User login
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 65‐year‐old immunocompetent man with a history of obesity, diabetes, and chronic lower extremity edema presents to the emergency room with a 1‐day history of right lower extremity pain and increased swelling. He reports no antecedent trauma and states he just noticed the symptoms that morning. On examination, he appears generally well. His temperature is 100F, pulse 92 beats per minute, blood pressure 120/60 mm Hg, and respiratory rate 16 breaths per minute. The rest of the exam is notable for right lower extremity erythema and swelling extending from his right shin to his right medial thigh without associated fluctuance or drainage. Labs reveal a mildly elevated white blood cell count of 13,000/L and normal serum creatinine. Are broad‐spectrum antibiotics like vancomycin and piperacillin/tazobactam the preferred regimen?
BACKGROUND
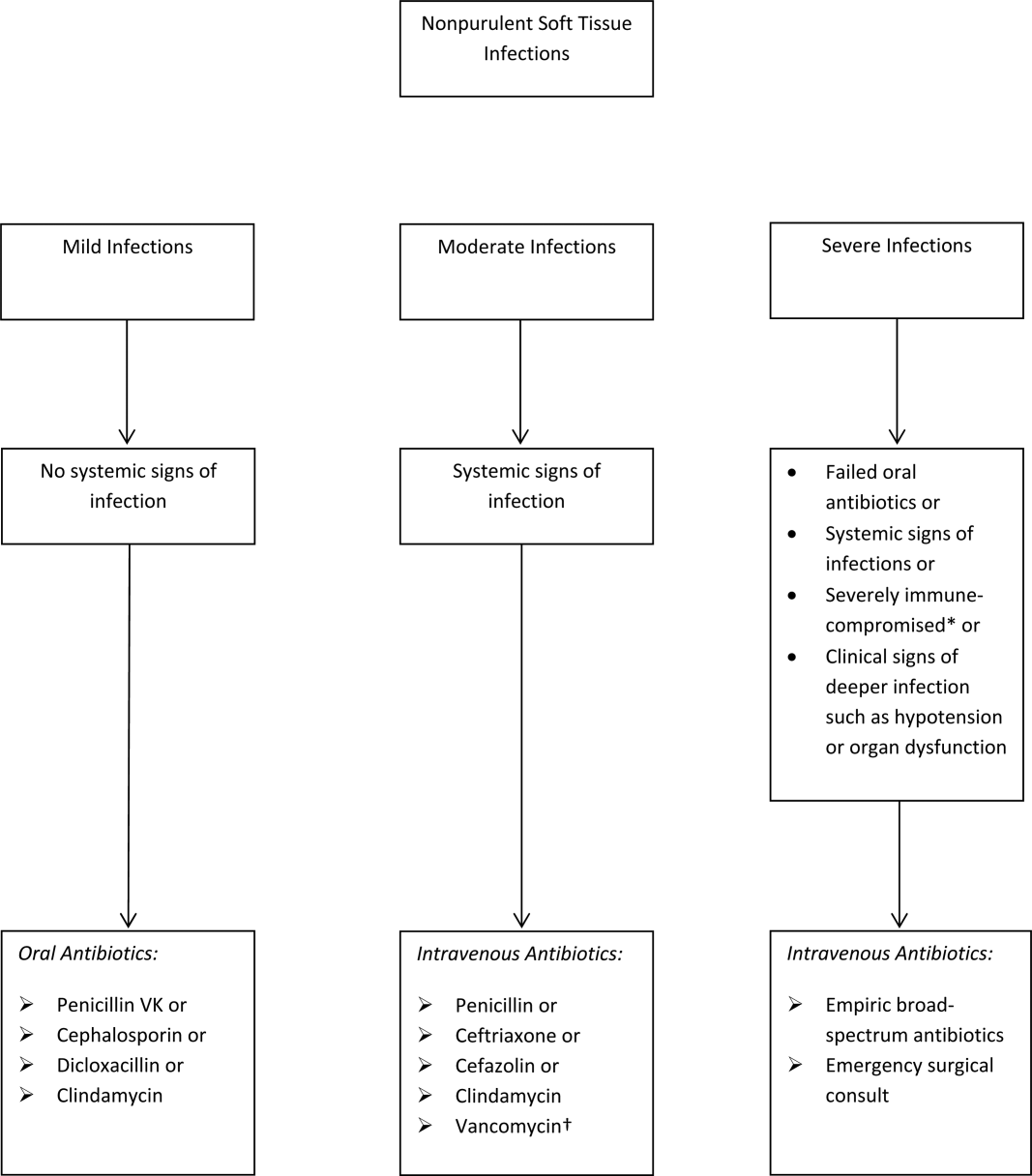
The term skin and soft tissue infection (SSTI) includes a heterogeneous group of infections including cellulitis, cutaneous abscess, diabetic foot infections, surgical site infections, and necrotizing soft tissue infections. As a group, SSTIs are the second most common type of infection in hospitalized adults in the United States behind pneumonia and result in more than 600,000 admissions per year.[1] The current guideline on SSTIs by the Infectious Disease Society of America (IDSA) makes the distinction between purulent and nonpurulent soft tissue infections based on the presence or absence of purulent drainage or abscess and between mild, moderate, and severe infections based on the presence and severity of systemic signs of infection.[2] Figure 1 provides an overview of the IDSA recommendations.
