User login
Tapping Electronic Medical Records to Improve Quality of Care
Electronic medical records have the potential to improve quality of care among patients with epilepsy. With that goal in mind, Jaishree Narayanan et al have created a digital toolkit that allows providers to capture structured clinical data at the point of care. The software facilitates writing notes and inputting test scores in several domains, including Generalized Anxiety Disorder-7, Neurological Disorders Depression Inventory for Epilepsy, the Montreal Cognitive Assessment/Short Test of Mental Status, and the Medical Research Council Prognostic Index.
Narayanan J, Dobrin S, Choi J, et al. Structured clinical documentation in the electronic medical record to improve quality and to support practice-based research in epilepsy. Epilepsia. 2017;58(1):68-76.
Electronic medical records have the potential to improve quality of care among patients with epilepsy. With that goal in mind, Jaishree Narayanan et al have created a digital toolkit that allows providers to capture structured clinical data at the point of care. The software facilitates writing notes and inputting test scores in several domains, including Generalized Anxiety Disorder-7, Neurological Disorders Depression Inventory for Epilepsy, the Montreal Cognitive Assessment/Short Test of Mental Status, and the Medical Research Council Prognostic Index.
Narayanan J, Dobrin S, Choi J, et al. Structured clinical documentation in the electronic medical record to improve quality and to support practice-based research in epilepsy. Epilepsia. 2017;58(1):68-76.
Electronic medical records have the potential to improve quality of care among patients with epilepsy. With that goal in mind, Jaishree Narayanan et al have created a digital toolkit that allows providers to capture structured clinical data at the point of care. The software facilitates writing notes and inputting test scores in several domains, including Generalized Anxiety Disorder-7, Neurological Disorders Depression Inventory for Epilepsy, the Montreal Cognitive Assessment/Short Test of Mental Status, and the Medical Research Council Prognostic Index.
Narayanan J, Dobrin S, Choi J, et al. Structured clinical documentation in the electronic medical record to improve quality and to support practice-based research in epilepsy. Epilepsia. 2017;58(1):68-76.
Finding the Best Oxygen Saturation Setting for Seizure Monitoring
To determine the value of pulse oximetry in detecting seizures, Goldenholz et al evaluated 193 seizures among 45 patients who were being monitored with video EEG, SpO2, and EKG. Their analysis found that SpO2 thresholds of 80%-86% were able to detect 63%-73% of generalized convulsions and 20%-28% of focal seizures. While the researchers concluded that continuous SpO2 monitoring requires a tradeoff between false alarms and accurate detection of seizures, they believe an alarm setting of 86% is likely to be worthwhile in patients who experience fewer false alarms and a setting of 80% would be better for those who have more false alarms.
Goldenholz DM, Kuhn A, Austermuehle A, et al. Long-term monitoring of cardiorespiratory patterns in drug-resistant epilepsy. Epilepsia. 2017;58(1):77-84.
To determine the value of pulse oximetry in detecting seizures, Goldenholz et al evaluated 193 seizures among 45 patients who were being monitored with video EEG, SpO2, and EKG. Their analysis found that SpO2 thresholds of 80%-86% were able to detect 63%-73% of generalized convulsions and 20%-28% of focal seizures. While the researchers concluded that continuous SpO2 monitoring requires a tradeoff between false alarms and accurate detection of seizures, they believe an alarm setting of 86% is likely to be worthwhile in patients who experience fewer false alarms and a setting of 80% would be better for those who have more false alarms.
Goldenholz DM, Kuhn A, Austermuehle A, et al. Long-term monitoring of cardiorespiratory patterns in drug-resistant epilepsy. Epilepsia. 2017;58(1):77-84.
To determine the value of pulse oximetry in detecting seizures, Goldenholz et al evaluated 193 seizures among 45 patients who were being monitored with video EEG, SpO2, and EKG. Their analysis found that SpO2 thresholds of 80%-86% were able to detect 63%-73% of generalized convulsions and 20%-28% of focal seizures. While the researchers concluded that continuous SpO2 monitoring requires a tradeoff between false alarms and accurate detection of seizures, they believe an alarm setting of 86% is likely to be worthwhile in patients who experience fewer false alarms and a setting of 80% would be better for those who have more false alarms.
Goldenholz DM, Kuhn A, Austermuehle A, et al. Long-term monitoring of cardiorespiratory patterns in drug-resistant epilepsy. Epilepsia. 2017;58(1):77-84.
Office-based survey characterizes seborrheic keratosis lesions in adults
WAILEA, HAWAII – More than half of the patients with seborrheic keratosis had more than 16 lesions, in a prospective study of 406 adults at 10 dermatology practices.
The results were presented in a poster at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
The study population was 62% female, with an average age of 58 years. Patients were seen at 10 community dermatology clinics across the United States, where they completed questionnaires about their seborrheic keratosis.
Of those surveyed, 54% had more than 16 lesions, while 18% had 5 or fewer; another 18% had 6-10 lesions, and 10% had 11-15 lesions. The mean number of lesions by location ranged from 3.6 on the face to 26 on the upper body. Approximately 31% of patients reported picking at their lesions to cause them to fall off.
Most of those surveyed said they were either interested or somewhat interested in an office-based treatment. Female patients and patients with lesions on the head and neck were more likely to report interest in an office-based treatment.
The findings were limited by the inclusion only of patients who presented to private dermatology clinics and therefore may not generalize to the overall population of seborrheic keratosis patients.
The author of the study was James Del Rosso, MD, of Touro University, Nevada.
The study was supported by Aclaris Therapeutics. Dr. Del Rosso has received consulting fees from Aclaris.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – More than half of the patients with seborrheic keratosis had more than 16 lesions, in a prospective study of 406 adults at 10 dermatology practices.
The results were presented in a poster at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
The study population was 62% female, with an average age of 58 years. Patients were seen at 10 community dermatology clinics across the United States, where they completed questionnaires about their seborrheic keratosis.
Of those surveyed, 54% had more than 16 lesions, while 18% had 5 or fewer; another 18% had 6-10 lesions, and 10% had 11-15 lesions. The mean number of lesions by location ranged from 3.6 on the face to 26 on the upper body. Approximately 31% of patients reported picking at their lesions to cause them to fall off.
Most of those surveyed said they were either interested or somewhat interested in an office-based treatment. Female patients and patients with lesions on the head and neck were more likely to report interest in an office-based treatment.
The findings were limited by the inclusion only of patients who presented to private dermatology clinics and therefore may not generalize to the overall population of seborrheic keratosis patients.
The author of the study was James Del Rosso, MD, of Touro University, Nevada.
The study was supported by Aclaris Therapeutics. Dr. Del Rosso has received consulting fees from Aclaris.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – More than half of the patients with seborrheic keratosis had more than 16 lesions, in a prospective study of 406 adults at 10 dermatology practices.
The results were presented in a poster at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
The study population was 62% female, with an average age of 58 years. Patients were seen at 10 community dermatology clinics across the United States, where they completed questionnaires about their seborrheic keratosis.
Of those surveyed, 54% had more than 16 lesions, while 18% had 5 or fewer; another 18% had 6-10 lesions, and 10% had 11-15 lesions. The mean number of lesions by location ranged from 3.6 on the face to 26 on the upper body. Approximately 31% of patients reported picking at their lesions to cause them to fall off.
Most of those surveyed said they were either interested or somewhat interested in an office-based treatment. Female patients and patients with lesions on the head and neck were more likely to report interest in an office-based treatment.
The findings were limited by the inclusion only of patients who presented to private dermatology clinics and therefore may not generalize to the overall population of seborrheic keratosis patients.
The author of the study was James Del Rosso, MD, of Touro University, Nevada.
The study was supported by Aclaris Therapeutics. Dr. Del Rosso has received consulting fees from Aclaris.
SDEF and this news organization are owned by the same parent company.
AT SDEF HAWAII DERMATOLOGY SEMINAR
How is Epilepsy Treated?
The association of geriatric syndromes with hospital outcomes
Geriatric syndromes are multifactorial health conditions that affect older people and include dementia, delirium, impaired mobility, falls, frailty, poor nutrition, weight loss, incontinence, and difficulties with activities of daily living.1 These syndromes are highly prevalent among older patients admitted to acute-care hospitals2,3 and often add complexity to the clinical status of hospitalized older adults with multiple comorbid conditions.4 In the English National Health Service (NHS), the proportion of older people admitted to acute-care hospitals with geriatric syndromes has increased dramatically.5
The recognition and management of geriatric syndromes by hospitalists requires specific knowledge and skill sets.6 However, geriatricians are a scarce resource in many settings, including the NHS. A challenge for service evaluation and research is the generally poor capture of information about geriatric syndromes compared to specific comorbidities in discharge summaries and hospital coding.7 Steps are being taken in the NHS to address this issue, and in 2013 our center started the routine collection of data on clinical frailty, history of dementia (HoD) and acute confusional state (ACS) in all patients 75 years or older admitted nonelectively to the hospital.8The presence of geriatric syndromes in older inpatients is an important driver of adverse outcomes, particularly length of stay (LOS) and admission to institutional care.9 However, acute illness severity (AIS) is also an important determinant of poor outcomes in the inpatient population and may drive disproportionate changes in health status in the most vulnerable.10 Research studies with geriatric syndromes in acute settings have not been able to simultaneously consider AIS.11 In addition, comorbidity is not always associated with an increased number of geriatric syndromes.12
We aimed to study the association of geriatric syndromes such as frailty, HoD and ACS that are measured in routine clinical care with hospital outcomes (prolonged LOS, inpatient mortality, delayed discharge, institutionalization, and 30-day readmission), while controlling for demographics (age, gender), AIS, comorbidity, and discharging specialty (general medicine, geriatric medicine, surgery).
PATIENTS AND METHODS
Study Design and Setting
This retrospective observational study was conducted in a large tertiary university hospital in England with 1000 acute beds receiving more than 102,000 visits to the emergency department (ED) and admitting over 73,000 patients per year; among the latter, more than 12,000 are 75 years and older.
Sample
We analyzed all first nonelective inpatient episodes (ie, from ED admission to discharge) of people 75 years and older (all specialties) between the October 26, 2014 and the October 26, 2015. Data were obtained via the hospital’s information systems following the implementation of a new electronic patient record on October 26, 2014.
Patients’ Characteristics
The following anonymized variables were extracted:
- Age and gender
- AIS information is routinely collected in our ED using a Modified Early Warning Score (ED-MEWS). The components and scoring of ED-MEWS are shown in Table 1. Where more than 1 ED-MEWS was collected, the highest was used in the analyses.
- Charlson Comorbidity Index (CCI, without age adjustment).13 The CCI is based on the discharge diagnoses, as coded according to WHO International Classification of Diseases, v 10 (ICD-10). The CCI was calculated retrospectively and would have not been available to clinicians early during the patients’ admission.
- Clinical Frailty Scale (CFS). The scoring of CFS is based on a global assessment of patients’ comorbidity symptoms, and their level of physical activity and dependency on activities of daily living, estimated to reflect the status immediately before the onset of the acute illness leading to hospitalization. The possible scores are: 1 (very fit), 2 (well), 3 (managing well), 4 (vulnerable), 5 (mildly frail), 6 (moderately frail), 7 (severely frail), 8 (very severely frail), and 9 (terminally ill) ().14 The use of the CFS in admissions of people 75 years and older was introduced in our center in 2013 under a local Commissioning for Quality and Innovation (CQUIN) scheme.8 The CQUIN required that all patients 75 years and older admitted to the hospital, via the ED, be screened for frailty using the CFS within 72 hours of admission. The admitting doctor usually scores the CFS on the electronic admission record, but it can also be completed by ED nurses or by nursing or therapy staff from the trust-wide Specialist Advice for the Frail Elderly team. Training on CFS scoring is provided to staff at a hiring orientation and at regular educational meetings. Permission to use CFS for clinical purposes was obtained from the principal investigator at Geriatric Medicine Research, Dalhousie University, Halifax, Canada.
- Cognitive variables were collected early during the admission in patients 75 years and older, thanks to a parallel local CQUIN scheme. The cognitive CQUIN variables are screening variables, not gold standard. The admission clerking is designed to clinically classify patients within 72 hours of admission into the following 3 mutually exclusive categories:
○ Known HoD (in the database: no = 0; yes = 1)
○ ACS, without HoD (in the database: no = 0; yes = 1)
○ Neither HoD nor ACS
- The cognitive CQUIN assessment does not intend to diagnose dementia in those who are not known to have it, but tries to separate the dementias that general practitioners (GPs) know from hospital-identified acute cognitive concerns that GPs may need to assess or investigate after discharge. The latter may include delirium and/or undiagnosed dementia.
- In our routine hospital practice, the initial cognitive assessment is performed by a clinician in the following fashion: if the patient is known to have dementia (ie, based on clinical history and/or chart review), the clinician selects the “known history of dementia” option in the admission navigator, and no further cognitive screening is conducted. If the patient has no known dementia, the clinician administers the 4-item Abbreviated Mental Test (AMT4): (1) age, (2) date of birth, (3) place, and (4) year, with impaired cognition indicated by an AMT4 of less than 4 and triggering the selection of “ACS without known HoD” option. If the AMT4 is normal, the clinician selects the “neither HoD nor ACS” option.
- Due to the service evaluation nature of our work, these measures could not be assessed for reliability within the electronic medical records system (eg, regarding sensitivity and specificity against a gold standard or inter-rater reliability).
- Discharged from geriatric medicine (no = 0; yes = 1). Every year, our hospital admits over 12,000 patients 75 years and older, of which 25% are managed by the Department of Medicine for the Elderly (DME). The DME specialist bed base consists of 5 core wards, which specialize in ward-based comprehensive geriatric assessment (CGA) and are supported by dedicated nursing, physiotherapy, occupational therapy, and social work teams, as well as by readily available input from speech and language therapy, clinical nutrition, psychogeriatric, pharmacy and palliative care teams. Formal multidisciplinary team meetings occur at least twice weekly. A sixth specialist DME ward with a more acute perspective has been operational for 7 years; this ward was renamed the Frailty and Acute Medicine for the Elderly (FAME) ward in 2014 and has daily multidisciplinary team meetings. Although admission to FAME is through the ED, admission to core DME wards can occur from FAME (ie, within-DME transfer), via the ED, or from other inpatient specialty areas if older patients are perceived to be in high need of CGA after screening by the Specialist Advice for the Frail Elderly team. An audit in our center showed that up to 20% of patients discharged by DME were not initially admitted by DME, underscoring the significant role of core specialist DME wards in absorbing complex cases, especially from the general medical wards.8
- Discharged from general medicine (no = 0; yes = 1). In our setting, virtually all patients discharged by general medicine were first admitted by general medicine.8
- Discharged by a surgical specialty (no = 0; yes = 1)
Hospital Outcomes
The following anonymized variables were identified:
- LOS (days). Prolonged LOS was defined as 10 or more days (no = 0; yes = 1)
- Inpatient mortality (no = 0; yes = 1)
- Delayed discharge (no = 0; yes = 1). This was defined as the total LOS being at least 1 day longer than the LOS up to the last recorded clinically fit date. This date is used in NHS hospitals to indicate that the acute medical episode has finished and discharge-planning arrangements (often via social care providers) can commence.
- Institutionalization (no = 0; yes = 1). This was defined as the discharge destination being a care home, when a care home was not the usual place of residence.
- 30-day readmission (no = 0; yes = 1)
Statistical Analyses
Anonymized data were analyzed with IBM SPSS Statistics (v 22, Armonk, New York) software. Descriptive statistics were given as count (with percentage) or mean (with standard deviation.
To avoid potential problems with multicollinearity in the multivariate regression models, the correlations among the predictor variables were checked using a correlation matrix of 2-sided Spearman’s rho correlation coefficients. Correlations of 0.50 or more were considered large.15,16
Because all outcomes in the study were binary, multivariate binary logistic regression models were computed. In these models, the odds ratio (OR) reflects the effect size of each predictor; 95% confidence intervals (CI) were requested for each OR. Predictors with P < 0.01 were considered as statistically significant. The classification performance of each logistic regression model was assessed calculating its area under the curve (AUC).
Sensitivity analyses were conducted after imputing missing data (SPSS multiple imputation procedure) and after fitting interaction terms between geriatric syndromes and discharge by geriatric medicine.
RESULTS
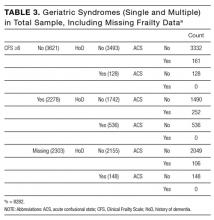
The initial database contained 12,282 nonelective admission and discharge episodes (all specialties) of patients 75 years and older between October 26, 2014 and October 26, 2015. Among those, 8202 (66.8%) were first episodes. Table 2 shows the sample descriptives, and Table 3 shows the breakdown of geriatric syndromes (single and multiple) in the total sample (n = 8282), including missing frailty data.
In the correlation matrix of 2-sided Spearman’s rho correlation coefficients, no correlations with large-effect size were found to suggest issues with multicollinearity; the largest correlation coefficients were between age and CFS (rho = 0.35), HoD and CFS (rho = 0.32), and CCI and CFS (rho = 0.26).
The results of the multivariate regression models are shown in Table 4. The best performing models were the ones for inpatient mortality (AUC = 0.80), followed by institutionalization (AUC = 0.76), and prolonged LOS (AUC = 0.71). After full adjustment, clinical frailty was an independent predictor of prolonged LOS, inpatient mortality, delayed discharge, and institutionalization. HoD was an independent predictor of prolonged LOS, delayed discharge, and institutionalization; and ACS was an independent predictor of prolonged LOS, delayed discharge, institutionalization, and 30-day readmission (Table 4). Results did not significantly change in sensitivity analyses conducted after multiple imputation of missing data and after inclusion of interaction terms (see Supplemental Table 1 and Supplemental Table
DISCUSSION
Our aim was to study the association of geriatric syndromes (measured in routine clinical care) with hospital outcomes. We found that geriatric syndromes such as clinical frailty, HoD, and ACS were strong independent predictors. Concerning prolonged LOS, delayed discharge, and institutionalization, geriatric syndromes had ORs that were greater than those of traditionally measured factors such as demographics, comorbidity and acute illness severity. Our findings add to the body of knowledge in this area because we accounted for the latter effects. Our experience shows that metrics on geriatric syndromes can be successfully collected in the routine hospital setting and add clear value to the prediction of operational outcomes. This may encourage other hospitals to do the same.
Our findings are consistent with suggestions that accounting for chronic conditions alone may be less informative than also accounting for the co-occurrence of geriatric syndromes.17 The focus of CFS is on the pre-admission level of physical activity and dependency on activities of daily living, and poorer scores may confer vulnerability to adverse outcomes due to reduced physiological reserve and ability to withstand acute stressors.18 Other studies have also found CFS to be a good predictor of inpatient outcomes,19-22 and it has been recommended as a possible means to identify vulnerable older adults in acute-care settings.23
HoD and ACS had independent effects beyond frailty, particularly in prolonging LOS, delaying discharge, and requiring institutionalization. Dementia prolongs LOS,24 and delirium prolongs hospitalization for persons with dementia.25 Older people with cognitive impairment may have an increased risk of acquiring new geriatric syndromes during hospitalization, particularly if it is prolonged.26 One study showed that the risk of poor functional recovery can be as high as 70% in complex delirious patients in hospital.27 All too often, delirium is neither benign nor reversible, with a significant proportion of patients not experiencing restoration ad integrum of cognition and function.28
Our results are consistent with observations that geriatric syndromes are associated with higher risk of institutionalization.29 It was interesting that female gender seemed to be an independent predictor of institutionalization, which is consistent with the results of a systematic review showing that the male-to-female ratio of admission rates ranged between 1 to 1.4 and 1 to 1.6.30
Discharge by general medicine appeared to be associated with a lower likelihood of prolonged LOS, and discharge by geriatric medicine seemed to be associated with a higher likelihood of delayed discharge and institutionalization. Unsurprisingly, geriatric medicine wards tend to absorb the most complex cases, often with complex discharge planning needs.8 In that light, CGA in geriatric wards may not be associated with reduced LOS (and it is possible that the LOS of complex patients might have been higher in nongeriatric wards). In addition, inpatient CGA increases frail patients’ likelihood of survival.31
Our study suggests that routinely collected metrics on frailty, HoD and ACS may be helpful to better adapt hospital care to the real requirements of aged people. The proportion of older people admitted to acute hospitals with geriatric syndromes continues to increase5 and geriatricians are a scarce resource. It will be increasingly important to upskill nongeriatric hospitalists in the recognition and management of geriatric syndromes. Frail older people are becoming the core business of acute hospitals,32 making geriatrics “too important to be left to geriatricians.”33 Therefore, easily collected metrics on geriatric syndromes may help nongeriatricians identify these syndromes and address them early during admission.
Our study has important limitations. Firstly, geriatric syndromes were not identified with gold-standard measures. For example, ACS in the absence of known dementia should be seen only as a surrogate for delirium. ACS as a proxy measure is likely to underestimate the diagnosis of delirium, because the hypoactive type is commonly missed without valid measures. In addition, a patient with delirium superimposed upon dementia would have been coded as a ‘known dementia.’ The geriatric syndromes’ measures could not be assessed for reliability within the electronic medical records system (eg, regarding sensitivity and specificity against a gold standard, or interrater reliability).
About the potential limitations of CFS, there have been concerns that an interobserver discrepancy in CFS scoring may occur between health professionals. However, 1 study investigated the interrater reliability of CFS between clinicians in 107 community-dwelling older adults 75 years and older, finding a substantial agreement with a weighted
Another limitation of our study is that we treated geriatric syndromes and the other predictors in the models as independent variables. However, many of the factors may be interrelated, and they present simultaneously in many patients. Indeed, the bivariate correlation between CFS and HoD was of moderate strength, because worsening cognition should score higher on CFS according to the scoring protocol. As expected, there was also a medium-sized correlation between CFS and CCI. It has been suggested that physical and cognitive frailty may be more informative as a single complex phenotype.36 Indeed, the problems of old age tend to come as a package.37
For 30-day readmission, the AUC of the model was small, suggesting the existence of unmeasured explanatory variables. For example, although our results agree that AIS and chronic illness predict readmission,38 the latter still remains an elusive outcome, and a more accurate prediction may be attained by adding socioeconomic variables to models.39Our study echoes the potential utility of incorporating common geriatric clinical features in routine clinical examination and disposition planning for older patients in acute settings.40 Hospitals may find it informative to undertake large-scale screening for geriatric syndromes including frailty, dementia, and delirium in all older adults admitted via the ED. When combined with other routinely collected variables such as demographics, AIS, and comorbidity data, this process may provide hospitals with information that will help define the acute needs of the local population and aid in the development of care pathways for the growing population of older adults.
Acknowledgments
The authors wish to thank all members of the acute teams in our hospital, without which this initiative would have not been possible. Licensed access to the NHS Foundation Trust’s information systems is also gratefully acknowledged.
Disclosure
The authors report no financial conflicts of interest.
1. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780-791. PubMed
2. Lakhan P, Jones M, Wilson A, Courtney M, Hirdes J, Gray LC. A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc. 2011;59:2001-2008. PubMed
3. Flood KL, Rohlfing A, Le CV, Carr DB, Rich MW. Geriatric syndromes in elderly patients admitted to an inpatient cardiology ward. J Hosp Med. 2007;2:394-400. PubMed
4. Clerencia-Sierra M, Calderon-Larranaga A, Martinez-Velilla N, et al. Multimorbidity patterns in hospitalized older patients: associations among chronic diseases and geriatric syndromes. PLoS One. 2015;10:e0132909. PubMed
5. Soong J, Poots AJ, Scott S, et al. Quantifying the prevalence of frailty in English hospitals. BMJ Open. 2015;5:e008456. PubMed
6. Warshaw GA, Bragg EJ, Fried LP, Hall WJ. Which patients benefit the most from a geriatrician’s care? Consensus among directors of geriatrics academic programs. J Am Geriatr Soc. 2008;56:1796-1801. PubMed
7. Ugboma I, Syddall HE, Cox V, Cooper C, Briggs R, Sayer AA. Coding geriatric syndromes: How good are we? CME J Geriatr Med. 2008;10:34-36. PubMed
8. Wallis SJ, Wall J, Biram RW, Romero-Ortuno R. Association of the clinical frailty scale with hospital outcomes. QJM. 2015;108:943-949. PubMed
9. Anpalahan M, Gibson SJ. Geriatric syndromes as predictors of adverse outcomes of hospitalization. Intern Med J. 2008;38:16-23. PubMed
10. Cournane S, Byrne D, O’Riordan D, Fitzgerald B, Silke B. Chronic disabling disease--impact on outcomes and costs in emergency medical admissions. QJM. 2015;108:387-396. PubMed
11. Soong J, Poots AJ, Scott S, Donald K, Bell D. Developing and validating a risk prediction model for acute care based on frailty syndromes. BMJ Open. 2015;5:e008457. PubMed
12. Vetrano DL, Foebel AD, Marengoni A, et al. Chronic diseases and geriatric syndromes: The different weight of comorbidity. Eur J Intern Med. 2016;27:62-67. PubMed
13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. PubMed
14. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489-495. PubMed
15. Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141:2-18. PubMed
16. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988.
17. Koroukian SM, Schiltz N, Warner DF, et al. Combinations of chronic conditions, functional limitations, and geriatric syndromes that predict health outcomes. J Gen Intern Med. 2016;31:630-637. PubMed
18. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752-762. PubMed
19. Romanowski KS, Barsun A, Pamlieri TL, Greenhalgh DG, Sen S. Frailty score on admission predicts outcomes in elderly burn injury. J Burn Care Res. 2015;36:1-6. PubMed
20. Ritt M, Schwarz C, Kronawitter V, et al. Analysis of Rockwood et al’s clinical frailty scale and Fried et al’s frailty phenotype as predictors of mortality and other clinical outcomes in older patients who were admitted to a geriatric ward. J Nutr Health Aging. 2015;19:1043-1048. PubMed
21. Murali-Krishnan R, Iqbal J, Rowe R, et al. Impact of frailty on outcomes after percutaneous coronary intervention: a prospective cohort study. Open Heart. 2015;2:e000294. PubMed
22. Kang L, Zhang SY, Zhu WL, et al. Is frailty associated with short-term outcomes for elderly patients with acute coronary syndrome? J Geriatr Cardiol. 2015;12:662-667.
23. Conroy S, Chikura G. Emergency care for frail older people-urgent AND important-but what works? Age Ageing. 2015;44:724-725. PubMed
24. Connolly S, O’Shea E. The impact of dementia on length of stay in acute hospitals in Ireland. Dementia (London). 2015;14:650-658. PubMed
25. Fick DM, Steis MR, Waller JL, Inouye SK. Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults. J Hosp Med. 2013;8:500-505. PubMed
26. Mecocci P, von Strauss E, Cherubini A, et al. Cognitive impairment is the major risk factor for development of geriatric syndromes during hospitalization: results from the GIFA study. Dement Geriatr Cogn Disord. 2005;20:262-269. PubMed
27. Dasgupta M, Brymer C. Poor functional recovery after delirium is associated with other geriatric syndromes and additional illnesses. Int Psychogeriatr. 2015;27:793-802. PubMed
28. Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30-39.
29. Wang SY, Shamliyan TA, Talley KM, Ramakrishnan R, Kane RL. Not just specific diseases: systematic review of the association of geriatric syndromes with hospitalization or nursing home admission. Arch Gerontol Geriatr. 2013;57:16-26. PubMed
30. Luppa M, Luck T, Weyerer S, Konig HH, Riedel-Heller SG. Gender differences in predictors of nursing home placement in the elderly: a systematic review. Int Psychogeriatr. 2009;21:1015-1025. PubMed
31. Ellis G, Whitehead MA, O’Neill D, Langhorne P, Robinson D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2011;(7):CD006211. PubMed
32. HSJ/SERCO. Commission on Hospital Care for Frail Older People. Main Report. Available at: http://www.hsj.co.uk/Journals/2014/11/18/l/q/r/HSJ141121_FRAILOLDERPEOPLE_LO-RES.pdf. 2014.
33. Coni N. The unlikely geriatricians. J R Soc Med. 1996;89:587-589. PubMed
34. Islam A. Gait variability is an independent marker of frailty. Electronic thesis and dissertation repository, the University of Western Ontario, 2012. Available at: http://ir.lib.uwo.ca/etd/558. Accessed July 23, 2016.
35. Grossman D, Rootenberg M, Perri GA, et al. Enhancing communication in end-of-life care: a clinical tool translating between the Clinical Frailty Scale and the Palliative Performance Scale. J Am Geriatr Soc. 2014;62:1562-1567. PubMed
36. Panza F, Seripa D, Solfrizzi V, et al. Targeting cognitive frailty: clinical and neurobiological roadmap for a single complex phenotype. J Alzheimers Dis. 2015;47:793-813. PubMed
37. Fontana L, Kennedy BK, Longo VD, Seals D, Melov S. Medical research: treat ageing. Nature. 2014;511:405-407. PubMed
38. Conway R, Byrne D, O’Riordan D, Silke B. Emergency readmissions are substantially determined by acute illness severity and chronic debilitating illness: a single centre cohort study. Eur J Intern Med. 2015;26:12-17. PubMed
39. Cournane S, Byrne D, Conway R, O’Riordan D, Coveney S, Silke B. Social deprivation and hospital admission rates, length of stay and readmissions in emergency medical admissions. Eur J Intern Med. 2015;26:766-771. PubMed
40. Costa AP, Hirdes JP, Heckman GA, et al. Geriatric syndromes predict postdischarge outcomes among older emergency department patients: findings from the interRAI Multinational Emergency Department Study. Acad Emerg Med. 2014;21:422-433. PubMed
Geriatric syndromes are multifactorial health conditions that affect older people and include dementia, delirium, impaired mobility, falls, frailty, poor nutrition, weight loss, incontinence, and difficulties with activities of daily living.1 These syndromes are highly prevalent among older patients admitted to acute-care hospitals2,3 and often add complexity to the clinical status of hospitalized older adults with multiple comorbid conditions.4 In the English National Health Service (NHS), the proportion of older people admitted to acute-care hospitals with geriatric syndromes has increased dramatically.5
The recognition and management of geriatric syndromes by hospitalists requires specific knowledge and skill sets.6 However, geriatricians are a scarce resource in many settings, including the NHS. A challenge for service evaluation and research is the generally poor capture of information about geriatric syndromes compared to specific comorbidities in discharge summaries and hospital coding.7 Steps are being taken in the NHS to address this issue, and in 2013 our center started the routine collection of data on clinical frailty, history of dementia (HoD) and acute confusional state (ACS) in all patients 75 years or older admitted nonelectively to the hospital.8The presence of geriatric syndromes in older inpatients is an important driver of adverse outcomes, particularly length of stay (LOS) and admission to institutional care.9 However, acute illness severity (AIS) is also an important determinant of poor outcomes in the inpatient population and may drive disproportionate changes in health status in the most vulnerable.10 Research studies with geriatric syndromes in acute settings have not been able to simultaneously consider AIS.11 In addition, comorbidity is not always associated with an increased number of geriatric syndromes.12
We aimed to study the association of geriatric syndromes such as frailty, HoD and ACS that are measured in routine clinical care with hospital outcomes (prolonged LOS, inpatient mortality, delayed discharge, institutionalization, and 30-day readmission), while controlling for demographics (age, gender), AIS, comorbidity, and discharging specialty (general medicine, geriatric medicine, surgery).
