User login
Nontraumatic Disc Herniation as a Cause of Unusual Cervical Spondylotic Myelopathy
Case
A 55-year-old previously healthy woman with an insignificant medical history presented to the ED for evaluation of right-sided numbness, tingling, and inability to sense temperature. The patient stated the numbness and tingling first began in her right leg and thigh 2 months earlier, and had progressively worsened to her entire right-side. She said she first experienced the thermoanesthesia while taking a shower the morning of presentation. While showering, the patient noted that she could not feel any hot or cold sensation on the right side of her body, including her right leg and arm. She also reported decreased sensation to her extremities on the right side.
She denied any new weakness, headache, chest pain, shortness of breath, fever, chills, nausea, vomiting, back pain, neck pain, or any other symptoms. In addition, she denied any difficulty swallowing, speaking, blurry vision, or double vision. Regarding her social history, the patient denied a history of sexually transmitted diseases, including syphilis; or any tobacco, alcohol, or illicit drug use. The patient confirmed that she had never experienced any of the presenting symptoms prior to 2 months ago. There was no history of trauma or falls. A review of systems was otherwise negative.
Vital signs at presentation were: blood pressure, 129/88 mm Hg; heart rate, 99 beats/min; respiratory rate, 18 breaths/min; and temperature, 98.5°F. Oxygen saturation was 98% on room air. Physical examination revealed a middle-aged woman who was awake, alert, and oriented. Her head was normocephalic and atraumatic, and her pupils were 5 mm, equal, round, and reactive to light bilaterally. Her cranial nerves II through XII were intact. She had normal 5/5 strength in both her upper and lower extremities bilaterally, and had 2+ and equal bilateral patella and Achilles deep tendon reflexes. The patient had no truncal ataxia, and she had a normal gait on ambulation. She was unable to sense temperature (assessed by touching a cold metal tray with her right hand). There was no neck or back midline tenderness to palpation of her spine.
Initial laboratory studies included a complete blood count (CBC); basic metabolic panel (BMP), including blood urea nitrogen; and urine drug screen (UDS). The CBC and BMP were within normal limits, except for an elevated creatine kinase of 249 U/L. The UDS was positive for cocaine. A head computed tomography (CT) scan without contrast was unremarkable.
The patient was admitted to the hospital for further evaluation. Additional laboratory workup during the inpatient stay included nonreactive treponemal immunoglobulin G/immunoglobulin M; nonreactive HIV antigen antibody assay; normal thyroid stimulating hormone; normal free thyroxine, folate, and vitamin B12 levels; normal erythrocyte sedimentation rate, and C-reactive protein levels. The patient’s hemoglobin A1C was also within normal range.
Other imaging studies, which included MR angiography (MRA) of the head and neck, and MRI of the thoracic and lumbar spine, were unremarkable, with the exception of some chronic spondylitic changes.
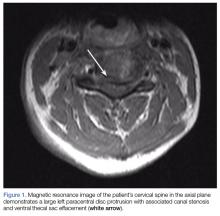
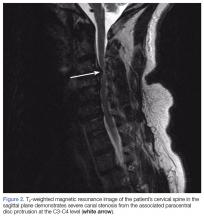
Due to the significant C3-C4 stenosis, orthopedic surgery services were consulted for a spinal surgery workup. The orthopedic examination identified a few beats of clonus, intact proprioception, and no dysmetria. The patient had decreased sensation to fine touch in the distribution of C7 at the level of the triceps, midphalangeal joints to distal fingertips on the right, fourth, and fifth fingers on the left and right lateral lower extremity. A Hoffmann sign was positive bilaterally. A CT scan of the cervical spine showed severe canal stenosis at the C3-C4 level secondary to a large C3-C4 left paracentral disc protrusion with AP dimensions of the canal measured at 4 to 5 mm. There was no evidence of acute cervical spine fracture or subluxation.
The patient was offered operative and nonoperative management options, including anterior cervical discectomy and fusion vs conservative management with corticosteroid therapy. She agreed to conservative management and received intravenous (IV) dexamethasone with subjective improvement in her symptoms. The patient was discharged home on hospital day 3, with instructions to follow-up with a spine surgeon in 2 weeks. She was also counseled on abstaining from further cocaine or other illicit drug use.
The patient eventually returned for an elective anterior cervical discectomy and fusion 2 months later, after several outpatient visits and progression of symptoms. She was discharged home on postoperative day 1 with pain well-controlled and was able to ambulate without assistance. On follow-up, she reported 15% improvement in her symptoms.
Discussion
Cervical spondylotic myelopathy (CSM) is the most common myelopathy in patients aged 55 years and older. Immediate neuroimaging studies followed by spinal surgery consultation are recommended for patients presenting with acute symptoms suggestive of cord compression.1,2
Diagnosis and Differential Diagnosis
Diagnosis of CSM can be made with a thorough patient history, neurological examination, and MRI/MRA. However, because cases of cervical disc herniation (CDH) are often atraumatic, the patient history may not always be contributory to the diagnosis and severity of the offending cause.
During our patient’s hospital course, there was initially a concern for Brown-Séquard syndrome (BSS) due to the lateralizing symptoms and radiographic findings. This is a rare condition that can occur in the setting of spinal trauma, unilateral disc herniation, tumors, epidural hematomas, and spinal cord ischemia.3,4 In one retrospective case review by Sayer et al,4 the incidence of CDH causing BSS was only 0.21% (5 per 2,350 patients), and 67% of the cases involved C5-C6 or C6-C7.
Although disc herniation usually presents with symptoms on the ipsilateral side in patients with BSS, there are rare case reports of patients with contralateral symptoms in the form of complete or incomplete BSS manifesting as ipsilateral motor deficit and/or loss of contralateral pain and temperature due to an incomplete spinal cord compression.5-7 We were able to rule-out BSS in our patient due to the absence of motor symptoms.
Treatment
Corticosteroid Therapy. High-dose IV corticosteroids should be given to all patients with CSM prior to surgery to reduce cord edema caused by spinal cord injury. In one randomized control trial by Bracken et al,8 patients given methylprednisolone within 8 hours of spinal cord injury had improvement in motor function, sensation to pinprick, and touch at 6 months when compared to placebo. When the aforementioned steps are taken in the emergent care setting, they may significantly improve patient outcomes.
Surgical Intervention. All cases of CSM in the review literature were treated surgically with laminectomy or hemilaminectomy, anterior discectomy with or without fusion, or corpectomy followed by interbody fusion, with the goal of achieving cord decompression. A large majority of patients underwent anterior discectomy with interbody fusion, and all of the cases recommend early surgical intervention in severe CSM to prevent rapidly worsening symptoms, including permanent hemiparesis.
Early surgical intervention is positively correlated with better outcomes, most often resulting in significant improvement of symptoms to full recovery.3,4,6,7,9-12 In one case report, surgical intervention did not result in a significant improvement, and the patient had been suffering from progressive symptoms for 7 years prior to diagnosis and treatment.11
Conservative Management. Conservative management of CSM includes immobilization, activity modification, pain management, and/or corticosteroids therapy.13 However, for patients undergoing surgical decompression, 50% to 80% reported symptom improvement.14,15 This evidence strongly supports management of CSM with early diagnosis and surgical intervention. Despite delays in diagnosis and treatment, surgical intervention can still offer significant relief of weakness and sensory deficits associated with severe CSM.11
Conclusion
Cervical spondylotic myelopathy is the most common myelopathy in patients aged 55 years and older. Common symptoms involve upper extremity sensation, gait disturbances, and deterioration of hand use16; however, there is a large differential for patients presenting to the ED with these symptoms, including mass effect, infection, vascular conditions, metabolic disorders, inflammatory conditions, and trauma.
Our patient with CSM presented with signs of an incomplete cord syndrome with lateralizing features caused by asymmetric disc herniation. This case is unique in that though our patient had some symptom resolution with corticosteroids therapy alone, she ultimately returned for definitive surgical decompression after symptom progression.
1. Chen TY, Dickman CA, Eleraky M, Sonntag VK. The role of decompression for acute incomplete cervical spinal cord injury in cervical spondylosis. Spine (Phila Pa 1976). 1998;23(22):2398-2403.
2. Ishida Y, Tominaga T. Predictors of neurologic recovery in acute central cervical cord injury with only upper extremity impairment. Spine (Phila Pa 1976). 2002;27(15):1652-1658. discussion 1658.
3. Porto GB, Tan LA, Kasliwal MK, Traynelis VC. Progressive Brown-Séquard syndrome: a rare manifestation of cervical disc herniation. J Clin Neurosci. 2016;29:196-198. doi:10.1016/j.jocn.2015.12.021
4. Sayer FT, Vitali AM, Low HL, Paquette S, Honey CR. Brown-Sèquard syndrome produced by C3-C4 cervical disc herniation: a case report and review of the literature. Spine (Phila Pa 1976). 2008;33(9):E279-E282. doi:10.1097/BRS.0b013e31816c835d.
5. Urrutia J, Fadic R. Cervical disc herniation producing acute Brown-Sequard syndrome: dynamic changes documented by intraoperative neuromonitoring. Eur Spine J. 2012;21 Suppl 4:S418-S421. doi:10.1007/s00586-011-1881-8.
6. Choi KB, Lee CD, Chung DJ, Lee SH. Cervical disc herniation as a cause of Brown-Séquard syndrome. J Korean Neurosurg Soc. 2009;46(5):505-510. doi:10.3340/jkns.2009.46.5.505. doi:10.3340/jkns.2009.46.5.505.
7. Kobayashi N, Asamoto S, Doi H, Sugiyama H. Brown-Sèquard syndrome produced by cervical disc herniation: report of two cases and review of the literature. Spine J. 2003;3(6):530-533.
8. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990; 322(20):1405-1411. doi:10.1056/NEJM199005173222001.
9. Stookey B. Compression of the spinal cord due to ventral extradural cervical chondromas: diagnosis and surgical treatment. Arch Neurol Psychiatry. 1928;20:275-291.