THE PROBLEM: OVERUSE OF BROAD‐SPECTRUM ANTIBIOTICS
Studies over the past decade have shown that the majority of patients hospitalized with SSTI receive broad‐spectrum antibiotics, usually with combinations of antibiotics active against gram‐positive (including methicillin‐resistant Staphylococcus aureus [MRSA]), gram‐negative (often including Pseudomonas aeruginosa), and anaerobic organisms. Broad‐spectrum treatment occurs despite guidelines from the IDSA, which state that the most common pathogens for nonpurulent cellulitis are ‐hemolytic streptococci, which remain susceptible to penicillin.[2, 3] One multicenter study of hospitalized adults with nonpurulent cellulitis, for example, reported that 85% of patients received therapy effective against MRSA (primarily vancomycin), 61% received broad gram‐negative coverage (primarily ‐lactam with ‐lactamase inhibitor), and 74% received anaerobic coverage.[4] Another multicenter study reported that the most common antibiotics given for cellulitis (excluding cases associated with cutaneous abscess) were vancomycin (60%), ‐lactam/‐lactamase combinations (32%), and clindamycin (19%). Only 13% of patients with cellulitis were treated with cefazolin, and only 1.1% of patients were treated with nafcillin or oxacillin.[5] According to the Centers for Disease Control and Prevention, unnecessary antibiotic use is associated with increased cost, development of antibiotic resistance, and increased rates of Clostridium difficile.[6]
The current use of broad‐spectrum antibiotics for nonpurulent cellulitis is likely due to several factors, including the emergence of community‐associated (CA)‐MRSA, confusion due to the heterogeneity of SSTI, and the limited data regarding the microbiology of nonpurulent cellulitis. The resulting uncertainty about cellulitis has been termed an existential crisis for the treating physician and is likely the single biggest factor behind the out‐of‐control prescribing.[7]
The Emergence of CA‐MRSA
Over the past decade, numerous studies have reported the increasing frequency of CA‐MRSA soft tissue infections, predominantly with the pulsed‐field gel electrophoresis type USA‐300. Originally, MRSA infections were limited to nosocomial infections. Subsequent multicenter studies from the United States have shown that CA‐MRSA is the most frequent pathogen isolated from purulent soft tissue infections presenting to emergency rooms[8] and the most frequent pathogen isolated from SSTI specimens in labs.[9] Many authors have therefore concluded that empiric antibiotics for SSTI should include coverage for MRSA.[8, 9]
Heterogeneity of SSTI
As already discussed, the term SSTI is an umbrella term that encompasses several types of clinically distinct infections. The only commonality between the SSTI is that that they all involve the skin and soft tissues in some way. Diabetic foot infections, cutaneous abscesses, surgical site infections, and nonpurulent cellulitis have different hosts, pathophysiology, clinical presentations, and microbiology. At one end of the spectrum is the cutaneous abscess, which is readily culturable through incision and drainage. At the other end of the spectrum is cellulitis, which is typically nonculturable. Unfortunately, studies of SSTI tend to lump all of these entities together when reporting microbiology. The landmark study by Moran et al., for example, described the microbiology of purulent soft tissue infections presenting to a network of emergency rooms across the county. Although all patients had by definition purulent infections, and 81% were abscesses, the authors made broad conclusions about skin and soft tissue infections in general and recommended antimicrobials effective against MRSA for empiric coverage for SSTIs.[8]
Uncertainty About the Microbiology of Nonpurulent Cellulitis
What then is the microbiology of nonpurulent cellulitis? As stated in the 2005 and 2014 IDSA guidelines, traditional teaching remains that nonpurulent cellulitis is primarily due to ‐hemolytic streptococci.[2, 3] Studies using needle aspiration have yielded conflicting results, although a systematic review of these studies concluded that S aureus was the most common pathogen.[10] On the other hand, a systematic review of positive blood cultures of patients identified as having cellulitis found that 61% were due to ‐hemolytic streptococci, and only 15% were due to S aureus.[11] Both reviews, however, comment on the limited quality of the included studies. Ultimately, because nonpurulent soft tissue infections are basically nonculturable, their true microbiologic etiology remains uncertain. Given this uncertainty, as well as the impressive evidence for CA‐MRSA causing cutaneous abscesses, along with the confusion about types of SSTI, it is not surprising that front‐line clinicians have resorted to prescribing broad‐spectrum antibiotics.
