User login
VIDEO: Helping cancer patients cope with psychological side effects
CHICAGO – Oncologists are highly skilled at minimizing side effects associated with toxic but curative therapies, but are less adept at helping patients cope with the distress, anxiety, fear, and other emotions associated with cancer.
Three studies presented at the annual meeting of the American Society of Clinical Oncology detail randomized, controlled trials of psychological interventions aimed at helping patients cope with a new cancer diagnosis, reduce fears of a recurrence, and come to grips with the realities of advanced disease, including fears of death or disability.
Don S. Dizon, MD, from the Massachusetts General Hospital Cancer Center, Boston, discusses the social and financial barriers that cancer patients face when they experience distress, and the difficulties that providers face with limited time and financial resources to help patients cope in this video interview.
Dr. Dizon reported having no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Oncologists are highly skilled at minimizing side effects associated with toxic but curative therapies, but are less adept at helping patients cope with the distress, anxiety, fear, and other emotions associated with cancer.
Three studies presented at the annual meeting of the American Society of Clinical Oncology detail randomized, controlled trials of psychological interventions aimed at helping patients cope with a new cancer diagnosis, reduce fears of a recurrence, and come to grips with the realities of advanced disease, including fears of death or disability.
Don S. Dizon, MD, from the Massachusetts General Hospital Cancer Center, Boston, discusses the social and financial barriers that cancer patients face when they experience distress, and the difficulties that providers face with limited time and financial resources to help patients cope in this video interview.
Dr. Dizon reported having no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Oncologists are highly skilled at minimizing side effects associated with toxic but curative therapies, but are less adept at helping patients cope with the distress, anxiety, fear, and other emotions associated with cancer.
Three studies presented at the annual meeting of the American Society of Clinical Oncology detail randomized, controlled trials of psychological interventions aimed at helping patients cope with a new cancer diagnosis, reduce fears of a recurrence, and come to grips with the realities of advanced disease, including fears of death or disability.
Don S. Dizon, MD, from the Massachusetts General Hospital Cancer Center, Boston, discusses the social and financial barriers that cancer patients face when they experience distress, and the difficulties that providers face with limited time and financial resources to help patients cope in this video interview.
Dr. Dizon reported having no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASCO 2017
VIDEO: Childhood cancer survivors living longer with fewer severe health problems
CHICAGO – Severe health problems occurring 5 or more years after diagnosis of a childhood cancer have been steadily declining, based on an analysis of 23,600 participants in the Childhood Cancer Survivor Study (CCSS), funded by the National Institutes of Health.
Watch our video interview with lead author Todd M. Gibson, PhD, of St. Jude Children’s Research Hospital, Memphis, who reported the data at a press conference at the annual meeting of the American Society of Clinical Oncology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @maryjodales
CHICAGO – Severe health problems occurring 5 or more years after diagnosis of a childhood cancer have been steadily declining, based on an analysis of 23,600 participants in the Childhood Cancer Survivor Study (CCSS), funded by the National Institutes of Health.
Watch our video interview with lead author Todd M. Gibson, PhD, of St. Jude Children’s Research Hospital, Memphis, who reported the data at a press conference at the annual meeting of the American Society of Clinical Oncology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @maryjodales
CHICAGO – Severe health problems occurring 5 or more years after diagnosis of a childhood cancer have been steadily declining, based on an analysis of 23,600 participants in the Childhood Cancer Survivor Study (CCSS), funded by the National Institutes of Health.
Watch our video interview with lead author Todd M. Gibson, PhD, of St. Jude Children’s Research Hospital, Memphis, who reported the data at a press conference at the annual meeting of the American Society of Clinical Oncology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @maryjodales
AT ASCO 2017
Differences emerge in new guidelines for managing FN in kids
A multidisciplinary, international panel of experts has updated earlier clinical practice guidelines on managing fever and neutropenia (FN) in children with cancer and in those undergoing hematopoietic stem cell transplantation (HSCT). And while most of the recommendations remained unchanged from the 2012 guidelines, a few key differences emerged. The changes included addition of a 4th generation cephalosporin for empirical antifungal therapy and refinements in risk stratification for invasive fungal disease (IFD), among others.
The new guidelines were published by The International Pediatric Fever and Neutropenia Guideline Panel in the Journal of Clinical Oncology.
The recommendations were organized into 3 major sections: initial presentation, ongoing management, and empirical antifungal therapy. The guidelines panel followed procedures previously validated for creating evidence-based guidelines and used the Appraisal of Guidelines for Research & Evaluation II instrument as a framework.
For the initial presentation of FN, the panel increased the quality of evidence from low to moderate in the recommendation to obtain peripheral blood cultures concurrent with central venous catheter cultures.
In the treatment of FN, the panel added a 4th-generation cephalosporin as empirical therapy in high-risk FN.
The panel refined the IFD risk factors and decreased the quality of evidence from moderate to low. Children with acute myeloid leukemia (AML), high-risk acute lymphoblastic leukemia (ALL), relapsed acute leukemia, those undergoing allogeneic HSCT, those with prolonged neutropenia, and those receiving high-dose corticosteroids are at high risk of IFD. All others should be categorized as IFD low risk.
The panel suggested serum galactomannan not be used to guide empirical antifungal management for prolonged FN lasting 96 hours or more in high-risk IFD patients. GM does not rule out non-Aspergillus molds, and therefore high negative values provide less useful predictions. Previously, the use of galactomannan was a weak recommendation.
The panel added a new recommendation against using fungal polymerase chain reaction (PCR) testing in blood. They explained PCR testing provides poor positive predictive values and negative predictive values are not sufficiently high to be clinically useful. Also, PCR testing is not yet standardized.
Another new recommendation is the addition of imaging of the abdomen in patients without localizing signs or symptoms. Even though the ideal imaging modality is not known, ultrasound is readily available, not associated with radiation exposure, and usually does not require sedation. For these reasons, the panel said it is preferable to computed tomography or magnetic resonance imaging.
The panel also changed a previously weak recommendation to administer empirical therapy for IFD low-risk patients with prolonged FN to a weak recommendation against administering therapy for these patients.
The panel's recommendations and their rationale can be found in the JCO article.
The guidelines update was supported by meeting grants from the Canadian Institutes of Health Research and the Garron Comprehensive Cancer Centre. ![]()
A multidisciplinary, international panel of experts has updated earlier clinical practice guidelines on managing fever and neutropenia (FN) in children with cancer and in those undergoing hematopoietic stem cell transplantation (HSCT). And while most of the recommendations remained unchanged from the 2012 guidelines, a few key differences emerged. The changes included addition of a 4th generation cephalosporin for empirical antifungal therapy and refinements in risk stratification for invasive fungal disease (IFD), among others.
The new guidelines were published by The International Pediatric Fever and Neutropenia Guideline Panel in the Journal of Clinical Oncology.
The recommendations were organized into 3 major sections: initial presentation, ongoing management, and empirical antifungal therapy. The guidelines panel followed procedures previously validated for creating evidence-based guidelines and used the Appraisal of Guidelines for Research & Evaluation II instrument as a framework.
For the initial presentation of FN, the panel increased the quality of evidence from low to moderate in the recommendation to obtain peripheral blood cultures concurrent with central venous catheter cultures.
In the treatment of FN, the panel added a 4th-generation cephalosporin as empirical therapy in high-risk FN.
The panel refined the IFD risk factors and decreased the quality of evidence from moderate to low. Children with acute myeloid leukemia (AML), high-risk acute lymphoblastic leukemia (ALL), relapsed acute leukemia, those undergoing allogeneic HSCT, those with prolonged neutropenia, and those receiving high-dose corticosteroids are at high risk of IFD. All others should be categorized as IFD low risk.
The panel suggested serum galactomannan not be used to guide empirical antifungal management for prolonged FN lasting 96 hours or more in high-risk IFD patients. GM does not rule out non-Aspergillus molds, and therefore high negative values provide less useful predictions. Previously, the use of galactomannan was a weak recommendation.
The panel added a new recommendation against using fungal polymerase chain reaction (PCR) testing in blood. They explained PCR testing provides poor positive predictive values and negative predictive values are not sufficiently high to be clinically useful. Also, PCR testing is not yet standardized.
Another new recommendation is the addition of imaging of the abdomen in patients without localizing signs or symptoms. Even though the ideal imaging modality is not known, ultrasound is readily available, not associated with radiation exposure, and usually does not require sedation. For these reasons, the panel said it is preferable to computed tomography or magnetic resonance imaging.
