User login
FDA opens abbreviated approval pathway for interchangeable biosimilars
The Food and Drug Administration has proposed a regulatory path for biosimilar biologics that are interchangeable with the reference product, paving the way for a new generation of less-expensive versions of these unique treatments.
But bringing an interchangeable biosimilar to market won’t be easy. The bar for interchangeability will be high, requiring that manufacturers prove switching between the new and older products is safe. And clinicians, while cautiously optimistic, aren’t thrilled with the industry payoff that could come with the designation: freedom for insurance companies and pharmacies to switch products at the dispensing level without requiring a new prescription.
The draft FDA guidance for industry, “Considerations in Demonstrating Interchangeability With a Reference Product,” arises from the Biologics Price Competition and Innovation Act of 2009. That section of the Affordable Care Act provides for abbreviated approval pathways for biological products that are demonstrated to be “highly similar” (biosimilar) to or “interchangeable” with an FDA-approved biological product.
The difference between these appellations is subtle but critical to the regulatory process – and perhaps to patient safety. Regulators recognize that the structure of these large, highly complex molecules can never precisely replicate the reference product. But to be labeled a “biosimilar,” developers must prove that the new product functions essentially the same; there can be no clinically meaningful differences in terms of safety, purity, and potency. Unlike a generic medication, a biosimilar can’t be substituted for its reference product at the pharmacy level. If a physician wants the patient on that biosimilar, the script must specify it.
Interchangeables jump a higher regulatory bar
An “interchangeable biosimilar,” though, would have to jump a higher regulatory bar. Not only must it produce the same clinical result as the reference product, it also must be benignly interchangeable with it, conferring no additional risk if a patient switches from the reference to the biosimilar and back again. A pharmacist could, if permitted by state law, substitute an interchangeable product for the reference product without going through the prescriber.
Like biosimilars, interchangeable products need not be tested in every disease for which the reference product is approved, according to the document. Once they are proved safe for one indication, those data can be extrapolated to allow approval for the other indications as well. Nor do biosimilars need to prove efficacy per se, as their molecular similarity to the reference product ensures that they bind to the same receptor and exert the same therapeutic effect.
The biosimilar/interchangeable market has been slow to take off in the United States. There are no approved interchangeable biosimilars, and only four biosimilars – three of which were approved in 2016:
• Sandoz’ filgrastim-sndz (Zarxio).
• Pfizer’s and Celltrion’s infliximab-dyyb (Inflectra).
• Sandoz’ etanercept-szzs (Erelzi).
• Amgen’s adalimumab-atto (Amjevita).
Switching studies is the key to achieving the interchangeable designation, according to the FDA document. They must include at least two full switches between the candidate product and the reference product, which must be licensed in the United States.
But because these products are so structurally diverse, the FDA isn’t imposing a one-size-fits-all process on them. Instead, the molecular complexity and immunogenicity of each product will dictate its approval requirements.
Those with relatively low structural complexity, high molecular similarity to the reference product, and a low incidence of immunogenic adverse events may only need a single switching study to achieve the “interchangeability” designation.
The bar will be higher for a product with high structural complexity that is not as similar to the reference product, or which has been associated with immunogenic adverse events. For this product, FDA might also require extensive safety postmarketing data for the product as a licensed biosimilar, as well as a switching study.
Pharmacokinetics, pharmacodynamics, immunogenicity, and safety will be the primary endpoints of a switching study. Efficacy data are not necessary but can be used as supportive endpoints. Any safety signals in a switching study would raise regulatory eyebrows whether they came from the candidate product or the reference product. Since the study replicates what could happen if the two were used sequentially, it makes little difference from which product the event might arise.
“If an apparent difference in immune response or adverse events is noticed between the switching and nonswitching arms of the study ... it would raise concerns as to whether the proposed interchangeable product is interchangeable, regardless of whether the proposed interchangeable product or the reference product or the switching of the two products actually caused the event,” the document notes.
The E.U. vs. U.S. experience
The United States is only now getting a taste of what has become common fare in the European Union, said Angus Worthing, MD, chair of the American College of Rheumatology’s Government Affairs Committee. The European Medicines Agency approved its first biosimilar in 2006. Since then, 23 such biosimilars have come on the market, at an average price of about 30% less than the reference product. Prices have dropped as much as 70% in countries in which national health care systems abandoned the reference product in favor of the competing biosimilar, Dr. Worthing said in an interview.
“But the U.S. doesn’t have a national health care system, so it won’t work like that here.” In fact, he noted, brand-new data show that Medicare actually paid 22% more for the infliximab biosimilar Inflectra than it did for Remicade in the last quarter of 2016.
It’s not immediately apparent why this is the case, but it’s probably related to company discounts and rebates on these very expensive treatments. According to the report in Inside Health Policy, Janssen Biotech may have increased its discount to compete with Inflectra’s launch price of 15% below Remicade’s wholesale cost. Prices won’t moderate as much in the United States as in the European Union until several biosimilars of the same class appear, Dr. Worthing said.
There have already been allegations that big pharma manipulates international and national pricing to reduce biosimilar competition.
In June, Russian biotech company Biocad filed a lawsuit in New York charging Roche/Genentech with price fixing. The suit alleges that the companies cut the cost of three cancer treatments (Avastin, Herceptin, and Rituxan/MabThera) in Russia, where Biocad markets biosimilars for each. At the same time, Biocad alleges, the companies raised U.S. prices on those products to make up for the money they were losing on the Russian market.
“I think most of the cost benefits will accrue to insurance plans and pharmacy managers, but maybe not to the patients themselves,” he said in an interview. “The most important beneficiaries may not see a single penny of benefit.”
It may be difficult to extrapolate the European economic experience into the U.S. health care market, but the safety record of its biosimilar armamentarium is solid. None of the biosimilars approved in the E.U. have ever been recalled or removed from the European market because of regulatory or safety concerns.
Nonmedical switching raises concerns
Academic medical societies and clinicians interviewed for this article view the proposed approval pathway with cautious optimism. While acknowledging the potential benefit of reducing the costs of prohibitively expensive treatments, they uniformly insist that patient safety – not economic pressure – should be the driving force here.
“I was initially skeptical, and I do believe that we need very close pharmacovigilance in monitoring these for safety,” said Gideon Smith, MD, PhD, a dermatologist at Massachusetts General Hospital, Boston. “But there has been huge uptake of these products in the E.U., and the data are so extensive that we can be reasonably confident these drugs are effective, and no good reason to believe the safety will be any different.”
He is not as comfortable with the prospect of pharmacy-level substitution of an interchangeable biosimilar with the reference product – a feeling that other clinicians echoed.
“I think this is a fundamental issue that should have been dealt with on a federal level. Physicians should always be involved in the decision,” said Dr. Smith, who spoke at an FDA advisory committee meeting last summer on behalf of the American Academy of Dermatology (AAD).
“In general, the GI field is OK with the idea of starting someone on a new prescription [of an interchangeable biosimilar], but not so much with the idea of switching around,” said Dr. Hanauer, who is the Clifford Joseph Barborka Professor of Gastroenterology at Northwestern University, Chicago. “In these biologic compounds, very small differences can be amplified” and alter therapeutic response.
The possibility of switching from the reference to the biosimilar and maybe back again worries him. He hearkened back to the approval of Remicade, when patients who had taken it during clinical trials only were finally able to obtain it on the market. Dr. Hanauer explained that, “20% of them developed serum sickness reactions after the reexposure.”
He also expressed some concern about quality control in international manufacturing plants, citing a 2005 epidemic of immune-mediated pure red cell anemia in patients who received an epoetin alfa biosimilar manufactured in Thailand. The prefilled syringes had an uncoated rubber stopper that apparently reacted with polysorbate 60 in the solution – an interaction that increased immunogenicity when the product was administered subcutaneously.
Dr. Smith concurred. “We know that some patients produce antibodies to biologics if they come on and off, and so we discourage that. The concern is that switching may lead to an increased rate of medication failure, if you have to switch back. This is especially troubling in the case of a hard-to-control patient with severe flares. If they’re being well controlled on a medication, the last thing you want to do is change it for no good clinical reason. And we may well be forced to do that.”
Neither the AAD nor the American Gastroenterological Association has a published stand on the FDA’s proposed guidance for interchangeable biosimilars. The preliminary view of the American College of Rheumatology is a positive one, Dr. Worthing said. However, ACR feels pharmacy-level switching should be a joint, not unilateral, decision.
“Our position statement on biosimilars has been that if it’s legal for a pharmacy to make that switch then we want the doctor and the patient to know, so we can track for safety signals.”
Bringing any biosimilar to market, though, takes a lot of money and a lot of time. And while companies are growing cell lines and producing new molecules that mimic existing treatments, science marches on, said Dr. Smith.
“If we keep dragging our feet on this issue, it might end up being a moot point,” he said. Newer products are achieving better results, raising the bar for therapeutic success. An example is the monoclonal antibody secukinumab (Cosentyx), an inhibitor of interleukin 17A. In October 2016, late-breaking data released at the annual meeting of the European Academy of Dermatology and Venereology impressed the dermatology community. In psoriasis patients, the treatment maintained 90% skin clearance for 4 years in 66% of patients, and 100% clearance for 4 years in 43%.
Not only does this kind of efficacy provide symptomatic relief, it also prevents the expensive long-term morbidity associated with psoriasis, Dr. Smith said.
“Even if these new medications are considerably more expensive upfront than a biosimilar for an older product, they may end up being less expensive in the long run.”
Dr. Krant and Dr. Worthing had no financial disclosures. Dr. Smith has received grants from Allergan and Cipher Pharmaceuticals. Dr. Hanauer has received grants from numerous pharmaceutical companies that manufacture biologics.
[email protected]
On Twitter @alz_gal
The Food and Drug Administration has proposed a regulatory path for biosimilar biologics that are interchangeable with the reference product, paving the way for a new generation of less-expensive versions of these unique treatments.
But bringing an interchangeable biosimilar to market won’t be easy. The bar for interchangeability will be high, requiring that manufacturers prove switching between the new and older products is safe. And clinicians, while cautiously optimistic, aren’t thrilled with the industry payoff that could come with the designation: freedom for insurance companies and pharmacies to switch products at the dispensing level without requiring a new prescription.
The draft FDA guidance for industry, “Considerations in Demonstrating Interchangeability With a Reference Product,” arises from the Biologics Price Competition and Innovation Act of 2009. That section of the Affordable Care Act provides for abbreviated approval pathways for biological products that are demonstrated to be “highly similar” (biosimilar) to or “interchangeable” with an FDA-approved biological product.
The difference between these appellations is subtle but critical to the regulatory process – and perhaps to patient safety. Regulators recognize that the structure of these large, highly complex molecules can never precisely replicate the reference product. But to be labeled a “biosimilar,” developers must prove that the new product functions essentially the same; there can be no clinically meaningful differences in terms of safety, purity, and potency. Unlike a generic medication, a biosimilar can’t be substituted for its reference product at the pharmacy level. If a physician wants the patient on that biosimilar, the script must specify it.
Interchangeables jump a higher regulatory bar
An “interchangeable biosimilar,” though, would have to jump a higher regulatory bar. Not only must it produce the same clinical result as the reference product, it also must be benignly interchangeable with it, conferring no additional risk if a patient switches from the reference to the biosimilar and back again. A pharmacist could, if permitted by state law, substitute an interchangeable product for the reference product without going through the prescriber.
Like biosimilars, interchangeable products need not be tested in every disease for which the reference product is approved, according to the document. Once they are proved safe for one indication, those data can be extrapolated to allow approval for the other indications as well. Nor do biosimilars need to prove efficacy per se, as their molecular similarity to the reference product ensures that they bind to the same receptor and exert the same therapeutic effect.
The biosimilar/interchangeable market has been slow to take off in the United States. There are no approved interchangeable biosimilars, and only four biosimilars – three of which were approved in 2016:
• Sandoz’ filgrastim-sndz (Zarxio).
• Pfizer’s and Celltrion’s infliximab-dyyb (Inflectra).
• Sandoz’ etanercept-szzs (Erelzi).
• Amgen’s adalimumab-atto (Amjevita).
Switching studies is the key to achieving the interchangeable designation, according to the FDA document. They must include at least two full switches between the candidate product and the reference product, which must be licensed in the United States.
But because these products are so structurally diverse, the FDA isn’t imposing a one-size-fits-all process on them. Instead, the molecular complexity and immunogenicity of each product will dictate its approval requirements.
Those with relatively low structural complexity, high molecular similarity to the reference product, and a low incidence of immunogenic adverse events may only need a single switching study to achieve the “interchangeability” designation.
The bar will be higher for a product with high structural complexity that is not as similar to the reference product, or which has been associated with immunogenic adverse events. For this product, FDA might also require extensive safety postmarketing data for the product as a licensed biosimilar, as well as a switching study.
Pharmacokinetics, pharmacodynamics, immunogenicity, and safety will be the primary endpoints of a switching study. Efficacy data are not necessary but can be used as supportive endpoints. Any safety signals in a switching study would raise regulatory eyebrows whether they came from the candidate product or the reference product. Since the study replicates what could happen if the two were used sequentially, it makes little difference from which product the event might arise.
“If an apparent difference in immune response or adverse events is noticed between the switching and nonswitching arms of the study ... it would raise concerns as to whether the proposed interchangeable product is interchangeable, regardless of whether the proposed interchangeable product or the reference product or the switching of the two products actually caused the event,” the document notes.
The E.U. vs. U.S. experience
The United States is only now getting a taste of what has become common fare in the European Union, said Angus Worthing, MD, chair of the American College of Rheumatology’s Government Affairs Committee. The European Medicines Agency approved its first biosimilar in 2006. Since then, 23 such biosimilars have come on the market, at an average price of about 30% less than the reference product. Prices have dropped as much as 70% in countries in which national health care systems abandoned the reference product in favor of the competing biosimilar, Dr. Worthing said in an interview.
“But the U.S. doesn’t have a national health care system, so it won’t work like that here.” In fact, he noted, brand-new data show that Medicare actually paid 22% more for the infliximab biosimilar Inflectra than it did for Remicade in the last quarter of 2016.
It’s not immediately apparent why this is the case, but it’s probably related to company discounts and rebates on these very expensive treatments. According to the report in Inside Health Policy, Janssen Biotech may have increased its discount to compete with Inflectra’s launch price of 15% below Remicade’s wholesale cost. Prices won’t moderate as much in the United States as in the European Union until several biosimilars of the same class appear, Dr. Worthing said.
There have already been allegations that big pharma manipulates international and national pricing to reduce biosimilar competition.
In June, Russian biotech company Biocad filed a lawsuit in New York charging Roche/Genentech with price fixing. The suit alleges that the companies cut the cost of three cancer treatments (Avastin, Herceptin, and Rituxan/MabThera) in Russia, where Biocad markets biosimilars for each. At the same time, Biocad alleges, the companies raised U.S. prices on those products to make up for the money they were losing on the Russian market.
“I think most of the cost benefits will accrue to insurance plans and pharmacy managers, but maybe not to the patients themselves,” he said in an interview. “The most important beneficiaries may not see a single penny of benefit.”
It may be difficult to extrapolate the European economic experience into the U.S. health care market, but the safety record of its biosimilar armamentarium is solid. None of the biosimilars approved in the E.U. have ever been recalled or removed from the European market because of regulatory or safety concerns.
Nonmedical switching raises concerns
Academic medical societies and clinicians interviewed for this article view the proposed approval pathway with cautious optimism. While acknowledging the potential benefit of reducing the costs of prohibitively expensive treatments, they uniformly insist that patient safety – not economic pressure – should be the driving force here.
“I was initially skeptical, and I do believe that we need very close pharmacovigilance in monitoring these for safety,” said Gideon Smith, MD, PhD, a dermatologist at Massachusetts General Hospital, Boston. “But there has been huge uptake of these products in the E.U., and the data are so extensive that we can be reasonably confident these drugs are effective, and no good reason to believe the safety will be any different.”
He is not as comfortable with the prospect of pharmacy-level substitution of an interchangeable biosimilar with the reference product – a feeling that other clinicians echoed.
“I think this is a fundamental issue that should have been dealt with on a federal level. Physicians should always be involved in the decision,” said Dr. Smith, who spoke at an FDA advisory committee meeting last summer on behalf of the American Academy of Dermatology (AAD).
“In general, the GI field is OK with the idea of starting someone on a new prescription [of an interchangeable biosimilar], but not so much with the idea of switching around,” said Dr. Hanauer, who is the Clifford Joseph Barborka Professor of Gastroenterology at Northwestern University, Chicago. “In these biologic compounds, very small differences can be amplified” and alter therapeutic response.
The possibility of switching from the reference to the biosimilar and maybe back again worries him. He hearkened back to the approval of Remicade, when patients who had taken it during clinical trials only were finally able to obtain it on the market. Dr. Hanauer explained that, “20% of them developed serum sickness reactions after the reexposure.”
He also expressed some concern about quality control in international manufacturing plants, citing a 2005 epidemic of immune-mediated pure red cell anemia in patients who received an epoetin alfa biosimilar manufactured in Thailand. The prefilled syringes had an uncoated rubber stopper that apparently reacted with polysorbate 60 in the solution – an interaction that increased immunogenicity when the product was administered subcutaneously.
Dr. Smith concurred. “We know that some patients produce antibodies to biologics if they come on and off, and so we discourage that. The concern is that switching may lead to an increased rate of medication failure, if you have to switch back. This is especially troubling in the case of a hard-to-control patient with severe flares. If they’re being well controlled on a medication, the last thing you want to do is change it for no good clinical reason. And we may well be forced to do that.”
Neither the AAD nor the American Gastroenterological Association has a published stand on the FDA’s proposed guidance for interchangeable biosimilars. The preliminary view of the American College of Rheumatology is a positive one, Dr. Worthing said. However, ACR feels pharmacy-level switching should be a joint, not unilateral, decision.
