User login
2016 Update on contraception
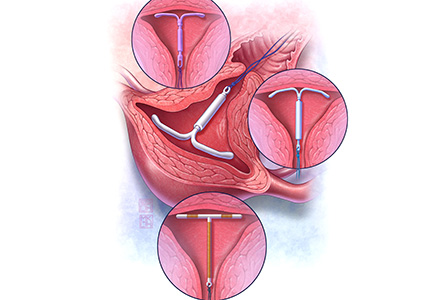
Contraception is an important tool that allows patients to carry out their reproductive-life plans. In the United States, the average woman desires 2 children.1 To achieve this goal, she will spend more than 30 years of her reproductive life avoiding pregnancy.1 The most effective reversible contraceptive methods, the intrauterine device (IUD) and the contraceptive implant, offer an efficient way to cover this significant period. Currently, American women more commonly choose an IUD than an implant by a factor 8 to 1.2 Between 2002 and 2012, the percentage of US contraceptive users aged 15 to 44 using the IUD rose from 2% to 10%.2
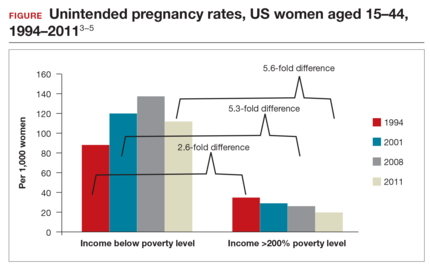
Significant barriers to contraceptive access still exist, however. Although widespread reports have lauded the decrease in unintended pregnancies in the United States, improvement only has been marginal for women who live below the poverty level. In fact, for unintended pregnancy the gap between women above and below the poverty level has increased from a 2.6-fold difference in 1994 to a 5.6-fold difference in 2011 (FIGURE).3−5 Since the decrease in the unintended pregnancy rate is most likely related to an increase in contraceptive use, particularly the IUD, we are not providing equal contraceptive access to all women.5
Both the copper IUD and the 3 available levonorgestrel (LNG)-releasing intrauterine system (IUS) products provide safe and effective contraception. As IUD research expands, it is imperative for providers to stay up to date so patients can have full access to these devices.
In this article, we present important updates regarding IUD use that will help break down some continuing barriers to contraceptive access, including:
- clinical trial data demonstrating efficacy of LNG 52-mg IUS for 7 years
- a novel emergency contraception (EC) regimen of same-day oral LNG and the LNG 52-mg IUS
- a large prospective trial demonstrating that women can safely have IUS placement without known sexually transmitted infection (STI) screening results and that pelvic infection rates are not higher in the time shortly after IUS placement.

WHO study demonstrates LNG 52-mg IUS is highly effective for up to 7 years of use
Rowe P, Farley T, Peregoudov A, et al; IUD Research Group of the UNDP/UNFPA/WHO/World Bank Special Programme of Research; Development and Research Training in Human Reproduction. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93(6):498−506.
Currently, the 2 LNG 52-mg IUS products (Liletta, Mirena) approved by the US Food and Drug Administration (FDA) are available for use for 3 and 5 years, respectively. The pivotal approval trial for Liletta is still ongoing and is planned to continue for up to 7 years.6 The TCu380A (ParaGard) copper IUD is FDA approved for up to 10 years of use; however, this product initially was approved for only 4 years. The duration of use was expanded to 10 years based on continued clinical trials.
Based on current data, we will not need to wait for the Liletta pivotal trial to have clinical evidence of a longer duration of efficacy for the LNG 52-mg IUS. A collaborative group as part of the UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction performed a multicenter, open-label randomized controlled trial to evaluate outcomes through 7 years of use of the LNG 52-mg IUS and the TCu380A IUD.
Details of the study
A total of 3,836 women were enrolled at 20 centers in Europe, Asia, South America, and China and were randomly assigned to one of the 2 products. Eligible women were aged 16 to 40 years, parous, and without known leiomyoma or recent pelvic infection. After excluding 15 failed IUD insertions, 1,910 women received an LNG 52-mg IUS and 1,911 received a TCu380A. Ultimately, 398 women in the LNG 52-mg IUS group and 682 in the TCu380A group completed 7-year follow-up with the IUD in place. Women were surveyed regarding pregnancy and method discontinuation.
Lower pregnancy rate, higher discontinuation with LNG IUS
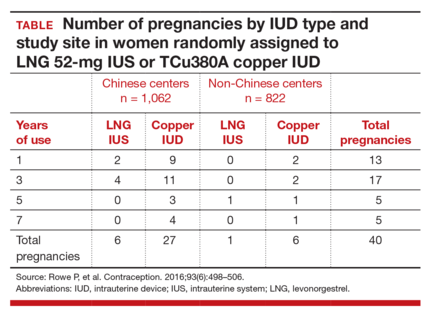
The cumulative 7-year pregnancy rate among LNG 52-mg IUS users was significantly lower than among TCu380A users (0.53 per 100 women vs 2.45 per 100 women, respectively). All pregnancies in the LNG 52-mg IUS group occurred in the first 5 years of study follow-up--with no pregnancies in years 6 through 7 (TABLE). The cumulative pregnancy rate in the TCu380A group in this study is consistent with that in a previous long-term trial of this IUD.7
Early removal was significantly higher in the LNG 52-mg IUS group, with a cumulative discontinuation rate of 70.6 per 100 women, compared with 40.8 per 100 women in the TCu380A group. Significant cultural variation existed when it came to both rate and reason for discontinuation. Most women at Chinese centers cited amenorrhea and decreased bleeding as the primary reason for discontinuation, and they did so at twice the rate of women at non-Chinese centers.
The patterns of method discontinuation in this study were different from those found among US women. By comparison, a recent study in the United States had lower overall discontinuation rates and did not find decreased bleeding to be among the main reasons for LNG 52-mg IUS removal.7 In fact, most women who discontinued the LNG 52-mg IUS cited concerns about upcoming expiration as their reason for removal.
The results of this large study also generally corroborated the low-risk profile of IUDs. Only 1 reported IUD perforation occurred, for a rate of 0.03 per 1,000 women. Device expulsion rates were similar between the IUDs and were uncommon overall with 7-year rates of 8 to 9 per 100 women. Pelvic infection was cited as reason for removal in only 7 women (0.18 per 100 women) over 7 years.
What this evidence means for practiceThis exciting study is the first large-scale clinical trial demonstrating continued high efficacy with LNG 52-mg IUS use through 7 years. This information affords women extended contraceptive coverage. While additional research will be welcome, particularly in younger women who will maintain greater fertility across the IUS’s 7-year life span, we are confident in extending the 7-year duration for this IUS to our patients.
Additionally, the method discontinuation findings in this study highlight the importance of discussing the expected menstrual changes of hormonal IUS use with women prior to insertion so they can determine if the potential changes would be satisfactory. As the acceptability of medical menstrual suppression may be new to many women, providers should frame the adverse effects in this context. Providers can use this opportunity to review the noncontraceptive benefits of the hormonal IUS as well.
Novel combination of LNG 52-mg IUS and oral LNG 1.5 mg is promising for emergency contraception
Turok DK, Sanders JN, Thompson IS, Royer PA, Eggebroten J, Gawron LM. Preference for and efficacy of oral levonorgestrel for emergency contraception with concomitant placement of a levonorgestrel IUD: a prospective cohort study. Contraception. 2016;93(6):526−532.
The copper IUD is superior for EC relative to oral agents and has the added benefit of providing ongoing highly effective contraception after placement.8 Despite this strong evidence, the copper IUD remains underutilized for this indication. Turok and colleagues noted that women in their clinic seeking IUDs for non-EC purposes preferred the LNG 52-mg IUS over the copper IUD. It is understandable that women might carry these preferences into EC encounters as well. Thus, the investigators devised a novel combination of LNG 52-mg IUS and oral LNG 1.5 mg, which provided both known EC benefit and same-day access to a more popular contraceptive device.
Details of the study
Women presenting for EC who desired same-day IUD placement were enrolled in the prospective cohort study. Eligible women had a negative urine pregnancy test, known last menstrual period (LMP), regular menstrual cycle, and reported unprotected intercourse within 120 hours prior to presentation. Importantly, women with multiple episodes of unprotected intercourse in the weeks prior to presentation were also included to provide a population more comparable to that encountered clinically. The women were then offered the choice of a TCu380A copper IUD or oral LNG EC with LNG 52-mg IUS placement. They were counseled on the potential increased risk of pregnancy with the novel oral LNG EC plus LNG IUS combination compared with the copper IUD. Participants were given a home pregnancy test that they were to complete in 2 weeks and then report the results to the clinic.
Of the 1,004 women presenting to the clinic for EC over the 16-month study period, 188 (18%) desired same-day IUD insertion. Of these, more opted for the oral LNG EC plus LNG IUS combination (n = 121, 64%) than the copper IUD (n = 67, 36%), demonstrating that women were often willing to accept a possible decrease in EC efficacy with the goal of obtaining their preferred lUD type.
Excluding failed insertion, undiagnosed uterine didelphys, and patient withdrawal, 110 women received the oral LNG EC plus LNG IUS and 66 received the copper IUD. Demographics were comparable between groups except for body mass index (BMI). Of note, more than half (61%) of the women who opted for the oral LNG EC plus LNG IUS combination were overweight or obese.
Both EC methods are effective, broadening options
All women who received the copper IUD followed up at 2 weeks, and no pregnancies were reported. Of the women who received oral LNG EC plus the LNG IUS, 107 (97%) had follow-up at 2 weeks. In this group, there was 1 reported ectopic pregnancy that ultimately required surgical management. However, further review of the patient's coital history suggested that conception occurred prior to IUD insertion, and the case was not classified by the investigators as an EC failure.
Although this study was not powered to detect pregnancy rate differences between the traditional copper IUD and the oral LNG EC plus LNG IUS combination, the results are promising. An important strength of this study is the presence of 2 high-risk groups for EC failure in the oral LNG EC plus LNG IUS group (elevated BMI and multiple episodes of unprotected intercourse).
What this evidence means for practiceEncounters for EC are important opportunities in which to discuss a woman’s reproductive goals. For women who are interested in an IUD, this study opens up the option of same-day placement of the LNG 52-mg IUS. While larger trials need to be conducted to obtain more information regarding pregnancy rates with an oral EC plus LNG IUS combination versus the TCu380A, offering such a combination is reasonable. Given that ulipristal acetate (UPA) is a more effective EC product, especially for women who are overweight or obese,9 offering a UPA EC plus LNG IUS combination may be a better alternative.
It is time to remove the STI screening barrier to same-day IUD insertion
Turok DK, Eisenberg DL, Teal SB, Keder LM, Creinin MD. A prospective assessment of pelvic infection risk following same-day sexually transmitted infection testing and levonorgestrel intrauterine system placement [published online ahead of print May 12, 2016]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2016.05017.
Provider concerns regarding the presence of pelvic infection remain a significant barrier to same-day IUD insertion. Older studies suggested a higher risk of pelvic infection in the first 20 days after IUD placement and extrapolated that pelvic infection was related to inserting an IUD in a woman at risk for STI.10 As a result, patients may be restricted from same-day IUD insertion by providers who think that obtaining results of STI testing is required prior to placement. Recently, a systematic review suggested, based on limited evidence, that IUD placement does not increase the risk of pelvic infection in asymptomatic women compared with those without an IUD.11
Turok and colleagues (including M.D.C., coauthor of this article) reported results from a planned secondary analysis of A Comprehensive Contraceptive Efficacy and Safety Study of an IUS (ACCESS IUS), a component of the regulatory approval of the Liletta LNG 52-mg IUS. This analysis represents the first large-scale prospective investigation of pelvic infection rates during the first 2 years after IUD placement in US women.
Details of the study
Of the 1,751 women enrolled in the study, 1,714 had successful IUS insertions. Infection was assessed via baseline pelvic visual and bimanual examination and Chlamydia testing in all women. Gonorrhea testing was also performed in women who had not been tested with their current sexual partner. STI test results were not required for IUS insertion. Participants were assessed in person at 1, 3, 6, 12, and 24 months after insertion. Additional pelvic exams were performed as needed based on reported symptoms. At 6 months, 1 year, and 2 years, IUS continuation was reported in 1,553 (90.6%), 1,401 (81.7%), and 1,157 (67.3%) women, respectively.
Nearly all women received baseline STI testing (98.4%); however, results were not available prior to same-day IUS insertion for 79.6% of participants. Twenty-nine (1.7%) women had positive baseline STI tests (25 for Chlamydia, 3 for gonorrhea, 1 for both). Of these, only 6 women had results available prior to IUS placement. All women with a positive STI test were treated, and the IUS was left in place. Importantly, none of these women developed pelvic infection in the subsequent 2 years of follow-up and none requested IUS removal.
Infection risk is low with IUD placement
Among women with negative baseline STI tests, there were only 9 (0.5%) clinical diagnoses of pelvic infections over the first 2 years of follow-up. Diagnosis was typically made based on physical examination findings. Most women underwent repeat Chlamydia and gonorrhea testing at the time of pelvic infection diagnosis, and none had positive results. There were no medically recommended IUS removals; 2 women with pelvic infection requested IUS removal per their preference.
Three of the 9 women with pelvic infections were diagnosed within 1 week of IUS placement, 1 at 39 days after placement, and the remaining 5 more than 6 months after placement, suggesting that pelvic infection is not temporally related to IUS placement. Most women were successfully treated as outpatients.
What this evidence means for practiceThis study provides further reassurance regarding the low risk of pelvic infection among women with an LNG IUS. Insertion of an IUS should not be delayed to await results of Chlamydia or gonorrhea testing in a woman without clinical evidence of pelvic infection. Risk-based, as opposed to universal testing, is imperative.12 These recommendations are in agreement with current recommendations of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists.13,14 Practices that employ 2-visit protocols unnecessarily limit women’s access to the IUS, as research has shown that nearly half of women desiring an IUD do not return for device placement if a second encounter is required.15
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Office of Population Affairs, Department of Health and Human Services. Family planning program, FY 1999 service program grants by state. Bethesda, MD: Office of Population Affairs; 1999.
- Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among US women, 2009-2012. Obstet Gynecol. 2015;126(5):917–927.
- Ventura SJ, Abma JC, Mosher WD, Henshaw S. Revised pregnancy rates, 1990-97, and new rates for 1998-99: United States. Natl Vital Stat Rep. 2003;52(7):1−14.
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001-2008. Am J Public Health. 2014;104(suppl 1):S43–S48.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843–852.
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD; ACCESS IUS Investigators. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
- United Nations Development Programme, United Nations Population Fund, World Health Organization, World Bank, Special Programme of Research, Development, and Research Training in Human Reproduction. Long-term reversible contraception: twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–352.
- Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27(7):1994–2000.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339(8796):785–788.
- Jatlaoui TC, Simmons KB, Curtis KM. The safety of intrauterine contraception initiation among women with current asymptomatic cervical infections or at increased risk of sexually transmitted infections [published online ahead of print June 1, 2016]. Contraception. doi:10.1016/j.contracep tion.2016.05.013.
- Grentzer JM, Peipert JF, Zhao Q, McNicholas C, Secura GM, Madden T. Risk-based screening for Chlamydia trachomatis and Neisseria gonorrhoeae prior to intrauterine device insertion. Contraception. 2015;92(4):313–318.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
- Centers for Disease Control and Prevention. US selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd ed. MMWR Recomm Rep. 2013;62(RR05):1–46.
- Bergin A, Tristan S, Terplan M, Gilliam ML, Whitaker AK. A missed opportunity for care: two-visit IUD insertion protocols inhibit placement. Contraception. 2012;86(6):694–697.
Contraception is an important tool that allows patients to carry out their reproductive-life plans. In the United States, the average woman desires 2 children.1 To achieve this goal, she will spend more than 30 years of her reproductive life avoiding pregnancy.1 The most effective reversible contraceptive methods, the intrauterine device (IUD) and the contraceptive implant, offer an efficient way to cover this significant period. Currently, American women more commonly choose an IUD than an implant by a factor 8 to 1.2 Between 2002 and 2012, the percentage of US contraceptive users aged 15 to 44 using the IUD rose from 2% to 10%.2
Significant barriers to contraceptive access still exist, however. Although widespread reports have lauded the decrease in unintended pregnancies in the United States, improvement only has been marginal for women who live below the poverty level. In fact, for unintended pregnancy the gap between women above and below the poverty level has increased from a 2.6-fold difference in 1994 to a 5.6-fold difference in 2011 (FIGURE).3−5 Since the decrease in the unintended pregnancy rate is most likely related to an increase in contraceptive use, particularly the IUD, we are not providing equal contraceptive access to all women.5

Both the copper IUD and the 3 available levonorgestrel (LNG)-releasing intrauterine system (IUS) products provide safe and effective contraception. As IUD research expands, it is imperative for providers to stay up to date so patients can have full access to these devices.
In this article, we present important updates regarding IUD use that will help break down some continuing barriers to contraceptive access, including:
- clinical trial data demonstrating efficacy of LNG 52-mg IUS for 7 years
- a novel emergency contraception (EC) regimen of same-day oral LNG and the LNG 52-mg IUS
- a large prospective trial demonstrating that women can safely have IUS placement without known sexually transmitted infection (STI) screening results and that pelvic infection rates are not higher in the time shortly after IUS placement.

WHO study demonstrates LNG 52-mg IUS is highly effective for up to 7 years of use
Rowe P, Farley T, Peregoudov A, et al; IUD Research Group of the UNDP/UNFPA/WHO/World Bank Special Programme of Research; Development and Research Training in Human Reproduction. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93(6):498−506.
Currently, the 2 LNG 52-mg IUS products (Liletta, Mirena) approved by the US Food and Drug Administration (FDA) are available for use for 3 and 5 years, respectively. The pivotal approval trial for Liletta is still ongoing and is planned to continue for up to 7 years.6 The TCu380A (ParaGard) copper IUD is FDA approved for up to 10 years of use; however, this product initially was approved for only 4 years. The duration of use was expanded to 10 years based on continued clinical trials.
Based on current data, we will not need to wait for the Liletta pivotal trial to have clinical evidence of a longer duration of efficacy for the LNG 52-mg IUS. A collaborative group as part of the UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction performed a multicenter, open-label randomized controlled trial to evaluate outcomes through 7 years of use of the LNG 52-mg IUS and the TCu380A IUD.
Details of the study
A total of 3,836 women were enrolled at 20 centers in Europe, Asia, South America, and China and were randomly assigned to one of the 2 products. Eligible women were aged 16 to 40 years, parous, and without known leiomyoma or recent pelvic infection. After excluding 15 failed IUD insertions, 1,910 women received an LNG 52-mg IUS and 1,911 received a TCu380A. Ultimately, 398 women in the LNG 52-mg IUS group and 682 in the TCu380A group completed 7-year follow-up with the IUD in place. Women were surveyed regarding pregnancy and method discontinuation.
Lower pregnancy rate, higher discontinuation with LNG IUS
The cumulative 7-year pregnancy rate among LNG 52-mg IUS users was significantly lower than among TCu380A users (0.53 per 100 women vs 2.45 per 100 women, respectively). All pregnancies in the LNG 52-mg IUS group occurred in the first 5 years of study follow-up--with no pregnancies in years 6 through 7 (TABLE). The cumulative pregnancy rate in the TCu380A group in this study is consistent with that in a previous long-term trial of this IUD.7

