User login
In memoriam
Sylvan Lee Weinberg, MD, FCCP, MACC, a Past President of the American College of Chest Physicians (1983-1984), died Jan 17, 2017, in Dayton, Ohio. Dr. Weinberg was born in Nashville, TN, and received both his bachelor of science and doctor of medicine degrees from Northwestern University in Evanston, IL. He spent his time as an intern, medical resident, and fellow in cardiology at the Michael Reese Hospital in Chicago and went on to serve as a physician at Good Samaritan Hospital in Dayton, Ohio, for more than 40 years, ultimately becoming chief of cardiology and founder of the first coronary care unit in Ohio. Dr. Weinberg was also a clinical professor of medicine at the Wright State University School of Medicine in Dayton, and led a group cardiology practice until his retirement in 2000.
CHEST extends its heartfelt condolences to Dr. Weinberg’s family and friends.
Sylvan Lee Weinberg, MD, FCCP, MACC, a Past President of the American College of Chest Physicians (1983-1984), died Jan 17, 2017, in Dayton, Ohio. Dr. Weinberg was born in Nashville, TN, and received both his bachelor of science and doctor of medicine degrees from Northwestern University in Evanston, IL. He spent his time as an intern, medical resident, and fellow in cardiology at the Michael Reese Hospital in Chicago and went on to serve as a physician at Good Samaritan Hospital in Dayton, Ohio, for more than 40 years, ultimately becoming chief of cardiology and founder of the first coronary care unit in Ohio. Dr. Weinberg was also a clinical professor of medicine at the Wright State University School of Medicine in Dayton, and led a group cardiology practice until his retirement in 2000.
CHEST extends its heartfelt condolences to Dr. Weinberg’s family and friends.
Sylvan Lee Weinberg, MD, FCCP, MACC, a Past President of the American College of Chest Physicians (1983-1984), died Jan 17, 2017, in Dayton, Ohio. Dr. Weinberg was born in Nashville, TN, and received both his bachelor of science and doctor of medicine degrees from Northwestern University in Evanston, IL. He spent his time as an intern, medical resident, and fellow in cardiology at the Michael Reese Hospital in Chicago and went on to serve as a physician at Good Samaritan Hospital in Dayton, Ohio, for more than 40 years, ultimately becoming chief of cardiology and founder of the first coronary care unit in Ohio. Dr. Weinberg was also a clinical professor of medicine at the Wright State University School of Medicine in Dayton, and led a group cardiology practice until his retirement in 2000.
CHEST extends its heartfelt condolences to Dr. Weinberg’s family and friends.
No rise in CV events seen with tocilizumab
Patients with refractory rheumatoid arthritis who switched to tocilizumab showed no increased cardiovascular risk when compared with those who switched to a tumor necrosis factor inhibitor in a large cohort study.
Rheumatoid arthritis is known to approximately double the risk of cardiovascular morbidity and mortality partly because of its associated chronic systemic inflammation. Several small trials and observational studies have reported that tocilizumab, an interleukin-6 receptor antagonist typically used as a second-line treatment for RA, elevates serum lipids, including LDL cholesterol. These serum lipid elevations caused by tocilizumab have brought concerns that the drug may further heighten CV risk in people with RA, said Seoyoung C. Kim, MD, ScD, of the division of pharmacoepidemiology and pharmacoeconomics and the division of rheumatology, immunology, and allergy at Brigham and Women’s Hospital, Boston, and her associates.
To minimize the effect of confounding by the severity of RA and the baseline CV risk, the researchers adjusted the data to account for more than 90 variables related to CV events and to RA severity.
The primary outcome — a composite of MI and stroke — occurred in 125 patients, 36 taking tocilizumab and 89 taking TNF inhibitors. The rate of this composite outcome was 0.52 per 100 person-years with tocilizumab and 0.59 per 100 person-years for TNF inhibitors, a nonsignificant difference, Dr. Kim and her associates reported (Arthritis Rheumatol. 2017 Feb 28. doi: 10.1002/art.40084).
There also were no significant differences between the two study groups in secondary endpoints, including rates of coronary revascularization, acute coronary syndrome, heart failure, and all-cause mortality. In addition, all subgroup analyses confirmed that tocilizumab did not raise CV risk, regardless of patient age (younger than or older than 60 years), the presence of cardiovascular disease at baseline, the presence of diabetes, the use of methotrexate, the use of oral steroids, or the use of statins.
These “reassuring” findings show that even though tocilizumab appears to raise LDL levels, “such increases do not appear to be associated with an increased risk of clinical CV events,” the investigators said.
The results confirm those reported at the 2016 American College of Rheumatology annual meeting for the 5-year, randomized, postmarketing ENTRACTE trial in which the lipid changes induced by tocilizumab did not translate into an increased risk of heart attack or stroke in RA patients.
This cohort study was sponsored by Genentech, which markets tocilizumab (Actemra). Dr. Kim reported ties to Genentech, Lilly, Pfizer, Bristol-Myers Squibb, and AstraZeneca. Her associates reported ties to Genentech, Lilly, Pfizer, AstraZeneca, Amgen, Corrona, Whiscon, Aetion, and Boehringer Ingelheim. Three of the seven authors were employees of Genentech.
Patients with refractory rheumatoid arthritis who switched to tocilizumab showed no increased cardiovascular risk when compared with those who switched to a tumor necrosis factor inhibitor in a large cohort study.
Rheumatoid arthritis is known to approximately double the risk of cardiovascular morbidity and mortality partly because of its associated chronic systemic inflammation. Several small trials and observational studies have reported that tocilizumab, an interleukin-6 receptor antagonist typically used as a second-line treatment for RA, elevates serum lipids, including LDL cholesterol. These serum lipid elevations caused by tocilizumab have brought concerns that the drug may further heighten CV risk in people with RA, said Seoyoung C. Kim, MD, ScD, of the division of pharmacoepidemiology and pharmacoeconomics and the division of rheumatology, immunology, and allergy at Brigham and Women’s Hospital, Boston, and her associates.
To minimize the effect of confounding by the severity of RA and the baseline CV risk, the researchers adjusted the data to account for more than 90 variables related to CV events and to RA severity.
The primary outcome — a composite of MI and stroke — occurred in 125 patients, 36 taking tocilizumab and 89 taking TNF inhibitors. The rate of this composite outcome was 0.52 per 100 person-years with tocilizumab and 0.59 per 100 person-years for TNF inhibitors, a nonsignificant difference, Dr. Kim and her associates reported (Arthritis Rheumatol. 2017 Feb 28. doi: 10.1002/art.40084).
There also were no significant differences between the two study groups in secondary endpoints, including rates of coronary revascularization, acute coronary syndrome, heart failure, and all-cause mortality. In addition, all subgroup analyses confirmed that tocilizumab did not raise CV risk, regardless of patient age (younger than or older than 60 years), the presence of cardiovascular disease at baseline, the presence of diabetes, the use of methotrexate, the use of oral steroids, or the use of statins.
These “reassuring” findings show that even though tocilizumab appears to raise LDL levels, “such increases do not appear to be associated with an increased risk of clinical CV events,” the investigators said.
The results confirm those reported at the 2016 American College of Rheumatology annual meeting for the 5-year, randomized, postmarketing ENTRACTE trial in which the lipid changes induced by tocilizumab did not translate into an increased risk of heart attack or stroke in RA patients.
This cohort study was sponsored by Genentech, which markets tocilizumab (Actemra). Dr. Kim reported ties to Genentech, Lilly, Pfizer, Bristol-Myers Squibb, and AstraZeneca. Her associates reported ties to Genentech, Lilly, Pfizer, AstraZeneca, Amgen, Corrona, Whiscon, Aetion, and Boehringer Ingelheim. Three of the seven authors were employees of Genentech.
Patients with refractory rheumatoid arthritis who switched to tocilizumab showed no increased cardiovascular risk when compared with those who switched to a tumor necrosis factor inhibitor in a large cohort study.
Rheumatoid arthritis is known to approximately double the risk of cardiovascular morbidity and mortality partly because of its associated chronic systemic inflammation. Several small trials and observational studies have reported that tocilizumab, an interleukin-6 receptor antagonist typically used as a second-line treatment for RA, elevates serum lipids, including LDL cholesterol. These serum lipid elevations caused by tocilizumab have brought concerns that the drug may further heighten CV risk in people with RA, said Seoyoung C. Kim, MD, ScD, of the division of pharmacoepidemiology and pharmacoeconomics and the division of rheumatology, immunology, and allergy at Brigham and Women’s Hospital, Boston, and her associates.
To minimize the effect of confounding by the severity of RA and the baseline CV risk, the researchers adjusted the data to account for more than 90 variables related to CV events and to RA severity.
The primary outcome — a composite of MI and stroke — occurred in 125 patients, 36 taking tocilizumab and 89 taking TNF inhibitors. The rate of this composite outcome was 0.52 per 100 person-years with tocilizumab and 0.59 per 100 person-years for TNF inhibitors, a nonsignificant difference, Dr. Kim and her associates reported (Arthritis Rheumatol. 2017 Feb 28. doi: 10.1002/art.40084).
There also were no significant differences between the two study groups in secondary endpoints, including rates of coronary revascularization, acute coronary syndrome, heart failure, and all-cause mortality. In addition, all subgroup analyses confirmed that tocilizumab did not raise CV risk, regardless of patient age (younger than or older than 60 years), the presence of cardiovascular disease at baseline, the presence of diabetes, the use of methotrexate, the use of oral steroids, or the use of statins.
These “reassuring” findings show that even though tocilizumab appears to raise LDL levels, “such increases do not appear to be associated with an increased risk of clinical CV events,” the investigators said.
The results confirm those reported at the 2016 American College of Rheumatology annual meeting for the 5-year, randomized, postmarketing ENTRACTE trial in which the lipid changes induced by tocilizumab did not translate into an increased risk of heart attack or stroke in RA patients.
This cohort study was sponsored by Genentech, which markets tocilizumab (Actemra). Dr. Kim reported ties to Genentech, Lilly, Pfizer, Bristol-Myers Squibb, and AstraZeneca. Her associates reported ties to Genentech, Lilly, Pfizer, AstraZeneca, Amgen, Corrona, Whiscon, Aetion, and Boehringer Ingelheim. Three of the seven authors were employees of Genentech.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Patients with refractory RA who switch to tocilizumab show no increased cardiovascular risk, compared with those who switch to a TNF inhibitor.
Major finding: The primary outcome – the rate of a composite of MI and stroke – was 0.52 per 100 person-years with tocilizumab and 0.59 per 100 person-years for TNF inhibitors, a nonsignificant difference.
Data source: A cohort study involving 28,028 adults with RA enrolled in three large health care claims databases from all 50 states who were followed for a median of 1 year.
Disclosures: This study was sponsored by Genentech, which markets tocilizumab (Actemra). Dr. Kim reported ties to Genentech, Lilly, Pfizer, Bristol-Myers Squibb, and AstraZeneca. Her associates reported ties to Genentech, Lilly, Pfizer, AstraZeneca, Amgen, Corrona, Whiscon, Aetion, and Boehringer Ingelheim. Three of the seven authors were employees of Genentech.
