User login
Iododerma Simulating Cryptococcal Infection
To the Editor:
A woman in her 40s presented with acute onset of rapidly spreading lesions on the face, trunk, and extremities. She reported high fever and endorsed malaise. She had a history of end-stage renal disease and was on renal dialysis. She recently underwent revision of an arteriovenous fistula.
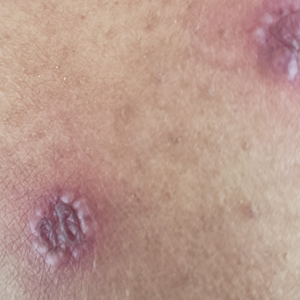
Physical examination revealed diffuse, erythematous, firm papules and plaques with central hemorrhage and umbilication on the dorsal aspect of the nose, forehead, temples, and cheeks. There also were purpuric papules and plaques with a peripheral rim of vesiculation (Figure 1) on the medial and posterior thighs and buttocks. Histopathology of a biopsy specimen revealed an interstitial neutrophilic infiltrate in the superficial dermis and mid dermis with scattered, haloed, acellular structures simulating cryptococcal organisms (Figure 2). Periodic acid–Schiff (PAS), Grocott methenamine-silver, and mucicarmine staining was negative. Repeat biopsy showed similar findings. A (1-3)-β-

The findings compatible with a diagnosis of iododerma included umbilicated hemorrhagic papules and plaques, cryptococcal-like structures with negative staining on histopathology, and elevated iodine levels with a negative infectious workup. The patient was treated with topical corticosteroids. At 1-month follow-up, the lesions had resolved.

Iododerma is a halogenoderma, a skin eruption that occurs after ingestion of or exposure to a halogen-containing substance (eg, iodine, bromine, fluorine) or medication (eg, lithium).1 Common sources of iodine include iodinated contrast media, potassium iodide ingestion, topical application of povidone–iodine, radioactive iodine administration, and the antiarrhythmic amiodarone. Excess exposure to iodine-containing compounds typically occurs in the setting of kidney disease or failure as well as due to reduced iodine clearance.1 Although the pathogenesis of iododerma is unknown, the most common hypothesis is that lesions are delayed hypersensitivity reactions secondary to formation of a protein-halogen complex.2
The presentation of iododerma is polymorphous and includes acneform, vegetative, or pustular eruptions; umbilicated papules and plaques can be present.2,3 Lesions can be either asymptomatic or painful and pruritic. Timing between iodine exposure and onset of lesions varies from hours to days to years.2,4
Systemic symptoms of iododerma can occur, including salivary gland swelling, hypotension and bradycardia, kidney injury, or thyroid and liver abnormalities. Histopathologic analysis demonstrates a dense neutrophilic dermatitis with negative staining for infectious causes.4,5 Cryptococcal-like structures have been described in iododerma3; neutrophilic dermatoses of various causes that mimic cryptococcal infection have been reported.6 Ultimately, iododerma remains a diagnosis of exclusion.
Withdrawal of an offending compound is remedial. Dialysis is beneficial in end-stage renal disease. Topical, intralesional, and systemic corticosteroids, as well as antibiotics, provide variable benefit.4,7 Lesions can take 4 to 6 weeks to clear after withdrawal of the offending agent. It is unclear whether recurrences happen; iodine-containing compounds need to be avoided after a patient has been affected.
Iododerma has a broad differential diagnosis due to the polymorphous presentation of the disorder, including acute febrile neutrophilic dermatosis (also known as Sweet syndrome), cutaneous cryptococcosis, and cutaneous histoplasmosis. Sweet syndrome presents as abrupt onset of edematous erythematous plaques with fever and leukocytosis. It is associated with infection, inflammatory disorders, medication, and malignancy.8 Histopathologic analysis reveals papillary dermal edema and a neutrophilic dermatosis. Cytoplasmic vacuolization resembling C neoformans has been reported.9 The diagnosis is less favored in the presence of renal disease, temporal association of the eruption with iodine exposure, and elevated blood and urine iodine levels, as in our patient.
Cutaneous cryptococcosis, an infection caused by C neoformans, typically occurs secondary to dissemination from the lungs; rarely, the disease is primary. Acneform plaques, vegetative plaques, and umbilicated lesions are seen.10 Histopathologic analysis shows characteristic yeast forms of cryptococcosis surrounded by gelatinous edema, which create a haloed effect, typically throughout the dermis. Capsules are positive for PAS or mucicarmine staining. Although C neoformans can closely mimic iododerma both clinically and histopathologically, negative infectious staining, localization of haloed structures to the upper dermis, a negative test for cryptococcal antigen, and elevated blood and urine iodine levels in this case all favored iododerma.
Cutaneous histoplasmosis is an infection caused by Histoplasma capsulatum, most commonly as secondary dissemination from pulmonary infection but rarely from direct inoculation of the skin.11 Presentation includes erythematous to hemorrhagic, umbilicated papules and plaques. Histopathologic findings are round to oval, narrow-based, budding yeasts that stain positive for PAS or mucicarmine. Although histoplasmosis can clinically mimic iododerma, the disease is distinguished histologically by the presence of fungal microorganisms that lack the gelatinous edema and haloed effect of iododerma.
We presented a unique case of iododerma simulating cryptococcal infection both clinically and histopathologically. Prompt recognition of histologic mimickers of true infectious microorganisms is essential to prevent unnecessary delay of withdrawal of the offending substance and to initiate appropriate therapy.
- Alagheband M, Engineer L. Lithium and halogenoderma. Arch Dermatol. 2000;136:126-127. doi:10.1001/archderm.136.1.126
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377-1379. doi:10.1111/bjd.12852
- Runge M, Williams K, Scharnitz T, et al. Iodine toxicity after iodinated contrast: new observations in iododerma. JAAD Case Rep. 2020;6:319-322. doi:10.1016/j.jdcr.2020.02.006
- Chalela JG, Aguilar L. Iododerma from contrast material. N Engl J Med. 2016;374:2477. doi:10.1056/NEJMicm1512512
- Chang MW, Miner JE, Moiin A, et al. Iododerma after computed tomographic scan with intravenous radiopaque contrast media. J Am Acad Dermatol. 1997;36:1014-1016. doi:10.1016/s0190-9622(97)80291-5
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi:10.1111/cup.12019
- Pranteda G, Grimaldi M, Salzetta M, et al. Vegetating iododerma and pulmonary eosinophilic infiltration. a simple co-occurrence? Acta Derm Venereol. 2004;84:480-481.
- Nelson CA, Stephen S, Ashchyan HJ, et al. M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.064
- Wilson J, Gleghorn K, Kelly B. Cryptococcoid Sweet’s syndrome: two reports of Sweet’s syndrome mimicking cutaneous cryptococcosis. J Cutan Pathol. 2017;44:413-419. doi:10.1111/cup.12921
- Beatson M, Harwood M, Reese V, et al. Primary cutaneous cryptococcosis in an elderly pigeon breeder. JAAD Case Rep. 2019;5:433-435. doi:10.1016/j.jdcr.2019.03.006
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348. doi:10.1177/0145561318097010-1108
To the Editor:
A woman in her 40s presented with acute onset of rapidly spreading lesions on the face, trunk, and extremities. She reported high fever and endorsed malaise. She had a history of end-stage renal disease and was on renal dialysis. She recently underwent revision of an arteriovenous fistula.
Physical examination revealed diffuse, erythematous, firm papules and plaques with central hemorrhage and umbilication on the dorsal aspect of the nose, forehead, temples, and cheeks. There also were purpuric papules and plaques with a peripheral rim of vesiculation (Figure 1) on the medial and posterior thighs and buttocks. Histopathology of a biopsy specimen revealed an interstitial neutrophilic infiltrate in the superficial dermis and mid dermis with scattered, haloed, acellular structures simulating cryptococcal organisms (Figure 2). Periodic acid–Schiff (PAS), Grocott methenamine-silver, and mucicarmine staining was negative. Repeat biopsy showed similar findings. A (1-3)-β-

The findings compatible with a diagnosis of iododerma included umbilicated hemorrhagic papules and plaques, cryptococcal-like structures with negative staining on histopathology, and elevated iodine levels with a negative infectious workup. The patient was treated with topical corticosteroids. At 1-month follow-up, the lesions had resolved.

Iododerma is a halogenoderma, a skin eruption that occurs after ingestion of or exposure to a halogen-containing substance (eg, iodine, bromine, fluorine) or medication (eg, lithium).1 Common sources of iodine include iodinated contrast media, potassium iodide ingestion, topical application of povidone–iodine, radioactive iodine administration, and the antiarrhythmic amiodarone. Excess exposure to iodine-containing compounds typically occurs in the setting of kidney disease or failure as well as due to reduced iodine clearance.1 Although the pathogenesis of iododerma is unknown, the most common hypothesis is that lesions are delayed hypersensitivity reactions secondary to formation of a protein-halogen complex.2
The presentation of iododerma is polymorphous and includes acneform, vegetative, or pustular eruptions; umbilicated papules and plaques can be present.2,3 Lesions can be either asymptomatic or painful and pruritic. Timing between iodine exposure and onset of lesions varies from hours to days to years.2,4
Systemic symptoms of iododerma can occur, including salivary gland swelling, hypotension and bradycardia, kidney injury, or thyroid and liver abnormalities. Histopathologic analysis demonstrates a dense neutrophilic dermatitis with negative staining for infectious causes.4,5 Cryptococcal-like structures have been described in iododerma3; neutrophilic dermatoses of various causes that mimic cryptococcal infection have been reported.6 Ultimately, iododerma remains a diagnosis of exclusion.
Withdrawal of an offending compound is remedial. Dialysis is beneficial in end-stage renal disease. Topical, intralesional, and systemic corticosteroids, as well as antibiotics, provide variable benefit.4,7 Lesions can take 4 to 6 weeks to clear after withdrawal of the offending agent. It is unclear whether recurrences happen; iodine-containing compounds need to be avoided after a patient has been affected.
Iododerma has a broad differential diagnosis due to the polymorphous presentation of the disorder, including acute febrile neutrophilic dermatosis (also known as Sweet syndrome), cutaneous cryptococcosis, and cutaneous histoplasmosis. Sweet syndrome presents as abrupt onset of edematous erythematous plaques with fever and leukocytosis. It is associated with infection, inflammatory disorders, medication, and malignancy.8 Histopathologic analysis reveals papillary dermal edema and a neutrophilic dermatosis. Cytoplasmic vacuolization resembling C neoformans has been reported.9 The diagnosis is less favored in the presence of renal disease, temporal association of the eruption with iodine exposure, and elevated blood and urine iodine levels, as in our patient.
Cutaneous cryptococcosis, an infection caused by C neoformans, typically occurs secondary to dissemination from the lungs; rarely, the disease is primary. Acneform plaques, vegetative plaques, and umbilicated lesions are seen.10 Histopathologic analysis shows characteristic yeast forms of cryptococcosis surrounded by gelatinous edema, which create a haloed effect, typically throughout the dermis. Capsules are positive for PAS or mucicarmine staining. Although C neoformans can closely mimic iododerma both clinically and histopathologically, negative infectious staining, localization of haloed structures to the upper dermis, a negative test for cryptococcal antigen, and elevated blood and urine iodine levels in this case all favored iododerma.
Cutaneous histoplasmosis is an infection caused by Histoplasma capsulatum, most commonly as secondary dissemination from pulmonary infection but rarely from direct inoculation of the skin.11 Presentation includes erythematous to hemorrhagic, umbilicated papules and plaques. Histopathologic findings are round to oval, narrow-based, budding yeasts that stain positive for PAS or mucicarmine. Although histoplasmosis can clinically mimic iododerma, the disease is distinguished histologically by the presence of fungal microorganisms that lack the gelatinous edema and haloed effect of iododerma.
We presented a unique case of iododerma simulating cryptococcal infection both clinically and histopathologically. Prompt recognition of histologic mimickers of true infectious microorganisms is essential to prevent unnecessary delay of withdrawal of the offending substance and to initiate appropriate therapy.
To the Editor:
A woman in her 40s presented with acute onset of rapidly spreading lesions on the face, trunk, and extremities. She reported high fever and endorsed malaise. She had a history of end-stage renal disease and was on renal dialysis. She recently underwent revision of an arteriovenous fistula.
Physical examination revealed diffuse, erythematous, firm papules and plaques with central hemorrhage and umbilication on the dorsal aspect of the nose, forehead, temples, and cheeks. There also were purpuric papules and plaques with a peripheral rim of vesiculation (Figure 1) on the medial and posterior thighs and buttocks. Histopathology of a biopsy specimen revealed an interstitial neutrophilic infiltrate in the superficial dermis and mid dermis with scattered, haloed, acellular structures simulating cryptococcal organisms (Figure 2). Periodic acid–Schiff (PAS), Grocott methenamine-silver, and mucicarmine staining was negative. Repeat biopsy showed similar findings. A (1-3)-β-

The findings compatible with a diagnosis of iododerma included umbilicated hemorrhagic papules and plaques, cryptococcal-like structures with negative staining on histopathology, and elevated iodine levels with a negative infectious workup. The patient was treated with topical corticosteroids. At 1-month follow-up, the lesions had resolved.

Iododerma is a halogenoderma, a skin eruption that occurs after ingestion of or exposure to a halogen-containing substance (eg, iodine, bromine, fluorine) or medication (eg, lithium).1 Common sources of iodine include iodinated contrast media, potassium iodide ingestion, topical application of povidone–iodine, radioactive iodine administration, and the antiarrhythmic amiodarone. Excess exposure to iodine-containing compounds typically occurs in the setting of kidney disease or failure as well as due to reduced iodine clearance.1 Although the pathogenesis of iododerma is unknown, the most common hypothesis is that lesions are delayed hypersensitivity reactions secondary to formation of a protein-halogen complex.2
The presentation of iododerma is polymorphous and includes acneform, vegetative, or pustular eruptions; umbilicated papules and plaques can be present.2,3 Lesions can be either asymptomatic or painful and pruritic. Timing between iodine exposure and onset of lesions varies from hours to days to years.2,4
Systemic symptoms of iododerma can occur, including salivary gland swelling, hypotension and bradycardia, kidney injury, or thyroid and liver abnormalities. Histopathologic analysis demonstrates a dense neutrophilic dermatitis with negative staining for infectious causes.4,5 Cryptococcal-like structures have been described in iododerma3; neutrophilic dermatoses of various causes that mimic cryptococcal infection have been reported.6 Ultimately, iododerma remains a diagnosis of exclusion.
Withdrawal of an offending compound is remedial. Dialysis is beneficial in end-stage renal disease. Topical, intralesional, and systemic corticosteroids, as well as antibiotics, provide variable benefit.4,7 Lesions can take 4 to 6 weeks to clear after withdrawal of the offending agent. It is unclear whether recurrences happen; iodine-containing compounds need to be avoided after a patient has been affected.
Iododerma has a broad differential diagnosis due to the polymorphous presentation of the disorder, including acute febrile neutrophilic dermatosis (also known as Sweet syndrome), cutaneous cryptococcosis, and cutaneous histoplasmosis. Sweet syndrome presents as abrupt onset of edematous erythematous plaques with fever and leukocytosis. It is associated with infection, inflammatory disorders, medication, and malignancy.8 Histopathologic analysis reveals papillary dermal edema and a neutrophilic dermatosis. Cytoplasmic vacuolization resembling C neoformans has been reported.9 The diagnosis is less favored in the presence of renal disease, temporal association of the eruption with iodine exposure, and elevated blood and urine iodine levels, as in our patient.
