User login
More pushback from docs after CBO scores GOP health care plan
Physician groups continue to push back against a Republican plan to repeal and replace the Affordable Care Act following a Congressional Budget Office analysis that showed up to 24 million patients would not be insured under the plan.
According to the CBO analysis, 14 million more patients would become uninsured in the first year of the American Health Care Act than under current law, with most dropping coverage because of the repeal of the ACA’s individual health insurance mandate.
By 2020, an additional 7 million patients would lose coverage largely because of the bill’s rollback of expansion in favor of a per capita allotment to states to cover their Medicaid population.
“In 2026, an estimated 52 million would be uninsured, compared with 28 million who would lack insurance that year under current law,” according to the CBO analysis.
American Medical Association President Andrew Gurman, MD, called the uptick in uninsured “unacceptable.”
“While the Affordable Care Act was an imperfect law, it was a significant improvement on the status quo at the time, and the AMA believes we need continued progress to expand coverage for the uninsured. Unfortunately, the current proposal – as the CBO analysis shows – would result in the most vulnerable population losing their coverage,” Dr. Gurman said in a statement.
Likewise, the American Osteopathic Association voiced its objections to the GOP proposal.
“The AOA urges Congress to halt any further progression of the American Health Care Act as written and take a more comprehensive approach that addresses such systemic issues while providing stability for the insurance marketplace and the millions of Americans who rely on it for coverage,” President Boyd Buser, DO, said in a statement.
According to the CBO analysis, the individual health insurance market “would probably be stable in most areas under either current law or the legislation.” However, there could be large premium increases for some patients, based on age group.
Tom Price, MD, secretary of the Department of Health & Human Services, criticized the CBO projections.
“The CBO report’s coverage numbers defy logic,” he said in a statement. “They project that zeroing out the individual mandate – allowing Americans to choose whether to have insurance – will result in 14 million Americans opting out of coverage in 1 year. For there to be the reductions in coverage they project in just the first year, they assume 5 million Americans on Medicaid will drop off of health insurance for which they pay very little, and another 9 million will stop participating in the individual and employer markets. These types of assumptions do not translate to the real world, and they do not accurately estimate the effects of this bill.”
Rep. Kevin Brady (R-Texas), chairman of the House Ways & Means Committee, also disputed the increase in the uninsured population.
“The American Health Care Act is a dramatic departure from Obamacare, which forced Americans to buy expensive, one-size-fits-all health insurance,” Chairman Brady said in a statement. “Our legislation gives individuals and families the freedom to access health care options that are tailored to the needs, not Washington’s.”
Rep. Brady and Dr. Price also pointed out that the AHCA is just the first step of a three-step process, which will include a review of ACA regulations as well as passage of further legislation aimed at providing high quality care at lower costs. However, those cannot be scored by CBO as those details have yet to be released, the GOP leaders pointed out.
The CBO analysis predicts that premiums for those buying insurance in the individual marketplace would increase of 15%-20% from 2018 to 2019, but starting in 2020, average premiums are expected to decrease from states using federal government funds to help offset costs from high users of health care and more younger people coming into the health insurance market.
“By 2026, average premiums for single policyholders in the nongroup market under the legislation would be roughly 10% lower than under current law,” according to the analysis, although since the law allows for higher premiums for older individuals, the congressional budget watchdog sees the provisions of the AHCA as “substantially reducing premiums for young adults and substantially raising premiums for older people.”
The CBO estimates that enacting this legislation would reduce federal deficits by $337 billion over the 2017-2026 period, mainly from reductions in Medicaid spending and the elimination of the current premium subsidies, though the deficit reduction is somewhat offset by the refundable premium tax credits that replace the ACA’s subsidies.
Physician groups continue to push back against a Republican plan to repeal and replace the Affordable Care Act following a Congressional Budget Office analysis that showed up to 24 million patients would not be insured under the plan.
According to the CBO analysis, 14 million more patients would become uninsured in the first year of the American Health Care Act than under current law, with most dropping coverage because of the repeal of the ACA’s individual health insurance mandate.
By 2020, an additional 7 million patients would lose coverage largely because of the bill’s rollback of expansion in favor of a per capita allotment to states to cover their Medicaid population.
“In 2026, an estimated 52 million would be uninsured, compared with 28 million who would lack insurance that year under current law,” according to the CBO analysis.
American Medical Association President Andrew Gurman, MD, called the uptick in uninsured “unacceptable.”
“While the Affordable Care Act was an imperfect law, it was a significant improvement on the status quo at the time, and the AMA believes we need continued progress to expand coverage for the uninsured. Unfortunately, the current proposal – as the CBO analysis shows – would result in the most vulnerable population losing their coverage,” Dr. Gurman said in a statement.
Likewise, the American Osteopathic Association voiced its objections to the GOP proposal.
“The AOA urges Congress to halt any further progression of the American Health Care Act as written and take a more comprehensive approach that addresses such systemic issues while providing stability for the insurance marketplace and the millions of Americans who rely on it for coverage,” President Boyd Buser, DO, said in a statement.
According to the CBO analysis, the individual health insurance market “would probably be stable in most areas under either current law or the legislation.” However, there could be large premium increases for some patients, based on age group.
Tom Price, MD, secretary of the Department of Health & Human Services, criticized the CBO projections.
“The CBO report’s coverage numbers defy logic,” he said in a statement. “They project that zeroing out the individual mandate – allowing Americans to choose whether to have insurance – will result in 14 million Americans opting out of coverage in 1 year. For there to be the reductions in coverage they project in just the first year, they assume 5 million Americans on Medicaid will drop off of health insurance for which they pay very little, and another 9 million will stop participating in the individual and employer markets. These types of assumptions do not translate to the real world, and they do not accurately estimate the effects of this bill.”
Rep. Kevin Brady (R-Texas), chairman of the House Ways & Means Committee, also disputed the increase in the uninsured population.
“The American Health Care Act is a dramatic departure from Obamacare, which forced Americans to buy expensive, one-size-fits-all health insurance,” Chairman Brady said in a statement. “Our legislation gives individuals and families the freedom to access health care options that are tailored to the needs, not Washington’s.”
Rep. Brady and Dr. Price also pointed out that the AHCA is just the first step of a three-step process, which will include a review of ACA regulations as well as passage of further legislation aimed at providing high quality care at lower costs. However, those cannot be scored by CBO as those details have yet to be released, the GOP leaders pointed out.
The CBO analysis predicts that premiums for those buying insurance in the individual marketplace would increase of 15%-20% from 2018 to 2019, but starting in 2020, average premiums are expected to decrease from states using federal government funds to help offset costs from high users of health care and more younger people coming into the health insurance market.
“By 2026, average premiums for single policyholders in the nongroup market under the legislation would be roughly 10% lower than under current law,” according to the analysis, although since the law allows for higher premiums for older individuals, the congressional budget watchdog sees the provisions of the AHCA as “substantially reducing premiums for young adults and substantially raising premiums for older people.”
The CBO estimates that enacting this legislation would reduce federal deficits by $337 billion over the 2017-2026 period, mainly from reductions in Medicaid spending and the elimination of the current premium subsidies, though the deficit reduction is somewhat offset by the refundable premium tax credits that replace the ACA’s subsidies.
Physician groups continue to push back against a Republican plan to repeal and replace the Affordable Care Act following a Congressional Budget Office analysis that showed up to 24 million patients would not be insured under the plan.
According to the CBO analysis, 14 million more patients would become uninsured in the first year of the American Health Care Act than under current law, with most dropping coverage because of the repeal of the ACA’s individual health insurance mandate.
By 2020, an additional 7 million patients would lose coverage largely because of the bill’s rollback of expansion in favor of a per capita allotment to states to cover their Medicaid population.
“In 2026, an estimated 52 million would be uninsured, compared with 28 million who would lack insurance that year under current law,” according to the CBO analysis.
American Medical Association President Andrew Gurman, MD, called the uptick in uninsured “unacceptable.”
“While the Affordable Care Act was an imperfect law, it was a significant improvement on the status quo at the time, and the AMA believes we need continued progress to expand coverage for the uninsured. Unfortunately, the current proposal – as the CBO analysis shows – would result in the most vulnerable population losing their coverage,” Dr. Gurman said in a statement.
Likewise, the American Osteopathic Association voiced its objections to the GOP proposal.
“The AOA urges Congress to halt any further progression of the American Health Care Act as written and take a more comprehensive approach that addresses such systemic issues while providing stability for the insurance marketplace and the millions of Americans who rely on it for coverage,” President Boyd Buser, DO, said in a statement.
According to the CBO analysis, the individual health insurance market “would probably be stable in most areas under either current law or the legislation.” However, there could be large premium increases for some patients, based on age group.
Tom Price, MD, secretary of the Department of Health & Human Services, criticized the CBO projections.
“The CBO report’s coverage numbers defy logic,” he said in a statement. “They project that zeroing out the individual mandate – allowing Americans to choose whether to have insurance – will result in 14 million Americans opting out of coverage in 1 year. For there to be the reductions in coverage they project in just the first year, they assume 5 million Americans on Medicaid will drop off of health insurance for which they pay very little, and another 9 million will stop participating in the individual and employer markets. These types of assumptions do not translate to the real world, and they do not accurately estimate the effects of this bill.”
Rep. Kevin Brady (R-Texas), chairman of the House Ways & Means Committee, also disputed the increase in the uninsured population.
“The American Health Care Act is a dramatic departure from Obamacare, which forced Americans to buy expensive, one-size-fits-all health insurance,” Chairman Brady said in a statement. “Our legislation gives individuals and families the freedom to access health care options that are tailored to the needs, not Washington’s.”
Rep. Brady and Dr. Price also pointed out that the AHCA is just the first step of a three-step process, which will include a review of ACA regulations as well as passage of further legislation aimed at providing high quality care at lower costs. However, those cannot be scored by CBO as those details have yet to be released, the GOP leaders pointed out.
The CBO analysis predicts that premiums for those buying insurance in the individual marketplace would increase of 15%-20% from 2018 to 2019, but starting in 2020, average premiums are expected to decrease from states using federal government funds to help offset costs from high users of health care and more younger people coming into the health insurance market.
“By 2026, average premiums for single policyholders in the nongroup market under the legislation would be roughly 10% lower than under current law,” according to the analysis, although since the law allows for higher premiums for older individuals, the congressional budget watchdog sees the provisions of the AHCA as “substantially reducing premiums for young adults and substantially raising premiums for older people.”
The CBO estimates that enacting this legislation would reduce federal deficits by $337 billion over the 2017-2026 period, mainly from reductions in Medicaid spending and the elimination of the current premium subsidies, though the deficit reduction is somewhat offset by the refundable premium tax credits that replace the ACA’s subsidies.
VIDEO: Cell-free DNA mutations linked with recurrence of ovarian cancer
NATIONAL HARBOR, MD. – Next-generation sequencing of high-grade serous ovarian tumor specimens taken during interval debulking identified several mutations that matched those in cell-free DNA (cfDNA), Rebecca C. Arend, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Furthermore, three of four patients whose ovarian cancer recurred had mutations in cfDNA that were previously detected in tumors, said Dr. Arend of the University of Alabama at Birmingham.
Researchers continue to search for ways to spare patients with ovarian cancer from serial biopsies, Dr. Arend noted during a video interview. As part of that work, she and her associates performed longitudinal next-generation sequencing of 50 genes in tumor and plasma specimens from 14 patients with high-grade serous ovarian cancer.
Mutations found only in tumors were relatively consistent before and after neoadjuvant chemotherapy, while mutations found only in cell-free DNA varied substantially.
The researchers also sequenced plasma samples from four patients at cancer recurrence. Three patients had at least one mutation that was previously detected in their tumor sample. Implicated genes included PIK3CA, TP53, KIT, and KDR.
Many studies have sought circulating tumor markers that reliably predict tumor recurrence. When it comes to cfDNA, “we are not there yet,” but the work is worthwhile, Dr. Arend stressed. Sequencing tumor and cfDNA specimens from patients at multiple points during their journey might one day help pinpoint mutations that reliably predict cancer recurrence, sparing patients from repeated biopsies and helping them efficiently enter clinical trials that target their specific mutation, she added.
Dr. Arend cited no funding sources and reported having no conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NATIONAL HARBOR, MD. – Next-generation sequencing of high-grade serous ovarian tumor specimens taken during interval debulking identified several mutations that matched those in cell-free DNA (cfDNA), Rebecca C. Arend, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Furthermore, three of four patients whose ovarian cancer recurred had mutations in cfDNA that were previously detected in tumors, said Dr. Arend of the University of Alabama at Birmingham.
Researchers continue to search for ways to spare patients with ovarian cancer from serial biopsies, Dr. Arend noted during a video interview. As part of that work, she and her associates performed longitudinal next-generation sequencing of 50 genes in tumor and plasma specimens from 14 patients with high-grade serous ovarian cancer.