Calls for faculty participation in the CHEST PREP program
About PREP
The CHEST PREP Clinical Immersion program is an unbranded, disease-state program that educates industry members and partners to advance their knowledge into understanding that builds their confidence for engagement in clinical
conversations with health-care teams. We are seeking faculty for the following initiatives:
1. The CHEST PREP program is embarking on a curriculum and content development initiative and is seeking interested faculty members to consider participating in the development of content in the areas of CTEPH, Alpha-1 Antitrypsin, and Bronchiectasis.
2. The CHEST PREP program is seeking interested CHEST members in Chicago-based institutions to consider participating as faculty presenters in the following disease areas: COPD, Asthma, PAH, CTEPH, IPF, SCLC, and NSCLC.
Requirements for participation
1. PREP welcomes faculty who would be interested in creating two or more presentations and/or cases on an assigned topic using a flipped classroom, interactive design. A minimum of four faculty experts will be needed per disease state indicated previously. Honorarium provided.
2. PREP welcomes faculty from Chicago-based institutions who would be interested in participating as faculty presenters in the disease states indicated previously. Honorarium provided.
Selection criteria
To be considered, please indicate the disease area in which you are interested in participating as content developer or faculty presenter, as well as providing the best way to contact you. For the asthma curriculum, we have a specific need for expertise/interest in moderate to severe asthma and the use of biologics in treatment.
If you are interested in participating in this initiative, please contact Jasmine Turner ([email protected]). Thank you.
About PREP
The CHEST PREP Clinical Immersion program is an unbranded, disease-state program that educates industry members and partners to advance their knowledge into understanding that builds their confidence for engagement in clinical
conversations with health-care teams. We are seeking faculty for the following initiatives:
1. The CHEST PREP program is embarking on a curriculum and content development initiative and is seeking interested faculty members to consider participating in the development of content in the areas of CTEPH, Alpha-1 Antitrypsin, and Bronchiectasis.
2. The CHEST PREP program is seeking interested CHEST members in Chicago-based institutions to consider participating as faculty presenters in the following disease areas: COPD, Asthma, PAH, CTEPH, IPF, SCLC, and NSCLC.
Requirements for participation
1. PREP welcomes faculty who would be interested in creating two or more presentations and/or cases on an assigned topic using a flipped classroom, interactive design. A minimum of four faculty experts will be needed per disease state indicated previously. Honorarium provided.
2. PREP welcomes faculty from Chicago-based institutions who would be interested in participating as faculty presenters in the disease states indicated previously. Honorarium provided.
Selection criteria
To be considered, please indicate the disease area in which you are interested in participating as content developer or faculty presenter, as well as providing the best way to contact you. For the asthma curriculum, we have a specific need for expertise/interest in moderate to severe asthma and the use of biologics in treatment.
If you are interested in participating in this initiative, please contact Jasmine Turner ([email protected]). Thank you.
About PREP
The CHEST PREP Clinical Immersion program is an unbranded, disease-state program that educates industry members and partners to advance their knowledge into understanding that builds their confidence for engagement in clinical
conversations with health-care teams. We are seeking faculty for the following initiatives:
1. The CHEST PREP program is embarking on a curriculum and content development initiative and is seeking interested faculty members to consider participating in the development of content in the areas of CTEPH, Alpha-1 Antitrypsin, and Bronchiectasis.
2. The CHEST PREP program is seeking interested CHEST members in Chicago-based institutions to consider participating as faculty presenters in the following disease areas: COPD, Asthma, PAH, CTEPH, IPF, SCLC, and NSCLC.
Requirements for participation
1. PREP welcomes faculty who would be interested in creating two or more presentations and/or cases on an assigned topic using a flipped classroom, interactive design. A minimum of four faculty experts will be needed per disease state indicated previously. Honorarium provided.
2. PREP welcomes faculty from Chicago-based institutions who would be interested in participating as faculty presenters in the disease states indicated previously. Honorarium provided.
Selection criteria
To be considered, please indicate the disease area in which you are interested in participating as content developer or faculty presenter, as well as providing the best way to contact you. For the asthma curriculum, we have a specific need for expertise/interest in moderate to severe asthma and the use of biologics in treatment.
If you are interested in participating in this initiative, please contact Jasmine Turner ([email protected]). Thank you.
Pulmonary Hypertension Care Center initiative moves forward
The Pulmonary Hypertension Association (PHA) launched its Pulmonary Hypertension Care Center (PHCC) initiative 2 years ago. This initiative was designed to raise the quality of care, as well as long-term outcomes for this disease that is often misdiagnosed and progressive. The PHCC program has designated 41 adult and 6 pediatric sites as Comprehensive Care Centers with ongoing accreditation of new sites. As part of this program, the PHA Registry was established to provide input to improve the care of PH patients. The PHA Registry (PHAR) is a multicenter, prospective observational registry of newly evaluated patients with pulmonary arterial hypertension (PAH) and has enrolled 200 patients to date. PHAR participation is open to any PHCC-accredited center.
PHCC accreditation has two pathways: Comprehensive Care Centers and Regional Care Centers. Accreditation is based on adherence “to consensus guidelines for the diagnosis and treatment of PH, the scope of PH-related services provided at the center, and the expertise of the center’s PH Care Team members.” PHCC accreditation is potentially available to all PH centers that meet the established criteria that can be found at the PHCC website.
Additional information may be found at the PHCC website (https://phassociation.org/PHCareCenters).
The Pulmonary Hypertension Association (PHA) launched its Pulmonary Hypertension Care Center (PHCC) initiative 2 years ago. This initiative was designed to raise the quality of care, as well as long-term outcomes for this disease that is often misdiagnosed and progressive. The PHCC program has designated 41 adult and 6 pediatric sites as Comprehensive Care Centers with ongoing accreditation of new sites. As part of this program, the PHA Registry was established to provide input to improve the care of PH patients. The PHA Registry (PHAR) is a multicenter, prospective observational registry of newly evaluated patients with pulmonary arterial hypertension (PAH) and has enrolled 200 patients to date. PHAR participation is open to any PHCC-accredited center.
PHCC accreditation has two pathways: Comprehensive Care Centers and Regional Care Centers. Accreditation is based on adherence “to consensus guidelines for the diagnosis and treatment of PH, the scope of PH-related services provided at the center, and the expertise of the center’s PH Care Team members.” PHCC accreditation is potentially available to all PH centers that meet the established criteria that can be found at the PHCC website.
Additional information may be found at the PHCC website (https://phassociation.org/PHCareCenters).
The Pulmonary Hypertension Association (PHA) launched its Pulmonary Hypertension Care Center (PHCC) initiative 2 years ago. This initiative was designed to raise the quality of care, as well as long-term outcomes for this disease that is often misdiagnosed and progressive. The PHCC program has designated 41 adult and 6 pediatric sites as Comprehensive Care Centers with ongoing accreditation of new sites. As part of this program, the PHA Registry was established to provide input to improve the care of PH patients. The PHA Registry (PHAR) is a multicenter, prospective observational registry of newly evaluated patients with pulmonary arterial hypertension (PAH) and has enrolled 200 patients to date. PHAR participation is open to any PHCC-accredited center.
PHCC accreditation has two pathways: Comprehensive Care Centers and Regional Care Centers. Accreditation is based on adherence “to consensus guidelines for the diagnosis and treatment of PH, the scope of PH-related services provided at the center, and the expertise of the center’s PH Care Team members.” PHCC accreditation is potentially available to all PH centers that meet the established criteria that can be found at the PHCC website.
Additional information may be found at the PHCC website (https://phassociation.org/PHCareCenters).
Alternative to 10-year ABIM exam starts 2018
On December 14, the American Board of Internal Medicine (ABIM) announced an alternative to the 10-year Internal Medicine recertification exam, effective 2018. Currently, ABIM board–certified physicians can participate in Maintenance of Certification (MOC) by earning 100 MOC points every 5 years and passing a maintenance of certification exam every 10 years.
Beginning in 2018, physicians who are certified by the ABIM in Internal Medicine will have the option to take a lower-stakes exam every 2 years, rather than taking the current high-stakes exam every 10 years. The low-stakes exam option provides greater flexibility to the diplomate by allowing one to complete the examination at a convenient time set by the physician at home or in the office. While this new option will initially be available only to Internal Medicine diplomates, the ABIM intends to extend this alternative recertification model to subspecialties in the future.
CHEST is exploring how our education will evolve to address these key changes. For additional information, please visit ABIM’s website.
On December 14, the American Board of Internal Medicine (ABIM) announced an alternative to the 10-year Internal Medicine recertification exam, effective 2018. Currently, ABIM board–certified physicians can participate in Maintenance of Certification (MOC) by earning 100 MOC points every 5 years and passing a maintenance of certification exam every 10 years.
Beginning in 2018, physicians who are certified by the ABIM in Internal Medicine will have the option to take a lower-stakes exam every 2 years, rather than taking the current high-stakes exam every 10 years. The low-stakes exam option provides greater flexibility to the diplomate by allowing one to complete the examination at a convenient time set by the physician at home or in the office. While this new option will initially be available only to Internal Medicine diplomates, the ABIM intends to extend this alternative recertification model to subspecialties in the future.
CHEST is exploring how our education will evolve to address these key changes. For additional information, please visit ABIM’s website.
On December 14, the American Board of Internal Medicine (ABIM) announced an alternative to the 10-year Internal Medicine recertification exam, effective 2018. Currently, ABIM board–certified physicians can participate in Maintenance of Certification (MOC) by earning 100 MOC points every 5 years and passing a maintenance of certification exam every 10 years.
Beginning in 2018, physicians who are certified by the ABIM in Internal Medicine will have the option to take a lower-stakes exam every 2 years, rather than taking the current high-stakes exam every 10 years. The low-stakes exam option provides greater flexibility to the diplomate by allowing one to complete the examination at a convenient time set by the physician at home or in the office. While this new option will initially be available only to Internal Medicine diplomates, the ABIM intends to extend this alternative recertification model to subspecialties in the future.
CHEST is exploring how our education will evolve to address these key changes. For additional information, please visit ABIM’s website.
