User login
Hidden burdens, limited follow-up complicate refugee mental health
SAN DIEGO – Psychiatrists may encounter refugee patients from war-torn countries in virtually every part of the United States with complex mental health needs, including high rates of posttraumatic stress disorder, chronic pain, and somatic symptoms, according to two presenters at the annual meeting of the American Psychiatric Association.
Over the past decade, refugees from Middle Eastern counties – particularly Iraq, Syria, and Afghanistan – have increased fourfold as a percentage of all refugees in the United States, while those from Sub-Saharan Africa continue to make up a large share. Despite heated political wrangling, the U.S. Department of State recently increased limits on the number of refugees that can be accepted. California, Texas, New York, Michigan, Ohio, and Washington are the states resettling the most new arrivals.
Refugees with trauma exposure have high rates of posttraumatic stress disorder, chronic pain, and somatic symptoms. In addition, recent research suggests, these refugees may have poorly understood stressors related to migration and adjustment that also may be significant contributors to mental illness risk. Despite this, refugees generally have less access to mental health care than does the general population.
The presenters shared their perspectives on refugee mental health with findings that could inform the timing and nature of interventions in these potentially vulnerable populations.
Cynthia L. Arfken, PhD, of Wayne State University in Detroit, presented results from an ongoing cohort study of Syrian families presenting to a primary care clinic as part of their State Department–mandated health check upon resettlement. Arash Javanbakht, MD, also of the university, led the research.
The investigators recruited families at a primary care clinic in southeastern Michigan, where refugees receive health assessments within the first month of arrival in the United States.
The researchers consecutively enrolled and evaluated 297 individuals, including 59 children aged 6 and older (mean age, 11.3) from Syria. These families represented 95% of refugees seen at the clinic during the study period, from June to December 2016.
The researchers also collected hair and saliva samples from consenting families for a separate study looking at biomarkers and mental health outcomes.
Adults were screened for PTSD using the PTSD checklist for adults, and children for anxiety using the Screen for Child Anxiety Related Emotional Disorders, or SCARED, measure. Psychiatric nurses and bilingual health care workers helped the team obtain consent and conduct assessments.
The researchers found that 61% of the children had a probable anxiety diagnosis, and nearly 85% had probable separation anxiety. Higher child anxiety scores were associated with higher PTSD scores in mothers (P = .05).
Dr. Arfken said in an interview that she and her team were “shocked” at the high prevalence of probable anxiety disorders in the cohort, in part because they’d conducted an earlier study enrolling adult Iraqi refugees and “found hardly any psychiatric symptoms at all.”
The high levels of anxiety seen among the Syrian refugees may be related to the severity of the ongoing conflict, Dr. Arfken said. The children’s results were sufficiently jarring to the team that “we changed our whole plan,” she said, “to concentrate on following up both the children who showed distress and those who did not.” They also attempted some nonmedical interventions, such as dance and mindfulness groups.
Also at the conference, Christopher Morrow, MD, of the University of Maryland in Baltimore, presented findings from a case study that illuminates some of the potential mental health risks for resettled refugees.
Dr. Morrow described a 31-year-old man from Afghanistan who had worked for the U.S. Special Forces in Afghanistan as a translator and subsequently entered the United States as a refugee. About a year later he was admitted to an inpatient psychiatric unit after a violent suicide attempt and was treated for depression.
The researchers noted that the patient had no previous history of depression or other mental illness prior to arriving in the United States. “His symptoms developed over the course of the first year of resettlement,” Dr. Morrow said in an interview.
This patient, Dr. Morrow said, was single and was not religious, leaving him not inclined to join a mosque or other Islamic community group. He was placed in an unskilled work assignment, despite his well-developed skills as a translator. Over the course of a year, he became increasingly isolated and “decompensated to the point where there was a really violent suicide attempt.
“We think that some kind of programmed follow-up – be it a community resource or through primary care – could have helped stabilize him before he got to a point of real hopelessness,” Dr. Morrow said.
Dr. Morrow and his colleagues proposed two interventions as adjustments to current health policy for refugees: adding universal mental health screening to each refugee’s health check in the first month after arrival, and scheduling follow-up later in the resettlement process.
“If there is active follow-up, a way that you could check in with these individuals as they’re acclimating, that’s probably the point where you could intervene best,” he said.
Dr. Morrow and Dr. Arfken disclosed no conflicts of interest related to their research.
SAN DIEGO – Psychiatrists may encounter refugee patients from war-torn countries in virtually every part of the United States with complex mental health needs, including high rates of posttraumatic stress disorder, chronic pain, and somatic symptoms, according to two presenters at the annual meeting of the American Psychiatric Association.
Over the past decade, refugees from Middle Eastern counties – particularly Iraq, Syria, and Afghanistan – have increased fourfold as a percentage of all refugees in the United States, while those from Sub-Saharan Africa continue to make up a large share. Despite heated political wrangling, the U.S. Department of State recently increased limits on the number of refugees that can be accepted. California, Texas, New York, Michigan, Ohio, and Washington are the states resettling the most new arrivals.
Refugees with trauma exposure have high rates of posttraumatic stress disorder, chronic pain, and somatic symptoms. In addition, recent research suggests, these refugees may have poorly understood stressors related to migration and adjustment that also may be significant contributors to mental illness risk. Despite this, refugees generally have less access to mental health care than does the general population.
The presenters shared their perspectives on refugee mental health with findings that could inform the timing and nature of interventions in these potentially vulnerable populations.
Cynthia L. Arfken, PhD, of Wayne State University in Detroit, presented results from an ongoing cohort study of Syrian families presenting to a primary care clinic as part of their State Department–mandated health check upon resettlement. Arash Javanbakht, MD, also of the university, led the research.
The investigators recruited families at a primary care clinic in southeastern Michigan, where refugees receive health assessments within the first month of arrival in the United States.
The researchers consecutively enrolled and evaluated 297 individuals, including 59 children aged 6 and older (mean age, 11.3) from Syria. These families represented 95% of refugees seen at the clinic during the study period, from June to December 2016.
The researchers also collected hair and saliva samples from consenting families for a separate study looking at biomarkers and mental health outcomes.
Adults were screened for PTSD using the PTSD checklist for adults, and children for anxiety using the Screen for Child Anxiety Related Emotional Disorders, or SCARED, measure. Psychiatric nurses and bilingual health care workers helped the team obtain consent and conduct assessments.
The researchers found that 61% of the children had a probable anxiety diagnosis, and nearly 85% had probable separation anxiety. Higher child anxiety scores were associated with higher PTSD scores in mothers (P = .05).
Dr. Arfken said in an interview that she and her team were “shocked” at the high prevalence of probable anxiety disorders in the cohort, in part because they’d conducted an earlier study enrolling adult Iraqi refugees and “found hardly any psychiatric symptoms at all.”
The high levels of anxiety seen among the Syrian refugees may be related to the severity of the ongoing conflict, Dr. Arfken said. The children’s results were sufficiently jarring to the team that “we changed our whole plan,” she said, “to concentrate on following up both the children who showed distress and those who did not.” They also attempted some nonmedical interventions, such as dance and mindfulness groups.
Also at the conference, Christopher Morrow, MD, of the University of Maryland in Baltimore, presented findings from a case study that illuminates some of the potential mental health risks for resettled refugees.
Dr. Morrow described a 31-year-old man from Afghanistan who had worked for the U.S. Special Forces in Afghanistan as a translator and subsequently entered the United States as a refugee. About a year later he was admitted to an inpatient psychiatric unit after a violent suicide attempt and was treated for depression.
The researchers noted that the patient had no previous history of depression or other mental illness prior to arriving in the United States. “His symptoms developed over the course of the first year of resettlement,” Dr. Morrow said in an interview.
This patient, Dr. Morrow said, was single and was not religious, leaving him not inclined to join a mosque or other Islamic community group. He was placed in an unskilled work assignment, despite his well-developed skills as a translator. Over the course of a year, he became increasingly isolated and “decompensated to the point where there was a really violent suicide attempt.
“We think that some kind of programmed follow-up – be it a community resource or through primary care – could have helped stabilize him before he got to a point of real hopelessness,” Dr. Morrow said.
Dr. Morrow and his colleagues proposed two interventions as adjustments to current health policy for refugees: adding universal mental health screening to each refugee’s health check in the first month after arrival, and scheduling follow-up later in the resettlement process.
“If there is active follow-up, a way that you could check in with these individuals as they’re acclimating, that’s probably the point where you could intervene best,” he said.
Dr. Morrow and Dr. Arfken disclosed no conflicts of interest related to their research.
SAN DIEGO – Psychiatrists may encounter refugee patients from war-torn countries in virtually every part of the United States with complex mental health needs, including high rates of posttraumatic stress disorder, chronic pain, and somatic symptoms, according to two presenters at the annual meeting of the American Psychiatric Association.
Over the past decade, refugees from Middle Eastern counties – particularly Iraq, Syria, and Afghanistan – have increased fourfold as a percentage of all refugees in the United States, while those from Sub-Saharan Africa continue to make up a large share. Despite heated political wrangling, the U.S. Department of State recently increased limits on the number of refugees that can be accepted. California, Texas, New York, Michigan, Ohio, and Washington are the states resettling the most new arrivals.
Refugees with trauma exposure have high rates of posttraumatic stress disorder, chronic pain, and somatic symptoms. In addition, recent research suggests, these refugees may have poorly understood stressors related to migration and adjustment that also may be significant contributors to mental illness risk. Despite this, refugees generally have less access to mental health care than does the general population.
The presenters shared their perspectives on refugee mental health with findings that could inform the timing and nature of interventions in these potentially vulnerable populations.
Cynthia L. Arfken, PhD, of Wayne State University in Detroit, presented results from an ongoing cohort study of Syrian families presenting to a primary care clinic as part of their State Department–mandated health check upon resettlement. Arash Javanbakht, MD, also of the university, led the research.