“Our position statement on biosimilars has been that if it’s legal for a pharmacy to make that switch then we want the doctor and the patient to know, so we can track for safety signals.”
Bringing any biosimilar to market, though, takes a lot of money and a lot of time. And while companies are growing cell lines and producing new molecules that mimic existing treatments, science marches on, said Dr. Smith.
“If we keep dragging our feet on this issue, it might end up being a moot point,” he said. Newer products are achieving better results, raising the bar for therapeutic success. An example is the monoclonal antibody secukinumab (Cosentyx), an inhibitor of interleukin 17A. In October 2016, late-breaking data released at the annual meeting of the European Academy of Dermatology and Venereology impressed the dermatology community. In psoriasis patients, the treatment maintained 90% skin clearance for 4 years in 66% of patients, and 100% clearance for 4 years in 43%.
Not only does this kind of efficacy provide symptomatic relief, it also prevents the expensive long-term morbidity associated with psoriasis, Dr. Smith said.
“Even if these new medications are considerably more expensive upfront than a biosimilar for an older product, they may end up being less expensive in the long run.”
Dr. Krant and Dr. Worthing had no financial disclosures. Dr. Smith has received grants from Allergan and Cipher Pharmaceuticals. Dr. Hanauer has received grants from numerous pharmaceutical companies that manufacture biologics.
[email protected]
On Twitter @alz_gal
The Food and Drug Administration has proposed a regulatory path for biosimilar biologics that are interchangeable with the reference product, paving the way for a new generation of less-expensive versions of these unique treatments.
But bringing an interchangeable biosimilar to market won’t be easy. The bar for interchangeability will be high, requiring that manufacturers prove switching between the new and older products is safe. And clinicians, while cautiously optimistic, aren’t thrilled with the industry payoff that could come with the designation: freedom for insurance companies and pharmacies to switch products at the dispensing level without requiring a new prescription.
The draft FDA guidance for industry, “Considerations in Demonstrating Interchangeability With a Reference Product,” arises from the Biologics Price Competition and Innovation Act of 2009. That section of the Affordable Care Act provides for abbreviated approval pathways for biological products that are demonstrated to be “highly similar” (biosimilar) to or “interchangeable” with an FDA-approved biological product.
The difference between these appellations is subtle but critical to the regulatory process – and perhaps to patient safety. Regulators recognize that the structure of these large, highly complex molecules can never precisely replicate the reference product. But to be labeled a “biosimilar,” developers must prove that the new product functions essentially the same; there can be no clinically meaningful differences in terms of safety, purity, and potency. Unlike a generic medication, a biosimilar can’t be substituted for its reference product at the pharmacy level. If a physician wants the patient on that biosimilar, the script must specify it.
Interchangeables jump a higher regulatory bar
An “interchangeable biosimilar,” though, would have to jump a higher regulatory bar. Not only must it produce the same clinical result as the reference product, it also must be benignly interchangeable with it, conferring no additional risk if a patient switches from the reference to the biosimilar and back again. A pharmacist could, if permitted by state law, substitute an interchangeable product for the reference product without going through the prescriber.
Like biosimilars, interchangeable products need not be tested in every disease for which the reference product is approved, according to the document. Once they are proved safe for one indication, those data can be extrapolated to allow approval for the other indications as well. Nor do biosimilars need to prove efficacy per se, as their molecular similarity to the reference product ensures that they bind to the same receptor and exert the same therapeutic effect.
The biosimilar/interchangeable market has been slow to take off in the United States. There are no approved interchangeable biosimilars, and only four biosimilars – three of which were approved in 2016:
• Sandoz’ filgrastim-sndz (Zarxio).
• Pfizer’s and Celltrion’s infliximab-dyyb (Inflectra).
• Sandoz’ etanercept-szzs (Erelzi).
• Amgen’s adalimumab-atto (Amjevita).
Switching studies is the key to achieving the interchangeable designation, according to the FDA document. They must include at least two full switches between the candidate product and the reference product, which must be licensed in the United States.
But because these products are so structurally diverse, the FDA isn’t imposing a one-size-fits-all process on them. Instead, the molecular complexity and immunogenicity of each product will dictate its approval requirements.
Those with relatively low structural complexity, high molecular similarity to the reference product, and a low incidence of immunogenic adverse events may only need a single switching study to achieve the “interchangeability” designation.
The bar will be higher for a product with high structural complexity that is not as similar to the reference product, or which has been associated with immunogenic adverse events. For this product, FDA might also require extensive safety postmarketing data for the product as a licensed biosimilar, as well as a switching study.
Pharmacokinetics, pharmacodynamics, immunogenicity, and safety will be the primary endpoints of a switching study. Efficacy data are not necessary but can be used as supportive endpoints. Any safety signals in a switching study would raise regulatory eyebrows whether they came from the candidate product or the reference product. Since the study replicates what could happen if the two were used sequentially, it makes little difference from which product the event might arise.
“If an apparent difference in immune response or adverse events is noticed between the switching and nonswitching arms of the study ... it would raise concerns as to whether the proposed interchangeable product is interchangeable, regardless of whether the proposed interchangeable product or the reference product or the switching of the two products actually caused the event,” the document notes.
The E.U. vs. U.S. experience
The United States is only now getting a taste of what has become common fare in the European Union, said Angus Worthing, MD, chair of the American College of Rheumatology’s Government Affairs Committee. The European Medicines Agency approved its first biosimilar in 2006. Since then, 23 such biosimilars have come on the market, at an average price of about 30% less than the reference product. Prices have dropped as much as 70% in countries in which national health care systems abandoned the reference product in favor of the competing biosimilar, Dr. Worthing said in an interview.
“But the U.S. doesn’t have a national health care system, so it won’t work like that here.” In fact, he noted, brand-new data show that Medicare actually paid 22% more for the infliximab biosimilar Inflectra than it did for Remicade in the last quarter of 2016.
It’s not immediately apparent why this is the case, but it’s probably related to company discounts and rebates on these very expensive treatments. According to the report in Inside Health Policy, Janssen Biotech may have increased its discount to compete with Inflectra’s launch price of 15% below Remicade’s wholesale cost. Prices won’t moderate as much in the United States as in the European Union until several biosimilars of the same class appear, Dr. Worthing said.
There have already been allegations that big pharma manipulates international and national pricing to reduce biosimilar competition.
In June, Russian biotech company Biocad filed a lawsuit in New York charging Roche/Genentech with price fixing. The suit alleges that the companies cut the cost of three cancer treatments (Avastin, Herceptin, and Rituxan/MabThera) in Russia, where Biocad markets biosimilars for each. At the same time, Biocad alleges, the companies raised U.S. prices on those products to make up for the money they were losing on the Russian market.
“I think most of the cost benefits will accrue to insurance plans and pharmacy managers, but maybe not to the patients themselves,” he said in an interview. “The most important beneficiaries may not see a single penny of benefit.”
It may be difficult to extrapolate the European economic experience into the U.S. health care market, but the safety record of its biosimilar armamentarium is solid. None of the biosimilars approved in the E.U. have ever been recalled or removed from the European market because of regulatory or safety concerns.
Nonmedical switching raises concerns
Academic medical societies and clinicians interviewed for this article view the proposed approval pathway with cautious optimism. While acknowledging the potential benefit of reducing the costs of prohibitively expensive treatments, they uniformly insist that patient safety – not economic pressure – should be the driving force here.
“I was initially skeptical, and I do believe that we need very close pharmacovigilance in monitoring these for safety,” said Gideon Smith, MD, PhD, a dermatologist at Massachusetts General Hospital, Boston. “But there has been huge uptake of these products in the E.U., and the data are so extensive that we can be reasonably confident these drugs are effective, and no good reason to believe the safety will be any different.”
He is not as comfortable with the prospect of pharmacy-level substitution of an interchangeable biosimilar with the reference product – a feeling that other clinicians echoed.
“I think this is a fundamental issue that should have been dealt with on a federal level. Physicians should always be involved in the decision,” said Dr. Smith, who spoke at an FDA advisory committee meeting last summer on behalf of the American Academy of Dermatology (AAD).
“In general, the GI field is OK with the idea of starting someone on a new prescription [of an interchangeable biosimilar], but not so much with the idea of switching around,” said Dr. Hanauer, who is the Clifford Joseph Barborka Professor of Gastroenterology at Northwestern University, Chicago. “In these biologic compounds, very small differences can be amplified” and alter therapeutic response.
The possibility of switching from the reference to the biosimilar and maybe back again worries him. He hearkened back to the approval of Remicade, when patients who had taken it during clinical trials only were finally able to obtain it on the market. Dr. Hanauer explained that, “20% of them developed serum sickness reactions after the reexposure.”
He also expressed some concern about quality control in international manufacturing plants, citing a 2005 epidemic of immune-mediated pure red cell anemia in patients who received an epoetin alfa biosimilar manufactured in Thailand. The prefilled syringes had an uncoated rubber stopper that apparently reacted with polysorbate 60 in the solution – an interaction that increased immunogenicity when the product was administered subcutaneously.
Dr. Smith concurred. “We know that some patients produce antibodies to biologics if they come on and off, and so we discourage that. The concern is that switching may lead to an increased rate of medication failure, if you have to switch back. This is especially troubling in the case of a hard-to-control patient with severe flares. If they’re being well controlled on a medication, the last thing you want to do is change it for no good clinical reason. And we may well be forced to do that.”
Neither the AAD nor the American Gastroenterological Association has a published stand on the FDA’s proposed guidance for interchangeable biosimilars. The preliminary view of the American College of Rheumatology is a positive one, Dr. Worthing said. However, ACR feels pharmacy-level switching should be a joint, not unilateral, decision.
“Our position statement on biosimilars has been that if it’s legal for a pharmacy to make that switch then we want the doctor and the patient to know, so we can track for safety signals.”
Bringing any biosimilar to market, though, takes a lot of money and a lot of time. And while companies are growing cell lines and producing new molecules that mimic existing treatments, science marches on, said Dr. Smith.
“If we keep dragging our feet on this issue, it might end up being a moot point,” he said. Newer products are achieving better results, raising the bar for therapeutic success. An example is the monoclonal antibody secukinumab (Cosentyx), an inhibitor of interleukin 17A. In October 2016, late-breaking data released at the annual meeting of the European Academy of Dermatology and Venereology impressed the dermatology community. In psoriasis patients, the treatment maintained 90% skin clearance for 4 years in 66% of patients, and 100% clearance for 4 years in 43%.
Not only does this kind of efficacy provide symptomatic relief, it also prevents the expensive long-term morbidity associated with psoriasis, Dr. Smith said.
“Even if these new medications are considerably more expensive upfront than a biosimilar for an older product, they may end up being less expensive in the long run.”
Dr. Krant and Dr. Worthing had no financial disclosures. Dr. Smith has received grants from Allergan and Cipher Pharmaceuticals. Dr. Hanauer has received grants from numerous pharmaceutical companies that manufacture biologics.
[email protected]
On Twitter @alz_gal
Ventilator use in patients with advanced dementia
Clinical question: Does the increasing number of ICU beds in the U.S. affect the use of mechanical ventilation in nursing home patients with advanced dementia?
Background: Some physicians are concerned that increases in ICU beds in the U.S. will translate to increased treatment of advanced dementia in the ICU, which might not line up with their preferences, nor improve mortality.
Study design: Retrospective cohort study.
Setting: Hospitals that completed the American Hospital Association (AHA) annual survey.
Synopsis: From 2000 to 2013, there were 635,008 hospitalizations of 380,060 Medicare patients with advanced dementia who had been in a nursing home in the 120 days prior to hospital admission. ICU admissions increased to 38.5% from 16.9% during the same period. The rate of mechanical ventilation per 1,000 hospital admissions increased to 78 from 39, and 1-year mortality for ventilation was unchanged.
For each increase in 10 ICU beds within a hospital, the adjusted odds ratio for receiving mechanical ventilation was 1.06 (95% CI, 1.05-1.07).
Limitations of the study include that only hospitals completing the AHA annual survey were studied, and also lacked information on individual patients.
Bottom line: The use of mechanical ventilation increased in hospitalized nursing home patients with advanced dementia, correlating with increased ICU bed capacity, yet with no changes in survival.
Citation: Teno JM, Gozalo P, Khandelwal N, et al. Association of increasing use of mechanical ventilation among nursing home residents with advanced dementia and intensive care unit beds [published online ahead of print, Oct. 10, 2016]. JAMA Int Med. 2016;176(12):1809-16.
Dr. Balch is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical question: Does the increasing number of ICU beds in the U.S. affect the use of mechanical ventilation in nursing home patients with advanced dementia?
Background: Some physicians are concerned that increases in ICU beds in the U.S. will translate to increased treatment of advanced dementia in the ICU, which might not line up with their preferences, nor improve mortality.
Study design: Retrospective cohort study.
Setting: Hospitals that completed the American Hospital Association (AHA) annual survey.
Synopsis: From 2000 to 2013, there were 635,008 hospitalizations of 380,060 Medicare patients with advanced dementia who had been in a nursing home in the 120 days prior to hospital admission. ICU admissions increased to 38.5% from 16.9% during the same period. The rate of mechanical ventilation per 1,000 hospital admissions increased to 78 from 39, and 1-year mortality for ventilation was unchanged.
For each increase in 10 ICU beds within a hospital, the adjusted odds ratio for receiving mechanical ventilation was 1.06 (95% CI, 1.05-1.07).
Limitations of the study include that only hospitals completing the AHA annual survey were studied, and also lacked information on individual patients.
Bottom line: The use of mechanical ventilation increased in hospitalized nursing home patients with advanced dementia, correlating with increased ICU bed capacity, yet with no changes in survival.
Citation: Teno JM, Gozalo P, Khandelwal N, et al. Association of increasing use of mechanical ventilation among nursing home residents with advanced dementia and intensive care unit beds [published online ahead of print, Oct. 10, 2016]. JAMA Int Med. 2016;176(12):1809-16.
Dr. Balch is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical question: Does the increasing number of ICU beds in the U.S. affect the use of mechanical ventilation in nursing home patients with advanced dementia?
Background: Some physicians are concerned that increases in ICU beds in the U.S. will translate to increased treatment of advanced dementia in the ICU, which might not line up with their preferences, nor improve mortality.
Study design: Retrospective cohort study.
Setting: Hospitals that completed the American Hospital Association (AHA) annual survey.
Synopsis: From 2000 to 2013, there were 635,008 hospitalizations of 380,060 Medicare patients with advanced dementia who had been in a nursing home in the 120 days prior to hospital admission. ICU admissions increased to 38.5% from 16.9% during the same period. The rate of mechanical ventilation per 1,000 hospital admissions increased to 78 from 39, and 1-year mortality for ventilation was unchanged.
For each increase in 10 ICU beds within a hospital, the adjusted odds ratio for receiving mechanical ventilation was 1.06 (95% CI, 1.05-1.07).
Limitations of the study include that only hospitals completing the AHA annual survey were studied, and also lacked information on individual patients.
Bottom line: The use of mechanical ventilation increased in hospitalized nursing home patients with advanced dementia, correlating with increased ICU bed capacity, yet with no changes in survival.
Citation: Teno JM, Gozalo P, Khandelwal N, et al. Association of increasing use of mechanical ventilation among nursing home residents with advanced dementia and intensive care unit beds [published online ahead of print, Oct. 10, 2016]. JAMA Int Med. 2016;176(12):1809-16.
Dr. Balch is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Consensus guidelines for calcium channel blocker poisoning
Clinical question: What is the best management approach for adults who are admitted to the hospital with a calcium channel blocker (CCB) overdose?
Background: There is significant morbidity and mortality from cardiac drug poisoning. Overall, the level of evidence in the literature on the treatment of CCB toxicity is very low. Prior to the current publication there were no guidelines for treating patients admitted to the hospital with a CCB overdose.
Setting: Panel members participated in online votes, telephone meetings, and two face-to-face meetings to develop the guidelines.
Synopsis: In symptomatic CCB poisoning, the following first-line measures are strongly recommended: IV calcium, with norepinephrine or epinephrine in the presence of shock, and high-dose IV insulin (with other first-line treatments) if there is myocardial dysfunction.
Further lower-strength suggestions were made: insulin therapy as monotherapy if cardiac dysfunction present, or in combination with other therapies if there is no cardiac dysfunction; atropine in the setting of symptomatic bradycardia; and dobutamine or epinephrine in the presence of cardiogenic shock.
For refractory CCB, toxicity suggestions included incremental doses of high-dose insulin (if myocardial dysfunction is present, or even if it is not present in periarrest situations), IV lipid emulsion therapy, and pacemaker for unstable bradycardia (if there is no evidence of cardiac dysfunction). If the patient is in refractory shock or periarrest, the panel suggests the use of venoarterial extracorporeal membrane oxygenation (VA-ECMO).
Limitations included the limited availability of evidence.
Bottom line: Management of CCB toxicity should include IV calcium and high-dose IV insulin, with vasopressors for shock, and other additional therapies for refractory cases.
Citation: St-Onge M, Anseeuw K, Cantrell FL, et al. Experts’ consensus recommendations for the management of calcium channel blocker poisoning in adults [published online ahead of print, Oct. 3, 2016]. Crit Care Med. doi: 10.1097/CCM.0000000000002087.
Dr. Balch is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical question: What is the best management approach for adults who are admitted to the hospital with a calcium channel blocker (CCB) overdose?
Background: There is significant morbidity and mortality from cardiac drug poisoning. Overall, the level of evidence in the literature on the treatment of CCB toxicity is very low. Prior to the current publication there were no guidelines for treating patients admitted to the hospital with a CCB overdose.
Setting: Panel members participated in online votes, telephone meetings, and two face-to-face meetings to develop the guidelines.
Synopsis: In symptomatic CCB poisoning, the following first-line measures are strongly recommended: IV calcium, with norepinephrine or epinephrine in the presence of shock, and high-dose IV insulin (with other first-line treatments) if there is myocardial dysfunction.