The panel also changed a previously weak recommendation to administer empirical therapy for IFD low-risk patients with prolonged FN to a weak recommendation against administering therapy for these patients.
The panel's recommendations and their rationale can be found in the JCO article.
The guidelines update was supported by meeting grants from the Canadian Institutes of Health Research and the Garron Comprehensive Cancer Centre. ![]()
A multidisciplinary, international panel of experts has updated earlier clinical practice guidelines on managing fever and neutropenia (FN) in children with cancer and in those undergoing hematopoietic stem cell transplantation (HSCT). And while most of the recommendations remained unchanged from the 2012 guidelines, a few key differences emerged. The changes included addition of a 4th generation cephalosporin for empirical antifungal therapy and refinements in risk stratification for invasive fungal disease (IFD), among others.
The new guidelines were published by The International Pediatric Fever and Neutropenia Guideline Panel in the Journal of Clinical Oncology.
The recommendations were organized into 3 major sections: initial presentation, ongoing management, and empirical antifungal therapy. The guidelines panel followed procedures previously validated for creating evidence-based guidelines and used the Appraisal of Guidelines for Research & Evaluation II instrument as a framework.
For the initial presentation of FN, the panel increased the quality of evidence from low to moderate in the recommendation to obtain peripheral blood cultures concurrent with central venous catheter cultures.
In the treatment of FN, the panel added a 4th-generation cephalosporin as empirical therapy in high-risk FN.
The panel refined the IFD risk factors and decreased the quality of evidence from moderate to low. Children with acute myeloid leukemia (AML), high-risk acute lymphoblastic leukemia (ALL), relapsed acute leukemia, those undergoing allogeneic HSCT, those with prolonged neutropenia, and those receiving high-dose corticosteroids are at high risk of IFD. All others should be categorized as IFD low risk.
The panel suggested serum galactomannan not be used to guide empirical antifungal management for prolonged FN lasting 96 hours or more in high-risk IFD patients. GM does not rule out non-Aspergillus molds, and therefore high negative values provide less useful predictions. Previously, the use of galactomannan was a weak recommendation.
The panel added a new recommendation against using fungal polymerase chain reaction (PCR) testing in blood. They explained PCR testing provides poor positive predictive values and negative predictive values are not sufficiently high to be clinically useful. Also, PCR testing is not yet standardized.
Another new recommendation is the addition of imaging of the abdomen in patients without localizing signs or symptoms. Even though the ideal imaging modality is not known, ultrasound is readily available, not associated with radiation exposure, and usually does not require sedation. For these reasons, the panel said it is preferable to computed tomography or magnetic resonance imaging.
The panel also changed a previously weak recommendation to administer empirical therapy for IFD low-risk patients with prolonged FN to a weak recommendation against administering therapy for these patients.
The panel's recommendations and their rationale can be found in the JCO article.
The guidelines update was supported by meeting grants from the Canadian Institutes of Health Research and the Garron Comprehensive Cancer Centre. ![]()
Access to ‘the little blue book’ just got a lot more expensive
“The little blue book” has been an office standard as long as I’ve been in practice. Every practice has a dog-eared copy in a drawer somewhere that’s constantly being pulled out to look up hospitals, other doctors, and pharmacies.
As small as it is, it’s pretty useful in the daily flow of my office routine.
Until now.
Sadly, 2016 was apparently the year I ordered my last copies. The publisher’s marketing people inform me that the paper version has been discontinued, and I can now get the digital version for only ... $500 per year.
Thanks, but no thanks.
I have nothing against digital editions. In fact, if it was the same price as the paper one, I’d get it. If I were a big practice that needed, say, 50 copies for the staff, the $500 per practice fee is a deal, compared with the $998 I’d pay for 50 paper copies.
But for my dinky little two-person practice? The difference between $39.90 and $500 just isn’t worth all the advantages a digital version may offer. For that kind of money, I’ll use Google.
This is another part of a gradual, and disturbing, trend in medicine: ignoring small practices. Large corporate practices are worth a lot more in sales than little one-to-three doctor groups, so companies, such as “the little blue book,” have no incentive to tailor their products to us. We have become medical persona non grata.
I understand this is a business decision. It’s not specifically directed at me.
Yet ...
I’ve been in practice for almost 20 years now, and buying two copies of the LBB is something I’ve done annually, in good and bad economic years. I’ve supported the publisher because it was a good product at a fair price. Sadly, they no longer find my little practice to be worth the effort or profit margin.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
“The little blue book” has been an office standard as long as I’ve been in practice. Every practice has a dog-eared copy in a drawer somewhere that’s constantly being pulled out to look up hospitals, other doctors, and pharmacies.
As small as it is, it’s pretty useful in the daily flow of my office routine.
Until now.
Sadly, 2016 was apparently the year I ordered my last copies. The publisher’s marketing people inform me that the paper version has been discontinued, and I can now get the digital version for only ... $500 per year.
Thanks, but no thanks.
I have nothing against digital editions. In fact, if it was the same price as the paper one, I’d get it. If I were a big practice that needed, say, 50 copies for the staff, the $500 per practice fee is a deal, compared with the $998 I’d pay for 50 paper copies.
But for my dinky little two-person practice? The difference between $39.90 and $500 just isn’t worth all the advantages a digital version may offer. For that kind of money, I’ll use Google.
This is another part of a gradual, and disturbing, trend in medicine: ignoring small practices. Large corporate practices are worth a lot more in sales than little one-to-three doctor groups, so companies, such as “the little blue book,” have no incentive to tailor their products to us. We have become medical persona non grata.
I understand this is a business decision. It’s not specifically directed at me.
Yet ...
I’ve been in practice for almost 20 years now, and buying two copies of the LBB is something I’ve done annually, in good and bad economic years. I’ve supported the publisher because it was a good product at a fair price. Sadly, they no longer find my little practice to be worth the effort or profit margin.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
“The little blue book” has been an office standard as long as I’ve been in practice. Every practice has a dog-eared copy in a drawer somewhere that’s constantly being pulled out to look up hospitals, other doctors, and pharmacies.
As small as it is, it’s pretty useful in the daily flow of my office routine.
Until now.
Sadly, 2016 was apparently the year I ordered my last copies. The publisher’s marketing people inform me that the paper version has been discontinued, and I can now get the digital version for only ... $500 per year.
Thanks, but no thanks.
I have nothing against digital editions. In fact, if it was the same price as the paper one, I’d get it. If I were a big practice that needed, say, 50 copies for the staff, the $500 per practice fee is a deal, compared with the $998 I’d pay for 50 paper copies.
But for my dinky little two-person practice? The difference between $39.90 and $500 just isn’t worth all the advantages a digital version may offer. For that kind of money, I’ll use Google.
This is another part of a gradual, and disturbing, trend in medicine: ignoring small practices. Large corporate practices are worth a lot more in sales than little one-to-three doctor groups, so companies, such as “the little blue book,” have no incentive to tailor their products to us. We have become medical persona non grata.
I understand this is a business decision. It’s not specifically directed at me.
Yet ...
I’ve been in practice for almost 20 years now, and buying two copies of the LBB is something I’ve done annually, in good and bad economic years. I’ve supported the publisher because it was a good product at a fair price. Sadly, they no longer find my little practice to be worth the effort or profit margin.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Effective monitoring of DMTs for multiple sclerosis can be tricky
NEW ORLEANS – Safety and efficacy laboratory monitoring of adherence to disease-modifying therapies for multiple sclerosis remains challenging, results from a small pilot study showed.
In an effort to determine if select patients on specific DMTs are receiving appropriate and adequate monitoring as outlined by each DMT’s internal guidance document, Felecia Hart, PharmD, and her associates retrospectively reviewed existing patients treated for MS at the Rocky Mountain Multiple Sclerosis Center at the University of Colorado, Denver, between June 1, 2013, and June 1, 2016.
“What we wanted to know was, if patients are getting their infusions or lab work done elsewhere, are we properly documenting it and keeping track of it?” Dr. Hart said in an interview at the meeting. “We’re not looking for core outcomes yet.”
Dr. Hart, a clinical pharmacy neurology fellow at the Skaggs School of Pharmacy and Pharmaceutical Sciences on the University of Colorado Anschutz Medical Campus, reported preliminary results from 50 patients treated with natalizumab and 50 treated with fingolimod. Among those treated with natalizumab, 49 had vitamin D measured within 6 months of drug initiation, 49 had a complete blood count measured within 1 year of drug initiation, and 49 had a comprehensive metabolic panel (CMP) measured within 1 year of drug initiation. “Interestingly, the absent CBC and CMP were from different patients,” she said.
