User login
Engage parents in treatment as part of family-based therapy for anorexia
SAN FRANCISCO – Parents have to stay level-headed if family-based therapy is going to work for anorexia nervosa, according to an expert on the technique, James Lock, MD, PhD, director of the Child and Adolescent Eating Disorder Program at Stanford (Calif.) University.
The idea of family-based therapy (FBT) is to guide parents to change behaviors – the patients’, but also their own – that undermine weight gain. Early on, the therapist has a meal with the family to observe dynamics that need to be addressed and encourage behaviors that help. It’s a nonauthoritarian approach, where the therapist helps families help themselves.
Heated emotion just opens the door to argument and resistance; patients aren’t rational when it comes to body image and eating, at least at first. Parents also have to learn not to be provoked by the child.
“You just have to ignore it. You don’t argue with the kids,” and “right now, ‘we love you’ just doesn’t go anywhere,” Dr. Lock said at a psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
Instead, caregivers are simply supportive. The only messages that matter initially, when weight gain is critical, are along the lines of, “I know you need to eat this. You need to eat it.” Parents “need to support the behavior change and really be kind of neutral about everything else,” he said.
FBT is one of many therapeutic options for anorexia. It has a strong evidence base going back about 30 years. Dr. Lock and his colleagues have been involved in many of the more recent studies and reported a 12-month remission rate of 49% in one (Arch Gen Psychiatry. 2010 Oct; 67[10]:1025-32). There’s growing support for FBT in bulimia, as well.
The earliest goal is to engage the parents in treatment. They are complimented on what they are doing well and told not to blame themselves or their child for the illness. The seriousness of anorexia is also impressed upon the parents if they are in denial about the illness.
The family meal comes early, too. They’re stressful but necessary to learn what parents are trying to do to help and to coach them about what needs improvement. “You, as a therapist, need” to be at the meal and “join the family in their dilemmas,” Dr. Lock said.
Parents are responsible for weight restoration at first, but when steady weight gain occurs, they are taught to hand control of eating and weight back to the child. In time, therapists help with normal adolescent developmental issues and healthy family relationships.
There are maybe 20 sessions over 6-12 months, more or less depending on how it’s going; each one lasts an hour. Single-parent families seem to need more sessions, likely because there’s no spouse to share in the work. There are no meal plans in FBT, because meal plans “are not a normal way to eat. When you try to empower parents to make reasonable decisions about food, a 24-hour meal plan that the adolescent is aware of is just an opportunity for fighting over what it says,” Dr. Lock said.
Dr. Lock has coauthored a treatment manual on using FBT for anorexia.
SAN FRANCISCO – Parents have to stay level-headed if family-based therapy is going to work for anorexia nervosa, according to an expert on the technique, James Lock, MD, PhD, director of the Child and Adolescent Eating Disorder Program at Stanford (Calif.) University.
The idea of family-based therapy (FBT) is to guide parents to change behaviors – the patients’, but also their own – that undermine weight gain. Early on, the therapist has a meal with the family to observe dynamics that need to be addressed and encourage behaviors that help. It’s a nonauthoritarian approach, where the therapist helps families help themselves.
Heated emotion just opens the door to argument and resistance; patients aren’t rational when it comes to body image and eating, at least at first. Parents also have to learn not to be provoked by the child.
“You just have to ignore it. You don’t argue with the kids,” and “right now, ‘we love you’ just doesn’t go anywhere,” Dr. Lock said at a psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
Instead, caregivers are simply supportive. The only messages that matter initially, when weight gain is critical, are along the lines of, “I know you need to eat this. You need to eat it.” Parents “need to support the behavior change and really be kind of neutral about everything else,” he said.
FBT is one of many therapeutic options for anorexia. It has a strong evidence base going back about 30 years. Dr. Lock and his colleagues have been involved in many of the more recent studies and reported a 12-month remission rate of 49% in one (Arch Gen Psychiatry. 2010 Oct; 67[10]:1025-32). There’s growing support for FBT in bulimia, as well.
The earliest goal is to engage the parents in treatment. They are complimented on what they are doing well and told not to blame themselves or their child for the illness. The seriousness of anorexia is also impressed upon the parents if they are in denial about the illness.
The family meal comes early, too. They’re stressful but necessary to learn what parents are trying to do to help and to coach them about what needs improvement. “You, as a therapist, need” to be at the meal and “join the family in their dilemmas,” Dr. Lock said.
Parents are responsible for weight restoration at first, but when steady weight gain occurs, they are taught to hand control of eating and weight back to the child. In time, therapists help with normal adolescent developmental issues and healthy family relationships.
There are maybe 20 sessions over 6-12 months, more or less depending on how it’s going; each one lasts an hour. Single-parent families seem to need more sessions, likely because there’s no spouse to share in the work. There are no meal plans in FBT, because meal plans “are not a normal way to eat. When you try to empower parents to make reasonable decisions about food, a 24-hour meal plan that the adolescent is aware of is just an opportunity for fighting over what it says,” Dr. Lock said.
Dr. Lock has coauthored a treatment manual on using FBT for anorexia.
SAN FRANCISCO – Parents have to stay level-headed if family-based therapy is going to work for anorexia nervosa, according to an expert on the technique, James Lock, MD, PhD, director of the Child and Adolescent Eating Disorder Program at Stanford (Calif.) University.
The idea of family-based therapy (FBT) is to guide parents to change behaviors – the patients’, but also their own – that undermine weight gain. Early on, the therapist has a meal with the family to observe dynamics that need to be addressed and encourage behaviors that help. It’s a nonauthoritarian approach, where the therapist helps families help themselves.
Heated emotion just opens the door to argument and resistance; patients aren’t rational when it comes to body image and eating, at least at first. Parents also have to learn not to be provoked by the child.
“You just have to ignore it. You don’t argue with the kids,” and “right now, ‘we love you’ just doesn’t go anywhere,” Dr. Lock said at a psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
Instead, caregivers are simply supportive. The only messages that matter initially, when weight gain is critical, are along the lines of, “I know you need to eat this. You need to eat it.” Parents “need to support the behavior change and really be kind of neutral about everything else,” he said.
FBT is one of many therapeutic options for anorexia. It has a strong evidence base going back about 30 years. Dr. Lock and his colleagues have been involved in many of the more recent studies and reported a 12-month remission rate of 49% in one (Arch Gen Psychiatry. 2010 Oct; 67[10]:1025-32). There’s growing support for FBT in bulimia, as well.
The earliest goal is to engage the parents in treatment. They are complimented on what they are doing well and told not to blame themselves or their child for the illness. The seriousness of anorexia is also impressed upon the parents if they are in denial about the illness.
The family meal comes early, too. They’re stressful but necessary to learn what parents are trying to do to help and to coach them about what needs improvement. “You, as a therapist, need” to be at the meal and “join the family in their dilemmas,” Dr. Lock said.
Parents are responsible for weight restoration at first, but when steady weight gain occurs, they are taught to hand control of eating and weight back to the child. In time, therapists help with normal adolescent developmental issues and healthy family relationships.
There are maybe 20 sessions over 6-12 months, more or less depending on how it’s going; each one lasts an hour. Single-parent families seem to need more sessions, likely because there’s no spouse to share in the work. There are no meal plans in FBT, because meal plans “are not a normal way to eat. When you try to empower parents to make reasonable decisions about food, a 24-hour meal plan that the adolescent is aware of is just an opportunity for fighting over what it says,” Dr. Lock said.
Dr. Lock has coauthored a treatment manual on using FBT for anorexia.
EXPERT ANALYSIS FROM THE PSYCHOPHARMACOLOGY UPDATE INSTITUTE
Survival better with breast-conserving therapy for early cancers
AMSTERDAM – In real-life practice, women with early, localized breast cancer who underwent breast conserving therapy had better breast cancer–specific and overall survival compared with women who underwent mastectomy, according to investigators in the Netherlands.
Among nearly 130,000 patients treated over two different time periods, breast-conserving surgery and radiation (BCT) was associated with superior survival for women older than 50, patients who did not receive adjuvant chemotherapy, and those with comorbidities – irrespective of either hormonal or human epidermal growth factor receptor 2 (HER2) status, reported Mirelle Lagendijk, MD, of Erasmus Medical Center Cancer Institute in Rotterdam, the Netherlands.
For patients 50 and younger, overall survival (OS), but not breast cancer–specific survival (BCSS), was superior with the more conservative approach.
“Breast conserving therapy in these identified subgroups seems to be the preferable treatment when both treatments are optional,” Dr. Lagendijk said at an annual congress sponsored by the European Cancer Organisation.
Although recent observational studies have shown survival with BCT to be at least equivalent for women with early stage disease, there is still a lack of sufficient data on BCSS, potential confounders such as systemic therapies and comorbidities, and on the relative effects of BCT or mastectomy on subgroups, she said.
The investigators drew data from the Netherlands Cancer Registry on 129,692 patients with early, primary invasive breast cancer without metastases other than to regional lymph nodes (T1-2NO-2MO).
They compared BCT to mastectomy for BCSS and OS in the population as a whole and in subgroups based on prognostic factors. They controlled for age, tumor and nodal stage, comorbidities, systemic therapy, hormone receptor and HER2 status, differentiation grade, morphology, year of treatment, axillary lymph node dissection, and contralateral breast cancer.
They divided patients into two treatment time periods. The older cohort consisted of 60,381 patients treated from 1999 through 2005, 48% of whom underwent mastectomy, with a median follow-up of 11.1 years, and 52% of whom had BCT, with a median follow-up of 12 years.
The more recent cohort consisted of 69,311 patients, 40% of whom had mastectomy with a median follow-up of 5.9 years, and 60% of whom had BCT with a median follow-up of 6.1 years.
In both time periods, deaths from all causes were lower among patients treated with BCT. In the older cohort, 13,960 of 28,968 patients (48.2%) who underwent mastectomy had died, compared with 8,915 of 31,413 patients (28.4%) who underwent BCT. In the more recent cohort, 5,504 of 27,731 (19.8%) of patients who had mastectomies had died, compared with 3,702 of 41,580 (8.9%) who underwent BCT.
“Irrespective of the time cohort and irrespective of the treatment, around 50% of the events were breast cancer related,” Dr. Lagendijk said.
BCSS was superior with BCT in each time cohort (log-rank P less than .001 for each). In the earlier cohort, BCT was significantly superior for BCSS across all disease stages; in the later cohort, it was significant for all but stages T1N1 and T1-2N2.
BCSS was superior for patients in all age categories in the early cohort, and for patients 50 and older in the later cohort.
“The final stratification performed for comorbidities present in the patients evaluated showed, surprisingly, that especially for those patients with comorbidity, there was significantly better breast cancer-specific survival when treated by breast conserving therapy as compared to a mastectomy,” Dr. Lagendijk said.
The investigators acknowledged that the study was limited by its retrospective design, potential confounding by severity, and the inability to show causal relationship between survival and treatment type.
Dutch health agencies sponsored the study. Dr. Lagendijk and Dr. Naredi reported no conflicts of interest.
AMSTERDAM – In real-life practice, women with early, localized breast cancer who underwent breast conserving therapy had better breast cancer–specific and overall survival compared with women who underwent mastectomy, according to investigators in the Netherlands.
Among nearly 130,000 patients treated over two different time periods, breast-conserving surgery and radiation (BCT) was associated with superior survival for women older than 50, patients who did not receive adjuvant chemotherapy, and those with comorbidities – irrespective of either hormonal or human epidermal growth factor receptor 2 (HER2) status, reported Mirelle Lagendijk, MD, of Erasmus Medical Center Cancer Institute in Rotterdam, the Netherlands.
For patients 50 and younger, overall survival (OS), but not breast cancer–specific survival (BCSS), was superior with the more conservative approach.
“Breast conserving therapy in these identified subgroups seems to be the preferable treatment when both treatments are optional,” Dr. Lagendijk said at an annual congress sponsored by the European Cancer Organisation.
Although recent observational studies have shown survival with BCT to be at least equivalent for women with early stage disease, there is still a lack of sufficient data on BCSS, potential confounders such as systemic therapies and comorbidities, and on the relative effects of BCT or mastectomy on subgroups, she said.
The investigators drew data from the Netherlands Cancer Registry on 129,692 patients with early, primary invasive breast cancer without metastases other than to regional lymph nodes (T1-2NO-2MO).
They compared BCT to mastectomy for BCSS and OS in the population as a whole and in subgroups based on prognostic factors. They controlled for age, tumor and nodal stage, comorbidities, systemic therapy, hormone receptor and HER2 status, differentiation grade, morphology, year of treatment, axillary lymph node dissection, and contralateral breast cancer.
They divided patients into two treatment time periods. The older cohort consisted of 60,381 patients treated from 1999 through 2005, 48% of whom underwent mastectomy, with a median follow-up of 11.1 years, and 52% of whom had BCT, with a median follow-up of 12 years.
The more recent cohort consisted of 69,311 patients, 40% of whom had mastectomy with a median follow-up of 5.9 years, and 60% of whom had BCT with a median follow-up of 6.1 years.
In both time periods, deaths from all causes were lower among patients treated with BCT. In the older cohort, 13,960 of 28,968 patients (48.2%) who underwent mastectomy had died, compared with 8,915 of 31,413 patients (28.4%) who underwent BCT. In the more recent cohort, 5,504 of 27,731 (19.8%) of patients who had mastectomies had died, compared with 3,702 of 41,580 (8.9%) who underwent BCT.
“Irrespective of the time cohort and irrespective of the treatment, around 50% of the events were breast cancer related,” Dr. Lagendijk said.
BCSS was superior with BCT in each time cohort (log-rank P less than .001 for each). In the earlier cohort, BCT was significantly superior for BCSS across all disease stages; in the later cohort, it was significant for all but stages T1N1 and T1-2N2.
BCSS was superior for patients in all age categories in the early cohort, and for patients 50 and older in the later cohort.
“The final stratification performed for comorbidities present in the patients evaluated showed, surprisingly, that especially for those patients with comorbidity, there was significantly better breast cancer-specific survival when treated by breast conserving therapy as compared to a mastectomy,” Dr. Lagendijk said.
The investigators acknowledged that the study was limited by its retrospective design, potential confounding by severity, and the inability to show causal relationship between survival and treatment type.
Dutch health agencies sponsored the study. Dr. Lagendijk and Dr. Naredi reported no conflicts of interest.
AMSTERDAM – In real-life practice, women with early, localized breast cancer who underwent breast conserving therapy had better breast cancer–specific and overall survival compared with women who underwent mastectomy, according to investigators in the Netherlands.
Among nearly 130,000 patients treated over two different time periods, breast-conserving surgery and radiation (BCT) was associated with superior survival for women older than 50, patients who did not receive adjuvant chemotherapy, and those with comorbidities – irrespective of either hormonal or human epidermal growth factor receptor 2 (HER2) status, reported Mirelle Lagendijk, MD, of Erasmus Medical Center Cancer Institute in Rotterdam, the Netherlands.
For patients 50 and younger, overall survival (OS), but not breast cancer–specific survival (BCSS), was superior with the more conservative approach.
“Breast conserving therapy in these identified subgroups seems to be the preferable treatment when both treatments are optional,” Dr. Lagendijk said at an annual congress sponsored by the European Cancer Organisation.
Although recent observational studies have shown survival with BCT to be at least equivalent for women with early stage disease, there is still a lack of sufficient data on BCSS, potential confounders such as systemic therapies and comorbidities, and on the relative effects of BCT or mastectomy on subgroups, she said.
The investigators drew data from the Netherlands Cancer Registry on 129,692 patients with early, primary invasive breast cancer without metastases other than to regional lymph nodes (T1-2NO-2MO).