PATIENTS AND METHODS
Study Design and Setting
This retrospective observational study was conducted in a large tertiary university hospital in England with 1000 acute beds receiving more than 102,000 visits to the emergency department (ED) and admitting over 73,000 patients per year; among the latter, more than 12,000 are 75 years and older.
Sample
We analyzed all first nonelective inpatient episodes (ie, from ED admission to discharge) of people 75 years and older (all specialties) between the October 26, 2014 and the October 26, 2015. Data were obtained via the hospital’s information systems following the implementation of a new electronic patient record on October 26, 2014.
Patients’ Characteristics
The following anonymized variables were extracted:
- Age and gender
- AIS information is routinely collected in our ED using a Modified Early Warning Score (ED-MEWS). The components and scoring of ED-MEWS are shown in Table 1. Where more than 1 ED-MEWS was collected, the highest was used in the analyses.
- Charlson Comorbidity Index (CCI, without age adjustment).13 The CCI is based on the discharge diagnoses, as coded according to WHO International Classification of Diseases, v 10 (ICD-10). The CCI was calculated retrospectively and would have not been available to clinicians early during the patients’ admission.
- Clinical Frailty Scale (CFS). The scoring of CFS is based on a global assessment of patients’ comorbidity symptoms, and their level of physical activity and dependency on activities of daily living, estimated to reflect the status immediately before the onset of the acute illness leading to hospitalization. The possible scores are: 1 (very fit), 2 (well), 3 (managing well), 4 (vulnerable), 5 (mildly frail), 6 (moderately frail), 7 (severely frail), 8 (very severely frail), and 9 (terminally ill) ().14 The use of the CFS in admissions of people 75 years and older was introduced in our center in 2013 under a local Commissioning for Quality and Innovation (CQUIN) scheme.8 The CQUIN required that all patients 75 years and older admitted to the hospital, via the ED, be screened for frailty using the CFS within 72 hours of admission. The admitting doctor usually scores the CFS on the electronic admission record, but it can also be completed by ED nurses or by nursing or therapy staff from the trust-wide Specialist Advice for the Frail Elderly team. Training on CFS scoring is provided to staff at a hiring orientation and at regular educational meetings. Permission to use CFS for clinical purposes was obtained from the principal investigator at Geriatric Medicine Research, Dalhousie University, Halifax, Canada.
- Cognitive variables were collected early during the admission in patients 75 years and older, thanks to a parallel local CQUIN scheme. The cognitive CQUIN variables are screening variables, not gold standard. The admission clerking is designed to clinically classify patients within 72 hours of admission into the following 3 mutually exclusive categories:
○ Known HoD (in the database: no = 0; yes = 1)
○ ACS, without HoD (in the database: no = 0; yes = 1)
○ Neither HoD nor ACS
- The cognitive CQUIN assessment does not intend to diagnose dementia in those who are not known to have it, but tries to separate the dementias that general practitioners (GPs) know from hospital-identified acute cognitive concerns that GPs may need to assess or investigate after discharge. The latter may include delirium and/or undiagnosed dementia.
- In our routine hospital practice, the initial cognitive assessment is performed by a clinician in the following fashion: if the patient is known to have dementia (ie, based on clinical history and/or chart review), the clinician selects the “known history of dementia” option in the admission navigator, and no further cognitive screening is conducted. If the patient has no known dementia, the clinician administers the 4-item Abbreviated Mental Test (AMT4): (1) age, (2) date of birth, (3) place, and (4) year, with impaired cognition indicated by an AMT4 of less than 4 and triggering the selection of “ACS without known HoD” option. If the AMT4 is normal, the clinician selects the “neither HoD nor ACS” option.
- Due to the service evaluation nature of our work, these measures could not be assessed for reliability within the electronic medical records system (eg, regarding sensitivity and specificity against a gold standard or inter-rater reliability).
- Discharged from geriatric medicine (no = 0; yes = 1). Every year, our hospital admits over 12,000 patients 75 years and older, of which 25% are managed by the Department of Medicine for the Elderly (DME). The DME specialist bed base consists of 5 core wards, which specialize in ward-based comprehensive geriatric assessment (CGA) and are supported by dedicated nursing, physiotherapy, occupational therapy, and social work teams, as well as by readily available input from speech and language therapy, clinical nutrition, psychogeriatric, pharmacy and palliative care teams. Formal multidisciplinary team meetings occur at least twice weekly. A sixth specialist DME ward with a more acute perspective has been operational for 7 years; this ward was renamed the Frailty and Acute Medicine for the Elderly (FAME) ward in 2014 and has daily multidisciplinary team meetings. Although admission to FAME is through the ED, admission to core DME wards can occur from FAME (ie, within-DME transfer), via the ED, or from other inpatient specialty areas if older patients are perceived to be in high need of CGA after screening by the Specialist Advice for the Frail Elderly team. An audit in our center showed that up to 20% of patients discharged by DME were not initially admitted by DME, underscoring the significant role of core specialist DME wards in absorbing complex cases, especially from the general medical wards.8
- Discharged from general medicine (no = 0; yes = 1). In our setting, virtually all patients discharged by general medicine were first admitted by general medicine.8
- Discharged by a surgical specialty (no = 0; yes = 1)
Hospital Outcomes
The following anonymized variables were identified:
- LOS (days). Prolonged LOS was defined as 10 or more days (no = 0; yes = 1)
- Inpatient mortality (no = 0; yes = 1)
- Delayed discharge (no = 0; yes = 1). This was defined as the total LOS being at least 1 day longer than the LOS up to the last recorded clinically fit date. This date is used in NHS hospitals to indicate that the acute medical episode has finished and discharge-planning arrangements (often via social care providers) can commence.
- Institutionalization (no = 0; yes = 1). This was defined as the discharge destination being a care home, when a care home was not the usual place of residence.
- 30-day readmission (no = 0; yes = 1)
Statistical Analyses
Anonymized data were analyzed with IBM SPSS Statistics (v 22, Armonk, New York) software. Descriptive statistics were given as count (with percentage) or mean (with standard deviation.
To avoid potential problems with multicollinearity in the multivariate regression models, the correlations among the predictor variables were checked using a correlation matrix of 2-sided Spearman’s rho correlation coefficients. Correlations of 0.50 or more were considered large.15,16
Because all outcomes in the study were binary, multivariate binary logistic regression models were computed. In these models, the odds ratio (OR) reflects the effect size of each predictor; 95% confidence intervals (CI) were requested for each OR. Predictors with P < 0.01 were considered as statistically significant. The classification performance of each logistic regression model was assessed calculating its area under the curve (AUC).
Sensitivity analyses were conducted after imputing missing data (SPSS multiple imputation procedure) and after fitting interaction terms between geriatric syndromes and discharge by geriatric medicine.
RESULTS
The initial database contained 12,282 nonelective admission and discharge episodes (all specialties) of patients 75 years and older between October 26, 2014 and October 26, 2015. Among those, 8202 (66.8%) were first episodes. Table 2 shows the sample descriptives, and Table 3 shows the breakdown of geriatric syndromes (single and multiple) in the total sample (n = 8282), including missing frailty data.
In the correlation matrix of 2-sided Spearman’s rho correlation coefficients, no correlations with large-effect size were found to suggest issues with multicollinearity; the largest correlation coefficients were between age and CFS (rho = 0.35), HoD and CFS (rho = 0.32), and CCI and CFS (rho = 0.26).
The results of the multivariate regression models are shown in Table 4. The best performing models were the ones for inpatient mortality (AUC = 0.80), followed by institutionalization (AUC = 0.76), and prolonged LOS (AUC = 0.71). After full adjustment, clinical frailty was an independent predictor of prolonged LOS, inpatient mortality, delayed discharge, and institutionalization. HoD was an independent predictor of prolonged LOS, delayed discharge, and institutionalization; and ACS was an independent predictor of prolonged LOS, delayed discharge, institutionalization, and 30-day readmission (Table 4). Results did not significantly change in sensitivity analyses conducted after multiple imputation of missing data and after inclusion of interaction terms (see Supplemental Table 1 and Supplemental Table
DISCUSSION
Our aim was to study the association of geriatric syndromes (measured in routine clinical care) with hospital outcomes. We found that geriatric syndromes such as clinical frailty, HoD, and ACS were strong independent predictors. Concerning prolonged LOS, delayed discharge, and institutionalization, geriatric syndromes had ORs that were greater than those of traditionally measured factors such as demographics, comorbidity and acute illness severity. Our findings add to the body of knowledge in this area because we accounted for the latter effects. Our experience shows that metrics on geriatric syndromes can be successfully collected in the routine hospital setting and add clear value to the prediction of operational outcomes. This may encourage other hospitals to do the same.
Our findings are consistent with suggestions that accounting for chronic conditions alone may be less informative than also accounting for the co-occurrence of geriatric syndromes.17 The focus of CFS is on the pre-admission level of physical activity and dependency on activities of daily living, and poorer scores may confer vulnerability to adverse outcomes due to reduced physiological reserve and ability to withstand acute stressors.18 Other studies have also found CFS to be a good predictor of inpatient outcomes,19-22 and it has been recommended as a possible means to identify vulnerable older adults in acute-care settings.23
HoD and ACS had independent effects beyond frailty, particularly in prolonging LOS, delaying discharge, and requiring institutionalization. Dementia prolongs LOS,24 and delirium prolongs hospitalization for persons with dementia.25 Older people with cognitive impairment may have an increased risk of acquiring new geriatric syndromes during hospitalization, particularly if it is prolonged.26 One study showed that the risk of poor functional recovery can be as high as 70% in complex delirious patients in hospital.27 All too often, delirium is neither benign nor reversible, with a significant proportion of patients not experiencing restoration ad integrum of cognition and function.28
Our results are consistent with observations that geriatric syndromes are associated with higher risk of institutionalization.29 It was interesting that female gender seemed to be an independent predictor of institutionalization, which is consistent with the results of a systematic review showing that the male-to-female ratio of admission rates ranged between 1 to 1.4 and 1 to 1.6.30
Discharge by general medicine appeared to be associated with a lower likelihood of prolonged LOS, and discharge by geriatric medicine seemed to be associated with a higher likelihood of delayed discharge and institutionalization. Unsurprisingly, geriatric medicine wards tend to absorb the most complex cases, often with complex discharge planning needs.8 In that light, CGA in geriatric wards may not be associated with reduced LOS (and it is possible that the LOS of complex patients might have been higher in nongeriatric wards). In addition, inpatient CGA increases frail patients’ likelihood of survival.31
Our study suggests that routinely collected metrics on frailty, HoD and ACS may be helpful to better adapt hospital care to the real requirements of aged people. The proportion of older people admitted to acute hospitals with geriatric syndromes continues to increase5 and geriatricians are a scarce resource. It will be increasingly important to upskill nongeriatric hospitalists in the recognition and management of geriatric syndromes. Frail older people are becoming the core business of acute hospitals,32 making geriatrics “too important to be left to geriatricians.”33 Therefore, easily collected metrics on geriatric syndromes may help nongeriatricians identify these syndromes and address them early during admission.
Our study has important limitations. Firstly, geriatric syndromes were not identified with gold-standard measures. For example, ACS in the absence of known dementia should be seen only as a surrogate for delirium. ACS as a proxy measure is likely to underestimate the diagnosis of delirium, because the hypoactive type is commonly missed without valid measures. In addition, a patient with delirium superimposed upon dementia would have been coded as a ‘known dementia.’ The geriatric syndromes’ measures could not be assessed for reliability within the electronic medical records system (eg, regarding sensitivity and specificity against a gold standard, or interrater reliability).
About the potential limitations of CFS, there have been concerns that an interobserver discrepancy in CFS scoring may occur between health professionals. However, 1 study investigated the interrater reliability of CFS between clinicians in 107 community-dwelling older adults 75 years and older, finding a substantial agreement with a weighted
Another limitation of our study is that we treated geriatric syndromes and the other predictors in the models as independent variables. However, many of the factors may be interrelated, and they present simultaneously in many patients. Indeed, the bivariate correlation between CFS and HoD was of moderate strength, because worsening cognition should score higher on CFS according to the scoring protocol. As expected, there was also a medium-sized correlation between CFS and CCI. It has been suggested that physical and cognitive frailty may be more informative as a single complex phenotype.36 Indeed, the problems of old age tend to come as a package.37
For 30-day readmission, the AUC of the model was small, suggesting the existence of unmeasured explanatory variables. For example, although our results agree that AIS and chronic illness predict readmission,38 the latter still remains an elusive outcome, and a more accurate prediction may be attained by adding socioeconomic variables to models.39Our study echoes the potential utility of incorporating common geriatric clinical features in routine clinical examination and disposition planning for older patients in acute settings.40 Hospitals may find it informative to undertake large-scale screening for geriatric syndromes including frailty, dementia, and delirium in all older adults admitted via the ED. When combined with other routinely collected variables such as demographics, AIS, and comorbidity data, this process may provide hospitals with information that will help define the acute needs of the local population and aid in the development of care pathways for the growing population of older adults.
Acknowledgments
The authors wish to thank all members of the acute teams in our hospital, without which this initiative would have not been possible. Licensed access to the NHS Foundation Trust’s information systems is also gratefully acknowledged.
Disclosure
The authors report no financial conflicts of interest.
Geriatric syndromes are multifactorial health conditions that affect older people and include dementia, delirium, impaired mobility, falls, frailty, poor nutrition, weight loss, incontinence, and difficulties with activities of daily living.1 These syndromes are highly prevalent among older patients admitted to acute-care hospitals2,3 and often add complexity to the clinical status of hospitalized older adults with multiple comorbid conditions.4 In the English National Health Service (NHS), the proportion of older people admitted to acute-care hospitals with geriatric syndromes has increased dramatically.5
The recognition and management of geriatric syndromes by hospitalists requires specific knowledge and skill sets.6 However, geriatricians are a scarce resource in many settings, including the NHS. A challenge for service evaluation and research is the generally poor capture of information about geriatric syndromes compared to specific comorbidities in discharge summaries and hospital coding.7 Steps are being taken in the NHS to address this issue, and in 2013 our center started the routine collection of data on clinical frailty, history of dementia (HoD) and acute confusional state (ACS) in all patients 75 years or older admitted nonelectively to the hospital.8The presence of geriatric syndromes in older inpatients is an important driver of adverse outcomes, particularly length of stay (LOS) and admission to institutional care.9 However, acute illness severity (AIS) is also an important determinant of poor outcomes in the inpatient population and may drive disproportionate changes in health status in the most vulnerable.10 Research studies with geriatric syndromes in acute settings have not been able to simultaneously consider AIS.11 In addition, comorbidity is not always associated with an increased number of geriatric syndromes.12
We aimed to study the association of geriatric syndromes such as frailty, HoD and ACS that are measured in routine clinical care with hospital outcomes (prolonged LOS, inpatient mortality, delayed discharge, institutionalization, and 30-day readmission), while controlling for demographics (age, gender), AIS, comorbidity, and discharging specialty (general medicine, geriatric medicine, surgery).
PATIENTS AND METHODS
Study Design and Setting
This retrospective observational study was conducted in a large tertiary university hospital in England with 1000 acute beds receiving more than 102,000 visits to the emergency department (ED) and admitting over 73,000 patients per year; among the latter, more than 12,000 are 75 years and older.
Sample
We analyzed all first nonelective inpatient episodes (ie, from ED admission to discharge) of people 75 years and older (all specialties) between the October 26, 2014 and the October 26, 2015. Data were obtained via the hospital’s information systems following the implementation of a new electronic patient record on October 26, 2014.
Patients’ Characteristics
The following anonymized variables were extracted:
- Age and gender
- AIS information is routinely collected in our ED using a Modified Early Warning Score (ED-MEWS). The components and scoring of ED-MEWS are shown in Table 1. Where more than 1 ED-MEWS was collected, the highest was used in the analyses.
- Charlson Comorbidity Index (CCI, without age adjustment).13 The CCI is based on the discharge diagnoses, as coded according to WHO International Classification of Diseases, v 10 (ICD-10). The CCI was calculated retrospectively and would have not been available to clinicians early during the patients’ admission.
- Clinical Frailty Scale (CFS). The scoring of CFS is based on a global assessment of patients’ comorbidity symptoms, and their level of physical activity and dependency on activities of daily living, estimated to reflect the status immediately before the onset of the acute illness leading to hospitalization. The possible scores are: 1 (very fit), 2 (well), 3 (managing well), 4 (vulnerable), 5 (mildly frail), 6 (moderately frail), 7 (severely frail), 8 (very severely frail), and 9 (terminally ill) ().14 The use of the CFS in admissions of people 75 years and older was introduced in our center in 2013 under a local Commissioning for Quality and Innovation (CQUIN) scheme.8 The CQUIN required that all patients 75 years and older admitted to the hospital, via the ED, be screened for frailty using the CFS within 72 hours of admission. The admitting doctor usually scores the CFS on the electronic admission record, but it can also be completed by ED nurses or by nursing or therapy staff from the trust-wide Specialist Advice for the Frail Elderly team. Training on CFS scoring is provided to staff at a hiring orientation and at regular educational meetings. Permission to use CFS for clinical purposes was obtained from the principal investigator at Geriatric Medicine Research, Dalhousie University, Halifax, Canada.
- Cognitive variables were collected early during the admission in patients 75 years and older, thanks to a parallel local CQUIN scheme. The cognitive CQUIN variables are screening variables, not gold standard. The admission clerking is designed to clinically classify patients within 72 hours of admission into the following 3 mutually exclusive categories:
○ Known HoD (in the database: no = 0; yes = 1)
○ ACS, without HoD (in the database: no = 0; yes = 1)
○ Neither HoD nor ACS
- The cognitive CQUIN assessment does not intend to diagnose dementia in those who are not known to have it, but tries to separate the dementias that general practitioners (GPs) know from hospital-identified acute cognitive concerns that GPs may need to assess or investigate after discharge. The latter may include delirium and/or undiagnosed dementia.
- In our routine hospital practice, the initial cognitive assessment is performed by a clinician in the following fashion: if the patient is known to have dementia (ie, based on clinical history and/or chart review), the clinician selects the “known history of dementia” option in the admission navigator, and no further cognitive screening is conducted. If the patient has no known dementia, the clinician administers the 4-item Abbreviated Mental Test (AMT4): (1) age, (2) date of birth, (3) place, and (4) year, with impaired cognition indicated by an AMT4 of less than 4 and triggering the selection of “ACS without known HoD” option. If the AMT4 is normal, the clinician selects the “neither HoD nor ACS” option.
- Due to the service evaluation nature of our work, these measures could not be assessed for reliability within the electronic medical records system (eg, regarding sensitivity and specificity against a gold standard or inter-rater reliability).
- Discharged from geriatric medicine (no = 0; yes = 1). Every year, our hospital admits over 12,000 patients 75 years and older, of which 25% are managed by the Department of Medicine for the Elderly (DME). The DME specialist bed base consists of 5 core wards, which specialize in ward-based comprehensive geriatric assessment (CGA) and are supported by dedicated nursing, physiotherapy, occupational therapy, and social work teams, as well as by readily available input from speech and language therapy, clinical nutrition, psychogeriatric, pharmacy and palliative care teams. Formal multidisciplinary team meetings occur at least twice weekly. A sixth specialist DME ward with a more acute perspective has been operational for 7 years; this ward was renamed the Frailty and Acute Medicine for the Elderly (FAME) ward in 2014 and has daily multidisciplinary team meetings. Although admission to FAME is through the ED, admission to core DME wards can occur from FAME (ie, within-DME transfer), via the ED, or from other inpatient specialty areas if older patients are perceived to be in high need of CGA after screening by the Specialist Advice for the Frail Elderly team. An audit in our center showed that up to 20% of patients discharged by DME were not initially admitted by DME, underscoring the significant role of core specialist DME wards in absorbing complex cases, especially from the general medical wards.8
- Discharged from general medicine (no = 0; yes = 1). In our setting, virtually all patients discharged by general medicine were first admitted by general medicine.8
- Discharged by a surgical specialty (no = 0; yes = 1)
Hospital Outcomes
The following anonymized variables were identified:
- LOS (days). Prolonged LOS was defined as 10 or more days (no = 0; yes = 1)
- Inpatient mortality (no = 0; yes = 1)
- Delayed discharge (no = 0; yes = 1). This was defined as the total LOS being at least 1 day longer than the LOS up to the last recorded clinically fit date. This date is used in NHS hospitals to indicate that the acute medical episode has finished and discharge-planning arrangements (often via social care providers) can commence.
- Institutionalization (no = 0; yes = 1). This was defined as the discharge destination being a care home, when a care home was not the usual place of residence.
- 30-day readmission (no = 0; yes = 1)
Statistical Analyses
Anonymized data were analyzed with IBM SPSS Statistics (v 22, Armonk, New York) software. Descriptive statistics were given as count (with percentage) or mean (with standard deviation.
To avoid potential problems with multicollinearity in the multivariate regression models, the correlations among the predictor variables were checked using a correlation matrix of 2-sided Spearman’s rho correlation coefficients. Correlations of 0.50 or more were considered large.15,16
Because all outcomes in the study were binary, multivariate binary logistic regression models were computed. In these models, the odds ratio (OR) reflects the effect size of each predictor; 95% confidence intervals (CI) were requested for each OR. Predictors with P < 0.01 were considered as statistically significant. The classification performance of each logistic regression model was assessed calculating its area under the curve (AUC).
Sensitivity analyses were conducted after imputing missing data (SPSS multiple imputation procedure) and after fitting interaction terms between geriatric syndromes and discharge by geriatric medicine.
RESULTS
The initial database contained 12,282 nonelective admission and discharge episodes (all specialties) of patients 75 years and older between October 26, 2014 and October 26, 2015. Among those, 8202 (66.8%) were first episodes. Table 2 shows the sample descriptives, and Table 3 shows the breakdown of geriatric syndromes (single and multiple) in the total sample (n = 8282), including missing frailty data.
In the correlation matrix of 2-sided Spearman’s rho correlation coefficients, no correlations with large-effect size were found to suggest issues with multicollinearity; the largest correlation coefficients were between age and CFS (rho = 0.35), HoD and CFS (rho = 0.32), and CCI and CFS (rho = 0.26).
The results of the multivariate regression models are shown in Table 4. The best performing models were the ones for inpatient mortality (AUC = 0.80), followed by institutionalization (AUC = 0.76), and prolonged LOS (AUC = 0.71). After full adjustment, clinical frailty was an independent predictor of prolonged LOS, inpatient mortality, delayed discharge, and institutionalization. HoD was an independent predictor of prolonged LOS, delayed discharge, and institutionalization; and ACS was an independent predictor of prolonged LOS, delayed discharge, institutionalization, and 30-day readmission (Table 4). Results did not significantly change in sensitivity analyses conducted after multiple imputation of missing data and after inclusion of interaction terms (see Supplemental Table 1 and Supplemental Table
DISCUSSION
Our aim was to study the association of geriatric syndromes (measured in routine clinical care) with hospital outcomes. We found that geriatric syndromes such as clinical frailty, HoD, and ACS were strong independent predictors. Concerning prolonged LOS, delayed discharge, and institutionalization, geriatric syndromes had ORs that were greater than those of traditionally measured factors such as demographics, comorbidity and acute illness severity. Our findings add to the body of knowledge in this area because we accounted for the latter effects. Our experience shows that metrics on geriatric syndromes can be successfully collected in the routine hospital setting and add clear value to the prediction of operational outcomes. This may encourage other hospitals to do the same.
Our findings are consistent with suggestions that accounting for chronic conditions alone may be less informative than also accounting for the co-occurrence of geriatric syndromes.17 The focus of CFS is on the pre-admission level of physical activity and dependency on activities of daily living, and poorer scores may confer vulnerability to adverse outcomes due to reduced physiological reserve and ability to withstand acute stressors.18 Other studies have also found CFS to be a good predictor of inpatient outcomes,19-22 and it has been recommended as a possible means to identify vulnerable older adults in acute-care settings.23
HoD and ACS had independent effects beyond frailty, particularly in prolonging LOS, delaying discharge, and requiring institutionalization. Dementia prolongs LOS,24 and delirium prolongs hospitalization for persons with dementia.25 Older people with cognitive impairment may have an increased risk of acquiring new geriatric syndromes during hospitalization, particularly if it is prolonged.26 One study showed that the risk of poor functional recovery can be as high as 70% in complex delirious patients in hospital.27 All too often, delirium is neither benign nor reversible, with a significant proportion of patients not experiencing restoration ad integrum of cognition and function.28
Our results are consistent with observations that geriatric syndromes are associated with higher risk of institutionalization.29 It was interesting that female gender seemed to be an independent predictor of institutionalization, which is consistent with the results of a systematic review showing that the male-to-female ratio of admission rates ranged between 1 to 1.4 and 1 to 1.6.30
Discharge by general medicine appeared to be associated with a lower likelihood of prolonged LOS, and discharge by geriatric medicine seemed to be associated with a higher likelihood of delayed discharge and institutionalization. Unsurprisingly, geriatric medicine wards tend to absorb the most complex cases, often with complex discharge planning needs.8 In that light, CGA in geriatric wards may not be associated with reduced LOS (and it is possible that the LOS of complex patients might have been higher in nongeriatric wards). In addition, inpatient CGA increases frail patients’ likelihood of survival.31
Our study suggests that routinely collected metrics on frailty, HoD and ACS may be helpful to better adapt hospital care to the real requirements of aged people. The proportion of older people admitted to acute hospitals with geriatric syndromes continues to increase5 and geriatricians are a scarce resource. It will be increasingly important to upskill nongeriatric hospitalists in the recognition and management of geriatric syndromes. Frail older people are becoming the core business of acute hospitals,32 making geriatrics “too important to be left to geriatricians.”33 Therefore, easily collected metrics on geriatric syndromes may help nongeriatricians identify these syndromes and address them early during admission.
Our study has important limitations. Firstly, geriatric syndromes were not identified with gold-standard measures. For example, ACS in the absence of known dementia should be seen only as a surrogate for delirium. ACS as a proxy measure is likely to underestimate the diagnosis of delirium, because the hypoactive type is commonly missed without valid measures. In addition, a patient with delirium superimposed upon dementia would have been coded as a ‘known dementia.’ The geriatric syndromes’ measures could not be assessed for reliability within the electronic medical records system (eg, regarding sensitivity and specificity against a gold standard, or interrater reliability).
About the potential limitations of CFS, there have been concerns that an interobserver discrepancy in CFS scoring may occur between health professionals. However, 1 study investigated the interrater reliability of CFS between clinicians in 107 community-dwelling older adults 75 years and older, finding a substantial agreement with a weighted
Another limitation of our study is that we treated geriatric syndromes and the other predictors in the models as independent variables. However, many of the factors may be interrelated, and they present simultaneously in many patients. Indeed, the bivariate correlation between CFS and HoD was of moderate strength, because worsening cognition should score higher on CFS according to the scoring protocol. As expected, there was also a medium-sized correlation between CFS and CCI. It has been suggested that physical and cognitive frailty may be more informative as a single complex phenotype.36 Indeed, the problems of old age tend to come as a package.37
For 30-day readmission, the AUC of the model was small, suggesting the existence of unmeasured explanatory variables. For example, although our results agree that AIS and chronic illness predict readmission,38 the latter still remains an elusive outcome, and a more accurate prediction may be attained by adding socioeconomic variables to models.39Our study echoes the potential utility of incorporating common geriatric clinical features in routine clinical examination and disposition planning for older patients in acute settings.40 Hospitals may find it informative to undertake large-scale screening for geriatric syndromes including frailty, dementia, and delirium in all older adults admitted via the ED. When combined with other routinely collected variables such as demographics, AIS, and comorbidity data, this process may provide hospitals with information that will help define the acute needs of the local population and aid in the development of care pathways for the growing population of older adults.
Acknowledgments
The authors wish to thank all members of the acute teams in our hospital, without which this initiative would have not been possible. Licensed access to the NHS Foundation Trust’s information systems is also gratefully acknowledged.
Disclosure
The authors report no financial conflicts of interest.
1. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780-791. PubMed
2. Lakhan P, Jones M, Wilson A, Courtney M, Hirdes J, Gray LC. A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc. 2011;59:2001-2008. PubMed
3. Flood KL, Rohlfing A, Le CV, Carr DB, Rich MW. Geriatric syndromes in elderly patients admitted to an inpatient cardiology ward. J Hosp Med. 2007;2:394-400. PubMed
4. Clerencia-Sierra M, Calderon-Larranaga A, Martinez-Velilla N, et al. Multimorbidity patterns in hospitalized older patients: associations among chronic diseases and geriatric syndromes. PLoS One. 2015;10:e0132909. PubMed
5. Soong J, Poots AJ, Scott S, et al. Quantifying the prevalence of frailty in English hospitals. BMJ Open. 2015;5:e008456. PubMed
6. Warshaw GA, Bragg EJ, Fried LP, Hall WJ. Which patients benefit the most from a geriatrician’s care? Consensus among directors of geriatrics academic programs. J Am Geriatr Soc. 2008;56:1796-1801. PubMed
7. Ugboma I, Syddall HE, Cox V, Cooper C, Briggs R, Sayer AA. Coding geriatric syndromes: How good are we? CME J Geriatr Med. 2008;10:34-36. PubMed
8. Wallis SJ, Wall J, Biram RW, Romero-Ortuno R. Association of the clinical frailty scale with hospital outcomes. QJM. 2015;108:943-949. PubMed
9. Anpalahan M, Gibson SJ. Geriatric syndromes as predictors of adverse outcomes of hospitalization. Intern Med J. 2008;38:16-23. PubMed
10. Cournane S, Byrne D, O’Riordan D, Fitzgerald B, Silke B. Chronic disabling disease--impact on outcomes and costs in emergency medical admissions. QJM. 2015;108:387-396. PubMed
11. Soong J, Poots AJ, Scott S, Donald K, Bell D. Developing and validating a risk prediction model for acute care based on frailty syndromes. BMJ Open. 2015;5:e008457. PubMed
12. Vetrano DL, Foebel AD, Marengoni A, et al. Chronic diseases and geriatric syndromes: The different weight of comorbidity. Eur J Intern Med. 2016;27:62-67. PubMed
13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. PubMed
14. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489-495. PubMed
15. Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141:2-18. PubMed
16. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988.