Optimal gestational age for cell-free DNA sampling in obese women
cfDNA screening failures occur in 1% to 12% of samples, a rate that has an inverse relationship to gestational age. Recent studies have shown an increased risk for screening failures among obese women. To determine the optimal gestational age for cfDNA testing among obese women, Mary C. Livergood, MD, and colleagues at the Mercy Hospital in St. Louis, Missouri, performed a retrospective cohort study of those undergoing cfDNA testing at one center from 2011 through 2016. Study results recently were published online in the American Journal of Obstetrics and Gynecology.1
Details of the study
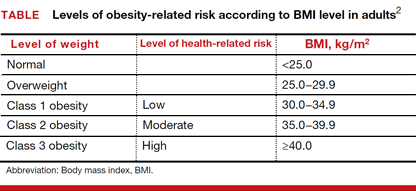
Adjusted odds ratios (aORs) with 95% confidence interval (CI) for a cfDNA screening failure (referred to as a “no call” in the study) were determined for each body mass index (BMI) weight class (TABLE). Each BMI weight class also was compared with the aOR of normal-weight women (BMI <25.0 kg/m2). The predicted probability of a no call was determined for each week of gestational age for normal weight and obese women and the results were compared.1
Among the 2,385 patients meeting inclusion criteria, 4.4% (n = 105) received a no call. Compared with normal weight women, the aOR of no call increased as weight increased from overweight (aOR, 2.31 [95% CI, 1.21–4.42]) to obesity class III (aOR, 8.55 [95% CI, 4.16–17.56]).1
At 21 weeks’ gestation, a cut-point was identified for obesity class II/III women (ie, there was no longer a significant difference in the probability of no call when compared with normal-weight women). From 8 to 16 weeks’ gestation, there was a 4.5% reduction in the probability of a no call for obesity class II/III women (aOR, 14.9; 95% CI, 8.95–20.78 and aOR, 10.4; 95% CI, 7.20–13.61; Ptrend<.01).1
Although the authors conclude that a cut-point of 21 weeks’ gestation allowed for optimal sampling of cfDNA in obese women, they also acknowledge that this cut-point limits a woman’s reproductive choices. However, they say that delaying cfDNA testing in obese women is a reasonable strategy to reduce the probability of screening failure.1
- Livergood MC, Lechien KA, Trudell AS. Obesity and cell-free DNA “no calls”: is there an optimal gestational age at time of sampling? [published online ahead of print January 28, 2017]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2017.01.011.
- Health risks of obesity. MedlinePlus website. https://medlineplus.gov/ency/patientinstructions/000348.htm. Updated February 7, 2017. Accessed March 10, 2017.
cfDNA screening failures occur in 1% to 12% of samples, a rate that has an inverse relationship to gestational age. Recent studies have shown an increased risk for screening failures among obese women. To determine the optimal gestational age for cfDNA testing among obese women, Mary C. Livergood, MD, and colleagues at the Mercy Hospital in St. Louis, Missouri, performed a retrospective cohort study of those undergoing cfDNA testing at one center from 2011 through 2016. Study results recently were published online in the American Journal of Obstetrics and Gynecology.1
Details of the study
Adjusted odds ratios (aORs) with 95% confidence interval (CI) for a cfDNA screening failure (referred to as a “no call” in the study) were determined for each body mass index (BMI) weight class (TABLE). Each BMI weight class also was compared with the aOR of normal-weight women (BMI <25.0 kg/m2). The predicted probability of a no call was determined for each week of gestational age for normal weight and obese women and the results were compared.1

Among the 2,385 patients meeting inclusion criteria, 4.4% (n = 105) received a no call. Compared with normal weight women, the aOR of no call increased as weight increased from overweight (aOR, 2.31 [95% CI, 1.21–4.42]) to obesity class III (aOR, 8.55 [95% CI, 4.16–17.56]).1
At 21 weeks’ gestation, a cut-point was identified for obesity class II/III women (ie, there was no longer a significant difference in the probability of no call when compared with normal-weight women). From 8 to 16 weeks’ gestation, there was a 4.5% reduction in the probability of a no call for obesity class II/III women (aOR, 14.9; 95% CI, 8.95–20.78 and aOR, 10.4; 95% CI, 7.20–13.61; Ptrend<.01).1
Although the authors conclude that a cut-point of 21 weeks’ gestation allowed for optimal sampling of cfDNA in obese women, they also acknowledge that this cut-point limits a woman’s reproductive choices. However, they say that delaying cfDNA testing in obese women is a reasonable strategy to reduce the probability of screening failure.1
cfDNA screening failures occur in 1% to 12% of samples, a rate that has an inverse relationship to gestational age. Recent studies have shown an increased risk for screening failures among obese women. To determine the optimal gestational age for cfDNA testing among obese women, Mary C. Livergood, MD, and colleagues at the Mercy Hospital in St. Louis, Missouri, performed a retrospective cohort study of those undergoing cfDNA testing at one center from 2011 through 2016. Study results recently were published online in the American Journal of Obstetrics and Gynecology.1
Details of the study
Adjusted odds ratios (aORs) with 95% confidence interval (CI) for a cfDNA screening failure (referred to as a “no call” in the study) were determined for each body mass index (BMI) weight class (TABLE). Each BMI weight class also was compared with the aOR of normal-weight women (BMI <25.0 kg/m2). The predicted probability of a no call was determined for each week of gestational age for normal weight and obese women and the results were compared.1

Among the 2,385 patients meeting inclusion criteria, 4.4% (n = 105) received a no call. Compared with normal weight women, the aOR of no call increased as weight increased from overweight (aOR, 2.31 [95% CI, 1.21–4.42]) to obesity class III (aOR, 8.55 [95% CI, 4.16–17.56]).1
At 21 weeks’ gestation, a cut-point was identified for obesity class II/III women (ie, there was no longer a significant difference in the probability of no call when compared with normal-weight women). From 8 to 16 weeks’ gestation, there was a 4.5% reduction in the probability of a no call for obesity class II/III women (aOR, 14.9; 95% CI, 8.95–20.78 and aOR, 10.4; 95% CI, 7.20–13.61; Ptrend<.01).1
Although the authors conclude that a cut-point of 21 weeks’ gestation allowed for optimal sampling of cfDNA in obese women, they also acknowledge that this cut-point limits a woman’s reproductive choices. However, they say that delaying cfDNA testing in obese women is a reasonable strategy to reduce the probability of screening failure.1
- Livergood MC, Lechien KA, Trudell AS. Obesity and cell-free DNA “no calls”: is there an optimal gestational age at time of sampling? [published online ahead of print January 28, 2017]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2017.01.011.
- Health risks of obesity. MedlinePlus website. https://medlineplus.gov/ency/patientinstructions/000348.htm. Updated February 7, 2017. Accessed March 10, 2017.
- Livergood MC, Lechien KA, Trudell AS. Obesity and cell-free DNA “no calls”: is there an optimal gestational age at time of sampling? [published online ahead of print January 28, 2017]. Am J Obstet Gynecol. doi:10.1016/j.ajog.2017.01.011.
- Health risks of obesity. MedlinePlus website. https://medlineplus.gov/ency/patientinstructions/000348.htm. Updated February 7, 2017. Accessed March 10, 2017.
Phacomatosis Cesioflammea in Association With von Recklinghausen Disease (Neurofibromatosis Type I)
To the Editor:
Vascular lesions associated with melanocytic nevi were first described by Ota et al1 in 1947 and given the name phacomatosis pigmentovascularis. In 2005, Happle2 reclassified phacomatosis pigmentovascularis into 3 well-defined types: (1) phacomatosis cesioflammea: blue spots (caesius means bluish gray in Latin) and nevus flammeus; (2) phacomatosis spilorosea: nevus spilus coexisting with a pale pink telangiectatic nevus; and (3) phacomatosis cesiomarmorata: blue spots and cutis marmorata telangiectatica congenita. In 2011 Joshi et al3 described a case of a 31-year-old woman who had a port-wine stain in association with neurofibromatosis type I (NF-1). We present a case of phacomatosis cesioflammea in association with NF-1.
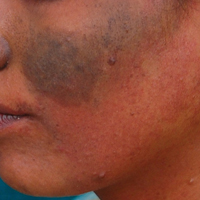
A 20-year-old woman presented to our outpatient section with a bluish black birthmark on the left side of the face since birth with the onset of multiple painless flesh-colored nodules on the trunk and arms of 1 year’s duration. She reported having occasional pruritus over the nodular lesions. Cutaneous examination showed multiple well-defined café au lait macules (0.5–3.0 cm) with regular margins. Multiple flesh-colored nodules were evident on the upper arms (Figure 1) and trunk. The nodules were firm in consistency and showed buttonholing phenomenon with some of the lesions demonstrating bag-of-worms consistency on palpation. Both palms showed multiple brownish frecklelike macules (Figure 2). A single bluish patch extended from the left ala of the nose to the sideburns. Adjoining the bluish patch was a subtle, ill-defined, nonblanchable red patch extending from the lower margin of the bluish patch to the mandibular ridge (Figure 3). Ocular examination showed melanosis bulbi of the left sclera and a few iris hamartomas (Lisch nodules) in both eyes. A biopsy of the skin nodule was obtained under local anesthesia after obtaining the patient’s informed consent; the specimen was fixed in 10% buffered formalin. A hematoxylin and eosin–stained section showed a well-circumscribed nonencapsulated tumor in the dermis composed of loosely spaced spindle cells and wavy collagenous strands (Figure 4). Routine hemogram and blood biochemistry including urinalysis were within reference range. Radiologic examination of the long bones was unremarkable. Our patient had 3 of 6 criteria defined by the National Institutes of Health for diagnosis of NF-1.4 On clinicopathological correlation we made a diagnosis of phacomatosis cesioflammea in association with NF-1. We have reassured the patient about the benign nature of vascular nevus. She was informed that the skin nodules could increase in size during pregnancy and to regularly follow-up with an eye specialist if any visual abnormalities occur.
