User login
Intermediate nature of ‘incomplete’ lupus shown in large study
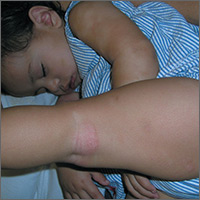
People with incomplete lupus erythematosus exhibit nearly as many disease-related symptoms as do those with systemic lupus erythematosus and often receive many of the same treatments, according to findings reported from a cohort study of 3,837 patients registered with the Lupus Family Registry and Repository.
The study found that while individuals with incomplete lupus erythematosus (ILE) generally have fewer biomarkers of lupus, a subset are at risk of permanent organ damage and may transition to systemic lupus erythematosus (SLE).
“No risk stratification protocols or treatment recommendations for ILE currently exist, and clinical care is largely derived from SLE experience, in part due to the lack of consensus on what constitutes ILE,” wrote Teresa Aberle of the Oklahoma Medical Research Foundation, Oklahoma City, and her coauthors.
The treatments that ILE patients received also ran the gamut from hydroxychloroquine and steroids to immunosuppressants of varying potency.
The investigators classified participants as having ILE if they met three of the 1997 American College of Rheumatology SLE classification criteria, and were classified as having SLE if they met four or more of those criteria. Overall, 440 individuals had ILE and 3,397 had SLE, with ILE patients on average older than SLE patients and less likely to be African American (Arthritis Care Res. 2017 Jan 24. doi: 10.1002/acr.23201).
Among those with ILE, 97.3% were ANA positive, compared with 99.3% of patients with SLE, but fewer ILE patients had ANA titers of 1:1,080 or higher (10.5% vs. 19.5%; odds ratio, 2.03; P less than .0001). ILE patients were also more than three times less likely to show anti-dsDNA antibodies than were SLE patients, but a similar percentage – approximately 42% – of both groups had anti-cardiolipin antibodies.
The researchers also examined plasma levels of B-lymphocyte stimulator, hypothesizing that lower antibody levels and fewer autoantibody specificities in ILE patients might connect with autoantibody production. In a subset of 72 ILE patients, 100 SLE patients, and 172 healthy controls, they observed that median B-lymphocyte stimulator levels were significantly lower in ILE patients, compared with SLE patients, although they were still higher than those in healthy controls. Around one-third of ILE patients had B-lymphocyte stimulator levels that exceeded the positive cutoff value, compared with 54% of SLE patients and 9% of healthy controls.
When it came to symptoms, the researchers found that each lupus classification criterion was seen in at least some ILE patients, although SLE patients were significantly more likely to have protein in urine (65.1% vs. 50%; OR, 1.9; P less than .0001), low blood counts (76.6% vs. 66%; OR, 1.7; P less than .0001), and experience seizures (19.4% vs. 14.4%; OR, 1.4; P = .012). Patients with SLE were also more likely to have pleurisy (58.2% vs. 51.9%; P = .012) and exhibit rapid hair loss (52.2% vs. 47.2%; P = .049) than were those with ILE.
Nearly one in five patients with ILE were affected by at least one clinical criterion of the disease linked to multiple indicators of disease severity, which included criteria such as serositis, renal symptoms, neurologic symptoms, hemolytic anemia, and thrombocytopenia. The investigators also noted that African Americans with ILE showed a higher rate of major clinical manifestations than did European Americans with ILE (25.7% vs. 14.9%).
“Given the association between renal disease and future transition to classified SLE, these observations suggest that patients of non-European American ancestry would likely benefit from careful monitoring and inclusion in future longitudinal studies of early or incomplete lupus,” they wrote.
While ILE patients used fewer medication types and were less likely to have used each type of medication, 65.2% of them had been treated with hydroxychloroquine and 70.7% had been treated with steroids. More than a quarter of ILE patients had used immunosuppressants and 14.8% had used major immunosuppressants, such as mycophenolate mofetil and cyclophosphamide.
“These results show that in clinical practice, ILE is often treated with hydroxychloroquine and/or steroids and occasionally may warrant treatment with potent immunosuppressants,” the authors wrote.
The authors also noted that while some patients with ILE have more severe clinical presentations and require more aggressive treatments, others with less severe disease who are at low risk of transitioning to SLE may be at risk of overtreatment.
“These results reinforce the need for new strategies to personalize disease management in ILE based on individual presentation and risk factors.”
The National Institutes of Health and the U.S. Department of Veterans Affairs supported the study. One author reported grants from pharmaceutical companies outside of the submitted work, and there were no other conflicts of interest declared.
Where the study from Ms. Aberle and her colleagues most advances knowledge of these intermediate phenotype ILE patients is in the more sophisticated interrogation of the immunologic features of ILE vs. SLE patients than has been performed in past studies. These findings are consistent with the conception of ILE as an intermediate state between health and “complete lupus” with similar, but less immune dysregulation than patients classified with SLE.
Karen H. Costenbader, MD, is with the division of rheumatology, immunology and allergy at Brigham and Women’s Hospital, Boston, and David I. Daikh, MD, is with the division of rheumatology at the University of California, San Francisco. These comments are adapted from an accompanying editorial (Arthritis Care Res. 2017 Jan 24. doi: 10.1002/acr.23196). No conflicts of interest were declared.
Where the study from Ms. Aberle and her colleagues most advances knowledge of these intermediate phenotype ILE patients is in the more sophisticated interrogation of the immunologic features of ILE vs. SLE patients than has been performed in past studies. These findings are consistent with the conception of ILE as an intermediate state between health and “complete lupus” with similar, but less immune dysregulation than patients classified with SLE.
Karen H. Costenbader, MD, is with the division of rheumatology, immunology and allergy at Brigham and Women’s Hospital, Boston, and David I. Daikh, MD, is with the division of rheumatology at the University of California, San Francisco. These comments are adapted from an accompanying editorial (Arthritis Care Res. 2017 Jan 24. doi: 10.1002/acr.23196). No conflicts of interest were declared.
Where the study from Ms. Aberle and her colleagues most advances knowledge of these intermediate phenotype ILE patients is in the more sophisticated interrogation of the immunologic features of ILE vs. SLE patients than has been performed in past studies. These findings are consistent with the conception of ILE as an intermediate state between health and “complete lupus” with similar, but less immune dysregulation than patients classified with SLE.
Karen H. Costenbader, MD, is with the division of rheumatology, immunology and allergy at Brigham and Women’s Hospital, Boston, and David I. Daikh, MD, is with the division of rheumatology at the University of California, San Francisco. These comments are adapted from an accompanying editorial (Arthritis Care Res. 2017 Jan 24. doi: 10.1002/acr.23196). No conflicts of interest were declared.
People with incomplete lupus erythematosus exhibit nearly as many disease-related symptoms as do those with systemic lupus erythematosus and often receive many of the same treatments, according to findings reported from a cohort study of 3,837 patients registered with the Lupus Family Registry and Repository.
The study found that while individuals with incomplete lupus erythematosus (ILE) generally have fewer biomarkers of lupus, a subset are at risk of permanent organ damage and may transition to systemic lupus erythematosus (SLE).
“No risk stratification protocols or treatment recommendations for ILE currently exist, and clinical care is largely derived from SLE experience, in part due to the lack of consensus on what constitutes ILE,” wrote Teresa Aberle of the Oklahoma Medical Research Foundation, Oklahoma City, and her coauthors.
The treatments that ILE patients received also ran the gamut from hydroxychloroquine and steroids to immunosuppressants of varying potency.
The investigators classified participants as having ILE if they met three of the 1997 American College of Rheumatology SLE classification criteria, and were classified as having SLE if they met four or more of those criteria. Overall, 440 individuals had ILE and 3,397 had SLE, with ILE patients on average older than SLE patients and less likely to be African American (Arthritis Care Res. 2017 Jan 24. doi: 10.1002/acr.23201).
Among those with ILE, 97.3% were ANA positive, compared with 99.3% of patients with SLE, but fewer ILE patients had ANA titers of 1:1,080 or higher (10.5% vs. 19.5%; odds ratio, 2.03; P less than .0001). ILE patients were also more than three times less likely to show anti-dsDNA antibodies than were SLE patients, but a similar percentage – approximately 42% – of both groups had anti-cardiolipin antibodies.
The researchers also examined plasma levels of B-lymphocyte stimulator, hypothesizing that lower antibody levels and fewer autoantibody specificities in ILE patients might connect with autoantibody production. In a subset of 72 ILE patients, 100 SLE patients, and 172 healthy controls, they observed that median B-lymphocyte stimulator levels were significantly lower in ILE patients, compared with SLE patients, although they were still higher than those in healthy controls. Around one-third of ILE patients had B-lymphocyte stimulator levels that exceeded the positive cutoff value, compared with 54% of SLE patients and 9% of healthy controls.
When it came to symptoms, the researchers found that each lupus classification criterion was seen in at least some ILE patients, although SLE patients were significantly more likely to have protein in urine (65.1% vs. 50%; OR, 1.9; P less than .0001), low blood counts (76.6% vs. 66%; OR, 1.7; P less than .0001), and experience seizures (19.4% vs. 14.4%; OR, 1.4; P = .012). Patients with SLE were also more likely to have pleurisy (58.2% vs. 51.9%; P = .012) and exhibit rapid hair loss (52.2% vs. 47.2%; P = .049) than were those with ILE.
Nearly one in five patients with ILE were affected by at least one clinical criterion of the disease linked to multiple indicators of disease severity, which included criteria such as serositis, renal symptoms, neurologic symptoms, hemolytic anemia, and thrombocytopenia. The investigators also noted that African Americans with ILE showed a higher rate of major clinical manifestations than did European Americans with ILE (25.7% vs. 14.9%).
“Given the association between renal disease and future transition to classified SLE, these observations suggest that patients of non-European American ancestry would likely benefit from careful monitoring and inclusion in future longitudinal studies of early or incomplete lupus,” they wrote.
