User login
Bortezomib-based regimen led to durable remissions in mantle cell lymphoma
SAN DIEGO – For adults with mantle cell lymphoma, adding bortezomib to a modified hyper-CVAD (VcR-CVAD) regimen followed by rituximab maintenance induced durable remissions at rates resembling those seen with more intensive chemotherapy followed by autologous hematopoietic stem cell transplantation, according to long-term results from a multicenter phase II trial.
Two-thirds of patients were alive and 50% remained in remission after a median follow-up period of 7.8 years, said Julie E. Chang, MD, who reported the results of the study at the annual meeting of the American Society of Hematology. “VcR-CVAD is a moderate-intensity chemotherapy regimen that is tolerable for many older and less fit adult patients as first-line therapy of mantle cell lymphoma,” she emphasized.
Mantle cell lymphoma lacks a clear standard first-line therapy, noted Dr. Chang of the University of Wisconsin in Madison. “We hypothesized that the addition of bortezomib would improve the complete response rate, and maintenance rituximab would improve the remission duration,” she said.
To test that idea, she and her associates enrolled 30 adults with histologically confirmed mantle cell lymphoma who had received either no treatment or just one cycle of CHOP or CHOP-like chemotherapy.
Patients received six 21-day cycles of VcR-CVAD induction chemotherapy. This regimen consisted of rituximab (375 mg/m2 IV) on day 1; bortezomib (1.3 mg/m2 IV) on days 1 and 4; cyclophosphamide (300 mg/m2 IV every 12 hours) on days 1 through 3; doxorubicin (50 mg/m2 IV given as a continuous infusion) on days 1 and 2; vincristine (1 mg IV) on day 3; and dexamethasone (40 mg orally) on days 1 through 4.
Patients were permitted all supportive care measures, including prophylaxis for tumor lysis syndrome, transfusions, and antibiotics. Those with at least a partial response received rituximab consolidation (375 mg/m2 IV per week for 4 weeks) followed by rituximab maintenance (375 mg/m2 IV every 12 weeks for 5 years).
Median age was 61 years (range, 48-74 years), 80% of patients were male, all had advanced-stage disease, 60% were mantle cell lymphoma international prognostic index (MIPI) medium or high risk, and six had blastic morphology, the researchers noted.
Estimated 6-year rates of progression-free and overall survival were 53% (95% confidence interval, 38%-75%) and 70% (95% CI, 55%-84%), respectively. Neither age nor MIPI score significantly affected the chances of progression-free or overall survival, but there was a trend toward worse survival among MIPI high-risk patients.
The 10 deaths included 5 from progressive disease, 3 from complications after allogeneic transplant, and 2 from unrelated causes. No patients who remained progression free for 5 years subsequently relapsed, nor were there late toxicities related to treatment.
A recent phase III trial (N Engl J Med. 2015 Mar 5;372[10]:944-53) confirmed the benefits of adding bortezomib to standard immunochemotherapy in mantle cell lymphoma, Dr. Chang noted. “VcR-CVAD remains an effective therapy choice for initial treatment of MCL, both in younger and older MCL populations,” she concluded.
Dr. Chang had no relevant disclosures. Senior author Brad S. Kahl, MD, disclosed ties to Celgene, Gilead, Infinity, Juno, Pharmacyclics, and Seattle Genetics.
SAN DIEGO – For adults with mantle cell lymphoma, adding bortezomib to a modified hyper-CVAD (VcR-CVAD) regimen followed by rituximab maintenance induced durable remissions at rates resembling those seen with more intensive chemotherapy followed by autologous hematopoietic stem cell transplantation, according to long-term results from a multicenter phase II trial.
Two-thirds of patients were alive and 50% remained in remission after a median follow-up period of 7.8 years, said Julie E. Chang, MD, who reported the results of the study at the annual meeting of the American Society of Hematology. “VcR-CVAD is a moderate-intensity chemotherapy regimen that is tolerable for many older and less fit adult patients as first-line therapy of mantle cell lymphoma,” she emphasized.
Mantle cell lymphoma lacks a clear standard first-line therapy, noted Dr. Chang of the University of Wisconsin in Madison. “We hypothesized that the addition of bortezomib would improve the complete response rate, and maintenance rituximab would improve the remission duration,” she said.
To test that idea, she and her associates enrolled 30 adults with histologically confirmed mantle cell lymphoma who had received either no treatment or just one cycle of CHOP or CHOP-like chemotherapy.
Patients received six 21-day cycles of VcR-CVAD induction chemotherapy. This regimen consisted of rituximab (375 mg/m2 IV) on day 1; bortezomib (1.3 mg/m2 IV) on days 1 and 4; cyclophosphamide (300 mg/m2 IV every 12 hours) on days 1 through 3; doxorubicin (50 mg/m2 IV given as a continuous infusion) on days 1 and 2; vincristine (1 mg IV) on day 3; and dexamethasone (40 mg orally) on days 1 through 4.
Patients were permitted all supportive care measures, including prophylaxis for tumor lysis syndrome, transfusions, and antibiotics. Those with at least a partial response received rituximab consolidation (375 mg/m2 IV per week for 4 weeks) followed by rituximab maintenance (375 mg/m2 IV every 12 weeks for 5 years).
Median age was 61 years (range, 48-74 years), 80% of patients were male, all had advanced-stage disease, 60% were mantle cell lymphoma international prognostic index (MIPI) medium or high risk, and six had blastic morphology, the researchers noted.
Estimated 6-year rates of progression-free and overall survival were 53% (95% confidence interval, 38%-75%) and 70% (95% CI, 55%-84%), respectively. Neither age nor MIPI score significantly affected the chances of progression-free or overall survival, but there was a trend toward worse survival among MIPI high-risk patients.
The 10 deaths included 5 from progressive disease, 3 from complications after allogeneic transplant, and 2 from unrelated causes. No patients who remained progression free for 5 years subsequently relapsed, nor were there late toxicities related to treatment.
A recent phase III trial (N Engl J Med. 2015 Mar 5;372[10]:944-53) confirmed the benefits of adding bortezomib to standard immunochemotherapy in mantle cell lymphoma, Dr. Chang noted. “VcR-CVAD remains an effective therapy choice for initial treatment of MCL, both in younger and older MCL populations,” she concluded.
Dr. Chang had no relevant disclosures. Senior author Brad S. Kahl, MD, disclosed ties to Celgene, Gilead, Infinity, Juno, Pharmacyclics, and Seattle Genetics.
SAN DIEGO – For adults with mantle cell lymphoma, adding bortezomib to a modified hyper-CVAD (VcR-CVAD) regimen followed by rituximab maintenance induced durable remissions at rates resembling those seen with more intensive chemotherapy followed by autologous hematopoietic stem cell transplantation, according to long-term results from a multicenter phase II trial.
Two-thirds of patients were alive and 50% remained in remission after a median follow-up period of 7.8 years, said Julie E. Chang, MD, who reported the results of the study at the annual meeting of the American Society of Hematology. “VcR-CVAD is a moderate-intensity chemotherapy regimen that is tolerable for many older and less fit adult patients as first-line therapy of mantle cell lymphoma,” she emphasized.
Mantle cell lymphoma lacks a clear standard first-line therapy, noted Dr. Chang of the University of Wisconsin in Madison. “We hypothesized that the addition of bortezomib would improve the complete response rate, and maintenance rituximab would improve the remission duration,” she said.
To test that idea, she and her associates enrolled 30 adults with histologically confirmed mantle cell lymphoma who had received either no treatment or just one cycle of CHOP or CHOP-like chemotherapy.
Patients received six 21-day cycles of VcR-CVAD induction chemotherapy. This regimen consisted of rituximab (375 mg/m2 IV) on day 1; bortezomib (1.3 mg/m2 IV) on days 1 and 4; cyclophosphamide (300 mg/m2 IV every 12 hours) on days 1 through 3; doxorubicin (50 mg/m2 IV given as a continuous infusion) on days 1 and 2; vincristine (1 mg IV) on day 3; and dexamethasone (40 mg orally) on days 1 through 4.
Patients were permitted all supportive care measures, including prophylaxis for tumor lysis syndrome, transfusions, and antibiotics. Those with at least a partial response received rituximab consolidation (375 mg/m2 IV per week for 4 weeks) followed by rituximab maintenance (375 mg/m2 IV every 12 weeks for 5 years).
Median age was 61 years (range, 48-74 years), 80% of patients were male, all had advanced-stage disease, 60% were mantle cell lymphoma international prognostic index (MIPI) medium or high risk, and six had blastic morphology, the researchers noted.
Estimated 6-year rates of progression-free and overall survival were 53% (95% confidence interval, 38%-75%) and 70% (95% CI, 55%-84%), respectively. Neither age nor MIPI score significantly affected the chances of progression-free or overall survival, but there was a trend toward worse survival among MIPI high-risk patients.
The 10 deaths included 5 from progressive disease, 3 from complications after allogeneic transplant, and 2 from unrelated causes. No patients who remained progression free for 5 years subsequently relapsed, nor were there late toxicities related to treatment.
A recent phase III trial (N Engl J Med. 2015 Mar 5;372[10]:944-53) confirmed the benefits of adding bortezomib to standard immunochemotherapy in mantle cell lymphoma, Dr. Chang noted. “VcR-CVAD remains an effective therapy choice for initial treatment of MCL, both in younger and older MCL populations,” she concluded.
Dr. Chang had no relevant disclosures. Senior author Brad S. Kahl, MD, disclosed ties to Celgene, Gilead, Infinity, Juno, Pharmacyclics, and Seattle Genetics.
Key clinical point: First-line therapy with bortezomib plus modified hyper-CVAD followed by rituximab maintenance induced durable remissions in mantle cell lymphoma.
Major finding: Two-thirds of patients were alive and 50% remained in remission after a median follow-up period of 7.8 years.
Data source: A multicenter phase II trial of 30 adults with mantle cell lymphoma who were treatment-naïve or had received only one cycle of CHOP or CHOP-like chemotherapy.
Disclosures: Dr. Chang had no relevant disclosures. Senior author Brad S. Kahl, MD, disclosed ties to Celgene, Gilead, Infinity, Juno, Pharmacyclics, and Seattle Genetics.
Model: Quadrivalent vaccine could cost effectively cut MSM’s HPV-related cancers
Use of a quadrivalent HPV vaccine in men who have sex with men, administered in genitourinary specialty clinics, would cost effectively provide herd immunity against the ill effects of the virus, particularly anogenital warts and male HPV-related cancers, according to an English mathematical model.
Mark Jit, PhD, a professor of vaccine epidemiology at the London School of Hygiene & Tropical Medicine, and his colleagues considered in which settings HPV vaccination delivery would have the greatest effect size; the patterns of sexual behavior in MSM leading to the transmission of HPV 6, 11, 16, and 18; and the costs and quality adjusted life year (QALY) implications of disease outcomes (Clin Infect Dis. 2016 Dec 23. doi: 10.1093/cid/ciw845).
Clinic attendance rates were based on genitourinary clinic returns in England recorded in public health surveillance data recorded between 2009 and 2012, stratified according to diagnosed HIV-positive status. Also modeled were the effects of vaccination in MSM between the ages of 16 and 40 years, according to groups aged 16-25 years, 16-30 years, 16-35 years, and 16-40 years. Models for ages on either side of 16 or 40 years were not considered because of confidentiality constraints in the former and limited specialty clinic use in the latter.
Herd protection likely would be notable in the first year, because of the breadth of the age ranges modeled, Dr. Jit and his colleagues determined. Specifically, the models predicted a 35% decline in incidence rates of anogenital warts within 5 years of initiating the vaccine across all MSM men seen in specialty clinics. If only HIV-positive MSM across the age groups were vaccinated, the models predicted a 5-year decline of 15%.
Declines predicted in HPV-related cancers would happen more slowly, because progression from infection to malignancies tends to occur over years. For example, there would be a 55% reduction over 100 years for anal cancer if all 16- to 40-year-old MSM attending specialty clinics are offered vaccination. However, the reduction rate would drop to 40% in that same time period if only HIV-positive men across the age groups were vaccinated.
Using a cost-effectiveness threshold of 20,000 British pounds (about $24,500) per QALY, with no more than a 10% probability that the incremental cost-effectiveness ratio would exceed 30,000 pounds per QALY ($36,710), Dr. Jit and his colleagues determined that using a quadrivalent HPV vaccination in MSM between ages 16 and 40 years in the specialty clinic setting would be cost effective if delivery cost an average of 63 pounds ($77) per dose.
By offering vaccination only to HIV-positive MSM between 16 and 40 years, even if the quadrivalent vaccine were to cost as much as 96.50 pounds ($118), it would still be cost effective. A nonavalent vaccine at the same price would also be cost effective, because nearly all HPV-related cancers are linked to HPV 16 and 18. However, a bivalent vaccine was not shown by the models to be cost effective in such a limited program.
