User login
CKD linked to cardiac arrest in Hispanic, Latinx patients
TOPLINE:
new data show, suggesting early identification of CKD may provide an opportunity to reduce the risk in these groups. Other predictors included heavy drinking, atrial fibrillation, coronary artery disease, heart failure and diabetes.
METHODOLOGY:
- The study included 295 Hispanic or Latinx patients with out-of-hospital SCA from the PRESTO study in Ventura County, California, and 590 frequency-matched controls from the San Diego site of the population-based HCHS/SOL (Hispanic Community Health Survey/Study of Latinos); in both cohorts, men made up 70% of participants, and the median age was about 63 years.
- Researchers collected data on demographics, medical history, and current health conditions. Of note, 51.2% of SCA cases and 8.8% of control participants had CKD, and 20.0% of cases and 0.7% of the control group were on dialysis.
- Pre-SCA echocardiograms were available for 48% of SCA cases and baseline echocardiograms for more than 99% of control participants.
TAKEAWAY:
- In analyses adjusted for age, sex, and clinical variables, predictors significantly associated with higher odds of SCA included: CKD (odds ratio, 7.3; 95% confidence interval, 3.8-14.3; P < .001), heavy drinking (OR, 4.5), stroke (OR, 3.1), atrial fibrillation (OR, 3.7), coronary artery disease (OR, 2.9), heart failure (OR, 2.5), and diabetes (OR, 1.5).
- Hypertension, hyperlipemia, body mass index, and current smoking status were not significantly associated with SCA.
- In adjusted analyses, heart rate (OR, 1.8 per one standard deviation [1-SD] increase), QTc interval (OR, 2.5 per 1-SD increase) and left ventricular ejection fraction (OR, 4.4 per 1-SD decrease) were significantly associated with SCA, suggesting echocardiogram evaluations could help identify Hispanic or Latinx individuals at increased risk for SCA, wrote the authors.
IN PRACTICE:
“Our study, the first to include feasible numbers of Hispanic or Latino individuals, highlights the importance of renal dysfunction as a risk factor for SCA in the community,” the authors wrote, adding that early identification and management of chronic kidney disease could reduce risk for SCA in this population.
SOURCE:
The study was conducted by Kyndaron Reinier, PhD, MPH, Cedars-Sinai Health System, Los Angeles, and colleagues. It was published online in the Journal of the American Heart Association.
LIMITATIONS:
Most participants from the HCHS/SOL study were born outside the United States, compared with about half the SCA cases, which could have influenced cardiovascular disease risk, although results did not change considerably when models were adjusted for place of birth. Study participants were predominantly of Mexican heritage, so results may not be generalizable to Hispanic or Latinx individuals from other regions. As medical history was assessed differently in the two studies, there could be some error in estimating the strength of associations. Results from echocardiographic data should be viewed as hypothesis generating because of the potential for residual bias.
DISCLOSURES:
The Ventura PRESTO study was funded, in part, by the National Institutes of Health, and National Heart, Lung, and Blood Institute. The HCHS/SOL was carried out as a collaborative study supported by contracts from the NHLBI.
A version of this article first appeared on Medscape.com.
TOPLINE:
new data show, suggesting early identification of CKD may provide an opportunity to reduce the risk in these groups. Other predictors included heavy drinking, atrial fibrillation, coronary artery disease, heart failure and diabetes.
METHODOLOGY:
- The study included 295 Hispanic or Latinx patients with out-of-hospital SCA from the PRESTO study in Ventura County, California, and 590 frequency-matched controls from the San Diego site of the population-based HCHS/SOL (Hispanic Community Health Survey/Study of Latinos); in both cohorts, men made up 70% of participants, and the median age was about 63 years.
- Researchers collected data on demographics, medical history, and current health conditions. Of note, 51.2% of SCA cases and 8.8% of control participants had CKD, and 20.0% of cases and 0.7% of the control group were on dialysis.
- Pre-SCA echocardiograms were available for 48% of SCA cases and baseline echocardiograms for more than 99% of control participants.
TAKEAWAY:
- In analyses adjusted for age, sex, and clinical variables, predictors significantly associated with higher odds of SCA included: CKD (odds ratio, 7.3; 95% confidence interval, 3.8-14.3; P < .001), heavy drinking (OR, 4.5), stroke (OR, 3.1), atrial fibrillation (OR, 3.7), coronary artery disease (OR, 2.9), heart failure (OR, 2.5), and diabetes (OR, 1.5).
- Hypertension, hyperlipemia, body mass index, and current smoking status were not significantly associated with SCA.
- In adjusted analyses, heart rate (OR, 1.8 per one standard deviation [1-SD] increase), QTc interval (OR, 2.5 per 1-SD increase) and left ventricular ejection fraction (OR, 4.4 per 1-SD decrease) were significantly associated with SCA, suggesting echocardiogram evaluations could help identify Hispanic or Latinx individuals at increased risk for SCA, wrote the authors.
IN PRACTICE:
“Our study, the first to include feasible numbers of Hispanic or Latino individuals, highlights the importance of renal dysfunction as a risk factor for SCA in the community,” the authors wrote, adding that early identification and management of chronic kidney disease could reduce risk for SCA in this population.
SOURCE:
The study was conducted by Kyndaron Reinier, PhD, MPH, Cedars-Sinai Health System, Los Angeles, and colleagues. It was published online in the Journal of the American Heart Association.
LIMITATIONS:
Most participants from the HCHS/SOL study were born outside the United States, compared with about half the SCA cases, which could have influenced cardiovascular disease risk, although results did not change considerably when models were adjusted for place of birth. Study participants were predominantly of Mexican heritage, so results may not be generalizable to Hispanic or Latinx individuals from other regions. As medical history was assessed differently in the two studies, there could be some error in estimating the strength of associations. Results from echocardiographic data should be viewed as hypothesis generating because of the potential for residual bias.
DISCLOSURES:
The Ventura PRESTO study was funded, in part, by the National Institutes of Health, and National Heart, Lung, and Blood Institute. The HCHS/SOL was carried out as a collaborative study supported by contracts from the NHLBI.
A version of this article first appeared on Medscape.com.
TOPLINE:
new data show, suggesting early identification of CKD may provide an opportunity to reduce the risk in these groups. Other predictors included heavy drinking, atrial fibrillation, coronary artery disease, heart failure and diabetes.
METHODOLOGY:
- The study included 295 Hispanic or Latinx patients with out-of-hospital SCA from the PRESTO study in Ventura County, California, and 590 frequency-matched controls from the San Diego site of the population-based HCHS/SOL (Hispanic Community Health Survey/Study of Latinos); in both cohorts, men made up 70% of participants, and the median age was about 63 years.
- Researchers collected data on demographics, medical history, and current health conditions. Of note, 51.2% of SCA cases and 8.8% of control participants had CKD, and 20.0% of cases and 0.7% of the control group were on dialysis.
- Pre-SCA echocardiograms were available for 48% of SCA cases and baseline echocardiograms for more than 99% of control participants.
TAKEAWAY:
- In analyses adjusted for age, sex, and clinical variables, predictors significantly associated with higher odds of SCA included: CKD (odds ratio, 7.3; 95% confidence interval, 3.8-14.3; P < .001), heavy drinking (OR, 4.5), stroke (OR, 3.1), atrial fibrillation (OR, 3.7), coronary artery disease (OR, 2.9), heart failure (OR, 2.5), and diabetes (OR, 1.5).
- Hypertension, hyperlipemia, body mass index, and current smoking status were not significantly associated with SCA.
- In adjusted analyses, heart rate (OR, 1.8 per one standard deviation [1-SD] increase), QTc interval (OR, 2.5 per 1-SD increase) and left ventricular ejection fraction (OR, 4.4 per 1-SD decrease) were significantly associated with SCA, suggesting echocardiogram evaluations could help identify Hispanic or Latinx individuals at increased risk for SCA, wrote the authors.
IN PRACTICE:
“Our study, the first to include feasible numbers of Hispanic or Latino individuals, highlights the importance of renal dysfunction as a risk factor for SCA in the community,” the authors wrote, adding that early identification and management of chronic kidney disease could reduce risk for SCA in this population.
SOURCE:
The study was conducted by Kyndaron Reinier, PhD, MPH, Cedars-Sinai Health System, Los Angeles, and colleagues. It was published online in the Journal of the American Heart Association.
LIMITATIONS:
Most participants from the HCHS/SOL study were born outside the United States, compared with about half the SCA cases, which could have influenced cardiovascular disease risk, although results did not change considerably when models were adjusted for place of birth. Study participants were predominantly of Mexican heritage, so results may not be generalizable to Hispanic or Latinx individuals from other regions. As medical history was assessed differently in the two studies, there could be some error in estimating the strength of associations. Results from echocardiographic data should be viewed as hypothesis generating because of the potential for residual bias.
DISCLOSURES:
The Ventura PRESTO study was funded, in part, by the National Institutes of Health, and National Heart, Lung, and Blood Institute. The HCHS/SOL was carried out as a collaborative study supported by contracts from the NHLBI.
A version of this article first appeared on Medscape.com.
Suits or joggers? A doctor’s dress code
Look at this guy – NFL Chargers jersey and shorts with a RVCA hat on backward. And next to him, a woman wearing her spin-class-Lulu gear. There’s also a guy sporting a 2016 San Diego Rock ‘n Roll Marathon Tee. And that young woman is actually wearing slippers. A visitor from the 1950s would be thunderstruck to see such casual wear on people waiting to board a plane. Photos from that era show men buttoned up in white shirt and tie and women wearing Chanel with hats and white gloves. This dramatic transformation from formal to unfussy wear cuts through all social situations, including in my office. As a new doc out of residency, I used to wear a tie and shoes that could hold a shine. Now I wear jogger scrubs and sneakers. Rather than be offended by the lack of formality though, patients seem to appreciate it. Should they?
At first glance this seems to be a modern phenomenon. The reasons for casual wear today are manifold: about one-third of people work from home, Millennials are taking over with their TikTok values and general irreverence, COVID made us all fat and lazy. Heck, even the U.S. Senate briefly abolished the requirement to wear suits on the Senate floor. But getting dressed up was never to signal that you are elite or superior to others. It’s the opposite. To get dressed is a signal that you are serving others, a tradition that is as old as society.
Think of Downton Abbey as an example. The servants were always required to be smartly dressed when working, whereas members of the family could be dressed up or not. It’s clear who is serving whom. This tradition lives today in the hospitality industry. When you mosey into the lobby of a luxury hotel in your Rainbow sandals you can expect everyone who greets you will be in finery, signaling that they put in effort to serve you. You’ll find the same for all staff at the Mayo Clinic in Rochester, Minn., which is no coincidence.
Suits used to be standard in medicine. In the 19th century, physicians wore formal black-tie when seeing patients. Unlike hospitality however, we had good reason to eschew the tradition: germs. Once we figured out that our pus-stained ties and jackets were doing harm, we switched to wearing sanitized uniforms. Casual wear for doctors isn’t a modern phenomenon after all, then. For proof, compare Thomas Eakins painting “The Gross Clinic” (1875) with his later “The Agnew Clinic” (1889). In the former, Dr. Gross is portrayed in formal black wear, bloody hand and all. In the latter, Dr. Agnew is wearing white FIGS (or the 1890’s equivalent anyway). Similarly, nurses uniforms traditionally resembled kitchen servants, with criss-cross aprons and floor length skirts. It wasn’t until the 1980’s that nurses stopped wearing dresses and white caps.

In the operating theater it’s obviously critical that we wear sanitized scrubs to mitigate the risk of infection. Originally white to signal cleanliness, scrubs were changed to blue-green because surgeons were blinded by the lights bouncing off the uniforms. (Green is also opposite red on the color wheel, supposedly enhancing the ability to distinguish shades of red).
But Over time we’ve lost significant autonomy in our practice and lost a little respect from our patients. Payers tell us what to do. Patients question our expertise. Choosing what we wear is one of the few bits of medicine we still have agency. Pewter or pink, joggers or cargo pants, we get to choose.
The last time I flew British Airways everyone was in lounge wear, except the flight crew, of course. They were all smartly dressed. Recently British Airways rolled out updated, slightly more relaxed dress codes. Very modern, but I wonder if in a way we’re not all just a bit worse off.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Look at this guy – NFL Chargers jersey and shorts with a RVCA hat on backward. And next to him, a woman wearing her spin-class-Lulu gear. There’s also a guy sporting a 2016 San Diego Rock ‘n Roll Marathon Tee. And that young woman is actually wearing slippers. A visitor from the 1950s would be thunderstruck to see such casual wear on people waiting to board a plane. Photos from that era show men buttoned up in white shirt and tie and women wearing Chanel with hats and white gloves. This dramatic transformation from formal to unfussy wear cuts through all social situations, including in my office. As a new doc out of residency, I used to wear a tie and shoes that could hold a shine. Now I wear jogger scrubs and sneakers. Rather than be offended by the lack of formality though, patients seem to appreciate it. Should they?
At first glance this seems to be a modern phenomenon. The reasons for casual wear today are manifold: about one-third of people work from home, Millennials are taking over with their TikTok values and general irreverence, COVID made us all fat and lazy. Heck, even the U.S. Senate briefly abolished the requirement to wear suits on the Senate floor. But getting dressed up was never to signal that you are elite or superior to others. It’s the opposite. To get dressed is a signal that you are serving others, a tradition that is as old as society.
Think of Downton Abbey as an example. The servants were always required to be smartly dressed when working, whereas members of the family could be dressed up or not. It’s clear who is serving whom. This tradition lives today in the hospitality industry. When you mosey into the lobby of a luxury hotel in your Rainbow sandals you can expect everyone who greets you will be in finery, signaling that they put in effort to serve you. You’ll find the same for all staff at the Mayo Clinic in Rochester, Minn., which is no coincidence.
Suits used to be standard in medicine. In the 19th century, physicians wore formal black-tie when seeing patients. Unlike hospitality however, we had good reason to eschew the tradition: germs. Once we figured out that our pus-stained ties and jackets were doing harm, we switched to wearing sanitized uniforms. Casual wear for doctors isn’t a modern phenomenon after all, then. For proof, compare Thomas Eakins painting “The Gross Clinic” (1875) with his later “The Agnew Clinic” (1889). In the former, Dr. Gross is portrayed in formal black wear, bloody hand and all. In the latter, Dr. Agnew is wearing white FIGS (or the 1890’s equivalent anyway). Similarly, nurses uniforms traditionally resembled kitchen servants, with criss-cross aprons and floor length skirts. It wasn’t until the 1980’s that nurses stopped wearing dresses and white caps.

In the operating theater it’s obviously critical that we wear sanitized scrubs to mitigate the risk of infection. Originally white to signal cleanliness, scrubs were changed to blue-green because surgeons were blinded by the lights bouncing off the uniforms. (Green is also opposite red on the color wheel, supposedly enhancing the ability to distinguish shades of red).
But Over time we’ve lost significant autonomy in our practice and lost a little respect from our patients. Payers tell us what to do. Patients question our expertise. Choosing what we wear is one of the few bits of medicine we still have agency. Pewter or pink, joggers or cargo pants, we get to choose.
The last time I flew British Airways everyone was in lounge wear, except the flight crew, of course. They were all smartly dressed. Recently British Airways rolled out updated, slightly more relaxed dress codes. Very modern, but I wonder if in a way we’re not all just a bit worse off.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Look at this guy – NFL Chargers jersey and shorts with a RVCA hat on backward. And next to him, a woman wearing her spin-class-Lulu gear. There’s also a guy sporting a 2016 San Diego Rock ‘n Roll Marathon Tee. And that young woman is actually wearing slippers. A visitor from the 1950s would be thunderstruck to see such casual wear on people waiting to board a plane. Photos from that era show men buttoned up in white shirt and tie and women wearing Chanel with hats and white gloves. This dramatic transformation from formal to unfussy wear cuts through all social situations, including in my office. As a new doc out of residency, I used to wear a tie and shoes that could hold a shine. Now I wear jogger scrubs and sneakers. Rather than be offended by the lack of formality though, patients seem to appreciate it. Should they?
At first glance this seems to be a modern phenomenon. The reasons for casual wear today are manifold: about one-third of people work from home, Millennials are taking over with their TikTok values and general irreverence, COVID made us all fat and lazy. Heck, even the U.S. Senate briefly abolished the requirement to wear suits on the Senate floor. But getting dressed up was never to signal that you are elite or superior to others. It’s the opposite. To get dressed is a signal that you are serving others, a tradition that is as old as society.
Think of Downton Abbey as an example. The servants were always required to be smartly dressed when working, whereas members of the family could be dressed up or not. It’s clear who is serving whom. This tradition lives today in the hospitality industry. When you mosey into the lobby of a luxury hotel in your Rainbow sandals you can expect everyone who greets you will be in finery, signaling that they put in effort to serve you. You’ll find the same for all staff at the Mayo Clinic in Rochester, Minn., which is no coincidence.
Suits used to be standard in medicine. In the 19th century, physicians wore formal black-tie when seeing patients. Unlike hospitality however, we had good reason to eschew the tradition: germs. Once we figured out that our pus-stained ties and jackets were doing harm, we switched to wearing sanitized uniforms. Casual wear for doctors isn’t a modern phenomenon after all, then. For proof, compare Thomas Eakins painting “The Gross Clinic” (1875) with his later “The Agnew Clinic” (1889). In the former, Dr. Gross is portrayed in formal black wear, bloody hand and all. In the latter, Dr. Agnew is wearing white FIGS (or the 1890’s equivalent anyway). Similarly, nurses uniforms traditionally resembled kitchen servants, with criss-cross aprons and floor length skirts. It wasn’t until the 1980’s that nurses stopped wearing dresses and white caps.

In the operating theater it’s obviously critical that we wear sanitized scrubs to mitigate the risk of infection. Originally white to signal cleanliness, scrubs were changed to blue-green because surgeons were blinded by the lights bouncing off the uniforms. (Green is also opposite red on the color wheel, supposedly enhancing the ability to distinguish shades of red).
But Over time we’ve lost significant autonomy in our practice and lost a little respect from our patients. Payers tell us what to do. Patients question our expertise. Choosing what we wear is one of the few bits of medicine we still have agency. Pewter or pink, joggers or cargo pants, we get to choose.
