User login
Drug can prevent bleeding in hemophilia A and B
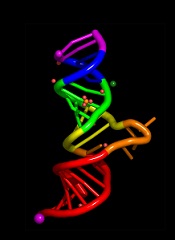
ORLANDO—Results from an ongoing phase 1 study suggest fitusiran, a small interfering RNA therapeutic targeting antithrombin (AT), can restore hemostasis and prevent bleeding in patients with hemophilia A or B, with or without inhibitors.
In patients with inhibitors, fitusiran exhibited preliminary evidence of reduced bleeding. In patients without inhibitors, fitusiran reduced the median estimated annualized bleeding rate (ABR) to 0.
In addition, researchers said fitusiran was generally well tolerated, and none of the patients developed anti-drug antibodies.
These results were presented at the World Federation of Hemophilia 2016 World Congress.* The study was sponsored by Alnylam Pharmaceuticals, Inc.
Study design
This phase 1 trial consists of 4 parts. Part A enrolled healthy volunteers who were randomized 3:1 to fitusiran or placebo. This part of the study was completed after the first dose cohort received a single subcutaneous dose of fitusiran at 30 mcg/kg.
Part B, which is also complete, enrolled 12 patients with severe hemophilia A or B. Patients received 3 weekly subcutaneous injections of fitusiran at doses of 15, 45, or 75 mcg/kg.
Part C, in which dosing is complete, enrolled 18 patients with moderate or severe hemophilia A or B without inhibitors. Twelve patients received 3 monthly subcutaneous doses of fitusiran at 225, 450, 900, or 1800 mcg/kg. Six patients received 3 fixed monthly subcutaneous doses of fitusiran at 80 mg.
Part D is designed to enroll up to 18 patients with inhibitors. The first cohort of 6 patients received a 50 mg, fixed, once-monthly, subcutaneous dose. The second cohort has completed enrollment, with 6 patients receiving an 80 mg, fixed, once-monthly, subcutaneous dose.
Results from Parts C and D were presented at the meeting.
Part C results
Treatment with fitusiran resulted in a dose-dependent, statistically significant decrease in AT and increase in thrombin generation. At the 80 mg monthly dose, fitusiran achieved 87±1% mean maximal AT lowering with low inter-patient variability.
Researchers assessed the association between AT lowering and increased thrombin generation in a post-hoc exploratory analysis in which AT lowering was grouped by 25% increments for completed patients in Parts B (n=12) and C (n=17) of the study.
In the highest quartile of ≥75% AT lowering (n=16), fitusiran administration resulted in mean increases in thrombin generation of approximately 290% relative to baseline (P<0.001, as compared to the lowest AT-lowering quartile).
There was a significant, AT-lowering-dependent reduction in bleeding frequency as well.
To obtain a comprehensive assessment of fitusiran’s potential effects on bleeding, researchers performed a post-hoc analysis in evaluable patients from Part C (n=17).
The team compared bleeding events that occurred over the 6-month period prior to study entry, bleeding events that were assessed prospectively during days 0-28 following the initial fitusiran dose (the onset period), and bleeding events that occurred beyond day 29 up to day 112 (the observation period).
Prior to study entry, the estimated median ABR was 28 for patients receiving on-demand factor therapy (n=4) and 2 for patients receiving prophylactic factor therapy (n=13).
The estimated median ABR was 13 among all evaluable patients during the onset period and 0 during the observation period.
In all Part C dose cohorts during the observation period, 53% of patients (9/17) were bleed-free, and 82% of patients reported no spontaneous bleeds.
Part D results
Prior to study entry, all patients in Part D utilized bypass agents, including recombinant factor VIIa and activated prothrombin complex concentrate, to manage their bleeds. They had a pre-study ABR of up to 80.
The first cohort (n=6) of patients received a once-monthly, fixed subcutaneous dose of 50 mg. Fitusiran achieved a mean maximal AT lowering of 81±2% and a mean maximal thrombin generation increase of approximately 368%.
In addition, there was preliminary evidence of reduced bleeding, with a 49% to 100% reduction in estimated ABR during the observation period compared with pre-study values.
Safety results
As of July 11, 2016, fitusiran appears to be generally well tolerated in hemophilia patients, with or without inhibitors (n=31, with 5 patients participating in both Parts B and C).
There have been no serious adverse events related to the drug and no thromboembolic events or laboratory evidence of pathologic clot formation.
One non-inhibitor patient in Part C treated with the 80 mg fixed dose discontinued treatment due to an adverse event that was considered severe and possibly related to the study drug.
This event was described as non-cardiac chest pain and was accompanied by transient elevations of ALT (10x upper limit of normal), AST (8x upper limit of normal), C-reactive protein, and D-dimer, without an increase in total bilirubin. The event resolved with symptomatic management, including antacids and analgesics.
Eleven patients (35%) reported mild, drug-related injection site reactions, which were mostly pain or erythema at the injection site.
Additional adverse events reported in at least 10% of patients included upper respiratory tract infection (10%) and arthralgia (10%). The majority of these events were mild or moderate in severity. ![]()

ORLANDO—Results from an ongoing phase 1 study suggest fitusiran, a small interfering RNA therapeutic targeting antithrombin (AT), can restore hemostasis and prevent bleeding in patients with hemophilia A or B, with or without inhibitors.
In patients with inhibitors, fitusiran exhibited preliminary evidence of reduced bleeding. In patients without inhibitors, fitusiran reduced the median estimated annualized bleeding rate (ABR) to 0.
In addition, researchers said fitusiran was generally well tolerated, and none of the patients developed anti-drug antibodies.
These results were presented at the World Federation of Hemophilia 2016 World Congress.* The study was sponsored by Alnylam Pharmaceuticals, Inc.
Study design
This phase 1 trial consists of 4 parts. Part A enrolled healthy volunteers who were randomized 3:1 to fitusiran or placebo. This part of the study was completed after the first dose cohort received a single subcutaneous dose of fitusiran at 30 mcg/kg.
Part B, which is also complete, enrolled 12 patients with severe hemophilia A or B. Patients received 3 weekly subcutaneous injections of fitusiran at doses of 15, 45, or 75 mcg/kg.
Part C, in which dosing is complete, enrolled 18 patients with moderate or severe hemophilia A or B without inhibitors. Twelve patients received 3 monthly subcutaneous doses of fitusiran at 225, 450, 900, or 1800 mcg/kg. Six patients received 3 fixed monthly subcutaneous doses of fitusiran at 80 mg.
Part D is designed to enroll up to 18 patients with inhibitors. The first cohort of 6 patients received a 50 mg, fixed, once-monthly, subcutaneous dose. The second cohort has completed enrollment, with 6 patients receiving an 80 mg, fixed, once-monthly, subcutaneous dose.
Results from Parts C and D were presented at the meeting.
Part C results
Treatment with fitusiran resulted in a dose-dependent, statistically significant decrease in AT and increase in thrombin generation. At the 80 mg monthly dose, fitusiran achieved 87±1% mean maximal AT lowering with low inter-patient variability.
Researchers assessed the association between AT lowering and increased thrombin generation in a post-hoc exploratory analysis in which AT lowering was grouped by 25% increments for completed patients in Parts B (n=12) and C (n=17) of the study.
In the highest quartile of ≥75% AT lowering (n=16), fitusiran administration resulted in mean increases in thrombin generation of approximately 290% relative to baseline (P<0.001, as compared to the lowest AT-lowering quartile).
There was a significant, AT-lowering-dependent reduction in bleeding frequency as well.
To obtain a comprehensive assessment of fitusiran’s potential effects on bleeding, researchers performed a post-hoc analysis in evaluable patients from Part C (n=17).
The team compared bleeding events that occurred over the 6-month period prior to study entry, bleeding events that were assessed prospectively during days 0-28 following the initial fitusiran dose (the onset period), and bleeding events that occurred beyond day 29 up to day 112 (the observation period).
Prior to study entry, the estimated median ABR was 28 for patients receiving on-demand factor therapy (n=4) and 2 for patients receiving prophylactic factor therapy (n=13).
The estimated median ABR was 13 among all evaluable patients during the onset period and 0 during the observation period.
In all Part C dose cohorts during the observation period, 53% of patients (9/17) were bleed-free, and 82% of patients reported no spontaneous bleeds.
Part D results
Prior to study entry, all patients in Part D utilized bypass agents, including recombinant factor VIIa and activated prothrombin complex concentrate, to manage their bleeds. They had a pre-study ABR of up to 80.
The first cohort (n=6) of patients received a once-monthly, fixed subcutaneous dose of 50 mg. Fitusiran achieved a mean maximal AT lowering of 81±2% and a mean maximal thrombin generation increase of approximately 368%.
In addition, there was preliminary evidence of reduced bleeding, with a 49% to 100% reduction in estimated ABR during the observation period compared with pre-study values.
Safety results
As of July 11, 2016, fitusiran appears to be generally well tolerated in hemophilia patients, with or without inhibitors (n=31, with 5 patients participating in both Parts B and C).
There have been no serious adverse events related to the drug and no thromboembolic events or laboratory evidence of pathologic clot formation.
One non-inhibitor patient in Part C treated with the 80 mg fixed dose discontinued treatment due to an adverse event that was considered severe and possibly related to the study drug.
This event was described as non-cardiac chest pain and was accompanied by transient elevations of ALT (10x upper limit of normal), AST (8x upper limit of normal), C-reactive protein, and D-dimer, without an increase in total bilirubin. The event resolved with symptomatic management, including antacids and analgesics.
Eleven patients (35%) reported mild, drug-related injection site reactions, which were mostly pain or erythema at the injection site.
Additional adverse events reported in at least 10% of patients included upper respiratory tract infection (10%) and arthralgia (10%). The majority of these events were mild or moderate in severity. ![]()

ORLANDO—Results from an ongoing phase 1 study suggest fitusiran, a small interfering RNA therapeutic targeting antithrombin (AT), can restore hemostasis and prevent bleeding in patients with hemophilia A or B, with or without inhibitors.
In patients with inhibitors, fitusiran exhibited preliminary evidence of reduced bleeding. In patients without inhibitors, fitusiran reduced the median estimated annualized bleeding rate (ABR) to 0.
In addition, researchers said fitusiran was generally well tolerated, and none of the patients developed anti-drug antibodies.
These results were presented at the World Federation of Hemophilia 2016 World Congress.* The study was sponsored by Alnylam Pharmaceuticals, Inc.
Study design
This phase 1 trial consists of 4 parts. Part A enrolled healthy volunteers who were randomized 3:1 to fitusiran or placebo. This part of the study was completed after the first dose cohort received a single subcutaneous dose of fitusiran at 30 mcg/kg.
Part B, which is also complete, enrolled 12 patients with severe hemophilia A or B. Patients received 3 weekly subcutaneous injections of fitusiran at doses of 15, 45, or 75 mcg/kg.
Part C, in which dosing is complete, enrolled 18 patients with moderate or severe hemophilia A or B without inhibitors. Twelve patients received 3 monthly subcutaneous doses of fitusiran at 225, 450, 900, or 1800 mcg/kg. Six patients received 3 fixed monthly subcutaneous doses of fitusiran at 80 mg.
Part D is designed to enroll up to 18 patients with inhibitors. The first cohort of 6 patients received a 50 mg, fixed, once-monthly, subcutaneous dose. The second cohort has completed enrollment, with 6 patients receiving an 80 mg, fixed, once-monthly, subcutaneous dose.
Results from Parts C and D were presented at the meeting.
Part C results
Treatment with fitusiran resulted in a dose-dependent, statistically significant decrease in AT and increase in thrombin generation. At the 80 mg monthly dose, fitusiran achieved 87±1% mean maximal AT lowering with low inter-patient variability.
Researchers assessed the association between AT lowering and increased thrombin generation in a post-hoc exploratory analysis in which AT lowering was grouped by 25% increments for completed patients in Parts B (n=12) and C (n=17) of the study.