The investigators theorized that HIV infection is associated with the rate of HPV-related disease progression. To simplify computation, however, their models only considered the overall cost effectiveness of offering HPV vaccination to either HIV-positive MSM or to MSM regardless of current HIV status. That could mean “the cost effectiveness of MSM vaccination may be even better than reported,” the researchers noted.
This study was funded primarily by the National Institute for Health Research and Public Health England in the United Kingdom. The authors had no relevant disclosures.
[email protected]On Twitter @whitneymcknight
Use of a quadrivalent HPV vaccine in men who have sex with men, administered in genitourinary specialty clinics, would cost effectively provide herd immunity against the ill effects of the virus, particularly anogenital warts and male HPV-related cancers, according to an English mathematical model.
Mark Jit, PhD, a professor of vaccine epidemiology at the London School of Hygiene & Tropical Medicine, and his colleagues considered in which settings HPV vaccination delivery would have the greatest effect size; the patterns of sexual behavior in MSM leading to the transmission of HPV 6, 11, 16, and 18; and the costs and quality adjusted life year (QALY) implications of disease outcomes (Clin Infect Dis. 2016 Dec 23. doi: 10.1093/cid/ciw845).
Clinic attendance rates were based on genitourinary clinic returns in England recorded in public health surveillance data recorded between 2009 and 2012, stratified according to diagnosed HIV-positive status. Also modeled were the effects of vaccination in MSM between the ages of 16 and 40 years, according to groups aged 16-25 years, 16-30 years, 16-35 years, and 16-40 years. Models for ages on either side of 16 or 40 years were not considered because of confidentiality constraints in the former and limited specialty clinic use in the latter.
Herd protection likely would be notable in the first year, because of the breadth of the age ranges modeled, Dr. Jit and his colleagues determined. Specifically, the models predicted a 35% decline in incidence rates of anogenital warts within 5 years of initiating the vaccine across all MSM men seen in specialty clinics. If only HIV-positive MSM across the age groups were vaccinated, the models predicted a 5-year decline of 15%.
Declines predicted in HPV-related cancers would happen more slowly, because progression from infection to malignancies tends to occur over years. For example, there would be a 55% reduction over 100 years for anal cancer if all 16- to 40-year-old MSM attending specialty clinics are offered vaccination. However, the reduction rate would drop to 40% in that same time period if only HIV-positive men across the age groups were vaccinated.
Using a cost-effectiveness threshold of 20,000 British pounds (about $24,500) per QALY, with no more than a 10% probability that the incremental cost-effectiveness ratio would exceed 30,000 pounds per QALY ($36,710), Dr. Jit and his colleagues determined that using a quadrivalent HPV vaccination in MSM between ages 16 and 40 years in the specialty clinic setting would be cost effective if delivery cost an average of 63 pounds ($77) per dose.
By offering vaccination only to HIV-positive MSM between 16 and 40 years, even if the quadrivalent vaccine were to cost as much as 96.50 pounds ($118), it would still be cost effective. A nonavalent vaccine at the same price would also be cost effective, because nearly all HPV-related cancers are linked to HPV 16 and 18. However, a bivalent vaccine was not shown by the models to be cost effective in such a limited program.
The investigators theorized that HIV infection is associated with the rate of HPV-related disease progression. To simplify computation, however, their models only considered the overall cost effectiveness of offering HPV vaccination to either HIV-positive MSM or to MSM regardless of current HIV status. That could mean “the cost effectiveness of MSM vaccination may be even better than reported,” the researchers noted.
This study was funded primarily by the National Institute for Health Research and Public Health England in the United Kingdom. The authors had no relevant disclosures.
[email protected]On Twitter @whitneymcknight
Use of a quadrivalent HPV vaccine in men who have sex with men, administered in genitourinary specialty clinics, would cost effectively provide herd immunity against the ill effects of the virus, particularly anogenital warts and male HPV-related cancers, according to an English mathematical model.
Mark Jit, PhD, a professor of vaccine epidemiology at the London School of Hygiene & Tropical Medicine, and his colleagues considered in which settings HPV vaccination delivery would have the greatest effect size; the patterns of sexual behavior in MSM leading to the transmission of HPV 6, 11, 16, and 18; and the costs and quality adjusted life year (QALY) implications of disease outcomes (Clin Infect Dis. 2016 Dec 23. doi: 10.1093/cid/ciw845).
Clinic attendance rates were based on genitourinary clinic returns in England recorded in public health surveillance data recorded between 2009 and 2012, stratified according to diagnosed HIV-positive status. Also modeled were the effects of vaccination in MSM between the ages of 16 and 40 years, according to groups aged 16-25 years, 16-30 years, 16-35 years, and 16-40 years. Models for ages on either side of 16 or 40 years were not considered because of confidentiality constraints in the former and limited specialty clinic use in the latter.
Herd protection likely would be notable in the first year, because of the breadth of the age ranges modeled, Dr. Jit and his colleagues determined. Specifically, the models predicted a 35% decline in incidence rates of anogenital warts within 5 years of initiating the vaccine across all MSM men seen in specialty clinics. If only HIV-positive MSM across the age groups were vaccinated, the models predicted a 5-year decline of 15%.
Declines predicted in HPV-related cancers would happen more slowly, because progression from infection to malignancies tends to occur over years. For example, there would be a 55% reduction over 100 years for anal cancer if all 16- to 40-year-old MSM attending specialty clinics are offered vaccination. However, the reduction rate would drop to 40% in that same time period if only HIV-positive men across the age groups were vaccinated.
Using a cost-effectiveness threshold of 20,000 British pounds (about $24,500) per QALY, with no more than a 10% probability that the incremental cost-effectiveness ratio would exceed 30,000 pounds per QALY ($36,710), Dr. Jit and his colleagues determined that using a quadrivalent HPV vaccination in MSM between ages 16 and 40 years in the specialty clinic setting would be cost effective if delivery cost an average of 63 pounds ($77) per dose.
By offering vaccination only to HIV-positive MSM between 16 and 40 years, even if the quadrivalent vaccine were to cost as much as 96.50 pounds ($118), it would still be cost effective. A nonavalent vaccine at the same price would also be cost effective, because nearly all HPV-related cancers are linked to HPV 16 and 18. However, a bivalent vaccine was not shown by the models to be cost effective in such a limited program.
The investigators theorized that HIV infection is associated with the rate of HPV-related disease progression. To simplify computation, however, their models only considered the overall cost effectiveness of offering HPV vaccination to either HIV-positive MSM or to MSM regardless of current HIV status. That could mean “the cost effectiveness of MSM vaccination may be even better than reported,” the researchers noted.
This study was funded primarily by the National Institute for Health Research and Public Health England in the United Kingdom. The authors had no relevant disclosures.
[email protected]On Twitter @whitneymcknight
Key clinical point:
Major finding: Substantial declines in HPV-related events in MSM were projected within 5 years of vaccination between ages 16 and 40 years in this cohort.
Data source: Mathematical modeling of HPV 6, 11, 16, and 18 sexual transmission in the MSM population of England.
Disclosures: This study was funded primarily by the National Institute for Health Research and Public Health England in the United Kingdom. The authors had no relevant disclosures.
Everything We Say and Do: Use familiar terminology to allay patients’ fears
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM ’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each article will focus on how the contributor applies one or more of the “key communication” tactics in practice to maintain provider accountability for “everything we say and do that affects our patients’ thoughts, feelings, and well-being.”
What I say and do
I clearly explain diagnoses and treatment plans in plain terms.
Why I do it
We hear repeatedly from patients and families that a major source of their fear comes from “not knowing.” Fear of the unknown. If our patients and their families do not understand the message we are trying to communicate, these fears will be realized. It is our responsibility to explain their medical situation(s) to them in plain terms that they can comprehend, so as to allay those fears and enable them to become active, informed participants in their care.
How I do it
I start by reminding myself that I want to treat each patient as I would want a member of my own family to be treated. No one else in my family is in the medical field, so this means I must avoid medical terminology and use more familiar, everyday phrases. For example, I say “heart doctor” or “lung doctor” instead of “cardiologist” or “pulmonologist.” I also prefer “sonogram” to “ultrasound” because most people have heard that term in relation to a pregnancy. Even “EEG” and “EKG” need more plain descriptions.
I also try to use common, relatable analogies when explaining diseases. My favorite is to describe COPD (or any restrictive lung disease) like an old, hard sponge as compared with normal lungs, which are like a new, soft sponge.
I use the Teach-Back Method (which has already been well-discussed in this column by Dr. Trina Dorrah) to check for comprehension. If there are still issues with my message not being received as I had hoped, then I try again to find the terminology or an analogy that will connect with that patient.
Hopefully, using familiar, relatable language in this manner gives my patients and their families a better understanding of their diagnoses and care plans, quells their fears, and enhances their experience.
Dr. Sharp is a chief hospitalist with Sound Physicians at UF Health in Jacksonville, Fla., and a member of SHM's Patient Experience Committee.
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM ’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each article will focus on how the contributor applies one or more of the “key communication” tactics in practice to maintain provider accountability for “everything we say and do that affects our patients’ thoughts, feelings, and well-being.”
What I say and do
I clearly explain diagnoses and treatment plans in plain terms.
Why I do it
We hear repeatedly from patients and families that a major source of their fear comes from “not knowing.” Fear of the unknown. If our patients and their families do not understand the message we are trying to communicate, these fears will be realized. It is our responsibility to explain their medical situation(s) to them in plain terms that they can comprehend, so as to allay those fears and enable them to become active, informed participants in their care.
How I do it
I start by reminding myself that I want to treat each patient as I would want a member of my own family to be treated. No one else in my family is in the medical field, so this means I must avoid medical terminology and use more familiar, everyday phrases. For example, I say “heart doctor” or “lung doctor” instead of “cardiologist” or “pulmonologist.” I also prefer “sonogram” to “ultrasound” because most people have heard that term in relation to a pregnancy. Even “EEG” and “EKG” need more plain descriptions.
I also try to use common, relatable analogies when explaining diseases. My favorite is to describe COPD (or any restrictive lung disease) like an old, hard sponge as compared with normal lungs, which are like a new, soft sponge.
I use the Teach-Back Method (which has already been well-discussed in this column by Dr. Trina Dorrah) to check for comprehension. If there are still issues with my message not being received as I had hoped, then I try again to find the terminology or an analogy that will connect with that patient.
Hopefully, using familiar, relatable language in this manner gives my patients and their families a better understanding of their diagnoses and care plans, quells their fears, and enhances their experience.
Dr. Sharp is a chief hospitalist with Sound Physicians at UF Health in Jacksonville, Fla., and a member of SHM's Patient Experience Committee.
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM ’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each article will focus on how the contributor applies one or more of the “key communication” tactics in practice to maintain provider accountability for “everything we say and do that affects our patients’ thoughts, feelings, and well-being.”
What I say and do
I clearly explain diagnoses and treatment plans in plain terms.
Why I do it
We hear repeatedly from patients and families that a major source of their fear comes from “not knowing.” Fear of the unknown. If our patients and their families do not understand the message we are trying to communicate, these fears will be realized. It is our responsibility to explain their medical situation(s) to them in plain terms that they can comprehend, so as to allay those fears and enable them to become active, informed participants in their care.
How I do it
I start by reminding myself that I want to treat each patient as I would want a member of my own family to be treated. No one else in my family is in the medical field, so this means I must avoid medical terminology and use more familiar, everyday phrases. For example, I say “heart doctor” or “lung doctor” instead of “cardiologist” or “pulmonologist.” I also prefer “sonogram” to “ultrasound” because most people have heard that term in relation to a pregnancy. Even “EEG” and “EKG” need more plain descriptions.
I also try to use common, relatable analogies when explaining diseases. My favorite is to describe COPD (or any restrictive lung disease) like an old, hard sponge as compared with normal lungs, which are like a new, soft sponge.
I use the Teach-Back Method (which has already been well-discussed in this column by Dr. Trina Dorrah) to check for comprehension. If there are still issues with my message not being received as I had hoped, then I try again to find the terminology or an analogy that will connect with that patient.
Hopefully, using familiar, relatable language in this manner gives my patients and their families a better understanding of their diagnoses and care plans, quells their fears, and enhances their experience.
Dr. Sharp is a chief hospitalist with Sound Physicians at UF Health in Jacksonville, Fla., and a member of SHM's Patient Experience Committee.
Cabozantinib shows promise for carcinoid tumors, pNET
SAN FRANCISCO – Treatment with cabozantinib was associated with objective tumor responses and encouraging progression-free survival in patients with advanced carcinoid and pancreatic neuroendocrine tumors in a two-cohort phase II trial.
Of 41 patients in the carcinoid tumor cohort, 6 achieved RECIST-defined partial response (objective response rate, 15%), and 26 had stable disease. Median progression-free survival was 31.4 months.