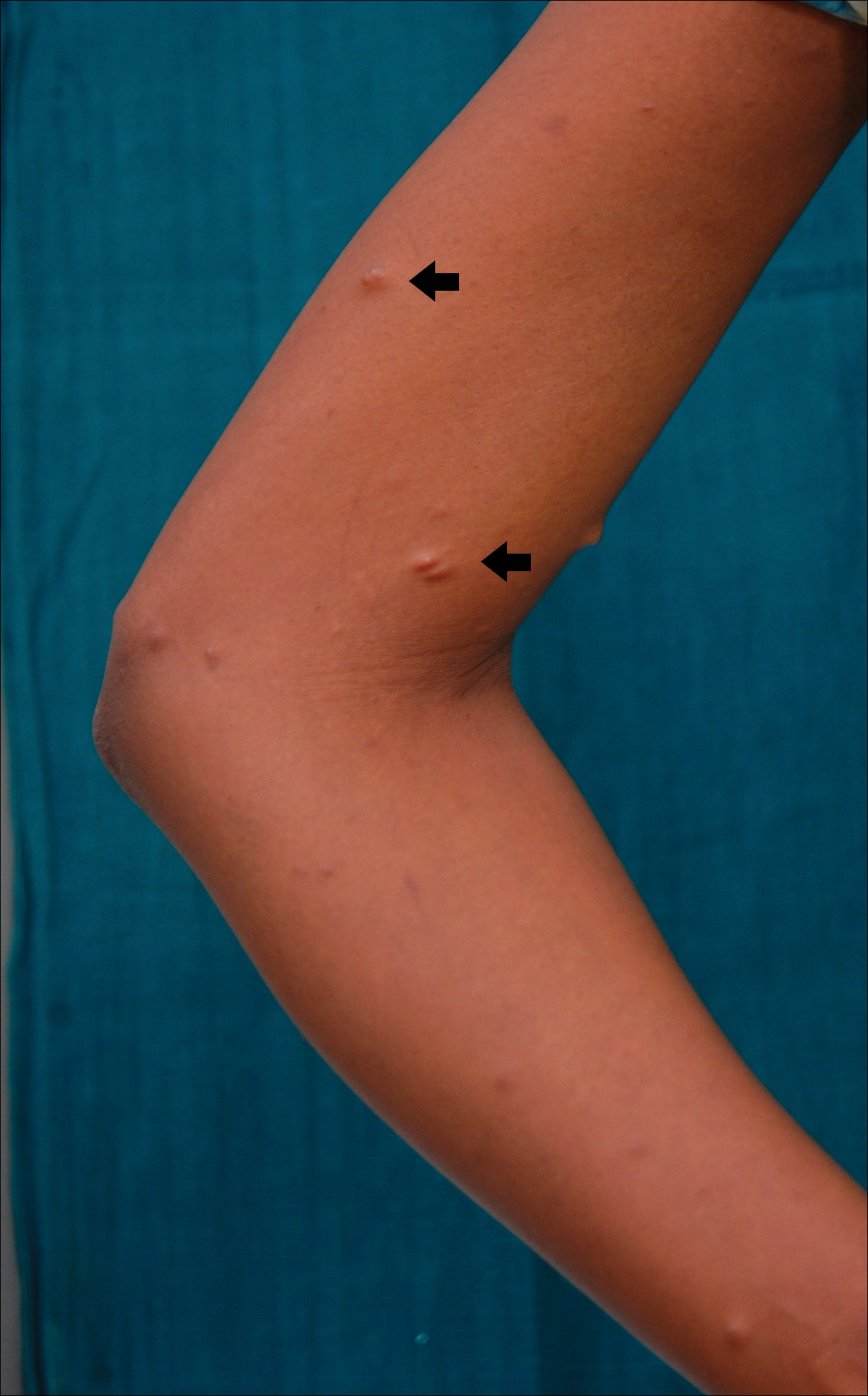
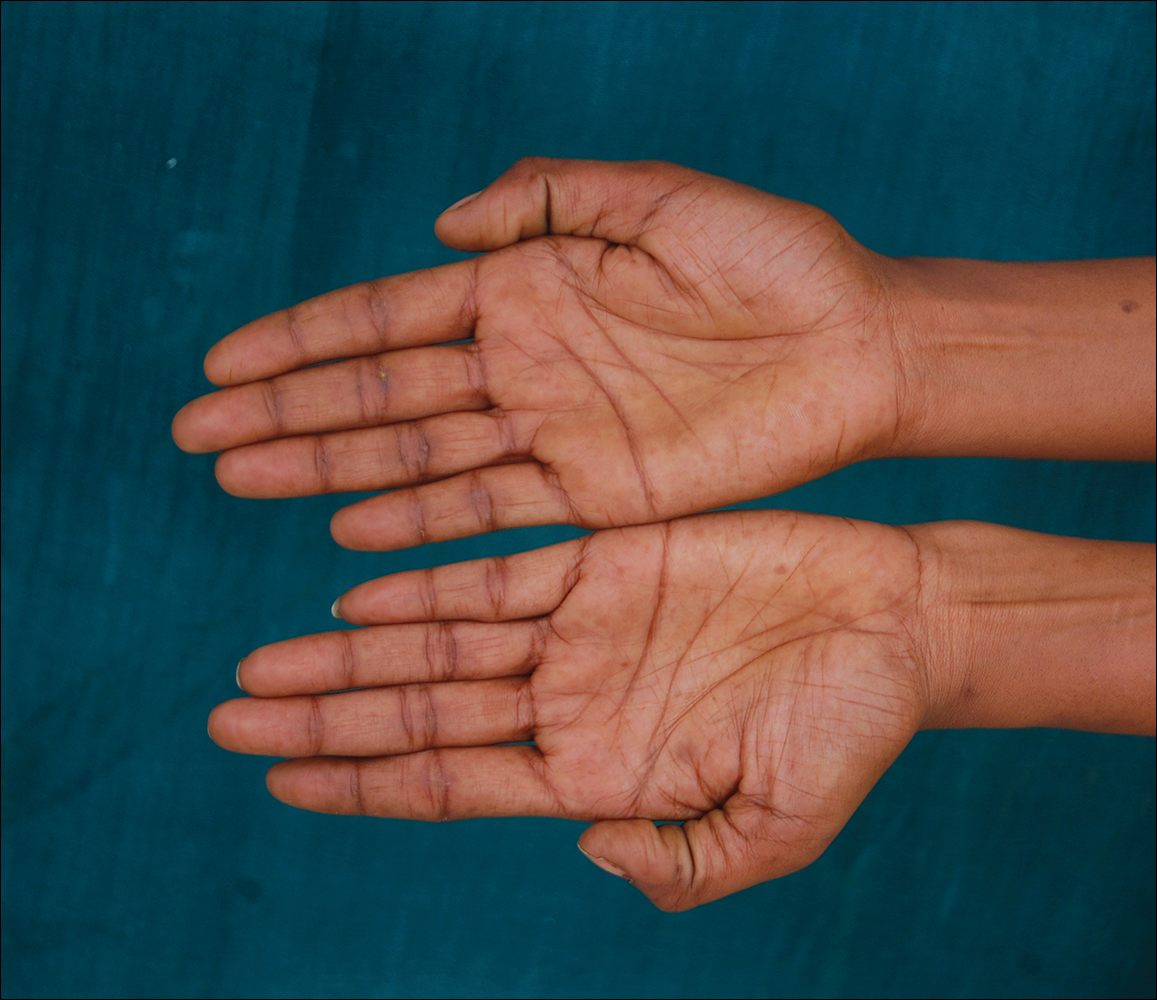
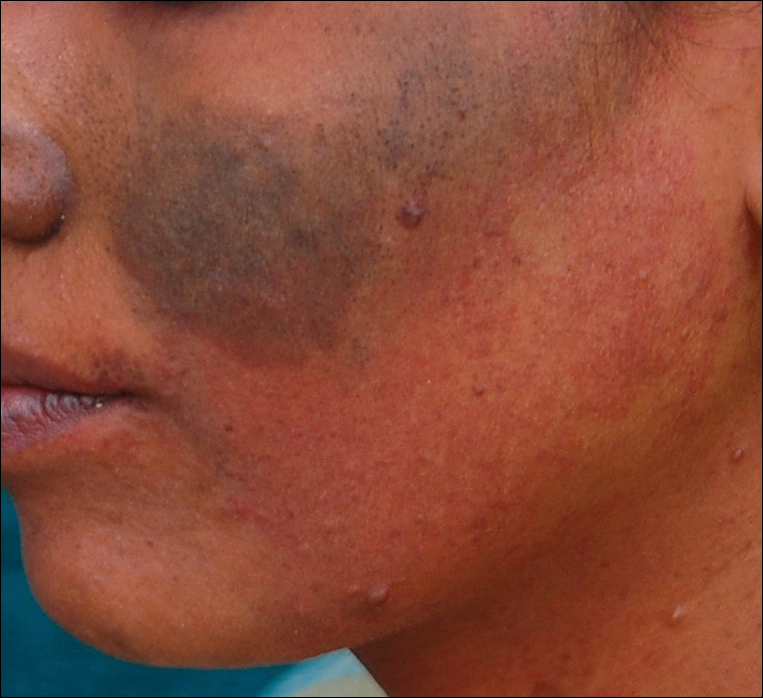
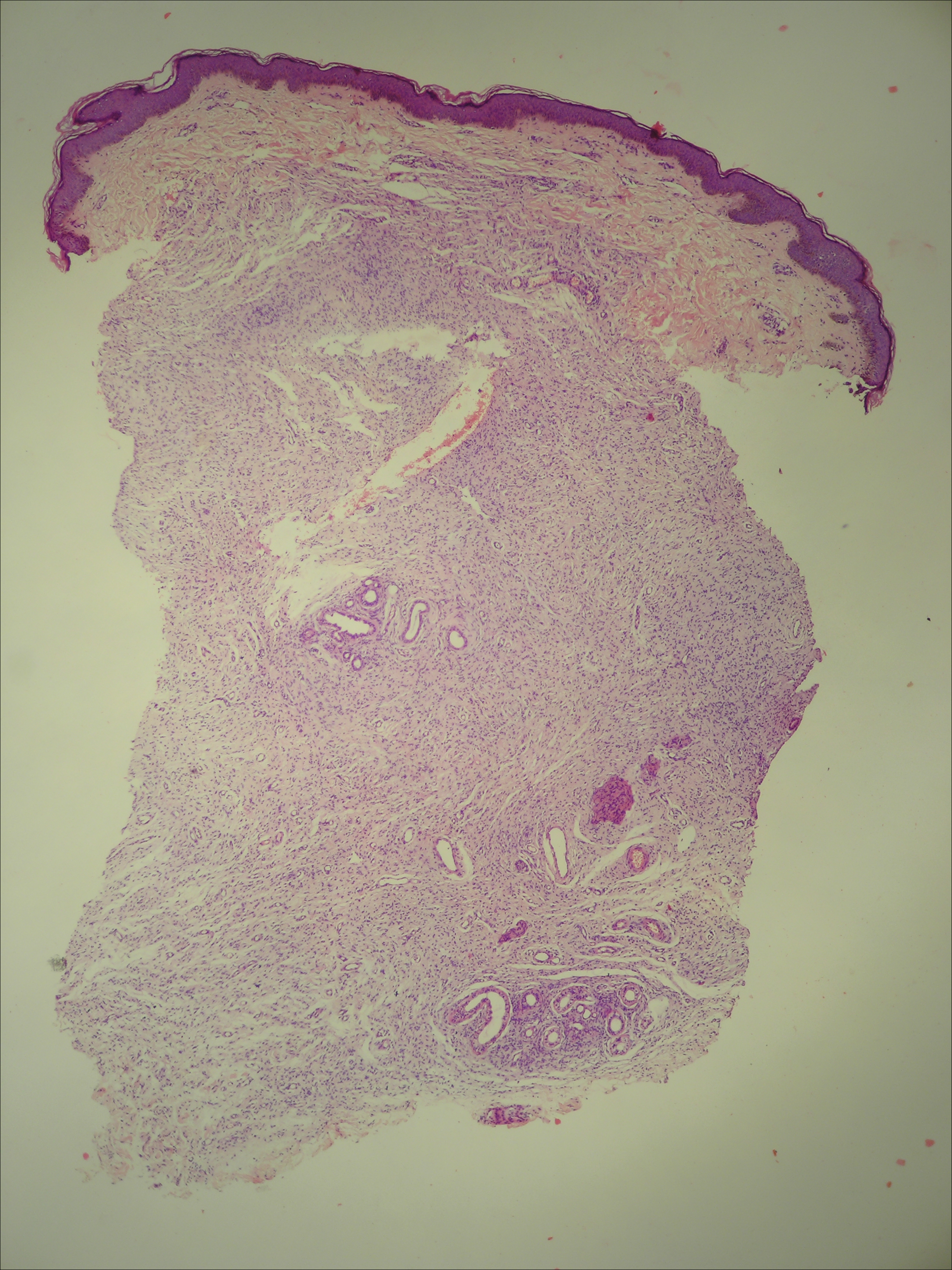
The term phacomatosis is applied to genetically determined disorders of tissue derived from ectodermal origin (eg, skin, central nervous system, eyes) and commonly includes NF-1, tuberous sclerosis, and von Hippel-Lindau syndrome. Neurofibromatosis type I was first described by German pathologist Friedrich Daniel von Recklinghausen.5 Phacomatosis pigmentovascularis has been defined as the association of vascular nevus with a pigmentary nevus. Its pathogenesis can be explained by the twin spotting phenomenon.6 Twin spots are paired patches of mutant tissue that differ from each other and from the surrounding normal background skin. They can occur as 2 clinical types: allelic and nonallelic twin spotting. Our patient had nonallelic twin spots for 2 nevoid conditions: vascular (nevus flammeus) and pigmentary (nevus of Ota). Nevus of Ota was distributed in the V2 segment (maxillary nerve) of the fifth cranial nerve along with classical melanosis bulbi, which is considered a characteristic clinical feature of nevus of Ota (nevus cesius).7 Nevus flammeus (port-wine stain) is a vascular malformation presenting with flat lesions that persists throughout a patient’s life. The phenomenon of twin spotting, or didymosis (didymos means twin in Greek), has been proposed for co-occurrence of vascular and pigmented nevi.8 The association of NF-1 along with phacomatosis cesioflammea (a twin spot) could be explained from mosaicism of tissues derived from neuroectodermal and mesenchymal elements. Neurofibromatosis type I can occur as a mosaic disorder due to either postzygotic germ line or somatic mutations in the NF1 gene located on the proximal long arm of chromosome 17.9 Irrespective of the mutational event, a mosaic patient has a mixture of cells, some have normal copies of a particular gene and others have an abnormal copy of the same gene. Somatic mutation can lead to segmental (localized), generalized, or gonadal mosaicism. Somatic mutations occurring early during embryonic development produce generalized mosaicism, and generalized mosaics clinically appear similar to nonmosaic NF-1 cases.10,11 However, due to a lack of adequate facilities for mutation analysis and financial constraints, we were unable to confirm our case as generalized somatic mosaic for NF1 gene.