The last time I flew British Airways everyone was in lounge wear, except the flight crew, of course. They were all smartly dressed. Recently British Airways rolled out updated, slightly more relaxed dress codes. Very modern, but I wonder if in a way we’re not all just a bit worse off.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected]
Doublet therapy ups survival in metastatic prostate cancer
TOPLINE:
Swedish registry data show.
METHODOLOGY:
- The use of doublet therapy has increased significantly in Sweden in recent years given the growing body of evidence demonstrating that doublet therapy improves survival in individuals with de novo metastatic castration-sensitive prostate cancer.
- Investigators wanted to see whether the increasing use of doublet therapy in this patient population has improved survival when taking various other factors into consideration.
- The analysis, which included 11,382 men diagnosed with metastatic castration-sensitive prostate cancer in Sweden from 2008-2020 and registered in the country’s National Prostate Cancer Register, explored the use of doublet therapy over time and its association with survival, adjusting for age, comorbidities, and cancer characteristics.
- The researchers estimated average 5-year and 10-year survival over time using a survival model.
TAKEAWAY:
- During the study period, patients exhibited a shift toward less advanced prostate cancer, with median prostate-specific antigen (PSA) levels at diagnosis decreasing from 145 to 107 ng/mL in men with metastatic disease.
- Upfront treatment with doublet therapy in these men simultaneously increased from 1% in 2016 to 44% in 2020.
- Adjusted 5-year overall survival increased from 26% between 2008-2012 to 35% in the period 2017-2020; in the 5 years following diagnosis, patients’ mean survival increased by about 6 months between 2008-2012 and 2017-2020.
- The percentage of patients still alive at 10 years doubled from 9% in 2008 to 18% in 2020. Improvements were greater in men younger than 80 years old.
IN PRACTICE:
“A clinically meaningful increase in long-term survival was observed in men diagnosed with de novo [metastatic castration-sensitive prostate cancer] between 2008 and 2020 in Sweden. We argue that the main reason for this improvement was the increased upfront use of doublet therapy,” the authors concluded.
SOURCE:
The study, with first author Christian Corsini, MD, of Uppsala (Sweden) University, was published online in JAMA Network Open.
LIMITATIONS:
Although there were no substantial changes in the diagnostic workup, unmeasured and unknown changes over the years may have affected survival. The researchers lacked information on PSA levels during follow-up, and therefore could not assess progression-free survival. Some upfront docetaxel use was not captured before 2017.
DISCLOSURES:
The study received funding from the Swedish Cancer Society and Region Uppsala. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Swedish registry data show.
METHODOLOGY:
- The use of doublet therapy has increased significantly in Sweden in recent years given the growing body of evidence demonstrating that doublet therapy improves survival in individuals with de novo metastatic castration-sensitive prostate cancer.
- Investigators wanted to see whether the increasing use of doublet therapy in this patient population has improved survival when taking various other factors into consideration.
- The analysis, which included 11,382 men diagnosed with metastatic castration-sensitive prostate cancer in Sweden from 2008-2020 and registered in the country’s National Prostate Cancer Register, explored the use of doublet therapy over time and its association with survival, adjusting for age, comorbidities, and cancer characteristics.
- The researchers estimated average 5-year and 10-year survival over time using a survival model.
TAKEAWAY:
- During the study period, patients exhibited a shift toward less advanced prostate cancer, with median prostate-specific antigen (PSA) levels at diagnosis decreasing from 145 to 107 ng/mL in men with metastatic disease.
- Upfront treatment with doublet therapy in these men simultaneously increased from 1% in 2016 to 44% in 2020.
- Adjusted 5-year overall survival increased from 26% between 2008-2012 to 35% in the period 2017-2020; in the 5 years following diagnosis, patients’ mean survival increased by about 6 months between 2008-2012 and 2017-2020.
- The percentage of patients still alive at 10 years doubled from 9% in 2008 to 18% in 2020. Improvements were greater in men younger than 80 years old.
IN PRACTICE:
“A clinically meaningful increase in long-term survival was observed in men diagnosed with de novo [metastatic castration-sensitive prostate cancer] between 2008 and 2020 in Sweden. We argue that the main reason for this improvement was the increased upfront use of doublet therapy,” the authors concluded.
SOURCE:
The study, with first author Christian Corsini, MD, of Uppsala (Sweden) University, was published online in JAMA Network Open.
LIMITATIONS:
Although there were no substantial changes in the diagnostic workup, unmeasured and unknown changes over the years may have affected survival. The researchers lacked information on PSA levels during follow-up, and therefore could not assess progression-free survival. Some upfront docetaxel use was not captured before 2017.
DISCLOSURES:
The study received funding from the Swedish Cancer Society and Region Uppsala. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Swedish registry data show.
METHODOLOGY:
- The use of doublet therapy has increased significantly in Sweden in recent years given the growing body of evidence demonstrating that doublet therapy improves survival in individuals with de novo metastatic castration-sensitive prostate cancer.
- Investigators wanted to see whether the increasing use of doublet therapy in this patient population has improved survival when taking various other factors into consideration.
- The analysis, which included 11,382 men diagnosed with metastatic castration-sensitive prostate cancer in Sweden from 2008-2020 and registered in the country’s National Prostate Cancer Register, explored the use of doublet therapy over time and its association with survival, adjusting for age, comorbidities, and cancer characteristics.
- The researchers estimated average 5-year and 10-year survival over time using a survival model.
TAKEAWAY:
- During the study period, patients exhibited a shift toward less advanced prostate cancer, with median prostate-specific antigen (PSA) levels at diagnosis decreasing from 145 to 107 ng/mL in men with metastatic disease.
- Upfront treatment with doublet therapy in these men simultaneously increased from 1% in 2016 to 44% in 2020.
- Adjusted 5-year overall survival increased from 26% between 2008-2012 to 35% in the period 2017-2020; in the 5 years following diagnosis, patients’ mean survival increased by about 6 months between 2008-2012 and 2017-2020.
- The percentage of patients still alive at 10 years doubled from 9% in 2008 to 18% in 2020. Improvements were greater in men younger than 80 years old.
IN PRACTICE:
“A clinically meaningful increase in long-term survival was observed in men diagnosed with de novo [metastatic castration-sensitive prostate cancer] between 2008 and 2020 in Sweden. We argue that the main reason for this improvement was the increased upfront use of doublet therapy,” the authors concluded.
SOURCE:
The study, with first author Christian Corsini, MD, of Uppsala (Sweden) University, was published online in JAMA Network Open.
LIMITATIONS:
Although there were no substantial changes in the diagnostic workup, unmeasured and unknown changes over the years may have affected survival. The researchers lacked information on PSA levels during follow-up, and therefore could not assess progression-free survival. Some upfront docetaxel use was not captured before 2017.
DISCLOSURES:
The study received funding from the Swedish Cancer Society and Region Uppsala. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Metformin, weight management to stop type 2 diabetes in kids
TOPLINE:
Nearly one in five adolescents are living with prediabetes, a condition where blood glucose levels are elevated, but are not high enough for a type 2 diabetes (T2D) diagnosis. According to a new study, higher levels of nonfasting glucose and hemoglobin A1c, and worsening obesity are important predictors of progression to T2D. In addition, metformin and weight stabilization may prove to be important interventions for preventing T2D in kids.
METHODOLOGY:
- Researchers did a retrospective chart review of patient data from Vanderbilt University Medical Center Pediatric Prediabetes Clinic, Nashville, Tenn., from May 2015 to August 2022.
- The study included 552 children with prediabetes, defined as abnormal blood glucose (fasting plasma glucose [FPG] ≥ 100 mg/dL, random glucose ≥ 150 mg/dL), or hemoglobin A1c equal to or greater than 5.9%.
- Based on follow-up visits, patients were classified as having progressed to T2D, or nonprogression.
- Researchers analyzed the patients’ initial visit A1c, fasting C-peptide, 2-hour glucose, fasting glucose, and body mass index (BMI), among other baseline characteristics.
TAKEAWAY:
- Thirty-six children (6.5%) progressed to T2D during the duration of the study period.
- The average time to T2D diagnosis was much longer in patients taking metformin (43 months), compared with those not taking the prescribed medication (28 months).
- Worsening obesity was strongly associated with T2D progression – patients who progressed to T2D had a higher BMI at baseline and had continued weight gain.
- A higher baseline A1c, fasting C-peptide, and 2-hour glucose were also associated with progression to T2D.
- In the multivariable analysis, both A1c and 2-hour glucose were strong independent predictors of progression.
- Fasting plasma glucose was not associated with progression to T2D.
IN PRACTICE:
“Weight stabilization and metformin therapy could be important interventions for diabetes prevention in children,” study author Ashley H. Shoemaker, MD, MSci, a pediatric endocrinologist at Vanderbilt University Medical Center in Nashville, Tenn., said in a press release.
In addition, A1c plus a nonfasting glucose may be a feasible way to identify high-risk pediatric patients in a clinical setting.
SOURCE:
This study was performed by Natasha Belsky, Jaclyn Tamaroff, and Ashley H. Shoemaker of the Vanderbilt University Medical Center and the Vanderbilt University School of Medicine in Nashville, Tenn. It was published October 12, 2023, in the Journal of the Endocrine Society
LIMITATIONS:
Additional patients who developed T2D may have been lost to follow-up, since the authors did not contact patients to confirm their disease status. The authors were also unable to establish racial differences in the progression to T2D because of missing data.
DISCLOSURES:
Funding for this study was provided by the National Center for Advancing Translational Sciences. One author has research contracts with Novo Nordisk and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
TOPLINE:
Nearly one in five adolescents are living with prediabetes, a condition where blood glucose levels are elevated, but are not high enough for a type 2 diabetes (T2D) diagnosis. According to a new study, higher levels of nonfasting glucose and hemoglobin A1c, and worsening obesity are important predictors of progression to T2D. In addition, metformin and weight stabilization may prove to be important interventions for preventing T2D in kids.
METHODOLOGY:
- Researchers did a retrospective chart review of patient data from Vanderbilt University Medical Center Pediatric Prediabetes Clinic, Nashville, Tenn., from May 2015 to August 2022.
- The study included 552 children with prediabetes, defined as abnormal blood glucose (fasting plasma glucose [FPG] ≥ 100 mg/dL, random glucose ≥ 150 mg/dL), or hemoglobin A1c equal to or greater than 5.9%.
- Based on follow-up visits, patients were classified as having progressed to T2D, or nonprogression.
- Researchers analyzed the patients’ initial visit A1c, fasting C-peptide, 2-hour glucose, fasting glucose, and body mass index (BMI), among other baseline characteristics.
TAKEAWAY:
- Thirty-six children (6.5%) progressed to T2D during the duration of the study period.
- The average time to T2D diagnosis was much longer in patients taking metformin (43 months), compared with those not taking the prescribed medication (28 months).
- Worsening obesity was strongly associated with T2D progression – patients who progressed to T2D had a higher BMI at baseline and had continued weight gain.
- A higher baseline A1c, fasting C-peptide, and 2-hour glucose were also associated with progression to T2D.
- In the multivariable analysis, both A1c and 2-hour glucose were strong independent predictors of progression.
- Fasting plasma glucose was not associated with progression to T2D.
IN PRACTICE:
“Weight stabilization and metformin therapy could be important interventions for diabetes prevention in children,” study author Ashley H. Shoemaker, MD, MSci, a pediatric endocrinologist at Vanderbilt University Medical Center in Nashville, Tenn., said in a press release.
In addition, A1c plus a nonfasting glucose may be a feasible way to identify high-risk pediatric patients in a clinical setting.
SOURCE:
This study was performed by Natasha Belsky, Jaclyn Tamaroff, and Ashley H. Shoemaker of the Vanderbilt University Medical Center and the Vanderbilt University School of Medicine in Nashville, Tenn. It was published October 12, 2023, in the Journal of the Endocrine Society
LIMITATIONS:
Additional patients who developed T2D may have been lost to follow-up, since the authors did not contact patients to confirm their disease status. The authors were also unable to establish racial differences in the progression to T2D because of missing data.
DISCLOSURES:
Funding for this study was provided by the National Center for Advancing Translational Sciences. One author has research contracts with Novo Nordisk and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
TOPLINE:
Nearly one in five adolescents are living with prediabetes, a condition where blood glucose levels are elevated, but are not high enough for a type 2 diabetes (T2D) diagnosis. According to a new study, higher levels of nonfasting glucose and hemoglobin A1c, and worsening obesity are important predictors of progression to T2D. In addition, metformin and weight stabilization may prove to be important interventions for preventing T2D in kids.
METHODOLOGY:
- Researchers did a retrospective chart review of patient data from Vanderbilt University Medical Center Pediatric Prediabetes Clinic, Nashville, Tenn., from May 2015 to August 2022.
- The study included 552 children with prediabetes, defined as abnormal blood glucose (fasting plasma glucose [FPG] ≥ 100 mg/dL, random glucose ≥ 150 mg/dL), or hemoglobin A1c equal to or greater than 5.9%.
- Based on follow-up visits, patients were classified as having progressed to T2D, or nonprogression.
- Researchers analyzed the patients’ initial visit A1c, fasting C-peptide, 2-hour glucose, fasting glucose, and body mass index (BMI), among other baseline characteristics.
TAKEAWAY:
- Thirty-six children (6.5%) progressed to T2D during the duration of the study period.
- The average time to T2D diagnosis was much longer in patients taking metformin (43 months), compared with those not taking the prescribed medication (28 months).
- Worsening obesity was strongly associated with T2D progression – patients who progressed to T2D had a higher BMI at baseline and had continued weight gain.
- A higher baseline A1c, fasting C-peptide, and 2-hour glucose were also associated with progression to T2D.
- In the multivariable analysis, both A1c and 2-hour glucose were strong independent predictors of progression.
- Fasting plasma glucose was not associated with progression to T2D.
IN PRACTICE:
“Weight stabilization and metformin therapy could be important interventions for diabetes prevention in children,” study author Ashley H. Shoemaker, MD, MSci, a pediatric endocrinologist at Vanderbilt University Medical Center in Nashville, Tenn., said in a press release.
In addition, A1c plus a nonfasting glucose may be a feasible way to identify high-risk pediatric patients in a clinical setting.
SOURCE:
This study was performed by Natasha Belsky, Jaclyn Tamaroff, and Ashley H. Shoemaker of the Vanderbilt University Medical Center and the Vanderbilt University School of Medicine in Nashville, Tenn. It was published October 12, 2023, in the Journal of the Endocrine Society
LIMITATIONS:
Additional patients who developed T2D may have been lost to follow-up, since the authors did not contact patients to confirm their disease status. The authors were also unable to establish racial differences in the progression to T2D because of missing data.
DISCLOSURES:
Funding for this study was provided by the National Center for Advancing Translational Sciences. One author has research contracts with Novo Nordisk and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
New ‘twincretin’ pemvidutide: Another option for obesity
HAMBURG, GERMANY – , adding a different type of “twincretin” to a growing mix of incretin-based weight-loss drugs in development that also offer additional benefits.
Pemvidutide (Altimmune Inc) is a long-acting “balanced” dual agonist of both glucagon-like peptide 1 (GLP-1) and glucagon that is in development for the treatment of obesity and nonalcoholic steatohepatitis (NASH) but not type 2 diabetes, as its effect on glucose is neutral. Phase 1 data for pemvidutide’s liver effect were presented in 2022.
In contrast, the dual GLP-1-glucose-dependent insulinotropic polypeptide (GIP) agonist tirzepatide (Mounjaro, Lilly) has been approved for the treatment of type 2 diabetes. It awaits an indication for obesity.
“When you look [at] the results for any given agent, think about obesity as a series of problems. Some overlap, and some don’t. While about 20%-25% of people with obesity also have type 2 diabetes, not everybody does. So the compounds that don’t lower glucose ... those will be great for others who have [fatty liver disease] or hyperlipidemia. ... It’s not going to be one compound for everybody,” said Louis J. Aronne, MD, director of the center for weight management and metabolic clinical research, Weill Cornell Medicine, New York.
Results of a new 24-week interim analysis of data from the phase 2 pemvidutide trial, called MOMENTUM, were presented at the annual meeting of the European Association for the Study of Diabetes by Dr. Aronne.
Included in that session were encore presentations of data for another GLP-1-glucagon dual agonist, survodutide, as well as data for Eli Lilly’s GLP-1-GIP-glucagon “triagonist,” retatrutide. Retatrutide is in development to induce weight loss, while survodutide (Boehringer Ingelheim and Zealand Pharma), like pemvidutide, is in development to induce weight loss and treat fatty liver disease.
Added Dr. Aronne, “As good as [the triple agonist] retatrutide looks, I doubt that every single person with obesity in the world will be treated with it. ... Think about this as a field, the way you treat diabetes and every other chronic illness.”
Asked to comment, session moderator Rajna Golubic, PhD, of the Oxford (England) Centre for Diabetes, Endocrinology and Metabolism, told this news organization, “We need to think in terms of treating beyond weight loss. ... We need to look at the person holistically and at other aspects of cardiometabolic health and treat in a personalized way and choose treatments according to the comorbidities people have.”
Regarding the dual GLP-1-glucagon agonists, including pemvidutide, Dr. Golubic pointed out that the glucagon agonism does the opposite of glucose-lowering agents but that the compound is “balanced for greater affinity for the GLP-1 receptor vs. glucagon, so that the beneficial effects outweigh the effect for glucose but it still harnesses the benefits of glucagon on liver with a decrease in liver fat, with positive effects on heart, positive effects on kidneys, and other beneficial metabolic effects.”
Pemvidutide lowers weight, LDL cholesterol, triglycerides, and blood pressure
Dr. Aronne began his presentation by noting that dyslipidemia, fatty liver disease, and hypertension are the most significant comorbidities of obesity, occurring in 66%-70%, 58%-75%, and 45%-55% of patients, respectively, while type 2 diabetes is less common, at 19%-23%.
Pemvidutide’s GLP-1 receptor agonism reduces appetite, inflammation, and gastric emptying, while glucagon agonism increases lipolysis, mobilizes fat, and increases energy expenditure, Dr. Aronne explained.
