User login
‘Inadequate’ anticoagulation found in AFib patients with stroke
A large study has revealed an association between stroke and “inadequate” anticoagulation among patients with atrial fibrillation.
More than 80% of the patients studied did not receive guideline-recommended anticoagulant therapy prior to having a stroke.
The study also showed that when patients did receive recommended anticoagulants, they had less severe stroke outcomes and a lower risk of death.
These findings were published in JAMA.
“Atrial fibrillation is very common, and people with the condition are at a much higher risk of having stroke,” said study author Ying Xian, MD, PhD, of the Duke University Medical Center in Durham, North Carolina.
“Treatment guidelines call for these patients to receive an anticoagulant such as warfarin at a therapeutic dose or a non-vitamin K antagonist oral anticoagulant (NOAC), so it’s surprising that this is not occurring in the vast majority of cases that occur in community settings.”
The study included 94,474 patients who had an acute ischemic stroke and known history of atrial fibrillation.
The patients were admitted from October 2012 through March 2015 to 1622 hospitals participating in the “Get with the Guidelines-Stroke” program, a national stroke registry sponsored by the American Heart Association and American Stroke Association.
In analyzing data from these patients, the researchers found the following:
- 83.6% of patients were not receiving therapeutic anticoagulation prior to stroke
- 30.3% were not receiving any antithrombotic treatment
- 39.9% were receiving antiplatelet therapy only
- 13.5% were receiving sub-therapeutic warfarin
- 7.6% were receiving therapeutic warfarin
- 8.8% were receiving NOACs.
“While some of these patients may have had reasons for not being anticoagulated, such as high bleeding or fall risk, more than two-thirds had no documented reason for receiving inadequate stroke prevention therapy,” Dr Xian said.
Dr Xian added that, in those cases where anticoagulation failed to prevent a stroke, patients who were on anticoagulant therapy tended to have less severe strokes, with less disability and death.
The researchers said the unadjusted rates of moderate or severe stroke were lower among patients receiving therapeutic warfarin (15.8%) or NOACs (17.5%) than among patients receiving no antithrombotic therapy (27.1%), antiplatelet therapy alone (24.8%), or sub-therapeutic warfarin (25.8%).
The same was true for the unadjusted rates of in-hospital mortality. Patients receiving therapeutic warfarin (6.4%) or NOACs (6.3%) had lower rates than patients receiving no antithrombotic therapy (9.3%), antiplatelet therapy alone (8.1%), or sub-therapeutic warfarin (8.8%).
In an adjusted analysis, the use of therapeutic warfarin, NOACs, or antiplatelet therapy was associated with lower odds of moderate or severe stroke (adjusted odds ratio=0.56, 0.65, and 0.88, respectively) and in-hospital mortality (adjusted odds ratio=0.75, 0.79, and 0.83, respectively), when compared to no antithrombotic treatment.
“These findings highlight the human costs of atrial fibrillation and the importance of appropriate anticoagulation,” Dr Xian said. “Broader adherence to these atrial fibrillation treatment guidelines could substantially reduce both the number and severity of strokes in the US. We estimate that between 58,000 to 88,000 strokes might be preventable per year if the treatment guidelines are followed appropriately.” ![]()
A large study has revealed an association between stroke and “inadequate” anticoagulation among patients with atrial fibrillation.
More than 80% of the patients studied did not receive guideline-recommended anticoagulant therapy prior to having a stroke.
The study also showed that when patients did receive recommended anticoagulants, they had less severe stroke outcomes and a lower risk of death.
These findings were published in JAMA.
“Atrial fibrillation is very common, and people with the condition are at a much higher risk of having stroke,” said study author Ying Xian, MD, PhD, of the Duke University Medical Center in Durham, North Carolina.
“Treatment guidelines call for these patients to receive an anticoagulant such as warfarin at a therapeutic dose or a non-vitamin K antagonist oral anticoagulant (NOAC), so it’s surprising that this is not occurring in the vast majority of cases that occur in community settings.”
The study included 94,474 patients who had an acute ischemic stroke and known history of atrial fibrillation.
The patients were admitted from October 2012 through March 2015 to 1622 hospitals participating in the “Get with the Guidelines-Stroke” program, a national stroke registry sponsored by the American Heart Association and American Stroke Association.
In analyzing data from these patients, the researchers found the following:
- 83.6% of patients were not receiving therapeutic anticoagulation prior to stroke
- 30.3% were not receiving any antithrombotic treatment
- 39.9% were receiving antiplatelet therapy only
- 13.5% were receiving sub-therapeutic warfarin
- 7.6% were receiving therapeutic warfarin
- 8.8% were receiving NOACs.
“While some of these patients may have had reasons for not being anticoagulated, such as high bleeding or fall risk, more than two-thirds had no documented reason for receiving inadequate stroke prevention therapy,” Dr Xian said.
Dr Xian added that, in those cases where anticoagulation failed to prevent a stroke, patients who were on anticoagulant therapy tended to have less severe strokes, with less disability and death.
The researchers said the unadjusted rates of moderate or severe stroke were lower among patients receiving therapeutic warfarin (15.8%) or NOACs (17.5%) than among patients receiving no antithrombotic therapy (27.1%), antiplatelet therapy alone (24.8%), or sub-therapeutic warfarin (25.8%).
The same was true for the unadjusted rates of in-hospital mortality. Patients receiving therapeutic warfarin (6.4%) or NOACs (6.3%) had lower rates than patients receiving no antithrombotic therapy (9.3%), antiplatelet therapy alone (8.1%), or sub-therapeutic warfarin (8.8%).
In an adjusted analysis, the use of therapeutic warfarin, NOACs, or antiplatelet therapy was associated with lower odds of moderate or severe stroke (adjusted odds ratio=0.56, 0.65, and 0.88, respectively) and in-hospital mortality (adjusted odds ratio=0.75, 0.79, and 0.83, respectively), when compared to no antithrombotic treatment.
“These findings highlight the human costs of atrial fibrillation and the importance of appropriate anticoagulation,” Dr Xian said. “Broader adherence to these atrial fibrillation treatment guidelines could substantially reduce both the number and severity of strokes in the US. We estimate that between 58,000 to 88,000 strokes might be preventable per year if the treatment guidelines are followed appropriately.” ![]()
A large study has revealed an association between stroke and “inadequate” anticoagulation among patients with atrial fibrillation.
More than 80% of the patients studied did not receive guideline-recommended anticoagulant therapy prior to having a stroke.
The study also showed that when patients did receive recommended anticoagulants, they had less severe stroke outcomes and a lower risk of death.
These findings were published in JAMA.
“Atrial fibrillation is very common, and people with the condition are at a much higher risk of having stroke,” said study author Ying Xian, MD, PhD, of the Duke University Medical Center in Durham, North Carolina.
“Treatment guidelines call for these patients to receive an anticoagulant such as warfarin at a therapeutic dose or a non-vitamin K antagonist oral anticoagulant (NOAC), so it’s surprising that this is not occurring in the vast majority of cases that occur in community settings.”
The study included 94,474 patients who had an acute ischemic stroke and known history of atrial fibrillation.
The patients were admitted from October 2012 through March 2015 to 1622 hospitals participating in the “Get with the Guidelines-Stroke” program, a national stroke registry sponsored by the American Heart Association and American Stroke Association.
In analyzing data from these patients, the researchers found the following:
- 83.6% of patients were not receiving therapeutic anticoagulation prior to stroke
- 30.3% were not receiving any antithrombotic treatment
- 39.9% were receiving antiplatelet therapy only
- 13.5% were receiving sub-therapeutic warfarin
- 7.6% were receiving therapeutic warfarin
- 8.8% were receiving NOACs.
“While some of these patients may have had reasons for not being anticoagulated, such as high bleeding or fall risk, more than two-thirds had no documented reason for receiving inadequate stroke prevention therapy,” Dr Xian said.
Dr Xian added that, in those cases where anticoagulation failed to prevent a stroke, patients who were on anticoagulant therapy tended to have less severe strokes, with less disability and death.
The researchers said the unadjusted rates of moderate or severe stroke were lower among patients receiving therapeutic warfarin (15.8%) or NOACs (17.5%) than among patients receiving no antithrombotic therapy (27.1%), antiplatelet therapy alone (24.8%), or sub-therapeutic warfarin (25.8%).
The same was true for the unadjusted rates of in-hospital mortality. Patients receiving therapeutic warfarin (6.4%) or NOACs (6.3%) had lower rates than patients receiving no antithrombotic therapy (9.3%), antiplatelet therapy alone (8.1%), or sub-therapeutic warfarin (8.8%).
In an adjusted analysis, the use of therapeutic warfarin, NOACs, or antiplatelet therapy was associated with lower odds of moderate or severe stroke (adjusted odds ratio=0.56, 0.65, and 0.88, respectively) and in-hospital mortality (adjusted odds ratio=0.75, 0.79, and 0.83, respectively), when compared to no antithrombotic treatment.
“These findings highlight the human costs of atrial fibrillation and the importance of appropriate anticoagulation,” Dr Xian said. “Broader adherence to these atrial fibrillation treatment guidelines could substantially reduce both the number and severity of strokes in the US. We estimate that between 58,000 to 88,000 strokes might be preventable per year if the treatment guidelines are followed appropriately.” ![]()
FDA approves pembrolizumab to treat cHL
The US Food and Drug Administration (FDA) has granted accelerated approval for pembrolizumab (Keytruda) as a treatment for adult and pediatric patients with relapsed or refractory classical Hodgkin lymphoma (cHL).
Pembrolizumab is a monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the antitumor immune response.
The drug, which is being developed by Merck, previously received FDA approval as a treatment for melanoma, lung cancer, and head and neck cancer.
Now, pembrolizumab has received accelerated approval to treat adult and pediatric patients with refractory cHL or those with cHL who have relapsed after 3 or more prior lines of therapy.
The accelerated approval was based on tumor response rate and durability of response. Continued approval of pembrolizumab for cHL patients may be contingent upon the verification and description of clinical benefit in confirmatory trials.
In adults with cHL, pembrolizumab is administered at a fixed dose of 200 mg every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
In pediatric patients with cHL, pembrolizumab is administered at a dose of 2 mg/kg (up to a maximum of 200 mg) every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
Pembrolizumab trials
The FDA’s approval of pembrolizumab in adults with cHL is based on data from the phase 2 KEYNOTE-087 trial. (The following data were provided by Merck.)
The trial enrolled 210 patients who received pembrolizumab at a dose of 200 mg every 3 weeks until unacceptable toxicity or documented disease progression, or for up to 24 months in patients who did not progress.
Fifty-eight percent of patients were refractory to their last prior therapy, including 35% with primary refractory disease and 14% whose disease was refractory to all prior regimens.
Sixty-one percent of patients had undergone prior autologous hematopoietic stem cell transplant, 83% had prior brentuximab use, and 36% had prior radiation therapy.
At a median follow-up of 9.4 months, the overall response rate was 69%, and the complete response rate was 22%. The median duration of response was 11.1 months (range, 0.0+ to 11.1 months).
Five percent of patients discontinued pembrolizumab due to adverse events (AEs), and 26% had dose interruptions due to AEs. Fifteen percent of patients had an AE requiring systemic corticosteroid therapy.
The most common AEs (occurring in ≥20% of patients) were fatigue (26%), pyrexia (24%), cough (24%), musculoskeletal pain (21%), diarrhea (20%), and rash (20%).
Serious AEs occurred in 16% of patients. The most frequent serious AEs (≥1%) were pneumonia, pneumonitis, pyrexia, dyspnea, graft-vs-host disease, and herpes zoster.
Two patients died from causes other than disease progression. One death was a result of graft-vs-host disease after subsequent allogeneic transplant, and the other was from septic shock.
There is limited experience with pembrolizumab in pediatric patients. The efficacy of the drug for pediatric patients was extrapolated from the results in the adult cHL population.