Of 20 patients in the pancreatic neuroendocrine tumors (pNET) cohort, 3 achieved a partial response (objective response rate, 15%), and 15 had stable disease. Median progression-free survival was 21.8 months, Jennifer A. Chan, MD, reported at the symposium, sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology.
Although dose reduction was common – occurring in 81% of 53 patients who completed at least one treatment cycle – treatment was tolerable, said Dr. Chan of Dana-Farber Cancer Institute, Boston.
Study patients had progressive, well-differentiated grade 1-2 carcinoid tumors or pNET, and were treated with 60 mg of oral cabozantinib daily. There were no limits on prior therapy, and patients were restaged every 2 months for the first 6 months, then every 3 months thereafter.
The 41 patients with carcinoid tumors had a median age of 63 years and ECOG performance status of 0 or 1. Only 20 patients were enrolled in the pNET group, because accrual was halted, in part because of funding considerations. Patients in that group had a median age of 55 years, and also had ECOG performance status of 0 or 1, Dr. Chan said.
Carcinoid tumor patients completed a median of 8 28-day treatment cycles (range, 0-44), and pNET patients completed a median of 10 cycles (range, 0-25).
The most common reasons for discontinuation were progression or death in 51% of patients, withdrawal of consent or investigator decision in 28%, and adverse events in 21%, she noted.
The most common grade 3/4 toxicities included hypertension in 13% of patients, hypophosphatemia in 11%, and diarrhea in 10%.
Unexpected grade 3/4 events included heart failure and autoimmune hemolytic anemia, which each occurred in 1 patient.
This phase II study was initiated in light of promising preclinical work, Dr. Chan said.
“There has been much progress in recent years in the treatment of advanced neuroendocrine tumors,” she said. “The VEGF pathway inhibitors have been demonstrated to show activity, and the tyrosine kinase inhibitor sunitinib is approved for patients with progressive pancreatic neuroendocrine tumors.”
Recent studies also suggest that inhibition of MET may be an effective treatment strategy. MET activation has been shown to be associated with tumor growth, expression of MET has been observed in a significant proportion of neuroendocrine tumors, and increased expression of MET has been associated with decreased overall survival in pancreatic neuroendocrine tumors, she noted.
“Cabozantinib is a tyrosine kinase inhibitor that targets multiple receptors, including the VEGF receptors MET, ASL, and RET,” Dr. Chan explained. “It improves progression-free survival in advanced renal cell carcinoma in the first-line setting, compared with sunitinib, and also in the second-line setting, compared with everolimus in patients previously treated with anti-angiogenic therapy.” Cabozantinib is also approved for use in patients with progressive, metastatic medullary thyroid carcinoma, she added.
In preclinical models of neuroendocrine tumors, cabozantinib inhibited cell viability, and in a mouse model it decreased metastasis and invasion of pNET.
The current findings provide further evidence of cabozantinib’s safety and efficacy, Dr. Chan said. The progression-free survival in this phase II study is of particularly interest in the context of historical results, she added.
“Recognizing the limitations of interpreting progression-free survival data in an uncontrolled phase II trial, as well as comparing data to previous clinical trials, the progression-free survival results that we observed do appear encouraging,” she said. “It will be important to confirm the activity of cabozantinib in a randomized phase III setting.”
Dr. Chan reported having stock or other ownership interests in Merck; having a consulting or advisory role with Bayer, Ipsen, Novartis, Pfizer, and Pozen; and receiving research funding from Novartis and Sanofi.
SAN FRANCISCO – Treatment with cabozantinib was associated with objective tumor responses and encouraging progression-free survival in patients with advanced carcinoid and pancreatic neuroendocrine tumors in a two-cohort phase II trial.
Of 41 patients in the carcinoid tumor cohort, 6 achieved RECIST-defined partial response (objective response rate, 15%), and 26 had stable disease. Median progression-free survival was 31.4 months.
Of 20 patients in the pancreatic neuroendocrine tumors (pNET) cohort, 3 achieved a partial response (objective response rate, 15%), and 15 had stable disease. Median progression-free survival was 21.8 months, Jennifer A. Chan, MD, reported at the symposium, sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology.
Although dose reduction was common – occurring in 81% of 53 patients who completed at least one treatment cycle – treatment was tolerable, said Dr. Chan of Dana-Farber Cancer Institute, Boston.
Study patients had progressive, well-differentiated grade 1-2 carcinoid tumors or pNET, and were treated with 60 mg of oral cabozantinib daily. There were no limits on prior therapy, and patients were restaged every 2 months for the first 6 months, then every 3 months thereafter.
The 41 patients with carcinoid tumors had a median age of 63 years and ECOG performance status of 0 or 1. Only 20 patients were enrolled in the pNET group, because accrual was halted, in part because of funding considerations. Patients in that group had a median age of 55 years, and also had ECOG performance status of 0 or 1, Dr. Chan said.
Carcinoid tumor patients completed a median of 8 28-day treatment cycles (range, 0-44), and pNET patients completed a median of 10 cycles (range, 0-25).
The most common reasons for discontinuation were progression or death in 51% of patients, withdrawal of consent or investigator decision in 28%, and adverse events in 21%, she noted.
The most common grade 3/4 toxicities included hypertension in 13% of patients, hypophosphatemia in 11%, and diarrhea in 10%.
Unexpected grade 3/4 events included heart failure and autoimmune hemolytic anemia, which each occurred in 1 patient.
This phase II study was initiated in light of promising preclinical work, Dr. Chan said.
“There has been much progress in recent years in the treatment of advanced neuroendocrine tumors,” she said. “The VEGF pathway inhibitors have been demonstrated to show activity, and the tyrosine kinase inhibitor sunitinib is approved for patients with progressive pancreatic neuroendocrine tumors.”
Recent studies also suggest that inhibition of MET may be an effective treatment strategy. MET activation has been shown to be associated with tumor growth, expression of MET has been observed in a significant proportion of neuroendocrine tumors, and increased expression of MET has been associated with decreased overall survival in pancreatic neuroendocrine tumors, she noted.
“Cabozantinib is a tyrosine kinase inhibitor that targets multiple receptors, including the VEGF receptors MET, ASL, and RET,” Dr. Chan explained. “It improves progression-free survival in advanced renal cell carcinoma in the first-line setting, compared with sunitinib, and also in the second-line setting, compared with everolimus in patients previously treated with anti-angiogenic therapy.” Cabozantinib is also approved for use in patients with progressive, metastatic medullary thyroid carcinoma, she added.
In preclinical models of neuroendocrine tumors, cabozantinib inhibited cell viability, and in a mouse model it decreased metastasis and invasion of pNET.
The current findings provide further evidence of cabozantinib’s safety and efficacy, Dr. Chan said. The progression-free survival in this phase II study is of particularly interest in the context of historical results, she added.
“Recognizing the limitations of interpreting progression-free survival data in an uncontrolled phase II trial, as well as comparing data to previous clinical trials, the progression-free survival results that we observed do appear encouraging,” she said. “It will be important to confirm the activity of cabozantinib in a randomized phase III setting.”
Dr. Chan reported having stock or other ownership interests in Merck; having a consulting or advisory role with Bayer, Ipsen, Novartis, Pfizer, and Pozen; and receiving research funding from Novartis and Sanofi.
SAN FRANCISCO – Treatment with cabozantinib was associated with objective tumor responses and encouraging progression-free survival in patients with advanced carcinoid and pancreatic neuroendocrine tumors in a two-cohort phase II trial.
Of 41 patients in the carcinoid tumor cohort, 6 achieved RECIST-defined partial response (objective response rate, 15%), and 26 had stable disease. Median progression-free survival was 31.4 months.
Of 20 patients in the pancreatic neuroendocrine tumors (pNET) cohort, 3 achieved a partial response (objective response rate, 15%), and 15 had stable disease. Median progression-free survival was 21.8 months, Jennifer A. Chan, MD, reported at the symposium, sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology.
Although dose reduction was common – occurring in 81% of 53 patients who completed at least one treatment cycle – treatment was tolerable, said Dr. Chan of Dana-Farber Cancer Institute, Boston.
Study patients had progressive, well-differentiated grade 1-2 carcinoid tumors or pNET, and were treated with 60 mg of oral cabozantinib daily. There were no limits on prior therapy, and patients were restaged every 2 months for the first 6 months, then every 3 months thereafter.
The 41 patients with carcinoid tumors had a median age of 63 years and ECOG performance status of 0 or 1. Only 20 patients were enrolled in the pNET group, because accrual was halted, in part because of funding considerations. Patients in that group had a median age of 55 years, and also had ECOG performance status of 0 or 1, Dr. Chan said.
Carcinoid tumor patients completed a median of 8 28-day treatment cycles (range, 0-44), and pNET patients completed a median of 10 cycles (range, 0-25).
The most common reasons for discontinuation were progression or death in 51% of patients, withdrawal of consent or investigator decision in 28%, and adverse events in 21%, she noted.
The most common grade 3/4 toxicities included hypertension in 13% of patients, hypophosphatemia in 11%, and diarrhea in 10%.
Unexpected grade 3/4 events included heart failure and autoimmune hemolytic anemia, which each occurred in 1 patient.
This phase II study was initiated in light of promising preclinical work, Dr. Chan said.
“There has been much progress in recent years in the treatment of advanced neuroendocrine tumors,” she said. “The VEGF pathway inhibitors have been demonstrated to show activity, and the tyrosine kinase inhibitor sunitinib is approved for patients with progressive pancreatic neuroendocrine tumors.”
Recent studies also suggest that inhibition of MET may be an effective treatment strategy. MET activation has been shown to be associated with tumor growth, expression of MET has been observed in a significant proportion of neuroendocrine tumors, and increased expression of MET has been associated with decreased overall survival in pancreatic neuroendocrine tumors, she noted.
“Cabozantinib is a tyrosine kinase inhibitor that targets multiple receptors, including the VEGF receptors MET, ASL, and RET,” Dr. Chan explained. “It improves progression-free survival in advanced renal cell carcinoma in the first-line setting, compared with sunitinib, and also in the second-line setting, compared with everolimus in patients previously treated with anti-angiogenic therapy.” Cabozantinib is also approved for use in patients with progressive, metastatic medullary thyroid carcinoma, she added.
In preclinical models of neuroendocrine tumors, cabozantinib inhibited cell viability, and in a mouse model it decreased metastasis and invasion of pNET.
The current findings provide further evidence of cabozantinib’s safety and efficacy, Dr. Chan said. The progression-free survival in this phase II study is of particularly interest in the context of historical results, she added.
“Recognizing the limitations of interpreting progression-free survival data in an uncontrolled phase II trial, as well as comparing data to previous clinical trials, the progression-free survival results that we observed do appear encouraging,” she said. “It will be important to confirm the activity of cabozantinib in a randomized phase III setting.”
Dr. Chan reported having stock or other ownership interests in Merck; having a consulting or advisory role with Bayer, Ipsen, Novartis, Pfizer, and Pozen; and receiving research funding from Novartis and Sanofi.
Key clinical point:
Major finding: The objective response rate was 15% in both groups; median progression-free survival was 31.4 and 21.8 months in the carcinoid and pNET groups, respectively.
Data source: A phase II trial involving 61 patients.
Disclosures: Dr. Chan reported having stock or other ownership interests in Merck; having a consulting or advisory role with Bayer, Ipsen, Novartis, Pfizer, and Pozen; and receiving research funding from Novartis and Sanofi.
In ICU, pair MRSA testing method with isolation protocol
An ICU’s method of testing for methicillin-resistant Staphylococcus aureus (MRSA) should be paired with its patient isolation policy, according to researchers at the University of Colorado at Denver.
In an ICU with all patients preemptively isolated, it is worth the added expense to opt for the polymerase chain reaction (PCR) test – which generates results in a few hours – so that patients negative for the infection can be moved out of isolation more quickly, wrote Melanie D. Whittington, PhD, and her coauthors. But if the ICU is only isolating MRSA-positive patients, the authors instead recommend the less expensive but slower chromogenic agar 24-hour testing.
The other two MRSA tests the researchers assessed – conventional culture and chromogenic agar 48-hour testing – are less expensive. But when paired with either ICU isolation policy, those tests lead to excessive inappropriate isolation costs while waiting for the results, the study investigators cautioned (Am J Infect Control. 2017 Jan 23. doi: 10.1016/j.ajic.2016.12.014).
Adding together the cost per patient of the test, the “appropriate isolation costs,” and “inappropriate isolation costs,” the universal isolation policy is least expensive per patient with PCR, at $82.51 per patient. With conventional culture, which can take several days, this cost ballooned to $290.11 per patient, with high inappropriate isolation costs.
Doing the same math with the more targeted isolation policy, the least expensive screening method was the 24-hour chromogenic agar, at $8.54 per patient, while the expense of the PCR test made it the most expensive method when paired with this isolation policy, at $30.95 per patient.
