User login
Here’s what’s trending at SHM
HM17 On Demand now available
Couldn’t make it to Las Vegas for SHM’s annual meeting, Hospital Medicine 2017? HM17 On Demand gives you access to over 80 online audio and slide recordings from the hottest tracks, including clinical updates, rapid fire, pediatrics, comanagement, quality, and high-value care.
Additionally, you can earn up to 70 American Medical Association Physician Recognition Award Category 1 Credit(s) and up to 30 American Board of Internal Medicine Maintenance of Certification credits. HM17 attendees can also benefit by earning additional credits on the sessions you missed out on.
To easily access content through SHM’s Learning Portal, visit shmlearningportal.org/hm17-demand to learn more.
Chapter Excellence Awards
SHM is proud to recognize outstanding chapters for the fourth annual Chapter Excellence Awards. Each year, chapters strive to demonstrate growth, sustenance, and innovation within their chapter activities.
View more at www.hospitalmedicine.org/chapterexcellence. Please join SHM in congratulating the following chapters on their success!
Silver Chapters
Boston Association of Academic Hospital Medicine (BAAHM)
Charlotte Metro Area
Houston
Kentucky
Los Angeles
Minnesota
North Jersey
Pacific Northwest
Philadelphia Tri-State
Rocky Mountain
San Francisco Bay
South Central PA
Gold Chapters
New Mexico
Wiregrass
Platinum Chapters
IowaMaryland
Michigan
NYC/Westchester
St. Louis
Outstanding Chapter of the Year
Michigan
Rising Star Chapter
Wiregrass
Student Hospitalist Scholar grant winners
SHM’s Student Hospitalist Scholar Grant provides funds with which medical students can conduct mentored scholarly projects related to quality improvement and patient safety in the field of hospital medicine. The program offers a summer and a longitudinal option.
Congratulations to the 2017-2018 Student Hospitalist Scholar Grant recipients:Summer Program
Anton Garazha
Rosalind Franklin University of Medicine and Science
“Effectiveness of Communication During ICU to Ward Transfer and Association with Medical ICU Readmission”
Cole Hirschfeld
Weill Cornell Medical College
“The Role of Diagnostic Bone Biopsies in the Management of Osteomyelitis”
Farah Hussain
University of Cincinnati College of Medicine
“Better Understanding Clinical Deterioration in a Children’s Hospital”
Longitudinal Program
Monisha Bhatia
Vanderbilt University School of Medicine
“Using Electronic Medical Record Phenotypic Data to Predict Discharge Destination”
Victor Ekuta
University of California, San Diego School of Medicine
“Reducing CAUTI with Noninvasive UC Alternatives and Measure-vention”
Yun Li
Geisel School of Medicine at Dartmouth
“Developing and implementing clinical pathway(s) for hospitalized injection drug users due to injection-related infection sequelae”
Learn more about the Student Hospitalist Scholar Grant at hospitalmedicine.org/scholargrant.
SPARK ONE: A tool to teach residents
SPARK ONE is a comprehensive, online self-assessment tool created specifically for hospital medicine professionals. The activity contains 450+ vignette-style multiple-choice questions covering 100% of the American Board of Internal Medicine’s Focused Practice in Hospital Medicine (FPHM) exam blueprint. This online tool can be utilized as a training mechanism for resident education on hospital medicine.
As a benefit of SHM membership, residents will receive a free subscription. SPARK ONE provides in-depth review of the following content areas:
- Cardiology
- Pulmonary Disease and Critical Care Medicine
- Gastroenterology and Hepatology
- Nephrology and Urology
- Endocrinology
- Hematology and Oncology
- Neurology
- Allergy, Immunology, Dermatology, Rheumatology and Transitions in Care
- Palliative Care, Medical Ethics and Decision-making
- Perioperative Medicine and Consultative Co-management
- Patient Safety
- Quality, Cost and Clinical Reasoning
“SPARK ONE provides a unique platform for academic institutions, engaging learners in directed learning sessions, reinforcing teaching points as we encounter specific conditions.” – Rachel E. Thompson, MD, MPH, SFHM
Visit hospitalmedicine.org/sparkone to learn more.
Sharpen your coding with the updated CODE-H
SHM’s Coding Optimally by Documenting Effectively for Hospitalists (CODE-H) has launched an updated program with all new content. It will now include eight recorded webinar sessions presented by expert faculty, downloadable resources, and an interactive discussion forum through the Hospital Medicine Exchange (HMX), enabling participants to ask questions and learn the most relevant best practices.
Following each webinar, learners will have the opportunity to complete an evaluation to redeem continuing medical education credits.
Webinars in the series include:
- E/M Basics Part I
- E/M Basics Part II
- Utilizing Other Providers in Your Practice
- EMR and Mitigating Risk
- Putting Time into Critical Care Documentation
- Time Based Services
- Navigating the Rules for Hospitalist Visits
- Challenges of Concurrent Care
To purchase CODE-H, visit hospitalmedicine.org/CODEH. If you have questions about the new program, please contact [email protected].
Set yourself apart as a Fellow in Hospital Medicine
The Fellow in Hospital Medicine (FHM) designation signals your commitment to the hospital medicine specialty and dedication to quality improvement and patient safety. This designation is available for hospital medicine practitioners, including practice administrators, nurse practitioners, and physician assistants. If you meet the prerequisites and complete the requirements, which are rooted in the Core Competencies in Hospital Medicine, you can apply for this prestigious designation and join more than 1,100 FHMs who are dedicated to the field of hospital medicine. Learn more and apply at hospitalmedicine.org/fellow.
New guide & modules on multimodal pain strategies for postoperative pain management
Pain management can pose multiple challenges in the acute care setting for hospitalists and front-line prescribers. While their first priority is to optimally manage pain in their patients, they also face the challenges of treating diverse patient populations, managing patient expectations, and considering how pain control and perceptions affect Hospital Consumer Assessment of Healthcare Providers and Systems scores. Furthermore, because of the ongoing opioid epidemic, prescribers must ensure that pain is managed responsibly and ethically.
To address these issues, SHM developed a guide to address how to work in an interdisciplinary team, identify impediments to implementation, and provide examples of appropriate pain management. In accompaniment with this Multimodal Pain Strategies Guide for Postoperative Pain Management, there are three modules presented by the authors which supplement the electronic guide.
To download the guide or view the modules, visit hospitalmedicine.org/pain.
Proven excellence through a unique education style: Academic Hospitalist Academy
Don’t miss the eighth annual Academic Hospitalist Academy (AHA), Sept. 25-28, 2017, at the Lakeway Resort and Spa in Austin, Texas. AHA attendees experience an energizing, interactive learning environment featuring didactics, small-group exercise and skill-building breakout sessions. Each full day of learning is facilitated by leading clinician-educators, hospitalist researchers, and clinical administrators in a 1 to 10 faculty to student ratio.
The Principal Goals of the Academy are to:
- Develop junior academic hospitalists as the premier teachers and educational leaders at their institutions
- Help academic hospitalists develop scholarly work and increase scholarly output
- Enhance awareness of the value of quality improvement and patient safety work
- Support academic promotion of all attendees
Don’t miss out on this unique, hands-on experience. Register before July 18, 2017, to receive the early-bird rates. Visit academichospitalist.org to learn more.
Choosing Wisely Case Study compendium now available
The Choosing Wisely Case Study Competition, hosted by SHM, sought submissions from hospitalists on innovative improvement initiatives implemented in their respective institutions. These initiatives reflect and promote movement toward reducing unnecessary medical tests and procedures and changing a culture that dictates, “More care is better care.”
Submissions were judged by the Choosing Wisely Subcommittee, a panel of SHM members, under adult and pediatric categories. One grand prize winner and three honorable mentions were selected from these categories. The compendium includes these case studies along with additional exemplary submissions.
View the Choosing Wisely Case Study Compendium at hospitalmedicine.org/choosingwisely.
Strengthen your interactions with the 5 Rs of Cultural Humility
Look inside this issue for your 5 Rs of Cultural Humility pocket card. It can be easily referenced on rounds and shared with colleagues. We hope to achieve heightened awareness of effective interactions. In addition to the definitions of each of the Rs, the card features questions to ask yourself before, during, and after every interaction to aid in attaining cultural humility.
For more information, visit hospitalmedicine.org/5Rs.
Brett Radler is communications specialist at the Society of Hospital Medicine.
HM17 On Demand now available
Couldn’t make it to Las Vegas for SHM’s annual meeting, Hospital Medicine 2017? HM17 On Demand gives you access to over 80 online audio and slide recordings from the hottest tracks, including clinical updates, rapid fire, pediatrics, comanagement, quality, and high-value care.
Additionally, you can earn up to 70 American Medical Association Physician Recognition Award Category 1 Credit(s) and up to 30 American Board of Internal Medicine Maintenance of Certification credits. HM17 attendees can also benefit by earning additional credits on the sessions you missed out on.
To easily access content through SHM’s Learning Portal, visit shmlearningportal.org/hm17-demand to learn more.
Chapter Excellence Awards
SHM is proud to recognize outstanding chapters for the fourth annual Chapter Excellence Awards. Each year, chapters strive to demonstrate growth, sustenance, and innovation within their chapter activities.
View more at www.hospitalmedicine.org/chapterexcellence. Please join SHM in congratulating the following chapters on their success!
Silver Chapters
Boston Association of Academic Hospital Medicine (BAAHM)
Charlotte Metro Area
Houston
Kentucky
Los Angeles
Minnesota
North Jersey
Pacific Northwest
Philadelphia Tri-State
Rocky Mountain
San Francisco Bay
South Central PA
Gold Chapters
New Mexico
Wiregrass
Platinum Chapters
IowaMaryland
Michigan
NYC/Westchester
St. Louis
Outstanding Chapter of the Year
Michigan
Rising Star Chapter
Wiregrass
Student Hospitalist Scholar grant winners
SHM’s Student Hospitalist Scholar Grant provides funds with which medical students can conduct mentored scholarly projects related to quality improvement and patient safety in the field of hospital medicine. The program offers a summer and a longitudinal option.
Congratulations to the 2017-2018 Student Hospitalist Scholar Grant recipients:Summer Program
Anton Garazha
Rosalind Franklin University of Medicine and Science
“Effectiveness of Communication During ICU to Ward Transfer and Association with Medical ICU Readmission”
Cole Hirschfeld
Weill Cornell Medical College
“The Role of Diagnostic Bone Biopsies in the Management of Osteomyelitis”
Farah Hussain
University of Cincinnati College of Medicine
“Better Understanding Clinical Deterioration in a Children’s Hospital”
Longitudinal Program
Monisha Bhatia
Vanderbilt University School of Medicine
“Using Electronic Medical Record Phenotypic Data to Predict Discharge Destination”
Victor Ekuta
University of California, San Diego School of Medicine
“Reducing CAUTI with Noninvasive UC Alternatives and Measure-vention”
Yun Li
Geisel School of Medicine at Dartmouth
“Developing and implementing clinical pathway(s) for hospitalized injection drug users due to injection-related infection sequelae”
Learn more about the Student Hospitalist Scholar Grant at hospitalmedicine.org/scholargrant.
SPARK ONE: A tool to teach residents
SPARK ONE is a comprehensive, online self-assessment tool created specifically for hospital medicine professionals. The activity contains 450+ vignette-style multiple-choice questions covering 100% of the American Board of Internal Medicine’s Focused Practice in Hospital Medicine (FPHM) exam blueprint. This online tool can be utilized as a training mechanism for resident education on hospital medicine.
As a benefit of SHM membership, residents will receive a free subscription. SPARK ONE provides in-depth review of the following content areas:
- Cardiology
- Pulmonary Disease and Critical Care Medicine
- Gastroenterology and Hepatology
- Nephrology and Urology
- Endocrinology
- Hematology and Oncology
- Neurology
- Allergy, Immunology, Dermatology, Rheumatology and Transitions in Care
- Palliative Care, Medical Ethics and Decision-making
- Perioperative Medicine and Consultative Co-management
- Patient Safety
- Quality, Cost and Clinical Reasoning
“SPARK ONE provides a unique platform for academic institutions, engaging learners in directed learning sessions, reinforcing teaching points as we encounter specific conditions.” – Rachel E. Thompson, MD, MPH, SFHM
Visit hospitalmedicine.org/sparkone to learn more.
Sharpen your coding with the updated CODE-H
SHM’s Coding Optimally by Documenting Effectively for Hospitalists (CODE-H) has launched an updated program with all new content. It will now include eight recorded webinar sessions presented by expert faculty, downloadable resources, and an interactive discussion forum through the Hospital Medicine Exchange (HMX), enabling participants to ask questions and learn the most relevant best practices.
Following each webinar, learners will have the opportunity to complete an evaluation to redeem continuing medical education credits.
Webinars in the series include:
- E/M Basics Part I
- E/M Basics Part II
- Utilizing Other Providers in Your Practice
- EMR and Mitigating Risk
- Putting Time into Critical Care Documentation
- Time Based Services
- Navigating the Rules for Hospitalist Visits
- Challenges of Concurrent Care
To purchase CODE-H, visit hospitalmedicine.org/CODEH. If you have questions about the new program, please contact [email protected].
Set yourself apart as a Fellow in Hospital Medicine
The Fellow in Hospital Medicine (FHM) designation signals your commitment to the hospital medicine specialty and dedication to quality improvement and patient safety. This designation is available for hospital medicine practitioners, including practice administrators, nurse practitioners, and physician assistants. If you meet the prerequisites and complete the requirements, which are rooted in the Core Competencies in Hospital Medicine, you can apply for this prestigious designation and join more than 1,100 FHMs who are dedicated to the field of hospital medicine. Learn more and apply at hospitalmedicine.org/fellow.
New guide & modules on multimodal pain strategies for postoperative pain management
Pain management can pose multiple challenges in the acute care setting for hospitalists and front-line prescribers. While their first priority is to optimally manage pain in their patients, they also face the challenges of treating diverse patient populations, managing patient expectations, and considering how pain control and perceptions affect Hospital Consumer Assessment of Healthcare Providers and Systems scores. Furthermore, because of the ongoing opioid epidemic, prescribers must ensure that pain is managed responsibly and ethically.
To address these issues, SHM developed a guide to address how to work in an interdisciplinary team, identify impediments to implementation, and provide examples of appropriate pain management. In accompaniment with this Multimodal Pain Strategies Guide for Postoperative Pain Management, there are three modules presented by the authors which supplement the electronic guide.
To download the guide or view the modules, visit hospitalmedicine.org/pain.
Proven excellence through a unique education style: Academic Hospitalist Academy
Don’t miss the eighth annual Academic Hospitalist Academy (AHA), Sept. 25-28, 2017, at the Lakeway Resort and Spa in Austin, Texas. AHA attendees experience an energizing, interactive learning environment featuring didactics, small-group exercise and skill-building breakout sessions. Each full day of learning is facilitated by leading clinician-educators, hospitalist researchers, and clinical administrators in a 1 to 10 faculty to student ratio.
The Principal Goals of the Academy are to:
- Develop junior academic hospitalists as the premier teachers and educational leaders at their institutions
- Help academic hospitalists develop scholarly work and increase scholarly output
- Enhance awareness of the value of quality improvement and patient safety work
- Support academic promotion of all attendees
Don’t miss out on this unique, hands-on experience. Register before July 18, 2017, to receive the early-bird rates. Visit academichospitalist.org to learn more.
Choosing Wisely Case Study compendium now available
The Choosing Wisely Case Study Competition, hosted by SHM, sought submissions from hospitalists on innovative improvement initiatives implemented in their respective institutions. These initiatives reflect and promote movement toward reducing unnecessary medical tests and procedures and changing a culture that dictates, “More care is better care.”
Submissions were judged by the Choosing Wisely Subcommittee, a panel of SHM members, under adult and pediatric categories. One grand prize winner and three honorable mentions were selected from these categories. The compendium includes these case studies along with additional exemplary submissions.
View the Choosing Wisely Case Study Compendium at hospitalmedicine.org/choosingwisely.
Strengthen your interactions with the 5 Rs of Cultural Humility
Look inside this issue for your 5 Rs of Cultural Humility pocket card. It can be easily referenced on rounds and shared with colleagues. We hope to achieve heightened awareness of effective interactions. In addition to the definitions of each of the Rs, the card features questions to ask yourself before, during, and after every interaction to aid in attaining cultural humility.
For more information, visit hospitalmedicine.org/5Rs.
Brett Radler is communications specialist at the Society of Hospital Medicine.
HM17 On Demand now available
Couldn’t make it to Las Vegas for SHM’s annual meeting, Hospital Medicine 2017? HM17 On Demand gives you access to over 80 online audio and slide recordings from the hottest tracks, including clinical updates, rapid fire, pediatrics, comanagement, quality, and high-value care.
Additionally, you can earn up to 70 American Medical Association Physician Recognition Award Category 1 Credit(s) and up to 30 American Board of Internal Medicine Maintenance of Certification credits. HM17 attendees can also benefit by earning additional credits on the sessions you missed out on.
To easily access content through SHM’s Learning Portal, visit shmlearningportal.org/hm17-demand to learn more.
Chapter Excellence Awards
SHM is proud to recognize outstanding chapters for the fourth annual Chapter Excellence Awards. Each year, chapters strive to demonstrate growth, sustenance, and innovation within their chapter activities.
View more at www.hospitalmedicine.org/chapterexcellence. Please join SHM in congratulating the following chapters on their success!
Silver Chapters
Boston Association of Academic Hospital Medicine (BAAHM)
Charlotte Metro Area
Houston
Kentucky
Los Angeles
Minnesota
North Jersey
Pacific Northwest
Philadelphia Tri-State
Rocky Mountain
San Francisco Bay
South Central PA
Gold Chapters
New Mexico
Wiregrass
Platinum Chapters
IowaMaryland
Michigan
NYC/Westchester
St. Louis
Outstanding Chapter of the Year
Michigan
Rising Star Chapter
Wiregrass
Student Hospitalist Scholar grant winners
SHM’s Student Hospitalist Scholar Grant provides funds with which medical students can conduct mentored scholarly projects related to quality improvement and patient safety in the field of hospital medicine. The program offers a summer and a longitudinal option.
Congratulations to the 2017-2018 Student Hospitalist Scholar Grant recipients:Summer Program
Anton Garazha
Rosalind Franklin University of Medicine and Science
“Effectiveness of Communication During ICU to Ward Transfer and Association with Medical ICU Readmission”
Cole Hirschfeld
Weill Cornell Medical College
“The Role of Diagnostic Bone Biopsies in the Management of Osteomyelitis”
Farah Hussain
University of Cincinnati College of Medicine
“Better Understanding Clinical Deterioration in a Children’s Hospital”
Longitudinal Program
Monisha Bhatia
Vanderbilt University School of Medicine
“Using Electronic Medical Record Phenotypic Data to Predict Discharge Destination”
Victor Ekuta
University of California, San Diego School of Medicine
“Reducing CAUTI with Noninvasive UC Alternatives and Measure-vention”
Yun Li
Geisel School of Medicine at Dartmouth
“Developing and implementing clinical pathway(s) for hospitalized injection drug users due to injection-related infection sequelae”
Learn more about the Student Hospitalist Scholar Grant at hospitalmedicine.org/scholargrant.
SPARK ONE: A tool to teach residents
SPARK ONE is a comprehensive, online self-assessment tool created specifically for hospital medicine professionals. The activity contains 450+ vignette-style multiple-choice questions covering 100% of the American Board of Internal Medicine’s Focused Practice in Hospital Medicine (FPHM) exam blueprint. This online tool can be utilized as a training mechanism for resident education on hospital medicine.
As a benefit of SHM membership, residents will receive a free subscription. SPARK ONE provides in-depth review of the following content areas:
- Cardiology
- Pulmonary Disease and Critical Care Medicine
- Gastroenterology and Hepatology
- Nephrology and Urology
- Endocrinology
- Hematology and Oncology
- Neurology
- Allergy, Immunology, Dermatology, Rheumatology and Transitions in Care
- Palliative Care, Medical Ethics and Decision-making
- Perioperative Medicine and Consultative Co-management
- Patient Safety
- Quality, Cost and Clinical Reasoning
“SPARK ONE provides a unique platform for academic institutions, engaging learners in directed learning sessions, reinforcing teaching points as we encounter specific conditions.” – Rachel E. Thompson, MD, MPH, SFHM
Visit hospitalmedicine.org/sparkone to learn more.
Sharpen your coding with the updated CODE-H
SHM’s Coding Optimally by Documenting Effectively for Hospitalists (CODE-H) has launched an updated program with all new content. It will now include eight recorded webinar sessions presented by expert faculty, downloadable resources, and an interactive discussion forum through the Hospital Medicine Exchange (HMX), enabling participants to ask questions and learn the most relevant best practices.
Following each webinar, learners will have the opportunity to complete an evaluation to redeem continuing medical education credits.
Webinars in the series include:
- E/M Basics Part I
- E/M Basics Part II
- Utilizing Other Providers in Your Practice
- EMR and Mitigating Risk
- Putting Time into Critical Care Documentation
- Time Based Services
- Navigating the Rules for Hospitalist Visits
- Challenges of Concurrent Care
To purchase CODE-H, visit hospitalmedicine.org/CODEH. If you have questions about the new program, please contact [email protected].
Set yourself apart as a Fellow in Hospital Medicine
The Fellow in Hospital Medicine (FHM) designation signals your commitment to the hospital medicine specialty and dedication to quality improvement and patient safety. This designation is available for hospital medicine practitioners, including practice administrators, nurse practitioners, and physician assistants. If you meet the prerequisites and complete the requirements, which are rooted in the Core Competencies in Hospital Medicine, you can apply for this prestigious designation and join more than 1,100 FHMs who are dedicated to the field of hospital medicine. Learn more and apply at hospitalmedicine.org/fellow.
New guide & modules on multimodal pain strategies for postoperative pain management
Pain management can pose multiple challenges in the acute care setting for hospitalists and front-line prescribers. While their first priority is to optimally manage pain in their patients, they also face the challenges of treating diverse patient populations, managing patient expectations, and considering how pain control and perceptions affect Hospital Consumer Assessment of Healthcare Providers and Systems scores. Furthermore, because of the ongoing opioid epidemic, prescribers must ensure that pain is managed responsibly and ethically.
To address these issues, SHM developed a guide to address how to work in an interdisciplinary team, identify impediments to implementation, and provide examples of appropriate pain management. In accompaniment with this Multimodal Pain Strategies Guide for Postoperative Pain Management, there are three modules presented by the authors which supplement the electronic guide.
To download the guide or view the modules, visit hospitalmedicine.org/pain.
Proven excellence through a unique education style: Academic Hospitalist Academy
Don’t miss the eighth annual Academic Hospitalist Academy (AHA), Sept. 25-28, 2017, at the Lakeway Resort and Spa in Austin, Texas. AHA attendees experience an energizing, interactive learning environment featuring didactics, small-group exercise and skill-building breakout sessions. Each full day of learning is facilitated by leading clinician-educators, hospitalist researchers, and clinical administrators in a 1 to 10 faculty to student ratio.
The Principal Goals of the Academy are to:
- Develop junior academic hospitalists as the premier teachers and educational leaders at their institutions
- Help academic hospitalists develop scholarly work and increase scholarly output
- Enhance awareness of the value of quality improvement and patient safety work
- Support academic promotion of all attendees
Don’t miss out on this unique, hands-on experience. Register before July 18, 2017, to receive the early-bird rates. Visit academichospitalist.org to learn more.
Choosing Wisely Case Study compendium now available
The Choosing Wisely Case Study Competition, hosted by SHM, sought submissions from hospitalists on innovative improvement initiatives implemented in their respective institutions. These initiatives reflect and promote movement toward reducing unnecessary medical tests and procedures and changing a culture that dictates, “More care is better care.”
Submissions were judged by the Choosing Wisely Subcommittee, a panel of SHM members, under adult and pediatric categories. One grand prize winner and three honorable mentions were selected from these categories. The compendium includes these case studies along with additional exemplary submissions.
View the Choosing Wisely Case Study Compendium at hospitalmedicine.org/choosingwisely.
Strengthen your interactions with the 5 Rs of Cultural Humility
Look inside this issue for your 5 Rs of Cultural Humility pocket card. It can be easily referenced on rounds and shared with colleagues. We hope to achieve heightened awareness of effective interactions. In addition to the definitions of each of the Rs, the card features questions to ask yourself before, during, and after every interaction to aid in attaining cultural humility.
For more information, visit hospitalmedicine.org/5Rs.
Brett Radler is communications specialist at the Society of Hospital Medicine.
A man with progressive dysphagia
A 71-year-old man was referred to the gastroenterology department for evaluation of 9 months of progressive swallowing difficulties associated with epigastric and chest discomfort.
He was a previous smoker (17 pack-years), with a history of coronary artery disease, hypertension, and cervical spinal stenosis requiring decompressive laminectomy with a postoperative course complicated by episodes of aspiration.
DYSPHAGIA: OROPHARYNGEAL OR ESOPHAGEAL
Difficulty swallowing (dysphagia) can be caused by problems in the oropharynx or in the esophagus. Difficulty initiating a swallow can be thought of as oropharyngeal dysphagia, whereas the intermittent sensation of food stuck in the neck or chest is considered esophageal dysphagia.
Focused questioning can help differentiate oropharyngeal symptoms from esophageal symptoms. For example, difficulty clearing secretions or passing the food bolus beyond the mouth or frequent coughing spells while eating is consistent with oropharyngeal dysphagia and suggests a neurologic cause. Our patient, however, presented with a constellation of symptoms more suggestive of esophageal dysphagia.
When eliciting a history of esophageal symptoms, it is crucial to determine the progression of swallowing difficulty, as well as how it directly relates to eating solids or liquids, or both. Difficulty swallowing solid foods that has progressed over time to include liquids would raise concern for an obstruction such as a stricture, ring, or malignancy. On the other hand, abrupt onset of intermittent dysphagia to both solids and liquids would raise concern for a motility disorder of the esophagus. This patient presented with an abrupt onset of intermittent symptoms to both solids and liquids that was associated with substernal chest pain.
Once coronary disease was ruled out by cardiac biomarker testing, electrocardiography, and a pharmacologic stress test, our patient underwent upper endoscopy, which showed a normal esophageal mucosa without masses or obstruction and no evidence of peptic ulcer disease.
WHAT IS THE NEXT STEP?
When upper endoscopy is negative and cardiac causes and gastroesophageal reflux disease have been ruled out, an esophageal motility disorder should be considered.
1. After obstruction has been ruled out with upper endoscopy, which should be the next step in the investigation of esophageal dysphagia?
- A 24-hour pH recording
- Barium esophagography
- Modified barium swallow
- Computed tomography of the chest
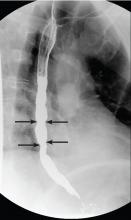
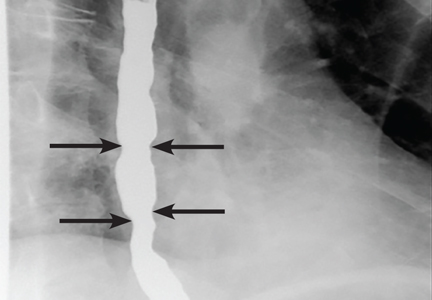
Barium esophagography is the optimal fluoroscopic study to evaluate the esophageal phase of the swallow. This study requires the patient to swallow a thick barium solution and a 13-mm barium pill under video analysis. It is useful early in the investigation of esophageal dysphagia because it can potentially reveal areas of esophageal luminal narrowing not detected endoscopically, as well as detail the rate of esophageal emptying.1
The modified barium swallow, which is performed with the assistance of a speech pathologist, is similar but only shows the oropharynx as far as the cervical esophagus. Therefore, it would be the best fluoroscopic test to assess patients with possible aspiration or oropharyngeal dysphagia, whereas barium esophagography would be the test of choice in evaluating esophageal dysmotility or mechanical obstruction.
pH testing may be helpful in diagnosing gastroesophageal reflux disease but is less helpful in the evaluation of dysphagia.
Computed tomography of the chest may be useful to evaluate for extrinsic compression of the esophagus, but it is not the best next step in the evaluation of dysphagia.
Our patient underwent barium esophagography, which revealed tertiary contractions in the mid and distal esophagus with slight narrowing of the lower cervical esophagus (Figure 1). (Primary contractions are elicited when initiating a swallow that propels the food bolus through the esophagus, while secondary contractions follow in response to esophageal distention to move all remaining esophageal contents from the thoracic esophagus. Tertiary contractions are abnormal, nonpropulsive, spontaneous contractions of the esophageal body that are initiated without swallowing.2)
EOSINOPHILIC ESOPHAGITIS
Histologic study of biopsies of the mid and distal esophagus from our patient’s upper endoscopy revealed 5 eosinophils per high-power field.
2. Does this patient meet the criteria for the diagnosis of eosinophilic esophagitis?
- Yes
- No
No. Having eosinophils in the esophagus is not enough to diagnose eosinophilic esophagitis, as eosinophils are also common in patients with gastroesophageal reflux disease.
Eosinophilic esophagitis is defined as a chronic immune-mediated esophageal disease with histologically eosinophil-predominant inflammation (with more than 15 eosinophils per high-power field). The diagnosis is additionally based on symptoms and endoscopic appearance.3 When investigating possible eosinophilic esophagitis, it is recommended that 2 to 4 samples be obtained from at least 2 different locations in the esophagus (eg, proximal and distal), because the inflammatory changes can be patchy.
WHAT DOES THE PATIENT HAVE?
3. What is the likely cause of this patient’s dysphagia?
- Eosinophilic esophagitis
- Achalasia
- Esophageal spasm
- Extrinsic compression
- Esophageal malignancy
Eosinophilic esophagitis causes characteristic symptoms that include difficulty swallowing, chest pain that does not respond to antisecretory therapy, and regurgitation of undigested food. As we discussed above, this patient has only 5 eosinophils per high-power field and does not meet the histologic criteria for eosinophilic esophagitis.
Achalasia has a characteristic “bird’s beak” appearance on esophagography that results from distal tapering of the esophagus to the gastroesophageal junction,1 and this is not apparent on our patient’s study.
Review of this patient’s esophagogram also does not reveal any extrinsic compression, esophageal malignancy, or distal tapering suggesting achalasia. In light of the abrupt onset of symptoms related to both solids and liquids associated with atypical chest pain, the primary concern should be for esophageal spasm.
ONE MORE TEST
4. What study would you order next to better elucidate the cause of this patient’s esophageal disorder?
- High-resolution esophageal manometry
- Esophagogastroduodenoscopy (EGD) with endoscopic ultrasonography
- 24-hour pH and impedance testing
- Wireless motility capsule
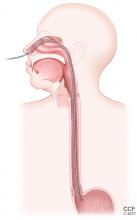
Esophageal manometry (Figure 2) is used to evaluate the function and coordination of the muscles of the esophagus, as in disorders of esophageal motility.
High-resolution manometry is the gold standard for evaluation of esophageal motility. It is appropriate in evaluating dysphagia or noncardiac chest pain without evidence of mechanical obstruction, ulceration, or inflammation.4,5
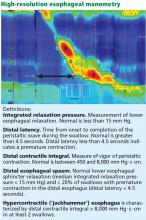
High-resolution manometry differs from conventional manometry in that the catheter has more sensors to measure intraluminal pressure (36 rather than the usual 7 to 12). The data are translated into pressure topography plots (Figure 3).6,7
Updated guidelines on how to interpret the findings of high-resolution manometry are known as the Chicago 3.0 criteria.4 According to this system, esophageal motility disorders are grouped on the basis of lower esophageal sphincter relaxation and then further subdivided based on the character of peristalsis.
EGD with endoscopic ultrasonography would not be appropriate at this time because there is little suspicion of an extraluminal mass that needs to be investigated.
A 24-hour pH and impedance study is helpful in determining the presence of esophageal acid exposure in patients presenting with gastroesophageal reflux disease. This patient does not have symptoms of heartburn or regurgitation; therefore, this investigation would not be of value.
A wireless motility capsule would help in investigating gastric and small-bowel motility and may be useful in the future for this patient, but at this point it would provide little additional utility.
ESOPHAGEAL SPASM
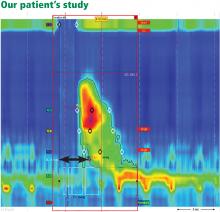
Our patient underwent high-resolution esophageal manometry. The results (Figure 4) revealed a normal resting pressure in the lower esophageal sphincter and complete relaxation in all swallows. The body of the esophagus demonstrated premature contractions in 90% of swallows. Overall, these findings were consistent with the diagnosis of distal esophageal spasm.
TREATMENTS FOR ESOPHAGEAL SPASM
In addition to incorporating data obtained from endoscopy, esophagography, and manometry, it is crucial to identify the patient’s predominant symptom when planning treatment. For example, is the prevailing symptom dysphagia or chest pain? Additional consideration must be given to medical, surgical, and psychiatric comorbidities.
5. Which of the following is appropriate medical therapy for esophageal spasm?
- Calcium channel blockers
- Nitrates
- Hydralazine
- Phosphodiesterase 5 (PDE5) inhibitors
- All of the above
All of these have been used to treat distal esophageal spasm as well as other hypercontractile esophageal motility disorders.8–20
Calcium channel blockers have proven to be effective in randomized controlled trials. Diltiazem has been shown to be beneficial at doses ranging from 60 to 90 mg, as has nifedipine 10 to 20 mg 3 times daily. Although different drugs of this class tend to relax the lower esophageal sphincter to different degrees, when choosing among them in patients with hypercontractile disorders there is little concern for potentially precipitating reflux.8–13
Nitrates, hydralazine, and PDE5 inhibitors have been effective in uncontrolled studies but have not been studied in randomized trials.14–17
Other treatments. Patients may also benefit from neuromodulators such as trazodone and imipramine for chest pain and optimization of antisecretory therapy if they have concomitant gastroesophageal reflux disease.18–20
Patients who have documented esophageal hypercontractility along with reflux disease confirmed by an abnormal pH study show significant improvement in their chest pain symptoms with high doses of a proton pump inhibitor (PPI). As our patient presented with chest pain and dysphagia, a dedicated pH study was not needed, and we could progress straight to manometry and a trial of a PPI.
Our patient was started on a PPI and nifedipine but developed a pruritic rash. As rash does not preclude using another medication in the same class, his treatment was changed to diltiazem 30 mg by mouth 3 times a day, and his dysphagia improved. However, he continued to experience intermittent chest pain with swallowing. After discussion of neuromodulator therapy, he declined additional pharmacologic therapy.
A NONPHARMACOLOGIC TREATMENT?
6. Which of the following would you offer this patient as a nonpharmacologic alternative for his esophageal pain?
- St. John’s wort
- Ginkgo biloba
- Ginseng
- Peppermint extract
- Eucalyptus oil
In a small, open-label study in patients with esophageal spasm, the use of 5 drops of commercially available 11% peppermint extract in 10 mL of water significantly decreased simultaneous contractions and resolved chest pain.21 Esophageal manometry was performed 10 minutes after the peppermint solution was consumed, and the results showed improvement in esophageal spasm. While the authors of this study did not make any formal recommendations, the findings suggest that peppermint extract should be given 10 minutes before meals.
There is no evidence for or against the use of the other nonpharmacologic treatments mentioned here.
PAIN RELIEF
7. If a pharmacologic approach were chosen, which would be the best option for pain relief in this patient?
- Oxycodone 5 mg every 8 hours
- Acetaminophen 650 mg every 8 hours
- Ibuprofen 400 mg every evening at bedtime
- Trazodone 100 mg every evening at bedtime
- Imipramine 50 mg every evening at bedtime
- Aripiprazole 5 mg by mouth every day
Trazodone would be the most appropriate of these options. Doses of 100 mg to 150 mg every evening at bedtime have been shown to significantly improve global assessment scores of pain at 6 weeks.18
Imipramine 50 mg every evening at bedtime would be another option and also has been shown to reduce chest pain.19
Even though these were the doses that were investigated, in clinical practice it is common to start at lower doses (trazodone 50 mg or imipramine 10 mg) and to then titrate every 4 weeks based on the patient’s response.
Opiates (eg, oxycodone) should be avoided, as they can cause esophageal motility disorders such as spasm or achalasia.22
Acetaminophen and aripiprazole have not been studied exclusively for their effect on chest pain related to esophageal spasm.
RECURRENT SYMPTOMS
The patient’s dysphagia initially decreased while he was taking diltiazem 30 mg 3 times a day, but it recurred after 6 months. The dosage was increased to 60 mg 3 times a day over the course of the next year, with minimal response. (The maximum dose is 90 mg 4 times a day, but because of side effects of lightheadedness and dizziness, out patient could not tolerate more than 60 mg 3 times a day).
ENDOSCOPIC THERAPY
8. What endoscopic therapies are appropriate for patients with esophageal spasm that does not respond to medication?
- Bougie dilation
- Balloon dilation
- Onabotulinum toxin injection
- Expandable mesh stent placement
- Mucosal sclerotherapy
Onabotulinum toxin injections have been shown to improve dysphagia when given in a linear pattern.23
Endoscopic dilation has not been shown to be beneficial in this setting, as a study found no difference in efficacy between therapeutic (54-French) and sham (24-French) bougie dilation.24
Our patient received 100 units of onabotulinum toxin (10 units every centimeter in the distal 10 cm of the esophagus). Afterward, he experienced resolution of dysphagia, with only mild intermittent chest pain, which was controlled by taking peppermint extract as needed. The symptoms returned approximately 1 year later but responded to repeat endoscopy with onabotulinum toxin injections.23,25
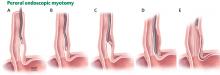
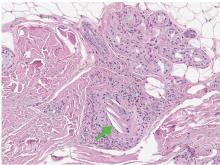
Peroral endoscopic myotomy
Another relatively new endoscopic treatment for esophageal motility disorders is peroral endoscopic myotomy (Figure 5). During this procedure a tiny incision is made in the esophageal mucosa, permitting the endoscope to tunnel within the lining. The smooth muscle of the distal esophagus and lower esophageal sphincter is then cut, thereby freeing either the spastic muscle (in distal esophageal spasm) or the hyperactive lower esophageal sphincter (in achalasia).26,27
In an open trial, after undergoing peroral endoscopic myotomy for esophageal spasm and hypercontractile esophagus, 89% of patients had complete relief of dysphagia, and 92% had palliation of chest pain.28 Of note, the rate of relief of dysphagia was higher for patients with achalasia (98%) than for nonachalasia patients (71%).
- Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol 2013; 108:1238–1249;
- Hellemans J, Vantrappen G. Physiology. In: Vantrappen G, Hellemans J, eds. Diseases of the esophagus. New York, NY: Springer-Verlag Berlin, Heidelberg; 1974:40–102.
- Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA; American College of Gastroenterology. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013; 108:679–692.
- Kahrilas PJ, Bredenoord AJ, Fox M, et al; International High Resolution Manometry Working Group. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015; 27:160–174.
- Pandolfino JE, Kahrilas PJ; American Gastroenterological Association. AGA technical review on the clinical use of esophageal manometry. Gastroenterology 2005; 128:209–224.
- Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Shah N, Kahrilas PJ. Quantifying esophageal peristalsis with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol 2006; 290:G988–G997.
- Kahrilas PJ, Sifrim D. High-resolution manometry and impedance-pH/manometry: valuable tools in clinical and investigational esophagology. Gastroenterology 2008; 135:756–769.
- Cattau EL Jr, Castell DO, Johnson DA, et al. Diltiazem therapy for symptoms associated with nutcracker esophagus. Am J Gastroenterol 1991; 86:272–276.
- Richter JE, Dalton CB, Bradley LA, Castell DO. Oral nifedipine in the treatment of noncardiac chest pain in patients with the nutcracker esophagus. Gastroenterology 1987; 93:21–28.
- Drenth JP, Bos LP, Engels LG. Efficacy of diltiazem in the treatment of diffuse oesophageal spasm. Aliment Pharmacol Ther 1990; 4:411–416.
- Thomas E, Witt P, Willis M, Morse J. Nifedipine therapy for diffuse esophageal spasm. South Med J 1986; 79:847–849.
- Davies HA, Lewis MJ, Rhodes J, Henderson AH. Trial of nifedipine for prevention of oesophageal spasm. Digestion 1987; 36:81–83.
- Richter JE, Dalton CB, Bradley LA, Castell DO. Oral nifedipine in the treatment of noncardiac chest pain in patients with the nutcracker esophagus. Gastroenterology 1987; 93:21–28.
- Tursi A, Brandimarte G, Gasbarrini G. Transdermal slow-release long-acting isosorbide dinitrate for ‘nutcracker’ oesophagus: an open study. Eur J Gastroenterol Hepatol 2000; 12:1061–1062.
- Mellow MH. Effect of isosorbide and hydralazine in painful primary esophageal motility disorders. Gastroenterology 1982; 83:364–370.
- Fox M, Sweis R, Wong T, Anggiansah A. Sildenafil relieves symptoms and normalizes motility in patients with oesophageal spasm: a report of two cases. Neurogastroenterol Motil 2007; 19:798–803.
- Orlando RC, Bozymski EM. Clinical and manometric effects of nitroglycerin in diffuse esophageal spasm. N Engl J Med 1973; 289:23–25.
- Clouse RE, Lustman PJ, Eckert TC, Ferney DM, Griffith LS. Low-dose trazodone for symptomatic patients with esophageal contraction abnormalities. A double-blind, placebo-controlled trial. Gastroenterology 1987; 92:1027–1036.
- Cannon RO 3rd, Quyyumi AA, Mincemoyer R, et al. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med 1994; 330:1411–1417.
- Achem SR, Kolts BE, Wears R, Burton L, Richter JE. Chest pain associated with nutcracker esophagus: a preliminary study of the role of gastroesophageal reflux. Am J Gastroenterol 1993; 88:187–192.
- Pimentel M, Bonorris GG, Chow EJ, Lin HC. Peppermint oil improves the manometric findings in diffuse esophageal spasm. J Clin Gastroenterol 2001; 33:27–31.
- Kraichely RE, Arora AS, Murray JA. Opiate-induced oesophageal dysmotility. Aliment Pharmacol Ther 2010; 31:601–606.
- Storr M, Allescher HD, Rösch T, Born P, Weigert N, Classen M. Treatment of symptomatic diffuse esophageal spasm by endoscopic injections of botulinum toxin: a prospective study with long-term follow-up. Gastrointest Endosc 2001; 54:754–759.
- Winters C, Artnak EJ, Benjamin SB, Castell DO. Esophageal bougienage in symptomatic patients with the nutcracker esophagus. A primary esophageal motility disorder. JAMA 1984; 252:363–366.
- Vanuytsel T, Bisschops R, Farré R, et al. Botulinum toxin reduces dysphagia in patients with nonachalasia primary esophageal motility disorders. Clin Gastroenterol Hepatol 2013; 11:1115–1121.e2.
- Khashab MA, Messallam AA, Onimaru M, et al. International multicenter experience with peroral endoscopic myotomy for the treatment of spastic esophageal disorders refractory to medical therapy (with video). Gastrointest Endosc 2015; 81:1170–1177.
- Leconte M, Douard R, Gaudric M, Dumontier I, Chaussade S, Dousset B. Functional results after extended myotomy for diffuse oesophageal spasm. Br J Surg 2007; 94:1113–1118.
- Sharata AM, Dunst CM, Pescarus R, et al. Peroral endoscopic myotomy (POEM) for esophageal primary motility disorders: analysis of 100 consecutive patients. J Gastrointest Surg 2015; 19:161–170.
A 71-year-old man was referred to the gastroenterology department for evaluation of 9 months of progressive swallowing difficulties associated with epigastric and chest discomfort.
He was a previous smoker (17 pack-years), with a history of coronary artery disease, hypertension, and cervical spinal stenosis requiring decompressive laminectomy with a postoperative course complicated by episodes of aspiration.
DYSPHAGIA: OROPHARYNGEAL OR ESOPHAGEAL
Difficulty swallowing (dysphagia) can be caused by problems in the oropharynx or in the esophagus. Difficulty initiating a swallow can be thought of as oropharyngeal dysphagia, whereas the intermittent sensation of food stuck in the neck or chest is considered esophageal dysphagia.
Focused questioning can help differentiate oropharyngeal symptoms from esophageal symptoms. For example, difficulty clearing secretions or passing the food bolus beyond the mouth or frequent coughing spells while eating is consistent with oropharyngeal dysphagia and suggests a neurologic cause. Our patient, however, presented with a constellation of symptoms more suggestive of esophageal dysphagia.
When eliciting a history of esophageal symptoms, it is crucial to determine the progression of swallowing difficulty, as well as how it directly relates to eating solids or liquids, or both. Difficulty swallowing solid foods that has progressed over time to include liquids would raise concern for an obstruction such as a stricture, ring, or malignancy. On the other hand, abrupt onset of intermittent dysphagia to both solids and liquids would raise concern for a motility disorder of the esophagus. This patient presented with an abrupt onset of intermittent symptoms to both solids and liquids that was associated with substernal chest pain.
Once coronary disease was ruled out by cardiac biomarker testing, electrocardiography, and a pharmacologic stress test, our patient underwent upper endoscopy, which showed a normal esophageal mucosa without masses or obstruction and no evidence of peptic ulcer disease.
WHAT IS THE NEXT STEP?
When upper endoscopy is negative and cardiac causes and gastroesophageal reflux disease have been ruled out, an esophageal motility disorder should be considered.
1. After obstruction has been ruled out with upper endoscopy, which should be the next step in the investigation of esophageal dysphagia?
- A 24-hour pH recording
- Barium esophagography
- Modified barium swallow
- Computed tomography of the chest
Barium esophagography is the optimal fluoroscopic study to evaluate the esophageal phase of the swallow. This study requires the patient to swallow a thick barium solution and a 13-mm barium pill under video analysis. It is useful early in the investigation of esophageal dysphagia because it can potentially reveal areas of esophageal luminal narrowing not detected endoscopically, as well as detail the rate of esophageal emptying.1
The modified barium swallow, which is performed with the assistance of a speech pathologist, is similar but only shows the oropharynx as far as the cervical esophagus. Therefore, it would be the best fluoroscopic test to assess patients with possible aspiration or oropharyngeal dysphagia, whereas barium esophagography would be the test of choice in evaluating esophageal dysmotility or mechanical obstruction.
pH testing may be helpful in diagnosing gastroesophageal reflux disease but is less helpful in the evaluation of dysphagia.
Computed tomography of the chest may be useful to evaluate for extrinsic compression of the esophagus, but it is not the best next step in the evaluation of dysphagia.
Our patient underwent barium esophagography, which revealed tertiary contractions in the mid and distal esophagus with slight narrowing of the lower cervical esophagus (Figure 1). (Primary contractions are elicited when initiating a swallow that propels the food bolus through the esophagus, while secondary contractions follow in response to esophageal distention to move all remaining esophageal contents from the thoracic esophagus. Tertiary contractions are abnormal, nonpropulsive, spontaneous contractions of the esophageal body that are initiated without swallowing.2)
EOSINOPHILIC ESOPHAGITIS
Histologic study of biopsies of the mid and distal esophagus from our patient’s upper endoscopy revealed 5 eosinophils per high-power field.
2. Does this patient meet the criteria for the diagnosis of eosinophilic esophagitis?
- Yes
- No
No. Having eosinophils in the esophagus is not enough to diagnose eosinophilic esophagitis, as eosinophils are also common in patients with gastroesophageal reflux disease.
Eosinophilic esophagitis is defined as a chronic immune-mediated esophageal disease with histologically eosinophil-predominant inflammation (with more than 15 eosinophils per high-power field). The diagnosis is additionally based on symptoms and endoscopic appearance.3 When investigating possible eosinophilic esophagitis, it is recommended that 2 to 4 samples be obtained from at least 2 different locations in the esophagus (eg, proximal and distal), because the inflammatory changes can be patchy.
WHAT DOES THE PATIENT HAVE?
3. What is the likely cause of this patient’s dysphagia?
- Eosinophilic esophagitis
- Achalasia
- Esophageal spasm
- Extrinsic compression
- Esophageal malignancy
Eosinophilic esophagitis causes characteristic symptoms that include difficulty swallowing, chest pain that does not respond to antisecretory therapy, and regurgitation of undigested food. As we discussed above, this patient has only 5 eosinophils per high-power field and does not meet the histologic criteria for eosinophilic esophagitis.
Achalasia has a characteristic “bird’s beak” appearance on esophagography that results from distal tapering of the esophagus to the gastroesophageal junction,1 and this is not apparent on our patient’s study.
Review of this patient’s esophagogram also does not reveal any extrinsic compression, esophageal malignancy, or distal tapering suggesting achalasia. In light of the abrupt onset of symptoms related to both solids and liquids associated with atypical chest pain, the primary concern should be for esophageal spasm.
ONE MORE TEST
4. What study would you order next to better elucidate the cause of this patient’s esophageal disorder?
- High-resolution esophageal manometry
- Esophagogastroduodenoscopy (EGD) with endoscopic ultrasonography
- 24-hour pH and impedance testing
- Wireless motility capsule
Esophageal manometry (Figure 2) is used to evaluate the function and coordination of the muscles of the esophagus, as in disorders of esophageal motility.
High-resolution manometry is the gold standard for evaluation of esophageal motility. It is appropriate in evaluating dysphagia or noncardiac chest pain without evidence of mechanical obstruction, ulceration, or inflammation.4,5
High-resolution manometry differs from conventional manometry in that the catheter has more sensors to measure intraluminal pressure (36 rather than the usual 7 to 12). The data are translated into pressure topography plots (Figure 3).6,7
Updated guidelines on how to interpret the findings of high-resolution manometry are known as the Chicago 3.0 criteria.4 According to this system, esophageal motility disorders are grouped on the basis of lower esophageal sphincter relaxation and then further subdivided based on the character of peristalsis.
EGD with endoscopic ultrasonography would not be appropriate at this time because there is little suspicion of an extraluminal mass that needs to be investigated.
A 24-hour pH and impedance study is helpful in determining the presence of esophageal acid exposure in patients presenting with gastroesophageal reflux disease. This patient does not have symptoms of heartburn or regurgitation; therefore, this investigation would not be of value.
A wireless motility capsule would help in investigating gastric and small-bowel motility and may be useful in the future for this patient, but at this point it would provide little additional utility.
ESOPHAGEAL SPASM
Our patient underwent high-resolution esophageal manometry. The results (Figure 4) revealed a normal resting pressure in the lower esophageal sphincter and complete relaxation in all swallows. The body of the esophagus demonstrated premature contractions in 90% of swallows. Overall, these findings were consistent with the diagnosis of distal esophageal spasm.
TREATMENTS FOR ESOPHAGEAL SPASM
In addition to incorporating data obtained from endoscopy, esophagography, and manometry, it is crucial to identify the patient’s predominant symptom when planning treatment. For example, is the prevailing symptom dysphagia or chest pain? Additional consideration must be given to medical, surgical, and psychiatric comorbidities.
5. Which of the following is appropriate medical therapy for esophageal spasm?
- Calcium channel blockers
- Nitrates
- Hydralazine
- Phosphodiesterase 5 (PDE5) inhibitors
- All of the above
All of these have been used to treat distal esophageal spasm as well as other hypercontractile esophageal motility disorders.8–20
Calcium channel blockers have proven to be effective in randomized controlled trials. Diltiazem has been shown to be beneficial at doses ranging from 60 to 90 mg, as has nifedipine 10 to 20 mg 3 times daily. Although different drugs of this class tend to relax the lower esophageal sphincter to different degrees, when choosing among them in patients with hypercontractile disorders there is little concern for potentially precipitating reflux.8–13
Nitrates, hydralazine, and PDE5 inhibitors have been effective in uncontrolled studies but have not been studied in randomized trials.14–17
Other treatments. Patients may also benefit from neuromodulators such as trazodone and imipramine for chest pain and optimization of antisecretory therapy if they have concomitant gastroesophageal reflux disease.18–20
Patients who have documented esophageal hypercontractility along with reflux disease confirmed by an abnormal pH study show significant improvement in their chest pain symptoms with high doses of a proton pump inhibitor (PPI). As our patient presented with chest pain and dysphagia, a dedicated pH study was not needed, and we could progress straight to manometry and a trial of a PPI.
Our patient was started on a PPI and nifedipine but developed a pruritic rash. As rash does not preclude using another medication in the same class, his treatment was changed to diltiazem 30 mg by mouth 3 times a day, and his dysphagia improved. However, he continued to experience intermittent chest pain with swallowing. After discussion of neuromodulator therapy, he declined additional pharmacologic therapy.
A NONPHARMACOLOGIC TREATMENT?
6. Which of the following would you offer this patient as a nonpharmacologic alternative for his esophageal pain?
- St. John’s wort
- Ginkgo biloba
- Ginseng
- Peppermint extract
- Eucalyptus oil
In a small, open-label study in patients with esophageal spasm, the use of 5 drops of commercially available 11% peppermint extract in 10 mL of water significantly decreased simultaneous contractions and resolved chest pain.21 Esophageal manometry was performed 10 minutes after the peppermint solution was consumed, and the results showed improvement in esophageal spasm. While the authors of this study did not make any formal recommendations, the findings suggest that peppermint extract should be given 10 minutes before meals.
There is no evidence for or against the use of the other nonpharmacologic treatments mentioned here.
PAIN RELIEF
7. If a pharmacologic approach were chosen, which would be the best option for pain relief in this patient?
- Oxycodone 5 mg every 8 hours
- Acetaminophen 650 mg every 8 hours
- Ibuprofen 400 mg every evening at bedtime
- Trazodone 100 mg every evening at bedtime
- Imipramine 50 mg every evening at bedtime
- Aripiprazole 5 mg by mouth every day
Trazodone would be the most appropriate of these options. Doses of 100 mg to 150 mg every evening at bedtime have been shown to significantly improve global assessment scores of pain at 6 weeks.18
Imipramine 50 mg every evening at bedtime would be another option and also has been shown to reduce chest pain.19
Even though these were the doses that were investigated, in clinical practice it is common to start at lower doses (trazodone 50 mg or imipramine 10 mg) and to then titrate every 4 weeks based on the patient’s response.
Opiates (eg, oxycodone) should be avoided, as they can cause esophageal motility disorders such as spasm or achalasia.22
Acetaminophen and aripiprazole have not been studied exclusively for their effect on chest pain related to esophageal spasm.
RECURRENT SYMPTOMS
The patient’s dysphagia initially decreased while he was taking diltiazem 30 mg 3 times a day, but it recurred after 6 months. The dosage was increased to 60 mg 3 times a day over the course of the next year, with minimal response. (The maximum dose is 90 mg 4 times a day, but because of side effects of lightheadedness and dizziness, out patient could not tolerate more than 60 mg 3 times a day).
ENDOSCOPIC THERAPY
8. What endoscopic therapies are appropriate for patients with esophageal spasm that does not respond to medication?
- Bougie dilation
- Balloon dilation
- Onabotulinum toxin injection
- Expandable mesh stent placement
- Mucosal sclerotherapy
Onabotulinum toxin injections have been shown to improve dysphagia when given in a linear pattern.23
Endoscopic dilation has not been shown to be beneficial in this setting, as a study found no difference in efficacy between therapeutic (54-French) and sham (24-French) bougie dilation.24
Our patient received 100 units of onabotulinum toxin (10 units every centimeter in the distal 10 cm of the esophagus). Afterward, he experienced resolution of dysphagia, with only mild intermittent chest pain, which was controlled by taking peppermint extract as needed. The symptoms returned approximately 1 year later but responded to repeat endoscopy with onabotulinum toxin injections.23,25
Peroral endoscopic myotomy
Another relatively new endoscopic treatment for esophageal motility disorders is peroral endoscopic myotomy (Figure 5). During this procedure a tiny incision is made in the esophageal mucosa, permitting the endoscope to tunnel within the lining. The smooth muscle of the distal esophagus and lower esophageal sphincter is then cut, thereby freeing either the spastic muscle (in distal esophageal spasm) or the hyperactive lower esophageal sphincter (in achalasia).26,27
In an open trial, after undergoing peroral endoscopic myotomy for esophageal spasm and hypercontractile esophagus, 89% of patients had complete relief of dysphagia, and 92% had palliation of chest pain.28 Of note, the rate of relief of dysphagia was higher for patients with achalasia (98%) than for nonachalasia patients (71%).
A 71-year-old man was referred to the gastroenterology department for evaluation of 9 months of progressive swallowing difficulties associated with epigastric and chest discomfort.
He was a previous smoker (17 pack-years), with a history of coronary artery disease, hypertension, and cervical spinal stenosis requiring decompressive laminectomy with a postoperative course complicated by episodes of aspiration.
DYSPHAGIA: OROPHARYNGEAL OR ESOPHAGEAL
Difficulty swallowing (dysphagia) can be caused by problems in the oropharynx or in the esophagus. Difficulty initiating a swallow can be thought of as oropharyngeal dysphagia, whereas the intermittent sensation of food stuck in the neck or chest is considered esophageal dysphagia.
Focused questioning can help differentiate oropharyngeal symptoms from esophageal symptoms. For example, difficulty clearing secretions or passing the food bolus beyond the mouth or frequent coughing spells while eating is consistent with oropharyngeal dysphagia and suggests a neurologic cause. Our patient, however, presented with a constellation of symptoms more suggestive of esophageal dysphagia.
When eliciting a history of esophageal symptoms, it is crucial to determine the progression of swallowing difficulty, as well as how it directly relates to eating solids or liquids, or both. Difficulty swallowing solid foods that has progressed over time to include liquids would raise concern for an obstruction such as a stricture, ring, or malignancy. On the other hand, abrupt onset of intermittent dysphagia to both solids and liquids would raise concern for a motility disorder of the esophagus. This patient presented with an abrupt onset of intermittent symptoms to both solids and liquids that was associated with substernal chest pain.
Once coronary disease was ruled out by cardiac biomarker testing, electrocardiography, and a pharmacologic stress test, our patient underwent upper endoscopy, which showed a normal esophageal mucosa without masses or obstruction and no evidence of peptic ulcer disease.
WHAT IS THE NEXT STEP?
When upper endoscopy is negative and cardiac causes and gastroesophageal reflux disease have been ruled out, an esophageal motility disorder should be considered.
1. After obstruction has been ruled out with upper endoscopy, which should be the next step in the investigation of esophageal dysphagia?
- A 24-hour pH recording
- Barium esophagography
- Modified barium swallow
- Computed tomography of the chest
Barium esophagography is the optimal fluoroscopic study to evaluate the esophageal phase of the swallow. This study requires the patient to swallow a thick barium solution and a 13-mm barium pill under video analysis. It is useful early in the investigation of esophageal dysphagia because it can potentially reveal areas of esophageal luminal narrowing not detected endoscopically, as well as detail the rate of esophageal emptying.1
The modified barium swallow, which is performed with the assistance of a speech pathologist, is similar but only shows the oropharynx as far as the cervical esophagus. Therefore, it would be the best fluoroscopic test to assess patients with possible aspiration or oropharyngeal dysphagia, whereas barium esophagography would be the test of choice in evaluating esophageal dysmotility or mechanical obstruction.
pH testing may be helpful in diagnosing gastroesophageal reflux disease but is less helpful in the evaluation of dysphagia.
Computed tomography of the chest may be useful to evaluate for extrinsic compression of the esophagus, but it is not the best next step in the evaluation of dysphagia.
Our patient underwent barium esophagography, which revealed tertiary contractions in the mid and distal esophagus with slight narrowing of the lower cervical esophagus (Figure 1). (Primary contractions are elicited when initiating a swallow that propels the food bolus through the esophagus, while secondary contractions follow in response to esophageal distention to move all remaining esophageal contents from the thoracic esophagus. Tertiary contractions are abnormal, nonpropulsive, spontaneous contractions of the esophageal body that are initiated without swallowing.2)
EOSINOPHILIC ESOPHAGITIS
Histologic study of biopsies of the mid and distal esophagus from our patient’s upper endoscopy revealed 5 eosinophils per high-power field.
2. Does this patient meet the criteria for the diagnosis of eosinophilic esophagitis?
- Yes
- No
No. Having eosinophils in the esophagus is not enough to diagnose eosinophilic esophagitis, as eosinophils are also common in patients with gastroesophageal reflux disease.
Eosinophilic esophagitis is defined as a chronic immune-mediated esophageal disease with histologically eosinophil-predominant inflammation (with more than 15 eosinophils per high-power field). The diagnosis is additionally based on symptoms and endoscopic appearance.3 When investigating possible eosinophilic esophagitis, it is recommended that 2 to 4 samples be obtained from at least 2 different locations in the esophagus (eg, proximal and distal), because the inflammatory changes can be patchy.
WHAT DOES THE PATIENT HAVE?
3. What is the likely cause of this patient’s dysphagia?
- Eosinophilic esophagitis
- Achalasia
- Esophageal spasm
- Extrinsic compression
- Esophageal malignancy
Eosinophilic esophagitis causes characteristic symptoms that include difficulty swallowing, chest pain that does not respond to antisecretory therapy, and regurgitation of undigested food. As we discussed above, this patient has only 5 eosinophils per high-power field and does not meet the histologic criteria for eosinophilic esophagitis.
Achalasia has a characteristic “bird’s beak” appearance on esophagography that results from distal tapering of the esophagus to the gastroesophageal junction,1 and this is not apparent on our patient’s study.
Review of this patient’s esophagogram also does not reveal any extrinsic compression, esophageal malignancy, or distal tapering suggesting achalasia. In light of the abrupt onset of symptoms related to both solids and liquids associated with atypical chest pain, the primary concern should be for esophageal spasm.
ONE MORE TEST
4. What study would you order next to better elucidate the cause of this patient’s esophageal disorder?
- High-resolution esophageal manometry
- Esophagogastroduodenoscopy (EGD) with endoscopic ultrasonography
- 24-hour pH and impedance testing
- Wireless motility capsule
Esophageal manometry (Figure 2) is used to evaluate the function and coordination of the muscles of the esophagus, as in disorders of esophageal motility.
High-resolution manometry is the gold standard for evaluation of esophageal motility. It is appropriate in evaluating dysphagia or noncardiac chest pain without evidence of mechanical obstruction, ulceration, or inflammation.4,5
High-resolution manometry differs from conventional manometry in that the catheter has more sensors to measure intraluminal pressure (36 rather than the usual 7 to 12). The data are translated into pressure topography plots (Figure 3).6,7
Updated guidelines on how to interpret the findings of high-resolution manometry are known as the Chicago 3.0 criteria.4 According to this system, esophageal motility disorders are grouped on the basis of lower esophageal sphincter relaxation and then further subdivided based on the character of peristalsis.
EGD with endoscopic ultrasonography would not be appropriate at this time because there is little suspicion of an extraluminal mass that needs to be investigated.
A 24-hour pH and impedance study is helpful in determining the presence of esophageal acid exposure in patients presenting with gastroesophageal reflux disease. This patient does not have symptoms of heartburn or regurgitation; therefore, this investigation would not be of value.
A wireless motility capsule would help in investigating gastric and small-bowel motility and may be useful in the future for this patient, but at this point it would provide little additional utility.
ESOPHAGEAL SPASM
Our patient underwent high-resolution esophageal manometry. The results (Figure 4) revealed a normal resting pressure in the lower esophageal sphincter and complete relaxation in all swallows. The body of the esophagus demonstrated premature contractions in 90% of swallows. Overall, these findings were consistent with the diagnosis of distal esophageal spasm.
TREATMENTS FOR ESOPHAGEAL SPASM
In addition to incorporating data obtained from endoscopy, esophagography, and manometry, it is crucial to identify the patient’s predominant symptom when planning treatment. For example, is the prevailing symptom dysphagia or chest pain? Additional consideration must be given to medical, surgical, and psychiatric comorbidities.
5. Which of the following is appropriate medical therapy for esophageal spasm?
- Calcium channel blockers
- Nitrates
- Hydralazine
- Phosphodiesterase 5 (PDE5) inhibitors
- All of the above
All of these have been used to treat distal esophageal spasm as well as other hypercontractile esophageal motility disorders.8–20
Calcium channel blockers have proven to be effective in randomized controlled trials. Diltiazem has been shown to be beneficial at doses ranging from 60 to 90 mg, as has nifedipine 10 to 20 mg 3 times daily. Although different drugs of this class tend to relax the lower esophageal sphincter to different degrees, when choosing among them in patients with hypercontractile disorders there is little concern for potentially precipitating reflux.8–13
Nitrates, hydralazine, and PDE5 inhibitors have been effective in uncontrolled studies but have not been studied in randomized trials.14–17
Other treatments. Patients may also benefit from neuromodulators such as trazodone and imipramine for chest pain and optimization of antisecretory therapy if they have concomitant gastroesophageal reflux disease.18–20
Patients who have documented esophageal hypercontractility along with reflux disease confirmed by an abnormal pH study show significant improvement in their chest pain symptoms with high doses of a proton pump inhibitor (PPI). As our patient presented with chest pain and dysphagia, a dedicated pH study was not needed, and we could progress straight to manometry and a trial of a PPI.
Our patient was started on a PPI and nifedipine but developed a pruritic rash. As rash does not preclude using another medication in the same class, his treatment was changed to diltiazem 30 mg by mouth 3 times a day, and his dysphagia improved. However, he continued to experience intermittent chest pain with swallowing. After discussion of neuromodulator therapy, he declined additional pharmacologic therapy.
A NONPHARMACOLOGIC TREATMENT?
6. Which of the following would you offer this patient as a nonpharmacologic alternative for his esophageal pain?
- St. John’s wort
- Ginkgo biloba
- Ginseng
- Peppermint extract
- Eucalyptus oil
In a small, open-label study in patients with esophageal spasm, the use of 5 drops of commercially available 11% peppermint extract in 10 mL of water significantly decreased simultaneous contractions and resolved chest pain.21 Esophageal manometry was performed 10 minutes after the peppermint solution was consumed, and the results showed improvement in esophageal spasm. While the authors of this study did not make any formal recommendations, the findings suggest that peppermint extract should be given 10 minutes before meals.
There is no evidence for or against the use of the other nonpharmacologic treatments mentioned here.
PAIN RELIEF
7. If a pharmacologic approach were chosen, which would be the best option for pain relief in this patient?
- Oxycodone 5 mg every 8 hours
- Acetaminophen 650 mg every 8 hours
- Ibuprofen 400 mg every evening at bedtime
- Trazodone 100 mg every evening at bedtime
- Imipramine 50 mg every evening at bedtime
- Aripiprazole 5 mg by mouth every day
Trazodone would be the most appropriate of these options. Doses of 100 mg to 150 mg every evening at bedtime have been shown to significantly improve global assessment scores of pain at 6 weeks.18
Imipramine 50 mg every evening at bedtime would be another option and also has been shown to reduce chest pain.19
Even though these were the doses that were investigated, in clinical practice it is common to start at lower doses (trazodone 50 mg or imipramine 10 mg) and to then titrate every 4 weeks based on the patient’s response.
Opiates (eg, oxycodone) should be avoided, as they can cause esophageal motility disorders such as spasm or achalasia.22
Acetaminophen and aripiprazole have not been studied exclusively for their effect on chest pain related to esophageal spasm.
RECURRENT SYMPTOMS
The patient’s dysphagia initially decreased while he was taking diltiazem 30 mg 3 times a day, but it recurred after 6 months. The dosage was increased to 60 mg 3 times a day over the course of the next year, with minimal response. (The maximum dose is 90 mg 4 times a day, but because of side effects of lightheadedness and dizziness, out patient could not tolerate more than 60 mg 3 times a day).
ENDOSCOPIC THERAPY
8. What endoscopic therapies are appropriate for patients with esophageal spasm that does not respond to medication?
- Bougie dilation
- Balloon dilation
- Onabotulinum toxin injection
- Expandable mesh stent placement
- Mucosal sclerotherapy
Onabotulinum toxin injections have been shown to improve dysphagia when given in a linear pattern.23
Endoscopic dilation has not been shown to be beneficial in this setting, as a study found no difference in efficacy between therapeutic (54-French) and sham (24-French) bougie dilation.24
Our patient received 100 units of onabotulinum toxin (10 units every centimeter in the distal 10 cm of the esophagus). Afterward, he experienced resolution of dysphagia, with only mild intermittent chest pain, which was controlled by taking peppermint extract as needed. The symptoms returned approximately 1 year later but responded to repeat endoscopy with onabotulinum toxin injections.23,25
Peroral endoscopic myotomy
Another relatively new endoscopic treatment for esophageal motility disorders is peroral endoscopic myotomy (Figure 5). During this procedure a tiny incision is made in the esophageal mucosa, permitting the endoscope to tunnel within the lining. The smooth muscle of the distal esophagus and lower esophageal sphincter is then cut, thereby freeing either the spastic muscle (in distal esophageal spasm) or the hyperactive lower esophageal sphincter (in achalasia).26,27
In an open trial, after undergoing peroral endoscopic myotomy for esophageal spasm and hypercontractile esophagus, 89% of patients had complete relief of dysphagia, and 92% had palliation of chest pain.28 Of note, the rate of relief of dysphagia was higher for patients with achalasia (98%) than for nonachalasia patients (71%).
- Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol 2013; 108:1238–1249;
- Hellemans J, Vantrappen G. Physiology. In: Vantrappen G, Hellemans J, eds. Diseases of the esophagus. New York, NY: Springer-Verlag Berlin, Heidelberg; 1974:40–102.
- Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA; American College of Gastroenterology. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013; 108:679–692.
- Kahrilas PJ, Bredenoord AJ, Fox M, et al; International High Resolution Manometry Working Group. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015; 27:160–174.
- Pandolfino JE, Kahrilas PJ; American Gastroenterological Association. AGA technical review on the clinical use of esophageal manometry. Gastroenterology 2005; 128:209–224.
- Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Shah N, Kahrilas PJ. Quantifying esophageal peristalsis with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol 2006; 290:G988–G997.
- Kahrilas PJ, Sifrim D. High-resolution manometry and impedance-pH/manometry: valuable tools in clinical and investigational esophagology. Gastroenterology 2008; 135:756–769.
- Cattau EL Jr, Castell DO, Johnson DA, et al. Diltiazem therapy for symptoms associated with nutcracker esophagus. Am J Gastroenterol 1991; 86:272–276.
- Richter JE, Dalton CB, Bradley LA, Castell DO. Oral nifedipine in the treatment of noncardiac chest pain in patients with the nutcracker esophagus. Gastroenterology 1987; 93:21–28.
- Drenth JP, Bos LP, Engels LG. Efficacy of diltiazem in the treatment of diffuse oesophageal spasm. Aliment Pharmacol Ther 1990; 4:411–416.
- Thomas E, Witt P, Willis M, Morse J. Nifedipine therapy for diffuse esophageal spasm. South Med J 1986; 79:847–849.
- Davies HA, Lewis MJ, Rhodes J, Henderson AH. Trial of nifedipine for prevention of oesophageal spasm. Digestion 1987; 36:81–83.
- Richter JE, Dalton CB, Bradley LA, Castell DO. Oral nifedipine in the treatment of noncardiac chest pain in patients with the nutcracker esophagus. Gastroenterology 1987; 93:21–28.
- Tursi A, Brandimarte G, Gasbarrini G. Transdermal slow-release long-acting isosorbide dinitrate for ‘nutcracker’ oesophagus: an open study. Eur J Gastroenterol Hepatol 2000; 12:1061–1062.
- Mellow MH. Effect of isosorbide and hydralazine in painful primary esophageal motility disorders. Gastroenterology 1982; 83:364–370.
- Fox M, Sweis R, Wong T, Anggiansah A. Sildenafil relieves symptoms and normalizes motility in patients with oesophageal spasm: a report of two cases. Neurogastroenterol Motil 2007; 19:798–803.
- Orlando RC, Bozymski EM. Clinical and manometric effects of nitroglycerin in diffuse esophageal spasm. N Engl J Med 1973; 289:23–25.
- Clouse RE, Lustman PJ, Eckert TC, Ferney DM, Griffith LS. Low-dose trazodone for symptomatic patients with esophageal contraction abnormalities. A double-blind, placebo-controlled trial. Gastroenterology 1987; 92:1027–1036.
- Cannon RO 3rd, Quyyumi AA, Mincemoyer R, et al. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med 1994; 330:1411–1417.
- Achem SR, Kolts BE, Wears R, Burton L, Richter JE. Chest pain associated with nutcracker esophagus: a preliminary study of the role of gastroesophageal reflux. Am J Gastroenterol 1993; 88:187–192.
- Pimentel M, Bonorris GG, Chow EJ, Lin HC. Peppermint oil improves the manometric findings in diffuse esophageal spasm. J Clin Gastroenterol 2001; 33:27–31.
- Kraichely RE, Arora AS, Murray JA. Opiate-induced oesophageal dysmotility. Aliment Pharmacol Ther 2010; 31:601–606.
- Storr M, Allescher HD, Rösch T, Born P, Weigert N, Classen M. Treatment of symptomatic diffuse esophageal spasm by endoscopic injections of botulinum toxin: a prospective study with long-term follow-up. Gastrointest Endosc 2001; 54:754–759.
- Winters C, Artnak EJ, Benjamin SB, Castell DO. Esophageal bougienage in symptomatic patients with the nutcracker esophagus. A primary esophageal motility disorder. JAMA 1984; 252:363–366.
- Vanuytsel T, Bisschops R, Farré R, et al. Botulinum toxin reduces dysphagia in patients with nonachalasia primary esophageal motility disorders. Clin Gastroenterol Hepatol 2013; 11:1115–1121.e2.
- Khashab MA, Messallam AA, Onimaru M, et al. International multicenter experience with peroral endoscopic myotomy for the treatment of spastic esophageal disorders refractory to medical therapy (with video). Gastrointest Endosc 2015; 81:1170–1177.
- Leconte M, Douard R, Gaudric M, Dumontier I, Chaussade S, Dousset B. Functional results after extended myotomy for diffuse oesophageal spasm. Br J Surg 2007; 94:1113–1118.
- Sharata AM, Dunst CM, Pescarus R, et al. Peroral endoscopic myotomy (POEM) for esophageal primary motility disorders: analysis of 100 consecutive patients. J Gastrointest Surg 2015; 19:161–170.
- Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol 2013; 108:1238–1249;
- Hellemans J, Vantrappen G. Physiology. In: Vantrappen G, Hellemans J, eds. Diseases of the esophagus. New York, NY: Springer-Verlag Berlin, Heidelberg; 1974:40–102.
- Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA; American College of Gastroenterology. ACG clinical guideline: evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol 2013; 108:679–692.
- Kahrilas PJ, Bredenoord AJ, Fox M, et al; International High Resolution Manometry Working Group. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil 2015; 27:160–174.
- Pandolfino JE, Kahrilas PJ; American Gastroenterological Association. AGA technical review on the clinical use of esophageal manometry. Gastroenterology 2005; 128:209–224.
- Ghosh SK, Pandolfino JE, Zhang Q, Jarosz A, Shah N, Kahrilas PJ. Quantifying esophageal peristalsis with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol 2006; 290:G988–G997.
- Kahrilas PJ, Sifrim D. High-resolution manometry and impedance-pH/manometry: valuable tools in clinical and investigational esophagology. Gastroenterology 2008; 135:756–769.
- Cattau EL Jr, Castell DO, Johnson DA, et al. Diltiazem therapy for symptoms associated with nutcracker esophagus. Am J Gastroenterol 1991; 86:272–276.
- Richter JE, Dalton CB, Bradley LA, Castell DO. Oral nifedipine in the treatment of noncardiac chest pain in patients with the nutcracker esophagus. Gastroenterology 1987; 93:21–28.
- Drenth JP, Bos LP, Engels LG. Efficacy of diltiazem in the treatment of diffuse oesophageal spasm. Aliment Pharmacol Ther 1990; 4:411–416.
- Thomas E, Witt P, Willis M, Morse J. Nifedipine therapy for diffuse esophageal spasm. South Med J 1986; 79:847–849.
- Davies HA, Lewis MJ, Rhodes J, Henderson AH. Trial of nifedipine for prevention of oesophageal spasm. Digestion 1987; 36:81–83.
- Richter JE, Dalton CB, Bradley LA, Castell DO. Oral nifedipine in the treatment of noncardiac chest pain in patients with the nutcracker esophagus. Gastroenterology 1987; 93:21–28.
- Tursi A, Brandimarte G, Gasbarrini G. Transdermal slow-release long-acting isosorbide dinitrate for ‘nutcracker’ oesophagus: an open study. Eur J Gastroenterol Hepatol 2000; 12:1061–1062.
- Mellow MH. Effect of isosorbide and hydralazine in painful primary esophageal motility disorders. Gastroenterology 1982; 83:364–370.
- Fox M, Sweis R, Wong T, Anggiansah A. Sildenafil relieves symptoms and normalizes motility in patients with oesophageal spasm: a report of two cases. Neurogastroenterol Motil 2007; 19:798–803.