However, there is safety data on pembrolizumab in pediatric patients enrolled in the phase 1/2 KEYNOTE-051 trial. (These data were also provided by Merck.)
The trial included 40 pediatric patients with advanced melanoma or PD-L1–positive advanced, relapsed, or refractory solid tumors or lymphoma. Patients in this trial received pembrolizumab for a median of 43 days (range, 1-414 days).
The safety profile in these patients was similar to the profile in adults. Toxicities that occurred at a higher rate (≥15% difference) in pediatric patients than in adults under age 65 were fatigue (45%), vomiting (38%), abdominal pain (28%), hypertransaminasemia (28%), and hyponatremia (18%). ![]()
The US Food and Drug Administration (FDA) has granted accelerated approval for pembrolizumab (Keytruda) as a treatment for adult and pediatric patients with relapsed or refractory classical Hodgkin lymphoma (cHL).
Pembrolizumab is a monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the antitumor immune response.
The drug, which is being developed by Merck, previously received FDA approval as a treatment for melanoma, lung cancer, and head and neck cancer.
Now, pembrolizumab has received accelerated approval to treat adult and pediatric patients with refractory cHL or those with cHL who have relapsed after 3 or more prior lines of therapy.
The accelerated approval was based on tumor response rate and durability of response. Continued approval of pembrolizumab for cHL patients may be contingent upon the verification and description of clinical benefit in confirmatory trials.
In adults with cHL, pembrolizumab is administered at a fixed dose of 200 mg every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
In pediatric patients with cHL, pembrolizumab is administered at a dose of 2 mg/kg (up to a maximum of 200 mg) every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
Pembrolizumab trials
The FDA’s approval of pembrolizumab in adults with cHL is based on data from the phase 2 KEYNOTE-087 trial. (The following data were provided by Merck.)
The trial enrolled 210 patients who received pembrolizumab at a dose of 200 mg every 3 weeks until unacceptable toxicity or documented disease progression, or for up to 24 months in patients who did not progress.
Fifty-eight percent of patients were refractory to their last prior therapy, including 35% with primary refractory disease and 14% whose disease was refractory to all prior regimens.
Sixty-one percent of patients had undergone prior autologous hematopoietic stem cell transplant, 83% had prior brentuximab use, and 36% had prior radiation therapy.
At a median follow-up of 9.4 months, the overall response rate was 69%, and the complete response rate was 22%. The median duration of response was 11.1 months (range, 0.0+ to 11.1 months).
Five percent of patients discontinued pembrolizumab due to adverse events (AEs), and 26% had dose interruptions due to AEs. Fifteen percent of patients had an AE requiring systemic corticosteroid therapy.
The most common AEs (occurring in ≥20% of patients) were fatigue (26%), pyrexia (24%), cough (24%), musculoskeletal pain (21%), diarrhea (20%), and rash (20%).
Serious AEs occurred in 16% of patients. The most frequent serious AEs (≥1%) were pneumonia, pneumonitis, pyrexia, dyspnea, graft-vs-host disease, and herpes zoster.
Two patients died from causes other than disease progression. One death was a result of graft-vs-host disease after subsequent allogeneic transplant, and the other was from septic shock.
There is limited experience with pembrolizumab in pediatric patients. The efficacy of the drug for pediatric patients was extrapolated from the results in the adult cHL population.
However, there is safety data on pembrolizumab in pediatric patients enrolled in the phase 1/2 KEYNOTE-051 trial. (These data were also provided by Merck.)
The trial included 40 pediatric patients with advanced melanoma or PD-L1–positive advanced, relapsed, or refractory solid tumors or lymphoma. Patients in this trial received pembrolizumab for a median of 43 days (range, 1-414 days).
The safety profile in these patients was similar to the profile in adults. Toxicities that occurred at a higher rate (≥15% difference) in pediatric patients than in adults under age 65 were fatigue (45%), vomiting (38%), abdominal pain (28%), hypertransaminasemia (28%), and hyponatremia (18%). ![]()
The US Food and Drug Administration (FDA) has granted accelerated approval for pembrolizumab (Keytruda) as a treatment for adult and pediatric patients with relapsed or refractory classical Hodgkin lymphoma (cHL).
Pembrolizumab is a monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the antitumor immune response.
The drug, which is being developed by Merck, previously received FDA approval as a treatment for melanoma, lung cancer, and head and neck cancer.
Now, pembrolizumab has received accelerated approval to treat adult and pediatric patients with refractory cHL or those with cHL who have relapsed after 3 or more prior lines of therapy.
The accelerated approval was based on tumor response rate and durability of response. Continued approval of pembrolizumab for cHL patients may be contingent upon the verification and description of clinical benefit in confirmatory trials.
In adults with cHL, pembrolizumab is administered at a fixed dose of 200 mg every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
In pediatric patients with cHL, pembrolizumab is administered at a dose of 2 mg/kg (up to a maximum of 200 mg) every 3 weeks until disease progression or unacceptable toxicity, or up to 24 months in patients without disease progression.
Pembrolizumab trials
The FDA’s approval of pembrolizumab in adults with cHL is based on data from the phase 2 KEYNOTE-087 trial. (The following data were provided by Merck.)
The trial enrolled 210 patients who received pembrolizumab at a dose of 200 mg every 3 weeks until unacceptable toxicity or documented disease progression, or for up to 24 months in patients who did not progress.
Fifty-eight percent of patients were refractory to their last prior therapy, including 35% with primary refractory disease and 14% whose disease was refractory to all prior regimens.
Sixty-one percent of patients had undergone prior autologous hematopoietic stem cell transplant, 83% had prior brentuximab use, and 36% had prior radiation therapy.
At a median follow-up of 9.4 months, the overall response rate was 69%, and the complete response rate was 22%. The median duration of response was 11.1 months (range, 0.0+ to 11.1 months).
Five percent of patients discontinued pembrolizumab due to adverse events (AEs), and 26% had dose interruptions due to AEs. Fifteen percent of patients had an AE requiring systemic corticosteroid therapy.
The most common AEs (occurring in ≥20% of patients) were fatigue (26%), pyrexia (24%), cough (24%), musculoskeletal pain (21%), diarrhea (20%), and rash (20%).
Serious AEs occurred in 16% of patients. The most frequent serious AEs (≥1%) were pneumonia, pneumonitis, pyrexia, dyspnea, graft-vs-host disease, and herpes zoster.
Two patients died from causes other than disease progression. One death was a result of graft-vs-host disease after subsequent allogeneic transplant, and the other was from septic shock.
There is limited experience with pembrolizumab in pediatric patients. The efficacy of the drug for pediatric patients was extrapolated from the results in the adult cHL population.
However, there is safety data on pembrolizumab in pediatric patients enrolled in the phase 1/2 KEYNOTE-051 trial. (These data were also provided by Merck.)
The trial included 40 pediatric patients with advanced melanoma or PD-L1–positive advanced, relapsed, or refractory solid tumors or lymphoma. Patients in this trial received pembrolizumab for a median of 43 days (range, 1-414 days).
The safety profile in these patients was similar to the profile in adults. Toxicities that occurred at a higher rate (≥15% difference) in pediatric patients than in adults under age 65 were fatigue (45%), vomiting (38%), abdominal pain (28%), hypertransaminasemia (28%), and hyponatremia (18%). ![]()
HDAC6 inhibitors reverse CIPN in rodents
Histone deacetylase (HDAC) inhibitors can treat chemotherapy-induced peripheral neuropathy (CIPN) in mice and rats, according to research published in PAIN.
The animals developed CIPN after treatment with cisplatin or paclitaxel.
But treatment with an HDAC6 inhibitor—ACY-1083 or ricolinostat (ACY-1215)—was able to reverse and sometimes prevent the symptoms of CIPN in the animals.
The researchers said these findings are “especially promising” because one of the HDAC6 inhibitors, ricolinostat, is already being tested in clinical trials.
This research was funded, in part, by Acetylon Pharmaceuticals Inc., and employees of the company were involved in the research.
The HDAC6 inhibitors are being developed by Regenacy Pharmaceuticals, LLC, a spinout of Acetylon Pharmaceuticals.
The researchers found that ACY-1083 was able to reverse cisplatin-induced mechanical allodynia (pain in response to touch) in mice.
ACY-1083 also prevented mechanical allodynia when given an hour prior to treatment with cisplatin.
The team noted that ricolinostat, which is less selective for HDAC6 than ACY-1083, also reversed cisplatin-induced mechanical allodynia.
The beneficial effect of ricolinostat was still seen a week after treatment, which was the last time point tested.
The researchers also found that ACY-1083 was able to reverse paclitaxel-induced mechanical allodynia in rats.
And ACY-1083 could reverse cisplatin-induced spontaneous pain and numbness in mice.
“These data demonstrate that HDAC6 is a novel therapeutic target for the treatment of chemotherapy-induced neuropathies, for which there are currently no FDA-approved drugs,” said Matthew B. Jarpe, PhD, associate vice-president of biology at Regenacy Pharmaceuticals.
Dr Jarpe said this research suggests an HDAC6 inhibitor could potentially be used in conjunction with platinum- or taxane-based chemotherapy to allow for higher and/or longer chemotherapy dosing regimens.
“Additionally, the reversal of both pain and numbness in this preclinical model suggests that an HDAC6 inhibitor could be used after a course of chemotherapy is completed to treat and potentially reverse resultant debilitating CIPN symptoms that impact function and quality of life for many patients,” he said.
“Preclinical results seen with the clinical candidate ricolinostat highlight the translational potential of these findings to the clinic.” ![]()
Histone deacetylase (HDAC) inhibitors can treat chemotherapy-induced peripheral neuropathy (CIPN) in mice and rats, according to research published in PAIN.
The animals developed CIPN after treatment with cisplatin or paclitaxel.
But treatment with an HDAC6 inhibitor—ACY-1083 or ricolinostat (ACY-1215)—was able to reverse and sometimes prevent the symptoms of CIPN in the animals.
The researchers said these findings are “especially promising” because one of the HDAC6 inhibitors, ricolinostat, is already being tested in clinical trials.
This research was funded, in part, by Acetylon Pharmaceuticals Inc., and employees of the company were involved in the research.
The HDAC6 inhibitors are being developed by Regenacy Pharmaceuticals, LLC, a spinout of Acetylon Pharmaceuticals.
The researchers found that ACY-1083 was able to reverse cisplatin-induced mechanical allodynia (pain in response to touch) in mice.
ACY-1083 also prevented mechanical allodynia when given an hour prior to treatment with cisplatin.
The team noted that ricolinostat, which is less selective for HDAC6 than ACY-1083, also reversed cisplatin-induced mechanical allodynia.
The beneficial effect of ricolinostat was still seen a week after treatment, which was the last time point tested.
The researchers also found that ACY-1083 was able to reverse paclitaxel-induced mechanical allodynia in rats.
And ACY-1083 could reverse cisplatin-induced spontaneous pain and numbness in mice.
“These data demonstrate that HDAC6 is a novel therapeutic target for the treatment of chemotherapy-induced neuropathies, for which there are currently no FDA-approved drugs,” said Matthew B. Jarpe, PhD, associate vice-president of biology at Regenacy Pharmaceuticals.
Dr Jarpe said this research suggests an HDAC6 inhibitor could potentially be used in conjunction with platinum- or taxane-based chemotherapy to allow for higher and/or longer chemotherapy dosing regimens.
“Additionally, the reversal of both pain and numbness in this preclinical model suggests that an HDAC6 inhibitor could be used after a course of chemotherapy is completed to treat and potentially reverse resultant debilitating CIPN symptoms that impact function and quality of life for many patients,” he said.
“Preclinical results seen with the clinical candidate ricolinostat highlight the translational potential of these findings to the clinic.” ![]()
Histone deacetylase (HDAC) inhibitors can treat chemotherapy-induced peripheral neuropathy (CIPN) in mice and rats, according to research published in PAIN.
