User login
When a doctor becomes a patient
An individual’s identity is a learned response to social stimuli, modeling oneself to the expectations of others. Doctors are perceived to be benevolent, knowledgeable, and powerful in matters of life and death. However, a complex concept of reverse hierarchy and role disorientation can take place when a doctor becomes a patient. Because doctors dedicate much of their lives to ensuring the well-being of patients, they may have a skewed perception of their personal health risks and fail to acknowledge that they, too, can fall victim to illness.
‘Them, not me’
Studies have found that doctors often do not advocate the same treatments for themselves than they would for their patients:
- Although most doctors recommend annual check-ups for their patients, 70% of physicians do not get one themselves.1
- Doctors are more likely to recommend potentially life-saving treatment with severe side effects to their patients than for themselves.2
These studies highlight how objectivity may be absent when doctors make decisions about their own treatment, as well as the complexity associated with treating a doctor as a patient.
A doctor’s sense of identity often is strongest in a health care setting. However, becoming a patient precipitates a drastic change in authority, duty, privacy, and even attire. Earlier this year, a colleague was in the hospital for workup of a cluster of symptoms. On a personal level, she experienced a momentary loss of identity, increased anxiety, and loss of self-esteem, which reduced her ability to connect with those who, in her professional role as “doctor,” were her colleagues. Trust became a matter of contention, especially in the context of understanding the inner workings of the health care system—its limitations, risks, and the possibility of human error. Professionally, she thought some management procedures were objectionable, but quickly assumed the passive role to avoid being labeled as “difficult.”
My colleague relayed 2 interesting viewpoints. First, doctors’ detached communication style seemed to evaporate when she revealed that she also is a physician. Perhaps it was the feeling of pride or competition that comes with being responsible for a colleague’s welfare or the camaraderie that lessened the divide. Slowness to relay clinical information or disregard for transparency—sometimes seen in the inpatient setting—were not apparent during my colleague’s care. However, aspects considered trivial from a doctor’s point of view, such as pre-procedural fasting, lack of privacy, and room changes became acutely intrusive.
Second, my colleague observed that her treatment team was overly solicitous. They wanted her to be pain-free and organized “urgent” tests to minimize waiting time. She recognized that there was an overt obligation to procure excessive investigations and treatment compared with a usual patient, because there was wariness of her vigilance of when things go wrong or are overlooked.
This situation was a reminder that clinicians should be mindful of finding the middle ground between unnecessary treatment for a “doctor as patient” and uninformed treatment for a “standard patient.”
Seek to understand
Role reversal represents the fundamental skill of connecting with others through self-awareness, self-regulation, and empathy.3 Studies have found that doctors who have assumed the patient role show more empathy and possess better communication skills.4 The situation for the doctor who becomes a patient may be disconcerting, but the “do no harm” nature of medicine and the generally accepting demeanor of patients render the relationship between empathy and role reversal especially harmonious. Although it is comfortable and convenient to stay on one side of the relationship, grasping an emotional representation of the other side is essential. It is the process of overcoming egocentricity and perceiving the subjective experience of the other role that is rewarding. We all desire to be understood, but to be understood, we must first seek to understand.
1. Schreiber SC. The sick doctor: medical school preparation. Psychiatr Forum. 1978;7(2):11-16.
2. Ubel PA, Angott AM, Zikmund-Fisher BJ. Physicians recommend different treatments for patients than they would choose for themselves. Arch Intern Med. 2011;171(7):630-634.
3. Yaniv D. Dynamics of creativity and empathy in role reversal: contributions from neuroscience. Rev Gen Psychol. 2012;16(1):70-77.
4. Fox FE, Rodham KJ, Harris MF, et al. Experiencing “the other side”: a study of empathy and empowerment in general practitioners who have been patients. Qualitative Health Research. 2009;19(11):1580-1588.
An individual’s identity is a learned response to social stimuli, modeling oneself to the expectations of others. Doctors are perceived to be benevolent, knowledgeable, and powerful in matters of life and death. However, a complex concept of reverse hierarchy and role disorientation can take place when a doctor becomes a patient. Because doctors dedicate much of their lives to ensuring the well-being of patients, they may have a skewed perception of their personal health risks and fail to acknowledge that they, too, can fall victim to illness.
‘Them, not me’
Studies have found that doctors often do not advocate the same treatments for themselves than they would for their patients:
- Although most doctors recommend annual check-ups for their patients, 70% of physicians do not get one themselves.1
- Doctors are more likely to recommend potentially life-saving treatment with severe side effects to their patients than for themselves.2
These studies highlight how objectivity may be absent when doctors make decisions about their own treatment, as well as the complexity associated with treating a doctor as a patient.
A doctor’s sense of identity often is strongest in a health care setting. However, becoming a patient precipitates a drastic change in authority, duty, privacy, and even attire. Earlier this year, a colleague was in the hospital for workup of a cluster of symptoms. On a personal level, she experienced a momentary loss of identity, increased anxiety, and loss of self-esteem, which reduced her ability to connect with those who, in her professional role as “doctor,” were her colleagues. Trust became a matter of contention, especially in the context of understanding the inner workings of the health care system—its limitations, risks, and the possibility of human error. Professionally, she thought some management procedures were objectionable, but quickly assumed the passive role to avoid being labeled as “difficult.”
My colleague relayed 2 interesting viewpoints. First, doctors’ detached communication style seemed to evaporate when she revealed that she also is a physician. Perhaps it was the feeling of pride or competition that comes with being responsible for a colleague’s welfare or the camaraderie that lessened the divide. Slowness to relay clinical information or disregard for transparency—sometimes seen in the inpatient setting—were not apparent during my colleague’s care. However, aspects considered trivial from a doctor’s point of view, such as pre-procedural fasting, lack of privacy, and room changes became acutely intrusive.
Second, my colleague observed that her treatment team was overly solicitous. They wanted her to be pain-free and organized “urgent” tests to minimize waiting time. She recognized that there was an overt obligation to procure excessive investigations and treatment compared with a usual patient, because there was wariness of her vigilance of when things go wrong or are overlooked.
This situation was a reminder that clinicians should be mindful of finding the middle ground between unnecessary treatment for a “doctor as patient” and uninformed treatment for a “standard patient.”
Seek to understand
Role reversal represents the fundamental skill of connecting with others through self-awareness, self-regulation, and empathy.3 Studies have found that doctors who have assumed the patient role show more empathy and possess better communication skills.4 The situation for the doctor who becomes a patient may be disconcerting, but the “do no harm” nature of medicine and the generally accepting demeanor of patients render the relationship between empathy and role reversal especially harmonious. Although it is comfortable and convenient to stay on one side of the relationship, grasping an emotional representation of the other side is essential. It is the process of overcoming egocentricity and perceiving the subjective experience of the other role that is rewarding. We all desire to be understood, but to be understood, we must first seek to understand.
An individual’s identity is a learned response to social stimuli, modeling oneself to the expectations of others. Doctors are perceived to be benevolent, knowledgeable, and powerful in matters of life and death. However, a complex concept of reverse hierarchy and role disorientation can take place when a doctor becomes a patient. Because doctors dedicate much of their lives to ensuring the well-being of patients, they may have a skewed perception of their personal health risks and fail to acknowledge that they, too, can fall victim to illness.
‘Them, not me’
Studies have found that doctors often do not advocate the same treatments for themselves than they would for their patients:
- Although most doctors recommend annual check-ups for their patients, 70% of physicians do not get one themselves.1
- Doctors are more likely to recommend potentially life-saving treatment with severe side effects to their patients than for themselves.2
These studies highlight how objectivity may be absent when doctors make decisions about their own treatment, as well as the complexity associated with treating a doctor as a patient.
A doctor’s sense of identity often is strongest in a health care setting. However, becoming a patient precipitates a drastic change in authority, duty, privacy, and even attire. Earlier this year, a colleague was in the hospital for workup of a cluster of symptoms. On a personal level, she experienced a momentary loss of identity, increased anxiety, and loss of self-esteem, which reduced her ability to connect with those who, in her professional role as “doctor,” were her colleagues. Trust became a matter of contention, especially in the context of understanding the inner workings of the health care system—its limitations, risks, and the possibility of human error. Professionally, she thought some management procedures were objectionable, but quickly assumed the passive role to avoid being labeled as “difficult.”
My colleague relayed 2 interesting viewpoints. First, doctors’ detached communication style seemed to evaporate when she revealed that she also is a physician. Perhaps it was the feeling of pride or competition that comes with being responsible for a colleague’s welfare or the camaraderie that lessened the divide. Slowness to relay clinical information or disregard for transparency—sometimes seen in the inpatient setting—were not apparent during my colleague’s care. However, aspects considered trivial from a doctor’s point of view, such as pre-procedural fasting, lack of privacy, and room changes became acutely intrusive.
Second, my colleague observed that her treatment team was overly solicitous. They wanted her to be pain-free and organized “urgent” tests to minimize waiting time. She recognized that there was an overt obligation to procure excessive investigations and treatment compared with a usual patient, because there was wariness of her vigilance of when things go wrong or are overlooked.
This situation was a reminder that clinicians should be mindful of finding the middle ground between unnecessary treatment for a “doctor as patient” and uninformed treatment for a “standard patient.”
Seek to understand
Role reversal represents the fundamental skill of connecting with others through self-awareness, self-regulation, and empathy.3 Studies have found that doctors who have assumed the patient role show more empathy and possess better communication skills.4 The situation for the doctor who becomes a patient may be disconcerting, but the “do no harm” nature of medicine and the generally accepting demeanor of patients render the relationship between empathy and role reversal especially harmonious. Although it is comfortable and convenient to stay on one side of the relationship, grasping an emotional representation of the other side is essential. It is the process of overcoming egocentricity and perceiving the subjective experience of the other role that is rewarding. We all desire to be understood, but to be understood, we must first seek to understand.
1. Schreiber SC. The sick doctor: medical school preparation. Psychiatr Forum. 1978;7(2):11-16.
2. Ubel PA, Angott AM, Zikmund-Fisher BJ. Physicians recommend different treatments for patients than they would choose for themselves. Arch Intern Med. 2011;171(7):630-634.
3. Yaniv D. Dynamics of creativity and empathy in role reversal: contributions from neuroscience. Rev Gen Psychol. 2012;16(1):70-77.
4. Fox FE, Rodham KJ, Harris MF, et al. Experiencing “the other side”: a study of empathy and empowerment in general practitioners who have been patients. Qualitative Health Research. 2009;19(11):1580-1588.
1. Schreiber SC. The sick doctor: medical school preparation. Psychiatr Forum. 1978;7(2):11-16.
2. Ubel PA, Angott AM, Zikmund-Fisher BJ. Physicians recommend different treatments for patients than they would choose for themselves. Arch Intern Med. 2011;171(7):630-634.
3. Yaniv D. Dynamics of creativity and empathy in role reversal: contributions from neuroscience. Rev Gen Psychol. 2012;16(1):70-77.
4. Fox FE, Rodham KJ, Harris MF, et al. Experiencing “the other side”: a study of empathy and empowerment in general practitioners who have been patients. Qualitative Health Research. 2009;19(11):1580-1588.
VIDEO: No disease progression in endometrial cancer patients with isolated tumor cells
AT THE ANNUAL MEETING ON WOMEN’S CANCER
NATIONAL HARBOR, MD. – Patients with endometrial cancer and isolated tumor cells in sentinel lymph nodes had excellent short-term outcomes, even if they opted out of adjuvant chemotherapy or radiation therapy.