In the highest quartile of ≥75% AT lowering (n=16), fitusiran administration resulted in mean increases in thrombin generation of approximately 290% relative to baseline (P<0.001, as compared to the lowest AT-lowering quartile).
There was a significant, AT-lowering-dependent reduction in bleeding frequency as well.
To obtain a comprehensive assessment of fitusiran’s potential effects on bleeding, researchers performed a post-hoc analysis in evaluable patients from Part C (n=17).
The team compared bleeding events that occurred over the 6-month period prior to study entry, bleeding events that were assessed prospectively during days 0-28 following the initial fitusiran dose (the onset period), and bleeding events that occurred beyond day 29 up to day 112 (the observation period).
Prior to study entry, the estimated median ABR was 28 for patients receiving on-demand factor therapy (n=4) and 2 for patients receiving prophylactic factor therapy (n=13).
The estimated median ABR was 13 among all evaluable patients during the onset period and 0 during the observation period.
In all Part C dose cohorts during the observation period, 53% of patients (9/17) were bleed-free, and 82% of patients reported no spontaneous bleeds.
Part D results
Prior to study entry, all patients in Part D utilized bypass agents, including recombinant factor VIIa and activated prothrombin complex concentrate, to manage their bleeds. They had a pre-study ABR of up to 80.
The first cohort (n=6) of patients received a once-monthly, fixed subcutaneous dose of 50 mg. Fitusiran achieved a mean maximal AT lowering of 81±2% and a mean maximal thrombin generation increase of approximately 368%.
In addition, there was preliminary evidence of reduced bleeding, with a 49% to 100% reduction in estimated ABR during the observation period compared with pre-study values.
Safety results
As of July 11, 2016, fitusiran appears to be generally well tolerated in hemophilia patients, with or without inhibitors (n=31, with 5 patients participating in both Parts B and C).
There have been no serious adverse events related to the drug and no thromboembolic events or laboratory evidence of pathologic clot formation.
One non-inhibitor patient in Part C treated with the 80 mg fixed dose discontinued treatment due to an adverse event that was considered severe and possibly related to the study drug.
This event was described as non-cardiac chest pain and was accompanied by transient elevations of ALT (10x upper limit of normal), AST (8x upper limit of normal), C-reactive protein, and D-dimer, without an increase in total bilirubin. The event resolved with symptomatic management, including antacids and analgesics.
Eleven patients (35%) reported mild, drug-related injection site reactions, which were mostly pain or erythema at the injection site.
Additional adverse events reported in at least 10% of patients included upper respiratory tract infection (10%) and arthralgia (10%). The majority of these events were mild or moderate in severity. ![]()
Sickle cell trait doesn’t increase risk of death, study suggests

Results of a large study contradict the view that having sickle cell trait increases a person’s risk of death.
Health records of nearly 50,000 active-duty US Army soldiers showed no significant difference in the risk of death between soldiers who had sickle cell trait and those who did not.
The risk of exertional rhabdomyolysis (ER) was 54% higher among soldiers with sickle cell trait than those without it.
But the study suggested that tobacco use, obesity, and taking certain drugs also incur a heightened risk of ER.
Lianne Kurina, PhD, of Stanford University School of Medicine in California, and her colleagues reported these findings in NEJM.
Previous studies have suggested the health consequences of sickle cell trait might be dire, including higher mortality from ER. ER is characterized by the severe breakdown of skeletal-muscle tissue due to extreme physical exertion. The condition has been known to affect athletes and soldiers.
To assess the risk of ER and death among people with sickle cell trait, Dr Kurina and her colleagues reviewed the health records of 47,944 African-American soldiers who served on active duty between 2011 and 2014 and for whom sickle cell status was known.
The team found no significant difference in the risk of death among soldiers with sickle cell trait and those without. The hazard ratio (HR) was 0.99 (95% confidence interval [CI], 0.46 to 2.13; P=0.97).
Sickle cell trait was associated with a significantly higher adjusted risk of ER, with an HR of 1.54 (95% CI, 1.12 to 2.12; P=0.008).
However, the risk of ER was also higher for the following groups:
- Soldiers who used tobacco (HR=1.54, 95% CI, 1.23 to 1.94; P<0.001)
- Those with a body mass index of 30 or higher, as compared to 25 or lower (HR=1.39, 95% CI, 1.04 to 1.86; P=0.03)
- Those who recently used a statin (HR=2.89, 95% CI, 1.51 to 5.55; P=0.001)
- Those who recently used an antipsychotic agent (HR=3.02, 95% CI, 1.34 to 6.82; P=0.008).
Dr Kurina said the reason the results of this study differ from those of previous studies may be better safety for active-duty soldiers.
As of 2003, soldiers who are engaged in strenuous exercise are required to drink plenty of fluids, build up to strenuous exercise gradually, and take regular rests when it’s hot. All of these measures are known to reduce exercise-related fatality rates, regardless of whether individuals have sickle cell trait, the researchers said.
“Another critical difference between our study and the earlier, population-based studies is that, in our study, we knew the sickle cell status of everyone in the population,” Dr Kurina said.
She and her team looked only at soldiers whose sickle cell status was confirmed by blood tests taken during their years of service, instead of from self-reported sickle cell status or past medical history, as had been done in the other studies.
“The most important thing to come out of this study is the really reassuring news that, under conditions of universal precautions against dehydration and overheating, we don’t see an elevation in the risk of mortality in people with sickle cell trait,” Dr Kurina said.
She added that the study’s results call into question the need to screen service members with sickle cell trait, especially with better safety precautions during intense exertion. ![]()

Results of a large study contradict the view that having sickle cell trait increases a person’s risk of death.
Health records of nearly 50,000 active-duty US Army soldiers showed no significant difference in the risk of death between soldiers who had sickle cell trait and those who did not.
The risk of exertional rhabdomyolysis (ER) was 54% higher among soldiers with sickle cell trait than those without it.
But the study suggested that tobacco use, obesity, and taking certain drugs also incur a heightened risk of ER.
Lianne Kurina, PhD, of Stanford University School of Medicine in California, and her colleagues reported these findings in NEJM.
Previous studies have suggested the health consequences of sickle cell trait might be dire, including higher mortality from ER. ER is characterized by the severe breakdown of skeletal-muscle tissue due to extreme physical exertion. The condition has been known to affect athletes and soldiers.
To assess the risk of ER and death among people with sickle cell trait, Dr Kurina and her colleagues reviewed the health records of 47,944 African-American soldiers who served on active duty between 2011 and 2014 and for whom sickle cell status was known.
The team found no significant difference in the risk of death among soldiers with sickle cell trait and those without. The hazard ratio (HR) was 0.99 (95% confidence interval [CI], 0.46 to 2.13; P=0.97).
Sickle cell trait was associated with a significantly higher adjusted risk of ER, with an HR of 1.54 (95% CI, 1.12 to 2.12; P=0.008).
However, the risk of ER was also higher for the following groups:
- Soldiers who used tobacco (HR=1.54, 95% CI, 1.23 to 1.94; P<0.001)
- Those with a body mass index of 30 or higher, as compared to 25 or lower (HR=1.39, 95% CI, 1.04 to 1.86; P=0.03)
- Those who recently used a statin (HR=2.89, 95% CI, 1.51 to 5.55; P=0.001)
- Those who recently used an antipsychotic agent (HR=3.02, 95% CI, 1.34 to 6.82; P=0.008).
Dr Kurina said the reason the results of this study differ from those of previous studies may be better safety for active-duty soldiers.
As of 2003, soldiers who are engaged in strenuous exercise are required to drink plenty of fluids, build up to strenuous exercise gradually, and take regular rests when it’s hot. All of these measures are known to reduce exercise-related fatality rates, regardless of whether individuals have sickle cell trait, the researchers said.
“Another critical difference between our study and the earlier, population-based studies is that, in our study, we knew the sickle cell status of everyone in the population,” Dr Kurina said.
She and her team looked only at soldiers whose sickle cell status was confirmed by blood tests taken during their years of service, instead of from self-reported sickle cell status or past medical history, as had been done in the other studies.
“The most important thing to come out of this study is the really reassuring news that, under conditions of universal precautions against dehydration and overheating, we don’t see an elevation in the risk of mortality in people with sickle cell trait,” Dr Kurina said.
She added that the study’s results call into question the need to screen service members with sickle cell trait, especially with better safety precautions during intense exertion. ![]()

Results of a large study contradict the view that having sickle cell trait increases a person’s risk of death.
Health records of nearly 50,000 active-duty US Army soldiers showed no significant difference in the risk of death between soldiers who had sickle cell trait and those who did not.
The risk of exertional rhabdomyolysis (ER) was 54% higher among soldiers with sickle cell trait than those without it.
But the study suggested that tobacco use, obesity, and taking certain drugs also incur a heightened risk of ER.
Lianne Kurina, PhD, of Stanford University School of Medicine in California, and her colleagues reported these findings in NEJM.
Previous studies have suggested the health consequences of sickle cell trait might be dire, including higher mortality from ER. ER is characterized by the severe breakdown of skeletal-muscle tissue due to extreme physical exertion. The condition has been known to affect athletes and soldiers.
To assess the risk of ER and death among people with sickle cell trait, Dr Kurina and her colleagues reviewed the health records of 47,944 African-American soldiers who served on active duty between 2011 and 2014 and for whom sickle cell status was known.
The team found no significant difference in the risk of death among soldiers with sickle cell trait and those without. The hazard ratio (HR) was 0.99 (95% confidence interval [CI], 0.46 to 2.13; P=0.97).
Sickle cell trait was associated with a significantly higher adjusted risk of ER, with an HR of 1.54 (95% CI, 1.12 to 2.12; P=0.008).
However, the risk of ER was also higher for the following groups:
- Soldiers who used tobacco (HR=1.54, 95% CI, 1.23 to 1.94; P<0.001)
- Those with a body mass index of 30 or higher, as compared to 25 or lower (HR=1.39, 95% CI, 1.04 to 1.86; P=0.03)
- Those who recently used a statin (HR=2.89, 95% CI, 1.51 to 5.55; P=0.001)
- Those who recently used an antipsychotic agent (HR=3.02, 95% CI, 1.34 to 6.82; P=0.008).
Dr Kurina said the reason the results of this study differ from those of previous studies may be better safety for active-duty soldiers.
As of 2003, soldiers who are engaged in strenuous exercise are required to drink plenty of fluids, build up to strenuous exercise gradually, and take regular rests when it’s hot. All of these measures are known to reduce exercise-related fatality rates, regardless of whether individuals have sickle cell trait, the researchers said.
“Another critical difference between our study and the earlier, population-based studies is that, in our study, we knew the sickle cell status of everyone in the population,” Dr Kurina said.
She and her team looked only at soldiers whose sickle cell status was confirmed by blood tests taken during their years of service, instead of from self-reported sickle cell status or past medical history, as had been done in the other studies.
“The most important thing to come out of this study is the really reassuring news that, under conditions of universal precautions against dehydration and overheating, we don’t see an elevation in the risk of mortality in people with sickle cell trait,” Dr Kurina said.
She added that the study’s results call into question the need to screen service members with sickle cell trait, especially with better safety precautions during intense exertion. ![]()
Improving upon results with checkpoint inhibitors

Photo by Rob Press
Manipulating metabolic events might reinvigorate exhausted T cells and complement the effects of checkpoint inhibitors in the treatment of cancers, according to research published in Immunity.
When T cells are activated because of a tumor or microbe, they transition from a catabolic existence of slow metabolic burn to an anabolic one in which the body revs up to generate chemical intermediates to build new cells.
However, T cells are hard-wired to stop the anabolic mode at a certain point because functioning at that level is unsustainable.
PD-1, a cell surface receptor and target of checkpoint inhibitors, tells T cells to turn off the anabolic pathway, but other molecular signals attempt to keep this pathway turned on because growing tumors or chronic infection are still present.