The investigators recruited families at a primary care clinic in southeastern Michigan, where refugees receive health assessments within the first month of arrival in the United States.
The researchers consecutively enrolled and evaluated 297 individuals, including 59 children aged 6 and older (mean age, 11.3) from Syria. These families represented 95% of refugees seen at the clinic during the study period, from June to December 2016.
The researchers also collected hair and saliva samples from consenting families for a separate study looking at biomarkers and mental health outcomes.
Adults were screened for PTSD using the PTSD checklist for adults, and children for anxiety using the Screen for Child Anxiety Related Emotional Disorders, or SCARED, measure. Psychiatric nurses and bilingual health care workers helped the team obtain consent and conduct assessments.
The researchers found that 61% of the children had a probable anxiety diagnosis, and nearly 85% had probable separation anxiety. Higher child anxiety scores were associated with higher PTSD scores in mothers (P = .05).
Dr. Arfken said in an interview that she and her team were “shocked” at the high prevalence of probable anxiety disorders in the cohort, in part because they’d conducted an earlier study enrolling adult Iraqi refugees and “found hardly any psychiatric symptoms at all.”
The high levels of anxiety seen among the Syrian refugees may be related to the severity of the ongoing conflict, Dr. Arfken said. The children’s results were sufficiently jarring to the team that “we changed our whole plan,” she said, “to concentrate on following up both the children who showed distress and those who did not.” They also attempted some nonmedical interventions, such as dance and mindfulness groups.
Also at the conference, Christopher Morrow, MD, of the University of Maryland in Baltimore, presented findings from a case study that illuminates some of the potential mental health risks for resettled refugees.
Dr. Morrow described a 31-year-old man from Afghanistan who had worked for the U.S. Special Forces in Afghanistan as a translator and subsequently entered the United States as a refugee. About a year later he was admitted to an inpatient psychiatric unit after a violent suicide attempt and was treated for depression.
The researchers noted that the patient had no previous history of depression or other mental illness prior to arriving in the United States. “His symptoms developed over the course of the first year of resettlement,” Dr. Morrow said in an interview.
This patient, Dr. Morrow said, was single and was not religious, leaving him not inclined to join a mosque or other Islamic community group. He was placed in an unskilled work assignment, despite his well-developed skills as a translator. Over the course of a year, he became increasingly isolated and “decompensated to the point where there was a really violent suicide attempt.
“We think that some kind of programmed follow-up – be it a community resource or through primary care – could have helped stabilize him before he got to a point of real hopelessness,” Dr. Morrow said.
Dr. Morrow and his colleagues proposed two interventions as adjustments to current health policy for refugees: adding universal mental health screening to each refugee’s health check in the first month after arrival, and scheduling follow-up later in the resettlement process.
“If there is active follow-up, a way that you could check in with these individuals as they’re acclimating, that’s probably the point where you could intervene best,” he said.
Dr. Morrow and Dr. Arfken disclosed no conflicts of interest related to their research.
EXPERT ANALYSIS FROM APA
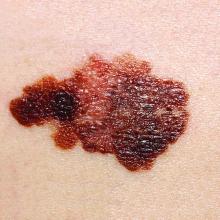
How to best evaluate children’s melanocytic lesions for melanoma
Children often present for evaluation of a melanocytic lesion that is new, evolving, or worrisome to parents and caregivers.
“In the pediatric health care system, malignant melanoma is considered an especially heinous crime. In San Diego, dedicated pediatric providers who identify this disease are members of an elite squad known as the mole patrol unit.” Dr. Sheila Fallon Friedlander opened her presentation on pediatric moles with this tongue-in-cheek statement, adapted from the popular TV show “Law and Order SVU,” at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and University of California, San Diego.
Only 104 cases were diagnosed in children aged less than 10 years, and the melanoma incidence in this age group was relatively unchanging from 1973 to 2009. Dr. Friedlander further emphasized, “Pediatric melanoma is extremely uncommon in patients less than 10 years of age, but more likely to be atypical.”
She continued by describing a group of surgical oncologists at MD Anderson Cancer Center in Houston, who conducted a retrospective review of children with cutaneous melanoma between 1988 and 2007 included in the SEER database, to determine the influence of age on disease presentation. Preadolescents younger than age 10 years were more ethnically diverse (nonwhite), more frequently presented with nontruncal primary melanocytic lesions, and increasingly were diagnosed with advanced disease, compared with their adolescent counterparts (J Pediatr Surg. 2013 Nov;48[11]:2207-13).
Congenital melanocytic nevi (CMN) may have increased risk for malignant potential, and can be challenging for pediatric providers to manage. Among all CMN, the increase in melanoma risk is estimated as less than 1%. The risk for malignant melanoma is further increased in individuals with large or giant CMN (greater than 20 cm diameter adult size), with an absolute risk of approximately 2%-5%. The number of satellite nevi also is considered in risk stratification. The presence of greater than 20 satellite nevi is associated with a greater than fivefold risk of neurocutaneous melanosis. There is no documented association between an increased quantity of satellite nevi and malignant melanoma.
“One particularly challenging pigmented lesion identified among pediatric patients is a Spitz nevus,” according to Dr. Friedlander. This lesion presents with greater cytologic atypia than other benign congenital and acquired nevi, and often clinically mimics malignant melanoma if identified in adults. There also exists a subset of atypical Spitz nevi, consisting of lesions with greater cytologic atypia than benign Spitz nevi. A retrospective review at Massachusetts General Hospital, Boston, of 157 cases of Spitz-type melanocytic lesions identified between 1987 and 2002 revealed increased melanoma risk, minimal mortality, and moderate risk of regional lymph node metastasis (Arch Dermatol. 2011;147[10]:1173-9).
“Classic pediatric Spitz nevi with typical clinical features and history may be managed conservatively with clinical monitoring alone, but those with concerning features such as bleeding, asymmetry, or ulceration should be excised with clear margins,” Dr. Friedlander emphasized. She discouraged sentinel lymph node biopsy, however, given the positive outcomes of 24 patients at Boston Children’s Hospital with atypical Spitz nevi treated with excision alone, published by Cerrato et al. (Pediatr Dermatol. 2011 Dec 30;29[4]:448-53).
“In light of the rising incidence of pediatric melanoma, we need to identify high-risk patients, educate about mole surveillance, and encourage sun protection,” Dr. Friedlander stressed. Children with phenotype of Fitzpatrick I (fair skin, blonde or red hair, and blue eye color) are at highest risk, as are those with a high density of freckles who burn easily and tan poorly. Further risk factors highlighted include excessive sun exposure, indoor tanning, use of phototoxic medications, immunosuppression, and genetics. The first and best line of defense against harmful ultraviolet radiation is covering up (clothing with a tight weave, wet suits, and hats).
The American Academy of Pediatrics encourages staying in the shade when possible, and limiting sun exposure during the peak sun intensity hours, between 10 a.m. and 4 p.m. When physical protection is not possible, the American Academy of Dermatology endorses the application of water resistant, broad spectrum SPF of greater than 30 at least every 2 hours.
Children often present for evaluation of a melanocytic lesion that is new, evolving, or worrisome to parents and caregivers.
“In the pediatric health care system, malignant melanoma is considered an especially heinous crime. In San Diego, dedicated pediatric providers who identify this disease are members of an elite squad known as the mole patrol unit.” Dr. Sheila Fallon Friedlander opened her presentation on pediatric moles with this tongue-in-cheek statement, adapted from the popular TV show “Law and Order SVU,” at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and University of California, San Diego.
Only 104 cases were diagnosed in children aged less than 10 years, and the melanoma incidence in this age group was relatively unchanging from 1973 to 2009. Dr. Friedlander further emphasized, “Pediatric melanoma is extremely uncommon in patients less than 10 years of age, but more likely to be atypical.”
She continued by describing a group of surgical oncologists at MD Anderson Cancer Center in Houston, who conducted a retrospective review of children with cutaneous melanoma between 1988 and 2007 included in the SEER database, to determine the influence of age on disease presentation. Preadolescents younger than age 10 years were more ethnically diverse (nonwhite), more frequently presented with nontruncal primary melanocytic lesions, and increasingly were diagnosed with advanced disease, compared with their adolescent counterparts (J Pediatr Surg. 2013 Nov;48[11]:2207-13).
Congenital melanocytic nevi (CMN) may have increased risk for malignant potential, and can be challenging for pediatric providers to manage. Among all CMN, the increase in melanoma risk is estimated as less than 1%. The risk for malignant melanoma is further increased in individuals with large or giant CMN (greater than 20 cm diameter adult size), with an absolute risk of approximately 2%-5%. The number of satellite nevi also is considered in risk stratification. The presence of greater than 20 satellite nevi is associated with a greater than fivefold risk of neurocutaneous melanosis. There is no documented association between an increased quantity of satellite nevi and malignant melanoma.
“One particularly challenging pigmented lesion identified among pediatric patients is a Spitz nevus,” according to Dr. Friedlander. This lesion presents with greater cytologic atypia than other benign congenital and acquired nevi, and often clinically mimics malignant melanoma if identified in adults. There also exists a subset of atypical Spitz nevi, consisting of lesions with greater cytologic atypia than benign Spitz nevi. A retrospective review at Massachusetts General Hospital, Boston, of 157 cases of Spitz-type melanocytic lesions identified between 1987 and 2002 revealed increased melanoma risk, minimal mortality, and moderate risk of regional lymph node metastasis (Arch Dermatol. 2011;147[10]:1173-9).
“Classic pediatric Spitz nevi with typical clinical features and history may be managed conservatively with clinical monitoring alone, but those with concerning features such as bleeding, asymmetry, or ulceration should be excised with clear margins,” Dr. Friedlander emphasized. She discouraged sentinel lymph node biopsy, however, given the positive outcomes of 24 patients at Boston Children’s Hospital with atypical Spitz nevi treated with excision alone, published by Cerrato et al. (Pediatr Dermatol. 2011 Dec 30;29[4]:448-53).