10. Antich PA, Sanjuan AC, Girvent FM, Simó JD. High cervical disc herniation and Brown-Sequard syndrome. A case report and review of the literature. J Bone Joint Surg Br. 1999;81(3):462-463.
11. Guan D, Wang G, Claire M, Kuang Z. Brown-Sequard syndrome produced by calcified herniated cervical disc and posterior vertebral osteophyte: case report.
J Orthop. 2015;12(Suppl 2):S260-S263. doi:10.1016/j.jor.2015.10.007.
12. Abouhashem S, Ammar M, Barakat M, Abdelhameed E. Management of Brown-Sequard syndrome in cervical disc diseases. Turk Neurosurg. 2013;23(4):470-475. doi:10.5137/1019-5149.JTN.7433-12.0.
13. Mazanec D, Reddy A. Medical management of cervical spondylosis. Neurosurgery. 2007;60(1 Suppl 1):S43-S50. doi:10.1227/01.NEU.0000215386.05760.6D.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64(Suppl 1):S1:30-S1:35; discussion S1:35-S1:36. doi:10.1016/j.surneu.2005.02.016.
15. Cheung WY, Arvinte D, Wong YW, Luk KD, Cheung KM. Neurological recovery after surgical decompression in patients with cervical spondylotic myelopathy - a prospective study. Int Orthop. 2008;32(2):273-378. doi:10.1007/s00264-006-0315-4.
16. Chiles BW 3rd, Leonard MA, Choudhri HF, Cooper PR. Cervical spondylotic myelopathy: patterns of neurological deficit and recovery after anterior cervical decompression. Neurosurgery. 1999;44(4):762-769; discussion 769-770
Case
A 55-year-old previously healthy woman with an insignificant medical history presented to the ED for evaluation of right-sided numbness, tingling, and inability to sense temperature. The patient stated the numbness and tingling first began in her right leg and thigh 2 months earlier, and had progressively worsened to her entire right-side. She said she first experienced the thermoanesthesia while taking a shower the morning of presentation. While showering, the patient noted that she could not feel any hot or cold sensation on the right side of her body, including her right leg and arm. She also reported decreased sensation to her extremities on the right side.
She denied any new weakness, headache, chest pain, shortness of breath, fever, chills, nausea, vomiting, back pain, neck pain, or any other symptoms. In addition, she denied any difficulty swallowing, speaking, blurry vision, or double vision. Regarding her social history, the patient denied a history of sexually transmitted diseases, including syphilis; or any tobacco, alcohol, or illicit drug use. The patient confirmed that she had never experienced any of the presenting symptoms prior to 2 months ago. There was no history of trauma or falls. A review of systems was otherwise negative.
Vital signs at presentation were: blood pressure, 129/88 mm Hg; heart rate, 99 beats/min; respiratory rate, 18 breaths/min; and temperature, 98.5°F. Oxygen saturation was 98% on room air. Physical examination revealed a middle-aged woman who was awake, alert, and oriented. Her head was normocephalic and atraumatic, and her pupils were 5 mm, equal, round, and reactive to light bilaterally. Her cranial nerves II through XII were intact. She had normal 5/5 strength in both her upper and lower extremities bilaterally, and had 2+ and equal bilateral patella and Achilles deep tendon reflexes. The patient had no truncal ataxia, and she had a normal gait on ambulation. She was unable to sense temperature (assessed by touching a cold metal tray with her right hand). There was no neck or back midline tenderness to palpation of her spine.
Initial laboratory studies included a complete blood count (CBC); basic metabolic panel (BMP), including blood urea nitrogen; and urine drug screen (UDS). The CBC and BMP were within normal limits, except for an elevated creatine kinase of 249 U/L. The UDS was positive for cocaine. A head computed tomography (CT) scan without contrast was unremarkable.
The patient was admitted to the hospital for further evaluation. Additional laboratory workup during the inpatient stay included nonreactive treponemal immunoglobulin G/immunoglobulin M; nonreactive HIV antigen antibody assay; normal thyroid stimulating hormone; normal free thyroxine, folate, and vitamin B12 levels; normal erythrocyte sedimentation rate, and C-reactive protein levels. The patient’s hemoglobin A1C was also within normal range.
Other imaging studies, which included MR angiography (MRA) of the head and neck, and MRI of the thoracic and lumbar spine, were unremarkable, with the exception of some chronic spondylitic changes.
Due to the significant C3-C4 stenosis, orthopedic surgery services were consulted for a spinal surgery workup. The orthopedic examination identified a few beats of clonus, intact proprioception, and no dysmetria. The patient had decreased sensation to fine touch in the distribution of C7 at the level of the triceps, midphalangeal joints to distal fingertips on the right, fourth, and fifth fingers on the left and right lateral lower extremity. A Hoffmann sign was positive bilaterally. A CT scan of the cervical spine showed severe canal stenosis at the C3-C4 level secondary to a large C3-C4 left paracentral disc protrusion with AP dimensions of the canal measured at 4 to 5 mm. There was no evidence of acute cervical spine fracture or subluxation.
The patient was offered operative and nonoperative management options, including anterior cervical discectomy and fusion vs conservative management with corticosteroid therapy. She agreed to conservative management and received intravenous (IV) dexamethasone with subjective improvement in her symptoms. The patient was discharged home on hospital day 3, with instructions to follow-up with a spine surgeon in 2 weeks. She was also counseled on abstaining from further cocaine or other illicit drug use.
The patient eventually returned for an elective anterior cervical discectomy and fusion 2 months later, after several outpatient visits and progression of symptoms. She was discharged home on postoperative day 1 with pain well-controlled and was able to ambulate without assistance. On follow-up, she reported 15% improvement in her symptoms.
Discussion
Cervical spondylotic myelopathy (CSM) is the most common myelopathy in patients aged 55 years and older. Immediate neuroimaging studies followed by spinal surgery consultation are recommended for patients presenting with acute symptoms suggestive of cord compression.1,2
Diagnosis and Differential Diagnosis
Diagnosis of CSM can be made with a thorough patient history, neurological examination, and MRI/MRA. However, because cases of cervical disc herniation (CDH) are often atraumatic, the patient history may not always be contributory to the diagnosis and severity of the offending cause.
During our patient’s hospital course, there was initially a concern for Brown-Séquard syndrome (BSS) due to the lateralizing symptoms and radiographic findings. This is a rare condition that can occur in the setting of spinal trauma, unilateral disc herniation, tumors, epidural hematomas, and spinal cord ischemia.3,4 In one retrospective case review by Sayer et al,4 the incidence of CDH causing BSS was only 0.21% (5 per 2,350 patients), and 67% of the cases involved C5-C6 or C6-C7.
Although disc herniation usually presents with symptoms on the ipsilateral side in patients with BSS, there are rare case reports of patients with contralateral symptoms in the form of complete or incomplete BSS manifesting as ipsilateral motor deficit and/or loss of contralateral pain and temperature due to an incomplete spinal cord compression.5-7 We were able to rule-out BSS in our patient due to the absence of motor symptoms.
Treatment
Corticosteroid Therapy. High-dose IV corticosteroids should be given to all patients with CSM prior to surgery to reduce cord edema caused by spinal cord injury. In one randomized control trial by Bracken et al,8 patients given methylprednisolone within 8 hours of spinal cord injury had improvement in motor function, sensation to pinprick, and touch at 6 months when compared to placebo. When the aforementioned steps are taken in the emergent care setting, they may significantly improve patient outcomes.
Surgical Intervention. All cases of CSM in the review literature were treated surgically with laminectomy or hemilaminectomy, anterior discectomy with or without fusion, or corpectomy followed by interbody fusion, with the goal of achieving cord decompression. A large majority of patients underwent anterior discectomy with interbody fusion, and all of the cases recommend early surgical intervention in severe CSM to prevent rapidly worsening symptoms, including permanent hemiparesis.
Early surgical intervention is positively correlated with better outcomes, most often resulting in significant improvement of symptoms to full recovery.3,4,6,7,9-12 In one case report, surgical intervention did not result in a significant improvement, and the patient had been suffering from progressive symptoms for 7 years prior to diagnosis and treatment.11
Conservative Management. Conservative management of CSM includes immobilization, activity modification, pain management, and/or corticosteroids therapy.13 However, for patients undergoing surgical decompression, 50% to 80% reported symptom improvement.14,15 This evidence strongly supports management of CSM with early diagnosis and surgical intervention. Despite delays in diagnosis and treatment, surgical intervention can still offer significant relief of weakness and sensory deficits associated with severe CSM.11
Conclusion
Cervical spondylotic myelopathy is the most common myelopathy in patients aged 55 years and older. Common symptoms involve upper extremity sensation, gait disturbances, and deterioration of hand use16; however, there is a large differential for patients presenting to the ED with these symptoms, including mass effect, infection, vascular conditions, metabolic disorders, inflammatory conditions, and trauma.
Our patient with CSM presented with signs of an incomplete cord syndrome with lateralizing features caused by asymmetric disc herniation. This case is unique in that though our patient had some symptom resolution with corticosteroids therapy alone, she ultimately returned for definitive surgical decompression after symptom progression.
Case
A 55-year-old previously healthy woman with an insignificant medical history presented to the ED for evaluation of right-sided numbness, tingling, and inability to sense temperature. The patient stated the numbness and tingling first began in her right leg and thigh 2 months earlier, and had progressively worsened to her entire right-side. She said she first experienced the thermoanesthesia while taking a shower the morning of presentation. While showering, the patient noted that she could not feel any hot or cold sensation on the right side of her body, including her right leg and arm. She also reported decreased sensation to her extremities on the right side.
She denied any new weakness, headache, chest pain, shortness of breath, fever, chills, nausea, vomiting, back pain, neck pain, or any other symptoms. In addition, she denied any difficulty swallowing, speaking, blurry vision, or double vision. Regarding her social history, the patient denied a history of sexually transmitted diseases, including syphilis; or any tobacco, alcohol, or illicit drug use. The patient confirmed that she had never experienced any of the presenting symptoms prior to 2 months ago. There was no history of trauma or falls. A review of systems was otherwise negative.