In a single-center prospective study, all 10 such patients remained alive and free of disease progression at 3 years, Marie Plante, MD, said in a video interview at the annual meeting of the Society of Gynecologic Oncology.
The finding suggests that isolated tumor cells in sentinel lymph nodes are not, by themselves, an indication for adjuvant therapy in women with endometrial cancer, said Dr. Plante of Laval University in Quebec City. The issue remains controversial, however, and merits larger cohort studies with longer-term follow-up, she acknowledged.
Ultrastaging of sentinel lymph node biopsies that are negative on hematoxylin and eosin staining has boosted the detection of low-volume metastases. In the case of endometrial cancer with isolated tumor cells, there is a dilemma about whether to recommend adjuvant therapy. Very few studies have examined this relatively rare patient subgroup.
This study included 519 patients undergoing hysterectomy, salpingo-oophorectomy, lymphadenectomy, and sentinel lymph node mapping for endometrial cancer. Pathologic ultrastaging identified 31 patients with isolated tumor cells (6%), of whom 11 received adjuvant chemotherapy, 14 received pelvic radiation therapy, and 10 received brachytherapy or observation only.
Only one patient with isolated tumor cells developed recurrent disease within 3 years. This patient had a 7-cm carcinosarcoma that recurred despite adjuvant chemotherapy and radiation therapy. There were no recurrences among patients with endometrioid histology, Dr. Plante noted.
Dr. Plante cited no funding sources and reported having no conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ANNUAL MEETING ON WOMEN’S CANCER
NATIONAL HARBOR, MD. – Patients with endometrial cancer and isolated tumor cells in sentinel lymph nodes had excellent short-term outcomes, even if they opted out of adjuvant chemotherapy or radiation therapy.
In a single-center prospective study, all 10 such patients remained alive and free of disease progression at 3 years, Marie Plante, MD, said in a video interview at the annual meeting of the Society of Gynecologic Oncology.
The finding suggests that isolated tumor cells in sentinel lymph nodes are not, by themselves, an indication for adjuvant therapy in women with endometrial cancer, said Dr. Plante of Laval University in Quebec City. The issue remains controversial, however, and merits larger cohort studies with longer-term follow-up, she acknowledged.
Ultrastaging of sentinel lymph node biopsies that are negative on hematoxylin and eosin staining has boosted the detection of low-volume metastases. In the case of endometrial cancer with isolated tumor cells, there is a dilemma about whether to recommend adjuvant therapy. Very few studies have examined this relatively rare patient subgroup.
This study included 519 patients undergoing hysterectomy, salpingo-oophorectomy, lymphadenectomy, and sentinel lymph node mapping for endometrial cancer. Pathologic ultrastaging identified 31 patients with isolated tumor cells (6%), of whom 11 received adjuvant chemotherapy, 14 received pelvic radiation therapy, and 10 received brachytherapy or observation only.
Only one patient with isolated tumor cells developed recurrent disease within 3 years. This patient had a 7-cm carcinosarcoma that recurred despite adjuvant chemotherapy and radiation therapy. There were no recurrences among patients with endometrioid histology, Dr. Plante noted.
Dr. Plante cited no funding sources and reported having no conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ANNUAL MEETING ON WOMEN’S CANCER
NATIONAL HARBOR, MD. – Patients with endometrial cancer and isolated tumor cells in sentinel lymph nodes had excellent short-term outcomes, even if they opted out of adjuvant chemotherapy or radiation therapy.
In a single-center prospective study, all 10 such patients remained alive and free of disease progression at 3 years, Marie Plante, MD, said in a video interview at the annual meeting of the Society of Gynecologic Oncology.
The finding suggests that isolated tumor cells in sentinel lymph nodes are not, by themselves, an indication for adjuvant therapy in women with endometrial cancer, said Dr. Plante of Laval University in Quebec City. The issue remains controversial, however, and merits larger cohort studies with longer-term follow-up, she acknowledged.
Ultrastaging of sentinel lymph node biopsies that are negative on hematoxylin and eosin staining has boosted the detection of low-volume metastases. In the case of endometrial cancer with isolated tumor cells, there is a dilemma about whether to recommend adjuvant therapy. Very few studies have examined this relatively rare patient subgroup.
This study included 519 patients undergoing hysterectomy, salpingo-oophorectomy, lymphadenectomy, and sentinel lymph node mapping for endometrial cancer. Pathologic ultrastaging identified 31 patients with isolated tumor cells (6%), of whom 11 received adjuvant chemotherapy, 14 received pelvic radiation therapy, and 10 received brachytherapy or observation only.
Only one patient with isolated tumor cells developed recurrent disease within 3 years. This patient had a 7-cm carcinosarcoma that recurred despite adjuvant chemotherapy and radiation therapy. There were no recurrences among patients with endometrioid histology, Dr. Plante noted.
Dr. Plante cited no funding sources and reported having no conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Key clinical point: Isolated tumor cells in sentinel lymph nodes did not signal disease progression or death among patients with low-risk endometrial cancer.
Major finding: After 3 years of follow-up, the rate of progression-free survival was 100% among patients who opted out of adjuvant chemotherapy or radiation therapy.
Data source: A single-center prospective study of 519 patients undergoing hysterectomy, salpingo-oophorectomy, lymphadenectomy, and sentinel lymph node mapping for endometrial cancer, including 31 patients with isolated tumor cells identified in sentinel lymph nodes.
Disclosures: Dr. Plante cited no funding sources and had no conflicts of interest.
VIDEO: Sexuality, fertility are focus of cancer education website
MIAMI BEACH – Often people living with cancer hesitate to ask their providers about sensitive and important issues surrounding sexuality and fertility. At the same time, some clinicians remain uncomfortable raising questions regarding sexual function or simply lack the time to appropriately address the issues during a patient encounter.
A new online resource aims to solve both problems simultaneously, giving both patients and providers the tools to meaningfully address sexuality and fertility issues, Leslie R. Schover, PhD, said in a video interview at the annual Miami Breast Cancer Conference, held by Physicians’ Education Resource.
The Will2love.com site features first-person patient account videos from women and men who faced similar concerns, said Dr. Schover, founder of the Will2Love digital health company based in Houston. In addition, vignettes with actors inform patients and also model how oncologists, oncology nurses, and other staff could effectively communicate with concerned patients. A professional portal offers online skills training for clinicians.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MIAMI BEACH – Often people living with cancer hesitate to ask their providers about sensitive and important issues surrounding sexuality and fertility. At the same time, some clinicians remain uncomfortable raising questions regarding sexual function or simply lack the time to appropriately address the issues during a patient encounter.
A new online resource aims to solve both problems simultaneously, giving both patients and providers the tools to meaningfully address sexuality and fertility issues, Leslie R. Schover, PhD, said in a video interview at the annual Miami Breast Cancer Conference, held by Physicians’ Education Resource.
The Will2love.com site features first-person patient account videos from women and men who faced similar concerns, said Dr. Schover, founder of the Will2Love digital health company based in Houston. In addition, vignettes with actors inform patients and also model how oncologists, oncology nurses, and other staff could effectively communicate with concerned patients. A professional portal offers online skills training for clinicians.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MIAMI BEACH – Often people living with cancer hesitate to ask their providers about sensitive and important issues surrounding sexuality and fertility. At the same time, some clinicians remain uncomfortable raising questions regarding sexual function or simply lack the time to appropriately address the issues during a patient encounter.
A new online resource aims to solve both problems simultaneously, giving both patients and providers the tools to meaningfully address sexuality and fertility issues, Leslie R. Schover, PhD, said in a video interview at the annual Miami Breast Cancer Conference, held by Physicians’ Education Resource.
The Will2love.com site features first-person patient account videos from women and men who faced similar concerns, said Dr. Schover, founder of the Will2Love digital health company based in Houston. In addition, vignettes with actors inform patients and also model how oncologists, oncology nurses, and other staff could effectively communicate with concerned patients. A professional portal offers online skills training for clinicians.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
U.S. chikungunya epidemic would likely put rheumatologists on front line
SNOWMASS, COLO. – The continental United States is vulnerable to an epidemic of chikungunya virus disease, an event which would have profound consequences for rheumatologists, Robert T. Schoen, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“We certainly have all the factors in place where we could have a major epidemic of chikungunya, particularly in Florida, Texas, and neighboring states. As rheumatologists, I think we can own this infection if we want to because it causes an arthritis that is a true arthritis in a significant percentage of patients,” said Dr. Schoen, a rheumatologist at Yale University in New Haven, Conn.
“That’s a major impact when you think that these epidemics affect tens of thousands of individuals. If 25% of them develop long-term arthritis disability, that’s a whole new world for us,” the rheumatologist observed.
Chikungunya is a single-stranded RNA virus in the togaviridae family, which also contains rubella. The infection is transmitted by Aedes aegypti and A. albopictus, also known as the yellow fever and Asian tiger mosquitoes, respectively. Both mosquitoes are established inhabitants of much of the United States.
“This infection is a one-bite deal. These are very aggressive mosquitoes,” Dr. Schoen observed. “If you haven’t seen a case of chikungunya yet, you will soon,” he added.
In 2014, at the height of a massive Caribbean epidemic which included half a million cases in Puerto Rico, roughly 2,800 cases of chikungunya were imported into 46 U.S. states. In 2015, however, as the Caribbean epidemic waned and herd immunity developed, that figure fell to 653 imported cases. But the epidemic in India, which began in 2008, remains ongoing with no end in sight. Several of Dr. Schoen’s patients with chikungunya had recently returned from India when they first became ill.
“India provides an unlimited reservoir of immunologically naive patients to perpetuate the infection,” he observed.
Course of illness
The rate of asymptomatic infection has been variously estimated at 3%-25%. Symptomatic chikungunya is a biphasic illness. After a 2- to 6-day incubation period, patients develop rapid-onset fever with severe joint pain and muscle aches. Indeed, chikungunya means “bent over” in Makonde, an African Bantu language.
Roughly 60% of patients develop a rash during the acute febrile phase of the illness, which lasts about a week. The dermatitis is most often a maculopapular rash on the trunk which is “absolutely indistinguishable” from the rash caused by Zika virus infection, transmitted by the same vectors, Dr. Schoen noted.
During this acute phase, almost all patients develop a severe polyarthritis which can last for weeks or, less commonly, for months or years. This polyarthritis mimics seronegative rheumatoid arthritis. It is usually symmetric and often affects the hands, wrists, and feet.
The acute febrile phase is characterized by high levels of viremia, with up to 1 billion viral particles per milliliter of blood. During this period, definitive diagnosis of chikungunya can be made through reverse transcriptase–polymerase chain reaction testing or viral culture.
Typically, though, patients make their way to a rheumatologist only well after the viremic period is over. In that situation, the diagnostic mainstay is serologic testing. Antichikungunya virus Immunoglobulin M is detectable starting on about day 5 after symptom onset, and it persists for the next 1-3 months. Immunoglobulin G (IgG) antibodies become detectable in the same time frame and remain elevated for years.
Dr. Schoen highlighted a small but intriguing study from Singapore that suggests that the early appearance of chikungunya-specific neutralizing IgG3–antibodies may constitute a marker for favorable long-term prognosis. The observational study involved 30 patients hospitalized for severe acute chikungunya infection. The investigators classified them into two groups: 14 patients had low levels of acute viremia, a less severe acute illness, and late development of IgG3 antibodies; the other 16 had a high initial viral load, a more severe acute phase, and a rapid IgG3 and interleukin-6 response to infection.