Early removal was significantly higher in the LNG 52-mg IUS group, with a cumulative discontinuation rate of 70.6 per 100 women, compared with 40.8 per 100 women in the TCu380A group. Significant cultural variation existed when it came to both rate and reason for discontinuation. Most women at Chinese centers cited amenorrhea and decreased bleeding as the primary reason for discontinuation, and they did so at twice the rate of women at non-Chinese centers.
The patterns of method discontinuation in this study were different from those found among US women. By comparison, a recent study in the United States had lower overall discontinuation rates and did not find decreased bleeding to be among the main reasons for LNG 52-mg IUS removal.7 In fact, most women who discontinued the LNG 52-mg IUS cited concerns about upcoming expiration as their reason for removal.
The results of this large study also generally corroborated the low-risk profile of IUDs. Only 1 reported IUD perforation occurred, for a rate of 0.03 per 1,000 women. Device expulsion rates were similar between the IUDs and were uncommon overall with 7-year rates of 8 to 9 per 100 women. Pelvic infection was cited as reason for removal in only 7 women (0.18 per 100 women) over 7 years.
What this evidence means for practiceThis exciting study is the first large-scale clinical trial demonstrating continued high efficacy with LNG 52-mg IUS use through 7 years. This information affords women extended contraceptive coverage. While additional research will be welcome, particularly in younger women who will maintain greater fertility across the IUS’s 7-year life span, we are confident in extending the 7-year duration for this IUS to our patients.
Additionally, the method discontinuation findings in this study highlight the importance of discussing the expected menstrual changes of hormonal IUS use with women prior to insertion so they can determine if the potential changes would be satisfactory. As the acceptability of medical menstrual suppression may be new to many women, providers should frame the adverse effects in this context. Providers can use this opportunity to review the noncontraceptive benefits of the hormonal IUS as well.
Novel combination of LNG 52-mg IUS and oral LNG 1.5 mg is promising for emergency contraception
Turok DK, Sanders JN, Thompson IS, Royer PA, Eggebroten J, Gawron LM. Preference for and efficacy of oral levonorgestrel for emergency contraception with concomitant placement of a levonorgestrel IUD: a prospective cohort study. Contraception. 2016;93(6):526−532.
The copper IUD is superior for EC relative to oral agents and has the added benefit of providing ongoing highly effective contraception after placement.8 Despite this strong evidence, the copper IUD remains underutilized for this indication. Turok and colleagues noted that women in their clinic seeking IUDs for non-EC purposes preferred the LNG 52-mg IUS over the copper IUD. It is understandable that women might carry these preferences into EC encounters as well. Thus, the investigators devised a novel combination of LNG 52-mg IUS and oral LNG 1.5 mg, which provided both known EC benefit and same-day access to a more popular contraceptive device.
Details of the study
Women presenting for EC who desired same-day IUD placement were enrolled in the prospective cohort study. Eligible women had a negative urine pregnancy test, known last menstrual period (LMP), regular menstrual cycle, and reported unprotected intercourse within 120 hours prior to presentation. Importantly, women with multiple episodes of unprotected intercourse in the weeks prior to presentation were also included to provide a population more comparable to that encountered clinically. The women were then offered the choice of a TCu380A copper IUD or oral LNG EC with LNG 52-mg IUS placement. They were counseled on the potential increased risk of pregnancy with the novel oral LNG EC plus LNG IUS combination compared with the copper IUD. Participants were given a home pregnancy test that they were to complete in 2 weeks and then report the results to the clinic.
Of the 1,004 women presenting to the clinic for EC over the 16-month study period, 188 (18%) desired same-day IUD insertion. Of these, more opted for the oral LNG EC plus LNG IUS combination (n = 121, 64%) than the copper IUD (n = 67, 36%), demonstrating that women were often willing to accept a possible decrease in EC efficacy with the goal of obtaining their preferred lUD type.
Excluding failed insertion, undiagnosed uterine didelphys, and patient withdrawal, 110 women received the oral LNG EC plus LNG IUS and 66 received the copper IUD. Demographics were comparable between groups except for body mass index (BMI). Of note, more than half (61%) of the women who opted for the oral LNG EC plus LNG IUS combination were overweight or obese.
Both EC methods are effective, broadening options
All women who received the copper IUD followed up at 2 weeks, and no pregnancies were reported. Of the women who received oral LNG EC plus the LNG IUS, 107 (97%) had follow-up at 2 weeks. In this group, there was 1 reported ectopic pregnancy that ultimately required surgical management. However, further review of the patient's coital history suggested that conception occurred prior to IUD insertion, and the case was not classified by the investigators as an EC failure.
Although this study was not powered to detect pregnancy rate differences between the traditional copper IUD and the oral LNG EC plus LNG IUS combination, the results are promising. An important strength of this study is the presence of 2 high-risk groups for EC failure in the oral LNG EC plus LNG IUS group (elevated BMI and multiple episodes of unprotected intercourse).
What this evidence means for practiceEncounters for EC are important opportunities in which to discuss a woman’s reproductive goals. For women who are interested in an IUD, this study opens up the option of same-day placement of the LNG 52-mg IUS. While larger trials need to be conducted to obtain more information regarding pregnancy rates with an oral EC plus LNG IUS combination versus the TCu380A, offering such a combination is reasonable. Given that ulipristal acetate (UPA) is a more effective EC product, especially for women who are overweight or obese,9 offering a UPA EC plus LNG IUS combination may be a better alternative.
It is time to remove the STI screening barrier to same-day IUD insertion
Turok DK, Eisenberg DL, Teal SB, Keder LM, Creinin MD. A prospective assessment of pelvic infection risk following same-day sexually transmitted infection testing and levonorgestrel intrauterine system placement [published online ahead of print May 12, 2016]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2016.05017.
Provider concerns regarding the presence of pelvic infection remain a significant barrier to same-day IUD insertion. Older studies suggested a higher risk of pelvic infection in the first 20 days after IUD placement and extrapolated that pelvic infection was related to inserting an IUD in a woman at risk for STI.10 As a result, patients may be restricted from same-day IUD insertion by providers who think that obtaining results of STI testing is required prior to placement. Recently, a systematic review suggested, based on limited evidence, that IUD placement does not increase the risk of pelvic infection in asymptomatic women compared with those without an IUD.11
Turok and colleagues (including M.D.C., coauthor of this article) reported results from a planned secondary analysis of A Comprehensive Contraceptive Efficacy and Safety Study of an IUS (ACCESS IUS), a component of the regulatory approval of the Liletta LNG 52-mg IUS. This analysis represents the first large-scale prospective investigation of pelvic infection rates during the first 2 years after IUD placement in US women.
Details of the study
Of the 1,751 women enrolled in the study, 1,714 had successful IUS insertions. Infection was assessed via baseline pelvic visual and bimanual examination and Chlamydia testing in all women. Gonorrhea testing was also performed in women who had not been tested with their current sexual partner. STI test results were not required for IUS insertion. Participants were assessed in person at 1, 3, 6, 12, and 24 months after insertion. Additional pelvic exams were performed as needed based on reported symptoms. At 6 months, 1 year, and 2 years, IUS continuation was reported in 1,553 (90.6%), 1,401 (81.7%), and 1,157 (67.3%) women, respectively.
Nearly all women received baseline STI testing (98.4%); however, results were not available prior to same-day IUS insertion for 79.6% of participants. Twenty-nine (1.7%) women had positive baseline STI tests (25 for Chlamydia, 3 for gonorrhea, 1 for both). Of these, only 6 women had results available prior to IUS placement. All women with a positive STI test were treated, and the IUS was left in place. Importantly, none of these women developed pelvic infection in the subsequent 2 years of follow-up and none requested IUS removal.
Infection risk is low with IUD placement
Among women with negative baseline STI tests, there were only 9 (0.5%) clinical diagnoses of pelvic infections over the first 2 years of follow-up. Diagnosis was typically made based on physical examination findings. Most women underwent repeat Chlamydia and gonorrhea testing at the time of pelvic infection diagnosis, and none had positive results. There were no medically recommended IUS removals; 2 women with pelvic infection requested IUS removal per their preference.
Three of the 9 women with pelvic infections were diagnosed within 1 week of IUS placement, 1 at 39 days after placement, and the remaining 5 more than 6 months after placement, suggesting that pelvic infection is not temporally related to IUS placement. Most women were successfully treated as outpatients.
What this evidence means for practiceThis study provides further reassurance regarding the low risk of pelvic infection among women with an LNG IUS. Insertion of an IUS should not be delayed to await results of Chlamydia or gonorrhea testing in a woman without clinical evidence of pelvic infection. Risk-based, as opposed to universal testing, is imperative.12 These recommendations are in agreement with current recommendations of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists.13,14 Practices that employ 2-visit protocols unnecessarily limit women’s access to the IUS, as research has shown that nearly half of women desiring an IUD do not return for device placement if a second encounter is required.15
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Contraception is an important tool that allows patients to carry out their reproductive-life plans. In the United States, the average woman desires 2 children.1 To achieve this goal, she will spend more than 30 years of her reproductive life avoiding pregnancy.1 The most effective reversible contraceptive methods, the intrauterine device (IUD) and the contraceptive implant, offer an efficient way to cover this significant period. Currently, American women more commonly choose an IUD than an implant by a factor 8 to 1.2 Between 2002 and 2012, the percentage of US contraceptive users aged 15 to 44 using the IUD rose from 2% to 10%.2
Significant barriers to contraceptive access still exist, however. Although widespread reports have lauded the decrease in unintended pregnancies in the United States, improvement only has been marginal for women who live below the poverty level. In fact, for unintended pregnancy the gap between women above and below the poverty level has increased from a 2.6-fold difference in 1994 to a 5.6-fold difference in 2011 (FIGURE).3−5 Since the decrease in the unintended pregnancy rate is most likely related to an increase in contraceptive use, particularly the IUD, we are not providing equal contraceptive access to all women.5

Both the copper IUD and the 3 available levonorgestrel (LNG)-releasing intrauterine system (IUS) products provide safe and effective contraception. As IUD research expands, it is imperative for providers to stay up to date so patients can have full access to these devices.
In this article, we present important updates regarding IUD use that will help break down some continuing barriers to contraceptive access, including:
- clinical trial data demonstrating efficacy of LNG 52-mg IUS for 7 years
- a novel emergency contraception (EC) regimen of same-day oral LNG and the LNG 52-mg IUS
- a large prospective trial demonstrating that women can safely have IUS placement without known sexually transmitted infection (STI) screening results and that pelvic infection rates are not higher in the time shortly after IUS placement.

WHO study demonstrates LNG 52-mg IUS is highly effective for up to 7 years of use
Rowe P, Farley T, Peregoudov A, et al; IUD Research Group of the UNDP/UNFPA/WHO/World Bank Special Programme of Research; Development and Research Training in Human Reproduction. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93(6):498−506.
Currently, the 2 LNG 52-mg IUS products (Liletta, Mirena) approved by the US Food and Drug Administration (FDA) are available for use for 3 and 5 years, respectively. The pivotal approval trial for Liletta is still ongoing and is planned to continue for up to 7 years.6 The TCu380A (ParaGard) copper IUD is FDA approved for up to 10 years of use; however, this product initially was approved for only 4 years. The duration of use was expanded to 10 years based on continued clinical trials.
Based on current data, we will not need to wait for the Liletta pivotal trial to have clinical evidence of a longer duration of efficacy for the LNG 52-mg IUS. A collaborative group as part of the UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction performed a multicenter, open-label randomized controlled trial to evaluate outcomes through 7 years of use of the LNG 52-mg IUS and the TCu380A IUD.
Details of the study
A total of 3,836 women were enrolled at 20 centers in Europe, Asia, South America, and China and were randomly assigned to one of the 2 products. Eligible women were aged 16 to 40 years, parous, and without known leiomyoma or recent pelvic infection. After excluding 15 failed IUD insertions, 1,910 women received an LNG 52-mg IUS and 1,911 received a TCu380A. Ultimately, 398 women in the LNG 52-mg IUS group and 682 in the TCu380A group completed 7-year follow-up with the IUD in place. Women were surveyed regarding pregnancy and method discontinuation.
Lower pregnancy rate, higher discontinuation with LNG IUS
The cumulative 7-year pregnancy rate among LNG 52-mg IUS users was significantly lower than among TCu380A users (0.53 per 100 women vs 2.45 per 100 women, respectively). All pregnancies in the LNG 52-mg IUS group occurred in the first 5 years of study follow-up--with no pregnancies in years 6 through 7 (TABLE). The cumulative pregnancy rate in the TCu380A group in this study is consistent with that in a previous long-term trial of this IUD.7

