User login
PARP inhibitors: New developments in ovarian cancer treatment
Ovarian cancer remains the leading cause of death from gynecologic cancer worldwide and one of the five leading causes of death from cancer in women in the United States. In addition to surgery, treatment consists of combination platinum and taxane chemotherapy that offers a high response rate; however, a majority of women will develop persistent or recurrent disease.
A clinical practice statement released by the Society of Gynecologic Oncology in October 2014 states that “women diagnosed with epithelial ovarian, tubal, and peritoneal cancers should receive genetic counseling and be offered genetic testing, even in the absence of family history.” Patients should be informed that this genetic testing serves to prognosticate, inform about personal and familial cancer risk, but also aids in choices of novel therapeutic agents, specifically Poly (ADP-ribose) polymerase (PARP) inhibitors.
Genetic involvement of BRCA
A small proportion of ovarian cancers are attributable to genetic mutations, with approximately 10%-15% of cases caused by germline mutations of BRCA1 and BRCA2. BRCA1 deleterious mutations confer an ovarian cancer risk of approximately 39%-46%; and the risk of ovarian cancer is roughly 12%-20% for patients with BRCA2 deleterious mutations. As a tumor suppressor gene, BRCA is involved in the DNA repair process. Specifically, it is involved in homologous recombination (a form of double-stranded DNA repair mechanism). Thus, cells with defective BRCA proteins cannot repair double-stranded breaks (DSB) in DNA.
The homologous recombination pathway is complex and involves a number of genes. Deficiencies in this pathway confer a sensitivity to PARP inhibition. Tumors that share dysfunction in the homologous recombination pathway, but do not contain mutations in the BRCA gene, are classified as tumors with “BRCAness.”
Generally, the inheritance of a defective BRCA1 or BRCA2 allele (a germline mutation) alone is not enough to cause the development of cancer. Instead, once the second, functioning allele becomes nonfunctional, cancer can arise through an accumulation of mutations in the genetic code.
Furthermore, regardless of germline BRCA status, cancers have high rates of genetic mutation. As a result of the mutation rate, tumors can develop noninherited, noninheritable alterations in BRCA1 or BRCA2 genes (a somatic mutation).
Mechanism of PARP inhibitor activity
The PARP family of enzymes hold a vital role in the repair of DNA and the stabilization of the human genome through the repair of single-stranded breaks (SSB) in DNA. PARP inhibitors were originally developed as a chemosensitizing agent for other cytotoxic agents. It was only later discovered that ovarian cancer cells and mouse models that were deficient in BRCA proteins were especially sensitive to PARP inhibition. Eventually, the clinical development strategy became to employ PARP inhibitors in selected patients with BRCA mutations.
As previously mentioned, cells deficient in the tumor suppressor genes (BRCA1 and BRCA2) have an inability to repair DSBs. Inhibiting PARP enzymes will therefore cause an increase in SSB. During cell replication, these SSBs are converted to DSBs. Ultimately, the accumulation of DSBs leads to cell death. The concept that these two deficiencies – which alone are nonlethal – can be combined to induce cell death is described as synthetic lethality.
The exact mechanism through which PARP inhibitors function is not fully understood; however, four models currently exist to explain how PARP inhibitors instigate synthetic lethality. PARP inhibitors may block base excision repair mechanisms, trap PARP enzymes on damaged DNA, reduce the affinity of functioning BRCA enzymes to damaged DNA, and suppress nonhomologous end joining repair mechanisms.1 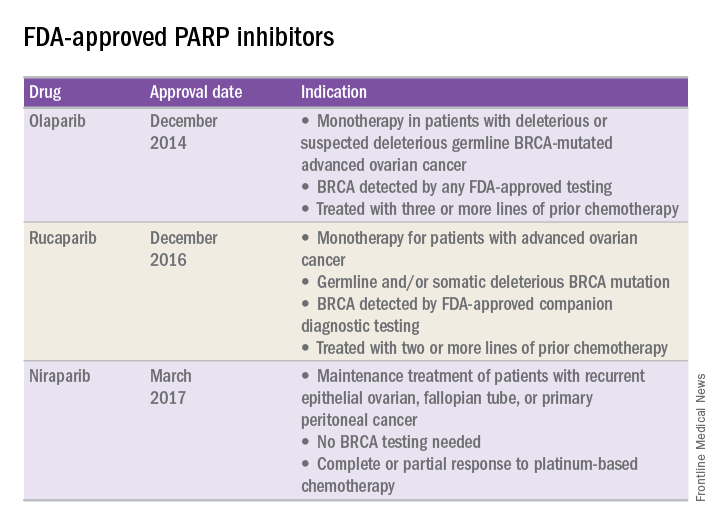
FDA approval of PARP inhibitors
In recent years, the Food and Drug Administration has approved three PARP inhibitors in the treatment of ovarian cancer in slightly different clinical scenarios.
Olaparib was tested in a trial of 193 patients who harbored a deleterious or suspected deleterious germline BRCA-associated ovarian cancer who had received prior therapies.2 Overall, the response rate in this population was 41% (95% confidence interval, 28-54) with a median duration of response of 8.0 months. These results led to the FDA approval of olaparib for ovarian cancer treatment as fourth-line therapy in patients with BRCA mutations.
Two separate trials using rucaparib showed an overall response rate of 54% and a duration of response of 9.2 months.3,4 These early results allowed the FDA to grant accelerated approval to another PARP inhibitor for use in ovarian cancer.
More recently, a phase III trial of niraparib maintenance therapy versus placebo enrolled 553 women with recurrent epithelial ovarian cancer.5 Women with germline BRCA mutations had recurrence-free intervals of 21 months on niraparib, compared with 5.5 months for those on placebo. Even without germline BRCA mutations, women benefited from a recurrence-free interval of 9.3 months, compared with 3.9 months for placebo.
PARP inhibitors represent a novel targeted therapy for ovarian cancer, particularly those with deleterious germline or somatic BRCA mutations. When combined with genetic testing for BRCA mutations, PARP inhibitors represent an example of a predictive biomarker paired with a tailored therapeutic. Maturing data from ongoing trials will likely expand the opportunity to use PARP inhibitors for the treatment of ovarian cancer.
References
1. Br J Cancer. 2016 Nov 8;115(10):1157-73.
2. J Clin Oncol. 2015 Jan 20;33(3):244-50.
3. Clin Cancer Res. 2017 Mar 6. pii: clincanres.2796.2016. doi: 10.1158/1078-0432.CCR-16-2796.
4. Lancet Oncol. 2017 Jan;18(1):75-87.
5. N Engl J Med 2016; 375:2154-64.
Dr. Tran is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
Ovarian cancer remains the leading cause of death from gynecologic cancer worldwide and one of the five leading causes of death from cancer in women in the United States. In addition to surgery, treatment consists of combination platinum and taxane chemotherapy that offers a high response rate; however, a majority of women will develop persistent or recurrent disease.
A clinical practice statement released by the Society of Gynecologic Oncology in October 2014 states that “women diagnosed with epithelial ovarian, tubal, and peritoneal cancers should receive genetic counseling and be offered genetic testing, even in the absence of family history.” Patients should be informed that this genetic testing serves to prognosticate, inform about personal and familial cancer risk, but also aids in choices of novel therapeutic agents, specifically Poly (ADP-ribose) polymerase (PARP) inhibitors.
Genetic involvement of BRCA
A small proportion of ovarian cancers are attributable to genetic mutations, with approximately 10%-15% of cases caused by germline mutations of BRCA1 and BRCA2. BRCA1 deleterious mutations confer an ovarian cancer risk of approximately 39%-46%; and the risk of ovarian cancer is roughly 12%-20% for patients with BRCA2 deleterious mutations. As a tumor suppressor gene, BRCA is involved in the DNA repair process. Specifically, it is involved in homologous recombination (a form of double-stranded DNA repair mechanism). Thus, cells with defective BRCA proteins cannot repair double-stranded breaks (DSB) in DNA.
The homologous recombination pathway is complex and involves a number of genes. Deficiencies in this pathway confer a sensitivity to PARP inhibition. Tumors that share dysfunction in the homologous recombination pathway, but do not contain mutations in the BRCA gene, are classified as tumors with “BRCAness.”
Generally, the inheritance of a defective BRCA1 or BRCA2 allele (a germline mutation) alone is not enough to cause the development of cancer. Instead, once the second, functioning allele becomes nonfunctional, cancer can arise through an accumulation of mutations in the genetic code.
Furthermore, regardless of germline BRCA status, cancers have high rates of genetic mutation. As a result of the mutation rate, tumors can develop noninherited, noninheritable alterations in BRCA1 or BRCA2 genes (a somatic mutation).
Mechanism of PARP inhibitor activity
The PARP family of enzymes hold a vital role in the repair of DNA and the stabilization of the human genome through the repair of single-stranded breaks (SSB) in DNA. PARP inhibitors were originally developed as a chemosensitizing agent for other cytotoxic agents. It was only later discovered that ovarian cancer cells and mouse models that were deficient in BRCA proteins were especially sensitive to PARP inhibition. Eventually, the clinical development strategy became to employ PARP inhibitors in selected patients with BRCA mutations.
As previously mentioned, cells deficient in the tumor suppressor genes (BRCA1 and BRCA2) have an inability to repair DSBs. Inhibiting PARP enzymes will therefore cause an increase in SSB. During cell replication, these SSBs are converted to DSBs. Ultimately, the accumulation of DSBs leads to cell death. The concept that these two deficiencies – which alone are nonlethal – can be combined to induce cell death is described as synthetic lethality.
The exact mechanism through which PARP inhibitors function is not fully understood; however, four models currently exist to explain how PARP inhibitors instigate synthetic lethality. PARP inhibitors may block base excision repair mechanisms, trap PARP enzymes on damaged DNA, reduce the affinity of functioning BRCA enzymes to damaged DNA, and suppress nonhomologous end joining repair mechanisms.1 
FDA approval of PARP inhibitors
In recent years, the Food and Drug Administration has approved three PARP inhibitors in the treatment of ovarian cancer in slightly different clinical scenarios.
Olaparib was tested in a trial of 193 patients who harbored a deleterious or suspected deleterious germline BRCA-associated ovarian cancer who had received prior therapies.2 Overall, the response rate in this population was 41% (95% confidence interval, 28-54) with a median duration of response of 8.0 months. These results led to the FDA approval of olaparib for ovarian cancer treatment as fourth-line therapy in patients with BRCA mutations.
Two separate trials using rucaparib showed an overall response rate of 54% and a duration of response of 9.2 months.3,4 These early results allowed the FDA to grant accelerated approval to another PARP inhibitor for use in ovarian cancer.
More recently, a phase III trial of niraparib maintenance therapy versus placebo enrolled 553 women with recurrent epithelial ovarian cancer.5 Women with germline BRCA mutations had recurrence-free intervals of 21 months on niraparib, compared with 5.5 months for those on placebo. Even without germline BRCA mutations, women benefited from a recurrence-free interval of 9.3 months, compared with 3.9 months for placebo.
PARP inhibitors represent a novel targeted therapy for ovarian cancer, particularly those with deleterious germline or somatic BRCA mutations. When combined with genetic testing for BRCA mutations, PARP inhibitors represent an example of a predictive biomarker paired with a tailored therapeutic. Maturing data from ongoing trials will likely expand the opportunity to use PARP inhibitors for the treatment of ovarian cancer.
References
1. Br J Cancer. 2016 Nov 8;115(10):1157-73.
2. J Clin Oncol. 2015 Jan 20;33(3):244-50.
3. Clin Cancer Res. 2017 Mar 6. pii: clincanres.2796.2016. doi: 10.1158/1078-0432.CCR-16-2796.
4. Lancet Oncol. 2017 Jan;18(1):75-87.
5. N Engl J Med 2016; 375:2154-64.
Dr. Tran is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
Ovarian cancer remains the leading cause of death from gynecologic cancer worldwide and one of the five leading causes of death from cancer in women in the United States. In addition to surgery, treatment consists of combination platinum and taxane chemotherapy that offers a high response rate; however, a majority of women will develop persistent or recurrent disease.
A clinical practice statement released by the Society of Gynecologic Oncology in October 2014 states that “women diagnosed with epithelial ovarian, tubal, and peritoneal cancers should receive genetic counseling and be offered genetic testing, even in the absence of family history.” Patients should be informed that this genetic testing serves to prognosticate, inform about personal and familial cancer risk, but also aids in choices of novel therapeutic agents, specifically Poly (ADP-ribose) polymerase (PARP) inhibitors.
Genetic involvement of BRCA
A small proportion of ovarian cancers are attributable to genetic mutations, with approximately 10%-15% of cases caused by germline mutations of BRCA1 and BRCA2. BRCA1 deleterious mutations confer an ovarian cancer risk of approximately 39%-46%; and the risk of ovarian cancer is roughly 12%-20% for patients with BRCA2 deleterious mutations. As a tumor suppressor gene, BRCA is involved in the DNA repair process. Specifically, it is involved in homologous recombination (a form of double-stranded DNA repair mechanism). Thus, cells with defective BRCA proteins cannot repair double-stranded breaks (DSB) in DNA.
The homologous recombination pathway is complex and involves a number of genes. Deficiencies in this pathway confer a sensitivity to PARP inhibition. Tumors that share dysfunction in the homologous recombination pathway, but do not contain mutations in the BRCA gene, are classified as tumors with “BRCAness.”
Generally, the inheritance of a defective BRCA1 or BRCA2 allele (a germline mutation) alone is not enough to cause the development of cancer. Instead, once the second, functioning allele becomes nonfunctional, cancer can arise through an accumulation of mutations in the genetic code.
Furthermore, regardless of germline BRCA status, cancers have high rates of genetic mutation. As a result of the mutation rate, tumors can develop noninherited, noninheritable alterations in BRCA1 or BRCA2 genes (a somatic mutation).
Mechanism of PARP inhibitor activity
The PARP family of enzymes hold a vital role in the repair of DNA and the stabilization of the human genome through the repair of single-stranded breaks (SSB) in DNA. PARP inhibitors were originally developed as a chemosensitizing agent for other cytotoxic agents. It was only later discovered that ovarian cancer cells and mouse models that were deficient in BRCA proteins were especially sensitive to PARP inhibition. Eventually, the clinical development strategy became to employ PARP inhibitors in selected patients with BRCA mutations.
As previously mentioned, cells deficient in the tumor suppressor genes (BRCA1 and BRCA2) have an inability to repair DSBs. Inhibiting PARP enzymes will therefore cause an increase in SSB. During cell replication, these SSBs are converted to DSBs. Ultimately, the accumulation of DSBs leads to cell death. The concept that these two deficiencies – which alone are nonlethal – can be combined to induce cell death is described as synthetic lethality.
The exact mechanism through which PARP inhibitors function is not fully understood; however, four models currently exist to explain how PARP inhibitors instigate synthetic lethality. PARP inhibitors may block base excision repair mechanisms, trap PARP enzymes on damaged DNA, reduce the affinity of functioning BRCA enzymes to damaged DNA, and suppress nonhomologous end joining repair mechanisms.1 
FDA approval of PARP inhibitors
In recent years, the Food and Drug Administration has approved three PARP inhibitors in the treatment of ovarian cancer in slightly different clinical scenarios.
Olaparib was tested in a trial of 193 patients who harbored a deleterious or suspected deleterious germline BRCA-associated ovarian cancer who had received prior therapies.2 Overall, the response rate in this population was 41% (95% confidence interval, 28-54) with a median duration of response of 8.0 months. These results led to the FDA approval of olaparib for ovarian cancer treatment as fourth-line therapy in patients with BRCA mutations.
Two separate trials using rucaparib showed an overall response rate of 54% and a duration of response of 9.2 months.3,4 These early results allowed the FDA to grant accelerated approval to another PARP inhibitor for use in ovarian cancer.