Further lower-strength suggestions were made: insulin therapy as monotherapy if cardiac dysfunction present, or in combination with other therapies if there is no cardiac dysfunction; atropine in the setting of symptomatic bradycardia; and dobutamine or epinephrine in the presence of cardiogenic shock.
For refractory CCB, toxicity suggestions included incremental doses of high-dose insulin (if myocardial dysfunction is present, or even if it is not present in periarrest situations), IV lipid emulsion therapy, and pacemaker for unstable bradycardia (if there is no evidence of cardiac dysfunction). If the patient is in refractory shock or periarrest, the panel suggests the use of venoarterial extracorporeal membrane oxygenation (VA-ECMO).
Limitations included the limited availability of evidence.
Bottom line: Management of CCB toxicity should include IV calcium and high-dose IV insulin, with vasopressors for shock, and other additional therapies for refractory cases.
Citation: St-Onge M, Anseeuw K, Cantrell FL, et al. Experts’ consensus recommendations for the management of calcium channel blocker poisoning in adults [published online ahead of print, Oct. 3, 2016]. Crit Care Med. doi: 10.1097/CCM.0000000000002087.
Dr. Balch is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical question: What is the best management approach for adults who are admitted to the hospital with a calcium channel blocker (CCB) overdose?
Background: There is significant morbidity and mortality from cardiac drug poisoning. Overall, the level of evidence in the literature on the treatment of CCB toxicity is very low. Prior to the current publication there were no guidelines for treating patients admitted to the hospital with a CCB overdose.
Setting: Panel members participated in online votes, telephone meetings, and two face-to-face meetings to develop the guidelines.
Synopsis: In symptomatic CCB poisoning, the following first-line measures are strongly recommended: IV calcium, with norepinephrine or epinephrine in the presence of shock, and high-dose IV insulin (with other first-line treatments) if there is myocardial dysfunction.
Further lower-strength suggestions were made: insulin therapy as monotherapy if cardiac dysfunction present, or in combination with other therapies if there is no cardiac dysfunction; atropine in the setting of symptomatic bradycardia; and dobutamine or epinephrine in the presence of cardiogenic shock.
For refractory CCB, toxicity suggestions included incremental doses of high-dose insulin (if myocardial dysfunction is present, or even if it is not present in periarrest situations), IV lipid emulsion therapy, and pacemaker for unstable bradycardia (if there is no evidence of cardiac dysfunction). If the patient is in refractory shock or periarrest, the panel suggests the use of venoarterial extracorporeal membrane oxygenation (VA-ECMO).
Limitations included the limited availability of evidence.
Bottom line: Management of CCB toxicity should include IV calcium and high-dose IV insulin, with vasopressors for shock, and other additional therapies for refractory cases.
Citation: St-Onge M, Anseeuw K, Cantrell FL, et al. Experts’ consensus recommendations for the management of calcium channel blocker poisoning in adults [published online ahead of print, Oct. 3, 2016]. Crit Care Med. doi: 10.1097/CCM.0000000000002087.
Dr. Balch is a clinical instructor at the University of Utah School of Medicine and an academic hospitalist at the University of Utah Hospital.
Clinical Pearl: Early Diagnosis of Nail Psoriasis and Psoriatic Arthritis
Practice Gap
Early diagnosis of nail psoriasis is challenging because nail changes, including pitting, subungual hyperkeratosis, crumbling, oil spots, salmon patches, onycholysis, and splinter hemorrhages, may be subtle and nonspecific. Furthermore, 5% to 10% of psoriasis patients do not have skin findings, making the diagnosis of nail psoriasis even more difficult. Psoriatic arthritis (PsA) is more common in patients with nail psoriasis than in those with cutaneous psoriasis, and early joint damage may be asymptomatic.1 Both nail psoriasis and PsA may progress rapidly, leading to functional impairment with poor quality of life.2
Diagnostic Tool
A 36-year-old man presented with a 4-year history of abnormal fingernails. He denied nail pain but stated that the nails felt sensitive at times and it was difficult to pick up small objects. His medical history was notable for type 2 diabetes mellitus, hypertension, and attention deficit disorder. He denied joint pain or skin rash.
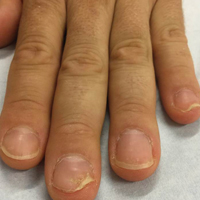
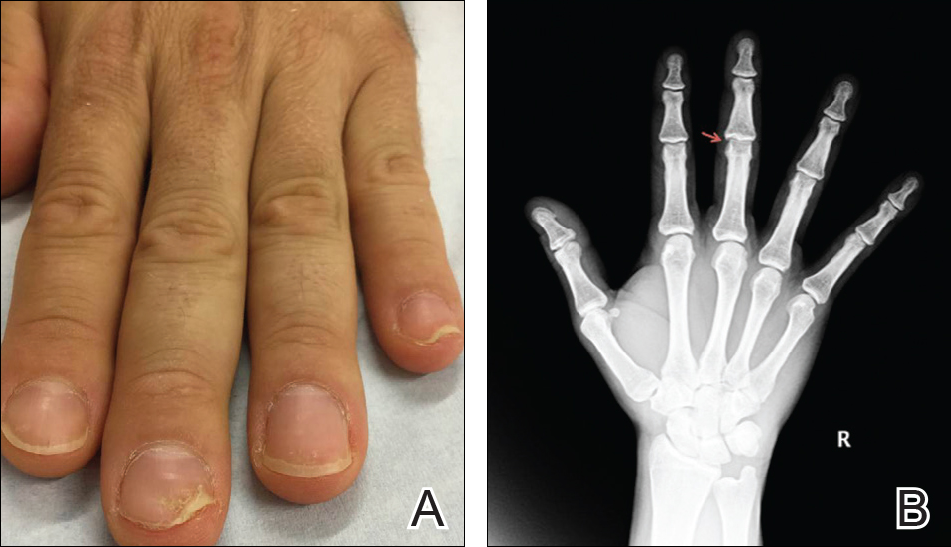
Physical examination revealed pitting and onycholysis of the fingernails (Figure, A) without involvement of the toenails. A nail clipping was negative for fungus but revealed an incompletely keratinized nail plate with subungual parakeratotic scale, consistent with nail psoriasis. A radiograph showed erosive changes of the third finger of the right hand that were compatible with PsA (Figure, B).
Practice Implications
A nail clipping may be performed to diagnose nail psoriasis. Imaging and/or referral to a rheumatologist should be performed in all patients with isolated nail psoriasis to evaluate for early arthritic changes. If present, appropriate therapy is initiated to prevent further joint damage. In patients with nail psoriasis with or without associated joint pain, dermatologists should consider using radiograph imaging to screen patients for PsA.
- 1. Balestri R, Rech G, Rossi E, et al. Natural history of isolated nail psoriasis and its role as a risk factor for the development of psoriatic arthritis: a single center cross sectional study [published online September 2, 2016]. Br J Dermatol. doi:10.1111/bjd.15026.
- Klaassen KM, van de Kerkhof PC, Pasch MC. Nail psoriasis, the unknown burden of disease [published online January 15, 2014]. J Eur Acad Dermatol Venereol. 2014;28:1690-1695.
Practice Gap
Early diagnosis of nail psoriasis is challenging because nail changes, including pitting, subungual hyperkeratosis, crumbling, oil spots, salmon patches, onycholysis, and splinter hemorrhages, may be subtle and nonspecific. Furthermore, 5% to 10% of psoriasis patients do not have skin findings, making the diagnosis of nail psoriasis even more difficult. Psoriatic arthritis (PsA) is more common in patients with nail psoriasis than in those with cutaneous psoriasis, and early joint damage may be asymptomatic.1 Both nail psoriasis and PsA may progress rapidly, leading to functional impairment with poor quality of life.2
Diagnostic Tool
A 36-year-old man presented with a 4-year history of abnormal fingernails. He denied nail pain but stated that the nails felt sensitive at times and it was difficult to pick up small objects. His medical history was notable for type 2 diabetes mellitus, hypertension, and attention deficit disorder. He denied joint pain or skin rash.
Physical examination revealed pitting and onycholysis of the fingernails (Figure, A) without involvement of the toenails. A nail clipping was negative for fungus but revealed an incompletely keratinized nail plate with subungual parakeratotic scale, consistent with nail psoriasis. A radiograph showed erosive changes of the third finger of the right hand that were compatible with PsA (Figure, B).

Practice Implications
A nail clipping may be performed to diagnose nail psoriasis. Imaging and/or referral to a rheumatologist should be performed in all patients with isolated nail psoriasis to evaluate for early arthritic changes. If present, appropriate therapy is initiated to prevent further joint damage. In patients with nail psoriasis with or without associated joint pain, dermatologists should consider using radiograph imaging to screen patients for PsA.
Practice Gap
Early diagnosis of nail psoriasis is challenging because nail changes, including pitting, subungual hyperkeratosis, crumbling, oil spots, salmon patches, onycholysis, and splinter hemorrhages, may be subtle and nonspecific. Furthermore, 5% to 10% of psoriasis patients do not have skin findings, making the diagnosis of nail psoriasis even more difficult. Psoriatic arthritis (PsA) is more common in patients with nail psoriasis than in those with cutaneous psoriasis, and early joint damage may be asymptomatic.1 Both nail psoriasis and PsA may progress rapidly, leading to functional impairment with poor quality of life.2
Diagnostic Tool
A 36-year-old man presented with a 4-year history of abnormal fingernails. He denied nail pain but stated that the nails felt sensitive at times and it was difficult to pick up small objects. His medical history was notable for type 2 diabetes mellitus, hypertension, and attention deficit disorder. He denied joint pain or skin rash.
Physical examination revealed pitting and onycholysis of the fingernails (Figure, A) without involvement of the toenails. A nail clipping was negative for fungus but revealed an incompletely keratinized nail plate with subungual parakeratotic scale, consistent with nail psoriasis. A radiograph showed erosive changes of the third finger of the right hand that were compatible with PsA (Figure, B).

Practice Implications
A nail clipping may be performed to diagnose nail psoriasis. Imaging and/or referral to a rheumatologist should be performed in all patients with isolated nail psoriasis to evaluate for early arthritic changes. If present, appropriate therapy is initiated to prevent further joint damage. In patients with nail psoriasis with or without associated joint pain, dermatologists should consider using radiograph imaging to screen patients for PsA.
- 1. Balestri R, Rech G, Rossi E, et al. Natural history of isolated nail psoriasis and its role as a risk factor for the development of psoriatic arthritis: a single center cross sectional study [published online September 2, 2016]. Br J Dermatol. doi:10.1111/bjd.15026.
- Klaassen KM, van de Kerkhof PC, Pasch MC. Nail psoriasis, the unknown burden of disease [published online January 15, 2014]. J Eur Acad Dermatol Venereol. 2014;28:1690-1695.
- 1. Balestri R, Rech G, Rossi E, et al. Natural history of isolated nail psoriasis and its role as a risk factor for the development of psoriatic arthritis: a single center cross sectional study [published online September 2, 2016]. Br J Dermatol. doi:10.1111/bjd.15026.
- Klaassen KM, van de Kerkhof PC, Pasch MC. Nail psoriasis, the unknown burden of disease [published online January 15, 2014]. J Eur Acad Dermatol Venereol. 2014;28:1690-1695.
More risk factors boost mortality in home births
LAS VEGAS – Analysis of nearly 13 million U.S. deliveries during 2009-2013 identified two new, significant dangers posed to neonates delivered by planned home births: nulliparous pregnancies and deliveries at 41 weeks gestational age or older.
Both conditions linked with a substantially increased risk for neonatal mortality, compared with babies delivered at a hospital, either by a nurse midwife or a physician, said Amos Grünebaum, MD, at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
The critical difference between a home birth–like setting at a hospital and home birth in the field is distance from a hospital when emergency care is needed, he said.
“Women want less intervention during delivery and should get less intervention,” but a midwife run, home birth–like clinic should operate adjacent to a hospital able to handle obstetrical and neonatal emergencies, Dr. Grünebaum said in an interview. “Women need to understand the risks of home births.”
He and his associates used data collected by the Centers for Disease Control and Prevention on 12,953,671 U.S. deliveries during 2009-2013 for singleton, nonanomalous neonates with at least 37 weeks gestation at birth and weighing at least 2,500 grams. The total included 91% hospital deliveries by a physician, 8% hospital deliveries by a nurse-midwife, and 96,815 home births or 0.75% of U.S. deliveries during this period. Despite that low percentage, the number of U.S. home births nearly tripled from 2007 to 2015, he noted.
The rate of neonatal deaths for each 10,000 live births was 3 among infants delivered by nurse midwives at hospitals, 5 for infants delivered by physicians at hospitals, and 12 for infants delivered by home births. The standard mortality ratio was 66% higher for physicians at hospitals, compared with nurse-midwives at hospitals, because physicians handle higher-risk deliveries, and more than fourfold higher for home births, compared with hospital deliveries by nurse-midwives, Dr. Grünebaum reported.
Further analysis showed that the death rate per 10,000 neonates for pregnancies that continued to a gestational age of 41 weeks or more was 17.2, and for deliveries among nulliparous women, neonatal mortality was 22.5 deaths per 10,000 births. These rates were in the same ballpark as three conditions cited by an ACOG committee in a 2016 report as contraindications for home birth: prior cesarean delivery, which had home birth mortality of 18.9 per 10,000 neonates in the current study, multiple gestations, and breach presentation, with home birth mortality in the current study of 127.5 per 10,000.Maternal age of 35 years or greater at the time of delivery linked with a death rate of 13.6 per 10,000 births, a rate that Dr. Grünebaum did not consider high enough to specifically label it a contraindication to home birth. But Dr. Grünebaum took a dim view of home births in general. For any type of pregnancy, a birth center not adjacent to a hospital is “unprofessional,” he declared.
A journal article with this report also appeared online (Am J Ob Gyn. 2017 Jan 29. doi: 10.1016/j.ajog.2017.01.012).
[email protected]
On Twitter @mitchelzoler
LAS VEGAS – Analysis of nearly 13 million U.S. deliveries during 2009-2013 identified two new, significant dangers posed to neonates delivered by planned home births: nulliparous pregnancies and deliveries at 41 weeks gestational age or older.
Both conditions linked with a substantially increased risk for neonatal mortality, compared with babies delivered at a hospital, either by a nurse midwife or a physician, said Amos Grünebaum, MD, at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
The critical difference between a home birth–like setting at a hospital and home birth in the field is distance from a hospital when emergency care is needed, he said.
“Women want less intervention during delivery and should get less intervention,” but a midwife run, home birth–like clinic should operate adjacent to a hospital able to handle obstetrical and neonatal emergencies, Dr. Grünebaum said in an interview. “Women need to understand the risks of home births.”
He and his associates used data collected by the Centers for Disease Control and Prevention on 12,953,671 U.S. deliveries during 2009-2013 for singleton, nonanomalous neonates with at least 37 weeks gestation at birth and weighing at least 2,500 grams. The total included 91% hospital deliveries by a physician, 8% hospital deliveries by a nurse-midwife, and 96,815 home births or 0.75% of U.S. deliveries during this period. Despite that low percentage, the number of U.S. home births nearly tripled from 2007 to 2015, he noted.
The rate of neonatal deaths for each 10,000 live births was 3 among infants delivered by nurse midwives at hospitals, 5 for infants delivered by physicians at hospitals, and 12 for infants delivered by home births. The standard mortality ratio was 66% higher for physicians at hospitals, compared with nurse-midwives at hospitals, because physicians handle higher-risk deliveries, and more than fourfold higher for home births, compared with hospital deliveries by nurse-midwives, Dr. Grünebaum reported.
Further analysis showed that the death rate per 10,000 neonates for pregnancies that continued to a gestational age of 41 weeks or more was 17.2, and for deliveries among nulliparous women, neonatal mortality was 22.5 deaths per 10,000 births. These rates were in the same ballpark as three conditions cited by an ACOG committee in a 2016 report as contraindications for home birth: prior cesarean delivery, which had home birth mortality of 18.9 per 10,000 neonates in the current study, multiple gestations, and breach presentation, with home birth mortality in the current study of 127.5 per 10,000.Maternal age of 35 years or greater at the time of delivery linked with a death rate of 13.6 per 10,000 births, a rate that Dr. Grünebaum did not consider high enough to specifically label it a contraindication to home birth. But Dr. Grünebaum took a dim view of home births in general. For any type of pregnancy, a birth center not adjacent to a hospital is “unprofessional,” he declared.
A journal article with this report also appeared online (Am J Ob Gyn. 2017 Jan 29. doi: 10.1016/j.ajog.2017.01.012).
[email protected]
On Twitter @mitchelzoler
LAS VEGAS – Analysis of nearly 13 million U.S. deliveries during 2009-2013 identified two new, significant dangers posed to neonates delivered by planned home births: nulliparous pregnancies and deliveries at 41 weeks gestational age or older.
Both conditions linked with a substantially increased risk for neonatal mortality, compared with babies delivered at a hospital, either by a nurse midwife or a physician, said Amos Grünebaum, MD, at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
The critical difference between a home birth–like setting at a hospital and home birth in the field is distance from a hospital when emergency care is needed, he said.
“Women want less intervention during delivery and should get less intervention,” but a midwife run, home birth–like clinic should operate adjacent to a hospital able to handle obstetrical and neonatal emergencies, Dr. Grünebaum said in an interview. “Women need to understand the risks of home births.”
He and his associates used data collected by the Centers for Disease Control and Prevention on 12,953,671 U.S. deliveries during 2009-2013 for singleton, nonanomalous neonates with at least 37 weeks gestation at birth and weighing at least 2,500 grams. The total included 91% hospital deliveries by a physician, 8% hospital deliveries by a nurse-midwife, and 96,815 home births or 0.75% of U.S. deliveries during this period. Despite that low percentage, the number of U.S. home births nearly tripled from 2007 to 2015, he noted.