Vital signs at presentation were: blood pressure, 129/88 mm Hg; heart rate, 99 beats/min; respiratory rate, 18 breaths/min; and temperature, 98.5°F. Oxygen saturation was 98% on room air. Physical examination revealed a middle-aged woman who was awake, alert, and oriented. Her head was normocephalic and atraumatic, and her pupils were 5 mm, equal, round, and reactive to light bilaterally. Her cranial nerves II through XII were intact. She had normal 5/5 strength in both her upper and lower extremities bilaterally, and had 2+ and equal bilateral patella and Achilles deep tendon reflexes. The patient had no truncal ataxia, and she had a normal gait on ambulation. She was unable to sense temperature (assessed by touching a cold metal tray with her right hand). There was no neck or back midline tenderness to palpation of her spine.
Initial laboratory studies included a complete blood count (CBC); basic metabolic panel (BMP), including blood urea nitrogen; and urine drug screen (UDS). The CBC and BMP were within normal limits, except for an elevated creatine kinase of 249 U/L. The UDS was positive for cocaine. A head computed tomography (CT) scan without contrast was unremarkable.
The patient was admitted to the hospital for further evaluation. Additional laboratory workup during the inpatient stay included nonreactive treponemal immunoglobulin G/immunoglobulin M; nonreactive HIV antigen antibody assay; normal thyroid stimulating hormone; normal free thyroxine, folate, and vitamin B12 levels; normal erythrocyte sedimentation rate, and C-reactive protein levels. The patient’s hemoglobin A1C was also within normal range.
Other imaging studies, which included MR angiography (MRA) of the head and neck, and MRI of the thoracic and lumbar spine, were unremarkable, with the exception of some chronic spondylitic changes.
Due to the significant C3-C4 stenosis, orthopedic surgery services were consulted for a spinal surgery workup. The orthopedic examination identified a few beats of clonus, intact proprioception, and no dysmetria. The patient had decreased sensation to fine touch in the distribution of C7 at the level of the triceps, midphalangeal joints to distal fingertips on the right, fourth, and fifth fingers on the left and right lateral lower extremity. A Hoffmann sign was positive bilaterally. A CT scan of the cervical spine showed severe canal stenosis at the C3-C4 level secondary to a large C3-C4 left paracentral disc protrusion with AP dimensions of the canal measured at 4 to 5 mm. There was no evidence of acute cervical spine fracture or subluxation.
The patient was offered operative and nonoperative management options, including anterior cervical discectomy and fusion vs conservative management with corticosteroid therapy. She agreed to conservative management and received intravenous (IV) dexamethasone with subjective improvement in her symptoms. The patient was discharged home on hospital day 3, with instructions to follow-up with a spine surgeon in 2 weeks. She was also counseled on abstaining from further cocaine or other illicit drug use.
The patient eventually returned for an elective anterior cervical discectomy and fusion 2 months later, after several outpatient visits and progression of symptoms. She was discharged home on postoperative day 1 with pain well-controlled and was able to ambulate without assistance. On follow-up, she reported 15% improvement in her symptoms.
Discussion
Cervical spondylotic myelopathy (CSM) is the most common myelopathy in patients aged 55 years and older. Immediate neuroimaging studies followed by spinal surgery consultation are recommended for patients presenting with acute symptoms suggestive of cord compression.1,2
Diagnosis and Differential Diagnosis
Diagnosis of CSM can be made with a thorough patient history, neurological examination, and MRI/MRA. However, because cases of cervical disc herniation (CDH) are often atraumatic, the patient history may not always be contributory to the diagnosis and severity of the offending cause.
During our patient’s hospital course, there was initially a concern for Brown-Séquard syndrome (BSS) due to the lateralizing symptoms and radiographic findings. This is a rare condition that can occur in the setting of spinal trauma, unilateral disc herniation, tumors, epidural hematomas, and spinal cord ischemia.3,4 In one retrospective case review by Sayer et al,4 the incidence of CDH causing BSS was only 0.21% (5 per 2,350 patients), and 67% of the cases involved C5-C6 or C6-C7.
Although disc herniation usually presents with symptoms on the ipsilateral side in patients with BSS, there are rare case reports of patients with contralateral symptoms in the form of complete or incomplete BSS manifesting as ipsilateral motor deficit and/or loss of contralateral pain and temperature due to an incomplete spinal cord compression.5-7 We were able to rule-out BSS in our patient due to the absence of motor symptoms.
Treatment
Corticosteroid Therapy. High-dose IV corticosteroids should be given to all patients with CSM prior to surgery to reduce cord edema caused by spinal cord injury. In one randomized control trial by Bracken et al,8 patients given methylprednisolone within 8 hours of spinal cord injury had improvement in motor function, sensation to pinprick, and touch at 6 months when compared to placebo. When the aforementioned steps are taken in the emergent care setting, they may significantly improve patient outcomes.
Surgical Intervention. All cases of CSM in the review literature were treated surgically with laminectomy or hemilaminectomy, anterior discectomy with or without fusion, or corpectomy followed by interbody fusion, with the goal of achieving cord decompression. A large majority of patients underwent anterior discectomy with interbody fusion, and all of the cases recommend early surgical intervention in severe CSM to prevent rapidly worsening symptoms, including permanent hemiparesis.
Early surgical intervention is positively correlated with better outcomes, most often resulting in significant improvement of symptoms to full recovery.3,4,6,7,9-12 In one case report, surgical intervention did not result in a significant improvement, and the patient had been suffering from progressive symptoms for 7 years prior to diagnosis and treatment.11
Conservative Management. Conservative management of CSM includes immobilization, activity modification, pain management, and/or corticosteroids therapy.13 However, for patients undergoing surgical decompression, 50% to 80% reported symptom improvement.14,15 This evidence strongly supports management of CSM with early diagnosis and surgical intervention. Despite delays in diagnosis and treatment, surgical intervention can still offer significant relief of weakness and sensory deficits associated with severe CSM.11
Conclusion
Cervical spondylotic myelopathy is the most common myelopathy in patients aged 55 years and older. Common symptoms involve upper extremity sensation, gait disturbances, and deterioration of hand use16; however, there is a large differential for patients presenting to the ED with these symptoms, including mass effect, infection, vascular conditions, metabolic disorders, inflammatory conditions, and trauma.
Our patient with CSM presented with signs of an incomplete cord syndrome with lateralizing features caused by asymmetric disc herniation. This case is unique in that though our patient had some symptom resolution with corticosteroids therapy alone, she ultimately returned for definitive surgical decompression after symptom progression.
1. Chen TY, Dickman CA, Eleraky M, Sonntag VK. The role of decompression for acute incomplete cervical spinal cord injury in cervical spondylosis. Spine (Phila Pa 1976). 1998;23(22):2398-2403.
2. Ishida Y, Tominaga T. Predictors of neurologic recovery in acute central cervical cord injury with only upper extremity impairment. Spine (Phila Pa 1976). 2002;27(15):1652-1658. discussion 1658.
3. Porto GB, Tan LA, Kasliwal MK, Traynelis VC. Progressive Brown-Séquard syndrome: a rare manifestation of cervical disc herniation. J Clin Neurosci. 2016;29:196-198. doi:10.1016/j.jocn.2015.12.021
4. Sayer FT, Vitali AM, Low HL, Paquette S, Honey CR. Brown-Sèquard syndrome produced by C3-C4 cervical disc herniation: a case report and review of the literature. Spine (Phila Pa 1976). 2008;33(9):E279-E282. doi:10.1097/BRS.0b013e31816c835d.
5. Urrutia J, Fadic R. Cervical disc herniation producing acute Brown-Sequard syndrome: dynamic changes documented by intraoperative neuromonitoring. Eur Spine J. 2012;21 Suppl 4:S418-S421. doi:10.1007/s00586-011-1881-8.
6. Choi KB, Lee CD, Chung DJ, Lee SH. Cervical disc herniation as a cause of Brown-Séquard syndrome. J Korean Neurosurg Soc. 2009;46(5):505-510. doi:10.3340/jkns.2009.46.5.505. doi:10.3340/jkns.2009.46.5.505.
7. Kobayashi N, Asamoto S, Doi H, Sugiyama H. Brown-Sèquard syndrome produced by cervical disc herniation: report of two cases and review of the literature. Spine J. 2003;3(6):530-533.
8. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990; 322(20):1405-1411. doi:10.1056/NEJM199005173222001.
9. Stookey B. Compression of the spinal cord due to ventral extradural cervical chondromas: diagnosis and surgical treatment. Arch Neurol Psychiatry. 1928;20:275-291.
10. Antich PA, Sanjuan AC, Girvent FM, Simó JD. High cervical disc herniation and Brown-Sequard syndrome. A case report and review of the literature. J Bone Joint Surg Br. 1999;81(3):462-463.
11. Guan D, Wang G, Claire M, Kuang Z. Brown-Sequard syndrome produced by calcified herniated cervical disc and posterior vertebral osteophyte: case report.
J Orthop. 2015;12(Suppl 2):S260-S263. doi:10.1016/j.jor.2015.10.007.
12. Abouhashem S, Ammar M, Barakat M, Abdelhameed E. Management of Brown-Sequard syndrome in cervical disc diseases. Turk Neurosurg. 2013;23(4):470-475. doi:10.5137/1019-5149.JTN.7433-12.0.
13. Mazanec D, Reddy A. Medical management of cervical spondylosis. Neurosurgery. 2007;60(1 Suppl 1):S43-S50. doi:10.1227/01.NEU.0000215386.05760.6D.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64(Suppl 1):S1:30-S1:35; discussion S1:35-S1:36. doi:10.1016/j.surneu.2005.02.016.
15. Cheung WY, Arvinte D, Wong YW, Luk KD, Cheung KM. Neurological recovery after surgical decompression in patients with cervical spondylotic myelopathy - a prospective study. Int Orthop. 2008;32(2):273-378. doi:10.1007/s00264-006-0315-4.