17. Koroukian SM, Schiltz N, Warner DF, et al. Combinations of chronic conditions, functional limitations, and geriatric syndromes that predict health outcomes. J Gen Intern Med. 2016;31:630-637. PubMed
18. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752-762. PubMed
19. Romanowski KS, Barsun A, Pamlieri TL, Greenhalgh DG, Sen S. Frailty score on admission predicts outcomes in elderly burn injury. J Burn Care Res. 2015;36:1-6. PubMed
20. Ritt M, Schwarz C, Kronawitter V, et al. Analysis of Rockwood et al’s clinical frailty scale and Fried et al’s frailty phenotype as predictors of mortality and other clinical outcomes in older patients who were admitted to a geriatric ward. J Nutr Health Aging. 2015;19:1043-1048. PubMed
21. Murali-Krishnan R, Iqbal J, Rowe R, et al. Impact of frailty on outcomes after percutaneous coronary intervention: a prospective cohort study. Open Heart. 2015;2:e000294. PubMed
22. Kang L, Zhang SY, Zhu WL, et al. Is frailty associated with short-term outcomes for elderly patients with acute coronary syndrome? J Geriatr Cardiol. 2015;12:662-667.
23. Conroy S, Chikura G. Emergency care for frail older people-urgent AND important-but what works? Age Ageing. 2015;44:724-725. PubMed
24. Connolly S, O’Shea E. The impact of dementia on length of stay in acute hospitals in Ireland. Dementia (London). 2015;14:650-658. PubMed
25. Fick DM, Steis MR, Waller JL, Inouye SK. Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults. J Hosp Med. 2013;8:500-505. PubMed
26. Mecocci P, von Strauss E, Cherubini A, et al. Cognitive impairment is the major risk factor for development of geriatric syndromes during hospitalization: results from the GIFA study. Dement Geriatr Cogn Disord. 2005;20:262-269. PubMed
27. Dasgupta M, Brymer C. Poor functional recovery after delirium is associated with other geriatric syndromes and additional illnesses. Int Psychogeriatr. 2015;27:793-802. PubMed
28. Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30-39.
29. Wang SY, Shamliyan TA, Talley KM, Ramakrishnan R, Kane RL. Not just specific diseases: systematic review of the association of geriatric syndromes with hospitalization or nursing home admission. Arch Gerontol Geriatr. 2013;57:16-26. PubMed
30. Luppa M, Luck T, Weyerer S, Konig HH, Riedel-Heller SG. Gender differences in predictors of nursing home placement in the elderly: a systematic review. Int Psychogeriatr. 2009;21:1015-1025. PubMed
31. Ellis G, Whitehead MA, O’Neill D, Langhorne P, Robinson D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2011;(7):CD006211. PubMed
32. HSJ/SERCO. Commission on Hospital Care for Frail Older People. Main Report. Available at: http://www.hsj.co.uk/Journals/2014/11/18/l/q/r/HSJ141121_FRAILOLDERPEOPLE_LO-RES.pdf. 2014.
33. Coni N. The unlikely geriatricians. J R Soc Med. 1996;89:587-589. PubMed
34. Islam A. Gait variability is an independent marker of frailty. Electronic thesis and dissertation repository, the University of Western Ontario, 2012. Available at: http://ir.lib.uwo.ca/etd/558. Accessed July 23, 2016.
35. Grossman D, Rootenberg M, Perri GA, et al. Enhancing communication in end-of-life care: a clinical tool translating between the Clinical Frailty Scale and the Palliative Performance Scale. J Am Geriatr Soc. 2014;62:1562-1567. PubMed
36. Panza F, Seripa D, Solfrizzi V, et al. Targeting cognitive frailty: clinical and neurobiological roadmap for a single complex phenotype. J Alzheimers Dis. 2015;47:793-813. PubMed
37. Fontana L, Kennedy BK, Longo VD, Seals D, Melov S. Medical research: treat ageing. Nature. 2014;511:405-407. PubMed
38. Conway R, Byrne D, O’Riordan D, Silke B. Emergency readmissions are substantially determined by acute illness severity and chronic debilitating illness: a single centre cohort study. Eur J Intern Med. 2015;26:12-17. PubMed
39. Cournane S, Byrne D, Conway R, O’Riordan D, Coveney S, Silke B. Social deprivation and hospital admission rates, length of stay and readmissions in emergency medical admissions. Eur J Intern Med. 2015;26:766-771. PubMed
40. Costa AP, Hirdes JP, Heckman GA, et al. Geriatric syndromes predict postdischarge outcomes among older emergency department patients: findings from the interRAI Multinational Emergency Department Study. Acad Emerg Med. 2014;21:422-433. PubMed
1. Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780-791. PubMed
2. Lakhan P, Jones M, Wilson A, Courtney M, Hirdes J, Gray LC. A prospective cohort study of geriatric syndromes among older medical patients admitted to acute care hospitals. J Am Geriatr Soc. 2011;59:2001-2008. PubMed
3. Flood KL, Rohlfing A, Le CV, Carr DB, Rich MW. Geriatric syndromes in elderly patients admitted to an inpatient cardiology ward. J Hosp Med. 2007;2:394-400. PubMed
4. Clerencia-Sierra M, Calderon-Larranaga A, Martinez-Velilla N, et al. Multimorbidity patterns in hospitalized older patients: associations among chronic diseases and geriatric syndromes. PLoS One. 2015;10:e0132909. PubMed
5. Soong J, Poots AJ, Scott S, et al. Quantifying the prevalence of frailty in English hospitals. BMJ Open. 2015;5:e008456. PubMed
6. Warshaw GA, Bragg EJ, Fried LP, Hall WJ. Which patients benefit the most from a geriatrician’s care? Consensus among directors of geriatrics academic programs. J Am Geriatr Soc. 2008;56:1796-1801. PubMed
7. Ugboma I, Syddall HE, Cox V, Cooper C, Briggs R, Sayer AA. Coding geriatric syndromes: How good are we? CME J Geriatr Med. 2008;10:34-36. PubMed
8. Wallis SJ, Wall J, Biram RW, Romero-Ortuno R. Association of the clinical frailty scale with hospital outcomes. QJM. 2015;108:943-949. PubMed
9. Anpalahan M, Gibson SJ. Geriatric syndromes as predictors of adverse outcomes of hospitalization. Intern Med J. 2008;38:16-23. PubMed
10. Cournane S, Byrne D, O’Riordan D, Fitzgerald B, Silke B. Chronic disabling disease--impact on outcomes and costs in emergency medical admissions. QJM. 2015;108:387-396. PubMed
11. Soong J, Poots AJ, Scott S, Donald K, Bell D. Developing and validating a risk prediction model for acute care based on frailty syndromes. BMJ Open. 2015;5:e008457. PubMed
12. Vetrano DL, Foebel AD, Marengoni A, et al. Chronic diseases and geriatric syndromes: The different weight of comorbidity. Eur J Intern Med. 2016;27:62-67. PubMed
13. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. PubMed
14. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489-495. PubMed
15. Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012;141:2-18. PubMed
16. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988.
17. Koroukian SM, Schiltz N, Warner DF, et al. Combinations of chronic conditions, functional limitations, and geriatric syndromes that predict health outcomes. J Gen Intern Med. 2016;31:630-637. PubMed
18. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752-762. PubMed
19. Romanowski KS, Barsun A, Pamlieri TL, Greenhalgh DG, Sen S. Frailty score on admission predicts outcomes in elderly burn injury. J Burn Care Res. 2015;36:1-6. PubMed
20. Ritt M, Schwarz C, Kronawitter V, et al. Analysis of Rockwood et al’s clinical frailty scale and Fried et al’s frailty phenotype as predictors of mortality and other clinical outcomes in older patients who were admitted to a geriatric ward. J Nutr Health Aging. 2015;19:1043-1048. PubMed
21. Murali-Krishnan R, Iqbal J, Rowe R, et al. Impact of frailty on outcomes after percutaneous coronary intervention: a prospective cohort study. Open Heart. 2015;2:e000294. PubMed
22. Kang L, Zhang SY, Zhu WL, et al. Is frailty associated with short-term outcomes for elderly patients with acute coronary syndrome? J Geriatr Cardiol. 2015;12:662-667.
23. Conroy S, Chikura G. Emergency care for frail older people-urgent AND important-but what works? Age Ageing. 2015;44:724-725. PubMed
24. Connolly S, O’Shea E. The impact of dementia on length of stay in acute hospitals in Ireland. Dementia (London). 2015;14:650-658. PubMed
25. Fick DM, Steis MR, Waller JL, Inouye SK. Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults. J Hosp Med. 2013;8:500-505. PubMed
26. Mecocci P, von Strauss E, Cherubini A, et al. Cognitive impairment is the major risk factor for development of geriatric syndromes during hospitalization: results from the GIFA study. Dement Geriatr Cogn Disord. 2005;20:262-269. PubMed
27. Dasgupta M, Brymer C. Poor functional recovery after delirium is associated with other geriatric syndromes and additional illnesses. Int Psychogeriatr. 2015;27:793-802. PubMed
28. Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367:30-39.
29. Wang SY, Shamliyan TA, Talley KM, Ramakrishnan R, Kane RL. Not just specific diseases: systematic review of the association of geriatric syndromes with hospitalization or nursing home admission. Arch Gerontol Geriatr. 2013;57:16-26. PubMed
30. Luppa M, Luck T, Weyerer S, Konig HH, Riedel-Heller SG. Gender differences in predictors of nursing home placement in the elderly: a systematic review. Int Psychogeriatr. 2009;21:1015-1025. PubMed
31. Ellis G, Whitehead MA, O’Neill D, Langhorne P, Robinson D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2011;(7):CD006211. PubMed
32. HSJ/SERCO. Commission on Hospital Care for Frail Older People. Main Report. Available at: http://www.hsj.co.uk/Journals/2014/11/18/l/q/r/HSJ141121_FRAILOLDERPEOPLE_LO-RES.pdf. 2014.
33. Coni N. The unlikely geriatricians. J R Soc Med. 1996;89:587-589. PubMed
34. Islam A. Gait variability is an independent marker of frailty. Electronic thesis and dissertation repository, the University of Western Ontario, 2012. Available at: http://ir.lib.uwo.ca/etd/558. Accessed July 23, 2016.
35. Grossman D, Rootenberg M, Perri GA, et al. Enhancing communication in end-of-life care: a clinical tool translating between the Clinical Frailty Scale and the Palliative Performance Scale. J Am Geriatr Soc. 2014;62:1562-1567. PubMed
36. Panza F, Seripa D, Solfrizzi V, et al. Targeting cognitive frailty: clinical and neurobiological roadmap for a single complex phenotype. J Alzheimers Dis. 2015;47:793-813. PubMed
37. Fontana L, Kennedy BK, Longo VD, Seals D, Melov S. Medical research: treat ageing. Nature. 2014;511:405-407. PubMed
38. Conway R, Byrne D, O’Riordan D, Silke B. Emergency readmissions are substantially determined by acute illness severity and chronic debilitating illness: a single centre cohort study. Eur J Intern Med. 2015;26:12-17. PubMed
39. Cournane S, Byrne D, Conway R, O’Riordan D, Coveney S, Silke B. Social deprivation and hospital admission rates, length of stay and readmissions in emergency medical admissions. Eur J Intern Med. 2015;26:766-771. PubMed
40. Costa AP, Hirdes JP, Heckman GA, et al. Geriatric syndromes predict postdischarge outcomes among older emergency department patients: findings from the interRAI Multinational Emergency Department Study. Acad Emerg Med. 2014;21:422-433. PubMed
© 2017 Society of Hospital Medicine
Patient-level exclusions from mHealth in a safety-net health system
Interest in mHealth—the use of mobile communication devices for clinical and public health—has exploded among clinicians and researchers for its potential to efficiently improve patient health. Recent studies have used mHealth’s asynchronous receptive and expressive communication functions in interventions targeted to managing care transitions and hospital readmissions.1-3 We also recently published on improved readmission risk assessments using postdischarge measures of patient reported outcomes, which could be collected through mobile devices. 4 But persistent disparities in access to5 and engagement with6 smartphones may threaten validity and equity when mHealth strategies do not fully address its own limitations.
Disparities introduced by uneven access to technology are well known, but the rapid, albeit belated, adoption of mobile devices by racial minority groups in the United States has allowed authors of recent thoughtful publications to recast mHealth as itself offering solutions to the disparities’ problem.7,8 Others have cautioned the emergence of disparities along domains other than race, such as low literacy and limited English proficiency (LEP).9 In this paper, we assessed the impact of inadequate reading health literacy (IRHL) and LEP on factors related to access and engagement with mHealth. We conducted our study among urban low-income adults in whom IRHL and LEP are common.
METHODS
We surveyed patients in a large public safety-net health system serving 132 municipalities, including the city of Chicago, in northeastern Illinois. In 2015, nearly 90% of patients were racial-ethnic minorities with more than one-third insured by Medicaid and another one-third uninsured. We sampled adult inpatients and outpatients separately by nonselectively approaching patients in November 2015 to complete an in-person questionnaire in a 464-bed hospital and in 2 primary-care clinics. All inpatients occupied a nonisolation room in a general medical-surgical ward that had been sampled for data collection for that day in 9-day cycles with 8 other similar units. All outpatients in the clinic waiting areas were approached on consecutive days until a predetermined recruitment target was met. Each participant was surveyed once in his/her preferred language (English or Spanish), was 18 years and older, consented verbally, and received no compensation. Sample size provided 80% power to detect a device ownership rate of 50% in an evenly allocated low literacy population compared to a reference rate of 66% assuming a 2-sided α of 0.05 using the Fisher exact test.
The 18-item questionnaire was informed by constructs addressed in the 2015 Pew Research Center smartphone survey.10 However, in addition to device ownership, we inquired about device capabilities, service-plan details, service interruptions due to difficulty paying bills in the previous year, home-Internet access, an active e-mail account, and self-assigned demographics. Self-reported reading health literacy,11 more directly measured than e-health literacy, was screened using a parsimonious instrument validated as a dichotomized measure.12 Instruments in English and Spanish were tested for appropriate and comprehensible word choices and syntax through pilot testing. We inferred LEP among patients preferring to complete the survey in Spanish based on our familiarity with the population. We defined any Internet access as having a mobile data-service plan or having home-Internet access. In addition, we inquired about primary insurance provider and offered Medicaid patients an informational brochure about the federal Lifeline Program (https://www.fcc.gov/lifeline) that subsidizes text-messaging-enabled cellular telephone service for low-income patients. Notably, we assessed engagement by asking about the extent of patients’ interest in “new ways of communicating with your doctor, clinic, or pharmacy using” text, e-mail, or mobile apps with a 5-level response scale ranging from “not at all interested” to “very interested”.
Participant characteristics were confirmed to be similar to the Cook County Health and Hospitals System patient population in 2015 with regards to age, gender, and race/ethnicity. We calculated unadjusted and adjusted odds ratios for IRHL and LEP’s association with each dependent measure of access (to smartphone, Internet, or e-mail) and engagement (using text messaging, e-mail, or mobile apps) controlling for age, gender, primary payer, recruitment location, IRHL, and LEP. Because we oversampled inpatients, we estimated sampling-weight-adjusted proportions and 95% confidence intervals (CI) of the entire CCHHS patient population with access to smartphone, data/text plan, non-prepaid plan, and service interruptions using STATA v13 (StataCorp LP, College Station Texas). The project received a waiver upon review by the local Institutional Review Board.
RESULTS
Participation rate was 65% (302/464). Differences in patients by site are shown in Table 1. IRHL was more frequent and LEP less frequent among hospitalized patients. As shown in Table 2, patients with IRHL were less likely to have any Internet access, to have an active e-mail account, and to be interested in using e-mail for healthcare communications. Patients with LEP were less likely than English speakers to be interested in using mobile apps. Inpatients were less likely than outpatients to be interested in text messaging for healthcare communications.
The estimated proportion (95% CI) of the health system’s patients owning a text-enabled mobile device was 87% (75%-94%) and an Internet-enabled mobile device was 64% (47%-78%). The proportion with no data service interruptions in the previous year was 40% (31%-50%).
DISCUSSION
In this cross-section of urban low-income adult patients, IRHL and LEP were factors associated with potential disparities introduced by mHealth. Even as access to smartphones becomes ubiquitous, lagging access to Internet and e-mail among low literacy patients, and low levels of technology engagement for healthcare communications among patients with IRHL or LEP, underscore concerns about equity in health systems’ adoption of mHealth strategies. Hospitalized patients were found to have diminished engagement with mHealth independent of IRHL and LEP.
Regarding engagement, significantly fewer patients with IRHL or LEP were interested in using technology for healthcare communications. Our finding suggests that health disparities already associated with these conditions13 may not be reduced by mobile device outreach alone and may even be worsened by it. Touch screens, audio-enabled questionnaires, and language translation engines are innovations that may be helpful to mitigate IRHL and LEP, but evidence is scarce. Privacy and security concerns, and lack of experience with technology, may also lower engagement. A contemporaneous study found lower apps’ usage among Latinos, also suggesting that language concordance between apps, their source, and targeted users is important.14 Low-tech solutions involving mobile telephone or even lower tech in-person communications targeted to the estimated 26% of the US population with low literacy15 and 20% with LEP16 may be practical stopgap measures. Even as disparities in access to technology across race-ethnicity are diminishing,10 equity across poverty levels, low levels of education, cultural norms, and disabilities may be more challenging to overcome. Our assessment indicates that large exclusions of a safety-net population in 2015 are a legitimate concern in communication strategies that rely too heavily on mHealth. These findings underscore the CONSORT-EHEALTH recommendation that investigators report web-based recruitment strategies and data-collection methods comprehensively.17
Regarding access, our estimates suggest that historical disparities in smartphone ownership are diminishing, but access to Internet capabilities may still be lower among the urban poor compared to the nation as a whole. The Pew Research Center found that 64% of Americans owned a smartphone in 2015 (respondents defined smartphone).10 In comparison, 87% (95% CI, 75%, 94%) of our study participants owned a text-enabled mobile device and 64% (47%, 78%) owned an Internet-enabled mobile device. However, the 40% (31%, 50%) of our safety-net population with an uninterrupted data plan over the previous year may be lower than the 50% of Americans reporting uninterrupted data plans over their lifetime.10 The impact of expense-related data plan interruptions is magnified by the 40% of our study population—compared to 15% of Americans—who are dependent on mobile devices for Internet access.10 The association between Internet connectivity and literacy evokes multiple bidirectional pathways yet to be elucidated. But if mHealth can reduce health disparities, closing the gap in device ownership is only a partial accomplishment, and future work also needs to expand Internet connectivity to allow literacy-enhancing and literacy-naïve technologies to flourish.
This study has limitations. Our study population was a consecutive sample and participation rate was less than 100%. However, we recruited participants into the study the way we may also have approached patients to introduce mHealth options in our clinical settings. Our sampling method proved adequate for our primary goal to explain differences in technology access and engagement using regression analysis. Although our patient population may not directly generalize to many healthcare systems, including other safety-net systems serving regions with variable technology uptake,18 our findings reflect the capacities and the preferences of the most disadvantaged segments of urban populations. We systematically excluded LEP non-Spanish speakers, but they consisted of less than 5% of inpatients and no outpatients. We did not assess current technology use. Finally, as discussed earlier, access and use of new technologies change rapidly and frequent updates are necessary.
mHealth is a promising tool because it may increase healthcare access, improve care quality, and promote research. All these potential benefits will be obtained with accompanying efforts to reduce healthcare disparities, especially where some technologies themselves are exclusionary.
Disclosures
The authors report no financial conflicts of interest.
1. Khosravi P, Ghapanchi AH. Investigating the effectiveness of technologies applied to assist seniors: a systematic literature review. Int J Med Inform. 2016;85:17-26. PubMed
2. Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160:774-784. PubMed
3. Prieto-Centurion V, Gussin HA, Rolle AJ, Krishnan JA. Chronic obstructive pulmonary disease readmissions at minority-serving institutions. Ann Am Thorac Soc. 2013;10:680-684. PubMed
4. Hinami K, Smith J, Deamant CD, BuBeshter K, Trick WE. When do patient-reported outcome measures inform readmission risk? J Hosp Med. 2015;10:294-300. PubMed
5. Gibbons MC. A historical overview of health disparities and the potential of eHealth solutions. J Med Internet Res. 2005;7:e50. PubMed
6. Nelson LA, Mulvaney SA, Gebretsadik T, Ho YX, Johnson KB, Osborn CY. Disparities in the use of a mHealth medication adherence promotion intervention for low-income adults with type2 diabetes. J Am Med Inform Assoc. 2016;23:12-18. PubMed
7. Martin T. Assessing mHealth: opportunities and barriers to patient engagement. J Health Care Poor Underserved. 2012;23:935-941. PubMed
8. Horn IB, Mendoza FS. Reframing the disparities agenda: a time to rethink, a time to focus. Acad Pediatr. 2013;14:115-116. PubMed
9. Viswanath K, Nagler RH, Bigman-Galimore CA, McCauley MP, Jung M, Ramanadhan S. The communications revolution and health inequalities in the 21st century: implications for cancer control. Cancer Epidemiol, BiomarkersPrev. 2012;21:1701-1708. PubMed
10. Pew Research Center. The Smartphone Difference. Pew Research Center; April 2015. Available at: http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015/. Accessed January 12, 2017.
11. Baker DW. The meaning and measure of health literacy. J Gen Intern Med. 2011;21:878-883. PubMed
12. Morris NS, MacLean CD, Chew LD, Littenberg B. The single item literacy screener: evalution of a brief instrument to identify limited reading ability. BMC Fam Pract. 2006;7:21. PubMed
13. Sentell T, Braun K. Low health literacy, limited English proficiency, and health status in Asians, Latinos, and other racial/ethnic groups in California. J Health Commun. 2012;17:82-99. PubMed
14. Arora S, Ford K, Terp S, et al. Describing the evolution of mobile technology usage for Latino patients and comparing findings to national mHealth estimates. J Am Med Inform Assoc. 2016;23:979-983. PubMed
15. Paasche-Orlow MK, Parker RM, Gazmararian JA, Nielsen-Bohlman LT, Rudd RR. The prevalence of limited health literacy. J Gen Intern Med. 2005;20:175-184. PubMed
16. US Census Bureau. Detailed languages spoken at home and ability to speak English for the population 5 years and over: 2009-2013. Published 2015. Available at: http://www.census.gov/data/tables/2013/demo/2009-2013-lang-tables.html. Accessed March 31, 2016.
17. Eysenbach G, CONSORT-EHEALTH Group. CONSORT-EHEALTH: improving and standardizing evaluation reports of Web-based and mobile health intervention. J Med Internet Res. 2011;13:e126. PubMed
18. Schickedanz A, Huang D, Lopex A, et al. Access, interest, and attitudes toward electronic communication for health care among patients in the medical safety net. J Gen Intern Med. 2013;28:914-920. PubMed
Interest in mHealth—the use of mobile communication devices for clinical and public health—has exploded among clinicians and researchers for its potential to efficiently improve patient health. Recent studies have used mHealth’s asynchronous receptive and expressive communication functions in interventions targeted to managing care transitions and hospital readmissions.1-3 We also recently published on improved readmission risk assessments using postdischarge measures of patient reported outcomes, which could be collected through mobile devices. 4 But persistent disparities in access to5 and engagement with6 smartphones may threaten validity and equity when mHealth strategies do not fully address its own limitations.
Disparities introduced by uneven access to technology are well known, but the rapid, albeit belated, adoption of mobile devices by racial minority groups in the United States has allowed authors of recent thoughtful publications to recast mHealth as itself offering solutions to the disparities’ problem.7,8 Others have cautioned the emergence of disparities along domains other than race, such as low literacy and limited English proficiency (LEP).9 In this paper, we assessed the impact of inadequate reading health literacy (IRHL) and LEP on factors related to access and engagement with mHealth. We conducted our study among urban low-income adults in whom IRHL and LEP are common.
METHODS
We surveyed patients in a large public safety-net health system serving 132 municipalities, including the city of Chicago, in northeastern Illinois. In 2015, nearly 90% of patients were racial-ethnic minorities with more than one-third insured by Medicaid and another one-third uninsured. We sampled adult inpatients and outpatients separately by nonselectively approaching patients in November 2015 to complete an in-person questionnaire in a 464-bed hospital and in 2 primary-care clinics. All inpatients occupied a nonisolation room in a general medical-surgical ward that had been sampled for data collection for that day in 9-day cycles with 8 other similar units. All outpatients in the clinic waiting areas were approached on consecutive days until a predetermined recruitment target was met. Each participant was surveyed once in his/her preferred language (English or Spanish), was 18 years and older, consented verbally, and received no compensation. Sample size provided 80% power to detect a device ownership rate of 50% in an evenly allocated low literacy population compared to a reference rate of 66% assuming a 2-sided α of 0.05 using the Fisher exact test.
The 18-item questionnaire was informed by constructs addressed in the 2015 Pew Research Center smartphone survey.10 However, in addition to device ownership, we inquired about device capabilities, service-plan details, service interruptions due to difficulty paying bills in the previous year, home-Internet access, an active e-mail account, and self-assigned demographics. Self-reported reading health literacy,11 more directly measured than e-health literacy, was screened using a parsimonious instrument validated as a dichotomized measure.12 Instruments in English and Spanish were tested for appropriate and comprehensible word choices and syntax through pilot testing. We inferred LEP among patients preferring to complete the survey in Spanish based on our familiarity with the population. We defined any Internet access as having a mobile data-service plan or having home-Internet access. In addition, we inquired about primary insurance provider and offered Medicaid patients an informational brochure about the federal Lifeline Program (https://www.fcc.gov/lifeline) that subsidizes text-messaging-enabled cellular telephone service for low-income patients. Notably, we assessed engagement by asking about the extent of patients’ interest in “new ways of communicating with your doctor, clinic, or pharmacy using” text, e-mail, or mobile apps with a 5-level response scale ranging from “not at all interested” to “very interested”.
Participant characteristics were confirmed to be similar to the Cook County Health and Hospitals System patient population in 2015 with regards to age, gender, and race/ethnicity. We calculated unadjusted and adjusted odds ratios for IRHL and LEP’s association with each dependent measure of access (to smartphone, Internet, or e-mail) and engagement (using text messaging, e-mail, or mobile apps) controlling for age, gender, primary payer, recruitment location, IRHL, and LEP. Because we oversampled inpatients, we estimated sampling-weight-adjusted proportions and 95% confidence intervals (CI) of the entire CCHHS patient population with access to smartphone, data/text plan, non-prepaid plan, and service interruptions using STATA v13 (StataCorp LP, College Station Texas). The project received a waiver upon review by the local Institutional Review Board.
RESULTS
Participation rate was 65% (302/464). Differences in patients by site are shown in Table 1. IRHL was more frequent and LEP less frequent among hospitalized patients. As shown in Table 2, patients with IRHL were less likely to have any Internet access, to have an active e-mail account, and to be interested in using e-mail for healthcare communications. Patients with LEP were less likely than English speakers to be interested in using mobile apps. Inpatients were less likely than outpatients to be interested in text messaging for healthcare communications.
The estimated proportion (95% CI) of the health system’s patients owning a text-enabled mobile device was 87% (75%-94%) and an Internet-enabled mobile device was 64% (47%-78%). The proportion with no data service interruptions in the previous year was 40% (31%-50%).
DISCUSSION
In this cross-section of urban low-income adult patients, IRHL and LEP were factors associated with potential disparities introduced by mHealth. Even as access to smartphones becomes ubiquitous, lagging access to Internet and e-mail among low literacy patients, and low levels of technology engagement for healthcare communications among patients with IRHL or LEP, underscore concerns about equity in health systems’ adoption of mHealth strategies. Hospitalized patients were found to have diminished engagement with mHealth independent of IRHL and LEP.
Regarding engagement, significantly fewer patients with IRHL or LEP were interested in using technology for healthcare communications. Our finding suggests that health disparities already associated with these conditions13 may not be reduced by mobile device outreach alone and may even be worsened by it. Touch screens, audio-enabled questionnaires, and language translation engines are innovations that may be helpful to mitigate IRHL and LEP, but evidence is scarce. Privacy and security concerns, and lack of experience with technology, may also lower engagement. A contemporaneous study found lower apps’ usage among Latinos, also suggesting that language concordance between apps, their source, and targeted users is important.14 Low-tech solutions involving mobile telephone or even lower tech in-person communications targeted to the estimated 26% of the US population with low literacy15 and 20% with LEP16 may be practical stopgap measures. Even as disparities in access to technology across race-ethnicity are diminishing,10 equity across poverty levels, low levels of education, cultural norms, and disabilities may be more challenging to overcome. Our assessment indicates that large exclusions of a safety-net population in 2015 are a legitimate concern in communication strategies that rely too heavily on mHealth. These findings underscore the CONSORT-EHEALTH recommendation that investigators report web-based recruitment strategies and data-collection methods comprehensively.17
Regarding access, our estimates suggest that historical disparities in smartphone ownership are diminishing, but access to Internet capabilities may still be lower among the urban poor compared to the nation as a whole. The Pew Research Center found that 64% of Americans owned a smartphone in 2015 (respondents defined smartphone).10 In comparison, 87% (95% CI, 75%, 94%) of our study participants owned a text-enabled mobile device and 64% (47%, 78%) owned an Internet-enabled mobile device. However, the 40% (31%, 50%) of our safety-net population with an uninterrupted data plan over the previous year may be lower than the 50% of Americans reporting uninterrupted data plans over their lifetime.10 The impact of expense-related data plan interruptions is magnified by the 40% of our study population—compared to 15% of Americans—who are dependent on mobile devices for Internet access.10 The association between Internet connectivity and literacy evokes multiple bidirectional pathways yet to be elucidated. But if mHealth can reduce health disparities, closing the gap in device ownership is only a partial accomplishment, and future work also needs to expand Internet connectivity to allow literacy-enhancing and literacy-naïve technologies to flourish.
This study has limitations. Our study population was a consecutive sample and participation rate was less than 100%. However, we recruited participants into the study the way we may also have approached patients to introduce mHealth options in our clinical settings. Our sampling method proved adequate for our primary goal to explain differences in technology access and engagement using regression analysis. Although our patient population may not directly generalize to many healthcare systems, including other safety-net systems serving regions with variable technology uptake,18 our findings reflect the capacities and the preferences of the most disadvantaged segments of urban populations. We systematically excluded LEP non-Spanish speakers, but they consisted of less than 5% of inpatients and no outpatients. We did not assess current technology use. Finally, as discussed earlier, access and use of new technologies change rapidly and frequent updates are necessary.
mHealth is a promising tool because it may increase healthcare access, improve care quality, and promote research. All these potential benefits will be obtained with accompanying efforts to reduce healthcare disparities, especially where some technologies themselves are exclusionary.
Disclosures
The authors report no financial conflicts of interest.
Interest in mHealth—the use of mobile communication devices for clinical and public health—has exploded among clinicians and researchers for its potential to efficiently improve patient health. Recent studies have used mHealth’s asynchronous receptive and expressive communication functions in interventions targeted to managing care transitions and hospital readmissions.1-3 We also recently published on improved readmission risk assessments using postdischarge measures of patient reported outcomes, which could be collected through mobile devices. 4 But persistent disparities in access to5 and engagement with6 smartphones may threaten validity and equity when mHealth strategies do not fully address its own limitations.