“With knowledge of the screening test that minimizes inappropriate and total costs, hospitals can maximize the efficiency of their resource use and improve the health of their patients,” Dr. Whittington and her coauthors wrote.
The authors reported no conflicts of interest.
An ICU’s method of testing for methicillin-resistant Staphylococcus aureus (MRSA) should be paired with its patient isolation policy, according to researchers at the University of Colorado at Denver.
In an ICU with all patients preemptively isolated, it is worth the added expense to opt for the polymerase chain reaction (PCR) test – which generates results in a few hours – so that patients negative for the infection can be moved out of isolation more quickly, wrote Melanie D. Whittington, PhD, and her coauthors. But if the ICU is only isolating MRSA-positive patients, the authors instead recommend the less expensive but slower chromogenic agar 24-hour testing.
The other two MRSA tests the researchers assessed – conventional culture and chromogenic agar 48-hour testing – are less expensive. But when paired with either ICU isolation policy, those tests lead to excessive inappropriate isolation costs while waiting for the results, the study investigators cautioned (Am J Infect Control. 2017 Jan 23. doi: 10.1016/j.ajic.2016.12.014).
Adding together the cost per patient of the test, the “appropriate isolation costs,” and “inappropriate isolation costs,” the universal isolation policy is least expensive per patient with PCR, at $82.51 per patient. With conventional culture, which can take several days, this cost ballooned to $290.11 per patient, with high inappropriate isolation costs.
Doing the same math with the more targeted isolation policy, the least expensive screening method was the 24-hour chromogenic agar, at $8.54 per patient, while the expense of the PCR test made it the most expensive method when paired with this isolation policy, at $30.95 per patient.
“With knowledge of the screening test that minimizes inappropriate and total costs, hospitals can maximize the efficiency of their resource use and improve the health of their patients,” Dr. Whittington and her coauthors wrote.
The authors reported no conflicts of interest.
An ICU’s method of testing for methicillin-resistant Staphylococcus aureus (MRSA) should be paired with its patient isolation policy, according to researchers at the University of Colorado at Denver.
In an ICU with all patients preemptively isolated, it is worth the added expense to opt for the polymerase chain reaction (PCR) test – which generates results in a few hours – so that patients negative for the infection can be moved out of isolation more quickly, wrote Melanie D. Whittington, PhD, and her coauthors. But if the ICU is only isolating MRSA-positive patients, the authors instead recommend the less expensive but slower chromogenic agar 24-hour testing.
The other two MRSA tests the researchers assessed – conventional culture and chromogenic agar 48-hour testing – are less expensive. But when paired with either ICU isolation policy, those tests lead to excessive inappropriate isolation costs while waiting for the results, the study investigators cautioned (Am J Infect Control. 2017 Jan 23. doi: 10.1016/j.ajic.2016.12.014).
Adding together the cost per patient of the test, the “appropriate isolation costs,” and “inappropriate isolation costs,” the universal isolation policy is least expensive per patient with PCR, at $82.51 per patient. With conventional culture, which can take several days, this cost ballooned to $290.11 per patient, with high inappropriate isolation costs.
Doing the same math with the more targeted isolation policy, the least expensive screening method was the 24-hour chromogenic agar, at $8.54 per patient, while the expense of the PCR test made it the most expensive method when paired with this isolation policy, at $30.95 per patient.
“With knowledge of the screening test that minimizes inappropriate and total costs, hospitals can maximize the efficiency of their resource use and improve the health of their patients,” Dr. Whittington and her coauthors wrote.
The authors reported no conflicts of interest.
Capping gestational weight gain didn’t deliver better pregnancy outcomes
LAS VEGAS – Two similar behavioral interventions during pregnancy both succeeded in capping gestational weight gain in two independent randomized trials, but neither intervention produced improvements in obstetrical outcomes.
Results from several prior studies linked excess gestational weight gain (GWG) with adverse outcomes, including gestational diabetes, hypertension, macrosomia, and cesarean delivery. But none of the rates of these complications fell among women in the study groups that received intervention and had reduced GWG, compared with controls.
“The clinical significance of the difference in GWG we saw is not known,” said Alison G. Cahill, MD, who presented one of the two reports at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine. In the study she led, women who received a behavioral intervention averaged about 3.5 pounds less GWG through 36 weeks of pregnancy.
“Our findings call into question the association between GWG and adverse pregnancy outcomes,” said Alan M. Peaceman, MD, who presented the second study, in which women receiving the behavioral intervention averaged 4 pounds less in GWG, compared with control women.
Dr. Peaceman reported results from the Maternal Offspring Metabolics: Family Intervention Trial (MOMFIT), a trial run at Northwestern University in Chicago that randomized 263 pregnant women. The women had to be at less than 16 weeks singleton gestation with a body mass index of 25-40 kg/m2, no pregestational diabetes, and a first trimester weight gain of no more than 15 pounds.
The researchers randomized participants to receive either an intervention that included an individualized diet, Internet-based self monitoring of diet adherence, recommendations on physical activity, and weekly coaching calls and opportunities for group meetings, webinars and podcasts; or a control regimen of electronic newsletters and website access that dispensed pregnancy information without mentioning diet. The participants averaged 33 years old, their average body mass index was 31 kg/m2, and about 55% were obese, with a body mass index of 30 kg/m2 or greater.
The study’s primary outcome was weight gain from enrollment through 36 weeks of gestation, which averaged 19.1 pounds among women who received the intervention and 23.7 pounds among controls, an average 4.6 pounds difference that was statistically significant, Dr. Peaceman reported.
The percentage of patients exceeding the GWG recommendations made in 2009 by the Institute of Medicine (IOM) was 68% in the intervention group and 86% among the controls, an 18 percentage-point difference that was statistically significant.
Despite these differences, the two groups showed very similar rates for the incidence of gestational diabetes, preeclampsia or hypertension, birth weight above 4,000 g, and gestational age at delivery (39 weeks on average for both subgroups).
The rate of cesarean delivery (40%) was higher in the women who received the intervention and had less GWG, compared with 27% among the control women. Despite meeting the statistical test for significance, it is most likely a chance result, said Dr. Peaceman, chief of maternal fetal medicine at Northwestern.
He stressed that while no benefit from reduced GWG has yet been found in the MOMFIT results, additional endpoints are under study, such as neonatal metabolism, infant metabolism at 1 year, and maternal weight retention.
Dr. Cahill reported very similar findings from her study, run as part of the Weight Management in Obese Pregnant Underserved African American Women (LIFE-Moms) trial. She enrolled 267 socioeconomically disadvantaged African American women with singleton, normal-anatomy pregnancies who presented for prenatal care at her clinic at less than 16 weeks gestation and were overweight or obese.
The study randomized these women to receive either an exercise and lifestyle intervention along with home visits from the Parents as Teachers program, or just home visits without the exercise and lifestyle component. The enrolled women averaged about 25 years old, and their average body mass index was about 32 kg/m2, with two-thirds of patients being obese.
The study’s primary endpoint, the percentage of women who exceeded the IOM’s 2009 recommendations on GWG, was 37% among women who received the exercise and lifestyle intervention and 46% among those who did not, a difference that was not statistically significant in the full intention-to-treat analysis, Dr. Cahill reported.
A subgroup analysis showed that most of the benefit focused in obese participants, where 34% of women who received the extra intervention had a GWG greater than the IOM recommendation, compared with a 49% rate among controls, a 15 percentage-point difference that fell just short of statistical significance.
For the secondary endpoint of average amount of GWG, women in the intervention arm had a 8.05 kg average, compared with 9.64 kg among the controls, an average GWG reduction of 1.59 kg (3.5 pounds) in the intervention arm. This difference was statistically significant, said Dr. Cahill, chief of maternal fetal medicine at Washington University in St. Louis.
Dr. Cahill also ran a modified intention-to-treat analysis that excluded women with missing GWG data at term, those with fetal death or miscarriage, and one women mistakenly enrolled who was of normal weight. Among the remaining 240 women, the impact of the exercise and lifestyle intervention was even more pronounced, resulting in an average reduction in GWG of 4 pounds and a 12 percentage-point reduction in women exceeding the IOM’s GWG recommendations.
Despite these favorable effects on GWG, the two study arms showed no significant differences in the incidence of gestational diabetes, gestational hypertension, preterm births, or cesarean delivery.
Dr. Cahill said that she too planned to look at additional outcomes that might be affected by controlling GWG, including maternal weight retention and neurodevelopment in the children.
[email protected] On Twitter @mitchelzoler
LAS VEGAS – Two similar behavioral interventions during pregnancy both succeeded in capping gestational weight gain in two independent randomized trials, but neither intervention produced improvements in obstetrical outcomes.
Results from several prior studies linked excess gestational weight gain (GWG) with adverse outcomes, including gestational diabetes, hypertension, macrosomia, and cesarean delivery. But none of the rates of these complications fell among women in the study groups that received intervention and had reduced GWG, compared with controls.
“The clinical significance of the difference in GWG we saw is not known,” said Alison G. Cahill, MD, who presented one of the two reports at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine. In the study she led, women who received a behavioral intervention averaged about 3.5 pounds less GWG through 36 weeks of pregnancy.
“Our findings call into question the association between GWG and adverse pregnancy outcomes,” said Alan M. Peaceman, MD, who presented the second study, in which women receiving the behavioral intervention averaged 4 pounds less in GWG, compared with control women.
Dr. Peaceman reported results from the Maternal Offspring Metabolics: Family Intervention Trial (MOMFIT), a trial run at Northwestern University in Chicago that randomized 263 pregnant women. The women had to be at less than 16 weeks singleton gestation with a body mass index of 25-40 kg/m2, no pregestational diabetes, and a first trimester weight gain of no more than 15 pounds.
The researchers randomized participants to receive either an intervention that included an individualized diet, Internet-based self monitoring of diet adherence, recommendations on physical activity, and weekly coaching calls and opportunities for group meetings, webinars and podcasts; or a control regimen of electronic newsletters and website access that dispensed pregnancy information without mentioning diet. The participants averaged 33 years old, their average body mass index was 31 kg/m2, and about 55% were obese, with a body mass index of 30 kg/m2 or greater.
The study’s primary outcome was weight gain from enrollment through 36 weeks of gestation, which averaged 19.1 pounds among women who received the intervention and 23.7 pounds among controls, an average 4.6 pounds difference that was statistically significant, Dr. Peaceman reported.
The percentage of patients exceeding the GWG recommendations made in 2009 by the Institute of Medicine (IOM) was 68% in the intervention group and 86% among the controls, an 18 percentage-point difference that was statistically significant.
Despite these differences, the two groups showed very similar rates for the incidence of gestational diabetes, preeclampsia or hypertension, birth weight above 4,000 g, and gestational age at delivery (39 weeks on average for both subgroups).
The rate of cesarean delivery (40%) was higher in the women who received the intervention and had less GWG, compared with 27% among the control women. Despite meeting the statistical test for significance, it is most likely a chance result, said Dr. Peaceman, chief of maternal fetal medicine at Northwestern.
He stressed that while no benefit from reduced GWG has yet been found in the MOMFIT results, additional endpoints are under study, such as neonatal metabolism, infant metabolism at 1 year, and maternal weight retention.
Dr. Cahill reported very similar findings from her study, run as part of the Weight Management in Obese Pregnant Underserved African American Women (LIFE-Moms) trial. She enrolled 267 socioeconomically disadvantaged African American women with singleton, normal-anatomy pregnancies who presented for prenatal care at her clinic at less than 16 weeks gestation and were overweight or obese.
The study randomized these women to receive either an exercise and lifestyle intervention along with home visits from the Parents as Teachers program, or just home visits without the exercise and lifestyle component. The enrolled women averaged about 25 years old, and their average body mass index was about 32 kg/m2, with two-thirds of patients being obese.
The study’s primary endpoint, the percentage of women who exceeded the IOM’s 2009 recommendations on GWG, was 37% among women who received the exercise and lifestyle intervention and 46% among those who did not, a difference that was not statistically significant in the full intention-to-treat analysis, Dr. Cahill reported.
A subgroup analysis showed that most of the benefit focused in obese participants, where 34% of women who received the extra intervention had a GWG greater than the IOM recommendation, compared with a 49% rate among controls, a 15 percentage-point difference that fell just short of statistical significance.
For the secondary endpoint of average amount of GWG, women in the intervention arm had a 8.05 kg average, compared with 9.64 kg among the controls, an average GWG reduction of 1.59 kg (3.5 pounds) in the intervention arm. This difference was statistically significant, said Dr. Cahill, chief of maternal fetal medicine at Washington University in St. Louis.
Dr. Cahill also ran a modified intention-to-treat analysis that excluded women with missing GWG data at term, those with fetal death or miscarriage, and one women mistakenly enrolled who was of normal weight. Among the remaining 240 women, the impact of the exercise and lifestyle intervention was even more pronounced, resulting in an average reduction in GWG of 4 pounds and a 12 percentage-point reduction in women exceeding the IOM’s GWG recommendations.