16. Chiles BW 3rd, Leonard MA, Choudhri HF, Cooper PR. Cervical spondylotic myelopathy: patterns of neurological deficit and recovery after anterior cervical decompression. Neurosurgery. 1999;44(4):762-769; discussion 769-770
1. Chen TY, Dickman CA, Eleraky M, Sonntag VK. The role of decompression for acute incomplete cervical spinal cord injury in cervical spondylosis. Spine (Phila Pa 1976). 1998;23(22):2398-2403.
2. Ishida Y, Tominaga T. Predictors of neurologic recovery in acute central cervical cord injury with only upper extremity impairment. Spine (Phila Pa 1976). 2002;27(15):1652-1658. discussion 1658.
3. Porto GB, Tan LA, Kasliwal MK, Traynelis VC. Progressive Brown-Séquard syndrome: a rare manifestation of cervical disc herniation. J Clin Neurosci. 2016;29:196-198. doi:10.1016/j.jocn.2015.12.021
4. Sayer FT, Vitali AM, Low HL, Paquette S, Honey CR. Brown-Sèquard syndrome produced by C3-C4 cervical disc herniation: a case report and review of the literature. Spine (Phila Pa 1976). 2008;33(9):E279-E282. doi:10.1097/BRS.0b013e31816c835d.
5. Urrutia J, Fadic R. Cervical disc herniation producing acute Brown-Sequard syndrome: dynamic changes documented by intraoperative neuromonitoring. Eur Spine J. 2012;21 Suppl 4:S418-S421. doi:10.1007/s00586-011-1881-8.
6. Choi KB, Lee CD, Chung DJ, Lee SH. Cervical disc herniation as a cause of Brown-Séquard syndrome. J Korean Neurosurg Soc. 2009;46(5):505-510. doi:10.3340/jkns.2009.46.5.505. doi:10.3340/jkns.2009.46.5.505.
7. Kobayashi N, Asamoto S, Doi H, Sugiyama H. Brown-Sèquard syndrome produced by cervical disc herniation: report of two cases and review of the literature. Spine J. 2003;3(6):530-533.
8. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990; 322(20):1405-1411. doi:10.1056/NEJM199005173222001.
9. Stookey B. Compression of the spinal cord due to ventral extradural cervical chondromas: diagnosis and surgical treatment. Arch Neurol Psychiatry. 1928;20:275-291.
10. Antich PA, Sanjuan AC, Girvent FM, Simó JD. High cervical disc herniation and Brown-Sequard syndrome. A case report and review of the literature. J Bone Joint Surg Br. 1999;81(3):462-463.
11. Guan D, Wang G, Claire M, Kuang Z. Brown-Sequard syndrome produced by calcified herniated cervical disc and posterior vertebral osteophyte: case report.
J Orthop. 2015;12(Suppl 2):S260-S263. doi:10.1016/j.jor.2015.10.007.
12. Abouhashem S, Ammar M, Barakat M, Abdelhameed E. Management of Brown-Sequard syndrome in cervical disc diseases. Turk Neurosurg. 2013;23(4):470-475. doi:10.5137/1019-5149.JTN.7433-12.0.
13. Mazanec D, Reddy A. Medical management of cervical spondylosis. Neurosurgery. 2007;60(1 Suppl 1):S43-S50. doi:10.1227/01.NEU.0000215386.05760.6D.
14. Chagas H, Domingues F, Aversa A, Vidal Fonseca AL, de Souza JM. Cervical spondylotic myelopathy: 10 years of prospective outcome analysis of anterior decompression and fusion. Surg Neurol. 2005;64(Suppl 1):S1:30-S1:35; discussion S1:35-S1:36. doi:10.1016/j.surneu.2005.02.016.
15. Cheung WY, Arvinte D, Wong YW, Luk KD, Cheung KM. Neurological recovery after surgical decompression in patients with cervical spondylotic myelopathy - a prospective study. Int Orthop. 2008;32(2):273-378. doi:10.1007/s00264-006-0315-4.
16. Chiles BW 3rd, Leonard MA, Choudhri HF, Cooper PR. Cervical spondylotic myelopathy: patterns of neurological deficit and recovery after anterior cervical decompression. Neurosurgery. 1999;44(4):762-769; discussion 769-770
Idiopathic Intracranial Hypertension in a 24-Year-Old Woman
Case
A 24-year-old woman presented to the ED for evaluation of a 3-week history of worsening headache and a 5-day history of increasingly blurry vision. The patient stated that she had initially contacted her primary care physician, but instead presented to the ED because he had no open appointments until the following week and recommended that she go to the ED.
The patient described her headache as a pulsating and throbbing pain over her entire head, which only mildly improved after taking over-the-counter (OTC) ibuprofen. She further noted that her headache was somewhat worse when lying down, and reported the sensation of hearing her own pulsating heartbeat in her ears.
The patient had no personal or family history of migraines, tension headaches, aneurysms, clotting disorders, bleeding disorders, or renal disease, and stated that she had never experienced this type of headache before. She denied photophobia, phonophobia, neck stiffness, fever, vomiting, cough, numbness or weakness in her extremities, or pain anywhere else in her body.
Over the past 5 days, the patient noticed her vision had become increasingly blurry. She was not on any prescription medications, stating the only medication she used was occasional OTC ibuprofen. She had no known allergy to medications and denied smoking or recreational drug use; she admitted to occasional alcohol consumption.
The patient resided with her husband, who had no similar symptoms. Physical examination showed an obese woman (height, 5 ft 6 in; weight, 195 lb; body mass index, 32 kg/m2), lying supine in apparent discomfort. Vital signs at presentation were all normal, and oxygen saturation was normal on room air.
A bedside ocular examination showed 20/100 in both eyes while using glasses; no visual field cuts or obvious central scotoma was present. The patient was alert and oriented to time and place. The neurological examination showed intact cranial nerves, 5/5 strength in all extremities, intact sensation in all extremities, no pronator drift, negative Romberg test, and a normal gait. Fundoscopic examination revealed mildly blurred medial optic discs bilaterally. The rest of the physical examination was normal.
Discussion
Pseudotumor cerebri, more commonly referred to as idiopathic intracranial hypertension (IIH), is characterized by increased intracranial pressure (ICP) with no explanatory findings on imaging studies or in cerebrospinal fluid (CSF) analysis, and may be accompanied by symptoms of chronic headache, tinnitus, papilledema and progressive vision loss caused by optic nerve damage.1 Though historically IIH was referred to by several other names, including “benign intracranial hypertension,” the condition is not benign—when untreated, IIH can cause chronic disabling headaches and permanent vision loss.1
Clinical Course
The clinical course of IIH is unpredictable: In some patients, vision loss occurs gradually over the course of several weeks, while in others, loss occurs over a several month period. There are also patients with IIH who do not experience any alteration or loss of vision. Furthermore, some patients will experience permanent resolution of symptoms after a single lumbar puncture (LP); others have symptom recurrence after less than 24 hours; and some patients spontaneously remit on their own with no treatment whatsoever.1-4
Etiology
In the United States, IIH is a rare cause of headache, occurring in just 1 person per 100,000 annually.1 Though 90% of IIH cases occur in obese women of childbearing age, the etiology of IIH is unknown. Lumbar puncture usually alleviates the patient’s headache, but the CSF pressure typically returns to its pre-tap levels after a few hours.4,5 Neither CSF overproduction nor insufficient CSF resorption is responsible for causing IIH. One theory on the etiology of IIH proposes its cause to be due to a congenital malformation of the venous sinuses. This theory would explain why the symptoms so closely mimic those of venous sinus thrombosis, and why some IIH patients experience relief of symptoms after placement of a venous sinus stent.2
Symptoms
As noted previously, the most common symptom of IIH is headache, which patients usually describe as pressure-like and throbbing, and often involving retro-ocular pain. One feature in over half of patients is pulse-synchronous tinnitus (ie, hearing their own heartbeat in their ears). Eye pain, photophobia, blurry vision, and nausea/vomiting are all common symptoms in IIH, but these symptoms are also present in other causes of headache. The IIH headache might be relapsing and remitting, and can last from a few hours to weeks.2-4,6
Diagnosis
Imaging Studies. Noncontrast computed tomography (CT) imaging studies do not typically demonstrate any abnormal findings.1 Magnetic resonance imaging (MRI) studies show some inconsistent and subtle findings, such as flattening of the backs of the eyeballs, empty sella, or tortuous optic nerves.1
Lumbar Puncture. On LP, a very high opening pressure is a hallmark of IIH. An opening pressure <20 cm H2O is generally considered normal, 20 cm to 25 cm H2O is “equivocal,” and >25 cm H2O is abnormal.7 Patients presenting with IIH commonly have an opening pressure that exceeds 200 cm H2O.1-3 Extremely high pressures, however, are not required for the diagnosis, but some elevations in opening pressure will always be present.2,5 With the exception of a high opening pressure, the patient’s CSF analysis is normal.
Differential Diagnosis
Idiopathic intracranial hypertension is essentially a diagnosis of exclusion, one that is made after exclusion of all other potential causes of increased ICP (Table). Since contrast CT and MRI can identify subtle anatomical deformities and small lesions, their absence on these studies can help establish a diagnosis of IIH.
Venous Sinus Thrombosis. Venous sinus thrombosis is a rare but devastating condition that also cannot be diagnosed from a noncontrast CT but must always be considered in the differential diagnosis of IIH.8-10 Venous sinus thrombosis is characterized by a clot in one of the large venous sinuses that drain blood from the brain; the clot causes pressure to back up into the smaller cerebral vasculature, eventually inducing either a hemorrhagic stroke from a stressed vessel rupturing, or an ischemic stroke from lack of blood flow to the affected area of the brain. This condition is even more rare than IIH (0.5 cases per 100,000 population), but it can be devastating if missed, carrying a mortality rate as high as 15% in some studies.11
Risk Factors
Risk factors known to cause cerebral venous clots include genetic thrombophilias, pregnancy or recent pregnancy, oral contraceptive use, inflammatory bowel disease, severe dehydration, local infection/trauma, and substance abuse. Regardless of risk factors, the most recent guidelines of the American Heart Association/American Stroke Association recommend imaging studies of the cerebral venous sinuses for any patient presenting with new-onset symptoms suggestive of IIH (Class 1, Level of Evidence C).11 The two imaging options for evaluation of the cerebral venous sinuses are CT venography or MR venography. Since the 2013 American College of Radiology Appropriateness Criteria do not indicate a preference of one modality over the other, the choice of can be left to your radiologist.12
Patient Disposition
Patients with IIH typically do not require inpatient admission. Only about 3% of IIH patients will have a fulminant course of rapid-onset of vision loss, but even the most severe and acute cases will deteriorate over weeks, not hours or days.13 Nevertheless, close neurology follow-up is essential. If rapid and thorough outpatient neurological care is unavailable, admission is required.