Disparities introduced by uneven access to technology are well known, but the rapid, albeit belated, adoption of mobile devices by racial minority groups in the United States has allowed authors of recent thoughtful publications to recast mHealth as itself offering solutions to the disparities’ problem.7,8 Others have cautioned the emergence of disparities along domains other than race, such as low literacy and limited English proficiency (LEP).9 In this paper, we assessed the impact of inadequate reading health literacy (IRHL) and LEP on factors related to access and engagement with mHealth. We conducted our study among urban low-income adults in whom IRHL and LEP are common.
METHODS
We surveyed patients in a large public safety-net health system serving 132 municipalities, including the city of Chicago, in northeastern Illinois. In 2015, nearly 90% of patients were racial-ethnic minorities with more than one-third insured by Medicaid and another one-third uninsured. We sampled adult inpatients and outpatients separately by nonselectively approaching patients in November 2015 to complete an in-person questionnaire in a 464-bed hospital and in 2 primary-care clinics. All inpatients occupied a nonisolation room in a general medical-surgical ward that had been sampled for data collection for that day in 9-day cycles with 8 other similar units. All outpatients in the clinic waiting areas were approached on consecutive days until a predetermined recruitment target was met. Each participant was surveyed once in his/her preferred language (English or Spanish), was 18 years and older, consented verbally, and received no compensation. Sample size provided 80% power to detect a device ownership rate of 50% in an evenly allocated low literacy population compared to a reference rate of 66% assuming a 2-sided α of 0.05 using the Fisher exact test.
The 18-item questionnaire was informed by constructs addressed in the 2015 Pew Research Center smartphone survey.10 However, in addition to device ownership, we inquired about device capabilities, service-plan details, service interruptions due to difficulty paying bills in the previous year, home-Internet access, an active e-mail account, and self-assigned demographics. Self-reported reading health literacy,11 more directly measured than e-health literacy, was screened using a parsimonious instrument validated as a dichotomized measure.12 Instruments in English and Spanish were tested for appropriate and comprehensible word choices and syntax through pilot testing. We inferred LEP among patients preferring to complete the survey in Spanish based on our familiarity with the population. We defined any Internet access as having a mobile data-service plan or having home-Internet access. In addition, we inquired about primary insurance provider and offered Medicaid patients an informational brochure about the federal Lifeline Program (https://www.fcc.gov/lifeline) that subsidizes text-messaging-enabled cellular telephone service for low-income patients. Notably, we assessed engagement by asking about the extent of patients’ interest in “new ways of communicating with your doctor, clinic, or pharmacy using” text, e-mail, or mobile apps with a 5-level response scale ranging from “not at all interested” to “very interested”.
Participant characteristics were confirmed to be similar to the Cook County Health and Hospitals System patient population in 2015 with regards to age, gender, and race/ethnicity. We calculated unadjusted and adjusted odds ratios for IRHL and LEP’s association with each dependent measure of access (to smartphone, Internet, or e-mail) and engagement (using text messaging, e-mail, or mobile apps) controlling for age, gender, primary payer, recruitment location, IRHL, and LEP. Because we oversampled inpatients, we estimated sampling-weight-adjusted proportions and 95% confidence intervals (CI) of the entire CCHHS patient population with access to smartphone, data/text plan, non-prepaid plan, and service interruptions using STATA v13 (StataCorp LP, College Station Texas). The project received a waiver upon review by the local Institutional Review Board.
RESULTS
Participation rate was 65% (302/464). Differences in patients by site are shown in Table 1. IRHL was more frequent and LEP less frequent among hospitalized patients. As shown in Table 2, patients with IRHL were less likely to have any Internet access, to have an active e-mail account, and to be interested in using e-mail for healthcare communications. Patients with LEP were less likely than English speakers to be interested in using mobile apps. Inpatients were less likely than outpatients to be interested in text messaging for healthcare communications.
The estimated proportion (95% CI) of the health system’s patients owning a text-enabled mobile device was 87% (75%-94%) and an Internet-enabled mobile device was 64% (47%-78%). The proportion with no data service interruptions in the previous year was 40% (31%-50%).
DISCUSSION
In this cross-section of urban low-income adult patients, IRHL and LEP were factors associated with potential disparities introduced by mHealth. Even as access to smartphones becomes ubiquitous, lagging access to Internet and e-mail among low literacy patients, and low levels of technology engagement for healthcare communications among patients with IRHL or LEP, underscore concerns about equity in health systems’ adoption of mHealth strategies. Hospitalized patients were found to have diminished engagement with mHealth independent of IRHL and LEP.
Regarding engagement, significantly fewer patients with IRHL or LEP were interested in using technology for healthcare communications. Our finding suggests that health disparities already associated with these conditions13 may not be reduced by mobile device outreach alone and may even be worsened by it. Touch screens, audio-enabled questionnaires, and language translation engines are innovations that may be helpful to mitigate IRHL and LEP, but evidence is scarce. Privacy and security concerns, and lack of experience with technology, may also lower engagement. A contemporaneous study found lower apps’ usage among Latinos, also suggesting that language concordance between apps, their source, and targeted users is important.14 Low-tech solutions involving mobile telephone or even lower tech in-person communications targeted to the estimated 26% of the US population with low literacy15 and 20% with LEP16 may be practical stopgap measures. Even as disparities in access to technology across race-ethnicity are diminishing,10 equity across poverty levels, low levels of education, cultural norms, and disabilities may be more challenging to overcome. Our assessment indicates that large exclusions of a safety-net population in 2015 are a legitimate concern in communication strategies that rely too heavily on mHealth. These findings underscore the CONSORT-EHEALTH recommendation that investigators report web-based recruitment strategies and data-collection methods comprehensively.17
Regarding access, our estimates suggest that historical disparities in smartphone ownership are diminishing, but access to Internet capabilities may still be lower among the urban poor compared to the nation as a whole. The Pew Research Center found that 64% of Americans owned a smartphone in 2015 (respondents defined smartphone).10 In comparison, 87% (95% CI, 75%, 94%) of our study participants owned a text-enabled mobile device and 64% (47%, 78%) owned an Internet-enabled mobile device. However, the 40% (31%, 50%) of our safety-net population with an uninterrupted data plan over the previous year may be lower than the 50% of Americans reporting uninterrupted data plans over their lifetime.10 The impact of expense-related data plan interruptions is magnified by the 40% of our study population—compared to 15% of Americans—who are dependent on mobile devices for Internet access.10 The association between Internet connectivity and literacy evokes multiple bidirectional pathways yet to be elucidated. But if mHealth can reduce health disparities, closing the gap in device ownership is only a partial accomplishment, and future work also needs to expand Internet connectivity to allow literacy-enhancing and literacy-naïve technologies to flourish.
This study has limitations. Our study population was a consecutive sample and participation rate was less than 100%. However, we recruited participants into the study the way we may also have approached patients to introduce mHealth options in our clinical settings. Our sampling method proved adequate for our primary goal to explain differences in technology access and engagement using regression analysis. Although our patient population may not directly generalize to many healthcare systems, including other safety-net systems serving regions with variable technology uptake,18 our findings reflect the capacities and the preferences of the most disadvantaged segments of urban populations. We systematically excluded LEP non-Spanish speakers, but they consisted of less than 5% of inpatients and no outpatients. We did not assess current technology use. Finally, as discussed earlier, access and use of new technologies change rapidly and frequent updates are necessary.
mHealth is a promising tool because it may increase healthcare access, improve care quality, and promote research. All these potential benefits will be obtained with accompanying efforts to reduce healthcare disparities, especially where some technologies themselves are exclusionary.
Disclosures
The authors report no financial conflicts of interest.
1. Khosravi P, Ghapanchi AH. Investigating the effectiveness of technologies applied to assist seniors: a systematic literature review. Int J Med Inform. 2016;85:17-26. PubMed
2. Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160:774-784. PubMed
3. Prieto-Centurion V, Gussin HA, Rolle AJ, Krishnan JA. Chronic obstructive pulmonary disease readmissions at minority-serving institutions. Ann Am Thorac Soc. 2013;10:680-684. PubMed
4. Hinami K, Smith J, Deamant CD, BuBeshter K, Trick WE. When do patient-reported outcome measures inform readmission risk? J Hosp Med. 2015;10:294-300. PubMed
5. Gibbons MC. A historical overview of health disparities and the potential of eHealth solutions. J Med Internet Res. 2005;7:e50. PubMed
6. Nelson LA, Mulvaney SA, Gebretsadik T, Ho YX, Johnson KB, Osborn CY. Disparities in the use of a mHealth medication adherence promotion intervention for low-income adults with type2 diabetes. J Am Med Inform Assoc. 2016;23:12-18. PubMed
7. Martin T. Assessing mHealth: opportunities and barriers to patient engagement. J Health Care Poor Underserved. 2012;23:935-941. PubMed
8. Horn IB, Mendoza FS. Reframing the disparities agenda: a time to rethink, a time to focus. Acad Pediatr. 2013;14:115-116. PubMed
9. Viswanath K, Nagler RH, Bigman-Galimore CA, McCauley MP, Jung M, Ramanadhan S. The communications revolution and health inequalities in the 21st century: implications for cancer control. Cancer Epidemiol, BiomarkersPrev. 2012;21:1701-1708. PubMed
10. Pew Research Center. The Smartphone Difference. Pew Research Center; April 2015. Available at: http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015/. Accessed January 12, 2017.
11. Baker DW. The meaning and measure of health literacy. J Gen Intern Med. 2011;21:878-883. PubMed
12. Morris NS, MacLean CD, Chew LD, Littenberg B. The single item literacy screener: evalution of a brief instrument to identify limited reading ability. BMC Fam Pract. 2006;7:21. PubMed
13. Sentell T, Braun K. Low health literacy, limited English proficiency, and health status in Asians, Latinos, and other racial/ethnic groups in California. J Health Commun. 2012;17:82-99. PubMed
14. Arora S, Ford K, Terp S, et al. Describing the evolution of mobile technology usage for Latino patients and comparing findings to national mHealth estimates. J Am Med Inform Assoc. 2016;23:979-983. PubMed
15. Paasche-Orlow MK, Parker RM, Gazmararian JA, Nielsen-Bohlman LT, Rudd RR. The prevalence of limited health literacy. J Gen Intern Med. 2005;20:175-184. PubMed
16. US Census Bureau. Detailed languages spoken at home and ability to speak English for the population 5 years and over: 2009-2013. Published 2015. Available at: http://www.census.gov/data/tables/2013/demo/2009-2013-lang-tables.html. Accessed March 31, 2016.
17. Eysenbach G, CONSORT-EHEALTH Group. CONSORT-EHEALTH: improving and standardizing evaluation reports of Web-based and mobile health intervention. J Med Internet Res. 2011;13:e126. PubMed
18. Schickedanz A, Huang D, Lopex A, et al. Access, interest, and attitudes toward electronic communication for health care among patients in the medical safety net. J Gen Intern Med. 2013;28:914-920. PubMed
1. Khosravi P, Ghapanchi AH. Investigating the effectiveness of technologies applied to assist seniors: a systematic literature review. Int J Med Inform. 2016;85:17-26. PubMed
2. Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160:774-784. PubMed
3. Prieto-Centurion V, Gussin HA, Rolle AJ, Krishnan JA. Chronic obstructive pulmonary disease readmissions at minority-serving institutions. Ann Am Thorac Soc. 2013;10:680-684. PubMed
4. Hinami K, Smith J, Deamant CD, BuBeshter K, Trick WE. When do patient-reported outcome measures inform readmission risk? J Hosp Med. 2015;10:294-300. PubMed
5. Gibbons MC. A historical overview of health disparities and the potential of eHealth solutions. J Med Internet Res. 2005;7:e50. PubMed
6. Nelson LA, Mulvaney SA, Gebretsadik T, Ho YX, Johnson KB, Osborn CY. Disparities in the use of a mHealth medication adherence promotion intervention for low-income adults with type2 diabetes. J Am Med Inform Assoc. 2016;23:12-18. PubMed
7. Martin T. Assessing mHealth: opportunities and barriers to patient engagement. J Health Care Poor Underserved. 2012;23:935-941. PubMed
8. Horn IB, Mendoza FS. Reframing the disparities agenda: a time to rethink, a time to focus. Acad Pediatr. 2013;14:115-116. PubMed
9. Viswanath K, Nagler RH, Bigman-Galimore CA, McCauley MP, Jung M, Ramanadhan S. The communications revolution and health inequalities in the 21st century: implications for cancer control. Cancer Epidemiol, BiomarkersPrev. 2012;21:1701-1708. PubMed
10. Pew Research Center. The Smartphone Difference. Pew Research Center; April 2015. Available at: http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015/. Accessed January 12, 2017.
11. Baker DW. The meaning and measure of health literacy. J Gen Intern Med. 2011;21:878-883. PubMed
12. Morris NS, MacLean CD, Chew LD, Littenberg B. The single item literacy screener: evalution of a brief instrument to identify limited reading ability. BMC Fam Pract. 2006;7:21. PubMed
13. Sentell T, Braun K. Low health literacy, limited English proficiency, and health status in Asians, Latinos, and other racial/ethnic groups in California. J Health Commun. 2012;17:82-99. PubMed
14. Arora S, Ford K, Terp S, et al. Describing the evolution of mobile technology usage for Latino patients and comparing findings to national mHealth estimates. J Am Med Inform Assoc. 2016;23:979-983. PubMed
15. Paasche-Orlow MK, Parker RM, Gazmararian JA, Nielsen-Bohlman LT, Rudd RR. The prevalence of limited health literacy. J Gen Intern Med. 2005;20:175-184. PubMed
16. US Census Bureau. Detailed languages spoken at home and ability to speak English for the population 5 years and over: 2009-2013. Published 2015. Available at: http://www.census.gov/data/tables/2013/demo/2009-2013-lang-tables.html. Accessed March 31, 2016.
17. Eysenbach G, CONSORT-EHEALTH Group. CONSORT-EHEALTH: improving and standardizing evaluation reports of Web-based and mobile health intervention. J Med Internet Res. 2011;13:e126. PubMed
18. Schickedanz A, Huang D, Lopex A, et al. Access, interest, and attitudes toward electronic communication for health care among patients in the medical safety net. J Gen Intern Med. 2013;28:914-920. PubMed
© 2017 Society of Hospital Medicine
Medical and economic burden of heparin-induced thrombocytopenia: A retrospective nationwide inpatient sample (NIS) study
Each year, approximately one-third of all hospitalized medical and surgical patients in the United States (about 12 million patients) are exposed to heparin products for the prevention or treatment of thromboembolism.1 Although generally safe, heparin can trigger an immune response in which platelet factor 4–heparin complexes set off an antibody-mediated cascade that can result in heparin-induced thrombocytopenia (HIT) and paradoxical arterial and venous thromboses, or heparin-induced thrombocytopenia with thrombosis (HITT). The incidence of HIT appears to be significantly higher with the more immunogenic unfractionated heparin (UFH) (2%-3% if treated for ≥5 days) than with low-molecular-weight heparin (LMWH) (0.2%-0.6%)2 and is significantly higher in postoperative patients (1%-5%) than in medical patients.3 Older patients and female patients, especially those who undergo surgery, are thought to be at higher risk.4 Progression from HIT to HITT can occur in up to 50% of surgical patients,5 and HITT can significantly increase mortality.4
In the United States, LMWH use has increased 5-fold since 2000—an increase attributed to the 2010 release of generic enoxaparin.6 As US hospitals switch from UFH to LMWH with its significantly lower risk of HIT, up-to-date HIT incidence data may help physicians and payers better understand the impact of the disorder on mortality and hospital length of stay (LOS) for medical patients and subsets of surgical patients and subsequently direct screening efforts to those at highest risk. Therefore, in the present study, we used national data to determine the latest incidence and economic implications of HIT overall and for high-risk surgical groups.
METHODS
In this study, we analyzed data from the Nationwide Inpatient Sample (NIS) database, part of the Healthcare Cost and Utilization Project (HCUP) of the Agency for Healthcare Research and Quality (AHRQ). The period studied was 2009-2011. We used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 289.84, introduced in 2009, to identify patients who were at least 18 years old and had a primary or secondary diagnosis of HIT. Validated Clinical Classifications Software (CCS) was used to identify those who underwent cardiac, vascular, or orthopedic surgery, and ICD-9-CM codes for various thromboses were used to identify those with HITT (Supplemental Figure, Supplemental Table 1). Baseline patient and hospital characteristics were compared using the Pearson’s Chi-square test for categorical variables and the Student t test for continuous variables (2-sided P < 0.05 for statistical significance) (Table 1). We calculated the incidence of HIT overall and for the 3 surgical subgroups and compared the cohorts on their mean hospital LOS, mean hospital charge, and in-hospital mortality (Table 2).
Statistical analysis was performed with Stata Version 13.1 (Stata Corp, College Station, TX). Survey commands were used to account for the complex survey design in NIS. Reading Health System’s Institutional Review Board determined that our study protocol was exempt.
RESULTS
Of 98,636,364 hospitalizations, 72,515 (0.07%) involved HIT. There were no significant differences in the annual incidence of HIT during the study period (0.06% in 2009, 0.05% in 2010, 0.06% in 2011).
Regarding HIT, the death rate was 4-fold higher for patients with the disorder (9.63%) than for those without it (2.19%); hospital LOS and costs were significantly higher, too (Table 2). In addition, in-hospital mortality was higher (P < 0.001) for patients with HITT (12.28%) than for patients with HIT without thrombosis (8.24%); HITT patients’ hospital LOS and costs were higher as well. In patients who had cardiac, vascular, or orthopedic surgery, development of HIT was also associated with significantly higher in-hospital mortality, mean hospital LOS, and mean hospital charge. In patients with HITT, deep vein thrombosis (DVT) and pulmonary embolism represented the majority of reported cases (Supplemental Table 2). However, in patients who had cardiac surgery, acute arterial thromboses of coronary and cerebral vessels were more common.
DISCUSSION
In this national database survey, the overall incidence of HIT during the study period 2009-2011 was 0.07%, or 1 in 1350 hospitalized patients. Although earlier studies reported rates as high as 5% for high-risk subgroups of surgical patients,7 our data are more in line with more recently reported rates: about 0.02% for hospital admissions8 and from less than 0.1% to 0.4% for patients who received heparin.9 Older studies, which predominantly involved postoperative patients and were conducted when UFH often was the first-line heparin product used, may account for higher rates relative to ours. Of the 3 types of surgeries we evaluated, cardiac surgery had the highest HIT rate (0.5%), consistent with other studies.4 The higher HIT/HITT rates found for larger urban hospitals in our study might be attributable to increased awareness and testing, availability of hematology consultation, and higher risk of heparin use in this setting, where patients are sicker and cases and procedures more complicated.
Age was an important determinant of HIT risk in our study and in similar large-database series.4 Whether increased UFH use in the elderly (because of age or kidney disease) was a causative factor in this finding is unknown. In our study, although men and women had a nearly equal incidence of HIT, women had a significantly higher risk of HIT after both cardiac surgery and vascular surgery. Immune-mediated mechanisms that are more common in females may play a causative role in these settings.10
Our study results showed HIT associated with increased hospital LOS and an almost 4-fold increase in inpatient mortality and costs. The increased economic burden in HIT cases may be driven by the diagnostic work-up cost and expensive alternative anticoagulation.11,12 Similarly, compared with HIT without thrombosis, HITT was associated with significantly increased hospital LOS (3.7 days), total hospital charge ($64,279) and mortality (49% increase, to 12.2% from 8.2%), consistent with prior studies.13 In addition, 34.1% (24,704) of our HIT patients developed at least 1 thrombotic complication, with venous thromboses more common than arterial thromboses, as previously reported.13 Lower extremity DVT was the most common thrombosis in orthopedic and vascular surgery. However, in cardiac surgery, acute coronary occlusion was the most common thrombotic complication. We postulate that the difference stems from the increased propensity of HIT-related thrombosis to occur in areas of vascular injury.14
The strengths of our study include its large size, which increases the generalizability of its results and avoids the biases inherent in small, single-center studies. As with any administrative dataset, the NIS may include coding errors related to underdiagnosis and overdiagnosis (eg, a HIT/HITT diagnosis carried forward from prior episodes). In our study, we inferred the HITT diagnosis in HIT cases with a vascular complication, but we could have missed HIT cases that had not been coded for vascular complications, and we could have overassociated vascular complications that had predated HIT and been treated with heparin. Although HIT and HITT were associated with worse clinical outcomes and increased hospital LOS, it is possible patients who were hospitalized longer had more opportunities for heparin use, and this exposure led to HIT or HITT. The lack of details regarding prior heparin use, including type of heparin (UFH or LMWH), prevented us from inferring the actual risks of individual heparin products.
In conclusion, in cardiac, vascular, and orthopedic surgery, HIT and especially HITT can significantly increase hospital LOS, inpatient costs, and mortality. Lower extremity DVT and acute coronary artery occlusion are the most common thrombotic complications in these cases. HIT screening strategies that incorporate platelet counts are recommended only in patients at highest risk (>1%), according to the most recent American College of Chest Physicians guidelines, but this recommendation was made on the basis of the high cost of alternative anticoagulants. Given our more recent data regarding the very high costs of HIT and especially HITT, screening strategies with platelet counts may prove more cost-effective. Recent genome-wide studies that found higher rates of HIT in patients with T-cell death–associated gene 8 (TDAG8) may help explain sex differences in postoperative patients and identify patients at highest risk so alternative anticoagulants can be used.15
Disclosures
This study was funded by the Reading Health System (grant RHS0010). Dr. Bhatt is supported by the Physician-Scientist Training Program (grant 2015-2016), College of Medicine, University of Nebraska Medical Center. The other authors report no financial conflicts of interest.
1. Fahey VA. Heparin-induced thrombocytopenia. J Vasc Nurs. 1995;13(4):112-116. PubMed
2. TE, Greinacher A. Heparin-induced thrombocytopenia and cardiac surgery. Ann Thorac Surg. 2003;76(6):2121-2131.
3. Junqueira DR, Perini E, Penholati RR, Carvalho MG. Unfractionated heparin versus low molecular weight heparin for avoiding heparin-induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev. 2012;(9):CD007557. PubMed
4. Seigerman M, Cavallaro P, Itagaki S, Chung I, Chikwe J. Incidence and outcomes of heparin-induced thrombocytopenia in patients undergoing cardiac surgery in North America: an analysis of the Nationwide Inpatient Sample. J Cardiothorac Vasc Anesth. 2014;28(1):98-102. PubMed
5. Greinacher A. Heparin-induced thrombocytopenia. N Engl J Med. 2015;373(3):252-261. PubMed
6. Grabowski HG, Guha R, Salgado M. Regulatory and cost barriers are likely to limit biosimilar development and expected savings in the near future. Health Aff (Millwood). 2014;33(6):1048-1057. PubMed
7. Prandoni P, Siragusa S, Girolami B, Fabris F; BELZONI Investigators Group. The incidence of heparin-induced thrombocytopenia in medical patients treated with low-molecular-weight heparin: a prospective cohort study. Blood. 2005;106(9):3049-3054. PubMed
8. Jenkins I, Helmons PJ, Martin-Armstrong LM, Montazeri ME, Renvall M. High rates of venous thromboembolism prophylaxis did not increase the incidence of heparin-induced thrombocytopenia. Jt Comm J Qual Patient Saf. 2011;37(4):163-169. PubMed
9. Zhou A, Winkler A, Emamifar A, et al. Is the incidence of heparin-induced thrombocytopenia affected by the increased use of heparin for VTE prophylaxis? Chest. 2012;142(5):1175-1178. PubMed
10. Warkentin TE, Sheppard JA, Sigouin CS, Kohlmann T, Eichler P, Greinacher A. Gender imbalance and risk factor interactions in heparin-induced thrombocytopenia. Blood. 2006;108(9):2937-2941. PubMed
11. Baroletti S, Piovella C, Fanikos J, Labreche M, Lin J, Goldhaber SZ. Heparin-induced thrombocytopenia (HIT): clinical and economic outcomes. Thromb Haemost. 2008;100(6):1130-1135. PubMed
12. Smythe MA, Koerber JM, Fitzgerald M, Mattson JC. The financial impact of heparin-induced thrombocytopenia. Chest. 2008;134(3):568-573. PubMed
13. Nand S, Wong W, Yuen B, Yetter A, Schmulbach E, Gross Fisher S. Heparin-induced thrombocytopenia with thrombosis: Incidence, analysis of risk factors, and clinical outcomes in 108 consecutive patients treated at a single institution. Am J Hematol. 1997;56(1):12-16. PubMed
14. Hong AP, Cook DJ, Sigouin CS, Warkentin TE. Central venous catheters and upper-extremity deep-vein thrombosis complicating immune heparin-induced thrombocytopenia. Blood. 2003;101(8):3049-3051. PubMed
15. Karnes JH, Cronin RM, Rollin J, et al. A genome-wide association study of heparin-induced thrombocytopenia using an electronic medical record. Thromb Haemost. 2015;113(4):772-781. PubMed
Each year, approximately one-third of all hospitalized medical and surgical patients in the United States (about 12 million patients) are exposed to heparin products for the prevention or treatment of thromboembolism.1 Although generally safe, heparin can trigger an immune response in which platelet factor 4–heparin complexes set off an antibody-mediated cascade that can result in heparin-induced thrombocytopenia (HIT) and paradoxical arterial and venous thromboses, or heparin-induced thrombocytopenia with thrombosis (HITT). The incidence of HIT appears to be significantly higher with the more immunogenic unfractionated heparin (UFH) (2%-3% if treated for ≥5 days) than with low-molecular-weight heparin (LMWH) (0.2%-0.6%)2 and is significantly higher in postoperative patients (1%-5%) than in medical patients.3 Older patients and female patients, especially those who undergo surgery, are thought to be at higher risk.4 Progression from HIT to HITT can occur in up to 50% of surgical patients,5 and HITT can significantly increase mortality.4
In the United States, LMWH use has increased 5-fold since 2000—an increase attributed to the 2010 release of generic enoxaparin.6 As US hospitals switch from UFH to LMWH with its significantly lower risk of HIT, up-to-date HIT incidence data may help physicians and payers better understand the impact of the disorder on mortality and hospital length of stay (LOS) for medical patients and subsets of surgical patients and subsequently direct screening efforts to those at highest risk. Therefore, in the present study, we used national data to determine the latest incidence and economic implications of HIT overall and for high-risk surgical groups.
METHODS
In this study, we analyzed data from the Nationwide Inpatient Sample (NIS) database, part of the Healthcare Cost and Utilization Project (HCUP) of the Agency for Healthcare Research and Quality (AHRQ). The period studied was 2009-2011. We used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 289.84, introduced in 2009, to identify patients who were at least 18 years old and had a primary or secondary diagnosis of HIT. Validated Clinical Classifications Software (CCS) was used to identify those who underwent cardiac, vascular, or orthopedic surgery, and ICD-9-CM codes for various thromboses were used to identify those with HITT (Supplemental Figure, Supplemental Table 1). Baseline patient and hospital characteristics were compared using the Pearson’s Chi-square test for categorical variables and the Student t test for continuous variables (2-sided P < 0.05 for statistical significance) (Table 1). We calculated the incidence of HIT overall and for the 3 surgical subgroups and compared the cohorts on their mean hospital LOS, mean hospital charge, and in-hospital mortality (Table 2).
Statistical analysis was performed with Stata Version 13.1 (Stata Corp, College Station, TX). Survey commands were used to account for the complex survey design in NIS. Reading Health System’s Institutional Review Board determined that our study protocol was exempt.
RESULTS
Of 98,636,364 hospitalizations, 72,515 (0.07%) involved HIT. There were no significant differences in the annual incidence of HIT during the study period (0.06% in 2009, 0.05% in 2010, 0.06% in 2011).
Regarding HIT, the death rate was 4-fold higher for patients with the disorder (9.63%) than for those without it (2.19%); hospital LOS and costs were significantly higher, too (Table 2). In addition, in-hospital mortality was higher (P < 0.001) for patients with HITT (12.28%) than for patients with HIT without thrombosis (8.24%); HITT patients’ hospital LOS and costs were higher as well. In patients who had cardiac, vascular, or orthopedic surgery, development of HIT was also associated with significantly higher in-hospital mortality, mean hospital LOS, and mean hospital charge. In patients with HITT, deep vein thrombosis (DVT) and pulmonary embolism represented the majority of reported cases (Supplemental Table 2). However, in patients who had cardiac surgery, acute arterial thromboses of coronary and cerebral vessels were more common.
DISCUSSION
In this national database survey, the overall incidence of HIT during the study period 2009-2011 was 0.07%, or 1 in 1350 hospitalized patients. Although earlier studies reported rates as high as 5% for high-risk subgroups of surgical patients,7 our data are more in line with more recently reported rates: about 0.02% for hospital admissions8 and from less than 0.1% to 0.4% for patients who received heparin.9 Older studies, which predominantly involved postoperative patients and were conducted when UFH often was the first-line heparin product used, may account for higher rates relative to ours. Of the 3 types of surgeries we evaluated, cardiac surgery had the highest HIT rate (0.5%), consistent with other studies.4 The higher HIT/HITT rates found for larger urban hospitals in our study might be attributable to increased awareness and testing, availability of hematology consultation, and higher risk of heparin use in this setting, where patients are sicker and cases and procedures more complicated.
Age was an important determinant of HIT risk in our study and in similar large-database series.4 Whether increased UFH use in the elderly (because of age or kidney disease) was a causative factor in this finding is unknown. In our study, although men and women had a nearly equal incidence of HIT, women had a significantly higher risk of HIT after both cardiac surgery and vascular surgery. Immune-mediated mechanisms that are more common in females may play a causative role in these settings.10
Our study results showed HIT associated with increased hospital LOS and an almost 4-fold increase in inpatient mortality and costs. The increased economic burden in HIT cases may be driven by the diagnostic work-up cost and expensive alternative anticoagulation.11,12 Similarly, compared with HIT without thrombosis, HITT was associated with significantly increased hospital LOS (3.7 days), total hospital charge ($64,279) and mortality (49% increase, to 12.2% from 8.2%), consistent with prior studies.13 In addition, 34.1% (24,704) of our HIT patients developed at least 1 thrombotic complication, with venous thromboses more common than arterial thromboses, as previously reported.13 Lower extremity DVT was the most common thrombosis in orthopedic and vascular surgery. However, in cardiac surgery, acute coronary occlusion was the most common thrombotic complication. We postulate that the difference stems from the increased propensity of HIT-related thrombosis to occur in areas of vascular injury.14
The strengths of our study include its large size, which increases the generalizability of its results and avoids the biases inherent in small, single-center studies. As with any administrative dataset, the NIS may include coding errors related to underdiagnosis and overdiagnosis (eg, a HIT/HITT diagnosis carried forward from prior episodes). In our study, we inferred the HITT diagnosis in HIT cases with a vascular complication, but we could have missed HIT cases that had not been coded for vascular complications, and we could have overassociated vascular complications that had predated HIT and been treated with heparin. Although HIT and HITT were associated with worse clinical outcomes and increased hospital LOS, it is possible patients who were hospitalized longer had more opportunities for heparin use, and this exposure led to HIT or HITT. The lack of details regarding prior heparin use, including type of heparin (UFH or LMWH), prevented us from inferring the actual risks of individual heparin products.