Mutations found only in tumors were relatively consistent before and after neoadjuvant chemotherapy, while mutations found only in cell-free DNA varied substantially.
The researchers also sequenced plasma samples from four patients at cancer recurrence. Three patients had at least one mutation that was previously detected in their tumor sample. Implicated genes included PIK3CA, TP53, KIT, and KDR.
Many studies have sought circulating tumor markers that reliably predict tumor recurrence. When it comes to cfDNA, “we are not there yet,” but the work is worthwhile, Dr. Arend stressed. Sequencing tumor and cfDNA specimens from patients at multiple points during their journey might one day help pinpoint mutations that reliably predict cancer recurrence, sparing patients from repeated biopsies and helping them efficiently enter clinical trials that target their specific mutation, she added.
Dr. Arend cited no funding sources and reported having no conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NATIONAL HARBOR, MD. – Next-generation sequencing of high-grade serous ovarian tumor specimens taken during interval debulking identified several mutations that matched those in cell-free DNA (cfDNA), Rebecca C. Arend, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Furthermore, three of four patients whose ovarian cancer recurred had mutations in cfDNA that were previously detected in tumors, said Dr. Arend of the University of Alabama at Birmingham.
Researchers continue to search for ways to spare patients with ovarian cancer from serial biopsies, Dr. Arend noted during a video interview. As part of that work, she and her associates performed longitudinal next-generation sequencing of 50 genes in tumor and plasma specimens from 14 patients with high-grade serous ovarian cancer.
Mutations found only in tumors were relatively consistent before and after neoadjuvant chemotherapy, while mutations found only in cell-free DNA varied substantially.
The researchers also sequenced plasma samples from four patients at cancer recurrence. Three patients had at least one mutation that was previously detected in their tumor sample. Implicated genes included PIK3CA, TP53, KIT, and KDR.
Many studies have sought circulating tumor markers that reliably predict tumor recurrence. When it comes to cfDNA, “we are not there yet,” but the work is worthwhile, Dr. Arend stressed. Sequencing tumor and cfDNA specimens from patients at multiple points during their journey might one day help pinpoint mutations that reliably predict cancer recurrence, sparing patients from repeated biopsies and helping them efficiently enter clinical trials that target their specific mutation, she added.
Dr. Arend cited no funding sources and reported having no conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: Next-generation sequencing of cell-free DNA specimens (liquid biopsies) might one day help identify tumor recurrence in patients with high-grade serous ovarian cancer.
Major finding: Three of four patients whose cancer recurred had mutations in cell-free DNA that were previously detected in tumor specimens collected during interval debulking.
Data source: Next-generation sequencing of tumor and cell-free (plasma) DNA from 14 patients with high-grade serous ovarian cancer before and after platinum-based neoadjuvant chemotherapy.
Disclosures: Dr. Arend cited no funding sources and reported having no conflicts of interest.
U.S. flu activity continues to decline
The 2016-2017 U.S. influenza season appears to have peaked, as activity measures dropped for the third consecutive week, the Centers for Disease Control and Prevention reported.
For the week ending March 4, there were 11 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity, with another three in the “high” range at levels 8 and 9. The previous week (Feb. 25), there were 22 states at level 10, with a total of 27 in the high range of ILI activity. At the peak of activity during the week of Feb. 11, there were 25 states at level 10, data from the CDC’s Outpatient ILI Surveillance Network show.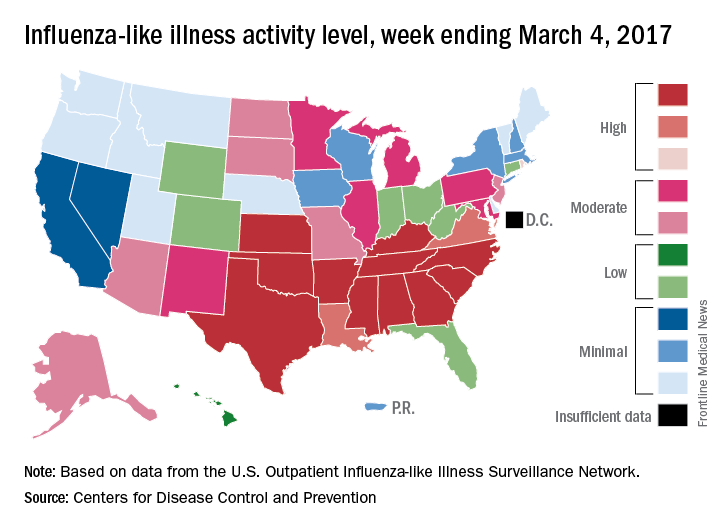
There were eight ILI-related pediatric deaths reported during the week ending March 4, although all occurred in earlier weeks. For the 2016-2017 season so far, 48 ILI-related pediatric deaths have been reported, the CDC said.
For the 70 counties in 13 states that report to the Influenza Hospitalization Surveillance Network, the flu-related hospitalization rate for the season is 43.5 per 100,000 population. The highest rate by age group is for those 65 years and over at 198.8 per 100,000, followed by 50- to 64-year-olds at 42.2 per 100,000 and children aged 0-4 years at 28.8 per 100,000, according to the CDC.
The 2016-2017 U.S. influenza season appears to have peaked, as activity measures dropped for the third consecutive week, the Centers for Disease Control and Prevention reported.
For the week ending March 4, there were 11 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity, with another three in the “high” range at levels 8 and 9. The previous week (Feb. 25), there were 22 states at level 10, with a total of 27 in the high range of ILI activity. At the peak of activity during the week of Feb. 11, there were 25 states at level 10, data from the CDC’s Outpatient ILI Surveillance Network show.
There were eight ILI-related pediatric deaths reported during the week ending March 4, although all occurred in earlier weeks. For the 2016-2017 season so far, 48 ILI-related pediatric deaths have been reported, the CDC said.
For the 70 counties in 13 states that report to the Influenza Hospitalization Surveillance Network, the flu-related hospitalization rate for the season is 43.5 per 100,000 population. The highest rate by age group is for those 65 years and over at 198.8 per 100,000, followed by 50- to 64-year-olds at 42.2 per 100,000 and children aged 0-4 years at 28.8 per 100,000, according to the CDC.
The 2016-2017 U.S. influenza season appears to have peaked, as activity measures dropped for the third consecutive week, the Centers for Disease Control and Prevention reported.
For the week ending March 4, there were 11 states at level 10 on the CDC’s 1-10 scale of influenza-like illness (ILI) activity, with another three in the “high” range at levels 8 and 9. The previous week (Feb. 25), there were 22 states at level 10, with a total of 27 in the high range of ILI activity. At the peak of activity during the week of Feb. 11, there were 25 states at level 10, data from the CDC’s Outpatient ILI Surveillance Network show.
There were eight ILI-related pediatric deaths reported during the week ending March 4, although all occurred in earlier weeks. For the 2016-2017 season so far, 48 ILI-related pediatric deaths have been reported, the CDC said.
For the 70 counties in 13 states that report to the Influenza Hospitalization Surveillance Network, the flu-related hospitalization rate for the season is 43.5 per 100,000 population. The highest rate by age group is for those 65 years and over at 198.8 per 100,000, followed by 50- to 64-year-olds at 42.2 per 100,000 and children aged 0-4 years at 28.8 per 100,000, according to the CDC.
No benefit found for routine inpatient rehab after knee replacement
A new randomized study from Australia suggests that for many patients, brief inpatient rehabilitation after knee replacement provides no benefits in several measures, compared with rehab at home.
However, “we are not concluding that there is no role for inpatient rehabilitation after knee arthroplasty,” said study coauthor Justine Naylor, PhD, of the University of New South Wales, Liverpool, Australia. “We are simply saying that for people who are otherwise well enough to be discharged directly home, inpatient rehabilitation does not provide more superior recovery across a range of outcomes.”
For the new study, conducted at two Australian hospitals during 2012-2015, researchers recruited patients aged 40 years or older with a primary diagnosis of osteoarthritis who were undergoing a primary unilateral knee arthroplasty and did not have complications such as a need for inpatient care in recovery beyond an initial 5 days after surgery.
The researchers randomly assigned 165 knee arthroplasty patients with uncomplicated cases to undergo either inpatient rehabilitation for 10 days and then recover at home for 6 weeks or a monitored 6-week home rehab program that began 2 weeks after surgery (JAMA. 2017;317[10]:1037-46).
A third group of 112 patients, 87 of whom were included in the primary analysis, were observed as they entered the home rehab protocol. They were in an initial group of 215 who declined to be randomized, mostly because they wanted to get home quickly after surgery.
The average age of all participants was 67 years, and just over two-thirds were women.
At 26 weeks, the researchers found that there was no significant difference in how the patients in the three groups fared on a 6-minute walk test (mean difference, −1.01 meters; 95% confidence interval, −25.56 to 23.55). They also found no significant difference in measurements of patient-reported pain and function (knee score mean difference, 2.06; 95% CI, −0.59 to 4.71), and quality of life (EQ-5D visual analog scale mean difference, 1.41; 95% CI, −6.42 to 3.60).
However, the patients did seem to have a preference. “Satisfaction with rehabilitation was significantly higher with inpatient rehab, average 92% vs. 83%, though both modes were well received overall,” Dr. Naylor said in an interview.
“Many patients who went to inpatient rehabilitation really enjoyed it,” she added. “We have observed that patients and carers like the convenience of inpatient rehab – a one-stop shop where patients get access to multiple clinicians, gyms, and other patients and do not have to prepare meals.”
However, she said, while “we have no doubt the inpatient rehabilitation environment is nurturing, there must also be advantages to being discharged directly home, otherwise we would have seen differences between the groups. It is possible that monitored home programs like the one provided in this study help people gain independence quickly and empower patients in their recovery.”
Dr. Naylor cautioned that inpatient rehabilitation does have potential benefits for certain patients, such as those who are the most impaired and those without someone available to care for them at home. “Future research could focus on what is best in those situations or, at least, design community-based programs [that] offer some of the perceived benefits of inpatient therapy,” she said. “In addition, we also need to know what the best rehabilitation approach is after hip arthroplasty.”
The study was funded by various sources, including a foundation grant. The study authors reported no relevant disclosures.
A new randomized study from Australia suggests that for many patients, brief inpatient rehabilitation after knee replacement provides no benefits in several measures, compared with rehab at home.
However, “we are not concluding that there is no role for inpatient rehabilitation after knee arthroplasty,” said study coauthor Justine Naylor, PhD, of the University of New South Wales, Liverpool, Australia. “We are simply saying that for people who are otherwise well enough to be discharged directly home, inpatient rehabilitation does not provide more superior recovery across a range of outcomes.”
For the new study, conducted at two Australian hospitals during 2012-2015, researchers recruited patients aged 40 years or older with a primary diagnosis of osteoarthritis who were undergoing a primary unilateral knee arthroplasty and did not have complications such as a need for inpatient care in recovery beyond an initial 5 days after surgery.
The researchers randomly assigned 165 knee arthroplasty patients with uncomplicated cases to undergo either inpatient rehabilitation for 10 days and then recover at home for 6 weeks or a monitored 6-week home rehab program that began 2 weeks after surgery (JAMA. 2017;317[10]:1037-46).
A third group of 112 patients, 87 of whom were included in the primary analysis, were observed as they entered the home rehab protocol. They were in an initial group of 215 who declined to be randomized, mostly because they wanted to get home quickly after surgery.
The average age of all participants was 67 years, and just over two-thirds were women.
At 26 weeks, the researchers found that there was no significant difference in how the patients in the three groups fared on a 6-minute walk test (mean difference, −1.01 meters; 95% confidence interval, −25.56 to 23.55). They also found no significant difference in measurements of patient-reported pain and function (knee score mean difference, 2.06; 95% CI, −0.59 to 4.71), and quality of life (EQ-5D visual analog scale mean difference, 1.41; 95% CI, −6.42 to 3.60).
However, the patients did seem to have a preference. “Satisfaction with rehabilitation was significantly higher with inpatient rehab, average 92% vs. 83%, though both modes were well received overall,” Dr. Naylor said in an interview.
“Many patients who went to inpatient rehabilitation really enjoyed it,” she added. “We have observed that patients and carers like the convenience of inpatient rehab – a one-stop shop where patients get access to multiple clinicians, gyms, and other patients and do not have to prepare meals.”
However, she said, while “we have no doubt the inpatient rehabilitation environment is nurturing, there must also be advantages to being discharged directly home, otherwise we would have seen differences between the groups. It is possible that monitored home programs like the one provided in this study help people gain independence quickly and empower patients in their recovery.”
Dr. Naylor cautioned that inpatient rehabilitation does have potential benefits for certain patients, such as those who are the most impaired and those without someone available to care for them at home. “Future research could focus on what is best in those situations or, at least, design community-based programs [that] offer some of the perceived benefits of inpatient therapy,” she said. “In addition, we also need to know what the best rehabilitation approach is after hip arthroplasty.”
