus (OR, 1.30) remained after adjusting for age, gender, smoking, obesity, and steroid use. Risk of hypothyroidism (OR, 1.61) remained after adjusting for age and gender; risk of osteoporosis (OR, 1.50) after adjusting for age, gender, steroid use, and smoking; and risk of Cushing’s disease (OR, 3.79) after adjustment for age, gender, and steroid use.
The large, population-based cohort is a strength of the study. “We are now going back to see how many of these patients were seen by rheumatologists,” Dr. Haddad said. A lack of association with disease burden is a potential limitation, he added.
Thirty percent of patients were treated with biologics and about 67% with steroids. “That number treated with steroids seems high,” a meeting attendee commented. Dr. Haddad explained that it is the percentage ever treated with steroids, not necessarily currently on steroids.
In a separate session at the GRAPPA meeting addressing psoriatic disease treatment recommendations, an attendee asked about specific recommendations for comorbidities. For now, GRAPPA plans to include comorbidities within its overall recommendations, as it did in its most recent update, released in January 2016. A limited amount of data is a primary reason.
“As the evidence on comorbidities gets better, we may someday have separate recommendations for comorbidities,” said Laura Coates, MD, a clinical lecturer in rheumatology at the University of Leeds (England).
“The comorbidities are very important,” said Arthur F. Kavanaugh, MD, professor of medicine at the University of California, San Diego. “That’s trickier and deals with the international nature of GRAPPA. It’s hard to say, ‘Go see this specialist,’ because that might not be standard of care in that country.”
Dr. Haddad, Dr. Coates, and Dr. Kavanaugh reported having no relevant financial disclosures.
AT 2016 GRAPPA ANNUAL MEETING
Key clinical point:Patients with psoriatic disease had a significantly higher prevalence of diabetes mellitus and some other endocrine comorbidities.
Major finding: In a univariate analysis, the risk for Cushing’s disease was notably higher among psoriatic arthritis patients, compared with controls (odds ratio, 5.31).
Data source: Retrospective, cross-sectional comparison of 3,161 patients with psoriatic arthritis and 31,610 matched controls.
Disclosures: Dr. Haddad, Dr. Coates, and Dr. Kavanaugh reported having no relevant financial disclosures.
Hemophilia drugs top Medicaid spending per prescription
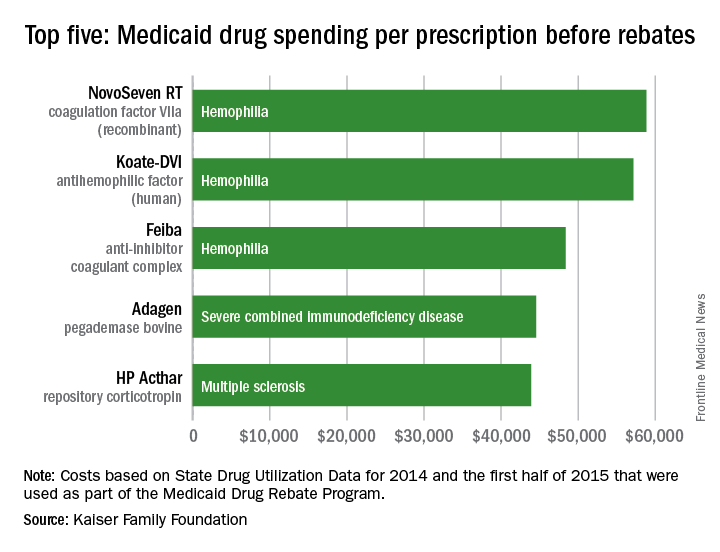
Medicaid’s three most expensive drugs by spending per prescription were for treatment of hemophilia, with the cost leader coming in at almost $59,000, according to an analysis by the Kaiser Family Foundation covering 2014 and the first half of 2015.