They compared BCT to mastectomy for BCSS and OS in the population as a whole and in subgroups based on prognostic factors. They controlled for age, tumor and nodal stage, comorbidities, systemic therapy, hormone receptor and HER2 status, differentiation grade, morphology, year of treatment, axillary lymph node dissection, and contralateral breast cancer.
They divided patients into two treatment time periods. The older cohort consisted of 60,381 patients treated from 1999 through 2005, 48% of whom underwent mastectomy, with a median follow-up of 11.1 years, and 52% of whom had BCT, with a median follow-up of 12 years.
The more recent cohort consisted of 69,311 patients, 40% of whom had mastectomy with a median follow-up of 5.9 years, and 60% of whom had BCT with a median follow-up of 6.1 years.
In both time periods, deaths from all causes were lower among patients treated with BCT. In the older cohort, 13,960 of 28,968 patients (48.2%) who underwent mastectomy had died, compared with 8,915 of 31,413 patients (28.4%) who underwent BCT. In the more recent cohort, 5,504 of 27,731 (19.8%) of patients who had mastectomies had died, compared with 3,702 of 41,580 (8.9%) who underwent BCT.
“Irrespective of the time cohort and irrespective of the treatment, around 50% of the events were breast cancer related,” Dr. Lagendijk said.
BCSS was superior with BCT in each time cohort (log-rank P less than .001 for each). In the earlier cohort, BCT was significantly superior for BCSS across all disease stages; in the later cohort, it was significant for all but stages T1N1 and T1-2N2.
BCSS was superior for patients in all age categories in the early cohort, and for patients 50 and older in the later cohort.
“The final stratification performed for comorbidities present in the patients evaluated showed, surprisingly, that especially for those patients with comorbidity, there was significantly better breast cancer-specific survival when treated by breast conserving therapy as compared to a mastectomy,” Dr. Lagendijk said.
The investigators acknowledged that the study was limited by its retrospective design, potential confounding by severity, and the inability to show causal relationship between survival and treatment type.
Dutch health agencies sponsored the study. Dr. Lagendijk and Dr. Naredi reported no conflicts of interest.
AT ECCO2017
Key clinical point: Breast cancer–specific survival and overall survival were better among women who had breast-conserving therapy (BCT) compared with mastectomy.
Major finding: BCT was associated with superior survival for women older than 50, patients who did not receive adjuvant chemotherapy, and those with comorbidities.
Data source: Retrospective registry data study of 129,692 women treated for early breast cancer in the Netherlands during 1999-2005 and 2006-2012.
Disclosures: Dutch health agencies sponsored the study. Dr. Langendijk and Dr. Naredi reported no conflicts of interest.
The PERT Movement – Vascular surgeons must answer the call
Pulmonary embolism (PE) is the third most common cause of cardiovascular death in the United States and remains the most common preventable cause of in-hospital death. One might think that, in 2017, such a life-threatening cardiovascular emergency would be managed by guideline-driven care based upon robust evidence gathered through clinical trials and large observational studies. Yet, unlike stroke and myocardial infarction (STEMI), a true consensus for best management of acute PE has not been reached.
Management of PE has received increasing attention recently at major cardiovascular meetings such as VEITH, TCT, and national and regional societies. This excitement has been driven by recent trial data demonstrating that right ventricular failure with an acute PE is associated with poor outcomes and rapid clot debulking can reduce mortality not only in patients with high risk (massive) PE but even in intermediate risk (submassive) PE.
Systemic thrombolysis has been the standard of care for higher risk PEs but multiple contraindications and high complication rates shifted care toward catheter interventions assumed to have a safer profile. The huge gap of past decades between therapeutic anticoagulation alone versus systemic thrombolysis or surgical pulmonary embolectomy has been filled with an array of catheter-based techniques. Contemporary management of intermediate and high-risk PE employs thrombolytic infusion catheters at the clot site with or without ultrasound technology, mechanical fragmentation, and aspiration/suction thrombectomy devices. These techniques may lack robust evidence supporting them, still their use has been exponentially growing over the past 2 years. Yet, there is no clear consensus guiding management. Who needs to be treated? What’s the best technique for any given patient? What are the therapeutic endpoints? A myriad of questions remain to be answered.
Uncertainty about best management for an individual patient with acute PE stimulated formation of a multidisciplinary, collaborative approach beginning in 2012 at Massachusetts General Hospital in Boston.
This approach, led by Kenneth Rosenfield, MD, involved the formal development of a call team of various specialists who would be rapidly activated to develop a care plan for any patient with acute PE. The term “Pulmonary Embolism Response Team” (PERT) was coined by MGH pulmonologist Richard Channick, MD, and since then, the PERT approach has been adopted by more than 100 centers across the United States and internationally.
Rapid communication between frontline physicians who diagnose PE and those who can offer definitive management is the hallmark of the PERT approach. PERTs at both Piedmont Atlanta Hospital (care plan shown below) and the University of Pittsburgh Medical Center (UPMC) function similarly. Patients are triaged by a critical care pulmonologist. For those with massive PE (hemodynamically unstable), the emergency call center establishes a cellular link between the bedside emergency physician, triaging critical care pulmonologist, the PE interventionalist, cardiothoracic surgeon, and ECMO team. CTAs may be viewed electronically, management plans initiated, and teams rapidly mobilized. For patients with submassive PE, the triaging critical care pulmonologist initiates management discussion with the “PE interventionalist” on call. Patient presentation, physiologic data, biomarkers, and preexisting comorbidities are discussed. CTAs are viewed. Multiple treatment plans are considered from traditional medical management to catheter-based techniques for more peripheral emboli to surgical pulmonary embolectomy for centrally located thrombus. Treatment plans are influenced by factors such as patient age and comorbidity and are collaboratively tailored to each individual patient.
Management decisions for acute PE are driven by risk stratification. Most patients who present with PE are considered “low risk” for PE-related death and are managed with therapeutic anticoagulation. Five percent of patients present with massive PE characterized by shock and are at “high-risk” for PE-related death. These patients require intervention. Up to 40% of patients present with submassive PE. These patients are hemodynamically stable (not hypotensive) but have evidence on CTA or echocardiogram for right ventricular dysfunction and are at “intermediate risk” for PE-related death. This group is further stratified as intermediate low-risk vs. intermediate high-risk by biomarkers that indicate myocardial damage, primarily troponin and BNP. In this intermediate-risk or submassive group, intervention is more commonly offered to those patients stratified as intermediate “high-risk.”
Significant variability in the management of both massive and submassive PE patients beyond therapeutic anticoagulation, e.g., upon whom to offer intervention and how, is the point where a PERT may have greatest impact. Rapid, collaborative decision making between physicians/surgeons from multiple specialties offers hope for minimizing morbidity and achievement of best outcome.
Who is the “Pulmonary Interventionalist” and where does the contemporarily-trained vascular surgeon with catheter and critical-care skills fit into this new paradigm? The answer to this question depends on each individual institution.
At the University of Pittsburgh Medical Center and Piedmont Heart and Vascular Institute at Piedmont Atlanta Hospital, catheter-based intervention programs for PE were initiated by vascular surgeons. As their interest developed over time, interventional cardiologists joined th “PE Interventional” call as full participants in the PERT programs.
PE interventions at other institutions such as Emory Midtown Medical Center in Atlanta have been driven by interventional cardiology in partnership with cardiothoracic surgery. Still, at others, such as Miami Heart and Vascular Institute, vascular and interventional radiology has led the charge. PE intervention, not owned by any single specialty, has been taken on by those groups interested in answering the call. In the case of the two programs that we represent, it was our established involvement in major venous interventions that followed a natural progression to PE intervention.
Management of PE may be challenging. Multidisciplinary collaboration is key. Recognition of the importance of collaboration in moving the field forward (and saving lives) led Dr. Rosenfield and others to host the first PERT Consortium Meeting in Boston 2015. This clarion call was answered by 80 individuals representing 40 institutions.
In 2016, the PERT Consortium was incorporated, and in June 2016, more than 140 people from nearly 80 institutions attended the second annual meeting. We attended the meeting last June, and with concern we noted only a few vascular surgeons representing other institutions. A participant survey, later published in a letter in CHEST (December 2016), suggested little involvement of vascular surgeons in PERT programs. Was this an artifact based on a survey of “those registered in the PERT mailing list” or does this represent the true interest of vascular surgeons in managing PE in this country? Whether the meeting survey accurately reflects the current involvement of vascular surgeons in the care of PE or not, one thing is certain: Vascular surgical visibility, as a specialty, in PE is poor.
Failure to be involved in the PERT movement deprives patients of the experience of vascular surgeons and potentially threatens the venous intervention practice of nonparticipating surgeons. Forty percent or more of cases of iliofemoral venous thrombosis have associated PE. Vena cava thrombosis may present with PE. Inferior vena cava tumors such as leiomyomatosis may be mistaken for PE. It is foreseeable that PERT activation will represent the gateway to care for many of these patients, who may in turn receive their care from others new to the management of venous and VTE disease.
Participation in a collaborative decision among colleagues about the best way to treat an individual patient is a gratifying experience. When the treatment fits, relieving the struggle of a patient with an acute PE through catheter-directed thrombus dissolution and debulking represents an opportunity to save productive lives using techniques that lie within the skill set of the contemporarily trained vascular surgeon. A save in the case of a challenging PE can be every bit as rewarding as successful management of a ruptured aneurysm.
Even in metropolitan regions, patients with acute PE are underserved because specialized care is frequently unavailable or PE programs nonexistent. In communities and hospitals where vascular surgeons represent the lead interventionalists, involvement in this field might even be considered a solemn responsibility.
Vascular surgeons such as Peter Lin, MD, previously at the University of Texas in Houston, and the group led by Rabih Chaer, MD, at UPMC, have advanced the science of PE intervention over the past 7 years. It is time for more vascular surgeons to enter the field and embrace pulmonary interventions. To achieve this, we need to embrace collaboration with pulmonary and critical care as well as emergency medicine since these are the main referral specialties. We need to promote initiatives participating in our local PERTs or bringing specialists together to start one where nonexistent. There is no reason for exclusivity, and collaboration with other interventionalists is essential for smooth interspecialty relations, multidisciplinary approaches, and optimal outcomes.
Academic and large community vascular centers need to include the vascular surgeons’ role in their descriptions of their PERTs. Toward this direction the vascular division of UPMC is consistently presenting and publishing results and outcomes of PE catheter interventions; very recently a randomized trial (SUNSET sPE) comparing lysis outcomes with and without ultrasound acceleration has been launched by the UPMC PERT, led and coordinated by vascular surgeons, and has already stimulated national interest. Vascular surgeons at Piedmont Heart and Vascular Institute are participating in national clinical trials (OPTALYSE) and actively collaborating with other PERT programs to advance the management of acute PE in Georgia and the southeast. Both UPMC and Piedmont Heart and Vascular Institute are founding institutional members of the PERT Consortium.
The third annual meeting of the PERT Consortium will occur in Boston in June 2017. Vascular surgeons who attend will assuredly be welcome. Answer the call.
Charles B. Ross, MD, is chief, Vascular and Endovascular Services, Piedmont Heart Institute, Atlanta. Efthymios Avgerinos, MD, is associate professor of surgery, Division of Vascular Surgery, Heart and Vascular Institute, University of Pittsburgh Medical Center. They had no relevant disclosures.
Pulmonary embolism (PE) is the third most common cause of cardiovascular death in the United States and remains the most common preventable cause of in-hospital death. One might think that, in 2017, such a life-threatening cardiovascular emergency would be managed by guideline-driven care based upon robust evidence gathered through clinical trials and large observational studies. Yet, unlike stroke and myocardial infarction (STEMI), a true consensus for best management of acute PE has not been reached.
Management of PE has received increasing attention recently at major cardiovascular meetings such as VEITH, TCT, and national and regional societies. This excitement has been driven by recent trial data demonstrating that right ventricular failure with an acute PE is associated with poor outcomes and rapid clot debulking can reduce mortality not only in patients with high risk (massive) PE but even in intermediate risk (submassive) PE.
Systemic thrombolysis has been the standard of care for higher risk PEs but multiple contraindications and high complication rates shifted care toward catheter interventions assumed to have a safer profile. The huge gap of past decades between therapeutic anticoagulation alone versus systemic thrombolysis or surgical pulmonary embolectomy has been filled with an array of catheter-based techniques. Contemporary management of intermediate and high-risk PE employs thrombolytic infusion catheters at the clot site with or without ultrasound technology, mechanical fragmentation, and aspiration/suction thrombectomy devices. These techniques may lack robust evidence supporting them, still their use has been exponentially growing over the past 2 years. Yet, there is no clear consensus guiding management. Who needs to be treated? What’s the best technique for any given patient? What are the therapeutic endpoints? A myriad of questions remain to be answered.
Uncertainty about best management for an individual patient with acute PE stimulated formation of a multidisciplinary, collaborative approach beginning in 2012 at Massachusetts General Hospital in Boston.
This approach, led by Kenneth Rosenfield, MD, involved the formal development of a call team of various specialists who would be rapidly activated to develop a care plan for any patient with acute PE. The term “Pulmonary Embolism Response Team” (PERT) was coined by MGH pulmonologist Richard Channick, MD, and since then, the PERT approach has been adopted by more than 100 centers across the United States and internationally.
Rapid communication between frontline physicians who diagnose PE and those who can offer definitive management is the hallmark of the PERT approach. PERTs at both Piedmont Atlanta Hospital (care plan shown below) and the University of Pittsburgh Medical Center (UPMC) function similarly. Patients are triaged by a critical care pulmonologist. For those with massive PE (hemodynamically unstable), the emergency call center establishes a cellular link between the bedside emergency physician, triaging critical care pulmonologist, the PE interventionalist, cardiothoracic surgeon, and ECMO team. CTAs may be viewed electronically, management plans initiated, and teams rapidly mobilized. For patients with submassive PE, the triaging critical care pulmonologist initiates management discussion with the “PE interventionalist” on call. Patient presentation, physiologic data, biomarkers, and preexisting comorbidities are discussed. CTAs are viewed. Multiple treatment plans are considered from traditional medical management to catheter-based techniques for more peripheral emboli to surgical pulmonary embolectomy for centrally located thrombus. Treatment plans are influenced by factors such as patient age and comorbidity and are collaboratively tailored to each individual patient.
Management decisions for acute PE are driven by risk stratification. Most patients who present with PE are considered “low risk” for PE-related death and are managed with therapeutic anticoagulation. Five percent of patients present with massive PE characterized by shock and are at “high-risk” for PE-related death. These patients require intervention. Up to 40% of patients present with submassive PE. These patients are hemodynamically stable (not hypotensive) but have evidence on CTA or echocardiogram for right ventricular dysfunction and are at “intermediate risk” for PE-related death. This group is further stratified as intermediate low-risk vs. intermediate high-risk by biomarkers that indicate myocardial damage, primarily troponin and BNP. In this intermediate-risk or submassive group, intervention is more commonly offered to those patients stratified as intermediate “high-risk.”
Significant variability in the management of both massive and submassive PE patients beyond therapeutic anticoagulation, e.g., upon whom to offer intervention and how, is the point where a PERT may have greatest impact. Rapid, collaborative decision making between physicians/surgeons from multiple specialties offers hope for minimizing morbidity and achievement of best outcome.
Who is the “Pulmonary Interventionalist” and where does the contemporarily-trained vascular surgeon with catheter and critical-care skills fit into this new paradigm? The answer to this question depends on each individual institution.