THE SOLUTION: NARROW‐SPECTRUM ANTIBIOTICS FOR MOST
Although studies of the microbiology of cellulitis remain inconclusive, several recent clinical trials have indicated that treatment with antimicrobials limited to ‐hemolytic streptococci and methicillin‐susceptible S aureus (MSSA) are as effective as antimicrobials against MRSA. A prospective study from 2010 of consecutive hospitalized adults with nonpurulent cellulitis found that 73% had serologic evidence for streptococcal infection, and overall 95.8% responded to cefazolin monotherapy.[12] More recently, a study of emergency room patients with nonpurulent cellulitis randomized patients to cephalexin alone or cephalexin plus trimethoprim‐sulfamethoxazole. These authors found no difference in response rates and concluded that the addition of anti‐MRSA therapy (trimethoprim‐sulfamethoxazole, in this study) for uncomplicated cellulitis was unnecessary.[13] This later study is the only randomized controlled study to assess the need for MRSA coverage for cellulitis, and the answer for outpatients, at least, is that MRSA coverage is unnecessary. Both of these studies are cited by the IDSA guideline from 2014, which recommends antibiotics for mild‐moderate cellulitis to be limited to antimicrobials effective against ‐hemolytic streptococci and MSSA. The guideline specifically does not recommend routinely treating for MRSA, gram‐negative, or anaerobic organisms citing lack of benefit as well as risks of antibiotic resistance and C difficile infection. A recent study from the University of Utah reported the development of a cellulitis order set, which included a pathway for nonpurulent cellulitis based on the use of cefazolin. These authors reported that the use of the pathway was associated with a 59% decrease in the use of broad‐spectrum antibiotics, a 23% decrease in pharmacy costs, a 13% decrease in total facility cost, with no change in hospital length of stay or readmission rate.[14] One important caveat to the use of clinical pathways is that they are often underused. In the study from the University of Utah, for example, only 55% of eligible patients had the clinical pathway ordered.
WHEN BROAD‐SPECTRUM ANTIBIOTICS ARE RECOMMENDED
The IDSA does recommend empiric broad‐spectrum antibiotics with combination gram‐positive and gram‐negative coverage in several situations, including severe infections in which necrotizing soft tissue infection is suspected, animal bites, immersion injuries, as well as for severely immunocompromised patients or those who have failed limited spectrum antibiotics. Additionally, the IDSA recommends antimicrobials effective against MRSA for purulent infections with systemic signs of inflammation as well as severe nonpurulent infections or those associated with penetrating trauma, injection drug use, and nasal colonization with MRSA (Figure 1).
RECOMMENDATIONS
Our patient has no associated purulence and no abscess and therefore has nonpurulent cellulitis. Based on his mild tachycardia and leukocytosis but intact immune system and lack of suspicion for necrotizing soft tissue infection, he would be classified as moderate‐severity cellulitis by the IDSA. In patients hospitalized with nonpurulent cellulitis who are not severely immunocompromised or severely ill and for whom necrotizing soft tissue infection is not suspected:
- Antibiotics should be directed at ‐hemolytic streptococci and MSSA, with 1 of the suggested antibiotics by the IDSA including penicillin, ceftriaxone, cefazolin, or clindamycin.
- Antibiotics effective against MRSA should be limited to situations described by the IDSA.
- If the cellulitis has not improved within 48 hours, then consider broader‐spectrum antibiotics.
- Hospitals should strongly consider implementation of a cellulitis pathway based on the IDSA recommendations to improve antibiotic stewardship as well as costs.
Disclosure
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
- , , . Most frequent conditions in U.S. hospitals, 2011. HCUP statistical brief #162. Healthcare Cost and Utilization Project statistical briefs. Rockville, MD: Agency for Health Care Policy and Research; 2013.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft‐tissue infections. Clin Infect Dis. 2005;41(10):1373–1406.
- , , , , , . Skin and soft‐tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis. 2010;51(8):895–903.
- , , , , , . A prospective, multicenter, observational study of complicated skin and soft tissue infections in hospitalized patients: clinical characteristics, medical treatment, and outcomes. BMC Infect Dis. 2012;12:227.
- Centers for Disease Control and Prevention. Overview and evidence to support stewardship. Available at: http://www.cdc.gov/getsmart/healthcare/evidence.html. Accessed March 2, 2016.
- . Cellulitis, by any other name. Clin Infect Dis. 2013;56(12):1763–1764.
- , , , et al. Methicillin‐resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666–674.
- , , , , , . Emergence of community‐acquired methicillin‐resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft‐tissue infections. Ann Intern Med. 2006;144(5):309–317.
- , . Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138(3):313–317.
- , . A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64(2):148–155.
- , , , . The role of beta‐hemolytic streptococci in causing diffuse, nonculturable cellulitis: a prospective investigation. Medicine (Baltimore). 2010;89(4):217–226.
- , , , et al. Clinical trial: comparative effectiveness of cephalexin plus trimethoprim‐sulfamethoxazole versus cephalexin alone for treatment of uncomplicated cellulitis: a randomized controlled trial. Clin Infect Dis. 2013;56(12):1754–1762.
- , , , , . Evidence‐based care pathway for cellulitis improves process, clinical, and cost outcomes. J Hosp Med. 2015;10:780–786.
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 65‐year‐old immunocompetent man with a history of obesity, diabetes, and chronic lower extremity edema presents to the emergency room with a 1‐day history of right lower extremity pain and increased swelling. He reports no antecedent trauma and states he just noticed the symptoms that morning. On examination, he appears generally well. His temperature is 100F, pulse 92 beats per minute, blood pressure 120/60 mm Hg, and respiratory rate 16 breaths per minute. The rest of the exam is notable for right lower extremity erythema and swelling extending from his right shin to his right medial thigh without associated fluctuance or drainage. Labs reveal a mildly elevated white blood cell count of 13,000/L and normal serum creatinine. Are broad‐spectrum antibiotics like vancomycin and piperacillin/tazobactam the preferred regimen?