Despite these favorable effects on GWG, the two study arms showed no significant differences in the incidence of gestational diabetes, gestational hypertension, preterm births, or cesarean delivery.
Dr. Cahill said that she too planned to look at additional outcomes that might be affected by controlling GWG, including maternal weight retention and neurodevelopment in the children.
[email protected] On Twitter @mitchelzoler
LAS VEGAS – Two similar behavioral interventions during pregnancy both succeeded in capping gestational weight gain in two independent randomized trials, but neither intervention produced improvements in obstetrical outcomes.
Results from several prior studies linked excess gestational weight gain (GWG) with adverse outcomes, including gestational diabetes, hypertension, macrosomia, and cesarean delivery. But none of the rates of these complications fell among women in the study groups that received intervention and had reduced GWG, compared with controls.
“The clinical significance of the difference in GWG we saw is not known,” said Alison G. Cahill, MD, who presented one of the two reports at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine. In the study she led, women who received a behavioral intervention averaged about 3.5 pounds less GWG through 36 weeks of pregnancy.
“Our findings call into question the association between GWG and adverse pregnancy outcomes,” said Alan M. Peaceman, MD, who presented the second study, in which women receiving the behavioral intervention averaged 4 pounds less in GWG, compared with control women.
Dr. Peaceman reported results from the Maternal Offspring Metabolics: Family Intervention Trial (MOMFIT), a trial run at Northwestern University in Chicago that randomized 263 pregnant women. The women had to be at less than 16 weeks singleton gestation with a body mass index of 25-40 kg/m2, no pregestational diabetes, and a first trimester weight gain of no more than 15 pounds.
The researchers randomized participants to receive either an intervention that included an individualized diet, Internet-based self monitoring of diet adherence, recommendations on physical activity, and weekly coaching calls and opportunities for group meetings, webinars and podcasts; or a control regimen of electronic newsletters and website access that dispensed pregnancy information without mentioning diet. The participants averaged 33 years old, their average body mass index was 31 kg/m2, and about 55% were obese, with a body mass index of 30 kg/m2 or greater.
The study’s primary outcome was weight gain from enrollment through 36 weeks of gestation, which averaged 19.1 pounds among women who received the intervention and 23.7 pounds among controls, an average 4.6 pounds difference that was statistically significant, Dr. Peaceman reported.
The percentage of patients exceeding the GWG recommendations made in 2009 by the Institute of Medicine (IOM) was 68% in the intervention group and 86% among the controls, an 18 percentage-point difference that was statistically significant.
Despite these differences, the two groups showed very similar rates for the incidence of gestational diabetes, preeclampsia or hypertension, birth weight above 4,000 g, and gestational age at delivery (39 weeks on average for both subgroups).
The rate of cesarean delivery (40%) was higher in the women who received the intervention and had less GWG, compared with 27% among the control women. Despite meeting the statistical test for significance, it is most likely a chance result, said Dr. Peaceman, chief of maternal fetal medicine at Northwestern.
He stressed that while no benefit from reduced GWG has yet been found in the MOMFIT results, additional endpoints are under study, such as neonatal metabolism, infant metabolism at 1 year, and maternal weight retention.
Dr. Cahill reported very similar findings from her study, run as part of the Weight Management in Obese Pregnant Underserved African American Women (LIFE-Moms) trial. She enrolled 267 socioeconomically disadvantaged African American women with singleton, normal-anatomy pregnancies who presented for prenatal care at her clinic at less than 16 weeks gestation and were overweight or obese.
The study randomized these women to receive either an exercise and lifestyle intervention along with home visits from the Parents as Teachers program, or just home visits without the exercise and lifestyle component. The enrolled women averaged about 25 years old, and their average body mass index was about 32 kg/m2, with two-thirds of patients being obese.
The study’s primary endpoint, the percentage of women who exceeded the IOM’s 2009 recommendations on GWG, was 37% among women who received the exercise and lifestyle intervention and 46% among those who did not, a difference that was not statistically significant in the full intention-to-treat analysis, Dr. Cahill reported.
A subgroup analysis showed that most of the benefit focused in obese participants, where 34% of women who received the extra intervention had a GWG greater than the IOM recommendation, compared with a 49% rate among controls, a 15 percentage-point difference that fell just short of statistical significance.
For the secondary endpoint of average amount of GWG, women in the intervention arm had a 8.05 kg average, compared with 9.64 kg among the controls, an average GWG reduction of 1.59 kg (3.5 pounds) in the intervention arm. This difference was statistically significant, said Dr. Cahill, chief of maternal fetal medicine at Washington University in St. Louis.
Dr. Cahill also ran a modified intention-to-treat analysis that excluded women with missing GWG data at term, those with fetal death or miscarriage, and one women mistakenly enrolled who was of normal weight. Among the remaining 240 women, the impact of the exercise and lifestyle intervention was even more pronounced, resulting in an average reduction in GWG of 4 pounds and a 12 percentage-point reduction in women exceeding the IOM’s GWG recommendations.
Despite these favorable effects on GWG, the two study arms showed no significant differences in the incidence of gestational diabetes, gestational hypertension, preterm births, or cesarean delivery.
Dr. Cahill said that she too planned to look at additional outcomes that might be affected by controlling GWG, including maternal weight retention and neurodevelopment in the children.
[email protected] On Twitter @mitchelzoler
Key clinical point:
Major finding: Behavioral interventions linked with average reductions in gestational weight gain of 4.6 pounds in MOMFIT and 3.5 pounds in LIFE-Moms.
Data source: MOMFIT and LIFE-Moms, two single-center randomized trials with 263 and 267 mothers, respectively.
Disclosures: Neither trial had commercial support. Dr. Peaceman and Dr. Cahill had no relevant disclosures.
After TAVR, 1 in 10 Medicare patients need permanent pacemaker
HOUSTON – About 1 in 10 Medicare patients require implantation of a permanent pacemaker following transcatheter aortic valve replacement, results from a large analysis showed.
“There is conflicting evidence and some debate over permanent pacemaker placement following transcatheter aortic valve replacement – whether it has a protective or adverse effect, and how often it takes place,” study investigator Fenton H. McCarthy, MD, said in an interview at the annual meeting of the Society of Thoracic Surgeons.
One recent study found that permanent pacemaker placement within 30 days post TAVR was found in 6.7% of patients undergoing balloon-expanding or self-expanding valve implantation, and is associated with increased mortality and hospitalizations (JACC Cardiovasc Interv. 2016 Nov 14;9[21]:2189-2199).
To evaluate the relationship between permanent pacemaker implantation and long-term patient outcomes among Medicare beneficiaries undergoing TAVR, Dr. McCarthy, a cardiothoracic surgery fellow at the University of Pennsylvania, Philadelphia, and his associates used Medicare carrier claims and Medicare Provider Analysis and Review files to identify 14,305 TAVR patients between January 2011 and December 2013.
The researchers used univariate Kaplan survival estimates and multivariable models to analyze survival, readmission and risk factors for pacemaker implantation.
The mean age of the 14,305 TAVR patients studied was 83 years, and 11% received a permanent pacemaker after TAVR. Of these, 9% received the pacemaker at index hospitalization, 1% at 30 days after implant, 0.5% at 90 days after implant, and 1% at 1 year after implant. Patient age of greater than 90 years was a significant predictor of pacemaker placement, with an odds ratio of 1.7 (P less than .01).
Dr. McCarthy and his associates observed that the readmission rates for pacemaker placement and no pacemaker placement at index hospitalization were similar at 30 days (21% vs. 19%, respectively), at 90 days (33% vs. 31%) and at 1 year (43% in both groups of patients).
In addition, Kaplan Meier estimates revealed no significant difference in long-term survival for patients with pacemaker placement within 30 days of TAVR, while multivariate Cox proportional hazard modeling revealed that pacemaker placement is not a predictor of long-term mortality (hazard ratio, 1.03; P = .65).
“This was the largest study to evaluate the question of incidence and effect of permanent pacemaker in the transcatheter aortic valve replacement population in the United States,” Dr. McCarthy said. “The size of our data set and the fact that the Medicare database includes all types of patients, regardless of trial participation, study or registry, is a strength of this study. Some other studies have used different inclusion and exclusion criteria. We used broad inclusion criteria and evaluated patients from as many different centers as possible.”
A key limitation of the study, he said, was that the researchers were unable to determine whether a patient received a balloon-expanding or self-expanding TAVR.
Dr. McCarthy reported having no financial disclosures.
HOUSTON – About 1 in 10 Medicare patients require implantation of a permanent pacemaker following transcatheter aortic valve replacement, results from a large analysis showed.
“There is conflicting evidence and some debate over permanent pacemaker placement following transcatheter aortic valve replacement – whether it has a protective or adverse effect, and how often it takes place,” study investigator Fenton H. McCarthy, MD, said in an interview at the annual meeting of the Society of Thoracic Surgeons.
One recent study found that permanent pacemaker placement within 30 days post TAVR was found in 6.7% of patients undergoing balloon-expanding or self-expanding valve implantation, and is associated with increased mortality and hospitalizations (JACC Cardiovasc Interv. 2016 Nov 14;9[21]:2189-2199).
To evaluate the relationship between permanent pacemaker implantation and long-term patient outcomes among Medicare beneficiaries undergoing TAVR, Dr. McCarthy, a cardiothoracic surgery fellow at the University of Pennsylvania, Philadelphia, and his associates used Medicare carrier claims and Medicare Provider Analysis and Review files to identify 14,305 TAVR patients between January 2011 and December 2013.
The researchers used univariate Kaplan survival estimates and multivariable models to analyze survival, readmission and risk factors for pacemaker implantation.
The mean age of the 14,305 TAVR patients studied was 83 years, and 11% received a permanent pacemaker after TAVR. Of these, 9% received the pacemaker at index hospitalization, 1% at 30 days after implant, 0.5% at 90 days after implant, and 1% at 1 year after implant. Patient age of greater than 90 years was a significant predictor of pacemaker placement, with an odds ratio of 1.7 (P less than .01).
Dr. McCarthy and his associates observed that the readmission rates for pacemaker placement and no pacemaker placement at index hospitalization were similar at 30 days (21% vs. 19%, respectively), at 90 days (33% vs. 31%) and at 1 year (43% in both groups of patients).
In addition, Kaplan Meier estimates revealed no significant difference in long-term survival for patients with pacemaker placement within 30 days of TAVR, while multivariate Cox proportional hazard modeling revealed that pacemaker placement is not a predictor of long-term mortality (hazard ratio, 1.03; P = .65).
“This was the largest study to evaluate the question of incidence and effect of permanent pacemaker in the transcatheter aortic valve replacement population in the United States,” Dr. McCarthy said. “The size of our data set and the fact that the Medicare database includes all types of patients, regardless of trial participation, study or registry, is a strength of this study. Some other studies have used different inclusion and exclusion criteria. We used broad inclusion criteria and evaluated patients from as many different centers as possible.”
A key limitation of the study, he said, was that the researchers were unable to determine whether a patient received a balloon-expanding or self-expanding TAVR.
Dr. McCarthy reported having no financial disclosures.
HOUSTON – About 1 in 10 Medicare patients require implantation of a permanent pacemaker following transcatheter aortic valve replacement, results from a large analysis showed.
“There is conflicting evidence and some debate over permanent pacemaker placement following transcatheter aortic valve replacement – whether it has a protective or adverse effect, and how often it takes place,” study investigator Fenton H. McCarthy, MD, said in an interview at the annual meeting of the Society of Thoracic Surgeons.
One recent study found that permanent pacemaker placement within 30 days post TAVR was found in 6.7% of patients undergoing balloon-expanding or self-expanding valve implantation, and is associated with increased mortality and hospitalizations (JACC Cardiovasc Interv. 2016 Nov 14;9[21]:2189-2199).
To evaluate the relationship between permanent pacemaker implantation and long-term patient outcomes among Medicare beneficiaries undergoing TAVR, Dr. McCarthy, a cardiothoracic surgery fellow at the University of Pennsylvania, Philadelphia, and his associates used Medicare carrier claims and Medicare Provider Analysis and Review files to identify 14,305 TAVR patients between January 2011 and December 2013.
The researchers used univariate Kaplan survival estimates and multivariable models to analyze survival, readmission and risk factors for pacemaker implantation.
The mean age of the 14,305 TAVR patients studied was 83 years, and 11% received a permanent pacemaker after TAVR. Of these, 9% received the pacemaker at index hospitalization, 1% at 30 days after implant, 0.5% at 90 days after implant, and 1% at 1 year after implant. Patient age of greater than 90 years was a significant predictor of pacemaker placement, with an odds ratio of 1.7 (P less than .01).