The patients with a robust early IgG3 response cleared the virus faster and none of them developed chronic arthritis. In contrast, those without early, chikungunya-specific IgG3 had a high rate of persistent arthralgia (J Infect Dis. 2012 Apr 1;205[7]:1147-54).
Treatment
No evidence-based treatment for chikungunya exists. Treatment in the acute phase is primarily supportive care. Rheumatologists on the Caribbean island of Martinique have reported a favorable experience using methotrexate at doses up to 25 mg/week or with standard doses of anti–tumor necrosis factor agents in chikungunya patients with severe bilateral symmetric chronic inflammatory joint disease arising during the 2013-2015 epidemic. The treatment response and tolerability were comparable with the results typically obtained in rheumatoid arthritis patients, according to the rheumatologists (Arthritis Rheumatol. 2016 Nov;68[11]:2817-24).
Dr. Schoen found the report from Martinique to be reassuring. He too has resorted to familiar rheumatologic medications in his severely affected patients. Anecdotally, his greatest success involved a patient with a 5-year history of disabling chikungunya polyarthritis who showed marked improvement after several months on hydroxychloroquine.
The Martinique investigators also reported on 22 patients who developed chikungunya while on biologic agents for rheumatoid arthritis, Crohn’s disease, psoriatic arthritis, or systemic lupus erythematosus. The biologics weren’t protective against the viral disease in these patients. All 22 of them developed severe acute chikungunya polyarthritis with a mean swollen joint count of 9.6 (Joint Bone Spine. 2016 Mar;83[2]:245-6).
Infection during pregnancy
There is a legitimate concern about chikungunya infection occurring during pregnancy, but, based on the experiences documented on Reunion Island, the risk to the baby is confined to intrapartum exposure in a viremic mother.
Reunion Island is a French region in the Indian Ocean off the eastern coast of Africa. It has first-world medical care. Much of the best documented early work on chikungunya outbreaks grew out of the 2005-2006 epidemic there, which affected more than one-third of the island’s 800,000-plus residents.
In a prospective study of 7,504 deliveries on the island during a 22-month period, mother-to-child transmission of chikungunya occurred only in the context of intrapartum viremia, with 19 cases of vertical transmission occurring among 39 affected mothers who delivered at a median 38 weeks of gestation. Cesarean section had no protective effect. All 19 infected neonates were asymptomatic at birth, with median onset of pain, fever, and thrombocytopenia on day 4. Nine of the 19 infected neonates developed encephalopathy with pathologic MRI findings, including cerebral hemorrhage in two cases (PLoS Med. 2008 Mar 18;5[3]:e60).
The case fatality rate for chikungunya is low at 0.1%. Most deaths occur in young children or elderly individuals with major comorbid conditions. Despite this low mortality, interest in chikungunya vaccine development is being driven by the infection’s impact on tourism, its spread to temperate climates, the economic impact of the considerable time lost from work, and military need. A promising attenuated, virus-like, particle-based vaccine is currently in phase II testing in the Caribbean.
Dr. Schoen reported having no financial conflicts.
SNOWMASS, COLO. – The continental United States is vulnerable to an epidemic of chikungunya virus disease, an event which would have profound consequences for rheumatologists, Robert T. Schoen, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“We certainly have all the factors in place where we could have a major epidemic of chikungunya, particularly in Florida, Texas, and neighboring states. As rheumatologists, I think we can own this infection if we want to because it causes an arthritis that is a true arthritis in a significant percentage of patients,” said Dr. Schoen, a rheumatologist at Yale University in New Haven, Conn.
“That’s a major impact when you think that these epidemics affect tens of thousands of individuals. If 25% of them develop long-term arthritis disability, that’s a whole new world for us,” the rheumatologist observed.
Chikungunya is a single-stranded RNA virus in the togaviridae family, which also contains rubella. The infection is transmitted by Aedes aegypti and A. albopictus, also known as the yellow fever and Asian tiger mosquitoes, respectively. Both mosquitoes are established inhabitants of much of the United States.
“This infection is a one-bite deal. These are very aggressive mosquitoes,” Dr. Schoen observed. “If you haven’t seen a case of chikungunya yet, you will soon,” he added.
In 2014, at the height of a massive Caribbean epidemic which included half a million cases in Puerto Rico, roughly 2,800 cases of chikungunya were imported into 46 U.S. states. In 2015, however, as the Caribbean epidemic waned and herd immunity developed, that figure fell to 653 imported cases. But the epidemic in India, which began in 2008, remains ongoing with no end in sight. Several of Dr. Schoen’s patients with chikungunya had recently returned from India when they first became ill.
“India provides an unlimited reservoir of immunologically naive patients to perpetuate the infection,” he observed.
Course of illness
The rate of asymptomatic infection has been variously estimated at 3%-25%. Symptomatic chikungunya is a biphasic illness. After a 2- to 6-day incubation period, patients develop rapid-onset fever with severe joint pain and muscle aches. Indeed, chikungunya means “bent over” in Makonde, an African Bantu language.
Roughly 60% of patients develop a rash during the acute febrile phase of the illness, which lasts about a week. The dermatitis is most often a maculopapular rash on the trunk which is “absolutely indistinguishable” from the rash caused by Zika virus infection, transmitted by the same vectors, Dr. Schoen noted.
During this acute phase, almost all patients develop a severe polyarthritis which can last for weeks or, less commonly, for months or years. This polyarthritis mimics seronegative rheumatoid arthritis. It is usually symmetric and often affects the hands, wrists, and feet.
The acute febrile phase is characterized by high levels of viremia, with up to 1 billion viral particles per milliliter of blood. During this period, definitive diagnosis of chikungunya can be made through reverse transcriptase–polymerase chain reaction testing or viral culture.
Typically, though, patients make their way to a rheumatologist only well after the viremic period is over. In that situation, the diagnostic mainstay is serologic testing. Antichikungunya virus Immunoglobulin M is detectable starting on about day 5 after symptom onset, and it persists for the next 1-3 months. Immunoglobulin G (IgG) antibodies become detectable in the same time frame and remain elevated for years.
Dr. Schoen highlighted a small but intriguing study from Singapore that suggests that the early appearance of chikungunya-specific neutralizing IgG3–antibodies may constitute a marker for favorable long-term prognosis. The observational study involved 30 patients hospitalized for severe acute chikungunya infection. The investigators classified them into two groups: 14 patients had low levels of acute viremia, a less severe acute illness, and late development of IgG3 antibodies; the other 16 had a high initial viral load, a more severe acute phase, and a rapid IgG3 and interleukin-6 response to infection.
The patients with a robust early IgG3 response cleared the virus faster and none of them developed chronic arthritis. In contrast, those without early, chikungunya-specific IgG3 had a high rate of persistent arthralgia (J Infect Dis. 2012 Apr 1;205[7]:1147-54).
Treatment
No evidence-based treatment for chikungunya exists. Treatment in the acute phase is primarily supportive care. Rheumatologists on the Caribbean island of Martinique have reported a favorable experience using methotrexate at doses up to 25 mg/week or with standard doses of anti–tumor necrosis factor agents in chikungunya patients with severe bilateral symmetric chronic inflammatory joint disease arising during the 2013-2015 epidemic. The treatment response and tolerability were comparable with the results typically obtained in rheumatoid arthritis patients, according to the rheumatologists (Arthritis Rheumatol. 2016 Nov;68[11]:2817-24).
Dr. Schoen found the report from Martinique to be reassuring. He too has resorted to familiar rheumatologic medications in his severely affected patients. Anecdotally, his greatest success involved a patient with a 5-year history of disabling chikungunya polyarthritis who showed marked improvement after several months on hydroxychloroquine.
The Martinique investigators also reported on 22 patients who developed chikungunya while on biologic agents for rheumatoid arthritis, Crohn’s disease, psoriatic arthritis, or systemic lupus erythematosus. The biologics weren’t protective against the viral disease in these patients. All 22 of them developed severe acute chikungunya polyarthritis with a mean swollen joint count of 9.6 (Joint Bone Spine. 2016 Mar;83[2]:245-6).
Infection during pregnancy
There is a legitimate concern about chikungunya infection occurring during pregnancy, but, based on the experiences documented on Reunion Island, the risk to the baby is confined to intrapartum exposure in a viremic mother.
Reunion Island is a French region in the Indian Ocean off the eastern coast of Africa. It has first-world medical care. Much of the best documented early work on chikungunya outbreaks grew out of the 2005-2006 epidemic there, which affected more than one-third of the island’s 800,000-plus residents.
In a prospective study of 7,504 deliveries on the island during a 22-month period, mother-to-child transmission of chikungunya occurred only in the context of intrapartum viremia, with 19 cases of vertical transmission occurring among 39 affected mothers who delivered at a median 38 weeks of gestation. Cesarean section had no protective effect. All 19 infected neonates were asymptomatic at birth, with median onset of pain, fever, and thrombocytopenia on day 4. Nine of the 19 infected neonates developed encephalopathy with pathologic MRI findings, including cerebral hemorrhage in two cases (PLoS Med. 2008 Mar 18;5[3]:e60).
The case fatality rate for chikungunya is low at 0.1%. Most deaths occur in young children or elderly individuals with major comorbid conditions. Despite this low mortality, interest in chikungunya vaccine development is being driven by the infection’s impact on tourism, its spread to temperate climates, the economic impact of the considerable time lost from work, and military need. A promising attenuated, virus-like, particle-based vaccine is currently in phase II testing in the Caribbean.
Dr. Schoen reported having no financial conflicts.
SNOWMASS, COLO. – The continental United States is vulnerable to an epidemic of chikungunya virus disease, an event which would have profound consequences for rheumatologists, Robert T. Schoen, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“We certainly have all the factors in place where we could have a major epidemic of chikungunya, particularly in Florida, Texas, and neighboring states. As rheumatologists, I think we can own this infection if we want to because it causes an arthritis that is a true arthritis in a significant percentage of patients,” said Dr. Schoen, a rheumatologist at Yale University in New Haven, Conn.
“That’s a major impact when you think that these epidemics affect tens of thousands of individuals. If 25% of them develop long-term arthritis disability, that’s a whole new world for us,” the rheumatologist observed.
Chikungunya is a single-stranded RNA virus in the togaviridae family, which also contains rubella. The infection is transmitted by Aedes aegypti and A. albopictus, also known as the yellow fever and Asian tiger mosquitoes, respectively. Both mosquitoes are established inhabitants of much of the United States.
“This infection is a one-bite deal. These are very aggressive mosquitoes,” Dr. Schoen observed. “If you haven’t seen a case of chikungunya yet, you will soon,” he added.
In 2014, at the height of a massive Caribbean epidemic which included half a million cases in Puerto Rico, roughly 2,800 cases of chikungunya were imported into 46 U.S. states. In 2015, however, as the Caribbean epidemic waned and herd immunity developed, that figure fell to 653 imported cases. But the epidemic in India, which began in 2008, remains ongoing with no end in sight. Several of Dr. Schoen’s patients with chikungunya had recently returned from India when they first became ill.
“India provides an unlimited reservoir of immunologically naive patients to perpetuate the infection,” he observed.
Course of illness
The rate of asymptomatic infection has been variously estimated at 3%-25%. Symptomatic chikungunya is a biphasic illness. After a 2- to 6-day incubation period, patients develop rapid-onset fever with severe joint pain and muscle aches. Indeed, chikungunya means “bent over” in Makonde, an African Bantu language.