The animals developed CIPN after treatment with cisplatin or paclitaxel.
But treatment with an HDAC6 inhibitor—ACY-1083 or ricolinostat (ACY-1215)—was able to reverse and sometimes prevent the symptoms of CIPN in the animals.
The researchers said these findings are “especially promising” because one of the HDAC6 inhibitors, ricolinostat, is already being tested in clinical trials.
This research was funded, in part, by Acetylon Pharmaceuticals Inc., and employees of the company were involved in the research.
The HDAC6 inhibitors are being developed by Regenacy Pharmaceuticals, LLC, a spinout of Acetylon Pharmaceuticals.
The researchers found that ACY-1083 was able to reverse cisplatin-induced mechanical allodynia (pain in response to touch) in mice.
ACY-1083 also prevented mechanical allodynia when given an hour prior to treatment with cisplatin.
The team noted that ricolinostat, which is less selective for HDAC6 than ACY-1083, also reversed cisplatin-induced mechanical allodynia.
The beneficial effect of ricolinostat was still seen a week after treatment, which was the last time point tested.
The researchers also found that ACY-1083 was able to reverse paclitaxel-induced mechanical allodynia in rats.
And ACY-1083 could reverse cisplatin-induced spontaneous pain and numbness in mice.
“These data demonstrate that HDAC6 is a novel therapeutic target for the treatment of chemotherapy-induced neuropathies, for which there are currently no FDA-approved drugs,” said Matthew B. Jarpe, PhD, associate vice-president of biology at Regenacy Pharmaceuticals.
Dr Jarpe said this research suggests an HDAC6 inhibitor could potentially be used in conjunction with platinum- or taxane-based chemotherapy to allow for higher and/or longer chemotherapy dosing regimens.
“Additionally, the reversal of both pain and numbness in this preclinical model suggests that an HDAC6 inhibitor could be used after a course of chemotherapy is completed to treat and potentially reverse resultant debilitating CIPN symptoms that impact function and quality of life for many patients,” he said.
“Preclinical results seen with the clinical candidate ricolinostat highlight the translational potential of these findings to the clinic.” ![]()
Steroids During Late Preterm Labor? Better Later Than Never

A 21-year-old G1P0 at 35 weeks, 2 days of gestation presents to labor and delivery reporting a “gush of clear fluid.” On exam, you confirm she has preterm rupture of membranes. She is contracting every three minutes and has a cervix dilated to 3 cm. Is there any neonatal benefit to using corticosteroids in this late preterm period?
Approximately 12% of all births in the United States are the result of preterm labor, and 8% take place in the late preterm period, defined as 34 to 36 weeks’ gestation.2,3 To reduce risk for neonatal death and respiratory complications, both the American College of Obstetricians and Gynecologists and the National Institutes of Health recommend a course of corticosteroids between 24 and 34 weeks’ gestation for women at increased risk for preterm delivery.2,4 Due to a lack of evidence from RCTs on the benefit of corticosteroids in late preterm labor, there are no recommendations to extend this period.5 However, multiple studies have shown that babies born during the late preterm period have more neonatal complications than term newborns.6-8
A retrospective chart review of more than 130,000 live births found that newborns who were delivered between 34 and 36 weeks had higher rates of respiratory distress than those delivered at 39 weeks (ventilator use dropped from 3.3% at 34 weeks to 0.3% at 39 weeks, and transient tachypnea decreased from 2.4% at 34 weeks to 0.4% at 39 weeks).6 Another retrospective review of more than 230,000 newborns, 19,000 of whom were born in the late preterm period, revealed that more neonates born between 34 and 36 weeks’ gestation had respiratory distress syndrome than neonates delivered at 39 weeks (10.5% at 34 weeks, 6% at 35 weeks, 2.8% at 36 weeks vs 0.3% at 39 weeks).8
STUDY SUMMARY
Late preterm newborns breathe better with antenatal betamethasone
This RCT examined the effectiveness of betamethasone in preventing neonatal respiratory complications for 2,831 women at high probability of preterm delivery between 34 weeks and 36 weeks, 6 days of gestation. “High probability of preterm delivery” was defined as preterm labor with intact membranes and at least 3 cm dilation or 75% cervical effacement; spontaneous rupture of membranes; or anticipated preterm delivery for any other indication either through induction or cesarean section between 24 hours and seven days after the planned randomization.
Patients were randomly assigned to receive two intramuscular injections (12 mg each) of either betamethasone or placebo, 24 hours apart. The two doses were successfully given in 60% of the betamethasone group and 59% of the placebo group. In 95% of the cases in which the second dose was not given, it was because delivery occurred within 24 hours of the first dose.
The primary outcome was the need for respiratory support within 72 hours of birth, defined as one or more of the following: the use of continuous positive airway pressure (CPAP) or high-flow nasal cannula for at least two consecutive hours, supplemental oxygen for at least four continuous hours, extracorporeal membrane oxygenation (ECMO), or mechanical ventilation.
The median length of time from enrollment to delivery was 31 to 33 hours, and 31.4% underwent cesarean delivery. In the intention-to-treat analysis, the primary outcome was significantly lower in the betamethasone group than in the placebo group (11.6% vs 14.4%; relative risk [RR], 0.80; number needed to treat [NNT], 35). Secondary outcomes (severe complications, representing a composite of the use of CPAP or high-flow nasal cannula for at least 12 continuous hours, supplemental oxygen for at least 24 continuous hours, ECMO, mechanical ventilation, stillbirth, or neonatal death within 72 hours after delivery) were also lower in the betamethasone group (8.1% vs 12.1%; RR, 0.67; NNT, 25). The betamethasone group also had a lower risk for transient tachypnea of the newborn (6.7% vs 9.9%; RR, 0.68).
There were no significant differences in the occurrence of maternal chorioamnionitis or endometritis between the groups. Hypoglycemia in the newborn occurred more in the betamethasone group (24% vs 15%; RR, 1.6; number needed to harm [NNH], 11). The betamethasone group had two neonatal deaths: one from septic shock, and the other from a structural cardiac anomaly and arrhythmia.
WHAT’S NEW
Betamethasone effective even in the late, late preterm period
This study demonstrated an improvement in neonatal respiratory outcomes when betamethasone versus placebo was used in the late preterm period. The findings were similar to those from the Antenatal Steroids for Term Elective Caesarean Section Research Team.9 Their trial showed a reduction in respiratory complications in term neonates delivered via elective cesarean section to mothers who received antenatal betamethasone (NNT, 37, to prevent admission to a special care nursery with respiratory distress). The findings were also consistent with those of a recent meta-analysis evaluating the occurrence of respiratory complications with the use of antenatal betamethasone in women expected to deliver in the late preterm period or with a planned cesarean delivery at ≥ 37 weeks’ gestation.10
CAVEATS
Neonates may develop hypoglycemia
The authors of the study reported an increased risk for hypoglycemia in the neonates receiving antenatal betamethasone. The long-term implications of this are unclear, however, given that there was a reduction in intermediate care nursery and neonatal ICU stays that were three days or longer in the betamethasone group. There was also no difference in hospital length of stay between the two groups. Additionally, it’s unclear if there are any long-term neonatal complications of betamethasone use in the late preterm period.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible
There are minimal challenges to implementing this strategy, as betamethasone is routinely used for preterm labor and is readily available on labor and delivery units.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2017;66(2):104-106.
1. Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
2. Practice Bulletin No. 159 Summary: Management of Preterm Labor. Obstet Gynecol. 2016;127:190-191.
3. Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
4. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12:1-24.
5. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13-B15.
6. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35-41.
7. Yoder BA, Gordon MC, Barth WH Jr. Late-preterm birth: does the changing obstetric paradigm alter the epidemiology of respiratory complications? Obstet Gynecol. 2008;111:814-822.
8. Consortium on Safe Labor, Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419-425.
9. Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331:662.
10. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.

A 21-year-old G1P0 at 35 weeks, 2 days of gestation presents to labor and delivery reporting a “gush of clear fluid.” On exam, you confirm she has preterm rupture of membranes. She is contracting every three minutes and has a cervix dilated to 3 cm. Is there any neonatal benefit to using corticosteroids in this late preterm period?
Approximately 12% of all births in the United States are the result of preterm labor, and 8% take place in the late preterm period, defined as 34 to 36 weeks’ gestation.2,3 To reduce risk for neonatal death and respiratory complications, both the American College of Obstetricians and Gynecologists and the National Institutes of Health recommend a course of corticosteroids between 24 and 34 weeks’ gestation for women at increased risk for preterm delivery.2,4 Due to a lack of evidence from RCTs on the benefit of corticosteroids in late preterm labor, there are no recommendations to extend this period.5 However, multiple studies have shown that babies born during the late preterm period have more neonatal complications than term newborns.6-8
A retrospective chart review of more than 130,000 live births found that newborns who were delivered between 34 and 36 weeks had higher rates of respiratory distress than those delivered at 39 weeks (ventilator use dropped from 3.3% at 34 weeks to 0.3% at 39 weeks, and transient tachypnea decreased from 2.4% at 34 weeks to 0.4% at 39 weeks).6 Another retrospective review of more than 230,000 newborns, 19,000 of whom were born in the late preterm period, revealed that more neonates born between 34 and 36 weeks’ gestation had respiratory distress syndrome than neonates delivered at 39 weeks (10.5% at 34 weeks, 6% at 35 weeks, 2.8% at 36 weeks vs 0.3% at 39 weeks).8
STUDY SUMMARY
Late preterm newborns breathe better with antenatal betamethasone
This RCT examined the effectiveness of betamethasone in preventing neonatal respiratory complications for 2,831 women at high probability of preterm delivery between 34 weeks and 36 weeks, 6 days of gestation. “High probability of preterm delivery” was defined as preterm labor with intact membranes and at least 3 cm dilation or 75% cervical effacement; spontaneous rupture of membranes; or anticipated preterm delivery for any other indication either through induction or cesarean section between 24 hours and seven days after the planned randomization.
Patients were randomly assigned to receive two intramuscular injections (12 mg each) of either betamethasone or placebo, 24 hours apart. The two doses were successfully given in 60% of the betamethasone group and 59% of the placebo group. In 95% of the cases in which the second dose was not given, it was because delivery occurred within 24 hours of the first dose.
The primary outcome was the need for respiratory support within 72 hours of birth, defined as one or more of the following: the use of continuous positive airway pressure (CPAP) or high-flow nasal cannula for at least two consecutive hours, supplemental oxygen for at least four continuous hours, extracorporeal membrane oxygenation (ECMO), or mechanical ventilation.
The median length of time from enrollment to delivery was 31 to 33 hours, and 31.4% underwent cesarean delivery. In the intention-to-treat analysis, the primary outcome was significantly lower in the betamethasone group than in the placebo group (11.6% vs 14.4%; relative risk [RR], 0.80; number needed to treat [NNT], 35). Secondary outcomes (severe complications, representing a composite of the use of CPAP or high-flow nasal cannula for at least 12 continuous hours, supplemental oxygen for at least 24 continuous hours, ECMO, mechanical ventilation, stillbirth, or neonatal death within 72 hours after delivery) were also lower in the betamethasone group (8.1% vs 12.1%; RR, 0.67; NNT, 25). The betamethasone group also had a lower risk for transient tachypnea of the newborn (6.7% vs 9.9%; RR, 0.68).
There were no significant differences in the occurrence of maternal chorioamnionitis or endometritis between the groups. Hypoglycemia in the newborn occurred more in the betamethasone group (24% vs 15%; RR, 1.6; number needed to harm [NNH], 11). The betamethasone group had two neonatal deaths: one from septic shock, and the other from a structural cardiac anomaly and arrhythmia.