At the University of Pittsburgh Medical Center and Piedmont Heart and Vascular Institute at Piedmont Atlanta Hospital, catheter-based intervention programs for PE were initiated by vascular surgeons. As their interest developed over time, interventional cardiologists joined th “PE Interventional” call as full participants in the PERT programs.
PE interventions at other institutions such as Emory Midtown Medical Center in Atlanta have been driven by interventional cardiology in partnership with cardiothoracic surgery. Still, at others, such as Miami Heart and Vascular Institute, vascular and interventional radiology has led the charge. PE intervention, not owned by any single specialty, has been taken on by those groups interested in answering the call. In the case of the two programs that we represent, it was our established involvement in major venous interventions that followed a natural progression to PE intervention.
Management of PE may be challenging. Multidisciplinary collaboration is key. Recognition of the importance of collaboration in moving the field forward (and saving lives) led Dr. Rosenfield and others to host the first PERT Consortium Meeting in Boston 2015. This clarion call was answered by 80 individuals representing 40 institutions.
In 2016, the PERT Consortium was incorporated, and in June 2016, more than 140 people from nearly 80 institutions attended the second annual meeting. We attended the meeting last June, and with concern we noted only a few vascular surgeons representing other institutions. A participant survey, later published in a letter in CHEST (December 2016), suggested little involvement of vascular surgeons in PERT programs. Was this an artifact based on a survey of “those registered in the PERT mailing list” or does this represent the true interest of vascular surgeons in managing PE in this country? Whether the meeting survey accurately reflects the current involvement of vascular surgeons in the care of PE or not, one thing is certain: Vascular surgical visibility, as a specialty, in PE is poor.
Failure to be involved in the PERT movement deprives patients of the experience of vascular surgeons and potentially threatens the venous intervention practice of nonparticipating surgeons. Forty percent or more of cases of iliofemoral venous thrombosis have associated PE. Vena cava thrombosis may present with PE. Inferior vena cava tumors such as leiomyomatosis may be mistaken for PE. It is foreseeable that PERT activation will represent the gateway to care for many of these patients, who may in turn receive their care from others new to the management of venous and VTE disease.
Participation in a collaborative decision among colleagues about the best way to treat an individual patient is a gratifying experience. When the treatment fits, relieving the struggle of a patient with an acute PE through catheter-directed thrombus dissolution and debulking represents an opportunity to save productive lives using techniques that lie within the skill set of the contemporarily trained vascular surgeon. A save in the case of a challenging PE can be every bit as rewarding as successful management of a ruptured aneurysm.
Even in metropolitan regions, patients with acute PE are underserved because specialized care is frequently unavailable or PE programs nonexistent. In communities and hospitals where vascular surgeons represent the lead interventionalists, involvement in this field might even be considered a solemn responsibility.
Vascular surgeons such as Peter Lin, MD, previously at the University of Texas in Houston, and the group led by Rabih Chaer, MD, at UPMC, have advanced the science of PE intervention over the past 7 years. It is time for more vascular surgeons to enter the field and embrace pulmonary interventions. To achieve this, we need to embrace collaboration with pulmonary and critical care as well as emergency medicine since these are the main referral specialties. We need to promote initiatives participating in our local PERTs or bringing specialists together to start one where nonexistent. There is no reason for exclusivity, and collaboration with other interventionalists is essential for smooth interspecialty relations, multidisciplinary approaches, and optimal outcomes.
Academic and large community vascular centers need to include the vascular surgeons’ role in their descriptions of their PERTs. Toward this direction the vascular division of UPMC is consistently presenting and publishing results and outcomes of PE catheter interventions; very recently a randomized trial (SUNSET sPE) comparing lysis outcomes with and without ultrasound acceleration has been launched by the UPMC PERT, led and coordinated by vascular surgeons, and has already stimulated national interest. Vascular surgeons at Piedmont Heart and Vascular Institute are participating in national clinical trials (OPTALYSE) and actively collaborating with other PERT programs to advance the management of acute PE in Georgia and the southeast. Both UPMC and Piedmont Heart and Vascular Institute are founding institutional members of the PERT Consortium.
The third annual meeting of the PERT Consortium will occur in Boston in June 2017. Vascular surgeons who attend will assuredly be welcome. Answer the call.
Charles B. Ross, MD, is chief, Vascular and Endovascular Services, Piedmont Heart Institute, Atlanta. Efthymios Avgerinos, MD, is associate professor of surgery, Division of Vascular Surgery, Heart and Vascular Institute, University of Pittsburgh Medical Center. They had no relevant disclosures.
Pulmonary embolism (PE) is the third most common cause of cardiovascular death in the United States and remains the most common preventable cause of in-hospital death. One might think that, in 2017, such a life-threatening cardiovascular emergency would be managed by guideline-driven care based upon robust evidence gathered through clinical trials and large observational studies. Yet, unlike stroke and myocardial infarction (STEMI), a true consensus for best management of acute PE has not been reached.
Management of PE has received increasing attention recently at major cardiovascular meetings such as VEITH, TCT, and national and regional societies. This excitement has been driven by recent trial data demonstrating that right ventricular failure with an acute PE is associated with poor outcomes and rapid clot debulking can reduce mortality not only in patients with high risk (massive) PE but even in intermediate risk (submassive) PE.
Systemic thrombolysis has been the standard of care for higher risk PEs but multiple contraindications and high complication rates shifted care toward catheter interventions assumed to have a safer profile. The huge gap of past decades between therapeutic anticoagulation alone versus systemic thrombolysis or surgical pulmonary embolectomy has been filled with an array of catheter-based techniques. Contemporary management of intermediate and high-risk PE employs thrombolytic infusion catheters at the clot site with or without ultrasound technology, mechanical fragmentation, and aspiration/suction thrombectomy devices. These techniques may lack robust evidence supporting them, still their use has been exponentially growing over the past 2 years. Yet, there is no clear consensus guiding management. Who needs to be treated? What’s the best technique for any given patient? What are the therapeutic endpoints? A myriad of questions remain to be answered.
Uncertainty about best management for an individual patient with acute PE stimulated formation of a multidisciplinary, collaborative approach beginning in 2012 at Massachusetts General Hospital in Boston.
This approach, led by Kenneth Rosenfield, MD, involved the formal development of a call team of various specialists who would be rapidly activated to develop a care plan for any patient with acute PE. The term “Pulmonary Embolism Response Team” (PERT) was coined by MGH pulmonologist Richard Channick, MD, and since then, the PERT approach has been adopted by more than 100 centers across the United States and internationally.
Rapid communication between frontline physicians who diagnose PE and those who can offer definitive management is the hallmark of the PERT approach. PERTs at both Piedmont Atlanta Hospital (care plan shown below) and the University of Pittsburgh Medical Center (UPMC) function similarly. Patients are triaged by a critical care pulmonologist. For those with massive PE (hemodynamically unstable), the emergency call center establishes a cellular link between the bedside emergency physician, triaging critical care pulmonologist, the PE interventionalist, cardiothoracic surgeon, and ECMO team. CTAs may be viewed electronically, management plans initiated, and teams rapidly mobilized. For patients with submassive PE, the triaging critical care pulmonologist initiates management discussion with the “PE interventionalist” on call. Patient presentation, physiologic data, biomarkers, and preexisting comorbidities are discussed. CTAs are viewed. Multiple treatment plans are considered from traditional medical management to catheter-based techniques for more peripheral emboli to surgical pulmonary embolectomy for centrally located thrombus. Treatment plans are influenced by factors such as patient age and comorbidity and are collaboratively tailored to each individual patient.
Management decisions for acute PE are driven by risk stratification. Most patients who present with PE are considered “low risk” for PE-related death and are managed with therapeutic anticoagulation. Five percent of patients present with massive PE characterized by shock and are at “high-risk” for PE-related death. These patients require intervention. Up to 40% of patients present with submassive PE. These patients are hemodynamically stable (not hypotensive) but have evidence on CTA or echocardiogram for right ventricular dysfunction and are at “intermediate risk” for PE-related death. This group is further stratified as intermediate low-risk vs. intermediate high-risk by biomarkers that indicate myocardial damage, primarily troponin and BNP. In this intermediate-risk or submassive group, intervention is more commonly offered to those patients stratified as intermediate “high-risk.”
Significant variability in the management of both massive and submassive PE patients beyond therapeutic anticoagulation, e.g., upon whom to offer intervention and how, is the point where a PERT may have greatest impact. Rapid, collaborative decision making between physicians/surgeons from multiple specialties offers hope for minimizing morbidity and achievement of best outcome.
Who is the “Pulmonary Interventionalist” and where does the contemporarily-trained vascular surgeon with catheter and critical-care skills fit into this new paradigm? The answer to this question depends on each individual institution.
At the University of Pittsburgh Medical Center and Piedmont Heart and Vascular Institute at Piedmont Atlanta Hospital, catheter-based intervention programs for PE were initiated by vascular surgeons. As their interest developed over time, interventional cardiologists joined th “PE Interventional” call as full participants in the PERT programs.
PE interventions at other institutions such as Emory Midtown Medical Center in Atlanta have been driven by interventional cardiology in partnership with cardiothoracic surgery. Still, at others, such as Miami Heart and Vascular Institute, vascular and interventional radiology has led the charge. PE intervention, not owned by any single specialty, has been taken on by those groups interested in answering the call. In the case of the two programs that we represent, it was our established involvement in major venous interventions that followed a natural progression to PE intervention.
Management of PE may be challenging. Multidisciplinary collaboration is key. Recognition of the importance of collaboration in moving the field forward (and saving lives) led Dr. Rosenfield and others to host the first PERT Consortium Meeting in Boston 2015. This clarion call was answered by 80 individuals representing 40 institutions.
In 2016, the PERT Consortium was incorporated, and in June 2016, more than 140 people from nearly 80 institutions attended the second annual meeting. We attended the meeting last June, and with concern we noted only a few vascular surgeons representing other institutions. A participant survey, later published in a letter in CHEST (December 2016), suggested little involvement of vascular surgeons in PERT programs. Was this an artifact based on a survey of “those registered in the PERT mailing list” or does this represent the true interest of vascular surgeons in managing PE in this country? Whether the meeting survey accurately reflects the current involvement of vascular surgeons in the care of PE or not, one thing is certain: Vascular surgical visibility, as a specialty, in PE is poor.
Failure to be involved in the PERT movement deprives patients of the experience of vascular surgeons and potentially threatens the venous intervention practice of nonparticipating surgeons. Forty percent or more of cases of iliofemoral venous thrombosis have associated PE. Vena cava thrombosis may present with PE. Inferior vena cava tumors such as leiomyomatosis may be mistaken for PE. It is foreseeable that PERT activation will represent the gateway to care for many of these patients, who may in turn receive their care from others new to the management of venous and VTE disease.
Participation in a collaborative decision among colleagues about the best way to treat an individual patient is a gratifying experience. When the treatment fits, relieving the struggle of a patient with an acute PE through catheter-directed thrombus dissolution and debulking represents an opportunity to save productive lives using techniques that lie within the skill set of the contemporarily trained vascular surgeon. A save in the case of a challenging PE can be every bit as rewarding as successful management of a ruptured aneurysm.
Even in metropolitan regions, patients with acute PE are underserved because specialized care is frequently unavailable or PE programs nonexistent. In communities and hospitals where vascular surgeons represent the lead interventionalists, involvement in this field might even be considered a solemn responsibility.
Vascular surgeons such as Peter Lin, MD, previously at the University of Texas in Houston, and the group led by Rabih Chaer, MD, at UPMC, have advanced the science of PE intervention over the past 7 years. It is time for more vascular surgeons to enter the field and embrace pulmonary interventions. To achieve this, we need to embrace collaboration with pulmonary and critical care as well as emergency medicine since these are the main referral specialties. We need to promote initiatives participating in our local PERTs or bringing specialists together to start one where nonexistent. There is no reason for exclusivity, and collaboration with other interventionalists is essential for smooth interspecialty relations, multidisciplinary approaches, and optimal outcomes.
Academic and large community vascular centers need to include the vascular surgeons’ role in their descriptions of their PERTs. Toward this direction the vascular division of UPMC is consistently presenting and publishing results and outcomes of PE catheter interventions; very recently a randomized trial (SUNSET sPE) comparing lysis outcomes with and without ultrasound acceleration has been launched by the UPMC PERT, led and coordinated by vascular surgeons, and has already stimulated national interest. Vascular surgeons at Piedmont Heart and Vascular Institute are participating in national clinical trials (OPTALYSE) and actively collaborating with other PERT programs to advance the management of acute PE in Georgia and the southeast. Both UPMC and Piedmont Heart and Vascular Institute are founding institutional members of the PERT Consortium.
The third annual meeting of the PERT Consortium will occur in Boston in June 2017. Vascular surgeons who attend will assuredly be welcome. Answer the call.
Charles B. Ross, MD, is chief, Vascular and Endovascular Services, Piedmont Heart Institute, Atlanta. Efthymios Avgerinos, MD, is associate professor of surgery, Division of Vascular Surgery, Heart and Vascular Institute, University of Pittsburgh Medical Center. They had no relevant disclosures.
VIDEO: Nonsteroidal topical expands options for pediatric AD
WAILEA, HAWAII – In an interview, pediatric dermatologist Lawrence F. Eichenfield, MD, discusses a recently approved topical therapy for atopic dermatitis, which provides a nonsteroidal option for treating the disease in young patients.
“We’re really excited to have a new topical agent” for AD, Dr. Eichenfield said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
The product, crisaborole (Eucrisa), is a phosphodiesterase 4 (PDE-4) inhibitor, a new type of chemical entity “based on a different pathway of decreasing inflammation,” said Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego. Crisaborole, the first new chemical entity to become available for treating AD since 2001, blocks PDE-4 and decreases cytokines, thereby reducing the inflammation in AD, he explained.
In the United States, the product is approved for the topical treatment of mild to moderate AD for patients aged 2 years and older. No serious adverse events attributed to crisaborole have been reported so far, in phase II and III studies and in a 1-year study, he said.
Dr. Eichenfield disclosed relationships with companies including Anacor/Pfizer, Genentech, Lilly, Regeneron/Sanofi, Medimetriks, and Otsuka. Crisaborole is manufactured by Anacor. SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – In an interview, pediatric dermatologist Lawrence F. Eichenfield, MD, discusses a recently approved topical therapy for atopic dermatitis, which provides a nonsteroidal option for treating the disease in young patients.
“We’re really excited to have a new topical agent” for AD, Dr. Eichenfield said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
The product, crisaborole (Eucrisa), is a phosphodiesterase 4 (PDE-4) inhibitor, a new type of chemical entity “based on a different pathway of decreasing inflammation,” said Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego. Crisaborole, the first new chemical entity to become available for treating AD since 2001, blocks PDE-4 and decreases cytokines, thereby reducing the inflammation in AD, he explained.
In the United States, the product is approved for the topical treatment of mild to moderate AD for patients aged 2 years and older. No serious adverse events attributed to crisaborole have been reported so far, in phase II and III studies and in a 1-year study, he said.
Dr. Eichenfield disclosed relationships with companies including Anacor/Pfizer, Genentech, Lilly, Regeneron/Sanofi, Medimetriks, and Otsuka. Crisaborole is manufactured by Anacor. SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – In an interview, pediatric dermatologist Lawrence F. Eichenfield, MD, discusses a recently approved topical therapy for atopic dermatitis, which provides a nonsteroidal option for treating the disease in young patients.
“We’re really excited to have a new topical agent” for AD, Dr. Eichenfield said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
The product, crisaborole (Eucrisa), is a phosphodiesterase 4 (PDE-4) inhibitor, a new type of chemical entity “based on a different pathway of decreasing inflammation,” said Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego. Crisaborole, the first new chemical entity to become available for treating AD since 2001, blocks PDE-4 and decreases cytokines, thereby reducing the inflammation in AD, he explained.