Cutaneous cryptococcosis, an infection caused by C neoformans, typically occurs secondary to dissemination from the lungs; rarely, the disease is primary. Acneform plaques, vegetative plaques, and umbilicated lesions are seen.10 Histopathologic analysis shows characteristic yeast forms of cryptococcosis surrounded by gelatinous edema, which create a haloed effect, typically throughout the dermis. Capsules are positive for PAS or mucicarmine staining. Although C neoformans can closely mimic iododerma both clinically and histopathologically, negative infectious staining, localization of haloed structures to the upper dermis, a negative test for cryptococcal antigen, and elevated blood and urine iodine levels in this case all favored iododerma.
Cutaneous histoplasmosis is an infection caused by Histoplasma capsulatum, most commonly as secondary dissemination from pulmonary infection but rarely from direct inoculation of the skin.11 Presentation includes erythematous to hemorrhagic, umbilicated papules and plaques. Histopathologic findings are round to oval, narrow-based, budding yeasts that stain positive for PAS or mucicarmine. Although histoplasmosis can clinically mimic iododerma, the disease is distinguished histologically by the presence of fungal microorganisms that lack the gelatinous edema and haloed effect of iododerma.
We presented a unique case of iododerma simulating cryptococcal infection both clinically and histopathologically. Prompt recognition of histologic mimickers of true infectious microorganisms is essential to prevent unnecessary delay of withdrawal of the offending substance and to initiate appropriate therapy.
- Alagheband M, Engineer L. Lithium and halogenoderma. Arch Dermatol. 2000;136:126-127. doi:10.1001/archderm.136.1.126
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377-1379. doi:10.1111/bjd.12852
- Runge M, Williams K, Scharnitz T, et al. Iodine toxicity after iodinated contrast: new observations in iododerma. JAAD Case Rep. 2020;6:319-322. doi:10.1016/j.jdcr.2020.02.006
- Chalela JG, Aguilar L. Iododerma from contrast material. N Engl J Med. 2016;374:2477. doi:10.1056/NEJMicm1512512
- Chang MW, Miner JE, Moiin A, et al. Iododerma after computed tomographic scan with intravenous radiopaque contrast media. J Am Acad Dermatol. 1997;36:1014-1016. doi:10.1016/s0190-9622(97)80291-5
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi:10.1111/cup.12019
- Pranteda G, Grimaldi M, Salzetta M, et al. Vegetating iododerma and pulmonary eosinophilic infiltration. a simple co-occurrence? Acta Derm Venereol. 2004;84:480-481.
- Nelson CA, Stephen S, Ashchyan HJ, et al. M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.064
- Wilson J, Gleghorn K, Kelly B. Cryptococcoid Sweet’s syndrome: two reports of Sweet’s syndrome mimicking cutaneous cryptococcosis. J Cutan Pathol. 2017;44:413-419. doi:10.1111/cup.12921
- Beatson M, Harwood M, Reese V, et al. Primary cutaneous cryptococcosis in an elderly pigeon breeder. JAAD Case Rep. 2019;5:433-435. doi:10.1016/j.jdcr.2019.03.006
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348. doi:10.1177/0145561318097010-1108
- Alagheband M, Engineer L. Lithium and halogenoderma. Arch Dermatol. 2000;136:126-127. doi:10.1001/archderm.136.1.126
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377-1379. doi:10.1111/bjd.12852
- Runge M, Williams K, Scharnitz T, et al. Iodine toxicity after iodinated contrast: new observations in iododerma. JAAD Case Rep. 2020;6:319-322. doi:10.1016/j.jdcr.2020.02.006
- Chalela JG, Aguilar L. Iododerma from contrast material. N Engl J Med. 2016;374:2477. doi:10.1056/NEJMicm1512512
- Chang MW, Miner JE, Moiin A, et al. Iododerma after computed tomographic scan with intravenous radiopaque contrast media. J Am Acad Dermatol. 1997;36:1014-1016. doi:10.1016/s0190-9622(97)80291-5
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi:10.1111/cup.12019
- Pranteda G, Grimaldi M, Salzetta M, et al. Vegetating iododerma and pulmonary eosinophilic infiltration. a simple co-occurrence? Acta Derm Venereol. 2004;84:480-481.
- Nelson CA, Stephen S, Ashchyan HJ, et al. M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.064
- Wilson J, Gleghorn K, Kelly B. Cryptococcoid Sweet’s syndrome: two reports of Sweet’s syndrome mimicking cutaneous cryptococcosis. J Cutan Pathol. 2017;44:413-419. doi:10.1111/cup.12921
- Beatson M, Harwood M, Reese V, et al. Primary cutaneous cryptococcosis in an elderly pigeon breeder. JAAD Case Rep. 2019;5:433-435. doi:10.1016/j.jdcr.2019.03.006
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348. doi:10.1177/0145561318097010-1108
Practice Points
- Halogenodermas are rare cutaneous reactions to excess exposure to or ingestion of halogen-containing drugs or substances such as bromine, iodine (iododerma), fluorine, and rarely lithium.
- The clinical presentation of a halogenoderma varies; the most characteristic manifestation is a vegetative or exudative plaque with a peripheral rim of pustules.
- Histologically, lesions of a halogenoderma are characterized by pseudoepitheliomatous hyperplasia associated with numerous intraepidermal microabscesses overlying a dense mixed inflammatory infiltrate of neutrophils, plasma cells, eosinophils, histiocytes, and scattered multinucleated giant cells.
- Rarely, the dermal infiltrate of a halogenoderma contains abundant acellular bodies surrounded by capsulelike vacuolated spaces mimicking Cryptococcus neoformans.
Metabolic effects of estetrol are promising in postmenopausal women
PHILADELPHIA – presented at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
Participants taking estetrol experienced a decrease in hemoglobin A1c, fasting plasma glucose, total cholesterol, LDL and lipoprotein as well as an increase in HDL cholesterol, according to the findings presented by Wolf Utian, MD, PhD, DSC, a professor emeritus of reproductive biology at Case Western Reserve University, Cleveland, and medical director emeritus of the Menopause Society.
A separate poster at the conference from the same trial also reported significant improvements from estetrol in quality of life, including that related to vasomotor symptoms, and several psychosocial and sexual functioning areas.
E4 is already available as combination oral contraception and is now being considered for treating vasomotor symptoms, explained Chrisandra Shufelt, MD, professor and chair of general internal of medicine and associate director of the Women’s Health Research Center at Mayo Clinic Florida, who was not involved in the study.
Background on estetrol
E4 is a human fetal liver estrogen produced during pregnancy that’s synthesized from plants for pharmaceutical use, including as the oral contraceptive drospirenone, Dr. Utian told attendees. It’s classified as a native estrogen with selective tissue activity (NEST), he said.
“E4 is a completely different native estrogen with oral administration mimicking the benefits of transdermals and hence safe and effective,” Dr. Utian said in an interview. “It would be a significant new addition to the pharmaceutical armamentarium.”
Two phase 3 trials presented by Dr. Utian at the same conference last year found estetrol reduced the frequency and severity of moderate to severe vasomotor symptoms, and a previous phase 2 trial finding vasomotor and genitourinary symptom benefits suggested it had potential benefits for lipids, carbohydrate metabolism, and bone turnover.
“In summary, E4 at a daily dose of 15 mg exhibited estrogenic effects in the vagina, leading to improved vaginal health and reduced signs of atrophy, emerging as a promising treatment option not only for vasomotor symptoms but also for other significant menopausal symptoms,” Dr. Utian said. “E4 could offer comprehensive relief for women experiencing a range of menopause-related discomforts.”
Dr. Utian also referenced a 2017 trial in which estetrol positively impacted lipid profiles, “lowering low-density lipoprotein cholesterol, increasing high-density lipoprotein cholesterol, and showing minimal influence on triglycerides,” he said. “Importantly, estetrol was associated with a significant decrease in osteocalcin levels in the higher dose groups, suggesting a potential preventive effect on bone loss,” he added. A recent review of the overall evidence on estetrol suggests its use is “promising,” Dr. Utian noted.
Current trial
His current randomized controlled phase 3 trial included postmenopausal women ages 40-65 from 151 sites in 14 countries in Europe, Latin America, and North America, and Russia. Among the 640 participants in the trial, 213 women randomly received 15 mg of estetrol, 213 women received 20 mg of estetrol, and 214 women received a placebo every day for 3 months. All women without hysterectomies also received 200 mg of progesterone once daily for two weeks after completing the estetrol treatment to protect the endometrium.
Researchers took blood samples from the participants at baseline and week 12 to assess total cholesterol, LDL, HDL, the total cholesterol/HDL ratio, triglycerides, lipoprotein A, fasting plasma glucose, insulin, and A1c.
Compared with women in the placebo group, women in both the 15 mg and 20 mg groups saw a statistically significant decrease in lipoprotein A and in the ratio of total cholesterol to HDL, and a statistically significant increase in HDL. Only the women in the 15 mg group saw a statistically significant decrease in LDL and increase in triglycerides; an increase in triglycerides in the 20 mg group did not reach statistical significance.
Statistically significant decreases in fasting plasma glucose and A1c also occurred in both treatment groups, but a decrease in insulin levels and in the homeostasis model-assessment-estimated insulin resistance (HOMA-IR) seen in both treatment arms did not reach significance.
“While the mean changes after 12 weeks from baseline overall were small changes to the cholesterol and blood sugar profiles, they are clinically meaningful because it suggests that E4 does not have any adverse effects to these measures,” Dr. Shufelt said in an interview. “An advantage is that this gives us another hormone option for vasomotor symptoms since it is a native estrogen with selective tissue.”
It’s too early, however, to determine whether estetrol offers benefits in terms of its safety profile, compared with currently available therapies, Dr. Shufelt said.
”These findings of E4 are similar to how oral estradiol changes lipids, which finds an increase in high-density lipoprotein cholesterol, and decreases plasma concentrations of total and low-density lipoprotein cholesterol. an increase in HDL-C and triglycerides and decrease in LDL-C,” she said.
Poster findings also promising
For the findings reported in the poster, researchers assessed quality of life and the clinical meaningfulness of vasomotor symptoms’ reduction at baseline and 12 weeks using the Menopause-Specific Quality of Life (MENQOL) questionnaire and the Clinical Global Impression questionnaire, respectively. They also assessed women’s self-reported genitourinary symptoms, including vaginal dryness, pain during urination, vaginal pain and bleeding related to sex, and vaginal or vulvar irritation or itching. Most of these findings primarily confirmed previous positive effects from E4 in other trials.
Women in both the 15 mg and 20 mg estetrol groups reported a statistically significant improvement at 12 weeks, compared with placebo, in their total MENQOL score and in the vasomotor, psychosocial, and sexual functioning domain scores (P < .05). Those in the 20 mg group also had a statistically significant improvement in their physical domain score (P < .05).
Although numerical improvements in genitourinary symptoms occurred at 12 weeks across all three groups, the only statistically significant difference from baseline occurred in patients taking 15 mg of estetrol, who experienced a decrease in vaginal dryness and vaginal pain during sex (P = .0142 and P = .003, respectively).
The Clinical Global Impression questionnaire asked women at 4 and 12 weeks to rate on a seven-item Likert scale their response to this question: “Rate the total improvement, whether or not in your judgment it is due entirely to drug treatment. Compared to your condition at admission to the study, how much has it changed?” Responses of “very much improved” and “much improved” counted as a clinically meaningful difference.
Compared with 27.9% of patients in the placebo group, 52.9% of patients in the 15 mg group and 59.8% of patients in the 20 mg group rated the weekly frequency of moderate to severe vasomotor symptoms as “much improved” or “very much improved” at 4 weeks (P < .0001). At 12 weeks, those numbers rose to 47% in the placebo group, 73.3% in the 15 mg group and 77.8% in the 20 mg group (P < .0001).
The trial’s primary limitation at this point is having only a 12-week follow-up, Dr. Shufelt said, though a few other questions remain.
“Because the two phase 3 RCTs included hysterectomized and nonhysterectomized women, it was unclear how many women in the study had E4 alone versus E4 with progesterone, as that might play a role in both cholesterol and carbohydrate metabolism,” Dr. Shufelt said. “While baseline data was not presented, it would also be important to know baseline values for the women and confirm that none were on lipid-lowering medications.”
The research was funded by Estetra SRL, an affiliate of Mithra Pharmaceuticals. Dr. Utian is a member of the Mithra and Elektra Scientific Advisory Boards. Dr. Shufelt has no disclosures.
PHILADELPHIA – presented at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
Participants taking estetrol experienced a decrease in hemoglobin A1c, fasting plasma glucose, total cholesterol, LDL and lipoprotein as well as an increase in HDL cholesterol, according to the findings presented by Wolf Utian, MD, PhD, DSC, a professor emeritus of reproductive biology at Case Western Reserve University, Cleveland, and medical director emeritus of the Menopause Society.
A separate poster at the conference from the same trial also reported significant improvements from estetrol in quality of life, including that related to vasomotor symptoms, and several psychosocial and sexual functioning areas.
E4 is already available as combination oral contraception and is now being considered for treating vasomotor symptoms, explained Chrisandra Shufelt, MD, professor and chair of general internal of medicine and associate director of the Women’s Health Research Center at Mayo Clinic Florida, who was not involved in the study.
Background on estetrol
E4 is a human fetal liver estrogen produced during pregnancy that’s synthesized from plants for pharmaceutical use, including as the oral contraceptive drospirenone, Dr. Utian told attendees. It’s classified as a native estrogen with selective tissue activity (NEST), he said.
“E4 is a completely different native estrogen with oral administration mimicking the benefits of transdermals and hence safe and effective,” Dr. Utian said in an interview. “It would be a significant new addition to the pharmaceutical armamentarium.”
Two phase 3 trials presented by Dr. Utian at the same conference last year found estetrol reduced the frequency and severity of moderate to severe vasomotor symptoms, and a previous phase 2 trial finding vasomotor and genitourinary symptom benefits suggested it had potential benefits for lipids, carbohydrate metabolism, and bone turnover.
“In summary, E4 at a daily dose of 15 mg exhibited estrogenic effects in the vagina, leading to improved vaginal health and reduced signs of atrophy, emerging as a promising treatment option not only for vasomotor symptoms but also for other significant menopausal symptoms,” Dr. Utian said. “E4 could offer comprehensive relief for women experiencing a range of menopause-related discomforts.”
Dr. Utian also referenced a 2017 trial in which estetrol positively impacted lipid profiles, “lowering low-density lipoprotein cholesterol, increasing high-density lipoprotein cholesterol, and showing minimal influence on triglycerides,” he said. “Importantly, estetrol was associated with a significant decrease in osteocalcin levels in the higher dose groups, suggesting a potential preventive effect on bone loss,” he added. A recent review of the overall evidence on estetrol suggests its use is “promising,” Dr. Utian noted.
Current trial
His current randomized controlled phase 3 trial included postmenopausal women ages 40-65 from 151 sites in 14 countries in Europe, Latin America, and North America, and Russia. Among the 640 participants in the trial, 213 women randomly received 15 mg of estetrol, 213 women received 20 mg of estetrol, and 214 women received a placebo every day for 3 months. All women without hysterectomies also received 200 mg of progesterone once daily for two weeks after completing the estetrol treatment to protect the endometrium.
