User login
EMA warns that omega-3-acid ethyl esters may cause AFib
In its September meeting, the Should atrial fibrillation develop, intake of the medication must be stopped permanently.
Omega-3-acid ethyl esters are used to treat hypertriglyceridemia if lifestyle changes, particularly those related to nutrition, have not been sufficient to lower the blood triglyceride level. Hypertriglyceridemia is a risk factor for coronary heart disease.
During a Periodic Safety Update Single Assessment Procedure, the EMA safety committee analyzed systematic overviews and meta-analyses of randomized, controlled clinical studies. Experts found a dose-dependent increase in the risk for atrial fibrillation in patients with cardiovascular diseases or cardiovascular risk factors who were being treated with omega-3-acid ethyl esters, compared with those treated with placebo. The observed risk was at its highest at a dose of 4 g/d.
The PRAC will recommend an update to the Summary of Product Characteristics for preparations that contain omega-3-acid ethyl esters. The aim is to inform physicians, pharmacists, and patients of the risk for atrial fibrillation. A notification will be sent to health care professionals soon to inform them of further details.
This article was translated from the Medscape German Edition. A version appeared on Medscape.com.
In its September meeting, the Should atrial fibrillation develop, intake of the medication must be stopped permanently.
Omega-3-acid ethyl esters are used to treat hypertriglyceridemia if lifestyle changes, particularly those related to nutrition, have not been sufficient to lower the blood triglyceride level. Hypertriglyceridemia is a risk factor for coronary heart disease.
During a Periodic Safety Update Single Assessment Procedure, the EMA safety committee analyzed systematic overviews and meta-analyses of randomized, controlled clinical studies. Experts found a dose-dependent increase in the risk for atrial fibrillation in patients with cardiovascular diseases or cardiovascular risk factors who were being treated with omega-3-acid ethyl esters, compared with those treated with placebo. The observed risk was at its highest at a dose of 4 g/d.
The PRAC will recommend an update to the Summary of Product Characteristics for preparations that contain omega-3-acid ethyl esters. The aim is to inform physicians, pharmacists, and patients of the risk for atrial fibrillation. A notification will be sent to health care professionals soon to inform them of further details.
This article was translated from the Medscape German Edition. A version appeared on Medscape.com.
In its September meeting, the Should atrial fibrillation develop, intake of the medication must be stopped permanently.
Omega-3-acid ethyl esters are used to treat hypertriglyceridemia if lifestyle changes, particularly those related to nutrition, have not been sufficient to lower the blood triglyceride level. Hypertriglyceridemia is a risk factor for coronary heart disease.
During a Periodic Safety Update Single Assessment Procedure, the EMA safety committee analyzed systematic overviews and meta-analyses of randomized, controlled clinical studies. Experts found a dose-dependent increase in the risk for atrial fibrillation in patients with cardiovascular diseases or cardiovascular risk factors who were being treated with omega-3-acid ethyl esters, compared with those treated with placebo. The observed risk was at its highest at a dose of 4 g/d.
The PRAC will recommend an update to the Summary of Product Characteristics for preparations that contain omega-3-acid ethyl esters. The aim is to inform physicians, pharmacists, and patients of the risk for atrial fibrillation. A notification will be sent to health care professionals soon to inform them of further details.
This article was translated from the Medscape German Edition. A version appeared on Medscape.com.
Painful heels
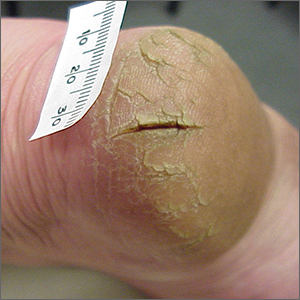
This patient was given a diagnosis of xerosis of the feet, commonly called fissured or cracked heels. Scaling and fissuring are also common in tinea pedis, but the location is often between the toes and there are finer splits and scale.
Xerosis is severely dry skin with hyperkeratosis due to abnormal keratinization;1 it leads to inflexibility and subsequent fissuring of the heel pads. The cracks can be painful and even bleed.
Although the condition is common, well-controlled trials and definitive evidence in the literature are sparse. The authors of one systematic review were unable to draw conclusions regarding the efficacy of various treatments due to wide variation in research methodologies and outcome measures; they did, however, note that urea-containing products (followed by ammonium lactate products) were studied the most.2
In clinical practice, frequently applied topical emollients are recommended. Exfoliating products, including prescription Lac-Hydrin (ammonium lactate 12% cream) and the over-the-counter version, Am-Lactin, may be helpful. Mechanical debridement with a file or pumice stone can be used (with caution) to reduce the hyperkeratotic plaques. If these measures fail, topical steroids may be added to the emollients. In addition, patients have used cyanoacrylate glues to hold the fissures together with a reported reduction in pain.3
This patient had already tried standard topical emollients. She was prescribed ammonium lactate cream to be used as an exfoliating moisturizer topically twice daily along with triamcinolone acetonide (TAC) 0.1% ointment to be applied twice daily. She was instructed to wean off the TAC once the xerosis was controlled with the ammonium lactate cream.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Mazereeuw J, Bonafé JL. La xérose [Xerosis]. Ann Dermatol Venereol. 2002;129(1 Pt 2):137-142
2. Parker J, Scharfbillig R, Jones S. Moisturisers for the treatment of foot xerosis: a systematic review. J Foot Ankle Res. 2017;10:9. doi: 10.1186/s13047-017-0190-9
3. Hashimoto H. Superglue for the treatment of heel fissures. J Am Podiatr Med Assoc. 1999;89:434-435. doi: 10.7547/87507315-89-8-434

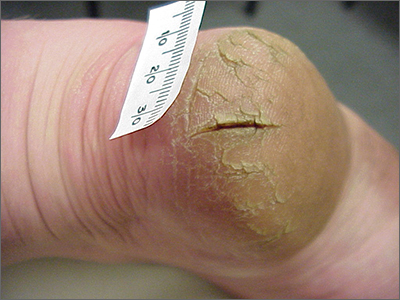
This patient was given a diagnosis of xerosis of the feet, commonly called fissured or cracked heels. Scaling and fissuring are also common in tinea pedis, but the location is often between the toes and there are finer splits and scale.
Xerosis is severely dry skin with hyperkeratosis due to abnormal keratinization;1 it leads to inflexibility and subsequent fissuring of the heel pads. The cracks can be painful and even bleed.
Although the condition is common, well-controlled trials and definitive evidence in the literature are sparse. The authors of one systematic review were unable to draw conclusions regarding the efficacy of various treatments due to wide variation in research methodologies and outcome measures; they did, however, note that urea-containing products (followed by ammonium lactate products) were studied the most.2
In clinical practice, frequently applied topical emollients are recommended. Exfoliating products, including prescription Lac-Hydrin (ammonium lactate 12% cream) and the over-the-counter version, Am-Lactin, may be helpful. Mechanical debridement with a file or pumice stone can be used (with caution) to reduce the hyperkeratotic plaques. If these measures fail, topical steroids may be added to the emollients. In addition, patients have used cyanoacrylate glues to hold the fissures together with a reported reduction in pain.3
This patient had already tried standard topical emollients. She was prescribed ammonium lactate cream to be used as an exfoliating moisturizer topically twice daily along with triamcinolone acetonide (TAC) 0.1% ointment to be applied twice daily. She was instructed to wean off the TAC once the xerosis was controlled with the ammonium lactate cream.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

This patient was given a diagnosis of xerosis of the feet, commonly called fissured or cracked heels. Scaling and fissuring are also common in tinea pedis, but the location is often between the toes and there are finer splits and scale.
Xerosis is severely dry skin with hyperkeratosis due to abnormal keratinization;1 it leads to inflexibility and subsequent fissuring of the heel pads. The cracks can be painful and even bleed.
Although the condition is common, well-controlled trials and definitive evidence in the literature are sparse. The authors of one systematic review were unable to draw conclusions regarding the efficacy of various treatments due to wide variation in research methodologies and outcome measures; they did, however, note that urea-containing products (followed by ammonium lactate products) were studied the most.2
In clinical practice, frequently applied topical emollients are recommended. Exfoliating products, including prescription Lac-Hydrin (ammonium lactate 12% cream) and the over-the-counter version, Am-Lactin, may be helpful. Mechanical debridement with a file or pumice stone can be used (with caution) to reduce the hyperkeratotic plaques. If these measures fail, topical steroids may be added to the emollients. In addition, patients have used cyanoacrylate glues to hold the fissures together with a reported reduction in pain.3
This patient had already tried standard topical emollients. She was prescribed ammonium lactate cream to be used as an exfoliating moisturizer topically twice daily along with triamcinolone acetonide (TAC) 0.1% ointment to be applied twice daily. She was instructed to wean off the TAC once the xerosis was controlled with the ammonium lactate cream.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Mazereeuw J, Bonafé JL. La xérose [Xerosis]. Ann Dermatol Venereol. 2002;129(1 Pt 2):137-142
2. Parker J, Scharfbillig R, Jones S. Moisturisers for the treatment of foot xerosis: a systematic review. J Foot Ankle Res. 2017;10:9. doi: 10.1186/s13047-017-0190-9
3. Hashimoto H. Superglue for the treatment of heel fissures. J Am Podiatr Med Assoc. 1999;89:434-435. doi: 10.7547/87507315-89-8-434
1. Mazereeuw J, Bonafé JL. La xérose [Xerosis]. Ann Dermatol Venereol. 2002;129(1 Pt 2):137-142
2. Parker J, Scharfbillig R, Jones S. Moisturisers for the treatment of foot xerosis: a systematic review. J Foot Ankle Res. 2017;10:9. doi: 10.1186/s13047-017-0190-9
3. Hashimoto H. Superglue for the treatment of heel fissures. J Am Podiatr Med Assoc. 1999;89:434-435. doi: 10.7547/87507315-89-8-434
High and low HDL cholesterol levels linked to dementia risk
TOPLINE:
High and low levels of HDL cholesterol but not levels of LDL cholesterol are associated with an increased risk for dementia in older adults, a new study found.
METHODOLOGY:
- Electronic health record and survey data on 184,367 Kaiser Permanente Northern California participants (median age, 69.5 years) with no history of dementia were taken.
- Cholesterol levels were measured within 2 years of survey completion.
TAKEAWAY:
- There were 25,214 incident cases of dementia reported over an average follow-up of 8.77 years.
- Dementia risk was significantly higher in people with low HDL cholesterol (11-41 mg/dL; adjusted hazard ratio, 1.07; 95% confidence interval, 1.03-1.11) and high HDL cholesterol (> 65 mg/dL; aHR, 1.15; 95% CI, 1.11-1.20).
- The study demonstrates an association between low and high levels of “good” cholesterol but not a causal link.
- There was no significant association between LDL cholesterol and dementia risk.
IN PRACTICE:
“These results support the conclusion that some lipoproteins may be modifiable risk factors for dementia, even in late life,” the authors wrote.
SOURCE:
The study was conducted by Erin L. Ferguson, MPH, department of epidemiology & biostatistics, University of California, San Francisco, and was funded by the National Institutes of Health. It was published online in Neurology.
LIMITATIONS:
There were no adjustments for apo E status and confounding and selection bias.
DISCLOSURES:
The authors report no relevant disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
High and low levels of HDL cholesterol but not levels of LDL cholesterol are associated with an increased risk for dementia in older adults, a new study found.
METHODOLOGY:
- Electronic health record and survey data on 184,367 Kaiser Permanente Northern California participants (median age, 69.5 years) with no history of dementia were taken.
- Cholesterol levels were measured within 2 years of survey completion.
TAKEAWAY:
- There were 25,214 incident cases of dementia reported over an average follow-up of 8.77 years.
- Dementia risk was significantly higher in people with low HDL cholesterol (11-41 mg/dL; adjusted hazard ratio, 1.07; 95% confidence interval, 1.03-1.11) and high HDL cholesterol (> 65 mg/dL; aHR, 1.15; 95% CI, 1.11-1.20).
- The study demonstrates an association between low and high levels of “good” cholesterol but not a causal link.
- There was no significant association between LDL cholesterol and dementia risk.
IN PRACTICE:
“These results support the conclusion that some lipoproteins may be modifiable risk factors for dementia, even in late life,” the authors wrote.
SOURCE:
The study was conducted by Erin L. Ferguson, MPH, department of epidemiology & biostatistics, University of California, San Francisco, and was funded by the National Institutes of Health. It was published online in Neurology.
LIMITATIONS:
There were no adjustments for apo E status and confounding and selection bias.
DISCLOSURES:
The authors report no relevant disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
High and low levels of HDL cholesterol but not levels of LDL cholesterol are associated with an increased risk for dementia in older adults, a new study found.