Among patients treated with fingolimod, all had CBC/CMP measured within 1 year of drug initiation, 48 had vitamin D measured within 6 months of drug initiation, and 47 had documented macular optical coherence tomography at baseline, but the proportion of patients who had adequate documentation for other recommended assessments declined significantly. For example, only 19 of 50 had a documented repeat echocardiography within 3 months of drug initiation. Also, several baseline measurements required prior to drug initiation were documented poorly or in an untimely manner. Four patients had their HIV-1 and -2 antibody measured after drug initiation, three had hepatitis B virus surface antigen measured after drug initiation, and three had varicella measured after drug initiation.
Even though the MS center has an electronic medical record system, Dr. Hart and her associates found it difficult to obtain and monitor the parameters of interest. “I’ve been working with our EMR for 6 years, so I know how to navigate it well,” she said. “But I found it difficult to do a simple chart review and find what I wanted to. We have a labs tab in our chart, but the difficulty became including patients who were coming from outside of our center. The lab and the order were referenced in a note but there was never any documentation after that. There are too many holes in getting it documented correctly.”
The findings suggest that having a dedicated clinician such as a clinical pharmacist to oversee pharmacovigilance may improve patient outcomes and ensure that safety and efficacy monitoring doesn’t inadvertently get overlooked because of difficulties with adequate documentation. “That would be ideal,” Dr. Hart said.
She reported having no financial disclosures.
NEW ORLEANS – Safety and efficacy laboratory monitoring of adherence to disease-modifying therapies for multiple sclerosis remains challenging, results from a small pilot study showed.
In an effort to determine if select patients on specific DMTs are receiving appropriate and adequate monitoring as outlined by each DMT’s internal guidance document, Felecia Hart, PharmD, and her associates retrospectively reviewed existing patients treated for MS at the Rocky Mountain Multiple Sclerosis Center at the University of Colorado, Denver, between June 1, 2013, and June 1, 2016.
“What we wanted to know was, if patients are getting their infusions or lab work done elsewhere, are we properly documenting it and keeping track of it?” Dr. Hart said in an interview at the meeting. “We’re not looking for core outcomes yet.”
Dr. Hart, a clinical pharmacy neurology fellow at the Skaggs School of Pharmacy and Pharmaceutical Sciences on the University of Colorado Anschutz Medical Campus, reported preliminary results from 50 patients treated with natalizumab and 50 treated with fingolimod. Among those treated with natalizumab, 49 had vitamin D measured within 6 months of drug initiation, 49 had a complete blood count measured within 1 year of drug initiation, and 49 had a comprehensive metabolic panel (CMP) measured within 1 year of drug initiation. “Interestingly, the absent CBC and CMP were from different patients,” she said.
Among patients treated with fingolimod, all had CBC/CMP measured within 1 year of drug initiation, 48 had vitamin D measured within 6 months of drug initiation, and 47 had documented macular optical coherence tomography at baseline, but the proportion of patients who had adequate documentation for other recommended assessments declined significantly. For example, only 19 of 50 had a documented repeat echocardiography within 3 months of drug initiation. Also, several baseline measurements required prior to drug initiation were documented poorly or in an untimely manner. Four patients had their HIV-1 and -2 antibody measured after drug initiation, three had hepatitis B virus surface antigen measured after drug initiation, and three had varicella measured after drug initiation.
Even though the MS center has an electronic medical record system, Dr. Hart and her associates found it difficult to obtain and monitor the parameters of interest. “I’ve been working with our EMR for 6 years, so I know how to navigate it well,” she said. “But I found it difficult to do a simple chart review and find what I wanted to. We have a labs tab in our chart, but the difficulty became including patients who were coming from outside of our center. The lab and the order were referenced in a note but there was never any documentation after that. There are too many holes in getting it documented correctly.”
The findings suggest that having a dedicated clinician such as a clinical pharmacist to oversee pharmacovigilance may improve patient outcomes and ensure that safety and efficacy monitoring doesn’t inadvertently get overlooked because of difficulties with adequate documentation. “That would be ideal,” Dr. Hart said.
She reported having no financial disclosures.
NEW ORLEANS – Safety and efficacy laboratory monitoring of adherence to disease-modifying therapies for multiple sclerosis remains challenging, results from a small pilot study showed.
In an effort to determine if select patients on specific DMTs are receiving appropriate and adequate monitoring as outlined by each DMT’s internal guidance document, Felecia Hart, PharmD, and her associates retrospectively reviewed existing patients treated for MS at the Rocky Mountain Multiple Sclerosis Center at the University of Colorado, Denver, between June 1, 2013, and June 1, 2016.
“What we wanted to know was, if patients are getting their infusions or lab work done elsewhere, are we properly documenting it and keeping track of it?” Dr. Hart said in an interview at the meeting. “We’re not looking for core outcomes yet.”
Dr. Hart, a clinical pharmacy neurology fellow at the Skaggs School of Pharmacy and Pharmaceutical Sciences on the University of Colorado Anschutz Medical Campus, reported preliminary results from 50 patients treated with natalizumab and 50 treated with fingolimod. Among those treated with natalizumab, 49 had vitamin D measured within 6 months of drug initiation, 49 had a complete blood count measured within 1 year of drug initiation, and 49 had a comprehensive metabolic panel (CMP) measured within 1 year of drug initiation. “Interestingly, the absent CBC and CMP were from different patients,” she said.
Among patients treated with fingolimod, all had CBC/CMP measured within 1 year of drug initiation, 48 had vitamin D measured within 6 months of drug initiation, and 47 had documented macular optical coherence tomography at baseline, but the proportion of patients who had adequate documentation for other recommended assessments declined significantly. For example, only 19 of 50 had a documented repeat echocardiography within 3 months of drug initiation. Also, several baseline measurements required prior to drug initiation were documented poorly or in an untimely manner. Four patients had their HIV-1 and -2 antibody measured after drug initiation, three had hepatitis B virus surface antigen measured after drug initiation, and three had varicella measured after drug initiation.
Even though the MS center has an electronic medical record system, Dr. Hart and her associates found it difficult to obtain and monitor the parameters of interest. “I’ve been working with our EMR for 6 years, so I know how to navigate it well,” she said. “But I found it difficult to do a simple chart review and find what I wanted to. We have a labs tab in our chart, but the difficulty became including patients who were coming from outside of our center. The lab and the order were referenced in a note but there was never any documentation after that. There are too many holes in getting it documented correctly.”
The findings suggest that having a dedicated clinician such as a clinical pharmacist to oversee pharmacovigilance may improve patient outcomes and ensure that safety and efficacy monitoring doesn’t inadvertently get overlooked because of difficulties with adequate documentation. “That would be ideal,” Dr. Hart said.
She reported having no financial disclosures.
AT THE CMSC ANNUAL MEETING
Key clinical point:
Major finding: Among patients treated with fingolimod, only 19 of 50 had a documented repeat ECG within 3 months of drug initiation. Also, several baseline measurements required prior to drug initiation were documented poorly or in an untimely manner.
Data source: Preliminary results from a retrospective review of 50 patients treated with natalizumab and 50 treated with fingolimod.
Disclosures: Dr. Hart reported having no financial disclosures.
Lactulose plus albumin is more effective than lactulose alone for treatment of hepatic encephalopathy
Clinical Question: Is the combination of lactulose plus albumin more effective than lactulose alone for treatment of hepatic encephalopathy?
Background: Hepatic encephalopathy is caused by the effect of toxins that build up in the bloodstream when the liver is not able to perform its normal functions. Lactulose is primarily directed at the reduction of blood ammonia levels. Albumin is thought to minimize oxidative injury and improve circulatory dysfunction present in cirrhosis.
Setting: Tertiary care centers in India.
Synopsis: 120 patients with overt hepatic encephalopathy were randomized to treatment with lactulose plus albumin (1.5 gm/kg/day; n = 60), versus lactulose alone (n = 60). Patients with serum creatinine greater than 1.5 mg/dL on admission, active alcohol intake less than 4 weeks prior to presentation, other metabolic encephalopathies, or hepatocellular carcinoma were excluded. Treatment was continued up to a maximum of 10 days until complete resolution of hepatic encephalopathy as assessed independently by two expert hepatologists.