In the United States, the product is approved for the topical treatment of mild to moderate AD for patients aged 2 years and older. No serious adverse events attributed to crisaborole have been reported so far, in phase II and III studies and in a 1-year study, he said.
Dr. Eichenfield disclosed relationships with companies including Anacor/Pfizer, Genentech, Lilly, Regeneron/Sanofi, Medimetriks, and Otsuka. Crisaborole is manufactured by Anacor. SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AGA, other medical organizations respond to Trump’s immigration order
Organizations representing physicians and medical students have expressed their concern regarding President Trump’s executive order of Jan. 27 that curtails entry into the United States by travelers from seven Muslim-majority countries. The order also suspends for 120 days entry into the United States for all persons seeking refugee status, and it bars refugees from Syria indefinitely.
Following are direct excerpts from statements issued by medical organizations.
American Gastroenterological Association
Science and illness ignore borders and political divides. That is why AGA is concerned that the recent U.S. executive order on immigration could limit scientific exchange, delay patient care, and impair medical training.
AGA is committed to diversity, which we define as inclusive of race, ethnicity, and national origin. Diversity within training programs and laboratories in the United States built today’s practice of gastroenterology. Scientists from around the world publish in our journals, work in our laboratories, train in our programs, and present data at Digestive Disease Week.® This exchange leads to better patient care, and very sick patients travel to the U.S. from around the world for the best digestive health care.
In light of these concerns, AGA adds our support to a growing number of medical institutions urging the administration to consider the devastating impact of the executive order on the health of the nation that will result from turning away patients, health professionals, and researchers. The recent immigration policy is clearly detrimental to America’s leadership role in advancing health care, and to the standing of the United States within the international community.
American Academy of Family Physicians
“We are deeply concerned that steps your Administration has taken will have a chilling effect on our nation’s physician workforce, biomedical research, and global health. It is often America’s physicians who answer the call to assist people around the world when a public health crisis occurs. Imagine a world where physicians fail to answer the call of the needy because they fear they may not be able to return to their home and families in the United States.
Many family physicians are international medical graduates (IMG), who have completed all or part of their education and training in the United States. They are professionals who dedicate their careers to the service of their patients in communities large and small, urban and rural. In fact, 20% of our membership and over 25% of family medicine residents [comprise] IMGs. The AAFP applauds and supports wholly the contributions of these individual family physicians to their patients and communities and we celebrate their diversity.
We recognize that one of your primary responsibilities as President is to ensure the safety and security of the country and its citizens. This is, without question, a daunting responsibility. But we strongly urge that the methods of doing so be examined carefully, so that the many people who can add so much to our country through immigration have the opportunity to do so, and those who are doing so already are treated with the respect and dignity they deserve.”
American Academy of Pediatrics
“The executive orders signed today are harmful to immigrant children and families throughout our country. Many of the children who will be most affected are the victims of unspeakable violence and have been exposed to trauma. Children do not immigrate, they flee. They are coming to the United States seeking safe haven in our country and they need our compassion and assistance. Broad scale expansion of family detention only exacerbates their suffering ... The AAP is non-partisan and pro-children. We urge President Trump and his administration to ensure that children and families who are fleeing violence and adversity can continue to seek refuge in our country. Immigrant children and families are an integral part of our communities and our nation, and they deserve to be cared for, treated with compassion, and celebrated. Most of all, they deserve to be healthy and safe. Pediatricians stand with the immigrant families we care for and will continue to advocate that their needs are met and prioritized.”
American Association of Medical Colleges
“The United States is facing a serious shortage of physicians. IMGs play an important role in U.S. health care, representing roughly 25% of the workforce. Current immigration pathways – including student, exchange-visitor, and employment visas – provide a balanced solution that improves health care access across the country through programs like the National Interest Waiver and the Conrad 30 J-1 Visa Waiver. In the last decade, Conrad 30 alone has directed nearly 10,000 physicians into rural and urban underserved communities. Impeding these U.S. immigration pathways jeopardizes critical access to high-quality physician care for our nation’s most vulnerable populations.
Our ability to attract top talent from around the world also enriches the research laboratories at medical schools and teaching hospitals that are working toward cures and has helped position the United States as a global leader in medical research, strengthening our economy and bolstering the public’s health. Because disease knows no geographic boundaries, it is essential to ensure that we continue to foster, rather than impede, scientific cooperation with physicians and researchers of all nationalities, as we strive to keep our country healthy.”
American College of Cardiology
“The ability to share ideas and knowledge necessary to address [the global epidemic of cardiovascular disease] is imperative. Policies that impede this free-flow of ideas will have a detrimental impact on scientific discovery, as well as the lives of patients around the world. If we are to realize a future where cardiovascular disease is no longer the number one killer of men and women worldwide we must ensure that our system of scientific exchange allows for health care professionals to learn from each other regardless of their nationality.
Additionally, IMGs, naturalized citizens, and legal residents make up a significant portion of the health care workforce in hospitals and practices across the country. More than 25% of current practicing physicians are IMGs, with cardiology ranking among the top when broken down by medical specialty. Policies that bring the immigration status of those already here into question, while also limiting the ability of others to legally train in the United States going forward, will only serve to exacerbate the already existing cardiovascular workforce shortage, especially in rural America. Such policies also threaten the care continuum of patients who rely on these providers for their medical care.”
American College of Physicians
“The executive order could deny entry or reentry to tens of thousands more persons, including medical students and physicians who are being trained in the United States and/or are delivering direct patient care. ... It also creates a precedent for barring entry of IMGs based on their religion and country of origin. ... Approximately 30% of ACP members are IMGs.”
American Society of Clinical Oncology
ASCO is deeply concerned about the potential impact of the recent executive order on cancer research, patient care, and international scientific collaboration.
Our more than 40,000 members in 148 countries lead the charge to conquer cancer in all its forms and in every nation. Tens of thousands of people from more than 100 countries participate in our scientific meetings to exchange advances and ideas to improve patient care. Millions of cancer survivors are alive today because of the progress made possible by scientific collaboration. Progress against this disease will falter if the close-knit global community of cancer care providers is divided by policies that bar members of certain nationalities from entering the United States to conduct research, care for people with cancer, or participate in scientific and medical conferences.
American Society of Hematology
We express our deep concern about the Administration’s executive order that has denied U.S. entry to people who bring unique expertise to the practice of medicine and the conduct of cancer and biomedical research. Our nation depends on the contributions of the greatest minds from around the world to maintain the high quality of our biomedical research enterprise and health care services.
The benefits of scientific collaborations are amplified by our diversity. Limiting the exchange of ideas, practices, and data across cultures has the potential to significantly retard scientific progress and adversely affect public health. Any loss of researchers and physicians will render the United States less competitive over time, and our traditionally strong research institutions and the patients they serve will be negatively affected.
We remain deeply concerned that restricting travel will prohibit participation in scientific meetings, where cutting-edge science and treatment methods are often first introduced. These in-person meetings and other global exchanges are vitally important because they provide unparalleled opportunities for collaborations and information-sharing. Such scientific and medical meetings are absolutely essential to the conquest of cancer and blood diseases.
(Statement issued on behalf of ASH, American Association for Cancer Research, Association of American Cancer Institutes, American Society for Radiation Oncology, The American Society for Pediatric Hematology/Oncology, and LUNGevity Foundation.)
The text of the executive order can be found on the White House website.
[email protected]
On Twitter @denisefulton
Organizations representing physicians and medical students have expressed their concern regarding President Trump’s executive order of Jan. 27 that curtails entry into the United States by travelers from seven Muslim-majority countries. The order also suspends for 120 days entry into the United States for all persons seeking refugee status, and it bars refugees from Syria indefinitely.
Following are direct excerpts from statements issued by medical organizations.
American Gastroenterological Association
Science and illness ignore borders and political divides. That is why AGA is concerned that the recent U.S. executive order on immigration could limit scientific exchange, delay patient care, and impair medical training.
AGA is committed to diversity, which we define as inclusive of race, ethnicity, and national origin. Diversity within training programs and laboratories in the United States built today’s practice of gastroenterology. Scientists from around the world publish in our journals, work in our laboratories, train in our programs, and present data at Digestive Disease Week.® This exchange leads to better patient care, and very sick patients travel to the U.S. from around the world for the best digestive health care.
In light of these concerns, AGA adds our support to a growing number of medical institutions urging the administration to consider the devastating impact of the executive order on the health of the nation that will result from turning away patients, health professionals, and researchers. The recent immigration policy is clearly detrimental to America’s leadership role in advancing health care, and to the standing of the United States within the international community.
American Academy of Family Physicians
“We are deeply concerned that steps your Administration has taken will have a chilling effect on our nation’s physician workforce, biomedical research, and global health. It is often America’s physicians who answer the call to assist people around the world when a public health crisis occurs. Imagine a world where physicians fail to answer the call of the needy because they fear they may not be able to return to their home and families in the United States.
Many family physicians are international medical graduates (IMG), who have completed all or part of their education and training in the United States. They are professionals who dedicate their careers to the service of their patients in communities large and small, urban and rural. In fact, 20% of our membership and over 25% of family medicine residents [comprise] IMGs. The AAFP applauds and supports wholly the contributions of these individual family physicians to their patients and communities and we celebrate their diversity.
We recognize that one of your primary responsibilities as President is to ensure the safety and security of the country and its citizens. This is, without question, a daunting responsibility. But we strongly urge that the methods of doing so be examined carefully, so that the many people who can add so much to our country through immigration have the opportunity to do so, and those who are doing so already are treated with the respect and dignity they deserve.”
American Academy of Pediatrics
“The executive orders signed today are harmful to immigrant children and families throughout our country. Many of the children who will be most affected are the victims of unspeakable violence and have been exposed to trauma. Children do not immigrate, they flee. They are coming to the United States seeking safe haven in our country and they need our compassion and assistance. Broad scale expansion of family detention only exacerbates their suffering ... The AAP is non-partisan and pro-children. We urge President Trump and his administration to ensure that children and families who are fleeing violence and adversity can continue to seek refuge in our country. Immigrant children and families are an integral part of our communities and our nation, and they deserve to be cared for, treated with compassion, and celebrated. Most of all, they deserve to be healthy and safe. Pediatricians stand with the immigrant families we care for and will continue to advocate that their needs are met and prioritized.”
American Association of Medical Colleges
“The United States is facing a serious shortage of physicians. IMGs play an important role in U.S. health care, representing roughly 25% of the workforce. Current immigration pathways – including student, exchange-visitor, and employment visas – provide a balanced solution that improves health care access across the country through programs like the National Interest Waiver and the Conrad 30 J-1 Visa Waiver. In the last decade, Conrad 30 alone has directed nearly 10,000 physicians into rural and urban underserved communities. Impeding these U.S. immigration pathways jeopardizes critical access to high-quality physician care for our nation’s most vulnerable populations.
Our ability to attract top talent from around the world also enriches the research laboratories at medical schools and teaching hospitals that are working toward cures and has helped position the United States as a global leader in medical research, strengthening our economy and bolstering the public’s health. Because disease knows no geographic boundaries, it is essential to ensure that we continue to foster, rather than impede, scientific cooperation with physicians and researchers of all nationalities, as we strive to keep our country healthy.”
American College of Cardiology
“The ability to share ideas and knowledge necessary to address [the global epidemic of cardiovascular disease] is imperative. Policies that impede this free-flow of ideas will have a detrimental impact on scientific discovery, as well as the lives of patients around the world. If we are to realize a future where cardiovascular disease is no longer the number one killer of men and women worldwide we must ensure that our system of scientific exchange allows for health care professionals to learn from each other regardless of their nationality.
Additionally, IMGs, naturalized citizens, and legal residents make up a significant portion of the health care workforce in hospitals and practices across the country. More than 25% of current practicing physicians are IMGs, with cardiology ranking among the top when broken down by medical specialty. Policies that bring the immigration status of those already here into question, while also limiting the ability of others to legally train in the United States going forward, will only serve to exacerbate the already existing cardiovascular workforce shortage, especially in rural America. Such policies also threaten the care continuum of patients who rely on these providers for their medical care.”
American College of Physicians
“The executive order could deny entry or reentry to tens of thousands more persons, including medical students and physicians who are being trained in the United States and/or are delivering direct patient care. ... It also creates a precedent for barring entry of IMGs based on their religion and country of origin. ... Approximately 30% of ACP members are IMGs.”
American Society of Clinical Oncology
ASCO is deeply concerned about the potential impact of the recent executive order on cancer research, patient care, and international scientific collaboration.
Our more than 40,000 members in 148 countries lead the charge to conquer cancer in all its forms and in every nation. Tens of thousands of people from more than 100 countries participate in our scientific meetings to exchange advances and ideas to improve patient care. Millions of cancer survivors are alive today because of the progress made possible by scientific collaboration. Progress against this disease will falter if the close-knit global community of cancer care providers is divided by policies that bar members of certain nationalities from entering the United States to conduct research, care for people with cancer, or participate in scientific and medical conferences.
American Society of Hematology
We express our deep concern about the Administration’s executive order that has denied U.S. entry to people who bring unique expertise to the practice of medicine and the conduct of cancer and biomedical research. Our nation depends on the contributions of the greatest minds from around the world to maintain the high quality of our biomedical research enterprise and health care services.
The benefits of scientific collaborations are amplified by our diversity. Limiting the exchange of ideas, practices, and data across cultures has the potential to significantly retard scientific progress and adversely affect public health. Any loss of researchers and physicians will render the United States less competitive over time, and our traditionally strong research institutions and the patients they serve will be negatively affected.
We remain deeply concerned that restricting travel will prohibit participation in scientific meetings, where cutting-edge science and treatment methods are often first introduced. These in-person meetings and other global exchanges are vitally important because they provide unparalleled opportunities for collaborations and information-sharing. Such scientific and medical meetings are absolutely essential to the conquest of cancer and blood diseases.
(Statement issued on behalf of ASH, American Association for Cancer Research, Association of American Cancer Institutes, American Society for Radiation Oncology, The American Society for Pediatric Hematology/Oncology, and LUNGevity Foundation.)
The text of the executive order can be found on the White House website.
[email protected]
On Twitter @denisefulton
Organizations representing physicians and medical students have expressed their concern regarding President Trump’s executive order of Jan. 27 that curtails entry into the United States by travelers from seven Muslim-majority countries. The order also suspends for 120 days entry into the United States for all persons seeking refugee status, and it bars refugees from Syria indefinitely.
Following are direct excerpts from statements issued by medical organizations.
American Gastroenterological Association
Science and illness ignore borders and political divides. That is why AGA is concerned that the recent U.S. executive order on immigration could limit scientific exchange, delay patient care, and impair medical training.
AGA is committed to diversity, which we define as inclusive of race, ethnicity, and national origin. Diversity within training programs and laboratories in the United States built today’s practice of gastroenterology. Scientists from around the world publish in our journals, work in our laboratories, train in our programs, and present data at Digestive Disease Week.® This exchange leads to better patient care, and very sick patients travel to the U.S. from around the world for the best digestive health care.
In light of these concerns, AGA adds our support to a growing number of medical institutions urging the administration to consider the devastating impact of the executive order on the health of the nation that will result from turning away patients, health professionals, and researchers. The recent immigration policy is clearly detrimental to America’s leadership role in advancing health care, and to the standing of the United States within the international community.