Roughly 60% of patients develop a rash during the acute febrile phase of the illness, which lasts about a week. The dermatitis is most often a maculopapular rash on the trunk which is “absolutely indistinguishable” from the rash caused by Zika virus infection, transmitted by the same vectors, Dr. Schoen noted.
During this acute phase, almost all patients develop a severe polyarthritis which can last for weeks or, less commonly, for months or years. This polyarthritis mimics seronegative rheumatoid arthritis. It is usually symmetric and often affects the hands, wrists, and feet.
The acute febrile phase is characterized by high levels of viremia, with up to 1 billion viral particles per milliliter of blood. During this period, definitive diagnosis of chikungunya can be made through reverse transcriptase–polymerase chain reaction testing or viral culture.
Typically, though, patients make their way to a rheumatologist only well after the viremic period is over. In that situation, the diagnostic mainstay is serologic testing. Antichikungunya virus Immunoglobulin M is detectable starting on about day 5 after symptom onset, and it persists for the next 1-3 months. Immunoglobulin G (IgG) antibodies become detectable in the same time frame and remain elevated for years.
Dr. Schoen highlighted a small but intriguing study from Singapore that suggests that the early appearance of chikungunya-specific neutralizing IgG3–antibodies may constitute a marker for favorable long-term prognosis. The observational study involved 30 patients hospitalized for severe acute chikungunya infection. The investigators classified them into two groups: 14 patients had low levels of acute viremia, a less severe acute illness, and late development of IgG3 antibodies; the other 16 had a high initial viral load, a more severe acute phase, and a rapid IgG3 and interleukin-6 response to infection.
The patients with a robust early IgG3 response cleared the virus faster and none of them developed chronic arthritis. In contrast, those without early, chikungunya-specific IgG3 had a high rate of persistent arthralgia (J Infect Dis. 2012 Apr 1;205[7]:1147-54).
Treatment
No evidence-based treatment for chikungunya exists. Treatment in the acute phase is primarily supportive care. Rheumatologists on the Caribbean island of Martinique have reported a favorable experience using methotrexate at doses up to 25 mg/week or with standard doses of anti–tumor necrosis factor agents in chikungunya patients with severe bilateral symmetric chronic inflammatory joint disease arising during the 2013-2015 epidemic. The treatment response and tolerability were comparable with the results typically obtained in rheumatoid arthritis patients, according to the rheumatologists (Arthritis Rheumatol. 2016 Nov;68[11]:2817-24).
Dr. Schoen found the report from Martinique to be reassuring. He too has resorted to familiar rheumatologic medications in his severely affected patients. Anecdotally, his greatest success involved a patient with a 5-year history of disabling chikungunya polyarthritis who showed marked improvement after several months on hydroxychloroquine.
The Martinique investigators also reported on 22 patients who developed chikungunya while on biologic agents for rheumatoid arthritis, Crohn’s disease, psoriatic arthritis, or systemic lupus erythematosus. The biologics weren’t protective against the viral disease in these patients. All 22 of them developed severe acute chikungunya polyarthritis with a mean swollen joint count of 9.6 (Joint Bone Spine. 2016 Mar;83[2]:245-6).
Infection during pregnancy
There is a legitimate concern about chikungunya infection occurring during pregnancy, but, based on the experiences documented on Reunion Island, the risk to the baby is confined to intrapartum exposure in a viremic mother.
Reunion Island is a French region in the Indian Ocean off the eastern coast of Africa. It has first-world medical care. Much of the best documented early work on chikungunya outbreaks grew out of the 2005-2006 epidemic there, which affected more than one-third of the island’s 800,000-plus residents.
In a prospective study of 7,504 deliveries on the island during a 22-month period, mother-to-child transmission of chikungunya occurred only in the context of intrapartum viremia, with 19 cases of vertical transmission occurring among 39 affected mothers who delivered at a median 38 weeks of gestation. Cesarean section had no protective effect. All 19 infected neonates were asymptomatic at birth, with median onset of pain, fever, and thrombocytopenia on day 4. Nine of the 19 infected neonates developed encephalopathy with pathologic MRI findings, including cerebral hemorrhage in two cases (PLoS Med. 2008 Mar 18;5[3]:e60).
The case fatality rate for chikungunya is low at 0.1%. Most deaths occur in young children or elderly individuals with major comorbid conditions. Despite this low mortality, interest in chikungunya vaccine development is being driven by the infection’s impact on tourism, its spread to temperate climates, the economic impact of the considerable time lost from work, and military need. A promising attenuated, virus-like, particle-based vaccine is currently in phase II testing in the Caribbean.
Dr. Schoen reported having no financial conflicts.
Odontogenic Sinusitis
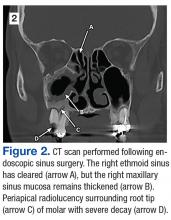
A 55-year-old man who had experienced discolored nasal drainage and mucus plugs in the right side of his nose for 5 years was referred to the ear, nose, and throat clinic. A computerized tomography (CT) scan showed opacification of the right ethmoid and maxillary sinuses and periapical radiolucency in the first and second right maxillary molars (Figure 1).
The patient was treated with antibiotics (amoxicillin and clavulanate; moxifloxacin) and nasal rinses but failed to improve.
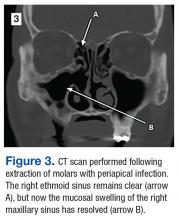
The maxillary molars were considered the source of the persistent sinus infection, and the patient was referred to oral surgery for extraction. Three months after oral surgery, the extraction sites were completely healed, and the right maxillary sinus appeared free of disease endoscopically (Figure 3).
1 .
2. Patel NA, Ferguson BJ. Odontogenic sinusitis: an ancient but under-appreciated cause of maxillary sinusitis. Curr Opin Otolaryngol Head Neck Surg. 2012;20(1):24-28.
A 55-year-old man who had experienced discolored nasal drainage and mucus plugs in the right side of his nose for 5 years was referred to the ear, nose, and throat clinic. A computerized tomography (CT) scan showed opacification of the right ethmoid and maxillary sinuses and periapical radiolucency in the first and second right maxillary molars (Figure 1).
The patient was treated with antibiotics (amoxicillin and clavulanate; moxifloxacin) and nasal rinses but failed to improve.
The maxillary molars were considered the source of the persistent sinus infection, and the patient was referred to oral surgery for extraction. Three months after oral surgery, the extraction sites were completely healed, and the right maxillary sinus appeared free of disease endoscopically (Figure 3).
A 55-year-old man who had experienced discolored nasal drainage and mucus plugs in the right side of his nose for 5 years was referred to the ear, nose, and throat clinic. A computerized tomography (CT) scan showed opacification of the right ethmoid and maxillary sinuses and periapical radiolucency in the first and second right maxillary molars (Figure 1).
The patient was treated with antibiotics (amoxicillin and clavulanate; moxifloxacin) and nasal rinses but failed to improve.
The maxillary molars were considered the source of the persistent sinus infection, and the patient was referred to oral surgery for extraction. Three months after oral surgery, the extraction sites were completely healed, and the right maxillary sinus appeared free of disease endoscopically (Figure 3).
1 .
2. Patel NA, Ferguson BJ. Odontogenic sinusitis: an ancient but under-appreciated cause of maxillary sinusitis. Curr Opin Otolaryngol Head Neck Surg. 2012;20(1):24-28.
1 .
2. Patel NA, Ferguson BJ. Odontogenic sinusitis: an ancient but under-appreciated cause of maxillary sinusitis. Curr Opin Otolaryngol Head Neck Surg. 2012;20(1):24-28.
MRD predicts outcome of HSCT in ALL, study suggests
ORLANDO, FL—Minimal residual disease (MRD) measurements before and after hematopoietic stem cell transplant (HSCT) can help predict outcomes in patients with childhood acute lymphoblastic leukemia (ALL), according to researchers.
Their work also suggests several other factors can be used to predict event-free survival (EFS) in this patient population, and the team developed risk scores incorporating these factors.
Michael A. Pulsipher, MD, of Children’s Hospital Los Angeles in California, presented this work as one of the “Best Abstracts” at the 2017 BMT Tandem Meetings (abstract 4*).
“The new risk scores that we were able to develop very nicely predict outcomes post-transplant and can guide study planning,” Dr Pulsipher said.
“MRD pre-transplant was a very powerful predictor of outcome, and MRD post-transplant highlights individual patients at risk.”
For this study, Dr Pulsipher and his colleagues retrospectively analyzed 747 patients treated in Europe, North America, and Australia. The patients received transplants between September 1999 and May 2015.
Most patients had pre-B ALL (78%, n=586), 19% (n=145) had T-cell ALL, 2% had “other” ALLs (n=8) or no data on ALL type (n=8). Sixty-two percent (n=466) were male.
Nearly half of patients were between the ages of 2 and 10 (49%, n=365), 47% (n=351) were older than 10, and 4% (n=31) were younger than 2.
Transplant details
Patients received grafts from matched unrelated donors (42%, n=314), matched sibling donors (30%, n=227), mismatched donors (10%, n=75), and cord blood from unrelated donors (17%, n=128). There was no data on donor type for 3 patients.
Most patients received bone marrow transplants (61%, n=458), 20% (n=147) received cord blood, and 18% (n=131) received peripheral blood stem cells. Eight patients received “other” types of transplants, and 3 patients had no data on stem cell source.
More than half of the patients (55%, n=410) were in their second complete remission (CR) at transplant. Thirty-seven percent were in their first CR (n=275), 7% were in their third or greater CR (n=53), and 1% were not in remission (n=7). Two patients had no data on remission status.
MRD
MRD was assessed before HSCT as well as after—on or near days 30, 60, 90, 180, 365, and beyond.
There were 4 MRD categories:
- MRD negative: No signal
- MRD low: >0 to <10-4 (<0.01%)
- MRD high: ≥10-4 to <10-3 (0.01 to 0.1%)
- MRD very high: ≥10-3 to <10-2 (>0.1%).
Dr Pulsipher noted that, when analyzing MRD pre-HSCT or at 30 days after HSCT, the estimated 5-year EFS was similar for patients in the MRD-negative and MRD-low groups. However, as time went on (at days 90, 180, and 365), any detectable level of MRD was associated with a poor prognosis.
“And patients arriving at day 365 with no detectable MRD had an exceptional prognosis, with survival approaching 90%,” Dr Pulsipher said.
He also pointed out an interaction between acute graft-vs-host disease (aGVHD) and MRD post-HSCT. He and his colleagues observed better survival for MRD-positive patients with aGVHD (grade 1-2) than for MRD-positive patients without aGVHD.
Pre-HSCT risk score
Via an adjusted Cox regression analysis, the researchers identified several pre-transplant factors that predicted EFS at 18 months.
These included remission status, donor type, immunophenotype, and MRD. The researchers assigned points to each of these factors to create a risk score.
Compared to patients in first CR, the hazard ratio (HR) for patients in early second CR was 2.53, and the score was 3. For patients in third CR or greater, the HR was 1.95, and the score was 2.
Compared to patients with a matched sibling donor, the HR for patients with a mismatched donor was 1.41, and the score was 1. For patients who received cord blood from an unrelated donor, the HR was 1.48, and the score was 1.
Compared to patients with T-cell ALL, the HR for patients with pre-B ALL was 1.35, and the score was 1.
Compared to patients with MRD <10-4, the HR for patients with MRD ≥10-4 was 2.32, and the score was 2.