WHAT’S NEW
Betamethasone effective even in the late, late preterm period
This study demonstrated an improvement in neonatal respiratory outcomes when betamethasone versus placebo was used in the late preterm period. The findings were similar to those from the Antenatal Steroids for Term Elective Caesarean Section Research Team.9 Their trial showed a reduction in respiratory complications in term neonates delivered via elective cesarean section to mothers who received antenatal betamethasone (NNT, 37, to prevent admission to a special care nursery with respiratory distress). The findings were also consistent with those of a recent meta-analysis evaluating the occurrence of respiratory complications with the use of antenatal betamethasone in women expected to deliver in the late preterm period or with a planned cesarean delivery at ≥ 37 weeks’ gestation.10
CAVEATS
Neonates may develop hypoglycemia
The authors of the study reported an increased risk for hypoglycemia in the neonates receiving antenatal betamethasone. The long-term implications of this are unclear, however, given that there was a reduction in intermediate care nursery and neonatal ICU stays that were three days or longer in the betamethasone group. There was also no difference in hospital length of stay between the two groups. Additionally, it’s unclear if there are any long-term neonatal complications of betamethasone use in the late preterm period.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible
There are minimal challenges to implementing this strategy, as betamethasone is routinely used for preterm labor and is readily available on labor and delivery units.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2017;66(2):104-106.

A 21-year-old G1P0 at 35 weeks, 2 days of gestation presents to labor and delivery reporting a “gush of clear fluid.” On exam, you confirm she has preterm rupture of membranes. She is contracting every three minutes and has a cervix dilated to 3 cm. Is there any neonatal benefit to using corticosteroids in this late preterm period?
Approximately 12% of all births in the United States are the result of preterm labor, and 8% take place in the late preterm period, defined as 34 to 36 weeks’ gestation.2,3 To reduce risk for neonatal death and respiratory complications, both the American College of Obstetricians and Gynecologists and the National Institutes of Health recommend a course of corticosteroids between 24 and 34 weeks’ gestation for women at increased risk for preterm delivery.2,4 Due to a lack of evidence from RCTs on the benefit of corticosteroids in late preterm labor, there are no recommendations to extend this period.5 However, multiple studies have shown that babies born during the late preterm period have more neonatal complications than term newborns.6-8
A retrospective chart review of more than 130,000 live births found that newborns who were delivered between 34 and 36 weeks had higher rates of respiratory distress than those delivered at 39 weeks (ventilator use dropped from 3.3% at 34 weeks to 0.3% at 39 weeks, and transient tachypnea decreased from 2.4% at 34 weeks to 0.4% at 39 weeks).6 Another retrospective review of more than 230,000 newborns, 19,000 of whom were born in the late preterm period, revealed that more neonates born between 34 and 36 weeks’ gestation had respiratory distress syndrome than neonates delivered at 39 weeks (10.5% at 34 weeks, 6% at 35 weeks, 2.8% at 36 weeks vs 0.3% at 39 weeks).8
STUDY SUMMARY
Late preterm newborns breathe better with antenatal betamethasone
This RCT examined the effectiveness of betamethasone in preventing neonatal respiratory complications for 2,831 women at high probability of preterm delivery between 34 weeks and 36 weeks, 6 days of gestation. “High probability of preterm delivery” was defined as preterm labor with intact membranes and at least 3 cm dilation or 75% cervical effacement; spontaneous rupture of membranes; or anticipated preterm delivery for any other indication either through induction or cesarean section between 24 hours and seven days after the planned randomization.
Patients were randomly assigned to receive two intramuscular injections (12 mg each) of either betamethasone or placebo, 24 hours apart. The two doses were successfully given in 60% of the betamethasone group and 59% of the placebo group. In 95% of the cases in which the second dose was not given, it was because delivery occurred within 24 hours of the first dose.
The primary outcome was the need for respiratory support within 72 hours of birth, defined as one or more of the following: the use of continuous positive airway pressure (CPAP) or high-flow nasal cannula for at least two consecutive hours, supplemental oxygen for at least four continuous hours, extracorporeal membrane oxygenation (ECMO), or mechanical ventilation.
The median length of time from enrollment to delivery was 31 to 33 hours, and 31.4% underwent cesarean delivery. In the intention-to-treat analysis, the primary outcome was significantly lower in the betamethasone group than in the placebo group (11.6% vs 14.4%; relative risk [RR], 0.80; number needed to treat [NNT], 35). Secondary outcomes (severe complications, representing a composite of the use of CPAP or high-flow nasal cannula for at least 12 continuous hours, supplemental oxygen for at least 24 continuous hours, ECMO, mechanical ventilation, stillbirth, or neonatal death within 72 hours after delivery) were also lower in the betamethasone group (8.1% vs 12.1%; RR, 0.67; NNT, 25). The betamethasone group also had a lower risk for transient tachypnea of the newborn (6.7% vs 9.9%; RR, 0.68).
There were no significant differences in the occurrence of maternal chorioamnionitis or endometritis between the groups. Hypoglycemia in the newborn occurred more in the betamethasone group (24% vs 15%; RR, 1.6; number needed to harm [NNH], 11). The betamethasone group had two neonatal deaths: one from septic shock, and the other from a structural cardiac anomaly and arrhythmia.
WHAT’S NEW
Betamethasone effective even in the late, late preterm period
This study demonstrated an improvement in neonatal respiratory outcomes when betamethasone versus placebo was used in the late preterm period. The findings were similar to those from the Antenatal Steroids for Term Elective Caesarean Section Research Team.9 Their trial showed a reduction in respiratory complications in term neonates delivered via elective cesarean section to mothers who received antenatal betamethasone (NNT, 37, to prevent admission to a special care nursery with respiratory distress). The findings were also consistent with those of a recent meta-analysis evaluating the occurrence of respiratory complications with the use of antenatal betamethasone in women expected to deliver in the late preterm period or with a planned cesarean delivery at ≥ 37 weeks’ gestation.10
CAVEATS
Neonates may develop hypoglycemia
The authors of the study reported an increased risk for hypoglycemia in the neonates receiving antenatal betamethasone. The long-term implications of this are unclear, however, given that there was a reduction in intermediate care nursery and neonatal ICU stays that were three days or longer in the betamethasone group. There was also no difference in hospital length of stay between the two groups. Additionally, it’s unclear if there are any long-term neonatal complications of betamethasone use in the late preterm period.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible
There are minimal challenges to implementing this strategy, as betamethasone is routinely used for preterm labor and is readily available on labor and delivery units.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice. 2017;66(2):104-106.
1. Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
2. Practice Bulletin No. 159 Summary: Management of Preterm Labor. Obstet Gynecol. 2016;127:190-191.
3. Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
4. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12:1-24.
5. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13-B15.
6. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35-41.
7. Yoder BA, Gordon MC, Barth WH Jr. Late-preterm birth: does the changing obstetric paradigm alter the epidemiology of respiratory complications? Obstet Gynecol. 2008;111:814-822.
8. Consortium on Safe Labor, Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419-425.
9. Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331:662.
10. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
1. Gyamfi-Bannerman C, Thom EA, Blackwell SC, et al; NICHD Maternal–Fetal Medicine Units Network. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374:1311-1320.
2. Practice Bulletin No. 159 Summary: Management of Preterm Labor. Obstet Gynecol. 2016;127:190-191.
3. Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
4. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12:1-24.
5. Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Implementation of the use of antenatal corticosteroids in the late preterm birth period in women at risk for preterm delivery. Am J Obstet Gynecol. 2016;215:B13-B15.
6. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35-41.
7. Yoder BA, Gordon MC, Barth WH Jr. Late-preterm birth: does the changing obstetric paradigm alter the epidemiology of respiratory complications? Obstet Gynecol. 2008;111:814-822.
8. Consortium on Safe Labor, Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419-425.
9. Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331:662.
10. Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044.
SPECIAL REPORT: SVS Takes Aim at Addressing Physician Burnout, Wellness
In my editorial in the November issue of Vascular Specialist I asked the question: Are we ok? I received many responses from members and I think the collective answer is best characterized as …maybe. Like nearly every other physician, I have witnessed the effects of burnout for my entire career. After high school, I enrolled in a BA/MD program with about 60 other students. Nearly half never matriculated. After medical school, I began a general surgery residency with seven other categorical interns. Six of them quit in the first year. What we now call burnout was once thought of as a process for weeding out those who could not “hack” it. Those students were not fit to be doctors; those interns were not fit to be surgeons. The process worked. Or so we thought.
The epidemic of physician burnout has raised alarm in many healthcare organizations. This mental state, characterized by emotional exhaustion, depersonalization and a diminished sense of personal accomplishment, has adverse consequences for both patient and clinician. The statistics are sobering. More than half of physicians, by self-report, have symptoms of burnout. No clinician is immune. Significant numbers of nurses and residents are similarly affected. The trend is worrisome. Compared to 2011, the percentage of physicians with burnout symptoms has increased, and the percentage satisfied with their work/life balance has decreased. Burnout affects physicians across all specialties, but the picture is particularly grim for surgeons, and even a bit more so for vascular surgeons. Compared with 13 other surgical specialties, vascular surgeons report the second highest rate of burnout. They also report the lowest level of career satisfaction, ranked first in stating they may have chosen an alternate career if they could choose again, and first in recommending an alternate career to medicine to their children.
In light of these sobering data, it must become an increasing concern of SVS as our professional home, as well as APDVS, as the professional home for Program Directors in Vascular Surgery, when almost a third of vascular surgeons report depression, and when compared to other surgical specialties, the highest incidence of suicidal ideation occurs in our own colleagues. Perhaps most importantly, 70 percent of physicians who score in the lower third on the Physician Well-Being Index report their well-being as average or above average. So relying on introspection offers limited hope.
Burnout is also a patient care-quality issue. Patients who are cared for by clinicians suffering from burnout do not fare well. A burned-out physician may appear to colleagues as frustrated, cynical and even callous, and to his or her patients as lacking empathy and compassion. Only 53 percent of patients, when surveyed, believe the health care system provides compassionate care. Diabetic patients of physicians with high empathy scores had improved control of HbA1c, fewer hospitalizations and fewer serious complications. Similarly, those with cancer had improved psychological adjustment, decreased ICU utilization and an improved immune response.
What does this landscape look like to those starting their medical careers – our future vascular surgeons? As a rule, students enter medical school altruistic and idealistic, excited about becoming a doctor, empathic and eager to care for their future patients. What follows is a decline in compassion, a crucial ingredient to mitigate burnout. Their curriculum focuses on emotional detachment and affective distancing for the purpose of clinical neutrality. Twenty percent of medical school graduates reported that they experienced disconnects between what they were taught about professional behaviors and attitudes and what they saw demonstrated by their faculty. Empathy is further eroded as residents experience “time-constrained” patient interactions in the clinic, the emergency department and the operating room. They often work with frustrated and overwhelmed faculty members who are experiencing increased pressure to generate greater clinical revenue and struggle with non-intuitive electronic medical record systems.
By almost any metric, vascular surgeons’ work is stressful. They work long hours, spend considerable hours in the operating room (average 20 hours a week) and average 2.7 nights of call a week. The vascular surgery workforce needs to continue to increase in light of the aging population that generally is sicker, yet demanding of more active lives. The demand will not be met without improving the health of our workforce.
Burnout also leads to early retirement. And burnout predicts the future. Each one-point change on the emotional exhaustion burnout scale is associated with a 43 percent higher likelihood that the affected physicians will reduce their FTE over the ensuing 24 months. Compounding supply further are the issues at the front end. An alarming 69 percent of U.S. general surgery trainees meet the criteria for burnout, and 44 percent of the 753 general surgery residents sampled considered dropping out of their training program; an even greater proportion considered doing so if they had symptoms of burnout. Women in training were at a higher risk of burnout compared to men. Although a study of burnout and job satisfaction in members of the American College of Surgeons found that private-practice surgeons were more likely to experience burnout than surgeons practicing in academic settings, Elmore et. al. observed that residents who planned to enter private practice reported higher levels of burnout.