American Academy of Family Physicians
“We are deeply concerned that steps your Administration has taken will have a chilling effect on our nation’s physician workforce, biomedical research, and global health. It is often America’s physicians who answer the call to assist people around the world when a public health crisis occurs. Imagine a world where physicians fail to answer the call of the needy because they fear they may not be able to return to their home and families in the United States.
Many family physicians are international medical graduates (IMG), who have completed all or part of their education and training in the United States. They are professionals who dedicate their careers to the service of their patients in communities large and small, urban and rural. In fact, 20% of our membership and over 25% of family medicine residents [comprise] IMGs. The AAFP applauds and supports wholly the contributions of these individual family physicians to their patients and communities and we celebrate their diversity.
We recognize that one of your primary responsibilities as President is to ensure the safety and security of the country and its citizens. This is, without question, a daunting responsibility. But we strongly urge that the methods of doing so be examined carefully, so that the many people who can add so much to our country through immigration have the opportunity to do so, and those who are doing so already are treated with the respect and dignity they deserve.”
American Academy of Pediatrics
“The executive orders signed today are harmful to immigrant children and families throughout our country. Many of the children who will be most affected are the victims of unspeakable violence and have been exposed to trauma. Children do not immigrate, they flee. They are coming to the United States seeking safe haven in our country and they need our compassion and assistance. Broad scale expansion of family detention only exacerbates their suffering ... The AAP is non-partisan and pro-children. We urge President Trump and his administration to ensure that children and families who are fleeing violence and adversity can continue to seek refuge in our country. Immigrant children and families are an integral part of our communities and our nation, and they deserve to be cared for, treated with compassion, and celebrated. Most of all, they deserve to be healthy and safe. Pediatricians stand with the immigrant families we care for and will continue to advocate that their needs are met and prioritized.”
American Association of Medical Colleges
“The United States is facing a serious shortage of physicians. IMGs play an important role in U.S. health care, representing roughly 25% of the workforce. Current immigration pathways – including student, exchange-visitor, and employment visas – provide a balanced solution that improves health care access across the country through programs like the National Interest Waiver and the Conrad 30 J-1 Visa Waiver. In the last decade, Conrad 30 alone has directed nearly 10,000 physicians into rural and urban underserved communities. Impeding these U.S. immigration pathways jeopardizes critical access to high-quality physician care for our nation’s most vulnerable populations.
Our ability to attract top talent from around the world also enriches the research laboratories at medical schools and teaching hospitals that are working toward cures and has helped position the United States as a global leader in medical research, strengthening our economy and bolstering the public’s health. Because disease knows no geographic boundaries, it is essential to ensure that we continue to foster, rather than impede, scientific cooperation with physicians and researchers of all nationalities, as we strive to keep our country healthy.”
American College of Cardiology
“The ability to share ideas and knowledge necessary to address [the global epidemic of cardiovascular disease] is imperative. Policies that impede this free-flow of ideas will have a detrimental impact on scientific discovery, as well as the lives of patients around the world. If we are to realize a future where cardiovascular disease is no longer the number one killer of men and women worldwide we must ensure that our system of scientific exchange allows for health care professionals to learn from each other regardless of their nationality.
Additionally, IMGs, naturalized citizens, and legal residents make up a significant portion of the health care workforce in hospitals and practices across the country. More than 25% of current practicing physicians are IMGs, with cardiology ranking among the top when broken down by medical specialty. Policies that bring the immigration status of those already here into question, while also limiting the ability of others to legally train in the United States going forward, will only serve to exacerbate the already existing cardiovascular workforce shortage, especially in rural America. Such policies also threaten the care continuum of patients who rely on these providers for their medical care.”
American College of Physicians
“The executive order could deny entry or reentry to tens of thousands more persons, including medical students and physicians who are being trained in the United States and/or are delivering direct patient care. ... It also creates a precedent for barring entry of IMGs based on their religion and country of origin. ... Approximately 30% of ACP members are IMGs.”
American Society of Clinical Oncology
ASCO is deeply concerned about the potential impact of the recent executive order on cancer research, patient care, and international scientific collaboration.
Our more than 40,000 members in 148 countries lead the charge to conquer cancer in all its forms and in every nation. Tens of thousands of people from more than 100 countries participate in our scientific meetings to exchange advances and ideas to improve patient care. Millions of cancer survivors are alive today because of the progress made possible by scientific collaboration. Progress against this disease will falter if the close-knit global community of cancer care providers is divided by policies that bar members of certain nationalities from entering the United States to conduct research, care for people with cancer, or participate in scientific and medical conferences.
American Society of Hematology
We express our deep concern about the Administration’s executive order that has denied U.S. entry to people who bring unique expertise to the practice of medicine and the conduct of cancer and biomedical research. Our nation depends on the contributions of the greatest minds from around the world to maintain the high quality of our biomedical research enterprise and health care services.
The benefits of scientific collaborations are amplified by our diversity. Limiting the exchange of ideas, practices, and data across cultures has the potential to significantly retard scientific progress and adversely affect public health. Any loss of researchers and physicians will render the United States less competitive over time, and our traditionally strong research institutions and the patients they serve will be negatively affected.
We remain deeply concerned that restricting travel will prohibit participation in scientific meetings, where cutting-edge science and treatment methods are often first introduced. These in-person meetings and other global exchanges are vitally important because they provide unparalleled opportunities for collaborations and information-sharing. Such scientific and medical meetings are absolutely essential to the conquest of cancer and blood diseases.
(Statement issued on behalf of ASH, American Association for Cancer Research, Association of American Cancer Institutes, American Society for Radiation Oncology, The American Society for Pediatric Hematology/Oncology, and LUNGevity Foundation.)
The text of the executive order can be found on the White House website.
[email protected]
On Twitter @denisefulton
Congenital Hemangioma
Hemangiomas are the most common benign tumors of childhood. In recent years, subsets of hemangiomas that are fully formed at birth have been recognized as clinically and biologically distinct from the classic infantile hemangioma (IH). Congenital hemangiomas (CHs) are classified based on clinical course as rapidly involuting CHs (RICHs) or noninvoluting CHs (NICHs). The aim of this retrospective study was to describe the epidemiology, clinical aspects, and clinical outcome of CH over a 5-year period.
Methods
Using electronic medical records from the department of dermatology (Hedi Chaker Hospital, Sfax, Tunisia) for a 5-year period (2008-2012), we searched for hemangioma. After collecting those records, we identified patients with CHs. We studied the epidemiologic (eg, sex, age), clinical course (eg, location, size, number, color, surrounding skin), and evolutionary aspects in these patients.
Results
Twenty IHs were identified, 6 (30%) of which were considered CHs. The clinical characteristics of the 6 patients are summarized in the Table. We identified 2 females and 4 males aged 2 to 60 days (mean age, 16 days). Four patients had CHs involving the limbs (knee [n=2]; ankle [n=1]; elbow [n=1]) and 2 patients had CHs involving the trunk. Congenital hemangiomas were singular, oval shaped, and surrounded by a clear halo in all 6 patients. They presented as exophytic masses (n=3) or bossed plaques (n=3). A blue hue was noted in 5 patients and a purple hue in 1 patient. In some cases, telangiectasia (n=2) or small areas of necrosis (n=1) were noted at the center of the CHs. The CHs ranged in size from 3 to 6 cm (mean, 4 cm). Doppler ultrasonography was performed in 2 patients and showed fast blood flow. It is well known that manipulating a CH when it is ulcerative may cause a fatal hemorrhage. Thus, parents/guardians should be cautious when cleaning and dressing the lesions. Regular follow-up was recommended to all patients as noted in the medical records. The lesion involuted in4 patients after a mean period of 6 months, which allowed us to classify the lesions as RICHs (Figure, A). Two CHs were persistent after 2-year (Figure, B) and 4-year (Figure, C) follow-up, which was consistent with NICH classification.
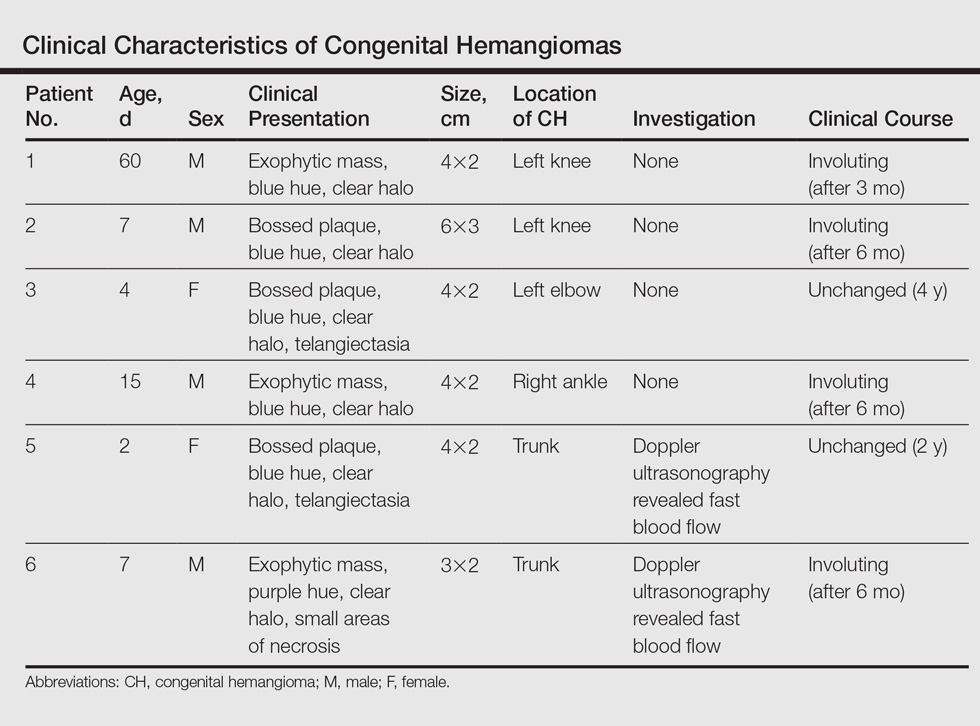
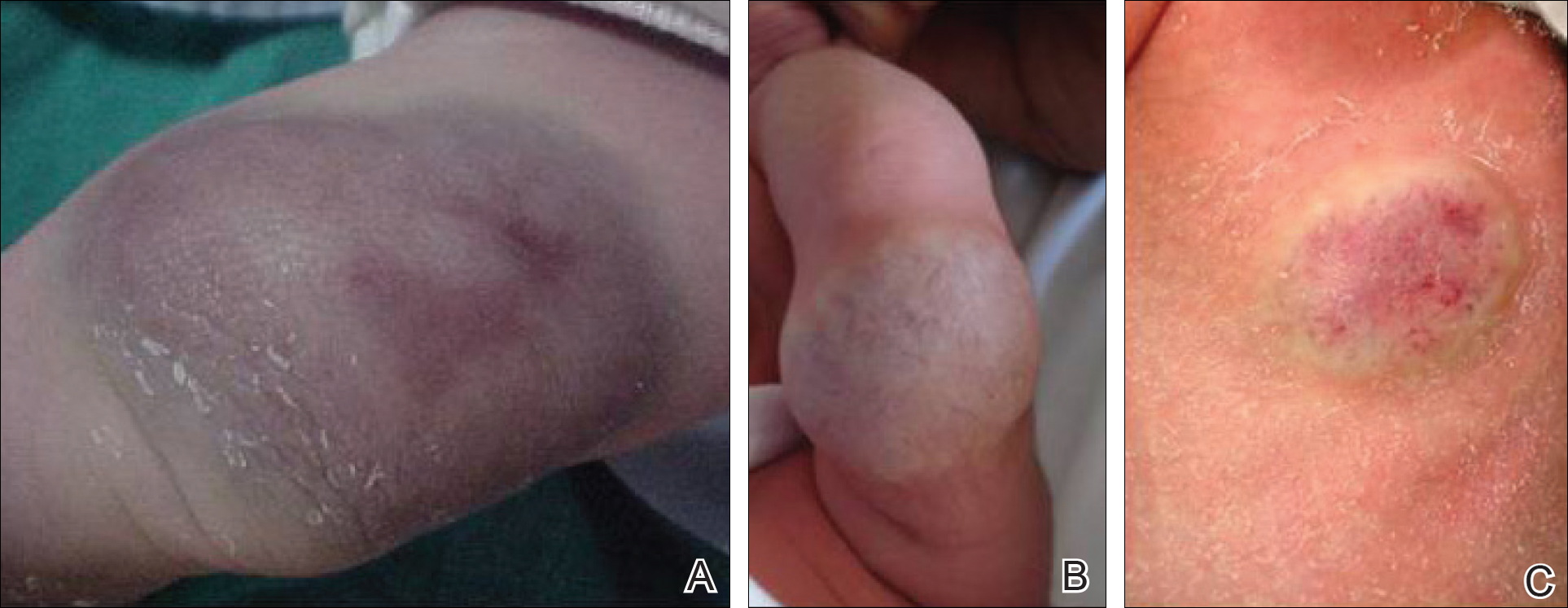
Comment
Since 1996, vascular anomalies have been classified either as tumors or malformations.1 Infantile hemangioma is the most common vascular tumor and presents as an endothelial cellular proliferation that develops within days after birth. Congenital hemangiomas are fully developed at birth2,3 and are classified as RICHs and NICHs according to their clinical outcome.
As expected, our analysis revealed that CH usually is solitary and may present as a small lesion (eg, a few millimeters) but also may be large in size.4 Congenital hemangioma has an equal sex distribution and a predilection for the head and limbs near a joint. In contrast, IH exhibits female predilection and can occur anywhere on the body.4-6 In our study, CHs were more common in males and had a predilection for the limbs. Three patients presented with exophytic masses with a clear halo and overlying telangiectasia, which are commonly described features in CH.4,6
In the classification of vascular anomalies, RICHs and NICHs are fast-flow lesions that are indistinguishable at birth.7,8 Untreated, RICHs usually resolve in the first 14 months of life, often resulting in an area of atrophic or excess skin.8,9 Noninvoluting CHs persist and grow in proportion with the patient.10-12
When Doppler ultrasonography findings are inconsistent with a CH, an early biopsy from the periphery of the lesion may be performed to exclude an uncommon soft-tissue tumor such as infantile myofibromatosis or sarcoma.8,9,12 Because of the presence of a clear halo in all cases and mainly rapid involution of CHs, these differential diagnoses were dismissed. The histologic appearance of RICH differed from NICH and common IH, but some overlap was noted among the 3 lesions. Rapidly involuting CH was composed of small to large lobules of capillaries with moderately plump endothelial cells and pericytes; the lobules were surrounded by abundant fibrous tissue.9
Despite the notable differences in natural history between RICHs and NICHs, as RICHs regress within months while NICHs do not, both classes of CH share an important immunohistochemical phenotype; they do not express glucose transporter 1, the marker of IH.13 Tests for this marker were not performed in our study. The prognosis of CH generally is good, and special management is not required.
Conclusion
Rapidly involuting CHs and NICHs have many similarities, such as appearance, location, and sex distribution. The obvious differences in behavior serve to differentiate RICHs, NICHs, and common IHs. Infantile hemangiomas are not fully developed at birth and need many years to regress.
- Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr. 1996;128:329-335.
- Neri I, Balestri R, Patrizi A. Hemangiomas: new insight and medical treatment. Dermatol Ther. 2012;25:322-334.
- Enjolras O, Mulliken JB. Vascular tumors and vascular malformations (new issues). Adv Dermatol. 1997;13:375-423.
- Mulliken JB, Enjolras O. Congenital hemangiomas and infantile hemangioma: missing links. J Am Acad Dermatol. 2004:50:875-882.