Early removal was significantly higher in the LNG 52-mg IUS group, with a cumulative discontinuation rate of 70.6 per 100 women, compared with 40.8 per 100 women in the TCu380A group. Significant cultural variation existed when it came to both rate and reason for discontinuation. Most women at Chinese centers cited amenorrhea and decreased bleeding as the primary reason for discontinuation, and they did so at twice the rate of women at non-Chinese centers.
The patterns of method discontinuation in this study were different from those found among US women. By comparison, a recent study in the United States had lower overall discontinuation rates and did not find decreased bleeding to be among the main reasons for LNG 52-mg IUS removal.7 In fact, most women who discontinued the LNG 52-mg IUS cited concerns about upcoming expiration as their reason for removal.
The results of this large study also generally corroborated the low-risk profile of IUDs. Only 1 reported IUD perforation occurred, for a rate of 0.03 per 1,000 women. Device expulsion rates were similar between the IUDs and were uncommon overall with 7-year rates of 8 to 9 per 100 women. Pelvic infection was cited as reason for removal in only 7 women (0.18 per 100 women) over 7 years.
What this evidence means for practiceThis exciting study is the first large-scale clinical trial demonstrating continued high efficacy with LNG 52-mg IUS use through 7 years. This information affords women extended contraceptive coverage. While additional research will be welcome, particularly in younger women who will maintain greater fertility across the IUS’s 7-year life span, we are confident in extending the 7-year duration for this IUS to our patients.
Additionally, the method discontinuation findings in this study highlight the importance of discussing the expected menstrual changes of hormonal IUS use with women prior to insertion so they can determine if the potential changes would be satisfactory. As the acceptability of medical menstrual suppression may be new to many women, providers should frame the adverse effects in this context. Providers can use this opportunity to review the noncontraceptive benefits of the hormonal IUS as well.
Novel combination of LNG 52-mg IUS and oral LNG 1.5 mg is promising for emergency contraception
Turok DK, Sanders JN, Thompson IS, Royer PA, Eggebroten J, Gawron LM. Preference for and efficacy of oral levonorgestrel for emergency contraception with concomitant placement of a levonorgestrel IUD: a prospective cohort study. Contraception. 2016;93(6):526−532.
The copper IUD is superior for EC relative to oral agents and has the added benefit of providing ongoing highly effective contraception after placement.8 Despite this strong evidence, the copper IUD remains underutilized for this indication. Turok and colleagues noted that women in their clinic seeking IUDs for non-EC purposes preferred the LNG 52-mg IUS over the copper IUD. It is understandable that women might carry these preferences into EC encounters as well. Thus, the investigators devised a novel combination of LNG 52-mg IUS and oral LNG 1.5 mg, which provided both known EC benefit and same-day access to a more popular contraceptive device.
Details of the study
Women presenting for EC who desired same-day IUD placement were enrolled in the prospective cohort study. Eligible women had a negative urine pregnancy test, known last menstrual period (LMP), regular menstrual cycle, and reported unprotected intercourse within 120 hours prior to presentation. Importantly, women with multiple episodes of unprotected intercourse in the weeks prior to presentation were also included to provide a population more comparable to that encountered clinically. The women were then offered the choice of a TCu380A copper IUD or oral LNG EC with LNG 52-mg IUS placement. They were counseled on the potential increased risk of pregnancy with the novel oral LNG EC plus LNG IUS combination compared with the copper IUD. Participants were given a home pregnancy test that they were to complete in 2 weeks and then report the results to the clinic.
Of the 1,004 women presenting to the clinic for EC over the 16-month study period, 188 (18%) desired same-day IUD insertion. Of these, more opted for the oral LNG EC plus LNG IUS combination (n = 121, 64%) than the copper IUD (n = 67, 36%), demonstrating that women were often willing to accept a possible decrease in EC efficacy with the goal of obtaining their preferred lUD type.
Excluding failed insertion, undiagnosed uterine didelphys, and patient withdrawal, 110 women received the oral LNG EC plus LNG IUS and 66 received the copper IUD. Demographics were comparable between groups except for body mass index (BMI). Of note, more than half (61%) of the women who opted for the oral LNG EC plus LNG IUS combination were overweight or obese.
Both EC methods are effective, broadening options
All women who received the copper IUD followed up at 2 weeks, and no pregnancies were reported. Of the women who received oral LNG EC plus the LNG IUS, 107 (97%) had follow-up at 2 weeks. In this group, there was 1 reported ectopic pregnancy that ultimately required surgical management. However, further review of the patient's coital history suggested that conception occurred prior to IUD insertion, and the case was not classified by the investigators as an EC failure.
Although this study was not powered to detect pregnancy rate differences between the traditional copper IUD and the oral LNG EC plus LNG IUS combination, the results are promising. An important strength of this study is the presence of 2 high-risk groups for EC failure in the oral LNG EC plus LNG IUS group (elevated BMI and multiple episodes of unprotected intercourse).
What this evidence means for practiceEncounters for EC are important opportunities in which to discuss a woman’s reproductive goals. For women who are interested in an IUD, this study opens up the option of same-day placement of the LNG 52-mg IUS. While larger trials need to be conducted to obtain more information regarding pregnancy rates with an oral EC plus LNG IUS combination versus the TCu380A, offering such a combination is reasonable. Given that ulipristal acetate (UPA) is a more effective EC product, especially for women who are overweight or obese,9 offering a UPA EC plus LNG IUS combination may be a better alternative.
It is time to remove the STI screening barrier to same-day IUD insertion
Turok DK, Eisenberg DL, Teal SB, Keder LM, Creinin MD. A prospective assessment of pelvic infection risk following same-day sexually transmitted infection testing and levonorgestrel intrauterine system placement [published online ahead of print May 12, 2016]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2016.05017.
Provider concerns regarding the presence of pelvic infection remain a significant barrier to same-day IUD insertion. Older studies suggested a higher risk of pelvic infection in the first 20 days after IUD placement and extrapolated that pelvic infection was related to inserting an IUD in a woman at risk for STI.10 As a result, patients may be restricted from same-day IUD insertion by providers who think that obtaining results of STI testing is required prior to placement. Recently, a systematic review suggested, based on limited evidence, that IUD placement does not increase the risk of pelvic infection in asymptomatic women compared with those without an IUD.11
Turok and colleagues (including M.D.C., coauthor of this article) reported results from a planned secondary analysis of A Comprehensive Contraceptive Efficacy and Safety Study of an IUS (ACCESS IUS), a component of the regulatory approval of the Liletta LNG 52-mg IUS. This analysis represents the first large-scale prospective investigation of pelvic infection rates during the first 2 years after IUD placement in US women.
Details of the study
Of the 1,751 women enrolled in the study, 1,714 had successful IUS insertions. Infection was assessed via baseline pelvic visual and bimanual examination and Chlamydia testing in all women. Gonorrhea testing was also performed in women who had not been tested with their current sexual partner. STI test results were not required for IUS insertion. Participants were assessed in person at 1, 3, 6, 12, and 24 months after insertion. Additional pelvic exams were performed as needed based on reported symptoms. At 6 months, 1 year, and 2 years, IUS continuation was reported in 1,553 (90.6%), 1,401 (81.7%), and 1,157 (67.3%) women, respectively.
Nearly all women received baseline STI testing (98.4%); however, results were not available prior to same-day IUS insertion for 79.6% of participants. Twenty-nine (1.7%) women had positive baseline STI tests (25 for Chlamydia, 3 for gonorrhea, 1 for both). Of these, only 6 women had results available prior to IUS placement. All women with a positive STI test were treated, and the IUS was left in place. Importantly, none of these women developed pelvic infection in the subsequent 2 years of follow-up and none requested IUS removal.
Infection risk is low with IUD placement
Among women with negative baseline STI tests, there were only 9 (0.5%) clinical diagnoses of pelvic infections over the first 2 years of follow-up. Diagnosis was typically made based on physical examination findings. Most women underwent repeat Chlamydia and gonorrhea testing at the time of pelvic infection diagnosis, and none had positive results. There were no medically recommended IUS removals; 2 women with pelvic infection requested IUS removal per their preference.
Three of the 9 women with pelvic infections were diagnosed within 1 week of IUS placement, 1 at 39 days after placement, and the remaining 5 more than 6 months after placement, suggesting that pelvic infection is not temporally related to IUS placement. Most women were successfully treated as outpatients.
What this evidence means for practiceThis study provides further reassurance regarding the low risk of pelvic infection among women with an LNG IUS. Insertion of an IUS should not be delayed to await results of Chlamydia or gonorrhea testing in a woman without clinical evidence of pelvic infection. Risk-based, as opposed to universal testing, is imperative.12 These recommendations are in agreement with current recommendations of the Centers for Disease Control and Prevention and the American College of Obstetricians and Gynecologists.13,14 Practices that employ 2-visit protocols unnecessarily limit women’s access to the IUS, as research has shown that nearly half of women desiring an IUD do not return for device placement if a second encounter is required.15
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Office of Population Affairs, Department of Health and Human Services. Family planning program, FY 1999 service program grants by state. Bethesda, MD: Office of Population Affairs; 1999.
- Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among US women, 2009-2012. Obstet Gynecol. 2015;126(5):917–927.
- Ventura SJ, Abma JC, Mosher WD, Henshaw S. Revised pregnancy rates, 1990-97, and new rates for 1998-99: United States. Natl Vital Stat Rep. 2003;52(7):1−14.
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001-2008. Am J Public Health. 2014;104(suppl 1):S43–S48.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843–852.
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD; ACCESS IUS Investigators. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
- United Nations Development Programme, United Nations Population Fund, World Health Organization, World Bank, Special Programme of Research, Development, and Research Training in Human Reproduction. Long-term reversible contraception: twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–352.
- Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27(7):1994–2000.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339(8796):785–788.
- Jatlaoui TC, Simmons KB, Curtis KM. The safety of intrauterine contraception initiation among women with current asymptomatic cervical infections or at increased risk of sexually transmitted infections [published online ahead of print June 1, 2016]. Contraception. doi:10.1016/j.contracep tion.2016.05.013.
- Grentzer JM, Peipert JF, Zhao Q, McNicholas C, Secura GM, Madden T. Risk-based screening for Chlamydia trachomatis and Neisseria gonorrhoeae prior to intrauterine device insertion. Contraception. 2015;92(4):313–318.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
- Centers for Disease Control and Prevention. US selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd ed. MMWR Recomm Rep. 2013;62(RR05):1–46.
- Bergin A, Tristan S, Terplan M, Gilliam ML, Whitaker AK. A missed opportunity for care: two-visit IUD insertion protocols inhibit placement. Contraception. 2012;86(6):694–697.
- Office of Population Affairs, Department of Health and Human Services. Family planning program, FY 1999 service program grants by state. Bethesda, MD: Office of Population Affairs; 1999.
- Kavanaugh ML, Jerman J, Finer LB. Changes in use of long-acting reversible contraceptive methods among US women, 2009-2012. Obstet Gynecol. 2015;126(5):917–927.
- Ventura SJ, Abma JC, Mosher WD, Henshaw S. Revised pregnancy rates, 1990-97, and new rates for 1998-99: United States. Natl Vital Stat Rep. 2003;52(7):1−14.
- Finer LB, Zolna MR. Shifts in intended and unintended pregnancies in the United States, 2001-2008. Am J Public Health. 2014;104(suppl 1):S43–S48.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843–852.
- Eisenberg DL, Schreiber CA, Turok DK, Teal SB, Westhoff CL, Creinin MD; ACCESS IUS Investigators. Three-year efficacy and safety of a new 52-mg levonorgestrel-releasing intrauterine system. Contraception. 2015;92(1):10–16.
- United Nations Development Programme, United Nations Population Fund, World Health Organization, World Bank, Special Programme of Research, Development, and Research Training in Human Reproduction. Long-term reversible contraception: twelve years of experience with the TCu380A and TCu220C. Contraception. 1997;56(6):341–352.
- Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27(7):1994–2000.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339(8796):785–788.
- Jatlaoui TC, Simmons KB, Curtis KM. The safety of intrauterine contraception initiation among women with current asymptomatic cervical infections or at increased risk of sexually transmitted infections [published online ahead of print June 1, 2016]. Contraception. doi:10.1016/j.contracep tion.2016.05.013.
- Grentzer JM, Peipert JF, Zhao Q, McNicholas C, Secura GM, Madden T. Risk-based screening for Chlamydia trachomatis and Neisseria gonorrhoeae prior to intrauterine device insertion. Contraception. 2015;92(4):313–318.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 121: Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2011;118(1):184–196.
- Centers for Disease Control and Prevention. US selected practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd ed. MMWR Recomm Rep. 2013;62(RR05):1–46.
- Bergin A, Tristan S, Terplan M, Gilliam ML, Whitaker AK. A missed opportunity for care: two-visit IUD insertion protocols inhibit placement. Contraception. 2012;86(6):694–697.
In this article
- Extended use of LNG IUS
- Oral LNG and LNG IUS combo for emergency contraception
- STI screening and same-day IUD placement
STOP using instruments to assist with delivery of the head at cesarean
Rates of cesarean delivery in the second stage of labor have increased dramatically over the past few years.1 Compared with cesarean delivery prior to labor, second-stage labor cesarean is associated with a higher risk to both the mother and the fetus; risks include excessive bleeding, lower uterine segment extensions, injuries to the maternal ureters or bladder, and injury to the fetus.2−4 The risk is increased even further if the fetal head is deeply impacted in the pelvis. What can we do to avoid and manage such situations?
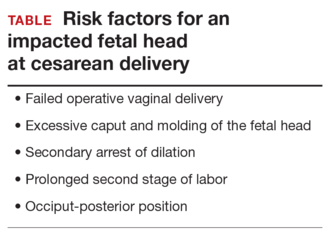
Anticipate an impacted fetal headThe true incidence of an impacted fetal head at the time of cesarean is not known, although a number of risk factors have been described (TABLE). Obstetric care providers should be aware of these risk factors and anticipate the likelihood of a difficult delivery of the fetal head at cesarean.
Options for managing an impacted fetal head at cesareanSeveral techniques have been reported in the literature for managing the delivery of a deeply engaged head, including:
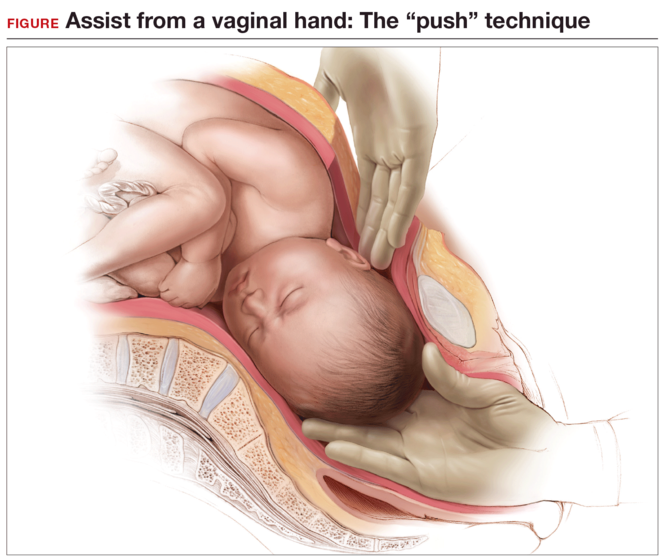
Using an assistant to push the fetus’s head up using a hand in the vagina (“push” technique). This can cause trauma to the fetus, since the force required to push the fetus up from below is uncontrolled.5,6
The reverse breech extraction (“pull” technique) involves pulling the infant out feet first through the uterine incision.7
Use of an instrument. The most common instrument used is a vacuum extractor,8 although a number of other devices have been developed, including the Murless fetal head extractor (an instrument with a hinged shaft and sliding collar lock),9 the C-Snorkel impacted fetal head release device (the device’s tip contains ventilation ports to facilitate airflow and release of the vacuum/suction created by the impacted fetal head),10 and the Fetal Pillow (a balloon device inserted in the vagina and inflated with sterile saline to disimpact an engaged fetal head before cesarean delivery).11
While all of these techniques can cause injury to the mother and the fetus, available data favor use of the reverse breech extraction (pull) technique, since it is associated with fewer maternal risks, including lower rates of uterine incision extension, infection, and postpartum hemorrhage and a shorter operative time.12−18
Stop use of vacuum to deliver the fetal head at cesarean
Placement of a vacuum can be effective in assisting with delivery of the fetal head at cesarean. For this reason, vacuum-assisted deliveries at cesarean are becoming more common. While the rate of complications caused by vacuum extraction of the fetal head at cesarean is not known, injuries have been reported.19,20 As such, routine use of vacuum extraction at the time of cesarean delivery cannot be recommended.
Start disengaging the fetal head prior to cesarean
One useful technique in planning a cesarean in the second stage of labor or when an impacted fetal head is anticipated is to disengage the fetal head vaginally prior to skin incision. This can be done in the delivery room or in the operating room immediately prior to surgery with the help of an assistant.
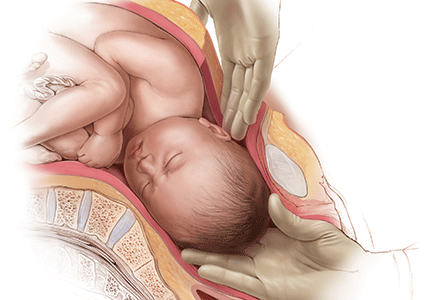
While supporting the patient’s legs, the assistant inserts a hand into the vagina and pushes upward on the fetal head with gentle, sustained effort. The assistant should use a cupped hand or the palm of the hand while attempting to both elevate and flex the fetal head. It is best to avoid using 1 or 2 fingers to elevate the head, as this may cause excessive pressure at a single point and lead to injury, such as a skull fracture (FIGURE). The assistant should disengage his or her hand only when the operating surgeon is able to reach down and secure the fetal head from above.
Elevating the fetal head prior to skin incision offers 3 major advantages:
- It avoids the embarrassing situation of having the fetus deliver vaginally before it can be pulled out through the abdominal incision. Although rare, this has been known to happen, because the dense regional anesthesia further relaxes the pelvic floor musculature, leading to flexion and rotation of the fetal head, which then descends and delivers. Performing a final bimanual examination in the operating room after the establishment of surgical level anesthesia and immediately prior to skin incision will avoid this situation.
- It elevates the fetal head, thereby creating additional space between the bony pelvis and fetal presenting part for the provider’s hand to fit. This helps minimize injury to the fetus and to the maternal soft tissues at the time of cesarean.
- Lastly, it provides additional information about the extent to which the fetal head is impacted in the pelvis and may influence decision making around the time of cesarean. For example, if the fetal head were deeply impacted in the pelvis and could not be disimpacted vaginally, the surgeon may choose to make a different uterine incision (such as a low vertical hysterotomy), administer a uterine relaxant (an inhaled anesthetic agent or nitric oxide), ask for additional instrumentation, and/or ask an assistant to be ready to elevate the fetal head vaginally should this be necessary.21
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Spencer C, Murphy D, Bewley S. Caesarean delivery in the second stage of labour. BMJ. 2006;333(7569):613–614.
- Häger RM, Daltviet AK, Hofoss D, et al. Complications of cesarean deliveries: rates and risk factors. Am J Obstet Gynecol. 2004;190(2):428–434.
- Murphy DJ, Liebling RE, Verity L, Swingler R, Patel R. Early maternal and neonatal morbidity associated with operative delivery in second stage of labour: a cohort study. Lancet. 2001;358(9289):1203–1207.
- Pergialiotis V, Vlachos DG, Rodolakis A, Haidopoulos D, Thomakos N, Vlachos GD. First versus second stage C/S maternal and neonatal morbidity: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;175:15–24.
- Lippert TH. Bimanual delivery of the fetal head at cesarean section with the fetal head in the midcavity. Arch Gynecol. 1983;234(1):59–60.
- Landesman R, Graber EA. Abdominovaginal delivery: modification of the cesarean section operation to facilitate delivery of the impacted head. Am J Obstet Gynecol. 1984;148(6):707–710.
- Fong YF, Arulkumaran S. Breech extraction—an alternative method of delivering a deeply engaged head at cesarean section. Int J Gynaecol Obstet. 1997;56(2):183–184.
- Arad I, Linder N, Bercovici B. Vacuum extraction at cesarean section—neonatal outcome. J Perinat Med. 1986;14(2):137–140.
- Murless BC. Lower-segment caesarean section; a new head extractor. BMJ. 1948;1(4564):1234.
- C-Snorkle impacted fetal head release device. Clinical Innovations website. http://clinicalinnovations.com /portfolio-items/c-snorkel/. Accessed July 22, 2016.
- Seal SL, Dey A, Barman SC, Kamilya G, Mukherji J, Onwude JL. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet. 2016;133(2):178–182.
- Fasubaa OB, Ezechi OC, Orji EO, et al. Delivery of the impacted head of the fetus at caesarean section after prolonged obstructed labour: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22(4):375–378.
- Levy R, Chernomoretz T, Appelman Z, Levin D, Or Y, Hagay ZJ. Head pushing versus reverse breech extraction in cases of impacted fetal head during Cesarean section. Eur J Obstet Gynecol Reprod Biol. 2005;121(1):24–26.
- Chopra S, Bagga R, Keepanasseril A, Jain V, Kalra J, Suri V. Disengagement of the deeply engaged fetal head during cesarean section in advanced labor: conventional method versus reverse breech extraction. Acta Obstet Gynecol Scand. 2009;88(10):1163–1166.
- Veisi F, Zangeneh M, Malekkhosravi S, Rezavand N. Comparison of “push” and “pull” methods for impacted fetal head extraction during cesarean delivery. Int J Gynaecol Obstet. 2012;118(1):4–6.
- Bastani P, Pourabolghasem S, Abbasalizadeh F, Motvalli L. ComparisonColor/Black of neonatal and maternal outcomes associated with head-pushing and head-pulling methods for impacted fetal head extraction during cesarean delivery. Int J Gynaecol Obstet. 2012;118(1):1–3.
- Waterfall H, Grivell RM, Dodd JM. Techniques for assisting difficult delivery at caesarean section. Cochrane Database Syst Rev. 2016;1:CD004944.
- Jeve YB, Navti OB, Konje JC. Comparison of techniques used to deliver a deeply impacted fetal head at full dilation: a systematic review and meta-analysis. BJOG. 2016;123(3): 337–345.
- Clark SL, Vines VL, Belfort MA. Fetal injury associated with routine vacuum use during cesarean delivery. Am J Obstet Gynecol. 2008;198(4):e4.
- Fareeduddin R, Schifrin BS. Subgaleal hemorrhage after the use of a vacuum extractor during elective cesarean delivery: a case report. J Reprod Med. 2008;53(10):809–810.
- Barbieri RL. Difficult fetal extraction at cesarean delivery: What should you do? OBG Manag. 2012;24(1):8–12.
Rates of cesarean delivery in the second stage of labor have increased dramatically over the past few years.1 Compared with cesarean delivery prior to labor, second-stage labor cesarean is associated with a higher risk to both the mother and the fetus; risks include excessive bleeding, lower uterine segment extensions, injuries to the maternal ureters or bladder, and injury to the fetus.2−4 The risk is increased even further if the fetal head is deeply impacted in the pelvis. What can we do to avoid and manage such situations?
Anticipate an impacted fetal headThe true incidence of an impacted fetal head at the time of cesarean is not known, although a number of risk factors have been described (TABLE). Obstetric care providers should be aware of these risk factors and anticipate the likelihood of a difficult delivery of the fetal head at cesarean.

Options for managing an impacted fetal head at cesareanSeveral techniques have been reported in the literature for managing the delivery of a deeply engaged head, including:
Using an assistant to push the fetus’s head up using a hand in the vagina (“push” technique). This can cause trauma to the fetus, since the force required to push the fetus up from below is uncontrolled.5,6
The reverse breech extraction (“pull” technique) involves pulling the infant out feet first through the uterine incision.7
Use of an instrument. The most common instrument used is a vacuum extractor,8 although a number of other devices have been developed, including the Murless fetal head extractor (an instrument with a hinged shaft and sliding collar lock),9 the C-Snorkel impacted fetal head release device (the device’s tip contains ventilation ports to facilitate airflow and release of the vacuum/suction created by the impacted fetal head),10 and the Fetal Pillow (a balloon device inserted in the vagina and inflated with sterile saline to disimpact an engaged fetal head before cesarean delivery).11
While all of these techniques can cause injury to the mother and the fetus, available data favor use of the reverse breech extraction (pull) technique, since it is associated with fewer maternal risks, including lower rates of uterine incision extension, infection, and postpartum hemorrhage and a shorter operative time.12−18
Stop use of vacuum to deliver the fetal head at cesarean
Placement of a vacuum can be effective in assisting with delivery of the fetal head at cesarean. For this reason, vacuum-assisted deliveries at cesarean are becoming more common. While the rate of complications caused by vacuum extraction of the fetal head at cesarean is not known, injuries have been reported.19,20 As such, routine use of vacuum extraction at the time of cesarean delivery cannot be recommended.
Start disengaging the fetal head prior to cesarean
One useful technique in planning a cesarean in the second stage of labor or when an impacted fetal head is anticipated is to disengage the fetal head vaginally prior to skin incision. This can be done in the delivery room or in the operating room immediately prior to surgery with the help of an assistant.
While supporting the patient’s legs, the assistant inserts a hand into the vagina and pushes upward on the fetal head with gentle, sustained effort. The assistant should use a cupped hand or the palm of the hand while attempting to both elevate and flex the fetal head. It is best to avoid using 1 or 2 fingers to elevate the head, as this may cause excessive pressure at a single point and lead to injury, such as a skull fracture (FIGURE). The assistant should disengage his or her hand only when the operating surgeon is able to reach down and secure the fetal head from above.

Elevating the fetal head prior to skin incision offers 3 major advantages:
- It avoids the embarrassing situation of having the fetus deliver vaginally before it can be pulled out through the abdominal incision. Although rare, this has been known to happen, because the dense regional anesthesia further relaxes the pelvic floor musculature, leading to flexion and rotation of the fetal head, which then descends and delivers. Performing a final bimanual examination in the operating room after the establishment of surgical level anesthesia and immediately prior to skin incision will avoid this situation.
- It elevates the fetal head, thereby creating additional space between the bony pelvis and fetal presenting part for the provider’s hand to fit. This helps minimize injury to the fetus and to the maternal soft tissues at the time of cesarean.
- Lastly, it provides additional information about the extent to which the fetal head is impacted in the pelvis and may influence decision making around the time of cesarean. For example, if the fetal head were deeply impacted in the pelvis and could not be disimpacted vaginally, the surgeon may choose to make a different uterine incision (such as a low vertical hysterotomy), administer a uterine relaxant (an inhaled anesthetic agent or nitric oxide), ask for additional instrumentation, and/or ask an assistant to be ready to elevate the fetal head vaginally should this be necessary.21
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Rates of cesarean delivery in the second stage of labor have increased dramatically over the past few years.1 Compared with cesarean delivery prior to labor, second-stage labor cesarean is associated with a higher risk to both the mother and the fetus; risks include excessive bleeding, lower uterine segment extensions, injuries to the maternal ureters or bladder, and injury to the fetus.2−4 The risk is increased even further if the fetal head is deeply impacted in the pelvis. What can we do to avoid and manage such situations?
Anticipate an impacted fetal headThe true incidence of an impacted fetal head at the time of cesarean is not known, although a number of risk factors have been described (TABLE). Obstetric care providers should be aware of these risk factors and anticipate the likelihood of a difficult delivery of the fetal head at cesarean.

Options for managing an impacted fetal head at cesareanSeveral techniques have been reported in the literature for managing the delivery of a deeply engaged head, including:
Using an assistant to push the fetus’s head up using a hand in the vagina (“push” technique). This can cause trauma to the fetus, since the force required to push the fetus up from below is uncontrolled.5,6
The reverse breech extraction (“pull” technique) involves pulling the infant out feet first through the uterine incision.7
Use of an instrument. The most common instrument used is a vacuum extractor,8 although a number of other devices have been developed, including the Murless fetal head extractor (an instrument with a hinged shaft and sliding collar lock),9 the C-Snorkel impacted fetal head release device (the device’s tip contains ventilation ports to facilitate airflow and release of the vacuum/suction created by the impacted fetal head),10 and the Fetal Pillow (a balloon device inserted in the vagina and inflated with sterile saline to disimpact an engaged fetal head before cesarean delivery).11
While all of these techniques can cause injury to the mother and the fetus, available data favor use of the reverse breech extraction (pull) technique, since it is associated with fewer maternal risks, including lower rates of uterine incision extension, infection, and postpartum hemorrhage and a shorter operative time.12−18
Stop use of vacuum to deliver the fetal head at cesarean
Placement of a vacuum can be effective in assisting with delivery of the fetal head at cesarean. For this reason, vacuum-assisted deliveries at cesarean are becoming more common. While the rate of complications caused by vacuum extraction of the fetal head at cesarean is not known, injuries have been reported.19,20 As such, routine use of vacuum extraction at the time of cesarean delivery cannot be recommended.
Start disengaging the fetal head prior to cesarean
One useful technique in planning a cesarean in the second stage of labor or when an impacted fetal head is anticipated is to disengage the fetal head vaginally prior to skin incision. This can be done in the delivery room or in the operating room immediately prior to surgery with the help of an assistant.
While supporting the patient’s legs, the assistant inserts a hand into the vagina and pushes upward on the fetal head with gentle, sustained effort. The assistant should use a cupped hand or the palm of the hand while attempting to both elevate and flex the fetal head. It is best to avoid using 1 or 2 fingers to elevate the head, as this may cause excessive pressure at a single point and lead to injury, such as a skull fracture (FIGURE). The assistant should disengage his or her hand only when the operating surgeon is able to reach down and secure the fetal head from above.