Management
Not every patient with IIH experiences amelioration or resolution of symptoms following an LP; moreover, there is no clear way to differentiate patients who will experience therapeutic effects from LP from those who will not. Serial LPs as treatment for IIH have been discussed in the literature, but a ventriculoperitoneal shunt is a more practical approach in patients who do not respond to an initial LP.2,14
CSF Volume. The volume of CSF that can be removed safely may be 15 to 25 mL or more. A 1974 paper by Johnston and Paterson15 described five pseudotumor patients whose CSF was drained until their pressure had normalized; the amount removed varied from 15 to 25 mL, without adverse effects. A 1975 case series by Weisberg6 described safe removal of up to 30 mL of CSF in pseudotumor patients—the precise amount removed was determined by that which was necessary to lower the CSF pressure into the normal range. In 2007, a case report by Aly and Lawther16 of a pregnant woman with IIH describes twice weekly LP drainage of 30 mL.
There is nothing in the current literature to suggest that removing 10 to 30 mL of CSF instead of the 4 to 8 mL typically drawn in a diagnostic LP is going to pose any risk to the patient. The main complication associated with therapeutic LP is post-LP headache.5,17,18 There are currently no studies documenting outcomes after specific amounts of CSF removal.
Lifestyle Modifications: Weight Loss. No prospective, randomized controlled trials have proven weight loss to be effective in ameliorating the symptoms of IIH; however, several studies have found that rapid weight loss—whether through aggressive dieting or gastric bypass surgery—can improve symptoms dramatically within several months.19,20 One small study by Johnson et al has suggested that a 6% weight reduction is associated with marked improvement in papilledema.21Pharmacotherapy. The accepted first-line medication to alleviate symptoms of IIH is acetazolamide, and its use is supported by a recent randomized controlled trial conducted by the Neuro-Ophthalmology Research Disease Investigator Consortium (NORDIC).22 Most neurologists will administer a starting dose of acetazolamide 500 mg twice a day, and then increase the dose until symptoms are controlled or adverse effects appear (eg, fatigue, nausea/vomiting/diarrhea, electrolyte abnormalities, kidney stones) that contraindicate further dosage increases. In the NORDIC trial, patients were given up to 4 g of acetazolamide daily.22
Other medications, including loop diuretics and corticosteroids, should not be used except under the direct supervision of a neurologist.2,14
Refractory Cases
A patient who fails conservative treatment should be referred to a neurosurgeon for placement of a CSF shunt, optic nerve sheath fenestration, or placement of a venous sinus stent.23
Case Conclusion
After a noncontrast CT of the head was interpreted as completely normal, an LP was performed with the patient in the left lateral recumbent position. The opening CSF pressure exceeded 55 cm H2O (the upper limit of the manometer). The CSF was clear, and opening pressure was rechecked after each 5 mL draw. After 15 mL had been removed, the patient reported a sudden, dramatic disappearance of her headache and clearing of her vision. After 19 mL of CSF had been removed, the CSF pressure had dropped into the normal range (<20 cm H2O), and the procedure was ended.
To definitively rule out venous sinus thrombosis, a CT venogram was performed in the ED, and interpreted as normal. All other CSF results (cell count, protein, glucose, and gram stain) were normal. After complete resolution of the patient’s symptoms, she was discharged home with a prescription for acetazolamide 500 mg twice daily and instructions to follow-up with a neurologist within 48 hours. At discharge, the patient also received weight-loss counseling and was instructed to return immediately to the ED if her headache recurred or if she experienced any new neurological symptoms.
Summary
Idiopathic intracranial hypertension, also referred to as pseudotumor cerebri, is a rare but potentially vision-threatening cause of headache. Patients with signs and symptoms of IIH often initially present to the ED for evaluation and management. While the etiology of IIH is poorly understood, its clinical picture is unique: elevated ICP (sometimes markedly so) with no other significant findings on noncontrast head CT or CSF analysis. Venous sinus thrombosis, a life-threatening mimic of IIH, must always be included in the differential diagnosis.
Idiopathic intracranial hypertension is initially treated with rapid weight loss and acetazolamide. Many patients experience instant, though sometimes only transient, symptom relief from LP. No definitive studies to support any specific approach, including “therapeutic lumbar punctures.” The condition is rarely fulminant, and hospital admission is not typically required as long as urgent outpatient neurology follow-up is available.
1. Degnan AJ, Levy LM. Pseudotumor cerebri: brief review of clinical syndrome and imaging findings. AJNR Am J Neuroradiol. 2011;32(11):1986-1893. doi:10.3174/ajnr.A2404.
2. Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. 2012;83(5):488-494. doi:10.1136/jnnp-2011-302029. 3. Wall M, George D. Idiopathic intracranial hypertension: a prospective study of 50 patients. Brain. 1991;114(Pt 1A):155-180.
4. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28(3):593-617. doi:10.1016/j.ncl.2010.03.003.
5. Friedman DI, Rausch EA. Headache diagnoses in patients with treated idiopathic intracranial hypertension. Neurology. 2002;58(10):1551-1553.
6. Weisberg LA. Benign intracranial hypertension. Medicine (Baltimore). 1975;54(3):197-207.
7. Whiteley W, Al-Shahi R, Warlow CP, Zeidler M, Lueck CJ. CSF opening pressure: reference interval and the effect of body mass index. Neurology. 2006;67(9):1690-1691.
8. Biousse V, Ameri A, Bousser MG. Isolated intracranial hypertension as the only sign of cerebral venous thrombosis. Neurology. 1999;53(7):1537-1542.
9. Leker RR, Steiner I. Features of dural sinus thrombosis simulating pseudotumor cerebri. Eur J Neurol. 1999;6(5):601-604.
10. Sylaja PN, Ahsan Moosa NV, Radhakrishnan K, Sankara Sarma P, Pradeep Kumar S. Differential diagnosis of patients with intracranial sinus venous thrombosis related isolated intracranial hypertension from those with idiopathic intracranial hypertension. J Neurol Sci. 2003;215(1-2):9-12.
11. Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192. doi:10.1161/STR.0b013e31820a8364.
12. American College of Radiology ACR Appropriateness Criteria: Headache. https://acsearch.acr.org/docs/69482/Narrative/. Updated 2013. Accessed January 19, 2017.
13. Thambisetty M, Lavin PJ, Newman NJ, Biousse V. Fulminant idiopathic intracranial hypertension. Neurology. 2007;68(3):229-232.
14. Mollan SP, Markey KA, Benzimra JD, et al. A practical approach to, diagnosis, assessment and management of idiopathic intracranial hypertension. Pract Neurol. 2014;14(6):380-390. doi:10.1136/practneurol-2014-000821.
15. Johnston I, Paterson A. Benign intracranial hypertension. II. CSF pressure and circulation. Brain. 1974;97(2):301-312.
16. Aly EE, Lawther BK. Anaesthetic management of uncontrolled idiopathic intracranial hypertension during labour and delivery using an intrathecal catheter. Anesthesia. 2007;62(2):178-181.
17. Panikkath R, Welker J, Johnston R, Lado-Abeal J. Intracranial hypertension and intracranial hypotension causing headache in the same patient. Proc (Bayl Univ Med Cent). 2014;27(3):217-218.
18. Nafiu OO, Monterosso D, Walton SR, Bradin S. Post dural puncture headache in a pediatric patient with idiopathic intracranial hypertension. Paediatr Anaesth. 2005;15(9):778-781. doi:10.1111/j.1460-9592.2004.01529.x.
19. Sinclair AJ, Burdon MA, Nightingale PG, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ. 2010;341:c2701. doi:10.1136/bmj.c2701.
20. Kupersmith MJ, Gamell L, Turbin R, Peck V, Spiegel P, Wall M. Effects of weight loss on the course of idiopathic intracranial hypertension in women. Neurology. 1998;50(4):1094-1098.
21. Johnson LN, Krohel GB, Madsen RW, March GA Jr. The role of weight loss and acetazolamide in the treatment of idiopathic intracranial hypertension (pseudotumor cerebri) Ophthalmology. 1998;105(12):2313-2317. doi:10.1016/S0161-6420(98)91234-9.
22. NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee; Wall M, McDermott MP, Kieburtz KD, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA. 2014;311(16):1641-1651. doi:10.1001/jama.2014.3312.
23. Fonseca PL, Rigamonti D, Miller NR, Subramanian PS. Visual outcomes of surgical intervention for pseudotumour cerebri: optic nerve sheath fenestration versus cerebrospinal fluid diversion. Br J Ophthalmol. 2014;98(10):1360-1363. doi:10.1136/bjophthalmol-2014-304953.
Case
A 24-year-old woman presented to the ED for evaluation of a 3-week history of worsening headache and a 5-day history of increasingly blurry vision. The patient stated that she had initially contacted her primary care physician, but instead presented to the ED because he had no open appointments until the following week and recommended that she go to the ED.
The patient described her headache as a pulsating and throbbing pain over her entire head, which only mildly improved after taking over-the-counter (OTC) ibuprofen. She further noted that her headache was somewhat worse when lying down, and reported the sensation of hearing her own pulsating heartbeat in her ears.