METHODOLOGY:
- Electronic health record and survey data on 184,367 Kaiser Permanente Northern California participants (median age, 69.5 years) with no history of dementia were taken.
- Cholesterol levels were measured within 2 years of survey completion.
TAKEAWAY:
- There were 25,214 incident cases of dementia reported over an average follow-up of 8.77 years.
- Dementia risk was significantly higher in people with low HDL cholesterol (11-41 mg/dL; adjusted hazard ratio, 1.07; 95% confidence interval, 1.03-1.11) and high HDL cholesterol (> 65 mg/dL; aHR, 1.15; 95% CI, 1.11-1.20).
- The study demonstrates an association between low and high levels of “good” cholesterol but not a causal link.
- There was no significant association between LDL cholesterol and dementia risk.
IN PRACTICE:
“These results support the conclusion that some lipoproteins may be modifiable risk factors for dementia, even in late life,” the authors wrote.
SOURCE:
The study was conducted by Erin L. Ferguson, MPH, department of epidemiology & biostatistics, University of California, San Francisco, and was funded by the National Institutes of Health. It was published online in Neurology.
LIMITATIONS:
There were no adjustments for apo E status and confounding and selection bias.
DISCLOSURES:
The authors report no relevant disclosures.
A version of this article first appeared on Medscape.com.
New RSV vaccine will cut hospitalizations, study shows
, according to research presented at an annual scientific meeting on infectious diseases.
“With RSV maternal vaccination that is associated with clinical efficacy of 69% against severe RSV disease at 6 months, we estimated that up to 200,000 cases can be averted, and that is associated with almost $800 million in total,” presenting author Amy W. Law, PharmD, director of global value and evidence at Pfizer, pointed out during a news briefing.
“RSV is associated with a significant burden in the U.S. and this newly approved and recommended maternal RSV vaccine can have substantial impact in easing some of that burden,” Dr. Law explained.
This study is “particularly timely as we head into RSV peak season,” said briefing moderator Natasha Halasa, MD, MPH, professor of pediatrics, division of pediatric infectious diseases at Vanderbilt University, Nashville, Tenn.
The challenge, said Dr. Halasa, is that uptake of maternal vaccines and vaccines in general is “not optimal,” making increased awareness of this new maternal RSV vaccine important.
Strong efficacy data
Most children are infected with RSV at least once by the time they reach age 2 years. Very young children are at particular risk of severe complications, such as pneumonia or bronchitis.
As reported previously by this news organization, in the randomized, double-blind, placebo-controlled phase 3 study, Pfizer’s maternal RSV vaccine had an almost 82% efficacy against severe RSV infection in infants from birth through the first 90 days of life.
The vaccine also had a 69% efficacy against severe disease through the first 6 months of life. As part of the trial, a total of 7,400 women received a single dose of the vaccine in the late second or third trimester of their pregnancy. There were no signs of safety issues for the mothers or infants.
Based on the results, the U.S. Food and Drug Administration approved the vaccine, known as Abrysvo, in August, to be given between weeks 32 and 36 of pregnancy.
New modeling study
Dr. Law and colleagues modeled the potential public health impact – both clinical and economic – of the maternal RSV vaccine among the population of all pregnant women and their infants born during a 12-month period in the United States. The model focused on severe RSV disease in babies that required medical attention.
According to their model, without widespread use of the maternal RSV vaccine, 48,246 hospitalizations, 144,495 emergency department encounters, and 399,313 outpatient clinic visits related to RSV are projected to occur annually among the U.S. birth cohort of 3.7 million infants younger than 12 months.
With widespread use of the vaccine, annual hospitalizations resulting from infant RSV would fall by 51%, emergency department encounters would decline by 32%, and outpatient clinic visits by 32% – corresponding to a decrease in direct medical costs of about $692 million and indirect nonmedical costs of roughly $110 million.
Dr. Law highlighted two important caveats to the data. “The protections are based on the year-round administration of the vaccine to pregnant women at 32 to 36 weeks’ gestational age, and this is also assuming 100% uptake. Of course, in reality, that most likely is not the case,” she told the briefing.
Dr. Halasa noted that the peak age for severe RSV illness is 3 months and it’s tough to identify infants at highest risk for severe RSV.
Nearly 80% of infants with RSV who are hospitalized do not have an underlying medical condition, “so we don’t even know who those high-risk infants are. That’s why having this vaccine is so exciting,” she told the briefing.
Dr. Halasa said it’s also important to note that infants with severe RSV typically make not just one but multiple visits to the clinic or emergency department, leading to missed days of work for the parent, not to mention the “emotional burden of having your otherwise healthy newborn or young infant in the hospital.”
In addition to Pfizer’s maternal RSV vaccine, the FDA in July approved AstraZeneca’s monoclonal antibody nirsevimab (Beyfortus) for the prevention of RSV in neonates and infants entering their first RSV season, and in children up to 24 months who remain vulnerable to severe RSV disease through their second RSV season.
The study was funded by Pfizer. Dr. Law is employed by Pfizer. Dr. Halasa has received grant and research support from Merck.
A version of this article first appeared on Medscape.com.
, according to research presented at an annual scientific meeting on infectious diseases.
“With RSV maternal vaccination that is associated with clinical efficacy of 69% against severe RSV disease at 6 months, we estimated that up to 200,000 cases can be averted, and that is associated with almost $800 million in total,” presenting author Amy W. Law, PharmD, director of global value and evidence at Pfizer, pointed out during a news briefing.
“RSV is associated with a significant burden in the U.S. and this newly approved and recommended maternal RSV vaccine can have substantial impact in easing some of that burden,” Dr. Law explained.
This study is “particularly timely as we head into RSV peak season,” said briefing moderator Natasha Halasa, MD, MPH, professor of pediatrics, division of pediatric infectious diseases at Vanderbilt University, Nashville, Tenn.
The challenge, said Dr. Halasa, is that uptake of maternal vaccines and vaccines in general is “not optimal,” making increased awareness of this new maternal RSV vaccine important.
Strong efficacy data
Most children are infected with RSV at least once by the time they reach age 2 years. Very young children are at particular risk of severe complications, such as pneumonia or bronchitis.
As reported previously by this news organization, in the randomized, double-blind, placebo-controlled phase 3 study, Pfizer’s maternal RSV vaccine had an almost 82% efficacy against severe RSV infection in infants from birth through the first 90 days of life.
The vaccine also had a 69% efficacy against severe disease through the first 6 months of life. As part of the trial, a total of 7,400 women received a single dose of the vaccine in the late second or third trimester of their pregnancy. There were no signs of safety issues for the mothers or infants.
Based on the results, the U.S. Food and Drug Administration approved the vaccine, known as Abrysvo, in August, to be given between weeks 32 and 36 of pregnancy.
New modeling study
Dr. Law and colleagues modeled the potential public health impact – both clinical and economic – of the maternal RSV vaccine among the population of all pregnant women and their infants born during a 12-month period in the United States. The model focused on severe RSV disease in babies that required medical attention.
According to their model, without widespread use of the maternal RSV vaccine, 48,246 hospitalizations, 144,495 emergency department encounters, and 399,313 outpatient clinic visits related to RSV are projected to occur annually among the U.S. birth cohort of 3.7 million infants younger than 12 months.
With widespread use of the vaccine, annual hospitalizations resulting from infant RSV would fall by 51%, emergency department encounters would decline by 32%, and outpatient clinic visits by 32% – corresponding to a decrease in direct medical costs of about $692 million and indirect nonmedical costs of roughly $110 million.
Dr. Law highlighted two important caveats to the data. “The protections are based on the year-round administration of the vaccine to pregnant women at 32 to 36 weeks’ gestational age, and this is also assuming 100% uptake. Of course, in reality, that most likely is not the case,” she told the briefing.
Dr. Halasa noted that the peak age for severe RSV illness is 3 months and it’s tough to identify infants at highest risk for severe RSV.
Nearly 80% of infants with RSV who are hospitalized do not have an underlying medical condition, “so we don’t even know who those high-risk infants are. That’s why having this vaccine is so exciting,” she told the briefing.
Dr. Halasa said it’s also important to note that infants with severe RSV typically make not just one but multiple visits to the clinic or emergency department, leading to missed days of work for the parent, not to mention the “emotional burden of having your otherwise healthy newborn or young infant in the hospital.”
In addition to Pfizer’s maternal RSV vaccine, the FDA in July approved AstraZeneca’s monoclonal antibody nirsevimab (Beyfortus) for the prevention of RSV in neonates and infants entering their first RSV season, and in children up to 24 months who remain vulnerable to severe RSV disease through their second RSV season.
The study was funded by Pfizer. Dr. Law is employed by Pfizer. Dr. Halasa has received grant and research support from Merck.
A version of this article first appeared on Medscape.com.
, according to research presented at an annual scientific meeting on infectious diseases.
“With RSV maternal vaccination that is associated with clinical efficacy of 69% against severe RSV disease at 6 months, we estimated that up to 200,000 cases can be averted, and that is associated with almost $800 million in total,” presenting author Amy W. Law, PharmD, director of global value and evidence at Pfizer, pointed out during a news briefing.
“RSV is associated with a significant burden in the U.S. and this newly approved and recommended maternal RSV vaccine can have substantial impact in easing some of that burden,” Dr. Law explained.
This study is “particularly timely as we head into RSV peak season,” said briefing moderator Natasha Halasa, MD, MPH, professor of pediatrics, division of pediatric infectious diseases at Vanderbilt University, Nashville, Tenn.
The challenge, said Dr. Halasa, is that uptake of maternal vaccines and vaccines in general is “not optimal,” making increased awareness of this new maternal RSV vaccine important.
Strong efficacy data
Most children are infected with RSV at least once by the time they reach age 2 years. Very young children are at particular risk of severe complications, such as pneumonia or bronchitis.
As reported previously by this news organization, in the randomized, double-blind, placebo-controlled phase 3 study, Pfizer’s maternal RSV vaccine had an almost 82% efficacy against severe RSV infection in infants from birth through the first 90 days of life.
The vaccine also had a 69% efficacy against severe disease through the first 6 months of life. As part of the trial, a total of 7,400 women received a single dose of the vaccine in the late second or third trimester of their pregnancy. There were no signs of safety issues for the mothers or infants.
Based on the results, the U.S. Food and Drug Administration approved the vaccine, known as Abrysvo, in August, to be given between weeks 32 and 36 of pregnancy.
New modeling study
Dr. Law and colleagues modeled the potential public health impact – both clinical and economic – of the maternal RSV vaccine among the population of all pregnant women and their infants born during a 12-month period in the United States. The model focused on severe RSV disease in babies that required medical attention.
According to their model, without widespread use of the maternal RSV vaccine, 48,246 hospitalizations, 144,495 emergency department encounters, and 399,313 outpatient clinic visits related to RSV are projected to occur annually among the U.S. birth cohort of 3.7 million infants younger than 12 months.
With widespread use of the vaccine, annual hospitalizations resulting from infant RSV would fall by 51%, emergency department encounters would decline by 32%, and outpatient clinic visits by 32% – corresponding to a decrease in direct medical costs of about $692 million and indirect nonmedical costs of roughly $110 million.
Dr. Law highlighted two important caveats to the data. “The protections are based on the year-round administration of the vaccine to pregnant women at 32 to 36 weeks’ gestational age, and this is also assuming 100% uptake. Of course, in reality, that most likely is not the case,” she told the briefing.
Dr. Halasa noted that the peak age for severe RSV illness is 3 months and it’s tough to identify infants at highest risk for severe RSV.
Nearly 80% of infants with RSV who are hospitalized do not have an underlying medical condition, “so we don’t even know who those high-risk infants are. That’s why having this vaccine is so exciting,” she told the briefing.
Dr. Halasa said it’s also important to note that infants with severe RSV typically make not just one but multiple visits to the clinic or emergency department, leading to missed days of work for the parent, not to mention the “emotional burden of having your otherwise healthy newborn or young infant in the hospital.”
In addition to Pfizer’s maternal RSV vaccine, the FDA in July approved AstraZeneca’s monoclonal antibody nirsevimab (Beyfortus) for the prevention of RSV in neonates and infants entering their first RSV season, and in children up to 24 months who remain vulnerable to severe RSV disease through their second RSV season.
The study was funded by Pfizer. Dr. Law is employed by Pfizer. Dr. Halasa has received grant and research support from Merck.
A version of this article first appeared on Medscape.com.