“In light of the rising incidence of pediatric melanoma, we need to identify high-risk patients, educate about mole surveillance, and encourage sun protection,” Dr. Friedlander stressed. Children with phenotype of Fitzpatrick I (fair skin, blonde or red hair, and blue eye color) are at highest risk, as are those with a high density of freckles who burn easily and tan poorly. Further risk factors highlighted include excessive sun exposure, indoor tanning, use of phototoxic medications, immunosuppression, and genetics. The first and best line of defense against harmful ultraviolet radiation is covering up (clothing with a tight weave, wet suits, and hats).
The American Academy of Pediatrics encourages staying in the shade when possible, and limiting sun exposure during the peak sun intensity hours, between 10 a.m. and 4 p.m. When physical protection is not possible, the American Academy of Dermatology endorses the application of water resistant, broad spectrum SPF of greater than 30 at least every 2 hours.
Children often present for evaluation of a melanocytic lesion that is new, evolving, or worrisome to parents and caregivers.
“In the pediatric health care system, malignant melanoma is considered an especially heinous crime. In San Diego, dedicated pediatric providers who identify this disease are members of an elite squad known as the mole patrol unit.” Dr. Sheila Fallon Friedlander opened her presentation on pediatric moles with this tongue-in-cheek statement, adapted from the popular TV show “Law and Order SVU,” at a pediatric dermatology meeting sponsored by Rady Children’s Hospital–San Diego and University of California, San Diego.
Only 104 cases were diagnosed in children aged less than 10 years, and the melanoma incidence in this age group was relatively unchanging from 1973 to 2009. Dr. Friedlander further emphasized, “Pediatric melanoma is extremely uncommon in patients less than 10 years of age, but more likely to be atypical.”
She continued by describing a group of surgical oncologists at MD Anderson Cancer Center in Houston, who conducted a retrospective review of children with cutaneous melanoma between 1988 and 2007 included in the SEER database, to determine the influence of age on disease presentation. Preadolescents younger than age 10 years were more ethnically diverse (nonwhite), more frequently presented with nontruncal primary melanocytic lesions, and increasingly were diagnosed with advanced disease, compared with their adolescent counterparts (J Pediatr Surg. 2013 Nov;48[11]:2207-13).
Congenital melanocytic nevi (CMN) may have increased risk for malignant potential, and can be challenging for pediatric providers to manage. Among all CMN, the increase in melanoma risk is estimated as less than 1%. The risk for malignant melanoma is further increased in individuals with large or giant CMN (greater than 20 cm diameter adult size), with an absolute risk of approximately 2%-5%. The number of satellite nevi also is considered in risk stratification. The presence of greater than 20 satellite nevi is associated with a greater than fivefold risk of neurocutaneous melanosis. There is no documented association between an increased quantity of satellite nevi and malignant melanoma.
“One particularly challenging pigmented lesion identified among pediatric patients is a Spitz nevus,” according to Dr. Friedlander. This lesion presents with greater cytologic atypia than other benign congenital and acquired nevi, and often clinically mimics malignant melanoma if identified in adults. There also exists a subset of atypical Spitz nevi, consisting of lesions with greater cytologic atypia than benign Spitz nevi. A retrospective review at Massachusetts General Hospital, Boston, of 157 cases of Spitz-type melanocytic lesions identified between 1987 and 2002 revealed increased melanoma risk, minimal mortality, and moderate risk of regional lymph node metastasis (Arch Dermatol. 2011;147[10]:1173-9).
“Classic pediatric Spitz nevi with typical clinical features and history may be managed conservatively with clinical monitoring alone, but those with concerning features such as bleeding, asymmetry, or ulceration should be excised with clear margins,” Dr. Friedlander emphasized. She discouraged sentinel lymph node biopsy, however, given the positive outcomes of 24 patients at Boston Children’s Hospital with atypical Spitz nevi treated with excision alone, published by Cerrato et al. (Pediatr Dermatol. 2011 Dec 30;29[4]:448-53).
“In light of the rising incidence of pediatric melanoma, we need to identify high-risk patients, educate about mole surveillance, and encourage sun protection,” Dr. Friedlander stressed. Children with phenotype of Fitzpatrick I (fair skin, blonde or red hair, and blue eye color) are at highest risk, as are those with a high density of freckles who burn easily and tan poorly. Further risk factors highlighted include excessive sun exposure, indoor tanning, use of phototoxic medications, immunosuppression, and genetics. The first and best line of defense against harmful ultraviolet radiation is covering up (clothing with a tight weave, wet suits, and hats).
The American Academy of Pediatrics encourages staying in the shade when possible, and limiting sun exposure during the peak sun intensity hours, between 10 a.m. and 4 p.m. When physical protection is not possible, the American Academy of Dermatology endorses the application of water resistant, broad spectrum SPF of greater than 30 at least every 2 hours.
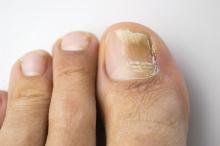
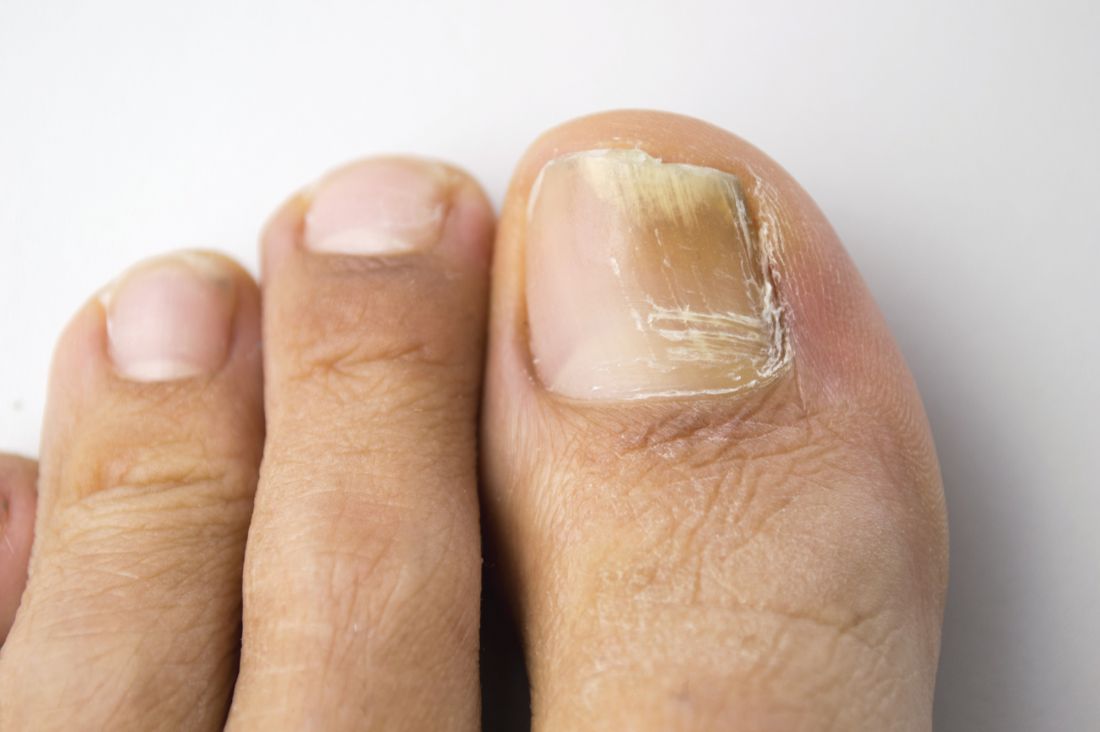
Direct microscopy plus nail clipping identifies onychomycosis
In the absence of a typical presentation, combining direct microscopy plus nail clipping histopathology – two diagnostic tests with different sensitivities and specificities – raises the likelihood of correctly diagnosing onychomycosis, according to a report published in Mycoses.
It is often difficult to diagnose nail diseases based solely on clinical features, and laboratory techniques for diagnosing onychomycoses in particular “remain a challenge,” said Fernanda G. Lavorato, MD, of Universidade do Estado do Rio de Janeiro, and her associates.
Isolating filamentous fungi in cultures is considered the preferred method for diagnosing the disorder, but this method lacks sensitivity and is not always accessible, since some dermatologic centers don’t have a mycology laboratory with personnel trained in sample collection and fungal processing.
The mean patient age was 58.8 years (range, 27-86 years). Most study participants (77.8%) had more than 1 affected nail. Many (29.7%) also had symptoms or signs of cutaneous lesions on the palm, sole, or interdigital region.
Direct microscopy was the most sensitive diagnostic test, correctly identifying 100% of the 122 cases of onychomycosis. In contrast, cultures identified only 34.4% of cases. This low sensitivity for culture testing was expected, and was “likely due to the rapid growth of fungi and bacteria comprising the local microbiota, which often prevents the growth of pathogenic fungi, particularly of slow-growing dermatophytes,” Dr. Lavorato and her associates said (Mycoses. 2017 May 15. doi:10.1111/myc.12633).
Histopathology of nail clippings was the most specific diagnostic test, correctly identifying 77% of cases. “Nail clipping histopathologic analysis complements the [microscopic] examination, particularly in cases of strong clinical suspicion but repeatedly negative mycological tests,” the investigators noted.
Direct microscopy showed greater accuracy with nondermatophytes, while nail clipping showed greater accuracy for dermatophytes, they added.