BACKGROUND
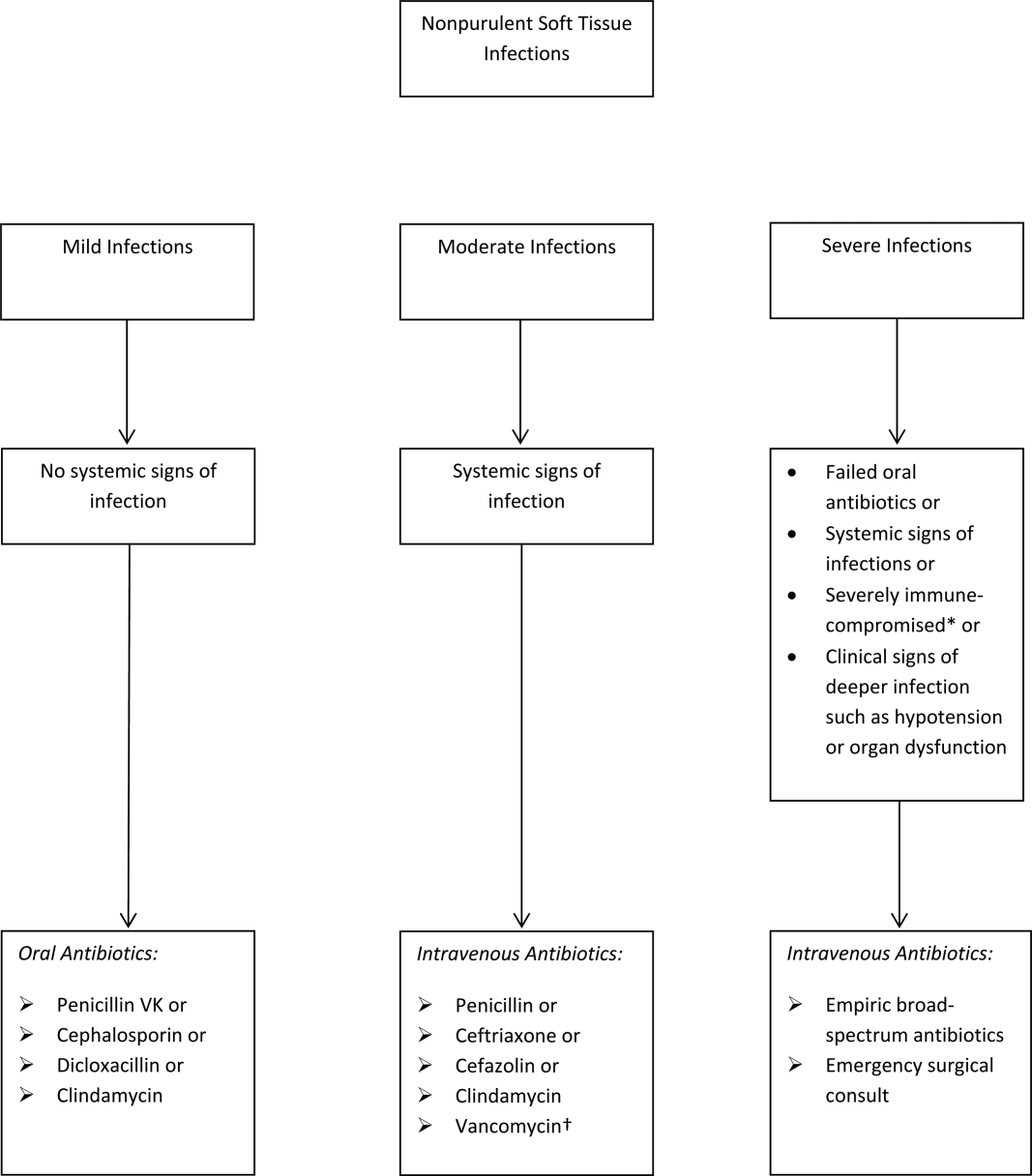
The term skin and soft tissue infection (SSTI) includes a heterogeneous group of infections including cellulitis, cutaneous abscess, diabetic foot infections, surgical site infections, and necrotizing soft tissue infections. As a group, SSTIs are the second most common type of infection in hospitalized adults in the United States behind pneumonia and result in more than 600,000 admissions per year.[1] The current guideline on SSTIs by the Infectious Disease Society of America (IDSA) makes the distinction between purulent and nonpurulent soft tissue infections based on the presence or absence of purulent drainage or abscess and between mild, moderate, and severe infections based on the presence and severity of systemic signs of infection.[2] Figure 1 provides an overview of the IDSA recommendations.