The rate of neonatal deaths for each 10,000 live births was 3 among infants delivered by nurse midwives at hospitals, 5 for infants delivered by physicians at hospitals, and 12 for infants delivered by home births. The standard mortality ratio was 66% higher for physicians at hospitals, compared with nurse-midwives at hospitals, because physicians handle higher-risk deliveries, and more than fourfold higher for home births, compared with hospital deliveries by nurse-midwives, Dr. Grünebaum reported.
Further analysis showed that the death rate per 10,000 neonates for pregnancies that continued to a gestational age of 41 weeks or more was 17.2, and for deliveries among nulliparous women, neonatal mortality was 22.5 deaths per 10,000 births. These rates were in the same ballpark as three conditions cited by an ACOG committee in a 2016 report as contraindications for home birth: prior cesarean delivery, which had home birth mortality of 18.9 per 10,000 neonates in the current study, multiple gestations, and breach presentation, with home birth mortality in the current study of 127.5 per 10,000.Maternal age of 35 years or greater at the time of delivery linked with a death rate of 13.6 per 10,000 births, a rate that Dr. Grünebaum did not consider high enough to specifically label it a contraindication to home birth. But Dr. Grünebaum took a dim view of home births in general. For any type of pregnancy, a birth center not adjacent to a hospital is “unprofessional,” he declared.
A journal article with this report also appeared online (Am J Ob Gyn. 2017 Jan 29. doi: 10.1016/j.ajog.2017.01.012).
[email protected]
On Twitter @mitchelzoler
AT THE PREGNANCY MEETING
Key clinical point:
Major finding: Home birth neonatal mortality per 10,000 births was 22.5 from nulliparous pregnancies and 17.2 with 41 weeks gestational age or greater.
Data source: Analysis of data from 12,953,671 selected full-term U.S. deliveries during 2009-2013, collected by the Centers for Disease Control and Prevention.
Disclosures: Dr. Grünebaum had no disclosures.
Cost of Diagnosing Psoriasis and Rosacea for Dermatologists Versus Primary Care Physicians
Growing incentives to control health care costs may cause accountable care organizations (ACOs) to reconsider how diseases are best managed. Few studies have examined the cost difference between primary care providers (PCPs) and specialists in managing the same disease. Limited data have suggested that management of some diseases by a PCP may be less costly compared to a specialist1,2; however, it is not clear if this finding extends to skin disease. This study sought to assess the cost of seeing a dermatologist versus a PCP for diagnosis of the common skin diseases psoriasis and rosacea.
Methods
Patient data were obtained from the Humana database, a large commercial data set for claims and reimbursed costs encompassing 18,162,539 patients covered between January 2007 and December 2014. Our study population consisted of 3,944,465 patients with claims that included International Classification of Diseases, Ninth Revision (ICD-9), codes for dermatological diagnoses (680.0–709.9). We searched by ICD-9 code for US patients with primary diagnoses of psoriasis (696.1) and rosacea (695.3). We narrowed the search to include patients aged 30 to 64 years, as the diagnoses for these diseases are most common in patients older than 30 years. Patients who were older than 64 years were not included in the study, as most are covered by Medicare and therefore costs covered by Humana in this age group would not be as representative as in younger age groups. Total and average diagnosis-related costs per patient were compared between dermatologists and PCPs. Diagnosis-related costs encompassed physician reimbursement; laboratory and imaging costs, including skin biopsies; inpatient hospitalization cost; and any other charge that could be coded or billed by providers and reimbursed by the insurance company. To be eligible for reimbursement from Humana, dermatologists and PCPs must be registered with the insurer according to specialty board certification and practice credentialing, and they are reimbursed differently based on specialty. Drug costs, which would possibly skew the data toward providers using more expensive systemic medications (ie, dermatologists), were not included in this study, as the discussion is better reserved for long-term management of disease rather than diagnosis-related costs. All diagnoses of psoriasis were included in the study, which likely includes all severities of psoriasis, though we did not have the ability to further break down these diagnoses by severity.
Results
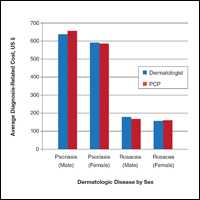
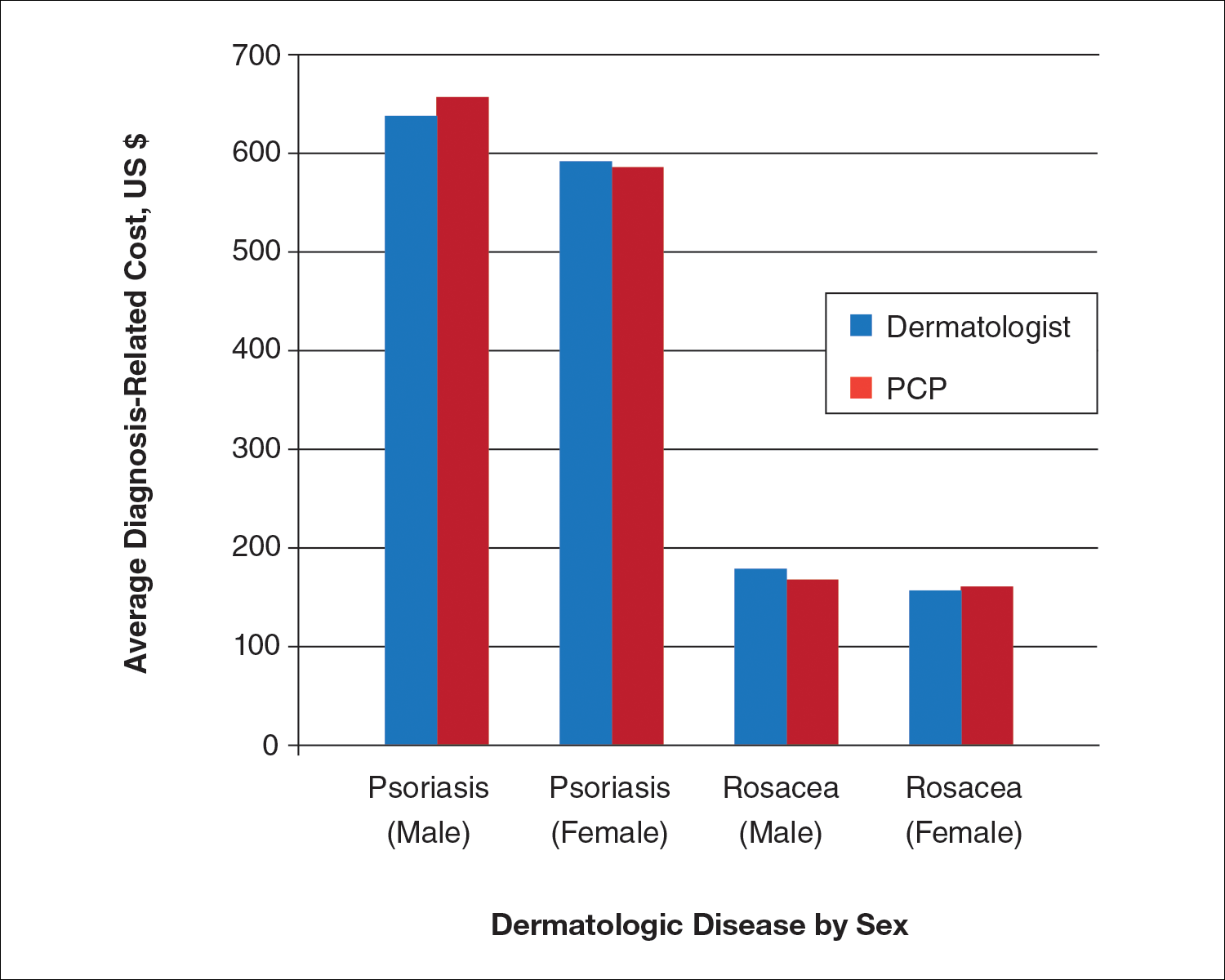
We identified 30,217 psoriasis patients and 37,561 rosacea patients. Of those patients with a primary diagnosis of psoriasis, 26,112 (86%) were seen by a dermatologist and 4105 (14%) were seen by a PCP (Table). Of those patients with a primary diagnosis of rosacea, 34,694 (92%) were seen by a dermatologist and 2867 (8%) were seen by a PCP (Table). There was little difference in the average diagnosis-related cost per patient for psoriasis in males (dermatologists, $638; PCPs, $657) versus females (dermatologists, $592; PCPs, $586) or between specialties (Figure). Findings were similar for rosacea in males (dermatologists, $179; PCPs, $168) versus females (dermatologists, $157; PCPs, $161). For these skin diseases, i

Comment
For the management of common skin disorders such as psoriasis and rosacea, there is little cost difference in seeing a dermatologist versus a PCP. Through extensive training and repeated exposure to many skin diseases, dermatologists are expected to be more comfortable in diagnosing and managing psoriasis and rosacea. Compared to PCPs, dermatologists have demonstrated increased diagnostic accuracy and efficiency when examining pigmented lesions and other dermatologic diseases in several studies.3-6 Although the current study shows that diagnosis-related costs for psoriasis and rosacea are essentially equal between dermatologists and PCPs, it actually may be less expensive for patients to see a dermatologist, as unnecessary tests, biopsies, or medications are more likely to be ordered/prescribed when there is less clinical diagnostic certainty.7,8 Additionally, seeing a PCP for diagnosis of a skin disease may be inefficient if subsequent referral to a dermatologist is needed, a common scenario that occurs when patients see a PCP for skin conditions.9
Our study had limitations, which is typical of a study using a claims database. We used ICD-9 codes recorded in patients’ medical claims to determine diagnosis of psoriasis and rosacea; therefore, our study and data are subject to coding errors. We could not assess the severity of disease, only the presence of disease. Further confirmation of diagnosis could have been made through searching for a second ICD-9 code in the patient’s history. Our data also are from a limited time period and may not represent costs from other time periods.
Conclusion
Given the lack of cost difference between both specialties, we conclude that ACOs should consider encouraging patients to seek care for dermatologic diseases by dermatologists who generally are more accurate and efficient skin diagnosticians, particularly if there is a shortage of PCPs within the ACO network.
- Wimo A, Religa D, Spångberg K, et al. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039-1044.
- Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705-710.
- Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682.
- Federman D, Hogan D, Taylor JR, et al. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. J Am Acad Dermatol. 1995;32:726-729.
- Feldman SR, Fleischer AB, Young AC, et al. Time-efficiency of nondermatologists compared with dermatologists in the care of skin disease. J Am Acad Dermatol. 1999;40:194-199.
- Feldman SR, Peterson SR, Fleischer AB Jr. Dermatologists meet the primary care standard for first contact management of skin disease. J Am Acad Dermatol. 1998;39(2, pt 1):182-186.
- Smith ES, Fleischer AB, Feldman SR. Nondermatologists are more likely than dermatologists to prescribe antifungal/corticosteroid products: an analysis of office visits for cutaneous fungal infections, 1990-1994. J Am Acad Dermatol. 1998;39:43-47.
- Shaffer MP, Feldman SR, Fleischer AB. Use of clotrimazole/betamethasone diproprionate by family physicians. Fam Med. 2000;32:561-565.
- Feldman SR, Fleischer AB, Chen JG. The gatekeeper model is inefficient for the delivery of dermatologic services. J Am Acad Dermatol. 1999;40:426-432.
Growing incentives to control health care costs may cause accountable care organizations (ACOs) to reconsider how diseases are best managed. Few studies have examined the cost difference between primary care providers (PCPs) and specialists in managing the same disease. Limited data have suggested that management of some diseases by a PCP may be less costly compared to a specialist1,2; however, it is not clear if this finding extends to skin disease. This study sought to assess the cost of seeing a dermatologist versus a PCP for diagnosis of the common skin diseases psoriasis and rosacea.
Methods
Patient data were obtained from the Humana database, a large commercial data set for claims and reimbursed costs encompassing 18,162,539 patients covered between January 2007 and December 2014. Our study population consisted of 3,944,465 patients with claims that included International Classification of Diseases, Ninth Revision (ICD-9), codes for dermatological diagnoses (680.0–709.9). We searched by ICD-9 code for US patients with primary diagnoses of psoriasis (696.1) and rosacea (695.3). We narrowed the search to include patients aged 30 to 64 years, as the diagnoses for these diseases are most common in patients older than 30 years. Patients who were older than 64 years were not included in the study, as most are covered by Medicare and therefore costs covered by Humana in this age group would not be as representative as in younger age groups. Total and average diagnosis-related costs per patient were compared between dermatologists and PCPs. Diagnosis-related costs encompassed physician reimbursement; laboratory and imaging costs, including skin biopsies; inpatient hospitalization cost; and any other charge that could be coded or billed by providers and reimbursed by the insurance company. To be eligible for reimbursement from Humana, dermatologists and PCPs must be registered with the insurer according to specialty board certification and practice credentialing, and they are reimbursed differently based on specialty. Drug costs, which would possibly skew the data toward providers using more expensive systemic medications (ie, dermatologists), were not included in this study, as the discussion is better reserved for long-term management of disease rather than diagnosis-related costs. All diagnoses of psoriasis were included in the study, which likely includes all severities of psoriasis, though we did not have the ability to further break down these diagnoses by severity.
Results
We identified 30,217 psoriasis patients and 37,561 rosacea patients. Of those patients with a primary diagnosis of psoriasis, 26,112 (86%) were seen by a dermatologist and 4105 (14%) were seen by a PCP (Table). Of those patients with a primary diagnosis of rosacea, 34,694 (92%) were seen by a dermatologist and 2867 (8%) were seen by a PCP (Table). There was little difference in the average diagnosis-related cost per patient for psoriasis in males (dermatologists, $638; PCPs, $657) versus females (dermatologists, $592; PCPs, $586) or between specialties (Figure). Findings were similar for rosacea in males (dermatologists, $179; PCPs, $168) versus females (dermatologists, $157; PCPs, $161). For these skin diseases, i


Comment
For the management of common skin disorders such as psoriasis and rosacea, there is little cost difference in seeing a dermatologist versus a PCP. Through extensive training and repeated exposure to many skin diseases, dermatologists are expected to be more comfortable in diagnosing and managing psoriasis and rosacea. Compared to PCPs, dermatologists have demonstrated increased diagnostic accuracy and efficiency when examining pigmented lesions and other dermatologic diseases in several studies.3-6 Although the current study shows that diagnosis-related costs for psoriasis and rosacea are essentially equal between dermatologists and PCPs, it actually may be less expensive for patients to see a dermatologist, as unnecessary tests, biopsies, or medications are more likely to be ordered/prescribed when there is less clinical diagnostic certainty.7,8 Additionally, seeing a PCP for diagnosis of a skin disease may be inefficient if subsequent referral to a dermatologist is needed, a common scenario that occurs when patients see a PCP for skin conditions.9
Our study had limitations, which is typical of a study using a claims database. We used ICD-9 codes recorded in patients’ medical claims to determine diagnosis of psoriasis and rosacea; therefore, our study and data are subject to coding errors. We could not assess the severity of disease, only the presence of disease. Further confirmation of diagnosis could have been made through searching for a second ICD-9 code in the patient’s history. Our data also are from a limited time period and may not represent costs from other time periods.
Conclusion
Given the lack of cost difference between both specialties, we conclude that ACOs should consider encouraging patients to seek care for dermatologic diseases by dermatologists who generally are more accurate and efficient skin diagnosticians, particularly if there is a shortage of PCPs within the ACO network.
Growing incentives to control health care costs may cause accountable care organizations (ACOs) to reconsider how diseases are best managed. Few studies have examined the cost difference between primary care providers (PCPs) and specialists in managing the same disease. Limited data have suggested that management of some diseases by a PCP may be less costly compared to a specialist1,2; however, it is not clear if this finding extends to skin disease. This study sought to assess the cost of seeing a dermatologist versus a PCP for diagnosis of the common skin diseases psoriasis and rosacea.
Methods
Patient data were obtained from the Humana database, a large commercial data set for claims and reimbursed costs encompassing 18,162,539 patients covered between January 2007 and December 2014. Our study population consisted of 3,944,465 patients with claims that included International Classification of Diseases, Ninth Revision (ICD-9), codes for dermatological diagnoses (680.0–709.9). We searched by ICD-9 code for US patients with primary diagnoses of psoriasis (696.1) and rosacea (695.3). We narrowed the search to include patients aged 30 to 64 years, as the diagnoses for these diseases are most common in patients older than 30 years. Patients who were older than 64 years were not included in the study, as most are covered by Medicare and therefore costs covered by Humana in this age group would not be as representative as in younger age groups. Total and average diagnosis-related costs per patient were compared between dermatologists and PCPs. Diagnosis-related costs encompassed physician reimbursement; laboratory and imaging costs, including skin biopsies; inpatient hospitalization cost; and any other charge that could be coded or billed by providers and reimbursed by the insurance company. To be eligible for reimbursement from Humana, dermatologists and PCPs must be registered with the insurer according to specialty board certification and practice credentialing, and they are reimbursed differently based on specialty. Drug costs, which would possibly skew the data toward providers using more expensive systemic medications (ie, dermatologists), were not included in this study, as the discussion is better reserved for long-term management of disease rather than diagnosis-related costs. All diagnoses of psoriasis were included in the study, which likely includes all severities of psoriasis, though we did not have the ability to further break down these diagnoses by severity.