Researchers took blood samples from the participants at baseline and week 12 to assess total cholesterol, LDL, HDL, the total cholesterol/HDL ratio, triglycerides, lipoprotein A, fasting plasma glucose, insulin, and A1c.
Compared with women in the placebo group, women in both the 15 mg and 20 mg groups saw a statistically significant decrease in lipoprotein A and in the ratio of total cholesterol to HDL, and a statistically significant increase in HDL. Only the women in the 15 mg group saw a statistically significant decrease in LDL and increase in triglycerides; an increase in triglycerides in the 20 mg group did not reach statistical significance.
Statistically significant decreases in fasting plasma glucose and A1c also occurred in both treatment groups, but a decrease in insulin levels and in the homeostasis model-assessment-estimated insulin resistance (HOMA-IR) seen in both treatment arms did not reach significance.
“While the mean changes after 12 weeks from baseline overall were small changes to the cholesterol and blood sugar profiles, they are clinically meaningful because it suggests that E4 does not have any adverse effects to these measures,” Dr. Shufelt said in an interview. “An advantage is that this gives us another hormone option for vasomotor symptoms since it is a native estrogen with selective tissue.”
It’s too early, however, to determine whether estetrol offers benefits in terms of its safety profile, compared with currently available therapies, Dr. Shufelt said.
”These findings of E4 are similar to how oral estradiol changes lipids, which finds an increase in high-density lipoprotein cholesterol, and decreases plasma concentrations of total and low-density lipoprotein cholesterol. an increase in HDL-C and triglycerides and decrease in LDL-C,” she said.
Poster findings also promising
For the findings reported in the poster, researchers assessed quality of life and the clinical meaningfulness of vasomotor symptoms’ reduction at baseline and 12 weeks using the Menopause-Specific Quality of Life (MENQOL) questionnaire and the Clinical Global Impression questionnaire, respectively. They also assessed women’s self-reported genitourinary symptoms, including vaginal dryness, pain during urination, vaginal pain and bleeding related to sex, and vaginal or vulvar irritation or itching. Most of these findings primarily confirmed previous positive effects from E4 in other trials.
Women in both the 15 mg and 20 mg estetrol groups reported a statistically significant improvement at 12 weeks, compared with placebo, in their total MENQOL score and in the vasomotor, psychosocial, and sexual functioning domain scores (P < .05). Those in the 20 mg group also had a statistically significant improvement in their physical domain score (P < .05).
Although numerical improvements in genitourinary symptoms occurred at 12 weeks across all three groups, the only statistically significant difference from baseline occurred in patients taking 15 mg of estetrol, who experienced a decrease in vaginal dryness and vaginal pain during sex (P = .0142 and P = .003, respectively).
The Clinical Global Impression questionnaire asked women at 4 and 12 weeks to rate on a seven-item Likert scale their response to this question: “Rate the total improvement, whether or not in your judgment it is due entirely to drug treatment. Compared to your condition at admission to the study, how much has it changed?” Responses of “very much improved” and “much improved” counted as a clinically meaningful difference.
Compared with 27.9% of patients in the placebo group, 52.9% of patients in the 15 mg group and 59.8% of patients in the 20 mg group rated the weekly frequency of moderate to severe vasomotor symptoms as “much improved” or “very much improved” at 4 weeks (P < .0001). At 12 weeks, those numbers rose to 47% in the placebo group, 73.3% in the 15 mg group and 77.8% in the 20 mg group (P < .0001).
The trial’s primary limitation at this point is having only a 12-week follow-up, Dr. Shufelt said, though a few other questions remain.
“Because the two phase 3 RCTs included hysterectomized and nonhysterectomized women, it was unclear how many women in the study had E4 alone versus E4 with progesterone, as that might play a role in both cholesterol and carbohydrate metabolism,” Dr. Shufelt said. “While baseline data was not presented, it would also be important to know baseline values for the women and confirm that none were on lipid-lowering medications.”
The research was funded by Estetra SRL, an affiliate of Mithra Pharmaceuticals. Dr. Utian is a member of the Mithra and Elektra Scientific Advisory Boards. Dr. Shufelt has no disclosures.
PHILADELPHIA – presented at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
Participants taking estetrol experienced a decrease in hemoglobin A1c, fasting plasma glucose, total cholesterol, LDL and lipoprotein as well as an increase in HDL cholesterol, according to the findings presented by Wolf Utian, MD, PhD, DSC, a professor emeritus of reproductive biology at Case Western Reserve University, Cleveland, and medical director emeritus of the Menopause Society.
A separate poster at the conference from the same trial also reported significant improvements from estetrol in quality of life, including that related to vasomotor symptoms, and several psychosocial and sexual functioning areas.
E4 is already available as combination oral contraception and is now being considered for treating vasomotor symptoms, explained Chrisandra Shufelt, MD, professor and chair of general internal of medicine and associate director of the Women’s Health Research Center at Mayo Clinic Florida, who was not involved in the study.
Background on estetrol
E4 is a human fetal liver estrogen produced during pregnancy that’s synthesized from plants for pharmaceutical use, including as the oral contraceptive drospirenone, Dr. Utian told attendees. It’s classified as a native estrogen with selective tissue activity (NEST), he said.
“E4 is a completely different native estrogen with oral administration mimicking the benefits of transdermals and hence safe and effective,” Dr. Utian said in an interview. “It would be a significant new addition to the pharmaceutical armamentarium.”
Two phase 3 trials presented by Dr. Utian at the same conference last year found estetrol reduced the frequency and severity of moderate to severe vasomotor symptoms, and a previous phase 2 trial finding vasomotor and genitourinary symptom benefits suggested it had potential benefits for lipids, carbohydrate metabolism, and bone turnover.
“In summary, E4 at a daily dose of 15 mg exhibited estrogenic effects in the vagina, leading to improved vaginal health and reduced signs of atrophy, emerging as a promising treatment option not only for vasomotor symptoms but also for other significant menopausal symptoms,” Dr. Utian said. “E4 could offer comprehensive relief for women experiencing a range of menopause-related discomforts.”
Dr. Utian also referenced a 2017 trial in which estetrol positively impacted lipid profiles, “lowering low-density lipoprotein cholesterol, increasing high-density lipoprotein cholesterol, and showing minimal influence on triglycerides,” he said. “Importantly, estetrol was associated with a significant decrease in osteocalcin levels in the higher dose groups, suggesting a potential preventive effect on bone loss,” he added. A recent review of the overall evidence on estetrol suggests its use is “promising,” Dr. Utian noted.
Current trial
His current randomized controlled phase 3 trial included postmenopausal women ages 40-65 from 151 sites in 14 countries in Europe, Latin America, and North America, and Russia. Among the 640 participants in the trial, 213 women randomly received 15 mg of estetrol, 213 women received 20 mg of estetrol, and 214 women received a placebo every day for 3 months. All women without hysterectomies also received 200 mg of progesterone once daily for two weeks after completing the estetrol treatment to protect the endometrium.
Researchers took blood samples from the participants at baseline and week 12 to assess total cholesterol, LDL, HDL, the total cholesterol/HDL ratio, triglycerides, lipoprotein A, fasting plasma glucose, insulin, and A1c.
Compared with women in the placebo group, women in both the 15 mg and 20 mg groups saw a statistically significant decrease in lipoprotein A and in the ratio of total cholesterol to HDL, and a statistically significant increase in HDL. Only the women in the 15 mg group saw a statistically significant decrease in LDL and increase in triglycerides; an increase in triglycerides in the 20 mg group did not reach statistical significance.
Statistically significant decreases in fasting plasma glucose and A1c also occurred in both treatment groups, but a decrease in insulin levels and in the homeostasis model-assessment-estimated insulin resistance (HOMA-IR) seen in both treatment arms did not reach significance.
“While the mean changes after 12 weeks from baseline overall were small changes to the cholesterol and blood sugar profiles, they are clinically meaningful because it suggests that E4 does not have any adverse effects to these measures,” Dr. Shufelt said in an interview. “An advantage is that this gives us another hormone option for vasomotor symptoms since it is a native estrogen with selective tissue.”
It’s too early, however, to determine whether estetrol offers benefits in terms of its safety profile, compared with currently available therapies, Dr. Shufelt said.
”These findings of E4 are similar to how oral estradiol changes lipids, which finds an increase in high-density lipoprotein cholesterol, and decreases plasma concentrations of total and low-density lipoprotein cholesterol. an increase in HDL-C and triglycerides and decrease in LDL-C,” she said.
Poster findings also promising
For the findings reported in the poster, researchers assessed quality of life and the clinical meaningfulness of vasomotor symptoms’ reduction at baseline and 12 weeks using the Menopause-Specific Quality of Life (MENQOL) questionnaire and the Clinical Global Impression questionnaire, respectively. They also assessed women’s self-reported genitourinary symptoms, including vaginal dryness, pain during urination, vaginal pain and bleeding related to sex, and vaginal or vulvar irritation or itching. Most of these findings primarily confirmed previous positive effects from E4 in other trials.
Women in both the 15 mg and 20 mg estetrol groups reported a statistically significant improvement at 12 weeks, compared with placebo, in their total MENQOL score and in the vasomotor, psychosocial, and sexual functioning domain scores (P < .05). Those in the 20 mg group also had a statistically significant improvement in their physical domain score (P < .05).
Although numerical improvements in genitourinary symptoms occurred at 12 weeks across all three groups, the only statistically significant difference from baseline occurred in patients taking 15 mg of estetrol, who experienced a decrease in vaginal dryness and vaginal pain during sex (P = .0142 and P = .003, respectively).
The Clinical Global Impression questionnaire asked women at 4 and 12 weeks to rate on a seven-item Likert scale their response to this question: “Rate the total improvement, whether or not in your judgment it is due entirely to drug treatment. Compared to your condition at admission to the study, how much has it changed?” Responses of “very much improved” and “much improved” counted as a clinically meaningful difference.
Compared with 27.9% of patients in the placebo group, 52.9% of patients in the 15 mg group and 59.8% of patients in the 20 mg group rated the weekly frequency of moderate to severe vasomotor symptoms as “much improved” or “very much improved” at 4 weeks (P < .0001). At 12 weeks, those numbers rose to 47% in the placebo group, 73.3% in the 15 mg group and 77.8% in the 20 mg group (P < .0001).
The trial’s primary limitation at this point is having only a 12-week follow-up, Dr. Shufelt said, though a few other questions remain.
“Because the two phase 3 RCTs included hysterectomized and nonhysterectomized women, it was unclear how many women in the study had E4 alone versus E4 with progesterone, as that might play a role in both cholesterol and carbohydrate metabolism,” Dr. Shufelt said. “While baseline data was not presented, it would also be important to know baseline values for the women and confirm that none were on lipid-lowering medications.”
The research was funded by Estetra SRL, an affiliate of Mithra Pharmaceuticals. Dr. Utian is a member of the Mithra and Elektra Scientific Advisory Boards. Dr. Shufelt has no disclosures.
AT NAMS 2023
Trials say start sacubitril-valsartan in hospital in HF with ‘below normal’ LVEF
CLEVELAND – suggests a combined analysis of two major studies.
Short-term risk for cardiovascular (CV) death or HF hospitalization fell 30% for such patients put on the angiotensin-receptor/neprilysin inhibitor (ARNI) at that early stage, compared with those assigned to receive an ACE inhibitor or angiotensin receptor blocker (ARB).
Of note, the risk-reduction benefit reached 41% among the overwhelming majority of patients with a left ventricular ejection fraction (LVEF) of 60% or lower across the two trials, PIONEER-HF and PARAGLIDE-HF. No such significant benefit was seen in patients with higher LVEF.
The prespecified analysis of 1,347 patients medically stabilized after a “worsening-HF event” was reported by Robert J. Mentz, MD, Duke Clinical Research Institute, Durham, N.C., at the annual scientific meeting of the Heart Failure Society of America.
Across both studies, levels of the prognostically telling biomarker NT-proBNP dropped further in the ARNI group, by almost a fourth, compared with those getting an ACE inhibitor or ARB. The difference emerged within a week and was “similar and consistent” throughout at least 8 weeks of follow-up, Dr. Mentz said.
Sacubitril-valsartan is approved in the United States for chronic HF, broadly but with labeling suggesting clearer efficacy at lower LVEF levels, based on the PARADIGM-HF and PARAGON-HF trials.
The PIONEER-HF and PARAGLIDE-HF trials lending patients to the current analysis demonstrated superiority for the drug vs. an ACE inhibitor or ARB when started in hospital in stabilized patients with HF.
Cautions about starting sacubitril-valsartan
In the pooled analysis, patients on sacubitril-valsartan were more likely to experience symptomatic hypotension, with a relative risk for drug’s known potential side effect reaching 1.35 (95% confidence interval, 1.05-1.72), compared with ACE inhibitor or ARB recipients.
But the hypotension risk when starting the drug is manageable to some extent, observed Dr. Mentz. “We can safely start sacubitril-valsartan in the hospital or early post discharge, but we need to make sure their volume status is okay” and keep track of their blood pressure trajectory, he told this news organization.
Those with initially low BP, unsurprisingly, seem more susceptible to the problem, Dr. Mentz said. In such patients “on antihypertensives or other therapies that aren’t going to give them a clinical outcome benefit,” those meds can be withdrawn or their dosages reduced before sacubitril-valsartan is added.
Such cautions are an “important take-home message” of the analysis, observed invited discussant Carolyn S. P. Lam, MBBS, PhD, National Heart Centre, Singapore, after the Dr. Mentz presentation.
Sacubitril-valsartan should be started only in stabilized patients, she emphasized. It should be delayed in those “with ongoing adjustments of antihypertensives, diuretics, and so on,” in whom premature initiation of the drug may promote symptomatic hypotension. Should that happen, Dr. Lam cautioned, there’s a risk that such patients would be “mislabeled as intolerant” of the ARNI and so wouldn’t be started on it later.
The pooled PIONEER-HF and PARAGLIDE-HF analysis, Dr. Lam proposed, might also help overcome the “clinical inertia and fear” that is slowing the uptake of early guideline-directed drug therapy initiation in patients hospitalized with HF.
LVEF spectrum across two studies
As Dr. Mentz reported, the analysis included 881 and 466 patients from PIONEER-HF and PARAGLIDE-HF, respectively. Of the total, 673 were assigned to receive valsartan and 674 to receive either enalapril or valsartan. Overall, 36% of the population were women.
Patients in PIONEER-HF, with an LVEF 40% or lower, were started on their assigned drug during an acute-HF hospitalization and followed a median of 8 weeks. PARAGLIDE-HF patients, with LVEF higher than 40%, started therapy either in hospital (in 70% of cases) or within 30 days of their HF event; they were followed a median of 6 months.
Hazard ratios for outcomes in the sacubitril-valsartan group vs. those on ACE inhibitors or ARBs were 0.76; 95% CI, 0.69-0.83; P < .0001 for change in NT-proBNP levels. For the composite of CV death or HF hospitalization, HRs were as follows:
- 0.70 (95% CI, 0.54-0.91; P = .0077) overall.
- 0.59 (95% CI, 0.44-0.79) for LVEF < 60%.