Elevating the fetal head prior to skin incision offers 3 major advantages:
- It avoids the embarrassing situation of having the fetus deliver vaginally before it can be pulled out through the abdominal incision. Although rare, this has been known to happen, because the dense regional anesthesia further relaxes the pelvic floor musculature, leading to flexion and rotation of the fetal head, which then descends and delivers. Performing a final bimanual examination in the operating room after the establishment of surgical level anesthesia and immediately prior to skin incision will avoid this situation.
- It elevates the fetal head, thereby creating additional space between the bony pelvis and fetal presenting part for the provider’s hand to fit. This helps minimize injury to the fetus and to the maternal soft tissues at the time of cesarean.
- Lastly, it provides additional information about the extent to which the fetal head is impacted in the pelvis and may influence decision making around the time of cesarean. For example, if the fetal head were deeply impacted in the pelvis and could not be disimpacted vaginally, the surgeon may choose to make a different uterine incision (such as a low vertical hysterotomy), administer a uterine relaxant (an inhaled anesthetic agent or nitric oxide), ask for additional instrumentation, and/or ask an assistant to be ready to elevate the fetal head vaginally should this be necessary.21
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Spencer C, Murphy D, Bewley S. Caesarean delivery in the second stage of labour. BMJ. 2006;333(7569):613–614.
- Häger RM, Daltviet AK, Hofoss D, et al. Complications of cesarean deliveries: rates and risk factors. Am J Obstet Gynecol. 2004;190(2):428–434.
- Murphy DJ, Liebling RE, Verity L, Swingler R, Patel R. Early maternal and neonatal morbidity associated with operative delivery in second stage of labour: a cohort study. Lancet. 2001;358(9289):1203–1207.
- Pergialiotis V, Vlachos DG, Rodolakis A, Haidopoulos D, Thomakos N, Vlachos GD. First versus second stage C/S maternal and neonatal morbidity: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;175:15–24.
- Lippert TH. Bimanual delivery of the fetal head at cesarean section with the fetal head in the midcavity. Arch Gynecol. 1983;234(1):59–60.
- Landesman R, Graber EA. Abdominovaginal delivery: modification of the cesarean section operation to facilitate delivery of the impacted head. Am J Obstet Gynecol. 1984;148(6):707–710.
- Fong YF, Arulkumaran S. Breech extraction—an alternative method of delivering a deeply engaged head at cesarean section. Int J Gynaecol Obstet. 1997;56(2):183–184.
- Arad I, Linder N, Bercovici B. Vacuum extraction at cesarean section—neonatal outcome. J Perinat Med. 1986;14(2):137–140.
- Murless BC. Lower-segment caesarean section; a new head extractor. BMJ. 1948;1(4564):1234.
- C-Snorkle impacted fetal head release device. Clinical Innovations website. http://clinicalinnovations.com /portfolio-items/c-snorkel/. Accessed July 22, 2016.
- Seal SL, Dey A, Barman SC, Kamilya G, Mukherji J, Onwude JL. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet. 2016;133(2):178–182.
- Fasubaa OB, Ezechi OC, Orji EO, et al. Delivery of the impacted head of the fetus at caesarean section after prolonged obstructed labour: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22(4):375–378.
- Levy R, Chernomoretz T, Appelman Z, Levin D, Or Y, Hagay ZJ. Head pushing versus reverse breech extraction in cases of impacted fetal head during Cesarean section. Eur J Obstet Gynecol Reprod Biol. 2005;121(1):24–26.
- Chopra S, Bagga R, Keepanasseril A, Jain V, Kalra J, Suri V. Disengagement of the deeply engaged fetal head during cesarean section in advanced labor: conventional method versus reverse breech extraction. Acta Obstet Gynecol Scand. 2009;88(10):1163–1166.
- Veisi F, Zangeneh M, Malekkhosravi S, Rezavand N. Comparison of “push” and “pull” methods for impacted fetal head extraction during cesarean delivery. Int J Gynaecol Obstet. 2012;118(1):4–6.
- Bastani P, Pourabolghasem S, Abbasalizadeh F, Motvalli L. ComparisonColor/Black of neonatal and maternal outcomes associated with head-pushing and head-pulling methods for impacted fetal head extraction during cesarean delivery. Int J Gynaecol Obstet. 2012;118(1):1–3.
- Waterfall H, Grivell RM, Dodd JM. Techniques for assisting difficult delivery at caesarean section. Cochrane Database Syst Rev. 2016;1:CD004944.
- Jeve YB, Navti OB, Konje JC. Comparison of techniques used to deliver a deeply impacted fetal head at full dilation: a systematic review and meta-analysis. BJOG. 2016;123(3): 337–345.
- Clark SL, Vines VL, Belfort MA. Fetal injury associated with routine vacuum use during cesarean delivery. Am J Obstet Gynecol. 2008;198(4):e4.
- Fareeduddin R, Schifrin BS. Subgaleal hemorrhage after the use of a vacuum extractor during elective cesarean delivery: a case report. J Reprod Med. 2008;53(10):809–810.
- Barbieri RL. Difficult fetal extraction at cesarean delivery: What should you do? OBG Manag. 2012;24(1):8–12.
- Spencer C, Murphy D, Bewley S. Caesarean delivery in the second stage of labour. BMJ. 2006;333(7569):613–614.
- Häger RM, Daltviet AK, Hofoss D, et al. Complications of cesarean deliveries: rates and risk factors. Am J Obstet Gynecol. 2004;190(2):428–434.
- Murphy DJ, Liebling RE, Verity L, Swingler R, Patel R. Early maternal and neonatal morbidity associated with operative delivery in second stage of labour: a cohort study. Lancet. 2001;358(9289):1203–1207.
- Pergialiotis V, Vlachos DG, Rodolakis A, Haidopoulos D, Thomakos N, Vlachos GD. First versus second stage C/S maternal and neonatal morbidity: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;175:15–24.
- Lippert TH. Bimanual delivery of the fetal head at cesarean section with the fetal head in the midcavity. Arch Gynecol. 1983;234(1):59–60.
- Landesman R, Graber EA. Abdominovaginal delivery: modification of the cesarean section operation to facilitate delivery of the impacted head. Am J Obstet Gynecol. 1984;148(6):707–710.
- Fong YF, Arulkumaran S. Breech extraction—an alternative method of delivering a deeply engaged head at cesarean section. Int J Gynaecol Obstet. 1997;56(2):183–184.
- Arad I, Linder N, Bercovici B. Vacuum extraction at cesarean section—neonatal outcome. J Perinat Med. 1986;14(2):137–140.
- Murless BC. Lower-segment caesarean section; a new head extractor. BMJ. 1948;1(4564):1234.
- C-Snorkle impacted fetal head release device. Clinical Innovations website. http://clinicalinnovations.com /portfolio-items/c-snorkel/. Accessed July 22, 2016.
- Seal SL, Dey A, Barman SC, Kamilya G, Mukherji J, Onwude JL. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet. 2016;133(2):178–182.
- Fasubaa OB, Ezechi OC, Orji EO, et al. Delivery of the impacted head of the fetus at caesarean section after prolonged obstructed labour: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22(4):375–378.
- Levy R, Chernomoretz T, Appelman Z, Levin D, Or Y, Hagay ZJ. Head pushing versus reverse breech extraction in cases of impacted fetal head during Cesarean section. Eur J Obstet Gynecol Reprod Biol. 2005;121(1):24–26.
- Chopra S, Bagga R, Keepanasseril A, Jain V, Kalra J, Suri V. Disengagement of the deeply engaged fetal head during cesarean section in advanced labor: conventional method versus reverse breech extraction. Acta Obstet Gynecol Scand. 2009;88(10):1163–1166.
- Veisi F, Zangeneh M, Malekkhosravi S, Rezavand N. Comparison of “push” and “pull” methods for impacted fetal head extraction during cesarean delivery. Int J Gynaecol Obstet. 2012;118(1):4–6.
- Bastani P, Pourabolghasem S, Abbasalizadeh F, Motvalli L. ComparisonColor/Black of neonatal and maternal outcomes associated with head-pushing and head-pulling methods for impacted fetal head extraction during cesarean delivery. Int J Gynaecol Obstet. 2012;118(1):1–3.
- Waterfall H, Grivell RM, Dodd JM. Techniques for assisting difficult delivery at caesarean section. Cochrane Database Syst Rev. 2016;1:CD004944.
- Jeve YB, Navti OB, Konje JC. Comparison of techniques used to deliver a deeply impacted fetal head at full dilation: a systematic review and meta-analysis. BJOG. 2016;123(3): 337–345.
- Clark SL, Vines VL, Belfort MA. Fetal injury associated with routine vacuum use during cesarean delivery. Am J Obstet Gynecol. 2008;198(4):e4.
- Fareeduddin R, Schifrin BS. Subgaleal hemorrhage after the use of a vacuum extractor during elective cesarean delivery: a case report. J Reprod Med. 2008;53(10):809–810.
- Barbieri RL. Difficult fetal extraction at cesarean delivery: What should you do? OBG Manag. 2012;24(1):8–12.
In this Article
- Risk factors for impacted fetal head
- Advantages to elevating fetal head
Does extending aromatase-inhibitor use from 5 to 10 years benefit menopausal women with hormone-positive breast cancer?
EXPERT COMMENTARY
Since the current treatment choice for hormone-receptor–positive early breast cancer in postmenopausal women is 5 years of aromatase inhibitor (AI) therapy, or AI therapy following initial tamoxifen treatment, could 10 years of an AI be beneficial to cancer recurrence? Goss and colleagues analyzed this question in the MA.17R trial, a North American Breast Cancer Group trial coordinated by the Canadian Cancer Trials Group. (Results of the prior MA.17 trial were published in 2003.1)
The randomized, double-blind, placebo-controlled trial evaluated the effect of 5 years of extended AI (letrozole 2.5 mg) treatment compared with placebo in menopausal women with hormone-receptor–positive breast cancer who had previously received 5 years of hormonal adjuvant therapy with tamoxifen alone or plus AIs. Of note, this study was funded in part by Novartis, the pharmaceutical manufacturer of letrozole, though the company had no role in either study design or writing of the manuscript. Seven of the 20 authors disclosed some sort of relationship with industry (some with the manufacturer of letrozole), including membership on advisory boards, board of directors, steering committees, or data and safety monitoring committees or receiving lecturer or consulting fees or grant support.
The trial’s primary end point was DFS. Secondary end points included overall survival, the incidence of contralateral breast cancer, quality of life (QOL), and long-term safety.
Details of the studyWomen were eligible to participate in the study if they were disease free after having completed 4.5 to 6 years of therapy with any AI and if their primary tumor was hormone-receptor positive. A total of 1,918 women were included in the trial and were randomly assigned to receive either letrozole treatment (n = 959) or placebo (n = 959).
Clinical evaluation was performed annually and included assessments of new bone fracture and new-onset osteoporosis, blood tests, mammography, and assessment of toxic effects. QOL measures were assessed with a validated health survey and a menopause-specific questionnaire. The Common Toxicity Criteria, version 2.0, was used to assess adverse events.
Impact on disease free, overall survivalThe rate of 5-year DFS was statistically improved in the letrozole group compared with the placebo group, 95% (95% confidence interval [CI], 93–96) versus 91% (95% CI, 89–93), respectively, a 4% improvement in DFS. However, there was no impact on disease-specific mortality and no benefit in overall survival (93% [95% CI, 92–95] with letrozole and 94% [95% CI, 92–95] with placebo), as competing causes of death become increasingly important in this older population. Among women who died during the study follow-up, more than half died of causes not related to breast cancer.
QOL measures. More than 85% of participants completed the QOL assessments at each time point. There was no difference in the various QOL measures between the letrozole and the placebo group.
Adverse effects. Expected adverse effects due to AIs were significantly higher in the letrozole group. For example, new-onset osteoporosis occurred in 109 (11%) of letrozole-treated women and in 54 (6%) of the placebo group (P<.001), and bone fracture occurred in 133 (14%) of the letrozole group and 88 (9%) of the placebo group (P = .001).
Of note, however, fewer toxicities/adverse effects were seen in the AI group in this study than in previously published reports. The authors suggested that these adverse effect data may be lower than expected because the majority of women eligible for this study likely had prior exposure to AIs, and those with significant adverse effects with aromatase inhibitor therapy may have self-selected out of this trial.
WHAT THIS EVIDENCE MEANS FOR PRACTICEWhile the study authors selected DFS as the primary outcome, the lack of overall survival, adverse effect profile, and the drug cost (average wholesale price, ~$33,050 for 5 years2) make the choice to routinely continue AIs in menopausal women with hormone-receptor–positive breast cancer less clear, and counseling on both the benefits and limitations of continuing hormonal adjuvant therapy will be important for these women.
Continued follow-up of the study participants over time would be useful to determine if, after 10 to 15 years, the benefit of extending AI therapy for an additional 5 years would provide an overall benefit in longevity, as competing causes of death (bone fracture, cardiovascular risk) actually may increase over time in the extended-treatment group compared with the placebo group.
— Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349(19):1793–1802.
- Average Wholesale Price (AWP) Policy. Truven Health Analytics. Red Book. http://sites.truvenhealth.com/redbook /awp/. Accessed July 18, 2016.
EXPERT COMMENTARY
Since the current treatment choice for hormone-receptor–positive early breast cancer in postmenopausal women is 5 years of aromatase inhibitor (AI) therapy, or AI therapy following initial tamoxifen treatment, could 10 years of an AI be beneficial to cancer recurrence? Goss and colleagues analyzed this question in the MA.17R trial, a North American Breast Cancer Group trial coordinated by the Canadian Cancer Trials Group. (Results of the prior MA.17 trial were published in 2003.1)
The randomized, double-blind, placebo-controlled trial evaluated the effect of 5 years of extended AI (letrozole 2.5 mg) treatment compared with placebo in menopausal women with hormone-receptor–positive breast cancer who had previously received 5 years of hormonal adjuvant therapy with tamoxifen alone or plus AIs. Of note, this study was funded in part by Novartis, the pharmaceutical manufacturer of letrozole, though the company had no role in either study design or writing of the manuscript. Seven of the 20 authors disclosed some sort of relationship with industry (some with the manufacturer of letrozole), including membership on advisory boards, board of directors, steering committees, or data and safety monitoring committees or receiving lecturer or consulting fees or grant support.
The trial’s primary end point was DFS. Secondary end points included overall survival, the incidence of contralateral breast cancer, quality of life (QOL), and long-term safety.
Details of the studyWomen were eligible to participate in the study if they were disease free after having completed 4.5 to 6 years of therapy with any AI and if their primary tumor was hormone-receptor positive. A total of 1,918 women were included in the trial and were randomly assigned to receive either letrozole treatment (n = 959) or placebo (n = 959).
Clinical evaluation was performed annually and included assessments of new bone fracture and new-onset osteoporosis, blood tests, mammography, and assessment of toxic effects. QOL measures were assessed with a validated health survey and a menopause-specific questionnaire. The Common Toxicity Criteria, version 2.0, was used to assess adverse events.
Impact on disease free, overall survivalThe rate of 5-year DFS was statistically improved in the letrozole group compared with the placebo group, 95% (95% confidence interval [CI], 93–96) versus 91% (95% CI, 89–93), respectively, a 4% improvement in DFS. However, there was no impact on disease-specific mortality and no benefit in overall survival (93% [95% CI, 92–95] with letrozole and 94% [95% CI, 92–95] with placebo), as competing causes of death become increasingly important in this older population. Among women who died during the study follow-up, more than half died of causes not related to breast cancer.
QOL measures. More than 85% of participants completed the QOL assessments at each time point. There was no difference in the various QOL measures between the letrozole and the placebo group.
Adverse effects. Expected adverse effects due to AIs were significantly higher in the letrozole group. For example, new-onset osteoporosis occurred in 109 (11%) of letrozole-treated women and in 54 (6%) of the placebo group (P<.001), and bone fracture occurred in 133 (14%) of the letrozole group and 88 (9%) of the placebo group (P = .001).
Of note, however, fewer toxicities/adverse effects were seen in the AI group in this study than in previously published reports. The authors suggested that these adverse effect data may be lower than expected because the majority of women eligible for this study likely had prior exposure to AIs, and those with significant adverse effects with aromatase inhibitor therapy may have self-selected out of this trial.
WHAT THIS EVIDENCE MEANS FOR PRACTICEWhile the study authors selected DFS as the primary outcome, the lack of overall survival, adverse effect profile, and the drug cost (average wholesale price, ~$33,050 for 5 years2) make the choice to routinely continue AIs in menopausal women with hormone-receptor–positive breast cancer less clear, and counseling on both the benefits and limitations of continuing hormonal adjuvant therapy will be important for these women.
Continued follow-up of the study participants over time would be useful to determine if, after 10 to 15 years, the benefit of extending AI therapy for an additional 5 years would provide an overall benefit in longevity, as competing causes of death (bone fracture, cardiovascular risk) actually may increase over time in the extended-treatment group compared with the placebo group.
— Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Since the current treatment choice for hormone-receptor–positive early breast cancer in postmenopausal women is 5 years of aromatase inhibitor (AI) therapy, or AI therapy following initial tamoxifen treatment, could 10 years of an AI be beneficial to cancer recurrence? Goss and colleagues analyzed this question in the MA.17R trial, a North American Breast Cancer Group trial coordinated by the Canadian Cancer Trials Group. (Results of the prior MA.17 trial were published in 2003.1)
The randomized, double-blind, placebo-controlled trial evaluated the effect of 5 years of extended AI (letrozole 2.5 mg) treatment compared with placebo in menopausal women with hormone-receptor–positive breast cancer who had previously received 5 years of hormonal adjuvant therapy with tamoxifen alone or plus AIs. Of note, this study was funded in part by Novartis, the pharmaceutical manufacturer of letrozole, though the company had no role in either study design or writing of the manuscript. Seven of the 20 authors disclosed some sort of relationship with industry (some with the manufacturer of letrozole), including membership on advisory boards, board of directors, steering committees, or data and safety monitoring committees or receiving lecturer or consulting fees or grant support.
The trial’s primary end point was DFS. Secondary end points included overall survival, the incidence of contralateral breast cancer, quality of life (QOL), and long-term safety.
Details of the studyWomen were eligible to participate in the study if they were disease free after having completed 4.5 to 6 years of therapy with any AI and if their primary tumor was hormone-receptor positive. A total of 1,918 women were included in the trial and were randomly assigned to receive either letrozole treatment (n = 959) or placebo (n = 959).
Clinical evaluation was performed annually and included assessments of new bone fracture and new-onset osteoporosis, blood tests, mammography, and assessment of toxic effects. QOL measures were assessed with a validated health survey and a menopause-specific questionnaire. The Common Toxicity Criteria, version 2.0, was used to assess adverse events.
Impact on disease free, overall survivalThe rate of 5-year DFS was statistically improved in the letrozole group compared with the placebo group, 95% (95% confidence interval [CI], 93–96) versus 91% (95% CI, 89–93), respectively, a 4% improvement in DFS. However, there was no impact on disease-specific mortality and no benefit in overall survival (93% [95% CI, 92–95] with letrozole and 94% [95% CI, 92–95] with placebo), as competing causes of death become increasingly important in this older population. Among women who died during the study follow-up, more than half died of causes not related to breast cancer.
QOL measures. More than 85% of participants completed the QOL assessments at each time point. There was no difference in the various QOL measures between the letrozole and the placebo group.
Adverse effects. Expected adverse effects due to AIs were significantly higher in the letrozole group. For example, new-onset osteoporosis occurred in 109 (11%) of letrozole-treated women and in 54 (6%) of the placebo group (P<.001), and bone fracture occurred in 133 (14%) of the letrozole group and 88 (9%) of the placebo group (P = .001).
Of note, however, fewer toxicities/adverse effects were seen in the AI group in this study than in previously published reports. The authors suggested that these adverse effect data may be lower than expected because the majority of women eligible for this study likely had prior exposure to AIs, and those with significant adverse effects with aromatase inhibitor therapy may have self-selected out of this trial.
WHAT THIS EVIDENCE MEANS FOR PRACTICEWhile the study authors selected DFS as the primary outcome, the lack of overall survival, adverse effect profile, and the drug cost (average wholesale price, ~$33,050 for 5 years2) make the choice to routinely continue AIs in menopausal women with hormone-receptor–positive breast cancer less clear, and counseling on both the benefits and limitations of continuing hormonal adjuvant therapy will be important for these women.
Continued follow-up of the study participants over time would be useful to determine if, after 10 to 15 years, the benefit of extending AI therapy for an additional 5 years would provide an overall benefit in longevity, as competing causes of death (bone fracture, cardiovascular risk) actually may increase over time in the extended-treatment group compared with the placebo group.
— Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349(19):1793–1802.
- Average Wholesale Price (AWP) Policy. Truven Health Analytics. Red Book. http://sites.truvenhealth.com/redbook /awp/. Accessed July 18, 2016.
- Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349(19):1793–1802.
- Average Wholesale Price (AWP) Policy. Truven Health Analytics. Red Book. http://sites.truvenhealth.com/redbook /awp/. Accessed July 18, 2016.
Protecting the newborn brain—the final frontier in obstetric and neonatal care
During the past 40 years neonatologists have discovered new treatments to improve pulmonary and cardiovascular care of preterm newborns, resulting in a dramatic reduction in newborn mortality and childhood morbidity. Important advances include glucocorticoid administration to mothers at risk for preterm birth, surfactant and nitric oxide administration to the newborn, kangaroo (or skin-to-skin) care, continuous positive airway pressure, and high-frequency ventilation.1 In 1960, only 5% of 1,000-g newborns survived. In 2000, 95% of 1,000-g newborns survive.1
The successes in pulmonary and cardiovascular care have revealed a new frontier in neonatal care: the prevention of long-term neurologic disability by the early treatment of newborn encephalpathy with therapeutic hypothermia. This novel undertaking is an important one; approximately 1 in 300 newborns are diagnosed with encephalopathy.2
Until recently there were no proven treatments for newborns with encephalopathy. However, therapeutic hypothermia now has been proven to be an effective intervention for the treatment of moderate and severe encephalopathy,3,4 and its use is expanding to include mild cases.
This increased use can lead to more complex situations arising for obstetricians, for when a neonatologist decides to initiate therapeutic hypothermia of a newborn the parents may wonder if the obstetrician’s management of labor and delivery was suboptimal, contributing to their baby’s brain injury.
Therapeutic hypothermia: The basics
First, we need to define therapeutic hypothermia. Both head hypothermia and whole-body hypothermia are effective techniques for the treatment of newborn encephalopathy.3,4 Most centers use whole-body (FIGURE) rather than head, hypothermia because it facilitates access to the head for placement of electroencephalogram (EEG) sensors.
The key principles of therapeutic hypothermia include5,6:
- Initiate hypothermia within 6 hours of birth.
- Cool the newborn to a core temperature of 33.5° to 34.5°C (92.3° to 94.1°F). Some centers focus on achieving consistent core temperatures of 33.5°C (92.3°F).
- Monitor core temperature every 5 to 15 minutes.
- Cool the newborn for 72 hours.
- Obtain head ultrasonography to detect intracranial hemorrhage.
- Initiate continuous or intermittent EEG monitoring.
- Treat seizures with phenobarbital, lorazepam, or phenytoin.
- Obtain blood cultures, a complete blood count, blood gas concentrations, alactate coagulation profile, and liver function tests.
- Sedate the newborn, if necessary.
- Minimize oral feedings during the initial phase of hypothermia.
- Obtain sequential magnetic resonance imaging (MRI) studies to assess brain structure and function.
- For all newborns with suspected encephalopathy, the placenta should be sent to pathology for histologic study.7
The data on therapy effectivenessTwo recent meta-analyses independently reported that therapeutic hypothermia reduced the risk of newborn death and major neurodevelopmental disability.3,4 The Cochrane meta-analysis reported that the therapy reduced the risk of neuromotor delay, developmental delay, cerebral palsy, and abnormal MRI results (TABLE).4 The study authors also reported that therapeutic hypothermia reduced the risk of blindness and deafness, although these effects did not reach statistical significance.4 Therapeutic hypothermia did increase the risk of newborn sinus bradycardia and thrombocytopenia.3,4 Compared with usual care, the therapy increased the average survival rate with a normal neurologic outcome at 18 months from 23% to 40%.3 It should be noted that even with therapeutic hypothermia treatment, many newborns with moderate to severe encephalopathy have long-term neurologic disabilities.
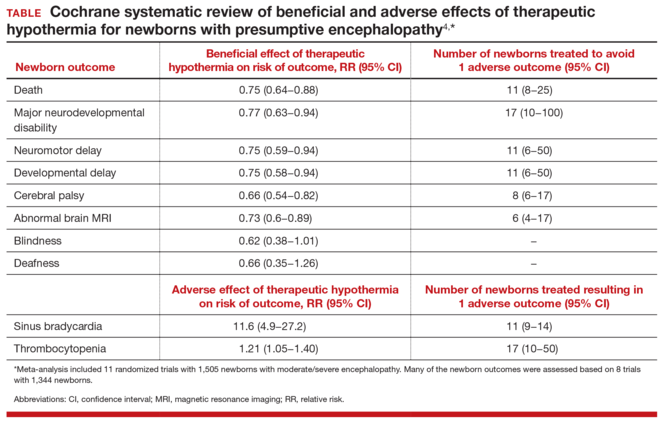
Indications for therapeutic hypothermia are expandingIn the initial clinical trials of therapeutic hypothermia, newborns with moderate to severe encephalopathy were enrolled. Typical inclusion criteria were: gestational age ≥35 or 36 weeks, initiation of therapeutic hypothermia within 6 hours of birth, pH ≤7.0 or base deficit of ≥16 mEq/L, 10-minute Apgar score <5 or ongoing resuscitation for 10 minutes, and moderate to severe encephalopathy on clinical examination.3,4 Typical exclusion criteria were: intrauterine growth restriction with birth weight less than 1,750 g, severe congenital anomalies or severe genetic or metabolic syndromes, major intracranial hemorrhage, sepsis, or persistent coagulopathy.
Given the success of therapeutic hypothermia for moderate to severe newborn encephalopathy, many neonatologists are expanding the indications for treatment. In some centers current indications for initiation of hypothermia include the following:
- gestational age ≥34 weeks
- suspicion of encephalopathy or a seizure event
- any obstetric sentinel event (including a bradycardia, umbilical cord prolapse, uterine rupture, placental abruption, Apgar score ≤5 at 10 minutes, pH ≤7.1 or base deficit of ≥10 mEq/L or Category III tracing, or fetal tachycardia with recurrent decelerations or fetal heart rate with minimal variability and recurrent decelerations).
Suspicion for encephalopathy might be triggered by any of a large number of newborn behaviors: lethargy, decreased activity, hypotonia, weak suck or incomplete Moro reflexes, constricted pupils, bradycardia, periodic breathing or apnea, hyperalertness, or irritability.8
Coordinate neonatology and obstetric communication with the familyGiven the expanding indications for therapeutic hypothermia, an increasing number of newborns will receive this treatment. This scenario makes enhanced communication vital. Consider this situation:
CASE Baby rushed for therapeutic hypothermia upon birthA baby is born limp and blue without a cry. Her hypotonia raises a concern for encephalopathy, and she is whisked off to the neonatal intensive care unit for 72 hours of therapeutic hypothermia. Stunned, the parents begin to wonder, “Will our baby be O.K.?” and “What went wrong?”
When neonatologists recommend therapeutic hypothermia for the newborn with presumptive encephalopathy, they may explain the situation to the parents with words such as brain injury, encephalopathy, hypoxia, and ischemia. Intrapartum events such as a Category II or III fetal heart rate tracing, operative vaginal delivery, or maternal sepsis or abruption might be mentioned as contributing factors. A consulting neurologist may mention injury of the cerebral cortex, subcortical white matter, or lateral thalami. The neonatologists and neurologists might not mention that less than 50% of cases of newborn encephalopathy are thought to be due to the management of labor.2
The obstetrician, as stunned by the events as the parents, may be at a loss about how to communicate effectively with their patient about the newborn’s encephalopathy. Obstetricians can help assure the parents of their continued involvement in the care and reinforce that the hospital’s neonatologists are superb clinicians who will do their best for the baby.
Challenges exist to effective communication. It is often difficult to optimally coordinate and align the communications of the neonatologists, neurologist, nurses, and obstetrician with the family. Communication with the family can be uncoordinated because interactions occur between the family and multiple specialists with unique perspectives and vocabularies. These conversations occur in sequence, separated in time and place. The communication between family and neonatologists typically occurs in the neonatal intensive care unit. Interactions between obstetrician and mother typically occur in the postpartum unit. The neonatologists and obstetricians are assigned to the hospital in rotating coverage shifts, increasing the number of hand-offs and physicians involved in the hospital care of the mother and newborn dyad.
A joint family meeting with the neonatologists, obstetrician, and family early in the course of newborn care might be an optimal approach to coordinating communication with the parents. Conflicting obligations certainly may make a joint meeting difficult to arrange, however.
Reducing the risk of permanent injury to the central and peripheral nervous system of the newborn is the goal of all obstetricians and neonatologists. Many authorities believe that therapeutic hypothermia can reduce the risk of death and major neurodevelopmental disorders in newborns with encephalopathy. Initial data are promising. If long-term follow-up studies prove that this therapy reduces neurologic disability, the treatment represents a major advance in maternal-child care. As we learn more about this novel, and potentially effective therapy, it should be on the minds of those involved with newborn care to involve the ObGyn in coordinated communication with the family and other medical staff.