More recently, a phase III trial of niraparib maintenance therapy versus placebo enrolled 553 women with recurrent epithelial ovarian cancer.5 Women with germline BRCA mutations had recurrence-free intervals of 21 months on niraparib, compared with 5.5 months for those on placebo. Even without germline BRCA mutations, women benefited from a recurrence-free interval of 9.3 months, compared with 3.9 months for placebo.
PARP inhibitors represent a novel targeted therapy for ovarian cancer, particularly those with deleterious germline or somatic BRCA mutations. When combined with genetic testing for BRCA mutations, PARP inhibitors represent an example of a predictive biomarker paired with a tailored therapeutic. Maturing data from ongoing trials will likely expand the opportunity to use PARP inhibitors for the treatment of ovarian cancer.
References
1. Br J Cancer. 2016 Nov 8;115(10):1157-73.
2. J Clin Oncol. 2015 Jan 20;33(3):244-50.
3. Clin Cancer Res. 2017 Mar 6. pii: clincanres.2796.2016. doi: 10.1158/1078-0432.CCR-16-2796.
4. Lancet Oncol. 2017 Jan;18(1):75-87.
5. N Engl J Med 2016; 375:2154-64.
Dr. Tran is a gynecologic oncology fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC-Chapel Hill. They reported having no relevant financial disclosures.
Pelvic organ prolapse: Effective treatments
Editor’s Note: This is the fifth installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte. This month’s edition of the Board Corner focuses on pelvic organ prolapse.
The American College of Obstetricians and Gynecologists’ “Practice Bulletins” are important practice management guidelines for ob.gyn. clinicians. The Practice Bulletins are rich sources of material that is often tested on board exams. Earlier this year, ACOG released a revised Practice Bulletin (#176) updating its advice on the diagnosis and management of pelvic organ prolapse (POP).1 It is a well-written document summarizing most of the landmark articles published in the field of female pelvic medicine and reconstructive surgery. We recommend you read this bulletin and review this topic carefully.
Let’s begin with a possible medical board question: Which of the following procedures is the most effective for a sexually-active patient with advanced prolapse?
A. Sacrospinous ligament suspension (SSLS)
B. Uterosacral ligament suspension (USLS)
C. Sacrocolpopexy (SCP)
D. Colpocleisis
E. Hysteropexy
A randomized trial comparing SSLS and USLS found the two apical procedures with native tissue repair are equally effective with comparable functional and adverse outcomes (answers A and B are incorrect). However, randomized trials comparing SCP to SSLS show that SCP with synthetic mesh has the lowest recurrence rate for prolapse. Colpocleisis is done for patients who are not sexually active (answer D is incorrect). Hysteropexy is performed for patients who desire preservation of the uterus. There is less available evidence on safety and efficacy, compared with hysterectomy at the time of prolapse repair (answer E is incorrect)
Key points
The key points to remember are:
1. SCP is the most effective prolapse repair technique.
2. USLS and SSLS fixation are equally effective when compared with one another.
3. Colpocleisis is a highly successful procedure for POP in patients who are not sexually active.
Literature summary
The lifetime risk for undergoing surgery for POP or stress incontinence is 20%. POP is the descent of one or more aspects of the vagina or uterus, which allows nearby organs to herniate into the vagina. POP should only be treated if it is symptomatic and bothersome for the patient. The pessary is an alternative to surgical treatment of prolapse.
Proven risk factors for POP are increased parity, vaginal delivery, age, obesity, chronic constipation, and certain congenital anomalies. A history should be taken to elucidate symptoms of prolapse, such as bulge, pressure, sexual dysfunction, lower urinary tract dysfunction, or defecatory dysfunction. It is also important to find out how much the POP is affecting her quality of life. A physical exam is best performed with a split speculum, with bladder empty, while the patient performs a Valsalva maneuver. We recommend using the POP-Q system to grade the severity of prolapse. The tone of the pelvic floor muscle should also be evaluated (absent, weak, normal, or strong) during pelvic exam.
The minimum testing necessary for a patient with POP is urinalysis and a postvoid residual. A stress test with a full bladder should also be done with and without reduction of the prolapse. If you’re considering surgery and the patient has advanced prolapse and/or other complicating factors – such as obstructive symptoms or significant neurologic disorder – you should consider performing urodynamic testing as well.
Native tissue, suture-based reconstructive repairs of the vagina include apical procedures, such as SSLS and USLS, in addition to anterior colporrhaphy and posterior repair. At 2-year follow-up, SSLS and USLS along with anterior colporrhaphy and posterior repair are equally effective for treatment of prolapse with comparable functional and adverse outcomes. SCP is more effective than SSLS but the abdominal procedure (not laparoscopic) may be associated with more complications. Currently, there are no published randomized trials comparing minimally-invasive SCP to USLS, but one is underway (clinicaltrials.gov).
Other procedures for POP include obliterative procedures such as colpocleisis, which is highly effective for patients who do not desire future vaginal intercourse and also has low morbidity. Preservation of the uterus by hysteropexy procedures (either transvaginal or transabdominal) are also options for women desiring to preserve their uterus, but these procedures have little safety and efficacy data. Regardless of the procedure performed, routine intraoperative cystoscopy should be done to assure ureteral patency and to rule out injury to the lower urinary tract.
Some type of prophylactic anti-incontinence procedure – retropubic or Burch – may be done at the time of vaginal prolapse repair or abdominal prolapse repair, respectively, in order to reduce the chance of postoperative stress urinary incontinence in a patient without symptoms of stress incontinence. The exception to this is in a patient who has an elevated postvoid residual or someone with a prior anti-incontinence procedure without symptoms of stress urinary incontinence.
Practice tips
Finally, here are some precautions and words of advice about the following POP procedures:
- Neither synthetic nor biologic grafts should be used to augment posterior repairs as these do not improve outcomes.
- Transvaginal repair of rectocele is superior to the transanal repair techniques.
- Synthetic mesh augmentation of the anterior vaginal wall may improve anatomic outcomes, but this comes at a cost (more reoperations and higher rate of complications). Thus, surgeons performing these procedures should have specialized training and the patient should have a unique indication and must undergo proper consent as recommended by ACOG.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Reference
Editor’s Note: This is the fifth installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte. This month’s edition of the Board Corner focuses on pelvic organ prolapse.
The American College of Obstetricians and Gynecologists’ “Practice Bulletins” are important practice management guidelines for ob.gyn. clinicians. The Practice Bulletins are rich sources of material that is often tested on board exams. Earlier this year, ACOG released a revised Practice Bulletin (#176) updating its advice on the diagnosis and management of pelvic organ prolapse (POP).1 It is a well-written document summarizing most of the landmark articles published in the field of female pelvic medicine and reconstructive surgery. We recommend you read this bulletin and review this topic carefully.
Let’s begin with a possible medical board question: Which of the following procedures is the most effective for a sexually-active patient with advanced prolapse?
A. Sacrospinous ligament suspension (SSLS)
B. Uterosacral ligament suspension (USLS)
C. Sacrocolpopexy (SCP)
D. Colpocleisis
E. Hysteropexy
A randomized trial comparing SSLS and USLS found the two apical procedures with native tissue repair are equally effective with comparable functional and adverse outcomes (answers A and B are incorrect). However, randomized trials comparing SCP to SSLS show that SCP with synthetic mesh has the lowest recurrence rate for prolapse. Colpocleisis is done for patients who are not sexually active (answer D is incorrect). Hysteropexy is performed for patients who desire preservation of the uterus. There is less available evidence on safety and efficacy, compared with hysterectomy at the time of prolapse repair (answer E is incorrect)
Key points
The key points to remember are:
1. SCP is the most effective prolapse repair technique.
2. USLS and SSLS fixation are equally effective when compared with one another.
3. Colpocleisis is a highly successful procedure for POP in patients who are not sexually active.
Literature summary
The lifetime risk for undergoing surgery for POP or stress incontinence is 20%. POP is the descent of one or more aspects of the vagina or uterus, which allows nearby organs to herniate into the vagina. POP should only be treated if it is symptomatic and bothersome for the patient. The pessary is an alternative to surgical treatment of prolapse.
Proven risk factors for POP are increased parity, vaginal delivery, age, obesity, chronic constipation, and certain congenital anomalies. A history should be taken to elucidate symptoms of prolapse, such as bulge, pressure, sexual dysfunction, lower urinary tract dysfunction, or defecatory dysfunction. It is also important to find out how much the POP is affecting her quality of life. A physical exam is best performed with a split speculum, with bladder empty, while the patient performs a Valsalva maneuver. We recommend using the POP-Q system to grade the severity of prolapse. The tone of the pelvic floor muscle should also be evaluated (absent, weak, normal, or strong) during pelvic exam.
The minimum testing necessary for a patient with POP is urinalysis and a postvoid residual. A stress test with a full bladder should also be done with and without reduction of the prolapse. If you’re considering surgery and the patient has advanced prolapse and/or other complicating factors – such as obstructive symptoms or significant neurologic disorder – you should consider performing urodynamic testing as well.
Native tissue, suture-based reconstructive repairs of the vagina include apical procedures, such as SSLS and USLS, in addition to anterior colporrhaphy and posterior repair. At 2-year follow-up, SSLS and USLS along with anterior colporrhaphy and posterior repair are equally effective for treatment of prolapse with comparable functional and adverse outcomes. SCP is more effective than SSLS but the abdominal procedure (not laparoscopic) may be associated with more complications. Currently, there are no published randomized trials comparing minimally-invasive SCP to USLS, but one is underway (clinicaltrials.gov).
Other procedures for POP include obliterative procedures such as colpocleisis, which is highly effective for patients who do not desire future vaginal intercourse and also has low morbidity. Preservation of the uterus by hysteropexy procedures (either transvaginal or transabdominal) are also options for women desiring to preserve their uterus, but these procedures have little safety and efficacy data. Regardless of the procedure performed, routine intraoperative cystoscopy should be done to assure ureteral patency and to rule out injury to the lower urinary tract.
Some type of prophylactic anti-incontinence procedure – retropubic or Burch – may be done at the time of vaginal prolapse repair or abdominal prolapse repair, respectively, in order to reduce the chance of postoperative stress urinary incontinence in a patient without symptoms of stress incontinence. The exception to this is in a patient who has an elevated postvoid residual or someone with a prior anti-incontinence procedure without symptoms of stress urinary incontinence.
Practice tips
Finally, here are some precautions and words of advice about the following POP procedures:
- Neither synthetic nor biologic grafts should be used to augment posterior repairs as these do not improve outcomes.
- Transvaginal repair of rectocele is superior to the transanal repair techniques.
- Synthetic mesh augmentation of the anterior vaginal wall may improve anatomic outcomes, but this comes at a cost (more reoperations and higher rate of complications). Thus, surgeons performing these procedures should have specialized training and the patient should have a unique indication and must undergo proper consent as recommended by ACOG.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Reference
Editor’s Note: This is the fifth installment of a six-part series that will review key concepts and articles that ob.gyns. can use to prepare for the American Board of Obstetrics and Gynecology Maintenance of Certification examination. The series is adapted from Ob/Gyn Board Master (obgynboardmaster.com), an online board review course created by Erudyte. This month’s edition of the Board Corner focuses on pelvic organ prolapse.
The American College of Obstetricians and Gynecologists’ “Practice Bulletins” are important practice management guidelines for ob.gyn. clinicians. The Practice Bulletins are rich sources of material that is often tested on board exams. Earlier this year, ACOG released a revised Practice Bulletin (#176) updating its advice on the diagnosis and management of pelvic organ prolapse (POP).1 It is a well-written document summarizing most of the landmark articles published in the field of female pelvic medicine and reconstructive surgery. We recommend you read this bulletin and review this topic carefully.
Let’s begin with a possible medical board question: Which of the following procedures is the most effective for a sexually-active patient with advanced prolapse?
A. Sacrospinous ligament suspension (SSLS)
B. Uterosacral ligament suspension (USLS)
C. Sacrocolpopexy (SCP)
D. Colpocleisis
E. Hysteropexy
A randomized trial comparing SSLS and USLS found the two apical procedures with native tissue repair are equally effective with comparable functional and adverse outcomes (answers A and B are incorrect). However, randomized trials comparing SCP to SSLS show that SCP with synthetic mesh has the lowest recurrence rate for prolapse. Colpocleisis is done for patients who are not sexually active (answer D is incorrect). Hysteropexy is performed for patients who desire preservation of the uterus. There is less available evidence on safety and efficacy, compared with hysterectomy at the time of prolapse repair (answer E is incorrect)
Key points
The key points to remember are:
1. SCP is the most effective prolapse repair technique.
2. USLS and SSLS fixation are equally effective when compared with one another.
3. Colpocleisis is a highly successful procedure for POP in patients who are not sexually active.
Literature summary
The lifetime risk for undergoing surgery for POP or stress incontinence is 20%. POP is the descent of one or more aspects of the vagina or uterus, which allows nearby organs to herniate into the vagina. POP should only be treated if it is symptomatic and bothersome for the patient. The pessary is an alternative to surgical treatment of prolapse.
Proven risk factors for POP are increased parity, vaginal delivery, age, obesity, chronic constipation, and certain congenital anomalies. A history should be taken to elucidate symptoms of prolapse, such as bulge, pressure, sexual dysfunction, lower urinary tract dysfunction, or defecatory dysfunction. It is also important to find out how much the POP is affecting her quality of life. A physical exam is best performed with a split speculum, with bladder empty, while the patient performs a Valsalva maneuver. We recommend using the POP-Q system to grade the severity of prolapse. The tone of the pelvic floor muscle should also be evaluated (absent, weak, normal, or strong) during pelvic exam.
The minimum testing necessary for a patient with POP is urinalysis and a postvoid residual. A stress test with a full bladder should also be done with and without reduction of the prolapse. If you’re considering surgery and the patient has advanced prolapse and/or other complicating factors – such as obstructive symptoms or significant neurologic disorder – you should consider performing urodynamic testing as well.
Native tissue, suture-based reconstructive repairs of the vagina include apical procedures, such as SSLS and USLS, in addition to anterior colporrhaphy and posterior repair. At 2-year follow-up, SSLS and USLS along with anterior colporrhaphy and posterior repair are equally effective for treatment of prolapse with comparable functional and adverse outcomes. SCP is more effective than SSLS but the abdominal procedure (not laparoscopic) may be associated with more complications. Currently, there are no published randomized trials comparing minimally-invasive SCP to USLS, but one is underway (clinicaltrials.gov).
Other procedures for POP include obliterative procedures such as colpocleisis, which is highly effective for patients who do not desire future vaginal intercourse and also has low morbidity. Preservation of the uterus by hysteropexy procedures (either transvaginal or transabdominal) are also options for women desiring to preserve their uterus, but these procedures have little safety and efficacy data. Regardless of the procedure performed, routine intraoperative cystoscopy should be done to assure ureteral patency and to rule out injury to the lower urinary tract.
Some type of prophylactic anti-incontinence procedure – retropubic or Burch – may be done at the time of vaginal prolapse repair or abdominal prolapse repair, respectively, in order to reduce the chance of postoperative stress urinary incontinence in a patient without symptoms of stress incontinence. The exception to this is in a patient who has an elevated postvoid residual or someone with a prior anti-incontinence procedure without symptoms of stress urinary incontinence.
Practice tips
Finally, here are some precautions and words of advice about the following POP procedures:
- Neither synthetic nor biologic grafts should be used to augment posterior repairs as these do not improve outcomes.
- Transvaginal repair of rectocele is superior to the transanal repair techniques.
- Synthetic mesh augmentation of the anterior vaginal wall may improve anatomic outcomes, but this comes at a cost (more reoperations and higher rate of complications). Thus, surgeons performing these procedures should have specialized training and the patient should have a unique indication and must undergo proper consent as recommended by ACOG.
Dr. Siddighi is editor-in-chief of the Ob/Gyn Board Master and director of female pelvic medicine and reconstructive surgery and director of grand rounds at Loma Linda University Health in California. Ob.Gyn. News and Ob/Gyn Board Master are owned by the same parent company, Frontline Medical Communications.