The patient had no personal or family history of migraines, tension headaches, aneurysms, clotting disorders, bleeding disorders, or renal disease, and stated that she had never experienced this type of headache before. She denied photophobia, phonophobia, neck stiffness, fever, vomiting, cough, numbness or weakness in her extremities, or pain anywhere else in her body.
Over the past 5 days, the patient noticed her vision had become increasingly blurry. She was not on any prescription medications, stating the only medication she used was occasional OTC ibuprofen. She had no known allergy to medications and denied smoking or recreational drug use; she admitted to occasional alcohol consumption.
The patient resided with her husband, who had no similar symptoms. Physical examination showed an obese woman (height, 5 ft 6 in; weight, 195 lb; body mass index, 32 kg/m2), lying supine in apparent discomfort. Vital signs at presentation were all normal, and oxygen saturation was normal on room air.
A bedside ocular examination showed 20/100 in both eyes while using glasses; no visual field cuts or obvious central scotoma was present. The patient was alert and oriented to time and place. The neurological examination showed intact cranial nerves, 5/5 strength in all extremities, intact sensation in all extremities, no pronator drift, negative Romberg test, and a normal gait. Fundoscopic examination revealed mildly blurred medial optic discs bilaterally. The rest of the physical examination was normal.
Discussion
Pseudotumor cerebri, more commonly referred to as idiopathic intracranial hypertension (IIH), is characterized by increased intracranial pressure (ICP) with no explanatory findings on imaging studies or in cerebrospinal fluid (CSF) analysis, and may be accompanied by symptoms of chronic headache, tinnitus, papilledema and progressive vision loss caused by optic nerve damage.1 Though historically IIH was referred to by several other names, including “benign intracranial hypertension,” the condition is not benign—when untreated, IIH can cause chronic disabling headaches and permanent vision loss.1
Clinical Course
The clinical course of IIH is unpredictable: In some patients, vision loss occurs gradually over the course of several weeks, while in others, loss occurs over a several month period. There are also patients with IIH who do not experience any alteration or loss of vision. Furthermore, some patients will experience permanent resolution of symptoms after a single lumbar puncture (LP); others have symptom recurrence after less than 24 hours; and some patients spontaneously remit on their own with no treatment whatsoever.1-4
Etiology
In the United States, IIH is a rare cause of headache, occurring in just 1 person per 100,000 annually.1 Though 90% of IIH cases occur in obese women of childbearing age, the etiology of IIH is unknown. Lumbar puncture usually alleviates the patient’s headache, but the CSF pressure typically returns to its pre-tap levels after a few hours.4,5 Neither CSF overproduction nor insufficient CSF resorption is responsible for causing IIH. One theory on the etiology of IIH proposes its cause to be due to a congenital malformation of the venous sinuses. This theory would explain why the symptoms so closely mimic those of venous sinus thrombosis, and why some IIH patients experience relief of symptoms after placement of a venous sinus stent.2
Symptoms
As noted previously, the most common symptom of IIH is headache, which patients usually describe as pressure-like and throbbing, and often involving retro-ocular pain. One feature in over half of patients is pulse-synchronous tinnitus (ie, hearing their own heartbeat in their ears). Eye pain, photophobia, blurry vision, and nausea/vomiting are all common symptoms in IIH, but these symptoms are also present in other causes of headache. The IIH headache might be relapsing and remitting, and can last from a few hours to weeks.2-4,6
Diagnosis
Imaging Studies. Noncontrast computed tomography (CT) imaging studies do not typically demonstrate any abnormal findings.1 Magnetic resonance imaging (MRI) studies show some inconsistent and subtle findings, such as flattening of the backs of the eyeballs, empty sella, or tortuous optic nerves.1
Lumbar Puncture. On LP, a very high opening pressure is a hallmark of IIH. An opening pressure <20 cm H2O is generally considered normal, 20 cm to 25 cm H2O is “equivocal,” and >25 cm H2O is abnormal.7 Patients presenting with IIH commonly have an opening pressure that exceeds 200 cm H2O.1-3 Extremely high pressures, however, are not required for the diagnosis, but some elevations in opening pressure will always be present.2,5 With the exception of a high opening pressure, the patient’s CSF analysis is normal.
Differential Diagnosis
Idiopathic intracranial hypertension is essentially a diagnosis of exclusion, one that is made after exclusion of all other potential causes of increased ICP (Table). Since contrast CT and MRI can identify subtle anatomical deformities and small lesions, their absence on these studies can help establish a diagnosis of IIH.
Venous Sinus Thrombosis. Venous sinus thrombosis is a rare but devastating condition that also cannot be diagnosed from a noncontrast CT but must always be considered in the differential diagnosis of IIH.8-10 Venous sinus thrombosis is characterized by a clot in one of the large venous sinuses that drain blood from the brain; the clot causes pressure to back up into the smaller cerebral vasculature, eventually inducing either a hemorrhagic stroke from a stressed vessel rupturing, or an ischemic stroke from lack of blood flow to the affected area of the brain. This condition is even more rare than IIH (0.5 cases per 100,000 population), but it can be devastating if missed, carrying a mortality rate as high as 15% in some studies.11
Risk Factors
Risk factors known to cause cerebral venous clots include genetic thrombophilias, pregnancy or recent pregnancy, oral contraceptive use, inflammatory bowel disease, severe dehydration, local infection/trauma, and substance abuse. Regardless of risk factors, the most recent guidelines of the American Heart Association/American Stroke Association recommend imaging studies of the cerebral venous sinuses for any patient presenting with new-onset symptoms suggestive of IIH (Class 1, Level of Evidence C).11 The two imaging options for evaluation of the cerebral venous sinuses are CT venography or MR venography. Since the 2013 American College of Radiology Appropriateness Criteria do not indicate a preference of one modality over the other, the choice of can be left to your radiologist.12
Patient Disposition
Patients with IIH typically do not require inpatient admission. Only about 3% of IIH patients will have a fulminant course of rapid-onset of vision loss, but even the most severe and acute cases will deteriorate over weeks, not hours or days.13 Nevertheless, close neurology follow-up is essential. If rapid and thorough outpatient neurological care is unavailable, admission is required.
Management
Not every patient with IIH experiences amelioration or resolution of symptoms following an LP; moreover, there is no clear way to differentiate patients who will experience therapeutic effects from LP from those who will not. Serial LPs as treatment for IIH have been discussed in the literature, but a ventriculoperitoneal shunt is a more practical approach in patients who do not respond to an initial LP.2,14
CSF Volume. The volume of CSF that can be removed safely may be 15 to 25 mL or more. A 1974 paper by Johnston and Paterson15 described five pseudotumor patients whose CSF was drained until their pressure had normalized; the amount removed varied from 15 to 25 mL, without adverse effects. A 1975 case series by Weisberg6 described safe removal of up to 30 mL of CSF in pseudotumor patients—the precise amount removed was determined by that which was necessary to lower the CSF pressure into the normal range. In 2007, a case report by Aly and Lawther16 of a pregnant woman with IIH describes twice weekly LP drainage of 30 mL.
There is nothing in the current literature to suggest that removing 10 to 30 mL of CSF instead of the 4 to 8 mL typically drawn in a diagnostic LP is going to pose any risk to the patient. The main complication associated with therapeutic LP is post-LP headache.5,17,18 There are currently no studies documenting outcomes after specific amounts of CSF removal.
Lifestyle Modifications: Weight Loss. No prospective, randomized controlled trials have proven weight loss to be effective in ameliorating the symptoms of IIH; however, several studies have found that rapid weight loss—whether through aggressive dieting or gastric bypass surgery—can improve symptoms dramatically within several months.19,20 One small study by Johnson et al has suggested that a 6% weight reduction is associated with marked improvement in papilledema.21Pharmacotherapy. The accepted first-line medication to alleviate symptoms of IIH is acetazolamide, and its use is supported by a recent randomized controlled trial conducted by the Neuro-Ophthalmology Research Disease Investigator Consortium (NORDIC).22 Most neurologists will administer a starting dose of acetazolamide 500 mg twice a day, and then increase the dose until symptoms are controlled or adverse effects appear (eg, fatigue, nausea/vomiting/diarrhea, electrolyte abnormalities, kidney stones) that contraindicate further dosage increases. In the NORDIC trial, patients were given up to 4 g of acetazolamide daily.22
Other medications, including loop diuretics and corticosteroids, should not be used except under the direct supervision of a neurologist.2,14
Refractory Cases
A patient who fails conservative treatment should be referred to a neurosurgeon for placement of a CSF shunt, optic nerve sheath fenestration, or placement of a venous sinus stent.23
Case Conclusion
After a noncontrast CT of the head was interpreted as completely normal, an LP was performed with the patient in the left lateral recumbent position. The opening CSF pressure exceeded 55 cm H2O (the upper limit of the manometer). The CSF was clear, and opening pressure was rechecked after each 5 mL draw. After 15 mL had been removed, the patient reported a sudden, dramatic disappearance of her headache and clearing of her vision. After 19 mL of CSF had been removed, the CSF pressure had dropped into the normal range (<20 cm H2O), and the procedure was ended.
To definitively rule out venous sinus thrombosis, a CT venogram was performed in the ED, and interpreted as normal. All other CSF results (cell count, protein, glucose, and gram stain) were normal. After complete resolution of the patient’s symptoms, she was discharged home with a prescription for acetazolamide 500 mg twice daily and instructions to follow-up with a neurologist within 48 hours. At discharge, the patient also received weight-loss counseling and was instructed to return immediately to the ED if her headache recurred or if she experienced any new neurological symptoms.
Summary
Idiopathic intracranial hypertension, also referred to as pseudotumor cerebri, is a rare but potentially vision-threatening cause of headache. Patients with signs and symptoms of IIH often initially present to the ED for evaluation and management. While the etiology of IIH is poorly understood, its clinical picture is unique: elevated ICP (sometimes markedly so) with no other significant findings on noncontrast head CT or CSF analysis. Venous sinus thrombosis, a life-threatening mimic of IIH, must always be included in the differential diagnosis.