- 1.53 (95% CI, 0.80-2.91) for LVEF > 60%.
Current guidelines, Dr. Mentz noted, recommend that sacubitril-valsartan “be initiated de novo” predischarge in patients without contraindications who are hospitalized with acute HF with reduced LVEF. The combined analysis of PIONEER-HF and PARAGLIDE-HF, he said, potentially extends the recommendation “across the ejection fraction spectrum.”
Dr. Mentz has received research support and honoraria from Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Fast BioMedical, Gilead, Innolife, Eli Lilly, Medtronic, Medable, Merck, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. Dr. Lam has reported financial relationships “with more than 25 pharmaceutical or device manufacturers, many of which produce therapies for heart failure,” as well as with Medscape/WebMD Global LLC.
A version of this article first appeared on Medscape.com.
CLEVELAND – suggests a combined analysis of two major studies.
Short-term risk for cardiovascular (CV) death or HF hospitalization fell 30% for such patients put on the angiotensin-receptor/neprilysin inhibitor (ARNI) at that early stage, compared with those assigned to receive an ACE inhibitor or angiotensin receptor blocker (ARB).
Of note, the risk-reduction benefit reached 41% among the overwhelming majority of patients with a left ventricular ejection fraction (LVEF) of 60% or lower across the two trials, PIONEER-HF and PARAGLIDE-HF. No such significant benefit was seen in patients with higher LVEF.
The prespecified analysis of 1,347 patients medically stabilized after a “worsening-HF event” was reported by Robert J. Mentz, MD, Duke Clinical Research Institute, Durham, N.C., at the annual scientific meeting of the Heart Failure Society of America.
Across both studies, levels of the prognostically telling biomarker NT-proBNP dropped further in the ARNI group, by almost a fourth, compared with those getting an ACE inhibitor or ARB. The difference emerged within a week and was “similar and consistent” throughout at least 8 weeks of follow-up, Dr. Mentz said.
Sacubitril-valsartan is approved in the United States for chronic HF, broadly but with labeling suggesting clearer efficacy at lower LVEF levels, based on the PARADIGM-HF and PARAGON-HF trials.
The PIONEER-HF and PARAGLIDE-HF trials lending patients to the current analysis demonstrated superiority for the drug vs. an ACE inhibitor or ARB when started in hospital in stabilized patients with HF.
Cautions about starting sacubitril-valsartan
In the pooled analysis, patients on sacubitril-valsartan were more likely to experience symptomatic hypotension, with a relative risk for drug’s known potential side effect reaching 1.35 (95% confidence interval, 1.05-1.72), compared with ACE inhibitor or ARB recipients.
But the hypotension risk when starting the drug is manageable to some extent, observed Dr. Mentz. “We can safely start sacubitril-valsartan in the hospital or early post discharge, but we need to make sure their volume status is okay” and keep track of their blood pressure trajectory, he told this news organization.
Those with initially low BP, unsurprisingly, seem more susceptible to the problem, Dr. Mentz said. In such patients “on antihypertensives or other therapies that aren’t going to give them a clinical outcome benefit,” those meds can be withdrawn or their dosages reduced before sacubitril-valsartan is added.
Such cautions are an “important take-home message” of the analysis, observed invited discussant Carolyn S. P. Lam, MBBS, PhD, National Heart Centre, Singapore, after the Dr. Mentz presentation.
Sacubitril-valsartan should be started only in stabilized patients, she emphasized. It should be delayed in those “with ongoing adjustments of antihypertensives, diuretics, and so on,” in whom premature initiation of the drug may promote symptomatic hypotension. Should that happen, Dr. Lam cautioned, there’s a risk that such patients would be “mislabeled as intolerant” of the ARNI and so wouldn’t be started on it later.
The pooled PIONEER-HF and PARAGLIDE-HF analysis, Dr. Lam proposed, might also help overcome the “clinical inertia and fear” that is slowing the uptake of early guideline-directed drug therapy initiation in patients hospitalized with HF.
LVEF spectrum across two studies
As Dr. Mentz reported, the analysis included 881 and 466 patients from PIONEER-HF and PARAGLIDE-HF, respectively. Of the total, 673 were assigned to receive valsartan and 674 to receive either enalapril or valsartan. Overall, 36% of the population were women.
Patients in PIONEER-HF, with an LVEF 40% or lower, were started on their assigned drug during an acute-HF hospitalization and followed a median of 8 weeks. PARAGLIDE-HF patients, with LVEF higher than 40%, started therapy either in hospital (in 70% of cases) or within 30 days of their HF event; they were followed a median of 6 months.
Hazard ratios for outcomes in the sacubitril-valsartan group vs. those on ACE inhibitors or ARBs were 0.76; 95% CI, 0.69-0.83; P < .0001 for change in NT-proBNP levels. For the composite of CV death or HF hospitalization, HRs were as follows:
- 0.70 (95% CI, 0.54-0.91; P = .0077) overall.
- 0.59 (95% CI, 0.44-0.79) for LVEF < 60%.
- 1.53 (95% CI, 0.80-2.91) for LVEF > 60%.
Current guidelines, Dr. Mentz noted, recommend that sacubitril-valsartan “be initiated de novo” predischarge in patients without contraindications who are hospitalized with acute HF with reduced LVEF. The combined analysis of PIONEER-HF and PARAGLIDE-HF, he said, potentially extends the recommendation “across the ejection fraction spectrum.”
Dr. Mentz has received research support and honoraria from Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Fast BioMedical, Gilead, Innolife, Eli Lilly, Medtronic, Medable, Merck, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. Dr. Lam has reported financial relationships “with more than 25 pharmaceutical or device manufacturers, many of which produce therapies for heart failure,” as well as with Medscape/WebMD Global LLC.
A version of this article first appeared on Medscape.com.
CLEVELAND – suggests a combined analysis of two major studies.
Short-term risk for cardiovascular (CV) death or HF hospitalization fell 30% for such patients put on the angiotensin-receptor/neprilysin inhibitor (ARNI) at that early stage, compared with those assigned to receive an ACE inhibitor or angiotensin receptor blocker (ARB).
Of note, the risk-reduction benefit reached 41% among the overwhelming majority of patients with a left ventricular ejection fraction (LVEF) of 60% or lower across the two trials, PIONEER-HF and PARAGLIDE-HF. No such significant benefit was seen in patients with higher LVEF.
The prespecified analysis of 1,347 patients medically stabilized after a “worsening-HF event” was reported by Robert J. Mentz, MD, Duke Clinical Research Institute, Durham, N.C., at the annual scientific meeting of the Heart Failure Society of America.
Across both studies, levels of the prognostically telling biomarker NT-proBNP dropped further in the ARNI group, by almost a fourth, compared with those getting an ACE inhibitor or ARB. The difference emerged within a week and was “similar and consistent” throughout at least 8 weeks of follow-up, Dr. Mentz said.
Sacubitril-valsartan is approved in the United States for chronic HF, broadly but with labeling suggesting clearer efficacy at lower LVEF levels, based on the PARADIGM-HF and PARAGON-HF trials.
The PIONEER-HF and PARAGLIDE-HF trials lending patients to the current analysis demonstrated superiority for the drug vs. an ACE inhibitor or ARB when started in hospital in stabilized patients with HF.
Cautions about starting sacubitril-valsartan
In the pooled analysis, patients on sacubitril-valsartan were more likely to experience symptomatic hypotension, with a relative risk for drug’s known potential side effect reaching 1.35 (95% confidence interval, 1.05-1.72), compared with ACE inhibitor or ARB recipients.
But the hypotension risk when starting the drug is manageable to some extent, observed Dr. Mentz. “We can safely start sacubitril-valsartan in the hospital or early post discharge, but we need to make sure their volume status is okay” and keep track of their blood pressure trajectory, he told this news organization.
Those with initially low BP, unsurprisingly, seem more susceptible to the problem, Dr. Mentz said. In such patients “on antihypertensives or other therapies that aren’t going to give them a clinical outcome benefit,” those meds can be withdrawn or their dosages reduced before sacubitril-valsartan is added.
Such cautions are an “important take-home message” of the analysis, observed invited discussant Carolyn S. P. Lam, MBBS, PhD, National Heart Centre, Singapore, after the Dr. Mentz presentation.
Sacubitril-valsartan should be started only in stabilized patients, she emphasized. It should be delayed in those “with ongoing adjustments of antihypertensives, diuretics, and so on,” in whom premature initiation of the drug may promote symptomatic hypotension. Should that happen, Dr. Lam cautioned, there’s a risk that such patients would be “mislabeled as intolerant” of the ARNI and so wouldn’t be started on it later.
The pooled PIONEER-HF and PARAGLIDE-HF analysis, Dr. Lam proposed, might also help overcome the “clinical inertia and fear” that is slowing the uptake of early guideline-directed drug therapy initiation in patients hospitalized with HF.
LVEF spectrum across two studies
As Dr. Mentz reported, the analysis included 881 and 466 patients from PIONEER-HF and PARAGLIDE-HF, respectively. Of the total, 673 were assigned to receive valsartan and 674 to receive either enalapril or valsartan. Overall, 36% of the population were women.
Patients in PIONEER-HF, with an LVEF 40% or lower, were started on their assigned drug during an acute-HF hospitalization and followed a median of 8 weeks. PARAGLIDE-HF patients, with LVEF higher than 40%, started therapy either in hospital (in 70% of cases) or within 30 days of their HF event; they were followed a median of 6 months.
Hazard ratios for outcomes in the sacubitril-valsartan group vs. those on ACE inhibitors or ARBs were 0.76; 95% CI, 0.69-0.83; P < .0001 for change in NT-proBNP levels. For the composite of CV death or HF hospitalization, HRs were as follows:
- 0.70 (95% CI, 0.54-0.91; P = .0077) overall.
- 0.59 (95% CI, 0.44-0.79) for LVEF < 60%.
- 1.53 (95% CI, 0.80-2.91) for LVEF > 60%.
Current guidelines, Dr. Mentz noted, recommend that sacubitril-valsartan “be initiated de novo” predischarge in patients without contraindications who are hospitalized with acute HF with reduced LVEF. The combined analysis of PIONEER-HF and PARAGLIDE-HF, he said, potentially extends the recommendation “across the ejection fraction spectrum.”
Dr. Mentz has received research support and honoraria from Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Fast BioMedical, Gilead, Innolife, Eli Lilly, Medtronic, Medable, Merck, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. Dr. Lam has reported financial relationships “with more than 25 pharmaceutical or device manufacturers, many of which produce therapies for heart failure,” as well as with Medscape/WebMD Global LLC.
A version of this article first appeared on Medscape.com.
AT HFSA 2023
Biomarkers may help women with RA to decide on medications in pregnancy
Rheumatoid arthritis can’t be cured, but it can significantly improve naturally during pregnancy in 50%-75% of women, prior research has established. It may worsen or stay the same during pregnancy in others.
As of yet, there’s no way to tell which experience a woman with RA will have. RA occurs in 1% of adults globally and is three times more likely to occur in women.
However, a novel study of 19 women with RA suggests that blood biomarkers before pregnancy may predict who will get better or worse during pregnancy. If confirmed with larger studies, the discovery could lead to personalizing medication choices for women with RA who are seeking to become pregnant and change prepregnancy counseling for physicians.
Findings from the research, conducted by first author Matthew Wright, MS, of Children’s Hospital Oakland (Calif.) Research Institute and colleagues were published online in Arthritis Research & Therapy.
A risky choice for women
Currently, the choice is difficult because stopping medications during pregnancy could cause disease flare and continuing could risk possible harm to the baby as some of the medications have toxic side effects.
This is the first study to analyze genetic differences in women with RA who plan to get pregnant, senior author Damini Jawaheer, PhD, research associate professor of medicine in rheumatology at Northwestern University, Chicago, said in an interview.
Identifying women who have the disease and confirming they were planning to get pregnant has been extremely difficult, she noted, especially since the start of their research predated electronic health records (EHRs).
The researchers were able to develop a cohort from work they were already doing with researchers in Denmark, which has a national registry that included both women with RA and women of reproductive age. From there they could contact women about their pregnancy intentions and build the cohort for this study.
Healthy women and women with RA of Danish descent who planned to get pregnant were enrolled and were prospectively followed.
Genetic differences at prepregnancy baseline
Researchers analyzed genetic differences through RNA sequencing before pregnancy from 19 women with RA and 13 healthy women.
Of the 19 women with RA, disease activity improved during pregnancy in 14 and worsened in 5.
Before pregnancy, the researchers found, several neutrophil-related genes were significantly overexpressed in women whose RA later improved during pregnancy. Genes related to B cells were highly expressed among women who worsened. Those elevated B-cell–related gene levels were not seen in the group who improved during pregnancy, Dr. Jawaheer added.
“We don’t understand at this point why that is,” she said.
They also compared the blood samples with women in the control group who did not have RA.
“Comparisons to healthy women revealed that the B-cell signature was specific” to women with worsened RA, the authors wrote. “Thus, at the prepregnancy stage, the two groups of RA women differed significantly from each other in terms of B-cell function.”
Information could help to eliminate fear
Dr. Jawaheer said almost all the women in the cohort who have RA said they were afraid to take medications during pregnancy even if the medications they are taking are considered safe.
“If we could reliably predict who’s going to improve, those women would not have to be scared,” she said. They could stop their medications if they know they’re going to improve naturally.
“Women who are predicted to worsen could then work together with their rheumatologist so that they get treatment to prevent them from getting worse,” Dr. Jawaheer said. “Treatment could be focused on that group only.”
Arthur Kavanaugh, MD, a rheumatologist at University of California San Diego Health and director of the UCSD Center for Innovative Therapy, who was not part of the study, said his patients planning pregnancy struggle with the choices the researchers describe and that investigating potential biomarkers is important.
“Ideally, people would not want to be on anything when they’re pregnant,” he says.
He found the results “intriguing and hypothesis-generating,” but he said the small sample size makes it hard to draw conclusions about the work before it is replicated on a larger scale.
Beth L. Jonas, MD, chief of the division of rheumatology, allergy, and immunology at the University of North Carolina, Chapel Hill, also not a part of the study, said the small study size must be considered, but if the findings are validated in larger studies, the potential is “huge.”
She said doctors used to tell their patients years ago that there’s an excellent chance they will be in remission in pregnancy.
Now, she says, “We’ve tempered our advice to say there’s a good chance you’ll still have disease activity during your pregnancy.”
Rheumatologists would be very interested in a predictive biomarker, she said, as would colleagues in obstetrics/gynecology and maternal-fetal medicine physicians who manage high-risk pregnancies and do prepregnancy counseling.
She said she would also like to see these data followed over multiple pregnancies for each woman, noting that some of her patients have seen RA improve in one pregnancy and worsen in another.
A question she has is, “with a single patient with RA, could you measure this multiple times and get different results?”
Tackling the unanswered questions
Next, the researchers want to conduct the study with a larger sample in the United States and one that is more diverse than the Danish cohort, which included only White patients. Now, Dr. Jawaheer and her team will have the help of EHRs.
A big part of Dr. Jawaheer’s lab’s focus is to find out why many with RA report “never feeling better” during pregnancy – some even experience remission – and why women who improve during pregnancy report that their disease flares 3-6 months after pregnancy, she said.
Her team is also studying what happens biologically when some women worsen in pregnancy.
Those answers “will give us an indication of what could be a potential drug target,” she said.