Reference
Disease-Modifying Drug Treatment Before, During, and After Pregnancy in Women With MS
Treatment Before, During, and After Pregnancy
To evaluate treatment patterns before, during, and after pregnancy in women with MS and a live birth, Dr. Houtchens and colleagues used a US administrative claims database to conduct a retrospective analysis of women ages 18 to 65 with MS, a claim indicative of a live birth, and one-year continuous eligibility before and after pregnancy in the IMS Health Real World Data Adjudicated Claims US database from January 1, 2006, to June 30, 2015. Disease-modifying drug treatment was evaluated during the year prior to pregnancy (at three-month intervals), the three trimesters of pregnancy, puerperium (six weeks post-pregnancy), and one year post pregnancy (seven to 12 weeks post pregnancy and three to six, six to nine, and nine to 12 months post pregnancy). The researchers evaluated the proportion of women exposed to disease-modifying drug treatment during the 12 time periods. Results were also stratified by the number of relapses women experienced in the year prior to pregnancy.
Of 190,475 women with MS, 2,158 met eligibility criteria. Mean age was 30.26. Most women had commercial health insurance (98%) and were from the Midwest (32%), South (30%), or Northeast (29%) regions of the US.
The proportion of women with MS and a live birth treated with any disease-modifying drug was 20.48% at nine to 12 months pre-pregnancy, 21.46% at six to nine months pre-pregnancy, 20.62% at three to six months pre-pregnancy, and 17.75% at three months pre-pregnancy. During pregnancy, the proportion of women treated with a disease-modifying drug decreased to 12.05% during the first trimester and 1.90% during the second trimester, and then increased slightly to 2.97% during the third trimester. The proportion of women treated with disease-modifying drugs increased to 8.34% during puerperium, 12.93% during seven to 12 weeks post partum, 21.97% during three to six months post partum, 24.47% during six to nine months post partum, and 25.49% during nine to 12 months post partum. The majority of women (81.9%) had received disease-modifying drug treatment by six to nine months post partum. The proportion of women with disease-modifying drug treatment before and after pregnancy increased numerically with the number of relapses experienced before pregnancy.
Treatment After a Live Birth
In a separate analysis using the same cohort, Dr. Houtchens and colleagues looked closer at the time to initiation of disease-modifying drug treatment after a live birth in women with MS. Of the 2,094 women included in this analysis, the proportion with a live birth initiating a disease-modifying drug treatment within one year was 28.46%, and the proportion with no disease-modifying treatment within one year was 71.54%.
For those initiating a disease-modifying treatment within one year, mean time from live birth to first treatment was 118.98 days, and median time to first treatment was 93.50 days. A total of 16.11% received a disease-modifying drug less than 30 days after live birth, approximately half initiated a treatment within 90 days (47.82%), and three-quarters initiated a disease-modifying drug within six months (75.5%). The proportion of patients initiating treatment within one year after live birth increased with higher numbers of pre-pregnancy relapses (zero relapses, n = 441, 24.53%; one relapse, n = 108, 50.94%; two relapses, n = 33, 54.10%; three or more relapses, n = 14, 60.87%). The mean number of days until disease-modifying drug initiation for those receiving treatment within one year who had zero pre-pregnancy relapses was 123.57 (median, 99); one relapse, 107.95 (median, 80); two relapses, 120.76 (median, 98); and three or more relapses, 55.57 (median, 49.5). Patients who received disease-modifying drug treatment one year pre-pregnancy were more likely to receive treatment within one year after delivery, compared with patients without exposure to treatment in the year before pregnancy (72.58% vs 12.44%).
This study was supported by EMD Serono.
Treatment Before, During, and After Pregnancy
To evaluate treatment patterns before, during, and after pregnancy in women with MS and a live birth, Dr. Houtchens and colleagues used a US administrative claims database to conduct a retrospective analysis of women ages 18 to 65 with MS, a claim indicative of a live birth, and one-year continuous eligibility before and after pregnancy in the IMS Health Real World Data Adjudicated Claims US database from January 1, 2006, to June 30, 2015. Disease-modifying drug treatment was evaluated during the year prior to pregnancy (at three-month intervals), the three trimesters of pregnancy, puerperium (six weeks post-pregnancy), and one year post pregnancy (seven to 12 weeks post pregnancy and three to six, six to nine, and nine to 12 months post pregnancy). The researchers evaluated the proportion of women exposed to disease-modifying drug treatment during the 12 time periods. Results were also stratified by the number of relapses women experienced in the year prior to pregnancy.
Of 190,475 women with MS, 2,158 met eligibility criteria. Mean age was 30.26. Most women had commercial health insurance (98%) and were from the Midwest (32%), South (30%), or Northeast (29%) regions of the US.
The proportion of women with MS and a live birth treated with any disease-modifying drug was 20.48% at nine to 12 months pre-pregnancy, 21.46% at six to nine months pre-pregnancy, 20.62% at three to six months pre-pregnancy, and 17.75% at three months pre-pregnancy. During pregnancy, the proportion of women treated with a disease-modifying drug decreased to 12.05% during the first trimester and 1.90% during the second trimester, and then increased slightly to 2.97% during the third trimester. The proportion of women treated with disease-modifying drugs increased to 8.34% during puerperium, 12.93% during seven to 12 weeks post partum, 21.97% during three to six months post partum, 24.47% during six to nine months post partum, and 25.49% during nine to 12 months post partum. The majority of women (81.9%) had received disease-modifying drug treatment by six to nine months post partum. The proportion of women with disease-modifying drug treatment before and after pregnancy increased numerically with the number of relapses experienced before pregnancy.
Treatment After a Live Birth
In a separate analysis using the same cohort, Dr. Houtchens and colleagues looked closer at the time to initiation of disease-modifying drug treatment after a live birth in women with MS. Of the 2,094 women included in this analysis, the proportion with a live birth initiating a disease-modifying drug treatment within one year was 28.46%, and the proportion with no disease-modifying treatment within one year was 71.54%.
For those initiating a disease-modifying treatment within one year, mean time from live birth to first treatment was 118.98 days, and median time to first treatment was 93.50 days. A total of 16.11% received a disease-modifying drug less than 30 days after live birth, approximately half initiated a treatment within 90 days (47.82%), and three-quarters initiated a disease-modifying drug within six months (75.5%). The proportion of patients initiating treatment within one year after live birth increased with higher numbers of pre-pregnancy relapses (zero relapses, n = 441, 24.53%; one relapse, n = 108, 50.94%; two relapses, n = 33, 54.10%; three or more relapses, n = 14, 60.87%). The mean number of days until disease-modifying drug initiation for those receiving treatment within one year who had zero pre-pregnancy relapses was 123.57 (median, 99); one relapse, 107.95 (median, 80); two relapses, 120.76 (median, 98); and three or more relapses, 55.57 (median, 49.5). Patients who received disease-modifying drug treatment one year pre-pregnancy were more likely to receive treatment within one year after delivery, compared with patients without exposure to treatment in the year before pregnancy (72.58% vs 12.44%).
This study was supported by EMD Serono.
Treatment Before, During, and After Pregnancy
To evaluate treatment patterns before, during, and after pregnancy in women with MS and a live birth, Dr. Houtchens and colleagues used a US administrative claims database to conduct a retrospective analysis of women ages 18 to 65 with MS, a claim indicative of a live birth, and one-year continuous eligibility before and after pregnancy in the IMS Health Real World Data Adjudicated Claims US database from January 1, 2006, to June 30, 2015. Disease-modifying drug treatment was evaluated during the year prior to pregnancy (at three-month intervals), the three trimesters of pregnancy, puerperium (six weeks post-pregnancy), and one year post pregnancy (seven to 12 weeks post pregnancy and three to six, six to nine, and nine to 12 months post pregnancy). The researchers evaluated the proportion of women exposed to disease-modifying drug treatment during the 12 time periods. Results were also stratified by the number of relapses women experienced in the year prior to pregnancy.
Of 190,475 women with MS, 2,158 met eligibility criteria. Mean age was 30.26. Most women had commercial health insurance (98%) and were from the Midwest (32%), South (30%), or Northeast (29%) regions of the US.
The proportion of women with MS and a live birth treated with any disease-modifying drug was 20.48% at nine to 12 months pre-pregnancy, 21.46% at six to nine months pre-pregnancy, 20.62% at three to six months pre-pregnancy, and 17.75% at three months pre-pregnancy. During pregnancy, the proportion of women treated with a disease-modifying drug decreased to 12.05% during the first trimester and 1.90% during the second trimester, and then increased slightly to 2.97% during the third trimester. The proportion of women treated with disease-modifying drugs increased to 8.34% during puerperium, 12.93% during seven to 12 weeks post partum, 21.97% during three to six months post partum, 24.47% during six to nine months post partum, and 25.49% during nine to 12 months post partum. The majority of women (81.9%) had received disease-modifying drug treatment by six to nine months post partum. The proportion of women with disease-modifying drug treatment before and after pregnancy increased numerically with the number of relapses experienced before pregnancy.
Treatment After a Live Birth
In a separate analysis using the same cohort, Dr. Houtchens and colleagues looked closer at the time to initiation of disease-modifying drug treatment after a live birth in women with MS. Of the 2,094 women included in this analysis, the proportion with a live birth initiating a disease-modifying drug treatment within one year was 28.46%, and the proportion with no disease-modifying treatment within one year was 71.54%.
For those initiating a disease-modifying treatment within one year, mean time from live birth to first treatment was 118.98 days, and median time to first treatment was 93.50 days. A total of 16.11% received a disease-modifying drug less than 30 days after live birth, approximately half initiated a treatment within 90 days (47.82%), and three-quarters initiated a disease-modifying drug within six months (75.5%). The proportion of patients initiating treatment within one year after live birth increased with higher numbers of pre-pregnancy relapses (zero relapses, n = 441, 24.53%; one relapse, n = 108, 50.94%; two relapses, n = 33, 54.10%; three or more relapses, n = 14, 60.87%). The mean number of days until disease-modifying drug initiation for those receiving treatment within one year who had zero pre-pregnancy relapses was 123.57 (median, 99); one relapse, 107.95 (median, 80); two relapses, 120.76 (median, 98); and three or more relapses, 55.57 (median, 49.5). Patients who received disease-modifying drug treatment one year pre-pregnancy were more likely to receive treatment within one year after delivery, compared with patients without exposure to treatment in the year before pregnancy (72.58% vs 12.44%).
This study was supported by EMD Serono.
CAR T cells elicit durable, potent responses in kids with EM relapse of ALL
CHICAGO—Outcomes for pediatric patients with relapsed acute lymphoblastic leukemia (ALL) are dismal, with the probability of event-free survival ranging from 15% to 70% after a first relapse to 15% to 20% after a second relapse.
“So novel therapies are obviously urgently needed,” Mala Kiran Talekar, MD, of the Children's Hospital of Philadelphia in Pennsylvania, affirmed. “And herein comes the role of CAR T cells as a breakthrough therapy for relapsed/refractory pediatric ALL.”
She presented the outcome of chimeric antigen receptor (CAR) T-cell therapy in pediatric patients with non-CNS extramedullary (EM) relapse at the ASCO 2017 Annual meeting as abstract 10507.
The investigators had drawn the patient population for this analysis from 2 CAR studies, CTL019 and CTL119.
CTL019, which had already been completed, employed a murine CAR, and CTL119 is ongoing and uses a humanized CAR.
Of the 60 patients enrolled in CTL019, 56 (93%) achieved a complete response (CR) at day 28, and 100% had a CNS remission. Their 12-month overall survival (OS) was 79%.
“[K]eep in mind, when the study first started,” Dr Talekar said, “the patient population that had been referred to us was patients who had suffered a second or greater relapse or had been refractory to forms of treatment available to them, and the majority had been refractory to multiple therapies.”
The humanized CAR study, CTL119, is divided into 2 cohorts—one with CAR-naïve patients (n=22) and the other a CAR-retreatment arm (n=15) with patients who had received previous CAR therapy and relapsed.
Dr Talekar explained that the humanized CAR was made with the intention of decreasing rejection or loss of persistence of the T cells related to murine antigenicity.
Nine patients (60%) in the CAR-retreatment arm achieved a CR at day 28, and at 6 months, 78% experienced relapse-free survival (RFS) with a median follow-up of 12 months.
All of the CAR-naïve patients achieved CR at day 28, with 86% achieving RFS at 6 months, with a median follow-up of 10 months.
ALL with EM involvement
The investigators identified 10 pediatric patients treated in the murine (n=6) or humanized (n=4) trials who had received CAR therapy for isolated extramedullary disease or for combined bone marrow extramedullary (BM/EM) relapse of ALL.
They defined EM relapse as involvement of a non-CNS site confirmed by imaging with or without pathology within 12 months of CAR T-cell infusion. After infusion, patients had diagnostic imaging performed at 1, 3, 6, 9, and 12 months.
Of the 10 patients, 5 had active EM involvement at the time of infusion, 2 had isolated EM relapse—1 with parotid and multifocal bony lesions and 1 with testis and sinus lesions—and 5 had multiple sites of EM relapse.
The patients had 2 to 4 prior ALL relapses, 2 had prior local radiation to the EM site, and all 10 had received prior bone marrow transplants.
Three patients had an MLL rearrangement, 1 had hypodiploid ALL, and 1 had trisomy 21.
Nine of the 10 patients achieved MRD-negative CR at day 28.
One patient was not evaluable because his disease progressed within 2 weeks of CAR therapy in both the bone marrow and EM site. He died 6 weeks after the infusion.
Five patients evaluated by serial imaging had objective responses. Two had no evidence of EM disease by day 28, 2 had resolution by 3 months, and 1 had continued decrease in the size of her uterine mass at 3 and 6 months. She underwent hysterectomy at 8 months with no evidence of disease on pathology.
Four patients with a prior history of skin or testicular involvement had no evidence of disease by exam at day 28.
Three of the 9 patients relapsed with CD19+ disease. One had skin/medullary involvement and died at 38 months after CAR T-cell infusion. And 2 had medullary disease: 1 died at 17 months and 1 is alive at 28 months.
The remaining 6 patients are alive and well at a median follow-up of 10 months (range, 3 – 16 months) without recurrence of disease.
The investigators therefore concluded that single agent CAR T-cell immunotherapy can induce potent and durable response in patients with EM relapse of their ALL. ![]()
CHICAGO—Outcomes for pediatric patients with relapsed acute lymphoblastic leukemia (ALL) are dismal, with the probability of event-free survival ranging from 15% to 70% after a first relapse to 15% to 20% after a second relapse.
“So novel therapies are obviously urgently needed,” Mala Kiran Talekar, MD, of the Children's Hospital of Philadelphia in Pennsylvania, affirmed. “And herein comes the role of CAR T cells as a breakthrough therapy for relapsed/refractory pediatric ALL.”
She presented the outcome of chimeric antigen receptor (CAR) T-cell therapy in pediatric patients with non-CNS extramedullary (EM) relapse at the ASCO 2017 Annual meeting as abstract 10507.
The investigators had drawn the patient population for this analysis from 2 CAR studies, CTL019 and CTL119.
CTL019, which had already been completed, employed a murine CAR, and CTL119 is ongoing and uses a humanized CAR.
Of the 60 patients enrolled in CTL019, 56 (93%) achieved a complete response (CR) at day 28, and 100% had a CNS remission. Their 12-month overall survival (OS) was 79%.
“[K]eep in mind, when the study first started,” Dr Talekar said, “the patient population that had been referred to us was patients who had suffered a second or greater relapse or had been refractory to forms of treatment available to them, and the majority had been refractory to multiple therapies.”
The humanized CAR study, CTL119, is divided into 2 cohorts—one with CAR-naïve patients (n=22) and the other a CAR-retreatment arm (n=15) with patients who had received previous CAR therapy and relapsed.