Of patients receiving lactulose plus albumin, 75% had complete reversal of hepatic encephalopathy within 10 days, compared with 53% of patients receiving lactulose alone (P = .03). Patients in lactulose plus albumin group had shorter hospital length-of-stay (6.4 vs. 8.6 days; P = .01). There was lower mortality at 10 days in the lactulose plus albumin group (18.3% vs. 31.6%; P = .04).
Limitations of the study include the noted exclusion factors, including presence of alcohol intake, limitation to a single country (India), and a relatively high mortality rate in both groups.
Bottom Line: Combination of lactulose plus albumin is more effective than lactulose alone at reversing hepatic encephalopathy and is also associated with decreased length-of-stay and mortality.
Reference: Sharma BC, Singh J, Srivastava S, et al. A randomized controlled trial comparing lactulose plus albumin with lactulose alone for treatment of hepatic encephalopathy. J Gastroenterol Hepatol. Published online Nov 25, 2016. doi: 10.1111/jgh.13666.
Dr. Huang is associate clinical professor in the division of hospital medicine, department of medicine, University of California, San Diego.
Clinical Question: Is the combination of lactulose plus albumin more effective than lactulose alone for treatment of hepatic encephalopathy?
Background: Hepatic encephalopathy is caused by the effect of toxins that build up in the bloodstream when the liver is not able to perform its normal functions. Lactulose is primarily directed at the reduction of blood ammonia levels. Albumin is thought to minimize oxidative injury and improve circulatory dysfunction present in cirrhosis.
Setting: Tertiary care centers in India.
Synopsis: 120 patients with overt hepatic encephalopathy were randomized to treatment with lactulose plus albumin (1.5 gm/kg/day; n = 60), versus lactulose alone (n = 60). Patients with serum creatinine greater than 1.5 mg/dL on admission, active alcohol intake less than 4 weeks prior to presentation, other metabolic encephalopathies, or hepatocellular carcinoma were excluded. Treatment was continued up to a maximum of 10 days until complete resolution of hepatic encephalopathy as assessed independently by two expert hepatologists.
Of patients receiving lactulose plus albumin, 75% had complete reversal of hepatic encephalopathy within 10 days, compared with 53% of patients receiving lactulose alone (P = .03). Patients in lactulose plus albumin group had shorter hospital length-of-stay (6.4 vs. 8.6 days; P = .01). There was lower mortality at 10 days in the lactulose plus albumin group (18.3% vs. 31.6%; P = .04).
Limitations of the study include the noted exclusion factors, including presence of alcohol intake, limitation to a single country (India), and a relatively high mortality rate in both groups.
Bottom Line: Combination of lactulose plus albumin is more effective than lactulose alone at reversing hepatic encephalopathy and is also associated with decreased length-of-stay and mortality.
Reference: Sharma BC, Singh J, Srivastava S, et al. A randomized controlled trial comparing lactulose plus albumin with lactulose alone for treatment of hepatic encephalopathy. J Gastroenterol Hepatol. Published online Nov 25, 2016. doi: 10.1111/jgh.13666.
Dr. Huang is associate clinical professor in the division of hospital medicine, department of medicine, University of California, San Diego.
Clinical Question: Is the combination of lactulose plus albumin more effective than lactulose alone for treatment of hepatic encephalopathy?
Background: Hepatic encephalopathy is caused by the effect of toxins that build up in the bloodstream when the liver is not able to perform its normal functions. Lactulose is primarily directed at the reduction of blood ammonia levels. Albumin is thought to minimize oxidative injury and improve circulatory dysfunction present in cirrhosis.
Setting: Tertiary care centers in India.
Synopsis: 120 patients with overt hepatic encephalopathy were randomized to treatment with lactulose plus albumin (1.5 gm/kg/day; n = 60), versus lactulose alone (n = 60). Patients with serum creatinine greater than 1.5 mg/dL on admission, active alcohol intake less than 4 weeks prior to presentation, other metabolic encephalopathies, or hepatocellular carcinoma were excluded. Treatment was continued up to a maximum of 10 days until complete resolution of hepatic encephalopathy as assessed independently by two expert hepatologists.
Of patients receiving lactulose plus albumin, 75% had complete reversal of hepatic encephalopathy within 10 days, compared with 53% of patients receiving lactulose alone (P = .03). Patients in lactulose plus albumin group had shorter hospital length-of-stay (6.4 vs. 8.6 days; P = .01). There was lower mortality at 10 days in the lactulose plus albumin group (18.3% vs. 31.6%; P = .04).
Limitations of the study include the noted exclusion factors, including presence of alcohol intake, limitation to a single country (India), and a relatively high mortality rate in both groups.
Bottom Line: Combination of lactulose plus albumin is more effective than lactulose alone at reversing hepatic encephalopathy and is also associated with decreased length-of-stay and mortality.
Reference: Sharma BC, Singh J, Srivastava S, et al. A randomized controlled trial comparing lactulose plus albumin with lactulose alone for treatment of hepatic encephalopathy. J Gastroenterol Hepatol. Published online Nov 25, 2016. doi: 10.1111/jgh.13666.
Dr. Huang is associate clinical professor in the division of hospital medicine, department of medicine, University of California, San Diego.
VIDEO: Hyperinflammatory ARDS responds to simvastatin
WASHINGTON – Acute respiratory distress syndrome (ARDS) appears to exist in at least two major forms, and one of these, the hyperinflammatory form, seemed responsive to simvastatin in a post-hoc analysis of trial data.
The other version of ARDs is a hypoinflammatory form, which occurred in 70% of ARDS patients in most of the analyses that have been done.
Researchers classified the 540 ARDS patients enrolled in a 2014 study of simvastatin as either hyperinflammatory or hypoinflammatory. Separating out the hyperinflammatory patients created a subclass that responded to simvastatin, with a 13% absolute reduction in mortality during follow-up, compared with no response among patients in the hypoinflammatory group, Carolyn S. Calfee, MD, said at an international conference of the American Thoracic Society.
“Hyperinflammatory patients treated with simvastatin may have improved outcomes, compared with hypoinflammatory* patients treated with placebo,” said Dr. Calfee, a pulmonologist at the University of California, San Francisco.
The finding raises the possibility that simvastatin, as well as other statins, may be an effective treatment for selected patients with ARDS, but proving this requires new prospective, randomized trials in hyperinflammatory patients, Dr. Calfee said in a video interview.
Currently, the tests Dr. Calfee uses to distinguish hyperinflammatory and hypoinflammatory ARDS patients take about 6-8 hours to complete. A “point of care test to stratify patients in real time,” is needed to further study the various forms of ARDs, Dr. Calfee noted. A critical next step would be the development of a “practical, rapid, bedside assay” to ease identification of hyperinflammatory ARDS patients. “The work we’ve done prior seems to indicate that we are going to definitely need to measure biomarkers in order to identity these subgroups,” she noted.
Hypoinflammatory patients also merit study, she added. Although hyperinflammatory patients have significant worse mortality rates, the hypoinflammatory subclass includes about 70% of ARDS patients, “so we need to better understand how to potentially treat this group.”
Dr. Calfee and her associates first reported finding the two ARDS subclasses, what they also call subphenotypes or endotypes, in two separate cohorts of ARDS patients in a 2014 report (Lancet Resp Med. 2014 Aug;2[8]:611-20). Then, they confirmed the finding in a third ARDS cohort in a 2017 report (Amer J Resp Crit Care Med. 2017 Feb 1;195[3]:331-8). These reports have documented other characteristics of the hyperinflammatory ARDS subclass: hypotension, metabolic acidosis, more frequent treatment with vasopressors, and a higher prevalence of sepsis and shock. Concurrent with the 2017 report, an editorial hailed the finding as “the dawn of personalized medicine for ARDS” (Amer J resp Crit Care Med. 2017 Feb 1;195[3]: 280-1).
To build on this, Dr. Calfee and her associates applied their method for identifying ARDS subclasses to a different cohort of 540 patients enrolled in the The HARP (Hydroxymethylglutaryl-CoA Reductase Inhibition with Simvastatin in Acute Lung Injury to Reduce Pulmonary Dysfunction)–2 study, a multicenter UK and Irish study designed to test the efficacy of daily simvastatin treatment in a heterogeneous group of ARDS patients. A 2014 report of the study’s primary results showed no significant effect from simvastatin for increasing the number of ventilator-free days nor did the drug improve any other measured efficacy endpoints (New Engl J Med. 2014 Oct 30;371[18]:1695-703).