The study was funded by various sources, including a foundation grant. The study authors reported no relevant disclosures.
A new randomized study from Australia suggests that for many patients, brief inpatient rehabilitation after knee replacement provides no benefits in several measures, compared with rehab at home.
However, “we are not concluding that there is no role for inpatient rehabilitation after knee arthroplasty,” said study coauthor Justine Naylor, PhD, of the University of New South Wales, Liverpool, Australia. “We are simply saying that for people who are otherwise well enough to be discharged directly home, inpatient rehabilitation does not provide more superior recovery across a range of outcomes.”
For the new study, conducted at two Australian hospitals during 2012-2015, researchers recruited patients aged 40 years or older with a primary diagnosis of osteoarthritis who were undergoing a primary unilateral knee arthroplasty and did not have complications such as a need for inpatient care in recovery beyond an initial 5 days after surgery.
The researchers randomly assigned 165 knee arthroplasty patients with uncomplicated cases to undergo either inpatient rehabilitation for 10 days and then recover at home for 6 weeks or a monitored 6-week home rehab program that began 2 weeks after surgery (JAMA. 2017;317[10]:1037-46).
A third group of 112 patients, 87 of whom were included in the primary analysis, were observed as they entered the home rehab protocol. They were in an initial group of 215 who declined to be randomized, mostly because they wanted to get home quickly after surgery.
The average age of all participants was 67 years, and just over two-thirds were women.
At 26 weeks, the researchers found that there was no significant difference in how the patients in the three groups fared on a 6-minute walk test (mean difference, −1.01 meters; 95% confidence interval, −25.56 to 23.55). They also found no significant difference in measurements of patient-reported pain and function (knee score mean difference, 2.06; 95% CI, −0.59 to 4.71), and quality of life (EQ-5D visual analog scale mean difference, 1.41; 95% CI, −6.42 to 3.60).
However, the patients did seem to have a preference. “Satisfaction with rehabilitation was significantly higher with inpatient rehab, average 92% vs. 83%, though both modes were well received overall,” Dr. Naylor said in an interview.
“Many patients who went to inpatient rehabilitation really enjoyed it,” she added. “We have observed that patients and carers like the convenience of inpatient rehab – a one-stop shop where patients get access to multiple clinicians, gyms, and other patients and do not have to prepare meals.”
However, she said, while “we have no doubt the inpatient rehabilitation environment is nurturing, there must also be advantages to being discharged directly home, otherwise we would have seen differences between the groups. It is possible that monitored home programs like the one provided in this study help people gain independence quickly and empower patients in their recovery.”
Dr. Naylor cautioned that inpatient rehabilitation does have potential benefits for certain patients, such as those who are the most impaired and those without someone available to care for them at home. “Future research could focus on what is best in those situations or, at least, design community-based programs [that] offer some of the perceived benefits of inpatient therapy,” she said. “In addition, we also need to know what the best rehabilitation approach is after hip arthroplasty.”
The study was funded by various sources, including a foundation grant. The study authors reported no relevant disclosures.
FROM JAMA
Key clinical point:
Major finding: There were no significant differences in the primary outcome of a 6-minute walk test at 26 weeks or in pain, function, and quality of life measures between patients randomized to inpatient or at-home rehab protocols.
Data source: A parallel, randomized controlled trial of 165 uncomplicated knee arthroplasty patients assigned to undergo either inpatient rehab for 10 days then in-home monitored rehab for 6 weeks or to a monitored 6-week home rehab 2 weeks after surgery. The study also included a third observational group of 112 patients (87 included in analysis) who underwent at-home rehab.
Disclosures: The study was funded by various sources, including a foundation grant. The study authors reported no relevant disclosures.
Send children who had anaphylaxis home after 4 hours
ATLANTA – Four hours in the ED are enough to observe most children after anaphylaxis; if they are doing well, they don’t need to be admitted for longer observation, according to Children’s Hospital of Philadelphia investigators.
The right amount of time for observation after anaphylaxis has been debated for years. The current recommendation is 4-8 hours for both children and adults, but it’s a broad range, and some still argue for 24 hours. At least at the Children’s Hospital of Philadelphia (CHOP), much more than 4 hours means that patients need to be admitted to an inpatient floor, or a special extend-care ED unit.
In the fall of 2014, “we decided as a compromise not to go all the way down to 2.5 hours” in the ED, “but to stay within guidelines and go for 4 hours,” so long as kids don’t have asthma or need two epinephrine injections in the ED or have other risks for biphasic reactions, said lead investigator and pediatrician Juhee Lee, MD.
The overall admission rate for pediatric anaphylaxis fell from 58% (106/182) when 8-hour observation was the norm, to 24% (63/257) after ED physicians opted for 4-hour observation in 2014, a near 60% reduction.
Shorter observation was safe; 1% of patients (1/76) came back to the ED within 72 hours before the change, versus 3% (5/194) afterward, a statistically insignificant difference. None of the revisits in either cohort was for severe anaphylaxis symptoms.
“It was surprising to see how significantly we could reduce the admission rate. A 4-hour length of observation appears safe and could drastically improve ED patient flow and decrease hospitalization rates,” Dr. Lee said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
About two-thirds of the ED visits in both groups were for food reactions, most commonly peanut and tree nut allergies.
The CHOP ED also changed how its clinicians treat anaphylaxis over the study period. Children used to get antihistamines and steroids in the ED, and were sent home with 5 days’ worth of both. They also left the ED with a prescription for epinephrine, but not an actual autoinjector.
Now, CHOP lets providers decide if children need antihistamines and steroids in the ED, and sends most home with an EpiPen.
Dr. Lee didn’t have any disclosures. There was no outside funding.
ATLANTA – Four hours in the ED are enough to observe most children after anaphylaxis; if they are doing well, they don’t need to be admitted for longer observation, according to Children’s Hospital of Philadelphia investigators.
The right amount of time for observation after anaphylaxis has been debated for years. The current recommendation is 4-8 hours for both children and adults, but it’s a broad range, and some still argue for 24 hours. At least at the Children’s Hospital of Philadelphia (CHOP), much more than 4 hours means that patients need to be admitted to an inpatient floor, or a special extend-care ED unit.
In the fall of 2014, “we decided as a compromise not to go all the way down to 2.5 hours” in the ED, “but to stay within guidelines and go for 4 hours,” so long as kids don’t have asthma or need two epinephrine injections in the ED or have other risks for biphasic reactions, said lead investigator and pediatrician Juhee Lee, MD.
The overall admission rate for pediatric anaphylaxis fell from 58% (106/182) when 8-hour observation was the norm, to 24% (63/257) after ED physicians opted for 4-hour observation in 2014, a near 60% reduction.
Shorter observation was safe; 1% of patients (1/76) came back to the ED within 72 hours before the change, versus 3% (5/194) afterward, a statistically insignificant difference. None of the revisits in either cohort was for severe anaphylaxis symptoms.
“It was surprising to see how significantly we could reduce the admission rate. A 4-hour length of observation appears safe and could drastically improve ED patient flow and decrease hospitalization rates,” Dr. Lee said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
About two-thirds of the ED visits in both groups were for food reactions, most commonly peanut and tree nut allergies.
The CHOP ED also changed how its clinicians treat anaphylaxis over the study period. Children used to get antihistamines and steroids in the ED, and were sent home with 5 days’ worth of both. They also left the ED with a prescription for epinephrine, but not an actual autoinjector.
Now, CHOP lets providers decide if children need antihistamines and steroids in the ED, and sends most home with an EpiPen.
Dr. Lee didn’t have any disclosures. There was no outside funding.
ATLANTA – Four hours in the ED are enough to observe most children after anaphylaxis; if they are doing well, they don’t need to be admitted for longer observation, according to Children’s Hospital of Philadelphia investigators.
The right amount of time for observation after anaphylaxis has been debated for years. The current recommendation is 4-8 hours for both children and adults, but it’s a broad range, and some still argue for 24 hours. At least at the Children’s Hospital of Philadelphia (CHOP), much more than 4 hours means that patients need to be admitted to an inpatient floor, or a special extend-care ED unit.
In the fall of 2014, “we decided as a compromise not to go all the way down to 2.5 hours” in the ED, “but to stay within guidelines and go for 4 hours,” so long as kids don’t have asthma or need two epinephrine injections in the ED or have other risks for biphasic reactions, said lead investigator and pediatrician Juhee Lee, MD.
The overall admission rate for pediatric anaphylaxis fell from 58% (106/182) when 8-hour observation was the norm, to 24% (63/257) after ED physicians opted for 4-hour observation in 2014, a near 60% reduction.
Shorter observation was safe; 1% of patients (1/76) came back to the ED within 72 hours before the change, versus 3% (5/194) afterward, a statistically insignificant difference. None of the revisits in either cohort was for severe anaphylaxis symptoms.
“It was surprising to see how significantly we could reduce the admission rate. A 4-hour length of observation appears safe and could drastically improve ED patient flow and decrease hospitalization rates,” Dr. Lee said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
About two-thirds of the ED visits in both groups were for food reactions, most commonly peanut and tree nut allergies.
The CHOP ED also changed how its clinicians treat anaphylaxis over the study period. Children used to get antihistamines and steroids in the ED, and were sent home with 5 days’ worth of both. They also left the ED with a prescription for epinephrine, but not an actual autoinjector.
Now, CHOP lets providers decide if children need antihistamines and steroids in the ED, and sends most home with an EpiPen.
Dr. Lee didn’t have any disclosures. There was no outside funding.
Key clinical point:
Major finding: The overall admission rate for pediatric anaphylaxis fell from 58% (106/182) when 8-hour observation was the norm, to 24% (63/257) after ED doctors opted for 4-hour observation in 2014, a near 60% reduction.
Data source: A study of 182 children who underwent 8-hour observation and 257 children who underwent 4-hour observation at the Children’s Hospital of Philadelphia ED.
Disclosures: The lead investigator didn’t have any disclosures. There was no outside funding.
Universal preterm birth screening not ready for prime time
Two screens to predict spontaneous preterm birth in low-risk women, which were rapidly adapted into clinical practice despite a lack of supportive evidence, proved to have little predictive value in a large cohort study.
Transvaginal ultrasound examination for short cervical length and quantitative cervicovaginal swabbing for fetal fibronectin are routinely used to predict spontaneous preterm birth. To assess the accuracy of both screens individually and in combination, researchers analyzed data for women participating in the prospective multicenter Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b). They focused on 9,410 nulliparous women with singleton pregnancies who were followed at eight clinical centers across the country. In addition to regular pregnancy visits, these women underwent both screening procedures at approximately 12 weeks, 19 weeks, and 28 weeks.
Both screens had relatively low sensitivity and low positive predictive value, regardless of when they were performed, which threshold values were used, and whether the results were considered individually or in combination (JAMA. 2017;317[10]:1047-56).
Transvaginal cervical length at 22-30 weeks’ gestation was the most accurate predictor of spontaneous preterm birth before 37 weeks, outperforming fetal fibronectin assessment alone. However, with a threshold of 25 mm or less – the most commonly used clinical cutoff – cervical length screening identified just 23.3% of spontaneous preterm births before 37 weeks. Use of that same threshold at 16-22 weeks – the most common time for screening in clinical practice – identified just 8% of subsequent spontaneous preterm births. The addition of fetal fibronectin did not increase the predictive performance of cervical length alone, according to the findings.
The researchers cited the low incidence of short cervix (1% at 16-22 weeks) as a potential reason why the ultrasound screen was not useful. “Using the most conservative threshold of 25 mm or less in the most common time for clinical screening (16-22 weeks’ gestation), 247 women would need to be screened to identify 1 case of spontaneous preterm birth,” the researchers wrote. Using a transvaginal cervical length of 15 mm or less during the same time period, the number needed to screen rose to 680 to identify a single case of spontaneous preterm birth.
These findings do not support the routine use of these two screens among nulliparous women with singleton pregnancies, the researchers wrote. Screening procedures with relatively poor predictive values “may sometimes be useful if they are inexpensive, lack serious adverse effects, and address a serious condition for which an effective intervention exists. Neither of these tests, alone or in combination, meets all of these criteria,” they added.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development supported the study. Dr. Esplin reported holding a patent for serum markers of preterm birth and ties to Sera Prognostics and Clinical Innovations. One of his coauthors reported ties to Natera, Sequenom, Illumina, March of Dimes, Ariosa Diagnostics/Roche, KellBenx, and LabCorp.
The report by Esplin and colleagues is important for at least two reasons. First, this large and well-executed prospective study provides compelling data that two popular screening modalities, either alone or in combination, do not accurately predict which women will deliver preterm. Second, this report provides provocative insights as to how medical practice determined through consensus, rather than reproducible evidence, contributes to the narrative in the United States regarding the cost and quality of health care.