In this study, Trichophyton rubrum and T. mentagrophytes were the most frequently isolated dermatophytes, found in 70% and 23% of cases, respectively. Neoscytalidium dimidatum and Fusarium species were the most frequently isolated nondermatophytes, found in 44% and 28% of cases, respectively. In addition, Candida yeasts were isolated in samples from 14% of patients, and bacterial colonies were isolated in 70%.
The Mycology Laboratory at Pedro Ernesto University Hospital supported the study. Dr. Lavorato and her associates reported having no relevant financial disclosures.
In the absence of a typical presentation, combining direct microscopy plus nail clipping histopathology – two diagnostic tests with different sensitivities and specificities – raises the likelihood of correctly diagnosing onychomycosis, according to a report published in Mycoses.
It is often difficult to diagnose nail diseases based solely on clinical features, and laboratory techniques for diagnosing onychomycoses in particular “remain a challenge,” said Fernanda G. Lavorato, MD, of Universidade do Estado do Rio de Janeiro, and her associates.
Isolating filamentous fungi in cultures is considered the preferred method for diagnosing the disorder, but this method lacks sensitivity and is not always accessible, since some dermatologic centers don’t have a mycology laboratory with personnel trained in sample collection and fungal processing.
The mean patient age was 58.8 years (range, 27-86 years). Most study participants (77.8%) had more than 1 affected nail. Many (29.7%) also had symptoms or signs of cutaneous lesions on the palm, sole, or interdigital region.
Direct microscopy was the most sensitive diagnostic test, correctly identifying 100% of the 122 cases of onychomycosis. In contrast, cultures identified only 34.4% of cases. This low sensitivity for culture testing was expected, and was “likely due to the rapid growth of fungi and bacteria comprising the local microbiota, which often prevents the growth of pathogenic fungi, particularly of slow-growing dermatophytes,” Dr. Lavorato and her associates said (Mycoses. 2017 May 15. doi:10.1111/myc.12633).
Histopathology of nail clippings was the most specific diagnostic test, correctly identifying 77% of cases. “Nail clipping histopathologic analysis complements the [microscopic] examination, particularly in cases of strong clinical suspicion but repeatedly negative mycological tests,” the investigators noted.
Direct microscopy showed greater accuracy with nondermatophytes, while nail clipping showed greater accuracy for dermatophytes, they added.
In this study, Trichophyton rubrum and T. mentagrophytes were the most frequently isolated dermatophytes, found in 70% and 23% of cases, respectively. Neoscytalidium dimidatum and Fusarium species were the most frequently isolated nondermatophytes, found in 44% and 28% of cases, respectively. In addition, Candida yeasts were isolated in samples from 14% of patients, and bacterial colonies were isolated in 70%.
The Mycology Laboratory at Pedro Ernesto University Hospital supported the study. Dr. Lavorato and her associates reported having no relevant financial disclosures.
In the absence of a typical presentation, combining direct microscopy plus nail clipping histopathology – two diagnostic tests with different sensitivities and specificities – raises the likelihood of correctly diagnosing onychomycosis, according to a report published in Mycoses.
It is often difficult to diagnose nail diseases based solely on clinical features, and laboratory techniques for diagnosing onychomycoses in particular “remain a challenge,” said Fernanda G. Lavorato, MD, of Universidade do Estado do Rio de Janeiro, and her associates.
Isolating filamentous fungi in cultures is considered the preferred method for diagnosing the disorder, but this method lacks sensitivity and is not always accessible, since some dermatologic centers don’t have a mycology laboratory with personnel trained in sample collection and fungal processing.
The mean patient age was 58.8 years (range, 27-86 years). Most study participants (77.8%) had more than 1 affected nail. Many (29.7%) also had symptoms or signs of cutaneous lesions on the palm, sole, or interdigital region.
Direct microscopy was the most sensitive diagnostic test, correctly identifying 100% of the 122 cases of onychomycosis. In contrast, cultures identified only 34.4% of cases. This low sensitivity for culture testing was expected, and was “likely due to the rapid growth of fungi and bacteria comprising the local microbiota, which often prevents the growth of pathogenic fungi, particularly of slow-growing dermatophytes,” Dr. Lavorato and her associates said (Mycoses. 2017 May 15. doi:10.1111/myc.12633).
Histopathology of nail clippings was the most specific diagnostic test, correctly identifying 77% of cases. “Nail clipping histopathologic analysis complements the [microscopic] examination, particularly in cases of strong clinical suspicion but repeatedly negative mycological tests,” the investigators noted.
Direct microscopy showed greater accuracy with nondermatophytes, while nail clipping showed greater accuracy for dermatophytes, they added.
In this study, Trichophyton rubrum and T. mentagrophytes were the most frequently isolated dermatophytes, found in 70% and 23% of cases, respectively. Neoscytalidium dimidatum and Fusarium species were the most frequently isolated nondermatophytes, found in 44% and 28% of cases, respectively. In addition, Candida yeasts were isolated in samples from 14% of patients, and bacterial colonies were isolated in 70%.
The Mycology Laboratory at Pedro Ernesto University Hospital supported the study. Dr. Lavorato and her associates reported having no relevant financial disclosures.
FROM MYCOSES
Key clinical point: In the absence of a typical clinical presentation, combining direct microscopy plus nail clipping histopathology – two diagnostic tests with different sensitivities and specificities – raises the likelihood of correctly diagnosing onychomycosis.
Major finding: Direct microscopy was the most sensitive diagnostic test, correctly identifying 100% of the 122 cases of onychomycosis, while histopathology of nail clippings was the most specific diagnostic test, correctly identifying 77% of cases.
Data source: A single-center prospective cross-sectional study involving 212 adults suspected of having onychomycosis during a 2-year period.
Disclosures: The Mycology Laboratory at Pedro Ernesto University Hospital supported the study. Dr. Lavorato and her associates reported having no relevant financial disclosures.
David Henry's JCSO podcast, May-June 2017
For the May-June issue of the Journal of Community and Supportive Oncology, the Editor in Chief, Dr David Henry, discusses an editorial by Kevin Knopf, a JCSO editor, about drawing on modern portfolio theory to improve cancer care. Side effects come under scrutiny this issue, with a How We Do It article on prehabilitation for lymphedema in head and neck cancer patients, a Review article that examines pancreatitis associated with newer classes of antineoplastic therapies, and a research article that looks at prescriber adherence to antiemetic guidelines with trifluridine-tipiracil. In other research articles, investigators report on physician attitudes and prevalence of molecular testing in lung cancer; a comprehensive assessment of cancer survivors’ concerns to inform program development; and perceived financial hardship among patients with advanced cancer. Two Case Reports address the treatment of Kaposi sarcoma in patients with AIDS, and a third describes a rare case of hypoglycemia induced by a classic gastrointestinal stromal tumor. Finally, Dr Henry summarizes an in-depth interview on cardiotoxicity, which he did with his colleague, Dr Joseph Carver.
Listen to the podcast below.
For the May-June issue of the Journal of Community and Supportive Oncology, the Editor in Chief, Dr David Henry, discusses an editorial by Kevin Knopf, a JCSO editor, about drawing on modern portfolio theory to improve cancer care. Side effects come under scrutiny this issue, with a How We Do It article on prehabilitation for lymphedema in head and neck cancer patients, a Review article that examines pancreatitis associated with newer classes of antineoplastic therapies, and a research article that looks at prescriber adherence to antiemetic guidelines with trifluridine-tipiracil. In other research articles, investigators report on physician attitudes and prevalence of molecular testing in lung cancer; a comprehensive assessment of cancer survivors’ concerns to inform program development; and perceived financial hardship among patients with advanced cancer. Two Case Reports address the treatment of Kaposi sarcoma in patients with AIDS, and a third describes a rare case of hypoglycemia induced by a classic gastrointestinal stromal tumor. Finally, Dr Henry summarizes an in-depth interview on cardiotoxicity, which he did with his colleague, Dr Joseph Carver.
Listen to the podcast below.
For the May-June issue of the Journal of Community and Supportive Oncology, the Editor in Chief, Dr David Henry, discusses an editorial by Kevin Knopf, a JCSO editor, about drawing on modern portfolio theory to improve cancer care. Side effects come under scrutiny this issue, with a How We Do It article on prehabilitation for lymphedema in head and neck cancer patients, a Review article that examines pancreatitis associated with newer classes of antineoplastic therapies, and a research article that looks at prescriber adherence to antiemetic guidelines with trifluridine-tipiracil. In other research articles, investigators report on physician attitudes and prevalence of molecular testing in lung cancer; a comprehensive assessment of cancer survivors’ concerns to inform program development; and perceived financial hardship among patients with advanced cancer. Two Case Reports address the treatment of Kaposi sarcoma in patients with AIDS, and a third describes a rare case of hypoglycemia induced by a classic gastrointestinal stromal tumor. Finally, Dr Henry summarizes an in-depth interview on cardiotoxicity, which he did with his colleague, Dr Joseph Carver.
Listen to the podcast below.
Risk tolerance to MS therapies varies widely
NEW ORLEANS – Risk tolerance to current disease modifying therapies by patients with multiple sclerosis varies widely, results from a large national survey demonstrated.
“We have therapies available with a wide range of risks,” study author Sneha Natarajan, PhD, said in an interview at the annual meeting of the Consortium of Multiple Sclerosis Centers. “Some of the risks are relatively minor like injection site reactions or flu-like symptoms and some are as bad as PML [progressive multifocal leukoencephalopathy], which can be fatal. We don’t know what kind of risks people tolerate.”
Dr. Natarajan, research coordinator at the Mellen Center for Multiple Sclerosis at the Cleveland Clinic, reported results from 3,371 survey respondents. Their mean age was 55 years, 93% were white, 61% had the relapsing-remitting form of MS, and 53% were currently taking a DMT. Overall, respondents reported the highest risk tolerance for infection or thyroid risks (1:1,000 for both) and lowest risk tolerance for PML and kidney injury risks (1:1,000,000 for both). Males reported a higher risk tolerance to all six risks (P less than .0001 for all). Females reported a risk tolerance to skin rash that was similar to kidney injury and PML.