THE PROBLEM: OVERUSE OF BROAD‐SPECTRUM ANTIBIOTICS
Studies over the past decade have shown that the majority of patients hospitalized with SSTI receive broad‐spectrum antibiotics, usually with combinations of antibiotics active against gram‐positive (including methicillin‐resistant Staphylococcus aureus [MRSA]), gram‐negative (often including Pseudomonas aeruginosa), and anaerobic organisms. Broad‐spectrum treatment occurs despite guidelines from the IDSA, which state that the most common pathogens for nonpurulent cellulitis are ‐hemolytic streptococci, which remain susceptible to penicillin.[2, 3] One multicenter study of hospitalized adults with nonpurulent cellulitis, for example, reported that 85% of patients received therapy effective against MRSA (primarily vancomycin), 61% received broad gram‐negative coverage (primarily ‐lactam with ‐lactamase inhibitor), and 74% received anaerobic coverage.[4] Another multicenter study reported that the most common antibiotics given for cellulitis (excluding cases associated with cutaneous abscess) were vancomycin (60%), ‐lactam/‐lactamase combinations (32%), and clindamycin (19%). Only 13% of patients with cellulitis were treated with cefazolin, and only 1.1% of patients were treated with nafcillin or oxacillin.[5] According to the Centers for Disease Control and Prevention, unnecessary antibiotic use is associated with increased cost, development of antibiotic resistance, and increased rates of Clostridium difficile.[6]
The current use of broad‐spectrum antibiotics for nonpurulent cellulitis is likely due to several factors, including the emergence of community‐associated (CA)‐MRSA, confusion due to the heterogeneity of SSTI, and the limited data regarding the microbiology of nonpurulent cellulitis. The resulting uncertainty about cellulitis has been termed an existential crisis for the treating physician and is likely the single biggest factor behind the out‐of‐control prescribing.[7]
The Emergence of CA‐MRSA
Over the past decade, numerous studies have reported the increasing frequency of CA‐MRSA soft tissue infections, predominantly with the pulsed‐field gel electrophoresis type USA‐300. Originally, MRSA infections were limited to nosocomial infections. Subsequent multicenter studies from the United States have shown that CA‐MRSA is the most frequent pathogen isolated from purulent soft tissue infections presenting to emergency rooms[8] and the most frequent pathogen isolated from SSTI specimens in labs.[9] Many authors have therefore concluded that empiric antibiotics for SSTI should include coverage for MRSA.[8, 9]
Heterogeneity of SSTI
As already discussed, the term SSTI is an umbrella term that encompasses several types of clinically distinct infections. The only commonality between the SSTI is that that they all involve the skin and soft tissues in some way. Diabetic foot infections, cutaneous abscesses, surgical site infections, and nonpurulent cellulitis have different hosts, pathophysiology, clinical presentations, and microbiology. At one end of the spectrum is the cutaneous abscess, which is readily culturable through incision and drainage. At the other end of the spectrum is cellulitis, which is typically nonculturable. Unfortunately, studies of SSTI tend to lump all of these entities together when reporting microbiology. The landmark study by Moran et al., for example, described the microbiology of purulent soft tissue infections presenting to a network of emergency rooms across the county. Although all patients had by definition purulent infections, and 81% were abscesses, the authors made broad conclusions about skin and soft tissue infections in general and recommended antimicrobials effective against MRSA for empiric coverage for SSTIs.[8]
Uncertainty About the Microbiology of Nonpurulent Cellulitis
What then is the microbiology of nonpurulent cellulitis? As stated in the 2005 and 2014 IDSA guidelines, traditional teaching remains that nonpurulent cellulitis is primarily due to ‐hemolytic streptococci.[2, 3] Studies using needle aspiration have yielded conflicting results, although a systematic review of these studies concluded that S aureus was the most common pathogen.[10] On the other hand, a systematic review of positive blood cultures of patients identified as having cellulitis found that 61% were due to ‐hemolytic streptococci, and only 15% were due to S aureus.[11] Both reviews, however, comment on the limited quality of the included studies. Ultimately, because nonpurulent soft tissue infections are basically nonculturable, their true microbiologic etiology remains uncertain. Given this uncertainty, as well as the impressive evidence for CA‐MRSA causing cutaneous abscesses, along with the confusion about types of SSTI, it is not surprising that front‐line clinicians have resorted to prescribing broad‐spectrum antibiotics.
THE SOLUTION: NARROW‐SPECTRUM ANTIBIOTICS FOR MOST
Although studies of the microbiology of cellulitis remain inconclusive, several recent clinical trials have indicated that treatment with antimicrobials limited to ‐hemolytic streptococci and methicillin‐susceptible S aureus (MSSA) are as effective as antimicrobials against MRSA. A prospective study from 2010 of consecutive hospitalized adults with nonpurulent cellulitis found that 73% had serologic evidence for streptococcal infection, and overall 95.8% responded to cefazolin monotherapy.[12] More recently, a study of emergency room patients with nonpurulent cellulitis randomized patients to cephalexin alone or cephalexin plus trimethoprim‐sulfamethoxazole. These authors found no difference in response rates and concluded that the addition of anti‐MRSA therapy (trimethoprim‐sulfamethoxazole, in this study) for uncomplicated cellulitis was unnecessary.[13] This later study is the only randomized controlled study to assess the need for MRSA coverage for cellulitis, and the answer for outpatients, at least, is that MRSA coverage is unnecessary. Both of these studies are cited by the IDSA guideline from 2014, which recommends antibiotics for mild‐moderate cellulitis to be limited to antimicrobials effective against ‐hemolytic streptococci and MSSA. The guideline specifically does not recommend routinely treating for MRSA, gram‐negative, or anaerobic organisms citing lack of benefit as well as risks of antibiotic resistance and C difficile infection. A recent study from the University of Utah reported the development of a cellulitis order set, which included a pathway for nonpurulent cellulitis based on the use of cefazolin. These authors reported that the use of the pathway was associated with a 59% decrease in the use of broad‐spectrum antibiotics, a 23% decrease in pharmacy costs, a 13% decrease in total facility cost, with no change in hospital length of stay or readmission rate.[14] One important caveat to the use of clinical pathways is that they are often underused. In the study from the University of Utah, for example, only 55% of eligible patients had the clinical pathway ordered.