In 2012, the publications committee of the Society for Maternal-Fetal Medicine provided a consensus clinical guideline for use of progesterone for the prevention of preterm birth. The guideline indicated that vaginal progesterone was beneficial for women at low risk for preterm birth who were discovered to have short cervical length during an ultrasound examination. This recommendation raised the issue of universal testing for short cervix in women such as those screened in the study by Esplin and colleagues. The authors of the clinical guideline, which was reaffirmed in 2016, stated that “cervical length screening in singleton gestations without prior preterm birth cannot yet be universally mandated. Nonetheless, implementation of such a screening strategy can be viewed as reasonable, and can be considered by individual practitioners.” Similarly, one of the recommendations – although based on limited or inconsistent scientific evidence – promulgated by the American College of Obstetricians and Gynecologists in its 2012 Practice Bulletin on Prediction and Prevention of Preterm Birth, and also reaffirmed in 2016, stated that “although this document does not mandate universal cervical length screening in women without a prior preterm birth, this screening strategy may be considered.”
The important study by Esplin and colleagues should serve to temper the use of two controversial screening approaches. While it is gratifying that such research takes place, that research should have preceded the adoption of this screening strategy into practice.
Steven L. Bloom, MD, and Kenneth J. Leveno, MD, are in the department of ob.gyn. at the University of Texas Southwestern Medical Center, Dallas. They reported having no relevant financial disclosures. These comments are excerpted from an editorial ( JAMA. 2017;317[10]:1025-26).
The report by Esplin and colleagues is important for at least two reasons. First, this large and well-executed prospective study provides compelling data that two popular screening modalities, either alone or in combination, do not accurately predict which women will deliver preterm. Second, this report provides provocative insights as to how medical practice determined through consensus, rather than reproducible evidence, contributes to the narrative in the United States regarding the cost and quality of health care.
In 2012, the publications committee of the Society for Maternal-Fetal Medicine provided a consensus clinical guideline for use of progesterone for the prevention of preterm birth. The guideline indicated that vaginal progesterone was beneficial for women at low risk for preterm birth who were discovered to have short cervical length during an ultrasound examination. This recommendation raised the issue of universal testing for short cervix in women such as those screened in the study by Esplin and colleagues. The authors of the clinical guideline, which was reaffirmed in 2016, stated that “cervical length screening in singleton gestations without prior preterm birth cannot yet be universally mandated. Nonetheless, implementation of such a screening strategy can be viewed as reasonable, and can be considered by individual practitioners.” Similarly, one of the recommendations – although based on limited or inconsistent scientific evidence – promulgated by the American College of Obstetricians and Gynecologists in its 2012 Practice Bulletin on Prediction and Prevention of Preterm Birth, and also reaffirmed in 2016, stated that “although this document does not mandate universal cervical length screening in women without a prior preterm birth, this screening strategy may be considered.”
The important study by Esplin and colleagues should serve to temper the use of two controversial screening approaches. While it is gratifying that such research takes place, that research should have preceded the adoption of this screening strategy into practice.
Steven L. Bloom, MD, and Kenneth J. Leveno, MD, are in the department of ob.gyn. at the University of Texas Southwestern Medical Center, Dallas. They reported having no relevant financial disclosures. These comments are excerpted from an editorial ( JAMA. 2017;317[10]:1025-26).
The report by Esplin and colleagues is important for at least two reasons. First, this large and well-executed prospective study provides compelling data that two popular screening modalities, either alone or in combination, do not accurately predict which women will deliver preterm. Second, this report provides provocative insights as to how medical practice determined through consensus, rather than reproducible evidence, contributes to the narrative in the United States regarding the cost and quality of health care.
In 2012, the publications committee of the Society for Maternal-Fetal Medicine provided a consensus clinical guideline for use of progesterone for the prevention of preterm birth. The guideline indicated that vaginal progesterone was beneficial for women at low risk for preterm birth who were discovered to have short cervical length during an ultrasound examination. This recommendation raised the issue of universal testing for short cervix in women such as those screened in the study by Esplin and colleagues. The authors of the clinical guideline, which was reaffirmed in 2016, stated that “cervical length screening in singleton gestations without prior preterm birth cannot yet be universally mandated. Nonetheless, implementation of such a screening strategy can be viewed as reasonable, and can be considered by individual practitioners.” Similarly, one of the recommendations – although based on limited or inconsistent scientific evidence – promulgated by the American College of Obstetricians and Gynecologists in its 2012 Practice Bulletin on Prediction and Prevention of Preterm Birth, and also reaffirmed in 2016, stated that “although this document does not mandate universal cervical length screening in women without a prior preterm birth, this screening strategy may be considered.”
The important study by Esplin and colleagues should serve to temper the use of two controversial screening approaches. While it is gratifying that such research takes place, that research should have preceded the adoption of this screening strategy into practice.
Steven L. Bloom, MD, and Kenneth J. Leveno, MD, are in the department of ob.gyn. at the University of Texas Southwestern Medical Center, Dallas. They reported having no relevant financial disclosures. These comments are excerpted from an editorial ( JAMA. 2017;317[10]:1025-26).
Two screens to predict spontaneous preterm birth in low-risk women, which were rapidly adapted into clinical practice despite a lack of supportive evidence, proved to have little predictive value in a large cohort study.
Transvaginal ultrasound examination for short cervical length and quantitative cervicovaginal swabbing for fetal fibronectin are routinely used to predict spontaneous preterm birth. To assess the accuracy of both screens individually and in combination, researchers analyzed data for women participating in the prospective multicenter Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b). They focused on 9,410 nulliparous women with singleton pregnancies who were followed at eight clinical centers across the country. In addition to regular pregnancy visits, these women underwent both screening procedures at approximately 12 weeks, 19 weeks, and 28 weeks.
Both screens had relatively low sensitivity and low positive predictive value, regardless of when they were performed, which threshold values were used, and whether the results were considered individually or in combination (JAMA. 2017;317[10]:1047-56).
Transvaginal cervical length at 22-30 weeks’ gestation was the most accurate predictor of spontaneous preterm birth before 37 weeks, outperforming fetal fibronectin assessment alone. However, with a threshold of 25 mm or less – the most commonly used clinical cutoff – cervical length screening identified just 23.3% of spontaneous preterm births before 37 weeks. Use of that same threshold at 16-22 weeks – the most common time for screening in clinical practice – identified just 8% of subsequent spontaneous preterm births. The addition of fetal fibronectin did not increase the predictive performance of cervical length alone, according to the findings.
The researchers cited the low incidence of short cervix (1% at 16-22 weeks) as a potential reason why the ultrasound screen was not useful. “Using the most conservative threshold of 25 mm or less in the most common time for clinical screening (16-22 weeks’ gestation), 247 women would need to be screened to identify 1 case of spontaneous preterm birth,” the researchers wrote. Using a transvaginal cervical length of 15 mm or less during the same time period, the number needed to screen rose to 680 to identify a single case of spontaneous preterm birth.
These findings do not support the routine use of these two screens among nulliparous women with singleton pregnancies, the researchers wrote. Screening procedures with relatively poor predictive values “may sometimes be useful if they are inexpensive, lack serious adverse effects, and address a serious condition for which an effective intervention exists. Neither of these tests, alone or in combination, meets all of these criteria,” they added.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development supported the study. Dr. Esplin reported holding a patent for serum markers of preterm birth and ties to Sera Prognostics and Clinical Innovations. One of his coauthors reported ties to Natera, Sequenom, Illumina, March of Dimes, Ariosa Diagnostics/Roche, KellBenx, and LabCorp.
Two screens to predict spontaneous preterm birth in low-risk women, which were rapidly adapted into clinical practice despite a lack of supportive evidence, proved to have little predictive value in a large cohort study.
Transvaginal ultrasound examination for short cervical length and quantitative cervicovaginal swabbing for fetal fibronectin are routinely used to predict spontaneous preterm birth. To assess the accuracy of both screens individually and in combination, researchers analyzed data for women participating in the prospective multicenter Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be (nuMoM2b). They focused on 9,410 nulliparous women with singleton pregnancies who were followed at eight clinical centers across the country. In addition to regular pregnancy visits, these women underwent both screening procedures at approximately 12 weeks, 19 weeks, and 28 weeks.
Both screens had relatively low sensitivity and low positive predictive value, regardless of when they were performed, which threshold values were used, and whether the results were considered individually or in combination (JAMA. 2017;317[10]:1047-56).
Transvaginal cervical length at 22-30 weeks’ gestation was the most accurate predictor of spontaneous preterm birth before 37 weeks, outperforming fetal fibronectin assessment alone. However, with a threshold of 25 mm or less – the most commonly used clinical cutoff – cervical length screening identified just 23.3% of spontaneous preterm births before 37 weeks. Use of that same threshold at 16-22 weeks – the most common time for screening in clinical practice – identified just 8% of subsequent spontaneous preterm births. The addition of fetal fibronectin did not increase the predictive performance of cervical length alone, according to the findings.
The researchers cited the low incidence of short cervix (1% at 16-22 weeks) as a potential reason why the ultrasound screen was not useful. “Using the most conservative threshold of 25 mm or less in the most common time for clinical screening (16-22 weeks’ gestation), 247 women would need to be screened to identify 1 case of spontaneous preterm birth,” the researchers wrote. Using a transvaginal cervical length of 15 mm or less during the same time period, the number needed to screen rose to 680 to identify a single case of spontaneous preterm birth.
These findings do not support the routine use of these two screens among nulliparous women with singleton pregnancies, the researchers wrote. Screening procedures with relatively poor predictive values “may sometimes be useful if they are inexpensive, lack serious adverse effects, and address a serious condition for which an effective intervention exists. Neither of these tests, alone or in combination, meets all of these criteria,” they added.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development supported the study. Dr. Esplin reported holding a patent for serum markers of preterm birth and ties to Sera Prognostics and Clinical Innovations. One of his coauthors reported ties to Natera, Sequenom, Illumina, March of Dimes, Ariosa Diagnostics/Roche, KellBenx, and LabCorp.
FROM JAMA
Key clinical point:
Major finding: With a threshold of transvaginal cervical length of 25 mm or less at 16-22 weeks’ gestation, 247 women would need to be screened to identify one case of spontaneous preterm birth before 37 weeks’ gestation.
Data source: A prospective multicenter observational cohort study involving 9,410 nulliparous women across the United States.
Disclosures: The Eunice Kennedy Shriver National Institute of Child Health and Human Development supported the study. Dr. Esplin reported holding a patent for serum markers of preterm birth and ties to Sera Prognostics and Clinical Innovations. An associate reported ties to Natera, Sequenom, Illumina, March of Dimes, Ariosa Diagnostics/Roche, KellBenx, and LabCorp.
Managing family differences
What is it about families that makes our patients so upset? Why can our patients not just walk away from conflict? Why do they get so bent out of shape when family members do not say or do what they expect them to do? We all have families that are less than ideal and struggle with how to manage difference.
This column gives psychiatrists a framework for thinking with families about the universal dilemma of managing difference. This dilemma can be viewed from the perspectives of the individual, the family, and society: Identity is formed in the crucible of the family, where parental introjects become a model for the child’s development and can be rejected as an adolescent or adult as individuals shape their own identity. Processes within the family shape family members’ relationships and, therefore, their expectations of one another. Strong boundaries provide safety for those inside the family versus those outside the family.
Individual perspective
Family members’ perspective and expectations of others depend on their family position. Children or young adults want to please the parent, and to be accepted and recognized for who they are. They want their unique qualities to be valued, they want to be loved, and they want to feel that they belong.
Parents want their young adult to reach what they consider a successful life, and to be fulfilled and healthy. When their child strikes out on his or her own, the parent may not understand, and may feel let down or angry. The parent may say: “She married him to get back at me.” “Why is my son so rejecting of the business our family spent generations to build?” “How can my child reject our family values that we brought from the old country?” “How did it happen that my son is gay?”
Siblings have an idea of who their sibling should be, and this idea often is fixed and immutable. They may ask, “Why won’t my sister help me out?” “Why can’t she be a good sister?” “Why is my brother so jealous of me?”
Family elders may wonder why their adult children do not want to return home to care for them or why they want their parents to go into a nursing home.
These dilemmas are easy to understand as conscious expectations. More difficult to understand are the unconscious projections that tangle up families.
Unconscious psychological processes
The two main unconscious psychological processes that tangle up families are projection and projective identification. Projective identification is an unconscious process in which aspects of the self are split off and projected onto another person. In 1946, Melanie Klein introduced the term “projective identification” as follows: “Much of the hatred against parts of the self is now directed toward the mother. This leads to a particular form of identification which establishes the prototype of an aggressive object-relation. I suggest for these processes the term ‘projective identification’ ” (Int J Psychoanal. 1946;27[pt 3-4]:99-110).
