User login
NIH launches trial of Zika vaccine candidate
A clinical trial to evaluate a candidate vaccine for Zika virus is underway, with preliminary results from the multisite phase I trial expected by the end of 2016.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health, announced the trial and gave an update about other Zika virus vaccine development efforts during an Aug. 3 telephone briefing with reporters. The announcement came just days after the continental United States saw its first cases of local transmission of Zika virus in Florida.
The phase I clinical trial began on Aug. 2 and will evaluate the safety and immunogenicity of an investigational DNA vaccine for Zika virus. This vaccine is similar to one that early-stage trials have shown to be safe and immunogenic for West Nile virus, a flavivirus closely related to Zika. The Zika virus vaccine had promising preclinical results, Dr. Fauci said. “Preliminary immune responses that we’ve seen in a variety of animal models have been rather robust.”
The DNA vaccine uses a plasmid to deliver genes that code for specific Zika virus proteins. “When the plasmid expresses the envelope protein from the Zika virus, it does so in a way that mimics the virus,” provoking a host immune response that includes neutralizing antibodies and T cells, according to lead trial investigator Julie Ledgerwood, DO, chief of the clinical trials program at the NIAID’s Vaccine Research Center.
The phase I clinical trial will enroll 80 healthy volunteers, aged 18-35 years. All participants will receive the investigational Zika virus vaccine at their first visit, with vaccine delivery via a needle-free system that injects the vaccine directly into the deltoid muscle.
Forty participants will receive one additional dose of the vaccine, with half of those people receiving the additional dose at 8 weeks after the first vaccine, and the other half receiving the additional dose at 12 weeks after the first dose. The other 40 participants will receive two additional doses, with half receiving them at 4 and 8 weeks after the first vaccination, and the other half receiving the extra doses at 4 and 20 weeks. All participants will receive the same dose at each vaccination.
Participants will be asked to monitor their temperature daily for a week after each vaccine dose is received; they will also report any adverse events. The trial design provides for several follow-up visits to track safety and to measure immune response in 44 weeks of short-term follow-up. Additionally, two follow-up visits at 18 months and 2 years post vaccination will measure the durability of the immune response.
The clinical trial will be run at the National Institutes of Health Clinical Center in Bethesda, Md., at Emory University in Atlanta, and at the University of Maryland’s Center for Vaccine Development in Baltimore.
Initial safety and immune response data should be available by the end of 2016, Dr. Fauci said. If these data are promising, a phase II clinical trial will be launched in early 2017 in several Zika-endemic countries. The NIH has the in-house capability to manufacture the 2,500-5,000 doses of vaccine that the phase II trial is expected to require.
However, said Dr. Fauci, without congressional approval of additional funding for Zika virus vaccine efforts, the transition to a phase II clinical trial is far from certain. Interruption in funding would be “effectively impeding our smooth process on the vaccine development front,” he said. “When I say we are going to run out of money soon, I mean really soon.”
Dr. Fauci, in response to questions, said that he continues to be bullish on such platform-based approaches to vaccines. The DNA strategy, in particular, represents “a very convenient and easily scalable vaccine,” he said. “We are moving much more toward platform-based vaccines … because of their ease and convenience, and scaling up and rapidity.”
The scope of a vaccination program will depend on the endemicity of the virus in a given area, said Dr. Fauci. In an endemic area, “Ultimately, you want to get women of childbearing age, as well as their sexual partners,” he said. This means that target populations will be as young as possible, to make sure women and their partners are vaccinated by the time pregnancy becomes a possibility.
Other Zika virus vaccine development efforts underway at NIAID include a strategy that uses a live attenuated virus, an approach used for a dengue virus vaccine that is currently in a phase 3 clinical trial in Brazil, according to the agency’s website. Dengue and Zika are closely related viruses. Another investigational Zika virus vaccine uses genetic engineering to create a vaccine derived from vesicular stomatitis virus, which infects cattle. This strategy, also used in one approach to Ebola vaccination, is in the pre-clinical stage.
On Twitter @karioakes
A clinical trial to evaluate a candidate vaccine for Zika virus is underway, with preliminary results from the multisite phase I trial expected by the end of 2016.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health, announced the trial and gave an update about other Zika virus vaccine development efforts during an Aug. 3 telephone briefing with reporters. The announcement came just days after the continental United States saw its first cases of local transmission of Zika virus in Florida.
The phase I clinical trial began on Aug. 2 and will evaluate the safety and immunogenicity of an investigational DNA vaccine for Zika virus. This vaccine is similar to one that early-stage trials have shown to be safe and immunogenic for West Nile virus, a flavivirus closely related to Zika. The Zika virus vaccine had promising preclinical results, Dr. Fauci said. “Preliminary immune responses that we’ve seen in a variety of animal models have been rather robust.”
The DNA vaccine uses a plasmid to deliver genes that code for specific Zika virus proteins. “When the plasmid expresses the envelope protein from the Zika virus, it does so in a way that mimics the virus,” provoking a host immune response that includes neutralizing antibodies and T cells, according to lead trial investigator Julie Ledgerwood, DO, chief of the clinical trials program at the NIAID’s Vaccine Research Center.
The phase I clinical trial will enroll 80 healthy volunteers, aged 18-35 years. All participants will receive the investigational Zika virus vaccine at their first visit, with vaccine delivery via a needle-free system that injects the vaccine directly into the deltoid muscle.
Forty participants will receive one additional dose of the vaccine, with half of those people receiving the additional dose at 8 weeks after the first vaccine, and the other half receiving the additional dose at 12 weeks after the first dose. The other 40 participants will receive two additional doses, with half receiving them at 4 and 8 weeks after the first vaccination, and the other half receiving the extra doses at 4 and 20 weeks. All participants will receive the same dose at each vaccination.
Participants will be asked to monitor their temperature daily for a week after each vaccine dose is received; they will also report any adverse events. The trial design provides for several follow-up visits to track safety and to measure immune response in 44 weeks of short-term follow-up. Additionally, two follow-up visits at 18 months and 2 years post vaccination will measure the durability of the immune response.
The clinical trial will be run at the National Institutes of Health Clinical Center in Bethesda, Md., at Emory University in Atlanta, and at the University of Maryland’s Center for Vaccine Development in Baltimore.
Initial safety and immune response data should be available by the end of 2016, Dr. Fauci said. If these data are promising, a phase II clinical trial will be launched in early 2017 in several Zika-endemic countries. The NIH has the in-house capability to manufacture the 2,500-5,000 doses of vaccine that the phase II trial is expected to require.
However, said Dr. Fauci, without congressional approval of additional funding for Zika virus vaccine efforts, the transition to a phase II clinical trial is far from certain. Interruption in funding would be “effectively impeding our smooth process on the vaccine development front,” he said. “When I say we are going to run out of money soon, I mean really soon.”
Dr. Fauci, in response to questions, said that he continues to be bullish on such platform-based approaches to vaccines. The DNA strategy, in particular, represents “a very convenient and easily scalable vaccine,” he said. “We are moving much more toward platform-based vaccines … because of their ease and convenience, and scaling up and rapidity.”
The scope of a vaccination program will depend on the endemicity of the virus in a given area, said Dr. Fauci. In an endemic area, “Ultimately, you want to get women of childbearing age, as well as their sexual partners,” he said. This means that target populations will be as young as possible, to make sure women and their partners are vaccinated by the time pregnancy becomes a possibility.
Other Zika virus vaccine development efforts underway at NIAID include a strategy that uses a live attenuated virus, an approach used for a dengue virus vaccine that is currently in a phase 3 clinical trial in Brazil, according to the agency’s website. Dengue and Zika are closely related viruses. Another investigational Zika virus vaccine uses genetic engineering to create a vaccine derived from vesicular stomatitis virus, which infects cattle. This strategy, also used in one approach to Ebola vaccination, is in the pre-clinical stage.
On Twitter @karioakes
A clinical trial to evaluate a candidate vaccine for Zika virus is underway, with preliminary results from the multisite phase I trial expected by the end of 2016.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health, announced the trial and gave an update about other Zika virus vaccine development efforts during an Aug. 3 telephone briefing with reporters. The announcement came just days after the continental United States saw its first cases of local transmission of Zika virus in Florida.
The phase I clinical trial began on Aug. 2 and will evaluate the safety and immunogenicity of an investigational DNA vaccine for Zika virus. This vaccine is similar to one that early-stage trials have shown to be safe and immunogenic for West Nile virus, a flavivirus closely related to Zika. The Zika virus vaccine had promising preclinical results, Dr. Fauci said. “Preliminary immune responses that we’ve seen in a variety of animal models have been rather robust.”
The DNA vaccine uses a plasmid to deliver genes that code for specific Zika virus proteins. “When the plasmid expresses the envelope protein from the Zika virus, it does so in a way that mimics the virus,” provoking a host immune response that includes neutralizing antibodies and T cells, according to lead trial investigator Julie Ledgerwood, DO, chief of the clinical trials program at the NIAID’s Vaccine Research Center.
The phase I clinical trial will enroll 80 healthy volunteers, aged 18-35 years. All participants will receive the investigational Zika virus vaccine at their first visit, with vaccine delivery via a needle-free system that injects the vaccine directly into the deltoid muscle.
Forty participants will receive one additional dose of the vaccine, with half of those people receiving the additional dose at 8 weeks after the first vaccine, and the other half receiving the additional dose at 12 weeks after the first dose. The other 40 participants will receive two additional doses, with half receiving them at 4 and 8 weeks after the first vaccination, and the other half receiving the extra doses at 4 and 20 weeks. All participants will receive the same dose at each vaccination.
Participants will be asked to monitor their temperature daily for a week after each vaccine dose is received; they will also report any adverse events. The trial design provides for several follow-up visits to track safety and to measure immune response in 44 weeks of short-term follow-up. Additionally, two follow-up visits at 18 months and 2 years post vaccination will measure the durability of the immune response.
The clinical trial will be run at the National Institutes of Health Clinical Center in Bethesda, Md., at Emory University in Atlanta, and at the University of Maryland’s Center for Vaccine Development in Baltimore.
Initial safety and immune response data should be available by the end of 2016, Dr. Fauci said. If these data are promising, a phase II clinical trial will be launched in early 2017 in several Zika-endemic countries. The NIH has the in-house capability to manufacture the 2,500-5,000 doses of vaccine that the phase II trial is expected to require.
However, said Dr. Fauci, without congressional approval of additional funding for Zika virus vaccine efforts, the transition to a phase II clinical trial is far from certain. Interruption in funding would be “effectively impeding our smooth process on the vaccine development front,” he said. “When I say we are going to run out of money soon, I mean really soon.”
Dr. Fauci, in response to questions, said that he continues to be bullish on such platform-based approaches to vaccines. The DNA strategy, in particular, represents “a very convenient and easily scalable vaccine,” he said. “We are moving much more toward platform-based vaccines … because of their ease and convenience, and scaling up and rapidity.”
The scope of a vaccination program will depend on the endemicity of the virus in a given area, said Dr. Fauci. In an endemic area, “Ultimately, you want to get women of childbearing age, as well as their sexual partners,” he said. This means that target populations will be as young as possible, to make sure women and their partners are vaccinated by the time pregnancy becomes a possibility.
Other Zika virus vaccine development efforts underway at NIAID include a strategy that uses a live attenuated virus, an approach used for a dengue virus vaccine that is currently in a phase 3 clinical trial in Brazil, according to the agency’s website. Dengue and Zika are closely related viruses. Another investigational Zika virus vaccine uses genetic engineering to create a vaccine derived from vesicular stomatitis virus, which infects cattle. This strategy, also used in one approach to Ebola vaccination, is in the pre-clinical stage.
On Twitter @karioakes
Clinical and Sonographic Evaluation of Bicortical Button for Proximal Biceps Tenodesis
The long head of the biceps (LHB) tendon is a recognized source of shoulder pain. LHB tendon pathology is commonly associated with other shoulder conditions, such as superior labral tears, rotator cuff tears, or subacromial impingement, whereas isolated pathology, such as traumatic ruptures, tendinosis, or medial subluxation, is rare.1 Treatment of LHB pathology ranges from conservative measures to surgical measures, including tenotomy or tenodesis.2 LHB tenodesis offers the advantage of maintaining the length–tension relationship of the biceps muscle to prevent atrophy and avoid the Popeye deformity incurred from tenotomy alone. Tenodesis also prevents muscle cramping associated with contracted biceps muscle and better maintains elbow flexion and supination strength, which may be decreased with tenotomy.3 In addition, when a subpectoral biceps tenodesis technique is used, pain from LHB tendinopathy in the intertubercular groove may be reduced.4
Open subpectoral biceps tenodesis is a reproducible, efficient method for LHB tenodesis.4,5 A variety of fixation devices has been used: bone tunnels,6 keyhole fixation,7 suture anchors,6-9 and interference screws.6-8,10,11 More recently, a bicortical button has been used for LHB tendon tenodesis.12 Biomechanical studies have shown that load to failure is comparable for bicortical button fixation and interference screw fixation.13,14 In other models of tendon repair, the bicortical button has strength and stability comparable to those of interference screw fixation and enables earlier rehabilitation.15-17 However, there is concern that bicortical button fixation may result in axillary nerve (AN) or posterior circumflex humeral artery (PCHA) compromise because of the proximity of these neurovascular structures to the bicortical button.13,18-21
We conducted a study to functionally and sonographically assess the outcomes of patients who underwent open subpectoral biceps tenodesis with a bicortical button. Functional outcomes were assessed with patient-reported outcomes and physician-reported outcomes. Sonographic studies were used to evaluate the integrity of the tenodesis and determine the proximity of the button to the AN and the PCHA along the posterior proximal humerus.
Methods
After obtaining Institutional Review Board approval for this study, we retrospectively identified 28 consecutive patients who had proximal biceps tenodesis performed by a single surgeon (Dr. K.E. Swanson) using a mini-open subpectoral biceps tenodesis technique with a bicortical button between March 2011 and January 2013. All 28 patients were asked to participate in the study. Twenty-four (86%) agreed to complete 2 surgical outcome surveys, and 18 (64%) completed a 3-part clinical examination at minimum 12-month follow-up.
One of the surveys was Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH), a validated comprehensive disability survey that scores upper extremity functionality on a scale ranging from 0 (none) to 100 (extreme difficulty).22,23 The other survey scored pain on a scale ranging from 0 (none) to 100 (worst pain).
The clinical examination was completed during a single visit by an orthopedic surgeon (Dr. Meadows or Dr. Diesselhorst) different from the primary surgeon (Dr. K.E. Swanson) and by a clinician-sonologist (Dr. Finnoff). The examination’s 3 parts were physical examination of arm, biceps supination strength test, and ultrasonographic evaluation.
Physical Examination of Arm. Physical examination included palpation of bicipital groove, range of motion (ROM) of shoulder and elbow, and clinical deformity of biceps. Patients were questioned regarding symptoms of AN damage, including sensory and motor findings. Bicipital groove tenderness was assessed with a visual analog scale rating pain 0 to 10. ROM was measured in degrees and was presented as a percentage of full elbow ROM (150°) and full shoulder ROM (180°).
Biceps Supination Strength Test. Biceps supination strength was tested with a baseline hydraulic wrist dynamometer with door handle attachment. Patients were seated with the elbow bent 90° and the forearm in a neutral position. In a series of 3 trials, the patient maintained grip of the dynamometer doorknob while supinating the forearm. The tenodesed (operated) arm and contralateral unaffected (nonoperated) arm were tested in random order and recorded in pounds.
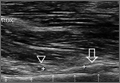
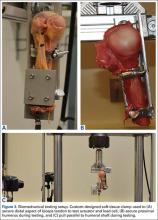
Ultrasonographic Evaluation. Ultrasonography was used to evaluate the tenodesis site. In each case, the biceps tendon was assessed to determine the location of the bicortical button in relation to the AN/PCHA neurovascular bundle. Whereas nerves are difficult to visualize with ultrasonography, arteries are readily seen. Dr. Finnoff used a CX50 ultrasound machine (Philips Medical Systems) with either a 12-3 MHz linear array or a 5-1 MHz curvilinear array transducer to measure the shortest distance from the PCHA to the button.
Each patient was placed in a lateral decubitus or prone position, and the skin of the upper arm was exposed. Tendon integrity was deemed either intact (continuity between biceps tendon and cortical button) or disrupted (lack of continuity between tendon and cortical button). The transducer was then placed in an anatomical sagittal plane over the posterior aspect of the proximal humerus. Power Doppler and cephalad and caudad transducer glides were used to identify the location of the PCHA. The transducer was then glided laterally and anteriorly around the humerus, following the course of the PCHA, until the cortical button was located. The narrowest interval between the PCHA and the cortical button was measured using the ultrasound machine’s software. A still image of each measurement was saved.
Surgical Technique
Biceps tenodesis indications included high-demand heavy laborers, athletes, and patients who preferred the cosmetic results of tenodesis over tenotomy. Most patients had acute symptomatic tears of the superior labrum with instability of the biceps anchor complex. Others had fraying and tenosynovitis of the LHB tendon. Any associated pathology was addressed during the same surgical period.
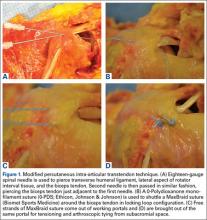
The surgical technique used was similar to that described by Snir and colleagues.12 Each patient was placed in the lateral decubitus position. Once pathology confirmed biceps tenodesis, the biceps tendon was tenotomized at the base of the superior labrum. A 3-cm incision was made along the axillary fold centered over the inferior border of the pectoralis major tendon. Blunt dissection was performed to define the inferior border of the pectoralis major tendon and to palpate the underlying biceps tendon as it exited the intertubercular groove. The LHB tendon was removed and prepared with No. 2 Fiberwire (Arthrex) in Krackow fashion starting 2 cm proximal to the musculotendinous junction. The excess tendon was excised.
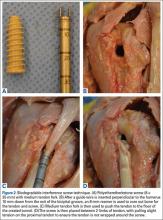
A 3.2-mm guide wire was centered along the most distal aspect of the biceps groove and then drilled through the anterior cortex and just through the posterior cortex. A cannulated reamer, selected on the basis of the biceps tendon diameter (typically, 5-7 mm), was then drilled over the guide wire through the anterior cortex only. The Food and Drug Administration–approved cortical button (BicepsButton; Arthrex) was then loaded by passing the tendon suture ends through each side of the button in alternating fashion, thus allowing the button to slide along the sutures.
The button was loaded onto the BicepsButton deployment device and inserted through the drilled tunnel of the anterior cortex and just through the posterior cortex. The deployment device was then removed, and 1 suture end was pulled to allow the button to engage the posterior humeral cortex. Pulling on both sutures allowed the biceps tendon to slide through the anterior cortex hole of the humerus until the tendon reached the posterior humeral cortex. Tension was verified, and the sutures were tied over the tendon. The wound was then irrigated and closed.
Rehabilitation Program
Patients completed a standard rehabilitation protocol for biceps tenodesis24 along with rehabilitation protocols for any additional procedures performed. In phase 1 (weeks 0-2), they focused on gradual restoration of passive ROM and remained in a sling. In phase 2 (weeks 2-6), they focused on gradual restoration of active ROM, and by week 3 were weaned out of the sling. In phase 3 (weeks 6-8), they continued ROM and strengthening exercises to normalize strength, endurance, and neuromuscular control. In phase 4 (weeks 8-12), they focused on advanced strengthening exercises and return to activities.
Statistical Analysis
Descriptive statistics included means, medians, and SDs. Comparisons between operated and nonoperated arms and between dominant and nondominant arms were performed by a statistician using paired t tests with P = .05. Confidence intervals were calculated for operated and nonoperated arms and for dominant and nondominant arms by using the differences between them.
Results
Functional Outcomes
Surgical outcome scores and pain scores were obtained from 24 patients (86%) at minimum 12-month follow-up. Mean (SD) DASH score was 15.15 (17.6; median, 9), and mean (median) pain score was 12.61 (7).
Eighteen patients (64%) completed the clinical examination: 16 men (88.9%) and 2 women (11.1%). Mean age was 48.3 years (age range, 33-59 years). Of these 18 patients, 9 (50%) had surgery on the dominant arm, and the other 9 had surgery on the nondominant arm. All patients were right-hand–dominant. In 3 patients, biceps tenodesis was performed with only minimal arthroscopic débridement (20%); in the other 15, biceps tenodesis was performed concomitantly with 1 or more additional arthroscopic procedures: acromioplasty (73%), rotator cuff repair (47%), distal clavicle resection (33%), subacromial bursectomy (13%), microfracture of glenoid (13%), and posterior labral repair (7%).
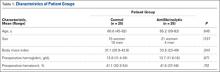
The clinical examination was performed a mean of 15.2 months (range, 12-26 months) after surgery. Physical examination findings are listed in Table 1.
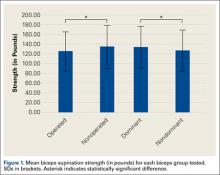
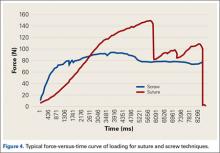
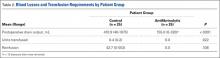
Forearm supination strength, averaged from 3 trials on each arm, was significantly (P = .01) greater in the nonoperated arm than in the operated arm (Table 2, Figure 1). A 95% confidence interval for the mean (SD) difference in strength was 9.35 (7.76) pounds, meaning that on average, the nonoperated arm will be 1.59 to 17.11 pounds stronger than the operated arm. In addition, strength of the dominant arm was greater than that of the nondominant arm (P = .05) regardless of which arm underwent surgery (Table 2, Figure 1). However, the mean (SD) difference in strength was 6.94 (8.39) pounds, indicating the observed difference was not statistically significant.
Sonographic Evaluation
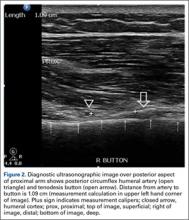
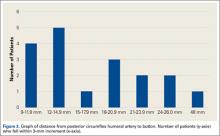
According to the sonographic evaluations, the tenodesis was intact in all 18 patients (Figure 2). Estimated mean (SD) distance from button to PCHA was 18.17 (9.0) mm (median, 16.1 mm; range, 9.4-48 mm) (Figure 2, Figure 3). No patient indicated any symptoms of AN damage.
Discussion
There are few studies of functional outcomes of biceps tenodesis. Pain is a common measure of patient satisfaction. Mazzocca and colleagues25 reported a mean follow-up pain score of 1.1 (range, 0.5-1.9) out of 10 for a group of 41 patients who had subpectoral tenodesis with an interference screw. Millett and colleagues26 reported a mean postoperative pain score of 2.5 out of 10 for patients who had subpectoral interference screw fixation. Our patients reported a mean pain score of 12.6 out of 100 after minimum 12-month follow-up. We also assessed for pain in the intertubercular groove during palpation. Although some studies have shown that groove pain was eliminated by subpectoral biceps tenodesis,5 3 patients in our study had pain on groove palpation. The cause of this residual pain is unclear, but some studies have suggested a chronic degenerative pathologic process that occurs while the tendon is within the biceps groove.27 Removing the tendon from the groove may not remove the underlying cause of pain.
Our patients’ mean DASH score was 15.15 (within the excellent range). Normative mean (SD) DASH score for the general population is 10.1 (14.68).28
Functional strength of forearm supination, shoulder ROM, and elbow ROM are objective measures of patient performance after fixation. On Cybex testing, Phillips and colleagues29 found no difference in forearm supination strength or elbow flexion (compared with contralateral arm) after biceps tenodesis or conservative treatment for proximal biceps ruptures. Shank and colleagues30 compared elbow flexion and supination strength of the affected and unaffected arms after suture anchor subpectoral biceps tenodesis. There was no significant difference in Cybex results, but there was a 14% to 15% loss of average strength in the tenodesed versus nonsurgical arm. In the present study, we found a significant difference in forearm supination strength between the operated and nonoperated arms, but with only a 7% loss of average strength in the operated arms. The difference in strength ranged from 1.59 to 17.11 pounds, which may not be clinically significant, as supination strength ranged from 60 to 270 pounds.
Of the 18 patients in this study, 9 had surgery on the dominant arm, and the other 9 had surgery on the nondominant arm. Examining the effect of arm dominance on results revealed that patients with surgery on the nondominant arm tended to have substantially reduced supination strength in that arm vs the dominant arm. There was an 11% loss of average strength for nondominant vs dominant arms that had surgery. Examining nondominant arms only revealed a 13% loss of strength for operated vs nonoperated arms. There was no difference in forearm supination strengths between nonoperated arms (dominant vs nondominant) or between dominant arms (operated vs nonoperated). This suggests that, though hand dominance may not play a significant role in control patients’ forearm supination strength,30 it may have a substantial effect on surgical patients’ ability to regain strength when the nondominant arm is the surgical arm. One objective of this study was to measure the distance between the biceps cortical button on the posterior humeral cortex and the AN/PCHA neurovascular bundle. The AN bundles with the PCHA posterior to the humeral neck.31-33 As the AN travels with the PCHA, and the PCHA has been reliably identified with Doppler ultrasonography,34-36 the PCHA was used as a marker for the AN in this study. Our bicortical button technique places the button on the posterior aspect of the humerus, making AN and PCHA the nearest at-risk neurovascular structures. None of our patients had symptoms of AN damage. However, 2 patients indicated pain in the posterior aspect of the humerus during deltoid activation. Distance from the neurovascular structures to the button was 48 mm in one patient and 13.6 mm in the other. DASH scores were 43 and 27, respectively. Both patients’ 1-year pain score was 30. The first patient underwent arthroscopic acromioplasty, distal clavicle resection, and microfracture of the glenoid surface in addition to the subpectoral biceps tenodesis; the second underwent subacromial decompression and distal clavicle resection in addition to the subpectoral biceps tenodesis. Whether the associated pathology contributed to their persistent pain is unknown. However, given the distance from AN/PCHA to button, it is unlikely that their pain was a result of neurovascular compromise from the procedure.
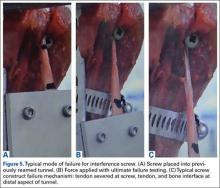
Advantages of the cortical button include the ability to drill a smaller hole in the humerus for fixation, compared with the hole drilled for an interference screw. Despite the biomechanical strength of the screw, large (8 mm) cortical violations have been associated with increased fracture risk of the proximal humerus.37,38 The tendon may experience less trauma than that caused by being twisted against an interference screw, the most common location of failure of which is the tendon–screw interface.39 In addition, tendon healing may be improved through circumferential healing in the cortical button tunnel.
A concern of using a bicortical button for fixation is drilling through the posterior cortex, because of the proximity of the posterior neurovascular structures. In a case in which the posterior cord was injured, Rhee and colleagues40 used a suture pullout technique whereby a Beath pin was passed out of the posterior humerus and soft tissues to then hold tension on the biceps tendon during the tenodesis. The radial nerve potentially could have been injured by pin overpenetration or by becoming wrapped up in the soft tissues as the pin was spinning through them. In our technique, the posterior humeral cortex is drilled cautiously to avoid overpenetration and possibly getting the posterior soft tissues wrapped up in the guide pin. No AN injuries have been reported with this technique. Mean distance from AN to posterior cortical button in this study was 18.17 mm. In 2 cadaver studies of bicortical drilling for subpectoral biceps tenodesis, the ANs were 25.1 mm and 36.7 mm from the posterior drill hole.41,21
Limitations of this study included its design (case series) and limited number of follow-up patients. Of the 28 consecutive patients identified for the study, 10 did not undergo the clinical examination, as they either lived more than 3 hours away (8 patients) or could not be contacted (2 patients). Another study limitation was the inability to directly image ANs with ultrasound. Therefore, measurements of the distance from the PCHA to the button were used to estimate the distance from the AN/PCHA neurovascular bundle to the button.
In this study, functional outcomes were excellent, and there were no tenodesis failures or neurovascular complications. These preliminary findings indicate that subpectoral biceps tenodesis with a bicortical button is a viable treatment option for patients with the appropriate indications for this procedure.
1. Khazzam M, George MS, Churchill RS, Kuhn JE. Disorders of the long head of biceps tendon. J Shoulder Elbow Surg. 2012;21(1):136-145.
2. Geaney LE, Mazzocca AD. Biceps brachii tendon ruptures: a review of diagnosis and treatment of proximal and distal biceps tendon ruptures. Phys Sportsmed. 2010;38(2):117-125.
3. Kelly AM, Drakos MC, Fealy S, Taylor SA, O’Brien SJ. Arthroscopic release of the long head of the biceps tendon: functional outcome and clinical results. Am J Sports Med. 2005;33(2):208-213.
4. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc Rev. 2008;16(3):170-176.
5. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
6. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
7. Ozalay, M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
8. Golish RS, Caldwell PE, Miller MD, et al. Interference screw versus suture anchor fixation for subpectoral tenodesis of the proximal biceps tendon: a cadaveric study. Arthroscopy. 2008;24(10):1103-1108.
9. Richards DP, Burkhart SS. A biomechanical analysis of two biceps tenodesis fixation techniques. Arthroscopy. 2005;21(7):861-866.
10. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
11. Wolf RS, Zheng N, Weichel D. Long head biceps tenotomy versus tenodesis: a cadaveric biomechanical analysis. Arthroscopy. 2005;21(2):182-185.
12. Snir N, Hamula M, Wolfson T, Laible C, Sherman O. Long head of the biceps tenodesis with cortical button technique. Arthrosc Tech. 2013;2(2):e95-e97.
13. Arora AS, Singh A, Koonce RC. Biomechanical evaluation of a unicortical button versus interference screw for subpectoral biceps tenodesis. Arthroscopy. 2013;29(4):638-644.
14. Buchholz A, Martetschläger F, Siebenlist S, et al. Biomechanical comparison of intramedullary cortical button fixation and interference screw technique for subpectoral biceps tenodesis. Arthroscopy. 2013;29(5):845-853.
15. Bain GI, Prem H, Heptinstall RJ, Verhellen R, Paix D. Repair of distal biceps tendon rupture: a new technique using the Endobutton. J Shoulder Elbow Surg. 2000;9(2):120-126.
16. Greenberg JA. Endobutton repair of distal biceps tendon ruptures. J Hand Surg Am. 2009;34(8):1541-1548.
17. Heinzelmann AD, Savoie FH 3rd, Ramsey JR, Field LD, Mazzocca AD. A combined technique for distal biceps repair using a soft tissue button and biotenodesis interference screw. Am J Sports Med. 2009;37(5):989-994.
18. DeAngelis JP, Chen A, Wexler M, et al. Biomechanical characterization of unicortical button fixation: a novel technique for proximal subpectoral biceps tenodesis. Knee Surg Sports Traumatol Arthrosc. 2015;23(5):1434-1441.
19. Dickens JF, Kilcoyne KG, Tintle SM, Giuliani J, Schaefer RA, Rue JP. Subpectoral biceps tenodesis: an anatomic study and evaluation of at-risk structures. Am J Sports Med. 2012;40(10):2337-2341.
20. Sethi PM, Rajaram A, Beitzel K, Hackett TR, Chowaniec DM, Mazzocca AD. Biomechanical performance of subpectoral biceps tenodesis: a comparison of interference screw fixation, cortical button fixation, and interference screw diameter. J Shoulder Elbow Surg. 2013;22(4):451-457.