While ILE patients used fewer medication types and were less likely to have used each type of medication, 65.2% of them had been treated with hydroxychloroquine and 70.7% had been treated with steroids. More than a quarter of ILE patients had used immunosuppressants and 14.8% had used major immunosuppressants, such as mycophenolate mofetil and cyclophosphamide.
“These results show that in clinical practice, ILE is often treated with hydroxychloroquine and/or steroids and occasionally may warrant treatment with potent immunosuppressants,” the authors wrote.
The authors also noted that while some patients with ILE have more severe clinical presentations and require more aggressive treatments, others with less severe disease who are at low risk of transitioning to SLE may be at risk of overtreatment.
“These results reinforce the need for new strategies to personalize disease management in ILE based on individual presentation and risk factors.”
The National Institutes of Health and the U.S. Department of Veterans Affairs supported the study. One author reported grants from pharmaceutical companies outside of the submitted work, and there were no other conflicts of interest declared.
People with incomplete lupus erythematosus exhibit nearly as many disease-related symptoms as do those with systemic lupus erythematosus and often receive many of the same treatments, according to findings reported from a cohort study of 3,837 patients registered with the Lupus Family Registry and Repository.
The study found that while individuals with incomplete lupus erythematosus (ILE) generally have fewer biomarkers of lupus, a subset are at risk of permanent organ damage and may transition to systemic lupus erythematosus (SLE).
“No risk stratification protocols or treatment recommendations for ILE currently exist, and clinical care is largely derived from SLE experience, in part due to the lack of consensus on what constitutes ILE,” wrote Teresa Aberle of the Oklahoma Medical Research Foundation, Oklahoma City, and her coauthors.
The treatments that ILE patients received also ran the gamut from hydroxychloroquine and steroids to immunosuppressants of varying potency.
The investigators classified participants as having ILE if they met three of the 1997 American College of Rheumatology SLE classification criteria, and were classified as having SLE if they met four or more of those criteria. Overall, 440 individuals had ILE and 3,397 had SLE, with ILE patients on average older than SLE patients and less likely to be African American (Arthritis Care Res. 2017 Jan 24. doi: 10.1002/acr.23201).
Among those with ILE, 97.3% were ANA positive, compared with 99.3% of patients with SLE, but fewer ILE patients had ANA titers of 1:1,080 or higher (10.5% vs. 19.5%; odds ratio, 2.03; P less than .0001). ILE patients were also more than three times less likely to show anti-dsDNA antibodies than were SLE patients, but a similar percentage – approximately 42% – of both groups had anti-cardiolipin antibodies.
The researchers also examined plasma levels of B-lymphocyte stimulator, hypothesizing that lower antibody levels and fewer autoantibody specificities in ILE patients might connect with autoantibody production. In a subset of 72 ILE patients, 100 SLE patients, and 172 healthy controls, they observed that median B-lymphocyte stimulator levels were significantly lower in ILE patients, compared with SLE patients, although they were still higher than those in healthy controls. Around one-third of ILE patients had B-lymphocyte stimulator levels that exceeded the positive cutoff value, compared with 54% of SLE patients and 9% of healthy controls.
When it came to symptoms, the researchers found that each lupus classification criterion was seen in at least some ILE patients, although SLE patients were significantly more likely to have protein in urine (65.1% vs. 50%; OR, 1.9; P less than .0001), low blood counts (76.6% vs. 66%; OR, 1.7; P less than .0001), and experience seizures (19.4% vs. 14.4%; OR, 1.4; P = .012). Patients with SLE were also more likely to have pleurisy (58.2% vs. 51.9%; P = .012) and exhibit rapid hair loss (52.2% vs. 47.2%; P = .049) than were those with ILE.
Nearly one in five patients with ILE were affected by at least one clinical criterion of the disease linked to multiple indicators of disease severity, which included criteria such as serositis, renal symptoms, neurologic symptoms, hemolytic anemia, and thrombocytopenia. The investigators also noted that African Americans with ILE showed a higher rate of major clinical manifestations than did European Americans with ILE (25.7% vs. 14.9%).
“Given the association between renal disease and future transition to classified SLE, these observations suggest that patients of non-European American ancestry would likely benefit from careful monitoring and inclusion in future longitudinal studies of early or incomplete lupus,” they wrote.
While ILE patients used fewer medication types and were less likely to have used each type of medication, 65.2% of them had been treated with hydroxychloroquine and 70.7% had been treated with steroids. More than a quarter of ILE patients had used immunosuppressants and 14.8% had used major immunosuppressants, such as mycophenolate mofetil and cyclophosphamide.
“These results show that in clinical practice, ILE is often treated with hydroxychloroquine and/or steroids and occasionally may warrant treatment with potent immunosuppressants,” the authors wrote.
The authors also noted that while some patients with ILE have more severe clinical presentations and require more aggressive treatments, others with less severe disease who are at low risk of transitioning to SLE may be at risk of overtreatment.
“These results reinforce the need for new strategies to personalize disease management in ILE based on individual presentation and risk factors.”
The National Institutes of Health and the U.S. Department of Veterans Affairs supported the study. One author reported grants from pharmaceutical companies outside of the submitted work, and there were no other conflicts of interest declared.
Key clinical point:
Major finding: Each lupus classification criterion was seen in at least some incomplete lupus erythematosus patients, although systemic lupus erythematosus patients were significantly more likely to have protein in urine, low blood counts, and experience seizures.
Data source: A cohort study of 3,837 patients registered with the Lupus Family Registry and Repository.
Disclosures: The National Institutes of Health and the U.S. Department of Veterans Affairs supported the study. One author reported grants from pharmaceutical companies outside of the submitted work, and there were no other conflicts of interest declared.
VIDEO: Dosing an important consideration when using neuromodulators in men
WAILEA, HAWAII – Using larger doses and avoiding overarching of the brows are among the considerations to keep in mind when using neuromodulators in men, advised Canadian dermatologist Katie Beleznay, MD.
In general, men have a very different anatomy with a different brow position, and have needs different from those of women, said Dr. Beleznay of the University of British Columbia, Vancouver.
One secret for successful injections of toxins in male patients: Dose matters. Men have stronger muscles in the face and need larger doses to overcome their muscular strength, Dr. Beleznay said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
On average, “if you use 20-30 units in a female glabella, maybe you’d use 40-50, maybe even 60 units in a male patient,” she said. However, the volume depends on the strength of the muscles in the area, and some men may need less to avoid a frozen look. “You can always treat less and bring them back in a couple of weeks and add more,” especially with patients who are new to treatment, Dr. Beleznay noted.
She disclosed financial relationships with Allergan, Revance, Evolus, Galderma, and Zeltiq.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – Using larger doses and avoiding overarching of the brows are among the considerations to keep in mind when using neuromodulators in men, advised Canadian dermatologist Katie Beleznay, MD.
In general, men have a very different anatomy with a different brow position, and have needs different from those of women, said Dr. Beleznay of the University of British Columbia, Vancouver.
One secret for successful injections of toxins in male patients: Dose matters. Men have stronger muscles in the face and need larger doses to overcome their muscular strength, Dr. Beleznay said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
On average, “if you use 20-30 units in a female glabella, maybe you’d use 40-50, maybe even 60 units in a male patient,” she said. However, the volume depends on the strength of the muscles in the area, and some men may need less to avoid a frozen look. “You can always treat less and bring them back in a couple of weeks and add more,” especially with patients who are new to treatment, Dr. Beleznay noted.
She disclosed financial relationships with Allergan, Revance, Evolus, Galderma, and Zeltiq.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – Using larger doses and avoiding overarching of the brows are among the considerations to keep in mind when using neuromodulators in men, advised Canadian dermatologist Katie Beleznay, MD.
In general, men have a very different anatomy with a different brow position, and have needs different from those of women, said Dr. Beleznay of the University of British Columbia, Vancouver.
One secret for successful injections of toxins in male patients: Dose matters. Men have stronger muscles in the face and need larger doses to overcome their muscular strength, Dr. Beleznay said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
On average, “if you use 20-30 units in a female glabella, maybe you’d use 40-50, maybe even 60 units in a male patient,” she said. However, the volume depends on the strength of the muscles in the area, and some men may need less to avoid a frozen look. “You can always treat less and bring them back in a couple of weeks and add more,” especially with patients who are new to treatment, Dr. Beleznay noted.
She disclosed financial relationships with Allergan, Revance, Evolus, Galderma, and Zeltiq.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
VIDEO: Patient survey data yield useful information on acne and different contraceptives
WAILEA, HAWAII – A study that used patient-reported outcomes to assess the impact of different contraceptive methods on acne provided “fascinating results,” Lawrence F. Eichenfield, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Progestins are present in birth control for the birth control, but they’re not usually good for the acne,” Dr. Eichenfield said in a video interview at the meeting.
Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and his colleagues reviewed patient reports from an Internet-based survey of more than 2,000 adolescent and young adult acne patients asking whether they were on any contraceptive, and if so, how they thought the contraceptive method affected their acne.
Overall, hormonal progestin-only IUDs and some progestin-only implants correlated with worse acne. Of the combined oral contraceptives, those on the OCs containing drospirenone performed best, he said. Also of interest, the triphasic OCs, with the progestin in three phases, had a better impact on the acne than did the dual ones, he added.
The review of patient-reported outcomes can not only inform clinicians on how they should prescribe hormonal therapy, but also raises awareness of the impact of contraceptives on acne in general, Dr. Eichenfield said.
The results were published last year (J Drugs Dermatol. 2016 Jun 1;15[6]:670-4).
Dr. Eichenfield disclosed relationships with Anacor/Pfizer, Genentech, Lilly, Regeneron/Sanofi, Medimetriks, and Otsuka.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – A study that used patient-reported outcomes to assess the impact of different contraceptive methods on acne provided “fascinating results,” Lawrence F. Eichenfield, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Progestins are present in birth control for the birth control, but they’re not usually good for the acne,” Dr. Eichenfield said in a video interview at the meeting.
Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and his colleagues reviewed patient reports from an Internet-based survey of more than 2,000 adolescent and young adult acne patients asking whether they were on any contraceptive, and if so, how they thought the contraceptive method affected their acne.
Overall, hormonal progestin-only IUDs and some progestin-only implants correlated with worse acne. Of the combined oral contraceptives, those on the OCs containing drospirenone performed best, he said. Also of interest, the triphasic OCs, with the progestin in three phases, had a better impact on the acne than did the dual ones, he added.
The review of patient-reported outcomes can not only inform clinicians on how they should prescribe hormonal therapy, but also raises awareness of the impact of contraceptives on acne in general, Dr. Eichenfield said.
The results were published last year (J Drugs Dermatol. 2016 Jun 1;15[6]:670-4).
Dr. Eichenfield disclosed relationships with Anacor/Pfizer, Genentech, Lilly, Regeneron/Sanofi, Medimetriks, and Otsuka.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – A study that used patient-reported outcomes to assess the impact of different contraceptive methods on acne provided “fascinating results,” Lawrence F. Eichenfield, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Progestins are present in birth control for the birth control, but they’re not usually good for the acne,” Dr. Eichenfield said in a video interview at the meeting.
Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and his colleagues reviewed patient reports from an Internet-based survey of more than 2,000 adolescent and young adult acne patients asking whether they were on any contraceptive, and if so, how they thought the contraceptive method affected their acne.
Overall, hormonal progestin-only IUDs and some progestin-only implants correlated with worse acne. Of the combined oral contraceptives, those on the OCs containing drospirenone performed best, he said. Also of interest, the triphasic OCs, with the progestin in three phases, had a better impact on the acne than did the dual ones, he added.
The review of patient-reported outcomes can not only inform clinicians on how they should prescribe hormonal therapy, but also raises awareness of the impact of contraceptives on acne in general, Dr. Eichenfield said.
The results were published last year (J Drugs Dermatol. 2016 Jun 1;15[6]:670-4).
Dr. Eichenfield disclosed relationships with Anacor/Pfizer, Genentech, Lilly, Regeneron/Sanofi, Medimetriks, and Otsuka.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
The Long Legacy of Agent Orange
For some veterans, exposure to Agent Orange and its many health ramifications is an ongoing concern—even though they might have been exposed more than 50 years ago. Clinicians from Sinai Hospital of Baltimore in Maryland report on a patient who reminded them that a current illness could be related to that long-ago exposure.
Related: Bladder Cancer and Hyperthyroidism Linked to Agent Orange
The 69-year-old man came to their clinic with a painful, enlarging mass of the right lateral thigh, which ultrasound revealed as a myxofibrosarcoma. He underwent radical excision of the sarcoma with adjuvant radiotherapy. Histologic examination revealed an 8.3 cm, grade 3 pleomorphic liposarcoma. The patient asked, “Was it related to Agent Orange?” It may have been, the clinicians decided.
Agent Orange tends to accumulate in the liver and adipose tissue; it also affects lipid metabolism and may lead to hyperlipidemia. Although in the early days after the Vietnam War, many pathologies were labeled as “stress induced,” a number of illnesses have since been linked to Agent Orange, including B-cell leukemia, Hodgkin and non-Hodgkin lymphoma, prostate cancer, and, perhaps, multiple myeloma. But the authors say the toxin’s role in sarcomagenesis has been controversial in part because of conflicting case-control studies and because large-scale clinical trials are not feasible.
Related: Link Found Between Agent Orange Exposure and Multiple Myeloma
There has been no well-established precipitating factor for liposarcoma, the authors note. But they suggest that clinicians should have a high degree of suspicion for persistent and evolving soft tissue masses in patients with a previous military background, which should prompt the search for a possible toxin exposure.
Their patient experienced the enlarging soft tissue mass over the course of a year. A simple question: “Have you ever been in the military or had any previous wartime toxin exposure?” early on would have impelled the physicians to do a swifter workup, the authors say, particularly in a case with high risk of metastasis and poor prognosis. The patient will continue to be monitored “for years,” the authors say.
“While these days we have access to a vast amount of diagnostic tests and imaging,” the authors conclude, “one cannot underestimate the importance of understanding a patient’s past history.”
Source:
Khan K, Wozniak SE, Coleman J, Didolkar MS. BMJ Case Rep. 2016;2016. pii: bcr2016217438.
doi: 10.1136/bcr-2016-217438.
For some veterans, exposure to Agent Orange and its many health ramifications is an ongoing concern—even though they might have been exposed more than 50 years ago. Clinicians from Sinai Hospital of Baltimore in Maryland report on a patient who reminded them that a current illness could be related to that long-ago exposure.
Related: Bladder Cancer and Hyperthyroidism Linked to Agent Orange
The 69-year-old man came to their clinic with a painful, enlarging mass of the right lateral thigh, which ultrasound revealed as a myxofibrosarcoma. He underwent radical excision of the sarcoma with adjuvant radiotherapy. Histologic examination revealed an 8.3 cm, grade 3 pleomorphic liposarcoma. The patient asked, “Was it related to Agent Orange?” It may have been, the clinicians decided.
Agent Orange tends to accumulate in the liver and adipose tissue; it also affects lipid metabolism and may lead to hyperlipidemia. Although in the early days after the Vietnam War, many pathologies were labeled as “stress induced,” a number of illnesses have since been linked to Agent Orange, including B-cell leukemia, Hodgkin and non-Hodgkin lymphoma, prostate cancer, and, perhaps, multiple myeloma. But the authors say the toxin’s role in sarcomagenesis has been controversial in part because of conflicting case-control studies and because large-scale clinical trials are not feasible.
Related: Link Found Between Agent Orange Exposure and Multiple Myeloma
There has been no well-established precipitating factor for liposarcoma, the authors note. But they suggest that clinicians should have a high degree of suspicion for persistent and evolving soft tissue masses in patients with a previous military background, which should prompt the search for a possible toxin exposure.
Their patient experienced the enlarging soft tissue mass over the course of a year. A simple question: “Have you ever been in the military or had any previous wartime toxin exposure?” early on would have impelled the physicians to do a swifter workup, the authors say, particularly in a case with high risk of metastasis and poor prognosis. The patient will continue to be monitored “for years,” the authors say.
“While these days we have access to a vast amount of diagnostic tests and imaging,” the authors conclude, “one cannot underestimate the importance of understanding a patient’s past history.”
Source:
Khan K, Wozniak SE, Coleman J, Didolkar MS. BMJ Case Rep. 2016;2016. pii: bcr2016217438.
doi: 10.1136/bcr-2016-217438.
For some veterans, exposure to Agent Orange and its many health ramifications is an ongoing concern—even though they might have been exposed more than 50 years ago. Clinicians from Sinai Hospital of Baltimore in Maryland report on a patient who reminded them that a current illness could be related to that long-ago exposure.
Related: Bladder Cancer and Hyperthyroidism Linked to Agent Orange
The 69-year-old man came to their clinic with a painful, enlarging mass of the right lateral thigh, which ultrasound revealed as a myxofibrosarcoma. He underwent radical excision of the sarcoma with adjuvant radiotherapy. Histologic examination revealed an 8.3 cm, grade 3 pleomorphic liposarcoma. The patient asked, “Was it related to Agent Orange?” It may have been, the clinicians decided.
Agent Orange tends to accumulate in the liver and adipose tissue; it also affects lipid metabolism and may lead to hyperlipidemia. Although in the early days after the Vietnam War, many pathologies were labeled as “stress induced,” a number of illnesses have since been linked to Agent Orange, including B-cell leukemia, Hodgkin and non-Hodgkin lymphoma, prostate cancer, and, perhaps, multiple myeloma. But the authors say the toxin’s role in sarcomagenesis has been controversial in part because of conflicting case-control studies and because large-scale clinical trials are not feasible.
Related: Link Found Between Agent Orange Exposure and Multiple Myeloma
There has been no well-established precipitating factor for liposarcoma, the authors note. But they suggest that clinicians should have a high degree of suspicion for persistent and evolving soft tissue masses in patients with a previous military background, which should prompt the search for a possible toxin exposure.
Their patient experienced the enlarging soft tissue mass over the course of a year. A simple question: “Have you ever been in the military or had any previous wartime toxin exposure?” early on would have impelled the physicians to do a swifter workup, the authors say, particularly in a case with high risk of metastasis and poor prognosis. The patient will continue to be monitored “for years,” the authors say.
“While these days we have access to a vast amount of diagnostic tests and imaging,” the authors conclude, “one cannot underestimate the importance of understanding a patient’s past history.”
Source:
Khan K, Wozniak SE, Coleman J, Didolkar MS. BMJ Case Rep. 2016;2016. pii: bcr2016217438.
doi: 10.1136/bcr-2016-217438.
Regimens seem similarly effective in ENKTL

Photo by Larry Young
SAN FRANCISCO—Interim results of a phase 3 trial suggest 2 treatment regimens may provide comparable efficacy in patients with extranodal natural killer/T-cell lymphoma (ENKTL), though 1 regimen appears more toxic than the other.
In this ongoing trial, investigators are comparing pegaspargase, gemcitabine, oxaliplatin, and thalidomide (P-Gemox+Thal) to pegaspargase, methotrexate, calcium folinate, and dexamethasone (AspaMetDex).
In some patients, either regimen may be followed by extensive involved-field radiotherapy (EIFRT) or autologous hematopoietic stem cell transplant (ASCT).
Thus far, P-Gemox+Thal and AspaMetDex have proven similarly effective for patients with newly diagnosed, stage I/II ENKTL.
And both regimens have produced unsatisfying survival outcomes in patients with advanced or relapsed/refractory ENKTL, according to investigator Huiqiang Huang, MD, of Sun Yat-sen Institute of Hematology in Guangzhou, China.
In addition, P-Gemox+Thal seems to be less toxic, overall, than AspaMetDex.