The trio of hemophilia drugs was topped by NovoSeven RT (coagulation factor VIIa [recombinant]), with Koate-DVI (antihemophilic factor [human]) second at $57,000 per prescription and Feiba (anti-inhibitor coagulant complex) third at $48,000 for each prescription.
None of the Medicaid costs include rebates since those data are unavailable to the public, Kaiser noted.
The fourth and fifth most expensive drugs were Adagen (pegademase bovine), which is used in the treatment of severe combined immunodeficiency disease associated with a deficiency of adenosine deaminase and cost Medicaid $45,000 per prescription, and the multiple sclerosis drug HP Acthar (repository corticotropin), which went for almost $44,000 a prescription, Kaiser said in its analysis, which used State Drug Utilization Data that are part of the Medicaid Drug Rebate Program.

Medicaid’s three most expensive drugs by spending per prescription were for treatment of hemophilia, with the cost leader coming in at almost $59,000, according to an analysis by the Kaiser Family Foundation covering 2014 and the first half of 2015.
The trio of hemophilia drugs was topped by NovoSeven RT (coagulation factor VIIa [recombinant]), with Koate-DVI (antihemophilic factor [human]) second at $57,000 per prescription and Feiba (anti-inhibitor coagulant complex) third at $48,000 for each prescription.
None of the Medicaid costs include rebates since those data are unavailable to the public, Kaiser noted.
The fourth and fifth most expensive drugs were Adagen (pegademase bovine), which is used in the treatment of severe combined immunodeficiency disease associated with a deficiency of adenosine deaminase and cost Medicaid $45,000 per prescription, and the multiple sclerosis drug HP Acthar (repository corticotropin), which went for almost $44,000 a prescription, Kaiser said in its analysis, which used State Drug Utilization Data that are part of the Medicaid Drug Rebate Program.

Medicaid’s three most expensive drugs by spending per prescription were for treatment of hemophilia, with the cost leader coming in at almost $59,000, according to an analysis by the Kaiser Family Foundation covering 2014 and the first half of 2015.
The trio of hemophilia drugs was topped by NovoSeven RT (coagulation factor VIIa [recombinant]), with Koate-DVI (antihemophilic factor [human]) second at $57,000 per prescription and Feiba (anti-inhibitor coagulant complex) third at $48,000 for each prescription.
None of the Medicaid costs include rebates since those data are unavailable to the public, Kaiser noted.
The fourth and fifth most expensive drugs were Adagen (pegademase bovine), which is used in the treatment of severe combined immunodeficiency disease associated with a deficiency of adenosine deaminase and cost Medicaid $45,000 per prescription, and the multiple sclerosis drug HP Acthar (repository corticotropin), which went for almost $44,000 a prescription, Kaiser said in its analysis, which used State Drug Utilization Data that are part of the Medicaid Drug Rebate Program.

Weight loss may improve fertility in anovulatory obese women
HELSINKI – A 6-month lifestyle intervention was associated with an increased natural conception rate in infertile anovulatory obese women, compared with infertile ovulatory obese women, although the rate of vaginal births of healthy singletons did not differ between the groups, according to subgroup analyses of the Lifestyle randomized controlled trial.
The findings, which confirm those in the overall study population and are likely explained by the beneficial effects of weight loss on the resumption of ovulation, have implications for managing obese women who are experiencing infertility, according to Anne van Oers, MD, of the University of Groningen (the Netherlands).
The Lifestyle trial – a multicenter study conducted in the Netherlands and published in 2016 – involved a 6-month lifestyle intervention preceding fertility treatment in obese infertile women. The intervention had no effect on the rate of vaginal births of healthy term singletons within 24 months versus immediate fertility treatment (relative risk, 0.77), although natural conceptions with an ongoing pregnancy did occur more often in the lifestyle intervention group (relative risk, 1.6).
For that study, conducted from 2009 to 2012, the investigators randomized 577 obese infertile women to either the 6-month lifestyle intervention followed by 18 months of infertility treatment or to immediate fertility treatment. Weight loss was 4.4 kg in the intervention group and 1.1 kg in the control group, Dr. van Oers said at the annual meeting of the European Society of Human Reproduction and Embryology.