Mutual projective processes can occur in committed relationships. The following scenario helps illustrate this: Ms. A. projects onto her husband her own feared and unwanted aggressive, dominating aspects of herself. The result is that she fears and respects him. He, in turn, comes to feel aggressive and dominating toward her, not only because of his own resources but because of her projections, which she forces onto him. He may, in turn, despise and disown timid and fearful aspects of his own personality and by a similar mechanism of projective identification force these unwanted aspects of himself onto his wife. Ms. A. is then composed of timid unaggressive parts of herself as well as his projections, and she carries these feelings as her part in the relationship. Some couples, like Mr. and Ms. A., live in such locked systems, dominated by mutual projections, with each not truly married to the other person but to the unwanted, split-off, and projected parts of themselves.
In this scenario, the husband becomes dominant and cruel, and the wife becomes stupidly timid and respectful. These marriages are stable, because each partner needs the other for narcissistic pathologic purposes (see “Some Psychodynamics of Large Groups” in “The Large Group: Dynamics and Therapy” [London: Karnac Books, 1975] and “The Ailment and Other Psychoanalytic Essays” [London: Free Association Books, 2015]).
Marriage offers an opportunity for individuals to work out these types of issues, or, in the case of Mr. and Ms. A, not work through them. Instead, they exist in tight mutual projections.
Family process perspective
Families function as a system or unit, and each person in the family has a role or function. When change occurs, basic rules of systems theory apply. For example, if the mother functions as the emotional barometer, no one else needs to pay attention to emotions, as that is the mother’s job. If she leaves or becomes ill, someone else will take on that role or the family will fall apart. If the father becomes depressed and unable to function in his role as a parent, the oldest child may have to step up to become the parent. When he gets better and his depression resolves, there may be tension – as the older child may not want to give up that role. There may be a disagreement in the family vision.
When the children grow and develop their own identities and lifestyles, the family has to adjust to include the adult children or cut them off. Individuals also may cut themselves off from the family if there are significant disagreements. There are variations, such as “semi-cutoffs,” where there is little contact except at ritualized holidays and significant family events. Therefore, tensions arise most clearly at these times when family members come together.
Boundaries protect the family
A family functions like a pack. As with most species, families and parents protect the young until they are able to care for themselves. The marriage contract specifies that spouses care for each other but additionally that they join extended families together. Family cares for family before caring for strangers. It is the elder’s role and responsibility to keep the family together, or the family members may drift apart or be subsumed into other family groups.
A clan is made up of related families that form a larger extended family unit. Historically, strong alliances, as in clans or family dynasties, become dominant socially. In recent history, the idea of clans has become less attractive as the idea of individualism has become the American ideal.
Modern families tend to be individually oriented and do not need their families for protection as much as primitive tribes did. Modern families have fairly loose boundaries, and problems can arise when the family tries to define boundaries and values.
Families also change composition with the impact of sociocultural influences, such as migration. However, the primitive social drive still forces us to form families and clans. This drive can explain much of the need for identifying people as “in or out” of the family. The Amish intentionally address this dilemma. At adolescence, the ritual of Rumspringa allows the young person to experience 1 year out of Amish life in Western life. The adolescent can then decide to be in or out. If the adolescent decides to be in, conformity to Amish lifestyle is required (“Serving the Amish,” Baltimore: Johns Hopkins University Press, 2014).
Lastly, our families provide memories of where we have come from and where we are going, both as individuals and as a clan. Powerful stories serve the next generation with a sense of belonging and a specific orientation to the world. The studies of third-generation Holocaust survivors attest to the power of family narratives. Individuals can choose to embrace the family narrative or alter it to allow individual growth.
Explaining families to families
When helping patients work through issues with their families, it is helpful to provide them with context. Among the important points we can make are:
● Families came into existence as a way to protect our young; this is true across the animal kingdom. Humans congregated into clans or tribes that demanded conformity and obedience to the chief. There was a clear sense of who was in and who was out. Many of the difficulties that we experience are tied to the primitive tension of needing to decide who is in and who is out. This is a normal function of families.
● These days, families have much looser boundaries, and individuals have the freedom to strike out on their own. Families have to grapple with their collective identity only when they get together at holiday times or transitional events like marriages, births, and deaths. So, is it worth getting upset about this? If so, ask patients what they would like to change – and why.
● With this background, the family can dive deeper. Ask your patients, “Is the issue a problem with roles within the family? Has there been a role transition? Has there been a death, serious illness, or birth? Has someone left, retired, or joined the family? How would you as a family like to proceed?”
● Lastly, is there a complicated tangled web or relationship that might be explained by mutual projective identifications? If so, refer to a colleague with family therapy skills.
Key points to keep in mind
1. Families should be placed in the context of clans and tribes.
2. Transitions and family events cause families to question their family identity, boundaries, and values.
3. Patients should explore their individual expectations about what families should do. This conversation can be extensive, and include cultural and generational flash points.
4. If there is a tangled web that makes no sense to you, refer to a colleague with family therapy skills.
Dr. Heru is professor of psychiatry at the University of Colorado Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose.
What is it about families that makes our patients so upset? Why can our patients not just walk away from conflict? Why do they get so bent out of shape when family members do not say or do what they expect them to do? We all have families that are less than ideal and struggle with how to manage difference.
This column gives psychiatrists a framework for thinking with families about the universal dilemma of managing difference. This dilemma can be viewed from the perspectives of the individual, the family, and society: Identity is formed in the crucible of the family, where parental introjects become a model for the child’s development and can be rejected as an adolescent or adult as individuals shape their own identity. Processes within the family shape family members’ relationships and, therefore, their expectations of one another. Strong boundaries provide safety for those inside the family versus those outside the family.
Individual perspective
Family members’ perspective and expectations of others depend on their family position. Children or young adults want to please the parent, and to be accepted and recognized for who they are. They want their unique qualities to be valued, they want to be loved, and they want to feel that they belong.
Parents want their young adult to reach what they consider a successful life, and to be fulfilled and healthy. When their child strikes out on his or her own, the parent may not understand, and may feel let down or angry. The parent may say: “She married him to get back at me.” “Why is my son so rejecting of the business our family spent generations to build?” “How can my child reject our family values that we brought from the old country?” “How did it happen that my son is gay?”
Siblings have an idea of who their sibling should be, and this idea often is fixed and immutable. They may ask, “Why won’t my sister help me out?” “Why can’t she be a good sister?” “Why is my brother so jealous of me?”
Family elders may wonder why their adult children do not want to return home to care for them or why they want their parents to go into a nursing home.
These dilemmas are easy to understand as conscious expectations. More difficult to understand are the unconscious projections that tangle up families.
Unconscious psychological processes
The two main unconscious psychological processes that tangle up families are projection and projective identification. Projective identification is an unconscious process in which aspects of the self are split off and projected onto another person. In 1946, Melanie Klein introduced the term “projective identification” as follows: “Much of the hatred against parts of the self is now directed toward the mother. This leads to a particular form of identification which establishes the prototype of an aggressive object-relation. I suggest for these processes the term ‘projective identification’ ” (Int J Psychoanal. 1946;27[pt 3-4]:99-110).
Mutual projective processes can occur in committed relationships. The following scenario helps illustrate this: Ms. A. projects onto her husband her own feared and unwanted aggressive, dominating aspects of herself. The result is that she fears and respects him. He, in turn, comes to feel aggressive and dominating toward her, not only because of his own resources but because of her projections, which she forces onto him. He may, in turn, despise and disown timid and fearful aspects of his own personality and by a similar mechanism of projective identification force these unwanted aspects of himself onto his wife. Ms. A. is then composed of timid unaggressive parts of herself as well as his projections, and she carries these feelings as her part in the relationship. Some couples, like Mr. and Ms. A., live in such locked systems, dominated by mutual projections, with each not truly married to the other person but to the unwanted, split-off, and projected parts of themselves.
In this scenario, the husband becomes dominant and cruel, and the wife becomes stupidly timid and respectful. These marriages are stable, because each partner needs the other for narcissistic pathologic purposes (see “Some Psychodynamics of Large Groups” in “The Large Group: Dynamics and Therapy” [London: Karnac Books, 1975] and “The Ailment and Other Psychoanalytic Essays” [London: Free Association Books, 2015]).
Marriage offers an opportunity for individuals to work out these types of issues, or, in the case of Mr. and Ms. A, not work through them. Instead, they exist in tight mutual projections.
Family process perspective
Families function as a system or unit, and each person in the family has a role or function. When change occurs, basic rules of systems theory apply. For example, if the mother functions as the emotional barometer, no one else needs to pay attention to emotions, as that is the mother’s job. If she leaves or becomes ill, someone else will take on that role or the family will fall apart. If the father becomes depressed and unable to function in his role as a parent, the oldest child may have to step up to become the parent. When he gets better and his depression resolves, there may be tension – as the older child may not want to give up that role. There may be a disagreement in the family vision.
When the children grow and develop their own identities and lifestyles, the family has to adjust to include the adult children or cut them off. Individuals also may cut themselves off from the family if there are significant disagreements. There are variations, such as “semi-cutoffs,” where there is little contact except at ritualized holidays and significant family events. Therefore, tensions arise most clearly at these times when family members come together.
Boundaries protect the family
A family functions like a pack. As with most species, families and parents protect the young until they are able to care for themselves. The marriage contract specifies that spouses care for each other but additionally that they join extended families together. Family cares for family before caring for strangers. It is the elder’s role and responsibility to keep the family together, or the family members may drift apart or be subsumed into other family groups.
A clan is made up of related families that form a larger extended family unit. Historically, strong alliances, as in clans or family dynasties, become dominant socially. In recent history, the idea of clans has become less attractive as the idea of individualism has become the American ideal.
Modern families tend to be individually oriented and do not need their families for protection as much as primitive tribes did. Modern families have fairly loose boundaries, and problems can arise when the family tries to define boundaries and values.
Families also change composition with the impact of sociocultural influences, such as migration. However, the primitive social drive still forces us to form families and clans. This drive can explain much of the need for identifying people as “in or out” of the family. The Amish intentionally address this dilemma. At adolescence, the ritual of Rumspringa allows the young person to experience 1 year out of Amish life in Western life. The adolescent can then decide to be in or out. If the adolescent decides to be in, conformity to Amish lifestyle is required (“Serving the Amish,” Baltimore: Johns Hopkins University Press, 2014).
Lastly, our families provide memories of where we have come from and where we are going, both as individuals and as a clan. Powerful stories serve the next generation with a sense of belonging and a specific orientation to the world. The studies of third-generation Holocaust survivors attest to the power of family narratives. Individuals can choose to embrace the family narrative or alter it to allow individual growth.
Explaining families to families
When helping patients work through issues with their families, it is helpful to provide them with context. Among the important points we can make are:
● Families came into existence as a way to protect our young; this is true across the animal kingdom. Humans congregated into clans or tribes that demanded conformity and obedience to the chief. There was a clear sense of who was in and who was out. Many of the difficulties that we experience are tied to the primitive tension of needing to decide who is in and who is out. This is a normal function of families.
● These days, families have much looser boundaries, and individuals have the freedom to strike out on their own. Families have to grapple with their collective identity only when they get together at holiday times or transitional events like marriages, births, and deaths. So, is it worth getting upset about this? If so, ask patients what they would like to change – and why.
● With this background, the family can dive deeper. Ask your patients, “Is the issue a problem with roles within the family? Has there been a role transition? Has there been a death, serious illness, or birth? Has someone left, retired, or joined the family? How would you as a family like to proceed?”
● Lastly, is there a complicated tangled web or relationship that might be explained by mutual projective identifications? If so, refer to a colleague with family therapy skills.
Key points to keep in mind
1. Families should be placed in the context of clans and tribes.
2. Transitions and family events cause families to question their family identity, boundaries, and values.
3. Patients should explore their individual expectations about what families should do. This conversation can be extensive, and include cultural and generational flash points.
4. If there is a tangled web that makes no sense to you, refer to a colleague with family therapy skills.
Dr. Heru is professor of psychiatry at the University of Colorado Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose.
What is it about families that makes our patients so upset? Why can our patients not just walk away from conflict? Why do they get so bent out of shape when family members do not say or do what they expect them to do? We all have families that are less than ideal and struggle with how to manage difference.
This column gives psychiatrists a framework for thinking with families about the universal dilemma of managing difference. This dilemma can be viewed from the perspectives of the individual, the family, and society: Identity is formed in the crucible of the family, where parental introjects become a model for the child’s development and can be rejected as an adolescent or adult as individuals shape their own identity. Processes within the family shape family members’ relationships and, therefore, their expectations of one another. Strong boundaries provide safety for those inside the family versus those outside the family.
Individual perspective
Family members’ perspective and expectations of others depend on their family position. Children or young adults want to please the parent, and to be accepted and recognized for who they are. They want their unique qualities to be valued, they want to be loved, and they want to feel that they belong.
Parents want their young adult to reach what they consider a successful life, and to be fulfilled and healthy. When their child strikes out on his or her own, the parent may not understand, and may feel let down or angry. The parent may say: “She married him to get back at me.” “Why is my son so rejecting of the business our family spent generations to build?” “How can my child reject our family values that we brought from the old country?” “How did it happen that my son is gay?”