This results in “metabolically confused” T cells, said study author E. John Wherry, PhD, of the University of Pennsylvania School of Medicine in Philadelphia.
To study this, Dr Wherry and his colleagues induced infection in mice using 2 different strains of the lymphocytic choriomeningitis virus, a model system for exploring T-cell biology.
“We found that, as early as the first week of a chronic viral infection, even before severe T-cell dysfunction becomes established, virus-specific T cells are already unable to match the bioenergetic demands of T cells generated during the height of fighting a well-contained viral infection in a mouse model,” Dr Wherry said.
In other words, these experiments revealed when PD-1 turns off the anabolic metabolism signal, and it’s earlier than previously thought.
The researchers said this finding is important because it identifies the altered metabolism as a distinct point in the development of exhausted T cells, rather than as a later consequence of exhausted T cells.
This research also revealed PD-1’s role as the metabolic switch in shutting down anabolic pathways and characterized downstream metabolic regulator targets of PD-1.
For example, restriction of glucose uptake and utilization, despite the upregulation of multiple backup metabolic pathways, was one metabolic defect in the exhausted T cells. PD-1 partially controls the development of this early defect in using glucose as a fuel, as well as the size and quality of mitochondria.
A second pathway repressed by PD-1 involved PGC-1α, a protein that regulates genes involved in metabolism. Correcting this PD-1-induced defect by overexpressing PGC-1α improved exhausted T-cell bioenergetics.
The researchers said these results have implications for therapeutic strategies aimed at the reinvigoration of exhausted T cells in chronic infections and cancer. And targeting exhausted T-cell metabolism could complement the effects of blocking PD-1 and other checkpoints. ![]()

Photo by Rob Press
Manipulating metabolic events might reinvigorate exhausted T cells and complement the effects of checkpoint inhibitors in the treatment of cancers, according to research published in Immunity.
When T cells are activated because of a tumor or microbe, they transition from a catabolic existence of slow metabolic burn to an anabolic one in which the body revs up to generate chemical intermediates to build new cells.
However, T cells are hard-wired to stop the anabolic mode at a certain point because functioning at that level is unsustainable.
PD-1, a cell surface receptor and target of checkpoint inhibitors, tells T cells to turn off the anabolic pathway, but other molecular signals attempt to keep this pathway turned on because growing tumors or chronic infection are still present.
This results in “metabolically confused” T cells, said study author E. John Wherry, PhD, of the University of Pennsylvania School of Medicine in Philadelphia.
To study this, Dr Wherry and his colleagues induced infection in mice using 2 different strains of the lymphocytic choriomeningitis virus, a model system for exploring T-cell biology.
“We found that, as early as the first week of a chronic viral infection, even before severe T-cell dysfunction becomes established, virus-specific T cells are already unable to match the bioenergetic demands of T cells generated during the height of fighting a well-contained viral infection in a mouse model,” Dr Wherry said.
In other words, these experiments revealed when PD-1 turns off the anabolic metabolism signal, and it’s earlier than previously thought.
The researchers said this finding is important because it identifies the altered metabolism as a distinct point in the development of exhausted T cells, rather than as a later consequence of exhausted T cells.
This research also revealed PD-1’s role as the metabolic switch in shutting down anabolic pathways and characterized downstream metabolic regulator targets of PD-1.
For example, restriction of glucose uptake and utilization, despite the upregulation of multiple backup metabolic pathways, was one metabolic defect in the exhausted T cells. PD-1 partially controls the development of this early defect in using glucose as a fuel, as well as the size and quality of mitochondria.
A second pathway repressed by PD-1 involved PGC-1α, a protein that regulates genes involved in metabolism. Correcting this PD-1-induced defect by overexpressing PGC-1α improved exhausted T-cell bioenergetics.
The researchers said these results have implications for therapeutic strategies aimed at the reinvigoration of exhausted T cells in chronic infections and cancer. And targeting exhausted T-cell metabolism could complement the effects of blocking PD-1 and other checkpoints. ![]()

Photo by Rob Press
Manipulating metabolic events might reinvigorate exhausted T cells and complement the effects of checkpoint inhibitors in the treatment of cancers, according to research published in Immunity.
When T cells are activated because of a tumor or microbe, they transition from a catabolic existence of slow metabolic burn to an anabolic one in which the body revs up to generate chemical intermediates to build new cells.
However, T cells are hard-wired to stop the anabolic mode at a certain point because functioning at that level is unsustainable.
PD-1, a cell surface receptor and target of checkpoint inhibitors, tells T cells to turn off the anabolic pathway, but other molecular signals attempt to keep this pathway turned on because growing tumors or chronic infection are still present.
This results in “metabolically confused” T cells, said study author E. John Wherry, PhD, of the University of Pennsylvania School of Medicine in Philadelphia.
To study this, Dr Wherry and his colleagues induced infection in mice using 2 different strains of the lymphocytic choriomeningitis virus, a model system for exploring T-cell biology.
“We found that, as early as the first week of a chronic viral infection, even before severe T-cell dysfunction becomes established, virus-specific T cells are already unable to match the bioenergetic demands of T cells generated during the height of fighting a well-contained viral infection in a mouse model,” Dr Wherry said.
In other words, these experiments revealed when PD-1 turns off the anabolic metabolism signal, and it’s earlier than previously thought.
The researchers said this finding is important because it identifies the altered metabolism as a distinct point in the development of exhausted T cells, rather than as a later consequence of exhausted T cells.
This research also revealed PD-1’s role as the metabolic switch in shutting down anabolic pathways and characterized downstream metabolic regulator targets of PD-1.
For example, restriction of glucose uptake and utilization, despite the upregulation of multiple backup metabolic pathways, was one metabolic defect in the exhausted T cells. PD-1 partially controls the development of this early defect in using glucose as a fuel, as well as the size and quality of mitochondria.
A second pathway repressed by PD-1 involved PGC-1α, a protein that regulates genes involved in metabolism. Correcting this PD-1-induced defect by overexpressing PGC-1α improved exhausted T-cell bioenergetics.
The researchers said these results have implications for therapeutic strategies aimed at the reinvigoration of exhausted T cells in chronic infections and cancer. And targeting exhausted T-cell metabolism could complement the effects of blocking PD-1 and other checkpoints. ![]()
Many pediatric trials go unfinished, unpublished

Photo by Logan Tuttle
Clinical trials in children too often go uncompleted or unpublished, according to a pair of researchers.
The duo evaluated nearly 560 pediatric trials and found that 19% were discontinued early. Of the trials that were completed, 30% remained unpublished several years later.
Industry-sponsored trials were more likely to be completed than trials sponsored by academic institutions. However, completed trials sponsored by industry were less likely to be published than trials sponsored by academia.
“Our findings are in line with previously published studies focusing on adult trials, which may speak to how commonplace discontinuation and non-publication are in medical research in general,” said study author Natalie Pica, MD, PhD, of Boston Children’s Hospital in Massachusetts.
She and Florence Bourgeois, MD, also of Boston Children’s Hospital, reported these findings in Pediatrics.
The researchers tracked 559 randomized, controlled pediatric trials registered on ClinicalTrials.gov from 2008 to 2010 and whose final status (completed or discontinued) was confirmed by the end of 2012.
The pair then searched for related peer-reviewed articles published through September 1, 2015. When no publication could be found, the researchers inquired with study investigators and sponsors via email.
Of the 559 trials, 104 (19%) were discontinued early. Two-thirds of these had enrolled participants.
Of the 455 completed trials, 136 (30%) remained unpublished after an average of 58 months post-completion. However, 42 of these (31%) did have results posted on ClinicalTrials.gov.
Of the 104 discontinued trials, 39% were sponsored by industry, and 55% were sponsored by academic institutions. (The rest were funded by other sources.)
Two years after trial completion, academia-sponsored trials accounted for 30% of unpublished trials, and industry-sponsored trials accounted for 63%.
Three years after trial completion, academia-sponsored trials accounted for 23% of unpublished trials, and industry-sponsored trials accounted for 70%.
In a multivariate analysis, the likelihood of non-publication was more than doubled for industry-sponsored trials 2 years after completion (odds ratio=2.21) and more than tripled 3 years after completion (odds ratio=3.12).
Overall, more than 8000 children were enrolled in trials that were never completed, and more than 69,000 children were enrolled in completed trials that were never published.
“This is the first study to look systematically at discontinuation and nonpublication of interventional pediatric clinical trials,” Dr Bourgeois said.
“A number of legislative initiatives have been implemented to increase the study of interventions in children. Now, we need to make sure that the proper resources are in place to ensure that information gleaned from these studies reaches the scientific community.”
One proposed initiative cited by Drs Bourgeois and Pica is RIAT (Restoring Invisible and Abandoned Trials), which is supported by some high-profile journals. RIAT invites researchers with unpublished trials to either commit to publish within a year or provide public access to their data.
“It’s hard to reanalyze others’ data,” Dr Pica noted, “but this may be a useful mechanism to make sure that findings from completed trials are disseminated in the medical literature.” ![]()

Photo by Logan Tuttle
Clinical trials in children too often go uncompleted or unpublished, according to a pair of researchers.
The duo evaluated nearly 560 pediatric trials and found that 19% were discontinued early. Of the trials that were completed, 30% remained unpublished several years later.
Industry-sponsored trials were more likely to be completed than trials sponsored by academic institutions. However, completed trials sponsored by industry were less likely to be published than trials sponsored by academia.
“Our findings are in line with previously published studies focusing on adult trials, which may speak to how commonplace discontinuation and non-publication are in medical research in general,” said study author Natalie Pica, MD, PhD, of Boston Children’s Hospital in Massachusetts.
She and Florence Bourgeois, MD, also of Boston Children’s Hospital, reported these findings in Pediatrics.
The researchers tracked 559 randomized, controlled pediatric trials registered on ClinicalTrials.gov from 2008 to 2010 and whose final status (completed or discontinued) was confirmed by the end of 2012.
The pair then searched for related peer-reviewed articles published through September 1, 2015. When no publication could be found, the researchers inquired with study investigators and sponsors via email.
Of the 559 trials, 104 (19%) were discontinued early. Two-thirds of these had enrolled participants.
Of the 455 completed trials, 136 (30%) remained unpublished after an average of 58 months post-completion. However, 42 of these (31%) did have results posted on ClinicalTrials.gov.
Of the 104 discontinued trials, 39% were sponsored by industry, and 55% were sponsored by academic institutions. (The rest were funded by other sources.)
Two years after trial completion, academia-sponsored trials accounted for 30% of unpublished trials, and industry-sponsored trials accounted for 63%.
Three years after trial completion, academia-sponsored trials accounted for 23% of unpublished trials, and industry-sponsored trials accounted for 70%.
In a multivariate analysis, the likelihood of non-publication was more than doubled for industry-sponsored trials 2 years after completion (odds ratio=2.21) and more than tripled 3 years after completion (odds ratio=3.12).
Overall, more than 8000 children were enrolled in trials that were never completed, and more than 69,000 children were enrolled in completed trials that were never published.
“This is the first study to look systematically at discontinuation and nonpublication of interventional pediatric clinical trials,” Dr Bourgeois said.
“A number of legislative initiatives have been implemented to increase the study of interventions in children. Now, we need to make sure that the proper resources are in place to ensure that information gleaned from these studies reaches the scientific community.”
One proposed initiative cited by Drs Bourgeois and Pica is RIAT (Restoring Invisible and Abandoned Trials), which is supported by some high-profile journals. RIAT invites researchers with unpublished trials to either commit to publish within a year or provide public access to their data.
“It’s hard to reanalyze others’ data,” Dr Pica noted, “but this may be a useful mechanism to make sure that findings from completed trials are disseminated in the medical literature.” ![]()

Photo by Logan Tuttle
Clinical trials in children too often go uncompleted or unpublished, according to a pair of researchers.