Several morphologic abnormalities have been reported with phacomatosis cesioflammea. Wu et al12 reported a single case of phacomatosis cesioflammea associated with pectus excavatum in a 9-month-old infant. Shields et al13 suggested that a thorough ocular examination on a periodic basis is essential to rule out melanoma of ocular tissues in patients with nevus flammeus and ocular melanosis.
Phacomatosis cesioflammea can occur in association with NF-1. The exact incidence of association is not known. The nevoid condition can be treated with appropriate lasers.
- Ota M, Kawamura T, Ito N. Phacomatosis pigmentovascularis (Ota). Jpn J Dermatol. 1947;52:1-3.
- Happle R. Phacomatosis pigmentovascularis revisited and reclassified. Arch Dermatol. 2005;141:385-388.
- Joshi A, Manchanda Y, Rijhwani M. Port-wine-stain with rare associations in two cases from Kuwait: phakomatosis pigmentovascularis redefined. Gulf J Dermatol Venereol. 2011;18:59-64.
- Neurofibromatosis. Conference Statement. National Institutes of Health Consensus. Arch Neurol. 1988;45:575-578.
- Gerber PA, Antal AS, Neumann NJ, et al. Neurofibromatosis. Eur J Med Res. 2009;14:102-105.
- Goyal T, Varshney A. Phacomatosis cesioflammea: first case report from India. Indian J Dermatol Venereol Leprol. 2010;76:307.
- Happle R. Didymosis cesioanemica: an unusual counterpart of phakomatosis cesioflammea. Eur J Dermatol. 2011;21:471.
- Happle R, Steijlen PM. Phacomatosis pigmentovascularis interpreted as a phenomenon of twin spots [in German]. Hautarzt. 1989;40:721-724.
- Adigun CG, Stein J. Segmental neurofibromatosis. Dermatol Online J. 2011;17:25.
- Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433-1443.
- Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 2009;61:1-14.
- Wu CY, Chen PH, Chen GS. Phacomatosis cesioflammea associated with pectus excavatum. Acta Derm Venereol. 2009;89:309-310.
- Shields CL, Kligman BE, Suriano M, et al. Phacomatosis pigmentovascularis of cesioflammea type in 7 patients: combination of ocular pigmentation (melanocytosis or melanosis) and nevus flammeus with risk for melanoma. Arch Ophthalmol. 2011;129:746-750.
To the Editor:
Vascular lesions associated with melanocytic nevi were first described by Ota et al1 in 1947 and given the name phacomatosis pigmentovascularis. In 2005, Happle2 reclassified phacomatosis pigmentovascularis into 3 well-defined types: (1) phacomatosis cesioflammea: blue spots (caesius means bluish gray in Latin) and nevus flammeus; (2) phacomatosis spilorosea: nevus spilus coexisting with a pale pink telangiectatic nevus; and (3) phacomatosis cesiomarmorata: blue spots and cutis marmorata telangiectatica congenita. In 2011 Joshi et al3 described a case of a 31-year-old woman who had a port-wine stain in association with neurofibromatosis type I (NF-1). We present a case of phacomatosis cesioflammea in association with NF-1.
A 20-year-old woman presented to our outpatient section with a bluish black birthmark on the left side of the face since birth with the onset of multiple painless flesh-colored nodules on the trunk and arms of 1 year’s duration. She reported having occasional pruritus over the nodular lesions. Cutaneous examination showed multiple well-defined café au lait macules (0.5–3.0 cm) with regular margins. Multiple flesh-colored nodules were evident on the upper arms (Figure 1) and trunk. The nodules were firm in consistency and showed buttonholing phenomenon with some of the lesions demonstrating bag-of-worms consistency on palpation. Both palms showed multiple brownish frecklelike macules (Figure 2). A single bluish patch extended from the left ala of the nose to the sideburns. Adjoining the bluish patch was a subtle, ill-defined, nonblanchable red patch extending from the lower margin of the bluish patch to the mandibular ridge (Figure 3). Ocular examination showed melanosis bulbi of the left sclera and a few iris hamartomas (Lisch nodules) in both eyes. A biopsy of the skin nodule was obtained under local anesthesia after obtaining the patient’s informed consent; the specimen was fixed in 10% buffered formalin. A hematoxylin and eosin–stained section showed a well-circumscribed nonencapsulated tumor in the dermis composed of loosely spaced spindle cells and wavy collagenous strands (Figure 4). Routine hemogram and blood biochemistry including urinalysis were within reference range. Radiologic examination of the long bones was unremarkable. Our patient had 3 of 6 criteria defined by the National Institutes of Health for diagnosis of NF-1.4 On clinicopathological correlation we made a diagnosis of phacomatosis cesioflammea in association with NF-1. We have reassured the patient about the benign nature of vascular nevus. She was informed that the skin nodules could increase in size during pregnancy and to regularly follow-up with an eye specialist if any visual abnormalities occur.




The term phacomatosis is applied to genetically determined disorders of tissue derived from ectodermal origin (eg, skin, central nervous system, eyes) and commonly includes NF-1, tuberous sclerosis, and von Hippel-Lindau syndrome. Neurofibromatosis type I was first described by German pathologist Friedrich Daniel von Recklinghausen.5 Phacomatosis pigmentovascularis has been defined as the association of vascular nevus with a pigmentary nevus. Its pathogenesis can be explained by the twin spotting phenomenon.6 Twin spots are paired patches of mutant tissue that differ from each other and from the surrounding normal background skin. They can occur as 2 clinical types: allelic and nonallelic twin spotting. Our patient had nonallelic twin spots for 2 nevoid conditions: vascular (nevus flammeus) and pigmentary (nevus of Ota). Nevus of Ota was distributed in the V2 segment (maxillary nerve) of the fifth cranial nerve along with classical melanosis bulbi, which is considered a characteristic clinical feature of nevus of Ota (nevus cesius).7 Nevus flammeus (port-wine stain) is a vascular malformation presenting with flat lesions that persists throughout a patient’s life. The phenomenon of twin spotting, or didymosis (didymos means twin in Greek), has been proposed for co-occurrence of vascular and pigmented nevi.8 The association of NF-1 along with phacomatosis cesioflammea (a twin spot) could be explained from mosaicism of tissues derived from neuroectodermal and mesenchymal elements. Neurofibromatosis type I can occur as a mosaic disorder due to either postzygotic germ line or somatic mutations in the NF1 gene located on the proximal long arm of chromosome 17.9 Irrespective of the mutational event, a mosaic patient has a mixture of cells, some have normal copies of a particular gene and others have an abnormal copy of the same gene. Somatic mutation can lead to segmental (localized), generalized, or gonadal mosaicism. Somatic mutations occurring early during embryonic development produce generalized mosaicism, and generalized mosaics clinically appear similar to nonmosaic NF-1 cases.10,11 However, due to a lack of adequate facilities for mutation analysis and financial constraints, we were unable to confirm our case as generalized somatic mosaic for NF1 gene.
Several morphologic abnormalities have been reported with phacomatosis cesioflammea. Wu et al12 reported a single case of phacomatosis cesioflammea associated with pectus excavatum in a 9-month-old infant. Shields et al13 suggested that a thorough ocular examination on a periodic basis is essential to rule out melanoma of ocular tissues in patients with nevus flammeus and ocular melanosis.
Phacomatosis cesioflammea can occur in association with NF-1. The exact incidence of association is not known. The nevoid condition can be treated with appropriate lasers.
To the Editor:
Vascular lesions associated with melanocytic nevi were first described by Ota et al1 in 1947 and given the name phacomatosis pigmentovascularis. In 2005, Happle2 reclassified phacomatosis pigmentovascularis into 3 well-defined types: (1) phacomatosis cesioflammea: blue spots (caesius means bluish gray in Latin) and nevus flammeus; (2) phacomatosis spilorosea: nevus spilus coexisting with a pale pink telangiectatic nevus; and (3) phacomatosis cesiomarmorata: blue spots and cutis marmorata telangiectatica congenita. In 2011 Joshi et al3 described a case of a 31-year-old woman who had a port-wine stain in association with neurofibromatosis type I (NF-1). We present a case of phacomatosis cesioflammea in association with NF-1.