- Orlando RC, Bozymski EM. Clinical and manometric effects of nitroglycerin in diffuse esophageal spasm. N Engl J Med 1973; 289:23–25.
- Clouse RE, Lustman PJ, Eckert TC, Ferney DM, Griffith LS. Low-dose trazodone for symptomatic patients with esophageal contraction abnormalities. A double-blind, placebo-controlled trial. Gastroenterology 1987; 92:1027–1036.
- Cannon RO 3rd, Quyyumi AA, Mincemoyer R, et al. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med 1994; 330:1411–1417.
- Achem SR, Kolts BE, Wears R, Burton L, Richter JE. Chest pain associated with nutcracker esophagus: a preliminary study of the role of gastroesophageal reflux. Am J Gastroenterol 1993; 88:187–192.
- Pimentel M, Bonorris GG, Chow EJ, Lin HC. Peppermint oil improves the manometric findings in diffuse esophageal spasm. J Clin Gastroenterol 2001; 33:27–31.
- Kraichely RE, Arora AS, Murray JA. Opiate-induced oesophageal dysmotility. Aliment Pharmacol Ther 2010; 31:601–606.
- Storr M, Allescher HD, Rösch T, Born P, Weigert N, Classen M. Treatment of symptomatic diffuse esophageal spasm by endoscopic injections of botulinum toxin: a prospective study with long-term follow-up. Gastrointest Endosc 2001; 54:754–759.
- Winters C, Artnak EJ, Benjamin SB, Castell DO. Esophageal bougienage in symptomatic patients with the nutcracker esophagus. A primary esophageal motility disorder. JAMA 1984; 252:363–366.
- Vanuytsel T, Bisschops R, Farré R, et al. Botulinum toxin reduces dysphagia in patients with nonachalasia primary esophageal motility disorders. Clin Gastroenterol Hepatol 2013; 11:1115–1121.e2.
- Khashab MA, Messallam AA, Onimaru M, et al. International multicenter experience with peroral endoscopic myotomy for the treatment of spastic esophageal disorders refractory to medical therapy (with video). Gastrointest Endosc 2015; 81:1170–1177.
- Leconte M, Douard R, Gaudric M, Dumontier I, Chaussade S, Dousset B. Functional results after extended myotomy for diffuse oesophageal spasm. Br J Surg 2007; 94:1113–1118.
- Sharata AM, Dunst CM, Pescarus R, et al. Peroral endoscopic myotomy (POEM) for esophageal primary motility disorders: analysis of 100 consecutive patients. J Gastrointest Surg 2015; 19:161–170.
Fighting the reflux reflex
More than 15 million patients in the United States have prescriptions for proton pump inhibitors (PPIs), in most cases for “heartburn,” reflux, and swallowing problems, and many more are taking over-the-counter PPIs or histamine 2 receptor antagonists.
Gastroesophageal reflux disease (GERD) seems to be the initial reflexive diagnosis given to most patients who complain of chest or epigastric burning or seemingly nonspecific swallowing difficulties. The prevalence of diagnosed GERD is high; in addition to causing “heartburn,” in my clinic it seems to be the most commonly attributed cause of chronic cough with a normal chest radiograph or hoarseness, and a frequent contributing comorbidity warranting treatment in patients with bronchospasm—diagnosed by my otolaryngology and pulmonary colleagues.
GERD is so common that it is no surprise that patients are increasingly diagnosed with it on the basis of a superficial history, or that patients diagnose and treat it themselves based on information they find on the Internet. Objective diagnostic tests are suggested when PPIs do not produce the expected response.
But long-term, high-dose PPI therapy may not be totally benign. Omeprazole, commonly prescribed and also available over the counter, may in some patients interfere with clopidogrel and increase the risk of coronary events, although this increase may actually be due to the underlying medical condition for which the PPI is prescribed—“confounding by indication.” PPI use is associated with decreased absorption of iron and vitamin B12, perhaps contributing to anemia. The estimated risks of vertebral and hip osteoporosis, interstitial nephritis, and dementia are slightly increased. Patients with severe liver disease seem to be at far higher risk of bacterial peritonitis. Clostridium difficile infection and some pneumonias may also be increased in chronic PPI users. Therefore, we should think twice when making a clinical diagnosis of GERD, a diagnosis that often leads us to prescribe antacid therapy (usually a PPI) for a long time, sometimes unnecessarily.1
Kichler and Gabbard, in this issue of the Journal, work through a clinical management scenario focusing on the evaluation of a patient with dysphagia, a common symptom described in many ways by patients who may have previously been diagnosed with GERD. The authors remind us of the value of a careful, focused, and detailed medical history, and provide updated information on the performance and utility of motility and endoscopic studies in diagnosing esophageal disorders.
- Benmassaoud A, McDonald EG, Lee TC. Potential harms of proton pump inhibitor therapy: rare adverse effects of commonly used drugs. CMAJ 2016; 188:657–662.
More than 15 million patients in the United States have prescriptions for proton pump inhibitors (PPIs), in most cases for “heartburn,” reflux, and swallowing problems, and many more are taking over-the-counter PPIs or histamine 2 receptor antagonists.
Gastroesophageal reflux disease (GERD) seems to be the initial reflexive diagnosis given to most patients who complain of chest or epigastric burning or seemingly nonspecific swallowing difficulties. The prevalence of diagnosed GERD is high; in addition to causing “heartburn,” in my clinic it seems to be the most commonly attributed cause of chronic cough with a normal chest radiograph or hoarseness, and a frequent contributing comorbidity warranting treatment in patients with bronchospasm—diagnosed by my otolaryngology and pulmonary colleagues.
GERD is so common that it is no surprise that patients are increasingly diagnosed with it on the basis of a superficial history, or that patients diagnose and treat it themselves based on information they find on the Internet. Objective diagnostic tests are suggested when PPIs do not produce the expected response.
But long-term, high-dose PPI therapy may not be totally benign. Omeprazole, commonly prescribed and also available over the counter, may in some patients interfere with clopidogrel and increase the risk of coronary events, although this increase may actually be due to the underlying medical condition for which the PPI is prescribed—“confounding by indication.” PPI use is associated with decreased absorption of iron and vitamin B12, perhaps contributing to anemia. The estimated risks of vertebral and hip osteoporosis, interstitial nephritis, and dementia are slightly increased. Patients with severe liver disease seem to be at far higher risk of bacterial peritonitis. Clostridium difficile infection and some pneumonias may also be increased in chronic PPI users. Therefore, we should think twice when making a clinical diagnosis of GERD, a diagnosis that often leads us to prescribe antacid therapy (usually a PPI) for a long time, sometimes unnecessarily.1
Kichler and Gabbard, in this issue of the Journal, work through a clinical management scenario focusing on the evaluation of a patient with dysphagia, a common symptom described in many ways by patients who may have previously been diagnosed with GERD. The authors remind us of the value of a careful, focused, and detailed medical history, and provide updated information on the performance and utility of motility and endoscopic studies in diagnosing esophageal disorders.
More than 15 million patients in the United States have prescriptions for proton pump inhibitors (PPIs), in most cases for “heartburn,” reflux, and swallowing problems, and many more are taking over-the-counter PPIs or histamine 2 receptor antagonists.
Gastroesophageal reflux disease (GERD) seems to be the initial reflexive diagnosis given to most patients who complain of chest or epigastric burning or seemingly nonspecific swallowing difficulties. The prevalence of diagnosed GERD is high; in addition to causing “heartburn,” in my clinic it seems to be the most commonly attributed cause of chronic cough with a normal chest radiograph or hoarseness, and a frequent contributing comorbidity warranting treatment in patients with bronchospasm—diagnosed by my otolaryngology and pulmonary colleagues.
GERD is so common that it is no surprise that patients are increasingly diagnosed with it on the basis of a superficial history, or that patients diagnose and treat it themselves based on information they find on the Internet. Objective diagnostic tests are suggested when PPIs do not produce the expected response.
But long-term, high-dose PPI therapy may not be totally benign. Omeprazole, commonly prescribed and also available over the counter, may in some patients interfere with clopidogrel and increase the risk of coronary events, although this increase may actually be due to the underlying medical condition for which the PPI is prescribed—“confounding by indication.” PPI use is associated with decreased absorption of iron and vitamin B12, perhaps contributing to anemia. The estimated risks of vertebral and hip osteoporosis, interstitial nephritis, and dementia are slightly increased. Patients with severe liver disease seem to be at far higher risk of bacterial peritonitis. Clostridium difficile infection and some pneumonias may also be increased in chronic PPI users. Therefore, we should think twice when making a clinical diagnosis of GERD, a diagnosis that often leads us to prescribe antacid therapy (usually a PPI) for a long time, sometimes unnecessarily.1
Kichler and Gabbard, in this issue of the Journal, work through a clinical management scenario focusing on the evaluation of a patient with dysphagia, a common symptom described in many ways by patients who may have previously been diagnosed with GERD. The authors remind us of the value of a careful, focused, and detailed medical history, and provide updated information on the performance and utility of motility and endoscopic studies in diagnosing esophageal disorders.
- Benmassaoud A, McDonald EG, Lee TC. Potential harms of proton pump inhibitor therapy: rare adverse effects of commonly used drugs. CMAJ 2016; 188:657–662.
- Benmassaoud A, McDonald EG, Lee TC. Potential harms of proton pump inhibitor therapy: rare adverse effects of commonly used drugs. CMAJ 2016; 188:657–662.
Is there a doctor on board? In-flight medical emergencies
It could happen. You are on a plane, perhaps on your way to a medical conference or a well-deserved vacation, when the flight attendant asks you to help a passenger experiencing an in-flight medical emergency. What is your role in this situation?
FLIGHT ATTENDANTS USED TO BE NURSES
Before World War II, nearly all American flight attendants were nurses, who could address most medical issues that arose during flights.1 Airlines eliminated this preferential hiring practice to support the war effort. Traveling healthcare providers thereafter often volunteered to assist when in-flight medical issues arose, but aircraft carried minimal medical equipment and volunteers’ liability was uncertain.
In 1998, Congress passed the Aviation Medical Assistance Act (AMAA), which provides liability protection for on-board healthcare providers who render medical assistance. It also required the Federal Aviation Administration (FAA) to improve its standards for in-flight medical equipment.2,3
HOW OFTEN DO EMERGENCIES ARISE?
How often medical events occur during flight is difficult to estimate because airlines are not mandated to report such issues.4 Based on data from a ground-based communications center that provides medical consultation service to airlines, medical events occur in approximately 1 in every 604 flights.5 This is likely an underestimate, as many medical events may be handled on board without involving a ground-based consultation center.
The most common emergencies are syncope or presyncope, representing 37.4% of consultations, followed by respiratory symptoms (12.1%), nausea or vomiting (9.5%), cardiac symptoms (7.7%), seizures (5.8%), and abdominal pain (4.1%).5 Very few in-flight medical emergencies progress to death; the reported mortality rate is 0.3%.5
CABIN PRESSURES ARE RELATIVELY LOW
The cabins of commercial airliners are pressurized, but the pressure is still lower than on the ground. The cabin pressure in flight is equivalent to that at an altitude of 6,000 to 8,000 feet,6,7 ie, about 23 or 24 mm Hg, compared with about 30 mm Hg at sea level. At this pressure, passengers have a partial pressure of arterial oxygen (Pao2) of 60 mm Hg (normal at sea level is > 80).8
This reduced oxygen pressure is typically not clinically meaningful in healthy people. However, people with underlying pulmonary or cardiac illness may be starting further to the left on the oxygen dissociation curve before gaining altitude, putting them at risk for acute exacerbations of underlying medical conditions. Many patients who rely on supplemental oxygen, such as those with chronic obstructive pulmonary disease, are advised to increase their oxygen support during flight.9
Boyle’s law says that the volume of a gas is inversely proportional to its pressure. As the pressure drops in the cabin after takeoff, air trapped in an enclosed space—eg, in some patient’s bodies—can increase in volume up to 30%,10 which can have medical ramifications. Clinically significant pneumothorax during flight has been reported.11–13 Partially because of these volumetric changes, patients who have undergone abdominal surgery are advised to avoid flying for at least 2 weeks after their procedure.10,14 Patients who have had recent ocular or intracranial surgery may also be at risk of in-flight complications.15
IN-FLIGHT MEDICAL RESOURCES
The limited medical supplies available on aircraft often challenge healthcare providers who offer to respond to in-flight medical events. However, several important medical resources are available.
Medical kits and defibrillators
FAA regulations require airlines based in the United States to carry basic first aid supplies such as bandages and splints.3 Airlines are also required to carry a medical kit containing the items listed in Table 1.
The FAA-mandated kit does not cover every circumstance that may arise. Although in-flight pediatric events occasionally occur,16 many of the available medications are inappropriate for young children. The FAA does not require sedative or antipsychotic agents, which could be useful for passengers who have acute psychiatric episodes. Obstetric supplies are absent. On international carriers, the contents of medical kits are highly variable,17 as are the names used for some medications.
The FAA requires at least 1 automated external defibrillator (AED) to be available on each commercial aircraft.3 The timely use of AEDs greatly improves survivability after out-of-hospital cardiac arrest.18,19 One study involving a major US airline found a 40% survival rate to hospital discharge in patients who received in-flight defibrillation.20 Without this intervention, very few of the patients would have been expected to survive. In addition to being clinically effective, placing AEDs aboard commercial aircraft is a cost-effective public health intervention.21
Consultation services
Most major airlines can contact ground-based medical consultation services during flight.10 These centers are staffed with healthcare providers who can provide flight crews with advice on how to handle medical events in real time. Healthcare providers can likewise discuss specific medical issues with these services if they respond to an in-flight medical event. Ground-based call centers can also communicate with prehospital providers should a flight need to be diverted.
Other on-board providers
Some medical events require the involvement of more than one medical provider. Other physicians, nurses, and prehospital providers are often also on board.22 Responding physicians can also request the assistance of these other healthcare providers. Flight attendants in the United States are required to be trained in cardiopulmonary resuscitation (CPR).23
Flight diversion
Critically ill patients or those with time-sensitive medical emergencies may require the aircraft to divert from its intended destination. As may be expected, medical emergencies suspected to involve the cardiovascular, neurologic, or respiratory system have been shown to most likely result in aircraft diversion.5,24 Approximately 7% of in-flight medical events in which a ground-based medical consultation service is contacted result in diversion.5
While an on-board responding physician can make a recommendation to divert based on the patient’s acute medical status, only the captain can make the ultimate decision.4 On-board healthcare providers should clearly state that a patient might benefit from an unscheduled landing if that is truly their assessment. In addition to communicating their clinical concerns with the flight crew, the responding physician may also be able to discuss the situation with the airline’s ground-based consultation service. On-board physicians can make important contributions to the assessment of illness severity and triage decisions.
MEDICOLEGAL ISSUES
No legal duty to assist
US healthcare providers are not legally required to respond to on-board medical emergencies on US-based airlines. Canada and the United Kingdom also do not require providers to render assistance. But the General Medical Council (the regulatory body for UK doctors) states that doctors have an ethical duty to respond in the event of a medical emergency, including one on board an aircraft. Other countries, notably Australia and some in the European Union, require healthcare professionals to respond to on-board medical emergencies.10
Regardless of potential legal duties to assist, healthcare providers are arguably ethically obliged to render assistance if they can.
Aviation Medical Assistance Act
The extent of an American healthcare provider’s liability risk for assisting in a medical emergency on a plane registered in the United States is limited by statute. The 1998 AMAA provides liability protection for on-board medical providers who are asked to assist during an in-flight medical emergency. This statute covers all US-certified air carriers on domestic flights and would likely be held to apply to US aircraft in foreign airspace because of the general rule that the law of the country where the air carrier is registered applies to in-flight events.
Under the AMAA, providers asked to assist with in-flight medical emergencies are not liable for malpractice as long as their actions are not “grossly” negligent or intended to cause the patient harm.25 This is distinguishable from a standard malpractice liability scenario, in which the plaintiff only needs to show ordinary negligence. In a traditional healthcare setting, a provider has to act within the “standard of care” when assessing and treating a patient. If the provider deviates from the standard of care, such as by making an error in judgment or diagnosis, the provider is legally negligent. Under traditional malpractice law, even if a provider is minimally negligent, he or she is liable for any damages resulting from that negligence. Under a gross negligence standard, providers are protected from liability unless they demonstrate flagrant disregard for the patient’s health and safety.
Postflight issues
A provider who undertakes care should continue to provide care until it is no longer necessary, either because the patient recovers or the responsibility has been transferred to another provider. At the point of transfer, the healthcare provider’s relationship with the patient terminates.
The provider should document the encounter, typically using airline-specific documentation. The responding physician needs to be mindful of the patient’s privacy, refraining from discussing the event with others without the patient’s authorization.26
SUGGESTED RESPONSE
Healthcare providers who wish to respond to in-flight medical emergencies must first determine if they are sufficiently capable of providing care. During a flight, providers do not expect to be on duty and so may have consumed alcoholic beverages to an extent that would potentially render them unsuitable to respond. When it is appropriate to become involved in a medical emergency during flight, the healthcare provider should state his or her qualifications to the passenger and to flight personnel.
If circumstances allow, the volunteer provider should obtain the patient’s consent for evaluation and treatment.10 Additionally, with the multilingual nature of commercial air travel, especially on international flights, the provider may need to enlist a translator’s assistance.
Providers may find it preferable to treat passengers in their seats.27 Given the confined space in an aircraft, keeping ill passengers out of the aisle allows others to move about the cabin. If it becomes necessary to move the patient, a location should be sought that minimally interferes with other passengers’ needs.
If a passenger has critical medical needs, in-flight medical volunteers can recommend flight diversion, which should also be discussed with ground-based medical staff. However, as emphasized earlier, the captain makes the ultimate decision to divert, taking into account other operational factors that affect the safety of the aircraft and its occupants. In-flight medical care providers should perform only the treatments they are qualified to provide and should operate within their scope of training.
After the aircraft lands, if the passenger must be transported to a hospital, providers should supply prehospital personnel with a requisite transfer-of-care communication. In-flight medical providers who have performed a significant medical intervention might find it appropriate to accompany the patient to the hospital.
SPECIFIC CONDITIONS
The list of possible acute medical issues that occur aboard aircraft is extensive. Here are a few of them.
Trauma
Passengers may experience injuries during flight, for example during periods of heavy air turbulence. Responding physicians should assess for potential life-threatening injuries, keeping in mind that some passengers may be at higher risk. For example, if a passenger on anticoagulation experiences a blunt head injury, this would raise suspicion for possible intracranial hemorrhage, and frequent reassessment of neurologic status may be necessary. If an extremity fracture is suspected, the physician should splint the affected limb. Analgesia may be provided from the medical kit, if appropriate.
Gastrointestinal issues
Acute gastrointestinal issues such as nausea and vomiting are often reported to ground-based medical consultation services.5 Responding on-board providers must consider if the passenger is simply experiencing gastrointestinal upset from a benign condition such as gastroenteritis or has a more serious condition. For some patients, vomiting may be a symptom of a myocardial infarction.28 Bilious emesis with abdominal distention may be associated with small-bowel obstruction. While antiemetics are not included in the FAA-mandated medical kit, providers can initiate intravenous fluid therapy for passengers who show signs of hypovolemia.
Cardiac arrest
Although cardiac arrest during flight is rare,5 medical providers should nonetheless be prepared to handle it. Upon recognition of cardiac arrest, the provider should immediately begin cardiopulmonary resuscitation and use the on-board AED to defibrillate a potentially shockable rhythm. Flight attendants are trained in cardiopulmonary resuscitation and therefore may assist with resuscitation efforts. If the patient is resuscitated, the responding physician should recommend diversion of the flight.
Anaphylaxis
In the event of a severe life-threatening allergic reaction, the FAA-mandated emergency medical kit contains both diphenhydramine and epinephrine. For an adult experiencing anaphylaxis, a responding on-board physician can administer diphenhydramine 50 mg and epinephrine 0.3 mg (using the 1:1000 formulation), both intramuscularly. For patients with bronchospasm, a metered-dose inhaler of albuterol can be given. As anaphylaxis is an acute and potentially lethal condition, diversion of the aircraft would also be appropriate.29
Myocardial infarction
When acute myocardial infarction is suspected, it is appropriate for the provider to give aspirin, with important exceptions for patients who are experiencing an acute hemorrhage or who have an aspirin allergy.30 Supplemental oxygen should likewise be provided if the responding physician suspects compromised oxygenation. As acute myocardial infarction is also a time-sensitive condition, the clinician who suspects this diagnosis should recommend diversion of the aircraft.
Acute psychiatric issues
While approximately 2.4% of on-board medical events are attributed to psychiatric issues,5 there are few tools at the clinician’s disposal in the FAA-mandated emergency medical kit. Antipsychotics and sedatives are not included. The responding physician may need to attempt verbal de-escalation of aggressive behavior. If the safety of the flight is compromised, the application of improvised physical restraints may be appropriate.
Altered mental status
The differential diagnosis for altered mental status is extensive. The on-board physician should try to identify reversible and potentially lethal conditions and determine the potential need for aircraft diversion.
If possible, a blood sugar level should be measured (although the FAA-mandated kit does not contain a glucometer). It may be appropriate to empirically give intravenous dextrose to patients strongly suspected of having hypoglycemia.
If respiratory or cerebrovascular compromise is suspected, supplemental oxygen should be provided.
Unless a reversible cause of altered mental status is identified and treated successfully, it will likely be appropriate to recommend diversion of the aircraft.
Acknowledgment: The authors acknowledge Linda J. Kesselring, MS, ELS, the technical editor/writer in the Department of Emergency Medicine University of Maryland School of Medicine, for her contributions as copy editor of a previous version of this manuscript.
- Gazdik M. Vault guide to flight attendant careers. New York, NY: Vault, Inc.; 2005.
- Stewart PH, Agin WS, Douglas SP. What does the law say to Good Samaritans? A review of Good Samaritan statutes in 50 states and on US airlines. Chest 2013; 143:1774–1783.
- Federal Aviation Administration (FAA), DOT. Emergency medical equipment. Final rule. Fed Regist 2001; 66:19028–19046.
- Goodwin T. In-flight medical emergencies: an overview. BMJ 2000; 321:1338–1341.
- Peterson DC, Martin-Gill C, Guyette FX, et al. Outcomes of medical emergencies on commercial airline flights. N Engl J Med 2013; 368:2075–2083.
- Aerospace Medical Association, Aviation Safety Committee, Civil Aviation Subcommittee. Cabin cruising altitudes for regular transport aircraft. Aviat Space Environ Med 2008; 79:433–439.
- Cottrell JJ. Altitude exposures during aircraft flight. Flying higher. Chest 1988; 93:81–84.
- Humphreys S, Deyermond R, Bali I, Stevenson M, Fee JP. The effect of high altitude commercial air travel on oxygen saturation. Anaesthesia 2005; 60:458–460.
- Shrikrishna D, Coker RK; Air Travel Working Party of the British Thoracic Society Standards of Care Committee. Managing passengers with stable respiratory disease planning air travel: British Thoracic Society recommendations. Thorax 2011; 66:831–833.
- Gendreau MA, DeJohn C. Responding to medical events during commercial airline flights. N Engl J Med 2002; 346:1067–1073.
- Hu X, Cowl CT, Baqir M, Ryu JH. Air travel and pneumothorax. Chest 2014; 145:688–694.
- Madan K, Vishwanath G, Singh N. In-flight spontaneous pneumothorax: congenital cystic adenomatoid malformation of the lung. Respiration 2012; 83:554–558.
- Wallace TW, Wong T, O’Bichere A, Ellis BW. Managing in flight emergencies. BMJ 1995; 311:374–376.
- Medical aspects of transportation aboard commercial aircraft. AMA commission on emergency medical services. JAMA 1982; 247:1007–1011.
- Mills MD, Devenyi RG, Lam WC, Berger AR, Beijer CD, Lam SR. An assessment of intraocular pressure rise in patients with gas-filled eyes during simulated air flight. Ophthalmology 2001; 108:40–44.
- Moore BR, Ping JM, Claypool DW. Pediatric emergencies on a US-based commercial airline. Pediatr Emerg Care 2005; 21:725–729.
- Sand M, Gambichler T, Sand D, Thrandorf C, Altmeyer P, Bechara FG. Emergency medical kits on board commercial aircraft: a comparative study. Travel Med Infect Dis 2010; 8:388–394.
- Auble TE, Menegazzi JJ, Paris PM. Effect of out-of-hospital defibrillation by basic life support providers on cardiac arrest mortality: a metaanalysis. Ann Emerg Med 1995; 25:642–648.
- Marenco JP, Wang PJ, Link MS, Homoud MK, Estes NA. Improving survival from sudden cardiac arrest: the role of the automated external defibrillator. JAMA 2001; 285:1193–1200.
- Page RL, Joglar JA, Kowal RC, et al. Use of automated external defibrillators by a US airline. N Engl J Med 2000; 343:1210–1216.
- Groeneveld PW, Kwong JL, Liu Y, et al. Cost-effectiveness of automated external defibrillators on airlines. JAMA 2001; 286:1482–1489.
- Baltsezak S. Clinic in the air? A retrospective study of medical emergency calls from a major international airline. J Travel Med 2008; 15:391–394.
- Federal Aviation Administration (FAA). Advisory circular: emergency medical equipment training AC 121-34B. www.faa.gov/documentLibrary/media/Advisory_Circular/AC121-34B.pdf. Accessed April 6, 2017.
- Cummins RO, Schubach JA. Medical emergencies among commercial air travelers. JAMA 1989; 261:1295–1299.
- US Government Publishing Office. Public Law 105-170. Aviation Medical Assistance Act of 1998.
- US Government Publishing Office. Public Law 104-191. Health Insurance Portability and Accountability Act of 1996.
- Chandra A, Conry S. In-flight medical emergencies. West J Emerg Med 2013; 14:499–504.
- Kirchberger I, Meisinger C, Heier M, et al. Patient-reported symptoms in acute myocardial infarction: differences related to ST-segment elevation: the MONICA/KORA Myocardial Infarction Registry. J Intern Med 2011; 270:58–64.
- Brady WJ Jr, Bright HL. Occurrence of multiphasic anaphylaxis during a transcontinental air flight. Am J Emerg Med 1999; 17:695–696.
- O’Connor RE, Brady W, Brooks SC, et al. Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122:(suppl 3):S787–S817.
It could happen. You are on a plane, perhaps on your way to a medical conference or a well-deserved vacation, when the flight attendant asks you to help a passenger experiencing an in-flight medical emergency. What is your role in this situation?
FLIGHT ATTENDANTS USED TO BE NURSES
Before World War II, nearly all American flight attendants were nurses, who could address most medical issues that arose during flights.1 Airlines eliminated this preferential hiring practice to support the war effort. Traveling healthcare providers thereafter often volunteered to assist when in-flight medical issues arose, but aircraft carried minimal medical equipment and volunteers’ liability was uncertain.
In 1998, Congress passed the Aviation Medical Assistance Act (AMAA), which provides liability protection for on-board healthcare providers who render medical assistance. It also required the Federal Aviation Administration (FAA) to improve its standards for in-flight medical equipment.2,3
HOW OFTEN DO EMERGENCIES ARISE?
How often medical events occur during flight is difficult to estimate because airlines are not mandated to report such issues.4 Based on data from a ground-based communications center that provides medical consultation service to airlines, medical events occur in approximately 1 in every 604 flights.5 This is likely an underestimate, as many medical events may be handled on board without involving a ground-based consultation center.
The most common emergencies are syncope or presyncope, representing 37.4% of consultations, followed by respiratory symptoms (12.1%), nausea or vomiting (9.5%), cardiac symptoms (7.7%), seizures (5.8%), and abdominal pain (4.1%).5 Very few in-flight medical emergencies progress to death; the reported mortality rate is 0.3%.5
CABIN PRESSURES ARE RELATIVELY LOW
The cabins of commercial airliners are pressurized, but the pressure is still lower than on the ground. The cabin pressure in flight is equivalent to that at an altitude of 6,000 to 8,000 feet,6,7 ie, about 23 or 24 mm Hg, compared with about 30 mm Hg at sea level. At this pressure, passengers have a partial pressure of arterial oxygen (Pao2) of 60 mm Hg (normal at sea level is > 80).8
This reduced oxygen pressure is typically not clinically meaningful in healthy people. However, people with underlying pulmonary or cardiac illness may be starting further to the left on the oxygen dissociation curve before gaining altitude, putting them at risk for acute exacerbations of underlying medical conditions. Many patients who rely on supplemental oxygen, such as those with chronic obstructive pulmonary disease, are advised to increase their oxygen support during flight.9
Boyle’s law says that the volume of a gas is inversely proportional to its pressure. As the pressure drops in the cabin after takeoff, air trapped in an enclosed space—eg, in some patient’s bodies—can increase in volume up to 30%,10 which can have medical ramifications. Clinically significant pneumothorax during flight has been reported.11–13 Partially because of these volumetric changes, patients who have undergone abdominal surgery are advised to avoid flying for at least 2 weeks after their procedure.10,14 Patients who have had recent ocular or intracranial surgery may also be at risk of in-flight complications.15
IN-FLIGHT MEDICAL RESOURCES
The limited medical supplies available on aircraft often challenge healthcare providers who offer to respond to in-flight medical events. However, several important medical resources are available.
Medical kits and defibrillators
FAA regulations require airlines based in the United States to carry basic first aid supplies such as bandages and splints.3 Airlines are also required to carry a medical kit containing the items listed in Table 1.
The FAA-mandated kit does not cover every circumstance that may arise. Although in-flight pediatric events occasionally occur,16 many of the available medications are inappropriate for young children. The FAA does not require sedative or antipsychotic agents, which could be useful for passengers who have acute psychiatric episodes. Obstetric supplies are absent. On international carriers, the contents of medical kits are highly variable,17 as are the names used for some medications.
The FAA requires at least 1 automated external defibrillator (AED) to be available on each commercial aircraft.3 The timely use of AEDs greatly improves survivability after out-of-hospital cardiac arrest.18,19 One study involving a major US airline found a 40% survival rate to hospital discharge in patients who received in-flight defibrillation.20 Without this intervention, very few of the patients would have been expected to survive. In addition to being clinically effective, placing AEDs aboard commercial aircraft is a cost-effective public health intervention.21
Consultation services
Most major airlines can contact ground-based medical consultation services during flight.10 These centers are staffed with healthcare providers who can provide flight crews with advice on how to handle medical events in real time. Healthcare providers can likewise discuss specific medical issues with these services if they respond to an in-flight medical event. Ground-based call centers can also communicate with prehospital providers should a flight need to be diverted.
Other on-board providers
Some medical events require the involvement of more than one medical provider. Other physicians, nurses, and prehospital providers are often also on board.22 Responding physicians can also request the assistance of these other healthcare providers. Flight attendants in the United States are required to be trained in cardiopulmonary resuscitation (CPR).23
Flight diversion
Critically ill patients or those with time-sensitive medical emergencies may require the aircraft to divert from its intended destination. As may be expected, medical emergencies suspected to involve the cardiovascular, neurologic, or respiratory system have been shown to most likely result in aircraft diversion.5,24 Approximately 7% of in-flight medical events in which a ground-based medical consultation service is contacted result in diversion.5
While an on-board responding physician can make a recommendation to divert based on the patient’s acute medical status, only the captain can make the ultimate decision.4 On-board healthcare providers should clearly state that a patient might benefit from an unscheduled landing if that is truly their assessment. In addition to communicating their clinical concerns with the flight crew, the responding physician may also be able to discuss the situation with the airline’s ground-based consultation service. On-board physicians can make important contributions to the assessment of illness severity and triage decisions.
MEDICOLEGAL ISSUES
No legal duty to assist
US healthcare providers are not legally required to respond to on-board medical emergencies on US-based airlines. Canada and the United Kingdom also do not require providers to render assistance. But the General Medical Council (the regulatory body for UK doctors) states that doctors have an ethical duty to respond in the event of a medical emergency, including one on board an aircraft. Other countries, notably Australia and some in the European Union, require healthcare professionals to respond to on-board medical emergencies.10
Regardless of potential legal duties to assist, healthcare providers are arguably ethically obliged to render assistance if they can.
Aviation Medical Assistance Act
The extent of an American healthcare provider’s liability risk for assisting in a medical emergency on a plane registered in the United States is limited by statute. The 1998 AMAA provides liability protection for on-board medical providers who are asked to assist during an in-flight medical emergency. This statute covers all US-certified air carriers on domestic flights and would likely be held to apply to US aircraft in foreign airspace because of the general rule that the law of the country where the air carrier is registered applies to in-flight events.
Under the AMAA, providers asked to assist with in-flight medical emergencies are not liable for malpractice as long as their actions are not “grossly” negligent or intended to cause the patient harm.25 This is distinguishable from a standard malpractice liability scenario, in which the plaintiff only needs to show ordinary negligence. In a traditional healthcare setting, a provider has to act within the “standard of care” when assessing and treating a patient. If the provider deviates from the standard of care, such as by making an error in judgment or diagnosis, the provider is legally negligent. Under traditional malpractice law, even if a provider is minimally negligent, he or she is liable for any damages resulting from that negligence. Under a gross negligence standard, providers are protected from liability unless they demonstrate flagrant disregard for the patient’s health and safety.
Postflight issues
A provider who undertakes care should continue to provide care until it is no longer necessary, either because the patient recovers or the responsibility has been transferred to another provider. At the point of transfer, the healthcare provider’s relationship with the patient terminates.
The provider should document the encounter, typically using airline-specific documentation. The responding physician needs to be mindful of the patient’s privacy, refraining from discussing the event with others without the patient’s authorization.26
SUGGESTED RESPONSE
Healthcare providers who wish to respond to in-flight medical emergencies must first determine if they are sufficiently capable of providing care. During a flight, providers do not expect to be on duty and so may have consumed alcoholic beverages to an extent that would potentially render them unsuitable to respond. When it is appropriate to become involved in a medical emergency during flight, the healthcare provider should state his or her qualifications to the passenger and to flight personnel.
If circumstances allow, the volunteer provider should obtain the patient’s consent for evaluation and treatment.10 Additionally, with the multilingual nature of commercial air travel, especially on international flights, the provider may need to enlist a translator’s assistance.
Providers may find it preferable to treat passengers in their seats.27 Given the confined space in an aircraft, keeping ill passengers out of the aisle allows others to move about the cabin. If it becomes necessary to move the patient, a location should be sought that minimally interferes with other passengers’ needs.
If a passenger has critical medical needs, in-flight medical volunteers can recommend flight diversion, which should also be discussed with ground-based medical staff. However, as emphasized earlier, the captain makes the ultimate decision to divert, taking into account other operational factors that affect the safety of the aircraft and its occupants. In-flight medical care providers should perform only the treatments they are qualified to provide and should operate within their scope of training.
After the aircraft lands, if the passenger must be transported to a hospital, providers should supply prehospital personnel with a requisite transfer-of-care communication. In-flight medical providers who have performed a significant medical intervention might find it appropriate to accompany the patient to the hospital.
SPECIFIC CONDITIONS
The list of possible acute medical issues that occur aboard aircraft is extensive. Here are a few of them.
Trauma
Passengers may experience injuries during flight, for example during periods of heavy air turbulence. Responding physicians should assess for potential life-threatening injuries, keeping in mind that some passengers may be at higher risk. For example, if a passenger on anticoagulation experiences a blunt head injury, this would raise suspicion for possible intracranial hemorrhage, and frequent reassessment of neurologic status may be necessary. If an extremity fracture is suspected, the physician should splint the affected limb. Analgesia may be provided from the medical kit, if appropriate.
Gastrointestinal issues
Acute gastrointestinal issues such as nausea and vomiting are often reported to ground-based medical consultation services.5 Responding on-board providers must consider if the passenger is simply experiencing gastrointestinal upset from a benign condition such as gastroenteritis or has a more serious condition. For some patients, vomiting may be a symptom of a myocardial infarction.28 Bilious emesis with abdominal distention may be associated with small-bowel obstruction. While antiemetics are not included in the FAA-mandated medical kit, providers can initiate intravenous fluid therapy for passengers who show signs of hypovolemia.
Cardiac arrest
Although cardiac arrest during flight is rare,5 medical providers should nonetheless be prepared to handle it. Upon recognition of cardiac arrest, the provider should immediately begin cardiopulmonary resuscitation and use the on-board AED to defibrillate a potentially shockable rhythm. Flight attendants are trained in cardiopulmonary resuscitation and therefore may assist with resuscitation efforts. If the patient is resuscitated, the responding physician should recommend diversion of the flight.
Anaphylaxis
In the event of a severe life-threatening allergic reaction, the FAA-mandated emergency medical kit contains both diphenhydramine and epinephrine. For an adult experiencing anaphylaxis, a responding on-board physician can administer diphenhydramine 50 mg and epinephrine 0.3 mg (using the 1:1000 formulation), both intramuscularly. For patients with bronchospasm, a metered-dose inhaler of albuterol can be given. As anaphylaxis is an acute and potentially lethal condition, diversion of the aircraft would also be appropriate.29
Myocardial infarction
When acute myocardial infarction is suspected, it is appropriate for the provider to give aspirin, with important exceptions for patients who are experiencing an acute hemorrhage or who have an aspirin allergy.30 Supplemental oxygen should likewise be provided if the responding physician suspects compromised oxygenation. As acute myocardial infarction is also a time-sensitive condition, the clinician who suspects this diagnosis should recommend diversion of the aircraft.
Acute psychiatric issues
While approximately 2.4% of on-board medical events are attributed to psychiatric issues,5 there are few tools at the clinician’s disposal in the FAA-mandated emergency medical kit. Antipsychotics and sedatives are not included. The responding physician may need to attempt verbal de-escalation of aggressive behavior. If the safety of the flight is compromised, the application of improvised physical restraints may be appropriate.
Altered mental status
The differential diagnosis for altered mental status is extensive. The on-board physician should try to identify reversible and potentially lethal conditions and determine the potential need for aircraft diversion.
If possible, a blood sugar level should be measured (although the FAA-mandated kit does not contain a glucometer). It may be appropriate to empirically give intravenous dextrose to patients strongly suspected of having hypoglycemia.
If respiratory or cerebrovascular compromise is suspected, supplemental oxygen should be provided.
Unless a reversible cause of altered mental status is identified and treated successfully, it will likely be appropriate to recommend diversion of the aircraft.
Acknowledgment: The authors acknowledge Linda J. Kesselring, MS, ELS, the technical editor/writer in the Department of Emergency Medicine University of Maryland School of Medicine, for her contributions as copy editor of a previous version of this manuscript.