For the subgroup analyses, the investigators focused on six groups based on age (those 36 years and older and those under age 36), ovulation status (those who were anovulatory and those who were ovulatory), and body mass index (those with a body-mass index (BMI) of 35 kg/m2 or greater and those with BMI under 35).
In the 564 women who completed follow-up, only the rate of natural conception was improved by the preconception lifestyle intervention: This was true in most of the subgroups, but was most pronounced among anovulatory women (28% vs. 11.4% in ovulatory women who received the intervention), she said.
Obese women are known to be at increased risk of infertility and are less likely than nonobese women to conceive after fertility treatment. In one prior study, ovulating subfertile women with a BMI of 29 kg/m2 or higher had a 4% lower pregnancy rate per kg/m2 increase per year, compared with ovulatory subfertile women with a BMI below 29, Dr. van Oers noted.
Although the current findings are limited by the nature of the subgroup analyses – the main study was not powered on analyses of subgroups or interaction tests – the findings do suggest a benefit of lifestyle intervention in some women, she noted.
“Our findings that lifestyle intervention in obese women more often leads to natural conception, specifically in anovulatory women, should be used in their counseling before fertility treatment and could reasonably be offered as first-line treatment for anovulation in obese women,” she said in a written statement.
Of note, 22% of the women in the main study were unable to adhere to the lifestyle intervention despite intensive coaching, according to the study’s project leader, Annemieke Hoek, MD, PhD, also from the University of Groningen.
The women who did not complete the program were significantly less likely to become pregnant, and those who did complete the program were more likely to conceive naturally, compared with the women in the control group who received immediate fertility treatment, Dr. Hoek said, noting that, again, this effect was most pronounced in anovulatory women.
Dr. van Oers and Dr. Hoek reported having no financial disclosures.
HELSINKI – A 6-month lifestyle intervention was associated with an increased natural conception rate in infertile anovulatory obese women, compared with infertile ovulatory obese women, although the rate of vaginal births of healthy singletons did not differ between the groups, according to subgroup analyses of the Lifestyle randomized controlled trial.
The findings, which confirm those in the overall study population and are likely explained by the beneficial effects of weight loss on the resumption of ovulation, have implications for managing obese women who are experiencing infertility, according to Anne van Oers, MD, of the University of Groningen (the Netherlands).
The Lifestyle trial – a multicenter study conducted in the Netherlands and published in 2016 – involved a 6-month lifestyle intervention preceding fertility treatment in obese infertile women. The intervention had no effect on the rate of vaginal births of healthy term singletons within 24 months versus immediate fertility treatment (relative risk, 0.77), although natural conceptions with an ongoing pregnancy did occur more often in the lifestyle intervention group (relative risk, 1.6).
For that study, conducted from 2009 to 2012, the investigators randomized 577 obese infertile women to either the 6-month lifestyle intervention followed by 18 months of infertility treatment or to immediate fertility treatment. Weight loss was 4.4 kg in the intervention group and 1.1 kg in the control group, Dr. van Oers said at the annual meeting of the European Society of Human Reproduction and Embryology.
For the subgroup analyses, the investigators focused on six groups based on age (those 36 years and older and those under age 36), ovulation status (those who were anovulatory and those who were ovulatory), and body mass index (those with a body-mass index (BMI) of 35 kg/m2 or greater and those with BMI under 35).
In the 564 women who completed follow-up, only the rate of natural conception was improved by the preconception lifestyle intervention: This was true in most of the subgroups, but was most pronounced among anovulatory women (28% vs. 11.4% in ovulatory women who received the intervention), she said.
Obese women are known to be at increased risk of infertility and are less likely than nonobese women to conceive after fertility treatment. In one prior study, ovulating subfertile women with a BMI of 29 kg/m2 or higher had a 4% lower pregnancy rate per kg/m2 increase per year, compared with ovulatory subfertile women with a BMI below 29, Dr. van Oers noted.