A 20-year-old woman presented to our outpatient section with a bluish black birthmark on the left side of the face since birth with the onset of multiple painless flesh-colored nodules on the trunk and arms of 1 year’s duration. She reported having occasional pruritus over the nodular lesions. Cutaneous examination showed multiple well-defined café au lait macules (0.5–3.0 cm) with regular margins. Multiple flesh-colored nodules were evident on the upper arms (Figure 1) and trunk. The nodules were firm in consistency and showed buttonholing phenomenon with some of the lesions demonstrating bag-of-worms consistency on palpation. Both palms showed multiple brownish frecklelike macules (Figure 2). A single bluish patch extended from the left ala of the nose to the sideburns. Adjoining the bluish patch was a subtle, ill-defined, nonblanchable red patch extending from the lower margin of the bluish patch to the mandibular ridge (Figure 3). Ocular examination showed melanosis bulbi of the left sclera and a few iris hamartomas (Lisch nodules) in both eyes. A biopsy of the skin nodule was obtained under local anesthesia after obtaining the patient’s informed consent; the specimen was fixed in 10% buffered formalin. A hematoxylin and eosin–stained section showed a well-circumscribed nonencapsulated tumor in the dermis composed of loosely spaced spindle cells and wavy collagenous strands (Figure 4). Routine hemogram and blood biochemistry including urinalysis were within reference range. Radiologic examination of the long bones was unremarkable. Our patient had 3 of 6 criteria defined by the National Institutes of Health for diagnosis of NF-1.4 On clinicopathological correlation we made a diagnosis of phacomatosis cesioflammea in association with NF-1. We have reassured the patient about the benign nature of vascular nevus. She was informed that the skin nodules could increase in size during pregnancy and to regularly follow-up with an eye specialist if any visual abnormalities occur.




The term phacomatosis is applied to genetically determined disorders of tissue derived from ectodermal origin (eg, skin, central nervous system, eyes) and commonly includes NF-1, tuberous sclerosis, and von Hippel-Lindau syndrome. Neurofibromatosis type I was first described by German pathologist Friedrich Daniel von Recklinghausen.5 Phacomatosis pigmentovascularis has been defined as the association of vascular nevus with a pigmentary nevus. Its pathogenesis can be explained by the twin spotting phenomenon.6 Twin spots are paired patches of mutant tissue that differ from each other and from the surrounding normal background skin. They can occur as 2 clinical types: allelic and nonallelic twin spotting. Our patient had nonallelic twin spots for 2 nevoid conditions: vascular (nevus flammeus) and pigmentary (nevus of Ota). Nevus of Ota was distributed in the V2 segment (maxillary nerve) of the fifth cranial nerve along with classical melanosis bulbi, which is considered a characteristic clinical feature of nevus of Ota (nevus cesius).7 Nevus flammeus (port-wine stain) is a vascular malformation presenting with flat lesions that persists throughout a patient’s life. The phenomenon of twin spotting, or didymosis (didymos means twin in Greek), has been proposed for co-occurrence of vascular and pigmented nevi.8 The association of NF-1 along with phacomatosis cesioflammea (a twin spot) could be explained from mosaicism of tissues derived from neuroectodermal and mesenchymal elements. Neurofibromatosis type I can occur as a mosaic disorder due to either postzygotic germ line or somatic mutations in the NF1 gene located on the proximal long arm of chromosome 17.9 Irrespective of the mutational event, a mosaic patient has a mixture of cells, some have normal copies of a particular gene and others have an abnormal copy of the same gene. Somatic mutation can lead to segmental (localized), generalized, or gonadal mosaicism. Somatic mutations occurring early during embryonic development produce generalized mosaicism, and generalized mosaics clinically appear similar to nonmosaic NF-1 cases.10,11 However, due to a lack of adequate facilities for mutation analysis and financial constraints, we were unable to confirm our case as generalized somatic mosaic for NF1 gene.
Several morphologic abnormalities have been reported with phacomatosis cesioflammea. Wu et al12 reported a single case of phacomatosis cesioflammea associated with pectus excavatum in a 9-month-old infant. Shields et al13 suggested that a thorough ocular examination on a periodic basis is essential to rule out melanoma of ocular tissues in patients with nevus flammeus and ocular melanosis.
Phacomatosis cesioflammea can occur in association with NF-1. The exact incidence of association is not known. The nevoid condition can be treated with appropriate lasers.
- Ota M, Kawamura T, Ito N. Phacomatosis pigmentovascularis (Ota). Jpn J Dermatol. 1947;52:1-3.
- Happle R. Phacomatosis pigmentovascularis revisited and reclassified. Arch Dermatol. 2005;141:385-388.
- Joshi A, Manchanda Y, Rijhwani M. Port-wine-stain with rare associations in two cases from Kuwait: phakomatosis pigmentovascularis redefined. Gulf J Dermatol Venereol. 2011;18:59-64.
- Neurofibromatosis. Conference Statement. National Institutes of Health Consensus. Arch Neurol. 1988;45:575-578.
- Gerber PA, Antal AS, Neumann NJ, et al. Neurofibromatosis. Eur J Med Res. 2009;14:102-105.
- Goyal T, Varshney A. Phacomatosis cesioflammea: first case report from India. Indian J Dermatol Venereol Leprol. 2010;76:307.
- Happle R. Didymosis cesioanemica: an unusual counterpart of phakomatosis cesioflammea. Eur J Dermatol. 2011;21:471.
- Happle R, Steijlen PM. Phacomatosis pigmentovascularis interpreted as a phenomenon of twin spots [in German]. Hautarzt. 1989;40:721-724.
- Adigun CG, Stein J. Segmental neurofibromatosis. Dermatol Online J. 2011;17:25.
- Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433-1443.
- Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 2009;61:1-14.
- Wu CY, Chen PH, Chen GS. Phacomatosis cesioflammea associated with pectus excavatum. Acta Derm Venereol. 2009;89:309-310.
- Shields CL, Kligman BE, Suriano M, et al. Phacomatosis pigmentovascularis of cesioflammea type in 7 patients: combination of ocular pigmentation (melanocytosis or melanosis) and nevus flammeus with risk for melanoma. Arch Ophthalmol. 2011;129:746-750.
- Ota M, Kawamura T, Ito N. Phacomatosis pigmentovascularis (Ota). Jpn J Dermatol. 1947;52:1-3.
- Happle R. Phacomatosis pigmentovascularis revisited and reclassified. Arch Dermatol. 2005;141:385-388.
- Joshi A, Manchanda Y, Rijhwani M. Port-wine-stain with rare associations in two cases from Kuwait: phakomatosis pigmentovascularis redefined. Gulf J Dermatol Venereol. 2011;18:59-64.
- Neurofibromatosis. Conference Statement. National Institutes of Health Consensus. Arch Neurol. 1988;45:575-578.
- Gerber PA, Antal AS, Neumann NJ, et al. Neurofibromatosis. Eur J Med Res. 2009;14:102-105.
- Goyal T, Varshney A. Phacomatosis cesioflammea: first case report from India. Indian J Dermatol Venereol Leprol. 2010;76:307.
- Happle R. Didymosis cesioanemica: an unusual counterpart of phakomatosis cesioflammea. Eur J Dermatol. 2011;21:471.
- Happle R, Steijlen PM. Phacomatosis pigmentovascularis interpreted as a phenomenon of twin spots [in German]. Hautarzt. 1989;40:721-724.
- Adigun CG, Stein J. Segmental neurofibromatosis. Dermatol Online J. 2011;17:25.
- Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433-1443.
- Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 2009;61:1-14.
- Wu CY, Chen PH, Chen GS. Phacomatosis cesioflammea associated with pectus excavatum. Acta Derm Venereol. 2009;89:309-310.
- Shields CL, Kligman BE, Suriano M, et al. Phacomatosis pigmentovascularis of cesioflammea type in 7 patients: combination of ocular pigmentation (melanocytosis or melanosis) and nevus flammeus with risk for melanoma. Arch Ophthalmol. 2011;129:746-750.
Practice Points
- Phacomatosis cesioflammea can be associated with neurofibromatosis type I.
- The port-wine stain component of phacomatosis cesioflammea may develop nodularity in long-standing cases.
- The Nd:YAG laser is beneficial for treating blue spots of phacomatosis cesioflammea.
HCV testing stagnant among baby boomers
Despite the urging of the United States Preventive Services Task Force and other organizations in 2013, the percentage of baby boomers who underwent testing for hepatitis C (HCV) infection had barely changed 2 years later – from 12.3% in 2013 to 13.8% in 2015.
The numbers are particularly troubling because new and improved antiviral drugs offer cures that could forestall liver cancer, cirrhosis, and other potential complications, with shorter regimens and fewer side effects than older regimens.
Other reactions were more forceful. “Kind of pathetic, isn’t it?” said John D. Scott, MD, assistant director of the Hepatitis and Liver Clinic at Harborview Medical Center, and an associate professor of medicine at the University of Washington, Seattle.
The researchers analyzed 2013 and 2015 data from the National Health Interview Survey, which included records for 21,827 baby boomers with HCV testing data.
The slight increase overall of 12.3% to 13.8% was small but also statistically significant (P = .013). Some populations fared better: Compared with the privately insured, those with Medicare plus Medicaid were more likely to have been tested (prevalence ratio, 1.83; 95% confidence interval, 1.32-2.53), as were those only on Medicaid (PR, 1.35; 95% CI, 1.04-1.76), and those with military insurance (PR, 1.62; 95% CI, 1.16-2.26).
The study could be subject to recall bias, since it relied on participants’ self-reports.
The authors speculate that the higher prevalence of testing in those with military insurance may reflect efforts by the Veterans Health Administration to reduce the high prevalence of HCV-associated disease among veterans.
It’s entirely possible to increase testing rates, according to Dr. Scott, who has a grant from the Centers for Disease Control and Prevention to study ways to increase uptake. “Probably the easiest thing to do is just incorporate this information into your electronic medical record and make it part of your alerts and standard preventative practices. Try to automate a lot of this rather than remind a very busy primary care doctor of all the things they have to do,” he said.
For example, one strategy that Seattle’s King County has employed is to automatically notify the testing laboratory if an antibody test is positive. “The lab knows to keep that blood and run a second (nucleic acid) test without the patient having to come back. That has helped to get our confirmatory rates up,” said Dr. Scott.
More broadly, the importance of testing needs to be emphasized, according to Paul J. Thuluvath, MD, medical director at the Institute of Digestive Health and Liver Disease at Mercy Medical Center, Baltimore, and a professor of medicine and surgery at the University of Maryland. “We need everybody to buy into this: the primary care physicians, internists, and gynecologists. If they are not convinced of the importance of this, it’s not going to happen. And I don’t think many primary care physicians and internists are convinced yet,” he said.
Despite the urging of the United States Preventive Services Task Force and other organizations in 2013, the percentage of baby boomers who underwent testing for hepatitis C (HCV) infection had barely changed 2 years later – from 12.3% in 2013 to 13.8% in 2015.
The numbers are particularly troubling because new and improved antiviral drugs offer cures that could forestall liver cancer, cirrhosis, and other potential complications, with shorter regimens and fewer side effects than older regimens.
Other reactions were more forceful. “Kind of pathetic, isn’t it?” said John D. Scott, MD, assistant director of the Hepatitis and Liver Clinic at Harborview Medical Center, and an associate professor of medicine at the University of Washington, Seattle.
The researchers analyzed 2013 and 2015 data from the National Health Interview Survey, which included records for 21,827 baby boomers with HCV testing data.
The slight increase overall of 12.3% to 13.8% was small but also statistically significant (P = .013). Some populations fared better: Compared with the privately insured, those with Medicare plus Medicaid were more likely to have been tested (prevalence ratio, 1.83; 95% confidence interval, 1.32-2.53), as were those only on Medicaid (PR, 1.35; 95% CI, 1.04-1.76), and those with military insurance (PR, 1.62; 95% CI, 1.16-2.26).