However, Dr Huang said it is still too early to draw any firm conclusions about these regimens.
He presented results from this trial at the 9th Annual T-cell Lymphoma Forum. Results were previously presented at the 2016 ASH Annual Meeting (abstract 1819).
Dr Huang noted that AspaMetDex and SMILE (dexamethasone, methotrexate, ifosfamide, lL-asparaginase, and etoposide) are frequently administered to patients with ENKTL. And P-Gemox is recommended in the 2016 NCCN guidelines.
“But optimal regimens still have not been fully defined,” he said.
Therefore, he and his colleagues decided to compare AspaMetDex to P-Gemox+Thal in this non-inferiority trial, which has enrolled 110 patients from 12 centers in China.
Treatment
Fifty-six patients have been randomized to receive P-Gemox+Thal. Every 3 weeks, they received pegaspargase at 2000 U/m2 on day 1, gemcitabine at 1000 mg/m2 on days 1 and 8, and oxaliplatin at 130 mg/m2 on days 1 and 8. They also received thalidomide at 100 mg every day for 1 year.
Fifty-four patients have been randomized to receive AspaMetDex. Every 3 weeks, they received pegaspargase at 2000 U/m2 on day 1, methotrexate at 3000 mg/m2 on day 1, calcium folinate at 30 mg every 6 hours until a safe serum methotrexate concentration was reached, and dexamethasone at 40 mg every day on days 1 to 4.
Newly diagnosed patients with stage I/II disease received either regimen as induction for a maximum of 4 cycles. Responders went on to receive EIFRT at 56 Gy in 28 fractions over 4 weeks.
Patients with newly diagnosed, stage III/IV ENKTL or relapsed/refractory ENKTL received either chemotherapy regimen for a maximum of 6 cycles. If they achieved a complete response (CR), these patients could proceed to ASCT.
Stage I/II ENKTL
Of the 63 stage I/II patients, 33 were randomized to P-Gemox+Thal, and 30 received AspaMetDex. In both arms, most patients were male (69.7% and 70%, respectively) and younger than 60 (78.8% and 90%, respectively).
Ninety-seven percent of patients in the P-Gemox+Thal arm had an ECOG status of 0-1, as did 100% of patients in the AspaMetDex arm.
The overall response rate (ORR) was 85.2% in the P-Gemox+Thal arm and 81.5% in the AspaMetDex arm. The CR rate was 59.3% in both arms. The rate of stable disease was 3.7% in the P-Gemox+Thal arm and 11.1% in the AspaMetDex arm.
After EIFRT, the ORR increased to 92.6% in the P-Gemox+Thal arm, and the CR rate increased to 88.8%. In the AspaMetDex arm, the ORR increased to 88.8%, and the CR rate increased to 85.1%.
At a median follow-up of 13.5 months, the 2-year progression-free survival rate was 82.9% in the P-Gemox+Thal arm and 84.5% in the AspaMetDex arm (P=0.791).
The 2-year overall survival rates were 95.0% in the P-Gemox+Thal arm and 75.8% in the AspaMetDex arm (P=0.089).
Advanced, rel/ref ENKTL
Of the 47 patients with stage III/IV or relapsed/refractory ENKTL, 24 were randomized to P-Gemox+Thal, and 23 to AspaMetDex. In both arms, most patients were male (75% and 87%, respectively) and younger than 60 (95.8% and 91.3%, respectively).
ECOG status was 0 for 62.5% of patients in the P-Gemox+Thal arm and 73.9% of those in the AspaMetDex arm. ECOG status was 1 for 33.3% and 17.4%, respectively.
The ORR was 86.3% in the P-Gemox+Thal arm and 70% in the AspaMetDex arm. The CR rate was 50% in both arms.
The partial response rate was 36.3% in the P-Gemox+Thal arm and 20% in the AspaMetDex arm. And the rate of stable disease was 13.6% and 15%, respectively.
Three patients in each treatment arm went on to ASCT after CR. A total of 3 patients relapsed within 6 months of ASCT—2 in the P-Gemox+Thal arm and 1 in the AspaMetDex arm. Two patients died of disease progression.
At a median follow-up of 14.5 months, the 2-year progression-free survival was 12.2 months in the P-Gemox+Thal arm and 7.6 months in the AspaMetDex arm (P=0.365).
The 2-year overall survival was 52.5% in the P-Gemox+Thal arm and 48.9% in the AspaMetDex arm (P=0.935).
Overall safety
Rates of leukopenia, thrombocytopenia, and ALT/AST increase were all significantly higher with P-Gemox+Thal than with AspaMetDex—100% vs 66.7% (P<0.001), 64.2% vs 35.2% (P=0.005), and 69.6% vs 64.8% (P=0.004), respectively.
Rates of anemia and edema were significantly higher with AspaMetDex than with P-Gemox+Thal—51.8% vs 77.8% (P=0.005) and 37.5% vs 66.7% (P=0.003), respectively.
There were 3 treatment-related deaths in the AspaMetDex arm but none in the P-Gemox+Thal arm. Two of the treatment-related deaths—from severe acute renal failure and sepsis—occurred in the first cycle, and 1 death—due to severe sepsis—occurred in the third cycle.
The median hospitalization time was significantly shorter in the P-Gemox+Thal arm than the AspaMetDex arm—1.9 days and 4.9 days, respectively (P<0.01).
Based on these results, Dr Huang said P-Gemox+Thal may be more tolerable and provide more convenient administration than AspaMetDex. ![]()

Photo by Larry Young
SAN FRANCISCO—Interim results of a phase 3 trial suggest 2 treatment regimens may provide comparable efficacy in patients with extranodal natural killer/T-cell lymphoma (ENKTL), though 1 regimen appears more toxic than the other.
In this ongoing trial, investigators are comparing pegaspargase, gemcitabine, oxaliplatin, and thalidomide (P-Gemox+Thal) to pegaspargase, methotrexate, calcium folinate, and dexamethasone (AspaMetDex).
In some patients, either regimen may be followed by extensive involved-field radiotherapy (EIFRT) or autologous hematopoietic stem cell transplant (ASCT).
Thus far, P-Gemox+Thal and AspaMetDex have proven similarly effective for patients with newly diagnosed, stage I/II ENKTL.
And both regimens have produced unsatisfying survival outcomes in patients with advanced or relapsed/refractory ENKTL, according to investigator Huiqiang Huang, MD, of Sun Yat-sen Institute of Hematology in Guangzhou, China.
In addition, P-Gemox+Thal seems to be less toxic, overall, than AspaMetDex.
However, Dr Huang said it is still too early to draw any firm conclusions about these regimens.
He presented results from this trial at the 9th Annual T-cell Lymphoma Forum. Results were previously presented at the 2016 ASH Annual Meeting (abstract 1819).
Dr Huang noted that AspaMetDex and SMILE (dexamethasone, methotrexate, ifosfamide, lL-asparaginase, and etoposide) are frequently administered to patients with ENKTL. And P-Gemox is recommended in the 2016 NCCN guidelines.
“But optimal regimens still have not been fully defined,” he said.
Therefore, he and his colleagues decided to compare AspaMetDex to P-Gemox+Thal in this non-inferiority trial, which has enrolled 110 patients from 12 centers in China.
Treatment
Fifty-six patients have been randomized to receive P-Gemox+Thal. Every 3 weeks, they received pegaspargase at 2000 U/m2 on day 1, gemcitabine at 1000 mg/m2 on days 1 and 8, and oxaliplatin at 130 mg/m2 on days 1 and 8. They also received thalidomide at 100 mg every day for 1 year.
Fifty-four patients have been randomized to receive AspaMetDex. Every 3 weeks, they received pegaspargase at 2000 U/m2 on day 1, methotrexate at 3000 mg/m2 on day 1, calcium folinate at 30 mg every 6 hours until a safe serum methotrexate concentration was reached, and dexamethasone at 40 mg every day on days 1 to 4.
Newly diagnosed patients with stage I/II disease received either regimen as induction for a maximum of 4 cycles. Responders went on to receive EIFRT at 56 Gy in 28 fractions over 4 weeks.
Patients with newly diagnosed, stage III/IV ENKTL or relapsed/refractory ENKTL received either chemotherapy regimen for a maximum of 6 cycles. If they achieved a complete response (CR), these patients could proceed to ASCT.
Stage I/II ENKTL
Of the 63 stage I/II patients, 33 were randomized to P-Gemox+Thal, and 30 received AspaMetDex. In both arms, most patients were male (69.7% and 70%, respectively) and younger than 60 (78.8% and 90%, respectively).
Ninety-seven percent of patients in the P-Gemox+Thal arm had an ECOG status of 0-1, as did 100% of patients in the AspaMetDex arm.
The overall response rate (ORR) was 85.2% in the P-Gemox+Thal arm and 81.5% in the AspaMetDex arm. The CR rate was 59.3% in both arms. The rate of stable disease was 3.7% in the P-Gemox+Thal arm and 11.1% in the AspaMetDex arm.
After EIFRT, the ORR increased to 92.6% in the P-Gemox+Thal arm, and the CR rate increased to 88.8%. In the AspaMetDex arm, the ORR increased to 88.8%, and the CR rate increased to 85.1%.
At a median follow-up of 13.5 months, the 2-year progression-free survival rate was 82.9% in the P-Gemox+Thal arm and 84.5% in the AspaMetDex arm (P=0.791).
The 2-year overall survival rates were 95.0% in the P-Gemox+Thal arm and 75.8% in the AspaMetDex arm (P=0.089).
Advanced, rel/ref ENKTL
Of the 47 patients with stage III/IV or relapsed/refractory ENKTL, 24 were randomized to P-Gemox+Thal, and 23 to AspaMetDex. In both arms, most patients were male (75% and 87%, respectively) and younger than 60 (95.8% and 91.3%, respectively).
ECOG status was 0 for 62.5% of patients in the P-Gemox+Thal arm and 73.9% of those in the AspaMetDex arm. ECOG status was 1 for 33.3% and 17.4%, respectively.