Dr. McCarthy and his associates observed that the readmission rates for pacemaker placement and no pacemaker placement at index hospitalization were similar at 30 days (21% vs. 19%, respectively), at 90 days (33% vs. 31%) and at 1 year (43% in both groups of patients).
In addition, Kaplan Meier estimates revealed no significant difference in long-term survival for patients with pacemaker placement within 30 days of TAVR, while multivariate Cox proportional hazard modeling revealed that pacemaker placement is not a predictor of long-term mortality (hazard ratio, 1.03; P = .65).
“This was the largest study to evaluate the question of incidence and effect of permanent pacemaker in the transcatheter aortic valve replacement population in the United States,” Dr. McCarthy said. “The size of our data set and the fact that the Medicare database includes all types of patients, regardless of trial participation, study or registry, is a strength of this study. Some other studies have used different inclusion and exclusion criteria. We used broad inclusion criteria and evaluated patients from as many different centers as possible.”
A key limitation of the study, he said, was that the researchers were unable to determine whether a patient received a balloon-expanding or self-expanding TAVR.
Dr. McCarthy reported having no financial disclosures.
Key clinical point:
Major finding: Pacemaker placement is not a predictor of long-term mortality (hazard ratio, 1.03; P = .65).
Data source: A study of 14,305 Medicare beneficiaries who underwent TAVR between January 2011 and December 2013.
Disclosures: Dr. McCarthy reported having no financial disclosures.
Travel ban affects international medical meetings
President Trump’s executive order blocking travelers from seven Muslim-majority countries from entering the United States has landed a damaging blow to global cooperation in scientific research and could impede assemblies of the world’s top medical experts.
“We are offering assistance as needed, and as each unique situation requires,” IAS-USA executive director and president Donna M. Jacobsen said in an interview. “Apart from that, we are establishing a list of individuals who may have contacts at the U.S. embassies, and will seek assistance from them as needed. Also, we are providing a letter documenting the individual as having been vetted as a legitimate scientist or researcher in the field, and having been approved to attend CROI.”
“We are monitoring the situation closely, however, and will offer whatever assistance we can to conference attendees, regardless of their point of origin, to ensure the free exchange of scientific information,” she said.
The travel ban could have detrimental effects on future collaboration between U.S. and international scientists and ultimately endanger the health and well-being of patients, Ms. Jacobsen said.
“Beyond the immediate possible impact on individuals intending to attend CROI who might be directly affected by the ban, there is serious reason for concern that the policy will dissuade other scientists and researchers from traveling to the U.S. in the future overall and sharing their work with colleagues here,” she said in an interview. “Such a response to the U.S. action would needlessly damage our global leadership in science and medicine.”
Thousands of academics from around the world, including physicians, researchers, and professors, have already vowed to boycott U.S.-based conferences until the travel ban is lifted. A Google Docs petition started shortly after the ban was announced has garnered more than 5,000 signatures by professionals acting in solidarity with those affected by the travel restrictions. It’s unclear who started the petition.
The academicians who signed the petition say they will not attend international conferences in the United States until those restricted from participating can rejoin their colleagues and freely share their ideas, said Charles R. Rogers, PhD, of the University of Minnesota, Minneapolis.
President Trump’s executive order, signed Jan. 27, bars refugees from entering the country for 120 days and bans immigrants from seven predominantly Muslim nations out for 3 months. The countries include Iran, Iraq, Syria, Sudan, Libya, Yemen and Somalia.
In a Feb. 1 press conference, White House press secretary Sean Spicer said the president’s intent is to ensure national security and prevent terrorists from entering the country.
“The president has been very clear that his No. 1 goal is not to target any one religion but places and areas where we believe that there is an issue,” Mr. Spicer said. “The president’s No. 1 goal has always been to focus on the safety of America, not the religion. He understands that it’s not a religious problem, it’s a radicalization problem. There’s a big difference between Islam, the religion, and radical Islamic terrorists that come here to seek to do us harm.”
[email protected]
On Twitter @legal_med
President Trump’s executive order blocking travelers from seven Muslim-majority countries from entering the United States has landed a damaging blow to global cooperation in scientific research and could impede assemblies of the world’s top medical experts.
“We are offering assistance as needed, and as each unique situation requires,” IAS-USA executive director and president Donna M. Jacobsen said in an interview. “Apart from that, we are establishing a list of individuals who may have contacts at the U.S. embassies, and will seek assistance from them as needed. Also, we are providing a letter documenting the individual as having been vetted as a legitimate scientist or researcher in the field, and having been approved to attend CROI.”
“We are monitoring the situation closely, however, and will offer whatever assistance we can to conference attendees, regardless of their point of origin, to ensure the free exchange of scientific information,” she said.
The travel ban could have detrimental effects on future collaboration between U.S. and international scientists and ultimately endanger the health and well-being of patients, Ms. Jacobsen said.
“Beyond the immediate possible impact on individuals intending to attend CROI who might be directly affected by the ban, there is serious reason for concern that the policy will dissuade other scientists and researchers from traveling to the U.S. in the future overall and sharing their work with colleagues here,” she said in an interview. “Such a response to the U.S. action would needlessly damage our global leadership in science and medicine.”
Thousands of academics from around the world, including physicians, researchers, and professors, have already vowed to boycott U.S.-based conferences until the travel ban is lifted. A Google Docs petition started shortly after the ban was announced has garnered more than 5,000 signatures by professionals acting in solidarity with those affected by the travel restrictions. It’s unclear who started the petition.
The academicians who signed the petition say they will not attend international conferences in the United States until those restricted from participating can rejoin their colleagues and freely share their ideas, said Charles R. Rogers, PhD, of the University of Minnesota, Minneapolis.
President Trump’s executive order, signed Jan. 27, bars refugees from entering the country for 120 days and bans immigrants from seven predominantly Muslim nations out for 3 months. The countries include Iran, Iraq, Syria, Sudan, Libya, Yemen and Somalia.
In a Feb. 1 press conference, White House press secretary Sean Spicer said the president’s intent is to ensure national security and prevent terrorists from entering the country.
“The president has been very clear that his No. 1 goal is not to target any one religion but places and areas where we believe that there is an issue,” Mr. Spicer said. “The president’s No. 1 goal has always been to focus on the safety of America, not the religion. He understands that it’s not a religious problem, it’s a radicalization problem. There’s a big difference between Islam, the religion, and radical Islamic terrorists that come here to seek to do us harm.”
[email protected]
On Twitter @legal_med
President Trump’s executive order blocking travelers from seven Muslim-majority countries from entering the United States has landed a damaging blow to global cooperation in scientific research and could impede assemblies of the world’s top medical experts.
“We are offering assistance as needed, and as each unique situation requires,” IAS-USA executive director and president Donna M. Jacobsen said in an interview. “Apart from that, we are establishing a list of individuals who may have contacts at the U.S. embassies, and will seek assistance from them as needed. Also, we are providing a letter documenting the individual as having been vetted as a legitimate scientist or researcher in the field, and having been approved to attend CROI.”
“We are monitoring the situation closely, however, and will offer whatever assistance we can to conference attendees, regardless of their point of origin, to ensure the free exchange of scientific information,” she said.
The travel ban could have detrimental effects on future collaboration between U.S. and international scientists and ultimately endanger the health and well-being of patients, Ms. Jacobsen said.
“Beyond the immediate possible impact on individuals intending to attend CROI who might be directly affected by the ban, there is serious reason for concern that the policy will dissuade other scientists and researchers from traveling to the U.S. in the future overall and sharing their work with colleagues here,” she said in an interview. “Such a response to the U.S. action would needlessly damage our global leadership in science and medicine.”
Thousands of academics from around the world, including physicians, researchers, and professors, have already vowed to boycott U.S.-based conferences until the travel ban is lifted. A Google Docs petition started shortly after the ban was announced has garnered more than 5,000 signatures by professionals acting in solidarity with those affected by the travel restrictions. It’s unclear who started the petition.
The academicians who signed the petition say they will not attend international conferences in the United States until those restricted from participating can rejoin their colleagues and freely share their ideas, said Charles R. Rogers, PhD, of the University of Minnesota, Minneapolis.
President Trump’s executive order, signed Jan. 27, bars refugees from entering the country for 120 days and bans immigrants from seven predominantly Muslim nations out for 3 months. The countries include Iran, Iraq, Syria, Sudan, Libya, Yemen and Somalia.
In a Feb. 1 press conference, White House press secretary Sean Spicer said the president’s intent is to ensure national security and prevent terrorists from entering the country.
“The president has been very clear that his No. 1 goal is not to target any one religion but places and areas where we believe that there is an issue,” Mr. Spicer said. “The president’s No. 1 goal has always been to focus on the safety of America, not the religion. He understands that it’s not a religious problem, it’s a radicalization problem. There’s a big difference between Islam, the religion, and radical Islamic terrorists that come here to seek to do us harm.”
[email protected]
On Twitter @legal_med
Company reports third death in SL-401 trial
Stemline Therapeutics, Inc. has announced another patient death in its ongoing phase 2 trial of SL-401 in patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN) and acute myeloid leukemia (AML).
The company became aware of this death, in a patient with BPDCN, on January 18.
The cause of death has not been determined, but the patient had developed capillary leak syndrome (CLS), a sometimes fatal and well-documented side effect of SL-401.
There have been 2 other deaths reported in patients with CLS in this trial. One of these deaths occurred in a patient with BPDCN and the other in a patient with AML.
Stemline said this study is ongoing, patient enrollment is ahead of schedule, and patients continue to receive SL-401 in the trial. Timelines for study completion and biologics licensing application submission remain on track.
The fact that CLS is an expected complication of SL-401 administration has been noted in filings with the Securities and Exchange Commission and US Food and Drug Administration (FDA), as well as in the study’s informed consent forms and other information provided to investigators.
Stemline said it has and will continue to report data to the FDA in accordance with the study protocol and applicable regulations. The company plans to provide a clinical and safety update on this cohort when the cohort and data are complete.
SL-410 in BPDCN
SL-401 is a targeted therapy directed to the interleukin-3 receptor (IL-3R), which is present in BPDCN and other hematologic malignancies. SL-401 is composed of human IL-3 coupled to a truncated diphtheria toxin payload that inhibits protein synthesis.
SL-401 is being tested in several clinical trials. Results from the phase 2 trial in BPDCN and AML patients were presented at the 2016 ASH Annual Meeting (abstract 342). However, the presentation only included data on the patients with BPDCN.
The trial consists of a lead-in dose-escalation stage (stage 1) and subsequent expansion stage (stage 2). In stage 1, patients received SL-401 as a daily intravenous infusion for up to 5 days (7, 9, 12, or 16 μg/kg/day) every 21 days. In stage 2, patients received SL-401 at the optimal stage 1 dose—12 μg/kg.
As of October 7, 2016, 32 BPDCN patients had been treated—9 in stage 1 and 23 in stage 2. Nineteen patients received SL-401 as first-line treatment, and 13 had relapsed/refractory disease. (Fourteen patients with relapsed/refractory AML were treated in stage 1.)
Efficacy
Of the 19 first-line BPDCN patients, 16 received the optimal dose (12 μg/kg). The overall response rate was 95% among all first-line patients (18/19) and 100% among those who received the optimal dose (16/16).
The complete response rates were 74% (14/19) and 81% (13/16), respectively. Six patients (all who received the optimal dose) proceeded to transplant (autologous and allogeneic).
All 13 relapsed/refractory BPDCN patients received the optimal dose. The overall response rate was 69% (9/13), and the complete response rate was 31% (4/13). One patient proceeded to allogeneic transplant.
Eleven first-line patients, including the 6 who went on to transplant, have ongoing responses. Six relapsed/refractory patients, including the patient who went on to transplant, have ongoing responses.
Among first-line patients treated at the optimal dose, the median progression-free and overall survival have not been reached. Among relapsed/refractory patients (all of whom were treated at the optimal dose), the median progression-free and overall survival are 8.5 months.
Safety
The most common treatment-related adverse events were transaminase elevation (52%), hypoalbuminemia (39%), chills (31%), pyrexia (27%), nausea (23%), fatigue (23%), peripheral edema (23%), thrombocytopenia (19%), hypotension (19%), weight increase (19%), anemia (19%), decreased appetite (19%), and CLS (19%).
In stage 1, two BPDCN patients had CLS—one grade 5 (7 μg/kg) and one grade 4 (12 μg/kg). After this, safety precautions were implemented to minimize the risk of severe CLS.
A second CLS-related death occurred in stage 1 in a patient with relapsed/refractory AML (16 μg/kg).
The other death in a BPDCN patient with CLS was reported after the ASH presentation. Stemline became aware of the death on January 18 and disclosed it to the public on February 2. ![]()

Stemline Therapeutics, Inc. has announced another patient death in its ongoing phase 2 trial of SL-401 in patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN) and acute myeloid leukemia (AML).
The company became aware of this death, in a patient with BPDCN, on January 18.