The 48-week phase 2 MOMENTUM trial randomly assigned 320 participants with overweight or obesity and at least one obesity-related comorbidity but not diabetes to receive weekly doses of 1.2 mg, 1.8 mg, or 2.4 mg of pemvidutide or placebo. The two lower pemvidutide doses were initiated immediately without titration, while the 2.4-mg dose was titrated rapidly over 4 weeks.
In a prespecified interim analysis of 160 participants, the percent body weight loss at 24 weeks was 10.7%, 9.4%, and 7.3% with the 2.4-mg, 1.8-mg, and 1.2-mg doses, respectively (P < .001). All weight loss values were significant; weight loss with placebo was a nonsignificant 1%.
The proportions of patients who lost at least 5% of their body weight were 84.6%, 66.7%, and 66.7%, respectively, vs. 25% with placebo. Half of the patients who received the 2.4-mg and 1.8-mg doses lost at least 10% of their body weight. Reductions in waist circumference followed suit; the patients who received the 2.4-mg dose lost an average of 10.2 cm, or “in the U.S., about 4 inches or 4 belt loops. That’s pretty good, you need a new belt,” Dr. Aronne commented.
Significant reductions in total cholesterol and triglyceride levels were also seen at week 24 by 16.5% and 25.0%, respectively, with the 2.4-mg dose. Low-density lipoprotein cholesterol levels also dropped, although not significantly; high-density lipoprotein levels dropped significantly.
Systolic blood pressure dropped by 5.5 mm Hg, and diastolic blood pressure dropped by 1.8 mm Hg in the 2.4-mg group and by lesser degrees among the patients who received lower doses. There were no significant changes in heart rate, Dr. Aronne noted.
Glucose homeostasis was preserved in all groups throughout the 24 weeks.
As with all drugs in the incretin class, gastrointestinal adverse events were common. Severe vomiting occurred in one person in the 1.8-mg group and in four with 2.4 mg. Efforts will be made to reduce that in subsequent trials, Dr. Aronne said.
“We have learned over time that going more gradually in titrating up these agents is a better strategy, allowing dose reduction may be a better strategy, and allowing antiemetics temporarily as we increase the dose is a lesson that many have learned doing these trials and of course in our clinical practices,” he commented.
Dr. Golubic told this news organization that the recent emergence of potent incretin-based weight loss drugs is “a huge paradigm shift. The prevalence of obesity will be 35% or higher by 2035. Bariatric surgery isn’t feasible for everyone, and it’s very expensive, so we need drugs to provide benefits in terms of lowering weight, glucose, and other cardiometabolic risk factors.”
The full 48-week data for MOMENTUM will be announced in the fourth quarter of 2023.
Dr. Aronne has received consulting fees from and serves on advisory boards for Allurion, Altimmune, Atria, Gelesis, Jamieson Wellness, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Novo Nordisk, Pfizer, Optum, Eli Lilly, Senda Biosciences, and Versanis; has received research funding from Allurion, AstraZeneca, Gelesis, Janssen Pharmaceuticals, Novo Nordisk, and Eli Lilly; has equity interests in Allurion, ERX Pharmaceuticals, Gelesis, Intellihealth, Jamieson Wellness, and Myos Corp; and serves on a board of directors for ERX Pharmaceuticals, Intellihealth, and Jamieson Wellness. Dr. Golubic has received research support from AstraZeneca.
A version of this article first appeared on Medscape.com.
HAMBURG, GERMANY – , adding a different type of “twincretin” to a growing mix of incretin-based weight-loss drugs in development that also offer additional benefits.
Pemvidutide (Altimmune Inc) is a long-acting “balanced” dual agonist of both glucagon-like peptide 1 (GLP-1) and glucagon that is in development for the treatment of obesity and nonalcoholic steatohepatitis (NASH) but not type 2 diabetes, as its effect on glucose is neutral. Phase 1 data for pemvidutide’s liver effect were presented in 2022.
In contrast, the dual GLP-1-glucose-dependent insulinotropic polypeptide (GIP) agonist tirzepatide (Mounjaro, Lilly) has been approved for the treatment of type 2 diabetes. It awaits an indication for obesity.
“When you look [at] the results for any given agent, think about obesity as a series of problems. Some overlap, and some don’t. While about 20%-25% of people with obesity also have type 2 diabetes, not everybody does. So the compounds that don’t lower glucose ... those will be great for others who have [fatty liver disease] or hyperlipidemia. ... It’s not going to be one compound for everybody,” said Louis J. Aronne, MD, director of the center for weight management and metabolic clinical research, Weill Cornell Medicine, New York.
Results of a new 24-week interim analysis of data from the phase 2 pemvidutide trial, called MOMENTUM, were presented at the annual meeting of the European Association for the Study of Diabetes by Dr. Aronne.
Included in that session were encore presentations of data for another GLP-1-glucagon dual agonist, survodutide, as well as data for Eli Lilly’s GLP-1-GIP-glucagon “triagonist,” retatrutide. Retatrutide is in development to induce weight loss, while survodutide (Boehringer Ingelheim and Zealand Pharma), like pemvidutide, is in development to induce weight loss and treat fatty liver disease.
Added Dr. Aronne, “As good as [the triple agonist] retatrutide looks, I doubt that every single person with obesity in the world will be treated with it. ... Think about this as a field, the way you treat diabetes and every other chronic illness.”
Asked to comment, session moderator Rajna Golubic, PhD, of the Oxford (England) Centre for Diabetes, Endocrinology and Metabolism, told this news organization, “We need to think in terms of treating beyond weight loss. ... We need to look at the person holistically and at other aspects of cardiometabolic health and treat in a personalized way and choose treatments according to the comorbidities people have.”
Regarding the dual GLP-1-glucagon agonists, including pemvidutide, Dr. Golubic pointed out that the glucagon agonism does the opposite of glucose-lowering agents but that the compound is “balanced for greater affinity for the GLP-1 receptor vs. glucagon, so that the beneficial effects outweigh the effect for glucose but it still harnesses the benefits of glucagon on liver with a decrease in liver fat, with positive effects on heart, positive effects on kidneys, and other beneficial metabolic effects.”
Pemvidutide lowers weight, LDL cholesterol, triglycerides, and blood pressure
Dr. Aronne began his presentation by noting that dyslipidemia, fatty liver disease, and hypertension are the most significant comorbidities of obesity, occurring in 66%-70%, 58%-75%, and 45%-55% of patients, respectively, while type 2 diabetes is less common, at 19%-23%.
Pemvidutide’s GLP-1 receptor agonism reduces appetite, inflammation, and gastric emptying, while glucagon agonism increases lipolysis, mobilizes fat, and increases energy expenditure, Dr. Aronne explained.
The 48-week phase 2 MOMENTUM trial randomly assigned 320 participants with overweight or obesity and at least one obesity-related comorbidity but not diabetes to receive weekly doses of 1.2 mg, 1.8 mg, or 2.4 mg of pemvidutide or placebo. The two lower pemvidutide doses were initiated immediately without titration, while the 2.4-mg dose was titrated rapidly over 4 weeks.
In a prespecified interim analysis of 160 participants, the percent body weight loss at 24 weeks was 10.7%, 9.4%, and 7.3% with the 2.4-mg, 1.8-mg, and 1.2-mg doses, respectively (P < .001). All weight loss values were significant; weight loss with placebo was a nonsignificant 1%.
The proportions of patients who lost at least 5% of their body weight were 84.6%, 66.7%, and 66.7%, respectively, vs. 25% with placebo. Half of the patients who received the 2.4-mg and 1.8-mg doses lost at least 10% of their body weight. Reductions in waist circumference followed suit; the patients who received the 2.4-mg dose lost an average of 10.2 cm, or “in the U.S., about 4 inches or 4 belt loops. That’s pretty good, you need a new belt,” Dr. Aronne commented.
Significant reductions in total cholesterol and triglyceride levels were also seen at week 24 by 16.5% and 25.0%, respectively, with the 2.4-mg dose. Low-density lipoprotein cholesterol levels also dropped, although not significantly; high-density lipoprotein levels dropped significantly.
Systolic blood pressure dropped by 5.5 mm Hg, and diastolic blood pressure dropped by 1.8 mm Hg in the 2.4-mg group and by lesser degrees among the patients who received lower doses. There were no significant changes in heart rate, Dr. Aronne noted.
Glucose homeostasis was preserved in all groups throughout the 24 weeks.
As with all drugs in the incretin class, gastrointestinal adverse events were common. Severe vomiting occurred in one person in the 1.8-mg group and in four with 2.4 mg. Efforts will be made to reduce that in subsequent trials, Dr. Aronne said.
“We have learned over time that going more gradually in titrating up these agents is a better strategy, allowing dose reduction may be a better strategy, and allowing antiemetics temporarily as we increase the dose is a lesson that many have learned doing these trials and of course in our clinical practices,” he commented.
Dr. Golubic told this news organization that the recent emergence of potent incretin-based weight loss drugs is “a huge paradigm shift. The prevalence of obesity will be 35% or higher by 2035. Bariatric surgery isn’t feasible for everyone, and it’s very expensive, so we need drugs to provide benefits in terms of lowering weight, glucose, and other cardiometabolic risk factors.”
The full 48-week data for MOMENTUM will be announced in the fourth quarter of 2023.
Dr. Aronne has received consulting fees from and serves on advisory boards for Allurion, Altimmune, Atria, Gelesis, Jamieson Wellness, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Novo Nordisk, Pfizer, Optum, Eli Lilly, Senda Biosciences, and Versanis; has received research funding from Allurion, AstraZeneca, Gelesis, Janssen Pharmaceuticals, Novo Nordisk, and Eli Lilly; has equity interests in Allurion, ERX Pharmaceuticals, Gelesis, Intellihealth, Jamieson Wellness, and Myos Corp; and serves on a board of directors for ERX Pharmaceuticals, Intellihealth, and Jamieson Wellness. Dr. Golubic has received research support from AstraZeneca.
A version of this article first appeared on Medscape.com.
HAMBURG, GERMANY – , adding a different type of “twincretin” to a growing mix of incretin-based weight-loss drugs in development that also offer additional benefits.
Pemvidutide (Altimmune Inc) is a long-acting “balanced” dual agonist of both glucagon-like peptide 1 (GLP-1) and glucagon that is in development for the treatment of obesity and nonalcoholic steatohepatitis (NASH) but not type 2 diabetes, as its effect on glucose is neutral. Phase 1 data for pemvidutide’s liver effect were presented in 2022.
In contrast, the dual GLP-1-glucose-dependent insulinotropic polypeptide (GIP) agonist tirzepatide (Mounjaro, Lilly) has been approved for the treatment of type 2 diabetes. It awaits an indication for obesity.
“When you look [at] the results for any given agent, think about obesity as a series of problems. Some overlap, and some don’t. While about 20%-25% of people with obesity also have type 2 diabetes, not everybody does. So the compounds that don’t lower glucose ... those will be great for others who have [fatty liver disease] or hyperlipidemia. ... It’s not going to be one compound for everybody,” said Louis J. Aronne, MD, director of the center for weight management and metabolic clinical research, Weill Cornell Medicine, New York.
Results of a new 24-week interim analysis of data from the phase 2 pemvidutide trial, called MOMENTUM, were presented at the annual meeting of the European Association for the Study of Diabetes by Dr. Aronne.
Included in that session were encore presentations of data for another GLP-1-glucagon dual agonist, survodutide, as well as data for Eli Lilly’s GLP-1-GIP-glucagon “triagonist,” retatrutide. Retatrutide is in development to induce weight loss, while survodutide (Boehringer Ingelheim and Zealand Pharma), like pemvidutide, is in development to induce weight loss and treat fatty liver disease.
Added Dr. Aronne, “As good as [the triple agonist] retatrutide looks, I doubt that every single person with obesity in the world will be treated with it. ... Think about this as a field, the way you treat diabetes and every other chronic illness.”
Asked to comment, session moderator Rajna Golubic, PhD, of the Oxford (England) Centre for Diabetes, Endocrinology and Metabolism, told this news organization, “We need to think in terms of treating beyond weight loss. ... We need to look at the person holistically and at other aspects of cardiometabolic health and treat in a personalized way and choose treatments according to the comorbidities people have.”
Regarding the dual GLP-1-glucagon agonists, including pemvidutide, Dr. Golubic pointed out that the glucagon agonism does the opposite of glucose-lowering agents but that the compound is “balanced for greater affinity for the GLP-1 receptor vs. glucagon, so that the beneficial effects outweigh the effect for glucose but it still harnesses the benefits of glucagon on liver with a decrease in liver fat, with positive effects on heart, positive effects on kidneys, and other beneficial metabolic effects.”
Pemvidutide lowers weight, LDL cholesterol, triglycerides, and blood pressure
Dr. Aronne began his presentation by noting that dyslipidemia, fatty liver disease, and hypertension are the most significant comorbidities of obesity, occurring in 66%-70%, 58%-75%, and 45%-55% of patients, respectively, while type 2 diabetes is less common, at 19%-23%.
Pemvidutide’s GLP-1 receptor agonism reduces appetite, inflammation, and gastric emptying, while glucagon agonism increases lipolysis, mobilizes fat, and increases energy expenditure, Dr. Aronne explained.
The 48-week phase 2 MOMENTUM trial randomly assigned 320 participants with overweight or obesity and at least one obesity-related comorbidity but not diabetes to receive weekly doses of 1.2 mg, 1.8 mg, or 2.4 mg of pemvidutide or placebo. The two lower pemvidutide doses were initiated immediately without titration, while the 2.4-mg dose was titrated rapidly over 4 weeks.
In a prespecified interim analysis of 160 participants, the percent body weight loss at 24 weeks was 10.7%, 9.4%, and 7.3% with the 2.4-mg, 1.8-mg, and 1.2-mg doses, respectively (P < .001). All weight loss values were significant; weight loss with placebo was a nonsignificant 1%.
The proportions of patients who lost at least 5% of their body weight were 84.6%, 66.7%, and 66.7%, respectively, vs. 25% with placebo. Half of the patients who received the 2.4-mg and 1.8-mg doses lost at least 10% of their body weight. Reductions in waist circumference followed suit; the patients who received the 2.4-mg dose lost an average of 10.2 cm, or “in the U.S., about 4 inches or 4 belt loops. That’s pretty good, you need a new belt,” Dr. Aronne commented.
Significant reductions in total cholesterol and triglyceride levels were also seen at week 24 by 16.5% and 25.0%, respectively, with the 2.4-mg dose. Low-density lipoprotein cholesterol levels also dropped, although not significantly; high-density lipoprotein levels dropped significantly.
Systolic blood pressure dropped by 5.5 mm Hg, and diastolic blood pressure dropped by 1.8 mm Hg in the 2.4-mg group and by lesser degrees among the patients who received lower doses. There were no significant changes in heart rate, Dr. Aronne noted.
Glucose homeostasis was preserved in all groups throughout the 24 weeks.
As with all drugs in the incretin class, gastrointestinal adverse events were common. Severe vomiting occurred in one person in the 1.8-mg group and in four with 2.4 mg. Efforts will be made to reduce that in subsequent trials, Dr. Aronne said.
“We have learned over time that going more gradually in titrating up these agents is a better strategy, allowing dose reduction may be a better strategy, and allowing antiemetics temporarily as we increase the dose is a lesson that many have learned doing these trials and of course in our clinical practices,” he commented.
Dr. Golubic told this news organization that the recent emergence of potent incretin-based weight loss drugs is “a huge paradigm shift. The prevalence of obesity will be 35% or higher by 2035. Bariatric surgery isn’t feasible for everyone, and it’s very expensive, so we need drugs to provide benefits in terms of lowering weight, glucose, and other cardiometabolic risk factors.”
The full 48-week data for MOMENTUM will be announced in the fourth quarter of 2023.
Dr. Aronne has received consulting fees from and serves on advisory boards for Allurion, Altimmune, Atria, Gelesis, Jamieson Wellness, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Novo Nordisk, Pfizer, Optum, Eli Lilly, Senda Biosciences, and Versanis; has received research funding from Allurion, AstraZeneca, Gelesis, Janssen Pharmaceuticals, Novo Nordisk, and Eli Lilly; has equity interests in Allurion, ERX Pharmaceuticals, Gelesis, Intellihealth, Jamieson Wellness, and Myos Corp; and serves on a board of directors for ERX Pharmaceuticals, Intellihealth, and Jamieson Wellness. Dr. Golubic has received research support from AstraZeneca.
A version of this article first appeared on Medscape.com.
AT EASD 2023
Lack of time is damaging women’s health
Various speakers at the VII National Conference of the Onda Foundation, Italy’s National Observatory for Women and Gender’s Health, focused on this topic. The conference was dedicated to the social factors that determine health within the context of gender medicine.
In our society, housework and raising a family are responsibilities placed predominantly on the shoulders of women. These responsibilities contribute significantly to women’s daily workload. The most overburdened women are working mothers (according to ISTAT, Italy’s Office for National Statistics, 2019), who are forced to combine their professional responsibilities with family life, dedicating 8 hours and 20 minutes per day to paid and unpaid work overall, compared with the 7 hours and 29 minutes spent by working fathers. Working mothers between ages 25 and 44 years have on average 2 hours and 35 minutes of free time per day.
Stress and sleep deprivation
“Under these conditions, the risk of chronic stress is raised, and stress leads to depression. The rate of depression in the female population is double that of the male population,” said Claudio Mencacci, MD, chair of the Italian Society of Neuropsychopharmacology and the Onda Foundation. “What’s more, stress increases the risk of cardiovascular and metabolic diseases, asthma, arthritis, and autoimmune diseases.”
The one thing that is especially damaging to physical and mental health is sleep deprivation, and working mothers get less sleep than do working fathers. “This is partially due to biological factors: hormonal changes that take place toward the end of adolescence in women during the premenstrual period are responsible for an increased rate of sleep disturbance and insomnia,” said Dr. Mencacci. “During pregnancy and the postpartum period, female sex hormones make sleep lighter, reducing time spent in the REM sleep stage. Then there’s the social aspect that plays a decisive role: by and large, it’s mothers who take care of the youngest children at night.”
According to a 2019 German study, during the first 6 years of life of the first child, a mother loses on average 44 minutes sleep per night, compared with the average time spent sleeping before pregnancy; a father loses 14 minutes.