21. Sethi PM, Vadasdi K, Greene RT, Vitale MA, Duong M, Miller SR. Safety of open suprapectoral and subpectoral biceps tenodesis: an anatomic assessment of risk for neurologic injury. J Shoulder Elbow Surg. 2015;24(1):138-142.
22. Gummesson C, Ward MM, Atroshi I. The shortened Disabilities of the Arm, Shoulder and Hand questionnaire (QuickDASH): validity and reliability based on responses within the full-length DASH. BMC Musculoskelet Disord. 2006;7:44.
23. Schmidt CC, Brown BT, Sawardeker PJ, DeGravelle M Jr, Miller MC. Factors affecting supination strength after a distal biceps rupture. J Shoulder Elbow Surg. 2014;23(1):68-75.
24. Brotzman SB, Wilk KE, eds. Handbook of Orthopaedic Rehabilitation. Philadelphia, PA: Mosby Elsevier; 2007.
25. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
26. Millett PJ, Snaders B, Gobezie R, Braun S, Warner JP. Interference screw versus suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9(121):1-6.
27. Streit JJ, Shishani Y, Rodgers M, Gobezie R. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extra-articular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;6:63-70.
28. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208-215.
29. Phillips BB, Canale ST, Sisk TD, Stralka SW, Wyatt KP. Rupture of the proximal biceps tendon in middle-aged patients. Orthop Rev. 1993;22(3):349-353.
30. Shank JR, Singleton SB, Braun S, et al. A comparison of forearm supination and elbow flexion strength in patients with long head of the biceps tenotomy or tenodesis. Arthroscopy. 2011;27(1):9-16.
31. Apaydin N, Tubbs RS, Loukas M, Duparc F. Review of the surgical anatomy of the axillary nerve and the anatomic basis of its iatrogenic and traumatic injury. Surg Radiol Anat. 2010;32(3):193-201.
32. Johnson D. Pectoral girdle and upper limp. In: Standring S, ed. Gray’s Anatomy. 40th ed. New York, NY: Elsevier; 2008:814-821.
33. Tubbs RS, Tyler-Kabara EC, Aikens AC, et al. Surgical anatomy of the axillary nerve within the quadrangular space. J Neurosurg. 2005;102(5):912-914.
34. Kim YA, Yoon KB, Kwon TD, Kim DH, Yoon DM. Evaluation of anatomic landmarks for axillary nerve block in the quadrilateral space. Acta Anaesthesiol Scand. 2014;58(5):567-571.
35. Robinson DJ, Marks P, Schneider-Kolsky ME. Ultrasound of the posterior circumflex humeral artery. J Med Imaging Radiat Oncol. 2010;54(3):219-223.
36. Rothe C, Asghar S, Andersen HL, Christensen JK, Lange KH. Ultrasound-guided block of the axillary nerve: a volunteer study of a new method. Acta Anaesthesiol Scand. 2011;55(5):565-570.
37. Reiff SN, Nho SJ, Romeo AA. Proximal humerus fracture after keyhole biceps tenodesis. Am J Orthop. 2010;39(7):E61-E63.
38. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
39. Koch BS, Burks RT. Failure of biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(5):735-740.
40. Rhee PC, Spinner RJ, Bishop AT, Shin AY. Iatrogenic brachial plexus injuries associated with open subpectoral biceps tenodesis. Am J Sports Med. 2013;41(9):2048-2053.
41. Ding DY, Gupta A, Snir N, Wolfson T, Meislin RJ. Nerve proximity during bicortical drilling for subpectoral biceps tenodesis: a cadaveric study. Arthroscopy. 2014;30(8):942-946.
The long head of the biceps (LHB) tendon is a recognized source of shoulder pain. LHB tendon pathology is commonly associated with other shoulder conditions, such as superior labral tears, rotator cuff tears, or subacromial impingement, whereas isolated pathology, such as traumatic ruptures, tendinosis, or medial subluxation, is rare.1 Treatment of LHB pathology ranges from conservative measures to surgical measures, including tenotomy or tenodesis.2 LHB tenodesis offers the advantage of maintaining the length–tension relationship of the biceps muscle to prevent atrophy and avoid the Popeye deformity incurred from tenotomy alone. Tenodesis also prevents muscle cramping associated with contracted biceps muscle and better maintains elbow flexion and supination strength, which may be decreased with tenotomy.3 In addition, when a subpectoral biceps tenodesis technique is used, pain from LHB tendinopathy in the intertubercular groove may be reduced.4
Open subpectoral biceps tenodesis is a reproducible, efficient method for LHB tenodesis.4,5 A variety of fixation devices has been used: bone tunnels,6 keyhole fixation,7 suture anchors,6-9 and interference screws.6-8,10,11 More recently, a bicortical button has been used for LHB tendon tenodesis.12 Biomechanical studies have shown that load to failure is comparable for bicortical button fixation and interference screw fixation.13,14 In other models of tendon repair, the bicortical button has strength and stability comparable to those of interference screw fixation and enables earlier rehabilitation.15-17 However, there is concern that bicortical button fixation may result in axillary nerve (AN) or posterior circumflex humeral artery (PCHA) compromise because of the proximity of these neurovascular structures to the bicortical button.13,18-21
We conducted a study to functionally and sonographically assess the outcomes of patients who underwent open subpectoral biceps tenodesis with a bicortical button. Functional outcomes were assessed with patient-reported outcomes and physician-reported outcomes. Sonographic studies were used to evaluate the integrity of the tenodesis and determine the proximity of the button to the AN and the PCHA along the posterior proximal humerus.
Methods
After obtaining Institutional Review Board approval for this study, we retrospectively identified 28 consecutive patients who had proximal biceps tenodesis performed by a single surgeon (Dr. K.E. Swanson) using a mini-open subpectoral biceps tenodesis technique with a bicortical button between March 2011 and January 2013. All 28 patients were asked to participate in the study. Twenty-four (86%) agreed to complete 2 surgical outcome surveys, and 18 (64%) completed a 3-part clinical examination at minimum 12-month follow-up.
One of the surveys was Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH), a validated comprehensive disability survey that scores upper extremity functionality on a scale ranging from 0 (none) to 100 (extreme difficulty).22,23 The other survey scored pain on a scale ranging from 0 (none) to 100 (worst pain).
The clinical examination was completed during a single visit by an orthopedic surgeon (Dr. Meadows or Dr. Diesselhorst) different from the primary surgeon (Dr. K.E. Swanson) and by a clinician-sonologist (Dr. Finnoff). The examination’s 3 parts were physical examination of arm, biceps supination strength test, and ultrasonographic evaluation.
Physical Examination of Arm. Physical examination included palpation of bicipital groove, range of motion (ROM) of shoulder and elbow, and clinical deformity of biceps. Patients were questioned regarding symptoms of AN damage, including sensory and motor findings. Bicipital groove tenderness was assessed with a visual analog scale rating pain 0 to 10. ROM was measured in degrees and was presented as a percentage of full elbow ROM (150°) and full shoulder ROM (180°).
Biceps Supination Strength Test. Biceps supination strength was tested with a baseline hydraulic wrist dynamometer with door handle attachment. Patients were seated with the elbow bent 90° and the forearm in a neutral position. In a series of 3 trials, the patient maintained grip of the dynamometer doorknob while supinating the forearm. The tenodesed (operated) arm and contralateral unaffected (nonoperated) arm were tested in random order and recorded in pounds.
Ultrasonographic Evaluation. Ultrasonography was used to evaluate the tenodesis site. In each case, the biceps tendon was assessed to determine the location of the bicortical button in relation to the AN/PCHA neurovascular bundle. Whereas nerves are difficult to visualize with ultrasonography, arteries are readily seen. Dr. Finnoff used a CX50 ultrasound machine (Philips Medical Systems) with either a 12-3 MHz linear array or a 5-1 MHz curvilinear array transducer to measure the shortest distance from the PCHA to the button.
Each patient was placed in a lateral decubitus or prone position, and the skin of the upper arm was exposed. Tendon integrity was deemed either intact (continuity between biceps tendon and cortical button) or disrupted (lack of continuity between tendon and cortical button). The transducer was then placed in an anatomical sagittal plane over the posterior aspect of the proximal humerus. Power Doppler and cephalad and caudad transducer glides were used to identify the location of the PCHA. The transducer was then glided laterally and anteriorly around the humerus, following the course of the PCHA, until the cortical button was located. The narrowest interval between the PCHA and the cortical button was measured using the ultrasound machine’s software. A still image of each measurement was saved.
Surgical Technique
Biceps tenodesis indications included high-demand heavy laborers, athletes, and patients who preferred the cosmetic results of tenodesis over tenotomy. Most patients had acute symptomatic tears of the superior labrum with instability of the biceps anchor complex. Others had fraying and tenosynovitis of the LHB tendon. Any associated pathology was addressed during the same surgical period.
The surgical technique used was similar to that described by Snir and colleagues.12 Each patient was placed in the lateral decubitus position. Once pathology confirmed biceps tenodesis, the biceps tendon was tenotomized at the base of the superior labrum. A 3-cm incision was made along the axillary fold centered over the inferior border of the pectoralis major tendon. Blunt dissection was performed to define the inferior border of the pectoralis major tendon and to palpate the underlying biceps tendon as it exited the intertubercular groove. The LHB tendon was removed and prepared with No. 2 Fiberwire (Arthrex) in Krackow fashion starting 2 cm proximal to the musculotendinous junction. The excess tendon was excised.
A 3.2-mm guide wire was centered along the most distal aspect of the biceps groove and then drilled through the anterior cortex and just through the posterior cortex. A cannulated reamer, selected on the basis of the biceps tendon diameter (typically, 5-7 mm), was then drilled over the guide wire through the anterior cortex only. The Food and Drug Administration–approved cortical button (BicepsButton; Arthrex) was then loaded by passing the tendon suture ends through each side of the button in alternating fashion, thus allowing the button to slide along the sutures.
The button was loaded onto the BicepsButton deployment device and inserted through the drilled tunnel of the anterior cortex and just through the posterior cortex. The deployment device was then removed, and 1 suture end was pulled to allow the button to engage the posterior humeral cortex. Pulling on both sutures allowed the biceps tendon to slide through the anterior cortex hole of the humerus until the tendon reached the posterior humeral cortex. Tension was verified, and the sutures were tied over the tendon. The wound was then irrigated and closed.
Rehabilitation Program
Patients completed a standard rehabilitation protocol for biceps tenodesis24 along with rehabilitation protocols for any additional procedures performed. In phase 1 (weeks 0-2), they focused on gradual restoration of passive ROM and remained in a sling. In phase 2 (weeks 2-6), they focused on gradual restoration of active ROM, and by week 3 were weaned out of the sling. In phase 3 (weeks 6-8), they continued ROM and strengthening exercises to normalize strength, endurance, and neuromuscular control. In phase 4 (weeks 8-12), they focused on advanced strengthening exercises and return to activities.
Statistical Analysis
Descriptive statistics included means, medians, and SDs. Comparisons between operated and nonoperated arms and between dominant and nondominant arms were performed by a statistician using paired t tests with P = .05. Confidence intervals were calculated for operated and nonoperated arms and for dominant and nondominant arms by using the differences between them.
Results
Functional Outcomes
Surgical outcome scores and pain scores were obtained from 24 patients (86%) at minimum 12-month follow-up. Mean (SD) DASH score was 15.15 (17.6; median, 9), and mean (median) pain score was 12.61 (7).
Eighteen patients (64%) completed the clinical examination: 16 men (88.9%) and 2 women (11.1%). Mean age was 48.3 years (age range, 33-59 years). Of these 18 patients, 9 (50%) had surgery on the dominant arm, and the other 9 had surgery on the nondominant arm. All patients were right-hand–dominant. In 3 patients, biceps tenodesis was performed with only minimal arthroscopic débridement (20%); in the other 15, biceps tenodesis was performed concomitantly with 1 or more additional arthroscopic procedures: acromioplasty (73%), rotator cuff repair (47%), distal clavicle resection (33%), subacromial bursectomy (13%), microfracture of glenoid (13%), and posterior labral repair (7%).
The clinical examination was performed a mean of 15.2 months (range, 12-26 months) after surgery. Physical examination findings are listed in Table 1.
Forearm supination strength, averaged from 3 trials on each arm, was significantly (P = .01) greater in the nonoperated arm than in the operated arm (Table 2, Figure 1). A 95% confidence interval for the mean (SD) difference in strength was 9.35 (7.76) pounds, meaning that on average, the nonoperated arm will be 1.59 to 17.11 pounds stronger than the operated arm. In addition, strength of the dominant arm was greater than that of the nondominant arm (P = .05) regardless of which arm underwent surgery (Table 2, Figure 1). However, the mean (SD) difference in strength was 6.94 (8.39) pounds, indicating the observed difference was not statistically significant.
Sonographic Evaluation
According to the sonographic evaluations, the tenodesis was intact in all 18 patients (Figure 2). Estimated mean (SD) distance from button to PCHA was 18.17 (9.0) mm (median, 16.1 mm; range, 9.4-48 mm) (Figure 2, Figure 3). No patient indicated any symptoms of AN damage.
Discussion
There are few studies of functional outcomes of biceps tenodesis. Pain is a common measure of patient satisfaction. Mazzocca and colleagues25 reported a mean follow-up pain score of 1.1 (range, 0.5-1.9) out of 10 for a group of 41 patients who had subpectoral tenodesis with an interference screw. Millett and colleagues26 reported a mean postoperative pain score of 2.5 out of 10 for patients who had subpectoral interference screw fixation. Our patients reported a mean pain score of 12.6 out of 100 after minimum 12-month follow-up. We also assessed for pain in the intertubercular groove during palpation. Although some studies have shown that groove pain was eliminated by subpectoral biceps tenodesis,5 3 patients in our study had pain on groove palpation. The cause of this residual pain is unclear, but some studies have suggested a chronic degenerative pathologic process that occurs while the tendon is within the biceps groove.27 Removing the tendon from the groove may not remove the underlying cause of pain.
Our patients’ mean DASH score was 15.15 (within the excellent range). Normative mean (SD) DASH score for the general population is 10.1 (14.68).28
Functional strength of forearm supination, shoulder ROM, and elbow ROM are objective measures of patient performance after fixation. On Cybex testing, Phillips and colleagues29 found no difference in forearm supination strength or elbow flexion (compared with contralateral arm) after biceps tenodesis or conservative treatment for proximal biceps ruptures. Shank and colleagues30 compared elbow flexion and supination strength of the affected and unaffected arms after suture anchor subpectoral biceps tenodesis. There was no significant difference in Cybex results, but there was a 14% to 15% loss of average strength in the tenodesed versus nonsurgical arm. In the present study, we found a significant difference in forearm supination strength between the operated and nonoperated arms, but with only a 7% loss of average strength in the operated arms. The difference in strength ranged from 1.59 to 17.11 pounds, which may not be clinically significant, as supination strength ranged from 60 to 270 pounds.
Of the 18 patients in this study, 9 had surgery on the dominant arm, and the other 9 had surgery on the nondominant arm. Examining the effect of arm dominance on results revealed that patients with surgery on the nondominant arm tended to have substantially reduced supination strength in that arm vs the dominant arm. There was an 11% loss of average strength for nondominant vs dominant arms that had surgery. Examining nondominant arms only revealed a 13% loss of strength for operated vs nonoperated arms. There was no difference in forearm supination strengths between nonoperated arms (dominant vs nondominant) or between dominant arms (operated vs nonoperated). This suggests that, though hand dominance may not play a significant role in control patients’ forearm supination strength,30 it may have a substantial effect on surgical patients’ ability to regain strength when the nondominant arm is the surgical arm. One objective of this study was to measure the distance between the biceps cortical button on the posterior humeral cortex and the AN/PCHA neurovascular bundle. The AN bundles with the PCHA posterior to the humeral neck.31-33 As the AN travels with the PCHA, and the PCHA has been reliably identified with Doppler ultrasonography,34-36 the PCHA was used as a marker for the AN in this study. Our bicortical button technique places the button on the posterior aspect of the humerus, making AN and PCHA the nearest at-risk neurovascular structures. None of our patients had symptoms of AN damage. However, 2 patients indicated pain in the posterior aspect of the humerus during deltoid activation. Distance from the neurovascular structures to the button was 48 mm in one patient and 13.6 mm in the other. DASH scores were 43 and 27, respectively. Both patients’ 1-year pain score was 30. The first patient underwent arthroscopic acromioplasty, distal clavicle resection, and microfracture of the glenoid surface in addition to the subpectoral biceps tenodesis; the second underwent subacromial decompression and distal clavicle resection in addition to the subpectoral biceps tenodesis. Whether the associated pathology contributed to their persistent pain is unknown. However, given the distance from AN/PCHA to button, it is unlikely that their pain was a result of neurovascular compromise from the procedure.
Advantages of the cortical button include the ability to drill a smaller hole in the humerus for fixation, compared with the hole drilled for an interference screw. Despite the biomechanical strength of the screw, large (8 mm) cortical violations have been associated with increased fracture risk of the proximal humerus.37,38 The tendon may experience less trauma than that caused by being twisted against an interference screw, the most common location of failure of which is the tendon–screw interface.39 In addition, tendon healing may be improved through circumferential healing in the cortical button tunnel.
A concern of using a bicortical button for fixation is drilling through the posterior cortex, because of the proximity of the posterior neurovascular structures. In a case in which the posterior cord was injured, Rhee and colleagues40 used a suture pullout technique whereby a Beath pin was passed out of the posterior humerus and soft tissues to then hold tension on the biceps tendon during the tenodesis. The radial nerve potentially could have been injured by pin overpenetration or by becoming wrapped up in the soft tissues as the pin was spinning through them. In our technique, the posterior humeral cortex is drilled cautiously to avoid overpenetration and possibly getting the posterior soft tissues wrapped up in the guide pin. No AN injuries have been reported with this technique. Mean distance from AN to posterior cortical button in this study was 18.17 mm. In 2 cadaver studies of bicortical drilling for subpectoral biceps tenodesis, the ANs were 25.1 mm and 36.7 mm from the posterior drill hole.41,21
Limitations of this study included its design (case series) and limited number of follow-up patients. Of the 28 consecutive patients identified for the study, 10 did not undergo the clinical examination, as they either lived more than 3 hours away (8 patients) or could not be contacted (2 patients). Another study limitation was the inability to directly image ANs with ultrasound. Therefore, measurements of the distance from the PCHA to the button were used to estimate the distance from the AN/PCHA neurovascular bundle to the button.
In this study, functional outcomes were excellent, and there were no tenodesis failures or neurovascular complications. These preliminary findings indicate that subpectoral biceps tenodesis with a bicortical button is a viable treatment option for patients with the appropriate indications for this procedure.
The long head of the biceps (LHB) tendon is a recognized source of shoulder pain. LHB tendon pathology is commonly associated with other shoulder conditions, such as superior labral tears, rotator cuff tears, or subacromial impingement, whereas isolated pathology, such as traumatic ruptures, tendinosis, or medial subluxation, is rare.1 Treatment of LHB pathology ranges from conservative measures to surgical measures, including tenotomy or tenodesis.2 LHB tenodesis offers the advantage of maintaining the length–tension relationship of the biceps muscle to prevent atrophy and avoid the Popeye deformity incurred from tenotomy alone. Tenodesis also prevents muscle cramping associated with contracted biceps muscle and better maintains elbow flexion and supination strength, which may be decreased with tenotomy.3 In addition, when a subpectoral biceps tenodesis technique is used, pain from LHB tendinopathy in the intertubercular groove may be reduced.4
Open subpectoral biceps tenodesis is a reproducible, efficient method for LHB tenodesis.4,5 A variety of fixation devices has been used: bone tunnels,6 keyhole fixation,7 suture anchors,6-9 and interference screws.6-8,10,11 More recently, a bicortical button has been used for LHB tendon tenodesis.12 Biomechanical studies have shown that load to failure is comparable for bicortical button fixation and interference screw fixation.13,14 In other models of tendon repair, the bicortical button has strength and stability comparable to those of interference screw fixation and enables earlier rehabilitation.15-17 However, there is concern that bicortical button fixation may result in axillary nerve (AN) or posterior circumflex humeral artery (PCHA) compromise because of the proximity of these neurovascular structures to the bicortical button.13,18-21
We conducted a study to functionally and sonographically assess the outcomes of patients who underwent open subpectoral biceps tenodesis with a bicortical button. Functional outcomes were assessed with patient-reported outcomes and physician-reported outcomes. Sonographic studies were used to evaluate the integrity of the tenodesis and determine the proximity of the button to the AN and the PCHA along the posterior proximal humerus.
Methods
After obtaining Institutional Review Board approval for this study, we retrospectively identified 28 consecutive patients who had proximal biceps tenodesis performed by a single surgeon (Dr. K.E. Swanson) using a mini-open subpectoral biceps tenodesis technique with a bicortical button between March 2011 and January 2013. All 28 patients were asked to participate in the study. Twenty-four (86%) agreed to complete 2 surgical outcome surveys, and 18 (64%) completed a 3-part clinical examination at minimum 12-month follow-up.
One of the surveys was Quick Disabilities of the Arm, Shoulder, and Hand (QuickDASH), a validated comprehensive disability survey that scores upper extremity functionality on a scale ranging from 0 (none) to 100 (extreme difficulty).22,23 The other survey scored pain on a scale ranging from 0 (none) to 100 (worst pain).
The clinical examination was completed during a single visit by an orthopedic surgeon (Dr. Meadows or Dr. Diesselhorst) different from the primary surgeon (Dr. K.E. Swanson) and by a clinician-sonologist (Dr. Finnoff). The examination’s 3 parts were physical examination of arm, biceps supination strength test, and ultrasonographic evaluation.
Physical Examination of Arm. Physical examination included palpation of bicipital groove, range of motion (ROM) of shoulder and elbow, and clinical deformity of biceps. Patients were questioned regarding symptoms of AN damage, including sensory and motor findings. Bicipital groove tenderness was assessed with a visual analog scale rating pain 0 to 10. ROM was measured in degrees and was presented as a percentage of full elbow ROM (150°) and full shoulder ROM (180°).
Biceps Supination Strength Test. Biceps supination strength was tested with a baseline hydraulic wrist dynamometer with door handle attachment. Patients were seated with the elbow bent 90° and the forearm in a neutral position. In a series of 3 trials, the patient maintained grip of the dynamometer doorknob while supinating the forearm. The tenodesed (operated) arm and contralateral unaffected (nonoperated) arm were tested in random order and recorded in pounds.
Ultrasonographic Evaluation. Ultrasonography was used to evaluate the tenodesis site. In each case, the biceps tendon was assessed to determine the location of the bicortical button in relation to the AN/PCHA neurovascular bundle. Whereas nerves are difficult to visualize with ultrasonography, arteries are readily seen. Dr. Finnoff used a CX50 ultrasound machine (Philips Medical Systems) with either a 12-3 MHz linear array or a 5-1 MHz curvilinear array transducer to measure the shortest distance from the PCHA to the button.
Each patient was placed in a lateral decubitus or prone position, and the skin of the upper arm was exposed. Tendon integrity was deemed either intact (continuity between biceps tendon and cortical button) or disrupted (lack of continuity between tendon and cortical button). The transducer was then placed in an anatomical sagittal plane over the posterior aspect of the proximal humerus. Power Doppler and cephalad and caudad transducer glides were used to identify the location of the PCHA. The transducer was then glided laterally and anteriorly around the humerus, following the course of the PCHA, until the cortical button was located. The narrowest interval between the PCHA and the cortical button was measured using the ultrasound machine’s software. A still image of each measurement was saved.
Surgical Technique
Biceps tenodesis indications included high-demand heavy laborers, athletes, and patients who preferred the cosmetic results of tenodesis over tenotomy. Most patients had acute symptomatic tears of the superior labrum with instability of the biceps anchor complex. Others had fraying and tenosynovitis of the LHB tendon. Any associated pathology was addressed during the same surgical period.
The surgical technique used was similar to that described by Snir and colleagues.12 Each patient was placed in the lateral decubitus position. Once pathology confirmed biceps tenodesis, the biceps tendon was tenotomized at the base of the superior labrum. A 3-cm incision was made along the axillary fold centered over the inferior border of the pectoralis major tendon. Blunt dissection was performed to define the inferior border of the pectoralis major tendon and to palpate the underlying biceps tendon as it exited the intertubercular groove. The LHB tendon was removed and prepared with No. 2 Fiberwire (Arthrex) in Krackow fashion starting 2 cm proximal to the musculotendinous junction. The excess tendon was excised.
A 3.2-mm guide wire was centered along the most distal aspect of the biceps groove and then drilled through the anterior cortex and just through the posterior cortex. A cannulated reamer, selected on the basis of the biceps tendon diameter (typically, 5-7 mm), was then drilled over the guide wire through the anterior cortex only. The Food and Drug Administration–approved cortical button (BicepsButton; Arthrex) was then loaded by passing the tendon suture ends through each side of the button in alternating fashion, thus allowing the button to slide along the sutures.
The button was loaded onto the BicepsButton deployment device and inserted through the drilled tunnel of the anterior cortex and just through the posterior cortex. The deployment device was then removed, and 1 suture end was pulled to allow the button to engage the posterior humeral cortex. Pulling on both sutures allowed the biceps tendon to slide through the anterior cortex hole of the humerus until the tendon reached the posterior humeral cortex. Tension was verified, and the sutures were tied over the tendon. The wound was then irrigated and closed.
Rehabilitation Program
Patients completed a standard rehabilitation protocol for biceps tenodesis24 along with rehabilitation protocols for any additional procedures performed. In phase 1 (weeks 0-2), they focused on gradual restoration of passive ROM and remained in a sling. In phase 2 (weeks 2-6), they focused on gradual restoration of active ROM, and by week 3 were weaned out of the sling. In phase 3 (weeks 6-8), they continued ROM and strengthening exercises to normalize strength, endurance, and neuromuscular control. In phase 4 (weeks 8-12), they focused on advanced strengthening exercises and return to activities.
Statistical Analysis
Descriptive statistics included means, medians, and SDs. Comparisons between operated and nonoperated arms and between dominant and nondominant arms were performed by a statistician using paired t tests with P = .05. Confidence intervals were calculated for operated and nonoperated arms and for dominant and nondominant arms by using the differences between them.
Results
Functional Outcomes
Surgical outcome scores and pain scores were obtained from 24 patients (86%) at minimum 12-month follow-up. Mean (SD) DASH score was 15.15 (17.6; median, 9), and mean (median) pain score was 12.61 (7).
Eighteen patients (64%) completed the clinical examination: 16 men (88.9%) and 2 women (11.1%). Mean age was 48.3 years (age range, 33-59 years). Of these 18 patients, 9 (50%) had surgery on the dominant arm, and the other 9 had surgery on the nondominant arm. All patients were right-hand–dominant. In 3 patients, biceps tenodesis was performed with only minimal arthroscopic débridement (20%); in the other 15, biceps tenodesis was performed concomitantly with 1 or more additional arthroscopic procedures: acromioplasty (73%), rotator cuff repair (47%), distal clavicle resection (33%), subacromial bursectomy (13%), microfracture of glenoid (13%), and posterior labral repair (7%).
The clinical examination was performed a mean of 15.2 months (range, 12-26 months) after surgery. Physical examination findings are listed in Table 1.
Forearm supination strength, averaged from 3 trials on each arm, was significantly (P = .01) greater in the nonoperated arm than in the operated arm (Table 2, Figure 1). A 95% confidence interval for the mean (SD) difference in strength was 9.35 (7.76) pounds, meaning that on average, the nonoperated arm will be 1.59 to 17.11 pounds stronger than the operated arm. In addition, strength of the dominant arm was greater than that of the nondominant arm (P = .05) regardless of which arm underwent surgery (Table 2, Figure 1). However, the mean (SD) difference in strength was 6.94 (8.39) pounds, indicating the observed difference was not statistically significant.
Sonographic Evaluation
According to the sonographic evaluations, the tenodesis was intact in all 18 patients (Figure 2). Estimated mean (SD) distance from button to PCHA was 18.17 (9.0) mm (median, 16.1 mm; range, 9.4-48 mm) (Figure 2, Figure 3). No patient indicated any symptoms of AN damage.
Discussion
There are few studies of functional outcomes of biceps tenodesis. Pain is a common measure of patient satisfaction. Mazzocca and colleagues25 reported a mean follow-up pain score of 1.1 (range, 0.5-1.9) out of 10 for a group of 41 patients who had subpectoral tenodesis with an interference screw. Millett and colleagues26 reported a mean postoperative pain score of 2.5 out of 10 for patients who had subpectoral interference screw fixation. Our patients reported a mean pain score of 12.6 out of 100 after minimum 12-month follow-up. We also assessed for pain in the intertubercular groove during palpation. Although some studies have shown that groove pain was eliminated by subpectoral biceps tenodesis,5 3 patients in our study had pain on groove palpation. The cause of this residual pain is unclear, but some studies have suggested a chronic degenerative pathologic process that occurs while the tendon is within the biceps groove.27 Removing the tendon from the groove may not remove the underlying cause of pain.
Our patients’ mean DASH score was 15.15 (within the excellent range). Normative mean (SD) DASH score for the general population is 10.1 (14.68).28
Functional strength of forearm supination, shoulder ROM, and elbow ROM are objective measures of patient performance after fixation. On Cybex testing, Phillips and colleagues29 found no difference in forearm supination strength or elbow flexion (compared with contralateral arm) after biceps tenodesis or conservative treatment for proximal biceps ruptures. Shank and colleagues30 compared elbow flexion and supination strength of the affected and unaffected arms after suture anchor subpectoral biceps tenodesis. There was no significant difference in Cybex results, but there was a 14% to 15% loss of average strength in the tenodesed versus nonsurgical arm. In the present study, we found a significant difference in forearm supination strength between the operated and nonoperated arms, but with only a 7% loss of average strength in the operated arms. The difference in strength ranged from 1.59 to 17.11 pounds, which may not be clinically significant, as supination strength ranged from 60 to 270 pounds.
Of the 18 patients in this study, 9 had surgery on the dominant arm, and the other 9 had surgery on the nondominant arm. Examining the effect of arm dominance on results revealed that patients with surgery on the nondominant arm tended to have substantially reduced supination strength in that arm vs the dominant arm. There was an 11% loss of average strength for nondominant vs dominant arms that had surgery. Examining nondominant arms only revealed a 13% loss of strength for operated vs nonoperated arms. There was no difference in forearm supination strengths between nonoperated arms (dominant vs nondominant) or between dominant arms (operated vs nonoperated). This suggests that, though hand dominance may not play a significant role in control patients’ forearm supination strength,30 it may have a substantial effect on surgical patients’ ability to regain strength when the nondominant arm is the surgical arm. One objective of this study was to measure the distance between the biceps cortical button on the posterior humeral cortex and the AN/PCHA neurovascular bundle. The AN bundles with the PCHA posterior to the humeral neck.31-33 As the AN travels with the PCHA, and the PCHA has been reliably identified with Doppler ultrasonography,34-36 the PCHA was used as a marker for the AN in this study. Our bicortical button technique places the button on the posterior aspect of the humerus, making AN and PCHA the nearest at-risk neurovascular structures. None of our patients had symptoms of AN damage. However, 2 patients indicated pain in the posterior aspect of the humerus during deltoid activation. Distance from the neurovascular structures to the button was 48 mm in one patient and 13.6 mm in the other. DASH scores were 43 and 27, respectively. Both patients’ 1-year pain score was 30. The first patient underwent arthroscopic acromioplasty, distal clavicle resection, and microfracture of the glenoid surface in addition to the subpectoral biceps tenodesis; the second underwent subacromial decompression and distal clavicle resection in addition to the subpectoral biceps tenodesis. Whether the associated pathology contributed to their persistent pain is unknown. However, given the distance from AN/PCHA to button, it is unlikely that their pain was a result of neurovascular compromise from the procedure.