Applying a statistical analysis called “latent class analysis,” which is designed to recognize subclass groupings that might not be readily apparent, Dr. Calfee and her team first confirmed that, in this fourth cohort, the ARDS patients again split into a hyperinflammatory subclass, in this case including 188 (35%) of the cohort, and a hypoinflammatory subclass with 352 (65%) patients. The next step was to see what impact simvastatin treatment had in each of the two patient subclasses. They focused the analysis on a secondary outcome in HARP-2, 28-day survival.
They found that simvastatin produced no significant difference in 28-day survival, compared with placebo among the hypoinflammatory patients, but, in the hyperinflammatory subclass, 28-day survival was 68% for patients on simvastatin and 55% for those on placebo, a statistically significant difference, Dr. Calfee reported (Am J Resp Crit Care Med. 2017;195:A6749).
“I’m excited that we are seeing, for the first time, a different response to pharmacotherapy” after dividing ARDS patients into these two subclasses, she said. But, the work remains in an early stage, she cautioned. “We need to test treatments [like statins] prospectively.” The new finding for simvastatin “is not the same as showing benefit in a prospective, randomized trial.”
In the meantime, Dr. Calfee plans to apply the same analytic approach to data collected in another failed statin trial in ARDS patients, the SAILS trial. That study failed to show benefit from rosuvastatin treatment in an unselected population of patients with sepsis-associated ARDS (New Engl J Med. 2014 June 5;370[23]:2191-200).
Dr. Calfee is a consultant to Bayer, Boehringer Ingelheim, and GlaxoSmithKline. She received research funding from GlaxoSmithKline.
[email protected]
On Twitter @mitchelzoler
*An earlier version of this article misquoted Dr. Calfee.
WASHINGTON – Acute respiratory distress syndrome (ARDS) appears to exist in at least two major forms, and one of these, the hyperinflammatory form, seemed responsive to simvastatin in a post-hoc analysis of trial data.
The other version of ARDs is a hypoinflammatory form, which occurred in 70% of ARDS patients in most of the analyses that have been done.
Researchers classified the 540 ARDS patients enrolled in a 2014 study of simvastatin as either hyperinflammatory or hypoinflammatory. Separating out the hyperinflammatory patients created a subclass that responded to simvastatin, with a 13% absolute reduction in mortality during follow-up, compared with no response among patients in the hypoinflammatory group, Carolyn S. Calfee, MD, said at an international conference of the American Thoracic Society.
“Hyperinflammatory patients treated with simvastatin may have improved outcomes, compared with hypoinflammatory* patients treated with placebo,” said Dr. Calfee, a pulmonologist at the University of California, San Francisco.
The finding raises the possibility that simvastatin, as well as other statins, may be an effective treatment for selected patients with ARDS, but proving this requires new prospective, randomized trials in hyperinflammatory patients, Dr. Calfee said in a video interview.
Currently, the tests Dr. Calfee uses to distinguish hyperinflammatory and hypoinflammatory ARDS patients take about 6-8 hours to complete. A “point of care test to stratify patients in real time,” is needed to further study the various forms of ARDs, Dr. Calfee noted. A critical next step would be the development of a “practical, rapid, bedside assay” to ease identification of hyperinflammatory ARDS patients. “The work we’ve done prior seems to indicate that we are going to definitely need to measure biomarkers in order to identity these subgroups,” she noted.
Hypoinflammatory patients also merit study, she added. Although hyperinflammatory patients have significant worse mortality rates, the hypoinflammatory subclass includes about 70% of ARDS patients, “so we need to better understand how to potentially treat this group.”
Dr. Calfee and her associates first reported finding the two ARDS subclasses, what they also call subphenotypes or endotypes, in two separate cohorts of ARDS patients in a 2014 report (Lancet Resp Med. 2014 Aug;2[8]:611-20). Then, they confirmed the finding in a third ARDS cohort in a 2017 report (Amer J Resp Crit Care Med. 2017 Feb 1;195[3]:331-8). These reports have documented other characteristics of the hyperinflammatory ARDS subclass: hypotension, metabolic acidosis, more frequent treatment with vasopressors, and a higher prevalence of sepsis and shock. Concurrent with the 2017 report, an editorial hailed the finding as “the dawn of personalized medicine for ARDS” (Amer J resp Crit Care Med. 2017 Feb 1;195[3]: 280-1).
To build on this, Dr. Calfee and her associates applied their method for identifying ARDS subclasses to a different cohort of 540 patients enrolled in the The HARP (Hydroxymethylglutaryl-CoA Reductase Inhibition with Simvastatin in Acute Lung Injury to Reduce Pulmonary Dysfunction)–2 study, a multicenter UK and Irish study designed to test the efficacy of daily simvastatin treatment in a heterogeneous group of ARDS patients. A 2014 report of the study’s primary results showed no significant effect from simvastatin for increasing the number of ventilator-free days nor did the drug improve any other measured efficacy endpoints (New Engl J Med. 2014 Oct 30;371[18]:1695-703).
Applying a statistical analysis called “latent class analysis,” which is designed to recognize subclass groupings that might not be readily apparent, Dr. Calfee and her team first confirmed that, in this fourth cohort, the ARDS patients again split into a hyperinflammatory subclass, in this case including 188 (35%) of the cohort, and a hypoinflammatory subclass with 352 (65%) patients. The next step was to see what impact simvastatin treatment had in each of the two patient subclasses. They focused the analysis on a secondary outcome in HARP-2, 28-day survival.
They found that simvastatin produced no significant difference in 28-day survival, compared with placebo among the hypoinflammatory patients, but, in the hyperinflammatory subclass, 28-day survival was 68% for patients on simvastatin and 55% for those on placebo, a statistically significant difference, Dr. Calfee reported (Am J Resp Crit Care Med. 2017;195:A6749).
“I’m excited that we are seeing, for the first time, a different response to pharmacotherapy” after dividing ARDS patients into these two subclasses, she said. But, the work remains in an early stage, she cautioned. “We need to test treatments [like statins] prospectively.” The new finding for simvastatin “is not the same as showing benefit in a prospective, randomized trial.”
In the meantime, Dr. Calfee plans to apply the same analytic approach to data collected in another failed statin trial in ARDS patients, the SAILS trial. That study failed to show benefit from rosuvastatin treatment in an unselected population of patients with sepsis-associated ARDS (New Engl J Med. 2014 June 5;370[23]:2191-200).
Dr. Calfee is a consultant to Bayer, Boehringer Ingelheim, and GlaxoSmithKline. She received research funding from GlaxoSmithKline.
[email protected]
On Twitter @mitchelzoler
*An earlier version of this article misquoted Dr. Calfee.
WASHINGTON – Acute respiratory distress syndrome (ARDS) appears to exist in at least two major forms, and one of these, the hyperinflammatory form, seemed responsive to simvastatin in a post-hoc analysis of trial data.
The other version of ARDs is a hypoinflammatory form, which occurred in 70% of ARDS patients in most of the analyses that have been done.
Researchers classified the 540 ARDS patients enrolled in a 2014 study of simvastatin as either hyperinflammatory or hypoinflammatory. Separating out the hyperinflammatory patients created a subclass that responded to simvastatin, with a 13% absolute reduction in mortality during follow-up, compared with no response among patients in the hypoinflammatory group, Carolyn S. Calfee, MD, said at an international conference of the American Thoracic Society.
“Hyperinflammatory patients treated with simvastatin may have improved outcomes, compared with hypoinflammatory* patients treated with placebo,” said Dr. Calfee, a pulmonologist at the University of California, San Francisco.
The finding raises the possibility that simvastatin, as well as other statins, may be an effective treatment for selected patients with ARDS, but proving this requires new prospective, randomized trials in hyperinflammatory patients, Dr. Calfee said in a video interview.
Currently, the tests Dr. Calfee uses to distinguish hyperinflammatory and hypoinflammatory ARDS patients take about 6-8 hours to complete. A “point of care test to stratify patients in real time,” is needed to further study the various forms of ARDs, Dr. Calfee noted. A critical next step would be the development of a “practical, rapid, bedside assay” to ease identification of hyperinflammatory ARDS patients. “The work we’ve done prior seems to indicate that we are going to definitely need to measure biomarkers in order to identity these subgroups,” she noted.