FROM IDWEEK 2023
Hopeful insights, no overall HFpEF gains from splanchnic nerve ablation: REBALANCE-HF
It’s still early days for a potential transcatheter technique that tones down sympathetic activation mediating blood volume shifts to the heart and lungs. Such volume transfers can contribute to congestion and acute decompensation in some patients with heart failure. But a randomized trial with negative overall results still may have moved the novel procedure a modest step forward.
The procedure, right-sided splanchnic-nerve ablation for volume management (SAVM), failed to show significant effects on hemodynamics, exercise capacity, natriuretic peptides, or quality of life in a trial covering a broad population of patients with heart failure with preserved ejection fraction (HFpEF).
The study, called REBALANCE-HF, compared ablation of the right greater splanchnic nerve with a sham version of the procedure for any effects on hemodynamic or functional outcomes.
Among such “potential responders,” those undergoing SAVM trended better than patients receiving the sham procedure with respect to hemodynamic, functional, natriuretic peptide, and quality of life endpoints.
The potential predictors of SAVM success included elevated or preserved cardiac output and pulse pressure with exercise or on standing up; appropriate heart-rate exercise responses; and little or no echocardiographic evidence of diastolic dysfunction.
The panel of features might potentially identify patients more likely to respond to the procedure and perhaps sharpen entry criteria in future clinical trials, Marat Fudim, MD, MHS, Duke University Medical Center, Durham, N.C., said in an interview.
Dr. Fudim presented the REBALANCE-HF findings at the annual meeting of the Heart Failure Society of America.
How SAVM works
Sympathetic activation can lead to acute or chronic constriction of vessels in the splanchnic bed within the upper and lower abdomen, one of the body’s largest blood reservoirs, Dr. Fudim explained. Resulting volume shifts to the general circulation, and therefore the heart and lungs, are a normal exercise response that, in HF, can fall out of balance and excessively raise cardiac filling pressure.
Lessened sympathetic tone after unilateral GNS ablation can promote splanchnic venous dilation that reduces intrathoracic blood volume, potentially averting congestion, and decompensation, observed Kavita Sharma, MD, invited discussant for Dr. Fudim’s presentation.
The trial’s potential-responder cohort “seemed able to augment cardiac output in response to stress” and to “maintain or augment their orthostatic pulse pressure,” more effectively than the other participants, said Dr. Sharma, of Johns Hopkins University, Baltimore.
Although the trial was overall negative for 1-month change in pulmonary capillary wedge pressure (PCWP), the primary efficacy endpoint, Dr. Sharma said, it confirmed SAVM as a safe procedure in HFpEF and “ensured its replicability and technical success.”
Future studies should explore ways to characterize unlikely SAVM responders, she proposed. “I would argue these patients are probably more important than even the responders.”
Yet it’s unknown why, for example, cardiac output wouldn’t increase with exercise in a patient with HFpEF. “Is it related to preload insufficiency, right ventricular failure, atrial myopathy, perhaps more restrictive physiology, chronotropic incompetence, or medications – or a combination of the above?”
REBALANCE-HF assigned 90 patients with HFpEF to either the active or sham SAVM groups, 44 and 46 patients, respectively. To be eligible, patients were stable on HF meds and had either elevated natriuretic peptides or, within the past year, at least one HF hospitalization or escalation of intravenous diuretics for worsening HF.
The active and sham control groups fared similarly for the primary PCWP endpoint and for the secondary endpoints of Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score, 6-minute walk distance (6MWD), and natriuretic peptide levels at 6 and 12 months.
Predicting SAVM response
In analysis limited to potential responders, PCWP, KCCQ, 6MWD, and natriuretic peptide outcomes for patients were combined into z scores, a single metric that reflects multiple outcomes, Dr. Fudim explained.
The z scores were derived for tertiles of patients in subgroups defined by a range of parameters that included demographics, medical history, and hemodynamic and echocardiographic variables.
Four such variables were found to interact across tertiles in a way that suggested their value as SAVM outcome predictors and were then used to select the cohort of potential responders. The variables were exertion-related changes in cardiac index, pulse pressure, and heart rate, and mitral E/A ratio – the latter a measure of diastolic dysfunction.
Among potential responders, those who underwent SAVM showed a 2.9–mm Hg steeper drop in peak PCWP at 1 month (P = .02), compared with patients getting the sham procedure.
They also bested control patients at both 6 and 12 months for KCCQ score, 6MWD, and natriuretic peptide levels, the latter of which fell in the SAVM group and climbed in control patients at both follow-ups.
“Hypothetically, it makes sense” to target the splanchnic nerve in HFpEF, and indeed in HF with reduced ejection fraction, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, said in an interview.
And should SAVM enter the mainstream, it would definitely be important to identify “the right” patients for such an invasive procedure, those likely to show “efficacy with a good safety margin,” said Dr. Bozkurt, who was not associated with REBALANCE-HF.
But the trial, she said, “unfortunately did not give real signals of outcome benefit.”
REBALANCE-HF was supported by Axon Therapies. Dr. Fudim disclosed consulting, receiving royalties, or having ownership or equity in Axon Therapies. Dr. Sharma disclosed receiving honoraria for speaking from Novartis and Janssen and serving on an advisory board or consulting for Novartis, Janssen, and Bayer. Dr. Bozkurt disclosed receiving honoraria from AstraZeneca, Baxter Health Care, and Sanofi Aventis and having other relationships with Renovacor, Respicardia, Abbott Vascular, Liva Nova, Vifor, and Cardurion.
A version of this article first appeared on Medscape.com.
It’s still early days for a potential transcatheter technique that tones down sympathetic activation mediating blood volume shifts to the heart and lungs. Such volume transfers can contribute to congestion and acute decompensation in some patients with heart failure. But a randomized trial with negative overall results still may have moved the novel procedure a modest step forward.
The procedure, right-sided splanchnic-nerve ablation for volume management (SAVM), failed to show significant effects on hemodynamics, exercise capacity, natriuretic peptides, or quality of life in a trial covering a broad population of patients with heart failure with preserved ejection fraction (HFpEF).
The study, called REBALANCE-HF, compared ablation of the right greater splanchnic nerve with a sham version of the procedure for any effects on hemodynamic or functional outcomes.
Among such “potential responders,” those undergoing SAVM trended better than patients receiving the sham procedure with respect to hemodynamic, functional, natriuretic peptide, and quality of life endpoints.
The potential predictors of SAVM success included elevated or preserved cardiac output and pulse pressure with exercise or on standing up; appropriate heart-rate exercise responses; and little or no echocardiographic evidence of diastolic dysfunction.
The panel of features might potentially identify patients more likely to respond to the procedure and perhaps sharpen entry criteria in future clinical trials, Marat Fudim, MD, MHS, Duke University Medical Center, Durham, N.C., said in an interview.
Dr. Fudim presented the REBALANCE-HF findings at the annual meeting of the Heart Failure Society of America.
How SAVM works
Sympathetic activation can lead to acute or chronic constriction of vessels in the splanchnic bed within the upper and lower abdomen, one of the body’s largest blood reservoirs, Dr. Fudim explained. Resulting volume shifts to the general circulation, and therefore the heart and lungs, are a normal exercise response that, in HF, can fall out of balance and excessively raise cardiac filling pressure.
Lessened sympathetic tone after unilateral GNS ablation can promote splanchnic venous dilation that reduces intrathoracic blood volume, potentially averting congestion, and decompensation, observed Kavita Sharma, MD, invited discussant for Dr. Fudim’s presentation.
The trial’s potential-responder cohort “seemed able to augment cardiac output in response to stress” and to “maintain or augment their orthostatic pulse pressure,” more effectively than the other participants, said Dr. Sharma, of Johns Hopkins University, Baltimore.
Although the trial was overall negative for 1-month change in pulmonary capillary wedge pressure (PCWP), the primary efficacy endpoint, Dr. Sharma said, it confirmed SAVM as a safe procedure in HFpEF and “ensured its replicability and technical success.”
Future studies should explore ways to characterize unlikely SAVM responders, she proposed. “I would argue these patients are probably more important than even the responders.”
Yet it’s unknown why, for example, cardiac output wouldn’t increase with exercise in a patient with HFpEF. “Is it related to preload insufficiency, right ventricular failure, atrial myopathy, perhaps more restrictive physiology, chronotropic incompetence, or medications – or a combination of the above?”
REBALANCE-HF assigned 90 patients with HFpEF to either the active or sham SAVM groups, 44 and 46 patients, respectively. To be eligible, patients were stable on HF meds and had either elevated natriuretic peptides or, within the past year, at least one HF hospitalization or escalation of intravenous diuretics for worsening HF.
The active and sham control groups fared similarly for the primary PCWP endpoint and for the secondary endpoints of Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score, 6-minute walk distance (6MWD), and natriuretic peptide levels at 6 and 12 months.
Predicting SAVM response
In analysis limited to potential responders, PCWP, KCCQ, 6MWD, and natriuretic peptide outcomes for patients were combined into z scores, a single metric that reflects multiple outcomes, Dr. Fudim explained.
The z scores were derived for tertiles of patients in subgroups defined by a range of parameters that included demographics, medical history, and hemodynamic and echocardiographic variables.
Four such variables were found to interact across tertiles in a way that suggested their value as SAVM outcome predictors and were then used to select the cohort of potential responders. The variables were exertion-related changes in cardiac index, pulse pressure, and heart rate, and mitral E/A ratio – the latter a measure of diastolic dysfunction.
Among potential responders, those who underwent SAVM showed a 2.9–mm Hg steeper drop in peak PCWP at 1 month (P = .02), compared with patients getting the sham procedure.
They also bested control patients at both 6 and 12 months for KCCQ score, 6MWD, and natriuretic peptide levels, the latter of which fell in the SAVM group and climbed in control patients at both follow-ups.
“Hypothetically, it makes sense” to target the splanchnic nerve in HFpEF, and indeed in HF with reduced ejection fraction, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, said in an interview.
And should SAVM enter the mainstream, it would definitely be important to identify “the right” patients for such an invasive procedure, those likely to show “efficacy with a good safety margin,” said Dr. Bozkurt, who was not associated with REBALANCE-HF.
But the trial, she said, “unfortunately did not give real signals of outcome benefit.”
REBALANCE-HF was supported by Axon Therapies. Dr. Fudim disclosed consulting, receiving royalties, or having ownership or equity in Axon Therapies. Dr. Sharma disclosed receiving honoraria for speaking from Novartis and Janssen and serving on an advisory board or consulting for Novartis, Janssen, and Bayer. Dr. Bozkurt disclosed receiving honoraria from AstraZeneca, Baxter Health Care, and Sanofi Aventis and having other relationships with Renovacor, Respicardia, Abbott Vascular, Liva Nova, Vifor, and Cardurion.
A version of this article first appeared on Medscape.com.
It’s still early days for a potential transcatheter technique that tones down sympathetic activation mediating blood volume shifts to the heart and lungs. Such volume transfers can contribute to congestion and acute decompensation in some patients with heart failure. But a randomized trial with negative overall results still may have moved the novel procedure a modest step forward.
The procedure, right-sided splanchnic-nerve ablation for volume management (SAVM), failed to show significant effects on hemodynamics, exercise capacity, natriuretic peptides, or quality of life in a trial covering a broad population of patients with heart failure with preserved ejection fraction (HFpEF).
The study, called REBALANCE-HF, compared ablation of the right greater splanchnic nerve with a sham version of the procedure for any effects on hemodynamic or functional outcomes.
Among such “potential responders,” those undergoing SAVM trended better than patients receiving the sham procedure with respect to hemodynamic, functional, natriuretic peptide, and quality of life endpoints.
The potential predictors of SAVM success included elevated or preserved cardiac output and pulse pressure with exercise or on standing up; appropriate heart-rate exercise responses; and little or no echocardiographic evidence of diastolic dysfunction.
The panel of features might potentially identify patients more likely to respond to the procedure and perhaps sharpen entry criteria in future clinical trials, Marat Fudim, MD, MHS, Duke University Medical Center, Durham, N.C., said in an interview.
Dr. Fudim presented the REBALANCE-HF findings at the annual meeting of the Heart Failure Society of America.
How SAVM works
Sympathetic activation can lead to acute or chronic constriction of vessels in the splanchnic bed within the upper and lower abdomen, one of the body’s largest blood reservoirs, Dr. Fudim explained. Resulting volume shifts to the general circulation, and therefore the heart and lungs, are a normal exercise response that, in HF, can fall out of balance and excessively raise cardiac filling pressure.
Lessened sympathetic tone after unilateral GNS ablation can promote splanchnic venous dilation that reduces intrathoracic blood volume, potentially averting congestion, and decompensation, observed Kavita Sharma, MD, invited discussant for Dr. Fudim’s presentation.