Results
We identified 30,217 psoriasis patients and 37,561 rosacea patients. Of those patients with a primary diagnosis of psoriasis, 26,112 (86%) were seen by a dermatologist and 4105 (14%) were seen by a PCP (Table). Of those patients with a primary diagnosis of rosacea, 34,694 (92%) were seen by a dermatologist and 2867 (8%) were seen by a PCP (Table). There was little difference in the average diagnosis-related cost per patient for psoriasis in males (dermatologists, $638; PCPs, $657) versus females (dermatologists, $592; PCPs, $586) or between specialties (Figure). Findings were similar for rosacea in males (dermatologists, $179; PCPs, $168) versus females (dermatologists, $157; PCPs, $161). For these skin diseases, i


Comment
For the management of common skin disorders such as psoriasis and rosacea, there is little cost difference in seeing a dermatologist versus a PCP. Through extensive training and repeated exposure to many skin diseases, dermatologists are expected to be more comfortable in diagnosing and managing psoriasis and rosacea. Compared to PCPs, dermatologists have demonstrated increased diagnostic accuracy and efficiency when examining pigmented lesions and other dermatologic diseases in several studies.3-6 Although the current study shows that diagnosis-related costs for psoriasis and rosacea are essentially equal between dermatologists and PCPs, it actually may be less expensive for patients to see a dermatologist, as unnecessary tests, biopsies, or medications are more likely to be ordered/prescribed when there is less clinical diagnostic certainty.7,8 Additionally, seeing a PCP for diagnosis of a skin disease may be inefficient if subsequent referral to a dermatologist is needed, a common scenario that occurs when patients see a PCP for skin conditions.9
Our study had limitations, which is typical of a study using a claims database. We used ICD-9 codes recorded in patients’ medical claims to determine diagnosis of psoriasis and rosacea; therefore, our study and data are subject to coding errors. We could not assess the severity of disease, only the presence of disease. Further confirmation of diagnosis could have been made through searching for a second ICD-9 code in the patient’s history. Our data also are from a limited time period and may not represent costs from other time periods.
Conclusion
Given the lack of cost difference between both specialties, we conclude that ACOs should consider encouraging patients to seek care for dermatologic diseases by dermatologists who generally are more accurate and efficient skin diagnosticians, particularly if there is a shortage of PCPs within the ACO network.
- Wimo A, Religa D, Spångberg K, et al. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039-1044.
- Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705-710.
- Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682.
- Federman D, Hogan D, Taylor JR, et al. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. J Am Acad Dermatol. 1995;32:726-729.
- Feldman SR, Fleischer AB, Young AC, et al. Time-efficiency of nondermatologists compared with dermatologists in the care of skin disease. J Am Acad Dermatol. 1999;40:194-199.
- Feldman SR, Peterson SR, Fleischer AB Jr. Dermatologists meet the primary care standard for first contact management of skin disease. J Am Acad Dermatol. 1998;39(2, pt 1):182-186.
- Smith ES, Fleischer AB, Feldman SR. Nondermatologists are more likely than dermatologists to prescribe antifungal/corticosteroid products: an analysis of office visits for cutaneous fungal infections, 1990-1994. J Am Acad Dermatol. 1998;39:43-47.
- Shaffer MP, Feldman SR, Fleischer AB. Use of clotrimazole/betamethasone diproprionate by family physicians. Fam Med. 2000;32:561-565.
- Feldman SR, Fleischer AB, Chen JG. The gatekeeper model is inefficient for the delivery of dermatologic services. J Am Acad Dermatol. 1999;40:426-432.
- Wimo A, Religa D, Spångberg K, et al. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039-1044.
- Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705-710.
- Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682.
- Federman D, Hogan D, Taylor JR, et al. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. J Am Acad Dermatol. 1995;32:726-729.
- Feldman SR, Fleischer AB, Young AC, et al. Time-efficiency of nondermatologists compared with dermatologists in the care of skin disease. J Am Acad Dermatol. 1999;40:194-199.
- Feldman SR, Peterson SR, Fleischer AB Jr. Dermatologists meet the primary care standard for first contact management of skin disease. J Am Acad Dermatol. 1998;39(2, pt 1):182-186.
- Smith ES, Fleischer AB, Feldman SR. Nondermatologists are more likely than dermatologists to prescribe antifungal/corticosteroid products: an analysis of office visits for cutaneous fungal infections, 1990-1994. J Am Acad Dermatol. 1998;39:43-47.
- Shaffer MP, Feldman SR, Fleischer AB. Use of clotrimazole/betamethasone diproprionate by family physicians. Fam Med. 2000;32:561-565.
- Feldman SR, Fleischer AB, Chen JG. The gatekeeper model is inefficient for the delivery of dermatologic services. J Am Acad Dermatol. 1999;40:426-432.
Practice Points
- Growing health care costs are causing accountable care organizations (ACOs) to reconsider how to best manage skin disease.
- There is little difference in average diagnosis-related cost between primary care physicians and dermatologists in diagnosing psoriasis or rosacea.
- With diagnosis costs essentially equal and increased dermatologist diagnostic accuracy, ACOs may encourage skin disease to be managed by dermatologists.
New Biologics in Psoriasis: An Update on IL-23 and IL-17 Inhibitors
The role of current biologic therapies in psoriasis predicates on the pathogenic role of upregulated, immune-related mechanisms that result in the activation of myeloid dendritic cells, which release IL-17, IL-23, and other cytokines to activate T cells, including helper T cell TH17. Along with other immune cells, TH17 produces IL-17. This proinflammatory cascade results in keratinocyte proliferation, angiogenesis, and migration of immune cells toward psoriatic lesions.1 Thus, the newest classes of biologics target IL-12, IL-23, and IL-17 to disrupt this inflammatory cascade.
We provide an updated review of the most recent clinical efficacy and safety data on the newest IL-23 and IL-17 inhibitors in the pipeline or approved for psoriasis, including risankizumab, guselkumab, tildrakizumab, ixekizumab, and brodalumab (Table). Ustekinumab and adalimumab, which have been previously approved by the US Food and Drug Administration (FDA), will be discussed here only as comparators.
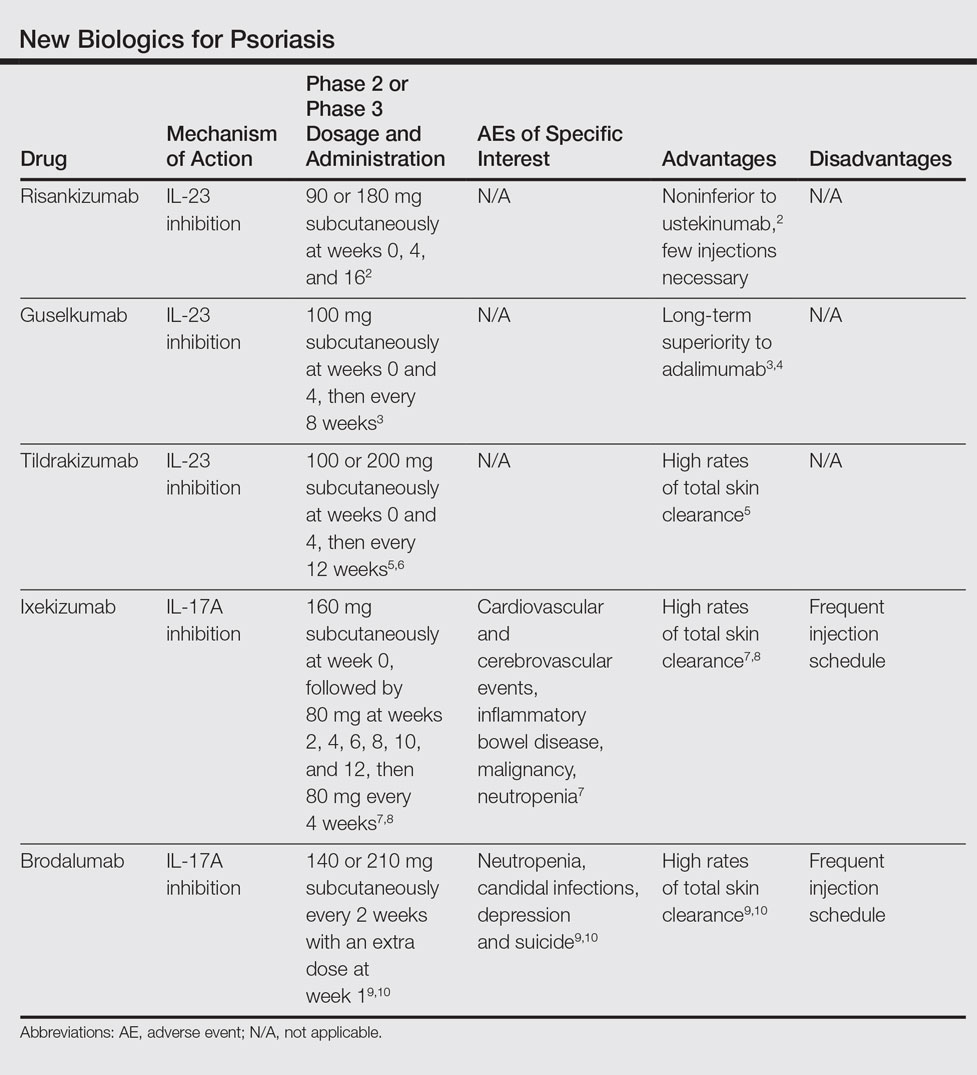
IL-23 Inhibitors
Risankizumab
Risankizumab (formerly known as BI 655066)(Boehringer Ingelheim) is a selective human monoclonal antibody targeting the p19 subunit of IL-23 and currently is undergoing phase 3 trials for psoriasis. A proof-of-concept phase 1 study of 39 participants demonstrated efficacy after 12 weeks of treatment at varying subcutaneous and intravenous doses with placebo control.11 At week 12, 87% (27/31)(P<.001) of all risankizumab-treated participants achieved 75% reduction in psoriasis area and severity index (PASI) score compared to 0% of 8 placebo-treated participants. Common adverse effects (AEs) occurred in 65% (20/31) of risankizumab-treated participants, including non–dose-dependent upper respiratory tract infections, nasopharyngitis, and headache. Serious adverse events (SAEs) that occurred were considered unrelated to the study medication.11
A phase 2 trial of 166 participants compared 3 dosing regimens of subcutaneous risankizumab (single 18-mg dose at week 0; single 90-mg dose at weeks 0, 4, and 16; or single 180-mg dose at weeks 0, 4, and 16) and ustekinumab (weight-based single 45- or 90-mg dose at weeks 0, 4, and 16), demonstrating noninferiority at higher doses of risankizumab.2 Preliminary primary end point results at week 12 showed PASI 90 in 32.6% (P=.4667), 73.2% (P=.0013), 81.0% (P<.0001), and 40.0% of the treatment groups, respectively. Participants in the 180-mg risankizumab group achieved PASI 90 eight weeks faster than those on ustekinumab, lasting more than 2 months longer. Adverse effects were similar across all treatment groups and SAEs were unrelated to the study medications.2
Guselkumab
Guselkumab (Janssen Biotech, Inc) is a selective human monoclonal antibody against the p19 subunit of IL-23. The 52-week phase 2 X-PLORE trial compared dose-ranging subcutaneous guselkumab (5 mg at weeks 0 and 4, then every 12 weeks; 15 mg every 8 weeks; 50 mg at weeks 0 and 4, then every 12 weeks; 100 mg every 8 weeks; or 200 mg at weeks 0 and 4, then every 12 weeks), adalimumab (80-mg loading dose, followed by 40 mg at week 1, then every other week), and placebo in 293 randomized participants.4 At week 16, 34% (P=.002) of participants in the 5-mg guselkumab group, 61% (P<.001) in the 15-mg group, 79% (P<.001) in the 50-mg group, 86% (P<.001) in the 100-mg group, 83% (P<.001) in the 200-mg group, and 58% (P<.001) in the adalimumab group achieved physician global assessment (PGA) scores of 0 (clear) or 1 (minimal psoriasis) compared to 7% of the placebo group. Achievement of PASI 75 similarly favored the guselkumab (44% [P<.001]; 76% [no P value given]; 81% [P<.001]; 79% [P<.001]; and 81% [P<.001], respectively) and adalimumab treatment arms (70% [P<.001]) compared to 5% in the placebo group. In longer-term comparisons to week 40, participants in the 50-, 100-, and 200-mg guselkumab groups showed significantly greater remission of psoriatic lesions, measured by a PGA score of 0 or 1, than participants in the adalimumab group (71% [P=.05]; 77% [P=.005]; 81% [P=.01]; and 49%, respectively).4
Preliminary results from VOYAGE 1 (N=837), the first of several phase 3 trials, further demonstrate the superiority of guselkumab 100 mg at weeks 0 and 4 and then every 8 weeks over adalimumab (standard dosing) and placebo; at week 16, 73.3% (P<.001 for both comparisons) versus 49.7% and 2.9% of participants, respectively, achieved PASI 90, with sustained superiority of skin clearance in guselkumab-treated participants compared to adalimumab and placebo through week 48.3
Long-term safety data showed no dose dependence or trend from 0 to 16 weeks and 16 to 52 weeks of treatment regarding rates of AEs, SAEs, or serious infections.4 Between weeks 16 and 52, 48.9% of all guselkumab-treated participants exhibited AEs compared to 60.5% of adalimumab-treated participants and 51.3% of placebo participants. Overall infection rates also were lowest in the guselkumab group at 29.8% compared to 36.8% and 35.9%, respectively. Three participants treated with guselkumab had major cardiovascular events, including a fatal myocardial infarction. No cases of tuberculosis or serious opportunistic infections were reported.4
Tildrakizumab
Tildrakizumab (formerly known as MK-3222)(Sun Pharmaceutical Industries Ltd) is a human monoclonal antibody also targeting the p19 subunit of IL-23. In a phase 2 study of 355 participants with chronic plaque psoriasis, participants received 5-, 25-, 100-, or 200-mg subcutaneous tildrakizumab or placebo at weeks 0 and 4 and then every 12 weeks for a total of 52 weeks.6 At week 16, PASI 75 results were 33.3%, 64.4%, 66.3%, 74.4%, and 4.4%, respectively (P<.001 for each comparison). Improvement began within the first month of treatment, with median times to PASI 75 of 57 days at 200-mg dosing and 84 days at 100-mg dosing. Of those participants achieving PASI 75 by drug discontinuation at week 52, 96% of the 100-mg group and 93% of the 200-mg group maintained PASI 75 through week 72, suggesting low relapse rates after treatment cessation.6
In October 2016, the efficacy results of 2 pivotal phase 3 trials (reSURFACE 1 and reSURFACE 2) involving more than 1800 participants combined revealed PASI 90 achievement in an average of 54% of participants on tildrakizumab 100 mg and 59% of participants on tildrakizumab 200 mg at week 28.5 Achievement of PASI 100 occurred in 24% and 30% of participants at week 28, respectively. The second of these trials included an etanercept comparison group and demonstrated head-to-head superiority of 100 and 200 mg subcutaneous tildrakizumab at week 12 by end point measures.5
Treatment-related AEs occurred at rates of 25% in tildrakizumab-treated participants and 22% in placebo-treated participants, most frequently nasopharyngitis and headache.6 At least 1 AE occurred in 64% of tildrakizumab-treated participants without dose dependence compared to 69% of placebo-treated participants. Severe AEs thought to be drug treatment related were bacterial arthritis, lymphedema, melanoma, stroke, and epiglottitis.6
IL-17 Inhibitors
Ixekizumab
Ixekizumab (Eli Lilly and Company), a monoclonal inhibitor of IL-17A, is the most recently approved psoriasis biologic on the market and has been cleared for use in adults with moderate to severe plaque psoriasis. Recommended dosing is 160 mg (given in two 80-mg subcutaneous injections via an autoinjector or prefilled syringe) at week 0, followed by an 80-mg injection at weeks 2, 4, 6, 8, 10, and 12, and then 80 mg every 4 weeks thereafter. The FDA approved ixekizumab in March 2016 following favorable results of several phase 3 trials: UNCOVER-1, UNCOVER-2, and UNCOVER-3.7,8
In UNCOVER-1, 1296 participants were randomized to 1 of 2 ixekizumab treatment arms—160 mg starting dose at week 0, 80 mg every 2 or 4 weeks thereafter—or placebo.7 At week 12, 89.1%, 82.6%, and 3.9% achieved PASI 75, respectively (P<.001 for both). Importantly, high numbers of participants also achieved PASI 90 (70.9% in the 2-week group and 64.6% in the 4-week group vs 0.5% in the placebo group [P<.001]) and PASI 100 (35.3% and 33.6% vs 0%, respectively [P<.001]), suggesting high rates of disease clearance.7
UNCOVER-2 (N=1224) and UNCOVER-3 (N=1346) investigated the same 2 dosing regimens of ixekizumab compared to etanercept 50 mg biweekly and placebo.8 At week 12, the percentage of participants achieving PASI 90 in UNCOVER-2 was 70.7%, 59.7%, 18.7%, and 0.6%, respectively, and 68.1%, 65.3%, 25.7%, and 3.1%, respectively, in UNCOVER-3 (P<.0001 for all comparisons to placebo and etanercept). At week 12, PASI 100 results also showed striking superiority, with 40.5%, 30.8%, 5.3%, and 0.6% of participants, respectively, in UNCOVER-2, and 37.7%, 35%, 7.3%, and 0%, respectively, in UNCOVER-3, achieving complete clearance of disease (P<.0001 for all comparisons to placebo and etanercept). Responses to ixekizumab were observed as early as weeks 1 and 2, while no participants in the etanercept and placebo treatment groups achieved comparative efficiency.8
In an extension of UNCOVER-3, efficacy increased from week 12 to week 60 according to PASI 90 (68%–73% in the 2-week group; 65%–72% in the 4-week group) and PASI 100 measures (38%–55% in the 2-week group; 35%–52% in the 4-week group).7
The most common AEs associated with ixekizumab treatment from weeks 0 to 12 occurred at higher rates in the 2-week and 4-week ixekizumab groups compared to placebo, including nasopharyngitis (9.5% and 9% vs 8.7%, respectively), upper respiratory tract infection (4.4% and 3.9% vs 3.5%, respectively), injection-site reaction (10% and 7.7% vs 1%, respectively), arthralgia (4.4% and 4.3% vs 2.9%, respectively), and headache (2.5% and 1.9% vs 2.1%, respectively). Infections, including candidal, oral, vulvovaginal, and cutaneous, occurred in 27% of the 2-week dosing group and 27.4% of the 4-week dosing group compared to 22.9% of the placebo group during weeks 0 to 12, with candidal infections in particular occurring more frequently in the active treatment groups and exhibiting dose dependence. Other AEs of special interest that occurred among all ixekizumab-treated participants (n=3736) from weeks 0 to 60 were cardiovascular and cerebrovascular events (22 [0.6%]), inflammatory bowel disease (11 [0.3%]), non–skin cancer malignancy (14 [0.4%]), and nonmelanoma skin cancer (20 [0.5%]). Neutropenia occurred at higher rates in ixekizumab-treated participants (9.3% in the 2-week group and 8.6% in the 4-week group) compared to placebo (3.3%) and occurred in 11.5% of all ixekizumab participants over 60 weeks.7
Brodalumab
Brodalumab (Valeant Pharmaceuticals International, Inc) is a human monoclonal antibody targeting the IL-17A receptor currently under review for FDA approval after undergoing phase 3 trials. The first of these trials, AMAGINE-1, showed efficacy of subcutaneous brodalumab (140 or 210 mg administered every 2 weeks with an extra dose at week 1) compared to placebo in 661 participants.9 At week 12, 60%, 83%, and 3%, respectively, achieved PASI 75; 43%, 70%, and 1%, respectively, achieved PASI 90; and 23%, 42%, and 1%, respectively, achieved PASI 100 (P<.001 for all respective comparisons to placebo). These effects were retained through 52 weeks of treatment. The median time to complete disease clearance in participants reaching PASI 100 was 12 weeks. Conversely, participants who were re-randomized to placebo after week 12 of brodalumab treatment relapsed within weeks to months.9
AMAGINE-2 and AMAGINE-3 further demonstrated the efficacy of brodalumab (140 or 210 mg every 2 weeks with extra dose at week 1) compared to ustekinumab (45 or 90 mg weight-based standard dosing) and placebo in 1831 participants, respectively.10 In AMAGINE-2, 49% of participants in the 140-mg group (P<.001 vs placebo), 70% in the 210-mg group (P<.001 vs placebo), 47% in the ustekinumab group, and 3% in the placebo group achieved PASI 90 at week 12. Similarly, in AMAGINE-3, 52% of participants in the 140-mg group (P<.001), 69% in the 210-mg group (P<.001), 48% in the ustekinumab group, and 2% in the placebo group achieved PASI 90. Impressively, complete clearance (PASI 100) at week 12 occurred in 26% of the 140-mg group (P<.001 vs placebo), 44% of the 210-mg group (P<.001 vs placebo), and 22% of the ustekinumab group compared to 2% of the placebo group in AMAGINE-2, with similar rates in AMAGINE-3. Brodalumab was significantly superior to ustekinumab at the 210-mg dose by PASI 90 measures (P<.001) in both studies and at the 140-mg dose by PASI 100 measures (P=.007) in AMAGINE-3 only.10
Common AEs were nasopharyngitis, upper respiratory tract infection, headache, and arthralgia, all occurring at grossly similar rates (49%–60%) across all experimental groups in AMAGINE-1, AMAGINE-2, and AMAGINE-3 during the first 12-week treatment period.9,10 Brodalumab treatment groups had high rates of specific interest AEs compared to ustekinumab and placebo groups, including neutropenia (0.8%, 1.1%, 0.3%, and 0%, respectively) and candidal infections (0.8%, 1.3%, 0.3%, and 0.3%, respectively). Induction phase (weeks 0–12) depression rates were concerning, with 6 cases each in AMAGINE-2 (4 [0.7%] in the 140-mg group, 2 [0.3%] in the 210-mg group) and AMAGINE-3 (4 [0.6%] in the 140-mg group, 2 [0.3%] in the 210-mg group). Cases of neutropenia were mild, were not associated with major infection, and were transient or reversible. Depression rates after 52 weeks of treatment were 1.7% (23/1567) of brodalumab participants in AMAGINE-2 and 1.8% (21/1613) in AMAGINE-3. Three participants, all on constant 210-mg dosing through week 52, attempted suicide with 1 completion10; however, because no other IL-17 inhibitors were associated with depression or suicide in other trials, it has been suggested that these cases were incidental and not treatment related.12 An FDA advisory panel recommended approval of brodalumab in July 2016 despite ongoing concerns of depression and suicide.13
Conclusion
The robust investigation into IL-23 and IL-17 inhibitors to treat plaque psoriasis has yielded promising results, including the unprecedented rates of PASI 100 achievement with these new biologics. Risankizumab, ixekizumab, and brodalumab have demonstrated superior efficacy in trials compared to ustekinumab. Tildrakizumab has shown low disease relapse after drug cessation. Ixekizumab and brodalumab have shown high rates of total disease clearance. Thus far, safety findings for these pipeline biologics have been consistent with those of ustekinumab. With ixekizumab approved in 2016 and brodalumab under review, new options in biologic therapy will offer patients and clinicians greater choices in treating severe and recalcitrant psoriasis.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Papp K, Menter A, Sofen H, et al. Efficacy and safety of different dose regimens of a selective IL-23p19 inhibitor (BI 655066) compared with ustekinumab in patients with moderate-to-severe plaque psoriasis with and without psoriatic arthritis. Paper presented at: 2015 American College of Rheumatology/Association of Rheumatology Health Professionals Annual Meeting; November 6-11, 2015; San Francisco, CA.