The probability of EFS at 18 months was 78% ± 2% for patients with 0 to 1 points, 54% ± 3% for those with 2 to 3 points, and 46% ± 5% for patients with 4 or more points.
Day 30 post-HSCT risk score
When considering patients at day 30 post-HSCT, factors that predicted 18-month EFS included remission status, donor type, immunophenotype, aGVHD status, and MRD.
The HR for patients in early second CR was 2.51, and the score was 3. For patients in third CR or greater, the HR was 2.09, and the score was 2.
The HR for patients with a mismatched donor was 1.75, and the score was 2. The HR for patients with pre-B ALL was 1.40, and the score was 1.
Compared to patients with grade 1-2 aGVHD, the HR was 2.02 for patients with grade 0 aGVHD, and the score was 2. For patients with grade 3 aGVHD, the HR was 1.44, and the score was 1. For patients with grade 4 aGVHD, the HR was 7.12, and the score was 7.
The researchers evaluated MRD prior to HSCT and MRD at day 30, using a reference of MRD <10-4 at both time points. For patients with MRD <10-4 pre-HSCT and ≥10-4 at day 30, the HR was 2.29, and the score was 2.
For patients with MRD ≥10-4 pre-HSCT and <10-4 at day 30, the HR was 3.17, and the score was 3. For patients with MRD ≥10-4 pre-HSCT and at day 30, the HR was 3.63, and the score was 4.
The probability of EFS at 18 months was 80% ± 2% for patients with 0 to 3 points, 54% ± 4% for those with 4 to 6 points, and 25% ± 6% for those with 7 or more points.
Day 90 post-HSCT risk score
When considering patients at day 90 post-HSCT, factors that predicted 18-month EFS included remission status, aGVHD status, and MRD.
For patients in early second CR, the HR was 2.81, and the score was 3. For those in third CR or greater, the HR was 1.85, and the score was 2.
Compared to patients with grade 1-2 aGVHD, the HR was 1.60 for patients with grade 0 aGVHD, and the score was 2. For patients with grade 4 aGVHD, the HR was 2.49, and the score was 2.
The researchers assessed MRD prior to HSCT and MRD at day 90, using a reference of MRD <10-4 at both time points. For patients with MRD <10-4 pre-HSCT and ≥10-4 at day 90, the HR was 6.03, and the score was 6.
For patients with MRD ≥10-4 pre-HSCT and <10-4 at day 90, the HR was 3.11, and the score was 3. For patients with MRD ≥10-4 pre-HSCT and at day 90, the HR was 4.59, and the score was 5.
The probability of EFS at 18 months was 83% ± 2% for patients with 0 to 2 points, 60% ± 4% for those with 3 to 5 points, and 17% ± 11 for those with 6 or more points. ![]()
*Information in the abstract differs from the presentation.
ORLANDO, FL—Minimal residual disease (MRD) measurements before and after hematopoietic stem cell transplant (HSCT) can help predict outcomes in patients with childhood acute lymphoblastic leukemia (ALL), according to researchers.
Their work also suggests several other factors can be used to predict event-free survival (EFS) in this patient population, and the team developed risk scores incorporating these factors.
Michael A. Pulsipher, MD, of Children’s Hospital Los Angeles in California, presented this work as one of the “Best Abstracts” at the 2017 BMT Tandem Meetings (abstract 4*).
“The new risk scores that we were able to develop very nicely predict outcomes post-transplant and can guide study planning,” Dr Pulsipher said.
“MRD pre-transplant was a very powerful predictor of outcome, and MRD post-transplant highlights individual patients at risk.”
For this study, Dr Pulsipher and his colleagues retrospectively analyzed 747 patients treated in Europe, North America, and Australia. The patients received transplants between September 1999 and May 2015.
Most patients had pre-B ALL (78%, n=586), 19% (n=145) had T-cell ALL, 2% had “other” ALLs (n=8) or no data on ALL type (n=8). Sixty-two percent (n=466) were male.
Nearly half of patients were between the ages of 2 and 10 (49%, n=365), 47% (n=351) were older than 10, and 4% (n=31) were younger than 2.
Transplant details
Patients received grafts from matched unrelated donors (42%, n=314), matched sibling donors (30%, n=227), mismatched donors (10%, n=75), and cord blood from unrelated donors (17%, n=128). There was no data on donor type for 3 patients.
Most patients received bone marrow transplants (61%, n=458), 20% (n=147) received cord blood, and 18% (n=131) received peripheral blood stem cells. Eight patients received “other” types of transplants, and 3 patients had no data on stem cell source.
More than half of the patients (55%, n=410) were in their second complete remission (CR) at transplant. Thirty-seven percent were in their first CR (n=275), 7% were in their third or greater CR (n=53), and 1% were not in remission (n=7). Two patients had no data on remission status.
MRD
MRD was assessed before HSCT as well as after—on or near days 30, 60, 90, 180, 365, and beyond.
There were 4 MRD categories:
- MRD negative: No signal
- MRD low: >0 to <10-4 (<0.01%)
- MRD high: ≥10-4 to <10-3 (0.01 to 0.1%)
- MRD very high: ≥10-3 to <10-2 (>0.1%).
Dr Pulsipher noted that, when analyzing MRD pre-HSCT or at 30 days after HSCT, the estimated 5-year EFS was similar for patients in the MRD-negative and MRD-low groups. However, as time went on (at days 90, 180, and 365), any detectable level of MRD was associated with a poor prognosis.
“And patients arriving at day 365 with no detectable MRD had an exceptional prognosis, with survival approaching 90%,” Dr Pulsipher said.
He also pointed out an interaction between acute graft-vs-host disease (aGVHD) and MRD post-HSCT. He and his colleagues observed better survival for MRD-positive patients with aGVHD (grade 1-2) than for MRD-positive patients without aGVHD.
Pre-HSCT risk score
Via an adjusted Cox regression analysis, the researchers identified several pre-transplant factors that predicted EFS at 18 months.
These included remission status, donor type, immunophenotype, and MRD. The researchers assigned points to each of these factors to create a risk score.
Compared to patients in first CR, the hazard ratio (HR) for patients in early second CR was 2.53, and the score was 3. For patients in third CR or greater, the HR was 1.95, and the score was 2.
Compared to patients with a matched sibling donor, the HR for patients with a mismatched donor was 1.41, and the score was 1. For patients who received cord blood from an unrelated donor, the HR was 1.48, and the score was 1.
Compared to patients with T-cell ALL, the HR for patients with pre-B ALL was 1.35, and the score was 1.
Compared to patients with MRD <10-4, the HR for patients with MRD ≥10-4 was 2.32, and the score was 2.
The probability of EFS at 18 months was 78% ± 2% for patients with 0 to 1 points, 54% ± 3% for those with 2 to 3 points, and 46% ± 5% for patients with 4 or more points.
Day 30 post-HSCT risk score
When considering patients at day 30 post-HSCT, factors that predicted 18-month EFS included remission status, donor type, immunophenotype, aGVHD status, and MRD.
The HR for patients in early second CR was 2.51, and the score was 3. For patients in third CR or greater, the HR was 2.09, and the score was 2.
The HR for patients with a mismatched donor was 1.75, and the score was 2. The HR for patients with pre-B ALL was 1.40, and the score was 1.
Compared to patients with grade 1-2 aGVHD, the HR was 2.02 for patients with grade 0 aGVHD, and the score was 2. For patients with grade 3 aGVHD, the HR was 1.44, and the score was 1. For patients with grade 4 aGVHD, the HR was 7.12, and the score was 7.
The researchers evaluated MRD prior to HSCT and MRD at day 30, using a reference of MRD <10-4 at both time points. For patients with MRD <10-4 pre-HSCT and ≥10-4 at day 30, the HR was 2.29, and the score was 2.
For patients with MRD ≥10-4 pre-HSCT and <10-4 at day 30, the HR was 3.17, and the score was 3. For patients with MRD ≥10-4 pre-HSCT and at day 30, the HR was 3.63, and the score was 4.
The probability of EFS at 18 months was 80% ± 2% for patients with 0 to 3 points, 54% ± 4% for those with 4 to 6 points, and 25% ± 6% for those with 7 or more points.
Day 90 post-HSCT risk score
When considering patients at day 90 post-HSCT, factors that predicted 18-month EFS included remission status, aGVHD status, and MRD.
For patients in early second CR, the HR was 2.81, and the score was 3. For those in third CR or greater, the HR was 1.85, and the score was 2.
Compared to patients with grade 1-2 aGVHD, the HR was 1.60 for patients with grade 0 aGVHD, and the score was 2. For patients with grade 4 aGVHD, the HR was 2.49, and the score was 2.
The researchers assessed MRD prior to HSCT and MRD at day 90, using a reference of MRD <10-4 at both time points. For patients with MRD <10-4 pre-HSCT and ≥10-4 at day 90, the HR was 6.03, and the score was 6.
For patients with MRD ≥10-4 pre-HSCT and <10-4 at day 90, the HR was 3.11, and the score was 3. For patients with MRD ≥10-4 pre-HSCT and at day 90, the HR was 4.59, and the score was 5.
The probability of EFS at 18 months was 83% ± 2% for patients with 0 to 2 points, 60% ± 4% for those with 3 to 5 points, and 17% ± 11 for those with 6 or more points. ![]()
*Information in the abstract differs from the presentation.
ORLANDO, FL—Minimal residual disease (MRD) measurements before and after hematopoietic stem cell transplant (HSCT) can help predict outcomes in patients with childhood acute lymphoblastic leukemia (ALL), according to researchers.
Their work also suggests several other factors can be used to predict event-free survival (EFS) in this patient population, and the team developed risk scores incorporating these factors.
Michael A. Pulsipher, MD, of Children’s Hospital Los Angeles in California, presented this work as one of the “Best Abstracts” at the 2017 BMT Tandem Meetings (abstract 4*).
“The new risk scores that we were able to develop very nicely predict outcomes post-transplant and can guide study planning,” Dr Pulsipher said.
“MRD pre-transplant was a very powerful predictor of outcome, and MRD post-transplant highlights individual patients at risk.”
For this study, Dr Pulsipher and his colleagues retrospectively analyzed 747 patients treated in Europe, North America, and Australia. The patients received transplants between September 1999 and May 2015.
Most patients had pre-B ALL (78%, n=586), 19% (n=145) had T-cell ALL, 2% had “other” ALLs (n=8) or no data on ALL type (n=8). Sixty-two percent (n=466) were male.
Nearly half of patients were between the ages of 2 and 10 (49%, n=365), 47% (n=351) were older than 10, and 4% (n=31) were younger than 2.
Transplant details
Patients received grafts from matched unrelated donors (42%, n=314), matched sibling donors (30%, n=227), mismatched donors (10%, n=75), and cord blood from unrelated donors (17%, n=128). There was no data on donor type for 3 patients.
Most patients received bone marrow transplants (61%, n=458), 20% (n=147) received cord blood, and 18% (n=131) received peripheral blood stem cells. Eight patients received “other” types of transplants, and 3 patients had no data on stem cell source.
More than half of the patients (55%, n=410) were in their second complete remission (CR) at transplant. Thirty-seven percent were in their first CR (n=275), 7% were in their third or greater CR (n=53), and 1% were not in remission (n=7). Two patients had no data on remission status.
MRD
MRD was assessed before HSCT as well as after—on or near days 30, 60, 90, 180, 365, and beyond.