“Another aspect to bear in mind is that, for cultural reasons, women tend to overlook the issue and not seek help, deeming sleep deprivation normal,” said Dr. Mencacci.
Caregivers at greatest risk
The negative effects of stress are evident in people continuously caring for a dependent older or disabled family member, so-called caregivers. This is, “A group predominantly made up of women aged between 45 and 55 years,” said Marina Petrini, PhD, of the Italian Health Institute’s Gender Medicine Center of Excellence. Dr. Petrini coordinated a study on stress and health in family caregivers.
“The results obtained reveal a high level of stress, especially among female caregivers, who are more exposed to the risk of severe symptoms of depression, physical disorders, especially those affecting the nervous and immune systems, and who tend to adopt irregular eating patterns and sedentary habits,” said Dr. Petrini.
Limited treatment access
Another study presented at the Onda Foundation’s conference, which shows just how much a lack of “me time” can damage your health, is the Access to Diagnostic Medicine and Treatment by Region: the Patient’s Perspective Survey, conducted by market research agency Elma Research on a sample of cancer patients requiring specialist treatment.
“Forty percent of them had to move to a different region from the one they live in to get the care they needed,” said Massimo Massagrande, CEO of Elma Research. “Of that group, 40% had to move to an area not neighboring their own. The impact of area of residence is heavy, in terms of money and logistics – so much so that a large proportion of patients interviewed were forced to turn their back on the best available treatments. For women responding to our survey, the biggest obstacle is the impossibility of reconciling the effects of a move or the prospective of a temporary transfer to another region with their responsibilities for looking after their family.”
This article was translated from Univadis Italy. A version appeared on Medscape.com.
Various speakers at the VII National Conference of the Onda Foundation, Italy’s National Observatory for Women and Gender’s Health, focused on this topic. The conference was dedicated to the social factors that determine health within the context of gender medicine.
In our society, housework and raising a family are responsibilities placed predominantly on the shoulders of women. These responsibilities contribute significantly to women’s daily workload. The most overburdened women are working mothers (according to ISTAT, Italy’s Office for National Statistics, 2019), who are forced to combine their professional responsibilities with family life, dedicating 8 hours and 20 minutes per day to paid and unpaid work overall, compared with the 7 hours and 29 minutes spent by working fathers. Working mothers between ages 25 and 44 years have on average 2 hours and 35 minutes of free time per day.
Stress and sleep deprivation
“Under these conditions, the risk of chronic stress is raised, and stress leads to depression. The rate of depression in the female population is double that of the male population,” said Claudio Mencacci, MD, chair of the Italian Society of Neuropsychopharmacology and the Onda Foundation. “What’s more, stress increases the risk of cardiovascular and metabolic diseases, asthma, arthritis, and autoimmune diseases.”
The one thing that is especially damaging to physical and mental health is sleep deprivation, and working mothers get less sleep than do working fathers. “This is partially due to biological factors: hormonal changes that take place toward the end of adolescence in women during the premenstrual period are responsible for an increased rate of sleep disturbance and insomnia,” said Dr. Mencacci. “During pregnancy and the postpartum period, female sex hormones make sleep lighter, reducing time spent in the REM sleep stage. Then there’s the social aspect that plays a decisive role: by and large, it’s mothers who take care of the youngest children at night.”
According to a 2019 German study, during the first 6 years of life of the first child, a mother loses on average 44 minutes sleep per night, compared with the average time spent sleeping before pregnancy; a father loses 14 minutes.
“Another aspect to bear in mind is that, for cultural reasons, women tend to overlook the issue and not seek help, deeming sleep deprivation normal,” said Dr. Mencacci.
Caregivers at greatest risk
The negative effects of stress are evident in people continuously caring for a dependent older or disabled family member, so-called caregivers. This is, “A group predominantly made up of women aged between 45 and 55 years,” said Marina Petrini, PhD, of the Italian Health Institute’s Gender Medicine Center of Excellence. Dr. Petrini coordinated a study on stress and health in family caregivers.
“The results obtained reveal a high level of stress, especially among female caregivers, who are more exposed to the risk of severe symptoms of depression, physical disorders, especially those affecting the nervous and immune systems, and who tend to adopt irregular eating patterns and sedentary habits,” said Dr. Petrini.
Limited treatment access
Another study presented at the Onda Foundation’s conference, which shows just how much a lack of “me time” can damage your health, is the Access to Diagnostic Medicine and Treatment by Region: the Patient’s Perspective Survey, conducted by market research agency Elma Research on a sample of cancer patients requiring specialist treatment.
“Forty percent of them had to move to a different region from the one they live in to get the care they needed,” said Massimo Massagrande, CEO of Elma Research. “Of that group, 40% had to move to an area not neighboring their own. The impact of area of residence is heavy, in terms of money and logistics – so much so that a large proportion of patients interviewed were forced to turn their back on the best available treatments. For women responding to our survey, the biggest obstacle is the impossibility of reconciling the effects of a move or the prospective of a temporary transfer to another region with their responsibilities for looking after their family.”
This article was translated from Univadis Italy. A version appeared on Medscape.com.
Various speakers at the VII National Conference of the Onda Foundation, Italy’s National Observatory for Women and Gender’s Health, focused on this topic. The conference was dedicated to the social factors that determine health within the context of gender medicine.
In our society, housework and raising a family are responsibilities placed predominantly on the shoulders of women. These responsibilities contribute significantly to women’s daily workload. The most overburdened women are working mothers (according to ISTAT, Italy’s Office for National Statistics, 2019), who are forced to combine their professional responsibilities with family life, dedicating 8 hours and 20 minutes per day to paid and unpaid work overall, compared with the 7 hours and 29 minutes spent by working fathers. Working mothers between ages 25 and 44 years have on average 2 hours and 35 minutes of free time per day.
Stress and sleep deprivation
“Under these conditions, the risk of chronic stress is raised, and stress leads to depression. The rate of depression in the female population is double that of the male population,” said Claudio Mencacci, MD, chair of the Italian Society of Neuropsychopharmacology and the Onda Foundation. “What’s more, stress increases the risk of cardiovascular and metabolic diseases, asthma, arthritis, and autoimmune diseases.”
The one thing that is especially damaging to physical and mental health is sleep deprivation, and working mothers get less sleep than do working fathers. “This is partially due to biological factors: hormonal changes that take place toward the end of adolescence in women during the premenstrual period are responsible for an increased rate of sleep disturbance and insomnia,” said Dr. Mencacci. “During pregnancy and the postpartum period, female sex hormones make sleep lighter, reducing time spent in the REM sleep stage. Then there’s the social aspect that plays a decisive role: by and large, it’s mothers who take care of the youngest children at night.”
According to a 2019 German study, during the first 6 years of life of the first child, a mother loses on average 44 minutes sleep per night, compared with the average time spent sleeping before pregnancy; a father loses 14 minutes.
“Another aspect to bear in mind is that, for cultural reasons, women tend to overlook the issue and not seek help, deeming sleep deprivation normal,” said Dr. Mencacci.
Caregivers at greatest risk
The negative effects of stress are evident in people continuously caring for a dependent older or disabled family member, so-called caregivers. This is, “A group predominantly made up of women aged between 45 and 55 years,” said Marina Petrini, PhD, of the Italian Health Institute’s Gender Medicine Center of Excellence. Dr. Petrini coordinated a study on stress and health in family caregivers.
“The results obtained reveal a high level of stress, especially among female caregivers, who are more exposed to the risk of severe symptoms of depression, physical disorders, especially those affecting the nervous and immune systems, and who tend to adopt irregular eating patterns and sedentary habits,” said Dr. Petrini.
Limited treatment access
Another study presented at the Onda Foundation’s conference, which shows just how much a lack of “me time” can damage your health, is the Access to Diagnostic Medicine and Treatment by Region: the Patient’s Perspective Survey, conducted by market research agency Elma Research on a sample of cancer patients requiring specialist treatment.
“Forty percent of them had to move to a different region from the one they live in to get the care they needed,” said Massimo Massagrande, CEO of Elma Research. “Of that group, 40% had to move to an area not neighboring their own. The impact of area of residence is heavy, in terms of money and logistics – so much so that a large proportion of patients interviewed were forced to turn their back on the best available treatments. For women responding to our survey, the biggest obstacle is the impossibility of reconciling the effects of a move or the prospective of a temporary transfer to another region with their responsibilities for looking after their family.”
This article was translated from Univadis Italy. A version appeared on Medscape.com.
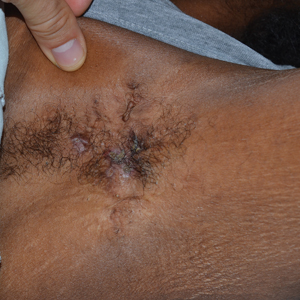
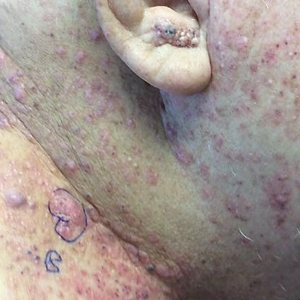
Tender Nodular Lesions in the Axilla and Vulva
The Diagnosis: Cutaneous Langerhans Cell Histiocytosis
Histopathologic findings of the left axillary lesion included a diffuse infiltrate of irregular hematolymphoid cells with reniform nuclei that strongly and diffusely stained positively with CD1a and S-100 but were negative for CD138 and CD163 (Figure). Numerous eosinophils also were present. The surrounding lymphocytic infiltrate stained positively with CD45. Polymerase chain reaction of the vaginal lesion was negative for herpes simplex virus types 1 and 2. Biopsy of the vaginal lesion revealed a mildly acanthotic epidermis and an aggregation of epithelioid cells with reniform nuclei in the papillary dermis. Positron emission tomography revealed widely disseminated disease. Sequencing of the mitogen-activated protein kinase/extracellular signalregulated kinase pathway showed amplified expression of these genes but found no mutations. These results led to a diagnosis of cutaneous Langerhans cell histiocytosis (LCH) with a background of hidradenitis suppurativa (HS). Our patient has since initiated therapy with trametinib leading to disease improvement without known recurrence.

Langerhans cell histiocytosis is a rare disease of clonal dendritic cells (Langerhans cells) that can present in any organ.1 Most LCH diagnoses are made in pediatric patients, most often presenting in the bones, with other presentations in the skin, hypophysis, liver, lymph nodes, lungs, and spleen occurring less commonly.2 Proto-oncogene BRAF V600E mutations are a common determinant of LCH, with half of cases linked with this mutation that leads to enhanced activation of the mitogen-activated protein kinase pathway, though other mutations have been reported.3,4 These genetic alterations suggest LCH is neoplastic in nature; however, this is controversial, as spontaneous regression among pulmonary LCH has been observed, pointing to a reactive inflammatory process.5 Cutaneous LCH can present as a distinct papular or nodular lesion or multiple lesions with possible ulceration, but it is rare that LCH first presents on the skin.2,6 There is a substantial association of cutaneous LCH with the development of systemically disseminated LCH as well as other blood tumors, such as myelomonocytic leukemia, histiocytic sarcoma, and multiple lymphomas; this association is thought to be due to the common origin of LCH and other blood diseases in the bone marrow.6
Histopathology of LCH shows a diffuse papillary dermal infiltrate of clonal proliferation of reniform or cleaved histiocytes.5 Epidermal ulceration and epidermotropism also are common. Neoplastic cells are found admixed with variable levels of eosinophils, lymphocytes, plasma cells, and neutrophils, though eosinophils typically are elevated. Immunohistochemistry characteristically shows the expression of CD1a, S-100, and/or CD207, and the absence of CD163 expression.
Treatment of LCH is primarily dependent on disease dissemination status, with splenic and hepatic involvement, genetic panel results, and central nervous system risk considered in the treatment plan.5 Langerhans cell histiocytosis localized to the skin may require follow-up and monitoring, as spontaneous regression of cutaneous LCH is common. However, topical steroids or psoralen and long-wave UV radiation are potential treatments. Physicians who diagnose unifocal cutaneous LCH should have high clinical suspicion of disseminated LCH, and laboratory and radiographic evaluation may be necessary to rule out systemic disease, as more than 40% of patients with cutaneous LCH have systemic disease upon full evaluation.7 With systemic involvement, systemic chemotherapy may reduce morbidity and mortality, but clinical response should be monitored after 6 weeks of treatment, as results are variably effective. Vinblastine is the most common chemotherapy regimen, with an 84% survival rate and 51.5% event-free survival rate after 8 years.8 Targeted therapy for common genetic mutations also is possible, as vemurafenib has been used to treat patients with the BRAF V600E mutation.
Due to the variable clinical presentation of cutaneous LCH, the lesions can mimic other common skin diseases such as eczema or seborrheic dermatitis.7 However, there are limited data on LCH presenting in infiltrative skin disease. Langerhans cell histiocytosis that was misdiagnosed as HS has been reported,9-11 but LCH presenting alongside long-standing HS is rare. Although LCH often mimics infiltrative skin diseases, its simultaneous presentation with a previously confirmed diagnosis of HS was notable in our patient.
In our patient, the differential diagnosis included HS, Actinomyces infection, lymphomatoid papulosis, and dermatofibrosarcoma protuberans. Cutaneous findings in HS include chronic acneform nodules with follicular plugging, ruptured ducts leading to epithelized sinuses, inflammation, and abscesses in the axillae or inguinal and perineal areas.11 Histopathology reveals follicular occlusion and hyperkeratinization, which cause destruction of the pilosebaceous glands. Hidradenitis suppurativa features on immunohistochemistry often are conflicting, but there consistently is co-localization of keratinocyte hyperplasia with CD3-, CD4-, CD8-, and CD68-positive staining of cells that produce tumor necrosis factor α, IL-12, IL-23, and IL-32, with CD1a staining variable.12 An infection with Actinomyces, a slow-progressing anaerobic or microaerophilic bacteria, may present in the skin with chronic suppurative inflammation on the neck, trunk, and abdomen. The classic presentation is subcutaneous nodules with localized infiltration of abscesses, fistulas, and draining sinuses.13 Morphologically, Actinomyces causes chronic granulomatous infection with 0.1- to 1-mm sulfur granules, which are seen as basophilic masses with eosinophilic terminal clubs on hematoxylin and eosin staining.14 Histopathology reveals grampositive filamentous Actinomyces bacteria that branch at the edge of the granules. Lymphomatoid papulosis, a nonaggressive T-cell lymphoma, presents as papulonodular and sometimes necrotic disseminated lesions that spontaneously can regress or can cause a higher risk for the development of more aggressive lymphomas.15 Histopathology shows consistently dense, dermal, lymphocytic infiltration. Immunohistochemistry is characterized by lymphocytes expressing CD30 of varying degrees: type A with many CD30 staining cells, type B presenting similar to mycosis fungoides with little CD30 staining, and type C with lymphocytic CD30-staining plaques. Dermatofibrosarcoma protuberans is a low-grade soft-tissue malignant tumor with extensive local infiltration characterized by asymptomatic plaques on the trunk and proximal extremities that are indurated and adhered to the skin.16 Histopathology shows extensive invasion into the adjacent tissue far from the original focus of the tumor.
- Girschikofsky M, Arico M, Castillo D, et al. Management of adult patients with Langerhans cell histiocytosis: recommendations from an expert panel on behalf of Euro-Histio-Net. Orphanet J Rare Dis. 2013;8:72. doi:10.1186/1750-1172-8-72
- Flores-Terry MA, Sanz-Trenado JL, García-Arpa M, et al. Cutaneous Langerhans cell histiocytosis presenting in adulthood. Actas Dermosifiliogr (Engl Ed). 2019;110:167-169. doi:10.1016/j .adengl.2018.12.005
- Emile J-F, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. doi:10.1182/blood-2016-01-690636
- Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
- Bohn OL, Teruya-Feldstein J, Sanchez-Sosa S. Skin biopsy diagnosis of Langerhans cell neoplasms. In: Fernando S, ed. Skin Biopsy: Diagnosis and Treatment [Internet]. InTechOpen; 2013. http://dx.doi .org/10.5772/55893
- Edelbroek JR, Vermeer MH, Jansen PM, et al. Langerhans cell histiocytosis first presenting in the skin in adults: frequent association with a second haematological malignancy. Br J Dermatol. 2012;167:1287-1294. doi:10.1111/j.1365-2133.2012.11169.x
- Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165: 990-996. doi:10.1016/j.jpeds.2014.07.063
- Yag˘ ci B, Varan A, Cag˘ lar M, et al. Langerhans cell histiocytosis: retrospective analysis of 217 cases in a single center. Pediatr Hematol Oncol. 2008;25:399-408. doi:10.1080/08880010802107356
- Kalen JE, Shokeen D, Mislankar M, et al. Langerhans cell histiocytosis with clinical and histologic features of hidradenitis suppurativa: brief report and review. Am J Dermatopathol. 2018;40:502-505. doi:10.1097/dad.0000000000001005
- Chertoff J, Chung J, Ataya A. Adult Langerhans cell histiocytosis masquerading as hidradenitis suppurativa. Am J Respir Crit Care Med. 2017;195:E34-E36. doi:10.1164/rccm.201610-2082IM
- St. Claire K, Bunney R, Ashack KA, et al. Langerhans cell histiocytosis: a great imitator. Clin Dermatol. 2020;38:223-234. doi:10.1016/j.clindermatol.2019.10.007
- Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of immunohistochemical associations in hidradenitis suppurativa. F1000Research. 2019;7:1923. doi:10.12688/f1000research.17268.2
- Robati RM, Niknezhad N, Bidari-Zerehpoush F, et al. Primary cutaneous actinomycosis along with the surgical scar on the hand [published online November 9, 2016]. Case Rep Infect Dis. doi:10.1155/2016/5943932
- Ferry T, Valour F, Karsenty J, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Res. 2014;2014:183-197. doi:10.2147/idr.s39601
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182 /blood-2004-09-3502
- Tsai Y, Lin P, Chew K, et al. Dermatofibrosarcoma protuberans in children and adolescents: clinical presentation, histology, treatment, and review of the literature. J Plast Reconstr Aesthet Surg. 2014;67:1222-1229. doi:10.1016/j.bjps.2014.05.03
The Diagnosis: Cutaneous Langerhans Cell Histiocytosis
Histopathologic findings of the left axillary lesion included a diffuse infiltrate of irregular hematolymphoid cells with reniform nuclei that strongly and diffusely stained positively with CD1a and S-100 but were negative for CD138 and CD163 (Figure). Numerous eosinophils also were present. The surrounding lymphocytic infiltrate stained positively with CD45. Polymerase chain reaction of the vaginal lesion was negative for herpes simplex virus types 1 and 2. Biopsy of the vaginal lesion revealed a mildly acanthotic epidermis and an aggregation of epithelioid cells with reniform nuclei in the papillary dermis. Positron emission tomography revealed widely disseminated disease. Sequencing of the mitogen-activated protein kinase/extracellular signalregulated kinase pathway showed amplified expression of these genes but found no mutations. These results led to a diagnosis of cutaneous Langerhans cell histiocytosis (LCH) with a background of hidradenitis suppurativa (HS). Our patient has since initiated therapy with trametinib leading to disease improvement without known recurrence.