- New phase 3 data show significant efficacy versus placebo and superiority of guselkumab versus Humira in treatment of moderate to severe plaque psoriasis [press release]. Vienna, Austria; Janssen Research & Development, LLC: October 1, 2016.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Sun Pharma to announce late-breaking results for investigational IL-23p19 inhibitor, Tildrakizumab, achieves primary end point in both phase-3 studies in patients with moderate-to-severe plaque psoriasis [press release]. Mumbai, India; Sun Pharmaceutical Industries Ltd: October 1, 2016.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 Study Group, UNCOVER-2 Study Group, UNCOVER-3 Study Group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis [published online June 23, 2016]. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial [published online March 1, 2015]. J Allergy Clin Immunol. 2015;136:116-124.e7.
- Chiricozzi A, Romanelli M, Saraceno R, et al. No meaningful association between suicidal behavior and the use of IL-17A-neutralizing or IL-17RA-blocking agents [published online August 31, 2016]. Expert Opin Drug Saf. 2016;15:1653-1659.
- FDA advisory committee recommends approval of brodalumab for treatment of moderate-to-severe plaque psoriasis [news release]. Laval, Quebec: Valeant Pharmaceuticals International, Inc; July 19, 2016.
The role of current biologic therapies in psoriasis predicates on the pathogenic role of upregulated, immune-related mechanisms that result in the activation of myeloid dendritic cells, which release IL-17, IL-23, and other cytokines to activate T cells, including helper T cell TH17. Along with other immune cells, TH17 produces IL-17. This proinflammatory cascade results in keratinocyte proliferation, angiogenesis, and migration of immune cells toward psoriatic lesions.1 Thus, the newest classes of biologics target IL-12, IL-23, and IL-17 to disrupt this inflammatory cascade.
We provide an updated review of the most recent clinical efficacy and safety data on the newest IL-23 and IL-17 inhibitors in the pipeline or approved for psoriasis, including risankizumab, guselkumab, tildrakizumab, ixekizumab, and brodalumab (Table). Ustekinumab and adalimumab, which have been previously approved by the US Food and Drug Administration (FDA), will be discussed here only as comparators.

IL-23 Inhibitors
Risankizumab
Risankizumab (formerly known as BI 655066)(Boehringer Ingelheim) is a selective human monoclonal antibody targeting the p19 subunit of IL-23 and currently is undergoing phase 3 trials for psoriasis. A proof-of-concept phase 1 study of 39 participants demonstrated efficacy after 12 weeks of treatment at varying subcutaneous and intravenous doses with placebo control.11 At week 12, 87% (27/31)(P<.001) of all risankizumab-treated participants achieved 75% reduction in psoriasis area and severity index (PASI) score compared to 0% of 8 placebo-treated participants. Common adverse effects (AEs) occurred in 65% (20/31) of risankizumab-treated participants, including non–dose-dependent upper respiratory tract infections, nasopharyngitis, and headache. Serious adverse events (SAEs) that occurred were considered unrelated to the study medication.11
A phase 2 trial of 166 participants compared 3 dosing regimens of subcutaneous risankizumab (single 18-mg dose at week 0; single 90-mg dose at weeks 0, 4, and 16; or single 180-mg dose at weeks 0, 4, and 16) and ustekinumab (weight-based single 45- or 90-mg dose at weeks 0, 4, and 16), demonstrating noninferiority at higher doses of risankizumab.2 Preliminary primary end point results at week 12 showed PASI 90 in 32.6% (P=.4667), 73.2% (P=.0013), 81.0% (P<.0001), and 40.0% of the treatment groups, respectively. Participants in the 180-mg risankizumab group achieved PASI 90 eight weeks faster than those on ustekinumab, lasting more than 2 months longer. Adverse effects were similar across all treatment groups and SAEs were unrelated to the study medications.2
Guselkumab
Guselkumab (Janssen Biotech, Inc) is a selective human monoclonal antibody against the p19 subunit of IL-23. The 52-week phase 2 X-PLORE trial compared dose-ranging subcutaneous guselkumab (5 mg at weeks 0 and 4, then every 12 weeks; 15 mg every 8 weeks; 50 mg at weeks 0 and 4, then every 12 weeks; 100 mg every 8 weeks; or 200 mg at weeks 0 and 4, then every 12 weeks), adalimumab (80-mg loading dose, followed by 40 mg at week 1, then every other week), and placebo in 293 randomized participants.4 At week 16, 34% (P=.002) of participants in the 5-mg guselkumab group, 61% (P<.001) in the 15-mg group, 79% (P<.001) in the 50-mg group, 86% (P<.001) in the 100-mg group, 83% (P<.001) in the 200-mg group, and 58% (P<.001) in the adalimumab group achieved physician global assessment (PGA) scores of 0 (clear) or 1 (minimal psoriasis) compared to 7% of the placebo group. Achievement of PASI 75 similarly favored the guselkumab (44% [P<.001]; 76% [no P value given]; 81% [P<.001]; 79% [P<.001]; and 81% [P<.001], respectively) and adalimumab treatment arms (70% [P<.001]) compared to 5% in the placebo group. In longer-term comparisons to week 40, participants in the 50-, 100-, and 200-mg guselkumab groups showed significantly greater remission of psoriatic lesions, measured by a PGA score of 0 or 1, than participants in the adalimumab group (71% [P=.05]; 77% [P=.005]; 81% [P=.01]; and 49%, respectively).4
Preliminary results from VOYAGE 1 (N=837), the first of several phase 3 trials, further demonstrate the superiority of guselkumab 100 mg at weeks 0 and 4 and then every 8 weeks over adalimumab (standard dosing) and placebo; at week 16, 73.3% (P<.001 for both comparisons) versus 49.7% and 2.9% of participants, respectively, achieved PASI 90, with sustained superiority of skin clearance in guselkumab-treated participants compared to adalimumab and placebo through week 48.3
Long-term safety data showed no dose dependence or trend from 0 to 16 weeks and 16 to 52 weeks of treatment regarding rates of AEs, SAEs, or serious infections.4 Between weeks 16 and 52, 48.9% of all guselkumab-treated participants exhibited AEs compared to 60.5% of adalimumab-treated participants and 51.3% of placebo participants. Overall infection rates also were lowest in the guselkumab group at 29.8% compared to 36.8% and 35.9%, respectively. Three participants treated with guselkumab had major cardiovascular events, including a fatal myocardial infarction. No cases of tuberculosis or serious opportunistic infections were reported.4
Tildrakizumab
Tildrakizumab (formerly known as MK-3222)(Sun Pharmaceutical Industries Ltd) is a human monoclonal antibody also targeting the p19 subunit of IL-23. In a phase 2 study of 355 participants with chronic plaque psoriasis, participants received 5-, 25-, 100-, or 200-mg subcutaneous tildrakizumab or placebo at weeks 0 and 4 and then every 12 weeks for a total of 52 weeks.6 At week 16, PASI 75 results were 33.3%, 64.4%, 66.3%, 74.4%, and 4.4%, respectively (P<.001 for each comparison). Improvement began within the first month of treatment, with median times to PASI 75 of 57 days at 200-mg dosing and 84 days at 100-mg dosing. Of those participants achieving PASI 75 by drug discontinuation at week 52, 96% of the 100-mg group and 93% of the 200-mg group maintained PASI 75 through week 72, suggesting low relapse rates after treatment cessation.6
In October 2016, the efficacy results of 2 pivotal phase 3 trials (reSURFACE 1 and reSURFACE 2) involving more than 1800 participants combined revealed PASI 90 achievement in an average of 54% of participants on tildrakizumab 100 mg and 59% of participants on tildrakizumab 200 mg at week 28.5 Achievement of PASI 100 occurred in 24% and 30% of participants at week 28, respectively. The second of these trials included an etanercept comparison group and demonstrated head-to-head superiority of 100 and 200 mg subcutaneous tildrakizumab at week 12 by end point measures.5
Treatment-related AEs occurred at rates of 25% in tildrakizumab-treated participants and 22% in placebo-treated participants, most frequently nasopharyngitis and headache.6 At least 1 AE occurred in 64% of tildrakizumab-treated participants without dose dependence compared to 69% of placebo-treated participants. Severe AEs thought to be drug treatment related were bacterial arthritis, lymphedema, melanoma, stroke, and epiglottitis.6
IL-17 Inhibitors
Ixekizumab
Ixekizumab (Eli Lilly and Company), a monoclonal inhibitor of IL-17A, is the most recently approved psoriasis biologic on the market and has been cleared for use in adults with moderate to severe plaque psoriasis. Recommended dosing is 160 mg (given in two 80-mg subcutaneous injections via an autoinjector or prefilled syringe) at week 0, followed by an 80-mg injection at weeks 2, 4, 6, 8, 10, and 12, and then 80 mg every 4 weeks thereafter. The FDA approved ixekizumab in March 2016 following favorable results of several phase 3 trials: UNCOVER-1, UNCOVER-2, and UNCOVER-3.7,8
In UNCOVER-1, 1296 participants were randomized to 1 of 2 ixekizumab treatment arms—160 mg starting dose at week 0, 80 mg every 2 or 4 weeks thereafter—or placebo.7 At week 12, 89.1%, 82.6%, and 3.9% achieved PASI 75, respectively (P<.001 for both). Importantly, high numbers of participants also achieved PASI 90 (70.9% in the 2-week group and 64.6% in the 4-week group vs 0.5% in the placebo group [P<.001]) and PASI 100 (35.3% and 33.6% vs 0%, respectively [P<.001]), suggesting high rates of disease clearance.7
UNCOVER-2 (N=1224) and UNCOVER-3 (N=1346) investigated the same 2 dosing regimens of ixekizumab compared to etanercept 50 mg biweekly and placebo.8 At week 12, the percentage of participants achieving PASI 90 in UNCOVER-2 was 70.7%, 59.7%, 18.7%, and 0.6%, respectively, and 68.1%, 65.3%, 25.7%, and 3.1%, respectively, in UNCOVER-3 (P<.0001 for all comparisons to placebo and etanercept). At week 12, PASI 100 results also showed striking superiority, with 40.5%, 30.8%, 5.3%, and 0.6% of participants, respectively, in UNCOVER-2, and 37.7%, 35%, 7.3%, and 0%, respectively, in UNCOVER-3, achieving complete clearance of disease (P<.0001 for all comparisons to placebo and etanercept). Responses to ixekizumab were observed as early as weeks 1 and 2, while no participants in the etanercept and placebo treatment groups achieved comparative efficiency.8
In an extension of UNCOVER-3, efficacy increased from week 12 to week 60 according to PASI 90 (68%–73% in the 2-week group; 65%–72% in the 4-week group) and PASI 100 measures (38%–55% in the 2-week group; 35%–52% in the 4-week group).7
The most common AEs associated with ixekizumab treatment from weeks 0 to 12 occurred at higher rates in the 2-week and 4-week ixekizumab groups compared to placebo, including nasopharyngitis (9.5% and 9% vs 8.7%, respectively), upper respiratory tract infection (4.4% and 3.9% vs 3.5%, respectively), injection-site reaction (10% and 7.7% vs 1%, respectively), arthralgia (4.4% and 4.3% vs 2.9%, respectively), and headache (2.5% and 1.9% vs 2.1%, respectively). Infections, including candidal, oral, vulvovaginal, and cutaneous, occurred in 27% of the 2-week dosing group and 27.4% of the 4-week dosing group compared to 22.9% of the placebo group during weeks 0 to 12, with candidal infections in particular occurring more frequently in the active treatment groups and exhibiting dose dependence. Other AEs of special interest that occurred among all ixekizumab-treated participants (n=3736) from weeks 0 to 60 were cardiovascular and cerebrovascular events (22 [0.6%]), inflammatory bowel disease (11 [0.3%]), non–skin cancer malignancy (14 [0.4%]), and nonmelanoma skin cancer (20 [0.5%]). Neutropenia occurred at higher rates in ixekizumab-treated participants (9.3% in the 2-week group and 8.6% in the 4-week group) compared to placebo (3.3%) and occurred in 11.5% of all ixekizumab participants over 60 weeks.7
Brodalumab
Brodalumab (Valeant Pharmaceuticals International, Inc) is a human monoclonal antibody targeting the IL-17A receptor currently under review for FDA approval after undergoing phase 3 trials. The first of these trials, AMAGINE-1, showed efficacy of subcutaneous brodalumab (140 or 210 mg administered every 2 weeks with an extra dose at week 1) compared to placebo in 661 participants.9 At week 12, 60%, 83%, and 3%, respectively, achieved PASI 75; 43%, 70%, and 1%, respectively, achieved PASI 90; and 23%, 42%, and 1%, respectively, achieved PASI 100 (P<.001 for all respective comparisons to placebo). These effects were retained through 52 weeks of treatment. The median time to complete disease clearance in participants reaching PASI 100 was 12 weeks. Conversely, participants who were re-randomized to placebo after week 12 of brodalumab treatment relapsed within weeks to months.9
AMAGINE-2 and AMAGINE-3 further demonstrated the efficacy of brodalumab (140 or 210 mg every 2 weeks with extra dose at week 1) compared to ustekinumab (45 or 90 mg weight-based standard dosing) and placebo in 1831 participants, respectively.10 In AMAGINE-2, 49% of participants in the 140-mg group (P<.001 vs placebo), 70% in the 210-mg group (P<.001 vs placebo), 47% in the ustekinumab group, and 3% in the placebo group achieved PASI 90 at week 12. Similarly, in AMAGINE-3, 52% of participants in the 140-mg group (P<.001), 69% in the 210-mg group (P<.001), 48% in the ustekinumab group, and 2% in the placebo group achieved PASI 90. Impressively, complete clearance (PASI 100) at week 12 occurred in 26% of the 140-mg group (P<.001 vs placebo), 44% of the 210-mg group (P<.001 vs placebo), and 22% of the ustekinumab group compared to 2% of the placebo group in AMAGINE-2, with similar rates in AMAGINE-3. Brodalumab was significantly superior to ustekinumab at the 210-mg dose by PASI 90 measures (P<.001) in both studies and at the 140-mg dose by PASI 100 measures (P=.007) in AMAGINE-3 only.10
Common AEs were nasopharyngitis, upper respiratory tract infection, headache, and arthralgia, all occurring at grossly similar rates (49%–60%) across all experimental groups in AMAGINE-1, AMAGINE-2, and AMAGINE-3 during the first 12-week treatment period.9,10 Brodalumab treatment groups had high rates of specific interest AEs compared to ustekinumab and placebo groups, including neutropenia (0.8%, 1.1%, 0.3%, and 0%, respectively) and candidal infections (0.8%, 1.3%, 0.3%, and 0.3%, respectively). Induction phase (weeks 0–12) depression rates were concerning, with 6 cases each in AMAGINE-2 (4 [0.7%] in the 140-mg group, 2 [0.3%] in the 210-mg group) and AMAGINE-3 (4 [0.6%] in the 140-mg group, 2 [0.3%] in the 210-mg group). Cases of neutropenia were mild, were not associated with major infection, and were transient or reversible. Depression rates after 52 weeks of treatment were 1.7% (23/1567) of brodalumab participants in AMAGINE-2 and 1.8% (21/1613) in AMAGINE-3. Three participants, all on constant 210-mg dosing through week 52, attempted suicide with 1 completion10; however, because no other IL-17 inhibitors were associated with depression or suicide in other trials, it has been suggested that these cases were incidental and not treatment related.12 An FDA advisory panel recommended approval of brodalumab in July 2016 despite ongoing concerns of depression and suicide.13
Conclusion
The robust investigation into IL-23 and IL-17 inhibitors to treat plaque psoriasis has yielded promising results, including the unprecedented rates of PASI 100 achievement with these new biologics. Risankizumab, ixekizumab, and brodalumab have demonstrated superior efficacy in trials compared to ustekinumab. Tildrakizumab has shown low disease relapse after drug cessation. Ixekizumab and brodalumab have shown high rates of total disease clearance. Thus far, safety findings for these pipeline biologics have been consistent with those of ustekinumab. With ixekizumab approved in 2016 and brodalumab under review, new options in biologic therapy will offer patients and clinicians greater choices in treating severe and recalcitrant psoriasis.