The authors and Dr. Kavanaugh and Dr. Jonas reported no relevant financial relationships.
Rheumatoid arthritis can’t be cured, but it can significantly improve naturally during pregnancy in 50%-75% of women, prior research has established. It may worsen or stay the same during pregnancy in others.
As of yet, there’s no way to tell which experience a woman with RA will have. RA occurs in 1% of adults globally and is three times more likely to occur in women.
However, a novel study of 19 women with RA suggests that blood biomarkers before pregnancy may predict who will get better or worse during pregnancy. If confirmed with larger studies, the discovery could lead to personalizing medication choices for women with RA who are seeking to become pregnant and change prepregnancy counseling for physicians.
Findings from the research, conducted by first author Matthew Wright, MS, of Children’s Hospital Oakland (Calif.) Research Institute and colleagues were published online in Arthritis Research & Therapy.
A risky choice for women
Currently, the choice is difficult because stopping medications during pregnancy could cause disease flare and continuing could risk possible harm to the baby as some of the medications have toxic side effects.
This is the first study to analyze genetic differences in women with RA who plan to get pregnant, senior author Damini Jawaheer, PhD, research associate professor of medicine in rheumatology at Northwestern University, Chicago, said in an interview.
Identifying women who have the disease and confirming they were planning to get pregnant has been extremely difficult, she noted, especially since the start of their research predated electronic health records (EHRs).
The researchers were able to develop a cohort from work they were already doing with researchers in Denmark, which has a national registry that included both women with RA and women of reproductive age. From there they could contact women about their pregnancy intentions and build the cohort for this study.
Healthy women and women with RA of Danish descent who planned to get pregnant were enrolled and were prospectively followed.
Genetic differences at prepregnancy baseline
Researchers analyzed genetic differences through RNA sequencing before pregnancy from 19 women with RA and 13 healthy women.
Of the 19 women with RA, disease activity improved during pregnancy in 14 and worsened in 5.
Before pregnancy, the researchers found, several neutrophil-related genes were significantly overexpressed in women whose RA later improved during pregnancy. Genes related to B cells were highly expressed among women who worsened. Those elevated B-cell–related gene levels were not seen in the group who improved during pregnancy, Dr. Jawaheer added.
“We don’t understand at this point why that is,” she said.
They also compared the blood samples with women in the control group who did not have RA.
“Comparisons to healthy women revealed that the B-cell signature was specific” to women with worsened RA, the authors wrote. “Thus, at the prepregnancy stage, the two groups of RA women differed significantly from each other in terms of B-cell function.”
Information could help to eliminate fear
Dr. Jawaheer said almost all the women in the cohort who have RA said they were afraid to take medications during pregnancy even if the medications they are taking are considered safe.
“If we could reliably predict who’s going to improve, those women would not have to be scared,” she said. They could stop their medications if they know they’re going to improve naturally.
“Women who are predicted to worsen could then work together with their rheumatologist so that they get treatment to prevent them from getting worse,” Dr. Jawaheer said. “Treatment could be focused on that group only.”
Arthur Kavanaugh, MD, a rheumatologist at University of California San Diego Health and director of the UCSD Center for Innovative Therapy, who was not part of the study, said his patients planning pregnancy struggle with the choices the researchers describe and that investigating potential biomarkers is important.
“Ideally, people would not want to be on anything when they’re pregnant,” he says.
He found the results “intriguing and hypothesis-generating,” but he said the small sample size makes it hard to draw conclusions about the work before it is replicated on a larger scale.
Beth L. Jonas, MD, chief of the division of rheumatology, allergy, and immunology at the University of North Carolina, Chapel Hill, also not a part of the study, said the small study size must be considered, but if the findings are validated in larger studies, the potential is “huge.”
She said doctors used to tell their patients years ago that there’s an excellent chance they will be in remission in pregnancy.
Now, she says, “We’ve tempered our advice to say there’s a good chance you’ll still have disease activity during your pregnancy.”
Rheumatologists would be very interested in a predictive biomarker, she said, as would colleagues in obstetrics/gynecology and maternal-fetal medicine physicians who manage high-risk pregnancies and do prepregnancy counseling.
She said she would also like to see these data followed over multiple pregnancies for each woman, noting that some of her patients have seen RA improve in one pregnancy and worsen in another.
A question she has is, “with a single patient with RA, could you measure this multiple times and get different results?”
Tackling the unanswered questions
Next, the researchers want to conduct the study with a larger sample in the United States and one that is more diverse than the Danish cohort, which included only White patients. Now, Dr. Jawaheer and her team will have the help of EHRs.
A big part of Dr. Jawaheer’s lab’s focus is to find out why many with RA report “never feeling better” during pregnancy – some even experience remission – and why women who improve during pregnancy report that their disease flares 3-6 months after pregnancy, she said.
Her team is also studying what happens biologically when some women worsen in pregnancy.
Those answers “will give us an indication of what could be a potential drug target,” she said.
The authors and Dr. Kavanaugh and Dr. Jonas reported no relevant financial relationships.
Rheumatoid arthritis can’t be cured, but it can significantly improve naturally during pregnancy in 50%-75% of women, prior research has established. It may worsen or stay the same during pregnancy in others.
As of yet, there’s no way to tell which experience a woman with RA will have. RA occurs in 1% of adults globally and is three times more likely to occur in women.
However, a novel study of 19 women with RA suggests that blood biomarkers before pregnancy may predict who will get better or worse during pregnancy. If confirmed with larger studies, the discovery could lead to personalizing medication choices for women with RA who are seeking to become pregnant and change prepregnancy counseling for physicians.
Findings from the research, conducted by first author Matthew Wright, MS, of Children’s Hospital Oakland (Calif.) Research Institute and colleagues were published online in Arthritis Research & Therapy.
A risky choice for women
Currently, the choice is difficult because stopping medications during pregnancy could cause disease flare and continuing could risk possible harm to the baby as some of the medications have toxic side effects.
This is the first study to analyze genetic differences in women with RA who plan to get pregnant, senior author Damini Jawaheer, PhD, research associate professor of medicine in rheumatology at Northwestern University, Chicago, said in an interview.
Identifying women who have the disease and confirming they were planning to get pregnant has been extremely difficult, she noted, especially since the start of their research predated electronic health records (EHRs).
The researchers were able to develop a cohort from work they were already doing with researchers in Denmark, which has a national registry that included both women with RA and women of reproductive age. From there they could contact women about their pregnancy intentions and build the cohort for this study.
Healthy women and women with RA of Danish descent who planned to get pregnant were enrolled and were prospectively followed.
Genetic differences at prepregnancy baseline
Researchers analyzed genetic differences through RNA sequencing before pregnancy from 19 women with RA and 13 healthy women.
Of the 19 women with RA, disease activity improved during pregnancy in 14 and worsened in 5.
Before pregnancy, the researchers found, several neutrophil-related genes were significantly overexpressed in women whose RA later improved during pregnancy. Genes related to B cells were highly expressed among women who worsened. Those elevated B-cell–related gene levels were not seen in the group who improved during pregnancy, Dr. Jawaheer added.
“We don’t understand at this point why that is,” she said.
They also compared the blood samples with women in the control group who did not have RA.
“Comparisons to healthy women revealed that the B-cell signature was specific” to women with worsened RA, the authors wrote. “Thus, at the prepregnancy stage, the two groups of RA women differed significantly from each other in terms of B-cell function.”
Information could help to eliminate fear
Dr. Jawaheer said almost all the women in the cohort who have RA said they were afraid to take medications during pregnancy even if the medications they are taking are considered safe.
“If we could reliably predict who’s going to improve, those women would not have to be scared,” she said. They could stop their medications if they know they’re going to improve naturally.
“Women who are predicted to worsen could then work together with their rheumatologist so that they get treatment to prevent them from getting worse,” Dr. Jawaheer said. “Treatment could be focused on that group only.”
Arthur Kavanaugh, MD, a rheumatologist at University of California San Diego Health and director of the UCSD Center for Innovative Therapy, who was not part of the study, said his patients planning pregnancy struggle with the choices the researchers describe and that investigating potential biomarkers is important.
“Ideally, people would not want to be on anything when they’re pregnant,” he says.
He found the results “intriguing and hypothesis-generating,” but he said the small sample size makes it hard to draw conclusions about the work before it is replicated on a larger scale.
Beth L. Jonas, MD, chief of the division of rheumatology, allergy, and immunology at the University of North Carolina, Chapel Hill, also not a part of the study, said the small study size must be considered, but if the findings are validated in larger studies, the potential is “huge.”
She said doctors used to tell their patients years ago that there’s an excellent chance they will be in remission in pregnancy.
Now, she says, “We’ve tempered our advice to say there’s a good chance you’ll still have disease activity during your pregnancy.”
Rheumatologists would be very interested in a predictive biomarker, she said, as would colleagues in obstetrics/gynecology and maternal-fetal medicine physicians who manage high-risk pregnancies and do prepregnancy counseling.
She said she would also like to see these data followed over multiple pregnancies for each woman, noting that some of her patients have seen RA improve in one pregnancy and worsen in another.
A question she has is, “with a single patient with RA, could you measure this multiple times and get different results?”
Tackling the unanswered questions
Next, the researchers want to conduct the study with a larger sample in the United States and one that is more diverse than the Danish cohort, which included only White patients. Now, Dr. Jawaheer and her team will have the help of EHRs.
A big part of Dr. Jawaheer’s lab’s focus is to find out why many with RA report “never feeling better” during pregnancy – some even experience remission – and why women who improve during pregnancy report that their disease flares 3-6 months after pregnancy, she said.
Her team is also studying what happens biologically when some women worsen in pregnancy.
Those answers “will give us an indication of what could be a potential drug target,” she said.
The authors and Dr. Kavanaugh and Dr. Jonas reported no relevant financial relationships.
FROM ARTHRITIS RESEARCH & THERAPY
Returning Low-Level Cancer Care to VA Might Save $10k/Year per Patient
CHICAGO—The VA could save nearly $10,000 annually per patient in the Mountain West region referral area by administering lower-level hematology/oncology care within the system instead of in the community, according to a new analysis.
The findings reflect that the community care encouraged by the MISSION Act is more expensive than US Department of Veterans Affairs (VA) care, said report lead author Alyson Clough, PharmD, a clinical pharmacy specialist with the George E. Wahlen Veterans Affairs Medical Center (VAMC) in Salt Lake City, Utah, in an interview. Clough and colleagues presented the poster at the 2023 annual meeting of the Association of VA Hematology/Oncology.
The 2018 MISSION Act expanded the eligibility for non-VA community services within the VA. “If a veteran requires care that a VA cannot provide in-house, lives in a state/territory without a full-service VAMC, and/or lives further than a certain distance/time from a VA Medical Center, then the veteran may be eligible to receive care in the community,” Clough said.
For the new analysis, the researchers focused on the Salt Lake City VAMC referral area, which spans about 125,000 square miles in Utah, Idaho, and Nevada. “While the MISSION Act helped give those veterans more options for specialty care—including hematology/oncology—many veterans are still driving several hours on a routine basis for chemotherapy treatment and injection therapies, even in the community setting,” Clough explained.
The researchers looked at the costs of “low-risk cancer care,” which Clough said consists of “chemotherapy or immunotherapy that is less likely to cause significant side effects during the infusion, is typically nonhazardous, and/or can be administered via a short infusion [30 minutes or less] or an injection.”
In fiscal year 2022, the VA paid more than $5.7 million for community care services for 380 veterans in the referral area, about $15,060 each, according to the analysis. In contrast, the average cost for 1774 veterans within the VA system was $5424. “By retaining or re-establishing hematology/oncology veteran care within VA, we estimate cost savings of approximately $9636 per unique veteran.”
Specifically, the researchers wrote that the average care costs were $5297 per veteran in the community vs $1143 at the VA, and average drug costs were $9763 per veteran in the community vs $4281 in the VA. These amount to total costs of $15,060 per veteran in the community vs $5424 in the VA.
Low-risk services “are ideal to bring to more isolated regions,” Clough noted. “Traveling and/or finding accommodations for pets can be very difficult for veterans during chemotherapy treatments. Bringing care to the veteran increases veteran convenience, reduces need for transportation, reduces out-of-pocket cost to the veteran, and can improve care coordination.”
It’s not clear why VA care is cheaper than community care, she said, “but it may be related to the [higher] patient volumes we see in our VA facilities.” Lower overhead costs and government pricing contracts for chemotherapies/injectables could also be factors, Clough explained. In an interview, Todd Wagner, PhD, Stanford University Professor in the Department of Surgery and Director of the Health Economics Research Center at the VA, said the analysis needs risk adjustment for cancer severity and other illnesses and comorbidities that could affect cancer treatment. “In our work, sicker veterans get care in VA, and healthier veterans are choosing VA-purchased care [in the community].”
He noted that in the new analysis, the VA care looks much cheaper. “I'm struggling with that result: It flies in the face of our past work,” he said. He added that an analysis should also look at surgery and radiation. “VA has a tradition of providing high-value medications at a lower cost. But VA’s costs of surgery and radiation tend to be more expensive.”
Clough does not plan more research in this area. For now, she said, her site is working to expand to include infusion clinics at local VA community-based outpatient clinics as part of the Close to Me program. The program, launched by the VA in 2022, aims to provide cancer services at community-based outpatient clinics, mobile infusion units, and patient homes.
“As part of this service, we are looking to maximize video-based and phone visits between our existing oncology staff and veterans living in more isolated areas, coordinate labs/imaging locally, and deliver infusion and/or injectable therapies to the veteran,” Clough said.
In addition, she said, “We currently have a dedicated hematology/oncology trained pharmacist and 2 infusion nurses working to expand the service and deliver veteran care in these remote areas.”
There was no funding for this study, and the authors have no disclosures. Wagner discloses grant funding to his institutions from the VA, the National Institutes of Health, the Agency for Healthcare Research and Quality, the Robert Wood Johnson Foundation, and the Heinz Foundation.
CHICAGO—The VA could save nearly $10,000 annually per patient in the Mountain West region referral area by administering lower-level hematology/oncology care within the system instead of in the community, according to a new analysis.
The findings reflect that the community care encouraged by the MISSION Act is more expensive than US Department of Veterans Affairs (VA) care, said report lead author Alyson Clough, PharmD, a clinical pharmacy specialist with the George E. Wahlen Veterans Affairs Medical Center (VAMC) in Salt Lake City, Utah, in an interview. Clough and colleagues presented the poster at the 2023 annual meeting of the Association of VA Hematology/Oncology.
The 2018 MISSION Act expanded the eligibility for non-VA community services within the VA. “If a veteran requires care that a VA cannot provide in-house, lives in a state/territory without a full-service VAMC, and/or lives further than a certain distance/time from a VA Medical Center, then the veteran may be eligible to receive care in the community,” Clough said.
For the new analysis, the researchers focused on the Salt Lake City VAMC referral area, which spans about 125,000 square miles in Utah, Idaho, and Nevada. “While the MISSION Act helped give those veterans more options for specialty care—including hematology/oncology—many veterans are still driving several hours on a routine basis for chemotherapy treatment and injection therapies, even in the community setting,” Clough explained.
The researchers looked at the costs of “low-risk cancer care,” which Clough said consists of “chemotherapy or immunotherapy that is less likely to cause significant side effects during the infusion, is typically nonhazardous, and/or can be administered via a short infusion [30 minutes or less] or an injection.”