There were 4 MRD categories:
- MRD negative: No signal
- MRD low: >0 to <10-4 (<0.01%)
- MRD high: ≥10-4 to <10-3 (0.01 to 0.1%)
- MRD very high: ≥10-3 to <10-2 (>0.1%).
Dr Pulsipher noted that, when analyzing MRD pre-HSCT or at 30 days after HSCT, the estimated 5-year EFS was similar for patients in the MRD-negative and MRD-low groups. However, as time went on (at days 90, 180, and 365), any detectable level of MRD was associated with a poor prognosis.
“And patients arriving at day 365 with no detectable MRD had an exceptional prognosis, with survival approaching 90%,” Dr Pulsipher said.
He also pointed out an interaction between acute graft-vs-host disease (aGVHD) and MRD post-HSCT. He and his colleagues observed better survival for MRD-positive patients with aGVHD (grade 1-2) than for MRD-positive patients without aGVHD.
Pre-HSCT risk score
Via an adjusted Cox regression analysis, the researchers identified several pre-transplant factors that predicted EFS at 18 months.
These included remission status, donor type, immunophenotype, and MRD. The researchers assigned points to each of these factors to create a risk score.
Compared to patients in first CR, the hazard ratio (HR) for patients in early second CR was 2.53, and the score was 3. For patients in third CR or greater, the HR was 1.95, and the score was 2.
Compared to patients with a matched sibling donor, the HR for patients with a mismatched donor was 1.41, and the score was 1. For patients who received cord blood from an unrelated donor, the HR was 1.48, and the score was 1.
Compared to patients with T-cell ALL, the HR for patients with pre-B ALL was 1.35, and the score was 1.
Compared to patients with MRD <10-4, the HR for patients with MRD ≥10-4 was 2.32, and the score was 2.
The probability of EFS at 18 months was 78% ± 2% for patients with 0 to 1 points, 54% ± 3% for those with 2 to 3 points, and 46% ± 5% for patients with 4 or more points.
Day 30 post-HSCT risk score
When considering patients at day 30 post-HSCT, factors that predicted 18-month EFS included remission status, donor type, immunophenotype, aGVHD status, and MRD.
The HR for patients in early second CR was 2.51, and the score was 3. For patients in third CR or greater, the HR was 2.09, and the score was 2.
The HR for patients with a mismatched donor was 1.75, and the score was 2. The HR for patients with pre-B ALL was 1.40, and the score was 1.
Compared to patients with grade 1-2 aGVHD, the HR was 2.02 for patients with grade 0 aGVHD, and the score was 2. For patients with grade 3 aGVHD, the HR was 1.44, and the score was 1. For patients with grade 4 aGVHD, the HR was 7.12, and the score was 7.
The researchers evaluated MRD prior to HSCT and MRD at day 30, using a reference of MRD <10-4 at both time points. For patients with MRD <10-4 pre-HSCT and ≥10-4 at day 30, the HR was 2.29, and the score was 2.
For patients with MRD ≥10-4 pre-HSCT and <10-4 at day 30, the HR was 3.17, and the score was 3. For patients with MRD ≥10-4 pre-HSCT and at day 30, the HR was 3.63, and the score was 4.
The probability of EFS at 18 months was 80% ± 2% for patients with 0 to 3 points, 54% ± 4% for those with 4 to 6 points, and 25% ± 6% for those with 7 or more points.
Day 90 post-HSCT risk score
When considering patients at day 90 post-HSCT, factors that predicted 18-month EFS included remission status, aGVHD status, and MRD.
For patients in early second CR, the HR was 2.81, and the score was 3. For those in third CR or greater, the HR was 1.85, and the score was 2.
Compared to patients with grade 1-2 aGVHD, the HR was 1.60 for patients with grade 0 aGVHD, and the score was 2. For patients with grade 4 aGVHD, the HR was 2.49, and the score was 2.
The researchers assessed MRD prior to HSCT and MRD at day 90, using a reference of MRD <10-4 at both time points. For patients with MRD <10-4 pre-HSCT and ≥10-4 at day 90, the HR was 6.03, and the score was 6.
For patients with MRD ≥10-4 pre-HSCT and <10-4 at day 90, the HR was 3.11, and the score was 3. For patients with MRD ≥10-4 pre-HSCT and at day 90, the HR was 4.59, and the score was 5.
The probability of EFS at 18 months was 83% ± 2% for patients with 0 to 2 points, 60% ± 4% for those with 3 to 5 points, and 17% ± 11 for those with 6 or more points. ![]()
*Information in the abstract differs from the presentation.
Drug receives orphan designation for DLBCL
The US Food and Drug Administration (FDA) has granted orphan drug designation for eFT508 to treat diffuse large B-cell lymphoma (DLBCL).
eFT508 is a highly selective inhibitor of MNK1 and MNK2, enzymes that integrate signals from several oncogenic and immune signaling pathways.
The FDA grants orphan designation to drugs or biologics intended to treat a disease or condition affecting fewer than 200,000 patients in the US.
The orphan designation for eFT508 provides several incentives for eFFECTOR Therapeutics, the company developing eFT508.
These incentives include increased access to FDA reviewers to discuss clinical trial designs, the ability to qualify for tax credits for certain clinical research costs, the ability to apply for annual grant funding, a waiver of Prescription Drug User Fee Act filing fees, and the potential for 7 years of US marketing exclusivity if eFT508 is approved.
eFFECTOR has dosed the first subject in a phase 1/2 trial of eFT508 in patients with B-cell hematologic malignancies. The study is designed to evaluate the safety, pharmacokinetics, pharmacodynamics, and antitumor activity of eFT508.
eFFECTOR presented preclinical research of eFT508 in DLBCL at the 2015 ASH Annual Meeting. The poster is available for download from the eFFECTOR website.
The researchers reported that eFT508 demonstrated anti-proliferative activity against multiple DLBCL cell lines, including the TMD8, OCI-Ly3, and HBL1 cell lines.
eFT508 also exhibited “significant anti-tumor activity” in mouse models of TMD8 and HBL-1 ABC-DLBCL.
Finally, the researchers found that eFT508 synergized with everolimus, ibrutinib, and venetoclax both in vitro and in vivo. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation for eFT508 to treat diffuse large B-cell lymphoma (DLBCL).
eFT508 is a highly selective inhibitor of MNK1 and MNK2, enzymes that integrate signals from several oncogenic and immune signaling pathways.
The FDA grants orphan designation to drugs or biologics intended to treat a disease or condition affecting fewer than 200,000 patients in the US.
The orphan designation for eFT508 provides several incentives for eFFECTOR Therapeutics, the company developing eFT508.
These incentives include increased access to FDA reviewers to discuss clinical trial designs, the ability to qualify for tax credits for certain clinical research costs, the ability to apply for annual grant funding, a waiver of Prescription Drug User Fee Act filing fees, and the potential for 7 years of US marketing exclusivity if eFT508 is approved.
eFFECTOR has dosed the first subject in a phase 1/2 trial of eFT508 in patients with B-cell hematologic malignancies. The study is designed to evaluate the safety, pharmacokinetics, pharmacodynamics, and antitumor activity of eFT508.
eFFECTOR presented preclinical research of eFT508 in DLBCL at the 2015 ASH Annual Meeting. The poster is available for download from the eFFECTOR website.
The researchers reported that eFT508 demonstrated anti-proliferative activity against multiple DLBCL cell lines, including the TMD8, OCI-Ly3, and HBL1 cell lines.
eFT508 also exhibited “significant anti-tumor activity” in mouse models of TMD8 and HBL-1 ABC-DLBCL.
Finally, the researchers found that eFT508 synergized with everolimus, ibrutinib, and venetoclax both in vitro and in vivo. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation for eFT508 to treat diffuse large B-cell lymphoma (DLBCL).
eFT508 is a highly selective inhibitor of MNK1 and MNK2, enzymes that integrate signals from several oncogenic and immune signaling pathways.
The FDA grants orphan designation to drugs or biologics intended to treat a disease or condition affecting fewer than 200,000 patients in the US.
The orphan designation for eFT508 provides several incentives for eFFECTOR Therapeutics, the company developing eFT508.
These incentives include increased access to FDA reviewers to discuss clinical trial designs, the ability to qualify for tax credits for certain clinical research costs, the ability to apply for annual grant funding, a waiver of Prescription Drug User Fee Act filing fees, and the potential for 7 years of US marketing exclusivity if eFT508 is approved.
eFFECTOR has dosed the first subject in a phase 1/2 trial of eFT508 in patients with B-cell hematologic malignancies. The study is designed to evaluate the safety, pharmacokinetics, pharmacodynamics, and antitumor activity of eFT508.
eFFECTOR presented preclinical research of eFT508 in DLBCL at the 2015 ASH Annual Meeting. The poster is available for download from the eFFECTOR website.
The researchers reported that eFT508 demonstrated anti-proliferative activity against multiple DLBCL cell lines, including the TMD8, OCI-Ly3, and HBL1 cell lines.
eFT508 also exhibited “significant anti-tumor activity” in mouse models of TMD8 and HBL-1 ABC-DLBCL.
Finally, the researchers found that eFT508 synergized with everolimus, ibrutinib, and venetoclax both in vitro and in vivo. ![]()
Infectious Penile Lesions
1. A 63-year-old man complains of a mildly painful and tender rash on his penis that has been there for almost two years. The patient is uncircumcised; when the foreskin is retracted, a bright red, erythematous, nonscaly, circumferential plaque is visible on the glans penis, spreading to the foreskin. He denies pain on urination, discharge, fever, malaise, arthralgias, and sexual contact outside of his marriage.
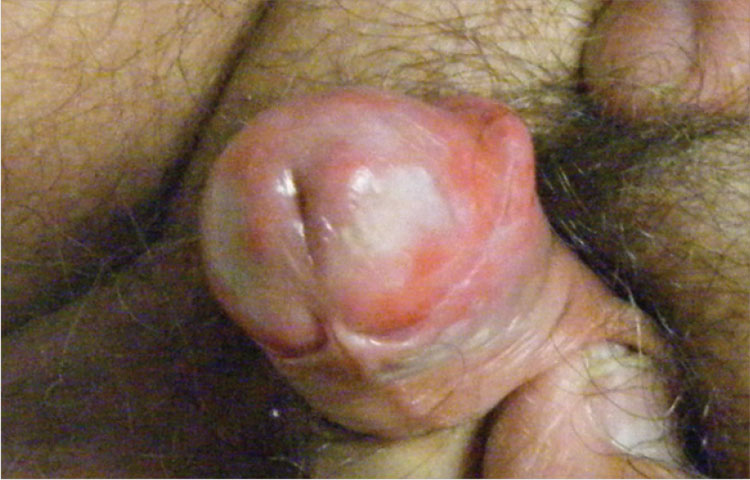
Diagnosis: Zoon balanitis is a benign condition that typically affects uncircumcised middle-aged to elderly men.1,2 Worldwide prevalence among uncircumcised men is approximately 3%.2 The etiology is unknown; it’s thought that this condition may be caused by friction, trauma, heat, lack of hygiene, exogenous or infectious agents, an IgE hypersensitivity, or a chronic infection with Mycobacterium smegmatis.1,2
Typically, the appearance of the lesion precedes diagnosis by about one to 2 years.1 The patient usually complains of mild pruritus and tenderness. Undergarments may be bloodstained.
The lesion associated with Zoon balanitis is a solitary, glistening, shiny, red-to-orange plaque of the glans penis or prepuce of an uncircumcised male. Pinpoint erythematous spots or “cayenne pepper spots” may also be associated with this condition.