The trial’s potential-responder cohort “seemed able to augment cardiac output in response to stress” and to “maintain or augment their orthostatic pulse pressure,” more effectively than the other participants, said Dr. Sharma, of Johns Hopkins University, Baltimore.
Although the trial was overall negative for 1-month change in pulmonary capillary wedge pressure (PCWP), the primary efficacy endpoint, Dr. Sharma said, it confirmed SAVM as a safe procedure in HFpEF and “ensured its replicability and technical success.”
Future studies should explore ways to characterize unlikely SAVM responders, she proposed. “I would argue these patients are probably more important than even the responders.”
Yet it’s unknown why, for example, cardiac output wouldn’t increase with exercise in a patient with HFpEF. “Is it related to preload insufficiency, right ventricular failure, atrial myopathy, perhaps more restrictive physiology, chronotropic incompetence, or medications – or a combination of the above?”
REBALANCE-HF assigned 90 patients with HFpEF to either the active or sham SAVM groups, 44 and 46 patients, respectively. To be eligible, patients were stable on HF meds and had either elevated natriuretic peptides or, within the past year, at least one HF hospitalization or escalation of intravenous diuretics for worsening HF.
The active and sham control groups fared similarly for the primary PCWP endpoint and for the secondary endpoints of Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score, 6-minute walk distance (6MWD), and natriuretic peptide levels at 6 and 12 months.
Predicting SAVM response
In analysis limited to potential responders, PCWP, KCCQ, 6MWD, and natriuretic peptide outcomes for patients were combined into z scores, a single metric that reflects multiple outcomes, Dr. Fudim explained.
The z scores were derived for tertiles of patients in subgroups defined by a range of parameters that included demographics, medical history, and hemodynamic and echocardiographic variables.
Four such variables were found to interact across tertiles in a way that suggested their value as SAVM outcome predictors and were then used to select the cohort of potential responders. The variables were exertion-related changes in cardiac index, pulse pressure, and heart rate, and mitral E/A ratio – the latter a measure of diastolic dysfunction.
Among potential responders, those who underwent SAVM showed a 2.9–mm Hg steeper drop in peak PCWP at 1 month (P = .02), compared with patients getting the sham procedure.
They also bested control patients at both 6 and 12 months for KCCQ score, 6MWD, and natriuretic peptide levels, the latter of which fell in the SAVM group and climbed in control patients at both follow-ups.
“Hypothetically, it makes sense” to target the splanchnic nerve in HFpEF, and indeed in HF with reduced ejection fraction, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, said in an interview.
And should SAVM enter the mainstream, it would definitely be important to identify “the right” patients for such an invasive procedure, those likely to show “efficacy with a good safety margin,” said Dr. Bozkurt, who was not associated with REBALANCE-HF.
But the trial, she said, “unfortunately did not give real signals of outcome benefit.”
REBALANCE-HF was supported by Axon Therapies. Dr. Fudim disclosed consulting, receiving royalties, or having ownership or equity in Axon Therapies. Dr. Sharma disclosed receiving honoraria for speaking from Novartis and Janssen and serving on an advisory board or consulting for Novartis, Janssen, and Bayer. Dr. Bozkurt disclosed receiving honoraria from AstraZeneca, Baxter Health Care, and Sanofi Aventis and having other relationships with Renovacor, Respicardia, Abbott Vascular, Liva Nova, Vifor, and Cardurion.
A version of this article first appeared on Medscape.com.
FROM HFSA 2023
Ocrelizumab benefit confirmed in older patients with MS
MILAN – they say.
The researchers studied about 700 patients with MS aged 60 years and older from an international database, comparing outcomes with the anti-CD20 monoclonal antibody ocrelizumab versus those for interferon/glatiramer acetate (BRACE). They found ocrelizumab significantly reduced the annual rate of relapses, although after adjustments, patients overall faced a relapse rate of less than 0.1 per year. There were also no significant differences in either disability progression or improvement between the two treatments.
“We believe this study is unique in that ocrelizumab demonstrates a very clear differential treatment benefit in this age group,” said study presenter Yi Chao Foong, MD, department of neuroscience, Monash University, Melbourne. “However, this has to be balanced against the fact that overall relapse activity is extremely low in people with MS over the age of 60. We believe that this study adds valuable, real-world data for nuanced benefit versus risk DMT discussions with for older adults with MS.”
The findings were presented at the 9th Joint ECTRIMS-ACTRIMS meeting.
Lack of data in older patients
Dr. Fong explained the comparative efficacy of disease-modifying therapies (DMTs) has not been demonstrated in older people with MS, as all landmark trials to date have excluded people older than age 60 years. He underlined, however, that the inflammatory aspect of MS reduces with age, when neurodegenerative processes begin to predominate.
“This, combined with increased risk of acute infections in older adults have raised concerns over the benefit ratios of DMTs in this age group,” Dr. Fong said.
This has led to several de-escalation studies in older patients already on treatment for MS, but with “varied results.”
One study, published earlier in 2023, was unable to conclude whether DMT discontinuation was noninferior to continuation in older patients with no recent relapse or new MRI activity.
To investigate further, the Australian team used the MSBase database to study patients with a confirmed MS diagnosis who had started or switched to ocrelizumab or BRACE when older than 60 years of age.
They were also required to have undergone an Expanded Disability Status Scale (EDSS) assessment around the time of the initiation of DMT. In all, 675 patients met the inclusion criteria, of whom 248 started with ocrelizumab and 427 with BRACE.
The treatment groups were well balanced, although baseline EDSS scores were higher in patients given ocrelizumab, at 5.22 versus 3.89 with BRACE (P = .05), and they had a lower relapse rate prior in the year (P = .01) and 2 years (P = .02) prior to baseline.
Only relapse rates reduced
With more than 571 patient-years of follow-up, there were eight relapses in patients treated with ocrelizumab, compared with 182 relapses during 2238 patient-years among those given BRACE.
The team then performed propensity matching based on patient age, disease duration, sex, baseline EDSS, prior relapses, and prior DMTs.
They found that, over a median follow-up of 2.47 years for ocrelizumab and 4.48 years for BRACE, there was a lower rate of relapse with ocrelizumab, at a weighted annualized relapse rate of 0.01 versus 0.08 (P < .0001). This, they calculated, equated to an ARR ratio in favor of ocrelizumab of 0.15 (P < .01).
The time to first relapse was also longer for ocrelizumab versus BRACE, at a weighted hazard ratio for relapse of 0.11 (P < .001) and with, as Dr. Fong highlighted, separation of the curves at 5 months.
Over a follow-up duration of 3.6 years, there was, however, no significant difference in confirmed disability progression between the two treatments (P = .31), with similar results seen for confirmed disability improvement (P = .92).
Dr. Fong noted the study was limited by an inherent treatment indication bias, affecting the sensitivity analysis and weighing, while assessment of confirmed disability progression and confirmed disability improvement was hampered by the relatively short follow-up period and the lack of data on comorbidities.
He also highlighted the lack of safety data for the study population, as well as the lack of MRI.
Muddling the data
Approached for comment, Pavan Bhargava, MBBS, MD, associate professor of neurology, Johns Hopkins Precision Medicine Center of Excellence for Multiple Sclerosis, Baltimore, pointed out the study is based on retrospective data.
“The main question that we normally come up against in clinical practice, once people are older, is: What do you do with their treatment?” he asked.
This, Dr. Bhargava said, was the question that was addressed in the previous de-escalation studies.
The current study “actually answered a completely different question: If you were starting or changing a treatment after 60, which one would be better to choose?” This is a “much rarer scenario,” he said.
The results nevertheless showed what is seen in younger patients; in other words, “a more efficacious treatment is more effective at reducing relapses than a less efficacious treatment, even though overall the number of relapses is quite low,” Dr. Bhargava said.
“The other problem,” he added, is the study included “not just relapsing but also progressive patients, so that kind of muddles the data a little bit.”
Consequently, “it’s hard to really make a definitive conclusion” from the results, Dr. Bhargava concluded.
No funding was declared. Dr. Fong declares relationships with Biogen, National Health and Medical Research Council, Multiple Sclerosis Research Australia, and the Australian and New Zealand Association of Neurologists. Several coauthors also declared financial relationships with industry.
A version of this article first appeared on Medscape.com.
MILAN – they say.
The researchers studied about 700 patients with MS aged 60 years and older from an international database, comparing outcomes with the anti-CD20 monoclonal antibody ocrelizumab versus those for interferon/glatiramer acetate (BRACE). They found ocrelizumab significantly reduced the annual rate of relapses, although after adjustments, patients overall faced a relapse rate of less than 0.1 per year. There were also no significant differences in either disability progression or improvement between the two treatments.
“We believe this study is unique in that ocrelizumab demonstrates a very clear differential treatment benefit in this age group,” said study presenter Yi Chao Foong, MD, department of neuroscience, Monash University, Melbourne. “However, this has to be balanced against the fact that overall relapse activity is extremely low in people with MS over the age of 60. We believe that this study adds valuable, real-world data for nuanced benefit versus risk DMT discussions with for older adults with MS.”
The findings were presented at the 9th Joint ECTRIMS-ACTRIMS meeting.
Lack of data in older patients
Dr. Fong explained the comparative efficacy of disease-modifying therapies (DMTs) has not been demonstrated in older people with MS, as all landmark trials to date have excluded people older than age 60 years. He underlined, however, that the inflammatory aspect of MS reduces with age, when neurodegenerative processes begin to predominate.
“This, combined with increased risk of acute infections in older adults have raised concerns over the benefit ratios of DMTs in this age group,” Dr. Fong said.
This has led to several de-escalation studies in older patients already on treatment for MS, but with “varied results.”
One study, published earlier in 2023, was unable to conclude whether DMT discontinuation was noninferior to continuation in older patients with no recent relapse or new MRI activity.
To investigate further, the Australian team used the MSBase database to study patients with a confirmed MS diagnosis who had started or switched to ocrelizumab or BRACE when older than 60 years of age.
They were also required to have undergone an Expanded Disability Status Scale (EDSS) assessment around the time of the initiation of DMT. In all, 675 patients met the inclusion criteria, of whom 248 started with ocrelizumab and 427 with BRACE.
The treatment groups were well balanced, although baseline EDSS scores were higher in patients given ocrelizumab, at 5.22 versus 3.89 with BRACE (P = .05), and they had a lower relapse rate prior in the year (P = .01) and 2 years (P = .02) prior to baseline.
Only relapse rates reduced
With more than 571 patient-years of follow-up, there were eight relapses in patients treated with ocrelizumab, compared with 182 relapses during 2238 patient-years among those given BRACE.
The team then performed propensity matching based on patient age, disease duration, sex, baseline EDSS, prior relapses, and prior DMTs.
They found that, over a median follow-up of 2.47 years for ocrelizumab and 4.48 years for BRACE, there was a lower rate of relapse with ocrelizumab, at a weighted annualized relapse rate of 0.01 versus 0.08 (P < .0001). This, they calculated, equated to an ARR ratio in favor of ocrelizumab of 0.15 (P < .01).
The time to first relapse was also longer for ocrelizumab versus BRACE, at a weighted hazard ratio for relapse of 0.11 (P < .001) and with, as Dr. Fong highlighted, separation of the curves at 5 months.
Over a follow-up duration of 3.6 years, there was, however, no significant difference in confirmed disability progression between the two treatments (P = .31), with similar results seen for confirmed disability improvement (P = .92).
Dr. Fong noted the study was limited by an inherent treatment indication bias, affecting the sensitivity analysis and weighing, while assessment of confirmed disability progression and confirmed disability improvement was hampered by the relatively short follow-up period and the lack of data on comorbidities.
He also highlighted the lack of safety data for the study population, as well as the lack of MRI.
Muddling the data
Approached for comment, Pavan Bhargava, MBBS, MD, associate professor of neurology, Johns Hopkins Precision Medicine Center of Excellence for Multiple Sclerosis, Baltimore, pointed out the study is based on retrospective data.
“The main question that we normally come up against in clinical practice, once people are older, is: What do you do with their treatment?” he asked.
This, Dr. Bhargava said, was the question that was addressed in the previous de-escalation studies.
The current study “actually answered a completely different question: If you were starting or changing a treatment after 60, which one would be better to choose?” This is a “much rarer scenario,” he said.
The results nevertheless showed what is seen in younger patients; in other words, “a more efficacious treatment is more effective at reducing relapses than a less efficacious treatment, even though overall the number of relapses is quite low,” Dr. Bhargava said.
“The other problem,” he added, is the study included “not just relapsing but also progressive patients, so that kind of muddles the data a little bit.”
Consequently, “it’s hard to really make a definitive conclusion” from the results, Dr. Bhargava concluded.