It could happen. You are on a plane, perhaps on your way to a medical conference or a well-deserved vacation, when the flight attendant asks you to help a passenger experiencing an in-flight medical emergency. What is your role in this situation?
FLIGHT ATTENDANTS USED TO BE NURSES
Before World War II, nearly all American flight attendants were nurses, who could address most medical issues that arose during flights.1 Airlines eliminated this preferential hiring practice to support the war effort. Traveling healthcare providers thereafter often volunteered to assist when in-flight medical issues arose, but aircraft carried minimal medical equipment and volunteers’ liability was uncertain.
In 1998, Congress passed the Aviation Medical Assistance Act (AMAA), which provides liability protection for on-board healthcare providers who render medical assistance. It also required the Federal Aviation Administration (FAA) to improve its standards for in-flight medical equipment.2,3
HOW OFTEN DO EMERGENCIES ARISE?
How often medical events occur during flight is difficult to estimate because airlines are not mandated to report such issues.4 Based on data from a ground-based communications center that provides medical consultation service to airlines, medical events occur in approximately 1 in every 604 flights.5 This is likely an underestimate, as many medical events may be handled on board without involving a ground-based consultation center.
The most common emergencies are syncope or presyncope, representing 37.4% of consultations, followed by respiratory symptoms (12.1%), nausea or vomiting (9.5%), cardiac symptoms (7.7%), seizures (5.8%), and abdominal pain (4.1%).5 Very few in-flight medical emergencies progress to death; the reported mortality rate is 0.3%.5
CABIN PRESSURES ARE RELATIVELY LOW
The cabins of commercial airliners are pressurized, but the pressure is still lower than on the ground. The cabin pressure in flight is equivalent to that at an altitude of 6,000 to 8,000 feet,6,7 ie, about 23 or 24 mm Hg, compared with about 30 mm Hg at sea level. At this pressure, passengers have a partial pressure of arterial oxygen (Pao2) of 60 mm Hg (normal at sea level is > 80).8
This reduced oxygen pressure is typically not clinically meaningful in healthy people. However, people with underlying pulmonary or cardiac illness may be starting further to the left on the oxygen dissociation curve before gaining altitude, putting them at risk for acute exacerbations of underlying medical conditions. Many patients who rely on supplemental oxygen, such as those with chronic obstructive pulmonary disease, are advised to increase their oxygen support during flight.9
Boyle’s law says that the volume of a gas is inversely proportional to its pressure. As the pressure drops in the cabin after takeoff, air trapped in an enclosed space—eg, in some patient’s bodies—can increase in volume up to 30%,10 which can have medical ramifications. Clinically significant pneumothorax during flight has been reported.11–13 Partially because of these volumetric changes, patients who have undergone abdominal surgery are advised to avoid flying for at least 2 weeks after their procedure.10,14 Patients who have had recent ocular or intracranial surgery may also be at risk of in-flight complications.15
IN-FLIGHT MEDICAL RESOURCES
The limited medical supplies available on aircraft often challenge healthcare providers who offer to respond to in-flight medical events. However, several important medical resources are available.
Medical kits and defibrillators
FAA regulations require airlines based in the United States to carry basic first aid supplies such as bandages and splints.3 Airlines are also required to carry a medical kit containing the items listed in Table 1.
The FAA-mandated kit does not cover every circumstance that may arise. Although in-flight pediatric events occasionally occur,16 many of the available medications are inappropriate for young children. The FAA does not require sedative or antipsychotic agents, which could be useful for passengers who have acute psychiatric episodes. Obstetric supplies are absent. On international carriers, the contents of medical kits are highly variable,17 as are the names used for some medications.
The FAA requires at least 1 automated external defibrillator (AED) to be available on each commercial aircraft.3 The timely use of AEDs greatly improves survivability after out-of-hospital cardiac arrest.18,19 One study involving a major US airline found a 40% survival rate to hospital discharge in patients who received in-flight defibrillation.20 Without this intervention, very few of the patients would have been expected to survive. In addition to being clinically effective, placing AEDs aboard commercial aircraft is a cost-effective public health intervention.21
Consultation services
Most major airlines can contact ground-based medical consultation services during flight.10 These centers are staffed with healthcare providers who can provide flight crews with advice on how to handle medical events in real time. Healthcare providers can likewise discuss specific medical issues with these services if they respond to an in-flight medical event. Ground-based call centers can also communicate with prehospital providers should a flight need to be diverted.
Other on-board providers
Some medical events require the involvement of more than one medical provider. Other physicians, nurses, and prehospital providers are often also on board.22 Responding physicians can also request the assistance of these other healthcare providers. Flight attendants in the United States are required to be trained in cardiopulmonary resuscitation (CPR).23
Flight diversion
Critically ill patients or those with time-sensitive medical emergencies may require the aircraft to divert from its intended destination. As may be expected, medical emergencies suspected to involve the cardiovascular, neurologic, or respiratory system have been shown to most likely result in aircraft diversion.5,24 Approximately 7% of in-flight medical events in which a ground-based medical consultation service is contacted result in diversion.5
While an on-board responding physician can make a recommendation to divert based on the patient’s acute medical status, only the captain can make the ultimate decision.4 On-board healthcare providers should clearly state that a patient might benefit from an unscheduled landing if that is truly their assessment. In addition to communicating their clinical concerns with the flight crew, the responding physician may also be able to discuss the situation with the airline’s ground-based consultation service. On-board physicians can make important contributions to the assessment of illness severity and triage decisions.
MEDICOLEGAL ISSUES
No legal duty to assist
US healthcare providers are not legally required to respond to on-board medical emergencies on US-based airlines. Canada and the United Kingdom also do not require providers to render assistance. But the General Medical Council (the regulatory body for UK doctors) states that doctors have an ethical duty to respond in the event of a medical emergency, including one on board an aircraft. Other countries, notably Australia and some in the European Union, require healthcare professionals to respond to on-board medical emergencies.10
Regardless of potential legal duties to assist, healthcare providers are arguably ethically obliged to render assistance if they can.
Aviation Medical Assistance Act
The extent of an American healthcare provider’s liability risk for assisting in a medical emergency on a plane registered in the United States is limited by statute. The 1998 AMAA provides liability protection for on-board medical providers who are asked to assist during an in-flight medical emergency. This statute covers all US-certified air carriers on domestic flights and would likely be held to apply to US aircraft in foreign airspace because of the general rule that the law of the country where the air carrier is registered applies to in-flight events.
Under the AMAA, providers asked to assist with in-flight medical emergencies are not liable for malpractice as long as their actions are not “grossly” negligent or intended to cause the patient harm.25 This is distinguishable from a standard malpractice liability scenario, in which the plaintiff only needs to show ordinary negligence. In a traditional healthcare setting, a provider has to act within the “standard of care” when assessing and treating a patient. If the provider deviates from the standard of care, such as by making an error in judgment or diagnosis, the provider is legally negligent. Under traditional malpractice law, even if a provider is minimally negligent, he or she is liable for any damages resulting from that negligence. Under a gross negligence standard, providers are protected from liability unless they demonstrate flagrant disregard for the patient’s health and safety.
Postflight issues
A provider who undertakes care should continue to provide care until it is no longer necessary, either because the patient recovers or the responsibility has been transferred to another provider. At the point of transfer, the healthcare provider’s relationship with the patient terminates.
The provider should document the encounter, typically using airline-specific documentation. The responding physician needs to be mindful of the patient’s privacy, refraining from discussing the event with others without the patient’s authorization.26
SUGGESTED RESPONSE
Healthcare providers who wish to respond to in-flight medical emergencies must first determine if they are sufficiently capable of providing care. During a flight, providers do not expect to be on duty and so may have consumed alcoholic beverages to an extent that would potentially render them unsuitable to respond. When it is appropriate to become involved in a medical emergency during flight, the healthcare provider should state his or her qualifications to the passenger and to flight personnel.
If circumstances allow, the volunteer provider should obtain the patient’s consent for evaluation and treatment.10 Additionally, with the multilingual nature of commercial air travel, especially on international flights, the provider may need to enlist a translator’s assistance.
Providers may find it preferable to treat passengers in their seats.27 Given the confined space in an aircraft, keeping ill passengers out of the aisle allows others to move about the cabin. If it becomes necessary to move the patient, a location should be sought that minimally interferes with other passengers’ needs.
If a passenger has critical medical needs, in-flight medical volunteers can recommend flight diversion, which should also be discussed with ground-based medical staff. However, as emphasized earlier, the captain makes the ultimate decision to divert, taking into account other operational factors that affect the safety of the aircraft and its occupants. In-flight medical care providers should perform only the treatments they are qualified to provide and should operate within their scope of training.
After the aircraft lands, if the passenger must be transported to a hospital, providers should supply prehospital personnel with a requisite transfer-of-care communication. In-flight medical providers who have performed a significant medical intervention might find it appropriate to accompany the patient to the hospital.
SPECIFIC CONDITIONS
The list of possible acute medical issues that occur aboard aircraft is extensive. Here are a few of them.
Trauma
Passengers may experience injuries during flight, for example during periods of heavy air turbulence. Responding physicians should assess for potential life-threatening injuries, keeping in mind that some passengers may be at higher risk. For example, if a passenger on anticoagulation experiences a blunt head injury, this would raise suspicion for possible intracranial hemorrhage, and frequent reassessment of neurologic status may be necessary. If an extremity fracture is suspected, the physician should splint the affected limb. Analgesia may be provided from the medical kit, if appropriate.
Gastrointestinal issues
Acute gastrointestinal issues such as nausea and vomiting are often reported to ground-based medical consultation services.5 Responding on-board providers must consider if the passenger is simply experiencing gastrointestinal upset from a benign condition such as gastroenteritis or has a more serious condition. For some patients, vomiting may be a symptom of a myocardial infarction.28 Bilious emesis with abdominal distention may be associated with small-bowel obstruction. While antiemetics are not included in the FAA-mandated medical kit, providers can initiate intravenous fluid therapy for passengers who show signs of hypovolemia.
Cardiac arrest
Although cardiac arrest during flight is rare,5 medical providers should nonetheless be prepared to handle it. Upon recognition of cardiac arrest, the provider should immediately begin cardiopulmonary resuscitation and use the on-board AED to defibrillate a potentially shockable rhythm. Flight attendants are trained in cardiopulmonary resuscitation and therefore may assist with resuscitation efforts. If the patient is resuscitated, the responding physician should recommend diversion of the flight.
Anaphylaxis
In the event of a severe life-threatening allergic reaction, the FAA-mandated emergency medical kit contains both diphenhydramine and epinephrine. For an adult experiencing anaphylaxis, a responding on-board physician can administer diphenhydramine 50 mg and epinephrine 0.3 mg (using the 1:1000 formulation), both intramuscularly. For patients with bronchospasm, a metered-dose inhaler of albuterol can be given. As anaphylaxis is an acute and potentially lethal condition, diversion of the aircraft would also be appropriate.29
Myocardial infarction
When acute myocardial infarction is suspected, it is appropriate for the provider to give aspirin, with important exceptions for patients who are experiencing an acute hemorrhage or who have an aspirin allergy.30 Supplemental oxygen should likewise be provided if the responding physician suspects compromised oxygenation. As acute myocardial infarction is also a time-sensitive condition, the clinician who suspects this diagnosis should recommend diversion of the aircraft.
Acute psychiatric issues
While approximately 2.4% of on-board medical events are attributed to psychiatric issues,5 there are few tools at the clinician’s disposal in the FAA-mandated emergency medical kit. Antipsychotics and sedatives are not included. The responding physician may need to attempt verbal de-escalation of aggressive behavior. If the safety of the flight is compromised, the application of improvised physical restraints may be appropriate.
Altered mental status
The differential diagnosis for altered mental status is extensive. The on-board physician should try to identify reversible and potentially lethal conditions and determine the potential need for aircraft diversion.
If possible, a blood sugar level should be measured (although the FAA-mandated kit does not contain a glucometer). It may be appropriate to empirically give intravenous dextrose to patients strongly suspected of having hypoglycemia.
If respiratory or cerebrovascular compromise is suspected, supplemental oxygen should be provided.
Unless a reversible cause of altered mental status is identified and treated successfully, it will likely be appropriate to recommend diversion of the aircraft.
Acknowledgment: The authors acknowledge Linda J. Kesselring, MS, ELS, the technical editor/writer in the Department of Emergency Medicine University of Maryland School of Medicine, for her contributions as copy editor of a previous version of this manuscript.
- Gazdik M. Vault guide to flight attendant careers. New York, NY: Vault, Inc.; 2005.
- Stewart PH, Agin WS, Douglas SP. What does the law say to Good Samaritans? A review of Good Samaritan statutes in 50 states and on US airlines. Chest 2013; 143:1774–1783.
- Federal Aviation Administration (FAA), DOT. Emergency medical equipment. Final rule. Fed Regist 2001; 66:19028–19046.
- Goodwin T. In-flight medical emergencies: an overview. BMJ 2000; 321:1338–1341.
- Peterson DC, Martin-Gill C, Guyette FX, et al. Outcomes of medical emergencies on commercial airline flights. N Engl J Med 2013; 368:2075–2083.
- Aerospace Medical Association, Aviation Safety Committee, Civil Aviation Subcommittee. Cabin cruising altitudes for regular transport aircraft. Aviat Space Environ Med 2008; 79:433–439.
- Cottrell JJ. Altitude exposures during aircraft flight. Flying higher. Chest 1988; 93:81–84.
- Humphreys S, Deyermond R, Bali I, Stevenson M, Fee JP. The effect of high altitude commercial air travel on oxygen saturation. Anaesthesia 2005; 60:458–460.
- Shrikrishna D, Coker RK; Air Travel Working Party of the British Thoracic Society Standards of Care Committee. Managing passengers with stable respiratory disease planning air travel: British Thoracic Society recommendations. Thorax 2011; 66:831–833.
- Gendreau MA, DeJohn C. Responding to medical events during commercial airline flights. N Engl J Med 2002; 346:1067–1073.
- Hu X, Cowl CT, Baqir M, Ryu JH. Air travel and pneumothorax. Chest 2014; 145:688–694.
- Madan K, Vishwanath G, Singh N. In-flight spontaneous pneumothorax: congenital cystic adenomatoid malformation of the lung. Respiration 2012; 83:554–558.
- Wallace TW, Wong T, O’Bichere A, Ellis BW. Managing in flight emergencies. BMJ 1995; 311:374–376.
- Medical aspects of transportation aboard commercial aircraft. AMA commission on emergency medical services. JAMA 1982; 247:1007–1011.
- Mills MD, Devenyi RG, Lam WC, Berger AR, Beijer CD, Lam SR. An assessment of intraocular pressure rise in patients with gas-filled eyes during simulated air flight. Ophthalmology 2001; 108:40–44.
- Moore BR, Ping JM, Claypool DW. Pediatric emergencies on a US-based commercial airline. Pediatr Emerg Care 2005; 21:725–729.
- Sand M, Gambichler T, Sand D, Thrandorf C, Altmeyer P, Bechara FG. Emergency medical kits on board commercial aircraft: a comparative study. Travel Med Infect Dis 2010; 8:388–394.
- Auble TE, Menegazzi JJ, Paris PM. Effect of out-of-hospital defibrillation by basic life support providers on cardiac arrest mortality: a metaanalysis. Ann Emerg Med 1995; 25:642–648.
- Marenco JP, Wang PJ, Link MS, Homoud MK, Estes NA. Improving survival from sudden cardiac arrest: the role of the automated external defibrillator. JAMA 2001; 285:1193–1200.
- Page RL, Joglar JA, Kowal RC, et al. Use of automated external defibrillators by a US airline. N Engl J Med 2000; 343:1210–1216.
- Groeneveld PW, Kwong JL, Liu Y, et al. Cost-effectiveness of automated external defibrillators on airlines. JAMA 2001; 286:1482–1489.
- Baltsezak S. Clinic in the air? A retrospective study of medical emergency calls from a major international airline. J Travel Med 2008; 15:391–394.
- Federal Aviation Administration (FAA). Advisory circular: emergency medical equipment training AC 121-34B. www.faa.gov/documentLibrary/media/Advisory_Circular/AC121-34B.pdf. Accessed April 6, 2017.
- Cummins RO, Schubach JA. Medical emergencies among commercial air travelers. JAMA 1989; 261:1295–1299.
- US Government Publishing Office. Public Law 105-170. Aviation Medical Assistance Act of 1998.
- US Government Publishing Office. Public Law 104-191. Health Insurance Portability and Accountability Act of 1996.
- Chandra A, Conry S. In-flight medical emergencies. West J Emerg Med 2013; 14:499–504.
- Kirchberger I, Meisinger C, Heier M, et al. Patient-reported symptoms in acute myocardial infarction: differences related to ST-segment elevation: the MONICA/KORA Myocardial Infarction Registry. J Intern Med 2011; 270:58–64.
- Brady WJ Jr, Bright HL. Occurrence of multiphasic anaphylaxis during a transcontinental air flight. Am J Emerg Med 1999; 17:695–696.
- O’Connor RE, Brady W, Brooks SC, et al. Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122:(suppl 3):S787–S817.
- Gazdik M. Vault guide to flight attendant careers. New York, NY: Vault, Inc.; 2005.
- Stewart PH, Agin WS, Douglas SP. What does the law say to Good Samaritans? A review of Good Samaritan statutes in 50 states and on US airlines. Chest 2013; 143:1774–1783.
- Federal Aviation Administration (FAA), DOT. Emergency medical equipment. Final rule. Fed Regist 2001; 66:19028–19046.
- Goodwin T. In-flight medical emergencies: an overview. BMJ 2000; 321:1338–1341.
- Peterson DC, Martin-Gill C, Guyette FX, et al. Outcomes of medical emergencies on commercial airline flights. N Engl J Med 2013; 368:2075–2083.
- Aerospace Medical Association, Aviation Safety Committee, Civil Aviation Subcommittee. Cabin cruising altitudes for regular transport aircraft. Aviat Space Environ Med 2008; 79:433–439.
- Cottrell JJ. Altitude exposures during aircraft flight. Flying higher. Chest 1988; 93:81–84.
- Humphreys S, Deyermond R, Bali I, Stevenson M, Fee JP. The effect of high altitude commercial air travel on oxygen saturation. Anaesthesia 2005; 60:458–460.
- Shrikrishna D, Coker RK; Air Travel Working Party of the British Thoracic Society Standards of Care Committee. Managing passengers with stable respiratory disease planning air travel: British Thoracic Society recommendations. Thorax 2011; 66:831–833.
- Gendreau MA, DeJohn C. Responding to medical events during commercial airline flights. N Engl J Med 2002; 346:1067–1073.
- Hu X, Cowl CT, Baqir M, Ryu JH. Air travel and pneumothorax. Chest 2014; 145:688–694.
- Madan K, Vishwanath G, Singh N. In-flight spontaneous pneumothorax: congenital cystic adenomatoid malformation of the lung. Respiration 2012; 83:554–558.
- Wallace TW, Wong T, O’Bichere A, Ellis BW. Managing in flight emergencies. BMJ 1995; 311:374–376.
- Medical aspects of transportation aboard commercial aircraft. AMA commission on emergency medical services. JAMA 1982; 247:1007–1011.
- Mills MD, Devenyi RG, Lam WC, Berger AR, Beijer CD, Lam SR. An assessment of intraocular pressure rise in patients with gas-filled eyes during simulated air flight. Ophthalmology 2001; 108:40–44.
- Moore BR, Ping JM, Claypool DW. Pediatric emergencies on a US-based commercial airline. Pediatr Emerg Care 2005; 21:725–729.
- Sand M, Gambichler T, Sand D, Thrandorf C, Altmeyer P, Bechara FG. Emergency medical kits on board commercial aircraft: a comparative study. Travel Med Infect Dis 2010; 8:388–394.
- Auble TE, Menegazzi JJ, Paris PM. Effect of out-of-hospital defibrillation by basic life support providers on cardiac arrest mortality: a metaanalysis. Ann Emerg Med 1995; 25:642–648.
- Marenco JP, Wang PJ, Link MS, Homoud MK, Estes NA. Improving survival from sudden cardiac arrest: the role of the automated external defibrillator. JAMA 2001; 285:1193–1200.
- Page RL, Joglar JA, Kowal RC, et al. Use of automated external defibrillators by a US airline. N Engl J Med 2000; 343:1210–1216.
- Groeneveld PW, Kwong JL, Liu Y, et al. Cost-effectiveness of automated external defibrillators on airlines. JAMA 2001; 286:1482–1489.
- Baltsezak S. Clinic in the air? A retrospective study of medical emergency calls from a major international airline. J Travel Med 2008; 15:391–394.
- Federal Aviation Administration (FAA). Advisory circular: emergency medical equipment training AC 121-34B. www.faa.gov/documentLibrary/media/Advisory_Circular/AC121-34B.pdf. Accessed April 6, 2017.
- Cummins RO, Schubach JA. Medical emergencies among commercial air travelers. JAMA 1989; 261:1295–1299.
- US Government Publishing Office. Public Law 105-170. Aviation Medical Assistance Act of 1998.
- US Government Publishing Office. Public Law 104-191. Health Insurance Portability and Accountability Act of 1996.
- Chandra A, Conry S. In-flight medical emergencies. West J Emerg Med 2013; 14:499–504.
- Kirchberger I, Meisinger C, Heier M, et al. Patient-reported symptoms in acute myocardial infarction: differences related to ST-segment elevation: the MONICA/KORA Myocardial Infarction Registry. J Intern Med 2011; 270:58–64.
- Brady WJ Jr, Bright HL. Occurrence of multiphasic anaphylaxis during a transcontinental air flight. Am J Emerg Med 1999; 17:695–696.
- O’Connor RE, Brady W, Brooks SC, et al. Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010; 122:(suppl 3):S787–S817.
KEY POINTS
- The exact incidence of medical emergencies aboard airplanes is unknown, but they occurred in 1 in 604 flights in 1 study, which is likely an underestimate.
- The relatively low air pressure in the cabin can contribute to the development of acute medical issues.
- In the United States, the Federal Aviation Administration mandates that airlines carry a limited set of medical resources.
- The Aviation Medical Assistance Act protects responding providers against liability except in cases of “gross negligence.”
- You the physician can recommend that the flight be diverted to the closest airport, but only the captain can make the actual decision.
The USPSTF and screening for obstructive sleep apnea: Dispelling misconceptions
Recent guidelines from the United States Preventive Services Task Force (USPSTF) say that there is insufficient evidence to recommend screening for obstructive sleep apnea in people who have no symptoms of it.1–3
The USPSTF committee systematically reviewed the evidence, sifting through 1,315 articles,3 and found no randomized controlled trials that compared screening with no screening in adults who have no symptoms (or no recognized symptoms) of obstructive sleep apnea. Conclusion: “The current evidence is insufficient to assess the balance of benefits and harms of screening for [obstructive sleep apnea] in asymptomatic adults.”1
This is logical, rigorous, and evidence-based. However, the conclusions might be misinterpreted and need to be put into context.
SCREENING IS WARRANTED IF PATIENTS HAVE SYMPTOMS
First, note that the USPSTF is referring to people who have no symptoms. The American Academy of Sleep Medicine has issued recommendations about screening and diagnostic testing in people who do have symptoms,4 in whom it is important to pursue screening and diagnostic testing.
Symptoms of obstructive sleep apnea include excessive daytime sleepiness, fatigue, drowsy driving, disrupted or fragmented sleep, nocturia, witnessed apnea, snoring, restless sleep, neurocognitive deficits, and depressed mood. Treating it improves these symptoms, as clinical trials have shown unequivocally and consistently.5
Moreover, the third edition of the International Classification of Sleep Disorders defines obstructive sleep apnea as an obstructive apnea-hypopnea index of 15 or more events per hour even in the absence of symptoms. This threshold recognizes the risk of adverse health outcomes observed in population-based studies (ie, in participants recruited irrespective of symptoms).6
ABSENCE OF EVIDENCE, NOT EVIDENCE OF ABSENCE
Second, the absence of sufficient evidence cited by the USPSTF does not necessarily mean that screening for obstructive sleep apnea in asymptomatic people is not beneficial—it has just not been systematically studied. There was insufficient evidence available to make a recommendation to allocate resources to screen all patients irrespective of symptoms.
The Sleep Heart Health Study suggested that few people with obstructive sleep apnea were diagnosed with it and that even fewer were treated for it.7 More recent data indicate that this underdiagnosis persists and is more pervasive in underserved minority groups.8,9
SCREENING VS CASE-FINDING
Moreover, screening is not the same as case-finding. The purpose of screening, as defined 50 years ago by Wilson and Jungner in a report for the World Health Organization, is “to discover those among the apparently well who are in fact suffering from disease.”10
Case-finding, on the other hand, focuses on those suspected of being at risk of the disease. In the case of obstructive sleep apnea, this is a lot of people. The overall prevalence of obstructive sleep apnea is about 26% by one estimate,11 and many more people have risk factors for it. For example, in one study, 69% of patients presenting to a primary care clinic were overweight or obese,12 and many primary care patients have diseases that obstructive sleep apnea can exacerbate. One can therefore argue that in clinical practice, testing for obstructive sleep apnea is more like case-finding than screening—most patients that you see have unrecognized symptoms of it or risk factors for it.
CRITERIA FOR A GOOD SCREENING TEST
Principles for screening outlined by Wilson and Jungner10 were:
- The condition we are trying to detect should be important
- There should be an accepted treatment for it
- Facilities for diagnosis and treatment should be available
- Testing should be acceptable to the population
- There should be cost benefit to the expense of case-finding
- There should be an agreed-upon policy on whom to treat as patients.
Screening for obstructive sleep apnea meets many of these criteria.
Obstructive sleep apnea is important
Solid evidence exists that obstructive sleep apnea exerts a bad effect on health and quality of life. Population-based studies that enrolled participants irrespective of symptoms indicate that the risk of death is about twice as high in those with severe obstructive sleep apnea as in those without, and treatment exerts benefit especially in those with cardiovascular risk.13,14 Therefore, the criterion for screening that says the disease must be important is met.
Pathophysiologic pathways by which obstructive sleep apnea causes harm include intermittent hypoxia, hypercapnia, intrathoracic pressure swings, and autonomic nervous system fluctuations.
Treatment is beneficial
The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recognized obstructive sleep apnea as a cause of hypertension.15
Treating obstructive sleep apnea lowers blood pressure, which in turn improves cardiovascular outcomes. Effects are most pronounced in those with resistant hypertension. The reduction in blood pressure is only about 2 to 3 mm Hg, but this translates to a 4% to 8% reduction in future risk of stroke and coronary heart disease.16,17
The Continuous Positive Airway Pressure Treatment of Obstructive Sleep Apnea to Prevent Cardiovascular Disease multicenter randomized clinical trial investigated the impact of treating obstructive sleep apnea with continuous positive airway pressure (CPAP) compared with usual care.18 Although no statistically significant difference was seen in the composite cardiovascular outcome, propensity-score analysis in the subgroup adherent to CPAP demonstrated a lower composite of cerebral events in those who used CPAP for at least 4 hours a day.
The findings from this trial are difficult to interpret for several reasons. Adherence to CPAP was suboptimal, the severity of obstructive sleep apnea might not have been bad enough to permit observation of a significant treatment effect, and the generalizability of the findings is unclear, given that many of the participants were from underresourced regions.19
In a meta-analysis of cohort studies comprising more than 3 million participants, Fu et al found that the cardiovascular mortality rate was 63% lower in those with obstructive sleep apnea using CPAP than in untreated patients.20
APPLY CLINICAL JUDGMENT
Overall, the USPSTF report is intended to guide healthcare decision-makers. However, it includes a caveat to not substitute the findings for clinical judgment and to interpret the findings in the context of collateral pertinent information.2
Although no high-quality data exist to support or refute global screening for obstructive sleep apnea in the primary care setting, the high prevalence of this disease and its detrimental effects on health and quality of life if left untreated should not be dismissed.
Arguably, most patients who present to primary care clinics are not healthy, are not free of symptoms, and are at risk of obstructive sleep apnea because they are obese. Testing for it is therefore more like case-finding than screening.
In view of the serious consequences of obstructive sleep apnea, we should view the situation as an opportunity to examine the impact of screening. Perhaps using electronic medical records, we could collect sleep-specific measures, implement case-finding strategies, and perform pragmatic clinical trials to inform and guide optimal and cost-effective screening approaches.
Patients with common disorders such as obstructive sleep apnea are often considered asymptomatic until asked about symptoms. Therefore, careful review of systems incorporating sleep health is important, particularly as patients do not typically volunteer this information. Obtaining this history does not necessarily fall under the USPSTF’s recommendation not to screen.
Future efforts should focus on leveraging the electronic medical record platform to collect sleep-specific measures, implementing case-finding strategies, and performing pragmatic clinical trials in the primary care setting to inform and guide optimal and cost-effective approaches to screening.
- US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force Recommendation Statement. JAMA 2017; 317:407–414.
- Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2017; 317:415–433.
- Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: an evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 146. AHRQ Publication No. 14-05216-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2017. www.uspreventiveservicestaskforce.org/Page/Document/final-evidence-review152/obstructive-sleep-apnea-in-adults-screening. Accessed May 2, 2017.
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13:479–504.
- Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003; 163:565–571.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6:49–54.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38:877–888.
- Redline S, Sotres-Alvarez D, Loredo J, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med 2014; 189:335–344.
- Wilson JMG, Jungner G. Principles and practice of screening for disease. Geneva: WHO; 1968. www.who.int/ionizing_radiation/medical_radiation_exposure/munich-WHO-1968-Screening-Disease.pdf?ua=1. Accessed May 2, 2017.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Stecker T, Sparks S. Prevalence of obese patients in a primary care setting. Obesity (Silver Spring) 2006; 14:373–376.
- Zhao YY, Wang R, Gleason KJ, et al; BestAIR Investigators. Effect of continuous positive airway pressure treatment on health-related quality of life and sleepiness in high cardiovascular risk individuals with sleep apnea: Best Apnea Interventions for Research (BestAIR) Trial. Sleep 2017; Apr 17. doi: 10.1093/sleep/zsx040. [Epub ahead of print].
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009 Aug;6(8) e1000132. doi: 10.1371/journal.pmed.1000132. Epub 2009 Aug 18.
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Schein AS, Kerkhoff AC, Coronel CC, Plentz RD, Sbruzzi G. Continuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea; a systematic review and meta-analysis with 1000 patients. J Hypertens 2014; 32:1762–1773.
- He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J 1999; 138:211–219.
- McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375:919–931.
- Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017; 69:841–858.
- Fu Y, Xia Y, Yi H, Xu H, Guan J, Yin S. Meta-analysis of all-cause and cardiovascular mortality in obstructive sleep apnea with or without continuous positive airway pressure treatment. Sleep Breath 2017; 21:181–189.
Recent guidelines from the United States Preventive Services Task Force (USPSTF) say that there is insufficient evidence to recommend screening for obstructive sleep apnea in people who have no symptoms of it.1–3
The USPSTF committee systematically reviewed the evidence, sifting through 1,315 articles,3 and found no randomized controlled trials that compared screening with no screening in adults who have no symptoms (or no recognized symptoms) of obstructive sleep apnea. Conclusion: “The current evidence is insufficient to assess the balance of benefits and harms of screening for [obstructive sleep apnea] in asymptomatic adults.”1
This is logical, rigorous, and evidence-based. However, the conclusions might be misinterpreted and need to be put into context.
SCREENING IS WARRANTED IF PATIENTS HAVE SYMPTOMS
First, note that the USPSTF is referring to people who have no symptoms. The American Academy of Sleep Medicine has issued recommendations about screening and diagnostic testing in people who do have symptoms,4 in whom it is important to pursue screening and diagnostic testing.
Symptoms of obstructive sleep apnea include excessive daytime sleepiness, fatigue, drowsy driving, disrupted or fragmented sleep, nocturia, witnessed apnea, snoring, restless sleep, neurocognitive deficits, and depressed mood. Treating it improves these symptoms, as clinical trials have shown unequivocally and consistently.5
Moreover, the third edition of the International Classification of Sleep Disorders defines obstructive sleep apnea as an obstructive apnea-hypopnea index of 15 or more events per hour even in the absence of symptoms. This threshold recognizes the risk of adverse health outcomes observed in population-based studies (ie, in participants recruited irrespective of symptoms).6
ABSENCE OF EVIDENCE, NOT EVIDENCE OF ABSENCE
Second, the absence of sufficient evidence cited by the USPSTF does not necessarily mean that screening for obstructive sleep apnea in asymptomatic people is not beneficial—it has just not been systematically studied. There was insufficient evidence available to make a recommendation to allocate resources to screen all patients irrespective of symptoms.
The Sleep Heart Health Study suggested that few people with obstructive sleep apnea were diagnosed with it and that even fewer were treated for it.7 More recent data indicate that this underdiagnosis persists and is more pervasive in underserved minority groups.8,9
SCREENING VS CASE-FINDING
Moreover, screening is not the same as case-finding. The purpose of screening, as defined 50 years ago by Wilson and Jungner in a report for the World Health Organization, is “to discover those among the apparently well who are in fact suffering from disease.”10
Case-finding, on the other hand, focuses on those suspected of being at risk of the disease. In the case of obstructive sleep apnea, this is a lot of people. The overall prevalence of obstructive sleep apnea is about 26% by one estimate,11 and many more people have risk factors for it. For example, in one study, 69% of patients presenting to a primary care clinic were overweight or obese,12 and many primary care patients have diseases that obstructive sleep apnea can exacerbate. One can therefore argue that in clinical practice, testing for obstructive sleep apnea is more like case-finding than screening—most patients that you see have unrecognized symptoms of it or risk factors for it.
CRITERIA FOR A GOOD SCREENING TEST
Principles for screening outlined by Wilson and Jungner10 were:
- The condition we are trying to detect should be important
- There should be an accepted treatment for it
- Facilities for diagnosis and treatment should be available
- Testing should be acceptable to the population
- There should be cost benefit to the expense of case-finding
- There should be an agreed-upon policy on whom to treat as patients.
Screening for obstructive sleep apnea meets many of these criteria.
Obstructive sleep apnea is important
Solid evidence exists that obstructive sleep apnea exerts a bad effect on health and quality of life. Population-based studies that enrolled participants irrespective of symptoms indicate that the risk of death is about twice as high in those with severe obstructive sleep apnea as in those without, and treatment exerts benefit especially in those with cardiovascular risk.13,14 Therefore, the criterion for screening that says the disease must be important is met.
Pathophysiologic pathways by which obstructive sleep apnea causes harm include intermittent hypoxia, hypercapnia, intrathoracic pressure swings, and autonomic nervous system fluctuations.
Treatment is beneficial
The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recognized obstructive sleep apnea as a cause of hypertension.15
Treating obstructive sleep apnea lowers blood pressure, which in turn improves cardiovascular outcomes. Effects are most pronounced in those with resistant hypertension. The reduction in blood pressure is only about 2 to 3 mm Hg, but this translates to a 4% to 8% reduction in future risk of stroke and coronary heart disease.16,17
The Continuous Positive Airway Pressure Treatment of Obstructive Sleep Apnea to Prevent Cardiovascular Disease multicenter randomized clinical trial investigated the impact of treating obstructive sleep apnea with continuous positive airway pressure (CPAP) compared with usual care.18 Although no statistically significant difference was seen in the composite cardiovascular outcome, propensity-score analysis in the subgroup adherent to CPAP demonstrated a lower composite of cerebral events in those who used CPAP for at least 4 hours a day.
The findings from this trial are difficult to interpret for several reasons. Adherence to CPAP was suboptimal, the severity of obstructive sleep apnea might not have been bad enough to permit observation of a significant treatment effect, and the generalizability of the findings is unclear, given that many of the participants were from underresourced regions.19
In a meta-analysis of cohort studies comprising more than 3 million participants, Fu et al found that the cardiovascular mortality rate was 63% lower in those with obstructive sleep apnea using CPAP than in untreated patients.20
APPLY CLINICAL JUDGMENT
Overall, the USPSTF report is intended to guide healthcare decision-makers. However, it includes a caveat to not substitute the findings for clinical judgment and to interpret the findings in the context of collateral pertinent information.2
Although no high-quality data exist to support or refute global screening for obstructive sleep apnea in the primary care setting, the high prevalence of this disease and its detrimental effects on health and quality of life if left untreated should not be dismissed.
Arguably, most patients who present to primary care clinics are not healthy, are not free of symptoms, and are at risk of obstructive sleep apnea because they are obese. Testing for it is therefore more like case-finding than screening.
In view of the serious consequences of obstructive sleep apnea, we should view the situation as an opportunity to examine the impact of screening. Perhaps using electronic medical records, we could collect sleep-specific measures, implement case-finding strategies, and perform pragmatic clinical trials to inform and guide optimal and cost-effective screening approaches.
Patients with common disorders such as obstructive sleep apnea are often considered asymptomatic until asked about symptoms. Therefore, careful review of systems incorporating sleep health is important, particularly as patients do not typically volunteer this information. Obtaining this history does not necessarily fall under the USPSTF’s recommendation not to screen.
Future efforts should focus on leveraging the electronic medical record platform to collect sleep-specific measures, implementing case-finding strategies, and performing pragmatic clinical trials in the primary care setting to inform and guide optimal and cost-effective approaches to screening.
Recent guidelines from the United States Preventive Services Task Force (USPSTF) say that there is insufficient evidence to recommend screening for obstructive sleep apnea in people who have no symptoms of it.1–3
The USPSTF committee systematically reviewed the evidence, sifting through 1,315 articles,3 and found no randomized controlled trials that compared screening with no screening in adults who have no symptoms (or no recognized symptoms) of obstructive sleep apnea. Conclusion: “The current evidence is insufficient to assess the balance of benefits and harms of screening for [obstructive sleep apnea] in asymptomatic adults.”1
This is logical, rigorous, and evidence-based. However, the conclusions might be misinterpreted and need to be put into context.
SCREENING IS WARRANTED IF PATIENTS HAVE SYMPTOMS
First, note that the USPSTF is referring to people who have no symptoms. The American Academy of Sleep Medicine has issued recommendations about screening and diagnostic testing in people who do have symptoms,4 in whom it is important to pursue screening and diagnostic testing.