In fiscal year 2022, the VA paid more than $5.7 million for community care services for 380 veterans in the referral area, about $15,060 each, according to the analysis. In contrast, the average cost for 1774 veterans within the VA system was $5424. “By retaining or re-establishing hematology/oncology veteran care within VA, we estimate cost savings of approximately $9636 per unique veteran.”
Specifically, the researchers wrote that the average care costs were $5297 per veteran in the community vs $1143 at the VA, and average drug costs were $9763 per veteran in the community vs $4281 in the VA. These amount to total costs of $15,060 per veteran in the community vs $5424 in the VA.
Low-risk services “are ideal to bring to more isolated regions,” Clough noted. “Traveling and/or finding accommodations for pets can be very difficult for veterans during chemotherapy treatments. Bringing care to the veteran increases veteran convenience, reduces need for transportation, reduces out-of-pocket cost to the veteran, and can improve care coordination.”
It’s not clear why VA care is cheaper than community care, she said, “but it may be related to the [higher] patient volumes we see in our VA facilities.” Lower overhead costs and government pricing contracts for chemotherapies/injectables could also be factors, Clough explained. In an interview, Todd Wagner, PhD, Stanford University Professor in the Department of Surgery and Director of the Health Economics Research Center at the VA, said the analysis needs risk adjustment for cancer severity and other illnesses and comorbidities that could affect cancer treatment. “In our work, sicker veterans get care in VA, and healthier veterans are choosing VA-purchased care [in the community].”
He noted that in the new analysis, the VA care looks much cheaper. “I'm struggling with that result: It flies in the face of our past work,” he said. He added that an analysis should also look at surgery and radiation. “VA has a tradition of providing high-value medications at a lower cost. But VA’s costs of surgery and radiation tend to be more expensive.”
Clough does not plan more research in this area. For now, she said, her site is working to expand to include infusion clinics at local VA community-based outpatient clinics as part of the Close to Me program. The program, launched by the VA in 2022, aims to provide cancer services at community-based outpatient clinics, mobile infusion units, and patient homes.
“As part of this service, we are looking to maximize video-based and phone visits between our existing oncology staff and veterans living in more isolated areas, coordinate labs/imaging locally, and deliver infusion and/or injectable therapies to the veteran,” Clough said.
In addition, she said, “We currently have a dedicated hematology/oncology trained pharmacist and 2 infusion nurses working to expand the service and deliver veteran care in these remote areas.”
There was no funding for this study, and the authors have no disclosures. Wagner discloses grant funding to his institutions from the VA, the National Institutes of Health, the Agency for Healthcare Research and Quality, the Robert Wood Johnson Foundation, and the Heinz Foundation.
CHICAGO—The VA could save nearly $10,000 annually per patient in the Mountain West region referral area by administering lower-level hematology/oncology care within the system instead of in the community, according to a new analysis.
The findings reflect that the community care encouraged by the MISSION Act is more expensive than US Department of Veterans Affairs (VA) care, said report lead author Alyson Clough, PharmD, a clinical pharmacy specialist with the George E. Wahlen Veterans Affairs Medical Center (VAMC) in Salt Lake City, Utah, in an interview. Clough and colleagues presented the poster at the 2023 annual meeting of the Association of VA Hematology/Oncology.
The 2018 MISSION Act expanded the eligibility for non-VA community services within the VA. “If a veteran requires care that a VA cannot provide in-house, lives in a state/territory without a full-service VAMC, and/or lives further than a certain distance/time from a VA Medical Center, then the veteran may be eligible to receive care in the community,” Clough said.
For the new analysis, the researchers focused on the Salt Lake City VAMC referral area, which spans about 125,000 square miles in Utah, Idaho, and Nevada. “While the MISSION Act helped give those veterans more options for specialty care—including hematology/oncology—many veterans are still driving several hours on a routine basis for chemotherapy treatment and injection therapies, even in the community setting,” Clough explained.
The researchers looked at the costs of “low-risk cancer care,” which Clough said consists of “chemotherapy or immunotherapy that is less likely to cause significant side effects during the infusion, is typically nonhazardous, and/or can be administered via a short infusion [30 minutes or less] or an injection.”
In fiscal year 2022, the VA paid more than $5.7 million for community care services for 380 veterans in the referral area, about $15,060 each, according to the analysis. In contrast, the average cost for 1774 veterans within the VA system was $5424. “By retaining or re-establishing hematology/oncology veteran care within VA, we estimate cost savings of approximately $9636 per unique veteran.”
Specifically, the researchers wrote that the average care costs were $5297 per veteran in the community vs $1143 at the VA, and average drug costs were $9763 per veteran in the community vs $4281 in the VA. These amount to total costs of $15,060 per veteran in the community vs $5424 in the VA.
Low-risk services “are ideal to bring to more isolated regions,” Clough noted. “Traveling and/or finding accommodations for pets can be very difficult for veterans during chemotherapy treatments. Bringing care to the veteran increases veteran convenience, reduces need for transportation, reduces out-of-pocket cost to the veteran, and can improve care coordination.”
It’s not clear why VA care is cheaper than community care, she said, “but it may be related to the [higher] patient volumes we see in our VA facilities.” Lower overhead costs and government pricing contracts for chemotherapies/injectables could also be factors, Clough explained. In an interview, Todd Wagner, PhD, Stanford University Professor in the Department of Surgery and Director of the Health Economics Research Center at the VA, said the analysis needs risk adjustment for cancer severity and other illnesses and comorbidities that could affect cancer treatment. “In our work, sicker veterans get care in VA, and healthier veterans are choosing VA-purchased care [in the community].”
He noted that in the new analysis, the VA care looks much cheaper. “I'm struggling with that result: It flies in the face of our past work,” he said. He added that an analysis should also look at surgery and radiation. “VA has a tradition of providing high-value medications at a lower cost. But VA’s costs of surgery and radiation tend to be more expensive.”
Clough does not plan more research in this area. For now, she said, her site is working to expand to include infusion clinics at local VA community-based outpatient clinics as part of the Close to Me program. The program, launched by the VA in 2022, aims to provide cancer services at community-based outpatient clinics, mobile infusion units, and patient homes.
“As part of this service, we are looking to maximize video-based and phone visits between our existing oncology staff and veterans living in more isolated areas, coordinate labs/imaging locally, and deliver infusion and/or injectable therapies to the veteran,” Clough said.
In addition, she said, “We currently have a dedicated hematology/oncology trained pharmacist and 2 infusion nurses working to expand the service and deliver veteran care in these remote areas.”
There was no funding for this study, and the authors have no disclosures. Wagner discloses grant funding to his institutions from the VA, the National Institutes of Health, the Agency for Healthcare Research and Quality, the Robert Wood Johnson Foundation, and the Heinz Foundation.
Tackling the Maternal Health Crisis
The US Department of Veterans Affairs (VA) provides health care to about 600,000 women veterans—half are of child-bearing age. Pregnancies in women veterans using VA care have increased by more than 80% since 2014, from 6950 in 2014 to 12,524 in 2022.
Until recently, VA maternity care coordinators would help women navigate health care from the beginning of pregnancy to 8 weeks postpartum. But “[e]vidence shows that new mothers often need support and care coordination long after 8 weeks postpartum, which is why VA is taking action to support veteran mothers for much longer after they give birth,” said Under Secretary for Health Shereef Elnahal, MD. As of October 1, 2023, the maternity support is now extended to 12 months postpartum.
The full range of maternity care services includes primary care, examinations, tests, ultrasounds, newborn care, and screening for social determinants of health, mental health risk factors, and relationship health and safety. Maternity care coordinators also connect veterans with care after delivery and ensure access to follow-up screenings.
The VA says expanding access to maternity care coordinators is part of the work it’s doing to implement the White House Blueprint for Addressing the Maternal Health Crisis, released last year. The US maternal mortality rate is the highest of any developed nation in the world and more than double the rate of peer countries, the report says. According to the Centers for Disease Control and Prevention (CDC), from 2018 to 2021, the maternal death rate in the US increased from 17.4 to 32.9 per 100,000 live births.
Moreover, “[t]housands of women experience unexpected outcomes of labor and delivery that result in significant short- or long-term consequences to their health,” the White House report says, “such as heart issues, the need for blood transfusions, eclampsia, and blood infections.” Disturbingly, more than 80% of pregnancy-related deaths are preventable. Black and American Indian/Alaska Native women, regardless of income or education, are most likely to experience poor outcomes. Women who live in rural America—where there are many maternal care “deserts”—are about 60% more likely to die, the White House report says.
Quality care requires “care organized for and provided to all women in a manner that maintains their dignity, privacy, and confidentiality, ensures freedom from harm and mistreatment, and enables informed choice and continuous support during labor and childbirth,” say CDC researchers who surveyed 2407 women about their maternity care experiences. One in 5 respondents reported instances of mistreatment. Roughly one-third of Black, Hispanic, and multiracial women reported, for instance, receiving no response to requests for help, being shouted at or scolded, not having their physical privacy protected, and being threatened with withholding treatment or made to accept unwanted treatment.
The White House Blueprint delineates several goals. One is to ”ensure those giving birth are heard and are decisionmakers in accountable systems of care…to improve quality of care, hold providers accountable, and prioritize patient needs and their experience before, during, and after pregnancy.”
The Blueprint advises, for instance, expanding the Hear Her campaign to include culturally relevant materials to raise awareness of urgent maternal warning signs and improve communication between patients and clinicians. It also urges addressing social determinants of maternal health, supporting projects to expand maternal mental health access, increasing access to digital tools, and expanding models that train maternal health care practitoners and students on how to address implicit bias and racism and screen for social determinants of health.
Answering its question of “how we get there,” the Blueprint says, “In working toward this vision, the Biden-Harris Administration has developed, for the first time, a national, whole-of-government strategy to address our maternal health crisis. This strategy starts with the recognition that a concerted national effort to solve the crisis must begin with clear leadership and action from across the federal government. Addressing the maternal health crisis is not limited to a single health care policy or federal agency but should include experts across the government, including: the US Departments of Health and Human Services, Agriculture, Defense, Housing and Urban Development, Labor, Justice, Environmental Protection Agency, Office of Personnel Management, as well as the VA.
“The Biden-Harris Administration believes that only through this whole-of-government approach—one that considers the entirety of a person’s health and experiences over the course of their full life—will we finally be able to make real progress in tackling this longstanding challenge.”
The US Department of Veterans Affairs (VA) provides health care to about 600,000 women veterans—half are of child-bearing age. Pregnancies in women veterans using VA care have increased by more than 80% since 2014, from 6950 in 2014 to 12,524 in 2022.
Until recently, VA maternity care coordinators would help women navigate health care from the beginning of pregnancy to 8 weeks postpartum. But “[e]vidence shows that new mothers often need support and care coordination long after 8 weeks postpartum, which is why VA is taking action to support veteran mothers for much longer after they give birth,” said Under Secretary for Health Shereef Elnahal, MD. As of October 1, 2023, the maternity support is now extended to 12 months postpartum.
The full range of maternity care services includes primary care, examinations, tests, ultrasounds, newborn care, and screening for social determinants of health, mental health risk factors, and relationship health and safety. Maternity care coordinators also connect veterans with care after delivery and ensure access to follow-up screenings.
The VA says expanding access to maternity care coordinators is part of the work it’s doing to implement the White House Blueprint for Addressing the Maternal Health Crisis, released last year. The US maternal mortality rate is the highest of any developed nation in the world and more than double the rate of peer countries, the report says. According to the Centers for Disease Control and Prevention (CDC), from 2018 to 2021, the maternal death rate in the US increased from 17.4 to 32.9 per 100,000 live births.
Moreover, “[t]housands of women experience unexpected outcomes of labor and delivery that result in significant short- or long-term consequences to their health,” the White House report says, “such as heart issues, the need for blood transfusions, eclampsia, and blood infections.” Disturbingly, more than 80% of pregnancy-related deaths are preventable. Black and American Indian/Alaska Native women, regardless of income or education, are most likely to experience poor outcomes. Women who live in rural America—where there are many maternal care “deserts”—are about 60% more likely to die, the White House report says.
Quality care requires “care organized for and provided to all women in a manner that maintains their dignity, privacy, and confidentiality, ensures freedom from harm and mistreatment, and enables informed choice and continuous support during labor and childbirth,” say CDC researchers who surveyed 2407 women about their maternity care experiences. One in 5 respondents reported instances of mistreatment. Roughly one-third of Black, Hispanic, and multiracial women reported, for instance, receiving no response to requests for help, being shouted at or scolded, not having their physical privacy protected, and being threatened with withholding treatment or made to accept unwanted treatment.
The White House Blueprint delineates several goals. One is to ”ensure those giving birth are heard and are decisionmakers in accountable systems of care…to improve quality of care, hold providers accountable, and prioritize patient needs and their experience before, during, and after pregnancy.”
The Blueprint advises, for instance, expanding the Hear Her campaign to include culturally relevant materials to raise awareness of urgent maternal warning signs and improve communication between patients and clinicians. It also urges addressing social determinants of maternal health, supporting projects to expand maternal mental health access, increasing access to digital tools, and expanding models that train maternal health care practitoners and students on how to address implicit bias and racism and screen for social determinants of health.
Answering its question of “how we get there,” the Blueprint says, “In working toward this vision, the Biden-Harris Administration has developed, for the first time, a national, whole-of-government strategy to address our maternal health crisis. This strategy starts with the recognition that a concerted national effort to solve the crisis must begin with clear leadership and action from across the federal government. Addressing the maternal health crisis is not limited to a single health care policy or federal agency but should include experts across the government, including: the US Departments of Health and Human Services, Agriculture, Defense, Housing and Urban Development, Labor, Justice, Environmental Protection Agency, Office of Personnel Management, as well as the VA.
“The Biden-Harris Administration believes that only through this whole-of-government approach—one that considers the entirety of a person’s health and experiences over the course of their full life—will we finally be able to make real progress in tackling this longstanding challenge.”
The US Department of Veterans Affairs (VA) provides health care to about 600,000 women veterans—half are of child-bearing age. Pregnancies in women veterans using VA care have increased by more than 80% since 2014, from 6950 in 2014 to 12,524 in 2022.
Until recently, VA maternity care coordinators would help women navigate health care from the beginning of pregnancy to 8 weeks postpartum. But “[e]vidence shows that new mothers often need support and care coordination long after 8 weeks postpartum, which is why VA is taking action to support veteran mothers for much longer after they give birth,” said Under Secretary for Health Shereef Elnahal, MD. As of October 1, 2023, the maternity support is now extended to 12 months postpartum.
The full range of maternity care services includes primary care, examinations, tests, ultrasounds, newborn care, and screening for social determinants of health, mental health risk factors, and relationship health and safety. Maternity care coordinators also connect veterans with care after delivery and ensure access to follow-up screenings.
The VA says expanding access to maternity care coordinators is part of the work it’s doing to implement the White House Blueprint for Addressing the Maternal Health Crisis, released last year. The US maternal mortality rate is the highest of any developed nation in the world and more than double the rate of peer countries, the report says. According to the Centers for Disease Control and Prevention (CDC), from 2018 to 2021, the maternal death rate in the US increased from 17.4 to 32.9 per 100,000 live births.