Although the current findings are limited by the nature of the subgroup analyses – the main study was not powered on analyses of subgroups or interaction tests – the findings do suggest a benefit of lifestyle intervention in some women, she noted.
“Our findings that lifestyle intervention in obese women more often leads to natural conception, specifically in anovulatory women, should be used in their counseling before fertility treatment and could reasonably be offered as first-line treatment for anovulation in obese women,” she said in a written statement.
Of note, 22% of the women in the main study were unable to adhere to the lifestyle intervention despite intensive coaching, according to the study’s project leader, Annemieke Hoek, MD, PhD, also from the University of Groningen.
The women who did not complete the program were significantly less likely to become pregnant, and those who did complete the program were more likely to conceive naturally, compared with the women in the control group who received immediate fertility treatment, Dr. Hoek said, noting that, again, this effect was most pronounced in anovulatory women.
Dr. van Oers and Dr. Hoek reported having no financial disclosures.
HELSINKI – A 6-month lifestyle intervention was associated with an increased natural conception rate in infertile anovulatory obese women, compared with infertile ovulatory obese women, although the rate of vaginal births of healthy singletons did not differ between the groups, according to subgroup analyses of the Lifestyle randomized controlled trial.
The findings, which confirm those in the overall study population and are likely explained by the beneficial effects of weight loss on the resumption of ovulation, have implications for managing obese women who are experiencing infertility, according to Anne van Oers, MD, of the University of Groningen (the Netherlands).
The Lifestyle trial – a multicenter study conducted in the Netherlands and published in 2016 – involved a 6-month lifestyle intervention preceding fertility treatment in obese infertile women. The intervention had no effect on the rate of vaginal births of healthy term singletons within 24 months versus immediate fertility treatment (relative risk, 0.77), although natural conceptions with an ongoing pregnancy did occur more often in the lifestyle intervention group (relative risk, 1.6).
For that study, conducted from 2009 to 2012, the investigators randomized 577 obese infertile women to either the 6-month lifestyle intervention followed by 18 months of infertility treatment or to immediate fertility treatment. Weight loss was 4.4 kg in the intervention group and 1.1 kg in the control group, Dr. van Oers said at the annual meeting of the European Society of Human Reproduction and Embryology.
For the subgroup analyses, the investigators focused on six groups based on age (those 36 years and older and those under age 36), ovulation status (those who were anovulatory and those who were ovulatory), and body mass index (those with a body-mass index (BMI) of 35 kg/m2 or greater and those with BMI under 35).
In the 564 women who completed follow-up, only the rate of natural conception was improved by the preconception lifestyle intervention: This was true in most of the subgroups, but was most pronounced among anovulatory women (28% vs. 11.4% in ovulatory women who received the intervention), she said.
Obese women are known to be at increased risk of infertility and are less likely than nonobese women to conceive after fertility treatment. In one prior study, ovulating subfertile women with a BMI of 29 kg/m2 or higher had a 4% lower pregnancy rate per kg/m2 increase per year, compared with ovulatory subfertile women with a BMI below 29, Dr. van Oers noted.
Although the current findings are limited by the nature of the subgroup analyses – the main study was not powered on analyses of subgroups or interaction tests – the findings do suggest a benefit of lifestyle intervention in some women, she noted.
“Our findings that lifestyle intervention in obese women more often leads to natural conception, specifically in anovulatory women, should be used in their counseling before fertility treatment and could reasonably be offered as first-line treatment for anovulation in obese women,” she said in a written statement.
Of note, 22% of the women in the main study were unable to adhere to the lifestyle intervention despite intensive coaching, according to the study’s project leader, Annemieke Hoek, MD, PhD, also from the University of Groningen.