The duo evaluated nearly 560 pediatric trials and found that 19% were discontinued early. Of the trials that were completed, 30% remained unpublished several years later.
Industry-sponsored trials were more likely to be completed than trials sponsored by academic institutions. However, completed trials sponsored by industry were less likely to be published than trials sponsored by academia.
“Our findings are in line with previously published studies focusing on adult trials, which may speak to how commonplace discontinuation and non-publication are in medical research in general,” said study author Natalie Pica, MD, PhD, of Boston Children’s Hospital in Massachusetts.
She and Florence Bourgeois, MD, also of Boston Children’s Hospital, reported these findings in Pediatrics.
The researchers tracked 559 randomized, controlled pediatric trials registered on ClinicalTrials.gov from 2008 to 2010 and whose final status (completed or discontinued) was confirmed by the end of 2012.
The pair then searched for related peer-reviewed articles published through September 1, 2015. When no publication could be found, the researchers inquired with study investigators and sponsors via email.
Of the 559 trials, 104 (19%) were discontinued early. Two-thirds of these had enrolled participants.
Of the 455 completed trials, 136 (30%) remained unpublished after an average of 58 months post-completion. However, 42 of these (31%) did have results posted on ClinicalTrials.gov.
Of the 104 discontinued trials, 39% were sponsored by industry, and 55% were sponsored by academic institutions. (The rest were funded by other sources.)
Two years after trial completion, academia-sponsored trials accounted for 30% of unpublished trials, and industry-sponsored trials accounted for 63%.
Three years after trial completion, academia-sponsored trials accounted for 23% of unpublished trials, and industry-sponsored trials accounted for 70%.
In a multivariate analysis, the likelihood of non-publication was more than doubled for industry-sponsored trials 2 years after completion (odds ratio=2.21) and more than tripled 3 years after completion (odds ratio=3.12).
Overall, more than 8000 children were enrolled in trials that were never completed, and more than 69,000 children were enrolled in completed trials that were never published.
“This is the first study to look systematically at discontinuation and nonpublication of interventional pediatric clinical trials,” Dr Bourgeois said.
“A number of legislative initiatives have been implemented to increase the study of interventions in children. Now, we need to make sure that the proper resources are in place to ensure that information gleaned from these studies reaches the scientific community.”
One proposed initiative cited by Drs Bourgeois and Pica is RIAT (Restoring Invisible and Abandoned Trials), which is supported by some high-profile journals. RIAT invites researchers with unpublished trials to either commit to publish within a year or provide public access to their data.
“It’s hard to reanalyze others’ data,” Dr Pica noted, “but this may be a useful mechanism to make sure that findings from completed trials are disseminated in the medical literature.” ![]()
Gene profile predicts RCC response to nivolumab
Many patients with advanced renal cell carcinoma have tumors that do not respond to immune checkpoint inhibitors targeted against the programmed death-1 (PD-1) pathway, despite expression of the target PD ligand 1 (PD-L1) on their tumors. Now investigators think they know why, and hope to use the information to predict which patients are likely to benefit and identify potential new therapies or combinations.
A study of renal cell carcinoma (RCC) samples from tumors with both good and poor clinical responses to treatment with the anti–PD-1 agent nivolumab (Opdivo) showed that a tumor gene–expression profile tipped more toward genes for controlling metabolic functions rather than immune functions was associated with a lack of response to anti-PD-1 therapy, reported Suzanne L. Topalian, MD, and her colleagues from Johns Hopkins University and the Sidney Kimmel Comprehensive Cancer Center, both in Baltimore.
“These findings suggest that tumor cell–intrinsic metabolic factors may contribute to treatment resistance in RCC, thus serving as predictive markers for treatment outcomes and potential new targets for combination therapy regimens with anti–PD-1,” they wrote in a study published online in Cancer Immunology Research.
The investigators obtained tumor samples from 13 patients with unresectable metastatic RCC treated in one of four clinical trials. They used radiographic staging to classify each patient as either a responder or nonresponder to anti–PD-1 therapy according to RECIST (Response Evaluation Criteria in Solid Tumors). The samples were evaluated with whole genome microarray and multiplex quantitative reverse-transcription polymerase chain reaction (qRT-PCR) profiling and analysis, and the results were compared with those from eight renal cell carcinoma cell lines.
They looked for expression of nearly 30,000 gene targets in samples from responders and nonresponders and found a pattern of differential expression of genes encoding for metabolic pathways and immune functions.
Specifically, they found that the expression of genes involved in metabolic and solute transport functions (for example, UGT1A) were associated with poor response to nivolumab, whereas overexpression of genes for immune markers involved in T-cell differentiation (BACH2) and leukocyte migration (CCL3) were associated with a good response.
The investigators acknowledge that the study was retrospective and limited by the analysis of only a small number of tumor samples but suggest that their findings point the way to further investigations in larger groups of patients with RCC tumors, including those both positive and negative for PD-L1 expression.
“The general approach to identifying biomarkers of clinical response to PD-1–targeted therapies has so far focused on immunologic factors in the [tumor microenvironment]. However, a deeper level of investigation may be warranted for individual tumor types, and intersections of tumor cell–intrinsic factors with immunologic factors may be particularly revealing,” they wrote.
The study was supported by research grants from the Bloomberg-Kimmel Institute for Cancer Immunotherapy at Johns Hopkins, Bristol-Myers Squibb, the National Cancer Institute, and Stand Up To Cancer. Dr. Topalian has served as a consultant/advisory board member for Five Prime Therapeutics, MedImmune, Merck, and Pfizer, and has an ownership interest in Bristol-Myers Squibb, Five Prime Therapeutics,and Potenza Therapeutics. Other coauthors reported similar potential conflicts of interest.
Many patients with advanced renal cell carcinoma have tumors that do not respond to immune checkpoint inhibitors targeted against the programmed death-1 (PD-1) pathway, despite expression of the target PD ligand 1 (PD-L1) on their tumors. Now investigators think they know why, and hope to use the information to predict which patients are likely to benefit and identify potential new therapies or combinations.
A study of renal cell carcinoma (RCC) samples from tumors with both good and poor clinical responses to treatment with the anti–PD-1 agent nivolumab (Opdivo) showed that a tumor gene–expression profile tipped more toward genes for controlling metabolic functions rather than immune functions was associated with a lack of response to anti-PD-1 therapy, reported Suzanne L. Topalian, MD, and her colleagues from Johns Hopkins University and the Sidney Kimmel Comprehensive Cancer Center, both in Baltimore.
“These findings suggest that tumor cell–intrinsic metabolic factors may contribute to treatment resistance in RCC, thus serving as predictive markers for treatment outcomes and potential new targets for combination therapy regimens with anti–PD-1,” they wrote in a study published online in Cancer Immunology Research.
The investigators obtained tumor samples from 13 patients with unresectable metastatic RCC treated in one of four clinical trials. They used radiographic staging to classify each patient as either a responder or nonresponder to anti–PD-1 therapy according to RECIST (Response Evaluation Criteria in Solid Tumors). The samples were evaluated with whole genome microarray and multiplex quantitative reverse-transcription polymerase chain reaction (qRT-PCR) profiling and analysis, and the results were compared with those from eight renal cell carcinoma cell lines.
They looked for expression of nearly 30,000 gene targets in samples from responders and nonresponders and found a pattern of differential expression of genes encoding for metabolic pathways and immune functions.
Specifically, they found that the expression of genes involved in metabolic and solute transport functions (for example, UGT1A) were associated with poor response to nivolumab, whereas overexpression of genes for immune markers involved in T-cell differentiation (BACH2) and leukocyte migration (CCL3) were associated with a good response.
The investigators acknowledge that the study was retrospective and limited by the analysis of only a small number of tumor samples but suggest that their findings point the way to further investigations in larger groups of patients with RCC tumors, including those both positive and negative for PD-L1 expression.
“The general approach to identifying biomarkers of clinical response to PD-1–targeted therapies has so far focused on immunologic factors in the [tumor microenvironment]. However, a deeper level of investigation may be warranted for individual tumor types, and intersections of tumor cell–intrinsic factors with immunologic factors may be particularly revealing,” they wrote.
The study was supported by research grants from the Bloomberg-Kimmel Institute for Cancer Immunotherapy at Johns Hopkins, Bristol-Myers Squibb, the National Cancer Institute, and Stand Up To Cancer. Dr. Topalian has served as a consultant/advisory board member for Five Prime Therapeutics, MedImmune, Merck, and Pfizer, and has an ownership interest in Bristol-Myers Squibb, Five Prime Therapeutics,and Potenza Therapeutics. Other coauthors reported similar potential conflicts of interest.
Many patients with advanced renal cell carcinoma have tumors that do not respond to immune checkpoint inhibitors targeted against the programmed death-1 (PD-1) pathway, despite expression of the target PD ligand 1 (PD-L1) on their tumors. Now investigators think they know why, and hope to use the information to predict which patients are likely to benefit and identify potential new therapies or combinations.
A study of renal cell carcinoma (RCC) samples from tumors with both good and poor clinical responses to treatment with the anti–PD-1 agent nivolumab (Opdivo) showed that a tumor gene–expression profile tipped more toward genes for controlling metabolic functions rather than immune functions was associated with a lack of response to anti-PD-1 therapy, reported Suzanne L. Topalian, MD, and her colleagues from Johns Hopkins University and the Sidney Kimmel Comprehensive Cancer Center, both in Baltimore.
“These findings suggest that tumor cell–intrinsic metabolic factors may contribute to treatment resistance in RCC, thus serving as predictive markers for treatment outcomes and potential new targets for combination therapy regimens with anti–PD-1,” they wrote in a study published online in Cancer Immunology Research.
The investigators obtained tumor samples from 13 patients with unresectable metastatic RCC treated in one of four clinical trials. They used radiographic staging to classify each patient as either a responder or nonresponder to anti–PD-1 therapy according to RECIST (Response Evaluation Criteria in Solid Tumors). The samples were evaluated with whole genome microarray and multiplex quantitative reverse-transcription polymerase chain reaction (qRT-PCR) profiling and analysis, and the results were compared with those from eight renal cell carcinoma cell lines.
They looked for expression of nearly 30,000 gene targets in samples from responders and nonresponders and found a pattern of differential expression of genes encoding for metabolic pathways and immune functions.
Specifically, they found that the expression of genes involved in metabolic and solute transport functions (for example, UGT1A) were associated with poor response to nivolumab, whereas overexpression of genes for immune markers involved in T-cell differentiation (BACH2) and leukocyte migration (CCL3) were associated with a good response.
The investigators acknowledge that the study was retrospective and limited by the analysis of only a small number of tumor samples but suggest that their findings point the way to further investigations in larger groups of patients with RCC tumors, including those both positive and negative for PD-L1 expression.
“The general approach to identifying biomarkers of clinical response to PD-1–targeted therapies has so far focused on immunologic factors in the [tumor microenvironment]. However, a deeper level of investigation may be warranted for individual tumor types, and intersections of tumor cell–intrinsic factors with immunologic factors may be particularly revealing,” they wrote.
The study was supported by research grants from the Bloomberg-Kimmel Institute for Cancer Immunotherapy at Johns Hopkins, Bristol-Myers Squibb, the National Cancer Institute, and Stand Up To Cancer. Dr. Topalian has served as a consultant/advisory board member for Five Prime Therapeutics, MedImmune, Merck, and Pfizer, and has an ownership interest in Bristol-Myers Squibb, Five Prime Therapeutics,and Potenza Therapeutics. Other coauthors reported similar potential conflicts of interest.
FROM CANCER IMMUNOLOGY RESEARCH
Key clinical point: Many renal cell carcinoma tumors do not respond to therapy with an anti-PD-1 agent, despite being positive for the PD-L1 target.
Major finding: A gene expression profile favoring genes associated with metabolic and solute transport functions was associated with poor response to nivolumab.