The study could be subject to recall bias, since it relied on participants’ self-reports.
The authors speculate that the higher prevalence of testing in those with military insurance may reflect efforts by the Veterans Health Administration to reduce the high prevalence of HCV-associated disease among veterans.
It’s entirely possible to increase testing rates, according to Dr. Scott, who has a grant from the Centers for Disease Control and Prevention to study ways to increase uptake. “Probably the easiest thing to do is just incorporate this information into your electronic medical record and make it part of your alerts and standard preventative practices. Try to automate a lot of this rather than remind a very busy primary care doctor of all the things they have to do,” he said.
For example, one strategy that Seattle’s King County has employed is to automatically notify the testing laboratory if an antibody test is positive. “The lab knows to keep that blood and run a second (nucleic acid) test without the patient having to come back. That has helped to get our confirmatory rates up,” said Dr. Scott.
More broadly, the importance of testing needs to be emphasized, according to Paul J. Thuluvath, MD, medical director at the Institute of Digestive Health and Liver Disease at Mercy Medical Center, Baltimore, and a professor of medicine and surgery at the University of Maryland. “We need everybody to buy into this: the primary care physicians, internists, and gynecologists. If they are not convinced of the importance of this, it’s not going to happen. And I don’t think many primary care physicians and internists are convinced yet,” he said.
Despite the urging of the United States Preventive Services Task Force and other organizations in 2013, the percentage of baby boomers who underwent testing for hepatitis C (HCV) infection had barely changed 2 years later – from 12.3% in 2013 to 13.8% in 2015.
The numbers are particularly troubling because new and improved antiviral drugs offer cures that could forestall liver cancer, cirrhosis, and other potential complications, with shorter regimens and fewer side effects than older regimens.
Other reactions were more forceful. “Kind of pathetic, isn’t it?” said John D. Scott, MD, assistant director of the Hepatitis and Liver Clinic at Harborview Medical Center, and an associate professor of medicine at the University of Washington, Seattle.
The researchers analyzed 2013 and 2015 data from the National Health Interview Survey, which included records for 21,827 baby boomers with HCV testing data.
The slight increase overall of 12.3% to 13.8% was small but also statistically significant (P = .013). Some populations fared better: Compared with the privately insured, those with Medicare plus Medicaid were more likely to have been tested (prevalence ratio, 1.83; 95% confidence interval, 1.32-2.53), as were those only on Medicaid (PR, 1.35; 95% CI, 1.04-1.76), and those with military insurance (PR, 1.62; 95% CI, 1.16-2.26).
The study could be subject to recall bias, since it relied on participants’ self-reports.
The authors speculate that the higher prevalence of testing in those with military insurance may reflect efforts by the Veterans Health Administration to reduce the high prevalence of HCV-associated disease among veterans.
It’s entirely possible to increase testing rates, according to Dr. Scott, who has a grant from the Centers for Disease Control and Prevention to study ways to increase uptake. “Probably the easiest thing to do is just incorporate this information into your electronic medical record and make it part of your alerts and standard preventative practices. Try to automate a lot of this rather than remind a very busy primary care doctor of all the things they have to do,” he said.
For example, one strategy that Seattle’s King County has employed is to automatically notify the testing laboratory if an antibody test is positive. “The lab knows to keep that blood and run a second (nucleic acid) test without the patient having to come back. That has helped to get our confirmatory rates up,” said Dr. Scott.
More broadly, the importance of testing needs to be emphasized, according to Paul J. Thuluvath, MD, medical director at the Institute of Digestive Health and Liver Disease at Mercy Medical Center, Baltimore, and a professor of medicine and surgery at the University of Maryland. “We need everybody to buy into this: the primary care physicians, internists, and gynecologists. If they are not convinced of the importance of this, it’s not going to happen. And I don’t think many primary care physicians and internists are convinced yet,” he said.
FROM AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Key clinical point: Primary care physicians are not yet convinced of HCV test’s value.
Major finding: Between 2013 and 2015, HCV testing rates rose from 12.3% to 13.8%.
Data source: Retrospective analysis of 21,827 baby boomers who were part of the National Health Interview Survey.
Disclosures: The study was funded by the American Cancer Society. Dr. Fedewa reported having no financial disclosures. Dr. Scott has received research funding from Merck and serves on the data safety and monitoring board for Tacere Therapeutics. Dr. Thuluvath has received funding from Gilead and has received speaking fees from Gilead and AbbVie.
Federal judge blocks Anthem-Cigna merger
A federal district court judge has blocked health insurer Anthem from acquiring Cigna, ruling the megamerger would violate antitrust laws and stifle competition.
The decision came weeks after another U.S. district court judge barred a merger between health insurance giants Aetna and Humana.
The U.S. Department of Justice praised the latest ruling, calling the decision a victory for patients.
“This merger would have stifled competition, harming consumers by increasing health insurance prices and slowing innovation aimed at lowering the costs of health care,” Acting Assistant Attorney General Brent Snyder said in a statement.
Anthem intends to appeal the decision, said Joseph R. Swedish, Anthem’s chair, president, and chief executive officer. “Anthem is significantly disappointed by the decision, as combining Anthem and Cigna would positively impact the health and well-being of millions of Americans – saving them more than $2 billion in medical costs annually,” Mr. Swedish said in a statement. “If not overturned, the consequences of the decision are far reaching and will hurt American consumers by limiting their access to high-quality affordable care, slowing the industry’s shift to value-based care and improved outcomes for patients, and restricting innovation, which is critical to meeting the evolving needs of health care consumers.”
In a statement, a Cigna official said the company intends to carefully review the opinion and evaluate its options in accordance with the merger agreement.
“Cigna remains focused on helping to improve health care by delivering value to our customers and clients and expanding our business around the world,” the statement said.
The DOJ, 11 states, and the District of Columbia sued Anthem and Cigna in July over their proposed $54 billion consolidation in what would have been the largest merger in history.
The DOJ argued the merger would substantially harm competition and negatively impact the entire insurance industry if allowed to proceed. The consolidation would enhance Anthem’s power to profit at the expense of consumers and the doctors and hospitals who provide their medical care, DOJ attorneys said in their complaint.
Anthem and Cigna argued the proposed acquisition was “procompetitive,” and that the merger would result in efficiencies that would directly benefit consumers via greater access to affordable health care. The benefits of the merger outweigh any alleged anticompetitive effects, according to Anthem.
A trial before Judge Amy Berman Jackson of the U.S. District Court for the District of Columbia ran from November through January.
Judge Berman’s opinion is temporarily under seal to allow parties to review for confidentiality.
The ruling is the second victory for the DOJ in as many weeks. In a Jan. 23 decision, Judge John D. Bates of the U.S. District Court for the District of Columbia denied Aetna’s $37 billion plan to purchase Humana, following a month-long trial that began in early December. Judge Bates ruled the consolidation would violate antitrust laws and reduce competition.
Aetna and Humana did not respond to requests for comment.
On Twitter @legal_med
Michael E. Nelson, MD, FCCP, comments: Any business owner who has been required to absorb yearly double-digit increases in employee health insurance costs cannot help but wonder where Mr. Swedish learned his “new math.” His second statement is even more incogitable – since when were insurers known for expanding access to health care.
Michael E. Nelson, MD, FCCP, comments: Any business owner who has been required to absorb yearly double-digit increases in employee health insurance costs cannot help but wonder where Mr. Swedish learned his “new math.” His second statement is even more incogitable – since when were insurers known for expanding access to health care.
Michael E. Nelson, MD, FCCP, comments: Any business owner who has been required to absorb yearly double-digit increases in employee health insurance costs cannot help but wonder where Mr. Swedish learned his “new math.” His second statement is even more incogitable – since when were insurers known for expanding access to health care.
A federal district court judge has blocked health insurer Anthem from acquiring Cigna, ruling the megamerger would violate antitrust laws and stifle competition.
The decision came weeks after another U.S. district court judge barred a merger between health insurance giants Aetna and Humana.
The U.S. Department of Justice praised the latest ruling, calling the decision a victory for patients.
“This merger would have stifled competition, harming consumers by increasing health insurance prices and slowing innovation aimed at lowering the costs of health care,” Acting Assistant Attorney General Brent Snyder said in a statement.
Anthem intends to appeal the decision, said Joseph R. Swedish, Anthem’s chair, president, and chief executive officer. “Anthem is significantly disappointed by the decision, as combining Anthem and Cigna would positively impact the health and well-being of millions of Americans – saving them more than $2 billion in medical costs annually,” Mr. Swedish said in a statement. “If not overturned, the consequences of the decision are far reaching and will hurt American consumers by limiting their access to high-quality affordable care, slowing the industry’s shift to value-based care and improved outcomes for patients, and restricting innovation, which is critical to meeting the evolving needs of health care consumers.”
In a statement, a Cigna official said the company intends to carefully review the opinion and evaluate its options in accordance with the merger agreement.
“Cigna remains focused on helping to improve health care by delivering value to our customers and clients and expanding our business around the world,” the statement said.
The DOJ, 11 states, and the District of Columbia sued Anthem and Cigna in July over their proposed $54 billion consolidation in what would have been the largest merger in history.
The DOJ argued the merger would substantially harm competition and negatively impact the entire insurance industry if allowed to proceed. The consolidation would enhance Anthem’s power to profit at the expense of consumers and the doctors and hospitals who provide their medical care, DOJ attorneys said in their complaint.
Anthem and Cigna argued the proposed acquisition was “procompetitive,” and that the merger would result in efficiencies that would directly benefit consumers via greater access to affordable health care. The benefits of the merger outweigh any alleged anticompetitive effects, according to Anthem.
A trial before Judge Amy Berman Jackson of the U.S. District Court for the District of Columbia ran from November through January.
Judge Berman’s opinion is temporarily under seal to allow parties to review for confidentiality.
The ruling is the second victory for the DOJ in as many weeks. In a Jan. 23 decision, Judge John D. Bates of the U.S. District Court for the District of Columbia denied Aetna’s $37 billion plan to purchase Humana, following a month-long trial that began in early December. Judge Bates ruled the consolidation would violate antitrust laws and reduce competition.
Aetna and Humana did not respond to requests for comment.
On Twitter @legal_med
A federal district court judge has blocked health insurer Anthem from acquiring Cigna, ruling the megamerger would violate antitrust laws and stifle competition.
The decision came weeks after another U.S. district court judge barred a merger between health insurance giants Aetna and Humana.
The U.S. Department of Justice praised the latest ruling, calling the decision a victory for patients.
“This merger would have stifled competition, harming consumers by increasing health insurance prices and slowing innovation aimed at lowering the costs of health care,” Acting Assistant Attorney General Brent Snyder said in a statement.
Anthem intends to appeal the decision, said Joseph R. Swedish, Anthem’s chair, president, and chief executive officer. “Anthem is significantly disappointed by the decision, as combining Anthem and Cigna would positively impact the health and well-being of millions of Americans – saving them more than $2 billion in medical costs annually,” Mr. Swedish said in a statement. “If not overturned, the consequences of the decision are far reaching and will hurt American consumers by limiting their access to high-quality affordable care, slowing the industry’s shift to value-based care and improved outcomes for patients, and restricting innovation, which is critical to meeting the evolving needs of health care consumers.”