Advantages of the cortical button include the ability to drill a smaller hole in the humerus for fixation, compared with the hole drilled for an interference screw. Despite the biomechanical strength of the screw, large (8 mm) cortical violations have been associated with increased fracture risk of the proximal humerus.37,38 The tendon may experience less trauma than that caused by being twisted against an interference screw, the most common location of failure of which is the tendon–screw interface.39 In addition, tendon healing may be improved through circumferential healing in the cortical button tunnel.
A concern of using a bicortical button for fixation is drilling through the posterior cortex, because of the proximity of the posterior neurovascular structures. In a case in which the posterior cord was injured, Rhee and colleagues40 used a suture pullout technique whereby a Beath pin was passed out of the posterior humerus and soft tissues to then hold tension on the biceps tendon during the tenodesis. The radial nerve potentially could have been injured by pin overpenetration or by becoming wrapped up in the soft tissues as the pin was spinning through them. In our technique, the posterior humeral cortex is drilled cautiously to avoid overpenetration and possibly getting the posterior soft tissues wrapped up in the guide pin. No AN injuries have been reported with this technique. Mean distance from AN to posterior cortical button in this study was 18.17 mm. In 2 cadaver studies of bicortical drilling for subpectoral biceps tenodesis, the ANs were 25.1 mm and 36.7 mm from the posterior drill hole.41,21
Limitations of this study included its design (case series) and limited number of follow-up patients. Of the 28 consecutive patients identified for the study, 10 did not undergo the clinical examination, as they either lived more than 3 hours away (8 patients) or could not be contacted (2 patients). Another study limitation was the inability to directly image ANs with ultrasound. Therefore, measurements of the distance from the PCHA to the button were used to estimate the distance from the AN/PCHA neurovascular bundle to the button.
In this study, functional outcomes were excellent, and there were no tenodesis failures or neurovascular complications. These preliminary findings indicate that subpectoral biceps tenodesis with a bicortical button is a viable treatment option for patients with the appropriate indications for this procedure.
1. Khazzam M, George MS, Churchill RS, Kuhn JE. Disorders of the long head of biceps tendon. J Shoulder Elbow Surg. 2012;21(1):136-145.
2. Geaney LE, Mazzocca AD. Biceps brachii tendon ruptures: a review of diagnosis and treatment of proximal and distal biceps tendon ruptures. Phys Sportsmed. 2010;38(2):117-125.
3. Kelly AM, Drakos MC, Fealy S, Taylor SA, O’Brien SJ. Arthroscopic release of the long head of the biceps tendon: functional outcome and clinical results. Am J Sports Med. 2005;33(2):208-213.
4. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc Rev. 2008;16(3):170-176.
5. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
6. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
7. Ozalay, M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
8. Golish RS, Caldwell PE, Miller MD, et al. Interference screw versus suture anchor fixation for subpectoral tenodesis of the proximal biceps tendon: a cadaveric study. Arthroscopy. 2008;24(10):1103-1108.
9. Richards DP, Burkhart SS. A biomechanical analysis of two biceps tenodesis fixation techniques. Arthroscopy. 2005;21(7):861-866.
10. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
11. Wolf RS, Zheng N, Weichel D. Long head biceps tenotomy versus tenodesis: a cadaveric biomechanical analysis. Arthroscopy. 2005;21(2):182-185.
12. Snir N, Hamula M, Wolfson T, Laible C, Sherman O. Long head of the biceps tenodesis with cortical button technique. Arthrosc Tech. 2013;2(2):e95-e97.
13. Arora AS, Singh A, Koonce RC. Biomechanical evaluation of a unicortical button versus interference screw for subpectoral biceps tenodesis. Arthroscopy. 2013;29(4):638-644.
14. Buchholz A, Martetschläger F, Siebenlist S, et al. Biomechanical comparison of intramedullary cortical button fixation and interference screw technique for subpectoral biceps tenodesis. Arthroscopy. 2013;29(5):845-853.
15. Bain GI, Prem H, Heptinstall RJ, Verhellen R, Paix D. Repair of distal biceps tendon rupture: a new technique using the Endobutton. J Shoulder Elbow Surg. 2000;9(2):120-126.
16. Greenberg JA. Endobutton repair of distal biceps tendon ruptures. J Hand Surg Am. 2009;34(8):1541-1548.
17. Heinzelmann AD, Savoie FH 3rd, Ramsey JR, Field LD, Mazzocca AD. A combined technique for distal biceps repair using a soft tissue button and biotenodesis interference screw. Am J Sports Med. 2009;37(5):989-994.
18. DeAngelis JP, Chen A, Wexler M, et al. Biomechanical characterization of unicortical button fixation: a novel technique for proximal subpectoral biceps tenodesis. Knee Surg Sports Traumatol Arthrosc. 2015;23(5):1434-1441.
19. Dickens JF, Kilcoyne KG, Tintle SM, Giuliani J, Schaefer RA, Rue JP. Subpectoral biceps tenodesis: an anatomic study and evaluation of at-risk structures. Am J Sports Med. 2012;40(10):2337-2341.
20. Sethi PM, Rajaram A, Beitzel K, Hackett TR, Chowaniec DM, Mazzocca AD. Biomechanical performance of subpectoral biceps tenodesis: a comparison of interference screw fixation, cortical button fixation, and interference screw diameter. J Shoulder Elbow Surg. 2013;22(4):451-457.
21. Sethi PM, Vadasdi K, Greene RT, Vitale MA, Duong M, Miller SR. Safety of open suprapectoral and subpectoral biceps tenodesis: an anatomic assessment of risk for neurologic injury. J Shoulder Elbow Surg. 2015;24(1):138-142.
22. Gummesson C, Ward MM, Atroshi I. The shortened Disabilities of the Arm, Shoulder and Hand questionnaire (QuickDASH): validity and reliability based on responses within the full-length DASH. BMC Musculoskelet Disord. 2006;7:44.
23. Schmidt CC, Brown BT, Sawardeker PJ, DeGravelle M Jr, Miller MC. Factors affecting supination strength after a distal biceps rupture. J Shoulder Elbow Surg. 2014;23(1):68-75.
24. Brotzman SB, Wilk KE, eds. Handbook of Orthopaedic Rehabilitation. Philadelphia, PA: Mosby Elsevier; 2007.
25. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
26. Millett PJ, Snaders B, Gobezie R, Braun S, Warner JP. Interference screw versus suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9(121):1-6.
27. Streit JJ, Shishani Y, Rodgers M, Gobezie R. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extra-articular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;6:63-70.
28. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208-215.
29. Phillips BB, Canale ST, Sisk TD, Stralka SW, Wyatt KP. Rupture of the proximal biceps tendon in middle-aged patients. Orthop Rev. 1993;22(3):349-353.
30. Shank JR, Singleton SB, Braun S, et al. A comparison of forearm supination and elbow flexion strength in patients with long head of the biceps tenotomy or tenodesis. Arthroscopy. 2011;27(1):9-16.
31. Apaydin N, Tubbs RS, Loukas M, Duparc F. Review of the surgical anatomy of the axillary nerve and the anatomic basis of its iatrogenic and traumatic injury. Surg Radiol Anat. 2010;32(3):193-201.
32. Johnson D. Pectoral girdle and upper limp. In: Standring S, ed. Gray’s Anatomy. 40th ed. New York, NY: Elsevier; 2008:814-821.
33. Tubbs RS, Tyler-Kabara EC, Aikens AC, et al. Surgical anatomy of the axillary nerve within the quadrangular space. J Neurosurg. 2005;102(5):912-914.
34. Kim YA, Yoon KB, Kwon TD, Kim DH, Yoon DM. Evaluation of anatomic landmarks for axillary nerve block in the quadrilateral space. Acta Anaesthesiol Scand. 2014;58(5):567-571.
35. Robinson DJ, Marks P, Schneider-Kolsky ME. Ultrasound of the posterior circumflex humeral artery. J Med Imaging Radiat Oncol. 2010;54(3):219-223.
36. Rothe C, Asghar S, Andersen HL, Christensen JK, Lange KH. Ultrasound-guided block of the axillary nerve: a volunteer study of a new method. Acta Anaesthesiol Scand. 2011;55(5):565-570.
37. Reiff SN, Nho SJ, Romeo AA. Proximal humerus fracture after keyhole biceps tenodesis. Am J Orthop. 2010;39(7):E61-E63.
38. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
39. Koch BS, Burks RT. Failure of biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(5):735-740.
40. Rhee PC, Spinner RJ, Bishop AT, Shin AY. Iatrogenic brachial plexus injuries associated with open subpectoral biceps tenodesis. Am J Sports Med. 2013;41(9):2048-2053.
41. Ding DY, Gupta A, Snir N, Wolfson T, Meislin RJ. Nerve proximity during bicortical drilling for subpectoral biceps tenodesis: a cadaveric study. Arthroscopy. 2014;30(8):942-946.
1. Khazzam M, George MS, Churchill RS, Kuhn JE. Disorders of the long head of biceps tendon. J Shoulder Elbow Surg. 2012;21(1):136-145.
2. Geaney LE, Mazzocca AD. Biceps brachii tendon ruptures: a review of diagnosis and treatment of proximal and distal biceps tendon ruptures. Phys Sportsmed. 2010;38(2):117-125.
3. Kelly AM, Drakos MC, Fealy S, Taylor SA, O’Brien SJ. Arthroscopic release of the long head of the biceps tendon: functional outcome and clinical results. Am J Sports Med. 2005;33(2):208-213.
4. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc Rev. 2008;16(3):170-176.
5. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
6. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
7. Ozalay, M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
8. Golish RS, Caldwell PE, Miller MD, et al. Interference screw versus suture anchor fixation for subpectoral tenodesis of the proximal biceps tendon: a cadaveric study. Arthroscopy. 2008;24(10):1103-1108.
9. Richards DP, Burkhart SS. A biomechanical analysis of two biceps tenodesis fixation techniques. Arthroscopy. 2005;21(7):861-866.
10. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
11. Wolf RS, Zheng N, Weichel D. Long head biceps tenotomy versus tenodesis: a cadaveric biomechanical analysis. Arthroscopy. 2005;21(2):182-185.
12. Snir N, Hamula M, Wolfson T, Laible C, Sherman O. Long head of the biceps tenodesis with cortical button technique. Arthrosc Tech. 2013;2(2):e95-e97.
13. Arora AS, Singh A, Koonce RC. Biomechanical evaluation of a unicortical button versus interference screw for subpectoral biceps tenodesis. Arthroscopy. 2013;29(4):638-644.
14. Buchholz A, Martetschläger F, Siebenlist S, et al. Biomechanical comparison of intramedullary cortical button fixation and interference screw technique for subpectoral biceps tenodesis. Arthroscopy. 2013;29(5):845-853.
15. Bain GI, Prem H, Heptinstall RJ, Verhellen R, Paix D. Repair of distal biceps tendon rupture: a new technique using the Endobutton. J Shoulder Elbow Surg. 2000;9(2):120-126.
16. Greenberg JA. Endobutton repair of distal biceps tendon ruptures. J Hand Surg Am. 2009;34(8):1541-1548.
17. Heinzelmann AD, Savoie FH 3rd, Ramsey JR, Field LD, Mazzocca AD. A combined technique for distal biceps repair using a soft tissue button and biotenodesis interference screw. Am J Sports Med. 2009;37(5):989-994.
18. DeAngelis JP, Chen A, Wexler M, et al. Biomechanical characterization of unicortical button fixation: a novel technique for proximal subpectoral biceps tenodesis. Knee Surg Sports Traumatol Arthrosc. 2015;23(5):1434-1441.
19. Dickens JF, Kilcoyne KG, Tintle SM, Giuliani J, Schaefer RA, Rue JP. Subpectoral biceps tenodesis: an anatomic study and evaluation of at-risk structures. Am J Sports Med. 2012;40(10):2337-2341.
20. Sethi PM, Rajaram A, Beitzel K, Hackett TR, Chowaniec DM, Mazzocca AD. Biomechanical performance of subpectoral biceps tenodesis: a comparison of interference screw fixation, cortical button fixation, and interference screw diameter. J Shoulder Elbow Surg. 2013;22(4):451-457.
21. Sethi PM, Vadasdi K, Greene RT, Vitale MA, Duong M, Miller SR. Safety of open suprapectoral and subpectoral biceps tenodesis: an anatomic assessment of risk for neurologic injury. J Shoulder Elbow Surg. 2015;24(1):138-142.
22. Gummesson C, Ward MM, Atroshi I. The shortened Disabilities of the Arm, Shoulder and Hand questionnaire (QuickDASH): validity and reliability based on responses within the full-length DASH. BMC Musculoskelet Disord. 2006;7:44.
23. Schmidt CC, Brown BT, Sawardeker PJ, DeGravelle M Jr, Miller MC. Factors affecting supination strength after a distal biceps rupture. J Shoulder Elbow Surg. 2014;23(1):68-75.
24. Brotzman SB, Wilk KE, eds. Handbook of Orthopaedic Rehabilitation. Philadelphia, PA: Mosby Elsevier; 2007.
25. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
26. Millett PJ, Snaders B, Gobezie R, Braun S, Warner JP. Interference screw versus suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9(121):1-6.
27. Streit JJ, Shishani Y, Rodgers M, Gobezie R. Tendinopathy of the long head of the biceps tendon: histopathologic analysis of the extra-articular biceps tendon and tenosynovium. Open Access J Sports Med. 2015;6:63-70.
28. Hunsaker FG, Cioffi DA, Amadio PC, Wright JG, Caughlin B. The American Academy of Orthopaedic Surgeons outcomes instruments: normative values from the general population. J Bone Joint Surg Am. 2002;84(2):208-215.
29. Phillips BB, Canale ST, Sisk TD, Stralka SW, Wyatt KP. Rupture of the proximal biceps tendon in middle-aged patients. Orthop Rev. 1993;22(3):349-353.
30. Shank JR, Singleton SB, Braun S, et al. A comparison of forearm supination and elbow flexion strength in patients with long head of the biceps tenotomy or tenodesis. Arthroscopy. 2011;27(1):9-16.
31. Apaydin N, Tubbs RS, Loukas M, Duparc F. Review of the surgical anatomy of the axillary nerve and the anatomic basis of its iatrogenic and traumatic injury. Surg Radiol Anat. 2010;32(3):193-201.
32. Johnson D. Pectoral girdle and upper limp. In: Standring S, ed. Gray’s Anatomy. 40th ed. New York, NY: Elsevier; 2008:814-821.
33. Tubbs RS, Tyler-Kabara EC, Aikens AC, et al. Surgical anatomy of the axillary nerve within the quadrangular space. J Neurosurg. 2005;102(5):912-914.
34. Kim YA, Yoon KB, Kwon TD, Kim DH, Yoon DM. Evaluation of anatomic landmarks for axillary nerve block in the quadrilateral space. Acta Anaesthesiol Scand. 2014;58(5):567-571.
35. Robinson DJ, Marks P, Schneider-Kolsky ME. Ultrasound of the posterior circumflex humeral artery. J Med Imaging Radiat Oncol. 2010;54(3):219-223.
36. Rothe C, Asghar S, Andersen HL, Christensen JK, Lange KH. Ultrasound-guided block of the axillary nerve: a volunteer study of a new method. Acta Anaesthesiol Scand. 2011;55(5):565-570.
37. Reiff SN, Nho SJ, Romeo AA. Proximal humerus fracture after keyhole biceps tenodesis. Am J Orthop. 2010;39(7):E61-E63.
38. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
39. Koch BS, Burks RT. Failure of biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(5):735-740.
40. Rhee PC, Spinner RJ, Bishop AT, Shin AY. Iatrogenic brachial plexus injuries associated with open subpectoral biceps tenodesis. Am J Sports Med. 2013;41(9):2048-2053.
41. Ding DY, Gupta A, Snir N, Wolfson T, Meislin RJ. Nerve proximity during bicortical drilling for subpectoral biceps tenodesis: a cadaveric study. Arthroscopy. 2014;30(8):942-946.
Biomechanical Evaluation of Two Arthroscopic Biceps Tenodesis Techniques: Proximal Interference Screw and Modified Percutaneous Intra-Articular Transtendon
Over the years, operative treatment of biceps pathology has escalated, likely secondary to increased identification and successful clinical outcomes. Although its true function remains controversial, the biceps tendon has been well accepted as a primary pain generator in the anterior aspect of the shoulder.1,2 Biceps pathology involves a spectrum of often overlapping findings—varying degrees of tearing, tendinitis, and instability. Pathology may be isolated or may present in association with other shoulder conditions, including impingement, bursitis, rotator cuff tears, SLAP (superior labral tear anterior to posterior) lesions, and acromioclavicular disorders.3
Operative treatment of disease of the long head of the biceps mandates an initial choice of tenotomy or tenodesis. Which approach is superior is controversial.4-6 Although tenotomy and tenodesis have comparably favorable clinical results, tenodesis is often recommended, particularly for younger, active patients, mostly because cosmetic deformity is possible with tenotomy.
Tenodesis may be performed arthroscopically or through an open incision, and the biceps tendon may be placed anywhere from in the joint to under the tendon of the pectoralis major tendon. In many recent biomechanical studies, interference screws had higher load to failure and improved stiffness in comparison with other fixation methods.7-19 Most of those studies focused on fixation in a subpectoral location. To our knowledge, only 2 studies of soft-tissue fixation have compared the percutaneous intra-articular transtendon (PITT) technique with other popular tenodesis techniques.20,21 The PITT technique demonstrated a common failure point, with sutures pulling through the tendon substance. It was hypothesized that adding a locking loop to the PITT suture configuration would further improve fixation.
We conducted a study to compare the biomechanical characteristics of 2 techniques for all-arthroscopic proximal biceps tenodesis: bioabsorbable interference screw (Biceptor; Smith & Nephew) and a locking-loop PITT modification developed at our institution.
Methods
Sixteen nonembalmed fresh-frozen human cadaveric shoulders (8 pairs: 3 male, 5 female) were used in this study. Mean specimen age was 55 years (range, 51-59 years). The specimens showed no evidence of high-grade osteoarthritic changes, biceps tendon fraying or tearing, biceps pulley lesions, or full-thickness rotator cuff tears. They were thawed at room temperature for 24 hours before the procedure.
In each pair, 1 shoulder was randomized to be treated with 1 of 2 arthroscopic biceps tenodesis techniques—modified PITT or Biceptor interference screw—and the other shoulder was treated with the other technique. Surgery was performed in an open fashion, and every attempt was made to simulate the arthroscopic approach. In all shoulders, biomechanical testing was completed immediately after tenodesis.
Modified PITT Technique
In an outside-in fashion, an 18-gauge spinal needle was used to pierce the transverse humeral ligament, the lateral aspect of the rotator interval tissue, and the biceps tendon. A second needle was then passed in similar fashion, piercing the biceps tendon just adjacent to the first needle (Figure 1A). A 0-polydioxanone monofilament suture (0-PDS; Ethicon, Johnson & Johnson) was threaded through the first needle and used to shuttle a single No. 2 braided nonabsorbable polyethylene suture (MaxBraid; Biomet Sports Medicine) back through the biceps tendon.
At this point, the free end of the nonabsorbable suture, which comes out of the anterior cannula during an arthroscopic procedure, was passed back into the glenohumeral joint (using a suture grasper), looped over the top of the biceps tendon, and brought back out of the joint anteriorly, thereby creating a locking loop around the tendon (Figure 1B). A shuttle suture (0-PDS) passed through the second needle was used to bring that anterior limb of nonabsorbable suture back through the biceps tendon, completing the stitch configuration (Figure 1C).
This process was repeated with another nonabsorbable suture. After suture passing was completed, the biceps was detached from its insertion at the superior labrum. The 2 nonabsorbable sutures, which would later be retrieved from the subacromial space, were then tied in standard fashion, securing the biceps tendon to the transverse humeral ligament/rotator interval tissue (Figure 1D).
Biceptor Interference Screw Technique
The interference screw technique was performed in accordance with the manufacturer’s operative instructions.22 An 8 × 25-mm polyetheretherketone interference screw was used in all specimens, and the medium tendon fork was used to maintain tension on the biceps tendon during fixation (Figure 2A).
A 2.4-mm guide wire was inserted perpendicular to the humeral shaft, at the planned site of tenodesis, 10 mm distal to the entrance of the bicipital groove. An 8-mm cannulated reamer was passed over the wire, and a 30-mm tunnel was drilled (Figure 2B). The proximal part of the tendon was advanced into the center of the tunnel using the tendon fork (Figure 2C), and the tendon was held at the bottom of the tunnel with a 1.5-mm guide pin. The tendon fork was removed, and the cannulated interference screw was inserted over the guide pin between the 2 limbs of the biceps tendon (Figure 2D). The tendon was closely monitored to ensure it was not wrapped up when the screw was placed.
Biomechanical Testing
After each tenodesis, the humerus was amputated 5 inches distal to the fixation site. All extraneous soft tissue was dissected away, leaving the distal aspect of the biceps tendon as a free graft. Each proximal humerus–biceps tendon construct was then mounted on a materials testing machine. A custom-designed soft-tissue clamp was used to secure the distal aspect of the biceps tendon to the test actuator and load cell (Figure 3A). A custom-designed jig was used to stabilize the proximal humerus to the platform of the materials testing machine (Figure 3B). The specimens were mounted so that the line of pull throughout the testing protocol was applied parallel to the long axis of the humerus, thereby approximating the in vivo biceps force vector (Figure 3C). Digital cameras recorded each test for analysis of the mechanism of failure for each specimen. Marker dots were drawn on each tendon to assess tendon stretch before construct failure.
The tendons were preloaded to 10 N and then cycled at 0 to 50 N for 100 cycles at 1 Hz. After cyclic loading, axial load to failure was performed at a rate of 1.0 mm per second until a peak load was observed and subsequent loading led to tendon elongation with no further increase in load. Displacement and force applied during cyclic loading and load to failure were recorded. Stiffness was then calculated as the slope of the linear portion of the force-displacement curve using the least mean squares approach. Mechanism of failure was documented for each specimen.
Statistical Analysis
Analyzing our preliminary data with G*Power, we determined that a total sample size of 8 would be required (effect size [Cohen dz] was 1, α error probability was .05, power was .8). We hypothesized that the ultimate strength and stiffness of one group would be less than 1 SD above those of the other group. Paired t test with significance set at P < .05 was used to compare the techniques.
Results
Both repair constructs exhibited standard load-displacement curves, with a linear increase in load with displacement until the point of failure, at which time further displacement occurred with no discernible increase in load (Figure 4). Ultimate load to failure is determined as the highest point of the curve, and stiffness is calculated as the slope of the load-displacement curve.
Mean axial displacement parallel to the shaft of the humerus with cyclic loading was 7.1 mm with the modified PITT technique and 7.9 mm with the interference screw technique. There was no macroscopically visible high-grade tearing or slippage of the biceps tendon in any specimen with cyclic loading for either repair construct.
Mean (SD) ultimate load to failure was significantly (P = .003) higher with the modified PITT technique, 157 (41) N, than with the interference screw technique, 107 (29) N; actual effect size (Cohen dz) was 1.19, difference of means was 50 N, and pooled SD was 42 N. The interference screw technique yielded significantly (P = .010) more mean (SD) stiffness, 38.7 (14.7) N/mm, than the modified PITT technique, 15.8 (9.1) N/mm; actual effect size (Cohen dz) was 1.37, difference of means was 22.9 N/mm, and pooled SD was 16.7 N/mm.
In the interference screw technique, the mode of failure was consistent. Of the 8 specimens, 7 failed at the screw–tendon interface at the distal aspect of the tunnel; in the eighth specimen, the entire tendon pulled out from under the screw construct. In the modified PITT technique, there was more variability in failure: tendon slipped through suture (4 specimens), tendon/suture construct as unit pulled through transverse ligament/rotator interval tissue (3), and suture failure (1).
Discussion
This study was the first to directly compare a bony interference screw technique with a soft-tissue technique (modified PITT). Fixation strength is crucial. A load of 112 N is applied to the long head of the biceps tendon when a person holds 1 kg of weight in the hand with the elbow at 90° flexion.22 As mean (SD) ultimate load to failure was 157 (41) N with the modified PITT technique and 107 (29) N with the interference screw technique in this study, the interference screw can be recommended only with some hesitation.
The interference screw was stiffer with cyclic loading—an expected outcome, as it was secured to rigid bone—vs soft tissue, as in the modified PITT technique. Although the clinical implications for the modified PITT technique are unknown, more than likely, with the tendon being secured to soft tissue, there will be scarring over time.
In laboratory testing of biceps tenodesis constructs, interference screw fixation has had superior load-to-failure characteristics in comparisons with other fixation methods. Golish and colleagues13 found significantly higher load to failure with a biotenodesis screw than with a double-loaded suture anchor for subpectoral tenodesis. Testing similar implants, in a location more proximal in the bicipital groove, Richards and Burkhart14 likewise found superior fixation strength with an interference screw. Ozalay and colleagues16 found superior strength in an interference screw compared with suture anchor, keyhole, and bone tunnel in sheep. In a pig model, the highest ultimate load to failure was found in an interference screw—vs keyhole, bone tunnel, suture anchor, and ligament washer.19 Load to failure for the interference screw in these studies ranged from 170 N to over 400 N.13,14,16,19
A few other investigators have studied the Biceptor interference screw. Slabaugh and colleagues15 found a mean (SD) load to failure of 173.9 (27.2) N for all specimens tested. Patzer and colleagues9,17 found that the mean (SD) ultimate load to failure with the Biceptor proximal interference screw, 173.9 (27.2) N, was superior to that of a suture anchor.
The mean (SD) ultimate load to failure reported for the Biceptor interference screw in the present study, 107 (29) N, is lower than the values reported in the other studies—not only for the Biceptor screw but for interference screws in general. Nevertheless, we performed the technique as the manufacturer recommended.22 Our results were consistent across all specimens studied. Interestingly, in the study by Slabaugh and colleagues,15 7 specimens failed at the tendon–screw interface during cyclic testing and were not included in the analysis of ultimate load to failure. As these specimens failed at a load between 5 N and 70 N, including their data would have significantly lowered the mean load to failure.
Concern over the Biceptor interference screw’s lower failure load relative to that of other interference screws has been raised before.9,17 A major issue is possible overstuffing of the humeral tunnel, as the hole is reamed the same size as the screw. With the Biceptor, the proximal and distal portions of the tendon are placed in the tunnel in a U-shaped configuration with the screw between these limbs. The idea is that the 2 biceps tendon limbs might become abraded and consecutively weaken as the screw is inserted between the tendon limbs, more so than with a single loop. This idea was suggested by the typical longitudinal tendon splitting that occurs at the screw–tendon interface at the distal aspect of the tunnel.23 In the present study, consistent failure (Figures 5A-5C) at the distal aspect of the screw–tendon junction supported the idea that the tendon is abraded during placement of the interference screw or during the friction-causing 90° turn the tendon takes into the bone on loading. There is no way to quantitatively examine tendon quality before interference screw placement, but on gross inspection all the tendons were of good quality. Slabaugh and colleagues15 also found consistent failure at the screw–tunnel interface.
The PITT technique has been described as a simple all-arthroscopic soft-tissue technique for biceps tenodesis.23,24 Subsequently developed soft-tissue techniques have demonstrated clinical benefits.25-27 Proposed advantages of these techniques are lower cost associated with decreased implant needs, no reliance on quality of bone for fixation, suturing while biceps tendon is still attached to anchor (anatomical tension is closely reproduced), and less interference with any subsequent use of magnetic resonance imaging for diagnostic purposes in the shoulder.
There have been only 2 biomechanical studies of the PITT technique. Lopez-Vidriero and colleagues20 compared the biomechanical properties of the PITT and suture anchor techniques in a human cadaveric laboratory study and found that the PITT technique had mean (SD) ultimate load to failure of 142.7 (30.9) N and mean (SD) stiffness of 13.3 (3) N/mm. They observed consistent suture pullout through the tendon substance during failure, which suggests the most important factor for strength is the quality of the biceps tendon. Su and colleagues21 found biomechanically inferior results of the classic PITT technique as compared with the interference screw technique.
This article provides the first description of the modified PITT technique. Our mean (SD) load to failure of the modified PITT technique was 157 (41) N, slightly higher than that reported for the classic PITT technique, albeit under a different setup.20 There was more variation in ultimate load to failure in our study than in previous studies, which could be secondary to tissue quality. As the modified PITT technique relies on surrounding tissue holding the biceps in place, this tissue would need to be of good quality and strength to obtain strong fixation. A possible concern is that placing stitches in the rotator interval could increase the risk of shoulder stiffness, but this has not been encountered clinically.
A more variable mechanism of failure was also found in the present study. Although half the specimens failed by suture pullout through the tendon, similar to what Lopez-Vidriero and colleagues20 described, 3 of our 8 specimens failed with the entire biceps tendon–suture construct pulling through the transverse ligament tissue, and 1 specimen failed by suture breakage. Although these numbers are too small for making definitive statements, our modified PITT technique may add some security to the tendon–suture construct. Such added security may be of particular value in the setting of poor-quality, diseased tendon tissue, and the construct may be more limited by the strength of surrounding tissues. In addition, if failure occurs at the suture transverse humeral ligament–rotator interval interface, more surrounding rotator interval tissue can be incorporated into the tenodesis to decrease the likelihood of failure through this mechanism.
This study had several limitations. First, it was a time zero study in a cadaveric model with simulated biomechanical loading. As such, it provided information only on initial fixation strength and could not prove any superior clinical outcomes or account for any biological changes with healing that occurred over time. Second, the study may have been underpowered, though sample size was chosen in accordance with other cadaveric biomechanical studies. Third, all procedures were performed in an open manner, simulating the arthroscopic approach. Particularly in the setting of the modified PITT technique, this represented a best case scenario. Spinal needles and subsequent sutures were easily passed under direct visualization through the transverse humeral ligament, rotator interval, and biceps tendon. There is likely marked variability in this step during arthroscopy in which visualization is more limited, as in the setting of concomitant procedures, such as subacromial decompression or rotator cuff repair. In addition, all tendons tested were normal in appearance and gave no indication of chronic degenerative changes.
Another study limitation is that we did not quantify bone mineral density, which if poor would have affected interference screw strength. However, mean specimen age was 55 years, minimizing chances of poor bone quality. In addition, 7 of the 8 failures in the interference screw group occurred not with pullout but at the screw–tendon junction, suggesting poor bone quality was not a significant factor. As tendon diameter was not measured before the procedures were performed, there is the possibility it could have been better in the modified PITT group and worse in the interference screw group because of tunnel crowding, as noted.
1. Alpantaki K, McLaughlin D, Karagogeos D, Hadjipavlou A, Kontakis G. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J Bone Joint Surg Am. 2005;87(7):1580-1583.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Khazzam M, George MS, Churchill RS, Kuhn JE. Disorders of the long head of biceps tendon. J Shoulder Elbow Surg. 2012;21(1):136-145.
4. Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of pathologic lesions of the tendon of the long head of the biceps brachii. Am J Sports Med. 2009;37(4):828-833.
5. Hsu AR, Ghodadra NS, Provencher MT, Lewis PB, Bach BR. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20(2):326-332.
6. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
7. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
8. Millett PJ, Sanders B, Gobezie R, Braun S, Warner JJ. Interference screw vs. suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9:121.
9. Patzer T, Rundic JM, Bobrowitsch E, Olender GD, Hurschler C, Schofer MD. Biomechanical comparison of arthroscopically performable techniques for suprapectoral biceps tenodesis. Arthroscopy. 2011;27(8):1036-1047.
10. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
11. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
12. Klepps S, Hazrati Y, Flatow E. Arthroscopic biceps tenodesis. Arthroscopy. 2002;18(9):1040-1045.
13. Golish SR, Caldwell PE 3rd, Miller MD, et al. Interference screw versus suture anchor fixation for subpectoral tenodesis of the proximal biceps tendon: a cadaveric study. Arthroscopy. 2008;24(10):1103-1108.
14. Richards DP, Burkhart SS. A biomechanical analysis of two biceps tenodesis fixation techniques. Arthroscopy. 2005;21(7):861-866.
15. Slabaugh MA, Frank RM, Van Thiel GS, et al. Biceps tenodesis with interference screw fixation: a biomechanical comparison of screw length and diameter. Arthroscopy. 2011;27(2):161-166.
16. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
17. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
18. Jayamoorthy T, Field JR, Costi JJ, Martin DK, Stanley RM, Hearn TC. Biceps tenodesis: a biomechanical study of fixation methods. J Shoulder Elbow Surg. 2004;13(2):160-164.
19. Kusma M, Dienst M, Eckert J, Steimer O, Kohn D. Tenodesis of the long head of biceps brachii: cyclic testing of five methods of fixation in a porcine model. J Shoulder Elbow Surg. 2008;17(6):967-973.
20. Lopez-Vidriero E, Costic RS, Fu FH, Rodosky MW. Biomechanical evaluation of 2 arthroscopic biceps tenodeses: double anchor versus percutaneous intra-articular transtendon (PITT) techniques. Am J Sports Med. 2010;38(1):146-152.
21. Su WR, Budoff JE, Chiang CH, Lee CJ, Lin CL. Biomechanical study comparing biceps wedge tenodesis with other proximal long head of the biceps tenodesis techniques. Arthroscopy. 2013;29(9):1498-1505.
22. Trenhaile SW. Biceptor Tenodesis System, Arthroscopic Biceps Tenodesis [operational instructions]. Andover, MA: Smith & Nephew; 2009:1-8.
23. Sekiya LC, Elkousy HA, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous intra-articular transtendon technique. Arthroscopy. 2003;19(10):1137-1141.
24. Elkousy HA, Fluhme DJ, O’Connor DP, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous, intra-articular trans-tendon technique: preliminary results. Orthopedics. 2005;28(11):1316-1319.
25. Castagna A, Conti M, Mouhsine E, Bungaro P, Garofalo R. Arthroscopic biceps tendon tenodesis; the anchorage technical note. Knee Surg Sports Traumatol Arthrosc. 2006;14(6):581-585.
26. Checchia SL, Doneux PS, Miyazaki AN, et al. Biceps tenodesis associated with arthroscopic repair of rotator cuff tears. J Shoulder Elbow Surg. 2005;14(2):138-144.
27. Moros C, Levine WN, Ahmad CS. Suture anchor and percutaneous intra-articular transtendon biceps tenodesis. Sports Med Arthrosc. 2008;16(3):177-179.
Over the years, operative treatment of biceps pathology has escalated, likely secondary to increased identification and successful clinical outcomes. Although its true function remains controversial, the biceps tendon has been well accepted as a primary pain generator in the anterior aspect of the shoulder.1,2 Biceps pathology involves a spectrum of often overlapping findings—varying degrees of tearing, tendinitis, and instability. Pathology may be isolated or may present in association with other shoulder conditions, including impingement, bursitis, rotator cuff tears, SLAP (superior labral tear anterior to posterior) lesions, and acromioclavicular disorders.3
Operative treatment of disease of the long head of the biceps mandates an initial choice of tenotomy or tenodesis. Which approach is superior is controversial.4-6 Although tenotomy and tenodesis have comparably favorable clinical results, tenodesis is often recommended, particularly for younger, active patients, mostly because cosmetic deformity is possible with tenotomy.
Tenodesis may be performed arthroscopically or through an open incision, and the biceps tendon may be placed anywhere from in the joint to under the tendon of the pectoralis major tendon. In many recent biomechanical studies, interference screws had higher load to failure and improved stiffness in comparison with other fixation methods.7-19 Most of those studies focused on fixation in a subpectoral location. To our knowledge, only 2 studies of soft-tissue fixation have compared the percutaneous intra-articular transtendon (PITT) technique with other popular tenodesis techniques.20,21 The PITT technique demonstrated a common failure point, with sutures pulling through the tendon substance. It was hypothesized that adding a locking loop to the PITT suture configuration would further improve fixation.
We conducted a study to compare the biomechanical characteristics of 2 techniques for all-arthroscopic proximal biceps tenodesis: bioabsorbable interference screw (Biceptor; Smith & Nephew) and a locking-loop PITT modification developed at our institution.
Methods
Sixteen nonembalmed fresh-frozen human cadaveric shoulders (8 pairs: 3 male, 5 female) were used in this study. Mean specimen age was 55 years (range, 51-59 years). The specimens showed no evidence of high-grade osteoarthritic changes, biceps tendon fraying or tearing, biceps pulley lesions, or full-thickness rotator cuff tears. They were thawed at room temperature for 24 hours before the procedure.
In each pair, 1 shoulder was randomized to be treated with 1 of 2 arthroscopic biceps tenodesis techniques—modified PITT or Biceptor interference screw—and the other shoulder was treated with the other technique. Surgery was performed in an open fashion, and every attempt was made to simulate the arthroscopic approach. In all shoulders, biomechanical testing was completed immediately after tenodesis.
Modified PITT Technique
In an outside-in fashion, an 18-gauge spinal needle was used to pierce the transverse humeral ligament, the lateral aspect of the rotator interval tissue, and the biceps tendon. A second needle was then passed in similar fashion, piercing the biceps tendon just adjacent to the first needle (Figure 1A). A 0-polydioxanone monofilament suture (0-PDS; Ethicon, Johnson & Johnson) was threaded through the first needle and used to shuttle a single No. 2 braided nonabsorbable polyethylene suture (MaxBraid; Biomet Sports Medicine) back through the biceps tendon.
At this point, the free end of the nonabsorbable suture, which comes out of the anterior cannula during an arthroscopic procedure, was passed back into the glenohumeral joint (using a suture grasper), looped over the top of the biceps tendon, and brought back out of the joint anteriorly, thereby creating a locking loop around the tendon (Figure 1B). A shuttle suture (0-PDS) passed through the second needle was used to bring that anterior limb of nonabsorbable suture back through the biceps tendon, completing the stitch configuration (Figure 1C).
This process was repeated with another nonabsorbable suture. After suture passing was completed, the biceps was detached from its insertion at the superior labrum. The 2 nonabsorbable sutures, which would later be retrieved from the subacromial space, were then tied in standard fashion, securing the biceps tendon to the transverse humeral ligament/rotator interval tissue (Figure 1D).
Biceptor Interference Screw Technique
The interference screw technique was performed in accordance with the manufacturer’s operative instructions.22 An 8 × 25-mm polyetheretherketone interference screw was used in all specimens, and the medium tendon fork was used to maintain tension on the biceps tendon during fixation (Figure 2A).
A 2.4-mm guide wire was inserted perpendicular to the humeral shaft, at the planned site of tenodesis, 10 mm distal to the entrance of the bicipital groove. An 8-mm cannulated reamer was passed over the wire, and a 30-mm tunnel was drilled (Figure 2B). The proximal part of the tendon was advanced into the center of the tunnel using the tendon fork (Figure 2C), and the tendon was held at the bottom of the tunnel with a 1.5-mm guide pin. The tendon fork was removed, and the cannulated interference screw was inserted over the guide pin between the 2 limbs of the biceps tendon (Figure 2D). The tendon was closely monitored to ensure it was not wrapped up when the screw was placed.
Biomechanical Testing
After each tenodesis, the humerus was amputated 5 inches distal to the fixation site. All extraneous soft tissue was dissected away, leaving the distal aspect of the biceps tendon as a free graft. Each proximal humerus–biceps tendon construct was then mounted on a materials testing machine. A custom-designed soft-tissue clamp was used to secure the distal aspect of the biceps tendon to the test actuator and load cell (Figure 3A). A custom-designed jig was used to stabilize the proximal humerus to the platform of the materials testing machine (Figure 3B). The specimens were mounted so that the line of pull throughout the testing protocol was applied parallel to the long axis of the humerus, thereby approximating the in vivo biceps force vector (Figure 3C). Digital cameras recorded each test for analysis of the mechanism of failure for each specimen. Marker dots were drawn on each tendon to assess tendon stretch before construct failure.
The tendons were preloaded to 10 N and then cycled at 0 to 50 N for 100 cycles at 1 Hz. After cyclic loading, axial load to failure was performed at a rate of 1.0 mm per second until a peak load was observed and subsequent loading led to tendon elongation with no further increase in load. Displacement and force applied during cyclic loading and load to failure were recorded. Stiffness was then calculated as the slope of the linear portion of the force-displacement curve using the least mean squares approach. Mechanism of failure was documented for each specimen.
Statistical Analysis
Analyzing our preliminary data with G*Power, we determined that a total sample size of 8 would be required (effect size [Cohen dz] was 1, α error probability was .05, power was .8). We hypothesized that the ultimate strength and stiffness of one group would be less than 1 SD above those of the other group. Paired t test with significance set at P < .05 was used to compare the techniques.
Results
Both repair constructs exhibited standard load-displacement curves, with a linear increase in load with displacement until the point of failure, at which time further displacement occurred with no discernible increase in load (Figure 4). Ultimate load to failure is determined as the highest point of the curve, and stiffness is calculated as the slope of the load-displacement curve.
Mean axial displacement parallel to the shaft of the humerus with cyclic loading was 7.1 mm with the modified PITT technique and 7.9 mm with the interference screw technique. There was no macroscopically visible high-grade tearing or slippage of the biceps tendon in any specimen with cyclic loading for either repair construct.
Mean (SD) ultimate load to failure was significantly (P = .003) higher with the modified PITT technique, 157 (41) N, than with the interference screw technique, 107 (29) N; actual effect size (Cohen dz) was 1.19, difference of means was 50 N, and pooled SD was 42 N. The interference screw technique yielded significantly (P = .010) more mean (SD) stiffness, 38.7 (14.7) N/mm, than the modified PITT technique, 15.8 (9.1) N/mm; actual effect size (Cohen dz) was 1.37, difference of means was 22.9 N/mm, and pooled SD was 16.7 N/mm.
In the interference screw technique, the mode of failure was consistent. Of the 8 specimens, 7 failed at the screw–tendon interface at the distal aspect of the tunnel; in the eighth specimen, the entire tendon pulled out from under the screw construct. In the modified PITT technique, there was more variability in failure: tendon slipped through suture (4 specimens), tendon/suture construct as unit pulled through transverse ligament/rotator interval tissue (3), and suture failure (1).
Discussion
This study was the first to directly compare a bony interference screw technique with a soft-tissue technique (modified PITT). Fixation strength is crucial. A load of 112 N is applied to the long head of the biceps tendon when a person holds 1 kg of weight in the hand with the elbow at 90° flexion.22 As mean (SD) ultimate load to failure was 157 (41) N with the modified PITT technique and 107 (29) N with the interference screw technique in this study, the interference screw can be recommended only with some hesitation.
The interference screw was stiffer with cyclic loading—an expected outcome, as it was secured to rigid bone—vs soft tissue, as in the modified PITT technique. Although the clinical implications for the modified PITT technique are unknown, more than likely, with the tendon being secured to soft tissue, there will be scarring over time.
In laboratory testing of biceps tenodesis constructs, interference screw fixation has had superior load-to-failure characteristics in comparisons with other fixation methods. Golish and colleagues13 found significantly higher load to failure with a biotenodesis screw than with a double-loaded suture anchor for subpectoral tenodesis. Testing similar implants, in a location more proximal in the bicipital groove, Richards and Burkhart14 likewise found superior fixation strength with an interference screw. Ozalay and colleagues16 found superior strength in an interference screw compared with suture anchor, keyhole, and bone tunnel in sheep. In a pig model, the highest ultimate load to failure was found in an interference screw—vs keyhole, bone tunnel, suture anchor, and ligament washer.19 Load to failure for the interference screw in these studies ranged from 170 N to over 400 N.13,14,16,19
A few other investigators have studied the Biceptor interference screw. Slabaugh and colleagues15 found a mean (SD) load to failure of 173.9 (27.2) N for all specimens tested. Patzer and colleagues9,17 found that the mean (SD) ultimate load to failure with the Biceptor proximal interference screw, 173.9 (27.2) N, was superior to that of a suture anchor.
The mean (SD) ultimate load to failure reported for the Biceptor interference screw in the present study, 107 (29) N, is lower than the values reported in the other studies—not only for the Biceptor screw but for interference screws in general. Nevertheless, we performed the technique as the manufacturer recommended.22 Our results were consistent across all specimens studied. Interestingly, in the study by Slabaugh and colleagues,15 7 specimens failed at the tendon–screw interface during cyclic testing and were not included in the analysis of ultimate load to failure. As these specimens failed at a load between 5 N and 70 N, including their data would have significantly lowered the mean load to failure.
Concern over the Biceptor interference screw’s lower failure load relative to that of other interference screws has been raised before.9,17 A major issue is possible overstuffing of the humeral tunnel, as the hole is reamed the same size as the screw. With the Biceptor, the proximal and distal portions of the tendon are placed in the tunnel in a U-shaped configuration with the screw between these limbs. The idea is that the 2 biceps tendon limbs might become abraded and consecutively weaken as the screw is inserted between the tendon limbs, more so than with a single loop. This idea was suggested by the typical longitudinal tendon splitting that occurs at the screw–tendon interface at the distal aspect of the tunnel.23 In the present study, consistent failure (Figures 5A-5C) at the distal aspect of the screw–tendon junction supported the idea that the tendon is abraded during placement of the interference screw or during the friction-causing 90° turn the tendon takes into the bone on loading. There is no way to quantitatively examine tendon quality before interference screw placement, but on gross inspection all the tendons were of good quality. Slabaugh and colleagues15 also found consistent failure at the screw–tunnel interface.
The PITT technique has been described as a simple all-arthroscopic soft-tissue technique for biceps tenodesis.23,24 Subsequently developed soft-tissue techniques have demonstrated clinical benefits.25-27 Proposed advantages of these techniques are lower cost associated with decreased implant needs, no reliance on quality of bone for fixation, suturing while biceps tendon is still attached to anchor (anatomical tension is closely reproduced), and less interference with any subsequent use of magnetic resonance imaging for diagnostic purposes in the shoulder.
There have been only 2 biomechanical studies of the PITT technique. Lopez-Vidriero and colleagues20 compared the biomechanical properties of the PITT and suture anchor techniques in a human cadaveric laboratory study and found that the PITT technique had mean (SD) ultimate load to failure of 142.7 (30.9) N and mean (SD) stiffness of 13.3 (3) N/mm. They observed consistent suture pullout through the tendon substance during failure, which suggests the most important factor for strength is the quality of the biceps tendon. Su and colleagues21 found biomechanically inferior results of the classic PITT technique as compared with the interference screw technique.
This article provides the first description of the modified PITT technique. Our mean (SD) load to failure of the modified PITT technique was 157 (41) N, slightly higher than that reported for the classic PITT technique, albeit under a different setup.20 There was more variation in ultimate load to failure in our study than in previous studies, which could be secondary to tissue quality. As the modified PITT technique relies on surrounding tissue holding the biceps in place, this tissue would need to be of good quality and strength to obtain strong fixation. A possible concern is that placing stitches in the rotator interval could increase the risk of shoulder stiffness, but this has not been encountered clinically.
A more variable mechanism of failure was also found in the present study. Although half the specimens failed by suture pullout through the tendon, similar to what Lopez-Vidriero and colleagues20 described, 3 of our 8 specimens failed with the entire biceps tendon–suture construct pulling through the transverse ligament tissue, and 1 specimen failed by suture breakage. Although these numbers are too small for making definitive statements, our modified PITT technique may add some security to the tendon–suture construct. Such added security may be of particular value in the setting of poor-quality, diseased tendon tissue, and the construct may be more limited by the strength of surrounding tissues. In addition, if failure occurs at the suture transverse humeral ligament–rotator interval interface, more surrounding rotator interval tissue can be incorporated into the tenodesis to decrease the likelihood of failure through this mechanism.
This study had several limitations. First, it was a time zero study in a cadaveric model with simulated biomechanical loading. As such, it provided information only on initial fixation strength and could not prove any superior clinical outcomes or account for any biological changes with healing that occurred over time. Second, the study may have been underpowered, though sample size was chosen in accordance with other cadaveric biomechanical studies. Third, all procedures were performed in an open manner, simulating the arthroscopic approach. Particularly in the setting of the modified PITT technique, this represented a best case scenario. Spinal needles and subsequent sutures were easily passed under direct visualization through the transverse humeral ligament, rotator interval, and biceps tendon. There is likely marked variability in this step during arthroscopy in which visualization is more limited, as in the setting of concomitant procedures, such as subacromial decompression or rotator cuff repair. In addition, all tendons tested were normal in appearance and gave no indication of chronic degenerative changes.
Another study limitation is that we did not quantify bone mineral density, which if poor would have affected interference screw strength. However, mean specimen age was 55 years, minimizing chances of poor bone quality. In addition, 7 of the 8 failures in the interference screw group occurred not with pullout but at the screw–tendon junction, suggesting poor bone quality was not a significant factor. As tendon diameter was not measured before the procedures were performed, there is the possibility it could have been better in the modified PITT group and worse in the interference screw group because of tunnel crowding, as noted.
Over the years, operative treatment of biceps pathology has escalated, likely secondary to increased identification and successful clinical outcomes. Although its true function remains controversial, the biceps tendon has been well accepted as a primary pain generator in the anterior aspect of the shoulder.1,2 Biceps pathology involves a spectrum of often overlapping findings—varying degrees of tearing, tendinitis, and instability. Pathology may be isolated or may present in association with other shoulder conditions, including impingement, bursitis, rotator cuff tears, SLAP (superior labral tear anterior to posterior) lesions, and acromioclavicular disorders.3
Operative treatment of disease of the long head of the biceps mandates an initial choice of tenotomy or tenodesis. Which approach is superior is controversial.4-6 Although tenotomy and tenodesis have comparably favorable clinical results, tenodesis is often recommended, particularly for younger, active patients, mostly because cosmetic deformity is possible with tenotomy.
Tenodesis may be performed arthroscopically or through an open incision, and the biceps tendon may be placed anywhere from in the joint to under the tendon of the pectoralis major tendon. In many recent biomechanical studies, interference screws had higher load to failure and improved stiffness in comparison with other fixation methods.7-19 Most of those studies focused on fixation in a subpectoral location. To our knowledge, only 2 studies of soft-tissue fixation have compared the percutaneous intra-articular transtendon (PITT) technique with other popular tenodesis techniques.20,21 The PITT technique demonstrated a common failure point, with sutures pulling through the tendon substance. It was hypothesized that adding a locking loop to the PITT suture configuration would further improve fixation.
We conducted a study to compare the biomechanical characteristics of 2 techniques for all-arthroscopic proximal biceps tenodesis: bioabsorbable interference screw (Biceptor; Smith & Nephew) and a locking-loop PITT modification developed at our institution.
Methods
Sixteen nonembalmed fresh-frozen human cadaveric shoulders (8 pairs: 3 male, 5 female) were used in this study. Mean specimen age was 55 years (range, 51-59 years). The specimens showed no evidence of high-grade osteoarthritic changes, biceps tendon fraying or tearing, biceps pulley lesions, or full-thickness rotator cuff tears. They were thawed at room temperature for 24 hours before the procedure.
In each pair, 1 shoulder was randomized to be treated with 1 of 2 arthroscopic biceps tenodesis techniques—modified PITT or Biceptor interference screw—and the other shoulder was treated with the other technique. Surgery was performed in an open fashion, and every attempt was made to simulate the arthroscopic approach. In all shoulders, biomechanical testing was completed immediately after tenodesis.
Modified PITT Technique
In an outside-in fashion, an 18-gauge spinal needle was used to pierce the transverse humeral ligament, the lateral aspect of the rotator interval tissue, and the biceps tendon. A second needle was then passed in similar fashion, piercing the biceps tendon just adjacent to the first needle (Figure 1A). A 0-polydioxanone monofilament suture (0-PDS; Ethicon, Johnson & Johnson) was threaded through the first needle and used to shuttle a single No. 2 braided nonabsorbable polyethylene suture (MaxBraid; Biomet Sports Medicine) back through the biceps tendon.
At this point, the free end of the nonabsorbable suture, which comes out of the anterior cannula during an arthroscopic procedure, was passed back into the glenohumeral joint (using a suture grasper), looped over the top of the biceps tendon, and brought back out of the joint anteriorly, thereby creating a locking loop around the tendon (Figure 1B). A shuttle suture (0-PDS) passed through the second needle was used to bring that anterior limb of nonabsorbable suture back through the biceps tendon, completing the stitch configuration (Figure 1C).
This process was repeated with another nonabsorbable suture. After suture passing was completed, the biceps was detached from its insertion at the superior labrum. The 2 nonabsorbable sutures, which would later be retrieved from the subacromial space, were then tied in standard fashion, securing the biceps tendon to the transverse humeral ligament/rotator interval tissue (Figure 1D).
Biceptor Interference Screw Technique
The interference screw technique was performed in accordance with the manufacturer’s operative instructions.22 An 8 × 25-mm polyetheretherketone interference screw was used in all specimens, and the medium tendon fork was used to maintain tension on the biceps tendon during fixation (Figure 2A).
A 2.4-mm guide wire was inserted perpendicular to the humeral shaft, at the planned site of tenodesis, 10 mm distal to the entrance of the bicipital groove. An 8-mm cannulated reamer was passed over the wire, and a 30-mm tunnel was drilled (Figure 2B). The proximal part of the tendon was advanced into the center of the tunnel using the tendon fork (Figure 2C), and the tendon was held at the bottom of the tunnel with a 1.5-mm guide pin. The tendon fork was removed, and the cannulated interference screw was inserted over the guide pin between the 2 limbs of the biceps tendon (Figure 2D). The tendon was closely monitored to ensure it was not wrapped up when the screw was placed.
Biomechanical Testing
After each tenodesis, the humerus was amputated 5 inches distal to the fixation site. All extraneous soft tissue was dissected away, leaving the distal aspect of the biceps tendon as a free graft. Each proximal humerus–biceps tendon construct was then mounted on a materials testing machine. A custom-designed soft-tissue clamp was used to secure the distal aspect of the biceps tendon to the test actuator and load cell (Figure 3A). A custom-designed jig was used to stabilize the proximal humerus to the platform of the materials testing machine (Figure 3B). The specimens were mounted so that the line of pull throughout the testing protocol was applied parallel to the long axis of the humerus, thereby approximating the in vivo biceps force vector (Figure 3C). Digital cameras recorded each test for analysis of the mechanism of failure for each specimen. Marker dots were drawn on each tendon to assess tendon stretch before construct failure.
The tendons were preloaded to 10 N and then cycled at 0 to 50 N for 100 cycles at 1 Hz. After cyclic loading, axial load to failure was performed at a rate of 1.0 mm per second until a peak load was observed and subsequent loading led to tendon elongation with no further increase in load. Displacement and force applied during cyclic loading and load to failure were recorded. Stiffness was then calculated as the slope of the linear portion of the force-displacement curve using the least mean squares approach. Mechanism of failure was documented for each specimen.
Statistical Analysis
Analyzing our preliminary data with G*Power, we determined that a total sample size of 8 would be required (effect size [Cohen dz] was 1, α error probability was .05, power was .8). We hypothesized that the ultimate strength and stiffness of one group would be less than 1 SD above those of the other group. Paired t test with significance set at P < .05 was used to compare the techniques.
Results
Both repair constructs exhibited standard load-displacement curves, with a linear increase in load with displacement until the point of failure, at which time further displacement occurred with no discernible increase in load (Figure 4). Ultimate load to failure is determined as the highest point of the curve, and stiffness is calculated as the slope of the load-displacement curve.
Mean axial displacement parallel to the shaft of the humerus with cyclic loading was 7.1 mm with the modified PITT technique and 7.9 mm with the interference screw technique. There was no macroscopically visible high-grade tearing or slippage of the biceps tendon in any specimen with cyclic loading for either repair construct.
Mean (SD) ultimate load to failure was significantly (P = .003) higher with the modified PITT technique, 157 (41) N, than with the interference screw technique, 107 (29) N; actual effect size (Cohen dz) was 1.19, difference of means was 50 N, and pooled SD was 42 N. The interference screw technique yielded significantly (P = .010) more mean (SD) stiffness, 38.7 (14.7) N/mm, than the modified PITT technique, 15.8 (9.1) N/mm; actual effect size (Cohen dz) was 1.37, difference of means was 22.9 N/mm, and pooled SD was 16.7 N/mm.
In the interference screw technique, the mode of failure was consistent. Of the 8 specimens, 7 failed at the screw–tendon interface at the distal aspect of the tunnel; in the eighth specimen, the entire tendon pulled out from under the screw construct. In the modified PITT technique, there was more variability in failure: tendon slipped through suture (4 specimens), tendon/suture construct as unit pulled through transverse ligament/rotator interval tissue (3), and suture failure (1).
Discussion
This study was the first to directly compare a bony interference screw technique with a soft-tissue technique (modified PITT). Fixation strength is crucial. A load of 112 N is applied to the long head of the biceps tendon when a person holds 1 kg of weight in the hand with the elbow at 90° flexion.22 As mean (SD) ultimate load to failure was 157 (41) N with the modified PITT technique and 107 (29) N with the interference screw technique in this study, the interference screw can be recommended only with some hesitation.
The interference screw was stiffer with cyclic loading—an expected outcome, as it was secured to rigid bone—vs soft tissue, as in the modified PITT technique. Although the clinical implications for the modified PITT technique are unknown, more than likely, with the tendon being secured to soft tissue, there will be scarring over time.
In laboratory testing of biceps tenodesis constructs, interference screw fixation has had superior load-to-failure characteristics in comparisons with other fixation methods. Golish and colleagues13 found significantly higher load to failure with a biotenodesis screw than with a double-loaded suture anchor for subpectoral tenodesis. Testing similar implants, in a location more proximal in the bicipital groove, Richards and Burkhart14 likewise found superior fixation strength with an interference screw. Ozalay and colleagues16 found superior strength in an interference screw compared with suture anchor, keyhole, and bone tunnel in sheep. In a pig model, the highest ultimate load to failure was found in an interference screw—vs keyhole, bone tunnel, suture anchor, and ligament washer.19 Load to failure for the interference screw in these studies ranged from 170 N to over 400 N.13,14,16,19
A few other investigators have studied the Biceptor interference screw. Slabaugh and colleagues15 found a mean (SD) load to failure of 173.9 (27.2) N for all specimens tested. Patzer and colleagues9,17 found that the mean (SD) ultimate load to failure with the Biceptor proximal interference screw, 173.9 (27.2) N, was superior to that of a suture anchor.
The mean (SD) ultimate load to failure reported for the Biceptor interference screw in the present study, 107 (29) N, is lower than the values reported in the other studies—not only for the Biceptor screw but for interference screws in general. Nevertheless, we performed the technique as the manufacturer recommended.22 Our results were consistent across all specimens studied. Interestingly, in the study by Slabaugh and colleagues,15 7 specimens failed at the tendon–screw interface during cyclic testing and were not included in the analysis of ultimate load to failure. As these specimens failed at a load between 5 N and 70 N, including their data would have significantly lowered the mean load to failure.
Concern over the Biceptor interference screw’s lower failure load relative to that of other interference screws has been raised before.9,17 A major issue is possible overstuffing of the humeral tunnel, as the hole is reamed the same size as the screw. With the Biceptor, the proximal and distal portions of the tendon are placed in the tunnel in a U-shaped configuration with the screw between these limbs. The idea is that the 2 biceps tendon limbs might become abraded and consecutively weaken as the screw is inserted between the tendon limbs, more so than with a single loop. This idea was suggested by the typical longitudinal tendon splitting that occurs at the screw–tendon interface at the distal aspect of the tunnel.23 In the present study, consistent failure (Figures 5A-5C) at the distal aspect of the screw–tendon junction supported the idea that the tendon is abraded during placement of the interference screw or during the friction-causing 90° turn the tendon takes into the bone on loading. There is no way to quantitatively examine tendon quality before interference screw placement, but on gross inspection all the tendons were of good quality. Slabaugh and colleagues15 also found consistent failure at the screw–tunnel interface.
The PITT technique has been described as a simple all-arthroscopic soft-tissue technique for biceps tenodesis.23,24 Subsequently developed soft-tissue techniques have demonstrated clinical benefits.25-27 Proposed advantages of these techniques are lower cost associated with decreased implant needs, no reliance on quality of bone for fixation, suturing while biceps tendon is still attached to anchor (anatomical tension is closely reproduced), and less interference with any subsequent use of magnetic resonance imaging for diagnostic purposes in the shoulder.
There have been only 2 biomechanical studies of the PITT technique. Lopez-Vidriero and colleagues20 compared the biomechanical properties of the PITT and suture anchor techniques in a human cadaveric laboratory study and found that the PITT technique had mean (SD) ultimate load to failure of 142.7 (30.9) N and mean (SD) stiffness of 13.3 (3) N/mm. They observed consistent suture pullout through the tendon substance during failure, which suggests the most important factor for strength is the quality of the biceps tendon. Su and colleagues21 found biomechanically inferior results of the classic PITT technique as compared with the interference screw technique.
This article provides the first description of the modified PITT technique. Our mean (SD) load to failure of the modified PITT technique was 157 (41) N, slightly higher than that reported for the classic PITT technique, albeit under a different setup.20 There was more variation in ultimate load to failure in our study than in previous studies, which could be secondary to tissue quality. As the modified PITT technique relies on surrounding tissue holding the biceps in place, this tissue would need to be of good quality and strength to obtain strong fixation. A possible concern is that placing stitches in the rotator interval could increase the risk of shoulder stiffness, but this has not been encountered clinically.
A more variable mechanism of failure was also found in the present study. Although half the specimens failed by suture pullout through the tendon, similar to what Lopez-Vidriero and colleagues20 described, 3 of our 8 specimens failed with the entire biceps tendon–suture construct pulling through the transverse ligament tissue, and 1 specimen failed by suture breakage. Although these numbers are too small for making definitive statements, our modified PITT technique may add some security to the tendon–suture construct. Such added security may be of particular value in the setting of poor-quality, diseased tendon tissue, and the construct may be more limited by the strength of surrounding tissues. In addition, if failure occurs at the suture transverse humeral ligament–rotator interval interface, more surrounding rotator interval tissue can be incorporated into the tenodesis to decrease the likelihood of failure through this mechanism.