Symptoms of obstructive sleep apnea include excessive daytime sleepiness, fatigue, drowsy driving, disrupted or fragmented sleep, nocturia, witnessed apnea, snoring, restless sleep, neurocognitive deficits, and depressed mood. Treating it improves these symptoms, as clinical trials have shown unequivocally and consistently.5
Moreover, the third edition of the International Classification of Sleep Disorders defines obstructive sleep apnea as an obstructive apnea-hypopnea index of 15 or more events per hour even in the absence of symptoms. This threshold recognizes the risk of adverse health outcomes observed in population-based studies (ie, in participants recruited irrespective of symptoms).6
ABSENCE OF EVIDENCE, NOT EVIDENCE OF ABSENCE
Second, the absence of sufficient evidence cited by the USPSTF does not necessarily mean that screening for obstructive sleep apnea in asymptomatic people is not beneficial—it has just not been systematically studied. There was insufficient evidence available to make a recommendation to allocate resources to screen all patients irrespective of symptoms.
The Sleep Heart Health Study suggested that few people with obstructive sleep apnea were diagnosed with it and that even fewer were treated for it.7 More recent data indicate that this underdiagnosis persists and is more pervasive in underserved minority groups.8,9
SCREENING VS CASE-FINDING
Moreover, screening is not the same as case-finding. The purpose of screening, as defined 50 years ago by Wilson and Jungner in a report for the World Health Organization, is “to discover those among the apparently well who are in fact suffering from disease.”10
Case-finding, on the other hand, focuses on those suspected of being at risk of the disease. In the case of obstructive sleep apnea, this is a lot of people. The overall prevalence of obstructive sleep apnea is about 26% by one estimate,11 and many more people have risk factors for it. For example, in one study, 69% of patients presenting to a primary care clinic were overweight or obese,12 and many primary care patients have diseases that obstructive sleep apnea can exacerbate. One can therefore argue that in clinical practice, testing for obstructive sleep apnea is more like case-finding than screening—most patients that you see have unrecognized symptoms of it or risk factors for it.
CRITERIA FOR A GOOD SCREENING TEST
Principles for screening outlined by Wilson and Jungner10 were:
- The condition we are trying to detect should be important
- There should be an accepted treatment for it
- Facilities for diagnosis and treatment should be available
- Testing should be acceptable to the population
- There should be cost benefit to the expense of case-finding
- There should be an agreed-upon policy on whom to treat as patients.
Screening for obstructive sleep apnea meets many of these criteria.
Obstructive sleep apnea is important
Solid evidence exists that obstructive sleep apnea exerts a bad effect on health and quality of life. Population-based studies that enrolled participants irrespective of symptoms indicate that the risk of death is about twice as high in those with severe obstructive sleep apnea as in those without, and treatment exerts benefit especially in those with cardiovascular risk.13,14 Therefore, the criterion for screening that says the disease must be important is met.
Pathophysiologic pathways by which obstructive sleep apnea causes harm include intermittent hypoxia, hypercapnia, intrathoracic pressure swings, and autonomic nervous system fluctuations.
Treatment is beneficial
The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure recognized obstructive sleep apnea as a cause of hypertension.15
Treating obstructive sleep apnea lowers blood pressure, which in turn improves cardiovascular outcomes. Effects are most pronounced in those with resistant hypertension. The reduction in blood pressure is only about 2 to 3 mm Hg, but this translates to a 4% to 8% reduction in future risk of stroke and coronary heart disease.16,17
The Continuous Positive Airway Pressure Treatment of Obstructive Sleep Apnea to Prevent Cardiovascular Disease multicenter randomized clinical trial investigated the impact of treating obstructive sleep apnea with continuous positive airway pressure (CPAP) compared with usual care.18 Although no statistically significant difference was seen in the composite cardiovascular outcome, propensity-score analysis in the subgroup adherent to CPAP demonstrated a lower composite of cerebral events in those who used CPAP for at least 4 hours a day.
The findings from this trial are difficult to interpret for several reasons. Adherence to CPAP was suboptimal, the severity of obstructive sleep apnea might not have been bad enough to permit observation of a significant treatment effect, and the generalizability of the findings is unclear, given that many of the participants were from underresourced regions.19
In a meta-analysis of cohort studies comprising more than 3 million participants, Fu et al found that the cardiovascular mortality rate was 63% lower in those with obstructive sleep apnea using CPAP than in untreated patients.20
APPLY CLINICAL JUDGMENT
Overall, the USPSTF report is intended to guide healthcare decision-makers. However, it includes a caveat to not substitute the findings for clinical judgment and to interpret the findings in the context of collateral pertinent information.2
Although no high-quality data exist to support or refute global screening for obstructive sleep apnea in the primary care setting, the high prevalence of this disease and its detrimental effects on health and quality of life if left untreated should not be dismissed.
Arguably, most patients who present to primary care clinics are not healthy, are not free of symptoms, and are at risk of obstructive sleep apnea because they are obese. Testing for it is therefore more like case-finding than screening.
In view of the serious consequences of obstructive sleep apnea, we should view the situation as an opportunity to examine the impact of screening. Perhaps using electronic medical records, we could collect sleep-specific measures, implement case-finding strategies, and perform pragmatic clinical trials to inform and guide optimal and cost-effective screening approaches.
Patients with common disorders such as obstructive sleep apnea are often considered asymptomatic until asked about symptoms. Therefore, careful review of systems incorporating sleep health is important, particularly as patients do not typically volunteer this information. Obtaining this history does not necessarily fall under the USPSTF’s recommendation not to screen.
Future efforts should focus on leveraging the electronic medical record platform to collect sleep-specific measures, implementing case-finding strategies, and performing pragmatic clinical trials in the primary care setting to inform and guide optimal and cost-effective approaches to screening.
- US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force Recommendation Statement. JAMA 2017; 317:407–414.
- Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2017; 317:415–433.
- Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: an evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 146. AHRQ Publication No. 14-05216-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2017. www.uspreventiveservicestaskforce.org/Page/Document/final-evidence-review152/obstructive-sleep-apnea-in-adults-screening. Accessed May 2, 2017.
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13:479–504.
- Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003; 163:565–571.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6:49–54.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38:877–888.
- Redline S, Sotres-Alvarez D, Loredo J, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med 2014; 189:335–344.
- Wilson JMG, Jungner G. Principles and practice of screening for disease. Geneva: WHO; 1968. www.who.int/ionizing_radiation/medical_radiation_exposure/munich-WHO-1968-Screening-Disease.pdf?ua=1. Accessed May 2, 2017.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Stecker T, Sparks S. Prevalence of obese patients in a primary care setting. Obesity (Silver Spring) 2006; 14:373–376.
- Zhao YY, Wang R, Gleason KJ, et al; BestAIR Investigators. Effect of continuous positive airway pressure treatment on health-related quality of life and sleepiness in high cardiovascular risk individuals with sleep apnea: Best Apnea Interventions for Research (BestAIR) Trial. Sleep 2017; Apr 17. doi: 10.1093/sleep/zsx040. [Epub ahead of print].
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009 Aug;6(8) e1000132. doi: 10.1371/journal.pmed.1000132. Epub 2009 Aug 18.
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Schein AS, Kerkhoff AC, Coronel CC, Plentz RD, Sbruzzi G. Continuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea; a systematic review and meta-analysis with 1000 patients. J Hypertens 2014; 32:1762–1773.
- He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J 1999; 138:211–219.
- McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375:919–931.
- Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017; 69:841–858.
- Fu Y, Xia Y, Yi H, Xu H, Guan J, Yin S. Meta-analysis of all-cause and cardiovascular mortality in obstructive sleep apnea with or without continuous positive airway pressure treatment. Sleep Breath 2017; 21:181–189.
- US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults: US Preventive Services Task Force Recommendation Statement. JAMA 2017; 317:407–414.
- Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2017; 317:415–433.
- Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: an evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 146. AHRQ Publication No. 14-05216-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2017. www.uspreventiveservicestaskforce.org/Page/Document/final-evidence-review152/obstructive-sleep-apnea-in-adults-screening. Accessed May 2, 2017.
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13:479–504.
- Patel SR, White DP, Malhotra A, Stanchina ML, Ayas NT. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med 2003; 163:565–571.
- American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
- Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6:49–54.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38:877–888.
- Redline S, Sotres-Alvarez D, Loredo J, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med 2014; 189:335–344.
- Wilson JMG, Jungner G. Principles and practice of screening for disease. Geneva: WHO; 1968. www.who.int/ionizing_radiation/medical_radiation_exposure/munich-WHO-1968-Screening-Disease.pdf?ua=1. Accessed May 2, 2017.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177:1006–1014.
- Stecker T, Sparks S. Prevalence of obese patients in a primary care setting. Obesity (Silver Spring) 2006; 14:373–376.
- Zhao YY, Wang R, Gleason KJ, et al; BestAIR Investigators. Effect of continuous positive airway pressure treatment on health-related quality of life and sleepiness in high cardiovascular risk individuals with sleep apnea: Best Apnea Interventions for Research (BestAIR) Trial. Sleep 2017; Apr 17. doi: 10.1093/sleep/zsx040. [Epub ahead of print].
- Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009 Aug;6(8) e1000132. doi: 10.1371/journal.pmed.1000132. Epub 2009 Aug 18.
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289:2560–2572.
- Schein AS, Kerkhoff AC, Coronel CC, Plentz RD, Sbruzzi G. Continuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea; a systematic review and meta-analysis with 1000 patients. J Hypertens 2014; 32:1762–1773.
- He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J 1999; 138:211–219.
- McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375:919–931.
- Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017; 69:841–858.
- Fu Y, Xia Y, Yi H, Xu H, Guan J, Yin S. Meta-analysis of all-cause and cardiovascular mortality in obstructive sleep apnea with or without continuous positive airway pressure treatment. Sleep Breath 2017; 21:181–189.
Black hairy tongue cured concurrently with respiratory infection
A 54-year-old female smoker was admitted to the hospital for fever and respiratory infection. On the day of admission, she reported lesions of the oral mucosa for the past several months. She denied taking any medications recently.
Physical examination showed brownish papillary lesions spread across the dorsum of the tongue; the lesions were a darker shade proximally (Figure 1), leading to the diagnosis of black hairy tongue. Hygienic measures were recommended, with other treatment options to be considered later, if necessary. Of note, during her 1-week hospital stay, she was treated with levofloxacin for the respiratory infection, and she did not smoke during this period. One week after her admission, the lesions had disappeared (Figure 2).
LINGUA VILLOSA NIGRA
Black hairy tongue (lingua villosa nigra) is a rare but benign condition caused by defective desquamation and reactive hypertrophy of the filiform papillae of the tongue. Causes that have been proposed include medications, hyposalivation, poor oral hygiene, oxidizing mouthwashes (eg, hydrogen peroxide), alcohol, smoking, and infection.1
The differential diagnosis includes acanthosis nigricans, oral hairy leukoplakia, oral candidiasis, pigmented fungiform papillae, Addison disease, and black staining over a normal tongue from bismuth or food colorings.
DIAGNOSIS AND TREATMENT
Visual inspection and a detailed history are often sufficient for diagnosis. The optimal treatment is unclear, but the condition can improve with hygienic measures alone, topical or oral retinoids,2 topical triamcinolone acetonide, salicylic acid, vitamin B complex, or antifungals. There are isolated cases in the literature in which improvement occurred with antibiotics, but their role is unclear.1 Rarely, surgical excision of the filliform papilla in black hairy tongue has been done for symptomatic relief and cosmetic purposes.3
While our patient presented with typical features of black hairy tongue, which resolved with hygienic measures and smoking cessation, we could not completely rule out the contribution of antibiotics given for the respiratory infection. It is important to keep this disease in mind to avoid unnecessary tests and to apply the most appropriate treatment according to the patient’s symptoms.
- Nakajima M, Mizooka M, Tazuma S. Black hairy tongue treated with oral antibiotics: a case report. J Am Geriatr Soc 2015; 63:412–413.
- Gurvits GE, Tan A. Black hairy tongue syndrome. World J Gastroenterol 2014; 20:10845–10850.
- Stringer LL, Zitella L. Hyperpigmentation of the tongue. J Adv Pract Oncol 2014; 5:71–72.
A 54-year-old female smoker was admitted to the hospital for fever and respiratory infection. On the day of admission, she reported lesions of the oral mucosa for the past several months. She denied taking any medications recently.
Physical examination showed brownish papillary lesions spread across the dorsum of the tongue; the lesions were a darker shade proximally (Figure 1), leading to the diagnosis of black hairy tongue. Hygienic measures were recommended, with other treatment options to be considered later, if necessary. Of note, during her 1-week hospital stay, she was treated with levofloxacin for the respiratory infection, and she did not smoke during this period. One week after her admission, the lesions had disappeared (Figure 2).
LINGUA VILLOSA NIGRA
Black hairy tongue (lingua villosa nigra) is a rare but benign condition caused by defective desquamation and reactive hypertrophy of the filiform papillae of the tongue. Causes that have been proposed include medications, hyposalivation, poor oral hygiene, oxidizing mouthwashes (eg, hydrogen peroxide), alcohol, smoking, and infection.1
The differential diagnosis includes acanthosis nigricans, oral hairy leukoplakia, oral candidiasis, pigmented fungiform papillae, Addison disease, and black staining over a normal tongue from bismuth or food colorings.
DIAGNOSIS AND TREATMENT
Visual inspection and a detailed history are often sufficient for diagnosis. The optimal treatment is unclear, but the condition can improve with hygienic measures alone, topical or oral retinoids,2 topical triamcinolone acetonide, salicylic acid, vitamin B complex, or antifungals. There are isolated cases in the literature in which improvement occurred with antibiotics, but their role is unclear.1 Rarely, surgical excision of the filliform papilla in black hairy tongue has been done for symptomatic relief and cosmetic purposes.3
While our patient presented with typical features of black hairy tongue, which resolved with hygienic measures and smoking cessation, we could not completely rule out the contribution of antibiotics given for the respiratory infection. It is important to keep this disease in mind to avoid unnecessary tests and to apply the most appropriate treatment according to the patient’s symptoms.
A 54-year-old female smoker was admitted to the hospital for fever and respiratory infection. On the day of admission, she reported lesions of the oral mucosa for the past several months. She denied taking any medications recently.
Physical examination showed brownish papillary lesions spread across the dorsum of the tongue; the lesions were a darker shade proximally (Figure 1), leading to the diagnosis of black hairy tongue. Hygienic measures were recommended, with other treatment options to be considered later, if necessary. Of note, during her 1-week hospital stay, she was treated with levofloxacin for the respiratory infection, and she did not smoke during this period. One week after her admission, the lesions had disappeared (Figure 2).
LINGUA VILLOSA NIGRA
Black hairy tongue (lingua villosa nigra) is a rare but benign condition caused by defective desquamation and reactive hypertrophy of the filiform papillae of the tongue. Causes that have been proposed include medications, hyposalivation, poor oral hygiene, oxidizing mouthwashes (eg, hydrogen peroxide), alcohol, smoking, and infection.1
The differential diagnosis includes acanthosis nigricans, oral hairy leukoplakia, oral candidiasis, pigmented fungiform papillae, Addison disease, and black staining over a normal tongue from bismuth or food colorings.
DIAGNOSIS AND TREATMENT
Visual inspection and a detailed history are often sufficient for diagnosis. The optimal treatment is unclear, but the condition can improve with hygienic measures alone, topical or oral retinoids,2 topical triamcinolone acetonide, salicylic acid, vitamin B complex, or antifungals. There are isolated cases in the literature in which improvement occurred with antibiotics, but their role is unclear.1 Rarely, surgical excision of the filliform papilla in black hairy tongue has been done for symptomatic relief and cosmetic purposes.3
While our patient presented with typical features of black hairy tongue, which resolved with hygienic measures and smoking cessation, we could not completely rule out the contribution of antibiotics given for the respiratory infection. It is important to keep this disease in mind to avoid unnecessary tests and to apply the most appropriate treatment according to the patient’s symptoms.
- Nakajima M, Mizooka M, Tazuma S. Black hairy tongue treated with oral antibiotics: a case report. J Am Geriatr Soc 2015; 63:412–413.
- Gurvits GE, Tan A. Black hairy tongue syndrome. World J Gastroenterol 2014; 20:10845–10850.
- Stringer LL, Zitella L. Hyperpigmentation of the tongue. J Adv Pract Oncol 2014; 5:71–72.
- Nakajima M, Mizooka M, Tazuma S. Black hairy tongue treated with oral antibiotics: a case report. J Am Geriatr Soc 2015; 63:412–413.
- Gurvits GE, Tan A. Black hairy tongue syndrome. World J Gastroenterol 2014; 20:10845–10850.
- Stringer LL, Zitella L. Hyperpigmentation of the tongue. J Adv Pract Oncol 2014; 5:71–72.
ERAAs for menopause treatment: Welcome the ‘designer estrogens’
Estrogen receptor agonist-antagonists (ERAAs), previously called selective estrogen receptor modulators (SERMs), have extended the options for treating the various conditions that menopausal women suffer from. These drugs act differently on estrogen receptors in different tissues, stimulating receptors in some tissues but inhibiting them in others. This allows selective inhibition or stimulation of estrogen-like action in various target tissues.1
This article highlights the use of ERAAs to treat menopausal vasomotor symptoms (eg, hot flashes, night sweats), genitourinary syndrome of menopause, osteoporosis, breast cancer (and the risk of breast cancer), and other health concerns unique to women at midlife.
SYMPTOMS OF MENOPAUSE: COMMON AND TROUBLESOME
Vasomotor symptoms such as hot flashes and night sweats are common during perimenopause—most women experience them. They are most frequent during the menopause transition but can persist for 10 years or more afterward.2
Genitourinary syndrome of menopause is also common and often worsens with years after menopause.3 It can lead to dyspareunia and vaginal dryness, which may in turn result in lower libido, vaginismus, and hypoactive sexual desire disorder, problems that often arise at the same time as vaginal dryness and atrophy.4
Osteopenia and osteoporosis. A drop in systemic estrogen leads to a decline in bone mineral density, increasing the risk of fractures.5
ESTROGEN-PROGESTIN TREATMENT: THE GOLD STANDARD, BUT NOT IDEAL
The current gold standard for treating moderate to severe hot flashes is estrogen, available in oral, transdermal, and vaginal formulations.6 Estrogen also has antiresorptive effects on bone and is approved for preventing osteoporosis. Systemic estrogen may also be prescribed for genitourinary syndrome of menopause if local vaginal treatment alone is insufficient.
If women who have an intact uterus receive estrogen, they should also receive a progestin to protect against endometrial hyperplasia and reduce the risk of endometrial cancer.
Despite its status as the gold standard, estrogen-progestin therapy presents challenges. In some women, progestins cause side effects such as breast tenderness, bloating, fatigue, and depression.7 Estrogen-progestin therapy often causes vaginal bleeding, which for some women is troublesome or distressing; bleeding may be the reason for repeated evaluations, can increase anxiety, and can lead to poor adherence with hormonal treatment. Women who carry a higher-than-normal risk of developing breast cancer or fear that taking hormones will lead to breast cancer may show decreased adherence to therapy. Women who have estrogen receptor-positive breast cancer cannot take estrogen.
Individualized options are needed for women who have progestin-related side effects, unwanted vaginal bleeding, or a higher risk of breast cancer.
WELCOME THE ERAAs
An ideal treatment for menopause would relieve vasomotor symptoms and genitourinary syndrome of menopause and increase bone mineral density without causing breast tenderness, vaginal bleeding, or endometrial proliferation.
The “designer estrogens,” or ERAAs, have specific positive effects on the bone, heart, and brain with neutral or antagonist effects on estrogen receptors in other tissues such as the breasts and endometrium.8 While not entirely free of adverse effects, these agents have been developed with the aim of minimizing the most common ones related to estrogen and progestin.
Several ERAAs are currently approved by the US Food and Drug Administration (FDA)for various indications, each having a unique profile. Clomifene was the first agent of this class, and it is still used clinically to induce ovulation. This article highlights subsequently approved agents, ie, tamoxifen, raloxifene, ospemifene, and the combination of conjugated estrogens and bazedoxifene (Table 1).
All ERAAs increase the risk of venous thromboembolism, and therefore none of them should be used in women with known venous thromboembolism or at high risk of it.
TAMOXIFEN: CANCER TREATMENT AND PREVENTION
After clomiphene, tamoxifen was the second ERAA on the market. Although researchers were looking for a new contraceptive drug, they found tamoxifen to be useful as a chemotherapeutic agent for breast cancer. First used in 1971, tamoxifen continues to be one of the most commonly prescribed chemotherapeutic medications today.
The FDA has approved tamoxifen to treat breast cancer as well as to prevent breast cancer in pre- and postmenopausal women at risk. It may also have beneficial effects on bone and on cardiovascular risk factors, but these are not approved uses for it.
Trials of tamoxifen for cancer treatment
The Early Breast Cancer Trialists’ Collaborative Group9 performed a meta-analysis and found that 5 years of adjuvant treatment with tamoxifen is associated with a 26% reduction in mortality and a 47% reduction in breast cancer recurrence at 10 years. In absolute terms, we estimate that 21 women would need to be treated to prevent 1 death and 8 would need to be treated to prevent 1 recurrence.
The ATLAS Trial (Adjuvant Tamoxifen Longer Against Shorter)10 and later the UK ATTOM (Adjuvant Tamoxifen Treatment to Offer More)11 trial confirmed an even greater reduction in recurrence and mortality after a total of 10 years of treatment.
Trials of tamoxifen for cancer prevention
Cuzik et al12 performed a meta-analysis of 4 trials of tamoxifen’s effectiveness in preventing breast cancer for women at elevated risk. The incidence of estrogen receptor-positive breast cancer was 48% lower with tamoxifen use, but there was no effect on estrogen-negative breast cancer. From their data, we estimate that 77 women would need to be treated to prevent 1 case of breast cancer.
The IBIS-I trial (International Breast Cancer Intervention Study I)13 found that, in healthy women at high risk of breast cancer, the benefit of taking tamoxifen for 5 years as preventive treatment persisted long afterward. The investigators estimated that at 20 years of follow-up the risk of breast cancer would be 12.3% in placebo recipients and 7.8% in tamoxifen recipients, a 4.5% absolute risk reduction; number needed to treat (NNT) 22.
Data on tamoxifen and osteoporosis
The Breast Cancer Prevention Trial revealed a 19% reduction in the incidence of osteoporotic fractures with tamoxifen, but the difference was not statistically significant.14 The 1-year rates of fracture in women age 50 and older were 0.727% with placebo and 0.567% with tamoxifen, an absolute difference of 0.151%; therefore, if the effect is real, 662 women age 50 or older would need to be treated for 1 year to prevent 1 fracture. Tamoxifen is not FDA-approved to treat osteoporosis.
Data on tamoxifen and cardiovascular risk reduction
Chang et al,15 in a study in women at risk of breast cancer, incidentally found that tamoxifen was associated with a 13% reduction in total cholesterol compared with placebo.
Herrington and Klein,16 in a systematic review, noted similar findings in multiple studies of tamoxifen, with decreases in total cholesterol ranging from 7% to 17% and decreases in low-density lipoprotein cholesterol ranging from 10% to 28%. However, they found no change in high-density lipoprotein cholesterol concentrations or in the cardiovascular mortality rate.
The ATLAS trial10 revealed a relative risk reduction of 0.76 (95% confidence interval [CI] 0.60–0.95, P = .02) in ischemic heart disease for women who took tamoxifen for 10 years compared with 5 years. We calculate that ischemic heart disease occurred in 163 (2.5%) of 6,440 women who took tamoxifen for 5 years compared with 127 (1.9%) of 6,454 women who took it for 10 years, a 0.6% absolute risk reduction, NNT = 167.
Adverse effects of tamoxifen
Uterine neoplasia. Women taking tamoxifen have a 2.5-fold increased risk of endometrial cancer.14 Tamoxifen also increases the risk of benign uterine disease such as endometrial hyperplasia and polyps. As many as 39% of women taking tamoxifen will have evidence of benign uterine changes on pathology.17 Other adverse effects:
Venous thromboembolism (the risk of pulmonary embolism is increased approximately threefold14)
Cataracts (there is a slight increase in cataract diagnosis in tamoxifen users)
Vasomotor symptoms, which limit the use of tamoxifen in many women.
Ideal candidate for tamoxifen
The ideal candidate for tamoxifen is a woman with breast cancer that is estrogen receptor-positive and who has a history of osteopenia or osteoporosis and no risk factors for venous thromboembolism.
RALOXIFENE: FOR OSTEOPOROSIS AND FOR CANCER PREVENTION
Raloxifene, a second-generation ERAA, was first approved for preventing and treating osteoporosis and later for reducing the risk of invasive estrogen receptor-positive breast cancer in postmenopausal women.
Trials of raloxifene for osteoporosis
The MORE trial (Multiple Outcomes of Raloxifene)18 was a large multicenter randomized double-blind study. Raloxifene recipients showed a significant increase in bone mineral density in the lumbar spine and femoral neck at year 3 (P < .001) compared with those receiving placebo. Even after only 1 year of treatment, raloxifene significantly reduced the risk of new fractures, despite only modest gains in bone mineral density. After 3 years of treatment, new clinical vertebral fractures had occurred in 3.5% of the placebo group compared with 2.1% of the group receiving raloxifene 60 mg.19 Relative risk reductions were similar in women who had already had a clinical vertebral fracture at baseline, whose absolute risk is higher. However, no significant effect was seen on the incidence of hip or nonvertebral fractures.
The CORE trial (Continuing Outcomes Relevant to Raloxifene)20 extended the treatment of the women enrolled in the MORE trial another 4 years and found that the benefit of raloxifene with regard to bone mineral density persisted with continued use.
Trials of raloxifene for breast cancer prevention
The MORE trial,21 in postmenopausal women with osteoporosis included breast cancer as a secondary end point, and raloxifene was shown to decrease the incidence of invasive breast cancer. At a median of 40 months, invasive breast cancer had arisen in 13 (0.25%) of the 5,129 women assigned to raloxifene and 27 (1.0%) of the 2,576 women assigned to placebo. The authors calculated that 126 women would need to be treated to prevent 1 case of breast cancer.
The CORE trial,22 as noted, extended the treatment of the women enrolled in the MORE trial another 4 years. The risk of any invasive breast cancer in postmenopausal women with osteoporosis was significantly reduced by 59% after 8 years, and the risk of estrogen receptor-positive invasive breast cancer was reduced by 66%.
There is evidence that raloxifene’s effect on breast cancer risk persists after discontinuation of use.23
Does raloxifene reduce mortality?
Grady et al24 studied the effect of raloxifene on all-cause mortality in a pooled analysis of mortality data from the MORE, CORE, and Raloxifene Use for the Heart (RUTH)25 trials. In older postmenopausal women, the rate of all-cause mortality was 8.65% in those taking placebo compared with 7.88% in those taking raloxifene 60 mg daily—10% lower. The mechanism behind the lower mortality rate is unclear, and Grady et al recommend that the finding be interpreted with caution.
Trials of raloxifene for heart protection
The RUTH trial25 was a 5.6-year study undertaken to study the effects of raloxifene on coronary outcomes and invasive breast cancer in postmenopausal women. Results were mixed. Active treatment:
- Did not significantly affect the risk of coronary artery disease compared with placebo
- Significantly decreased the risk of invasive breast cancer
- Significantly decreased the risk of clinical vertebral fractures
- Increased the risk of fatal stroke (59 vs 39 events, hazard ratio 1.49, 95% CI 1.00–2.24) and venous thromboembolism (103 vs 71 events, hazard ratio 1.44, 95% CI 1.06–1.95).
The STAR trial (Study of Tamoxifen and Raloxifene)26,27 compared raloxifene and tamoxifen in postmenopausal women at increased risk of breast cancer. Women were randomized to receive either tamoxifen 20 mg or raloxifene 60 mg for 5 years. Results:
- No difference in the number of new cases of invasive breast cancer between the groups
- Fewer cases of noninvasive breast cancer in the tamoxifen group, but the difference was not statistically significant
- Fewer cases of uterine cancer in the raloxifene group, annual incidence rates 0.125% vs 0.199%, absolute risk reduction 0.74%, NNT 1,351, relative risk with raloxifene 0.62, 95% CI 0.30–0.50
- Fewer thromboembolic events with raloxifene
- Fewer cataracts with raloxifene.
Adverse effects of raloxifene
Raloxifene increases the risk of venous thromboembolism and stroke in women at high risk of coronary artery disease.19
Ideal candidates for raloxifene
Postmenopausal women with osteopenia or osteoporosis and a higher risk of breast cancer who have minimal to no vasomotor symptoms or genitourinary syndrome of menopause are good candidates for raloxifene. Raloxifene is also a good choice for women who have genitourinary syndrome of menopause treated with local vaginal estrogen. Raloxifene has no effect on vasomotor symptoms or genitourinary syndrome of menopause.
OSPEMIFENE: FOR GENITOURINARY SYNDROME OF MENOPAUSE
Although ospemifene does not have the steroid structure of estrogen, it acts as an estrogen agonist specifically in the vaginal mucosa and an antagonist in other tissues.28 It has been shown on Papanicolaou smears to reduce the number of parabasal cells and increase the number of intermediate and superficial cells after 3 months of treatment.29
Ospemifene 60 mg taken orally with food is approved by the FDA to treat genitourinary syndrome of menopause.
Why ospemifene is needed
First-line treatment options for genitourinary syndrome of menopause include over-the-counter lubricants. However, there is no evidence that these products reverse vaginal atrophy,30 and many women report no relief of symptoms with them.
While various local estrogen preparations positively affect genitourinary syndrome of menopause, some of them can be messy, which can limit-long term adherence.
In one of the largest surveys on genitourinary syndrome of menopause (the REVIVE survey—the Real Women’s View of Treatment Options for Menopausal Vaginal Changes29), 59% of women reported that their vaginal symptoms negatively affected sexual activity. The problem affects not only the patient but also her sexual partner.31 Another large study showed that 38% of women and 39% of male partners reported that it had a worse-than-expected impact on their intimate relationships.31
Genitourinary syndrome of menopause also makes pelvic examinations difficult, may worsen or exacerbate cystitis, and may increase urinary tract infections.
Trials of ospemifene for genitourinary syndrome of menopause
To date, 3 randomized, double-blind clinical trials have demonstrated ospemifene 60 mg to be superior to placebo in treating genitourinary syndrome of menopause. Two were short-term (12-week) and showed significant positive changes in the percent of superficial cells, vaginal pH (lower is better), and number of parabasal cells, along with improvements in the Likert rating of both vaginal dryness and dyspareunia.32,33
A long-term (52-week) randomized placebo-controlled trial compared ospemifene and placebo and showed significant improvement in vaginal maturation index and pH at weeks 12 and 52.34 Other outcome measures included petechiae, pallor, friability, erythema, and dryness, all of which improved from baseline (P < .001). At the end of the trial, 80% of the patients who received ospemifene had no vaginal atrophy.
No serious adverse events were noted in any of the clinical trials to date, and a systemic review and meta-analysis demonstrated ospemifene to be safe and efficacious.35 The most frequently reported reasons for discontinuation were hot flashes, vaginal discharge, muscle spasms, and hyperhidrosis, but the rates of these effects were similar to those with placebo.
Trial of ospemifene’s effect on bone turnover
As an estrogen receptor agonist in bone, ospemifene decreases the levels of bone turnover markers in postmenopausal women.36 A study found ospemifene to be about as effective as raloxifene in suppressing bone turnover,37 but ospemifene does not carry FDA approval for preventing or treating osteoporosis.
Other effects
In experiments in rats, the incidence of breast cancer appears to be lower with ospemifene, and the higher the dose, the lower the incidence.38
Ospemifene also has antagonistic effects on uterine tissue, and no cases of endometrial hyperplasia or carcinoma have been reported in short-term or long-term studies.35
Ospemifene has no effect however on vasomotor symptoms and may in fact worsen vasomotor symptoms in women suffering with hot flashes and night sweats. Further investigation into its long-term safety and effects on breast tissue and bone would provide more insight.
Ideal candidates for ospemifene
Ospemifene could help postmenopausal women with genitourinary syndrome of menopause for whom over-the-counter lubricants fail, who dislike local vaginal estrogen, or who decline systemic hormone therapy, and who do not meet the criteria for treatment with systemic hormone therapy.
CONJUGATED ESTROGENS AND BAZEDOXIFENE COMBINATION
A combination agent consisting of conjugated estrogens 0.45 mg plus bazedoxifene 20 mg has been approved by the FDA for treating moderate to severe vasomotor symptoms associated with menopause and also for preventing postmenopausal osteoporosis in women who have an intact uterus.
Trials of estrogen-bazedoxifene for vasomotor symptoms
The Selective Estrogen Menopause and Response to Therapy (SMART) trials39,40 were a series of randomized, double-blind, placebo-controlled phase 3 studies evaluating the efficacy and safety of the estrogen-bazedoxifene combination in postmenopausal women.
The SMART-2 trial39 evaluated the combination of conjugated estrogens (either 0.45 mg or 0.625) plus bazedoxifene 20 mg and found both dosages significantly reduced the number and severity of hot flashes at weeks 4 and 12 (P < .001). At week 12, the combination with 0.45 mg of estrogen reduced vasomotor symptoms from baseline by 74% (10.3 hot flashes per week at baseline vs 2.8 at week 12); the combination with 0.625 mg of estrogen reduced vasomotor symptoms by 80% (10.4 vs 2.4 flashes); and placebo reduced them by 51% (10.5 vs 5.4 flashes).
For bone density. The SMART-1 trial40 showed that the estrogen-bazedoxifene combination in both estrogen dosages significantly increased mean lumbar spine bone mineral density (P < .001) and total hip bone mineral density (P < .05) from baseline at 12 and 24 months compared with placebo. Increases in density tended to be higher with the higher estrogen dose (0.625 mg), but less with higher doses of bazedoxifene.41 At 24 months, the increase in bone mineral density was even greater than in women treated with raloxifene.42 However, the effect of estrogen-bazedoxifene on the incidence of fractures remains to be studied.
For genitourinary syndrome of menopause. The SMART-3 trial showed that treatment with conjugated estrogens plus bazedoxifene (0.45/20 mg or 0.625/20 mg) was more effective than placebo in increasing the percent of superficial and intermediate cells and decreased the number of parabasal cells at 12 weeks compared with placebo (P < .01).43 Both doses also significantly decreased the mean vaginal pH and improved vaginal dryness.
Patients treated with estrogen-bazedoxifene for a minimum of 12 weeks in a double-blind placebo-controlled study also showed a significant improvement in sexual function and quality-of-life measurements based on 3 well-defined scales, which included ease of lubrication, satisfaction with treatment, control of hot flashes, and sleep parameters.43
Low rates of side effects
To evaluate this regimen’s antagonistic effects on uterine tissue, endometrial hyperplasia was diagnosed by blinded pathologists using endometrial biopsies taken at 6, 12, and 24 months or more if cancer was a suspected diagnosis. At 12 and 24 months of treatment, the incidence of hyperplasia with bazedoxifene 20 or 40 mg at doses of either 0.45 or 0.625 mg of conjugated estrogens was less than 1%, which was similar to placebo rates over the 24 months.44 The lowest dose studied, bazedoxifene 10 mg, did not prevent hyperplasia with conjugated estrogens 0.45 or 0.625 mg, and its use was discontinued.
Rates of amenorrhea with bazedoxifene 20 or 40 mg and conjugated estrogens 0.45 or 0.625 mg were very favorable (83%–93%) and similar to those with placebo.45 For women with continued bleeding on hormone therapy requiring multiple evaluations, or for women who won’t accept the risk of bleeding on hormone therapy, conjugated estrogens and bazedoxifene may be a sustainable option. However, any woman with abnormal bleeding should undergo prompt immediate evaluation.
A typical side effect of estrogen replacement therapy is breast tenderness. For women seeking vasomotor symptom treatment but who experience breast tenderness, this may be a deterrent from continuing hormone therapy. As shown in the SMART-1 and SMART-2 trials,46 conjugated estrogens and bazedoxifene did not cause an increase in breast tenderness, which may enhance medication adherence.
Ideal candidates for conjugated estrogens plus bazedoxifene
This product could help postmenopausal women who have an intact uterus and are suffering with moderate to severe vasomotor symptoms and genitourinary syndrome of menopause who cannot tolerate the side effects of hormone therapy such as bleeding, bloating, or breast tenderness, or who prefer to take an estrogen but without a progestin. It is also ideal for women at higher risk of osteoporosis.
WHO SHOULD GET WHAT?
Not all postmenopausal women have vasomotor symptoms, genitourinary syndrome of menopause, or bone loss. For those who do, standard hormone therapy is an option.
For those who have symptoms and a lower threshold of side effects such as breast tenderness and vaginal bleeding, a combination of an estrogen plus an ERAA (eg, bazedoxifene) is an option.
For women who have no vasomotor symptoms but do have genitourinary syndrome of menopause and don’t want local vaginal treatment, ospemifene is an option.
For women with no vasomotor symptoms but who have bone loss and increased risk of estrogen receptor-positive breast cancer, raloxifene is a good option.
Both premenopausal and postmenopausal women who are at increased risk for breast cancer should be considered for tamoxifen chemoprevention. Postmenopausal women with a uterus at increased risk for breast cancer should be considered for raloxifene, as it has no uterine effect. Raloxifene is not indicated in premenopausal women.
No woman at increased risk of venous thromboembolism is a candidate for ERAA treatment or for oral estrogen. However, the clinician has multiple options to improve quality of life and work productivity and reduce office visits of women at midlife, especially when they are individually assessed and treated.
- Giannini A, Russo E, Mannella P, Simoncini T. Selective steroid receptor modulators in reproductive medicine. Minerva Ginecol 2015; 67:431–455.
- Feldman BM, Voda A, Gronseth E. The prevalence of hot flash and associated variables among perimenopausal women. Res Nurs Health 1985; 8:261–268.
- Versi E, Harvey MA, Cardozo L, Brincat M, Studd JW. Urogenital prolapse and atrophy at menopause: a prevalence study. Int Urogynecol J Pelvic Floor Dysfunct 2001; 12:107–110.
- Hess R, Chang CC, Conigliaro J, McNeil M. Understanding physicians’ attitudes towards hormone therapy. Womens Health Issues 2005; 15:31–38.
- Melton LJ 3rd, Khosla S, Atkinson EJ, O’Fallon WM, Riggs BL. Relationship of bone turnover to bone density and fractures. J Bone Miner Res 1997; 12:1083–1091.
- Sikon A, Thacker HL. Treatment options for menopausal hot flashes. Cleve Clin J Med 2004; 71:578–582.
- Levine JP. Treating menopausal symptoms with a tissue-selective estrogen complex. Gend Med 2011; 8:57–68.
- Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. J Steroid Biochem Mol Biol 2014; 142:142–154.
- Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 1998; 351:1451–1467.
- Davies C, Pan H, Godwin J, et al; Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013; 381:805–816.
- Gray RG, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 2013; (suppl): abstract 5.
- Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet 2003; 361:296–300.
- Cuzick J, Sestak I, Cawthorn S, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol 2015; 16:67–75.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388.
- Chang J, Powles TJ, Ashley SE, et al. The effect of tamoxifen and hormone replacement therapy on serum cholesterol, bone mineral density and coagulation factors in healthy postmenopausal women participating in a randomised, controlled tamoxifen prevention study. Ann Oncol 1996; 7:671–675.
- Herrington DM, Klein KP. Effects of SERMs on important indicators of cardiovascular health: lipoproteins, hemostatic factors and endothelial function. Womens Health Issues 2001; 11:95–102.
- Kedar RP, Bourne TH, Powles TJ, et al. Effects of tamoxifen on uterus and ovaries of postmenopausal women in a randomized breast cancer prevention trial. Lancet 1994; 343:1318–1321.
- Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999; 282:637–645.
- Maricic M, Adachi JD, Sarkar S, Wu W, Wong M, Harper KD. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med 2002; 162:1140–1143.
- Recker RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin 2011; 27:1755–1761.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 1999; 281:2189–2197.
- Martino S, Cauley JA, Barrett-Connor E, et al; CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst 2004; 96:1751–1761.
- Vogel VG, Qu Y, Wong M, Mitchell B, Mershon JL. Incidence of invasive breast cancer in postmenopausal women after discontinuation of long-term raloxifene administration. Clin Breast Cancer 2009; 9:45–50.
- Grady D, Cauley JA, Stock JL, et al. Effect of raloxifene on all-cause mortality. Am J Med 2010; 123:469.e1–e7.
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006; 355:125–137.
- Vogel VG. The NSABP Study of Tamoxifen and Raloxifene (STAR) trial. Expert Rev Anticancer Ther 2009; 9:51–60.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006; 295:2727–2741.
- Barnes KN, Pearce EF, Yancey AM, Forinash AB. Ospemifene in the treatment of vulvovaginal atrophy. Ann Pharmacother 2014; 48:752–757.
- Rutanen EM, Heikkinen J, Halonen K, Komi J, Lammintausta R, Ylikorkala O. Effects of ospemifene, a novel SERM, on hormones, genital tract, climacteric symptoms, and quality of life in postmenopausal women: a double-blind, randomized trial. Menopause 2003; 10:433–439.
- Constantine G, Graham S, Koltun WD, Kingsberg SA. Assessment of ospemifene or lubricants on clinical signs of VVA. J Sex Med 2014; 11:1033–1041.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE survey. J Sex Med 2013; 10:1790–1799.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause 2013; 20:623–630.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause 2010; 17:480–486.
- Goldstein SR, Bachmann GA, Koninckx PR, Lin VH, Portman DJ, Ylikorkala O; Ospemifene Study Group. Ospemifene 12-month safety and efficacy in postmenopausal women with vulvar and vaginal atrophy. Climacteric 2014; 17:173–182.
- Cui Y, Zong H, Yan H, Li N, Zhang Y. The efficacy and safety of ospemifene in treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy: a systematic review and meta-analysis. J Sex Med 2014; 11:487–497.
- Komi J, Heikkinen J, Rutanen EM, Halonen K, Lammintausta R, Ylikorkala O. Effects of ospemifene, a novel SERM, on biochemical markers of bone turnover in healthy postmenopausal women. Gynecal Endocrinol 2004; 18:152–158.
- Komi J, Lankinen KS, DeGregorio M, et al. Effects of ospemifene and raloxifene on biochemical markers of bone turnover in postmenopausal women. J Bone Miner Metab 2006; 24:314–318.
- Wurz GT, Read KC, Marchisano-Karpman C, et al. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol 2005; 97:230–240.
- Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009; 16:1116–1124.
- Pickar JH, Mirkin S. Tissue-selective agents: selective estrogen receptor modulators and the tissue-selective estrogen complex. Menopause Int 2010; 16:121–128.
- Levine JP. Treating menopausal symptoms with a tissue-selective estrogen complex. Gend Med 2011; 8:57–68.
- Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrange complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril 2009; 92:1045–1052.
- Bachmann G, Bobula J, Mirkin S. Effects of bazedoxifene/conjugated estrogens on quality of life in postmenopausal women with symptoms of vulvar/vaginal atrophy. Climacteric 2010; 13:132–140.
- Pickar JH, Yeh IT, Bachmann G, Speroff L. Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril 2009; 92:1018–1024.
- Archer DF, Lewis V, Carr BR, Olivier S, Pickar JH. Bazedoxifene/conjugated estrogens (BZA/CE): incidence of uterine bleeding in postmenopausal women. Fertil Steril 2009: 92:1039–1044.
- Pinkerton JV, Abraham L, Bushmakin AG, et al. Evaluation of the efficacy and safety of bazedoxifene/conjugated estrogens for secondary outcomes including vasomotor symptoms in postmenopausal women by years since menopause in the Selective estrogens, Menopause and Response to Therapy (SMART) trials. J Womens Health (Larchmt) 2014; 23:18–28.
Estrogen receptor agonist-antagonists (ERAAs), previously called selective estrogen receptor modulators (SERMs), have extended the options for treating the various conditions that menopausal women suffer from. These drugs act differently on estrogen receptors in different tissues, stimulating receptors in some tissues but inhibiting them in others. This allows selective inhibition or stimulation of estrogen-like action in various target tissues.1
This article highlights the use of ERAAs to treat menopausal vasomotor symptoms (eg, hot flashes, night sweats), genitourinary syndrome of menopause, osteoporosis, breast cancer (and the risk of breast cancer), and other health concerns unique to women at midlife.
SYMPTOMS OF MENOPAUSE: COMMON AND TROUBLESOME
Vasomotor symptoms such as hot flashes and night sweats are common during perimenopause—most women experience them. They are most frequent during the menopause transition but can persist for 10 years or more afterward.2
Genitourinary syndrome of menopause is also common and often worsens with years after menopause.3 It can lead to dyspareunia and vaginal dryness, which may in turn result in lower libido, vaginismus, and hypoactive sexual desire disorder, problems that often arise at the same time as vaginal dryness and atrophy.4
Osteopenia and osteoporosis. A drop in systemic estrogen leads to a decline in bone mineral density, increasing the risk of fractures.5
ESTROGEN-PROGESTIN TREATMENT: THE GOLD STANDARD, BUT NOT IDEAL
The current gold standard for treating moderate to severe hot flashes is estrogen, available in oral, transdermal, and vaginal formulations.6 Estrogen also has antiresorptive effects on bone and is approved for preventing osteoporosis. Systemic estrogen may also be prescribed for genitourinary syndrome of menopause if local vaginal treatment alone is insufficient.
If women who have an intact uterus receive estrogen, they should also receive a progestin to protect against endometrial hyperplasia and reduce the risk of endometrial cancer.
Despite its status as the gold standard, estrogen-progestin therapy presents challenges. In some women, progestins cause side effects such as breast tenderness, bloating, fatigue, and depression.7 Estrogen-progestin therapy often causes vaginal bleeding, which for some women is troublesome or distressing; bleeding may be the reason for repeated evaluations, can increase anxiety, and can lead to poor adherence with hormonal treatment. Women who carry a higher-than-normal risk of developing breast cancer or fear that taking hormones will lead to breast cancer may show decreased adherence to therapy. Women who have estrogen receptor-positive breast cancer cannot take estrogen.
Individualized options are needed for women who have progestin-related side effects, unwanted vaginal bleeding, or a higher risk of breast cancer.
WELCOME THE ERAAs
An ideal treatment for menopause would relieve vasomotor symptoms and genitourinary syndrome of menopause and increase bone mineral density without causing breast tenderness, vaginal bleeding, or endometrial proliferation.
The “designer estrogens,” or ERAAs, have specific positive effects on the bone, heart, and brain with neutral or antagonist effects on estrogen receptors in other tissues such as the breasts and endometrium.8 While not entirely free of adverse effects, these agents have been developed with the aim of minimizing the most common ones related to estrogen and progestin.
Several ERAAs are currently approved by the US Food and Drug Administration (FDA)for various indications, each having a unique profile. Clomifene was the first agent of this class, and it is still used clinically to induce ovulation. This article highlights subsequently approved agents, ie, tamoxifen, raloxifene, ospemifene, and the combination of conjugated estrogens and bazedoxifene (Table 1).
All ERAAs increase the risk of venous thromboembolism, and therefore none of them should be used in women with known venous thromboembolism or at high risk of it.
TAMOXIFEN: CANCER TREATMENT AND PREVENTION
After clomiphene, tamoxifen was the second ERAA on the market. Although researchers were looking for a new contraceptive drug, they found tamoxifen to be useful as a chemotherapeutic agent for breast cancer. First used in 1971, tamoxifen continues to be one of the most commonly prescribed chemotherapeutic medications today.
The FDA has approved tamoxifen to treat breast cancer as well as to prevent breast cancer in pre- and postmenopausal women at risk. It may also have beneficial effects on bone and on cardiovascular risk factors, but these are not approved uses for it.
Trials of tamoxifen for cancer treatment
The Early Breast Cancer Trialists’ Collaborative Group9 performed a meta-analysis and found that 5 years of adjuvant treatment with tamoxifen is associated with a 26% reduction in mortality and a 47% reduction in breast cancer recurrence at 10 years. In absolute terms, we estimate that 21 women would need to be treated to prevent 1 death and 8 would need to be treated to prevent 1 recurrence.
The ATLAS Trial (Adjuvant Tamoxifen Longer Against Shorter)10 and later the UK ATTOM (Adjuvant Tamoxifen Treatment to Offer More)11 trial confirmed an even greater reduction in recurrence and mortality after a total of 10 years of treatment.
Trials of tamoxifen for cancer prevention
Cuzik et al12 performed a meta-analysis of 4 trials of tamoxifen’s effectiveness in preventing breast cancer for women at elevated risk. The incidence of estrogen receptor-positive breast cancer was 48% lower with tamoxifen use, but there was no effect on estrogen-negative breast cancer. From their data, we estimate that 77 women would need to be treated to prevent 1 case of breast cancer.
The IBIS-I trial (International Breast Cancer Intervention Study I)13 found that, in healthy women at high risk of breast cancer, the benefit of taking tamoxifen for 5 years as preventive treatment persisted long afterward. The investigators estimated that at 20 years of follow-up the risk of breast cancer would be 12.3% in placebo recipients and 7.8% in tamoxifen recipients, a 4.5% absolute risk reduction; number needed to treat (NNT) 22.
Data on tamoxifen and osteoporosis
The Breast Cancer Prevention Trial revealed a 19% reduction in the incidence of osteoporotic fractures with tamoxifen, but the difference was not statistically significant.14 The 1-year rates of fracture in women age 50 and older were 0.727% with placebo and 0.567% with tamoxifen, an absolute difference of 0.151%; therefore, if the effect is real, 662 women age 50 or older would need to be treated for 1 year to prevent 1 fracture. Tamoxifen is not FDA-approved to treat osteoporosis.
Data on tamoxifen and cardiovascular risk reduction
Chang et al,15 in a study in women at risk of breast cancer, incidentally found that tamoxifen was associated with a 13% reduction in total cholesterol compared with placebo.
Herrington and Klein,16 in a systematic review, noted similar findings in multiple studies of tamoxifen, with decreases in total cholesterol ranging from 7% to 17% and decreases in low-density lipoprotein cholesterol ranging from 10% to 28%. However, they found no change in high-density lipoprotein cholesterol concentrations or in the cardiovascular mortality rate.
The ATLAS trial10 revealed a relative risk reduction of 0.76 (95% confidence interval [CI] 0.60–0.95, P = .02) in ischemic heart disease for women who took tamoxifen for 10 years compared with 5 years. We calculate that ischemic heart disease occurred in 163 (2.5%) of 6,440 women who took tamoxifen for 5 years compared with 127 (1.9%) of 6,454 women who took it for 10 years, a 0.6% absolute risk reduction, NNT = 167.
Adverse effects of tamoxifen
Uterine neoplasia. Women taking tamoxifen have a 2.5-fold increased risk of endometrial cancer.14 Tamoxifen also increases the risk of benign uterine disease such as endometrial hyperplasia and polyps. As many as 39% of women taking tamoxifen will have evidence of benign uterine changes on pathology.17 Other adverse effects:
Venous thromboembolism (the risk of pulmonary embolism is increased approximately threefold14)
Cataracts (there is a slight increase in cataract diagnosis in tamoxifen users)
Vasomotor symptoms, which limit the use of tamoxifen in many women.
Ideal candidate for tamoxifen
The ideal candidate for tamoxifen is a woman with breast cancer that is estrogen receptor-positive and who has a history of osteopenia or osteoporosis and no risk factors for venous thromboembolism.
RALOXIFENE: FOR OSTEOPOROSIS AND FOR CANCER PREVENTION
Raloxifene, a second-generation ERAA, was first approved for preventing and treating osteoporosis and later for reducing the risk of invasive estrogen receptor-positive breast cancer in postmenopausal women.
Trials of raloxifene for osteoporosis
The MORE trial (Multiple Outcomes of Raloxifene)18 was a large multicenter randomized double-blind study. Raloxifene recipients showed a significant increase in bone mineral density in the lumbar spine and femoral neck at year 3 (P < .001) compared with those receiving placebo. Even after only 1 year of treatment, raloxifene significantly reduced the risk of new fractures, despite only modest gains in bone mineral density. After 3 years of treatment, new clinical vertebral fractures had occurred in 3.5% of the placebo group compared with 2.1% of the group receiving raloxifene 60 mg.19 Relative risk reductions were similar in women who had already had a clinical vertebral fracture at baseline, whose absolute risk is higher. However, no significant effect was seen on the incidence of hip or nonvertebral fractures.
The CORE trial (Continuing Outcomes Relevant to Raloxifene)20 extended the treatment of the women enrolled in the MORE trial another 4 years and found that the benefit of raloxifene with regard to bone mineral density persisted with continued use.
Trials of raloxifene for breast cancer prevention
The MORE trial,21 in postmenopausal women with osteoporosis included breast cancer as a secondary end point, and raloxifene was shown to decrease the incidence of invasive breast cancer. At a median of 40 months, invasive breast cancer had arisen in 13 (0.25%) of the 5,129 women assigned to raloxifene and 27 (1.0%) of the 2,576 women assigned to placebo. The authors calculated that 126 women would need to be treated to prevent 1 case of breast cancer.
The CORE trial,22 as noted, extended the treatment of the women enrolled in the MORE trial another 4 years. The risk of any invasive breast cancer in postmenopausal women with osteoporosis was significantly reduced by 59% after 8 years, and the risk of estrogen receptor-positive invasive breast cancer was reduced by 66%.
There is evidence that raloxifene’s effect on breast cancer risk persists after discontinuation of use.23
Does raloxifene reduce mortality?
Grady et al24 studied the effect of raloxifene on all-cause mortality in a pooled analysis of mortality data from the MORE, CORE, and Raloxifene Use for the Heart (RUTH)25 trials. In older postmenopausal women, the rate of all-cause mortality was 8.65% in those taking placebo compared with 7.88% in those taking raloxifene 60 mg daily—10% lower. The mechanism behind the lower mortality rate is unclear, and Grady et al recommend that the finding be interpreted with caution.
Trials of raloxifene for heart protection
The RUTH trial25 was a 5.6-year study undertaken to study the effects of raloxifene on coronary outcomes and invasive breast cancer in postmenopausal women. Results were mixed. Active treatment:
- Did not significantly affect the risk of coronary artery disease compared with placebo
- Significantly decreased the risk of invasive breast cancer
- Significantly decreased the risk of clinical vertebral fractures
- Increased the risk of fatal stroke (59 vs 39 events, hazard ratio 1.49, 95% CI 1.00–2.24) and venous thromboembolism (103 vs 71 events, hazard ratio 1.44, 95% CI 1.06–1.95).
The STAR trial (Study of Tamoxifen and Raloxifene)26,27 compared raloxifene and tamoxifen in postmenopausal women at increased risk of breast cancer. Women were randomized to receive either tamoxifen 20 mg or raloxifene 60 mg for 5 years. Results:
- No difference in the number of new cases of invasive breast cancer between the groups
- Fewer cases of noninvasive breast cancer in the tamoxifen group, but the difference was not statistically significant
- Fewer cases of uterine cancer in the raloxifene group, annual incidence rates 0.125% vs 0.199%, absolute risk reduction 0.74%, NNT 1,351, relative risk with raloxifene 0.62, 95% CI 0.30–0.50
- Fewer thromboembolic events with raloxifene
- Fewer cataracts with raloxifene.
Adverse effects of raloxifene
Raloxifene increases the risk of venous thromboembolism and stroke in women at high risk of coronary artery disease.19
Ideal candidates for raloxifene
Postmenopausal women with osteopenia or osteoporosis and a higher risk of breast cancer who have minimal to no vasomotor symptoms or genitourinary syndrome of menopause are good candidates for raloxifene. Raloxifene is also a good choice for women who have genitourinary syndrome of menopause treated with local vaginal estrogen. Raloxifene has no effect on vasomotor symptoms or genitourinary syndrome of menopause.
OSPEMIFENE: FOR GENITOURINARY SYNDROME OF MENOPAUSE
Although ospemifene does not have the steroid structure of estrogen, it acts as an estrogen agonist specifically in the vaginal mucosa and an antagonist in other tissues.28 It has been shown on Papanicolaou smears to reduce the number of parabasal cells and increase the number of intermediate and superficial cells after 3 months of treatment.29
Ospemifene 60 mg taken orally with food is approved by the FDA to treat genitourinary syndrome of menopause.
Why ospemifene is needed
First-line treatment options for genitourinary syndrome of menopause include over-the-counter lubricants. However, there is no evidence that these products reverse vaginal atrophy,30 and many women report no relief of symptoms with them.
While various local estrogen preparations positively affect genitourinary syndrome of menopause, some of them can be messy, which can limit-long term adherence.
In one of the largest surveys on genitourinary syndrome of menopause (the REVIVE survey—the Real Women’s View of Treatment Options for Menopausal Vaginal Changes29), 59% of women reported that their vaginal symptoms negatively affected sexual activity. The problem affects not only the patient but also her sexual partner.31 Another large study showed that 38% of women and 39% of male partners reported that it had a worse-than-expected impact on their intimate relationships.31
Genitourinary syndrome of menopause also makes pelvic examinations difficult, may worsen or exacerbate cystitis, and may increase urinary tract infections.
Trials of ospemifene for genitourinary syndrome of menopause
To date, 3 randomized, double-blind clinical trials have demonstrated ospemifene 60 mg to be superior to placebo in treating genitourinary syndrome of menopause. Two were short-term (12-week) and showed significant positive changes in the percent of superficial cells, vaginal pH (lower is better), and number of parabasal cells, along with improvements in the Likert rating of both vaginal dryness and dyspareunia.32,33
A long-term (52-week) randomized placebo-controlled trial compared ospemifene and placebo and showed significant improvement in vaginal maturation index and pH at weeks 12 and 52.34 Other outcome measures included petechiae, pallor, friability, erythema, and dryness, all of which improved from baseline (P < .001). At the end of the trial, 80% of the patients who received ospemifene had no vaginal atrophy.
No serious adverse events were noted in any of the clinical trials to date, and a systemic review and meta-analysis demonstrated ospemifene to be safe and efficacious.35 The most frequently reported reasons for discontinuation were hot flashes, vaginal discharge, muscle spasms, and hyperhidrosis, but the rates of these effects were similar to those with placebo.
Trial of ospemifene’s effect on bone turnover
As an estrogen receptor agonist in bone, ospemifene decreases the levels of bone turnover markers in postmenopausal women.36 A study found ospemifene to be about as effective as raloxifene in suppressing bone turnover,37 but ospemifene does not carry FDA approval for preventing or treating osteoporosis.
Other effects
In experiments in rats, the incidence of breast cancer appears to be lower with ospemifene, and the higher the dose, the lower the incidence.38
Ospemifene also has antagonistic effects on uterine tissue, and no cases of endometrial hyperplasia or carcinoma have been reported in short-term or long-term studies.35
Ospemifene has no effect however on vasomotor symptoms and may in fact worsen vasomotor symptoms in women suffering with hot flashes and night sweats. Further investigation into its long-term safety and effects on breast tissue and bone would provide more insight.
Ideal candidates for ospemifene
Ospemifene could help postmenopausal women with genitourinary syndrome of menopause for whom over-the-counter lubricants fail, who dislike local vaginal estrogen, or who decline systemic hormone therapy, and who do not meet the criteria for treatment with systemic hormone therapy.
CONJUGATED ESTROGENS AND BAZEDOXIFENE COMBINATION
A combination agent consisting of conjugated estrogens 0.45 mg plus bazedoxifene 20 mg has been approved by the FDA for treating moderate to severe vasomotor symptoms associated with menopause and also for preventing postmenopausal osteoporosis in women who have an intact uterus.
Trials of estrogen-bazedoxifene for vasomotor symptoms
The Selective Estrogen Menopause and Response to Therapy (SMART) trials39,40 were a series of randomized, double-blind, placebo-controlled phase 3 studies evaluating the efficacy and safety of the estrogen-bazedoxifene combination in postmenopausal women.
The SMART-2 trial39 evaluated the combination of conjugated estrogens (either 0.45 mg or 0.625) plus bazedoxifene 20 mg and found both dosages significantly reduced the number and severity of hot flashes at weeks 4 and 12 (P < .001). At week 12, the combination with 0.45 mg of estrogen reduced vasomotor symptoms from baseline by 74% (10.3 hot flashes per week at baseline vs 2.8 at week 12); the combination with 0.625 mg of estrogen reduced vasomotor symptoms by 80% (10.4 vs 2.4 flashes); and placebo reduced them by 51% (10.5 vs 5.4 flashes).
For bone density. The SMART-1 trial40 showed that the estrogen-bazedoxifene combination in both estrogen dosages significantly increased mean lumbar spine bone mineral density (P < .001) and total hip bone mineral density (P < .05) from baseline at 12 and 24 months compared with placebo. Increases in density tended to be higher with the higher estrogen dose (0.625 mg), but less with higher doses of bazedoxifene.41 At 24 months, the increase in bone mineral density was even greater than in women treated with raloxifene.42 However, the effect of estrogen-bazedoxifene on the incidence of fractures remains to be studied.
For genitourinary syndrome of menopause. The SMART-3 trial showed that treatment with conjugated estrogens plus bazedoxifene (0.45/20 mg or 0.625/20 mg) was more effective than placebo in increasing the percent of superficial and intermediate cells and decreased the number of parabasal cells at 12 weeks compared with placebo (P < .01).43 Both doses also significantly decreased the mean vaginal pH and improved vaginal dryness.
Patients treated with estrogen-bazedoxifene for a minimum of 12 weeks in a double-blind placebo-controlled study also showed a significant improvement in sexual function and quality-of-life measurements based on 3 well-defined scales, which included ease of lubrication, satisfaction with treatment, control of hot flashes, and sleep parameters.43
Low rates of side effects
To evaluate this regimen’s antagonistic effects on uterine tissue, endometrial hyperplasia was diagnosed by blinded pathologists using endometrial biopsies taken at 6, 12, and 24 months or more if cancer was a suspected diagnosis. At 12 and 24 months of treatment, the incidence of hyperplasia with bazedoxifene 20 or 40 mg at doses of either 0.45 or 0.625 mg of conjugated estrogens was less than 1%, which was similar to placebo rates over the 24 months.44 The lowest dose studied, bazedoxifene 10 mg, did not prevent hyperplasia with conjugated estrogens 0.45 or 0.625 mg, and its use was discontinued.
Rates of amenorrhea with bazedoxifene 20 or 40 mg and conjugated estrogens 0.45 or 0.625 mg were very favorable (83%–93%) and similar to those with placebo.45 For women with continued bleeding on hormone therapy requiring multiple evaluations, or for women who won’t accept the risk of bleeding on hormone therapy, conjugated estrogens and bazedoxifene may be a sustainable option. However, any woman with abnormal bleeding should undergo prompt immediate evaluation.
A typical side effect of estrogen replacement therapy is breast tenderness. For women seeking vasomotor symptom treatment but who experience breast tenderness, this may be a deterrent from continuing hormone therapy. As shown in the SMART-1 and SMART-2 trials,46 conjugated estrogens and bazedoxifene did not cause an increase in breast tenderness, which may enhance medication adherence.
Ideal candidates for conjugated estrogens plus bazedoxifene
This product could help postmenopausal women who have an intact uterus and are suffering with moderate to severe vasomotor symptoms and genitourinary syndrome of menopause who cannot tolerate the side effects of hormone therapy such as bleeding, bloating, or breast tenderness, or who prefer to take an estrogen but without a progestin. It is also ideal for women at higher risk of osteoporosis.
WHO SHOULD GET WHAT?
Not all postmenopausal women have vasomotor symptoms, genitourinary syndrome of menopause, or bone loss. For those who do, standard hormone therapy is an option.
For those who have symptoms and a lower threshold of side effects such as breast tenderness and vaginal bleeding, a combination of an estrogen plus an ERAA (eg, bazedoxifene) is an option.
For women who have no vasomotor symptoms but do have genitourinary syndrome of menopause and don’t want local vaginal treatment, ospemifene is an option.
For women with no vasomotor symptoms but who have bone loss and increased risk of estrogen receptor-positive breast cancer, raloxifene is a good option.
Both premenopausal and postmenopausal women who are at increased risk for breast cancer should be considered for tamoxifen chemoprevention. Postmenopausal women with a uterus at increased risk for breast cancer should be considered for raloxifene, as it has no uterine effect. Raloxifene is not indicated in premenopausal women.
No woman at increased risk of venous thromboembolism is a candidate for ERAA treatment or for oral estrogen. However, the clinician has multiple options to improve quality of life and work productivity and reduce office visits of women at midlife, especially when they are individually assessed and treated.
Estrogen receptor agonist-antagonists (ERAAs), previously called selective estrogen receptor modulators (SERMs), have extended the options for treating the various conditions that menopausal women suffer from. These drugs act differently on estrogen receptors in different tissues, stimulating receptors in some tissues but inhibiting them in others. This allows selective inhibition or stimulation of estrogen-like action in various target tissues.1
This article highlights the use of ERAAs to treat menopausal vasomotor symptoms (eg, hot flashes, night sweats), genitourinary syndrome of menopause, osteoporosis, breast cancer (and the risk of breast cancer), and other health concerns unique to women at midlife.
SYMPTOMS OF MENOPAUSE: COMMON AND TROUBLESOME
Vasomotor symptoms such as hot flashes and night sweats are common during perimenopause—most women experience them. They are most frequent during the menopause transition but can persist for 10 years or more afterward.2
Genitourinary syndrome of menopause is also common and often worsens with years after menopause.3 It can lead to dyspareunia and vaginal dryness, which may in turn result in lower libido, vaginismus, and hypoactive sexual desire disorder, problems that often arise at the same time as vaginal dryness and atrophy.4
Osteopenia and osteoporosis. A drop in systemic estrogen leads to a decline in bone mineral density, increasing the risk of fractures.5
ESTROGEN-PROGESTIN TREATMENT: THE GOLD STANDARD, BUT NOT IDEAL
The current gold standard for treating moderate to severe hot flashes is estrogen, available in oral, transdermal, and vaginal formulations.6 Estrogen also has antiresorptive effects on bone and is approved for preventing osteoporosis. Systemic estrogen may also be prescribed for genitourinary syndrome of menopause if local vaginal treatment alone is insufficient.
If women who have an intact uterus receive estrogen, they should also receive a progestin to protect against endometrial hyperplasia and reduce the risk of endometrial cancer.
Despite its status as the gold standard, estrogen-progestin therapy presents challenges. In some women, progestins cause side effects such as breast tenderness, bloating, fatigue, and depression.7 Estrogen-progestin therapy often causes vaginal bleeding, which for some women is troublesome or distressing; bleeding may be the reason for repeated evaluations, can increase anxiety, and can lead to poor adherence with hormonal treatment. Women who carry a higher-than-normal risk of developing breast cancer or fear that taking hormones will lead to breast cancer may show decreased adherence to therapy. Women who have estrogen receptor-positive breast cancer cannot take estrogen.
Individualized options are needed for women who have progestin-related side effects, unwanted vaginal bleeding, or a higher risk of breast cancer.
WELCOME THE ERAAs
An ideal treatment for menopause would relieve vasomotor symptoms and genitourinary syndrome of menopause and increase bone mineral density without causing breast tenderness, vaginal bleeding, or endometrial proliferation.
The “designer estrogens,” or ERAAs, have specific positive effects on the bone, heart, and brain with neutral or antagonist effects on estrogen receptors in other tissues such as the breasts and endometrium.8 While not entirely free of adverse effects, these agents have been developed with the aim of minimizing the most common ones related to estrogen and progestin.
Several ERAAs are currently approved by the US Food and Drug Administration (FDA)for various indications, each having a unique profile. Clomifene was the first agent of this class, and it is still used clinically to induce ovulation. This article highlights subsequently approved agents, ie, tamoxifen, raloxifene, ospemifene, and the combination of conjugated estrogens and bazedoxifene (Table 1).
All ERAAs increase the risk of venous thromboembolism, and therefore none of them should be used in women with known venous thromboembolism or at high risk of it.
TAMOXIFEN: CANCER TREATMENT AND PREVENTION
After clomiphene, tamoxifen was the second ERAA on the market. Although researchers were looking for a new contraceptive drug, they found tamoxifen to be useful as a chemotherapeutic agent for breast cancer. First used in 1971, tamoxifen continues to be one of the most commonly prescribed chemotherapeutic medications today.
The FDA has approved tamoxifen to treat breast cancer as well as to prevent breast cancer in pre- and postmenopausal women at risk. It may also have beneficial effects on bone and on cardiovascular risk factors, but these are not approved uses for it.
Trials of tamoxifen for cancer treatment
The Early Breast Cancer Trialists’ Collaborative Group9 performed a meta-analysis and found that 5 years of adjuvant treatment with tamoxifen is associated with a 26% reduction in mortality and a 47% reduction in breast cancer recurrence at 10 years. In absolute terms, we estimate that 21 women would need to be treated to prevent 1 death and 8 would need to be treated to prevent 1 recurrence.
The ATLAS Trial (Adjuvant Tamoxifen Longer Against Shorter)10 and later the UK ATTOM (Adjuvant Tamoxifen Treatment to Offer More)11 trial confirmed an even greater reduction in recurrence and mortality after a total of 10 years of treatment.
Trials of tamoxifen for cancer prevention
Cuzik et al12 performed a meta-analysis of 4 trials of tamoxifen’s effectiveness in preventing breast cancer for women at elevated risk. The incidence of estrogen receptor-positive breast cancer was 48% lower with tamoxifen use, but there was no effect on estrogen-negative breast cancer. From their data, we estimate that 77 women would need to be treated to prevent 1 case of breast cancer.
The IBIS-I trial (International Breast Cancer Intervention Study I)13 found that, in healthy women at high risk of breast cancer, the benefit of taking tamoxifen for 5 years as preventive treatment persisted long afterward. The investigators estimated that at 20 years of follow-up the risk of breast cancer would be 12.3% in placebo recipients and 7.8% in tamoxifen recipients, a 4.5% absolute risk reduction; number needed to treat (NNT) 22.
Data on tamoxifen and osteoporosis
The Breast Cancer Prevention Trial revealed a 19% reduction in the incidence of osteoporotic fractures with tamoxifen, but the difference was not statistically significant.14 The 1-year rates of fracture in women age 50 and older were 0.727% with placebo and 0.567% with tamoxifen, an absolute difference of 0.151%; therefore, if the effect is real, 662 women age 50 or older would need to be treated for 1 year to prevent 1 fracture. Tamoxifen is not FDA-approved to treat osteoporosis.
Data on tamoxifen and cardiovascular risk reduction
Chang et al,15 in a study in women at risk of breast cancer, incidentally found that tamoxifen was associated with a 13% reduction in total cholesterol compared with placebo.
Herrington and Klein,16 in a systematic review, noted similar findings in multiple studies of tamoxifen, with decreases in total cholesterol ranging from 7% to 17% and decreases in low-density lipoprotein cholesterol ranging from 10% to 28%. However, they found no change in high-density lipoprotein cholesterol concentrations or in the cardiovascular mortality rate.
The ATLAS trial10 revealed a relative risk reduction of 0.76 (95% confidence interval [CI] 0.60–0.95, P = .02) in ischemic heart disease for women who took tamoxifen for 10 years compared with 5 years. We calculate that ischemic heart disease occurred in 163 (2.5%) of 6,440 women who took tamoxifen for 5 years compared with 127 (1.9%) of 6,454 women who took it for 10 years, a 0.6% absolute risk reduction, NNT = 167.
Adverse effects of tamoxifen
Uterine neoplasia. Women taking tamoxifen have a 2.5-fold increased risk of endometrial cancer.14 Tamoxifen also increases the risk of benign uterine disease such as endometrial hyperplasia and polyps. As many as 39% of women taking tamoxifen will have evidence of benign uterine changes on pathology.17 Other adverse effects:
Venous thromboembolism (the risk of pulmonary embolism is increased approximately threefold14)
Cataracts (there is a slight increase in cataract diagnosis in tamoxifen users)
Vasomotor symptoms, which limit the use of tamoxifen in many women.
Ideal candidate for tamoxifen
The ideal candidate for tamoxifen is a woman with breast cancer that is estrogen receptor-positive and who has a history of osteopenia or osteoporosis and no risk factors for venous thromboembolism.
RALOXIFENE: FOR OSTEOPOROSIS AND FOR CANCER PREVENTION
Raloxifene, a second-generation ERAA, was first approved for preventing and treating osteoporosis and later for reducing the risk of invasive estrogen receptor-positive breast cancer in postmenopausal women.
Trials of raloxifene for osteoporosis
The MORE trial (Multiple Outcomes of Raloxifene)18 was a large multicenter randomized double-blind study. Raloxifene recipients showed a significant increase in bone mineral density in the lumbar spine and femoral neck at year 3 (P < .001) compared with those receiving placebo. Even after only 1 year of treatment, raloxifene significantly reduced the risk of new fractures, despite only modest gains in bone mineral density. After 3 years of treatment, new clinical vertebral fractures had occurred in 3.5% of the placebo group compared with 2.1% of the group receiving raloxifene 60 mg.19 Relative risk reductions were similar in women who had already had a clinical vertebral fracture at baseline, whose absolute risk is higher. However, no significant effect was seen on the incidence of hip or nonvertebral fractures.
The CORE trial (Continuing Outcomes Relevant to Raloxifene)20 extended the treatment of the women enrolled in the MORE trial another 4 years and found that the benefit of raloxifene with regard to bone mineral density persisted with continued use.
Trials of raloxifene for breast cancer prevention
The MORE trial,21 in postmenopausal women with osteoporosis included breast cancer as a secondary end point, and raloxifene was shown to decrease the incidence of invasive breast cancer. At a median of 40 months, invasive breast cancer had arisen in 13 (0.25%) of the 5,129 women assigned to raloxifene and 27 (1.0%) of the 2,576 women assigned to placebo. The authors calculated that 126 women would need to be treated to prevent 1 case of breast cancer.
The CORE trial,22 as noted, extended the treatment of the women enrolled in the MORE trial another 4 years. The risk of any invasive breast cancer in postmenopausal women with osteoporosis was significantly reduced by 59% after 8 years, and the risk of estrogen receptor-positive invasive breast cancer was reduced by 66%.
There is evidence that raloxifene’s effect on breast cancer risk persists after discontinuation of use.23
Does raloxifene reduce mortality?
Grady et al24 studied the effect of raloxifene on all-cause mortality in a pooled analysis of mortality data from the MORE, CORE, and Raloxifene Use for the Heart (RUTH)25 trials. In older postmenopausal women, the rate of all-cause mortality was 8.65% in those taking placebo compared with 7.88% in those taking raloxifene 60 mg daily—10% lower. The mechanism behind the lower mortality rate is unclear, and Grady et al recommend that the finding be interpreted with caution.
Trials of raloxifene for heart protection
The RUTH trial25 was a 5.6-year study undertaken to study the effects of raloxifene on coronary outcomes and invasive breast cancer in postmenopausal women. Results were mixed. Active treatment:
- Did not significantly affect the risk of coronary artery disease compared with placebo
- Significantly decreased the risk of invasive breast cancer
- Significantly decreased the risk of clinical vertebral fractures
- Increased the risk of fatal stroke (59 vs 39 events, hazard ratio 1.49, 95% CI 1.00–2.24) and venous thromboembolism (103 vs 71 events, hazard ratio 1.44, 95% CI 1.06–1.95).
The STAR trial (Study of Tamoxifen and Raloxifene)26,27 compared raloxifene and tamoxifen in postmenopausal women at increased risk of breast cancer. Women were randomized to receive either tamoxifen 20 mg or raloxifene 60 mg for 5 years. Results:
- No difference in the number of new cases of invasive breast cancer between the groups
- Fewer cases of noninvasive breast cancer in the tamoxifen group, but the difference was not statistically significant
- Fewer cases of uterine cancer in the raloxifene group, annual incidence rates 0.125% vs 0.199%, absolute risk reduction 0.74%, NNT 1,351, relative risk with raloxifene 0.62, 95% CI 0.30–0.50
- Fewer thromboembolic events with raloxifene
- Fewer cataracts with raloxifene.
Adverse effects of raloxifene
Raloxifene increases the risk of venous thromboembolism and stroke in women at high risk of coronary artery disease.19
Ideal candidates for raloxifene
Postmenopausal women with osteopenia or osteoporosis and a higher risk of breast cancer who have minimal to no vasomotor symptoms or genitourinary syndrome of menopause are good candidates for raloxifene. Raloxifene is also a good choice for women who have genitourinary syndrome of menopause treated with local vaginal estrogen. Raloxifene has no effect on vasomotor symptoms or genitourinary syndrome of menopause.
OSPEMIFENE: FOR GENITOURINARY SYNDROME OF MENOPAUSE
Although ospemifene does not have the steroid structure of estrogen, it acts as an estrogen agonist specifically in the vaginal mucosa and an antagonist in other tissues.28 It has been shown on Papanicolaou smears to reduce the number of parabasal cells and increase the number of intermediate and superficial cells after 3 months of treatment.29
Ospemifene 60 mg taken orally with food is approved by the FDA to treat genitourinary syndrome of menopause.