- Philip AG. The evolution of neonatology. Pediatr Res. 2005;58(4):799−815.
- Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischemic encephalopathy. Early Hum Dev. 2010;86(6):329−338.
- Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(6):558−566.
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Syst Rev. 2013;(1):CD003311.
- Papile LA, Baley JE, Benitz W, et al; American Academy of Pediatrics Committee on Fetus and Newborn. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133(6):1146−1150.
- Azzopardi D, Strohm B, Edwards AD, et al; Steering Group and TOBY Cooling Register participants. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Arch Dis Child Fetal Neonatal Ed. 2009;94(4):F260−F264.
- Mir IN, Johnson-Welch SF, Nelson DB, Brown LS, Rosenfeld CR, Chalak LF. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am J Obstet Gynecol. 2015;213(6):849.e1−e7.
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86(7):757−761.
During the past 40 years neonatologists have discovered new treatments to improve pulmonary and cardiovascular care of preterm newborns, resulting in a dramatic reduction in newborn mortality and childhood morbidity. Important advances include glucocorticoid administration to mothers at risk for preterm birth, surfactant and nitric oxide administration to the newborn, kangaroo (or skin-to-skin) care, continuous positive airway pressure, and high-frequency ventilation.1 In 1960, only 5% of 1,000-g newborns survived. In 2000, 95% of 1,000-g newborns survive.1
The successes in pulmonary and cardiovascular care have revealed a new frontier in neonatal care: the prevention of long-term neurologic disability by the early treatment of newborn encephalpathy with therapeutic hypothermia. This novel undertaking is an important one; approximately 1 in 300 newborns are diagnosed with encephalopathy.2
Until recently there were no proven treatments for newborns with encephalopathy. However, therapeutic hypothermia now has been proven to be an effective intervention for the treatment of moderate and severe encephalopathy,3,4 and its use is expanding to include mild cases.
This increased use can lead to more complex situations arising for obstetricians, for when a neonatologist decides to initiate therapeutic hypothermia of a newborn the parents may wonder if the obstetrician’s management of labor and delivery was suboptimal, contributing to their baby’s brain injury.
Therapeutic hypothermia: The basics
First, we need to define therapeutic hypothermia. Both head hypothermia and whole-body hypothermia are effective techniques for the treatment of newborn encephalopathy.3,4 Most centers use whole-body (FIGURE) rather than head, hypothermia because it facilitates access to the head for placement of electroencephalogram (EEG) sensors.

The key principles of therapeutic hypothermia include5,6:
- Initiate hypothermia within 6 hours of birth.
- Cool the newborn to a core temperature of 33.5° to 34.5°C (92.3° to 94.1°F). Some centers focus on achieving consistent core temperatures of 33.5°C (92.3°F).
- Monitor core temperature every 5 to 15 minutes.
- Cool the newborn for 72 hours.
- Obtain head ultrasonography to detect intracranial hemorrhage.
- Initiate continuous or intermittent EEG monitoring.
- Treat seizures with phenobarbital, lorazepam, or phenytoin.
- Obtain blood cultures, a complete blood count, blood gas concentrations, alactate coagulation profile, and liver function tests.
- Sedate the newborn, if necessary.
- Minimize oral feedings during the initial phase of hypothermia.
- Obtain sequential magnetic resonance imaging (MRI) studies to assess brain structure and function.
- For all newborns with suspected encephalopathy, the placenta should be sent to pathology for histologic study.7
The data on therapy effectivenessTwo recent meta-analyses independently reported that therapeutic hypothermia reduced the risk of newborn death and major neurodevelopmental disability.3,4 The Cochrane meta-analysis reported that the therapy reduced the risk of neuromotor delay, developmental delay, cerebral palsy, and abnormal MRI results (TABLE).4 The study authors also reported that therapeutic hypothermia reduced the risk of blindness and deafness, although these effects did not reach statistical significance.4 Therapeutic hypothermia did increase the risk of newborn sinus bradycardia and thrombocytopenia.3,4 Compared with usual care, the therapy increased the average survival rate with a normal neurologic outcome at 18 months from 23% to 40%.3 It should be noted that even with therapeutic hypothermia treatment, many newborns with moderate to severe encephalopathy have long-term neurologic disabilities.

Indications for therapeutic hypothermia are expandingIn the initial clinical trials of therapeutic hypothermia, newborns with moderate to severe encephalopathy were enrolled. Typical inclusion criteria were: gestational age ≥35 or 36 weeks, initiation of therapeutic hypothermia within 6 hours of birth, pH ≤7.0 or base deficit of ≥16 mEq/L, 10-minute Apgar score <5 or ongoing resuscitation for 10 minutes, and moderate to severe encephalopathy on clinical examination.3,4 Typical exclusion criteria were: intrauterine growth restriction with birth weight less than 1,750 g, severe congenital anomalies or severe genetic or metabolic syndromes, major intracranial hemorrhage, sepsis, or persistent coagulopathy.
Given the success of therapeutic hypothermia for moderate to severe newborn encephalopathy, many neonatologists are expanding the indications for treatment. In some centers current indications for initiation of hypothermia include the following:
- gestational age ≥34 weeks
- suspicion of encephalopathy or a seizure event
- any obstetric sentinel event (including a bradycardia, umbilical cord prolapse, uterine rupture, placental abruption, Apgar score ≤5 at 10 minutes, pH ≤7.1 or base deficit of ≥10 mEq/L or Category III tracing, or fetal tachycardia with recurrent decelerations or fetal heart rate with minimal variability and recurrent decelerations).
Suspicion for encephalopathy might be triggered by any of a large number of newborn behaviors: lethargy, decreased activity, hypotonia, weak suck or incomplete Moro reflexes, constricted pupils, bradycardia, periodic breathing or apnea, hyperalertness, or irritability.8
Coordinate neonatology and obstetric communication with the familyGiven the expanding indications for therapeutic hypothermia, an increasing number of newborns will receive this treatment. This scenario makes enhanced communication vital. Consider this situation:
CASE Baby rushed for therapeutic hypothermia upon birthA baby is born limp and blue without a cry. Her hypotonia raises a concern for encephalopathy, and she is whisked off to the neonatal intensive care unit for 72 hours of therapeutic hypothermia. Stunned, the parents begin to wonder, “Will our baby be O.K.?” and “What went wrong?”
When neonatologists recommend therapeutic hypothermia for the newborn with presumptive encephalopathy, they may explain the situation to the parents with words such as brain injury, encephalopathy, hypoxia, and ischemia. Intrapartum events such as a Category II or III fetal heart rate tracing, operative vaginal delivery, or maternal sepsis or abruption might be mentioned as contributing factors. A consulting neurologist may mention injury of the cerebral cortex, subcortical white matter, or lateral thalami. The neonatologists and neurologists might not mention that less than 50% of cases of newborn encephalopathy are thought to be due to the management of labor.2
The obstetrician, as stunned by the events as the parents, may be at a loss about how to communicate effectively with their patient about the newborn’s encephalopathy. Obstetricians can help assure the parents of their continued involvement in the care and reinforce that the hospital’s neonatologists are superb clinicians who will do their best for the baby.
Challenges exist to effective communication. It is often difficult to optimally coordinate and align the communications of the neonatologists, neurologist, nurses, and obstetrician with the family. Communication with the family can be uncoordinated because interactions occur between the family and multiple specialists with unique perspectives and vocabularies. These conversations occur in sequence, separated in time and place. The communication between family and neonatologists typically occurs in the neonatal intensive care unit. Interactions between obstetrician and mother typically occur in the postpartum unit. The neonatologists and obstetricians are assigned to the hospital in rotating coverage shifts, increasing the number of hand-offs and physicians involved in the hospital care of the mother and newborn dyad.
A joint family meeting with the neonatologists, obstetrician, and family early in the course of newborn care might be an optimal approach to coordinating communication with the parents. Conflicting obligations certainly may make a joint meeting difficult to arrange, however.
Reducing the risk of permanent injury to the central and peripheral nervous system of the newborn is the goal of all obstetricians and neonatologists. Many authorities believe that therapeutic hypothermia can reduce the risk of death and major neurodevelopmental disorders in newborns with encephalopathy. Initial data are promising. If long-term follow-up studies prove that this therapy reduces neurologic disability, the treatment represents a major advance in maternal-child care. As we learn more about this novel, and potentially effective therapy, it should be on the minds of those involved with newborn care to involve the ObGyn in coordinated communication with the family and other medical staff.

During the past 40 years neonatologists have discovered new treatments to improve pulmonary and cardiovascular care of preterm newborns, resulting in a dramatic reduction in newborn mortality and childhood morbidity. Important advances include glucocorticoid administration to mothers at risk for preterm birth, surfactant and nitric oxide administration to the newborn, kangaroo (or skin-to-skin) care, continuous positive airway pressure, and high-frequency ventilation.1 In 1960, only 5% of 1,000-g newborns survived. In 2000, 95% of 1,000-g newborns survive.1
The successes in pulmonary and cardiovascular care have revealed a new frontier in neonatal care: the prevention of long-term neurologic disability by the early treatment of newborn encephalpathy with therapeutic hypothermia. This novel undertaking is an important one; approximately 1 in 300 newborns are diagnosed with encephalopathy.2
Until recently there were no proven treatments for newborns with encephalopathy. However, therapeutic hypothermia now has been proven to be an effective intervention for the treatment of moderate and severe encephalopathy,3,4 and its use is expanding to include mild cases.
This increased use can lead to more complex situations arising for obstetricians, for when a neonatologist decides to initiate therapeutic hypothermia of a newborn the parents may wonder if the obstetrician’s management of labor and delivery was suboptimal, contributing to their baby’s brain injury.
Therapeutic hypothermia: The basics
First, we need to define therapeutic hypothermia. Both head hypothermia and whole-body hypothermia are effective techniques for the treatment of newborn encephalopathy.3,4 Most centers use whole-body (FIGURE) rather than head, hypothermia because it facilitates access to the head for placement of electroencephalogram (EEG) sensors.

The key principles of therapeutic hypothermia include5,6:
- Initiate hypothermia within 6 hours of birth.
- Cool the newborn to a core temperature of 33.5° to 34.5°C (92.3° to 94.1°F). Some centers focus on achieving consistent core temperatures of 33.5°C (92.3°F).
- Monitor core temperature every 5 to 15 minutes.
- Cool the newborn for 72 hours.
- Obtain head ultrasonography to detect intracranial hemorrhage.
- Initiate continuous or intermittent EEG monitoring.
- Treat seizures with phenobarbital, lorazepam, or phenytoin.
- Obtain blood cultures, a complete blood count, blood gas concentrations, alactate coagulation profile, and liver function tests.
- Sedate the newborn, if necessary.
- Minimize oral feedings during the initial phase of hypothermia.
- Obtain sequential magnetic resonance imaging (MRI) studies to assess brain structure and function.
- For all newborns with suspected encephalopathy, the placenta should be sent to pathology for histologic study.7
The data on therapy effectivenessTwo recent meta-analyses independently reported that therapeutic hypothermia reduced the risk of newborn death and major neurodevelopmental disability.3,4 The Cochrane meta-analysis reported that the therapy reduced the risk of neuromotor delay, developmental delay, cerebral palsy, and abnormal MRI results (TABLE).4 The study authors also reported that therapeutic hypothermia reduced the risk of blindness and deafness, although these effects did not reach statistical significance.4 Therapeutic hypothermia did increase the risk of newborn sinus bradycardia and thrombocytopenia.3,4 Compared with usual care, the therapy increased the average survival rate with a normal neurologic outcome at 18 months from 23% to 40%.3 It should be noted that even with therapeutic hypothermia treatment, many newborns with moderate to severe encephalopathy have long-term neurologic disabilities.

Indications for therapeutic hypothermia are expandingIn the initial clinical trials of therapeutic hypothermia, newborns with moderate to severe encephalopathy were enrolled. Typical inclusion criteria were: gestational age ≥35 or 36 weeks, initiation of therapeutic hypothermia within 6 hours of birth, pH ≤7.0 or base deficit of ≥16 mEq/L, 10-minute Apgar score <5 or ongoing resuscitation for 10 minutes, and moderate to severe encephalopathy on clinical examination.3,4 Typical exclusion criteria were: intrauterine growth restriction with birth weight less than 1,750 g, severe congenital anomalies or severe genetic or metabolic syndromes, major intracranial hemorrhage, sepsis, or persistent coagulopathy.
Given the success of therapeutic hypothermia for moderate to severe newborn encephalopathy, many neonatologists are expanding the indications for treatment. In some centers current indications for initiation of hypothermia include the following:
- gestational age ≥34 weeks
- suspicion of encephalopathy or a seizure event
- any obstetric sentinel event (including a bradycardia, umbilical cord prolapse, uterine rupture, placental abruption, Apgar score ≤5 at 10 minutes, pH ≤7.1 or base deficit of ≥10 mEq/L or Category III tracing, or fetal tachycardia with recurrent decelerations or fetal heart rate with minimal variability and recurrent decelerations).
Suspicion for encephalopathy might be triggered by any of a large number of newborn behaviors: lethargy, decreased activity, hypotonia, weak suck or incomplete Moro reflexes, constricted pupils, bradycardia, periodic breathing or apnea, hyperalertness, or irritability.8
Coordinate neonatology and obstetric communication with the familyGiven the expanding indications for therapeutic hypothermia, an increasing number of newborns will receive this treatment. This scenario makes enhanced communication vital. Consider this situation:
CASE Baby rushed for therapeutic hypothermia upon birthA baby is born limp and blue without a cry. Her hypotonia raises a concern for encephalopathy, and she is whisked off to the neonatal intensive care unit for 72 hours of therapeutic hypothermia. Stunned, the parents begin to wonder, “Will our baby be O.K.?” and “What went wrong?”
When neonatologists recommend therapeutic hypothermia for the newborn with presumptive encephalopathy, they may explain the situation to the parents with words such as brain injury, encephalopathy, hypoxia, and ischemia. Intrapartum events such as a Category II or III fetal heart rate tracing, operative vaginal delivery, or maternal sepsis or abruption might be mentioned as contributing factors. A consulting neurologist may mention injury of the cerebral cortex, subcortical white matter, or lateral thalami. The neonatologists and neurologists might not mention that less than 50% of cases of newborn encephalopathy are thought to be due to the management of labor.2
The obstetrician, as stunned by the events as the parents, may be at a loss about how to communicate effectively with their patient about the newborn’s encephalopathy. Obstetricians can help assure the parents of their continued involvement in the care and reinforce that the hospital’s neonatologists are superb clinicians who will do their best for the baby.
Challenges exist to effective communication. It is often difficult to optimally coordinate and align the communications of the neonatologists, neurologist, nurses, and obstetrician with the family. Communication with the family can be uncoordinated because interactions occur between the family and multiple specialists with unique perspectives and vocabularies. These conversations occur in sequence, separated in time and place. The communication between family and neonatologists typically occurs in the neonatal intensive care unit. Interactions between obstetrician and mother typically occur in the postpartum unit. The neonatologists and obstetricians are assigned to the hospital in rotating coverage shifts, increasing the number of hand-offs and physicians involved in the hospital care of the mother and newborn dyad.
A joint family meeting with the neonatologists, obstetrician, and family early in the course of newborn care might be an optimal approach to coordinating communication with the parents. Conflicting obligations certainly may make a joint meeting difficult to arrange, however.
Reducing the risk of permanent injury to the central and peripheral nervous system of the newborn is the goal of all obstetricians and neonatologists. Many authorities believe that therapeutic hypothermia can reduce the risk of death and major neurodevelopmental disorders in newborns with encephalopathy. Initial data are promising. If long-term follow-up studies prove that this therapy reduces neurologic disability, the treatment represents a major advance in maternal-child care. As we learn more about this novel, and potentially effective therapy, it should be on the minds of those involved with newborn care to involve the ObGyn in coordinated communication with the family and other medical staff.