In conclusion, in cardiac, vascular, and orthopedic surgery, HIT and especially HITT can significantly increase hospital LOS, inpatient costs, and mortality. Lower extremity DVT and acute coronary artery occlusion are the most common thrombotic complications in these cases. HIT screening strategies that incorporate platelet counts are recommended only in patients at highest risk (>1%), according to the most recent American College of Chest Physicians guidelines, but this recommendation was made on the basis of the high cost of alternative anticoagulants. Given our more recent data regarding the very high costs of HIT and especially HITT, screening strategies with platelet counts may prove more cost-effective. Recent genome-wide studies that found higher rates of HIT in patients with T-cell death–associated gene 8 (TDAG8) may help explain sex differences in postoperative patients and identify patients at highest risk so alternative anticoagulants can be used.15
Disclosures
This study was funded by the Reading Health System (grant RHS0010). Dr. Bhatt is supported by the Physician-Scientist Training Program (grant 2015-2016), College of Medicine, University of Nebraska Medical Center. The other authors report no financial conflicts of interest.
Each year, approximately one-third of all hospitalized medical and surgical patients in the United States (about 12 million patients) are exposed to heparin products for the prevention or treatment of thromboembolism.1 Although generally safe, heparin can trigger an immune response in which platelet factor 4–heparin complexes set off an antibody-mediated cascade that can result in heparin-induced thrombocytopenia (HIT) and paradoxical arterial and venous thromboses, or heparin-induced thrombocytopenia with thrombosis (HITT). The incidence of HIT appears to be significantly higher with the more immunogenic unfractionated heparin (UFH) (2%-3% if treated for ≥5 days) than with low-molecular-weight heparin (LMWH) (0.2%-0.6%)2 and is significantly higher in postoperative patients (1%-5%) than in medical patients.3 Older patients and female patients, especially those who undergo surgery, are thought to be at higher risk.4 Progression from HIT to HITT can occur in up to 50% of surgical patients,5 and HITT can significantly increase mortality.4
In the United States, LMWH use has increased 5-fold since 2000—an increase attributed to the 2010 release of generic enoxaparin.6 As US hospitals switch from UFH to LMWH with its significantly lower risk of HIT, up-to-date HIT incidence data may help physicians and payers better understand the impact of the disorder on mortality and hospital length of stay (LOS) for medical patients and subsets of surgical patients and subsequently direct screening efforts to those at highest risk. Therefore, in the present study, we used national data to determine the latest incidence and economic implications of HIT overall and for high-risk surgical groups.
METHODS
In this study, we analyzed data from the Nationwide Inpatient Sample (NIS) database, part of the Healthcare Cost and Utilization Project (HCUP) of the Agency for Healthcare Research and Quality (AHRQ). The period studied was 2009-2011. We used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 289.84, introduced in 2009, to identify patients who were at least 18 years old and had a primary or secondary diagnosis of HIT. Validated Clinical Classifications Software (CCS) was used to identify those who underwent cardiac, vascular, or orthopedic surgery, and ICD-9-CM codes for various thromboses were used to identify those with HITT (Supplemental Figure, Supplemental Table 1). Baseline patient and hospital characteristics were compared using the Pearson’s Chi-square test for categorical variables and the Student t test for continuous variables (2-sided P < 0.05 for statistical significance) (Table 1). We calculated the incidence of HIT overall and for the 3 surgical subgroups and compared the cohorts on their mean hospital LOS, mean hospital charge, and in-hospital mortality (Table 2).
Statistical analysis was performed with Stata Version 13.1 (Stata Corp, College Station, TX). Survey commands were used to account for the complex survey design in NIS. Reading Health System’s Institutional Review Board determined that our study protocol was exempt.
RESULTS
Of 98,636,364 hospitalizations, 72,515 (0.07%) involved HIT. There were no significant differences in the annual incidence of HIT during the study period (0.06% in 2009, 0.05% in 2010, 0.06% in 2011).
Regarding HIT, the death rate was 4-fold higher for patients with the disorder (9.63%) than for those without it (2.19%); hospital LOS and costs were significantly higher, too (Table 2). In addition, in-hospital mortality was higher (P < 0.001) for patients with HITT (12.28%) than for patients with HIT without thrombosis (8.24%); HITT patients’ hospital LOS and costs were higher as well. In patients who had cardiac, vascular, or orthopedic surgery, development of HIT was also associated with significantly higher in-hospital mortality, mean hospital LOS, and mean hospital charge. In patients with HITT, deep vein thrombosis (DVT) and pulmonary embolism represented the majority of reported cases (Supplemental Table 2). However, in patients who had cardiac surgery, acute arterial thromboses of coronary and cerebral vessels were more common.
DISCUSSION
In this national database survey, the overall incidence of HIT during the study period 2009-2011 was 0.07%, or 1 in 1350 hospitalized patients. Although earlier studies reported rates as high as 5% for high-risk subgroups of surgical patients,7 our data are more in line with more recently reported rates: about 0.02% for hospital admissions8 and from less than 0.1% to 0.4% for patients who received heparin.9 Older studies, which predominantly involved postoperative patients and were conducted when UFH often was the first-line heparin product used, may account for higher rates relative to ours. Of the 3 types of surgeries we evaluated, cardiac surgery had the highest HIT rate (0.5%), consistent with other studies.4 The higher HIT/HITT rates found for larger urban hospitals in our study might be attributable to increased awareness and testing, availability of hematology consultation, and higher risk of heparin use in this setting, where patients are sicker and cases and procedures more complicated.
Age was an important determinant of HIT risk in our study and in similar large-database series.4 Whether increased UFH use in the elderly (because of age or kidney disease) was a causative factor in this finding is unknown. In our study, although men and women had a nearly equal incidence of HIT, women had a significantly higher risk of HIT after both cardiac surgery and vascular surgery. Immune-mediated mechanisms that are more common in females may play a causative role in these settings.10
Our study results showed HIT associated with increased hospital LOS and an almost 4-fold increase in inpatient mortality and costs. The increased economic burden in HIT cases may be driven by the diagnostic work-up cost and expensive alternative anticoagulation.11,12 Similarly, compared with HIT without thrombosis, HITT was associated with significantly increased hospital LOS (3.7 days), total hospital charge ($64,279) and mortality (49% increase, to 12.2% from 8.2%), consistent with prior studies.13 In addition, 34.1% (24,704) of our HIT patients developed at least 1 thrombotic complication, with venous thromboses more common than arterial thromboses, as previously reported.13 Lower extremity DVT was the most common thrombosis in orthopedic and vascular surgery. However, in cardiac surgery, acute coronary occlusion was the most common thrombotic complication. We postulate that the difference stems from the increased propensity of HIT-related thrombosis to occur in areas of vascular injury.14
The strengths of our study include its large size, which increases the generalizability of its results and avoids the biases inherent in small, single-center studies. As with any administrative dataset, the NIS may include coding errors related to underdiagnosis and overdiagnosis (eg, a HIT/HITT diagnosis carried forward from prior episodes). In our study, we inferred the HITT diagnosis in HIT cases with a vascular complication, but we could have missed HIT cases that had not been coded for vascular complications, and we could have overassociated vascular complications that had predated HIT and been treated with heparin. Although HIT and HITT were associated with worse clinical outcomes and increased hospital LOS, it is possible patients who were hospitalized longer had more opportunities for heparin use, and this exposure led to HIT or HITT. The lack of details regarding prior heparin use, including type of heparin (UFH or LMWH), prevented us from inferring the actual risks of individual heparin products.
In conclusion, in cardiac, vascular, and orthopedic surgery, HIT and especially HITT can significantly increase hospital LOS, inpatient costs, and mortality. Lower extremity DVT and acute coronary artery occlusion are the most common thrombotic complications in these cases. HIT screening strategies that incorporate platelet counts are recommended only in patients at highest risk (>1%), according to the most recent American College of Chest Physicians guidelines, but this recommendation was made on the basis of the high cost of alternative anticoagulants. Given our more recent data regarding the very high costs of HIT and especially HITT, screening strategies with platelet counts may prove more cost-effective. Recent genome-wide studies that found higher rates of HIT in patients with T-cell death–associated gene 8 (TDAG8) may help explain sex differences in postoperative patients and identify patients at highest risk so alternative anticoagulants can be used.15
Disclosures
This study was funded by the Reading Health System (grant RHS0010). Dr. Bhatt is supported by the Physician-Scientist Training Program (grant 2015-2016), College of Medicine, University of Nebraska Medical Center. The other authors report no financial conflicts of interest.
1. Fahey VA. Heparin-induced thrombocytopenia. J Vasc Nurs. 1995;13(4):112-116. PubMed
2. TE, Greinacher A. Heparin-induced thrombocytopenia and cardiac surgery. Ann Thorac Surg. 2003;76(6):2121-2131.
3. Junqueira DR, Perini E, Penholati RR, Carvalho MG. Unfractionated heparin versus low molecular weight heparin for avoiding heparin-induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev. 2012;(9):CD007557. PubMed
4. Seigerman M, Cavallaro P, Itagaki S, Chung I, Chikwe J. Incidence and outcomes of heparin-induced thrombocytopenia in patients undergoing cardiac surgery in North America: an analysis of the Nationwide Inpatient Sample. J Cardiothorac Vasc Anesth. 2014;28(1):98-102. PubMed
5. Greinacher A. Heparin-induced thrombocytopenia. N Engl J Med. 2015;373(3):252-261. PubMed
6. Grabowski HG, Guha R, Salgado M. Regulatory and cost barriers are likely to limit biosimilar development and expected savings in the near future. Health Aff (Millwood). 2014;33(6):1048-1057. PubMed
7. Prandoni P, Siragusa S, Girolami B, Fabris F; BELZONI Investigators Group. The incidence of heparin-induced thrombocytopenia in medical patients treated with low-molecular-weight heparin: a prospective cohort study. Blood. 2005;106(9):3049-3054. PubMed
8. Jenkins I, Helmons PJ, Martin-Armstrong LM, Montazeri ME, Renvall M. High rates of venous thromboembolism prophylaxis did not increase the incidence of heparin-induced thrombocytopenia. Jt Comm J Qual Patient Saf. 2011;37(4):163-169. PubMed
9. Zhou A, Winkler A, Emamifar A, et al. Is the incidence of heparin-induced thrombocytopenia affected by the increased use of heparin for VTE prophylaxis? Chest. 2012;142(5):1175-1178. PubMed
10. Warkentin TE, Sheppard JA, Sigouin CS, Kohlmann T, Eichler P, Greinacher A. Gender imbalance and risk factor interactions in heparin-induced thrombocytopenia. Blood. 2006;108(9):2937-2941. PubMed
11. Baroletti S, Piovella C, Fanikos J, Labreche M, Lin J, Goldhaber SZ. Heparin-induced thrombocytopenia (HIT): clinical and economic outcomes. Thromb Haemost. 2008;100(6):1130-1135. PubMed
12. Smythe MA, Koerber JM, Fitzgerald M, Mattson JC. The financial impact of heparin-induced thrombocytopenia. Chest. 2008;134(3):568-573. PubMed
13. Nand S, Wong W, Yuen B, Yetter A, Schmulbach E, Gross Fisher S. Heparin-induced thrombocytopenia with thrombosis: Incidence, analysis of risk factors, and clinical outcomes in 108 consecutive patients treated at a single institution. Am J Hematol. 1997;56(1):12-16. PubMed
14. Hong AP, Cook DJ, Sigouin CS, Warkentin TE. Central venous catheters and upper-extremity deep-vein thrombosis complicating immune heparin-induced thrombocytopenia. Blood. 2003;101(8):3049-3051. PubMed
15. Karnes JH, Cronin RM, Rollin J, et al. A genome-wide association study of heparin-induced thrombocytopenia using an electronic medical record. Thromb Haemost. 2015;113(4):772-781. PubMed
1. Fahey VA. Heparin-induced thrombocytopenia. J Vasc Nurs. 1995;13(4):112-116. PubMed
2. TE, Greinacher A. Heparin-induced thrombocytopenia and cardiac surgery. Ann Thorac Surg. 2003;76(6):2121-2131.
3. Junqueira DR, Perini E, Penholati RR, Carvalho MG. Unfractionated heparin versus low molecular weight heparin for avoiding heparin-induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev. 2012;(9):CD007557. PubMed
4. Seigerman M, Cavallaro P, Itagaki S, Chung I, Chikwe J. Incidence and outcomes of heparin-induced thrombocytopenia in patients undergoing cardiac surgery in North America: an analysis of the Nationwide Inpatient Sample. J Cardiothorac Vasc Anesth. 2014;28(1):98-102. PubMed
5. Greinacher A. Heparin-induced thrombocytopenia. N Engl J Med. 2015;373(3):252-261. PubMed
6. Grabowski HG, Guha R, Salgado M. Regulatory and cost barriers are likely to limit biosimilar development and expected savings in the near future. Health Aff (Millwood). 2014;33(6):1048-1057. PubMed
7. Prandoni P, Siragusa S, Girolami B, Fabris F; BELZONI Investigators Group. The incidence of heparin-induced thrombocytopenia in medical patients treated with low-molecular-weight heparin: a prospective cohort study. Blood. 2005;106(9):3049-3054. PubMed
8. Jenkins I, Helmons PJ, Martin-Armstrong LM, Montazeri ME, Renvall M. High rates of venous thromboembolism prophylaxis did not increase the incidence of heparin-induced thrombocytopenia. Jt Comm J Qual Patient Saf. 2011;37(4):163-169. PubMed
9. Zhou A, Winkler A, Emamifar A, et al. Is the incidence of heparin-induced thrombocytopenia affected by the increased use of heparin for VTE prophylaxis? Chest. 2012;142(5):1175-1178. PubMed
10. Warkentin TE, Sheppard JA, Sigouin CS, Kohlmann T, Eichler P, Greinacher A. Gender imbalance and risk factor interactions in heparin-induced thrombocytopenia. Blood. 2006;108(9):2937-2941. PubMed
11. Baroletti S, Piovella C, Fanikos J, Labreche M, Lin J, Goldhaber SZ. Heparin-induced thrombocytopenia (HIT): clinical and economic outcomes. Thromb Haemost. 2008;100(6):1130-1135. PubMed
12. Smythe MA, Koerber JM, Fitzgerald M, Mattson JC. The financial impact of heparin-induced thrombocytopenia. Chest. 2008;134(3):568-573. PubMed
13. Nand S, Wong W, Yuen B, Yetter A, Schmulbach E, Gross Fisher S. Heparin-induced thrombocytopenia with thrombosis: Incidence, analysis of risk factors, and clinical outcomes in 108 consecutive patients treated at a single institution. Am J Hematol. 1997;56(1):12-16. PubMed
14. Hong AP, Cook DJ, Sigouin CS, Warkentin TE. Central venous catheters and upper-extremity deep-vein thrombosis complicating immune heparin-induced thrombocytopenia. Blood. 2003;101(8):3049-3051. PubMed
15. Karnes JH, Cronin RM, Rollin J, et al. A genome-wide association study of heparin-induced thrombocytopenia using an electronic medical record. Thromb Haemost. 2015;113(4):772-781. PubMed
© 2017 Society of Hospital Medicine
Assessment of readability, understandability, and completeness of pediatric hospital medicine discharge instructions
The average American adult reads at an 8th-grade level.1 Limited general literacy can affect health literacy, which is defined as the “degree to which individuals have the capacity to obtain, process and understand basic health information and services needed to make appropriate health decisions.”2,3 Adults with limited health literacy are at risk for poorer outcomes, including overuse of the emergency department and lower adherence to preventive care recommendations.4
Children transitioning from hospital to home depend on their adult caregivers (and their caregivers’ health literacy) to carry out discharge instructions. During the immediate postdischarge period, complex care needs can involve new or changed medications, follow-up instructions, home care instructions, and suggestions regarding when and why to seek additional care.
The discharge education provided to patients in the hospital is often subpar because of lack of standardization and divided responsibility among providers.5 Communication of vital information to patients with low health literacy has been noted to be particularly poor,6 as many patient education materials are written at 10th-, 11th-, and 12th-grade reading levels.4 Evidence supports providing materials written at 6th-grade level or lower to increase comprehension.7 Several studies have evaluated the quality and readability of discharge instructions for hospitalized adults,8,9 and one study found a link between poorly written instructions for adult patients and readmission risk.10 Less is known about readability in pediatrics, in which education may be more important for families of children most commonly hospitalized for acute illness.
We conducted a study to describe readability levels, understandability scores, and completeness of written instructions given to families at hospital discharge.
METHODS
Study Design and Setting
In this study, we performed a cross-sectional review of discharge instructions within electronic health records at Cincinnati Children’s Hospital Medical Center (CCHMC). The study was reviewed and approved by CCHMC’s Institutional Review Board. Charts were randomly selected from all hospital medicine service discharges during two 3-month periods of high patient volume: January-March 2014 and January-March 2015.
CCHMC is a large urban academic referral center that is the sole provider of general, subspecialty, and critical pediatric inpatient care for a large geographical area. CCHMC, which has 600 beds, provides cares for many children who live in impoverished settings. Its hospital medicine service consists of 4 teams that care for approximately 7000 children hospitalized with general pediatric illnesses each year. Each team consists of 5 or 6 pediatric residents supervised by a hospital medicine attending.
Providers, most commonly pediatric interns, generate discharge instructions in electronic health records. In this nonautomated process, they use free-text or nonstandardized templates to create content. At discharge, instructions are printed as part of the postvisit summary, which includes updates on medications and scheduled follow-up appointments. Bedside nurses verbally review the instructions with families and provide printed copies for home use.
Data Collection and Analysis
A random sequence generator was used to select charts for review. Instructions written in a language other than English were excluded. Written discharge instructions and clinical information, including age, sex, primary diagnosis, insurance type, number of discharge medications, number of scheduled appointments at discharge, and hospital length of stay, were abstracted from electronic health records and anonymized before analysis. The primary outcomes assessed were discharge instruction readability, understandability, and completeness. Readability was calculated with Fry Readability Scale (FRS) scores,11 which range from 1 to 17 and correspond to reading levels (score 1 = 1st-grade reading level). Health literacy experts have used the FRS to assess readability in health care environments.12
Understandability was measured with the Patient Education Materials Assessment Tool (PEMAT), a validated scoring system provided by the Agency for Healthcare Research and Quality.13 The PEMAT measures the understandability of print materials on a scale ranging from 0% to 100%. Higher scores indicate increased understandability, and scores under 70% indicate instructions are difficult to understand.
Although recent efforts have focused on the development of quality metrics for hospital-to-home transitions of pediatric patients,14 during our study there were no standard items to include in pediatric discharge instructions. Five criteria for completeness were determined by consensus of 3 pediatric hospital medicine faculty and were informed by qualitative results of work performed at our institution—work in which families noted challenges with information overload and a desire for pertinent and usable information that would enhance caregiver confidence and discharge preparedness.15 The criteria included statement of diagnosis, description of diagnosis, signs and symptoms indicative of the need for escalation of care (warning signs), the person caregivers should call if worried, and contact information for the primary care provider, subspecialist, and/or emergency department. Each set of discharge instructions was manually evaluated for completeness (presence of each individual component, number of components present, presence of all components). All charts were scored by the same investigator. A convenience sample of 20 charts was evaluated by a different investigator to ensure rating parameters were clear and classification was consistent (defined as perfect agreement). If the primary rater was undecided on a discharge instruction score, the secondary rater rated the instruction, and consensus was reached.
Means, medians, and ranges were calculated to enumerate the distribution of readability levels, understandability scores, and completeness of discharge instructions. Instructions were classified as readable if the FRS score was 6 or under, as understandable if the PEMAT score was under 70%, and as complete if all 5 criteria were satisfied. Descriptive statistics were generated for all demographic and clinical variables.
RESULTS
Of the study period’s 3819 discharges, 200 were randomly selected for review. Table 1 lists the demographic and clinical information of patients included in the analyses. Median FRS score was 10, indicating a 10th-grade reading level (interquartile range, 8-12; range, 1-13) (Table 2). Only 14 (7%) of 200 discharge instructions had a score of 6 or under. Median PEMAT understandability score was 73% (interquartile range, 64%-82%), and 36% of instructions had a PEMAT score under 70%. No instruction satisfied all 5 of the defined characteristics of complete discharge instructions (Table 2).
DISCUSSION
To our knowledge, this is the first study of the readability, understandability, and completeness of discharge instructions in a pediatric population. We found that the majority of discharge instruction readability levels were 10th grade or higher, that many instructions were difficult to understand, and that important information was missing from many instructions.
Discharge instruction readability levels were higher than the literacy level of many families in surrounding communities. The high school dropout rates in Cincinnati are staggering; they range from 22% to 64% in the 10 neighborhoods with the largest proportion of residents not completing high school.16 However, such findings are not unique to Cincinnati; low literacy is prevalent throughout the United States. Caregivers with limited literacy skills may struggle to navigate complex health systems, understand medical instructions and anticipatory guidance, perform child care and self-care tasks, and understand issues related to consent, medical authorization, and risk communication.17
Although readability is important, other factors also correlate with comprehension and execution of discharge tasks.18 Information must be understandable, or presented in a way that makes sense and can inform appropriate action. In many cases in our study, instructions were incomplete, despite previous investigators’ emphasizing caregivers’ desire and need for written instructions that are complete, informative, and inclusive of clearly outlined contingency plans.15,19 In addition, families may differ in the level of support needed after discharge; standardizing elements and including families in the development of discharge instructions may improve communication.8
This study had several limitations. First, the discharge instructions randomly selected for review were all written during the winter months. As the census on the hospital medicine teams is particularly high during that time, authors with competing responsibilities may not have had enough time to write effective discharge instructions then. We selected the winter period in order to capture real-world instructions written during a busy clinical time, when providers care for a high volume of patients. Second, caregiver health literacy and English-language proficiency were not assessed, and information regarding caregivers’ race/ethnicity, educational attainment, and socioeconomic status was unavailable. Third, interrater agreement was not formally evaluated. Fourth, this was a single-center study with results that may not be generalizable.
In conclusion, discharge instructions for pediatric patients are often difficult to read and understand, and incomplete. Efforts to address these communication gaps—including educational initiatives for physician trainees focused on health literacy, and quality improvement work directed at standardization and creation of readable, understandable, and complete discharge instructions—are crucial in providing safe, high-value care. Researchers need to evaluate the relationship between discharge instruction quality and outcomes, including unplanned office visits, emergency department visits, and readmissions.
Disclosure
Nothing to report.
1. Kutner MA, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America’s Adults: Results From the 2003 National Assessment of Adult Literacy. Washington, DC: US Dept of Education, National Center for Education Statistics; 2006. NCES publication 2006-483. https://nces.ed.gov/pubs2006/2006483.pdf. Published September 2006. Accessed December 21, 2016.
2. Ratzan SC, Parker RM. Introduction. In: Selden CR, Zorn M, Ratzan S, Parker RM, eds. National Library of Medicine Current Bibliographies in Medicine: Health Literacy. Bethesda, MD: US Dept of Health and Human Services, National Institutes of Health; 2000:v-vi. NLM publication CBM 2000-1. https://www.nlm.nih.gov/archive//20061214/pubs/cbm/hliteracy.pdf. Published February 2000. Accessed December 21, 2016.
3. Arora VM, Schaninger C, D’Arcy M, et al. Improving inpatients’ identification of their doctors: use of FACE cards. Jt Comm J Qual Patient Saf. 2009;35(12):613-619. PubMed
4. Berkman ND, Sheridan SL, Donahue KE, et al. Health literacy interventions and outcomes: an updated systematic review. Evid Rep Technol Assess (Full Rep). 2011;(199):1-941. PubMed
5. Ashbrook L, Mourad M, Sehgal N. Communicating discharge instructions to patients: a survey of nurse, intern, and hospitalist practices. J Hosp Med. 2013;8(1):36-41. PubMed
6. Kripalani S, Jacobson TA, Mugalla IC, Cawthon CR, Niesner KJ, Vaccarino V. Health literacy and the quality of physician–patient communication during hospitalization. J Hosp Med. 2010;5(5):269-275. PubMed
7. Nielsen-Bohlman L, Panzer AM, Kindig DA, eds; Committee on Health Literacy, Board on Neuroscience and Behavioral Health, Institute of Medicine. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004.
8. Hahn-Goldberg S, Okrainec K, Huynh T, Zahr N, Abrams H. Co-creating patient-oriented discharge instructions with patients, caregivers, and healthcare providers. J Hosp Med. 2015;10(12):804-807. PubMed
9. Lauster CD, Gibson JM, DiNella JV, DiNardo M, Korytkowski MT, Donihi AC. Implementation of standardized instructions for insulin at hospital discharge. J Hosp Med. 2009;4(8):E41-E42. PubMed
10. Howard-Anderson J, Busuttil A, Lonowski S, Vangala S, Afsar-Manesh N. From discharge to readmission: understanding the process from the patient perspective. J Hosp Med. 2016;11(6):407-412. PubMed
11. Fry E. A readability formula that saves time. J Reading. 1968;11:513-516, 575-578.
12. D’Alessandro DM, Kingsley P, Johnson-West J. The readability of pediatric patient education materials on the World Wide Web. Arch Pediatr Adolesc Med. 2001;155(7):807-812. PubMed
13. Shoemaker SJ, Wolf MS, Brach C. The Patient Education Materials Assessment Tool (PEMAT) and User’s Guide: An Instrument to Assess the Understandability and Actionability of Print and Audiovisual Patient Education Materials. Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.ahrq.gov/professionals/prevention-chronic-care/improve/self-mgmt/pemat/index.html. Published October 2013. Accessed November 27, 2013.
14. Leyenaar JK, Desai AD, Burkhart Q, et al. Quality measures to assess care transitions for hospitalized children. Pediatrics. 2016;138(2). PubMed
15. Solan LG, Beck AF, Brunswick SA, et al; H2O Study Group. The family perspective on hospital to home transitions: a qualitative study. Pediatrics. 2015;136(6):e1539-e1549. PubMed
16. Maloney M, Auffrey C. The Social Areas of Cincinnati: An Analysis of Social Needs: Patterns for Five Census Decades. 5th ed. Cincinnati, OH: University of Cincinnati School of Planning/United Way/University of Cincinnati Community Research Collaborative; 2013. http://www.socialareasofcincinnati.org/files/FifthEdition/SASBook.pdf. Published April 2013. Accessed December 21, 2016.
17. Rothman RL, Yin HS, Mulvaney S, Co JP, Homer C, Lannon C. Health literacy and quality: focus on chronic illness care and patient safety. Pediatrics. 2009;124(suppl 3):S315-S326. PubMed
18. Moon RY, Cheng TL, Patel KM, Baumhaft K, Scheidt PC. Parental literacy level and understanding of medical information. Pediatrics. 1998;102(2):e25. PubMed
19. Desai AD, Durkin LK, Jacob-Files EA, Mangione-Smith R. Caregiver perceptions of hospital to home transitions according to medical complexity: a qualitative study. Acad Pediatr. 2016;16(2):136-144. PubMed
The average American adult reads at an 8th-grade level.1 Limited general literacy can affect health literacy, which is defined as the “degree to which individuals have the capacity to obtain, process and understand basic health information and services needed to make appropriate health decisions.”2,3 Adults with limited health literacy are at risk for poorer outcomes, including overuse of the emergency department and lower adherence to preventive care recommendations.4
Children transitioning from hospital to home depend on their adult caregivers (and their caregivers’ health literacy) to carry out discharge instructions. During the immediate postdischarge period, complex care needs can involve new or changed medications, follow-up instructions, home care instructions, and suggestions regarding when and why to seek additional care.
The discharge education provided to patients in the hospital is often subpar because of lack of standardization and divided responsibility among providers.5 Communication of vital information to patients with low health literacy has been noted to be particularly poor,6 as many patient education materials are written at 10th-, 11th-, and 12th-grade reading levels.4 Evidence supports providing materials written at 6th-grade level or lower to increase comprehension.7 Several studies have evaluated the quality and readability of discharge instructions for hospitalized adults,8,9 and one study found a link between poorly written instructions for adult patients and readmission risk.10 Less is known about readability in pediatrics, in which education may be more important for families of children most commonly hospitalized for acute illness.
We conducted a study to describe readability levels, understandability scores, and completeness of written instructions given to families at hospital discharge.
METHODS
Study Design and Setting
In this study, we performed a cross-sectional review of discharge instructions within electronic health records at Cincinnati Children’s Hospital Medical Center (CCHMC). The study was reviewed and approved by CCHMC’s Institutional Review Board. Charts were randomly selected from all hospital medicine service discharges during two 3-month periods of high patient volume: January-March 2014 and January-March 2015.
CCHMC is a large urban academic referral center that is the sole provider of general, subspecialty, and critical pediatric inpatient care for a large geographical area. CCHMC, which has 600 beds, provides cares for many children who live in impoverished settings. Its hospital medicine service consists of 4 teams that care for approximately 7000 children hospitalized with general pediatric illnesses each year. Each team consists of 5 or 6 pediatric residents supervised by a hospital medicine attending.
Providers, most commonly pediatric interns, generate discharge instructions in electronic health records. In this nonautomated process, they use free-text or nonstandardized templates to create content. At discharge, instructions are printed as part of the postvisit summary, which includes updates on medications and scheduled follow-up appointments. Bedside nurses verbally review the instructions with families and provide printed copies for home use.
Data Collection and Analysis
A random sequence generator was used to select charts for review. Instructions written in a language other than English were excluded. Written discharge instructions and clinical information, including age, sex, primary diagnosis, insurance type, number of discharge medications, number of scheduled appointments at discharge, and hospital length of stay, were abstracted from electronic health records and anonymized before analysis. The primary outcomes assessed were discharge instruction readability, understandability, and completeness. Readability was calculated with Fry Readability Scale (FRS) scores,11 which range from 1 to 17 and correspond to reading levels (score 1 = 1st-grade reading level). Health literacy experts have used the FRS to assess readability in health care environments.12
Understandability was measured with the Patient Education Materials Assessment Tool (PEMAT), a validated scoring system provided by the Agency for Healthcare Research and Quality.13 The PEMAT measures the understandability of print materials on a scale ranging from 0% to 100%. Higher scores indicate increased understandability, and scores under 70% indicate instructions are difficult to understand.
Although recent efforts have focused on the development of quality metrics for hospital-to-home transitions of pediatric patients,14 during our study there were no standard items to include in pediatric discharge instructions. Five criteria for completeness were determined by consensus of 3 pediatric hospital medicine faculty and were informed by qualitative results of work performed at our institution—work in which families noted challenges with information overload and a desire for pertinent and usable information that would enhance caregiver confidence and discharge preparedness.15 The criteria included statement of diagnosis, description of diagnosis, signs and symptoms indicative of the need for escalation of care (warning signs), the person caregivers should call if worried, and contact information for the primary care provider, subspecialist, and/or emergency department. Each set of discharge instructions was manually evaluated for completeness (presence of each individual component, number of components present, presence of all components). All charts were scored by the same investigator. A convenience sample of 20 charts was evaluated by a different investigator to ensure rating parameters were clear and classification was consistent (defined as perfect agreement). If the primary rater was undecided on a discharge instruction score, the secondary rater rated the instruction, and consensus was reached.