This study had several limitations. First, it was a time zero study in a cadaveric model with simulated biomechanical loading. As such, it provided information only on initial fixation strength and could not prove any superior clinical outcomes or account for any biological changes with healing that occurred over time. Second, the study may have been underpowered, though sample size was chosen in accordance with other cadaveric biomechanical studies. Third, all procedures were performed in an open manner, simulating the arthroscopic approach. Particularly in the setting of the modified PITT technique, this represented a best case scenario. Spinal needles and subsequent sutures were easily passed under direct visualization through the transverse humeral ligament, rotator interval, and biceps tendon. There is likely marked variability in this step during arthroscopy in which visualization is more limited, as in the setting of concomitant procedures, such as subacromial decompression or rotator cuff repair. In addition, all tendons tested were normal in appearance and gave no indication of chronic degenerative changes.
Another study limitation is that we did not quantify bone mineral density, which if poor would have affected interference screw strength. However, mean specimen age was 55 years, minimizing chances of poor bone quality. In addition, 7 of the 8 failures in the interference screw group occurred not with pullout but at the screw–tendon junction, suggesting poor bone quality was not a significant factor. As tendon diameter was not measured before the procedures were performed, there is the possibility it could have been better in the modified PITT group and worse in the interference screw group because of tunnel crowding, as noted.
1. Alpantaki K, McLaughlin D, Karagogeos D, Hadjipavlou A, Kontakis G. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J Bone Joint Surg Am. 2005;87(7):1580-1583.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Khazzam M, George MS, Churchill RS, Kuhn JE. Disorders of the long head of biceps tendon. J Shoulder Elbow Surg. 2012;21(1):136-145.
4. Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of pathologic lesions of the tendon of the long head of the biceps brachii. Am J Sports Med. 2009;37(4):828-833.
5. Hsu AR, Ghodadra NS, Provencher MT, Lewis PB, Bach BR. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20(2):326-332.
6. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
7. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
8. Millett PJ, Sanders B, Gobezie R, Braun S, Warner JJ. Interference screw vs. suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9:121.
9. Patzer T, Rundic JM, Bobrowitsch E, Olender GD, Hurschler C, Schofer MD. Biomechanical comparison of arthroscopically performable techniques for suprapectoral biceps tenodesis. Arthroscopy. 2011;27(8):1036-1047.
10. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
11. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
12. Klepps S, Hazrati Y, Flatow E. Arthroscopic biceps tenodesis. Arthroscopy. 2002;18(9):1040-1045.
13. Golish SR, Caldwell PE 3rd, Miller MD, et al. Interference screw versus suture anchor fixation for subpectoral tenodesis of the proximal biceps tendon: a cadaveric study. Arthroscopy. 2008;24(10):1103-1108.
14. Richards DP, Burkhart SS. A biomechanical analysis of two biceps tenodesis fixation techniques. Arthroscopy. 2005;21(7):861-866.
15. Slabaugh MA, Frank RM, Van Thiel GS, et al. Biceps tenodesis with interference screw fixation: a biomechanical comparison of screw length and diameter. Arthroscopy. 2011;27(2):161-166.
16. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
17. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
18. Jayamoorthy T, Field JR, Costi JJ, Martin DK, Stanley RM, Hearn TC. Biceps tenodesis: a biomechanical study of fixation methods. J Shoulder Elbow Surg. 2004;13(2):160-164.
19. Kusma M, Dienst M, Eckert J, Steimer O, Kohn D. Tenodesis of the long head of biceps brachii: cyclic testing of five methods of fixation in a porcine model. J Shoulder Elbow Surg. 2008;17(6):967-973.
20. Lopez-Vidriero E, Costic RS, Fu FH, Rodosky MW. Biomechanical evaluation of 2 arthroscopic biceps tenodeses: double anchor versus percutaneous intra-articular transtendon (PITT) techniques. Am J Sports Med. 2010;38(1):146-152.
21. Su WR, Budoff JE, Chiang CH, Lee CJ, Lin CL. Biomechanical study comparing biceps wedge tenodesis with other proximal long head of the biceps tenodesis techniques. Arthroscopy. 2013;29(9):1498-1505.
22. Trenhaile SW. Biceptor Tenodesis System, Arthroscopic Biceps Tenodesis [operational instructions]. Andover, MA: Smith & Nephew; 2009:1-8.
23. Sekiya LC, Elkousy HA, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous intra-articular transtendon technique. Arthroscopy. 2003;19(10):1137-1141.
24. Elkousy HA, Fluhme DJ, O’Connor DP, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous, intra-articular trans-tendon technique: preliminary results. Orthopedics. 2005;28(11):1316-1319.
25. Castagna A, Conti M, Mouhsine E, Bungaro P, Garofalo R. Arthroscopic biceps tendon tenodesis; the anchorage technical note. Knee Surg Sports Traumatol Arthrosc. 2006;14(6):581-585.
26. Checchia SL, Doneux PS, Miyazaki AN, et al. Biceps tenodesis associated with arthroscopic repair of rotator cuff tears. J Shoulder Elbow Surg. 2005;14(2):138-144.
27. Moros C, Levine WN, Ahmad CS. Suture anchor and percutaneous intra-articular transtendon biceps tenodesis. Sports Med Arthrosc. 2008;16(3):177-179.
1. Alpantaki K, McLaughlin D, Karagogeos D, Hadjipavlou A, Kontakis G. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J Bone Joint Surg Am. 2005;87(7):1580-1583.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Khazzam M, George MS, Churchill RS, Kuhn JE. Disorders of the long head of biceps tendon. J Shoulder Elbow Surg. 2012;21(1):136-145.
4. Frost A, Zafar MS, Maffulli N. Tenotomy versus tenodesis in the management of pathologic lesions of the tendon of the long head of the biceps brachii. Am J Sports Med. 2009;37(4):828-833.
5. Hsu AR, Ghodadra NS, Provencher MT, Lewis PB, Bach BR. Biceps tenotomy versus tenodesis: a review of clinical outcomes and biomechanical results. J Shoulder Elbow Surg. 2011;20(2):326-332.
6. Slenker NR, Lawson K, Ciccotti MG, Dodson CC, Cohen SB. Biceps tenotomy versus tenodesis: clinical outcomes. Arthroscopy. 2012;28(4):576-582.
7. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
8. Millett PJ, Sanders B, Gobezie R, Braun S, Warner JJ. Interference screw vs. suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9:121.
9. Patzer T, Rundic JM, Bobrowitsch E, Olender GD, Hurschler C, Schofer MD. Biomechanical comparison of arthroscopically performable techniques for suprapectoral biceps tenodesis. Arthroscopy. 2011;27(8):1036-1047.
10. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
11. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
12. Klepps S, Hazrati Y, Flatow E. Arthroscopic biceps tenodesis. Arthroscopy. 2002;18(9):1040-1045.
13. Golish SR, Caldwell PE 3rd, Miller MD, et al. Interference screw versus suture anchor fixation for subpectoral tenodesis of the proximal biceps tendon: a cadaveric study. Arthroscopy. 2008;24(10):1103-1108.
14. Richards DP, Burkhart SS. A biomechanical analysis of two biceps tenodesis fixation techniques. Arthroscopy. 2005;21(7):861-866.
15. Slabaugh MA, Frank RM, Van Thiel GS, et al. Biceps tenodesis with interference screw fixation: a biomechanical comparison of screw length and diameter. Arthroscopy. 2011;27(2):161-166.
16. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
17. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
18. Jayamoorthy T, Field JR, Costi JJ, Martin DK, Stanley RM, Hearn TC. Biceps tenodesis: a biomechanical study of fixation methods. J Shoulder Elbow Surg. 2004;13(2):160-164.
19. Kusma M, Dienst M, Eckert J, Steimer O, Kohn D. Tenodesis of the long head of biceps brachii: cyclic testing of five methods of fixation in a porcine model. J Shoulder Elbow Surg. 2008;17(6):967-973.
20. Lopez-Vidriero E, Costic RS, Fu FH, Rodosky MW. Biomechanical evaluation of 2 arthroscopic biceps tenodeses: double anchor versus percutaneous intra-articular transtendon (PITT) techniques. Am J Sports Med. 2010;38(1):146-152.
21. Su WR, Budoff JE, Chiang CH, Lee CJ, Lin CL. Biomechanical study comparing biceps wedge tenodesis with other proximal long head of the biceps tenodesis techniques. Arthroscopy. 2013;29(9):1498-1505.
22. Trenhaile SW. Biceptor Tenodesis System, Arthroscopic Biceps Tenodesis [operational instructions]. Andover, MA: Smith & Nephew; 2009:1-8.
23. Sekiya LC, Elkousy HA, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous intra-articular transtendon technique. Arthroscopy. 2003;19(10):1137-1141.
24. Elkousy HA, Fluhme DJ, O’Connor DP, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous, intra-articular trans-tendon technique: preliminary results. Orthopedics. 2005;28(11):1316-1319.
25. Castagna A, Conti M, Mouhsine E, Bungaro P, Garofalo R. Arthroscopic biceps tendon tenodesis; the anchorage technical note. Knee Surg Sports Traumatol Arthrosc. 2006;14(6):581-585.
26. Checchia SL, Doneux PS, Miyazaki AN, et al. Biceps tenodesis associated with arthroscopic repair of rotator cuff tears. J Shoulder Elbow Surg. 2005;14(2):138-144.
27. Moros C, Levine WN, Ahmad CS. Suture anchor and percutaneous intra-articular transtendon biceps tenodesis. Sports Med Arthrosc. 2008;16(3):177-179.
New HIV antiretroviral guidelines embrace PrEP, early ART
The International Antiviral Society–USA has updated its recommendations for the use of antiretroviral drugs to treat or to prevent HIV infection in adults.
In light of new evidence emerging since its 2014 report on antiretroviral drugs (ARVs), the International Antiviral Society–USA convened a panel of HIV experts to review data published in peer-reviewed journals, presented by regulatory agencies, or presented as conference abstracts at peer-reviewed scientific conferences that would change previous recommendations or ratings. The resulting recommendations were published online in the Journal of the American Medical Association. (JAMA. 2016;316[2]:191-210. doi:10.1001/jama.2016.8900)
The panel concluded that newer data support the “widely accepted” recommendation that antiretroviral therapy (ART) should be started in all individuals with HIV infection with detectable viremia regardless of CD4 cell count. They said optimal initial regimens for most patients should include two nucleoside reverse transcriptase inhibitors (NRTIs) plus an integrase strand transfer inhibitor (InSTI). The panel concluded that other effective regimens may include nonnucleoside reverse transcriptase inhibitors or boosted protease inhibitors with two NRTIs.
Also of note are the panel’s recommendations for certain special populations. The panel said HIV-infected pregnant women should initiate ART for their own health and to reduce the likelihood of HIV transmission to the infant. HIV-infected patients with hepatitis B virus (HBV) coinfection should initiate a recommended ART regimen that contains tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide (TAF), lamivudine or emtricitabine, and a third component.
In addition, the panel said ART should be started within the first 2 weeks after diagnosis for most acute opportunistic infections, with the possible exception of acute cryptococcal meningitis, and ART should be started within the first 2 weeks of initiation of tuberculosis treatment for those with CD4 cell counts of 50/mcL or less and within the first 2-8 weeks for those with CD4 cell counts above 50/mcL.
For prevention of HIV infection, the panel concluded that preexposure prophylaxis (PrEP) should be considered for anyone from a population whose HIV incidence is at least 2% per year, or for HIV-seronegative partners of HIV-infected persons who do not have viral suppression. Daily tenofovir disoproxil fumarate/emtricitabine is recommended for use as preexposure prophylaxis to prevent HIV infection in persons at high risk. Also, postexposure prophylaxis should be started as soon as possible after exposure.
For the complete list of ARV recommendations, see the report in JAMA (doi:10.1001/jama.2016.8900).
On Twitter @richpizzi
The International Antiviral Society–USA has updated its recommendations for the use of antiretroviral drugs to treat or to prevent HIV infection in adults.
In light of new evidence emerging since its 2014 report on antiretroviral drugs (ARVs), the International Antiviral Society–USA convened a panel of HIV experts to review data published in peer-reviewed journals, presented by regulatory agencies, or presented as conference abstracts at peer-reviewed scientific conferences that would change previous recommendations or ratings. The resulting recommendations were published online in the Journal of the American Medical Association. (JAMA. 2016;316[2]:191-210. doi:10.1001/jama.2016.8900)
The panel concluded that newer data support the “widely accepted” recommendation that antiretroviral therapy (ART) should be started in all individuals with HIV infection with detectable viremia regardless of CD4 cell count. They said optimal initial regimens for most patients should include two nucleoside reverse transcriptase inhibitors (NRTIs) plus an integrase strand transfer inhibitor (InSTI). The panel concluded that other effective regimens may include nonnucleoside reverse transcriptase inhibitors or boosted protease inhibitors with two NRTIs.
Also of note are the panel’s recommendations for certain special populations. The panel said HIV-infected pregnant women should initiate ART for their own health and to reduce the likelihood of HIV transmission to the infant. HIV-infected patients with hepatitis B virus (HBV) coinfection should initiate a recommended ART regimen that contains tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide (TAF), lamivudine or emtricitabine, and a third component.
In addition, the panel said ART should be started within the first 2 weeks after diagnosis for most acute opportunistic infections, with the possible exception of acute cryptococcal meningitis, and ART should be started within the first 2 weeks of initiation of tuberculosis treatment for those with CD4 cell counts of 50/mcL or less and within the first 2-8 weeks for those with CD4 cell counts above 50/mcL.
For prevention of HIV infection, the panel concluded that preexposure prophylaxis (PrEP) should be considered for anyone from a population whose HIV incidence is at least 2% per year, or for HIV-seronegative partners of HIV-infected persons who do not have viral suppression. Daily tenofovir disoproxil fumarate/emtricitabine is recommended for use as preexposure prophylaxis to prevent HIV infection in persons at high risk. Also, postexposure prophylaxis should be started as soon as possible after exposure.
For the complete list of ARV recommendations, see the report in JAMA (doi:10.1001/jama.2016.8900).
On Twitter @richpizzi
The International Antiviral Society–USA has updated its recommendations for the use of antiretroviral drugs to treat or to prevent HIV infection in adults.
In light of new evidence emerging since its 2014 report on antiretroviral drugs (ARVs), the International Antiviral Society–USA convened a panel of HIV experts to review data published in peer-reviewed journals, presented by regulatory agencies, or presented as conference abstracts at peer-reviewed scientific conferences that would change previous recommendations or ratings. The resulting recommendations were published online in the Journal of the American Medical Association. (JAMA. 2016;316[2]:191-210. doi:10.1001/jama.2016.8900)
The panel concluded that newer data support the “widely accepted” recommendation that antiretroviral therapy (ART) should be started in all individuals with HIV infection with detectable viremia regardless of CD4 cell count. They said optimal initial regimens for most patients should include two nucleoside reverse transcriptase inhibitors (NRTIs) plus an integrase strand transfer inhibitor (InSTI). The panel concluded that other effective regimens may include nonnucleoside reverse transcriptase inhibitors or boosted protease inhibitors with two NRTIs.
Also of note are the panel’s recommendations for certain special populations. The panel said HIV-infected pregnant women should initiate ART for their own health and to reduce the likelihood of HIV transmission to the infant. HIV-infected patients with hepatitis B virus (HBV) coinfection should initiate a recommended ART regimen that contains tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide (TAF), lamivudine or emtricitabine, and a third component.
In addition, the panel said ART should be started within the first 2 weeks after diagnosis for most acute opportunistic infections, with the possible exception of acute cryptococcal meningitis, and ART should be started within the first 2 weeks of initiation of tuberculosis treatment for those with CD4 cell counts of 50/mcL or less and within the first 2-8 weeks for those with CD4 cell counts above 50/mcL.
For prevention of HIV infection, the panel concluded that preexposure prophylaxis (PrEP) should be considered for anyone from a population whose HIV incidence is at least 2% per year, or for HIV-seronegative partners of HIV-infected persons who do not have viral suppression. Daily tenofovir disoproxil fumarate/emtricitabine is recommended for use as preexposure prophylaxis to prevent HIV infection in persons at high risk. Also, postexposure prophylaxis should be started as soon as possible after exposure.
For the complete list of ARV recommendations, see the report in JAMA (doi:10.1001/jama.2016.8900).
On Twitter @richpizzi
FROM JAMA
Is a Persistent Vacuum Phenomenon a Sign of Pseudarthrosis After Posterolateral Spinal Fusion?
The spinal vacuum sign or vacuum phenomenon (VP) is the radiographic finding of an air-density linear radiolucency in the intervertebral disc or vertebral body. The result of a gaseous accumulation, it is often a diagnostic sign of disc degeneration as well as a rare sign of infection, Schmorl node formation, or osteonecrosis.1,2 Although the VP was first described on plain radiographs, it is better seen on computed tomography (CT).3 Multiple studies have found a possible association between the VP and nonunion in diaphyseal fractures,4 ankylosing spondylitis,5,6 and lumbar spinal fusion.7
To our knowledge, no one has studied whether the intervertebral VP resolves after posterolateral lumbar spinal fusion in adults with degenerative spinal pathology, and no one has investigated the association between the persistence of the intervertebral VP and pseudarthrosis after posterolateral spinal fusion.
We conducted a study to determine whether the VP resolves after posterolateral lumbar spinal fusion procedures and whether persistence of the VP after fusion surgery is indicative of pseudarthrosis.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of patients who had degenerative spinal stenosis with instability and the intervertebral vacuum sign on preoperative digital lumbar spine CT scans and who underwent posterolateral lumbar spinal fusion with or without instrumentation. Study inclusion criteria were lumbar spine CT at minimum 6-month follow-up after spinal fusion and preoperative and postoperative lumbar spine radiographs. Exclusion criteria were any type of interbody fusion procedure (anterior, posterior, transforaminal, lateral) at a level with the VP, age under 21 years, follow-up of less than 6 months, and incomplete radiographic records. As this was a retrospective study, patient consent was not required.
CT was performed with a 16-, 64-, or 128-slice multidetector CT scanner with effective tube current set at 250 to 320 mA, voltage set at 120 to 140 kV, and pitch set at 0.75 to 0.9. After axial acquisition of 3×3-mm isometric voxels, sagittal and coronal multiplanar images were reconstructed with a slice thickness of 2 mm. Patient demographics, diagnoses, and surgical details were recorded. All digital lumbar spine CT scans and radiographs were initially screened on PACS (picture archiving and communication system) by the orthopedic spine surgery fellow at an academic medical institution; then they were reviewed on a radiology reading room monitor by 3 observers (senior radiologist, senior orthopedic spine surgeon, orthopedic spine surgery fellow). Axial images and sagittal and coronal reconstructed images of the preoperative and postoperative follow-up lumbar CT scans—together with the lateral and anteroposterior lumbar spine radiographs—were evaluated for the intervertebral VP. Mean (SD) follow-up (with CT to assess fusion) was 1.6 (0.86) years (range, 0.75-3.38 years). Fusion at each level was evaluated on the postoperative follow-up CT on axial images and sagittal and coronal reconstructed images; criteria for fusion were continuous bridging bone across posterolateral gutters and facets on one or both sides at each intervertebral level.8 Pseudarthrosis was recorded if there was no continuity of bridging bone across both posterolateral gutters and facets, a complete radiolucent line on both sides across a level, or lysis or loosening around screws. All recordings were made by consensus, or by majority decision in case of disagreement.
Presence of the VP at the lumbar levels not included in the fusion was also recorded on the preoperative and follow-up CT scan and radiographs.
Descriptive and inferential statistical tests were performed as applicable. Pearson χ2 test and Fischer exact test were used to evaluate if there was a significant association between the groups where the VP disappeared and persisted and fusion and pseudarthrosis. Significance was set at P < .05. Statistical analysis was performed with Stata Version 10.0.
Results
Using the preoperative lumbar spine CT scans of 18 patients (10 men, 8 women), we identified 36 cases of intervertebral levels exhibiting the VP (median positive vacuum sign levels per patient, 2; minimum, 1; maximum, 5) at the levels included in the fusion (Table 1). Mean (SD) age at surgery was 67.6 (9.4) years (range, 46.5-79.6 years). Mean (SD) radiologic follow-up was 1.6 (0.86) years (range, 0.75-3.38 years). All patients underwent lumbar fusion with local autograft, allograft, and recombinant human bone morphogenetic protein 2. Spinal instrumentation was used in 16 of the 18 patients.
On preoperative CT, positive VP was diagnosed in the 36 cases as follows: L5–S1 (11 cases), L4–L5 (9 cases), L3–L4 (4 cases), L2–L3 (6 cases), L1–L2 (4 cases), and T12–L1 (2 cases). On follow-up CT, 15 cases showed persistence of the VP, and 21 cases showed disappearance of the VP (Table 1).
Evidence of spinal fusion was identified on follow-up CT in 32 (88.9%) of the 36 cases. In 3 of the 18 patients, nonunion was diagnosed. Of the 15 intervertebral cases in which the VP persisted, 13 (86.7%) showed evidence of fusion on CT, and 2 (13.3%) showed evidence of pseudarthrosis. Of the 21 intervertebral cases in which the VP disappeared, 19 (90.5%) showed evidence of fusion on CT, and 2 (9.5%) showed evidence of pseudarthrosis (Table 2). There was no significant difference in fusion rate or pseudarthrosis rate in the groups in which the VP persisted or disappeared (Fischer exact test, P = .99). There was no significant association between VP persistence or disappearance and sex, primary or revision surgery, or intervertebral level (Fischer exact test, P > .05). A case example is shown in the Figure.
At levels not included in spinal fusion, CT identified the VP at 6 lumbar intervertebral levels before surgery and 11 levels at follow-up. The VP did not disappear at any level not included in the fusion. At follow-up, no new VP was identified in a segment included in fusion. Results are summarized in Table 3.
Discussion
The association of radiologic intervertebral VP and disc degeneration, first recognized by Knutsson1 in 1942, refers to the presence of gas, mainly containing nitrogen, in the crevices between or within vertebrae.2 The VP is more often seen in patients older than 50 years, on plain radiographs in hyperextension.9 CT is more sensitive than radiography in detecting the VP; Lardé and colleagues3 found it in about 50% of 50 patients on CT scans but in only 12% of patients on radiographs. The VP is visible because of the nitrogen gas that accumulates when there is a negative pressure within the disc space. Nitrogen emerges from the blood and moves into the disc space; perhaps the disc space opens, causing the negative pressure.1-3 On T1- or T2-weighted magnetic resonance imaging (MRI), the VP is visible as a signal void. MRI, however, is less accurate than CT.10 In a study of 10 patients who had low back pain and more than 1 level of intradiscal VP, and who underwent supine MRI examinations at 0, 1, and 2 hours, Wang and colleagues11 found that, after prolonged supine positioning, the signal intensity of the vacuum was replaced by hyperintense fluid contents. D’Anastasi and colleagues,12 in a study of 20 patients who had lumbar vacuum phenomenon on CT and underwent MRI examinations, found a significant correlation between presence of intradiscal fluid and amount of bone marrow edema on MRI and degenerative endplate abnormalities on CT. In the present study, we found that, after the spinal fusion vacuum phenomenon disappeared in 58.3% of the lumbar levels and persisted in 41.7% on follow-up CT at the levels included in posterolateral fusion, there were 5 new levels, adjacent to the lumbar fusion, where the VP was seen on the follow-up CT.
We studied whether evidence of a persistent vacuum sign on CT is indicative of pseudarthrosis. Other authors have reported an association between the VP and nonunion in fractures4 and ankylosing spondylitis.5,6 In a study of 19 patients with diaphyseal fractures, Stallenberg and colleagues4 found that, in 7 of the 10 patients with nonunion, the VP was detected on CT at the nonunion site. Martel5 first reported on the intervertebral VP in a case of ankylosing spondylitis with spinal pseudarthrosis. Ten years later, in a study of 18 patients with advanced ankylosing spondylitis with spinal pseudarthrosis, Chan and colleagues6 identified the intervertebral VP on CT in 7 patients. Edwards and colleagues7 studied 15 patients with prior lumbar fusion with 17 positive intervertebral VP levels on CT and found that the vacuum disc sign was a strong predictor of lumbar nonunion as determined by surgical exploration. Mirovsky and colleagues13 identified the intravertebral vacuum cleft in 26 patients with an osteoporotic vertebral fracture treated with vertebroplasty and concluded that nonunion of the vertebral fracture could be identified by presence of the intravertebral vacuum cleft on radiography. In the present study, there was radiologic evidence of lumbar spinal fusion in 89% of disc levels with a preoperative positive intervertebral VP and pseudarthrosis in 11% of disc levels. The rate of fusion at levels with the VP was comparable to the rate at intervertebral levels without the phenomenon. These findings indicate that persistence of the VP after spinal fusion is not an indication that fusion has not been achieved. Preoperative VP also did not predispose to failure of fusion. That there is a persistent vacuum disc might imply that, even after successful fusion as seen on CT, some motion may be occurring at the disc level to cause a negative pressure phenomenon. Even in cases of facet fusion with bridging bone, there may still be motion at the disc level, as fusions can plastically deform (even with screws in), particularly in elderly osteopenic bone. We found no association between a persistent vacuum sign and pseudarthrosis. Our study findings are clinically useful even if the benefits are limited. These findings may help surgeons avoid misinterpreting this sign as an indication for additional surgery.
This study had some limitations. First, radiographs were used to determine presence or absence of fusion. Although CT is widely considered the gold standard for noninvasive assessment of fusion,14 even when both posterolateral gutters and facets have been found to be fused on CT, the probability of a solid fusion on exploration ranges from 69% to 96%.8,15 Second, detection of the VP on radiographs and CT may be affected by patient position.11 Third, this was a retrospective series with a small number of patients and limited follow-up with CT. Arthrodesis and the VP may take years to fully evolve. It is possible that fusion rates could be higher on longer follow-up, and resolution of the VP may occur with longer follow-up. Fourth, clinical outcomes were not evaluated, as there are other confounding factors, apart from successful fusion, that could affect clinical outcomes. A larger prospective controlled study would be helpful.
Conclusion
The radiologic intervertebral VP may persist after posterolateral lumbar spinal fusion. We did not find an association between the VP and pseudarthrosis. In addition, VP persistence on follow-up CT was not indicative of pseudarthrosis, and VP disappearance was not indicative of fusion. The vacuum sign should not be misinterpreted as an indication for additional surgery.
1. Knutsson F. The vacuum phenomenon in the intervertebral discs. Acta Radiol. 1942;23:173-179.
2. Resnick D, Niwayama G, Guerra J Jr, Vint V, Usselman J. Spinal vacuum phenomenon: anatomical study and review. Radiology. 1981;139(2):341-348.
3. Lardé D, Mathieu D, Frija J, Gaston A, Vasile N. Spinal vacuum phenomenon: CT diagnosis and significance. J Comput Assist Tomogr. 1982;6(4):671-676.
4. Stallenberg B, Madani A, Burny F, Gevenois PA. The vacuum phenomenon: a CT sign of nonunited fracture. AJR Am J Roentgenol. 2001;176(5):1161-1164.
5. Martel W. Spinal pseudarthrosis: a complication of ankylosing spondylitis. Arthritis Rheum. 1978;21(4):485-490.
6. Chan FL, Ho EK, Chau EM. Spinal pseudarthrosis complicating ankylosing spondylitis: comparison of CT and conventional tomography. AJR Am J Roentgenol. 1988;150(3):611-614.
7. Edwards CE, Antonoiades SB, Ford L, Crabster E. CT vacuum disc sign: a highly specific predictor of lumbar nonunion. Poster presented at: 41st Annual Meeting of the Scoliosis Research Society; September 2006; Monterey, CA.
8. Carreon LY, Djurasovic M, Glassman SD, Sailer P. Diagnostic accuracy and reliability of fine-cut CT scans with reconstructions to determine the status of an instrumented posterolateral fusion with surgical exploration as reference standard. Spine. 2007;32(8):892-895.
9. Goobar JE, Pate D, Resnick D, Sartoris DJ. Radiography of the hyperextended lumbar spine: an effective technique for the demonstration of discal vacuum phenomena. Can Assoc Radiol J. 1987;38(4):271-274.
10. Grenier N, Grossman RI, Schiebler ML, Yeager BA, Goldberg HI, Kressel HY. Degenerative lumbar disk disease: pitfalls and usefulness of MR imaging in detection of vacuum phenomenon. Radiology. 1987;164(3):861-865.
11. Wang HJ, Chen BB, Yu CW, Hsu CY, Shih TT. Alteration of disc vacuum contents during prolonged supine positioning: evaluation with MR Image. Spine. 2007;32(23):2610-2615.
12. D’Anastasi M, Birkenmaier C, Schmidt GP, Wegener B, Reiser MF, Baur-Melnyk A. Correlation between vacuum phenomenon on CT and fluid on MRI in degenerative disks. AJR Am J Roentgenol. 2011;197(5):1182-1189.
13. Mirovsky Y, Anekstein Y, Shalmon E, Peer A. Vacuum clefts of the vertebral bodies. AJNR Am J Neuroradiol. 2005;26(7):1634-1640.
14. Selby MD, Clark SR, Hall DJ, Freeman BJ. Radiologic assessment of spinal fusion. J Am Acad Orthop Surg. 2012;20(11):694-703.
15. Kanayama M, Hashimoto T, Shigenobu K, Yamane S, Bauer TW, Togawa D. A prospective randomized study of posterolateral lumbar fusion using osteogenic protein-1 (OP-1) versus local autograft with ceramic bone substitute: emphasis of surgical exploration and histologic assessment. Spine. 2006;31(10):1067-1074.
The spinal vacuum sign or vacuum phenomenon (VP) is the radiographic finding of an air-density linear radiolucency in the intervertebral disc or vertebral body. The result of a gaseous accumulation, it is often a diagnostic sign of disc degeneration as well as a rare sign of infection, Schmorl node formation, or osteonecrosis.1,2 Although the VP was first described on plain radiographs, it is better seen on computed tomography (CT).3 Multiple studies have found a possible association between the VP and nonunion in diaphyseal fractures,4 ankylosing spondylitis,5,6 and lumbar spinal fusion.7
To our knowledge, no one has studied whether the intervertebral VP resolves after posterolateral lumbar spinal fusion in adults with degenerative spinal pathology, and no one has investigated the association between the persistence of the intervertebral VP and pseudarthrosis after posterolateral spinal fusion.
We conducted a study to determine whether the VP resolves after posterolateral lumbar spinal fusion procedures and whether persistence of the VP after fusion surgery is indicative of pseudarthrosis.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of patients who had degenerative spinal stenosis with instability and the intervertebral vacuum sign on preoperative digital lumbar spine CT scans and who underwent posterolateral lumbar spinal fusion with or without instrumentation. Study inclusion criteria were lumbar spine CT at minimum 6-month follow-up after spinal fusion and preoperative and postoperative lumbar spine radiographs. Exclusion criteria were any type of interbody fusion procedure (anterior, posterior, transforaminal, lateral) at a level with the VP, age under 21 years, follow-up of less than 6 months, and incomplete radiographic records. As this was a retrospective study, patient consent was not required.