Siblings have an idea of who their sibling should be, and this idea often is fixed and immutable. They may ask, “Why won’t my sister help me out?” “Why can’t she be a good sister?” “Why is my brother so jealous of me?”
Family elders may wonder why their adult children do not want to return home to care for them or why they want their parents to go into a nursing home.
These dilemmas are easy to understand as conscious expectations. More difficult to understand are the unconscious projections that tangle up families.
Unconscious psychological processes
The two main unconscious psychological processes that tangle up families are projection and projective identification. Projective identification is an unconscious process in which aspects of the self are split off and projected onto another person. In 1946, Melanie Klein introduced the term “projective identification” as follows: “Much of the hatred against parts of the self is now directed toward the mother. This leads to a particular form of identification which establishes the prototype of an aggressive object-relation. I suggest for these processes the term ‘projective identification’ ” (Int J Psychoanal. 1946;27[pt 3-4]:99-110).
Mutual projective processes can occur in committed relationships. The following scenario helps illustrate this: Ms. A. projects onto her husband her own feared and unwanted aggressive, dominating aspects of herself. The result is that she fears and respects him. He, in turn, comes to feel aggressive and dominating toward her, not only because of his own resources but because of her projections, which she forces onto him. He may, in turn, despise and disown timid and fearful aspects of his own personality and by a similar mechanism of projective identification force these unwanted aspects of himself onto his wife. Ms. A. is then composed of timid unaggressive parts of herself as well as his projections, and she carries these feelings as her part in the relationship. Some couples, like Mr. and Ms. A., live in such locked systems, dominated by mutual projections, with each not truly married to the other person but to the unwanted, split-off, and projected parts of themselves.
In this scenario, the husband becomes dominant and cruel, and the wife becomes stupidly timid and respectful. These marriages are stable, because each partner needs the other for narcissistic pathologic purposes (see “Some Psychodynamics of Large Groups” in “The Large Group: Dynamics and Therapy” [London: Karnac Books, 1975] and “The Ailment and Other Psychoanalytic Essays” [London: Free Association Books, 2015]).
Marriage offers an opportunity for individuals to work out these types of issues, or, in the case of Mr. and Ms. A, not work through them. Instead, they exist in tight mutual projections.
Family process perspective
Families function as a system or unit, and each person in the family has a role or function. When change occurs, basic rules of systems theory apply. For example, if the mother functions as the emotional barometer, no one else needs to pay attention to emotions, as that is the mother’s job. If she leaves or becomes ill, someone else will take on that role or the family will fall apart. If the father becomes depressed and unable to function in his role as a parent, the oldest child may have to step up to become the parent. When he gets better and his depression resolves, there may be tension – as the older child may not want to give up that role. There may be a disagreement in the family vision.
When the children grow and develop their own identities and lifestyles, the family has to adjust to include the adult children or cut them off. Individuals also may cut themselves off from the family if there are significant disagreements. There are variations, such as “semi-cutoffs,” where there is little contact except at ritualized holidays and significant family events. Therefore, tensions arise most clearly at these times when family members come together.
Boundaries protect the family
A family functions like a pack. As with most species, families and parents protect the young until they are able to care for themselves. The marriage contract specifies that spouses care for each other but additionally that they join extended families together. Family cares for family before caring for strangers. It is the elder’s role and responsibility to keep the family together, or the family members may drift apart or be subsumed into other family groups.
A clan is made up of related families that form a larger extended family unit. Historically, strong alliances, as in clans or family dynasties, become dominant socially. In recent history, the idea of clans has become less attractive as the idea of individualism has become the American ideal.
Modern families tend to be individually oriented and do not need their families for protection as much as primitive tribes did. Modern families have fairly loose boundaries, and problems can arise when the family tries to define boundaries and values.
Families also change composition with the impact of sociocultural influences, such as migration. However, the primitive social drive still forces us to form families and clans. This drive can explain much of the need for identifying people as “in or out” of the family. The Amish intentionally address this dilemma. At adolescence, the ritual of Rumspringa allows the young person to experience 1 year out of Amish life in Western life. The adolescent can then decide to be in or out. If the adolescent decides to be in, conformity to Amish lifestyle is required (“Serving the Amish,” Baltimore: Johns Hopkins University Press, 2014).
Lastly, our families provide memories of where we have come from and where we are going, both as individuals and as a clan. Powerful stories serve the next generation with a sense of belonging and a specific orientation to the world. The studies of third-generation Holocaust survivors attest to the power of family narratives. Individuals can choose to embrace the family narrative or alter it to allow individual growth.
Explaining families to families
When helping patients work through issues with their families, it is helpful to provide them with context. Among the important points we can make are:
● Families came into existence as a way to protect our young; this is true across the animal kingdom. Humans congregated into clans or tribes that demanded conformity and obedience to the chief. There was a clear sense of who was in and who was out. Many of the difficulties that we experience are tied to the primitive tension of needing to decide who is in and who is out. This is a normal function of families.
● These days, families have much looser boundaries, and individuals have the freedom to strike out on their own. Families have to grapple with their collective identity only when they get together at holiday times or transitional events like marriages, births, and deaths. So, is it worth getting upset about this? If so, ask patients what they would like to change – and why.
● With this background, the family can dive deeper. Ask your patients, “Is the issue a problem with roles within the family? Has there been a role transition? Has there been a death, serious illness, or birth? Has someone left, retired, or joined the family? How would you as a family like to proceed?”
● Lastly, is there a complicated tangled web or relationship that might be explained by mutual projective identifications? If so, refer to a colleague with family therapy skills.
Key points to keep in mind
1. Families should be placed in the context of clans and tribes.
2. Transitions and family events cause families to question their family identity, boundaries, and values.
3. Patients should explore their individual expectations about what families should do. This conversation can be extensive, and include cultural and generational flash points.
4. If there is a tangled web that makes no sense to you, refer to a colleague with family therapy skills.
Dr. Heru is professor of psychiatry at the University of Colorado Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose.
WPR may benefit patients with stage IVB cervical cancer
NATIONAL HARBOR, MD. – Whole pelvic radiation delivered in addition to chemotherapy in women who present with stage IVB cervical cancer confers significant 12-month overall and progression-free survival benefits, according to findings from a multi-institutional retrospective review.
Of 127 patients diagnosed during 2005-2015 at four academic high-volume centers, 31 received no treatment or elected hospice care and, of the remaining patients, 34 received whole pelvic radiation (WPR) in addition to chemotherapy, and 62 received chemotherapy alone, which is the standard of care. The median overall survival was 14.1 vs. 6.9 months with vs. without WPR, and the median progression-free survival was 10 vs. 5 months, respectively, Victoria B. Perkins, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Of note, the rates of pelvic-related morbidity, including ureteral obstruction, vaginal or rectal bleeding, pelvic infection, pelvic pain, and fistula, did not differ significantly between the groups, said Dr. Perkins of the University of Oklahoma Health Sciences Center, Oklahoma City.
Study subjects were women with a median age of 54 years. A little more than a third (36%) were white, 35% were Hispanic, and 16% were black. Most (75%) had squamous cell carcinoma, and 95% were grade 2 or 3.
There were no significant differences in the demographics, location of [metastatic] disease, or distribution of chemotherapy type between the two groups, she said, noting that “interestingly, 26% of patients in the chemotherapy alone group received additional bevacizumab, compared to only 12% in the whole pelvic radiation with chemotherapy group.
“This could reflect the change in treatment strategy with the approval of bevacizumab during the treatment period,” Dr. Perkins noted.
About 5% of women have stage IVB cervical cancer at the time of diagnosis, but 5-year survival in these women is only about 15%.
“The mainstay of treatment is platinum and taxane with or without bevacizumab. In clinical practice, treatment of stage IVB disease varies,” Dr. Perkins said.
One strategy follows the guidance of phase III trials looking at chemotherapy with palliative radiation as needed.
“However, a significant number of patients experience morbidity and mortality directly related to their pelvic disease, such as vaginal bleeding, ureteral obstruction, fistulization, infections, and pain. Thus, an alternative approach is aimed at treating bulky pelvic disease with whole pelvic radiation followed by systemic chemotherapy with the goal to control symptoms and theoretically reduce recurrences in the pelvic field, which can be highly problematic in terms of symptomatic control,” she said, noting that this novel approach has not been formally studied.
The aim of the current review was to determine if WPR with chemotherapy would reduce pelvic morbidity and improve overall and progression-free survival.
“Survival in stage IVB disease remains extremely poor. Perhaps adding whole pelvic radiation to systemic chemotherapy has utility without increasing morbidity. However, the addition of whole pelvic radiation did not improve pelvic-related morbidity as previously hypothesized,” she said.
The study was limited by varied chemotherapy regimens in both groups and by changes in standard treatment practice during the span of study. It also was limited by the retrospective design, small sample size, and lack of quality of life data, but the findings support further study regarding subgroups of patients who could benefit the most from this treatment strategy, she concluded, noting, however, that such study is challenging because of the rarity of stage IVB disease.
Dr. Perkins reported having no disclosures.
NATIONAL HARBOR, MD. – Whole pelvic radiation delivered in addition to chemotherapy in women who present with stage IVB cervical cancer confers significant 12-month overall and progression-free survival benefits, according to findings from a multi-institutional retrospective review.
Of 127 patients diagnosed during 2005-2015 at four academic high-volume centers, 31 received no treatment or elected hospice care and, of the remaining patients, 34 received whole pelvic radiation (WPR) in addition to chemotherapy, and 62 received chemotherapy alone, which is the standard of care. The median overall survival was 14.1 vs. 6.9 months with vs. without WPR, and the median progression-free survival was 10 vs. 5 months, respectively, Victoria B. Perkins, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Of note, the rates of pelvic-related morbidity, including ureteral obstruction, vaginal or rectal bleeding, pelvic infection, pelvic pain, and fistula, did not differ significantly between the groups, said Dr. Perkins of the University of Oklahoma Health Sciences Center, Oklahoma City.
Study subjects were women with a median age of 54 years. A little more than a third (36%) were white, 35% were Hispanic, and 16% were black. Most (75%) had squamous cell carcinoma, and 95% were grade 2 or 3.
There were no significant differences in the demographics, location of [metastatic] disease, or distribution of chemotherapy type between the two groups, she said, noting that “interestingly, 26% of patients in the chemotherapy alone group received additional bevacizumab, compared to only 12% in the whole pelvic radiation with chemotherapy group.
“This could reflect the change in treatment strategy with the approval of bevacizumab during the treatment period,” Dr. Perkins noted.
About 5% of women have stage IVB cervical cancer at the time of diagnosis, but 5-year survival in these women is only about 15%.
“The mainstay of treatment is platinum and taxane with or without bevacizumab. In clinical practice, treatment of stage IVB disease varies,” Dr. Perkins said.
One strategy follows the guidance of phase III trials looking at chemotherapy with palliative radiation as needed.
“However, a significant number of patients experience morbidity and mortality directly related to their pelvic disease, such as vaginal bleeding, ureteral obstruction, fistulization, infections, and pain. Thus, an alternative approach is aimed at treating bulky pelvic disease with whole pelvic radiation followed by systemic chemotherapy with the goal to control symptoms and theoretically reduce recurrences in the pelvic field, which can be highly problematic in terms of symptomatic control,” she said, noting that this novel approach has not been formally studied.
The aim of the current review was to determine if WPR with chemotherapy would reduce pelvic morbidity and improve overall and progression-free survival.
“Survival in stage IVB disease remains extremely poor. Perhaps adding whole pelvic radiation to systemic chemotherapy has utility without increasing morbidity. However, the addition of whole pelvic radiation did not improve pelvic-related morbidity as previously hypothesized,” she said.
The study was limited by varied chemotherapy regimens in both groups and by changes in standard treatment practice during the span of study. It also was limited by the retrospective design, small sample size, and lack of quality of life data, but the findings support further study regarding subgroups of patients who could benefit the most from this treatment strategy, she concluded, noting, however, that such study is challenging because of the rarity of stage IVB disease.
Dr. Perkins reported having no disclosures.
NATIONAL HARBOR, MD. – Whole pelvic radiation delivered in addition to chemotherapy in women who present with stage IVB cervical cancer confers significant 12-month overall and progression-free survival benefits, according to findings from a multi-institutional retrospective review.
Of 127 patients diagnosed during 2005-2015 at four academic high-volume centers, 31 received no treatment or elected hospice care and, of the remaining patients, 34 received whole pelvic radiation (WPR) in addition to chemotherapy, and 62 received chemotherapy alone, which is the standard of care. The median overall survival was 14.1 vs. 6.9 months with vs. without WPR, and the median progression-free survival was 10 vs. 5 months, respectively, Victoria B. Perkins, MD, said at the annual meeting of the Society of Gynecologic Oncology.