Langerhans cell histiocytosis is a rare disease of clonal dendritic cells (Langerhans cells) that can present in any organ.1 Most LCH diagnoses are made in pediatric patients, most often presenting in the bones, with other presentations in the skin, hypophysis, liver, lymph nodes, lungs, and spleen occurring less commonly.2 Proto-oncogene BRAF V600E mutations are a common determinant of LCH, with half of cases linked with this mutation that leads to enhanced activation of the mitogen-activated protein kinase pathway, though other mutations have been reported.3,4 These genetic alterations suggest LCH is neoplastic in nature; however, this is controversial, as spontaneous regression among pulmonary LCH has been observed, pointing to a reactive inflammatory process.5 Cutaneous LCH can present as a distinct papular or nodular lesion or multiple lesions with possible ulceration, but it is rare that LCH first presents on the skin.2,6 There is a substantial association of cutaneous LCH with the development of systemically disseminated LCH as well as other blood tumors, such as myelomonocytic leukemia, histiocytic sarcoma, and multiple lymphomas; this association is thought to be due to the common origin of LCH and other blood diseases in the bone marrow.6
Histopathology of LCH shows a diffuse papillary dermal infiltrate of clonal proliferation of reniform or cleaved histiocytes.5 Epidermal ulceration and epidermotropism also are common. Neoplastic cells are found admixed with variable levels of eosinophils, lymphocytes, plasma cells, and neutrophils, though eosinophils typically are elevated. Immunohistochemistry characteristically shows the expression of CD1a, S-100, and/or CD207, and the absence of CD163 expression.
Treatment of LCH is primarily dependent on disease dissemination status, with splenic and hepatic involvement, genetic panel results, and central nervous system risk considered in the treatment plan.5 Langerhans cell histiocytosis localized to the skin may require follow-up and monitoring, as spontaneous regression of cutaneous LCH is common. However, topical steroids or psoralen and long-wave UV radiation are potential treatments. Physicians who diagnose unifocal cutaneous LCH should have high clinical suspicion of disseminated LCH, and laboratory and radiographic evaluation may be necessary to rule out systemic disease, as more than 40% of patients with cutaneous LCH have systemic disease upon full evaluation.7 With systemic involvement, systemic chemotherapy may reduce morbidity and mortality, but clinical response should be monitored after 6 weeks of treatment, as results are variably effective. Vinblastine is the most common chemotherapy regimen, with an 84% survival rate and 51.5% event-free survival rate after 8 years.8 Targeted therapy for common genetic mutations also is possible, as vemurafenib has been used to treat patients with the BRAF V600E mutation.
Due to the variable clinical presentation of cutaneous LCH, the lesions can mimic other common skin diseases such as eczema or seborrheic dermatitis.7 However, there are limited data on LCH presenting in infiltrative skin disease. Langerhans cell histiocytosis that was misdiagnosed as HS has been reported,9-11 but LCH presenting alongside long-standing HS is rare. Although LCH often mimics infiltrative skin diseases, its simultaneous presentation with a previously confirmed diagnosis of HS was notable in our patient.
In our patient, the differential diagnosis included HS, Actinomyces infection, lymphomatoid papulosis, and dermatofibrosarcoma protuberans. Cutaneous findings in HS include chronic acneform nodules with follicular plugging, ruptured ducts leading to epithelized sinuses, inflammation, and abscesses in the axillae or inguinal and perineal areas.11 Histopathology reveals follicular occlusion and hyperkeratinization, which cause destruction of the pilosebaceous glands. Hidradenitis suppurativa features on immunohistochemistry often are conflicting, but there consistently is co-localization of keratinocyte hyperplasia with CD3-, CD4-, CD8-, and CD68-positive staining of cells that produce tumor necrosis factor α, IL-12, IL-23, and IL-32, with CD1a staining variable.12 An infection with Actinomyces, a slow-progressing anaerobic or microaerophilic bacteria, may present in the skin with chronic suppurative inflammation on the neck, trunk, and abdomen. The classic presentation is subcutaneous nodules with localized infiltration of abscesses, fistulas, and draining sinuses.13 Morphologically, Actinomyces causes chronic granulomatous infection with 0.1- to 1-mm sulfur granules, which are seen as basophilic masses with eosinophilic terminal clubs on hematoxylin and eosin staining.14 Histopathology reveals grampositive filamentous Actinomyces bacteria that branch at the edge of the granules. Lymphomatoid papulosis, a nonaggressive T-cell lymphoma, presents as papulonodular and sometimes necrotic disseminated lesions that spontaneously can regress or can cause a higher risk for the development of more aggressive lymphomas.15 Histopathology shows consistently dense, dermal, lymphocytic infiltration. Immunohistochemistry is characterized by lymphocytes expressing CD30 of varying degrees: type A with many CD30 staining cells, type B presenting similar to mycosis fungoides with little CD30 staining, and type C with lymphocytic CD30-staining plaques. Dermatofibrosarcoma protuberans is a low-grade soft-tissue malignant tumor with extensive local infiltration characterized by asymptomatic plaques on the trunk and proximal extremities that are indurated and adhered to the skin.16 Histopathology shows extensive invasion into the adjacent tissue far from the original focus of the tumor.
The Diagnosis: Cutaneous Langerhans Cell Histiocytosis
Histopathologic findings of the left axillary lesion included a diffuse infiltrate of irregular hematolymphoid cells with reniform nuclei that strongly and diffusely stained positively with CD1a and S-100 but were negative for CD138 and CD163 (Figure). Numerous eosinophils also were present. The surrounding lymphocytic infiltrate stained positively with CD45. Polymerase chain reaction of the vaginal lesion was negative for herpes simplex virus types 1 and 2. Biopsy of the vaginal lesion revealed a mildly acanthotic epidermis and an aggregation of epithelioid cells with reniform nuclei in the papillary dermis. Positron emission tomography revealed widely disseminated disease. Sequencing of the mitogen-activated protein kinase/extracellular signalregulated kinase pathway showed amplified expression of these genes but found no mutations. These results led to a diagnosis of cutaneous Langerhans cell histiocytosis (LCH) with a background of hidradenitis suppurativa (HS). Our patient has since initiated therapy with trametinib leading to disease improvement without known recurrence.

Langerhans cell histiocytosis is a rare disease of clonal dendritic cells (Langerhans cells) that can present in any organ.1 Most LCH diagnoses are made in pediatric patients, most often presenting in the bones, with other presentations in the skin, hypophysis, liver, lymph nodes, lungs, and spleen occurring less commonly.2 Proto-oncogene BRAF V600E mutations are a common determinant of LCH, with half of cases linked with this mutation that leads to enhanced activation of the mitogen-activated protein kinase pathway, though other mutations have been reported.3,4 These genetic alterations suggest LCH is neoplastic in nature; however, this is controversial, as spontaneous regression among pulmonary LCH has been observed, pointing to a reactive inflammatory process.5 Cutaneous LCH can present as a distinct papular or nodular lesion or multiple lesions with possible ulceration, but it is rare that LCH first presents on the skin.2,6 There is a substantial association of cutaneous LCH with the development of systemically disseminated LCH as well as other blood tumors, such as myelomonocytic leukemia, histiocytic sarcoma, and multiple lymphomas; this association is thought to be due to the common origin of LCH and other blood diseases in the bone marrow.6
Histopathology of LCH shows a diffuse papillary dermal infiltrate of clonal proliferation of reniform or cleaved histiocytes.5 Epidermal ulceration and epidermotropism also are common. Neoplastic cells are found admixed with variable levels of eosinophils, lymphocytes, plasma cells, and neutrophils, though eosinophils typically are elevated. Immunohistochemistry characteristically shows the expression of CD1a, S-100, and/or CD207, and the absence of CD163 expression.
Treatment of LCH is primarily dependent on disease dissemination status, with splenic and hepatic involvement, genetic panel results, and central nervous system risk considered in the treatment plan.5 Langerhans cell histiocytosis localized to the skin may require follow-up and monitoring, as spontaneous regression of cutaneous LCH is common. However, topical steroids or psoralen and long-wave UV radiation are potential treatments. Physicians who diagnose unifocal cutaneous LCH should have high clinical suspicion of disseminated LCH, and laboratory and radiographic evaluation may be necessary to rule out systemic disease, as more than 40% of patients with cutaneous LCH have systemic disease upon full evaluation.7 With systemic involvement, systemic chemotherapy may reduce morbidity and mortality, but clinical response should be monitored after 6 weeks of treatment, as results are variably effective. Vinblastine is the most common chemotherapy regimen, with an 84% survival rate and 51.5% event-free survival rate after 8 years.8 Targeted therapy for common genetic mutations also is possible, as vemurafenib has been used to treat patients with the BRAF V600E mutation.
Due to the variable clinical presentation of cutaneous LCH, the lesions can mimic other common skin diseases such as eczema or seborrheic dermatitis.7 However, there are limited data on LCH presenting in infiltrative skin disease. Langerhans cell histiocytosis that was misdiagnosed as HS has been reported,9-11 but LCH presenting alongside long-standing HS is rare. Although LCH often mimics infiltrative skin diseases, its simultaneous presentation with a previously confirmed diagnosis of HS was notable in our patient.
In our patient, the differential diagnosis included HS, Actinomyces infection, lymphomatoid papulosis, and dermatofibrosarcoma protuberans. Cutaneous findings in HS include chronic acneform nodules with follicular plugging, ruptured ducts leading to epithelized sinuses, inflammation, and abscesses in the axillae or inguinal and perineal areas.11 Histopathology reveals follicular occlusion and hyperkeratinization, which cause destruction of the pilosebaceous glands. Hidradenitis suppurativa features on immunohistochemistry often are conflicting, but there consistently is co-localization of keratinocyte hyperplasia with CD3-, CD4-, CD8-, and CD68-positive staining of cells that produce tumor necrosis factor α, IL-12, IL-23, and IL-32, with CD1a staining variable.12 An infection with Actinomyces, a slow-progressing anaerobic or microaerophilic bacteria, may present in the skin with chronic suppurative inflammation on the neck, trunk, and abdomen. The classic presentation is subcutaneous nodules with localized infiltration of abscesses, fistulas, and draining sinuses.13 Morphologically, Actinomyces causes chronic granulomatous infection with 0.1- to 1-mm sulfur granules, which are seen as basophilic masses with eosinophilic terminal clubs on hematoxylin and eosin staining.14 Histopathology reveals grampositive filamentous Actinomyces bacteria that branch at the edge of the granules. Lymphomatoid papulosis, a nonaggressive T-cell lymphoma, presents as papulonodular and sometimes necrotic disseminated lesions that spontaneously can regress or can cause a higher risk for the development of more aggressive lymphomas.15 Histopathology shows consistently dense, dermal, lymphocytic infiltration. Immunohistochemistry is characterized by lymphocytes expressing CD30 of varying degrees: type A with many CD30 staining cells, type B presenting similar to mycosis fungoides with little CD30 staining, and type C with lymphocytic CD30-staining plaques. Dermatofibrosarcoma protuberans is a low-grade soft-tissue malignant tumor with extensive local infiltration characterized by asymptomatic plaques on the trunk and proximal extremities that are indurated and adhered to the skin.16 Histopathology shows extensive invasion into the adjacent tissue far from the original focus of the tumor.
- Girschikofsky M, Arico M, Castillo D, et al. Management of adult patients with Langerhans cell histiocytosis: recommendations from an expert panel on behalf of Euro-Histio-Net. Orphanet J Rare Dis. 2013;8:72. doi:10.1186/1750-1172-8-72
- Flores-Terry MA, Sanz-Trenado JL, García-Arpa M, et al. Cutaneous Langerhans cell histiocytosis presenting in adulthood. Actas Dermosifiliogr (Engl Ed). 2019;110:167-169. doi:10.1016/j .adengl.2018.12.005
- Emile J-F, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. doi:10.1182/blood-2016-01-690636
- Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
- Bohn OL, Teruya-Feldstein J, Sanchez-Sosa S. Skin biopsy diagnosis of Langerhans cell neoplasms. In: Fernando S, ed. Skin Biopsy: Diagnosis and Treatment [Internet]. InTechOpen; 2013. http://dx.doi .org/10.5772/55893
- Edelbroek JR, Vermeer MH, Jansen PM, et al. Langerhans cell histiocytosis first presenting in the skin in adults: frequent association with a second haematological malignancy. Br J Dermatol. 2012;167:1287-1294. doi:10.1111/j.1365-2133.2012.11169.x
- Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165: 990-996. doi:10.1016/j.jpeds.2014.07.063
- Yag˘ ci B, Varan A, Cag˘ lar M, et al. Langerhans cell histiocytosis: retrospective analysis of 217 cases in a single center. Pediatr Hematol Oncol. 2008;25:399-408. doi:10.1080/08880010802107356
- Kalen JE, Shokeen D, Mislankar M, et al. Langerhans cell histiocytosis with clinical and histologic features of hidradenitis suppurativa: brief report and review. Am J Dermatopathol. 2018;40:502-505. doi:10.1097/dad.0000000000001005
- Chertoff J, Chung J, Ataya A. Adult Langerhans cell histiocytosis masquerading as hidradenitis suppurativa. Am J Respir Crit Care Med. 2017;195:E34-E36. doi:10.1164/rccm.201610-2082IM
- St. Claire K, Bunney R, Ashack KA, et al. Langerhans cell histiocytosis: a great imitator. Clin Dermatol. 2020;38:223-234. doi:10.1016/j.clindermatol.2019.10.007
- Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of immunohistochemical associations in hidradenitis suppurativa. F1000Research. 2019;7:1923. doi:10.12688/f1000research.17268.2
- Robati RM, Niknezhad N, Bidari-Zerehpoush F, et al. Primary cutaneous actinomycosis along with the surgical scar on the hand [published online November 9, 2016]. Case Rep Infect Dis. doi:10.1155/2016/5943932
- Ferry T, Valour F, Karsenty J, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Res. 2014;2014:183-197. doi:10.2147/idr.s39601
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182 /blood-2004-09-3502
- Tsai Y, Lin P, Chew K, et al. Dermatofibrosarcoma protuberans in children and adolescents: clinical presentation, histology, treatment, and review of the literature. J Plast Reconstr Aesthet Surg. 2014;67:1222-1229. doi:10.1016/j.bjps.2014.05.03
- Girschikofsky M, Arico M, Castillo D, et al. Management of adult patients with Langerhans cell histiocytosis: recommendations from an expert panel on behalf of Euro-Histio-Net. Orphanet J Rare Dis. 2013;8:72. doi:10.1186/1750-1172-8-72
- Flores-Terry MA, Sanz-Trenado JL, García-Arpa M, et al. Cutaneous Langerhans cell histiocytosis presenting in adulthood. Actas Dermosifiliogr (Engl Ed). 2019;110:167-169. doi:10.1016/j .adengl.2018.12.005
- Emile J-F, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. doi:10.1182/blood-2016-01-690636
- Badalian-Very G, Vergilio J-A, Degar BA, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919-1923. doi:10.1182/blood-2010-04-279083
- Bohn OL, Teruya-Feldstein J, Sanchez-Sosa S. Skin biopsy diagnosis of Langerhans cell neoplasms. In: Fernando S, ed. Skin Biopsy: Diagnosis and Treatment [Internet]. InTechOpen; 2013. http://dx.doi .org/10.5772/55893
- Edelbroek JR, Vermeer MH, Jansen PM, et al. Langerhans cell histiocytosis first presenting in the skin in adults: frequent association with a second haematological malignancy. Br J Dermatol. 2012;167:1287-1294. doi:10.1111/j.1365-2133.2012.11169.x
- Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165: 990-996. doi:10.1016/j.jpeds.2014.07.063
- Yag˘ ci B, Varan A, Cag˘ lar M, et al. Langerhans cell histiocytosis: retrospective analysis of 217 cases in a single center. Pediatr Hematol Oncol. 2008;25:399-408. doi:10.1080/08880010802107356
- Kalen JE, Shokeen D, Mislankar M, et al. Langerhans cell histiocytosis with clinical and histologic features of hidradenitis suppurativa: brief report and review. Am J Dermatopathol. 2018;40:502-505. doi:10.1097/dad.0000000000001005
- Chertoff J, Chung J, Ataya A. Adult Langerhans cell histiocytosis masquerading as hidradenitis suppurativa. Am J Respir Crit Care Med. 2017;195:E34-E36. doi:10.1164/rccm.201610-2082IM
- St. Claire K, Bunney R, Ashack KA, et al. Langerhans cell histiocytosis: a great imitator. Clin Dermatol. 2020;38:223-234. doi:10.1016/j.clindermatol.2019.10.007
- Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of immunohistochemical associations in hidradenitis suppurativa. F1000Research. 2019;7:1923. doi:10.12688/f1000research.17268.2
- Robati RM, Niknezhad N, Bidari-Zerehpoush F, et al. Primary cutaneous actinomycosis along with the surgical scar on the hand [published online November 9, 2016]. Case Rep Infect Dis. doi:10.1155/2016/5943932
- Ferry T, Valour F, Karsenty J, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Res. 2014;2014:183-197. doi:10.2147/idr.s39601
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182 /blood-2004-09-3502
- Tsai Y, Lin P, Chew K, et al. Dermatofibrosarcoma protuberans in children and adolescents: clinical presentation, histology, treatment, and review of the literature. J Plast Reconstr Aesthet Surg. 2014;67:1222-1229. doi:10.1016/j.bjps.2014.05.03
A 28-year-old woman presented with tender burning lesions of the left axillary and vaginal skin that had worsened over the last year. Her medical history was notable for hidradenitis suppurativa, which had been present since adolescence, as well as pulmonary Langerhans cell histiocytosis diagnosed 7 years prior to the current presentation after a spontaneous pneumothorax that eventually led to a pulmonary transplantation 3 years prior. The patient’s Langerhans cell histiocytosis was believed to have resolved without treatment after smoking cessation. Physical examination revealed nodular inflammation and scarring with deep undermining along the left axilla as well as swelling of the mons pubis with erosive skin lesions in the surrounding vaginal area. Bilateral cervical, axillary, inguinal, supraclavicular, and femoral lymph node chains were negative for adenopathy. A shave biopsy was performed on the axillary nodule.