No funding was declared. Dr. Fong declares relationships with Biogen, National Health and Medical Research Council, Multiple Sclerosis Research Australia, and the Australian and New Zealand Association of Neurologists. Several coauthors also declared financial relationships with industry.
A version of this article first appeared on Medscape.com.
MILAN – they say.
The researchers studied about 700 patients with MS aged 60 years and older from an international database, comparing outcomes with the anti-CD20 monoclonal antibody ocrelizumab versus those for interferon/glatiramer acetate (BRACE). They found ocrelizumab significantly reduced the annual rate of relapses, although after adjustments, patients overall faced a relapse rate of less than 0.1 per year. There were also no significant differences in either disability progression or improvement between the two treatments.
“We believe this study is unique in that ocrelizumab demonstrates a very clear differential treatment benefit in this age group,” said study presenter Yi Chao Foong, MD, department of neuroscience, Monash University, Melbourne. “However, this has to be balanced against the fact that overall relapse activity is extremely low in people with MS over the age of 60. We believe that this study adds valuable, real-world data for nuanced benefit versus risk DMT discussions with for older adults with MS.”
The findings were presented at the 9th Joint ECTRIMS-ACTRIMS meeting.
Lack of data in older patients
Dr. Fong explained the comparative efficacy of disease-modifying therapies (DMTs) has not been demonstrated in older people with MS, as all landmark trials to date have excluded people older than age 60 years. He underlined, however, that the inflammatory aspect of MS reduces with age, when neurodegenerative processes begin to predominate.
“This, combined with increased risk of acute infections in older adults have raised concerns over the benefit ratios of DMTs in this age group,” Dr. Fong said.
This has led to several de-escalation studies in older patients already on treatment for MS, but with “varied results.”
One study, published earlier in 2023, was unable to conclude whether DMT discontinuation was noninferior to continuation in older patients with no recent relapse or new MRI activity.
To investigate further, the Australian team used the MSBase database to study patients with a confirmed MS diagnosis who had started or switched to ocrelizumab or BRACE when older than 60 years of age.
They were also required to have undergone an Expanded Disability Status Scale (EDSS) assessment around the time of the initiation of DMT. In all, 675 patients met the inclusion criteria, of whom 248 started with ocrelizumab and 427 with BRACE.
The treatment groups were well balanced, although baseline EDSS scores were higher in patients given ocrelizumab, at 5.22 versus 3.89 with BRACE (P = .05), and they had a lower relapse rate prior in the year (P = .01) and 2 years (P = .02) prior to baseline.
Only relapse rates reduced
With more than 571 patient-years of follow-up, there were eight relapses in patients treated with ocrelizumab, compared with 182 relapses during 2238 patient-years among those given BRACE.
The team then performed propensity matching based on patient age, disease duration, sex, baseline EDSS, prior relapses, and prior DMTs.
They found that, over a median follow-up of 2.47 years for ocrelizumab and 4.48 years for BRACE, there was a lower rate of relapse with ocrelizumab, at a weighted annualized relapse rate of 0.01 versus 0.08 (P < .0001). This, they calculated, equated to an ARR ratio in favor of ocrelizumab of 0.15 (P < .01).
The time to first relapse was also longer for ocrelizumab versus BRACE, at a weighted hazard ratio for relapse of 0.11 (P < .001) and with, as Dr. Fong highlighted, separation of the curves at 5 months.
Over a follow-up duration of 3.6 years, there was, however, no significant difference in confirmed disability progression between the two treatments (P = .31), with similar results seen for confirmed disability improvement (P = .92).
Dr. Fong noted the study was limited by an inherent treatment indication bias, affecting the sensitivity analysis and weighing, while assessment of confirmed disability progression and confirmed disability improvement was hampered by the relatively short follow-up period and the lack of data on comorbidities.
He also highlighted the lack of safety data for the study population, as well as the lack of MRI.
Muddling the data
Approached for comment, Pavan Bhargava, MBBS, MD, associate professor of neurology, Johns Hopkins Precision Medicine Center of Excellence for Multiple Sclerosis, Baltimore, pointed out the study is based on retrospective data.
“The main question that we normally come up against in clinical practice, once people are older, is: What do you do with their treatment?” he asked.
This, Dr. Bhargava said, was the question that was addressed in the previous de-escalation studies.
The current study “actually answered a completely different question: If you were starting or changing a treatment after 60, which one would be better to choose?” This is a “much rarer scenario,” he said.
The results nevertheless showed what is seen in younger patients; in other words, “a more efficacious treatment is more effective at reducing relapses than a less efficacious treatment, even though overall the number of relapses is quite low,” Dr. Bhargava said.
“The other problem,” he added, is the study included “not just relapsing but also progressive patients, so that kind of muddles the data a little bit.”
Consequently, “it’s hard to really make a definitive conclusion” from the results, Dr. Bhargava concluded.
No funding was declared. Dr. Fong declares relationships with Biogen, National Health and Medical Research Council, Multiple Sclerosis Research Australia, and the Australian and New Zealand Association of Neurologists. Several coauthors also declared financial relationships with industry.
A version of this article first appeared on Medscape.com.
AT ECTRIMS 2023
AI detects hidden, potentially curable pancreatic cancers
TOPLINE:
new research suggests.
METHODOLOGY:
- The researchers utilized a diverse dataset of 3,014 CT scans: 1,105 diagnostic CT scans with pancreatic ductal adenocarcinoma (PDA) and 1,909 control CT scans.
- Of the total, 696 diagnostic CT scans with PDA and 1,080 control CT scans were used as an AI model training subset, and 409 CT scans with PDA and 829 control CT scans were used as an intramural hold-out test subset.
- The model was also tested on a simulated cohort that evaluated the risk for PDA in new-onset diabetes; multicenter public datasets (194 CT scans with PDA and 80 controls); and a cohort of 100 prediagnostic CT scans, incidentally acquired 3-36 months prior to PDA being diagnosed, and 134 controls.
TAKEAWAY:
- The model correctly classified 360 CT scans with PDA (88%) and 783 control CT scans (94%) in the intramural test subset. The mean accuracy was 0.92, the area under the receiver operating characteristic curve was 0.97, sensitivity was 0.88, and specificity was 0.95.
- On heat maps, activation areas overlapped with the tumor in 350 of 360 CT scans (97%).
- Performance was high across tumor stages, with sensitivities of 0.80, 0.87, 0.95, and 1.0 on T1 through T4 stages, respectively. Performance was comparable for hypodense versus isodense tumors (sensitivity of 0.90 vs. 0.82, respectively), patient demographics, CT slice thicknesses, and vendors.
- Findings were generalizable on both the simulated cohort (accuracy, 0.95; area under the ROC curve, 0.97) and public datasets (accuracy, 0.86; AUROC, 0.9).
- Occult PDA was detected on prediagnostic CT scans at a median 475 days before clinical diagnosis. Accuracy was 0.84, AUROC was 0.91, sensitivity was 0.75, and specificity was 0.9.
IN PRACTICE:
“Artificial intelligence model could mitigate the inadequacies of imaging and the diagnostic errors in interpretation, which often contribute to delayed diagnosis of pancreas cancer. In combination with emerging blood-based biomarkers, such a model could be evaluated to screen for sporadic cancer in ongoing trials of high-risk cohorts such as the Early Detection Initiative (NCT04662879).”
SOURCE:
Panagiotis Korfiatis, PhD, of Mayo Clinic, Rochester, Minn., led the study, which was published online in Gastroenterology.
LIMITATIONS:
The retrospective design is prone to selection bias. Results are presented dichotomously as either cancer or control. These are preliminary insights, and prospective clinical trials that incorporate epidemiological risk factors and emerging blood-based biomarkers are needed to further evaluate the model’s performance.
DISCLOSURES:
The research was supported by the National Cancer Institute, the Centene Charitable Foundation, and the Champions for Hope Pancreatic Cancer Research Program of the Funk Zitiello Foundation. One author received an institutional research grant from Sofie Biosciences and Clovis Oncology, is on the BlueStar Genomics advisory board (ad hoc), and is a consultant for Bayer Healthcare, Candel Therapeutics, and UWorld. The remaining authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
new research suggests.
METHODOLOGY:
- The researchers utilized a diverse dataset of 3,014 CT scans: 1,105 diagnostic CT scans with pancreatic ductal adenocarcinoma (PDA) and 1,909 control CT scans.
- Of the total, 696 diagnostic CT scans with PDA and 1,080 control CT scans were used as an AI model training subset, and 409 CT scans with PDA and 829 control CT scans were used as an intramural hold-out test subset.
- The model was also tested on a simulated cohort that evaluated the risk for PDA in new-onset diabetes; multicenter public datasets (194 CT scans with PDA and 80 controls); and a cohort of 100 prediagnostic CT scans, incidentally acquired 3-36 months prior to PDA being diagnosed, and 134 controls.
TAKEAWAY:
- The model correctly classified 360 CT scans with PDA (88%) and 783 control CT scans (94%) in the intramural test subset. The mean accuracy was 0.92, the area under the receiver operating characteristic curve was 0.97, sensitivity was 0.88, and specificity was 0.95.
- On heat maps, activation areas overlapped with the tumor in 350 of 360 CT scans (97%).
- Performance was high across tumor stages, with sensitivities of 0.80, 0.87, 0.95, and 1.0 on T1 through T4 stages, respectively. Performance was comparable for hypodense versus isodense tumors (sensitivity of 0.90 vs. 0.82, respectively), patient demographics, CT slice thicknesses, and vendors.
- Findings were generalizable on both the simulated cohort (accuracy, 0.95; area under the ROC curve, 0.97) and public datasets (accuracy, 0.86; AUROC, 0.9).
- Occult PDA was detected on prediagnostic CT scans at a median 475 days before clinical diagnosis. Accuracy was 0.84, AUROC was 0.91, sensitivity was 0.75, and specificity was 0.9.
IN PRACTICE:
“Artificial intelligence model could mitigate the inadequacies of imaging and the diagnostic errors in interpretation, which often contribute to delayed diagnosis of pancreas cancer. In combination with emerging blood-based biomarkers, such a model could be evaluated to screen for sporadic cancer in ongoing trials of high-risk cohorts such as the Early Detection Initiative (NCT04662879).”
SOURCE:
Panagiotis Korfiatis, PhD, of Mayo Clinic, Rochester, Minn., led the study, which was published online in Gastroenterology.
LIMITATIONS:
The retrospective design is prone to selection bias. Results are presented dichotomously as either cancer or control. These are preliminary insights, and prospective clinical trials that incorporate epidemiological risk factors and emerging blood-based biomarkers are needed to further evaluate the model’s performance.
DISCLOSURES:
The research was supported by the National Cancer Institute, the Centene Charitable Foundation, and the Champions for Hope Pancreatic Cancer Research Program of the Funk Zitiello Foundation. One author received an institutional research grant from Sofie Biosciences and Clovis Oncology, is on the BlueStar Genomics advisory board (ad hoc), and is a consultant for Bayer Healthcare, Candel Therapeutics, and UWorld. The remaining authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
new research suggests.
METHODOLOGY:
- The researchers utilized a diverse dataset of 3,014 CT scans: 1,105 diagnostic CT scans with pancreatic ductal adenocarcinoma (PDA) and 1,909 control CT scans.
- Of the total, 696 diagnostic CT scans with PDA and 1,080 control CT scans were used as an AI model training subset, and 409 CT scans with PDA and 829 control CT scans were used as an intramural hold-out test subset.
- The model was also tested on a simulated cohort that evaluated the risk for PDA in new-onset diabetes; multicenter public datasets (194 CT scans with PDA and 80 controls); and a cohort of 100 prediagnostic CT scans, incidentally acquired 3-36 months prior to PDA being diagnosed, and 134 controls.
TAKEAWAY:
- The model correctly classified 360 CT scans with PDA (88%) and 783 control CT scans (94%) in the intramural test subset. The mean accuracy was 0.92, the area under the receiver operating characteristic curve was 0.97, sensitivity was 0.88, and specificity was 0.95.
- On heat maps, activation areas overlapped with the tumor in 350 of 360 CT scans (97%).
- Performance was high across tumor stages, with sensitivities of 0.80, 0.87, 0.95, and 1.0 on T1 through T4 stages, respectively. Performance was comparable for hypodense versus isodense tumors (sensitivity of 0.90 vs. 0.82, respectively), patient demographics, CT slice thicknesses, and vendors.
- Findings were generalizable on both the simulated cohort (accuracy, 0.95; area under the ROC curve, 0.97) and public datasets (accuracy, 0.86; AUROC, 0.9).
- Occult PDA was detected on prediagnostic CT scans at a median 475 days before clinical diagnosis. Accuracy was 0.84, AUROC was 0.91, sensitivity was 0.75, and specificity was 0.9.