Of note, the rates of pelvic-related morbidity, including ureteral obstruction, vaginal or rectal bleeding, pelvic infection, pelvic pain, and fistula, did not differ significantly between the groups, said Dr. Perkins of the University of Oklahoma Health Sciences Center, Oklahoma City.
Study subjects were women with a median age of 54 years. A little more than a third (36%) were white, 35% were Hispanic, and 16% were black. Most (75%) had squamous cell carcinoma, and 95% were grade 2 or 3.
There were no significant differences in the demographics, location of [metastatic] disease, or distribution of chemotherapy type between the two groups, she said, noting that “interestingly, 26% of patients in the chemotherapy alone group received additional bevacizumab, compared to only 12% in the whole pelvic radiation with chemotherapy group.
“This could reflect the change in treatment strategy with the approval of bevacizumab during the treatment period,” Dr. Perkins noted.
About 5% of women have stage IVB cervical cancer at the time of diagnosis, but 5-year survival in these women is only about 15%.
“The mainstay of treatment is platinum and taxane with or without bevacizumab. In clinical practice, treatment of stage IVB disease varies,” Dr. Perkins said.
One strategy follows the guidance of phase III trials looking at chemotherapy with palliative radiation as needed.
“However, a significant number of patients experience morbidity and mortality directly related to their pelvic disease, such as vaginal bleeding, ureteral obstruction, fistulization, infections, and pain. Thus, an alternative approach is aimed at treating bulky pelvic disease with whole pelvic radiation followed by systemic chemotherapy with the goal to control symptoms and theoretically reduce recurrences in the pelvic field, which can be highly problematic in terms of symptomatic control,” she said, noting that this novel approach has not been formally studied.
The aim of the current review was to determine if WPR with chemotherapy would reduce pelvic morbidity and improve overall and progression-free survival.
“Survival in stage IVB disease remains extremely poor. Perhaps adding whole pelvic radiation to systemic chemotherapy has utility without increasing morbidity. However, the addition of whole pelvic radiation did not improve pelvic-related morbidity as previously hypothesized,” she said.
The study was limited by varied chemotherapy regimens in both groups and by changes in standard treatment practice during the span of study. It also was limited by the retrospective design, small sample size, and lack of quality of life data, but the findings support further study regarding subgroups of patients who could benefit the most from this treatment strategy, she concluded, noting, however, that such study is challenging because of the rarity of stage IVB disease.
Dr. Perkins reported having no disclosures.
AT THE ANNUAL MEETING ON WOMEN’S CANCERS
Key clinical point:
Major finding: Median overall and progression-free survival with vs. without WPR: 14.1 vs. 6.9 months and 10 vs. 5 months, respectively, but no difference in pelvic-related morbidity.
Data source: A retrospective review of 127 cases.
Disclosures: Dr. Perkins reported having no disclosures.
Adjuvant chemotherapy alone may suffice for some high-risk early cervical cancers
National Harbor, MD. – Patients, even those with surgically-confirmed risk factors, fared well when they received adjuvant chemotherapy without radiotherapy for early-stage cervical cancer.
In a retrospective review of 101 patients, Kwang-Beom Lee, MD, and his associates found that patients with known surgical risk factors had a posttreatment disease-free survival rate of 94.6%, a 5-year overall survival rate of 90.6%. and a disease-specific 5-year survival rate of 96.2%. These figures compare with survival rates of 79.4%, 90.6%, and 90.6%, respectively, for early-stage cervical cancer patients with lymph node metastasis.
Dr. Lee, professor of obstetrics and gynecology at Gachon University School of Medicine, Incheon, South Korea, said that about 3,600 cases of cervical cancer are diagnosed each year in Korea, and a little more than half (58%) are early-stage cancers. Most Korean patients with International Federation of Gynecology and Obstetrics (FIGO) stage IA2-IIA cancer will receive a radical hysterectomy with lymphadenectomy, he said at the annual meeting of the Society of Gynecologic Oncology.
Dr. Lee and his colleagues sought to ascertain morbidity when patients with early cervical cancer received either adjuvant radiotherapy or concurrent chemoradiotherapy (CCRT), and to explore the potential role that chemotherapy alone might play in these patients.
Accordingly, one of the primary outcomes of the study was to determine outcomes of adjuvant chemotherapy alone for patients with FIGO stage IB-IIA cervical cancer who had surgically-confirmed risk factors, and who received radical surgery.
The surgically-confirmed factors included lymphovascular space invasion, depth of penetration, and tumor size.
Currently, Dr. Lee said, evidence indicates that CCRT for patients with high risk factors improves both progression-free survival and overall survival. For patients with intermediate risk factors, radiotherapy is associated with increased progression-free survival.
The researchers examined 101 patients in this category who were treated between March of 2006 and December of 2014 at two Korean academic medical centers, excluding patients who had positive tumor margins, who received neoadjuvant chemotherapy, or who had microscopic parametrium involvement.
The mean age of the patients was 47.1 years (range, 23-73 years). Their mean body mass index was 23.1, and two thirds of patients were premenopausal. Most patients (73.3%, n = 74) had stage IB1 cancer, while 23 (22.8%) had stage IB2 cancer, and 4 (3.9%) had stage IIA cancer. Most patients (74.3%, n = 75) had squamous cell cancer.
The radical procedure performed was a type C radical hysterectomy; patients underwent pelvic lymph node dissection, with or without para-aortic node dissection. Pelvic nodes included all of the common iliac nodes, the external and internal iliac chains, and the obturator nodes. Para-aortic node dissection included dissection up to the level of the inferior mesenteric artery.
A total of 50 patients received pelvic lymph node and para-aortic lymph node dissection, with a mean 54.5 tumors retrieved per patient. A mean of 4.58 pelvic nodes were assessed as metastatic. A mean of 11.2 para-aortic nodes were retrieved, and of these, a mean 5.3 were metastatic.
All together, 76 patients had a combination of three surgically-confirmed risk factors without positive lymph nodes; the remaining 25 patients had positive lymph nodes (4 with pelvic and para-aortic involvement, the remaining with pelvic involvement alone), and were included irrespective of whether they met other risk factor criteria.
Dr. Lee said that the protocol for chemotherapy paralelled Protocol 92 from the Gynecologic Oncology Group; patients received chemotherapy if the combination of tumor diameter, depth of stromal invasion, and lymphovascular space invasion met Protocol 92 criteria for treatment, or if more than one lymph node metastasis was found.
The chemotherapy regime for intermediate-risk patients called for six cycles of either platinum alone (n = 47), or platinum with paclitaxel (n = 54). High-risk patients received six cycles of paclitaxel and platinum.
Patients were followed for a median of 65 months, and a total of 14 patients had a recurrence.
Dr. Lee reported no conflicts of interest.
[email protected]
On Twitter @karioakes
National Harbor, MD. – Patients, even those with surgically-confirmed risk factors, fared well when they received adjuvant chemotherapy without radiotherapy for early-stage cervical cancer.
In a retrospective review of 101 patients, Kwang-Beom Lee, MD, and his associates found that patients with known surgical risk factors had a posttreatment disease-free survival rate of 94.6%, a 5-year overall survival rate of 90.6%. and a disease-specific 5-year survival rate of 96.2%. These figures compare with survival rates of 79.4%, 90.6%, and 90.6%, respectively, for early-stage cervical cancer patients with lymph node metastasis.
Dr. Lee, professor of obstetrics and gynecology at Gachon University School of Medicine, Incheon, South Korea, said that about 3,600 cases of cervical cancer are diagnosed each year in Korea, and a little more than half (58%) are early-stage cancers. Most Korean patients with International Federation of Gynecology and Obstetrics (FIGO) stage IA2-IIA cancer will receive a radical hysterectomy with lymphadenectomy, he said at the annual meeting of the Society of Gynecologic Oncology.
Dr. Lee and his colleagues sought to ascertain morbidity when patients with early cervical cancer received either adjuvant radiotherapy or concurrent chemoradiotherapy (CCRT), and to explore the potential role that chemotherapy alone might play in these patients.
Accordingly, one of the primary outcomes of the study was to determine outcomes of adjuvant chemotherapy alone for patients with FIGO stage IB-IIA cervical cancer who had surgically-confirmed risk factors, and who received radical surgery.
The surgically-confirmed factors included lymphovascular space invasion, depth of penetration, and tumor size.
Currently, Dr. Lee said, evidence indicates that CCRT for patients with high risk factors improves both progression-free survival and overall survival. For patients with intermediate risk factors, radiotherapy is associated with increased progression-free survival.
The researchers examined 101 patients in this category who were treated between March of 2006 and December of 2014 at two Korean academic medical centers, excluding patients who had positive tumor margins, who received neoadjuvant chemotherapy, or who had microscopic parametrium involvement.
The mean age of the patients was 47.1 years (range, 23-73 years). Their mean body mass index was 23.1, and two thirds of patients were premenopausal. Most patients (73.3%, n = 74) had stage IB1 cancer, while 23 (22.8%) had stage IB2 cancer, and 4 (3.9%) had stage IIA cancer. Most patients (74.3%, n = 75) had squamous cell cancer.
The radical procedure performed was a type C radical hysterectomy; patients underwent pelvic lymph node dissection, with or without para-aortic node dissection. Pelvic nodes included all of the common iliac nodes, the external and internal iliac chains, and the obturator nodes. Para-aortic node dissection included dissection up to the level of the inferior mesenteric artery.
A total of 50 patients received pelvic lymph node and para-aortic lymph node dissection, with a mean 54.5 tumors retrieved per patient. A mean of 4.58 pelvic nodes were assessed as metastatic. A mean of 11.2 para-aortic nodes were retrieved, and of these, a mean 5.3 were metastatic.
All together, 76 patients had a combination of three surgically-confirmed risk factors without positive lymph nodes; the remaining 25 patients had positive lymph nodes (4 with pelvic and para-aortic involvement, the remaining with pelvic involvement alone), and were included irrespective of whether they met other risk factor criteria.
Dr. Lee said that the protocol for chemotherapy paralelled Protocol 92 from the Gynecologic Oncology Group; patients received chemotherapy if the combination of tumor diameter, depth of stromal invasion, and lymphovascular space invasion met Protocol 92 criteria for treatment, or if more than one lymph node metastasis was found.
The chemotherapy regime for intermediate-risk patients called for six cycles of either platinum alone (n = 47), or platinum with paclitaxel (n = 54). High-risk patients received six cycles of paclitaxel and platinum.
Patients were followed for a median of 65 months, and a total of 14 patients had a recurrence.
Dr. Lee reported no conflicts of interest.
[email protected]
On Twitter @karioakes
National Harbor, MD. – Patients, even those with surgically-confirmed risk factors, fared well when they received adjuvant chemotherapy without radiotherapy for early-stage cervical cancer.
In a retrospective review of 101 patients, Kwang-Beom Lee, MD, and his associates found that patients with known surgical risk factors had a posttreatment disease-free survival rate of 94.6%, a 5-year overall survival rate of 90.6%. and a disease-specific 5-year survival rate of 96.2%. These figures compare with survival rates of 79.4%, 90.6%, and 90.6%, respectively, for early-stage cervical cancer patients with lymph node metastasis.
Dr. Lee, professor of obstetrics and gynecology at Gachon University School of Medicine, Incheon, South Korea, said that about 3,600 cases of cervical cancer are diagnosed each year in Korea, and a little more than half (58%) are early-stage cancers. Most Korean patients with International Federation of Gynecology and Obstetrics (FIGO) stage IA2-IIA cancer will receive a radical hysterectomy with lymphadenectomy, he said at the annual meeting of the Society of Gynecologic Oncology.
Dr. Lee and his colleagues sought to ascertain morbidity when patients with early cervical cancer received either adjuvant radiotherapy or concurrent chemoradiotherapy (CCRT), and to explore the potential role that chemotherapy alone might play in these patients.
Accordingly, one of the primary outcomes of the study was to determine outcomes of adjuvant chemotherapy alone for patients with FIGO stage IB-IIA cervical cancer who had surgically-confirmed risk factors, and who received radical surgery.
The surgically-confirmed factors included lymphovascular space invasion, depth of penetration, and tumor size.
Currently, Dr. Lee said, evidence indicates that CCRT for patients with high risk factors improves both progression-free survival and overall survival. For patients with intermediate risk factors, radiotherapy is associated with increased progression-free survival.
The researchers examined 101 patients in this category who were treated between March of 2006 and December of 2014 at two Korean academic medical centers, excluding patients who had positive tumor margins, who received neoadjuvant chemotherapy, or who had microscopic parametrium involvement.
The mean age of the patients was 47.1 years (range, 23-73 years). Their mean body mass index was 23.1, and two thirds of patients were premenopausal. Most patients (73.3%, n = 74) had stage IB1 cancer, while 23 (22.8%) had stage IB2 cancer, and 4 (3.9%) had stage IIA cancer. Most patients (74.3%, n = 75) had squamous cell cancer.