“There is a pattern to the risks that our patients accept,” Dr. Natarajan said. “I don’t think a doctor would not recommend a therapy benefit because of a skin rash [risk], but he may need to address the concerns of the patient upfront and have a talk with the patient.”
The researchers also found that current DMT users expressed increased risk tolerance for all outcomes, compared with those not using any DMT (P less than .001). Higher risk tolerance was also expressed by respondents who were older, more disabled, and by those taking infusion therapies.
The National Multiple Sclerosis Society funded the study. Dr. Natarajan reported having no financial disclosures.
NEW ORLEANS – Risk tolerance to current disease modifying therapies by patients with multiple sclerosis varies widely, results from a large national survey demonstrated.
“We have therapies available with a wide range of risks,” study author Sneha Natarajan, PhD, said in an interview at the annual meeting of the Consortium of Multiple Sclerosis Centers. “Some of the risks are relatively minor like injection site reactions or flu-like symptoms and some are as bad as PML [progressive multifocal leukoencephalopathy], which can be fatal. We don’t know what kind of risks people tolerate.”
Dr. Natarajan, research coordinator at the Mellen Center for Multiple Sclerosis at the Cleveland Clinic, reported results from 3,371 survey respondents. Their mean age was 55 years, 93% were white, 61% had the relapsing-remitting form of MS, and 53% were currently taking a DMT. Overall, respondents reported the highest risk tolerance for infection or thyroid risks (1:1,000 for both) and lowest risk tolerance for PML and kidney injury risks (1:1,000,000 for both). Males reported a higher risk tolerance to all six risks (P less than .0001 for all). Females reported a risk tolerance to skin rash that was similar to kidney injury and PML.
“There is a pattern to the risks that our patients accept,” Dr. Natarajan said. “I don’t think a doctor would not recommend a therapy benefit because of a skin rash [risk], but he may need to address the concerns of the patient upfront and have a talk with the patient.”
The researchers also found that current DMT users expressed increased risk tolerance for all outcomes, compared with those not using any DMT (P less than .001). Higher risk tolerance was also expressed by respondents who were older, more disabled, and by those taking infusion therapies.
The National Multiple Sclerosis Society funded the study. Dr. Natarajan reported having no financial disclosures.
NEW ORLEANS – Risk tolerance to current disease modifying therapies by patients with multiple sclerosis varies widely, results from a large national survey demonstrated.
“We have therapies available with a wide range of risks,” study author Sneha Natarajan, PhD, said in an interview at the annual meeting of the Consortium of Multiple Sclerosis Centers. “Some of the risks are relatively minor like injection site reactions or flu-like symptoms and some are as bad as PML [progressive multifocal leukoencephalopathy], which can be fatal. We don’t know what kind of risks people tolerate.”
Dr. Natarajan, research coordinator at the Mellen Center for Multiple Sclerosis at the Cleveland Clinic, reported results from 3,371 survey respondents. Their mean age was 55 years, 93% were white, 61% had the relapsing-remitting form of MS, and 53% were currently taking a DMT. Overall, respondents reported the highest risk tolerance for infection or thyroid risks (1:1,000 for both) and lowest risk tolerance for PML and kidney injury risks (1:1,000,000 for both). Males reported a higher risk tolerance to all six risks (P less than .0001 for all). Females reported a risk tolerance to skin rash that was similar to kidney injury and PML.
“There is a pattern to the risks that our patients accept,” Dr. Natarajan said. “I don’t think a doctor would not recommend a therapy benefit because of a skin rash [risk], but he may need to address the concerns of the patient upfront and have a talk with the patient.”
The researchers also found that current DMT users expressed increased risk tolerance for all outcomes, compared with those not using any DMT (P less than .001). Higher risk tolerance was also expressed by respondents who were older, more disabled, and by those taking infusion therapies.
The National Multiple Sclerosis Society funded the study. Dr. Natarajan reported having no financial disclosures.
AT THE CMSC ANNUAL MEETING
Key clinical point:
Major finding: Survey respondents reported the highest risk tolerance for infection or thyroid risks (1:1,000 for both) and lowest risk tolerance for PML and kidney injury risks (1:1,000,000 for both).
Data source: A survey of 3,371 people who reported having MS.
Disclosures: The National Multiple Sclerosis Society funded the study. Dr. Natarajan reported having no financial disclosures.
Family reports provide additional information regarding adverse events
Clinical Question: Do family reports of adverse events improve incident detection, compared with clinician reports and hospital incident reports?
Background: Hospital incident reports, which are voluntary and suffer from underreporting, capture only a fraction of errors and adverse events (defined as errors resulting in harm). Systematic, prospective surveillance by researchers is the gold standard but is time consuming and expensive. The authors hypothesized that family reports would improve error and adverse event detection.
Setting: Four U.S. pediatric hospitals.
Synopsis: The authors developed a Family Safety Interview, administered weekly and on discharge, and compared reporting of errors and adverse events to clinician reports, hospital incident reports, and systematic review of records by researchers. Of 989 hospitalized pediatric patients, 746 parents/caregivers completed interviews between December 2014 and July 2015. From all sources, the authors found a total of 179 errors and 113 adverse events. Families reported a total of 39 of these 179 errors (including 19 unique errors not reported elsewhere) and 33 of 113 adverse events (8 unique).
Overall, error rates with family-reported errors were 15.5% higher (95% confidence interval, 9.0%-22.3%) than without. Adverse event rates with family reporting were 9.8% higher (95% CI, 3.1%-16.9%) than without. Family-reported error rates were 5 times higher (95% CI, 1.9-13.0) than hospital incident report rates.
The study showed that family-reported error and adverse event rates were significantly higher than voluntary, clinician-only hospital incident report rates. The study was limited to pediatric hospitals on general pediatric and subspecialty services, though findings potentially may be applicable more broadly (for example, adult and surgical services).
Bottom Line: Using a structured interview, families report significantly higher rates of errors and adverse events, compared with other sources.
Reference: Khan A, Coffey M, Litterer KP, et al. Families as partners in hospital error and adverse event surveillance. JAMA Pediatrics. Published online Feb 27, 2017. doi: 10.1001/jamapediatrics.2016.4812.
Clinical Question: Do family reports of adverse events improve incident detection, compared with clinician reports and hospital incident reports?
Background: Hospital incident reports, which are voluntary and suffer from underreporting, capture only a fraction of errors and adverse events (defined as errors resulting in harm). Systematic, prospective surveillance by researchers is the gold standard but is time consuming and expensive. The authors hypothesized that family reports would improve error and adverse event detection.
Setting: Four U.S. pediatric hospitals.
Synopsis: The authors developed a Family Safety Interview, administered weekly and on discharge, and compared reporting of errors and adverse events to clinician reports, hospital incident reports, and systematic review of records by researchers. Of 989 hospitalized pediatric patients, 746 parents/caregivers completed interviews between December 2014 and July 2015. From all sources, the authors found a total of 179 errors and 113 adverse events. Families reported a total of 39 of these 179 errors (including 19 unique errors not reported elsewhere) and 33 of 113 adverse events (8 unique).
Overall, error rates with family-reported errors were 15.5% higher (95% confidence interval, 9.0%-22.3%) than without. Adverse event rates with family reporting were 9.8% higher (95% CI, 3.1%-16.9%) than without. Family-reported error rates were 5 times higher (95% CI, 1.9-13.0) than hospital incident report rates.
The study showed that family-reported error and adverse event rates were significantly higher than voluntary, clinician-only hospital incident report rates. The study was limited to pediatric hospitals on general pediatric and subspecialty services, though findings potentially may be applicable more broadly (for example, adult and surgical services).
Bottom Line: Using a structured interview, families report significantly higher rates of errors and adverse events, compared with other sources.
Reference: Khan A, Coffey M, Litterer KP, et al. Families as partners in hospital error and adverse event surveillance. JAMA Pediatrics. Published online Feb 27, 2017. doi: 10.1001/jamapediatrics.2016.4812.
Clinical Question: Do family reports of adverse events improve incident detection, compared with clinician reports and hospital incident reports?
Background: Hospital incident reports, which are voluntary and suffer from underreporting, capture only a fraction of errors and adverse events (defined as errors resulting in harm). Systematic, prospective surveillance by researchers is the gold standard but is time consuming and expensive. The authors hypothesized that family reports would improve error and adverse event detection.
Setting: Four U.S. pediatric hospitals.
Synopsis: The authors developed a Family Safety Interview, administered weekly and on discharge, and compared reporting of errors and adverse events to clinician reports, hospital incident reports, and systematic review of records by researchers. Of 989 hospitalized pediatric patients, 746 parents/caregivers completed interviews between December 2014 and July 2015. From all sources, the authors found a total of 179 errors and 113 adverse events. Families reported a total of 39 of these 179 errors (including 19 unique errors not reported elsewhere) and 33 of 113 adverse events (8 unique).
Overall, error rates with family-reported errors were 15.5% higher (95% confidence interval, 9.0%-22.3%) than without. Adverse event rates with family reporting were 9.8% higher (95% CI, 3.1%-16.9%) than without. Family-reported error rates were 5 times higher (95% CI, 1.9-13.0) than hospital incident report rates.
The study showed that family-reported error and adverse event rates were significantly higher than voluntary, clinician-only hospital incident report rates. The study was limited to pediatric hospitals on general pediatric and subspecialty services, though findings potentially may be applicable more broadly (for example, adult and surgical services).
Bottom Line: Using a structured interview, families report significantly higher rates of errors and adverse events, compared with other sources.
Reference: Khan A, Coffey M, Litterer KP, et al. Families as partners in hospital error and adverse event surveillance. JAMA Pediatrics. Published online Feb 27, 2017. doi: 10.1001/jamapediatrics.2016.4812.