Dr Talekar explained that the humanized CAR was made with the intention of decreasing rejection or loss of persistence of the T cells related to murine antigenicity.
Nine patients (60%) in the CAR-retreatment arm achieved a CR at day 28, and at 6 months, 78% experienced relapse-free survival (RFS) with a median follow-up of 12 months.
All of the CAR-naïve patients achieved CR at day 28, with 86% achieving RFS at 6 months, with a median follow-up of 10 months.
ALL with EM involvement
The investigators identified 10 pediatric patients treated in the murine (n=6) or humanized (n=4) trials who had received CAR therapy for isolated extramedullary disease or for combined bone marrow extramedullary (BM/EM) relapse of ALL.
They defined EM relapse as involvement of a non-CNS site confirmed by imaging with or without pathology within 12 months of CAR T-cell infusion. After infusion, patients had diagnostic imaging performed at 1, 3, 6, 9, and 12 months.
Of the 10 patients, 5 had active EM involvement at the time of infusion, 2 had isolated EM relapse—1 with parotid and multifocal bony lesions and 1 with testis and sinus lesions—and 5 had multiple sites of EM relapse.
The patients had 2 to 4 prior ALL relapses, 2 had prior local radiation to the EM site, and all 10 had received prior bone marrow transplants.
Three patients had an MLL rearrangement, 1 had hypodiploid ALL, and 1 had trisomy 21.
Nine of the 10 patients achieved MRD-negative CR at day 28.
One patient was not evaluable because his disease progressed within 2 weeks of CAR therapy in both the bone marrow and EM site. He died 6 weeks after the infusion.
Five patients evaluated by serial imaging had objective responses. Two had no evidence of EM disease by day 28, 2 had resolution by 3 months, and 1 had continued decrease in the size of her uterine mass at 3 and 6 months. She underwent hysterectomy at 8 months with no evidence of disease on pathology.
Four patients with a prior history of skin or testicular involvement had no evidence of disease by exam at day 28.
Three of the 9 patients relapsed with CD19+ disease. One had skin/medullary involvement and died at 38 months after CAR T-cell infusion. And 2 had medullary disease: 1 died at 17 months and 1 is alive at 28 months.
The remaining 6 patients are alive and well at a median follow-up of 10 months (range, 3 – 16 months) without recurrence of disease.
The investigators therefore concluded that single agent CAR T-cell immunotherapy can induce potent and durable response in patients with EM relapse of their ALL. ![]()
CHICAGO—Outcomes for pediatric patients with relapsed acute lymphoblastic leukemia (ALL) are dismal, with the probability of event-free survival ranging from 15% to 70% after a first relapse to 15% to 20% after a second relapse.
“So novel therapies are obviously urgently needed,” Mala Kiran Talekar, MD, of the Children's Hospital of Philadelphia in Pennsylvania, affirmed. “And herein comes the role of CAR T cells as a breakthrough therapy for relapsed/refractory pediatric ALL.”
She presented the outcome of chimeric antigen receptor (CAR) T-cell therapy in pediatric patients with non-CNS extramedullary (EM) relapse at the ASCO 2017 Annual meeting as abstract 10507.
The investigators had drawn the patient population for this analysis from 2 CAR studies, CTL019 and CTL119.
CTL019, which had already been completed, employed a murine CAR, and CTL119 is ongoing and uses a humanized CAR.
Of the 60 patients enrolled in CTL019, 56 (93%) achieved a complete response (CR) at day 28, and 100% had a CNS remission. Their 12-month overall survival (OS) was 79%.
“[K]eep in mind, when the study first started,” Dr Talekar said, “the patient population that had been referred to us was patients who had suffered a second or greater relapse or had been refractory to forms of treatment available to them, and the majority had been refractory to multiple therapies.”
The humanized CAR study, CTL119, is divided into 2 cohorts—one with CAR-naïve patients (n=22) and the other a CAR-retreatment arm (n=15) with patients who had received previous CAR therapy and relapsed.
Dr Talekar explained that the humanized CAR was made with the intention of decreasing rejection or loss of persistence of the T cells related to murine antigenicity.
Nine patients (60%) in the CAR-retreatment arm achieved a CR at day 28, and at 6 months, 78% experienced relapse-free survival (RFS) with a median follow-up of 12 months.
All of the CAR-naïve patients achieved CR at day 28, with 86% achieving RFS at 6 months, with a median follow-up of 10 months.
ALL with EM involvement
The investigators identified 10 pediatric patients treated in the murine (n=6) or humanized (n=4) trials who had received CAR therapy for isolated extramedullary disease or for combined bone marrow extramedullary (BM/EM) relapse of ALL.
They defined EM relapse as involvement of a non-CNS site confirmed by imaging with or without pathology within 12 months of CAR T-cell infusion. After infusion, patients had diagnostic imaging performed at 1, 3, 6, 9, and 12 months.
Of the 10 patients, 5 had active EM involvement at the time of infusion, 2 had isolated EM relapse—1 with parotid and multifocal bony lesions and 1 with testis and sinus lesions—and 5 had multiple sites of EM relapse.
The patients had 2 to 4 prior ALL relapses, 2 had prior local radiation to the EM site, and all 10 had received prior bone marrow transplants.
Three patients had an MLL rearrangement, 1 had hypodiploid ALL, and 1 had trisomy 21.
Nine of the 10 patients achieved MRD-negative CR at day 28.
One patient was not evaluable because his disease progressed within 2 weeks of CAR therapy in both the bone marrow and EM site. He died 6 weeks after the infusion.
Five patients evaluated by serial imaging had objective responses. Two had no evidence of EM disease by day 28, 2 had resolution by 3 months, and 1 had continued decrease in the size of her uterine mass at 3 and 6 months. She underwent hysterectomy at 8 months with no evidence of disease on pathology.
Four patients with a prior history of skin or testicular involvement had no evidence of disease by exam at day 28.
Three of the 9 patients relapsed with CD19+ disease. One had skin/medullary involvement and died at 38 months after CAR T-cell infusion. And 2 had medullary disease: 1 died at 17 months and 1 is alive at 28 months.
The remaining 6 patients are alive and well at a median follow-up of 10 months (range, 3 – 16 months) without recurrence of disease.
The investigators therefore concluded that single agent CAR T-cell immunotherapy can induce potent and durable response in patients with EM relapse of their ALL. ![]()
Azacitidine alone comparable to AZA combos for most MDS patients
A 3-arm phase 2 study of azacitidine alone or in combination with lenalidomide or vorinostat in patients with higher-risk myelodysplastic syndromes (MDS) or chronic myelomonocytic leukemia (CMML) has shown the combination therapies to have similar overall response rates (ORR) to azacitidine monotherapy. Based on these findings, investigators did not choose either combination arm for phase 3 testing of overall survival.
However, patients with CMML treated with the azacitidine-lenalidomide combination had twice the ORR compared with azacitidine monotherapy, they reported.
And patients with certain mutations, such as DNMT3A, BCOR, and NRAS, had higher overall response rates, although only those with the DNMT3A mutation were significant.
Mikkael A. Sekeres, MD, of the Cleveland Clinic in Cleveland, Ohio, and colleagues reported these findings in the Journal of Clinical Oncology on behalf of the North American Intergroup Study SWOG S117.
Doses of azacitidine were the same for monotherapy and combination arms: 75 mg/m2/day intravenously or subcutaneously on days 1 to 7 of a 28-day cycle.
Patients in the lenalidomide arm received 10 mg/day orally of that drug on days 1 to 21, and patients in the vorinostat arm received 300 mg twice daily orally on days 3 to 9.
Patient characteristics
Patients had MDS of IPSS Intermediate-2 or higher or bone marrow blasts 5% or greater. Patients with CMML had fewer than 20% blasts.
The investigators randomized 277 patients to receive either azacitidine alone (n=92), azacitidine plus lenalidomide (n=93), or azacitidine plus vorinostat (n=92).
Patients were a median age of 70 years (range, 28 to 93). Eighty-five patients (31%) were female, 53 (19%) had CMML, and 18 (6%) had treatment-related MDS. More than half the patients were transfusion-dependent at baseline.
Baseline characteristics were similar across the 3 arms. The investigators noted that the baseline characteristics were also similar across the 90 centers participating in the study, whether they were an MDS Center of Excellence or a high-volume center.
Adverse events
For the most part, therapy-related adverse events were similar across the arms.
Rates of grade 3 or higher febrile neutropenia and infection and infestations were similar for all 3 cohorts: 89% for azaciditine monotherapy, 91% for the lenalidomide combination, and 91% for the vorinostat combination.
However, the vorinostat arm had more grade 3 or higher gastrointestinal toxicities (14 patients, 15%) compared with the monotherapy arm (4 patients, 4%), P=0.02.
And patients receiving lenalidomide experienced more grade 3 or higher rash (14 patients, 16%) compared with patients receiving monotherapy (3 patients, 3%), P=0.005.
Patients in the combination arms stopped therapy at significantly higher rates than the monotherapy arm. Eight percent of patients receiving monotherapy stopped treatment compared with 20% in the lenalidomide arm and 21% in the vorinostat arm.
Patients in the combination arms also had more dose modifications not specified in the protocol than those in the monotherapy arm. Twenty-four percent receiving azacitidine monotherapy had non-protocol defined dose modifications, compared with 43% in the lenalidomide arm and 42% in the vorinostat arm.
Responses
The ORR for the entire study population was 38%.
Patients in the monotherapy arm had an ORR of 38%, those in the lenalidomide arm, 49%, and those in the vorinostate arm, 27%. Neither arm achieved significance compared with the monotherapy arm.
Patients who were treatment-naïve in the lenalidomide arm had a somewhat improved ORR compared with monotherapy, P=0.08.
The median duration of response for all cohorts was 15 months: 10 months for monotherapy, 14 months for lenalidomide, and 18 months for vorinostat.
Patients who were able to remain on therapy for 6 months or more in the lenalidomide arm achieved a higher ORR of 87% compared with monotherapy (62%, P=0.01). However, there was no difference in response duration with longer therapy.
The median overall survival (OS) was 17 months for all patients, 15 months for patients in the monotherapy group, 19 months for those in the lenalidomide arm, and 17 months for those in the vorinostat group.
CMML patients had similar OS across treatment arms, with the median not yet reached for patients in the monotherapy arm.
Subgroup responses
Patients with CMML in the lenalidomide arm had a significantly higher ORR than CMML patients in the monotherapy arm, 68% and 28%, respectively (P=0.02).
Median duration of response for CMML patients was 19 months, with no differences between the arms.
The investigators observed no differences in ORR for therapy-related MDS, IPSS subgroups, transfusion-dependent patients, or allogeneic transplant rates.
However, they noted ORR was better for patients with chromosome 5 abnormality regardless of treatment arm than for those without the abnormality (odds ratio, 2.17, P=0.008).
One hundred thirteen patients had mutational data available. They had a median number of 2 mutations (range, 0 to 7), with the most common being ASXL1 (n = 31), TET2 (n = 26), SRSF2 (n = 23), TP53 (n = 22), RUNX1 (n = 21), and U2AF1 (n = 19).
Patients with DNMT3A mutation had a significantly higher ORR than for patients without mutations, 67% and 34%, respectively P=0.025).
Patients with BCOR and NRAS mutations had numerically higher, but non-significant, ORR than non-mutated patients. Patients with BCOR mutation had a 57% ORR compared with 34% for non-mutated patients (P=0.23). Patients with NRAS mutation had a 60% ORR compared with 36% for non-mutated patients (P=0.28).
Patients with mutations in TET2 (P = .046) and TP53 (P = .003) had a worse response duration than those without mutations.
Response duration was significantly better with fewer mutations. For 2 or more mutations, the hazard ration was 6.86 versus no mutations (P=0.01).
The investigators believed under-dosing may have compromised response and survival in the combination arms. They suggested that studies focused on the subgroups that seemed to benefit from the combinations should be conducted. ![]()
A 3-arm phase 2 study of azacitidine alone or in combination with lenalidomide or vorinostat in patients with higher-risk myelodysplastic syndromes (MDS) or chronic myelomonocytic leukemia (CMML) has shown the combination therapies to have similar overall response rates (ORR) to azacitidine monotherapy. Based on these findings, investigators did not choose either combination arm for phase 3 testing of overall survival.
However, patients with CMML treated with the azacitidine-lenalidomide combination had twice the ORR compared with azacitidine monotherapy, they reported.
And patients with certain mutations, such as DNMT3A, BCOR, and NRAS, had higher overall response rates, although only those with the DNMT3A mutation were significant.
Mikkael A. Sekeres, MD, of the Cleveland Clinic in Cleveland, Ohio, and colleagues reported these findings in the Journal of Clinical Oncology on behalf of the North American Intergroup Study SWOG S117.
Doses of azacitidine were the same for monotherapy and combination arms: 75 mg/m2/day intravenously or subcutaneously on days 1 to 7 of a 28-day cycle.
Patients in the lenalidomide arm received 10 mg/day orally of that drug on days 1 to 21, and patients in the vorinostat arm received 300 mg twice daily orally on days 3 to 9.
Patient characteristics
Patients had MDS of IPSS Intermediate-2 or higher or bone marrow blasts 5% or greater. Patients with CMML had fewer than 20% blasts.
The investigators randomized 277 patients to receive either azacitidine alone (n=92), azacitidine plus lenalidomide (n=93), or azacitidine plus vorinostat (n=92).
Patients were a median age of 70 years (range, 28 to 93). Eighty-five patients (31%) were female, 53 (19%) had CMML, and 18 (6%) had treatment-related MDS. More than half the patients were transfusion-dependent at baseline.
Baseline characteristics were similar across the 3 arms. The investigators noted that the baseline characteristics were also similar across the 90 centers participating in the study, whether they were an MDS Center of Excellence or a high-volume center.
Adverse events
For the most part, therapy-related adverse events were similar across the arms.
Rates of grade 3 or higher febrile neutropenia and infection and infestations were similar for all 3 cohorts: 89% for azaciditine monotherapy, 91% for the lenalidomide combination, and 91% for the vorinostat combination.
However, the vorinostat arm had more grade 3 or higher gastrointestinal toxicities (14 patients, 15%) compared with the monotherapy arm (4 patients, 4%), P=0.02.
And patients receiving lenalidomide experienced more grade 3 or higher rash (14 patients, 16%) compared with patients receiving monotherapy (3 patients, 3%), P=0.005.
Patients in the combination arms stopped therapy at significantly higher rates than the monotherapy arm. Eight percent of patients receiving monotherapy stopped treatment compared with 20% in the lenalidomide arm and 21% in the vorinostat arm.
Patients in the combination arms also had more dose modifications not specified in the protocol than those in the monotherapy arm. Twenty-four percent receiving azacitidine monotherapy had non-protocol defined dose modifications, compared with 43% in the lenalidomide arm and 42% in the vorinostat arm.
Responses
The ORR for the entire study population was 38%.
Patients in the monotherapy arm had an ORR of 38%, those in the lenalidomide arm, 49%, and those in the vorinostate arm, 27%. Neither arm achieved significance compared with the monotherapy arm.
Patients who were treatment-naïve in the lenalidomide arm had a somewhat improved ORR compared with monotherapy, P=0.08.
The median duration of response for all cohorts was 15 months: 10 months for monotherapy, 14 months for lenalidomide, and 18 months for vorinostat.
Patients who were able to remain on therapy for 6 months or more in the lenalidomide arm achieved a higher ORR of 87% compared with monotherapy (62%, P=0.01). However, there was no difference in response duration with longer therapy.
The median overall survival (OS) was 17 months for all patients, 15 months for patients in the monotherapy group, 19 months for those in the lenalidomide arm, and 17 months for those in the vorinostat group.
CMML patients had similar OS across treatment arms, with the median not yet reached for patients in the monotherapy arm.