- Philip AG. The evolution of neonatology. Pediatr Res. 2005;58(4):799−815.
- Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischemic encephalopathy. Early Hum Dev. 2010;86(6):329−338.
- Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(6):558−566.
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Syst Rev. 2013;(1):CD003311.
- Papile LA, Baley JE, Benitz W, et al; American Academy of Pediatrics Committee on Fetus and Newborn. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133(6):1146−1150.
- Azzopardi D, Strohm B, Edwards AD, et al; Steering Group and TOBY Cooling Register participants. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Arch Dis Child Fetal Neonatal Ed. 2009;94(4):F260−F264.
- Mir IN, Johnson-Welch SF, Nelson DB, Brown LS, Rosenfeld CR, Chalak LF. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am J Obstet Gynecol. 2015;213(6):849.e1−e7.
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86(7):757−761.
- Philip AG. The evolution of neonatology. Pediatr Res. 2005;58(4):799−815.
- Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischemic encephalopathy. Early Hum Dev. 2010;86(6):329−338.
- Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(6):558−566.
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Syst Rev. 2013;(1):CD003311.
- Papile LA, Baley JE, Benitz W, et al; American Academy of Pediatrics Committee on Fetus and Newborn. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133(6):1146−1150.
- Azzopardi D, Strohm B, Edwards AD, et al; Steering Group and TOBY Cooling Register participants. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Arch Dis Child Fetal Neonatal Ed. 2009;94(4):F260−F264.
- Mir IN, Johnson-Welch SF, Nelson DB, Brown LS, Rosenfeld CR, Chalak LF. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am J Obstet Gynecol. 2015;213(6):849.e1−e7.
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86(7):757−761.
Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related articles:

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related articles:

For more videos from the Society of Gynecologic Surgeons, click here
Visit the Society of Gynecologic Surgeons online: sgsonline.org
Related articles:
This video is brought to you by ![]()
Large scar after multiple procedures
Large scar after multiple procedures
A woman with a history of 3 cesarean deliveries, a tubal ligation reversal, and an abdominoplasty discussed treatment for a large uterine fibroid with her ObGyn. She wanted to avoid a large scar. The ObGyn informed the patient that a laparoscopic hysterectomy could not be promised until her pelvic area was inspected to see if minimally invasive surgery safely could be performed.
During surgery, the ObGyn discovered that pelvic adhesions had distorted the patient’s anatomy; he converted to laparotomy, which left a larger scar.
Two days after surgery, the patient was found to have a bowel injury and underwent additional surgery that included placement of surgical mesh, leaving an enlarged scar.
PATIENT'S CLAIM:
The ObGyn was negligent in injuring the patient’s bowel during hysterectomy and not detecting the injury intraoperatively. Her scars were larger because of the additional repair operation.
PHYSICIAN'S DEFENSE:
Bowel injury is a known complication of the procedure. Many bowel injuries are not detected intraoperatively. The ObGyn made every effort to prevent and check for injury during the procedure.
VERDICT:
An Illinois defense verdict was returned.
Uterus and bowel injured during D&C: $1.5M verdict
A 56-year-old woman underwent hysteroscopy and dilation and curettage (D&C). During the procedure, the gynecologist recognized that he had perforated the uterus and injured the bowel and called in a general surgeon to resect 5 cm of the bowel and repair the uterus.
PATIENT'S CLAIM:
The patient has a large abdominal scar and a chronically distended abdomen. She experienced a year of daily pain and suffering. The D&C was unnecessary and improperly performed: the standard of care is for the gynecologist to operate in a gentle manner; that did not occur.
PHYSICIAN'S DEFENSE:
The D&C was medically necessary. The gynecologist exercised the proper standard of care.
VERDICT:
A $1.5 million New Jersey verdict was returned. The jury found the D&C necessary, but determined that the gynecologist deviated from the accepted standard of care in his performance of the procedure.
Injured ureter allegedly not treated
On December 6, a 42-year-old woman underwent hysterectomy. Postoperatively, she reported increasing dysuria with pain and fever.
On December 13, a computed tomography (CT) scan suggested a partial ureter obstruction. Despite test results, the gynecologist elected to continue to monitor the patient.
The patient’s symptoms continued to worsen and, on December 27, she underwent a second CT scan that identified an obstructed ureter. The gynecologist referred the patient to a urologist, who determined that the patient had sustained a significant ureter injury that required placement of a nephrostomy tube.
PATIENT'S CLAIM:
The gynecologist failed to identify the injury during surgery. The gynecologist was negligent in not consulting a urologist after results of the first CT scan.
PHYSICIAN'S DEFENSE:
Uterine injury is a known complication of the procedure. The gynecologist inspected adjacent organs during surgery but did not find an injury. Postoperative treatment was appropriate.
VERDICT:
The case was presented before a medical review board that concluded that there was no error after the first injury, there was no duty to trace the ureter, and a urology consult was not required after the first CT scan. A Louisiana defense verdict was returned.
Was FHR properly monitored?
After a failed nonstress test, a mother was admitted to triage for blood pressure monitoring. Fetal heart-rate (FHR) monitoring was discontinued at that time. Later that day, FHR monitoring was resumed, fetal distress was detected, and an emergency cesarean delivery was performed. Placental abruption resulted in hypoxia in the baby; she received a diagnosis of cerebral palsy.
PARENT'S CLAIM:
The pregnancy was at high risk because of the mother’s hypertension. The ObGyns misread the FHR at admission and discontinued FHR monitoring too early. If continuous FHR monitoring had occurred, fetal distress would have been detected earlier, resulting in a better outcome for the baby.
PHYSICIAN'S DEFENSE:
There were no signs of fetal distress when the FHR monitoring was discontinued. Placental abruption is an acute event that cannot be predicted.
VERDICT:
A Missouri defense verdict was returned.
Should the ObGyn have come to the hospital earlier?
At 39 weeks’ gestation, a mother arrived at the hospital for induction of labor. That evening, the ObGyn, who was not at the hospital, was notified that the mother had an elevated temperature and that the FHR indicated tachycardia. The ObGyn prescribed antibiotics, and the fever subsided. After an hour, the patient was fully dilated and started to push under a nurse’s supervision. Twenty minutes later, the ObGyn was notified that the fetus was experiencing variable decelerations. The ObGyn arrived in 30 minutes and ordered a cesarean delivery. The baby was born 24 minutes later.
The baby began to have seizures 10 hours after birth. He was transferred to another hospital and remained in the neonatal intensive care unit for 15 days. The child received a diagnosis of cerebral palsy.
PARENT'S CLAIM:
The ObGyn was negligent in not coming to the hospital when the mother was feverish and the fetus tachycardic. The baby experienced an acute hypoxic ischemic injury; an earlier cesarean delivery would have avoided brain injury.
PHYSICIAN'S DEFENSE:
There was no negligence. The infant did not meet all the criteria for an acute hypoxic ischemic injury. Based on a computed tomography scan taken after the seizures began, the infant’s brain injury most likely occurred hours before birth.
VERDICT:
A Virginia defense verdict was returned.
Large scar after multiple procedures
A woman with a history of 3 cesarean deliveries, a tubal ligation reversal, and an abdominoplasty discussed treatment for a large uterine fibroid with her ObGyn. She wanted to avoid a large scar. The ObGyn informed the patient that a laparoscopic hysterectomy could not be promised until her pelvic area was inspected to see if minimally invasive surgery safely could be performed.
During surgery, the ObGyn discovered that pelvic adhesions had distorted the patient’s anatomy; he converted to laparotomy, which left a larger scar.
Two days after surgery, the patient was found to have a bowel injury and underwent additional surgery that included placement of surgical mesh, leaving an enlarged scar.
PATIENT'S CLAIM:
The ObGyn was negligent in injuring the patient’s bowel during hysterectomy and not detecting the injury intraoperatively. Her scars were larger because of the additional repair operation.
PHYSICIAN'S DEFENSE:
Bowel injury is a known complication of the procedure. Many bowel injuries are not detected intraoperatively. The ObGyn made every effort to prevent and check for injury during the procedure.
VERDICT:
An Illinois defense verdict was returned.
Uterus and bowel injured during D&C: $1.5M verdict
A 56-year-old woman underwent hysteroscopy and dilation and curettage (D&C). During the procedure, the gynecologist recognized that he had perforated the uterus and injured the bowel and called in a general surgeon to resect 5 cm of the bowel and repair the uterus.
PATIENT'S CLAIM:
The patient has a large abdominal scar and a chronically distended abdomen. She experienced a year of daily pain and suffering. The D&C was unnecessary and improperly performed: the standard of care is for the gynecologist to operate in a gentle manner; that did not occur.
PHYSICIAN'S DEFENSE:
The D&C was medically necessary. The gynecologist exercised the proper standard of care.
VERDICT:
A $1.5 million New Jersey verdict was returned. The jury found the D&C necessary, but determined that the gynecologist deviated from the accepted standard of care in his performance of the procedure.
Injured ureter allegedly not treated
On December 6, a 42-year-old woman underwent hysterectomy. Postoperatively, she reported increasing dysuria with pain and fever.
On December 13, a computed tomography (CT) scan suggested a partial ureter obstruction. Despite test results, the gynecologist elected to continue to monitor the patient.
The patient’s symptoms continued to worsen and, on December 27, she underwent a second CT scan that identified an obstructed ureter. The gynecologist referred the patient to a urologist, who determined that the patient had sustained a significant ureter injury that required placement of a nephrostomy tube.
PATIENT'S CLAIM:
The gynecologist failed to identify the injury during surgery. The gynecologist was negligent in not consulting a urologist after results of the first CT scan.
PHYSICIAN'S DEFENSE:
Uterine injury is a known complication of the procedure. The gynecologist inspected adjacent organs during surgery but did not find an injury. Postoperative treatment was appropriate.
VERDICT:
The case was presented before a medical review board that concluded that there was no error after the first injury, there was no duty to trace the ureter, and a urology consult was not required after the first CT scan. A Louisiana defense verdict was returned.
Was FHR properly monitored?
After a failed nonstress test, a mother was admitted to triage for blood pressure monitoring. Fetal heart-rate (FHR) monitoring was discontinued at that time. Later that day, FHR monitoring was resumed, fetal distress was detected, and an emergency cesarean delivery was performed. Placental abruption resulted in hypoxia in the baby; she received a diagnosis of cerebral palsy.
PARENT'S CLAIM:
The pregnancy was at high risk because of the mother’s hypertension. The ObGyns misread the FHR at admission and discontinued FHR monitoring too early. If continuous FHR monitoring had occurred, fetal distress would have been detected earlier, resulting in a better outcome for the baby.
PHYSICIAN'S DEFENSE:
There were no signs of fetal distress when the FHR monitoring was discontinued. Placental abruption is an acute event that cannot be predicted.
VERDICT:
A Missouri defense verdict was returned.
Should the ObGyn have come to the hospital earlier?
At 39 weeks’ gestation, a mother arrived at the hospital for induction of labor. That evening, the ObGyn, who was not at the hospital, was notified that the mother had an elevated temperature and that the FHR indicated tachycardia. The ObGyn prescribed antibiotics, and the fever subsided. After an hour, the patient was fully dilated and started to push under a nurse’s supervision. Twenty minutes later, the ObGyn was notified that the fetus was experiencing variable decelerations. The ObGyn arrived in 30 minutes and ordered a cesarean delivery. The baby was born 24 minutes later.
The baby began to have seizures 10 hours after birth. He was transferred to another hospital and remained in the neonatal intensive care unit for 15 days. The child received a diagnosis of cerebral palsy.
PARENT'S CLAIM:
The ObGyn was negligent in not coming to the hospital when the mother was feverish and the fetus tachycardic. The baby experienced an acute hypoxic ischemic injury; an earlier cesarean delivery would have avoided brain injury.
PHYSICIAN'S DEFENSE:
There was no negligence. The infant did not meet all the criteria for an acute hypoxic ischemic injury. Based on a computed tomography scan taken after the seizures began, the infant’s brain injury most likely occurred hours before birth.
VERDICT:
A Virginia defense verdict was returned.
Large scar after multiple procedures
A woman with a history of 3 cesarean deliveries, a tubal ligation reversal, and an abdominoplasty discussed treatment for a large uterine fibroid with her ObGyn. She wanted to avoid a large scar. The ObGyn informed the patient that a laparoscopic hysterectomy could not be promised until her pelvic area was inspected to see if minimally invasive surgery safely could be performed.
During surgery, the ObGyn discovered that pelvic adhesions had distorted the patient’s anatomy; he converted to laparotomy, which left a larger scar.
Two days after surgery, the patient was found to have a bowel injury and underwent additional surgery that included placement of surgical mesh, leaving an enlarged scar.
PATIENT'S CLAIM:
The ObGyn was negligent in injuring the patient’s bowel during hysterectomy and not detecting the injury intraoperatively. Her scars were larger because of the additional repair operation.
PHYSICIAN'S DEFENSE:
Bowel injury is a known complication of the procedure. Many bowel injuries are not detected intraoperatively. The ObGyn made every effort to prevent and check for injury during the procedure.
VERDICT:
An Illinois defense verdict was returned.
Uterus and bowel injured during D&C: $1.5M verdict
A 56-year-old woman underwent hysteroscopy and dilation and curettage (D&C). During the procedure, the gynecologist recognized that he had perforated the uterus and injured the bowel and called in a general surgeon to resect 5 cm of the bowel and repair the uterus.
PATIENT'S CLAIM:
The patient has a large abdominal scar and a chronically distended abdomen. She experienced a year of daily pain and suffering. The D&C was unnecessary and improperly performed: the standard of care is for the gynecologist to operate in a gentle manner; that did not occur.
PHYSICIAN'S DEFENSE:
The D&C was medically necessary. The gynecologist exercised the proper standard of care.
VERDICT:
A $1.5 million New Jersey verdict was returned. The jury found the D&C necessary, but determined that the gynecologist deviated from the accepted standard of care in his performance of the procedure.
Injured ureter allegedly not treated
On December 6, a 42-year-old woman underwent hysterectomy. Postoperatively, she reported increasing dysuria with pain and fever.
On December 13, a computed tomography (CT) scan suggested a partial ureter obstruction. Despite test results, the gynecologist elected to continue to monitor the patient.
The patient’s symptoms continued to worsen and, on December 27, she underwent a second CT scan that identified an obstructed ureter. The gynecologist referred the patient to a urologist, who determined that the patient had sustained a significant ureter injury that required placement of a nephrostomy tube.
PATIENT'S CLAIM:
The gynecologist failed to identify the injury during surgery. The gynecologist was negligent in not consulting a urologist after results of the first CT scan.
PHYSICIAN'S DEFENSE:
Uterine injury is a known complication of the procedure. The gynecologist inspected adjacent organs during surgery but did not find an injury. Postoperative treatment was appropriate.
VERDICT:
The case was presented before a medical review board that concluded that there was no error after the first injury, there was no duty to trace the ureter, and a urology consult was not required after the first CT scan. A Louisiana defense verdict was returned.
Was FHR properly monitored?
After a failed nonstress test, a mother was admitted to triage for blood pressure monitoring. Fetal heart-rate (FHR) monitoring was discontinued at that time. Later that day, FHR monitoring was resumed, fetal distress was detected, and an emergency cesarean delivery was performed. Placental abruption resulted in hypoxia in the baby; she received a diagnosis of cerebral palsy.
PARENT'S CLAIM:
The pregnancy was at high risk because of the mother’s hypertension. The ObGyns misread the FHR at admission and discontinued FHR monitoring too early. If continuous FHR monitoring had occurred, fetal distress would have been detected earlier, resulting in a better outcome for the baby.
PHYSICIAN'S DEFENSE:
There were no signs of fetal distress when the FHR monitoring was discontinued. Placental abruption is an acute event that cannot be predicted.
VERDICT:
A Missouri defense verdict was returned.
Should the ObGyn have come to the hospital earlier?
At 39 weeks’ gestation, a mother arrived at the hospital for induction of labor. That evening, the ObGyn, who was not at the hospital, was notified that the mother had an elevated temperature and that the FHR indicated tachycardia. The ObGyn prescribed antibiotics, and the fever subsided. After an hour, the patient was fully dilated and started to push under a nurse’s supervision. Twenty minutes later, the ObGyn was notified that the fetus was experiencing variable decelerations. The ObGyn arrived in 30 minutes and ordered a cesarean delivery. The baby was born 24 minutes later.
The baby began to have seizures 10 hours after birth. He was transferred to another hospital and remained in the neonatal intensive care unit for 15 days. The child received a diagnosis of cerebral palsy.
PARENT'S CLAIM:
The ObGyn was negligent in not coming to the hospital when the mother was feverish and the fetus tachycardic. The baby experienced an acute hypoxic ischemic injury; an earlier cesarean delivery would have avoided brain injury.
PHYSICIAN'S DEFENSE:
There was no negligence. The infant did not meet all the criteria for an acute hypoxic ischemic injury. Based on a computed tomography scan taken after the seizures began, the infant’s brain injury most likely occurred hours before birth.
VERDICT:
A Virginia defense verdict was returned.
Letters to the Editor: Alternatives to DEET for pregnant patients; Tissue extraction
“What Insect repellents are safe during pregnancy?”
ANUSHKA CHELLIAH, MD, AND PATRICK DUFF, MD (JUNE 2016)
Alternatives to DEET
Picaridin is not mentioned in this brief report from Drs. Chelliah and Duff. I suggest reviewing the July 2015 Consumer Reports article on repellents; picaridin is a likely safer alternative to DEET, with the highest efficacy of all those tested, at least in Sawyer Fisherman’s Formula Picaridin Insect Repellent and Natrapel 8 Hour Insect Repellent. Products that have little or no efficacy also were not mentioned, including Avon Skin So Soft, Coleman Naturals Insect Repellent Snap Band, and SuperBand Wristband. In addition, the concentration of products is very important, as is the precise formulation within brands. For example, Off! Deep Woods VIII (with DEET 25%) is very effective versus Off! FamilyCare II Clean Feel (with picaridin 5%), which has very little benefit.
David H. Janowitz, MD
Houston, Texas
Drs. Chelliah and Duff respond
In our short discussion of mosquito repellents, we based our recommendations on publications from the Centers for Disease Control and Prevention (CDC) and the Florida Department of Health. Those publications presented DEET (N,N-diethyl-m-toluamide) at the top of the list for preferred repellents. A recent publication from the Organization of Teratology Information Specialists (MotherToBaby, September 2013) indicated that, in a concentration of 20% to 30%, DEET was safe in pregnancy and was effective in protecting against 90% of all mosquito bites and tick attachments. Increasing the concentration of DEET above 30% does not enhance the product’s effectiveness or prolong its duration of action.
However, Dr. Janowitz is correct in stating that other agents are also highly effective and safe in pregnancy. These agents include picaridin (20%) and oil of lemon/eucalyptus (30%). We thank Dr. Janowitz for directing us to the most recent testing program conducted by Consumer Reports.1 That testing program demonstrated that Sawyer Fisherman’s Formula Picaridin and Natrapel 8 Hour, which each contain 20% picaridin, and Off! Deep Woods VIII, which contains 25% DEET, kept Aedes mosquitoes from biting for approximately 8 hours. The Sawyer product was also effective in preventing bites from the Culex mosquitoes, which carry West Nile virus, and deer ticks, which can transmit Lyme disease. Repel Lemon Eucalyptus (30%) stopped Aedes mosquito bites for 7 hours.
In the Consumer Reports testing program, IR3535 products, which we recommended in our article, did not perform well, nor did repellents that contained only 7% DEET or less than 20% picaridin. Moreover, products made from natural plant oils—such as citronella, lemongrass oil, cedar oil, geraniol, rosemary oil, and cinnamon oil—were not particularly effective. Some did not last for more than 1 hour; some failed immediately.
When applying any of these products, individuals should observe the following guidelines:
- apply insect repellents only to exposed skin or clothing
- do not apply repellents on cuts, wounds, or abraded skin or immediately after shaving
- avoid the eyes and mouth when applying repellent to the face
- after exposure is over, wash the skin with soap and water
- clothing that has been treated with one of these agents or with permethrin should be washed separately before it is worn again.
Reference
- Byrne S. Mosquito repellents that best protect against Zika. Consumer Reports. http://www.consumerreports.org/insect-repellents/mosquito-repellents-that-best-protect-against-zika/. Updated April 16, 2016. Accessed July 25, 2016.
“Tissue extraction: Can the pendulum change direction?”
ARNOLD P. ADVINCULA, MD (JUNE 2016)
We have met the enemy and he is us
While I share the optimism Dr. Advincula expressed in his recent guest editorial regarding a change in the direction of the pendulum that swung away from use of the power morcellator, I feel compelled to express the opinion that this entire fiasco has been nothing other than an outrageous regulatory overreach.
Shortly after the US Food and Drug Administration (FDA) issued its proclamation in April 2014, the Society of Gynecologic Oncology repudiated the bogus statistics that were being used to describe the incidence of leiomyosarcoma and, further, stated that it would not matter how someone’s uterus containing this rare tumor was removed because the outcome would be poor. Similarly, the American Journal of Obstetrics and Gynecology published an article enumerating the expected significant increase in complications and the resulting misery that could be expected for patients whose management was diverted from minimally invasive to open hysterectomy.1 The AAGL also expressed opinions that this was an unnecessary, and counterproductive, policy—all to no avail.
My optimism, however, is tempered by a number of questions: 1) Why did it take more than a year for 36 nationally recognized gynecologic surgeons to write a letter to the FDA denouncing the warning, yet again, and reiterating the errors in analysis used to establish the policy? 2) Why are gynecologic surgeons only now being asked to serve in the FDA’s Network of Experts? Should not that have been the case before the warning was issued? 3) If the perioperative outcomes are similar using a containment bag compared with open morcellation, what is the benefit of using the containment system? I, for one, think that prolonging a procedure another half hour is significant.
The FDA’s egregious policy clearly has had a net negative impact on the welfare of our patients. The gynecologic surgeon community should have pushed back more forcefully and effectively. I hope the next time something like this happens (and it will) we can be better advocates for our patients.
Mark S. Finkelston, DO
Shawnee Mission, Kansas
Reference
- Siedhoff MT, Wheeler SB, Rutstein SE, et al. Laparoscopic hysterectomy with morcellation vs abdominal hysterectomy for presumed fibroid tumors in premenopausal women: a decision analysis. Am J Obstet Gynecol. 2015;212(5):591.e1−e8.
Dr. Advincula responds
I thank Dr. Finkelston for his thoughts regarding my editorial. There is no doubt that the issues surrounding tissue extraction have been heated. Although I do not have definitive answers that explain all of the various reactions, whether immediate or delayed, to the cascade of events surrounding morcellation, I do believe that much of it was a response to N-of-1 policy-making, as very nicely discussed in a New England Journal of Medicine article by Lisa Rosenbaum.1 We must continue to foster constructive dialogues with our regulatory bodies and cultivate the spirit of innovation that has brought so many advances to the field of surgery. Ultimately, going forward, it will be important for clinicians and other health care providers to speak up and not remain silent for fear of being vilified.
Reference
- Rosenbaum L. N-of-1 policymaking—tragedy, trade-offs, and the demise of morcellation. N Engl J Med. 2016;374(10):986−990.
Vaginal hysterectomy solves the tissue morcellation dilemma
Dr. Advincula starts his guest editorial with the statement, “With practical, evidence-based, sound clinical judgement, I believe that it can.”
In fact, what “practical, evidence-based, sound clinical judgement” supports is a return to vaginal hysterectomy with transvaginal extracorporeal morcellation techniques. As Dr. Carl Zimmerman said in a recent debate at the Society of Gynecologic Surgeons (SGS annual meeting), “There is no recorded case of a vaginal hysterectomy with morcellation upgrading a patient with leiomyosarcoma.” In addition, the majority of cases in which Dr. Advincula and others are performing robot-assisted laparoscopic hysterectomy or total laparoscopic hysterectomy have this clinical and demographic profile: average age, 42; average parity, G2; average body mass index, 30; most common diagnosis, abnormal uterine bleeding, fibroids; most common pathology, fibroids; average uterine weight, 165 g. The majority of these can be performed much more safely, quickly, and cost effectively by transvaginal hysterectomy/morcellation. Please see an excellent commentary by Dr. Andrew Walter, immediate past president of SGS, on “Why we should strive for a vaginal hysterectomy rate of 40%.”1
But the main reason Dr. Advincula should not be given a voice on this issue is because he has significant financial conflict of interest with the medical device industry. Should he even be on the OBG <scaps>Management</scaps> board of editors? I do not believe the rest of your editors have anywhere near his level of conflict of interest. Should he not be asked to recuse himself in this debate or abandon his financial connections with the medical device industry? Is this not the whole purpose of the Sunshine Act? Please, should you not be supporting what is in the best interest of our patients and payers?
R. Bruce Councell, MD
Asheville, North Carolina
Reference
- Walter AJ. Why we should strive for a vaginal hysterectomy rate of 40%. ObGyn News. http://www.obgynnews.com/?id=11146&tx_ttnews[tt_news]=505393&cHash=d0dd4348213d571a2dd0f7c6a6873091. Published May 6, 2016. Accessed July 27, 2016.
Dr. Barbieri responds
At OBG Management, we wholeheartedly agree with Dr. Councell that vaginal hysterectomy is an excellent approach to removing the uterus in most women with noncancer indications for surgery. Our recently featured articles focused on vaginal hysterectomy include: “Transforming vaginal hysterectomy: 7 solutions to the most daunting challenges,” “Is energy-based vessel sealing safer than suturing for vaginal hysterectomy?,” Is same-day discharge feasible and safe for women undergoing vaginal hysterectomy?,” and “Can we reduce the use of abdominal hysterectomy and increase the use of vaginal and laparoscopic approaches?” We plan to publish more content on advances in both vaginal and laparoscopic surgery.
We are proud to have Dr. Advincula, an internationally recognized leader in gynecologic surgery, serve on the OBG Management Editorial Board. His expertise and perspective is of great value to our readers. It is true that many leading surgeons, including Dr. Advincula, serve as consultants with manufacturers of surgical devices. Working together, clinical experts and device manufacturers help to advance medical care. In his editorial, Dr. Advincula did disclose these relationships. As a check on the quality and balance in our editorial material, I personally review all content and I have no financial relationships with any pharmaceutical or device manufacturer.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“What Insect repellents are safe during pregnancy?”
ANUSHKA CHELLIAH, MD, AND PATRICK DUFF, MD (JUNE 2016)
Alternatives to DEET
Picaridin is not mentioned in this brief report from Drs. Chelliah and Duff. I suggest reviewing the July 2015 Consumer Reports article on repellents; picaridin is a likely safer alternative to DEET, with the highest efficacy of all those tested, at least in Sawyer Fisherman’s Formula Picaridin Insect Repellent and Natrapel 8 Hour Insect Repellent. Products that have little or no efficacy also were not mentioned, including Avon Skin So Soft, Coleman Naturals Insect Repellent Snap Band, and SuperBand Wristband. In addition, the concentration of products is very important, as is the precise formulation within brands. For example, Off! Deep Woods VIII (with DEET 25%) is very effective versus Off! FamilyCare II Clean Feel (with picaridin 5%), which has very little benefit.
David H. Janowitz, MD
Houston, Texas
Drs. Chelliah and Duff respond
In our short discussion of mosquito repellents, we based our recommendations on publications from the Centers for Disease Control and Prevention (CDC) and the Florida Department of Health. Those publications presented DEET (N,N-diethyl-m-toluamide) at the top of the list for preferred repellents. A recent publication from the Organization of Teratology Information Specialists (MotherToBaby, September 2013) indicated that, in a concentration of 20% to 30%, DEET was safe in pregnancy and was effective in protecting against 90% of all mosquito bites and tick attachments. Increasing the concentration of DEET above 30% does not enhance the product’s effectiveness or prolong its duration of action.
However, Dr. Janowitz is correct in stating that other agents are also highly effective and safe in pregnancy. These agents include picaridin (20%) and oil of lemon/eucalyptus (30%). We thank Dr. Janowitz for directing us to the most recent testing program conducted by Consumer Reports.1 That testing program demonstrated that Sawyer Fisherman’s Formula Picaridin and Natrapel 8 Hour, which each contain 20% picaridin, and Off! Deep Woods VIII, which contains 25% DEET, kept Aedes mosquitoes from biting for approximately 8 hours. The Sawyer product was also effective in preventing bites from the Culex mosquitoes, which carry West Nile virus, and deer ticks, which can transmit Lyme disease. Repel Lemon Eucalyptus (30%) stopped Aedes mosquito bites for 7 hours.
In the Consumer Reports testing program, IR3535 products, which we recommended in our article, did not perform well, nor did repellents that contained only 7% DEET or less than 20% picaridin. Moreover, products made from natural plant oils—such as citronella, lemongrass oil, cedar oil, geraniol, rosemary oil, and cinnamon oil—were not particularly effective. Some did not last for more than 1 hour; some failed immediately.
When applying any of these products, individuals should observe the following guidelines:
- apply insect repellents only to exposed skin or clothing
- do not apply repellents on cuts, wounds, or abraded skin or immediately after shaving
- avoid the eyes and mouth when applying repellent to the face
- after exposure is over, wash the skin with soap and water
- clothing that has been treated with one of these agents or with permethrin should be washed separately before it is worn again.
Reference
- Byrne S. Mosquito repellents that best protect against Zika. Consumer Reports. http://www.consumerreports.org/insect-repellents/mosquito-repellents-that-best-protect-against-zika/. Updated April 16, 2016. Accessed July 25, 2016.
“Tissue extraction: Can the pendulum change direction?”
ARNOLD P. ADVINCULA, MD (JUNE 2016)
We have met the enemy and he is us
While I share the optimism Dr. Advincula expressed in his recent guest editorial regarding a change in the direction of the pendulum that swung away from use of the power morcellator, I feel compelled to express the opinion that this entire fiasco has been nothing other than an outrageous regulatory overreach.
Shortly after the US Food and Drug Administration (FDA) issued its proclamation in April 2014, the Society of Gynecologic Oncology repudiated the bogus statistics that were being used to describe the incidence of leiomyosarcoma and, further, stated that it would not matter how someone’s uterus containing this rare tumor was removed because the outcome would be poor. Similarly, the American Journal of Obstetrics and Gynecology published an article enumerating the expected significant increase in complications and the resulting misery that could be expected for patients whose management was diverted from minimally invasive to open hysterectomy.1 The AAGL also expressed opinions that this was an unnecessary, and counterproductive, policy—all to no avail.
My optimism, however, is tempered by a number of questions: 1) Why did it take more than a year for 36 nationally recognized gynecologic surgeons to write a letter to the FDA denouncing the warning, yet again, and reiterating the errors in analysis used to establish the policy? 2) Why are gynecologic surgeons only now being asked to serve in the FDA’s Network of Experts? Should not that have been the case before the warning was issued? 3) If the perioperative outcomes are similar using a containment bag compared with open morcellation, what is the benefit of using the containment system? I, for one, think that prolonging a procedure another half hour is significant.
The FDA’s egregious policy clearly has had a net negative impact on the welfare of our patients. The gynecologic surgeon community should have pushed back more forcefully and effectively. I hope the next time something like this happens (and it will) we can be better advocates for our patients.
Mark S. Finkelston, DO
Shawnee Mission, Kansas
Reference
- Siedhoff MT, Wheeler SB, Rutstein SE, et al. Laparoscopic hysterectomy with morcellation vs abdominal hysterectomy for presumed fibroid tumors in premenopausal women: a decision analysis. Am J Obstet Gynecol. 2015;212(5):591.e1−e8.
Dr. Advincula responds
I thank Dr. Finkelston for his thoughts regarding my editorial. There is no doubt that the issues surrounding tissue extraction have been heated. Although I do not have definitive answers that explain all of the various reactions, whether immediate or delayed, to the cascade of events surrounding morcellation, I do believe that much of it was a response to N-of-1 policy-making, as very nicely discussed in a New England Journal of Medicine article by Lisa Rosenbaum.1 We must continue to foster constructive dialogues with our regulatory bodies and cultivate the spirit of innovation that has brought so many advances to the field of surgery. Ultimately, going forward, it will be important for clinicians and other health care providers to speak up and not remain silent for fear of being vilified.
Reference
- Rosenbaum L. N-of-1 policymaking—tragedy, trade-offs, and the demise of morcellation. N Engl J Med. 2016;374(10):986−990.
Vaginal hysterectomy solves the tissue morcellation dilemma
Dr. Advincula starts his guest editorial with the statement, “With practical, evidence-based, sound clinical judgement, I believe that it can.”
In fact, what “practical, evidence-based, sound clinical judgement” supports is a return to vaginal hysterectomy with transvaginal extracorporeal morcellation techniques. As Dr. Carl Zimmerman said in a recent debate at the Society of Gynecologic Surgeons (SGS annual meeting), “There is no recorded case of a vaginal hysterectomy with morcellation upgrading a patient with leiomyosarcoma.” In addition, the majority of cases in which Dr. Advincula and others are performing robot-assisted laparoscopic hysterectomy or total laparoscopic hysterectomy have this clinical and demographic profile: average age, 42; average parity, G2; average body mass index, 30; most common diagnosis, abnormal uterine bleeding, fibroids; most common pathology, fibroids; average uterine weight, 165 g. The majority of these can be performed much more safely, quickly, and cost effectively by transvaginal hysterectomy/morcellation. Please see an excellent commentary by Dr. Andrew Walter, immediate past president of SGS, on “Why we should strive for a vaginal hysterectomy rate of 40%.”1
But the main reason Dr. Advincula should not be given a voice on this issue is because he has significant financial conflict of interest with the medical device industry. Should he even be on the OBG <scaps>Management</scaps> board of editors? I do not believe the rest of your editors have anywhere near his level of conflict of interest. Should he not be asked to recuse himself in this debate or abandon his financial connections with the medical device industry? Is this not the whole purpose of the Sunshine Act? Please, should you not be supporting what is in the best interest of our patients and payers?
R. Bruce Councell, MD
Asheville, North Carolina
Reference
- Walter AJ. Why we should strive for a vaginal hysterectomy rate of 40%. ObGyn News. http://www.obgynnews.com/?id=11146&tx_ttnews[tt_news]=505393&cHash=d0dd4348213d571a2dd0f7c6a6873091. Published May 6, 2016. Accessed July 27, 2016.
Dr. Barbieri responds
At OBG Management, we wholeheartedly agree with Dr. Councell that vaginal hysterectomy is an excellent approach to removing the uterus in most women with noncancer indications for surgery. Our recently featured articles focused on vaginal hysterectomy include: “Transforming vaginal hysterectomy: 7 solutions to the most daunting challenges,” “Is energy-based vessel sealing safer than suturing for vaginal hysterectomy?,” Is same-day discharge feasible and safe for women undergoing vaginal hysterectomy?,” and “Can we reduce the use of abdominal hysterectomy and increase the use of vaginal and laparoscopic approaches?” We plan to publish more content on advances in both vaginal and laparoscopic surgery.
We are proud to have Dr. Advincula, an internationally recognized leader in gynecologic surgery, serve on the OBG Management Editorial Board. His expertise and perspective is of great value to our readers. It is true that many leading surgeons, including Dr. Advincula, serve as consultants with manufacturers of surgical devices. Working together, clinical experts and device manufacturers help to advance medical care. In his editorial, Dr. Advincula did disclose these relationships. As a check on the quality and balance in our editorial material, I personally review all content and I have no financial relationships with any pharmaceutical or device manufacturer.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“What Insect repellents are safe during pregnancy?”
ANUSHKA CHELLIAH, MD, AND PATRICK DUFF, MD (JUNE 2016)
Alternatives to DEET
Picaridin is not mentioned in this brief report from Drs. Chelliah and Duff. I suggest reviewing the July 2015 Consumer Reports article on repellents; picaridin is a likely safer alternative to DEET, with the highest efficacy of all those tested, at least in Sawyer Fisherman’s Formula Picaridin Insect Repellent and Natrapel 8 Hour Insect Repellent. Products that have little or no efficacy also were not mentioned, including Avon Skin So Soft, Coleman Naturals Insect Repellent Snap Band, and SuperBand Wristband. In addition, the concentration of products is very important, as is the precise formulation within brands. For example, Off! Deep Woods VIII (with DEET 25%) is very effective versus Off! FamilyCare II Clean Feel (with picaridin 5%), which has very little benefit.
David H. Janowitz, MD
Houston, Texas
Drs. Chelliah and Duff respond
In our short discussion of mosquito repellents, we based our recommendations on publications from the Centers for Disease Control and Prevention (CDC) and the Florida Department of Health. Those publications presented DEET (N,N-diethyl-m-toluamide) at the top of the list for preferred repellents. A recent publication from the Organization of Teratology Information Specialists (MotherToBaby, September 2013) indicated that, in a concentration of 20% to 30%, DEET was safe in pregnancy and was effective in protecting against 90% of all mosquito bites and tick attachments. Increasing the concentration of DEET above 30% does not enhance the product’s effectiveness or prolong its duration of action.
However, Dr. Janowitz is correct in stating that other agents are also highly effective and safe in pregnancy. These agents include picaridin (20%) and oil of lemon/eucalyptus (30%). We thank Dr. Janowitz for directing us to the most recent testing program conducted by Consumer Reports.1 That testing program demonstrated that Sawyer Fisherman’s Formula Picaridin and Natrapel 8 Hour, which each contain 20% picaridin, and Off! Deep Woods VIII, which contains 25% DEET, kept Aedes mosquitoes from biting for approximately 8 hours. The Sawyer product was also effective in preventing bites from the Culex mosquitoes, which carry West Nile virus, and deer ticks, which can transmit Lyme disease. Repel Lemon Eucalyptus (30%) stopped Aedes mosquito bites for 7 hours.
In the Consumer Reports testing program, IR3535 products, which we recommended in our article, did not perform well, nor did repellents that contained only 7% DEET or less than 20% picaridin. Moreover, products made from natural plant oils—such as citronella, lemongrass oil, cedar oil, geraniol, rosemary oil, and cinnamon oil—were not particularly effective. Some did not last for more than 1 hour; some failed immediately.
When applying any of these products, individuals should observe the following guidelines:
- apply insect repellents only to exposed skin or clothing
- do not apply repellents on cuts, wounds, or abraded skin or immediately after shaving
- avoid the eyes and mouth when applying repellent to the face
- after exposure is over, wash the skin with soap and water
- clothing that has been treated with one of these agents or with permethrin should be washed separately before it is worn again.
Reference
- Byrne S. Mosquito repellents that best protect against Zika. Consumer Reports. http://www.consumerreports.org/insect-repellents/mosquito-repellents-that-best-protect-against-zika/. Updated April 16, 2016. Accessed July 25, 2016.
“Tissue extraction: Can the pendulum change direction?”
ARNOLD P. ADVINCULA, MD (JUNE 2016)
We have met the enemy and he is us
While I share the optimism Dr. Advincula expressed in his recent guest editorial regarding a change in the direction of the pendulum that swung away from use of the power morcellator, I feel compelled to express the opinion that this entire fiasco has been nothing other than an outrageous regulatory overreach.
Shortly after the US Food and Drug Administration (FDA) issued its proclamation in April 2014, the Society of Gynecologic Oncology repudiated the bogus statistics that were being used to describe the incidence of leiomyosarcoma and, further, stated that it would not matter how someone’s uterus containing this rare tumor was removed because the outcome would be poor. Similarly, the American Journal of Obstetrics and Gynecology published an article enumerating the expected significant increase in complications and the resulting misery that could be expected for patients whose management was diverted from minimally invasive to open hysterectomy.1 The AAGL also expressed opinions that this was an unnecessary, and counterproductive, policy—all to no avail.
My optimism, however, is tempered by a number of questions: 1) Why did it take more than a year for 36 nationally recognized gynecologic surgeons to write a letter to the FDA denouncing the warning, yet again, and reiterating the errors in analysis used to establish the policy? 2) Why are gynecologic surgeons only now being asked to serve in the FDA’s Network of Experts? Should not that have been the case before the warning was issued? 3) If the perioperative outcomes are similar using a containment bag compared with open morcellation, what is the benefit of using the containment system? I, for one, think that prolonging a procedure another half hour is significant.
The FDA’s egregious policy clearly has had a net negative impact on the welfare of our patients. The gynecologic surgeon community should have pushed back more forcefully and effectively. I hope the next time something like this happens (and it will) we can be better advocates for our patients.
Mark S. Finkelston, DO
Shawnee Mission, Kansas
Reference
- Siedhoff MT, Wheeler SB, Rutstein SE, et al. Laparoscopic hysterectomy with morcellation vs abdominal hysterectomy for presumed fibroid tumors in premenopausal women: a decision analysis. Am J Obstet Gynecol. 2015;212(5):591.e1−e8.
Dr. Advincula responds
I thank Dr. Finkelston for his thoughts regarding my editorial. There is no doubt that the issues surrounding tissue extraction have been heated. Although I do not have definitive answers that explain all of the various reactions, whether immediate or delayed, to the cascade of events surrounding morcellation, I do believe that much of it was a response to N-of-1 policy-making, as very nicely discussed in a New England Journal of Medicine article by Lisa Rosenbaum.1 We must continue to foster constructive dialogues with our regulatory bodies and cultivate the spirit of innovation that has brought so many advances to the field of surgery. Ultimately, going forward, it will be important for clinicians and other health care providers to speak up and not remain silent for fear of being vilified.
Reference
- Rosenbaum L. N-of-1 policymaking—tragedy, trade-offs, and the demise of morcellation. N Engl J Med. 2016;374(10):986−990.
Vaginal hysterectomy solves the tissue morcellation dilemma
Dr. Advincula starts his guest editorial with the statement, “With practical, evidence-based, sound clinical judgement, I believe that it can.”
In fact, what “practical, evidence-based, sound clinical judgement” supports is a return to vaginal hysterectomy with transvaginal extracorporeal morcellation techniques. As Dr. Carl Zimmerman said in a recent debate at the Society of Gynecologic Surgeons (SGS annual meeting), “There is no recorded case of a vaginal hysterectomy with morcellation upgrading a patient with leiomyosarcoma.” In addition, the majority of cases in which Dr. Advincula and others are performing robot-assisted laparoscopic hysterectomy or total laparoscopic hysterectomy have this clinical and demographic profile: average age, 42; average parity, G2; average body mass index, 30; most common diagnosis, abnormal uterine bleeding, fibroids; most common pathology, fibroids; average uterine weight, 165 g. The majority of these can be performed much more safely, quickly, and cost effectively by transvaginal hysterectomy/morcellation. Please see an excellent commentary by Dr. Andrew Walter, immediate past president of SGS, on “Why we should strive for a vaginal hysterectomy rate of 40%.”1
But the main reason Dr. Advincula should not be given a voice on this issue is because he has significant financial conflict of interest with the medical device industry. Should he even be on the OBG <scaps>Management</scaps> board of editors? I do not believe the rest of your editors have anywhere near his level of conflict of interest. Should he not be asked to recuse himself in this debate or abandon his financial connections with the medical device industry? Is this not the whole purpose of the Sunshine Act? Please, should you not be supporting what is in the best interest of our patients and payers?
R. Bruce Councell, MD
Asheville, North Carolina
Reference
- Walter AJ. Why we should strive for a vaginal hysterectomy rate of 40%. ObGyn News. http://www.obgynnews.com/?id=11146&tx_ttnews[tt_news]=505393&cHash=d0dd4348213d571a2dd0f7c6a6873091. Published May 6, 2016. Accessed July 27, 2016.
Dr. Barbieri responds
At OBG Management, we wholeheartedly agree with Dr. Councell that vaginal hysterectomy is an excellent approach to removing the uterus in most women with noncancer indications for surgery. Our recently featured articles focused on vaginal hysterectomy include: “Transforming vaginal hysterectomy: 7 solutions to the most daunting challenges,” “Is energy-based vessel sealing safer than suturing for vaginal hysterectomy?,” Is same-day discharge feasible and safe for women undergoing vaginal hysterectomy?,” and “Can we reduce the use of abdominal hysterectomy and increase the use of vaginal and laparoscopic approaches?” We plan to publish more content on advances in both vaginal and laparoscopic surgery.
We are proud to have Dr. Advincula, an internationally recognized leader in gynecologic surgery, serve on the OBG Management Editorial Board. His expertise and perspective is of great value to our readers. It is true that many leading surgeons, including Dr. Advincula, serve as consultants with manufacturers of surgical devices. Working together, clinical experts and device manufacturers help to advance medical care. In his editorial, Dr. Advincula did disclose these relationships. As a check on the quality and balance in our editorial material, I personally review all content and I have no financial relationships with any pharmaceutical or device manufacturer.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Product Update: RESECTR disposable handheld resector, PreTRM Test
DISPOSABLE HANDHELD RESECTOR