Why ospemifene is needed
First-line treatment options for genitourinary syndrome of menopause include over-the-counter lubricants. However, there is no evidence that these products reverse vaginal atrophy,30 and many women report no relief of symptoms with them.
While various local estrogen preparations positively affect genitourinary syndrome of menopause, some of them can be messy, which can limit-long term adherence.
In one of the largest surveys on genitourinary syndrome of menopause (the REVIVE survey—the Real Women’s View of Treatment Options for Menopausal Vaginal Changes29), 59% of women reported that their vaginal symptoms negatively affected sexual activity. The problem affects not only the patient but also her sexual partner.31 Another large study showed that 38% of women and 39% of male partners reported that it had a worse-than-expected impact on their intimate relationships.31
Genitourinary syndrome of menopause also makes pelvic examinations difficult, may worsen or exacerbate cystitis, and may increase urinary tract infections.
Trials of ospemifene for genitourinary syndrome of menopause
To date, 3 randomized, double-blind clinical trials have demonstrated ospemifene 60 mg to be superior to placebo in treating genitourinary syndrome of menopause. Two were short-term (12-week) and showed significant positive changes in the percent of superficial cells, vaginal pH (lower is better), and number of parabasal cells, along with improvements in the Likert rating of both vaginal dryness and dyspareunia.32,33
A long-term (52-week) randomized placebo-controlled trial compared ospemifene and placebo and showed significant improvement in vaginal maturation index and pH at weeks 12 and 52.34 Other outcome measures included petechiae, pallor, friability, erythema, and dryness, all of which improved from baseline (P < .001). At the end of the trial, 80% of the patients who received ospemifene had no vaginal atrophy.
No serious adverse events were noted in any of the clinical trials to date, and a systemic review and meta-analysis demonstrated ospemifene to be safe and efficacious.35 The most frequently reported reasons for discontinuation were hot flashes, vaginal discharge, muscle spasms, and hyperhidrosis, but the rates of these effects were similar to those with placebo.
Trial of ospemifene’s effect on bone turnover
As an estrogen receptor agonist in bone, ospemifene decreases the levels of bone turnover markers in postmenopausal women.36 A study found ospemifene to be about as effective as raloxifene in suppressing bone turnover,37 but ospemifene does not carry FDA approval for preventing or treating osteoporosis.
Other effects
In experiments in rats, the incidence of breast cancer appears to be lower with ospemifene, and the higher the dose, the lower the incidence.38
Ospemifene also has antagonistic effects on uterine tissue, and no cases of endometrial hyperplasia or carcinoma have been reported in short-term or long-term studies.35
Ospemifene has no effect however on vasomotor symptoms and may in fact worsen vasomotor symptoms in women suffering with hot flashes and night sweats. Further investigation into its long-term safety and effects on breast tissue and bone would provide more insight.
Ideal candidates for ospemifene
Ospemifene could help postmenopausal women with genitourinary syndrome of menopause for whom over-the-counter lubricants fail, who dislike local vaginal estrogen, or who decline systemic hormone therapy, and who do not meet the criteria for treatment with systemic hormone therapy.
CONJUGATED ESTROGENS AND BAZEDOXIFENE COMBINATION
A combination agent consisting of conjugated estrogens 0.45 mg plus bazedoxifene 20 mg has been approved by the FDA for treating moderate to severe vasomotor symptoms associated with menopause and also for preventing postmenopausal osteoporosis in women who have an intact uterus.
Trials of estrogen-bazedoxifene for vasomotor symptoms
The Selective Estrogen Menopause and Response to Therapy (SMART) trials39,40 were a series of randomized, double-blind, placebo-controlled phase 3 studies evaluating the efficacy and safety of the estrogen-bazedoxifene combination in postmenopausal women.
The SMART-2 trial39 evaluated the combination of conjugated estrogens (either 0.45 mg or 0.625) plus bazedoxifene 20 mg and found both dosages significantly reduced the number and severity of hot flashes at weeks 4 and 12 (P < .001). At week 12, the combination with 0.45 mg of estrogen reduced vasomotor symptoms from baseline by 74% (10.3 hot flashes per week at baseline vs 2.8 at week 12); the combination with 0.625 mg of estrogen reduced vasomotor symptoms by 80% (10.4 vs 2.4 flashes); and placebo reduced them by 51% (10.5 vs 5.4 flashes).
For bone density. The SMART-1 trial40 showed that the estrogen-bazedoxifene combination in both estrogen dosages significantly increased mean lumbar spine bone mineral density (P < .001) and total hip bone mineral density (P < .05) from baseline at 12 and 24 months compared with placebo. Increases in density tended to be higher with the higher estrogen dose (0.625 mg), but less with higher doses of bazedoxifene.41 At 24 months, the increase in bone mineral density was even greater than in women treated with raloxifene.42 However, the effect of estrogen-bazedoxifene on the incidence of fractures remains to be studied.
For genitourinary syndrome of menopause. The SMART-3 trial showed that treatment with conjugated estrogens plus bazedoxifene (0.45/20 mg or 0.625/20 mg) was more effective than placebo in increasing the percent of superficial and intermediate cells and decreased the number of parabasal cells at 12 weeks compared with placebo (P < .01).43 Both doses also significantly decreased the mean vaginal pH and improved vaginal dryness.
Patients treated with estrogen-bazedoxifene for a minimum of 12 weeks in a double-blind placebo-controlled study also showed a significant improvement in sexual function and quality-of-life measurements based on 3 well-defined scales, which included ease of lubrication, satisfaction with treatment, control of hot flashes, and sleep parameters.43
Low rates of side effects
To evaluate this regimen’s antagonistic effects on uterine tissue, endometrial hyperplasia was diagnosed by blinded pathologists using endometrial biopsies taken at 6, 12, and 24 months or more if cancer was a suspected diagnosis. At 12 and 24 months of treatment, the incidence of hyperplasia with bazedoxifene 20 or 40 mg at doses of either 0.45 or 0.625 mg of conjugated estrogens was less than 1%, which was similar to placebo rates over the 24 months.44 The lowest dose studied, bazedoxifene 10 mg, did not prevent hyperplasia with conjugated estrogens 0.45 or 0.625 mg, and its use was discontinued.
Rates of amenorrhea with bazedoxifene 20 or 40 mg and conjugated estrogens 0.45 or 0.625 mg were very favorable (83%–93%) and similar to those with placebo.45 For women with continued bleeding on hormone therapy requiring multiple evaluations, or for women who won’t accept the risk of bleeding on hormone therapy, conjugated estrogens and bazedoxifene may be a sustainable option. However, any woman with abnormal bleeding should undergo prompt immediate evaluation.
A typical side effect of estrogen replacement therapy is breast tenderness. For women seeking vasomotor symptom treatment but who experience breast tenderness, this may be a deterrent from continuing hormone therapy. As shown in the SMART-1 and SMART-2 trials,46 conjugated estrogens and bazedoxifene did not cause an increase in breast tenderness, which may enhance medication adherence.
Ideal candidates for conjugated estrogens plus bazedoxifene
This product could help postmenopausal women who have an intact uterus and are suffering with moderate to severe vasomotor symptoms and genitourinary syndrome of menopause who cannot tolerate the side effects of hormone therapy such as bleeding, bloating, or breast tenderness, or who prefer to take an estrogen but without a progestin. It is also ideal for women at higher risk of osteoporosis.
WHO SHOULD GET WHAT?
Not all postmenopausal women have vasomotor symptoms, genitourinary syndrome of menopause, or bone loss. For those who do, standard hormone therapy is an option.
For those who have symptoms and a lower threshold of side effects such as breast tenderness and vaginal bleeding, a combination of an estrogen plus an ERAA (eg, bazedoxifene) is an option.
For women who have no vasomotor symptoms but do have genitourinary syndrome of menopause and don’t want local vaginal treatment, ospemifene is an option.
For women with no vasomotor symptoms but who have bone loss and increased risk of estrogen receptor-positive breast cancer, raloxifene is a good option.
Both premenopausal and postmenopausal women who are at increased risk for breast cancer should be considered for tamoxifen chemoprevention. Postmenopausal women with a uterus at increased risk for breast cancer should be considered for raloxifene, as it has no uterine effect. Raloxifene is not indicated in premenopausal women.
No woman at increased risk of venous thromboembolism is a candidate for ERAA treatment or for oral estrogen. However, the clinician has multiple options to improve quality of life and work productivity and reduce office visits of women at midlife, especially when they are individually assessed and treated.
- Giannini A, Russo E, Mannella P, Simoncini T. Selective steroid receptor modulators in reproductive medicine. Minerva Ginecol 2015; 67:431–455.
- Feldman BM, Voda A, Gronseth E. The prevalence of hot flash and associated variables among perimenopausal women. Res Nurs Health 1985; 8:261–268.
- Versi E, Harvey MA, Cardozo L, Brincat M, Studd JW. Urogenital prolapse and atrophy at menopause: a prevalence study. Int Urogynecol J Pelvic Floor Dysfunct 2001; 12:107–110.
- Hess R, Chang CC, Conigliaro J, McNeil M. Understanding physicians’ attitudes towards hormone therapy. Womens Health Issues 2005; 15:31–38.
- Melton LJ 3rd, Khosla S, Atkinson EJ, O’Fallon WM, Riggs BL. Relationship of bone turnover to bone density and fractures. J Bone Miner Res 1997; 12:1083–1091.
- Sikon A, Thacker HL. Treatment options for menopausal hot flashes. Cleve Clin J Med 2004; 71:578–582.
- Levine JP. Treating menopausal symptoms with a tissue-selective estrogen complex. Gend Med 2011; 8:57–68.
- Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. J Steroid Biochem Mol Biol 2014; 142:142–154.
- Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 1998; 351:1451–1467.
- Davies C, Pan H, Godwin J, et al; Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013; 381:805–816.
- Gray RG, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 2013; (suppl): abstract 5.
- Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet 2003; 361:296–300.
- Cuzick J, Sestak I, Cawthorn S, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol 2015; 16:67–75.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388.
- Chang J, Powles TJ, Ashley SE, et al. The effect of tamoxifen and hormone replacement therapy on serum cholesterol, bone mineral density and coagulation factors in healthy postmenopausal women participating in a randomised, controlled tamoxifen prevention study. Ann Oncol 1996; 7:671–675.
- Herrington DM, Klein KP. Effects of SERMs on important indicators of cardiovascular health: lipoproteins, hemostatic factors and endothelial function. Womens Health Issues 2001; 11:95–102.
- Kedar RP, Bourne TH, Powles TJ, et al. Effects of tamoxifen on uterus and ovaries of postmenopausal women in a randomized breast cancer prevention trial. Lancet 1994; 343:1318–1321.
- Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999; 282:637–645.
- Maricic M, Adachi JD, Sarkar S, Wu W, Wong M, Harper KD. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med 2002; 162:1140–1143.
- Recker RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin 2011; 27:1755–1761.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 1999; 281:2189–2197.
- Martino S, Cauley JA, Barrett-Connor E, et al; CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst 2004; 96:1751–1761.
- Vogel VG, Qu Y, Wong M, Mitchell B, Mershon JL. Incidence of invasive breast cancer in postmenopausal women after discontinuation of long-term raloxifene administration. Clin Breast Cancer 2009; 9:45–50.
- Grady D, Cauley JA, Stock JL, et al. Effect of raloxifene on all-cause mortality. Am J Med 2010; 123:469.e1–e7.
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006; 355:125–137.
- Vogel VG. The NSABP Study of Tamoxifen and Raloxifene (STAR) trial. Expert Rev Anticancer Ther 2009; 9:51–60.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006; 295:2727–2741.
- Barnes KN, Pearce EF, Yancey AM, Forinash AB. Ospemifene in the treatment of vulvovaginal atrophy. Ann Pharmacother 2014; 48:752–757.
- Rutanen EM, Heikkinen J, Halonen K, Komi J, Lammintausta R, Ylikorkala O. Effects of ospemifene, a novel SERM, on hormones, genital tract, climacteric symptoms, and quality of life in postmenopausal women: a double-blind, randomized trial. Menopause 2003; 10:433–439.
- Constantine G, Graham S, Koltun WD, Kingsberg SA. Assessment of ospemifene or lubricants on clinical signs of VVA. J Sex Med 2014; 11:1033–1041.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE survey. J Sex Med 2013; 10:1790–1799.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause 2013; 20:623–630.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause 2010; 17:480–486.
- Goldstein SR, Bachmann GA, Koninckx PR, Lin VH, Portman DJ, Ylikorkala O; Ospemifene Study Group. Ospemifene 12-month safety and efficacy in postmenopausal women with vulvar and vaginal atrophy. Climacteric 2014; 17:173–182.
- Cui Y, Zong H, Yan H, Li N, Zhang Y. The efficacy and safety of ospemifene in treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy: a systematic review and meta-analysis. J Sex Med 2014; 11:487–497.
- Komi J, Heikkinen J, Rutanen EM, Halonen K, Lammintausta R, Ylikorkala O. Effects of ospemifene, a novel SERM, on biochemical markers of bone turnover in healthy postmenopausal women. Gynecal Endocrinol 2004; 18:152–158.
- Komi J, Lankinen KS, DeGregorio M, et al. Effects of ospemifene and raloxifene on biochemical markers of bone turnover in postmenopausal women. J Bone Miner Metab 2006; 24:314–318.
- Wurz GT, Read KC, Marchisano-Karpman C, et al. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol 2005; 97:230–240.
- Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009; 16:1116–1124.
- Pickar JH, Mirkin S. Tissue-selective agents: selective estrogen receptor modulators and the tissue-selective estrogen complex. Menopause Int 2010; 16:121–128.
- Levine JP. Treating menopausal symptoms with a tissue-selective estrogen complex. Gend Med 2011; 8:57–68.
- Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrange complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril 2009; 92:1045–1052.
- Bachmann G, Bobula J, Mirkin S. Effects of bazedoxifene/conjugated estrogens on quality of life in postmenopausal women with symptoms of vulvar/vaginal atrophy. Climacteric 2010; 13:132–140.
- Pickar JH, Yeh IT, Bachmann G, Speroff L. Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril 2009; 92:1018–1024.
- Archer DF, Lewis V, Carr BR, Olivier S, Pickar JH. Bazedoxifene/conjugated estrogens (BZA/CE): incidence of uterine bleeding in postmenopausal women. Fertil Steril 2009: 92:1039–1044.
- Pinkerton JV, Abraham L, Bushmakin AG, et al. Evaluation of the efficacy and safety of bazedoxifene/conjugated estrogens for secondary outcomes including vasomotor symptoms in postmenopausal women by years since menopause in the Selective estrogens, Menopause and Response to Therapy (SMART) trials. J Womens Health (Larchmt) 2014; 23:18–28.
- Giannini A, Russo E, Mannella P, Simoncini T. Selective steroid receptor modulators in reproductive medicine. Minerva Ginecol 2015; 67:431–455.
- Feldman BM, Voda A, Gronseth E. The prevalence of hot flash and associated variables among perimenopausal women. Res Nurs Health 1985; 8:261–268.
- Versi E, Harvey MA, Cardozo L, Brincat M, Studd JW. Urogenital prolapse and atrophy at menopause: a prevalence study. Int Urogynecol J Pelvic Floor Dysfunct 2001; 12:107–110.
- Hess R, Chang CC, Conigliaro J, McNeil M. Understanding physicians’ attitudes towards hormone therapy. Womens Health Issues 2005; 15:31–38.
- Melton LJ 3rd, Khosla S, Atkinson EJ, O’Fallon WM, Riggs BL. Relationship of bone turnover to bone density and fractures. J Bone Miner Res 1997; 12:1083–1091.
- Sikon A, Thacker HL. Treatment options for menopausal hot flashes. Cleve Clin J Med 2004; 71:578–582.
- Levine JP. Treating menopausal symptoms with a tissue-selective estrogen complex. Gend Med 2011; 8:57–68.
- Pinkerton JV, Thomas S. Use of SERMs for treatment in postmenopausal women. J Steroid Biochem Mol Biol 2014; 142:142–154.
- Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 1998; 351:1451–1467.
- Davies C, Pan H, Godwin J, et al; Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013; 381:805–816.
- Gray RG, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 2013; (suppl): abstract 5.
- Cuzick J, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet 2003; 361:296–300.
- Cuzick J, Sestak I, Cawthorn S, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol 2015; 16:67–75.
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90:1371–1388.
- Chang J, Powles TJ, Ashley SE, et al. The effect of tamoxifen and hormone replacement therapy on serum cholesterol, bone mineral density and coagulation factors in healthy postmenopausal women participating in a randomised, controlled tamoxifen prevention study. Ann Oncol 1996; 7:671–675.
- Herrington DM, Klein KP. Effects of SERMs on important indicators of cardiovascular health: lipoproteins, hemostatic factors and endothelial function. Womens Health Issues 2001; 11:95–102.
- Kedar RP, Bourne TH, Powles TJ, et al. Effects of tamoxifen on uterus and ovaries of postmenopausal women in a randomized breast cancer prevention trial. Lancet 1994; 343:1318–1321.
- Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 1999; 282:637–645.
- Maricic M, Adachi JD, Sarkar S, Wu W, Wong M, Harper KD. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med 2002; 162:1140–1143.
- Recker RR, Mitlak BH, Ni X, Krege JH. Long-term raloxifene for postmenopausal osteoporosis. Curr Med Res Opin 2011; 27:1755–1761.
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 1999; 281:2189–2197.
- Martino S, Cauley JA, Barrett-Connor E, et al; CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst 2004; 96:1751–1761.
- Vogel VG, Qu Y, Wong M, Mitchell B, Mershon JL. Incidence of invasive breast cancer in postmenopausal women after discontinuation of long-term raloxifene administration. Clin Breast Cancer 2009; 9:45–50.
- Grady D, Cauley JA, Stock JL, et al. Effect of raloxifene on all-cause mortality. Am J Med 2010; 123:469.e1–e7.
- Barrett-Connor E, Mosca L, Collins P, et al; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 2006; 355:125–137.
- Vogel VG. The NSABP Study of Tamoxifen and Raloxifene (STAR) trial. Expert Rev Anticancer Ther 2009; 9:51–60.
- Vogel VG, Costantino JP, Wickerham DL, et al; National Surgical Adjuvant Breast and Bowel Project (NSABP). Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006; 295:2727–2741.
- Barnes KN, Pearce EF, Yancey AM, Forinash AB. Ospemifene in the treatment of vulvovaginal atrophy. Ann Pharmacother 2014; 48:752–757.
- Rutanen EM, Heikkinen J, Halonen K, Komi J, Lammintausta R, Ylikorkala O. Effects of ospemifene, a novel SERM, on hormones, genital tract, climacteric symptoms, and quality of life in postmenopausal women: a double-blind, randomized trial. Menopause 2003; 10:433–439.
- Constantine G, Graham S, Koltun WD, Kingsberg SA. Assessment of ospemifene or lubricants on clinical signs of VVA. J Sex Med 2014; 11:1033–1041.
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE survey. J Sex Med 2013; 10:1790–1799.
- Portman DJ, Bachmann GA, Simon JA; Ospemifene Study Group. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause 2013; 20:623–630.
- Bachmann GA, Komi JO; Ospemifene Study Group. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause 2010; 17:480–486.
- Goldstein SR, Bachmann GA, Koninckx PR, Lin VH, Portman DJ, Ylikorkala O; Ospemifene Study Group. Ospemifene 12-month safety and efficacy in postmenopausal women with vulvar and vaginal atrophy. Climacteric 2014; 17:173–182.
- Cui Y, Zong H, Yan H, Li N, Zhang Y. The efficacy and safety of ospemifene in treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy: a systematic review and meta-analysis. J Sex Med 2014; 11:487–497.
- Komi J, Heikkinen J, Rutanen EM, Halonen K, Lammintausta R, Ylikorkala O. Effects of ospemifene, a novel SERM, on biochemical markers of bone turnover in healthy postmenopausal women. Gynecal Endocrinol 2004; 18:152–158.
- Komi J, Lankinen KS, DeGregorio M, et al. Effects of ospemifene and raloxifene on biochemical markers of bone turnover in postmenopausal women. J Bone Miner Metab 2006; 24:314–318.
- Wurz GT, Read KC, Marchisano-Karpman C, et al. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol 2005; 97:230–240.
- Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009; 16:1116–1124.
- Pickar JH, Mirkin S. Tissue-selective agents: selective estrogen receptor modulators and the tissue-selective estrogen complex. Menopause Int 2010; 16:121–128.
- Levine JP. Treating menopausal symptoms with a tissue-selective estrogen complex. Gend Med 2011; 8:57–68.
- Lindsay R, Gallagher JC, Kagan R, Pickar JH, Constantine G. Efficacy of tissue-selective estrange complex of bazedoxifene/conjugated estrogens for osteoporosis prevention in at-risk postmenopausal women. Fertil Steril 2009; 92:1045–1052.
- Bachmann G, Bobula J, Mirkin S. Effects of bazedoxifene/conjugated estrogens on quality of life in postmenopausal women with symptoms of vulvar/vaginal atrophy. Climacteric 2010; 13:132–140.
- Pickar JH, Yeh IT, Bachmann G, Speroff L. Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril 2009; 92:1018–1024.
- Archer DF, Lewis V, Carr BR, Olivier S, Pickar JH. Bazedoxifene/conjugated estrogens (BZA/CE): incidence of uterine bleeding in postmenopausal women. Fertil Steril 2009: 92:1039–1044.
- Pinkerton JV, Abraham L, Bushmakin AG, et al. Evaluation of the efficacy and safety of bazedoxifene/conjugated estrogens for secondary outcomes including vasomotor symptoms in postmenopausal women by years since menopause in the Selective estrogens, Menopause and Response to Therapy (SMART) trials. J Womens Health (Larchmt) 2014; 23:18–28.
KEY POINTS
- Tamoxifen is approved to prevent and treat breast cancer. It may also have beneficial effects on bone and on cardiovascular risk factors, but these are not approved uses.
- Raloxifene, a second-generation ERAA, was initially approved for preventing and treating osteoporosis and later received approval to reduce the risk of invasive estrogen receptor-positive breast cancer in postmenopausal women.
- Ospemifene is approved for treatment of genitourinary syndrome of menopause.
- The combination of conjugated estrogen and bazedoxifene is approved for treating moderate to severe vasomotor symptoms associated with menopause and also for preventing postmenopausal osteoporosis in women with an intact uterus.
Apps and fitness trackers that measure sleep: Are they useful?
More and more consumers are using wearable devices and smartphones to monitor and measure various body functions, including sleep. Many patients now present their providers with sleep data obtained from their phones and other devices. But can these devices provide valid, useful clinical information?
This article describes common sleep tracking devices available to consumers and the mechanisms the devices probably use to distinguish sleep from wakefulness (their algorithms are secret), the studies evaluating the validity of device manufacturers’ claims, and their clinical utility and limitations.
DEVICES ARE COMMON
Close to 1 in 10 adults over age 18 owns an activity tracker, and sales are projected to reach $50 billion by 2018.1 Even more impressive, close to 69% of Americans own a smartphone,1 and more than half use it as an alarm clock.2
At the same time that these devices have become so popular, sleep medicine has come of age, and experts have been pushing to improve people’s sleep and increase awareness of sleep disorders.3,4 While the technology has significantly advanced, adoption of data from these devices for clinical evaluation has been limited. Studies examining the validity of these devices have only recently been conducted, and companies that make the devices have not been forthcoming with details of the specific algorithms they use to tell if the patient is asleep or awake or what stage of sleep the patient is in.
WHAT ARE THESE DEVICES?
Consumer tracking devices that claim to measure sleep are easily available for purchase and include wearable fitness trackers such as Fitbit, Jawbone UP, and Nike+ Fuelband. Other sleep tracking devices are catalogued by Ko et al.5 Various smartphone applications (apps) are also available.
Fitness trackers, usually worn as a wrist band, are primarily designed to measure movement and activity, but manufacturers now claim the trackers can also measure sleep. Collected data are available for the user to review the following day. In most cases, these trackers display sleep and wake times; others also claim to record sound sleep, light sleep, and the number and duration of awakenings. Most fitness trackers have complementary apps available for download that graphically display the data on smartphones and interact with social media to allow users to post their sleep and activity data.
More than 500 sleep-related apps are available for download to smartphones in the iTunes app store5; the Sleep Cycle alarm clock app was among the top 5 sleep-tracking apps downloaded in 2014.6 Because sleep data collection relies on the smartphone being placed on the user’s mattress, movements of bed partners, pets, and bedding may interfere with results. In most cases, the apps display data in a format similar to that of fitness trackers. Some claim to determine the optimal sleep phase for the alarm to wake the user.
HOW DOES THE TECHNOLOGY WORK?
Older activity-tracking devices used single-channel electroencephalographic recordings or multiple physiologic channels such as galvanic skin response, skin temperature, and heat flux to measure activity to determine transitions between periods of sleep and wakefulness.7,8
None of the currently available consumer sleep tracking devices discloses the exact mechanisms used to measure sleep and wakefulness, but most appear to rely on 3-axis accelerometers,9 ie, microelectromechanical devices that measure front-to-back, side-to-side, and up-and-down motion and convert the data into an activity count. Activity counts are acquired over 30- or 60-second intervals and are entered into algorithms that determine if the pattern indicates that the patient is awake or asleep. This is the same method that actigraphy uses to evaluate sleep, but most actigraphs used in medicine disclose their mechanisms and provide clinicians with the option of using various validated algorithms to classify the activity counts into sleep or awake periods.9–11
ARE THE MEASURES VALID?
Only a few studies have examined the validity and accuracy of current fitness trackers and apps for measuring sleep.
The available studies are difficult to compare; most have been small and used different actigraphy devices for comparison. Some tested healthy volunteers, others included people with suspected or confirmed sleep disorders, and some had both types of participants. In many studies, the device was compared with polysomnography for only 1 night, making the “first-night effect” likely to be a confounding factor, as people tend to sleep worse during the first night of testing. Technical failures for the devices were noted in some studies.12 In addition, some currently used apps may use different platforms than the devices used in these studies, limiting the extrapolation of results.
As with fitness trackers, few studies have been done to examine the validity of smartphone apps.5 Findings of 3 studies are summarized in Table 2.17–19 In addition to tracking the duration and depth of sleep, some apps purport to detect snoring, sleep apnea, and periodic limb movements of sleep. Discussion of these apps is beyond the scope of this review.
ARE THE DEVICES CLINICALLY USEFUL?
Although a thorough history remains the cornerstone of a good evaluation of sleep problems, testing is sometimes essential, and in certain situations, objective data can complement the history and clarify the diagnosis.
Polysomnography remains the gold standard for telling when the patient is asleep vs awake, diagnosing sleep-disordered breathing, detecting periodic limb movements and parasomnias, and aiding in the diagnosis of narcolepsy.
Actigraphy, which uses technology similar to fitness trackers, can help distinguish sleep from wakefulness, reveal erratic sleep schedules, and help diagnose circadian rhythm sleep disorders. In patients with insomnia, actigraphy can help determine daily sleep patterns and response to treatment.20 It can be especially useful for patients who cannot provide a clear history, eg, children and those with developmental disabilities or cognitive dysfunction.
Consumer sleep tracking devices, like actigraphy, are portable and unobtrusive, providing a way to measure sleep duration and demonstrate sleep patterns in a patient’s natural environment. Being more accessible, cheaper, and less time-consuming than clinical tests, the commercially available devices could be clinically useful in some situations, eg, for monitoring overall sleep patterns to look for circadian sleep-wake disorders, commonly seen in shift workers (shift work disorder) or adolescents (delayed sleep-wake phase disorder); or in patients with poor motivation to maintain a sleep diary. Because of their poor performance in clinical trials, they should not be relied upon to distinguish sleep from wakefulness, quantify the amount of sleep, determine sleep stages, and awaken patients exclusively from light sleep.
Discerning poor sleep hygiene from insomnia
Patients with insomnia tend to take longer to go to sleep (have longer sleep latencies), wake up more (have more disturbed sleep with increased awakenings), and have shorter sleep times with reduced sleep efficiencies.21 Sleep tracking devices tend to be less accurate for patients with short sleep duration and disturbed sleep, limiting their usefulness in this group. Furthermore, patients with insomnia tend to underestimate their sleep time and overestimate sleep latency; some devices also tend to overestimate the time to fall asleep, reinforcing this common error made by patients.22,23
On the other hand, data from sleep tracking devices could help distinguish poor sleep hygiene from an insomnia disorder. For example, the data may indicate that a patient has poor sleep habits, such as taking long daytime naps or having significantly variable time in and out of bed from day to day. The total times asleep and awake in the middle of the night may also be substantially different on each night, which would also possibly indicate poor sleep hygiene.
Detecting circadian rhythms
A device may show that a patient has a clear circadian preference that is not in line with his or her daily routines, suggesting an underlying circadian rhythm sleep-wake disorder. This may be evident by bedtimes and wake times that are consistent but substantially out of sync with one’s social or occupational needs.
Measuring overall sleep duration
In people with normal sleep, fitness trackers perform reasonably well for measuring overall sleep duration. This information could be used to assess a patient with daytime sleepiness and fatigue to evaluate insufficient sleep as an etiologic factor.
Table 3 summarizes how to evaluate the data from sleep apps and fitness tracking devices for clinical use. While these features of consumer sleep tracking devices could conceivably help in the above clinical scenarios, further validation of devices in clinical populations is necessary before their use can be recommended without reservation.
ADVISING PATIENTS
Patients sometimes present to clinicians with concerns about the duration of sleep time and time spent in various sleep stages as delineated by their sleep tracking devices. Currently, these devices do not appear to be able to adequately distinguish various sleep stages, and in many users, they can substantially underestimate or overestimate sleep parameters such as time taken to fall asleep or duration of awakenings in the middle of the night. Patients can be reassured about this lack of evidence and should be advised to not place too much weight on such data alone.
Sleep “goals” set by many devices have not been scientifically validated. People without sleep problems should be discouraged from making substantial changes to their routines to accommodate sleep targets set by the devices. Patients should be counseled about the pitfalls of the data and can be reassured that little evidence suggests that time spent in various sleep stages correlates with adverse daytime consequences or with poor health outcomes.
Some of the apps used as alarm clocks claim to be able to tell what stage of sleep people are in and wait to awaken them until they are in a light stage, which is less jarring than being awakened from a deep stage, but the evidence for this is unclear. In the one study that tested this claim, the app did not awaken participants from light sleep more often than is likely to occur by chance.17 The utility of these apps as personalized alarm clocks is still extremely limited, and patients should be counseled to obtain an adequate amount of sleep rather than rely on devices to awaken them during specific sleep stages.
The rates for discontinuing the use of these devices are high, which could limit their utility. Some surveys have shown that close to 50% of users stop using fitness trackers; 33% stop using them within 6 months of obtaining the device.24 Also, there is little evidence that close monitoring of sleep results in behavior changes or improved sleep duration. Conversely, the potential harms of excessive monitoring of one’s sleep are currently unknown.
- Rock Health. The future of biosensing wearables. http://rockhealth.com/reports/the-future-of-biosensing-wearables/. Accessed March 16, 2017.
- Time, Inc. Your wireless life: results of Time’s mobility poll. http://content.time.com/time/interactive/0,31813,2122187,00.html. Accessed March 16, 2017.
- Office of Disease Prevention and Health Promotion (ODPHP). Healthy people 2020. Sleep health. www.healthypeople.gov/2020/topics-objectives/topic/sleep-health. Accessed March 16, 2017.
- Consensus Conference Panel; Watson NF, Badr MS, Belenky G, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. J Clin Sleep Med 2015; 11:931–952.
- Ko PT, Kientz JA, Choe EK, Kay M, Landis CA, Watson NF. Consumer sleep technologies: a review of the landscape. J Clin Sleep Med 2015; 11:1455–1461.
- Investor Place Media, LLC. Top iTunes picks: Apple names best apps of 2014. http://investorplace.com/2014/12/apple-best-apps-of-2014-aapl/#.VIYeE9LF98E/. Accessed April 13, 2017.
- Sunseri M, Liden CB, Farringdon J, et al. The SenseWear armband as a sleep detection device. Internal publication.
- Shambroom JR, Fábregas SE, Johnstone J. Validation of an automated wireless system to monitor sleep in healthy adults. J Sleep Res 2012; 21:221–230.
- John D, Freedson P. ActiGraph and Actical physical activity monitors: a peek under the hood. Med Sci Sports Exerc 2012; 44(suppl 1):S86–S89.
- Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep 1994; 17:201–207.
- Kripke DF, Hahn EK, Grizas AP, et al. Wrist actigraphic scoring for sleep laboratory patients: algorithm development. J Sleep Res 2010; 19:612–619.
- Meltzer LJ, Marcus CL. Reply: caffeine therapy for apnea of prematurity: long-term effect on sleep by actigraphy and polysomnography. Am J Respir Crit Care Med 2014; 190:1457–1458.
- Montgomery-Downs HE, Insana SP, Bond JA. Movement toward a novel activity monitoring device. Sleep Breath 2012; 16:913–917.
- Meltzer LJ, Hiruma LS, Avis K, Montgomery-Downs H, Valentin J. Comparison of a commercial accelerometer with polysomnography and actigraphy in children and adolescents. Sleep 2015; 38:1323–1330.
- de Zambotti M, Baker FC, Colrain IM. Validation of sleep-tracking technology compared with polysomnography in adolescents. Sleep 2015; 38:1461–1468.
- de Zambotti M, Claudatos S, Inkelis S, Colrain IM, Baker FC. Evaluation of a consumer fitness-tracking device to assess sleep in adults. Chronobiol Int 2015; 32:1024–1028.
- Toon E, Davey MJ, Hollis SL, Nixon GM, Horne RS, Biggs SN. Comparison of commercial wrist-based and smartphone accelerometers, actigraphy, and PSG in a clinical cohort of children and adolescents. J Clin Sleep Med 2016; 12:343–350.
- Bhat S, Ferraris A, Gupta D, et al. Is there a clinical role for smartphone sleep apps? Comparison of sleep cycle detection by a smartphone application to polysomnography. J Clin Sleep Med 2015; 11:709–715.
- Min JK, Doryab A, Wiese J, Amini S, Zimmerman J, Hong JI. Toss ‘n’ turn: smartphone as sleep and sleep quality detector. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems. Toronto, Ontario, Canada: ACM; 2014:477-486.
- Morgenthaler T, Alessi C, Friedman L, et al; Standards of Practice Committee; American Academy of Sleep Medicine. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep 2007; 30:519-529.
- Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther 2003; 41:427–445.
- Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry 1976; 133:1382–1388.
- Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res 1997; 6:179–188.
- Endeavour Partners LLC. Inside wearables: how the science of human behavior change offers the secret to long-term engagement. http://endeavourpartners.net/assets/Wearables-and-the-Science-of-Human-Behavior-Change-EP4.pdf. Accessed March 16, 2017.
More and more consumers are using wearable devices and smartphones to monitor and measure various body functions, including sleep. Many patients now present their providers with sleep data obtained from their phones and other devices. But can these devices provide valid, useful clinical information?
This article describes common sleep tracking devices available to consumers and the mechanisms the devices probably use to distinguish sleep from wakefulness (their algorithms are secret), the studies evaluating the validity of device manufacturers’ claims, and their clinical utility and limitations.
DEVICES ARE COMMON
Close to 1 in 10 adults over age 18 owns an activity tracker, and sales are projected to reach $50 billion by 2018.1 Even more impressive, close to 69% of Americans own a smartphone,1 and more than half use it as an alarm clock.2
At the same time that these devices have become so popular, sleep medicine has come of age, and experts have been pushing to improve people’s sleep and increase awareness of sleep disorders.3,4 While the technology has significantly advanced, adoption of data from these devices for clinical evaluation has been limited. Studies examining the validity of these devices have only recently been conducted, and companies that make the devices have not been forthcoming with details of the specific algorithms they use to tell if the patient is asleep or awake or what stage of sleep the patient is in.
WHAT ARE THESE DEVICES?
Consumer tracking devices that claim to measure sleep are easily available for purchase and include wearable fitness trackers such as Fitbit, Jawbone UP, and Nike+ Fuelband. Other sleep tracking devices are catalogued by Ko et al.5 Various smartphone applications (apps) are also available.
Fitness trackers, usually worn as a wrist band, are primarily designed to measure movement and activity, but manufacturers now claim the trackers can also measure sleep. Collected data are available for the user to review the following day. In most cases, these trackers display sleep and wake times; others also claim to record sound sleep, light sleep, and the number and duration of awakenings. Most fitness trackers have complementary apps available for download that graphically display the data on smartphones and interact with social media to allow users to post their sleep and activity data.
More than 500 sleep-related apps are available for download to smartphones in the iTunes app store5; the Sleep Cycle alarm clock app was among the top 5 sleep-tracking apps downloaded in 2014.6 Because sleep data collection relies on the smartphone being placed on the user’s mattress, movements of bed partners, pets, and bedding may interfere with results. In most cases, the apps display data in a format similar to that of fitness trackers. Some claim to determine the optimal sleep phase for the alarm to wake the user.
HOW DOES THE TECHNOLOGY WORK?
Older activity-tracking devices used single-channel electroencephalographic recordings or multiple physiologic channels such as galvanic skin response, skin temperature, and heat flux to measure activity to determine transitions between periods of sleep and wakefulness.7,8
None of the currently available consumer sleep tracking devices discloses the exact mechanisms used to measure sleep and wakefulness, but most appear to rely on 3-axis accelerometers,9 ie, microelectromechanical devices that measure front-to-back, side-to-side, and up-and-down motion and convert the data into an activity count. Activity counts are acquired over 30- or 60-second intervals and are entered into algorithms that determine if the pattern indicates that the patient is awake or asleep. This is the same method that actigraphy uses to evaluate sleep, but most actigraphs used in medicine disclose their mechanisms and provide clinicians with the option of using various validated algorithms to classify the activity counts into sleep or awake periods.9–11
ARE THE MEASURES VALID?
Only a few studies have examined the validity and accuracy of current fitness trackers and apps for measuring sleep.
The available studies are difficult to compare; most have been small and used different actigraphy devices for comparison. Some tested healthy volunteers, others included people with suspected or confirmed sleep disorders, and some had both types of participants. In many studies, the device was compared with polysomnography for only 1 night, making the “first-night effect” likely to be a confounding factor, as people tend to sleep worse during the first night of testing. Technical failures for the devices were noted in some studies.12 In addition, some currently used apps may use different platforms than the devices used in these studies, limiting the extrapolation of results.