Nautical metaphors build physician resilience, beat burnout
SCOTTSDALE, ARIZ. – Linda L.M. Worley, MD, was stunned when a meeting she’d requested with her supervisor to address a shortage of beds turned into a rebuke.
“You’re on the tenure track, Linda. If you want to keep your job 6 years from now, you’d best pick up the pace. You need to see 20 private patients a week, and get moving on your research and publications,” Dr. Worley remembers the supervisor saying. At the time, she was a 32-year-old mother of two, wife, academic faculty physician, and sole attending running a general hospital consultation liaison psychiatry department and the college of medicine student mental health service. She also worked as the 24/7 on-call psychiatrist for a week at a time, said Dr. Worley, now a staff psychiatrist in the Fayetteville, Ark., Veterans Health Care System of the Ozarks and chief mental health officer for South Central VA Health Care Network.
Over the decades of an academic medical career complete with tenure, and dozens of published articles and book chapters, Dr. Worley has developed a system for achieving success while avoiding burnout, based on nautical references. In a session cofacilitated by Cynthia M. Stonnington, MD, chair of psychiatry and psychology at the Mayo Clinic’s campus in Scottsdale, Ariz., Dr. Worley presented her tips for self-care at the annual meeting of the American College of Psychiatrists.
“I use the nautical framework as a bio-psycho-social-spiritual model,” Dr. Worley said in an interview. “I teach it to medical students; I teach it to residents; I teach it to distressed physicians. I even teach it to patients when I am explaining a framework for a necessary treatment approach. With sailing, you have to stay in balance. That’s the same with taking good care of ourselves so we are less likely to get sick physically and mentally,” said Dr. Worley, who commutes to Nashville, Tenn., several times a year as part of her appointment as an adjunct professor of medicine at Vanderbilt University.
Her “Smooth Sailing Life” seminars have evolved over the past 20 years and are rooted in her training in psychosomatic medicine, which she said emphasizes the complexity of the entire person. “It’s about the biology and about the emotions, and the bridge between them,” according to Dr. Worley, who has a website, SmoothSailingLife.com, and is working on a book aimed at helping to meet what she said has been a steadily growing thirst for her approach to developing resilience.
“I am not studying anyone, but I am helping people to self-diagnose. I teach people how to avoid having to see a psychiatrist or a mental health provider but also to feel good about reaching out for help when necessary,” she said. “Life is far too short to suffer needlessly.”
In the interview, Dr. Worley said she adapts her presentations to the venue and the time allowed. Key aspects of her system include:
• Care for your yacht, which is the body, including the brain. “You only get one, and if you’re going to have a chance of winning the regatta, you have to take care of it. This means getting good sleep, nutrition, exercise, preventive care, rest, and rejuvenation, including vacation,” Dr. Worley said.
• Chart your course; have a navigational plan that includes your life goals and aspirations. Identify and rely upon “landmarks,” such as being a good spouse, mother, physician, or friend for the most authentic definition of personal success. “These are like buoys that keep us sailing in the right direction,” she said.
• Reef in your sails, meaning mind the “winds that come at us from every side,” she said. This includes triaging tasks and not letting perfectionism get in the way. “Perfectionists take too long to tack; they don’t know when it’s time to turn in the other direction,” she said. “If you want to finish the race, you have to do the best you can in the time you have.” This was the lesson Dr. Worley said she learned that day when she was a young physician feeling overwhelmed.
• Empty your bilge, the nautical term for removing waste water from within the hull. Dr. Worley uses this as a metaphor for identifying and expressing negative emotions of fear, anxiety, sadness, and frustration. “These vital emotions are giving us important messages. It is important to recognize that they are present. Name and accept them, and understand what they are trying to tell us. Is it a symptom of an underlying illness that needs treatment? A conflict in a relationship? A need not being met? Are you living your deepest values? Express the emotions and sort through the best response,” she said. “It’s all part of emotional intelligence.”
• Keep an even keel, which is Dr. Worley’s way of stating the importance of being connected to love and to living your deepest values. “The keel is your character, your connection to meaning, a spiritual connection. In medicine, we shy away from that. I have only lately ventured into talking about this,” she said, noting that this connection can come in numerous ways, such as meditation, and being in nature or with animals. “It’s very personal. It’s hard to quantify, but I have witnessed it and its healing power within the therapeutic alliance.”
In break-out sessions during her well-attended talk at the meeting, Dr. Worley listened as psychiatrists of all levels of experience and responsibility, ranging from medical directors to those in private community practice, shared the kinds of concerns she said she often encounters in her role as a core faculty member of the Program for Distressed Physicians at the Vanderbilt Center for Professional Health.
“Changes in medicine have been so frustrating; physicians are at their wits’ end. We don’t recruit people into medicine because they have a skill set for expressing their emotions, or taking care of themselves, or dealing with conflict,” she said. “That’s okay. They can learn it.”
[email protected]
On Twitter @whitneymcknight
SCOTTSDALE, ARIZ. – Linda L.M. Worley, MD, was stunned when a meeting she’d requested with her supervisor to address a shortage of beds turned into a rebuke.
“You’re on the tenure track, Linda. If you want to keep your job 6 years from now, you’d best pick up the pace. You need to see 20 private patients a week, and get moving on your research and publications,” Dr. Worley remembers the supervisor saying. At the time, she was a 32-year-old mother of two, wife, academic faculty physician, and sole attending running a general hospital consultation liaison psychiatry department and the college of medicine student mental health service. She also worked as the 24/7 on-call psychiatrist for a week at a time, said Dr. Worley, now a staff psychiatrist in the Fayetteville, Ark., Veterans Health Care System of the Ozarks and chief mental health officer for South Central VA Health Care Network.
Over the decades of an academic medical career complete with tenure, and dozens of published articles and book chapters, Dr. Worley has developed a system for achieving success while avoiding burnout, based on nautical references. In a session cofacilitated by Cynthia M. Stonnington, MD, chair of psychiatry and psychology at the Mayo Clinic’s campus in Scottsdale, Ariz., Dr. Worley presented her tips for self-care at the annual meeting of the American College of Psychiatrists.
“I use the nautical framework as a bio-psycho-social-spiritual model,” Dr. Worley said in an interview. “I teach it to medical students; I teach it to residents; I teach it to distressed physicians. I even teach it to patients when I am explaining a framework for a necessary treatment approach. With sailing, you have to stay in balance. That’s the same with taking good care of ourselves so we are less likely to get sick physically and mentally,” said Dr. Worley, who commutes to Nashville, Tenn., several times a year as part of her appointment as an adjunct professor of medicine at Vanderbilt University.
Her “Smooth Sailing Life” seminars have evolved over the past 20 years and are rooted in her training in psychosomatic medicine, which she said emphasizes the complexity of the entire person. “It’s about the biology and about the emotions, and the bridge between them,” according to Dr. Worley, who has a website, SmoothSailingLife.com, and is working on a book aimed at helping to meet what she said has been a steadily growing thirst for her approach to developing resilience.
“I am not studying anyone, but I am helping people to self-diagnose. I teach people how to avoid having to see a psychiatrist or a mental health provider but also to feel good about reaching out for help when necessary,” she said. “Life is far too short to suffer needlessly.”
In the interview, Dr. Worley said she adapts her presentations to the venue and the time allowed. Key aspects of her system include:
• Care for your yacht, which is the body, including the brain. “You only get one, and if you’re going to have a chance of winning the regatta, you have to take care of it. This means getting good sleep, nutrition, exercise, preventive care, rest, and rejuvenation, including vacation,” Dr. Worley said.
• Chart your course; have a navigational plan that includes your life goals and aspirations. Identify and rely upon “landmarks,” such as being a good spouse, mother, physician, or friend for the most authentic definition of personal success. “These are like buoys that keep us sailing in the right direction,” she said.
• Reef in your sails, meaning mind the “winds that come at us from every side,” she said. This includes triaging tasks and not letting perfectionism get in the way. “Perfectionists take too long to tack; they don’t know when it’s time to turn in the other direction,” she said. “If you want to finish the race, you have to do the best you can in the time you have.” This was the lesson Dr. Worley said she learned that day when she was a young physician feeling overwhelmed.
• Empty your bilge, the nautical term for removing waste water from within the hull. Dr. Worley uses this as a metaphor for identifying and expressing negative emotions of fear, anxiety, sadness, and frustration. “These vital emotions are giving us important messages. It is important to recognize that they are present. Name and accept them, and understand what they are trying to tell us. Is it a symptom of an underlying illness that needs treatment? A conflict in a relationship? A need not being met? Are you living your deepest values? Express the emotions and sort through the best response,” she said. “It’s all part of emotional intelligence.”
• Keep an even keel, which is Dr. Worley’s way of stating the importance of being connected to love and to living your deepest values. “The keel is your character, your connection to meaning, a spiritual connection. In medicine, we shy away from that. I have only lately ventured into talking about this,” she said, noting that this connection can come in numerous ways, such as meditation, and being in nature or with animals. “It’s very personal. It’s hard to quantify, but I have witnessed it and its healing power within the therapeutic alliance.”
In break-out sessions during her well-attended talk at the meeting, Dr. Worley listened as psychiatrists of all levels of experience and responsibility, ranging from medical directors to those in private community practice, shared the kinds of concerns she said she often encounters in her role as a core faculty member of the Program for Distressed Physicians at the Vanderbilt Center for Professional Health.
“Changes in medicine have been so frustrating; physicians are at their wits’ end. We don’t recruit people into medicine because they have a skill set for expressing their emotions, or taking care of themselves, or dealing with conflict,” she said. “That’s okay. They can learn it.”
[email protected]
On Twitter @whitneymcknight
SCOTTSDALE, ARIZ. – Linda L.M. Worley, MD, was stunned when a meeting she’d requested with her supervisor to address a shortage of beds turned into a rebuke.