In summary, burnout out is real and is impacting the health, well-being, and careers of vascular surgeons. Burnout places both the surgeon and the patient at risk for a poor outcome. Burnout influences medical student and resident career choices. Burnout can no longer be ignored – doing nothing is not an option.
Can the Society of Vascular Surgery do anything to improve the well-being of its members? We will begin that discussion next month.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, M.D., Scholar in Residence, Schwartz Center for Compassionate Care, Boston, Mass., and Clinical Professor of Orthopedics at Seattle Children’s Hospital..
In my editorial in the November issue of Vascular Specialist I asked the question: Are we ok? I received many responses from members and I think the collective answer is best characterized as …maybe. Like nearly every other physician, I have witnessed the effects of burnout for my entire career. After high school, I enrolled in a BA/MD program with about 60 other students. Nearly half never matriculated. After medical school, I began a general surgery residency with seven other categorical interns. Six of them quit in the first year. What we now call burnout was once thought of as a process for weeding out those who could not “hack” it. Those students were not fit to be doctors; those interns were not fit to be surgeons. The process worked. Or so we thought.
The epidemic of physician burnout has raised alarm in many healthcare organizations. This mental state, characterized by emotional exhaustion, depersonalization and a diminished sense of personal accomplishment, has adverse consequences for both patient and clinician. The statistics are sobering. More than half of physicians, by self-report, have symptoms of burnout. No clinician is immune. Significant numbers of nurses and residents are similarly affected. The trend is worrisome. Compared to 2011, the percentage of physicians with burnout symptoms has increased, and the percentage satisfied with their work/life balance has decreased. Burnout affects physicians across all specialties, but the picture is particularly grim for surgeons, and even a bit more so for vascular surgeons. Compared with 13 other surgical specialties, vascular surgeons report the second highest rate of burnout. They also report the lowest level of career satisfaction, ranked first in stating they may have chosen an alternate career if they could choose again, and first in recommending an alternate career to medicine to their children.
In light of these sobering data, it must become an increasing concern of SVS as our professional home, as well as APDVS, as the professional home for Program Directors in Vascular Surgery, when almost a third of vascular surgeons report depression, and when compared to other surgical specialties, the highest incidence of suicidal ideation occurs in our own colleagues. Perhaps most importantly, 70 percent of physicians who score in the lower third on the Physician Well-Being Index report their well-being as average or above average. So relying on introspection offers limited hope.
Burnout is also a patient care-quality issue. Patients who are cared for by clinicians suffering from burnout do not fare well. A burned-out physician may appear to colleagues as frustrated, cynical and even callous, and to his or her patients as lacking empathy and compassion. Only 53 percent of patients, when surveyed, believe the health care system provides compassionate care. Diabetic patients of physicians with high empathy scores had improved control of HbA1c, fewer hospitalizations and fewer serious complications. Similarly, those with cancer had improved psychological adjustment, decreased ICU utilization and an improved immune response.
What does this landscape look like to those starting their medical careers – our future vascular surgeons? As a rule, students enter medical school altruistic and idealistic, excited about becoming a doctor, empathic and eager to care for their future patients. What follows is a decline in compassion, a crucial ingredient to mitigate burnout. Their curriculum focuses on emotional detachment and affective distancing for the purpose of clinical neutrality. Twenty percent of medical school graduates reported that they experienced disconnects between what they were taught about professional behaviors and attitudes and what they saw demonstrated by their faculty. Empathy is further eroded as residents experience “time-constrained” patient interactions in the clinic, the emergency department and the operating room. They often work with frustrated and overwhelmed faculty members who are experiencing increased pressure to generate greater clinical revenue and struggle with non-intuitive electronic medical record systems.
By almost any metric, vascular surgeons’ work is stressful. They work long hours, spend considerable hours in the operating room (average 20 hours a week) and average 2.7 nights of call a week. The vascular surgery workforce needs to continue to increase in light of the aging population that generally is sicker, yet demanding of more active lives. The demand will not be met without improving the health of our workforce.
Burnout also leads to early retirement. And burnout predicts the future. Each one-point change on the emotional exhaustion burnout scale is associated with a 43 percent higher likelihood that the affected physicians will reduce their FTE over the ensuing 24 months. Compounding supply further are the issues at the front end. An alarming 69 percent of U.S. general surgery trainees meet the criteria for burnout, and 44 percent of the 753 general surgery residents sampled considered dropping out of their training program; an even greater proportion considered doing so if they had symptoms of burnout. Women in training were at a higher risk of burnout compared to men. Although a study of burnout and job satisfaction in members of the American College of Surgeons found that private-practice surgeons were more likely to experience burnout than surgeons practicing in academic settings, Elmore et. al. observed that residents who planned to enter private practice reported higher levels of burnout.
In summary, burnout out is real and is impacting the health, well-being, and careers of vascular surgeons. Burnout places both the surgeon and the patient at risk for a poor outcome. Burnout influences medical student and resident career choices. Burnout can no longer be ignored – doing nothing is not an option.
Can the Society of Vascular Surgery do anything to improve the well-being of its members? We will begin that discussion next month.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, M.D., Scholar in Residence, Schwartz Center for Compassionate Care, Boston, Mass., and Clinical Professor of Orthopedics at Seattle Children’s Hospital..
In my editorial in the November issue of Vascular Specialist I asked the question: Are we ok? I received many responses from members and I think the collective answer is best characterized as …maybe. Like nearly every other physician, I have witnessed the effects of burnout for my entire career. After high school, I enrolled in a BA/MD program with about 60 other students. Nearly half never matriculated. After medical school, I began a general surgery residency with seven other categorical interns. Six of them quit in the first year. What we now call burnout was once thought of as a process for weeding out those who could not “hack” it. Those students were not fit to be doctors; those interns were not fit to be surgeons. The process worked. Or so we thought.
The epidemic of physician burnout has raised alarm in many healthcare organizations. This mental state, characterized by emotional exhaustion, depersonalization and a diminished sense of personal accomplishment, has adverse consequences for both patient and clinician. The statistics are sobering. More than half of physicians, by self-report, have symptoms of burnout. No clinician is immune. Significant numbers of nurses and residents are similarly affected. The trend is worrisome. Compared to 2011, the percentage of physicians with burnout symptoms has increased, and the percentage satisfied with their work/life balance has decreased. Burnout affects physicians across all specialties, but the picture is particularly grim for surgeons, and even a bit more so for vascular surgeons. Compared with 13 other surgical specialties, vascular surgeons report the second highest rate of burnout. They also report the lowest level of career satisfaction, ranked first in stating they may have chosen an alternate career if they could choose again, and first in recommending an alternate career to medicine to their children.
In light of these sobering data, it must become an increasing concern of SVS as our professional home, as well as APDVS, as the professional home for Program Directors in Vascular Surgery, when almost a third of vascular surgeons report depression, and when compared to other surgical specialties, the highest incidence of suicidal ideation occurs in our own colleagues. Perhaps most importantly, 70 percent of physicians who score in the lower third on the Physician Well-Being Index report their well-being as average or above average. So relying on introspection offers limited hope.
Burnout is also a patient care-quality issue. Patients who are cared for by clinicians suffering from burnout do not fare well. A burned-out physician may appear to colleagues as frustrated, cynical and even callous, and to his or her patients as lacking empathy and compassion. Only 53 percent of patients, when surveyed, believe the health care system provides compassionate care. Diabetic patients of physicians with high empathy scores had improved control of HbA1c, fewer hospitalizations and fewer serious complications. Similarly, those with cancer had improved psychological adjustment, decreased ICU utilization and an improved immune response.
What does this landscape look like to those starting their medical careers – our future vascular surgeons? As a rule, students enter medical school altruistic and idealistic, excited about becoming a doctor, empathic and eager to care for their future patients. What follows is a decline in compassion, a crucial ingredient to mitigate burnout. Their curriculum focuses on emotional detachment and affective distancing for the purpose of clinical neutrality. Twenty percent of medical school graduates reported that they experienced disconnects between what they were taught about professional behaviors and attitudes and what they saw demonstrated by their faculty. Empathy is further eroded as residents experience “time-constrained” patient interactions in the clinic, the emergency department and the operating room. They often work with frustrated and overwhelmed faculty members who are experiencing increased pressure to generate greater clinical revenue and struggle with non-intuitive electronic medical record systems.
By almost any metric, vascular surgeons’ work is stressful. They work long hours, spend considerable hours in the operating room (average 20 hours a week) and average 2.7 nights of call a week. The vascular surgery workforce needs to continue to increase in light of the aging population that generally is sicker, yet demanding of more active lives. The demand will not be met without improving the health of our workforce.
Burnout also leads to early retirement. And burnout predicts the future. Each one-point change on the emotional exhaustion burnout scale is associated with a 43 percent higher likelihood that the affected physicians will reduce their FTE over the ensuing 24 months. Compounding supply further are the issues at the front end. An alarming 69 percent of U.S. general surgery trainees meet the criteria for burnout, and 44 percent of the 753 general surgery residents sampled considered dropping out of their training program; an even greater proportion considered doing so if they had symptoms of burnout. Women in training were at a higher risk of burnout compared to men. Although a study of burnout and job satisfaction in members of the American College of Surgeons found that private-practice surgeons were more likely to experience burnout than surgeons practicing in academic settings, Elmore et. al. observed that residents who planned to enter private practice reported higher levels of burnout.
In summary, burnout out is real and is impacting the health, well-being, and careers of vascular surgeons. Burnout places both the surgeon and the patient at risk for a poor outcome. Burnout influences medical student and resident career choices. Burnout can no longer be ignored – doing nothing is not an option.
Can the Society of Vascular Surgery do anything to improve the well-being of its members? We will begin that discussion next month.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, M.D., Scholar in Residence, Schwartz Center for Compassionate Care, Boston, Mass., and Clinical Professor of Orthopedics at Seattle Children’s Hospital..
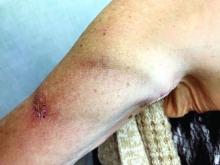
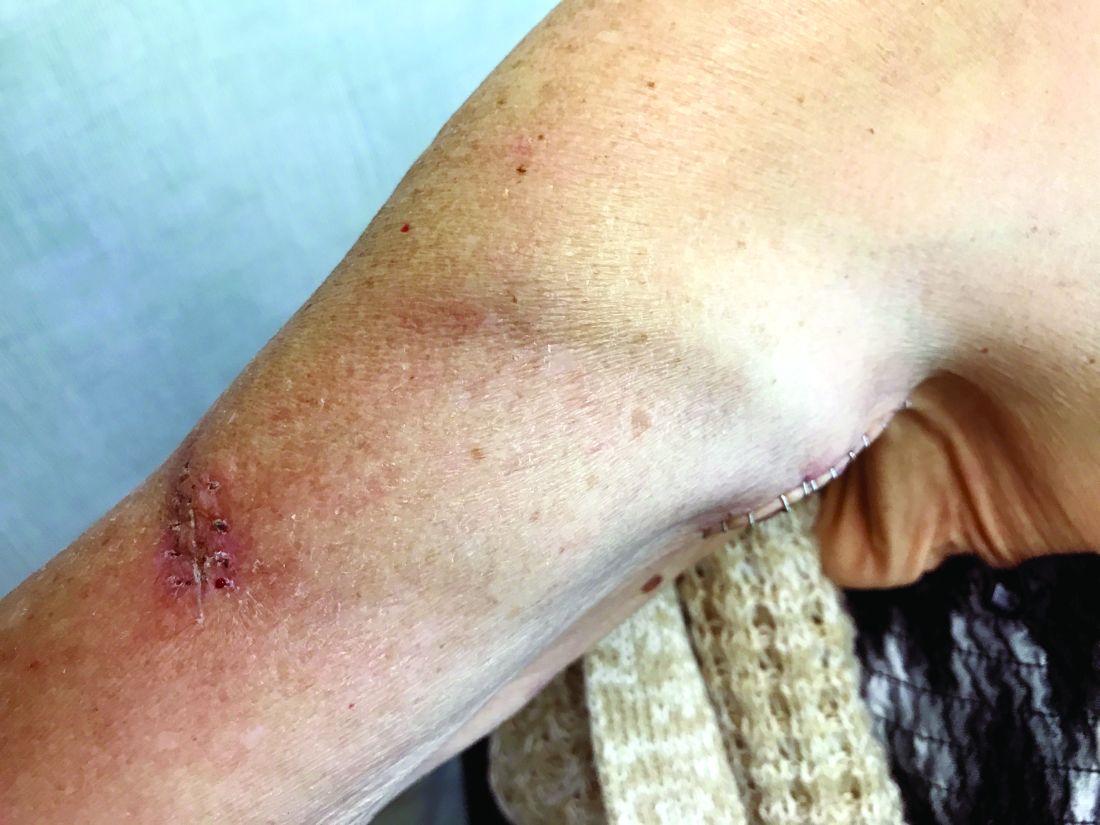
Upper arm loop grafts
Many forearm loop grafts have multiple outflow interventions before failure. These angioplasties and outflow stents create inflammation around the vein just above the antecubital area. This inflammation makes arterial exposure difficult for a standard upper arm graft configuration. Accordingly, surgeons may avoid this area by performing an upper arm loop graft, which originates and terminates in one high arm incision. Upper arm loop grafts are also used for high bifurcation of the radial and ulnar arteries and when prior access grafts make other configurations difficult. Surgeons appear to place these upper arm loops routed with one limb running subcutaneously parallel to the brachial artery, placing half of the loop in an awkward position for cannulation.