For more information on this case, see “Erythematous penile lesion.” J Fam Pract. 2012;61(12):753-755.
2. A 21-year-old man presents with bumps on his penis. He admits to having unprotected sexual intercourse with more than six women in the past year. He is otherwise healthy.
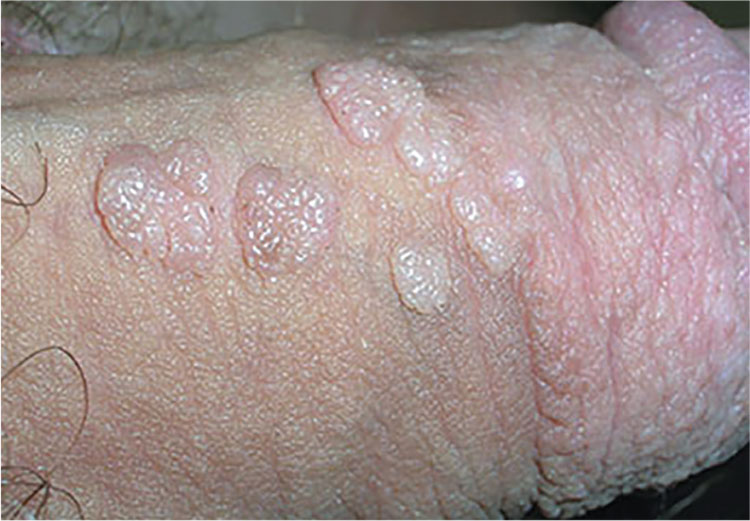
Diagnosis: Genital warts are caused by human papillomavirus infection. The incubation period after exposure ranges from three weeks to eight months. Anogenital warts represent the most common viral sexually transmitted infection in the United States; there are approximately 1 million new cases of genital warts per year. Most infections are transient and clear up within two years, but some infections persist and recur.
The diagnosis is usually clinical; genital warts are typically asymptomatic and present as flesh-colored, exophytic lesions on the genitalia, including the penis, vulva, vagina, scrotum, perineum, and perianal skin. External warts can appear as small bumps, or they may be flat, verrucous, or pedunculated. Treatment options include cryotherapy and prescription imiquimod cream.
For more information on this case, see “Genital bumps.”
3. A 27-year-old man is worried that he may have contracted chlamydia. About five days ago, he experienced pain and a burning feeling with urination. Three days ago, a painful, growing lump near the head of his penis developed. The patient has a purulent urethral discharge and fluctuant, yellowish white, tender swelling on the left side of the frenulum. There are no ulcers, but there is a single 2-cm lymph node in the right inguinal area that is of normal consistency, mobile, nontender, and nonfluctuant.
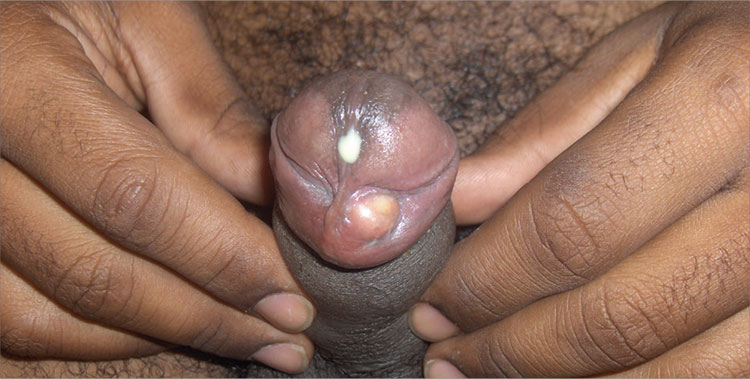
Diagnosis: The clinical history was consistent with a diagnosis of gonococcal urethritis complicated by a periurethral gland abscess. The location of the swelling was most consistent with an abscess in the Tyson’s gland (also known as tysonitis). The Tyson’s (or preputial) glands of the penis are sebaceous-type glands on either side of the frenulum at the balanopreputial sulcus.1 In women, an abscess of the periurethral Skene’s gland is an analogous gonorrheal complication.
The differential diagnosis of acute swelling on the penile shaft includes syphilis, chancroid, lymphogranuloma venereum, herpes simplex virus, Behçet’s syndrome, a drug reaction, erythema multiforme, Crohn’s disease, lichen planus, amebiasis, scabies, trauma, and cancer.
For more information on this case, see “Returning traveler with painful penile mass.” J Fam Pract. 2011 May;60(5):285-287.
4. A 32-year-old man presents with a week-long history of painful vesicles on the shaft of his penis associated with tender groin adenopathy. Two days ago, the vesicles broke and the pain worsened. The patient had similar lesions a year ago but did not seek medical care. He has had three different female sexual partners over the past two years but has no knowledge of them having any sores or diseases.
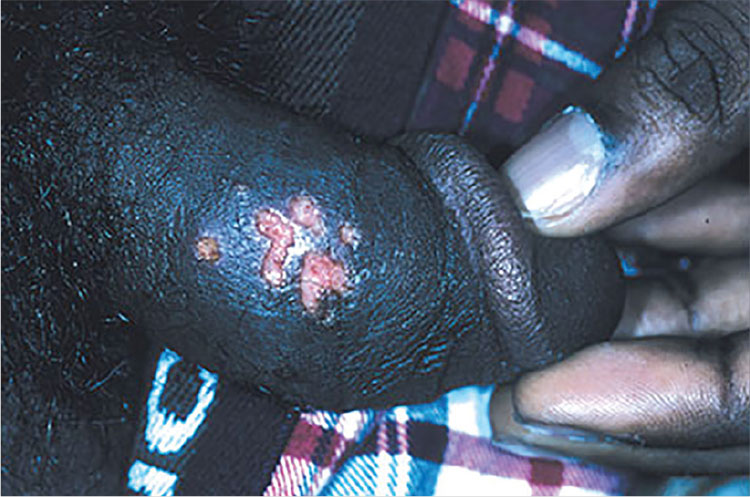
Diagnosis: Genital herpes presents with multiple transient, painful vesicles that appear on the penis, vulva, buttocks, perineum, vagina, or cervix. The vesicles break down and become ulcers that develop crusts while healing. Recurrences typically occur 2 to 3 times a year. The duration is shorter and less painful than in primary infections. The lesions often heal completely by 8 to 10 days.
Antiviral therapy is recommended for an initial genital herpes outbreak. Although systemic antiviral drugs can partially control the signs and symptoms of herpes episodes, they do not eradicate the latent virus.
For more information on this case, see “Painful vesicles on penis.”
1. A 63-year-old man complains of a mildly painful and tender rash on his penis that has been there for almost two years. The patient is uncircumcised; when the foreskin is retracted, a bright red, erythematous, nonscaly, circumferential plaque is visible on the glans penis, spreading to the foreskin. He denies pain on urination, discharge, fever, malaise, arthralgias, and sexual contact outside of his marriage.

Diagnosis: Zoon balanitis is a benign condition that typically affects uncircumcised middle-aged to elderly men.1,2 Worldwide prevalence among uncircumcised men is approximately 3%.2 The etiology is unknown; it’s thought that this condition may be caused by friction, trauma, heat, lack of hygiene, exogenous or infectious agents, an IgE hypersensitivity, or a chronic infection with Mycobacterium smegmatis.1,2
Typically, the appearance of the lesion precedes diagnosis by about one to 2 years.1 The patient usually complains of mild pruritus and tenderness. Undergarments may be bloodstained.
The lesion associated with Zoon balanitis is a solitary, glistening, shiny, red-to-orange plaque of the glans penis or prepuce of an uncircumcised male. Pinpoint erythematous spots or “cayenne pepper spots” may also be associated with this condition.
For more information on this case, see “Erythematous penile lesion.” J Fam Pract. 2012;61(12):753-755.
2. A 21-year-old man presents with bumps on his penis. He admits to having unprotected sexual intercourse with more than six women in the past year. He is otherwise healthy.

Diagnosis: Genital warts are caused by human papillomavirus infection. The incubation period after exposure ranges from three weeks to eight months. Anogenital warts represent the most common viral sexually transmitted infection in the United States; there are approximately 1 million new cases of genital warts per year. Most infections are transient and clear up within two years, but some infections persist and recur.
The diagnosis is usually clinical; genital warts are typically asymptomatic and present as flesh-colored, exophytic lesions on the genitalia, including the penis, vulva, vagina, scrotum, perineum, and perianal skin. External warts can appear as small bumps, or they may be flat, verrucous, or pedunculated. Treatment options include cryotherapy and prescription imiquimod cream.
For more information on this case, see “Genital bumps.”
3. A 27-year-old man is worried that he may have contracted chlamydia. About five days ago, he experienced pain and a burning feeling with urination. Three days ago, a painful, growing lump near the head of his penis developed. The patient has a purulent urethral discharge and fluctuant, yellowish white, tender swelling on the left side of the frenulum. There are no ulcers, but there is a single 2-cm lymph node in the right inguinal area that is of normal consistency, mobile, nontender, and nonfluctuant.

Diagnosis: The clinical history was consistent with a diagnosis of gonococcal urethritis complicated by a periurethral gland abscess. The location of the swelling was most consistent with an abscess in the Tyson’s gland (also known as tysonitis). The Tyson’s (or preputial) glands of the penis are sebaceous-type glands on either side of the frenulum at the balanopreputial sulcus.1 In women, an abscess of the periurethral Skene’s gland is an analogous gonorrheal complication.
The differential diagnosis of acute swelling on the penile shaft includes syphilis, chancroid, lymphogranuloma venereum, herpes simplex virus, Behçet’s syndrome, a drug reaction, erythema multiforme, Crohn’s disease, lichen planus, amebiasis, scabies, trauma, and cancer.
For more information on this case, see “Returning traveler with painful penile mass.” J Fam Pract. 2011 May;60(5):285-287.
4. A 32-year-old man presents with a week-long history of painful vesicles on the shaft of his penis associated with tender groin adenopathy. Two days ago, the vesicles broke and the pain worsened. The patient had similar lesions a year ago but did not seek medical care. He has had three different female sexual partners over the past two years but has no knowledge of them having any sores or diseases.

Diagnosis: Genital herpes presents with multiple transient, painful vesicles that appear on the penis, vulva, buttocks, perineum, vagina, or cervix. The vesicles break down and become ulcers that develop crusts while healing. Recurrences typically occur 2 to 3 times a year. The duration is shorter and less painful than in primary infections. The lesions often heal completely by 8 to 10 days.
Antiviral therapy is recommended for an initial genital herpes outbreak. Although systemic antiviral drugs can partially control the signs and symptoms of herpes episodes, they do not eradicate the latent virus.
For more information on this case, see “Painful vesicles on penis.”
1. A 63-year-old man complains of a mildly painful and tender rash on his penis that has been there for almost two years. The patient is uncircumcised; when the foreskin is retracted, a bright red, erythematous, nonscaly, circumferential plaque is visible on the glans penis, spreading to the foreskin. He denies pain on urination, discharge, fever, malaise, arthralgias, and sexual contact outside of his marriage.

Diagnosis: Zoon balanitis is a benign condition that typically affects uncircumcised middle-aged to elderly men.1,2 Worldwide prevalence among uncircumcised men is approximately 3%.2 The etiology is unknown; it’s thought that this condition may be caused by friction, trauma, heat, lack of hygiene, exogenous or infectious agents, an IgE hypersensitivity, or a chronic infection with Mycobacterium smegmatis.1,2
Typically, the appearance of the lesion precedes diagnosis by about one to 2 years.1 The patient usually complains of mild pruritus and tenderness. Undergarments may be bloodstained.