“You’re on the tenure track, Linda. If you want to keep your job 6 years from now, you’d best pick up the pace. You need to see 20 private patients a week, and get moving on your research and publications,” Dr. Worley remembers the supervisor saying. At the time, she was a 32-year-old mother of two, wife, academic faculty physician, and sole attending running a general hospital consultation liaison psychiatry department and the college of medicine student mental health service. She also worked as the 24/7 on-call psychiatrist for a week at a time, said Dr. Worley, now a staff psychiatrist in the Fayetteville, Ark., Veterans Health Care System of the Ozarks and chief mental health officer for South Central VA Health Care Network.
Over the decades of an academic medical career complete with tenure, and dozens of published articles and book chapters, Dr. Worley has developed a system for achieving success while avoiding burnout, based on nautical references. In a session cofacilitated by Cynthia M. Stonnington, MD, chair of psychiatry and psychology at the Mayo Clinic’s campus in Scottsdale, Ariz., Dr. Worley presented her tips for self-care at the annual meeting of the American College of Psychiatrists.
“I use the nautical framework as a bio-psycho-social-spiritual model,” Dr. Worley said in an interview. “I teach it to medical students; I teach it to residents; I teach it to distressed physicians. I even teach it to patients when I am explaining a framework for a necessary treatment approach. With sailing, you have to stay in balance. That’s the same with taking good care of ourselves so we are less likely to get sick physically and mentally,” said Dr. Worley, who commutes to Nashville, Tenn., several times a year as part of her appointment as an adjunct professor of medicine at Vanderbilt University.
Her “Smooth Sailing Life” seminars have evolved over the past 20 years and are rooted in her training in psychosomatic medicine, which she said emphasizes the complexity of the entire person. “It’s about the biology and about the emotions, and the bridge between them,” according to Dr. Worley, who has a website, SmoothSailingLife.com, and is working on a book aimed at helping to meet what she said has been a steadily growing thirst for her approach to developing resilience.
“I am not studying anyone, but I am helping people to self-diagnose. I teach people how to avoid having to see a psychiatrist or a mental health provider but also to feel good about reaching out for help when necessary,” she said. “Life is far too short to suffer needlessly.”
In the interview, Dr. Worley said she adapts her presentations to the venue and the time allowed. Key aspects of her system include:
• Care for your yacht, which is the body, including the brain. “You only get one, and if you’re going to have a chance of winning the regatta, you have to take care of it. This means getting good sleep, nutrition, exercise, preventive care, rest, and rejuvenation, including vacation,” Dr. Worley said.
• Chart your course; have a navigational plan that includes your life goals and aspirations. Identify and rely upon “landmarks,” such as being a good spouse, mother, physician, or friend for the most authentic definition of personal success. “These are like buoys that keep us sailing in the right direction,” she said.
• Reef in your sails, meaning mind the “winds that come at us from every side,” she said. This includes triaging tasks and not letting perfectionism get in the way. “Perfectionists take too long to tack; they don’t know when it’s time to turn in the other direction,” she said. “If you want to finish the race, you have to do the best you can in the time you have.” This was the lesson Dr. Worley said she learned that day when she was a young physician feeling overwhelmed.
• Empty your bilge, the nautical term for removing waste water from within the hull. Dr. Worley uses this as a metaphor for identifying and expressing negative emotions of fear, anxiety, sadness, and frustration. “These vital emotions are giving us important messages. It is important to recognize that they are present. Name and accept them, and understand what they are trying to tell us. Is it a symptom of an underlying illness that needs treatment? A conflict in a relationship? A need not being met? Are you living your deepest values? Express the emotions and sort through the best response,” she said. “It’s all part of emotional intelligence.”
• Keep an even keel, which is Dr. Worley’s way of stating the importance of being connected to love and to living your deepest values. “The keel is your character, your connection to meaning, a spiritual connection. In medicine, we shy away from that. I have only lately ventured into talking about this,” she said, noting that this connection can come in numerous ways, such as meditation, and being in nature or with animals. “It’s very personal. It’s hard to quantify, but I have witnessed it and its healing power within the therapeutic alliance.”
In break-out sessions during her well-attended talk at the meeting, Dr. Worley listened as psychiatrists of all levels of experience and responsibility, ranging from medical directors to those in private community practice, shared the kinds of concerns she said she often encounters in her role as a core faculty member of the Program for Distressed Physicians at the Vanderbilt Center for Professional Health.
“Changes in medicine have been so frustrating; physicians are at their wits’ end. We don’t recruit people into medicine because they have a skill set for expressing their emotions, or taking care of themselves, or dealing with conflict,” she said. “That’s okay. They can learn it.”
[email protected]
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM THE AMERICAN COLLEGE OF PSYCHIATRISTS MEETING
Cosmetic Corner: Dermatologists Weigh in on Face Scrubs
To improve patient care and outcomes, leading dermatologists offered their recommendations on face scrubs. Consideration must be given to:
- Crystal Peel Microdermabrasion Exfoliating Face Crème
Formulary for Physicians, Inc
“This product is a highly effective facial scrub for patients with thick skin. Its exfoliating ingredient is corundum, another name for aluminum oxide, the crystal used by most microabrasion machines.”— Mark G. Rubin, MD, Beverly Hills, California
- Facial Fuel Energizing Scrub
Kiehl’s
Recommended by Gary Goldenberg, MD, New York, New York
- Olay Regenerist Regenerating Cream Cleanser
Procter & Gamble
“Oxygenated beads in the creamy formula help to gently exfoliate the skin without overdrying and stripping the skin’s outer layer, leaving the skin soft and fresh.”—Jeannette Graf, MD, New York, New York
- PRESCRIBEDsolutions: Starting Up/Face, Surface Improvement
Biopelle, Inc
“I use Starting Up/Face as my daily cleanser, as it contains salicylic acid and helps improve the overall texture plus minimize bumps from shaving, and Surface Improvement about every other day on my face in the shower.”—Joel L. Cohen, MD, Greenwood Village, Colorado
- St. Ives Apricot Blemish Control Scrub
Unilever
“It exfoliates and has salicylic acid. After exfoliating, I recommend allowing it to sit on the skin for 5 minutes before washing off.”—Anthony M. Rossi, MD, New York, New York
Cutis invites readers to send us their recommendations. Athlete’s foot treatments, cleansing devices, and redness-reducing products will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on face scrubs. Consideration must be given to:
- Crystal Peel Microdermabrasion Exfoliating Face Crème
Formulary for Physicians, Inc
“This product is a highly effective facial scrub for patients with thick skin. Its exfoliating ingredient is corundum, another name for aluminum oxide, the crystal used by most microabrasion machines.”— Mark G. Rubin, MD, Beverly Hills, California
- Facial Fuel Energizing Scrub
Kiehl’s
Recommended by Gary Goldenberg, MD, New York, New York
- Olay Regenerist Regenerating Cream Cleanser
Procter & Gamble
“Oxygenated beads in the creamy formula help to gently exfoliate the skin without overdrying and stripping the skin’s outer layer, leaving the skin soft and fresh.”—Jeannette Graf, MD, New York, New York
- PRESCRIBEDsolutions: Starting Up/Face, Surface Improvement
Biopelle, Inc
“I use Starting Up/Face as my daily cleanser, as it contains salicylic acid and helps improve the overall texture plus minimize bumps from shaving, and Surface Improvement about every other day on my face in the shower.”—Joel L. Cohen, MD, Greenwood Village, Colorado
- St. Ives Apricot Blemish Control Scrub
Unilever
“It exfoliates and has salicylic acid. After exfoliating, I recommend allowing it to sit on the skin for 5 minutes before washing off.”—Anthony M. Rossi, MD, New York, New York
Cutis invites readers to send us their recommendations. Athlete’s foot treatments, cleansing devices, and redness-reducing products will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on face scrubs. Consideration must be given to:
- Crystal Peel Microdermabrasion Exfoliating Face Crème
Formulary for Physicians, Inc
“This product is a highly effective facial scrub for patients with thick skin. Its exfoliating ingredient is corundum, another name for aluminum oxide, the crystal used by most microabrasion machines.”— Mark G. Rubin, MD, Beverly Hills, California
- Facial Fuel Energizing Scrub
Kiehl’s
Recommended by Gary Goldenberg, MD, New York, New York
- Olay Regenerist Regenerating Cream Cleanser
Procter & Gamble
“Oxygenated beads in the creamy formula help to gently exfoliate the skin without overdrying and stripping the skin’s outer layer, leaving the skin soft and fresh.”—Jeannette Graf, MD, New York, New York
- PRESCRIBEDsolutions: Starting Up/Face, Surface Improvement
Biopelle, Inc
“I use Starting Up/Face as my daily cleanser, as it contains salicylic acid and helps improve the overall texture plus minimize bumps from shaving, and Surface Improvement about every other day on my face in the shower.”—Joel L. Cohen, MD, Greenwood Village, Colorado
- St. Ives Apricot Blemish Control Scrub
Unilever
“It exfoliates and has salicylic acid. After exfoliating, I recommend allowing it to sit on the skin for 5 minutes before washing off.”—Anthony M. Rossi, MD, New York, New York
Cutis invites readers to send us their recommendations. Athlete’s foot treatments, cleansing devices, and redness-reducing products will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
Public favors Obamacare over Trumpcare
The American Health Care Act, the House Republicans’ replacement for the Affordable Care Act, is currently viewed less favorably than its predecessor, according to a new poll by the Kaiser Family Foundation.
In the survey, 40% of respondents said that they had a “very unfavorable” opinion of the AHCA, compared with 29% for the ACA. The “very favorable” opinions also favored the ACA: 29% to 12%, according to a Kaiser report released May 31.