Subgroup responses
Patients with CMML in the lenalidomide arm had a significantly higher ORR than CMML patients in the monotherapy arm, 68% and 28%, respectively (P=0.02).
Median duration of response for CMML patients was 19 months, with no differences between the arms.
The investigators observed no differences in ORR for therapy-related MDS, IPSS subgroups, transfusion-dependent patients, or allogeneic transplant rates.
However, they noted ORR was better for patients with chromosome 5 abnormality regardless of treatment arm than for those without the abnormality (odds ratio, 2.17, P=0.008).
One hundred thirteen patients had mutational data available. They had a median number of 2 mutations (range, 0 to 7), with the most common being ASXL1 (n = 31), TET2 (n = 26), SRSF2 (n = 23), TP53 (n = 22), RUNX1 (n = 21), and U2AF1 (n = 19).
Patients with DNMT3A mutation had a significantly higher ORR than for patients without mutations, 67% and 34%, respectively P=0.025).
Patients with BCOR and NRAS mutations had numerically higher, but non-significant, ORR than non-mutated patients. Patients with BCOR mutation had a 57% ORR compared with 34% for non-mutated patients (P=0.23). Patients with NRAS mutation had a 60% ORR compared with 36% for non-mutated patients (P=0.28).
Patients with mutations in TET2 (P = .046) and TP53 (P = .003) had a worse response duration than those without mutations.
Response duration was significantly better with fewer mutations. For 2 or more mutations, the hazard ration was 6.86 versus no mutations (P=0.01).
The investigators believed under-dosing may have compromised response and survival in the combination arms. They suggested that studies focused on the subgroups that seemed to benefit from the combinations should be conducted. ![]()
A 3-arm phase 2 study of azacitidine alone or in combination with lenalidomide or vorinostat in patients with higher-risk myelodysplastic syndromes (MDS) or chronic myelomonocytic leukemia (CMML) has shown the combination therapies to have similar overall response rates (ORR) to azacitidine monotherapy. Based on these findings, investigators did not choose either combination arm for phase 3 testing of overall survival.
However, patients with CMML treated with the azacitidine-lenalidomide combination had twice the ORR compared with azacitidine monotherapy, they reported.
And patients with certain mutations, such as DNMT3A, BCOR, and NRAS, had higher overall response rates, although only those with the DNMT3A mutation were significant.
Mikkael A. Sekeres, MD, of the Cleveland Clinic in Cleveland, Ohio, and colleagues reported these findings in the Journal of Clinical Oncology on behalf of the North American Intergroup Study SWOG S117.
Doses of azacitidine were the same for monotherapy and combination arms: 75 mg/m2/day intravenously or subcutaneously on days 1 to 7 of a 28-day cycle.
Patients in the lenalidomide arm received 10 mg/day orally of that drug on days 1 to 21, and patients in the vorinostat arm received 300 mg twice daily orally on days 3 to 9.
Patient characteristics
Patients had MDS of IPSS Intermediate-2 or higher or bone marrow blasts 5% or greater. Patients with CMML had fewer than 20% blasts.
The investigators randomized 277 patients to receive either azacitidine alone (n=92), azacitidine plus lenalidomide (n=93), or azacitidine plus vorinostat (n=92).
Patients were a median age of 70 years (range, 28 to 93). Eighty-five patients (31%) were female, 53 (19%) had CMML, and 18 (6%) had treatment-related MDS. More than half the patients were transfusion-dependent at baseline.
Baseline characteristics were similar across the 3 arms. The investigators noted that the baseline characteristics were also similar across the 90 centers participating in the study, whether they were an MDS Center of Excellence or a high-volume center.
Adverse events
For the most part, therapy-related adverse events were similar across the arms.
Rates of grade 3 or higher febrile neutropenia and infection and infestations were similar for all 3 cohorts: 89% for azaciditine monotherapy, 91% for the lenalidomide combination, and 91% for the vorinostat combination.
However, the vorinostat arm had more grade 3 or higher gastrointestinal toxicities (14 patients, 15%) compared with the monotherapy arm (4 patients, 4%), P=0.02.
And patients receiving lenalidomide experienced more grade 3 or higher rash (14 patients, 16%) compared with patients receiving monotherapy (3 patients, 3%), P=0.005.
Patients in the combination arms stopped therapy at significantly higher rates than the monotherapy arm. Eight percent of patients receiving monotherapy stopped treatment compared with 20% in the lenalidomide arm and 21% in the vorinostat arm.
Patients in the combination arms also had more dose modifications not specified in the protocol than those in the monotherapy arm. Twenty-four percent receiving azacitidine monotherapy had non-protocol defined dose modifications, compared with 43% in the lenalidomide arm and 42% in the vorinostat arm.
Responses
The ORR for the entire study population was 38%.
Patients in the monotherapy arm had an ORR of 38%, those in the lenalidomide arm, 49%, and those in the vorinostate arm, 27%. Neither arm achieved significance compared with the monotherapy arm.
Patients who were treatment-naïve in the lenalidomide arm had a somewhat improved ORR compared with monotherapy, P=0.08.
The median duration of response for all cohorts was 15 months: 10 months for monotherapy, 14 months for lenalidomide, and 18 months for vorinostat.
Patients who were able to remain on therapy for 6 months or more in the lenalidomide arm achieved a higher ORR of 87% compared with monotherapy (62%, P=0.01). However, there was no difference in response duration with longer therapy.
The median overall survival (OS) was 17 months for all patients, 15 months for patients in the monotherapy group, 19 months for those in the lenalidomide arm, and 17 months for those in the vorinostat group.
CMML patients had similar OS across treatment arms, with the median not yet reached for patients in the monotherapy arm.
Subgroup responses
Patients with CMML in the lenalidomide arm had a significantly higher ORR than CMML patients in the monotherapy arm, 68% and 28%, respectively (P=0.02).
Median duration of response for CMML patients was 19 months, with no differences between the arms.
The investigators observed no differences in ORR for therapy-related MDS, IPSS subgroups, transfusion-dependent patients, or allogeneic transplant rates.
However, they noted ORR was better for patients with chromosome 5 abnormality regardless of treatment arm than for those without the abnormality (odds ratio, 2.17, P=0.008).
One hundred thirteen patients had mutational data available. They had a median number of 2 mutations (range, 0 to 7), with the most common being ASXL1 (n = 31), TET2 (n = 26), SRSF2 (n = 23), TP53 (n = 22), RUNX1 (n = 21), and U2AF1 (n = 19).
Patients with DNMT3A mutation had a significantly higher ORR than for patients without mutations, 67% and 34%, respectively P=0.025).
Patients with BCOR and NRAS mutations had numerically higher, but non-significant, ORR than non-mutated patients. Patients with BCOR mutation had a 57% ORR compared with 34% for non-mutated patients (P=0.23). Patients with NRAS mutation had a 60% ORR compared with 36% for non-mutated patients (P=0.28).
Patients with mutations in TET2 (P = .046) and TP53 (P = .003) had a worse response duration than those without mutations.
Response duration was significantly better with fewer mutations. For 2 or more mutations, the hazard ration was 6.86 versus no mutations (P=0.01).
The investigators believed under-dosing may have compromised response and survival in the combination arms. They suggested that studies focused on the subgroups that seemed to benefit from the combinations should be conducted. ![]()
Study contradicts AAP recommendations on infant room-sharing
Infants who slept in their own rooms by age 9 months slept significantly longer and better than those who continued sharing a room with their parents as recommended by the American Academy of Pediatrics, the authors of a prospective study of 279 mother-infant dyads reported June 5.
The findings “raise questions about the well-intended AAP recommendation that room-sharing should ideally occur for all infants until their first birthday,” wrote Ian M. Paul, MD, of Penn State University, Hershey, and his associates (Pediatrics. 2017 Jun 5. doi: 10.1542/peds.2017-0122). “Perhaps our most troubling finding was that room-sharing was associated with overnight transitions to bed-sharing, which is strongly discouraged by the AAP.”
Insufficient sleep leads to excess weight gain during infancy and sleep problems later in childhood and has negative implications for parents. “The desire to optimize infant sleep duration and consolidation, however, must be balanced with safe infant sleep,” the researchers emphasized. About 3,500 infants die annually of SIDS and other sleep-related deaths, about 90% of which occur before age 6 months. Currently, however, the AAP’s updated 2016 recommendations advise that infants sleep on a separate surface in their parents’ room for at least the first 6 months and ideally for 1 year (Pediatrics. 2016; 138:e20162938).
To examine relationships among where, how well, and how long infants slept, the researchers analyzed Brief Infant Sleep Questionnaires collected from first-time mothers of term singletons as part of the prospective, single-center Intervention Nurses Start Infants Growing on Healthy Trajectories (INSIGHT) study.
For 4-month-olds, average reported sleep duration was similar whether they slept alone or in the parental bedroom. Solo sleepers, however, had better sleep consolidation, averaging 46 more minutes of sleep at the longest stretch, compared with room sharers (P = .02). By age 9 months, infants who had slept alone by age 4 months averaged 40 more minutes of nightly sleep than room-sharers and 26 more minutes than infants who began sleeping alone after 4 months of age (P = .008). Furthermore, the average longest sleep span of early solo sleepers was 100 minutes more than that of room-sharers and 45 minutes more than that of infants who began sleeping alone between ages 4 and 9 months (P = .01).
At age 30 months, infants who had slept alone by age 9 months averaged 45 more minutes of nightly sleep than those who had shared a room (P = .004). Room-sharing at 4 months also was tied to a two-fold greater odds of having pillows, blankets, or other unsafe objects on the sleep surface, the researchers said. Together, the findings support revising the AAP recommendation until evidence conclusively supports it, they concluded.
The study’s funders included Penn State University, Hershey; Penn State Children’s Hospital; the U.S. Department of Agriculture; the Penn State Clinical and Translational Science Institute, and the National Institutes of Health/National Center for Advancing Translational Sciences. The investigators reported having no conflicts of interest.
The study by Dr. Paul and his associates is an important contribution to the literature, wrote Rachel Y. Moon, MD, and Fern R. Hauck, MD, in an accompanying editorial (Pediatrics. 2017 Jun 5. doi: 10.1542/peds.2017-1323). However, they said, more research is needed.
For example, it is unclear whether sleep consolidation in young infants is preferable from a physiologic perspective. “The ability to arouse is critical physiologically, and a leading hypothesis is that failure to arouse makes an infant vulnerable to SIDS,” they wrote.
Dr. Moon and Dr. Hauck also noted that the AAP’s recommendation on room-sharing without bed-sharing is based on studies conducted in England, New Zealand, and Scotland showing that room-sharing lowers the risk of SIDS, compared with solitary sleeping.
“More recent, unpublished data from the New Zealand Sudden and Unexplained Death in Infancy study show similar protection from room-sharing, with an adjusted odds ratio of 0.36 (95% confidence interval, 0.19-0.71) for room-sharing infants compared with solitary-sleeping infants (E. Mitchell, MBBS, personal communication, 2016),” they wrote. “Because none of these studies stratified the risk by infant age in months, it is difficult to determine the optimal endpoint for room-sharing.
“We strongly support more research, both about the physiology of infant sleep and arousal for room-sharing infants and about the consequences of room-sharing on parental and child sleep.”
Dr. Moon and Dr. Hauck are with the University of Virginia, Charlottesville. They had no relevant disclosures.
The study by Dr. Paul and his associates is an important contribution to the literature, wrote Rachel Y. Moon, MD, and Fern R. Hauck, MD, in an accompanying editorial (Pediatrics. 2017 Jun 5. doi: 10.1542/peds.2017-1323). However, they said, more research is needed.
For example, it is unclear whether sleep consolidation in young infants is preferable from a physiologic perspective. “The ability to arouse is critical physiologically, and a leading hypothesis is that failure to arouse makes an infant vulnerable to SIDS,” they wrote.
Dr. Moon and Dr. Hauck also noted that the AAP’s recommendation on room-sharing without bed-sharing is based on studies conducted in England, New Zealand, and Scotland showing that room-sharing lowers the risk of SIDS, compared with solitary sleeping.
“More recent, unpublished data from the New Zealand Sudden and Unexplained Death in Infancy study show similar protection from room-sharing, with an adjusted odds ratio of 0.36 (95% confidence interval, 0.19-0.71) for room-sharing infants compared with solitary-sleeping infants (E. Mitchell, MBBS, personal communication, 2016),” they wrote. “Because none of these studies stratified the risk by infant age in months, it is difficult to determine the optimal endpoint for room-sharing.
“We strongly support more research, both about the physiology of infant sleep and arousal for room-sharing infants and about the consequences of room-sharing on parental and child sleep.”
Dr. Moon and Dr. Hauck are with the University of Virginia, Charlottesville. They had no relevant disclosures.
The study by Dr. Paul and his associates is an important contribution to the literature, wrote Rachel Y. Moon, MD, and Fern R. Hauck, MD, in an accompanying editorial (Pediatrics. 2017 Jun 5. doi: 10.1542/peds.2017-1323). However, they said, more research is needed.
For example, it is unclear whether sleep consolidation in young infants is preferable from a physiologic perspective. “The ability to arouse is critical physiologically, and a leading hypothesis is that failure to arouse makes an infant vulnerable to SIDS,” they wrote.
Dr. Moon and Dr. Hauck also noted that the AAP’s recommendation on room-sharing without bed-sharing is based on studies conducted in England, New Zealand, and Scotland showing that room-sharing lowers the risk of SIDS, compared with solitary sleeping.
“More recent, unpublished data from the New Zealand Sudden and Unexplained Death in Infancy study show similar protection from room-sharing, with an adjusted odds ratio of 0.36 (95% confidence interval, 0.19-0.71) for room-sharing infants compared with solitary-sleeping infants (E. Mitchell, MBBS, personal communication, 2016),” they wrote. “Because none of these studies stratified the risk by infant age in months, it is difficult to determine the optimal endpoint for room-sharing.
“We strongly support more research, both about the physiology of infant sleep and arousal for room-sharing infants and about the consequences of room-sharing on parental and child sleep.”
Dr. Moon and Dr. Hauck are with the University of Virginia, Charlottesville. They had no relevant disclosures.
Infants who slept in their own rooms by age 9 months slept significantly longer and better than those who continued sharing a room with their parents as recommended by the American Academy of Pediatrics, the authors of a prospective study of 279 mother-infant dyads reported June 5.
The findings “raise questions about the well-intended AAP recommendation that room-sharing should ideally occur for all infants until their first birthday,” wrote Ian M. Paul, MD, of Penn State University, Hershey, and his associates (Pediatrics. 2017 Jun 5. doi: 10.1542/peds.2017-0122). “Perhaps our most troubling finding was that room-sharing was associated with overnight transitions to bed-sharing, which is strongly discouraged by the AAP.”
Insufficient sleep leads to excess weight gain during infancy and sleep problems later in childhood and has negative implications for parents. “The desire to optimize infant sleep duration and consolidation, however, must be balanced with safe infant sleep,” the researchers emphasized. About 3,500 infants die annually of SIDS and other sleep-related deaths, about 90% of which occur before age 6 months. Currently, however, the AAP’s updated 2016 recommendations advise that infants sleep on a separate surface in their parents’ room for at least the first 6 months and ideally for 1 year (Pediatrics. 2016; 138:e20162938).
To examine relationships among where, how well, and how long infants slept, the researchers analyzed Brief Infant Sleep Questionnaires collected from first-time mothers of term singletons as part of the prospective, single-center Intervention Nurses Start Infants Growing on Healthy Trajectories (INSIGHT) study.
For 4-month-olds, average reported sleep duration was similar whether they slept alone or in the parental bedroom. Solo sleepers, however, had better sleep consolidation, averaging 46 more minutes of sleep at the longest stretch, compared with room sharers (P = .02). By age 9 months, infants who had slept alone by age 4 months averaged 40 more minutes of nightly sleep than room-sharers and 26 more minutes than infants who began sleeping alone after 4 months of age (P = .008). Furthermore, the average longest sleep span of early solo sleepers was 100 minutes more than that of room-sharers and 45 minutes more than that of infants who began sleeping alone between ages 4 and 9 months (P = .01).