The ORR was 86.3% in the P-Gemox+Thal arm and 70% in the AspaMetDex arm. The CR rate was 50% in both arms.
The partial response rate was 36.3% in the P-Gemox+Thal arm and 20% in the AspaMetDex arm. And the rate of stable disease was 13.6% and 15%, respectively.
Three patients in each treatment arm went on to ASCT after CR. A total of 3 patients relapsed within 6 months of ASCT—2 in the P-Gemox+Thal arm and 1 in the AspaMetDex arm. Two patients died of disease progression.
At a median follow-up of 14.5 months, the 2-year progression-free survival was 12.2 months in the P-Gemox+Thal arm and 7.6 months in the AspaMetDex arm (P=0.365).
The 2-year overall survival was 52.5% in the P-Gemox+Thal arm and 48.9% in the AspaMetDex arm (P=0.935).
Overall safety
Rates of leukopenia, thrombocytopenia, and ALT/AST increase were all significantly higher with P-Gemox+Thal than with AspaMetDex—100% vs 66.7% (P<0.001), 64.2% vs 35.2% (P=0.005), and 69.6% vs 64.8% (P=0.004), respectively.
Rates of anemia and edema were significantly higher with AspaMetDex than with P-Gemox+Thal—51.8% vs 77.8% (P=0.005) and 37.5% vs 66.7% (P=0.003), respectively.
There were 3 treatment-related deaths in the AspaMetDex arm but none in the P-Gemox+Thal arm. Two of the treatment-related deaths—from severe acute renal failure and sepsis—occurred in the first cycle, and 1 death—due to severe sepsis—occurred in the third cycle.
The median hospitalization time was significantly shorter in the P-Gemox+Thal arm than the AspaMetDex arm—1.9 days and 4.9 days, respectively (P<0.01).
Based on these results, Dr Huang said P-Gemox+Thal may be more tolerable and provide more convenient administration than AspaMetDex. ![]()

Photo by Larry Young
SAN FRANCISCO—Interim results of a phase 3 trial suggest 2 treatment regimens may provide comparable efficacy in patients with extranodal natural killer/T-cell lymphoma (ENKTL), though 1 regimen appears more toxic than the other.
In this ongoing trial, investigators are comparing pegaspargase, gemcitabine, oxaliplatin, and thalidomide (P-Gemox+Thal) to pegaspargase, methotrexate, calcium folinate, and dexamethasone (AspaMetDex).
In some patients, either regimen may be followed by extensive involved-field radiotherapy (EIFRT) or autologous hematopoietic stem cell transplant (ASCT).
Thus far, P-Gemox+Thal and AspaMetDex have proven similarly effective for patients with newly diagnosed, stage I/II ENKTL.
And both regimens have produced unsatisfying survival outcomes in patients with advanced or relapsed/refractory ENKTL, according to investigator Huiqiang Huang, MD, of Sun Yat-sen Institute of Hematology in Guangzhou, China.
In addition, P-Gemox+Thal seems to be less toxic, overall, than AspaMetDex.
However, Dr Huang said it is still too early to draw any firm conclusions about these regimens.
He presented results from this trial at the 9th Annual T-cell Lymphoma Forum. Results were previously presented at the 2016 ASH Annual Meeting (abstract 1819).
Dr Huang noted that AspaMetDex and SMILE (dexamethasone, methotrexate, ifosfamide, lL-asparaginase, and etoposide) are frequently administered to patients with ENKTL. And P-Gemox is recommended in the 2016 NCCN guidelines.
“But optimal regimens still have not been fully defined,” he said.
Therefore, he and his colleagues decided to compare AspaMetDex to P-Gemox+Thal in this non-inferiority trial, which has enrolled 110 patients from 12 centers in China.
Treatment
Fifty-six patients have been randomized to receive P-Gemox+Thal. Every 3 weeks, they received pegaspargase at 2000 U/m2 on day 1, gemcitabine at 1000 mg/m2 on days 1 and 8, and oxaliplatin at 130 mg/m2 on days 1 and 8. They also received thalidomide at 100 mg every day for 1 year.
Fifty-four patients have been randomized to receive AspaMetDex. Every 3 weeks, they received pegaspargase at 2000 U/m2 on day 1, methotrexate at 3000 mg/m2 on day 1, calcium folinate at 30 mg every 6 hours until a safe serum methotrexate concentration was reached, and dexamethasone at 40 mg every day on days 1 to 4.
Newly diagnosed patients with stage I/II disease received either regimen as induction for a maximum of 4 cycles. Responders went on to receive EIFRT at 56 Gy in 28 fractions over 4 weeks.
Patients with newly diagnosed, stage III/IV ENKTL or relapsed/refractory ENKTL received either chemotherapy regimen for a maximum of 6 cycles. If they achieved a complete response (CR), these patients could proceed to ASCT.
Stage I/II ENKTL
Of the 63 stage I/II patients, 33 were randomized to P-Gemox+Thal, and 30 received AspaMetDex. In both arms, most patients were male (69.7% and 70%, respectively) and younger than 60 (78.8% and 90%, respectively).
Ninety-seven percent of patients in the P-Gemox+Thal arm had an ECOG status of 0-1, as did 100% of patients in the AspaMetDex arm.
The overall response rate (ORR) was 85.2% in the P-Gemox+Thal arm and 81.5% in the AspaMetDex arm. The CR rate was 59.3% in both arms. The rate of stable disease was 3.7% in the P-Gemox+Thal arm and 11.1% in the AspaMetDex arm.
After EIFRT, the ORR increased to 92.6% in the P-Gemox+Thal arm, and the CR rate increased to 88.8%. In the AspaMetDex arm, the ORR increased to 88.8%, and the CR rate increased to 85.1%.
At a median follow-up of 13.5 months, the 2-year progression-free survival rate was 82.9% in the P-Gemox+Thal arm and 84.5% in the AspaMetDex arm (P=0.791).
The 2-year overall survival rates were 95.0% in the P-Gemox+Thal arm and 75.8% in the AspaMetDex arm (P=0.089).
Advanced, rel/ref ENKTL
Of the 47 patients with stage III/IV or relapsed/refractory ENKTL, 24 were randomized to P-Gemox+Thal, and 23 to AspaMetDex. In both arms, most patients were male (75% and 87%, respectively) and younger than 60 (95.8% and 91.3%, respectively).
ECOG status was 0 for 62.5% of patients in the P-Gemox+Thal arm and 73.9% of those in the AspaMetDex arm. ECOG status was 1 for 33.3% and 17.4%, respectively.
The ORR was 86.3% in the P-Gemox+Thal arm and 70% in the AspaMetDex arm. The CR rate was 50% in both arms.
The partial response rate was 36.3% in the P-Gemox+Thal arm and 20% in the AspaMetDex arm. And the rate of stable disease was 13.6% and 15%, respectively.
Three patients in each treatment arm went on to ASCT after CR. A total of 3 patients relapsed within 6 months of ASCT—2 in the P-Gemox+Thal arm and 1 in the AspaMetDex arm. Two patients died of disease progression.
At a median follow-up of 14.5 months, the 2-year progression-free survival was 12.2 months in the P-Gemox+Thal arm and 7.6 months in the AspaMetDex arm (P=0.365).
The 2-year overall survival was 52.5% in the P-Gemox+Thal arm and 48.9% in the AspaMetDex arm (P=0.935).
Overall safety
Rates of leukopenia, thrombocytopenia, and ALT/AST increase were all significantly higher with P-Gemox+Thal than with AspaMetDex—100% vs 66.7% (P<0.001), 64.2% vs 35.2% (P=0.005), and 69.6% vs 64.8% (P=0.004), respectively.
Rates of anemia and edema were significantly higher with AspaMetDex than with P-Gemox+Thal—51.8% vs 77.8% (P=0.005) and 37.5% vs 66.7% (P=0.003), respectively.
There were 3 treatment-related deaths in the AspaMetDex arm but none in the P-Gemox+Thal arm. Two of the treatment-related deaths—from severe acute renal failure and sepsis—occurred in the first cycle, and 1 death—due to severe sepsis—occurred in the third cycle.
The median hospitalization time was significantly shorter in the P-Gemox+Thal arm than the AspaMetDex arm—1.9 days and 4.9 days, respectively (P<0.01).
Based on these results, Dr Huang said P-Gemox+Thal may be more tolerable and provide more convenient administration than AspaMetDex. ![]()
Gene therapy increases FIX expression in hemophilia B

Image courtesy of the
National Institute of
General Medical Sciences
A gene therapy known as DTX101 has shown early promise for the treatment of adults with hemophilia B, according to the company developing the therapy.
DTX101 is designed to deliver stable expression of factor IX (FIX) in patients with hemophilia B.
Preliminary results from a phase 1/2 trial showed that DTX101 can increase FIX expression in these patients, allowing some to forgo prophylactic and on-demand treatment.
However, 5 of the 6 patients enrolled in this trial experienced elevations in alanine aminotransferase (ALT), with 1 patient experiencing a grade 4 adverse event as a result.
These results were released by Dimension Therapeutics, Inc., the company developing DTX101.
DTX101 is a non-replicating, recombinant adeno-associated viral vector, AAVrh10, with a codon-optimized FIX gene expressing wild-type FIX protein.
The phase 1/2 study of DTX101 has enrolled 6 patients, ages 28 to 70, with moderate/severe to severe hemophilia B. They had baseline FIX expression of ≤ 2%, which requires either prophylactic or on-demand recombinant FIX therapy.
These 6 patients were divided into 2 dose cohorts. Cohort 1 (n=3) received DXT101 at 1.6 x 1012 GC/kg. And cohort 2 (n=3) received 5 x 1012 GC/kg.
All patients have been in post-treatment follow-up ranging from 6 weeks to 52 weeks.
Efficacy
Researchers observed evidence of efficient liver transduction of DTX101 across the 2 cohorts.