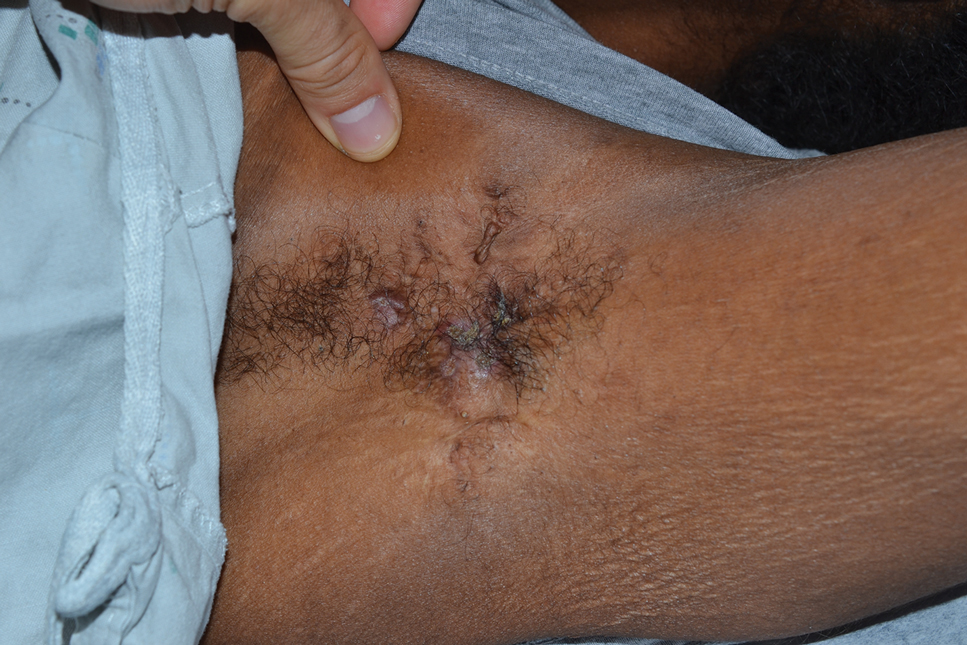
Cutaneous Presentation of Metastatic Salivary Duct Carcinoma
To the Editor:
Metastatic spread of salivary duct carcinoma (SDC) to the skin is rare. Diagnosing SDC can be challenging because the cutaneous manifestations of this disease are variable and include nodules, papules, and erysipelaslike inflammation (also known as shield sign) with purpuric papules and pseudovesicles. We describe a case of cutaneous metastatic SDC that originated from the parotid gland and presented with 2 distinct cutaneous findings: sharply demarcated erythematous plaques and focally hemorrhagic angiomatous papules.
A 60-year-old man presented with a persistent polymorphous pruritic eruption of several months’ duration involving the entire face, ears, neck, and upper chest. He had a history of unspecified adenocarcinoma of the parotid gland diagnosed 2 years prior and underwent multiple treatment cycles with several chemotherapeutic agents over the course of 18 months. Physical examination showed erythematous papules and nodules on the face and neck with slight overlying scale. Sharply demarcated, erythematous plaques studded with focally hemorrhagic, angiomatous papules were noted on the neck and chest (Figure 1). Two 4-mm punch biopsies were sampled from representative nodular areas. Histopathology showed multiple round solid-tumor nodules with central necrosis in the superficial and deep dermis that were not associated with the overlying epidermis (Figures 2A and 2B). The tumor cells appeared polygonal and contained ample eosinophilic cytoplasm. Tumor nuclei showed marked pleomorphism, and numerous atypical mitotic figures were readily identifiable (Figure 2C). There was diffuse cytoplasmic staining with cytokeratin 7 and nuclear staining with androgen receptor (Figure 2D). These findings were consistent with a diagnosis of SDC metastatic to the skin.
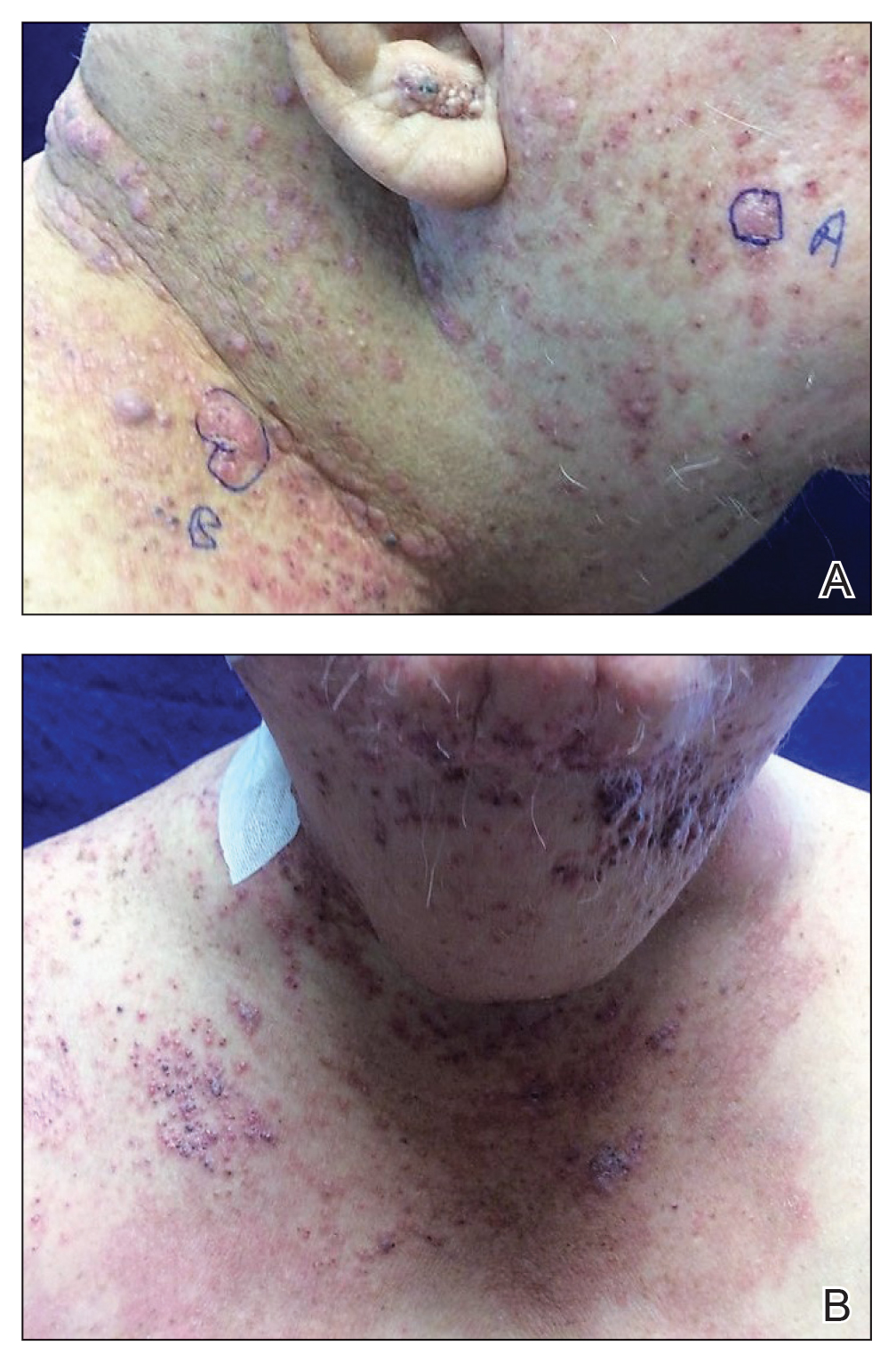
The patient underwent 8 cycles of docetaxel chemotherapy. With disease progression, the chemotherapy regimen was changed to gemcitabine and methotrexate. The patient continued to experience disease progression and died 9 months after diagnosis of skin metastases.
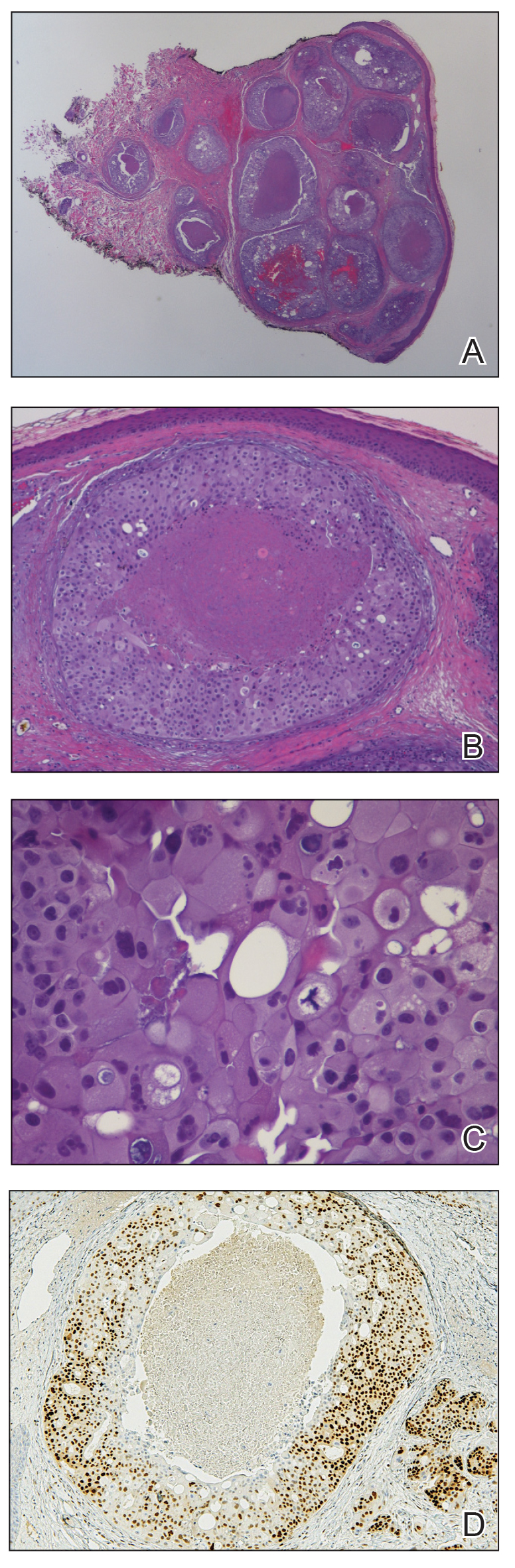
Salivary duct carcinoma is rare and is estimated to represent 1% to 3% of all salivary malignancies.1 It is a highly aggressive form of salivary gland carcinoma and is associated with a poor clinical outcome. The 3-year overall survival rate for stage I disease is 42% and only 23% for stage IV disease.2 Salivary duct carcinoma has a high rate of distant metastasis,3 but cases of cutaneous metastases are rare.3-8 Previously reported cases of SDC that metastasized to the skin originated from the parotid gland (n=6) and submandibular gland (n=1).3
The diagnosis of cutaneous metastases is challenging due to the variability of the skin manifestations. Three cases described small firm nodules in patients,3-5 while others presented with purpuric papules and pseudovesicles.6-8 Our patient presented with sharply demarcated, erythematous plaques studded with focally hemorrhagic, angiomatous papules, which further emphasizes the capricious nature of skin findings.
The morphology of SDC is strikingly similar to ductal adenocarcinoma of the breast, which can lead to diagnostic confusion. Both carcinomas may show oncocytic cells, ductal formations, and cribriform structures with central comedo necrosis. Moreover, immunohistochemical features overlap, including positive staining for cytokeratin 7 and gross cystic disease fluid protein 15. Positive immunohistochemistry with androgen receptor is consistent with SDC but also can be expressed in some cases of breast carcinoma.9,10 Therefore, the diagnosis of cutaneous involvement from metastatic SDC requires not just an evaluation of the pathologic features but careful attention to the clinical history and a thorough staging evaluation.
- D’heygere E, Meulemans J, Vander Poorten V. Salivary duct carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2018;26:142-151.
- Gilbert MR, Sharma A, Schmitt NC, et al. A 20-year review of 75 cases of salivary duct carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142:489-495.
- Chakari W, Andersen L, Andersen JL. Cutaneous metastases from salivary duct carcinoma of the submandibular gland. Case Rep Dermatol. 2017;9:254-258.
- Tok J, Kao GF, Berberian BJ, et al. Cutaneous metastasis from a parotid adenocarcinoma. Report of a case with immunohistochemical findings and review of the literature. Am J Dermatopathol. 1995;17:303-306.
- Aygit AC, Top H, Cakir B, et al. Salivary duct carcinoma of the parotid gland metastasizing to the skin: a case report and review of the literature. Am J Dermatopathol. 2005;27:48-50.
- Cohen PR, Prieto VG, Piha-Paul SA, et al. The “shield sign” in two men with metastatic salivary duct carcinoma to the skin: cutaneous metastases presenting as carcinoma hemorrhagiectoides. J Clin Aesthet Dermatol. 2012;5:27-36.
- Hafiji J, Rytina E, Jani P, et al. A rare cutaneous presentation of metastatic parotid adenocarcinoma. Australas J Dermatol. 2013;54:E40-E42.
- Zanca A, Ferracini U, Bertazzoni MG. Telangiectatic metastasis from ductal carcinoma of the parotid gland. J Am Acad Dermatol. 1993;28:113-114.
- Brys´ M, Wójcik M, Romanowicz-Makowska H, et al. Androgen receptor status in female breast cancer: RT-PCR and Western blot studies. J Cancer Res Clin Oncol. 2002;128:85-90.
- Udager AM, Chiosea SI. Salivary duct carcinoma: an update on morphologic mimics and diagnostic use of androgen receptor immunohistochemistry. Head Neck Pathol. 2017;11:288-294.
To the Editor:
Metastatic spread of salivary duct carcinoma (SDC) to the skin is rare. Diagnosing SDC can be challenging because the cutaneous manifestations of this disease are variable and include nodules, papules, and erysipelaslike inflammation (also known as shield sign) with purpuric papules and pseudovesicles. We describe a case of cutaneous metastatic SDC that originated from the parotid gland and presented with 2 distinct cutaneous findings: sharply demarcated erythematous plaques and focally hemorrhagic angiomatous papules.
A 60-year-old man presented with a persistent polymorphous pruritic eruption of several months’ duration involving the entire face, ears, neck, and upper chest. He had a history of unspecified adenocarcinoma of the parotid gland diagnosed 2 years prior and underwent multiple treatment cycles with several chemotherapeutic agents over the course of 18 months. Physical examination showed erythematous papules and nodules on the face and neck with slight overlying scale. Sharply demarcated, erythematous plaques studded with focally hemorrhagic, angiomatous papules were noted on the neck and chest (Figure 1). Two 4-mm punch biopsies were sampled from representative nodular areas. Histopathology showed multiple round solid-tumor nodules with central necrosis in the superficial and deep dermis that were not associated with the overlying epidermis (Figures 2A and 2B). The tumor cells appeared polygonal and contained ample eosinophilic cytoplasm. Tumor nuclei showed marked pleomorphism, and numerous atypical mitotic figures were readily identifiable (Figure 2C). There was diffuse cytoplasmic staining with cytokeratin 7 and nuclear staining with androgen receptor (Figure 2D). These findings were consistent with a diagnosis of SDC metastatic to the skin.

The patient underwent 8 cycles of docetaxel chemotherapy. With disease progression, the chemotherapy regimen was changed to gemcitabine and methotrexate. The patient continued to experience disease progression and died 9 months after diagnosis of skin metastases.

Salivary duct carcinoma is rare and is estimated to represent 1% to 3% of all salivary malignancies.1 It is a highly aggressive form of salivary gland carcinoma and is associated with a poor clinical outcome. The 3-year overall survival rate for stage I disease is 42% and only 23% for stage IV disease.2 Salivary duct carcinoma has a high rate of distant metastasis,3 but cases of cutaneous metastases are rare.3-8 Previously reported cases of SDC that metastasized to the skin originated from the parotid gland (n=6) and submandibular gland (n=1).3
The diagnosis of cutaneous metastases is challenging due to the variability of the skin manifestations. Three cases described small firm nodules in patients,3-5 while others presented with purpuric papules and pseudovesicles.6-8 Our patient presented with sharply demarcated, erythematous plaques studded with focally hemorrhagic, angiomatous papules, which further emphasizes the capricious nature of skin findings.
The morphology of SDC is strikingly similar to ductal adenocarcinoma of the breast, which can lead to diagnostic confusion. Both carcinomas may show oncocytic cells, ductal formations, and cribriform structures with central comedo necrosis. Moreover, immunohistochemical features overlap, including positive staining for cytokeratin 7 and gross cystic disease fluid protein 15. Positive immunohistochemistry with androgen receptor is consistent with SDC but also can be expressed in some cases of breast carcinoma.9,10 Therefore, the diagnosis of cutaneous involvement from metastatic SDC requires not just an evaluation of the pathologic features but careful attention to the clinical history and a thorough staging evaluation.
To the Editor:
Metastatic spread of salivary duct carcinoma (SDC) to the skin is rare. Diagnosing SDC can be challenging because the cutaneous manifestations of this disease are variable and include nodules, papules, and erysipelaslike inflammation (also known as shield sign) with purpuric papules and pseudovesicles. We describe a case of cutaneous metastatic SDC that originated from the parotid gland and presented with 2 distinct cutaneous findings: sharply demarcated erythematous plaques and focally hemorrhagic angiomatous papules.