The radical procedure performed was a type C radical hysterectomy; patients underwent pelvic lymph node dissection, with or without para-aortic node dissection. Pelvic nodes included all of the common iliac nodes, the external and internal iliac chains, and the obturator nodes. Para-aortic node dissection included dissection up to the level of the inferior mesenteric artery.
A total of 50 patients received pelvic lymph node and para-aortic lymph node dissection, with a mean 54.5 tumors retrieved per patient. A mean of 4.58 pelvic nodes were assessed as metastatic. A mean of 11.2 para-aortic nodes were retrieved, and of these, a mean 5.3 were metastatic.
All together, 76 patients had a combination of three surgically-confirmed risk factors without positive lymph nodes; the remaining 25 patients had positive lymph nodes (4 with pelvic and para-aortic involvement, the remaining with pelvic involvement alone), and were included irrespective of whether they met other risk factor criteria.
Dr. Lee said that the protocol for chemotherapy paralelled Protocol 92 from the Gynecologic Oncology Group; patients received chemotherapy if the combination of tumor diameter, depth of stromal invasion, and lymphovascular space invasion met Protocol 92 criteria for treatment, or if more than one lymph node metastasis was found.
The chemotherapy regime for intermediate-risk patients called for six cycles of either platinum alone (n = 47), or platinum with paclitaxel (n = 54). High-risk patients received six cycles of paclitaxel and platinum.
Patients were followed for a median of 65 months, and a total of 14 patients had a recurrence.
Dr. Lee reported no conflicts of interest.
[email protected]
On Twitter @karioakes
AT THE ANNUAL MEETING ON WOMEN’S CANCERS
Key clinical point:
Major finding: Patients with known surgical risk factors had a posttreatment disease-free survival rate of 94.6%.
Data source: Retrospective, two-center review of patients with early cervical cancer and multiple risk factors or pelvic node involvement.
Disclosures: Dr. Lee reported no conflicts of interest.
Stroke in AF patients often preceded by inadequate anticoagulation
Of the 94,474 ischemic strokes that occurred in a nationwide registry among patients with known atrial fibrillation, 84% were preceded by inadequate anticoagulation, according to a report published online March 14 in JAMA.
Fully 30% of the patients in this retrospective cohort study weren’t taking any form of antithrombotic therapy before their stroke, even though all of them had atrial fibrillation (AF) and most of them had additional risk factors as well. And although nearly 20,000 of the study participants were taking warfarin before their stroke, 64% of them were taking subtherapeutic doses, said Ying Xian, MD, PhD, of the department of neurology at the Duke Clinical Research Institute, Durham, N.C., and his associates.
These findings highlight tens of thousands of missed opportunities for preventing stroke simply by following existing guidelines, the investigators said.
In previous studies, researchers have consistently found underuse of oral anticoagulants in community practice. To examine current trends since the rapid adoption of non–vitamin-K antagonist oral anticoagulants (NOACs), Dr. Xian and his associates analyzed data from the AHA/ASA Get With the Guidelines-Stroke Registry. They determined the prevalence of antithrombotic treatment among AF patients who developed ischemic stroke, focusing on patients (mean age, 79.9 years) enrolled in the registry after being admitted for ischemic stroke to 1,622 participating U.S. hospitals during a 2.5-year period.
A total of 79,008 (83.6%) were not receiving therapeutic anticoagulation at the time of their stroke. This included 28,583 who were not taking any antithrombotic treatment, 12,751 who were taking subtherapeutic doses of warfarin (international normalized ratio,or INR, of less than 2 at the time of their stroke), and 37,674 who were taking only antiplatelet drugs when additional treatment was indicated, the investigators said (JAMA. 2017 Mar 14;317[10]:1057-67).
Among the minority of AF patients who were receiving therapeutic anticoagulation at the time of their stroke, 7,176 were taking adequate warfarin and 8,290 were taking adequate NOACs such as dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis).
Stroke severity, as measured by median scores on the National Institutes of Health Stroke Scale, was significantly greater among patients receiving no antithrombotic medication, antiplatelet agents alone, or subtherapeutic levels of warfarin, compared with patients receiving therapeutic levels of warfarin or NOACs. Similarly, patients who had adequate anticoagulation before their stroke were significantly more likely to have better functional outcomes than those who did not. “These findings reinforce the importance of INR monitoring and dose adjustment to ... keep the INR in the therapeutic range,” Dr. Xian and his associates wrote.
The reasons why patients weren’t receiving adequate anticoagulation before their stroke – including any direct contraindications to treatment – were not available from the registry data. But such reasons are likely to persist after a stroke, and study clinicians were required to document such reasons in the medical records at hospital discharge. So the investigators examined reasons listed for not prescribing oral anticoagulation at hospital discharge in 58,084 patients.
A striking 38,249 AF patients (65.8%) in this large subgroup of study participants had no documented reason for not receiving oral anticoagulation, even after they were hospitalized for ischemic stroke and even though such documentation was “required.” Among the minority of patients whose records did show such reasons, the most common reason cited was bleeding risk (16.3%), followed by risk of falling (10.3%), the presence of a terminal illness (6.2%), patient or family refusal of the medication (4.3%), the patient’s impaired mental status (1.1%), adverse effects of the medication (1.0%), and allergy to the medication (0.6%).
This study was supported by the Patient-Centered Outcomes Research Institute. Dr. Xian reported that his institution received research funding from the American Heart Association, Daiichi Sankyo, Janssen, and Genentech. His associates reported ties to numerous industry sources.
Of the 94,474 ischemic strokes that occurred in a nationwide registry among patients with known atrial fibrillation, 84% were preceded by inadequate anticoagulation, according to a report published online March 14 in JAMA.
Fully 30% of the patients in this retrospective cohort study weren’t taking any form of antithrombotic therapy before their stroke, even though all of them had atrial fibrillation (AF) and most of them had additional risk factors as well. And although nearly 20,000 of the study participants were taking warfarin before their stroke, 64% of them were taking subtherapeutic doses, said Ying Xian, MD, PhD, of the department of neurology at the Duke Clinical Research Institute, Durham, N.C., and his associates.
These findings highlight tens of thousands of missed opportunities for preventing stroke simply by following existing guidelines, the investigators said.
In previous studies, researchers have consistently found underuse of oral anticoagulants in community practice. To examine current trends since the rapid adoption of non–vitamin-K antagonist oral anticoagulants (NOACs), Dr. Xian and his associates analyzed data from the AHA/ASA Get With the Guidelines-Stroke Registry. They determined the prevalence of antithrombotic treatment among AF patients who developed ischemic stroke, focusing on patients (mean age, 79.9 years) enrolled in the registry after being admitted for ischemic stroke to 1,622 participating U.S. hospitals during a 2.5-year period.
A total of 79,008 (83.6%) were not receiving therapeutic anticoagulation at the time of their stroke. This included 28,583 who were not taking any antithrombotic treatment, 12,751 who were taking subtherapeutic doses of warfarin (international normalized ratio,or INR, of less than 2 at the time of their stroke), and 37,674 who were taking only antiplatelet drugs when additional treatment was indicated, the investigators said (JAMA. 2017 Mar 14;317[10]:1057-67).
Among the minority of AF patients who were receiving therapeutic anticoagulation at the time of their stroke, 7,176 were taking adequate warfarin and 8,290 were taking adequate NOACs such as dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis).
Stroke severity, as measured by median scores on the National Institutes of Health Stroke Scale, was significantly greater among patients receiving no antithrombotic medication, antiplatelet agents alone, or subtherapeutic levels of warfarin, compared with patients receiving therapeutic levels of warfarin or NOACs. Similarly, patients who had adequate anticoagulation before their stroke were significantly more likely to have better functional outcomes than those who did not. “These findings reinforce the importance of INR monitoring and dose adjustment to ... keep the INR in the therapeutic range,” Dr. Xian and his associates wrote.
The reasons why patients weren’t receiving adequate anticoagulation before their stroke – including any direct contraindications to treatment – were not available from the registry data. But such reasons are likely to persist after a stroke, and study clinicians were required to document such reasons in the medical records at hospital discharge. So the investigators examined reasons listed for not prescribing oral anticoagulation at hospital discharge in 58,084 patients.
A striking 38,249 AF patients (65.8%) in this large subgroup of study participants had no documented reason for not receiving oral anticoagulation, even after they were hospitalized for ischemic stroke and even though such documentation was “required.” Among the minority of patients whose records did show such reasons, the most common reason cited was bleeding risk (16.3%), followed by risk of falling (10.3%), the presence of a terminal illness (6.2%), patient or family refusal of the medication (4.3%), the patient’s impaired mental status (1.1%), adverse effects of the medication (1.0%), and allergy to the medication (0.6%).
This study was supported by the Patient-Centered Outcomes Research Institute. Dr. Xian reported that his institution received research funding from the American Heart Association, Daiichi Sankyo, Janssen, and Genentech. His associates reported ties to numerous industry sources.
Of the 94,474 ischemic strokes that occurred in a nationwide registry among patients with known atrial fibrillation, 84% were preceded by inadequate anticoagulation, according to a report published online March 14 in JAMA.
Fully 30% of the patients in this retrospective cohort study weren’t taking any form of antithrombotic therapy before their stroke, even though all of them had atrial fibrillation (AF) and most of them had additional risk factors as well. And although nearly 20,000 of the study participants were taking warfarin before their stroke, 64% of them were taking subtherapeutic doses, said Ying Xian, MD, PhD, of the department of neurology at the Duke Clinical Research Institute, Durham, N.C., and his associates.
These findings highlight tens of thousands of missed opportunities for preventing stroke simply by following existing guidelines, the investigators said.
In previous studies, researchers have consistently found underuse of oral anticoagulants in community practice. To examine current trends since the rapid adoption of non–vitamin-K antagonist oral anticoagulants (NOACs), Dr. Xian and his associates analyzed data from the AHA/ASA Get With the Guidelines-Stroke Registry. They determined the prevalence of antithrombotic treatment among AF patients who developed ischemic stroke, focusing on patients (mean age, 79.9 years) enrolled in the registry after being admitted for ischemic stroke to 1,622 participating U.S. hospitals during a 2.5-year period.
A total of 79,008 (83.6%) were not receiving therapeutic anticoagulation at the time of their stroke. This included 28,583 who were not taking any antithrombotic treatment, 12,751 who were taking subtherapeutic doses of warfarin (international normalized ratio,or INR, of less than 2 at the time of their stroke), and 37,674 who were taking only antiplatelet drugs when additional treatment was indicated, the investigators said (JAMA. 2017 Mar 14;317[10]:1057-67).
Among the minority of AF patients who were receiving therapeutic anticoagulation at the time of their stroke, 7,176 were taking adequate warfarin and 8,290 were taking adequate NOACs such as dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis).
Stroke severity, as measured by median scores on the National Institutes of Health Stroke Scale, was significantly greater among patients receiving no antithrombotic medication, antiplatelet agents alone, or subtherapeutic levels of warfarin, compared with patients receiving therapeutic levels of warfarin or NOACs. Similarly, patients who had adequate anticoagulation before their stroke were significantly more likely to have better functional outcomes than those who did not. “These findings reinforce the importance of INR monitoring and dose adjustment to ... keep the INR in the therapeutic range,” Dr. Xian and his associates wrote.
The reasons why patients weren’t receiving adequate anticoagulation before their stroke – including any direct contraindications to treatment – were not available from the registry data. But such reasons are likely to persist after a stroke, and study clinicians were required to document such reasons in the medical records at hospital discharge. So the investigators examined reasons listed for not prescribing oral anticoagulation at hospital discharge in 58,084 patients.
A striking 38,249 AF patients (65.8%) in this large subgroup of study participants had no documented reason for not receiving oral anticoagulation, even after they were hospitalized for ischemic stroke and even though such documentation was “required.” Among the minority of patients whose records did show such reasons, the most common reason cited was bleeding risk (16.3%), followed by risk of falling (10.3%), the presence of a terminal illness (6.2%), patient or family refusal of the medication (4.3%), the patient’s impaired mental status (1.1%), adverse effects of the medication (1.0%), and allergy to the medication (0.6%).
This study was supported by the Patient-Centered Outcomes Research Institute. Dr. Xian reported that his institution received research funding from the American Heart Association, Daiichi Sankyo, Janssen, and Genentech. His associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: 84% of ischemic strokes that occurred in patients with known atrial fibrillation were preceded by inadequate anticoagulation.
Major finding: A total of 30% of AF patients were not taking any antithrombotic treatment before their ischemic stroke, 14% were taking subtherapeutic doses of warfarin, and 40% were taking only antiplatelet drugs when additional treatment was indicated.
Data source: A retrospective observational cohort study involving 94,474 patients with AF enrolled in a nationwide stroke outcomes registry.
Disclosures: This study was supported by the Patient-Centered Outcomes Research Institute. Dr. Xian reported that his institution received research funding from the American Heart Association, Daiichi Sankyo, Janssen, and Genentech. His associates reported ties to numerous industry sources.