Hypoinflammatory patients also merit study, she added. Although hyperinflammatory patients have significant worse mortality rates, the hypoinflammatory subclass includes about 70% of ARDS patients, “so we need to better understand how to potentially treat this group.”
Dr. Calfee and her associates first reported finding the two ARDS subclasses, what they also call subphenotypes or endotypes, in two separate cohorts of ARDS patients in a 2014 report (Lancet Resp Med. 2014 Aug;2[8]:611-20). Then, they confirmed the finding in a third ARDS cohort in a 2017 report (Amer J Resp Crit Care Med. 2017 Feb 1;195[3]:331-8). These reports have documented other characteristics of the hyperinflammatory ARDS subclass: hypotension, metabolic acidosis, more frequent treatment with vasopressors, and a higher prevalence of sepsis and shock. Concurrent with the 2017 report, an editorial hailed the finding as “the dawn of personalized medicine for ARDS” (Amer J resp Crit Care Med. 2017 Feb 1;195[3]: 280-1).
To build on this, Dr. Calfee and her associates applied their method for identifying ARDS subclasses to a different cohort of 540 patients enrolled in the The HARP (Hydroxymethylglutaryl-CoA Reductase Inhibition with Simvastatin in Acute Lung Injury to Reduce Pulmonary Dysfunction)–2 study, a multicenter UK and Irish study designed to test the efficacy of daily simvastatin treatment in a heterogeneous group of ARDS patients. A 2014 report of the study’s primary results showed no significant effect from simvastatin for increasing the number of ventilator-free days nor did the drug improve any other measured efficacy endpoints (New Engl J Med. 2014 Oct 30;371[18]:1695-703).
Applying a statistical analysis called “latent class analysis,” which is designed to recognize subclass groupings that might not be readily apparent, Dr. Calfee and her team first confirmed that, in this fourth cohort, the ARDS patients again split into a hyperinflammatory subclass, in this case including 188 (35%) of the cohort, and a hypoinflammatory subclass with 352 (65%) patients. The next step was to see what impact simvastatin treatment had in each of the two patient subclasses. They focused the analysis on a secondary outcome in HARP-2, 28-day survival.
They found that simvastatin produced no significant difference in 28-day survival, compared with placebo among the hypoinflammatory patients, but, in the hyperinflammatory subclass, 28-day survival was 68% for patients on simvastatin and 55% for those on placebo, a statistically significant difference, Dr. Calfee reported (Am J Resp Crit Care Med. 2017;195:A6749).
“I’m excited that we are seeing, for the first time, a different response to pharmacotherapy” after dividing ARDS patients into these two subclasses, she said. But, the work remains in an early stage, she cautioned. “We need to test treatments [like statins] prospectively.” The new finding for simvastatin “is not the same as showing benefit in a prospective, randomized trial.”
In the meantime, Dr. Calfee plans to apply the same analytic approach to data collected in another failed statin trial in ARDS patients, the SAILS trial. That study failed to show benefit from rosuvastatin treatment in an unselected population of patients with sepsis-associated ARDS (New Engl J Med. 2014 June 5;370[23]:2191-200).
Dr. Calfee is a consultant to Bayer, Boehringer Ingelheim, and GlaxoSmithKline. She received research funding from GlaxoSmithKline.
[email protected]
On Twitter @mitchelzoler
*An earlier version of this article misquoted Dr. Calfee.
AT ATS 2017
Key clinical point:
Major finding: Among hyperinflammatory ARDS patients, 28-day survival was 68% with simvastatin and 55% with placebo, a statistically significant difference.
Data source: A post-hoc analysis of a multicenter randomized trial of 540 patients.
Disclosures: Dr. Calfee is a consultant to Bayer, Boehringer Ingelheim, and GlaxoSmithKline. She received research funding from GlaxoSmithKline.
Coexisting Autoimmune Disorders May Influence Clinical Outcomes in MS
NEW ORLEANS—Patients with multiple sclerosis (MS) had increased rates of in-hospital mortality and discharge to nursing facilities, compared with patients with MS plus a coexisting autoimmune disorder, according to research presented at the 31st Annual Meeting of the Consortium of MS Centers.
“The findings in this study suggest that a coexisting autoimmune disorder may in fact lessen the severity of the immune dysregulation of MS, which leads to improved health outcomes,” said Malik M. Adil, MD, a neurology resident at the Ochsner Clinic Foundation in New Orleans, and colleagues.
Studies have suggested an association between MS and autoimmune disorders, but research concerning clinical outcomes is limited. To determine whether coexisting autoimmune disorders affect outcomes in patients with MS, Dr. Adil and colleagues analyzed Nationwide Inpatient Survey data files from 2006 to 2010. The researchers classified patients with MS into two groups: an MS group (without coexisting autoimmune disorders) and an MS Plus group (with coexisting autoimmune disorders).
The investigators then compared the rate of clinical outcomes (ie, in-hospital mortality, discharge to home, discharge to nursing facilities, length of stay, and total charges) between the MS and MS Plus groups. Dr. Adil and colleagues used multivariate analysis to adjust for potential confounders when assessing in-hospital outcomes.
Of 115,120 patients with MS, 18,796 were in the MS Plus group. The adjusted odds of in-hospital mortality and of discharge to nursing facilities were significantly higher in the MS group, compared with the MS Plus group (odds ratios, 4.67 and 1.16, respectively). Length of stay and mean hospital charges were significantly higher in the MS Plus group, compared with the MS group.
A possible explanation for the findings is that coexisting autoimmune diseases involve different effector T cells that may regulate the effect of other effector T cells, said the authors. “Other possible explanations of better outcome in the MS Plus group might be the use of chronic immune suppressive agents in addition to MS immunotherapy, and multidisciplinary management,” they added.
NEW ORLEANS—Patients with multiple sclerosis (MS) had increased rates of in-hospital mortality and discharge to nursing facilities, compared with patients with MS plus a coexisting autoimmune disorder, according to research presented at the 31st Annual Meeting of the Consortium of MS Centers.
“The findings in this study suggest that a coexisting autoimmune disorder may in fact lessen the severity of the immune dysregulation of MS, which leads to improved health outcomes,” said Malik M. Adil, MD, a neurology resident at the Ochsner Clinic Foundation in New Orleans, and colleagues.
Studies have suggested an association between MS and autoimmune disorders, but research concerning clinical outcomes is limited. To determine whether coexisting autoimmune disorders affect outcomes in patients with MS, Dr. Adil and colleagues analyzed Nationwide Inpatient Survey data files from 2006 to 2010. The researchers classified patients with MS into two groups: an MS group (without coexisting autoimmune disorders) and an MS Plus group (with coexisting autoimmune disorders).
The investigators then compared the rate of clinical outcomes (ie, in-hospital mortality, discharge to home, discharge to nursing facilities, length of stay, and total charges) between the MS and MS Plus groups. Dr. Adil and colleagues used multivariate analysis to adjust for potential confounders when assessing in-hospital outcomes.
Of 115,120 patients with MS, 18,796 were in the MS Plus group. The adjusted odds of in-hospital mortality and of discharge to nursing facilities were significantly higher in the MS group, compared with the MS Plus group (odds ratios, 4.67 and 1.16, respectively). Length of stay and mean hospital charges were significantly higher in the MS Plus group, compared with the MS group.
A possible explanation for the findings is that coexisting autoimmune diseases involve different effector T cells that may regulate the effect of other effector T cells, said the authors. “Other possible explanations of better outcome in the MS Plus group might be the use of chronic immune suppressive agents in addition to MS immunotherapy, and multidisciplinary management,” they added.
NEW ORLEANS—Patients with multiple sclerosis (MS) had increased rates of in-hospital mortality and discharge to nursing facilities, compared with patients with MS plus a coexisting autoimmune disorder, according to research presented at the 31st Annual Meeting of the Consortium of MS Centers.
“The findings in this study suggest that a coexisting autoimmune disorder may in fact lessen the severity of the immune dysregulation of MS, which leads to improved health outcomes,” said Malik M. Adil, MD, a neurology resident at the Ochsner Clinic Foundation in New Orleans, and colleagues.
Studies have suggested an association between MS and autoimmune disorders, but research concerning clinical outcomes is limited. To determine whether coexisting autoimmune disorders affect outcomes in patients with MS, Dr. Adil and colleagues analyzed Nationwide Inpatient Survey data files from 2006 to 2010. The researchers classified patients with MS into two groups: an MS group (without coexisting autoimmune disorders) and an MS Plus group (with coexisting autoimmune disorders).