The women who did not complete the program were significantly less likely to become pregnant, and those who did complete the program were more likely to conceive naturally, compared with the women in the control group who received immediate fertility treatment, Dr. Hoek said, noting that, again, this effect was most pronounced in anovulatory women.
Dr. van Oers and Dr. Hoek reported having no financial disclosures.
AT ESHRE 2016
Key clinical point: A lifestyle intervention involving weight loss was associated with an increased natural conception rate in infertile anovulatory obese women, compared with infertile ovulatory obese women.
Major finding: The postintervention natural conception rate was 28% in anovulatory obese women, compared with 11.4% in ovulatory obese women.
Data source: Subgroup analyses in 564 infertile obese women from a randomized controlled trial.
Disclosures: Dr. van Oers and Dr. Hoek reported having no financial disclosures.
Diabetes Prevalence in US Adolescents Is Under 1%
The prevalence of diabetes among adolescents in the United States is estimated to be 0.8%, with more than a quarter of that group being undiagnosed, according to a research letter published in JAMA.
Using data from the National Health and Nutrition Examination Survey 2005-2014, Andy Menke, PhD, and colleagues estimated the prevalence of diabetes overall, undiagnosed disease, and prediabetes in adolescents aged 12-19 years.
Of the 2,606 adolescents included in the analysis, 62 had diabetes, 20 were undiagnosed, and 512 had prediabetes. The weighted prevalence of diabetes was 0.8% (95% confidence interval, 0.6%-1.1%), of which 28.5% (95% CI, 16.4%-44.8%) was undiagnosed. The researchers defined undiagnosed diabetes as having no report of previous diagnosis but having a hemoglobin A1c level of 6.5% or greater, a fasting plasma glucose level of 126 mg/dL or greater, or a 2-hour plasma glucose level of 200 mg/dL or greater.
The prevalence of prediabetes among the adolescent sample was 17.7% (95% CI, 15.8%-19.8%). Prediabetes was defined as a hemoglobin A1c level of 5.7%-6.4%, a fasting plasma glucose level of 100-125 mg/dL, or a 2-hour plasma glucose level of 140-199 mg/dL among adolescents who did not have diagnosed or undiagnosed diabetes.
“A relatively large proportion was unaware of the condition, particularly among non-Hispanic black participants and Hispanic participants, indicating a need for improved diabetes screening among adolescents,” the researchers wrote. “These findings may have important public health implications because diabetes in youth is associated with early onset of risk factors and complications.”
The researchers were unable to distinguish between types of diabetes, but they noted that previous research in adolescents found that 87% had type 1.
Read the full research letter in JAMA (2016;316[3]:344-5. doi:10.1001/jama.2016.8544).
The prevalence of diabetes among adolescents in the United States is estimated to be 0.8%, with more than a quarter of that group being undiagnosed, according to a research letter published in JAMA.
Using data from the National Health and Nutrition Examination Survey 2005-2014, Andy Menke, PhD, and colleagues estimated the prevalence of diabetes overall, undiagnosed disease, and prediabetes in adolescents aged 12-19 years.
Of the 2,606 adolescents included in the analysis, 62 had diabetes, 20 were undiagnosed, and 512 had prediabetes. The weighted prevalence of diabetes was 0.8% (95% confidence interval, 0.6%-1.1%), of which 28.5% (95% CI, 16.4%-44.8%) was undiagnosed. The researchers defined undiagnosed diabetes as having no report of previous diagnosis but having a hemoglobin A1c level of 6.5% or greater, a fasting plasma glucose level of 126 mg/dL or greater, or a 2-hour plasma glucose level of 200 mg/dL or greater.
The prevalence of prediabetes among the adolescent sample was 17.7% (95% CI, 15.8%-19.8%). Prediabetes was defined as a hemoglobin A1c level of 5.7%-6.4%, a fasting plasma glucose level of 100-125 mg/dL, or a 2-hour plasma glucose level of 140-199 mg/dL among adolescents who did not have diagnosed or undiagnosed diabetes.