CT was performed with a 16-, 64-, or 128-slice multidetector CT scanner with effective tube current set at 250 to 320 mA, voltage set at 120 to 140 kV, and pitch set at 0.75 to 0.9. After axial acquisition of 3×3-mm isometric voxels, sagittal and coronal multiplanar images were reconstructed with a slice thickness of 2 mm. Patient demographics, diagnoses, and surgical details were recorded. All digital lumbar spine CT scans and radiographs were initially screened on PACS (picture archiving and communication system) by the orthopedic spine surgery fellow at an academic medical institution; then they were reviewed on a radiology reading room monitor by 3 observers (senior radiologist, senior orthopedic spine surgeon, orthopedic spine surgery fellow). Axial images and sagittal and coronal reconstructed images of the preoperative and postoperative follow-up lumbar CT scans—together with the lateral and anteroposterior lumbar spine radiographs—were evaluated for the intervertebral VP. Mean (SD) follow-up (with CT to assess fusion) was 1.6 (0.86) years (range, 0.75-3.38 years). Fusion at each level was evaluated on the postoperative follow-up CT on axial images and sagittal and coronal reconstructed images; criteria for fusion were continuous bridging bone across posterolateral gutters and facets on one or both sides at each intervertebral level.8 Pseudarthrosis was recorded if there was no continuity of bridging bone across both posterolateral gutters and facets, a complete radiolucent line on both sides across a level, or lysis or loosening around screws. All recordings were made by consensus, or by majority decision in case of disagreement.
Presence of the VP at the lumbar levels not included in the fusion was also recorded on the preoperative and follow-up CT scan and radiographs.
Descriptive and inferential statistical tests were performed as applicable. Pearson χ2 test and Fischer exact test were used to evaluate if there was a significant association between the groups where the VP disappeared and persisted and fusion and pseudarthrosis. Significance was set at P < .05. Statistical analysis was performed with Stata Version 10.0.
Results
Using the preoperative lumbar spine CT scans of 18 patients (10 men, 8 women), we identified 36 cases of intervertebral levels exhibiting the VP (median positive vacuum sign levels per patient, 2; minimum, 1; maximum, 5) at the levels included in the fusion (Table 1). Mean (SD) age at surgery was 67.6 (9.4) years (range, 46.5-79.6 years). Mean (SD) radiologic follow-up was 1.6 (0.86) years (range, 0.75-3.38 years). All patients underwent lumbar fusion with local autograft, allograft, and recombinant human bone morphogenetic protein 2. Spinal instrumentation was used in 16 of the 18 patients.
On preoperative CT, positive VP was diagnosed in the 36 cases as follows: L5–S1 (11 cases), L4–L5 (9 cases), L3–L4 (4 cases), L2–L3 (6 cases), L1–L2 (4 cases), and T12–L1 (2 cases). On follow-up CT, 15 cases showed persistence of the VP, and 21 cases showed disappearance of the VP (Table 1).
Evidence of spinal fusion was identified on follow-up CT in 32 (88.9%) of the 36 cases. In 3 of the 18 patients, nonunion was diagnosed. Of the 15 intervertebral cases in which the VP persisted, 13 (86.7%) showed evidence of fusion on CT, and 2 (13.3%) showed evidence of pseudarthrosis. Of the 21 intervertebral cases in which the VP disappeared, 19 (90.5%) showed evidence of fusion on CT, and 2 (9.5%) showed evidence of pseudarthrosis (Table 2). There was no significant difference in fusion rate or pseudarthrosis rate in the groups in which the VP persisted or disappeared (Fischer exact test, P = .99). There was no significant association between VP persistence or disappearance and sex, primary or revision surgery, or intervertebral level (Fischer exact test, P > .05). A case example is shown in the Figure.
At levels not included in spinal fusion, CT identified the VP at 6 lumbar intervertebral levels before surgery and 11 levels at follow-up. The VP did not disappear at any level not included in the fusion. At follow-up, no new VP was identified in a segment included in fusion. Results are summarized in Table 3.
Discussion
The association of radiologic intervertebral VP and disc degeneration, first recognized by Knutsson1 in 1942, refers to the presence of gas, mainly containing nitrogen, in the crevices between or within vertebrae.2 The VP is more often seen in patients older than 50 years, on plain radiographs in hyperextension.9 CT is more sensitive than radiography in detecting the VP; Lardé and colleagues3 found it in about 50% of 50 patients on CT scans but in only 12% of patients on radiographs. The VP is visible because of the nitrogen gas that accumulates when there is a negative pressure within the disc space. Nitrogen emerges from the blood and moves into the disc space; perhaps the disc space opens, causing the negative pressure.1-3 On T1- or T2-weighted magnetic resonance imaging (MRI), the VP is visible as a signal void. MRI, however, is less accurate than CT.10 In a study of 10 patients who had low back pain and more than 1 level of intradiscal VP, and who underwent supine MRI examinations at 0, 1, and 2 hours, Wang and colleagues11 found that, after prolonged supine positioning, the signal intensity of the vacuum was replaced by hyperintense fluid contents. D’Anastasi and colleagues,12 in a study of 20 patients who had lumbar vacuum phenomenon on CT and underwent MRI examinations, found a significant correlation between presence of intradiscal fluid and amount of bone marrow edema on MRI and degenerative endplate abnormalities on CT. In the present study, we found that, after the spinal fusion vacuum phenomenon disappeared in 58.3% of the lumbar levels and persisted in 41.7% on follow-up CT at the levels included in posterolateral fusion, there were 5 new levels, adjacent to the lumbar fusion, where the VP was seen on the follow-up CT.
We studied whether evidence of a persistent vacuum sign on CT is indicative of pseudarthrosis. Other authors have reported an association between the VP and nonunion in fractures4 and ankylosing spondylitis.5,6 In a study of 19 patients with diaphyseal fractures, Stallenberg and colleagues4 found that, in 7 of the 10 patients with nonunion, the VP was detected on CT at the nonunion site. Martel5 first reported on the intervertebral VP in a case of ankylosing spondylitis with spinal pseudarthrosis. Ten years later, in a study of 18 patients with advanced ankylosing spondylitis with spinal pseudarthrosis, Chan and colleagues6 identified the intervertebral VP on CT in 7 patients. Edwards and colleagues7 studied 15 patients with prior lumbar fusion with 17 positive intervertebral VP levels on CT and found that the vacuum disc sign was a strong predictor of lumbar nonunion as determined by surgical exploration. Mirovsky and colleagues13 identified the intravertebral vacuum cleft in 26 patients with an osteoporotic vertebral fracture treated with vertebroplasty and concluded that nonunion of the vertebral fracture could be identified by presence of the intravertebral vacuum cleft on radiography. In the present study, there was radiologic evidence of lumbar spinal fusion in 89% of disc levels with a preoperative positive intervertebral VP and pseudarthrosis in 11% of disc levels. The rate of fusion at levels with the VP was comparable to the rate at intervertebral levels without the phenomenon. These findings indicate that persistence of the VP after spinal fusion is not an indication that fusion has not been achieved. Preoperative VP also did not predispose to failure of fusion. That there is a persistent vacuum disc might imply that, even after successful fusion as seen on CT, some motion may be occurring at the disc level to cause a negative pressure phenomenon. Even in cases of facet fusion with bridging bone, there may still be motion at the disc level, as fusions can plastically deform (even with screws in), particularly in elderly osteopenic bone. We found no association between a persistent vacuum sign and pseudarthrosis. Our study findings are clinically useful even if the benefits are limited. These findings may help surgeons avoid misinterpreting this sign as an indication for additional surgery.
This study had some limitations. First, radiographs were used to determine presence or absence of fusion. Although CT is widely considered the gold standard for noninvasive assessment of fusion,14 even when both posterolateral gutters and facets have been found to be fused on CT, the probability of a solid fusion on exploration ranges from 69% to 96%.8,15 Second, detection of the VP on radiographs and CT may be affected by patient position.11 Third, this was a retrospective series with a small number of patients and limited follow-up with CT. Arthrodesis and the VP may take years to fully evolve. It is possible that fusion rates could be higher on longer follow-up, and resolution of the VP may occur with longer follow-up. Fourth, clinical outcomes were not evaluated, as there are other confounding factors, apart from successful fusion, that could affect clinical outcomes. A larger prospective controlled study would be helpful.
Conclusion
The radiologic intervertebral VP may persist after posterolateral lumbar spinal fusion. We did not find an association between the VP and pseudarthrosis. In addition, VP persistence on follow-up CT was not indicative of pseudarthrosis, and VP disappearance was not indicative of fusion. The vacuum sign should not be misinterpreted as an indication for additional surgery.
The spinal vacuum sign or vacuum phenomenon (VP) is the radiographic finding of an air-density linear radiolucency in the intervertebral disc or vertebral body. The result of a gaseous accumulation, it is often a diagnostic sign of disc degeneration as well as a rare sign of infection, Schmorl node formation, or osteonecrosis.1,2 Although the VP was first described on plain radiographs, it is better seen on computed tomography (CT).3 Multiple studies have found a possible association between the VP and nonunion in diaphyseal fractures,4 ankylosing spondylitis,5,6 and lumbar spinal fusion.7
To our knowledge, no one has studied whether the intervertebral VP resolves after posterolateral lumbar spinal fusion in adults with degenerative spinal pathology, and no one has investigated the association between the persistence of the intervertebral VP and pseudarthrosis after posterolateral spinal fusion.
We conducted a study to determine whether the VP resolves after posterolateral lumbar spinal fusion procedures and whether persistence of the VP after fusion surgery is indicative of pseudarthrosis.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we retrospectively reviewed the medical records of patients who had degenerative spinal stenosis with instability and the intervertebral vacuum sign on preoperative digital lumbar spine CT scans and who underwent posterolateral lumbar spinal fusion with or without instrumentation. Study inclusion criteria were lumbar spine CT at minimum 6-month follow-up after spinal fusion and preoperative and postoperative lumbar spine radiographs. Exclusion criteria were any type of interbody fusion procedure (anterior, posterior, transforaminal, lateral) at a level with the VP, age under 21 years, follow-up of less than 6 months, and incomplete radiographic records. As this was a retrospective study, patient consent was not required.
CT was performed with a 16-, 64-, or 128-slice multidetector CT scanner with effective tube current set at 250 to 320 mA, voltage set at 120 to 140 kV, and pitch set at 0.75 to 0.9. After axial acquisition of 3×3-mm isometric voxels, sagittal and coronal multiplanar images were reconstructed with a slice thickness of 2 mm. Patient demographics, diagnoses, and surgical details were recorded. All digital lumbar spine CT scans and radiographs were initially screened on PACS (picture archiving and communication system) by the orthopedic spine surgery fellow at an academic medical institution; then they were reviewed on a radiology reading room monitor by 3 observers (senior radiologist, senior orthopedic spine surgeon, orthopedic spine surgery fellow). Axial images and sagittal and coronal reconstructed images of the preoperative and postoperative follow-up lumbar CT scans—together with the lateral and anteroposterior lumbar spine radiographs—were evaluated for the intervertebral VP. Mean (SD) follow-up (with CT to assess fusion) was 1.6 (0.86) years (range, 0.75-3.38 years). Fusion at each level was evaluated on the postoperative follow-up CT on axial images and sagittal and coronal reconstructed images; criteria for fusion were continuous bridging bone across posterolateral gutters and facets on one or both sides at each intervertebral level.8 Pseudarthrosis was recorded if there was no continuity of bridging bone across both posterolateral gutters and facets, a complete radiolucent line on both sides across a level, or lysis or loosening around screws. All recordings were made by consensus, or by majority decision in case of disagreement.
Presence of the VP at the lumbar levels not included in the fusion was also recorded on the preoperative and follow-up CT scan and radiographs.
Descriptive and inferential statistical tests were performed as applicable. Pearson χ2 test and Fischer exact test were used to evaluate if there was a significant association between the groups where the VP disappeared and persisted and fusion and pseudarthrosis. Significance was set at P < .05. Statistical analysis was performed with Stata Version 10.0.
Results
Using the preoperative lumbar spine CT scans of 18 patients (10 men, 8 women), we identified 36 cases of intervertebral levels exhibiting the VP (median positive vacuum sign levels per patient, 2; minimum, 1; maximum, 5) at the levels included in the fusion (Table 1). Mean (SD) age at surgery was 67.6 (9.4) years (range, 46.5-79.6 years). Mean (SD) radiologic follow-up was 1.6 (0.86) years (range, 0.75-3.38 years). All patients underwent lumbar fusion with local autograft, allograft, and recombinant human bone morphogenetic protein 2. Spinal instrumentation was used in 16 of the 18 patients.
On preoperative CT, positive VP was diagnosed in the 36 cases as follows: L5–S1 (11 cases), L4–L5 (9 cases), L3–L4 (4 cases), L2–L3 (6 cases), L1–L2 (4 cases), and T12–L1 (2 cases). On follow-up CT, 15 cases showed persistence of the VP, and 21 cases showed disappearance of the VP (Table 1).
Evidence of spinal fusion was identified on follow-up CT in 32 (88.9%) of the 36 cases. In 3 of the 18 patients, nonunion was diagnosed. Of the 15 intervertebral cases in which the VP persisted, 13 (86.7%) showed evidence of fusion on CT, and 2 (13.3%) showed evidence of pseudarthrosis. Of the 21 intervertebral cases in which the VP disappeared, 19 (90.5%) showed evidence of fusion on CT, and 2 (9.5%) showed evidence of pseudarthrosis (Table 2). There was no significant difference in fusion rate or pseudarthrosis rate in the groups in which the VP persisted or disappeared (Fischer exact test, P = .99). There was no significant association between VP persistence or disappearance and sex, primary or revision surgery, or intervertebral level (Fischer exact test, P > .05). A case example is shown in the Figure.
At levels not included in spinal fusion, CT identified the VP at 6 lumbar intervertebral levels before surgery and 11 levels at follow-up. The VP did not disappear at any level not included in the fusion. At follow-up, no new VP was identified in a segment included in fusion. Results are summarized in Table 3.
Discussion
The association of radiologic intervertebral VP and disc degeneration, first recognized by Knutsson1 in 1942, refers to the presence of gas, mainly containing nitrogen, in the crevices between or within vertebrae.2 The VP is more often seen in patients older than 50 years, on plain radiographs in hyperextension.9 CT is more sensitive than radiography in detecting the VP; Lardé and colleagues3 found it in about 50% of 50 patients on CT scans but in only 12% of patients on radiographs. The VP is visible because of the nitrogen gas that accumulates when there is a negative pressure within the disc space. Nitrogen emerges from the blood and moves into the disc space; perhaps the disc space opens, causing the negative pressure.1-3 On T1- or T2-weighted magnetic resonance imaging (MRI), the VP is visible as a signal void. MRI, however, is less accurate than CT.10 In a study of 10 patients who had low back pain and more than 1 level of intradiscal VP, and who underwent supine MRI examinations at 0, 1, and 2 hours, Wang and colleagues11 found that, after prolonged supine positioning, the signal intensity of the vacuum was replaced by hyperintense fluid contents. D’Anastasi and colleagues,12 in a study of 20 patients who had lumbar vacuum phenomenon on CT and underwent MRI examinations, found a significant correlation between presence of intradiscal fluid and amount of bone marrow edema on MRI and degenerative endplate abnormalities on CT. In the present study, we found that, after the spinal fusion vacuum phenomenon disappeared in 58.3% of the lumbar levels and persisted in 41.7% on follow-up CT at the levels included in posterolateral fusion, there were 5 new levels, adjacent to the lumbar fusion, where the VP was seen on the follow-up CT.
We studied whether evidence of a persistent vacuum sign on CT is indicative of pseudarthrosis. Other authors have reported an association between the VP and nonunion in fractures4 and ankylosing spondylitis.5,6 In a study of 19 patients with diaphyseal fractures, Stallenberg and colleagues4 found that, in 7 of the 10 patients with nonunion, the VP was detected on CT at the nonunion site. Martel5 first reported on the intervertebral VP in a case of ankylosing spondylitis with spinal pseudarthrosis. Ten years later, in a study of 18 patients with advanced ankylosing spondylitis with spinal pseudarthrosis, Chan and colleagues6 identified the intervertebral VP on CT in 7 patients. Edwards and colleagues7 studied 15 patients with prior lumbar fusion with 17 positive intervertebral VP levels on CT and found that the vacuum disc sign was a strong predictor of lumbar nonunion as determined by surgical exploration. Mirovsky and colleagues13 identified the intravertebral vacuum cleft in 26 patients with an osteoporotic vertebral fracture treated with vertebroplasty and concluded that nonunion of the vertebral fracture could be identified by presence of the intravertebral vacuum cleft on radiography. In the present study, there was radiologic evidence of lumbar spinal fusion in 89% of disc levels with a preoperative positive intervertebral VP and pseudarthrosis in 11% of disc levels. The rate of fusion at levels with the VP was comparable to the rate at intervertebral levels without the phenomenon. These findings indicate that persistence of the VP after spinal fusion is not an indication that fusion has not been achieved. Preoperative VP also did not predispose to failure of fusion. That there is a persistent vacuum disc might imply that, even after successful fusion as seen on CT, some motion may be occurring at the disc level to cause a negative pressure phenomenon. Even in cases of facet fusion with bridging bone, there may still be motion at the disc level, as fusions can plastically deform (even with screws in), particularly in elderly osteopenic bone. We found no association between a persistent vacuum sign and pseudarthrosis. Our study findings are clinically useful even if the benefits are limited. These findings may help surgeons avoid misinterpreting this sign as an indication for additional surgery.
This study had some limitations. First, radiographs were used to determine presence or absence of fusion. Although CT is widely considered the gold standard for noninvasive assessment of fusion,14 even when both posterolateral gutters and facets have been found to be fused on CT, the probability of a solid fusion on exploration ranges from 69% to 96%.8,15 Second, detection of the VP on radiographs and CT may be affected by patient position.11 Third, this was a retrospective series with a small number of patients and limited follow-up with CT. Arthrodesis and the VP may take years to fully evolve. It is possible that fusion rates could be higher on longer follow-up, and resolution of the VP may occur with longer follow-up. Fourth, clinical outcomes were not evaluated, as there are other confounding factors, apart from successful fusion, that could affect clinical outcomes. A larger prospective controlled study would be helpful.
Conclusion
The radiologic intervertebral VP may persist after posterolateral lumbar spinal fusion. We did not find an association between the VP and pseudarthrosis. In addition, VP persistence on follow-up CT was not indicative of pseudarthrosis, and VP disappearance was not indicative of fusion. The vacuum sign should not be misinterpreted as an indication for additional surgery.
1. Knutsson F. The vacuum phenomenon in the intervertebral discs. Acta Radiol. 1942;23:173-179.
2. Resnick D, Niwayama G, Guerra J Jr, Vint V, Usselman J. Spinal vacuum phenomenon: anatomical study and review. Radiology. 1981;139(2):341-348.
3. Lardé D, Mathieu D, Frija J, Gaston A, Vasile N. Spinal vacuum phenomenon: CT diagnosis and significance. J Comput Assist Tomogr. 1982;6(4):671-676.
4. Stallenberg B, Madani A, Burny F, Gevenois PA. The vacuum phenomenon: a CT sign of nonunited fracture. AJR Am J Roentgenol. 2001;176(5):1161-1164.
5. Martel W. Spinal pseudarthrosis: a complication of ankylosing spondylitis. Arthritis Rheum. 1978;21(4):485-490.
6. Chan FL, Ho EK, Chau EM. Spinal pseudarthrosis complicating ankylosing spondylitis: comparison of CT and conventional tomography. AJR Am J Roentgenol. 1988;150(3):611-614.
7. Edwards CE, Antonoiades SB, Ford L, Crabster E. CT vacuum disc sign: a highly specific predictor of lumbar nonunion. Poster presented at: 41st Annual Meeting of the Scoliosis Research Society; September 2006; Monterey, CA.
8. Carreon LY, Djurasovic M, Glassman SD, Sailer P. Diagnostic accuracy and reliability of fine-cut CT scans with reconstructions to determine the status of an instrumented posterolateral fusion with surgical exploration as reference standard. Spine. 2007;32(8):892-895.
9. Goobar JE, Pate D, Resnick D, Sartoris DJ. Radiography of the hyperextended lumbar spine: an effective technique for the demonstration of discal vacuum phenomena. Can Assoc Radiol J. 1987;38(4):271-274.
10. Grenier N, Grossman RI, Schiebler ML, Yeager BA, Goldberg HI, Kressel HY. Degenerative lumbar disk disease: pitfalls and usefulness of MR imaging in detection of vacuum phenomenon. Radiology. 1987;164(3):861-865.
11. Wang HJ, Chen BB, Yu CW, Hsu CY, Shih TT. Alteration of disc vacuum contents during prolonged supine positioning: evaluation with MR Image. Spine. 2007;32(23):2610-2615.
12. D’Anastasi M, Birkenmaier C, Schmidt GP, Wegener B, Reiser MF, Baur-Melnyk A. Correlation between vacuum phenomenon on CT and fluid on MRI in degenerative disks. AJR Am J Roentgenol. 2011;197(5):1182-1189.
13. Mirovsky Y, Anekstein Y, Shalmon E, Peer A. Vacuum clefts of the vertebral bodies. AJNR Am J Neuroradiol. 2005;26(7):1634-1640.
14. Selby MD, Clark SR, Hall DJ, Freeman BJ. Radiologic assessment of spinal fusion. J Am Acad Orthop Surg. 2012;20(11):694-703.
15. Kanayama M, Hashimoto T, Shigenobu K, Yamane S, Bauer TW, Togawa D. A prospective randomized study of posterolateral lumbar fusion using osteogenic protein-1 (OP-1) versus local autograft with ceramic bone substitute: emphasis of surgical exploration and histologic assessment. Spine. 2006;31(10):1067-1074.
1. Knutsson F. The vacuum phenomenon in the intervertebral discs. Acta Radiol. 1942;23:173-179.
2. Resnick D, Niwayama G, Guerra J Jr, Vint V, Usselman J. Spinal vacuum phenomenon: anatomical study and review. Radiology. 1981;139(2):341-348.
3. Lardé D, Mathieu D, Frija J, Gaston A, Vasile N. Spinal vacuum phenomenon: CT diagnosis and significance. J Comput Assist Tomogr. 1982;6(4):671-676.
4. Stallenberg B, Madani A, Burny F, Gevenois PA. The vacuum phenomenon: a CT sign of nonunited fracture. AJR Am J Roentgenol. 2001;176(5):1161-1164.
5. Martel W. Spinal pseudarthrosis: a complication of ankylosing spondylitis. Arthritis Rheum. 1978;21(4):485-490.
6. Chan FL, Ho EK, Chau EM. Spinal pseudarthrosis complicating ankylosing spondylitis: comparison of CT and conventional tomography. AJR Am J Roentgenol. 1988;150(3):611-614.
7. Edwards CE, Antonoiades SB, Ford L, Crabster E. CT vacuum disc sign: a highly specific predictor of lumbar nonunion. Poster presented at: 41st Annual Meeting of the Scoliosis Research Society; September 2006; Monterey, CA.
8. Carreon LY, Djurasovic M, Glassman SD, Sailer P. Diagnostic accuracy and reliability of fine-cut CT scans with reconstructions to determine the status of an instrumented posterolateral fusion with surgical exploration as reference standard. Spine. 2007;32(8):892-895.
9. Goobar JE, Pate D, Resnick D, Sartoris DJ. Radiography of the hyperextended lumbar spine: an effective technique for the demonstration of discal vacuum phenomena. Can Assoc Radiol J. 1987;38(4):271-274.
10. Grenier N, Grossman RI, Schiebler ML, Yeager BA, Goldberg HI, Kressel HY. Degenerative lumbar disk disease: pitfalls and usefulness of MR imaging in detection of vacuum phenomenon. Radiology. 1987;164(3):861-865.
11. Wang HJ, Chen BB, Yu CW, Hsu CY, Shih TT. Alteration of disc vacuum contents during prolonged supine positioning: evaluation with MR Image. Spine. 2007;32(23):2610-2615.
12. D’Anastasi M, Birkenmaier C, Schmidt GP, Wegener B, Reiser MF, Baur-Melnyk A. Correlation between vacuum phenomenon on CT and fluid on MRI in degenerative disks. AJR Am J Roentgenol. 2011;197(5):1182-1189.
13. Mirovsky Y, Anekstein Y, Shalmon E, Peer A. Vacuum clefts of the vertebral bodies. AJNR Am J Neuroradiol. 2005;26(7):1634-1640.
14. Selby MD, Clark SR, Hall DJ, Freeman BJ. Radiologic assessment of spinal fusion. J Am Acad Orthop Surg. 2012;20(11):694-703.
15. Kanayama M, Hashimoto T, Shigenobu K, Yamane S, Bauer TW, Togawa D. A prospective randomized study of posterolateral lumbar fusion using osteogenic protein-1 (OP-1) versus local autograft with ceramic bone substitute: emphasis of surgical exploration and histologic assessment. Spine. 2006;31(10):1067-1074.
Driving skills already affected with mild cognitive impairment
TORONTO – Patients with amnestic mild cognitive impairment made significantly more errors during a complex test of driving skills and showed altered brain activity while performing this test in a two-part study.
The findings are the first to show a correlation between physiologic brain activity and driving and suggest that patients with mild cognitive impairment (MCI) may already be experiencing potentially dangerous changes in their ability to operate a motor vehicle, Megan Hird said at the Alzheimer’s Association International Conference 2016.
“Driving is a highly complex behavior that requires the integration of cognitive, motor function, and perceptual abilities,” said Ms. Hird, a researcher at the University of Toronto. “Individuals with multidomain MCI can express memory, visuospatial, and executive function deficits” even before their less demanding activities of daily living are affected. Nevertheless, Ms. Hird said, “these deficits can affect someone’s ability to drive safely.”
The decision to hang up the car keys is always a tough one for patients with dementia, their families, and their physicians, and there are no guidelines in the United States or Canada to help in that decision. But Ms. Hird and her colleagues recently published a meta-analysis of driving studies in which patients with very mild Alzheimer’s were 10 times more likely to fail an on-road driving test than were cognitively healthy drivers (J Alz Dis. 2016;53[2]:713-29).
Nevertheless, both the American and Canadian Medical Associations state that a diagnosis of cognitive impairment is not sufficient to withdraw driving privileges, and no cognitive test is capable of determining driving ability.
Ms. Hird used a combination of a driving simulator and functional MRI to study driving skill and the neural correlates of that activity. There were two parts to the study. The first comprised 20 healthy controls and 24 amnestic MCI patients (11 with single-domain MCI and 13 with multidomain MCI). The driving tasks were routine right and left turns, plus left turns against traffic and left turns against traffic with auditory distraction.
Overall, those with MCI (both single- and multi-domain patients combined) committed significantly more risky driving errors than the control group (mean of 9 vs. 3). Risky errors were considered errors that could result in an imminent collision, crossing the center line into oncoming traffic, or crossing onto the sidewalk.
When the researchers examined the MCI groups separately, they determined that only those with multi-domain MCI performed significantly worse than did controls (mean of 14 vs. 2.7 errors). These errors were most often committed during the more complicated left turns. There were no significant between-group differences in routine right and left turns.
Differences in brain activation revealed
The second part comprised 32 patients: 16 with MCI and 16 age-matched, cognitively normal controls. These subjects undertook both the driving simulation and the functional MRI test.
On the uncomplicated right turns, there were no between-group differences in errors, Ms. Hird said. However, there were some differences in the way the subjects’ brains reacted to the task. “On the brain activation patterns, the healthy controls showed some negative activation in the temporal and occipital regions, areas that actually were activated in the patients. The patients also showed some increased signal in the posterior medial regions, compared with the healthy controls. Overall, however, the activation patterns looked quite similar between the two groups.”
The left turn into traffic task showed quite different results. “In the MCI group, there was more anterior activation. They also showed significantly higher activation than did the control group in the orbitofrontal, medial superior, and midfrontal cortex – all of which are involved in planning, higher-order attention, and cognitive control.”
Recognizing that there are demonstrable differences in brain activation that correlate with poor driving performance could spark the creation of an objective clinical measurement tool for determining driving status, Ms. Hird said.
“This is an important fundamental step in the development of a tool that is sensitive and specific for addressing the driving safety of these patients.”
During discussion after her presentation, she fielded a question from an Australian clinician, who said his country likewise has no objective measure of when driving is no longer safe for a dementia patient.
“We accept the fact that people do drive with some degree of cognitive impairment,” said the physician, who did not identify himself. “But there are regions where having a license is important for social engagement. And we know that social disengagement drives rapid progression of cognitive decline. I think there is a real tension here between the withdrawal of licensure and the preservation of social engagement.”
Ms. Hird agreed. “Obviously, many MCI patients do retain the ability to drive safely. But we need to achieve a balance between maintaining patient autonomy and the safety of the general public.”
Ms. Hird had no financial disclosures.
On Twitter @alz_gal
TORONTO – Patients with amnestic mild cognitive impairment made significantly more errors during a complex test of driving skills and showed altered brain activity while performing this test in a two-part study.
The findings are the first to show a correlation between physiologic brain activity and driving and suggest that patients with mild cognitive impairment (MCI) may already be experiencing potentially dangerous changes in their ability to operate a motor vehicle, Megan Hird said at the Alzheimer’s Association International Conference 2016.
“Driving is a highly complex behavior that requires the integration of cognitive, motor function, and perceptual abilities,” said Ms. Hird, a researcher at the University of Toronto. “Individuals with multidomain MCI can express memory, visuospatial, and executive function deficits” even before their less demanding activities of daily living are affected. Nevertheless, Ms. Hird said, “these deficits can affect someone’s ability to drive safely.”
The decision to hang up the car keys is always a tough one for patients with dementia, their families, and their physicians, and there are no guidelines in the United States or Canada to help in that decision. But Ms. Hird and her colleagues recently published a meta-analysis of driving studies in which patients with very mild Alzheimer’s were 10 times more likely to fail an on-road driving test than were cognitively healthy drivers (J Alz Dis. 2016;53[2]:713-29).
Nevertheless, both the American and Canadian Medical Associations state that a diagnosis of cognitive impairment is not sufficient to withdraw driving privileges, and no cognitive test is capable of determining driving ability.
Ms. Hird used a combination of a driving simulator and functional MRI to study driving skill and the neural correlates of that activity. There were two parts to the study. The first comprised 20 healthy controls and 24 amnestic MCI patients (11 with single-domain MCI and 13 with multidomain MCI). The driving tasks were routine right and left turns, plus left turns against traffic and left turns against traffic with auditory distraction.
Overall, those with MCI (both single- and multi-domain patients combined) committed significantly more risky driving errors than the control group (mean of 9 vs. 3). Risky errors were considered errors that could result in an imminent collision, crossing the center line into oncoming traffic, or crossing onto the sidewalk.
When the researchers examined the MCI groups separately, they determined that only those with multi-domain MCI performed significantly worse than did controls (mean of 14 vs. 2.7 errors). These errors were most often committed during the more complicated left turns. There were no significant between-group differences in routine right and left turns.
Differences in brain activation revealed
The second part comprised 32 patients: 16 with MCI and 16 age-matched, cognitively normal controls. These subjects undertook both the driving simulation and the functional MRI test.