Idiopathic intracranial hypertension is initially treated with rapid weight loss and acetazolamide. Many patients experience instant, though sometimes only transient, symptom relief from LP. No definitive studies to support any specific approach, including “therapeutic lumbar punctures.” The condition is rarely fulminant, and hospital admission is not typically required as long as urgent outpatient neurology follow-up is available.
Case
A 24-year-old woman presented to the ED for evaluation of a 3-week history of worsening headache and a 5-day history of increasingly blurry vision. The patient stated that she had initially contacted her primary care physician, but instead presented to the ED because he had no open appointments until the following week and recommended that she go to the ED.
The patient described her headache as a pulsating and throbbing pain over her entire head, which only mildly improved after taking over-the-counter (OTC) ibuprofen. She further noted that her headache was somewhat worse when lying down, and reported the sensation of hearing her own pulsating heartbeat in her ears.
The patient had no personal or family history of migraines, tension headaches, aneurysms, clotting disorders, bleeding disorders, or renal disease, and stated that she had never experienced this type of headache before. She denied photophobia, phonophobia, neck stiffness, fever, vomiting, cough, numbness or weakness in her extremities, or pain anywhere else in her body.
Over the past 5 days, the patient noticed her vision had become increasingly blurry. She was not on any prescription medications, stating the only medication she used was occasional OTC ibuprofen. She had no known allergy to medications and denied smoking or recreational drug use; she admitted to occasional alcohol consumption.
The patient resided with her husband, who had no similar symptoms. Physical examination showed an obese woman (height, 5 ft 6 in; weight, 195 lb; body mass index, 32 kg/m2), lying supine in apparent discomfort. Vital signs at presentation were all normal, and oxygen saturation was normal on room air.
A bedside ocular examination showed 20/100 in both eyes while using glasses; no visual field cuts or obvious central scotoma was present. The patient was alert and oriented to time and place. The neurological examination showed intact cranial nerves, 5/5 strength in all extremities, intact sensation in all extremities, no pronator drift, negative Romberg test, and a normal gait. Fundoscopic examination revealed mildly blurred medial optic discs bilaterally. The rest of the physical examination was normal.
Discussion
Pseudotumor cerebri, more commonly referred to as idiopathic intracranial hypertension (IIH), is characterized by increased intracranial pressure (ICP) with no explanatory findings on imaging studies or in cerebrospinal fluid (CSF) analysis, and may be accompanied by symptoms of chronic headache, tinnitus, papilledema and progressive vision loss caused by optic nerve damage.1 Though historically IIH was referred to by several other names, including “benign intracranial hypertension,” the condition is not benign—when untreated, IIH can cause chronic disabling headaches and permanent vision loss.1
Clinical Course
The clinical course of IIH is unpredictable: In some patients, vision loss occurs gradually over the course of several weeks, while in others, loss occurs over a several month period. There are also patients with IIH who do not experience any alteration or loss of vision. Furthermore, some patients will experience permanent resolution of symptoms after a single lumbar puncture (LP); others have symptom recurrence after less than 24 hours; and some patients spontaneously remit on their own with no treatment whatsoever.1-4
Etiology
In the United States, IIH is a rare cause of headache, occurring in just 1 person per 100,000 annually.1 Though 90% of IIH cases occur in obese women of childbearing age, the etiology of IIH is unknown. Lumbar puncture usually alleviates the patient’s headache, but the CSF pressure typically returns to its pre-tap levels after a few hours.4,5 Neither CSF overproduction nor insufficient CSF resorption is responsible for causing IIH. One theory on the etiology of IIH proposes its cause to be due to a congenital malformation of the venous sinuses. This theory would explain why the symptoms so closely mimic those of venous sinus thrombosis, and why some IIH patients experience relief of symptoms after placement of a venous sinus stent.2
Symptoms
As noted previously, the most common symptom of IIH is headache, which patients usually describe as pressure-like and throbbing, and often involving retro-ocular pain. One feature in over half of patients is pulse-synchronous tinnitus (ie, hearing their own heartbeat in their ears). Eye pain, photophobia, blurry vision, and nausea/vomiting are all common symptoms in IIH, but these symptoms are also present in other causes of headache. The IIH headache might be relapsing and remitting, and can last from a few hours to weeks.2-4,6
Diagnosis
Imaging Studies. Noncontrast computed tomography (CT) imaging studies do not typically demonstrate any abnormal findings.1 Magnetic resonance imaging (MRI) studies show some inconsistent and subtle findings, such as flattening of the backs of the eyeballs, empty sella, or tortuous optic nerves.1
Lumbar Puncture. On LP, a very high opening pressure is a hallmark of IIH. An opening pressure <20 cm H2O is generally considered normal, 20 cm to 25 cm H2O is “equivocal,” and >25 cm H2O is abnormal.7 Patients presenting with IIH commonly have an opening pressure that exceeds 200 cm H2O.1-3 Extremely high pressures, however, are not required for the diagnosis, but some elevations in opening pressure will always be present.2,5 With the exception of a high opening pressure, the patient’s CSF analysis is normal.
Differential Diagnosis
Idiopathic intracranial hypertension is essentially a diagnosis of exclusion, one that is made after exclusion of all other potential causes of increased ICP (Table). Since contrast CT and MRI can identify subtle anatomical deformities and small lesions, their absence on these studies can help establish a diagnosis of IIH.
Venous Sinus Thrombosis. Venous sinus thrombosis is a rare but devastating condition that also cannot be diagnosed from a noncontrast CT but must always be considered in the differential diagnosis of IIH.8-10 Venous sinus thrombosis is characterized by a clot in one of the large venous sinuses that drain blood from the brain; the clot causes pressure to back up into the smaller cerebral vasculature, eventually inducing either a hemorrhagic stroke from a stressed vessel rupturing, or an ischemic stroke from lack of blood flow to the affected area of the brain. This condition is even more rare than IIH (0.5 cases per 100,000 population), but it can be devastating if missed, carrying a mortality rate as high as 15% in some studies.11
Risk Factors
Risk factors known to cause cerebral venous clots include genetic thrombophilias, pregnancy or recent pregnancy, oral contraceptive use, inflammatory bowel disease, severe dehydration, local infection/trauma, and substance abuse. Regardless of risk factors, the most recent guidelines of the American Heart Association/American Stroke Association recommend imaging studies of the cerebral venous sinuses for any patient presenting with new-onset symptoms suggestive of IIH (Class 1, Level of Evidence C).11 The two imaging options for evaluation of the cerebral venous sinuses are CT venography or MR venography. Since the 2013 American College of Radiology Appropriateness Criteria do not indicate a preference of one modality over the other, the choice of can be left to your radiologist.12
Patient Disposition
Patients with IIH typically do not require inpatient admission. Only about 3% of IIH patients will have a fulminant course of rapid-onset of vision loss, but even the most severe and acute cases will deteriorate over weeks, not hours or days.13 Nevertheless, close neurology follow-up is essential. If rapid and thorough outpatient neurological care is unavailable, admission is required.
Management
Not every patient with IIH experiences amelioration or resolution of symptoms following an LP; moreover, there is no clear way to differentiate patients who will experience therapeutic effects from LP from those who will not. Serial LPs as treatment for IIH have been discussed in the literature, but a ventriculoperitoneal shunt is a more practical approach in patients who do not respond to an initial LP.2,14
CSF Volume. The volume of CSF that can be removed safely may be 15 to 25 mL or more. A 1974 paper by Johnston and Paterson15 described five pseudotumor patients whose CSF was drained until their pressure had normalized; the amount removed varied from 15 to 25 mL, without adverse effects. A 1975 case series by Weisberg6 described safe removal of up to 30 mL of CSF in pseudotumor patients—the precise amount removed was determined by that which was necessary to lower the CSF pressure into the normal range. In 2007, a case report by Aly and Lawther16 of a pregnant woman with IIH describes twice weekly LP drainage of 30 mL.
There is nothing in the current literature to suggest that removing 10 to 30 mL of CSF instead of the 4 to 8 mL typically drawn in a diagnostic LP is going to pose any risk to the patient. The main complication associated with therapeutic LP is post-LP headache.5,17,18 There are currently no studies documenting outcomes after specific amounts of CSF removal.
Lifestyle Modifications: Weight Loss. No prospective, randomized controlled trials have proven weight loss to be effective in ameliorating the symptoms of IIH; however, several studies have found that rapid weight loss—whether through aggressive dieting or gastric bypass surgery—can improve symptoms dramatically within several months.19,20 One small study by Johnson et al has suggested that a 6% weight reduction is associated with marked improvement in papilledema.21Pharmacotherapy. The accepted first-line medication to alleviate symptoms of IIH is acetazolamide, and its use is supported by a recent randomized controlled trial conducted by the Neuro-Ophthalmology Research Disease Investigator Consortium (NORDIC).22 Most neurologists will administer a starting dose of acetazolamide 500 mg twice a day, and then increase the dose until symptoms are controlled or adverse effects appear (eg, fatigue, nausea/vomiting/diarrhea, electrolyte abnormalities, kidney stones) that contraindicate further dosage increases. In the NORDIC trial, patients were given up to 4 g of acetazolamide daily.22
Other medications, including loop diuretics and corticosteroids, should not be used except under the direct supervision of a neurologist.2,14
Refractory Cases
A patient who fails conservative treatment should be referred to a neurosurgeon for placement of a CSF shunt, optic nerve sheath fenestration, or placement of a venous sinus stent.23
Case Conclusion
After a noncontrast CT of the head was interpreted as completely normal, an LP was performed with the patient in the left lateral recumbent position. The opening CSF pressure exceeded 55 cm H2O (the upper limit of the manometer). The CSF was clear, and opening pressure was rechecked after each 5 mL draw. After 15 mL had been removed, the patient reported a sudden, dramatic disappearance of her headache and clearing of her vision. After 19 mL of CSF had been removed, the CSF pressure had dropped into the normal range (<20 cm H2O), and the procedure was ended.