Data source: Retrospective study of tumor gene expression in 13 patients with advanced RCC and 8 RCC cell lines.
Disclosures: The study was supported by research grants from the Bloomberg-Kimmel Institute for Cancer Immunotherapy at Johns Hopkins, Baltimore; Bristol-Myers Squibb; the National Cancer Institute; and Stand Up To Cancer. Dr. Topalian has served as a consultant/advisory board member for Five Prime Therapeutics, MedImmune, Merck, and Pfizer, and has an ownership interest in Bristol-Myers Squibb, Five Prime Therapeutics,and Potenza Therapeutics. Other coauthors reported similar potential conflicts of interest.
Psychotropic drug use similar in cosmetic and medical dermatology patients
The use of psychotropic medications was similar between patients seeking cosmetic dermatology treatment and those presenting with medical dermatologic conditions in a study published in the Journal of Drugs in Dermatology.
A retrospective chart review of 154 adult female patients presenting for cosmetic dermatology and 156 presenting for medical reasons to a suburban dermatology private practice found that 26.8% of the cosmetic group and 22.2% of the medical group reported taking psychotropic medications (P = .09).
The most common medication type was a selective serotonin reuptake inhibitor antidepressant, reported Dr. Heather K. Hamilton, a dermatologist in Chestnut Hill, Mass., at the time of the study, and her associates. An SSRI was used by 23 patients (56.1%) in the cosmetic group and 25 (71.4%) of the general dermatology patients, followed by benzodiazepines, tricyclic antidepressants, and attention-deficit/hyperactivity disorder medications (J Drugs Dermatol. 2016; 15 9[7]:858-61).
The study also found no significant difference between the two groups in self-reported record of a psychiatric disorder, with four such cases in the medical group and six in the cosmetic group (P = .139).
Cosmetic patients presenting for appearance-related dermatologic therapy are often perceived as being more difficult to satisfy than those with dermatologic problems, the authors noted. “While the reasons for this perception are many, some have hypothesized that this may be related to a higher rate of anxiety, mild depression, or body image issues among this patient population,” they wrote.
The authors referred to the high proportion of patients in both groups taking psychotropic medications in the study, a finding that was “even more striking” since the study excluded patients with conditions known to be associated with psychopathology, such as vitiligo, psoriasis, and neurotic excoriations.
“This finding serves as a reminder that we should take full medical histories as mental health may play a role in compliance and satisfaction with treatment,” the authors stated.
They acknowledged that there were limitations of the study, including using psychotropic medication as a marker for mental health problems and the use of self-reported data.
No conflicts of interest were declared. Dr. Hamilton now practices outside of New York.
The use of psychotropic medications was similar between patients seeking cosmetic dermatology treatment and those presenting with medical dermatologic conditions in a study published in the Journal of Drugs in Dermatology.
A retrospective chart review of 154 adult female patients presenting for cosmetic dermatology and 156 presenting for medical reasons to a suburban dermatology private practice found that 26.8% of the cosmetic group and 22.2% of the medical group reported taking psychotropic medications (P = .09).
The most common medication type was a selective serotonin reuptake inhibitor antidepressant, reported Dr. Heather K. Hamilton, a dermatologist in Chestnut Hill, Mass., at the time of the study, and her associates. An SSRI was used by 23 patients (56.1%) in the cosmetic group and 25 (71.4%) of the general dermatology patients, followed by benzodiazepines, tricyclic antidepressants, and attention-deficit/hyperactivity disorder medications (J Drugs Dermatol. 2016; 15 9[7]:858-61).
The study also found no significant difference between the two groups in self-reported record of a psychiatric disorder, with four such cases in the medical group and six in the cosmetic group (P = .139).
Cosmetic patients presenting for appearance-related dermatologic therapy are often perceived as being more difficult to satisfy than those with dermatologic problems, the authors noted. “While the reasons for this perception are many, some have hypothesized that this may be related to a higher rate of anxiety, mild depression, or body image issues among this patient population,” they wrote.
The authors referred to the high proportion of patients in both groups taking psychotropic medications in the study, a finding that was “even more striking” since the study excluded patients with conditions known to be associated with psychopathology, such as vitiligo, psoriasis, and neurotic excoriations.
“This finding serves as a reminder that we should take full medical histories as mental health may play a role in compliance and satisfaction with treatment,” the authors stated.
They acknowledged that there were limitations of the study, including using psychotropic medication as a marker for mental health problems and the use of self-reported data.
No conflicts of interest were declared. Dr. Hamilton now practices outside of New York.
The use of psychotropic medications was similar between patients seeking cosmetic dermatology treatment and those presenting with medical dermatologic conditions in a study published in the Journal of Drugs in Dermatology.
A retrospective chart review of 154 adult female patients presenting for cosmetic dermatology and 156 presenting for medical reasons to a suburban dermatology private practice found that 26.8% of the cosmetic group and 22.2% of the medical group reported taking psychotropic medications (P = .09).
The most common medication type was a selective serotonin reuptake inhibitor antidepressant, reported Dr. Heather K. Hamilton, a dermatologist in Chestnut Hill, Mass., at the time of the study, and her associates. An SSRI was used by 23 patients (56.1%) in the cosmetic group and 25 (71.4%) of the general dermatology patients, followed by benzodiazepines, tricyclic antidepressants, and attention-deficit/hyperactivity disorder medications (J Drugs Dermatol. 2016; 15 9[7]:858-61).
The study also found no significant difference between the two groups in self-reported record of a psychiatric disorder, with four such cases in the medical group and six in the cosmetic group (P = .139).
Cosmetic patients presenting for appearance-related dermatologic therapy are often perceived as being more difficult to satisfy than those with dermatologic problems, the authors noted. “While the reasons for this perception are many, some have hypothesized that this may be related to a higher rate of anxiety, mild depression, or body image issues among this patient population,” they wrote.
The authors referred to the high proportion of patients in both groups taking psychotropic medications in the study, a finding that was “even more striking” since the study excluded patients with conditions known to be associated with psychopathology, such as vitiligo, psoriasis, and neurotic excoriations.
“This finding serves as a reminder that we should take full medical histories as mental health may play a role in compliance and satisfaction with treatment,” the authors stated.
They acknowledged that there were limitations of the study, including using psychotropic medication as a marker for mental health problems and the use of self-reported data.
No conflicts of interest were declared. Dr. Hamilton now practices outside of New York.
FROM JOURNAL OF DRUGS IN DERMATOLOGY
Key clinical point: The use of psychotropic medications is high among cosmetic and medical dermatology patients.
Major finding: About one-quarter of patients presenting for cosmetic or medical dermatology consultations reported taking psychotropic medications.
Data source: A retrospective chart review of 154 adult female patients presenting for cosmetic dermatology and 156 presenting for medical reasons at a suburban comprehensive dermatology private practice.
Disclosures: No conflicts of interest were declared.
Better innate immunity in Amish vs. Hutterite children: Different allergen exposure?
Although related by their European origins in neighboring countries and similar cultural lifestyles, the immune profiles and prevalences of asthma and allergic sensitization differ between Amish and Hutterite school children, according to the results of a study published in the New England Journal of Medicine.
The predominant differences between the Amish and Hutterite populations today are region of concentration (Indiana and South Dakota, respectively) and agrarian style (traditional and industrialized farming, respectively). First author Michelle M. Stein of the University of Chicago and her colleagues conducted a study of 30 Amish age- and sex-matched children (aged 7-14 years) living in Indiana and 30 Hutterite children living in South Dakota during the months of November and December, respectively. Immune profile–related variables examined included levels of common allergens and endotoxins detectable in airborne dust, percentages of total peripheral blood leukocytes, cell-surface markers on neutrophils and monocytes, cytokine levels from peripheral-blood leukocytes with or without innate or adaptive stimuli, and gene expression levels from peripheral-blood leukocytes. In additional experiments using a murine model of experimental allergic asthma, house dust from Amish and Hutterite homes was administered intranasally to mice for 4-5 weeks to gauge their immune responses (N Engl J Med. 2016;375:411-21).
A genetic analysis revealed that the two populations shared a closer genetic relationship with each other than with populations from other countries in Europe. The study results also confirmed the known differences between the Amish and Hutterite prevalences of asthma (0% vs. 20%, respectively) and positivity for allergen-specific IgE (17% vs. 30% and 7% vs. 30% using cutoffs of greater than 0.7 and greater than 3.5 kUA/L, respectively). Also, median levels of endotoxins were found to be almost sevenfold greater in Amish homes (4,399 endotoxin units [EU] per square meter vs. 648 EU per square meter, P less than .001).
Compared with results generated using Hutterite peripheral-blood leukocytes, increased proportions of neutrophils, decreased proportions of eosinophils, and similar proportions of monocytes were observed in the Amish. In addition to proportional differences, the phenotypes of the neutrophils from the Amish children also differed by expressing lower levels of the chemokine receptor CXCR4 and the adhesion molecules CD11b and CD11c. The phenotypes of the monocytes differed as well, showing a suppressive phenotype in the Amish children. A specific network of differentially expressed innate immune genes in untreated peripheral-blood leukocytes was found to be associated with the observed differences in the relative amounts of neutrophils, eosinophils, and monocytes, as well as their phenotypes. The median levels of all 23 cytokines detected in peripheral-blood leukocytes following innate stimulation differed between the populations and showed lower levels among the Amish children (P less than .001). Adaptive stimulation, however, did not lead to differences in cytokine levels between the two groups. The results from the murine model indicated that exposure to the Amish conditions was associated with innate immune responses consistent with protective effects against asthma. Collectively, these results strongly suggest that the Amish environment affords protection against asthma via innate immune system signaling.
“A deeper understanding of the relevant stimuli and the innate immune pathways they engage may ultimately pave the way for the development of effective strategies for the prevention of asthma,” wrote Ms. Stein and her colleagues.
Funding for this project was provided by the National Institutes of Health, St. Vincent Foundation, and the American Academy of Allergy, Asthma, and Immunology Foundation. Several study authors disclosed grant support from the NIH; one received support from the European Research Council and the German Research Foundation; and several disclosed ties to industry sources.
Although related by their European origins in neighboring countries and similar cultural lifestyles, the immune profiles and prevalences of asthma and allergic sensitization differ between Amish and Hutterite school children, according to the results of a study published in the New England Journal of Medicine.
The predominant differences between the Amish and Hutterite populations today are region of concentration (Indiana and South Dakota, respectively) and agrarian style (traditional and industrialized farming, respectively). First author Michelle M. Stein of the University of Chicago and her colleagues conducted a study of 30 Amish age- and sex-matched children (aged 7-14 years) living in Indiana and 30 Hutterite children living in South Dakota during the months of November and December, respectively. Immune profile–related variables examined included levels of common allergens and endotoxins detectable in airborne dust, percentages of total peripheral blood leukocytes, cell-surface markers on neutrophils and monocytes, cytokine levels from peripheral-blood leukocytes with or without innate or adaptive stimuli, and gene expression levels from peripheral-blood leukocytes. In additional experiments using a murine model of experimental allergic asthma, house dust from Amish and Hutterite homes was administered intranasally to mice for 4-5 weeks to gauge their immune responses (N Engl J Med. 2016;375:411-21).
A genetic analysis revealed that the two populations shared a closer genetic relationship with each other than with populations from other countries in Europe. The study results also confirmed the known differences between the Amish and Hutterite prevalences of asthma (0% vs. 20%, respectively) and positivity for allergen-specific IgE (17% vs. 30% and 7% vs. 30% using cutoffs of greater than 0.7 and greater than 3.5 kUA/L, respectively). Also, median levels of endotoxins were found to be almost sevenfold greater in Amish homes (4,399 endotoxin units [EU] per square meter vs. 648 EU per square meter, P less than .001).