On the uncomplicated right turns, there were no between-group differences in errors, Ms. Hird said. However, there were some differences in the way the subjects’ brains reacted to the task. “On the brain activation patterns, the healthy controls showed some negative activation in the temporal and occipital regions, areas that actually were activated in the patients. The patients also showed some increased signal in the posterior medial regions, compared with the healthy controls. Overall, however, the activation patterns looked quite similar between the two groups.”
The left turn into traffic task showed quite different results. “In the MCI group, there was more anterior activation. They also showed significantly higher activation than did the control group in the orbitofrontal, medial superior, and midfrontal cortex – all of which are involved in planning, higher-order attention, and cognitive control.”
Recognizing that there are demonstrable differences in brain activation that correlate with poor driving performance could spark the creation of an objective clinical measurement tool for determining driving status, Ms. Hird said.
“This is an important fundamental step in the development of a tool that is sensitive and specific for addressing the driving safety of these patients.”
During discussion after her presentation, she fielded a question from an Australian clinician, who said his country likewise has no objective measure of when driving is no longer safe for a dementia patient.
“We accept the fact that people do drive with some degree of cognitive impairment,” said the physician, who did not identify himself. “But there are regions where having a license is important for social engagement. And we know that social disengagement drives rapid progression of cognitive decline. I think there is a real tension here between the withdrawal of licensure and the preservation of social engagement.”
Ms. Hird agreed. “Obviously, many MCI patients do retain the ability to drive safely. But we need to achieve a balance between maintaining patient autonomy and the safety of the general public.”
Ms. Hird had no financial disclosures.
On Twitter @alz_gal
TORONTO – Patients with amnestic mild cognitive impairment made significantly more errors during a complex test of driving skills and showed altered brain activity while performing this test in a two-part study.
The findings are the first to show a correlation between physiologic brain activity and driving and suggest that patients with mild cognitive impairment (MCI) may already be experiencing potentially dangerous changes in their ability to operate a motor vehicle, Megan Hird said at the Alzheimer’s Association International Conference 2016.
“Driving is a highly complex behavior that requires the integration of cognitive, motor function, and perceptual abilities,” said Ms. Hird, a researcher at the University of Toronto. “Individuals with multidomain MCI can express memory, visuospatial, and executive function deficits” even before their less demanding activities of daily living are affected. Nevertheless, Ms. Hird said, “these deficits can affect someone’s ability to drive safely.”
The decision to hang up the car keys is always a tough one for patients with dementia, their families, and their physicians, and there are no guidelines in the United States or Canada to help in that decision. But Ms. Hird and her colleagues recently published a meta-analysis of driving studies in which patients with very mild Alzheimer’s were 10 times more likely to fail an on-road driving test than were cognitively healthy drivers (J Alz Dis. 2016;53[2]:713-29).
Nevertheless, both the American and Canadian Medical Associations state that a diagnosis of cognitive impairment is not sufficient to withdraw driving privileges, and no cognitive test is capable of determining driving ability.
Ms. Hird used a combination of a driving simulator and functional MRI to study driving skill and the neural correlates of that activity. There were two parts to the study. The first comprised 20 healthy controls and 24 amnestic MCI patients (11 with single-domain MCI and 13 with multidomain MCI). The driving tasks were routine right and left turns, plus left turns against traffic and left turns against traffic with auditory distraction.
Overall, those with MCI (both single- and multi-domain patients combined) committed significantly more risky driving errors than the control group (mean of 9 vs. 3). Risky errors were considered errors that could result in an imminent collision, crossing the center line into oncoming traffic, or crossing onto the sidewalk.
When the researchers examined the MCI groups separately, they determined that only those with multi-domain MCI performed significantly worse than did controls (mean of 14 vs. 2.7 errors). These errors were most often committed during the more complicated left turns. There were no significant between-group differences in routine right and left turns.
Differences in brain activation revealed
The second part comprised 32 patients: 16 with MCI and 16 age-matched, cognitively normal controls. These subjects undertook both the driving simulation and the functional MRI test.
On the uncomplicated right turns, there were no between-group differences in errors, Ms. Hird said. However, there were some differences in the way the subjects’ brains reacted to the task. “On the brain activation patterns, the healthy controls showed some negative activation in the temporal and occipital regions, areas that actually were activated in the patients. The patients also showed some increased signal in the posterior medial regions, compared with the healthy controls. Overall, however, the activation patterns looked quite similar between the two groups.”
The left turn into traffic task showed quite different results. “In the MCI group, there was more anterior activation. They also showed significantly higher activation than did the control group in the orbitofrontal, medial superior, and midfrontal cortex – all of which are involved in planning, higher-order attention, and cognitive control.”
Recognizing that there are demonstrable differences in brain activation that correlate with poor driving performance could spark the creation of an objective clinical measurement tool for determining driving status, Ms. Hird said.
“This is an important fundamental step in the development of a tool that is sensitive and specific for addressing the driving safety of these patients.”
During discussion after her presentation, she fielded a question from an Australian clinician, who said his country likewise has no objective measure of when driving is no longer safe for a dementia patient.
“We accept the fact that people do drive with some degree of cognitive impairment,” said the physician, who did not identify himself. “But there are regions where having a license is important for social engagement. And we know that social disengagement drives rapid progression of cognitive decline. I think there is a real tension here between the withdrawal of licensure and the preservation of social engagement.”
Ms. Hird agreed. “Obviously, many MCI patients do retain the ability to drive safely. But we need to achieve a balance between maintaining patient autonomy and the safety of the general public.”
Ms. Hird had no financial disclosures.
On Twitter @alz_gal
AT AAIC 2016
Key clinical point: Patients with mild cognitive impairment may already be unsafe drivers.
Major finding: Patients with multidomain MCI made significantly more driving errors than did healthy controls (mean of 14 vs. 2.7 errors).
Data source: The studies comprised 40 patients with MCI and 36 age-matched cognitively healthy controls.
Disclosures: Megan Hird had no financial disclosures.
Using Aminocaproic Acid to Reduce Blood Loss After Primary Unilateral Total Knee Arthroplasty
During total knee arthroplasty (TKA), traditionally a thigh tourniquet is used to minimize blood loss. Although intraoperative blood loss is negligible, postoperative blood loss can be extensive, and patients often require blood transfusions. Transfusions expose patients to clinical risks and increase costs. Well-documented transfusion complications include allergic reaction, transfusion-related acute lung injury, transfusion-associated circulatory overload, venous thromboembolism, graft vs host disease, bloodborne infections, and immunomodulation.1 Although measures are taken to reduce these risks, the costs associated with transfusions continue to escalate.2
Postoperative bleeding is attributed to fibrinolytic system activation. The antifibrinolytic agent aminocaproic acid (ACA), a synthetic analogue of the amino acid lysine, acts by competitively blocking the lysine-binding site of plasminogen, inhibiting fibrinolysis.3 Multiple studies have shown that ACA and a similar drug, tranexamic acid, can reduce postoperative blood loss when used intravenously in unilateral TKA.4,5 However, more studies are needed to evaluate antifibrinolytic agents with comparative controls using standardized procedures and documented outcome measures. In addition, the majority of studies have used tranexamic acid rather than ACA, despite the lower cost and similar efficacy of ACA.1,4 ACA is an inexpensive medication with a low risk profile, making it an attractive alternative to historical post-TKA management (which has a higher rate of blood transfusions) and a viable replacement in protocols already implementing tranexamic acid, the more expensive antifibrinolytic.5,6 It has been proposed that ACA use reduces equipment (drain) costs, blood transfusion costs, exposure to complications of blood loss, and transfusion reactions and reduces or eliminates the need for costly medications, such as erythropoiesis-stimulating agents.
Kagoma and colleagues5 reported that antifibrinolytic agents may reduce bleeding by at least 300 mL and may reduce the need for transfusions by 50% or eliminate this need altogether. Other antifibrinolytic agents have been studied in unilateral TKA, with results showing decreased drainage and improved postoperative hemoglobin (Hb) levels.6
We conducted a study to evaluate the effectiveness of a single intraoperative dose of ACA in reducing postoperative blood loss and the need for blood transfusions with increased preservation of postoperative Hb levels.
Methods
In October 2011, Dr. Anderson initiated an intraoperative intravenous (IV) ACA protocol for primary unilateral TKA. Given the decreased drain output immediately observed, and patients’ increased postoperative Hb levels, a retrospective study was proposed. After obtaining full Institutional Review Board approval for the study, we retrospectively reviewed the medical charts of 50 consecutive patients who underwent primary unilateral TKA—the last 25 who had the surgery before the IV ACA protocol was initiated (control group) and the first 25 who were given the IV ACA medication during the surgery (antifibrinolytic group). Inclusion criteria were primary unilateral TKA, no bleeding dyscrasia, no history of anaphylactic response to antifibrinolytic agents, no history of deep vein thrombosis, and normal preoperative coagulation parameters, international normalized ratio (INR), and partial thromboplastin time. Exclusion criteria included lateral corner release, lateral retinacular release, combined extensive deep and superficial medial collateral ligament releases, and cardiac or peripheral stent in place.
Each surgery—a standard primary unilateral TKA with an intramedullary femoral component and an extramedullary tibial component—was performed by Dr. Anderson. Each component was cemented. Each patient underwent a posterior cruciate ligament release and/or a deep medial collateral ligament release. A well-padded thigh tourniquet was inflated before surgical incision, and it remained inflated until all postoperative surgical dressings were applied. Each patient in the antifibrinolytic group was given a 10-g dose of IV ACA at the start of implant cementation; the dose was administered over 10 minutes and was completely infused before tourniquet deflation. For each patient in the control group, a suction drain (Constavac, Stryker) was used. As postoperative drainage was so insignificant in the first 12 antifibrinolytic cases, use of the drain was then discontinued.
All patients received standard postoperative deep vein thrombosis prophylaxis in the form of warfarin in accordance with existing practice. Warfarin was given once a day starting the night of surgery and was continued until discharge based on daily INR values with an agreed-on target of 2.0. Thigh-high compression stockings and calf sequential compression devices were used in all cases. No patient in either group predonated blood or was given erythropoietin injections before or after surgery. Postoperative allogeneic transfusions were given to patients who were clinically symptomatic or short of breath; patients with hypotension uncorrectable with IV volume supplementation and an Hb level under 9.0 g/dL; and patients with an Hb level under 7.0 g/dL regardless of symptoms. All patients were monitored for postoperative adverse events and complications.
Postoperative blood loss (drain output), Hb levels on postoperative days 1 and 2 (POD-1, POD-2), blood transfusion amounts, and complications were recorded for all patients. Group means were compared with 2-sample t tests for independent samples. Data are reported as group means and SDs. All significance tests were 2-tailed, and statistical significance was set at P < .05.
Results
Fifty patients enrolled in the study: 25 in the control group and 25 in the antifibrinolytic group. Table 1 compares the main characteristics of the 2 groups. No significant differences were found between these groups for any of the characteristics considered.
There was significantly (P < .0001) more postoperative drainage in the control group: Mean drain output was 410.9 mL for the control group and 155.0 mL for the antifibrinolytic group (Table 2). Patients in the antifibrinolytic group did not receive any blood transfusions, whereas 40% of patients in the control group received transfusions (P = .022). On average, the transfused patients received 0.4 unit of packed red blood cells.
Although there was no statistically significant difference in POD-1 or POD-2 Hb levels between the antifibrinolytic and control groups, the antifibrinolytic group trended higher on POD-1 (11.1 g vs 10.7 g; P = .108) and POD-2 (11.5 g vs 10.2 g; P = .117) (Table 3). Mean Hb level was 8.1 g for control patients transfused on POD-1 and 7.9 g for control patients transfused on POD-2. For control patients who were not transfused, mean Hb level was 10.7 g on POD-1 and 10.2 g on POD-2.
There were no adverse events (eg, anaphylaxis, hypersensitivity) in either group, and there was no difference in incision drainage or returns to operating room between the groups.
Discussion
In TKA, a tourniquet is used to minimize intraoperative blood loss; postoperative bleeding, however, is often extensive. Both surgery and tourniquet use are reported to enhance local fibrinolytic activity within the limb.8 The synthetic antifibrinolytic ACA reduces blood loss by clot stabilization rather than by promotion of clot formation.8
In the present study, a single intraoperative dose of IV ACA administered in primary unilateral TKA significantly reduced postoperative wound drainage and eliminated the need for postoperative allogeneic blood transfusions. In addition, patients who received ACA had higher Hb levels on POD-1 and POD-2. These results are similar to those of other clinical trials in which external blood losses were measured.4-7 The postoperative drain output differences (~250 mL) in our study are clinically relevant, as they indicate significant reductions in postoperative blood loss with the implementation of an antifibrinolytic operative protocol.
In a study by Ponnusamy and colleagues,1 blood transfusion after orthopedic surgery accounted for 10% of all packed red blood cell transfusions, but use varied widely. National TKA transfusion rates vary from 4.3% to 63.8% among surgeons and hospitals.9 This evidence calls for standardization and critical review of practices to ensure more efficient use of blood products, effectively protecting patients from unneeded complications and reducing hospital costs. Mounting evidence supporting the efficacy of ACA in reducing perioperative blood loss and lowering postoperative blood transfusion rates points toward including antifibrinolytic therapy in standard TKA protocols. In our study, 40% of control patients and no antifibrinolytic patients required a transfusion—a stark contrast.
Although our antifibrinolytic group’s postoperative Hb levels were not statistically significantly higher, their being elevated illustrates the protective effect of intraoperative use of antifibrinolytics in TKA. This elevation in Hb levels is especially valid given the similarity of the antifibrinolytic and control patients’ preoperative Hb levels (P = .871) (Table 1). Other studies have shown similar upward trends in postoperative Hb levels, many of which were statistically significant.5-8,10
Conclusion
This study showed that a single intraoperative 10-g dose of IV ACA significantly reduced perioperative blood loss and lowered blood transfusion rates in TKA. In addition, postoperative Hb levels were higher in the patients who received ACA than in patients who did not receive an antifibrinolytic. The positive effects of ACA were obtained without adverse events or complications, making use of this antifibrinolytic a relevant addition to TKA protocols.
1. Ponnusamy KE, Kim TJ, Khanuja HS. Perioperative blood transfusions in orthopaedic surgery. J Bone Joint Surg Am. 2014;96(21):1836-1844.
2. Spahn DR, Casutt M. Eliminating blood transfusions: new aspects and perspectives. Anesthesiology. 2000;93(1):242-255.
3. Van Aelbrouck C, Englberger L, Faraoni D. Review of the fibrinolytic system: comparison of different antifibrinolytics used during cardiopulmonary bypass. Recent Pat Cardiovasc Drug Discov. 2012;7(3):175-179.
4. Sepah YJ, Umer M, Ahmad T, Nasim F, Chaudhry MU, Umar M. Use of tranexamic acid is a cost effective method in preventing blood loss during and after total knee replacement. J Orthop Surg Res. 2011;6:22.
5. Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009;123(5):687-696.
6. Zufferey P, Merquiol F, Laporte S, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. 2006;105(5):1034-1046.
7. Camarasa MA, Ollé G, Serra-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96(5):576-582.
8. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110.
9. Chen AF, Klatt BA, Yazer MH, Waters JH. Blood utilization after primary total joint arthroplasty in a large hospital network. HSS J. 2013;9(2):123-128.
10. Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007.
During total knee arthroplasty (TKA), traditionally a thigh tourniquet is used to minimize blood loss. Although intraoperative blood loss is negligible, postoperative blood loss can be extensive, and patients often require blood transfusions. Transfusions expose patients to clinical risks and increase costs. Well-documented transfusion complications include allergic reaction, transfusion-related acute lung injury, transfusion-associated circulatory overload, venous thromboembolism, graft vs host disease, bloodborne infections, and immunomodulation.1 Although measures are taken to reduce these risks, the costs associated with transfusions continue to escalate.2
Postoperative bleeding is attributed to fibrinolytic system activation. The antifibrinolytic agent aminocaproic acid (ACA), a synthetic analogue of the amino acid lysine, acts by competitively blocking the lysine-binding site of plasminogen, inhibiting fibrinolysis.3 Multiple studies have shown that ACA and a similar drug, tranexamic acid, can reduce postoperative blood loss when used intravenously in unilateral TKA.4,5 However, more studies are needed to evaluate antifibrinolytic agents with comparative controls using standardized procedures and documented outcome measures. In addition, the majority of studies have used tranexamic acid rather than ACA, despite the lower cost and similar efficacy of ACA.1,4 ACA is an inexpensive medication with a low risk profile, making it an attractive alternative to historical post-TKA management (which has a higher rate of blood transfusions) and a viable replacement in protocols already implementing tranexamic acid, the more expensive antifibrinolytic.5,6 It has been proposed that ACA use reduces equipment (drain) costs, blood transfusion costs, exposure to complications of blood loss, and transfusion reactions and reduces or eliminates the need for costly medications, such as erythropoiesis-stimulating agents.
Kagoma and colleagues5 reported that antifibrinolytic agents may reduce bleeding by at least 300 mL and may reduce the need for transfusions by 50% or eliminate this need altogether. Other antifibrinolytic agents have been studied in unilateral TKA, with results showing decreased drainage and improved postoperative hemoglobin (Hb) levels.6
We conducted a study to evaluate the effectiveness of a single intraoperative dose of ACA in reducing postoperative blood loss and the need for blood transfusions with increased preservation of postoperative Hb levels.
Methods
In October 2011, Dr. Anderson initiated an intraoperative intravenous (IV) ACA protocol for primary unilateral TKA. Given the decreased drain output immediately observed, and patients’ increased postoperative Hb levels, a retrospective study was proposed. After obtaining full Institutional Review Board approval for the study, we retrospectively reviewed the medical charts of 50 consecutive patients who underwent primary unilateral TKA—the last 25 who had the surgery before the IV ACA protocol was initiated (control group) and the first 25 who were given the IV ACA medication during the surgery (antifibrinolytic group). Inclusion criteria were primary unilateral TKA, no bleeding dyscrasia, no history of anaphylactic response to antifibrinolytic agents, no history of deep vein thrombosis, and normal preoperative coagulation parameters, international normalized ratio (INR), and partial thromboplastin time. Exclusion criteria included lateral corner release, lateral retinacular release, combined extensive deep and superficial medial collateral ligament releases, and cardiac or peripheral stent in place.
Each surgery—a standard primary unilateral TKA with an intramedullary femoral component and an extramedullary tibial component—was performed by Dr. Anderson. Each component was cemented. Each patient underwent a posterior cruciate ligament release and/or a deep medial collateral ligament release. A well-padded thigh tourniquet was inflated before surgical incision, and it remained inflated until all postoperative surgical dressings were applied. Each patient in the antifibrinolytic group was given a 10-g dose of IV ACA at the start of implant cementation; the dose was administered over 10 minutes and was completely infused before tourniquet deflation. For each patient in the control group, a suction drain (Constavac, Stryker) was used. As postoperative drainage was so insignificant in the first 12 antifibrinolytic cases, use of the drain was then discontinued.
All patients received standard postoperative deep vein thrombosis prophylaxis in the form of warfarin in accordance with existing practice. Warfarin was given once a day starting the night of surgery and was continued until discharge based on daily INR values with an agreed-on target of 2.0. Thigh-high compression stockings and calf sequential compression devices were used in all cases. No patient in either group predonated blood or was given erythropoietin injections before or after surgery. Postoperative allogeneic transfusions were given to patients who were clinically symptomatic or short of breath; patients with hypotension uncorrectable with IV volume supplementation and an Hb level under 9.0 g/dL; and patients with an Hb level under 7.0 g/dL regardless of symptoms. All patients were monitored for postoperative adverse events and complications.
Postoperative blood loss (drain output), Hb levels on postoperative days 1 and 2 (POD-1, POD-2), blood transfusion amounts, and complications were recorded for all patients. Group means were compared with 2-sample t tests for independent samples. Data are reported as group means and SDs. All significance tests were 2-tailed, and statistical significance was set at P < .05.
Results
Fifty patients enrolled in the study: 25 in the control group and 25 in the antifibrinolytic group. Table 1 compares the main characteristics of the 2 groups. No significant differences were found between these groups for any of the characteristics considered.
There was significantly (P < .0001) more postoperative drainage in the control group: Mean drain output was 410.9 mL for the control group and 155.0 mL for the antifibrinolytic group (Table 2). Patients in the antifibrinolytic group did not receive any blood transfusions, whereas 40% of patients in the control group received transfusions (P = .022). On average, the transfused patients received 0.4 unit of packed red blood cells.
Although there was no statistically significant difference in POD-1 or POD-2 Hb levels between the antifibrinolytic and control groups, the antifibrinolytic group trended higher on POD-1 (11.1 g vs 10.7 g; P = .108) and POD-2 (11.5 g vs 10.2 g; P = .117) (Table 3). Mean Hb level was 8.1 g for control patients transfused on POD-1 and 7.9 g for control patients transfused on POD-2. For control patients who were not transfused, mean Hb level was 10.7 g on POD-1 and 10.2 g on POD-2.
There were no adverse events (eg, anaphylaxis, hypersensitivity) in either group, and there was no difference in incision drainage or returns to operating room between the groups.
Discussion
In TKA, a tourniquet is used to minimize intraoperative blood loss; postoperative bleeding, however, is often extensive. Both surgery and tourniquet use are reported to enhance local fibrinolytic activity within the limb.8 The synthetic antifibrinolytic ACA reduces blood loss by clot stabilization rather than by promotion of clot formation.8
In the present study, a single intraoperative dose of IV ACA administered in primary unilateral TKA significantly reduced postoperative wound drainage and eliminated the need for postoperative allogeneic blood transfusions. In addition, patients who received ACA had higher Hb levels on POD-1 and POD-2. These results are similar to those of other clinical trials in which external blood losses were measured.4-7 The postoperative drain output differences (~250 mL) in our study are clinically relevant, as they indicate significant reductions in postoperative blood loss with the implementation of an antifibrinolytic operative protocol.
In a study by Ponnusamy and colleagues,1 blood transfusion after orthopedic surgery accounted for 10% of all packed red blood cell transfusions, but use varied widely. National TKA transfusion rates vary from 4.3% to 63.8% among surgeons and hospitals.9 This evidence calls for standardization and critical review of practices to ensure more efficient use of blood products, effectively protecting patients from unneeded complications and reducing hospital costs. Mounting evidence supporting the efficacy of ACA in reducing perioperative blood loss and lowering postoperative blood transfusion rates points toward including antifibrinolytic therapy in standard TKA protocols. In our study, 40% of control patients and no antifibrinolytic patients required a transfusion—a stark contrast.
Although our antifibrinolytic group’s postoperative Hb levels were not statistically significantly higher, their being elevated illustrates the protective effect of intraoperative use of antifibrinolytics in TKA. This elevation in Hb levels is especially valid given the similarity of the antifibrinolytic and control patients’ preoperative Hb levels (P = .871) (Table 1). Other studies have shown similar upward trends in postoperative Hb levels, many of which were statistically significant.5-8,10
Conclusion
This study showed that a single intraoperative 10-g dose of IV ACA significantly reduced perioperative blood loss and lowered blood transfusion rates in TKA. In addition, postoperative Hb levels were higher in the patients who received ACA than in patients who did not receive an antifibrinolytic. The positive effects of ACA were obtained without adverse events or complications, making use of this antifibrinolytic a relevant addition to TKA protocols.
During total knee arthroplasty (TKA), traditionally a thigh tourniquet is used to minimize blood loss. Although intraoperative blood loss is negligible, postoperative blood loss can be extensive, and patients often require blood transfusions. Transfusions expose patients to clinical risks and increase costs. Well-documented transfusion complications include allergic reaction, transfusion-related acute lung injury, transfusion-associated circulatory overload, venous thromboembolism, graft vs host disease, bloodborne infections, and immunomodulation.1 Although measures are taken to reduce these risks, the costs associated with transfusions continue to escalate.2
Postoperative bleeding is attributed to fibrinolytic system activation. The antifibrinolytic agent aminocaproic acid (ACA), a synthetic analogue of the amino acid lysine, acts by competitively blocking the lysine-binding site of plasminogen, inhibiting fibrinolysis.3 Multiple studies have shown that ACA and a similar drug, tranexamic acid, can reduce postoperative blood loss when used intravenously in unilateral TKA.4,5 However, more studies are needed to evaluate antifibrinolytic agents with comparative controls using standardized procedures and documented outcome measures. In addition, the majority of studies have used tranexamic acid rather than ACA, despite the lower cost and similar efficacy of ACA.1,4 ACA is an inexpensive medication with a low risk profile, making it an attractive alternative to historical post-TKA management (which has a higher rate of blood transfusions) and a viable replacement in protocols already implementing tranexamic acid, the more expensive antifibrinolytic.5,6 It has been proposed that ACA use reduces equipment (drain) costs, blood transfusion costs, exposure to complications of blood loss, and transfusion reactions and reduces or eliminates the need for costly medications, such as erythropoiesis-stimulating agents.
Kagoma and colleagues5 reported that antifibrinolytic agents may reduce bleeding by at least 300 mL and may reduce the need for transfusions by 50% or eliminate this need altogether. Other antifibrinolytic agents have been studied in unilateral TKA, with results showing decreased drainage and improved postoperative hemoglobin (Hb) levels.6
We conducted a study to evaluate the effectiveness of a single intraoperative dose of ACA in reducing postoperative blood loss and the need for blood transfusions with increased preservation of postoperative Hb levels.
Methods
In October 2011, Dr. Anderson initiated an intraoperative intravenous (IV) ACA protocol for primary unilateral TKA. Given the decreased drain output immediately observed, and patients’ increased postoperative Hb levels, a retrospective study was proposed. After obtaining full Institutional Review Board approval for the study, we retrospectively reviewed the medical charts of 50 consecutive patients who underwent primary unilateral TKA—the last 25 who had the surgery before the IV ACA protocol was initiated (control group) and the first 25 who were given the IV ACA medication during the surgery (antifibrinolytic group). Inclusion criteria were primary unilateral TKA, no bleeding dyscrasia, no history of anaphylactic response to antifibrinolytic agents, no history of deep vein thrombosis, and normal preoperative coagulation parameters, international normalized ratio (INR), and partial thromboplastin time. Exclusion criteria included lateral corner release, lateral retinacular release, combined extensive deep and superficial medial collateral ligament releases, and cardiac or peripheral stent in place.
Each surgery—a standard primary unilateral TKA with an intramedullary femoral component and an extramedullary tibial component—was performed by Dr. Anderson. Each component was cemented. Each patient underwent a posterior cruciate ligament release and/or a deep medial collateral ligament release. A well-padded thigh tourniquet was inflated before surgical incision, and it remained inflated until all postoperative surgical dressings were applied. Each patient in the antifibrinolytic group was given a 10-g dose of IV ACA at the start of implant cementation; the dose was administered over 10 minutes and was completely infused before tourniquet deflation. For each patient in the control group, a suction drain (Constavac, Stryker) was used. As postoperative drainage was so insignificant in the first 12 antifibrinolytic cases, use of the drain was then discontinued.
All patients received standard postoperative deep vein thrombosis prophylaxis in the form of warfarin in accordance with existing practice. Warfarin was given once a day starting the night of surgery and was continued until discharge based on daily INR values with an agreed-on target of 2.0. Thigh-high compression stockings and calf sequential compression devices were used in all cases. No patient in either group predonated blood or was given erythropoietin injections before or after surgery. Postoperative allogeneic transfusions were given to patients who were clinically symptomatic or short of breath; patients with hypotension uncorrectable with IV volume supplementation and an Hb level under 9.0 g/dL; and patients with an Hb level under 7.0 g/dL regardless of symptoms. All patients were monitored for postoperative adverse events and complications.
Postoperative blood loss (drain output), Hb levels on postoperative days 1 and 2 (POD-1, POD-2), blood transfusion amounts, and complications were recorded for all patients. Group means were compared with 2-sample t tests for independent samples. Data are reported as group means and SDs. All significance tests were 2-tailed, and statistical significance was set at P < .05.
Results
Fifty patients enrolled in the study: 25 in the control group and 25 in the antifibrinolytic group. Table 1 compares the main characteristics of the 2 groups. No significant differences were found between these groups for any of the characteristics considered.
There was significantly (P < .0001) more postoperative drainage in the control group: Mean drain output was 410.9 mL for the control group and 155.0 mL for the antifibrinolytic group (Table 2). Patients in the antifibrinolytic group did not receive any blood transfusions, whereas 40% of patients in the control group received transfusions (P = .022). On average, the transfused patients received 0.4 unit of packed red blood cells.
Although there was no statistically significant difference in POD-1 or POD-2 Hb levels between the antifibrinolytic and control groups, the antifibrinolytic group trended higher on POD-1 (11.1 g vs 10.7 g; P = .108) and POD-2 (11.5 g vs 10.2 g; P = .117) (Table 3). Mean Hb level was 8.1 g for control patients transfused on POD-1 and 7.9 g for control patients transfused on POD-2. For control patients who were not transfused, mean Hb level was 10.7 g on POD-1 and 10.2 g on POD-2.
There were no adverse events (eg, anaphylaxis, hypersensitivity) in either group, and there was no difference in incision drainage or returns to operating room between the groups.
Discussion
In TKA, a tourniquet is used to minimize intraoperative blood loss; postoperative bleeding, however, is often extensive. Both surgery and tourniquet use are reported to enhance local fibrinolytic activity within the limb.8 The synthetic antifibrinolytic ACA reduces blood loss by clot stabilization rather than by promotion of clot formation.8
In the present study, a single intraoperative dose of IV ACA administered in primary unilateral TKA significantly reduced postoperative wound drainage and eliminated the need for postoperative allogeneic blood transfusions. In addition, patients who received ACA had higher Hb levels on POD-1 and POD-2. These results are similar to those of other clinical trials in which external blood losses were measured.4-7 The postoperative drain output differences (~250 mL) in our study are clinically relevant, as they indicate significant reductions in postoperative blood loss with the implementation of an antifibrinolytic operative protocol.
In a study by Ponnusamy and colleagues,1 blood transfusion after orthopedic surgery accounted for 10% of all packed red blood cell transfusions, but use varied widely. National TKA transfusion rates vary from 4.3% to 63.8% among surgeons and hospitals.9 This evidence calls for standardization and critical review of practices to ensure more efficient use of blood products, effectively protecting patients from unneeded complications and reducing hospital costs. Mounting evidence supporting the efficacy of ACA in reducing perioperative blood loss and lowering postoperative blood transfusion rates points toward including antifibrinolytic therapy in standard TKA protocols. In our study, 40% of control patients and no antifibrinolytic patients required a transfusion—a stark contrast.
Although our antifibrinolytic group’s postoperative Hb levels were not statistically significantly higher, their being elevated illustrates the protective effect of intraoperative use of antifibrinolytics in TKA. This elevation in Hb levels is especially valid given the similarity of the antifibrinolytic and control patients’ preoperative Hb levels (P = .871) (Table 1). Other studies have shown similar upward trends in postoperative Hb levels, many of which were statistically significant.5-8,10
Conclusion
This study showed that a single intraoperative 10-g dose of IV ACA significantly reduced perioperative blood loss and lowered blood transfusion rates in TKA. In addition, postoperative Hb levels were higher in the patients who received ACA than in patients who did not receive an antifibrinolytic. The positive effects of ACA were obtained without adverse events or complications, making use of this antifibrinolytic a relevant addition to TKA protocols.