A 60-year-old man presented with a persistent polymorphous pruritic eruption of several months’ duration involving the entire face, ears, neck, and upper chest. He had a history of unspecified adenocarcinoma of the parotid gland diagnosed 2 years prior and underwent multiple treatment cycles with several chemotherapeutic agents over the course of 18 months. Physical examination showed erythematous papules and nodules on the face and neck with slight overlying scale. Sharply demarcated, erythematous plaques studded with focally hemorrhagic, angiomatous papules were noted on the neck and chest (Figure 1). Two 4-mm punch biopsies were sampled from representative nodular areas. Histopathology showed multiple round solid-tumor nodules with central necrosis in the superficial and deep dermis that were not associated with the overlying epidermis (Figures 2A and 2B). The tumor cells appeared polygonal and contained ample eosinophilic cytoplasm. Tumor nuclei showed marked pleomorphism, and numerous atypical mitotic figures were readily identifiable (Figure 2C). There was diffuse cytoplasmic staining with cytokeratin 7 and nuclear staining with androgen receptor (Figure 2D). These findings were consistent with a diagnosis of SDC metastatic to the skin.

The patient underwent 8 cycles of docetaxel chemotherapy. With disease progression, the chemotherapy regimen was changed to gemcitabine and methotrexate. The patient continued to experience disease progression and died 9 months after diagnosis of skin metastases.

Salivary duct carcinoma is rare and is estimated to represent 1% to 3% of all salivary malignancies.1 It is a highly aggressive form of salivary gland carcinoma and is associated with a poor clinical outcome. The 3-year overall survival rate for stage I disease is 42% and only 23% for stage IV disease.2 Salivary duct carcinoma has a high rate of distant metastasis,3 but cases of cutaneous metastases are rare.3-8 Previously reported cases of SDC that metastasized to the skin originated from the parotid gland (n=6) and submandibular gland (n=1).3
The diagnosis of cutaneous metastases is challenging due to the variability of the skin manifestations. Three cases described small firm nodules in patients,3-5 while others presented with purpuric papules and pseudovesicles.6-8 Our patient presented with sharply demarcated, erythematous plaques studded with focally hemorrhagic, angiomatous papules, which further emphasizes the capricious nature of skin findings.
The morphology of SDC is strikingly similar to ductal adenocarcinoma of the breast, which can lead to diagnostic confusion. Both carcinomas may show oncocytic cells, ductal formations, and cribriform structures with central comedo necrosis. Moreover, immunohistochemical features overlap, including positive staining for cytokeratin 7 and gross cystic disease fluid protein 15. Positive immunohistochemistry with androgen receptor is consistent with SDC but also can be expressed in some cases of breast carcinoma.9,10 Therefore, the diagnosis of cutaneous involvement from metastatic SDC requires not just an evaluation of the pathologic features but careful attention to the clinical history and a thorough staging evaluation.
- D’heygere E, Meulemans J, Vander Poorten V. Salivary duct carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2018;26:142-151.
- Gilbert MR, Sharma A, Schmitt NC, et al. A 20-year review of 75 cases of salivary duct carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142:489-495.
- Chakari W, Andersen L, Andersen JL. Cutaneous metastases from salivary duct carcinoma of the submandibular gland. Case Rep Dermatol. 2017;9:254-258.
- Tok J, Kao GF, Berberian BJ, et al. Cutaneous metastasis from a parotid adenocarcinoma. Report of a case with immunohistochemical findings and review of the literature. Am J Dermatopathol. 1995;17:303-306.
- Aygit AC, Top H, Cakir B, et al. Salivary duct carcinoma of the parotid gland metastasizing to the skin: a case report and review of the literature. Am J Dermatopathol. 2005;27:48-50.
- Cohen PR, Prieto VG, Piha-Paul SA, et al. The “shield sign” in two men with metastatic salivary duct carcinoma to the skin: cutaneous metastases presenting as carcinoma hemorrhagiectoides. J Clin Aesthet Dermatol. 2012;5:27-36.
- Hafiji J, Rytina E, Jani P, et al. A rare cutaneous presentation of metastatic parotid adenocarcinoma. Australas J Dermatol. 2013;54:E40-E42.
- Zanca A, Ferracini U, Bertazzoni MG. Telangiectatic metastasis from ductal carcinoma of the parotid gland. J Am Acad Dermatol. 1993;28:113-114.
- Brys´ M, Wójcik M, Romanowicz-Makowska H, et al. Androgen receptor status in female breast cancer: RT-PCR and Western blot studies. J Cancer Res Clin Oncol. 2002;128:85-90.
- Udager AM, Chiosea SI. Salivary duct carcinoma: an update on morphologic mimics and diagnostic use of androgen receptor immunohistochemistry. Head Neck Pathol. 2017;11:288-294.
- D’heygere E, Meulemans J, Vander Poorten V. Salivary duct carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2018;26:142-151.
- Gilbert MR, Sharma A, Schmitt NC, et al. A 20-year review of 75 cases of salivary duct carcinoma. JAMA Otolaryngol Head Neck Surg. 2016;142:489-495.
- Chakari W, Andersen L, Andersen JL. Cutaneous metastases from salivary duct carcinoma of the submandibular gland. Case Rep Dermatol. 2017;9:254-258.
- Tok J, Kao GF, Berberian BJ, et al. Cutaneous metastasis from a parotid adenocarcinoma. Report of a case with immunohistochemical findings and review of the literature. Am J Dermatopathol. 1995;17:303-306.
- Aygit AC, Top H, Cakir B, et al. Salivary duct carcinoma of the parotid gland metastasizing to the skin: a case report and review of the literature. Am J Dermatopathol. 2005;27:48-50.
- Cohen PR, Prieto VG, Piha-Paul SA, et al. The “shield sign” in two men with metastatic salivary duct carcinoma to the skin: cutaneous metastases presenting as carcinoma hemorrhagiectoides. J Clin Aesthet Dermatol. 2012;5:27-36.
- Hafiji J, Rytina E, Jani P, et al. A rare cutaneous presentation of metastatic parotid adenocarcinoma. Australas J Dermatol. 2013;54:E40-E42.
- Zanca A, Ferracini U, Bertazzoni MG. Telangiectatic metastasis from ductal carcinoma of the parotid gland. J Am Acad Dermatol. 1993;28:113-114.
- Brys´ M, Wójcik M, Romanowicz-Makowska H, et al. Androgen receptor status in female breast cancer: RT-PCR and Western blot studies. J Cancer Res Clin Oncol. 2002;128:85-90.
- Udager AM, Chiosea SI. Salivary duct carcinoma: an update on morphologic mimics and diagnostic use of androgen receptor immunohistochemistry. Head Neck Pathol. 2017;11:288-294.
Practice Points
- Skin manifestations of metastatic salivary duct carcinoma can be variable, ranging from nodules to erysipelaslike inflammation (also known as shield sign) with purpuric papules and pseudovesicles.
- The specific clinical findings as well as histologic and immunohistochemical characteristics can aid in the diagnosis of this rare disease.
News & Perspectives from Ob.Gyn. News
COMMENTARY
The safety of vaginal estrogen in breast cancer survivors
Currently, more than 3.8 million breast cancer survivors reside in the United States, reflecting high prevalence as well as cure rates for this common malignancy.
When over-the-counter measures including vaginal lubricants and moisturizers are not adequate, vaginal estrogen may be a highly effective treatment for genitourinary syndrome of menopause (GSM), a common condition associated with hypoestrogenism that impairs sexual function and quality of life.
Use of vaginal formulations does not result in systemic levels of estrogen above the normal postmenopausal range. Nonetheless, the U.S. Food and Drug Administration lists a history of breast cancer as a contraindication to the use of all systemic as well as vaginal estrogens.
In premenopausal women, chemotherapy for breast cancer often results in early menopause. Aromatase inhibitors, although effective in preventing recurrent disease in menopausal women, exacerbate GSM. These factors result in a high prevalence of GSM in breast cancer survivors.
Because the safety of vaginal estrogen in the setting of breast cancer is uncertain, investigators at Johns Hopkins conducted a cohort study using claims-based data from more than 200 million U.S. patients that identified women with GSM who had previously been diagnosed with breast cancer. Among some 42,000 women diagnosed with GSM after breast cancer, 5% had three or more prescriptions and were considered vaginal estrogen users.
No significant differences were noted in recurrence-free survival between the vaginal estrogen group and the no estrogen group. At 5 and 10 years of follow-up, use of vaginal estrogen was not associated with higher all-cause mortality. Among women with estrogen receptor–positive tumors, risk for breast cancer recurrence was similar between estrogen users and nonusers.
LATEST NEWS
Older women who get mammograms risk overdiagnosis
TOPLINE:
Women who continue breast cancer screening after age 70 face a considerable risk for overdiagnosis.
METHODOLOGY:
- Overdiagnosis – the risk of detecting and treating cancers that would never have caused issues in a person’s lifetime – is increasingly recognized as a harm of breast cancer screening; however, the scope of the problem among older women remains uncertain.
- To get an idea, investigators linked Medicare claims data with Surveillance, Epidemiology, and End Results (SEER) data for 54,635 women 70 years or older to compare the incidence of breast cancer and breast cancer–specific death among women who continued screening mammography with those who did not.
- The women all had undergone recent screening mammograms and had no history of breast cancer at study entry. Those who had a subsequent mammogram within 3 years were classified as undergoing continued screening while those who did not were classified as not undergoing continued screening.
- Overdiagnosis was defined as the difference in cumulative incidence of breast cancer between screened and unscreened women divided by the cumulative incidence among screened women.
- Results were adjusted for potential confounders, including age, race, and ethnicity.
Continue to: TAKEAWAY...
TAKEAWAY:
- Over 80% of women 70-84 years old and more than 60% of women 85 years or older continued screening.
- Among women 70-74 years old, the adjusted cumulative incidence of breast cancer was 6.1 cases per 100 screened women vs. 4.2 cases per 100 unscreened women; for women aged 75-84 years old, the cumulative incidence was 4.9 per 100 screened women vs. 2.6 per 100 unscreened women, and for women 85 years and older, the cumulative incidence was 2.8 vs. 1.3 per 100, respectively.
- Estimates of overdiagnosis ranged from 31% of breast cancer cases among screened women in the 70-74 age group to 54% of cases in the 85 and older group.
- The researchers found no statistically significant reduction in breast cancer–specific death associated with screening in any age or life-expectancy group. Overdiagnosis appeared to be driven by in situ and localized invasive breast cancer, not advanced breast cancer.
IN PRACTICE:
The proportion of older women who continue to receive screening mammograms and may experience breast cancer overdiagnosis is “considerable” and “increases with advancing age and with decreasing life expectancy,” the authors conclude. Given potential benefits and harms of screening in this population, “patient preferences, including risk tolerance, comfort with uncertainty, and willingness to undergo treatment, are important for informing screening decisions.”
SOURCE: https://www.acpjournals.org/doi/10.7326/M23-0133
https://www.mdedge.com/obgyn/latest-news
CONFERENCE COVERAGE
Offering HPV vaccine at age 9 linked to greater series completion
BALTIMORE—Receiving the first dose of the human papillomavirus (HPV) vaccine at age 9, rather than bundling it with the Tdap and meningitis vaccines, appears to increase the likelihood that children will complete the HPV vaccine series, according to a retrospective cohort study of commercially insured youth presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. The research was published ahead of print in Human Vaccines and Immunotherapeutics.
“These findings are novel because they emphasize starting at age 9, and that is different than prior studies that emphasize bundling of these vaccines,” Kevin Ault, MD, professor and chair of the department of obstetrics and gynecology at Western Michigan University Homer Stryker MD School of Medicine and a former member of the CDC’s Advisory Committee on Immunization Practices, said in an interview.
Dr. Ault was not involved in the study but noted that these findings support the AAP’s recommendation to start the HPV vaccine series at age 9. The Centers for Disease Control and Prevention currently recommends giving the first dose of the HPV vaccine at ages 11-12, at the same time as the Tdap and meningitis vaccines. This recommendation to “bundle” the HPV vaccine with the Tdap and meningitis vaccines aims to facilitate provider-family discussion about the HPV vaccine, ideally reducing parent hesitancy and concerns about the vaccines. Multiple studies have shown improved HPV vaccine uptake when providers offer the HPV vaccine at the same time as the Tdap and meningococcal vaccines.
However, shifts in parents’ attitudes have occurred toward the HPV vaccine since those studies on bundling: Concerns about sexual activity have receded while concerns about safety remain high. The American Academy of Pediatrics and the American Cancer Society both advise starting the HPV vaccine series at age 9, based on evidence showing that more children complete the series when they get the first shot before age 11 compared to getting it at 11 or 12.
COMMENTARY
The safety of vaginal estrogen in breast cancer survivors
Currently, more than 3.8 million breast cancer survivors reside in the United States, reflecting high prevalence as well as cure rates for this common malignancy.
When over-the-counter measures including vaginal lubricants and moisturizers are not adequate, vaginal estrogen may be a highly effective treatment for genitourinary syndrome of menopause (GSM), a common condition associated with hypoestrogenism that impairs sexual function and quality of life.
Use of vaginal formulations does not result in systemic levels of estrogen above the normal postmenopausal range. Nonetheless, the U.S. Food and Drug Administration lists a history of breast cancer as a contraindication to the use of all systemic as well as vaginal estrogens.
In premenopausal women, chemotherapy for breast cancer often results in early menopause. Aromatase inhibitors, although effective in preventing recurrent disease in menopausal women, exacerbate GSM. These factors result in a high prevalence of GSM in breast cancer survivors.
Because the safety of vaginal estrogen in the setting of breast cancer is uncertain, investigators at Johns Hopkins conducted a cohort study using claims-based data from more than 200 million U.S. patients that identified women with GSM who had previously been diagnosed with breast cancer. Among some 42,000 women diagnosed with GSM after breast cancer, 5% had three or more prescriptions and were considered vaginal estrogen users.
No significant differences were noted in recurrence-free survival between the vaginal estrogen group and the no estrogen group. At 5 and 10 years of follow-up, use of vaginal estrogen was not associated with higher all-cause mortality. Among women with estrogen receptor–positive tumors, risk for breast cancer recurrence was similar between estrogen users and nonusers.
LATEST NEWS
Older women who get mammograms risk overdiagnosis
TOPLINE:
Women who continue breast cancer screening after age 70 face a considerable risk for overdiagnosis.
METHODOLOGY:
- Overdiagnosis – the risk of detecting and treating cancers that would never have caused issues in a person’s lifetime – is increasingly recognized as a harm of breast cancer screening; however, the scope of the problem among older women remains uncertain.
- To get an idea, investigators linked Medicare claims data with Surveillance, Epidemiology, and End Results (SEER) data for 54,635 women 70 years or older to compare the incidence of breast cancer and breast cancer–specific death among women who continued screening mammography with those who did not.
- The women all had undergone recent screening mammograms and had no history of breast cancer at study entry. Those who had a subsequent mammogram within 3 years were classified as undergoing continued screening while those who did not were classified as not undergoing continued screening.
- Overdiagnosis was defined as the difference in cumulative incidence of breast cancer between screened and unscreened women divided by the cumulative incidence among screened women.
- Results were adjusted for potential confounders, including age, race, and ethnicity.
Continue to: TAKEAWAY...
TAKEAWAY:
- Over 80% of women 70-84 years old and more than 60% of women 85 years or older continued screening.
- Among women 70-74 years old, the adjusted cumulative incidence of breast cancer was 6.1 cases per 100 screened women vs. 4.2 cases per 100 unscreened women; for women aged 75-84 years old, the cumulative incidence was 4.9 per 100 screened women vs. 2.6 per 100 unscreened women, and for women 85 years and older, the cumulative incidence was 2.8 vs. 1.3 per 100, respectively.
- Estimates of overdiagnosis ranged from 31% of breast cancer cases among screened women in the 70-74 age group to 54% of cases in the 85 and older group.
- The researchers found no statistically significant reduction in breast cancer–specific death associated with screening in any age or life-expectancy group. Overdiagnosis appeared to be driven by in situ and localized invasive breast cancer, not advanced breast cancer.
IN PRACTICE:
The proportion of older women who continue to receive screening mammograms and may experience breast cancer overdiagnosis is “considerable” and “increases with advancing age and with decreasing life expectancy,” the authors conclude. Given potential benefits and harms of screening in this population, “patient preferences, including risk tolerance, comfort with uncertainty, and willingness to undergo treatment, are important for informing screening decisions.”
SOURCE: https://www.acpjournals.org/doi/10.7326/M23-0133
https://www.mdedge.com/obgyn/latest-news
CONFERENCE COVERAGE
Offering HPV vaccine at age 9 linked to greater series completion
BALTIMORE—Receiving the first dose of the human papillomavirus (HPV) vaccine at age 9, rather than bundling it with the Tdap and meningitis vaccines, appears to increase the likelihood that children will complete the HPV vaccine series, according to a retrospective cohort study of commercially insured youth presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. The research was published ahead of print in Human Vaccines and Immunotherapeutics.
“These findings are novel because they emphasize starting at age 9, and that is different than prior studies that emphasize bundling of these vaccines,” Kevin Ault, MD, professor and chair of the department of obstetrics and gynecology at Western Michigan University Homer Stryker MD School of Medicine and a former member of the CDC’s Advisory Committee on Immunization Practices, said in an interview.
Dr. Ault was not involved in the study but noted that these findings support the AAP’s recommendation to start the HPV vaccine series at age 9. The Centers for Disease Control and Prevention currently recommends giving the first dose of the HPV vaccine at ages 11-12, at the same time as the Tdap and meningitis vaccines. This recommendation to “bundle” the HPV vaccine with the Tdap and meningitis vaccines aims to facilitate provider-family discussion about the HPV vaccine, ideally reducing parent hesitancy and concerns about the vaccines. Multiple studies have shown improved HPV vaccine uptake when providers offer the HPV vaccine at the same time as the Tdap and meningococcal vaccines.