Compared with results generated using Hutterite peripheral-blood leukocytes, increased proportions of neutrophils, decreased proportions of eosinophils, and similar proportions of monocytes were observed in the Amish. In addition to proportional differences, the phenotypes of the neutrophils from the Amish children also differed by expressing lower levels of the chemokine receptor CXCR4 and the adhesion molecules CD11b and CD11c. The phenotypes of the monocytes differed as well, showing a suppressive phenotype in the Amish children. A specific network of differentially expressed innate immune genes in untreated peripheral-blood leukocytes was found to be associated with the observed differences in the relative amounts of neutrophils, eosinophils, and monocytes, as well as their phenotypes. The median levels of all 23 cytokines detected in peripheral-blood leukocytes following innate stimulation differed between the populations and showed lower levels among the Amish children (P less than .001). Adaptive stimulation, however, did not lead to differences in cytokine levels between the two groups. The results from the murine model indicated that exposure to the Amish conditions was associated with innate immune responses consistent with protective effects against asthma. Collectively, these results strongly suggest that the Amish environment affords protection against asthma via innate immune system signaling.
“A deeper understanding of the relevant stimuli and the innate immune pathways they engage may ultimately pave the way for the development of effective strategies for the prevention of asthma,” wrote Ms. Stein and her colleagues.
Funding for this project was provided by the National Institutes of Health, St. Vincent Foundation, and the American Academy of Allergy, Asthma, and Immunology Foundation. Several study authors disclosed grant support from the NIH; one received support from the European Research Council and the German Research Foundation; and several disclosed ties to industry sources.
Although related by their European origins in neighboring countries and similar cultural lifestyles, the immune profiles and prevalences of asthma and allergic sensitization differ between Amish and Hutterite school children, according to the results of a study published in the New England Journal of Medicine.
The predominant differences between the Amish and Hutterite populations today are region of concentration (Indiana and South Dakota, respectively) and agrarian style (traditional and industrialized farming, respectively). First author Michelle M. Stein of the University of Chicago and her colleagues conducted a study of 30 Amish age- and sex-matched children (aged 7-14 years) living in Indiana and 30 Hutterite children living in South Dakota during the months of November and December, respectively. Immune profile–related variables examined included levels of common allergens and endotoxins detectable in airborne dust, percentages of total peripheral blood leukocytes, cell-surface markers on neutrophils and monocytes, cytokine levels from peripheral-blood leukocytes with or without innate or adaptive stimuli, and gene expression levels from peripheral-blood leukocytes. In additional experiments using a murine model of experimental allergic asthma, house dust from Amish and Hutterite homes was administered intranasally to mice for 4-5 weeks to gauge their immune responses (N Engl J Med. 2016;375:411-21).
A genetic analysis revealed that the two populations shared a closer genetic relationship with each other than with populations from other countries in Europe. The study results also confirmed the known differences between the Amish and Hutterite prevalences of asthma (0% vs. 20%, respectively) and positivity for allergen-specific IgE (17% vs. 30% and 7% vs. 30% using cutoffs of greater than 0.7 and greater than 3.5 kUA/L, respectively). Also, median levels of endotoxins were found to be almost sevenfold greater in Amish homes (4,399 endotoxin units [EU] per square meter vs. 648 EU per square meter, P less than .001).
Compared with results generated using Hutterite peripheral-blood leukocytes, increased proportions of neutrophils, decreased proportions of eosinophils, and similar proportions of monocytes were observed in the Amish. In addition to proportional differences, the phenotypes of the neutrophils from the Amish children also differed by expressing lower levels of the chemokine receptor CXCR4 and the adhesion molecules CD11b and CD11c. The phenotypes of the monocytes differed as well, showing a suppressive phenotype in the Amish children. A specific network of differentially expressed innate immune genes in untreated peripheral-blood leukocytes was found to be associated with the observed differences in the relative amounts of neutrophils, eosinophils, and monocytes, as well as their phenotypes. The median levels of all 23 cytokines detected in peripheral-blood leukocytes following innate stimulation differed between the populations and showed lower levels among the Amish children (P less than .001). Adaptive stimulation, however, did not lead to differences in cytokine levels between the two groups. The results from the murine model indicated that exposure to the Amish conditions was associated with innate immune responses consistent with protective effects against asthma. Collectively, these results strongly suggest that the Amish environment affords protection against asthma via innate immune system signaling.
“A deeper understanding of the relevant stimuli and the innate immune pathways they engage may ultimately pave the way for the development of effective strategies for the prevention of asthma,” wrote Ms. Stein and her colleagues.
Funding for this project was provided by the National Institutes of Health, St. Vincent Foundation, and the American Academy of Allergy, Asthma, and Immunology Foundation. Several study authors disclosed grant support from the NIH; one received support from the European Research Council and the German Research Foundation; and several disclosed ties to industry sources.
Key clinical point: The innate immune responses and prevalences of asthma and allergic sensitization differ between populations of school children with similar regional and genetic origins and cultures.
Major finding: The two populations were highly genetically similar, but expressed a variety of differences in their immune profiles suggestive of enhanced immunity in the Amish, possibly attributable to differential allergen exposure.
Data sources: Sixty children (30 Amish, 30 Hutterite) were matched for age and sex. A murine model of experimental allergic asthma also was employed.
Disclosures: Funding for this project was provided by the National Institutes of Health, St. Vincent Foundation, and the American Academy of Allergy, Asthma, and Immunology Foundation. Several study authors disclosed grant support from the NIH; one received support from the European Research Council and the German Research Foundation; and several disclosed ties to industry sources.
Sickle cell trait raises exertional rhabdomyolysis risk
Sickle cell trait doesn’t raise the risk of death but does raise the risk of exertional rhabdomyolysis among black soldiers, “a population that is known to engage consistently in regular and strenuous exercise while protected by exertional-injury precautions,” investigators reported. The study was published online Aug. 4 in the New England Journal of Medicine.
A number of high-profile deaths among athletes and military personnel involving exertional rhabdomyolysis have been attributed to sickle cell trait. The National Collegiate Athletic Association, the U.S. Air Force, and the U.S. Navy all require universal screening for sickle cell trait.
“However, concerns have been raised by the American Society of Hematology and other professional organizations about the possibility of stigmatization and discrimination resulting from the mandated screening for sickle cell trait,” which predominantly affects people of African descent and some of Hispanic ancestry, but usually not whites, wrote D. Alan Nelson, PhD, of Stanford (Calif.) University, and his associates.
“These concerns warrant consideration, especially given the absence of published evidence that such screening is effective in preventing exertion-related events,” added the researchers.
The U.S. Army primarily screens for sickle cell trait among personnel deployed for combat and certain specialists whose work at high altitudes puts them at risk. To study a large enough population to generate accurate data about this rare blood abnormality, the investigators analyzed data for 47,944 black soldiers who had been tested for sickle cell trait and who served on active duty during a recent 3-year period.
The researchers assessed all inpatient and outpatient health care visits at military and civilian facilities. There were 391 exertional rhabdomyolysis events and 96 deaths from all causes during 1.61 million person-months of observation. A total of 7.4% of the study cohort carried sickle cell trait. These participants were similar to noncarriers in demographic characteristics and health predictors.
Soldiers with sickle cell trait did not have a greater risk of death than did those without sickle cell trait (hazard ratio, 0.99), and there also was no difference between carriers and noncarriers in either battle-related or non–battle-related mortality rates.
However, sickle cell trait was associated with a 54% greater risk of exertional rhabdomyolysis (HR, 1.54), Dr. Nelson and his associates noted (N Engl J Med. 2016 Aug 4. doi: 10.1056/NEJMoa1516257).
It is important to note that factors other than sickle cell trait raised the risk of exertional rhabdomyolysis to a similar or even greater degree, including obesity (HR, 1.39) and tobacco use (HR, 1.54). Recent use of antipsychotic medication tripled the risk for exertional rhabdomyolysis (HR, 3.02), and statin use nearly tripled it (HR, 2.89).
“These findings are compelling because case reports dominate the relevant literature and emphasize the presence of sickle cell trait as a risk factor for adverse outcomes, including exertional rhabdomyolysis and sudden death,” the researchers wrote. “A large longitudinal study involving a population fully tested for sickle cell trait that has formally investigated [these outcomes] while also examining other known major risk factors, such as medications, has been lacking.”
The study found that women were at much lower risk for exertional rhabdomyolysis than were men (HR, 0.51), and that risk increased with increasing age, so that soldiers aged 36 years and older had a 57% greater risk compared with those in the youngest age group.
The National Heart, Lung, and Blood Institute and the Uniformed Services University of the Health Sciences supported the study. Dr. Nelson and his associates reported having no relevant financial disclosures.
Sickle cell trait doesn’t raise the risk of death but does raise the risk of exertional rhabdomyolysis among black soldiers, “a population that is known to engage consistently in regular and strenuous exercise while protected by exertional-injury precautions,” investigators reported. The study was published online Aug. 4 in the New England Journal of Medicine.
A number of high-profile deaths among athletes and military personnel involving exertional rhabdomyolysis have been attributed to sickle cell trait. The National Collegiate Athletic Association, the U.S. Air Force, and the U.S. Navy all require universal screening for sickle cell trait.
“However, concerns have been raised by the American Society of Hematology and other professional organizations about the possibility of stigmatization and discrimination resulting from the mandated screening for sickle cell trait,” which predominantly affects people of African descent and some of Hispanic ancestry, but usually not whites, wrote D. Alan Nelson, PhD, of Stanford (Calif.) University, and his associates.
“These concerns warrant consideration, especially given the absence of published evidence that such screening is effective in preventing exertion-related events,” added the researchers.
The U.S. Army primarily screens for sickle cell trait among personnel deployed for combat and certain specialists whose work at high altitudes puts them at risk. To study a large enough population to generate accurate data about this rare blood abnormality, the investigators analyzed data for 47,944 black soldiers who had been tested for sickle cell trait and who served on active duty during a recent 3-year period.
The researchers assessed all inpatient and outpatient health care visits at military and civilian facilities. There were 391 exertional rhabdomyolysis events and 96 deaths from all causes during 1.61 million person-months of observation. A total of 7.4% of the study cohort carried sickle cell trait. These participants were similar to noncarriers in demographic characteristics and health predictors.
Soldiers with sickle cell trait did not have a greater risk of death than did those without sickle cell trait (hazard ratio, 0.99), and there also was no difference between carriers and noncarriers in either battle-related or non–battle-related mortality rates.
However, sickle cell trait was associated with a 54% greater risk of exertional rhabdomyolysis (HR, 1.54), Dr. Nelson and his associates noted (N Engl J Med. 2016 Aug 4. doi: 10.1056/NEJMoa1516257).
It is important to note that factors other than sickle cell trait raised the risk of exertional rhabdomyolysis to a similar or even greater degree, including obesity (HR, 1.39) and tobacco use (HR, 1.54). Recent use of antipsychotic medication tripled the risk for exertional rhabdomyolysis (HR, 3.02), and statin use nearly tripled it (HR, 2.89).
“These findings are compelling because case reports dominate the relevant literature and emphasize the presence of sickle cell trait as a risk factor for adverse outcomes, including exertional rhabdomyolysis and sudden death,” the researchers wrote. “A large longitudinal study involving a population fully tested for sickle cell trait that has formally investigated [these outcomes] while also examining other known major risk factors, such as medications, has been lacking.”
The study found that women were at much lower risk for exertional rhabdomyolysis than were men (HR, 0.51), and that risk increased with increasing age, so that soldiers aged 36 years and older had a 57% greater risk compared with those in the youngest age group.
The National Heart, Lung, and Blood Institute and the Uniformed Services University of the Health Sciences supported the study. Dr. Nelson and his associates reported having no relevant financial disclosures.
Sickle cell trait doesn’t raise the risk of death but does raise the risk of exertional rhabdomyolysis among black soldiers, “a population that is known to engage consistently in regular and strenuous exercise while protected by exertional-injury precautions,” investigators reported. The study was published online Aug. 4 in the New England Journal of Medicine.