WHEN BROAD‐SPECTRUM ANTIBIOTICS ARE RECOMMENDED
The IDSA does recommend empiric broad‐spectrum antibiotics with combination gram‐positive and gram‐negative coverage in several situations, including severe infections in which necrotizing soft tissue infection is suspected, animal bites, immersion injuries, as well as for severely immunocompromised patients or those who have failed limited spectrum antibiotics. Additionally, the IDSA recommends antimicrobials effective against MRSA for purulent infections with systemic signs of inflammation as well as severe nonpurulent infections or those associated with penetrating trauma, injection drug use, and nasal colonization with MRSA (Figure 1).
RECOMMENDATIONS
Our patient has no associated purulence and no abscess and therefore has nonpurulent cellulitis. Based on his mild tachycardia and leukocytosis but intact immune system and lack of suspicion for necrotizing soft tissue infection, he would be classified as moderate‐severity cellulitis by the IDSA. In patients hospitalized with nonpurulent cellulitis who are not severely immunocompromised or severely ill and for whom necrotizing soft tissue infection is not suspected:
- Antibiotics should be directed at ‐hemolytic streptococci and MSSA, with 1 of the suggested antibiotics by the IDSA including penicillin, ceftriaxone, cefazolin, or clindamycin.
- Antibiotics effective against MRSA should be limited to situations described by the IDSA.
- If the cellulitis has not improved within 48 hours, then consider broader‐spectrum antibiotics.
- Hospitals should strongly consider implementation of a cellulitis pathway based on the IDSA recommendations to improve antibiotic stewardship as well as costs.
Disclosure
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 65‐year‐old immunocompetent man with a history of obesity, diabetes, and chronic lower extremity edema presents to the emergency room with a 1‐day history of right lower extremity pain and increased swelling. He reports no antecedent trauma and states he just noticed the symptoms that morning. On examination, he appears generally well. His temperature is 100F, pulse 92 beats per minute, blood pressure 120/60 mm Hg, and respiratory rate 16 breaths per minute. The rest of the exam is notable for right lower extremity erythema and swelling extending from his right shin to his right medial thigh without associated fluctuance or drainage. Labs reveal a mildly elevated white blood cell count of 13,000/L and normal serum creatinine. Are broad‐spectrum antibiotics like vancomycin and piperacillin/tazobactam the preferred regimen?
BACKGROUND
The term skin and soft tissue infection (SSTI) includes a heterogeneous group of infections including cellulitis, cutaneous abscess, diabetic foot infections, surgical site infections, and necrotizing soft tissue infections. As a group, SSTIs are the second most common type of infection in hospitalized adults in the United States behind pneumonia and result in more than 600,000 admissions per year.[1] The current guideline on SSTIs by the Infectious Disease Society of America (IDSA) makes the distinction between purulent and nonpurulent soft tissue infections based on the presence or absence of purulent drainage or abscess and between mild, moderate, and severe infections based on the presence and severity of systemic signs of infection.[2] Figure 1 provides an overview of the IDSA recommendations.