At age 30 months, infants who had slept alone by age 9 months averaged 45 more minutes of nightly sleep than those who had shared a room (P = .004). Room-sharing at 4 months also was tied to a two-fold greater odds of having pillows, blankets, or other unsafe objects on the sleep surface, the researchers said. Together, the findings support revising the AAP recommendation until evidence conclusively supports it, they concluded.
The study’s funders included Penn State University, Hershey; Penn State Children’s Hospital; the U.S. Department of Agriculture; the Penn State Clinical and Translational Science Institute, and the National Institutes of Health/National Center for Advancing Translational Sciences. The investigators reported having no conflicts of interest.
Infants who slept in their own rooms by age 9 months slept significantly longer and better than those who continued sharing a room with their parents as recommended by the American Academy of Pediatrics, the authors of a prospective study of 279 mother-infant dyads reported June 5.
The findings “raise questions about the well-intended AAP recommendation that room-sharing should ideally occur for all infants until their first birthday,” wrote Ian M. Paul, MD, of Penn State University, Hershey, and his associates (Pediatrics. 2017 Jun 5. doi: 10.1542/peds.2017-0122). “Perhaps our most troubling finding was that room-sharing was associated with overnight transitions to bed-sharing, which is strongly discouraged by the AAP.”
Insufficient sleep leads to excess weight gain during infancy and sleep problems later in childhood and has negative implications for parents. “The desire to optimize infant sleep duration and consolidation, however, must be balanced with safe infant sleep,” the researchers emphasized. About 3,500 infants die annually of SIDS and other sleep-related deaths, about 90% of which occur before age 6 months. Currently, however, the AAP’s updated 2016 recommendations advise that infants sleep on a separate surface in their parents’ room for at least the first 6 months and ideally for 1 year (Pediatrics. 2016; 138:e20162938).
To examine relationships among where, how well, and how long infants slept, the researchers analyzed Brief Infant Sleep Questionnaires collected from first-time mothers of term singletons as part of the prospective, single-center Intervention Nurses Start Infants Growing on Healthy Trajectories (INSIGHT) study.
For 4-month-olds, average reported sleep duration was similar whether they slept alone or in the parental bedroom. Solo sleepers, however, had better sleep consolidation, averaging 46 more minutes of sleep at the longest stretch, compared with room sharers (P = .02). By age 9 months, infants who had slept alone by age 4 months averaged 40 more minutes of nightly sleep than room-sharers and 26 more minutes than infants who began sleeping alone after 4 months of age (P = .008). Furthermore, the average longest sleep span of early solo sleepers was 100 minutes more than that of room-sharers and 45 minutes more than that of infants who began sleeping alone between ages 4 and 9 months (P = .01).
At age 30 months, infants who had slept alone by age 9 months averaged 45 more minutes of nightly sleep than those who had shared a room (P = .004). Room-sharing at 4 months also was tied to a two-fold greater odds of having pillows, blankets, or other unsafe objects on the sleep surface, the researchers said. Together, the findings support revising the AAP recommendation until evidence conclusively supports it, they concluded.
The study’s funders included Penn State University, Hershey; Penn State Children’s Hospital; the U.S. Department of Agriculture; the Penn State Clinical and Translational Science Institute, and the National Institutes of Health/National Center for Advancing Translational Sciences. The investigators reported having no conflicts of interest.
FROM PEDIATRICS
Key clinical point: Infants who slept in their own room by age 4 months slept significantly longer and better than those who continued sharing a room with their parents as recommended by the American Academy of Pediatrics.
Major finding: By age 9 months, infants who had slept alone by age 4 months averaged 40 more minutes of nightly sleep than room sharers and 26 more minutes than infants who began sleeping alone after 4 months of age (P = .008). Furthermore, the average longest sleep span of early solo sleepers was 100 minutes more than that of room-sharers and 45 minutes more than that of infants who began sleeping alone between ages 4 and 9 months (P = .01).
Data source: Secondary analyses of 279 mother-infant dyads from the single-center, prospective Intervention Nurses Start Infants Growing on Healthy Trajectories (INSIGHT) study.
Disclosures: The study funders included Penn State University, Hershey; Penn State Children’s Hospital; the U.S. Department of Agriculture; the Penn State Clinical and Translational Science Institute, and the National Institutes of Health/National Center for Advancing Translational Sciences. The investigators reported having no conflicts of interest.
The Professional Doctorate: What Are We Waiting for?
The increasingly complex health care system in the United States relies heavily on quality improvement, interprofessional collaboration, patient outcomes, health policy legislation, and advocacy. While important, most of these factors are outside the scope of the traditional master’s-level education program—necessitating the development of methods to help advanced practice providers, including NPs and PAs, obtain additional skills. The solution of choice, for many professions, has been the introduction of the “professional doctorate” as a complementary alternative to the typical research-focused doctoral program, such as the PhD.
Traditional PhD curricula prepare individuals to perform research that is typically specialized and confined to their field of study.1 While this research does produce new knowledge, it usually remains in the realm of academia and often does not address any specific “real-world” problem.2 But to be recognized, compensated, and identified as a full professional in modern societ
Analysis by Taylor and Maxwell and by Lee, Green, and Brennan has shown that, rather than theory, the workplace demands the application of knowledge geared toward daily professional duties.3,4 They envisioned a doctorate-prepared practitioner who had less skill in pure research but who would be able to apply theory to everyday problems in the workplace.4,5 Rather than devalue the contributions of classical PhD training, this model proposed the creation of a hybrid curriculum that would prepare individuals to use “applied research.”3 As the professional doctorate gained acceptance, it matured from the “first-generation” concept (which was quite similar to the PhD in structure) to “second-generation,” which is more focused on discipline and workplace realities.3,5 In general, these professional doctorates can be earned in less time than a PhD and do not require original research.
Over the past two decades, more than 500 unique professional practice doctorate programs have emerged across the US, in fields ranging from nursing to bioethics. One of the most prominent is the Doctor of Nursing Practice (DNP), designed for RNs seeking a post-professional degree in nursing. In 2004, following three years of research by a task force, the American Association of Colleges of Nursing (AACN) endorsed the DNP, with the goal that it would become the minimum educational standard for advanced practice nurses by 2015.6 According to the AACN, there are 289 DNP programs in the US, with an additional 128 in development.6
The PA profession has lagged behind not only our NP colleagues, but also many other health professions, in the adoption of a discipline-specific, doctoral-level degree. Our counterparts in audiology, physical therapy, occupational therapy, and athletic training have been part of the exponential growth in second-generation health care doctorates.7 While these programs may differ in concept, they share several similarities: They do not require original research; they include a clinical component; and they promote knowledge in the context of the workplace.5-8
In the past five years, PAs have started considering (or debating, depending on your perspective) a professional/clinical doctorate as the next step in our post-professional journey. It’s about time, when you consider that 16.8% of newly certified PAs intend to pursue additional education or clinical training, according to a recent report from the National Commission on Certification of Physician Assistants.9
There are already a few doctoral programs for PAs. Among the earliest clinically focused doctorate programs was the US Army/Air Force-Baylor DScPAS-EM program, designed to educate military PAs at the doctoral level upon completion of an 18-month emergency medicine residency.10 Lincoln Memorial University has a Doctor of Medical Science (DMS) program, comprised of one year of online advanced clinical medicine coursework and one year of online coursework focused on primary care, hospital medicine, emergency medicine, or education.11 And Lynchburg College in Virginia has just launched a post-professional doctoral program for PAs; this DMS program includes a clinical fellowship, as well as coursework in leadership training, health care management and law, organizational behavior, disaster medicine, and global health.12
While not strictly created for PAs, the Doctor of Health Science programs at Nova Southeastern University and A.T. Still University have been educating PAs at the doctoral level for more than 10 years.13,14 Later this year, A.T. Still University plans to introduce a post-professional Doctor of Physician Assistant Studies that will provide a pathway for PAs wishing to become leaders and scholar-practitioners, develop core leadership abilities, and/or enter PA education without the location-specific requirement of a clinical or academic residency.
When the push for professional practice doctorates started, pundits claimed they were just an attempt at a “cash grab” by universities looking to bolster their rosters (and their coffers). But advocates have long argued that these degrees provide practitioners with the knowledge and training required to offer advanced services in increasingly complex social and technologic environments.7 No less than The Institute of Medicine, The Joint Commission, and the Robert Wood Johnson Foundation have called for the reinvention of education programs to equip today’s health professionals
Why? First and foremost, to ensure quality patient outcomes. Beyond that, better prepared clinicians can help to address provider shortages. Those with doctorates can also serve as faculty, educating the next generation of health care providers. And practically speaking, for those seeking advanced education, holding a doctorate will create opportunities for increased decision-making and upward mobility in the workplace.
There is no question that our current health care environment is driven by the regulations and costs of the Affordable Care Act, as well as quality management systems and strategies. NPs and PAs are in a unique position to cost-effectively direct the care of, and advocate for, diverse patient populations. NPs and PAs who recognize this opportunity to serve need doctoral-level training tailored to this milieu.
Do you agree? Share your thoughts on professional doctorates with me at [email protected].
1. Carnegie Project on the Education Doctorate. Founding literature. www.cpedinitiative.org/?. Accessed May 8, 2017.
2. Costley C, Lester S. Work-based doctorates: professional extension at the highest levels. Studies in Higher Education. 2012;37(3):257-269.
3. Taylor N, Maxwell T. Enhancing the relevance of a professional doctorate: the case of the doctor of education degree at the University of New England. Asia-Pacific J Coop Edu. 2004;5(1):60-69.
4. Lee A, Green B, Brennan M. Organisational knowledge, professional practice and the professional doctorate at work. In: Garrick J, Rhodes C (eds). Research and Knowledge at Work. Perspectives, Case-studies and Innovative Strategies. London: Routledge; 2000.
5. Maxwell T. From first to second generation professional doctorate. Studies in Higher Education. 2003;28(3):279-291.
6. American Association of Colleges of Nursing. DNP fact sheet. www.aacn.nche.edu/media-relations/fact-sheets/dnp. Accessed May 8, 2017.
7. Zusman A. Degrees of change: how new kinds of professional doctorates are changing higher education institutions. Research and Occasional Paper Series. 2013;8(13):1-20.
8. Kumar S, Dawson K. Exploring the impact of a professional practice education doctorate in educational environments. Studies in Continuing Education. 2013;35(2):165-178.
9. National Commission on Certification of Physician Assistants. 2014 statistical profile of recently certified physician assistants: an annual report of the NCCPA. www.nccpa.net/Uploads/docs/RecentlyCertifiedReport2014.pdf. Accessed May 8, 2017.
10. Baylor University. Army-Baylor emergency medicine physician assistant (EMPA) program. www.baylor.edu/graduate/pa/index.php?id=936090. Accessed May 8, 2017.
11. Lincoln Memorial University. Doctor of medical science announcement. www.lmunet.edu/academics/schools/debusk-college-of-osteopathic-medicine/dms. Accessed May 8, 2017.
12. Lynchburg College. Doctor of medical science. www.lynchburg.edu/graduate/physician-assistant-medicine/doctor-of-medical-science/. Accessed May 8, 2017.
13. Nova Southeastern University. Doctor of health science program. http://healthsciences.nova.edu/healthsciences/dhs/. Accessed May 8, 2017.
14. A.T. Still University. About the college of graduate health studies – online. www.atsu.edu/college-of-graduate-health-studies. Accessed May 8, 2017.
The increasingly complex health care system in the United States relies heavily on quality improvement, interprofessional collaboration, patient outcomes, health policy legislation, and advocacy. While important, most of these factors are outside the scope of the traditional master’s-level education program—necessitating the development of methods to help advanced practice providers, including NPs and PAs, obtain additional skills. The solution of choice, for many professions, has been the introduction of the “professional doctorate” as a complementary alternative to the typical research-focused doctoral program, such as the PhD.
Traditional PhD curricula prepare individuals to perform research that is typically specialized and confined to their field of study.1 While this research does produce new knowledge, it usually remains in the realm of academia and often does not address any specific “real-world” problem.2 But to be recognized, compensated, and identified as a full professional in modern societ
Analysis by Taylor and Maxwell and by Lee, Green, and Brennan has shown that, rather than theory, the workplace demands the application of knowledge geared toward daily professional duties.3,4 They envisioned a doctorate-prepared practitioner who had less skill in pure research but who would be able to apply theory to everyday problems in the workplace.4,5 Rather than devalue the contributions of classical PhD training, this model proposed the creation of a hybrid curriculum that would prepare individuals to use “applied research.”3 As the professional doctorate gained acceptance, it matured from the “first-generation” concept (which was quite similar to the PhD in structure) to “second-generation,” which is more focused on discipline and workplace realities.3,5 In general, these professional doctorates can be earned in less time than a PhD and do not require original research.
Over the past two decades, more than 500 unique professional practice doctorate programs have emerged across the US, in fields ranging from nursing to bioethics. One of the most prominent is the Doctor of Nursing Practice (DNP), designed for RNs seeking a post-professional degree in nursing. In 2004, following three years of research by a task force, the American Association of Colleges of Nursing (AACN) endorsed the DNP, with the goal that it would become the minimum educational standard for advanced practice nurses by 2015.6 According to the AACN, there are 289 DNP programs in the US, with an additional 128 in development.6
The PA profession has lagged behind not only our NP colleagues, but also many other health professions, in the adoption of a discipline-specific, doctoral-level degree. Our counterparts in audiology, physical therapy, occupational therapy, and athletic training have been part of the exponential growth in second-generation health care doctorates.7 While these programs may differ in concept, they share several similarities: They do not require original research; they include a clinical component; and they promote knowledge in the context of the workplace.5-8
In the past five years, PAs have started considering (or debating, depending on your perspective) a professional/clinical doctorate as the next step in our post-professional journey. It’s about time, when you consider that 16.8% of newly certified PAs intend to pursue additional education or clinical training, according to a recent report from the National Commission on Certification of Physician Assistants.9
There are already a few doctoral programs for PAs. Among the earliest clinically focused doctorate programs was the US Army/Air Force-Baylor DScPAS-EM program, designed to educate military PAs at the doctoral level upon completion of an 18-month emergency medicine residency.10 Lincoln Memorial University has a Doctor of Medical Science (DMS) program, comprised of one year of online advanced clinical medicine coursework and one year of online coursework focused on primary care, hospital medicine, emergency medicine, or education.11 And Lynchburg College in Virginia has just launched a post-professional doctoral program for PAs; this DMS program includes a clinical fellowship, as well as coursework in leadership training, health care management and law, organizational behavior, disaster medicine, and global health.12
While not strictly created for PAs, the Doctor of Health Science programs at Nova Southeastern University and A.T. Still University have been educating PAs at the doctoral level for more than 10 years.13,14 Later this year, A.T. Still University plans to introduce a post-professional Doctor of Physician Assistant Studies that will provide a pathway for PAs wishing to become leaders and scholar-practitioners, develop core leadership abilities, and/or enter PA education without the location-specific requirement of a clinical or academic residency.
When the push for professional practice doctorates started, pundits claimed they were just an attempt at a “cash grab” by universities looking to bolster their rosters (and their coffers). But advocates have long argued that these degrees provide practitioners with the knowledge and training required to offer advanced services in increasingly complex social and technologic environments.7 No less than The Institute of Medicine, The Joint Commission, and the Robert Wood Johnson Foundation have called for the reinvention of education programs to equip today’s health professionals
Why? First and foremost, to ensure quality patient outcomes. Beyond that, better prepared clinicians can help to address provider shortages. Those with doctorates can also serve as faculty, educating the next generation of health care providers. And practically speaking, for those seeking advanced education, holding a doctorate will create opportunities for increased decision-making and upward mobility in the workplace.