The investigators then compared the rate of clinical outcomes (ie, in-hospital mortality, discharge to home, discharge to nursing facilities, length of stay, and total charges) between the MS and MS Plus groups. Dr. Adil and colleagues used multivariate analysis to adjust for potential confounders when assessing in-hospital outcomes.
Of 115,120 patients with MS, 18,796 were in the MS Plus group. The adjusted odds of in-hospital mortality and of discharge to nursing facilities were significantly higher in the MS group, compared with the MS Plus group (odds ratios, 4.67 and 1.16, respectively). Length of stay and mean hospital charges were significantly higher in the MS Plus group, compared with the MS group.
A possible explanation for the findings is that coexisting autoimmune diseases involve different effector T cells that may regulate the effect of other effector T cells, said the authors. “Other possible explanations of better outcome in the MS Plus group might be the use of chronic immune suppressive agents in addition to MS immunotherapy, and multidisciplinary management,” they added.
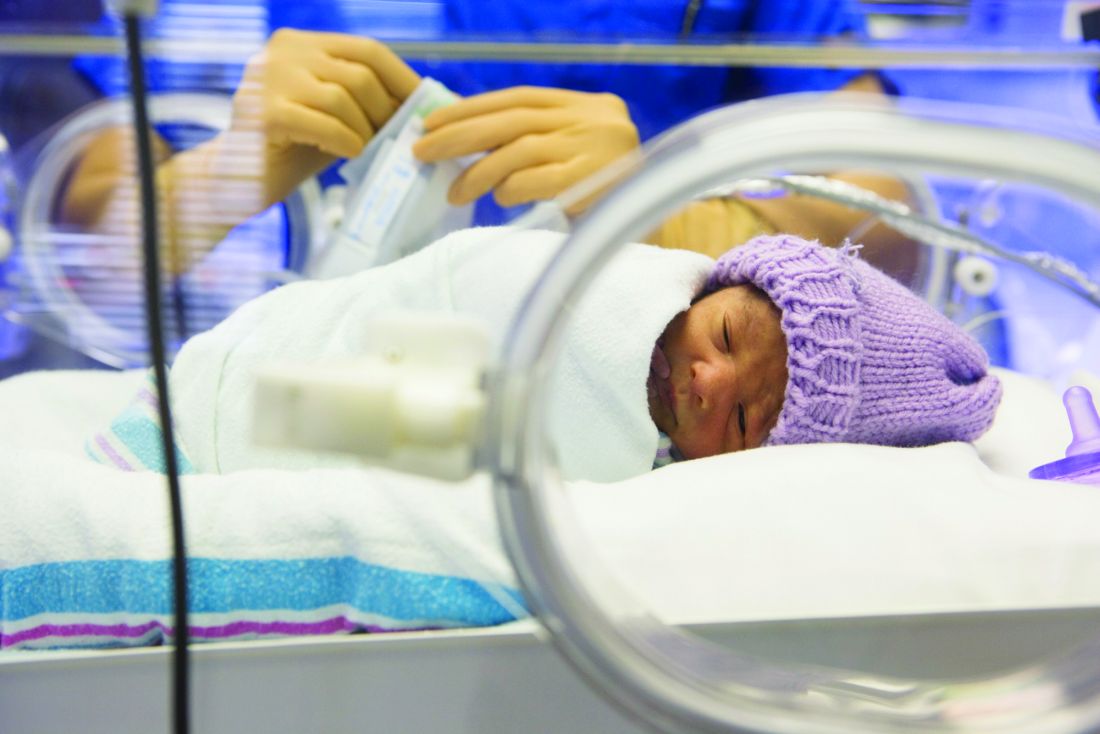
Caffeine therapy of VLBW neonates reduces later motor impairment
SAN FRANCISCO – The 11-year follow-up results of the Caffeine for Apnea of Prematurity randomized, placebo-controlled trial has established the benefits of methylated xanthine therapy in the form of caffeine for very-low-birth-weight (VLBW) neonates in lessening the risk of motor impairment.
“The latest findings bolster the value of caffeine therapy to address apnea of prematurity in VLBW neonates. The CAP trial has provided compelling evidence for the use of caffeine even before this latest finding of improved motor function at age 11 years,” said presenter and the CAP trial’s principal investigator Barbara Schmidt, MD, of McMaster University, Hamilton, Ont.
At least 5 of every 1,000 live-born babies are very premature with a VLBW. Up of 40% die or survive with lasting disabilities. One cause of mortality or disability is apnea. Apnea can be lessened by treatment with methylxanthines such as caffeine, she noted. However, at the time the CAP trial began, it was unclear whether the therapy posed a danger by worsening the damage caused by lack of oxygen.
“Very few studies have followed more recent cohorts of very preterm infants to middle school age. Therefore, it was difficult to anticipate actual rates of functional impairment, and the possible effect of caffeine on those rates,” said Dr. Schmidt.
As reported about a decade ago, the CAP trial involving VLBW infants randomized to caffeine therapy or placebo established the short-term safety and effectiveness of the therapy in terms of reducing cerebral palsy and cognitive delay (N Engl J Med. 2007;357:1893-902).
Follow-ups at 18 months and 5 years revealed the benefits of caffeine therapy in reducing the rates of bronchopulmonary dysplasia, severe retinopathy, and neurodevelopmental disability. The present follow-up data assessed academic performance, motor function, and behavior in 1,202 children with a median age of 11 years. Of these, 602 had received caffeine therapy and 600 had been randomized to the placebo group. Data at age 11 years was available for 457 and 463 of the children randomized to caffeine therapy or placebo, respectively.
The primary outcome was functional impairment, which was indicated by at least one of poor academic performance (with at least one standard score more than two standard deviations below the mean on the Wide Range Achievement Test, 4th edition), motor impairment (percentile rank of 5 or less on the Movement Assessment Battery for Children, Second Edition), and behavior problems (Total Problem T score more than two standard deviations above the mean on the Child Behavior Checklist).
Functional impairment was evident in 145 the 457 (32%) children who had received caffeine therapy and 174 of the 463 (38%) who had not. The rates of 32% and 38% were statistically similar (P = .07). In the individual functional outcomes, the caffeine and placebo groups also were similar in terms of poor academic performance (14% vs. 13%; P = .58) and behavior (11% vs. 8%, respectively; P = .22).
However, caffeine therapy was associated with a reduced risk of motor impairment at age 11 years, compared with those in the placebo group (20% vs. 28%, respectively; odds ratio [OR], 0.66; 95% confidence interval [CI], 0.48-0.90; P = .009). The number of children needed to treat to lessen motor impairment in one child was 13 (95% CI, 8-42).
“Caffeine therapy for apnea of prematurity did not significantly reduce the combined rate of academic, motor, and behavioral impairments at age 11 years. However, caffeine therapy reduced the risk of motor impairment 11 years later,” said Dr. Schmidt. Whether caffeine therapy using higher doses or longer treatment duration is equally risk free is uncertain and further studies, which are not planned, would be needed, she said.
These 11-year follow-up findings of the CAP trial were published online in JAMA Pediatrics (2017, Apr 24. doi: 10.1001/jamapediatrics.2017.0238).
The sponsor of study was McMaster University. The study was funded by the Canadian Institutes of Health Research and the National Health and Medical Research Council of Australia. Dr. Schmidt had no relevant financial disclosures to report.
SAN FRANCISCO – The 11-year follow-up results of the Caffeine for Apnea of Prematurity randomized, placebo-controlled trial has established the benefits of methylated xanthine therapy in the form of caffeine for very-low-birth-weight (VLBW) neonates in lessening the risk of motor impairment.
“The latest findings bolster the value of caffeine therapy to address apnea of prematurity in VLBW neonates. The CAP trial has provided compelling evidence for the use of caffeine even before this latest finding of improved motor function at age 11 years,” said presenter and the CAP trial’s principal investigator Barbara Schmidt, MD, of McMaster University, Hamilton, Ont.
At least 5 of every 1,000 live-born babies are very premature with a VLBW. Up of 40% die or survive with lasting disabilities. One cause of mortality or disability is apnea. Apnea can be lessened by treatment with methylxanthines such as caffeine, she noted. However, at the time the CAP trial began, it was unclear whether the therapy posed a danger by worsening the damage caused by lack of oxygen.