The role of current biologic therapies in psoriasis predicates on the pathogenic role of upregulated, immune-related mechanisms that result in the activation of myeloid dendritic cells, which release IL-17, IL-23, and other cytokines to activate T cells, including helper T cell TH17. Along with other immune cells, TH17 produces IL-17. This proinflammatory cascade results in keratinocyte proliferation, angiogenesis, and migration of immune cells toward psoriatic lesions.1 Thus, the newest classes of biologics target IL-12, IL-23, and IL-17 to disrupt this inflammatory cascade.
We provide an updated review of the most recent clinical efficacy and safety data on the newest IL-23 and IL-17 inhibitors in the pipeline or approved for psoriasis, including risankizumab, guselkumab, tildrakizumab, ixekizumab, and brodalumab (Table). Ustekinumab and adalimumab, which have been previously approved by the US Food and Drug Administration (FDA), will be discussed here only as comparators.

IL-23 Inhibitors
Risankizumab
Risankizumab (formerly known as BI 655066)(Boehringer Ingelheim) is a selective human monoclonal antibody targeting the p19 subunit of IL-23 and currently is undergoing phase 3 trials for psoriasis. A proof-of-concept phase 1 study of 39 participants demonstrated efficacy after 12 weeks of treatment at varying subcutaneous and intravenous doses with placebo control.11 At week 12, 87% (27/31)(P<.001) of all risankizumab-treated participants achieved 75% reduction in psoriasis area and severity index (PASI) score compared to 0% of 8 placebo-treated participants. Common adverse effects (AEs) occurred in 65% (20/31) of risankizumab-treated participants, including non–dose-dependent upper respiratory tract infections, nasopharyngitis, and headache. Serious adverse events (SAEs) that occurred were considered unrelated to the study medication.11
A phase 2 trial of 166 participants compared 3 dosing regimens of subcutaneous risankizumab (single 18-mg dose at week 0; single 90-mg dose at weeks 0, 4, and 16; or single 180-mg dose at weeks 0, 4, and 16) and ustekinumab (weight-based single 45- or 90-mg dose at weeks 0, 4, and 16), demonstrating noninferiority at higher doses of risankizumab.2 Preliminary primary end point results at week 12 showed PASI 90 in 32.6% (P=.4667), 73.2% (P=.0013), 81.0% (P<.0001), and 40.0% of the treatment groups, respectively. Participants in the 180-mg risankizumab group achieved PASI 90 eight weeks faster than those on ustekinumab, lasting more than 2 months longer. Adverse effects were similar across all treatment groups and SAEs were unrelated to the study medications.2
Guselkumab
Guselkumab (Janssen Biotech, Inc) is a selective human monoclonal antibody against the p19 subunit of IL-23. The 52-week phase 2 X-PLORE trial compared dose-ranging subcutaneous guselkumab (5 mg at weeks 0 and 4, then every 12 weeks; 15 mg every 8 weeks; 50 mg at weeks 0 and 4, then every 12 weeks; 100 mg every 8 weeks; or 200 mg at weeks 0 and 4, then every 12 weeks), adalimumab (80-mg loading dose, followed by 40 mg at week 1, then every other week), and placebo in 293 randomized participants.4 At week 16, 34% (P=.002) of participants in the 5-mg guselkumab group, 61% (P<.001) in the 15-mg group, 79% (P<.001) in the 50-mg group, 86% (P<.001) in the 100-mg group, 83% (P<.001) in the 200-mg group, and 58% (P<.001) in the adalimumab group achieved physician global assessment (PGA) scores of 0 (clear) or 1 (minimal psoriasis) compared to 7% of the placebo group. Achievement of PASI 75 similarly favored the guselkumab (44% [P<.001]; 76% [no P value given]; 81% [P<.001]; 79% [P<.001]; and 81% [P<.001], respectively) and adalimumab treatment arms (70% [P<.001]) compared to 5% in the placebo group. In longer-term comparisons to week 40, participants in the 50-, 100-, and 200-mg guselkumab groups showed significantly greater remission of psoriatic lesions, measured by a PGA score of 0 or 1, than participants in the adalimumab group (71% [P=.05]; 77% [P=.005]; 81% [P=.01]; and 49%, respectively).4
Preliminary results from VOYAGE 1 (N=837), the first of several phase 3 trials, further demonstrate the superiority of guselkumab 100 mg at weeks 0 and 4 and then every 8 weeks over adalimumab (standard dosing) and placebo; at week 16, 73.3% (P<.001 for both comparisons) versus 49.7% and 2.9% of participants, respectively, achieved PASI 90, with sustained superiority of skin clearance in guselkumab-treated participants compared to adalimumab and placebo through week 48.3
Long-term safety data showed no dose dependence or trend from 0 to 16 weeks and 16 to 52 weeks of treatment regarding rates of AEs, SAEs, or serious infections.4 Between weeks 16 and 52, 48.9% of all guselkumab-treated participants exhibited AEs compared to 60.5% of adalimumab-treated participants and 51.3% of placebo participants. Overall infection rates also were lowest in the guselkumab group at 29.8% compared to 36.8% and 35.9%, respectively. Three participants treated with guselkumab had major cardiovascular events, including a fatal myocardial infarction. No cases of tuberculosis or serious opportunistic infections were reported.4
Tildrakizumab
Tildrakizumab (formerly known as MK-3222)(Sun Pharmaceutical Industries Ltd) is a human monoclonal antibody also targeting the p19 subunit of IL-23. In a phase 2 study of 355 participants with chronic plaque psoriasis, participants received 5-, 25-, 100-, or 200-mg subcutaneous tildrakizumab or placebo at weeks 0 and 4 and then every 12 weeks for a total of 52 weeks.6 At week 16, PASI 75 results were 33.3%, 64.4%, 66.3%, 74.4%, and 4.4%, respectively (P<.001 for each comparison). Improvement began within the first month of treatment, with median times to PASI 75 of 57 days at 200-mg dosing and 84 days at 100-mg dosing. Of those participants achieving PASI 75 by drug discontinuation at week 52, 96% of the 100-mg group and 93% of the 200-mg group maintained PASI 75 through week 72, suggesting low relapse rates after treatment cessation.6
In October 2016, the efficacy results of 2 pivotal phase 3 trials (reSURFACE 1 and reSURFACE 2) involving more than 1800 participants combined revealed PASI 90 achievement in an average of 54% of participants on tildrakizumab 100 mg and 59% of participants on tildrakizumab 200 mg at week 28.5 Achievement of PASI 100 occurred in 24% and 30% of participants at week 28, respectively. The second of these trials included an etanercept comparison group and demonstrated head-to-head superiority of 100 and 200 mg subcutaneous tildrakizumab at week 12 by end point measures.5
Treatment-related AEs occurred at rates of 25% in tildrakizumab-treated participants and 22% in placebo-treated participants, most frequently nasopharyngitis and headache.6 At least 1 AE occurred in 64% of tildrakizumab-treated participants without dose dependence compared to 69% of placebo-treated participants. Severe AEs thought to be drug treatment related were bacterial arthritis, lymphedema, melanoma, stroke, and epiglottitis.6
IL-17 Inhibitors
Ixekizumab
Ixekizumab (Eli Lilly and Company), a monoclonal inhibitor of IL-17A, is the most recently approved psoriasis biologic on the market and has been cleared for use in adults with moderate to severe plaque psoriasis. Recommended dosing is 160 mg (given in two 80-mg subcutaneous injections via an autoinjector or prefilled syringe) at week 0, followed by an 80-mg injection at weeks 2, 4, 6, 8, 10, and 12, and then 80 mg every 4 weeks thereafter. The FDA approved ixekizumab in March 2016 following favorable results of several phase 3 trials: UNCOVER-1, UNCOVER-2, and UNCOVER-3.7,8
In UNCOVER-1, 1296 participants were randomized to 1 of 2 ixekizumab treatment arms—160 mg starting dose at week 0, 80 mg every 2 or 4 weeks thereafter—or placebo.7 At week 12, 89.1%, 82.6%, and 3.9% achieved PASI 75, respectively (P<.001 for both). Importantly, high numbers of participants also achieved PASI 90 (70.9% in the 2-week group and 64.6% in the 4-week group vs 0.5% in the placebo group [P<.001]) and PASI 100 (35.3% and 33.6% vs 0%, respectively [P<.001]), suggesting high rates of disease clearance.7
UNCOVER-2 (N=1224) and UNCOVER-3 (N=1346) investigated the same 2 dosing regimens of ixekizumab compared to etanercept 50 mg biweekly and placebo.8 At week 12, the percentage of participants achieving PASI 90 in UNCOVER-2 was 70.7%, 59.7%, 18.7%, and 0.6%, respectively, and 68.1%, 65.3%, 25.7%, and 3.1%, respectively, in UNCOVER-3 (P<.0001 for all comparisons to placebo and etanercept). At week 12, PASI 100 results also showed striking superiority, with 40.5%, 30.8%, 5.3%, and 0.6% of participants, respectively, in UNCOVER-2, and 37.7%, 35%, 7.3%, and 0%, respectively, in UNCOVER-3, achieving complete clearance of disease (P<.0001 for all comparisons to placebo and etanercept). Responses to ixekizumab were observed as early as weeks 1 and 2, while no participants in the etanercept and placebo treatment groups achieved comparative efficiency.8
In an extension of UNCOVER-3, efficacy increased from week 12 to week 60 according to PASI 90 (68%–73% in the 2-week group; 65%–72% in the 4-week group) and PASI 100 measures (38%–55% in the 2-week group; 35%–52% in the 4-week group).7
The most common AEs associated with ixekizumab treatment from weeks 0 to 12 occurred at higher rates in the 2-week and 4-week ixekizumab groups compared to placebo, including nasopharyngitis (9.5% and 9% vs 8.7%, respectively), upper respiratory tract infection (4.4% and 3.9% vs 3.5%, respectively), injection-site reaction (10% and 7.7% vs 1%, respectively), arthralgia (4.4% and 4.3% vs 2.9%, respectively), and headache (2.5% and 1.9% vs 2.1%, respectively). Infections, including candidal, oral, vulvovaginal, and cutaneous, occurred in 27% of the 2-week dosing group and 27.4% of the 4-week dosing group compared to 22.9% of the placebo group during weeks 0 to 12, with candidal infections in particular occurring more frequently in the active treatment groups and exhibiting dose dependence. Other AEs of special interest that occurred among all ixekizumab-treated participants (n=3736) from weeks 0 to 60 were cardiovascular and cerebrovascular events (22 [0.6%]), inflammatory bowel disease (11 [0.3%]), non–skin cancer malignancy (14 [0.4%]), and nonmelanoma skin cancer (20 [0.5%]). Neutropenia occurred at higher rates in ixekizumab-treated participants (9.3% in the 2-week group and 8.6% in the 4-week group) compared to placebo (3.3%) and occurred in 11.5% of all ixekizumab participants over 60 weeks.7
Brodalumab
Brodalumab (Valeant Pharmaceuticals International, Inc) is a human monoclonal antibody targeting the IL-17A receptor currently under review for FDA approval after undergoing phase 3 trials. The first of these trials, AMAGINE-1, showed efficacy of subcutaneous brodalumab (140 or 210 mg administered every 2 weeks with an extra dose at week 1) compared to placebo in 661 participants.9 At week 12, 60%, 83%, and 3%, respectively, achieved PASI 75; 43%, 70%, and 1%, respectively, achieved PASI 90; and 23%, 42%, and 1%, respectively, achieved PASI 100 (P<.001 for all respective comparisons to placebo). These effects were retained through 52 weeks of treatment. The median time to complete disease clearance in participants reaching PASI 100 was 12 weeks. Conversely, participants who were re-randomized to placebo after week 12 of brodalumab treatment relapsed within weeks to months.9
AMAGINE-2 and AMAGINE-3 further demonstrated the efficacy of brodalumab (140 or 210 mg every 2 weeks with extra dose at week 1) compared to ustekinumab (45 or 90 mg weight-based standard dosing) and placebo in 1831 participants, respectively.10 In AMAGINE-2, 49% of participants in the 140-mg group (P<.001 vs placebo), 70% in the 210-mg group (P<.001 vs placebo), 47% in the ustekinumab group, and 3% in the placebo group achieved PASI 90 at week 12. Similarly, in AMAGINE-3, 52% of participants in the 140-mg group (P<.001), 69% in the 210-mg group (P<.001), 48% in the ustekinumab group, and 2% in the placebo group achieved PASI 90. Impressively, complete clearance (PASI 100) at week 12 occurred in 26% of the 140-mg group (P<.001 vs placebo), 44% of the 210-mg group (P<.001 vs placebo), and 22% of the ustekinumab group compared to 2% of the placebo group in AMAGINE-2, with similar rates in AMAGINE-3. Brodalumab was significantly superior to ustekinumab at the 210-mg dose by PASI 90 measures (P<.001) in both studies and at the 140-mg dose by PASI 100 measures (P=.007) in AMAGINE-3 only.10
Common AEs were nasopharyngitis, upper respiratory tract infection, headache, and arthralgia, all occurring at grossly similar rates (49%–60%) across all experimental groups in AMAGINE-1, AMAGINE-2, and AMAGINE-3 during the first 12-week treatment period.9,10 Brodalumab treatment groups had high rates of specific interest AEs compared to ustekinumab and placebo groups, including neutropenia (0.8%, 1.1%, 0.3%, and 0%, respectively) and candidal infections (0.8%, 1.3%, 0.3%, and 0.3%, respectively). Induction phase (weeks 0–12) depression rates were concerning, with 6 cases each in AMAGINE-2 (4 [0.7%] in the 140-mg group, 2 [0.3%] in the 210-mg group) and AMAGINE-3 (4 [0.6%] in the 140-mg group, 2 [0.3%] in the 210-mg group). Cases of neutropenia were mild, were not associated with major infection, and were transient or reversible. Depression rates after 52 weeks of treatment were 1.7% (23/1567) of brodalumab participants in AMAGINE-2 and 1.8% (21/1613) in AMAGINE-3. Three participants, all on constant 210-mg dosing through week 52, attempted suicide with 1 completion10; however, because no other IL-17 inhibitors were associated with depression or suicide in other trials, it has been suggested that these cases were incidental and not treatment related.12 An FDA advisory panel recommended approval of brodalumab in July 2016 despite ongoing concerns of depression and suicide.13
Conclusion
The robust investigation into IL-23 and IL-17 inhibitors to treat plaque psoriasis has yielded promising results, including the unprecedented rates of PASI 100 achievement with these new biologics. Risankizumab, ixekizumab, and brodalumab have demonstrated superior efficacy in trials compared to ustekinumab. Tildrakizumab has shown low disease relapse after drug cessation. Ixekizumab and brodalumab have shown high rates of total disease clearance. Thus far, safety findings for these pipeline biologics have been consistent with those of ustekinumab. With ixekizumab approved in 2016 and brodalumab under review, new options in biologic therapy will offer patients and clinicians greater choices in treating severe and recalcitrant psoriasis.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Papp K, Menter A, Sofen H, et al. Efficacy and safety of different dose regimens of a selective IL-23p19 inhibitor (BI 655066) compared with ustekinumab in patients with moderate-to-severe plaque psoriasis with and without psoriatic arthritis. Paper presented at: 2015 American College of Rheumatology/Association of Rheumatology Health Professionals Annual Meeting; November 6-11, 2015; San Francisco, CA.