In a statement, a Cigna official said the company intends to carefully review the opinion and evaluate its options in accordance with the merger agreement.
“Cigna remains focused on helping to improve health care by delivering value to our customers and clients and expanding our business around the world,” the statement said.
The DOJ, 11 states, and the District of Columbia sued Anthem and Cigna in July over their proposed $54 billion consolidation in what would have been the largest merger in history.
The DOJ argued the merger would substantially harm competition and negatively impact the entire insurance industry if allowed to proceed. The consolidation would enhance Anthem’s power to profit at the expense of consumers and the doctors and hospitals who provide their medical care, DOJ attorneys said in their complaint.
Anthem and Cigna argued the proposed acquisition was “procompetitive,” and that the merger would result in efficiencies that would directly benefit consumers via greater access to affordable health care. The benefits of the merger outweigh any alleged anticompetitive effects, according to Anthem.
A trial before Judge Amy Berman Jackson of the U.S. District Court for the District of Columbia ran from November through January.
Judge Berman’s opinion is temporarily under seal to allow parties to review for confidentiality.
The ruling is the second victory for the DOJ in as many weeks. In a Jan. 23 decision, Judge John D. Bates of the U.S. District Court for the District of Columbia denied Aetna’s $37 billion plan to purchase Humana, following a month-long trial that began in early December. Judge Bates ruled the consolidation would violate antitrust laws and reduce competition.
Aetna and Humana did not respond to requests for comment.
On Twitter @legal_med
Trending at the Society of Hospital Medicine
Calling all pediatric hospitalists
Register for Pediatric Hospital Medicine 2017 (PHM17), the premier educational conference for pediatric hospitalists and other clinicians who care for hospitalized children. Re-energize your practice with the latest research, best practices, innovations, and more.
Register before June 7 to receive the early-bird rates. Visit www.peds2017.org for more information.
SHM can prepare you for MACRA
Visit www.macraforhm.org for general information and details in the MACRA FAQ and MIPS Tips links.
Don’t miss five new tracks at HM17
- Learn how to avoid diagnostic and therapeutic overuse, and how to move towards the right care for every hospital medicine patient with the High Value Care Track.
- Don’t miss the Clinical Updates Track, which provides evidence-based updates from recent literature published in medicine subspecialty fields and specific topic areas that all hospitalists need to know.
- Accurate and timely diagnosis are the two cornerstones of high-quality patient care. Find out what topics are in the Diagnostic Reasoning Track.
- Learn from experts during the Health Policy Track who will discuss the most current health care policy issues as they impact hospitalists and what we can expect from a new Presidential administration and changes in Congress.
- The Mini Medical Education Track is for hospitalists who are interested in improving their teaching skills.
Learn more about the HM17 schedule and offerings at www.hospitalmedicine2017.org/schedule.
Prepare for the entire Focused Practice in Hospital Medicine (FPHM) exam with SPARK ONE
This self-paced study guide engages learners through an open-book format, allowing users to review detailed learning objectives and discussion points and define individual areas of strengths and weaknesses. SHM members Save $150! Learn more at www.hospitalmedicine.org/sparkone.
Improve your treatment of VTE during Blood Clot Awareness Month
March is Blood Clot Awareness Month, and SHM recently introduced a new toolkit and guide surrounding treatment of venous thromboembolism (VTE) in the hospital setting. SHM has a history of providing cutting-edge resources in this space, and Steven B. Deitelzweig, MD, MMM, SFHM, FACP, FACC, system chairman of hospital medicine at Oschner Health System in New Orleans, was integral in editing SHM’s VTE treatment mentored implementation guide and online toolkit.
“SHM has an established track record of implementing evidence-based and guideline-driven learnings successfully, and we continue to see improvement across multiple facilities based on this work with this disease,” Dr. Deitelzweig says. “Whenever possible, I would strongly recommend taking full advantage of SHM’s outstanding programs as they are intensely developed by experts for adoption at hospitals of different sizes, including community and academic centers.”
SHM can help you and your hospital improve treatment of VTE as well – learn how at www.hospitalmedicine.org/vtetreatment.
Share patient experience success stories
Our Patient Experience Committee wants to showcase stories of when care teams or their counterparts in the hospital made a notable shift in a patient’s experience: a special moment or interaction; a successful improvement project; an award for excellence in practice; a memo of commendation; a letter from a patient. Email examples of success to Claudia Stahl at [email protected] by May 11. Submissions can include photos, letters, or videos. SHM will share these moments that “made all the difference” with members on its website via other channels soon to be announced.
Brett Radler is SHM’s communications specialist.
Not a member? Know someone who should be? Visit www.joinshm.org to learn about the opportunities we can offer hospital medicine professionals.
Calling all pediatric hospitalists
Register for Pediatric Hospital Medicine 2017 (PHM17), the premier educational conference for pediatric hospitalists and other clinicians who care for hospitalized children. Re-energize your practice with the latest research, best practices, innovations, and more.
Register before June 7 to receive the early-bird rates. Visit www.peds2017.org for more information.
SHM can prepare you for MACRA
Visit www.macraforhm.org for general information and details in the MACRA FAQ and MIPS Tips links.
Don’t miss five new tracks at HM17
- Learn how to avoid diagnostic and therapeutic overuse, and how to move towards the right care for every hospital medicine patient with the High Value Care Track.
- Don’t miss the Clinical Updates Track, which provides evidence-based updates from recent literature published in medicine subspecialty fields and specific topic areas that all hospitalists need to know.
- Accurate and timely diagnosis are the two cornerstones of high-quality patient care. Find out what topics are in the Diagnostic Reasoning Track.
- Learn from experts during the Health Policy Track who will discuss the most current health care policy issues as they impact hospitalists and what we can expect from a new Presidential administration and changes in Congress.
- The Mini Medical Education Track is for hospitalists who are interested in improving their teaching skills.
Learn more about the HM17 schedule and offerings at www.hospitalmedicine2017.org/schedule.
Prepare for the entire Focused Practice in Hospital Medicine (FPHM) exam with SPARK ONE
This self-paced study guide engages learners through an open-book format, allowing users to review detailed learning objectives and discussion points and define individual areas of strengths and weaknesses. SHM members Save $150! Learn more at www.hospitalmedicine.org/sparkone.
Improve your treatment of VTE during Blood Clot Awareness Month
March is Blood Clot Awareness Month, and SHM recently introduced a new toolkit and guide surrounding treatment of venous thromboembolism (VTE) in the hospital setting. SHM has a history of providing cutting-edge resources in this space, and Steven B. Deitelzweig, MD, MMM, SFHM, FACP, FACC, system chairman of hospital medicine at Oschner Health System in New Orleans, was integral in editing SHM’s VTE treatment mentored implementation guide and online toolkit.
“SHM has an established track record of implementing evidence-based and guideline-driven learnings successfully, and we continue to see improvement across multiple facilities based on this work with this disease,” Dr. Deitelzweig says. “Whenever possible, I would strongly recommend taking full advantage of SHM’s outstanding programs as they are intensely developed by experts for adoption at hospitals of different sizes, including community and academic centers.”
SHM can help you and your hospital improve treatment of VTE as well – learn how at www.hospitalmedicine.org/vtetreatment.
Share patient experience success stories
Our Patient Experience Committee wants to showcase stories of when care teams or their counterparts in the hospital made a notable shift in a patient’s experience: a special moment or interaction; a successful improvement project; an award for excellence in practice; a memo of commendation; a letter from a patient. Email examples of success to Claudia Stahl at [email protected] by May 11. Submissions can include photos, letters, or videos. SHM will share these moments that “made all the difference” with members on its website via other channels soon to be announced.
Brett Radler is SHM’s communications specialist.
Not a member? Know someone who should be? Visit www.joinshm.org to learn about the opportunities we can offer hospital medicine professionals.
Calling all pediatric hospitalists
Register for Pediatric Hospital Medicine 2017 (PHM17), the premier educational conference for pediatric hospitalists and other clinicians who care for hospitalized children. Re-energize your practice with the latest research, best practices, innovations, and more.
Register before June 7 to receive the early-bird rates. Visit www.peds2017.org for more information.
SHM can prepare you for MACRA
Visit www.macraforhm.org for general information and details in the MACRA FAQ and MIPS Tips links.
Don’t miss five new tracks at HM17
- Learn how to avoid diagnostic and therapeutic overuse, and how to move towards the right care for every hospital medicine patient with the High Value Care Track.
- Don’t miss the Clinical Updates Track, which provides evidence-based updates from recent literature published in medicine subspecialty fields and specific topic areas that all hospitalists need to know.
- Accurate and timely diagnosis are the two cornerstones of high-quality patient care. Find out what topics are in the Diagnostic Reasoning Track.
- Learn from experts during the Health Policy Track who will discuss the most current health care policy issues as they impact hospitalists and what we can expect from a new Presidential administration and changes in Congress.
- The Mini Medical Education Track is for hospitalists who are interested in improving their teaching skills.
Learn more about the HM17 schedule and offerings at www.hospitalmedicine2017.org/schedule.
Prepare for the entire Focused Practice in Hospital Medicine (FPHM) exam with SPARK ONE
This self-paced study guide engages learners through an open-book format, allowing users to review detailed learning objectives and discussion points and define individual areas of strengths and weaknesses. SHM members Save $150! Learn more at www.hospitalmedicine.org/sparkone.
Improve your treatment of VTE during Blood Clot Awareness Month
March is Blood Clot Awareness Month, and SHM recently introduced a new toolkit and guide surrounding treatment of venous thromboembolism (VTE) in the hospital setting. SHM has a history of providing cutting-edge resources in this space, and Steven B. Deitelzweig, MD, MMM, SFHM, FACP, FACC, system chairman of hospital medicine at Oschner Health System in New Orleans, was integral in editing SHM’s VTE treatment mentored implementation guide and online toolkit.
“SHM has an established track record of implementing evidence-based and guideline-driven learnings successfully, and we continue to see improvement across multiple facilities based on this work with this disease,” Dr. Deitelzweig says. “Whenever possible, I would strongly recommend taking full advantage of SHM’s outstanding programs as they are intensely developed by experts for adoption at hospitals of different sizes, including community and academic centers.”
SHM can help you and your hospital improve treatment of VTE as well – learn how at www.hospitalmedicine.org/vtetreatment.
Share patient experience success stories
Our Patient Experience Committee wants to showcase stories of when care teams or their counterparts in the hospital made a notable shift in a patient’s experience: a special moment or interaction; a successful improvement project; an award for excellence in practice; a memo of commendation; a letter from a patient. Email examples of success to Claudia Stahl at [email protected] by May 11. Submissions can include photos, letters, or videos. SHM will share these moments that “made all the difference” with members on its website via other channels soon to be announced.
Brett Radler is SHM’s communications specialist.
Not a member? Know someone who should be? Visit www.joinshm.org to learn about the opportunities we can offer hospital medicine professionals.