“A relatively large proportion was unaware of the condition, particularly among non-Hispanic black participants and Hispanic participants, indicating a need for improved diabetes screening among adolescents,” the researchers wrote. “These findings may have important public health implications because diabetes in youth is associated with early onset of risk factors and complications.”
The researchers were unable to distinguish between types of diabetes, but they noted that previous research in adolescents found that 87% had type 1.
Read the full research letter in JAMA (2016;316[3]:344-5. doi:10.1001/jama.2016.8544).
The prevalence of diabetes among adolescents in the United States is estimated to be 0.8%, with more than a quarter of that group being undiagnosed, according to a research letter published in JAMA.
Using data from the National Health and Nutrition Examination Survey 2005-2014, Andy Menke, PhD, and colleagues estimated the prevalence of diabetes overall, undiagnosed disease, and prediabetes in adolescents aged 12-19 years.
Of the 2,606 adolescents included in the analysis, 62 had diabetes, 20 were undiagnosed, and 512 had prediabetes. The weighted prevalence of diabetes was 0.8% (95% confidence interval, 0.6%-1.1%), of which 28.5% (95% CI, 16.4%-44.8%) was undiagnosed. The researchers defined undiagnosed diabetes as having no report of previous diagnosis but having a hemoglobin A1c level of 6.5% or greater, a fasting plasma glucose level of 126 mg/dL or greater, or a 2-hour plasma glucose level of 200 mg/dL or greater.
The prevalence of prediabetes among the adolescent sample was 17.7% (95% CI, 15.8%-19.8%). Prediabetes was defined as a hemoglobin A1c level of 5.7%-6.4%, a fasting plasma glucose level of 100-125 mg/dL, or a 2-hour plasma glucose level of 140-199 mg/dL among adolescents who did not have diagnosed or undiagnosed diabetes.
“A relatively large proportion was unaware of the condition, particularly among non-Hispanic black participants and Hispanic participants, indicating a need for improved diabetes screening among adolescents,” the researchers wrote. “These findings may have important public health implications because diabetes in youth is associated with early onset of risk factors and complications.”
The researchers were unable to distinguish between types of diabetes, but they noted that previous research in adolescents found that 87% had type 1.
Read the full research letter in JAMA (2016;316[3]:344-5. doi:10.1001/jama.2016.8544).
FROM JAMA
Diabetes prevalence in U.S. adolescents is under 1%
The prevalence of diabetes among adolescents in the United States is estimated to be 0.8%, with more than a quarter of that group being undiagnosed, according to a research letter published in JAMA.
Using data from the National Health and Nutrition Examination Survey 2005-2014, Andy Menke, PhD, and colleagues estimated the prevalence of diabetes overall, undiagnosed disease, and prediabetes in adolescents aged 12-19 years.
Of the 2,606 adolescents included in the analysis, 62 had diabetes, 20 were undiagnosed, and 512 had prediabetes. The weighted prevalence of diabetes was 0.8% (95% confidence interval, 0.6%-1.1%), of which 28.5% (95% CI, 16.4%-44.8%) was undiagnosed. The researchers defined undiagnosed diabetes as having no report of previous diagnosis but having a hemoglobin A1c level of 6.5% or greater, a fasting plasma glucose level of 126 mg/dL or greater, or a 2-hour plasma glucose level of 200 mg/dL or greater.
The prevalence of prediabetes among the adolescent sample was 17.7% (95% CI, 15.8%-19.8%). Prediabetes was defined as a hemoglobin A1c level of 5.7%-6.4%, a fasting plasma glucose level of 100-125 mg/dL, or a 2-hour plasma glucose level of 140-199 mg/dL among adolescents who did not have diagnosed or undiagnosed diabetes.
“A relatively large proportion was unaware of the condition, particularly among non-Hispanic black participants and Hispanic participants, indicating a need for improved diabetes screening among adolescents,” the researchers wrote. “These findings may have important public health implications because diabetes in youth is associated with early onset of risk factors and complications.”