“Very few studies have followed more recent cohorts of very preterm infants to middle school age. Therefore, it was difficult to anticipate actual rates of functional impairment, and the possible effect of caffeine on those rates,” said Dr. Schmidt.
As reported about a decade ago, the CAP trial involving VLBW infants randomized to caffeine therapy or placebo established the short-term safety and effectiveness of the therapy in terms of reducing cerebral palsy and cognitive delay (N Engl J Med. 2007;357:1893-902).
Follow-ups at 18 months and 5 years revealed the benefits of caffeine therapy in reducing the rates of bronchopulmonary dysplasia, severe retinopathy, and neurodevelopmental disability. The present follow-up data assessed academic performance, motor function, and behavior in 1,202 children with a median age of 11 years. Of these, 602 had received caffeine therapy and 600 had been randomized to the placebo group. Data at age 11 years was available for 457 and 463 of the children randomized to caffeine therapy or placebo, respectively.
The primary outcome was functional impairment, which was indicated by at least one of poor academic performance (with at least one standard score more than two standard deviations below the mean on the Wide Range Achievement Test, 4th edition), motor impairment (percentile rank of 5 or less on the Movement Assessment Battery for Children, Second Edition), and behavior problems (Total Problem T score more than two standard deviations above the mean on the Child Behavior Checklist).
Functional impairment was evident in 145 the 457 (32%) children who had received caffeine therapy and 174 of the 463 (38%) who had not. The rates of 32% and 38% were statistically similar (P = .07). In the individual functional outcomes, the caffeine and placebo groups also were similar in terms of poor academic performance (14% vs. 13%; P = .58) and behavior (11% vs. 8%, respectively; P = .22).
However, caffeine therapy was associated with a reduced risk of motor impairment at age 11 years, compared with those in the placebo group (20% vs. 28%, respectively; odds ratio [OR], 0.66; 95% confidence interval [CI], 0.48-0.90; P = .009). The number of children needed to treat to lessen motor impairment in one child was 13 (95% CI, 8-42).
“Caffeine therapy for apnea of prematurity did not significantly reduce the combined rate of academic, motor, and behavioral impairments at age 11 years. However, caffeine therapy reduced the risk of motor impairment 11 years later,” said Dr. Schmidt. Whether caffeine therapy using higher doses or longer treatment duration is equally risk free is uncertain and further studies, which are not planned, would be needed, she said.
These 11-year follow-up findings of the CAP trial were published online in JAMA Pediatrics (2017, Apr 24. doi: 10.1001/jamapediatrics.2017.0238).
The sponsor of study was McMaster University. The study was funded by the Canadian Institutes of Health Research and the National Health and Medical Research Council of Australia. Dr. Schmidt had no relevant financial disclosures to report.
SAN FRANCISCO – The 11-year follow-up results of the Caffeine for Apnea of Prematurity randomized, placebo-controlled trial has established the benefits of methylated xanthine therapy in the form of caffeine for very-low-birth-weight (VLBW) neonates in lessening the risk of motor impairment.
“The latest findings bolster the value of caffeine therapy to address apnea of prematurity in VLBW neonates. The CAP trial has provided compelling evidence for the use of caffeine even before this latest finding of improved motor function at age 11 years,” said presenter and the CAP trial’s principal investigator Barbara Schmidt, MD, of McMaster University, Hamilton, Ont.
At least 5 of every 1,000 live-born babies are very premature with a VLBW. Up of 40% die or survive with lasting disabilities. One cause of mortality or disability is apnea. Apnea can be lessened by treatment with methylxanthines such as caffeine, she noted. However, at the time the CAP trial began, it was unclear whether the therapy posed a danger by worsening the damage caused by lack of oxygen.
“Very few studies have followed more recent cohorts of very preterm infants to middle school age. Therefore, it was difficult to anticipate actual rates of functional impairment, and the possible effect of caffeine on those rates,” said Dr. Schmidt.
As reported about a decade ago, the CAP trial involving VLBW infants randomized to caffeine therapy or placebo established the short-term safety and effectiveness of the therapy in terms of reducing cerebral palsy and cognitive delay (N Engl J Med. 2007;357:1893-902).
Follow-ups at 18 months and 5 years revealed the benefits of caffeine therapy in reducing the rates of bronchopulmonary dysplasia, severe retinopathy, and neurodevelopmental disability. The present follow-up data assessed academic performance, motor function, and behavior in 1,202 children with a median age of 11 years. Of these, 602 had received caffeine therapy and 600 had been randomized to the placebo group. Data at age 11 years was available for 457 and 463 of the children randomized to caffeine therapy or placebo, respectively.
The primary outcome was functional impairment, which was indicated by at least one of poor academic performance (with at least one standard score more than two standard deviations below the mean on the Wide Range Achievement Test, 4th edition), motor impairment (percentile rank of 5 or less on the Movement Assessment Battery for Children, Second Edition), and behavior problems (Total Problem T score more than two standard deviations above the mean on the Child Behavior Checklist).
Functional impairment was evident in 145 the 457 (32%) children who had received caffeine therapy and 174 of the 463 (38%) who had not. The rates of 32% and 38% were statistically similar (P = .07). In the individual functional outcomes, the caffeine and placebo groups also were similar in terms of poor academic performance (14% vs. 13%; P = .58) and behavior (11% vs. 8%, respectively; P = .22).
However, caffeine therapy was associated with a reduced risk of motor impairment at age 11 years, compared with those in the placebo group (20% vs. 28%, respectively; odds ratio [OR], 0.66; 95% confidence interval [CI], 0.48-0.90; P = .009). The number of children needed to treat to lessen motor impairment in one child was 13 (95% CI, 8-42).
“Caffeine therapy for apnea of prematurity did not significantly reduce the combined rate of academic, motor, and behavioral impairments at age 11 years. However, caffeine therapy reduced the risk of motor impairment 11 years later,” said Dr. Schmidt. Whether caffeine therapy using higher doses or longer treatment duration is equally risk free is uncertain and further studies, which are not planned, would be needed, she said.
These 11-year follow-up findings of the CAP trial were published online in JAMA Pediatrics (2017, Apr 24. doi: 10.1001/jamapediatrics.2017.0238).
The sponsor of study was McMaster University. The study was funded by the Canadian Institutes of Health Research and the National Health and Medical Research Council of Australia. Dr. Schmidt had no relevant financial disclosures to report.
AT PAS 17
Key clinical point:
Major finding: At the 11-year follow-up, motor impairment was identified in 20% of those who received caffeine therapy versus 28% of those in the placebo group (P = .009).
Data source: Multicenter, randomized, placebo-controlled trial (NCT00182312).
Disclosures: The sponsor of study was McMaster University. The study was funded by the Canadian Institutes of Health Research and the National Health and Medical Research Council of Australia. Dr. Schmidt had no relevant financial disclosures to report.
FDA approves Rebinyn for hemophilia B treatment
The Food and Drug Administration has approved Rebinyn (nonacog beta pegol, N9-GP) for the treatment of hemophilia B, according to a statement from Novo Nordisk.
Caused by deficient blood clotting factor IX activity, hemophilia B affects about 5,000 people in the United States. The disease is chronic and inherited and causes prolonged and spontaneous bleeding, particularly into muscles, joints, or internal organs. Rebinyn is intended to control and treat bleeding episodes, as well as provide perioperative management of bleeding.
Find the full statement about Rebinyn’s approval on the Novo Nordisk website.
The Food and Drug Administration has approved Rebinyn (nonacog beta pegol, N9-GP) for the treatment of hemophilia B, according to a statement from Novo Nordisk.
Caused by deficient blood clotting factor IX activity, hemophilia B affects about 5,000 people in the United States. The disease is chronic and inherited and causes prolonged and spontaneous bleeding, particularly into muscles, joints, or internal organs. Rebinyn is intended to control and treat bleeding episodes, as well as provide perioperative management of bleeding.
Find the full statement about Rebinyn’s approval on the Novo Nordisk website.
The Food and Drug Administration has approved Rebinyn (nonacog beta pegol, N9-GP) for the treatment of hemophilia B, according to a statement from Novo Nordisk.
Caused by deficient blood clotting factor IX activity, hemophilia B affects about 5,000 people in the United States. The disease is chronic and inherited and causes prolonged and spontaneous bleeding, particularly into muscles, joints, or internal organs. Rebinyn is intended to control and treat bleeding episodes, as well as provide perioperative management of bleeding.
Find the full statement about Rebinyn’s approval on the Novo Nordisk website.