Dr. Showalter is clinical assistant professor of surgery, Florida State University Medical School, Tallahassee, and attending vascular surgeon, Sarasota Vascular Specialists.
Many forearm loop grafts have multiple outflow interventions before failure. These angioplasties and outflow stents create inflammation around the vein just above the antecubital area. This inflammation makes arterial exposure difficult for a standard upper arm graft configuration. Accordingly, surgeons may avoid this area by performing an upper arm loop graft, which originates and terminates in one high arm incision. Upper arm loop grafts are also used for high bifurcation of the radial and ulnar arteries and when prior access grafts make other configurations difficult. Surgeons appear to place these upper arm loops routed with one limb running subcutaneously parallel to the brachial artery, placing half of the loop in an awkward position for cannulation.
Dr. Showalter is clinical assistant professor of surgery, Florida State University Medical School, Tallahassee, and attending vascular surgeon, Sarasota Vascular Specialists.
Many forearm loop grafts have multiple outflow interventions before failure. These angioplasties and outflow stents create inflammation around the vein just above the antecubital area. This inflammation makes arterial exposure difficult for a standard upper arm graft configuration. Accordingly, surgeons may avoid this area by performing an upper arm loop graft, which originates and terminates in one high arm incision. Upper arm loop grafts are also used for high bifurcation of the radial and ulnar arteries and when prior access grafts make other configurations difficult. Surgeons appear to place these upper arm loops routed with one limb running subcutaneously parallel to the brachial artery, placing half of the loop in an awkward position for cannulation.
Dr. Showalter is clinical assistant professor of surgery, Florida State University Medical School, Tallahassee, and attending vascular surgeon, Sarasota Vascular Specialists.
Taking a leap of faith
After a grueling first two years of surgical residency, I welcomed with open arms my surgical research years. Junior surgical residency was arguably the toughest years of my training to date. Long hours at the hospital; the uncertainty of being called in to the hospital when on-call, which led to chronic anxiety and at times insomnia; and the pressures I put on myself to excel in all aspects of my training were draining, to say the least.
Of course, when it came time to leave my clinical responsibilities and pursue my Master’s degree, I was overcome with relief. First, I got my life back on track, leading a life of optimal nutrition, physical activity, and sleep and exploring different horizons in surgery.
Second, this time allowed me to grow as a person, learning techniques to remain calm in the face of adversity, to take at least 10 minutes a day for mindfulness, and to be cognizant and gauge when I am creeping upon that tipping point. I believe the key to success and happiness is to keep re-evaluating and being honest with ourselves, our happiness, our stresses, and our anxieties and to reach out to pillars of support, whoever they may be.
And finally, we are fundamentally teachers and inspirations to the next generation of surgeons who will follow in our footsteps. By being open, encouraging, and sharing our enthusiasm for our specialty, our patients, and our research, we may see the seeds of the future flourish under our wings.
That being said, I am terrified of returning to vascular surgery. I know it will be a challenge transitioning to senior resident, and I am scared that the progress I made over these years in terms of wellness and wellbeing will regress; however, in the end, I have to take a leap of faith and hope it all pulls together ... seamlessly.
Dr. Drudi is a vascular surgery resident at McGill University, Montreal, and the resident medical editor of Vascular Specialist.
After a grueling first two years of surgical residency, I welcomed with open arms my surgical research years. Junior surgical residency was arguably the toughest years of my training to date. Long hours at the hospital; the uncertainty of being called in to the hospital when on-call, which led to chronic anxiety and at times insomnia; and the pressures I put on myself to excel in all aspects of my training were draining, to say the least.
Of course, when it came time to leave my clinical responsibilities and pursue my Master’s degree, I was overcome with relief. First, I got my life back on track, leading a life of optimal nutrition, physical activity, and sleep and exploring different horizons in surgery.
Second, this time allowed me to grow as a person, learning techniques to remain calm in the face of adversity, to take at least 10 minutes a day for mindfulness, and to be cognizant and gauge when I am creeping upon that tipping point. I believe the key to success and happiness is to keep re-evaluating and being honest with ourselves, our happiness, our stresses, and our anxieties and to reach out to pillars of support, whoever they may be.
And finally, we are fundamentally teachers and inspirations to the next generation of surgeons who will follow in our footsteps. By being open, encouraging, and sharing our enthusiasm for our specialty, our patients, and our research, we may see the seeds of the future flourish under our wings.
That being said, I am terrified of returning to vascular surgery. I know it will be a challenge transitioning to senior resident, and I am scared that the progress I made over these years in terms of wellness and wellbeing will regress; however, in the end, I have to take a leap of faith and hope it all pulls together ... seamlessly.
Dr. Drudi is a vascular surgery resident at McGill University, Montreal, and the resident medical editor of Vascular Specialist.
After a grueling first two years of surgical residency, I welcomed with open arms my surgical research years. Junior surgical residency was arguably the toughest years of my training to date. Long hours at the hospital; the uncertainty of being called in to the hospital when on-call, which led to chronic anxiety and at times insomnia; and the pressures I put on myself to excel in all aspects of my training were draining, to say the least.
Of course, when it came time to leave my clinical responsibilities and pursue my Master’s degree, I was overcome with relief. First, I got my life back on track, leading a life of optimal nutrition, physical activity, and sleep and exploring different horizons in surgery.
Second, this time allowed me to grow as a person, learning techniques to remain calm in the face of adversity, to take at least 10 minutes a day for mindfulness, and to be cognizant and gauge when I am creeping upon that tipping point. I believe the key to success and happiness is to keep re-evaluating and being honest with ourselves, our happiness, our stresses, and our anxieties and to reach out to pillars of support, whoever they may be.
And finally, we are fundamentally teachers and inspirations to the next generation of surgeons who will follow in our footsteps. By being open, encouraging, and sharing our enthusiasm for our specialty, our patients, and our research, we may see the seeds of the future flourish under our wings.
That being said, I am terrified of returning to vascular surgery. I know it will be a challenge transitioning to senior resident, and I am scared that the progress I made over these years in terms of wellness and wellbeing will regress; however, in the end, I have to take a leap of faith and hope it all pulls together ... seamlessly.
Dr. Drudi is a vascular surgery resident at McGill University, Montreal, and the resident medical editor of Vascular Specialist.
Chronic rhinosinusitis associated with poor sleep quality
ATLANTA – Answers on a popular self-reported sleep questionnaire correlated positively with sinonasal inflammation, suggesting that patients with chronic rhinosinusitis should be assessed for sleep-related problems, results from a single-center study showed.
“We need to be recognizing the symptoms of chronic rhinosinusitis patients more in order to help them improve their quality of life,” lead study author Jessica Hui, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Asking them about sleep is important.”
Dr. Hui, who is a second-year pediatrics resident at Rush University Medical Center, reported that CRS patients had significant worse sleep quality, compared with controls (a mean PSQI score of 7.44 vs. 3.31, respectively) and that a higher Lund-Mackay Score correlated with greater PSQI (Pearson correlation coefficient of 0.25; P = .03).
The mean age of CRS cases without sleep disruption was 12.08 years, while the mean age of CRS cases with sleep disruption was 34.74 years.
Poor sleep quality was also associated with higher pain index scores (Pearson correlation coefficient of 0.35; P = .002) and higher scores on the SNOT-22 (Pearson correlation coefficient of 0.25; P = .025). The researchers observed that CRS patients without nasal polyps trended towards a higher PSQI, compared with controls (a mean of 8.14 vs. 6.36; P=0.10). Sinus histopathology variables and comorbid diseases did not correlate with PSQI scores.
Dr. Hui reported having no financial disclosures.
ATLANTA – Answers on a popular self-reported sleep questionnaire correlated positively with sinonasal inflammation, suggesting that patients with chronic rhinosinusitis should be assessed for sleep-related problems, results from a single-center study showed.
“We need to be recognizing the symptoms of chronic rhinosinusitis patients more in order to help them improve their quality of life,” lead study author Jessica Hui, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Asking them about sleep is important.”
Dr. Hui, who is a second-year pediatrics resident at Rush University Medical Center, reported that CRS patients had significant worse sleep quality, compared with controls (a mean PSQI score of 7.44 vs. 3.31, respectively) and that a higher Lund-Mackay Score correlated with greater PSQI (Pearson correlation coefficient of 0.25; P = .03).
The mean age of CRS cases without sleep disruption was 12.08 years, while the mean age of CRS cases with sleep disruption was 34.74 years.
Poor sleep quality was also associated with higher pain index scores (Pearson correlation coefficient of 0.35; P = .002) and higher scores on the SNOT-22 (Pearson correlation coefficient of 0.25; P = .025). The researchers observed that CRS patients without nasal polyps trended towards a higher PSQI, compared with controls (a mean of 8.14 vs. 6.36; P=0.10). Sinus histopathology variables and comorbid diseases did not correlate with PSQI scores.
Dr. Hui reported having no financial disclosures.
ATLANTA – Answers on a popular self-reported sleep questionnaire correlated positively with sinonasal inflammation, suggesting that patients with chronic rhinosinusitis should be assessed for sleep-related problems, results from a single-center study showed.
“We need to be recognizing the symptoms of chronic rhinosinusitis patients more in order to help them improve their quality of life,” lead study author Jessica Hui, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Asking them about sleep is important.”
Dr. Hui, who is a second-year pediatrics resident at Rush University Medical Center, reported that CRS patients had significant worse sleep quality, compared with controls (a mean PSQI score of 7.44 vs. 3.31, respectively) and that a higher Lund-Mackay Score correlated with greater PSQI (Pearson correlation coefficient of 0.25; P = .03).
The mean age of CRS cases without sleep disruption was 12.08 years, while the mean age of CRS cases with sleep disruption was 34.74 years.
Poor sleep quality was also associated with higher pain index scores (Pearson correlation coefficient of 0.35; P = .002) and higher scores on the SNOT-22 (Pearson correlation coefficient of 0.25; P = .025). The researchers observed that CRS patients without nasal polyps trended towards a higher PSQI, compared with controls (a mean of 8.14 vs. 6.36; P=0.10). Sinus histopathology variables and comorbid diseases did not correlate with PSQI scores.
Dr. Hui reported having no financial disclosures.