THE PROBLEM: OVERUSE OF BROAD‐SPECTRUM ANTIBIOTICS
Studies over the past decade have shown that the majority of patients hospitalized with SSTI receive broad‐spectrum antibiotics, usually with combinations of antibiotics active against gram‐positive (including methicillin‐resistant Staphylococcus aureus [MRSA]), gram‐negative (often including Pseudomonas aeruginosa), and anaerobic organisms. Broad‐spectrum treatment occurs despite guidelines from the IDSA, which state that the most common pathogens for nonpurulent cellulitis are ‐hemolytic streptococci, which remain susceptible to penicillin.[2, 3] One multicenter study of hospitalized adults with nonpurulent cellulitis, for example, reported that 85% of patients received therapy effective against MRSA (primarily vancomycin), 61% received broad gram‐negative coverage (primarily ‐lactam with ‐lactamase inhibitor), and 74% received anaerobic coverage.[4] Another multicenter study reported that the most common antibiotics given for cellulitis (excluding cases associated with cutaneous abscess) were vancomycin (60%), ‐lactam/‐lactamase combinations (32%), and clindamycin (19%). Only 13% of patients with cellulitis were treated with cefazolin, and only 1.1% of patients were treated with nafcillin or oxacillin.[5] According to the Centers for Disease Control and Prevention, unnecessary antibiotic use is associated with increased cost, development of antibiotic resistance, and increased rates of Clostridium difficile.[6]
The current use of broad‐spectrum antibiotics for nonpurulent cellulitis is likely due to several factors, including the emergence of community‐associated (CA)‐MRSA, confusion due to the heterogeneity of SSTI, and the limited data regarding the microbiology of nonpurulent cellulitis. The resulting uncertainty about cellulitis has been termed an existential crisis for the treating physician and is likely the single biggest factor behind the out‐of‐control prescribing.[7]
The Emergence of CA‐MRSA
Over the past decade, numerous studies have reported the increasing frequency of CA‐MRSA soft tissue infections, predominantly with the pulsed‐field gel electrophoresis type USA‐300. Originally, MRSA infections were limited to nosocomial infections. Subsequent multicenter studies from the United States have shown that CA‐MRSA is the most frequent pathogen isolated from purulent soft tissue infections presenting to emergency rooms[8] and the most frequent pathogen isolated from SSTI specimens in labs.[9] Many authors have therefore concluded that empiric antibiotics for SSTI should include coverage for MRSA.[8, 9]
Heterogeneity of SSTI
As already discussed, the term SSTI is an umbrella term that encompasses several types of clinically distinct infections. The only commonality between the SSTI is that that they all involve the skin and soft tissues in some way. Diabetic foot infections, cutaneous abscesses, surgical site infections, and nonpurulent cellulitis have different hosts, pathophysiology, clinical presentations, and microbiology. At one end of the spectrum is the cutaneous abscess, which is readily culturable through incision and drainage. At the other end of the spectrum is cellulitis, which is typically nonculturable. Unfortunately, studies of SSTI tend to lump all of these entities together when reporting microbiology. The landmark study by Moran et al., for example, described the microbiology of purulent soft tissue infections presenting to a network of emergency rooms across the county. Although all patients had by definition purulent infections, and 81% were abscesses, the authors made broad conclusions about skin and soft tissue infections in general and recommended antimicrobials effective against MRSA for empiric coverage for SSTIs.[8]
Uncertainty About the Microbiology of Nonpurulent Cellulitis
What then is the microbiology of nonpurulent cellulitis? As stated in the 2005 and 2014 IDSA guidelines, traditional teaching remains that nonpurulent cellulitis is primarily due to ‐hemolytic streptococci.[2, 3] Studies using needle aspiration have yielded conflicting results, although a systematic review of these studies concluded that S aureus was the most common pathogen.[10] On the other hand, a systematic review of positive blood cultures of patients identified as having cellulitis found that 61% were due to ‐hemolytic streptococci, and only 15% were due to S aureus.[11] Both reviews, however, comment on the limited quality of the included studies. Ultimately, because nonpurulent soft tissue infections are basically nonculturable, their true microbiologic etiology remains uncertain. Given this uncertainty, as well as the impressive evidence for CA‐MRSA causing cutaneous abscesses, along with the confusion about types of SSTI, it is not surprising that front‐line clinicians have resorted to prescribing broad‐spectrum antibiotics.
THE SOLUTION: NARROW‐SPECTRUM ANTIBIOTICS FOR MOST
Although studies of the microbiology of cellulitis remain inconclusive, several recent clinical trials have indicated that treatment with antimicrobials limited to ‐hemolytic streptococci and methicillin‐susceptible S aureus (MSSA) are as effective as antimicrobials against MRSA. A prospective study from 2010 of consecutive hospitalized adults with nonpurulent cellulitis found that 73% had serologic evidence for streptococcal infection, and overall 95.8% responded to cefazolin monotherapy.[12] More recently, a study of emergency room patients with nonpurulent cellulitis randomized patients to cephalexin alone or cephalexin plus trimethoprim‐sulfamethoxazole. These authors found no difference in response rates and concluded that the addition of anti‐MRSA therapy (trimethoprim‐sulfamethoxazole, in this study) for uncomplicated cellulitis was unnecessary.[13] This later study is the only randomized controlled study to assess the need for MRSA coverage for cellulitis, and the answer for outpatients, at least, is that MRSA coverage is unnecessary. Both of these studies are cited by the IDSA guideline from 2014, which recommends antibiotics for mild‐moderate cellulitis to be limited to antimicrobials effective against ‐hemolytic streptococci and MSSA. The guideline specifically does not recommend routinely treating for MRSA, gram‐negative, or anaerobic organisms citing lack of benefit as well as risks of antibiotic resistance and C difficile infection. A recent study from the University of Utah reported the development of a cellulitis order set, which included a pathway for nonpurulent cellulitis based on the use of cefazolin. These authors reported that the use of the pathway was associated with a 59% decrease in the use of broad‐spectrum antibiotics, a 23% decrease in pharmacy costs, a 13% decrease in total facility cost, with no change in hospital length of stay or readmission rate.[14] One important caveat to the use of clinical pathways is that they are often underused. In the study from the University of Utah, for example, only 55% of eligible patients had the clinical pathway ordered.