The researchers were unable to distinguish between types of diabetes, but they noted that previous research in adolescents found that 87% had type 1.
Read the full research letter in JAMA (2016;316[3]:344-5. doi:10.1001/jama.2016.8544).
The prevalence of diabetes among adolescents in the United States is estimated to be 0.8%, with more than a quarter of that group being undiagnosed, according to a research letter published in JAMA.
Using data from the National Health and Nutrition Examination Survey 2005-2014, Andy Menke, PhD, and colleagues estimated the prevalence of diabetes overall, undiagnosed disease, and prediabetes in adolescents aged 12-19 years.
Of the 2,606 adolescents included in the analysis, 62 had diabetes, 20 were undiagnosed, and 512 had prediabetes. The weighted prevalence of diabetes was 0.8% (95% confidence interval, 0.6%-1.1%), of which 28.5% (95% CI, 16.4%-44.8%) was undiagnosed. The researchers defined undiagnosed diabetes as having no report of previous diagnosis but having a hemoglobin A1c level of 6.5% or greater, a fasting plasma glucose level of 126 mg/dL or greater, or a 2-hour plasma glucose level of 200 mg/dL or greater.
The prevalence of prediabetes among the adolescent sample was 17.7% (95% CI, 15.8%-19.8%). Prediabetes was defined as a hemoglobin A1c level of 5.7%-6.4%, a fasting plasma glucose level of 100-125 mg/dL, or a 2-hour plasma glucose level of 140-199 mg/dL among adolescents who did not have diagnosed or undiagnosed diabetes.
“A relatively large proportion was unaware of the condition, particularly among non-Hispanic black participants and Hispanic participants, indicating a need for improved diabetes screening among adolescents,” the researchers wrote. “These findings may have important public health implications because diabetes in youth is associated with early onset of risk factors and complications.”
The researchers were unable to distinguish between types of diabetes, but they noted that previous research in adolescents found that 87% had type 1.
Read the full research letter in JAMA (2016;316[3]:344-5. doi:10.1001/jama.2016.8544).
The prevalence of diabetes among adolescents in the United States is estimated to be 0.8%, with more than a quarter of that group being undiagnosed, according to a research letter published in JAMA.
Using data from the National Health and Nutrition Examination Survey 2005-2014, Andy Menke, PhD, and colleagues estimated the prevalence of diabetes overall, undiagnosed disease, and prediabetes in adolescents aged 12-19 years.
Of the 2,606 adolescents included in the analysis, 62 had diabetes, 20 were undiagnosed, and 512 had prediabetes. The weighted prevalence of diabetes was 0.8% (95% confidence interval, 0.6%-1.1%), of which 28.5% (95% CI, 16.4%-44.8%) was undiagnosed. The researchers defined undiagnosed diabetes as having no report of previous diagnosis but having a hemoglobin A1c level of 6.5% or greater, a fasting plasma glucose level of 126 mg/dL or greater, or a 2-hour plasma glucose level of 200 mg/dL or greater.
The prevalence of prediabetes among the adolescent sample was 17.7% (95% CI, 15.8%-19.8%). Prediabetes was defined as a hemoglobin A1c level of 5.7%-6.4%, a fasting plasma glucose level of 100-125 mg/dL, or a 2-hour plasma glucose level of 140-199 mg/dL among adolescents who did not have diagnosed or undiagnosed diabetes.
“A relatively large proportion was unaware of the condition, particularly among non-Hispanic black participants and Hispanic participants, indicating a need for improved diabetes screening among adolescents,” the researchers wrote. “These findings may have important public health implications because diabetes in youth is associated with early onset of risk factors and complications.”
The researchers were unable to distinguish between types of diabetes, but they noted that previous research in adolescents found that 87% had type 1.
Read the full research letter in JAMA (2016;316[3]:344-5. doi:10.1001/jama.2016.8544).
FROM JAMA