IN PRACTICE:
“Artificial intelligence model could mitigate the inadequacies of imaging and the diagnostic errors in interpretation, which often contribute to delayed diagnosis of pancreas cancer. In combination with emerging blood-based biomarkers, such a model could be evaluated to screen for sporadic cancer in ongoing trials of high-risk cohorts such as the Early Detection Initiative (NCT04662879).”
SOURCE:
Panagiotis Korfiatis, PhD, of Mayo Clinic, Rochester, Minn., led the study, which was published online in Gastroenterology.
LIMITATIONS:
The retrospective design is prone to selection bias. Results are presented dichotomously as either cancer or control. These are preliminary insights, and prospective clinical trials that incorporate epidemiological risk factors and emerging blood-based biomarkers are needed to further evaluate the model’s performance.
DISCLOSURES:
The research was supported by the National Cancer Institute, the Centene Charitable Foundation, and the Champions for Hope Pancreatic Cancer Research Program of the Funk Zitiello Foundation. One author received an institutional research grant from Sofie Biosciences and Clovis Oncology, is on the BlueStar Genomics advisory board (ad hoc), and is a consultant for Bayer Healthcare, Candel Therapeutics, and UWorld. The remaining authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
GLP-1 agonists linked to higher risk for rare but serious GI complications
Patients taking either of these glucagonlike peptide-1 (GLP-1) receptor agonists had a 9-fold elevation in risk for pancreatitis. They were also 4 times more likely to develop bowel obstruction and over 3.5 times more likely to experience gastroparesis.
The research letter was published online in JAMA.
Investigators say their findings are not about scaring people off the weight-loss drugs, but instead about increasing awareness that these potential adverse outcomes can happen.
“Given the wide use of these drugs, these adverse events, although rare, must be considered by patients thinking about using them for weight loss,” said lead author Mohit Sodhi, MSc, in a news release about the study. Mr. Sodhi is a graduate of the experimental medicine program at the University of British Columbia in Vancouver, and also a 4th-year medical student at UBC.
People taking a GLP-1 agonist to treat diabetes might be more willing to accept the risks, given their potential advantages, especially that of lowering the risk for heart problems, said Mahyar Etminan, PharmD, MSc, the study’s senior author and an expert in drug safety and pharmacoepidemiology at UBC. “But those who are otherwise healthy and just taking them for weight loss might want to be more careful in weighing the risk–benefit equation.”
People taking these drugs for weight loss have an approximately 1%-2% chance of experiencing these events, including a 1% risk for gastroparesis, Dr. Etminan said.
Key findings
The study included 4,144 people taking liraglutide, 613 taking semaglutide, and 654 taking naltrexone/bupropion based on medical records between 2006 and 2020.
They included patients with a recent history of obesity but excluded those with diabetes or who had been prescribed another diabetes medication.
The use of GLP-1 agonists, compared with naltrexone/bupropion, was associated with an increased risk for pancreatitis (adjusted hazard ratio, 9.09; 95% confidence interval, 1.25-66.00), bowel obstruction (HR, 4.22; 95% CI, 1.02-17.40), and gastroparesis (HR, 3.67; 95% CI, 1.15-11.90).
The study also found a higher incidence of biliary disease, but the difference was not statistically significant (HR, 1.50; 95% CI, 0.89-2.53). The incidence of biliary disease (per 1,000 person-years) was 11.7 for semaglutide, 18.6 for liraglutide, and 12.6 for naltrexone/bupropion.
Not the first report of GI issues
“This important paper confirms the safety signals hinted at in previous randomized controlled trials,” said Carel Le Roux, MBChB, PhD, professor of metabolic medicine, Ulster University, Coleraine, Ireland, and professor of experimental pathology at University College Dublin.
“The limitations of the paper are acknowledged but do not detract from the value of the robust data,” Dr. Le Roux said. “Patients should be informed of the low risk of serious complications, such as pancreatitis, gastroparesis, and bowel obstruction, before they start semaglutide or liraglutide.”
This is not the first report of GI issues associated with GLP-1 agonists, but it’s one of the largest. Most reports have been anecdotal. The U.S. Food and Drug Administration announced on Sept. 28 that it would require manufacturers to include a warning about gastrointestinal ileus on the Ozempic (semaglutide) label.
“The results from this study highlight how important it is that patients access these drugs only through trusted medical professionals, and only with ongoing support and monitoring,” noted Simon Cork, PhD, senior lecturer in physiology, Anglia Ruskin University in Cambridge, England.
Dr. Cork added that “it’s important to look at this in the proper context.” Obesity significantly increases the risk for developing cardiovascular disease, type 2 diabetes, cancer, gallbladder disease, and stroke, risks that fall dramatically with clinically meaningful and sustained weight loss, he said.
“For the overwhelming majority of patients for whom these drugs are targeted (those with the most severe forms of obesity), the benefits of weight loss far outweigh the risks,” Dr. Cork said.
The study was independently supported. Mr. Sodhi, Dr. Etminan, and Dr. Cork report no relevant financial relationships. Dr. Le Roux is a consultant and has received research funding and reimbursement of travel expenses from Novo Nordisk.
A version of this article first appeared on Medscape.com.
Patients taking either of these glucagonlike peptide-1 (GLP-1) receptor agonists had a 9-fold elevation in risk for pancreatitis. They were also 4 times more likely to develop bowel obstruction and over 3.5 times more likely to experience gastroparesis.
The research letter was published online in JAMA.
Investigators say their findings are not about scaring people off the weight-loss drugs, but instead about increasing awareness that these potential adverse outcomes can happen.
“Given the wide use of these drugs, these adverse events, although rare, must be considered by patients thinking about using them for weight loss,” said lead author Mohit Sodhi, MSc, in a news release about the study. Mr. Sodhi is a graduate of the experimental medicine program at the University of British Columbia in Vancouver, and also a 4th-year medical student at UBC.
People taking a GLP-1 agonist to treat diabetes might be more willing to accept the risks, given their potential advantages, especially that of lowering the risk for heart problems, said Mahyar Etminan, PharmD, MSc, the study’s senior author and an expert in drug safety and pharmacoepidemiology at UBC. “But those who are otherwise healthy and just taking them for weight loss might want to be more careful in weighing the risk–benefit equation.”
People taking these drugs for weight loss have an approximately 1%-2% chance of experiencing these events, including a 1% risk for gastroparesis, Dr. Etminan said.
Key findings
The study included 4,144 people taking liraglutide, 613 taking semaglutide, and 654 taking naltrexone/bupropion based on medical records between 2006 and 2020.
They included patients with a recent history of obesity but excluded those with diabetes or who had been prescribed another diabetes medication.
The use of GLP-1 agonists, compared with naltrexone/bupropion, was associated with an increased risk for pancreatitis (adjusted hazard ratio, 9.09; 95% confidence interval, 1.25-66.00), bowel obstruction (HR, 4.22; 95% CI, 1.02-17.40), and gastroparesis (HR, 3.67; 95% CI, 1.15-11.90).
The study also found a higher incidence of biliary disease, but the difference was not statistically significant (HR, 1.50; 95% CI, 0.89-2.53). The incidence of biliary disease (per 1,000 person-years) was 11.7 for semaglutide, 18.6 for liraglutide, and 12.6 for naltrexone/bupropion.
Not the first report of GI issues
“This important paper confirms the safety signals hinted at in previous randomized controlled trials,” said Carel Le Roux, MBChB, PhD, professor of metabolic medicine, Ulster University, Coleraine, Ireland, and professor of experimental pathology at University College Dublin.
“The limitations of the paper are acknowledged but do not detract from the value of the robust data,” Dr. Le Roux said. “Patients should be informed of the low risk of serious complications, such as pancreatitis, gastroparesis, and bowel obstruction, before they start semaglutide or liraglutide.”
This is not the first report of GI issues associated with GLP-1 agonists, but it’s one of the largest. Most reports have been anecdotal. The U.S. Food and Drug Administration announced on Sept. 28 that it would require manufacturers to include a warning about gastrointestinal ileus on the Ozempic (semaglutide) label.
“The results from this study highlight how important it is that patients access these drugs only through trusted medical professionals, and only with ongoing support and monitoring,” noted Simon Cork, PhD, senior lecturer in physiology, Anglia Ruskin University in Cambridge, England.
Dr. Cork added that “it’s important to look at this in the proper context.” Obesity significantly increases the risk for developing cardiovascular disease, type 2 diabetes, cancer, gallbladder disease, and stroke, risks that fall dramatically with clinically meaningful and sustained weight loss, he said.
“For the overwhelming majority of patients for whom these drugs are targeted (those with the most severe forms of obesity), the benefits of weight loss far outweigh the risks,” Dr. Cork said.
The study was independently supported. Mr. Sodhi, Dr. Etminan, and Dr. Cork report no relevant financial relationships. Dr. Le Roux is a consultant and has received research funding and reimbursement of travel expenses from Novo Nordisk.
A version of this article first appeared on Medscape.com.
Patients taking either of these glucagonlike peptide-1 (GLP-1) receptor agonists had a 9-fold elevation in risk for pancreatitis. They were also 4 times more likely to develop bowel obstruction and over 3.5 times more likely to experience gastroparesis.
The research letter was published online in JAMA.
Investigators say their findings are not about scaring people off the weight-loss drugs, but instead about increasing awareness that these potential adverse outcomes can happen.
“Given the wide use of these drugs, these adverse events, although rare, must be considered by patients thinking about using them for weight loss,” said lead author Mohit Sodhi, MSc, in a news release about the study. Mr. Sodhi is a graduate of the experimental medicine program at the University of British Columbia in Vancouver, and also a 4th-year medical student at UBC.
People taking a GLP-1 agonist to treat diabetes might be more willing to accept the risks, given their potential advantages, especially that of lowering the risk for heart problems, said Mahyar Etminan, PharmD, MSc, the study’s senior author and an expert in drug safety and pharmacoepidemiology at UBC. “But those who are otherwise healthy and just taking them for weight loss might want to be more careful in weighing the risk–benefit equation.”
People taking these drugs for weight loss have an approximately 1%-2% chance of experiencing these events, including a 1% risk for gastroparesis, Dr. Etminan said.
Key findings
The study included 4,144 people taking liraglutide, 613 taking semaglutide, and 654 taking naltrexone/bupropion based on medical records between 2006 and 2020.
They included patients with a recent history of obesity but excluded those with diabetes or who had been prescribed another diabetes medication.
The use of GLP-1 agonists, compared with naltrexone/bupropion, was associated with an increased risk for pancreatitis (adjusted hazard ratio, 9.09; 95% confidence interval, 1.25-66.00), bowel obstruction (HR, 4.22; 95% CI, 1.02-17.40), and gastroparesis (HR, 3.67; 95% CI, 1.15-11.90).
The study also found a higher incidence of biliary disease, but the difference was not statistically significant (HR, 1.50; 95% CI, 0.89-2.53). The incidence of biliary disease (per 1,000 person-years) was 11.7 for semaglutide, 18.6 for liraglutide, and 12.6 for naltrexone/bupropion.
Not the first report of GI issues
“This important paper confirms the safety signals hinted at in previous randomized controlled trials,” said Carel Le Roux, MBChB, PhD, professor of metabolic medicine, Ulster University, Coleraine, Ireland, and professor of experimental pathology at University College Dublin.
“The limitations of the paper are acknowledged but do not detract from the value of the robust data,” Dr. Le Roux said. “Patients should be informed of the low risk of serious complications, such as pancreatitis, gastroparesis, and bowel obstruction, before they start semaglutide or liraglutide.”
This is not the first report of GI issues associated with GLP-1 agonists, but it’s one of the largest. Most reports have been anecdotal. The U.S. Food and Drug Administration announced on Sept. 28 that it would require manufacturers to include a warning about gastrointestinal ileus on the Ozempic (semaglutide) label.
“The results from this study highlight how important it is that patients access these drugs only through trusted medical professionals, and only with ongoing support and monitoring,” noted Simon Cork, PhD, senior lecturer in physiology, Anglia Ruskin University in Cambridge, England.
Dr. Cork added that “it’s important to look at this in the proper context.” Obesity significantly increases the risk for developing cardiovascular disease, type 2 diabetes, cancer, gallbladder disease, and stroke, risks that fall dramatically with clinically meaningful and sustained weight loss, he said.
“For the overwhelming majority of patients for whom these drugs are targeted (those with the most severe forms of obesity), the benefits of weight loss far outweigh the risks,” Dr. Cork said.