Means, medians, and ranges were calculated to enumerate the distribution of readability levels, understandability scores, and completeness of discharge instructions. Instructions were classified as readable if the FRS score was 6 or under, as understandable if the PEMAT score was under 70%, and as complete if all 5 criteria were satisfied. Descriptive statistics were generated for all demographic and clinical variables.
RESULTS
Of the study period’s 3819 discharges, 200 were randomly selected for review. Table 1 lists the demographic and clinical information of patients included in the analyses. Median FRS score was 10, indicating a 10th-grade reading level (interquartile range, 8-12; range, 1-13) (Table 2). Only 14 (7%) of 200 discharge instructions had a score of 6 or under. Median PEMAT understandability score was 73% (interquartile range, 64%-82%), and 36% of instructions had a PEMAT score under 70%. No instruction satisfied all 5 of the defined characteristics of complete discharge instructions (Table 2).
DISCUSSION
To our knowledge, this is the first study of the readability, understandability, and completeness of discharge instructions in a pediatric population. We found that the majority of discharge instruction readability levels were 10th grade or higher, that many instructions were difficult to understand, and that important information was missing from many instructions.
Discharge instruction readability levels were higher than the literacy level of many families in surrounding communities. The high school dropout rates in Cincinnati are staggering; they range from 22% to 64% in the 10 neighborhoods with the largest proportion of residents not completing high school.16 However, such findings are not unique to Cincinnati; low literacy is prevalent throughout the United States. Caregivers with limited literacy skills may struggle to navigate complex health systems, understand medical instructions and anticipatory guidance, perform child care and self-care tasks, and understand issues related to consent, medical authorization, and risk communication.17
Although readability is important, other factors also correlate with comprehension and execution of discharge tasks.18 Information must be understandable, or presented in a way that makes sense and can inform appropriate action. In many cases in our study, instructions were incomplete, despite previous investigators’ emphasizing caregivers’ desire and need for written instructions that are complete, informative, and inclusive of clearly outlined contingency plans.15,19 In addition, families may differ in the level of support needed after discharge; standardizing elements and including families in the development of discharge instructions may improve communication.8
This study had several limitations. First, the discharge instructions randomly selected for review were all written during the winter months. As the census on the hospital medicine teams is particularly high during that time, authors with competing responsibilities may not have had enough time to write effective discharge instructions then. We selected the winter period in order to capture real-world instructions written during a busy clinical time, when providers care for a high volume of patients. Second, caregiver health literacy and English-language proficiency were not assessed, and information regarding caregivers’ race/ethnicity, educational attainment, and socioeconomic status was unavailable. Third, interrater agreement was not formally evaluated. Fourth, this was a single-center study with results that may not be generalizable.
In conclusion, discharge instructions for pediatric patients are often difficult to read and understand, and incomplete. Efforts to address these communication gaps—including educational initiatives for physician trainees focused on health literacy, and quality improvement work directed at standardization and creation of readable, understandable, and complete discharge instructions—are crucial in providing safe, high-value care. Researchers need to evaluate the relationship between discharge instruction quality and outcomes, including unplanned office visits, emergency department visits, and readmissions.
Disclosure
Nothing to report.
The average American adult reads at an 8th-grade level.1 Limited general literacy can affect health literacy, which is defined as the “degree to which individuals have the capacity to obtain, process and understand basic health information and services needed to make appropriate health decisions.”2,3 Adults with limited health literacy are at risk for poorer outcomes, including overuse of the emergency department and lower adherence to preventive care recommendations.4
Children transitioning from hospital to home depend on their adult caregivers (and their caregivers’ health literacy) to carry out discharge instructions. During the immediate postdischarge period, complex care needs can involve new or changed medications, follow-up instructions, home care instructions, and suggestions regarding when and why to seek additional care.
The discharge education provided to patients in the hospital is often subpar because of lack of standardization and divided responsibility among providers.5 Communication of vital information to patients with low health literacy has been noted to be particularly poor,6 as many patient education materials are written at 10th-, 11th-, and 12th-grade reading levels.4 Evidence supports providing materials written at 6th-grade level or lower to increase comprehension.7 Several studies have evaluated the quality and readability of discharge instructions for hospitalized adults,8,9 and one study found a link between poorly written instructions for adult patients and readmission risk.10 Less is known about readability in pediatrics, in which education may be more important for families of children most commonly hospitalized for acute illness.
We conducted a study to describe readability levels, understandability scores, and completeness of written instructions given to families at hospital discharge.
METHODS
Study Design and Setting
In this study, we performed a cross-sectional review of discharge instructions within electronic health records at Cincinnati Children’s Hospital Medical Center (CCHMC). The study was reviewed and approved by CCHMC’s Institutional Review Board. Charts were randomly selected from all hospital medicine service discharges during two 3-month periods of high patient volume: January-March 2014 and January-March 2015.
CCHMC is a large urban academic referral center that is the sole provider of general, subspecialty, and critical pediatric inpatient care for a large geographical area. CCHMC, which has 600 beds, provides cares for many children who live in impoverished settings. Its hospital medicine service consists of 4 teams that care for approximately 7000 children hospitalized with general pediatric illnesses each year. Each team consists of 5 or 6 pediatric residents supervised by a hospital medicine attending.
Providers, most commonly pediatric interns, generate discharge instructions in electronic health records. In this nonautomated process, they use free-text or nonstandardized templates to create content. At discharge, instructions are printed as part of the postvisit summary, which includes updates on medications and scheduled follow-up appointments. Bedside nurses verbally review the instructions with families and provide printed copies for home use.
Data Collection and Analysis
A random sequence generator was used to select charts for review. Instructions written in a language other than English were excluded. Written discharge instructions and clinical information, including age, sex, primary diagnosis, insurance type, number of discharge medications, number of scheduled appointments at discharge, and hospital length of stay, were abstracted from electronic health records and anonymized before analysis. The primary outcomes assessed were discharge instruction readability, understandability, and completeness. Readability was calculated with Fry Readability Scale (FRS) scores,11 which range from 1 to 17 and correspond to reading levels (score 1 = 1st-grade reading level). Health literacy experts have used the FRS to assess readability in health care environments.12
Understandability was measured with the Patient Education Materials Assessment Tool (PEMAT), a validated scoring system provided by the Agency for Healthcare Research and Quality.13 The PEMAT measures the understandability of print materials on a scale ranging from 0% to 100%. Higher scores indicate increased understandability, and scores under 70% indicate instructions are difficult to understand.
Although recent efforts have focused on the development of quality metrics for hospital-to-home transitions of pediatric patients,14 during our study there were no standard items to include in pediatric discharge instructions. Five criteria for completeness were determined by consensus of 3 pediatric hospital medicine faculty and were informed by qualitative results of work performed at our institution—work in which families noted challenges with information overload and a desire for pertinent and usable information that would enhance caregiver confidence and discharge preparedness.15 The criteria included statement of diagnosis, description of diagnosis, signs and symptoms indicative of the need for escalation of care (warning signs), the person caregivers should call if worried, and contact information for the primary care provider, subspecialist, and/or emergency department. Each set of discharge instructions was manually evaluated for completeness (presence of each individual component, number of components present, presence of all components). All charts were scored by the same investigator. A convenience sample of 20 charts was evaluated by a different investigator to ensure rating parameters were clear and classification was consistent (defined as perfect agreement). If the primary rater was undecided on a discharge instruction score, the secondary rater rated the instruction, and consensus was reached.
Means, medians, and ranges were calculated to enumerate the distribution of readability levels, understandability scores, and completeness of discharge instructions. Instructions were classified as readable if the FRS score was 6 or under, as understandable if the PEMAT score was under 70%, and as complete if all 5 criteria were satisfied. Descriptive statistics were generated for all demographic and clinical variables.
RESULTS
Of the study period’s 3819 discharges, 200 were randomly selected for review. Table 1 lists the demographic and clinical information of patients included in the analyses. Median FRS score was 10, indicating a 10th-grade reading level (interquartile range, 8-12; range, 1-13) (Table 2). Only 14 (7%) of 200 discharge instructions had a score of 6 or under. Median PEMAT understandability score was 73% (interquartile range, 64%-82%), and 36% of instructions had a PEMAT score under 70%. No instruction satisfied all 5 of the defined characteristics of complete discharge instructions (Table 2).
DISCUSSION
To our knowledge, this is the first study of the readability, understandability, and completeness of discharge instructions in a pediatric population. We found that the majority of discharge instruction readability levels were 10th grade or higher, that many instructions were difficult to understand, and that important information was missing from many instructions.
Discharge instruction readability levels were higher than the literacy level of many families in surrounding communities. The high school dropout rates in Cincinnati are staggering; they range from 22% to 64% in the 10 neighborhoods with the largest proportion of residents not completing high school.16 However, such findings are not unique to Cincinnati; low literacy is prevalent throughout the United States. Caregivers with limited literacy skills may struggle to navigate complex health systems, understand medical instructions and anticipatory guidance, perform child care and self-care tasks, and understand issues related to consent, medical authorization, and risk communication.17
Although readability is important, other factors also correlate with comprehension and execution of discharge tasks.18 Information must be understandable, or presented in a way that makes sense and can inform appropriate action. In many cases in our study, instructions were incomplete, despite previous investigators’ emphasizing caregivers’ desire and need for written instructions that are complete, informative, and inclusive of clearly outlined contingency plans.15,19 In addition, families may differ in the level of support needed after discharge; standardizing elements and including families in the development of discharge instructions may improve communication.8
This study had several limitations. First, the discharge instructions randomly selected for review were all written during the winter months. As the census on the hospital medicine teams is particularly high during that time, authors with competing responsibilities may not have had enough time to write effective discharge instructions then. We selected the winter period in order to capture real-world instructions written during a busy clinical time, when providers care for a high volume of patients. Second, caregiver health literacy and English-language proficiency were not assessed, and information regarding caregivers’ race/ethnicity, educational attainment, and socioeconomic status was unavailable. Third, interrater agreement was not formally evaluated. Fourth, this was a single-center study with results that may not be generalizable.
In conclusion, discharge instructions for pediatric patients are often difficult to read and understand, and incomplete. Efforts to address these communication gaps—including educational initiatives for physician trainees focused on health literacy, and quality improvement work directed at standardization and creation of readable, understandable, and complete discharge instructions—are crucial in providing safe, high-value care. Researchers need to evaluate the relationship between discharge instruction quality and outcomes, including unplanned office visits, emergency department visits, and readmissions.
Disclosure
Nothing to report.
1. Kutner MA, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America’s Adults: Results From the 2003 National Assessment of Adult Literacy. Washington, DC: US Dept of Education, National Center for Education Statistics; 2006. NCES publication 2006-483. https://nces.ed.gov/pubs2006/2006483.pdf. Published September 2006. Accessed December 21, 2016.
2. Ratzan SC, Parker RM. Introduction. In: Selden CR, Zorn M, Ratzan S, Parker RM, eds. National Library of Medicine Current Bibliographies in Medicine: Health Literacy. Bethesda, MD: US Dept of Health and Human Services, National Institutes of Health; 2000:v-vi. NLM publication CBM 2000-1. https://www.nlm.nih.gov/archive//20061214/pubs/cbm/hliteracy.pdf. Published February 2000. Accessed December 21, 2016.
3. Arora VM, Schaninger C, D’Arcy M, et al. Improving inpatients’ identification of their doctors: use of FACE cards. Jt Comm J Qual Patient Saf. 2009;35(12):613-619. PubMed
4. Berkman ND, Sheridan SL, Donahue KE, et al. Health literacy interventions and outcomes: an updated systematic review. Evid Rep Technol Assess (Full Rep). 2011;(199):1-941. PubMed
5. Ashbrook L, Mourad M, Sehgal N. Communicating discharge instructions to patients: a survey of nurse, intern, and hospitalist practices. J Hosp Med. 2013;8(1):36-41. PubMed
6. Kripalani S, Jacobson TA, Mugalla IC, Cawthon CR, Niesner KJ, Vaccarino V. Health literacy and the quality of physician–patient communication during hospitalization. J Hosp Med. 2010;5(5):269-275. PubMed
7. Nielsen-Bohlman L, Panzer AM, Kindig DA, eds; Committee on Health Literacy, Board on Neuroscience and Behavioral Health, Institute of Medicine. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004.
8. Hahn-Goldberg S, Okrainec K, Huynh T, Zahr N, Abrams H. Co-creating patient-oriented discharge instructions with patients, caregivers, and healthcare providers. J Hosp Med. 2015;10(12):804-807. PubMed
9. Lauster CD, Gibson JM, DiNella JV, DiNardo M, Korytkowski MT, Donihi AC. Implementation of standardized instructions for insulin at hospital discharge. J Hosp Med. 2009;4(8):E41-E42. PubMed
10. Howard-Anderson J, Busuttil A, Lonowski S, Vangala S, Afsar-Manesh N. From discharge to readmission: understanding the process from the patient perspective. J Hosp Med. 2016;11(6):407-412. PubMed
11. Fry E. A readability formula that saves time. J Reading. 1968;11:513-516, 575-578.
12. D’Alessandro DM, Kingsley P, Johnson-West J. The readability of pediatric patient education materials on the World Wide Web. Arch Pediatr Adolesc Med. 2001;155(7):807-812. PubMed
13. Shoemaker SJ, Wolf MS, Brach C. The Patient Education Materials Assessment Tool (PEMAT) and User’s Guide: An Instrument to Assess the Understandability and Actionability of Print and Audiovisual Patient Education Materials. Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.ahrq.gov/professionals/prevention-chronic-care/improve/self-mgmt/pemat/index.html. Published October 2013. Accessed November 27, 2013.
14. Leyenaar JK, Desai AD, Burkhart Q, et al. Quality measures to assess care transitions for hospitalized children. Pediatrics. 2016;138(2). PubMed
15. Solan LG, Beck AF, Brunswick SA, et al; H2O Study Group. The family perspective on hospital to home transitions: a qualitative study. Pediatrics. 2015;136(6):e1539-e1549. PubMed
16. Maloney M, Auffrey C. The Social Areas of Cincinnati: An Analysis of Social Needs: Patterns for Five Census Decades. 5th ed. Cincinnati, OH: University of Cincinnati School of Planning/United Way/University of Cincinnati Community Research Collaborative; 2013. http://www.socialareasofcincinnati.org/files/FifthEdition/SASBook.pdf. Published April 2013. Accessed December 21, 2016.
17. Rothman RL, Yin HS, Mulvaney S, Co JP, Homer C, Lannon C. Health literacy and quality: focus on chronic illness care and patient safety. Pediatrics. 2009;124(suppl 3):S315-S326. PubMed
18. Moon RY, Cheng TL, Patel KM, Baumhaft K, Scheidt PC. Parental literacy level and understanding of medical information. Pediatrics. 1998;102(2):e25. PubMed
19. Desai AD, Durkin LK, Jacob-Files EA, Mangione-Smith R. Caregiver perceptions of hospital to home transitions according to medical complexity: a qualitative study. Acad Pediatr. 2016;16(2):136-144. PubMed
1. Kutner MA, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America’s Adults: Results From the 2003 National Assessment of Adult Literacy. Washington, DC: US Dept of Education, National Center for Education Statistics; 2006. NCES publication 2006-483. https://nces.ed.gov/pubs2006/2006483.pdf. Published September 2006. Accessed December 21, 2016.
2. Ratzan SC, Parker RM. Introduction. In: Selden CR, Zorn M, Ratzan S, Parker RM, eds. National Library of Medicine Current Bibliographies in Medicine: Health Literacy. Bethesda, MD: US Dept of Health and Human Services, National Institutes of Health; 2000:v-vi. NLM publication CBM 2000-1. https://www.nlm.nih.gov/archive//20061214/pubs/cbm/hliteracy.pdf. Published February 2000. Accessed December 21, 2016.
3. Arora VM, Schaninger C, D’Arcy M, et al. Improving inpatients’ identification of their doctors: use of FACE cards. Jt Comm J Qual Patient Saf. 2009;35(12):613-619. PubMed
4. Berkman ND, Sheridan SL, Donahue KE, et al. Health literacy interventions and outcomes: an updated systematic review. Evid Rep Technol Assess (Full Rep). 2011;(199):1-941. PubMed
5. Ashbrook L, Mourad M, Sehgal N. Communicating discharge instructions to patients: a survey of nurse, intern, and hospitalist practices. J Hosp Med. 2013;8(1):36-41. PubMed
6. Kripalani S, Jacobson TA, Mugalla IC, Cawthon CR, Niesner KJ, Vaccarino V. Health literacy and the quality of physician–patient communication during hospitalization. J Hosp Med. 2010;5(5):269-275. PubMed
7. Nielsen-Bohlman L, Panzer AM, Kindig DA, eds; Committee on Health Literacy, Board on Neuroscience and Behavioral Health, Institute of Medicine. Health Literacy: A Prescription to End Confusion. Washington, DC: National Academies Press; 2004.
8. Hahn-Goldberg S, Okrainec K, Huynh T, Zahr N, Abrams H. Co-creating patient-oriented discharge instructions with patients, caregivers, and healthcare providers. J Hosp Med. 2015;10(12):804-807. PubMed
9. Lauster CD, Gibson JM, DiNella JV, DiNardo M, Korytkowski MT, Donihi AC. Implementation of standardized instructions for insulin at hospital discharge. J Hosp Med. 2009;4(8):E41-E42. PubMed
10. Howard-Anderson J, Busuttil A, Lonowski S, Vangala S, Afsar-Manesh N. From discharge to readmission: understanding the process from the patient perspective. J Hosp Med. 2016;11(6):407-412. PubMed
11. Fry E. A readability formula that saves time. J Reading. 1968;11:513-516, 575-578.
12. D’Alessandro DM, Kingsley P, Johnson-West J. The readability of pediatric patient education materials on the World Wide Web. Arch Pediatr Adolesc Med. 2001;155(7):807-812. PubMed
13. Shoemaker SJ, Wolf MS, Brach C. The Patient Education Materials Assessment Tool (PEMAT) and User’s Guide: An Instrument to Assess the Understandability and Actionability of Print and Audiovisual Patient Education Materials. Rockville, MD: US Dept of Health and Human Services, Agency for Healthcare Research and Quality; 2013. http://www.ahrq.gov/professionals/prevention-chronic-care/improve/self-mgmt/pemat/index.html. Published October 2013. Accessed November 27, 2013.
14. Leyenaar JK, Desai AD, Burkhart Q, et al. Quality measures to assess care transitions for hospitalized children. Pediatrics. 2016;138(2). PubMed
15. Solan LG, Beck AF, Brunswick SA, et al; H2O Study Group. The family perspective on hospital to home transitions: a qualitative study. Pediatrics. 2015;136(6):e1539-e1549. PubMed
16. Maloney M, Auffrey C. The Social Areas of Cincinnati: An Analysis of Social Needs: Patterns for Five Census Decades. 5th ed. Cincinnati, OH: University of Cincinnati School of Planning/United Way/University of Cincinnati Community Research Collaborative; 2013. http://www.socialareasofcincinnati.org/files/FifthEdition/SASBook.pdf. Published April 2013. Accessed December 21, 2016.
17. Rothman RL, Yin HS, Mulvaney S, Co JP, Homer C, Lannon C. Health literacy and quality: focus on chronic illness care and patient safety. Pediatrics. 2009;124(suppl 3):S315-S326. PubMed
18. Moon RY, Cheng TL, Patel KM, Baumhaft K, Scheidt PC. Parental literacy level and understanding of medical information. Pediatrics. 1998;102(2):e25. PubMed
19. Desai AD, Durkin LK, Jacob-Files EA, Mangione-Smith R. Caregiver perceptions of hospital to home transitions according to medical complexity: a qualitative study. Acad Pediatr. 2016;16(2):136-144. PubMed
© 2017 Society of Hospital Medicine
Student perceptions of high-value care education in internal medicine clerkships
During internal medicine (IM) clerkships, course directors are responsible for ensuring that medical students attain basic competency in patient management through use of risk–benefit, cost–benefit, and evidence-based considerations.1 However, the students’ primary teachers—IM residents and attendings—consistently role-model high-value care (HVC) perhaps only half the time.2 The inconsistency may have a few sources, including unawareness of the costs of tests and treatments ordered and little formal training in HVC.3-5 In addition, the environment at some academic institutions may reward learners for performing tests that may be unnecessary.6
We conducted a study to assess medical students’ perceptions of unnecessary testing and the adequacy–inadequacy of HVC instruction, as well as their observations of behavior that may hinder the practice of HVC during the IM clerkship.
METHODS
When students completed their third-year IM clerkships at The Johns Hopkins University School of Medicine, the Icahn School of Medicine at Mount Sinai, the University of Alabama at Birmingham School of Medicine, and the Tulane University School of Medicine, we sent them a recruitment email asking them to complete an anonymous survey regarding their clerkship experiences with HVC. The clerkships’ directors, who collaborated on survey development, searched the literature to quantify behavior thought to decrease the practice of HVC. The survey was tested several times with different learners and faculty to increase response process validity.
The SurveyMonkey online platform was used to administer the survey. Students were given 1 week after the end of their clerkship to complete the survey. Data were collected for the period October 2013 to December 2014. Each student was offered a $10 gift certificate for survey completion. Each institution received exempt approval from its institutional review board.
Survey respondents were divided into those who perceived HVC education as adequate and those who perceived it as inadequate. Chi-square tests were performed with Stata Version 12 (College Station, TX) to determine whether a student’s perception of HVC education being adequate or inadequate was significantly associated with the other survey questions.
RESULTS
Of 577 eligible students, 307 (53%) completed the survey. About 83% of the respondents reported noticing the ordering of laboratory or radiologic tests they considered unnecessary, and a majority (81%) of those students noticed this activity at least once a week. Overall, 51% of the respondents thought their HVC education was inadequate. Significantly more of the students who perceived their HVC education as inadequate were uncomfortable bringing an unnecessary test to the attention of the ward team, rarely discussed costs, and rarely observed team members being praised for forgoing unnecessary tests (Table). Two significant associations were found: between institution attended and perceived adequacy–inadequacy of HVC education and between institution and frequency of cost discussions.
Most (78.5%) students thought an HVC curriculum should be added to the IM clerkship, and 34.5% thought the HVC curriculum should be incorporated into daily rounds. In regards to additions to the clerkship curriculum, most students wanted to round with phlebotomy (29%) or discuss costs of testing on patients (26%).
Students attributed the ordering of unnecessary tests and treatments to several factors: residents investigating “interesting diagnoses” (46%), teams practicing defensive medicine (43%), consultants making requests (40%), attendings investigating “interesting diagnoses” (27%), and patients making requests (8%).
DISCUSSION
About 51% of the students thought their HVC education was inadequate, and about 83% noticed unnecessary testing. Our study findings reaffirm those of a single-site study in which 93% of students noted unnecessary testing.7
In this study, many students perceived HVC education as inadequate and reported wanting HVC principles added to their training and an HVC curriculum incorporated into daily rounds. Students who perceived HVC education as inadequate were significantly less comfortable bringing an unnecessary test to the attention of the ward team and noticed less discussion about costs and less praise for avoiding unnecessary tests. One institution had a significantly higher proportion of students perceiving their HVC education as adequate and noticing more discussions about test costs. This institution was the only one that incorporated discussions about test costs into its curriculum during the study period—which may account for its students’ perceptions.
This study had a few limitations. First, as the survey was administered after the IM clerkships, students’ responses may have been subject to recall bias. We minimized this bias by allowing 1 week for survey completion. Second, given the 53% response rate, there may have been response bias. However, one institution’s demographics showed no significant differences between responders and nonresponders with respect to age, sex, ethnicity, or type of degree. Third, students’ understanding of what tests and treatments are necessary and unnecessary may be relatively underdeveloped, given their training level. One study found that medical students with minimal clinical experience were able to identify unnecessary tests and treatments, but this study has not been validated at other institutions.7
Efforts to increase HVC education and practice have focused on residents and attendings, but our study findings reaffirm that HVC training is much needed and wanted in undergraduate medical education. Studies are needed to test the effectiveness of HVC curricula in medical school and the ability of these curricula to give students the tools they need to practice HVC.
Disclosures
Dr. Pahwa received support from the Johns Hopkins Hospitalist Scholars Fund, and Dr. Cayea is supported by the Daniel and Jeanette Hendin Schapiro Geriatric Medical Education Center. The sponsors had no role in study design, methods, subject recruitment, data collection, data analysis, or manuscript preparation. The authors have no conflicts of interest to disclose.
1. Clerkship Directors in Internal Medicine, Society of General Internal Medicine. CDIM-SGIM Core Medicine Clerkship Curriculum Guide: A Resource for Teachers and Learners. Version 3.0. http://connect.im.org/p/cm/ld/fid=385. Published 2006. Accessed May 12, 2015.
2. Patel MS, Reed DA, Smith C, Arora VM. Role-modeling cost-conscious care—a national evaluation of perceptions of faculty at teaching hospitals in the United States. J Gen Intern Med. 2015;30(9):1294-1298. PubMed
3. Tek Sehgal R, Gorman P. Internal medicine physicians’ knowledge of health care charges. J Grad Med Educ. 2011;3(2):182-187. PubMed
4. Patel MS, Reed DA, Loertscher L, McDonald FS, Arora VM. Teaching residents to provide cost-conscious care: a national survey of residency program directors. JAMA Intern Med. 2014;174(3):470-472. PubMed
5. Graham JD, Potyk D, Raimi E. Hospitalists’ awareness of patient charges associated with inpatient care. J Hosp Med. 2010;5(5):295-297. PubMed
6. Detsky AS, Verma AA. A new model for medical education: celebrating restraint. JAMA. 2012;308(13):1329-1330. PubMed
7. Tartaglia KM, Kman N, Ledford C. Medical student perceptions of cost-conscious care in an internal medicine clerkship: a thematic analysis. J Gen Intern Med. 2015;30(10):1491-1496. PubMed
During internal medicine (IM) clerkships, course directors are responsible for ensuring that medical students attain basic competency in patient management through use of risk–benefit, cost–benefit, and evidence-based considerations.1 However, the students’ primary teachers—IM residents and attendings—consistently role-model high-value care (HVC) perhaps only half the time.2 The inconsistency may have a few sources, including unawareness of the costs of tests and treatments ordered and little formal training in HVC.3-5 In addition, the environment at some academic institutions may reward learners for performing tests that may be unnecessary.6
We conducted a study to assess medical students’ perceptions of unnecessary testing and the adequacy–inadequacy of HVC instruction, as well as their observations of behavior that may hinder the practice of HVC during the IM clerkship.
METHODS
When students completed their third-year IM clerkships at The Johns Hopkins University School of Medicine, the Icahn School of Medicine at Mount Sinai, the University of Alabama at Birmingham School of Medicine, and the Tulane University School of Medicine, we sent them a recruitment email asking them to complete an anonymous survey regarding their clerkship experiences with HVC. The clerkships’ directors, who collaborated on survey development, searched the literature to quantify behavior thought to decrease the practice of HVC. The survey was tested several times with different learners and faculty to increase response process validity.
The SurveyMonkey online platform was used to administer the survey. Students were given 1 week after the end of their clerkship to complete the survey. Data were collected for the period October 2013 to December 2014. Each student was offered a $10 gift certificate for survey completion. Each institution received exempt approval from its institutional review board.
Survey respondents were divided into those who perceived HVC education as adequate and those who perceived it as inadequate. Chi-square tests were performed with Stata Version 12 (College Station, TX) to determine whether a student’s perception of HVC education being adequate or inadequate was significantly associated with the other survey questions.
RESULTS
Of 577 eligible students, 307 (53%) completed the survey. About 83% of the respondents reported noticing the ordering of laboratory or radiologic tests they considered unnecessary, and a majority (81%) of those students noticed this activity at least once a week. Overall, 51% of the respondents thought their HVC education was inadequate. Significantly more of the students who perceived their HVC education as inadequate were uncomfortable bringing an unnecessary test to the attention of the ward team, rarely discussed costs, and rarely observed team members being praised for forgoing unnecessary tests (Table). Two significant associations were found: between institution attended and perceived adequacy–inadequacy of HVC education and between institution and frequency of cost discussions.
Most (78.5%) students thought an HVC curriculum should be added to the IM clerkship, and 34.5% thought the HVC curriculum should be incorporated into daily rounds. In regards to additions to the clerkship curriculum, most students wanted to round with phlebotomy (29%) or discuss costs of testing on patients (26%).
Students attributed the ordering of unnecessary tests and treatments to several factors: residents investigating “interesting diagnoses” (46%), teams practicing defensive medicine (43%), consultants making requests (40%), attendings investigating “interesting diagnoses” (27%), and patients making requests (8%).
DISCUSSION
About 51% of the students thought their HVC education was inadequate, and about 83% noticed unnecessary testing. Our study findings reaffirm those of a single-site study in which 93% of students noted unnecessary testing.7
In this study, many students perceived HVC education as inadequate and reported wanting HVC principles added to their training and an HVC curriculum incorporated into daily rounds. Students who perceived HVC education as inadequate were significantly less comfortable bringing an unnecessary test to the attention of the ward team and noticed less discussion about costs and less praise for avoiding unnecessary tests. One institution had a significantly higher proportion of students perceiving their HVC education as adequate and noticing more discussions about test costs. This institution was the only one that incorporated discussions about test costs into its curriculum during the study period—which may account for its students’ perceptions.
This study had a few limitations. First, as the survey was administered after the IM clerkships, students’ responses may have been subject to recall bias. We minimized this bias by allowing 1 week for survey completion. Second, given the 53% response rate, there may have been response bias. However, one institution’s demographics showed no significant differences between responders and nonresponders with respect to age, sex, ethnicity, or type of degree. Third, students’ understanding of what tests and treatments are necessary and unnecessary may be relatively underdeveloped, given their training level. One study found that medical students with minimal clinical experience were able to identify unnecessary tests and treatments, but this study has not been validated at other institutions.7
Efforts to increase HVC education and practice have focused on residents and attendings, but our study findings reaffirm that HVC training is much needed and wanted in undergraduate medical education. Studies are needed to test the effectiveness of HVC curricula in medical school and the ability of these curricula to give students the tools they need to practice HVC.
Disclosures
Dr. Pahwa received support from the Johns Hopkins Hospitalist Scholars Fund, and Dr. Cayea is supported by the Daniel and Jeanette Hendin Schapiro Geriatric Medical Education Center. The sponsors had no role in study design, methods, subject recruitment, data collection, data analysis, or manuscript preparation. The authors have no conflicts of interest to disclose.