The lesion associated with Zoon balanitis is a solitary, glistening, shiny, red-to-orange plaque of the glans penis or prepuce of an uncircumcised male. Pinpoint erythematous spots or “cayenne pepper spots” may also be associated with this condition.
For more information on this case, see “Erythematous penile lesion.” J Fam Pract. 2012;61(12):753-755.
2. A 21-year-old man presents with bumps on his penis. He admits to having unprotected sexual intercourse with more than six women in the past year. He is otherwise healthy.

Diagnosis: Genital warts are caused by human papillomavirus infection. The incubation period after exposure ranges from three weeks to eight months. Anogenital warts represent the most common viral sexually transmitted infection in the United States; there are approximately 1 million new cases of genital warts per year. Most infections are transient and clear up within two years, but some infections persist and recur.
The diagnosis is usually clinical; genital warts are typically asymptomatic and present as flesh-colored, exophytic lesions on the genitalia, including the penis, vulva, vagina, scrotum, perineum, and perianal skin. External warts can appear as small bumps, or they may be flat, verrucous, or pedunculated. Treatment options include cryotherapy and prescription imiquimod cream.
For more information on this case, see “Genital bumps.”
3. A 27-year-old man is worried that he may have contracted chlamydia. About five days ago, he experienced pain and a burning feeling with urination. Three days ago, a painful, growing lump near the head of his penis developed. The patient has a purulent urethral discharge and fluctuant, yellowish white, tender swelling on the left side of the frenulum. There are no ulcers, but there is a single 2-cm lymph node in the right inguinal area that is of normal consistency, mobile, nontender, and nonfluctuant.

Diagnosis: The clinical history was consistent with a diagnosis of gonococcal urethritis complicated by a periurethral gland abscess. The location of the swelling was most consistent with an abscess in the Tyson’s gland (also known as tysonitis). The Tyson’s (or preputial) glands of the penis are sebaceous-type glands on either side of the frenulum at the balanopreputial sulcus.1 In women, an abscess of the periurethral Skene’s gland is an analogous gonorrheal complication.
The differential diagnosis of acute swelling on the penile shaft includes syphilis, chancroid, lymphogranuloma venereum, herpes simplex virus, Behçet’s syndrome, a drug reaction, erythema multiforme, Crohn’s disease, lichen planus, amebiasis, scabies, trauma, and cancer.
For more information on this case, see “Returning traveler with painful penile mass.” J Fam Pract. 2011 May;60(5):285-287.
4. A 32-year-old man presents with a week-long history of painful vesicles on the shaft of his penis associated with tender groin adenopathy. Two days ago, the vesicles broke and the pain worsened. The patient had similar lesions a year ago but did not seek medical care. He has had three different female sexual partners over the past two years but has no knowledge of them having any sores or diseases.

Diagnosis: Genital herpes presents with multiple transient, painful vesicles that appear on the penis, vulva, buttocks, perineum, vagina, or cervix. The vesicles break down and become ulcers that develop crusts while healing. Recurrences typically occur 2 to 3 times a year. The duration is shorter and less painful than in primary infections. The lesions often heal completely by 8 to 10 days.
Antiviral therapy is recommended for an initial genital herpes outbreak. Although systemic antiviral drugs can partially control the signs and symptoms of herpes episodes, they do not eradicate the latent virus.
For more information on this case, see “Painful vesicles on penis.”
VIDEO: Residual cancer burden may be better outcome measure than pCR
MIAMI BEACH – The Food and Drug Administration has accepted pathological complete response rate (pCR) as a surrogate endpoint for disease-free and overall survival in clinical trials for neoadjuvant therapy of breast cancer.
Yet the specimen collection and histopathologic methods used to measure pCR have differed considerably across major neoadjuvant trials for breast cancer, said Michael F. Press, MD, PhD, of the USC/Norris Comprehensive Cancer Center at the University of California, Los Angeles.
In a video interview conducted at the annual Miami Breast Cancer Conference, held by Physicians’ Education Resource, Dr. Press outlined the problems associated with a lack of standardization of outcomes measures, and described how residual cancer burden may be a more effective, validated measures for comparing outcomes across clinical trials.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Press disclosed grant/research support from Cepheid, and consulting with Cepheid, Karyopharm Therapeutics, Eli Lilly, Puma Biotechnology, Halozyme Therapeutics, Biocartis SA, and ADC Therapeutics.
MIAMI BEACH – The Food and Drug Administration has accepted pathological complete response rate (pCR) as a surrogate endpoint for disease-free and overall survival in clinical trials for neoadjuvant therapy of breast cancer.
Yet the specimen collection and histopathologic methods used to measure pCR have differed considerably across major neoadjuvant trials for breast cancer, said Michael F. Press, MD, PhD, of the USC/Norris Comprehensive Cancer Center at the University of California, Los Angeles.
In a video interview conducted at the annual Miami Breast Cancer Conference, held by Physicians’ Education Resource, Dr. Press outlined the problems associated with a lack of standardization of outcomes measures, and described how residual cancer burden may be a more effective, validated measures for comparing outcomes across clinical trials.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Press disclosed grant/research support from Cepheid, and consulting with Cepheid, Karyopharm Therapeutics, Eli Lilly, Puma Biotechnology, Halozyme Therapeutics, Biocartis SA, and ADC Therapeutics.
MIAMI BEACH – The Food and Drug Administration has accepted pathological complete response rate (pCR) as a surrogate endpoint for disease-free and overall survival in clinical trials for neoadjuvant therapy of breast cancer.
Yet the specimen collection and histopathologic methods used to measure pCR have differed considerably across major neoadjuvant trials for breast cancer, said Michael F. Press, MD, PhD, of the USC/Norris Comprehensive Cancer Center at the University of California, Los Angeles.
In a video interview conducted at the annual Miami Breast Cancer Conference, held by Physicians’ Education Resource, Dr. Press outlined the problems associated with a lack of standardization of outcomes measures, and described how residual cancer burden may be a more effective, validated measures for comparing outcomes across clinical trials.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Press disclosed grant/research support from Cepheid, and consulting with Cepheid, Karyopharm Therapeutics, Eli Lilly, Puma Biotechnology, Halozyme Therapeutics, Biocartis SA, and ADC Therapeutics.
AT MBCC
Gender impacts risk of atopic diseases
ATLANTA – Obese females who live in urban settings are significantly more likely to develop atopic diseases, compared with their male counterparts, results from a single-center study showed.
“Some research has shown that obese girls are much more likely to be atopic, but many of them only look at one disease alone, such as atopic dermatitis or food allergies,” lead study author Sairaman Nagarajan, MD, MPH, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Most of the studies are in asthma.”
The mean age of patients was 9 years, 23% were obese, and 55% were male. The researchers observed no differences in laboratory biomarkers nor in the prevalence of individual/or cumulative atopic disease in obese children, compared with controls. When stratified by gender, obese females had a significantly higher mean atopic disease score, compared with controls (4.00 vs. 2.62, respectively; P less than .001), while males had a significantly lower mean atopic disease score, compared with controls (3 vs. 3.42; P less than .001). Regression models yielded similar results; obese females had a significantly higher mean atopic disease score, compared with controls (by a mean elevation of 1.37 points; P less than .005), while males had a significantly lower mean atopic disease score (by a mean decline of -0.42 points; P less than .006). “From this we can say that female gender is a positive risk factor for atopy, and urban obese females may be particularly likely to benefit from lifestyle modification therapy like exercise and diet in controlling weight, and thereby allergies,” Dr. Nagarajan said.
He noted that it remains unclear whether the findings would apply to children who live in nonurban settings. “It’s difficult to say because there are some variables which are unique to urban minority populations like the housing that they live in and the kind of stores they buy food from,” he said.
Dr. Nagarajan reported having no financial disclosures.
ATLANTA – Obese females who live in urban settings are significantly more likely to develop atopic diseases, compared with their male counterparts, results from a single-center study showed.
“Some research has shown that obese girls are much more likely to be atopic, but many of them only look at one disease alone, such as atopic dermatitis or food allergies,” lead study author Sairaman Nagarajan, MD, MPH, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Most of the studies are in asthma.”
The mean age of patients was 9 years, 23% were obese, and 55% were male. The researchers observed no differences in laboratory biomarkers nor in the prevalence of individual/or cumulative atopic disease in obese children, compared with controls. When stratified by gender, obese females had a significantly higher mean atopic disease score, compared with controls (4.00 vs. 2.62, respectively; P less than .001), while males had a significantly lower mean atopic disease score, compared with controls (3 vs. 3.42; P less than .001). Regression models yielded similar results; obese females had a significantly higher mean atopic disease score, compared with controls (by a mean elevation of 1.37 points; P less than .005), while males had a significantly lower mean atopic disease score (by a mean decline of -0.42 points; P less than .006). “From this we can say that female gender is a positive risk factor for atopy, and urban obese females may be particularly likely to benefit from lifestyle modification therapy like exercise and diet in controlling weight, and thereby allergies,” Dr. Nagarajan said.
He noted that it remains unclear whether the findings would apply to children who live in nonurban settings. “It’s difficult to say because there are some variables which are unique to urban minority populations like the housing that they live in and the kind of stores they buy food from,” he said.
Dr. Nagarajan reported having no financial disclosures.
ATLANTA – Obese females who live in urban settings are significantly more likely to develop atopic diseases, compared with their male counterparts, results from a single-center study showed.
“Some research has shown that obese girls are much more likely to be atopic, but many of them only look at one disease alone, such as atopic dermatitis or food allergies,” lead study author Sairaman Nagarajan, MD, MPH, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Most of the studies are in asthma.”
The mean age of patients was 9 years, 23% were obese, and 55% were male. The researchers observed no differences in laboratory biomarkers nor in the prevalence of individual/or cumulative atopic disease in obese children, compared with controls. When stratified by gender, obese females had a significantly higher mean atopic disease score, compared with controls (4.00 vs. 2.62, respectively; P less than .001), while males had a significantly lower mean atopic disease score, compared with controls (3 vs. 3.42; P less than .001). Regression models yielded similar results; obese females had a significantly higher mean atopic disease score, compared with controls (by a mean elevation of 1.37 points; P less than .005), while males had a significantly lower mean atopic disease score (by a mean decline of -0.42 points; P less than .006). “From this we can say that female gender is a positive risk factor for atopy, and urban obese females may be particularly likely to benefit from lifestyle modification therapy like exercise and diet in controlling weight, and thereby allergies,” Dr. Nagarajan said.
He noted that it remains unclear whether the findings would apply to children who live in nonurban settings. “It’s difficult to say because there are some variables which are unique to urban minority populations like the housing that they live in and the kind of stores they buy food from,” he said.
Dr. Nagarajan reported having no financial disclosures.
AT 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: When stratified by gender, obese females had a significantly higher mean atopic disease score, compared with controls (4 vs. 2.62, respectively; P less than .001), while males had a significantly lower mean atopic disease score, compared with controls (3 vs. 3.42; P less than .001).
Data source: A retrospective review of 113 children who were evaluated for a history of allergic rhinitis, eczema, asthma, food allergies, and IgE, the percentage of eosinophils, and absolute eosinophil counts.
Disclosures: Dr. Nagarajan reported having no financial disclosures.