The study was independently supported. Mr. Sodhi, Dr. Etminan, and Dr. Cork report no relevant financial relationships. Dr. Le Roux is a consultant and has received research funding and reimbursement of travel expenses from Novo Nordisk.
A version of this article first appeared on Medscape.com.
FROM JAMA
Obesity linked to multiple ills in MS study
MILAN – Swedish researchers reported at the 9th Joint ECTRIMS-ACTRIMS meeting.
In a group of 3,249 subjects tracked for up to 5 years (74% female; mean age, 37.8 years), patients who were obese at diagnosis were 1.41 times more likely than normal-weight patients to reach an Expanded Disability Status Scale (EDSS) score of 3. About 35% of 355 obese subjects (body mass index > 30 kg/m2) reached that level versus 29% of 713 overweight patients (BMI, 25-30) and 28% of 1,475 normal-weight patients (BMI, 18.5-24.99).
Among subjects whose BMI category didn’t change over follow-up, those who were obese at diagnosis were more likely to develop cognitive worsening than those who weren’t obese (hazard ratio, 1.47, 95% confidence interval, 1.08-2.01).
Lars Alfredsson, PhD, a professor at the Karolinska Institutet, Stockholm, who presented the study findings, said in an interview that they fill a gap in knowledge about obesity and MS. “It is known that obesity around the age of 20 or in adolescence is a risk factor for developing MS. But much less is known in regard to progression, and the studies have been very inconclusive.”
The researchers tracked patients via the Swedish MS registry: 1,475 of normal weight, 713 overweight, and 355 obese. Before adjustment for factors such as age, gender, and baseline EDSS, obese subjects were 1.51 times more likely to reach EDSS score 3 than normal-weight subjects.
Obese subjects whose BMI level didn’t change over time were 1.70 times more likely than the nonobese to develop physical worsening as measured by an increased Multiple Sclerosis Impact Scale physical score of 7.5 points or more, and they were 1.36 times more likely to have psychological worsening as measured by increased MSIS-28 psychological score of 7.5 points or more.
Also, among subjects whose BMI didn’t change over time, the likelihood of cognitive disability worsening was 1.47 times higher among obese participants versus nonobese participants. Worsening was defined as an increased Symbol Digit Modalities Test score of 8 points or more.
The level of excess cognitive decline “will affect people significantly,” Dr. Alfredsson said.
While obesity can counterintuitively provide a protective effect in some diseases, he said there’s no sign of such an effect in the subjects.
As for limitations, Dr. Alfredsson noted in his presentation that BMI data is self-reported, and it’s possible that the researchers didn’t adjust their statistics to reflect important confounders.
A 2023 German study of outcomes in MS patients with obesity came to similar conclusions. It tracked 1,066 subjects for up to 6 years and found that “median time to reach EDSS 3 was 0.99 years for patients with BMI of 30 or higher and 1.46 years for nonobese patients. Risk to reach EDSS 3 over 6 years was significantly increased in patients with BMI of at least 30, compared with patients with BMI less than 30 after adjustment for sex, age, smoking (HR, 1.87; 95% CI, 1.3-2.6; P < .001), and independent of disease-modifying therapies.”
However, the German researchers found no link between obesity and higher levels of relapse, contrast-enhancing MRI lesions, or MRI T2 lesion burden.
Interpretation and commentary
Could obesity be causing worse outcomes? The new study doesn’t provide insight into cause and effect. However, obesity may speed up progression via low-grade inflammation, Dr. Alfredsson said.
What can clinicians do with the information from the study? If patients are obese, it can be a good idea to more carefully monitor them and use reliable tools to improve their progression, Dr. Alfredsson said.
In an interview, Michael D. Kornberg, MD, PhD, an assistant professor of neurology at Johns Hopkins University, Baltimore, who was not involved with the study, agreed with Dr. Alfredsson that other research has linked obesity early in life to higher rates of MS. He added that “a number of studies have shown that comorbidities in general are usually associated with a higher rate of disability.”
Dr. Kornberg said the new research is important, and he noted that it has a “robust” cohort because of its larger size.
Could patients with MS reverse the risk of progression and other poor outcomes by losing weight? “It’s hard to say,” Dr. Kornberg said. “We have to be cautious when we assume causation. There’s a plausible rationale that obesity might worsen progression in MS, but it could just be a marker of some other factor that reflects a different phenotype of MS.”
He doesn’t think it’s likely that weight loss would “dramatically reverse the biology of MS,” but he said reversing the obesity epidemic would still be a good thing. An interventional study could examine the effects of weight-loss intervention on disability measures, he said, “and that’s the next step.”
Also contacted for commentary, Adil Harroud, MD, a neurologist at McGill University who studies obesity in MS, said research suggests that “obesity seems to exacerbate MS disability. While some studies show no effect, the majority indicate a detrimental impact.”
However, “the effect of obesity on MS progression remains unclear. Animal studies suggest that shifts in immune cell subsets and functions may play a role, but the relevance to humans is yet to be determined,” he said.
Dr. Harroud, who did not take part in the new study, said it’s “one of the largest examining the impact of obesity on MS disability.” He added that “the cohort was relatively early in their disease course, suggesting that obesity impacts even the early stages of MS. This underscores the importance of obesity as a modifiable risk factor for disability accumulation.”
As for why obesity affects MS, he said one theory is that obesity plays a role through its impact on vitamin D levels. “However, using a genetic approach, we have demonstrated that, at least for MS risk, the effect of obesity is independent of vitamin D. This is also likely true for MS progression, as recent trials of vitamin D supplementation have not shown a meaningful impact on MS outcomes.”
According to Dr. Harroud, “other theories suggest that obesity leads to a pro-inflammatory immune shift. Additionally, it has been proposed that obesity may influence the response to disease-modifying therapy by reducing drug bioavailability, potentially necessitating weight-based dosing for some therapies.”
Dr. Alfredsson reported receiving grants from the Swedish Research Council, the Swedish Research Council for Health Working Life and Welfare, and the Swedish Brain Foundation and personal fees from Teva and Biogene Idec. Some of the other study authors reported various disclosures. Dr. Kornberg and Dr. Harroud reported no relevant disclosures.
This article was updated 10/20/23.
MILAN – Swedish researchers reported at the 9th Joint ECTRIMS-ACTRIMS meeting.
In a group of 3,249 subjects tracked for up to 5 years (74% female; mean age, 37.8 years), patients who were obese at diagnosis were 1.41 times more likely than normal-weight patients to reach an Expanded Disability Status Scale (EDSS) score of 3. About 35% of 355 obese subjects (body mass index > 30 kg/m2) reached that level versus 29% of 713 overweight patients (BMI, 25-30) and 28% of 1,475 normal-weight patients (BMI, 18.5-24.99).
Among subjects whose BMI category didn’t change over follow-up, those who were obese at diagnosis were more likely to develop cognitive worsening than those who weren’t obese (hazard ratio, 1.47, 95% confidence interval, 1.08-2.01).
Lars Alfredsson, PhD, a professor at the Karolinska Institutet, Stockholm, who presented the study findings, said in an interview that they fill a gap in knowledge about obesity and MS. “It is known that obesity around the age of 20 or in adolescence is a risk factor for developing MS. But much less is known in regard to progression, and the studies have been very inconclusive.”
The researchers tracked patients via the Swedish MS registry: 1,475 of normal weight, 713 overweight, and 355 obese. Before adjustment for factors such as age, gender, and baseline EDSS, obese subjects were 1.51 times more likely to reach EDSS score 3 than normal-weight subjects.
Obese subjects whose BMI level didn’t change over time were 1.70 times more likely than the nonobese to develop physical worsening as measured by an increased Multiple Sclerosis Impact Scale physical score of 7.5 points or more, and they were 1.36 times more likely to have psychological worsening as measured by increased MSIS-28 psychological score of 7.5 points or more.
Also, among subjects whose BMI didn’t change over time, the likelihood of cognitive disability worsening was 1.47 times higher among obese participants versus nonobese participants. Worsening was defined as an increased Symbol Digit Modalities Test score of 8 points or more.
The level of excess cognitive decline “will affect people significantly,” Dr. Alfredsson said.
While obesity can counterintuitively provide a protective effect in some diseases, he said there’s no sign of such an effect in the subjects.
As for limitations, Dr. Alfredsson noted in his presentation that BMI data is self-reported, and it’s possible that the researchers didn’t adjust their statistics to reflect important confounders.
A 2023 German study of outcomes in MS patients with obesity came to similar conclusions. It tracked 1,066 subjects for up to 6 years and found that “median time to reach EDSS 3 was 0.99 years for patients with BMI of 30 or higher and 1.46 years for nonobese patients. Risk to reach EDSS 3 over 6 years was significantly increased in patients with BMI of at least 30, compared with patients with BMI less than 30 after adjustment for sex, age, smoking (HR, 1.87; 95% CI, 1.3-2.6; P < .001), and independent of disease-modifying therapies.”
However, the German researchers found no link between obesity and higher levels of relapse, contrast-enhancing MRI lesions, or MRI T2 lesion burden.
Interpretation and commentary
Could obesity be causing worse outcomes? The new study doesn’t provide insight into cause and effect. However, obesity may speed up progression via low-grade inflammation, Dr. Alfredsson said.
What can clinicians do with the information from the study? If patients are obese, it can be a good idea to more carefully monitor them and use reliable tools to improve their progression, Dr. Alfredsson said.
In an interview, Michael D. Kornberg, MD, PhD, an assistant professor of neurology at Johns Hopkins University, Baltimore, who was not involved with the study, agreed with Dr. Alfredsson that other research has linked obesity early in life to higher rates of MS. He added that “a number of studies have shown that comorbidities in general are usually associated with a higher rate of disability.”
Dr. Kornberg said the new research is important, and he noted that it has a “robust” cohort because of its larger size.
Could patients with MS reverse the risk of progression and other poor outcomes by losing weight? “It’s hard to say,” Dr. Kornberg said. “We have to be cautious when we assume causation. There’s a plausible rationale that obesity might worsen progression in MS, but it could just be a marker of some other factor that reflects a different phenotype of MS.”
He doesn’t think it’s likely that weight loss would “dramatically reverse the biology of MS,” but he said reversing the obesity epidemic would still be a good thing. An interventional study could examine the effects of weight-loss intervention on disability measures, he said, “and that’s the next step.”
Also contacted for commentary, Adil Harroud, MD, a neurologist at McGill University who studies obesity in MS, said research suggests that “obesity seems to exacerbate MS disability. While some studies show no effect, the majority indicate a detrimental impact.”
However, “the effect of obesity on MS progression remains unclear. Animal studies suggest that shifts in immune cell subsets and functions may play a role, but the relevance to humans is yet to be determined,” he said.
Dr. Harroud, who did not take part in the new study, said it’s “one of the largest examining the impact of obesity on MS disability.” He added that “the cohort was relatively early in their disease course, suggesting that obesity impacts even the early stages of MS. This underscores the importance of obesity as a modifiable risk factor for disability accumulation.”
As for why obesity affects MS, he said one theory is that obesity plays a role through its impact on vitamin D levels. “However, using a genetic approach, we have demonstrated that, at least for MS risk, the effect of obesity is independent of vitamin D. This is also likely true for MS progression, as recent trials of vitamin D supplementation have not shown a meaningful impact on MS outcomes.”
According to Dr. Harroud, “other theories suggest that obesity leads to a pro-inflammatory immune shift. Additionally, it has been proposed that obesity may influence the response to disease-modifying therapy by reducing drug bioavailability, potentially necessitating weight-based dosing for some therapies.”
Dr. Alfredsson reported receiving grants from the Swedish Research Council, the Swedish Research Council for Health Working Life and Welfare, and the Swedish Brain Foundation and personal fees from Teva and Biogene Idec. Some of the other study authors reported various disclosures. Dr. Kornberg and Dr. Harroud reported no relevant disclosures.
This article was updated 10/20/23.
MILAN – Swedish researchers reported at the 9th Joint ECTRIMS-ACTRIMS meeting.
In a group of 3,249 subjects tracked for up to 5 years (74% female; mean age, 37.8 years), patients who were obese at diagnosis were 1.41 times more likely than normal-weight patients to reach an Expanded Disability Status Scale (EDSS) score of 3. About 35% of 355 obese subjects (body mass index > 30 kg/m2) reached that level versus 29% of 713 overweight patients (BMI, 25-30) and 28% of 1,475 normal-weight patients (BMI, 18.5-24.99).
Among subjects whose BMI category didn’t change over follow-up, those who were obese at diagnosis were more likely to develop cognitive worsening than those who weren’t obese (hazard ratio, 1.47, 95% confidence interval, 1.08-2.01).