During internal medicine (IM) clerkships, course directors are responsible for ensuring that medical students attain basic competency in patient management through use of risk–benefit, cost–benefit, and evidence-based considerations.1 However, the students’ primary teachers—IM residents and attendings—consistently role-model high-value care (HVC) perhaps only half the time.2 The inconsistency may have a few sources, including unawareness of the costs of tests and treatments ordered and little formal training in HVC.3-5 In addition, the environment at some academic institutions may reward learners for performing tests that may be unnecessary.6
We conducted a study to assess medical students’ perceptions of unnecessary testing and the adequacy–inadequacy of HVC instruction, as well as their observations of behavior that may hinder the practice of HVC during the IM clerkship.
METHODS
When students completed their third-year IM clerkships at The Johns Hopkins University School of Medicine, the Icahn School of Medicine at Mount Sinai, the University of Alabama at Birmingham School of Medicine, and the Tulane University School of Medicine, we sent them a recruitment email asking them to complete an anonymous survey regarding their clerkship experiences with HVC. The clerkships’ directors, who collaborated on survey development, searched the literature to quantify behavior thought to decrease the practice of HVC. The survey was tested several times with different learners and faculty to increase response process validity.
The SurveyMonkey online platform was used to administer the survey. Students were given 1 week after the end of their clerkship to complete the survey. Data were collected for the period October 2013 to December 2014. Each student was offered a $10 gift certificate for survey completion. Each institution received exempt approval from its institutional review board.
Survey respondents were divided into those who perceived HVC education as adequate and those who perceived it as inadequate. Chi-square tests were performed with Stata Version 12 (College Station, TX) to determine whether a student’s perception of HVC education being adequate or inadequate was significantly associated with the other survey questions.
RESULTS
Of 577 eligible students, 307 (53%) completed the survey. About 83% of the respondents reported noticing the ordering of laboratory or radiologic tests they considered unnecessary, and a majority (81%) of those students noticed this activity at least once a week. Overall, 51% of the respondents thought their HVC education was inadequate. Significantly more of the students who perceived their HVC education as inadequate were uncomfortable bringing an unnecessary test to the attention of the ward team, rarely discussed costs, and rarely observed team members being praised for forgoing unnecessary tests (Table). Two significant associations were found: between institution attended and perceived adequacy–inadequacy of HVC education and between institution and frequency of cost discussions.
Most (78.5%) students thought an HVC curriculum should be added to the IM clerkship, and 34.5% thought the HVC curriculum should be incorporated into daily rounds. In regards to additions to the clerkship curriculum, most students wanted to round with phlebotomy (29%) or discuss costs of testing on patients (26%).
Students attributed the ordering of unnecessary tests and treatments to several factors: residents investigating “interesting diagnoses” (46%), teams practicing defensive medicine (43%), consultants making requests (40%), attendings investigating “interesting diagnoses” (27%), and patients making requests (8%).
DISCUSSION
About 51% of the students thought their HVC education was inadequate, and about 83% noticed unnecessary testing. Our study findings reaffirm those of a single-site study in which 93% of students noted unnecessary testing.7
In this study, many students perceived HVC education as inadequate and reported wanting HVC principles added to their training and an HVC curriculum incorporated into daily rounds. Students who perceived HVC education as inadequate were significantly less comfortable bringing an unnecessary test to the attention of the ward team and noticed less discussion about costs and less praise for avoiding unnecessary tests. One institution had a significantly higher proportion of students perceiving their HVC education as adequate and noticing more discussions about test costs. This institution was the only one that incorporated discussions about test costs into its curriculum during the study period—which may account for its students’ perceptions.
This study had a few limitations. First, as the survey was administered after the IM clerkships, students’ responses may have been subject to recall bias. We minimized this bias by allowing 1 week for survey completion. Second, given the 53% response rate, there may have been response bias. However, one institution’s demographics showed no significant differences between responders and nonresponders with respect to age, sex, ethnicity, or type of degree. Third, students’ understanding of what tests and treatments are necessary and unnecessary may be relatively underdeveloped, given their training level. One study found that medical students with minimal clinical experience were able to identify unnecessary tests and treatments, but this study has not been validated at other institutions.7
Efforts to increase HVC education and practice have focused on residents and attendings, but our study findings reaffirm that HVC training is much needed and wanted in undergraduate medical education. Studies are needed to test the effectiveness of HVC curricula in medical school and the ability of these curricula to give students the tools they need to practice HVC.
Disclosures
Dr. Pahwa received support from the Johns Hopkins Hospitalist Scholars Fund, and Dr. Cayea is supported by the Daniel and Jeanette Hendin Schapiro Geriatric Medical Education Center. The sponsors had no role in study design, methods, subject recruitment, data collection, data analysis, or manuscript preparation. The authors have no conflicts of interest to disclose.
1. Clerkship Directors in Internal Medicine, Society of General Internal Medicine. CDIM-SGIM Core Medicine Clerkship Curriculum Guide: A Resource for Teachers and Learners. Version 3.0. http://connect.im.org/p/cm/ld/fid=385. Published 2006. Accessed May 12, 2015.
2. Patel MS, Reed DA, Smith C, Arora VM. Role-modeling cost-conscious care—a national evaluation of perceptions of faculty at teaching hospitals in the United States. J Gen Intern Med. 2015;30(9):1294-1298. PubMed
3. Tek Sehgal R, Gorman P. Internal medicine physicians’ knowledge of health care charges. J Grad Med Educ. 2011;3(2):182-187. PubMed
4. Patel MS, Reed DA, Loertscher L, McDonald FS, Arora VM. Teaching residents to provide cost-conscious care: a national survey of residency program directors. JAMA Intern Med. 2014;174(3):470-472. PubMed
5. Graham JD, Potyk D, Raimi E. Hospitalists’ awareness of patient charges associated with inpatient care. J Hosp Med. 2010;5(5):295-297. PubMed
6. Detsky AS, Verma AA. A new model for medical education: celebrating restraint. JAMA. 2012;308(13):1329-1330. PubMed
7. Tartaglia KM, Kman N, Ledford C. Medical student perceptions of cost-conscious care in an internal medicine clerkship: a thematic analysis. J Gen Intern Med. 2015;30(10):1491-1496. PubMed
1. Clerkship Directors in Internal Medicine, Society of General Internal Medicine. CDIM-SGIM Core Medicine Clerkship Curriculum Guide: A Resource for Teachers and Learners. Version 3.0. http://connect.im.org/p/cm/ld/fid=385. Published 2006. Accessed May 12, 2015.
2. Patel MS, Reed DA, Smith C, Arora VM. Role-modeling cost-conscious care—a national evaluation of perceptions of faculty at teaching hospitals in the United States. J Gen Intern Med. 2015;30(9):1294-1298. PubMed
3. Tek Sehgal R, Gorman P. Internal medicine physicians’ knowledge of health care charges. J Grad Med Educ. 2011;3(2):182-187. PubMed
4. Patel MS, Reed DA, Loertscher L, McDonald FS, Arora VM. Teaching residents to provide cost-conscious care: a national survey of residency program directors. JAMA Intern Med. 2014;174(3):470-472. PubMed
5. Graham JD, Potyk D, Raimi E. Hospitalists’ awareness of patient charges associated with inpatient care. J Hosp Med. 2010;5(5):295-297. PubMed
6. Detsky AS, Verma AA. A new model for medical education: celebrating restraint. JAMA. 2012;308(13):1329-1330. PubMed
7. Tartaglia KM, Kman N, Ledford C. Medical student perceptions of cost-conscious care in an internal medicine clerkship: a thematic analysis. J Gen Intern Med. 2015;30(10):1491-1496. PubMed
© 2017 Society of Hospital Medicine
A shocking diagnosis
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 75-year-old man was brought by ambulance to the emergency department (ED) after the acute onset of palpitations, lightheadedness, and confusion. His medical history, provided by his wife, included osteoarthritis and remote cholecystectomy. He was not a smoker but drank 2 to 4 cans of beer daily. His medications were aspirin 162 mg daily and naproxen as needed. There was no history of bruising, diarrhea, melena, or bleeding.
Palpitations may represent an arrhythmia arising from an ischemic or alcoholic cardiomyopathy. Mental status changes usually have metabolic, infectious, structural (eg, hemorrhage, tumor), or toxic causes. Lightheadedness and confusion could occur with arrhythmia-associated cerebral hypoperfusion or a seizure. Daily alcohol use could cause confusion through acute intoxication, thiamine or B12 deficiency, repeated head trauma, or liver failure.
The patient’s systolic blood pressure (BP) was 60 mm Hg, heart rate (HR) was 120 beats per minute (bpm), and oral temperature was 98.4°F. Rousing him was difficult. There were no localizing neurologic abnormalities, and the rest of the physical examination findings were normal. Point-of-care blood glucose level was 155 mg/dL. Blood cultures were obtained and broad-spectrum antibiotics initiated. After fluid resuscitation, BP improved to 116/87 mm Hg, HR fell to 105 bpm, and the patient became alert and oriented. He denied chest pain, fever, or diaphoresis.
The patient’s improvement with intravenous (IV) fluids makes cardiogenic shock unlikely but does not exclude an underlying compensated cardiomyopathy that may be predisposing to arrhythmia. Hypotension, tachycardia, and somnolence may represent sepsis, but the near normalization of vital signs and mental status shortly after administration of IV fluids, the normal temperature, and the absence of localizing signs of infection favor withholding additional antibiotics. Other causes of hypotension are hypovolemia, medication effects, adrenal insufficiency, anaphylaxis, and autonomic insufficiency. There was no reported nausea, vomiting, diarrhea, bleeding, polyuria, or impaired oral intake to support hypovolemia, though the response to IV fluids suggests hypovolemia may still be playing a role.
White blood cell (WBC) count was 15,450/µL with a normal differential; hemoglobin level was 15.8 g/dL; and platelet count was 176,000/µL. Electrolytes, liver function tests, cardiac enzymes, and urinalysis were normal. Electrocardiogram showed sinus tachycardia with premature atrial complexes and no ST-segment abnormalities. Radiograph of the chest and computed tomography scan of the head were normal. Echocardiogram showed moderate left ventricular hypertrophy with a normal ejection fraction and no valvular abnormalities. Exercise nuclear cardiac stress test was negative for ischemia. Blood cultures were sterile. The patient quickly became asymptomatic and remained so during his 3-day hospitalization. There were no arrhythmias on telemetry. The patient was discharged with follow-up scheduled with his primary care physician.
The nonlocalizing history and physical examination findings, normal chest radiograph and urinalysis, absence of fevers, negative blood cultures, and quick recovery make infection unlikely, despite the moderate leukocytosis. Conditions that present with acute and transient hypotension and altered mental status include arrhythmias, seizures, and reactions to drugs or toxins. Given the cardiac test results, a chronic cardiomyopathy seems unlikely, but arrhythmia is still possible. Continuous outpatient monitoring is required to assess the palpitations and the frequency of the premature atrial complexes.
Two days after discharge, the patient suddenly became diaphoretic and lost consciousness while walking to the bathroom. He was taken to the ED, where his BP was 90/60 mm Hg and HR was 108 bpm. Family members reported that he had appeared flushed during the syncopal episode, showed no seizure activity, and been unconscious for 15 to 20 minutes. The patient denied chest pain, dyspnea, fever, bowel or bladder incontinence, focal weakness, slurred speech, visual changes, nausea or vomiting either before or after the episode. Physical examination revealed a tongue laceration and facial erythema; all other findings were normal. In the ED, there was an asymptomatic 7-beat run of nonsustained ventricular tachycardia, and the hypotension resolved after fluid resuscitation. The patient now reported 2 similar syncopal episodes in the past. The first occurred in a restaurant 6 years earlier, and the second occurred 3 years later, at which time he was hospitalized and no etiology was found.
The loss of consciousness is attributable to cerebral hypoperfusion. Hypotension has 3 principal categories: hypovolemic, cardiogenic, and distributive. With syncopal episodes recurring over several years, hypovolemia seems unlikely. Given the palpitations and ventricular tachycardia, it is reasonable to suspect a cardiogenic cause. Although his heart appears to be structurally normal on echocardiogram, genetic, electrophysiologic, or magnetic resonance imaging (MRI) testing will occasionally reveal an unsuspected substrate for arrhythmia.
The recurring yet self-limited nature, diaphoresis, flushing, and facial erythema suggest a non-sepsis distributive cause of hypotension. It is possible the patient is recurrently exposed to a toxin (eg, alcohol) that causes both flushing and dehydration. Flushing disorders include carcinoid syndrome, pheochromocytoma, drug reaction with eosinophilia and systemic symptoms (DRESS), and mastocytosis. Carcinoid syndrome is characterized by bronchospasm and diarrhea and, in some cases, right-sided valvulopathy, all of which are absent in this patient. Pheochromocytoma is associated with orthostasis, but patients typically are hypertensive at baseline. DRESS, which may arise from nonsteroidal anti-inflammatory drug (NSAID) or aspirin use, can cause facial erythema and swelling but is also characterized by liver, renal, and hematologic abnormalities, none of which was demonstrated. Furthermore, DRESS typically does not cause hypotension. Mastocytosis can manifest as isolated or recurrent anaphylaxis.
It is important to investigate antecedents of these syncopal episodes. If the earlier episodes were food-related—one occurred at a restaurant—then deglutition syncope (syncope precipitated by swallowing) should be considered. If an NSAID or aspirin was ingested before each episode, then medication hypersensitivity or mast cell degranulation (which can be triggered by these medications) should be further examined. Loss of consciousness lasting 20 minutes without causing any neurologic sequelae is unusual for most causes of recurrent syncope. This feature raises the possibility that a toxin or mediator might still be present in the patient’s system.
Serial cardiac enzymes and electrocardiogram were normal. A tilt-table study was negative. The cortisol response to ACTH (cosyntropin) stimulation was normal. The level of serum tryptase, drawn 2 days after syncope, was 18.4 ng/dL (normal, <11.5 ng/dL). Computed tomography scan of chest and abdomen was negative for pulmonary embolism but showed a 1.4×1.3-cm hypervascular lesion in the tail of pancreas. The following neuroendocrine tests were within normal limits: serum and urine catecholamines; urine 5-hydroxyindoleacetic acid (5-HIAA); and serum chromogranin A, insulin, serotonin, vasoactive intestinal polypeptide (VIP), and somatostatin (Table 1). The patient remained asymptomatic during his hospital stay and was discharged home with appointments for cardiology follow-up and endoscopic ultrasound-guided biopsy of the pancreatic mass.
Pheochromocytoma is unlikely with normal serum and urine catecholamine levels and normal adrenal images. The differential diagnosis for a pancreatic mass includes pancreatic carcinoma, lymphoma, cystic neoplasm, and neuroendocrine tumor. All markers of neuroendocrine excess are normal, though elevations can be episodic. The normal 5-HIAA level makes carcinoid syndrome unlikely. VIPomas are associated with flushing, but the absence of profound and protracted diarrhea makes a VIPoma unlikely.
As hypoglycemia from a pancreatic insulinoma is plausible as a cause of episodic loss of consciousness lasting 15 minutes or more, it is important to inquire if giving food or drink helped resolve previous episodes. The normal insulin level reported here is of limited value, because it is the combination of insulin and C-peptide levels at time of hypoglycemia that is diagnostic. The normal glucose level recorded during one of the earlier episodes and the hypotension argue against hypoglycemia.
The elevated tryptase level is an indicator of mast cell degranulation. Tryptase levels are transiently elevated during the initial 2 to 4 hours after an anaphylactic episode and then normalize. An elevated level many hours or days later is considered a sign of mast cell excess. Although there is no evidence of the multi-organ disease (eg, cytopenia, bone disease, hepatosplenomegaly) seen in patients with a high systemic burden of mast cells, mast cell disorders exist on a spectrum. There may be a focal excess of mast cells confined to one organ or an isolated mass.
The same day as discharge, the patient’s wife drove them to the grocery store. He remained in the car while she shopped. When she returned, she found him confused and minimally responsive with subsequent brief loss of consciousness. He was taken to an ED, where he was flushed and hypotensive (systolic BP, 60 mm Hg) and tachycardic. Other examination findings were normal. After fluid resuscitation he became alert and oriented. WBC count was 20,850/μL with 89% neutrophils, hemoglobin level was 14.6 g/dL, and platelet count was 168,000/μL. Serum lactate level was 3.7 mmol/L (normal, <2.3 mmol/L). Chest radiograph was normal. He was treated with broad-spectrum antibiotic therapy and admitted to the hospital. Blood and urine cultures were sterile. Fine-needle aspiration of the pancreatic mass demonstrated nonspecific inflammation. Four days after admission (3 days after pancreatic mass biopsy) the patient developed palpitations, felt unwell, and had marked flushing of the face and trunk, with concomitant BP of 90/50 mm Hg and HR of 140 bpm.
The salient features of this case are recurrent hypotension, tachycardia, and flushing. Autonomic insufficiency, to which elderly patients are prone, causes hemodynamic perturbations but rarely flushing. The patient does not have diabetes mellitus, Parkinson disease, or another condition that puts him at risk for dysautonomia. Pancreatic neuroendocrine tumors secrete mediators that lead to vasodilation and hypotension but are unlikely given the clinical and biochemical data.
The patient’s symptoms are consistent with anaphylaxis, though prototypical immunoglobulin E (IgE)–mediated anaphylaxis is usually accompanied by urticaria, angioedema, and wheezing, which have been absent during his presentations. There are no clear food, pharmacologic, or environmental precipitants.
Recurrent anaphylaxis can be a manifestation of mast cell excess (eg, cutaneous or systemic mastocytosis). A markedly elevated tryptase level during an anaphylactic episode is consistent with mastocytosis or IgE-mediated anaphylaxis. An elevated baseline tryptase level days after an anaphylactic episode signals increased mast cell burden. There may be a reservoir of mast cells in the bone marrow. Alternatively, the hypervascular pancreatic mass may be a mastocytoma or a mast cell sarcoma (missed because of inadequate sampling or staining).
The lactic acidosis likely reflects global tissue hypoperfusion from vasodilatory hypotension. The leukocytosis may reflect WBC mobilization secondary to endogenous corticosteroids and catecholamines in response to hypotension or may be a direct response to the release of mast cell–derived mediators of inflammation.
The patient was treated with diphenhydramine and ranitidine. Serum tryptase level was 46.8 ng/mL (normal, <11.5 ng/mL), and 24-hour urine histamine level was 95 µ g/dL (normal, <60 µ g/dL). Bone marrow biopsy results showed multifocal dense infiltrative aggregates of mast cells (>15 cells/aggregate), which were confirmed by CD117 (Kit) and tryptase positivity (Figure). Mutation analysis for Kit Asp816Val, which is present in 80% to 90% of patients with mastocytosis, was positive. He fulfilled the 2008 World Health Organization criteria for systemic mastocytosis (Table 2). Prednisone, histamine inhibitors, and montelukast were prescribed. Six months later, magnetic resonance imaging of the abdomen showed no change in the pancreatic mass, which was now characterized as a possible splenule. The patient had no additional episodes of flushing or syncope over 2 years.
DISCUSSION
Cardiovascular collapse (hypotension, tachycardia, syncope) in an elderly patient prompts clinicians to focus on life-threatening conditions, such as acute coronary syndrome, pulmonary embolus, arrhythmia, and sepsis. Each of these diagnoses was considered early in the course of this patient’s presentations, but each was deemed unlikely as it became apparent that the episodes were self-limited and recurrent over years. Incorporating flushing into the diagnostic problem representation allowed the clinicians to focus on a subset of causes of hypotension.
Flushing disorders may be classified by whether they are mediated by the autonomic nervous system (wet flushes, because they are usually accompanied by diaphoresis) or by exogenous or endogenous vasoactive substances (dry flushes).1 Autonomic nervous system flushing is triggered by emotions, fever, exercise, perimenopause (hot flashes), and neurologic conditions (eg, Parkinson disease, spinal cord injury, multiple sclerosis). Vasoactive flushing precipitants include drugs (eg, niacin); alcohol (secondary to cutaneous vasodilation, or acetaldehyde particularly in people with insufficient acetaldehyde dehydrogenase activity)2; foods that contain capsaicin, tyramine, sulfites, or histamine (eg, eating improperly handled fish can cause scombroid poisoning); and anaphylaxis. Rare causes of vasoactive flushing include carcinoid syndrome, pheochromocytoma, medullary thyroid carcinoma, VIPoma, and mastocytosis.2
Mastocytosis is a rare clonal disorder characterized by the accumulation of abnormal mast cells in the skin (cutaneous mastocytosis), in multiple organs (systemic mastocytosis), or in a solid tumor (mastocytoma). Urticaria pigmentosa is the most common form of cutaneous mastocytosis; it is seen more often in children than in adults and typically is associated with a maculopapular rash and dermatographism. Systemic mastocytosis is the most common form of the disorder in adults.3 Symptoms are related to mast cell infiltration or mast cell mediator–related effects, which range from itching, flushing, and diarrhea to hypotension and anaphylaxis. Other manifestations are fatigue, urticaria pigmentosa, osteoporosis, hepatosplenomegaly, bone pain, cytopenias, and lymphadenopathy.4
Systemic mastocytosis can occur at any age and should be considered in patients with recurrent unexplained flushing, syncope, or hypotension. Eighty percent to 90% of patients with systemic mastocytosis have a mutation in Kit,5 a transmembrane tyrosine kinase that is the receptor for stem cell factor. The Asp816Val mutation leads to increased proliferation and reduced apoptosis of mast cells.3,6,7 Proposed diagnostic algorithms8-11 involve measurement of serum tryptase levels and examination of bone marrow. Bone marrow biopsy and testing for the Asp816Val
The primary goals of treatment are managing mast cell–mediated symptoms and, in advanced cases, achieving cytoreduction. Alcohol can trigger mast cell degranulation in indolent systemic mastocytosis and should be avoided. Mast cell–mediated symptoms are managed with histamine blockers, leukotriene antagonists, and mast cell stabilizers.12 Targeted therapy with tyrosine kinase inhibitors (eg, imatinib) in patients with transmembrane Kit mutation (eg, Phe522Cys, Lys509Ile) associated with systemic mastocytosis has had promising results.13,14 However, this patient’s Asp816Val mutation is in the Kit catalytic domain, not the transmembrane region, and therefore would not be expected to respond to imatinib. A recent open-label trial of the multikinase inhibitor midostaurin demonstrated resolution of organ damage, reduced bone marrow burden, and lowered serum tryptase levels in patients with advanced systemic mastocytosis.15 Interferon, cladribine, and high-dose corticosteroids are prescribed in patients for whom other therapies have been ineffective.8
The differential diagnosis is broad for both hypotension and for flushing, but the differential diagnosis for recurrent hypotension and flushing is limited. Recognizing that flushing was an essential feature of this patient’s hypotensive condition, and not an epiphenomenon of syncope, allowed the clinicians to focus on the overlap and make a shocking diagnosis.
Acknowledgment
The authors thank David Bosler, MD (Cleveland Clinic) for interpreting the pathology image.
Disclosure
Nothing to report.
1. Wilkin JK. The red face: flushing disorders. Clin Dermatol. 1993;11(2):211-223. PubMed
2. Izikson L, English JC 3rd, Zirwas MJ. The flushing patient: differential diagnosis, workup, and treatment. J Am Acad Dermatol. 2006;55(2):193-208. PubMed
3. Valent P, Akin C, Escribano L, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37(6):435-453. PubMed
4. Hermans MA, Rietveld MJ, van Laar JA, et al. Systemic mastocytosis: a cohort study on clinical characteristics of 136 patients in a large tertiary centre. Eur J Intern Med. 2016;30:25-30. PubMed
5. Kristensen T, Vestergaard H, Bindslev-Jensen C, Møller MB, Broesby-Olsen S; Mastocytosis Centre, Odense University Hospital (MastOUH). Sensitive KIT D816V mutation analysis of blood as a diagnostic test in mastocytosis. Am J Hematol. 2014;89(5):493-498. PubMed
6. Verstovsek S. Advanced systemic mastocytosis: the impact of KIT mutations in diagnosis, treatment, and progression. Eur J Haematol. 2013;90(2):89-98. PubMed
7. Garcia-Montero AC, Jara-Acevedo M, Teodosio C, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108(7):2366-2372. PubMed
8. Pardanani A. Systemic mastocytosis in adults: 2015 update on diagnosis, risk stratification, and management. Am J Hematol. 2015;90(3):250-262. PubMed
9. Valent P, Aberer E, Beham-Schmid C, et al. Guidelines and diagnostic algorithm for patients with suspected systemic mastocytosis: a proposal of the Austrian Competence Network (AUCNM). Am J Blood Res. 2013;3(2):174-180. PubMed
10. Valent P, Escribano L, Broesby-Olsen S, et al; European Competence Network on Mastocytosis. Proposed diagnostic algorithm for patients with suspected mastocytosis: a proposal of the European Competence Network on Mastocytosis. Allergy. 2014;69(10):1267-1274. PubMed
11. Akin C, Soto D, Brittain E, et al. Tryptase haplotype in mastocytosis: relationship to disease variant and diagnostic utility of total tryptase levels. Clin Immunol. 2007;123(3):268-271. PubMed
12. Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373(19):1885-1886. PubMed
13. Akin C, Fumo G, Yavuz AS, Lipsky PE, Neckers L, Metcalfe DD. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood. 2004;103(8):3222-3225. PubMed
14. Zhang LY, Smith ML, Schultheis B, et al. A novel K509I mutation of KIT identified in familial mastocytosis—in vitro and in vivo responsiveness to imatinib therapy. Leuk Res. 2006;30(4):373-378. PubMed
15. Gotlib J, Kluin-Nelemans HC, George TI, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374(26):2530-2541. PubMed
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 75-year-old man was brought by ambulance to the emergency department (ED) after the acute onset of palpitations, lightheadedness, and confusion. His medical history, provided by his wife, included osteoarthritis and remote cholecystectomy. He was not a smoker but drank 2 to 4 cans of beer daily. His medications were aspirin 162 mg daily and naproxen as needed. There was no history of bruising, diarrhea, melena, or bleeding.
Palpitations may represent an arrhythmia arising from an ischemic or alcoholic cardiomyopathy. Mental status changes usually have metabolic, infectious, structural (eg, hemorrhage, tumor), or toxic causes. Lightheadedness and confusion could occur with arrhythmia-associated cerebral hypoperfusion or a seizure. Daily alcohol use could cause confusion through acute intoxication, thiamine or B12 deficiency, repeated head trauma, or liver failure.
The patient’s systolic blood pressure (BP) was 60 mm Hg, heart rate (HR) was 120 beats per minute (bpm), and oral temperature was 98.4°F. Rousing him was difficult. There were no localizing neurologic abnormalities, and the rest of the physical examination findings were normal. Point-of-care blood glucose level was 155 mg/dL. Blood cultures were obtained and broad-spectrum antibiotics initiated. After fluid resuscitation, BP improved to 116/87 mm Hg, HR fell to 105 bpm, and the patient became alert and oriented. He denied chest pain, fever, or diaphoresis.
The patient’s improvement with intravenous (IV) fluids makes cardiogenic shock unlikely but does not exclude an underlying compensated cardiomyopathy that may be predisposing to arrhythmia. Hypotension, tachycardia, and somnolence may represent sepsis, but the near normalization of vital signs and mental status shortly after administration of IV fluids, the normal temperature, and the absence of localizing signs of infection favor withholding additional antibiotics. Other causes of hypotension are hypovolemia, medication effects, adrenal insufficiency, anaphylaxis, and autonomic insufficiency. There was no reported nausea, vomiting, diarrhea, bleeding, polyuria, or impaired oral intake to support hypovolemia, though the response to IV fluids suggests hypovolemia may still be playing a role.
White blood cell (WBC) count was 15,450/µL with a normal differential; hemoglobin level was 15.8 g/dL; and platelet count was 176,000/µL. Electrolytes, liver function tests, cardiac enzymes, and urinalysis were normal. Electrocardiogram showed sinus tachycardia with premature atrial complexes and no ST-segment abnormalities. Radiograph of the chest and computed tomography scan of the head were normal. Echocardiogram showed moderate left ventricular hypertrophy with a normal ejection fraction and no valvular abnormalities. Exercise nuclear cardiac stress test was negative for ischemia. Blood cultures were sterile. The patient quickly became asymptomatic and remained so during his 3-day hospitalization. There were no arrhythmias on telemetry. The patient was discharged with follow-up scheduled with his primary care physician.
The nonlocalizing history and physical examination findings, normal chest radiograph and urinalysis, absence of fevers, negative blood cultures, and quick recovery make infection unlikely, despite the moderate leukocytosis. Conditions that present with acute and transient hypotension and altered mental status include arrhythmias, seizures, and reactions to drugs or toxins. Given the cardiac test results, a chronic cardiomyopathy seems unlikely, but arrhythmia is still possible. Continuous outpatient monitoring is required to assess the palpitations and the frequency of the premature atrial complexes.
Two days after discharge, the patient suddenly became diaphoretic and lost consciousness while walking to the bathroom. He was taken to the ED, where his BP was 90/60 mm Hg and HR was 108 bpm. Family members reported that he had appeared flushed during the syncopal episode, showed no seizure activity, and been unconscious for 15 to 20 minutes. The patient denied chest pain, dyspnea, fever, bowel or bladder incontinence, focal weakness, slurred speech, visual changes, nausea or vomiting either before or after the episode. Physical examination revealed a tongue laceration and facial erythema; all other findings were normal. In the ED, there was an asymptomatic 7-beat run of nonsustained ventricular tachycardia, and the hypotension resolved after fluid resuscitation. The patient now reported 2 similar syncopal episodes in the past. The first occurred in a restaurant 6 years earlier, and the second occurred 3 years later, at which time he was hospitalized and no etiology was found.
The loss of consciousness is attributable to cerebral hypoperfusion. Hypotension has 3 principal categories: hypovolemic, cardiogenic, and distributive. With syncopal episodes recurring over several years, hypovolemia seems unlikely. Given the palpitations and ventricular tachycardia, it is reasonable to suspect a cardiogenic cause. Although his heart appears to be structurally normal on echocardiogram, genetic, electrophysiologic, or magnetic resonance imaging (MRI) testing will occasionally reveal an unsuspected substrate for arrhythmia.
The recurring yet self-limited nature, diaphoresis, flushing, and facial erythema suggest a non-sepsis distributive cause of hypotension. It is possible the patient is recurrently exposed to a toxin (eg, alcohol) that causes both flushing and dehydration. Flushing disorders include carcinoid syndrome, pheochromocytoma, drug reaction with eosinophilia and systemic symptoms (DRESS), and mastocytosis. Carcinoid syndrome is characterized by bronchospasm and diarrhea and, in some cases, right-sided valvulopathy, all of which are absent in this patient. Pheochromocytoma is associated with orthostasis, but patients typically are hypertensive at baseline. DRESS, which may arise from nonsteroidal anti-inflammatory drug (NSAID) or aspirin use, can cause facial erythema and swelling but is also characterized by liver, renal, and hematologic abnormalities, none of which was demonstrated. Furthermore, DRESS typically does not cause hypotension. Mastocytosis can manifest as isolated or recurrent anaphylaxis.