To definitively rule out venous sinus thrombosis, a CT venogram was performed in the ED, and interpreted as normal. All other CSF results (cell count, protein, glucose, and gram stain) were normal. After complete resolution of the patient’s symptoms, she was discharged home with a prescription for acetazolamide 500 mg twice daily and instructions to follow-up with a neurologist within 48 hours. At discharge, the patient also received weight-loss counseling and was instructed to return immediately to the ED if her headache recurred or if she experienced any new neurological symptoms.
Summary
Idiopathic intracranial hypertension, also referred to as pseudotumor cerebri, is a rare but potentially vision-threatening cause of headache. Patients with signs and symptoms of IIH often initially present to the ED for evaluation and management. While the etiology of IIH is poorly understood, its clinical picture is unique: elevated ICP (sometimes markedly so) with no other significant findings on noncontrast head CT or CSF analysis. Venous sinus thrombosis, a life-threatening mimic of IIH, must always be included in the differential diagnosis.
Idiopathic intracranial hypertension is initially treated with rapid weight loss and acetazolamide. Many patients experience instant, though sometimes only transient, symptom relief from LP. No definitive studies to support any specific approach, including “therapeutic lumbar punctures.” The condition is rarely fulminant, and hospital admission is not typically required as long as urgent outpatient neurology follow-up is available.
1. Degnan AJ, Levy LM. Pseudotumor cerebri: brief review of clinical syndrome and imaging findings. AJNR Am J Neuroradiol. 2011;32(11):1986-1893. doi:10.3174/ajnr.A2404.
2. Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. 2012;83(5):488-494. doi:10.1136/jnnp-2011-302029. 3. Wall M, George D. Idiopathic intracranial hypertension: a prospective study of 50 patients. Brain. 1991;114(Pt 1A):155-180.
4. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28(3):593-617. doi:10.1016/j.ncl.2010.03.003.
5. Friedman DI, Rausch EA. Headache diagnoses in patients with treated idiopathic intracranial hypertension. Neurology. 2002;58(10):1551-1553.
6. Weisberg LA. Benign intracranial hypertension. Medicine (Baltimore). 1975;54(3):197-207.
7. Whiteley W, Al-Shahi R, Warlow CP, Zeidler M, Lueck CJ. CSF opening pressure: reference interval and the effect of body mass index. Neurology. 2006;67(9):1690-1691.
8. Biousse V, Ameri A, Bousser MG. Isolated intracranial hypertension as the only sign of cerebral venous thrombosis. Neurology. 1999;53(7):1537-1542.
9. Leker RR, Steiner I. Features of dural sinus thrombosis simulating pseudotumor cerebri. Eur J Neurol. 1999;6(5):601-604.
10. Sylaja PN, Ahsan Moosa NV, Radhakrishnan K, Sankara Sarma P, Pradeep Kumar S. Differential diagnosis of patients with intracranial sinus venous thrombosis related isolated intracranial hypertension from those with idiopathic intracranial hypertension. J Neurol Sci. 2003;215(1-2):9-12.
11. Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192. doi:10.1161/STR.0b013e31820a8364.
12. American College of Radiology ACR Appropriateness Criteria: Headache. https://acsearch.acr.org/docs/69482/Narrative/. Updated 2013. Accessed January 19, 2017.
13. Thambisetty M, Lavin PJ, Newman NJ, Biousse V. Fulminant idiopathic intracranial hypertension. Neurology. 2007;68(3):229-232.
14. Mollan SP, Markey KA, Benzimra JD, et al. A practical approach to, diagnosis, assessment and management of idiopathic intracranial hypertension. Pract Neurol. 2014;14(6):380-390. doi:10.1136/practneurol-2014-000821.
15. Johnston I, Paterson A. Benign intracranial hypertension. II. CSF pressure and circulation. Brain. 1974;97(2):301-312.
16. Aly EE, Lawther BK. Anaesthetic management of uncontrolled idiopathic intracranial hypertension during labour and delivery using an intrathecal catheter. Anesthesia. 2007;62(2):178-181.
17. Panikkath R, Welker J, Johnston R, Lado-Abeal J. Intracranial hypertension and intracranial hypotension causing headache in the same patient. Proc (Bayl Univ Med Cent). 2014;27(3):217-218.
18. Nafiu OO, Monterosso D, Walton SR, Bradin S. Post dural puncture headache in a pediatric patient with idiopathic intracranial hypertension. Paediatr Anaesth. 2005;15(9):778-781. doi:10.1111/j.1460-9592.2004.01529.x.
19. Sinclair AJ, Burdon MA, Nightingale PG, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ. 2010;341:c2701. doi:10.1136/bmj.c2701.
20. Kupersmith MJ, Gamell L, Turbin R, Peck V, Spiegel P, Wall M. Effects of weight loss on the course of idiopathic intracranial hypertension in women. Neurology. 1998;50(4):1094-1098.
21. Johnson LN, Krohel GB, Madsen RW, March GA Jr. The role of weight loss and acetazolamide in the treatment of idiopathic intracranial hypertension (pseudotumor cerebri) Ophthalmology. 1998;105(12):2313-2317. doi:10.1016/S0161-6420(98)91234-9.
22. NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee; Wall M, McDermott MP, Kieburtz KD, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA. 2014;311(16):1641-1651. doi:10.1001/jama.2014.3312.
23. Fonseca PL, Rigamonti D, Miller NR, Subramanian PS. Visual outcomes of surgical intervention for pseudotumour cerebri: optic nerve sheath fenestration versus cerebrospinal fluid diversion. Br J Ophthalmol. 2014;98(10):1360-1363. doi:10.1136/bjophthalmol-2014-304953.
1. Degnan AJ, Levy LM. Pseudotumor cerebri: brief review of clinical syndrome and imaging findings. AJNR Am J Neuroradiol. 2011;32(11):1986-1893. doi:10.3174/ajnr.A2404.
2. Biousse V, Bruce BB, Newman NJ. Update on the pathophysiology and management of idiopathic intracranial hypertension. J Neurol Neurosurg Psychiatry. 2012;83(5):488-494. doi:10.1136/jnnp-2011-302029. 3. Wall M, George D. Idiopathic intracranial hypertension: a prospective study of 50 patients. Brain. 1991;114(Pt 1A):155-180.
4. Wall M. Idiopathic intracranial hypertension. Neurol Clin. 2010;28(3):593-617. doi:10.1016/j.ncl.2010.03.003.
5. Friedman DI, Rausch EA. Headache diagnoses in patients with treated idiopathic intracranial hypertension. Neurology. 2002;58(10):1551-1553.
6. Weisberg LA. Benign intracranial hypertension. Medicine (Baltimore). 1975;54(3):197-207.
7. Whiteley W, Al-Shahi R, Warlow CP, Zeidler M, Lueck CJ. CSF opening pressure: reference interval and the effect of body mass index. Neurology. 2006;67(9):1690-1691.
8. Biousse V, Ameri A, Bousser MG. Isolated intracranial hypertension as the only sign of cerebral venous thrombosis. Neurology. 1999;53(7):1537-1542.
9. Leker RR, Steiner I. Features of dural sinus thrombosis simulating pseudotumor cerebri. Eur J Neurol. 1999;6(5):601-604.
10. Sylaja PN, Ahsan Moosa NV, Radhakrishnan K, Sankara Sarma P, Pradeep Kumar S. Differential diagnosis of patients with intracranial sinus venous thrombosis related isolated intracranial hypertension from those with idiopathic intracranial hypertension. J Neurol Sci. 2003;215(1-2):9-12.
11. Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192. doi:10.1161/STR.0b013e31820a8364.
12. American College of Radiology ACR Appropriateness Criteria: Headache. https://acsearch.acr.org/docs/69482/Narrative/. Updated 2013. Accessed January 19, 2017.
13. Thambisetty M, Lavin PJ, Newman NJ, Biousse V. Fulminant idiopathic intracranial hypertension. Neurology. 2007;68(3):229-232.
14. Mollan SP, Markey KA, Benzimra JD, et al. A practical approach to, diagnosis, assessment and management of idiopathic intracranial hypertension. Pract Neurol. 2014;14(6):380-390. doi:10.1136/practneurol-2014-000821.
15. Johnston I, Paterson A. Benign intracranial hypertension. II. CSF pressure and circulation. Brain. 1974;97(2):301-312.
16. Aly EE, Lawther BK. Anaesthetic management of uncontrolled idiopathic intracranial hypertension during labour and delivery using an intrathecal catheter. Anesthesia. 2007;62(2):178-181.
17. Panikkath R, Welker J, Johnston R, Lado-Abeal J. Intracranial hypertension and intracranial hypotension causing headache in the same patient. Proc (Bayl Univ Med Cent). 2014;27(3):217-218.
18. Nafiu OO, Monterosso D, Walton SR, Bradin S. Post dural puncture headache in a pediatric patient with idiopathic intracranial hypertension. Paediatr Anaesth. 2005;15(9):778-781. doi:10.1111/j.1460-9592.2004.01529.x.
19. Sinclair AJ, Burdon MA, Nightingale PG, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ. 2010;341:c2701. doi:10.1136/bmj.c2701.
20. Kupersmith MJ, Gamell L, Turbin R, Peck V, Spiegel P, Wall M. Effects of weight loss on the course of idiopathic intracranial hypertension in women. Neurology. 1998;50(4):1094-1098.
21. Johnson LN, Krohel GB, Madsen RW, March GA Jr. The role of weight loss and acetazolamide in the treatment of idiopathic intracranial hypertension (pseudotumor cerebri) Ophthalmology. 1998;105(12):2313-2317. doi:10.1016/S0161-6420(98)91234-9.
22. NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee; Wall M, McDermott MP, Kieburtz KD, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA. 2014;311(16):1641-1651. doi:10.1001/jama.2014.3312.
23. Fonseca PL, Rigamonti D, Miller NR, Subramanian PS. Visual outcomes of surgical intervention for pseudotumour cerebri: optic nerve sheath fenestration versus cerebrospinal fluid diversion. Br J Ophthalmol. 2014;98(10):1360-1363. doi:10.1136/bjophthalmol-2014-304953.