However, shifts in parents’ attitudes have occurred toward the HPV vaccine since those studies on bundling: Concerns about sexual activity have receded while concerns about safety remain high. The American Academy of Pediatrics and the American Cancer Society both advise starting the HPV vaccine series at age 9, based on evidence showing that more children complete the series when they get the first shot before age 11 compared to getting it at 11 or 12.
COMMENTARY
The safety of vaginal estrogen in breast cancer survivors
Currently, more than 3.8 million breast cancer survivors reside in the United States, reflecting high prevalence as well as cure rates for this common malignancy.
When over-the-counter measures including vaginal lubricants and moisturizers are not adequate, vaginal estrogen may be a highly effective treatment for genitourinary syndrome of menopause (GSM), a common condition associated with hypoestrogenism that impairs sexual function and quality of life.
Use of vaginal formulations does not result in systemic levels of estrogen above the normal postmenopausal range. Nonetheless, the U.S. Food and Drug Administration lists a history of breast cancer as a contraindication to the use of all systemic as well as vaginal estrogens.
In premenopausal women, chemotherapy for breast cancer often results in early menopause. Aromatase inhibitors, although effective in preventing recurrent disease in menopausal women, exacerbate GSM. These factors result in a high prevalence of GSM in breast cancer survivors.
Because the safety of vaginal estrogen in the setting of breast cancer is uncertain, investigators at Johns Hopkins conducted a cohort study using claims-based data from more than 200 million U.S. patients that identified women with GSM who had previously been diagnosed with breast cancer. Among some 42,000 women diagnosed with GSM after breast cancer, 5% had three or more prescriptions and were considered vaginal estrogen users.
No significant differences were noted in recurrence-free survival between the vaginal estrogen group and the no estrogen group. At 5 and 10 years of follow-up, use of vaginal estrogen was not associated with higher all-cause mortality. Among women with estrogen receptor–positive tumors, risk for breast cancer recurrence was similar between estrogen users and nonusers.
LATEST NEWS
Older women who get mammograms risk overdiagnosis
TOPLINE:
Women who continue breast cancer screening after age 70 face a considerable risk for overdiagnosis.
METHODOLOGY:
- Overdiagnosis – the risk of detecting and treating cancers that would never have caused issues in a person’s lifetime – is increasingly recognized as a harm of breast cancer screening; however, the scope of the problem among older women remains uncertain.
- To get an idea, investigators linked Medicare claims data with Surveillance, Epidemiology, and End Results (SEER) data for 54,635 women 70 years or older to compare the incidence of breast cancer and breast cancer–specific death among women who continued screening mammography with those who did not.
- The women all had undergone recent screening mammograms and had no history of breast cancer at study entry. Those who had a subsequent mammogram within 3 years were classified as undergoing continued screening while those who did not were classified as not undergoing continued screening.
- Overdiagnosis was defined as the difference in cumulative incidence of breast cancer between screened and unscreened women divided by the cumulative incidence among screened women.
- Results were adjusted for potential confounders, including age, race, and ethnicity.
Continue to: TAKEAWAY...
TAKEAWAY:
- Over 80% of women 70-84 years old and more than 60% of women 85 years or older continued screening.
- Among women 70-74 years old, the adjusted cumulative incidence of breast cancer was 6.1 cases per 100 screened women vs. 4.2 cases per 100 unscreened women; for women aged 75-84 years old, the cumulative incidence was 4.9 per 100 screened women vs. 2.6 per 100 unscreened women, and for women 85 years and older, the cumulative incidence was 2.8 vs. 1.3 per 100, respectively.
- Estimates of overdiagnosis ranged from 31% of breast cancer cases among screened women in the 70-74 age group to 54% of cases in the 85 and older group.
- The researchers found no statistically significant reduction in breast cancer–specific death associated with screening in any age or life-expectancy group. Overdiagnosis appeared to be driven by in situ and localized invasive breast cancer, not advanced breast cancer.
IN PRACTICE:
The proportion of older women who continue to receive screening mammograms and may experience breast cancer overdiagnosis is “considerable” and “increases with advancing age and with decreasing life expectancy,” the authors conclude. Given potential benefits and harms of screening in this population, “patient preferences, including risk tolerance, comfort with uncertainty, and willingness to undergo treatment, are important for informing screening decisions.”
SOURCE: https://www.acpjournals.org/doi/10.7326/M23-0133
https://www.mdedge.com/obgyn/latest-news
CONFERENCE COVERAGE
Offering HPV vaccine at age 9 linked to greater series completion
BALTIMORE—Receiving the first dose of the human papillomavirus (HPV) vaccine at age 9, rather than bundling it with the Tdap and meningitis vaccines, appears to increase the likelihood that children will complete the HPV vaccine series, according to a retrospective cohort study of commercially insured youth presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. The research was published ahead of print in Human Vaccines and Immunotherapeutics.
“These findings are novel because they emphasize starting at age 9, and that is different than prior studies that emphasize bundling of these vaccines,” Kevin Ault, MD, professor and chair of the department of obstetrics and gynecology at Western Michigan University Homer Stryker MD School of Medicine and a former member of the CDC’s Advisory Committee on Immunization Practices, said in an interview.
Dr. Ault was not involved in the study but noted that these findings support the AAP’s recommendation to start the HPV vaccine series at age 9. The Centers for Disease Control and Prevention currently recommends giving the first dose of the HPV vaccine at ages 11-12, at the same time as the Tdap and meningitis vaccines. This recommendation to “bundle” the HPV vaccine with the Tdap and meningitis vaccines aims to facilitate provider-family discussion about the HPV vaccine, ideally reducing parent hesitancy and concerns about the vaccines. Multiple studies have shown improved HPV vaccine uptake when providers offer the HPV vaccine at the same time as the Tdap and meningococcal vaccines.
However, shifts in parents’ attitudes have occurred toward the HPV vaccine since those studies on bundling: Concerns about sexual activity have receded while concerns about safety remain high. The American Academy of Pediatrics and the American Cancer Society both advise starting the HPV vaccine series at age 9, based on evidence showing that more children complete the series when they get the first shot before age 11 compared to getting it at 11 or 12.
What's the diagnosis?
The lesions on the heels are consistent with piezogenic pedal papules. They seem to be more common in women and have been described in families, though a genetic link hasn’t been elucidated. PPP manifests as small, soft, compressible papules on the lateral aspects of the skin on the heels, more noticeable when the patient is standing, and can also present on the wrists and legs. While they may not be a cause for serious concern, understanding their causes, associated conditions, and management is important.
Piezogenic pedal papules are flesh-colored or slightly reddish and can range in size from a few millimeters to a centimeter or more. They are described as benign herniations of elastic tissue and subcutaneous fat through the reticular dermis. The lesions are triggered by increased pressure and compression, such as standing or the application of pressure on the heel. The exact etiology is not known. While piezogenic pedal papules are often asymptomatic, some individuals experience discomfort, itching, or mild pain, particularly when walking or applying pressure to the affected area, especially in patients with Ehlers-Danlos syndrome (EDS).
Individuals who may be at risk of developing these lesions include obese patients, individuals with pes planus, and people who have occupations that require long periods of standing. It can also be seen in athletes who participate in long-distance running or high-impact sports. Piezogenic pedal papules have been described as one of the core skin findings in patients with hypermobile Ehlers-Danlos syndrome (hEDS), which also includes skin hyperextensibility, joint hypermobility, tissue fragility with atrophic cutaneous scars, and abnormal bruising and bleeding. Our patient presented with some of these characteristics (piezogenic papules, soft elastic skin, and some joint hypermobility) but did not fulfill all the criteria for the diagnosis of hEDS or other types of EDS.
The diagnosis of hEDS is based on the 2017 diagnostic criteria checklist. To be diagnosed with hEDS, the patient may have all three parts of the diagnostic criteria. The three domains include generalized joint hypermobility (partially met by our patient), evidence of syndromic features, musculoskeletal complications, and/or family history (she had a few of these criteria, including piezogenic papules and striae), and the exclusion of alternative diagnoses (see references for the PDF checklist). As she does have some features, we diagnosed her with hypermobility spectrum disorder. There is no genetic testing available for the hypermobile spectrum disorder or the hypermobile type of EDS. Given that these patients can present with mitral valve prolapse, she was referred to a cardiac echocardiogram, which was reported as normal.
The diagnosis of PPP is made clinically, and rarely a biopsy is required. Biopsies of the lesions show hyperkeratosis, degeneration of the thin fibrous septa between fat lobules, and subsequent coalescence of fat. If the presentation is atypical, a high-frequency ultrasound can be requested to confirm the physical exam findings.
If the lesions are fixed, firm, and solitary, a diagnosis to consider is juvenile aponeurotic fibroma, which occurs more often in children and adolescents on the wrists and is less common on the ankles. If there is suspicion for this condition, a plain radiograph will show stippled calcifications.
PPP are usually asymptomatic and need no further treatment. When they are symptomatic, conservative management should be considered first, which includes behavioral modifications, weight loss, avoidance of prolonged standing, and reduced foot trauma. If these are not successful, compression socks, heel cups, and other orthotics can be recommended. Intralesional injections of betamethasone and bupivacaine have been reported as an option in patients with symptomatic PPP and a history of EDS.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested reading
Edimo CO et al. Int J Womens Dermatol. 2021 Jan. doi: 10.1016/j.ijwd.2021.01.020. Erratum in: Int J Womens Dermatol. 2021 Jul 31;7(5Part B):869-70.
Brown F, Cook C. Piezogenic Pedal Papule. 2023 Aug 16. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing, 2023. PMID: 29489228.
Levy HP. Hypermobile Ehlers-Danlos Syndrome. 2004 Oct 22 [Updated 2018 Jun 21]. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle: University of Washington, Seattle; 1993-2023. Available from: www.ncbi.nlm.nih.gov/books/NBK1279/.
The International Consortium on Ehlers-Danlos Syndrome & Related Disorders. Diagnostic Criteria for Hypermobile Ehlers-Danlos Syndrome (hEDS). www.ehlers-danlos.com/wp-content/uploads/2017/05/hEDS-Dx-Criteria-checklist-1.pdf.
The lesions on the heels are consistent with piezogenic pedal papules. They seem to be more common in women and have been described in families, though a genetic link hasn’t been elucidated. PPP manifests as small, soft, compressible papules on the lateral aspects of the skin on the heels, more noticeable when the patient is standing, and can also present on the wrists and legs. While they may not be a cause for serious concern, understanding their causes, associated conditions, and management is important.
Piezogenic pedal papules are flesh-colored or slightly reddish and can range in size from a few millimeters to a centimeter or more. They are described as benign herniations of elastic tissue and subcutaneous fat through the reticular dermis. The lesions are triggered by increased pressure and compression, such as standing or the application of pressure on the heel. The exact etiology is not known. While piezogenic pedal papules are often asymptomatic, some individuals experience discomfort, itching, or mild pain, particularly when walking or applying pressure to the affected area, especially in patients with Ehlers-Danlos syndrome (EDS).
Individuals who may be at risk of developing these lesions include obese patients, individuals with pes planus, and people who have occupations that require long periods of standing. It can also be seen in athletes who participate in long-distance running or high-impact sports. Piezogenic pedal papules have been described as one of the core skin findings in patients with hypermobile Ehlers-Danlos syndrome (hEDS), which also includes skin hyperextensibility, joint hypermobility, tissue fragility with atrophic cutaneous scars, and abnormal bruising and bleeding. Our patient presented with some of these characteristics (piezogenic papules, soft elastic skin, and some joint hypermobility) but did not fulfill all the criteria for the diagnosis of hEDS or other types of EDS.
The diagnosis of hEDS is based on the 2017 diagnostic criteria checklist. To be diagnosed with hEDS, the patient may have all three parts of the diagnostic criteria. The three domains include generalized joint hypermobility (partially met by our patient), evidence of syndromic features, musculoskeletal complications, and/or family history (she had a few of these criteria, including piezogenic papules and striae), and the exclusion of alternative diagnoses (see references for the PDF checklist). As she does have some features, we diagnosed her with hypermobility spectrum disorder. There is no genetic testing available for the hypermobile spectrum disorder or the hypermobile type of EDS. Given that these patients can present with mitral valve prolapse, she was referred to a cardiac echocardiogram, which was reported as normal.
The diagnosis of PPP is made clinically, and rarely a biopsy is required. Biopsies of the lesions show hyperkeratosis, degeneration of the thin fibrous septa between fat lobules, and subsequent coalescence of fat. If the presentation is atypical, a high-frequency ultrasound can be requested to confirm the physical exam findings.
If the lesions are fixed, firm, and solitary, a diagnosis to consider is juvenile aponeurotic fibroma, which occurs more often in children and adolescents on the wrists and is less common on the ankles. If there is suspicion for this condition, a plain radiograph will show stippled calcifications.
PPP are usually asymptomatic and need no further treatment. When they are symptomatic, conservative management should be considered first, which includes behavioral modifications, weight loss, avoidance of prolonged standing, and reduced foot trauma. If these are not successful, compression socks, heel cups, and other orthotics can be recommended. Intralesional injections of betamethasone and bupivacaine have been reported as an option in patients with symptomatic PPP and a history of EDS.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested reading
Edimo CO et al. Int J Womens Dermatol. 2021 Jan. doi: 10.1016/j.ijwd.2021.01.020. Erratum in: Int J Womens Dermatol. 2021 Jul 31;7(5Part B):869-70.
Brown F, Cook C. Piezogenic Pedal Papule. 2023 Aug 16. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing, 2023. PMID: 29489228.
Levy HP. Hypermobile Ehlers-Danlos Syndrome. 2004 Oct 22 [Updated 2018 Jun 21]. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle: University of Washington, Seattle; 1993-2023. Available from: www.ncbi.nlm.nih.gov/books/NBK1279/.
The International Consortium on Ehlers-Danlos Syndrome & Related Disorders. Diagnostic Criteria for Hypermobile Ehlers-Danlos Syndrome (hEDS). www.ehlers-danlos.com/wp-content/uploads/2017/05/hEDS-Dx-Criteria-checklist-1.pdf.
The lesions on the heels are consistent with piezogenic pedal papules. They seem to be more common in women and have been described in families, though a genetic link hasn’t been elucidated. PPP manifests as small, soft, compressible papules on the lateral aspects of the skin on the heels, more noticeable when the patient is standing, and can also present on the wrists and legs. While they may not be a cause for serious concern, understanding their causes, associated conditions, and management is important.
Piezogenic pedal papules are flesh-colored or slightly reddish and can range in size from a few millimeters to a centimeter or more. They are described as benign herniations of elastic tissue and subcutaneous fat through the reticular dermis. The lesions are triggered by increased pressure and compression, such as standing or the application of pressure on the heel. The exact etiology is not known. While piezogenic pedal papules are often asymptomatic, some individuals experience discomfort, itching, or mild pain, particularly when walking or applying pressure to the affected area, especially in patients with Ehlers-Danlos syndrome (EDS).
Individuals who may be at risk of developing these lesions include obese patients, individuals with pes planus, and people who have occupations that require long periods of standing. It can also be seen in athletes who participate in long-distance running or high-impact sports. Piezogenic pedal papules have been described as one of the core skin findings in patients with hypermobile Ehlers-Danlos syndrome (hEDS), which also includes skin hyperextensibility, joint hypermobility, tissue fragility with atrophic cutaneous scars, and abnormal bruising and bleeding. Our patient presented with some of these characteristics (piezogenic papules, soft elastic skin, and some joint hypermobility) but did not fulfill all the criteria for the diagnosis of hEDS or other types of EDS.
The diagnosis of hEDS is based on the 2017 diagnostic criteria checklist. To be diagnosed with hEDS, the patient may have all three parts of the diagnostic criteria. The three domains include generalized joint hypermobility (partially met by our patient), evidence of syndromic features, musculoskeletal complications, and/or family history (she had a few of these criteria, including piezogenic papules and striae), and the exclusion of alternative diagnoses (see references for the PDF checklist). As she does have some features, we diagnosed her with hypermobility spectrum disorder. There is no genetic testing available for the hypermobile spectrum disorder or the hypermobile type of EDS. Given that these patients can present with mitral valve prolapse, she was referred to a cardiac echocardiogram, which was reported as normal.
The diagnosis of PPP is made clinically, and rarely a biopsy is required. Biopsies of the lesions show hyperkeratosis, degeneration of the thin fibrous septa between fat lobules, and subsequent coalescence of fat. If the presentation is atypical, a high-frequency ultrasound can be requested to confirm the physical exam findings.
If the lesions are fixed, firm, and solitary, a diagnosis to consider is juvenile aponeurotic fibroma, which occurs more often in children and adolescents on the wrists and is less common on the ankles. If there is suspicion for this condition, a plain radiograph will show stippled calcifications.
PPP are usually asymptomatic and need no further treatment. When they are symptomatic, conservative management should be considered first, which includes behavioral modifications, weight loss, avoidance of prolonged standing, and reduced foot trauma. If these are not successful, compression socks, heel cups, and other orthotics can be recommended. Intralesional injections of betamethasone and bupivacaine have been reported as an option in patients with symptomatic PPP and a history of EDS.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested reading
Edimo CO et al. Int J Womens Dermatol. 2021 Jan. doi: 10.1016/j.ijwd.2021.01.020. Erratum in: Int J Womens Dermatol. 2021 Jul 31;7(5Part B):869-70.
Brown F, Cook C. Piezogenic Pedal Papule. 2023 Aug 16. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing, 2023. PMID: 29489228.
Levy HP. Hypermobile Ehlers-Danlos Syndrome. 2004 Oct 22 [Updated 2018 Jun 21]. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle: University of Washington, Seattle; 1993-2023. Available from: www.ncbi.nlm.nih.gov/books/NBK1279/.
The International Consortium on Ehlers-Danlos Syndrome & Related Disorders. Diagnostic Criteria for Hypermobile Ehlers-Danlos Syndrome (hEDS). www.ehlers-danlos.com/wp-content/uploads/2017/05/hEDS-Dx-Criteria-checklist-1.pdf.
On a physical exam, she has several skin-colored soft papules on the heels when she stands up (Picture 1).
She is not able to touch the floor without bending her knees, and she has normal extension of her arms and knees. She has no bruises or abnormal scars and has some striae on her legs.