A number of high-profile deaths among athletes and military personnel involving exertional rhabdomyolysis have been attributed to sickle cell trait. The National Collegiate Athletic Association, the U.S. Air Force, and the U.S. Navy all require universal screening for sickle cell trait.
“However, concerns have been raised by the American Society of Hematology and other professional organizations about the possibility of stigmatization and discrimination resulting from the mandated screening for sickle cell trait,” which predominantly affects people of African descent and some of Hispanic ancestry, but usually not whites, wrote D. Alan Nelson, PhD, of Stanford (Calif.) University, and his associates.
“These concerns warrant consideration, especially given the absence of published evidence that such screening is effective in preventing exertion-related events,” added the researchers.
The U.S. Army primarily screens for sickle cell trait among personnel deployed for combat and certain specialists whose work at high altitudes puts them at risk. To study a large enough population to generate accurate data about this rare blood abnormality, the investigators analyzed data for 47,944 black soldiers who had been tested for sickle cell trait and who served on active duty during a recent 3-year period.
The researchers assessed all inpatient and outpatient health care visits at military and civilian facilities. There were 391 exertional rhabdomyolysis events and 96 deaths from all causes during 1.61 million person-months of observation. A total of 7.4% of the study cohort carried sickle cell trait. These participants were similar to noncarriers in demographic characteristics and health predictors.
Soldiers with sickle cell trait did not have a greater risk of death than did those without sickle cell trait (hazard ratio, 0.99), and there also was no difference between carriers and noncarriers in either battle-related or non–battle-related mortality rates.
However, sickle cell trait was associated with a 54% greater risk of exertional rhabdomyolysis (HR, 1.54), Dr. Nelson and his associates noted (N Engl J Med. 2016 Aug 4. doi: 10.1056/NEJMoa1516257).
It is important to note that factors other than sickle cell trait raised the risk of exertional rhabdomyolysis to a similar or even greater degree, including obesity (HR, 1.39) and tobacco use (HR, 1.54). Recent use of antipsychotic medication tripled the risk for exertional rhabdomyolysis (HR, 3.02), and statin use nearly tripled it (HR, 2.89).
“These findings are compelling because case reports dominate the relevant literature and emphasize the presence of sickle cell trait as a risk factor for adverse outcomes, including exertional rhabdomyolysis and sudden death,” the researchers wrote. “A large longitudinal study involving a population fully tested for sickle cell trait that has formally investigated [these outcomes] while also examining other known major risk factors, such as medications, has been lacking.”
The study found that women were at much lower risk for exertional rhabdomyolysis than were men (HR, 0.51), and that risk increased with increasing age, so that soldiers aged 36 years and older had a 57% greater risk compared with those in the youngest age group.
The National Heart, Lung, and Blood Institute and the Uniformed Services University of the Health Sciences supported the study. Dr. Nelson and his associates reported having no relevant financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Sickle cell trait doesn’t raise the risk of death but does raise the risk of exertional rhabdomyolysis among black soldiers.
Major finding: Soldiers with sickle cell trait did not have a greater risk of death than did those without sickle cell trait (hazard ratio, 0.99) but did have a higher risk of exertional rhabdomyolysis (HR, 1.54).
Data source: A retrospective longitudinal cohort study involving 47,944 black U.S. soldiers on active duty during 2011-2014.
Disclosures: The National Heart, Lung, and Blood Institute and the Uniformed Services University of the Health Sciences supported the study. Dr. Nelson and his associates reported having no relevant financial disclosures.
MCR-1 gene a growing concern for antibiotic resistance
The MCR-1 gene is quickly emerging as a powerful roadblock in the fight against antibiotic-resistant bacteria, according to experts from the Centers for Disease Control and Prevention.
Alex J. Kallen, MD, of the CDC in Atlanta, said in an Aug. 2 webinar – cohosted by CDC and the Partnership for Quality Care – that the CDC is closely monitoring the emergence of antibiotic resistant bacteria in the United States. The prevailing message of Dr. Kallen and his CDC colleague Arjun Srinivasan, MD was that the importance of the MCR-1 gene should not be underestimated.
First discovered in a human patient last year, the presence of the MCR-1 gene makes bacteria resistant to colistin, an antibiotic used often as a last resort to “treat patients with multidrug-resistant infections,” according to the CDC. Because the MCR-1 gene exists on a plasmid, or a small piece of DNA, it is easily transferable among bacteria, making it a problem for health care providers treating a patient infected with bacteria that has the gene.
“As of this year, all state and some local health departments will be funded to respond to [antibiotic resistance] threats, including emerging threats like [MCR-1], within their jurisdiction,” Dr. Kallen said. This funding could be used to support technical assistance, contact investigations, and laboratory testing, among other things.
In order to help prevent the spread of MCR-1 and mitigate cases of novel antimicrobial resistance, health care providers and facilities should institute recommended intensive care precautions as soon as resistance is identified, along with alerting their local public health office. Isolates should be saved, and prospective and retrospective surveillance should be implemented to “identify isolates with similar phenotypes.” If an infected patient is being transferred to another facility, that facility should be notified ahead of time about the patient and what protocols to follow.
Because antibiotic-resistant organisms do not spread through the air like influenza, the risk that these organisms pose to health care workers is relatively low, Dr. Srinivasan explained. However, he stressed that a multifaceted, team-based approach to antibiotic stewardship and decontamination of health care facilities is absolutely necessary to achieve the best results for both patients and staff.
“We are now beginning to recognize that the contamination of surfaces and items in our health care environment is increasingly a problem in the transmission of these drug-resistant organisms,” Dr. Srinivasan explained. He said that, given the complexity of a typical health care environment, such as a hospital room or operating theater, it’s perhaps not so surprising that keeping everything clean is not the top priority.
In addition to making sure hospital rooms and other areas are properly cleaned, simple things like washing hands and keeping surfaces clean are just as important. Dr. Srinivasan pointed to a 2006 study by Philip C. Carling, MD, and his associates which showed that educational interventions can lead to substantial increases in hygiene and cleanliness, and that training of new staff is also important. Furthermore, giving staff enough time to properly clean rooms could significantly contribute to curtailing MCR-1 bacteria from spreading, he said.
“The other thing to keep in mind is that if we lose effective therapy, if we lose antibiotic therapy, the infections that are currently very treatable and are seldom deadly could again become very, very serious threats to life,” Dr. Srinivasan warned, specifically citing Escherichia coli, which is the leading cause of urinary tract infections. If community strains of E.coli become resistant to typical antibiotic treatment, these cases could become “difficult, if not impossible to treat.”
According to data shared by Dr. Srinivasan, in 2013 there were 2,049,422 illnesses in the United States attributed to antibiotic resistance and 23,000 fatalities.
AGA resource
The AGA Center for Gut Microbiome Research and Education was created to serve as a virtual “home” for AGA activities related to the gut microbiome. The center is focused on advancing gut microbiome research, educating AGA members and other stakeholders on the latest microbiome breakthroughs, and working with FDA and others to ensure that emerging microbiome-based treatments are safe and appropriately evaluated. Learn more here.
The MCR-1 gene is quickly emerging as a powerful roadblock in the fight against antibiotic-resistant bacteria, according to experts from the Centers for Disease Control and Prevention.
Alex J. Kallen, MD, of the CDC in Atlanta, said in an Aug. 2 webinar – cohosted by CDC and the Partnership for Quality Care – that the CDC is closely monitoring the emergence of antibiotic resistant bacteria in the United States. The prevailing message of Dr. Kallen and his CDC colleague Arjun Srinivasan, MD was that the importance of the MCR-1 gene should not be underestimated.
First discovered in a human patient last year, the presence of the MCR-1 gene makes bacteria resistant to colistin, an antibiotic used often as a last resort to “treat patients with multidrug-resistant infections,” according to the CDC. Because the MCR-1 gene exists on a plasmid, or a small piece of DNA, it is easily transferable among bacteria, making it a problem for health care providers treating a patient infected with bacteria that has the gene.
“As of this year, all state and some local health departments will be funded to respond to [antibiotic resistance] threats, including emerging threats like [MCR-1], within their jurisdiction,” Dr. Kallen said. This funding could be used to support technical assistance, contact investigations, and laboratory testing, among other things.
In order to help prevent the spread of MCR-1 and mitigate cases of novel antimicrobial resistance, health care providers and facilities should institute recommended intensive care precautions as soon as resistance is identified, along with alerting their local public health office. Isolates should be saved, and prospective and retrospective surveillance should be implemented to “identify isolates with similar phenotypes.” If an infected patient is being transferred to another facility, that facility should be notified ahead of time about the patient and what protocols to follow.
Because antibiotic-resistant organisms do not spread through the air like influenza, the risk that these organisms pose to health care workers is relatively low, Dr. Srinivasan explained. However, he stressed that a multifaceted, team-based approach to antibiotic stewardship and decontamination of health care facilities is absolutely necessary to achieve the best results for both patients and staff.
“We are now beginning to recognize that the contamination of surfaces and items in our health care environment is increasingly a problem in the transmission of these drug-resistant organisms,” Dr. Srinivasan explained. He said that, given the complexity of a typical health care environment, such as a hospital room or operating theater, it’s perhaps not so surprising that keeping everything clean is not the top priority.
In addition to making sure hospital rooms and other areas are properly cleaned, simple things like washing hands and keeping surfaces clean are just as important. Dr. Srinivasan pointed to a 2006 study by Philip C. Carling, MD, and his associates which showed that educational interventions can lead to substantial increases in hygiene and cleanliness, and that training of new staff is also important. Furthermore, giving staff enough time to properly clean rooms could significantly contribute to curtailing MCR-1 bacteria from spreading, he said.
“The other thing to keep in mind is that if we lose effective therapy, if we lose antibiotic therapy, the infections that are currently very treatable and are seldom deadly could again become very, very serious threats to life,” Dr. Srinivasan warned, specifically citing Escherichia coli, which is the leading cause of urinary tract infections. If community strains of E.coli become resistant to typical antibiotic treatment, these cases could become “difficult, if not impossible to treat.”
According to data shared by Dr. Srinivasan, in 2013 there were 2,049,422 illnesses in the United States attributed to antibiotic resistance and 23,000 fatalities.
AGA resource
The AGA Center for Gut Microbiome Research and Education was created to serve as a virtual “home” for AGA activities related to the gut microbiome. The center is focused on advancing gut microbiome research, educating AGA members and other stakeholders on the latest microbiome breakthroughs, and working with FDA and others to ensure that emerging microbiome-based treatments are safe and appropriately evaluated. Learn more here.
The MCR-1 gene is quickly emerging as a powerful roadblock in the fight against antibiotic-resistant bacteria, according to experts from the Centers for Disease Control and Prevention.
Alex J. Kallen, MD, of the CDC in Atlanta, said in an Aug. 2 webinar – cohosted by CDC and the Partnership for Quality Care – that the CDC is closely monitoring the emergence of antibiotic resistant bacteria in the United States. The prevailing message of Dr. Kallen and his CDC colleague Arjun Srinivasan, MD was that the importance of the MCR-1 gene should not be underestimated.
First discovered in a human patient last year, the presence of the MCR-1 gene makes bacteria resistant to colistin, an antibiotic used often as a last resort to “treat patients with multidrug-resistant infections,” according to the CDC. Because the MCR-1 gene exists on a plasmid, or a small piece of DNA, it is easily transferable among bacteria, making it a problem for health care providers treating a patient infected with bacteria that has the gene.
“As of this year, all state and some local health departments will be funded to respond to [antibiotic resistance] threats, including emerging threats like [MCR-1], within their jurisdiction,” Dr. Kallen said. This funding could be used to support technical assistance, contact investigations, and laboratory testing, among other things.