Distal Access offers nationwide availability of the RESECTR™ 9 French / 3.0 mm High- Performance Disposable Tissue Resector, a disposable, nonpowered, handheld, and hand-driven system designed to combine the benefits of basic manual devices and complex electro-mechanical systems.
The RESECTR platform is “ready-to-use,” says Distal Access, giving clinicians an important tool to see-and-treat lesions in the hospital, clinic, or office. Starting at the cutting tip, aspiration pulls tissue samples into the cutting window where oscillating blades are controlled by the clinician’s index finger and hand. Clinicians can increase or decrease oscillation and cutting based on what they see and feel during the procedure.
According to Distal Access, for small tissue samples, resection time with the RESECTR can be similar to that with electromechanical devices, with a significantly lower cost. The RESECTR is compatible with available fluid management systems and endoscopic devices.
FOR MORE INFORMATION, VISIT: www.resectr.com
PREDICTIVE TOOL FOR PRETERM BIRTH RISK

Sera Prognostics announced that its PreTRM® Test is the first and only clinically validated blood test to predict preterm birth risk in asymptomatic, singleton pregnancies.
Premature birth, defined as birth before 37 weeks, is the leading cause of death and illness in newborns and is associated with an increased risk of major long-term complications. Previously, the 2 best traditional predictors of premature birth were prior preterm birth history and short cervical length, but these identify only a small percentage of women who deliver early, asserts Sera Prognostics. Implemented during gestational weeks 19 and 20, the PreTRM test uses proteomic technology to measure and analyze 2 proteins in the blood that are highly predictive of preterm birth: IBP4, insulin-like growth factor binding protein 4, and SHBG, sex-hormone binding globulin.
According to Sera Prognostics, data from the 5,501-patient Proteomic Assessment of Preterm Risk (PAPR) study, recently published in American Journal of Obstetrics & Gynecology, confirm that the test can help identify a high percentage of women who are at increased risk early in pregnancy before symptoms occur.
FOR MORE INFORMATION, VISIT: www.pretrm.com
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
DISPOSABLE HANDHELD RESECTOR

Distal Access offers nationwide availability of the RESECTR™ 9 French / 3.0 mm High- Performance Disposable Tissue Resector, a disposable, nonpowered, handheld, and hand-driven system designed to combine the benefits of basic manual devices and complex electro-mechanical systems.
The RESECTR platform is “ready-to-use,” says Distal Access, giving clinicians an important tool to see-and-treat lesions in the hospital, clinic, or office. Starting at the cutting tip, aspiration pulls tissue samples into the cutting window where oscillating blades are controlled by the clinician’s index finger and hand. Clinicians can increase or decrease oscillation and cutting based on what they see and feel during the procedure.
According to Distal Access, for small tissue samples, resection time with the RESECTR can be similar to that with electromechanical devices, with a significantly lower cost. The RESECTR is compatible with available fluid management systems and endoscopic devices.
FOR MORE INFORMATION, VISIT: www.resectr.com
PREDICTIVE TOOL FOR PRETERM BIRTH RISK

Sera Prognostics announced that its PreTRM® Test is the first and only clinically validated blood test to predict preterm birth risk in asymptomatic, singleton pregnancies.
Premature birth, defined as birth before 37 weeks, is the leading cause of death and illness in newborns and is associated with an increased risk of major long-term complications. Previously, the 2 best traditional predictors of premature birth were prior preterm birth history and short cervical length, but these identify only a small percentage of women who deliver early, asserts Sera Prognostics. Implemented during gestational weeks 19 and 20, the PreTRM test uses proteomic technology to measure and analyze 2 proteins in the blood that are highly predictive of preterm birth: IBP4, insulin-like growth factor binding protein 4, and SHBG, sex-hormone binding globulin.
According to Sera Prognostics, data from the 5,501-patient Proteomic Assessment of Preterm Risk (PAPR) study, recently published in American Journal of Obstetrics & Gynecology, confirm that the test can help identify a high percentage of women who are at increased risk early in pregnancy before symptoms occur.
FOR MORE INFORMATION, VISIT: www.pretrm.com
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
DISPOSABLE HANDHELD RESECTOR

Distal Access offers nationwide availability of the RESECTR™ 9 French / 3.0 mm High- Performance Disposable Tissue Resector, a disposable, nonpowered, handheld, and hand-driven system designed to combine the benefits of basic manual devices and complex electro-mechanical systems.
The RESECTR platform is “ready-to-use,” says Distal Access, giving clinicians an important tool to see-and-treat lesions in the hospital, clinic, or office. Starting at the cutting tip, aspiration pulls tissue samples into the cutting window where oscillating blades are controlled by the clinician’s index finger and hand. Clinicians can increase or decrease oscillation and cutting based on what they see and feel during the procedure.
According to Distal Access, for small tissue samples, resection time with the RESECTR can be similar to that with electromechanical devices, with a significantly lower cost. The RESECTR is compatible with available fluid management systems and endoscopic devices.
FOR MORE INFORMATION, VISIT: www.resectr.com
PREDICTIVE TOOL FOR PRETERM BIRTH RISK

Sera Prognostics announced that its PreTRM® Test is the first and only clinically validated blood test to predict preterm birth risk in asymptomatic, singleton pregnancies.
Premature birth, defined as birth before 37 weeks, is the leading cause of death and illness in newborns and is associated with an increased risk of major long-term complications. Previously, the 2 best traditional predictors of premature birth were prior preterm birth history and short cervical length, but these identify only a small percentage of women who deliver early, asserts Sera Prognostics. Implemented during gestational weeks 19 and 20, the PreTRM test uses proteomic technology to measure and analyze 2 proteins in the blood that are highly predictive of preterm birth: IBP4, insulin-like growth factor binding protein 4, and SHBG, sex-hormone binding globulin.
According to Sera Prognostics, data from the 5,501-patient Proteomic Assessment of Preterm Risk (PAPR) study, recently published in American Journal of Obstetrics & Gynecology, confirm that the test can help identify a high percentage of women who are at increased risk early in pregnancy before symptoms occur.
FOR MORE INFORMATION, VISIT: www.pretrm.com
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
VIDEO: Telecardiology improves chronic care management, reduces cost
MINNEAPOLIS – The use of telecardiology can vastly improve outcomes, reduce hospitalizations, and lower health care costs, explained Michael Shen, MD, a cardiologist and chief medical officer at Duxlink Health in Sunrise, Fla.
Dr. Shen recently spoke at the American Telemedicine Association annual meeting about the impact of telecardiology on the practice of cardiology.
“This is at the very beginning of the technology, and it will be very good for cardiologists to be early adopters – to be the early users – so they can engage the technology as leaders, rather than followers,” explained Dr. Shen.
In a video interview at the meeting, he discussed how telecardiology has advanced over the years and how the technology can improve chronic care management. Dr. Shen also shared details about a telecardiology program implemented in his practice, and he discussed how the program has affected patient care and hospital readmissions.
Dr. Shen had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @legal_med
MINNEAPOLIS – The use of telecardiology can vastly improve outcomes, reduce hospitalizations, and lower health care costs, explained Michael Shen, MD, a cardiologist and chief medical officer at Duxlink Health in Sunrise, Fla.
Dr. Shen recently spoke at the American Telemedicine Association annual meeting about the impact of telecardiology on the practice of cardiology.
“This is at the very beginning of the technology, and it will be very good for cardiologists to be early adopters – to be the early users – so they can engage the technology as leaders, rather than followers,” explained Dr. Shen.
In a video interview at the meeting, he discussed how telecardiology has advanced over the years and how the technology can improve chronic care management. Dr. Shen also shared details about a telecardiology program implemented in his practice, and he discussed how the program has affected patient care and hospital readmissions.
Dr. Shen had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @legal_med
MINNEAPOLIS – The use of telecardiology can vastly improve outcomes, reduce hospitalizations, and lower health care costs, explained Michael Shen, MD, a cardiologist and chief medical officer at Duxlink Health in Sunrise, Fla.
Dr. Shen recently spoke at the American Telemedicine Association annual meeting about the impact of telecardiology on the practice of cardiology.
“This is at the very beginning of the technology, and it will be very good for cardiologists to be early adopters – to be the early users – so they can engage the technology as leaders, rather than followers,” explained Dr. Shen.
In a video interview at the meeting, he discussed how telecardiology has advanced over the years and how the technology can improve chronic care management. Dr. Shen also shared details about a telecardiology program implemented in his practice, and he discussed how the program has affected patient care and hospital readmissions.
Dr. Shen had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @legal_med
Simple colon surgery bundle accelerated outcomes improvement
SAN DIEGO – Implementation of a simple colon bundle decreased the rate of colonic and enteric resections faster, compared with improvements seen for other procedures, according to a study that involved 23 hospitals in Tennessee.
At the American College of Surgeons/National Surgical Quality Improvement Program National Conference, Brian J. Daley, MD, discussed findings from an analysis conducted by members of the Tennessee Surgical Quality Collaborative (TSQC), which he described as “a collection of surgeons who put aside their hospital and regional affiliations to work together to help each other and to help our fellow Tennesseans.” Established in 2008 with member hospitals, the TSQC has grown to 23 member hospitals, including 18 community hospitals and 5 academic medical centers. It provides data on nearly 600 surgeons across the state. “While this only represents about half of the surgical procedures in the state, there is sufficient statistical power to make comments about our surgical performance,” said Dr. Daley of the department of surgery at the University of Tennessee Medical Center, Knoxville.
To quantify TSQC’s impact on surgical outcomes, surgeons at the member hospitals evaluated the TSQC colon bundle, which was developed in 2012 and implemented in 2013. It bundles four processes of care: maintaining intraoperative oxygen delivery, maintaining a temperature of 36° C, making sure the patient’s blood glucose is normal, and choosing the appropriate antibiotics. “We kept it simple: easy, not expensive, and hopefully helpful,” Dr. Daley said.
With other procedures as a baseline, they used statistical analyses to determine if implementation of the bundle led to an incremental acceleration of reduced complications, compared with other improvements observed in other procedures. “To understand our outcomes, we needed to prove three points: that the trend improved [a negative trend in the resection rate], that this negative trend was more negative than trends for other comparator procedures, and that the trend for intercept was not equal to the comparator in any way,” Dr. Daley explained.
Following adoption of the bundle, he and his associates observed that the rate of decrease in postoperative recurrences was greater in colectomy, compared with that for all other surgical procedures (P less than .001 for both the trend and the intercept statistical models). Adoption of the bundle also positively impacted decreases in postoperative recurrences among enterectomy cases (P less than .001 for both the trend and the intercept statistical models).
“We were able to demonstrate that our TSQC bundle paid dividends in improving colectomy outcomes,” Dr. Daley concluded. “We have seen these efforts spill over into enterectomy. From this we can also infer that participation in the collaborative improves outcomes and is imperative to maintain the acceleration in surgical improvement.”
Dr. Daley reported that he and his coauthors had no relevant financial disclosures.
SAN DIEGO – Implementation of a simple colon bundle decreased the rate of colonic and enteric resections faster, compared with improvements seen for other procedures, according to a study that involved 23 hospitals in Tennessee.
At the American College of Surgeons/National Surgical Quality Improvement Program National Conference, Brian J. Daley, MD, discussed findings from an analysis conducted by members of the Tennessee Surgical Quality Collaborative (TSQC), which he described as “a collection of surgeons who put aside their hospital and regional affiliations to work together to help each other and to help our fellow Tennesseans.” Established in 2008 with member hospitals, the TSQC has grown to 23 member hospitals, including 18 community hospitals and 5 academic medical centers. It provides data on nearly 600 surgeons across the state. “While this only represents about half of the surgical procedures in the state, there is sufficient statistical power to make comments about our surgical performance,” said Dr. Daley of the department of surgery at the University of Tennessee Medical Center, Knoxville.
To quantify TSQC’s impact on surgical outcomes, surgeons at the member hospitals evaluated the TSQC colon bundle, which was developed in 2012 and implemented in 2013. It bundles four processes of care: maintaining intraoperative oxygen delivery, maintaining a temperature of 36° C, making sure the patient’s blood glucose is normal, and choosing the appropriate antibiotics. “We kept it simple: easy, not expensive, and hopefully helpful,” Dr. Daley said.
With other procedures as a baseline, they used statistical analyses to determine if implementation of the bundle led to an incremental acceleration of reduced complications, compared with other improvements observed in other procedures. “To understand our outcomes, we needed to prove three points: that the trend improved [a negative trend in the resection rate], that this negative trend was more negative than trends for other comparator procedures, and that the trend for intercept was not equal to the comparator in any way,” Dr. Daley explained.
Following adoption of the bundle, he and his associates observed that the rate of decrease in postoperative recurrences was greater in colectomy, compared with that for all other surgical procedures (P less than .001 for both the trend and the intercept statistical models). Adoption of the bundle also positively impacted decreases in postoperative recurrences among enterectomy cases (P less than .001 for both the trend and the intercept statistical models).
“We were able to demonstrate that our TSQC bundle paid dividends in improving colectomy outcomes,” Dr. Daley concluded. “We have seen these efforts spill over into enterectomy. From this we can also infer that participation in the collaborative improves outcomes and is imperative to maintain the acceleration in surgical improvement.”
Dr. Daley reported that he and his coauthors had no relevant financial disclosures.
SAN DIEGO – Implementation of a simple colon bundle decreased the rate of colonic and enteric resections faster, compared with improvements seen for other procedures, according to a study that involved 23 hospitals in Tennessee.
At the American College of Surgeons/National Surgical Quality Improvement Program National Conference, Brian J. Daley, MD, discussed findings from an analysis conducted by members of the Tennessee Surgical Quality Collaborative (TSQC), which he described as “a collection of surgeons who put aside their hospital and regional affiliations to work together to help each other and to help our fellow Tennesseans.” Established in 2008 with member hospitals, the TSQC has grown to 23 member hospitals, including 18 community hospitals and 5 academic medical centers. It provides data on nearly 600 surgeons across the state. “While this only represents about half of the surgical procedures in the state, there is sufficient statistical power to make comments about our surgical performance,” said Dr. Daley of the department of surgery at the University of Tennessee Medical Center, Knoxville.
To quantify TSQC’s impact on surgical outcomes, surgeons at the member hospitals evaluated the TSQC colon bundle, which was developed in 2012 and implemented in 2013. It bundles four processes of care: maintaining intraoperative oxygen delivery, maintaining a temperature of 36° C, making sure the patient’s blood glucose is normal, and choosing the appropriate antibiotics. “We kept it simple: easy, not expensive, and hopefully helpful,” Dr. Daley said.
With other procedures as a baseline, they used statistical analyses to determine if implementation of the bundle led to an incremental acceleration of reduced complications, compared with other improvements observed in other procedures. “To understand our outcomes, we needed to prove three points: that the trend improved [a negative trend in the resection rate], that this negative trend was more negative than trends for other comparator procedures, and that the trend for intercept was not equal to the comparator in any way,” Dr. Daley explained.
Following adoption of the bundle, he and his associates observed that the rate of decrease in postoperative recurrences was greater in colectomy, compared with that for all other surgical procedures (P less than .001 for both the trend and the intercept statistical models). Adoption of the bundle also positively impacted decreases in postoperative recurrences among enterectomy cases (P less than .001 for both the trend and the intercept statistical models).
“We were able to demonstrate that our TSQC bundle paid dividends in improving colectomy outcomes,” Dr. Daley concluded. “We have seen these efforts spill over into enterectomy. From this we can also infer that participation in the collaborative improves outcomes and is imperative to maintain the acceleration in surgical improvement.”
Dr. Daley reported that he and his coauthors had no relevant financial disclosures.
AT THE ACS NSQIP NATIONAL CONFERENCE
Key clinical point: Adoption of a colon bundle by a collaborative of Tennessee hospitals improved certain colectomy outcomes.
Major finding: Following adoption of a colon bundle, the rate of decrease in postoperative recurrences was greater in colectomy than for all other surgical procedures (P less than .001 for both the trend and the intercept statistical models).
Data source: An analysis conducted by members of the Tennessee Surgical Quality Collaborative, which included 23 hospitals in the state.
Disclosures: The researchers reported having no relevant financial disclosures.