Moreover, “[t]housands of women experience unexpected outcomes of labor and delivery that result in significant short- or long-term consequences to their health,” the White House report says, “such as heart issues, the need for blood transfusions, eclampsia, and blood infections.” Disturbingly, more than 80% of pregnancy-related deaths are preventable. Black and American Indian/Alaska Native women, regardless of income or education, are most likely to experience poor outcomes. Women who live in rural America—where there are many maternal care “deserts”—are about 60% more likely to die, the White House report says.
Quality care requires “care organized for and provided to all women in a manner that maintains their dignity, privacy, and confidentiality, ensures freedom from harm and mistreatment, and enables informed choice and continuous support during labor and childbirth,” say CDC researchers who surveyed 2407 women about their maternity care experiences. One in 5 respondents reported instances of mistreatment. Roughly one-third of Black, Hispanic, and multiracial women reported, for instance, receiving no response to requests for help, being shouted at or scolded, not having their physical privacy protected, and being threatened with withholding treatment or made to accept unwanted treatment.
The White House Blueprint delineates several goals. One is to ”ensure those giving birth are heard and are decisionmakers in accountable systems of care…to improve quality of care, hold providers accountable, and prioritize patient needs and their experience before, during, and after pregnancy.”
The Blueprint advises, for instance, expanding the Hear Her campaign to include culturally relevant materials to raise awareness of urgent maternal warning signs and improve communication between patients and clinicians. It also urges addressing social determinants of maternal health, supporting projects to expand maternal mental health access, increasing access to digital tools, and expanding models that train maternal health care practitoners and students on how to address implicit bias and racism and screen for social determinants of health.
Answering its question of “how we get there,” the Blueprint says, “In working toward this vision, the Biden-Harris Administration has developed, for the first time, a national, whole-of-government strategy to address our maternal health crisis. This strategy starts with the recognition that a concerted national effort to solve the crisis must begin with clear leadership and action from across the federal government. Addressing the maternal health crisis is not limited to a single health care policy or federal agency but should include experts across the government, including: the US Departments of Health and Human Services, Agriculture, Defense, Housing and Urban Development, Labor, Justice, Environmental Protection Agency, Office of Personnel Management, as well as the VA.
“The Biden-Harris Administration believes that only through this whole-of-government approach—one that considers the entirety of a person’s health and experiences over the course of their full life—will we finally be able to make real progress in tackling this longstanding challenge.”
Pallor and weight loss
Ulcerative colitis is a chronic inflammatory condition that characteristically involves the large bowel. Disease activity usually follows a pattern of periods of active inflammation alternating with periods of remission. Approximately 15% of patients experience an aggressive course of ulcerative colitis. Acute severe ulcerative colitis (ASUC) is a life-threatening medical emergency, which may require hospitalization for prompt medical treatment and colectomy if medical treatment fails. Predictors of an aggressive disease course and colectomy include young age at the time of diagnosis, extensive disease, severe endoscopic disease activity, presence of extraintestinal manifestations, elevated inflammatory markers, and early need for corticosteroids.
The diagnosis of ASUC is based on the Mayo Clinic Score and the Truelove and Witts criteria which consists of the presence of six or more bloody stools per day and at least one of these signs of systemic toxicity:
• Pulse rate > 90 beats/min
• Temperature > 100.04 °F
• Hemoglobin < 10.5 g/dL
• Erythrocyte sedimentation rate (ESR) > 30 mm/h
Further evaluation in patients with suspected ASUC aims to exclude alternative diagnoses and to determine the severity and extent of disease. Abdominal radiographs are obtained to rule out colonic dilatation and to evaluate for the possibility of microperforations. Stool studies should be obtained to evaluate for infections such as C difficile. To assess for the severity of mucosal disease a limited lower endoscopy is usually performed in hospitalized patients with ASUC. In addition, it allows for the opportunity to perform a biopsy to rule out cytomegalovirus as the cause of the disease flare. However, a colonoscopy should be avoided in these patients because of the increased risk for colonic dilation and perforation; a carefully performed flexible sigmoidoscopy with minimal insufflation by an experienced operator is sufficient for most patients. Endoscopic features of ASUC include erythema, absent vascular pattern, friability, erosions, and ulcerations.
The mainstay of management of hospitalized individuals with ASUC is intravenous corticosteroids. However, up to one third of patients may not show improvement in clinical or biochemical markers after treatment with steroids. In hospitalized patients with ASUC refractory to 3 to 5 days of intravenous corticosteroids infliximab or cyclosporin are suggested. Colectomy is a treatment option for patients unresponsive to medical therapy or for patients who develop life-threatening complications (colonic perforation, toxic megacolon, etc.)
Leyla Ghazi, MD, Physician, Dartmouth Health, GI Associates, Concord, New Hampshire.
Leyla Ghazi, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Ulcerative colitis is a chronic inflammatory condition that characteristically involves the large bowel. Disease activity usually follows a pattern of periods of active inflammation alternating with periods of remission. Approximately 15% of patients experience an aggressive course of ulcerative colitis. Acute severe ulcerative colitis (ASUC) is a life-threatening medical emergency, which may require hospitalization for prompt medical treatment and colectomy if medical treatment fails. Predictors of an aggressive disease course and colectomy include young age at the time of diagnosis, extensive disease, severe endoscopic disease activity, presence of extraintestinal manifestations, elevated inflammatory markers, and early need for corticosteroids.
The diagnosis of ASUC is based on the Mayo Clinic Score and the Truelove and Witts criteria which consists of the presence of six or more bloody stools per day and at least one of these signs of systemic toxicity:
• Pulse rate > 90 beats/min
• Temperature > 100.04 °F
• Hemoglobin < 10.5 g/dL
• Erythrocyte sedimentation rate (ESR) > 30 mm/h
Further evaluation in patients with suspected ASUC aims to exclude alternative diagnoses and to determine the severity and extent of disease. Abdominal radiographs are obtained to rule out colonic dilatation and to evaluate for the possibility of microperforations. Stool studies should be obtained to evaluate for infections such as C difficile. To assess for the severity of mucosal disease a limited lower endoscopy is usually performed in hospitalized patients with ASUC. In addition, it allows for the opportunity to perform a biopsy to rule out cytomegalovirus as the cause of the disease flare. However, a colonoscopy should be avoided in these patients because of the increased risk for colonic dilation and perforation; a carefully performed flexible sigmoidoscopy with minimal insufflation by an experienced operator is sufficient for most patients. Endoscopic features of ASUC include erythema, absent vascular pattern, friability, erosions, and ulcerations.
The mainstay of management of hospitalized individuals with ASUC is intravenous corticosteroids. However, up to one third of patients may not show improvement in clinical or biochemical markers after treatment with steroids. In hospitalized patients with ASUC refractory to 3 to 5 days of intravenous corticosteroids infliximab or cyclosporin are suggested. Colectomy is a treatment option for patients unresponsive to medical therapy or for patients who develop life-threatening complications (colonic perforation, toxic megacolon, etc.)
Leyla Ghazi, MD, Physician, Dartmouth Health, GI Associates, Concord, New Hampshire.
Leyla Ghazi, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Ulcerative colitis is a chronic inflammatory condition that characteristically involves the large bowel. Disease activity usually follows a pattern of periods of active inflammation alternating with periods of remission. Approximately 15% of patients experience an aggressive course of ulcerative colitis. Acute severe ulcerative colitis (ASUC) is a life-threatening medical emergency, which may require hospitalization for prompt medical treatment and colectomy if medical treatment fails. Predictors of an aggressive disease course and colectomy include young age at the time of diagnosis, extensive disease, severe endoscopic disease activity, presence of extraintestinal manifestations, elevated inflammatory markers, and early need for corticosteroids.
The diagnosis of ASUC is based on the Mayo Clinic Score and the Truelove and Witts criteria which consists of the presence of six or more bloody stools per day and at least one of these signs of systemic toxicity:
• Pulse rate > 90 beats/min
• Temperature > 100.04 °F
• Hemoglobin < 10.5 g/dL
• Erythrocyte sedimentation rate (ESR) > 30 mm/h
Further evaluation in patients with suspected ASUC aims to exclude alternative diagnoses and to determine the severity and extent of disease. Abdominal radiographs are obtained to rule out colonic dilatation and to evaluate for the possibility of microperforations. Stool studies should be obtained to evaluate for infections such as C difficile. To assess for the severity of mucosal disease a limited lower endoscopy is usually performed in hospitalized patients with ASUC. In addition, it allows for the opportunity to perform a biopsy to rule out cytomegalovirus as the cause of the disease flare. However, a colonoscopy should be avoided in these patients because of the increased risk for colonic dilation and perforation; a carefully performed flexible sigmoidoscopy with minimal insufflation by an experienced operator is sufficient for most patients. Endoscopic features of ASUC include erythema, absent vascular pattern, friability, erosions, and ulcerations.
The mainstay of management of hospitalized individuals with ASUC is intravenous corticosteroids. However, up to one third of patients may not show improvement in clinical or biochemical markers after treatment with steroids. In hospitalized patients with ASUC refractory to 3 to 5 days of intravenous corticosteroids infliximab or cyclosporin are suggested. Colectomy is a treatment option for patients unresponsive to medical therapy or for patients who develop life-threatening complications (colonic perforation, toxic megacolon, etc.)
Leyla Ghazi, MD, Physician, Dartmouth Health, GI Associates, Concord, New Hampshire.
Leyla Ghazi, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 36-year-old man presents reporting bouts of bloody diarrhea up to 10 times per day for the past 6 weeks. The diarrhea is associated with asymmetric polyarthralgia in the elbows and knees; a skin rash on the lower extremities; fatigue, weakness, and pallor; and a 12-lb weight loss. One month before, the patient had a colonoscopy that revealed left-sided ulcerative colitis (UC); results were confirmed with biopsy and the patient was started on mesalamine and prednisone.
Vital signs at the time of presentation include blood pressure 90/58, heart rate 112 beats/min, respiratory rate 21 breaths/min, and body temperature 101.9 °F. Examination shows generalized pallor with pale conjunctiva and dry mucosa. No heart murmurs are heard on auscultation. Palpation of the abdomen reveals no palpable masses or organomegaly. Mild pain is present on palpation of the left lower quadrant but without signs of peritoneal irritation. Bowel sounds are present. The lower extremities display erythematous nodular lesions and no edema. Peripheral pulses are present. Laboratory results showed an erythrocyte sedimentation rate of 60 mm, C-reactive protein of 20.2 mg/L, and hemoglobin of 9.8 g/dL. Abdominal radiographs were within normal limits. Stool cultures are pending. Flexible sigmoidoscopy was performed shortly after admission and showed the results above. Biopsies were taken to rule out infection.
Working with industry in private practice gastroenterology
In this video, Dr. Nadeem Baig of Allied Digestive Health in West Long Branck, N.J., discusses why he chose private practice gastroenterology and how his organization works with industry to support its mission of providing the best care for patients.
He has no financial conflicts relative to the topics in this video.

In this video, Dr. Nadeem Baig of Allied Digestive Health in West Long Branck, N.J., discusses why he chose private practice gastroenterology and how his organization works with industry to support its mission of providing the best care for patients.
He has no financial conflicts relative to the topics in this video.

In this video, Dr. Nadeem Baig of Allied Digestive Health in West Long Branck, N.J., discusses why he chose private practice gastroenterology and how his organization works with industry to support its mission of providing the best care for patients.
He has no financial conflicts relative to the topics in this video.

Roflumilast side effect benefits patients with psoriasis and overweight/obesity
BERLIN – .
Reporting secondary outcomes from the investigator-led trial at the annual congress of the European Academy of Dermatology and Venereology, Alexander Egeberg, MD, PhD, DMSc, noted that “clinically significant weight loss” was seen among patients who were treated with oral roflumilast, 500 mcg once daily, versus those receiving placebo.
Indeed, after 12 weeks of therapy, one in three patients treated with oral roflumilast experienced at least a 5% drop in their baseline body weight vs no patients who received placebo (35% vs. 0%; P < .05).
Additionally, a respective 17% versus 0% of patients lost 10% or more of their body weight, and 4% versus 0% lost 15% or more of their baseline body weight at 12 weeks.
After 24 weeks’ treatment, a substantial percentage of patients still had greater than or equal to 5%, greater than or equal to 10%, or greater than or equal to 15% weight loss, at 30%, 17%, and 13% for oral roflumilast, compared with 9%, 0%, and 0% for placebo, respectively.
“We saw that the higher baseline weight correlated with the proportion of weight loss, so that the more heavy patients at baseline also were the ones who experienced the greatest weight loss,” said Dr. Egeberg, who is professor of dermatology at the University of Copenhagen and a senior consultant at the department of dermatology at Bispebjerg Hospital, Copenhagen.
A beneficial side effect in psoriasis?
“You may have heard in psoriasis about topical roflumilast, but oral roflumilast is actually also shown to be effective in treating psoriasis,” said Egeberg.
Topical roflumilast is approved in the United States and Canada for treating plaque psoriasis.
Efficacy results from the PSORRO study were published earlier this year and showed a significantly greater improvement in Psoriasis Area and Severity Index (PASI) 75 with oral roflumilast vs. placebo at 12 weeks (35% vs. 0%), with a sustained effect seen at 24 weeks (44% vs. 40%).
Weight loss was among the most common side effects seen, leading Dr. Egeberg and fellow PSORRO investigators to wonder whether this may actually be a beneficial effect in patients with psoriasis.
“Oral roflumilast is actually a drug that has been on the market for quite a number of years,” Dr. Egeberg said.
Although only currently licensed for chronic obstructive pulmonary disease (COPD) in the United States, oral roflumilast, a phosphodiesterase (PDE) 4 inhibitor, is available as a generic, “which also means that it is extremely affordable,” suggested Dr. Edeberg.
Weight loss may be a problem in patients with COPD, he acknowledged; these patients tend to be underweight as a result of their poor state of health caused by the lung condition. Weight loss could be an advantage in patients with psoriasis who are overweight or living with obesity and have poor cardiometabolic parameters.
The psoriasis treatment with oral roflumilast study
The PSORRO study was a phase 2, multicenter, placebo-controlled, randomized trial performed between 2021 and 2022. A total of 46 adults with plaque psoriasis participated; half were initially treated with oral roflumilast and half with placebo.
Treatment was double-blind for the first 12 weeks, with all patients then receiving open-label treatment with roflumilast for 12 weeks.
The primary endpoint was the proportion of patients achieving at least 75% reduction from baseline PASI (PASI75). A host of secondary endpoints were studied, including weight and cardiometabolic parameters, which Dr. Egeberg reported at the EADV meeting.
Looking at the baseline characteristics of the oral roflumilast and placebo groups, the mean age was a respective 38 and 39 years, 65% and 83% were men, and the mean starting body weight was 102 kg and 105.1 kg.
After 12 weeks of treatment, body weight fell by a mean of 5.4 kg in the oral roflumilast group, with a further decrease of 1.4 kg by 24 weeks, bringing the total average weight loss to 6.8 kg. By comparison, weight loss among those in the placebo group was 0 kg at 12 weeks and around 2 kg at 24 weeks.
The majority of participants in both groups had high baseline BMIs; 70% of those who received oral roflumilast and 61% of those who received placebo had a BMI of 30 or higher.