It is important to investigate antecedents of these syncopal episodes. If the earlier episodes were food-related—one occurred at a restaurant—then deglutition syncope (syncope precipitated by swallowing) should be considered. If an NSAID or aspirin was ingested before each episode, then medication hypersensitivity or mast cell degranulation (which can be triggered by these medications) should be further examined. Loss of consciousness lasting 20 minutes without causing any neurologic sequelae is unusual for most causes of recurrent syncope. This feature raises the possibility that a toxin or mediator might still be present in the patient’s system.
Serial cardiac enzymes and electrocardiogram were normal. A tilt-table study was negative. The cortisol response to ACTH (cosyntropin) stimulation was normal. The level of serum tryptase, drawn 2 days after syncope, was 18.4 ng/dL (normal, <11.5 ng/dL). Computed tomography scan of chest and abdomen was negative for pulmonary embolism but showed a 1.4×1.3-cm hypervascular lesion in the tail of pancreas. The following neuroendocrine tests were within normal limits: serum and urine catecholamines; urine 5-hydroxyindoleacetic acid (5-HIAA); and serum chromogranin A, insulin, serotonin, vasoactive intestinal polypeptide (VIP), and somatostatin (Table 1). The patient remained asymptomatic during his hospital stay and was discharged home with appointments for cardiology follow-up and endoscopic ultrasound-guided biopsy of the pancreatic mass.
Pheochromocytoma is unlikely with normal serum and urine catecholamine levels and normal adrenal images. The differential diagnosis for a pancreatic mass includes pancreatic carcinoma, lymphoma, cystic neoplasm, and neuroendocrine tumor. All markers of neuroendocrine excess are normal, though elevations can be episodic. The normal 5-HIAA level makes carcinoid syndrome unlikely. VIPomas are associated with flushing, but the absence of profound and protracted diarrhea makes a VIPoma unlikely.
As hypoglycemia from a pancreatic insulinoma is plausible as a cause of episodic loss of consciousness lasting 15 minutes or more, it is important to inquire if giving food or drink helped resolve previous episodes. The normal insulin level reported here is of limited value, because it is the combination of insulin and C-peptide levels at time of hypoglycemia that is diagnostic. The normal glucose level recorded during one of the earlier episodes and the hypotension argue against hypoglycemia.
The elevated tryptase level is an indicator of mast cell degranulation. Tryptase levels are transiently elevated during the initial 2 to 4 hours after an anaphylactic episode and then normalize. An elevated level many hours or days later is considered a sign of mast cell excess. Although there is no evidence of the multi-organ disease (eg, cytopenia, bone disease, hepatosplenomegaly) seen in patients with a high systemic burden of mast cells, mast cell disorders exist on a spectrum. There may be a focal excess of mast cells confined to one organ or an isolated mass.
The same day as discharge, the patient’s wife drove them to the grocery store. He remained in the car while she shopped. When she returned, she found him confused and minimally responsive with subsequent brief loss of consciousness. He was taken to an ED, where he was flushed and hypotensive (systolic BP, 60 mm Hg) and tachycardic. Other examination findings were normal. After fluid resuscitation he became alert and oriented. WBC count was 20,850/μL with 89% neutrophils, hemoglobin level was 14.6 g/dL, and platelet count was 168,000/μL. Serum lactate level was 3.7 mmol/L (normal, <2.3 mmol/L). Chest radiograph was normal. He was treated with broad-spectrum antibiotic therapy and admitted to the hospital. Blood and urine cultures were sterile. Fine-needle aspiration of the pancreatic mass demonstrated nonspecific inflammation. Four days after admission (3 days after pancreatic mass biopsy) the patient developed palpitations, felt unwell, and had marked flushing of the face and trunk, with concomitant BP of 90/50 mm Hg and HR of 140 bpm.
The salient features of this case are recurrent hypotension, tachycardia, and flushing. Autonomic insufficiency, to which elderly patients are prone, causes hemodynamic perturbations but rarely flushing. The patient does not have diabetes mellitus, Parkinson disease, or another condition that puts him at risk for dysautonomia. Pancreatic neuroendocrine tumors secrete mediators that lead to vasodilation and hypotension but are unlikely given the clinical and biochemical data.
The patient’s symptoms are consistent with anaphylaxis, though prototypical immunoglobulin E (IgE)–mediated anaphylaxis is usually accompanied by urticaria, angioedema, and wheezing, which have been absent during his presentations. There are no clear food, pharmacologic, or environmental precipitants.
Recurrent anaphylaxis can be a manifestation of mast cell excess (eg, cutaneous or systemic mastocytosis). A markedly elevated tryptase level during an anaphylactic episode is consistent with mastocytosis or IgE-mediated anaphylaxis. An elevated baseline tryptase level days after an anaphylactic episode signals increased mast cell burden. There may be a reservoir of mast cells in the bone marrow. Alternatively, the hypervascular pancreatic mass may be a mastocytoma or a mast cell sarcoma (missed because of inadequate sampling or staining).
The lactic acidosis likely reflects global tissue hypoperfusion from vasodilatory hypotension. The leukocytosis may reflect WBC mobilization secondary to endogenous corticosteroids and catecholamines in response to hypotension or may be a direct response to the release of mast cell–derived mediators of inflammation.
The patient was treated with diphenhydramine and ranitidine. Serum tryptase level was 46.8 ng/mL (normal, <11.5 ng/mL), and 24-hour urine histamine level was 95 µ g/dL (normal, <60 µ g/dL). Bone marrow biopsy results showed multifocal dense infiltrative aggregates of mast cells (>15 cells/aggregate), which were confirmed by CD117 (Kit) and tryptase positivity (Figure). Mutation analysis for Kit Asp816Val, which is present in 80% to 90% of patients with mastocytosis, was positive. He fulfilled the 2008 World Health Organization criteria for systemic mastocytosis (Table 2). Prednisone, histamine inhibitors, and montelukast were prescribed. Six months later, magnetic resonance imaging of the abdomen showed no change in the pancreatic mass, which was now characterized as a possible splenule. The patient had no additional episodes of flushing or syncope over 2 years.

DISCUSSION
Cardiovascular collapse (hypotension, tachycardia, syncope) in an elderly patient prompts clinicians to focus on life-threatening conditions, such as acute coronary syndrome, pulmonary embolus, arrhythmia, and sepsis. Each of these diagnoses was considered early in the course of this patient’s presentations, but each was deemed unlikely as it became apparent that the episodes were self-limited and recurrent over years. Incorporating flushing into the diagnostic problem representation allowed the clinicians to focus on a subset of causes of hypotension.
Flushing disorders may be classified by whether they are mediated by the autonomic nervous system (wet flushes, because they are usually accompanied by diaphoresis) or by exogenous or endogenous vasoactive substances (dry flushes).1 Autonomic nervous system flushing is triggered by emotions, fever, exercise, perimenopause (hot flashes), and neurologic conditions (eg, Parkinson disease, spinal cord injury, multiple sclerosis). Vasoactive flushing precipitants include drugs (eg, niacin); alcohol (secondary to cutaneous vasodilation, or acetaldehyde particularly in people with insufficient acetaldehyde dehydrogenase activity)2; foods that contain capsaicin, tyramine, sulfites, or histamine (eg, eating improperly handled fish can cause scombroid poisoning); and anaphylaxis. Rare causes of vasoactive flushing include carcinoid syndrome, pheochromocytoma, medullary thyroid carcinoma, VIPoma, and mastocytosis.2
Mastocytosis is a rare clonal disorder characterized by the accumulation of abnormal mast cells in the skin (cutaneous mastocytosis), in multiple organs (systemic mastocytosis), or in a solid tumor (mastocytoma). Urticaria pigmentosa is the most common form of cutaneous mastocytosis; it is seen more often in children than in adults and typically is associated with a maculopapular rash and dermatographism. Systemic mastocytosis is the most common form of the disorder in adults.3 Symptoms are related to mast cell infiltration or mast cell mediator–related effects, which range from itching, flushing, and diarrhea to hypotension and anaphylaxis. Other manifestations are fatigue, urticaria pigmentosa, osteoporosis, hepatosplenomegaly, bone pain, cytopenias, and lymphadenopathy.4
Systemic mastocytosis can occur at any age and should be considered in patients with recurrent unexplained flushing, syncope, or hypotension. Eighty percent to 90% of patients with systemic mastocytosis have a mutation in Kit,5 a transmembrane tyrosine kinase that is the receptor for stem cell factor. The Asp816Val mutation leads to increased proliferation and reduced apoptosis of mast cells.3,6,7 Proposed diagnostic algorithms8-11 involve measurement of serum tryptase levels and examination of bone marrow. Bone marrow biopsy and testing for the Asp816Val
The primary goals of treatment are managing mast cell–mediated symptoms and, in advanced cases, achieving cytoreduction. Alcohol can trigger mast cell degranulation in indolent systemic mastocytosis and should be avoided. Mast cell–mediated symptoms are managed with histamine blockers, leukotriene antagonists, and mast cell stabilizers.12 Targeted therapy with tyrosine kinase inhibitors (eg, imatinib) in patients with transmembrane Kit mutation (eg, Phe522Cys, Lys509Ile) associated with systemic mastocytosis has had promising results.13,14 However, this patient’s Asp816Val mutation is in the Kit catalytic domain, not the transmembrane region, and therefore would not be expected to respond to imatinib. A recent open-label trial of the multikinase inhibitor midostaurin demonstrated resolution of organ damage, reduced bone marrow burden, and lowered serum tryptase levels in patients with advanced systemic mastocytosis.15 Interferon, cladribine, and high-dose corticosteroids are prescribed in patients for whom other therapies have been ineffective.8
The differential diagnosis is broad for both hypotension and for flushing, but the differential diagnosis for recurrent hypotension and flushing is limited. Recognizing that flushing was an essential feature of this patient’s hypotensive condition, and not an epiphenomenon of syncope, allowed the clinicians to focus on the overlap and make a shocking diagnosis.
Acknowledgment
The authors thank David Bosler, MD (Cleveland Clinic) for interpreting the pathology image.
Disclosure
Nothing to report.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 75-year-old man was brought by ambulance to the emergency department (ED) after the acute onset of palpitations, lightheadedness, and confusion. His medical history, provided by his wife, included osteoarthritis and remote cholecystectomy. He was not a smoker but drank 2 to 4 cans of beer daily. His medications were aspirin 162 mg daily and naproxen as needed. There was no history of bruising, diarrhea, melena, or bleeding.
Palpitations may represent an arrhythmia arising from an ischemic or alcoholic cardiomyopathy. Mental status changes usually have metabolic, infectious, structural (eg, hemorrhage, tumor), or toxic causes. Lightheadedness and confusion could occur with arrhythmia-associated cerebral hypoperfusion or a seizure. Daily alcohol use could cause confusion through acute intoxication, thiamine or B12 deficiency, repeated head trauma, or liver failure.
The patient’s systolic blood pressure (BP) was 60 mm Hg, heart rate (HR) was 120 beats per minute (bpm), and oral temperature was 98.4°F. Rousing him was difficult. There were no localizing neurologic abnormalities, and the rest of the physical examination findings were normal. Point-of-care blood glucose level was 155 mg/dL. Blood cultures were obtained and broad-spectrum antibiotics initiated. After fluid resuscitation, BP improved to 116/87 mm Hg, HR fell to 105 bpm, and the patient became alert and oriented. He denied chest pain, fever, or diaphoresis.
The patient’s improvement with intravenous (IV) fluids makes cardiogenic shock unlikely but does not exclude an underlying compensated cardiomyopathy that may be predisposing to arrhythmia. Hypotension, tachycardia, and somnolence may represent sepsis, but the near normalization of vital signs and mental status shortly after administration of IV fluids, the normal temperature, and the absence of localizing signs of infection favor withholding additional antibiotics. Other causes of hypotension are hypovolemia, medication effects, adrenal insufficiency, anaphylaxis, and autonomic insufficiency. There was no reported nausea, vomiting, diarrhea, bleeding, polyuria, or impaired oral intake to support hypovolemia, though the response to IV fluids suggests hypovolemia may still be playing a role.
White blood cell (WBC) count was 15,450/µL with a normal differential; hemoglobin level was 15.8 g/dL; and platelet count was 176,000/µL. Electrolytes, liver function tests, cardiac enzymes, and urinalysis were normal. Electrocardiogram showed sinus tachycardia with premature atrial complexes and no ST-segment abnormalities. Radiograph of the chest and computed tomography scan of the head were normal. Echocardiogram showed moderate left ventricular hypertrophy with a normal ejection fraction and no valvular abnormalities. Exercise nuclear cardiac stress test was negative for ischemia. Blood cultures were sterile. The patient quickly became asymptomatic and remained so during his 3-day hospitalization. There were no arrhythmias on telemetry. The patient was discharged with follow-up scheduled with his primary care physician.
The nonlocalizing history and physical examination findings, normal chest radiograph and urinalysis, absence of fevers, negative blood cultures, and quick recovery make infection unlikely, despite the moderate leukocytosis. Conditions that present with acute and transient hypotension and altered mental status include arrhythmias, seizures, and reactions to drugs or toxins. Given the cardiac test results, a chronic cardiomyopathy seems unlikely, but arrhythmia is still possible. Continuous outpatient monitoring is required to assess the palpitations and the frequency of the premature atrial complexes.
Two days after discharge, the patient suddenly became diaphoretic and lost consciousness while walking to the bathroom. He was taken to the ED, where his BP was 90/60 mm Hg and HR was 108 bpm. Family members reported that he had appeared flushed during the syncopal episode, showed no seizure activity, and been unconscious for 15 to 20 minutes. The patient denied chest pain, dyspnea, fever, bowel or bladder incontinence, focal weakness, slurred speech, visual changes, nausea or vomiting either before or after the episode. Physical examination revealed a tongue laceration and facial erythema; all other findings were normal. In the ED, there was an asymptomatic 7-beat run of nonsustained ventricular tachycardia, and the hypotension resolved after fluid resuscitation. The patient now reported 2 similar syncopal episodes in the past. The first occurred in a restaurant 6 years earlier, and the second occurred 3 years later, at which time he was hospitalized and no etiology was found.
The loss of consciousness is attributable to cerebral hypoperfusion. Hypotension has 3 principal categories: hypovolemic, cardiogenic, and distributive. With syncopal episodes recurring over several years, hypovolemia seems unlikely. Given the palpitations and ventricular tachycardia, it is reasonable to suspect a cardiogenic cause. Although his heart appears to be structurally normal on echocardiogram, genetic, electrophysiologic, or magnetic resonance imaging (MRI) testing will occasionally reveal an unsuspected substrate for arrhythmia.
The recurring yet self-limited nature, diaphoresis, flushing, and facial erythema suggest a non-sepsis distributive cause of hypotension. It is possible the patient is recurrently exposed to a toxin (eg, alcohol) that causes both flushing and dehydration. Flushing disorders include carcinoid syndrome, pheochromocytoma, drug reaction with eosinophilia and systemic symptoms (DRESS), and mastocytosis. Carcinoid syndrome is characterized by bronchospasm and diarrhea and, in some cases, right-sided valvulopathy, all of which are absent in this patient. Pheochromocytoma is associated with orthostasis, but patients typically are hypertensive at baseline. DRESS, which may arise from nonsteroidal anti-inflammatory drug (NSAID) or aspirin use, can cause facial erythema and swelling but is also characterized by liver, renal, and hematologic abnormalities, none of which was demonstrated. Furthermore, DRESS typically does not cause hypotension. Mastocytosis can manifest as isolated or recurrent anaphylaxis.
It is important to investigate antecedents of these syncopal episodes. If the earlier episodes were food-related—one occurred at a restaurant—then deglutition syncope (syncope precipitated by swallowing) should be considered. If an NSAID or aspirin was ingested before each episode, then medication hypersensitivity or mast cell degranulation (which can be triggered by these medications) should be further examined. Loss of consciousness lasting 20 minutes without causing any neurologic sequelae is unusual for most causes of recurrent syncope. This feature raises the possibility that a toxin or mediator might still be present in the patient’s system.
Serial cardiac enzymes and electrocardiogram were normal. A tilt-table study was negative. The cortisol response to ACTH (cosyntropin) stimulation was normal. The level of serum tryptase, drawn 2 days after syncope, was 18.4 ng/dL (normal, <11.5 ng/dL). Computed tomography scan of chest and abdomen was negative for pulmonary embolism but showed a 1.4×1.3-cm hypervascular lesion in the tail of pancreas. The following neuroendocrine tests were within normal limits: serum and urine catecholamines; urine 5-hydroxyindoleacetic acid (5-HIAA); and serum chromogranin A, insulin, serotonin, vasoactive intestinal polypeptide (VIP), and somatostatin (Table 1). The patient remained asymptomatic during his hospital stay and was discharged home with appointments for cardiology follow-up and endoscopic ultrasound-guided biopsy of the pancreatic mass.
Pheochromocytoma is unlikely with normal serum and urine catecholamine levels and normal adrenal images. The differential diagnosis for a pancreatic mass includes pancreatic carcinoma, lymphoma, cystic neoplasm, and neuroendocrine tumor. All markers of neuroendocrine excess are normal, though elevations can be episodic. The normal 5-HIAA level makes carcinoid syndrome unlikely. VIPomas are associated with flushing, but the absence of profound and protracted diarrhea makes a VIPoma unlikely.
As hypoglycemia from a pancreatic insulinoma is plausible as a cause of episodic loss of consciousness lasting 15 minutes or more, it is important to inquire if giving food or drink helped resolve previous episodes. The normal insulin level reported here is of limited value, because it is the combination of insulin and C-peptide levels at time of hypoglycemia that is diagnostic. The normal glucose level recorded during one of the earlier episodes and the hypotension argue against hypoglycemia.
The elevated tryptase level is an indicator of mast cell degranulation. Tryptase levels are transiently elevated during the initial 2 to 4 hours after an anaphylactic episode and then normalize. An elevated level many hours or days later is considered a sign of mast cell excess. Although there is no evidence of the multi-organ disease (eg, cytopenia, bone disease, hepatosplenomegaly) seen in patients with a high systemic burden of mast cells, mast cell disorders exist on a spectrum. There may be a focal excess of mast cells confined to one organ or an isolated mass.
The same day as discharge, the patient’s wife drove them to the grocery store. He remained in the car while she shopped. When she returned, she found him confused and minimally responsive with subsequent brief loss of consciousness. He was taken to an ED, where he was flushed and hypotensive (systolic BP, 60 mm Hg) and tachycardic. Other examination findings were normal. After fluid resuscitation he became alert and oriented. WBC count was 20,850/μL with 89% neutrophils, hemoglobin level was 14.6 g/dL, and platelet count was 168,000/μL. Serum lactate level was 3.7 mmol/L (normal, <2.3 mmol/L). Chest radiograph was normal. He was treated with broad-spectrum antibiotic therapy and admitted to the hospital. Blood and urine cultures were sterile. Fine-needle aspiration of the pancreatic mass demonstrated nonspecific inflammation. Four days after admission (3 days after pancreatic mass biopsy) the patient developed palpitations, felt unwell, and had marked flushing of the face and trunk, with concomitant BP of 90/50 mm Hg and HR of 140 bpm.
The salient features of this case are recurrent hypotension, tachycardia, and flushing. Autonomic insufficiency, to which elderly patients are prone, causes hemodynamic perturbations but rarely flushing. The patient does not have diabetes mellitus, Parkinson disease, or another condition that puts him at risk for dysautonomia. Pancreatic neuroendocrine tumors secrete mediators that lead to vasodilation and hypotension but are unlikely given the clinical and biochemical data.
The patient’s symptoms are consistent with anaphylaxis, though prototypical immunoglobulin E (IgE)–mediated anaphylaxis is usually accompanied by urticaria, angioedema, and wheezing, which have been absent during his presentations. There are no clear food, pharmacologic, or environmental precipitants.
Recurrent anaphylaxis can be a manifestation of mast cell excess (eg, cutaneous or systemic mastocytosis). A markedly elevated tryptase level during an anaphylactic episode is consistent with mastocytosis or IgE-mediated anaphylaxis. An elevated baseline tryptase level days after an anaphylactic episode signals increased mast cell burden. There may be a reservoir of mast cells in the bone marrow. Alternatively, the hypervascular pancreatic mass may be a mastocytoma or a mast cell sarcoma (missed because of inadequate sampling or staining).
The lactic acidosis likely reflects global tissue hypoperfusion from vasodilatory hypotension. The leukocytosis may reflect WBC mobilization secondary to endogenous corticosteroids and catecholamines in response to hypotension or may be a direct response to the release of mast cell–derived mediators of inflammation.
The patient was treated with diphenhydramine and ranitidine. Serum tryptase level was 46.8 ng/mL (normal, <11.5 ng/mL), and 24-hour urine histamine level was 95 µ g/dL (normal, <60 µ g/dL). Bone marrow biopsy results showed multifocal dense infiltrative aggregates of mast cells (>15 cells/aggregate), which were confirmed by CD117 (Kit) and tryptase positivity (Figure). Mutation analysis for Kit Asp816Val, which is present in 80% to 90% of patients with mastocytosis, was positive. He fulfilled the 2008 World Health Organization criteria for systemic mastocytosis (Table 2). Prednisone, histamine inhibitors, and montelukast were prescribed. Six months later, magnetic resonance imaging of the abdomen showed no change in the pancreatic mass, which was now characterized as a possible splenule. The patient had no additional episodes of flushing or syncope over 2 years.

DISCUSSION
Cardiovascular collapse (hypotension, tachycardia, syncope) in an elderly patient prompts clinicians to focus on life-threatening conditions, such as acute coronary syndrome, pulmonary embolus, arrhythmia, and sepsis. Each of these diagnoses was considered early in the course of this patient’s presentations, but each was deemed unlikely as it became apparent that the episodes were self-limited and recurrent over years. Incorporating flushing into the diagnostic problem representation allowed the clinicians to focus on a subset of causes of hypotension.
Flushing disorders may be classified by whether they are mediated by the autonomic nervous system (wet flushes, because they are usually accompanied by diaphoresis) or by exogenous or endogenous vasoactive substances (dry flushes).1 Autonomic nervous system flushing is triggered by emotions, fever, exercise, perimenopause (hot flashes), and neurologic conditions (eg, Parkinson disease, spinal cord injury, multiple sclerosis). Vasoactive flushing precipitants include drugs (eg, niacin); alcohol (secondary to cutaneous vasodilation, or acetaldehyde particularly in people with insufficient acetaldehyde dehydrogenase activity)2; foods that contain capsaicin, tyramine, sulfites, or histamine (eg, eating improperly handled fish can cause scombroid poisoning); and anaphylaxis. Rare causes of vasoactive flushing include carcinoid syndrome, pheochromocytoma, medullary thyroid carcinoma, VIPoma, and mastocytosis.2
Mastocytosis is a rare clonal disorder characterized by the accumulation of abnormal mast cells in the skin (cutaneous mastocytosis), in multiple organs (systemic mastocytosis), or in a solid tumor (mastocytoma). Urticaria pigmentosa is the most common form of cutaneous mastocytosis; it is seen more often in children than in adults and typically is associated with a maculopapular rash and dermatographism. Systemic mastocytosis is the most common form of the disorder in adults.3 Symptoms are related to mast cell infiltration or mast cell mediator–related effects, which range from itching, flushing, and diarrhea to hypotension and anaphylaxis. Other manifestations are fatigue, urticaria pigmentosa, osteoporosis, hepatosplenomegaly, bone pain, cytopenias, and lymphadenopathy.4
Systemic mastocytosis can occur at any age and should be considered in patients with recurrent unexplained flushing, syncope, or hypotension. Eighty percent to 90% of patients with systemic mastocytosis have a mutation in Kit,5 a transmembrane tyrosine kinase that is the receptor for stem cell factor. The Asp816Val mutation leads to increased proliferation and reduced apoptosis of mast cells.3,6,7 Proposed diagnostic algorithms8-11 involve measurement of serum tryptase levels and examination of bone marrow. Bone marrow biopsy and testing for the Asp816Val
The primary goals of treatment are managing mast cell–mediated symptoms and, in advanced cases, achieving cytoreduction. Alcohol can trigger mast cell degranulation in indolent systemic mastocytosis and should be avoided. Mast cell–mediated symptoms are managed with histamine blockers, leukotriene antagonists, and mast cell stabilizers.12 Targeted therapy with tyrosine kinase inhibitors (eg, imatinib) in patients with transmembrane Kit mutation (eg, Phe522Cys, Lys509Ile) associated with systemic mastocytosis has had promising results.13,14 However, this patient’s Asp816Val mutation is in the Kit catalytic domain, not the transmembrane region, and therefore would not be expected to respond to imatinib. A recent open-label trial of the multikinase inhibitor midostaurin demonstrated resolution of organ damage, reduced bone marrow burden, and lowered serum tryptase levels in patients with advanced systemic mastocytosis.15 Interferon, cladribine, and high-dose corticosteroids are prescribed in patients for whom other therapies have been ineffective.8
The differential diagnosis is broad for both hypotension and for flushing, but the differential diagnosis for recurrent hypotension and flushing is limited. Recognizing that flushing was an essential feature of this patient’s hypotensive condition, and not an epiphenomenon of syncope, allowed the clinicians to focus on the overlap and make a shocking diagnosis.
Acknowledgment
The authors thank David Bosler, MD (Cleveland Clinic) for interpreting the pathology image.
Disclosure
Nothing to report.
1. Wilkin JK. The red face: flushing disorders. Clin Dermatol. 1993;11(2):211-223. PubMed
2. Izikson L, English JC 3rd, Zirwas MJ. The flushing patient: differential diagnosis, workup, and treatment. J Am Acad Dermatol. 2006;55(2):193-208. PubMed
3. Valent P, Akin C, Escribano L, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37(6):435-453. PubMed
4. Hermans MA, Rietveld MJ, van Laar JA, et al. Systemic mastocytosis: a cohort study on clinical characteristics of 136 patients in a large tertiary centre. Eur J Intern Med. 2016;30:25-30. PubMed
5. Kristensen T, Vestergaard H, Bindslev-Jensen C, Møller MB, Broesby-Olsen S; Mastocytosis Centre, Odense University Hospital (MastOUH). Sensitive KIT D816V mutation analysis of blood as a diagnostic test in mastocytosis. Am J Hematol. 2014;89(5):493-498. PubMed
6. Verstovsek S. Advanced systemic mastocytosis: the impact of KIT mutations in diagnosis, treatment, and progression. Eur J Haematol. 2013;90(2):89-98. PubMed
7. Garcia-Montero AC, Jara-Acevedo M, Teodosio C, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108(7):2366-2372. PubMed
8. Pardanani A. Systemic mastocytosis in adults: 2015 update on diagnosis, risk stratification, and management. Am J Hematol. 2015;90(3):250-262. PubMed
9. Valent P, Aberer E, Beham-Schmid C, et al. Guidelines and diagnostic algorithm for patients with suspected systemic mastocytosis: a proposal of the Austrian Competence Network (AUCNM). Am J Blood Res. 2013;3(2):174-180. PubMed
10. Valent P, Escribano L, Broesby-Olsen S, et al; European Competence Network on Mastocytosis. Proposed diagnostic algorithm for patients with suspected mastocytosis: a proposal of the European Competence Network on Mastocytosis. Allergy. 2014;69(10):1267-1274. PubMed
11. Akin C, Soto D, Brittain E, et al. Tryptase haplotype in mastocytosis: relationship to disease variant and diagnostic utility of total tryptase levels. Clin Immunol. 2007;123(3):268-271. PubMed
12. Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373(19):1885-1886. PubMed
13. Akin C, Fumo G, Yavuz AS, Lipsky PE, Neckers L, Metcalfe DD. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood. 2004;103(8):3222-3225. PubMed
14. Zhang LY, Smith ML, Schultheis B, et al. A novel K509I mutation of KIT identified in familial mastocytosis—in vitro and in vivo responsiveness to imatinib therapy. Leuk Res. 2006;30(4):373-378. PubMed
15. Gotlib J, Kluin-Nelemans HC, George TI, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374(26):2530-2541. PubMed
1. Wilkin JK. The red face: flushing disorders. Clin Dermatol. 1993;11(2):211-223. PubMed
2. Izikson L, English JC 3rd, Zirwas MJ. The flushing patient: differential diagnosis, workup, and treatment. J Am Acad Dermatol. 2006;55(2):193-208. PubMed
3. Valent P, Akin C, Escribano L, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007;37(6):435-453. PubMed
4. Hermans MA, Rietveld MJ, van Laar JA, et al. Systemic mastocytosis: a cohort study on clinical characteristics of 136 patients in a large tertiary centre. Eur J Intern Med. 2016;30:25-30. PubMed
5. Kristensen T, Vestergaard H, Bindslev-Jensen C, Møller MB, Broesby-Olsen S; Mastocytosis Centre, Odense University Hospital (MastOUH). Sensitive KIT D816V mutation analysis of blood as a diagnostic test in mastocytosis. Am J Hematol. 2014;89(5):493-498. PubMed
6. Verstovsek S. Advanced systemic mastocytosis: the impact of KIT mutations in diagnosis, treatment, and progression. Eur J Haematol. 2013;90(2):89-98. PubMed
7. Garcia-Montero AC, Jara-Acevedo M, Teodosio C, et al. KIT mutation in mast cells and other bone marrow hematopoietic cell lineages in systemic mast cell disorders: a prospective study of the Spanish Network on Mastocytosis (REMA) in a series of 113 patients. Blood. 2006;108(7):2366-2372. PubMed
8. Pardanani A. Systemic mastocytosis in adults: 2015 update on diagnosis, risk stratification, and management. Am J Hematol. 2015;90(3):250-262. PubMed
9. Valent P, Aberer E, Beham-Schmid C, et al. Guidelines and diagnostic algorithm for patients with suspected systemic mastocytosis: a proposal of the Austrian Competence Network (AUCNM). Am J Blood Res. 2013;3(2):174-180. PubMed
10. Valent P, Escribano L, Broesby-Olsen S, et al; European Competence Network on Mastocytosis. Proposed diagnostic algorithm for patients with suspected mastocytosis: a proposal of the European Competence Network on Mastocytosis. Allergy. 2014;69(10):1267-1274. PubMed
11. Akin C, Soto D, Brittain E, et al. Tryptase haplotype in mastocytosis: relationship to disease variant and diagnostic utility of total tryptase levels. Clin Immunol. 2007;123(3):268-271. PubMed
12. Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373(19):1885-1886. PubMed
13. Akin C, Fumo G, Yavuz AS, Lipsky PE, Neckers L, Metcalfe DD. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood. 2004;103(8):3222-3225. PubMed
14. Zhang LY, Smith ML, Schultheis B, et al. A novel K509I mutation of KIT identified in familial mastocytosis—in vitro and in vivo responsiveness to imatinib therapy. Leuk Res. 2006;30(4):373-378. PubMed
15. Gotlib J, Kluin-Nelemans HC, George TI, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016;374(26):2530-2541. PubMed
© 2017 Society of Hospital Medicine