As with fitness trackers, few studies have been done to examine the validity of smartphone apps.5 Findings of 3 studies are summarized in Table 2.17–19 In addition to tracking the duration and depth of sleep, some apps purport to detect snoring, sleep apnea, and periodic limb movements of sleep. Discussion of these apps is beyond the scope of this review.
ARE THE DEVICES CLINICALLY USEFUL?
Although a thorough history remains the cornerstone of a good evaluation of sleep problems, testing is sometimes essential, and in certain situations, objective data can complement the history and clarify the diagnosis.
Polysomnography remains the gold standard for telling when the patient is asleep vs awake, diagnosing sleep-disordered breathing, detecting periodic limb movements and parasomnias, and aiding in the diagnosis of narcolepsy.
Actigraphy, which uses technology similar to fitness trackers, can help distinguish sleep from wakefulness, reveal erratic sleep schedules, and help diagnose circadian rhythm sleep disorders. In patients with insomnia, actigraphy can help determine daily sleep patterns and response to treatment.20 It can be especially useful for patients who cannot provide a clear history, eg, children and those with developmental disabilities or cognitive dysfunction.
Consumer sleep tracking devices, like actigraphy, are portable and unobtrusive, providing a way to measure sleep duration and demonstrate sleep patterns in a patient’s natural environment. Being more accessible, cheaper, and less time-consuming than clinical tests, the commercially available devices could be clinically useful in some situations, eg, for monitoring overall sleep patterns to look for circadian sleep-wake disorders, commonly seen in shift workers (shift work disorder) or adolescents (delayed sleep-wake phase disorder); or in patients with poor motivation to maintain a sleep diary. Because of their poor performance in clinical trials, they should not be relied upon to distinguish sleep from wakefulness, quantify the amount of sleep, determine sleep stages, and awaken patients exclusively from light sleep.
Discerning poor sleep hygiene from insomnia
Patients with insomnia tend to take longer to go to sleep (have longer sleep latencies), wake up more (have more disturbed sleep with increased awakenings), and have shorter sleep times with reduced sleep efficiencies.21 Sleep tracking devices tend to be less accurate for patients with short sleep duration and disturbed sleep, limiting their usefulness in this group. Furthermore, patients with insomnia tend to underestimate their sleep time and overestimate sleep latency; some devices also tend to overestimate the time to fall asleep, reinforcing this common error made by patients.22,23
On the other hand, data from sleep tracking devices could help distinguish poor sleep hygiene from an insomnia disorder. For example, the data may indicate that a patient has poor sleep habits, such as taking long daytime naps or having significantly variable time in and out of bed from day to day. The total times asleep and awake in the middle of the night may also be substantially different on each night, which would also possibly indicate poor sleep hygiene.
Detecting circadian rhythms
A device may show that a patient has a clear circadian preference that is not in line with his or her daily routines, suggesting an underlying circadian rhythm sleep-wake disorder. This may be evident by bedtimes and wake times that are consistent but substantially out of sync with one’s social or occupational needs.
Measuring overall sleep duration
In people with normal sleep, fitness trackers perform reasonably well for measuring overall sleep duration. This information could be used to assess a patient with daytime sleepiness and fatigue to evaluate insufficient sleep as an etiologic factor.
Table 3 summarizes how to evaluate the data from sleep apps and fitness tracking devices for clinical use. While these features of consumer sleep tracking devices could conceivably help in the above clinical scenarios, further validation of devices in clinical populations is necessary before their use can be recommended without reservation.
ADVISING PATIENTS
Patients sometimes present to clinicians with concerns about the duration of sleep time and time spent in various sleep stages as delineated by their sleep tracking devices. Currently, these devices do not appear to be able to adequately distinguish various sleep stages, and in many users, they can substantially underestimate or overestimate sleep parameters such as time taken to fall asleep or duration of awakenings in the middle of the night. Patients can be reassured about this lack of evidence and should be advised to not place too much weight on such data alone.
Sleep “goals” set by many devices have not been scientifically validated. People without sleep problems should be discouraged from making substantial changes to their routines to accommodate sleep targets set by the devices. Patients should be counseled about the pitfalls of the data and can be reassured that little evidence suggests that time spent in various sleep stages correlates with adverse daytime consequences or with poor health outcomes.
Some of the apps used as alarm clocks claim to be able to tell what stage of sleep people are in and wait to awaken them until they are in a light stage, which is less jarring than being awakened from a deep stage, but the evidence for this is unclear. In the one study that tested this claim, the app did not awaken participants from light sleep more often than is likely to occur by chance.17 The utility of these apps as personalized alarm clocks is still extremely limited, and patients should be counseled to obtain an adequate amount of sleep rather than rely on devices to awaken them during specific sleep stages.
The rates for discontinuing the use of these devices are high, which could limit their utility. Some surveys have shown that close to 50% of users stop using fitness trackers; 33% stop using them within 6 months of obtaining the device.24 Also, there is little evidence that close monitoring of sleep results in behavior changes or improved sleep duration. Conversely, the potential harms of excessive monitoring of one’s sleep are currently unknown.
More and more consumers are using wearable devices and smartphones to monitor and measure various body functions, including sleep. Many patients now present their providers with sleep data obtained from their phones and other devices. But can these devices provide valid, useful clinical information?
This article describes common sleep tracking devices available to consumers and the mechanisms the devices probably use to distinguish sleep from wakefulness (their algorithms are secret), the studies evaluating the validity of device manufacturers’ claims, and their clinical utility and limitations.
DEVICES ARE COMMON
Close to 1 in 10 adults over age 18 owns an activity tracker, and sales are projected to reach $50 billion by 2018.1 Even more impressive, close to 69% of Americans own a smartphone,1 and more than half use it as an alarm clock.2
At the same time that these devices have become so popular, sleep medicine has come of age, and experts have been pushing to improve people’s sleep and increase awareness of sleep disorders.3,4 While the technology has significantly advanced, adoption of data from these devices for clinical evaluation has been limited. Studies examining the validity of these devices have only recently been conducted, and companies that make the devices have not been forthcoming with details of the specific algorithms they use to tell if the patient is asleep or awake or what stage of sleep the patient is in.
WHAT ARE THESE DEVICES?
Consumer tracking devices that claim to measure sleep are easily available for purchase and include wearable fitness trackers such as Fitbit, Jawbone UP, and Nike+ Fuelband. Other sleep tracking devices are catalogued by Ko et al.5 Various smartphone applications (apps) are also available.
Fitness trackers, usually worn as a wrist band, are primarily designed to measure movement and activity, but manufacturers now claim the trackers can also measure sleep. Collected data are available for the user to review the following day. In most cases, these trackers display sleep and wake times; others also claim to record sound sleep, light sleep, and the number and duration of awakenings. Most fitness trackers have complementary apps available for download that graphically display the data on smartphones and interact with social media to allow users to post their sleep and activity data.
More than 500 sleep-related apps are available for download to smartphones in the iTunes app store5; the Sleep Cycle alarm clock app was among the top 5 sleep-tracking apps downloaded in 2014.6 Because sleep data collection relies on the smartphone being placed on the user’s mattress, movements of bed partners, pets, and bedding may interfere with results. In most cases, the apps display data in a format similar to that of fitness trackers. Some claim to determine the optimal sleep phase for the alarm to wake the user.
HOW DOES THE TECHNOLOGY WORK?
Older activity-tracking devices used single-channel electroencephalographic recordings or multiple physiologic channels such as galvanic skin response, skin temperature, and heat flux to measure activity to determine transitions between periods of sleep and wakefulness.7,8
None of the currently available consumer sleep tracking devices discloses the exact mechanisms used to measure sleep and wakefulness, but most appear to rely on 3-axis accelerometers,9 ie, microelectromechanical devices that measure front-to-back, side-to-side, and up-and-down motion and convert the data into an activity count. Activity counts are acquired over 30- or 60-second intervals and are entered into algorithms that determine if the pattern indicates that the patient is awake or asleep. This is the same method that actigraphy uses to evaluate sleep, but most actigraphs used in medicine disclose their mechanisms and provide clinicians with the option of using various validated algorithms to classify the activity counts into sleep or awake periods.9–11
ARE THE MEASURES VALID?
Only a few studies have examined the validity and accuracy of current fitness trackers and apps for measuring sleep.
The available studies are difficult to compare; most have been small and used different actigraphy devices for comparison. Some tested healthy volunteers, others included people with suspected or confirmed sleep disorders, and some had both types of participants. In many studies, the device was compared with polysomnography for only 1 night, making the “first-night effect” likely to be a confounding factor, as people tend to sleep worse during the first night of testing. Technical failures for the devices were noted in some studies.12 In addition, some currently used apps may use different platforms than the devices used in these studies, limiting the extrapolation of results.
As with fitness trackers, few studies have been done to examine the validity of smartphone apps.5 Findings of 3 studies are summarized in Table 2.17–19 In addition to tracking the duration and depth of sleep, some apps purport to detect snoring, sleep apnea, and periodic limb movements of sleep. Discussion of these apps is beyond the scope of this review.
ARE THE DEVICES CLINICALLY USEFUL?
Although a thorough history remains the cornerstone of a good evaluation of sleep problems, testing is sometimes essential, and in certain situations, objective data can complement the history and clarify the diagnosis.
Polysomnography remains the gold standard for telling when the patient is asleep vs awake, diagnosing sleep-disordered breathing, detecting periodic limb movements and parasomnias, and aiding in the diagnosis of narcolepsy.
Actigraphy, which uses technology similar to fitness trackers, can help distinguish sleep from wakefulness, reveal erratic sleep schedules, and help diagnose circadian rhythm sleep disorders. In patients with insomnia, actigraphy can help determine daily sleep patterns and response to treatment.20 It can be especially useful for patients who cannot provide a clear history, eg, children and those with developmental disabilities or cognitive dysfunction.
Consumer sleep tracking devices, like actigraphy, are portable and unobtrusive, providing a way to measure sleep duration and demonstrate sleep patterns in a patient’s natural environment. Being more accessible, cheaper, and less time-consuming than clinical tests, the commercially available devices could be clinically useful in some situations, eg, for monitoring overall sleep patterns to look for circadian sleep-wake disorders, commonly seen in shift workers (shift work disorder) or adolescents (delayed sleep-wake phase disorder); or in patients with poor motivation to maintain a sleep diary. Because of their poor performance in clinical trials, they should not be relied upon to distinguish sleep from wakefulness, quantify the amount of sleep, determine sleep stages, and awaken patients exclusively from light sleep.
Discerning poor sleep hygiene from insomnia
Patients with insomnia tend to take longer to go to sleep (have longer sleep latencies), wake up more (have more disturbed sleep with increased awakenings), and have shorter sleep times with reduced sleep efficiencies.21 Sleep tracking devices tend to be less accurate for patients with short sleep duration and disturbed sleep, limiting their usefulness in this group. Furthermore, patients with insomnia tend to underestimate their sleep time and overestimate sleep latency; some devices also tend to overestimate the time to fall asleep, reinforcing this common error made by patients.22,23
On the other hand, data from sleep tracking devices could help distinguish poor sleep hygiene from an insomnia disorder. For example, the data may indicate that a patient has poor sleep habits, such as taking long daytime naps or having significantly variable time in and out of bed from day to day. The total times asleep and awake in the middle of the night may also be substantially different on each night, which would also possibly indicate poor sleep hygiene.
Detecting circadian rhythms
A device may show that a patient has a clear circadian preference that is not in line with his or her daily routines, suggesting an underlying circadian rhythm sleep-wake disorder. This may be evident by bedtimes and wake times that are consistent but substantially out of sync with one’s social or occupational needs.
Measuring overall sleep duration
In people with normal sleep, fitness trackers perform reasonably well for measuring overall sleep duration. This information could be used to assess a patient with daytime sleepiness and fatigue to evaluate insufficient sleep as an etiologic factor.
Table 3 summarizes how to evaluate the data from sleep apps and fitness tracking devices for clinical use. While these features of consumer sleep tracking devices could conceivably help in the above clinical scenarios, further validation of devices in clinical populations is necessary before their use can be recommended without reservation.
ADVISING PATIENTS
Patients sometimes present to clinicians with concerns about the duration of sleep time and time spent in various sleep stages as delineated by their sleep tracking devices. Currently, these devices do not appear to be able to adequately distinguish various sleep stages, and in many users, they can substantially underestimate or overestimate sleep parameters such as time taken to fall asleep or duration of awakenings in the middle of the night. Patients can be reassured about this lack of evidence and should be advised to not place too much weight on such data alone.
Sleep “goals” set by many devices have not been scientifically validated. People without sleep problems should be discouraged from making substantial changes to their routines to accommodate sleep targets set by the devices. Patients should be counseled about the pitfalls of the data and can be reassured that little evidence suggests that time spent in various sleep stages correlates with adverse daytime consequences or with poor health outcomes.
Some of the apps used as alarm clocks claim to be able to tell what stage of sleep people are in and wait to awaken them until they are in a light stage, which is less jarring than being awakened from a deep stage, but the evidence for this is unclear. In the one study that tested this claim, the app did not awaken participants from light sleep more often than is likely to occur by chance.17 The utility of these apps as personalized alarm clocks is still extremely limited, and patients should be counseled to obtain an adequate amount of sleep rather than rely on devices to awaken them during specific sleep stages.
The rates for discontinuing the use of these devices are high, which could limit their utility. Some surveys have shown that close to 50% of users stop using fitness trackers; 33% stop using them within 6 months of obtaining the device.24 Also, there is little evidence that close monitoring of sleep results in behavior changes or improved sleep duration. Conversely, the potential harms of excessive monitoring of one’s sleep are currently unknown.
- Rock Health. The future of biosensing wearables. http://rockhealth.com/reports/the-future-of-biosensing-wearables/. Accessed March 16, 2017.
- Time, Inc. Your wireless life: results of Time’s mobility poll. http://content.time.com/time/interactive/0,31813,2122187,00.html. Accessed March 16, 2017.
- Office of Disease Prevention and Health Promotion (ODPHP). Healthy people 2020. Sleep health. www.healthypeople.gov/2020/topics-objectives/topic/sleep-health. Accessed March 16, 2017.
- Consensus Conference Panel; Watson NF, Badr MS, Belenky G, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. J Clin Sleep Med 2015; 11:931–952.
- Ko PT, Kientz JA, Choe EK, Kay M, Landis CA, Watson NF. Consumer sleep technologies: a review of the landscape. J Clin Sleep Med 2015; 11:1455–1461.
- Investor Place Media, LLC. Top iTunes picks: Apple names best apps of 2014. http://investorplace.com/2014/12/apple-best-apps-of-2014-aapl/#.VIYeE9LF98E/. Accessed April 13, 2017.
- Sunseri M, Liden CB, Farringdon J, et al. The SenseWear armband as a sleep detection device. Internal publication.
- Shambroom JR, Fábregas SE, Johnstone J. Validation of an automated wireless system to monitor sleep in healthy adults. J Sleep Res 2012; 21:221–230.
- John D, Freedson P. ActiGraph and Actical physical activity monitors: a peek under the hood. Med Sci Sports Exerc 2012; 44(suppl 1):S86–S89.
- Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep 1994; 17:201–207.
- Kripke DF, Hahn EK, Grizas AP, et al. Wrist actigraphic scoring for sleep laboratory patients: algorithm development. J Sleep Res 2010; 19:612–619.
- Meltzer LJ, Marcus CL. Reply: caffeine therapy for apnea of prematurity: long-term effect on sleep by actigraphy and polysomnography. Am J Respir Crit Care Med 2014; 190:1457–1458.
- Montgomery-Downs HE, Insana SP, Bond JA. Movement toward a novel activity monitoring device. Sleep Breath 2012; 16:913–917.
- Meltzer LJ, Hiruma LS, Avis K, Montgomery-Downs H, Valentin J. Comparison of a commercial accelerometer with polysomnography and actigraphy in children and adolescents. Sleep 2015; 38:1323–1330.
- de Zambotti M, Baker FC, Colrain IM. Validation of sleep-tracking technology compared with polysomnography in adolescents. Sleep 2015; 38:1461–1468.
- de Zambotti M, Claudatos S, Inkelis S, Colrain IM, Baker FC. Evaluation of a consumer fitness-tracking device to assess sleep in adults. Chronobiol Int 2015; 32:1024–1028.
- Toon E, Davey MJ, Hollis SL, Nixon GM, Horne RS, Biggs SN. Comparison of commercial wrist-based and smartphone accelerometers, actigraphy, and PSG in a clinical cohort of children and adolescents. J Clin Sleep Med 2016; 12:343–350.
- Bhat S, Ferraris A, Gupta D, et al. Is there a clinical role for smartphone sleep apps? Comparison of sleep cycle detection by a smartphone application to polysomnography. J Clin Sleep Med 2015; 11:709–715.
- Min JK, Doryab A, Wiese J, Amini S, Zimmerman J, Hong JI. Toss ‘n’ turn: smartphone as sleep and sleep quality detector. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems. Toronto, Ontario, Canada: ACM; 2014:477-486.
- Morgenthaler T, Alessi C, Friedman L, et al; Standards of Practice Committee; American Academy of Sleep Medicine. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep 2007; 30:519-529.
- Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther 2003; 41:427–445.
- Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry 1976; 133:1382–1388.
- Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res 1997; 6:179–188.
- Endeavour Partners LLC. Inside wearables: how the science of human behavior change offers the secret to long-term engagement. http://endeavourpartners.net/assets/Wearables-and-the-Science-of-Human-Behavior-Change-EP4.pdf. Accessed March 16, 2017.
- Rock Health. The future of biosensing wearables. http://rockhealth.com/reports/the-future-of-biosensing-wearables/. Accessed March 16, 2017.
- Time, Inc. Your wireless life: results of Time’s mobility poll. http://content.time.com/time/interactive/0,31813,2122187,00.html. Accessed March 16, 2017.
- Office of Disease Prevention and Health Promotion (ODPHP). Healthy people 2020. Sleep health. www.healthypeople.gov/2020/topics-objectives/topic/sleep-health. Accessed March 16, 2017.
- Consensus Conference Panel; Watson NF, Badr MS, Belenky G, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. J Clin Sleep Med 2015; 11:931–952.
- Ko PT, Kientz JA, Choe EK, Kay M, Landis CA, Watson NF. Consumer sleep technologies: a review of the landscape. J Clin Sleep Med 2015; 11:1455–1461.
- Investor Place Media, LLC. Top iTunes picks: Apple names best apps of 2014. http://investorplace.com/2014/12/apple-best-apps-of-2014-aapl/#.VIYeE9LF98E/. Accessed April 13, 2017.
- Sunseri M, Liden CB, Farringdon J, et al. The SenseWear armband as a sleep detection device. Internal publication.
- Shambroom JR, Fábregas SE, Johnstone J. Validation of an automated wireless system to monitor sleep in healthy adults. J Sleep Res 2012; 21:221–230.
- John D, Freedson P. ActiGraph and Actical physical activity monitors: a peek under the hood. Med Sci Sports Exerc 2012; 44(suppl 1):S86–S89.
- Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep 1994; 17:201–207.
- Kripke DF, Hahn EK, Grizas AP, et al. Wrist actigraphic scoring for sleep laboratory patients: algorithm development. J Sleep Res 2010; 19:612–619.
- Meltzer LJ, Marcus CL. Reply: caffeine therapy for apnea of prematurity: long-term effect on sleep by actigraphy and polysomnography. Am J Respir Crit Care Med 2014; 190:1457–1458.
- Montgomery-Downs HE, Insana SP, Bond JA. Movement toward a novel activity monitoring device. Sleep Breath 2012; 16:913–917.
- Meltzer LJ, Hiruma LS, Avis K, Montgomery-Downs H, Valentin J. Comparison of a commercial accelerometer with polysomnography and actigraphy in children and adolescents. Sleep 2015; 38:1323–1330.
- de Zambotti M, Baker FC, Colrain IM. Validation of sleep-tracking technology compared with polysomnography in adolescents. Sleep 2015; 38:1461–1468.
- de Zambotti M, Claudatos S, Inkelis S, Colrain IM, Baker FC. Evaluation of a consumer fitness-tracking device to assess sleep in adults. Chronobiol Int 2015; 32:1024–1028.
- Toon E, Davey MJ, Hollis SL, Nixon GM, Horne RS, Biggs SN. Comparison of commercial wrist-based and smartphone accelerometers, actigraphy, and PSG in a clinical cohort of children and adolescents. J Clin Sleep Med 2016; 12:343–350.
- Bhat S, Ferraris A, Gupta D, et al. Is there a clinical role for smartphone sleep apps? Comparison of sleep cycle detection by a smartphone application to polysomnography. J Clin Sleep Med 2015; 11:709–715.
- Min JK, Doryab A, Wiese J, Amini S, Zimmerman J, Hong JI. Toss ‘n’ turn: smartphone as sleep and sleep quality detector. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems. Toronto, Ontario, Canada: ACM; 2014:477-486.
- Morgenthaler T, Alessi C, Friedman L, et al; Standards of Practice Committee; American Academy of Sleep Medicine. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep 2007; 30:519-529.
- Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther 2003; 41:427–445.
- Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry 1976; 133:1382–1388.
- Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res 1997; 6:179–188.
- Endeavour Partners LLC. Inside wearables: how the science of human behavior change offers the secret to long-term engagement. http://endeavourpartners.net/assets/Wearables-and-the-Science-of-Human-Behavior-Change-EP4.pdf. Accessed March 16, 2017.
KEY POINTS
- Wearable fitness trackers tend to perform better than smartphone applications, which are more prone to interference from bed partners and pets.
- Sleep data from tracking devices are less reliable in patients with fragmented sleep and insomnia.
- In normal sleepers, devices tend to measure sleep duration with reasonable accuracy, so that one can tell if a patient is getting too little sleep or reassure someone who is getting enough sleep.
- Devices may help identify patients with poor sleep hygiene or atypical circadian rhythms.
A 68-year-old man with a blue toe
A 68-year-old man presented with concern about a bluish toe. Several months earlier he had undergone total aortic arch replacement and coronary artery bypass grafting. Since then his renal function had declined and he had been losing weight.
He had hypercholesterolemia, hypertension, and a 20-pack-year smoking history. Physical examination confirmed that his right great toe was indeed bluish (Figure 1). Peripheral, neck, and abdominal vascular examinations were normal. Laboratory testing revealed:
- Serum creatinine concentration 5.15 mg/dL (reference range 0.61–1.04)
- C-reactive protein level 1.5 mg/dL (0–0.3)
- Eosinophil count 0.58 × 109/L (0–0.50)
- Serum complement level normal
- Urine sediment unremarkable.
Transthoracic echocardiography revealed no evidence of vegetation, and a series of blood cultures were negative. The right toe was biopsied, and study revealed cholesterol clefts (Figure 2), confirming the diagnosis of cholesterol crystal embolism.
He was treated with prednisolone 20 mg/day, and his weight loss and renal function improved.
CHOLESTEROL CRYSTAL EMBOLISM
Cholesterol embolization typically occurs after arteriography, cardiac catheterization, vascular surgery, or anticoagulant use in men over age 55 with atherosclerosis.1 It presents with renal failure, abdominal pain, systemic symptoms, or, most commonly (in 88% of cases), skin findings.2
“Blue-toe syndrome,” characterized by tissue ischemia, is seen in 65% of patients.2 Lesions can appear anywhere on the body, but most commonly on the lower extremities. Most are painful due to ischemia. The condition can progress to necrosis.
Patients may have elevated C-reactive protein, hypocomplementemia (39%), and eosinophilia (80%).3,4 The diagnosis is confirmed only with histopathologic findings of intravascular cholesterol crystals, seen as cholesterol clefts.
The differential diagnosis includes contrast nephropathy and infectious endocarditis. However, contrast nephropathy begins to recover within several days and is not accompanied by skin lesions. Repeated blood cultures and echocardiography are useful to rule out infectious endocarditis.
Treatment includes managing cardiovascular risk factors and end-organ ischemia and preventing recurrent embolization. Surgical or endovascular treatment has been shown to be effective in decreasing the rate of further embolism.2 Corticosteroid therapy is assumed to control the secondary inflammation associated with cholesterol crystal embolism.1,5
- Paraskevas KI, Koutsias S, Mikhailidis DP, Giannoukas AD. Cholesterol crystal embolization: a possible complication of peripheral endovascular interventions. J Endovasc Ther 2008; 15:614–625.
- Jucgla A, Moreso F, Muniesa C, Moreno A, Vidaller A. Cholesterol embolism: still an unrecognized entity with a high mortality rate. J Am Acad Dermatol 2006; 55:786–793.
- Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation 2010; 122:631–641.
- Lye WC, Cheah JS, Sinniah R. Renal cholesterol embolic disease. Case report and review of the literature. Am J Nephrol 1993; 13:489–493.
- Nakayama M, Izumaru K, Nagata M, et al. The effect of low-dose corticosteroids on short- and long-term renal outcome in patients with cholesterol crystal embolism. Ren Fail 2011; 33:298–306.
A 68-year-old man presented with concern about a bluish toe. Several months earlier he had undergone total aortic arch replacement and coronary artery bypass grafting. Since then his renal function had declined and he had been losing weight.
He had hypercholesterolemia, hypertension, and a 20-pack-year smoking history. Physical examination confirmed that his right great toe was indeed bluish (Figure 1). Peripheral, neck, and abdominal vascular examinations were normal. Laboratory testing revealed:
- Serum creatinine concentration 5.15 mg/dL (reference range 0.61–1.04)
- C-reactive protein level 1.5 mg/dL (0–0.3)
- Eosinophil count 0.58 × 109/L (0–0.50)
- Serum complement level normal
- Urine sediment unremarkable.
Transthoracic echocardiography revealed no evidence of vegetation, and a series of blood cultures were negative. The right toe was biopsied, and study revealed cholesterol clefts (Figure 2), confirming the diagnosis of cholesterol crystal embolism.
He was treated with prednisolone 20 mg/day, and his weight loss and renal function improved.
CHOLESTEROL CRYSTAL EMBOLISM
Cholesterol embolization typically occurs after arteriography, cardiac catheterization, vascular surgery, or anticoagulant use in men over age 55 with atherosclerosis.1 It presents with renal failure, abdominal pain, systemic symptoms, or, most commonly (in 88% of cases), skin findings.2
“Blue-toe syndrome,” characterized by tissue ischemia, is seen in 65% of patients.2 Lesions can appear anywhere on the body, but most commonly on the lower extremities. Most are painful due to ischemia. The condition can progress to necrosis.
Patients may have elevated C-reactive protein, hypocomplementemia (39%), and eosinophilia (80%).3,4 The diagnosis is confirmed only with histopathologic findings of intravascular cholesterol crystals, seen as cholesterol clefts.
The differential diagnosis includes contrast nephropathy and infectious endocarditis. However, contrast nephropathy begins to recover within several days and is not accompanied by skin lesions. Repeated blood cultures and echocardiography are useful to rule out infectious endocarditis.
Treatment includes managing cardiovascular risk factors and end-organ ischemia and preventing recurrent embolization. Surgical or endovascular treatment has been shown to be effective in decreasing the rate of further embolism.2 Corticosteroid therapy is assumed to control the secondary inflammation associated with cholesterol crystal embolism.1,5
A 68-year-old man presented with concern about a bluish toe. Several months earlier he had undergone total aortic arch replacement and coronary artery bypass grafting. Since then his renal function had declined and he had been losing weight.
He had hypercholesterolemia, hypertension, and a 20-pack-year smoking history. Physical examination confirmed that his right great toe was indeed bluish (Figure 1). Peripheral, neck, and abdominal vascular examinations were normal. Laboratory testing revealed:
- Serum creatinine concentration 5.15 mg/dL (reference range 0.61–1.04)
- C-reactive protein level 1.5 mg/dL (0–0.3)
- Eosinophil count 0.58 × 109/L (0–0.50)
- Serum complement level normal
- Urine sediment unremarkable.
Transthoracic echocardiography revealed no evidence of vegetation, and a series of blood cultures were negative. The right toe was biopsied, and study revealed cholesterol clefts (Figure 2), confirming the diagnosis of cholesterol crystal embolism.
He was treated with prednisolone 20 mg/day, and his weight loss and renal function improved.
CHOLESTEROL CRYSTAL EMBOLISM
Cholesterol embolization typically occurs after arteriography, cardiac catheterization, vascular surgery, or anticoagulant use in men over age 55 with atherosclerosis.1 It presents with renal failure, abdominal pain, systemic symptoms, or, most commonly (in 88% of cases), skin findings.2
“Blue-toe syndrome,” characterized by tissue ischemia, is seen in 65% of patients.2 Lesions can appear anywhere on the body, but most commonly on the lower extremities. Most are painful due to ischemia. The condition can progress to necrosis.
Patients may have elevated C-reactive protein, hypocomplementemia (39%), and eosinophilia (80%).3,4 The diagnosis is confirmed only with histopathologic findings of intravascular cholesterol crystals, seen as cholesterol clefts.
The differential diagnosis includes contrast nephropathy and infectious endocarditis. However, contrast nephropathy begins to recover within several days and is not accompanied by skin lesions. Repeated blood cultures and echocardiography are useful to rule out infectious endocarditis.
Treatment includes managing cardiovascular risk factors and end-organ ischemia and preventing recurrent embolization. Surgical or endovascular treatment has been shown to be effective in decreasing the rate of further embolism.2 Corticosteroid therapy is assumed to control the secondary inflammation associated with cholesterol crystal embolism.1,5
- Paraskevas KI, Koutsias S, Mikhailidis DP, Giannoukas AD. Cholesterol crystal embolization: a possible complication of peripheral endovascular interventions. J Endovasc Ther 2008; 15:614–625.
- Jucgla A, Moreso F, Muniesa C, Moreno A, Vidaller A. Cholesterol embolism: still an unrecognized entity with a high mortality rate. J Am Acad Dermatol 2006; 55:786–793.
- Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation 2010; 122:631–641.
- Lye WC, Cheah JS, Sinniah R. Renal cholesterol embolic disease. Case report and review of the literature. Am J Nephrol 1993; 13:489–493.
- Nakayama M, Izumaru K, Nagata M, et al. The effect of low-dose corticosteroids on short- and long-term renal outcome in patients with cholesterol crystal embolism. Ren Fail 2011; 33:298–306.
- Paraskevas KI, Koutsias S, Mikhailidis DP, Giannoukas AD. Cholesterol crystal embolization: a possible complication of peripheral endovascular interventions. J Endovasc Ther 2008; 15:614–625.
- Jucgla A, Moreso F, Muniesa C, Moreno A, Vidaller A. Cholesterol embolism: still an unrecognized entity with a high mortality rate. J Am Acad Dermatol 2006; 55:786–793.
- Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation 2010; 122:631–641.
- Lye WC, Cheah JS, Sinniah R. Renal cholesterol embolic disease. Case report and review of the literature. Am J Nephrol 1993; 13:489–493.
- Nakayama M, Izumaru K, Nagata M, et al. The effect of low-dose corticosteroids on short- and long-term renal outcome in patients with cholesterol crystal embolism. Ren Fail 2011; 33:298–306.
A dermatosis of pregnancy
On the eighth day after giving birth to monochorionic twins, a 33-year-old woman presented with pruritic erythematous papules and plaques that started on the striae of the lower abdomen and spread rapidly to the thighs and upper limbs, sparing the umbilical region (Figure 1). Histologic examination of a specimen of the abdominal plaques showed moderate superficial perivascular lymphohistiocytic infiltrate with eosinophils (Figure 2), leading to the diagnosis of polymorphic eruption of pregnancy. Oral cetirizine 10 mg/day and topical methylprednisolone cream brought complete regression of the lesions within 1 month.
DERMATOSES OF PREGNANCY
Polymorphic eruption of pregnancy—also known as pruritic urticarial papules and plaques of pregnancy1—is a specific dermatosis of pregnancy characterized by pruritic urticarial papules on abdominal striae that usually first appear during the latter portion of the third trimester or immediately postpartum. It is more frequent in primiparous women and does not represent a risk to the mother or fetus.2–4
The lesions tend to coalesce into plaques, spreading to the buttocks and proximal thighs. They then become more polymorphic and vesicular, with widespread nonurticated erythema and targetoid and eczematous lesions. Characteristically, the lesions spare the umbilical region. Their location within striae suggests that stretching of abdominal skin may damage the connective tissue, initiating an immune response with subsequent appearance of the eruption.2,4
The diagnosis is mainly clinical. In some cases, skin biopsy can help to confirm the diagnosis. Histologic features are nonspecific and vary with the stage of disease, showing a superficial to mid-dermal perivascular lymphohistiocytic infiltrate with eosinophils. At earlier stages, biopsy results can show a prominent dermal edema. In later stages, epidermal changes such as spongiosis, hyperkeratosis, and parakeratosis can occur.2
As an aid to diagnosis, Table 1 lists clinical differences between various dermatoses of pregnancy.
- Lawley TJ, Hertz KC, Wade TR, Ackerman AB, Katz SI. Pruritic urticarial papules and plaques of pregnancy. JAMA 1979; 241:1696–1699.
- Ambros-Rudolph CM. Dermatoses of pregnancy—clues to diagnosis, fetal risk and therapy. Ann Dermatol 2011; 23:265–275.
- Rudolph C, Shornick J. Pregnancy dermatoses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. London, UK: Saunders; 2012:441–443.
- Rudolph CM, Al-Fares S, Vaughan-Jones SA, Müllegger RR, Kerl H, Black MM. Polymorphic eruption of pregnancy: clinicopathology and potential trigger factors in 181 patients. Br J Dermatol 2006; 154:54–60.
On the eighth day after giving birth to monochorionic twins, a 33-year-old woman presented with pruritic erythematous papules and plaques that started on the striae of the lower abdomen and spread rapidly to the thighs and upper limbs, sparing the umbilical region (Figure 1). Histologic examination of a specimen of the abdominal plaques showed moderate superficial perivascular lymphohistiocytic infiltrate with eosinophils (Figure 2), leading to the diagnosis of polymorphic eruption of pregnancy. Oral cetirizine 10 mg/day and topical methylprednisolone cream brought complete regression of the lesions within 1 month.
DERMATOSES OF PREGNANCY
Polymorphic eruption of pregnancy—also known as pruritic urticarial papules and plaques of pregnancy1—is a specific dermatosis of pregnancy characterized by pruritic urticarial papules on abdominal striae that usually first appear during the latter portion of the third trimester or immediately postpartum. It is more frequent in primiparous women and does not represent a risk to the mother or fetus.2–4
The lesions tend to coalesce into plaques, spreading to the buttocks and proximal thighs. They then become more polymorphic and vesicular, with widespread nonurticated erythema and targetoid and eczematous lesions. Characteristically, the lesions spare the umbilical region. Their location within striae suggests that stretching of abdominal skin may damage the connective tissue, initiating an immune response with subsequent appearance of the eruption.2,4
The diagnosis is mainly clinical. In some cases, skin biopsy can help to confirm the diagnosis. Histologic features are nonspecific and vary with the stage of disease, showing a superficial to mid-dermal perivascular lymphohistiocytic infiltrate with eosinophils. At earlier stages, biopsy results can show a prominent dermal edema. In later stages, epidermal changes such as spongiosis, hyperkeratosis, and parakeratosis can occur.2
As an aid to diagnosis, Table 1 lists clinical differences between various dermatoses of pregnancy.
On the eighth day after giving birth to monochorionic twins, a 33-year-old woman presented with pruritic erythematous papules and plaques that started on the striae of the lower abdomen and spread rapidly to the thighs and upper limbs, sparing the umbilical region (Figure 1). Histologic examination of a specimen of the abdominal plaques showed moderate superficial perivascular lymphohistiocytic infiltrate with eosinophils (Figure 2), leading to the diagnosis of polymorphic eruption of pregnancy. Oral cetirizine 10 mg/day and topical methylprednisolone cream brought complete regression of the lesions within 1 month.
DERMATOSES OF PREGNANCY
Polymorphic eruption of pregnancy—also known as pruritic urticarial papules and plaques of pregnancy1—is a specific dermatosis of pregnancy characterized by pruritic urticarial papules on abdominal striae that usually first appear during the latter portion of the third trimester or immediately postpartum. It is more frequent in primiparous women and does not represent a risk to the mother or fetus.2–4
The lesions tend to coalesce into plaques, spreading to the buttocks and proximal thighs. They then become more polymorphic and vesicular, with widespread nonurticated erythema and targetoid and eczematous lesions. Characteristically, the lesions spare the umbilical region. Their location within striae suggests that stretching of abdominal skin may damage the connective tissue, initiating an immune response with subsequent appearance of the eruption.2,4
The diagnosis is mainly clinical. In some cases, skin biopsy can help to confirm the diagnosis. Histologic features are nonspecific and vary with the stage of disease, showing a superficial to mid-dermal perivascular lymphohistiocytic infiltrate with eosinophils. At earlier stages, biopsy results can show a prominent dermal edema. In later stages, epidermal changes such as spongiosis, hyperkeratosis, and parakeratosis can occur.2
As an aid to diagnosis, Table 1 lists clinical differences between various dermatoses of pregnancy.
- Lawley TJ, Hertz KC, Wade TR, Ackerman AB, Katz SI. Pruritic urticarial papules and plaques of pregnancy. JAMA 1979; 241:1696–1699.
- Ambros-Rudolph CM. Dermatoses of pregnancy—clues to diagnosis, fetal risk and therapy. Ann Dermatol 2011; 23:265–275.
- Rudolph C, Shornick J. Pregnancy dermatoses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. London, UK: Saunders; 2012:441–443.
- Rudolph CM, Al-Fares S, Vaughan-Jones SA, Müllegger RR, Kerl H, Black MM. Polymorphic eruption of pregnancy: clinicopathology and potential trigger factors in 181 patients. Br J Dermatol 2006; 154:54–60.
- Lawley TJ, Hertz KC, Wade TR, Ackerman AB, Katz SI. Pruritic urticarial papules and plaques of pregnancy. JAMA 1979; 241:1696–1699.
- Ambros-Rudolph CM. Dermatoses of pregnancy—clues to diagnosis, fetal risk and therapy. Ann Dermatol 2011; 23:265–275.
- Rudolph C, Shornick J. Pregnancy dermatoses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. London, UK: Saunders; 2012:441–443.
- Rudolph CM, Al-Fares S, Vaughan-Jones SA, Müllegger RR, Kerl H, Black MM. Polymorphic eruption of pregnancy: clinicopathology and potential trigger factors in 181 patients. Br J Dermatol 2006; 154:54–60.