There is no question that our current health care environment is driven by the regulations and costs of the Affordable Care Act, as well as quality management systems and strategies. NPs and PAs are in a unique position to cost-effectively direct the care of, and advocate for, diverse patient populations. NPs and PAs who recognize this opportunity to serve need doctoral-level training tailored to this milieu.
Do you agree? Share your thoughts on professional doctorates with me at [email protected].
The increasingly complex health care system in the United States relies heavily on quality improvement, interprofessional collaboration, patient outcomes, health policy legislation, and advocacy. While important, most of these factors are outside the scope of the traditional master’s-level education program—necessitating the development of methods to help advanced practice providers, including NPs and PAs, obtain additional skills. The solution of choice, for many professions, has been the introduction of the “professional doctorate” as a complementary alternative to the typical research-focused doctoral program, such as the PhD.
Traditional PhD curricula prepare individuals to perform research that is typically specialized and confined to their field of study.1 While this research does produce new knowledge, it usually remains in the realm of academia and often does not address any specific “real-world” problem.2 But to be recognized, compensated, and identified as a full professional in modern societ
Analysis by Taylor and Maxwell and by Lee, Green, and Brennan has shown that, rather than theory, the workplace demands the application of knowledge geared toward daily professional duties.3,4 They envisioned a doctorate-prepared practitioner who had less skill in pure research but who would be able to apply theory to everyday problems in the workplace.4,5 Rather than devalue the contributions of classical PhD training, this model proposed the creation of a hybrid curriculum that would prepare individuals to use “applied research.”3 As the professional doctorate gained acceptance, it matured from the “first-generation” concept (which was quite similar to the PhD in structure) to “second-generation,” which is more focused on discipline and workplace realities.3,5 In general, these professional doctorates can be earned in less time than a PhD and do not require original research.
Over the past two decades, more than 500 unique professional practice doctorate programs have emerged across the US, in fields ranging from nursing to bioethics. One of the most prominent is the Doctor of Nursing Practice (DNP), designed for RNs seeking a post-professional degree in nursing. In 2004, following three years of research by a task force, the American Association of Colleges of Nursing (AACN) endorsed the DNP, with the goal that it would become the minimum educational standard for advanced practice nurses by 2015.6 According to the AACN, there are 289 DNP programs in the US, with an additional 128 in development.6
The PA profession has lagged behind not only our NP colleagues, but also many other health professions, in the adoption of a discipline-specific, doctoral-level degree. Our counterparts in audiology, physical therapy, occupational therapy, and athletic training have been part of the exponential growth in second-generation health care doctorates.7 While these programs may differ in concept, they share several similarities: They do not require original research; they include a clinical component; and they promote knowledge in the context of the workplace.5-8
In the past five years, PAs have started considering (or debating, depending on your perspective) a professional/clinical doctorate as the next step in our post-professional journey. It’s about time, when you consider that 16.8% of newly certified PAs intend to pursue additional education or clinical training, according to a recent report from the National Commission on Certification of Physician Assistants.9
There are already a few doctoral programs for PAs. Among the earliest clinically focused doctorate programs was the US Army/Air Force-Baylor DScPAS-EM program, designed to educate military PAs at the doctoral level upon completion of an 18-month emergency medicine residency.10 Lincoln Memorial University has a Doctor of Medical Science (DMS) program, comprised of one year of online advanced clinical medicine coursework and one year of online coursework focused on primary care, hospital medicine, emergency medicine, or education.11 And Lynchburg College in Virginia has just launched a post-professional doctoral program for PAs; this DMS program includes a clinical fellowship, as well as coursework in leadership training, health care management and law, organizational behavior, disaster medicine, and global health.12
While not strictly created for PAs, the Doctor of Health Science programs at Nova Southeastern University and A.T. Still University have been educating PAs at the doctoral level for more than 10 years.13,14 Later this year, A.T. Still University plans to introduce a post-professional Doctor of Physician Assistant Studies that will provide a pathway for PAs wishing to become leaders and scholar-practitioners, develop core leadership abilities, and/or enter PA education without the location-specific requirement of a clinical or academic residency.
When the push for professional practice doctorates started, pundits claimed they were just an attempt at a “cash grab” by universities looking to bolster their rosters (and their coffers). But advocates have long argued that these degrees provide practitioners with the knowledge and training required to offer advanced services in increasingly complex social and technologic environments.7 No less than The Institute of Medicine, The Joint Commission, and the Robert Wood Johnson Foundation have called for the reinvention of education programs to equip today’s health professionals
Why? First and foremost, to ensure quality patient outcomes. Beyond that, better prepared clinicians can help to address provider shortages. Those with doctorates can also serve as faculty, educating the next generation of health care providers. And practically speaking, for those seeking advanced education, holding a doctorate will create opportunities for increased decision-making and upward mobility in the workplace.
There is no question that our current health care environment is driven by the regulations and costs of the Affordable Care Act, as well as quality management systems and strategies. NPs and PAs are in a unique position to cost-effectively direct the care of, and advocate for, diverse patient populations. NPs and PAs who recognize this opportunity to serve need doctoral-level training tailored to this milieu.
Do you agree? Share your thoughts on professional doctorates with me at [email protected].
1. Carnegie Project on the Education Doctorate. Founding literature. www.cpedinitiative.org/?. Accessed May 8, 2017.
2. Costley C, Lester S. Work-based doctorates: professional extension at the highest levels. Studies in Higher Education. 2012;37(3):257-269.
3. Taylor N, Maxwell T. Enhancing the relevance of a professional doctorate: the case of the doctor of education degree at the University of New England. Asia-Pacific J Coop Edu. 2004;5(1):60-69.
4. Lee A, Green B, Brennan M. Organisational knowledge, professional practice and the professional doctorate at work. In: Garrick J, Rhodes C (eds). Research and Knowledge at Work. Perspectives, Case-studies and Innovative Strategies. London: Routledge; 2000.
5. Maxwell T. From first to second generation professional doctorate. Studies in Higher Education. 2003;28(3):279-291.
6. American Association of Colleges of Nursing. DNP fact sheet. www.aacn.nche.edu/media-relations/fact-sheets/dnp. Accessed May 8, 2017.
7. Zusman A. Degrees of change: how new kinds of professional doctorates are changing higher education institutions. Research and Occasional Paper Series. 2013;8(13):1-20.
8. Kumar S, Dawson K. Exploring the impact of a professional practice education doctorate in educational environments. Studies in Continuing Education. 2013;35(2):165-178.
9. National Commission on Certification of Physician Assistants. 2014 statistical profile of recently certified physician assistants: an annual report of the NCCPA. www.nccpa.net/Uploads/docs/RecentlyCertifiedReport2014.pdf. Accessed May 8, 2017.
10. Baylor University. Army-Baylor emergency medicine physician assistant (EMPA) program. www.baylor.edu/graduate/pa/index.php?id=936090. Accessed May 8, 2017.
11. Lincoln Memorial University. Doctor of medical science announcement. www.lmunet.edu/academics/schools/debusk-college-of-osteopathic-medicine/dms. Accessed May 8, 2017.
12. Lynchburg College. Doctor of medical science. www.lynchburg.edu/graduate/physician-assistant-medicine/doctor-of-medical-science/. Accessed May 8, 2017.
13. Nova Southeastern University. Doctor of health science program. http://healthsciences.nova.edu/healthsciences/dhs/. Accessed May 8, 2017.
14. A.T. Still University. About the college of graduate health studies – online. www.atsu.edu/college-of-graduate-health-studies. Accessed May 8, 2017.
1. Carnegie Project on the Education Doctorate. Founding literature. www.cpedinitiative.org/?. Accessed May 8, 2017.
2. Costley C, Lester S. Work-based doctorates: professional extension at the highest levels. Studies in Higher Education. 2012;37(3):257-269.
3. Taylor N, Maxwell T. Enhancing the relevance of a professional doctorate: the case of the doctor of education degree at the University of New England. Asia-Pacific J Coop Edu. 2004;5(1):60-69.
4. Lee A, Green B, Brennan M. Organisational knowledge, professional practice and the professional doctorate at work. In: Garrick J, Rhodes C (eds). Research and Knowledge at Work. Perspectives, Case-studies and Innovative Strategies. London: Routledge; 2000.
5. Maxwell T. From first to second generation professional doctorate. Studies in Higher Education. 2003;28(3):279-291.
6. American Association of Colleges of Nursing. DNP fact sheet. www.aacn.nche.edu/media-relations/fact-sheets/dnp. Accessed May 8, 2017.
7. Zusman A. Degrees of change: how new kinds of professional doctorates are changing higher education institutions. Research and Occasional Paper Series. 2013;8(13):1-20.
8. Kumar S, Dawson K. Exploring the impact of a professional practice education doctorate in educational environments. Studies in Continuing Education. 2013;35(2):165-178.
9. National Commission on Certification of Physician Assistants. 2014 statistical profile of recently certified physician assistants: an annual report of the NCCPA. www.nccpa.net/Uploads/docs/RecentlyCertifiedReport2014.pdf. Accessed May 8, 2017.
10. Baylor University. Army-Baylor emergency medicine physician assistant (EMPA) program. www.baylor.edu/graduate/pa/index.php?id=936090. Accessed May 8, 2017.
11. Lincoln Memorial University. Doctor of medical science announcement. www.lmunet.edu/academics/schools/debusk-college-of-osteopathic-medicine/dms. Accessed May 8, 2017.
12. Lynchburg College. Doctor of medical science. www.lynchburg.edu/graduate/physician-assistant-medicine/doctor-of-medical-science/. Accessed May 8, 2017.
13. Nova Southeastern University. Doctor of health science program. http://healthsciences.nova.edu/healthsciences/dhs/. Accessed May 8, 2017.
14. A.T. Still University. About the college of graduate health studies – online. www.atsu.edu/college-of-graduate-health-studies. Accessed May 8, 2017.
Test goes wide and deep to detect free tumor DNA in blood
CHICAGO – An experimental liquid biopsy method plumbs the depths of the genome to detect a broad array of mutations in blood samples that closely correspond to variants in tumors.
The high-intensity method for detecting circulating free DNA (cfDNA) in plasma detected at least one significant genetic variant in samples from 89% of patients with advanced breast, lung, and prostate cancers.
If validated in further studies, the technique could inform more accurate drug selection in patients with refractory disease and eventually may form the basis for plasma-based assays to detect early-stage cancers, said Pedram Razavi, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York.
“This novel, high-intensity sequencing assay incorporates an unprecedented combination of depth and breadth of coverage compared to previous assays. High levels of concordance for variants between plasma and tissue provide strong evidence for high rates of tumor DNA detection in plasma,” he said at a press briefing at the annual meeting of the American Society of Clinical Oncology.
The technique also provides important insights into tumor biology, “including first exploration of mutational signatures in the plasma,” he added.
Deep scanning
The high-intensity sequencing method scans for 508 genes in a 2 million base-pair swath of genome, performing 60,000 repeat reads on each genome region to improve the assay’s sensitivity, and deep sequencing allows for detection of rare “needle-in-a-haystack” variants.
Dr. Razavi and his colleagues examined prospectively collected blood and tissues from 161 patients, 124 of whom had samples sufficient for concordance studies.
The samples were collected within 6 weeks of a de novo cancer diagnosis or evidence of disease progression, before the initiation of therapy.
Both cfDNA and genomic data from white blood cells (WBCs) of each patient were sequenced with the aforementioned 508-gene panel covering a broad range of known cancer variants and mutations.
Tumor tissues were sequenced with MSK-IMPACT, a 410 gene assay, with blinding of results in regard to plasma and WBC sequencing. Variants were classified as clonal or subclonal based on tumor sequencing in breast cancer and non–small cell lung cancer (NSCLC).
As noted before, 89% of patients had at least one genetic variant detected in both the tumor and in plasma, including 97% of patients with metastatic breast cancer, 85% of those with NSCLC, and 84% of patients with metastatic prostate cancer.
The investigators identified 864 clonal or subclonal variants in tissue samples from all three of these cancers, and 627 of the variants also were found in plasma.
In addition, 76% of clinically actionable somatic mutations identified in tumors also were found in plasma, which suggests that the high-intensity sequencing technique may be able to identify tumor heterogeneity that is not always evident in single tumor biopsies, Dr. Razavi said.
‘A clear advance’
“The work by Dr. Razavi and colleagues is a clear advance in the field because it surveys for the first time a much broader panel of genes – 508 genes in this case – and it does it with much deeper sequencing, which means it can detect much rarer alterations,” commented ASCO expert John Heymach, MD, PhD, from the University of Texas MD Anderson Cancer Center in Houston.
The study “helps illuminate a path toward a day when we will be using circulating tumor DNA assays for early detection of cancer, and not just for selecting certain therapies,” he added.
“We’re a long way from utilizing liquid biopsy for detecting cancers; already though, we’re seeing some utility of circulating tumor DNA in the domain of identifying novel alterations as a means of segmentation clinical trials,” commented ASCO expert Sumanta Kumar Pal, MD, from City of Hope in Duarte, Calif.
Dr. Heymach and Dr. Pal were not involved in the study, but were invited discussants at the briefing.
The study was funded in part by GRAIL. Dr. Razavi reported institutional research funding from the company. Multiple coauthors are employees of the company.
CHICAGO – An experimental liquid biopsy method plumbs the depths of the genome to detect a broad array of mutations in blood samples that closely correspond to variants in tumors.
The high-intensity method for detecting circulating free DNA (cfDNA) in plasma detected at least one significant genetic variant in samples from 89% of patients with advanced breast, lung, and prostate cancers.
If validated in further studies, the technique could inform more accurate drug selection in patients with refractory disease and eventually may form the basis for plasma-based assays to detect early-stage cancers, said Pedram Razavi, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York.
“This novel, high-intensity sequencing assay incorporates an unprecedented combination of depth and breadth of coverage compared to previous assays. High levels of concordance for variants between plasma and tissue provide strong evidence for high rates of tumor DNA detection in plasma,” he said at a press briefing at the annual meeting of the American Society of Clinical Oncology.
The technique also provides important insights into tumor biology, “including first exploration of mutational signatures in the plasma,” he added.
Deep scanning
The high-intensity sequencing method scans for 508 genes in a 2 million base-pair swath of genome, performing 60,000 repeat reads on each genome region to improve the assay’s sensitivity, and deep sequencing allows for detection of rare “needle-in-a-haystack” variants.
Dr. Razavi and his colleagues examined prospectively collected blood and tissues from 161 patients, 124 of whom had samples sufficient for concordance studies.
The samples were collected within 6 weeks of a de novo cancer diagnosis or evidence of disease progression, before the initiation of therapy.
Both cfDNA and genomic data from white blood cells (WBCs) of each patient were sequenced with the aforementioned 508-gene panel covering a broad range of known cancer variants and mutations.
Tumor tissues were sequenced with MSK-IMPACT, a 410 gene assay, with blinding of results in regard to plasma and WBC sequencing. Variants were classified as clonal or subclonal based on tumor sequencing in breast cancer and non–small cell lung cancer (NSCLC).
As noted before, 89% of patients had at least one genetic variant detected in both the tumor and in plasma, including 97% of patients with metastatic breast cancer, 85% of those with NSCLC, and 84% of patients with metastatic prostate cancer.
The investigators identified 864 clonal or subclonal variants in tissue samples from all three of these cancers, and 627 of the variants also were found in plasma.
In addition, 76% of clinically actionable somatic mutations identified in tumors also were found in plasma, which suggests that the high-intensity sequencing technique may be able to identify tumor heterogeneity that is not always evident in single tumor biopsies, Dr. Razavi said.
‘A clear advance’
“The work by Dr. Razavi and colleagues is a clear advance in the field because it surveys for the first time a much broader panel of genes – 508 genes in this case – and it does it with much deeper sequencing, which means it can detect much rarer alterations,” commented ASCO expert John Heymach, MD, PhD, from the University of Texas MD Anderson Cancer Center in Houston.