The cause of death has not been determined, but the patient had developed capillary leak syndrome (CLS), a sometimes fatal and well-documented side effect of SL-401.
There have been 2 other deaths reported in patients with CLS in this trial. One of these deaths occurred in a patient with BPDCN and the other in a patient with AML.
Stemline said this study is ongoing, patient enrollment is ahead of schedule, and patients continue to receive SL-401 in the trial. Timelines for study completion and biologics licensing application submission remain on track.
The fact that CLS is an expected complication of SL-401 administration has been noted in filings with the Securities and Exchange Commission and US Food and Drug Administration (FDA), as well as in the study’s informed consent forms and other information provided to investigators.
Stemline said it has and will continue to report data to the FDA in accordance with the study protocol and applicable regulations. The company plans to provide a clinical and safety update on this cohort when the cohort and data are complete.
SL-410 in BPDCN
SL-401 is a targeted therapy directed to the interleukin-3 receptor (IL-3R), which is present in BPDCN and other hematologic malignancies. SL-401 is composed of human IL-3 coupled to a truncated diphtheria toxin payload that inhibits protein synthesis.
SL-401 is being tested in several clinical trials. Results from the phase 2 trial in BPDCN and AML patients were presented at the 2016 ASH Annual Meeting (abstract 342). However, the presentation only included data on the patients with BPDCN.
The trial consists of a lead-in dose-escalation stage (stage 1) and subsequent expansion stage (stage 2). In stage 1, patients received SL-401 as a daily intravenous infusion for up to 5 days (7, 9, 12, or 16 μg/kg/day) every 21 days. In stage 2, patients received SL-401 at the optimal stage 1 dose—12 μg/kg.
As of October 7, 2016, 32 BPDCN patients had been treated—9 in stage 1 and 23 in stage 2. Nineteen patients received SL-401 as first-line treatment, and 13 had relapsed/refractory disease. (Fourteen patients with relapsed/refractory AML were treated in stage 1.)
Efficacy
Of the 19 first-line BPDCN patients, 16 received the optimal dose (12 μg/kg). The overall response rate was 95% among all first-line patients (18/19) and 100% among those who received the optimal dose (16/16).
The complete response rates were 74% (14/19) and 81% (13/16), respectively. Six patients (all who received the optimal dose) proceeded to transplant (autologous and allogeneic).
All 13 relapsed/refractory BPDCN patients received the optimal dose. The overall response rate was 69% (9/13), and the complete response rate was 31% (4/13). One patient proceeded to allogeneic transplant.
Eleven first-line patients, including the 6 who went on to transplant, have ongoing responses. Six relapsed/refractory patients, including the patient who went on to transplant, have ongoing responses.
Among first-line patients treated at the optimal dose, the median progression-free and overall survival have not been reached. Among relapsed/refractory patients (all of whom were treated at the optimal dose), the median progression-free and overall survival are 8.5 months.
Safety
The most common treatment-related adverse events were transaminase elevation (52%), hypoalbuminemia (39%), chills (31%), pyrexia (27%), nausea (23%), fatigue (23%), peripheral edema (23%), thrombocytopenia (19%), hypotension (19%), weight increase (19%), anemia (19%), decreased appetite (19%), and CLS (19%).
In stage 1, two BPDCN patients had CLS—one grade 5 (7 μg/kg) and one grade 4 (12 μg/kg). After this, safety precautions were implemented to minimize the risk of severe CLS.
A second CLS-related death occurred in stage 1 in a patient with relapsed/refractory AML (16 μg/kg).
The other death in a BPDCN patient with CLS was reported after the ASH presentation. Stemline became aware of the death on January 18 and disclosed it to the public on February 2. ![]()

Stemline Therapeutics, Inc. has announced another patient death in its ongoing phase 2 trial of SL-401 in patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN) and acute myeloid leukemia (AML).
The company became aware of this death, in a patient with BPDCN, on January 18.
The cause of death has not been determined, but the patient had developed capillary leak syndrome (CLS), a sometimes fatal and well-documented side effect of SL-401.
There have been 2 other deaths reported in patients with CLS in this trial. One of these deaths occurred in a patient with BPDCN and the other in a patient with AML.
Stemline said this study is ongoing, patient enrollment is ahead of schedule, and patients continue to receive SL-401 in the trial. Timelines for study completion and biologics licensing application submission remain on track.
The fact that CLS is an expected complication of SL-401 administration has been noted in filings with the Securities and Exchange Commission and US Food and Drug Administration (FDA), as well as in the study’s informed consent forms and other information provided to investigators.
Stemline said it has and will continue to report data to the FDA in accordance with the study protocol and applicable regulations. The company plans to provide a clinical and safety update on this cohort when the cohort and data are complete.
SL-410 in BPDCN
SL-401 is a targeted therapy directed to the interleukin-3 receptor (IL-3R), which is present in BPDCN and other hematologic malignancies. SL-401 is composed of human IL-3 coupled to a truncated diphtheria toxin payload that inhibits protein synthesis.
SL-401 is being tested in several clinical trials. Results from the phase 2 trial in BPDCN and AML patients were presented at the 2016 ASH Annual Meeting (abstract 342). However, the presentation only included data on the patients with BPDCN.
The trial consists of a lead-in dose-escalation stage (stage 1) and subsequent expansion stage (stage 2). In stage 1, patients received SL-401 as a daily intravenous infusion for up to 5 days (7, 9, 12, or 16 μg/kg/day) every 21 days. In stage 2, patients received SL-401 at the optimal stage 1 dose—12 μg/kg.
As of October 7, 2016, 32 BPDCN patients had been treated—9 in stage 1 and 23 in stage 2. Nineteen patients received SL-401 as first-line treatment, and 13 had relapsed/refractory disease. (Fourteen patients with relapsed/refractory AML were treated in stage 1.)
Efficacy
Of the 19 first-line BPDCN patients, 16 received the optimal dose (12 μg/kg). The overall response rate was 95% among all first-line patients (18/19) and 100% among those who received the optimal dose (16/16).
The complete response rates were 74% (14/19) and 81% (13/16), respectively. Six patients (all who received the optimal dose) proceeded to transplant (autologous and allogeneic).
All 13 relapsed/refractory BPDCN patients received the optimal dose. The overall response rate was 69% (9/13), and the complete response rate was 31% (4/13). One patient proceeded to allogeneic transplant.
Eleven first-line patients, including the 6 who went on to transplant, have ongoing responses. Six relapsed/refractory patients, including the patient who went on to transplant, have ongoing responses.
Among first-line patients treated at the optimal dose, the median progression-free and overall survival have not been reached. Among relapsed/refractory patients (all of whom were treated at the optimal dose), the median progression-free and overall survival are 8.5 months.
Safety
The most common treatment-related adverse events were transaminase elevation (52%), hypoalbuminemia (39%), chills (31%), pyrexia (27%), nausea (23%), fatigue (23%), peripheral edema (23%), thrombocytopenia (19%), hypotension (19%), weight increase (19%), anemia (19%), decreased appetite (19%), and CLS (19%).
In stage 1, two BPDCN patients had CLS—one grade 5 (7 μg/kg) and one grade 4 (12 μg/kg). After this, safety precautions were implemented to minimize the risk of severe CLS.
A second CLS-related death occurred in stage 1 in a patient with relapsed/refractory AML (16 μg/kg).
The other death in a BPDCN patient with CLS was reported after the ASH presentation. Stemline became aware of the death on January 18 and disclosed it to the public on February 2. ![]()
Obstetric perineal trauma prevented with SAFE PASSAGES
LAS VEGAS – Severe obstetric perineal trauma can often be avoided, even in operative deliveries, with the use of a suite of evidence-based interventions, according to findings from two prospective studies.
Collectively, these interventions resulted in significant reductions in third- and fourth-degree perineal lacerations in both military and civilian settings, according to research presented at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
Developed by the Military Health System, the SAFE PASSAGES protocol brings together interventions that help achieve a controlled delivery over a relaxed perineum, minimizing risk for maternal obstetric trauma.
The entire SAFE PASSAGES curriculum is available free online.
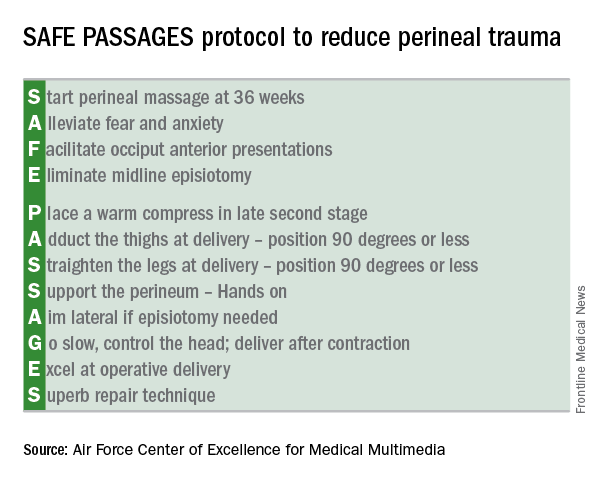
Military results

In a prospective cohort design, 272,161 deliveries conducted before the 2011 implementation of the SAFE PASSAGES training program were compared with 451,446 postimplementation deliveries. Primary outcome measures were the incidence of third- and fourth-degree perineal lacerations during vaginal deliveries with and without instrumentation.
For vaginal deliveries with instrumentation within one service branch of the military medical system (Service X), implementation of SAFE PASSAGES training was associated with a 63.6% reduction in third- and fourth-degree perineal lacerations, compared with preintervention rates (P less than .001).
The other two services – Service Y and Service Z – received just administrative encouragement and saw a 15.5% reduction and a 12.6% increase in significant obstetric trauma when instrumentation-assisted vaginal deliveries were performed (P = .04 and .30, respectively), according to Merlin Fausett, MD, an ob.gyn. currently in private practice in Missoula, Mont., who led the SAFE PASSAGES efforts before retiring from the Air Force.
For vaginal deliveries performed without instrumentation, the rates also fell for Service X, which saw a 41.8% reduction in third- and fourth-degree perineal lacerations (P less than .002). The other services saw a 16% increase and a 12% decrease with administrative encouragement alone (P = .48 and .08, respectively).
Though the military training program had initially been conducted in person, Dr. Fausett said that the program was switched to web-based simulations because of budget constraints. Efficacy remained high, he said.
Civilian results
When the team-based simulation that formed the core of the military SAFE PASSAGES training was rolled out in a large civilian health care system, similar improvements were seen.
Over an 18-month period, 675 nurses, midwives, and physicians received simulation-based training in the SAFE PASSAGES techniques. Overall, severe perineal laceration rates in the civilian facilities were down by 38.53% after adoption of SAFE PASSAGES.
“We have really achieved a culture shift,” said Emily Marko, MD, an ob.gyn. and clerkship director for the Virginia Commonwealth University School of Medicine Inova Campus in Falls Church, Va. “This really requires the whole delivery team to get involved: the patient, the patient’s support person, the nurses, any midwives or doulas that are there,” she said. “To tell you the truth, this whole program is about paying attention to the perineum, and not rushing delivery.”
Posttraining surveys showed that 95% of providers had changed their practice patterns after training in the delivery strategies.
Interventions
The emphasis in SAFE PASSAGES is to achieve a slow, controlled delivery and to minimize strain on the perineum by means of conditioning, relaxation, and positioning.
The first intervention is to have pregnant women “start” perineal massage at 36 weeks. Next, providers are urged to “alleviate” maternal fears. Providers are also encouraged to recognize posterior presentations, and to “facilitate” an anterior presentation through rotation. The “E” in SAFE stands for “eliminating” midline episiotomies – one of the more difficult practices to shift, according to both Dr. Fausett and Dr. Marko.
Despite a wealth of evidence showing fewer anal sphincter disruptions and better overall outcomes, it’s been difficult to convince U.S. physicians to adopt the mediolateral episiotomy technique that’s widely adopted in Europe, they said.
The protocol calls for “placing” a warm compress over the perineum during labor to encourage relaxation and stretching. Though prenatal perineal massage is encouraged, Dr. Marko said that intrapartum massage is not, as it’s thought to contribute to edema when performed during labor.
During delivery, leg positioning to reduce stretching of the perineum is also important: The thighs should be “adducted” to 90 degrees or less, and “straightened” to 90 degrees or less as well. Though this can make “a bit of a tight space” for the delivering physician, Dr. Marko said, it really “helps engage the pelvic muscles to support the perineum,” and a few technique adjustments make the position workable.
The perineum should be “supported” during delivery by one hand of the delivering practitioner forming a U shape with the thumb and forefinger, with the first webspace overlying the posterior fourchette. Reinforcing the importance of avoiding a midline episiotomy, the “A” of passages stands for “aiming” lateral when an episiotomy is needed.
During the delivery, the physician should “go” slow, controlling the head and delivering after, rather than during, a contraction.