AT 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: CRS patients had significant worse sleep quality, compared with controls (a mean Pittsburgh Sleep Quality Index score of 7.44 vs. 3.31, respectively).
Data source: A cohort study of 125 CRS patients with refractory disease and 41 controls.
Disclosures: Dr. Hui reported having no financial disclosures.
More time in aftercare improves abstinence
Patients treated for addiction for longer than 30 days showed a significantly higher abstinence success rate of 84%, compared with 55% for patients whose treatment stopped at 30 days, a study of 72 adults shows.
The findings were not significantly different among different kinds of addictions, such as alcohol and amphetamine addiction or opioid and benzodiazepine dependency (Open J Psychiatr. Jan 2017;7:51-60).
“Recovery is an ongoing process once a client leaves treatment,” wrote Akikur R. Mohammad, MD, CEO/founder of the Inspire Malibu drug and alcohol treatment center in Southern California, and his colleagues. “Clients who adhere to their discharge plan and immerse themselves in recovery-related activities and lifestyle are likely to achieve sobriety for longer periods of time, if not indefinitely.”
To assess the efficacy of treatment and the predictors of relapse, the researchers enrolled 32 men and 40 women who were undergoing clinical treatment for various types of addiction. The average age was 30 years for the men and 30.7 years for the women.
In addition, the researchers developed models of treatment outcomes. They found a relative risk of substance abuse relapse of 18.1 for patients who failed to answer the phone at least three times during a 12-month follow-up period, compared with patients who only failed to answer the phone either zero, one, or two times.
Although the results were limited by the use of self reports, the findings support the role of aftercare follow-up in identifying addiction patients at risk for relapse, noted Dr. Mohammad of the University of California, Los Angeles, and his colleagues.
The researchers had no financial conflicts to disclose.
Patients treated for addiction for longer than 30 days showed a significantly higher abstinence success rate of 84%, compared with 55% for patients whose treatment stopped at 30 days, a study of 72 adults shows.
The findings were not significantly different among different kinds of addictions, such as alcohol and amphetamine addiction or opioid and benzodiazepine dependency (Open J Psychiatr. Jan 2017;7:51-60).
“Recovery is an ongoing process once a client leaves treatment,” wrote Akikur R. Mohammad, MD, CEO/founder of the Inspire Malibu drug and alcohol treatment center in Southern California, and his colleagues. “Clients who adhere to their discharge plan and immerse themselves in recovery-related activities and lifestyle are likely to achieve sobriety for longer periods of time, if not indefinitely.”
To assess the efficacy of treatment and the predictors of relapse, the researchers enrolled 32 men and 40 women who were undergoing clinical treatment for various types of addiction. The average age was 30 years for the men and 30.7 years for the women.
In addition, the researchers developed models of treatment outcomes. They found a relative risk of substance abuse relapse of 18.1 for patients who failed to answer the phone at least three times during a 12-month follow-up period, compared with patients who only failed to answer the phone either zero, one, or two times.
Although the results were limited by the use of self reports, the findings support the role of aftercare follow-up in identifying addiction patients at risk for relapse, noted Dr. Mohammad of the University of California, Los Angeles, and his colleagues.
The researchers had no financial conflicts to disclose.
Patients treated for addiction for longer than 30 days showed a significantly higher abstinence success rate of 84%, compared with 55% for patients whose treatment stopped at 30 days, a study of 72 adults shows.
The findings were not significantly different among different kinds of addictions, such as alcohol and amphetamine addiction or opioid and benzodiazepine dependency (Open J Psychiatr. Jan 2017;7:51-60).
“Recovery is an ongoing process once a client leaves treatment,” wrote Akikur R. Mohammad, MD, CEO/founder of the Inspire Malibu drug and alcohol treatment center in Southern California, and his colleagues. “Clients who adhere to their discharge plan and immerse themselves in recovery-related activities and lifestyle are likely to achieve sobriety for longer periods of time, if not indefinitely.”
To assess the efficacy of treatment and the predictors of relapse, the researchers enrolled 32 men and 40 women who were undergoing clinical treatment for various types of addiction. The average age was 30 years for the men and 30.7 years for the women.
In addition, the researchers developed models of treatment outcomes. They found a relative risk of substance abuse relapse of 18.1 for patients who failed to answer the phone at least three times during a 12-month follow-up period, compared with patients who only failed to answer the phone either zero, one, or two times.
Although the results were limited by the use of self reports, the findings support the role of aftercare follow-up in identifying addiction patients at risk for relapse, noted Dr. Mohammad of the University of California, Los Angeles, and his colleagues.
The researchers had no financial conflicts to disclose.
FROM THE OPEN JOURNAL OF PSYCHIATRY
Decision support tool appears to safely reduce CSF use
ORLANDO – A decision support tool safely reduces use of colony-stimulating factors (CSFs) in patients undergoing chemotherapy for lung cancer, suggests a retrospective claims-based cohort study of nearly 3,500 patients across the country.
The rate of CSF use fell among patients treated in the nine states that implemented the tool – a library of chemotherapy regimens and their expected FN risk that uses preauthorization and an algorithm to promote risk-appropriate, guideline-adherent use – but it remained unchanged in the 39 states and the District of Columbia, where usual practice continued, investigators reported at a symposium on quality care sponsored by the American Society of Clinical Oncology and simultaneously published (J Oncol Pract. 2017 March 4. doi: 10.1200/JOP.2017.020867). The adjusted difference was nearly 9%.
“Decision support programs like the one highlighted here could be one way, definitely not the only way, of achieving guideline-adherent CSF use and reducing practice variation across the country,” commented coinvestigator Abiy Agiro, PhD, associate director of payer and provider research at HealthCore, a subsidiary of Anthem, in Wilmington, Delaware.
“Such efforts could also have unintended consequences, so it’s important to study relevant patient outcomes,” he added. “In this case, although it appears that the incidence of febrile neutropenia rising does not seem to relate with the program, the study does not establish the safety of CSF use reduction in lung cancer patients receiving chemotherapy. So, we should take the results with that caveat.”
Parsing the findings
Although the United States makes up just 4% of the world’s population, it uses nearly 80% of CSFs sold by a leading manufacturer, according to invited discussant Thomas J. Smith, MD, a professor of oncology and palliative medicine at Johns Hopkins University in Baltimore.
“When we rewrote the ASCO [American Society of Clinical Oncology] guidelines on CSF use in 2015, there were some specific indications: dose-intense chemo for adjuvant breast cancer and uroepithelial cancer and ... when the risk of febrile neutropenia is about 20% and dose reduction is not an appropriate strategy. We were quick to point out that most regimens have a risk of febrile neutropenia much less than that,” he noted.
Dr. Agiro and his colleagues’ findings are valid, real, and reproducible, Dr. Smith maintained. However, it is unclear to what extent the observed levels of CSF use represented overuse.
“In lung cancer, there are very few regimens that have a febrile neutropenia rate close to 20%,” he elaborated. “What we don’t know is how much of this [use] was actually justified. I would suspect it is 10% or 15%, rather than 40%.”
CSF use, as guided by the new tool, “might not support increased dose density [of chemotherapy], but I would challenge anybody in the audience to show me data in normal solid tumor patients that [show that] dose density maintained by CSFs makes a difference in overall survival,” he said.
Questions yet to be addressed include the difficulty and cost of using the decision support tool and the possible negative impact on practices’ finances, according to Dr. Smith.
“When ESAs [erythropoiesis-stimulating agents] came off being used so much, some of my friends’ practices took a 15% to 20% drop in their revenue, and this is an important source of revenue for a lot of practices,” he explained. “So, I hope that when we take this revenue away, that we are cognizant of that and realize that it’s just another stress on practices, many of which are under significant stress already.”
Study details
An estimated 26% of uses of CSFs in patients with lung cancer are not in accordance with the ASCO practice guidelines, according to Dr. Agiro. “Such variations from recommendations are sometimes the reason why different stakeholders take actions” to improve care, such as ASCO’s Quality Oncology Practice Initiative (QOPI) and the American Board of Internal Medicine’s Choosing Wisely initiative (J Oncol Pract. 2015;11:338-43).
The decision support tool evaluated in the study uses preauthorization before delivery of care and, therefore, differs from point-of-care interventions, he noted.
“The tool allows access to a library of chemotherapy regimens and their associated, expected febrile neutropenia risk based on the myelotoxicity of the planned regimens as indicated in published trials. The tool is accessible online and provides real-time recommendations that are tailored based on disease- and patient-specific factors for either the use of CSF or not,” he elaborated.
According to the tool’s algorithm, use is recommended for patients who are given a regimen with a high risk of febrile neutropenia (greater than 20%) and is not recommended for those given a low-risk regimen (less than 10%). It is tailored according to the presence of additional risk factors for the intermediate-risk group.
Oncologists use the tool only for patients starting a new chemotherapy and only in the first cycle, when the risk of febrile neutropenia is highest, according to Dr. Agiro. “Once the approval is given in the first cycle, it remains in effect for the next 6 months, so they don’t have to use it again and again in additional cycles,” he explained.
The decision support tool was implemented in nine states starting in July 2014. In the study, which was funded by Anthem, the investigators analyzed administrative claims data from commercially insured adult patients starting chemotherapy for lung cancer, assessing changes in outcomes between a preimplementation period (April 2013 to Dec. 2013) and a postimplementation period (July 2014 to March 2015).
Analyses were based on 1,857 patients in the states that implemented the tool and 1,610 patients in the states that did not.
The percentage of patients receiving CSFs in the 6 months after starting chemotherapy fell in states that implemented the decision support tool (from 48.4% to 35.6%) but remained stable in states that did not (43.2% and 44.4%), Dr. Agiro reported. The adjusted difference in differences was –8.7% (P less than .001).
Meanwhile, the percentage of patients admitted for febrile neutropenia or experiencing this outcome while hospitalized increased in both states implementing the tool (from 2.8% to 4.3%) and those not implementing it (from 3.1% to 5.1%). Although the magnitude of increase was smaller in the former (+1.5% vs. +2.0%), the difference was not significant. Findings were essentially the same among the subset of patients aged 65 years and older.
“It’s important to study both intended and unintended consequences of such interventions,” Dr. Agiro noted. “Our study goes beyond financial considerations by looking at unintended outcomes: in this case, focusing on the incidence of febrile neutropenia, an outcome that is of prime interest to patients and oncologists and payers alike.”
The study may have missed some cases of febrile neutropenia, he acknowledged. “Also, there are other important outcomes of concern. For example, were there any delays in chemotherapy administration or immune recovery that could have been triggered by the implementation of the decision support program?”
The impact, both intended and unintended, on practices warrants evaluation as well, he further noted. “An important question could be, ‘Does it take less time to use this decision support tool compared to the time taken with normal care processes?’ ”
Dr. Agiro disclosed that he is employed by, has stock or other ownership interests in, and receives research funding from Anthem.
ORLANDO – A decision support tool safely reduces use of colony-stimulating factors (CSFs) in patients undergoing chemotherapy for lung cancer, suggests a retrospective claims-based cohort study of nearly 3,500 patients across the country.
The rate of CSF use fell among patients treated in the nine states that implemented the tool – a library of chemotherapy regimens and their expected FN risk that uses preauthorization and an algorithm to promote risk-appropriate, guideline-adherent use – but it remained unchanged in the 39 states and the District of Columbia, where usual practice continued, investigators reported at a symposium on quality care sponsored by the American Society of Clinical Oncology and simultaneously published (J Oncol Pract. 2017 March 4. doi: 10.1200/JOP.2017.020867). The adjusted difference was nearly 9%.
“Decision support programs like the one highlighted here could be one way, definitely not the only way, of achieving guideline-adherent CSF use and reducing practice variation across the country,” commented coinvestigator Abiy Agiro, PhD, associate director of payer and provider research at HealthCore, a subsidiary of Anthem, in Wilmington, Delaware.