In order to help prevent the spread of MCR-1 and mitigate cases of novel antimicrobial resistance, health care providers and facilities should institute recommended intensive care precautions as soon as resistance is identified, along with alerting their local public health office. Isolates should be saved, and prospective and retrospective surveillance should be implemented to “identify isolates with similar phenotypes.” If an infected patient is being transferred to another facility, that facility should be notified ahead of time about the patient and what protocols to follow.
Because antibiotic-resistant organisms do not spread through the air like influenza, the risk that these organisms pose to health care workers is relatively low, Dr. Srinivasan explained. However, he stressed that a multifaceted, team-based approach to antibiotic stewardship and decontamination of health care facilities is absolutely necessary to achieve the best results for both patients and staff.
“We are now beginning to recognize that the contamination of surfaces and items in our health care environment is increasingly a problem in the transmission of these drug-resistant organisms,” Dr. Srinivasan explained. He said that, given the complexity of a typical health care environment, such as a hospital room or operating theater, it’s perhaps not so surprising that keeping everything clean is not the top priority.
In addition to making sure hospital rooms and other areas are properly cleaned, simple things like washing hands and keeping surfaces clean are just as important. Dr. Srinivasan pointed to a 2006 study by Philip C. Carling, MD, and his associates which showed that educational interventions can lead to substantial increases in hygiene and cleanliness, and that training of new staff is also important. Furthermore, giving staff enough time to properly clean rooms could significantly contribute to curtailing MCR-1 bacteria from spreading, he said.
“The other thing to keep in mind is that if we lose effective therapy, if we lose antibiotic therapy, the infections that are currently very treatable and are seldom deadly could again become very, very serious threats to life,” Dr. Srinivasan warned, specifically citing Escherichia coli, which is the leading cause of urinary tract infections. If community strains of E.coli become resistant to typical antibiotic treatment, these cases could become “difficult, if not impossible to treat.”
According to data shared by Dr. Srinivasan, in 2013 there were 2,049,422 illnesses in the United States attributed to antibiotic resistance and 23,000 fatalities.
AGA resource
The AGA Center for Gut Microbiome Research and Education was created to serve as a virtual “home” for AGA activities related to the gut microbiome. The center is focused on advancing gut microbiome research, educating AGA members and other stakeholders on the latest microbiome breakthroughs, and working with FDA and others to ensure that emerging microbiome-based treatments are safe and appropriately evaluated. Learn more here.
MCR-1 gene a growing concern for antibiotic resistance
The MCR-1 gene is quickly emerging as a powerful roadblock in the fight against antibiotic-resistant bacteria, according to experts from the Centers for Disease Control and Prevention.
Alex J. Kallen, MD, of the CDC in Atlanta, said in an Aug. 2 webinar – cohosted by CDC and the Partnership for Quality Care – that the CDC is closely monitoring the emergence of antibiotic resistant bacteria in the United States. The prevailing message of Dr. Kallen and his CDC colleague Arjun Srinivasan, MD was that the importance of the MCR-1 gene should not be underestimated.
First discovered in a human patient last year, the presence of the MCR-1 gene makes bacteria resistant to colistin, an antibiotic used often as a last resort to “treat patients with multidrug-resistant infections,” according to the CDC. Because the MCR-1 gene exists on a plasmid, or a small piece of DNA, it is easily transferable among bacteria, making it a problem for health care providers treating a patient infected with bacteria that has the gene.
“As of this year, all state and some local health departments will be funded to respond to [antibiotic resistance] threats, including emerging threats like [MCR-1], within their jurisdiction,” Dr. Kallen said. This funding could be used to support technical assistance, contact investigations, and laboratory testing, among other things.
In order to help prevent the spread of MCR-1 and mitigate cases of novel antimicrobial resistance, health care providers and facilities should institute recommended intensive care precautions as soon as resistance is identified, along with alerting their local public health office. Isolates should be saved, and prospective and retrospective surveillance should be implemented to “identify isolates with similar phenotypes.” If an infected patient is being transferred to another facility, that facility should be notified ahead of time about the patient and what protocols to follow.
Because antibiotic-resistant organisms do not spread through the air like influenza, the risk that these organisms pose to health care workers is relatively low, Dr. Srinivasan explained. However, he stressed that a multifaceted, team-based approach to antibiotic stewardship and decontamination of health care facilities is absolutely necessary to achieve the best results for both patients and staff.
“We are now beginning to recognize that the contamination of surfaces and items in our health care environment is increasingly a problem in the transmission of these drug-resistant organisms,” Dr. Srinivasan explained. He said that, given the complexity of a typical health care environment, such as a hospital room or operating theater, it’s perhaps not so surprising that keeping everything clean is not the top priority.
In addition to making sure hospital rooms and other areas are properly cleaned, simple things like washing hands and keeping surfaces clean are just as important. Dr. Srinivasan pointed to a 2006 study by Philip C. Carling, MD, and his associates which showed that educational interventions can lead to substantial increases in hygiene and cleanliness, and that training of new staff is also important. Furthermore, giving staff enough time to properly clean rooms could significantly contribute to curtailing MCR-1 bacteria from spreading, he said.
“The other thing to keep in mind is that if we lose effective therapy, if we lose antibiotic therapy, the infections that are currently very treatable and are seldom deadly could again become very, very serious threats to life,” Dr. Srinivasan warned, specifically citing Escherichia coli, which is the leading cause of urinary tract infections. If community strains of E.coli become resistant to typical antibiotic treatment, these cases could become “difficult, if not impossible to treat.”
According to data shared by Dr. Srinivasan, in 2013 there were 2,049,422 illnesses in the United States attributed to antibiotic resistance and 23,000 fatalities.
The MCR-1 gene is quickly emerging as a powerful roadblock in the fight against antibiotic-resistant bacteria, according to experts from the Centers for Disease Control and Prevention.
Alex J. Kallen, MD, of the CDC in Atlanta, said in an Aug. 2 webinar – cohosted by CDC and the Partnership for Quality Care – that the CDC is closely monitoring the emergence of antibiotic resistant bacteria in the United States. The prevailing message of Dr. Kallen and his CDC colleague Arjun Srinivasan, MD was that the importance of the MCR-1 gene should not be underestimated.
First discovered in a human patient last year, the presence of the MCR-1 gene makes bacteria resistant to colistin, an antibiotic used often as a last resort to “treat patients with multidrug-resistant infections,” according to the CDC. Because the MCR-1 gene exists on a plasmid, or a small piece of DNA, it is easily transferable among bacteria, making it a problem for health care providers treating a patient infected with bacteria that has the gene.
“As of this year, all state and some local health departments will be funded to respond to [antibiotic resistance] threats, including emerging threats like [MCR-1], within their jurisdiction,” Dr. Kallen said. This funding could be used to support technical assistance, contact investigations, and laboratory testing, among other things.
In order to help prevent the spread of MCR-1 and mitigate cases of novel antimicrobial resistance, health care providers and facilities should institute recommended intensive care precautions as soon as resistance is identified, along with alerting their local public health office. Isolates should be saved, and prospective and retrospective surveillance should be implemented to “identify isolates with similar phenotypes.” If an infected patient is being transferred to another facility, that facility should be notified ahead of time about the patient and what protocols to follow.
Because antibiotic-resistant organisms do not spread through the air like influenza, the risk that these organisms pose to health care workers is relatively low, Dr. Srinivasan explained. However, he stressed that a multifaceted, team-based approach to antibiotic stewardship and decontamination of health care facilities is absolutely necessary to achieve the best results for both patients and staff.
“We are now beginning to recognize that the contamination of surfaces and items in our health care environment is increasingly a problem in the transmission of these drug-resistant organisms,” Dr. Srinivasan explained. He said that, given the complexity of a typical health care environment, such as a hospital room or operating theater, it’s perhaps not so surprising that keeping everything clean is not the top priority.
In addition to making sure hospital rooms and other areas are properly cleaned, simple things like washing hands and keeping surfaces clean are just as important. Dr. Srinivasan pointed to a 2006 study by Philip C. Carling, MD, and his associates which showed that educational interventions can lead to substantial increases in hygiene and cleanliness, and that training of new staff is also important. Furthermore, giving staff enough time to properly clean rooms could significantly contribute to curtailing MCR-1 bacteria from spreading, he said.
“The other thing to keep in mind is that if we lose effective therapy, if we lose antibiotic therapy, the infections that are currently very treatable and are seldom deadly could again become very, very serious threats to life,” Dr. Srinivasan warned, specifically citing Escherichia coli, which is the leading cause of urinary tract infections. If community strains of E.coli become resistant to typical antibiotic treatment, these cases could become “difficult, if not impossible to treat.”
According to data shared by Dr. Srinivasan, in 2013 there were 2,049,422 illnesses in the United States attributed to antibiotic resistance and 23,000 fatalities.
The MCR-1 gene is quickly emerging as a powerful roadblock in the fight against antibiotic-resistant bacteria, according to experts from the Centers for Disease Control and Prevention.
Alex J. Kallen, MD, of the CDC in Atlanta, said in an Aug. 2 webinar – cohosted by CDC and the Partnership for Quality Care – that the CDC is closely monitoring the emergence of antibiotic resistant bacteria in the United States. The prevailing message of Dr. Kallen and his CDC colleague Arjun Srinivasan, MD was that the importance of the MCR-1 gene should not be underestimated.
First discovered in a human patient last year, the presence of the MCR-1 gene makes bacteria resistant to colistin, an antibiotic used often as a last resort to “treat patients with multidrug-resistant infections,” according to the CDC. Because the MCR-1 gene exists on a plasmid, or a small piece of DNA, it is easily transferable among bacteria, making it a problem for health care providers treating a patient infected with bacteria that has the gene.
“As of this year, all state and some local health departments will be funded to respond to [antibiotic resistance] threats, including emerging threats like [MCR-1], within their jurisdiction,” Dr. Kallen said. This funding could be used to support technical assistance, contact investigations, and laboratory testing, among other things.
In order to help prevent the spread of MCR-1 and mitigate cases of novel antimicrobial resistance, health care providers and facilities should institute recommended intensive care precautions as soon as resistance is identified, along with alerting their local public health office. Isolates should be saved, and prospective and retrospective surveillance should be implemented to “identify isolates with similar phenotypes.” If an infected patient is being transferred to another facility, that facility should be notified ahead of time about the patient and what protocols to follow.
Because antibiotic-resistant organisms do not spread through the air like influenza, the risk that these organisms pose to health care workers is relatively low, Dr. Srinivasan explained. However, he stressed that a multifaceted, team-based approach to antibiotic stewardship and decontamination of health care facilities is absolutely necessary to achieve the best results for both patients and staff.
“We are now beginning to recognize that the contamination of surfaces and items in our health care environment is increasingly a problem in the transmission of these drug-resistant organisms,” Dr. Srinivasan explained. He said that, given the complexity of a typical health care environment, such as a hospital room or operating theater, it’s perhaps not so surprising that keeping everything clean is not the top priority.
In addition to making sure hospital rooms and other areas are properly cleaned, simple things like washing hands and keeping surfaces clean are just as important. Dr. Srinivasan pointed to a 2006 study by Philip C. Carling, MD, and his associates which showed that educational interventions can lead to substantial increases in hygiene and cleanliness, and that training of new staff is also important. Furthermore, giving staff enough time to properly clean rooms could significantly contribute to curtailing MCR-1 bacteria from spreading, he said.
“The other thing to keep in mind is that if we lose effective therapy, if we lose antibiotic therapy, the infections that are currently very treatable and are seldom deadly could again become very, very serious threats to life,” Dr. Srinivasan warned, specifically citing Escherichia coli, which is the leading cause of urinary tract infections. If community strains of E.coli become resistant to typical antibiotic treatment, these cases could become “difficult, if not impossible to treat.”
According to data shared by Dr. Srinivasan, in 2013 there were 2,049,422 illnesses in the United States attributed to antibiotic resistance and 23,000 fatalities.