It’s important to be comfortable with forceps deliveries and vacuum extractions in order to minimize both maternal and fetal trauma; thus, physicians should “excel” at operative delivery, according to the protocol.
The SAFE PASSAGES website includes comprehensive explanations, with graphics and demonstrations using a model, of both forceps and vacuum delivery techniques.
Finally, should a laceration occur, the SAFE PASSAGES website provides detailed explanations of repair techniques, with an emphasis on understanding perineal, vaginal, and anal anatomy so “superb” approximation and repair can be achieved.
Though obstetric trauma may not be life threatening, it’s still associated with significant and persistent morbidity. When perineal and pelvic floor trauma disrupts the anal sphincter, anal incontinence can occur, even after a meticulous attempt at repair. Perineal and pelvic floor trauma can result in a host of urinary and sexual problems as well.
After the intensive training period, Dr. Fausett said, “laceration rates can creep back up if people forget about it and stop paying attention to it. But where it becomes a culture, the rates can stay low. Standardized training can reduce perineal trauma rates without increasing cesarean or neonatal trauma rates,” Dr. Fausett said.
Dr. Marko and Dr. Fausett reported having no conflicts of interest.
[email protected]
On Twitter @karioakes
LAS VEGAS – Severe obstetric perineal trauma can often be avoided, even in operative deliveries, with the use of a suite of evidence-based interventions, according to findings from two prospective studies.
Collectively, these interventions resulted in significant reductions in third- and fourth-degree perineal lacerations in both military and civilian settings, according to research presented at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
Developed by the Military Health System, the SAFE PASSAGES protocol brings together interventions that help achieve a controlled delivery over a relaxed perineum, minimizing risk for maternal obstetric trauma.
The entire SAFE PASSAGES curriculum is available free online.

Military results

In a prospective cohort design, 272,161 deliveries conducted before the 2011 implementation of the SAFE PASSAGES training program were compared with 451,446 postimplementation deliveries. Primary outcome measures were the incidence of third- and fourth-degree perineal lacerations during vaginal deliveries with and without instrumentation.
For vaginal deliveries with instrumentation within one service branch of the military medical system (Service X), implementation of SAFE PASSAGES training was associated with a 63.6% reduction in third- and fourth-degree perineal lacerations, compared with preintervention rates (P less than .001).
The other two services – Service Y and Service Z – received just administrative encouragement and saw a 15.5% reduction and a 12.6% increase in significant obstetric trauma when instrumentation-assisted vaginal deliveries were performed (P = .04 and .30, respectively), according to Merlin Fausett, MD, an ob.gyn. currently in private practice in Missoula, Mont., who led the SAFE PASSAGES efforts before retiring from the Air Force.
For vaginal deliveries performed without instrumentation, the rates also fell for Service X, which saw a 41.8% reduction in third- and fourth-degree perineal lacerations (P less than .002). The other services saw a 16% increase and a 12% decrease with administrative encouragement alone (P = .48 and .08, respectively).
Though the military training program had initially been conducted in person, Dr. Fausett said that the program was switched to web-based simulations because of budget constraints. Efficacy remained high, he said.
Civilian results
When the team-based simulation that formed the core of the military SAFE PASSAGES training was rolled out in a large civilian health care system, similar improvements were seen.
Over an 18-month period, 675 nurses, midwives, and physicians received simulation-based training in the SAFE PASSAGES techniques. Overall, severe perineal laceration rates in the civilian facilities were down by 38.53% after adoption of SAFE PASSAGES.
“We have really achieved a culture shift,” said Emily Marko, MD, an ob.gyn. and clerkship director for the Virginia Commonwealth University School of Medicine Inova Campus in Falls Church, Va. “This really requires the whole delivery team to get involved: the patient, the patient’s support person, the nurses, any midwives or doulas that are there,” she said. “To tell you the truth, this whole program is about paying attention to the perineum, and not rushing delivery.”
Posttraining surveys showed that 95% of providers had changed their practice patterns after training in the delivery strategies.
Interventions
The emphasis in SAFE PASSAGES is to achieve a slow, controlled delivery and to minimize strain on the perineum by means of conditioning, relaxation, and positioning.
The first intervention is to have pregnant women “start” perineal massage at 36 weeks. Next, providers are urged to “alleviate” maternal fears. Providers are also encouraged to recognize posterior presentations, and to “facilitate” an anterior presentation through rotation. The “E” in SAFE stands for “eliminating” midline episiotomies – one of the more difficult practices to shift, according to both Dr. Fausett and Dr. Marko.
Despite a wealth of evidence showing fewer anal sphincter disruptions and better overall outcomes, it’s been difficult to convince U.S. physicians to adopt the mediolateral episiotomy technique that’s widely adopted in Europe, they said.
The protocol calls for “placing” a warm compress over the perineum during labor to encourage relaxation and stretching. Though prenatal perineal massage is encouraged, Dr. Marko said that intrapartum massage is not, as it’s thought to contribute to edema when performed during labor.
During delivery, leg positioning to reduce stretching of the perineum is also important: The thighs should be “adducted” to 90 degrees or less, and “straightened” to 90 degrees or less as well. Though this can make “a bit of a tight space” for the delivering physician, Dr. Marko said, it really “helps engage the pelvic muscles to support the perineum,” and a few technique adjustments make the position workable.
The perineum should be “supported” during delivery by one hand of the delivering practitioner forming a U shape with the thumb and forefinger, with the first webspace overlying the posterior fourchette. Reinforcing the importance of avoiding a midline episiotomy, the “A” of passages stands for “aiming” lateral when an episiotomy is needed.
During the delivery, the physician should “go” slow, controlling the head and delivering after, rather than during, a contraction.
It’s important to be comfortable with forceps deliveries and vacuum extractions in order to minimize both maternal and fetal trauma; thus, physicians should “excel” at operative delivery, according to the protocol.
The SAFE PASSAGES website includes comprehensive explanations, with graphics and demonstrations using a model, of both forceps and vacuum delivery techniques.
Finally, should a laceration occur, the SAFE PASSAGES website provides detailed explanations of repair techniques, with an emphasis on understanding perineal, vaginal, and anal anatomy so “superb” approximation and repair can be achieved.
Though obstetric trauma may not be life threatening, it’s still associated with significant and persistent morbidity. When perineal and pelvic floor trauma disrupts the anal sphincter, anal incontinence can occur, even after a meticulous attempt at repair. Perineal and pelvic floor trauma can result in a host of urinary and sexual problems as well.
After the intensive training period, Dr. Fausett said, “laceration rates can creep back up if people forget about it and stop paying attention to it. But where it becomes a culture, the rates can stay low. Standardized training can reduce perineal trauma rates without increasing cesarean or neonatal trauma rates,” Dr. Fausett said.
Dr. Marko and Dr. Fausett reported having no conflicts of interest.
[email protected]
On Twitter @karioakes
LAS VEGAS – Severe obstetric perineal trauma can often be avoided, even in operative deliveries, with the use of a suite of evidence-based interventions, according to findings from two prospective studies.
Collectively, these interventions resulted in significant reductions in third- and fourth-degree perineal lacerations in both military and civilian settings, according to research presented at the annual Pregnancy Meeting sponsored by the Society for Maternal-Fetal Medicine.
Developed by the Military Health System, the SAFE PASSAGES protocol brings together interventions that help achieve a controlled delivery over a relaxed perineum, minimizing risk for maternal obstetric trauma.
The entire SAFE PASSAGES curriculum is available free online.

Military results

In a prospective cohort design, 272,161 deliveries conducted before the 2011 implementation of the SAFE PASSAGES training program were compared with 451,446 postimplementation deliveries. Primary outcome measures were the incidence of third- and fourth-degree perineal lacerations during vaginal deliveries with and without instrumentation.
For vaginal deliveries with instrumentation within one service branch of the military medical system (Service X), implementation of SAFE PASSAGES training was associated with a 63.6% reduction in third- and fourth-degree perineal lacerations, compared with preintervention rates (P less than .001).
The other two services – Service Y and Service Z – received just administrative encouragement and saw a 15.5% reduction and a 12.6% increase in significant obstetric trauma when instrumentation-assisted vaginal deliveries were performed (P = .04 and .30, respectively), according to Merlin Fausett, MD, an ob.gyn. currently in private practice in Missoula, Mont., who led the SAFE PASSAGES efforts before retiring from the Air Force.
For vaginal deliveries performed without instrumentation, the rates also fell for Service X, which saw a 41.8% reduction in third- and fourth-degree perineal lacerations (P less than .002). The other services saw a 16% increase and a 12% decrease with administrative encouragement alone (P = .48 and .08, respectively).
Though the military training program had initially been conducted in person, Dr. Fausett said that the program was switched to web-based simulations because of budget constraints. Efficacy remained high, he said.
Civilian results
When the team-based simulation that formed the core of the military SAFE PASSAGES training was rolled out in a large civilian health care system, similar improvements were seen.
Over an 18-month period, 675 nurses, midwives, and physicians received simulation-based training in the SAFE PASSAGES techniques. Overall, severe perineal laceration rates in the civilian facilities were down by 38.53% after adoption of SAFE PASSAGES.
“We have really achieved a culture shift,” said Emily Marko, MD, an ob.gyn. and clerkship director for the Virginia Commonwealth University School of Medicine Inova Campus in Falls Church, Va. “This really requires the whole delivery team to get involved: the patient, the patient’s support person, the nurses, any midwives or doulas that are there,” she said. “To tell you the truth, this whole program is about paying attention to the perineum, and not rushing delivery.”
Posttraining surveys showed that 95% of providers had changed their practice patterns after training in the delivery strategies.
Interventions
The emphasis in SAFE PASSAGES is to achieve a slow, controlled delivery and to minimize strain on the perineum by means of conditioning, relaxation, and positioning.
The first intervention is to have pregnant women “start” perineal massage at 36 weeks. Next, providers are urged to “alleviate” maternal fears. Providers are also encouraged to recognize posterior presentations, and to “facilitate” an anterior presentation through rotation. The “E” in SAFE stands for “eliminating” midline episiotomies – one of the more difficult practices to shift, according to both Dr. Fausett and Dr. Marko.
Despite a wealth of evidence showing fewer anal sphincter disruptions and better overall outcomes, it’s been difficult to convince U.S. physicians to adopt the mediolateral episiotomy technique that’s widely adopted in Europe, they said.
The protocol calls for “placing” a warm compress over the perineum during labor to encourage relaxation and stretching. Though prenatal perineal massage is encouraged, Dr. Marko said that intrapartum massage is not, as it’s thought to contribute to edema when performed during labor.
During delivery, leg positioning to reduce stretching of the perineum is also important: The thighs should be “adducted” to 90 degrees or less, and “straightened” to 90 degrees or less as well. Though this can make “a bit of a tight space” for the delivering physician, Dr. Marko said, it really “helps engage the pelvic muscles to support the perineum,” and a few technique adjustments make the position workable.
The perineum should be “supported” during delivery by one hand of the delivering practitioner forming a U shape with the thumb and forefinger, with the first webspace overlying the posterior fourchette. Reinforcing the importance of avoiding a midline episiotomy, the “A” of passages stands for “aiming” lateral when an episiotomy is needed.
During the delivery, the physician should “go” slow, controlling the head and delivering after, rather than during, a contraction.
It’s important to be comfortable with forceps deliveries and vacuum extractions in order to minimize both maternal and fetal trauma; thus, physicians should “excel” at operative delivery, according to the protocol.
The SAFE PASSAGES website includes comprehensive explanations, with graphics and demonstrations using a model, of both forceps and vacuum delivery techniques.
Finally, should a laceration occur, the SAFE PASSAGES website provides detailed explanations of repair techniques, with an emphasis on understanding perineal, vaginal, and anal anatomy so “superb” approximation and repair can be achieved.
Though obstetric trauma may not be life threatening, it’s still associated with significant and persistent morbidity. When perineal and pelvic floor trauma disrupts the anal sphincter, anal incontinence can occur, even after a meticulous attempt at repair. Perineal and pelvic floor trauma can result in a host of urinary and sexual problems as well.
After the intensive training period, Dr. Fausett said, “laceration rates can creep back up if people forget about it and stop paying attention to it. But where it becomes a culture, the rates can stay low. Standardized training can reduce perineal trauma rates without increasing cesarean or neonatal trauma rates,” Dr. Fausett said.
Dr. Marko and Dr. Fausett reported having no conflicts of interest.
[email protected]
On Twitter @karioakes
AT THE PREGNANCY MEETING
Key clinical point:
Major finding: Third- and fourth-degree perineal lacerations dropped by as much as 64% after SAFE PASSAGES training.
Data source: Prospective cohort study of 723,607 military deliveries; prospective study of 675 providers involved in labor and delivery at four civilian hospitals in a large health care system.
Disclosures: Dr. Marko and Dr. Fausett reported having no conflicts of interest.