The study “helps illuminate a path toward a day when we will be using circulating tumor DNA assays for early detection of cancer, and not just for selecting certain therapies,” he added.
“We’re a long way from utilizing liquid biopsy for detecting cancers; already though, we’re seeing some utility of circulating tumor DNA in the domain of identifying novel alterations as a means of segmentation clinical trials,” commented ASCO expert Sumanta Kumar Pal, MD, from City of Hope in Duarte, Calif.
Dr. Heymach and Dr. Pal were not involved in the study, but were invited discussants at the briefing.
The study was funded in part by GRAIL. Dr. Razavi reported institutional research funding from the company. Multiple coauthors are employees of the company.
CHICAGO – An experimental liquid biopsy method plumbs the depths of the genome to detect a broad array of mutations in blood samples that closely correspond to variants in tumors.
The high-intensity method for detecting circulating free DNA (cfDNA) in plasma detected at least one significant genetic variant in samples from 89% of patients with advanced breast, lung, and prostate cancers.
If validated in further studies, the technique could inform more accurate drug selection in patients with refractory disease and eventually may form the basis for plasma-based assays to detect early-stage cancers, said Pedram Razavi, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York.
“This novel, high-intensity sequencing assay incorporates an unprecedented combination of depth and breadth of coverage compared to previous assays. High levels of concordance for variants between plasma and tissue provide strong evidence for high rates of tumor DNA detection in plasma,” he said at a press briefing at the annual meeting of the American Society of Clinical Oncology.
The technique also provides important insights into tumor biology, “including first exploration of mutational signatures in the plasma,” he added.
Deep scanning
The high-intensity sequencing method scans for 508 genes in a 2 million base-pair swath of genome, performing 60,000 repeat reads on each genome region to improve the assay’s sensitivity, and deep sequencing allows for detection of rare “needle-in-a-haystack” variants.
Dr. Razavi and his colleagues examined prospectively collected blood and tissues from 161 patients, 124 of whom had samples sufficient for concordance studies.
The samples were collected within 6 weeks of a de novo cancer diagnosis or evidence of disease progression, before the initiation of therapy.
Both cfDNA and genomic data from white blood cells (WBCs) of each patient were sequenced with the aforementioned 508-gene panel covering a broad range of known cancer variants and mutations.
Tumor tissues were sequenced with MSK-IMPACT, a 410 gene assay, with blinding of results in regard to plasma and WBC sequencing. Variants were classified as clonal or subclonal based on tumor sequencing in breast cancer and non–small cell lung cancer (NSCLC).
As noted before, 89% of patients had at least one genetic variant detected in both the tumor and in plasma, including 97% of patients with metastatic breast cancer, 85% of those with NSCLC, and 84% of patients with metastatic prostate cancer.
The investigators identified 864 clonal or subclonal variants in tissue samples from all three of these cancers, and 627 of the variants also were found in plasma.
In addition, 76% of clinically actionable somatic mutations identified in tumors also were found in plasma, which suggests that the high-intensity sequencing technique may be able to identify tumor heterogeneity that is not always evident in single tumor biopsies, Dr. Razavi said.
‘A clear advance’
“The work by Dr. Razavi and colleagues is a clear advance in the field because it surveys for the first time a much broader panel of genes – 508 genes in this case – and it does it with much deeper sequencing, which means it can detect much rarer alterations,” commented ASCO expert John Heymach, MD, PhD, from the University of Texas MD Anderson Cancer Center in Houston.
The study “helps illuminate a path toward a day when we will be using circulating tumor DNA assays for early detection of cancer, and not just for selecting certain therapies,” he added.
“We’re a long way from utilizing liquid biopsy for detecting cancers; already though, we’re seeing some utility of circulating tumor DNA in the domain of identifying novel alterations as a means of segmentation clinical trials,” commented ASCO expert Sumanta Kumar Pal, MD, from City of Hope in Duarte, Calif.
Dr. Heymach and Dr. Pal were not involved in the study, but were invited discussants at the briefing.
The study was funded in part by GRAIL. Dr. Razavi reported institutional research funding from the company. Multiple coauthors are employees of the company.
AT ASCO 2017
Key clinical point: High-intensity sequencing of plasma samples appears capable of detecting actionable tumor mutations in a large proportion of samples.
Major finding: In 89% of patients with advanced cancers, genetic variants were identified in both tumor samples and circulating free DNA testing.
Data source: Prospective study of tissue and plasma samples from 124 patients with non–small cell lung cancer or metastatic breast and prostate cancers.
Disclosures: The study was funded in part by GRAIL. Dr. Razavi reported institutional research funding from the company. Multiple coauthors are employees of the company.
Cost, value of new cancer treatments rarely correlate
New high-priced cancer treatments rarely demonstrate value, compared with the prices being charged for them.
Researchers examined clinical trial data for new treatments using scoring frameworks developed by both the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) that assess the value of cancer therapies by taking into consideration survival gains in relation to toxicity and quality. Findings were simultaneously published online June 2 in The Lancet Oncology (2017 June 2. doi: 10.1016/S1470-2045(17)30415-1) and presented at the annual meeting of the American Society of Clinical Oncology.
One of the findings from the research quantified the magnitude of how much benefit a new treatment offers patients.
“We were able to compare that across different treatments and we were able to calculate the drug cost per month of treatment with each new cancer treatment,” Christopher Booth, MD, of Queen’s University Cancer Research Institute, Kingston, Ont., and one of the report’s authors, said in an interview.
“We looked to see whether there is a relationship between cost and benefit. In most elements of our economy, if something is of higher value and better quality, we often pay more for it as opposed to something that offers less quality or less value,” he continued. “We found that that relationship does not hold true in the world of cancer drugs. In fact, if anything, we saw an inverse association, meaning that the drugs that are the most expensive actually have the smallest benefit for patients. That is probably the most important finding of the work.”
Dr. Booth suggested that this has to do with the fact that value is never a factor in pricing.
“In very general terms, the way that a new drug is priced is effectively the highest price the market will bear,” he said. “There really isn’t an approach currently to price drugs based on the return that they offer to patients and society. There is a conversation under way within the oncology community about whether we need to be shifting that conversation to something perhaps called value-based pricing where treatments that offer greater benefit to patients are priced higher than treatments that offer negligible benefit.”
He also noted that “there is not a lot of incentive for the research community or pharmaceutical companies to identify drugs that have larger and larger benefits, being that the financial return on their investment does not appear to be related to how much benefit the drug offers. If we shifted that conversation, it would hopefully, at least in some way, drive the research community and push us to find treatments that have large or meaningful benefits to patients instead of some of these treatments that are very expensive with important side effects that really offer very small improvements in patient outcomes.”
Dr. Booth continued: “I think it’s inevitable and I think what these score cards are doing, and even though they are not perfect, are getting the conversation into the mainstream and for the first time allowing oncologists and researchers and policy makers to start some kind of comparative analyses looking at one treatment compared to the other in the same disease setting as offering a greater or lesser benefit to patients,” which is an important conversation as health care systems are faced with stretching their limited resources to help the populations they serve.
The other key finding of the report was the lack of correlation between the scores yielded by the ASCO Value Framework and the ESMO Magnitude of Clinical Benefit Scale.
“We looked at a number of randomized trials of new cancer treatments and we scored them using the European approach and the American approach and found that there is actually fairly little agreement between the two systems,” Dr. Booth said.
The cause, he suggested, was tied to differences in methodology between the two systems, though he noted that both frameworks are evolving and “I suspect there will be convergence over time.”
New high-priced cancer treatments rarely demonstrate value, compared with the prices being charged for them.
Researchers examined clinical trial data for new treatments using scoring frameworks developed by both the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) that assess the value of cancer therapies by taking into consideration survival gains in relation to toxicity and quality. Findings were simultaneously published online June 2 in The Lancet Oncology (2017 June 2. doi: 10.1016/S1470-2045(17)30415-1) and presented at the annual meeting of the American Society of Clinical Oncology.
One of the findings from the research quantified the magnitude of how much benefit a new treatment offers patients.
“We were able to compare that across different treatments and we were able to calculate the drug cost per month of treatment with each new cancer treatment,” Christopher Booth, MD, of Queen’s University Cancer Research Institute, Kingston, Ont., and one of the report’s authors, said in an interview.
“We looked to see whether there is a relationship between cost and benefit. In most elements of our economy, if something is of higher value and better quality, we often pay more for it as opposed to something that offers less quality or less value,” he continued. “We found that that relationship does not hold true in the world of cancer drugs. In fact, if anything, we saw an inverse association, meaning that the drugs that are the most expensive actually have the smallest benefit for patients. That is probably the most important finding of the work.”
Dr. Booth suggested that this has to do with the fact that value is never a factor in pricing.
“In very general terms, the way that a new drug is priced is effectively the highest price the market will bear,” he said. “There really isn’t an approach currently to price drugs based on the return that they offer to patients and society. There is a conversation under way within the oncology community about whether we need to be shifting that conversation to something perhaps called value-based pricing where treatments that offer greater benefit to patients are priced higher than treatments that offer negligible benefit.”
He also noted that “there is not a lot of incentive for the research community or pharmaceutical companies to identify drugs that have larger and larger benefits, being that the financial return on their investment does not appear to be related to how much benefit the drug offers. If we shifted that conversation, it would hopefully, at least in some way, drive the research community and push us to find treatments that have large or meaningful benefits to patients instead of some of these treatments that are very expensive with important side effects that really offer very small improvements in patient outcomes.”
Dr. Booth continued: “I think it’s inevitable and I think what these score cards are doing, and even though they are not perfect, are getting the conversation into the mainstream and for the first time allowing oncologists and researchers and policy makers to start some kind of comparative analyses looking at one treatment compared to the other in the same disease setting as offering a greater or lesser benefit to patients,” which is an important conversation as health care systems are faced with stretching their limited resources to help the populations they serve.
The other key finding of the report was the lack of correlation between the scores yielded by the ASCO Value Framework and the ESMO Magnitude of Clinical Benefit Scale.
“We looked at a number of randomized trials of new cancer treatments and we scored them using the European approach and the American approach and found that there is actually fairly little agreement between the two systems,” Dr. Booth said.
The cause, he suggested, was tied to differences in methodology between the two systems, though he noted that both frameworks are evolving and “I suspect there will be convergence over time.”
New high-priced cancer treatments rarely demonstrate value, compared with the prices being charged for them.
Researchers examined clinical trial data for new treatments using scoring frameworks developed by both the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) that assess the value of cancer therapies by taking into consideration survival gains in relation to toxicity and quality. Findings were simultaneously published online June 2 in The Lancet Oncology (2017 June 2. doi: 10.1016/S1470-2045(17)30415-1) and presented at the annual meeting of the American Society of Clinical Oncology.
One of the findings from the research quantified the magnitude of how much benefit a new treatment offers patients.
“We were able to compare that across different treatments and we were able to calculate the drug cost per month of treatment with each new cancer treatment,” Christopher Booth, MD, of Queen’s University Cancer Research Institute, Kingston, Ont., and one of the report’s authors, said in an interview.
“We looked to see whether there is a relationship between cost and benefit. In most elements of our economy, if something is of higher value and better quality, we often pay more for it as opposed to something that offers less quality or less value,” he continued. “We found that that relationship does not hold true in the world of cancer drugs. In fact, if anything, we saw an inverse association, meaning that the drugs that are the most expensive actually have the smallest benefit for patients. That is probably the most important finding of the work.”
Dr. Booth suggested that this has to do with the fact that value is never a factor in pricing.
“In very general terms, the way that a new drug is priced is effectively the highest price the market will bear,” he said. “There really isn’t an approach currently to price drugs based on the return that they offer to patients and society. There is a conversation under way within the oncology community about whether we need to be shifting that conversation to something perhaps called value-based pricing where treatments that offer greater benefit to patients are priced higher than treatments that offer negligible benefit.”
He also noted that “there is not a lot of incentive for the research community or pharmaceutical companies to identify drugs that have larger and larger benefits, being that the financial return on their investment does not appear to be related to how much benefit the drug offers. If we shifted that conversation, it would hopefully, at least in some way, drive the research community and push us to find treatments that have large or meaningful benefits to patients instead of some of these treatments that are very expensive with important side effects that really offer very small improvements in patient outcomes.”
Dr. Booth continued: “I think it’s inevitable and I think what these score cards are doing, and even though they are not perfect, are getting the conversation into the mainstream and for the first time allowing oncologists and researchers and policy makers to start some kind of comparative analyses looking at one treatment compared to the other in the same disease setting as offering a greater or lesser benefit to patients,” which is an important conversation as health care systems are faced with stretching their limited resources to help the populations they serve.
The other key finding of the report was the lack of correlation between the scores yielded by the ASCO Value Framework and the ESMO Magnitude of Clinical Benefit Scale.
“We looked at a number of randomized trials of new cancer treatments and we scored them using the European approach and the American approach and found that there is actually fairly little agreement between the two systems,” Dr. Booth said.
The cause, he suggested, was tied to differences in methodology between the two systems, though he noted that both frameworks are evolving and “I suspect there will be convergence over time.”
FROM ASCO 2017
VIDEO: Olaparib improves outlook in women with BRCA-related HER2-negative MBC
CHICAGO – Compared with single-agent chemotherapy of the oncologist’s choice, the PARP inhibitor olaparib reduced the risk of progression or death by 42% in women with BRCA-related HER2-negative metastatic breast cancer, the OlympiAD trialists reported in a plenary session at the annual meeting of the American Society of Clinical Oncology.
Lead author Mark E. Robson, MD, of Memorial Sloan Kettering Cancer Center, New York, discussed findings of the randomized phase III trial, as well as strategies for building on the trial’s success and what the future holds for this class of agents in breast cancer.
Dr. Robson disclosed that he has a consulting or advisory role with McKesson and AstraZeneca; receives travel, accommodations, and/or expenses from AstraZeneca; receives honoraria from AstraZeneca; and receives research funding (institutional) from AstraZeneca, Abbvie, Myriad Genetics, Biomarin, Medivation, and Tesaro. The trial was funded by AstraZeneca.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Compared with single-agent chemotherapy of the oncologist’s choice, the PARP inhibitor olaparib reduced the risk of progression or death by 42% in women with BRCA-related HER2-negative metastatic breast cancer, the OlympiAD trialists reported in a plenary session at the annual meeting of the American Society of Clinical Oncology.
Lead author Mark E. Robson, MD, of Memorial Sloan Kettering Cancer Center, New York, discussed findings of the randomized phase III trial, as well as strategies for building on the trial’s success and what the future holds for this class of agents in breast cancer.
Dr. Robson disclosed that he has a consulting or advisory role with McKesson and AstraZeneca; receives travel, accommodations, and/or expenses from AstraZeneca; receives honoraria from AstraZeneca; and receives research funding (institutional) from AstraZeneca, Abbvie, Myriad Genetics, Biomarin, Medivation, and Tesaro. The trial was funded by AstraZeneca.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Compared with single-agent chemotherapy of the oncologist’s choice, the PARP inhibitor olaparib reduced the risk of progression or death by 42% in women with BRCA-related HER2-negative metastatic breast cancer, the OlympiAD trialists reported in a plenary session at the annual meeting of the American Society of Clinical Oncology.
Lead author Mark E. Robson, MD, of Memorial Sloan Kettering Cancer Center, New York, discussed findings of the randomized phase III trial, as well as strategies for building on the trial’s success and what the future holds for this class of agents in breast cancer.
Dr. Robson disclosed that he has a consulting or advisory role with McKesson and AstraZeneca; receives travel, accommodations, and/or expenses from AstraZeneca; receives honoraria from AstraZeneca; and receives research funding (institutional) from AstraZeneca, Abbvie, Myriad Genetics, Biomarin, Medivation, and Tesaro. The trial was funded by AstraZeneca.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASCO 2017




