- Frieden IJ, Haggstrom AN, Drolet BA, et al. Infantile hemangiomas: current knowledge, future directions. proceedings of a research workshop on infantile hemangiomas, April 7-9, 2005, Bethesda, Maryland, USA. Pediatr Dermatol. 2005;22:383-406.
- Enjolras O, Picard A, Soupre V. Congenital haemangiomas and other rare infantile vascular tumours [in French]. Ann Chir Plast Esthet. 2006;51:339-346.
- Gorincour G, Kokta V, Rypens F, et al. Imaging characteristics of two subtypes of congenital hemangiomas: rapidly involuting congenital hemangiomas and non-involuting congenital hemangiomas. Pediatr Radiol. 2005;35:1178-1185.
- Rogers M, Lam A, Fischer G. Sonographic findings in a series of rapidly involuting congenital hemangiomas (RICH). Pediatr Dermatol. 2002;19:5-11.
- Berenguer B, Mulliken JB, Enjolras O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003;6:495-510.
- North PE, Waner M, James CA, et al. Congenital nonprogressive hemangioma: a distinct clinicopathologic entity unlike infantile hemangioma. Arch Dermatol. 2001;137:1607-1620.
- Chiavérini C, Kurzenne JY, Rogopoulos A, et al. Noninvoluting congenital hemangioma: 2 cases [in French]. Ann Dermatol Venerol. 2002;129:735-737.
- Enjolras O, Mulliken JB, Boon LM, et al. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg. 2001;107:1647-1654.
- North PE, Waner M, Mizeracki A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11-22.
Hemangiomas are the most common benign tumors of childhood. In recent years, subsets of hemangiomas that are fully formed at birth have been recognized as clinically and biologically distinct from the classic infantile hemangioma (IH). Congenital hemangiomas (CHs) are classified based on clinical course as rapidly involuting CHs (RICHs) or noninvoluting CHs (NICHs). The aim of this retrospective study was to describe the epidemiology, clinical aspects, and clinical outcome of CH over a 5-year period.
Methods
Using electronic medical records from the department of dermatology (Hedi Chaker Hospital, Sfax, Tunisia) for a 5-year period (2008-2012), we searched for hemangioma. After collecting those records, we identified patients with CHs. We studied the epidemiologic (eg, sex, age), clinical course (eg, location, size, number, color, surrounding skin), and evolutionary aspects in these patients.
Results
Twenty IHs were identified, 6 (30%) of which were considered CHs. The clinical characteristics of the 6 patients are summarized in the Table. We identified 2 females and 4 males aged 2 to 60 days (mean age, 16 days). Four patients had CHs involving the limbs (knee [n=2]; ankle [n=1]; elbow [n=1]) and 2 patients had CHs involving the trunk. Congenital hemangiomas were singular, oval shaped, and surrounded by a clear halo in all 6 patients. They presented as exophytic masses (n=3) or bossed plaques (n=3). A blue hue was noted in 5 patients and a purple hue in 1 patient. In some cases, telangiectasia (n=2) or small areas of necrosis (n=1) were noted at the center of the CHs. The CHs ranged in size from 3 to 6 cm (mean, 4 cm). Doppler ultrasonography was performed in 2 patients and showed fast blood flow. It is well known that manipulating a CH when it is ulcerative may cause a fatal hemorrhage. Thus, parents/guardians should be cautious when cleaning and dressing the lesions. Regular follow-up was recommended to all patients as noted in the medical records. The lesion involuted in4 patients after a mean period of 6 months, which allowed us to classify the lesions as RICHs (Figure, A). Two CHs were persistent after 2-year (Figure, B) and 4-year (Figure, C) follow-up, which was consistent with NICH classification.


Comment
Since 1996, vascular anomalies have been classified either as tumors or malformations.1 Infantile hemangioma is the most common vascular tumor and presents as an endothelial cellular proliferation that develops within days after birth. Congenital hemangiomas are fully developed at birth2,3 and are classified as RICHs and NICHs according to their clinical outcome.
As expected, our analysis revealed that CH usually is solitary and may present as a small lesion (eg, a few millimeters) but also may be large in size.4 Congenital hemangioma has an equal sex distribution and a predilection for the head and limbs near a joint. In contrast, IH exhibits female predilection and can occur anywhere on the body.4-6 In our study, CHs were more common in males and had a predilection for the limbs. Three patients presented with exophytic masses with a clear halo and overlying telangiectasia, which are commonly described features in CH.4,6
In the classification of vascular anomalies, RICHs and NICHs are fast-flow lesions that are indistinguishable at birth.7,8 Untreated, RICHs usually resolve in the first 14 months of life, often resulting in an area of atrophic or excess skin.8,9 Noninvoluting CHs persist and grow in proportion with the patient.10-12
When Doppler ultrasonography findings are inconsistent with a CH, an early biopsy from the periphery of the lesion may be performed to exclude an uncommon soft-tissue tumor such as infantile myofibromatosis or sarcoma.8,9,12 Because of the presence of a clear halo in all cases and mainly rapid involution of CHs, these differential diagnoses were dismissed. The histologic appearance of RICH differed from NICH and common IH, but some overlap was noted among the 3 lesions. Rapidly involuting CH was composed of small to large lobules of capillaries with moderately plump endothelial cells and pericytes; the lobules were surrounded by abundant fibrous tissue.9
Despite the notable differences in natural history between RICHs and NICHs, as RICHs regress within months while NICHs do not, both classes of CH share an important immunohistochemical phenotype; they do not express glucose transporter 1, the marker of IH.13 Tests for this marker were not performed in our study. The prognosis of CH generally is good, and special management is not required.
Conclusion
Rapidly involuting CHs and NICHs have many similarities, such as appearance, location, and sex distribution. The obvious differences in behavior serve to differentiate RICHs, NICHs, and common IHs. Infantile hemangiomas are not fully developed at birth and need many years to regress.
Hemangiomas are the most common benign tumors of childhood. In recent years, subsets of hemangiomas that are fully formed at birth have been recognized as clinically and biologically distinct from the classic infantile hemangioma (IH). Congenital hemangiomas (CHs) are classified based on clinical course as rapidly involuting CHs (RICHs) or noninvoluting CHs (NICHs). The aim of this retrospective study was to describe the epidemiology, clinical aspects, and clinical outcome of CH over a 5-year period.
Methods
Using electronic medical records from the department of dermatology (Hedi Chaker Hospital, Sfax, Tunisia) for a 5-year period (2008-2012), we searched for hemangioma. After collecting those records, we identified patients with CHs. We studied the epidemiologic (eg, sex, age), clinical course (eg, location, size, number, color, surrounding skin), and evolutionary aspects in these patients.
Results
Twenty IHs were identified, 6 (30%) of which were considered CHs. The clinical characteristics of the 6 patients are summarized in the Table. We identified 2 females and 4 males aged 2 to 60 days (mean age, 16 days). Four patients had CHs involving the limbs (knee [n=2]; ankle [n=1]; elbow [n=1]) and 2 patients had CHs involving the trunk. Congenital hemangiomas were singular, oval shaped, and surrounded by a clear halo in all 6 patients. They presented as exophytic masses (n=3) or bossed plaques (n=3). A blue hue was noted in 5 patients and a purple hue in 1 patient. In some cases, telangiectasia (n=2) or small areas of necrosis (n=1) were noted at the center of the CHs. The CHs ranged in size from 3 to 6 cm (mean, 4 cm). Doppler ultrasonography was performed in 2 patients and showed fast blood flow. It is well known that manipulating a CH when it is ulcerative may cause a fatal hemorrhage. Thus, parents/guardians should be cautious when cleaning and dressing the lesions. Regular follow-up was recommended to all patients as noted in the medical records. The lesion involuted in4 patients after a mean period of 6 months, which allowed us to classify the lesions as RICHs (Figure, A). Two CHs were persistent after 2-year (Figure, B) and 4-year (Figure, C) follow-up, which was consistent with NICH classification.


Comment
Since 1996, vascular anomalies have been classified either as tumors or malformations.1 Infantile hemangioma is the most common vascular tumor and presents as an endothelial cellular proliferation that develops within days after birth. Congenital hemangiomas are fully developed at birth2,3 and are classified as RICHs and NICHs according to their clinical outcome.
As expected, our analysis revealed that CH usually is solitary and may present as a small lesion (eg, a few millimeters) but also may be large in size.4 Congenital hemangioma has an equal sex distribution and a predilection for the head and limbs near a joint. In contrast, IH exhibits female predilection and can occur anywhere on the body.4-6 In our study, CHs were more common in males and had a predilection for the limbs. Three patients presented with exophytic masses with a clear halo and overlying telangiectasia, which are commonly described features in CH.4,6
In the classification of vascular anomalies, RICHs and NICHs are fast-flow lesions that are indistinguishable at birth.7,8 Untreated, RICHs usually resolve in the first 14 months of life, often resulting in an area of atrophic or excess skin.8,9 Noninvoluting CHs persist and grow in proportion with the patient.10-12
When Doppler ultrasonography findings are inconsistent with a CH, an early biopsy from the periphery of the lesion may be performed to exclude an uncommon soft-tissue tumor such as infantile myofibromatosis or sarcoma.8,9,12 Because of the presence of a clear halo in all cases and mainly rapid involution of CHs, these differential diagnoses were dismissed. The histologic appearance of RICH differed from NICH and common IH, but some overlap was noted among the 3 lesions. Rapidly involuting CH was composed of small to large lobules of capillaries with moderately plump endothelial cells and pericytes; the lobules were surrounded by abundant fibrous tissue.9
Despite the notable differences in natural history between RICHs and NICHs, as RICHs regress within months while NICHs do not, both classes of CH share an important immunohistochemical phenotype; they do not express glucose transporter 1, the marker of IH.13 Tests for this marker were not performed in our study. The prognosis of CH generally is good, and special management is not required.
Conclusion
Rapidly involuting CHs and NICHs have many similarities, such as appearance, location, and sex distribution. The obvious differences in behavior serve to differentiate RICHs, NICHs, and common IHs. Infantile hemangiomas are not fully developed at birth and need many years to regress.
- Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr. 1996;128:329-335.
- Neri I, Balestri R, Patrizi A. Hemangiomas: new insight and medical treatment. Dermatol Ther. 2012;25:322-334.
- Enjolras O, Mulliken JB. Vascular tumors and vascular malformations (new issues). Adv Dermatol. 1997;13:375-423.
- Mulliken JB, Enjolras O. Congenital hemangiomas and infantile hemangioma: missing links. J Am Acad Dermatol. 2004:50:875-882.
- Frieden IJ, Haggstrom AN, Drolet BA, et al. Infantile hemangiomas: current knowledge, future directions. proceedings of a research workshop on infantile hemangiomas, April 7-9, 2005, Bethesda, Maryland, USA. Pediatr Dermatol. 2005;22:383-406.
- Enjolras O, Picard A, Soupre V. Congenital haemangiomas and other rare infantile vascular tumours [in French]. Ann Chir Plast Esthet. 2006;51:339-346.
- Gorincour G, Kokta V, Rypens F, et al. Imaging characteristics of two subtypes of congenital hemangiomas: rapidly involuting congenital hemangiomas and non-involuting congenital hemangiomas. Pediatr Radiol. 2005;35:1178-1185.
- Rogers M, Lam A, Fischer G. Sonographic findings in a series of rapidly involuting congenital hemangiomas (RICH). Pediatr Dermatol. 2002;19:5-11.
- Berenguer B, Mulliken JB, Enjolras O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003;6:495-510.
- North PE, Waner M, James CA, et al. Congenital nonprogressive hemangioma: a distinct clinicopathologic entity unlike infantile hemangioma. Arch Dermatol. 2001;137:1607-1620.
- Chiavérini C, Kurzenne JY, Rogopoulos A, et al. Noninvoluting congenital hemangioma: 2 cases [in French]. Ann Dermatol Venerol. 2002;129:735-737.
- Enjolras O, Mulliken JB, Boon LM, et al. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg. 2001;107:1647-1654.
- North PE, Waner M, Mizeracki A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11-22.
- Boon LM, Enjolras O, Mulliken JB. Congenital hemangioma: evidence of accelerated involution. J Pediatr. 1996;128:329-335.
- Neri I, Balestri R, Patrizi A. Hemangiomas: new insight and medical treatment. Dermatol Ther. 2012;25:322-334.
- Enjolras O, Mulliken JB. Vascular tumors and vascular malformations (new issues). Adv Dermatol. 1997;13:375-423.
- Mulliken JB, Enjolras O. Congenital hemangiomas and infantile hemangioma: missing links. J Am Acad Dermatol. 2004:50:875-882.
- Frieden IJ, Haggstrom AN, Drolet BA, et al. Infantile hemangiomas: current knowledge, future directions. proceedings of a research workshop on infantile hemangiomas, April 7-9, 2005, Bethesda, Maryland, USA. Pediatr Dermatol. 2005;22:383-406.
- Enjolras O, Picard A, Soupre V. Congenital haemangiomas and other rare infantile vascular tumours [in French]. Ann Chir Plast Esthet. 2006;51:339-346.
- Gorincour G, Kokta V, Rypens F, et al. Imaging characteristics of two subtypes of congenital hemangiomas: rapidly involuting congenital hemangiomas and non-involuting congenital hemangiomas. Pediatr Radiol. 2005;35:1178-1185.
- Rogers M, Lam A, Fischer G. Sonographic findings in a series of rapidly involuting congenital hemangiomas (RICH). Pediatr Dermatol. 2002;19:5-11.
- Berenguer B, Mulliken JB, Enjolras O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003;6:495-510.
- North PE, Waner M, James CA, et al. Congenital nonprogressive hemangioma: a distinct clinicopathologic entity unlike infantile hemangioma. Arch Dermatol. 2001;137:1607-1620.
- Chiavérini C, Kurzenne JY, Rogopoulos A, et al. Noninvoluting congenital hemangioma: 2 cases [in French]. Ann Dermatol Venerol. 2002;129:735-737.
- Enjolras O, Mulliken JB, Boon LM, et al. Noninvoluting congenital hemangioma: a rare cutaneous vascular anomaly. Plast Reconstr Surg. 2001;107:1647-1654.
- North PE, Waner M, Mizeracki A, et al. GLUT1: a newly discovered immunohistochemical marker for juvenile hemangiomas. Hum Pathol. 2000;31:11-22.
Practice Points
- Congenital hemangiomas (CHs) are fully developed hemangiomas that are present at birth.
- In our study, CHs were more common in males, with a predilection for the limbs.
- Infantile hemangiomas are not fully developed at birth and need many years to regress.
Burnout: No laughing matter
Much has been written about burnout in U.S. physicians over the course of many years. Burnout is a syndrome that is exemplified by emotional exhaustion, depersonalization, and a low sense of personal accomplishment. It appears that hospitalists are particularly prone to burnout, being at the very front line of patient care. In addition, the prevalence of burnout appears to be getting worse. According to a survey from the American Medical Association, the prevalence of burnout in 2011 was 45%. Three years later in 2014 the prevalence was up to 55%.1,2
Although triggers for the onset and intensity of burnout likely vary by specialty, a recent Medscape Lifestyle Report found the most common causes of burnout among physicians included (see graphic):3
• Bureaucratic tasks.
• Work hours.
• Computerization.
• Compensation.

• Lower work satisfaction.
• Disrupted personal relationships.
• Substance misuse.
• Depression.
• Suicide.