Patients in the low-dose cohort achieved peak FIX expression levels of 10% to 11%, which stabilized to between 3% and 4% at last follow-up (weeks 24, 48, and 52).
Patients in the second dose cohort achieved peak FIX expression of 13%, 20%, and 12% at weeks 4, 8, and 8, respectively. FIX activity was 5% and 8% in 2 patients at 12 weeks of follow-up. It was 7% for the third patient at 7 weeks.
None of the patients in cohort 2 have required prophylactic or on-demand recombinant FIX therapy for spontaneous bleeds post-dosing.
Safety
None of the patients experienced a drug-related serious adverse event as of the January 28, 2017, data cutoff.
Five of the 6 patients had elevations in ALT. All elevated liver enzymes were clinically asymptomatic with no elevations of gamma-glutamyl transferase, alkaline phosphatase, or bilirubin.
Patient 3 in cohort 2 experienced a grade 4 adverse event due to an elevated laboratory ALT (defined as > 800 IU/L).
Preliminary findings from 2 patients in each cohort prompted the administration of a standard tapering course of corticosteroids to treat mild, asymptomatic elevations in ALT (52-98 IU/L).
The third patient in cohort 2 also received corticosteroids, experiencing a peak ALT of 914 IU/L, and was at 431 IU/L at 6 weeks post-dosing.
As of the January 28, 2017, data cutoff, 2 of 3 patients in cohort 2 had ALT levels in the normal range. Cohort 1 patients were all clinically stable and off steroids, with ALT levels in the normal range.
Dimension Therapeutics said it expects cohort 2 will continue to receive a standard tapering course of corticosteroid therapy.
As required by the trial protocol, the company reported the ALT levels for patient 3 in cohort 2 to the Data Safety Monitoring Committee, the US Food and Drug Administration, and the appropriate regulatory authorities.
The company said it will await their feedback prior to initiating dosing of cohort 3.
“We are encouraged by the apparent efficiency of gene transduction and the early trend we are seeing in sustained FIX activity across both cohorts with our wild-type FIX AAVrh10 vector in patients,” said Annalisa Jenkins, MBBS, chief executive officer of Dimension Therapeutics.
“We continue to explore the therapeutic window for DTX101 as our data mature and in light of the ALT rises that appear to be associated with a decline in FIX activity.” ![]()

Image courtesy of the
National Institute of
General Medical Sciences
A gene therapy known as DTX101 has shown early promise for the treatment of adults with hemophilia B, according to the company developing the therapy.
DTX101 is designed to deliver stable expression of factor IX (FIX) in patients with hemophilia B.
Preliminary results from a phase 1/2 trial showed that DTX101 can increase FIX expression in these patients, allowing some to forgo prophylactic and on-demand treatment.
However, 5 of the 6 patients enrolled in this trial experienced elevations in alanine aminotransferase (ALT), with 1 patient experiencing a grade 4 adverse event as a result.
These results were released by Dimension Therapeutics, Inc., the company developing DTX101.
DTX101 is a non-replicating, recombinant adeno-associated viral vector, AAVrh10, with a codon-optimized FIX gene expressing wild-type FIX protein.
The phase 1/2 study of DTX101 has enrolled 6 patients, ages 28 to 70, with moderate/severe to severe hemophilia B. They had baseline FIX expression of ≤ 2%, which requires either prophylactic or on-demand recombinant FIX therapy.
These 6 patients were divided into 2 dose cohorts. Cohort 1 (n=3) received DXT101 at 1.6 x 1012 GC/kg. And cohort 2 (n=3) received 5 x 1012 GC/kg.
All patients have been in post-treatment follow-up ranging from 6 weeks to 52 weeks.
Efficacy
Researchers observed evidence of efficient liver transduction of DTX101 across the 2 cohorts.
Patients in the low-dose cohort achieved peak FIX expression levels of 10% to 11%, which stabilized to between 3% and 4% at last follow-up (weeks 24, 48, and 52).
Patients in the second dose cohort achieved peak FIX expression of 13%, 20%, and 12% at weeks 4, 8, and 8, respectively. FIX activity was 5% and 8% in 2 patients at 12 weeks of follow-up. It was 7% for the third patient at 7 weeks.
None of the patients in cohort 2 have required prophylactic or on-demand recombinant FIX therapy for spontaneous bleeds post-dosing.
Safety
None of the patients experienced a drug-related serious adverse event as of the January 28, 2017, data cutoff.
Five of the 6 patients had elevations in ALT. All elevated liver enzymes were clinically asymptomatic with no elevations of gamma-glutamyl transferase, alkaline phosphatase, or bilirubin.
Patient 3 in cohort 2 experienced a grade 4 adverse event due to an elevated laboratory ALT (defined as > 800 IU/L).
Preliminary findings from 2 patients in each cohort prompted the administration of a standard tapering course of corticosteroids to treat mild, asymptomatic elevations in ALT (52-98 IU/L).
The third patient in cohort 2 also received corticosteroids, experiencing a peak ALT of 914 IU/L, and was at 431 IU/L at 6 weeks post-dosing.
As of the January 28, 2017, data cutoff, 2 of 3 patients in cohort 2 had ALT levels in the normal range. Cohort 1 patients were all clinically stable and off steroids, with ALT levels in the normal range.
Dimension Therapeutics said it expects cohort 2 will continue to receive a standard tapering course of corticosteroid therapy.
As required by the trial protocol, the company reported the ALT levels for patient 3 in cohort 2 to the Data Safety Monitoring Committee, the US Food and Drug Administration, and the appropriate regulatory authorities.
The company said it will await their feedback prior to initiating dosing of cohort 3.
“We are encouraged by the apparent efficiency of gene transduction and the early trend we are seeing in sustained FIX activity across both cohorts with our wild-type FIX AAVrh10 vector in patients,” said Annalisa Jenkins, MBBS, chief executive officer of Dimension Therapeutics.
“We continue to explore the therapeutic window for DTX101 as our data mature and in light of the ALT rises that appear to be associated with a decline in FIX activity.” ![]()

Image courtesy of the
National Institute of
General Medical Sciences
A gene therapy known as DTX101 has shown early promise for the treatment of adults with hemophilia B, according to the company developing the therapy.
DTX101 is designed to deliver stable expression of factor IX (FIX) in patients with hemophilia B.
Preliminary results from a phase 1/2 trial showed that DTX101 can increase FIX expression in these patients, allowing some to forgo prophylactic and on-demand treatment.
However, 5 of the 6 patients enrolled in this trial experienced elevations in alanine aminotransferase (ALT), with 1 patient experiencing a grade 4 adverse event as a result.
These results were released by Dimension Therapeutics, Inc., the company developing DTX101.
DTX101 is a non-replicating, recombinant adeno-associated viral vector, AAVrh10, with a codon-optimized FIX gene expressing wild-type FIX protein.
The phase 1/2 study of DTX101 has enrolled 6 patients, ages 28 to 70, with moderate/severe to severe hemophilia B. They had baseline FIX expression of ≤ 2%, which requires either prophylactic or on-demand recombinant FIX therapy.
These 6 patients were divided into 2 dose cohorts. Cohort 1 (n=3) received DXT101 at 1.6 x 1012 GC/kg. And cohort 2 (n=3) received 5 x 1012 GC/kg.
All patients have been in post-treatment follow-up ranging from 6 weeks to 52 weeks.
Efficacy
Researchers observed evidence of efficient liver transduction of DTX101 across the 2 cohorts.
Patients in the low-dose cohort achieved peak FIX expression levels of 10% to 11%, which stabilized to between 3% and 4% at last follow-up (weeks 24, 48, and 52).
Patients in the second dose cohort achieved peak FIX expression of 13%, 20%, and 12% at weeks 4, 8, and 8, respectively. FIX activity was 5% and 8% in 2 patients at 12 weeks of follow-up. It was 7% for the third patient at 7 weeks.
None of the patients in cohort 2 have required prophylactic or on-demand recombinant FIX therapy for spontaneous bleeds post-dosing.
Safety
None of the patients experienced a drug-related serious adverse event as of the January 28, 2017, data cutoff.
Five of the 6 patients had elevations in ALT. All elevated liver enzymes were clinically asymptomatic with no elevations of gamma-glutamyl transferase, alkaline phosphatase, or bilirubin.
Patient 3 in cohort 2 experienced a grade 4 adverse event due to an elevated laboratory ALT (defined as > 800 IU/L).
Preliminary findings from 2 patients in each cohort prompted the administration of a standard tapering course of corticosteroids to treat mild, asymptomatic elevations in ALT (52-98 IU/L).
The third patient in cohort 2 also received corticosteroids, experiencing a peak ALT of 914 IU/L, and was at 431 IU/L at 6 weeks post-dosing.
As of the January 28, 2017, data cutoff, 2 of 3 patients in cohort 2 had ALT levels in the normal range. Cohort 1 patients were all clinically stable and off steroids, with ALT levels in the normal range.
Dimension Therapeutics said it expects cohort 2 will continue to receive a standard tapering course of corticosteroid therapy.
As required by the trial protocol, the company reported the ALT levels for patient 3 in cohort 2 to the Data Safety Monitoring Committee, the US Food and Drug Administration, and the appropriate regulatory authorities.
The company said it will await their feedback prior to initiating dosing of cohort 3.
“We are encouraged by the apparent efficiency of gene transduction and the early trend we are seeing in sustained FIX activity across both cohorts with our wild-type FIX AAVrh10 vector in patients,” said Annalisa Jenkins, MBBS, chief executive officer of Dimension Therapeutics.
“We continue to explore the therapeutic window for DTX101 as our data mature and in light of the ALT rises that appear to be associated with a decline in FIX activity.” ![]()
Cheap manufacture of generic cancer drugs is feasible, study shows

Photo courtesy of FDA
AMSTERDAM—New research suggests some generic cancer drugs could be manufactured for less than 1% of the prices currently charged in the US and UK.