WHEN BROAD‐SPECTRUM ANTIBIOTICS ARE RECOMMENDED
The IDSA does recommend empiric broad‐spectrum antibiotics with combination gram‐positive and gram‐negative coverage in several situations, including severe infections in which necrotizing soft tissue infection is suspected, animal bites, immersion injuries, as well as for severely immunocompromised patients or those who have failed limited spectrum antibiotics. Additionally, the IDSA recommends antimicrobials effective against MRSA for purulent infections with systemic signs of inflammation as well as severe nonpurulent infections or those associated with penetrating trauma, injection drug use, and nasal colonization with MRSA (Figure 1).
RECOMMENDATIONS
Our patient has no associated purulence and no abscess and therefore has nonpurulent cellulitis. Based on his mild tachycardia and leukocytosis but intact immune system and lack of suspicion for necrotizing soft tissue infection, he would be classified as moderate‐severity cellulitis by the IDSA. In patients hospitalized with nonpurulent cellulitis who are not severely immunocompromised or severely ill and for whom necrotizing soft tissue infection is not suspected:
- Antibiotics should be directed at ‐hemolytic streptococci and MSSA, with 1 of the suggested antibiotics by the IDSA including penicillin, ceftriaxone, cefazolin, or clindamycin.
- Antibiotics effective against MRSA should be limited to situations described by the IDSA.
- If the cellulitis has not improved within 48 hours, then consider broader‐spectrum antibiotics.
- Hospitals should strongly consider implementation of a cellulitis pathway based on the IDSA recommendations to improve antibiotic stewardship as well as costs.
Disclosure
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
- , , . Most frequent conditions in U.S. hospitals, 2011. HCUP statistical brief #162. Healthcare Cost and Utilization Project statistical briefs. Rockville, MD: Agency for Health Care Policy and Research; 2013.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft‐tissue infections. Clin Infect Dis. 2005;41(10):1373–1406.
- , , , , , . Skin and soft‐tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis. 2010;51(8):895–903.
- , , , , , . A prospective, multicenter, observational study of complicated skin and soft tissue infections in hospitalized patients: clinical characteristics, medical treatment, and outcomes. BMC Infect Dis. 2012;12:227.
- Centers for Disease Control and Prevention. Overview and evidence to support stewardship. Available at: http://www.cdc.gov/getsmart/healthcare/evidence.html. Accessed March 2, 2016.
- . Cellulitis, by any other name. Clin Infect Dis. 2013;56(12):1763–1764.
- , , , et al. Methicillin‐resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666–674.
- , , , , , . Emergence of community‐acquired methicillin‐resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft‐tissue infections. Ann Intern Med. 2006;144(5):309–317.
- , . Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138(3):313–317.
- , . A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64(2):148–155.
- , , , . The role of beta‐hemolytic streptococci in causing diffuse, nonculturable cellulitis: a prospective investigation. Medicine (Baltimore). 2010;89(4):217–226.
- , , , et al. Clinical trial: comparative effectiveness of cephalexin plus trimethoprim‐sulfamethoxazole versus cephalexin alone for treatment of uncomplicated cellulitis: a randomized controlled trial. Clin Infect Dis. 2013;56(12):1754–1762.
- , , , , . Evidence‐based care pathway for cellulitis improves process, clinical, and cost outcomes. J Hosp Med. 2015;10:780–786.
- , , . Most frequent conditions in U.S. hospitals, 2011. HCUP statistical brief #162. Healthcare Cost and Utilization Project statistical briefs. Rockville, MD: Agency for Health Care Policy and Research; 2013.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft‐tissue infections. Clin Infect Dis. 2005;41(10):1373–1406.
- , , , , , . Skin and soft‐tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis. 2010;51(8):895–903.
- , , , , , . A prospective, multicenter, observational study of complicated skin and soft tissue infections in hospitalized patients: clinical characteristics, medical treatment, and outcomes. BMC Infect Dis. 2012;12:227.
- Centers for Disease Control and Prevention. Overview and evidence to support stewardship. Available at: http://www.cdc.gov/getsmart/healthcare/evidence.html. Accessed March 2, 2016.
- . Cellulitis, by any other name. Clin Infect Dis. 2013;56(12):1763–1764.
- , , , et al. Methicillin‐resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666–674.
- , , , , , . Emergence of community‐acquired methicillin‐resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft‐tissue infections. Ann Intern Med. 2006;144(5):309–317.
- , . Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138(3):313–317.
- , . A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64(2):148–155.
- , , , . The role of beta‐hemolytic streptococci in causing diffuse, nonculturable cellulitis: a prospective investigation. Medicine (Baltimore). 2010;89(4):217–226.
- , , , et al. Clinical trial: comparative effectiveness of cephalexin plus trimethoprim‐sulfamethoxazole versus cephalexin alone for treatment of uncomplicated cellulitis: a randomized controlled trial. Clin Infect Dis. 2013;56(12):1754–1762.
- , , , , . Evidence‐based care pathway for cellulitis improves process, clinical, and cost outcomes. J Hosp Med. 2015;10:780–786.
© 2016 Society of Hospital Medicine