“We wanted to investigate the impact of body weight, [so] we didn’t allow patients to be underweight when they were included,” Dr. Egeberg explained. Thus, for inclusion, patients had to have a BMI of 20 or higher.
An “extraordinary” finding was how some patients’ weight status based on their BMI changed throughout the study.
“We could see people that went from obese class 3, all the way to obese class 1. And we could see people going from being overweight to normal weight, which is really extraordinary for patients with psoriasis,” Dr. Egeberg said.
“But most importantly,” he added, “we didn’t have any patients who became underweight, suggesting that it actually is safe to use also in normal-weight patients.”
Reduced appetite behind benefit?
Trying to see why the weight loss occurred, Dr. Egeberg noted that it looked like it could be a result of a reduced appetite.
In common with other PDE-4 inhibitors, oral roflumilast treatment was associated with gastrointestinal symptoms – nausea, diarrhea, and abdominal pain – but all of these “decrease to placebo levels again, quite quickly,” he said.
“This really suggests that it’s not because of diarrhea, it’s not because of nausea and abdominal pain; it is because of a reduced appetite that patients actually lose weight when treated with roflumilast,” Dr. Egeberg said. It’s a potential bonus for the drug’s effects on the skin and could afford clinicians an opportunity to help motivate patients to eat well when they do eat, he observed.
Other cardiometabolic parameters assessed included blood pressure, glycated hemoglobin, total cholesterol and other key lipids, creatinine, alanine aminotransferase, and high-sensitivity C-reactive protein, but there were no noteworthy differences between the groups.
Roflumilast is an inexpensive drug because it is generic, Dr. Egeberg observed, but that also means that its use is likely to be off-label.
“It will be up to the treating physician to decide if this is an optimal therapy for their patients,” he suggested.
Cardiometabolic comorbidities important to target
Obesity is a cardiometabolic comorbidity that is important to consider when treating your patients with psoriasis, Paolo Gisondi, MD, of the University of Verona (Italy), said at a separate presentation at the EADV meeting.
While not directly commenting on the roflumilast study, he noted that moderate to severe psoriasis was “frequently associated” with metabolic disorders that put people at additional risk for cardiovascular and fatty liver diseases.
The PSORRO study was an investigator-initiated and investigator-led study and received no commercial funding. Research funding came from the Danish Psoriasis Foundation, Herlev and Gentofte Hospital, and several charitable and humanitarian organizations. Dr. Egeberg acknowledged acting as the principal investigator, speaker, and/or consultant to multiple pharma companies, all of which were unrelated to the study he presented. Dr. Gisondi’s comments were from a separate presentation, and he was not involved in the study.
A version of this article first appeared on Medscape.com.
BERLIN – .
Reporting secondary outcomes from the investigator-led trial at the annual congress of the European Academy of Dermatology and Venereology, Alexander Egeberg, MD, PhD, DMSc, noted that “clinically significant weight loss” was seen among patients who were treated with oral roflumilast, 500 mcg once daily, versus those receiving placebo.
Indeed, after 12 weeks of therapy, one in three patients treated with oral roflumilast experienced at least a 5% drop in their baseline body weight vs no patients who received placebo (35% vs. 0%; P < .05).
Additionally, a respective 17% versus 0% of patients lost 10% or more of their body weight, and 4% versus 0% lost 15% or more of their baseline body weight at 12 weeks.
After 24 weeks’ treatment, a substantial percentage of patients still had greater than or equal to 5%, greater than or equal to 10%, or greater than or equal to 15% weight loss, at 30%, 17%, and 13% for oral roflumilast, compared with 9%, 0%, and 0% for placebo, respectively.
“We saw that the higher baseline weight correlated with the proportion of weight loss, so that the more heavy patients at baseline also were the ones who experienced the greatest weight loss,” said Dr. Egeberg, who is professor of dermatology at the University of Copenhagen and a senior consultant at the department of dermatology at Bispebjerg Hospital, Copenhagen.
A beneficial side effect in psoriasis?
“You may have heard in psoriasis about topical roflumilast, but oral roflumilast is actually also shown to be effective in treating psoriasis,” said Egeberg.
Topical roflumilast is approved in the United States and Canada for treating plaque psoriasis.
Efficacy results from the PSORRO study were published earlier this year and showed a significantly greater improvement in Psoriasis Area and Severity Index (PASI) 75 with oral roflumilast vs. placebo at 12 weeks (35% vs. 0%), with a sustained effect seen at 24 weeks (44% vs. 40%).
Weight loss was among the most common side effects seen, leading Dr. Egeberg and fellow PSORRO investigators to wonder whether this may actually be a beneficial effect in patients with psoriasis.
“Oral roflumilast is actually a drug that has been on the market for quite a number of years,” Dr. Egeberg said.
Although only currently licensed for chronic obstructive pulmonary disease (COPD) in the United States, oral roflumilast, a phosphodiesterase (PDE) 4 inhibitor, is available as a generic, “which also means that it is extremely affordable,” suggested Dr. Edeberg.
Weight loss may be a problem in patients with COPD, he acknowledged; these patients tend to be underweight as a result of their poor state of health caused by the lung condition. Weight loss could be an advantage in patients with psoriasis who are overweight or living with obesity and have poor cardiometabolic parameters.
The psoriasis treatment with oral roflumilast study
The PSORRO study was a phase 2, multicenter, placebo-controlled, randomized trial performed between 2021 and 2022. A total of 46 adults with plaque psoriasis participated; half were initially treated with oral roflumilast and half with placebo.
Treatment was double-blind for the first 12 weeks, with all patients then receiving open-label treatment with roflumilast for 12 weeks.
The primary endpoint was the proportion of patients achieving at least 75% reduction from baseline PASI (PASI75). A host of secondary endpoints were studied, including weight and cardiometabolic parameters, which Dr. Egeberg reported at the EADV meeting.
Looking at the baseline characteristics of the oral roflumilast and placebo groups, the mean age was a respective 38 and 39 years, 65% and 83% were men, and the mean starting body weight was 102 kg and 105.1 kg.
After 12 weeks of treatment, body weight fell by a mean of 5.4 kg in the oral roflumilast group, with a further decrease of 1.4 kg by 24 weeks, bringing the total average weight loss to 6.8 kg. By comparison, weight loss among those in the placebo group was 0 kg at 12 weeks and around 2 kg at 24 weeks.
The majority of participants in both groups had high baseline BMIs; 70% of those who received oral roflumilast and 61% of those who received placebo had a BMI of 30 or higher.
“We wanted to investigate the impact of body weight, [so] we didn’t allow patients to be underweight when they were included,” Dr. Egeberg explained. Thus, for inclusion, patients had to have a BMI of 20 or higher.
An “extraordinary” finding was how some patients’ weight status based on their BMI changed throughout the study.
“We could see people that went from obese class 3, all the way to obese class 1. And we could see people going from being overweight to normal weight, which is really extraordinary for patients with psoriasis,” Dr. Egeberg said.
“But most importantly,” he added, “we didn’t have any patients who became underweight, suggesting that it actually is safe to use also in normal-weight patients.”
Reduced appetite behind benefit?
Trying to see why the weight loss occurred, Dr. Egeberg noted that it looked like it could be a result of a reduced appetite.
In common with other PDE-4 inhibitors, oral roflumilast treatment was associated with gastrointestinal symptoms – nausea, diarrhea, and abdominal pain – but all of these “decrease to placebo levels again, quite quickly,” he said.
“This really suggests that it’s not because of diarrhea, it’s not because of nausea and abdominal pain; it is because of a reduced appetite that patients actually lose weight when treated with roflumilast,” Dr. Egeberg said. It’s a potential bonus for the drug’s effects on the skin and could afford clinicians an opportunity to help motivate patients to eat well when they do eat, he observed.
Other cardiometabolic parameters assessed included blood pressure, glycated hemoglobin, total cholesterol and other key lipids, creatinine, alanine aminotransferase, and high-sensitivity C-reactive protein, but there were no noteworthy differences between the groups.
Roflumilast is an inexpensive drug because it is generic, Dr. Egeberg observed, but that also means that its use is likely to be off-label.
“It will be up to the treating physician to decide if this is an optimal therapy for their patients,” he suggested.
Cardiometabolic comorbidities important to target
Obesity is a cardiometabolic comorbidity that is important to consider when treating your patients with psoriasis, Paolo Gisondi, MD, of the University of Verona (Italy), said at a separate presentation at the EADV meeting.
While not directly commenting on the roflumilast study, he noted that moderate to severe psoriasis was “frequently associated” with metabolic disorders that put people at additional risk for cardiovascular and fatty liver diseases.
The PSORRO study was an investigator-initiated and investigator-led study and received no commercial funding. Research funding came from the Danish Psoriasis Foundation, Herlev and Gentofte Hospital, and several charitable and humanitarian organizations. Dr. Egeberg acknowledged acting as the principal investigator, speaker, and/or consultant to multiple pharma companies, all of which were unrelated to the study he presented. Dr. Gisondi’s comments were from a separate presentation, and he was not involved in the study.
A version of this article first appeared on Medscape.com.
BERLIN – .
Reporting secondary outcomes from the investigator-led trial at the annual congress of the European Academy of Dermatology and Venereology, Alexander Egeberg, MD, PhD, DMSc, noted that “clinically significant weight loss” was seen among patients who were treated with oral roflumilast, 500 mcg once daily, versus those receiving placebo.
Indeed, after 12 weeks of therapy, one in three patients treated with oral roflumilast experienced at least a 5% drop in their baseline body weight vs no patients who received placebo (35% vs. 0%; P < .05).
Additionally, a respective 17% versus 0% of patients lost 10% or more of their body weight, and 4% versus 0% lost 15% or more of their baseline body weight at 12 weeks.
After 24 weeks’ treatment, a substantial percentage of patients still had greater than or equal to 5%, greater than or equal to 10%, or greater than or equal to 15% weight loss, at 30%, 17%, and 13% for oral roflumilast, compared with 9%, 0%, and 0% for placebo, respectively.
“We saw that the higher baseline weight correlated with the proportion of weight loss, so that the more heavy patients at baseline also were the ones who experienced the greatest weight loss,” said Dr. Egeberg, who is professor of dermatology at the University of Copenhagen and a senior consultant at the department of dermatology at Bispebjerg Hospital, Copenhagen.
A beneficial side effect in psoriasis?
“You may have heard in psoriasis about topical roflumilast, but oral roflumilast is actually also shown to be effective in treating psoriasis,” said Egeberg.
Topical roflumilast is approved in the United States and Canada for treating plaque psoriasis.
Efficacy results from the PSORRO study were published earlier this year and showed a significantly greater improvement in Psoriasis Area and Severity Index (PASI) 75 with oral roflumilast vs. placebo at 12 weeks (35% vs. 0%), with a sustained effect seen at 24 weeks (44% vs. 40%).
Weight loss was among the most common side effects seen, leading Dr. Egeberg and fellow PSORRO investigators to wonder whether this may actually be a beneficial effect in patients with psoriasis.
“Oral roflumilast is actually a drug that has been on the market for quite a number of years,” Dr. Egeberg said.
Although only currently licensed for chronic obstructive pulmonary disease (COPD) in the United States, oral roflumilast, a phosphodiesterase (PDE) 4 inhibitor, is available as a generic, “which also means that it is extremely affordable,” suggested Dr. Edeberg.
Weight loss may be a problem in patients with COPD, he acknowledged; these patients tend to be underweight as a result of their poor state of health caused by the lung condition. Weight loss could be an advantage in patients with psoriasis who are overweight or living with obesity and have poor cardiometabolic parameters.
The psoriasis treatment with oral roflumilast study
The PSORRO study was a phase 2, multicenter, placebo-controlled, randomized trial performed between 2021 and 2022. A total of 46 adults with plaque psoriasis participated; half were initially treated with oral roflumilast and half with placebo.
Treatment was double-blind for the first 12 weeks, with all patients then receiving open-label treatment with roflumilast for 12 weeks.
The primary endpoint was the proportion of patients achieving at least 75% reduction from baseline PASI (PASI75). A host of secondary endpoints were studied, including weight and cardiometabolic parameters, which Dr. Egeberg reported at the EADV meeting.
Looking at the baseline characteristics of the oral roflumilast and placebo groups, the mean age was a respective 38 and 39 years, 65% and 83% were men, and the mean starting body weight was 102 kg and 105.1 kg.
After 12 weeks of treatment, body weight fell by a mean of 5.4 kg in the oral roflumilast group, with a further decrease of 1.4 kg by 24 weeks, bringing the total average weight loss to 6.8 kg. By comparison, weight loss among those in the placebo group was 0 kg at 12 weeks and around 2 kg at 24 weeks.
The majority of participants in both groups had high baseline BMIs; 70% of those who received oral roflumilast and 61% of those who received placebo had a BMI of 30 or higher.
“We wanted to investigate the impact of body weight, [so] we didn’t allow patients to be underweight when they were included,” Dr. Egeberg explained. Thus, for inclusion, patients had to have a BMI of 20 or higher.
An “extraordinary” finding was how some patients’ weight status based on their BMI changed throughout the study.
“We could see people that went from obese class 3, all the way to obese class 1. And we could see people going from being overweight to normal weight, which is really extraordinary for patients with psoriasis,” Dr. Egeberg said.
“But most importantly,” he added, “we didn’t have any patients who became underweight, suggesting that it actually is safe to use also in normal-weight patients.”
Reduced appetite behind benefit?
Trying to see why the weight loss occurred, Dr. Egeberg noted that it looked like it could be a result of a reduced appetite.
In common with other PDE-4 inhibitors, oral roflumilast treatment was associated with gastrointestinal symptoms – nausea, diarrhea, and abdominal pain – but all of these “decrease to placebo levels again, quite quickly,” he said.
“This really suggests that it’s not because of diarrhea, it’s not because of nausea and abdominal pain; it is because of a reduced appetite that patients actually lose weight when treated with roflumilast,” Dr. Egeberg said. It’s a potential bonus for the drug’s effects on the skin and could afford clinicians an opportunity to help motivate patients to eat well when they do eat, he observed.
Other cardiometabolic parameters assessed included blood pressure, glycated hemoglobin, total cholesterol and other key lipids, creatinine, alanine aminotransferase, and high-sensitivity C-reactive protein, but there were no noteworthy differences between the groups.
Roflumilast is an inexpensive drug because it is generic, Dr. Egeberg observed, but that also means that its use is likely to be off-label.
“It will be up to the treating physician to decide if this is an optimal therapy for their patients,” he suggested.
Cardiometabolic comorbidities important to target
Obesity is a cardiometabolic comorbidity that is important to consider when treating your patients with psoriasis, Paolo Gisondi, MD, of the University of Verona (Italy), said at a separate presentation at the EADV meeting.
While not directly commenting on the roflumilast study, he noted that moderate to severe psoriasis was “frequently associated” with metabolic disorders that put people at additional risk for cardiovascular and fatty liver diseases.
The PSORRO study was an investigator-initiated and investigator-led study and received no commercial funding. Research funding came from the Danish Psoriasis Foundation, Herlev and Gentofte Hospital, and several charitable and humanitarian organizations. Dr. Egeberg acknowledged acting as the principal investigator, speaker, and/or consultant to multiple pharma companies, all of which were unrelated to the study he presented. Dr. Gisondi’s comments were from a separate presentation, and he was not involved in the study.
A version of this article first appeared on Medscape.com.
AT THE EADV CONGRESS