For example, researchers calculated that manufacturing a 400 mg tablet of imatinib costs $0.92.
Charging $1.04 per tablet would cover costs and allow for a 10% profit margin.
However, the current price of imatinib is $84.36 per tablet in the UK and $247.74 per tablet in the US.
Melissa Barber, of the London School of Hygiene and Tropical Medicine in the UK, reported these findings at ECCO 2017: European Cancer Congress (abstract 1032).
Barber and her colleagues collected data on per-kilogram costs of exported active pharmaceutical ingredients (APIs) from an online database of Indian export logs.
The team then estimated generic prices for tablets through an established costing algorithm. They calculated per-dose API costs and added excipient costs of $2.63 per kg of finished pharmaceutical product and per-tablet costs of production of $0.01, plus a 10% profit margin accounting for a 26.6% average tax on profits (assuming manufacture in India.)
Finally, the researchers compared the calculated price to current unit prices in the US, UK, Spain, and India.
For imatinib, the team determined the cost of the API to be $2284 per kg and the API cost per tablet to be $0.91. They then added excipient cost ($0.002 per tablet), conversion cost ($0.01 per tablet), and a 10% profit margin accounting for a 26.6% tax on profits.
This resulted in the estimated generic price of $1.04 per tablet. The per-tablet price is below the estimated price in India ($0.22) but much higher than the estimated price in Spain ($57.53), the UK ($84.36), and the US ($247.74).
Barber noted that, according to her group’s calculations, imatinib could be produced for $54 a month.
Another drug that could be produced for a low cost is etoposide. Barber and her colleagues calculated a generic price for etoposide of $0.97 per 100 mg tablet.
However, the per-tablet price is $1.50 in India, $8.65 in Spain, $11.34 in the UK, and $87.14 in the US.
The researchers calculated a generic price for mercaptopurine of $0.03 per 50 mg tablet, which is the same as the per-tablet price in India. However, a 50 mg mercaptopurine tablet costs $3.14 in Spain, $2.56 in the UK, and $0.40 in the US.
“Showing that certain cancers could be treated for very low prices could transform the future of people with these cancers in very low-income countries where there are usually few or no treatment options,” Barber said. ![]()

Photo courtesy of FDA
AMSTERDAM—New research suggests some generic cancer drugs could be manufactured for less than 1% of the prices currently charged in the US and UK.
For example, researchers calculated that manufacturing a 400 mg tablet of imatinib costs $0.92.
Charging $1.04 per tablet would cover costs and allow for a 10% profit margin.
However, the current price of imatinib is $84.36 per tablet in the UK and $247.74 per tablet in the US.
Melissa Barber, of the London School of Hygiene and Tropical Medicine in the UK, reported these findings at ECCO 2017: European Cancer Congress (abstract 1032).
Barber and her colleagues collected data on per-kilogram costs of exported active pharmaceutical ingredients (APIs) from an online database of Indian export logs.
The team then estimated generic prices for tablets through an established costing algorithm. They calculated per-dose API costs and added excipient costs of $2.63 per kg of finished pharmaceutical product and per-tablet costs of production of $0.01, plus a 10% profit margin accounting for a 26.6% average tax on profits (assuming manufacture in India.)
Finally, the researchers compared the calculated price to current unit prices in the US, UK, Spain, and India.
For imatinib, the team determined the cost of the API to be $2284 per kg and the API cost per tablet to be $0.91. They then added excipient cost ($0.002 per tablet), conversion cost ($0.01 per tablet), and a 10% profit margin accounting for a 26.6% tax on profits.
This resulted in the estimated generic price of $1.04 per tablet. The per-tablet price is below the estimated price in India ($0.22) but much higher than the estimated price in Spain ($57.53), the UK ($84.36), and the US ($247.74).
Barber noted that, according to her group’s calculations, imatinib could be produced for $54 a month.
Another drug that could be produced for a low cost is etoposide. Barber and her colleagues calculated a generic price for etoposide of $0.97 per 100 mg tablet.
However, the per-tablet price is $1.50 in India, $8.65 in Spain, $11.34 in the UK, and $87.14 in the US.
The researchers calculated a generic price for mercaptopurine of $0.03 per 50 mg tablet, which is the same as the per-tablet price in India. However, a 50 mg mercaptopurine tablet costs $3.14 in Spain, $2.56 in the UK, and $0.40 in the US.
“Showing that certain cancers could be treated for very low prices could transform the future of people with these cancers in very low-income countries where there are usually few or no treatment options,” Barber said. ![]()

Photo courtesy of FDA
AMSTERDAM—New research suggests some generic cancer drugs could be manufactured for less than 1% of the prices currently charged in the US and UK.
For example, researchers calculated that manufacturing a 400 mg tablet of imatinib costs $0.92.
Charging $1.04 per tablet would cover costs and allow for a 10% profit margin.
However, the current price of imatinib is $84.36 per tablet in the UK and $247.74 per tablet in the US.
Melissa Barber, of the London School of Hygiene and Tropical Medicine in the UK, reported these findings at ECCO 2017: European Cancer Congress (abstract 1032).
Barber and her colleagues collected data on per-kilogram costs of exported active pharmaceutical ingredients (APIs) from an online database of Indian export logs.
The team then estimated generic prices for tablets through an established costing algorithm. They calculated per-dose API costs and added excipient costs of $2.63 per kg of finished pharmaceutical product and per-tablet costs of production of $0.01, plus a 10% profit margin accounting for a 26.6% average tax on profits (assuming manufacture in India.)
Finally, the researchers compared the calculated price to current unit prices in the US, UK, Spain, and India.
For imatinib, the team determined the cost of the API to be $2284 per kg and the API cost per tablet to be $0.91. They then added excipient cost ($0.002 per tablet), conversion cost ($0.01 per tablet), and a 10% profit margin accounting for a 26.6% tax on profits.
This resulted in the estimated generic price of $1.04 per tablet. The per-tablet price is below the estimated price in India ($0.22) but much higher than the estimated price in Spain ($57.53), the UK ($84.36), and the US ($247.74).
Barber noted that, according to her group’s calculations, imatinib could be produced for $54 a month.
Another drug that could be produced for a low cost is etoposide. Barber and her colleagues calculated a generic price for etoposide of $0.97 per 100 mg tablet.
However, the per-tablet price is $1.50 in India, $8.65 in Spain, $11.34 in the UK, and $87.14 in the US.
The researchers calculated a generic price for mercaptopurine of $0.03 per 50 mg tablet, which is the same as the per-tablet price in India. However, a 50 mg mercaptopurine tablet costs $3.14 in Spain, $2.56 in the UK, and $0.40 in the US.
“Showing that certain cancers could be treated for very low prices could transform the future of people with these cancers in very low-income countries where there are usually few or no treatment options,” Barber said. ![]()
Company withdraws MAA for pegfilgrastim biosimilar

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has announced that Sandoz GmbH withdrew its marketing authorization application (MAA) for Zioxtenzo.
The active ingredient of Zioxtenzo is pegfilgrastim, and the product was intended to be biosimilar to Amgen’s Neulasta.
The intended use for Zioxtenzo was to reduce the duration of neutropenia and the occurrence of febrile neutropenia in cancer patients.
In its application for Zioxtenzo, Sandoz presented results of studies designed to show the product is highly similar to Neulasta in terms of chemical structure, purity, the way it works, and how the body handles the drug.
In addition, there were 2 studies comparing the safety and effectiveness of Zioxtenzo and Neulasta in patients receiving cancer drugs.
Sandoz withdrew the MAA for Zioxtenzo after the CHMP had evaluated the initial documentation provided by the company and formulated a list of questions. The company had not responded to the questions at the time of the withdrawal.
Based on a review of the data, at the time of the withdrawal, the CHMP had 2 main concerns and was of the provisional opinion that Zioxtenzo could not have been approved as a biosimilar of Neulasta.
One concern was that study results were not able to show that the concentrations of pegfilgrastim in blood were the same after taking Zioxtenzo and Neulasta.
The other concern was the lack of a certificate of Good Manufacturing Practice for Zioxtenzo’s manufacturing site. An inspection of the site would therefore be needed before the drug could be approved.
At the time of the MAA withdrawal, Sandoz had not demonstrated that Zioxtenzo is highly similar to Neulasta, and an inspection to confirm that Zioxtenzo was being manufactured according to Good Manufacturing Practice standards had not yet taken place.
In its letter notifying the CHMP of the MAA withdrawal, Sandoz said it would not be able to provide the additional data required by the CHMP within the timeframe allowed for the procedure.
The company also said the withdrawal of Zioxtenzo will not impact ongoing clinical trials, and there are no compassionate use programs for Zioxtenzo. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has announced that Sandoz GmbH withdrew its marketing authorization application (MAA) for Zioxtenzo.
The active ingredient of Zioxtenzo is pegfilgrastim, and the product was intended to be biosimilar to Amgen’s Neulasta.
The intended use for Zioxtenzo was to reduce the duration of neutropenia and the occurrence of febrile neutropenia in cancer patients.
In its application for Zioxtenzo, Sandoz presented results of studies designed to show the product is highly similar to Neulasta in terms of chemical structure, purity, the way it works, and how the body handles the drug.
In addition, there were 2 studies comparing the safety and effectiveness of Zioxtenzo and Neulasta in patients receiving cancer drugs.
Sandoz withdrew the MAA for Zioxtenzo after the CHMP had evaluated the initial documentation provided by the company and formulated a list of questions. The company had not responded to the questions at the time of the withdrawal.
Based on a review of the data, at the time of the withdrawal, the CHMP had 2 main concerns and was of the provisional opinion that Zioxtenzo could not have been approved as a biosimilar of Neulasta.
One concern was that study results were not able to show that the concentrations of pegfilgrastim in blood were the same after taking Zioxtenzo and Neulasta.
The other concern was the lack of a certificate of Good Manufacturing Practice for Zioxtenzo’s manufacturing site. An inspection of the site would therefore be needed before the drug could be approved.