Burnout also leads to lower productivity, higher job turnover, and early retirement. In addition, from a systems perspective, burnout is associated with higher medical errors, reduced quality of patient care, and lower patient satisfaction. And, at its most extreme, burnout is deadly: Sadly, every year, 300-400 physicians in the United States commit suicide. Female physicians are 2.3 times more likely to commit suicide than are female nonphysicians; for males, the risk is 1.4 times higher among physicians compared to the general population.1
Proactive approaches
Despite all these sobering statistics on the prevalence and outcomes of burnout among physicians, the ongoing question is, what can we do about it? Although awareness and recognition of burnout has grown substantially over time, successful interventions to prevent or mitigate burnout have not. Many potential interventions and ideas have surfaced and have been published, but none have had impressive impacts or have been adopted widely within or across institutions. According to a Modern Healthcare survey of approximately 100 health care CEOs, only about one-third reported that their organization had programs to address physician burnout, although about another one-third reported attempts to develop such programs.1
The good news is that at least there is a lot of activity around trying new interventions to reduce burnout, including in medical schools and graduate training programs. The thought is that if you can employ healthy resilience tactics during training, these can be carried throughout a career to diminish the risk and/or severity of burnout, despite any challenges that arise along the way.
Some of these interventions are aimed at individuals (to enhance personal resilience and coping skills) while others are aimed at the level of organizations (to reduce organizational stress and/or workload). A recent Modern Healthcare article found several good examples:1
• New York’s Albert Einstein College of Medicine’s WellMed program has been designed to help students develop healthy and balanced habits and attitudes, and to enhance their personal resilience, for the short and the long term.
• Baystate Health in Massachusetts hosts a physician leadership academy that offers training in communication, unconscious bias, strategy, and other management skills, to enhance individual resilience and organizational engagement.
• HealthPartners, a not-for-profit, Minnesota-based health care organization, has specific programs to engage physicians and allow them to have organizational impact, as well as programs to simplify technology use.
Organization efforts are key to prevent, treat
The key to reducing burnout does seem to be employing a combination of self-directed and organization-directed interventions, each of which enhances resilience and reduces workplace stressors (i.e., administrative tasks and workload). Specific to hospitalists, Leslie Flores, MBA, recently wrote about burnout at The Hospital Leader blog. Her list included several specific examples to reduce the top causes of burnout among busy hospitalists:4
• Modifying the skill mix in hospital medicine groups so that less costly support staff are doing much of the work not requiring a physician’s expertise, freeing up hospitalists to provide better care to more patients.
• Reducing unnecessary interruptions and the stress they cause, via both technology and process improvement.
• Paying deliberate attention to hospitalist personal and professional well-being.
• Adjusting hospitalist schedules and work flow so that hospitalists can be more efficient (that is, do less low-value work and re-work) and have better work-life balance.
• Ensuring that hospitalists have the training, clinical competencies, and support to comfortably perform in expanded clinical roles.
Many of these systemic solutions were recently validated as likely able to have an impact on burnout (and seem to be more effective than interventions focused on individual resilience).5 A recent meta-analysis found that physician-directed interventions were associated with small but significant reductions in burnout; these were primarily mindfulness-based stress reduction techniques, educational interventions targeting physicians self-confidence and communication skills, exercise, or a combination of these features. More impactful were organization-directed interventions, which were associated with more significant reductions in burnout; these were primarily aimed at reducing workload and enhancing teamwork and leadership.
In sum
It is important for all of us hospitalists to understand and try to mitigate burnout within our teams. Although individual-focused interventions can have some effect, most efforts should primarily be focused on system-based interventions, to reduce administrative burdens and workload. Through such system design and redesign, we can likely reduce burnout amongst our teams, and therefore improve the sustainability of our specialty.
References
1.http://www.modernhealthcare.com/article/20161029/MAGAZINE/310299983
2. http://www.mayoclinicproceedings.org/article/S0025-6196(15)00716-8/abstract
3.http://www.medscape.com/features/slideshow/lifestyle/2016/public/overview#page=5
4. http://blogs.hospitalmedicine.org/Blog/making-hospital-medicine-a-sustainable-specialty/
5.http://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2588814
Much has been written about burnout in U.S. physicians over the course of many years. Burnout is a syndrome that is exemplified by emotional exhaustion, depersonalization, and a low sense of personal accomplishment. It appears that hospitalists are particularly prone to burnout, being at the very front line of patient care. In addition, the prevalence of burnout appears to be getting worse. According to a survey from the American Medical Association, the prevalence of burnout in 2011 was 45%. Three years later in 2014 the prevalence was up to 55%.1,2
Although triggers for the onset and intensity of burnout likely vary by specialty, a recent Medscape Lifestyle Report found the most common causes of burnout among physicians included (see graphic):3
• Bureaucratic tasks.
• Work hours.
• Computerization.
• Compensation.

• Lower work satisfaction.
• Disrupted personal relationships.
• Substance misuse.
• Depression.
• Suicide.
Burnout also leads to lower productivity, higher job turnover, and early retirement. In addition, from a systems perspective, burnout is associated with higher medical errors, reduced quality of patient care, and lower patient satisfaction. And, at its most extreme, burnout is deadly: Sadly, every year, 300-400 physicians in the United States commit suicide. Female physicians are 2.3 times more likely to commit suicide than are female nonphysicians; for males, the risk is 1.4 times higher among physicians compared to the general population.1
Proactive approaches
Despite all these sobering statistics on the prevalence and outcomes of burnout among physicians, the ongoing question is, what can we do about it? Although awareness and recognition of burnout has grown substantially over time, successful interventions to prevent or mitigate burnout have not. Many potential interventions and ideas have surfaced and have been published, but none have had impressive impacts or have been adopted widely within or across institutions. According to a Modern Healthcare survey of approximately 100 health care CEOs, only about one-third reported that their organization had programs to address physician burnout, although about another one-third reported attempts to develop such programs.1
The good news is that at least there is a lot of activity around trying new interventions to reduce burnout, including in medical schools and graduate training programs. The thought is that if you can employ healthy resilience tactics during training, these can be carried throughout a career to diminish the risk and/or severity of burnout, despite any challenges that arise along the way.
Some of these interventions are aimed at individuals (to enhance personal resilience and coping skills) while others are aimed at the level of organizations (to reduce organizational stress and/or workload). A recent Modern Healthcare article found several good examples:1
• New York’s Albert Einstein College of Medicine’s WellMed program has been designed to help students develop healthy and balanced habits and attitudes, and to enhance their personal resilience, for the short and the long term.
• Baystate Health in Massachusetts hosts a physician leadership academy that offers training in communication, unconscious bias, strategy, and other management skills, to enhance individual resilience and organizational engagement.
• HealthPartners, a not-for-profit, Minnesota-based health care organization, has specific programs to engage physicians and allow them to have organizational impact, as well as programs to simplify technology use.
Organization efforts are key to prevent, treat
The key to reducing burnout does seem to be employing a combination of self-directed and organization-directed interventions, each of which enhances resilience and reduces workplace stressors (i.e., administrative tasks and workload). Specific to hospitalists, Leslie Flores, MBA, recently wrote about burnout at The Hospital Leader blog. Her list included several specific examples to reduce the top causes of burnout among busy hospitalists:4
• Modifying the skill mix in hospital medicine groups so that less costly support staff are doing much of the work not requiring a physician’s expertise, freeing up hospitalists to provide better care to more patients.
• Reducing unnecessary interruptions and the stress they cause, via both technology and process improvement.
• Paying deliberate attention to hospitalist personal and professional well-being.
• Adjusting hospitalist schedules and work flow so that hospitalists can be more efficient (that is, do less low-value work and re-work) and have better work-life balance.
• Ensuring that hospitalists have the training, clinical competencies, and support to comfortably perform in expanded clinical roles.
Many of these systemic solutions were recently validated as likely able to have an impact on burnout (and seem to be more effective than interventions focused on individual resilience).5 A recent meta-analysis found that physician-directed interventions were associated with small but significant reductions in burnout; these were primarily mindfulness-based stress reduction techniques, educational interventions targeting physicians self-confidence and communication skills, exercise, or a combination of these features. More impactful were organization-directed interventions, which were associated with more significant reductions in burnout; these were primarily aimed at reducing workload and enhancing teamwork and leadership.
In sum
It is important for all of us hospitalists to understand and try to mitigate burnout within our teams. Although individual-focused interventions can have some effect, most efforts should primarily be focused on system-based interventions, to reduce administrative burdens and workload. Through such system design and redesign, we can likely reduce burnout amongst our teams, and therefore improve the sustainability of our specialty.
References
1.http://www.modernhealthcare.com/article/20161029/MAGAZINE/310299983
2. http://www.mayoclinicproceedings.org/article/S0025-6196(15)00716-8/abstract
3.http://www.medscape.com/features/slideshow/lifestyle/2016/public/overview#page=5
4. http://blogs.hospitalmedicine.org/Blog/making-hospital-medicine-a-sustainable-specialty/
5.http://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2588814
Much has been written about burnout in U.S. physicians over the course of many years. Burnout is a syndrome that is exemplified by emotional exhaustion, depersonalization, and a low sense of personal accomplishment. It appears that hospitalists are particularly prone to burnout, being at the very front line of patient care. In addition, the prevalence of burnout appears to be getting worse. According to a survey from the American Medical Association, the prevalence of burnout in 2011 was 45%. Three years later in 2014 the prevalence was up to 55%.1,2
Although triggers for the onset and intensity of burnout likely vary by specialty, a recent Medscape Lifestyle Report found the most common causes of burnout among physicians included (see graphic):3
• Bureaucratic tasks.
• Work hours.
• Computerization.
• Compensation.

• Lower work satisfaction.
• Disrupted personal relationships.
• Substance misuse.
• Depression.
• Suicide.
Burnout also leads to lower productivity, higher job turnover, and early retirement. In addition, from a systems perspective, burnout is associated with higher medical errors, reduced quality of patient care, and lower patient satisfaction. And, at its most extreme, burnout is deadly: Sadly, every year, 300-400 physicians in the United States commit suicide. Female physicians are 2.3 times more likely to commit suicide than are female nonphysicians; for males, the risk is 1.4 times higher among physicians compared to the general population.1
Proactive approaches
Despite all these sobering statistics on the prevalence and outcomes of burnout among physicians, the ongoing question is, what can we do about it? Although awareness and recognition of burnout has grown substantially over time, successful interventions to prevent or mitigate burnout have not. Many potential interventions and ideas have surfaced and have been published, but none have had impressive impacts or have been adopted widely within or across institutions. According to a Modern Healthcare survey of approximately 100 health care CEOs, only about one-third reported that their organization had programs to address physician burnout, although about another one-third reported attempts to develop such programs.1
The good news is that at least there is a lot of activity around trying new interventions to reduce burnout, including in medical schools and graduate training programs. The thought is that if you can employ healthy resilience tactics during training, these can be carried throughout a career to diminish the risk and/or severity of burnout, despite any challenges that arise along the way.
Some of these interventions are aimed at individuals (to enhance personal resilience and coping skills) while others are aimed at the level of organizations (to reduce organizational stress and/or workload). A recent Modern Healthcare article found several good examples:1
• New York’s Albert Einstein College of Medicine’s WellMed program has been designed to help students develop healthy and balanced habits and attitudes, and to enhance their personal resilience, for the short and the long term.
• Baystate Health in Massachusetts hosts a physician leadership academy that offers training in communication, unconscious bias, strategy, and other management skills, to enhance individual resilience and organizational engagement.
• HealthPartners, a not-for-profit, Minnesota-based health care organization, has specific programs to engage physicians and allow them to have organizational impact, as well as programs to simplify technology use.
Organization efforts are key to prevent, treat
The key to reducing burnout does seem to be employing a combination of self-directed and organization-directed interventions, each of which enhances resilience and reduces workplace stressors (i.e., administrative tasks and workload). Specific to hospitalists, Leslie Flores, MBA, recently wrote about burnout at The Hospital Leader blog. Her list included several specific examples to reduce the top causes of burnout among busy hospitalists:4
• Modifying the skill mix in hospital medicine groups so that less costly support staff are doing much of the work not requiring a physician’s expertise, freeing up hospitalists to provide better care to more patients.
• Reducing unnecessary interruptions and the stress they cause, via both technology and process improvement.
• Paying deliberate attention to hospitalist personal and professional well-being.
• Adjusting hospitalist schedules and work flow so that hospitalists can be more efficient (that is, do less low-value work and re-work) and have better work-life balance.
• Ensuring that hospitalists have the training, clinical competencies, and support to comfortably perform in expanded clinical roles.
Many of these systemic solutions were recently validated as likely able to have an impact on burnout (and seem to be more effective than interventions focused on individual resilience).5 A recent meta-analysis found that physician-directed interventions were associated with small but significant reductions in burnout; these were primarily mindfulness-based stress reduction techniques, educational interventions targeting physicians self-confidence and communication skills, exercise, or a combination of these features. More impactful were organization-directed interventions, which were associated with more significant reductions in burnout; these were primarily aimed at reducing workload and enhancing teamwork and leadership.
In sum
It is important for all of us hospitalists to understand and try to mitigate burnout within our teams. Although individual-focused interventions can have some effect, most efforts should primarily be focused on system-based interventions, to reduce administrative burdens and workload. Through such system design and redesign, we can likely reduce burnout amongst our teams, and therefore improve the sustainability of our specialty.
References
1.http://www.modernhealthcare.com/article/20161029/MAGAZINE/310299983
2. http://www.mayoclinicproceedings.org/article/S0025-6196(15)00716-8/abstract
3.http://www.medscape.com/features/slideshow/lifestyle/2016/public/overview#page=5
4. http://blogs.hospitalmedicine.org/Blog/making-hospital-medicine-a-sustainable-specialty/
5.http://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2588814
AATS Week 2017 Registration Now Open
AATS Week 2017 will bring together the world’s foremost CT surgery scientists and medical professionals for seven days at three important events:
Mitral Conclave
April 27 – 28, 2017
New York, NY
AATS Innovation Summit
April 29, 2017
Boston, MA
The AATS Centennial
April 29 – May 3
Boston, MA
Registration is now open.
Choose from one of several registration packages:
AATS Week 2017 Package
Register and receive a $100 discount*
*Discount does not apply to Residents/Fellows or Medical Students. The discount is applied to the AATS Mitral Conclave registration fee.
Genius Pass
The Genius Pass provides access to almost every offering at the AATS Centennial. The package includes:
** Access to Saturday Skills Courses and Sunday Symposia
** Access to all simulation sessions (Monday – Wednesday)
** Exhibit Hall admission
** Lunch in the Exhibit Hall (Monday & Tuesday)
** Welcome Reception
Innovation Pass
Ignite your spirit for innovation with Course Director W. Randolph Chitwood Jr. at this one-day program assisting cardiothoracic surgeons to develop new clinically applicable technology by obtaining the cross-specialty knowledge needed for novel idea generation, protecting intellectual property, developmental funding, clinical trials, regulatory pathways and industry relations.
Innovation Pass Registration includes:
** Registration for the Saturday AATS Innovation Summit
** Access to all Sunday Symposia
** Access to all simulations sessions (Monday- Wednesday)
** Exhibit Hall admission
** Lunch in the Exhibit Hall (Monday and Tuesday)
** Welcome Reception
Note: You can only attend the AATS Innovation Summit or the Saturday Skills Courses. You cannot choose to attend both events on Saturday.
Resident/Fellows and Medical Students: Can only attend the AATS Centennial by choosing the AATS Genius or Innovation Pass.
Separate registration is required for: Saturday hands-on sessions, Member for a Day session, Survival Guide for the Cardiothoracic Surgical Team, MOC Breakfast, Cardiothoracic Residents & Ethics Forum Luncheons, and all social events and tours.
Conference Flex Pass
This pass is best suited for those who want to attend some, but not all, offerings.