The Kaiser Health Tracking Poll involved 1,205 adults and was conducted May 16-22, 2017.
The American Health Care Act, the House Republicans’ replacement for the Affordable Care Act, is currently viewed less favorably than its predecessor, according to a new poll by the Kaiser Family Foundation.
In the survey, 40% of respondents said that they had a “very unfavorable” opinion of the AHCA, compared with 29% for the ACA. The “very favorable” opinions also favored the ACA: 29% to 12%, according to a Kaiser report released May 31.
The Kaiser Health Tracking Poll involved 1,205 adults and was conducted May 16-22, 2017.
The American Health Care Act, the House Republicans’ replacement for the Affordable Care Act, is currently viewed less favorably than its predecessor, according to a new poll by the Kaiser Family Foundation.
In the survey, 40% of respondents said that they had a “very unfavorable” opinion of the AHCA, compared with 29% for the ACA. The “very favorable” opinions also favored the ACA: 29% to 12%, according to a Kaiser report released May 31.
The Kaiser Health Tracking Poll involved 1,205 adults and was conducted May 16-22, 2017.
Immunization requirements, availability vary in U.S. universities
SAN FRANCISCO – in the enrollment process and vaccine availability through on-campus student health.
The policies of the institutions usually reflected the policies of the particular state or district.
The cross-sectional study surveyed two private and two publicly-funded 4-year degree-granting colleges or universities in each state and the District of Columbia – 216 institutions in total. The institutions were randomly selected to reflect the diversities in size, religious affiliations, and type of institution. The institutions’ websites were scrutinized for information on immunization requirements, vaccinations needed prior to enrollment, vaccination options available on-campus, and consequence of failure to obtain the necessary vaccinations.
A wide variation in vaccine requirements and on-campus availability was evident. MMR vaccination was an admission requirement of about 82% of the schools surveyed. Vaccination was best done prior to arrival on campus, as only 42% of the surveyed colleges and universities offered the vaccine through student health. Vaccination for hepatitis B was required by only 31% of colleges/universities, with 44% offering the vaccine through student health. Vaccination for hepatitis A was required by only about 1% of the surveyed institutions, although the vaccine was available on one-third of the campuses, Dr. Feemster said at the Pediatric Academic Societies annual meeting.
Meningococcal B (MenB) vaccination was required by 25 schools, of which 6 (24%) had experienced MenB illness outbreaks. Of the 191 schools that did not have a requirement for MenB vaccination, only 4 (2.0%) had experienced a MenB outbreak.
Of contemporary concern, vaccination for human papillomavirus was offered by one-third of the colleges/universities, but this vaccination was not a requirement for admission to any of the surveyed institutions. Vaccination for influenza, another disease with a high propensity to spread, also was not required by any school, with only 37% having influenza vaccination available as part of student health care.
Compliance with immunization requirements was enforced by 67% of the schools, with course registration not finalized until the necessary vaccinations had been received and documented. Of the 17% of schools that did not have an enforcement policy, 61% cited the vaccine requirements of their particular state, the assumption being that the incoming students from that state would have received the necessary vaccinations, reflecting a more reactive than proactive stance, according to Dr. Feemster. There was no difference in enforcement strategy between the public or private institutions.
Of the surveyed vaccines, at least some were available at just over 91% of the public institutions and at 76% of the private institutions
“The variation in requirements and enforcement suggest inconsistent vaccine uptake. Next steps include a mixed-methods study to measure attitudes, beliefs, and behaviors related to school vaccine policy among a national sample of college students and to identify facilitators and barriers to school vaccine policy implementation among school health administrators and providers,” said Dr. Feemster.
“The ultimate goal is to identify the best practices for implementation of college vaccine policies to optimize vaccine uptake and increase positive attitudes, beliefs, and future intentions about vaccines,” she added.
The sponsor of study was the Children’s Hospital of Philadelphia. The study was not funded. Dr. Feemster had no conflicts to disclose.
SAN FRANCISCO – in the enrollment process and vaccine availability through on-campus student health.
The policies of the institutions usually reflected the policies of the particular state or district.
The cross-sectional study surveyed two private and two publicly-funded 4-year degree-granting colleges or universities in each state and the District of Columbia – 216 institutions in total. The institutions were randomly selected to reflect the diversities in size, religious affiliations, and type of institution. The institutions’ websites were scrutinized for information on immunization requirements, vaccinations needed prior to enrollment, vaccination options available on-campus, and consequence of failure to obtain the necessary vaccinations.
A wide variation in vaccine requirements and on-campus availability was evident. MMR vaccination was an admission requirement of about 82% of the schools surveyed. Vaccination was best done prior to arrival on campus, as only 42% of the surveyed colleges and universities offered the vaccine through student health. Vaccination for hepatitis B was required by only 31% of colleges/universities, with 44% offering the vaccine through student health. Vaccination for hepatitis A was required by only about 1% of the surveyed institutions, although the vaccine was available on one-third of the campuses, Dr. Feemster said at the Pediatric Academic Societies annual meeting.
Meningococcal B (MenB) vaccination was required by 25 schools, of which 6 (24%) had experienced MenB illness outbreaks. Of the 191 schools that did not have a requirement for MenB vaccination, only 4 (2.0%) had experienced a MenB outbreak.
Of contemporary concern, vaccination for human papillomavirus was offered by one-third of the colleges/universities, but this vaccination was not a requirement for admission to any of the surveyed institutions. Vaccination for influenza, another disease with a high propensity to spread, also was not required by any school, with only 37% having influenza vaccination available as part of student health care.
Compliance with immunization requirements was enforced by 67% of the schools, with course registration not finalized until the necessary vaccinations had been received and documented. Of the 17% of schools that did not have an enforcement policy, 61% cited the vaccine requirements of their particular state, the assumption being that the incoming students from that state would have received the necessary vaccinations, reflecting a more reactive than proactive stance, according to Dr. Feemster. There was no difference in enforcement strategy between the public or private institutions.
Of the surveyed vaccines, at least some were available at just over 91% of the public institutions and at 76% of the private institutions
“The variation in requirements and enforcement suggest inconsistent vaccine uptake. Next steps include a mixed-methods study to measure attitudes, beliefs, and behaviors related to school vaccine policy among a national sample of college students and to identify facilitators and barriers to school vaccine policy implementation among school health administrators and providers,” said Dr. Feemster.
“The ultimate goal is to identify the best practices for implementation of college vaccine policies to optimize vaccine uptake and increase positive attitudes, beliefs, and future intentions about vaccines,” she added.
The sponsor of study was the Children’s Hospital of Philadelphia. The study was not funded. Dr. Feemster had no conflicts to disclose.
SAN FRANCISCO – in the enrollment process and vaccine availability through on-campus student health.
The policies of the institutions usually reflected the policies of the particular state or district.
The cross-sectional study surveyed two private and two publicly-funded 4-year degree-granting colleges or universities in each state and the District of Columbia – 216 institutions in total. The institutions were randomly selected to reflect the diversities in size, religious affiliations, and type of institution. The institutions’ websites were scrutinized for information on immunization requirements, vaccinations needed prior to enrollment, vaccination options available on-campus, and consequence of failure to obtain the necessary vaccinations.
A wide variation in vaccine requirements and on-campus availability was evident. MMR vaccination was an admission requirement of about 82% of the schools surveyed. Vaccination was best done prior to arrival on campus, as only 42% of the surveyed colleges and universities offered the vaccine through student health. Vaccination for hepatitis B was required by only 31% of colleges/universities, with 44% offering the vaccine through student health. Vaccination for hepatitis A was required by only about 1% of the surveyed institutions, although the vaccine was available on one-third of the campuses, Dr. Feemster said at the Pediatric Academic Societies annual meeting.
Meningococcal B (MenB) vaccination was required by 25 schools, of which 6 (24%) had experienced MenB illness outbreaks. Of the 191 schools that did not have a requirement for MenB vaccination, only 4 (2.0%) had experienced a MenB outbreak.
Of contemporary concern, vaccination for human papillomavirus was offered by one-third of the colleges/universities, but this vaccination was not a requirement for admission to any of the surveyed institutions. Vaccination for influenza, another disease with a high propensity to spread, also was not required by any school, with only 37% having influenza vaccination available as part of student health care.
Compliance with immunization requirements was enforced by 67% of the schools, with course registration not finalized until the necessary vaccinations had been received and documented. Of the 17% of schools that did not have an enforcement policy, 61% cited the vaccine requirements of their particular state, the assumption being that the incoming students from that state would have received the necessary vaccinations, reflecting a more reactive than proactive stance, according to Dr. Feemster. There was no difference in enforcement strategy between the public or private institutions.
Of the surveyed vaccines, at least some were available at just over 91% of the public institutions and at 76% of the private institutions
“The variation in requirements and enforcement suggest inconsistent vaccine uptake. Next steps include a mixed-methods study to measure attitudes, beliefs, and behaviors related to school vaccine policy among a national sample of college students and to identify facilitators and barriers to school vaccine policy implementation among school health administrators and providers,” said Dr. Feemster.
“The ultimate goal is to identify the best practices for implementation of college vaccine policies to optimize vaccine uptake and increase positive attitudes, beliefs, and future intentions about vaccines,” she added.
The sponsor of study was the Children’s Hospital of Philadelphia. The study was not funded. Dr. Feemster had no conflicts to disclose.
AT PAS 2017
Key clinical point: A survey of colleges and universities nationwide in the United States has revealed marked variation in vaccination requirements and vaccine availability.
Major finding: Of the two public and two private schools surveyed in each state and the District of Columbia, none require vaccination for human papillomavirus, with only one-third of schools having the vaccine available through student health.
Data source: Cross-sectional survey of 216 U.S. colleges and universities.
Disclosures: The sponsor of the study was the Children’s Hospital of Philadelphia. The study was not funded. Dr. Feemster had no conflicts to disclose.