At the time of the MAA withdrawal, Sandoz had not demonstrated that Zioxtenzo is highly similar to Neulasta, and an inspection to confirm that Zioxtenzo was being manufactured according to Good Manufacturing Practice standards had not yet taken place.
In its letter notifying the CHMP of the MAA withdrawal, Sandoz said it would not be able to provide the additional data required by the CHMP within the timeframe allowed for the procedure.
The company also said the withdrawal of Zioxtenzo will not impact ongoing clinical trials, and there are no compassionate use programs for Zioxtenzo. ![]()

The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has announced that Sandoz GmbH withdrew its marketing authorization application (MAA) for Zioxtenzo.
The active ingredient of Zioxtenzo is pegfilgrastim, and the product was intended to be biosimilar to Amgen’s Neulasta.
The intended use for Zioxtenzo was to reduce the duration of neutropenia and the occurrence of febrile neutropenia in cancer patients.
In its application for Zioxtenzo, Sandoz presented results of studies designed to show the product is highly similar to Neulasta in terms of chemical structure, purity, the way it works, and how the body handles the drug.
In addition, there were 2 studies comparing the safety and effectiveness of Zioxtenzo and Neulasta in patients receiving cancer drugs.
Sandoz withdrew the MAA for Zioxtenzo after the CHMP had evaluated the initial documentation provided by the company and formulated a list of questions. The company had not responded to the questions at the time of the withdrawal.
Based on a review of the data, at the time of the withdrawal, the CHMP had 2 main concerns and was of the provisional opinion that Zioxtenzo could not have been approved as a biosimilar of Neulasta.
One concern was that study results were not able to show that the concentrations of pegfilgrastim in blood were the same after taking Zioxtenzo and Neulasta.
The other concern was the lack of a certificate of Good Manufacturing Practice for Zioxtenzo’s manufacturing site. An inspection of the site would therefore be needed before the drug could be approved.
At the time of the MAA withdrawal, Sandoz had not demonstrated that Zioxtenzo is highly similar to Neulasta, and an inspection to confirm that Zioxtenzo was being manufactured according to Good Manufacturing Practice standards had not yet taken place.
In its letter notifying the CHMP of the MAA withdrawal, Sandoz said it would not be able to provide the additional data required by the CHMP within the timeframe allowed for the procedure.
The company also said the withdrawal of Zioxtenzo will not impact ongoing clinical trials, and there are no compassionate use programs for Zioxtenzo. ![]()
Child with various rashes
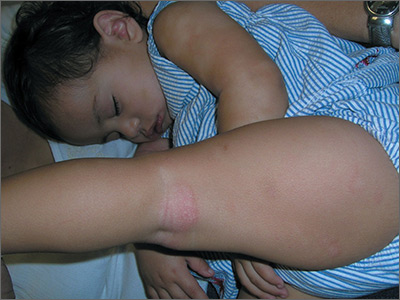
The FP made the diagnoses of atopic dermatitis (especially in the popliteal fossae) and pityriasis alba, which was seen on the child’s face and arms. Pityriasis alba is very common in children (especially in those who also have atopic dermatitis), but it can occur in children without atopic dermatitis, as well. The condition is characterized by hypopigmented patches of skin and is of cosmetic concern only.
The FP prescribed 2.5% hydrocortisone ointment for both conditions and told the mother that she would need to apply it once to twice daily, as needed. The FP explained that the hypopigmentation would take longer to resolve than the inflammatory erythema. Fortunately, atopic dermatitis in children typically resolves by adulthood.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Finklea L. Atopic dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill;2013:584-590.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP made the diagnoses of atopic dermatitis (especially in the popliteal fossae) and pityriasis alba, which was seen on the child’s face and arms. Pityriasis alba is very common in children (especially in those who also have atopic dermatitis), but it can occur in children without atopic dermatitis, as well. The condition is characterized by hypopigmented patches of skin and is of cosmetic concern only.
The FP prescribed 2.5% hydrocortisone ointment for both conditions and told the mother that she would need to apply it once to twice daily, as needed. The FP explained that the hypopigmentation would take longer to resolve than the inflammatory erythema. Fortunately, atopic dermatitis in children typically resolves by adulthood.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Finklea L. Atopic dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill;2013:584-590.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The FP made the diagnoses of atopic dermatitis (especially in the popliteal fossae) and pityriasis alba, which was seen on the child’s face and arms. Pityriasis alba is very common in children (especially in those who also have atopic dermatitis), but it can occur in children without atopic dermatitis, as well. The condition is characterized by hypopigmented patches of skin and is of cosmetic concern only.
The FP prescribed 2.5% hydrocortisone ointment for both conditions and told the mother that she would need to apply it once to twice daily, as needed. The FP explained that the hypopigmentation would take longer to resolve than the inflammatory erythema. Fortunately, atopic dermatitis in children typically resolves by adulthood.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Finklea L. Atopic dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill;2013:584-590.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
More Than a Spot of Bother
ANSWER
The correct answer is vitiligo (choice “b”).
DISCUSSION
Vitiligo develops when pigment cells (melanocytes) fail or die. Although there appears to be a hereditary component in some cases, as well as a connection to autoimmune disease, environmental factors (eg, intense sun exposure, stress) may also play a role.
This patient has nonsegmental vitiligo (NSV), the most common form. It is usually symmetrically distributed on high-friction areas, such as hands, knees, and elbows, as well as around the eyes and mouth. Segmental vitiligo, which affects only 10% of all vitiligo patients, tends to manifest during adolescence and typically remains confined to one area.
Unfortunately, for the majority of those with NSV (including the case patient), the condition tends to be progressive—and it responds poorly to treatment with topical steroids, calcineurin inhibitors, or phototherapy. As seen in this case, it can cause pigment loss in or around lesions; in fact, if left alone, the lesion may completely lose color. And NSV can encompass wider areas of involvement—to the extent that some patients lose all the pigment in their bodies. The resulting psychiatric fallout is considerable, especially in darker-skinned patients.
This patient's lesion will likely double in size by adulthood, which will not only subject him to ridicule but also increase the risk for malignant transformation. For this reason, he was advised to have the lesion excised under general anesthesia. He was also started on a regimen of a topical steroid cream and a calcineurin inhibitor on alternating days, but his prognosis is, in all honesty, poor.
ANSWER
The correct answer is vitiligo (choice “b”).
DISCUSSION
Vitiligo develops when pigment cells (melanocytes) fail or die. Although there appears to be a hereditary component in some cases, as well as a connection to autoimmune disease, environmental factors (eg, intense sun exposure, stress) may also play a role.
This patient has nonsegmental vitiligo (NSV), the most common form. It is usually symmetrically distributed on high-friction areas, such as hands, knees, and elbows, as well as around the eyes and mouth. Segmental vitiligo, which affects only 10% of all vitiligo patients, tends to manifest during adolescence and typically remains confined to one area.
Unfortunately, for the majority of those with NSV (including the case patient), the condition tends to be progressive—and it responds poorly to treatment with topical steroids, calcineurin inhibitors, or phototherapy. As seen in this case, it can cause pigment loss in or around lesions; in fact, if left alone, the lesion may completely lose color. And NSV can encompass wider areas of involvement—to the extent that some patients lose all the pigment in their bodies. The resulting psychiatric fallout is considerable, especially in darker-skinned patients.
This patient's lesion will likely double in size by adulthood, which will not only subject him to ridicule but also increase the risk for malignant transformation. For this reason, he was advised to have the lesion excised under general anesthesia. He was also started on a regimen of a topical steroid cream and a calcineurin inhibitor on alternating days, but his prognosis is, in all honesty, poor.
ANSWER
The correct answer is vitiligo (choice “b”).
DISCUSSION
Vitiligo develops when pigment cells (melanocytes) fail or die. Although there appears to be a hereditary component in some cases, as well as a connection to autoimmune disease, environmental factors (eg, intense sun exposure, stress) may also play a role.
This patient has nonsegmental vitiligo (NSV), the most common form. It is usually symmetrically distributed on high-friction areas, such as hands, knees, and elbows, as well as around the eyes and mouth. Segmental vitiligo, which affects only 10% of all vitiligo patients, tends to manifest during adolescence and typically remains confined to one area.
Unfortunately, for the majority of those with NSV (including the case patient), the condition tends to be progressive—and it responds poorly to treatment with topical steroids, calcineurin inhibitors, or phototherapy. As seen in this case, it can cause pigment loss in or around lesions; in fact, if left alone, the lesion may completely lose color. And NSV can encompass wider areas of involvement—to the extent that some patients lose all the pigment in their bodies. The resulting psychiatric fallout is considerable, especially in darker-skinned patients.
This patient's lesion will likely double in size by adulthood, which will not only subject him to ridicule but also increase the risk for malignant transformation. For this reason, he was advised to have the lesion excised under general anesthesia. He was also started on a regimen of a topical steroid cream and a calcineurin inhibitor on alternating days, but his prognosis is, in all honesty, poor.
A 5-year-old boy is referred to dermatology for evaluation of recent color changes to the skin around a congenital lesion. Located on his mid low back, the polygonal lesion measures 8 x 5 cm and is uniformly dark brown with a mammillated, hair-bearing surface. The plaque has grown proportionately with the child but otherwise remained stable.
A year ago, however, the patient’s family noticed that the normal brown skin around the lesion was turning white. This “halo” became noticeably larger over the span of the year—effectively doubling the size of the lesion.
The child’s type V skin is in sharp contrast to the porcelain white band that parallels the margins of his lesion. The surface of the depigmented skin is completely smooth, with no epidermal changes. Faint but definite depigmentation is noted on the periocular skin of both eyes, in addition to well-defined depigmentation on his fingertips and the perionychial areas of all 10 fingers.
The family asserts that the boy is otherwise healthy and that there is no family history of similar phenomena. The rest of the examination is unremarkable.