Lars Alfredsson, PhD, a professor at the Karolinska Institutet, Stockholm, who presented the study findings, said in an interview that they fill a gap in knowledge about obesity and MS. “It is known that obesity around the age of 20 or in adolescence is a risk factor for developing MS. But much less is known in regard to progression, and the studies have been very inconclusive.”
The researchers tracked patients via the Swedish MS registry: 1,475 of normal weight, 713 overweight, and 355 obese. Before adjustment for factors such as age, gender, and baseline EDSS, obese subjects were 1.51 times more likely to reach EDSS score 3 than normal-weight subjects.
Obese subjects whose BMI level didn’t change over time were 1.70 times more likely than the nonobese to develop physical worsening as measured by an increased Multiple Sclerosis Impact Scale physical score of 7.5 points or more, and they were 1.36 times more likely to have psychological worsening as measured by increased MSIS-28 psychological score of 7.5 points or more.
Also, among subjects whose BMI didn’t change over time, the likelihood of cognitive disability worsening was 1.47 times higher among obese participants versus nonobese participants. Worsening was defined as an increased Symbol Digit Modalities Test score of 8 points or more.
The level of excess cognitive decline “will affect people significantly,” Dr. Alfredsson said.
While obesity can counterintuitively provide a protective effect in some diseases, he said there’s no sign of such an effect in the subjects.
As for limitations, Dr. Alfredsson noted in his presentation that BMI data is self-reported, and it’s possible that the researchers didn’t adjust their statistics to reflect important confounders.
A 2023 German study of outcomes in MS patients with obesity came to similar conclusions. It tracked 1,066 subjects for up to 6 years and found that “median time to reach EDSS 3 was 0.99 years for patients with BMI of 30 or higher and 1.46 years for nonobese patients. Risk to reach EDSS 3 over 6 years was significantly increased in patients with BMI of at least 30, compared with patients with BMI less than 30 after adjustment for sex, age, smoking (HR, 1.87; 95% CI, 1.3-2.6; P < .001), and independent of disease-modifying therapies.”
However, the German researchers found no link between obesity and higher levels of relapse, contrast-enhancing MRI lesions, or MRI T2 lesion burden.
Interpretation and commentary
Could obesity be causing worse outcomes? The new study doesn’t provide insight into cause and effect. However, obesity may speed up progression via low-grade inflammation, Dr. Alfredsson said.
What can clinicians do with the information from the study? If patients are obese, it can be a good idea to more carefully monitor them and use reliable tools to improve their progression, Dr. Alfredsson said.
In an interview, Michael D. Kornberg, MD, PhD, an assistant professor of neurology at Johns Hopkins University, Baltimore, who was not involved with the study, agreed with Dr. Alfredsson that other research has linked obesity early in life to higher rates of MS. He added that “a number of studies have shown that comorbidities in general are usually associated with a higher rate of disability.”
Dr. Kornberg said the new research is important, and he noted that it has a “robust” cohort because of its larger size.
Could patients with MS reverse the risk of progression and other poor outcomes by losing weight? “It’s hard to say,” Dr. Kornberg said. “We have to be cautious when we assume causation. There’s a plausible rationale that obesity might worsen progression in MS, but it could just be a marker of some other factor that reflects a different phenotype of MS.”
He doesn’t think it’s likely that weight loss would “dramatically reverse the biology of MS,” but he said reversing the obesity epidemic would still be a good thing. An interventional study could examine the effects of weight-loss intervention on disability measures, he said, “and that’s the next step.”
Also contacted for commentary, Adil Harroud, MD, a neurologist at McGill University who studies obesity in MS, said research suggests that “obesity seems to exacerbate MS disability. While some studies show no effect, the majority indicate a detrimental impact.”
However, “the effect of obesity on MS progression remains unclear. Animal studies suggest that shifts in immune cell subsets and functions may play a role, but the relevance to humans is yet to be determined,” he said.
Dr. Harroud, who did not take part in the new study, said it’s “one of the largest examining the impact of obesity on MS disability.” He added that “the cohort was relatively early in their disease course, suggesting that obesity impacts even the early stages of MS. This underscores the importance of obesity as a modifiable risk factor for disability accumulation.”
As for why obesity affects MS, he said one theory is that obesity plays a role through its impact on vitamin D levels. “However, using a genetic approach, we have demonstrated that, at least for MS risk, the effect of obesity is independent of vitamin D. This is also likely true for MS progression, as recent trials of vitamin D supplementation have not shown a meaningful impact on MS outcomes.”
According to Dr. Harroud, “other theories suggest that obesity leads to a pro-inflammatory immune shift. Additionally, it has been proposed that obesity may influence the response to disease-modifying therapy by reducing drug bioavailability, potentially necessitating weight-based dosing for some therapies.”
Dr. Alfredsson reported receiving grants from the Swedish Research Council, the Swedish Research Council for Health Working Life and Welfare, and the Swedish Brain Foundation and personal fees from Teva and Biogene Idec. Some of the other study authors reported various disclosures. Dr. Kornberg and Dr. Harroud reported no relevant disclosures.
This article was updated 10/20/23.
AT ECTRIMS 2023
Repetitive primary care screenings may miss depression and anxiety
Routine screening for depression and anxiety at each primary care clinical encounter in order to meet performance metrics could compromise accuracy and clinical care, based on data from more than 380,000 individuals in primary care.
“Prioritizing repetition of intake screening questionnaires at primary care visits may have unintended consequences such as administrative burden, provision of low-value care, and reduced clinical capacity to deliver other, high-value services,” but the accuracy of workflow-based intake screening on subsequent diagnosis has not been explored, wrote Jodi Simon, DrPH, of AllianceChicago, Ill., and colleagues.
In a study published in the Annals of Family Medicine, the researchers reviewed data from screenings performed on 380,057 patients in primary care settings. They examined the accuracy and utility of the Patient Health Questionnaire (PHQ-2) for depression and the Generalized Anxiety Disorder 2 (GAD-2) for anxiety.
The data included 1,883,317 screenings with PHQ-2s and 1,573,107 with GAD-2s. Of these, 92.3% of PHQ-2 screenings and 91.4% of GAD-2 screenings indicated low likelihood of depression or anxiety (defined as cumulative scores of 0 or 1). Mean scores for the PHQ-2 and GAD-2 in the study population were 0.29 and 0.35, respectively.
In the current study, 11% of patients had positive PHQ-2 scores (defined as 2 or higher) vs. 47%-53% seen in previous studies and census data.
In an analysis of new diagnoses of depression and anxiety, the researchers found that 42.3% of patients with a new depression diagnosis were not identified on intake screening; they had scores of 0 or 1 on the PHQ-2 in the past 30 days. Similarly, 42.7% of patients with a new anxiety diagnosis had scores of 0 or 1 on the GAD-2 in the past 30 days.
In other words, “Screening only detected risk in 57.7% of patients subsequently diagnosed with depression and 57.3% of patients subsequently diagnosed with anxiety,” the researchers said. This low positivity rate in patients diagnosed within 30 days merits further research, they added.
More studies are needed, but preliminary interviews with patients, clinicians, and staff indicate that time constraints and variation in the administration of questionnaires are among the factors contributing to inaccurate screening, the researchers noted.
The current study results suggest that screenings for anxiety and depression may occur in a perfunctory or inconsistent manner that might compromise accuracy when they are part of the workflow for each clinical visit in order to meet performance metrics, they said. “Ineffective screening may unintentionally detract from clinical care because care teams and patients have less time and cognitive energy to focus on other priorities during busy clinical encounters,” they added.
Alternatively, , the researchers concluded.
The study was funded by the American Medical Association Transformation Initiative. The researchers had no financial conflicts to disclose.
Routine screening for depression and anxiety at each primary care clinical encounter in order to meet performance metrics could compromise accuracy and clinical care, based on data from more than 380,000 individuals in primary care.
“Prioritizing repetition of intake screening questionnaires at primary care visits may have unintended consequences such as administrative burden, provision of low-value care, and reduced clinical capacity to deliver other, high-value services,” but the accuracy of workflow-based intake screening on subsequent diagnosis has not been explored, wrote Jodi Simon, DrPH, of AllianceChicago, Ill., and colleagues.
In a study published in the Annals of Family Medicine, the researchers reviewed data from screenings performed on 380,057 patients in primary care settings. They examined the accuracy and utility of the Patient Health Questionnaire (PHQ-2) for depression and the Generalized Anxiety Disorder 2 (GAD-2) for anxiety.
The data included 1,883,317 screenings with PHQ-2s and 1,573,107 with GAD-2s. Of these, 92.3% of PHQ-2 screenings and 91.4% of GAD-2 screenings indicated low likelihood of depression or anxiety (defined as cumulative scores of 0 or 1). Mean scores for the PHQ-2 and GAD-2 in the study population were 0.29 and 0.35, respectively.
In the current study, 11% of patients had positive PHQ-2 scores (defined as 2 or higher) vs. 47%-53% seen in previous studies and census data.
In an analysis of new diagnoses of depression and anxiety, the researchers found that 42.3% of patients with a new depression diagnosis were not identified on intake screening; they had scores of 0 or 1 on the PHQ-2 in the past 30 days. Similarly, 42.7% of patients with a new anxiety diagnosis had scores of 0 or 1 on the GAD-2 in the past 30 days.
In other words, “Screening only detected risk in 57.7% of patients subsequently diagnosed with depression and 57.3% of patients subsequently diagnosed with anxiety,” the researchers said. This low positivity rate in patients diagnosed within 30 days merits further research, they added.
More studies are needed, but preliminary interviews with patients, clinicians, and staff indicate that time constraints and variation in the administration of questionnaires are among the factors contributing to inaccurate screening, the researchers noted.
The current study results suggest that screenings for anxiety and depression may occur in a perfunctory or inconsistent manner that might compromise accuracy when they are part of the workflow for each clinical visit in order to meet performance metrics, they said. “Ineffective screening may unintentionally detract from clinical care because care teams and patients have less time and cognitive energy to focus on other priorities during busy clinical encounters,” they added.
Alternatively, , the researchers concluded.
The study was funded by the American Medical Association Transformation Initiative. The researchers had no financial conflicts to disclose.
Routine screening for depression and anxiety at each primary care clinical encounter in order to meet performance metrics could compromise accuracy and clinical care, based on data from more than 380,000 individuals in primary care.
“Prioritizing repetition of intake screening questionnaires at primary care visits may have unintended consequences such as administrative burden, provision of low-value care, and reduced clinical capacity to deliver other, high-value services,” but the accuracy of workflow-based intake screening on subsequent diagnosis has not been explored, wrote Jodi Simon, DrPH, of AllianceChicago, Ill., and colleagues.
In a study published in the Annals of Family Medicine, the researchers reviewed data from screenings performed on 380,057 patients in primary care settings. They examined the accuracy and utility of the Patient Health Questionnaire (PHQ-2) for depression and the Generalized Anxiety Disorder 2 (GAD-2) for anxiety.
The data included 1,883,317 screenings with PHQ-2s and 1,573,107 with GAD-2s. Of these, 92.3% of PHQ-2 screenings and 91.4% of GAD-2 screenings indicated low likelihood of depression or anxiety (defined as cumulative scores of 0 or 1). Mean scores for the PHQ-2 and GAD-2 in the study population were 0.29 and 0.35, respectively.
In the current study, 11% of patients had positive PHQ-2 scores (defined as 2 or higher) vs. 47%-53% seen in previous studies and census data.
In an analysis of new diagnoses of depression and anxiety, the researchers found that 42.3% of patients with a new depression diagnosis were not identified on intake screening; they had scores of 0 or 1 on the PHQ-2 in the past 30 days. Similarly, 42.7% of patients with a new anxiety diagnosis had scores of 0 or 1 on the GAD-2 in the past 30 days.
In other words, “Screening only detected risk in 57.7% of patients subsequently diagnosed with depression and 57.3% of patients subsequently diagnosed with anxiety,” the researchers said. This low positivity rate in patients diagnosed within 30 days merits further research, they added.
More studies are needed, but preliminary interviews with patients, clinicians, and staff indicate that time constraints and variation in the administration of questionnaires are among the factors contributing to inaccurate screening, the researchers noted.
The current study results suggest that screenings for anxiety and depression may occur in a perfunctory or inconsistent manner that might compromise accuracy when they are part of the workflow for each clinical visit in order to meet performance metrics, they said. “Ineffective screening may unintentionally detract from clinical care because care teams and patients have less time and cognitive energy to focus on other priorities during busy clinical encounters,” they added.
Alternatively, , the researchers concluded.
The study was funded by the American Medical Association Transformation Initiative. The researchers had no financial conflicts to disclose.
FROM THE ANNALS OF FAMILY MEDICINE