1. Ponnusamy KE, Kim TJ, Khanuja HS. Perioperative blood transfusions in orthopaedic surgery. J Bone Joint Surg Am. 2014;96(21):1836-1844.
2. Spahn DR, Casutt M. Eliminating blood transfusions: new aspects and perspectives. Anesthesiology. 2000;93(1):242-255.
3. Van Aelbrouck C, Englberger L, Faraoni D. Review of the fibrinolytic system: comparison of different antifibrinolytics used during cardiopulmonary bypass. Recent Pat Cardiovasc Drug Discov. 2012;7(3):175-179.
4. Sepah YJ, Umer M, Ahmad T, Nasim F, Chaudhry MU, Umar M. Use of tranexamic acid is a cost effective method in preventing blood loss during and after total knee replacement. J Orthop Surg Res. 2011;6:22.
5. Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009;123(5):687-696.
6. Zufferey P, Merquiol F, Laporte S, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. 2006;105(5):1034-1046.
7. Camarasa MA, Ollé G, Serra-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96(5):576-582.
8. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110.
9. Chen AF, Klatt BA, Yazer MH, Waters JH. Blood utilization after primary total joint arthroplasty in a large hospital network. HSS J. 2013;9(2):123-128.
10. Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007.
1. Ponnusamy KE, Kim TJ, Khanuja HS. Perioperative blood transfusions in orthopaedic surgery. J Bone Joint Surg Am. 2014;96(21):1836-1844.
2. Spahn DR, Casutt M. Eliminating blood transfusions: new aspects and perspectives. Anesthesiology. 2000;93(1):242-255.
3. Van Aelbrouck C, Englberger L, Faraoni D. Review of the fibrinolytic system: comparison of different antifibrinolytics used during cardiopulmonary bypass. Recent Pat Cardiovasc Drug Discov. 2012;7(3):175-179.
4. Sepah YJ, Umer M, Ahmad T, Nasim F, Chaudhry MU, Umar M. Use of tranexamic acid is a cost effective method in preventing blood loss during and after total knee replacement. J Orthop Surg Res. 2011;6:22.
5. Kagoma YK, Crowther MA, Douketis J, Bhandari M, Eikelboom J, Lim W. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009;123(5):687-696.
6. Zufferey P, Merquiol F, Laporte S, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. 2006;105(5):1034-1046.
7. Camarasa MA, Ollé G, Serra-Prat M, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96(5):576-582.
8. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110.
9. Chen AF, Klatt BA, Yazer MH, Waters JH. Blood utilization after primary total joint arthroplasty in a large hospital network. HSS J. 2013;9(2):123-128.
10. Aguilera X, Martinez-Zapata MJ, Bosch A, et al. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95(22):2001-2007.
Study compares sterile vs. nonsterile gloves for outpatient derm procedures
The use of sterile or nonsterile gloves during outpatient dermatologic and dental procedures resulted in similar rates of postoperative surgical site infections (SSIs), results from a large systematic review and meta-analysis demonstrated.
“During the past few decades, the use of surgical gloves has become standard practice to prevent postoperative wound infections or surgical site infection,” researchers led by Dr. Jerry Brewer wrote in a study published online Aug. 3, 2016 in JAMA Dermatology. “However, whether the use of sterile vs. non-sterile gloves makes a difference in the development of postoperative SSIs in the setting of cutaneous and minor outpatient surgical procedures remains unclear.”
In an effort to examine that question, Dr. Brewer of the division of dermatologic surgery at Mayo Clinic, Rochester, Minn., and his associates conducted a systematic review and meta-analysis of randomized clinical trials and comparative studies with information on sterile vs. non-sterile gloves in outpatient surgical procedures (JAMA Dermatol. 2016 Aug. 3. doi: 10.1001/jamadermatol.2016.1965). Patients in the studies underwent outpatient cutaneous or mucosal surgical procedures, including Mohs micrographic surgery, repair of a laceration, standard excisions, and tooth extractions.
The final meta-analysis included 11,071 patients from 13 studies. Of these, 6,040 underwent procedures with sterile gloves and 5,031 underwent procedures with nonsterile gloves. The researchers reported that a total of 228 patients (2.1%) had a postoperative SSI, including 107 in the nonsterile glove group (2.1%), and 121 in the sterile glove group (2%). The overall relative risk for an SSI with nonsterile glove use was 1.06.
In an interview, Dr. Brewer estimated that sterile gloves cost anywhere from $0.27 to $1.29 per pair, compared with about 8 cents per pair for clean nonsterile gloves. “This cost difference may not seem like much, but if you think about all the surgeries that are done on a regular basis across the country, that’s a huge difference in cost,” he said.
The authors acknowledged certain limitations of the study, including the potential for selection bias, since many of the studies included in the meta-analysis were observational. They also noted that findings from some previous studies on the topic run counter to theirs (see Dermatol. Surg. 2010; 36[10]:1529-36 and J. Hosp. Infect. 2007;65[3]:258-63 ). “Although the broad use of nonsterile clean gloves may be justified, caution is advised in generalizing this justification to more advanced outpatient surgical procedures that may not pertain to the information summarized in this review and meta-analysis,” they concluded. “Future study could include whether duration of surgery and complexity of the repair influence postoperative SSI development in the setting of sterile vs. nonsterile gloves.”
The researchers reported having no financial disclosures.
The use of sterile or nonsterile gloves during outpatient dermatologic and dental procedures resulted in similar rates of postoperative surgical site infections (SSIs), results from a large systematic review and meta-analysis demonstrated.
“During the past few decades, the use of surgical gloves has become standard practice to prevent postoperative wound infections or surgical site infection,” researchers led by Dr. Jerry Brewer wrote in a study published online Aug. 3, 2016 in JAMA Dermatology. “However, whether the use of sterile vs. non-sterile gloves makes a difference in the development of postoperative SSIs in the setting of cutaneous and minor outpatient surgical procedures remains unclear.”
In an effort to examine that question, Dr. Brewer of the division of dermatologic surgery at Mayo Clinic, Rochester, Minn., and his associates conducted a systematic review and meta-analysis of randomized clinical trials and comparative studies with information on sterile vs. non-sterile gloves in outpatient surgical procedures (JAMA Dermatol. 2016 Aug. 3. doi: 10.1001/jamadermatol.2016.1965). Patients in the studies underwent outpatient cutaneous or mucosal surgical procedures, including Mohs micrographic surgery, repair of a laceration, standard excisions, and tooth extractions.
The final meta-analysis included 11,071 patients from 13 studies. Of these, 6,040 underwent procedures with sterile gloves and 5,031 underwent procedures with nonsterile gloves. The researchers reported that a total of 228 patients (2.1%) had a postoperative SSI, including 107 in the nonsterile glove group (2.1%), and 121 in the sterile glove group (2%). The overall relative risk for an SSI with nonsterile glove use was 1.06.
In an interview, Dr. Brewer estimated that sterile gloves cost anywhere from $0.27 to $1.29 per pair, compared with about 8 cents per pair for clean nonsterile gloves. “This cost difference may not seem like much, but if you think about all the surgeries that are done on a regular basis across the country, that’s a huge difference in cost,” he said.
The authors acknowledged certain limitations of the study, including the potential for selection bias, since many of the studies included in the meta-analysis were observational. They also noted that findings from some previous studies on the topic run counter to theirs (see Dermatol. Surg. 2010; 36[10]:1529-36 and J. Hosp. Infect. 2007;65[3]:258-63 ). “Although the broad use of nonsterile clean gloves may be justified, caution is advised in generalizing this justification to more advanced outpatient surgical procedures that may not pertain to the information summarized in this review and meta-analysis,” they concluded. “Future study could include whether duration of surgery and complexity of the repair influence postoperative SSI development in the setting of sterile vs. nonsterile gloves.”
The researchers reported having no financial disclosures.
The use of sterile or nonsterile gloves during outpatient dermatologic and dental procedures resulted in similar rates of postoperative surgical site infections (SSIs), results from a large systematic review and meta-analysis demonstrated.
“During the past few decades, the use of surgical gloves has become standard practice to prevent postoperative wound infections or surgical site infection,” researchers led by Dr. Jerry Brewer wrote in a study published online Aug. 3, 2016 in JAMA Dermatology. “However, whether the use of sterile vs. non-sterile gloves makes a difference in the development of postoperative SSIs in the setting of cutaneous and minor outpatient surgical procedures remains unclear.”
In an effort to examine that question, Dr. Brewer of the division of dermatologic surgery at Mayo Clinic, Rochester, Minn., and his associates conducted a systematic review and meta-analysis of randomized clinical trials and comparative studies with information on sterile vs. non-sterile gloves in outpatient surgical procedures (JAMA Dermatol. 2016 Aug. 3. doi: 10.1001/jamadermatol.2016.1965). Patients in the studies underwent outpatient cutaneous or mucosal surgical procedures, including Mohs micrographic surgery, repair of a laceration, standard excisions, and tooth extractions.
The final meta-analysis included 11,071 patients from 13 studies. Of these, 6,040 underwent procedures with sterile gloves and 5,031 underwent procedures with nonsterile gloves. The researchers reported that a total of 228 patients (2.1%) had a postoperative SSI, including 107 in the nonsterile glove group (2.1%), and 121 in the sterile glove group (2%). The overall relative risk for an SSI with nonsterile glove use was 1.06.
In an interview, Dr. Brewer estimated that sterile gloves cost anywhere from $0.27 to $1.29 per pair, compared with about 8 cents per pair for clean nonsterile gloves. “This cost difference may not seem like much, but if you think about all the surgeries that are done on a regular basis across the country, that’s a huge difference in cost,” he said.
The authors acknowledged certain limitations of the study, including the potential for selection bias, since many of the studies included in the meta-analysis were observational. They also noted that findings from some previous studies on the topic run counter to theirs (see Dermatol. Surg. 2010; 36[10]:1529-36 and J. Hosp. Infect. 2007;65[3]:258-63 ). “Although the broad use of nonsterile clean gloves may be justified, caution is advised in generalizing this justification to more advanced outpatient surgical procedures that may not pertain to the information summarized in this review and meta-analysis,” they concluded. “Future study could include whether duration of surgery and complexity of the repair influence postoperative SSI development in the setting of sterile vs. nonsterile gloves.”
The researchers reported having no financial disclosures.
FROM JAMA DERMATOLOGY
Key clinical point: No difference was observed in the rate of postoperative SSIs between outpatient surgical procedures performed with sterile versus nonsterile gloves.
Major finding: Overall, 2.1% of patients had a postoperative SSI, including 2.1% in the nonsterile glove group and 2% in the sterile glove group.
Data source: A meta-analysis that included 11,071 patients from 13 studies with information on sterile vs. nonsterile gloves in outpatient surgical procedures.
Disclosures: The researchers reported having no financial disclosures.
Extended-release naltrexone helps alcohol-dependent HIV-positive prisoners transition to community
DURBAN, SOUTH AFRICA – Extended-release naltrexone provides clinically meaningful benefits in HIV-infected prisoners with alcohol use disorder and multiple comorbid conditions as they transition back into the community, according to the findings of a double-blind randomized clinical trial.
“I think it’s important to know that a very effective medication, which has not previously been given to this population, was accepted by this group. It may be a feasible conduit to care as they transition to the community, even among those with severe psychosocial disparities like homelessness and mental illness,” Sandra A. Springer, MD, said in presenting the study findings at the 21st International AIDS Conference.
Extended-release naltrexone (Vivitrol) is a mu-opioid receptor antagonist approved for the treatment of alcohol use disorder, where it has been shown to decrease consumption. But prior to her study, the once-monthly injectable drug hadn’t been studied in alcohol-dependent prisoners living with HIV who are transitioning from jail or prison into the community, noted Dr. Springer, an infectious disease specialist at Yale University in New Haven, Conn.
This is a large, important, and seriously neglected patient population, she observed. The United States has the highest incarceration rate in the world. The prevalence of HIV infection is at least three times greater in U.S. criminal justice settings than in the general population. Alcohol use disorders are eightfold more common. Release from prison or jail in affected individuals often is complicated by relapse to alcohol use, which in turn is associated with poor HIV treatment outcomes.
Dr. Springer reported on 100 HIV-positive adult prisoners with alcohol use disorder diagnosed by DSM-IV criteria who were randomized double-blind two-to-one to 6 monthly 380-mg intramuscular injections of extended-release naltrexone or placebo, with the first dose given 3-7 days prior to release. Participants were required to have no baseline clinical evidence of cirrhosis or very high liver enzyme levels.
Half of participants had chronic hepatitis C. Eighty-seven percent of subjects scored 20 or higher on the Alcohol Use Disorders Identification test, indicating alcohol dependence. On the Mini International Neuropsychiatric Interview, 15% of participants met criteria for major depressive disorder, 16% for bipolar disorder, 59% for cocaine use disorder, 16% for narcotic use disorder, and 16% for cannabis use disorder. Most of the subjects were homeless or had an unstable housing situation.
Alcohol outcomes were assessed monthly during the 6-month trial. Not surprisingly, the better the treatment adherence, the better the outcomes. During the 90 days before incarceration, patients self-reported that 70% of those days were heavy drinking days, defined in men as having five or more drinks per day and in women as four or more. Their average consumption on those heavy drinking days was 28 drinks per day. In contrast, patients who accepted four or more extended-release naltrexone injections during 180 days of follow-up after release from custody drank heavily on just 7.6% of days, with an average of 8.6 drinks per day on those heavy drinking days. Subjects who received four or more placebo injections drank heavily on 11.6% of days, consuming an average of 12 drinks per heavy drinking day.
The time to first heavy drinking day was longer in patients who accepted 4-6 monthly injections of extended-release naltrexone than in those with 4-6 placebo injections. However, the difference achieved statistical significance only in the younger subgroup of participants aged 21-29 years. In that subgroup, the average time to the first heavy drinking day was 24.1 days, compared with 9.5 days with placebo.
On a composite alcohol consumption index comprised of time to first heavy drinking day after release, mean number of drinks per drinking day, change from before to after incarceration in average number of drinks per drinking day, alcohol craving score, and total number of drinking days, subjects who received four or more extended-release naltrexone injections had a significantly more favorable result, with a mean score of 3.15, compared with 2.93 in patients who took four or more placebo injections.
Moreover, consistent use of extended-release naltrexone was associated with significantly lower HIV viral load counts, compared with placebo-treated controls.
Treatment with extended-release naltrexone was safe. No serious side effects occurred, even in patients with comorbid hepatitis C who were on antiretroviral therapy. The most common side effects were the same as in seen in studies of the drug in other populations: mild to moderate nausea, headache, decreased appetite, fatigue, and dizziness.
Elsewhere at AIDS 2016, Chris Beyrer, MD, president of the International AIDS Society, included prisoners on his list of the populations most vulnerable to HIV because of discriminatory laws and policies in many parts of the world. Others on the list were transgender people, sex workers, men who have sex with men, and injection drug users.
“We’ll never be able to end AIDS without addressing the needs of these most vulnerable individuals and communities, and yet we know in 2016 far too many are being left behind,” said Dr. Beyrer, professor of epidemiology at Johns Hopkins University, Baltimore.
Transgender individuals, for example, are 49 times more likely to have HIV infection than other adults. Injection drug users and men who have sex with men are each 24-fold more likely to become HIV infected than the general population. Sex workers are 10 times more likely to acquire HIV infection than others in their reproductive years. And prisoners have a fivefold greater prevalence of HIV.
“In 2014 these vulnerable groups accounted for more than one-third of all new HIV infections. That’s an extraordinary proportion of HIV,” he observed. “This truly is the undone work of the HIV response. If there’s any silver lining in this cloud, it’s this: We’re talking about a relatively small number of people who are at high risk of infection relative to the world’s population. And that means that turning this around doesn’t require massive new commitments to very large populations. What it does require is an honest acknowledgment of where the epidemic is hitting hardest and directing resources to that need.”
Unfortunately, screening and treatment programs are rarely tailored to reach these highly vulnerable groups effectively, he added.
Dr. Beyrer was a contributor to a special issue of the Lancet devoted to HIV infection among prisoners published with the AIDS 2016 conference.
Dr. Springer’s study was funded by the National Institute on Alcohol Abuse and Alcoholism, and the National Institute on Drug Abuse. She reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Extended-release naltrexone provides clinically meaningful benefits in HIV-infected prisoners with alcohol use disorder and multiple comorbid conditions as they transition back into the community, according to the findings of a double-blind randomized clinical trial.
“I think it’s important to know that a very effective medication, which has not previously been given to this population, was accepted by this group. It may be a feasible conduit to care as they transition to the community, even among those with severe psychosocial disparities like homelessness and mental illness,” Sandra A. Springer, MD, said in presenting the study findings at the 21st International AIDS Conference.
Extended-release naltrexone (Vivitrol) is a mu-opioid receptor antagonist approved for the treatment of alcohol use disorder, where it has been shown to decrease consumption. But prior to her study, the once-monthly injectable drug hadn’t been studied in alcohol-dependent prisoners living with HIV who are transitioning from jail or prison into the community, noted Dr. Springer, an infectious disease specialist at Yale University in New Haven, Conn.
This is a large, important, and seriously neglected patient population, she observed. The United States has the highest incarceration rate in the world. The prevalence of HIV infection is at least three times greater in U.S. criminal justice settings than in the general population. Alcohol use disorders are eightfold more common. Release from prison or jail in affected individuals often is complicated by relapse to alcohol use, which in turn is associated with poor HIV treatment outcomes.
Dr. Springer reported on 100 HIV-positive adult prisoners with alcohol use disorder diagnosed by DSM-IV criteria who were randomized double-blind two-to-one to 6 monthly 380-mg intramuscular injections of extended-release naltrexone or placebo, with the first dose given 3-7 days prior to release. Participants were required to have no baseline clinical evidence of cirrhosis or very high liver enzyme levels.
Half of participants had chronic hepatitis C. Eighty-seven percent of subjects scored 20 or higher on the Alcohol Use Disorders Identification test, indicating alcohol dependence. On the Mini International Neuropsychiatric Interview, 15% of participants met criteria for major depressive disorder, 16% for bipolar disorder, 59% for cocaine use disorder, 16% for narcotic use disorder, and 16% for cannabis use disorder. Most of the subjects were homeless or had an unstable housing situation.
Alcohol outcomes were assessed monthly during the 6-month trial. Not surprisingly, the better the treatment adherence, the better the outcomes. During the 90 days before incarceration, patients self-reported that 70% of those days were heavy drinking days, defined in men as having five or more drinks per day and in women as four or more. Their average consumption on those heavy drinking days was 28 drinks per day. In contrast, patients who accepted four or more extended-release naltrexone injections during 180 days of follow-up after release from custody drank heavily on just 7.6% of days, with an average of 8.6 drinks per day on those heavy drinking days. Subjects who received four or more placebo injections drank heavily on 11.6% of days, consuming an average of 12 drinks per heavy drinking day.
The time to first heavy drinking day was longer in patients who accepted 4-6 monthly injections of extended-release naltrexone than in those with 4-6 placebo injections. However, the difference achieved statistical significance only in the younger subgroup of participants aged 21-29 years. In that subgroup, the average time to the first heavy drinking day was 24.1 days, compared with 9.5 days with placebo.
On a composite alcohol consumption index comprised of time to first heavy drinking day after release, mean number of drinks per drinking day, change from before to after incarceration in average number of drinks per drinking day, alcohol craving score, and total number of drinking days, subjects who received four or more extended-release naltrexone injections had a significantly more favorable result, with a mean score of 3.15, compared with 2.93 in patients who took four or more placebo injections.
Moreover, consistent use of extended-release naltrexone was associated with significantly lower HIV viral load counts, compared with placebo-treated controls.
Treatment with extended-release naltrexone was safe. No serious side effects occurred, even in patients with comorbid hepatitis C who were on antiretroviral therapy. The most common side effects were the same as in seen in studies of the drug in other populations: mild to moderate nausea, headache, decreased appetite, fatigue, and dizziness.
Elsewhere at AIDS 2016, Chris Beyrer, MD, president of the International AIDS Society, included prisoners on his list of the populations most vulnerable to HIV because of discriminatory laws and policies in many parts of the world. Others on the list were transgender people, sex workers, men who have sex with men, and injection drug users.
“We’ll never be able to end AIDS without addressing the needs of these most vulnerable individuals and communities, and yet we know in 2016 far too many are being left behind,” said Dr. Beyrer, professor of epidemiology at Johns Hopkins University, Baltimore.
Transgender individuals, for example, are 49 times more likely to have HIV infection than other adults. Injection drug users and men who have sex with men are each 24-fold more likely to become HIV infected than the general population. Sex workers are 10 times more likely to acquire HIV infection than others in their reproductive years. And prisoners have a fivefold greater prevalence of HIV.
“In 2014 these vulnerable groups accounted for more than one-third of all new HIV infections. That’s an extraordinary proportion of HIV,” he observed. “This truly is the undone work of the HIV response. If there’s any silver lining in this cloud, it’s this: We’re talking about a relatively small number of people who are at high risk of infection relative to the world’s population. And that means that turning this around doesn’t require massive new commitments to very large populations. What it does require is an honest acknowledgment of where the epidemic is hitting hardest and directing resources to that need.”
Unfortunately, screening and treatment programs are rarely tailored to reach these highly vulnerable groups effectively, he added.
Dr. Beyrer was a contributor to a special issue of the Lancet devoted to HIV infection among prisoners published with the AIDS 2016 conference.
Dr. Springer’s study was funded by the National Institute on Alcohol Abuse and Alcoholism, and the National Institute on Drug Abuse. She reported having no financial conflicts of interest.
DURBAN, SOUTH AFRICA – Extended-release naltrexone provides clinically meaningful benefits in HIV-infected prisoners with alcohol use disorder and multiple comorbid conditions as they transition back into the community, according to the findings of a double-blind randomized clinical trial.
“I think it’s important to know that a very effective medication, which has not previously been given to this population, was accepted by this group. It may be a feasible conduit to care as they transition to the community, even among those with severe psychosocial disparities like homelessness and mental illness,” Sandra A. Springer, MD, said in presenting the study findings at the 21st International AIDS Conference.
Extended-release naltrexone (Vivitrol) is a mu-opioid receptor antagonist approved for the treatment of alcohol use disorder, where it has been shown to decrease consumption. But prior to her study, the once-monthly injectable drug hadn’t been studied in alcohol-dependent prisoners living with HIV who are transitioning from jail or prison into the community, noted Dr. Springer, an infectious disease specialist at Yale University in New Haven, Conn.
This is a large, important, and seriously neglected patient population, she observed. The United States has the highest incarceration rate in the world. The prevalence of HIV infection is at least three times greater in U.S. criminal justice settings than in the general population. Alcohol use disorders are eightfold more common. Release from prison or jail in affected individuals often is complicated by relapse to alcohol use, which in turn is associated with poor HIV treatment outcomes.
Dr. Springer reported on 100 HIV-positive adult prisoners with alcohol use disorder diagnosed by DSM-IV criteria who were randomized double-blind two-to-one to 6 monthly 380-mg intramuscular injections of extended-release naltrexone or placebo, with the first dose given 3-7 days prior to release. Participants were required to have no baseline clinical evidence of cirrhosis or very high liver enzyme levels.
Half of participants had chronic hepatitis C. Eighty-seven percent of subjects scored 20 or higher on the Alcohol Use Disorders Identification test, indicating alcohol dependence. On the Mini International Neuropsychiatric Interview, 15% of participants met criteria for major depressive disorder, 16% for bipolar disorder, 59% for cocaine use disorder, 16% for narcotic use disorder, and 16% for cannabis use disorder. Most of the subjects were homeless or had an unstable housing situation.
Alcohol outcomes were assessed monthly during the 6-month trial. Not surprisingly, the better the treatment adherence, the better the outcomes. During the 90 days before incarceration, patients self-reported that 70% of those days were heavy drinking days, defined in men as having five or more drinks per day and in women as four or more. Their average consumption on those heavy drinking days was 28 drinks per day. In contrast, patients who accepted four or more extended-release naltrexone injections during 180 days of follow-up after release from custody drank heavily on just 7.6% of days, with an average of 8.6 drinks per day on those heavy drinking days. Subjects who received four or more placebo injections drank heavily on 11.6% of days, consuming an average of 12 drinks per heavy drinking day.
The time to first heavy drinking day was longer in patients who accepted 4-6 monthly injections of extended-release naltrexone than in those with 4-6 placebo injections. However, the difference achieved statistical significance only in the younger subgroup of participants aged 21-29 years. In that subgroup, the average time to the first heavy drinking day was 24.1 days, compared with 9.5 days with placebo.
On a composite alcohol consumption index comprised of time to first heavy drinking day after release, mean number of drinks per drinking day, change from before to after incarceration in average number of drinks per drinking day, alcohol craving score, and total number of drinking days, subjects who received four or more extended-release naltrexone injections had a significantly more favorable result, with a mean score of 3.15, compared with 2.93 in patients who took four or more placebo injections.
Moreover, consistent use of extended-release naltrexone was associated with significantly lower HIV viral load counts, compared with placebo-treated controls.
Treatment with extended-release naltrexone was safe. No serious side effects occurred, even in patients with comorbid hepatitis C who were on antiretroviral therapy. The most common side effects were the same as in seen in studies of the drug in other populations: mild to moderate nausea, headache, decreased appetite, fatigue, and dizziness.
Elsewhere at AIDS 2016, Chris Beyrer, MD, president of the International AIDS Society, included prisoners on his list of the populations most vulnerable to HIV because of discriminatory laws and policies in many parts of the world. Others on the list were transgender people, sex workers, men who have sex with men, and injection drug users.
“We’ll never be able to end AIDS without addressing the needs of these most vulnerable individuals and communities, and yet we know in 2016 far too many are being left behind,” said Dr. Beyrer, professor of epidemiology at Johns Hopkins University, Baltimore.
Transgender individuals, for example, are 49 times more likely to have HIV infection than other adults. Injection drug users and men who have sex with men are each 24-fold more likely to become HIV infected than the general population. Sex workers are 10 times more likely to acquire HIV infection than others in their reproductive years. And prisoners have a fivefold greater prevalence of HIV.
“In 2014 these vulnerable groups accounted for more than one-third of all new HIV infections. That’s an extraordinary proportion of HIV,” he observed. “This truly is the undone work of the HIV response. If there’s any silver lining in this cloud, it’s this: We’re talking about a relatively small number of people who are at high risk of infection relative to the world’s population. And that means that turning this around doesn’t require massive new commitments to very large populations. What it does require is an honest acknowledgment of where the epidemic is hitting hardest and directing resources to that need.”
Unfortunately, screening and treatment programs are rarely tailored to reach these highly vulnerable groups effectively, he added.
Dr. Beyrer was a contributor to a special issue of the Lancet devoted to HIV infection among prisoners published with the AIDS 2016 conference.
Dr. Springer’s study was funded by the National Institute on Alcohol Abuse and Alcoholism, and the National Institute on Drug Abuse. She reported having no financial conflicts of interest.
AT AIDS 2016
Key clinical point: Extended-release naltrexone helps HIV-infected prisoners with alcohol use disorder in transitioning to the community.
Major finding: The mean time to the first heavy drinking day among 21- to 29-year-old HIV-infected prisoners with an alcohol use disorder was 24.1 days following release from prison or jail in those on extended-release naltrexone versus 9.5 days with placebo.
Data source: This randomized, double-blind clinical trial included 100 HIV-positive prisoners with alcohol use disorder who were released into the community. Two-thirds received six monthly injections of extended-release naltrexone, the rest placebo.
Disclosures: The study was funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse. The presenter reported having no financial conflicts of interest.
FDA grants fast track status to volixibat
The Food and Drug Administration has granted fast track status to volixibat, an investigational treatment manufactured by Shire.
“The FDA’s fast track is a process designed to facilitate the development, and expedite the review, of drugs to treat serious conditions and fill an unmet medical need,” the Dublin-based pharmaceutical company explained in a statement. “However, it does not guarantee that the FDA will ultimately approve [volixibat] for NASH [nonalcoholic steatohepatitis] or the timing of any such approval.”
Volixibat, also known as SHP626, is meant to treat NASH with liver fibrosis in adult patients via an apical sodium-dependent bile acid transporter inhibitor, which is a protein that recycles bile acids from the intestine and into the liver. This orally administered, once-daily treatment would be the first ever treatment available for NASH.
“This fast track designation is further recognition of the critical need to develop new, effective therapeutic options for patients with this serious condition,” said Philip J. Vickers, PhD, head of research and development for Shire, in a statement.
Preclinical and phase I studies have already been completed, the results of which contributed to the FDA granting volixibat fast track status. A randomized, placebo-controlled, double-blind phase II trial is set to get underway shortly at centers in the United States, Canada, and the United Kingdom, which will examine safety, tolerability, and efficacy of a three-dose volixibat regimen administered over the course of 48 weeks.
The Food and Drug Administration has granted fast track status to volixibat, an investigational treatment manufactured by Shire.
“The FDA’s fast track is a process designed to facilitate the development, and expedite the review, of drugs to treat serious conditions and fill an unmet medical need,” the Dublin-based pharmaceutical company explained in a statement. “However, it does not guarantee that the FDA will ultimately approve [volixibat] for NASH [nonalcoholic steatohepatitis] or the timing of any such approval.”
Volixibat, also known as SHP626, is meant to treat NASH with liver fibrosis in adult patients via an apical sodium-dependent bile acid transporter inhibitor, which is a protein that recycles bile acids from the intestine and into the liver. This orally administered, once-daily treatment would be the first ever treatment available for NASH.
“This fast track designation is further recognition of the critical need to develop new, effective therapeutic options for patients with this serious condition,” said Philip J. Vickers, PhD, head of research and development for Shire, in a statement.
Preclinical and phase I studies have already been completed, the results of which contributed to the FDA granting volixibat fast track status. A randomized, placebo-controlled, double-blind phase II trial is set to get underway shortly at centers in the United States, Canada, and the United Kingdom, which will examine safety, tolerability, and efficacy of a three-dose volixibat regimen administered over the course of 48 weeks.
The Food and Drug Administration has granted fast track status to volixibat, an investigational treatment manufactured by Shire.
“The FDA’s fast track is a process designed to facilitate the development, and expedite the review, of drugs to treat serious conditions and fill an unmet medical need,” the Dublin-based pharmaceutical company explained in a statement. “However, it does not guarantee that the FDA will ultimately approve [volixibat] for NASH [nonalcoholic steatohepatitis] or the timing of any such approval.”
Volixibat, also known as SHP626, is meant to treat NASH with liver fibrosis in adult patients via an apical sodium-dependent bile acid transporter inhibitor, which is a protein that recycles bile acids from the intestine and into the liver. This orally administered, once-daily treatment would be the first ever treatment available for NASH.
“This fast track designation is further recognition of the critical need to develop new, effective therapeutic options for patients with this serious condition,” said Philip J. Vickers, PhD, head of research and development for Shire, in a statement.
Preclinical and phase I studies have already been completed, the results of which contributed to the FDA granting volixibat fast track status. A randomized, placebo-controlled, double-blind phase II trial is set to get underway shortly at centers in the United States, Canada, and the United Kingdom, which will examine safety, tolerability, and efficacy of a three-dose volixibat regimen administered over the course of 48 weeks.