“Such efforts could also have unintended consequences, so it’s important to study relevant patient outcomes,” he added. “In this case, although it appears that the incidence of febrile neutropenia rising does not seem to relate with the program, the study does not establish the safety of CSF use reduction in lung cancer patients receiving chemotherapy. So, we should take the results with that caveat.”
Parsing the findings
Although the United States makes up just 4% of the world’s population, it uses nearly 80% of CSFs sold by a leading manufacturer, according to invited discussant Thomas J. Smith, MD, a professor of oncology and palliative medicine at Johns Hopkins University in Baltimore.
“When we rewrote the ASCO [American Society of Clinical Oncology] guidelines on CSF use in 2015, there were some specific indications: dose-intense chemo for adjuvant breast cancer and uroepithelial cancer and ... when the risk of febrile neutropenia is about 20% and dose reduction is not an appropriate strategy. We were quick to point out that most regimens have a risk of febrile neutropenia much less than that,” he noted.
Dr. Agiro and his colleagues’ findings are valid, real, and reproducible, Dr. Smith maintained. However, it is unclear to what extent the observed levels of CSF use represented overuse.
“In lung cancer, there are very few regimens that have a febrile neutropenia rate close to 20%,” he elaborated. “What we don’t know is how much of this [use] was actually justified. I would suspect it is 10% or 15%, rather than 40%.”
CSF use, as guided by the new tool, “might not support increased dose density [of chemotherapy], but I would challenge anybody in the audience to show me data in normal solid tumor patients that [show that] dose density maintained by CSFs makes a difference in overall survival,” he said.
Questions yet to be addressed include the difficulty and cost of using the decision support tool and the possible negative impact on practices’ finances, according to Dr. Smith.
“When ESAs [erythropoiesis-stimulating agents] came off being used so much, some of my friends’ practices took a 15% to 20% drop in their revenue, and this is an important source of revenue for a lot of practices,” he explained. “So, I hope that when we take this revenue away, that we are cognizant of that and realize that it’s just another stress on practices, many of which are under significant stress already.”
Study details
An estimated 26% of uses of CSFs in patients with lung cancer are not in accordance with the ASCO practice guidelines, according to Dr. Agiro. “Such variations from recommendations are sometimes the reason why different stakeholders take actions” to improve care, such as ASCO’s Quality Oncology Practice Initiative (QOPI) and the American Board of Internal Medicine’s Choosing Wisely initiative (J Oncol Pract. 2015;11:338-43).
The decision support tool evaluated in the study uses preauthorization before delivery of care and, therefore, differs from point-of-care interventions, he noted.
“The tool allows access to a library of chemotherapy regimens and their associated, expected febrile neutropenia risk based on the myelotoxicity of the planned regimens as indicated in published trials. The tool is accessible online and provides real-time recommendations that are tailored based on disease- and patient-specific factors for either the use of CSF or not,” he elaborated.
According to the tool’s algorithm, use is recommended for patients who are given a regimen with a high risk of febrile neutropenia (greater than 20%) and is not recommended for those given a low-risk regimen (less than 10%). It is tailored according to the presence of additional risk factors for the intermediate-risk group.
Oncologists use the tool only for patients starting a new chemotherapy and only in the first cycle, when the risk of febrile neutropenia is highest, according to Dr. Agiro. “Once the approval is given in the first cycle, it remains in effect for the next 6 months, so they don’t have to use it again and again in additional cycles,” he explained.
The decision support tool was implemented in nine states starting in July 2014. In the study, which was funded by Anthem, the investigators analyzed administrative claims data from commercially insured adult patients starting chemotherapy for lung cancer, assessing changes in outcomes between a preimplementation period (April 2013 to Dec. 2013) and a postimplementation period (July 2014 to March 2015).
Analyses were based on 1,857 patients in the states that implemented the tool and 1,610 patients in the states that did not.
The percentage of patients receiving CSFs in the 6 months after starting chemotherapy fell in states that implemented the decision support tool (from 48.4% to 35.6%) but remained stable in states that did not (43.2% and 44.4%), Dr. Agiro reported. The adjusted difference in differences was –8.7% (P less than .001).
Meanwhile, the percentage of patients admitted for febrile neutropenia or experiencing this outcome while hospitalized increased in both states implementing the tool (from 2.8% to 4.3%) and those not implementing it (from 3.1% to 5.1%). Although the magnitude of increase was smaller in the former (+1.5% vs. +2.0%), the difference was not significant. Findings were essentially the same among the subset of patients aged 65 years and older.
“It’s important to study both intended and unintended consequences of such interventions,” Dr. Agiro noted. “Our study goes beyond financial considerations by looking at unintended outcomes: in this case, focusing on the incidence of febrile neutropenia, an outcome that is of prime interest to patients and oncologists and payers alike.”
The study may have missed some cases of febrile neutropenia, he acknowledged. “Also, there are other important outcomes of concern. For example, were there any delays in chemotherapy administration or immune recovery that could have been triggered by the implementation of the decision support program?”
The impact, both intended and unintended, on practices warrants evaluation as well, he further noted. “An important question could be, ‘Does it take less time to use this decision support tool compared to the time taken with normal care processes?’ ”
Dr. Agiro disclosed that he is employed by, has stock or other ownership interests in, and receives research funding from Anthem.
ORLANDO – A decision support tool safely reduces use of colony-stimulating factors (CSFs) in patients undergoing chemotherapy for lung cancer, suggests a retrospective claims-based cohort study of nearly 3,500 patients across the country.
The rate of CSF use fell among patients treated in the nine states that implemented the tool – a library of chemotherapy regimens and their expected FN risk that uses preauthorization and an algorithm to promote risk-appropriate, guideline-adherent use – but it remained unchanged in the 39 states and the District of Columbia, where usual practice continued, investigators reported at a symposium on quality care sponsored by the American Society of Clinical Oncology and simultaneously published (J Oncol Pract. 2017 March 4. doi: 10.1200/JOP.2017.020867). The adjusted difference was nearly 9%.
“Decision support programs like the one highlighted here could be one way, definitely not the only way, of achieving guideline-adherent CSF use and reducing practice variation across the country,” commented coinvestigator Abiy Agiro, PhD, associate director of payer and provider research at HealthCore, a subsidiary of Anthem, in Wilmington, Delaware.
“Such efforts could also have unintended consequences, so it’s important to study relevant patient outcomes,” he added. “In this case, although it appears that the incidence of febrile neutropenia rising does not seem to relate with the program, the study does not establish the safety of CSF use reduction in lung cancer patients receiving chemotherapy. So, we should take the results with that caveat.”
Parsing the findings
Although the United States makes up just 4% of the world’s population, it uses nearly 80% of CSFs sold by a leading manufacturer, according to invited discussant Thomas J. Smith, MD, a professor of oncology and palliative medicine at Johns Hopkins University in Baltimore.
“When we rewrote the ASCO [American Society of Clinical Oncology] guidelines on CSF use in 2015, there were some specific indications: dose-intense chemo for adjuvant breast cancer and uroepithelial cancer and ... when the risk of febrile neutropenia is about 20% and dose reduction is not an appropriate strategy. We were quick to point out that most regimens have a risk of febrile neutropenia much less than that,” he noted.
Dr. Agiro and his colleagues’ findings are valid, real, and reproducible, Dr. Smith maintained. However, it is unclear to what extent the observed levels of CSF use represented overuse.
“In lung cancer, there are very few regimens that have a febrile neutropenia rate close to 20%,” he elaborated. “What we don’t know is how much of this [use] was actually justified. I would suspect it is 10% or 15%, rather than 40%.”
CSF use, as guided by the new tool, “might not support increased dose density [of chemotherapy], but I would challenge anybody in the audience to show me data in normal solid tumor patients that [show that] dose density maintained by CSFs makes a difference in overall survival,” he said.
Questions yet to be addressed include the difficulty and cost of using the decision support tool and the possible negative impact on practices’ finances, according to Dr. Smith.
“When ESAs [erythropoiesis-stimulating agents] came off being used so much, some of my friends’ practices took a 15% to 20% drop in their revenue, and this is an important source of revenue for a lot of practices,” he explained. “So, I hope that when we take this revenue away, that we are cognizant of that and realize that it’s just another stress on practices, many of which are under significant stress already.”
Study details
An estimated 26% of uses of CSFs in patients with lung cancer are not in accordance with the ASCO practice guidelines, according to Dr. Agiro. “Such variations from recommendations are sometimes the reason why different stakeholders take actions” to improve care, such as ASCO’s Quality Oncology Practice Initiative (QOPI) and the American Board of Internal Medicine’s Choosing Wisely initiative (J Oncol Pract. 2015;11:338-43).
The decision support tool evaluated in the study uses preauthorization before delivery of care and, therefore, differs from point-of-care interventions, he noted.
“The tool allows access to a library of chemotherapy regimens and their associated, expected febrile neutropenia risk based on the myelotoxicity of the planned regimens as indicated in published trials. The tool is accessible online and provides real-time recommendations that are tailored based on disease- and patient-specific factors for either the use of CSF or not,” he elaborated.
According to the tool’s algorithm, use is recommended for patients who are given a regimen with a high risk of febrile neutropenia (greater than 20%) and is not recommended for those given a low-risk regimen (less than 10%). It is tailored according to the presence of additional risk factors for the intermediate-risk group.
Oncologists use the tool only for patients starting a new chemotherapy and only in the first cycle, when the risk of febrile neutropenia is highest, according to Dr. Agiro. “Once the approval is given in the first cycle, it remains in effect for the next 6 months, so they don’t have to use it again and again in additional cycles,” he explained.
The decision support tool was implemented in nine states starting in July 2014. In the study, which was funded by Anthem, the investigators analyzed administrative claims data from commercially insured adult patients starting chemotherapy for lung cancer, assessing changes in outcomes between a preimplementation period (April 2013 to Dec. 2013) and a postimplementation period (July 2014 to March 2015).
Analyses were based on 1,857 patients in the states that implemented the tool and 1,610 patients in the states that did not.
The percentage of patients receiving CSFs in the 6 months after starting chemotherapy fell in states that implemented the decision support tool (from 48.4% to 35.6%) but remained stable in states that did not (43.2% and 44.4%), Dr. Agiro reported. The adjusted difference in differences was –8.7% (P less than .001).
Meanwhile, the percentage of patients admitted for febrile neutropenia or experiencing this outcome while hospitalized increased in both states implementing the tool (from 2.8% to 4.3%) and those not implementing it (from 3.1% to 5.1%). Although the magnitude of increase was smaller in the former (+1.5% vs. +2.0%), the difference was not significant. Findings were essentially the same among the subset of patients aged 65 years and older.
“It’s important to study both intended and unintended consequences of such interventions,” Dr. Agiro noted. “Our study goes beyond financial considerations by looking at unintended outcomes: in this case, focusing on the incidence of febrile neutropenia, an outcome that is of prime interest to patients and oncologists and payers alike.”
The study may have missed some cases of febrile neutropenia, he acknowledged. “Also, there are other important outcomes of concern. For example, were there any delays in chemotherapy administration or immune recovery that could have been triggered by the implementation of the decision support program?”
The impact, both intended and unintended, on practices warrants evaluation as well, he further noted. “An important question could be, ‘Does it take less time to use this decision support tool compared to the time taken with normal care processes?’ ”
Dr. Agiro disclosed that he is employed by, has stock or other ownership interests in, and receives research funding from Anthem.
AT THE QUALITY CARE SYMPOSIUM
Key clinical point:
Major finding: The percentage of patients receiving CSFs fell in states that used the tool, versus those that did not (difference in differences, –8.7%), but changes in admissions for febrile neutropenia did not differ significantly.
Data source: A retrospective cohort study of 3,467 patients from 48 states starting chemotherapy for lung cancer.
Disclosures: Dr. Agiro disclosed that he is employed by, has stock or other ownership interests in, and receives research funding from Anthem. The study was funded by Anthem.