Customize your AATS Centennial participation by adding individual educational events to your cart — any combination of the Saturday Courses, Sunday Symposia, and Monday-Wednesday simultaneous sessions.
Saturday Courses and Sunday Symposium Registration
Register for a Saturday course and/or a Sunday symposium and receive access to all other courses/symposia taking place that day.
Note: Registration for the Saturday courses and/or Sunday symposium is separate from the Annual Meeting registration rate.
Registration & Housing information
AATS Week 2017 will bring together the world’s foremost CT surgery scientists and medical professionals for seven days at three important events:
Mitral Conclave
April 27 – 28, 2017
New York, NY
AATS Innovation Summit
April 29, 2017
Boston, MA
The AATS Centennial
April 29 – May 3
Boston, MA
Registration is now open.
Choose from one of several registration packages:
AATS Week 2017 Package
Register and receive a $100 discount*
*Discount does not apply to Residents/Fellows or Medical Students. The discount is applied to the AATS Mitral Conclave registration fee.
Genius Pass
The Genius Pass provides access to almost every offering at the AATS Centennial. The package includes:
** Access to Saturday Skills Courses and Sunday Symposia
** Access to all simulation sessions (Monday – Wednesday)
** Exhibit Hall admission
** Lunch in the Exhibit Hall (Monday & Tuesday)
** Welcome Reception
Innovation Pass
Ignite your spirit for innovation with Course Director W. Randolph Chitwood Jr. at this one-day program assisting cardiothoracic surgeons to develop new clinically applicable technology by obtaining the cross-specialty knowledge needed for novel idea generation, protecting intellectual property, developmental funding, clinical trials, regulatory pathways and industry relations.
Innovation Pass Registration includes:
** Registration for the Saturday AATS Innovation Summit
** Access to all Sunday Symposia
** Access to all simulations sessions (Monday- Wednesday)
** Exhibit Hall admission
** Lunch in the Exhibit Hall (Monday and Tuesday)
** Welcome Reception
Note: You can only attend the AATS Innovation Summit or the Saturday Skills Courses. You cannot choose to attend both events on Saturday.
Resident/Fellows and Medical Students: Can only attend the AATS Centennial by choosing the AATS Genius or Innovation Pass.
Separate registration is required for: Saturday hands-on sessions, Member for a Day session, Survival Guide for the Cardiothoracic Surgical Team, MOC Breakfast, Cardiothoracic Residents & Ethics Forum Luncheons, and all social events and tours.
Conference Flex Pass
This pass is best suited for those who want to attend some, but not all, offerings.
Customize your AATS Centennial participation by adding individual educational events to your cart — any combination of the Saturday Courses, Sunday Symposia, and Monday-Wednesday simultaneous sessions.
Saturday Courses and Sunday Symposium Registration
Register for a Saturday course and/or a Sunday symposium and receive access to all other courses/symposia taking place that day.
Note: Registration for the Saturday courses and/or Sunday symposium is separate from the Annual Meeting registration rate.
Registration & Housing information
AATS Week 2017 will bring together the world’s foremost CT surgery scientists and medical professionals for seven days at three important events:
Mitral Conclave
April 27 – 28, 2017
New York, NY
AATS Innovation Summit
April 29, 2017
Boston, MA
The AATS Centennial
April 29 – May 3
Boston, MA
Registration is now open.
Choose from one of several registration packages:
AATS Week 2017 Package
Register and receive a $100 discount*
*Discount does not apply to Residents/Fellows or Medical Students. The discount is applied to the AATS Mitral Conclave registration fee.
Genius Pass
The Genius Pass provides access to almost every offering at the AATS Centennial. The package includes:
** Access to Saturday Skills Courses and Sunday Symposia
** Access to all simulation sessions (Monday – Wednesday)
** Exhibit Hall admission
** Lunch in the Exhibit Hall (Monday & Tuesday)
** Welcome Reception
Innovation Pass
Ignite your spirit for innovation with Course Director W. Randolph Chitwood Jr. at this one-day program assisting cardiothoracic surgeons to develop new clinically applicable technology by obtaining the cross-specialty knowledge needed for novel idea generation, protecting intellectual property, developmental funding, clinical trials, regulatory pathways and industry relations.
Innovation Pass Registration includes:
** Registration for the Saturday AATS Innovation Summit
** Access to all Sunday Symposia
** Access to all simulations sessions (Monday- Wednesday)
** Exhibit Hall admission
** Lunch in the Exhibit Hall (Monday and Tuesday)
** Welcome Reception
Note: You can only attend the AATS Innovation Summit or the Saturday Skills Courses. You cannot choose to attend both events on Saturday.
Resident/Fellows and Medical Students: Can only attend the AATS Centennial by choosing the AATS Genius or Innovation Pass.
Separate registration is required for: Saturday hands-on sessions, Member for a Day session, Survival Guide for the Cardiothoracic Surgical Team, MOC Breakfast, Cardiothoracic Residents & Ethics Forum Luncheons, and all social events and tours.
Conference Flex Pass
This pass is best suited for those who want to attend some, but not all, offerings.
Customize your AATS Centennial participation by adding individual educational events to your cart — any combination of the Saturday Courses, Sunday Symposia, and Monday-Wednesday simultaneous sessions.
Saturday Courses and Sunday Symposium Registration
Register for a Saturday course and/or a Sunday symposium and receive access to all other courses/symposia taking place that day.
Note: Registration for the Saturday courses and/or Sunday symposium is separate from the Annual Meeting registration rate.
Registration & Housing information
Don’t Miss the Celebration: AATS Centennial
Help us celebrate the AATS Centennial. Experience activities, events, historical artifacts and memorabilia commemorating the 100th anniversary of the American Association for Thoracic Surgery.
April 29 – May 3, 2017
Boston Hynes Convention Center
Boston, MA
A unique aspect of this year’s meeting is our collaboration with the American Society of Extracorporeal Technology (AmSECT). During the didactic portion of the program, the two organizations will be conducting joint panel sessions of interest to all members of the team.
AATS President & Annual Meeting Chair
Thoralf M. Sundt, III
AATS Annual Meeting Co-Chairs
Robert D. Jaquiss & Bryan F. Meyers
AmSECT President
Kenneth Shann
AmSECT International Conference Co-Chairs
Emily Saulitis & Larissa M.V. Teresi
Help us celebrate the AATS Centennial. Experience activities, events, historical artifacts and memorabilia commemorating the 100th anniversary of the American Association for Thoracic Surgery.
April 29 – May 3, 2017
Boston Hynes Convention Center
Boston, MA
A unique aspect of this year’s meeting is our collaboration with the American Society of Extracorporeal Technology (AmSECT). During the didactic portion of the program, the two organizations will be conducting joint panel sessions of interest to all members of the team.
AATS President & Annual Meeting Chair
Thoralf M. Sundt, III
AATS Annual Meeting Co-Chairs
Robert D. Jaquiss & Bryan F. Meyers
AmSECT President
Kenneth Shann
AmSECT International Conference Co-Chairs
Emily Saulitis & Larissa M.V. Teresi
Help us celebrate the AATS Centennial. Experience activities, events, historical artifacts and memorabilia commemorating the 100th anniversary of the American Association for Thoracic Surgery.
April 29 – May 3, 2017
Boston Hynes Convention Center
Boston, MA
A unique aspect of this year’s meeting is our collaboration with the American Society of Extracorporeal Technology (AmSECT). During the didactic portion of the program, the two organizations will be conducting joint panel sessions of interest to all members of the team.
AATS President & Annual Meeting Chair
Thoralf M. Sundt, III
AATS Annual Meeting Co-Chairs
Robert D. Jaquiss & Bryan F. Meyers
AmSECT President
Kenneth Shann
AmSECT International Conference Co-Chairs
Emily Saulitis & Larissa M.V. Teresi
Hydroxychloroquine dosing guidelines have little impact in clinical practice
A substantial number of rheumatology patients are receiving excess starting and maintenance doses of hydroxychloroquine despite the existence of dosing guidelines, a group of ophthalmologists has reported.
The report raises some concerns because hydroxychloroquine has well-established ocular side effects and toxicities that makes screening by a ophthalmologist necessary for all patients on long-term therapy.
Rebekah A. Braslow, MD, and her colleagues in the division of ophthalmology at NorthShore University HealthSystem in Illinois conducted a retrospective review of the records of 554 rheumatology patients who were prescribed hydroxychloroquine during 2009-2016 and seen by a NorthShore ophthalmologist. They found that about half of the patients were on excess initial doses of the disease-modifying antirheumatic drug, according to retinal toxicity guidelines published by the American Academy of Ophthalmology (AAO) in 2011. There was no difference in the percentage of patients on an excess starting dose before the 2011 guidelines were published (54% of 92 patients) or after (49% of 462). The maintenance dose 1 year after beginning treatment was the same as the starting dose in 84% of patients, with reductions in 7% and increases in 9% (Ophthalmology. 2017 Jan 30. doi: 10.1016/j.ophtha.2016.12.021).
The AAO revised the guidelines in 2016 to define excess doses of hydroxychloroquine as greater than 5.0 mg/kg of actual body weight (Ophthalmology. 2016;123:1386-94). Based on these new guidelines, the investigators found that 56% of 527 patients who were currently receiving hydroxychloroquine were taking excess doses, and 43% of the 527 patients were still receiving doses that are more than 50 mg in excess of the calculated target dose that is recommended by the guidelines.
“The published ophthalmology screening guidelines have had no appreciable impact on clinical practice, highlighting a persistent deficiency in patient care and a significant medico-legal risk,” they concluded.
“Our findings are particularly concerning given that choosing a proper starting dose is the single safest, simplest, and most cost-effective measure available,” they wrote.
According to the authors, there were several reasons for the widespread inaccurate dosing. Firstly, the guidelines were published in ophthalmology literature and were therefore unlikely to be seen by rheumatologists. Secondly, the formulation of the drug, currently available only in 200-mg tablet form, made it difficult to adjust doses according to a patient’s weight.
This particular issue could be addressed if the drug manufacturers expanded formulation options, or by alternating daily dosing regimens or involving compound pharmacies, they suggested.
System-wide education and prompts via electronic medical records would also go some way toward ensuring that patients are prescribed the correct dose, they added.
The study was supported by intramural research funds within the division of ophthalmology in NorthShore University Health System.
A substantial number of rheumatology patients are receiving excess starting and maintenance doses of hydroxychloroquine despite the existence of dosing guidelines, a group of ophthalmologists has reported.
The report raises some concerns because hydroxychloroquine has well-established ocular side effects and toxicities that makes screening by a ophthalmologist necessary for all patients on long-term therapy.
Rebekah A. Braslow, MD, and her colleagues in the division of ophthalmology at NorthShore University HealthSystem in Illinois conducted a retrospective review of the records of 554 rheumatology patients who were prescribed hydroxychloroquine during 2009-2016 and seen by a NorthShore ophthalmologist. They found that about half of the patients were on excess initial doses of the disease-modifying antirheumatic drug, according to retinal toxicity guidelines published by the American Academy of Ophthalmology (AAO) in 2011. There was no difference in the percentage of patients on an excess starting dose before the 2011 guidelines were published (54% of 92 patients) or after (49% of 462). The maintenance dose 1 year after beginning treatment was the same as the starting dose in 84% of patients, with reductions in 7% and increases in 9% (Ophthalmology. 2017 Jan 30. doi: 10.1016/j.ophtha.2016.12.021).
The AAO revised the guidelines in 2016 to define excess doses of hydroxychloroquine as greater than 5.0 mg/kg of actual body weight (Ophthalmology. 2016;123:1386-94). Based on these new guidelines, the investigators found that 56% of 527 patients who were currently receiving hydroxychloroquine were taking excess doses, and 43% of the 527 patients were still receiving doses that are more than 50 mg in excess of the calculated target dose that is recommended by the guidelines.
“The published ophthalmology screening guidelines have had no appreciable impact on clinical practice, highlighting a persistent deficiency in patient care and a significant medico-legal risk,” they concluded.
“Our findings are particularly concerning given that choosing a proper starting dose is the single safest, simplest, and most cost-effective measure available,” they wrote.
According to the authors, there were several reasons for the widespread inaccurate dosing. Firstly, the guidelines were published in ophthalmology literature and were therefore unlikely to be seen by rheumatologists. Secondly, the formulation of the drug, currently available only in 200-mg tablet form, made it difficult to adjust doses according to a patient’s weight.
This particular issue could be addressed if the drug manufacturers expanded formulation options, or by alternating daily dosing regimens or involving compound pharmacies, they suggested.
System-wide education and prompts via electronic medical records would also go some way toward ensuring that patients are prescribed the correct dose, they added.
The study was supported by intramural research funds within the division of ophthalmology in NorthShore University Health System.
A substantial number of rheumatology patients are receiving excess starting and maintenance doses of hydroxychloroquine despite the existence of dosing guidelines, a group of ophthalmologists has reported.
The report raises some concerns because hydroxychloroquine has well-established ocular side effects and toxicities that makes screening by a ophthalmologist necessary for all patients on long-term therapy.
Rebekah A. Braslow, MD, and her colleagues in the division of ophthalmology at NorthShore University HealthSystem in Illinois conducted a retrospective review of the records of 554 rheumatology patients who were prescribed hydroxychloroquine during 2009-2016 and seen by a NorthShore ophthalmologist. They found that about half of the patients were on excess initial doses of the disease-modifying antirheumatic drug, according to retinal toxicity guidelines published by the American Academy of Ophthalmology (AAO) in 2011. There was no difference in the percentage of patients on an excess starting dose before the 2011 guidelines were published (54% of 92 patients) or after (49% of 462). The maintenance dose 1 year after beginning treatment was the same as the starting dose in 84% of patients, with reductions in 7% and increases in 9% (Ophthalmology. 2017 Jan 30. doi: 10.1016/j.ophtha.2016.12.021).
The AAO revised the guidelines in 2016 to define excess doses of hydroxychloroquine as greater than 5.0 mg/kg of actual body weight (Ophthalmology. 2016;123:1386-94). Based on these new guidelines, the investigators found that 56% of 527 patients who were currently receiving hydroxychloroquine were taking excess doses, and 43% of the 527 patients were still receiving doses that are more than 50 mg in excess of the calculated target dose that is recommended by the guidelines.
“The published ophthalmology screening guidelines have had no appreciable impact on clinical practice, highlighting a persistent deficiency in patient care and a significant medico-legal risk,” they concluded.
“Our findings are particularly concerning given that choosing a proper starting dose is the single safest, simplest, and most cost-effective measure available,” they wrote.
According to the authors, there were several reasons for the widespread inaccurate dosing. Firstly, the guidelines were published in ophthalmology literature and were therefore unlikely to be seen by rheumatologists. Secondly, the formulation of the drug, currently available only in 200-mg tablet form, made it difficult to adjust doses according to a patient’s weight.
This particular issue could be addressed if the drug manufacturers expanded formulation options, or by alternating daily dosing regimens or involving compound pharmacies, they suggested.
System-wide education and prompts via electronic medical records would also go some way toward ensuring that patients are prescribed the correct dose, they added.
The study was supported by intramural research funds within the division of ophthalmology in NorthShore University Health System.
FROM OPHTHALMOLOGY
Key clinical point:
Major finding: Nearly half of the patients seen by rheumatologist in a health system were prescribed excess initial doses of hydroxychloroquine and just over half of the patients (56%) were taking excess maintenance doses, according to 2016 dosing guidelines.
Data source: A retrospective review of the electronic medical records of 554 rheumatology patients.
Disclosures: The study was supported by intramural research funds within the division of ophthalmology in NorthShore University HealthSystem.