- New phase 3 data show significant efficacy versus placebo and superiority of guselkumab versus Humira in treatment of moderate to severe plaque psoriasis [press release]. Vienna, Austria; Janssen Research & Development, LLC: October 1, 2016.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Sun Pharma to announce late-breaking results for investigational IL-23p19 inhibitor, Tildrakizumab, achieves primary end point in both phase-3 studies in patients with moderate-to-severe plaque psoriasis [press release]. Mumbai, India; Sun Pharmaceutical Industries Ltd: October 1, 2016.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 Study Group, UNCOVER-2 Study Group, UNCOVER-3 Study Group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis [published online June 23, 2016]. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial [published online March 1, 2015]. J Allergy Clin Immunol. 2015;136:116-124.e7.
- Chiricozzi A, Romanelli M, Saraceno R, et al. No meaningful association between suicidal behavior and the use of IL-17A-neutralizing or IL-17RA-blocking agents [published online August 31, 2016]. Expert Opin Drug Saf. 2016;15:1653-1659.
- FDA advisory committee recommends approval of brodalumab for treatment of moderate-to-severe plaque psoriasis [news release]. Laval, Quebec: Valeant Pharmaceuticals International, Inc; July 19, 2016.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Papp K, Menter A, Sofen H, et al. Efficacy and safety of different dose regimens of a selective IL-23p19 inhibitor (BI 655066) compared with ustekinumab in patients with moderate-to-severe plaque psoriasis with and without psoriatic arthritis. Paper presented at: 2015 American College of Rheumatology/Association of Rheumatology Health Professionals Annual Meeting; November 6-11, 2015; San Francisco, CA.
- New phase 3 data show significant efficacy versus placebo and superiority of guselkumab versus Humira in treatment of moderate to severe plaque psoriasis [press release]. Vienna, Austria; Janssen Research & Development, LLC: October 1, 2016.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Sun Pharma to announce late-breaking results for investigational IL-23p19 inhibitor, Tildrakizumab, achieves primary end point in both phase-3 studies in patients with moderate-to-severe plaque psoriasis [press release]. Mumbai, India; Sun Pharmaceutical Industries Ltd: October 1, 2016.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 Study Group, UNCOVER-2 Study Group, UNCOVER-3 Study Group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis [published online June 23, 2016]. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial [published online March 1, 2015]. J Allergy Clin Immunol. 2015;136:116-124.e7.
- Chiricozzi A, Romanelli M, Saraceno R, et al. No meaningful association between suicidal behavior and the use of IL-17A-neutralizing or IL-17RA-blocking agents [published online August 31, 2016]. Expert Opin Drug Saf. 2016;15:1653-1659.
- FDA advisory committee recommends approval of brodalumab for treatment of moderate-to-severe plaque psoriasis [news release]. Laval, Quebec: Valeant Pharmaceuticals International, Inc; July 19, 2016.
Practice Points
- The newest biologics for treatment of moderate to severe plaque psoriasis are IL-23 and IL-17 inhibitors with unprecedented efficacy of complete skin clearance compared to older biologics.
- Risankizumab, guselkumab, and tildrakizumab are new IL-23 inhibitors currently in phase 3 trials with promising early efficacy and safety results.
- Ixekizumab, which recently was approved, and brodalumab, which is pending US Food and Drug Administration review, are new IL-17 inhibitors that achieved total skin clearance in more than one-quarter of phase 3 participants after 12 weeks of treatment.
Case series shows benefit of nonthermal atmospheric pressure plasma for actinic keratoses
Nonthermal atmospheric pressure plasma (NTAP) may serve as an effective, well-tolerated treatment for actinic keratoses, according to findings of a small study published in the February issue of Journal of the American Academy of Dermatology.
Dr. Peter C. Friedman and his associates at the Skin Center Dermatology Group, New York, wrote that prior studies suggest that NTAP – applied with a hand-held electrode – could selectively induce apoptosis in cancer cells in vitro, and may also up-regulate local and tumor-specific systemic immune response.
In this study, five patients with 5 biopsy-proven and 12 clinically diagnosed actinic keratoses (AKs) were treated with a single dose of NTAP on the target lesion. The procedure involved nanosecond pulses that were applied to the treatment area for 1-2 minutes, an approach designed to provide sufficient dose while avoiding tissue damage (J Am Acad Dermatol. 2017 Feb. doi: 10.1016/j.jaad.2016.09.004).
At the 1-month follow-up – in which photographs were used to compare before and after treatment – 9 of the 17 AK lesions showed full clinical resolution, with no visible or palpable lesions and only minimal site erythema. Three lesions showed significant improvement, with at least a 50% improvement, and five lesions should minor or no improvement.
Dr. Friedman and his associates noted that current treatment options for AKs all have significant downsides including side effects such as pain and inflammation, and frequent recurrences. There were no adverse effects, such as pain, inflammation, or site reaction, reported during treatment with NTAP or 1 month after treatment.
“Given its very impressive tolerability, NTAP may be an excellent alternative for our current treatment options for AKs, especially if its efficacy and treatment procedure time can be improved and if further, long-term studies demonstrate lasting effects,” the authors wrote. “Further studies are needed to optimize treatment parameters, provide histologic confirmation of treatment effect, and evaluate the long-term benefits of this modality.”
No conflicts of interest were declared.
Nonthermal atmospheric pressure plasma (NTAP) may serve as an effective, well-tolerated treatment for actinic keratoses, according to findings of a small study published in the February issue of Journal of the American Academy of Dermatology.
Dr. Peter C. Friedman and his associates at the Skin Center Dermatology Group, New York, wrote that prior studies suggest that NTAP – applied with a hand-held electrode – could selectively induce apoptosis in cancer cells in vitro, and may also up-regulate local and tumor-specific systemic immune response.
In this study, five patients with 5 biopsy-proven and 12 clinically diagnosed actinic keratoses (AKs) were treated with a single dose of NTAP on the target lesion. The procedure involved nanosecond pulses that were applied to the treatment area for 1-2 minutes, an approach designed to provide sufficient dose while avoiding tissue damage (J Am Acad Dermatol. 2017 Feb. doi: 10.1016/j.jaad.2016.09.004).
At the 1-month follow-up – in which photographs were used to compare before and after treatment – 9 of the 17 AK lesions showed full clinical resolution, with no visible or palpable lesions and only minimal site erythema. Three lesions showed significant improvement, with at least a 50% improvement, and five lesions should minor or no improvement.
Dr. Friedman and his associates noted that current treatment options for AKs all have significant downsides including side effects such as pain and inflammation, and frequent recurrences. There were no adverse effects, such as pain, inflammation, or site reaction, reported during treatment with NTAP or 1 month after treatment.
“Given its very impressive tolerability, NTAP may be an excellent alternative for our current treatment options for AKs, especially if its efficacy and treatment procedure time can be improved and if further, long-term studies demonstrate lasting effects,” the authors wrote. “Further studies are needed to optimize treatment parameters, provide histologic confirmation of treatment effect, and evaluate the long-term benefits of this modality.”
No conflicts of interest were declared.
Nonthermal atmospheric pressure plasma (NTAP) may serve as an effective, well-tolerated treatment for actinic keratoses, according to findings of a small study published in the February issue of Journal of the American Academy of Dermatology.
Dr. Peter C. Friedman and his associates at the Skin Center Dermatology Group, New York, wrote that prior studies suggest that NTAP – applied with a hand-held electrode – could selectively induce apoptosis in cancer cells in vitro, and may also up-regulate local and tumor-specific systemic immune response.
In this study, five patients with 5 biopsy-proven and 12 clinically diagnosed actinic keratoses (AKs) were treated with a single dose of NTAP on the target lesion. The procedure involved nanosecond pulses that were applied to the treatment area for 1-2 minutes, an approach designed to provide sufficient dose while avoiding tissue damage (J Am Acad Dermatol. 2017 Feb. doi: 10.1016/j.jaad.2016.09.004).
At the 1-month follow-up – in which photographs were used to compare before and after treatment – 9 of the 17 AK lesions showed full clinical resolution, with no visible or palpable lesions and only minimal site erythema. Three lesions showed significant improvement, with at least a 50% improvement, and five lesions should minor or no improvement.
Dr. Friedman and his associates noted that current treatment options for AKs all have significant downsides including side effects such as pain and inflammation, and frequent recurrences. There were no adverse effects, such as pain, inflammation, or site reaction, reported during treatment with NTAP or 1 month after treatment.
“Given its very impressive tolerability, NTAP may be an excellent alternative for our current treatment options for AKs, especially if its efficacy and treatment procedure time can be improved and if further, long-term studies demonstrate lasting effects,” the authors wrote. “Further studies are needed to optimize treatment parameters, provide histologic confirmation of treatment effect, and evaluate the long-term benefits of this modality.”
No conflicts of interest were declared.
FROM JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Nonthermal atmospheric pressure plasma may be an effective, well-tolerated treatment for actinic keratoses.
Major finding: One month after a single treatment with NTAP, 9 of 17 AK lesions showed full clinical resolution and 3 lesions showed significant improvement.
Data source: Case series in five patients with actinic keratoses.
Disclosures: No conflicts of interest were declared.
New Zika-infected pregnancies down for most of United States
New cases of Zika infection in pregnant women were down again for the 50 states over the 2-week reporting period ending Jan. 24, but U.S. territories saw a big increase, according to the Centers for Disease Control and Prevention.
It is important to note, however, that Puerto Rico, where most U.S. Zika cases are occurring, has been retroactively reporting cases for months, which can result in larger-than-normal increases.
The territories had 186 new cases of pregnant women with laboratory evidence of Zika virus infection for the 2 weeks ending Jan. 24, compared with 43 for the previous 2-week period. The corresponding numbers for the 50 states and the District of Columbia were 47 (Jan. 24) and 55 (Jan. 10), the CDC data show.
So far for 2016-2017, there have been 4,465 pregnant women reported to have Zika virus infection in the United States: 3,071 in the territories and 1,394 in the 50 states and D.C., according to the U.S. Zika Pregnancy Registry (states, territories, and the District of Columbia) and the U.S. Zika Active Pregnancy Surveillance System (Puerto Rico).
Of the nearly 1,400 state/D.C. Zika-infected pregnancies, 999 have been completed, with 38 infants born with birth defects and 5 defect-related pregnancy losses, the CDC said. There have been no Zika-related pregnancy losses reported in the states/D.C. since late June. The CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same inclusion criteria.
Among all Americans in 2015-2017, there have been 41,387 cases of Zika infection as of Feb. 1. Of that total, 35,334 cases, which is more than 85%, have occurred in Puerto Rico, according to data from the CDC’s Arboviral Disease Branch.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
New cases of Zika infection in pregnant women were down again for the 50 states over the 2-week reporting period ending Jan. 24, but U.S. territories saw a big increase, according to the Centers for Disease Control and Prevention.
It is important to note, however, that Puerto Rico, where most U.S. Zika cases are occurring, has been retroactively reporting cases for months, which can result in larger-than-normal increases.
The territories had 186 new cases of pregnant women with laboratory evidence of Zika virus infection for the 2 weeks ending Jan. 24, compared with 43 for the previous 2-week period. The corresponding numbers for the 50 states and the District of Columbia were 47 (Jan. 24) and 55 (Jan. 10), the CDC data show.
So far for 2016-2017, there have been 4,465 pregnant women reported to have Zika virus infection in the United States: 3,071 in the territories and 1,394 in the 50 states and D.C., according to the U.S. Zika Pregnancy Registry (states, territories, and the District of Columbia) and the U.S. Zika Active Pregnancy Surveillance System (Puerto Rico).
Of the nearly 1,400 state/D.C. Zika-infected pregnancies, 999 have been completed, with 38 infants born with birth defects and 5 defect-related pregnancy losses, the CDC said. There have been no Zika-related pregnancy losses reported in the states/D.C. since late June. The CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same inclusion criteria.
Among all Americans in 2015-2017, there have been 41,387 cases of Zika infection as of Feb. 1. Of that total, 35,334 cases, which is more than 85%, have occurred in Puerto Rico, according to data from the CDC’s Arboviral Disease Branch.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
New cases of Zika infection in pregnant women were down again for the 50 states over the 2-week reporting period ending Jan. 24, but U.S. territories saw a big increase, according to the Centers for Disease Control and Prevention.
It is important to note, however, that Puerto Rico, where most U.S. Zika cases are occurring, has been retroactively reporting cases for months, which can result in larger-than-normal increases.
The territories had 186 new cases of pregnant women with laboratory evidence of Zika virus infection for the 2 weeks ending Jan. 24, compared with 43 for the previous 2-week period. The corresponding numbers for the 50 states and the District of Columbia were 47 (Jan. 24) and 55 (Jan. 10), the CDC data show.
So far for 2016-2017, there have been 4,465 pregnant women reported to have Zika virus infection in the United States: 3,071 in the territories and 1,394 in the 50 states and D.C., according to the U.S. Zika Pregnancy Registry (states, territories, and the District of Columbia) and the U.S. Zika Active Pregnancy Surveillance System (Puerto Rico).
Of the nearly 1,400 state/D.C. Zika-infected pregnancies, 999 have been completed, with 38 infants born with birth defects and 5 defect-related pregnancy losses, the CDC said. There have been no Zika-related pregnancy losses reported in the states/D.C. since late June. The CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same inclusion criteria.
Among all Americans in 2015-2017, there have been 41,387 cases of Zika infection as of Feb. 1. Of that total, 35,334 cases, which is more than 85%, have occurred in Puerto Rico, according to data from the CDC’s Arboviral Disease Branch.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
VIDEO: Pediatric psoriasis patients prepare for biologics
WAILEA, Hawaii – The first approval of a biologic for pediatric psoriasis, and ongoing clinical trials of other biologics in children with psoriasis, are among the encouraging therapeutic developments for this patient population, Wynnis Tom, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“We are incredibly excited ... as pediatric dermatologists that we’re finally seeing breakthroughs” in terms of Food and Drug Administration activity regarding the use of biologics for treating psoriasis in children, Dr. Tom said in a video interview at the seminar.
Etanercept (Enbrel) is now approved for children with psoriasis, the first biologic indicated for pediatric psoriasis, and clinical trials of other biologics that have been available for adults and nonbiologic products for pediatric psoriasis are underway, she said.
However, getting insurance coverage can still be a challenge, although having long-term efficacy and safety data helps, noted Dr. Tom of the department of dermatology and pediatrics, University of California, San Diego, and Rady Children’s Hospital, San Diego. Also helpful is sending letters to insurers on behalf of the patient, describing the patient’s quality of life, descriptions of treatments that have been unsuccessful, and even photos documenting the disease in the child, she added.
Dr. Tom disclosed ties with Promius, Celgene, and Janssen.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, Hawaii – The first approval of a biologic for pediatric psoriasis, and ongoing clinical trials of other biologics in children with psoriasis, are among the encouraging therapeutic developments for this patient population, Wynnis Tom, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“We are incredibly excited ... as pediatric dermatologists that we’re finally seeing breakthroughs” in terms of Food and Drug Administration activity regarding the use of biologics for treating psoriasis in children, Dr. Tom said in a video interview at the seminar.
Etanercept (Enbrel) is now approved for children with psoriasis, the first biologic indicated for pediatric psoriasis, and clinical trials of other biologics that have been available for adults and nonbiologic products for pediatric psoriasis are underway, she said.
However, getting insurance coverage can still be a challenge, although having long-term efficacy and safety data helps, noted Dr. Tom of the department of dermatology and pediatrics, University of California, San Diego, and Rady Children’s Hospital, San Diego. Also helpful is sending letters to insurers on behalf of the patient, describing the patient’s quality of life, descriptions of treatments that have been unsuccessful, and even photos documenting the disease in the child, she added.
Dr. Tom disclosed ties with Promius, Celgene, and Janssen.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, Hawaii – The first approval of a biologic for pediatric psoriasis, and ongoing clinical trials of other biologics in children with psoriasis, are among the encouraging therapeutic developments for this patient population, Wynnis Tom, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“We are incredibly excited ... as pediatric dermatologists that we’re finally seeing breakthroughs” in terms of Food and Drug Administration activity regarding the use of biologics for treating psoriasis in children, Dr. Tom said in a video interview at the seminar.
Etanercept (Enbrel) is now approved for children with psoriasis, the first biologic indicated for pediatric psoriasis, and clinical trials of other biologics that have been available for adults and nonbiologic products for pediatric psoriasis are underway, she said.
However, getting insurance coverage can still be a challenge, although having long-term efficacy and safety data helps, noted Dr. Tom of the department of dermatology and pediatrics, University of California, San Diego, and Rady Children’s Hospital, San Diego. Also helpful is sending letters to insurers on behalf of the patient, describing the patient’s quality of life, descriptions of treatments that have been unsuccessful, and even photos documenting the disease in the child, she added.
Dr. Tom disclosed ties with Promius, Celgene, and Janssen.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR